Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/33/02. The contractual start date was in March 2012. The draft report began editorial review in May 2017 and was accepted for publication in January 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Marc Serfaty is a member of the National Institute for Health Research Health Technology Assessment General Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Serfaty et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
The CanTalk trial was devised in response to a specific call from the National Institute for Health Research (NIHR)’s Health Technology Assessment (HTA) programme to provide evidence on the clinical effectiveness and cost-effectiveness of cognitive–behavioural therapy (CBT) for the treatment of depression in patients with advanced cancer. Those who took part had an estimated minimum clinically assessed prognosis of 4 months so as to maximise the potential to retain participants in the trial, deliver the intervention and collect outcome data.
Definition of advanced cancer
For this study, we defined ‘advanced cancer’ as cancer that is considered by oncology experts not to be amenable to cure. People vary in terms of prognosis and the impact of the disease on their physical functioning. Advanced cancer may include metastatic disease for which standard curative therapies have failed, disease that is already widespread at the time the patient presents and that is unlikely to be cured, and cancers with a poor prognosis, such as lung or pancreatic cancers.
Depression and advanced cancer
In this study, we defined ‘depression’ using the Mini-International Neuropsychiatric Interview (MINI). It is distinct from adjustment disorders that might be expected in those with a terminal illness, for whom appropriate sadness is a common response.
Depression is one of the most common mental disorders in people with cancer. 1 Among those with advanced cancer, the prevalence of depression measured using structured clinical interviews ranges from 5% to 45%2–4 and may vary with type of cancer. 5,6 A meta-analysis3 of depression in advanced cancer found the pooled prevalence of clinical depression to be 16.5%. There is a considerable burden on public finances presented by depression; the overall economic cost of depression generally in England was estimated to be £9B in 2000. 7
Depression in people with cancer is associated with several negative health outcomes. It undermines quality of life (QoL) for both patients and their carers,8–10 can reduce adherence to medications and treatment,10 and may prolong episodes of hospitalisation and increase health-care costs. 11 Psychological distress,12 and a diagnosis of depression in particular, predicts elevated mortality among cancer patients,13 as do higher levels of depressive symptoms. Among those with advanced cancer, untreated depression is an independent predictor of early death. 14
Therapy for depression in advanced cancer
There is a scarcity of evidence to guide the management of depressive symptoms in advanced cancer. 15–17 Among cancer patients with mixed prognoses (not limited to advanced cancer), evidence suggests that a nurse-led intervention including education about depression, problem-solving and behavioural activation is effective in treating depression in people with cancer (not advanced). 18,19 However, this intervention was also found to be effective among patients with poor-prognosis (lung) cancer. 20 Guidelines developed for the European Palliative Care Research Collaborative suggest that patients whose cancer is unlikely to be cured and who present with depression should be referred to specialist palliative care. 21 Treatment will include psychosocial support and the use of antidepressants and/or psychosocial therapy. 22
Three Cochrane reviews23–25 have looked at which psychosocial therapies are effective in advanced cancer. One of these, a meta-analysis23 of psychosocial therapies for depression in advanced cancer, cited six studies: four26–29 used supportive psychotherapy, one30 used group CBT and one31 used problem solving. The results of this meta-analysis23 were heterogeneous, and the authors concluded that further, well-designed trials were needed to evaluate if CBT is effective at treating depression in patients with advanced cancer. The other two Cochrane reviews24,25 looked at psychological interventions in women with metastatic breast cancer. One24 identified five well-designed studies. 26,30–33 The first two of these studies30,32 suggested that CBT resulted in a short-term improvement in the Profile Of Mood States (POMS) score; however, the effects were lost at the 6-month follow-up, possibly because group therapy did not sufficiently address the needs of specific individuals. An updated review of psychological interventions in women with metastatic breast cancer and depression25 identified 10 well-designed studies. 26,30–38 Three of these studies26,31,33 showed evidence of a small improvement in the POMS score among patients receiving group CBT, although this finding did not reach statistical significance.
Cognitive–behavioural therapy
Cognitive–behavioural therapy is an empirically effective treatment for major depression. The rationale behind CBT is that depression is associated with negative ways of thinking and unhelpful ways of behaving, and teaching the individual to challenge and modify negative thoughts and unhelpful behaviours helps to improve mood. CBT for treating depression compares favourably with antidepressant treatments and has been shown to be associated with significant therapeutic gains over time. Trials of CBT have demonstrated some evidence of efficacy in treating depression in cancer patients. A recent Cochrane review39 of trials of psychosocial interventions for women with non-metastatic breast cancer indicated that patients receiving CBT were less depressed than patients in control groups. CBT is an approach that may be pertinent to treating a population who may experience significant symptom burden from advanced cancer and palliative treatments, such as nausea and pain. A review40 of treatments for depression in patients with advanced cancer has suggested that CBT approaches are the best evaluated and show the most encouraging results; the studies are summarised here.
Screening and recruitment of advanced cancer patients
The European Association for Palliative Care calls for the screening and treatment of depression in patients with advanced cancer. 41 A number of different methods have been used and recommended to screen for depression in cancer patients. 42 The simplest method asks two simple questions43 that have been shown to exclude depression in non-depressed individuals in cancer and palliative care with a 97% negative predictive value. 44,45 The first two questions of the Patient Health Questionnaire-9 (PHQ-9), known as the Patient Health Questionnaire-2 (PHQ-2), are routinely used to screen for depression in primary care. Such brief screening is acceptable if followed by a clinical interview to confirm the clinical diagnosis of depression. 46 The MINI47 provides a brief and reliable method for diagnosing depression according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) and International Classification of Diseases, Tenth Edition (ICD-10) criteria in cancer patients. 47–55 It has been validated against the Structured Clinical Interview for DSM,56 Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised57 and the Composite International Diagnostic Interview58 and has been widely used in cancer patients.
Recruitment into, and retention of participants in, palliative care studies can be difficult for several reasons. 59,60 First, associated mental and physical exhaustion present major hurdles to recruitment and retention. 61 Second, there may be high rates of attrition due to early death. 61 Third, well-meaning health professionals may be protective towards patients by discouraging them from making the extra effort required to participate in a study. For example, in previous trials of CBT for depression in advanced cancer, 9–19% of patients approached agreed to participate in the research. 30,62,63 Follow-up rates as low as 44% at 10 weeks have been reported in severely ill palliative care patients,62 although higher follow-up rates are possible and some studies have reported 75% follow-up at 3 months. 30,63 Previous research conducted by our research team within the London cancer networks have achieved follow-up rates of at least 65%. 64,65 A number of strategies and recommendations have since been made to increase recruitment of people with cancer in clinical trials. 59,60
In trials conducted in our research group to evaluate CBT in older people with depression,66 CBT in cancer patients67 and advance care planning discussions in cancer,68 follow-up rates of > 85% were recorded. In each of these studies we offered both treatment at home and telephone interview follow-ups, significantly minimising attrition. Telephone CBT has been shown to be both feasible and clinically effective and cost-effective. 69,70 Individualised, rather than group, CBT is likely to facilitate recruitment and minimise attrition. Individual CBT has been found to be preferred by patients with head and neck cancer. 15,71 Benefits of individualised CBT have also been reported in a study of CBT for depression in women with metastatic breast cancer. 63
Rationale for providing therapy for depression in advanced cancer through the NHS
The research outlined suggests that CBT is an effective treatment for depression and may be a promising treatment for depression among advanced cancer patients. However, advanced cancer patients are not routinely screened and treated for depression within the NHS, despite National Institute for Health and Care Excellence (NICE)’s recommendations that this be done. 72 There is currently no manual-based therapy aimed at treating depression in this patient group and there is not currently a sufficient evidence base to determine that CBT is clinically effective and cost-effective in advanced cancer patients.
Our therapy, consistent with the evidence given, consisted of individual as opposed to group CBT. The UK agenda for treating depression is to widen access to psychological treatment through Improving Access to Psychological Therapies (IAPT) centres that operate in primary care and provide a stepped care approach provided by trained mental health practitioners. In order to provide a pragmatic trial of the effectiveness of CBT in treating patients with advanced cancer, we utilised this existing IAPT infrastructure to provide CBT to advanced cancer patients who screen positive for clinical depression.
Evaluation of cognitive–behavioural therapy provided, fidelity to the intervention and its principles
Moncher and Prinz73 proposed guidelines to enhance treatment fidelity, which have been further developed by Lichstein et al. 74 and Bellg et al. 75 They recommend assessing whether or not a psychological treatment is delivered by the therapist and is understood and carried out by the client; these stages have been respectively described as delivery, receipt and enactment. The primary purpose of the CanTalk trial was to evaluate the addition of CBT to treatment as usual (TAU) compared with TAU alone. We adopted a pragmatic approach within the constraints of the resources available by focusing on evaluation of the treatment delivery, and assessing whether or not the patient seemed to have understood the principles of CBT.
The first question, of treatment delivery, was assessed using quantitative methods. The second question, about whether or not the treatment was received, was explored using qualitative semistructured interviews with 10 participants who had received CBT. The background to this is reported in Background to qualitative work and the methodology in Chapter 3, with a summary of the findings in Chapter 5. A fuller report on qualitative experience of therapy will be prepared for publication elsewhere. The third question, relating to enactment and determining whether or not the CBT model prompted behaviour change in the patient, was beyond the aims of the study and, therefore, was not evaluated.
Concerning treatment delivery, two areas are worthy of consideration. The first important consideration is whether or not the CBT is being delivered competently, including whether or not the therapist is sufficiently flexible and able to use a range of techniques to engage the patient. Competent delivery of CBT, evaluated using the Cognitive Therapy Scale (CTS), has been shown to be related to outcome,76 although the relationship between therapist adherence and competence in determining symptom change is less clear cut than originally thought. 77 The second important consideration is whether or not the therapist adheres to the treatment manual described by Moorey, Mannix and Serfaty. The CanTalk treatment manual was intended to be made available for download on the NIHR webpage for this project. However, the manual was based on work published in Moorey78 and it was not possible to obtain permission from the publisher to make this document available. Please contact the corresponding author for more information about the manual. Indeed, we would like to point out that the revised Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting non-pharmacological trials79 have suggested that a description of different components of the intervention is to be provided when evaluating non-pharmacological interventions.
With respect to the first question concerning treatment delivery, we decided that two issues were worthy of consideration. First, an assessment of the therapist’s competence and, second, a measure to confirm that adherence to the CanTalk manual had taken place. In effectiveness studies such as the current one, it can be more difficult to ensure competence and adherence owing to the challenges inherent in delivering the treatment in a routine clinical service. Therapists may be time pressured, there may be differences between services in how CBT is delivered and, crucially in a study of patients with advanced cancer, therapists may not have experience in treating people with physical conditions.
Training of therapist for CanTalk to deliver cognitive–behavioural therapy specific for people with advanced cancer
To maximise competence and adherence to the protocol, mental health therapists with existing CBT skills were selected and trained to apply these skills to people with advanced cancer. This seemed appropriate given that the Five Year Forward View for Mental Health80 aims to expand the IAPT services for people with long-term conditions (LTCs). However, we chose to stipulate that, within IAPT services, therapists would need to be accredited by the professional organisation of the British Association for Behavioural and Cognitive Psychotherapies (BABCP). Accreditation is not in itself sufficient assurance that high-quality treatment is delivered; however, it does improve the likelihood. When adherence is concerned, it needs to be acknowledged that accreditation cannot ensure that therapists deliver the therapy in accordance with a specific protocol for the treatment of depression associated with cancer. However, selecting high-level therapists minimises any distraction caused by needing to learn CBT techniques and allows them to apply their skills to cancer patients. This was done by training all therapists and some of the supervisors on how to assess and deliver the Moorey, Mannix and Serfaty model to advanced cancer patients; more details are provided in Chapter 3, Training Improving Access to Psychological Therapies therapists.
Background to qualitative work
In the CanTalk trial, we explored both patients’ experience of receiving CBT and and therapists’ experience of delivering it, as well as clinicians’ views of referring into CanTalk. As this work was not commissioned or funded by the HTA programme, we have included only a summary in this report. Nevertheless, we would suggest that findings from this qualitative work are likely to guide practice. The full background, methodology, results and discussion are to be reported elsewhere.
Clinicians’ experience of the CanTalk trial
During the course of the study, we became aware that cancer clinicians involved in identifying patients who did not come from a mental health background had a wide range of views about psychological research in people with advanced cancer. We informally detected a range of views about the relevance of psychological research in advanced cancer patients. Some clinicians were strongly supportive and others suggested that CanTalk was outside their remit or that they felt uncomfortable with the psychological nature of the trial. Further examination of the literature suggested that there was a dearth of research in the area. As cancer clinicians play an essential role in facilitating recruitment, we decided to conduct qualitative work to determine the views of clinicians about psychological research in advanced cancer, their experience of referring into the CanTalk trial, any obstacles they may have experienced and how to improve recruitment into similar trials.
Therapists’ views of treating people with advanced cancer
Both the client and therapist play a role in the outcome of CBT,81 with the therapeutic alliance having an impact on the outcome of therapy. 82 When randomised controlled trials (RCTs) have failed to demonstrate an impact on cancer populations,83,84 interpretations of the findings are purely theoretical. Empirical literature suggests that a number of therapist-specific factors play a major role in the outcome of CBT. 85,86 Despite the importance of the therapists’ role in this type of therapy, there is a dearth of qualitative research conducted from a therapist perspective that addresses their personal experiences of delivering therapy. Indeed, most of published research into experiences of CBT has used quantitative methods, and concerns about such methods have been raised,87 with the focus being purely on client perspectives of CBT with no attempts to explain things qualitatively and directly from a therapist perspective.
In order to determine whether or not the costs outweigh the benefits of this particular treatment in this population, we took a holistic approach so that, as well as quantitatively addressing the effectiveness of treatment, we undertook a qualitative assessment (not required in the brief) of treatment from the therapists’ perspectives on (1) their overall views and experiences of delivering CBT, (2) how services and therapy may be improved, (3) specific training requirements and (4) issues in delivering therapy sessions. This was to help inform the optimum use of resources.
Experience of cognitive–behavioural therapy in people with advanced cancer
There is evidence to suggest that CBT is an effective treatment for people with depression and advanced cancer63 but little is known about how patients in this group perceive CBT or about their thoughts and experiences of it.
For cancer patients attending group CBT, their experiences have been positive; patients enjoy the interpersonal and social environment of the group88 and learn skills to challenge and solve problems. 89 Feedback from patients receiving individual CBT has also been encouraging. Omylinska-Thurston and Cooper90 interviewed eight patients with primary cancers who had received a course of psychological therapy within a NHS service for cancer patients and found that participants found talking about their feelings to someone outside their family and problem solving helpful. In a study in Australia,91 cancer patients with metastatic disease commented that CBT allowed them to share their thoughts and feelings with an understanding, caring therapist. Finally, Anderson et al. 92 found that hospice patients reported CBT to be acceptable and effective.
Although qualitative work has focused on cancer patients’ experience of individual CBT, there is little information about the experience of advanced cancer patients. The remit of IAPT services in the UK is to be expanded to cover patients with chronic health conditions,93 including cancer. Despite the London Cancer Alliance94 having some reservations about the ability of IAPT therapists with brief training to treat older people with complex needs, there are few data evaluating how patients should best be managed.
The qualitative work was designed to elucidate what aspects of CBT participants found helpful, their thoughts about their therapist and the impact of CBT on their QoL, and patients’ views about the best way to support them emotionally.
Chapter 2 Objectives
The study aimed to test, within a RCT, the clinical effectiveness and cost-effectiveness of IAPT-delivered manualised individual CBT together with TAU for people with a depressive disorder and advanced cancer compared with TAU alone on depressive symptoms over a 6-month period.
An economic evaluation was undertaken using a cost-effectiveness analysis comparing differences in treatment costs for patients receiving the CBT intervention with quality-adjusted life-years (QALYs) computed from the EuroQol-5 Dimensions (EQ-5D) and societal weights over a 6-month follow-up.
Chapter 3 Methods
Acknowledgement
This chapter contains information previously published by Serfaty et al. 95 © 2016 Serfaty et al. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Trial design and governance
Trial registration
This trial is registered as ISRCTN07622709. The full protocol is available online and published in Trials. 95
Trial design
This is a parallel-group RCT, stratified by antidepressant prescribed at baseline (yes/no), comparing TAU with TAU plus up to 12 sessions of manualised CBT. Allocation ratio for the two trial arms was 1 : 1.
Patient and public involvement
The trial was designed in response to a HTA programme call. Janet St.John-Austen, a user of cancer services, was an active contributor to the design of the project, the preparation of the materials (including the layout and wording for clarity and sensitivity) and ethical considerations. She attended regular steering group meetings and was invaluable in commenting on methods to boost recruitment. She also contributed to the interpretation of the results and write-up.
Ethics approval
A favourable opinion for the conduct of the study was granted by the London–Camberwell St Giles National Research Ethics Service committee, Central London (REC3 reference number 11/LO/0376). This study formed part of the National Cancer Research Network (NCRN) clinical trials portfolio (registration number 10255, ISRCTN07622709). The trial was conducted in compliance with the Declaration of Helsinki. 96 Informed written consent was obtained from all participants.
Changes to protocol
Several amendments were made to the study’s procedures during the course of the study, including changes to streamline recruitment methods and the addition of three qualitative substudies. These amendments are outlined in Table 1.
| Amendment number | Date of amendment approval | Summary of changes |
|---|---|---|
| 1 | 4 December 2012 |
|
| 2 | 28 October 2014 |
|
| 3 | 22 January 2015 |
|
| 4 | 1 July 2015 |
|
| 5 | 14 September 2015 |
|
| 6 | 21 September 2015 |
|
Eligibility criteria for participants
Inclusion criteria
-
People with a diagnosis of cancer not amenable to curative treatment as assessed by their clinician and defined as those receiving palliative radiotherapy or chemotherapy, and those with metastatic disease or subsequent incurable recurrence. The diagnosis was verified by oncologists or general practitioners (GPs).
-
A DSM-IV diagnosis of depressive disorder using the MINI. 47
-
Sufficient understanding of English judged by clinic staff to enable them to engage in CBT.
-
Eligible for treatment in an IAPT centre. Either the patient or their GP had to be located in an appropriate IAPT catchment area.
Exclusion criteria
-
Clinician-estimated survival of < 4 months, verified by the patients’ oncologists or GPs.
-
People at high risk of suicide, established through module C of the MINI.
-
Currently receiving, or having received in the last 2 months, a psychological intervention recommended by NICE aimed at treating depression (e.g. interpersonal psychotherapy, CBT).
-
Suspected alcohol dependence using the Alcohol Use Disorders Identification Test. 97
We did not recruit in areas where the local palliative care service includes routine access to CBT, to avoid contamination of TAU arm by non-study CBT.
Recruitment methods and procedures
Timeline and setting
Recruitment of participants commenced on 1 September 2012. Recruitment finished on 14 December 2015 (the end of the study’s agreed recruitment period). The first participant was recruited into the study on 27 November 2012. The first 6-week follow-up took place on 15 January 2013 and the final 24-week follow-up took place on 19 May 2016.
Participants were identified in four ways: (1) from oncology centres, (2) through GP practices, (3) through the Marie Curie Hospice, Hampstead and (4) through self-referral using leaflets left in GP surgeries and oncology clinics.
Outpatient oncology clinics
National Cancer Research Network support staff working with University College London (UCL) researchers facilitated recruitment from oncology outpatient clinics. Patients’ GP addresses were checked to determine that they were eligible to be referred to an IAPT service before they were approached. UCL researchers collected accurate data about the number of patients screened and the proportion of whom satisfied the entry criteria. However, NCRN support staff or research nurses could not always commit to collecting data on the number of people screened for eligibility. In addition, UCL researchers attempted to collect patients’ reasons for not wishing to take part in the study; however, this information was not always available as the ethics application stipulated that patients did not need to give a reason.
We selected oncology services to represent a variety of patients from the main tumour groups: breast, gastrointestinal (GI), lung, haematology, prostate and other. Patients attending radiotherapy and chemotherapy clinics came from all tumour groups. Oncology centres were identified across England and represented a variety of services, and we recruited from the following hospitals and clinics. Screening data and numbers of participants recruited, when available, are presented in Chapter 5.
Unless otherwise specified, UCL research staff attended in the following clinics.
North London
-
Royal Free Hospital: breast, radiotherapy, lung, urology, lymphoma, melanoma, head and neck, and renal.
-
Whittington Hospital: the clinic research nurses approached, screened and then sent screen-positive details about patients from the following clinics – colorectal, upper GI, lung and breast.
-
University College London Hospital: myeloma, lymphoma, melanoma, gynaecology and radiotherapy, breast, lung, GI and sarcoma.
-
North Middlesex University Hospital: lung, chemotherapy (all tumours), GI and breast. A cancer researcher also screened from breast and lung clinics.
East London
-
Homerton University Hospital: lung clinics.
-
Barts and The London: prostate, GI, lung, breast (Barts) and melanoma (The Royal London Hospital).
South London
-
University Hospital Lewisham: the clinic research nurses approached, screened and then sent screen-positive details about patients from the following clinics – lung, colorectal and breast.
-
Guy’s and St Thomas’ Hospital: myeloma, breast, upper GI, colorectal, hepatopancreatobiliary (HPB), lung (Guy’s) and Neurology (St Thomas’).
-
Princess Royal University Hospital (PRUH): UCL research staff attended the lung clinic. Research nurses at the PRUH approached, screened and sent screen-positive patients to contact from the breast and haematology clinics.
-
King’s College Hospital: breast, myeloma and neurology, and a research nurse screened in the haematology, lung, breast and colorectal clinics.
-
Queen Elizabeth Hospital: the clinic research nurses approached, screened and then sent screen-positive details about patients from the following clinics – breast, lung, GI and colorectal, and urology.
Out-of-London sites
Patients in all of the out-of-London sites were identified, screened, recruited and followed up by research nurses. The following clinics participated in the study:
-
South-west England (Weston Super Mare) – participants were identified from the Weston General Hospital haematology and prostate oncology clinics.
-
Midlands (Coventry and Warwick) – oncology clinics (breast, colorectal, prostrate, GI, neurological, and head and neck).
-
The south of England (Brighton General Hospital) – identified patients for the study in the Midhurst Macmillan multidisciplinary team meeting. Patients identified were then approached in their clinics and screened.
-
The north of England (South Tyneside) – patients were identified from oncology clinics (colorectal and urology).
-
The north-west of England – patients were identified in oncology clinics (gynaecology, HPB, breast, palliative care and chemotherapy) at oncology centre 14.
General practitioner practices
Reeve et al. 98 have used methods to identify those patients from registers of people with advanced metastatic cancer who are receiving only palliative treatment. However, our preliminary examination of general practice data prior to the study suggested that < 10% of all cancer patients are placed on palliative care registers even though 60% of cancer patients may have advanced disease. Identifying patients from palliative care registers approaches a restricted population; for example, only the sickest patients may be placed on such registers and they may have been too ill to respond to the authors’ survey. Indeed, psychological and psychiatric morbidity associated with cancer goes undetected and undertreated in > 80% of people. 99,100 Given these varied data, for the purposes of our study, we assumed a more conservative prevalence rate for major depression in advanced cancer patients with rates of depression of 15% in oncology outpatients and 10% in GP patients.
General practitioner practices were identified from areas where collaborating IAPT/well-being services were located and were approached if they had previously expressed an interest in research and had ≥ 55 patients on their cancer register. We used our established links with the Primary Care Research Network (PCRN) in south London and the North Central London Research Consortium in north London to approach practices that expressed an interest in research. Cancer registers were used rather than palliative care registers as the latter include a significant number of people with non-cancer diagnoses.
Marie Curie Hospice, Hampstead
The Marie Curie Hospice, Hampstead, is purpose built and cares for around 450 registered patients. Hospice clinic staff identified potential participants attending the hospice day-care, outpatients services and the hospice gym and asked them if they could be approached by UCL researchers to see whether or not they were eligible to take part in a research study.
Self-referral
With the permission of clinical leads in each service, posters and leaflets about the study were placed in approved oncology clinics and GP practices.
Set-up procedures
The time taken for sites to become active was an important element of the research process as such procedures may delay the start of recruitment and escalate the costs of research. In Chapter 5, we will report when research and development (R&D) applications were made, when R&D approval was received and when sites became active. In one centre, approval was required by the hospital board prior to submitting an application for R&D approval.
Screening methods
Participants were screened for entry into the study between 1 September 2012 and 14 December 2015.
Oncology centres
Either support staff or UCL researchers screened suitable patients for depression using the PHQ-2,101,102 the first two questions of the PHQ-9,103 a valid screening measure for depression routinely used in general practice.
Patients who scored ≥ 3 points were provided with a pre-screening information pack and asked if they would be willing to be assessed for the study. If they scored ≥ 3 points but did not wish to participate, their permission was sought for their GP or oncology team to be informed that they may be depressed. If they agreed in principle to take part, a researcher undertook a further assessment, using the MINI to establish a DSM-IV diagnosis of depressive disorder. If a DSM-IV diagnosis of depression was confirmed, the patient was given an information pack. The patient was then given at least 48 hours to reflect on whether or not they wished to participate in the study before giving written consent for participation. If a patient consented to take part, the researcher then conducted baseline assessments and passed the participant’s details to an independent trial administrator, who arranged randomisation through the PRImary care and MENTal health (PRIMENT) Clinical Trials Unit (CTU). The study administrator informed the participant by telephone of their group allocation. For those randomised to the treatment arm, the administrator liaised with IAPT to set up the therapeutic sessions. Both administrator and PRIMENT were situated separately from the trial research team to maximise masking of trial arm allocation from the researchers.
General practitioner practices
University College London researchers attended the practice and trained a practice research nurse in the standard operating procedure on how to identify potential suitable participants. Practice administrators identified people on the cancer register and consulted the GP on whether or not the patient had advanced cancer as defined in the protocol. By mutual agreement according to availability at the practice, either the practice nurse or PCRN staff contacted the patient by telephone or face to face in practices to explore whether or not they were willing to answer the two PHQ-9 screening questions for depression. The procedure was then the same as for the oncology centres but, in this case, the practice nurses/PCRN nurses collected follow-up data at the relevant time points.
Marie Curie Hospice, Hampstead
University College London researchers consulted clinic records and checked the eligibility criteria for those identified as suitable by hospice staff. They then approached potential participants for screening as outlined above. Those suitable were then told about the trial and consented once they had had 48 hours to consider participation.
Self-referral
The leaflet contained the PHQ-2 for patients to conduct a quick assessment of their mood themselves, suggesting to people with a score of ≥ 3 points that they may have depression and that they should either (1) approach the clinical team within the site or (2) contact the study team directly using the reply slip attached to the leaflet. The process of recruitment was the same as that previously outlined.
Screening data
In instances in which the UCL researchers undertook the screening, they were able to collect data about the number of patients screened – the proportion of whom satisfied entry criteria – and, if patients declined to take part, the reasons (if given). It is important to highlight that the ethical principle that patients are free to decline to take part or withdraw from a study without giving a reason was included in the project’s ethics approval submission. When Comprehensive Local Research Networks (CLRNs) were undertaking identification, selecting and screening of patients, comprehensive data for numbers of people screened and reasons for declining to take part in research were not always available as CLRNs indicated that they did not have the resources to collect these data.
Randomisation
Participants were randomised to one of two conditions, (1) TAU or (2) TAU plus CBT, with an equal allocation to each treatment arm. Randomisation occurred after patients had been assessed to meet the eligibility criteria and had consented to participate and baseline measures had been collected. Once a participant had been randomised, the trial administrator called the participant to inform them of their group allocation. For participants randomised to the group receiving CBT, the trial administrator then sent an e-mail to a contact in the relevant treatment centre providing details of the participant.
Randomisation was conducted by the trial administrator using Sealed Envelope (Sealed Envelope Ltd, London, UK), an automated online randomisation system supplied by the PRIMENT CRU (a UK Clinical Research Collaboration registered CTU). This system was pre-populated with a randomisation list using a randomisation algorithm developed by the trial statisticians. The randomisation was tested using a test version of the Sealed Envelope randomisation system. Randomisation was conducted using permuted blocks with block sizes of four or six, stratified for antidepressant use (yes or no). Antidepressants are a predictor of outcome. 104 In cancer, tricyclic antidepressants (TCAs) may be preferentially prescribed over selective serotonin reuptake inhibitors because they have fewer relevant side effects, such as nausea, and may be used for both mood and, in lower doses, for pain. Indeed, even low doses of TCAs may be effective. 105 Therefore, we stratified our randomisation according to whether or not participants were prescribed an antidepressant, irrespective of dose. We did not have the resources to measure compliance with medication through pill counts or by taking blood levels, but we did ask participants what they were taking, estimated their antidepressant doses and converted them to equivalent doses of fluoxetine using methods previously described by Hayasaka et al. 106 to assess whether or not the doses of prescribed antidepressants were similar in both arms of the trial at randomisation (see Chapter 4, Analysis plan).
Masking
Once a participant had been randomised, the trial administrator unblinded that participant by clicking on an ‘unblind’ link on the Sealed Envelope system that generated an e-mail to themselves with details of the group allocation.
It is not possible for patients or therapists to remain blind to the treatment group. The trial manager was unblinded only if needed, for example if there was a problem referring a patient to therapy. The trial team worked at UCL and was based in a different location to the therapy teams that conducted the trial intervention.
Assessment of blindness
The UCL researchers who were blinded were asked to guess group allocation (TAU alone, TAU plus CBT or do not know) at 3 months (post intervention) and 6 months (follow-up). Although the PCRN assessors were blinded, they requested that any additional data collection was kept to a minimum and, therefore, they did not make an assessment of blindness.
Unmasking for those conducting the analysis did not occur until databases were closed.
Intervention
Treatment as usual
All participants received TAU from all clinicians involved in their care. This consisted of routine support, such as appointments with GPs, clinical nurse specialists, oncologists and palliative care clinicians. Participants’ physical health and medication were reviewed and treatment was modified according to symptoms, such as pain. Psychotropic medication was allowed to be prescribed as necessary, by either the GP or the oncologist. In line with NICE’s guidance,107 specific psychological support should have been available for those who presented with psychological needs at any time, and study participants were not exempt from receiving external psychological support. We discouraged specific psychological interventions aimed at treating symptoms of depression (e.g. CBT or interpersonal psychotherapy), but, ultimately, we could not interfere with usual care for ethical reasons. We recorded the numbers of participants receiving any psychological therapy during the trial, although we predicted that the numbers were likely to be small. 66 We did not stipulate post randomisation that antidepressant medication could not be used or that the dose should be fixed. Withholding a recognised treatment for depression would be unethical and would not reflect TAU.
Cognitive–behavioural therapy (in addition to treatment as usual)
The CBT was delivered through IAPT93 and well-being centres. IAPT/well-being centres train, supervise and supply therapists to treat people in primary care with mental health problems. For the purpose of the study, only step 3 and 4 (high-intensity) therapists who had experience of CBT were used. They were given 1 day’s training by the CanTalk team (SM, MS and KM) so that their existing CBT skills could be adapted to use a specially developed treatment manual for people with advanced cancer. The manual detailed modifications in the structure of therapy and its content; in particular, it took into account physical health problems, existential issues and communication with loved ones.
Structure of cognitive–behavioural therapy sessions
The National Institute for Health and Care Excellence recommends 16–20 sessions of CBT to treat severe depression in secondary care. Experience shows that, in primary care, considerably fewer sessions are taken up. People with advanced cancer may have difficulty coping with longer therapy as their health may be deteriorating. Our intervention consisted of up to 12 sessions of individual CBT, which was delivered either face to face or over the telephone over 3 months. Although telephone CBT was not delivered as a substitute for face-to-face treatment, it was used to facilitate engagement and minimise dropout. Twice-weekly sessions could be offered for the first 2 weeks, weekly sessions for weeks 3–9 and then two sessions within weeks 10–12. The timing of sessions was flexible and pragmatic to fit in with the existing commitments of the IAPT service and with patient availability, taking their other medical clinics and treatments into account.
In order to facilitate engagement for those who may not be able to attend sessions face to face, telephone CBT was offered if at least three sessions of face-to-face therapy had already been received. Telephone CBT was already being used by IAPT therapists. Stirling Moorey, Marc Serfaty and Kathryn Mannix taught CBT therapists how to adapt their CBT techniques for telephone-based therapy using similar methods to Tutty et al. 108
Content of cognitive–behavioural therapy sessions, guided by a written manual
Improving Access to Psychological Therapies guidelines recommend that patients with moderate to severe depression and complex needs receive high-intensity (step 3) work. This is consistent with the level 4 psychological interventions recommended by NICE72 for people with cancer. The CBT intervention used a flexible approach, adapted for use with people with advanced illness who face a poor prognosis. In the manual, developed by Stirling Moorey, Kathryn Mannix and Marc Serfaty, therapists adapted their work to patients with advanced cancer. The key shift was to identify whether thinking and behaviour are ‘helpful’ or ‘unhelpful’ rather than solely a reality-testing approach, thereby enabling patients to adopt adaptive strategies to cope with adverse and often unpredictable health circumstances.
The intervention broadly covered the following:
-
Session 1 – an assessment of problems, psychoeducation about depressive disorder and an introduction to the cognitive model was undertaken. A simple cross-sectional formulation of current emotional distress was established, and the triggers to emotional distress and how to manage them were identified, with steps towards one of the patient’s goals. A list of enjoyable activities was instigated, and unhelpful thinking styles were identified, using specific examples from recent events.
-
Session 2 – aimed to help patients develop an understanding of their problems within a cognitive behavioural framework and began the process of therapy using cross-sectional formulation. This included a discussion of past strengths and coping abilities. Behavioural activation techniques were used within the constraints of the person’s physical illness.
-
Session 3 – consisted of a review of the formulation, identifying any new insights/changes. Guided discovery, through a deeper discussion of the patient’s thoughts/beliefs around their illness and their resilience, was used to help them apply their resilience under current circumstances. A start was made on identifying ‘helpful’ versus ‘unhelpful’ thinking and behaviours.
-
Sessions 4 and 5 – helped the patient to apply new learning to current difficulties, recent successful experiences were reformulated and helpful changes were identified. Guided discovery was used to help the person notice successful experiences and build resilience. The triggers to emotional distress and strategies for responding were explored. These included thought-testing and an in-session experiment of allowing intrusive thoughts to pass.
-
Sessions 6 and 7 – focused on thought-testing and finding ‘helpful’ alternative thoughts. This was done within sessions, supplemented by homework completed by the patient between sessions, when logs of patients’ mood, the associated thoughts and behaviours were reviewed. Thoughts and behaviours could then be challenged and more helpful alternatives considered. Examples of recent success experiences were added to successes lists and exploration of these for their associated ‘helpful’ thoughts.
-
Session 8 – focused on problem solving and worry time. Confirmation was made that the thought-testing/’helpful’ thoughts concept had been understood. Examples of realistic concerns were identified to generate a ‘problems to address’ list. An example of one problem was taken to illustrate the problem-solving approach. The concept of ‘worry time’ was introduced to reduce rumination.
-
Session 9 – consolidated CBT strategies and reviewing and prioritising a problem list. Planning on how to tackle harder problems was undertaken, identifying unhelpful thoughts and behaviours with consideration of the pros and cons of potential solutions and the commitment to this process. The use of worry management strategies was also reviewed.
-
Session 10 – consisted of a review of the person’s perceived progress, including successes and difficulties.
-
Session 11 – consisted of relapse prevention. This included reviewing presenting difficulties, the progress and personal achievement made, personal resilience and successes and the development of a relapse prevention checklist.
-
Session 12 – consisted of future planning, reviewing a relapse-prevention checklist, making concrete plans for action if emotional distress recurred or unhelpful behaviours/thinking returned.
In addition, therapists were taught about materials contained in three sections in the manual, so that, if relevant, these may be addressed with participants. These sections covered (1) existential issues in addition to eliciting and discussing the patient’s fears about death, including their mode of dying, fears about the effect of their death on others and fears about what happens after death; (2) applying CBT when health is poor, which included running shorter sessions, and discussing how to deal with fatigue and coping with loss of function; and (3) facilitating communication with a partner, families and carers. This provided CBT therapists with confidence in adapting their skills to people with advanced cancer.
Please contact the corresponding author for more information about the CanTalk treatment manual.
Improving Access to Psychological Therapies involvement
Identifying and contacting Improving Access to Psychological Therapies centres
The following methods were used to identify IAPT/well-being collaborating centres where the intervention could take place. IAPT/well-being leads were approached for selected boroughs across London for the pilot stage and further areas within, and outside, London for the definitive trial. Areas were selected for several reasons: first, where study team personnel had existing links with oncology teams, hospices and GP centres for recruitment to be feasible; second, where study personnel had existing links with IAPT/well-being services and IAPT/well-being leads expressed an interest in research; third, IAPT/well-being services were approached if they had a mature service running for ≥ 2 years and, therefore, would be more likely to be able to participate in the delivery of specialist CBT; and fourth, we were also approached by a number of services who identified the CanTalk trial through trust research co-ordinators.
Our approaches were initially made by telephone to IAPT/well-being leads, identified from websites and by personal contact. These were followed by an e-mail summarising the project with the advantages to IAPT/well-being services highlighted as follows:
-
free training for IAPT/well-being high-level CBT practitioners
-
improvement in IAPT/well-being therapists’ CBT skills
-
developing the delivery of IAPT to patients with long-term physical health conditions, which is consistent with national aims
-
effective publicity for IAPT/well-being so that services are properly funded
-
demonstration that, if effective, CBT, delivered through IAPT, would represent a good model of care for LTCs.
Training Improving Access to Psychological Therapies therapists
The IAPT/well-being services were asked to supply at least two high-intensity IAPT/well-being therapists for manualised training. IAPT/well-being supervisors were also encouraged to attend.
Training was delivered by Stirling Moorey, Marc Serfaty and Kathryn Mannix. Therapists were supplied with a therapists’ manual, presented with Microsoft PowerPoint® 2010 (Microsoft Corporation, Redmond, WA, USA) slides giving an overview of sessions in the manual as well as videos and role plays and were asked to participate in practising skills using a variety of scenarios. How they could access the resources online was also explained. Finally, they were taken through processes required for, and associated with, the research.
Training took place at the following sites: UCL for London, the south of England, the Midlands and the west of England (SM and MS); Chester for the North West (MS and KM); and Newcastle upon Tyne for the North East (KM).
Training took place in 27 IAPT/well-being services. Of these, 25 participated in the study. Chester and Newcastle upon Tyne did not participate as we could not set up recruitment centres in these areas. Details of the 25 services engaged in the study, including the number of therapists (124 in total) represented from each service and the IAPT/well-being leads, are presented below.
The number of people trained in applying CBT skills to patients with cancer came from the following services.
-
London:
-
North London – 13 people from five IAPT services from Barnet, Camden and Islington, Enfield and Haringey.
-
East London – 19 people from nine IAPT services from City and Hackney, St Bartholomew’s and The London (Hospital), Tower Hamlets, Redbridge, Homerton, Newham, Waltham Forrest, Havering, Barking and Dagenham.
-
South London – 30 people from seven IAPT services from Lambeth, Lewisham, Bromley, Bexley, Croydon, Southwark and Greenwich.
-
-
Outside London:
-
the south of England – four people from two IAPT services (Sussex and Brighton and Hove).
-
the west of England – two people from one IAPT service (Avon and Wiltshire).
-
Midlands – four people from Coventry and Warwick IAPT services.
-
North East – 11 people from one IAPT service (South Tyneside).
-
North West – five people from one IAPT service (Stockport).
-
Evaluation of training
Training was evaluated using a feedback questionnaire with a Likert scale asking about specific aspects of training; boxes enabled additional free-text comments. These findings are presented in Chapter 5.
Location of therapy
Patients were offered the opportunity for face-to-face therapy in their local IAPT/well-being centre. In some cases, this was at a local GP practice, depending on the set-up of the service. We did consider delivering therapy in the patients’ own homes but were constrained by limits on safe lone working and, thus, therapy could not be delivered in this way through the IAPT/well-being service. When patients were too frail or reluctant to continue to attend therapy in an IAPT/well-being centre, we allowed for the delivery of telephone CBT, providing the patient had seen the therapist at least three times and it was deemed by the therapist to be consistent with safe working practices. We asked therapists to record whether therapy was delivered face to face or by telephone.
Supervision of Improving Access to Psychological Therapies therapists
Supervision structures are well set up within IAPT services. Routine supervision of therapy in IAPT takes place at least monthly but is flexible within this period. However, in this trial, we recommended flexibility, so that if any immediate issues needed attention, the therapists could consult their IAPT supervisors. Stirling Moorey, Kathryn Mannix and Marc Serfaty were also available, by e-mail or by telephone, to discuss any difficulties related to interventions in people with cancer. The CanTalk trial supervisors were also accessible to the local IAPT supervisors by e-mail to answer any additional queries that arose between supervision sessions. Flexibility in the ‘practice stage’ was used to learn about how clarification about the CBT intervention might be required. Audio recordings of all therapy sessions are routinely made in IAPT and these were also made available for the independent assessment of quality.
Delivery of cognitive–behavioural therapy
We decided that CanTalk should be a pragmatic approach, which aimed to determine whether or not our target population would benefit from CBT delivered through IAPT. If the CanTalk approach were to be rolled out across the UK, we decided that, for the findings to be generalisable, therapists should be managed in the usual way.
We kept a record of which IAPT services and which therapist within the service delivered the intervention, and of how many sessions were delivered to each patient.
Quantitative assessment of delivery of cognitive–behavioural therapy
The quality of therapy was assessed using mixed methods. Quantitatively, the Cognitive Therapy Scale – Revised (CTS-R) was used to assess the delivery of CBT and adherence to the therapy manual was assessed using an adherence checklist (described in Measure of adherence to cognitive–behavioural therapy in cancer manual). Qualitatively, the patients’ experience of therapy and the therapists’ experience of delivering CBT to people with advanced cancer with depression was explored through interview. Although this work was not commissioned by the HTA programme, we felt that this would provide a useful addition to this report and the findings are summarised accordingly in Chapter 5.
Measure to assess competence of delivery of cognitive–behavioural therapy
A scale for measuring therapist competence in cognitive therapy, based on the original CTS,109 is the 12-item CTS-R. 110
This revised version improves on the original CTS by eliminating the overlap between items, improves on the scaling system and defines items more clearly. In this trial, we assessed competence by having an independent rater listen to audio recordings of therapy sessions using the CTS-R.
Scoring
The CTS-R consists of 12 items: (1) agenda setting and adherence, (2) feedback, (3) collaboration, (4) pacing and efficient use of time, (5) interpersonal effectiveness, (6) eliciting of appropriate emotional expression, (7) eliciting key cognitions, (8) eliciting and planning behaviours, (9) guided discovery, (10) conceptual integration, (11) application of change methods and (12) homework setting. Although the CTS-R is more specific than the original CTS, in that therapist competence is defined very precisely, the CTS-R has poorer inter-rater reliability. In the CTS-R, each item is rated from 0 to 6 on a visual analogue scale (VAS) encompassing the following competencies: incompetent, novice, advanced beginner, competent, proficient and expert. The total score ranges from 0 to 72 points, with a minimum score of 36 points taken as competency for the delivery of therapy. We would expect therapists to achieve a minimum score of 36 points, which is the standard criterion for competence within IAPT services.
How the quantitative measures of delivery of therapy were collected
Assessment of the quality of therapy to the manualised treatment was assessed as follows:
-
Quality of delivery of CBT – a total of 194 therapy sessions were audio recorded. In accordance with our plan to sample 1 in 10 of the therapy recordings, we selected 55 out of 543 audio recordings to rate the therapy. As the sample was skewed, with the mode being one session, we purposefully sampled recordings to obtain a balance of therapy sessions from the different phases of the intervention (early, sessions 1–4; middle, sessions 5–8; or late, sessions 9–12). Tapes were allocated a random identification number, but it enabled identification of the therapy session number (1–12) so that a range of phases of therapy could be assessed.
-
Therapists were asked to upload recordings of therapy, when possible, onto a secure database using encryption software. Local health-care trust policy and therapists’ experience of information technology (IT) systems may limit this process. Recordings of therapy were rated by an accredited member of the BABCP using the updated version of the CTS109 (the CTS-R110), which is a reliable measure of the delivery of CBT. 76
Measure of adherence to cognitive–behavioural therapy in cancer manual
In this context, we defined therapist adherence as the extent to which the therapist adhered to the essential ingredients described within the treatment manual developed for use in the trial by Marc Serfaty, Stirling Moorey and Kathryn Mannix.
Detailed information about the content of the intervention was collected using a ‘Therapy Components Checklist’ (TCC) (Table 2). The role of the TCC was threefold. First, it provided the therapist with a guide to which elements were delivered (or not) from the manual. Second, it provided information about which elements had been delivered by therapists during the course of the trial. Third, it enabled us to evaluate whether a therapist’s self-report of what they said they did was consistent with what they actually did, judged by an independent rater.
| Component | Session the component was covered | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| General procedures | ||||||||||||
| Initial assessment | ||||||||||||
| Describe Beck’s model and concept of CBT | ||||||||||||
| Agree goals of therapy | ||||||||||||
| Present a shared formulation | ||||||||||||
| Goal-setting | ||||||||||||
| Review of shared formulation | ||||||||||||
| Review of success list | ||||||||||||
| Relapse prevention/future planning | ||||||||||||
| Behavioural techniques | ||||||||||||
| Relaxation training | ||||||||||||
| Breathing space | ||||||||||||
| Activity schedule | ||||||||||||
| Pleasure experiences sheet | ||||||||||||
| Cognitive techniques | ||||||||||||
| Refocusing techniques | ||||||||||||
| Mindfulness | ||||||||||||
| Four-step process for resilience and coping | ||||||||||||
| Coping map | ||||||||||||
| List of strengths and resources | ||||||||||||
| Reattribution | ||||||||||||
| Decatastrophising | ||||||||||||
| Advantages/disadvantages | ||||||||||||
| Success list | ||||||||||||
| Thoughts diary | ||||||||||||
| Personal rule (pros/cons) | ||||||||||||
| Managing worry (worry tree handout) | ||||||||||||
| Blueprint for coping | ||||||||||||
| Cognitive–behavioural techniques | ||||||||||||
| Guided discovery | ||||||||||||
| Pleasure prediction sheet | ||||||||||||
| Pleasure experiences sheet | ||||||||||||
| Negative triad/negative automatic thoughts | ||||||||||||
| Applying resilience | ||||||||||||
| Thinking traps handout | ||||||||||||
| Reality testing | ||||||||||||
| Searching for alternatives | ||||||||||||
| ABC form | ||||||||||||
| Specific cancer topics | ||||||||||||
| Impact of physical illness | ||||||||||||
| Beliefs and expectations about illness | ||||||||||||
| Plans and hopes for care as disease advances | ||||||||||||
| Relationship between emotions and physical symptoms | ||||||||||||
| Concerns about current and future ability to cope | ||||||||||||
| Concerns about loss of control | ||||||||||||
| Concerns about accepting help | ||||||||||||
| Concerns about dying (mode/afterwards/life expectancy) | ||||||||||||
| Impact of disease and mood on behaviour | ||||||||||||
| Impact of disease/death on loved ones | ||||||||||||
| Discussion of ‘the meaning’ of the illness | ||||||||||||
| Acceptance of unfinished business | ||||||||||||
The general procedures and main interventions for successful treatment are as follows:
-
general procedures (eight elements)
-
behavioural techniques (four elements)
-
cognitive techniques (13 elements)
-
cognitive–behavioural techniques (nine elements)
-
specific cancer topics (12 elements).
We also collected information from the therapist about what they thought were the three most important aspects of the therapy and why, whether or not they felt that there was anything missing from therapy and whether or not they had any general comments.
How adherence was assessed
Adherence to the therapist manual was undertaken using two methods:
-
Self-report by therapists – therapists were asked to upload the TCC (see Table 2), which they completed at the end of each therapy session. The therapy components were generated by the trial team (MS, SM, KM and MK) to help identify the main elements thought to be important in this intervention. A checklist was piloted in a previous study,66 and, in the present trial, was adapted for people with cancer.
-
Independent ratings of adherence – the assessor described in point 1 (above) was also asked to complete the TCC so that it could be compared with the therapists’ reports of what their intervention comprised.
Analysis
Cognitive Therapy Scale – Revised
For normally distributed data, we have presented the means and standard deviations (SDs) of the CTS-R. We chose a threshold of ≥ 36 points for competence on the 12-item CTS-R and also indicated the proportion of therapists who fall under this score. A score of 36 points is also the accepted pass mark for the postgraduate diploma in CBT that IAPT trainees take.
We have also presented the means and SDs for CTS-R scores for different phases of therapy (early, sessions 1–4; middle, sessions 5–8; and late, sessions 9–12).
Therapy Components Checklist
-
Elements of the adherence checklist that have been covered are presented as a proportion of the total score for each of the five subsets: (1) general procedures, (2) behavioural techniques, (3) cognitive techniques, (4) cognitive–behavioural techniques and (5) specific cancer topics.
We also provided a description of the various elements covered for all the TCCs submitted by therapists. An independent assessor conducted an objective rating of 1 in 10 therapy sessions to see what elements were delivered and we compared these with the therapists’ self-reports.
For each component of therapy, we calculated agreement between the independent assessor and the therapist’s own assessment of whether or not the component was covered, providing the four possible outcomes: (1) both rate that the component was delivered; (2) both rate that the component was not delivered; (3) the therapist, but not observer, rated the component as being delivered; and (4) the observer, but not therapist, rated the component as being delivered. Using these possible agreement outcomes, we then calculated the prevalence-adjusted and bias-adjusted kappa111 (PABAK) for each component.
Therapist supervision and workload
We recommended that two IAPT therapists would be required from each primary care trust to each treat approximately 4.5 participants per year. We have experience in delivering a training programme for palliative care nurses in CBT skills,112 which improves confidence in managing patients. 113 Relevant sections of this have been adapted to provide CBT therapists with confidence in adapting their skills to people with advanced cancer.
Ensuring safety
This study was not a drug trial and our main concern was centred around risk of self-harm or suicide. UCL researchers and the research nurses screening in oncology centres were given training by Marc Serfaty covering the serious adverse events (SAEs) protocol (see Appendix 1), detailing what action should be taken if patients assessed were considered to be high risk, even though they would be excluded from entry into the study. These appropriate governance procedures and good clinical practice applied to all patients seen, to ensure safety at all times. For those who were detected as being at high risk at follow-up, similar procedures were actioned.
Examples of good practice and the difficulties associated with risk were discussed, including ensuring that there was an opportunity for individual supervision on request with a senior member of the team who was always available. This culture of transparency ensures that researchers are able to always raise concerns about their participants. Time was also taken within supervision to highlight the importance of behaving ethically and safely in all aspects of clinical work.
We considered the possibility that patients being seen by IAPT/well-being therapists may be detected as being at increased risk. However, practitioners are bound by their own governance procedures in their assessment of risk and are very familiar with managing suicidal patients. Because of the number of IAPT/well-being centres collaborating in this study and minor variations in the procedures on how to manage risk, we stipulated that IAPT/well-being therapists adopt their own governance procedures, as this is what would happen if the intervention were to be rolled out across the country, but that they also complete and return a SAE form. The chief investigator, Marc Serfaty, would then contact the IAPT/well-being team within 2 working days to discuss the case and consider whether or not the participant should be withdrawn from the study.
Research staff support
It is recognised that working with patients with cancer and at the end of life could be distressing for field researchers, particularly when it is likely that staff may have had direct personal experience of cancer. Therefore, several systems were put in place to ensure the pastoral care of staff. First, scheduled weekly meetings took place with the chief investigator and the team to discuss particularly distressing cases, and research staff were also offered the opportunity to discuss any cases individually with the chief investigator. Second, there was good cohesiveness among team members for peer support, enabling sharing of difficult situations. Third, there was the opportunity for UCL researchers to meet monthly with Liz Cort, an experienced palliative care nurse, at oncology centre 13. Liz Cort is not only independent from UCL but is also very experienced in issues frequently faced with the often distressing day-to-day lives of people with cancer. An important dimension of this support was to help researchers develop self-reflexivity, exploring and making sense of their own responses to the people and situations that they were assessing. The combination of group and individual supervision meant that researchers felt that their experiences were validated by their shared experiences.
Cancer research nurses are very experienced in conducting research in this client group. Their support was provided in the usual way, through group and individual supervision within different services.
Qualitative methods
Embedded qualitative study
In this report, we have included information about a qualitative substudy exploring clinicians’ views on referring into the CanTalk trial, which we conducted independently. However, as qualitative research was not commissioned by the HTA programme, only a brief summary of the methodology and results has been provided (see relevant sections of this report):
-
an evaluation of clinicians’ experience of referring into the CanTalk trial evaluated through qualitative interviews (described in Clinicians’ views of the CanTalk trial)
-
an evaluation of the patient experience of therapy was evaluated through qualitative interviews (described in Patient interviews to determine experience of cognitive–behavioural therapy)
-
an evaluation of the therapists’ experience of delivering CBT to advanced cancer patients using semistructured interviews (described in Therapists’ views of delivering cognitive–behavioural therapy to people with advanced cancer).
General interview procedures for qualitative data capture
Semistructured, one-to-one interviews using topic guides (presented in Appendices 2–4) ensured that all participants within these three groups were asked the same questions to minimise researcher effects. The topic guide initially consisted of a number of open-ended, non-leading questions. We aimed to cover the six types of questions described by Patton114 for qualitative interviews. These include experience/behaviour questions, opinion/belief questions, feeling questions, knowledge questions, sensory questions and background/demographic questions.
The interview began with introductory questions in order to help establish a good relationship with interviewees to encourage rapport. The topic guide followed a logical sequence with topics being grouped together with corresponding categories. The questions were formulated to avoid influencing the participant. We attached probes to each question to aid rapport building. The topic guide ended with a closing question to encourage participants to discuss topics or issues that were not mentioned previously. Individual interviews were audio recorded and transcribed verbatim. Flexibility allowed the interviewer or interviewee to divert from questions to pursue other areas where necessary. 115
The interviews took place where there were minimum distractions for the participant and for the researcher to aid the dialogue. All interviews were audio recorded for transcription at a later date. We aimed to recruit up to 20 interviewees for each of the three areas of interest from the CanTalk trial to allow a sufficient number to generate themes from the data until no new themes emerged from the interviews.
Clinicians’ views of the CanTalk trial
Any clinician who had been involved in referring patients to the CanTalk trial was considered eligible for inclusion. The study aimed to obtain a good cross-section of participants including consultants, registrars, nurses and radiotherapists. The study also aimed to include the views of 15 clinicians from each of the referring sites.
Clinicians were asked to describe their role in oncology clinics, their previous involvement in research, their views on non-drug trials, the CanTalk trial, any patient feedback and their views about future psychological studies. A detailed topic guide is provided in Appendix 2.
Therapists’ views of delivering cognitive–behavioural therapy to people with advanced cancer
We used a purposive sampling frame and contacted all therapists who delivered CBT as part of the CanTalk trial. We aimed to recruit up to 20 IAPT therapists for one-to-one, face-to-face, semistructured interviews in a quiet setting agreed by both the researcher and therapist.
The therapists were asked to describe their role and how it applied to the CanTalk trial, what they knew about the patient, their views on working with patients with advanced cancer and, with CanTalk patients in particular, any components of CBT that were or were not useful, and any other important views. A detailed topic guide is given in Appendix 3.
Patient interviews to determine experience of cognitive–behavioural therapy
All participants recruited from London sites who had reached the 24-week follow-up who had not died or withdrawn from the study and who had received at least one session of CBT were approached by a member of their clinical team via post and asked for permission to contact them. Those agreeable to being contacted or who had already provided consent to contact were sent an invitation letter offering them the chance to provide feedback about their experiences of CBT.
Interviews took place in the participants’ homes or within the department where the research was taking place. The interview schedule was as follows: participants were asked to describe what therapy they had, what knowledge they had of CBT, what they found helpful or not, their views of the therapist, how CBT had an impact on their life and their views about CBT for low mood in cancer patients. The precise topic guide is provided in Appendix 4.
Qualitative analysis
All interviews were audio recorded and transcribed. Thematic analysis was used to analyse data using NVivo version 11 software (QSR International, Warrington, UK) by two researchers who were ‘immersed’ in the data before proceeding with data analysis, as this can strengthen data analysis. 116
Researchers used a matrix-based analytical method of framework analysis to analyse the data. 117 The method of ‘complete line-by-line coding’ was used to broadly identify any code or theme that emerged without restricting analyses to detecting particular themes. 116 Codes were then grouped into broader themes. For the purposes of triangulation, researchers compared their findings with each other once they had identified broader themes. Any discrepancies were discussed until agreement was reached.
Chapter 4 Outcomes
Acknowledgement
This chapter contains information previously published by Serfaty et al. 95 © 2016 Serfaty et al. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Assessments
Screening measures
Patient Health Questionnaire-2
The PHQ-2101 consists of the first two questions of the PHQ-9,103 a valid screening measure of depression that has also been used in cancer services.
Mini-International Neuropsychiatric Interview
The MINI is a short structured diagnostic interview that takes 15 minutes to complete. It was developed jointly by psychiatrists and clinicians in the USA and Europe for DSM-IV and ICD-10 psychiatric disorders47 and has been widely used in cancer patients.
The UCL researchers gave the assessors 1 day’s training on how to use the MINI. We did not have the resources to make assessments of inter-rater reliability.
Demographic information
A baseline assessment form was used to ensure that entry criteria were satisfied and that the patient had consented. It also included demographic information on sex, date of birth, marital status, ethnicity, employment status, highest level of education, previous history of depression, cancer diagnosis and whether the tumour was defined by clinicians as primary or secondary, along with date of diagnosis of the primary or secondary tumour type. We also noted other treatments and prescribed medication, dose and frequency.
Outcome measures
Quantitative
We collected a number of measures at the different time points summarised (see Table 3).
Primary outcome
Beck Depression Inventory, version 2
The Beck Depression Inventory, version 2 (BDI-II)118 is a 21-item self-report measure, with a maximum score of 63 points indicating severe depressive symptoms. It contains few items measuring affective-somatic symptoms, with 15 of the 21 items assessing negative cognitions, which are a target of cognitive interventions. The psychometric properties of the BDI-II are similar to the Beck Depression Inventory119 (BDI), the most widely used self-report instrument for depressive symptoms, which has also been used in trials of psychotherapy for people with advanced cancer. 63,120,121 The BDI-II also has a number of cognitive elements that are particularly useful for measuring change with CBT.
Secondary outcomes
Patient Health Questionnaire-9
The PHQ-9103 screens for depression. It is used in primary care settings including IAPT services. It has been validated as a measure of depression in primary care,102,122 and can be administered over the telephone. 123
EuroQol-5 Dimensions, five-level version
The EuroQol-5 Dimensions, five-level version (EQ-5D-5L),124,125 is a generic utility measure of QoL consisting of five domains and a VAS intended for use in the cost-effectiveness analysis.
Satisfaction with care
Collected using a VAS (scored 0–10 towards higher satisfaction). This method has been used in previous psychotherapy research. 64
Eastern Cooperative Oncology Group-Performance Status
The Eastern Cooperative Oncology Group-Performance Status (ECOG-PS)126 is a scale measuring physical functioning on five levels: 0 (asymptomatic normal activity), 1 (symptomatic but fully ambulatory), 2 (symptomatic and in bed < 50% of time), 3 (symptomatic and in bed > 50% of time), 4 (100% restricted to bed) and 5 (dead).
Client Service Receipt Inventory
A short, modified version of the Client Service Receipt Inventory127 (CSRI), which collects data directly from participants on use of hospital services, day centres, residential homes, rehabilitation centres, paramedic/ambulance, community nurses, occupational/physiotherapists and local GP/practice nurses, as well as social care/social housing use.
Measures to reduce bias at baseline
Antidepressant use
Participants were asked to provide information about all the medication they were taking. We were particularly interested in collecting information about antidepressant use as this may influence the outcome of depression. 128,129 Antidepressants were identified from a list of prescribed antidepressants and daily doses were recorded. 130,131 Although compliance with medication, for example through pill counts, may be important, this was beyond the resources of the study. We recorded the name and dose of any antidepressants prescribed to participants during the course of the study, as well as any changes in prescribing patterns. Mean equivalent doses of fluoxetine were calculated using data from the meta-analysis by Hayasaka et al. 106 Mean (SD) equivalent doses of fluoxetine at baseline were provided in the results section.
Other psychological therapies
We noted any psychological intervention reported by patients or recorded in their case notes during the period of the trial.
Expectations at baseline
Prior to randomisation, participants were asked to predict the degree to which they thought that their mood would improve or not on a 7-point Likert scale ranging from –3 to + 3. 132
Treatment preference
Patients’ preferences for treatment were collected on a 4-point Likert scale (0–3), as in Serfaty et al. 66
Timing of measures
Timing of outcome measures
The BDI-II (main outcome measure), PHQ-9, EQ-5D-5L, ECOG-PS and CSRIs were collected at baseline and at 12 and 24 weeks from baseline. In addition, we collected the PHQ-9 at 6 weeks (mid-intervention) and 18 weeks (mid-follow-up) (Table 3).
| Measures | Time point | ||||
|---|---|---|---|---|---|
| T3 | T4 (6 weeks) | T5 (12 weeks) | T6 (18 weeks) | T7 (24 weeks) | |
| Baseline | Mid-intervention | Post intervention | Follow-up | Follow-up | |
| PHQ-9 | ✓ | ✓ | ✓ | ||
| BDI-II | ✓ | ✓ | ✓ | ✓ | ✓ |
| EQ-5D-5L | ✓ | ✓ | ✓ | ||
| Satisfaction with care | ✓ | ||||
| ECOG-PS | ✓ | ✓ | ✓ | ||
| CSRI | ✓ | ✓ | ✓ | ||
| Antidepressant use | ✓ | ✓ | ✓ | ||
| Expectation of therapy | ✓ | ||||
| Blindness | ✓ | ✓ | |||
| Attrition | ✓ | ✓ | |||
Timing of sources of bias
Post intervention (12 weeks post baseline)
Reason for not attending therapy sessions (e.g. did not like therapy, recorded death).
Participants were asked to rate on a five-point scale (ranging from not at all to very much) whether or not they found CBT useful.
At follow-up (24 weeks post baseline)
Immediately prior to completing the BDI-II, the UCL researcher undertaking assessments was asked to guess the patients’ trial arm (TAU alone, TAU plus CBT or do not know).
Reason for missing follow-up data (e.g. patient too ill, died). We did not pursue people who dropped out with qualitative interviews to establish their reason for doing so. Although this may have generated useful information, the ethics committee stipulated that no pressure should be placed on this vulnerable group.
Statistical methods
Sample size and power calculations
Published data for trials of CBT suggest that initial reductions in BDI-II with time may not be linear. A separation in depression scores favouring therapy has been observed within 6 weeks of starting treatment133,134 and continues after the treatment phase has finished. 135,136
For ethical reasons, participants were entitled to withdraw from the study without giving a reason; however, we recorded the reason for withdrawal from the trial if known. We recorded the timing of any attrition. Differential dropout may occur early on because people are not satisfied with their trial arm allocation. Dropout at later phases is more likely to be due to factors such as advancing disease or death, which are less likely to be influenced by group allocation. Details of the assumptions made concerning attrition and the effect of treatment are described below and summarised (see Table 4).
Power
Clinical effectiveness: our primary outcome was an overall effect of treatment over the 24-week period of follow-up. We had powered the study to enable a detection of a difference in BDI-II of 6 points (SD 12 points) between the TAU and CBT groups measured at 12 weeks, assuming a treatment effect of 3 points after 6 weeks and a sustained 6-point difference after 18 and 24 weeks. We were cautious in assuming a sustained rather than an increasing treatment effect after 12 weeks. Follow-up at 12 weeks post baseline in other trials range from 44%62 to > 75%. 30,63 Although our client group may have been in the last year of life, we did not plan to recruit people who were about to die. We assumed a 70% follow-up rate after 6 weeks, decreasing to 65% at 12 weeks and 60% after 24 weeks. Our 6-point difference was chosen as a conservative estimate given that, for the BDI, which has similar psychometric properties, a 3-point difference has been quoted as being clinically significant. 137
The BDI-II manual reports that the correlation between BDI-II values from sessions 1 week apart is 0.93. 118 To estimate the correlation between measurements 6 weeks apart, the simplest assumption possible was that future values would depend only on the most recent past and not on any history prior to that (in technical terms, this is called an ‘autoregressive process of order 1’). In this case, the correlation between measurements would decay at a constant rate of 0.93 per week and our best estimate of the correlation between BDI-II measures taken 6 weeks apart is 0.936 = 0.65.
Sample size calculations taking account of the longitudinal nature of the design, with correlation, attrition and effect size, and pattern assumed to be as above, were undertaken following Equation 24 of Hedeker et al. 138 Assuming the given attrition rates and correlation, the sample size required to detect an overall difference between the groups at 90% power and 5% significance was 109 participants per trial arm (using a multilevel model adjusting for baseline BDI-II). To account for clustering by therapist, the sample size needed to be inflated by a factor of 1.10:
This was based on an intraclass correlation coefficient (ICC) of 0.02139,140 and an average of six participants per therapist post intervention. Therefore, we intended to recruit 120 participants per trial arm, with expected numbers available at each follow-up given in Table 4.
| Time (weeks) | CBT over TAU; difference in BDI-II score | Per cent remaining in the study | Randomisation group, n | |
|---|---|---|---|---|
| TAU (N = 120) | CBT (N = 120) | |||
| 0 (baseline) | 0 | 100 | 120 | 120 |
| 6 | 3 | 70 | 84 | 84 |
| 12 | 6 | 65 | 78 | 78 |
| 18 | 6 | 63 | 76 | 76 |
| 24 | 6 | 60 | 72 | 72 |
An important secondary outcome was to assess how the treatment effect changes over time. The proposed sample size of 120 would provide 90% power to detect a 6-point difference in BDI-II at 12 weeks and 80% power to detect a 6-point difference at 24 weeks if attrition rates were as assumed.
Analysis plan
Clinical data (overview)
Analyses of data were undertaken within the PRIMENT CTU and reported according to CONSORT guidelines. A flow chart presents the follow-up rate for each group, with the reason for non-completion of the BDI-II score (see Figure 2). Appropriate summary descriptive statistics (e.g. mean and SD for continuous data that are approximately normally distributed) are given for baseline demographic data and pre-and post-treatment outcome scores at each follow-up period by treatment group. Analyses are presented on an intention-to-treat (ITT) basis using multilevel (hierarchical) models. The levels of hierarchy in the data are as follows: first level – repeated measures; second level – participants; and third level – therapist.
The primary analysis tested for an overall treatment effect on BDI-II over the four follow-ups, controlling for baseline BDI-II score and baseline antidepressant use (which was used for stratification in the randomisation). Secondary analyses of BDI-II also looked at the effect of treatment separately at each time point and an analysis adjusted for compliance (specifically, the number of sessions attended) was performed to take into account the possible lack of adherence to CBT. Analyses were performed using the current version of Stata® version 14 (StataCorp LP, College Station, TX, USA).
Antidepressants, including TCAs, which are often used in small doses for symptom control in end-of-life care, were converted into a mean equivalent dose of fluoxetine following a method we used in another trial66 of CBT for depressed older people.
Scoring questionnaires
Outcome scores for each scale were calculated from the scale’s individual items using the standard recommended methods for the scale. For the BDI-II (primary outcome), PHQ-9 and the satisfaction with care questionnaire, a scale score was calculated as the sum of the scale’s individual items. When any individual items on these scales were missing, they were imputed using the mean of the completed items on that scale, provided that ≥ 50% of the items for the scale had been completed. If < 50% of items had been completed, the scale was treated as missing.
Analysis of the principal outcome variable: Beck Depression Inventory, version 2
Primary analysis
All analyses were pre-specified in the statistical analysis plan. The primary analysis was a comparison between the CBT and control arms for the BDI-II score measured at all four follow-up points: 6, 12, 18 and 24 weeks. The BDI-II score was analysed using multilevel modelling, allowing for repeated measurements with equal weighting for each time point. Clustering in the intervention group from the same therapist treating multiple patients was included as a level within the model.
The model comprised three levels: (1) repeated measures, (2) individuals and (3) therapists. In the intervention group, clusters were defined by therapist. For the control group, each individual was treated as an individual cluster (n = 1).
Baseline BDI-II score and baseline antidepressant use (yes/no) were included in the model as fixed effects. No other covariates were included. The model was fitted using a linear mixed effects model assuming a Gaussian error distribution. Model assumptions of normality were checked visually using a normal probability plot of standardised residuals, and assumptions of linearity and constant variance were checked using a scatterplot of standardised residuals against predicted values.
Supportive analysis
Supportive analysis included the primary analysis, which was repeated:
-
using clustering by IAPT service
-
ignoring the therapist clustering
-
including the following covariates (as fixed effects)
-
baseline previous history of depression (yes/no)
-
baseline EQ-5D total score
-
baseline duration of current episode of depression (number of weeks)
-
number of days between diagnosis of primary tumour and baseline visit
-
-
with separate analyses carried out for each post-baseline follow-up (6, 12, 18 and 24 weeks).
Exploratory and subgroup analyses
Exploratory and subgroup analyses included the following the primary analysis:
-
including a treatment by time interaction
-
repeated including a treatment by marital status (married/partner; divorced/separated/widowed; single/never married) interaction
-
including a treatment by education status [Advanced level (A level) and above vs. below A level] interaction.
Contamination-adjusted intention-to-treat analysis
The analysis adjusted for compliance that we undertook to account for possible lack of adherence to CBT was a ‘CAITT’ rather than the better-known ‘complier average causal effects’ analysis. Here, a ‘per session’ effect of treatment is estimated rather than the effect for ‘compliers’ (which would require a binary definition of ‘complier’ in terms of number of sessions attended). CAITT is the slightly more sophisticated analysis141 and was carried out as follows.
The outcome used was the 18- and/or 24-week total BDI-II score. Only individuals with a post-CBT BDI-II score available at either 18 and/or 24 weeks were included in this analysis. When an individual had both 18- and 24-week scores, the average of the two values was used. The measure of compliance was the number of CBT sessions attended (when available) before the latest follow-up (18 or 24 weeks) for which the individual had outcome data.
Instrumental variable regression using two-stage least squares was undertaken with the randomisation group as an instrumental variable for the number of CBT sessions. The outcome was the total BDI-II score (as described earlier). The model also included the baseline total BDI-II score as a covariate. In addition, baseline antidepressant prescribed (yes/no) was included in the model as this was used as the stratification variable for the randomisation while, for simplicity, clustering by therapist was ignored.
Analysis of secondary outcome variables
-
Patient Health Questionnaire-9: depression severity.
-
Satisfaction with care.
Both these variables were analysed in the same way, using multilevel modelling that allows for repeated measures (at 12 and 24 weeks). As with the primary analysis, an additional level was included to allow for therapist clustering within the intervention arm. Baseline score and antidepressant use (yes/no) were included as fixed effects. The model was fitted using a linear mixed effects model assuming a Gaussian error distribution, mirroring the principal analysis of the primary outcome variable (BDI-II). Additional separate analyses were carried out for the 12- and 24-week time points.
-
Eastern Cooperative Oncology Group Performance Status: physical functioning.
The ECOG-PS is an ordered categorical variable that cannot be regarded as approximately continuous. The numbers and percentages (by group) falling into each category at baseline, 12 and 24 weeks were tabulated and a simple (unstratified and unclustered) non-parametric comparison was made between groups of the change in ECOG-PS score from baseline at each time point.
Analysis for bias
We compared participant data at baseline for those randomised to TAU with data for those randomised to TAU plus CBT for the following factors: whether or not they were using any non-pharmacological treatment for depression, their treatment preference (CBT group/TAU group/no preference) and expectations of improvement if they were to receive CBT [rated from 1 (not at all) to 10 (completely)].
As previously indicated, even low doses of antidepressants may be effective. The therapeutic dose of an antidepressant varies depending on which antidepressant is prescribed. In order to compare doses, the medications were standardised by converting them into equivalent doses of fluoxetine, using dose equivalents from data from Hayasaka et al. 106
Health economics
A health and social care perspective was adopted, in line with NICE’s recommendations. 142
Outcomes
Quality-adjusted life-years were calculated from EQ-5D scores at baseline, 12 and 24 weeks’ follow-up. The EQ-5D is a non-disease-specific measure for describing and valuing health-related QoL. 143 The measure includes a rating of own health in five domains (1, mobility; 2, self-care; 3, usual activities; 4; pain/discomfort; and 5, anxiety/depression) and a rating of own health by means of a VAS [a ‘thermometer’ (score of 0–100)]. The EQ-5D is widely used in economic evaluations for common mental health disorders. The health states from the EQ-5D were given a utility score using responses from a representative sample of adults in the UK. 144 From these, QALYs were calculated using the area under the curve approach as defined by the utility values at baseline and at each follow-up.
Costs
Study participants
Resource data cover community health and social services, although the use of hospital services has been omitted in a modified version of the CSRI. 145 The calculation of costs was separated into the identification, measurement and valuation of relevant resources. A unit cost was applied to each resource use to calculate the total cost of resources used by each study participant. For NHS primary care services, and social care and voluntary services, we used costs from Curtis. 146
Trial intervention
Recorded details of attendance and non-attendance at sessions for each study participant were used as the basis for the calculation of the total cost of the intervention. Furthermore, costs associated with therapists attending a CBT coaching session (including subsistence and travel) were incorporated. It was assumed that, in those instances in which a session was offered but not attended, this still constituted IAPT’s therapist opportunity cost. We assumed that trial therapists were on band 7 or 8 on the Agenda for Change salary scale,147 and employer’s national insurance, superannuation contributions and overheads were added to the average salary. 148
Cost–utility and cost-effectiveness analyses
Costs were compared for the groups using a bootstrap regression model to account for non-normality in the distribution of cost data.
A cost–utility analysis was undertaken using QALYs calculated from the EQ-5D measure of QoL.
Cost-effectiveness was assessed by estimating an incremental cost-effectiveness ratio (ICER) to show the extra cost incurred by CBT to generate one extra QALY. 149 This is defined as the cost difference divided by the outcome difference, after adjusting for costs and outcomes measured at baseline. However, there will inevitably be uncertainty around the cost and outcome differences. In the ICER calculation, we adjusted for baseline costs and utility in the correlation structure between costs and effects the variance – covariance matrix is generated via Cholesky’s decomposition.
To deal with uncertainty around the ICER, a cost-effectiveness plane (CEP) and cost-effectiveness acceptability curves (CEACs) were created. 150 We used the standard net benefit regression to produce the CEAC, using participant-level effect and incorporating associated cost data in the estimation. For the CEP, 1000 bootstrapped estimates of cost and outcome differences were produced, adjusted for baseline and plotted against each other. This aimed to show the probability that CBT had (1) higher costs and better outcomes, (2) higher costs and worse outcomes, (3) lower costs and worse outcomes or (4) lower costs and better outcomes than TAU. The CEAC was produced using the net benefit approach, for which the QALY difference is multiplied by the societal value (threshold) placed on a QALY and the incremental service cost is subtracted. A positive incremental net benefit means that CBT is more cost-effective and the proportion of positive values for each societal QALY value gives the probability that CBT is cost-effective at that threshold. In this study, the cost-effectiveness of receiving CBT rather than TAU on its own was examined. Models for analysing incremental cost-effectiveness were fitted using Stata® version 12.0 (StataCorp LP, College Station, TX, USA).
Qualitative data
We used purposive sampling and wrote to 20 participants in the trial who had received CBT at the end of their follow-up period, inviting them to take part in qualitative interviews to explore their experiences of the trial and their therapy. We planned to interview 12 therapists to explore their experience of delivering therapy to this population. Semistructured, one-to-one interviews were conducted using a topic guide either in the participants’ homes or within the department where the research was taking place. All interviews were audio recorded for transcription at a later date. Data were analysed using thematic content analysis. Findings from these data were used to generate recommendations on what may be improved in the delivery of CBT to people with advanced cancer.
Chapter 5 Results
Project set-up
Permissions
Table 5 summarises the process taken for research to take place.
| Trial site | Year | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | 2015 | |||||||||||||||||||||||||||||||||||||||||||||
| Quarter | |||||||||||||||||||||||||||||||||||||||||||||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | ||||||||||||||||||||||||||||||||||
| Oncology centre 1 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 2 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 3 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Marie Curie Hospice | R | A | |||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 4 | D | S | R | A | |||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 5 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 6 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 7 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 8 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 9 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 10 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 11 | S | R | A | Withdrew | |||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 12 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 13 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 14 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 15 | R | A | |||||||||||||||||||||||||||||||||||||||||||||||
| Oncology centre 16 | S | R | A | ||||||||||||||||||||||||||||||||||||||||||||||
Project set-up started in December 2011. Because of limited resources, some services were not able to complete data for the number of people assessed for eligibility, namely oncology centres 7, 9 and 16 (see Table 5).
Furthermore, the time taken from the submission of documentation to the approval for research to start is shown in Table 5. The time taken for submission and documentation varied between as little as 1 month for oncology centre 16 and 9 months for oncology centres 1, 2, 3 and 6.
Oncology centres 4 and 14 encouraged early involvement to prepare the documentation for review and discussion prior to submission to R&D, for approval for research to proceed. As this process would normally be considered part of the submission and approval process, we have included it in Table 5 in order to make a comparison with other centres. As the research nurse undertaking recruitment from oncology centre 11 left her role, oncology centre 11 had to withdraw from the study.
Screening and recruitment
Where available, the number of people assessed for eligibility, screened and recruited from primary care and hospice settings (Table 6) and from oncology clinics (Table 7) is shown. The proportion of those recruited was lowest for GPs (2.6%) and greatest for the hospice (35.3%). There was considerable variability in the percentage of people screened and recruited within a particular recruitment source (GP and oncology) and caution needs to be exercised because of the small sample population in the GP sample. However, as shown for the oncology service, the proportion of people recruited varied considerably between hospices. Oncology centre 2 recruited > 50% of participants screened and oncology centre 1 recruited 2.4%, although it should be noted that each site was made up of a different combination of clinics described in Chapter 3, Recruitment methods and procedures. Also shown is the number of people recruited and screened per month; these methods did not apply to primary care recruitment, as only a one-off sweep of records was undertaken at one time point and, therefore, the last two columns of Table 6 are blank. The proportion of participants randomised to each arm was similar from each site.
| Practice | Number assessed for eligibility | Number screened | Number recruited | Percentage of screened patients recruited | Number of database searches | Number screened per month of recruitment | Number recruited per month of recruitment |
|---|---|---|---|---|---|---|---|
| Primary care recruitment | |||||||
| Primary care centre 1 | 415 | 16 | 0 | 0.0 | 1 | ||
| Primary care centre 2 | 81 | 7 | 0 | 0.0 | 1 | ||
| Primary care centre 3 | 26 | 20 | 0 | 0.0 | 1 | ||
| Primary care centre 4 | 220 | 13 | 1 | 7.7 | 1 | ||
| Primary care centre 5 | 155 | 10 | 2 | 20.0 | 2 | ||
| Primary care centre 6 | 69 | 105 | 0 | 0.0 | 1 | ||
| Primary care centre 7 | 26 | 26 | 1 | 3.8 | 1 | ||
| Primary care centre 8 | 266 | 20 | 0 | 0.0 | 1 | ||
| Primary care centre 9 | 123 | 9 | 0 | 0.0 | 1 | ||
| Primary care centre 10 | 1 | 1 | 1 | 100.0 | 1 | ||
| Primary care centre 11 | 2 | 1 | 0 | 0.0 | 1 | ||
| Primary care centre 12 | 20 | 4 | 1 | 25.0 | 1 | ||
| Total | 1404 | 232 | 6 | 2.6 | |||
| Hospice recruitment | |||||||
| Marie Curie Hospice | 336 | 79 | 28 | 35.4 | 39 | 1.6 | 0.7 |
| Oncology centre | Number of patients | Percentage of screened patients recruited | Months of recruitment | Number of patients | |||
|---|---|---|---|---|---|---|---|
| Assessed for eligibility | Screened | Recruited | Screened per month of recruitment | Recruited per month of recruitment | |||
| 1 | 167 | 167 | 4 | 2.4 | 40 | 4.2 | 0.1 |
| 2 | 182 | 8 | 4 | 50.0 | 39 | 4.4 | 0.1 |
| 3 | 173 | 82 | 13 | 15.9 | 39 | 5.2 | 0.3 |
| 4 | 1501 | 477 | 26 | 5.5 | 32 | 38.3 | 0.8 |
| 5 | 235 | 68 | 16 | 23.5 | 32 | 4.5 | 0.5 |
| 6 | 263 | 81 | 11 | 13.6 | 29 | 3.0 | 0.4 |
| 7 | N/A | N/A | 9 | N/A | 35 | N/A | 0.3 |
| 8 | 71 | 45 | 8 | 17.8 | 35 | 1.9 | 0.2 |
| 9 | N/A | N/A | 3 | N/A | 34 | N/A | 0.1 |
| 10 | 535 | 319 | 15 | 4.7 | 30 | 17.8 | 0.5 |
| 11 | 541 | 13 | 10 | 76.9 | 29 | 0.2 | 0.3 |
| 12 | 976 | 353 | 39 | 11.0 | 31 | 21.0 | 1.3 |
| 13 | 414 | 196 | 26 | 13.3 | 31 | 10.8 | 0.8 |
| 14 | 1738 | 20 | 2 | 10.0 | 11 | 1.8 | 0.2 |
| 15 | 160 | 84 | 8 | 9.5 | 15 | 14.7 | 0.5 |
| 16 | N/A | N/A | 2 | N/A | 6 | N/A | 0.3 |
| Total | 6956 | 1913 | 196 | 10.2 | |||
Table 8 shows the reasons that people were not screened and the reasons they were not referred to the study, although data were limited. The commonest reasons were: in GP practices, people did not have a diagnosis of advanced cancer; in oncology clinics, patients were not in the IAPT area; and, in hospices, there was a slower turnover of patients so people had already been screened. In oncology clinics, staff often indicated that people did not wish to be approached; however, informal feedback from researchers suggested that there was some gatekeeping taking place.
| Reason for exclusion | Recruitment site, n | ||
|---|---|---|---|
| GP practices | Hospital oncology clinics | Hospices | |
| Total register list | 1404 | 6956 | 352 |
| Reason for not screeninga | |||
| Aged < 18 years | 10 | 5 | 122 |
| IAPT area | 0 | 2612 | 2 |
| Diagnosis | 979 | 665 | 24 |
| Prognosis | 44 | 197 | 0 |
| Difficulty with English | 43 | 156 | 3 |
| Problems with alcohol | 9 | 3 | 0 |
| Screened already | 0 | 112 | 172 |
| Not screened: other reason | 138 | 392 | 26 |
| Not screened: no reason recorded | 0 | 694 | 0 |
| Missed | 0 | 207 | 26 |
| Approached for screening | 232 | 1913 | 79 |
| Reason for not referring | |||
| Declined | 49 | 959 | 13 |
| PHQ-2 score of < 3 points | 8 | 501 | 23 |
| MINI negative diagnosis | 1 | 23 | 3 |
| Screened out (measure unspecified) | 0 | 34 | 0 |
| High suicidality | 0 | 3 | 2 |
| Other reason | 168 | 43 | 10 |
| Not referred: no reason recorded | 0 | 144 | 0 |
| Referred for baseline | 6 | 206b | 28 |
| Conversion rate (% approached for screening referred for baseline) | 2.6 | 10.8 | 35.4 |
We had limited available information on screening by tumour group (Table 9). Not all clinics were able to provide these data, as staff indicated that they were too busy to consider anything but referral into the study. As shown, the highest proportion of people recruited came from neurological clinics and the lowest proportion came from chest clinics. Unfortunately, we were not able to collect data specifically from prostate clinics.
| Tumour group | Number of patients | Percentage of screened patients recruited | |
|---|---|---|---|
| Screened | Recruited | ||
| Breast | 127 | 19 | 15.0 |
| Colorectal/GI | 369 | 27 | 7.3 |
| Lung | 247 | 17 | 6.9 |
| Haematological | 380 | 31 | 8.2 |
| HPB | 61 | 6 | 9.8 |
| Neurological | 83 | 14 | 16.9 |
| Total | 1267 | 114 | 9.0 |
Recruitment rate
Figure 1 shows cumulative original, amended and actual recruitment by month and year from the start of the project in August 2012. This figure is supplemented by the data in Table 5. No participants were recruited until December 2012 because of the delay in permissions; this is represented by the relatively flat recruitment line from August 2012 to February 2013, when recruitment was initially slow.
FIGURE 1.
Cumulative original, amended and actual recruitment time.
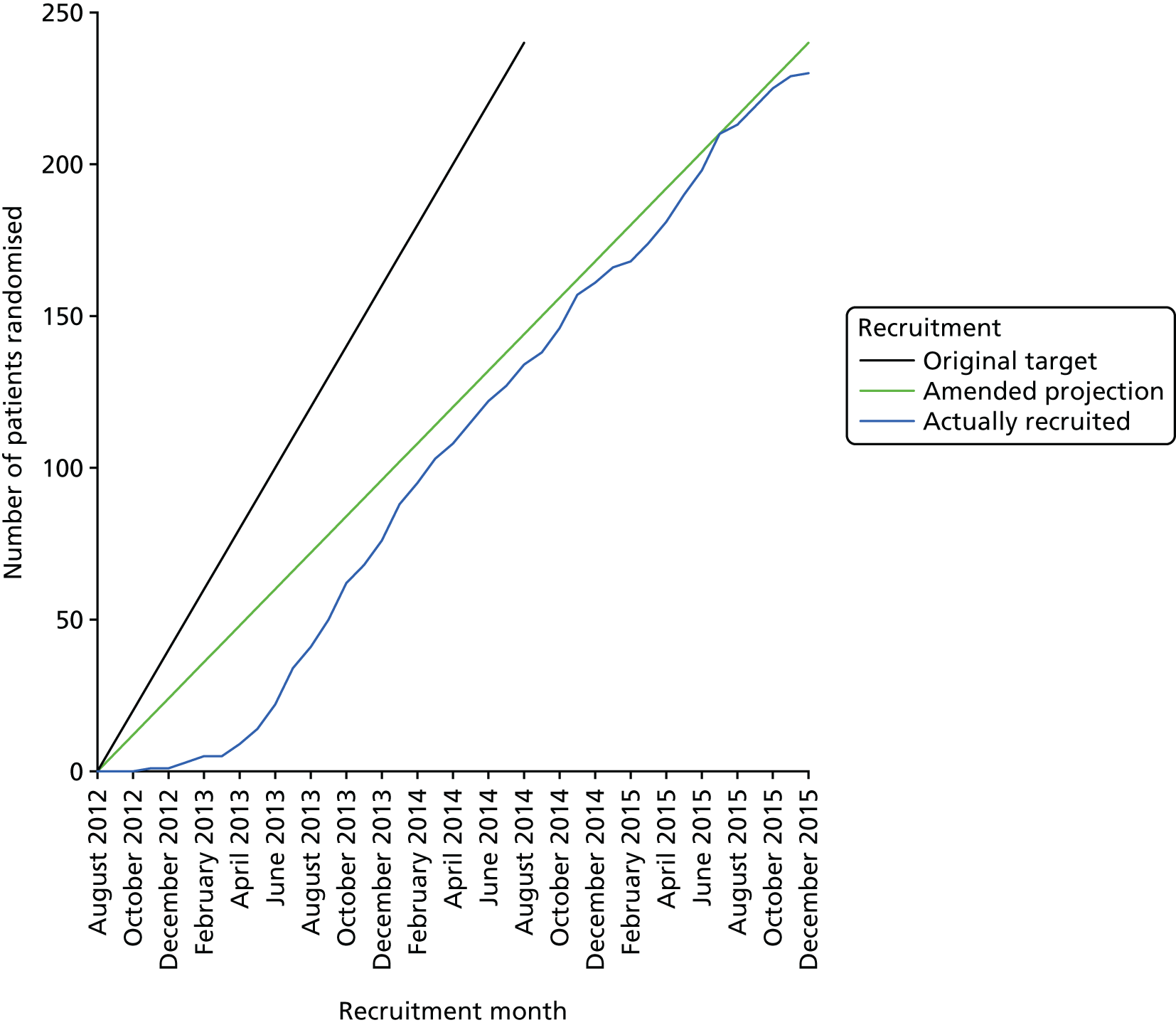
As permissions came through, recruitment accelerated to around 7.8 participants per month (93 participants over 12 months for the period from June 2013 to the end of May 2014).
Our recruitment target and methods had to be revised to allow for delays in permissions that were beyond our control. We overcame these hurdles by increasing the number of clinics within a locality from which recruitment was taking place and enrolling other areas of the UK into the study. This required us to establish whether or not IAPT services had the capacity to take on additional cases and whether or not IAPT services from different regions could actually participate, especially as changes in the configuration of IAPT services nationally were taking place. This made them cautious about being able to commit to the trial.
It became evident that the same patients were being seen in both the oncology clinics and hospices. Therefore, we chose to expand the project to centres with large numbers of oncology patients, such as oncology centre 14. It is also notable that, in the final 6 months, the recruitment rate fell to 3.3 participants per month (20 participants were recruited in the final 6 months). This is because the reservoir of patients available became depleted.
A CONSORT flow diagram is provided in Figure 2. Of those assessed for eligibility, only 2.7% (230/8398) were suitable for the trial. However, at least one outcome measure was available on 80.4% (185/230) of participants.
FIGURE 2.
Participant flow and recruitment; a CONSORT flow diagram.
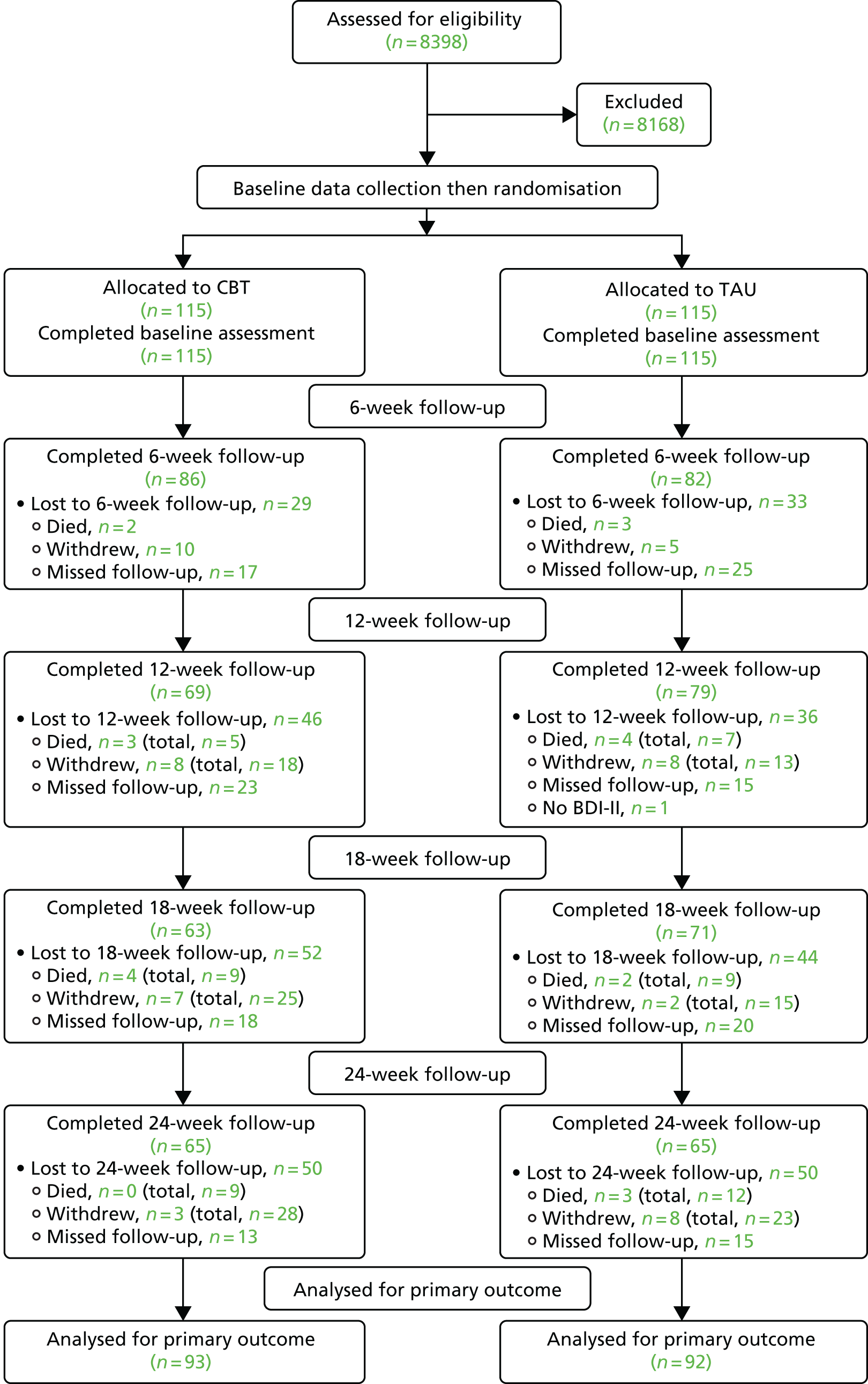
Reasons for withdrawal from the study
Twenty-one (9.1%) of the 230 recruited participants died and 51 (22.2%) participants withdrew. Although the ethics committee stipulated that patients did not need to give a reason for withdrawal from the study, we aimed to collect this information when possible (these reasons are summarised in Table 10). The number of participants who withdrew from the study during the first 6 weeks was twice as high in the CBT group as in the TAU group.
| Reason for withdrawal | Number by group prior to each follow-up point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 weeks | 12 weeks | 18 weeks | 24 weeks | Total (%) | |||||
| CBT | TAU | CBT | TAU | CBT | TAU | CBT | TAU | ||
| No reason given | 4 | 2 | 2 | 1 | 1 | 1 | 11 (21.6) | ||
| Ill health | 2 | 1 | 3 | 3 | 3 | 1 | 5 | 18 (35.3) | |
| Too busy | 1 | 1 | 1 | 3 (5.9) | |||||
| Moved away | 1 | 1 | 2 (3.9) | ||||||
| Unhappy with group allocation | 2 | 1 | 1 | 4 (7.8) | |||||
| Did not want to answer questions | 2 | 1 | 3 (5.9) | ||||||
| Did not think study would help them | 1 | 1 (2.0) | |||||||
| Felt that therapy did not suit them | 2 | 1 | 3 (5.9) | ||||||
| Difficulties with IAPT service | 1 | 2 | 3 (5.9) | ||||||
| Did not like therapist | 1 | 1 (2.0) | |||||||
| No longer wanted therapy | 1 | 1 (2.0) | |||||||
| Could not receive therapy as outside IAPT area | 1 | 1 (2.0) | |||||||
| Total | 10 | 5 | 8 | 8 | 7 | 2 | 3 | 8 | 51 (100.0) |
Reason for missed follow-up
The CONSORT flow diagram (see Figure 2) provides participant flow. It is also of note that follow-up was missed at different time points. The specific reasons why participants were not followed up at 6, 12, 18 and 24 weeks are given in Table 11.
| Reason for missed follow-up | Number by group that missed each follow-up point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6 weeks | 12 weeks | 18 weeks | 24 weeks | Total (%) | |||||
| CBT | TAU | CBT | TAU | CBT | TAU | CBT | TAU | ||
| No reason recorded | 6 | 9 | 6 | 3 | 3 | 7 | 2 | 2 | 38 (26.2) |
| Could not contact participant | 4 | 5 | 4 | 5 | 6 | 3 | 5 | 6 | 38 (26.2) |
| Ill health | 2 | 9 | 10 | 5 | 5 | 6 | 4 | 6 | 47 (32.2) |
| Ill health of family member | 1 | 1 (0.7) | |||||||
| Too busy | 1 | 1 | 1 | 2 | 5 (3.4) | ||||
| Away at the time of follow-up | 1 | 1 | 1 | 1 | 1 | 5 (3.4) | |||
| Did not want to attend or answer questions | 1 | 1 | 1 | 1 | 1 | 5 (3.4) | |||
| Unable to answer questions | 1 | 1 (0.7) | |||||||
| Issues with IAPT service | 2 | 1 | 1 | 4 (2.7) | |||||
| Did not like therapist | 1 | 1 (0.7) | |||||||
| Total | 16 | 25 | 23 | 15 | 18 | 20 | 13 | 15 | 145 (100.0) |
Demographics
Demographic information concerning sex, marital status, ethnicity and employment status is illustrated in Table 12, in which it can be seen that two-thirds of the sample population were female. The mean age of participants was 60 years. Around three-quarters of the participants were white, with the remaining population being of a variety of ethnicities. The 19 participants whose ethnicity was described as ‘other’ were white Jewish (one), white Irish (two), European (two), white other (one), Greek Cypriot (two), Turkish/Cypriot (one), Mediterranean (one), British Bangladeshi (one), South American (one), North African (one), white/black Caribbean (one), Iraqi (one), Filipino (one) and Armenian (one), with two unknown. Around two-fifths of participants were retired and one-fifth of participants were unable to work because of illness disability. The employment status of six participants was reported as ‘other’: two were on sick leave, two were disabled, one was medically retired and in one the reason was unknown. There was a balance of participants from different educational backgrounds. The two trial arms, (1) TAU plus CBT and (2) CBT alone, were similar with respect to demographic factors.
| Demographic characteristics | Randomisation group | Total | |
|---|---|---|---|
| TAU | CBT | ||
| Age (years), mean (SD); min., max. | 59.5 (12.4); 27, 93 (n = 115) | 59.5 (10.3); 37, 81 (n = 115) | 59.5 (11.4); 27, 93, (n = 230) |
| Sex, n (%) | |||
| Male | 37 (32.2) | 41 (35.7) | 78 (33.9) |
| Female | 78 (67.8) | 74 (64.3) | 152 (66.1) |
| Total | 115 (100.0) | 115 (100.0) | 230 (100.0) |
| Marital status, n (%) | |||
| Married | 55 (48.2) | 59 (51.3) | 114 (49.8) |
| Partner – living with | 9 (7.9) | 9 (7.8) | 18 (7.9) |
| Partner – not living with | 1 (0.9) | 2 (1.7) | 3 (1.3) |
| Divorced/separated | 18 (15.8) | 13 (11.3) | 31 (13.5) |
| Widowed | 9 (7.9) | 10 (8.7) | 19 (8.3) |
| Single, never married | 20 (17.5) | 22 (19.1) | 42 (18.3) |
| Other | 2 (1.8) | 0 (0.0) | 2 (0.9) |
| Total | 114 (100.0) | 115 (100.0) | 229 (100.0) |
| Ethnicity, n (%) | |||
| White | 84 (73.0) | 83 (72.2) | 167 (72.6) |
| Black – British/African/Caribbean | 17 (14.8) | 14 (12.2) | 31 (13.5) |
| Indian/Pakistani/Bangladeshi | 6 (5.2) | 7 (6.1) | 13 (5.6) |
| Other | 8 (7.0) | 11 (9.6) | 19 (8.3) |
| Total | 115 (100.0) | 115 (100.0) | 230 (100.0) |
| Employment, n (%) | |||
| Employed | 16 (14.3) | 27 (23.7) | 43 (19.0) |
| Self-employed | 5 (4.5) | 13 (11.4) | 18 (8.0) |
| Unemployed – seeking work | 2 (1.8) | 1 (0.9) | 3 (1.3) |
| Unemployed – not seeking work | 10 (8.9) | 13 (11.4) | 23 (10.2) |
| Homemaker | 2 (1.8) | 0 (0.0) | 2 (0.9) |
| Retired | 49 (43.8) | 38 (33.3) | 87(38.5) |
| Unable to work due to illness/disability | 24 (21.4) | 20 (17.5) | 44 (19.5) |
| Other | 4 (3.6) | 2 (1.8) | 6 (2.7) |
| Total | 112 (100.0) | 114 (100.0) | 226 (100.0) |
| Education, n (%) | |||
| Higher degree | 15 (13.0) | 13 (11.3) | 28 (12.2) |
| Degree | 27 (23.5) | 31 (27.0) | 58 (25.2) |
| A Level (or equivalent) | 15 (13.0) | 9 (7.8) | 24 (10.4) |
| HNC (or equivalent) | 8 (7.0) | 4 (3.5) | 12 (5.2) |
| NVQ (or equivalent) | 13 (11.3) | 13 (11.3) | 26 (11.3) |
| GCSE (or equivalent) | 16 (13.9) | 24 (20.9) | 40 (17.4) |
| No qualification | 8 (7.0) | 7 (6.1) | 15 (6.5) |
| Other | 13 (11.3) | 14 (12.2) | 27 (11.7) |
| Total | 115 (100.0) | 115 (100.0) | 230 (100.0) |
Demographics of cancer
Two-thirds of patients had tumours of one of the five main groups (breast, colorectal, lung, prostate and haematological), with the majority, around one-third, having a primary diagnosis of breast cancer. Of the 41 ‘other’, 14 were neurological, four were oesophageal, three were bowel, two cervical, two liver, two bone, two anal, two sarcoma, one nose, one throat, one ovarian, one testicular, one parotid gland, one mesothelioma, one myelofibrosis and three patients had cancer of unknown primary diagnosis (Table 13). The mean time since diagnosis of cancer was skewed by 44 participants with a haematological cancer, among whom the mean time since diagnosis was 2970 days. The median length of time since the primary diagnosis was just over 2 years (770 days).
| Randomisation group | Total | ||
|---|---|---|---|
| TAU | CBT | ||
| Time since primary diagnosis (days), mean (SD); min., max. | 1484 (1680); 16, 8548 (n = 99) | 1386 (2235); 12, 17,644 (n = 104) | 1433 (1975); 13, 17,644 (n = 203) |
| Primary tumour site, n (%) | |||
| Breast | 36 (31.3) | 36 (31.3) | 72 (31.3) |
| Colon/rectal | 17 (14.8) | 12 (10.4) | 29 (12.6) |
| Endometrial | 1 (0.9) | 0 (0.0) | 1 (0.4) |
| Kidney | 1 (0.9) | 1 (0.9) | 2 (0.9) |
| Leukaemia | 3 (2.6) | 2 (1.7) | 5 (2.2) |
| Lung | 10 (8.7) | 17 (14.8) | 27 (11.7) |
| Melanoma | 1(0.9) | 0 (0.0) | 1 (0.4) |
| Lymphoma | 9 (7.8) | 8 (7.0) | 17 (14.8) |
| Myeloma | 8 (7.0) | 13 (11.3) | 21 (18.3) |
| Pancreatic | 2 (1.7) | 0 (0.0) | 2 (0.9) |
| Prostate | 5 (4.3) | 7 (6.1) | 12 (5.2) |
| Other | 22 (19.1) | 19 (16.5) | 41 (17.8) |
| Total | 115 (100.0) | 115 (100.0) | 230 (100.0) |
| Secondary tumour site, n (%) | |||
| None | 25 (24.3) | 30 (29.7) | 55 (27.0) |
| Breast | 8 (7.8) | 7 (6.9) | 15 (7.4) |
| Colon/rectal | 2 (1.9) | 4 (4.0) | 6 (2.9) |
| Endometrial | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Kidney | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Leukaemia | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Lung | 10 (9.7) | 5 (5.0) | 15 (7.4) |
| Non-Hodgkin lymphoma | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Prostate | 2 (1.9) | 2 (2.0) | 4 (2.0) |
| Other | 52 (50.5) | 51 (50.5) | 103 (50.5) |
| Total | 103 (100.0) | 101 (100.0) | 204 (100.0) |
Diagnosis of depression, previous psychiatric history and treatment
All participants included in the study satisfied a MINI diagnosis of depressive disorder. Three-fifths of participants had a previous history of depression, with the mean number of previous episodes being just over two. The duration of the current episode of depression was also skewed, with the median duration being around 12 weeks. One-tenth of participants had previously received CBT, with less than one-third receiving treatment for depression (Table 14).
| Randomisation group | Total | ||
|---|---|---|---|
| TAU | CBT | ||
| Number of previous episodes of depression (those with previous depression), mean (SD); min., max. | 2.2 (1.9); 1, 10 (n = 63) | 2.6 (2.4); 1, 12 (n = 59) | 2.4 (2.1); 1, 12 (n = 122) |
| Duration of current depression (weeks), mean (SD); min., max. | 74.3 (242.7); 0, 2080 (n = 90) | 86.6 (266.5); 0, 2080 (n = 84) | 80.3 (253.8); 0, 2080 (n = 174) |
| Previous depression, n (%) | |||
| Yes | 69 (60.0) | 68 (59.1) | 137 (59.6) |
| Total | 115 (100.0) | 115 (100.0) | 230 (100.0) |
| Ever had CBT before, n (%) | |||
| Yes | 12 (10.4) | 12 (10.4) | 24 (10.4) |
| Total | 115 (100.0) | 115 (100.0) | 230 (100.0) |
| On current depression treatment, n (%) | |||
| Yes | 33 (29.2) | 33 (29.2) | 66 (29.2) |
| Total | 113 (100.0) | 113 (100.0) | 226 (100.0) |
| On antidepressant treatment, n (%) | |||
| Yes | 27 (23.5) | 28 (24.3) | 55 (23.9) |
| Total | 115 (100.0) | 115 (100.0) | 230 (100.0) |
| Treatment preference, n (%) | |||
| The CBT group | 92 (80.0) | 87 (75.7) | 179 (77.8) |
| The group with no CBT | 3 (2.6) | 2 (1.7) | 5 (2.2) |
| Do not have a preference | 20 (17.4) | 26 (22.6) | 46 (20.0) |
| Total | 115 (100.0) | 115 (100.0) | 230 (100.0) |
Potential sources of bias
One-quarter of participants were receiving an antidepressant, three-quarters desired CBT and there was a 70% expectation of improvement from CBT that was equally distributed in participants allocated to either TAU or TAU plus CBT (Table 15).
| Randomisation group | Total | ||
|---|---|---|---|
| TAU | CBT | ||
| CBT treatment expectation, mean (SD); min., max. | 7.0 (1.9); 1, 10 (n = 111) | 7.2 (1.8); 4, 10 (n = 113) | 7.1 (1.9); 1, 10 (n = 224) |
| Treatment preference, n (%) | |||
| CBT group | 92 (80.0) | 87 (75.7) | 179 (77.8) |
| TAU group | 3 (2.6) | 2 (1.7) | 5 (2.2) |
| No preference | 20 (17.4) | 26 (22.6) | 46 (20.0) |
| Previously received CBT, n (%) | 12 (10.4) | 12 (10.4) | 24 (10.4) |
| Antidepressant use, n (%) | |||
| Baseline | 26 (22.6) | 29 (25.2) | 55 (23.9) |
| 12-week follow-up | 20 (17.4) | 22(19.1) | 42 (18.3) |
| 24-week follow-up | 16 (13.9) | 20 (17.4) | 36 (15.7) |
| Other psychological therapy, n (%) | |||
| Baseline | 5 (4.3) | 3 (2.6) | 8 (3.5) |
| 12-week follow-up | 6 (5.2) | 1 (0.9) | 7 (6.1) |
| 24-week follow-up | 5 (4.3) | 3 (2.6) | 8 (7.0) |
Antidepressant usage
Fifty-five (23.9%) of the 230 participants were taking an antidepressant, 26 out of 115 in the TAU group and 29 out of 115 in the CBT group. There was no significant between-group difference as to whether or not an antidepressant was prescribed. Although the names of the antidepressants were recorded, the dose was available in only 35 cases. The mean dose-equivalent of fluoxetine prescribed was 23.4 mg (SD 8.2 mg) for the TAU group (n = 17) and 31.8 mg (SD 8.2 mg) for the CBT group (n = 18). There was no significant difference in the mean dose by group allocation.
Other psychological therapies
The record of any psychological intervention reported by patients or recorded in their case notes during the period of the trial is summarised in Table 15.
Expectations at baseline132
Prior to randomisation, participants were asked to predict the degree to which they thought that their mood would or would not improve on a 7-point Likert scale ranging from –3 to 3.
Treatment preference
Patients’ preferences for treatment were collected on a 4-point Likert scale (0–3), as in Serfaty et al. 66
Clinical outcomes
All participants met caseness for depressive disorder using the MINI. We present clinical outcomes (see Tables 16–19) for the main outcome (BDI-II) and secondary outcomes (PHQ-9, EQ-5D, satisfaction with care and ECOG-PS).
Main outcome
Data for the main outcome measure, the BDI-II, are shown in Table 16 and on the histograms of Figure 3. Mean BDI-II score by time and group is also presented (Figure 4).
| Time point and randomisation group | Summary statistic | |||||||
|---|---|---|---|---|---|---|---|---|
| Min. | Q1 | Median | Q3 | Max. | Mean | SD | n | |
| Baseline | ||||||||
| TAU | 4 | 18 | 24 | 30 | 52 | 24.5 | 9.7 | 115 |
| CBT | 2 | 17 | 24 | 32 | 53 | 25.2 | 10.4 | 115 |
| Total | 2 | 18 | 24 | 31 | 53 | 24.9 | 10.0 | 230 |
| 6 weeks | ||||||||
| TAU | 0 | 15 | 21 | 30 | 50 | 23.1 | 10.8 | 82 |
| CBT | 1 | 16 | 23 | 30 | 57 | 23.6 | 10.8 | 86 |
| Total | 0 | 15 | 23 | 30 | 57 | 23.3 | 10.8 | 168 |
| 12 weeks | ||||||||
| TAU | 0 | 13 | 20 | 29 | 49 | 21.4 | 11.1 | 79 |
| CBT | 3 | 14 | 20 | 26 | 54 | 21.3 | 11.0 | 69 |
| Total | 0 | 14 | 20 | 27 | 54 | 21.3 | 11.0 | 148 |
| 18 weeks | ||||||||
| TAU | 0 | 12 | 20 | 27 | 54 | 21.2 | 12.5 | 71 |
| CBT | 5 | 12 | 17 | 28 | 56 | 20.6 | 11.9 | 63 |
| Total | 0 | 12 | 19 | 27 | 56 | 20.9 | 12.2 | 134 |
| 24 weeks | ||||||||
| TAU | 0 | 13 | 19 | 27 | 48 | 20.4 | 11.4 | 65 |
| CBT | 2 | 11 | 18 | 25 | 54 | 19.4 | 11.4 | 65 |
| Total | 0 | 12 | 19 | 27 | 54 | 19.9 | 11.4 | 130 |
FIGURE 3.
Histograms of BDI-II score at baseline and at 6, 12, 18 and 24 weeks. (a) TAU, baseline; (b) TAU, 6 weeks; (c) TAU, 12 weeks; (d) TAU, 18 weeks; (e) TAU, 24 weeks; (f) CBT, baseline; (g) CBT, 6 weeks; (h) CBT, 12 weeks; (i) CBT, 18 weeks; and (j) CBT, 24 weeks.
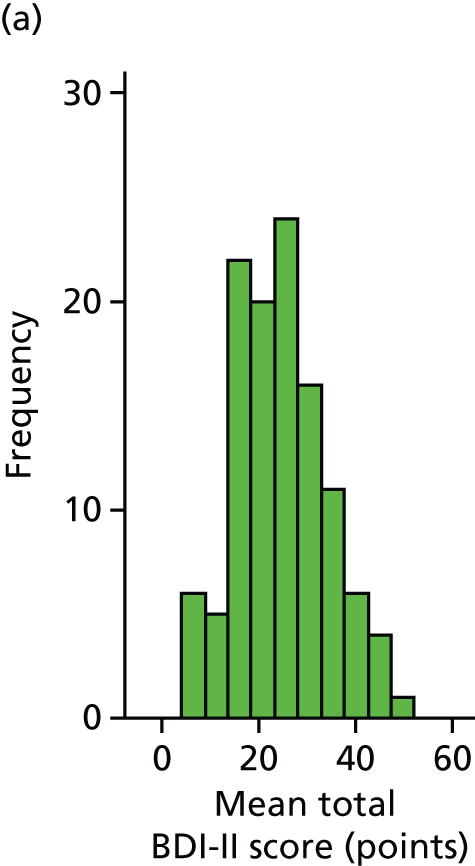
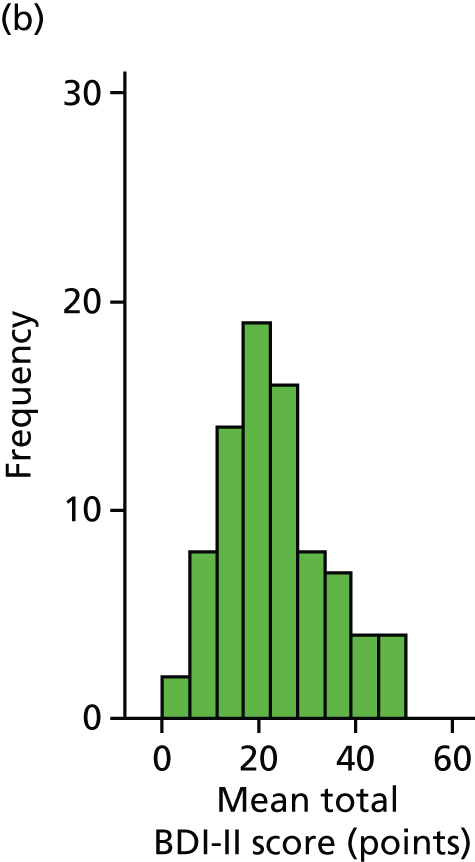
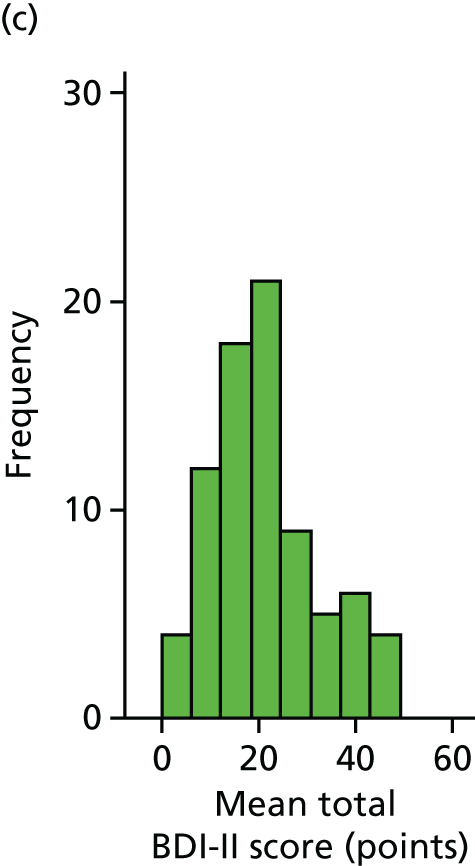
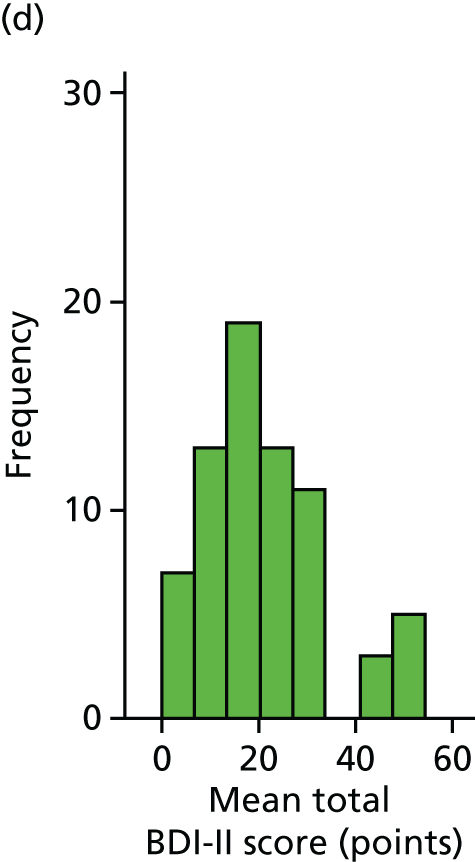
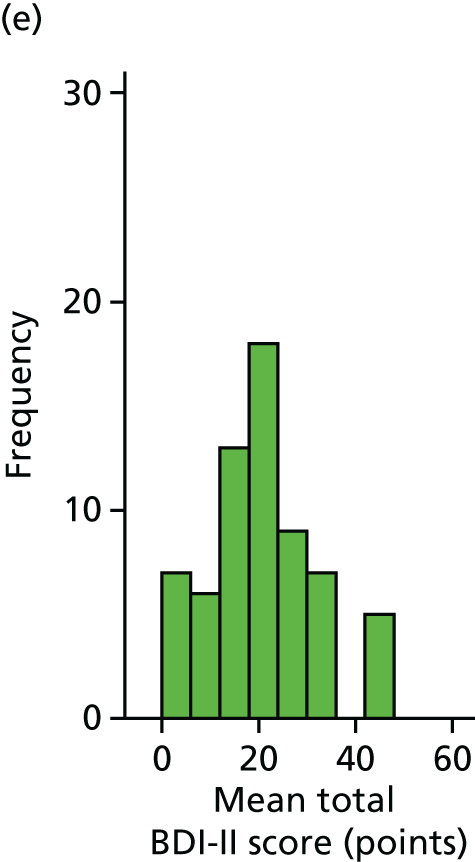
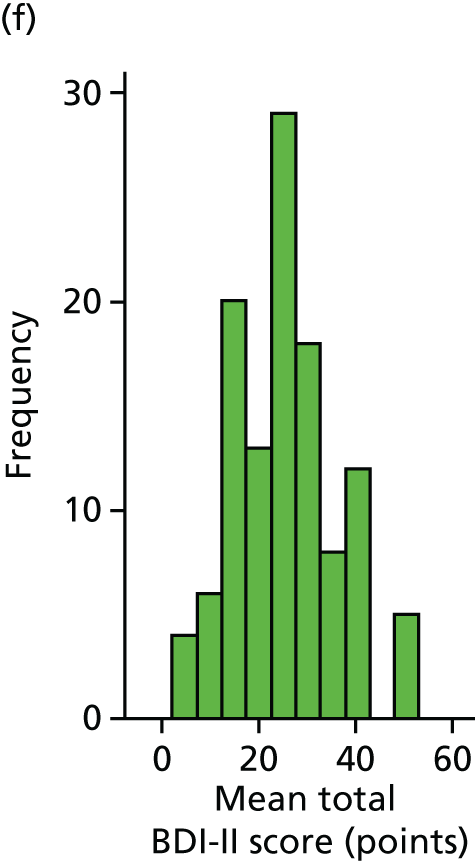
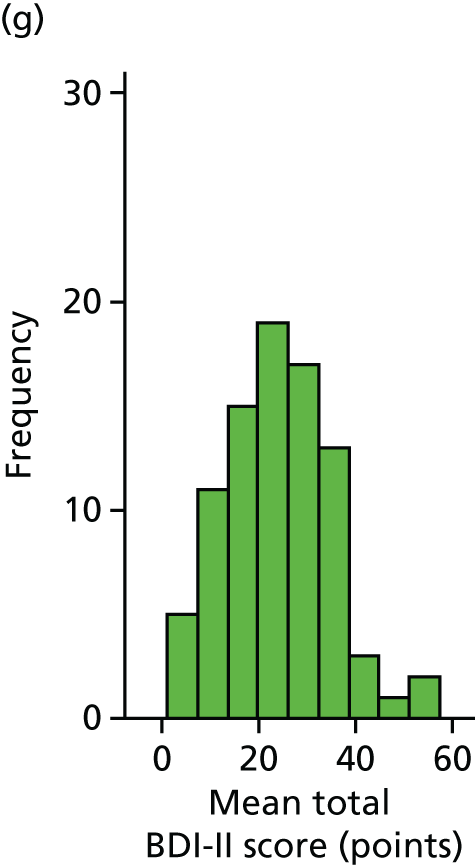
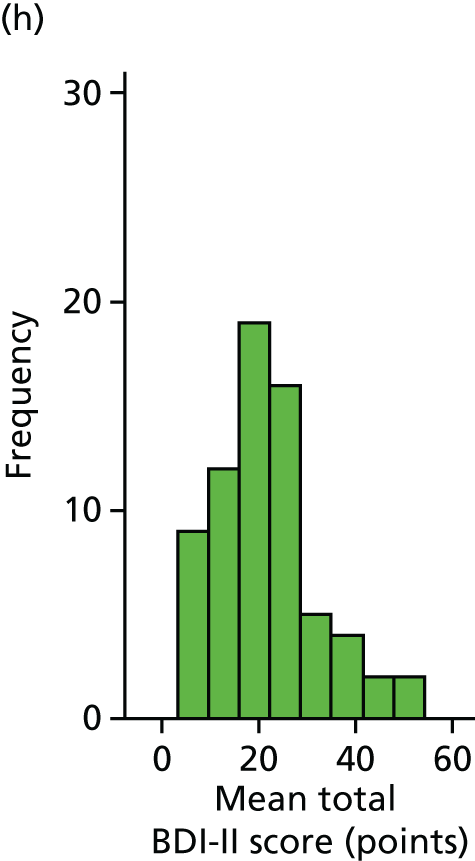
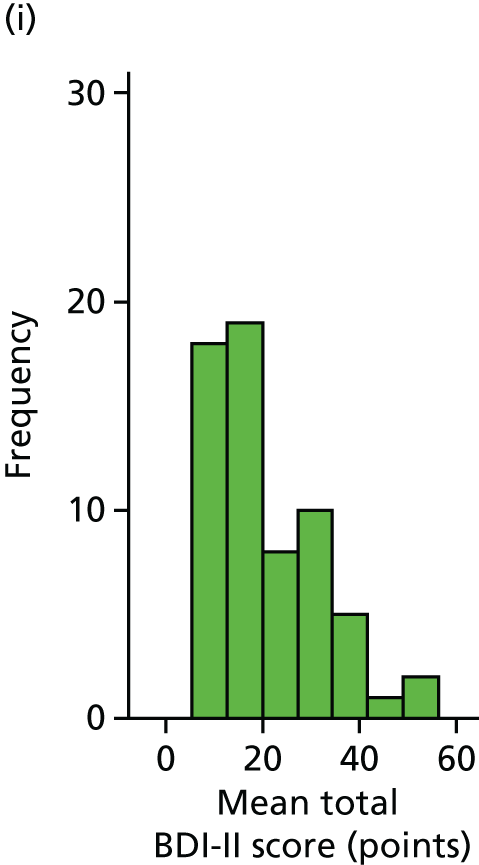
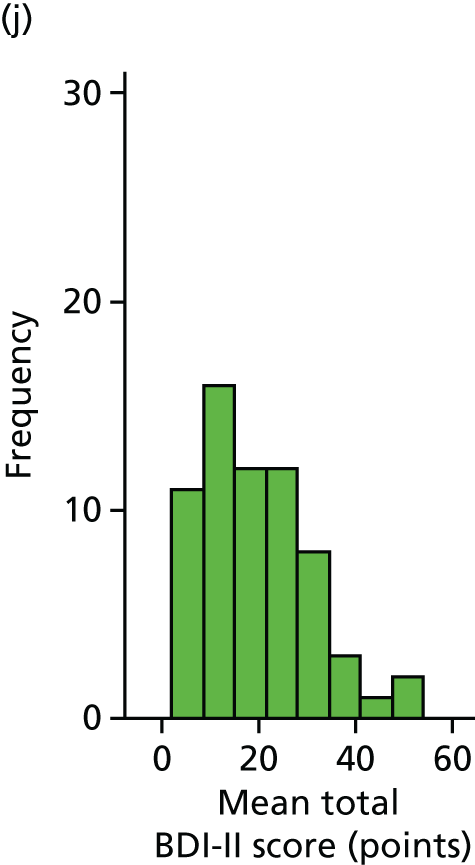
FIGURE 4.
Mean BDI-II score, by time and randomisation group.
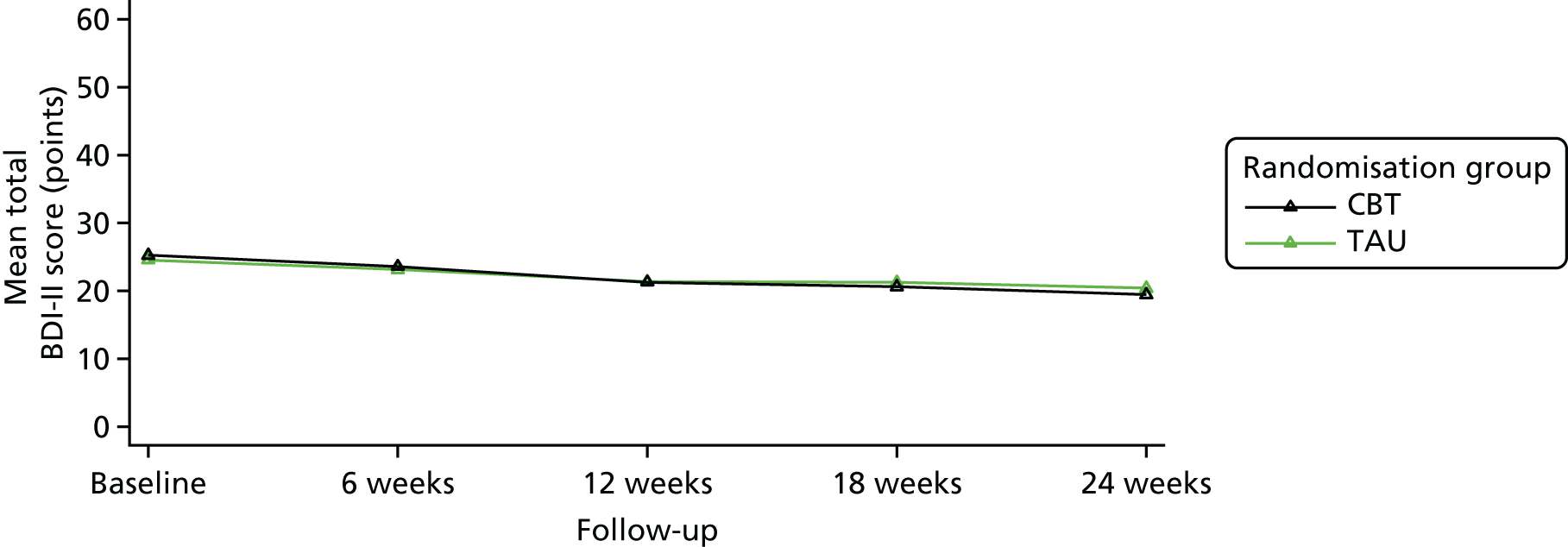
At baseline, the data were normally distributed, as exemplified by the similar mean and median scores (see Table 16). A BDI-II score of 20–28 points suggests the severity of depression to be moderate and a score of 29–63 points suggests severe depression. A total of 194 out of the 230 participants had at least moderate depression, 17 participants had mild mood disturbance (a BDI-II score of 14–19 points) and 19 participants had minimal depression (a BDI-II score of 0–13 points). BDI-II scores decreased for both groups by around 5 points during the course of the study. There appears to be a slight skewing of the data for CBT at 18 and 24 weeks. This is shown in Table 16 and Figure 3. The histograms show that there appears to be a shift to the left by week 12 for the CBT group with respect to the BDI-II score with TAU plus CBT (see Figure 3). The BDI-II scores for CBT and TAU, from baseline to 24 weeks, are also shown (see Figure 4).
Statistical modelling was conducted in accordance with the methods described in the original analysis plan (Table 17). There were no baseline differences for BDI-II, antidepressant use or clustering by therapist or IAPT service. Nor, using this model, were there any differences in previous history of depression, baseline EQ-5D, baseline duration of depression, or length between primary diagnosis and baseline visits. It is noteworthy that the ICC for clustering by therapist was low. Indeed, the analyses of the primary outcome variable (BDI-II) over all time points were identical, whether clustering by therapist or IAPT service, or whether no clustering at all was included in the model. This is because, averaged over time points, there was no evidence that the components of variance associated with therapist and IAPT service were other than zero.
| Model | Treatment effect (intervention – TAU) | 95% CI | p-value |
|---|---|---|---|
| Model with baseline BDI-II, baseline antidepressant use, time and group: clustering by therapista | |||
| Number in model = 185 | |||
| Estimates | –0.836 | –2.755 to 1.083 | 0.393 |
| Model with baseline BDI-II, baseline antidepressant use, time and group: clustering by IAPT service | |||
| Number in model = 185 | |||
| Estimates | –0.836 | –2.755 to 1.083 | 0.393 |
| Model with baseline BDI-II, baseline antidepressant use, time and group: no clustering by therapist | |||
| Number in model = 185 | |||
| Estimates | –0.836 | –2.755 to 1.083 | 0.393 |
| Model with baseline BDI, baseline antidepressant use, time and group: clustering by therapist. Plus baseline previous history of depression, baseline EQ-5D health score, baseline duration (weeks) of current depression and length (days) between primary diagnosis and baseline visit | |||
| Number in model = 122 | |||
| Estimates | 0.105 | –2.273 to 2.483 | 0.931 |
| Model with baseline BDI-II, baseline antidepressant use and group: clustering by therapist. 6 weeks’ follow-up only | |||
| Number in model = 168 | |||
| Estimates | –0.136 | –2.157 to 1.884 | 0.895 |
| Model with baseline BDI, baseline antidepressant use and group: clustering by therapist. 12 weeks’ follow-up only | |||
| Number in model = 148 | |||
| Estimates | –1.504 | –3.714 to 0.707 | 0.182 |
| Model with baseline BDI, baseline antidepressant use and group: clustering by therapist. 18 weeks’ follow-up only | |||
| Number in model = 134 | |||
| Estimates | –0.964 | –4.133 to 2.205 | 0.551 |
| Model with baseline BDI, baseline antidepressant use and group: clustering by therapist. 24 weeks’ follow-up only | |||
| Number in model = 130 | |||
| Estimates | –1.875 | –4.845 to 1.096 | 0.216 |
Although we did not find a significant benefit of CBT plus TAU versus TAU alone for the treatment of depression, participants who were widowed, divorced or separated had significantly poorer outcomes on the BDI-II without the addition of CBT to TAU (Table 18 and Figure 5). There was no clustering by therapist or IAPT service.
| Model | Treatment effect (intervention – TAU) | 95% CI | p-value |
|---|---|---|---|
| Model with baseline BDI, baseline antidepressant use, time and group: clustering by therapist. Plus group by time interaction | |||
| Number in model = 185, p-value for interaction = 0.471 | |||
| Estimates | 0.127 | –2.202 to 2.456 | 0.915 |
| 6 weeks | |||
| 12 weeks | –0.847 | –3.281 to 1.586 | 0.495 |
| 18 weeks | –1.365 | –3.875 to 1.146 | 0.287 |
| 24 weeks | –1.728 | –4.262 to 0.806 | 0.181 |
| Model with baseline BDI, baseline antidepressant use, time and group: clustering by therapist. Plus group by marital status interaction | |||
| Number in model = 183, p-value for interaction = 0.002 | |||
| Estimates | 0.645 | –1.791 to 3.081 | 0.604 |
| Married/partner | |||
| Widowed/divorced/separated | –7.211 | –11.147 to –3.276 | < 0.001 |
| Single, never married | 0.836 | –3.372 to 5.044 | 0.697 |
| Model with baseline BDI, baseline antidepressant use, time and group: clustering by therapist. Plus group by education status interaction | |||
| Number in model = 170, p-value for interaction = 0.710 | |||
| Estimates | –0.463 | –3.558 to 2.631 | 0.769 |
| Below A Level | |||
| A Level and above | –1.234 | –3.862 to 1.395 | 0.358 |
FIGURE 5.
Mean BDI-II score by marital status and randomisation group with time.
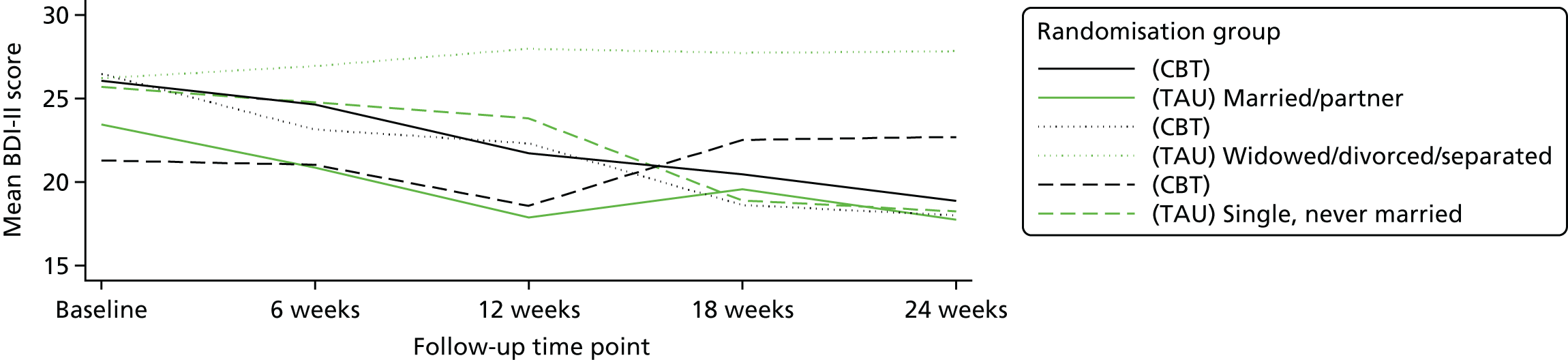
As shown in Figure 5, those in the TAU group who were widowed, divorced or separated remained unwell, whereas those who received CBT behaved similarly to the other participants, with some improvement in BDI-II scores.
Contamination-adjusted intention-to-treat analysis
Table 19 presents the frequency and percentage of the total number of CBT sessions attended by the 24-week follow-up in the intervention group. A number of patients were entered as ‘not included in the analysis’ because they did not have an 18- or a 24-week follow-up that was used to calculate the CAITT, the rationale being that, even though CBT sessions may have been received, the outcome data used to calculate the CAITT were missing for these individuals.
| CBT session (pre 24 weeks) | Frequency, n | % | Number of sessions |
|---|---|---|---|
| 0 | 15 | 13.0 | 0 |
| 1 | 4 | 3.5 | 4 |
| 2 | 4 | 3.5 | 8 |
| 3 | 5 | 4.4 | 15 |
| 4 | 4 | 3.5 | 16 |
| 5 | 3 | 2.6 | 15 |
| 6 | 1 | 0.9 | 6 |
| 7 | 5 | 4.4 | 35 |
| 8 | 3 | 2.6 | 24 |
| 9 | 13 | 11.3 | 27 |
| 10 | 4 | 3.5 | 40 |
| 11 | 5 | 4.4 | 55 |
| 12 | 6 | 5.2 | 72 |
| Total in the CAITT analysis | 43 | 62.6 | 317 |
| Not included in analysis | 37.4 | 226 |
For the modelling, a total of 153 individuals were included in the model [those with relevant outcome data (for the control and intervention group) and number of CBT sessions available (for the intervention group)].
The estimated ‘per-session’ effect on the BDI-II was –0.295 points (95% CI –0.760 to 0.170 points; p = 0.213). Thus, on average, every session of CBT would be expected to decrease the total BDI-II score by 0.3 points (compared with no sessions). However, this effect was not found to be statistically significant (p = 0.213).
Secondary outcomes
Secondary outcomes for the PHQ-9, ECOG-PS and satisfaction with care are shown (see Tables 20–22).
Patient Health Questionnaire-9 scores and the changes observed were similar to the BDI-II. Data for the PHQ-9 are shown in Table 20.
| Time point and randomisation group | Summary statistic | |||||||
|---|---|---|---|---|---|---|---|---|
| Min. | Q1 | Median | Q3 | Max. | Mean | SD | n | |
| Baseline | ||||||||
| TAU | 1 | 10 | 14 | 17 | 24 | 13.5 | 4.8 | 115 |
| CBT | 3 | 10 | 15 | 18 | 27 | 14.0 | 5.3 | 115 |
| Total | 1 | 10 | 14 | 17 | 27 | 13.8 | 5.1 | 230 |
| 12 weeks | ||||||||
| TAU | 0 | 8 | 11 | 15 | 26 | 11.4 | 5.8 | 79 |
| CBT | 0 | 6 | 9 | 15 | 26 | 10.3 | 5.7 | 68 |
| Total | 0 | 6 | 10 | 15 | 26 | 10.9 | 5.8 | 147 |
| 24 weeks | ||||||||
| TAU | 0 | 5 | 9 | 14 | 24 | 9.9 | 6.3 | 64 |
| CBT | 1 | 5 | 9 | 14 | 25 | 10.0 | 6.2 | 64 |
| Total | 0 | 5 | 9 | 14 | 25 | 9.9 | 6.2 | 128 |
Selection criteria stipulated that participants needed to have a PHQ-2 score of ≥ 3 points to enter into the trial. It is of note that one participant in the TAU group had a score of only 1 point on the PHQ-9 at baseline. This is because the baseline measures were repeated immediately prior to randomisation and scores may change in the time from the week at screening for entry into the trial to when the baseline measures were collected. Recovery in IAPT services is taken as < 10 points on the PHQ-9. The number of participants with scores of < 10 points in the TAU group at baseline, 12 and 24 weeks was 20, 33 and 35, respectively, and in the TAU plus CBT group at baseline, 12 and 24 weeks the number was 26, 37 and 33, respectively.
The PHQ-9 suggests that a score of 10–14 points indicates moderate depression and a score of 15–19 indicates moderately severe depression. As shown in Table 20, the scores are not skewed and consistent with our target population, namely people with a diagnosis of depression of moderate severity. At the end of treatment, both groups had median depressive symptoms at the mild end of the severity range (range 5–9 points).
Eastern Cooperative Oncology Group Performance Status scores suggested that only one-fifth of participants were fully active, with two-fifths being of restricted mobility (Table 21). Participants were equally balanced between the groups with respect to disability and this appeared to remain constant with time. Although the ECOG-PS questionnaire contains the category ‘dead’, these data were excluded in the analysis to maintain consistency with the primary outcome measure, as it is not possible to collect mood ratings from deceased participants. The CanTalk data suggest that participants’ physical functioning was towards the active end of the scale, with the greatest proportion being fully active or restricted, a similar portion being classed as ambulatory, fewer participants being limited and no participants being classed as disabled.
| Time point | Randomisation group | Total | ||||
|---|---|---|---|---|---|---|
| TAU | CBT | |||||
| n | % | n | % | n | % | |
| Baseline | ||||||
| Fully active | 23 | 20.0 | 22 | 19.1 | 45 | 19.6 |
| Restricted | 45 | 39.1 | 52 | 45.2 | 97 | 42.2 |
| Ambulatory | 34 | 29.6 | 29 | 25.2 | 63 | 27.4 |
| Limited | 13 | 11.3 | 12 | 10.4 | 25 | 10.9 |
| Disabled | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Dead | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total | 115 | 100.0 | 115 | 100.0 | 230 | 100.0 |
| 12 weeks | ||||||
| Fully active | 12 | 15.6 | 9 | 13.2 | 21 | 14.5 |
| Restricted | 33 | 42.9 | 30 | 44.1 | 63 | 43.4 |
| Ambulatory | 24 | 31.2 | 23 | 33.8 | 47 | 32.4 |
| Limited | 7 | 9.1 | 5 | 7.4 | 12 | 8.3 |
| Disabled | 1 | 1.3 | 1 | 1.5 | 2 | 1.4 |
| Dead | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total | 77 | 100.0 | 68 | 100.0 | 145 | 100.0 |
| 24 weeks | ||||||
| Fully active | 9 | 14.3 | 15 | 23.1 | 24 | 18.8 |
| Restricted | 25 | 39.7 | 25 | 38.5 | 50 | 39.1 |
| Ambulatory | 19 | 30.2 | 21 | 32.3 | 40 | 31.3 |
| Limited | 10 | 15.9 | 4 | 6.2 | 14 | 10.9 |
| Disabled | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Dead | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total | 63 | 100.0 | 65 | 100.0 | 128 | 100.0 |
The mean satisfaction with care was 80% (40 out of a total score of 50 points) at baseline (prior to randomisation) and did not change with time or by treatment group (Table 22). This finding is also represented graphically (Figure 6).
| Time point and randomisation group | Summary statistic | |||||||
|---|---|---|---|---|---|---|---|---|
| Min. | Q1 | Median | Q3 | Max. | Mean | SD | n | |
| Baseline | ||||||||
| TAU | 11 | 36 | 40 | 47 | 50 | 39.7 | 9.2 | 114 |
| CBT | 14 | 35 | 41 | 47 | 50 | 39.6 | 8.3 | 115 |
| Total | 11 | 35 | 40 | 47 | 50 | 39.6 | 8.7 | 229 |
| 12 weeks | ||||||||
| TAU | 16 | 38 | 42 | 46 | 50 | 40.8 | 7.3 | 79 |
| CBT | 4 | 33 | 43 | 48 | 50 | 39.5 | 10.1 | 68 |
| Total | 4 | 36 | 43 | 47 | 50 | 40.2 | 8.7 | 147 |
| 24 weeks | ||||||||
| TAU | 11 | 36 | 43 | 47 | 50 | 40.8 | 8.4 | 64 |
| CBT | 11 | 33 | 44 | 47 | 50 | 39.4 | 10.1 | 65 |
| Total | 11 | 35 | 43 | 47 | 50 | 40.1 | 9.3 | 129 |
FIGURE 6.
Satisfaction with care total score, by randomisation group and time for each follow-up.
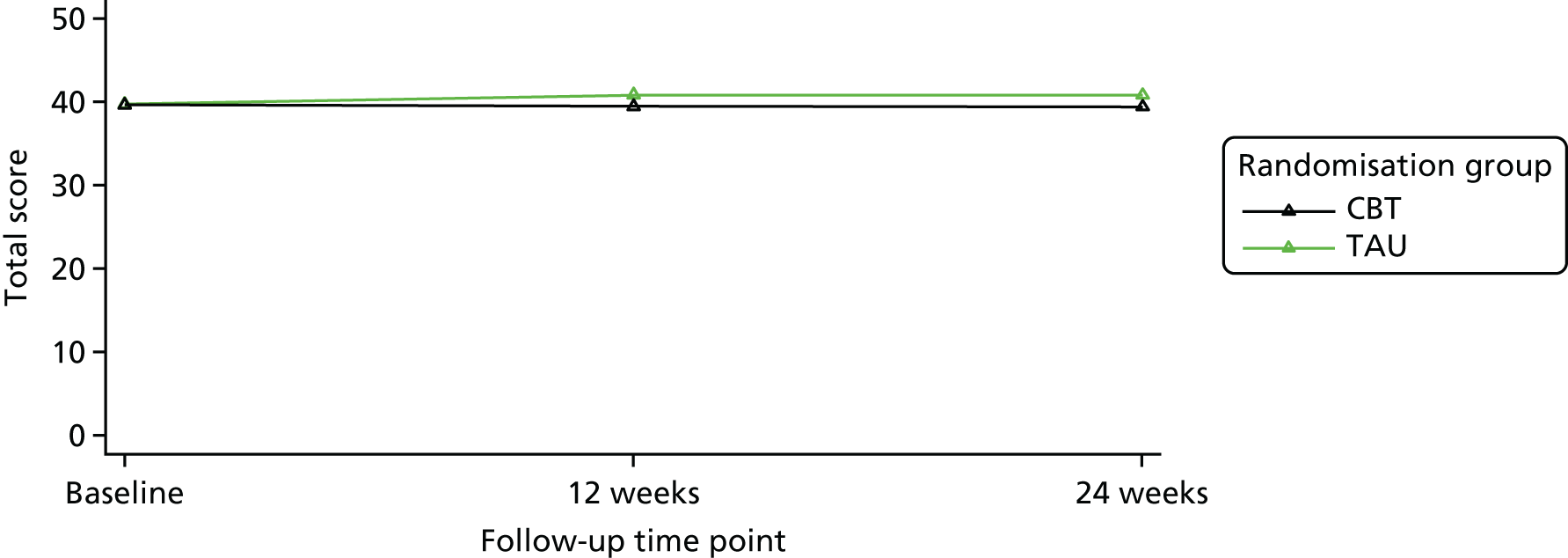
Intervention
Training for the intervention
Training was delivered to 129 therapists and information regarding their experience of the training session was collected from 22% of them (29/129) by asking them to rate the questions on a 4-point Likert scale (Table 23).
| Section | Feedback, mean score (SD) | ||
|---|---|---|---|
| Helped my understanding (0–3) | Helped my skills (0–3) | Helped my confidence (0–3) | |
| Psychological distress in cancer and the application of CBT | 2.82 (0.39) | 2.69 (0.47) | 2.62 (0.50) |
| Overview of CanTalk trial | 2.80 (0.48) | 2.62 (0.56) | 2.67 (0.56) |
| Overview of CBT in CanTalk | 2.93 (0.26) | 2.85 (0.37) | 2.88 (0.34) |
| First phase of therapy (sessions 1–4) | 2.89 (0.31) | 2.77 (0.51) | 2.77 (0.51) |
| Demonstration video: hot cross bun | 2.82 (0.66) | 2.95 (0.22) | 2.85 (0.37) |
| Small group exercise: formulation and treatment planning | 2.71 (0.56) | 2.67 (0.58) | 2.7 (0.57) |
| Middle phase of therapy (sessions 5–8) | 2.93 (0.27) | 2.73 (0.45) | 2.80 (0.41) |
| Demonstration video: working with patient with severe physical symptoms | 2.91 (0.30) | 2.91 (0.30) | 2.80 (0.42) |
| Final phase of therapy (sessions 9–12) | 2.89 (0.32) | 2.73 (0.45) | 2.76 (0.44) |
| Existential issues | 2.85 (0.36) | 2.81 (0.40) | 2.64 (0.49) |
| Specific topics decided on the day | 2.86 (0.38) | 3.00 (0.00) | 2.67 (0.52) |
Take-up and engagement with cognitive–behavioural therapy
A total of 543 (39.3%) sessions out of a potential total of 1380 sessions (12 × 115) were taken up, of which 32 were by telephone (5.9%): seven participants took up one telephone session, three participants took up two sessions, one participant took up three sessions, one participant took up four sessions and one participant took up 12 sessions. The mean time from being referred to being seen by IAPT was 29.4 days (SD 26.7 days).
Among the total of 115 participants randomised to CBT, the mean number of sessions received was 4.7 (SD 4.9). It is notable that, of the 115 participants allocated to CBT, 41 (35.6%) did not take up any sessions and 36 (31.3%) had at least eight sessions. The reason for sessions not being received was predominantly participants withdrawing after randomisation (Table 24). The median time from being referred to being seen by IAPT was 21 days [quartile range (QR) 13–37 days].
| Therapy status | n (%) |
|---|---|
| Not referred | 6 (5.2) |
| Withdrew prior to commencing therapy | 26 (22.6) |
| Deceased prior to commencing therapy | 2 (1.7) |
| Discharged prior to commencing therapy | 5 (4.3) |
| Withdrew after commencing therapy | 21 (18.3) |
| Deceased after commencing therapy | 4 (3.5) |
| Discharged after commencing therapy | 13 (11.3) |
| Completed therapya | 36 (31.3) |
| No information available | 2 (1.7) |
| Total | 115 (100.0) |
Not referred
Six participants were not referred to therapy: two moved out of the IAPT area, two were not referred to the therapist by the IAPT administrator, one declined to be referred and one could not be contacted. A total of 26 participants withdrew prior to commencing therapy: 12 had physical problems, five were no longer depressed, four were too busy, two were unable to travel, one declined therapy, one received therapy outside the study and one gave no reason. Five participants were discharged by IAPT services prior to starting therapy: four were reported to be no longer depressed and one was receiving therapy elsewhere. A total of 21 participants withdrew after commencing therapy without giving a reason. Thirteen participants were discharged by IAPT service after commencing therapy: six did not attend on two or more occasions, three were too unwell to continue attending, three did not feel that CBT was beneficial and one could not be contacted to continue therapy. A total of 36 participants completed 8–12 sessions of therapy.
Withdrawal and time to referral
Participants commented in the qualitative interviews that the delay between the time of assessment and referral was an issue. Thus, although not included in the original analysis plan, we were interested to know whether or not this may have had an impact on retention. As shown in Table 24, 47 participants withdrew early (26 before commencing therapy and 21 soon after commencing therapy), whereas 36 participants demonstrated engagement with therapy, having received 8–12 sessions. Data were available for 30 out of 47 (63.8%) participants who withdrew early and for 27 out of 36 (75%) who completed therapy. The median time from referral to seeing a therapist for those who withdrew early was 26 days (QR 16.0–41.24 days) and, for the 36 who were well engaged, was 16.0 days (QR 11.0–35.0 days).
Quality of cognitive–behavioural therapy and adherence to manual
Quality of cognitive–behavioural therapy using the Cognitive Therapy Scale – Revised
Of the 543 therapy sessions delivered, 55 tapes, namely 1 in 10, were rated. Of these, 21 (38%) were from the initial phase of therapy (sessions 1–4), 19 (35%) were from the middle phase (sessions 5–8) and 15 (27%) were from the final phase of therapy (sessions 9–12). The mean CTS-R score was 47.6 points (SD 13.8 points) (the upper end of the ‘proficient’ range). Forty-seven (90%) out of 52 tapes scored ≥ 36 points on independent ratings of the CTS-R. The mean CTS-R score by phase of therapy was 47.9 points (SD 10.6 points) for 21 early sessions, 48.1 points (SD 18.8 points) for 19 middle sessions and 46.7 points (SD 10.9 points) for 15 late sessions. These scores indicate strong adherence to the therapeutic model in CBT.
Elements delivered in therapy recorded in the Therapy Components Checklist
Subjective TCCs from therapists were returned for 293 out of 543 (54%) therapy sessions delivered. The total number of the elements ticked by the therapist and the proportion of times a particular intervention was used is shown in Table 25. Guided discovery, activity scheduling, discussion about specific cancer topics, covering the impact of the physical illness, and beliefs and expectations about the illness were most the most common interventions. It also needs to be noted that general procedures ranged from 9.9% to 19.5% of sessions. However, general procedures would depend very much on the stage of therapy.
| Component | Frequency with which the component was used,a n | Percentage of sessions in which the component was used |
|---|---|---|
| General procedures | ||
| Total | 178 | 60.8 |
| Initial assessment | 48 | 16.4 |
| Describe Beck’s model and concept of CBT | 47 | 16.0 |
| Agree goals of therapy | 57 | 19.5 |
| Present a shared formulation | 35 | 11.9 |
| Goal-setting | 55 | 18.8 |
| Review of shared formulation | 35 | 11.9 |
| Review of success list | 29 | 9.9 |
| Relapse prevention/future planning | 33 | 11.3 |
| Behavioural techniques | ||
| Total | 109 | 37.2 |
| Relaxation training | 21 | 7.2 |
| Breathing space | 9 | 3.1 |
| Activity schedule | 86 | 29.4 |
| Pleasure experiences sheet | 23 | 7.8 |
| Cognitive techniques | ||
| Total | 168 | 57.3 |
| Refocusing techniques | 25 | 8.5 |
| Mindfulness | 12 | 4.1 |
| Four-step process for resilience and coping | 18 | 6.1 |
| Coping map | 12 | 4.1 |
| List of strengths and resources | 47 | 16.0 |
| Reattribution | 30 | 10.2 |
| Decatastrophising | 30 | 10.2 |
| Advantages/disadvantages | 27 | 9.2 |
| Success list | 26 | 8.9 |
| Thoughts diary | 38 | 13.0 |
| Personal rule (pros/cons) | 26 | 8.9 |
| Managing worry (worry tree handout) | 17 | 5.8 |
| Blueprint for coping | 28 | 9.6 |
| Cognitive–behavioural techniques | ||
| Total | 169 | 57.7 |
| Guided discovery | 117 | 39.9 |
| Pleasure prediction sheet | 8 | 2.7 |
| Pleasure experiences sheet | 16 | 5.5 |
| Negative triad/negative automatic thoughts | 46 | 15.7 |
| Applying resilience | 43 | 14.7 |
| Thinking traps handout | 36 | 12.3 |
| Reality testing | 30 | 10.2 |
| Searching for alternatives | 43 | 14.7 |
| ABC form | 16 | 5.5 |
| Specific cancer topics | ||
| Total | 205 | 70.0 |
| Impact of physical illness | 123 | 42.0 |
| Beliefs and expectations about illness | 104 | 35.5 |
| Plans and hopes for care as disease advances | 58 | 19.8 |
| Relationship between emotional and physical symptoms | 59 | 20.1 |
| Concerns about current and future ability to cope | 75 | 25.6 |
| Concerns about loss of control | 40 | 13.7 |
| Concerns about accepting help | 57 | 19.5 |
| Concerns about dying (mode/afterwards/life expectancy) | 40 | 13.7 |
| Impact of disease and mood on behaviour | 85 | 29.0 |
| Impact of disease/death on loved ones | 90 | 30.7 |
| Discussion of ‘the meaning’ of the illness | 30 | 10.2 |
| Acceptance of unfinished business | 8 | 2.7 |
Validity of therapists’ self-rating using the Therapy Components Checklist
We present the comparison of therapists’ self-reports of what their intervention was and the observer rating for that particular therapy session (Table 26). The TCC has 46 potential responses. Given that we had paired objective and subjective ratings in 39 TCC responses, a potential of 1794 data points were available. The frequencies are as follows: both the therapist and observer agreed that the intervention took place in 66 data points; neither the therapist nor the independent rater believed that an intervention took place in 1431 data points; the therapist considered that an intervention took place but the independent rater did not in 212 data points; and the therapist considered that the intervention was not delivered but the independent rater felt that it was in 85 data points. Data with Kappa scores of ≥ 0.7 are considered significant.
| Intervention used | Agreement | PABAK score | |||
|---|---|---|---|---|---|
| Ticked by therapist and observer | Not ticked by therapist or observer | Ticked by therapist but not observer | Ticked by observer but not therapist | ||
| General procedures | |||||
| Initial assessment | 0 | 36 | 3 | 0 | 0.85 |
| Describe Beck’s model and concept of CBT | 5 | 31 | 3 | 0 | 0.85 |
| Agree goals of therapy | 3 | 29 | 5 | 2 | 0.64 |
| Present a shared formulation | 1 | 29 | 4 | 5 | 0.54 |
| Goal-setting | 1 | 28 | 9 | 1 | 0.49 |
| Review of shared formulation | 0 | 33 | 4 | 2 | 0.69 |
| Review of success list | 1 | 32 | 6 | 0 | 0.69 |
| Relapse prevention/future planning | 5 | 32 | 1 | 1 | 0.90 |
| Behavioural techniques | |||||
| Relaxation training | 2 | 36 | 1 | 0 | 0.95 |
| Breathing space | 1 | 37 | 1 | 0 | 0.95 |
| Activity schedule | 4 | 23 | 9 | 3 | 0.38 |
| Pleasure experiences sheet | 0 | 37 | 2 | 0 | 0.90 |
| Cognitive techniques | |||||
| Refocusing techniques | 1 | 35 | 2 | 1 | 0.85 |
| Mindfulness | 0 | 37 | 2 | 0 | 0.90 |
| Four-step process for resilience and coping | 0 | 32 | 6 | 1 | 0.64 |
| Coping map | 0 | 34 | 2 | 3 | 0.74 |
| List of strengths and resources | 0 | 28 | 7 | 4 | 0.44 |
| Reattribution | 0 | 35 | 1 | 3 | 0.79 |
| Decatastrophising | 0 | 38 | 1 | 0 | 0.95 |
| Advantages/disadvantages | 1 | 35 | 3 | 0 | 0.85 |
| Success list | 1 | 32 | 3 | 3 | 0.69 |
| Thoughts diary | 1 | 29 | 6 | 3 | 0.54 |
| Personal rule (pros/cons) | 0 | 37 | 1 | 1 | 0.90 |
| Managing worry (worry tree handout) | 1 | 34 | 2 | 2 | 0.79 |
| Blueprint for coping | 1 | 31 | 4 | 3 | 0.64 |
| Cognitive–behavioural techniques | |||||
| Guided discovery | 9 | 11 | 5 | 14 | 0.03 |
| Pleasure prediction sheet | 0 | 39 | 0 | 0 | 1.00 |
| Pleasure experiences sheet | 0 | 37 | 1 | 1 | 0.90 |
| Negative triad/negative automatic thoughts | 0 | 32 | 5 | 2 | 0.64 |
| Applying resilience | 0 | 33 | 6 | 0 | 0.69 |
| Thinking traps handout | 4 | 26 | 3 | 6 | 0.54 |
| Reality testing | 3 | 29 | 2 | 5 | 0.64 |
| Searching for alternatives | 0 | 33 | 4 | 2 | 0.69 |
| ABC form | 1 | 35 | 2 | 1 | 0.85 |
| Specific cancer topics | |||||
| Impact of physical illness | 2 | 18 | 15 | 4 | 0.03 |
| Beliefs and expectations about illness | 5 | 21 | 10 | 3 | 0.33 |
| Plans and hopes for care as disease advances | 2 | 27 | 10 | 0 | 0.49 |
| Relationship between emotional/physical symptoms | 0 | 34 | 5 | 0 | 0.74 |
| Concerns about current/future ability to cope | 0 | 27 | 12 | 0 | 0.38 |
| Concerns about loss of control | 1 | 32 | 5 | 1 | 0.69 |
| Concerns about accepting help | 1 | 31 | 6 | 1 | 0.64 |
| Concerns about dying | 3 | 29 | 4 | 3 | 0.64 |
| Impact of disease and mood on behaviour | 2 | 22 | 14 | 1 | 0.23 |
| Impact of disease/death on loved ones | 3 | 24 | 10 | 2 | 0.38 |
| Discussion of ‘the meaning’ of the illness | 1 | 33 | 4 | 1 | 0.74 |
| Acceptance of unfinished business | 0 | 38 | 1 | 0 | 0.95 |
The findings suggest that there was a strong relationship between therapist’s self-report and the independent rater’s report for most of the components delivered or not delivered, with the majority of PABAK scores being > 0.70. Notably low PABAK agreement scores were for the items ‘Guided discovery’ and ‘Impact of physical illness’ (both of which had a PABAK score of 0.03, indicating very little agreement). We would like to point out that guided discovery, believed to be an essential component of CBT, was reported by therapists as being delivered in 39.9% of sessions. However, the observer’s report suggested that therapists were indeed using more guided discovery than they reported. By contrast, therapists reported more discussion about the patients’ physical illness than reported by the observer.
Qualitative findings
Health-care workers’ views about the CanTalk trial
An additional qualitative substudy was conducted, looking at the views of 14 health-care workers. This study looked at health-care workers’ views of psychological [non-Clinical Trial of Investigative Medicinal Product (CTIMP)] research in general and of the CanTalk trial more specifically. An initial analysis of the data generated the following emerging themes.
Recruitment issues
Themes include issues with (1) finding the time to conduct these trials, (2) money (with the perception that CTIMPs provide better financial incentives), (3) the competing interests of CTIMP trials and (4) geographical catchment areas, in that not all participants were eligible to participate in CanTalk owing to the limitations of IAPT catchment areas.
The role of the clinician
Themes include (1) the influence of the clinician on their team’s involvement with the study, (2) the influence of the clinician on patients in encouraging participation, (3) ‘gatekeeping’, in which some clinicians may try to protect participants from research studies and (4) the importance of the clinicians’ initial agreement to involvement in the study.
The sensitive nature of the research
Themes include (1) psychological research ‘opening a can of worms’ with health-care workers who are unsure how to help patients if they become distressed, (2) issues about how the study fits in or clashes with existing psychological services and teams that operate within the hospital and (3) concerns regarding people with significant psychological issues who may not be eligible to be referred to the trial.
The role of the trial team
Issues include (1) the study being ‘mis-sold’ to hospital research units, with the trial team presenting the study as easier to recruit for than it turned out to be and (2) members of the trial team recruiting at clinics being important for the success of the study, given the limited research resources of hospitals and the trial team’s more detailed knowledge of the study.
Therapists’ views of treating patients with advanced cancer
Sixteen therapists were interviewed qualitatively using semistructured questionnaires and their repsonses transcribed and coded onto a Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA) spreadsheet. Although such interviews were not required for the purpose of this report, a brief summary is presented here.
Background information
The range of experience of therapists interviewed was 1–18 years (median 8 years). The 16 therapists had treated 29 out of 33 assessed participants (four had dropped out or were withdrawn for health reasons). A total of 193 sessions were delivered, 14 of which were by telephone contact.
Knowledge of cancer
Nine out of 16 therapists had treated a patient with cancer previously and five indicated that they had direct experience of cancer with family or a friend. General themes suggested that therapists would have liked more knowledge about cancer, but had gained this from talking to the patient and most did not feel that it adversely affected the therapeutic process.
Experience of training
The overall experience of training on how to apply CBT skills to people with cancer was felt to be excellent, although therapists would have liked to have had more information about the type of cancer and how treatment may have affected the patient.
Concerns about treating advanced cancer patients
Therapists described feeling anxious about treating cancer patients and were concerned that applying CBT to this patient group may appear to be trivialising their situation and feared not having sufficient expertise. Therapists had mixed views about the degree of physical ill heath to expect, but were surprised how receptive patients were to the CBT model and how they could work psychologically.
Support and supervision
Therapists reported variability about the quality of support from their regular supervisors and thought that it would be helpful for more supervisors to have attended the CanTalk training. Therapists would also have liked more specialist support from the CanTalk supervisors.
Experience of working with patients
Therapists generally found it easy to co-ordinate meetings with patients, but recognised that their physical problems posed a challenge. They tried to be more flexible in their delivery of care and felt that patients were motivated and could challenge unhelpful beliefs in therapy. They found their experience of working with this patient group more positive than expected. However, a number of therapists indicated that the issues that came up in therapy were not always cancer related.
Therapy materials
Therapists generally liked the materials, but felt that it was not always easy to stick to the manual. They found themselves having to tailor the treatment to the needs of the patient, requiring more flexibility than they thought they were allowed.
Patients’ views of cognitive–behavioural therapy
Background of patients interviewed for qualitative element
Qualitative interviews were conducted with 10 patients who had received CBT. The mean number of sessions received was 9.4. Of these patients, eight were female and three came from one IAPT service, with the remaining seven coming from seven different IAPT services. Four were diagnosed with lung cancer, four with breast cancer, one with colorectal cancer and one with Non-Hodgkin lymphoma. The majority had some awareness that they were depressed.
Expectations and knowledge of cognitive–behavioural therapy
Most patients had no prior experience of CBT but had some limited knowledge about what the therapy would involve. Most people wanted to participate in the study because they wanted to seek support for depression or because they had an interest in psychological therapies and research. The patients who had previously received psychodynamic therapy found CBT to be more clinically effective, with more time being appropriately given to their diagnosis.
Structure and delivery of cognitive–behavioural therapy
The mean number of CBT sessions received by those interviewed was 9.4. There was a general view that the location of the IAPT service was convenient and that the appointments were well organised and flexible. Some patients were dependent on a taxi service to travel to the appointments (which was funded for by the study). Transport problems could be a barrier to patients attending therapy at an IAPT service. The average waiting time between referral and starting therapy varied largely between participants. Some participants were very happy with the referral process, whereas others felt that it was problematic, especially because of the delay between being referred and being seen.
Experience of cognitive–behavioural therapy
Patients’ experience of CBT was that it was helpful, especially as it was practical and non-threatening. They also reported that CBT facilitated their ability to talk to family and friends about cancer and that it had a positive impact on their social life and helped them to cope. Participants felt that they developed effective cognitive skills to help them deal with negative or unhelpful thinking.
Although patients would recommend CBT to others, they wondered whether or not simple emotional support may be what is needed. Most patients felt that it would have been helpful for therapists to know more about their diagnosis and treatment, as they often had to inform them of this. All of the participants spoke positively about their therapist and identified them as being professional, empathetic and genuinely interested in them.
Although the general view was that CBT was helpful and covered all the areas that participants felt was necessary, they reported that the opportunity to just talk about their feelings was useful in itself. Participants largely felt that CBT had a positive impact on their life, with only one participant commenting on a negative impact of CBT, namely that they found completing homework tasks difficult. The majority of participants who had completed therapy were aware of how to seek help in the future and how to make use of relapse prevention techniques.
Other therapeutic options for advanced cancer patients
Although participants stated that CBT should be offered, it was generally felt that CBT is not apprpriate for everyone and that therapy should be tailored to the needs of the patient. There was a sense that patients should be given space to talk, but that this need not necessarily be through the medium of CBT, and that other interventions, such as counselling, may be more suitable. The majority of participants said that they would recommend CBT to other cancer patients. Participants thought that psychological therapy should be offered to all patients with advanced cancer.
Improvements to therapy
Suggestions for improvement were varied. One participant felt that therapy would be better placed in the hospital where the patient is receiving cancer treatment as this would be more convenient and would fit around their existing hospital appointments. Two patients highlighted that it would have been useful to have been given a manual/workbook to take away from therapy to enable them to reflect on the session at home. One participant suggested that individual therapy for carers would be beneficial to help them deal with some of the emotional difficulties that come with the caring role. Some patients felt that weekly sessions were too time demanding and that bi-monthly appointments would have been more suitable.
Adverse events
The only adverse event (AE) stipulated in the protocol was an increase in suicidality or a death being under investigation for possible suicide by a coroner. No AEs were recorded.
The study protocol did not require the routine reporting of deaths and instances of hospitalisation as ‘SAEs’, given their expectedness within this study population. Despite this, clinicians reported five events as SAEs over the course of the study. These were two SAEs for patients in the CBT group (one death and one hospitalisation) and three SAEs in the TAU group (two deaths and one hospitalisation). However, these deaths were not unexpected and not SAEs as defined in the protocol. We did, of course, record deaths in each arm over the course of the study. Of a total of 21 recorded deaths, nine were in the CBT arm and 12 were in the TAU arm.
Chapter 6 Health economics results
A health and social care perspective was adopted in line with NICE’s recommendations. 151 Service use costs were calculated from resource data to calculate the total cost of resources used by each study participant. QALYs were calculated from EQ-5D scores at baseline, 12 and 24 weeks’ follow-up. Costs were compared for the groups using a bootstrap regression model to account for non-normality in the distribution of cost data. A cost–utility analysis was undertaken using QALYs calculated from the EQ-5D measure. Cost-effectiveness was assessed by estimating an ICER to show the extra cost incurred by CBT to generate one extra QALY. To deal with uncertainty around the ICER, a CEP and CEACs were created.
Sample size
Full economic data were available for 230 participants at baseline, 148 participants at 12 weeks’ and 128 participants at 24 weeks’ follow-up.
Service use
As there was no expectation that hospital utilisation would be affected by the intervention, its inclusion would have introduced more variability to the costs. Hence, the proportion of those who used different community health services and the mean number of contracts that the participants made are presented in Table 27.
| Service | Time point | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-week follow-up | 24-week follow-up | ||||||||||||||||
| CBT (n = 115) | TAU (n = 115) | CBT (n = 68) | TAU (n = 80) | CBT (n = 64) | TAU (n = 64) | |||||||||||||
| n (%) | Mean | SD | n (%) | Mean | SD | n (%) | Mean | SD | n (%) | Mean | SD | n (%) | Mean | SD | n (%) | Mean | SD | |
| Number of contacts | ||||||||||||||||||
| GP | 99 (86) | 3.1 | 2.4 | 108 (94) | 3.4 | 2.8 | 64 (94) | 2.6 | 1.8 | 73 (91) | 2.9 | 2.3 | 52 (81) | 2.9 | 2.2 | 51 (80) | 2.8 | 2.2 |
| District nurse | 36 (31) | 2.2 | 1.7 | 31 (27) | 2.2 | 2.5 | 34 (50) | 1.3 | 0.6 | 28 (35) | 1.5 | 0.6 | 24 (38) | 1.8 | 1.3 | 26 (41) | 1.4 | 0.6 |
| Practice nurse | 77 (67) | 4.79 | 9.21 | 72 (63) | 4.29 | 6.29 | 48 (71) | 3.67 | 2.33 | 53 (66) | 4.39 | 3.43 | 41 (64) | 3.34 | 5.56 | 39 (61) | 3.16 | 5.27 |
| Specialist nurse | 54 (47) | 6 | 4.5 | 40 (35) | 5.9 | 3.5 | 42 (62) | 3.5 | 3.9 | 37 (46) | 2.9 | 2.4 | 40 (63) | 3.3 | 3 | 39 (61) | 2.8 | 1.8 |
| Occupational therapist | 43 (37) | 4.8 | 3.2 | 42 (37) | 6.7 | 12 | 55 (81) | 3.6 | 5.5 | 51 (64) | 3.5 | 3 | 42 (66) | 2.6 | 1.3 | 43 (67) | 2.4 | 1.5 |
| Physiotherapist | 37 (32) | 11.8 | 18.1 | 40 (35) | 10 | 12.8 | 43 (63) | 6.8 | 8.6 | 40 (50) | 6.8 | 9 | 39 (61) | 5.6 | 6.2 | 37 (58) | 9.9 | 14.7 |
| Community matron | 16 (14) | 2.3 | 1.3 | 17 (15) | 4.6 | 4 | 16 (24) | 2.8 | 2.9 | 15 (19) | 2.9 | 2.5 | 14 (19) | 3.4 | 3.8 | 13 (20) | 4.6 | 5.3 |
| Mental health services | 73 (63) | 2.9 | 3 | 82 (71) | 2.7 | 1.4 | 52 (76) | 2.8 | 2.9 | 55 (69) | 2.9 | 2.5 | 51 (80) | 2.5 | 2.1 | 50 (78) | 2.8 | 2.2 |
| Social care | 47 (41) | 4.6 | 3.3 | 66 (57) | 4.7 | 3.9 | 42 (62) | 3.1 | 3.5 | 50 (63) | 3.5 | 3.4 | 37 (58) | 3.5 | 2.6 | 43 (67) | 3.1 | 2.8 |
| Palliative care | 0 | – | – | 0 | – | – | 3 (4) | 1 | 0 | 2 (3) | 3 | 2.7 | 3 (5) | 1.5 | 1.6 | 1 (2) | 1 | – |
At baseline, service use was, for the most part, similar between the two groups. Although more participants in the TAU group than the CBT group (94% vs. 86%) accessed their GP and had slightly more appointments, the difference was not significant. Similar non-significant differences were observed in the proportions of participants reporting regular contact with a psychiatrist (TAU 71% vs. CBT 63%) and social services (TAU 57% vs. CBT 41%). Participants in the CBT group were more likely to report being in contact with a specialist nurse (CBT 47% vs. TAU 35%).
At both 12- and 24-week follow-up stages, no significant differences in the service use between the two groups were apparent. Use of GP services decreased slightly at 24-week follow-up for both groups.
Baseline access to community mental health services was similar in both the CBT plus TAU group (78%) and the TAU alone group (71%). The frequency of contacts with mental health community services (excluding IAPT) increased at the 12-week follow-up for both groups (89% for CBT and 90% for TAU) and slightly decreased for CBT but increased for TAU at the 24-week follow-up (80% and 94%, respectively). There were no between-group difference with time with respect to the BDI-II and PHQ-9.
The number of contacts with social care declined steadily in both groups throughout the follow-up period, while the demand for palliative care services increased during that period.
The mean service costs for participants (not including the costs of the interventions) are presented in Table 28.
| Service | Time point, cost (£) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-week follow-up | 24-week follow-up | ||||||||||
| CBT (n = 115) | TAU (n = 115) | CBT (n = 68) | TAU (n = 80) | CBT (n = 64) | TAU (n = 64) | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| GP | 81 | 118 | 92 | 108 | 53 | 63 | 63 | 101 | 64 | 100 | 76 | 90 |
| District nurse | 21 | 39 | 21 | 41 | 14 | 18 | 15 | 20 | 14 | 20 | 15 | 23 |
| Practice nurse | 16 | 20 | 17 | 28 | 30 | 58 | 38 | 77 | 21 | 30 | 23 | 34 |
| Specialist nurse | 72 | 151 | 40 | 83 | 33 | 83 | 22 | 39 | 28 | 60 | 24 | 40 |
| Occupational therapist | 46 | 143 | 62 | 211 | 30 | 94 | 30 | 93 | 28 | 98 | 34 | 117 |
| Physiotherapist | 86 | 160 | 74 | 160 | 43 | 89 | 65 | 118 | 77 | 231 | 53 | 92 |
| Community matron | 26 | 98 | 65 | 180 | 35 | 153 | 22 | 60 | 17 | 38 | 17 | 29 |
| Psychiatrist/mental health services | 90 | 162 | 82 | 114 | 61 | 133 | 72 | 125 | 51 | 90 | 60 | 104 |
| Social care | 64 | 129 | 43 | 84 | 29 | 63 | 37 | 65 | 22 | 51 | 26 | 122 |
| Palliative care | 0 | 0 | 0 | 0 | 45 | 78 | 30 | 68 | 38 | 74 | 27 | 62 |
| Total costs | 502 | 1020 | 496 | 1009 | 373 | 832 | 394 | 766 | 360 | 792 | 355 | 713 |
The figures were similar across the two randomisation groups. Compared with baseline, costs associated with service use increased for both randomisation groups with follow-up. The differences in costs between the two randomisation groups at both follow-ups were marginal.
Costing the intervention
In total, 1380 sessions were available for participants in the CBT arm; however, fewer than half (n = 543) were taken up. A total of 65% of CBT arm participants attended at least one session and 31% attended at least eight sessions. The mean number of sessions attended in the CBT arm per participant was 4.8. It was assumed that when a session was offered but a participant did not attend it still constituted the IAPT’s therapist opportunity cost. The mean cost of CBT intervention was £948.75 (SD £427.00) per participant.
Quality-adjusted life-years
Line plots for EQ-5D VAS and histograms for EQ-5D index scores are presented in Figures 7 and 8, respectively.
FIGURE 7.
Median EQ-5D VAS scores at baseline, 12 and 24 weeks, by time and randomisation group.
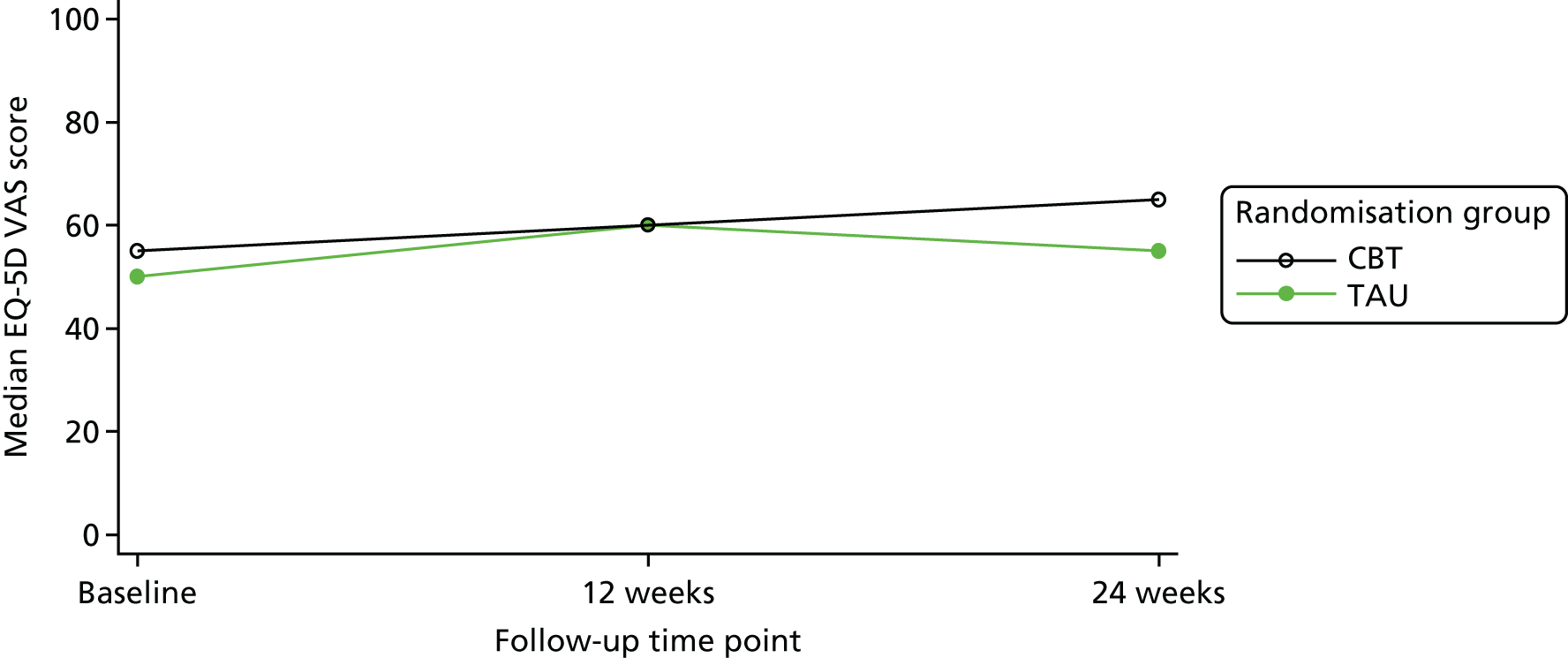
FIGURE 8.
Mean EQ-5D values at baseline, 12 and 24 weeks, by randomisation group.
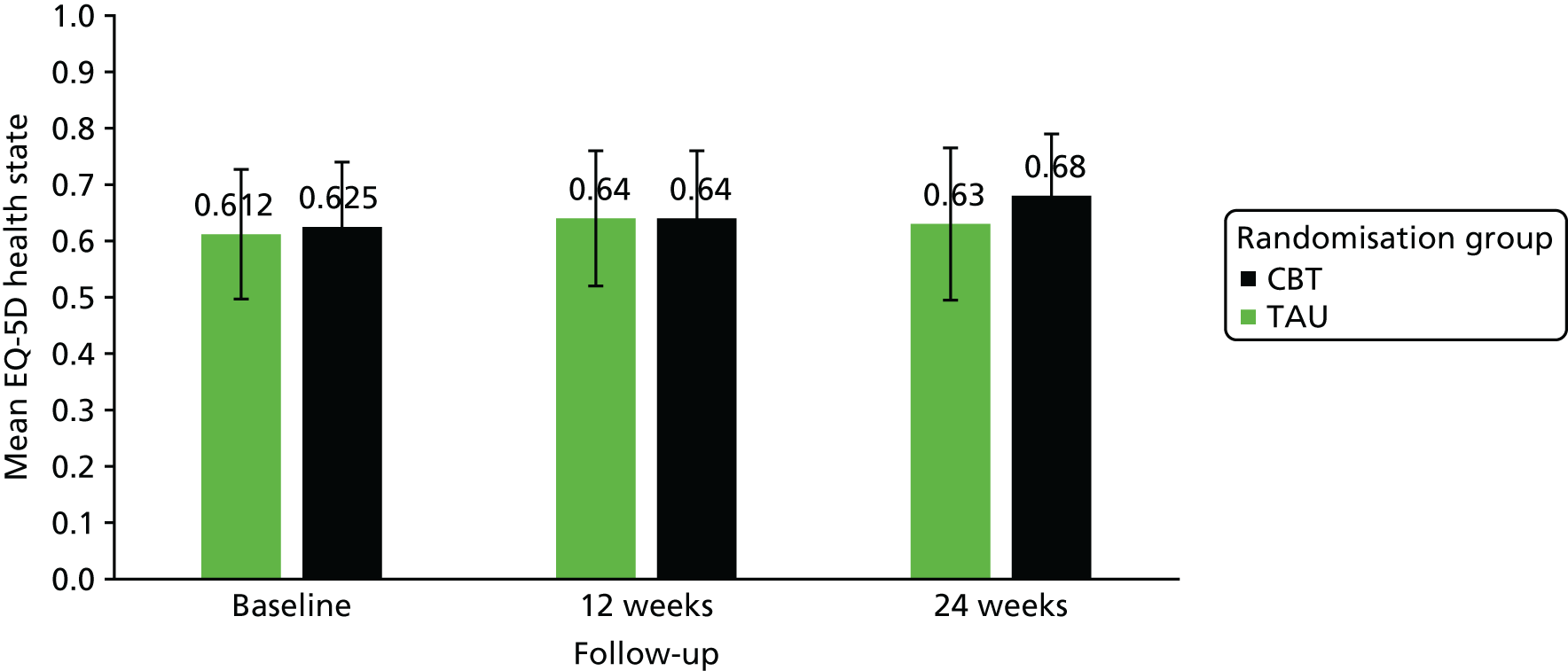
There were no differences in EQ-5D median scores at baseline, nor was there any advantage of CBT over TAU at 12 weeks. At 24 weeks, there was a trend towards improvement with CBT; however, this was not significant. A similar pattern was shown for the VAS on the EQ-5D. There was no statistically significant improvement in QALYs at 24 weeks. Both these figures and the data on which they are based show that any mean changes over time or mean differences between trial arms in either of these scores were minimal.
In the CBT group, the mean EQ-5D-5L values were 0.64 (SD 0.24) at 12-week follow-up and 0.68 (SD 0.22) at 24-week follow-up. The figures for TAU were similar: 0.64 (SD 0.24) and 0.63 (SD 0.27), respectively.
Summary statistics are presented in Table 29 and the results of the statistical analysis in Table 30.
| Time point and randomisation group | Summary statistic | |||||||
|---|---|---|---|---|---|---|---|---|
| Min. | Q1 | Median | Q3 | Max. | Mean | SD | n | |
| Baseline | ||||||||
| TAU | 0 | 0.43 | 0.673 | 0.77 | 1 | 0.612 | 0.23 | 115 |
| CBT | –0.22 | 0.53 | 0.696 | 0.79 | 0.95 | 0.625 | 0.23 | 115 |
| 12 weeks | ||||||||
| TAU | –0.19 | 0.55 | 0.68 | 0.81 | 1 | 0.64 | 0.24 | 80 |
| CBT | –0.17 | 0.5 | 0.71 | 0.82 | 1 | 0.64 | 0.24 | 68 |
| 24 weeks | ||||||||
| TAU | –0.12 | 0.56 | 0.69 | 0.81 | 1 | 0.63 | 0.27 | 64 |
| CBT | –0.12 | 0.58 | 0.73 | 0.83 | 1 | 0.68 | 0.22 | 64 |
| QALYs | ||||||||
| TAU | –0.16 | 0.28 | 0.44 | 0.56 | 0.69 | 0.39 | 0.19 | 64 |
| CBT | –0.11 | 0.36 | 0.57 | 0.74 | 0.82 | 0.5 | 0.21 | 64 |
| Cost of outcome category | Randomisation group | Incremental difference (adjusted) | p-value | |
|---|---|---|---|---|
| CBT (n = 64) | TAU (n = 64) | |||
| Health and social care cost (NHS/PSS), £ (mean) | 4437 | 3755 | 682 (457) | 0.08 |
| QALY (EQ-5D) | 0.504 | 0.386 | 0.118 (0.105) | 0.12 |
| BDI-II score (points) | 19.4 | 20.4 | 1 (0.295) | 0.21 |
Table 29 shows the EQ-5D scores at baseline and at each follow-up period, and total QALYs over the 24-week follow-up. The data are presented as both median scores and lower and upper QRs (Q1 and Q3), as well as mean, SD and the number of participants available.
The maximum QALY accrual was 0.82. The mean QALY accrual was 0.504 for the CBT group and 0.386 for the TAU group. The difference adjusting for baseline was 0.118 in favour of CBT; this difference in QALYS was not statistically significant (p = 0.12).
Cost–utility analysis
The cost–utility results are presented in Table 30, showing the incremental cost-effectiveness for each CBT session compared with TAU for the period of the trial.
Analysis suggests that CBT has higher costs and produces more QALYs than TAU. To reduce the degree of uncertainty around the estimates, bootstrapping is applied.
Cost-effectiveness
Figure 9 is a scatterplot of incremental costs and QALYs and shows the cost-effectiveness plane of 1000 bootstrap-replicated ICERs for CBT compared with TAU, based on health and social care costs and QALYs over 24 weeks, adjusted for baseline costs and utility.
FIGURE 9.
Cost-effectiveness plane: CBT vs. TAU, ratio of incremental cost to QALY gained.
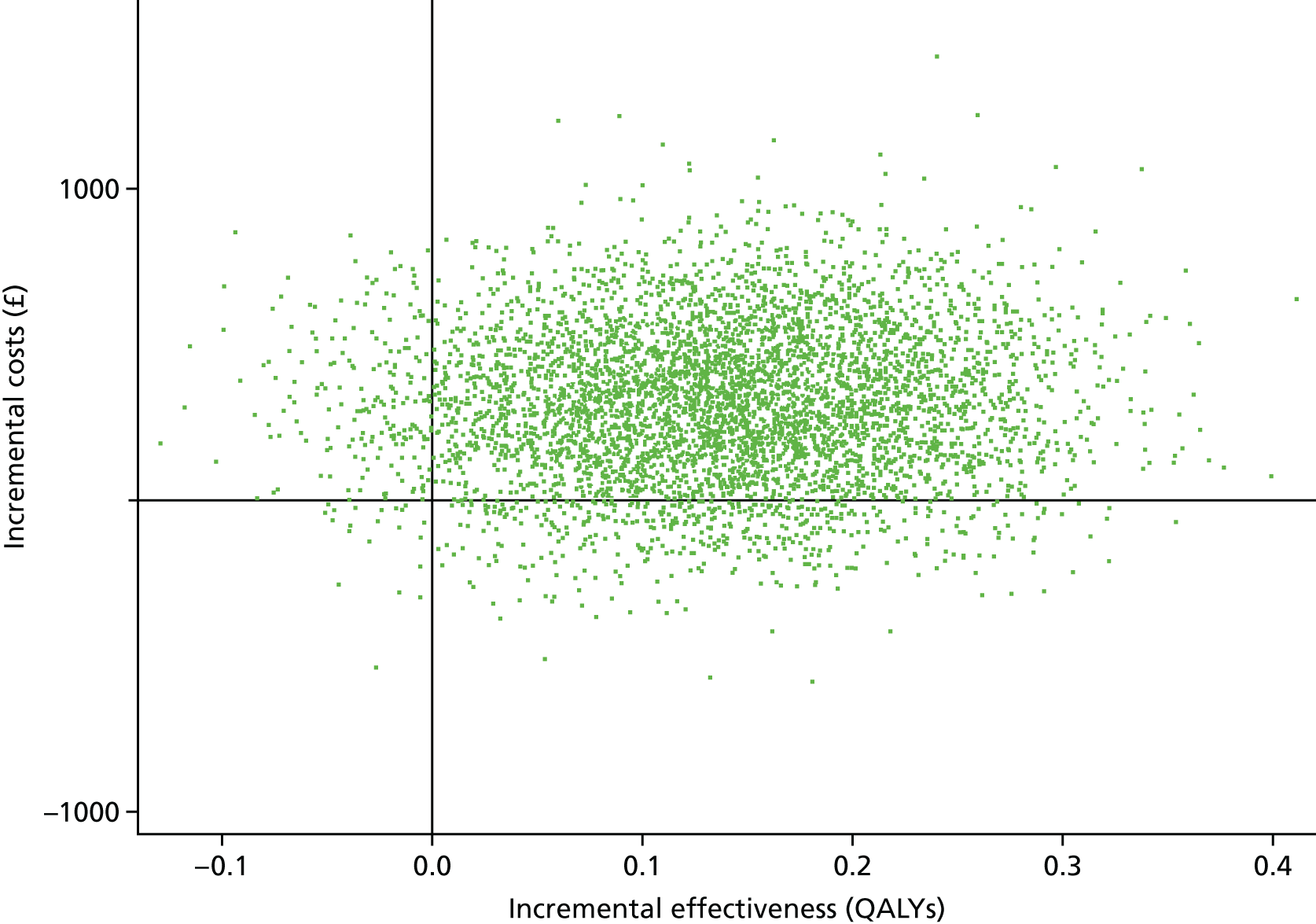
The CEP indicates that there is a 15.5% chance that CBT is cheaper and produces more QALYs, and a 74% chance that CBT is more expensive and produces more QALYs.
The CEAC indicating the probability that CBT is cost-effective compared with TAU, based on health and social care costs and QALYs over 24 weeks, is shown in Figure 10.
FIGURE 10.
Cost-effectiveness acceptability curve.
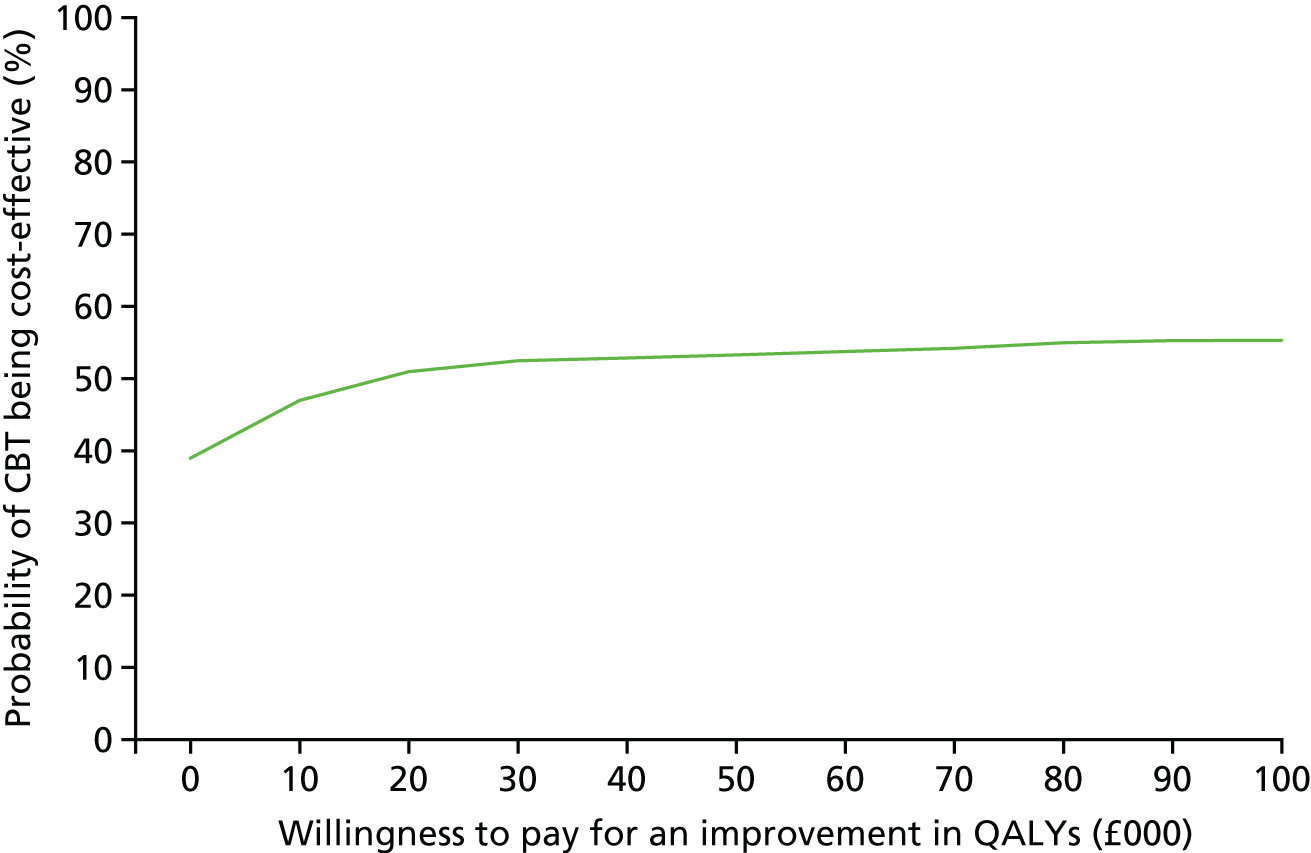
Willingness to pay (WTP) for an improvement in QoL per participant per year is around £20,000–30,000. 151 As illustrated by the CEAC (see Figure 10), at a WTP for an improvement in QALY of up to £20,000, the probability that CBT is cost-effective compared with TAU is not > 51%. At a WTP for an improvement in QALY of up to £30,000, the probability is at most 52.5% and it never rises above 56% at a WTP of up to £100,000.
Furthermore, translating ICER results into monetary terms from the adjusted analysis, we can estimate that an additional £47,985 would need to be invested to generate a unit increase in the participant’s QoL.
Cost-effectiveness of improving negative symptoms
In comparison with the TAU group, participants in the CBT group showed an improvement in QoL at 24 weeks; however, the difference was not deemed statistically significant at the 0.05 level.
Complier average intention-to-treat analysis has estimated that, on average, every CBT session would be expected to decrease the total BDI-II score by 0.3 points compared with TAU. Although this effect was not found to be statistically significant (p = 0.213), dividing the cost by the difference-in-change scores shows that CBT had higher costs and better outcomes; however, the difference between the groups was marginal. Taking account of the ICER, which is the cost incurred by CBT to produce an extra unit improvement on this scale, Figure 9 indicates there was a 14.5% probability that CBT was dominant (i.e. less costly and more effective) compared with TAU. The north-east quadrant, defining a scenario of CBT having higher costs and better outcomes, has a 62% proportion of ICERs, whereas the south-west quadrant, indicating lower costs and worse outcome, is represented by the lower proportion of bootstrapped ICERs.
Chapter 7 Discussion
Main findings
In the CanTalk trial, we aimed to determine the clinical effectiveness and cost-effectiveness of CBT for the treatment of depression in people with advanced cancer that was no longer amenable to cure.
The CanTalk trial was designed and reported in accordance with CONSORT guidelines and was powered to detect a significant difference between TAU and TAU plus CBT, delivered by IAPT therapists in a RCT. Our sample specifically included participants with tumours from as wide a range of tumour groups as possible and in whom disease was advanced and cure no longer probable. Caseness for depression was carefully assessed through a multistage screening and measurement procedure using standardised tools.
Results in the light of previous findings
Our main finding is that CBT (plus TAU) did not show any benefit over TAU in terms of either our primary outcome or our other measures. Although CAITT analysis suggested that the estimated mean change in BDI-II score per therapy session was –0.3 points for CBT compared with TAU, the wide CI (95% CI –0.760 to 0.170 points) was consistent with the main result, showing no significant benefit of CBT (p = 0.21). A subanalysis has suggested that for a particular subgroup of participants, those who were widowed, divorced or separated, there may be a benefit from CBT (mean change –7.21, 95% CI –11.15 to –3.28; p < 0.001).
Previous work reviewing the use of psychological interventions for depression in advanced cancer has raised questions concerning research design, generalisability of the findings and, more specifically, uncertainty about whether or not CBT, either group or individual, is effective in an advanced cancer population. Although three published Cochrane reviews23–25 have suggested that psychosocial therapies are effective for advanced cancer, the last two24,25 covered women with metastatic breast cancer only. The largest study30 that used CBT for low mood in metastatic breast cancer had only 62 participants and suggested no effect of CBT at 3 and 6 months. A more recent study by Savard et al. 63 suggested a benefit of CBT for treating depression in a similar population, but numbers were limited to a small sample size of 45 patients. The small sample size calls into question how much authority these results should hold.
In addition to the finding by Savard et al. 63 that cognitive therapy (as opposed to CBT) was effective for depression in metastatic breast cancer, the SMaRT (Smart Management Research Trials) Oncology-3 trial20 found that integrated collaborative care delivered in a secondary care setting for people with lung cancer was substantially more efficacious than usual care. However, both of these studies20,63 delivered interventions to specific populations of oncology outpatients.
It is generally agreed that CBT is an effective treatment for depression, so what might explain our findings in people with advanced cancer? There are two broad possibilities. One is that limitations in the CanTalk trial obscured a treatment effect. The other is that CBT, as delivered in accordance with the CanTalk manual, is ineffective in this patient group. We will consider these possibilities in turn.
Reason for a treatment effect in widowed, divorced and separated individuals
The CanTalk trial demonstrated a significant effect of CBT for people who were widowed, divorced or separated. Although we found a highly significant effect, the trial was not powered to detect a significant difference by marital group and it is possible that this was a type I error.
The effect of demographic variables as moderators of response to CBT in adults has been previously reported. 152–154 Fournier et al. 153 found that being married, unemployed and having more antecedent life events were associated with a better response to CBT than to antidepressants.
A study by Button et al. 152 was consistent with our findings, suggesting that being widowed, divorced or separated was associated with better responses to CBT. Although no explanation was offered by Button et al. 152 for this finding, we considered two main possibilities. The first is that this is a random finding. However, our finding is a highly significant effect (p < 0.01) and is consistent with previous reports. Second, people who are emotionally isolated or have suffered a bereavement or separation may benefit from the non-specific component of CBT, by having a warm and friendly person to talk to, with positive effects on their mood. We have not explored the outcome when broken down into separate categories for the subgroup (widowed, divorced or separated) as this was not a part of our pre-analysis plan. It might have also been useful to know whether or not the participants’ status was a recent event. Although there appears to be some indication that CBT is helpful, it would be premature to specifically recommend CBT to people who are widowed, divorced or separated until further research validates these findings.
Establishing whether improvement is associated with the specific effects of CBT or, rather, a non-specific treatment effect such as having a warm, friendly person to talk to remains to be evaluated. There are very few studies that examine the impact of non-specific interventions with CBT in depression. Although older people with depressive disorder have reported feeling that just talking helped, this did not improve their depression scores. 66,67 However, preliminary findings from more recent feasibility work, comparing acceptance and commitment therapy (a new-wave therapy derived from CBT that uses cognitive diffusion and mindfulness methods rather than attempting problem-solving) with a talking control for people with advanced cancer155 indicates that being given the space to simply talk and be heard is well received. It is possible that additional psychosocial support, possibly in the form of CBT, should be considered in this demographic group.
Subsidiary findings
Eastern Cooperative Oncology Group Performance Status
In a previous study156 that measured ECOG-PS in 1000 patients with advanced cancer, the proportions of patients found to be fully active, restricted, ambulatory, limited and disabled were 3%, 16%, 26%, 42% and 12%, respectively, compared with 20%, 42%, 27%, 11% and 0%, respectively, in this study, suggesting that our population was less disabled, despite having advanced cancer. This finding could be explained by our recruitment of outpatient attenders rather than a mixed inpatient and outpatient sample, as was the case in the other study.
Possible limitations of the study
Power
We recruited a total of 230 participants. Our original power analysis suggested that 240 participants (accounting for attrition at follow-up) would provide 90% power to detect a 6-point difference at 12 weeks and 80% power to detect a 6-point difference at 24 weeks (see Table 4). Retention in the trial exceeded our original estimates so that, with 230 participants, we had sufficient numbers to detect a 3-point change on the BDI-II at 90% power, suggesting that our trial was overpowered. Savard et al. 63 reported a clinically significant improvement with CBT in metastatic breast cancer patients but these findings should be treated with caution as the sample size was small, with 21 in the CBT group and 16 in the ‘wait list’ group. One of the strengths of our study is that it is sufficiently powered to produce robust findings and to answer the question of the benefit of CBT delivered by an IAPT service for people with advanced cancer.
Attrition is common and unavoidable in studies of those with advanced, progressive disease. It is always a serious consideration when considering the validity of the findings, as differential dropout in the two study arms may reduce the internal validity of the findings. We conducted a sensitivity analysis, assuming that all people in the CBT group who dropped out recovered and we still could not demonstrate any benefit of CBT.
Representativeness of the sample population
Screening data
The proportion of participants recruited as a proportion of those screened in the CanTalk trial varied between 35.4% for hospice, 10.4% for oncology outpatients and 2.6% for GPs. Data for oncology outpatients with good prognosis cancer19 showed that 500 out of 1428 (35%) were recruited, with 142 out of 490 (29.0%) patients with poor prognosis (lung) cancer being recruited. 20 In the CanTalk trial, participants were selected from all cancer types. These factors might have accounted for the difference in recruitment rates. Furthermore, our recruitment methods relied, in part, on busy clinic staff, who varied in their commitment to the research process. Qualitative interviews suggested that clinic staff did not have time to engage with this study and often viewed drug trials as more worthy of research. Recruitment rates are also likely to be better when research is led and conducted in one service, such as in the SMaRT oncology trials. 18–20 This was not feasible in the CanTalk trial because of the UK-wide recruitment base required.
Demographics
The mean age of our sample population was 59.5 years, as participants were people with ‘advanced cancer’, which is predominantly a disease of people in the second half of life. In the UK, over one-third of new cancer diagnoses are in people aged ≥ 75 years. 157 Breast, prostate, lung and bowel cancers together accounted for over half (53%) of all new cancers in the UK in 2013. 157 Among females the highest lifetime risks are associated with breast, lung and bowel cancers and and among males the highest lifetime risk is of prostate, lung and bowel cancers. These tumour groups made up 60.8% of our sample population. The slightly higher rate is because we chose to recruit from the major tumour groups as a priority to ensure that we recruited people with the most common cancers. However, the rates of depression are lower in genitourinary cancers20 and, therefore, the lower rate of people with prostate tumours and depression is consistent with findings.
Reliability of the diagnosis of depression
Typically, the CanTalk trial targeted people with a depressive episode (ICD-10 F32), a recurrent depressive disorder (ICD-10 F33) or a mixed anxiety and depressive disorder (ICD-10 F41.2), as identified by the MINI. However, it is not always easy to distinguish depressive disorder from an adjustment disorder with a prolonged depressive reaction (ICD-10 F43.21).
Rayner et al. 22 found that 69% (27/39) of patients in palliative care who were diagnosed with major depressive disorder at baseline had remitted 4 weeks later. Our target population, after adjusting to a recent major life event around their cancer diagnosis, could experience remission of their symptoms. This is consistent with the observation that people allocated to TAU alone also improved. This is also a common finding in RCTs because of regression to the mean.
The mean time from the initial diagnosis was almost 4 years in our study population. Therefore, it is unlikely that a diagnosis of cancer per se was the trigger for distress; however, it is possible that people were adjusting to the realisation that cure was no longer likely. Unfortunately, the entry definition for the study was for ‘people with cancer not amenable to cure’ and this group is more heterogeneous in term of adjustment disorders. For example, patients with lung cancer that is not amenable to cure, which is often known from the time of diagnosis, may have had more time to adjust than people who entered into the study with a recent 1 month reoccurrence of breast cancer. In hindsight, it may have been useful to ask participants whether or not they had had recent upsetting information about their disease prognosis or recurrence.
Reliability of the main outcome measure
The BDI-II was used as the main outcome measure. The BDI has been used in trials of psychotherapy for patients with advanced cancer. 63,120,121 However, in the light of our findings, we need to consider whether or not the BDI/BDI-II is a valid scale for measuring change in depressive symptoms in advanced cancer. The difficulty with assessing severity of depression in cancer is that there are a number of items on depression scales that complicate estimations of severity. For example, people may lose weight because of the cancer itself or nausea associated with treatment, sleep may be increased or decreased because of the use of opiates or decreased because of associated physical disease, such as pain.
Prior to setting up the study, we considered the different scales to be used for measuring depression in cancer and also reflected that people with advanced cancer, our target population, are likely to be older than average.
Unfortunately, there is no ideal scale for measuring depression in cancer158 and a variety of scales have been used. 159,160 These include the BDI-II,118 Brief Symptom Inventory,161 Centre for Epidemiologic Studies–Depression Scale,162 Geriatric Depression Scale,163 Hospital Anxiety and Depression Scale,164 PHQ-9,102 Profile of Mood States – Short Form,165 Zung Self-Rating Depression Scale166 and the Modified Edinburgh Postnatal depression Scale. 167
As we were evaluating the effects of CBT, we decided on the BDI-II for two main reasons. First, the BDI-II, or its previous version (the BDI), has been used in previous studies to measure depressive symptoms in advanced cancer. Second, the BDI-II contains a balance of cognitive and somato-affective elements. The rationale behind CBT is that targeting cognitions associated with depression results in an improvement in mood. Although other scales may ask some cognitive-related questions, such as suicidal thoughts and hopelessness, they tend to be less weighted towards the measurement of cognitive elements. The BDI-II includes thoughts about being punished, worthless, being a failure, self-dislike and self-criticism, all of which, along with addressing existential issues, were a target of the CanTalk intervention. Nevertheless, like all measures of depression, the BDI-II also includes somatic symptoms, for example cachexia, which, in this population, are related to the underlying physical disease, rather than to depression per se. Even with CBT, these elements may be less amenable to change, making it harder to detect a significant treatment effect using the BDI-II.
Finally, a criticism associated with the BDI-II is that it takes quite a long time to complete, and this may increase the number of missing items, which makes it unreliable. This was not the case in this study, as we found that the number of missing items was low.
Screening using the PHQ-2 suggested significant depressive symptoms, and this was confirmed with the MINI, giving a DSM-IV diagnosis of depression in all participants who entered the trial. Nevertheless, in 36 out of 230 (15.6%) cases, the BDI-II score was < 17 points, suggesting mild mood disturbance (score 11–16 points) or normal ups and downs (0–10 points). Even though our analysis adjusted for baseline depression score, the inclusion of participants with low depression scores generally reduces the ability to influence significant change. This observation was also consistent with qualitative feedback from some therapists, who indicated that, by the time some patients were seen, they were no longer ‘depressed’.
Randomisation
In this randomised trial, various demographic and other factors that are known to predict depression outcome were well balanced in both arms.
However, more people were employed in the CBT group. Unemployment is known to be associated with poorer prognosis of depression. This imbalance would be expected to favour CBT, but we did not find this to be the case. Although our population included a lower proportion of single, never married people (17%) than in the total UK adult population (33%), this may be explained by the fact that the mean age of our population was higher than the UK average, and the proportion of single and never married people is generally lower among the older population. This age distribution may also explain why people with a degree or higher degree in our population was lower than for the UK population as a whole.
Both groups were also balanced on our recorded potential sources of bias, such as antidepressant use, use of other psychological therapies, predicted improvement and treatment preference. Therefore, these factors are unlikely to account for our lack of apparent treatment effect.
Dropout
There was considerable morbidity among our population, which accounts for the fact that almost one-third of people were not able to provide complete data at any any particular time point. However, this finding may be misleading because, as shown by Figure 2 for example, although 24-week follow-up data were available for 130 out of 230 (56%) participants, people dip in and out of follow-up. Analysis was possible because at least one post-baseline data point was available for 185 out of 230 (80.4%) participants. Furthermore, missing data for people who died can, in this study, be regarded as missing completely at random,168 with respect to whether or not they have received CBT (potential cases of suicide excluded; none occurred).
The patterns of early and late dropout may be relevant, especially as they may not be completely random. Dropout early on (within 6 weeks) may occur because people are unhappy about their group allocation, whereas dropout late in the treatment may occur because people are unable to comply with the rigours of having to attend therapy sessions. Over 75% of participants indicated a preference for CBT; however, withdrawal in the early stages was greatest for the CBT group. It is unlikely that dropout from the CBT group is explained by people receiving their preferred treatment. One explanation for the greater dropout in those allocated to CBT is that their expectations were not met because they had to wait to be seen by IAPT services. It is notable that people who engaged well with therapy were seen within a median of 16 days, whereas those who engaged poorly had to wait a median of 26 days. We are aware of the need for caution when interpreting these data, especially as it could be argued that a delay for people to be seen by IAPT services occurs because people are physically unwell. However, using ECOG-PS, which is a measure of disability, we found no significant differences in physical function at baseline between those who were seen quickly and those who were not. Furthermore, overall satisfaction with care was equally high in both groups. Our belief that delays in being seen by IAPT services explained some of these findings was supported by qualitative interviews, in which participants voiced dissatisfaction at ‘having to wait’ to be seen. If waiting to get CBT elicits dissatisfaction, then reduction in waiting time must be considered. This observation is further supported by our finding that attrition later in the study (weeks 12–24) was balanced across the two arms.
Satisfaction with care was high and this did not differ between the TAU and TAU plus CBT group. Satisfaction with care as measured in this study related to a general satisfaction. In hindsight, it may have been more helpful to collect quantitative data specifically related to participants’ satisfaction with CBT.
Engagement with therapy is an important issue when considering whether or not a particular intervention can be delivered in a NHS setting. A total of 22% (25/115) of participants withdrew before starting therapy. Data from IAPT for 2014/15169 suggest that just under 70% of participants referred to IAPT enter treatment. The CanTalk trial found that, although 65% (75/115) of participants attended at least one therapy session, only 31.3% (36/115) completed at least eight therapy sessions. A major reason for dropout was that participants had significant physical ill health. Our quantitative and qualitative data suggested that a proportion of participants withdrew either because of health reasons or because of the rigidity of IAPT practice, the policy of which tends to be to discharge people if they fail to attend on two occasions. Although ethics stipulated that patients did not need to give a reason for not taking up treatment, it was perceived by the study researchers that patients felt that attending therapy sessions for some was burdensome. 170 Although it is costly, attrition may be minimised by seeing people in their own homes and by allowing great flexibility for those with chronic physical health problems. Unfortunately, we were not able to offer treatment in a home setting because of restrictions on lone working within IAPT. Although we did offer the opportunity for telephone CBT, few sessions (5.9%) were taken up. Moreover, these telephone sessions were accounted for by one participant who received all 12 sessions over the telephone as they were unable to attend face-to-face therapy. Although our protocol recommended that at least three sessions were offered face to face, we decided to be flexible. Although the alternative option of offering computerised CBT should be considered, the benefit of these packages is questionable170 and we cannot make recommendations on these interventions based on the results of our study.
A total of 36 out of 115 (31.3%) participants referred for therapy were recorded as having ‘completed’ it. However, the proportion of those who engaged was slightly higher (32.7%), as only 110 out of the 115 would have been able to take up therapy, with five participants dying by 3 months’ follow-up. This figure compares with recent IAPT data171 suggesting that 41% of people complete therapy. Of the 110 participants who could have received all 12 therapy sessions, 41 participants had withdrawn or data were missing. Although for a proportion of these patients the decision to terminate therapy was agreed between the patient and the therapist, it would have been useful to have had data on why these termination decisions were made.
Seventeen out of 115 (14.8%) patients in our population were discharged from IAPT, in keeping with the IAPT policy of discharging people who fail to attend. Qualitative interviews suggested that therapists tried to be more flexible with our patient population. If IAPT is to treat patients with LTCs, such as advanced cancer, consideration must be given to physical problems and attendances. Furthermore, six people were not referred for therapy; despite us sending an e-mail to IAPT and confirming receipt in a follow-up telephone call, we were informed by IAPT that a referral had never been received. Patients were subsequently re-referred for ethical reasons, but the delay in this process meant that they had reached the final follow-up point in the trial. Such administrative errors may be minimised by closer tracking of participants. However, changes in staffing, IT systems and administrative errors do occur and remain potential sources of error. These errors could be minimised in future trials by asking patients allocated to CBT to be proactive in contacting the trial team and IAPT if they have not heard from a therapist within 3 weeks of allocation.
The quality of the intervention
We are aware that research-active sites tend to deliver higher-quality care. This could be reflected in improved outcomes from IAPT. However, as 25 IAPT services from across England were involved, we would argue that these were representative of a range of IAPT services. Engaging IAPT centres that have an interest in participating in the study is a prerequisite for the study and, if anything, would be likely to bias the outcome in a positive way.
Although a meta-analysis on therapist adherence and competence suggests that these factors play little role in determining symptom change,77 competence is routinely measured in IAPT, and adherence to treatment protocol is considered essential in evaluating trials of CBT. Therefore, we have to consider the possibility that CBT failed to effect an improvement in depressive symptoms because the quality of therapy was poor and that therapists did not adhere to the treatment manual. However, the CTS-R suggests that the quality of delivery of CBT was at the upper end of the proficient range.
Findings from the Cognitive Therapy Scale – Revised
We are aware that not all therapy tapes were uploaded and it is possible that therapists uploaded what they felt were the best therapy sessions. This would introduce a bias. The major reason given was that therapists did not have time to upload tapes or they could not access IT systems because of governance issues. However, we would argue that an assessment of the quality of therapy is justifiable despite the limitations.
It is widely assumed that therapist adherence and competence predicts outcome; however, there may be little relationship between adherence and competence and symptom change. 77 Nevertheless, the CTS-R is routinely used as a measure of competence of the therapy delivered. Despite competent delivery of therapy in this study, we failed to observe a change in BDI-II with CBT. We chose to rate tapes of therapy by period (beginning, middle or end). With practice, the quality of therapy, evaluated by the CTS-R, may be expected to improve. However, a greater number of early therapy sessions are likely to occur, as people often do not complete all sessions, and this may be associated with a skewing of the CTS-R score by phase of therapy. However, as there were no differences in therapy CTS-R scores by session, the data suggest that it is unlikely that poor delivery of treatment accounts for the lack of improvement from therapy. Lichstein et al. 74 suggest that, for successful treatment, therapy needs to be delivered and also received and enacted. Although we did exclude participants on the basis of cognitive impairment, it is possible that some participants had difficulty in understanding the therapeutic model. However, this suggestion is negated by our finding that higher scores were not associated with educational status. Furthermore, there is no evidence to suggest that educational attainment, age and history of depression moderates a treatment response to CBT. 152 It is possible that, although the therapy was competently delivered and the patients understood this, they did not act on it. Although the independent ratings of therapy using the CTS-R suggest that therapists did set homework, we did not have the resources to evaluate whether or not patients completed the tasks set out.
Adherence to treatment using the Therapy Components Checklist
Fidelity to the treatment manual appeared to be good, with therapists reporting the use of a wide range of elements, subsequently confirmed by an independent rater. Although we acknowledge that limited resources did not make it possible for an independent rater to assess all sessions for adherence using the TCC, it did provide a useful measure of adherence.
If anything, the therapists underestimated some of their skills, such as the use of guided discovery, which was reported by the rater using the TCC. Our findings suggest that CBT was well adapted to treating an advanced cancer population and that it can be used to deliver a challenge to unhelpful beliefs and explore existential issues. For example, cognitive techniques were reported as being used in 58% of assessed sessions and specific cancer topics were discussed in 70%. What is not clear is how many of these techniques were received and then enacted on. 74 Evaluating these processes was beyond the scope of this work.
We acknowledge that the psychometric properties of the TCC remain to be evaluated. With greater resources this could be done through a study measuring inter-rater reliability and whether or not particular components, correlated with each other, could be dropped. Further research could then explore whether or not particular delivered components are associated with improved outcomes.
Finally, although assessors were asked to confirm that participants had a sufficient understanding of English to engage in CBT, it is possible that a small number of participants suffered from cognitive problems associated with cerebral involvement of cancer or, indeed, the side effects of medication. This may have impaired people’s capacity to retain the contents of therapy.
Number of therapy sessions
National Institute for Health and Care Excellence’s guidelines142 for the treatment of depression suggest that a range in number of sessions may be required, with 16–20 individual CBT sessions over 3–4 months. When planning the study, the consensus view was that fewer sessions would be needed as it was felt that most patients with cancer would have reasonable pre-morbid adjustment and that one of the main hurdles in their lives is adjusting to a life-threatening illness. In CanTalk, a mean of 4.7 sessions (SD 4.9 sessions) were taken up, which is lower than a mean of 6.3 treatment appointments attended overall for IAPT in 2014/15. 171 However, the number of people with physical health problems treated by IAPT, although increasing, remains low.
Complier average intention-to-treat analysis found a change in BDI-II score with CBT of 0.3 points per therapy session and the wide CI suggests no benefit of CBT. We chose to adhere to a consensus opinion among clinicians, notably that a change of 6 points is considered clinically significant on the BDI-II. It is possible that a 6-point difference would be achieved by simply delivering more sessions. Although speculative, as a linear relationship between the number of CBT sessions and a reduction in the BDI-II score cannot be assumed, one could envisage a 6-point change with around 20 CBT sessions. More cautiously, scaling up the ‘per-session’ estimate by the average number of sessions experienced within the treatment group for those who took up at least one session (this comes to 7.14 sessions) produces an estimate of –2.11 points (95% CI –5.43 to 1.21 points); this is an estimate of the average benefit in the treatment group for ‘compliers’ (defined here as those who had at least one session of CBT), which can be compared with the principal ITT analysis estimate (–0.84, 95% CI –2.76 to 1.08).
Our original assumptions were that our sample population had better pre-morbid resilience than a population with repeated depressive episodes. In hindsight, it may be that the severe physical consequences of their disease necessitate considerably more sessions for clinically significant change. In reality, it is going to be very difficult and there may not be enough time to engage people for 20 sessions. Furthermore, even if this were possible, resources are not currently available in the NHS to deliver sufficient therapy for treatment to be successful. It follows that CBT is not a pragmatic treatment for people with depression and advanced cancer.
It is notable that, when the CanTalk project was set up, we stipulated that, when possible, IAPT should fast track people into therapy, ideally within 2 weeks. Our study findings suggested that there was an association between the time for the IAPT appointment to be received and the number of therapy sessions completed; the faster an appointment with IAPT, the more sessions were taken up. People with more physical problems may be less able to attend appointments and may also be less likely to engage in therapy. However, attendance was not found to be related to physical health status judged by the ECOG-PS. Furthermore, IAPT services send out appointments irrespective of an individual’s health status. It follows that, in order to encourage people to take up therapy sessions, rapid referral into therapy is desirable.
Although we requested that participants be fast tracked into therapy within 2 weeks of referral by the research team, in reality this was not possible because of service constraints. Nevertheless, the mean time for participants to be seen was 29 days, which is better than routine IAPT referral, for which waiting times for a course of treatment (for those finishing a course of treatment in March 2015) are quoted as being < 6 weeks to enter treatment for 37,097 (78.0%) people and < 18 weeks for 45,589 (95.9%) people. In contrast, the presence of a CBT practitioner within a palliative care service ensures rapid access to treatment. We deliberately excluded areas that offer this service from recruitment to avoid contamination from the TAU group.
We are confident that the CanTalk trial is one of the largest and methodologically most robust trials of therapy in psycho-oncology. So, if the results are sound, the outstanding question is why CBT delivered using the CanTalk manual was ineffective in the IAPT setting.
Possible reasons for ineffectiveness of cognitive–behavioural therapy in CanTalk
Depression in advanced cancer is more treatment resistant
A previous study62 of CBT in palliative care found an effect for anxiety but not depression. The nature of depression in advanced disease may be different (e.g. depression in advanced disease may have a biological underpinning that makes it less amenable to treatment). Despite the lack of effect on depressive symptoms, therapists reported useful work with other problems and goals that patients brought to therapy. CBT, as a collaborative effort in problem solving, works with what the patient brings and it may be that problems other than depression were shifted by the therapy. Unfortunately, CanTalk did not use measures of personal problems or anxiety, so it is not possible to determine if CBT had an impact on areas other than depression. For many people with advanced cancer, anxiety may be a more disabling symptom than depression, but we do not know if our treatment improved anxiety. CBT in palliative care is less disorder focused and much more problem focused. For instance, symptoms of fatigue or pain may be the main ones addressed in therapy and, although these are related to depressive symptoms, they may change without effecting changes in depression. This raises questions of whether or not a disorder-specific approach and disorder-specific measures are the best outcome measures in advanced diseases.
The intervention was not sufficiently well designed
There was no difference in mean satisfaction with care between patients allocated to TAU and TAU plus CBT. This is probably because questions referred broadly to their total care and CBT made up only a small element. However, the mean score of around 40 on a scale ranging from 0 to 50 suggests that considerable improvement could not be made with the care delivered. Engagement with therapy may provide an indication of whether or not the intervention was acceptable. Although 65% of people took up the offer of therapy, it is notable that around one-third completed 8–12 sessions. Data from IAPT for 2014/15169 suggest that the rates of uptake were similar to IAPT. However, our population had many more physical problems and, therefore, continued engagement as health deteriorates is likely to be challenging. Although telephone CBT was offered to participants, the majority elected to be seen face to face. Qualitative interviews with therapists and patients suggested that the treatment could be delivered and that it was well received. Generally the therapists and the patients liked the materials used and we were pleased that they adhered to the protocol by showing flexibility in which elements were tackled and when.
The CanTalk manual was constructed on the basis of considerable clinical experience in working with cancer patients and is geared towards addressing the cancer-related issues faced by patients with advanced disease. The entry criteria for the study did not differentiate between depression resulting from the disease and pre-existing depression or even depression subsequent to the disease but not related to cancer. Some therapists reported that the problems with which patients presented were not cancer related. Thus, the manual may have restricted them in their delivery of therapy.
An alternative explanation is that the therapists did not focus enough on cancer-related issues. It is interesting that the TCC reveals a discrepancy between the observer and therapist ratings in the areas of impact of illness, impact of disease and mood on behaviour, beliefs and expectations about illness, concerns about current and future ability to cope, and impact of disease and death on loved ones. Therapists were more likely than raters to have believed that they covered these issues in a session.
Although the problem-solving focus of CBT can help patients with early-stage cancer to develop effective coping strategies and evaluate unrealistic hopeless and helpless beliefs, in advanced disease there is less scope for problem solving. It may be that ‘third wave’ therapies, such as acceptance and commitment therapy, which are based more on acceptance, could be more relevant. However, this is not the clinical experience expressed by clinicians working in palliative care, in which problem-solving approaches to fatigue, pain and avoidance of activity for example seem to work very effectively.
We need to consider whether or not a cognitive–behavioural approach is not the treatment of choice in this target population. More recent work has been conducted by Walker et al. 20 in the SMaRT Oncology-3 trial, in which 142 people with lung cancer (poor prognosis) were randomised to integrated collaborative care, which uses elements of CBT or usual care. This found that the active arm was substantially more efficacious than usual care alone, although data were available for 113 (80%) and 97 people at (68%) at 12 and 24 weeks, respectively. It may be that the use of CBT in combination with this integrated collaborative care approach is more suitable for advanced cancer patients. However, this remains to be evaluated for cancer patients with different tumour types.
Engagement with the intervention
A low uptake of therapy may explain failure to find a treatment effect. However, the CanTalk trial was a trial of the clinical effectiveness, not efficacy, of CBT. The uptake of therapy for people referred to IAPT is around 70%. In the CanTalk trial, of 99 people referred to therapy, 66 (67%) took up at least one therapy session, which is similar to IAPT. Although we offered people the opportunity to tell us their reasons for withdrawal, it is not ethical to pressurise people into providing a response. In qualitative interviews with participants and therapists, there was a suggestion that CBT was well received. Physical problems meant that people were not always able to attend therapy in a local IAPT centre or GP premises. In addition, there may be stigma associated with attending a centre that is known to address mental health problems. Despite this, only a small proportion of participants took up the option of telephone CBT. A strategy to boost engagement could be to offer patients the opportunity to receive therapy in their homes or at the oncology centre, but it is unclear whether or not this would be taken up. Delivering integrated care through palliative care teams may facilitate engagement with psychological interventions and is recommended by the Improving Outcomes in Supportive and Palliative Care for Adults with Cancer guidance. 72
Psychological interventions are less effective than previously thought
Cuijpers et al. 172 suggest that the effectiveness of CBT for depression generally may have been overestimated, possibly owing to publication bias, small sample size and a lack of suitable control groups. Although the therapeutic outcomes from various psychological interventions are broadly similar173 and the effectiveness of an intervention may be enhanced by improving retention with treatment, the findings from CanTalk trial suggest a lack of treatment effect in patients with advanced cancer.
Procedural issues
It is notable that there was wide variation between centres for permission to start recruitment, despite all the relevant documentation being provided. We allocated a maximum of 6 months for the set-up of the project. Some centres and clinics were outstanding in quickly approving research and recruiting patients. At others, there were hurdles that impeded us in recruiting participants promptly. We would suggest that if some centres are able to set up permissions for research within 1 month, a duration of no more than 2 months should be expected in others, with possible financial incentives awarded to those who reach this target and with these decreasing gradually to zero if a centre does not recruit within 6 months.
Differences in recruitment rates may depend on referral source (e.g. lung clinics were high recruiters); however, these variations cannot be explained by recruitment source alone. Indeed, it came to our attention that some services would be rewarded if they became research active and recruited at least two participants within the first month. After this was achieved, recruitment was minimal, suggesting a degree of gaming in the system.
Recruitment issues
Recruitment in the CanTalk trial suggested that GP practices yielded low recruitment rates, and, once a GP database was screened, rescreening within the next year was unlikely to yield additional numbers. Although our original proposal aimed to recruit participants through the General Practice Research Framework, this was abolished at the start of the study because of a change in government funding priorities. This may have been fortunate, as recruitment from GP practices yielded few patients. Screening directly in secondary care was a more efficient way to identify and recruit patients with advanced cancer.
Qualitative interviews with health-care workers who helped to recruit for the CanTalk trial suggested that there were difficulties with finding the time to recruit for the study. These included competing interests with recruiting for CTIMPs, which were perceived as providing better incentives. Providing boosted incentives for psychological studies may be a way of redressing this imbalance.
Furthermore, addressing the difficulty of conducting empirical research in a palliative care setting, regular research briefing and education sessions held with local palliative care teams can help to emphasise the value of research and address individual concerns. 174
In oncology outpatient departments and hospices, there were reservoir effects, so that, over time, the numbers of eligible participants waned, as all those eligible had been approached. There is a greater turnover of patients when the prognosis is poor. This will be reflected in the service type (e.g. hospice) and the tumour type (e.g. pancreatic cancer). Researchers need to pay close attention to monthly recruitment figures and if there is a consistent reduction in people identified during a 3-month period, alternative strategies need to be employed. Extended recruitment within the same clinics can be used for people whose survival time is likely to be relatively short (4–12 months). However, for those who have a relatively longer survival (e.g. haematology), for example at least 12 months, this strategy is problematic because of the low turnover of cases.
Finally, there is a possibility that recruitment tails off because of fatigue in clinic staff. However, in this case, one would expect recruitment to fall off equally in people with good or poor prognosis.
Health economics
Service use
General practitioner services
Two main reasons people access their GP is for help with physical or psychological problems. The reason for the decrease in GP consultations we witnessed in the trial is that people were less distressed or were less physically unwell. Our data suggest that our population was less distressed with time, in both groups. The explanation that people were less physically unwell seems unlikely. Data from the ECOG-PS suggested poorer function with time. It is possible that, when people become more ill, they are less likely to consult their GP but, rather, access secondary care services.
Specialist nurse
Part of the CBT intervention was to empower participants so that they were more likely to ask for help. This may explain why increased contact with the specialist nurse was observed.
Mental health services
Participants in both groups had more frequent contact with mental health community services than at baseline. One possible explanation is that CBT increased the demand by participants for psychological help. At 24 weeks, we can speculate that 94% of participants not allocated to CBT had contact with community mental health partially because they lacked the required level of psychological support.
Social care
Although participants in both arms had a decrease in demand for social care, use of palliative care services increased for both arms, suggesting that participants were becoming more physically unwell.
Costs
The increase in costs throughout both follow-ups for both groups may be partially explained by more frequent inpatient admissions and general uptake of secondary care.
When planning services, one needs to take into account the full costs of an intervention, including the IAPT therapist’s time allocated to a participant who may not, for example, attend a therapy session, as was the case in this trial.
Quality-adjusted life-year
Although there was some QALY accrual for the CBT group compared with control, the difference was not statistically significant. Because of the nature of the population, it is unlikely that there would be a significant improvement in QALYs. It is more likely that psychological treatment would slow the expected deterioration in QoL associated with poor psychological well-being. This suggests that there is no significant benefit of CBT to patients’ QoL for people with depression and advanced cancer.
Cost-effectiveness acceptability curve
The CEAC is used in the health economic analysis to indicate the probability that an intervention is cost-effective versus a control. The CEAC indicates that at a WTP for an improvement in a participant’s QoL of up to £20,000, the probability that CBT is cost-effective compared with TAU is not greater than 51%. This probability never rises above 56% at a WTP for an improvement in QALY of up to £100,000. Therefore, this makes the intervention equivocal at the NICE threshold.
Translating the ICER to monetary terms indicates that significantly more needs to be spent to generate a unit increase in a participant’s QoL, as shown in Chapter 6. This is higher than the normally accepted WTP for QALYs when they relate to the adoption of health technologies within the NHS. Furthermore, there is uncertainty around the result as the 95% CI surrounding this ratio is not definable. The small QALY gain and the considerable uncertainty surrounding this estimate are consistent with the overall effectiveness results of the trial in relation to BDI-II outcome.
We have also considered the cost-effectiveness of improving negative symptoms. As reported in Chapter 6, in addition to the statistically insignificant effectiveness result for nearly all comparisons and analyses performed, it can be concluded that CBT is not cost-effective compared with TAU in this population.
Chapter 8 Conclusions and implications for practice
Summary of key findings
-
This is the largest study to evaluate individual CBT, delivered through IAPT, for people with advanced cancer not amenable to cure who had depressive disorder. Findings suggest that for people with a range of cancers, CBT is not clinically effective for treating depression.
-
The health economic analysis suggests that more financial resources are needed to generate a unit increase in participants’ QoL than the normally accepted WTP for a QALY within the NHS. CBT for depression in this population is, therefore, not sufficiently cost-effective to be recommended under the current NICE guidelines. 151
-
CBT appeared to be effective for a subgroup of patients who were widowed, divorced or separated.
-
The demographics of our population were typical of those treated in a NHS service and represented a range of cancers including the common cancers (breast, lung, colorectal and prostate).
-
Attrition occurred in around one-third of participants, with withdrawal in 22% and death in 9%. Ill health was the main reason for withdrawal.
The intervention and supervision
-
It was possible for CBT therapists to be trained to deliver CBT to an advanced cancer population.
-
IAPT therapists may have been reluctant to explore some of the physical health, deterioration and death issues with patients.
-
A variety of CBT methods were used, with predominantly cognitive and cognitive–behavioural methods, as well as discussion of specific cancer topics, being undertaken.
-
The number of sessions taken up was consistent with other studies.
-
Independent ratings of therapy, judged by the CTS-R as proficient, suggested that high-quality therapy was delivered.
-
Qualitative interviews suggested that therapy was well received.
-
Although this was a pragmatic study, qualitative interviews suggested that therapists would have preferred more specialist supervision than currently exists in IAPT services.
-
Therapists’ self-report appeared to be an accurate reflection of what was delivered.
-
No AEs were recorded.
Structure of services
-
The lowest proportion of people recruited was from GP databases (2.6%), with the numbers of people available for screening being small; the greatest proportion was from oncology clinics (10.2%).
-
IAPT discharged 11% of participants because they did not satisfy requirements of IAPT for therapy.
-
There was considerable replication of documentation required for the research to be approved by local trusts, which held up the trial in some centres. The time taken from submission to approval for the research to proceed varied from 2 to 9 months.
Implications for practice
Clinical
The intervention
People with advanced cancer and depression who received an appointment quickly for CBT were more likely to remain in treatment than people who experienced a delay. We know that people with physical health problems are more likely to drop out from therapy. As IAPT is aiming to treat people with long-term health conditions, retention may be facilitated by fast-tracking patients into therapy within 2 weeks, so as to minimise attrition.
Although CBT has been shown to be effective for people with a cancer diagnosis, we found that it was not effective for the treatment of depression in advanced cancer. The delivery of CBT in the CanTalk trial was typical of CBT routinely delivered within an IAPT service. We do not recommend routinely offering CBT delivered through IAPT, which is a relatively costly therapy, to people with advanced cancer.
As there is a significant DSM-IV prevalence of depression in people with advanced cancer, we would support NICE’s guidelines142 to consider people’s psychological needs. However, only those with advanced cancer who are attending oncology outpatients and/or GP practices, who are widowed, divorced or separated and have significant depression should be considered for referral to IAPT for CBT. The optimum clinical management of people who are not from this demographic group should be left to the discretion of the managing team.
Supervision
Even though it is possible to train IAPT therapists to apply their existing CBT skills to specific conditions, therapists desired more specialist support and supervision in order to deliver the treatment. IAPT services should consider identifying specialists able to support CBT therapists so that they can tap into additional knowledge about the condition being targeted.
Therapists also had their own concerns about existential issues and dying. The training prior to their seeing patients was helpful in addressing some of these issues and we would consider this essential to providing pastoral care for therapists.
The TCC provided a simple, useful guide to help therapists identify the elements of therapy that they did or did not deliver. Should IAPT choose to measure adherence to treatment protocols, this approach could be considered within an IAPT setting.
Research implications
Governance
The CanTalk team experienced considerable difficulty with the complexity of research governance. The replication of submissions and long delay for responses were major hurdles to the project and accounted for delayed recruitment. We support the NHS Health Services Research Authority aims for a more streamlined approval system.
Recruitment issues
To ensure that participating health-care trusts screen or recruit for the trial, it will be important to agree with the trust early on (ideally during site set-up) what time slots the trust can allocate to screening for the study or which clinics it will be able to screen, and to monitor screening logs to ensure that these clinics are screened on an ongoing basis. This can ensure that commitments to the study are maintained, as competing demands on staff time may mean that trials perceived as providing better incentives such as CTIMP trials take priority and inadvertently ‘push out’ time spent screening for the study.
As described Chapter 7, Recruitment issues, there is a depletion in the numbers of patients available in clinics in which the turnover of patients is slow. We recommend expanding or changing the source of recruitment to similar oncology groups in different centres.
The recruitment setting
The number of patients with advanced cancer on GP lists was small. Although this group could be recruited from GP practices, the yield was low. Significant numbers of GP practices would need to be engaged to recruit sufficient patients for a study such as CanTalk. We would recommend that screening directly in secondary care is a more cost-effective way to identify and recruit patients with advanced cancer.
Target population
Potentially, further research could involve identifying people with advanced cancer who are widowed, divorced or separated, screen them for depression and randomise them to a RCT of CBT, delivered through IAPT or a talking control, in order to determine whether or not change is likely to have been effected by non-specific factors in therapy.
Although there is a policy of promoting the use of CBT, delivered through IAPT, for the treatment of chronic health conditions, including cancer, we found that treating our physically ill population with CBT, delivered through IAPT, was not effective. People with chronic health conditions have many of the same characteristics as our population. Even though clinicians, therapists and patients may advocate the use of CBT for chronic conditions, further research to generate a robust evidence base is required to determine whether or not CBT delivered through IAPT is effective.
The intervention
It is unclear whether or not the setting where therapy was delivered contributed to a lack of treatment effect. Further work could involve evaluating CBT delivered by cognitive therapists embedded in an oncology rather than an IAPT setting. However, given the lack of even a trend towards benefit, we do not think the use of a pure CBT approach warrants further research.
The Walker et al. 20 approach using integrated collaborative care in an oncology service setting appeared to be effective and was a sufficiently powered trial for people with lung cancer. Further research should involve testing a collaborative care approach in people with a range of cancers delivered in a secondary care setting.
Final recommendations
Summary of service implications
-
CBT delivered through IAPT is neither clinically effective nor cost-effective for people with depression and advanced cancer.
-
People who are widowed, divorced or separated and who have advanced cancer may benefit from screening and referral to IAPT for CBT.
-
Clinical supervision may benefit from involving people with additional expertise for specialist client groups, possibly through a centralised supervision system.
-
Therapist’s self-report sheets may be helpful in guiding the components of therapy delivered.
Research recommendations
-
Recruitment should be considered over a wide recruitment base from oncology and hospices, but this should be no longer than 6–12 months to avoid reservoir effects.
-
Further studies should consider researching the use of an integrative care model, such as that described in the SMaRT oncology trials,18–20 for all people with advanced cancer.
-
The mechanism of change in the treatment of people who are widowed, divorced or separated and who have advanced cancer and depression needs to be evaluated to see whether specific or non-specific effects bring about a change with CBT.
-
We support the NHS Health Research Authority aims of making it easier for research studies to be set up and the elimination of duplicate application. We would recommend a target for approvals to be processed within 2 months.
Acknowledgements
We would like to thank the participants who kindly took part in the study:
-
The principal investigators in all participating IAPT centres and oncology clinics, the therapists who treated participants as part of the trial, and all oncology and hospice staff who helped to screen and recruit participants for the study.
-
Janet St.John-Austen, a user representative, who was invaluable in contributing towards the planning of the project and preparing patient materials, and who also helped with the interpretation of the results.
-
The members of the Trial Steering Committee – Mathew Hotopf (chairperson), David Ekers, Sarah Purdy, Janet St.John-Austen, Sarah White, Louise Jones, Marc Serfaty and Vari Drennan.
-
The members of the Trial Data Monitoring Committee – John Norrie (chairperson), Nicola Wiles, Galina Velikova and Paddy Stone.
-
The researchers who collected data for the trial – Brendan Hore, Nicola White, Georgina Forden, Deborah Haworth, Cate Barlow, Kirsty Bennett, Suzan Hassan, Ekaterina Cooper and Andrea Beetison, and Terrie Fiawoo, who helped to co-ordinate the trial.
We would also like to thank Steve Pilling for helping with IAPT service involvement, Baptiste Laurent, for helping with the statistical design at the start of the project, Marc Griffin for conducting the statistical analysis, Faye Gishen for overseeing the trial and recruitment of patients in the Marie Curie Hospice, Hampstead, and Liz Court for the mentoring, supervision and support of trial researchers.
Contributions of authors
Dr Marc Serfaty was the chief investigator of the CanTalk trial. He was involved in the planning, management, analysis and write-up of the project.
Professor Michael King was involved in the design, planning and management of the project. He also provided an input into the analysis plan and interpretation of the statistics/findings, as well as the design of the treatment manual and the training of therapists.
Professor Irwin Nazareth was involved in the design and planning management of the project. He also provided an input into the analysis plan and interpretation of the statistics/findings.
Dr Stirling Moorey was involved in the design and planning management of the project. He was involved in the design of the treatment manual and the training of therapists. He was also involved in the interpretation of the results and write-up.
Dr Trefor Aspden was involved in the project management, interpretation of the results and write-up.
Dr Adrian Tookman was involved in the design and planning of the project. He helped to engage the hospices to recruit patients and was also involved in the write-up.
Dr Kathryn Mannix was involved in the design and planning management of the project. She was involved in the design of the treatment manual and the training of therapists. She was also involved in the interpretation of the results and write-up.
Dr Anna Gola was involved in the analysis and interpretation of the health economics, as well as the write-up and discussion.
Ms Sarah Davis was involved in the design and planning management of the project, participant identification, follow-up and write-up.
Mr John Wood was involved in the management of the project, the analysis plan and the interpretation of findings.
Dr Louise Jones was essential to the design and planning management of the project. She was actively involved with the day-to-day running of the project, as well as the interpretation of the results and write-up.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- McDaniel JS, Musselman DL, Porter MR, Reed DA, Nemeroff CB. Depression in patients with cancer. Diagnosis, biology, and treatment. Arch Gen Psychiatry 1995;52:89-9. https://doi.org/10.1001/archpsyc.1995.03950140007002.
- Hotopf M, Chidgey J, Addington-Hall J, Ly KL. Depression in advanced disease: a systematic review Part 1. Prevalence and case finding. Palliat Med 2002;16:81-97. https://doi.org/10.1191/0269216302pm507oa.
- Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 2011;12:160-74. https://doi.org/10.1016/S1470-2045(11)70002-X.
- Potash M, Breitbart W. Affective disorders in advanced cancer. Hematol Oncol Clin North Am 2002;16:671-700. https://doi.org/10.1016/S0889-8588(02)00013-8.
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monographs 2004;32:57-71. https://doi.org/10.1093/jncimonographs/lgh014.
- Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord 2012;141:343-51. https://doi.org/10.1016/j.jad.2012.03.025.
- Thomas CM, Morris S. Cost of depression among adults in England in 2000. Br J Psychiatry 2003;183:514-19. https://doi.org/10.1192/bjp.183.6.514.
- Alexander PJ, Dinesh N, Vidyasagar MS. Psychiatric morbidity among cancer patients and its relationship with awareness of illness and expectations about treatment outcome. Acta Oncol 1993;32:623-6. https://doi.org/10.3109/02841869309092441.
- Brown LF, Kroenke K, Theobald DE, Wu J, Tu W. The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psycho-Oncology 2010;19:734-41. https://doi.org/10.1002/pon.1627.
- Arrieta O, Angulo LP, Núñez-Valencia C, Dorantes-Gallareta Y, Macedo EO, Martínez-López D, et al. Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer. Ann Surg Oncol 2013;20:1941-8. https://doi.org/10.1245/s10434-012-2793-5.
- Bowers L, Boyle DA. Depression in patients with advanced cancer. Clin J Oncol Nurs 2003;7:281-8. https://doi.org/10.1188/03.CJON.281-288.
- Batty GD, Russ TC, Stamatakis E, Kivimäki M. Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. BMJ 2017;356. https://doi.org/10.1136/bmj.j108.
- Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med 2010;40:1797-810. https://doi.org/10.1017/S0033291709992285.
- Lloyd-Williams M, Shiels C, Taylor F, Dennis M. Depression – an independent predictor of early death in patients with advanced cancer. J Affect Disord 2009;113:127-32. https://doi.org/10.1016/j.jad.2008.04.002.
- Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analyses. Int J Psychiatry Med 2006;36:13-34. https://doi.org/10.2190/EUFN-RV1K-Y3TR-FK0L.
- Williams S, Dale J. The effectiveness of treatment for depression/depressive symptoms in adults with cancer: a systematic review. Br J Cancer 2006;94:372-90. https://doi.org/10.1038/sj.bjc.6602949.
- Newell SA, Sanson-Fisher RW, Savolainen NJ. Systematic review of psychological therapies for cancer patients: overview and recommendations for future research. J Natl Cancer Inst 2002;94:558-84. https://doi.org/10.1093/jnci/94.8.558.
- Strong V, Waters R, Hibberd C, Murray G, Wall L, Walker J, et al. Management of depression for people with cancer (SMaRT oncology 1): a randomised trial. Lancet 2008;372:40-8. https://doi.org/10.1016/S0140-6736(08)60991-5.
- Sharpe M, Walker J, Holm Hansen C, Martin P, Symeonides S, Gourley C, et al. Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology-2): a multicentre randomised controlled effectiveness trial. Lancet 2014;384:1099-108. https://doi.org/10.1016/S0140-6736(14)61231-9.
- Walker J, Hansen CH, Martin P, Symeonides S, Gourley C, Wall L, et al. Integrated collaborative care for major depression comorbid with a poor prognosis cancer (SMaRT Oncology-3): a multicentre randomised controlled trial in patients with lung cancer. Lancet Oncol 2014;15:1168-76. https://doi.org/10.1016/S1470-2045(14)70343-2.
- Rayner L, Higginson IJ, Price A, Hotopf M. The Management of Depression in Palliative Care: European Clinical Guidelines. London: Department of Palliative Care, Policy & Rehabilitation and European Palliative Care Research Collaborative; 2010.
- Rayner L, Lee W, Price A, Monroe B, Sykes N, Hansford P, et al. The clinical epidemiology of depression in palliative care and the predictive value of somatic symptoms: cross-sectional survey with four-week follow-up. Palliat Med 2011;25:229-41. https://doi.org/10.1177/0269216310387458.
- Akechi T, Okuyama T, Onishi J, Morita T, Furukawa TA. Psychotherapy for depression among incurable cancer patients. Cochrane Database Syst Rev 2008;2. https://doi.org/10.1002/14651858.CD005537.pub2.
- Edwards AG, Hulbert-Williams N, Neal RD. Psychological interventions for women with metastatic breast cancer. Cochrane Database Syst Rev 2008;3. https://doi.org/10.1002/14651858.CD004253.pub3.
- Mustafa M, Carson-Stevens A, Gillespie D, Edwards AG. Psychological interventions for women with metastatic breast cancer. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD004253.pub4.
- Goodwin PJ, Leszcz M, Ennis M, Koopmans J, Vincent L, Guther H, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med 2001;345:1719-26. https://doi.org/10.1056/NEJMoa011871.
- Linn MW, Linn BS, Harris R. Effects of counseling for late stage cancer patients. Cancer 1982;49:1048-55. https://doi.org/10.1002/1097-0142(19820301)49:5<1048::AID-CNCR2820490534>3.0.CO;2-G.
- Spiegel D, Bloom JR, Yalom I. Group support for patients with metastatic cancer. A randomized outcome study. Arch Gen Psychiatry 1981;38:527-33. https://doi.org/10.1001/archpsyc.1980.01780300039004.
- Wood BC, Mynors-Wallis LM. Problem-solving therapy in palliative care. Palliat Med 1997;11:49-54. https://doi.org/10.1177/026921639701100106.
- Edelman S, Bell DR, Kidman AD. A group cognitive behaviour therapy programme with metastatic breast cancer patients. Psycho-Oncology 1999;8:295-30. https://doi.org/10.1002/(SICI)1099-1611(199907/08)8:4<295::AID-PON386>3.0.CO;2-Y.
- Classen C, Butler LD, Koopman C, Miller E, DiMiceli S, Giese-Davis J, et al. Supportive-expressive group therapy and distress in patients with metastatic breast cancer: a randomized clinical intervention trial. Arch Gen Psychiatry 2001;58:494-501. https://doi.org/10.1001/archpsyc.58.5.494.
- Cunningham AJ, Edmonds CV, Jenkins GP, Pollack H, Lockwood GA, Warr D. A randomized controlled trial of the effects of group psychological therapy on survival in women with metastatic breast cancer. Psycho-Oncology 1998;7:508-17. https://doi.org/10.1002/(SICI)1099-1611(199811/12)7:6<508::AID-PON376>3.0.CO;2-7.
- Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet 1989;2:888-91. https://doi.org/10.1016/S0140-6736(89)91551-1.
- Gotay CC, Moinpour CM, Unger JM, Jiang CS, Coleman D, Martino S, et al. Impact of a peer-delivered telephone intervention for women experiencing a breast cancer recurrence. J Clin Oncol 2007;25:2093-9. https://doi.org/10.1200/JCO.2006.07.4674.
- Kissane DW, Grabsch B, Clarke DM, Smith GC, Love AW, Bloch S, et al. Supportive-expressive group therapy for women with metastatic breast cancer: survival and psychosocial outcome from a randomized controlled trial. Psycho-Oncology 2007;16:277-86. https://doi.org/10.1002/pon.1185.
- Low CA, Stanton AL, Bower JE, Gyllenhammer L. A randomized controlled trial of emotionally expressive writing for women with metastatic breast cancer. Health Psychol 2010;29:460-6. https://doi.org/10.1037/a0020153.
- Scholten C, Weinländer G, Krainer M, Frischenschlager O, Zielinski CC. Difference in patient’s acceptance of early versus late initiation of psychosocial support in breast cancer. Support Care Cancer 2001;9:459-64. https://doi.org/10.1007/s005200000233.
- Aranda S, Schofield P, Weih L, Milne D, Yates P, Faulkner R. Meeting the support and information needs of women with advanced breast cancer: a randomised controlled trial. Br J Cancer 2006;95:667-73. https://doi.org/10.1038/sj.bjc.6603320.
- Jassim GA, Whitford DL, Hickey A, Carter B. Psychological interventions for women with non-metastatic breast cancer. Cochrane Database Syst Rev 2015;5. https://doi.org/10.1002/14651858.CD008729.pub2.
- Price A, Hotopf M. The treatment of depression in patients with advanced cancer undergoing palliative care. Curr Opin Support Palliat Care 2009;3:61-6. https://doi.org/10.1097/SPC.0b013e328325d17a.
- Stiefel F, Die Trill M, Berney A, Olarte JM, Razavi A. Depression in palliative care: a pragmatic report from the Expert Working Group of the European Association for Palliative Care. Support Care Cancer 2001;9:477-88. https://doi.org/10.1007/s005200100244.
- Thekkumpurath P, Venkateswaran C, Kumar M, Bennett MI. Screening for psychological distress in palliative care: a systematic review. J Pain Symptom Manage 2008;36:520-8. https://doi.org/10.1016/j.jpainsymman.2007.11.010.
- Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ 2003;327:1144-6. https://doi.org/10.1136/bmj.327.7424.1144.
- Mitchell A. Are one or two simple questions sufficient to detect depression in cancer and palliative care? A Bayesian meta-analysis. Br J Cancer 2008;98:1934-43. https://doi.org/10.1038/sj.bjc.6604396.
- Low J, Gessler S, Williams R, Daniells E, Brough V, Tookman A, et al. Screening for distress and depression in cancer patients: is ultrashort depression screening a valid measure in the UK? A prospective validation study. J Pain Symptom Manage 2009;38:234-43. https://doi.org/10.1016/j.jpainsymman.2008.08.006.
- Pessin H, Olden M, Jacobson C, Kosinski A. Clinical assessment of depression in terminally ill cancer patients: a practical guide. Palliat Support Care 2005;3:319-24. https://doi.org/10.1017/S1478951505050480.
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59:22-33.
- Passik SD, Kirsh KL, Donaghy KB, Theobald DE, Lundberg JC, Holtsclaw E, et al. An attempt to employ the Zung Self-Rating Depression Scale as a ‘lab test’ to trigger follow-up in ambulatory oncology clinics: criterion validity and detection. J Pain Symptom Manage 2001;21:273-81. https://doi.org/10.1016/S0885-3924(00)00264-5.
- Passik SD, Lundberg JC, Rosenfeld B, Kirsh KL, Donaghy K, Theobald D, et al. Factor analysis of the Zung Self-Rating Depression Scale in a large ambulatory oncology sample. Psychosomatics 2000;41:121-7. https://doi.org/10.1176/appi.psy.41.2.121.
- Passik SD, Kirsh KL, Donaghy K, Holtsclaw E, Theobald D, Cella D, et al. Fatigue Coalition . Patient-related barriers to fatigue communication: initial validation of the fatigue management barriers questionnaire. J Pain Symptom Manage 2002;24:481-93. https://doi.org/10.1016/S0885-3924(02)00518-3.
- Manzanera C, Lafay N, Papet N, Senon J. Depression, anxiety and cancer. Ann Méd-Psychol 2003;98:140-6. https://doi.org/10.1016/S0003-4487(03)00020-9.
- Drabe N, Zwahlen D, Büchi S, Moergeli H, Zwahlen RA, Jenewein J. Psychiatric morbidity and quality of life in wives of men with long-term head and neck cancer. Psycho-Oncology 2008;17:199-204. https://doi.org/10.1002/pon.1199.
- Sukegawa A, Miyagi E, Asai-Sato M, Saji H, Sugiura K, Matsumura T, et al. Anxiety and prevalence of psychiatric disorders among patients awaiting surgery for suspected ovarian cancer. J Obstet Gynaecol Res 2008;34:543-51. https://doi.org/10.1111/j.1447-0756.2008.00738.x.
- Gandubert C, Carrière I, Escot C, Soulier M, Hermès A, Boulet P, et al. Onset and relapse of psychiatric disorders following early breast cancer: a case-control study. Psycho-Oncology 2009;18:1029-37. https://doi.org/10.1002/pon.1469.
- Zwahlen RA, Dannemann C, Grätz KW, Studer G, Zwahlen D, Moergeli H, et al. Quality of life and psychiatric morbidity in patients successfully treated for oral cavity squamous cell cancer and their wives. J Oral Maxillofac Surg 2008;66:1125-32. https://doi.org/10.1016/j.joms.2007.09.003.
- Sheehan D, Lecrubier Y, Sheehan K, Janavs J, Weiller E. Comparison of the Mini International Neuropsychiatric Interview (MINI) with the SCID-P and the CIDI: a validity study. Psychopharmacol Bull 1995;31.
- Diagnostic and Statistical Manual of Mental Disorders, Third Edition. Washington, DC: American Psychiatric Association; 1980.
- Amorim P, Lecrubier Y, Weiller E, Hergueta T, Sheehan D. DSM-IH-R Psychotic Disorders: procedural validity of the Mini International Neuropsychiatric Interview (MINI). Concordance and causes for discordance with the CIDI. Eur Psychiatry 1998;13:26-34. https://doi.org/10.1016/S0924-9338(97)86748-X.
- Shelby-James TM, Hardy J, Agar M, Yates P, Mitchell G, Sanderson C, et al. Designing and conducting randomized controlled trials in palliative care: a summary of discussions from the 2010 clinical research forum of the Australian Palliative Care Clinical Studies Collaborative. Palliat Med 2012;26:1042-7. https://doi.org/10.1177/0269216311417036.
- Boland J, Currow DC, Wilcock A, Tieman J, Hussain JA, Pitsillides C, et al. A systematic review of strategies used to increase recruitment of people with cancer or organ failure into clinical trials: implications for palliative care research. J Pain Symptom Manage 2015;49:762-72.e5. https://doi.org/10.1016/j.jpainsymman.2014.09.018.
- Jordhøy MS, Kaasa S, Fayers P, Ovreness T, Underland G, Ahlner-Elmqvist M. Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliat Med 1999;13:299-310. https://doi.org/10.1191/026921699668963873.
- Moorey S, Cort E, Kapari M, Monroe B, Hansford P, Mannix K, et al. A cluster randomized controlled trial of cognitive behaviour therapy for common mental disorders in patients with advanced cancer. Psychol Med 2009;39:713-23. https://doi.org/10.1017/S0033291708004169.
- Savard J, Simard S, Giguère I, Ivers H, Morin CM, Maunsell E, et al. Randomized clinical trial on cognitive therapy for depression in women with metastatic breast cancer: psychological and immunological effects. Palliat Support Care 2006;4:219-37. https://doi.org/10.1017/S1478951506060305.
- King M, Jones L, Richardson A, Murad S, Irving A, Aslett H, et al. The relationship between patients’ experiences of continuity of cancer care and health outcomes: a mixed methods study. Br J Cancer 2008;98:529-36. https://doi.org/10.1038/sj.bjc.6604164.
- King M, Jones L, McCarthy O, Rogers M, Richardson A, Williams R, et al. Development and pilot evaluation of a complex intervention to improve experienced continuity of care in patients with cancer. Br J Cancer 2009;100:274-80. https://doi.org/10.1038/sj.bjc.6604836.
- Serfaty MA, Haworth D, Blanchard M, Buszewicz M, Murad S, King M. Clinical effectiveness of individual cognitive behavioral therapy for depressed older people in primary care: a randomized controlled trial. Arch Gen Psychiatry 2009;66:1332-40. https://doi.org/10.1001/archgenpsychiatry.2009.165.
- Serfaty M, Wilkinson S, Freeman C, Mannix K, King M. The ToT study: helping with Touch or Talk (ToT): a pilot randomised controlled trial to examine the clinical effectiveness of aromatherapy massage versus cognitive behaviour therapy for emotional distress in patients in cancer/palliative care. Psycho-Oncology 2012;21:563-9. https://doi.org/10.1002/pon.1921.
- Jones L, Harrington J, Barlow CA, Tookman A, Drake R, Barnes K, et al. Advance care planning in advanced cancer: can it be achieved? An exploratory randomized patient preference trial of a care planning discussion. Palliat Support Care 2011;9:3-13. https://doi.org/10.1017/S1478951510000490.
- Simon GE, Ludman EJ, Tutty S, Operskalski B, Von Korff M. Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: a randomized controlled trial. JAMA 2004;292:935-42. https://doi.org/10.1001/jama.292.8.935.
- Wang PS, Simon GE, Avorn J, Azocar F, Ludman EJ, McCulloch J, et al. Telephone screening, outreach, and care management for depressed workers and impact on clinical and work productivity outcomes: a randomized controlled trial. JAMA 2007;298:1401-11. https://doi.org/10.1001/jama.298.12.1401.
- Semple CJ, Dunwoody L, Sullivan K, Kernohan WG. Patients with head and neck cancer prefer individualized cognitive behavioural therapy. Eur J Cancer Care 2006;15:220-7. https://doi.org/10.1111/j.1365-2354.2005.00643.x.
- Improving Outcomes in Supportive and Palliative Care for Adults with Cancer. London: NICE; 2004.
- Moncher FJ, Prinz RJ. Treatment fidelity in outcome studies. Clin Psychol Rev 1991;11:247-66. https://doi.org/10.1016/0272-7358(91)90103-2.
- Lichstein KL, Riedel BW, Grieve R. Fair tests of clinical trials: a treatment implementation model. Adv Behav Res Ther 1994;16:1-29. https://doi.org/10.1016/0146-6402(94)90001-9.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- Shaw BF, Elkin I, Yamaguchi J, Olmsted M, Vallis TM, Dobson KS, et al. Therapist competence ratings in relation to clinical outcome in cognitive therapy of depression. J Consult Clin Psychol 1999;67:837-46. https://doi.org/10.1037/0022-006X.67.6.837.
- Webb CA, Derubeis RJ, Barber JP. Therapist adherence/competence and treatment outcome: a meta-analytic review. J Consult Clin Psychol 2010;78:200-11. https://doi.org/10.1037/a0018912.
- Moorey S, Greer S. Cognitive Behaviour Therapy for People with Cancer. Oxford: Oxford University Press; 2002.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. CONSORT Group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. https://doi.org/10.7326/0003-4819-148-4-200802190-00008.
- The Five Year Forward View for Mental Health. A report from the independent Mental Health Taskforce to the NHS in England. London: NHS England; 2016.
- Baldwin SA, Wampold BE, Imel ZE. Untangling the alliance-outcome correlation: exploring the relative importance of therapist and patient variability in the alliance. J Consult Clin Psychol 2007;75:842-52. https://doi.org/10.1037/0022-006X.75.6.842.
- Weerasekera P, Linder B, Greenberg L, Watson J. The working alliance in client-centered and process-experiential therapy of depression. Psychothe Res 2001;11:221-33. https://doi.org/10.1093/ptr/11.2.221.
- Fukui S, Kugaya A, Okamura H, Kamiya M, Koike M, Nakanishi T, et al. A psychosocial group intervention for Japanese women with primary breast carcinoma. Cancer 2000;89:1026-36. https://doi.org/10.1002/1097-0142(20000901)89:5<1026::AID-CNCR12>3.0.CO;2-5.
- Sandgren AK, McCaul KD, King B, O’Donnell S, Foreman G. Telephone therapy for patients with breast cancer. Oncol Nurs Forum 2000;27:683-8.
- Keijsers GP, Schaap CP, Hoogduin CA. The impact of interpersonal patient and therapist behavior on outcome in cognitive-behavior therapy. A review of empirical studies. Behav Modif 2000;24:264-97. https://doi.org/10.1177/0145445500242006.
- Huppert JD, Bufka LF, Barlow DH, Gorman JM, Shear MK, Woods SW. Therapists, therapist variables, and cognitive-behavioral therapy outcome in a multicenter trial for panic disorder. J Consult Clin Psychol 2001;69:747-55. https://doi.org/10.1037/0022-006X.69.5.747.
- Kuller AM, Ott BD, Goisman RM, Wainwright LD, Rabin RJ. Cognitive behavioral therapy and schizophrenia: a survey of clinical practices and views on efficacy in the United States and United kingdom. Community Ment Health J 2010;46:2-9. https://doi.org/10.1007/s10597-009-9223-6.
- Edelman S, Lemon J, Kidman A. Group cognitive behavior therapy for breast cancer patients: a qualitative evaluation. Psychol Health Med 2005;10:139-44. https://doi.org/10.1080/13548500412331334172.
- Bottomley A. Group cognitive behavioural therapy with cancer patients: the views of women participants on a short-term intervention. Eur J Cancer Care 1998;7:23-30. https://doi.org/10.1046/j.1365-2354.1998.00064.x.
- Omylinska-Thurston J, Cooper M. Helpful processes in psychological therapy for patients with primary cancers: a qualitative interview study. Couns Psychother Res 2013;14:84-92. https://doi.org/10.1080/14733145.2013.813952.
- MacCormack T, Simonian J, Lim J, Remond L, Roets D, Dunn S, et al. ‘Someone who cares:’ a qualitative investigation of cancer patients’ experiences of psychotherapy. Psycho-Oncology 2001;10:52-65. https://doi.org/10.1002/1099-1611(200101/02)10:1<52::AID-PON489>3.0.CO;2-V.
- Anderson T, Watson M, Davidson R. The use of cognitive behavioural therapy techniques for anxiety and depression in hospice patients: a feasibility study. Palliat Med 2008;22:814-21. https://doi.org/10.1177/0269216308095157.
- Talking Therapies: A Four-Year Plan of Action. London: DHSC; 2011.
- Developing a Pathway for Mental Health and Psychological Support Services for Adults. London Cancer Alliance West and South; 2014.
- Serfaty M, King M, Nazareth I, Tookman A, Wood J, Gola A, et al. The clinical and cost effectiveness of cognitive behavioural therapy plus treatment as usual for the treatment of depression in advanced cancer (CanTalk): study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1223-6.
- World Medical Association . Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction 1993;88:791-804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x.
- Reeve J, Lloyd-Williams M, Dowrick C. Depression in terminal illness: the need for primary care-specific research. Fam Pract 2007;24:263-8. https://doi.org/10.1093/fampra/cmm017.
- Wilson KG, Chochinov H, De Faye B, Breitbart W, Chochinov HM, Breitbart W. Handbook of Psychiatry in Palliative Medicine. Oxford: Oxford University Press; 2000.
- Maguire P. Improving the detection of psychiatric problems in cancer patients. Soc Sci Med 1985;20:819-23. https://doi.org/10.1016/0277-9536(85)90336-3.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284-92. https://doi.org/10.1097/01.MLR.0000093487.78664.3C.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 2002;32:509-15. https://doi.org/10.3928/0048-5713-20020901-06.
- Kamboj SK, Tookman A, Jones L, Curran HV. The effects of immediate-release morphine on cognitive functioning in patients receiving chronic opioid therapy in palliative care. Pain 2005;117:388-95. https://doi.org/10.1016/j.pain.2005.06.022.
- Furukawa TA, McGuire H, Barbui C. Meta-analysis of effects and side effects of low dosage tricyclic antidepressants in depression: systematic review. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7371.991.
- Hayasaka Y, Purgato M, Magni LR, Ogawa Y, Takeshima N, Cipriani A, et al. Dose equivalents of antidepressants: Evidence-based recommendations from randomized controlled trials. J Affect Disord 2015;180:179-84. https://doi.org/10.1016/j.jad.2015.03.021.
- Ward S, Salazano S, Sampson F, Cowan J. Improving Supportive and Palliative Care for Adults with Cancer. London: NICE; 2004.
- Tutty S, Simon G, Ludman E. Telephone counseling as an adjunct to antidepressant treatment in the primary care system. A pilot study. Eff Clin Pract 2000;3:170-8.
- Young J, Beck AT. Cognitive therapy scale: Rating manual. Philadelphia, PA: University of Pennsylvania; 1980.
- Blackburn I-M, James IA, Milne DL, Baker C, Standart S, Garland A, et al. The revised cognitive therapy scale (CTS-R): psychometric properties. Behav Cogn Psychother 2001;29:431-46. https://doi.org/10.1017/S1352465801004040.
- Chen G, Faris P, Hemmelgarn B, Walker RL, Quan H. Measuring agreement of administrative data with chart data using prevalence unadjusted and adjusted kappa. BMC Med Res Methodol 2009;9. https://doi.org/10.1186/1471-2288-9-5.
- Mannix KA, Blackburn IM, Garland A, Gracie J, Moorey S, Reid B, et al. Effectiveness of brief training in cognitive behaviour therapy techniques for palliative care practitioners. Palliat Med 2006;20:579-84. https://doi.org/10.1177/0269216306071058.
- Cort E, Moorey S, Hotopf M, Kapari M, Monroe B, Hansford P. Palliative care nurses’ experiences of training in cognitive behaviour therapy and taking part in a randomized controlled trial. Int J Palliat Nurs 2009;15:290-8. https://doi.org/10.12968/ijpn.2009.15.6.42988.
- Patton MQ. How to Use Qualitative Methods in Evaluation. Thousand Oaks, CA: Sage Publications Inc; 1987.
- Britten N. Qualitative interviews in medical research. BMJ 1995;311:251-3. https://doi.org/10.1136/bmj.311.6999.251.
- Braun V, Clarke V. Successful Qualitative Research: a Practical Guide for Beginners. Thousand Oaks, CA: Sage Publications Inc; 2013.
- Ritchie J, Lewis J. Qualitative Research Practice: a Guide for Social Science Students and Researchers. Thousand Oaks, CA: Sage Publications Inc; 2003.
- Beck A, Steer R, Brown G. BDI-II, Beck Depression Inventory. San Antonio, TX: Psychological Corp.; 1996.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-71. https://doi.org/10.1001/archpsyc.1961.01710120031004.
- McLean LM, Jones JM, Rydall AC, Walsh A, Esplen MJ, Zimmermann C, et al. A couples intervention for patients facing advanced cancer and their spouse caregivers: outcomes of a pilot study. Psycho-Oncology 2008;17:1152-6. https://doi.org/10.1002/pon.1319.
- Laidlaw T, Bennett BM, Dwivedi P, Naito A, Gruzelier J. Quality of life and mood changes in metastatic breast cancer after training in self-hypnosis or Johrei: a short report. Contemp Hypn 2005;22:84-93. https://doi.org/10.1002/ch.27.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737-44. https://doi.org/10.1001/jama.282.18.1737.
- Pinto-Meza A, Serrano-Blanco A, Penarrubia MT, Blanco E, Haro JM. Assessing depression in primary care with the PHQ-9: can it be carried out over the telephone?. J Gen Intern Med 2005;20:738-42. https://doi.org/10.1111/j.1525-1497.2005.0144.x.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- EuroQol Group . EuroQol-a new facility for the measurement of health-related quality of life. Health Pol 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. https://doi.org/10.1097/00000421-198212000-00014.
- Beecham J, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring Mental Health Needs. Oxford: Oxford University Press; 1992.
- Patten SB. The comparative efficacy of trazodone and imipramine in the treatment of depression. CMAJ 1992;146:1177-82.
- Bollini P, Pampallona S, Tibaldi G, Kupelnick B, Munizza C. Effectiveness of antidepressants. Meta-analysis of dose-effect relationships in randomised clinical trials. Br J Psychiatry 1999;174:297-303. https://doi.org/10.1192/bjp.174.4.297.
- Bazire S. Psychotropic Drug Directory: The Professional’s Pocket Handbook and Aide Memoire. Salisbury: Fivepin; 2005.
- British National Formulary. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2016.
- Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry 1972;3:257-60. https://doi.org/10.1016/0005-7916(72)90045-6.
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry 2005;62:409-16. https://doi.org/10.1001/archpsyc.62.4.409.
- Oei TP, Free ML. Do cognitive behaviour therapies validate cognitive models of mood disorders? A review of the empirical evidence. Int J Psychol 1995;30:145-80. https://doi.org/10.1080/00207599508246564.
- Scott J. Cognitive therapy for depression. Br Med Bull 2001;57:101-13. https://doi.org/10.1093/bmb/57.1.101.
- Tang TZ, DeRubeis RJ, Beberman R, Pham T. Cognitive changes, critical sessions, and sudden gains in cognitive-behavioral therapy for depression. J Consult Clin Psychol 2005;73:168-72. https://doi.org/10.1037/0022-006X.73.1.168.
- Depression: Management of Depression in Primary and Secondary Care. London: NICE; 2004.
- Hedeker D, Gibbons RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related contrasts between two groups. J Educ Behav Stat 1999;24:70-93. https://doi.org/10.3102/10769986024001070.
- Kessler D, Lewis G, Kaur S, Wiles N, King M, Weich S, et al. Therapist-delivered Internet psychotherapy for depression in primary care: a randomised controlled trial. Lancet 2009;374:628-34. https://doi.org/10.1016/S0140-6736(09)61257-5.
- King M, Davidson O, Taylor F, Haines A, Sharp D, Turner R. Effectiveness of teaching general practitioners skills in brief cognitive behaviour therapy to treat patients with depression: randomised controlled trial. BMJ 2002;324:947-50. https://doi.org/10.1136/bmj.324.7343.947.
- Sussman JB, Hayward RA. An IV for the RCT: using instrumental variables to adjust for treatment contamination in randomised controlled trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c2073.
- Clinical Depression in Adults: Recognition and Management. Clinical Guideline [CG90]. London: NICE; 2009.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- Kind PH, Hardman G, Macran S. UK Population Norms for EQ-5D. York: The University of York Centre for Health Economics; 1999.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Curtis L. Unit Cost of Health and Social Care 2009. Canterbury: PSSRU, University of Kent; 2010.
- NHS Reference Costs 2009–10. London: DHSC; 2011.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Gomes M, Grieve R, Nixon R, Edmunds WJ. Statistical methods for cost-effectiveness analyses that use data from cluster randomized trials: a systematic review and checklist for critical appraisal. Med Decis Making 2012;32:209-20. https://doi.org/10.1177/0272989X11407341.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Button KS, Wiles NJ, Lewis G, Peters TJ, Kessler D. Factors associated with differential response to online cognitive behavioural therapy. Soc Psychiatry Psychiatr Epidemiol 2012;47:827-33. https://doi.org/10.1007/s00127-011-0389-1.
- Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J Consult Clin Psychol 2009;77:775-87. https://doi.org/10.1037/a0015401.
- Sotsky SM, Glass DR, Shea MT, Pilkonis PA, Collins JF, Elkin I, et al. Patient predictors of response to psychotherapy and pharmacotherapy: findings in the NIMH Treatment of Depression Collaborative Research Program. Am J Psychiatry 1991;148:997-1008. https://doi.org/10.1176/ajp.148.8.997.
- Low J, Serfaty M, Davis S, Vickerstaff V, Gola A, Omar RZ, et al. Acceptance and commitment therapy for adults with advanced cancer (CanACT): study protocol for a feasibility randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1169-8.
- Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer 2000;8:175-9. https://doi.org/10.1007/s005200050281.
- Cancer Research UK . Cancer Incidence Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/incidence (accessed 20 March 2018).
- Lloyd-Williams M, Spiller J, Ward J. Which depression screening tools should be used in palliative care?. Palliat Med 2003;17:40-3. https://doi.org/10.1191/0269216303pm664oa.
- Nelson CJ, Cho C, Berk AR, Holland J, Roth AJ. Are gold standard depression measures appropriate for use in geriatric cancer patients? A systematic evaluation of self-report depression instruments used with geriatric, cancer, and geriatric cancer samples. J Clin Oncol 2010;28:348-56. https://doi.org/10.1200/JCO.2009.23.0201.
- Massie MJ, Holland JC. Depression and the cancer patient. J Clin Psychiatry 1990;51:12-7.
- Derogatis L. Brief Symptom Inventory (BSI): Administration, Scoring, and Procedures Manual. Minneapolis, MN: NCS Pearson; 2000.
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385-401. https://doi.org/10.1177/014662167700100306.
- Sheikh JI, Yesavage JA. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: Haworth Press; 1986.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Shacham S. A shortened version of the Profile of Mood States. J Pers Assess 1983;47:305-6. https://doi.org/10.1207/s15327752jpa4703_14.
- Zung WW. A self-rating depression scale. Arch Gen Psychiatry 1965;12:63-70. https://doi.org/10.1001/archpsyc.1965.01720310065008.
- Lloyd-Williams M, Friedman T, Rudd N. Criterion validation of the Edinburgh postnatal depression scale as a screening tool for depression in patients with advanced metastatic cancer. J Pain Symptom Manage 2000;20:259-65. https://doi.org/10.1016/S0885-3924(00)00182-2.
- Streiner DL. The case of the missing data: methods of dealing with dropouts and other research vagaries. Can J Psychiatry 2002;47:68-75. https://doi.org/10.1177/070674370204700111.
- NHS Digital . Improving Access to Psychological Therapies Report, March 2015 Final 2015. http://content.digital.nhs.uk/catalogue/PUB17755 (accessed 4 November 2016).
- Gilbody S, Littlewood E, Hewitt C, Brierley G, Tharmanathan P, Araya R, et al. Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial. BMJ 2015;351. https://doi.org/10.1136/bmj.h5627.
- NHS Digital . Psychological Therapies, Annual Report on the Use of IAPT Services – England, 2014–15 n.d. https://digital.nhs.uk/catalogue/PUB19098 (accessed 23 May 2018).
- Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry 2013;58:376-85. https://doi.org/10.1177/070674371305800702.
- Cuijpers P, van Straten A, Andersson G, van Oppen P. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol 2008;76:909-22. https://doi.org/10.1037/a0013075.
- King M, Llewellyn H, Leurent B, Owen F, Leavey G, Tookman A, et al. Spiritual beliefs near the end of life: a prospective cohort study of people with cancer receiving palliative care. Psycho-Oncology 2013;22:2505-12. https://doi.org/10.1002/pon.3313.
- Joint Project Guideline notes on Pharmacovigilance . Workstream 6: Pharmacovigilance n.d. www.ct-toolkit.ac.uk/routemap/risk-assessment/downloads/joint-project-pharmacovigilance.pdf (accessed 26 March 2018).
Appendix 1 Serious adverse events protocol
Serious adverse event (adapted from the Joint Project Guideline notes on Pharmacovigilance)175
Any AE or adverse reaction that:
-
results in death
-
is life-threatening
-
requires hospitalisation or prolongation of existing hospitalisation
-
results in persistent or significant disability or incapacity
-
consists of a congenital anomaly or birth defect.
Serious adverse events
Suicide intent, self-harm and overdose are highly unlikely but are associated with major depression and, therefore, would be expected SAEs. We do not expect treatment to cause these events. Therefore, if any of the following SAEs are reported or observed then the SAE procedure should be implemented as soon as participant safety has been assured.
1. Suicide ideation or intention
Suicide ideation is characterised by thoughts of suicide without clear intention or plan to attempt suicide. Suicide intention is characterised by intentions or plans to attempt suicide. If a participant reports possible suicide ideation or intention they will be assessed for suicide risk using the MINI Section C: Suicidality at screening or the BDI-II during follow-ups.
-
Low to moderate risk:
-
At home – they will be encouraged to contact the GP if their mood continues to deteriorate. They will be asked for consent to call their GP on their behalf and a family member. If they refuse contact with a GP or family, but are still at high risk to themselves or others, proceed to call GP.
-
In a health-care setting – ask the patient to speak with their GP/oncologist or see the psychological support services available.
-
-
High risk (i.e. the participant reports immediate suicide intent):
-
At home – they will be asked to contact their GP immediately, either themselves or by someone on their behalf. Check if a relative can be contacted. Remain with them until a family member or the GP arrives. Follow up with the research site to inform them of event (see lone worker policy). When the participant refuses help and risk is deemed immediately life-threatening then the police should be notified.
-
In a health-care setting – they will be encouraged to contact their GP immediately. Remain with them until a family member or the GP arrives. If in the patient’s home, inform research site of event (see lone worker policy). When the participant refuses help and risk is deemed immediately life-threatening then the police may also be notified.
-
2. Self-harm
-
If participants exhibit any signs of self-harm, they will be encouraged to contact their GP that day. In the event that they refuse to seek help and their safety is a concern, their GP will be notified.
-
When the participant refuses help and risk is deemed immediately life-threatening then the police should also be notified.
3. Overdose
-
If the participant reports or exhibits signs of excessive drug consumption/intention to overdose they will be encouraged to contact their GP that day.
-
In the event that they refuse to seek help and their safety is a concern, their GP will be notified.
Appendix 2 Topic guide: clinicians’ experiences
During this interview I am going to ask you a number of questions to help to explore your experiences of referring patients to psychological research studies in general and then more specifically to the CanTalk trial. You have read the information sheet for the study. Are there any questions you have? So you know that CanTalk is a research trial recruiting patients with incurable cancer and low mood, then randomising these patients into either care as usual or 12 sessions of cognitive–behavioural therapy plus care as usual. I am going to ask you a number of questions about your experiences of helping to recruit to CanTalk and this conversation will be audio recorded. We may also use quotes from the interview in publications however these will be anonymised.
My first question will focus on you and your role in the oncology clinic . . .
1. Describe your role in the oncology clinics.
-
Can you describe your current role in this clinic to me?
-
How did you come to be in this role?
-
How do you feel about your role?
My next question will look at your research participation to date . . .
2. Research participation.
-
Have you been involved in any type of research in your role and can you tell me about it?
-
What type of methodology
-
Duration of trial
-
CTIMP or non-CTIMP
-
Personal research or study (PhD, dissertation, etc.)
-
I would like to ask you a little bit now on your views on trials that are not of medicinal products or drug trials . . .
3. Views on non-drug trials.
-
How often do you participate in this type of research? [e.g. psychological research, complex interventions and non-CTIMPS (non-drug trials)]
-
To what extent do you feel these trials are part of your remit?
I would like to ask you more specific questions now about your views on the CanTalk trial itself . . .
4. Specific to CanTalk.
-
What do you know about CanTalk study?
-
(Preamble: as you are aware the CanTalk trial was conducted by researchers from UCL.) What are your views about the research being carried out by the university and not embedded in the clinic team?
-
What are your views on CanTalk’s comparison of active treatment with usual care (e.g. randomised trials)?
Now I will ask you questions about your participation in the CanTalk trial . . .
5. Participation in CanTalk.
-
How do you think one’s feelings about research influence their involvement?
-
How do both your patients’ reactions to research and experiences of participation affect your involvement in the research?
-
What factors do you think may influence recruitment? (Prompts – anything that might increase recruitment? Factors influencing poor recruitment?)
I would like to ask you now to tell me about any patient feedback you have had about CanTalk . . .
6. Feedback about CanTalk.
-
Have any of your patients mentioned the trial? (prompt positive or negative responses)
-
How do you feel the trial affected your patients?
Lastly, I would like to ask you about your opinions on psychological research studies like this in the future . . .
7. Future for psychosocial studies.
-
Would you participate in this type of research in the future?
-
Why do you say this?
-
(Depending on response, if negative) What might make you invest more in these types of trials? (More CPD points, professional development or topic you are passionate about)
-
Overall what did you think about the CanTalk study and do you have any suggestions for improvements to CanTalk and studies like these in the future?
Appendix 3 Topic guide: therapists’ experience
Prompts
1. Describe your role/involvement in the CanTalk study. – How long have you been a therapist? – Have you seen any other patients with chronic health conditions aside from the CanTalk study? – Is your role as part of the study ongoing or have you finished seeing patients? – How many patients have/did you see as part of the study? – Where did you see patients (e.g. office or at home)?
2. What did you know about the patient’s diagnosis prior to their first appointment? – What was your level of knowledge in cancer? Did you understand the patient’s condition (e.g. less well-known cancers such as myeloma)?
-
Would it have been useful to know more? If so, what do you suggest would have been the best way of providing therapists with more information? (Online training facilities, workshops, information booklets?)
3. How did you feel about working with patients with advanced cancer? – What were your prior expectations?
-
How did working with these patients match or not match your expectations?
-
What do you think about the training required to work with these patients?
-
What other training would have been beneficial (prior to seeing patients)?
4. What was your experience of working with patients from the CanTalk study? – What problems or issues, if any, did you encounter working with this patient group (e.g. personally – emotional issues)? How do you think these may have impacted therapy sessions? – Issues with patient (e.g. not being able to attend therapy, etc.)? How do you think this may have impacted therapy sessions? – How well supported and supervised did you feel?
-
Issues in the setting/structure: can you tell me how easy/difficult it was to coordinate your appointments among all your other appointments? How much space and time did you have to conduct therapy sessions?
5. Were there any components of CBT that you thought were most helpful/useful for this patient group? Were there any components of CBT that you found challenging to deliver to this particular patient group? – If they suggest some helpful/useful ones – does this differ from patients without chronic health conditions?
-
If they suggest challenging ones – how did you tackle these challenges?
6. What do you think about CBT for this patient group?
-
In what ways do you think patients should be supported psychologically? If not, why not?
-
What do you think if future IAPT services were required to treat cancer patients for psychological issues as part of normal services? If you were able to make any changes beforehand what changes or improvements would you suggest?
7. Other – please feel free to share with us any other comments you have in relation to this topic. Is there anything else that you would like to add that we have not discussed?
Appendix 4 Topic guide: patients’ experience of cognitive–behavioural therapy
Prompts
1. Describe the therapy you had?
Face-to-face/telephone therapy?
How many sessions did you have?
How did it end? Do you know how to get support in the future?
Where was it held?
How long did you wait to start therapy?
Were the appointments flexible?
2. What did you know about cognitive–behavioural therapy before you started the CanTalk study?
Had you or someone you knew had it before?
What was your expectation of the therapy?
Why did you want to try this type of therapy?
3. What aspects of the cognitive–behavioural therapy did you find helpful and why?
Did it meet your needs?
Was there anything you learnt that you think is especially helpful for cancer patients?
Was there anything that was not discussed that you think should have been?
4. What did you think of your therapist?
Were they knowledgeable about your cancer diagnosis and treatment?
5. What impact has having cognitive–behavioural therapy had for your life?
Improvement on physical symptoms?
Coping with treatment/medication?
Social activities/work?
Relationships with family and friends?
Thinking/planning for the future?
6. What do you think about cognitive–behavioural therapy as a treatment for cancer patients with low mood?
Would you recommend it to other cancer patients?
Do you think it is the best way of supporting patients psychologically?
How do you think the therapy could be improved?
How do you think the service could be improved?
List of abbreviations
- AE
- adverse event
- A level
- Advanced level
- BABCP
- British Association for Behavioural and Cognitive Psychotherapies
- BDI
- Beck Depression Inventory
- BDI-II
- Beck Depression Inventory, version 2
- CAITT
- contamination adjusted intention to treat
- CBT
- cognitive–behavioural therapy
- CEAC
- cost-effectiveness acceptability curve
- CEP
- cost-effectiveness plane
- CI
- confidence interval
- CLRN
- Comprehensive Local Research Network
- CONSORT
- Consolidated Standards of Reporting Trials
- CSRI
- Client Services Receipt Inventory
- CTIMP
- Clinical Trial of Investigational Medicinal Product
- CTS
- Cognitive Therapy Scale
- CTS-R
- Cognitive Therapy Scale – Revised
- CTU
- Clinical Trials Unit
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- ECOG-PS
- Eastern Cooperative Oncology Group Performance Status
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GI
- gastrointestinal
- GP
- general practitioner
- HPB
- hepatopancreatobiliary
- HTA
- Health Technology Assessment
- IAPT
- Improving Access to Psychological Therapies
- ICC
- intraclass correlation coefficient
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- IT
- information technology
- ITT
- intention to treat
- LTC
- long-term condition
- MINI
- Mini-International Neuropsychiatric Interview
- NCRN
- National Cancer Research Network
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PABAK
- prevalence-adjusted and bias-adjusted kappa
- PCRN
- Primary Care Research Network
- PHQ-2
- Patient Health Questionnaire-2
- PHQ-9
- Patient Health Questionnaire-9
- POMS
- Profile of Mood States
- PRIMENT
- PRImary care and MENTal health
- PRUH
- Princess Royal University Hospital
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QR
- quartile range
- R&D
- research and development
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- TAU
- treatment as usual
- TCA
- tricyclic antidepressant
- TCC
- Therapy Components Checklist
- UCL
- University College London
- VAS
- visual analogue scale
- WTP
- willingness to pay
