Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/127/10. The contractual start date was in July 2014. The draft report began editorial review in August 2017 and was accepted for publication in October 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jonathan DC Ross reports personal fees from GlaxoSmithKline (GSK) Pharma, Hologic, Inc. and Janssen Pharmaceutica outside the submitted work as well as ownership of shares in GSK Pharma and AstraZeneca Pharmaceuticals. In addition, he is author of the UK and European Guidelines on Pelvic Inflammatory Disease, is a member of the European Sexually Transmitted Infections Guidelines Editorial Board, is a member of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Commissioning Board, was previously a member of the NIHR HTA Primary Care, Community and Preventative Interventions Panel (2013–16) and is a member of the NIHR Journals Library Editorial Group. Alan A Montgomery is a member of the NIHR HTA Clinical Evaluation and Trials Board. Janet Wilson reports non-financial support from Hologic/Gen-Probe and personal fees from Becton, Dickinson and Company (BD) outside the submitted work. John White reports personal fees from Hologic, GSK Pharma and BD UK Pty Ltd outside the submitted work, as well as personal fees from SAGE publishing, and is Editor-in-Chief of the International Journal of STD & AIDS. Trish Hepburn reports ownership of shares in AstraZeneca Pharmaceuticals. During the trial, Lelia Duley was the Director of the Nottingham Clinical Trials Unit, a unit with NIHR clinical trials unit support funding.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Ross et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Gonorrhoea is the second most common bacterial sexually transmitted infection (STI) in the UK, with 36,244 infections reported in 2016. 1 A disproportionate burden of infection is seen in young adults (36% of infections occur in those aged < 25 years) and minority ethnic groups (23% of infections occur in non-white people). The highest rates of infection are in large urban areas and are concentrated in core groups that include men who have sex with men (MSM), black people and minority ethnic groups, and those reporting multiple sexual partners. Over the past few years, there has been a significant rise in rectal gonorrhoea in MSM, thought to reflect an increase in detection using sensitive nucleic acid amplification tests (NAATs)2 and an increase in unsafe sexual behaviour. 1,3
Gonorrhoea leads to local inflammation, causing genital pain and discomfort; the localised immune activation associated with infection also facilitates the acquisition and transmission of human immunodeficiency virus (HIV). 4 In women, infection can spread to the fallopian tubes and ovaries, causing pelvic inflammatory disease, with resultant tubal scarring, infertility and chronic pelvic pain and an increased risk of ectopic pregnancy. In men, gonorrhoea can lead to epididymo-orchitis and, in MSM, gonococcal proctitis can lead to abscess and fistula formation. Pharyngeal infection, although usually asymptomatic, is an important reservoir of onward transmission in both women and MSM. It is also harder to treat with antibiotics and can persist even when antimicrobial susceptibility testing suggests that it should be susceptible. 5 It is, therefore, important to know whether or not treatment is effective for infection at all anatomical sites.
Antibiotic treatment and resistance
Neisseria gonorrhoeae readily develops resistance to antibiotic regimens. Globally, there are now high levels of resistance against penicillins, sulphonamides, tetracyclines and quinolones, all of which are no longer recommended for routine use. 6 A real possibility of multidrug-resistant gonorrhoea and the lack of alternative treatment options has been highlighted. 7 Guidance from the British Association for Sexual Health and HIV (BASHH) recommends treating gonorrhoea with ceftriaxone (given with adjunctive azithromycin) and this currently cures > 95% of patients. 8 Recent surveillance data show a reduction in sensitivity to ceftriaxone with an upwards drift in the minimum inhibitory concentration (MIC); that is, the proportion of cases that remain highly sensitive to ceftriaxone has decreased over time. 3 Sporadic treatment failure of cephalosporins has been reported from a number of countries9–14 and a patient who failed dual therapy with ceftriaxone plus azithromycin has recently been documented in the UK. 15 The same reduction in antibiotic susceptibility was followed by widespread treatment failure within a few years for other antimicrobials (penicillin, tetracyclines and quinolones) used to treat gonorrhoea. An outbreak of azithromycin-resistant gonorrhoea has been reported in England since November 2014, further highlighting the need to identify other effective treatment regimens. 16 Despite this recent outbreak, azithromycin resistance remains uncommon in England and, overall, levels of azithromycin resistance have probably not increased over the past 3 years since the outbreak was first identified, although a change in laboratory procedures in 2015 makes direct comparisons over time difficult. Current national and international treatment guidelines continue to recommend dual antibiotic therapy, including azithromycin, for the treatment of gonorrhoea.
Alternative treatments
The options for treating gonorrhoea are limited if cephalosporins become ineffective. With the exception of gentamicin, alternative agents have not been fully assessed in vivo [such as ertapenem (Invanz;® Merck, Sharpe & Dohme, Kenilworth, NJ, USA), solithromycin, gepotidicin],17–20 are reserved for specific infections (e.g. rifampicin for tuberculosis)21 or have the potential to rapidly develop resistance (e.g. spectinomycin). 22
Two systematic reviews on gentamicin monotherapy23,24 reported cure rates for gentamicin of 62–98% in patients with gonorrhoea, but available studies were generally small and of low quality. No adverse events (AEs) were reported in these studies. A more recent non-comparative prospective trial25 evaluated single-dose gentamicin combined with 2 g of oral azithromycin with a reported cure rate of 100% in mostly genital infections. Limited data are available on the efficacy of gentamicin when treating gonorrhoea in the pharynx or rectum, although antibiotics are sometimes less effective at these sites.
As the susceptibility of N. gonorrhoeae to currently recommended antibiotics decreases and multidrug-resistant strains become more common, it is important to demonstrate the efficacy and safety of alternative treatment regimens in patients with gonorrhoea. Gentamicin is a relatively cheap and widely available antibiotic. Despite an apparent dose-related association with renal and vestibulocochlear toxicity, a single one-off dose appears to be well tolerated. 26 In vitro antimicrobial susceptibility data support the use of gentamicin, but there is a need for clinical trial data to assess its efficacy and safety, particularly in pharyngeal and rectal infections. 27
Research question
Our hypothesis was that gentamicin was not clinically worse than ceftriaxone in the treatment of gonorrhoea. This randomised controlled trial (RCT) tested this hypothesis by comparing the microbiological clearance of N. gonorrhoeae following treatment with either gentamicin or ceftriaxone.
Objectives
Primary objective
The primary objective was to determine whether or not gentamicin is an acceptable alternative to ceftriaxone in the treatment of gonorrhoea. This was addressed by determining whether or not the rate of microbiological clearance of N. gonorrhoeae in participants treated with gentamicin was non-inferior to the rate of clearance in participants treated with ceftriaxone.
Secondary objectives
The secondary objectives were to determine:
-
whether or not a single intramuscular (i.m.) dose of gentamicin is safe and well tolerated
-
whether or not a single i.m. dose of gentamicin is cost-effective for the NHS in comparison to ceftriaxone for the treatment of gonorrhoea
-
the relationship between clinical effectiveness and the laboratory measurement of antibiotic effectiveness (the MIC required to inhibit growth of N. gonorrhoeae).
Chapter 2 Methods
Study design
The Gentamicin in the Treatment Of Gonorrhoea (G-TOG) trial was a blinded, two-arm, multicentre, non-inferiority, randomised trial comparing the clinical effectiveness and safety of gentamicin with that of ceftriaxone in the treatment of gonorrhoea.
Participants were randomised to receive a single i.m. injection of either gentamicin or ceftriaxone. In addition, all participants received 1 g of oral azithromycin as standard treatment. The primary outcome was clearance of N. gonorrhoeae at all infected sites, indicated by a negative NAAT, 2 weeks post treatment. The secondary outcomes included clearance of N. gonorrhoeae at genital, pharyngeal and rectal sites; clinical resolution of symptoms; frequency of AEs; tolerability of therapy; the relationship between clinical effectiveness and antibiotic MIC for N. gonorrhoeae; and cost-effectiveness.
Trial setting and participants
Recruiting centres
The trial was conducted in 14 sexual health clinics in England. Seven sites were originally planned to meet the recruitment target and a further seven sites were added during the trial. Sites were opened between September 2014 and February 2016, with participants being recruited from October 2014 to November 2016.
Identification of participants
Patients with a provisional (microscopy identification of Gram-negative cocci on a Gram stain of genital secretions) or confirmed (indicated by a positive NAAT) diagnosis of gonorrhoea were screened for the trial and approached by a member of the site research team to determine whether or not they were interested in participating. They were provided with a patient information sheet (PIS) and a verbal explanation of the trial and were given the opportunity to ask any questions that they might have. In addition, trial posters were on display in relevant areas of the clinic. These helped to introduce the trial and, if patients were interested, they could ask clinic staff for additional details. All participants gave written informed consent.
To avoid delaying treatment for a transmissible infection with serious sequelae, patients with either a provisional (on microscopy) or confirmed (on NAAT) diagnosis of untreated gonorrhoea were invited to participate and provide written consent at the same clinic visit.
Eligibility criteria
Patients were eligible for inclusion if they were aged 16–70 years and had a positive diagnosis in the previous 4 weeks of uncomplicated, untreated (i.e. they had not received any antibiotic in the previous 28 days that could have treated gonorrhoea, either partially or completely) genital, pharyngeal and/or rectal gonorrhoea. The exclusion criteria were having known concurrent STI(s) (excluding chlamydia); bacterial vaginosis and/or Trichomonas vaginalis infection; having contraindications or an allergy to gentamicin, ceftriaxone, azithromycin or lidocaine; being pregnant or breastfeeding; having complicated gonorrhoeal infection, for example pelvic inflammatory disease or epididymo-orchitis; weighing < 40 kg; and having used ceftriaxone, gentamicin or azithromycin in the preceding 28 days.
Assessment of feasibility
At 9 months after the start of recruitment, accrual was reviewed by the Trial Steering Committee (TSC) to determine whether or not the following feasibility criteria had been met for progression:
-
Recruitment at 80% of target. If this was not achieved, the Trial Management Group (TMG) would implement effective and realistic strategies to increase recruitment and retention for the study to proceed.
-
Percentage of completed follow-ups of > 50%.
If the study did proceed, an additional assessment of recruitment would be carried out.
On 22 June 2015, the trial received approval for progression, having met the feasibility criteria. At 9 months, 88% of target recruitment had been achieved, with follow-up of 74%. Recruitment was assessed regularly at monthly TMG meetings and at further TSC meetings.
Trial procedures
Baseline visit
Demographic information and details of a participant’s sexual history and symptoms were collected during the baseline visit. Symptomatic and asymptomatic individuals were eligible for inclusion in the trial. For symptomatic participants, the baseline visit usually took place on the same day as diagnosis, which was based on the microscopy appearances of Gram-stained genital discharge. Asymptomatic participants were recalled to the clinic for treatment after a positive test result for gonorrhoea had been received; once they had given consent to participate in the trial, this second visit (the first following diagnosis) was considered as the baseline visit.
Each participant had swabs taken for NAAT and culture testing to determine the site(s) of infection. A urine sample could be taken in place of a NAAT urethral sample for men. A full sampling profile was required, taking account of a participant’s sex and sexual orientation, to reflect potential sites of exposure. This allowed the efficacy of treatment to be assessed at each infected site. When swabs or urine specimens were not part of routine clinical care or had not been taken already during the baseline clinic visit (e.g. symptomatic participants who had only swabs taken for routine care on the same day prior to consent), additional swabs were taken to complete the full sampling profile, as defined in Table 1.
| Sex/reported sexual orientation | Sample | ||
|---|---|---|---|
| Genital | Pharyngeal | Rectal | |
| Females | ✓a | ✓c | ✓c |
| Heterosexual men | ✓b | Not required | Not required |
| MSM | ✓b | ✓c | ✓c |
All specimens collected were sent to local site laboratories for the identification of N. gonorrhoeae and results were reported back to the clinics in the usual manner. The results from the baseline visit informed subsequent testing of previously infected sites at the follow-up visit.
A number of licensed NAATs are available to detect N. gonorrhoeae, including the Hologic Aptima Combo 2® (AC2) assay (Hologic, Inc., Marlborough, MA, USA). In centres where the AC2 assay was not used locally for testing, an additional set of swabs (or urine) was taken for the AC2 assay to be performed at Public Health England (PHE). These results were not reported back to the clinic but were reported in batches to the Nottingham Clinical Trials Unit (NCTU). The AC2 assay was, therefore, considered as the reference standard for the trial and the AC2 NAAT result was used as the primary outcome measure. The management of participants was based only on the results of local testing.
Additional blood samples were taken for future measurement of the pre-treatment immune response to gonococcal infection and for measurement of creatinine level.
Follow-up
Participants were asked to return to the clinic 2 weeks post treatment (which was also 2 weeks post randomisation) for a follow-up visit. Participants were reminded of their appointment using the individual clinics’ existing recall procedures, such as Short Message Service (SMS) and telephone reminders. During the follow-up visit, swabs (or urine) from previously infected sites were taken for NAAT and culture testing to assess the clearance of N. gonorrhoeae. A blood sample was taken to measure the post-treatment immune response and creatinine level. Each participant remained in the trial until this follow-up visit was completed. Participants were considered lost to follow-up if they had not returned for their follow-up appointment within 60 days of the baseline visit; this time point was chosen pragmatically to balance flexibility over when participants could return to the clinic against the potential increased risk of reinfection over a more prolonged time period. The recruitment flow diagram is shown in Figure 1.
FIGURE 1.
The G-TOG trial flow diagram. VAS, visual analogue scale. Reproduced from Brittain et al. 28 © The Author(s). 2016 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.

If follow-up test results at 2 weeks post treatment showed that a participant had gonorrhoea, he or she was offered further investigation and treatment in accordance with local clinic guidelines. This treatment was not considered to be part of the trial.
Randomisation
After providing consent, and after confirmation of eligibility, participants were registered in the trial using a secure web-based registration and randomisation system. Participants were randomised to receive either gentamicin or ceftriaxone by a member of the research team. Staff who performed randomisation had no role in administering trial treatments and remained blinded to the treatment allocation, thereby minimising the risk of selection bias through prediction of the allocation sequence.
Randomisation was based on a computer-generated pseudorandom code using permuted blocks of randomly varying size. The code was created by NCTU in accordance with standard operating procedures and held on a secure server. Randomisation was carried out in a 1 : 1 ratio, stratified by recruiting centre.
The web-based system generated a blinded prescription for G-TOG trial treatment, which required signature by the prescribing doctor. Site staff recorded only ‘G-TOG trial drug’ in the participants’ medical notes. The signed prescription was then passed to an injecting nurse who determined the actual treatment that a participant was randomised to and then administered the injection. The injecting nurse was the only member of the research team who was unblinded to treatment allocation.
Interventions
Participants were randomised to receive either 240 mg of gentamicin (intervention) or 500 mg of ceftriaxone (current standard treatment). Both treatments were administered from routine clinic stock as a single i.m. injection. Any European Union-licensed brands of gentamicin and ceftriaxone were permitted to be used.
The 240 mg of gentamicin was made up from three 2-ml (80-mg) vials, in accordance with the Summary of Product Characteristics (SmPC), and administered as a single 6-ml i.m. injection. For the ceftriaxone arm, 500 mg in a powder formulation was dissolved in 1% lidocaine, in accordance with the SmPC, and administered as a single 2-ml i.m. injection. In addition, all participants received a single oral dose of 1 g of azithromycin, which is currently given as standard treatment alongside ceftriaxone.
In previous trials, a 240-mg dose of gentamicin was most commonly used and the use of different doses has not demonstrated a significant dose–response effect across studies. 23,24 In vitro antimicrobial susceptibility testing also suggests that isolates remain sensitive to gentamicin. 27,29 The dose of ceftriaxone was chosen to be consistent with current UK gonorrhoea treatment guidelines. 30
Blinding
Nurses administering trial treatments were required to know each participant’s allocation as they prepared the drug for injection. Details of the nurses administering treatment at each centre were obtained during the trial set-up stage and access to the online randomisation system and treatment allocation was granted depending on the delegated role. All other staff at the recruiting centres remained blinded to treatment allocation.
Preparation and administration of trial treatments was undertaken in a separate area, away from the blinded research team and participants. In addition, nurses administering treatments were given guidance to provide standardised information to participants at the time of injection, which was the same regardless of treatment allocation, to prevent inadvertent unblinding. This two-step approach maintained blinding for members of the research team who were subsequently involved in the assessment of participants.
To ensure that assessment of outcome was not influenced by knowledge of the allocated treatment, nurses administering trial treatments were not permitted any role in the collection of outcome data.
End of the trial
Participants left the trial when they completed their 2-week follow-up visit.
Failure to receive the allocated treatment and withdrawal from follow-up were reported and reasons for withdrawal (if given) were documented. If a participant did not receive his or her allocated treatment but agreed to remain in the trial, outcome data collection continued in accordance with the protocol. 28 Participants were informed at the start of the trial that data collected up to the point of withdrawal would be retained and used in the final analysis.
Outcome measures
Primary outcome
The primary outcome measure was clearance of N. gonorrhoeae at all infected sites, confirmed by a negative NAAT, at 2 weeks post treatment (as recommended by BASHH).
The NAAT is an automated laboratory test and, therefore, not subject to bias through knowledge of treatment allocation. Different licensed NAAT assays for the diagnosis of gonorrhoea are available from different manufacturers [e.g. AC2 NAAT; Becton, Dickinson and Company (BD) NAAT (BD, Franklin Lakes, NJ, USA); Cobas® NAAT (Roche Diagnostics, Basel, Switzerland)]. Sexual health clinics participating in the G-TOG trial used either the AC2 NAAT or the BD NAAT; therefore, in order to ensure consistency and standardisation in diagnostic and follow-up tests, additional samples were taken from participants recruited at centres where the AC2 NAAT method was not used by the local laboratory. Testing of these additional samples by AC2 NAAT was performed by PHE. The results from the AC2 NAAT were used to assess clearance for the primary end point.
Secondary outcomes
The secondary outcomes were:
-
clinical resolution of symptoms
-
frequency of nausea/vomiting, hearing loss, dizziness and rash
-
frequency of any other AEs reported by participants
-
tolerability of injection as assessed by participants on a visual analogue scale (VAS)
-
cost-effectiveness.
The relationship between clearance of N. gonorrhoeae and in vitro measurement of antibiotic MIC to inhibit N. gonorrhoeae growth was also assessed.
Effectiveness, tolerability and safety were assessed at the follow-up visit 2 weeks post treatment.
Clinical resolution of symptoms
Resolution of each individual clinical symptom was defined as absence of the symptom at 2 weeks post treatment in those participants with symptoms present at baseline.
Frequency of nausea/vomiting, hearing loss, dizziness and rash
Information recorded for each symptom comprised whether or not the participant had experienced the symptom, the severity of that symptom, the time to the start of the symptom from injection with trial medication, the duration of that symptom and whether or not it had resolved.
Frequency of any other adverse events
An AE is defined as any untoward medical occurrence in a participant administered a medicinal product that does not necessarily have a causal relationship with the treatment. Participants were asked if they had any AEs in addition to the potential side effects collected. The information collected comprised a verbatim description of the event, the severity of the event and the duration of the event. These AEs were then coded using MedDRA (Medical Dictionary for Regulatory Activities; version 17.1). 31 All AEs were assessed for seriousness. A serious adverse event (SAE) is defined as any untoward medical occurrence that results in death, is life-threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability/incapacity or is a congenital anomaly/birth defect.
Additional safety information
In addition to the collection of data on side effects and AEs, participants had a blood sample taken to measure their creatinine levels at baseline and at 2 weeks post treatment. These levels were used to calculate the estimated glomerular filtration rate (eGFR).
Tolerability of the intramuscular injection
Tolerability of the injection was measured immediately after the injection and then at the 2-week clinic visit. It was measured using a 100-mm VAS that asked ‘How severe was the pain of the injection when at its worst?’. A score of 0 denoted no pain and a score of 100 denoted the worst imaginable pain.
Measurement of antibiotic minimum inhibitory concentrations
Minimum inhibitory concentrations were established at PHE using Etests® (BioMérieux UK Ltd, Basingstoke, UK) for gentamicin (0.016–256 mg/l), ceftriaxone (0.002–32 mg/l) and azithromycin (0.016–256 mg/l) on gonococcal agar with Vitox [gonococcal agar base (BD Difco™; BD, Wokingham, UK) and 1% Vitox (Oxoid Ltd, Basingstoke, UK)]. Before the Etest, the gonococcal isolates were confirmed to be N. gonorrhoeae by Gram stain, oxidase test and matrix-assisted laser desorption/ionisation time of flight (MALDI-TOF; Bruker, Billerica, MA, USA).
Research governance
The trial was conducted in accordance with the recommendations adopted by the 18th World Medical Association General Assembly, Helsinki, 196432 and later revisions; the NHS Research Governance Framework for Health and Social Care (2nd edition);33 and the principles of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice guidelines. 34
The National Research Ethics Service Oxford C – South Central Research Ethics Committee (reference: 14/SC/1030) gave ethics approval for the trial for NHS participants (reference: 155423).
The final protocol, approved on 17 June 2015, was version 2.0. There were a number of administrative and procedural changes made to the protocol during the trial, which are outlined in Appendix 1.
Protocol deviations
A protocol deviation was defined as an unanticipated or unintentional divergence or departure from the expected conduct of the trial, inconsistent with the protocol, consent document or other trial procedures. Protocol deviations were recorded by site staff. Protocol violations were defined as deviations that affected eligibility or outcome measures, as assessed by the TMG.
Trial oversight
A number of oversight groups monitored ongoing progress for the duration of the trial and also contributed to interpretation of findings. Roles and responsibilities were defined including the use of charters for the independent TSC and Data Monitoring Committee (DMC).
Trial Management Group
The TMG, which was responsible for the day-to-day running of the trial, comprised the chief investigator, members of NCTU and other core members of the trial team, for example health economists.
Trial Steering Committee
The TSC, which met approximately every 6 months, included an independent chairperson and five independent members and was responsible for overseeing the conduct of the trial. Independent members were professionals in reproductive and sexual health as well as clinical epidemiology. The TSC also had two independent patient and public involvement (PPI) representatives and the chief investigator and trial manager. The TSC monitored trial progress, specifically, advising on recruitment and follow-up strategies and ensuring adherence to the trial protocol. The trial funder and sponsor were invited to attend meetings as observers.
Data Monitoring Committee
The DMC, which met approximately every 6 months, was an independent group with expertise in statistics, primary care and sexual health. They had access, in confidence, to unblinded data by allocated group. The role of the DMC was to safeguard the interests of trial participants, with particular reference to the safety of the intervention; to monitor the overall progress and conduct of the trial; and to assist and advise the TSC and the investigators. The DMC reported to the TSC and, therefore, met shortly before TSC meetings and reported to the TSC on trial safety and recommendations for continuation or stopping of the trial.
Risk assessment and safety monitoring
A risk assessment was conducted as part of protocol development; there was regular monitoring throughout the trial for new risks. The main risks to the trial were loss to follow-up in a young, sometimes transient, population and poor data collection owing to the very short time frame for trial participation of just 2 weeks. However, recruitment sites were well supported by NCTU in obtaining high-quality data and regular central monitoring checks were performed to identify any issues with data collection that could be followed up at individual site level. Data on AEs and SAEs were collected during the trial. As agreed by the sponsor, the Research Ethics Committee, the DMC and the TSC, the DMC was provided with a list of all AEs and SAEs, including any deaths, for review at each meeting.
Patient and public involvement
Twenty-four patients from three sexual health clinics (Birmingham, London and Manchester) commented on the trial design in June and July 2012. A further 25 patients in Birmingham reviewed a PIS for the trial in April 2013. Input was requested on the trial concept and design, whether or not patients with gonorrhoea would be likely to consent to take part in the proposed trial design and the clarity of the patient information provided. Patients’ main concerns following the initial consultation exercise were around the amount of time that participation would involve and whether or not the therapies being offered were safe. In response, the trial procedures were reviewed to optimise patient flow and the draft PIS was revised to expand the information on safety and AEs. Based on the revised patient information, 24 out of 25 patients in the second consultation exercise would have been happy to consider participation in the trial.
Two members of the public joined the TSC and provided input into the design and management of the trial. Specifically, they were invited to comment on all aspects of the trial design and conduct and to contribute to the design and review of documentation given to trial participants to ensure understanding and acceptability. This greatly benefited the research by helping to ensure that our material was acceptable and comprehensible, thus increasing our response rate and reducing the number of missing data. The public members of the TSC commented on the best way of sharing trial findings with the public and contributed as part of the research team to the interpretation of the trial findings. At each stage of the trial, the G-TOG trial team aimed to provide the PPI representatives with clear information in lay terms to allow them to participate in discussing the research, but not to bias their perspective towards that of the researchers/clinicians.
A lay summary of the trial findings, informed by our public members, will also be disseminated to participants who consented to receive the results.
Payments to participants
Participants were not paid to participate in the trial; however, at the end of their follow-up visit they were provided with a £15 voucher to compensate for the additional time associated with taking part in the trial.
Statistical methods
Sample size
Based on a clearance rate of 96% for the ceftriaxone regimen, consistent with previous trials, it was estimated that a total sample size of 646 participants for analysis (323 in each group) would achieve 90% power at the 2.5% one-sided significance level to detect non-inferiority with a lower 95% confidence interval (CI) for the absolute risk difference of 5%. It was planned to randomise a total of 720 participants to allow for a loss to follow-up rate of ≤ 10%.
Analysis plan
A statistical analysis plan (SAP) was finalised before database lock and release of treatment codes to the statistician. All summaries and statistical analyses were conducted using Stata® version 13.1 (StataCorp LP, College Station, TX, USA).
Analysis data sets
Intention-to-treat data set
This data set comprised participants as randomised, regardless of adherence to their allocated group and without imputation for missing data [intention-to-treat (ITT) principle].
Safety data set
The safety data set comprised participants as per the treatment that they actually received.
No specific per-protocol analysis data set was required as several sensitivity analyses were planned to investigate the robustness of the primary outcome.
Data derivations
Clearance of Neisseria gonorrhoeae
Clearance of N. gonorrhoeae at all sites was derived from a post-treatment negative AC2 NAAT at the sites that were positive pre treatment. All sites that were positive pre treatment for an individual participant had to be negative post treatment for gonorrhoea to be considered cleared.
Sensitivity analyses were performed when the AC2 NAAT data were not available and when other data were missing.
Resolution of clinical symptoms
Resolution of each individual clinical symptom was defined as absence of the symptom at 2 weeks post treatment in those participants who had symptoms present at baseline. Each symptom was summarised individually.
Changes in creatinine and estimated glomerular filtration rate
The absolute change in creatinine level from baseline to 2 weeks post treatment for each participant was calculated. In addition, the following variables were derived:
-
Whether or not each participant had a clinically important change between baseline and 2 weeks post treatment. A clinically important change was defined by the study team as an increase or decrease of > 30% from baseline.
-
Whether or not the creatinine level at 2 weeks post treatment exceeded the upper normal limit value. These upper limits were determined by the local laboratory that analysed the samples.
-
eGFR was calculated using the following formula (Chronic Kidney Disease Epidemiology Collaboration). 35 The change in eGFR was then calculated by subtracting the eGFR at 2 weeks post treatment from the eGFR at baseline, where min is minimum, max is maximum, Scr is serum creatinine (mg/dl), κ = 0.7 (female) or 0.9 (male) and α = –0.329 (female) or –0.411 (male):
Minimum inhibitory concentration data
Data were summarised on a per-participant basis. For overall summaries, when a participant had more than one value for MIC (e.g. when samples had been taken from more than one site), the largest value was used. When data were summarised by clearance, the data derived for the primary end point (i.e. cleared of infection at all sites as determined by the AC2 NAAT) were used.
Missing data
The primary analysis was performed on the ITT data set without imputation of missing data for clearance of N. gonorrhoeae at 2 weeks. When the baseline tests did not show any sites positive for N. gonorrhoeae or the baseline test results were missing, the results of positive pre-trial tests were used.
Sensitivity analyses of the primary outcome were performed on the ITT data set to check the robustness of the conclusions to missing outcome data. The pattern of missing data was explored overall and in each of the two treatment groups. When clearance at 2 weeks post treatment using the AC2 NAAT was missing, but there were data for the BD NAAT, the result of the BD NAAT was used for a sensitivity analysis.
Three imputation methods were applied when data for the clearance of N. gonorrhoeae (both the AC2 and the BD NAAT) at 2 weeks post treatment were missing:
-
multiple imputation using chained equations
-
assume that all missing data show clearance of N. gonorrhoeae
-
assume that all missing data show non-clearance of N. gonorrhoeae.
Table 2 outlines possible scenarios when a value for the primary outcome could be derived. Scenarios not included in this table resulted in missing primary outcome data.
| Visit | Primary outcome available? | |||
|---|---|---|---|---|
| Baseline | Follow-up | |||
| Tests | Results | Tests | Results | |
| All required samples taken | ≥ 1 positive | All positives retested | + or – | Yes |
| No positivea | All pre-trial positives retested | + or – | Yes | |
| Not all required samples taken | ≥ 1 positive | All positives (from pre trial and baseline) retested | + or – | Yes |
| No positive | All pre-trial positives retested | + or – | Yes | |
| Any positive follow-up AC2 NAAT | Yes | |||
Analysis of the primary outcome
The analysis of the primary outcome was modified from that specified in the protocol. This amendment was made before the database was locked and the treatment codes revealed.
The initial planned method of analysis was to use a general linear model for binary outcome adjusted by clinic site, with the primary efficacy parameter comparing gentamicin with ceftriaxone being the risk difference in the proportion of participants clear of infection at 2 weeks’ follow-up, along with the 95% CI. However, during the trial, additional centres were introduced, some of which could recruit only small numbers of participants. This meant that there was a chance that some centres would have no participants who had ‘failed’ treatment, making the inclusion of centre as a fixed effect inappropriate. Therefore, the primary approach to the between-group comparative analyses was modified to use generalised estimating equations (GEEs) for binary outcomes adjusted by recruiting centre as a random effect with robust standard errors. The GEE model used an identity link function to enable estimation of adjusted risk difference. The primary efficacy parameter comparing gentamicin with ceftriaxone was the risk difference in the proportion of participants clear of infection at all sites, determined by the AC2 NAAT at the 2-week follow-up, along with the 95% CI. Gentamicin was to be regarded as non-inferior if the lower 95% confidence limit for the risk difference (gentamicin group vs. ceftriaxone group) in confirmed clearance was ≥ –5 percentage points (i.e. closer to zero).
Sensitivity analyses for the primary outcome
In addition to the sensitivity analyses outlined in Missing data, we also investigated the treatment efficacy by performing the following sensitivity analyses:
-
exclude participants who did not have any positive samples at baseline
-
exclude participants who did not receive the allocated treatment
-
exclude participants who did not have full baseline samples taken, that is, females and MSM should have had genital, rectal and pharyngeal samples taken and heterosexual men should have had genital samples taken.
It was planned that there would be an additional analysis further adjusting for baseline variables with a marked imbalance between treatment groups identified after the treatment codes were revealed. However, there were no marked imbalances considered likely to influence the results of the trial; therefore, this additional analysis was not appropriate.
Secondary outcomes
Clearance of Neisseria gonorrhoeae by infection site
Participants may have had infection at multiple sites on entry to the trial, with up to seven different combinations of one, two and three sites possible. For each of the three infection sites, we separately estimated clearance by treatment arm along with 95% CIs, rather than formally fitting an interaction term for different combinations of infection site in the regression model. Any suggestion of a differential effect according to infected site would require confirmation in future research.
Clinical resolution of symptoms
The evaluation of clinical resolution was performed using GEEs for binary outcomes adjusted by recruiting centre as a random effect. The efficacy parameter comparing gentamicin with ceftriaxone was the risk difference in the proportion of participants clear of clinical symptoms at the 2-week follow up, along with the 95% CI. These symptoms were genital discharge, dysuria, sore throat, anorectal pain, rectal bleeding, rectal discharge, tenesmus, constipation, intermenstrual bleeding and post-coital bleeding. The assessment of all symptoms at baseline was recorded for all participants, irrespective of their site(s) of infection.
Creatinine level at 2 weeks
The creatinine-related binary outcomes [number of participants having a clinically important change and number of participants exceeding the upper limit of normal (using the local laboratory ranges) at 2 weeks post treatment] and change in eGFR were summarised using basic descriptive statistics. Shift plots were presented to identify extreme values.
Minimal inhibitory concentration for trial medications
The MIC distribution data were plotted and summarised overall and, separately, by infection site for each antimicrobial. It was expected that some data values would be below or above quantifiable limits; therefore, plots of the MIC value distribution for each medication (gentamicin, ceftriaxone and azithromycin) were produced. For overall summaries, when a participant had more than one MIC (e.g. when they had samples taken from more than one site), the largest of these MICs was used.
Concomitant medications
Additional antibiotics and other concomitant medications taken during the trial were listed by treatment group.
Side effects/adverse events
Descriptive summaries of side effects and AEs by treatment group were provided:
-
Number and percentage of participants who reported each of the following – nausea, vomiting, hearing loss, dizziness and rash. The total numbers of times that these side effects were reported are also summarised.
-
Severity and time in hours or days from injection to onset of each of the following – nausea, vomiting, hearing loss, dizziness and rash.
-
Visual analogue scale pain score immediately following injection and recollection of injection pain at the 2-week follow-up visit.
-
All non-serious AEs and all SAEs were coded using MedDRA. 31 The number and proportion of participants who experienced any AE or SAE were summarised.
Chapter 3 Results
Recruitment
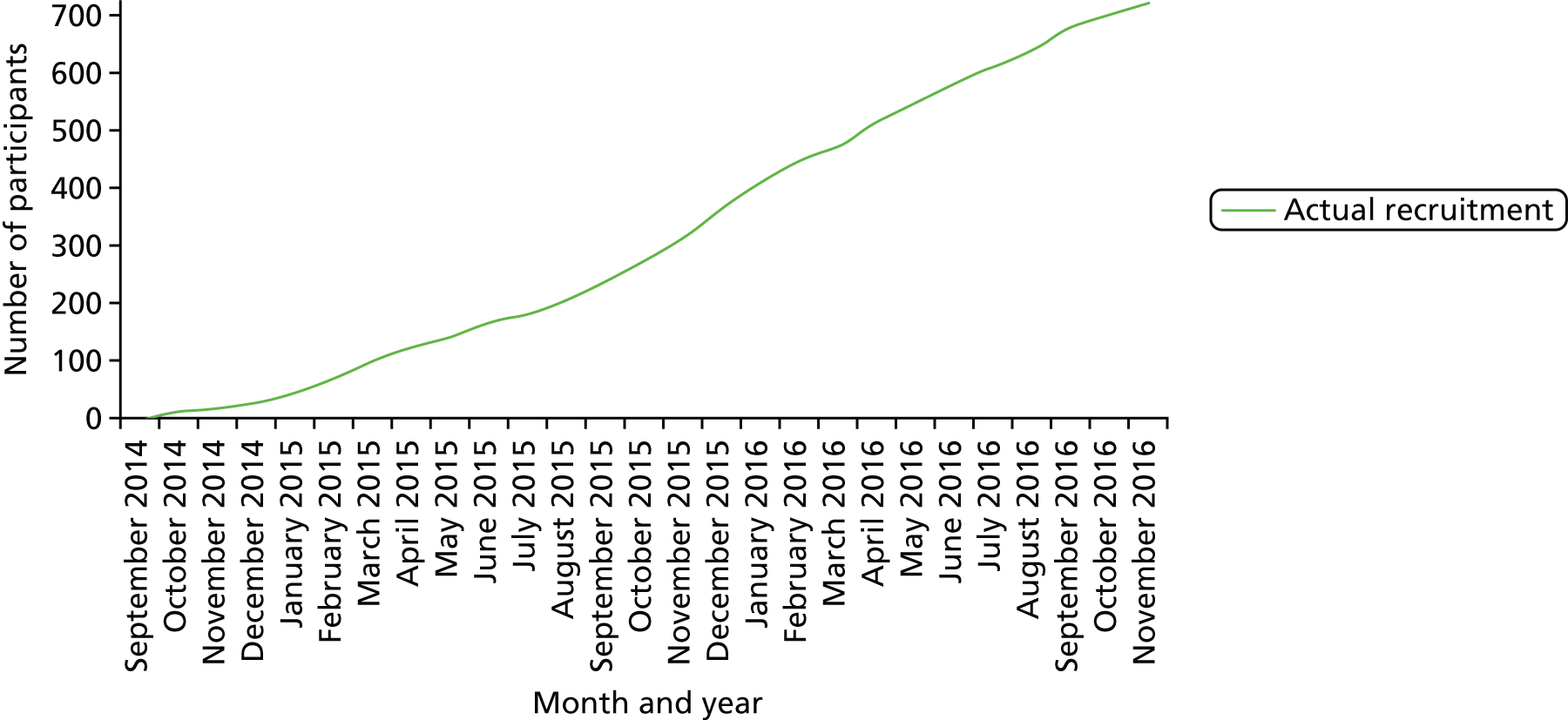
Recruitment commenced in October 2014 and continued until November 2016 when the recruitment target was met (Figure 2).
FIGURE 2.
Monthly actual recruitment.
Participant flow is shown in Figure 3.
FIGURE 3.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
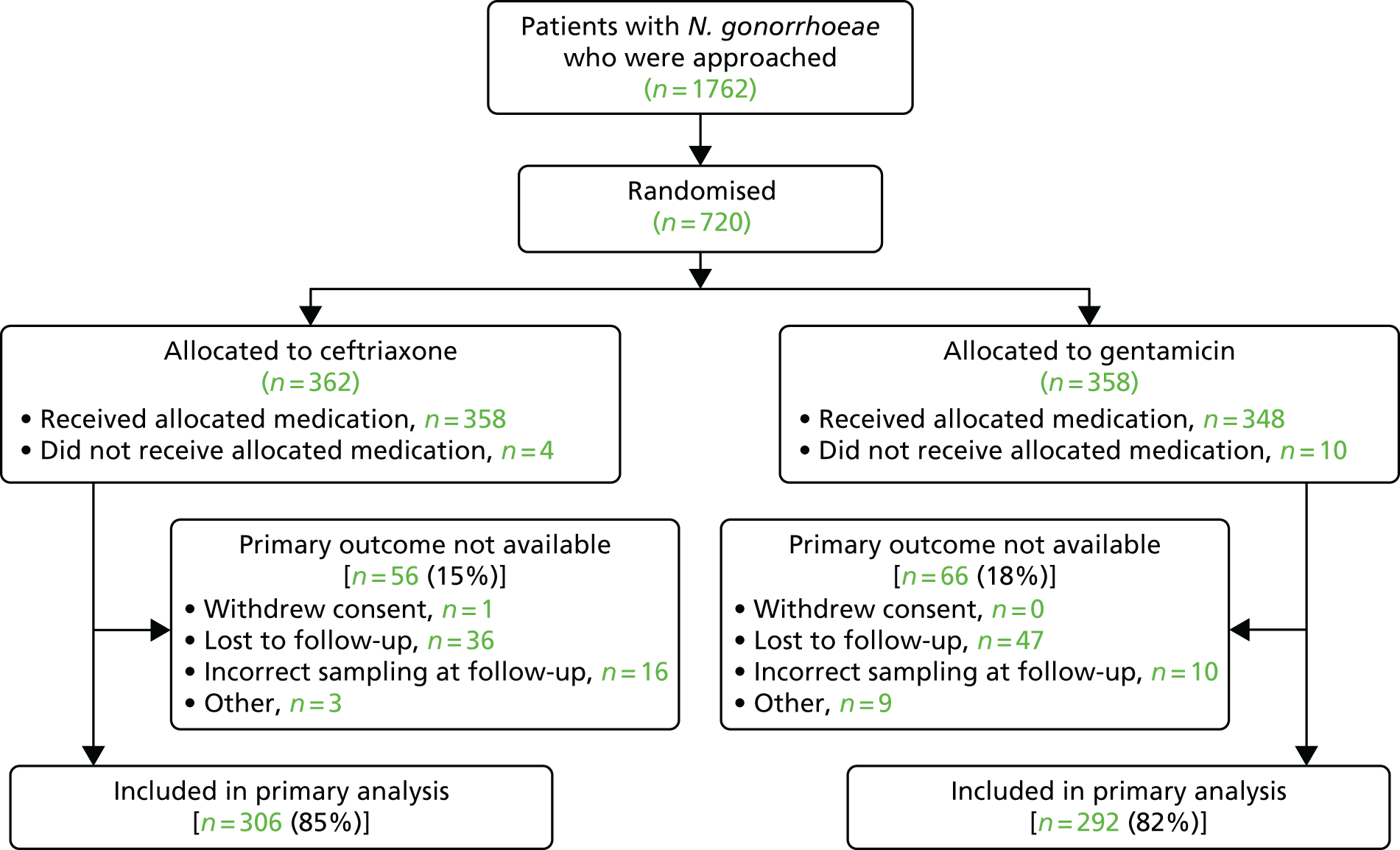
Between 7 October 2014 and 14 November 2016, 1762 patients were approached, of whom, 1042 (59%) were not randomised: 331 were not eligible, 174 declined to participate, 169 felt that participation in the trial would take too much time and 368 were not randomised because of ‘other’ reasons. The main ‘other’ reasons included having a needle phobia, having taken antibiotics in the preceding 4 weeks, not being able to attend follow-up and appropriate staff not being available.
In total, 720 patients were randomised from 14 sexual health clinics in England: 362 (50%) were randomised to receive ceftriaxone and 358 (50%) were randomised to receive gentamicin (Table 3). All participants were randomised on the day of their clinic visit.
| Site | Treatment group (n) | Total (N) | |
|---|---|---|---|
| Ceftriaxone | Gentamicin | ||
| Whittall Street Clinic, Birmingham | 87 | 86 | 173 |
| Barts Sexual Health Centre | 35 | 34 | 69 |
| Burrell Street Clinic, Guy’s and St Thomas Hospital | 45 | 47 | 92 |
| Leeds Sexual Health | 50 | 50 | 100 |
| Manchester Centre for Sexual Health | 14 | 16 | 30 |
| Sheffield | 46 | 44 | 90 |
| Southampton Department of Sexual Health | 14 | 13 | 27 |
| Chelsea and Westminster | 19 | 19 | 38 |
| Brighton | 8 | 8 | 16 |
| Coventry | 18 | 17 | 35 |
| Royal Free | 3 | 2 | 5 |
| Royal Berkshire | 7 | 6 | 13 |
| St Mary’s | 12 | 13 | 25 |
| John Hunter Clinic | 4 | 3 | 7 |
| All sites | 362 | 358 | 720 |
Baseline characteristics
The baseline characteristics of the 720 participants are provided in Table 4. The population comprised 585 (81%) men, 134 (19%) women and one participant classified as ‘other’. The mean age was 30 years with a range of 16–70 years. Sixty-nine per cent of participants were white and 13% were HIV positive.
| Characteristic | Treatment group | Total (N = 720) | |
|---|---|---|---|
| Ceftriaxone (N = 362) | Gentamicin (N = 358) | ||
| Age at randomisation (years) | |||
| Mean (SD) | 30.2 (10.1) | 30.4 (9.9) | 30.3 (10) |
| Median (25th percentile, 75th percentile) | 27.5 (22.6, 34.9) | 28.2 (22.9, 35.1) | 27.9 (22.7, 35.0) |
| Minimum, maximum | 16.1, 70.2 | 16.5, 68.4 | 16.1, 70.2 |
| Sex, n (%) | |||
| Male | 293 (81) | 292 (82) | 585 (81) |
| Female | 69 (19) | 65 (18) | 134 (19) |
| Other | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Ethnicity, n (%) | |||
| White | 241 (67) | 255 (71) | 496 (69) |
| Black | 53 (15) | 48 (13) | 101 (14) |
| Asian | 26 (7) | 18 (5) | 44 (6) |
| Mixed | 27 (7) | 26 (7) | 53 (7) |
| Other | 15 (4) | 11 (3) | 26 (4) |
| Country of birth, n (%) | |||
| UK | 258 (71) | 253 (71) | 511 (71) |
| Other | 104 (29) | 105 (29) | 209 (29) |
| If other, region | |||
| Europe (non-UK) | 51 (14) | 56 (16) | 107 (15) |
| North America | 8 (2) | 5 (1) | 13 (2) |
| Asia Pacific | 18 (5) | 14 (4) | 32 (4) |
| Latin America | 7 (2) | 11 (3) | 18 (3) |
| Middle East | 2 (1) | 5 (1) | 7 (1) |
| Africa | 18 (5) | 14 (4) | 32 (4) |
| Creatinine level (µmol/l) | |||
| Mean (SD) | 78.6 (15.4) | 78.3 (15.8) | 78.5 (15.6) |
| Median (25th percentile, 75th percentile) | 78 (69, 88) | 77 (67.5, 86.0) | 77 (68, 87) |
| Minimum, maximum | 42, 137 | 26, 154 | 26, 154 |
| N | 343 | 332 | 675 |
| Medical history, n (%) | |||
| Diabetes mellitus | 3 (1) | 1 (< 0.5) | 4 (1) |
| Otitis media | 9 (2) | 7 (2) | 16 (2) |
| Renal disease | 3 (1) | 4 (1) | 7 (1) |
| Liver disease | 8 (2) | 5 (1) | 13 (2) |
| Immunodeficiency | 34 (9) | 24 (7) | 58 (8) |
| Any known drug allergies | 17 (5) | 25 (7) | 42 (6) |
| HIV infection status,a n (%) | |||
| Negative | 299 (83) | 307 (86) | 606 (84) |
| Positive | 53 (15) | 43 (12) | 96 (13) |
| Unknown | 10 (3) | 8 (2) | 18 (3) |
Most (n = 633, 88%) participants had a positive diagnosis of N. gonorrhoeae at their baseline attendance by either NAAT or Gram stain (Table 5). There was a slight imbalance between treatment groups in terms of a positive diagnosis using Gram stain (38% in the ceftriaxone group vs. 46% in the gentamicin group), but there was balance between groups in those diagnosed using NAAT and overall. Slightly more participants had infection at the genital site in the gentamicin group than in the ceftriaxone group (61% vs. 52%). Similar percentages of participants in each group had pharyngeal and rectal infections. Fifty-one per cent of participants were infected at only one site, 26% at two sites and 10% at three sites. Overall, baseline characteristics between the two treatment groups were well balanced.
| Baseline diagnosis and infection | Treatment group, n (%) | Total (N = 720), n (%) | |
|---|---|---|---|
| Ceftriaxone (N = 362) | Gentamicin (N = 358) | ||
| Participants with a positive diagnosis at baseline attendancea | 317 (87) | 316 (88) | 633 (88) |
| Participants with infection at each sitea | |||
| Genital | 190 (52) | 219 (61) | 409 (57) |
| Pharyngeal | 128 (35) | 128 (36) | 256 (36) |
| Rectal | 159 (44) | 147 (41) | 306 (43) |
| Number of sites infecteda | |||
| 1 | 189 (52) | 180 (50) | 369 (51) |
| 2 | 96 (27) | 94 (26) | 190 (26) |
| 3 | 32 (9) | 42 (12) | 74 (10) |
| Positive diagnosis of N. gonorrhoeae using Gram stainb | 139 (38) | 166 (46) | 305 (42) |
| Positive diagnosis of N. gonorrhoeae using AC2 NAATc | 308 (85) | 309 (86) | 617 (86) |
The treatment groups appeared to be balanced with respect to participants’ history of STIs [41% of participants had had at least one previous diagnosis of gonorrhoea, 34% of participants had previously had chlamydia, 14% had had syphilis and 3% had had pelvic inflammatory disease (women only)] (Table 6). The number and type of sexual partners were similar in the two treatment groups, as were other details of their sexual history (Tables 7 and 8).
| STI history | Treatment group | Total (N = 720) | |
|---|---|---|---|
| Ceftriaxone (N = 362) | Gentamicin (N = 358) | ||
| Previously had a positive diagnosis for gonorrhoea, n (%) | |||
| No | 205 (57) | 214 (60) | 419 (58) |
| Yes | 152 (42) | 142 (40) | 294 (41) |
| Not known | 5 (1) | 2 (1) | 7 (1) |
| Number of previous episodes experienced | |||
| Median (25th percentile, 75th percentile) | 1 (1, 2) | 1 (1, 3) | 1 (1, 2) |
| Minimum, maximum | 1, 25 | 1, 6 | 1, 25 |
| n | 152 | 142 | 294 |
| Previously had a positive diagnosis for chlamydia, n (%) | |||
| No | 235 (65) | 228 (64) | 463 (64) |
| Yes | 121 (33) | 127 (35) | 248 (34) |
| Not known | 6 (2) | 3 (1) | 9 (1) |
| Number of episodes experienced | |||
| Median (25th percentile, 75th percentile) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) |
| Minimum, maximum | 1, 20 | 1, 7 | 1, 20 |
| n | 121 | 127 | 248 |
| Previously had a positive diagnosis for syphilis, n (%) | |||
| No | 311 (86) | 302 (84) | 613 (85) |
| Yes | 48 (13) | 53 (15) | 101 (14) |
| Not known | 3 (1) | 3 (1) | 6 (1) |
| Number of episodes experienced | |||
| Median (25th percentile, 75th percentile) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) |
| Minimum, maximum | 1, 2 | 1, 4 | 1, 4 |
| n | 48 | 53 | 101 |
| Previously had a positive diagnosis for pelvic inflammatory disease (female only), n (%) | |||
| No | 67 (97) | 64 (97) | 131 (97) |
| Yes | 2 (3) | 2 (3) | 4 (3) |
| Unknown | 0 | 0 | 0 |
| Number of episodes experienced | |||
| Median (25th percentile, 75th percentile) | 1.5 (1, 12) | 2 (1, 3) | 1.5 (1, 2.5) |
| Minimum, maximum | 1, 2 | 1, 3 | 1, 3 |
| n | 2 | 2 | 4 |
| Previous HIV test, n (%) | |||
| No | 69 (19) | 66 (18) | 135 (19) |
| Yes | 289 (80) | 286 (80) | 575 (80) |
| Not known | 4 (1) | 6 (2) | 10 (1) |
| If yes | |||
| Positive | 52 (14) | 42 (12) | 94 (13) |
| Negative | 237 (65) | 244 (68) | 481 (67) |
| Baseline sexual history | Treatment group | Total (N = 585) | |
|---|---|---|---|
| Ceftriaxone (N = 293) | Gentamicin (N = 292) | ||
| Number of partners in the previous 3 months | |||
| Median (25th percentile, 75th percentile) | 3 (2, 6) | 3 (2, 5) | 3 (2, 5) |
| Minimum, maximum | 0, 50 | 0, 99 | 0, 99 |
| Number of partners in the previous 12 months | |||
| Median (25th percentile, 75th percentile) | 7 (3, 20) | 6 (3, 18) | 6 (3, 20) |
| Minimum, maximum | 1, 192 | 1, 500 | 1, 500 |
| Previous history of same-sex partner (ever), n (%) | |||
| No | 77 (26) | 75 (26) | 152 (26) |
| Yes | 216 (74) | 216 (74) | 432 (74) |
| Not known | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Previous history of receptive anal sexual intercourse (ever), n (%) | |||
| No | 93 (32) | 98 (34) | 191 (33) |
| Yes | 200 (68) | 193 (66) | 393 (67) |
| Not known | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Previous history of receptive oral sexual intercourse (ever), n (%) | |||
| No | 55 (19) | 47 (16) | 102 (17) |
| Yes | 238 (81) | 243 (83) | 481 (82) |
| Not known | 0 | 2 (1) | 2 (< 0.5) |
| Previous history of partner born outside the UK (ever), n (%) | |||
| No | 105 (36) | 89 (30) | 194 (33) |
| Yes | 181 (62) | 196 (67) | 377 (64) |
| Not known | 7 (2) | 7 (2) | 14 (2) |
| In the previous 3 months, for approximately what proportion of sexual contacts were condoms used? | |||
| Median (25th percentile, 75th percentile) | 50 (0, 93) | 50 (5, 95) | 50 (0, 95) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| Latest partner | |||
| Sex, n (%) | |||
| Male | 212 (72) | 211 (72) | 423 (72) |
| Female | 81 (28) | 80 (27) | 161 (28) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Time (days) since last sexual intercourse | |||
| Median (25th percentile, 75th percentile) | 10 (6, 21) | 10 (5, 18) | 10 (6, 21) |
| Minimum, maximum | 1, 196 | 1, 210 | 1, 210 |
| Duration of last relationship, n (%) | |||
| One-off | 128 (44) | 131 (45) | 286 (40) |
| Occasional | 56 (19) | 60 (21) | 134 (19) |
| Regular | 95 (32) | 89 (30) | 251 (35) |
| Previous regular | 14 (5) | 9 (3) | 45 (6) |
| Not known | 0 | 3 (1) | 3 (1) |
| Type of sexual contacta, n (%) | |||
| Genital–genital | 152 (52) | 144 (49) | 296 (51) |
| Anal–genital | 119 (41) | 122 (42) | 241 (41) |
| Genital–anal | 143 (49) | 149 (51) | 292 (50) |
| Oral–genital | 214 (73) | 211 (72) | 425 (73) |
| Genital–oral | 228 (78) | 239 (82) | 467 (80) |
| Oral–anal | 85 (29) | 87 (30) | 172 (29) |
| Anal–oral | 87 (30) | 74 (25) | 161 (28) |
| Digital–anal | 81 (28) | 86 (29) | 167 (29) |
| Anal–digital | 72 (25) | 68 (23) | 140 (24) |
| Use of condoms, n (%) | |||
| No | 159 (54) | 142 (49) | 301 (51) |
| Yes, partially | 49 (17) | 52 (18) | 101 (17) |
| Yes, consistently, including for oral sex | 7 (2) | 8 (3) | 15 (3) |
| Yes, consistently, but not for oral sex | 78 (27) | 89 (30) | 167 (29) |
| Missing | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Partner known to have gonorrhoea, n (%) | |||
| No | 257 (88) | 250 (86) | 507 (87) |
| Yes | 36 (12) | 41 (14) | 77 (13) |
| Not known | 0 | 1 (< 0.5) | 1 (< 0.5) |
| Baseline sexual history | Treatment group | Total (N = 134) | |
|---|---|---|---|
| Ceftriaxone (N = 69) | Gentamicin (N = 65) | ||
| Number of partners in the previous 3 months | |||
| Median (25th percentile, 75th percentile) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) |
| Minimum, maximum | 0, 120 | 0, 100 | 0, 120 |
| Number of partners in the previous 12 months | |||
| Median (25th percentile, 75th percentile) | 3 (1, 4) | 2 (1, 4) | 2 (1, 4) |
| Minimum, maximum | 1, 120 | 1, 300 | 1, 300 |
| Previous history of same-sex partner (ever), n (%) | |||
| No | 65 (94) | 62 (95) | 127 (95) |
| Yes | 4 (6) | 2 (3) | 6 (4) |
| Not known | 0 | 1 (2) | 1 (1) |
| Previous history of receptive anal sexual intercourse (ever), n (%) | |||
| No | 48 (70) | 45 (69) | 93 (69) |
| Yes | 21 (30) | 19 (29) | 40 (30) |
| Not known | 0 | 1 (2) | 1 (1) |
| Previous history of receptive oral sexual intercourse (ever), n (%) | |||
| No | 15 (22) | 17 (26) | 32 (24) |
| Yes | 54 (78) | 47 (72) | 101 (75) |
| Not known | 0 | 1 (2) | 1 (1) |
| Previous history of partner born outside the UK (ever), n (%) | |||
| No | 42 (61) | 46 (71) | 88 (66) |
| Yes | 24 (35) | 17 (26) | 41 (31) |
| Not known | 3 (4) | 2 (3) | 5 (4) |
| In the previous 3 months, for approximately what proportion of sexual contacts were condoms used? | |||
| Median (25th percentile, 75th percentile) | 0 (0, 50) | 0 (0, 50) | 0 (0, 50) |
| Minimum, maximum | 0, 100 | 0, 100 | 0, 100 |
| Latest partner | |||
| Sex of latest sexual partner, n (%) | |||
| Male | 69 (100) | 63 (97) | 132 (98) |
| Female | 0 | 1 (2) | 1 (1) |
| Missing | 0 | 1 (2) | 1 (1) |
| Time (days) since last sexual intercourse | |||
| Median (25th percentile, 75th percentile) | 14 (7, 21) | 14 (7, 28) | 14 (7, 28) |
| Minimum, maximum | 1, 112 | 1, 112 | 1, 112 |
| Duration of last relationship, n (%) | |||
| One-off | 15 (22) | 12 (18) | 27 (20) |
| Occasional | 8 (12) | 10 (15) | 18 (13) |
| Regular | 33 (48) | 33 (51) | 66 (49) |
| Previous regular | 13 (19) | 9 (14) | 22 (16) |
| Not known | 0 | 1 (2) | 1 (1) |
| Type of sexual contact,a n (%) | |||
| Genital–genital | 67 (97) | 63 (97) | 130 (97) |
| Anal–genital | 7 (10) | 5 (8) | 12 (9) |
| Genital–anal | 0 | 2 (3) | 2 (1) |
| Oral–genital | 48 (70) | 37 (57) | 85 (63) |
| Genital–oral | 43 (62) | 36 (55) | 79 (59) |
| Oral–anal | 3 (4) | 1 (2) | 4 (3) |
| Anal–oral | 4 (6) | 2 (3) | 6 (4) |
| Digital–anal | 4 (6) | 3 (5) | 7 (5) |
| Anal–digital | 3 (4) | 6 (9) | 9 (7) |
| Use of condoms, n (%) | |||
| No | 49 (71) | 46 (71) | 95 (71) |
| Yes, partially | 15 (22) | 10 (15) | 25 (19) |
| Yes, consistently, including for oral sex | 0 | 2 (3) | 2 (1) |
| Yes, consistently, but not for oral sex | 0 | 6 (9) | 11 (8) |
| Missing | 5 (7) | 1 (1) | 1 (1) |
| Partner known to have gonorrhoea, n (%) | |||
| No | 56 (81) | 55 (85) | 111 (83) |
| Yes | 13 (19) | 9 (14) | 22 (16) |
| Not known | 0 | 1 (2) | 1 (1) |
The results of the clinical examination and symptom assessment at baseline were similar between the treatment groups. Slightly fewer participants in the ceftriaxone group than in the gentamicin group had evidence of genital discharge on clinical examination (45% vs. 54%; Table 9). Similarly, at the baseline symptom assessment, 42% of participants in the ceftriaxone group and 50% in the gentamicin group reported the presence of genital discharge (Table 10). Few participants had hearing impairment at baseline [8 out of 362 (2%) in the ceftriaxone group and 11 out of 358 (3%) in the gentamicin group]. All impairments were mild with the exception of two participants in the ceftriaxone group who reported severe hearing impairment and one participant in the gentamicin group who reported moderate hearing impairment.
| Baseline examination | Treatment group | Total (N = 720) | |
|---|---|---|---|
| Ceftriaxone (N = 362) | Gentamicin (N = 358) | ||
| Height (cm) | |||
| Mean (SD) | 176.1 (9.3) | 176.4 (9.2) | 176.3 (9.3) |
| Median (25th percentile, 75th percentile) | 177 (170, 183) | 178 (171, 183) | 177 (170, 183) |
| Minimum, maximum | 147, 198 | 106, 197 | 106, 198 |
| Weight (kg) | |||
| Mean (SD) | 77 (17.7) | 76.2 (13.7) | 76.6 (15.8) |
| Median (25th percentile, 75th percentile) | 75 (66, 84.1) | 75 (67, 83) | 75 (66.6, 83) |
| Minimum, maximum | 41, 193 | 49.2, 135 | 41, 193 |
| BMI (kg/m2) | |||
| Mean (SD) | 24.8 (5.2) | 24.5 (4.5) | 24.7 (4.9) |
| Median (25th percentile, 75th percentile) | 23.7 (21.9, 26.9) | 23.7 (21.5, 26.5) | 23.7 (21.6, 26.7) |
| Minimum, maximum | 16.7, 59.5 | 16.6, 46.4 | 16.6, 59.5 |
| Women | |||
| Cervicitis, n (%) | |||
| No | 58 (84) | 56 (86) | 114 (85) |
| Yes | 8 (12) | 5 (8) | 13 (10) |
| Not known | 3 (4) | 4 (7) | 7 (5) |
| Men and women | |||
| Evidence of genital discharge, n (%) | |||
| No | 195 (54) | 164 (46) | 359 (50) |
| Yes | 164 (45) | 192 (54) | 356 (49) |
| Not known | 3 (1) | 2 (1) | 5 (1) |
| If yes, colour | |||
| Clear | 19 (12) | 21 (11) | 40 (11) |
| Mucopurulent | 62 (38) | 79 (41) | 141 (40) |
| Purulent | 83 (51) | 92 (48) | 175 (49) |
| If yes, amount | |||
| Scanty | 34 (21) | 37 (19) | 71 (20) |
| Average | 68 (41) | 87 (45) | 155 (44) |
| Profuse | 59 (36) | 68 (35) | 127 (36) |
| Missing | 3 (1) | 0 | 3 (1) |
| Other abnormality, n (%) | 28 (8) | 22 (6) | 50 (7) |
| Baseline symptoms | Treatment group | Total (N = 720) | |
|---|---|---|---|
| Ceftriaxone (N = 362) | Gentamicin (N = 358) | ||
| Presence of any symptom at baseline, n (%) | 230 (64) | 241 (67) | 471 (65) |
| Presence of symptom at baseline, n (%) | |||
| Genital discharge | 153 (42) | 179 (50) | 332 (46) |
| Dysuria | 125 (35) | 154 (43) | 279 (39) |
| Anorectal pain | 15 (4) | 8 (2) | 23 (3) |
| Sore throat | 53 (15) | 52 (15) | 105 (15) |
| Rectal discharge | 12 (3) | 10 (3) | 22 (3) |
| Rectal bleeding | 9 (2) | 8 (2) | 17 (2) |
| Tenesmus | 8 (2) | 4 (1) | 12 (2) |
| Constipation | 11 (3) | 4 (1) | 15 (2) |
| Intermenstrual bleeding (women only) | 9 (2) | 7 (2) | 16 (2) |
| Post-coital bleeding (women only) | 5 (1) | 7 (2) | 12 (2) |
| Other | 26 (7) | 26 (7) | 52 (7) |
| Duration (days) of symptom at baseline, median (IQR) | |||
| Genital discharge | 4.5 (2.5–8) | 4 (2–7) | 4 (2–7) |
| Dysuria | 4 (2–7) | 4 (2–7) | 4 (2–7) |
| Anorectal pain | 7 (5–21) | 9.5 (3.5–21) | 9 (4–21) |
| Sore throat | 7 (3–14) | 5.5 (2–14) | 7 (2–14) |
| Rectal discharge | 10.5 (3.5–24.5) | 17.5 (10–28) | 14 (4–28) |
| Rectal bleeding | 28 (7–77) | 17.5 (4.5–80.5) | 21 (7–77) |
| Tenesmus | 10.5 (3.5–24.5) | 2 (2–11.5) | 4 (2.5–21) |
| Constipation | 14 (3–112) | 45.5 (4–126) | 14 (3–112) |
| Intermenstrual bleeding (women only) | 14 (5–14) | 14 (4–28) | 14 (5–21) |
| Post-coital bleeding (women only) | 7 (1–14) | 14 (2–112) | 10.5 (2–70) |
| Other | 7 (2–28) | 6.5 (3–28) | 7 (2.5–28) |
| Hearing impairment, n (%) | |||
| No | 354 (98) | 347 (97) | 701 (97) |
| Yes | 8 (2) | 11 (3) | 19 (3) |
| If yes, severity | |||
| Grade 1 (mild) | 6 (75) | 10 (91) | 16 (84) |
| Grade 2 (moderate) | 0 | 1 (9) | 1 (5) |
| Grade 3 (severe) | 2 (25) | 0 | 2 (11) |
Compliance with the allocated intervention
Fourteen participants did not receive their allocated treatment: four (1%) in the ceftriaxone arm and 10 (3%) in the gentamicin arm. The reasons for this are given in Table 11. These 14 participants also did not receive azithromycin. In addition, three participants were recorded as not receiving azithromycin alongside their allocated treatment: one participant was recorded as not receiving azithromycin as they vomited within 50 minutes of taking it; the other two participants had already been prescribed/provided with azithromycin by another nurse practitioner and so did not receive it as part of trial treatment.
| Compliance | Treatment group, n (%) | |
|---|---|---|
| Ceftriaxone (N = 362) | Gentamicin (N = 358) | |
| Full injection of trial medication administered | ||
| No | 4 (1) | 10 (3) |
| Yes | 358 (99) | 348 (97) |
| Reason for not taking trial medication, n | ||
| Allergic to penicillin | 1 | 1 |
| Allergic to azithromycin | 0 | 1 |
| Diagnosis of PID after randomisation | 0 | 2 |
| Diagnosis of BV after randomisation | 1 | 1 |
| Trial drug could not be found/out of stock | 0 | 2 |
| No injecting nurse available | 1 | 1 |
| Found to have hearing impairment after randomisation | 1 | 0 |
| Participant refused injection because of needle phobia | 0 | 1 |
| Unknown | 0 | 1 |
| Azithromycin taken | ||
| No | 5 (1) | 12 (3) |
| Yes | 357 (99) | 346 (97) |
Of the 720 participants randomised, 624 (87%) attended their follow-up visit, 89% in the ceftriaxone group and 84% in the gentamicin group (Table 12). The median time from randomisation to follow-up was similar in both treatment groups, at 16 and 15 days in the ceftriaxone and gentamicin groups, respectively, with an interquartile range (IQR) of 14–20 days.
| Follow-up attendance | Treatment group | |
|---|---|---|
| Ceftriaxone (N = 362) | Gentamicin (N = 358) | |
| Attended follow-up visit, n (%) | 322 (89) | 302 (84) |
| Time from randomisation to follow-up visit, n (%) | ||
| < 14 days | 4 (1) | 3 (1) |
| 14 days | 101 (31) | 124 (41) |
| 15–21 days | 162 (50) | 121 (40) |
| 22–28 days | 27 (8) | 34 (11) |
| 5–6 weeks | 24 (7) | 12 (4) |
| > 6 weeks | 4 (1) | 8 (3) |
| Time (days) from randomisation to follow-up | ||
| Mean (SD) | 18.5 (6.9) | 18.4 (8.4) |
| Median (25th percentile, 75th percentile) | 16 (14, 20) | 15 (14, 20) |
| Primary outcome data available, n (%) | 306 (85) | 292 (82) |
Data sets
The ITT data set was defined as ‘participants as randomised’, regardless of adherence to the allocated group and without imputation for missing data. There were 720 participants included in the ITT data set: 362 who were randomised to ceftriaxone and 358 who were randomised to gentamicin. All baseline summaries and summaries/analyses of efficacy data are based on this data set. Follow-up data were available for 624 participants: 322 in the ceftriaxone group and 302 in the gentamicin group.
The safety data set was defined as ‘all participants according to the treatment that they actually received’. It comprised 706 participants: 358 who received ceftriaxone and 348 who received gentamicin. The 14 participants who had not received ceftriaxone or gentamicin were excluded from this data set but the participants who did not receive azithromycin remained in this data set as they had received either ceftriaxone or gentamicin. This data set was used to summarise safety data at follow-up. Follow-up data were available for 618 participants: 320 who received ceftriaxone and 298 who received gentamicin.
Primary outcome
The primary outcome – clearance of N. gonorrhoeae at all infected sites confirmed by a negative NAAT 2 weeks post treatment – was available for 598 (83%) participants overall: 306 (85%) and 292 (82%) in the ceftriaxone and gentamicin groups, respectively.
The main reasons for not having evaluable data were participants not returning for their follow-up visit and incorrect sampling at the follow-up visit (Table 13). At the start of the trial, a small number of participants were not asked by the recruiting site to return for follow-up, in error. This occurred after they reported exclusion criteria post randomisation (e.g. penicillin allergy), tested negative for gonorrhoea at baseline or did not receive trial medication. This was corrected by site training. Slightly more participants in the gentamicin group failed to come back for their follow-up visit.
| Primary outcome | Treatment group | |
|---|---|---|
| Ceftriaxone group (N = 362) | Gentamicin group (N = 358) | |
| Primary outcome available, n (%) | 306 (85) | 292 (82) |
| Primary outcome not available, n (%) | 56 (15) | 66 (18) |
| Reason primary outcome not available, n | ||
| Participant withdrew consent | 1 | 0 |
| Loss to follow-up | 36 | 47 |
| Incorrect sampling at follow-up | 16 | 10 |
| Other | 3 | 9 |
| Other reasons, n | ||
| Penicillin allergy | 1 | 1 |
| Ineligible post randomisation | 1 | 2 |
| Baseline test for gonorrhoea negative | 1 | 1 |
| No trial medication given | 0 | 2 |
| Did not attend appointments | 0 | 3 |
In total, of those with evaluable data for the primary outcome, 299 out of 306 (98%) participants in the ceftriaxone group and 267 out of 292 (91%) participants in the gentamicin group had clearance of gonorrhoea from all sites (Table 14).
| Clearance | Treatment group, n (%) | |
|---|---|---|
| Ceftriaxone (N = 362) | Gentamicin (N = 358) | |
| Participants with clearance data | 306 (85) | 292 (82) |
| Results of clearance data | ||
| Clearance of N. gonorrhoeae | 299 (98) | 267 (91) |
| Not cleared of N. gonorrhoeae | 7 (2) | 25 (9) |
The baseline characteristics for participants who had evaluable primary outcome data were similar between the two treatment groups, with the exception of site of infection: 50% of participants in the ceftriaxone group had a genital infection compared with 60% in the gentamicin group. For those randomised to ceftriaxone, there were more men (89% vs. 79%) and more genital infections (64% vs. 50%) in the group without clearance data than in the group with clearance data. For those randomised to gentamicin, there were fewer men (71% vs. 84%) in the group without clearance data than in the group with clearance data (see Appendix 3, Table 34).
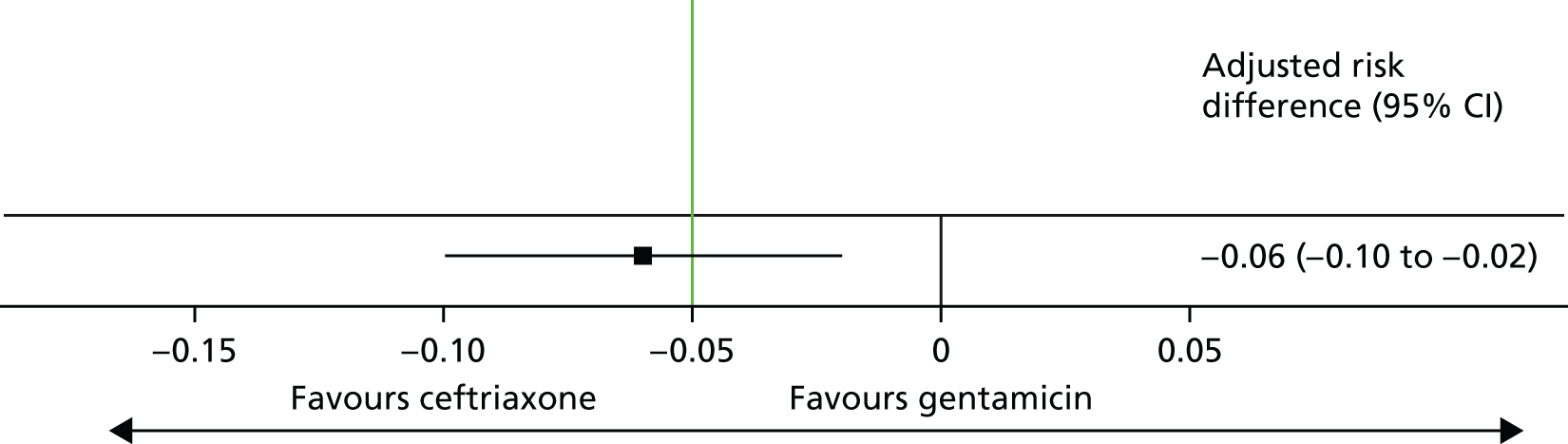
The adjusted risk difference was –6.4% (gentamicin vs. ceftriaxone), with a 95% CI of –10.4% to –2.4% (Table 15 and Figure 4). The lower 95% confidence limit (–10.4%) was < –5%, the predefined threshold for determining non-inferiority. Therefore, non-inferiority was not demonstrated.
| Treatment group | Clearance rate of N. gonorrhoeae (%) | Adjusted risk difference of gentamicin vs. ceftriaxonea (%) | 95% CI (%) |
|---|---|---|---|
| Ceftriaxone | 98 | –6.4 | –10.4 to –2.4 |
| Gentamicin | 91 |
FIGURE 4.
Clearance of N. gonorrhoeae at 2 weeks post randomisation. The non-inferiority margin is –0.05.
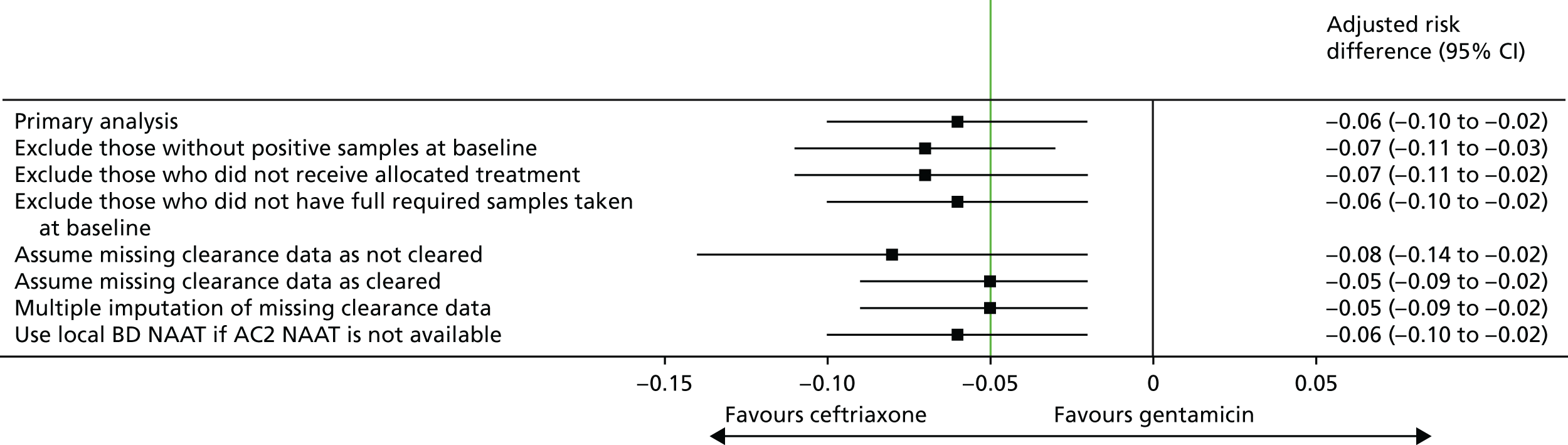
Sensitivity analyses for the primary outcome
Sensitivity analyses for the primary outcome are presented in Table 16 and Figure 5. Although the adjusted risk differences varied between the different sensitivity analyses, all analyses performed were supportive of the primary analysis, with the lower 95% confidence limits of the 95% CIs all being < –5%.
| Scenario | Treatment group | Number of participants included in analysis | Clearance rate of N. gonorrhoeae (%) | Adjusted risk differencea (%) | 95% CI (%) |
|---|---|---|---|---|---|
| Exclude those without any positive samples at baseline | Ceftriaxone | 268 | 98 | –7.1 | –11.4 to –2.8 |
| Gentamicin | 261 | 91 | |||
| Exclude those who did not receive allocated treatment | Ceftriaxone | 304 | 98 | –6.5 | –10.5 to –2.4 |
| Gentamicin | 289 | 91 | |||
| Exclude those who did not have full required samples taken at baseline | Ceftriaxone | 269 | 98 | –5.9 | –10.0 to –1.8 |
| Gentamicin | 260 | 92 | |||
| Assume missing clearance data as not cleared | Ceftriaxone | 362 | 83 | –8.1 | –14.1 to –2.1 |
| Gentamicin | 358 | 75 | |||
| Assume missing clearance data as cleared | Ceftriaxone | 362 | 98 | –5.3 | –8.6 to –1.9 |
| Gentamicin | 358 | 93 | |||
| Multiple imputation of missing clearance data | Ceftriaxone | 362 | 97 | –5.1 | –8.7 to –1.5 |
| Gentamicin | 358 | 92 | |||
| Use local BD NAAT if AC2 NAAT not available | Ceftriaxone | 317 | 97 | –6.2 | –10.2 to –2.2 |
| Gentamicin | 295 | 91 |
FIGURE 5.
Sensitivity analyses for clearance of N. gonorrhoeae at 2 weeks post randomisation. The non-inferiority margin is –0.05.
Secondary outcomes
Clearance of N. gonorrhoeae by infection site
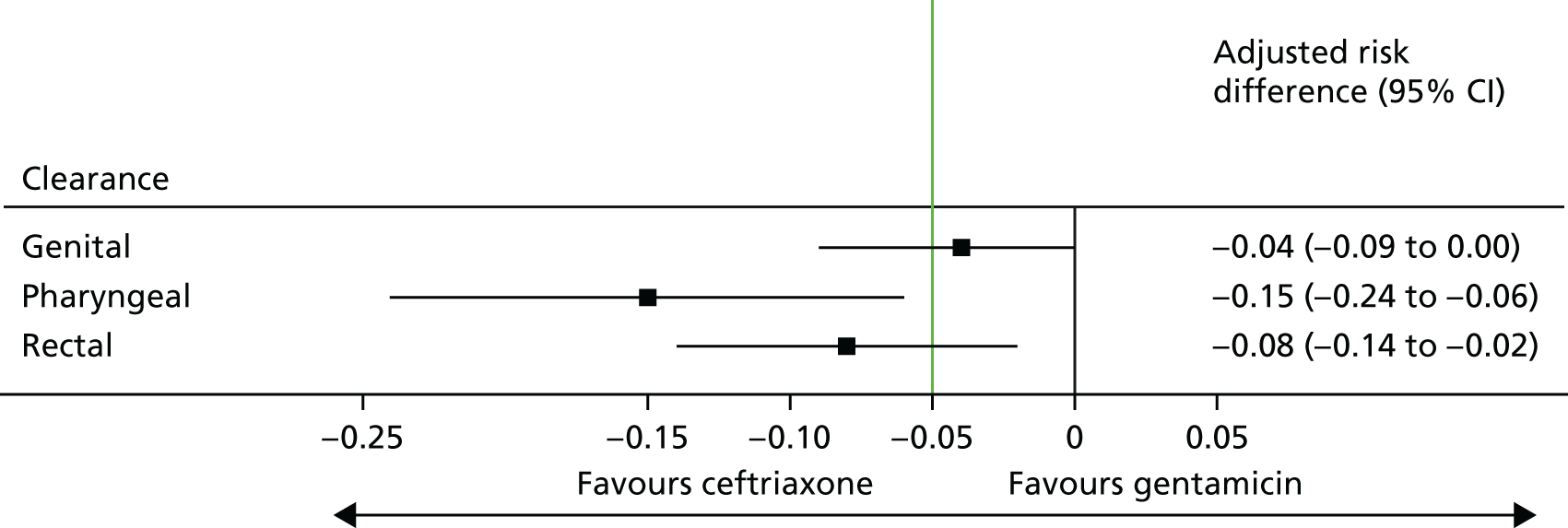
Of the participants who had a genital infection, 98% in the ceftriaxone group and 94% in the gentamicin group were cleared at their follow-up visit (Table 17 and Figure 6). The adjusted risk difference was –4.4% (95% CI –8.7% to 0%). A greater proportion of participants with pharyngeal infection receiving ceftriaxone had clearance at their follow-up visit (96%) than participants receiving gentamicin (80%). The adjusted risk difference was –15.3% (95% CI –24.0% to –6.5%). Similarly, a greater proportion of participants with rectal infection in the ceftriaxone group showed clearance (98%) than patients in the gentamicin group (90%) (adjusted risk difference –7.8%, 95% CI –13.6% to –2.0%). Clearance at genital site by sex is also provided in Appendix 3, Table 34.
| Clearance at infection site | Treatment group | |
|---|---|---|
| Ceftriaxone | Gentamicin | |
| Genital infection | N = 154 | N = 174 |
| Cleared of N. gonorrhoeae, n (%) | 151 (98) | 163 (94) |
| 95% CI (%) | 96 to 100 | 90 to 97 |
| Not cleared of N. gonorrhoeae, n (%) | 3 (2) | 11 (6) |
| Risk difference (95% CI) for clearance (%) | –4.4 (–8.7 to 0) | |
| Pharyngeal infection | N = 113 | N = 102 |
| Cleared of N. gonorrhoeae, n (%) | 108 (96) | 82 (80) |
| 95% CI (%) | 92 to 99 | 72 to 88 |
| Not cleared of N. gonorrhoeae, n (%) | 5 (4) | 20 (20) |
| Risk difference (95% CI) for clearance (%) | –15.3 (–24.0 to –6.5) | |
| Rectal infection | N = 137 | N = 119 |
| Cleared of N. gonorrhoeae, n (%) | 134 (98) | 107 (90) |
| 95% CI | 95 to 100 | 84 to 95 |
| Not cleared of N. gonorrhoeae, n (%) | 3 (2) | 12 (10) |
| Risk difference (95% CI) for clearance (%) | –7.8 (–13.6 to –2.0) | |
FIGURE 6.
Clearance of N. gonorrhoeae by infection site. The non-inferiority margin is –0.05.
Clinical resolution of symptoms
There was no evidence of any difference between the treatment groups in terms of resolution of symptoms (Table 18). The 95% CIs for the adjusted risk differences are wide for those cases when the number of participants experiencing some symptoms at baseline was small. For all 12 participants who had post-coital bleeding, this symptom had resolved at 2 weeks post randomisation. These data are therefore not included in Table 18. The complete summary data of symptoms at baseline and follow-up are included in Appendix 3, Table 35.
| Symptom | Number of participants included in analysis | Adjusted risk difference (gentamicin vs. ceftriaxone)a (%) | 95% CI (%) |
|---|---|---|---|
| Genital discharge | 276 | –0.1 | –5.5 to 5.2 |
| Dysuria | 234 | –7.7 | –13.6 to 1.9 |
| Sore throat | 92 | 4.0 | –7.4 to 15.4 |
| Anorectal pain | 20 | –24.4 | –62.5 to 13.7 |
| Rectal bleeding | 15 | 12.5 | –10.4 to 35.4 |
| Rectal discharge | 20 | –9.9 | –43.7 to 23.9 |
| Tenesmus | 10 | 12.5 | –10.4 to 35.4 |
| Constipation | 15 | –12.6 | –57.8 to 32.6 |
| Intermenstrual bleeding (female only) | 14 | 11.1 | –9.0 to 31.6 |
Frequency of nausea, vomiting, reduction in hearing, dizziness and rash
The frequencies of the expected side effects of gentamicin and ceftriaxone were summarised based on the safety data set. This data set excluded the 14 participants who did not receive either ceftriaxone or gentamicin. There were 358 participants who received ceftriaxone and 348 participants who received gentamicin included in the safety data set. Of these participants, follow-up data were available for 618 participants: 320 who had received ceftriaxone and 298 who had received gentamicin.
Nausea
The percentages of participants experiencing nausea were similar in the ceftriaxone group [12% (38/320)] and the gentamicin group [14% (41/298)]. In total, 2% of participants in each group had grade 2 nausea (oral intake significantly decreased). All other reports of nausea were grade 1 (able to eat normally). The time to onset of nausea from the time of injection was similar in both treatment groups, as was the duration of nausea and the percentage of participants fully recovered by follow-up (95% in both treatment groups).
Vomiting
The incidence of vomiting was low, with three participants (1%) in the ceftriaxone group and 12 (4%) in the gentamicin group experiencing at least one episode. All participants in the ceftriaxone group experienced grade 1 vomiting (one episode in 24 hours), whereas, in the gentamicin group, eight participants (3% of the total number of participants) experienced grade 1 and four (1%) experienced grade 2 vomiting (2–5 episodes in 24 hours).
Reduction in hearing
Five participants in the ceftriaxone group (2%) and three (1%) in the gentamicin group reported a mild reduction in their hearing. Of these participants, one in each group had not fully recovered by their follow-up visit.
Dizziness/unsteadiness
A total of 24 participants in the ceftriaxone group (7%) and 21 in the gentamicin group (7%) reported dizziness or unsteadiness. In the ceftriaxone group, 20 reported grade 1 severity (not interfering with function), three reported grade 2 severity (interfering with function but not interfering with daily activity) and one reported grade 4 severity (bedridden or disabled) events. The grade 4 severity event was reported as a SAE and was not considered to be related to the trial medication. In the gentamicin group, 19 participants reported grade 1 severity and two participants reported grade 2 severity events.
Skin rashes
Five participants in the ceftriaxone group (2%) and 12 participants in the gentamicin group (4%) reported skin rashes; all five participants in the ceftriaxone group and 11 out of the 12 participants in the gentamicin group reported a grade 1 skin rash (localised skin eruption). One participant in the gentamicin group reported a grade 2 skin rash (diffuse skin eruption covering ≤ 50% of the body surface area). All participants in the ceftriaxone group had fully recovered by their follow-up visit, whereas 6 out of the 12 participants in the gentamicin group had not fully recovered.
Injection pain
In total, 315 out of the 320 participants in the ceftriaxone group (98%) and 294 of the 298 participants in the gentamicin group (99%) recorded injection pain. The median time for the pain to completely resolve was slightly longer in the gentamicin group than in the ceftriaxone group (1.5 hours vs. 1 hour; IQR: 0–24 hours for the gentamicin group and 0–12 hours for the ceftriaxone group).
The results for all expected side effects of ceftriaxone and gentamicin are summarised in Table 19.
| Side effect | Treatment group | |
|---|---|---|
| Ceftriaxone (N = 320) | Gentamicin (N = 298) | |
| Any nausea, n (%) | 38 (12) | 41 (14) |
| Severity, n (%) | ||
| Grade 1 (able to eat normally) | 30 (9) | 36 (12) |
| Grade 2 (oral intake significantly decreased) | 8 (2) | 5 (2) |
| Grade 3 (no significant intake or requiring i.v. fluids) | 0 (0) | 0 (0) |
| Time from injection to onset, n (%) | ||
| < 2 hours | 22 (58) | 24 (59) |
| 2–6 hours | 8 (21) | 10 (24) |
| 6–24 hours | 4 (11) | 3 (7) |
| 1–3 days | 2 (5) | 2 (5) |
| > 3 days | 2 (5) | 2 (5) |
| Duration (hours) | ||
| Median (25th percentile, 75th percentile) | 3 (1–9) | 3 (1–24) |
| Minimum, maximum | 1, 240 | 1, 240 |
| Fully recovered, n (%) | ||
| No | 2 (5) | 2 (5) |
| Yes | 36 (95) | 39 (95) |
| Any vomiting, n (%) | 3 (1) | 12 (4) |
| Severity, n (%) | ||
| Grade 1 (1 episode in 24 hours) | 3 (1) | 8 (3) |
| Grade 2 (2–5 episodes in 24 hours) | 0 (0) | 4 (1) |
| Grade 3 (≥ 6 episodes in 24 hours or need for i.v. fluids) | 0 (0) | 0 (0) |
| Time from injection to onset, n (%) | ||
| < 2 hours | 1 (33) | 5 (42) |
| 2–6 hours | 0 (0) | 1 (8) |
| 6–24 hours | 0 (0) | 1 (8) |
| 1–3 days | 1 (33) | 3 (25) |
| > 3 days | 1 (33) | 2 (17) |
| Duration (hours) | ||
| Median (25th percentile, 75th percentile) | 1 (1–3) | 1.5 (1–36) |
| Minimum, maximum | 1, 3 | 1, 72 |
| Fully recovered, n (%) | ||
| No | 0 | 0 |
| Yes | 3 (100) | 12 (100) |
| Any reported reduction in hearing, n (%) | 5 (2) | 3 (1) |
| Severity, n (%) | ||
| Mild | 5 (2) | 3 (1) |
| Moderate | 0 (0) | 0 (0) |
| Severe | 0 (0) | 0 (0) |
| Duration (hours) | ||
| Median (25th percentile, 75th percentile) | 144 (72–264) | 96 (2–336) |
| Minimum, maximum | 12, 504 | 2, 336 |
| Time from injection to onset, n (%) | ||
| < 2 hours | 1 (20) | 0 (0) |
| 2–6 hours | 0 (0) | 0 (0) |
| 6–24 hours | 0 (0) | 1 (33) |
| 1–3 days | 2 (40) | 1 (33) |
| > 3 days | 2 (40) | 1 (33) |
| Fully recovered, n (%) | ||
| No | 1 (20) | 1 (33) |
| Yes | 4 (80) | 2 (67) |
| Any reported dizziness or unsteadiness, n (%) | 24 (7) | 21 (7) |
| Severity, n (%) | ||
| Grade 1 (not interfering with function) | 20 (6) | 19 (6) |
| Grade 2 (interfering with function but not interfering with daily activities) | 3 (1) | 2 (1) |
| Grade 3 (interfering with daily activities) | 0 (0) | 0 (0) |
| Grade 4 (bedridden or disabled) | 1 (0) | 0 (0) |
| Duration (hours) | ||
| Median (25th percentile, 75th percentile) | 2 (1–15) | 4 (1–24) |
| Minimum, maximum | 1, 72 | 0, 168 |
| Time from injection to onset, n (%) | ||
| < 2 hours | 13 (54) | 10 (48) |
| 2–6 hours | 5 (21) | 5 (24) |
| 6–24 hours | 2 (8) | 4 (19) |
| 1–3 days | 0 (0) | 1 (5) |
| > 3 days | 4 (17) | 1 (5) |
| Fully recovered, n (%) | ||
| No | 1 (4) | 2 (10) |
| Yes | 23 (96) | 19 (90) |
| Any new skin rash, n (%) | 5 (2) | 12 (4) |
| Severity, n (%) | ||
| Grade 1 (localised skin eruption) | 5 (2) | 11 (4) |
| Grade 2 (diffuse skin eruption covering ≤ 50% of body surface area) | 0 (0) | 1 (0) |
| Grade 3 (generalised skin eruption covering > 50% of body surface area) | 0 (0) | 0 (0) |
| Duration (hours) | ||
| Median (25th percentile, 75th percentile) | 24 (24–24) | 72 (36–324) |
| Minimum, maximum | 6, 72 | 4, 744 |
| Fully recovered, n (%) | ||
| No | 0 (0) | 6 (50) |
| Yes | 5 (100) | 6 (50) |
| Injection pain, n (%) | 315 (98) | 294 (99) |
| How long (hours) did it take to completely resolve the pain associated with injection? | ||
| Median (25th percentile, 75th percentile) | 1 (0–12) | 2 (0–24) |
| Minimum, maximum | 0, 240 | 0, 432 |
Other adverse events
Table 20 shows that the percentage of participants reporting other AEs at their follow-up visit was similar in both treatment groups: 15% (48/320) in the ceftriaxone group and 13% (38/298) in the gentamicin group, with the majority of AEs being mild. The most frequent class of AEs reported was gastrointestinal disorders: 14 participants reported these in the ceftriaxone group (4%) and 22 participants reported these in the gentamicin group (7%). The majority of these gastrointestinal disorders consisted of diarrhoea. Nervous system disorders were reported by 10 participants in the ceftriaxone group and three participants in the gentamicin group. The events that coded to nervous system disorders were headaches (six in the ceftriaxone group and one in the gentamicin group), dizziness (one in the ceftriaxone group and one in the gentamicin group), migraine (one in the ceftriaxone group), lethargy (one in the ceftriaxone group and one in the gentamicin group) and Bell’s palsy (one in the ceftriaxone group).
| AEs | Treatment group | |
|---|---|---|
| Ceftriaxone (N = 320) | Gentamicin (N = 298) | |
| Total number of AEs | 54 | 43 |
| Total number of AEs thought to be related to trial medication | 48 | 38 |
| Participants with any AE, n (%) | 48 (15) | 38 (13) |
| Participants with AE related to trial medication, n (%) | 15 (5) | 17 (6) |
| Participants with any SAE, n (%) | 1 (<0.5) | 0 (0) |
| Severity of AE, n (%) | ||
| Mild | 45 (83) | 35 (81) |
| Moderate | 8 (15) | 6 (14) |
| Severe | 1 (2) | 2 (5) |
| MedDRA31 SOC codes,a n (%) | ||
| Ear and labyrinth disorders | 3 (3) | 0 (0) |
| Gastrointestinal disorders | 14 (14) | 22 (22) |
| General disorders and administration site conditions | 6 (6) | 3 (3) |
| Infections and infestations | 7 (7) | 5 (5) |
| Investigations | 5 (5) | 2 (2) |
| Metabolism and nutrition disorders | 0 (0) | 2 (2) |
| Musculoskeletal and connective tissue disorders | 2 (1) | 4 (4) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 1 (1) | 0 (0) |
| Nervous system disorders | 10 (10) | 3 (3) |
| Pregnancy, puerperium and perinatal conditions | 1 (1) | 0 (0) |
| Psychiatric disorders | 1 (1) | 0 (0) |
| Respiratory, thoracic and mediastinal disorders | 3 (3) | 1 (1) |
| Skin and subcutaneous tissue disorders | 1 (1) | 1 (1) |
Five per cent of AEs in the ceftriaxone group and 6% in the gentamicin group were reported to be related to the trial medication. Three AEs (only one of which was related to the trial medication) were reported to be severe: one in the ceftriaxone group and two in the gentamicin group. These were ‘diarrhoea’, ‘grade 4 dizziness’ and ‘sickness’. Of these, the grade 4 dizziness was also classified as a SAE. All were resolved by the follow-up visit.
The SAE was grade 4 dizziness in a participant in the ceftriaxone group, which had occurred 4 days after randomisation. It was considered to be unrelated to the trial medication and was resolved before the follow-up visit.
A full list of AEs is provided in Appendix 3.
Creatinine level and estimated glomerular filtration rate
The percentage of participants who had an increase in creatinine level of > 30% was similar in both treatment groups: 3% (10/358) in the ceftriaxone group and 3% (9/348) in the gentamicin group (Table 21). The follow-up creatinine value for 9% (28/304) of participants in the ceftriaxone group and 13% (38/289) of participants in the gentamicin group exceeded the upper limit of normal, as defined by their local laboratory. Changes in eGFR between baseline and follow-up were similar in both arms (see Table 21).
| Creatinine levels and eGFR | Treatment group | |
|---|---|---|
| Ceftriaxone (N = 358) | Gentamicin (N = 348) | |
| Creatinine level | ||
| At baseline (µmol/l) | ||
| Mean (SD) | 78.7 (15.4) | 78.3 (15.8) |
| N | 341 | 329 |
| At 2 weeks post treatment (µmol/l) | ||
| Mean (SD) | 79.7 (14.4) | 80.2 (15.6) |
| N | 304 | 289 |
| Change in creatinine level, n (%) | ||
| Number of participants who met clinically important change from baseline to 2 weeks post treatmenta | 10 (3) | 9 (3) |
| Number of participants who exceeded upper limit of normal value at 2 weeks post treatmentb | 27 (7) | 38 (11) |
| eGFR (ml/minute/1.73m2) | ||
| At baseline, mean (SD) | 110.6 (18.2) | 111.5 (17.7) |
| At 2 weeks post treatment, mean (SD) | 109.2 (17.4) | 108.7 (17.9) |
| Change in eGFR at 2 weeks post treatment, median (IQR) | –1.3 (–6.7 to 4.3) | –1.4 (–6.9 to 3.7) |
The shift plots in Figures 7 and 8 demonstrate the shifts in creatinine level from baseline to visit 2. AEs relating to creatinine were reported for seven participants: five who had received ceftriaxone and two who had received gentamicin. All were mild and five out of the seven AEs were not thought to be related to trial medication. The grid lines represent the lowest of the lower bounds and highest of the upper bounds of normality between all of the local laboratories. The diagonal line represents the line of unity (no change).
FIGURE 7.
Shift plot of pre- and post-treatment creatinine levels in males.
FIGURE 8.
Shift plot of pre- and post-treatment creatinine levels in females.
Tolerability of injection
Injection site pain
The VAS score (1–100) completed immediately after injection with the trial treatment showed higher mean and median values (i.e. more pain) for the gentamicin group (Table 22). The pain scores recalled at the 2-week follow-up visit were also higher in the gentamicin group.
| VAS score | Treatment group | |
|---|---|---|
| Ceftriaxone (N = 362) | Gentamicin (N = 358) | |
| VAS score for injection site pain immediately after injection | ||
| Mean (SD) | 21.2 (19.4) | 36 (23.2) |
| Median (25th percentile, 75th percentile) | 15 (6, 30) | 31.5 (18, 53.5) |
| Minimum, maximum | 0, 86 | 0, 100 |
| N | 353 | 348 |
| Recalled VAS score for pain at baseline injection after 2 weeks | ||
| Mean (SD) | 20.4 (20.5) | 34.3 (25) |
| Median (25th percentile, 75th percentile) | 15 (4, 30) | 31 (13, 55) |
| Minimum, maximum | 0, 100 | 0, 100 |
| N | 313 | 295 |
Minimum inhibitory concentrations
Gentamicin
Figure 9 shows the MIC distribution for gentamicin for the 367 participants for whom isolate data were available.
FIGURE 9.
Gentamicin overall MIC distribution (mg/l) at baseline (all participants with sample data).
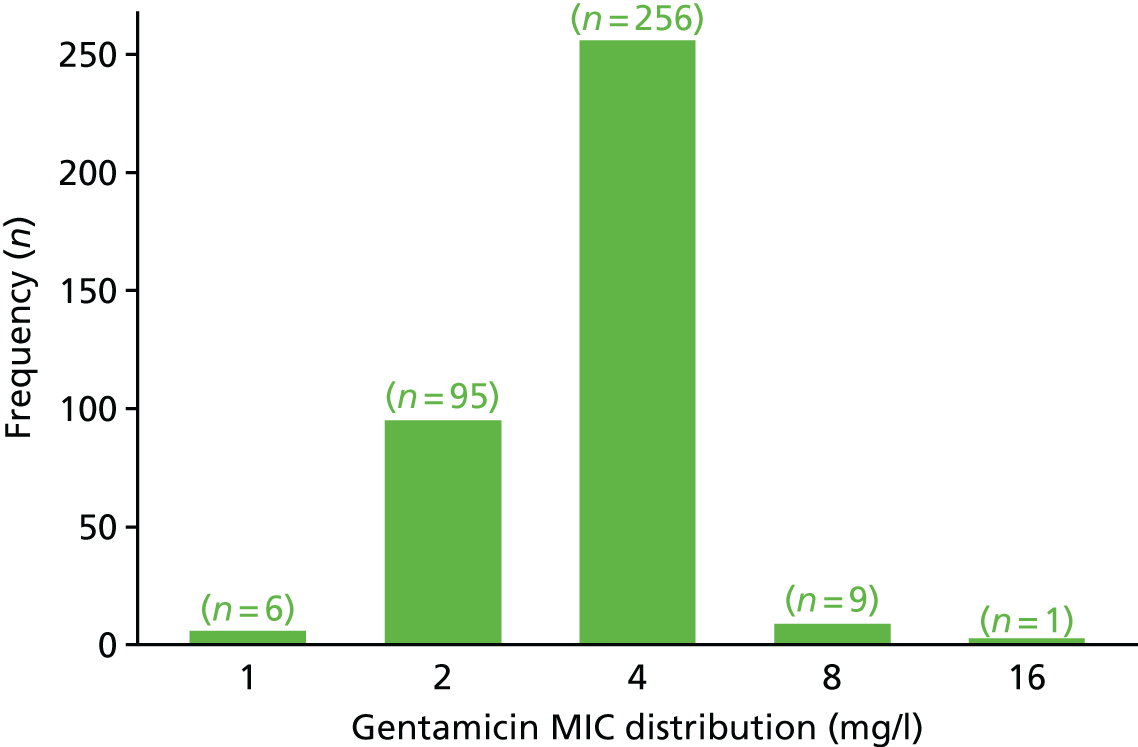
Figure 10 shows the gentamicin MICs of isolates from participants who received gentamicin and achieved microbiological clearance (as defined by a negative AC2 NAAT at all previously infected sites, 2 weeks after treatment). Data were available for 149 participants. The proportion who had clearance of N. gonorrhoeae at all sites was 90%. All isolates from non-responders had a MIC of 4 mg/l.
FIGURE 10.
Distribution of gentamicin MIC (mg/l) for participants who received gentamicin, by clearance at 2 weeks post treatment.
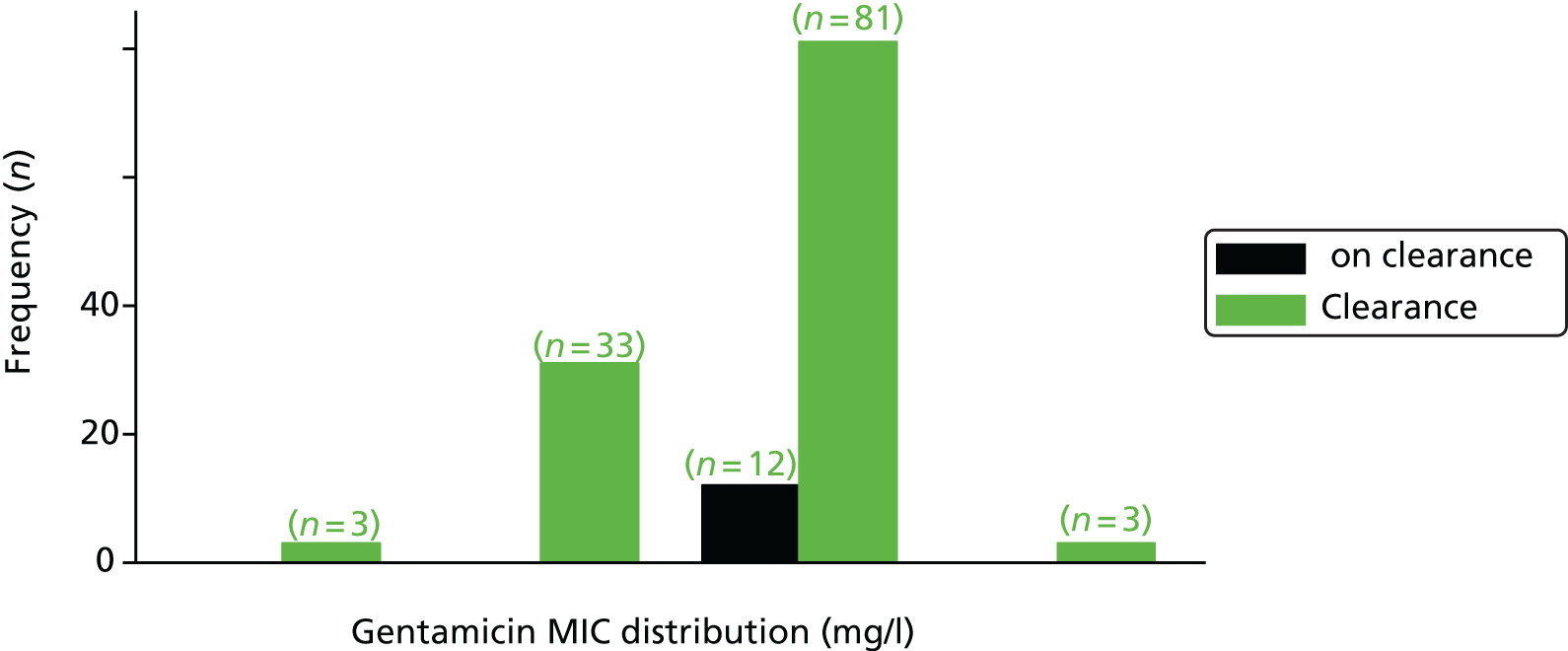
Ceftriaxone
Figure 11 shows the MIC distribution for ceftriaxone for the 364 participants for whom isolate data were available. Fifty-nine participants (16%) harboured isolates with MIC values of ≤ 0.002 mg/l.
FIGURE 11.
Ceftriaxone overall MIC distribution (mg/l) at baseline (all participants with sample data).
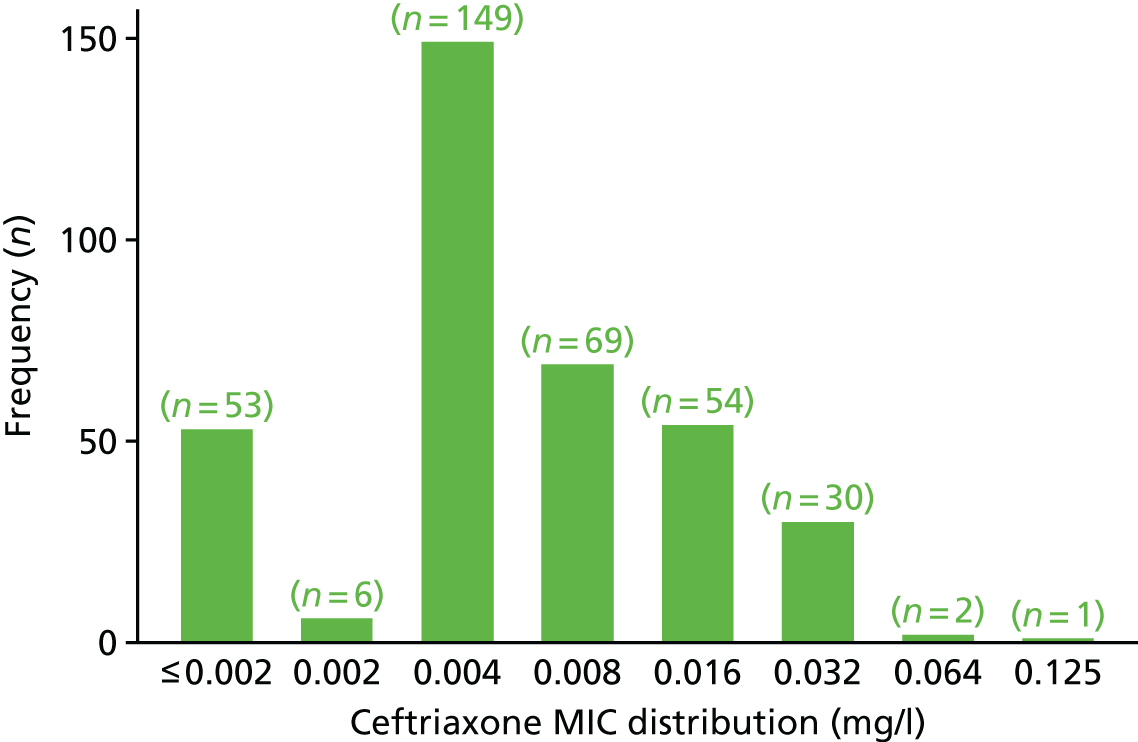
Figure 12 shows the ceftriaxone MICs of isolates from participants who received ceftriaxone and achieved microbiological clearance (as defined by a negative AC2 NAAT at all previously infected sites, 2 weeks after treatment). Data were available for 170 participants. The proportion of these participants who had clearance of N. gonorrhoeae at all sites was 96%. The six non-responders had MICs ranging from ≤ 0.002 to 0.008 mg/l.
FIGURE 12.
Distribution of ceftriaxone MIC (mg/l) for participants who received ceftriaxone, by clearance at 2 weeks post treatment.
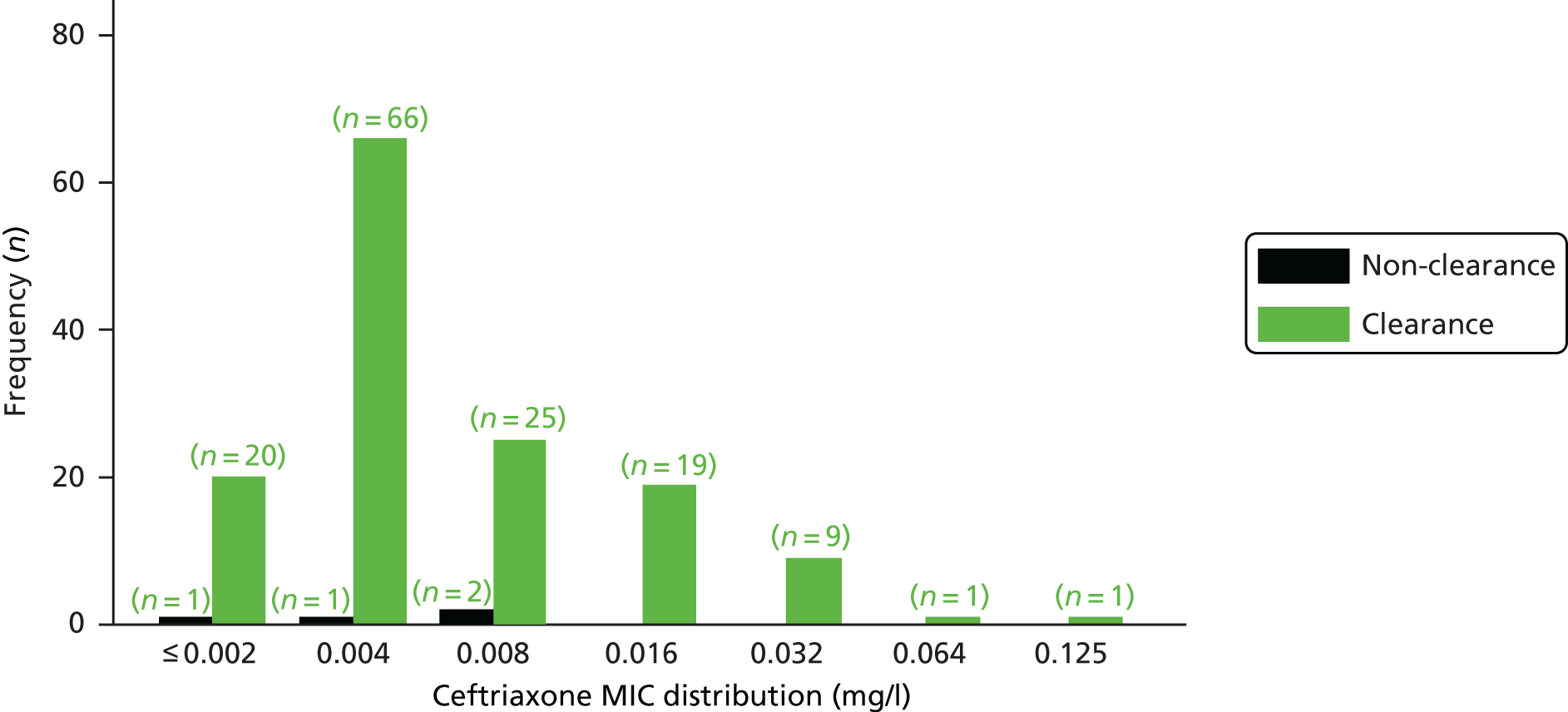
Azithromycin
Figure 13 shows the MIC distribution for azithromycin for the 357 participants for whom isolate data were available. The distribution of MICs ranged from 0.032 to 4 mg/l.
FIGURE 13.
Azithromycin overall MIC distribution (mg/l) at baseline (all participants with sample data).
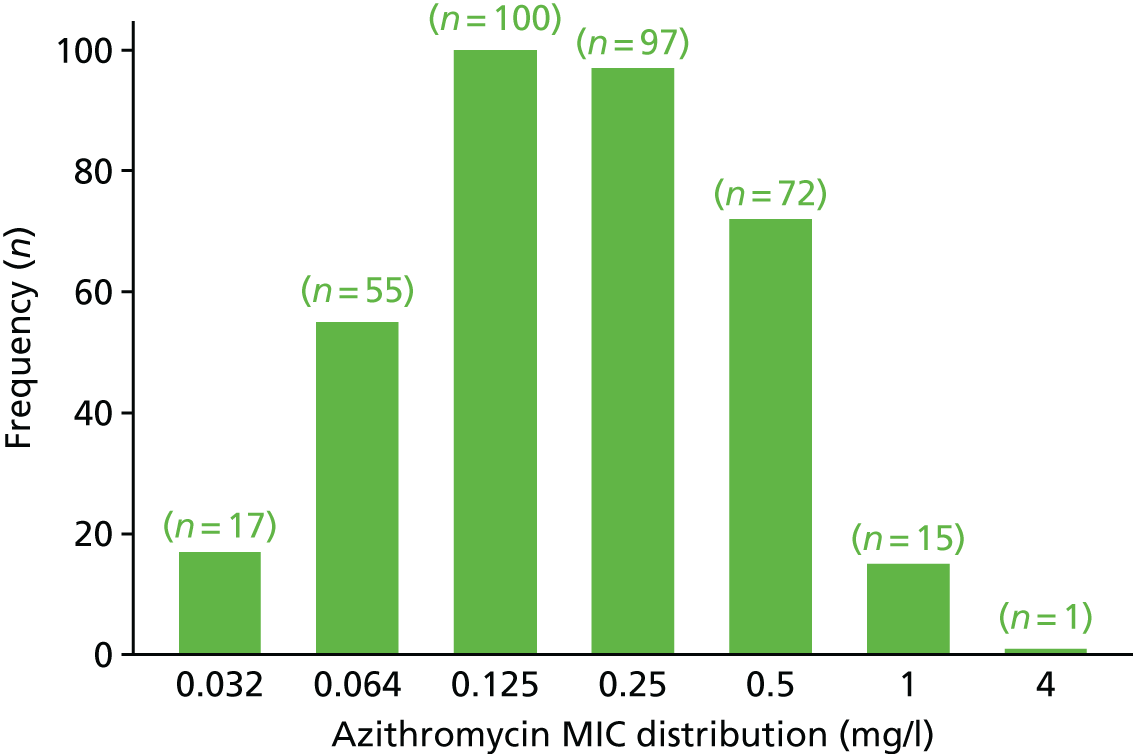
Figure 14 shows the azithromycin MICs of isolates from participants who received azithromycin and achieved microbiological clearance (as defined by a negative AC2 NAAT at all previously infected sites, 2 weeks after treatment). Data were available for 305 participants. The proportion of these participants who had clearance of N. gonorrhoeae at all sites was 93%. The two non-respondents had MICs ranging from 0.064 to 1 mg/l.
FIGURE 14.
Distribution of azithromycin MIC (mg/l) for all participants, by clearance at 2 weeks post treatment.
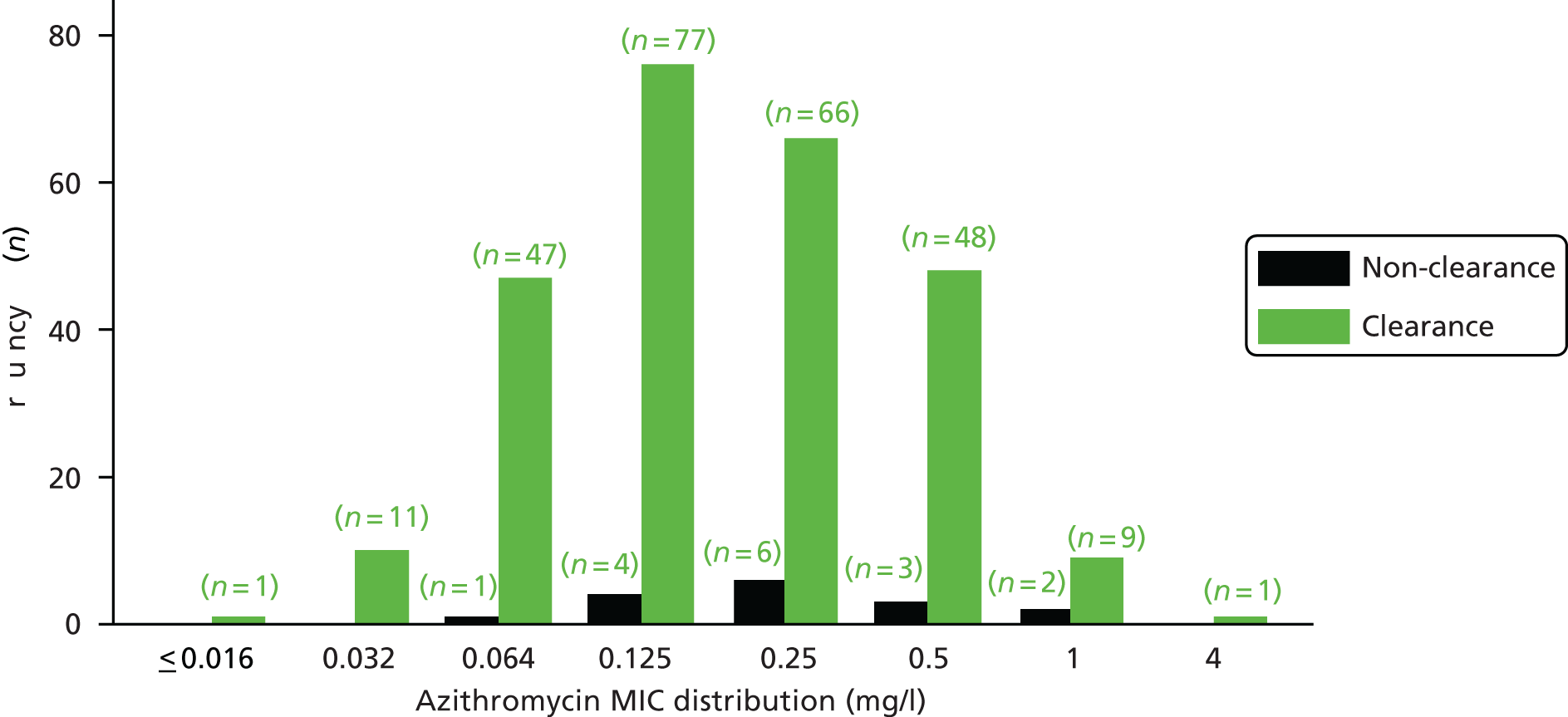
Further plots showing the MIC distribution by infection site are available in Appendix 3.
Medications taken during the trial
A similar proportion of participants in both treatment arms took additional antibiotics in the period from randomisation to follow-up (Table 23).
| Antibiotic | Treatment group, (n) | |
|---|---|---|
| Ceftriaxone (N = 362) | Gentamicin (N = 358) | |
| Taken at least one non-trial antibiotic | 13 | 16 |
| Azithromycin | 2 | 1 |
| Doxycycline | 9 | 9 |
| Ceftriaxone | 0 | 3 |
| Metronidazole | 1 | 1 |
| Trimethoprim | 0 | 1 |
| Ofloxacin | 0 | 1 |
| Clarithromycin | 1 | 0 |
Other antibiotics taken during the trial
A listing of other antibiotics that participants took during the trial is provided in Appendix 3.
Sexual behaviour and condom use during the trial
Tables 24 and 25 show the sexual behaviour of males and females, respectively, after receiving their trial medication. Out of 257 males in the ceftriaxone group and 251 males in the gentamicin group, 98 (38%) and 88 (35%), respectively, reported having had sex between receiving their randomised treatment and the 2-week follow-up. Of these, 40 males out of 98 (41%) and 37 males out of 88 (42%), respectively, never used a condom. Out of 65 females in the ceftriaxone group and 50 females in the gentamicin group, 20 (31%) and 16 (32%), respectively, reported having had sex between receiving trial treatment and the 2-week follow-up visit. Of these, 7 females out of 20 (35%) and 10 females out of 16 (63%) reported never using a condom. The risk of reinfection as a result of having sex in the follow-up period and the failure to use condoms in this period was therefore deemed similar in the two treatment groups.
| Sexual history | Treatment group | |
|---|---|---|
| Ceftriaxone (N = 257) | Gentamicin (N = 251) | |
| Sexual contact since receiving treatment for gonorrhoea | ||
| No, n (%) | 157 (61) | 163 (65) |
| Yes, n (%) | 98 (38) | 88 (35) |
| Not known, n (%) | 2 (1) | 0 |
| If yes, number of partners | ||
| Median (25th percentile, 75th percentile) | 1 (1, 2) | 1 (1, 2) |
| Minimum, maximum | 1, 6 | 1, 13 |
| Previous partners | ||
| Median (25th percentile, 75th percentile) | 1 (0, 1) | 1 (0, 1) |
| Minimum, maximum | 0, 3 | 0, 5 |
| New partners | ||
| Median (25th percentile, 75th percentile) | 1 (0, 1) | 0 (0, 1) |
| Minimum, maximum | 0, 6 | 0, 12 |
| Number of episodes of sexual contact since receiving treatment for gonorrhoea | ||
| Median (25th percentile, 75th percentile) | 2 (1, 3) | 2 (1, 4) |
| Minimum, maximum | 1, 15 | 1, 20 |
| Type of sexual contact,a n (%) | ||
| Genital–genital | 48 (19) | 43 (17) |
| Genital–oral | 73 (28) | 67 (27) |
| Oral–genital | 67 (26) | 61 (24) |
| Genital–anal | 51 (20) | 47 (19) |
| Anal–genital | 36 (14) | 42 (17) |
| Oral–anal | 24 (9) | 29 (12) |
| Anal–oral | 22 (9) | 25 (10) |
| Digital–anal | 26 (10) | 26 (10) |
| Anal–digital | 21 (8) | 21 (8) |
| Use of condoms since receiving treatment for gonorrhoea, n (%) | ||
| No | 40 (15) | 37 (15) |
| Yes, partially | 6 (2) | 11 (4) |
| Yes, consistently, including for oral sex | 15 (6) | 7 (3) |
| Yes, consistently, but not for oral sex | 37 (14) | 33 (13) |
| Unknown | 2 (1) | 0 |
| N/A | 157 (61) | 163 (65) |
| Sexual history | Treatment group | |
|---|---|---|
| Ceftriaxone (N = 65) | Gentamicin (N = 50) | |
| Sexual contact since receiving treatment for gonorrhoea | ||
| No, n (%) | 45 (69) | 34 (68) |
| Yes, n (%) | 20 (31) | 16 (32) |
| If yes, number of partners | ||
| Median (25th percentile, 75th percentile) | 1 (1, 1) | 1 (1, 1) |
| Minimum, maximum | 1, 5 | 1, 2 |
| Previous partners | ||
| Median (25th percentile, 75th percentile) | 1 (0.5, 1) | 1 (1, 1) |
| Minimum, maximum | 0, 1 | 0, 1 |
| New partners | ||
| Median (25th percentile, 75th percentile) | 0 (0, 1) | 0 (0, 0.5) |
| Minimum, maximum | 0, 5 | 0, 1 |
| Number of episodes of sexual contact since receiving treatment for gonorrhoea | ||
| Median (25th percentile, 75th percentile) | 1 (1, 2.5) | 1 (1, 2.5) |
| Minimum, maximum | 1, 14 | 1, 4 |
| Type of sexual contact,a n (%) | ||
| Genital–genital | 19 (29) | 13 (26) |
| Genital–oral | 5 (8) | 3 (6) |
| Oral genital | 8 (12) | 3 (6) |
| Genital–anal | 0 | 0 |
| Anal–genital | 1 (2) | 1 (2) |
| Oral–anal | 0 | 0 |
| Anal–oral | 0 | 0 |
| Digital–anal | 0 | 0 |
| Anal–digital | 0 | 0 |
| Use of condoms since receiving treatment for gonorrhoea, n (%) | ||
| No | 7 (11) | 10 (20) |
| Yes, partially | 5 (8) | 2 (4) |
| Yes, consistently, including for oral sex | 3 (5) | 2 (4) |
| Yes, consistently, but not for oral sex | 5 (8) | 2 (4) |
| Unknown | 0 | 0 |
| N/A | 45 (69) | 34 (68) |
Protocol deviations
Protocol deviations were reported in 121 out of 362 (33%) participants receiving ceftriaxone and 124 out of 358 (35%) participants receiving gentamicin; the majority of deviations were considered minor. There were only two major deviation categories: not receiving treatment according to randomisation and not fulfilling the eligibility criteria. Fourteen participants did not receive treatment according to randomisation and 20 deviations from the eligibility criteria were reported for 18 participants. These included participants being found to have contraindications to trial medication and having a disallowed concomitant disease. A list of the protocol deviations recorded on the deviation log can be found in Appendix 3.
Chapter 4 Economic evaluation of gentamicin compared with ceftriaxone in the treatment of gonorrhoea
Introduction and aims
The aim of the economic evaluation was to compare the costs and outcomes associated with the current standard treatment, ceftriaxone, in the treatment of gonorrhoea with those of the proposed alternative treatment, gentamicin. The primary aim of the G-TOG trial was to determine whether or not gentamicin is an acceptable alternative to ceftriaxone in the treatment of gonorrhoea. There are several scenarios in which a decision might be taken to use an alternative treatment. For example, the use of an alternative antibiotic might be recommended to preserve the effectiveness of the current standard treatment, as antibiotic resistance is linked to consumption. 36 In addition, an alternative treatment might be needed in the context of developing antimicrobial resistance (AMR) to the current standard treatment, as has happened repeatedly with gonorrhoea over the last 70–80 years. 37
The G-TOG trial was designed as a non-inferiority trial to assess whether or not the rate of microbiological clearance of N. gonorrhoeae in participants treated with gentamicin is non-inferior to the rate of microbiological clearance in participants treated with ceftriaxone. The economic analysis, therefore, focused on establishing whether or not the use of gentamicin rather than ceftriaxone is cost neutral in the treatment of gonorrhoea, in which case gentamicin could be used as a substitute for ceftriaxone without additional resource implications. This involved the examination of costs and resource use to determine whether or not there were any differences between the two treatments.
Methods
Overview
A cost-effectiveness analysis (CEA) was undertaken from the perspective of the NHS. The main outcome considered was the clearance of gonorrhoea, as this was the primary outcome of the trial. A health-care perspective was deemed to be the most relevant as the RCT is concerned with the non-inferiority of gentamicin in the treatment of gonorrhoea; hence, the costs to the NHS associated with the two treatments need to be assessed. A within-trial economic evaluation was undertaken that reflected the objectives of the trial and the short follow-up period.
A simple decision tree was deemed to be the most suitable way of presenting the alternative patient pathways and synthesising the available data. The model was necessary in order to analyse alternative patient pathways, particularly in the sensitivity analyses, and collate available data. A decision-analytic model involves using mathematical relationships to set out the consequences that are associated with the different policy options under consideration. 38 This allows the costs and outcomes for each option to be evaluated, taking into account the probability of the consequences. Data on resource use and costs were collected prospectively via trial reporting mechanisms and additional data were sourced from the literature.
Model structure
A decision tree model was developed using TreeAge Pro 2016 (TreeAge Software, Inc., Williamstown, MA, USA). The structure was informed by the trial objectives and patient pathways were indicated by the clinical data. As shown in Figure 15, participants entered the model at the point of randomisation to receive the alternative treatment (gentamicin) or the standard treatment (ceftriaxone). Following the initial course of antibiotic treatment, participants either received additional NHS care [e.g. general practitioner (GP) visit] or did not access care. At 2 weeks post treatment, participants either were cleared of N. gonorrhoeae at all sites, confirmed by a negative NAAT, or were not cleared and required further treatment.
FIGURE 15.
Model structure.
If clearance of N. gonorrhoeae at all sites was not confirmed (at 2 weeks post treatment), participants were treated with a further course of antibiotics. For the purposes of the economic evaluation, the base-case analysis followed current guidelines and it was assumed that all participants who were not cleared of infection would be treated with a course of ceftriaxone. However, in the sensitivity analyses, alternative scenarios were also explored, including the use of alternative antibiotic treatments when participants were not cleared of the infection at all sites.
Clinical data
The primary outcome of the economic evaluation reflected the primary outcome of the trial – the clearance of N. gonorrhoeae at all infected sites (confirmed by a negative NAAT) at 2 weeks post treatment (Table 26). The primary approach to analysis was ITT without imputation of missing outcome data, as specified in the SAP. Data were also collected on whether or not participants required further NHS care following the initial treatment.
| Treatment group description | Trial data, n/N | Probability | Distribution |
|---|---|---|---|
| Ceftriaxone | |||
| Requiring NHS treatment after the initial visit | 10/322 | 0.03 | Beta |
| Not requiring NHS treatment after the initial visit | 312/322 | 0.97 | Beta |
| Clearance of N. gonorrhoeae | 299/306 | 0.98 | Beta |
| Not cleared of N. gonorrhoeae | 7/306 | 0.02 | Beta |
| Gentamicin | |||
| Requiring NHS treatment after the initial visit | 8/302 | 0.03 | Beta |
| Not requiring NHS treatment after the initial visit | 294/302 | 0.96 | Beta |
| Clearance of N. gonorrhoeae | 267/292 | 0.91 | Beta |
| Not cleared of N. gonorrhoeae | 25/292 | 0.09 | Beta |
Treatment costs
Trial participants allocated to the gentamicin arm received 240 mg of gentamicin as a single i.m. injection. This was obtained from three 80-mg ampoules, with an estimated cost of £1 per ampoule [£3 overall, estimated using the British National Formulary (BNF)],39 as shown in Table 27. Participants allocated to the ceftriaxone arm received a 500-mg dose. Ceftriaxone was purchased in units of 1 g and mixed with 4 ml (1%) of lidocaine solution. Only half of the preparation was administered to the participant (half was discarded); we therefore included costs for one vial of 1 g of ceftriaxone powder (£1.10) per participant and two 2-ml lidocaine ampoules (£0.22 per ampoule). 39 Hence, the initial costs associated with gentamicin treatment were higher than those associated with the current standard treatment, ceftriaxone. Other equipment (such as syringes) would be required by both arms of the trial and so these costs were not included. In both trial arms, participants received a single oral dose of azithromycin, an initial consultation with a health-care professional and a follow-up visit. These costs were excluded from the analysis as they were incurred equally across the trial arms.
| Resource use | Cost item | Base-case value (£) | Distribution | Source |
|---|---|---|---|---|
| Gentamicin treatment | Per participant | 3.00 | Gamma | BNF39 |
| Ceftriaxone treatment | Per participant | 1.54 | Gamma | BNF39 |
To check that there was no difference in the length of time required for delivery of the treatment therapy, a survey was undertaken with research nurses involved in the delivery of the two treatments. The survey aimed to check whether or not the delivery of the treatment and the length of the consultation were affected by the drug that was being administered. The survey received responses from 21 nurses who were administering injections. The majority of nurses (17 respondents, 81%) stated that there was no difference in the time taken to administer the two treatments. Just four nurses (19%) responded that there was a difference, all of whom stated that appointments to administer gentamicin were longer than those to administer ceftriaxone. The main reason given for the longer time needed to administer gentamicin was the increased pain associated with gentamicin injections (given as a reason by three of the nurses who felt that gentamicin took longer to administer). Two nurses stated that appointments to administer gentamicin took 0–5 minutes longer and two stated that an additional time of up to 10 minutes was needed. Given the survey results, for the base-case analysis additional time was not included to administer the gentamicin treatment; however, increased time was explored as part of the sensitivity analysis.
NHS resource use and costs incurred after initial treatment
NHS resource use by participants was collected during the trial at all participating sites using a form completed by a nurse/assessor. Data were collected on whether or not participants had visited their GP/nurse at their general practice, a GP out-of-hours service, health professionals at a sexual health clinic or an accident and emergency (A&E) department and on the use of other services. Data were also collected on whether or not participants were prescribed other medication (other than the trial treatment). In addition, details of any AEs and associated resource use were recorded. Unit cost estimates were applied to resource use data to generate individual-level cost estimates. The sources of unit costs included routine and published literature (e.g. Unit Costs of Health and Social Care 201640).
Resource use was similar across trial arms, with the main resources that were used relating to GP visits and sexual health centre visits (Table 28). No participants reported that they had been hospitalised during the trial or attended A&E.
| Resource use | Cost item | Unit cost (£) | Treatment group (n) | Total cost (£) | ||
|---|---|---|---|---|---|---|
| Ceftriaxone | Gentamicin | Ceftriaxone | Gentamicin | |||
| GP consultation | Per visit | 36.00 | 6 | 3 | 216.00 | 108.00 |
| Sexual health clinic: health advisor consultation | Per visit | 93.00a | 1 | 2 | 93.00 | 186.00 |
| Sexual health clinic: doctor consultation | Per visit | 130.00b | 5 | 4 | 650.00 | 520.00 |
| NHS 111 call | Per call | 6.10c | 1 | 1 | 6.10 | 6.10 |
| Total costs | 965.00 | 820.10 | ||||
| Total number of patients accessing additional treatment | 10 | 8 | ||||
| Total cost per patient accessing additional treatment | 95.60 | 102.51 | ||||
Further treatment costs owing to non-clearance of infection
Within the trial, when infection was not cleared at all sites (as indicated by a NAAT), further treatment was given. Participants in the gentamicin arm were assumed to be treated with ceftriaxone (unless there was a contraindication for this treatment). Participants in the ceftriaxone arm were assumed to have been given a second course of ceftriaxone unless the culture demonstrated resistance to this treatment. For the purposes of the economic evaluation, it was assumed that in both trial arms, when infection was not cleared at 2 weeks post treatment, a sexual health clinic appointment would be needed and that a course of the standard treatment (ceftriaxone) would be given (Table 29). This was to reflect current guidelines;8 other scenarios were explored in the sensitivity analysis.
| Resource use | Cost item | Unit cost (£) | Source |
|---|---|---|---|
| Sexual health clinic: nurse/health advisor consultation | Per visit | 93.00a | NHS Reference Costs 2015 to 2016 41 |
| Second course of antibiotic treatment | Per participant | 1.54b | NHS Reference Costs 2015 to 2016 41 |
| Total costs | 94.54 |
Analysis
The within-trial analysis took the form of a CEA, with results reported in terms of the cost per participant successfully treated (measured in terms of microbial clearance of N. gonorrhoeae at all infected sites, 2 weeks post treatment). As the trial was concerned with the immediate post-treatment period of 2 weeks, discounting was not undertaken. All costs are given in Great British pounds for 2015/16.
Sensitivity analysis
Both deterministic and probabilistic sensitivity analyses were undertaken to explore the uncertainty in the parameter estimates on the results produced by the model. 42 In the deterministic sensitivity analysis, one or more parameters were varied while keeping the others at their baseline value. Deterministic analysis can help to identify which values are important in leading to a particular decision and can help to identify threshold values. 43,44
A range of deterministic sensitivity analyses was carried out. First, the cost of the interventions was varied to reflect the purchase of different solutions/equipment based on prices reported in the BNF. 39 Second, the costs of additional treatment for those without clearance of N. gonorrhoeae was varied to take into account different scenarios for the development of AMR in gonorrhoea. The cost was increased to reflect a scenario in which two further treatments of ceftriaxone were given and two follow-up visits to a clinic. Third, the rates of clearance for gonorrhoea were varied to reflect different rates at different sites, using the lowest and highest 95% CIs reported in Table 17 for different sites. Fourth, the time taken to administer the gentamicin treatment was varied to reflect the responses of a minority of nurses in the survey who indicated that gentamicin injections took longer to administer. The cost of treatment was increased to take into account a scenario in which an additional 10 minutes would be needed to administer gentamicin than to administer ceftriaxone, assuming that the cost of the time of the nurse administering the treatment would be equivalent to that of a GP nurse. 40
A probabilistic sensitivity analysis (PSA) was also undertaken to allow uncertainty to be explored more comprehensively. A PSA involves varying all parameters simultaneously and sampling multiple sets of parameter values from defined probability distributions. A Monte Carlo simulation was used to sample from the distributions; this involved 1000 repeated random draws to analyse how variation in the parameters used in the model would affect the results. Beta distributions were used for binomial data and gamma distributions were used for costs, in line with recommendations for specifying distributions for parameters. 42
Results
The results of the analysis are shown in Table 30. A higher proportion of participants treated with ceftriaxone were cleared of infection with N. gonorrhoeae (98%) than participants treated with gentamicin (91%). The average participant costs associated with gentamicin were higher than those associated with ceftriaxone. The main difference in costs related to the need for additional consultations and treatment for participants who were not successfully cleared of infection at all sites (at 2 weeks post treatment). Hence, the analysis found that treatment with gentamicin is not non-inferior to ceftriaxone and that it is not cost neutral. The average cost per participant treated was £13.90 for gentamicin, compared with £6.72 for ceftriaxone.
| Treatment group | Average cost (£) per participant (95% CI) | % cleared of infection at 2 weeks post treatment | ICER |
|---|---|---|---|
| Ceftriaxone | 6.72 (1.36 to 17.84) | 98 | Dominates |
| Gentamicin | 13.90 (2.47 to 37.34) | 91 |
The results of the one-way deterministic sensitivity analyses are shown in Table 31. First, the results arising from varying the costs associated with the antibiotic treatments are presented. The costs associated with antibiotics might increase if penalties were introduced to discourage their use to slow down the development of AMR. As expected, increasing the costs of the treatments increased the overall costs per participant treated in both arms. Second, results from increasing the costs of additional treatment for those without clearance of N. gonorrhoeae 2 weeks after treatment are shown. It is evident that this increased overall costs for both trial arms, but particularly for the gentamicin arm as a result of the higher proportion of participants in this arm who were not cleared of infection. The results for the third scenario show that varying the rates of clearance to reflect the results at different sites (see Table 31) affects the relative cost-effectiveness of the treatments. If the rates for clearance of genital infection are used, ceftriaxone still dominates, but the difference in cost per participant between the groups is smaller. Clearance rates for gentamicin would need to be higher than those for ceftriaxone for gentamicin to be cost neutral, owing to the higher costs associated with gentamicin treatment. In the final scenario, increasing the costs associated with administering the gentamicin treatment led to increased costs for the gentamicin arm.
| Deterministic sensitivity analysis scenarios | Value | Treatment group, average cost per participant (£) | ||
|---|---|---|---|---|
| Original | Revised | Ceftriaxone | Gentamicin | |
| Base case | – | – | 6.72 | 13.90 |
| (1) Varying the cost (£) of antibiotics | ||||
| Gentamicin | 3.00 | 1.54–12.00 | 12.44–22.90 | |
| Ceftriaxone | 1.54 | 1.00–9.58 | 6.15–14.92 | |
| (2) Increasing the cost (£) of additional treatment for those without clearance of N. gonorrhoeae | 94.54 | 247.62 | 10.20 | 26.92 |
| (3) Varying the rates of clearance of N. gonorrhoeae | ||||
| Gentamicin arm (%) | 91 | 72–97 | 32.55–8.66 | |
| Ceftriaxone arm (%) | 98 | 92–100 | 10.16–4.54 | |
| (4) Increasing the cost (£) of gentamicin treatment (longer consultation time) | 3.00 | 9.00 | 19.90 | |
The scatterplot in Figure 16 shows the results of the PSA involving 1000 simulations. It is evident that the majority of the simulations generated did not show that gentamicin was non-inferior to ceftriaxone and that the costs of gentamicin were shown to be higher. A large proportion of the points are in the north-west quadrant, indicating that treatment with ceftriaxone dominates treatment with gentamicin (gentamicin is not shown to be non-inferior and is unlikely to be cost neutral).
FIGURE 16.
Incremental cost-effectiveness scatterplot for clearance of infection: gentamicin vs. ceftriaxone.
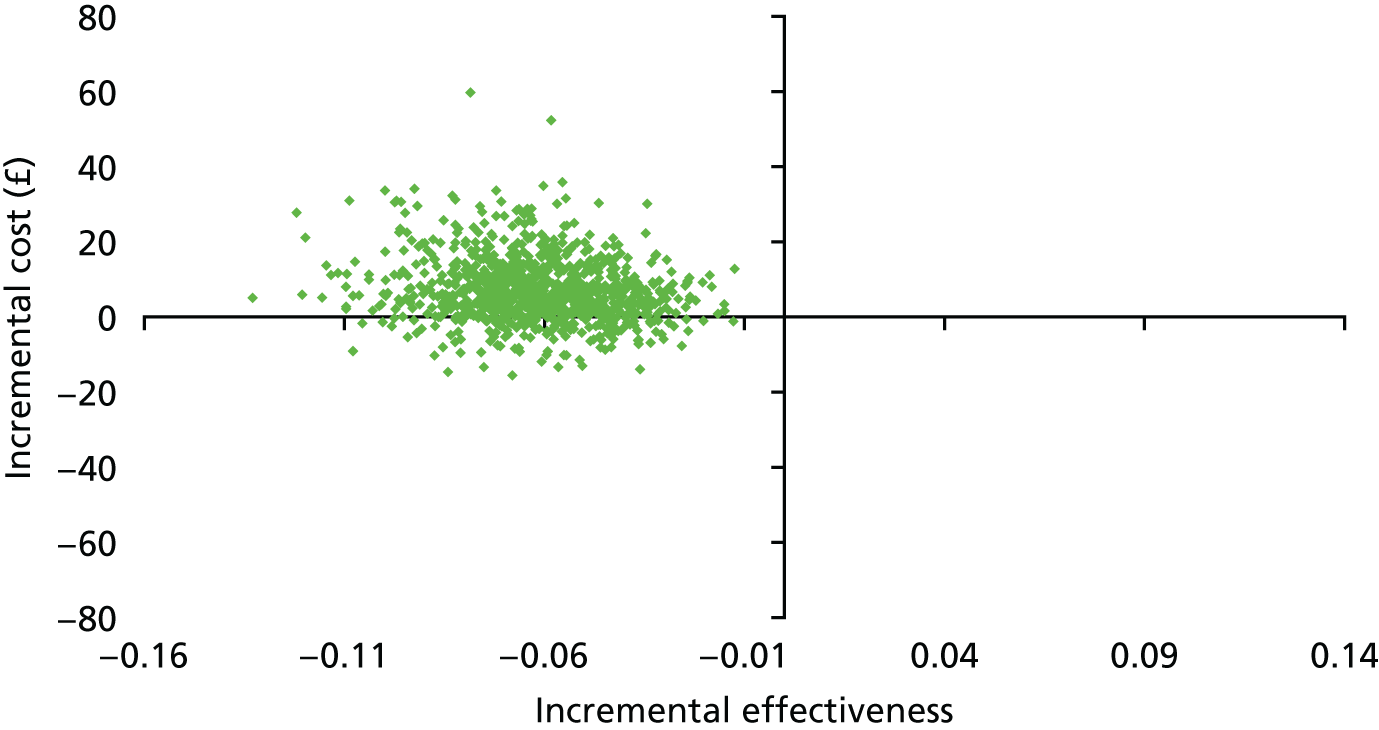
Discussion
The results show that gentamicin is likely to be more costly than ceftriaxone in the treatment of gonorrhoea. Currently, it is not shown that gentamicin is non-inferior to ceftriaxone in the treatment of gonorrhoea, nor is there evidence that it is cost neutral.
A major limitation of the economic analysis is that it was necessarily restricted because it was not possible to fully take into account the potential costs associated with AMR in gonorrhoea, nor the potential value associated with preserving the effectiveness of ceftriaxone. These issues were outside the scope of the current trial but may warrant further investigation. A strength of the economic evaluation is that it draws on prospective data collected during the RCT. Although assumptions were made about treatment strategies when infection was not cleared, these were based on published clinical guidelines.
There is currently a lack of evidence about how economic evaluations should be undertaken for interventions addressing AMR. 45 Very few economic evaluations have been undertaken that assess different antibiotic strategies in the context of developing AMR. 46,47 The costs associated with AMR in gonorrhoea require further analysis and there is an urgent need to develop appropriate methods for economic evaluations of interventions to address AMR in gonorrhoea and other disease areas. 48
Chapter 5 Discussion
The main trial finding is that single-dose gentamicin (240 mg) is not shown to be non-inferior to single-dose ceftriaxone (500 mg) for the treatment of gonorrhoea, when both drugs are combined with a single dose of oral azithromycin (1 g). The trial was not designed to assess superiority, but the magnitude of the risk difference (–6.4%, 95% CI –10.4% to –2.4%) and the consistency of the findings on sensitivity analyses suggest that gentamicin may be less effective than ceftriaxone for the microbiological cure of gonorrhoea. The difference in efficacy was most marked for pharyngeal gonorrhoea (risk difference –15.3%, 95% CI –24.0% to –6.5%) and rectal gonorrhoea (risk difference –7.8%, 95% CI –13.6% to –2.0%). For genital gonorrhoea, gentamicin achieved microbiological cure in 94% of infections compared with a 98% clearance rate for ceftriaxone (risk difference –4.4%, 95% CI –8.7% to 0.0%).
Both ceftriaxone and gentamicin were generally well tolerated. Nausea was the most commonly reported side effect of treatment, occurring in 12% of participants receiving ceftriaxone and 14% receiving gentamicin. Few participants reported vestibulocochlear symptoms; these symptoms, when reported, occurred similarly between treatment arms: reduction in hearing in 2% of participants receiving ceftriaxone and 1% receiving gentamicin and dizziness or unsteadiness in 7% (ceftriaxone) and 7% (gentamicin). The proportion of participants with either clinically important changes in creatinine level from baseline or a creatinine level exceeding the upper limit of normal was similar in both groups; a high/increased or abnormal creatinine level was recorded as an AE for only seven participants. All AEs were mild and in three out of the seven cases they were thought by the local principal investigator (PI) to be related to treatment. The mean change in eGFR [median (25th percentile, 75th percentile)] was similar in the two treatment groups and not considered of clinical importance, being –1.3 ml/minute/1.73 m2 [median (–6.7 ml/minute/1.73 m2, 4.3 ml/minute/1.73 m2)] for the ceftriaxone group and –1.4 ml/minute/1.73 m2 [median (–6.9 ml/minute/1.73 m2, 3.8 ml/minute/1.73 m2)] for the gentamicin group. Almost all participants receiving either ceftriaxone or gentamicin reported injection site pain (98% and 99%, respectively) but the severity of the pain was less for ceftriaxone (median VAS score immediately after injection 15.0) than for gentamicin (median VAS score 31.5). Injection site pain resolved within a median of 1 hour (IQR 0–12 days) for ceftriaxone and 1.5 hours (IQR 0–24 days) for gentamicin.
A single SAE occurred in a participant who developed severe dizziness 4 days after receiving ceftriaxone. On subsequent review, he gave a history of eating biscuits containing cannabis shortly before developing these symptoms.
Efficacy of gentamicin for the treatment of gonorrhoea
The trial was designed to assess the efficacy of gentamicin compared with ceftriaxone and the primary end point was microbiological cure 14 days after treatment. There are a number of factors that could affect the response to gentamicin treatment.
Antimicrobial resistance
The mechanisms leading to gentamicin resistance have not been fully elucidated but include reduced uptake into infected cells as a result of decreased cell membrane permeability to the antibiotic49 or modification of the drug by cellular enzymes, which reduce its activity. 50 For spectinomycin, which is in the same drug class of aminoglycosides and has a similar mechanism of action, mutations within 16S ribosomal ribonucleic acid that inhibit binding of the drug to the ribosome have been associated with high-level resistance. 51 In vitro measurement of the MIC for gentamicin in cultured isolates of N. gonorrhoeae provides a phenotypic assessment of antimicrobial susceptibility, but the ‘breakpoint’ MIC value, below which clinical cure occurs and above which treatment is ineffective, has not been established. It has been tentatively suggested that an isolate with a MIC of < 8 mg/l is susceptible, with a MIC of 8–16 mg/l is intermediate and with a MIC of > 16 mg/l is resistant. 29,52 The European Network for STI Surveillance found that 95% of isolates had gentamicin MICs in the range of 4–8 mg/l,27 using the agar dilution technique. Isolates of N. gonorrhoeae from participants in the G-TOG trial had a similar susceptibility profile, with 70% having a MIC of 4–8 mg/l using the Etest technique.
Previously proposed MIC ‘cut-offs’ points associated with AMR in N. gonorrhoeae for gentamicin, ceftriaxone and azithromycin are shown in Table 32.
| Drug | Proposed MIC cut-off points (mg/l) | |||
|---|---|---|---|---|
| UK Gonococcal Resistance to Antimicrobials Surveillance Programme3 | European Committee on Antimicrobial Susceptibility Testing53 | US Centers for Disease Control and Prevention54 | Other29,52 | |
| Gentamicin | – | – | – | > 16 |
| Ceftriaxone | > 0.064a | > 0.12 | > 0.25 | – |
| Azithromycin | > 0.5 | > 0.5 | – | – |
The measurement of MICs requires a positive N. gonorrhoeae culture sample. Participants in the G-TOG trial had samples taken for culture at their baseline attendance and 2-week follow-up but culture is less sensitive than NAAT, particularly for extragenital sites, and, therefore, the data on in vitro susceptibility (using MICs) are limited to the subset of individuals who had a positive culture sample available. For genital infections, culture isolates were available for 356 out of 402 (88%) NAAT-positive samples at baseline; for rectal infections, this number was 146 out of 301 (47%) and for pharyngeal infections it was 88 out of 256 (34%).
Caution is, therefore, required in the interpretation of the antibiotic susceptibility profiles, which represent only a subgroup of the overall trial population, especially for those with pharyngeal and rectal infections. Overall, the culture-positive and culture-negative groups were similar with respect to age, sex and ethnicity, although there was a greater proportion of males among those with no culture result available (94% compared with 79% in the culture-positive group and 78% in the culture-negative group) and there were fewer white participants (59% compared with 66% and 74% in the culture-positive and culture-negative groups, respectively).
The extent of tissue penetration of different antimicrobials at the genital, rectal and pharyngeal sites is not known, nor is the extent of interindividual variation in tissue drug levels. This makes it difficult to interpret how the MIC values measured in vitro relate to the relative antibiotic susceptibility of gonococcal isolates in individual trial participants.
There did not appear to be any association between the in vitro gentamicin MIC and response to treatment.
The structurally similar antibiotic spectinomycin has been reported to be less effective for the treatment of pharyngeal gonorrhoea,55 probably because it fails to achieve bactericidal concentrations within the infected tissues for a sufficiently long time period to clear infection. 5 It is possible that this mechanism is also relevant for gentamicin.
Participants in both treatment groups received oral azithromycin and treatment failure was associated with a reduction in in vitro susceptibility (shift of the azithromycin MIC distribution to the right). Overall, treatment failure occurred in 14 out of 262 (5%) participants who had isolates with an azithromycin MIC of ≤ 0.5 mg/l compared with 2 out of 12 (17%) participants who had isolates with an azithromycin MIC of ≥ 1 mg/l.
In summary, we did not find that gentamicin MICs, as assessed by in vitro antimicrobial susceptibility testing, accurately predicted treatment failure. This suggests that in vitro laboratory assessment of MICs for gentamicin is not likely to be helpful in selecting those patients with gonorrhoea who can be successfully treated with gentamicin.
Reinfection
If a patient is reinfected after treatment, then their follow-up test for gonorrhoea will be positive even after successful initial therapy. If this occurs following sex with an existing infected partner, then it is likely that the same subtype of N. gonorrhoeae will be present at baseline and follow-up. If sexual contact was with a new partner, then the same subtype of N. gonorrhoeae could be present at follow-up (e.g. if this is a common subtype within the population) or a different subtype may occur.
Sexual contact following treatment for gonorrhoea was well balanced between the treatment groups: it was reported by 37% (118/322) of participants receiving ceftriaxone [15% (47/322) of whom reported not using condoms] and 34% (104/302) receiving gentamicin [16% (47/302) of whom reported not using condoms]. For both groups, a median of two episodes of sexual contact occurred in the interval between treatment and follow-up. There were similar clearance rates for participants who had and those who had not had sex following randomisation, and these rates appeared to be similar between treatment groups.
Reinfection of participants with gonorrhoea is, therefore, not likely to explain the difference in treatment success between participants receiving ceftriaxone and those receiving gentamicin.
Interval between treatment and follow-up assessment
Microbiological cure following treatment was assessed using NAAT and occurred at ≤ 21 days in 81% of participants in both treatment groups. The median time to assessment of cure did not differ between the two treatment groups (ceftriaxone 16 days, gentamicin 15 days). The median time to follow-up was 15 days from randomisation for those who were cleared of infection and 15.5 days for those who were not cleared of infection.
Persistence of bacterial nucleic acid following effective treatment, even in the absence of viable bacteria, has the potential to cause false-positive test results. Current UK national guidance is to assess cure by performing a NAAT 2 weeks after treatment, by which time a false-positive result is unlikely. 56–60 It has been suggested that pharyngeal infection may take longer to clear following treatment, with a greater possibility of a false-positive result 2 weeks after therapy. 61 The high treatment success rate at all anatomical sites in participants receiving ceftriaxone [genital 98% (151/154), pharyngeal 96% (108/113) and rectal 98% (134/137)] suggests that persistence of bacterial nucleic acid (in the absence of viable bacteria) leading to false-positive results did not occur frequently in the G-TOG trial participants. However, the pharmacodynamics of gentamicin for the treatment of pharyngeal gonorrhoea are largely unknown and it remains possible that the rate of bacterial clearance may be slower with gentamicin than with ceftriaxone.
Negative interaction between gentamicin and azithromycin
An antagonistic interaction between gentamicin and azithromycin could potentially reduce the efficacy of the combination of these two drugs. However, in vitro testing suggests that there is neither antagonism nor synergy when the two antibiotics are combined;62 furthermore, a recent trial using this combination demonstrated the potential for a high cure rate using dual therapy, at least in a subset of patients with genital infection diagnosed by culture. 25 An antagonistic interaction between gentamicin and azithromycin would, therefore, be an unlikely explanation for the difference in cure rates between ceftriaxone and gentamicin.
Protocol deviations
The proportion of participants reporting protocol deviations was similar between treatment groups and most deviations were considered to be minor. There was an imbalance in the proportion of participants who did not receive their randomised medication. However, it was considered unlikely that this was as a result of selection bias or knowledge of the treatment allocation. It is therefore believed that the protocol deviations did not affect the validity of the trial.
Results in context
Two systematic reviews23,24 have reported wide variation in the efficacy of gentamicin for the treatment of gonorrhoea and highlighted the low quality of the previous studies, which had a significant risk of bias. A more recent high-quality non-comparative trial evaluated i.m. gentamicin (240 mg) combined with oral azithromycin (2 g) and reported a cure rate of 100% (lower 95% CI 98.5%). 25 The design of this trial is compared with that of the G-TOG trial in Table 33. An updated literature search (see Appendix 2) was performed but no further studies evaluating gentamicin for the treatment of gonorrhoea were identified.
| Design | Study | |
|---|---|---|
| Kirkcaldy et al.25 | G-TOG trial | |
| Number of participants assigned to gentamicin treatment | 309 | 358 |
| Number of participants receiving gentamicin who were included in the primary analysis | 157 | 292 |
| Gentamicin dose (mg) | 240 | 240 |
| Azithromycin dose (g) | 2 | 1 |
| Diagnostic criteria | Positive culture for N. gonorrhoeae | Positive NAAT for N. gonorrhoeae or positive Gram stain on microscopy |
| Primary end point | Negative culture 10–17 days after treatment | Negative NAAT 14 days after treatment |
| Number of participants with pharyngeal infection receiving gentamicin | 10 | 128 |
| Number of participants with rectal infection receiving gentamicin | 1 | 147 |
There are a number of possible explanations for the higher cure rate reported by Kirkcaldy et al. 25 In the diagnosis of gonorrhoea, culture is less sensitive (≈80%) than NAAT; this is most apparent for pharyngeal infections (30% sensitivity) and rectal infections (50% sensitivity). 63,64 This reduced ability to isolate extragenital N. gonorrhoeae is likely to be the reason for the small number of pharyngeal and rectal infections that met the inclusion criteria for the Kirkcaldy et al. 25 study.
In the G-TOG trial, we found the efficacy of gentamicin to be higher for genital infections (94%) than for extragenital infections (pharynx 80% and rectum 90%), which may partially explain the higher overall cure rate in the Kirkcaldy et al. 25 study (100%, 95% CI 98.5% to 100%), in which the majority of infections were genital.
There is also a theoretical possibility that the lower sensitivity of culture could fail to identify persistent infection following unsuccessful treatment if transient suppression of N. gonorrhoeae occurred (below the level detectable by culture) but without cure.
The role of azithromycin in the treatment of gonorrhoea
Participants in the G-TOG trial received dual treatment: 1 g of azithromycin combined with either ceftriaxone or gentamicin. Azithromycin has previously been shown to be effective for the treatment of gonorrhoea using a single dose of either 1 g or 2 g,65–68 although these previous studies used culture to diagnose infection and assess cure, which, as outlined in the previous section, is less sensitive than NAAT. Azithromycin resistance and treatment failure has subsequently been reported in a number of geographical locations. 69–73 In the UK, azithromycin-resistant N. gonorrhoeae has been detected intermittently since 2004,74,75 with an ongoing outbreak of high-level azithromycin-resistant isolates identified in England since 2014. 76 The Gonococcal Resistance to Antimicrobials Surveillance Programme reported that 10% of gonococcal isolates in England and Wales had a MIC of > 0.5 mg/l in 2015, indicating probable resistance. 3
In the G-TOG trial, participant treatment failure occurred in 6% of genital infections, 10% of rectal infections and 20% of pharyngeal infections in those who received gentamicin plus azithromycin, suggesting that oral azithromycin given as a 1-g dose may be suboptimal, particularly for extragenital gonorrhoea. The large majority of gonococcal isolates from participants in the G-TOG trial [290/305 (95%)] had azithromycin MICs within the non-resistant range (≤ 0.5 mg/l). Of the 15 isolates with a MIC of > 0.5 mg/l, two (13%) were from participants who had treatment failure. The majority of treatment failures overall [14/20 (70%)] occurred in participants who had isolates with a MIC of ≤ 0.25 mg/l. Sixty participants harboured an isolate with an azithromycin intermediate MIC of 0.5 mg/l, of whom four (7%) had treatment failure. Thus, we found in vitro azithromycin resistance was only partially predictive of treatment failure. The limited association between MIC and treatment efficacy has been reported by others. 77,78 It is possible that a higher dose of azithromycin (2 g), as was used in the Kirkcaldy et al. 25 study, would have been more effective, although without a direct comparative study this is speculative, especially for the treatment of pharyngeal gonorrhoea.
The use of a 1-g dose of azithromycin as monotherapy has also been reported to potentially induce resistance in N. gonorrhoeae, with a substantial increase in the MIC following treatment. 79,80
The use of a 2-g dose of azithromycin combined with gentamicin was associated with a high incidence of gastrointestinal side effects in the Kirkcaldy et al. 25 study, with nausea reported by 26% of participants and vomiting by 10%. In the G-TOG trial, the 1-g dose was better tolerated: 14% of participants reported nausea and 4% reported vomiting, which is consistent with previous studies. 68 An extended-release formulation of azithromycin is available that may reduce the incidence of side effects and improve tolerability but there are limited data directly comparing its AE profile with that of the immediate-release formulation. 81,82
The current UK and US gonorrhoea treatment guidelines recommend dual therapy with a regimen containing oral azithromycin as a 1-g single dose. 6 Our findings, which are the first to use the more sensitive NAATs to assess microbiological clearance, suggest that this component of treatment may be suboptimal, particularly for extragenital gonococcal infections.
Injection site pain
Participants receiving gentamicin reported more severe injection site pain than those receiving ceftriaxone (median VAS score 31.5 and 15.0, respectively), with resolution of pain occurring within a median of 1 hour for ceftriaxone and 1.5 hours for gentamicin. The site of injection and the needle gauge were not prespecified in the trial but would usually be the same for both antibiotics. Ceftriaxone is manufactured as a powder preparation that is reconstituted with lidocaine, resulting in an injection volume of approximately 2 ml for a 500-mg dose. 83 Gentamicin is manufactured as a solution: a 240-mg dose equates to an injection volume of 6 ml. 84 It is likely that this larger volume of injection led to participants who received gentamicin reporting more severe local site pain. In addition, the local anaesthetic effect of lidocaine in those receiving ceftriaxone is likely to have reduced the discomfort following injection.
Safety
Ceftriaxone and gentamicin were generally well tolerated; with a similar proportion of AEs reported in both treatment arms. Gastrointestinal side effects were the most commonly reported: nausea was reported by 12% (38/320) of those who received ceftriaxone and 14% (41/298) of those who received gentamicin. The majority of these side effects were classified as grade 1 (able to eat normally), with only 2% in each treatment group (ceftriaxone 8/320, gentamicin 5/298) being classified as grade 2 (oral intake significantly decreased). There were no grade 3 events (no significant oral intake or requiring intravenous fluids) and nausea resolved within a median of 3 hours following treatment in both treatment groups. Vomiting was reported by 1% (3/320) of those who received ceftriaxone and 4% (12/298) of those who received gentamicin.
Ceftriaxone and gentamicin are licensed medications that have well-recognised safety profiles. Nausea and vomiting are uncommon side effects of ceftriaxone (range of incidence rate: ≥ 1/1000 to < 1/100)83 and have been reported in association with gentamicin,84 but are common following the use of oral azithromycin (≥ 1/100 to < 1/10). 85 It is, therefore, probable that the gastrointestinal side effects reported were principally caused by azithromycin, although the higher reported rate of vomiting in those receiving gentamicin (4%, compared with 1% for ceftriaxone) suggests that gentamicin may also have been a contributing factor.
Dizziness or unsteadiness was reported in 7% of participants who received their allocated treatment (ceftriaxone 24/302, gentamicin 21/298) but was classified as grade 1 (not interfering with function) in the large majority (ceftriaxone 20/24, gentamicin 19/21). One participant who received ceftriaxone reported grade 4 dizziness (bedridden or disabled).
Dizziness is uncommon (≥ 1/1000 to < 1/100) following treatment with azithromycin or ceftriaxone. 83,85 Gentamicin is potentially vestibulotoxic;86 it can cause damage to the vestibular apparatus, initially affecting the cristae and progressing to the striolar regions of the maculae,87 and can cause dizziness, ataxia and nystagmus. Most gentamicin studies have used a prolonged course of treatment and the safety of a single dose is less well characterised, but a recent systematic review of single-dose therapy found vestibulotoxicity to be rare,88 which is consistent with the G-TOG trial findings.
Gentamicin can also cause renal impairment following reuptake of the drug in the proximal renal tubule, where it leads to a locally high renal drug concentration. 89 The recent review of AEs of gentamicin reported that a transient rise in creatinine was common following a single dose of gentamicin, although many of the relevant studies were in elderly, surgical patients, which may not be directly applicable to the G-TOG trial population. 88 In the G-TOG trial participants, we found a median change in eGFR that was similar in both treatment groups [ceftriaxone –1.3 ml/minute/1.73 m2 (IQR –6.7–4.3 ml/minute/1.73 m2), gentamicin –1.4 ml/minute/1.73 m2 (IQR –6.9–3.8 ml/minute/1.73 m2)]. This magnitude of change is not considered to be of clinical importance. A small number of participants (2%) experienced a change of > 30% in creatinine level following treatment but, despite this, remained within or just above the upper limit of normal.
The results of the trial were presented to the PPI representatives and their feedback was sought. They considered the study results to be reassuring regarding the safety of gentamicin and that using gentamicin as a second-line therapy in those unable to receive ceftriaxone would be acceptable.
Strengths and limitations
The G-TOG trial was delivered through a registered clinical trials unit and had a robust design resulting in well-balanced treatment arms and a low risk of bias. The trial was pragmatic in design and likely to be relevant to clinical practice in the UK and other countries with similar health-care systems. It included symptomatic and asymptomatic patients, patients with a wide age range, HIV-positive and HIV-negative individuals, men and women, heterosexual men and MSM and a wide variety of ethnic groups, which provides generalisability for our findings. Recruitment was completed to time and target and the sample size was large enough to provide a clear result.
The potential for treatment unblinding was low. It is possible that a participant who had previously received treatment with ceftriaxone (41% of participants were known to have had at least one previous episode of gonorrhoea) may have recognised their therapy and that this could have affected the reporting of subjective outcomes. However, both treatments were given by injection and we would consider it unlikely that participants distinguished between treatments based on their preparation or administration. The reporting of side effects was similar in both treatment arms. We were not aware of any clinicians being unblinded.
The trial design allowed for a loss to follow-up of ≤ 10% of participants (90% of participants were to be available for analysis) but data were available for analysis of the primary end point in only 85% (306/362) of participants allocated to ceftriaxone and 82% (292/358) of those allocated to gentamicin. The sample size for the trial had been calculated to provide 90% power and it was felt that, despite fewer participants having data for the primary outcome measure, the result was robust as all sensitivity analyses were supportive. The G-TOG trial was designed and powered as a non-inferiority trial, which prevents us making a firm conclusion about the superiority of ceftriaxone, but the size of the risk difference and consistency of our findings in secondary sensitivity analyses suggest that it is likely that gentamicin is less effective than ceftriaxone. The pre-trial estimate of 10% for loss to follow-up was based on a patient return rate of 80–85% in routine clinical practice, with an expectation that with closer monitoring, visit reminders and a monetary incentive this could be increased to 90%. Our experience suggests that during trial design greater allowance should have been made for incorrect sampling at follow-up when estimating the number of patients with available primary end-point data.
The main factor limiting recruitment was research capacity within sexual health centres, for example availability of research nurses and experienced PIs, which was exacerbated by widespread structural reform in the clinical service that occurred during the time of the G-TOG trial. This resulted in some centres not meeting their pre-trial estimates for recruitment. The trial has demonstrated a need to improve engagement in research and increase research expertise in the area of sexual health.
Unexpectedly, a number of participants who were recruited to the trial were found to have a negative AC2 NAAT at their baseline visit [ceftriaxone arm 12% (45/362); gentamicin arm 12% (42/358)]. These were all individuals who had been previously tested and found to be positive on NAAT, but who had not yet received treatment and had been recalled to the clinic to be given antibiotic therapy. In normal clinical practice, a repeat NAAT would not be performed before treatment if a previous test result was positive. The apparently spontaneous reversion from positive to negative NAAT observed in some trial participants could result from one of the following:
-
An initial false-positive NAAT before trial entry. NAAT for N. gonorrhoeae has a high specificity, particularly for genital specimens, which makes this unlikely. 90
-
A false-negative NAAT at the baseline trial visit. NAAT for N. gonorrhoeae has a high sensitivity, which makes this also unlikely. 90
-
Natural clearance of gonorrhoea without antibiotic therapy. It is not known how often this occurs because it would be unethical to knowingly leave gonorrhoea untreated and, as noted above, repeat testing following a positive test result before treatment is not routine practice. In one natural history study, none of 16 women with gonorrhoea had spontaneous clearance over a 5- to 11-week period. 91 In contrast, van Liere et al. 92 reported natural clearance of 20% (5/25) of gonococcal infections in a median interval of 11 days between initial testing and returning for follow-up.
The occurrence of 12% of participants with negative tests at their baseline visit does not bias our results since they were equally distributed between the treatment arms. A lack of bias is also supported by the secondary sensitivity analysis that excluded these participants and that was consistent in demonstrating that gentamicin was not non-inferior to ceftriaxone (risk difference –7.1%, 95% CI –11.4% to –2.8%).
The efficacy of antibiotic therapy for gonorrhoea varies over time as different resistant subtypes of infection develop and circulate within a population. Our findings are therefore applicable only to the UK or similar settings at the present time; any future interpretation will need to account for changes in antimicrobial susceptibility. For example, if high-level resistance to ceftriaxone subsequently develops, resulting in a high rate of treatment failure, then gentamicin may become appropriate first-line therapy. Equally, if future circulating subtypes of gonorrhoea become highly resistant to gentamicin, its use would be inappropriate.
Chapter 6 Conclusions
The G-TOG trial compared single-dose gentamicin (240 mg) with single-dose ceftriaxone (500 mg) for the treatment of gonorrhoea and found that non-inferiority of gentamicin to ceftriaxone could not be demonstrated. Gentamicin led to microbiological cure in 94% of participants with genital infection, suggesting that its use would be appropriate as second-line therapy, but cure rates were lower for infections in the rectum (90% cure rate) and pharynx (80% cure rate). Single-dose gentamicin was well tolerated.
Implications for health care
The G-TOG trial has clearly demonstrated that gentamicin cannot be considered to be non-inferior to ceftriaxone, with the largest risk differences in cure being observed in patients with extragenital gonorrhoea. It is, therefore, probable that clinicians will wish to continue to use ceftriaxone (plus azithromycin) as their preferred first-line therapy. However, gentamicin (plus azithromycin) achieved a cure rate of 94% for genital gonorrhoea and its use in patients who are allergic, intolerant or resistant to ceftriaxone would be acceptable. The lower cure rates for rectal (90%) and pharyngeal (80%) infections make gentamicin a less attractive treatment option, but antibiotics are generally less effective at these sites and gentamicin may still be useful as a second- or third-line therapy. A repeat test for gonorrhoea to ensure microbiological cure would be advisable following gentamicin therapy.
The lack of correlation between gentamicin MIC values, obtained on in vitro sensitivity testing, and microbiological cure suggests that these should be interpreted with caution and that they have limited predictive value in clinical practice. There were too few failures with ceftriaxone to comment on any association with in vitro MIC testing. For azithromycin, there was an association between treatment failure and in vitro MICs but it was relatively weak, which limits its utility in clinical practice.
A clinically significant proportion of participants in the gentamicin treatment arm failed therapy, despite also receiving 1 g of oral azithromycin; this suggests that, independent of the efficacy of gentamicin, azithromycin at this dose may be suboptimal, especially for extragenital gonorrhoea. Although the number of culture isolates was limited, a modest trend towards a higher azithromycin MIC in this group suggests that a higher dose of azithromycin (e.g. 2 g) might be more effective but with the caveat that the tolerability of this dose is poor as a result of gastrointestinal side effects. Azithromycin is currently used to reduce the development of resistance and ‘protect’ ceftriaxone by providing microbiological cover if cephalosporin resistance develops. The observation in the G-TOG trial that a 1-g dose of azithromycin, even in combination with gentamicin, has a significant failure rate raises concerns about the effectiveness of this approach.
The results of the economic evaluation demonstrate that gentamicin is likely to be more costly than ceftriaxone in the treatment of gonorrhoea. This means that there is no evidence that gentamicin is non-inferior to ceftriaxone in the treatment of gonorrhoea, nor is there evidence that it is cost neutral. However, the economic analysis was necessarily restricted because it was not possible to fully take into account the potential costs associated with AMR in gonorrhoea, nor the potential value associated with preserving the effectiveness of ceftriaxone.
A single dose of 240 mg of gentamicin was found to cause few AEs and had a safety profile comparable to that of ceftriaxone. In particular, there was no increase in the frequency of vestibulocochlear or renal side effects, which have been associated with prolonged courses of gentamicin. This provides reassurance for the safe use of gentamicin in clinical practice. Participants receiving i.m. gentamicin reported more injection site pain than those receiving ceftriaxone, most probably related to the larger volume of injection. The pain resolved fully within a few hours for the majority of individuals but an alternative would be to give two separate 3-ml injections of gentamicin intramuscularly into each buttock rather than a single 6-ml injection, although PPI input during trial design suggested that a single injection might be preferred.
Recommendations for research
The lack of a strong association between the in vitro assessment of gonococcal resistance to gentamicin and clinical response highlights a need to explore further why gentamicin treatment is not effective in some patients and whether or not its efficacy can predicted. Whole-genome sequencing will allow the identification of specific subtypes of N. gonorrhoeae, which may provide insights into the mechanisms of resistance and detect potential markers of resistance.
Antibiotic resistance in N. gonorrhoeae is common and treatment options remain limited. The development of a preventative or therapeutic vaccine is therefore a priority and greater understanding of the immune response to infection is required to facilitate this. The collection and storage of before-and-after serum samples, and matched NAAT and culture samples in the G-TOG trial, will facilitate this future work.
The role of multiple different gonococcal infections within a single individual in the transmission of resistance is poorly understood. Transfer of resistance genes between N. gonorrhoeae is common and the archive of isolates collected in the G-TOG trial will allow an exploration of how frequently multiple infections occur and their potential role in the spread of resistance.
Azithromycin used as a 1-g dose to treat gonorrhoea was associated with a significant treatment failure rate, suggesting that it is not the optimal antibiotic to use as part of dual therapy designed to slow the spread of resistance. Further studies are required to evaluate alternative ‘second agents’, including a 2-g dose of azithromycin using an extended spectrum formulation that might improve tolerability.
Further research is needed to evaluate the potential costs associated with AMR in gonorrhoea. In addition, there needs to be further development of appropriate methods for economic evaluations when AMR is likely to be an issue. Such work will benefit from case studies, and examining the wider implications associated with alternative treatments for gonorrhoea would provide a valuable exemplar to aid in the development of general approaches.
Acknowledgements
We thank all those who took part in the trial and clinical staff at the participating sites.
Sponsor
University Hospitals Birmingham NHS Foundation Trust acted as sponsor for the research and the trial.
Role of funder
The National Institute for Health Research had input into the trial design through peer review of the funding proposal and viewed the report before publication, with the opportunity to comment.
The G-TOG Collaborative Group
Trial and data management: Nottingham Clinical Trials Unit
Lelia Duley, Professor of Clinical Trials Research; Alan Montgomery, Professor of Medical Statistics and Clinical Trials; Claire Brittan, Trial Manager to June 2016, then Senior Trial Manager to March 2017; Kirsty Sprange, Senior Trial Manager from March 2017; Margo Childs, Senior Trial Manager to March 2016; Trish Hepburn, Clinical Research Facilitator, Senior Statistician; Wei Tan, Medical Statistician; Sukhwinder Thandi, Trial Manager from June 2016; Garry Meakin, Trial Coordinator to September 2015; John Watson, Trial Administrator from December 15; Dan Simpkins, Senior Data Manager; Sarah Walker, Data Coordinator; Matt Foster, Data Administrator from April 2016; and Chris Rumsey, Information Technology (IT) Programmer.
Co-applicants
Jonathan Ross, Professor of Sexual Health and HIV, University Hospitals Birmingham NHS Foundation Trust; Christine Bowman, Consultant Physician in Genitourinary Medicine, Sheffield Teaching Hospitals NHS Foundation Trust; Stephanie Chisholm, Clinical Scientist, formerly of Public Health England; Michelle Cole, Health-care Scientist, Antimicrobial Resistance and Healthcare Associated Infections, National Infection Service, Public Health England; Lelia Duley, Professor of Clinical Trials Research, NCTU, University of Nottingham; Claudia Estcourt, Reader in Sexual Health and HIV, Barts and The London School of Medicine and Dentistry; Jan Harding, Research Co-ordinator in Sexual Health and HIV, University Hospitals Birmingham NHS Foundation Trust; Philip Hay, Reader, University of London; Louise Jackson, Research Fellow, University of Birmingham; Lucy Land, Reader in Nursing, Birmingham City University; Alan A Montgomery, Professor of Medical Statistics and Clinical Trials, NCTU, University of Nottingham; Rajul Patel, Senior Lecturer, University of Southampton; Gabriel Schembri, Consultant in Sexual Health and HIV Medicine/Research Lead, Central Manchester University Hospitals NHS Foundation Trust; John White, Consultant Physician in Sexual Health and HIV, Guy’s and St Thomas’ NHS Foundation Trust; and Janet Wilson, Consultant in Genitourinary Medicine, Leeds Teaching Hospitals NHS Trust.
Health economics
Louise Jackson and Tracy Roberts at the University of Birmingham provided health economics expertise to the trial.
Trial Management Group
Jonathan Ross, Professor of Sexual Health and HIV (chairperson); Lelia Duley, Professor of Clinical Trials Research; Alan A Montgomery, Professor of Medical Statistics and Clinical Trials; Clare Brittain, Trial Manager/Senior Trial Manager; Kirsty Sprange, Senior Trial Manager; Margo Childs, Senior Trial Manager; Trish Hepburn, Clinical Research Facilitator, Senior Statistician; Wei Tan, Medical Statistician; Tessa Lawrence, Sexual Health and HIV Research Manager; Sukhwinder Thandi, Trial Manager; Garry Meakin, Trial Co-ordinator; John Watson, Trial Administrator; and Jan Harding, Research Co-ordinator in Sexual Health and HIV.
Trial Steering Committee (independent members)
Professor Judith Stevenson (chairperson), Professor of Reproductive and Sexual Health, University College London; Professor John McLeod, Professor in Clinical Epidemiology and Primary Care, University of Bristol; Professor Andy Winter, Consultant in Sexual Health and HIV, NHS Greater Glasgow and Clyde; Mr David Roberts-Jones, Patient Representative; and Mr Matthew Keogh, Patient Representative.
Data Monitoring Committee (independent members)
Professor Chris Butler (chairperson), Professor of Primary Care, University of Oxford; Dr Mike Bradburn, Senior Statistician, University of Sheffield; Dr Danielle Mercey, Senior Clinical Lecturer, Farr Institute; and Professor Charles Lacey, Professor of Medicine, University of York.
Public Health England
Dr Colin Churchward and Mr Francesco Tripodo.
Sites
Presented in alphabetical order.
Barts Health NHS Trust
Vanessa Apea, Pauline Curnock, Kimberly Dzvova, Margaret Feeney, Stuart Flanagan, James Hand, Anna Hartley, Helena Miras, Andrew Motherwell, Jackie O’Connell, Laura Parry, Thomas Pasvol, Margaret Portman, Liat Sarner (PI), Athavan Umaipalan, Dayan Vijeratnam, Ryan Whyte, Andy Williams, Elizabeth Williams and Samantha Wu.
Brighton and Sussex University Hospitals NHS Trust
Lisa Barbour, Andrew Bexley, Tom Brittain, Marion Campbell, Sarah Cavilla, Maggie Cole, Gillian Dean (PI), Stewart Eastwood, Fionnuala Finnerty, Colin Fitzpatrick, Yvonne Gilleece, Alyson Knott, Celia Richardson, Daniel Richardson, Suneeta Soni, Fearghal Tucker and Deborah Williams.
Burrell Street Clinic, Guy’s and St Thomas’ NHS Foundation Trust
Jacqueline Aregbe, Ruslan Artykov, Claire Broad, Irene Cheah, Naomi Fitzgerald, Nina Francia, Momta Gurung, Katie Holmes, Helen Iveson, Amy Jack, Sarah Thompson (née Lovell), Juliana Lwanga, Tiffany Martin, Anatole Menon-Johansson, Sabelo Meyrick, Aoife Moylan, Achyuta Nori, Patrick O’Rourke, Rudiger Pitroff, Rashidat Rabiu, Tometta Roberts, Hannah Schwarz, Rebecca Simons, Andrew Skingsley and John White (PI).
Chelsea and Westminster Hospital NHS Foundation Trust
Elizabeth Byrne, Sharanjit Dhoot, Sweenie Goonesekera, Sophie Hannay, Sapna Harish, Rachael Jones, Sarah Keeley, Georgie Kendall, Jay McBain, Oxana Mutalak, Michael Rayment (PI), Christopher Scott, Mini Thankachen and Clare Turvey.
Coventry and Warwickshire Partnership NHS Trust
Amine Abbas, Sris Allan, Anne Bassinder, Kerrie Beasley, Billikanti Kumari (PI), Heather Carter, Steven Clay, Satyajit Das, Mercheter Farrell, Victoria Hardy, Charlotte Hubbard, Natalie Jackson, Saleha Karim, Nicholas March, Nyamayaro Nyaradzo, Katie Peck, Rajiner Singh, Katie Smith, Huda Taha, Shyamalie Bopitiya and Kerry Flahive.
John Hunter Clinic, Chelsea and Westminster Hospital NHS Foundation Trust
Tristan Barber, Carina Bautista, Elna Cifra, Rachael McIntosh, Kate Nulty, Michael Rayment (PI), Harriet Stevenson, Ella Svensson and Mini Thankachen.
Manchester Royal Infirmary, Central Manchester University Hospitals NHS Foundation Trust
James Boateng, Brynn Chappell, Susanna Currie, Denise Donahue, Pamela Hackney, Helen Holt, Sally Jewsbury, Denise Kadiu, Alison Kelly, Clare Langan, Jennifer Leigton, Bronagh McBrien, Julie Melville, Elizabeth Okecha, Gabriel Schembri (PI), Vian Shafiq, Jonathan Shaw, Lisa Southon, Cheryl Stott, Chris Ward, Clare Warren, Clare Wood and Stephanie Yau.
Royal Berkshire NHS Foundation Trust
Christina Ambrose, Candy Brown, Fabian Chen (PI), Elaine Fernandes, Nyla Hague, Katie Keating-Fedders, Sheila O’Connor, Sanjeeva Pallawela, Ruth Reakes, Tracey Staughton, Julia Tassano-Smith, Emily Ward, Hannah Whetnall, Ruth Wilson and Alice Wright.
Royal Free London NHS Foundation Trust
Silvia Belmondo, Joanna Damm, Barbara Danielski, Mirelle Harris, Dan Ivens (PI), Adrian Lyons and Louie Pong.
Sheffield Teaching Hospitals NHS Foundation Trust
Deborah Allen, Sarah Berry, Kathryn Birchall, Aparna Briggs, Leisa Broadhurst, Lesley Campbell, Ashleigh Devine, Claire Dewsnap (PI), Claire Erskine, Helen Jackson, Hannah Loftus, Laura Makey, Eleanor Marks, Lisa Moat, Danielle Ned, Olofunso Olarinde, Rasha Omar, John Savas, Rebecca Schatzberger, Lynne Smart, Naomi Sutton, Beruwalage Swaris, Cheryl Taylor, Lauren Theaker and Vincent Tucker.
St James’s University Hospital, Leeds Teaching Hospitals NHS Trust
Tooba Ahmed, Emma Barron, Jane Brown, Joanna Bulman, Charlie Burland, Marshall Tim Coates, Barbara Davies, Nadia Ekong, Jayne Fisher, Debbie Goode, Michelle Loftus-Keeling, Melanie Marson, Joanne McGregor, Eric Monteiro, Jennifer Murira, Isabel Okpaluba, Sarah Schoeman, Angela Talbot, Siew Yen Teo, Karina Veitch, Harriet Wallace, Rachel Westmorland, Sue Williamson and Janet Wilson (PI).
St Mary’s Hospital, Imperial College Healthcare NHS Trust
Wilbert Ayap, Olamide Dosekun, Ladan Farah, Naomi Goodhand, Matthew Grundy-Bowers, Ken Legg, Victoria Manns, Scott Mullaney, John Walsh, Dawn Wilkinson (PI), Ajerico Del Rosario and Jasmini Alagaratnam.
Whittall Street Clinic, University Hospitals Birmingham NHS Foundation Trust
Faye Andrews, Ruth Bacani, Prita Bannerjee, Meg Boothby, Anthony Brierley, Anne Campbell, Christine Carter, Rachael Caswell, Hannah Church, Amisha Desai, Amita Gill, Emma Goodhead, Penny Goold, Celsa Gumpic, Nutan Gupta, Sharon Hackett, Rebecca Harding, Christine Hardwick, Leisa-Kay Harris, Rachel Hayward, Mia Huensberg, Hapiloe Hunter, Lisa James, Alicess Joe, Jaishree Joshi, Matthew Keighly, Catherine Khan, Bilques Khawaja, Vinod Kumar, Lakshmi Kumarasingha, Michael Langford, Tessa Lawrence, Mar Mar Lwin, Kaveh Manhavi, Robert Molloy, Charlotte Newcombe, Sara Newell, Andrea Ng, Magda Nowacka, Catherine O’Brien, Matilda O’Donovan, Monika Okriak, Fayofunmi Olonilua, John Peterson, Jara Phattey, Keith Radcliffe, Rukhsana Raza, Jonathan Ross (PI), Nicola Thorley, Kelly Walker-Reed, Aaron Williams, Rotina Willis-Richards and Louise Wright.
Western Community Hospital, Solent NHS Trust
Lesley-Ann Castle, Emily Clarke, Catherine Elliot, Sarah Lawson, Rajul Patel (PI), Catherine Thomas, Jo Turpitt and Jane Whitehead.
West Middlesex University Hospital, Chelsea and Westminster Hospital NHS Trust
Shamela De Silva, Katie Dodds, Ursula Kirwan, Gemma McNamara, Metod Oblak, Michael Rayment (PI), Chelsea Richardson, Marie-Louise Svensson, Caroline Turner and Zoe Wiggins.
Contributions of authors
Jonathan DC Ross (Professor of Sexual Health and HIV) was the chief investigator and co-authored the final report.
Jan Harding (Research Co-ordinator) supported the trial delivery.
Lelia Duley (Professor of Clinical Trials Research) contributed to the protocol, provided oversight of the NCTU input and contributed to the final report.
Alan A Montgomery (Professor of Medical Statistics and Clinical Trials) oversaw the clinical effectiveness analysis and contributed to the final report.
Trish Hepburn (Senior Medical Statistician) oversaw the clinical effectiveness analysis and prepared the results for publication.
Wei Tan (Medical Statistician, NCTU) analysed the clinical effectiveness data and prepared the results for publication.
Clare Brittain (Clinical Trial Manager, then Senior Trial Manager, NCTU) managed the trial.
Garry Meakin (Clinical Trial Co-ordinator, NCTU) supported the trial delivery.
Kirsty Sprange (Senior Trial Manager, NCTU) contributed to the trial delivery and preparation of the final report.
Sukhwinder Thandi (Clinical Trial Manager, NCTU) managed the trial.
Louise Jackson (Research Fellow) analysed the cost-effectiveness analysis and prepared the results for publication.
Tracy Roberts (Professor of Health Economics) oversaw the cost-effectiveness analysis and prepared the results for publication.
Janet Wilson (Consultant in Genitourinary Medicine and HIV) was a PI and supported the trial delivery.
John White (Consultant Physician in Sexual Health and HIV) was a PI and supported the trial delivery.
Claire Dewsnap (Consultant Physician in Sexual Health and HIV) was a PI and supported the trial delivery.
Michelle Cole (Healthcare Scientist) oversaw the analysis of the trial samples, prepared the results for publication and contributed to the final report.
Tessa Lawrence (Sexual Health and HIV Research Manager) supported the trial delivery.
Publications
Ross JD, Lewis DA. Cephalosporin resistant Neisseria gonorrhoeae: time to consider gentamicin? Sex Transm Infect 2012;88:6–8.
Hathorn E, Dhasmana D, Duley L, Ross JD. The effectiveness of gentamicin in the treatment of Neisseria gonorrhoeae: a systematic review. Syst Rev 2014;3:104.
Ross JDC, Brittain C, Cole M, Dewsnap C, Harding J, Hepburn T, et al. Gentamicin compared with Ceftriaxone for the treatment of gonorrhoea: a randomised trial (G-ToG Trial) [published online ahead of print May 2 2019]. Lancet 2019.
Data-sharing statement
All requests for anonymised data should be addressed to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Public Health England . Sexually Transmitted Infections (STIs): Annual Data Tables 2016. www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables (accessed 5 June 2017).
- Haidari G, Perry ME, White JA. Are we seeing a true rise in Neisseria gonorrhoeae and Chlamydia trachomatis in men who have sex with men in the UK?. Sex Transm Infect 2014;90. https://doi.org/10.1136/sextrans-2014-051532.
- Public Health England . Surveillance of Antimicrobial Resistance in Neisseria Gonorrhoeae: Key Findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) 2016. www.gov.uk/government/uploads/system/uploads/attachment_data/file/567602/GRASP_Report_2016.pdf (accessed 5 June 2017).
- Jarvis GA, Chang TL. Modulation of HIV transmission by Neisseria gonorrhoeae: molecular and immunological aspects. Curr HIV Res 2012;10:211-7. https://doi.org/10.2174/157016212800618138.
- Moran JS, Levine WC. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis 1995;20:S47-65. https://doi.org/10.1093/clinids/20.Supplement_1.S47.
- World Health Organization . WHO Guidelines for the Treatment of Neisseria Gonorrhoeae 2016. http://apps.who.int/iris/bitstream/10665/246114/1/9789241549691-eng.pdf?ua=1 (accessed 11 June 2017).
- Duncan S, Duncan CJ. The emerging threat of untreatable gonococcal infection. N Engl J Med 2012;366. https://doi.org/10.1056/NEJMc1203138.
- Bignell C, Fitzgerald M. Guideline Development Group . UK national guideline for the management of gonorrhoea in adults, 2011. Int J STD AIDS 2011;22:541-7. https://doi.org/10.1258/ijsa.2011.011267.
- Forsyth S, Penney P, Rooney G. Cefixime-resistant Neisseria gonorrhoeae in the UK: a time to reflect on practice and recommendations. Int J STD AIDS 2011;22:296-7. https://doi.org/10.1258/ijsa.2009.009191.
- Ison CA, Hussey J, Sankar KN, Evans J, Alexander S. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Euro Surveill 2011;16.
- Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, et al. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 2011;55:3538-45. https://doi.org/10.1128/AAC.00325-11.
- Unemo M, Golparian D, Hestner A. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill 2011;16.
- Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 2012;56:1273-80. https://doi.org/10.1128/AAC.05760-11.
- Whiley DM, Lahra MM, Unemo M. Prospects of untreatable gonorrhea and ways forward. Future Microbiol 2015;10:313-16. https://doi.org/10.2217/fmb.14.138.
- Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, et al. Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 2016;374:2504-6. https://doi.org/10.1056/NEJMc1512757.
- Public Health England . Health Protection Report. Outbreak of High Level Azithromycin Resistant Gonorrhoea in England: An Update 2016. www.gov.uk/government/uploads/system/uploads/attachment_data/file/552058/hpr3016_hlzrg.pdf (accessed 11 June 2017).
- Golparian D, Fernandes P, Ohnishi M, Jensen JS, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains, including those with high-level antimicrobial resistance: potential treatment option for gonorrhea?. Antimicrob Agents Chemother 2012;56:2739-42. https://doi.org/10.1128/AAC.00036-12.
- Livermore DM, Alexander S, Marsden B, James D, Warner M, Rudd E, et al. Activity of ertapenem against Neisseria gonorrhoeae. J Antimicrob Chemother 2004;54:280-1. https://doi.org/10.1093/jac/dkh272.
- Olsen B, Pham TL, Golparian D, Johansson E, Tran HK, Unemo M. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011. BMC Infect Dis 2013;13. https://doi.org/10.1186/1471-2334-13-40.
- Unemo M, Golparian D, Limnios A, Whiley D, Ohnishi M, Lahra MM, et al. In vitro activity of ertapenem versus ceftriaxone against Neisseria gonorrhoeae isolates with highly diverse ceftriaxone MIC values and effects of ceftriaxone resistance determinants: ertapenem for treatment of gonorrhea?. Antimicrob Agents Chemother 2012;56:3603-9. https://doi.org/10.1128/AAC.00326-12.
- Panduro J. Treatment of acute gonorrhoea with a single oral dose of rifampicin. Br J Vener Dis 1971;47:440-2. https://doi.org/10.1136/sti.47.6.440.
- Boslego JW, Tramont EC, Takafuji ET, Diniega BM, Mitchell BS, Small JW, et al. Effect of spectinomycin use on the prevalence of spectinomycin-resistant and of penicillinase-producing Neisseria gonorrhoeae. N Engl J Med 1987;317:272-8. https://doi.org/10.1056/NEJM198707303170504.
- Dowell D, Kirkcaldy RD. Effectiveness of gentamicin for gonorrhoea treatment: systematic review and meta-analysis. Sex Transm Infect 2012;88:589-94. https://doi.org/10.1136/sextrans-2012-050604.
- Hathorn E, Dhasmana D, Duley L, Ross JD. The effectiveness of gentamicin in the treatment of Neisseria gonorrhoeae: a systematic review. Syst Rev 2014;3. https://doi.org/10.1186/2046-4053-3-104.
- Kirkcaldy RD, Weinstock HS, Moore PC, Philip SS, Wiesenfeld HC, Papp JR, et al. The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis 2014;59:1083-91. https://doi.org/10.1093/cid/ciu521.
- Hayward S, Harding J, Molloy R, Land L, Longcroft-Neal K, Moore D, et al. Adverse Effects of Single Dose Gentamicin in Adults: A Systematic Review n.d.
- Chisholm SA, Quaye N, Cole MJ, Fredlund H, Hoffmann S, Jensen JS, et al. An evaluation of gentamicin susceptibility of Neisseria gonorrhoeae isolates in Europe. J Antimicrob Chemother 2011;66:592-5. https://doi.org/10.1093/jac/dkq476.
- Brittain C, Childs M, Duley L, Harding J, Hepburn T, Meakin G, et al. Gentamicin versus ceftriaxone for the treatment of gonorrhoea (G-TOG trial): study protocol for a randomised trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1683-8.
- Brown LB, Krysiak R, Kamanga G, Mapanje C, Kanyamula H, Banda B, et al. Neisseria gonorrhoeae antimicrobial susceptibility in Lilongwe, Malawi, 2007. Sex Transm Dis 2010;37:169-72. https://doi.org/10.1097/OLQ.0b013e3181bf575c.
- British Association for Sexual Health and HIV . Clinical Effectiveness Group Guidelines n.d. www.bashh.org/guidelines (accessed 20 June 2017).
- Medical Dictionary for Regulatory Activities (MedDRA) . Introductory Guide MedDRA Version 17.1 2014. https://www.meddra.org/sites/default/files/guidance/file/intguide_17_1_english.pdf (accessed 16 March 2018).
- World Medical Association . WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects n.d. www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed 16 March 2018).
- Department of Heath and Social Care . Research Governance Framework for Health and Social Care. Second Edition 2005. www.gov.uk/government/uploads/system/uploads/attachment_data/file/139565/dh_4122427.pdf (accessed 16 March 2018).
- International Conference on Harmonisation for Better Health . ICH E6 Good Clinical Practice n.d. www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html (accessed 16 March 2018).
- Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) . GFR Calculator 2017. http://ckdepi.org/equations/gfr-calculator/ (accessed 26 June 2017).
- Gaynes RP. Preserving the effectiveness of antibiotics. JAMA 2010;303:2293-4. https://doi.org/10.1001/jama.2010.766.
- Unemo M, del Rio C, Shafer WM. Antimicrobial resistance expressed by Neisseria gonorrhoeae: a major global public health problem in the 21st century. Microbiol Spectr 2016;4. https://doi.org/10.1128/microbiolspec.EI10-0009-2015.
- Briggs A, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press; 2006.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; n.d.
- Department of Health and Social Care . NHS Reference Costs 2015 to 2016 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 21 May 2018).
- Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. ISPOR–SMDM (International Society For Pharmacoeconomics and Outcomes Research–Society for Medical Decision Making) Modeling Good Research Practices Task Force . Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making 2012;32:722-32. https://doi.org/10.1177/0272989X12458348.
- Andronis L, Barton P, Bryan S. Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13290.
- Drummond M, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2015.
- Oppong R, Smith RD, Little P, Verheij T, Butler CC, Goossens H, et al. Cost effectiveness of amoxicillin for lower respiratory tract infections in primary care: an economic evaluation accounting for the cost of antimicrobial resistance. Br J Gen Pract 2016;66:e633-9. https://doi.org/10.3399/bjgp16X686533.
- Patel DA, Shorr AF, Chastre J, Niederman M, Simor A, Stephens JM, et al. Modeling the economic impact of linezolid versus vancomycin in confirmed nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus. Crit Care 2014;18. https://doi.org/10.1186/cc13996.
- van Werkhoven CH, Postma DF, Mangen MJ, Oosterheert JJ, Bonten MJ. CAP-START (Community-Acquired Pneumonia – Study on the Initial Treatment With Antibiotics of Lower Respiratory Tract Infections) Study Group . Cost-effectiveness of antibiotic treatment strategies for community-acquired pneumonia: results from a cluster randomized cross-over trial. BMC Infect Dis 2017;17. https://doi.org/10.1186/s12879-016-2179-6.
- Smith R, Coast J. The true cost of antimicrobial resistance. BMJ 2013;346. https://doi.org/10.1136/bmj.f1493.
- Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. Aminoglycosides: activity and resistance. Antimicrob Agents Chemother 1999;43:727-37.
- Davies J, Wright GD. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol 1997;5:234-40. https://doi.org/10.1016/S0966-842X(97)01033-0.
- Davies JK, Kahler CM. Pathogenic Neisseria: Genomics, Molecular Biology and Disease Intervention. Poole: Caister Academic Press; 2014.
- Bala M, Singh V, Philipova I, Bhargava A, Chandra Joshi N, Unemo M. Gentamicin in vitro activity and tentative gentamicin interpretation criteria for the CLSI and calibrated dichotomous sensitivity disc diffusion methods for Neisseria gonorrhoeae. J Antimicrob Chemother 2016;71:1856-9. https://doi.org/10.1093/jac/dkw102.
- European Committee on Antimicrobial Susceptibility Testing . Breakpoint Tables for Interpretation of MICs and Zone Diameters 2017. www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf (accessed 30 April 2017).
- Centers for Disease Control and Prevention . Interpretive Criteria for Neisseria Gonorrhoeae Susceptibility Testing 2013. www.cdc.gov/std/gonorrhea/arg/criteria.htm (accessed 30 April 2017).
- Judson FN, Ehret JM, Handsfield HH. Comparative study of ceftriaxone and spectinomycin for treatment of pharyngeal and anorectal gonorrhea. JAMA 1985;253:1417-19. https://doi.org/10.1001/jama.1985.03350340069019.
- British Association for Sexual Health and HIV . UK National Guideline for the Management of Gonorrhoea in Adults 2011. www.bashh.org/documents/3920.pdf (accessed 30 April 2017).
- Nguyen TQ, Cohen SE, Noohi T, Kohn RP, Philip SS. Time to clearance for molecular test-of-cure among men treated for urethral, pharyngeal, or rectal gonorrhoea in San Francisco, 2013–2014. Sex Transm Infect 2015;91. https://doi.org/10.1136/sextrans-2015-052270.399.
- Hjelmevoll SO, Olsen ME, Sollid JU, Haaheim H, Melby KK, Moi H, et al. Appropriate time for test-of-cure when diagnosing gonorrhoea with a nucleic acid amplification test. Acta Derm Venereol 2012;92:316-19. https://doi.org/10.2340/00015555-1275.
- Bell N, Drayton R. Test of cure for gonorrhoea infection: when is the optimal time?. Int J STD AIDS 2013;24:21-2. https://doi.org/10.1177/0956462413484421.
- Wind CM, Van Der Loeff MFS, Unemo M, Schuurman R, Van Dam AP, De Vries HJC. Test of cure for anogenital gonorrhoea using modern RNA-based and DNA-based nucleic acid amplification tests: a prospective cohort study. Clin Infect Dis 2016;62:1348-55. https://doi.org/10.1093/cid/ciw141.
- Bissessor M, Whiley DM, Fairley CK, Bradshaw CS, Lee DM, Snow AS, et al. Persistence of Neisseria gonorrhoeae DNA following treatment for pharyngeal and rectal gonorrhea is influenced by antibiotic susceptibility and reinfection. Clin Infect Dis 2015;60:557-63. https://doi.org/10.1093/cid/ciu873.
- Wind CM, de Vries HJ, van Dam AP. Determination of in vitro synergy for dual antimicrobial therapy against resistant Neisseria gonorrhoeae using Etest and agar dilution. Int J Antimicrob Agents 2015;45:305-8. https://doi.org/10.1016/j.ijantimicag.2014.10.020.
- Page-Shafer K, Graves A, Kent C, Balls JE, Zapitz VM, Klausner JD. Increased sensitivity of DNA amplification testing for the detection of pharyngeal gonorrhea in men who have sex with men. Clin Infect Dis 2002;34:173-6. https://doi.org/10.1086/338236.
- Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis 2008;35:637-42. https://doi.org/10.1097/OLQ.0b013e31817bdd7e.
- Handsfield HH, Dalu ZA, Martin DH, Douglas JM, McCarty JM, Schlossberg D. Multicenter trial of single-dose azithromycin vs. ceftriaxone in the treatment of uncomplicated gonorrhea. Azithromycin Gonorrhea Study Group. Sex Transm Dis 1994;21:107-11. https://doi.org/10.1097/00007435-199403000-00010.
- Habib AR, Fernando R. Efficacy of azithromycin 1 g single dose in the management of uncomplicated gonorrhoea. Int J STD AIDS 2004;15:240-2. https://doi.org/10.1258/095646204773557767.
- Waugh MA. Open study of the safety and efficacy of a single oral dose of azithromycin for the treatment of uncomplicated gonorrhoea in men and women. J Antimicrob Chemother 1993;31:193-8. https://doi.org/10.1093/jac/31.suppl_E.193.
- Bignell C, Garley J. Azithromycin in the treatment of infection with Neisseria gonorrhoeae. Sex Transm Infect 2010;86:422-6. https://doi.org/10.1136/sti.2010.044586.
- Barbee LA, Soge OO, Dombrowski JC, Katz DA, Holmes KK, Golden MR. Azithromycin-resistant Neisseria gonorrhoeae in men who have sex with men (MSM) in Seattle, Washington: 2014–2015. Sex Transm Infect 2015;91. https://doi.org/10.1136/sextrans-2015-052270.80.
- Galarza PG, Alcalá B, Salcedo C, Canigia LF, Buscemi L, Pagano I, et al. Emergence of high level azithromycin-resistant Neisseria gonorrhoeae strain isolated in Argentina. Sex Transm Dis 2009;36:787-8. https://doi.org/10.1097/OLQ.0b013e3181b61bb1.
- Ni C, Xue J, Zhang C, Zhou H, van der Veen S. High prevalence of Neisseria gonorrhoeae with high-level resistance to azithromycin in Hangzhou, China. J Antimicrob Chemother 2016;71:2355-7. https://doi.org/10.1093/jac/dkw131.
- Martin I, Sawatzky P, Liu G, Allen V, Lefebvre B, Hoang L, et al. Decline in decreased cephalosporin susceptibility and increase in azithromycin resistance in Neisseria gonorrhoeae, Canada. Emerging Infect Dis 2016;22:65-7. https://doi.org/10.3201/eid2201.151247.
- Belkacem A, Jacquier H, Goubard A, Mougari F, La Ruche G, Patey O, et al. Molecular epidemiology and mechanisms of resistance of azithromycin-resistant Neisseria gonorrhoeae isolated in France during 2013–14. J Antimicrob Chemother 2016;71:2471-8. https://doi.org/10.1093/jac/dkw182.
- Palmer HM, Young H, Winter A, Dave J. Emergence and spread of azithromycin-resistant Neisseria gonorrhoeae in Scotland. J Antimicrob Chemother 2008;62:490-4. https://doi.org/10.1093/jac/dkn235.
- Chisholm SA, Neal TJ, Alawattegama AB, Birley HD, Howe RA, Ison CA. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J Antimicrob Chemother 2009;64:353-8. https://doi.org/10.1093/jac/dkp188.
- Chisholm SA, Wilson J, Alexander S, Tripodo F, Al-Shahib A, Schaefer U, et al. An outbreak of high-level azithromycin resistant Neisseria gonorrhoeae in England. Sex Transm Infect 2016;92:365-7. https://doi.org/10.1136/sextrans-2015-052312.
- Tapsall JW, Shultz TR, Limnios EA, Donovan B, Lum G, Mulhall BP. Failure of azithromycin therapy in gonorrhea and discorrelation with laboratory test parameters. Sex Transm Dis 1998;25:505-8. https://doi.org/10.1097/00007435-199811000-00002.
- Steingrimsson O, Olafsson JH, Thorarinsson H, Ryan RW, Johnson RB, Tilton RC. Azithromycin in the treatment of sexually transmitted disease. J Antimicrob Chemother 1990;25:109-14. https://doi.org/10.1093/jac/25.suppl_A.109.
- Tanaka M, Furuya R, Irie S, Kanayama A, Kobayashi I. High prevalence of azithromycin-resistant Neisseria gonorrhoeae isolates with a multidrug resistance phenotype in Fukuoka, Japan. Sex Transm Dis 2015;42:337-41. https://doi.org/10.1097/OLQ.0000000000000279.
- Young H, Moyes A, McMillan A. Azithromycin and erythromycin resistant Neisseria gonorrhoeae following treatment with azithromycin. Int J STD AIDS 1997;8:299-302. https://doi.org/10.1258/0956462971920127.
- Liu P, Allaudeen H, Chandra R, Phillips K, Jungnik A, Breen JD, et al. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob Agents Chemother 2007;51:103-9. https://doi.org/10.1128/AAC.00852-06.
- Yasuda M, Ito S, Kido A, Hamano K, Uchijima Y, Uwatoko N, et al. A single 2 g oral dose of extended-release azithromycin for treatment of gonococcal urethritis. J Antimicrob Chemother 2014;69:3116-18. https://doi.org/10.1093/jac/dku221.
- Roche Products Limited . Summary of Product Characteristics for Ceftriaxone 2017. www.medicines.org.uk/emc/medicine/1729 (accessed 30 April 2017).
- Sanofi . Summary of Product Characteristics for Gentamicin 2015. www.medicines.org.uk/emc/medicine/28271 (accessed 30 April 2017).
- Sandoz . Summary of Product Characteristics for Azithromycin 2015. www.medicines.org.uk/emc/medicine/26131 (accessed 30 April 2017).
- Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol 2000;5:3-22. https://doi.org/10.1159/000013861.
- Rybak L. Aminoglycoside Antibiotics. Philadelphia, PA: Elsevier; 2005.
- Hayward R, Harding J, Molloy R, Land L, Longcroft-Neal K, Moore D, et al. Safety of single dose gentamicin compared with multiple dose regimens. Sex Transm Infect 2016;92:A39-40. https://doi.org/10.1136/sextrans-2016-052718.115.
- Vandewalle A, Farman N, Morin JP, Fillastre JP, Hatt PY, Bonvalet JP. Gentamicin incorporation along the nephron: autoradiographic study on isolated tubules. Kidney Int 1981;19:529-39. https://doi.org/10.1038/ki.1981.50.
- Golparian D, Tabrizi S, Jacobsson S, Stezcko Nilsson C, Fredlund H, Unemo M. Analytical specificity and sensitivity of the APTIMA Combo 2 and APTIMA GC assays for detection of Neisseria gonorrhoeae on the Gen-Probe PANTHER instrument and verification of specimens positive for N. gonorrhoeae using other commercial diagnostic NAATs. Sex Transm Infect 2013;89. https://doi.org/10.1136/sextrans-2013-051184.0284.
- Stupiansky NW, Van Der Pol B, Williams JA, Weaver B, Taylor SE, Fortenberry JD. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis 2011;38:750-4. https://doi.org/10.1097/OLQ.0b013e31820ff9a4.
- Van Liere GAFS, Dukers-Muijrers NHTM, Wolffs PFG, Hoebe CJPA. Substantial natural clearance of genital and extragenital Chlamydia trachomatis and Neisseria gonorrhoeae in STD clinic attendees. Sex Transm Infect 2013;89. https://doi.org/10.1136/sextrans-2013-051184.0643.
Appendix 1 Summary of trial amendments
| Amendment reference and date | Amendment details | Previous version number and date | New version number and date |
|---|---|---|---|
| Substantial amendment 06, 18 June 2015 | Removal of PK substudy and amendment to eligibility criteria | 1.0, 27 May 2014 | 2.0, 17 June 2015 |
| PIS | 1.0, 4 July 2014 | 2.0, 4 June 2015 | |
| Informed consent form | 1.0, 27 May 2014 | 2.0, 4 June 2015 | |
| Substantial amendment 08, 22 October 2015 | Change of RSI | 80 mg of cidomycin or a 2-ml solution for injection (date of revision of text: 19 June 2013) | 80 mg of cidomycin or a 2-ml solution for injection (date of revision of text: 29 June 2015) |
| Substantial amendment 09, 21 December 2015 | Amendment to PIS to clarify sending of AC2 NAAT swabs to STBRUa | 2.0, 4 June 2015 | 3.0, 21 December 2015 |
Appendix 2 Search strategy for studies assessing the treatment of gonorrhoea with gentamicin
MEDLINE and EMBASE
Date range searched: 1 March 2013 to 22 April 2017.
Date searched: 22 April 2017
One hundred and fifty-six references identified and reviewed
Search strategy
-
gonorrhoea.mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui]
-
gonorrhea.mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui]
-
Neisseria gonorrhoeae.mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui]
-
N gonorrhoeae.mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui]
-
1 or 2 or 3 or 4
-
gentamicin.mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui]
-
gentamycin.mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui]
-
6 or 7
-
5 and 8
-
limit 9 to yr =‘2013-Current’
-
remove duplicates from 10
Appendix 3 Additional tables, listings and figures
| Clearance | Sex, n (%) | |||
|---|---|---|---|---|
| Male | Female | |||
| Ceftriaxone | Gentamicin | Ceftriaxone | Gentamicin | |
| Number with infection at genital site | 107 | 142 | 47 | 32 |
| Cleared of N. gonorrhoeae | 106 (99) | 132 (93) | 45 (96) | 31 (97) |
| Not cleared of N. gonorrhoeae | 1 (1) | 10 (7) | 2 (4) | 1 (3) |
| Symptom | Treatment group, n (%) | |||||
|---|---|---|---|---|---|---|
| Ceftriaxone (N = 362) | Gentamicin (N = 358) | |||||
| Symptom present at baseline | Symptom present at baseline | |||||
| No | Yes | Not known | No | Yes | Not known | |
| Genital discharge at 2 weeks | ||||||
| No | 191 (53) | 122 (34) | 0 (0) | 154 (43) | 139 (39) | 0 (0) |
| Yes | 1 (< 0.5) | 7 (2) | 0 (0) | 1 (< 0.5) | 8 (2) | 0 (0) |
| Not known | 17 (5) | 24 (7) | 0 (0) | 24 (7) | 32 (9) | 0 (0) |
| Dysuria at 2 weeks | ||||||
| No | 214 (59) | 104 (29) | 0 (0) | 174 (49) | 116 (32) | 0 (0) |
| Yes | 1 (< 0.5) | 2 (1) | 0 (0) | 0 (0) | 12 (3) | 0 (0) |
| Not known | 22 (6) | 19 (5) | 0 (0) | 30 (8) | 26 (7) | 0 (0) |
| Anorectal pain at 2 weeks | ||||||
| No | 306 (85) | 12 (3) | 0 (0) | 294 (82) | 5 (1) | 0 (0) |
| Yes | 2 (1) | 1 (< 0.5) | 0 (0) | 1 (< 0.5) | 2 (1) | 0 (0) |
| Not known | 39 (11) | 2 (1) | 0 (0) | 55 (15) | 1 (< 0.5) | 0 (0) |
| Rectal discharge at 2 weeks | ||||||
| No | 308 (85) | 11 (3) | 0 (0) | 294 (82) | 6 (2) | 0 (0) |
| Yes | 1 (< 0.5) | 1 (< 0.5) | 0 (0) | 0 (0) | 2 (1) | 0 (0) |
| Not known | 41 (11) | 0 (0) | 0 (0) | 54 (15) | 2 (1) | 0 (0) |
| Sore throat at 2 weeks | ||||||
| No | 269 (74) | 42 (12) | 0 (0) | 253 (71) | 42 (12) | 0 (0) |
| Yes | 5 (2) | 5 (1) | 0 (0) | 4 (1) | 3 (1) | 0 (0) |
| Not known | 35 (10) | 6 (2) | 0 (0) | 49 (14) | 7 (2) | 0 (0) |
| Rectal bleeding at 2 weeks | ||||||
| No | 310 (86) | 7 (2) | 0 (0) | 294 (82) | 7 (2) | 0 (0) |
| Yes | 3 (1) | 1 (< 0.5) | 0 (0) | 1 (< 0.5) | 0 (0) | 0 (0) |
| Not known | 40 (11) | 1 (< 0.5) | 0 (0) | 55 (15) | 1 (< 0.5) | 0 (0) |
| Tenesmus at 2 weeks | ||||||
| No | 313 (86) | 7 (2) | 0 (0) | 298 (83) | 3 (1) | 0 (0) |
| Yes | 0 (0) | 1 (< 0.5) | 0 (0) | 1 (< 0.5) | 0 (0) | 0 (0) |
| Not known | 41 (11) | 0 (0) | 0 (0) | 55 (15) | 1 (< 0.5) | 0 (0) |
| Constipation at 2 weeks | ||||||
| No | 302 (83) | 10 (3) | 0 (0) | 293 (82) | 3 (1) | 0 (0) |
| Yes | 8 (2) | 1 (< 0.5) | 0 (0) | 5 (1) | 1 (< 0.5) | 0 (0) |
| Not known | 41 (11) | 0 (0) | 0 (0) | 56 (16) | 0 (0) | 0 (0) |
| Intermenstrual bleeding at 2 weeks | ||||||
| No | 53 (15) | 8 (2) | 1 (< 0.5) | 41 (11) | 5 (1) | 0 (0) |
| Yes | 2 (1) | 1 (< 0.5) | 0 (0) | 2 (1) | 0 (0) | 0 (0) |
| Not known | 4 (1) | 0 (0) | 0 (0) | 15 (4) | 2 (1) | 0 (0) |
| Post-coital bleeding at 2 weeks | ||||||
| No | 58 (16) | 5 (1) | 0 (0) | 40 (11) | 5 (1) | 0 (0) |
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Not known | 6 (2) | 0 (0) | 0 (0) | 18 (5) | 2 (1) | 0 (0) |
| Symptom | Treatment group, n (%) | |||||
|---|---|---|---|---|---|---|
| Ceftriaxone (N = 69) | Gentamicin (N = 65) | |||||
| Symptom present at baseline | Symptom present at baseline | |||||
| No | Yes | Not known | No | Yes | Not known | |
| Genital discharge at 2 weeks | ||||||
| No | 35 (51) | 24 (35) | 0 (0) | 30 (46) | 15 (23) | 0 (0) |
| Yes | 1 (1) | 5 (7) | 0 (0) | 1 (2) | 4 (6) | 0 (0) |
| Not known | 3 (4) | 1 (1) | 0 (0) | 8 (12) | 7 (11) | 0 (0) |
| Dysuria at 2 weeks | ||||||
| No | 45 (65) | 20 (29) | 0 (0) | 42 (65) | 6 (9) | 0 (0) |
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 0 (0) |
| Not known | 3 (4) | 1 (1) | 0 (0) | 11 (17) | 4 (6) | 0 (0) |
| Anorectal pain at 2 weeks | ||||||
| No | 63 (91) | 1 (1) | 0 (0) | 48 (74) | 2 (3) | 0 (0) |
| Yes | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Not known | 4 (6) | 0 (0) | 0 (0) | 15 (23) | 0 (0) | 0 (0) |
| Rectal discharge at 2 weeks | ||||||
| No | 64 (93) | 1 (1) | 0 (0) | 49 (75) | 1 (2) | 0 (0) |
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Not known | 4 (6) | 0 (0) | 0 (0) | 15 (23) | 0 (0) | 0 (0) |
| Sore throat at 2 weeks | ||||||
| No | 56 (81) | 8 (12) | 0 (0) | 41 (63) | 9 (14) | 0 (0) |
| Yes | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Not known | 4 (6) | 0 (0) | 0 (0) | 12 (18) | 3 (5) | 0 (0) |
| Rectal bleeding at 2 weeks | ||||||
| No | 63 (91) | 2 (3) | 0 (0) | 50 (77) | 0 (0) | 0 (0) |
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Not known | 4 (6) | 0 (0) | 0 (0) | 15 (23) | 0 (0) | 0 (0) |
| Tenesmus at 2 weeks | ||||||
| No | 64 (93) | 1 (1) | 0 (0) | 49 (75) | 0 (0) | 0 (0) |
| Yes | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
| Not known | 4 (6) | 0 (0) | 0 (0) | 15 (23) | 0 (0) | 0 (0) |
| Constipation at 2 weeks | ||||||
| No | 58 (84) | 4 (6) | 0 (0) | 48 (74) | 0 (0) | 0 (0) |
| Yes | 3 (4) | 0 (0) | 0 (0) | 1 (2) | 1 (2) | 0 (0) |
| Not known | 4 (6) | 0 (0) | 0 (0) | 15 (23) | 0 (0) | 0 (0) |
| Intermenstrual bleeding at 2 weeks | ||||||
| No | 53 (77) | 8 (12) | 1 (1) | 41 (63) | 5 (8) | 0 (0) |
| Yes | 2 (3) | 1 (1) | 0 (0) | 2 (3) | 0 (0) | 0 (0) |
| Not known | 4 (6) | 0 (0) | 0 (0) | 15 (23) | 2 (3) | 0 (0) |
| Post-coital bleeding at 2 weeks | ||||||
| No | 58 (84) | 5 (7) | 0 (0) | 40 (62) | 5 (8) | 0 (0) |
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Not known | 6 (9) | 0 (0) | 0 (0) | 18 (28) | 2 (3) | 0 (0) |
| Symptom | Treatment group, n (%) | |||
|---|---|---|---|---|
| Ceftriaxone (N = 293) | Gentamicin (N = 292) | |||
| Symptom present at baseline | Symptom present at baseline | |||
| No | Yes | No | Yes | |
| Genital discharge at 2 weeks | ||||
| No | 156 (53) | 98 (33) | 123 (42) | 124 (42) |
| Yes | 0 (0) | 2 (1) | 0 (0) | 4 (1) |
| Not known | 14 (5) | 23 (8) | 16 (5) | 25 (9) |
| Dysuria at 2 weeks | ||||
| No | 169 (58) | 84 (29) | 131 (45) | 110 (38) |
| Yes | 1 (< 0.5) | 2 (1) | 0 (0) | 10 (3) |
| Not known | 19 (6) | 18 (6) | 19 (7) | 22 (8) |
| Anorectal pain at 2 weeks | ||||
| No | 243 (83) | 11 (4) | 245 (84) | 3 (1) |
| Yes | 1 (< 0.5) | 1 (< 0.5) | 1 (< 0.5) | 2 (1) |
| Not known | 35 (12) | 2 (1) | 40 (14) | 1 (< 0.5) |
| Rectal discharge at 2 weeks | ||||
| No | 244 (83) | 10 (3) | 244 (84) | 5 (2) |
| Yes | 1 (< 0.5) | 1 (< 0.5) | 0 (0) | 2 (1) |
| Not known | 37 (13) | 0 (0) | 39 (13) | 2 (1) |
| Sore throat at 2 weeks | ||||
| No | 213 (73) | 34 (12) | 211 (72) | 33 (11) |
| Yes | 4 (1) | 5 (2) | 4 (1) | 3 (1) |
| Not known | 31 (11) | 6 (2) | 37 (13) | 4 (1) |
| Rectal bleeding at 2 weeks | ||||
| No | 247 (84) | 5 (2) | 243 (83) | 7 (2) |
| Yes | 3 (1) | 1 (< 0.5) | 1 (< 0.5) | 0 (0) |
| Not known | 36 (12) | 1 (< 0.5) | 40 (14) | 1 (< 0.5) |
| Tenesmus at 2 weeks | ||||
| No | 249 (85) | 6 (2) | 248 (85) | 3 (1) |
| Yes | 0 (0) | 1 (< 0.5) | 0 (0) | 0 (0) |
| Not known | 37 (13) | 0 (0) | 40 (14) | 1 (< 0.5) |
| Constipation at 2 weeks | ||||
| No | 244 (83) | 6 (2) | 244 (84) | 3 (1) |
| Yes | 5 (1) | 1 (< 0.5) | 4 (1) | 0 (0) |
| Not known | 37 (13) | 0 (0) | 41 (14) | 0 (0) |
| Characteristic | Treatment group | |||
|---|---|---|---|---|
| Ceftriaxone | Gentamicin | |||
| Without clearance data (N = 56) | With clearance data (N = 306) | Without clearance data (N = 66) | With clearance data (N = 292) | |
| Age at randomisation (years) | ||||
| Mean (SD) | 28.6 (8.9) | 30.5 (10.3) | 27.8 (8.7) | 31.1 (10.1) |
| Median (25th percentile, 75th percentile) | 26.2 (22.2, 32.1) | 27.7 (22.7, 35.2) | 25.8 (21.7, 31.6) | 29 (23.2, 35.9) |
| Minimum, maximum | 18.7, 57.4 | 16.1, 70.2 | 16.5, 51.1 | 17.1, 68.4 |
| Sex, n (%) | ||||
| Male | 50 (89) | 243 (79) | 47 (71) | 245 (84) |
| Female | 6 (11) | 63 (21) | 19 (29) | 46 (16) |
| Other | 0 (0) | 0 (0) | 0 (0) | 1 (< 0.5) |
| Ethnicity, n (%) | ||||
| White | 34 (61) | 207 (68) | 47 (71) | 208 (71) |
| Black | 11 (20) | 42 (14) | 9 (14) | 39 (13) |
| Asian | 5 (9) | 21 (7) | 3 (5) | 15 (5) |
| Mixed race | 4 (7) | 23 (8) | 7 (11) | 19 (7) |
| Other | 2 (4) | 13 (4) | 0 (0) | 11 (4) |
| Country of birth, n (%) | ||||
| UK | 38 (68) | 220 (72) | 51 (77) | 202 (69) |
| Other | 18 (32) | 86 (28) | 15 (23) | 90 (31) |
| If other, region | ||||
| Europe (non-UK) | 4 (7) | 14 (5) | 4 (6) | 10 (3) |
| North America | 1 (2) | 17 (6) | 3 (5) | 11 (4) |
| Asia Pacific | 8 (14) | 43 (14) | 5 (8) | 51 (17) |
| Latin America | 1 (2) | 6 (2) | 1 (2) | 10 (3) |
| Middle East | 1 (2) | 1 (< 0.5) | 1 (2) | 4 (1) |
| Africa | 3 (5) | 5 (2) | 1 (2) | 4 (1) |
| Creatinine level (µmol/l) | ||||
| Mean (SD) | 78.6 (18) | 78.6 (14.9) | 74.2 (13) | 79.1 (16.2) |
| Median (25th percentile, 75th percentile) | 76 (67, 87) | 78 (69, 88) | 72 (63.5, 84) | 77 (69, 87) |
| Minimum, maximum | 42, 124 | 45, 137 | 51, 104 | 26, 154 |
| n | 51 | 292 | 52 | 280 |
| Medical history, n (%) | ||||
| Diabetes mellitus | 0 (0) | 3 (1) | 0 (0) | 1 (< 0.5) |
| Otitis media | 0 (0) | 9 (3) | 1 (2) | 6 (2) |
| Renal disease | 0 (0) | 3 (1) | 1 (2) | 3 (1) |
| Liver disease | 1 (2) | 7 (2) | 2 (3) | 3 (1) |
| Immunodeficiency | 5 (9) | 29 (9) | 2 (3) | 22 (8) |
| Any known drug allergies | 2 (4) | 15 (5) | 7 (11) | 18 (6) |
| Participants with infection at each site, n (%) | ||||
| Genital | 36 (64) | 154 (50) | 45 (68) | 174 (60) |
| Pharyngeal | 15 (27) | 113 (37) | 26 (39) | 102 (35) |
| Rectal | 22 (39) | 137 (45) | 28 (42) | 119 (41) |
| Description | Severity | Outcome | Treatment group |
|---|---|---|---|
| Pain radiating down left leg since injection. Difficulty standing | Moderate | Recovered/resolved | Ceftriaxone |
| Folliculitis | Mild | Recovered/resolved | Ceftriaxone |
| Very strong stomach ache | Mild | Recovered/resolved | Ceftriaxone |
| Ear pain | Mild | Unknown | Ceftriaxone |
| Right inner ear pain | Mild | Recovered/resolved | Ceftriaxone |
| Headache | Mild | Recovered/resolved | Ceftriaxone |
| Dyspepsia | Mild | Ongoing | Ceftriaxone |
| Occasional diarrhoea | Mild | Ongoing | Ceftriaxone |
| Headache | Mild | Recovered/resolved | Ceftriaxone |
| Cold/flu-like symptoms | Mild | Ongoing | Ceftriaxone |
| Headache | Mild | Recovered/resolved | Ceftriaxone |
| Diarrhoea | Moderate | Ongoing | Ceftriaxone |
| Headache | Mild | Recovered/resolved | Ceftriaxone |
| Tinitus | Mild | Recovered/resolved | Ceftriaxone |
| Creatinine level increased from 87 µmol/l in v1 on 15 January 2016 to 123 µmol/l in v2 on 3 February 2016 | Mild | Ongoing | Ceftriaxone |
| Vulval thrush (candida) | Mild | Ongoing | Ceftriaxone |
| Loose stools | Mild | Recovered/resolved | Ceftriaxone |
| Headache | Mild | Recovered/resolved | Ceftriaxone |
| Diarrhoea | Mild | Recovered/resolved | Ceftriaxone |
| Grade 4 dizziness | Severe | Recovered/resolved | Ceftriaxone |
| Felt like been kicked by a horse | Mild | Recovered/resolved | Ceftriaxone |
| Bell’s palsy | Mild | Ongoing | Ceftriaxone |
| Tonsillitis | Mild | Recovered/resolved | Ceftriaxone |
| Discomfort in left upper abdomen | Mild | Recovered/resolved | Ceftriaxone |
| Lethargy | Moderate | Recovered/resolved | Ceftriaxone |
| Nausea | Mild | Recovered/resolved | Ceftriaxone |
| Running nose for 2 days | Mild | Recovered/resolved | Ceftriaxone |
| Fatigue | Moderate | Ongoing | Ceftriaxone |
| Chesty cough | Moderate | Ongoing | Ceftriaxone |
| Fatigue | Mild | Recovered/resolved | Ceftriaxone |
| Cellulitis | Mild | Ongoing | Ceftriaxone |
| Skin itch and bumps | Moderate | Ongoing | Ceftriaxone |
| Septic tonsillitis | Moderate | Recovered/resolved | Ceftriaxone |
| Pregnancy at visit 2 | Mild | Recovered/resolved | Ceftriaxone |
| High creatinine level | Mild | Ongoing | Ceftriaxone |
| Migraine | Mild | Ongoing | Ceftriaxone |
| Diarrhoea | Mild | Ongoing | Ceftriaxone |
| Toothache | Mild | Ongoing | Ceftriaxone |
| Rectal bleeding | Mild | Recovered/resolved | Ceftriaxone |
| Right lower-leg cellulitis | Moderate | Ongoing | Ceftriaxone |
| Diarrhoea | Mild | Recovered/resolved | Ceftriaxone |
| Aching back | Mild | Ongoing | Ceftriaxone |
| Aching neck | Mild | Ongoing | Ceftriaxone |
| Bleeding on wiping post-BO (perianal warts) | Mild | Ongoing | Ceftriaxone |
| Fever (39.6 °C) | Mild | Recovered/resolved | Ceftriaxone |
| Loose stool | Mild | Recovered/resolved | Ceftriaxone |
| Increased anxiety | Mild | Recovered/resolved | Ceftriaxone |
| Flu-like symptoms | Mild | Recovered/resolved | Ceftriaxone |
| Sore throat | Mild | Recovered/resolved | Ceftriaxone |
| Headache | Mild | Recovered/resolved | Ceftriaxone |
| Creatinine level increased from 76 to 124 mg/dl | Mild | Recovered/resolved | Ceftriaxone |
| Raised creatinine level | Mild | Recovered/resolved | Ceftriaxone |
| Anorectal pain | Mild | Recovered/resolved | Ceftriaxone |
| Deranged creatinine level | Mild | Ongoing | Ceftriaxone |
| Diarrhoea | Mild | Recovered/resolved | Gentamicin |
| Diarrhoea | Mild | Recovered/resolved | Gentamicin |
| Diarrhoea | Mild | Recovered/resolved | Gentamicin |
| Cold symptoms | Mild | Ongoing | Gentamicin |
| Abdominal pain | Mild | Recovered/resolved | Gentamicin |
| Lethargy | Mild | Recovered/resolved | Gentamicin |
| Mouth ulcers | Mild | Ongoing | Gentamicin |
| Diarrhoea | Mild | Recovered/resolved | Gentamicin |
| Headache | Moderate | Recovered/resolved | Gentamicin |
| Diarrhoea | Mild | Recovered/resolved | Gentamicin |
| Bloated | Mild | Recovered/resolved | Gentamicin |
| Diarrhoea | Severe | Recovered/resolved | Gentamicin |
| Viral URTI | Mild | Ongoing | Gentamicin |
| Visit 2 bloods: hyperkalaemia (potassium level 6.1). Rpt = 5.1. Likely to be artefactual | Moderate | Recovered/resolved | Gentamicin |
| Nausea | Mild | Recovered/resolved | Gentamicin |
| Dizziness | Mild | Recovered/resolved | Gentamicin |
| Nausea | Mild | Recovered/resolved | Gentamicin |
| Rash | Mild | Ongoing | Gentamicin |
| Sore throat | Mild | Ongoing | Gentamicin |
| Raised creatinine level at visit 2: 106 umol/l (grade: mild) | Mild | Ongoing | Gentamicin |
| Raised potassium level result at visit 1: 5.6 mmol/l | Mild | Recovered/resolved | Gentamicin |
| Rectal itching | Mild | Recovered/resolved | Gentamicin |
| Stomach cramps | Mild | Recovered/resolved | Gentamicin |
| Heartburn | Mild | Recovered/resolved | Gentamicin |
| Pain at injection site (buttock) | Mild | Ongoing | Gentamicin |
| Sickness | Severe | Recovered/resolved | Gentamicin |
| Pain at site of injection | Mild | Ongoing | Gentamicin |
| Raised creatinine level | Mild | Ongoing | Gentamicin |
| Left eye conjunctivitis | Mild | Unknown | Gentamicin |
| Diarrhoea | Mild | Recovered/resolved | Gentamicin |
| Diarrhoea | Mild | Recovered/resolved | Gentamicin |
| Diarrhoea | Mild | Ongoing | Gentamicin |
| Tonsillitis | Moderate | Recovered/resolved | Gentamicin |
| URTI | Moderate | Recovered/resolved | Gentamicin |
| Diarrhoea | Mild | Recovered/resolved | Gentamicin |
| Stiff arms | Moderate | Ongoing | Gentamicin |
| Diarrhoea (one episode, type 6, no blood or mucous) | Mild | Recovered/resolved | Gentamicin |
| Diarrhoea (one episode, type 7, no blood or mucous) | Mild | Recovered/resolved | Gentamicin |
| Myalgia | Mild | Recovered/resolved | Gentamicin |
| Diarrhoea | Mild | Recovered/resolved | Gentamicin |
| Diarrhoea | Mild | Recovered/resolved | Gentamicin |
| Bilateral flank pain – intermittent/stabbing | Mild | Recovered/resolved | Gentamicin |
| Back pain | Moderate | Recovered/resolved | Gentamicin |
| Drug name | Treatment group |
|---|---|
| Ceftriaxone, 500 mg stat | Gentamicin |
| Azithromycin, 1 g stat p.o. | Ceftriaxone |
| Azithromycin, 1 g p.o. | Ceftriaxone |
| Doxycycline | Ceftriaxone |
| Doxycycline | Ceftriaxone |
| Doxycycline | Gentamicin |
| Trimethoprim | Gentamicin |
| Ceftriaxone | Gentamicin |
| Doxycycline | Gentamicin |
| Doxycycline | Gentamicin |
| Doxycycline, 100 mg | Ceftriaxone |
| Metronidazole | Gentamicin |
| Ceftriazone | Gentamicin |
| Clarithromycin | Ceftriaxone |
| Doxycycline | Ceftriaxone |
| Doxycycline | Ceftriaxone |
| Ofloxacin | Gentamicin |
| Doxycycline | Ceftriaxone |
| Doxycycline | Gentamicin |
| Doxycycline, 100 mg b.i.d. | Gentamicin |
| Doxycycline | Gentamicin |
| Doxycycline | Gentamicin |
| Doxycycline | Ceftriaxone |
| Doxycycline | Ceftriaxone |
| Doxycycline | Gentamicin |
| Metronidazole, 400-mg tablet | Ceftriaxone |
| Azithromycin | Gentamicin |
| Doxycycline | Gentamicin |
| Doxycycline | Ceftriaxone |
| Details | Treatment group |
|---|---|
| Participant is heterosexual and a rectal swab was taken and there were no indications to take this | Gentamicin |
| Participant is heterosexual & a pharyngael swab was taken and there were no indications to take this | Gentamicin |
| wrong blood test requested = no creatinine done on pt | Ceftriaxone |
| No microscopy done on FU- NAAT and cultures taken only which were negative | Ceftriaxone |
| Creatinine not done as wrong test requested on form- too late to add on as blood destroyed after 7d | Ceftriaxone |
| Patient did not have full examiniation done at FU as was only oral GC pos | Gentamicin |
| Clinical examination not done at FU appt as patient asymptomatic needed for all patients though | Ceftriaxone |
| Examination not performed on Follow Up Visit | Ceftriaxone |
| Rectal GC culture not obtained on Visit 2 | Ceftriaxone |
| Pt allergic to penicillin wrongly randomized | Ceftriaxone |
| Pt withdrawn due to change of clinicians decision. Pt treated for PID | Gentamicin |
| All tests on V1 obtained as per protocol partly discarded as Pt could not continue the study | Gentamicin |
| Pt randomized into the trial before microscopy results available – not eligible due to BV infection | Ceftriaxone |
| Creatinine test not performed by LAB CK done instead. Called the Lab the samples already destroyed | Gentamicin |
| Creatinine blood test not performed by Lab CK test done instead-contacting the Lab-sample destroyed | Ceftriaxone |
| oral NAAT & culture tests obtained on Visit 2 – while not required | Gentamicin |
| participant declined clinical examination at follow-up | Gentamicin |
| Creatinin result unavailable due to unlabled specimen | Ceftriaxone |
| Dose Administration Details not entered on Randomisation system by the injecting nurse | Ceftriaxone |
| isolate not saved | Gentamicin |
| VAS not done at baseline | Ceftriaxone |
| test result of recal culture not obtainable from lab | Gentamicin |
| test result of pharyngeal culture not obtainable from lab | Gentamicin |
| isolate not saved | Ceftriaxone |
| isolate not saved | Ceftriaxone |
| Pt declined urethral GC culture test on FU visit | Ceftriaxone |
| Gc not isolated | Ceftriaxone |
| Incorrect labelling of sample | Ceftriaxone |
| Oral NAAT not repeated on visit 2 | Gentamicin |
| Oral Culture not taken on 2nd visit | Gentamicin |
| no creatinine results sample request lost in transit | Ceftriaxone |
| Patient declined rectal swab as no risk of infection he percieved | Gentamicin |
| Visit 2 culture sample not taken | Ceftriaxone |
| Creatine result unavailable sample not recieved by QEHB lab | Ceftriaxone |
| Study team aware pregnancy test not taken before randomisation. Patient given treatment when pregna[nt] | Gentamicin |
| unable to obtain blood samples creatinine result available from 05.10.16 = 80 | Ceftriaxone |
| only creatinine blood taken unable to bleed patient for immune response samples | Ceftriaxone |
| BD NAATs from × 3 sites accidently discarded at baseline | Ceftriaxone |
| Pharangeal culture not sent at baseline in error | Ceftriaxone |
| There were not freezing isolates at this particular time | Gentamicin |
| U + E not sent for serum creatinine in error | Ceftriaxone |
| Urethral and rectal cultures not sent in error | Ceftriaxone |
| No throat NAAT/culture sent at visit 2 in error | Ceftriaxone |
| lab stopped freezing isolate at this time | Ceftriaxone |
| Visit 1 U + E sample not labelled so result unavailable | Gentamicin |
| Immune samples not sent at visit 2 as no kits available | Ceftriaxone |
| Aptima combo samples not sent at visit 2 in error | Ceftriaxone |
| VAS not completed by patient at visit 1 in error | Ceftriaxone |
| Lab was not keeping isolates | Gentamicin |
| U + E not sent at visit 2 in error | Gentamicin |
| VAS not done at visit 2 in error | Gentamicin |
| Urethral GC culture unable to exclude GC due to lab error | Gentamicin |
| follow-up was performed 13 post baseline thus fell before the 14 days post baseline required | Ceftriaxone |
| U + Es not taken. Pt IVDU and refused after immune samples taken | Gentamicin |
| Lab was not keeping isolates | Gentamicin |
| lab Stopped frezzing isolate at this tiem | Ceftriaxone |
| RECTAL SAMPLING NOT SEN IN ERROR | Gentamicin |
| Rectal testing not done at baseline in error | Gentamicin |
| rectal sampling not done in error | Ceftriaxone |
| VAS not completed at visit 2 in error | Ceftriaxone |
| Clinical lab did not save | Ceftriaxone |
| Patients clinic number not on tracking sheet so lab could not follow up result | Gentamicin |
| lost on subculture | Gentamicin |
| VAS not completed at visit 1 in error | Gentamicin |
| VAS not complete | Gentamicin |
| immune response not taken | Gentamicin |
| creatinine not taken | Gentamicin |
| Sample left in incubator for too long – unable to process in lab | Gentamicin |
| v 2 ocurred day12 as patietn unable to coemanother day | Gentamicin |
| Creatinine resutls not available due to lab error | Gentamicin |
| Follow up: incorrectly sampled pharynx in place of rectum | Ceftriaxone |
| Visual Analogue Scale not done at this visit | Ceftriaxone |
| Visit 2 2 weeks post visit 1 but not scheduled. No G-TOG delegated Dr available. Seen by clinic staf | Ceftriaxone |
| Immune study bloods not performed in error | Ceftriaxone |
| Couldnt access MACRO db. Paper CRF not available some data not collected. Immune visit 2 blood ND | Ceftriaxone |
| Culture sample was not transferred to STBRUa – lab error | Ceftriaxone |
| No Side effects or use of NHS service info collected in error | Gentamicin |
| No Visit 2 immune study bloods collected in error | Gentamicin |
| baseline NAAT tests not obtained only pre-trial rectal NAAT +ve for GC. baseline culture was taken | Gentamicin |
| Culture sample was not transferred to STBRUa – lab error | Gentamicin |
| Throat sample not taken. Rectal sample not taken as patient did not admit to anal sex | Ceftriaxone |
| VAS not completed in error | Ceftriaxone |
| doxycycline prescribed in addition to azithromycin | Gentamicin |
| Doxycycline erroneously prescribed in addition to G-TOG drug/azithromycin | Gentamicin |
| failed to send urine sample for lab analysis | Ceftriaxone |
| Full sampling profile not taken on baseline visit. Rectal and urethral NAAT and cultures missing | Ceftriaxone |
| Culture sample was not transferred to STBRU.a Lab error | Gentamicin |
| rectal and urethral NAAT/ culture not taken at baseline. PI error | Gentamicin |
| Full sampling profile on baseline incomplete. Pharynx and urine NAAT/cultures missing. PI error | Ceftriaxone |
| Culture sample was not transferred to STBRU.a Lab error | Ceftriaxone |
| Full sampling profile on visit 1 incomplete. Urine and rectal NAAT/culture not taken | Ceftriaxone |
| rectal and throat culture not taken at baseline | Gentamicin |
| urine NAAT was missing. No results from the laboratory | Gentamicin |
| at TOC visit throat culture but not TMA was obtained. Pt was recalled TMA taken 4 days later | Gentamicin |
| lost to follow up. pt reattended for screen 23/1/16 but too late for study. All GC swabs negative | Gentamicin |
| urine sample was missing from the laboratory | Gentamicin |
| patient not keen on signing pt. 5 of consent. Discussed with [name] happy for data to be included | Ceftriaxone |
| incomplete sampling profile. Rectal NAAT/ culture not taken | Ceftriaxone |
| Culture sample was not transferred to STBRUa – lab error | Gentamicin |
| lost to follow up | Ceftriaxone |
| sampling error. rectal sample was not taken by mistake | Ceftriaxone |
| Culture sample was not transferred to STBRUa – lab error | Ceftriaxone |
| Culture sample was not transferred to STBRUa – lab error | Gentamicin |
| Patient decline rectal swab to be taken as never had rectal swabs before. No history of anal sex | Gentamicin |
| Culture sample was not transferred to STBRUa – lab error | Gentamicin |
| Culture sample was not transferred to STBRU.a Lab error | Ceftriaxone |
| lost to follow up | Ceftriaxone |
| lost to follow up | Gentamicin |
| patient declined rectal sample to be taken. no rectal contact on baseline | Gentamicin |
| Prescribed doxcycline from [clinic name]. Has had 3 doses before coming for G-TOG follow up appt | Gentamicin |
| lost to follow up | Gentamicin |
| Patient withdrew consent for study | Ceftriaxone |
| patient declined questionnaire and bloods at follow up visit due to time constraints | Ceftriaxone |
| pt received doxycyline for 1 week due to rectal chlamydia positive result | Ceftriaxone |
| Pt DNA TOC visit. emaield and siad he went to [clinic name] for tests on 17/8/16 & was all clear. No SEs | Gentamicin |
| Blood sample taken from participant on 20-FEB-2015 and deliverd to the lab for creatinine testing. M | Gentamicin |
| Some culture plates were incorrectly discarded in the laboratory and this subjects plate was amongst | Gentamicin |
| rectal culture sample was not transferred to STBRU.a ISOLATE WAS NOT SAVED | Gentamicin |
| NO CREATINE RESULT MARKED AS DONE | Gentamicin |
| NO REPEAT VVS DONE AT FOLLOW UP | Gentamicin |
| No result for urethral culture as marked as done but no result on server | Ceftriaxone |
| PHARYNX POSITIVE ON RESULT BUT ISOLOTE WAS NOT SAVED BY THE LABORATORY | Gentamicin |
| no result for creatitine marked as done but no result on server | Ceftriaxone |
| uretral sample not done two cultures were sent for pharynx both were reported as negative | Ceftriaxone |
| THE ISOLATE WAS NOT SAVED BY THE LAB FOROM THE CERVIX CULTURE THAT CAME BACK POSITIVE FOR GC | Gentamicin |
| patient did not attend follow up | Gentamicin |
| Blood sample too old to process | Ceftriaxone |
| pharynx result culture not available lab had discarded sample and apologized as this was a mistake | Ceftriaxone |
| CREATITINE BLOOD TOO OLD TO PROCESS | Gentamicin |
| culture not obtained at visit 2 my mistake sorry | Gentamicin |
| NO BLOOD KITS AVAILABLE FOR IMMUNE RESPONSE | Ceftriaxone |
| patient reattended on the 3NOV 2015 as she tested too early on follow up her results came back NEG | Ceftriaxone |
| Isolate was not saved from the cervix and sent to STBRUa | Ceftriaxone |
| DID NOT ATTEND HIS FOLLOW UP APPOINTMENT | Gentamicin |
| Did not attend follow up appointment | Gentamicin |
| NO BLOOD KITS AVAILABLE SO BLOODS NOT DONE FOR IMMUNE RESPONSE | Gentamicin |
| NO RESULT ON SERVER FOR CREATITNE BLOODS | Gentamicin |
| culture only taken from Pharynx at this visit | Gentamicin |
| patient attended follow up at 13 days as she could not attend aftre this date | Ceftriaxone |
| vvs not repeated as already had a positive result now aware a n additional one should have been don | Ceftriaxone |
| culture not repeated at follow up as all were negative sorry should have repeated | Ceftriaxone |
| natts were not repeated at this visit as were all done 05-10-2015 al cultures were done | Gentamicin |
| urethral sample positive but isilate was not saved by the labs and sent to mSTBTU | Ceftriaxone |
| NO RESULT AVAIALBLE FOR UREATHRAL CULTURE AS PLATE WAS UNLABELED | Ceftriaxone |
| creatitne blood taken but reported as too old to test | Ceftriaxone |
| ISOLATE WAS NOT SAVED BY THE LABS AND WAS NOT SENT TO STBRUa | Ceftriaxone |
| lost to follow up | Gentamicin |
| INSUFFICIENT SAMPLE FOR CREATITINE PATIENT DIFFICULT TO BLEED | Ceftriaxone |
| only pharynx sample was obtained at visit 1 | Ceftriaxone |
| sample lost in transport. Urine | Gentamicin |
| BLOODS DISPOSED AS UNBLINDED | Gentamicin |
| PROBLEM AT FIRST VISIT WITH CULTURES NOT INCUBATED FOR THE RIGHT AMOUNT OF TIME SO THESE WERE REPEAT | Ceftriaxone |
| result not available reported as plate not inoculated | Ceftriaxone |
| rectal sample not done | Ceftriaxone |
| did not attend follow up | Gentamicin |
| sample reported as unlabelled so not processed creatitine creatitne sample | Ceftriaxone |
| UREATHRAL SAMPLE ISOLATE WAS NOT SAVED AND SENT TO STBRUa | Ceftriaxone |
| RECTAL CULTURE ISOLATE WAS NOT SAVED AND SENT TO STBRUa BY THE LABS | Gentamicin |
| unable to obtain bloods 2 × attempts .Follow up visit 28-JAN-2016 CREATITNE AND IMMUNE RESPONSE BLOO | Ceftriaxone |
| creatine kinase result given not creatitne as requested | Ceftriaxone |
| THE ISOLATE WAS NOT SAVED BY THE LAB | Ceftriaxone |
| BLOOD WAS TAKEN BUT NOT PROCESSED AS ARRIVED AT LAB LATE | Ceftriaxone |
| no immune kits available | Gentamicin |
| creatitine result not available as wasnt recieved in lab for 24 hours and too old to process | Gentamicin |
| no culture result for cervix report states plate not innoculated | Gentamicin |
| At visit 1 patient left department with his urine so no sample collected at this visit | Gentamicin |
| lost to follow up | Gentamicin |
| the pharyngeal culture isolate was not saved by the lab | Ceftriaxone |
| DID NOT ATTEND FOLLOW UP | Ceftriaxone |
| patient declined to have bloods done | Ceftriaxone |
| patient declined to have bloods done | Ceftriaxone |
| did not attend follow up | Ceftriaxone |
| no creatitine result as patient declined bloods | Gentamicin |
| did not attend follow up | Ceftriaxone |
| did not attend follow up | Gentamicin |
| blood creatitine taken but no result on server | Gentamicin |
| did not attend follow up | Gentamicin |
| PATIENT ATTENDED FOR TOC AT 7 DAYS THEN ATTENDED DAY AFTER THAT AND WAS RETREATED AS HAD SEXUAL CON | Gentamicin |
| no creatitne result on server sample lost in transit or not processed | Gentamicin |
| unable to obtain bloods 3 attempts became distressed | Ceftriaxone |
| CREATITINE TOO OLD TO TEST BY THE TIME IT REACHEDCTHE LAB | Ceftriaxone |
| NO BLOOD KITS AVAILABLE | Gentamicin |
| NO BLOOD KITS AVAILABLE | Ceftriaxone |
| no blood kits available | Gentamicin |
| patient did not attend follow up | Gentamicin |
| ISOLATE WAS NOT SAVED AS ORGANISM DIED ON PHARNX PLATE | Gentamicin |
| no result for creatinine on server may have been lost in transit | Gentamicin |
| DECLINED BLOODS AT VISIT 2 | Gentamicin |
| VAS SCORE NOT DONE AS PATIENT DECLINED DID NOT HAVE THE INJECTION. DECLINED | Gentamicin |
| No VAS sheet for visit 2 verbal from patient no change and documented on VAS sheet visit 1 by nurse | Gentamicin |
| Microscopy not done in error | Gentamicin |
| NAAT visit1 no result on system & pt notes no longer on site therefore pre randomistion input on mac | Gentamicin |
| VAS sheet not given @visit2 pt asked by nurse re:changes and written on VAS sheet visit 1 | Gentamicin |
| unable to clarify if doxycycline px and taken notes no longer on site no written info on system | Gentamicin |
| Urine TMA sample lost so no result | Ceftriaxone |
| No creatinine result – sent to lab? lost no result | Ceftriaxone |
| VASsheet visit 2 not given to pt documented on VAS sheet visit 1 same value as per pt says | Ceftriaxone |
| Visit 2 was done on day 13 and not after day 14 as included day of visit as day1 so visit early | Ceftriaxone |
| VAS sheet not given @ visit2 documented on VAS sheet visit 1 not change in value as per patient | Ceftriaxone |
| In error- incorporated pretrial blood with visit1 as complete screen so only NAAT pretrial results | Ceftriaxone |
| Vas sheet visit 2 not given documented on vas sheet Visit1 same value as per patient stated | Ceftriaxone |
| Vas score for visit 2 not completed in error | Gentamicin |
| No creatinine result from visit 1. Lab error they performed hepatic screen and not renal screen | Gentamicin |
| Vas sheet not given visit 2 written on vas sheet from visit 1 so 1 vas sheet in total | Gentamicin |
| No creatinine result form baseline visit check with cmft labs no listed sample so ?? lost in transit | Ceftriaxone |
| No Creatinine result sample not done in error.PI aware | Gentamicin |
| Urethral microscopy not performed at visit 2 in error | Ceftriaxone |
| Visual Analogue scale not completed in error | Gentamicin |
| Retreated for GC by GUM 17/11/2015 as non compliance | Ceftriaxone |
| Never attended for visit 2 | Ceftriaxone |
| Creatinine not done in error so no result for this visit | Ceftriaxone |
| Culture plates not taken from sites 5 and 2 dr decision | Ceftriaxone |
| Naat testinfg not taken from site 2 & 5 dr decision | Ceftriaxone |
| No NAAT for PHarynx taken at baseline. Unknown reason why as culture was obtained | Ceftriaxone |
| Culture not taken in error | Gentamicin |
| No follow up examination made. – patient believed to be a false pos on micro | Gentamicin |
| No RECtal NAAT or Culture taken at follow up- patient believed to be negative | Gentamicin |
| dr decision not to test UR culture | Gentamicin |
| VAS scale not completed by participant at visit 2 | Gentamicin |
| patient declined some baseline swabs -no UR culture taken | Ceftriaxone |
| Unable to obtain visit 2 immunology bloods due to poor blood flow | Ceftriaxone |
| source data missing for weight assume visually patient weight greater than 40 kg | Ceftriaxone |
| Clinician unaware immunology samples required at follow up. Education provided | Gentamicin |
| Patient reports no receptive anal sex therefore original assessing clinician did not take | Ceftriaxone |
| VAS score not taken at follow up | Ceftriaxone |
| Immunology bloods not taken at follow up | Gentamicin |
| VAS not taken at Follow up | Gentamicin |
| Bacterial Vaginosis at visit 1 | Ceftriaxone |
| Creatinine blood not taken at visit 2 | Ceftriaxone |
| Cultures not taken visit 2 – user error – education provided | Ceftriaxone |
| VAS score not taken visit 2. Busy clinic | Ceftriaxone |
| Participant diagnosed with bacterial vaginosis at baseline | Gentamicin |
| Immunology bloods not taken at visit 2 | Gentamicin |
| Duration of pain not asked of patient. User Error – education provided | Gentamicin |
| Rectal swabs not taken at baseline- patient declined swabs | Gentamicin |
| Rectal NAAT not taken and urine taken at drs discretion | Gentamicin |
| Creatine blood not obtained at visit 1. The request was made but not recieved in labs | Ceftriaxone |
| Newly trained nurse was not aware that CREATINE WAS required at visit 1 – training provided | Ceftriaxone |
| Rectal and pharangeal swabs not taken at v1. this was a user error and a reminder was made | Gentamicin |
| Rec and additional swabs not taken on date of visit 1 – user error and training offered | Gentamicin |
| Rec and Ph cultures not taken at visit 2. User error and reminders were provided | Ceftriaxone |
| PH and REC swab not taken at visit 2 in error | Gentamicin |
| U and E blood taken at visit 1 but not recieved in labs. Local investigation taking place | Ceftriaxone |
| Rec swabs not taken in error | Gentamicin |
| Vas score not taken at visit two patient fainted so limited for time | Ceftriaxone |
| disgnosis of BV | Gentamicin |
| GRAM STAIN NOT REPEATED FOR RECTAL SLIDE POSITIVE AT BASELINE | Gentamicin |
| NAAT sample for pharynx result not available ? lost retaken pon 6th may | Gentamicin |
| Culture sample for phaynx result not available ?lost retaken on 6th May | Gentamicin |
| urethral culture plate sample not taken | Ceftriaxone |
| patient disclosed long standing chronic conditon Dr diagnosed PID | Gentamicin |
| Pharynx naat sample not taken no Oral Sex | Ceftriaxone |
| Pharynx culture not taken No oral sex | Ceftriaxone |
| No Rectal Naat done ? No Anal Sex | Gentamicin |
| No Rectal culture done ? No anal sex | Gentamicin |
| Urethral swab not done No penetrative sex | Ceftriaxone |
| NAAT Rectum sample not performed | Ceftriaxone |
| CULTURE Rectum sample not performed | Ceftriaxone |
| VISUAL ANALOGUE SCALE NOT COMPLETED | Ceftriaxone |
| phyical exam not done in error | Gentamicin |
| Pencillin allergy | Gentamicin |
| Follow-up visual analgue scale not done in error | Ceftriaxone |
| no rectal Naats result available for visit 1 | Gentamicin |
| pt left clinic before clinical examination swabs and bloods taken | Gentamicin |
| Rectal Naats and culture was not repeated at Visit 2 | Gentamicin |
| serum creatine not done in error | Gentamicin |
| The culture sample was not taken at visit 2 | Ceftriaxone |
| Culture not taken at Visit 2 | Ceftriaxone |
| Naat Testing performed as routine clinic appointment and not at V2 | Ceftriaxone |
| Culture testing not performed at routine visit also not performed at V2 | Ceftriaxone |
| Unable to obtain blood sampling at V2 – 3 × attempts | Ceftriaxone |
| Rectal culture not performed at Baseline | Ceftriaxone |
| GC culture (R) isolate not saved by laboratory | Ceftriaxone |
| Positive result for visit 2 Ur GC culture samples reported on Lastword – sample not stored by lab | Gentamicin |
| No creatinine result available from lab (lab error) | Gentamicin |
| Noted after patient left that GC diagnosis over 4/52 ago. PI & G-TOG team informed immediately | Ceftriaxone |
| Cepheid machine error – TH. Previous BD NAAT neg. Culture neg. AC NAAT sent. Will repeat at v2 for safety | Ceftriaxone |
| Error result on Cepheid machine for GC Th NAAT. Returned 09-May-2016 to repeat | Gentamicin |
| Triple site AC NAATs and cultures sent at visit 1 but local NAATs not repeated | Gentamicin |
| Site not clear from culture samples- will repeat triple site at visit 2 for completeness | Gentamicin |
| AC NAATs and cultures sent at visit 1 on 01-Jul-2016. Local NAATs not repeated | Ceftriaxone |
| Creatinine result not available-?mismatched sample/labelling error | Ceftriaxone |
| throat swab result shown as error on 22/08/16 at local lab. patient will be recalled to reswab throa | Ceftriaxone |
| Patient didnt come for repeat throat swab after multiple attempts to contact as dated on 05/09/16 | Ceftriaxone |
| technical error results with the machine for rectal swabs. patient will be recalled to reswab site | Ceftriaxone |
| PRE BASELINE SYPHILIS TEST WAS NEGATIVE BUT CONFIRMED RESULTS OF 12/09/2016 CAME AS POSITIVE | Gentamicin |
| Culture sample not taken from infected site at visit 2 - Oversight by nurse covering study visit | Ceftriaxone |
| Culture sample not taken at follow-up visit. Oversight by nurse covering visit | Gentamicin |
| No Creatinine visit 2 - porter failed to deliver sample in time for processing | Gentamicin |
| CREATININE SAMPLE NOT PROCESSED DUE TO PORTER FAILING TO DELIVER TO LAB | Gentamicin |
| Porter failed to deliver sample to path intime and sample was no processed | Ceftriaxone |
| CREATININE NOT TAKEN IN ERROR | Gentamicin |
| Copy of positive GC lab result not available at time of recruitment as tested at London clinic | Gentamicin |
| Symptom assessment /Follow up:genital discharge negative but the symptom resolution period unknown | Gentamicin |
| VISUAL ANALOGUE SCALE WAS NOT DONE ON VISIT 1 – BASELINE | Gentamicin |
| PATIENT ALLERGIC TO AZITHROMYCIN | Gentamicin |
| BV DIAGNOSED ON LOCAL SLIDE NOT SEEN | Gentamicin |
| PATIENT DECLINED NAAT AND CULTURE SET OF SCREENING SWABS RECTAL AND PHARYNX ONCE RANDOMISED | Gentamicin |
| PARTICIPANT DID NOT ATTEND HIS FOLLOW UP VISIT LOST TO FOLLOW UP AS PER PROTOCOL | Ceftriaxone |
| LOCAL LABORATORY URINE NAAT TEST NOT DONE/BASELINE VISIT | Gentamicin |
| PHARYNX LOCAL LABORATORY CULTURE TEST/FOLLOW UP VISIT NOT DONE | Gentamicin |
| FOLLOW UP VISIT DONE 13 DAYS AFTER THE BASELINE VISIT BY MISTAKE | Gentamicin |
| VISUAL ANALOGUE SCALE NOT DONE AT THE BASELINE VISIT | Ceftriaxone |
| VISUAL ANALOGUE SCALE AT THE FOLLOW UP VISIT NOT DONE | Ceftriaxone |
| VISUAL ANALOGUE SCALE NOT DONE | Ceftriaxone |
| CREATININE RESULT NOT AVAILABLE AT VISIT 1 | Gentamicin |
| NAAT AND CULTURE PHARYNGEAL TESTING NOT DONE IN THE F/U VISITNAAT POSITIVE ONLY IN SCREENING VISIT | Ceftriaxone |
| Pharynx NAAT negative on visit one retested by mistake in visit 2 | Gentamicin |
| PARTICIPANT WAS NOT ELIGIBLE | Gentamicin |
| Blood sample for creatinine issed in error. Nurses are aware they need to take this sample for futur | Gentamicin |
| culture of urethreal sample only taken in error. Aware need sample from all sites for next patient | Gentamicin |
| NAAT Pharyngeal sample taken in error at baseline visit | Ceftriaxone |
| Pharyngeal culture omitted in error at Follow up visit | Ceftriaxone |
| Rectal NAAT sample taken in error at follow up visit. Removed from eCRF as requested | Gentamicin |
| Visit 1 NAAT samples are missing from local IT system | Ceftriaxone |
| Pt tested in London told +ve came here for Rx & enrolled.Later found result equivocal | Gentamicin |
| Samples taken from rectum and pharynx for NAAT testing but no results available from lab | Ceftriaxone |
| Rectal sample not done | Ceftriaxone |
| Pharyngeal result not done (NAAT testing) | Ceftriaxone |
| Baseline urethra sample not available | Ceftriaxone |
| Blood sample not taken due to venepuncture difficulties | Ceftriaxone |
| Additional swab not taken for AC testing. Unknown reason | Ceftriaxone |
| No GC culture taken at Visit 1 from urethral site | Gentamicin |
| No GC CULTURE AT VISIT 1 FROM THE THROAT | Gentamicin |
| NO URETHRAL GC CULTURE TAKEN ON VISIT 1 | Ceftriaxone |
| DATE ON CHEMICAL PATHOLOGY REQUEST FORM MISREAD BY LAB STAFF AND SAMPLE NOT PROCESSED | Gentamicin |
| URETHRAL GC CULTURE NOT TAKEN | Ceftriaxone |
| THROAT CULTURE NOT TAKEN BY GUM STAFF PRIOR TO RANDOMISATION | Ceftriaxone |
| AC swab missed and not obtained at Enrolment visit | Gentamicin |
| Throat swab for NAAT not done at visit 2. Patient came back to have the sample taken on 2 Sept 2016 | Gentamicin |
| VAS not completed on baseline visit | Gentamicin |
| VAS form missed during the Visit 1 | Ceftriaxone |
| Weight not done on Visit 1 | Ceftriaxone |
FIGURE 17.
Distribution of genital gentamicin MIC. (a) All participants; and (b) by treatment response.
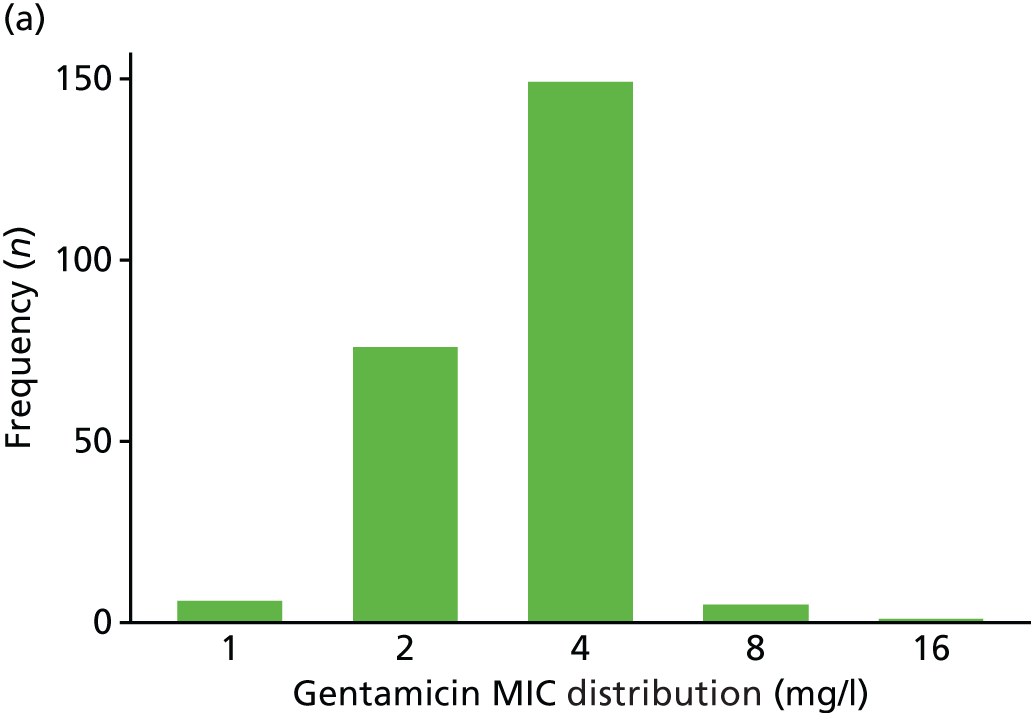
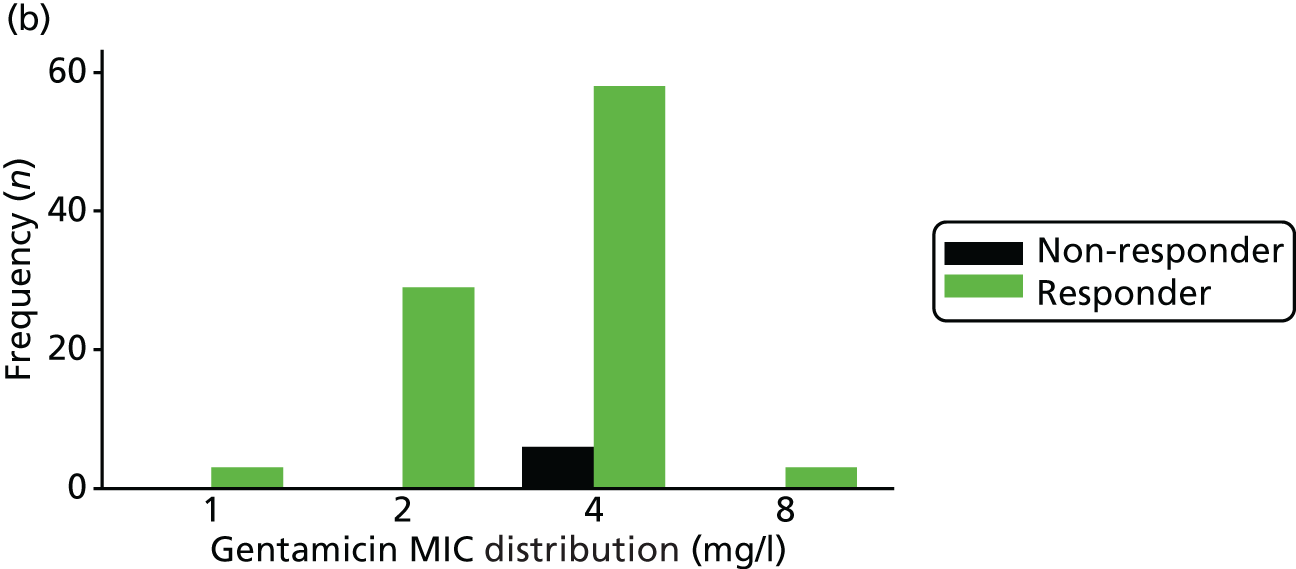
FIGURE 18.
Distribution of pharyngeal gentamicin MIC. (a) All participants; and (b) by treatment response.
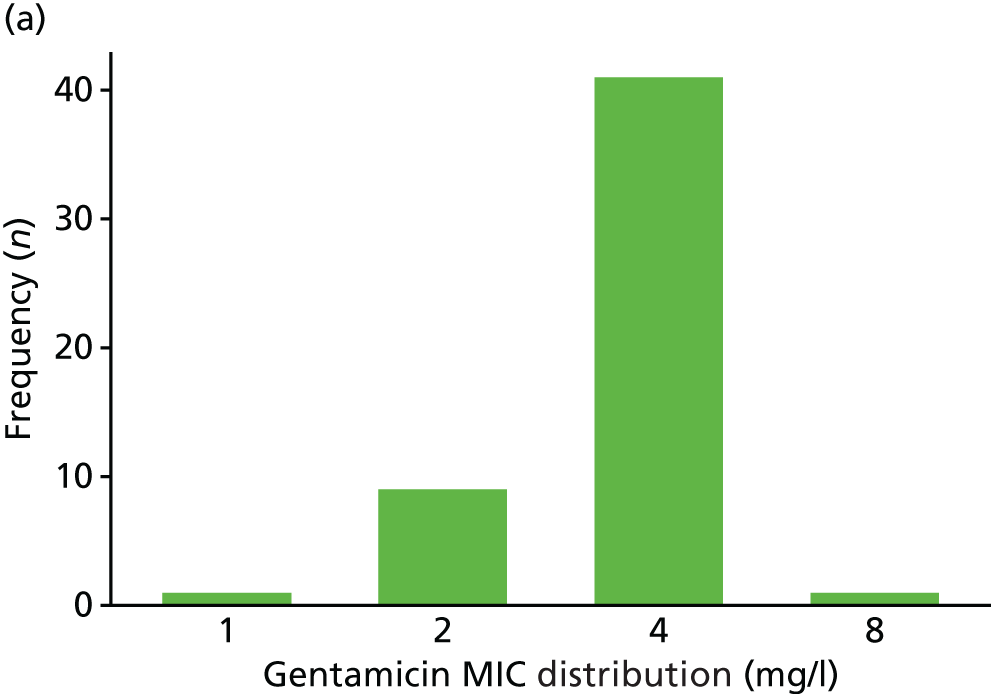
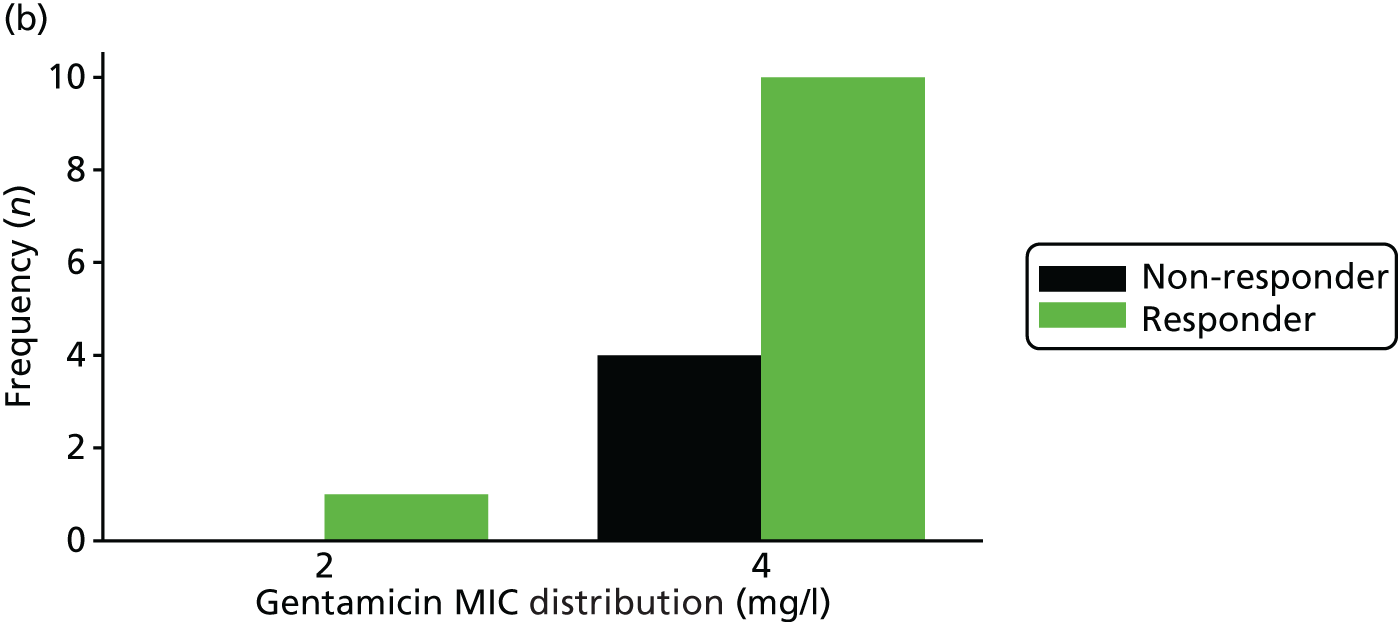
FIGURE 19.
Distribution of rectal gentamicin MIC. (a) All participants; and (b) by treatment response.
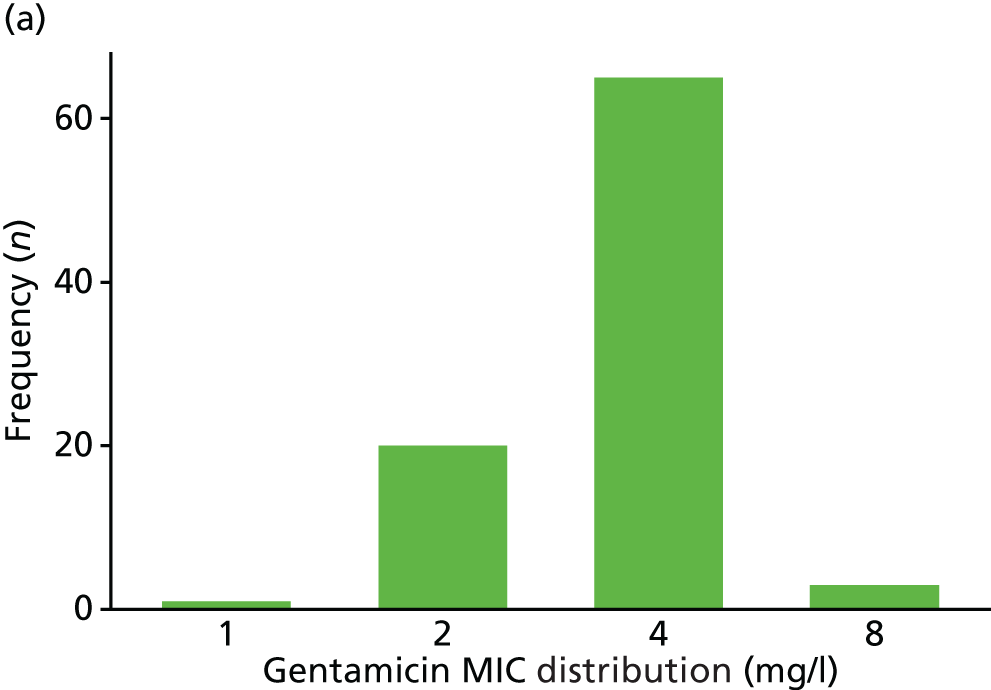
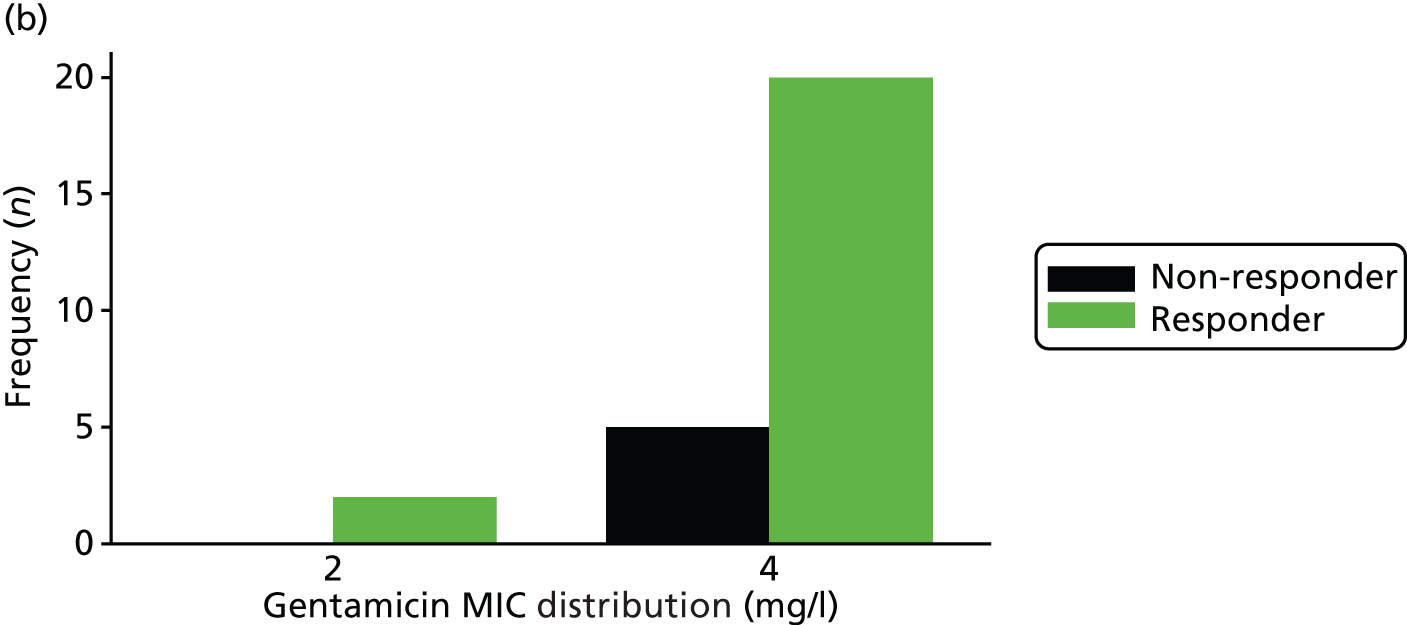
FIGURE 20.
Distribution of genital ceftriaxone MIC. (a) All participants; and (b) by treatment response.
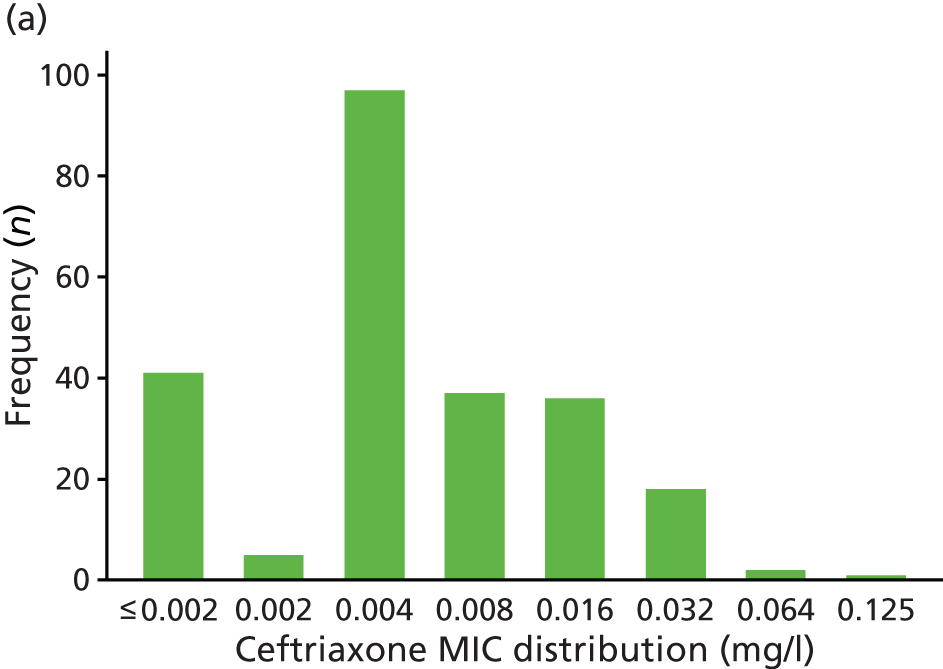
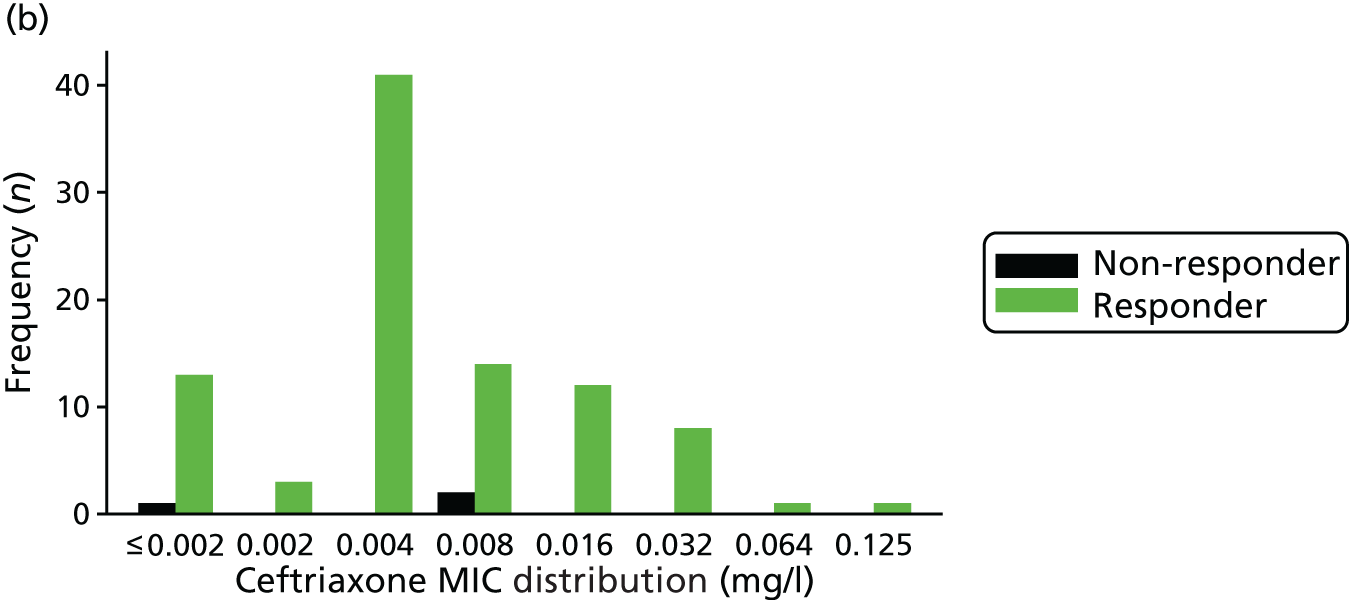
FIGURE 21.
Distribution of pharyngeal ceftriaxone MIC. (a) All participants; and (b) by treatment response.
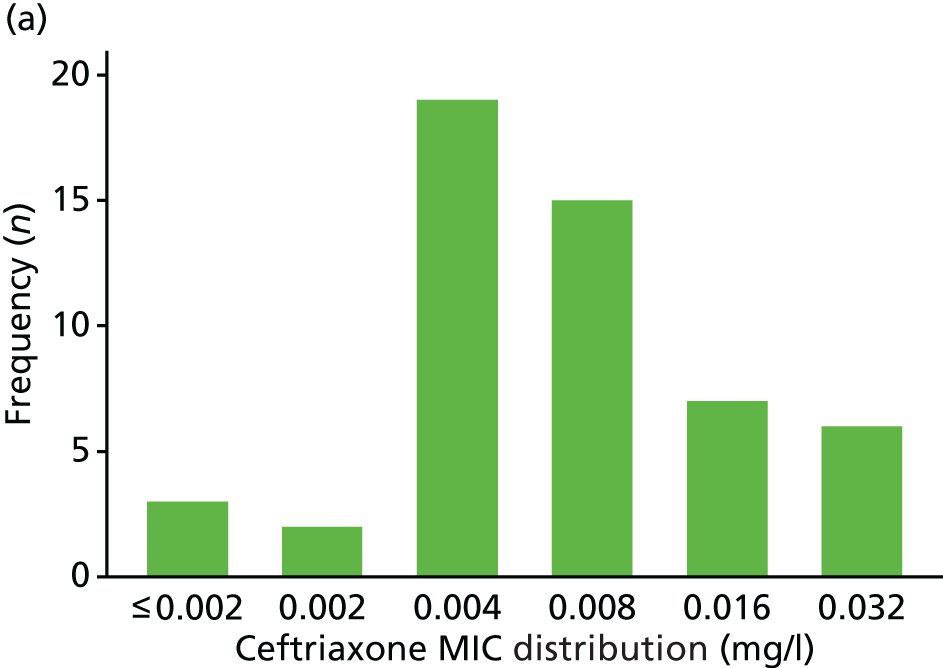
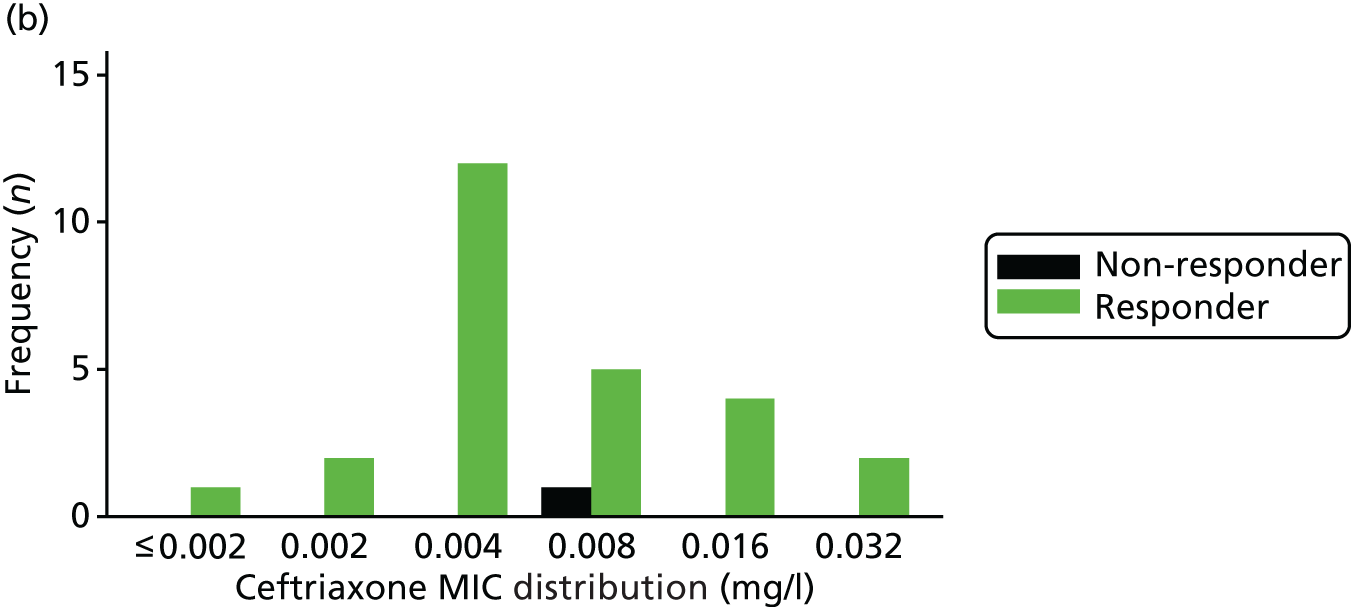
FIGURE 22.
Distribution of rectal ceftriaxone MIC. (a) All participants; and (b) by treatment response.
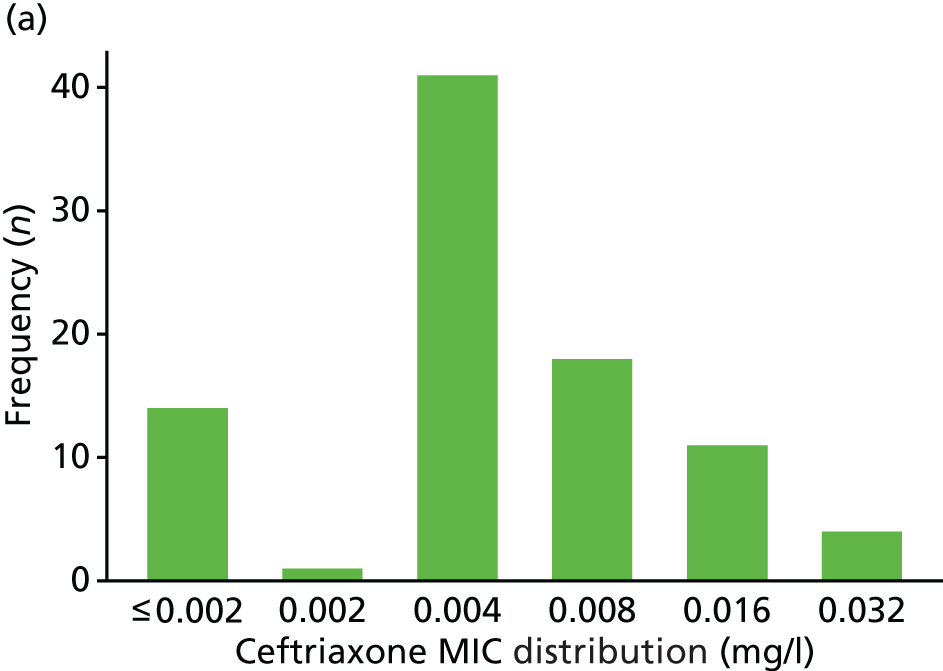
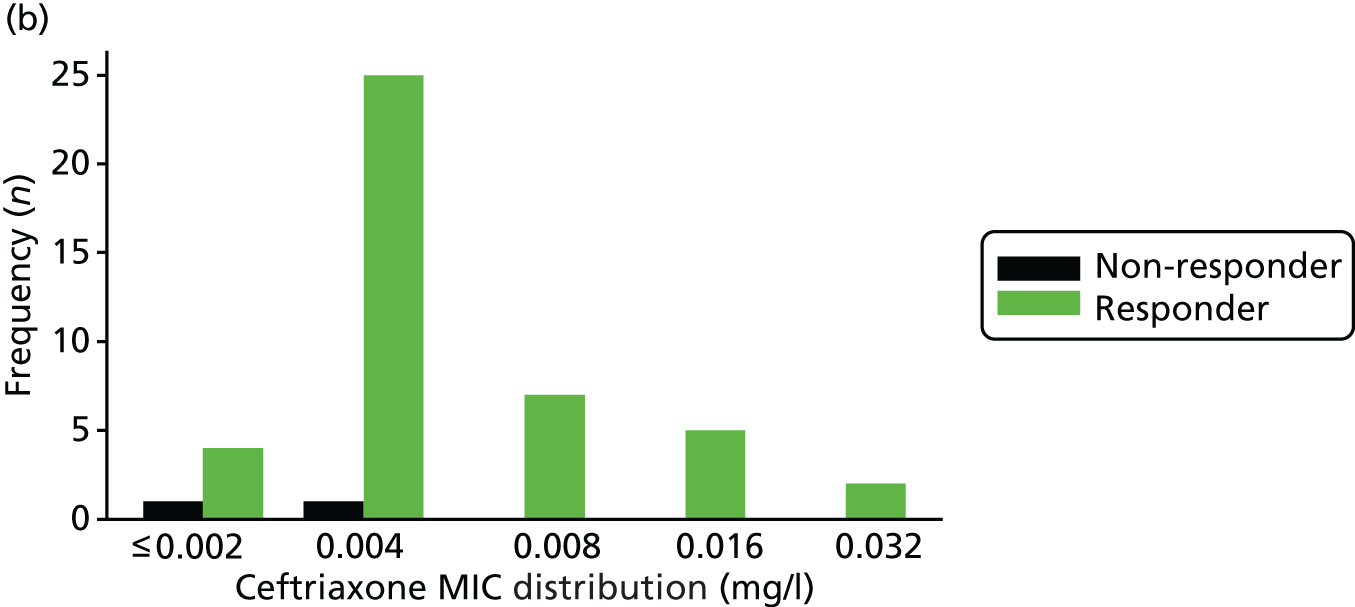
FIGURE 23.
Distribution of genital azithromycin MIC. (a) All participants; and (b) by treatment response.
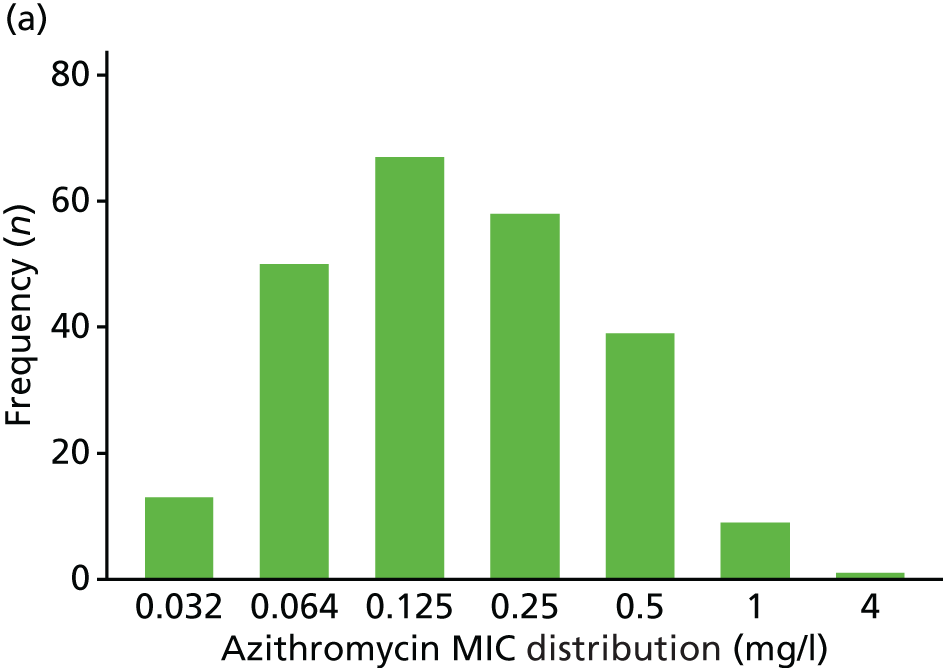
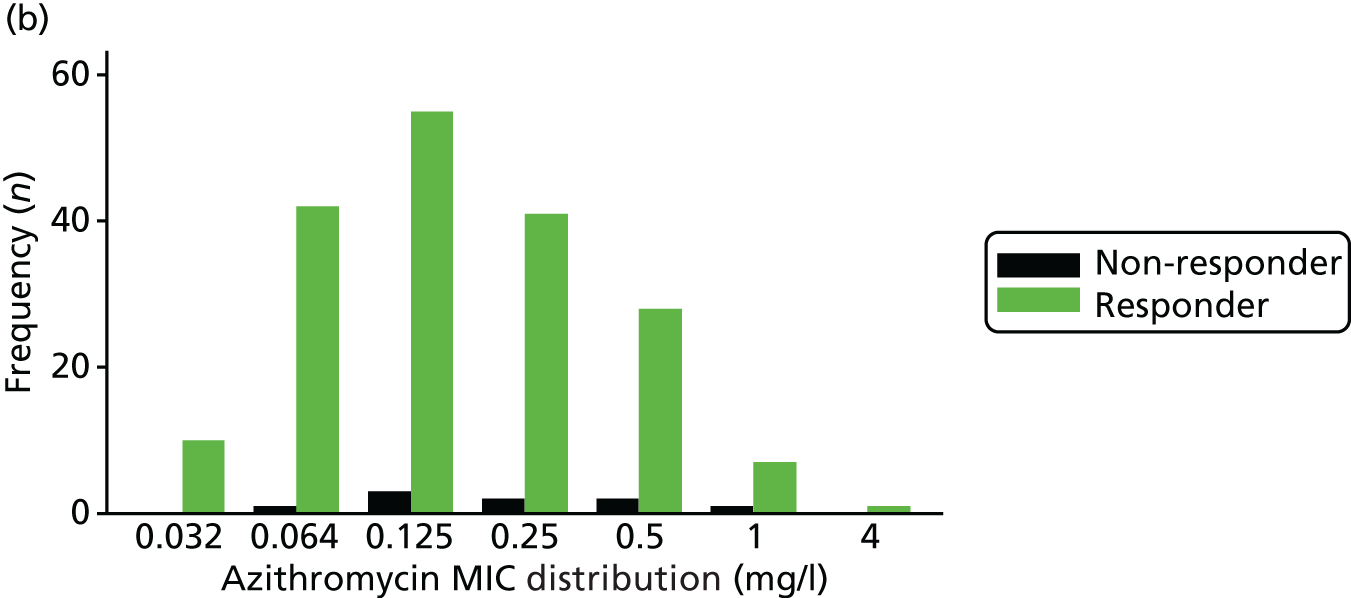
FIGURE 24.
Distribution of pharyngeal azithromycin MIC. (a) All participants; and (b) by treatment response.
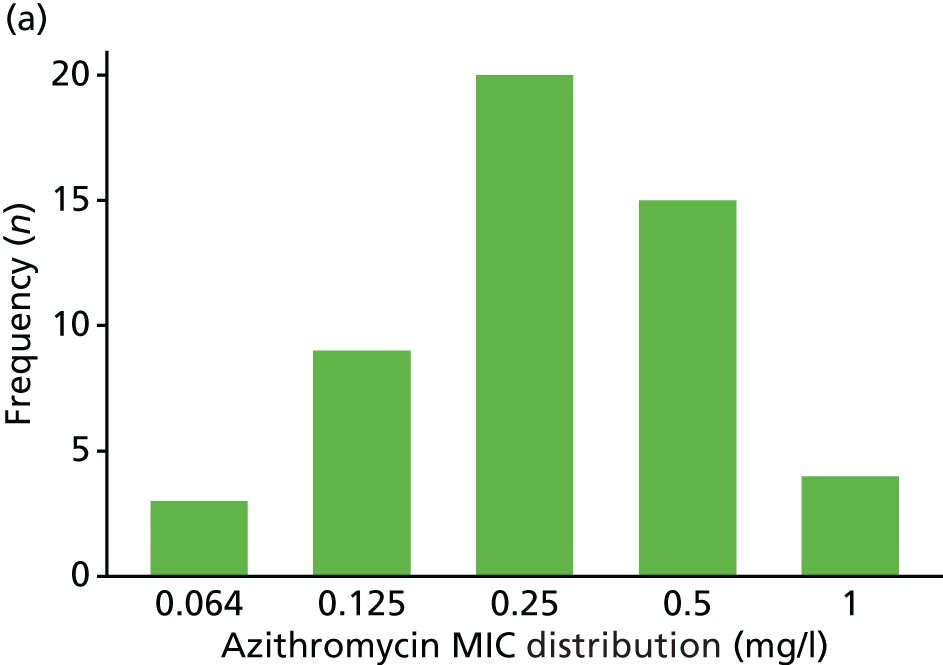
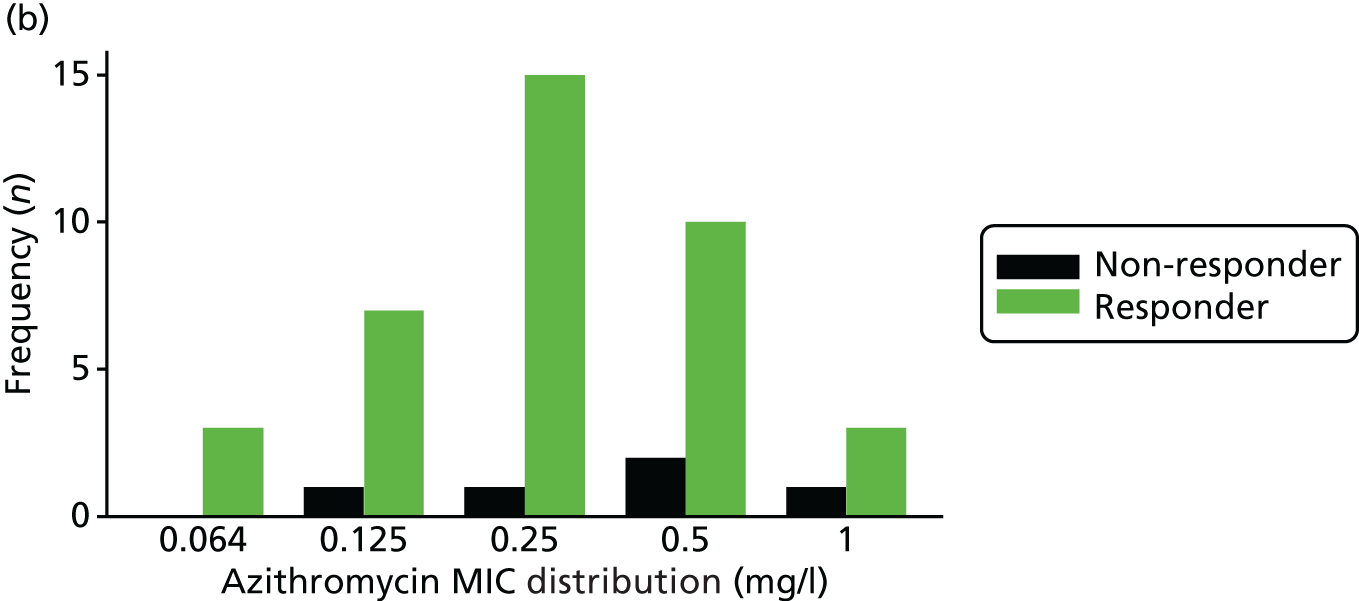
FIGURE 25.
Distribution of rectal azithromycin MIC. (a) All participants; and (b) by treatment response.
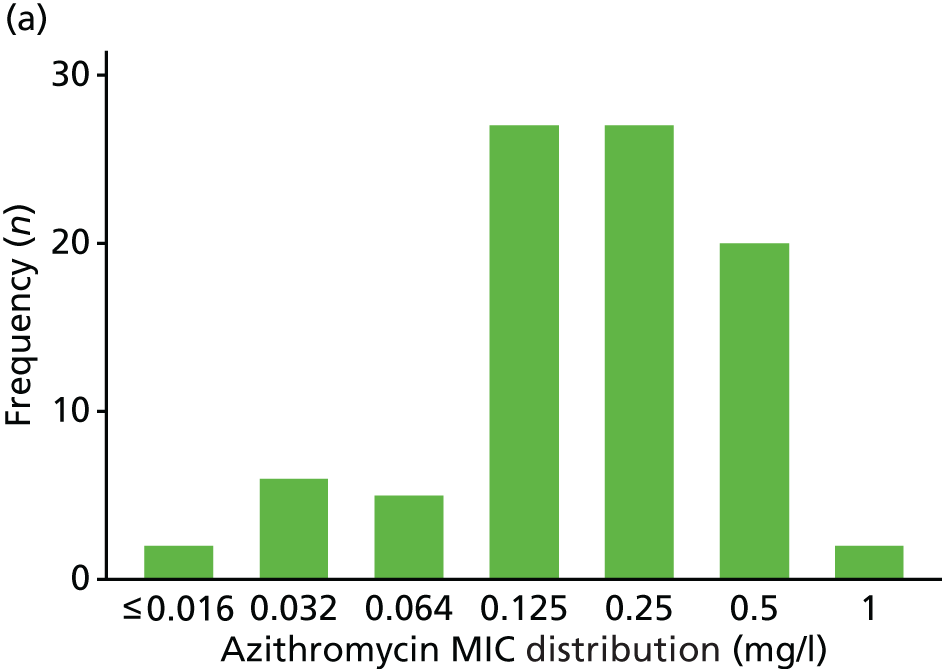
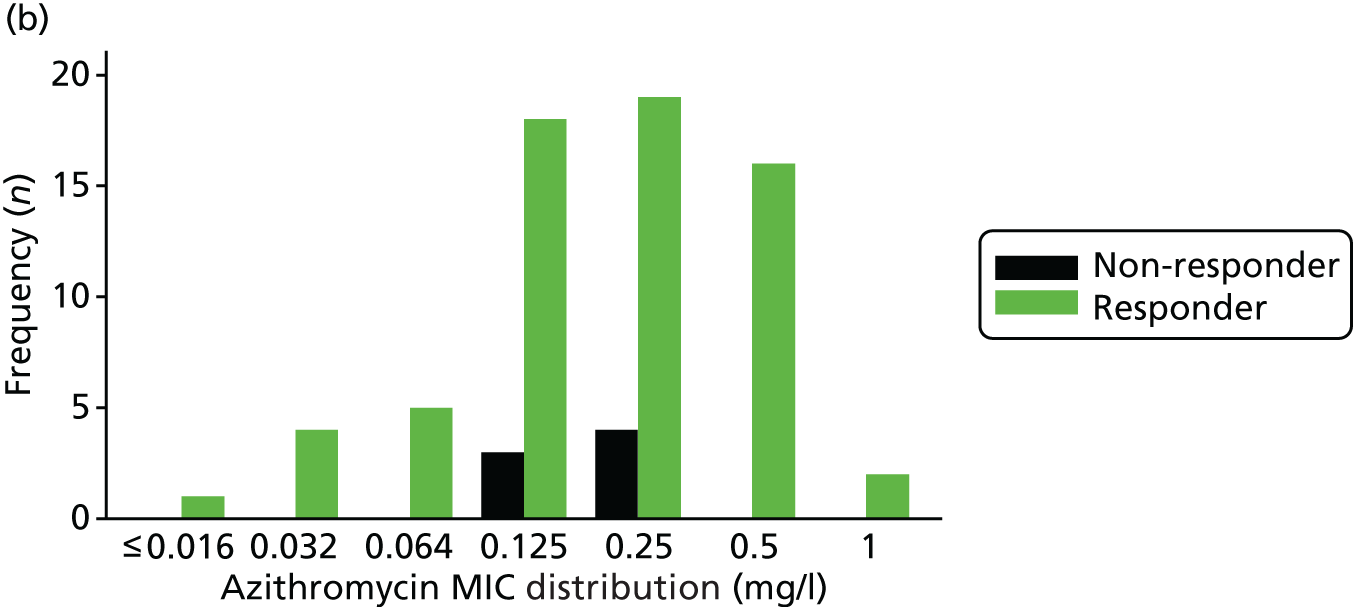
List of abbreviations
- A&E
- accident and emergency
- AC2
- Aptima Combo 2
- AE
- adverse event
- AMR
- antimicrobial resistance
- BASHH
- British Association for Sexual Health and HIV
- BD
- Becton, Dickinson and Company
- BNF
- British National Formulary
- CEA
- cost-effectiveness analysis
- CI
- confidence interval
- DMC
- Data Monitoring Committee
- eGFR
- estimated glomerular filtration rate
- G-TOG
- Gentamicin in the Treatment Of Gonorrhoea
- GEE
- generalised estimating equation
- GP
- general practitioner
- HIV
- human immunodeficiency virus
- i.m.
- intramuscular
- IQR
- interquartile range
- IT
- information technology
- ITT
- intention to treat
- MedDRA
- Medical Dictionary for Regulatory Activities
- MIC
- minimum inhibitory concentration
- MSM
- men who have sex with men
- NAAT
- nucleic acid amplification test
- NCTU
- Nottingham Clinical Trials Unit
- PHE
- Public Health England
- PI
- principal investigator
- PIS
- patient information sheet
- PPI
- patient and public involvement
- PSA
- probabilistic sensitivity analysis
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SmPC
- Summary of Product Characteristics
- STI
- sexually transmitted infection
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
