Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/176/12. The contractual start date was in September 2016. The draft report began editorial review in May 2018 and was accepted for publication in October 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Paul Ewings is a member of the National Institute for Health Research Health Technology Assessment Clinical Evaluation and Trials Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Gunn et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Multiple sclerosis (MS) is an incurable, unpredictable but typically progressive, life-long neurological condition, affecting approximately 100,000 people in the UK. 1 It is the most common cause of neurological disability in young adults, with an estimated cost of £1.4B per annum to the NHS and society. 2 More recently, new insights into the burden and costs of MS in Europe have demonstrated that, on average, costs are €22,800 in mild, €37,100 in moderate and €57,500 in severe disease (adjusted for purchasing power parity). 3 Although most people start with a relapsing–remitting (RR) disease course, approximately two-thirds move to a progressive phase, with a gradual rise in the total percentage of progressive cases as the disease advances. 4 At this point, medical interventions are limited and further disease progression is usually inevitable. 5 People in this phase, the majority of whom have limited mobility, are often excluded from clinical trials, which tend to focus on the RR stage. 6
Balance and falls in multiple sclerosis
Eighty-five per cent of people with MS report gait disturbance as their main problem. 7 Within approximately 15 years of diagnosis, 50% of people are unable to walk unaided, and over time an estimated 25% are dependent on a wheelchair. 8 It is, therefore, unsurprising that mobility is a major concern for people with MS and the health professionals involved in their care, and an area that is consistently highlighted by research, policy and service user fora. Surveys of people with MS consistently rank mobility as their highest priority9 and the most important, yet most challenging, daily function. 10 Furthermore, mobility has been correlated with employment status, earnings and quality of life (QoL). 11,12 An important contributor to difficulties in mobility is impaired balance, which is reported by approximately 75% of people with MS13 and has been shown to be more compromised in people with secondary progressive multiple sclerosis (SPMS) than in those with relapsing–remitting multiple sclerosis (RRMS). 14 Our own work suggests that falls may be an early marker of mobility deterioration associated with disease progression. 15 Rehabilitation interventions that improve balance and physical activity and decrease the risk of falls may slow this deterioration, providing a persuasive argument for ensuring that optimal physical management is a clinical priority. With only limited medical interventions available for this patient group, such rehabilitation programmes are considered key to the treatment of SPMS but currently lack a robust evidence base. 5
Our research,16 in line with that of others, demonstrates that, alongside impaired mobility and balance, falls are a common issue for people with SPMS, who are twice as likely to fall as those with RRMS. 17 The evidence shows that approximately 70% of people with MS fall regularly,15 at a rate of > 26 falls per person per year with SPMS. 16 More than 10% of these falls lead to injuries18 and people with MS are three times more likely to sustain a fracture than the general population. 19 Falling and fear of falling have a profound impact on individuals, leading to activity curtailment, social isolation and a downwards spiral of immobility, deconditioning and disability accumulation. 20 This has significant implications for an individual’s health, well-being and QoL. Unsurprisingly, 4 out of the 10 research priorities identified by the James Lind MS Priority Setting Partnership relate directly to this area. 21 This trial has provided an opportunity for people with SPMS, whose limited mobility often excludes them from clinical trials, to participate in a trial targeting what they themselves consider to be a key concern.
Implications of impaired mobility and falls for society and for health practice
There are substantial economic and social costs related to increasing immobility, impaired balance and falls in people with MS. Costs of health and social care have been shown to increase steeply with increasing disease severity/immobility. 2 By this stage of the disease, rehabilitation interventions form the mainstay of treatment and drug therapy options are limited. The mean cost per wheelchair-dependent patient is four to five times higher than that for an ambulatory patient. 22 This, together with the associated costs of falls for those who continue to ambulate, underlines the importance of optimising safe mobility for as long as possible: a key aim of our work. This is particularly relevant given recent evidence that people with MS are living longer, leading to a rising population living with the disease. 23 This has important implications for resource provision in the UK and for the NHS, as highlighted in a national audit of neurological services,24 which demonstrated a significant increase in emergency hospital admissions in people with progressive neurological disability, including MS. The importance of mobility and falls is emphasised by their consistent prominence in recent policy documents for long-term neurological conditions. 25
An overwhelming proportion of a physiotherapist’s MS caseload comprises people who have balance and mobility impairments, many of whom are falling. Improving balance and mobility in people with SPMS and reducing falls is likely to have a significant impact on QoL and independence. Our recent literature reviews, however, demonstrate that there is a lack of available evidence to support this assertion, although data from mixed samples of patients with RRMS or SPMS are suggestive. 5,26 Furthermore, professionals and patients have identified serious problems with the feasibility and sustainability of a traditional weekly outpatient model of programme delivery. 27 The emphasis on evidence-based practice heavily influences whether or not interventions are provided, as does local policy. These decisions rely on the availability of robust evidence of clinical effectiveness and cost-effectiveness. Currently, there is minimal evidence-based guidance to inform optimal mobility management and none to inform falls management in people with MS. This paucity of evidence is highlighted in the National Institute for Health and Care Excellence (NICE) clinical guideline 186,25 which nominates the rehabilitation of mobility as one of its five key research recommendations. Although evidence is available for older people and for other neurological conditions, research suggests that translating existing interventions to people with MS is likely to be ineffective. 28,29 Small, limited-duration studies have evaluated single elements of MS balance and falls interventions, individually demonstrating short-term improvements in mobility, balance or falls awareness,13,30,31 but these elements have not yet been implemented or evaluated collectively. Moreover, no studies have focused on people with SPMS.
The BRiMS programme
Development of the BRiMS programme
Health-care policy prioritises the need to empower patients to self-manage through partnership working and self-management programmes,32 with emphasis placed on a future NHS that implements interventions that promote self-care and lifestyle behavioural change and are community based. 33
In partnership with service users, providers, other key stakeholders (including commissioners) and international collaborators, our ongoing research programme systematically developed Balance Right in MS (BRiMS), an innovative, evidence-based, user-focused self-management programme, designed to improve safe mobility and reduce falls for people with MS. The development of BRiMS has been based on the Medical Research Council framework34 for the development and evaluation of complex interventions and supplementary guidance identifying specific tasks to be undertaken in the development process. 35 It was informed by input from a number of internationally recognised experts,36 which is explicitly acknowledged in the programme documentation. The programme’s underlying philosophy is based on the premise that interventions must promote lifestyle behavioural change, be community based and focus on prevention and self-care, an approach in line with the future direction of the NHS. 33
Overview of the BRiMS programme
BRiMS is a novel 13-week, therapy-led personalised education and exercise programme structured to maximise the development of self-efficacy and support participant engagement (Figure 1). It addresses modifiable risk factors, enabling self-management by individualised mobility, safety and falls risk management strategies.
FIGURE 1.
The BRiMS programme delivery plan.
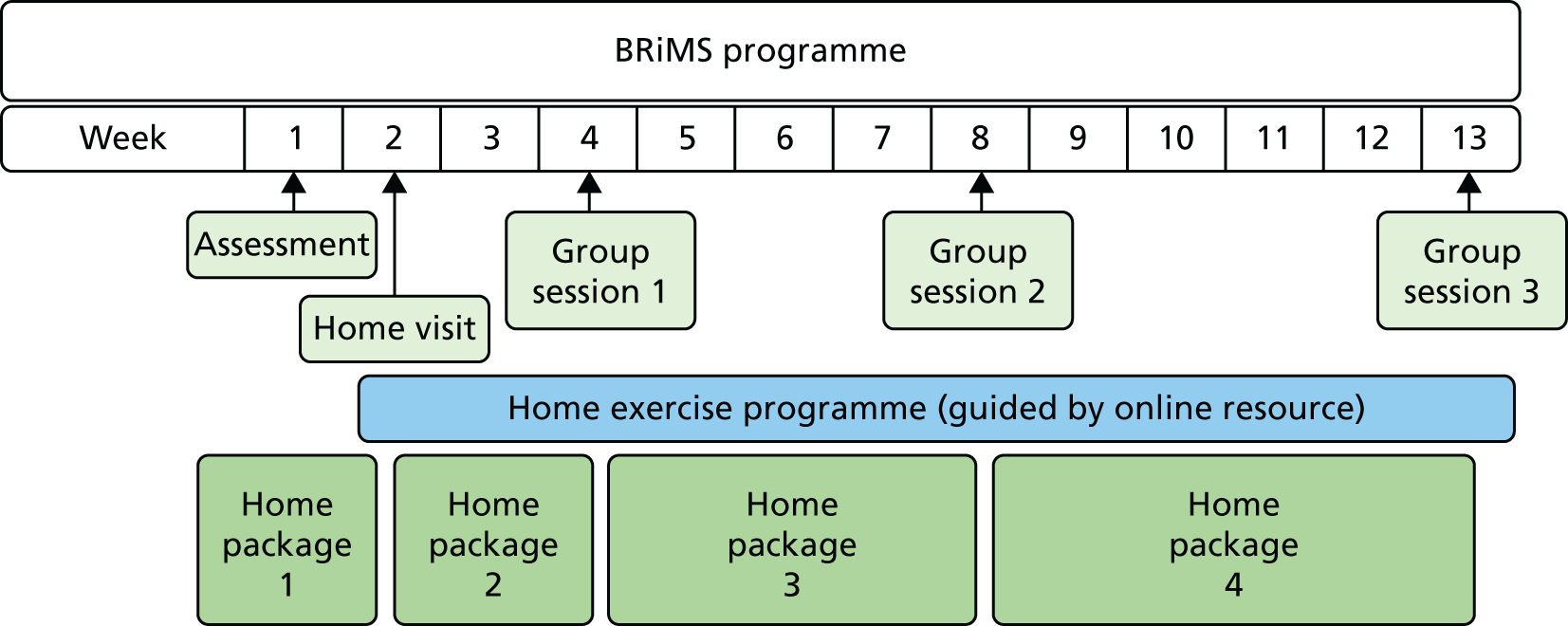
The programme includes two individual and three group sessions addressing physical, behavioural and environmental aspects of mobility and falls management. These are supplemented by a home-based package delivered via an established web-based interactive resource to ensure integration into daily life from the outset. As emphasised in the NICE guideline,25 this combined approach (which was developed in collaboration with people with MS, physical therapists, sports scientists, occupational therapists and psychologists) aims to equip the person with MS with the knowledge, skills and motivation to sustain long-term behaviour change. Developing and supporting motivation is addressed throughout using new functional imagery techniques37,38 to supplement established motivational techniques.
The BRiMS education component aims to improve exercise self-efficacy and develop individualised falls prevention and management practices through the acquisition and application of relevant knowledge and skills. 39 This component is delivered through a mix of home and group activities embedded throughout the programme. It utilises a number of evidence-based self-management practices, specifically group brainstorming, problem-solving and action-planning. 40 It also applies the principles of cognitive–behavioural therapy to facilitate self-efficacy enhancement. In group sessions, BRiMS utilises peer modelling, vicarious learning, social persuasion and guided mastery to boost self-efficacy41 and encourages the setting and imagery of short-term exercise goals to boost the desire to achieve them. 42
The BRiMS exercise component is designed to achieve a minimum of 120 minutes of individualised, progressive, gait, balance and functional training per week. The content is guided by a comprehensive literature review of MS balance exercise interventions,43 while the structure and format are informed by comprehensive stakeholder input. 27
The BRiMS exercise component is designed to be predominantly home-based, with exercise planning and progression undertaken in partnership between the participant and the programme leader. The group sessions include exercise activities to encourage peer support and problem-solving. Motivational support is built into both elements. Additionally, BRiMS integrates an online exercise prescription resource (https://webbasedphysio.com)44 to support and guide participants’ home-based practice. The resource can be customised to the participant’s individual exercise prescription and remotely amended during the programme to maintain an appropriate level of challenge.
Intervention description and standardisation
The BRiMS programme has been manualised to provide a detailed description of the intervention and to ensure consistency of content, approach and delivery of sessions across time, region and groups. The manual includes identification of the critical elements of each part of the programme, key objectives of each session and detailed guidance/scripts for programme leaders along with accompanying participant resources.
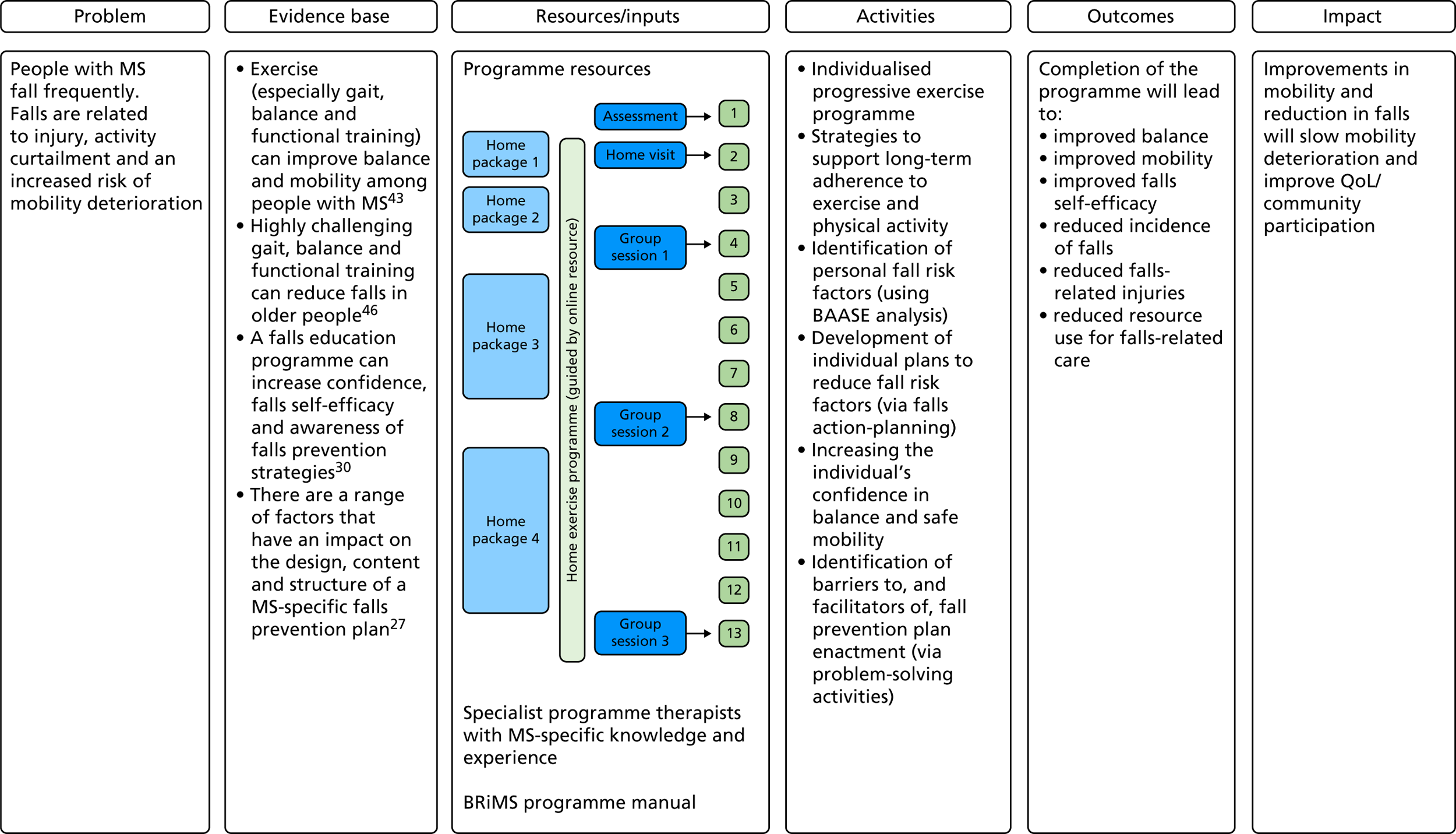
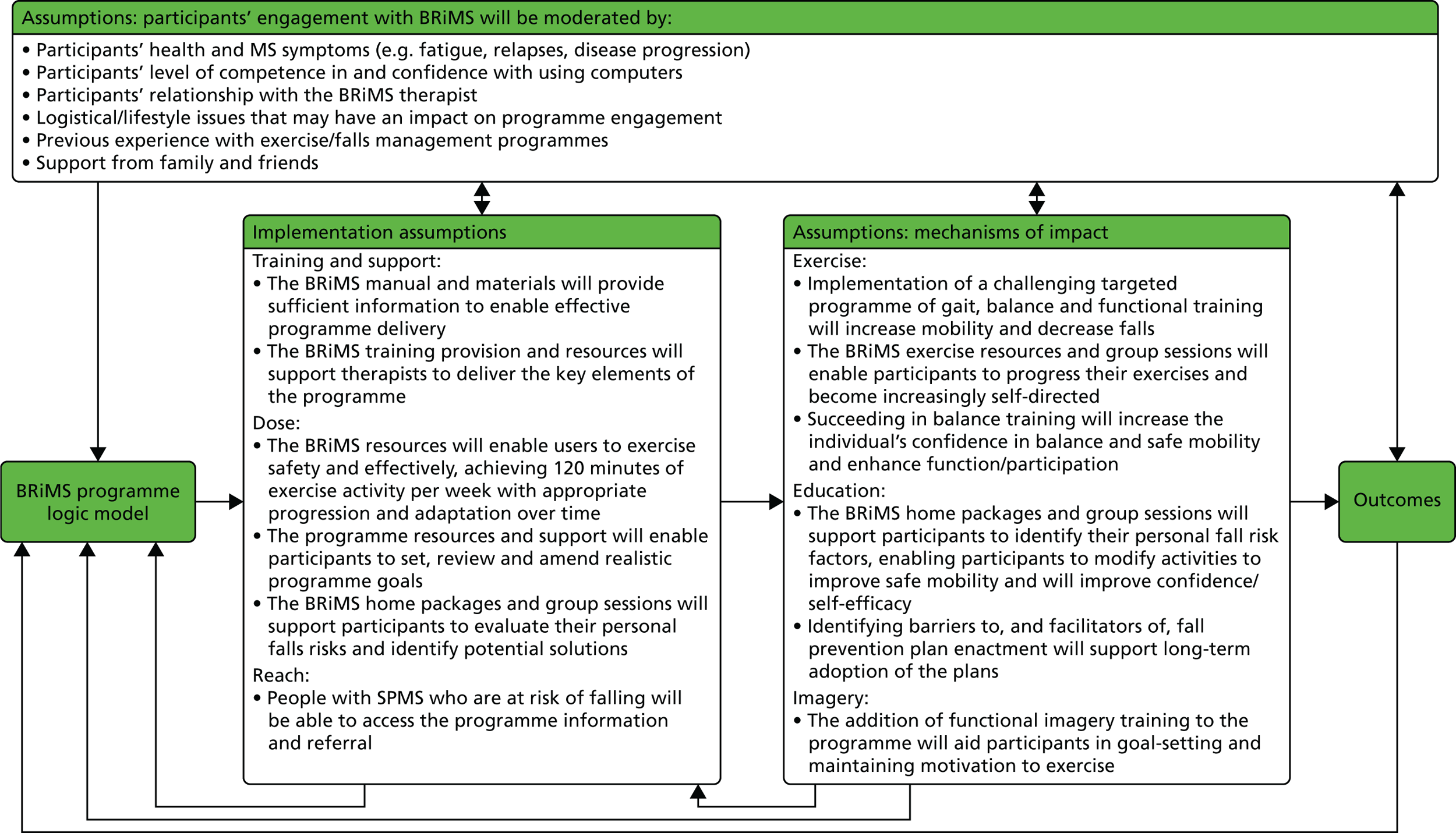
Figure 2 shows the BRiMS ‘logic model’,45 which maps the programme content and delivery methods, along with causal assumptions, the mechanisms that are theorised to drive the programme and any expected external factors that may affect the outcomes. Table 1 provides an overview of the schedule of content delivery.
FIGURE 2.
The BRiMS programme logic model. BAASE, Behaviours and Attitudes, Activities, MS Symptoms, Environment.
| Week | BRiMS activities |
|---|---|
| 1 |
Session 1: individual assessment and introduction to the programme. The therapist develops a personalised exercise programme based on assessment findings and agreed outcomes Takes place at a local health-care establishment Home activities: patient receives BRiMS manual and website login, and completes first home package |
| 2 | Session 2: home visit by BRiMS therapist to explain and set up the exercise programme |
| 2–4 |
Home-based individual practice of exercise programme, plus education activities, with online support from the BRiMS therapist Therapist undertakes online review and adjustment of web-based exercise prescription every 2 weeks |
| 4 |
Group session 1: group exercise and education activities Takes place at a local health-care establishment |
| 5–8 | Home-based practice of exercise programme, plus education activities |
| 8 |
Group session 2: group exercise and education activities Takes place at a local health-care establishment |
| 9–13 | Home-based practice of exercise programme, plus education activities |
| 13 |
Group session 3: group exercise and education activities Takes place at a local health-care establishment |
Trial rationale and objectives
The National Institute for Health Research (NIHR) commissioning brief (Health Technology Assessment 15/47) requested applications for studies undertaking primary research in rehabilitation therapies to improve QoL in patients with SPMS. Having previously developed BRiMS, it was critical, and timely, to assess the feasibility of delivering this programme and the proposed evaluation methods before undertaking a definitive trial to assess the clinical effectiveness and cost-effectiveness of the programme.
There were a number of uncertainties about the optimal parameters for the definitive trial. This current trial tested the feasibility of conducting such a trial, providing estimates of recruitment, attrition and concordance, completion rates of measures, and baseline scores and standard deviations (SDs) of proposed outcomes. We also assessed the acceptability of the intervention, and of participating in the trial, from both the participants’ and the health professionals’ perspectives, and the process of delivering BRiMS.
This feasibility trial aimed to obtain the necessary data and operational experience to finalise the planning of an intended future definitive multicentre randomised controlled trial (RCT) to compare a manualised 13-week education and exercise programme (BRiMS) plus usual care with usual care alone in improving mobility, improving QoL and reducing falls in people with SPMS. The intention was to learn lessons to enable a definitive trial to be successfully delivered with confidence. The objectives were divided into four areas, as follows.
Trial feasibility objectives
To determine the:
-
feasibility, utility and acceptability of the trial procedures
-
suitability and feasibility of eligibility criteria
-
numbers of eligible participants from the target population
-
willingness of clinicians to recruit patients
-
willingness of patients to be randomised
-
likely recruitment and retention rates as participants move through the trial.
Potential full trial outcome objectives
To determine the:
-
completion and performance of proposed outcome measures, including rates of outcome measure completion, baseline scores, distributional properties and SDs of outcome measures, and responsiveness to inform selection of primary outcome (and refine the number of secondary outcomes) for a definitive trial
-
baseline factors most strongly associated with outcomes, as potential stratification factors in a definitive trial
-
sample size required for a fully powered RCT to evaluate the effectiveness of the BRiMS intervention.
BRiMS programme feasibility (process evaluation) objectives
To determine:
-
the optimum way of delivering the BRiMS programme
-
intervention fidelity and application between sites
-
the acceptability of, and adherence to, the 13-week BRiMS programme.
Health economics objectives
To determine:
-
estimates of resource use and related costs associated with delivery of the BRiMS intervention
-
a framework for assessing the cost-effectiveness of the BRiMS intervention in a future economic evaluation alongside a full trial.
Chapter 2 Trial design and methods
This was a pragmatic, multicentre, feasibility RCT with blinded outcome assessment. Participants were randomised either to a manualised 13-week education and exercise programme (BRiMS) plus usual care (intervention group), or to usual care alone (usual-care group). The protocol for the trial has been published previously. 47
Trial participants
The target population was English-speaking men and women aged ≥ 18 years who had a confirmed diagnosis of SPMS and reported having walking difficulties and experiencing falls. This population constitutes an estimated 70% of all individuals with SPMS, as, by the time this phase has been reached, balance, mobility and physical activity levels are usually compromised.
The trial was explicitly designed to have high applicability to people with SPMS, and so it used broad inclusion criteria and relatively few exclusion criteria.
Inclusion criteria
The patient:
-
Had a confirmed diagnosis of MS as determined by a neurologist; and, in the secondary progressive phase, as confirmed by a MS specialist clinician.
-
Was aged ≥ 18 years.
-
Was willing and able to understand/comply with all trial activities.
-
Had an Expanded Disability Status Scale (EDSS) score of between ≥ 4.0 and ≤ 7.0 points.
-
Had self-reported two or more falls in the past 6 months.
-
Was willing and able to travel to and participate in BRiMS group sessions at local sites and to commit to undertaking their individualised home-based programme.
-
Had access to a computer or tablet and to the internet.
Exclusion criteria
The patient:
-
Had reported relapse or receiving steroid treatment within the past month (patient-reported relapse is defined as ‘the appearance of new symptoms, or the return of old symptoms, for a period of 24 hours or more – in the absence of a change in core body temperature or infection’). 48
-
Had any recent changes in disease-modifying therapies. More specifically, patients were excluded if they:
-
had ever had previous treatment with alemtuzumab (Lemtrada®, Sanofi Genzyme, Cambridge, MA, USA) because it was felt to be a major disease modifier that had long-term effects after the usual courses given 12 months apart; or
-
had ceased nataluzimab (Tysabri®, Biogen, Cambridge, MA, USA) in the previous 6 months; or
-
were within 3 months of ceasing any other MS disease-modifying drug.
-
-
Had participated in a falls management programme (e.g. for older people) within the previous 6 months.
-
Had comorbidities that may have influenced their ability to participate safely in the programme or that were likely to have an impact on the trial (e.g. uncontrolled epilepsy).
-
Had been recruited to a concurrent interventional trial.
Trial settings
The sites involved were based in two geographical regions of the UK: south-west England (Devon/Cornwall) and Ayrshire.
Research activity took place at four sites:
-
Plymouth
-
Exeter
-
Cornwall
-
Ayrshire and Arran.
All sites implemented the trial protocol in the same manner. Physiotherapists (treating therapists) from each of these sites performed the interventions (as part of their NHS role) and two BRiMS research therapists (employed specifically for the trial) undertook the blinded assessments.
Sample size
In accordance with relevant best-practice,49 we wanted to test processes within and across the three sites to ensure that this feasibility trial gave a realistic indication of the practicalities for conducting the intended full trial. This included gaining robust information on likely recruitment and retention rates and full testing of the procedures involved in all trial processes. Therefore, the more common sample size calculation, based on considerations of power for detecting a between-group clinically meaningful difference in a primary clinical outcome, was not appropriate. 18
From other studies in similar settings, it was estimated that retention rates would be in the region of 80%. 19,20 A target sample size of 60 participants in total would allow estimation of the overall retention rate with precision of at least ± 13%; for example, if the 6-month follow-up rate was around 80%, the estimate would have precision of ± 10%. Assuming a non-differential follow-up rate at 6 months of 80%, it was estimated that recruiting 60 participants would provide outcome data on a minimum of 24 participants in each of the two allocated groups, enabling reasonable estimates to be made of the variability (i.e. SD) in each of the proposed outcomes.
Recruitment and screening
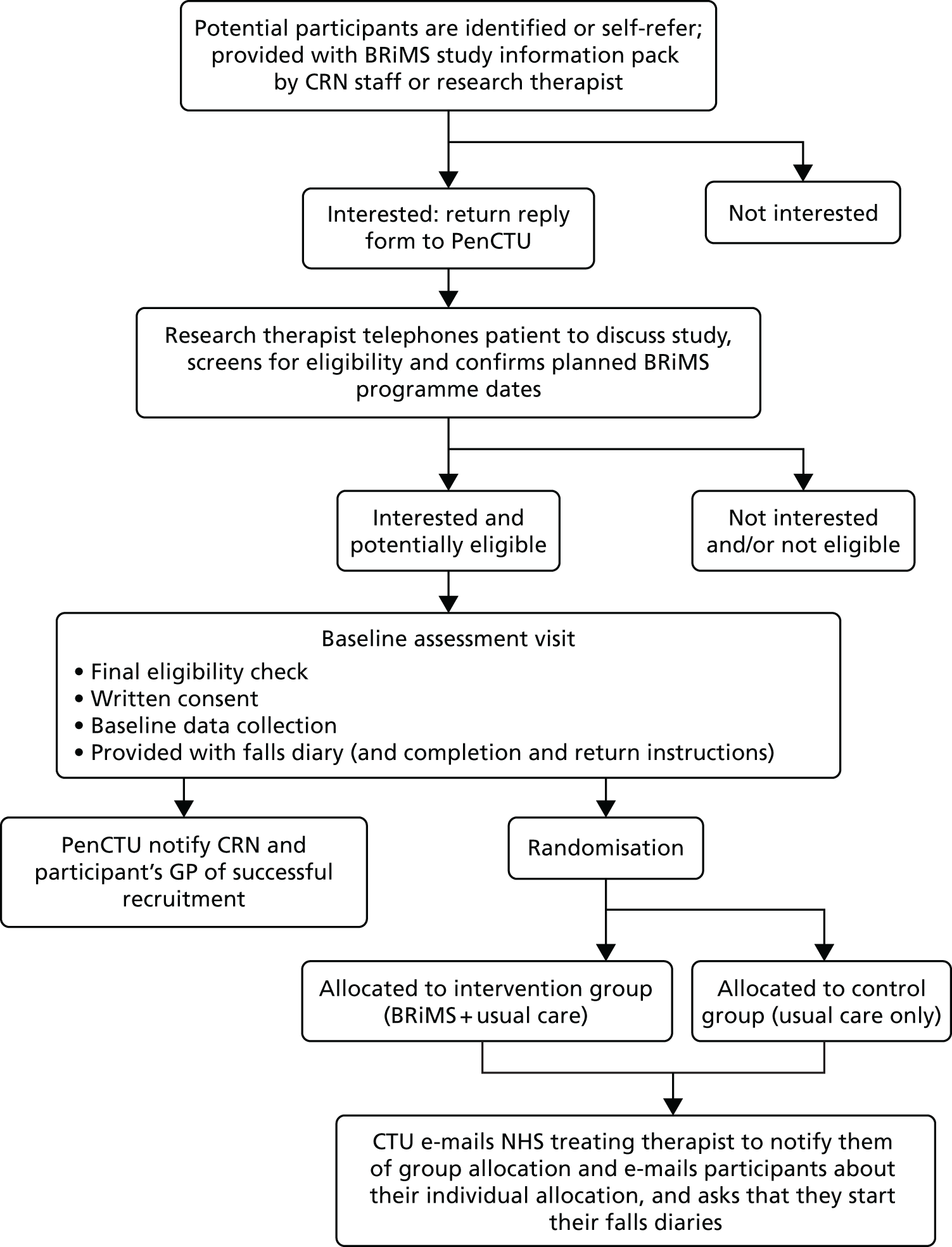
A summary of the recruitment and screening process is shown in Figure 3.
FIGURE 3.
Recruitment pathway. CRN, Clinical Research Network; GP, general practitioner; PenCTU, Peninsula Clinical Trials Unit.
Recruitment
To our knowledge, there are no data that report recruitment rates to MS clinical trials from national sources and so it was difficult to estimate what these would be. This feasibility trial aimed to elucidate this, as we recorded the sources of recruitment for all participants. However, for this feasibility trial, we could be more confident of recruitment rates from the local sites involved. For example, our previous work15,50 in ambulant individuals with MS, using similar local recruitment methods, demonstrated high recruitment rates, with 60–65% of those who were eligible participating. However, given that BRiMS involved attendance at three group sessions and the commitment to undertake a home-based exercise programme and work package, we believed that a 50% local recruitment rate was more realistic. Based on this, and on a conservative estimate of approximately 600 eligible local participants (Devon/Cornwall, n = 284; Ayrshire, n = 330), our data demonstrated that sufficient numbers of eligible people would be available within our two recruiting regions from whom to recruit our target sample.
For this feasibility trial, the aim was to recruit 60 participants across the four sites (40 in the south-west and 20 in Ayrshire) over 6 months. This meant running six intervention groups, each with approximately five participants. It was anticipated that, to recruit these 60 participants, around 240 people would need to be screened, assuming that around 80% would agree to participate (n = 190), of whom approximately 35% (n = 70) would be found to be ineligible after screening, leaving around 120 eligible people. Following this screening, it was anticipated that approximately 50% of eligible people would consent to participate, leaving around 60 participants to be randomised.
Our recruitment period was relatively short, at 6 months. Our rationale for this was efficiency: to minimise the overall length and, therefore, the cost of this feasibility trial. Trial awareness-raising activities commenced during the 4-month set-up period. In line with recommendations from Treweek et al. ’s51 systematic review, a multifaceted recruitment approach was undertaken using both national and local routes. In doing this, we anticipated that we would have a list of potential participants to screen as soon as sites confirmed research capacity and capability. As necessary, during the recruitment period we complemented this approach by utilising direct recruitment strategies. Our first priority was to send out consultant invitation letters, as this has been demonstrated to be a cost-effective strategy for recruitment in other MS rehabilitation trials. 52–54
Nationally, recruitment was promoted via a number of sources:
-
the UK MS Register via their quarterly newsletter and social networking sites
-
promotion through the MS charitable bodies’ regular open access newsletters (i.e. MS Society ‘Research Matters’)
-
promotion through the MS Society online resources that alert people with MS to studies they may be interested in participating in
-
promotion and support from the NIHR Clinical Research Network (CRN) and national specialty lead for neurology
-
the trial’s website (www.brims.org.uk), which included generic e-mail contact details.
All promotional materials included information inviting people to make contact via the trial’s generic e-mail address. These e-mails were monitored by the Peninsula Clinical Trials Unit (PenCTU), which triaged enquiries and redirected them to the appropriate research therapist.
Local recruitment was also promoted via a number of routes, which included:
-
adoption onto the UK CRN Portfolio; the local Clinical Research specialty lead also promoted the trial through existing clinical networks
-
through the caseloads of neurologists, MS specialist nurses and NHS therapists who discussed the trial with interested patients, or wrote a letter of invitation to potential participants
-
leaflets and posters placed in relevant outpatient clinics of the participating health establishments
-
promotion through local initiatives where they existed, for example the South West Impact of MS (SWIMS) project55
-
local MS centres and MS Society branches, via posters, newsletters, personal contact with the regional and branch leaders, and informal presentations at local MS branch meetings.
All people with MS who expressed an interest in participating were sent a BRiMS trial information pack by the CRN staff or research therapists at the appropriate site. The pack consisted of a letter of invitation, the participant information sheet, a list of local pre-scheduled BRiMS programme dates and venues, a reply form and a reply-paid envelope. Where packs were distributed through direct contact (either face to face or by telephone), potential participants were given the option to be contacted by the local research therapist to verbally discuss the project and ask any questions. If the potential participant opted in to this option, the member of staff passed the person’s name and contact details (e-mail address or telephone number) to the local research therapist.
All patients were asked to read the participant information sheet and return the reply form to indicate their interest, to confirm that they felt they were eligible, and to give consent for the staff undertaking screening to contact their treating team to confirm their diagnosis of SPMS. This also gave the CRN staff or research therapists permission to contact the potential participant to establish fully whether or not the individual met the trial’s eligibility criteria.
Screening
On receipt of the completed reply form, the research therapist telephoned the person with MS to answer any further questions and to screen them for eligibility using a pre-formatted screening checklist based on the eligibility criteria. This included determining the person’s disability level using a telephone version of the EDSS. 56 During this screening telephone call, the participant’s preferred contact details were confirmed and the planned dates of the BRiMS programme were discussed to ensure that they were able to attend if they were allocated to the BRiMS group. Screen failures (i.e. patients who did not meet the eligibility criteria at time of screening) were informed if they were eligible for re-screening at a later date and, in this case, the research therapist arranged a follow-up screening call for a suitable date.
Clinical Research Network staff and research therapists maintained a screening log of potential participants who made contact with the research team to be considered for entry to the trial. Anonymised data from the screening log were transferred to PenCTU as required for the purpose of monitoring recruitment. These data included the reason individuals were not eligible for trial participation, if they were eligible but declined, and their reason(s) for declining if they were happy to divulge this. Furthermore, a record was kept of all people who were sent invitation letters to determine the proportion of those who expressed an interest in the trial.
Once initial eligibility checks were completed, the individual’s details were forwarded on to the research therapist (if screening had been undertaken by CRN staff). The research therapist telephoned the participant to confirm eligibility and send an appointment e-mail for the baseline assessment. It was the intention that the final face-to-face screening and the baseline assessment would be undertaken up to 2 weeks before the participant commenced the pre-scheduled BRiMS programme, should they subsequently be allocated to the intervention group. To minimise travel costs and burden on the participants, this baseline assessment was undertaken at a local health-care establishment. Reasonable travel expenses were reimbursed for all visits additional to normal care, namely for the three research assessments at the local health-care establishments at day 0, 15 weeks (± 1 week) after randomisation and 27 weeks (± 1 week) after randomisation. All participants were reminded that the allocation to either group of the trial was by chance and would occur after baseline assessment (see the next section).
Randomisation, concealment and blinding
The group-based element of the BRiMS intervention necessitated the confirmed recruitment and participation of a sufficient number of patients within a recruiting site before randomisation was undertaken. Each of the four sites aimed to recruit 10 participants per block/BRiMS delivery, but there was flexibility to recruit 8–12 participants. After the completion of the block of participants, each participant within the block was randomised (i.e. all participants within a block were simultaneously randomised). A participant was deemed recruited once they had provided written informed consent, confirmed their ability to attend a BRiMS group and completed the baseline assessment.
Once the decision was taken to declare a block of participants complete, randomisation was undertaken a minimum of 3 and a maximum of 7 working days before the BRiMS programme commenced. Individuals in the block were randomised approximately 1 : 1 to the intervention or usual-care arm following a strict and auditable protocol. When the block size was 9 or 11, allocation was forced to have one more participant in the intervention group than the usual-care group to maximise learning opportunities in this feasibility trial. Randomisation was conducted via a secure web-based system. The randomised allocations were computer-generated by PenCTU in conjunction with a statistician independent to the trial team, in accordance with PenCTU’s relevant standard operating procedure. An automatic e-mail was sent by PenCTU to the NHS treating therapist leading the BRiMS programme to notify them of those participants allocated to the BRiMS programme plus usual care (intervention).
Access to the randomisation process was confined to the PenCTU data programmer only. This ensured effective allocation concealment from every other member of the trial team. Following randomisation, only appropriate members of the trial team were made aware of the allocations to the intervention or usual-care arm [e.g. clinical trials unit (CTU) trial manager, chief investigator and site principal investigators]. Clearly, the participants and the NHS treating therapists leading the BRiMS programme could not be blinded to which treatment the participants were receiving. However, the research therapist (outcome assessor) remained blinded to the allocated treatment arm at all stages. In addition, the trial statisticians remained blinded until the database was locked for analyses.
Treatment
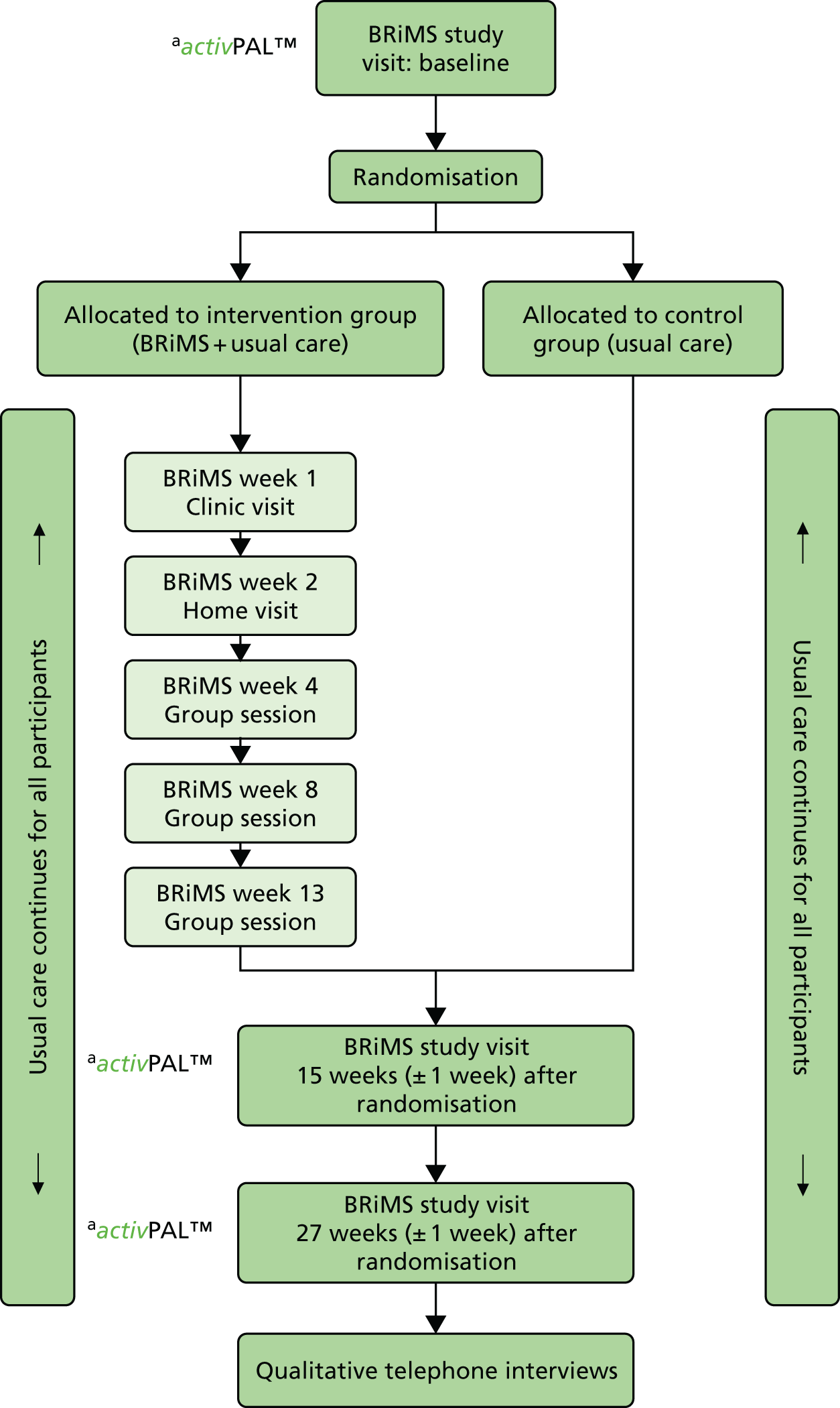
The two trial groups are shown in Figure 4, which includes a summary of the participant pathway.
FIGURE 4.
Participant pathway. activPAL™ (Paltechnologies Ltd, Glasgow, UK). a, All participants wear the activity monitor for 1 week from visit and then return it to the research therapist.
Usual care (usual-care group)
All participants allocated to this group continued to receive their usual clinical care; thus, with the exception of the trial assessments, they were not asked to attend any additional visits or sessions. Although usual care varies across the country,57 it rarely involves regular ongoing physiotherapy intervention in the community or as an outpatient on either an individual or a group basis. As a general rule, for those with SPMS, physiotherapy input is provided when an event has caused a significant deterioration in the person’s ability to function (e.g. a respiratory infection or an injurious fall). The standard physiotherapy care pathway usually comprises short, intermittent episodes of face-to-face intervention, which are generally limited to a few sessions. The typical approach and content of these sessions is one wherein presenting problems are managed (e.g. providing mobility aids, a written home exercise programme and advice) rather than focusing on the promotion of long-term self-management strategies.
For people with mobility impairment who are at risk of falling, physiotherapy regimes typically consist of gait re-education and the provision of a written home exercise programme, which is aimed at strengthening muscles and/or optimising balance. In line with NICE guidelines,7 for individuals with mild to moderate disability, advice may also be given to enhance cardiovascular fitness, as this has been demonstrated to optimise general physical and emotional well-being and minimise deconditioning. For individuals whose mobility is more severely impaired, advice and information to support their carer in terms of facilitating movement (e.g. manual handling advice to enhance the safety of assisted transfers) may be given.
Usual care may also involve appointments with a variety of other health professionals (e.g. an occupational therapist, a general practitioner, a MS nurse specialist, a neurologist or a rehabilitation consultant). As with physiotherapy, multidisciplinary interventions are usually short term as resource restrictions limit the provision of long-term maintenance therapy.
Falls programmes for older people exist in most locations across the UK;58 however, anecdotal evidence highlights that people with MS are seldom referred to these services. 27 Some programmes specifically exclude those with neurological conditions from attending, and have lower minimum age restrictions, which present further barriers.
This trial recorded the content of usual care on the participant resource-use questionnaire at each trial assessment. Details of actual use for both groups are in see Table 41.
Usual care plus BRiMS (intervention group)
During the trial, six deliveries of the BRiMS programme were undertaken. The dates were planned in advance to secure the services of the NHS treating therapists at each site and to facilitate participants’ advance diary planning. The intervention groups were conducted by band 7 NHS physiotherapists who were experienced clinical specialist neurological therapists (see Table 25). The therapist training took the form of a 1-day workshop delivered by two members of the research team (HG and JA), which all treating therapists attended. The workshop included an overview of the key elements of the programme, interactive sessions to introduce therapists to less familiar activities and an opportunity to practise setting up and amending the online exercise activities. Ongoing support was provided in the form of an online forum, promoting peer support and with additional input from a member of the research team (JA).
As participants were recruited in blocks that were related to each programme delivery, they were already aware of the expected programme dates. Therefore, when participants received confirmation that they had been allocated to the intervention group, they were simply contacted by the relevant treating therapist to arrange the one-to-one sessions and to confirm their ongoing availability for the group sessions. All BRiMS programmes were delivered in accordance with the programme manual and plan (see Chapter 1, The BRiMS programme). In addition to participating in the BRiMS programme, all of those allocated to this group were encouraged to continue utilising their usual clinical care and services.
Data collection and outcome measures
The BRiMS trial had four main analytical strands:
-
evaluation of trial feasibility
-
clinical outcomes
-
health economics evaluation
-
BRiMS programme process evaluation.
All of the required data, assessment tools, collection time points and processes are summarised in Table 2.
| Outcome group/measure | Objective | Evaluation time point(s) | |||
|---|---|---|---|---|---|
| Baseline | 13 weeks ± 1 weeka | 27 weeks ± 1 weeka | Post trial | ||
| Trial feasibility | |||||
|
i–vi | ✗ | ✗ | ✗ | |
|
✗ | ||||
|
→ | ||||
| Potential full trial outcomes | |||||
| Participant characteristics | |||||
|
✗ | ||||
|
✗ | ✗ | ✗ | ||
| Primary outcomes | |||||
|
vii–ix | ✗ | ✗ | ✗ | |
|
✗ | ✗ | ✗ | ||
|
✗ | ✗ | ✗ | ||
| Secondary outcomes | |||||
|
vii–ix | → | |||
|
✗ | ✗ | ✗ | ||
|
✗ | ✗ | ✗ | ||
|
✗ | ✗ | ✗ | ||
|
✗ | ✗ | ✗ | ||
|
✗ | ✗ | ✗ | ||
|
✗ | ✗ | ✗ | ||
| BRiMS programme feasibility (process evaluation) | |||||
| Programme acceptability and feasibility (participant and therapist interviews) | x, xii | ✗ | |||
|
x–xii | → | |||
|
|||||
|
|||||
| Health economics | |||||
|
xiii–xv | ✗ | ✗ | ✗ | |
|
✗ | ✗ | ✗ | ||
Evaluation of trial feasibility (objectives i–vi)
Feasibility outcomes
-
Recruitment, retention and attrition rates [Consolidated Standards of Reporting Trials (CONSORT) data]: number of patients assessed for eligibility, reasons for exclusion, numbers lost to follow-up, numbers discontinuing (with reasons) and numbers analysed and excluded from the analysis. Research staff invited participants who withdrew from the intervention or research procedures to provide a reason.
-
Participants’ and therapists’ views on the acceptability of the trial procedures were obtained through qualitative methods (see Methods of evaluation for details).
Participant safety and adverse events
Adverse events
An adverse event (AE) was defined as any unfavourable and unintended sign (e.g. including an abnormal laboratory finding), symptom or disease that developed or worsened during the trial, whether or not it was considered to be related to the trial intervention. The risk of an AE from participating in this trial was assessed to be low. 1 AEs such as chest infections and urinary tract infections, which are common in people with MS, were not intentionally monitored for any participants (intervention or usual-care group). However, all participants were asked to report any new or worsening problems that they perceived to be related to participation in activity and/or exercise, as well as any relapses and falls, in the daily pre-formatted paper diaries. These were completed from the day of randomisation until the final assessment (27 weeks ± 1 week following randomisation), and returned in the reply-paid envelope on a fortnightly basis to PenCTU for data entry. AEs may also have been discovered by treating therapists or research therapists during questioning, physical examinations or during another intervention. When this was the case, the therapist took appropriate action and also asked the participant to record the AE in their diary to ensure it was reported as part of the trial data. To avoid double counting AEs, the therapist was requested not to report these to the PenCTU.
On receipt of the diary returns, PenCTU recorded any AEs in the trial database, with collated reports being (of the whole group, i.e. not according to group allocation) regularly presented at the Trial Management Group (TMG) meeting for review.
Adverse events considered related to the trial intervention were followed until resolution or until the event was considered stable.
Serious adverse events
A serious adverse event (SAE) was defined as an untoward occurrence that:
-
resulted in death
-
was life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
consisted of a congenital anomaly or birth defect, or
-
was otherwise considered medically significant by the investigator.
It was not anticipated that there would be any SAEs related to this feasibility trial. Any SAE, whether or not thought to be related to any trial intervention, was reported to the CTU by the local principal investigator or another member of the research team by telephone or e-mail within 24 hours of the research team becoming aware of it. SAEs were recorded from the time of the baseline assessment until the date the participant completed follow-up or withdrew from the trial. SAEs could be directly reported by the participant or another informant (e.g. by telephone) or discovered by the treating therapist or research therapist through questioning, physical examination or another investigation. In addition, the 2-weekly diaries were reviewed to check for potential SAEs that had not otherwise been reported. Within 7 days of a local research team becoming aware of such an event, it was required that a SAE form was completed, signed by the principal investigator and returned to PenCTU. Completion of the SAE form included the principal investigator’s assessment of causality (i.e. whether or not there was a reasonable causal relationship between the SAE and the trial intervention). If the available information was incomplete at the time of reporting, all appropriate information relating to the SAE was forwarded to PenCTU as soon as possible.
If the principal investigator considered that the SAE was not, or was unlikely to be, related to the trial, PenCTU obtained a second assessment of causality either from the Scottish regional co-ordinator (for SAEs at the Plymouth site) or from the chief investigator (for SAEs at other participating sites).
It was protocolised that, if SAEs were adjudicated as being possibly related to the trial intervention and unexpected, then they would be reported by PenCTU to the Research Ethics Committee within 15 days of the local research team becoming aware of the event. This situation did not arise in this feasibility trial.
All SAEs were followed until resolution. PenCTU routinely notified the chief investigator by e-mail of all reported SAEs as they occurred and reported organ system listings of all SAEs to the Trial Steering Committee (TSC) and sponsor on a quarterly basis. PenCTU was responsible for the preparation and submission of an annual safety report to the Research Ethics Committee.
Trial outcome objectives (vii–ix)
Potential primary outcome for the definitive trial
A key aim of this feasibility trial was to inform the selection of a primary outcome measure and provide potential sample size estimates for the definitive trial. In line with the remit of a feasibility trial, a variety of outcome measures were undertaken to determine their performance and to identify those that may be most appropriate to use in the definitive trial. The selection of potential outcomes was informed by best practice guidance,59,60 and refined by the findings of our previous stakeholder activities (which involved service users, providers and commissioners),27 collaboration with our trial patient and public involvement (PPI) representatives and guidance from methodological experts. This led to the decision to propose mobility and quality-of-life measures as potential primary outcomes rather than as falls outcomes. There were two main reasons for this: (1) the recognition that a reduction in falls without a corresponding improvement in mobility or QoL outcomes would be undesirable, and (2) concern about possible issues associated with relying on self-report falls diaries as a data collection mechanism.
As this process led to the proposal of several potential primary outcomes, one of the key objectives was to establish what would be the most sensible choice for use in a main trial. In particular, our aim was to select a primary outcome that, as well as being meaningful to patients, ensured that the main trial would be feasible and provide good value for money; for example, the selected outcome should demonstrate good completion rates and lead to a realistic sample size requirement.
Outcome time points
Standardised, validated, clinician-rated and patient self-reported clinical outcomes were measured at baseline, at 15 ± 1 weeks after randomisation (coinciding with the end of intervention period for participants allocated to receive BRiMS) and at 27 ± 1 weeks after randomisation (coinciding with the 12-week post-intervention period). In a definitive trial, we would ideally like to follow up participants for 6 months post intervention to determine whether long-term behaviour change (such as sustained engagement in exercise) occurs once therapy support is withdrawn.
Procedures
All research procedures were protocolised and any deviations were documented. A research therapist (this was always a physiotherapist) in each region undertook all of the outcome assessments, independently of treatment, at separate research visits at local health-care establishments. Before recruitment commenced, the research therapists from the two regions met with each other and members of the research team to ensure a consistent approach and to standardise procedures. Every effort was made to ensure that these assessments were blinded, and participants were asked to not discuss whether they were attending a group session or undertaking any exercise programme. We carefully chose the battery of measures on the basis of proven psychometric properties, our previous research in this area, recommendations from the International Multiple Sclerosis Falls Prevention Network, and discussions held with people with MS/carers, as well as health professionals working in this field, to ensure acceptability and relevance. Previous trials of the assessment protocol demonstrated that the assessment would take approximately 60 minutes. This is the typical duration for NHS physiotherapy assessments for complex neurological conditions and well within the maximum 90 minutes that people with MS have told us is acceptable for such assessments.
Baseline measures
The following demographic clinical characteristic data were collected by participant self-report: age, sex, educational attainment, marital status, employment status, length of time since diagnosis, disease course, MS relapse history (within the past 3 months), number of falls/related injuries in the past 6 months, currently prescribed drugs and comorbid medical conditions. The diagnosis was corroborated by the research therapists or CRN staff with the participant’s medical records.
Information about changes to health and medication use was also collected at the follow-up assessments.
Potential primary outcomes
Multiple Sclerosis Walking Scale (12-item) version 2
An important outcome to evaluate in this study was walking from the patient’s perspective. We chose the MS Walking Scale (12-item) version 2 (MSWS-12vs2)61 to achieve this because it is a widely used, psychometrically robust, patient-reported questionnaire that assesses the impact of MS on walking ability, which is a key goal of the BRiMS programme. This questionnaire evaluates the impact on 12 aspects of walking function and quality (walking, running, climbing stairs, standing, balance, distance, effort, support needed indoors, support needed outdoors, speed, smoothness, and concentration needed to walk) identified as important by people with MS. The original Multiple Sclerosis Walking Scale (MSWS-12) has been robustly evaluated in terms of its psychometric properties,62–65 and the revised version 250,61,66,67 has minor modifications to response options. Of the 12 items, three are scored 1–3 and the other nine are scored 1–5. The category descriptors range from 1 (‘not at all limited’) to 5 (‘extremely limited’). Scores on the 12 items are summed, giving a total raw score whose range is 12–54. To ease interpretation, this raw score is transformed to 0–100 (minimum to maximum walking disability) by subtracting the minimum score from the sum, dividing the result by 42 and then multiplying by 100. 68
Quality of life (EuroQol-5 Dimensions, five-level version)
The EuroQol-5 Dimensions, five-level version (EQ-5D-5L)69 is a standardised self-report measure of health status, recommended for use in UK health technology appraisals and health policy decision-making. 70 Taking approximately 5 minutes to complete, the EQ-5D-5L collects data across five dimensions; mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension is scored by the participant as no problems, slight problems, moderate problems, severe problems or extreme problems. From the initial collection of the EQ-5D-5L, data can be mapped to be reported as the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), in accordance with relevant guidance (see Health economics analyses). The EQ-5D-5L would also be used to inform the economic evaluation in a follow-on definitive trial, wherein it is expected that it would be used in combination with a UK tariff of health state values71 to estimate quality-adjusted life-years (QALYs) for use as the primary economic end point.
Quality of life [Multiple Sclerosis Impact Scale (29-item) version 2]
The MS Impact Scale (29-item) version 2 (MSIS-29vs2)61,72,73 is a condition-specific measure of health-related QoL. This widely used self-report questionnaire was devised specifically for people with MS and was founded on interviews with patients exploring how MS affects their QoL. The original Multiple Sclerosis Impact Scale (MSIS-29) has been robustly evaluated in terms of its psychometric properties,61,74–77 and the revised version 2 has minor modifications to response options. 67,73 It consists of 29 items, all of which have four response categories numbered 1 (‘not at all limited’) to 4 (‘extremely limited’); a higher score indicates greater impact on the individual’s life. The MSIS-29vs2 provides domain scores for QoL and summary scales for physical and psychological elements. There is an accumulating body of evidence to support the internal consistency, reproducibility, validity and responsiveness of this later version. 67,73 Furthermore, the MSIS-29vs2 data can be used to derive a MS-specific preference-based measure, the Multiple Sclerosis Impact Scale (8-dimensions) (MSIS-8D),73,78,79 which may be used in assessment of cost-effectiveness.
Potential secondary outcomes
Falls frequency and injury rates
Evaluation of falls status is important, as there is a known link between falls status and activity curtailment20 that may, in turn, have an impact on mobility and QoL. In this trial, falls were assessed by prospective direct measurement, as it is widely acknowledged that other methods lack reliability and validity. 80,81 In line with best practice guidance,59 participants were asked to complete the pre-formatted daily paper diaries throughout the trial to record falls and any related injuries (see Appendix 1). A fall was defined as ‘an unexpected event in which you come to rest on the floor or ground or lower level’. 60 In addition, participants were asked to record any injuries and the related use of medical services as a result of each fall. In line with recommendations from Coote et al. ,59 participants were asked to complete the diary daily and return a batch of 14 completed daily diaries in a reply-paid envelope every 2 weeks throughout the trial. Participants received an automatic e-mail on six occasions throughout the trial to thank them for their engagement and to prompt ongoing diary returns. Returned diaries were reviewed by the trial managers and the data were entered into the trial database. Automatic e-mail reminders were triggered when diary returns fell more than 2 weeks behind schedule. Up to two reminders were sent to participants to remind them to complete and return their diary. Pre-formatted diaries to record falls and related injuries have been used in a number of MS studies. 16,82,83 Previous studies using this method have indicated that high completion and return rates are consistently gained (75–99%), with our own previous study demonstrating a 93% completed diary return rate. 16
Physical activity level
It is important to measure levels of physical activity, as these reflect much more than simply walking, and potentially provide a clearer indication of the impact of both the disease and the intervention on the physical dimensions of an individual’s daily life. For instance, although falls may reduce if people remain sitting (appearing to be a positive outcome from a falls perspective), this would not generally be considered a positive outcome for the person with MS. The intention of BRiMS is to encourage increased levels of safe mobility/physical activity, which has been demonstrated to enhance people’s QoL and minimise secondary complications such as deconditioning.
The level of physical activity was measured objectively over 7 days using an activity monitor (activPAL™, Paltechnologies Ltd, Glasgow, UK). 84 The activPAL is a tri-axial accelerometer, worn on the thigh, which can record data continuously for up to 21 days. It is smaller and lighter than other accelerometers and attaches securely to the skin. The activPAL, and its accompanying manufacturer’s software, classifies activity in terms of the time spent sitting or lying, standing and stepping, the number of steps taken, the cadence, and the number of sit-to-stand and stand-to-sit transitions. To our knowledge, these are features that no other comparable device offers. To ensure that the data collected are a true representation of the individual’s physical activity, 5 consecutive days of data are required. 85 Thus, to improve the likelihood of achieving 5 full days of data, participants were asked to wear the monitor continuously (24 hours a day) for 7 consecutive days and to undertake normal daily activity. The device was not removed during the data collection period. The time spent in each posture and the number of steps were averaged over the 5-day period. The activity monitor was fitted to the participant at each assessment session and participants were instructed to remove the device after 7 days and post it back to the research team in the pre-paid addressed envelope provided. This methodology has been successfully used in previous studies,86 including several undertaken by some of the co-applicants. 87,88
Walking capacity
To complement the self-report MSWS-12vs2, we also undertook an objective clinician-rated measure of walking capacity: the Two-Minute Walk Test (2MWT). This determines the longest distance an individual can walk (using walking aids if required) over 2 minutes on a hard, flat surface. 89 This measure was chosen as it is strongly correlated with community mobility levels,90 which is a potentially important outcome of the BRiMS programme. There is evidence to support the reliability, validity and responsiveness of the 2MWT in a range of rehabilitation studies, and reference values are available regarding clinically meaningful improvement, according to disability level, in people with MS. 50,90,91 The 2MWT has been recommended as the timed walking measure of choice for evaluating rehabilitation interventions in people with MS who have moderate disability. 91 In this study, the 2MWT was undertaken using a 5-metre track set up in a quiet corridor. Participants were instructed to walk as many lengths as possible in the time allowed, taking breaks if required.
Balance
Poor balance has been identified as a key modifiable risk factor for falls in MS15 and is one of the primary targets of the BRiMS exercise component. The multidimensional nature of balance means that some measures have been criticised for lacking responsiveness. 92 In this trial, balance was evaluated using the following two measures:
-
The Mini-Balance Evaluation Systems Test (Mini-BEST),93 a 14-item clinician rated balance assessment tool that aims to target and identify the contributions of six different balance control systems to functional stability: anticipatory postural adjustments, reactive postural correction and dynamic balance during gait (including cognitive effects). Each item is scored on a three-level ordinal scale (0–2), with higher scores indicating better performance; the maximum possible score is 28 points. The Mini-BEST has established psychometric properties, including excellent internal consistency, reliability, validity and responsiveness. 94 It is recommended for inclusion as part of a core outcome set for measuring balance in adult populations. 95
-
The Functional Reach and Lateral Reach Tests are clinician-rated measures of standing balance that mirror the everyday activity of reaching for objects beyond arm’s length. The person stands adjacent to a wall with their shoulder flexed (forwards reach) or abducted (lateral reach) to 90 degrees, and leans forward (or laterally) as far as possible without stepping, thereby testing the limits of stability. Measurements are taken with a metre rule and an average of three repetitions is used. 96 The Functional Reach and Lateral Reach Tests are considered psychometrically robust for use in neurological clinical practice97 and have been used in a number of studies to evaluate the effect of exercise interventions that aim to improve the balance of people with MS. 66,98
Fear of falling
Fear of falling has been highlighted as a risk factor for falls99 and is also associated with activity curtailment in MS. 20 A reduction in fear of falling is, therefore, a potentially important outcome of BRiMS, given the recognised association between physical activity levels and QoL. 100 We measured fear of falling with the Falls Efficacy Scale – International (FES-I),101 which has been recommended by the European falls network ProFane (www.profane.eu.org) as the preferred measure of fear of falling for clinical and research use because of its speed and simplicity of completion. 60 The FES-I has also been validated for use in ambulant people with MS, demonstrating excellent internal reliability and construct validity. 102 The FES-I produces a single score based on the summed total of the individual responses to the 16 questions; the maximum possible score is 64, with higher scores indicating a greater degree of anxiety.
Community Integration
The World Health Organization’s focus on participation as a key construct in the International Classification of Functioning, Disability and Health103 recognises that high-quality health care looks beyond mortality and disease to focus on how people live with their conditions within their environments. As a result, participation is increasingly a focus of measurement in rehabilitation studies. 104,105 We measured participation using the Community Participation Indicators (CPI),105 a self-report measure that evaluates participation using three key indicators: engagement (20 items), involvement in life situations (14 items) and control over participation (13 items). There is preliminary evidence supporting the validity of the CPI; however, its use has been relatively small-scale to date. The CPI was recommended for use in an expert review paper published by members of the International MS Falls Prevention Research Network;106 this feasibility trial provided an opportunity to evaluate its performance before its potential inclusion in the definitive trial.
Programme feasibility objectives (x–xii)
Evaluation of programme feasibility: BRiMS process evaluation
There is growing recognition of the complexity of designing, implementing and evaluating rehabilitation interventions and the challenges of moving programmes from the research setting into clinical practice. The updated Medical Research Council guidance on the evaluation of complex interventions calls for researchers to combine assessment of outcomes alongside evaluation of process. 34 This reflects the recognition that for evaluations to inform policy and practice, emphasis is needed not only on whether interventions ‘work’ but also on how they are implemented, their causal mechanisms and the impact of the ‘real world’ on programme uptake, delivery and engagement.
This trial provided an opportunity to evaluate the implementation of BRiMS as the next step in the complex intervention development process. 35 The BRiMS process evaluation was developed in accordance with best practice guidelines45 and with reference to key theoretical and evaluation frameworks. The overarching approach to the BRiMS PE is one of realist evaluation, as laid down by Pawson and Tilley. 107 The paradigm driving the evaluation is one of pragmatism, recognising the influence of historical, cultural and political contexts on programme design, implementation and user experience. 108
The process evaluation explored three main areas of programme delivery (Figure 5):
-
intervention implementation
-
mechanisms of impact
-
context.
FIGURE 5.
The BRiMS process evaluation framework.
The process evaluation plan
The overarching methodological approach to the BRiMS process evaluation is that of mixed methods. A mixed-methods approach allows a number of strategies to be employed concurrently or sequentially. Each element supports the findings of the other methods to create a coherent whole109 to inform the development of holistic, realistic recommendations. 110
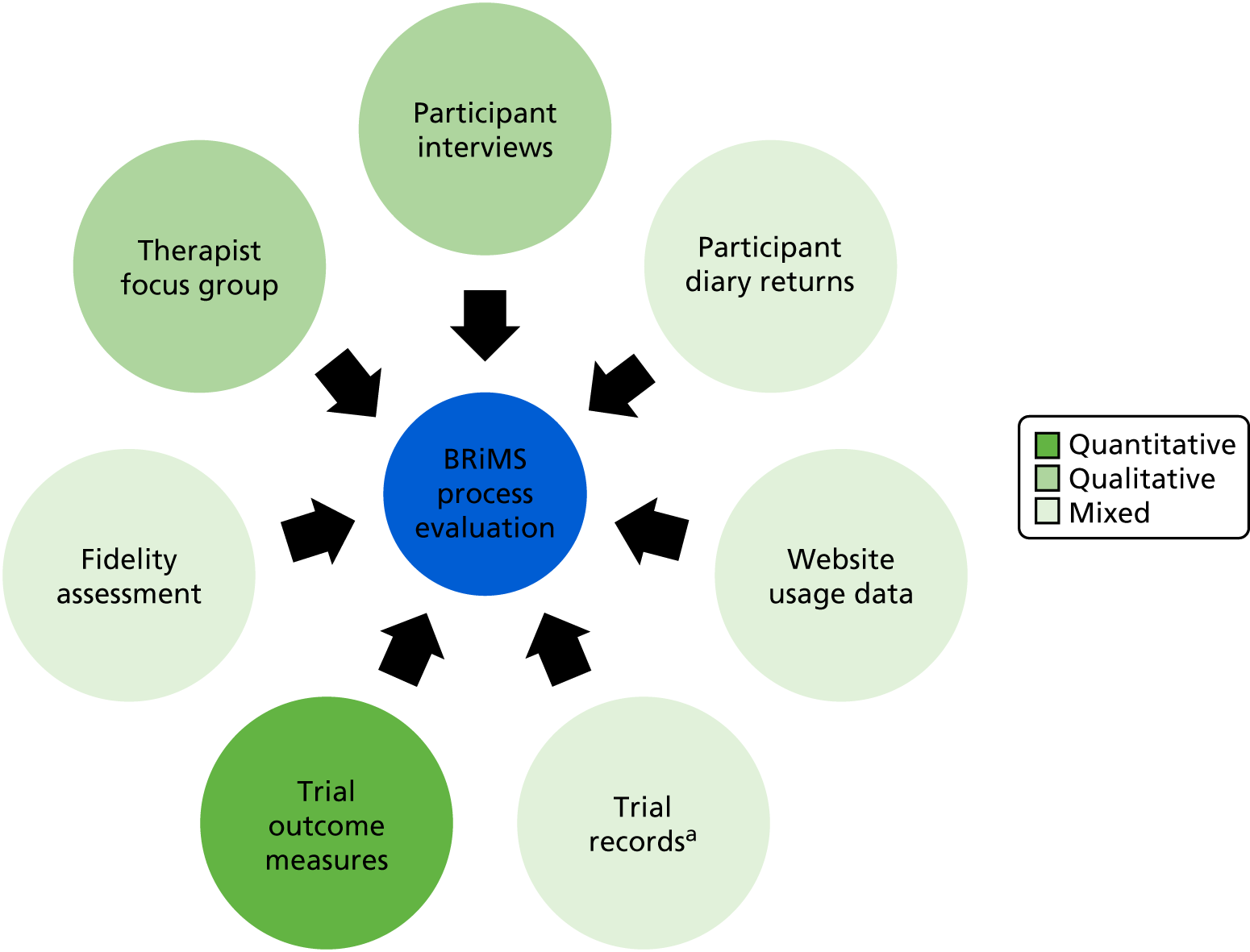
Sources of data
The sources of data for the process evaluation are detailed in Figure 6.
FIGURE 6.
Sources of data for the BRiMS process evaluation. a, Treating therapist contact sheets, withdrawal and adverse event data.
Methods of evaluation
Intervention fidelity
The assessment of intervention fidelity aimed to measure the degree of concordance between the BRiMS manual and the actual programme delivery. The BRiMS fidelity assessment was carried out by three members of the research team who had a range of expertise relevant to the programme:
-
assessor 1 – consultant neurological physiotherapist; expertise in clinical management of MS, site principal investigator for the Ayrshire site
-
assessor 2 – professor of psychology; lead for the development of functional imagery and motivational support aspects of BRiMS
-
assessor 3 – lecturer in physiotherapy; developer of BRiMS programme and BRiMS trial co-ordinator.
Fidelity assessments, customised according to the content of each session, were undertaken by scoring audio-recordings of a sample of BRiMS sessions against the relevant checklist rating scale. The items scored included both generic behaviours and session-specific content. The checklists were based on the Dreyfus system for assessing skill acquisition111 and an adaptation of the Motivational Interviewing Treatment Integrity scale. 112
The fidelity checklist rating scales
The fidelity assessment for every session type included four common items that were scored according to the criteria specific to the item (see Fidelity assessment; full manual available from the authors on request). In addition, session-specific items were included that were scored according to the generic criteria (see Appendix 2). Thus, the scale assessed adherence to the content of the intended intervention techniques, the depth of coverage and the delivery approach. Each item was rated according to a four-point Likert scale; to aid with the rating of items, an outline of the key features of each item was provided. The tool was piloted, and scoring comparisons were made between the three reviewers, for three session recordings. This enabled a consensus to be reached about its application before its use in the full evaluation.
Method
With the participants’ permission, all face-to-face BRiMS sessions were audio-recorded (clinic assessment, home visit and all group sessions). The recorded sessions were used to measure fidelity by comparing the session audio recording against the checklist for that session. A random sample of 25% of the audio-recordings was scored. No patient identifiable information was recorded on the fidelity checklist and the audio-recordings were destroyed at the earliest opportunity after the checklist was completed. The recorded sessions were used to evaluate the quality of the intervention delivery and the degree of concordance with the BRiMS manual.
Participant engagement and adherence to the BRiMS programme
The BRiMS programme comprises five face-to-face sessions (two individual and three group based); attendance at these face-to-face sessions was monitored and recorded. Participants were also advised to undertake a minimum of 120 minutes of home-based exercise per week for a minimum of 12 weeks, utilising a web-based physiotherapy programme, completing a web-based exercise diary each time. Thus, adherence to the home-based programme was monitored based on the number of web-based log-in sessions and the exercise diary data recorded online by the participant during the programme; adherence to each element was calculated as a percentage. For example, with regard to adherence to the requested time spent exercising, the optimum duration of exercise was 24 hours (2 hours per week for 12 weeks); if a participant reported completing 18 hours, then they had 75% adherence. In addition, the weekly adherence was monitored throughout the programme, as evidence suggests that adherence to internet-delivered interventions reduces over time. 113
Participants’ and therapists’ views on acceptability of the BRiMS intervention
The BRiMS programme has not yet been trialled for use by therapists in the clinical setting, and nor has it been fully evaluated in a clinical trial. Therefore, a qualitative component was included in the process evaluation to enable an exploration about the experience of participating in the trial (for both allocated groups) and engaging in the BRiMS intervention (for the intervention group only), from the perspective of both people with MS and the therapists delivering the intervention.
Procedures
The qualitative data from BRiMS participants were collected through one-to-one telephone interviews, and a telephone focus group114 with treating therapists was held at the end of the programme. For the participant interviews, a purposive sample of 13 participants was recruited, including people from different regions and different BRiMS intervention groups, together with a sample of usual-care group participants. At the end of the trial period, participants were contacted and a mutually convenient time was agreed to undertake a telephone interview within 2 weeks of the completion of the BRiMS final trial visit.
All four treating therapists were invited to participate in the telephone focus group, which was convened within 10 weeks of the completion of the final BRiMS programme delivery. All qualitative interviews were facilitated by members of the research team (participant interviews, HG or JF; therapist focus group, HG and JA). All interviews were digitally recorded and transcribed verbatim before being thematically analysed (see Qualitative analyses) and synthesised into the process evaluation.
Health economics objectives (xiii–xiv)
Health economics outcomes
The main aims of the health economics analyses were to (1) provide an estimate of the resource use and associated cost of delivering the BRiMS intervention; (2) develop a framework for collecting data on costs and outcomes, and (3) develop methods for conducting cost-effectiveness analyses (CEA) in a future full trial.
Methods for future conduct of economic evaluation were developed and tested, within this feasibility trial, on the collection of resource use, cost and outcome data. The data on resource use associated with the set-up and delivery of the BRiMS intervention were collected via within trial reporting, including participant-level contact and non-contact time for staffing input on delivery, equipment and consumable costs, training and supervision for delivery staff. Data on health and social care resource use were collected at participant level using a resource-use questionnaire, developed for this trial based on resource-use forms used successfully in previous studies in people with MS. 67
The EQ-5D-5L was used to assess health outcomes from an economic viewpoint. The EQ-5D-5L is used to derive health state values associated with the health status (states) described by trial participants. A future economic evaluation would be expected to use the EQ-5D-5L as the primary economic end point (over a6-month follow-up) to estimate the cost per QALY and, accordingly, EQ-5D-5L data were collected in this trial. However, given some debate and uncertainty over the appropriateness of the EQ-5D-5L for people with MS, the MSIS-29vs2 data collected during the trial were also used to estimate health state values (QALYs) via the MS-specific preference-based measure developed by Goodwin and Green. 79
Progression to definitive trial
We pre-defined a number of progression criteria for consideration as part of the decision about whether we would either progress to a full trial application, or need to undertake further developmental work. These criteria, described below, were designed to address the key aims and objectives of this feasibility trial. The progression criteria were finalised in discussion with the TSC, with whom we were also able to discuss whether or not there was sufficient evidence, with appropriate changes, to move forwards to a definitive trial. The criteria were:
-
A minimum of 80% recruitment of the intended 60 participants within the planned 6-month recruitment window.
-
A minimum of 80% of consented participants randomised to the intervention group fulfilling the minimum engagement criteria of engaging with the 13-week BRiMS intervention, which was to attend the initial face-to-face clinic appointment where the participant was assessed, their individualised home programme was designed and explained, and the paper-based BRiMS participant manual was provided to them.
-
A minimum of 80% completion rate of at least one of the proposed primary outcome measures among participants attending the planned primary end point of 27 weeks (± 1 week).
-
The total resource estimated to conduct the definitive trial at a level that is likely to attract funding.
We considered that there would most likely be unforeseen issues raised during the trial that could affect the decision to progress, but we anticipated that the process evaluation data and input from PPI team members would be helpful in finding potential solutions and indicating remedial action.
Trial governance
Ethics and research governance
Application for ethics review via NHS and university ethics was submitted during the pre-trial period (January–September 2016) in time for the commencement of the trial in September 2016. During this period, relevant NHS trust research and development department and Clinical Commissioning Group approvals were also obtained.
Patient and public involvement
From the outset, the programme was informed by people with SPMS. In 2010, this topic was identified by participants in the chief investigator’s longitudinal study evaluating mobility. A survey (n = 116) asked for views about what future research should focus on: safe mobility and falls were priorities. Subsequently, two discussion groups confirmed this and were used to focus research questions, determine acceptable study designs and problem-solve implementation issues. People with SPMS have sat on advisory committees in our subsequent studies: the BRiMS programme is a direct output. They were intimately involved in the development of BRiMS through participation in the nominal group study [n = 36 (50%) people with MS],27 which was innovative in how it facilitated people with MS, health professionals, commissioners and the research team to work together. Dedicated training sessions (facilitated by the local NIHR user involvement group) supported people with MS to engage fully and confidently in the process. This high level of user engagement continued throughout this feasibility trial: Ben Marshall (who has MS) was a co-applicant on the trial and had input on the development of the qualitative interview schedule/evaluation/validation checks. John Kendrick, who also has SPMS, sat on the TSC. They provided advice on issues such as recruitment, on participant materials such as the information sheet and plain English summary, and on publications designed for patient/general public consumption such as MS organisation newsletters. Participants from the nominal group phase, who helped us to design the BRiMS intervention, gave permission for us to involve them in related discussion groups, and we contacted some of these individuals again to use them as a ‘sounding board’ for materials during this study. In line with INVOLVE guidance, lay members were financially reimbursed for attending discussion groups, TSC and TMG meetings, as well as for the associated preparation time (included in the trial costs). The project team has a well-established relationship with the MS Trust and the MS Society; both organisations were consulted about the development of the trial protocol application and were highly supportive, playing a key role in ensuring that it was possible to access people with SPMS through local branches.
Human and data management
As chief investigator, Jenny Freeman assumed overall responsibility for the trial, ensuring that it finished on time and within budget. The trial was sponsored by University Hospitals Plymouth NHS Trust (formerly Plymouth Hospitals NHS Trust). It was managed by the UKCRC-registered PenCTU, which co-ordinated the development of the trial protocol and trial-specific documentation, managed the approvals process, liaised with both trial sites, monitored recruitment and was responsible for the day-to-day conducting of the trial in conjunction with the chief investigator. The CTU also provided a bespoke web-based randomisation system, database and data management services for the trial.
Trial Management Group
A TMG, chaired by Jenny Freeman and including the co-applicants, the PenCTU trial manager, the PenCTU data manager, patient representatives and sponsor representative, met monthly to monitor general progress/timelines, recruitment, retention, adherence to the trial intervention and budgetary issues and to discuss any problems as they arose.
Trial Steering Committee
Make-up
The TSC was a group of experienced triallists with majority independent representation: the chairperson (independent), an external statistician (independent), the chief investigator (non-independent) and a lay member (independent).
Frequency of meetings
The TSC met before the start of the trial in person and subsequently by teleconference on four occasions. In addition, the TSC received a quarterly report of SAEs and injurious falls.
Responsibilities
The responsibility for calling and organising the TSC meetings lay with PenCTU in association with the chairperson.
Degree of independence from sponsor and investigators
Confirmation that independent members of the TSC were unconnected to either the trial sponsor or the investigators was made through the completion of conflict of interests documents by all TSC members.
Minutes of meetings were sent to all members, the sponsor and the funder, and were retained in the trial master file.
Data Monitoring Committee
Make-up
The group comprised an independent statistician and two experienced methodologists, one of whom was a MS specialist consultant neurologist (the chairperson).
Frequency of meetings
The Data Monitoring Committee (DMC) first met at a telephone conference (November 2016) to agree the terms of reference. There was no planned interim analysis for this trial, and thus there were no definitive plans for the DMC to meet again. The TSC was charged with reviewing a quarterly report of all SAEs and injurious falls, pooled across allocated groups (thus avoiding unblinding the TSC). It was agreed that the TSC chairperson would trigger a DMC meeting to review the unblinded safety data in the event of more than four fall-related injuries that required medical care in any consecutive 3-month period. This was based on data from Peterson et al. ,18 who reported rates of injurious falls requiring medical care of 0.23 falls per person per year. This equates to a rate of 0.05 per person over 3 months, which, when rounded to 60 participants, equates to a rate of 3.45 falls per 3-month period. Therefore, a rate of four or more reports of injurious falls requiring medical care represented a rate in excess of the known average. For the purposes of this analysis, the number of falls that required medical care was used to calculate the rate, and not the number of times that the person saw a medical practitioner.
A DMC teleconference was triggered on one occasion because there were five such falls reported between May and July 2017. A DMC teleconference was held in early November 2017, during which the committee reviewed the unblinded data and concluded that there were no concerns, recommending that the trial continue through what remained of the follow-up period.
Responsibilities
The DMC maintained the interests of trial participants, with particular reference to safety.
Degree of independence from sponsor and investigators
This committee was independent of the trial organisers and the TSC. The TSC and the DMC met independently of each other.
Data management
Peninsula Clinical Trials Unit was responsible for data management in this trial.
Data protection
Data were collected and stored in accordance with the Data Protection Act 1998. 115 Electronic trial records were stored in a Microsoft SQL Server (Microsoft Corporation, Redmond, VA, USA) database, stored on a restricted-access, secure server maintained by the University of Plymouth. Data were entered into the database via a bespoke web-based data-entry system encrypted using SSL.
Participant numbering
Each participant was allocated a unique trial number by the CRN staff/research therapists when they were registered on the data collection website.
Data collection tools and source document identification
Case report form (CRF) entries were considered source data. These were printed and collated into booklets. The research therapists completed the CRFs for all participants. Completeness of data was maximised by the research therapists, who:
-
checked all forms at each assessment to ensure that there were no missing items
-
arranged another assessment session, wherever possible, if the pre-scheduled session was cancelled or participants did not attend.
PenCTU prompted the participants if they failed to return their diaries (within 2 weeks of each due date). In addition, periodic e-mail reminders were sent to all participants to maximise the completion of diary data.
Data handling and record-keeping
Completed CRFs were checked and signed by the research therapists before being scanned and transmitted to the Plymouth-based PenCTU on a participant-by-participant basis; the original CRF was retained at site. When PenCTU received the completed CRFs and diaries, all data were double-entered into a password-protected database. Double-entered data were compared for discrepancies. Discrepant data were verified using the original paper data sheets.
Treating therapists completed contact sheets following every contact they had with BRiMS programme participants. These were sent to the PenCTU on completion of each BRiMS delivery for data entry as above.
Qualitative data in the form of telephone interview and focus group audio-recordings were transcribed and anonymised as soon as practicable; original recordings were held securely until completion of the qualitative data analysis process, and then deleted.
The research teams ensured that participants’ anonymity was maintained on all documents. All paper forms (including all original signed informed consent forms and copies of the CRF pages) were archived by each site in a secure location and will be stored for a minimum of 5 years after trial closure, in line with the sponsor’s archiving requirements. Records remain accessible for the purposes of monitoring and auditing, or at the request of regulatory bodies.
Trial data were analysed by Siobhan Creanor and Kara Stevens (trial statisticians), Hilary Gunn (trial co-ordinator), Jenny Freeman (chief investigator) and Colin Green (in charge of economic data).
Archiving
The sponsor was responsible for archiving the original trial data (in paper and electronic formats) and essential documentation (the contents of the trial master file) in a secure location for a minimum period of 5 years after the end of the trial. Archiving was authorised by the sponsor following submission of the end-of-trial report. Each individual trial site was responsible for archiving copies of local trial data (as applicable where copies exist) and essential documentation (the contents of the investigator site file) in a secure location for the same period. No essential documents will be destroyed unless or until the sponsor gives authorisation to do so.
Monitoring, audit and inspection
The PenCTU trial manager devised a monitoring plan specific to the trial, based on an initial pre-trial risk assessment that was updated as required throughout the trial. The monitoring plan included both central monitoring strategies and trial site visits as appropriate, and was reviewed and agreed by the TMG. Monitoring included oversight of processes relating to the safety of participants and the integrity/reliability of the trial data, including AE reporting, participant enrolment, consent, eligibility and allocation to trial groups, adherence to trial interventions and policies to promote the accuracy, and timeliness of data collection.
All trial procedures were conducted in accordance with the protocol and according to the principles of good clinical practice. Procedures specifically conducted by the CTU team (e.g. randomisation, data management, trial management and trial monitoring) were conducted in compliance with PenCTU standard operating procedures. Principal investigators and the participating NHS trusts were required to permit the CTU trial manager or deputy to undertake trial-related monitoring to ensure compliance with the approved trial protocol and applicable standard operating procedures, providing direct access to source data and documents as requested.
Protocol amendments
No substantial amendments were made to the protocol during the lifetime of the trial, however, a number of non-substantial amendments were made. These included minor changes to improve consistency and clarity throughout; an update to the sponsor contact details; an update to the title of the chief investigator; and the removal of the clause ‘and adherence to’ from a sentence in section 14.4 that relates to participants engaging with the trial. This was removed on the advice of the TSC members, who felt that it would be overly optimistic to expect that the research team could reliably and comprehensively measure participants’ adherence to the programme. The TSC members also emphasised the complexity in understanding the level of engagement required in programmes of this nature to instigate behavioural change. They cautioned, therefore, about being very specific about required levels of adherence.
Data analyses
Statistical methods
A detailed statistical analysis plan was written by the team statisticians and approved by the TSC (statistical analysis plan version 1.0, dated 15 March 2018) prior to trial database lock.
Analytical approach
All analyses were undertaken in accordance with appropriate analytical and reporting guidelines. 116 Primary analysis (in the form of summary statistics, not formal/inferential analysis) was undertaken on an intention-to-treat basis, whereby participants were analysed according to their allocated group, regardless of adherence to the protocol or lack of participation or completion if allocated to the intervention group.
Statistical significance levels
As this was a feasibility trial, no inferential between-group comparisons were undertaken (i.e. there was no between-group hypothesis testing). When presented, confidence intervals are at the 95% level, unless otherwise stated.
Interim analysis
There was no planned interim analysis for this trial.
Time points of statistical analysis
Statistical analysis was undertaken once the final group of participants completed the final assessment at 27 (± 1) weeks post randomisation and the database was locked following final approval and sign-off of the statistical analysis plan by the TSC.
Data sources and data quality
The data from this trial came from information entered onto CRFs completed by a blinded research therapist at baseline, 15 (± 1) and 27 (± 1) weeks post randomisation. All participants were asked to complete a 2-weekly self-reported pre-formatted paper diary. In addition, intervention participants were asked to complete an online exercise diary to record their adherence to the programme. Attendance at the BRiMS face-to-face sessions and the number of log-ins to the online exercise portal were also recorded.
Missing data
One of the objectives of this feasibility trial was to assess the completeness of potential outcome measures for the definitive trial, at the level of both item and outcome measure. Missing outcome data were noted and used to inform the likely pattern of missing data in a full-scale trial.
Imputation methods
For the validated outcome measures MSWS-12vs2,61 MSIS-29vs261,72,73 and FES-I,101 the established methods for imputing missing item-level data were implemented, when the minimum number of items required to impute the missing data was met.
If the participants completed at least 11 of the 14 Mini-BEST test components, the final score was imputed by replacing missing values with the mean of the non-missing test component scores.
The mean of the three Functional Reach Test values (forwards and lateral) was calculated and analysed. If participants were missing any of the three repeated test components of the Functional Reach Tests, the mean of the successful attempts was used.
If there were up to four missing items from the FES-I score, the total score was imputed by replacing the missing items with the mean score. 117
A validated imputation method was not available for any CPI score. Therefore, if a participant was missing at least one item, he or she was excluded from the analysis.
Statistical software
The statistical analyses were undertaken using Stata/SETM version 14 (StataCorp LP, College Station, TX, USA), supplemented, where required, by R (The R Foundation for Statistical Computing, Vienna, Austria).
Statistical analyses
As this was a feasibility trial, it was not powered to be able to support or justify any conclusions regarding treatment effectiveness and efficacy realised from hypothesis testing,23 and, indeed, that was not the purpose of the trial. As such, the analysis of the results did not involve formal/inferential statistical comparisons between groups, but rather it was descriptive with the view to informing the design of a fully powered BRiMS RCT.
Continuous measures were summarised as means, SDs and ranges when the distribution appeared normal, and as medians, interquartile ranges (IQRs) and ranges when the distribution appeared otherwise. Categorical data were summarised by frequencies and percentages. When appropriate, parameter estimates (e.g. between-group differences) were presented with 95% confidence intervals (CIs). With the exception of the falls diary analyses (see Analysis of patient-reported and clinician-rated outcome measures), any potential outliers were identified and reported but not removed from the descriptive statistics of this feasibility trial unless stated. Analyses of quantitative data were conducted to summarise feasibility outcomes (objectives i–vi), evaluate acceptability and adherence to BRiMS (objective xii), and the completion and summary statistics of the planned primary and secondary patient-reported and clinical outcomes measures (objective vii). In addition, appropriate plots were used to illustrate key data and assess potential relationships.
Trial population
Data from the screening process through to the completion of the trial were recorded and presented in a CONSORT-style flow diagram. 116
Baseline characteristics and demographics
Baseline characteristics, collected before randomisation, were summarised by allocated group to informally check for balance between groups (by visual inspection) and provide an exploratory overview of the trial population.
Analysis of randomised groups at baseline is not good practice118 and so this was not undertaken, but any considerable imbalances were noted to inform the design of the full trial.
Participants who discontinued, withdrew or were lost to follow-up
It was possible that participants would withdraw consent part-way through the trial, or that their treatment would be discontinued for medical reasons. It was unlikely that a participant would be discontinued on medical grounds (in either allocated group), but for reasons such as injury, some participants may not have been able to complete the trial. Participants who discontinued were categorised as follows:
-
continued to consent for follow-up and data collection
-
consented to use pre-collected data only
-
complete withdrawal of consent to use any data.
Reasons for withdrawal or loss to follow-up were summarised, where these were reported, at each stage of the process. These included ‘participant withdrew before randomisation’, ‘participant did not receive their allocated treatment’, ‘participant did not complete treatment’ and ‘participant was lost to follow-up’.
Participants who withdrew from the trial, or whose treatment was discontinued on medical grounds, were not replaced. No participant who withdrew from the trial requested that their previously collected data be removed from the trial database. The extent of discontinuation, withdrawal and loss to follow-up will be used to inform the design of the anticipated fully powered trial, predominantly to ensure a sufficiently powered trial after allowing for losses to follow-up.
Trial feasibility outcome analyses
In addition to the summary statistics detailed in Statistical analyses, data pertaining to a range of feasibility issues were summarised, including the time to recruit each block of individuals; the number of completed assessments within the pre-defined assessment window; a detailed breakdown on attendance at each BRiMS face-to-face session; and the recorded web-based log-ins and diary completions.
Analysis of patient-reported and clinician-rated outcome measures
Summary statistics were calculated for each of the patient-reported and clinician-rated outcome measures at each time point, including CIs for the SDs. Between-group differences at 15 weeks (± 1 week) and 27 weeks (± 1 week) post randomisation were calculated, together with 95% CIs (no p-values are presented). The correlation between baseline and follow-up scores was calculated across all participants with available data, with corresponding CIs, for each of the candidate primary outcome measures for use in future sample size calculations.
Visual displays, such as box plots and scatterplots, with point and interval estimates, were used to identify any baseline characteristics that have a strong association with each or all of the candidate primary outcomes.
Analysis of EuroQol-5 Dimensions, five-level version, data
The advice from NICE is to use the EQ-5D-3L rather than the EQ-5D-5L,119 so the EQ-5D-5L was mapped to the EQ-5D-3L using the ‘crosswalk’ technique. 120 Therefore, we quote the EQ-5D-3L in the results tables throughout.
Analysis of activity monitor (activPAL) data
Data cleaning
Initial data cleaning was undertaken using visual inspection of each activity summary sheet to remove any incomplete days of data at the start and end of the recording period (e.g. data from the assessment day or the day of the removal of the activPAL).
Decisions relating to the classification of incomplete days at the end of the recording were informed by reference to the typical daily activity patterns recorded by the individual participant (through visual inspection of the summary sheets), and lack of event recordings in the following 24-hour periods (indicating prolonged non-use). Any uncertainties were addressed by checking appointment dates for the individual participant to inform the scheduled removal date.
Data analysis
All complete days of activity data were included. Initial analysis reported the time spent in the three activity classifications (sitting/lying, standing/incidental stepping, and purposeful stepping), plus step count and sit-to-stand transitions per day averaged over the number of full days of collected data.
Analysis of falls diary data
Participants returned data reporting falls and related injuries every 2 weeks for the duration of the study. As the diary return rate and completeness of diary returns were below what we expected, these data were analysed and presented in three ways.
Analysis 1 (reported)
Falls/injurious falls rate was calculated using the actual number of days of data available as the denominator (i.e. valid days only):
Analysis 2 (expected)
Falls/injurious falls rate was calculated according to the number of days available had all those who submitted any diary entries (n = 48) done so fully (i.e. returned 100% of their expected diaries):
Analysis 3 (randomised)
Intention-to-treat analysis: falls/injurious falls rate was calculated according to the number of days available had all those randomised (n = 56) done so fully (i.e. returned 100% of their expected diaries):
Safety data
Data on AEs were collected at the participant level as part of the fortnightly participant diary returns (see Potential secondary outcomes). In preparation for the analysis, any diary entries that were not for dates within the specified trial period (i.e. 28 weeks after randomisation) were excluded from the analysis. Potentially duplicated entries were not removed unless the diary start date was the same on both returns (i.e. obvious duplicates), and therefore it is possible that a small proportion of duplicated data remains. As with the falls data, AE reports were analysed and presented in three ways.
Analysis 1 (reported)
Analysis based on actual diaries returned (denoted as ‘reported’ in see Tables 20 and 21):
-
255 diary returns from 22 participants in the usual-care group
-
234 diary returns from 26 participants in the intervention group
-
489 diary returns in the combined group (48 participants in total).
Analysis 2 (expected)
Analysis based on expected number of diary returns (for those who completed any diary returns):
-
22 (participants) × 14 (two weekly diary returns) = 308 in the usual-care group
-
26 (participants) × 14 (two weekly diary returns) = 364 in the intervention group
-
48 (participants) × 14 (two weekly diary returns) = 672 in the combined group.
Analysis 3 (randomised)
Analysis based on number of diary returns expected for all randomised participants (intention to treat):
-
26 (participants) × 14 (two weekly diary returns) = 364 in the usual-care group
-
30 (participants) × 14 (two weekly diary returns) = 420 in the intervention group
-
56 (participants) × 14 (two weekly diary returns) = 784 in the combined group.
Subsequently, all reported AEs and SAEs were cross-tabulated by group and assessed for clinical relevance to inform the design and conduct of a full trial. They were also categorised according to Medical Dictionary for Regulatory Activities (MedDRA)’s System Organ Classification (Version 14). 121
Process evaluation analyses
Fidelity assessment
Analysis of scoring of the fidelity checklist rating scales was undertaken by combining the scoring for each session type and calculating the median and IQR for each. Each score was recorded individually, and, once collated, each individual entry was allocated a record number to ensure that a clear audit trail was maintained. Qualitative feedback, in the form of written annotations, was provided by the fidelity assessors to support scoring judgements where those judgements had been less than straightforward, rather than to provide a comprehensive qualitative overview of delivery fidelity.
Qualitative analyses
Approach and theoretical underpinnings
The qualitative data for analysis included transcripts from one-to-one participant telephone interviews, and the telephone focus group of staff involved in the delivery of BRiMS. Analysis aimed to achieve an in-depth investigation and critical analysis of the data to explore the underpinning concepts and emergent themes, rather than using the data as a simple representation of the phenomenon.
Five members of the research team contributed to the qualitative analysis, which was co-ordinated by the BRiMS trial co-ordinator (HG). The analysis was underpinned by a pragmatic paradigm, which recognises the influence of historical, cultural and political contexts on programme design, implementation and user experience. 108 As this analysis formed part of an evaluation of a pre-existing programme, bracketing of previous knowledge was neither possible nor advisable. 122 In addition, the need for the analysis to achieve the specific aims of the process evaluation meant that some alignment of the data to the broad process evaluation framework was necessary to aid synthesis of the data. Thus, the chosen qualitative analytical framework was template analysis. 123 Template analysis is a form of thematic analysis that emphasises the use of hierarchical coding but balances a relatively high degree of structure in the process of analysing textual data with the flexibility to adapt it to the needs of a particular trial. Unlike framework analysis, template analysis allows the integration of a priori themes into an initial coding template, which is then developed and refined through analysis. 123
Procedures
The data analysis was undertaken in accordance with Brook et al. ’s guidance. 123 Anonymised transcribed data of the participant interviews and therapist focus group were entered into NVivo software, version 12 (QSR International, Southport, UK), and preliminary coding was undertaken to split the data into the three categories that make up level 1 of the coding template (see Figure 6). Subsequently, a pragmatic process of data immersion, coding and clustering was undertaken on a subset of three interview transcripts to develop a more detailed coding template informed by the proposed themes within each of the categories, before the template was applied to the rest of the transcripts. Ongoing review of the developing categories and themes was undertaken as analysis continued to aid implementation of the final coding template, agreement of findings and generation of recommendations.
Credibility and trustworthiness
Analysis was undertaken collaboratively by two researchers (HG and AD). Initially, three interview transcripts were coded by both researchers and the outcomes were discussed and compared to agree and refine the basic coding template. Subsequently, further transcripts were coded separately, and similarities and differences in themes and clusters were compared and refined to develop a more detailed template.
The researchers kept reflexive diaries, and regular discussions between them were undertaken to explore their assumptions and the potential impact of these on the analytical process. Other members of the research team were also involved in the process of refinement of the themes and coding template, with the aim of maximising credibility within the process.
Owing to the variety of data included in the analysis, simple respondent validation was unlikely to be beneficial; however, interview and focus group participants were invited to review an initial draft to ensure that the analysis represented an accurate overview of participants’ views, experiences and recommendations. Once this was verified, we used the data to (where necessary) recommend revisions to the BRiMS operational manual and the trial procedures to optimise the success of the proposed future definitive trial.
Health economics analyses
The economic analyses undertaken as part of this feasibility trial is informed by expectations that any future economic evaluation of the BRiMS programme will be undertaken primarily from the perspective of the third-party payer (NHS and Personal Social Care/Services). A broader perspective was also considered by incorporating into data collection a wider participant/societal perspective.
Aligned with the main statistical analysis plan, the economic analysis considers BRiMS plus usual care versus usual care alone, and the time horizon reported is consistent with the trial assessments over a 27-week (± 1 week) post-randomisation follow-up.
General principles of the health economics analyses
Data are presented descriptively, and no formal statistical analyses were undertaken/presented (consistent with methods for a feasibility RCT). As analyses were over a 27-week (± 1 week) period, no discounting of future costs was required.
Analysis of resource use and cost of intervention delivery
The additional (incremental) costs associated with delivery of the BRiMS intervention, when added to usual care, were estimated using resource-use data collected within the trial, and unit costs for resource use from national published/NHS source. 124,125 Resource use consisted of time input from BRiMS treating therapists (contact time, non-contact time, travel), training (set-up) costs for therapists, and consumables. Data on treating therapist input (time) were captured/reported at the participant level using treating therapist contact sheets. Other resource-use data were collected by research therapists during the feasibility trial. Each component of resource use was collated and presented in a tabular format (mean), together with the unit cost data (national estimates, e.g. by staff grade/level, with standardised currency year, e.g. 2015/16 Great British pounds). A mean cost per participant of BRiMS intervention delivery is presented.
Analysis of data on health, social care and other resource use
We report the resource use and associated estimated costs for health and social care service use. We also report estimates of the resource use and costs associated with reported use of informal care and time off work for those providing informal care and support. Data were collected using the CRF at trial assessments [baseline, week 15 (± 1 week) and week 27 (± 1 week)]. The primary objective of this feasibility trial was to test the methods of data collection to ensure that participants were able to report resource use using the form supplied in the trial, and that the methods were practical, feasible and successful. We combine participant-reported data on resource use with estimates of unit costs associated with resource use (see Appendix 3). Mean (SD) resource use, by item, is presented for baseline assessment, and for resource use during the follow-up period. Unit costs are applied to items of resource use, taken from national/credible tariffs,124–127 and mean (SD) cost data are presented, by treatment arm, for the baseline assessment and 6-month (27 weeks ± 1 week) follow-up data. Cost data for resource use are presented using appropriate subgroupings (categories) of data (e.g. primary care, hospital care). In this feasibility trial, we present descriptive statistics based on complete-case data to inform any future economic evaluation.
Analysis of data on health outcomes
The proposed primary economic end point is the QALY. QALY data were derived from trial data on EQ-5D-5L using the UK algorithms/tariffs, in the first instance those derived from Dolan128 (via van Hout et al. 120), which is the current recommendation of NICE in the UK. Derived health state values were used to estimate QALYs through the application of standard area-under-the-curve methods129 using baseline, 13-week (± 1 week) and 27-week (± 1 week) assessments. We present descriptive data on EQ-5D-3L health state values (primary analyses),130 as well as descriptive data on health state values derived from the MSIS-29vs2, using the tariff for the MSIS-8D. 79 The descriptive statistics presented are based on complete-case data to inform any future economic evaluation.
Chapter 3 Trial results
Trial feasibility objectives (i–vi)
Trial procedures and implementation
Trial set-up
The original trial timeline anticipated that the approval process would commence in June 2016, predicting that all approvals would be in place by October 2016 (see Appendix 4). However, this was reliant on commencing the process during the pre-funding period, which proved difficult because of limited staff availability. As outlined in Table 3, the initial ethics application was submitted in August 2016, with final approvals gained in February 2017.
| Approving organisation | Type of approval | Date of submission | Date of approval | Time taken (days) |
|---|---|---|---|---|
| NRES Exeter Research Ethics Committee | Ethics | 18 August 2016 | 8 November 2016 | 82 |
| Health Research Authority | National approval | 21 October 2016 | 13 December 2016 | 53 |
| University of Plymouth Faculty Ethics Committee | Ethics | 29 November 2016 | 30 November 2016 | 1 |
| University of Plymouth | PAHC site approval | 13 December 2016 | 22 December 2016 | 9 |
| University Hospitals Plymouth NHS Trust | C&C PIC site | 5 January 2017 | 11 January 2017 | 6 |
| Cornwall Partnership NHS Foundation Trust | C&C recruiting site | 5 January 2017 | 13 February 2017 | 39 |
| Royal Cornwall Hospitals NHS Trust | C&C PIC site | 6 January 2017 | 13 February 2017 | 38 |
| Royal Devon & Exeter NHS Foundation Trust | C&C recruiting site | 5 January 2017 | 1 February 2018 | 26 |
| NHS Ayrshire & Arran | R&D approval for recruiting site | 23 November 2016 | 25 January 2016 | 70 |
Once the final approvals had been granted, all subsequent activities took place in line with the trial Gantt chart (see Appendix 4).
Summary
The submission of approval documents took longer than expected, which was mainly a result of staff capacity in the pre-funded period when these activities were scheduled to be undertaken. Nevertheless, this did not have an impact on the trial timeline.
NHS treatment costs and intervention delivery
The total excess treatment costs (ETCs) for delivery of the BRiMS intervention were calculated as £236.79 per patient. ETCs for 20 patients in the South-West were provided by two Clinical Commissioning Groups [Northern Eastern and Western Devon, and Kernow (Cornwall)]. The costs for intervention delivery for 10 patients in Ayrshire fell below the threshold for requiring application for ETCs and were agreed to be absorbed by NHS Ayrshire and Arran.
In all centres, the ETCs provided funding for treating physiotherapist time and service support costs (e.g.travel expenses), which allowed the BRiMS programme to be delivered according to plan.
Summary
Close communication with the local CRN was key to facilitating the ETC approval process. The availability of funding for ETCs was essential as confirmed by feedback from clinical teams who highlighted that there was no spare capacity to deliver the BRiMS intervention, for the purposes of a research project, in addition to their usual workload. Close communication with managers of local therapy services was important to ensure that the additional requirements of the project fitted in with existing service commitments.
Recruitment methods
A variety of recruitment methods were used in this feasibility trial, including personal approach from clinicians and research staff, invitation letters and enquiries prompted through media advertisements. Social media activities included publicity via the BRiMS website and Twitter feed and university social media accounts, and asking local and national MS support groups to share details of the study via their social media links (predominantly Facebook and Twitter). Data on the sources of recruitment to the trial are detailed in Table 4. Overall, 232 people are known to have been approached about taking part in the trial; from these, we recruited 56 participants, which represents a conversion rate of 24% (objective iii). The conversion rates vary by recruitment approach; by far the most effective method was personal approach by clinicians or research staff, thus providing evidence of the importance of the willingness of clinicians to recruit patients (Figure 7) (objective iv).
| Recruitment method | Invitations (n) | Reponses,a n (%) | Recruited,b n (%) | Conversion ratec (%) | |
|---|---|---|---|---|---|
| Media (including support groups, social media, trial website) | 10 | 10 (100) | Yes: 10 (100) | 2 (20) | 20 |
| No: 0 | |||||
| Letter | 139 | 36 (26) | Yes: 23 (64) | 17 (74) | 12 |
| No: 13 (36) | |||||
| Personal approach | 38 | 35 (92) | Yes: 23 (66) | 21 (91) | 55 |
| No: 12 (34) | |||||
| Personal approach with letter follow-up | 45 | 39 (87) | Yes: 20 (51) | 16 (80) | 36 |
| No: 19 (49) | |||||
FIGURE 7.
Recruitment sources.
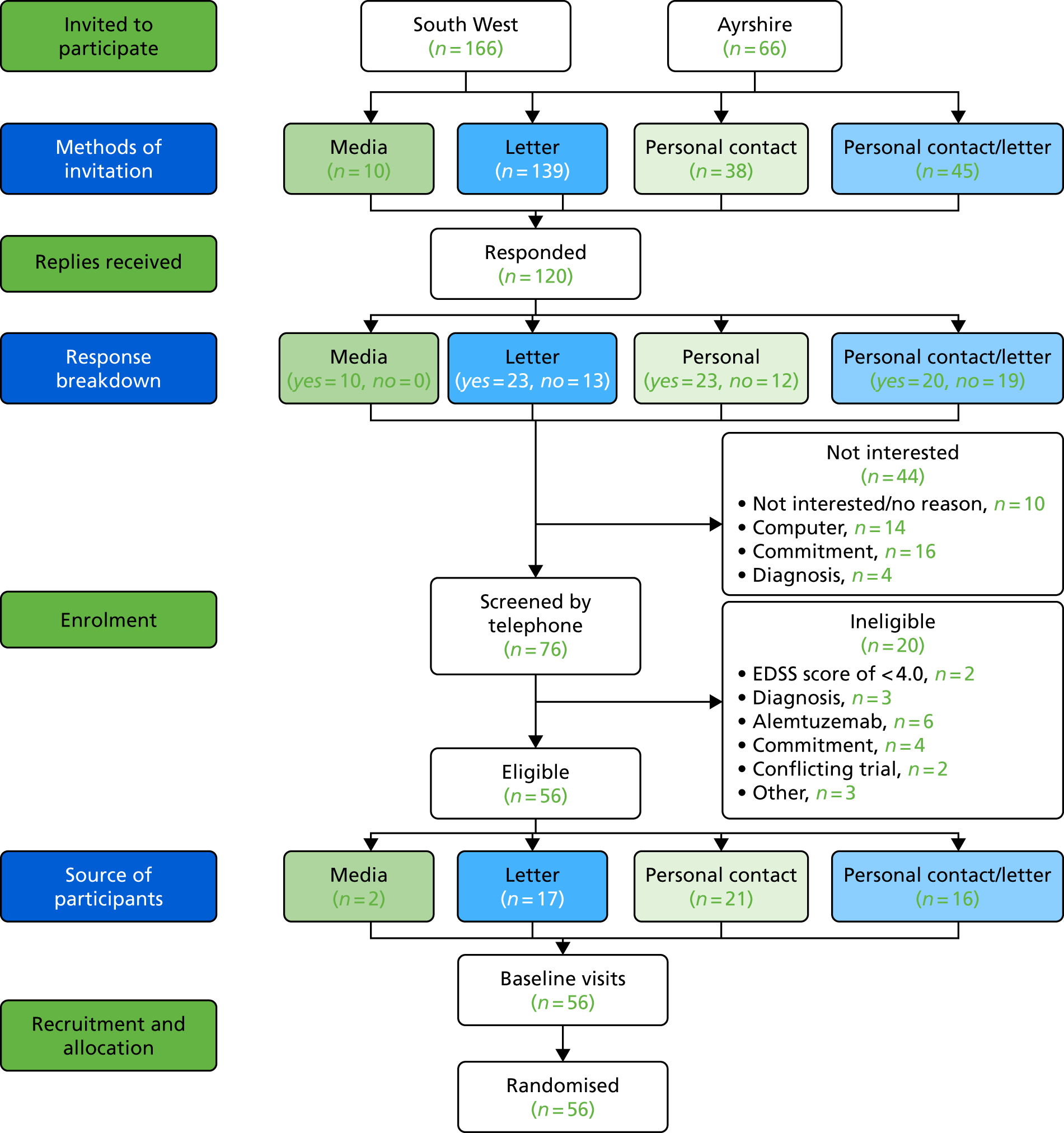
Figure 8 shows the flow of participants from invitation through to recruitment. The main reasons for not wishing to participate were the time commitment required (n = 16) and not having access to a computer or tablet/having poor information technology (IT) literacy (n = 14). The most common reason for patients failing the screening process was that they had previously had treatment with alemtuzumab (Lemtrada); all of these patients were in the South West. There were also a number of people who were keen to participate but were excluded because they had a diagnosis of primary progressive MS (n = 3) or because they lived outside the Clinical Commissioning Group catchment area that had agreed the ETCs for the site (objective ii).
FIGURE 8.
BRiMS recruitment: January–June 2017.
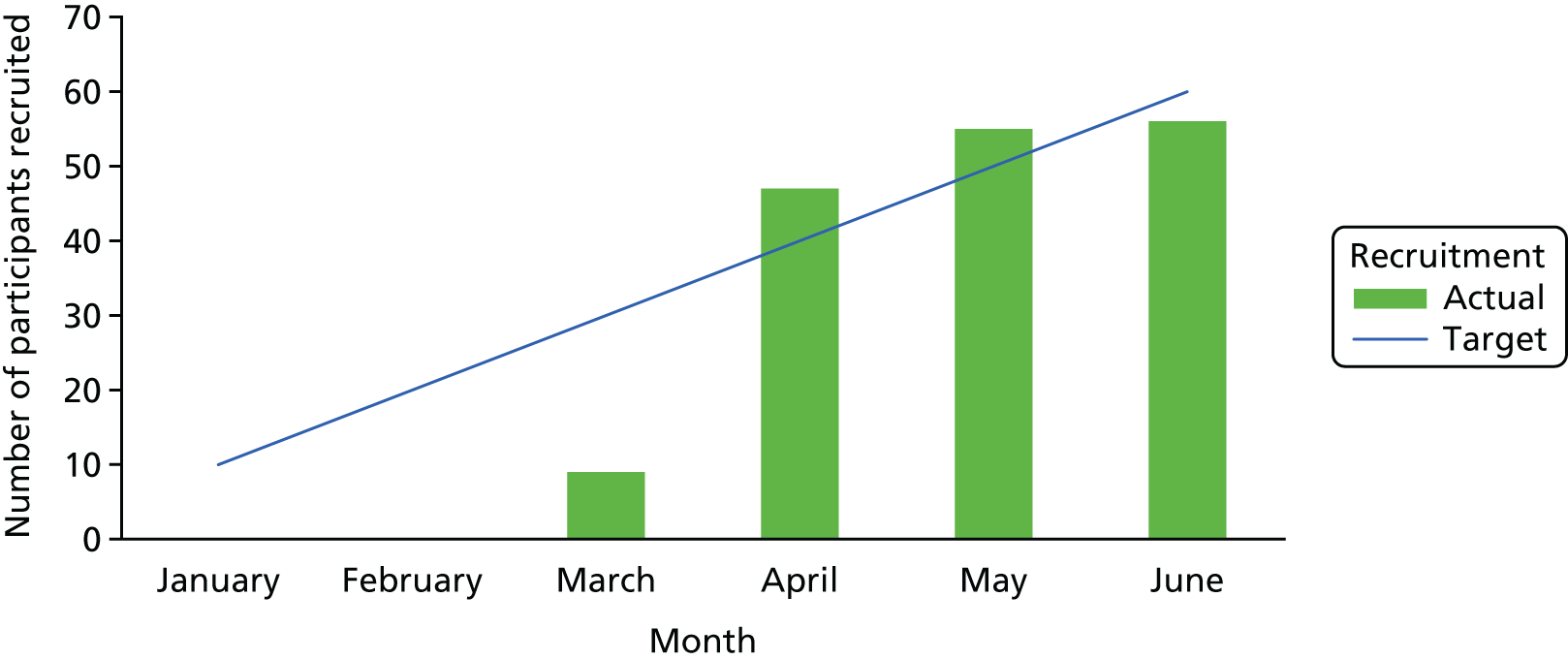
There were no instances in which patients declined to participate in the trial because they were unwilling to be randomised (objective v).
Summary
The most effective method of recruitment was personal approach by clinicians or research staff. Our local recruiting colleagues were extremely supportive, but it is acknowledged that recruitment to multicentre RCTs can be particularly challenging. 131 It will be important for a future large-scale definitive trial to include measures to facilitate engagement and active involvement from clinicians at all sites in order to optimise recruitment.
Current exclusion criteria include participants who had previously had treatment with alemtuzumab, were within 3 months of switching disease-modifying therapies or were within 6 months of ceasing Tysabri. In the south-west, previous treatment with alemtuzumab was the most common reason why a patient failed the screening process. Given the potential for an increasing number of people to be treated with alemtuzumab, the move towards more frequently switching disease-modifying therapies,132 and the potential for new drugs to be licensed for people in the progressive phase of MS, this exclusion criterion should be carefully considered when determining the recruitment rate of a future trial.
Recruitment of blocks of participants
In this trial, the recruitment window for each block of participants was fixed, as BRiMS treatment sessions needed to be pre-scheduled to enable the group elements to go ahead. According to the original timeline, a minimum of 2 months was allowed from the commencement of screening to the dates scheduled for baseline visits for each block. However, delays in the ethics and Health Research Authority approval process (see Trial set-up) reduced the recruitment window for the first block to < 4 weeks and, as a result, this had to be rescheduled to allow sufficient time for recruitment. This change did not affect the overall trial milestones, with all programmes taking place during the originally planned trial recruitment period (Figure 8). This timescale enabled us to recruit 56 of the anticipated 60 participants (93% of the proposed sample).
The time to recruit each block was calculated by subtracting the date of the first screening telephone interview from the date of the block randomisation (Table 5). The variation in the length of times of screening to recruitment reflected the fact that the screening of patients had begun as soon as governance permissions had been granted at each site. As a result, the sites with fewer set-up difficulties had longer time periods between screening and randomisation. Despite the time to recruit being high for several blocks, participants were aware of the pre-scheduled timescales from the outset and did not express any concerns about these, with only one participant withdrawing between screening and baseline as a result of a relapse.
| Block | Time to recruit (days) | Number of participants (n) | ||
|---|---|---|---|---|
| Usual care | Intervention | Total | ||
| Ayrshire 1 | 26 | 5 | 6 | 11 |
| Ayrshire 2 | 22 | 4 | 5 | 9 |
| Cornwall 1 | 57 | 4 | 5 | 9 |
| East Devon 1 | 83 | 5 | 5 | 10 |
| Plymouth 1 | 86 | 4 | 4 | 8 |
| Plymouth 2 | 72 | 4 | 5 | 9 |
Summary
The pre-scheduling of deliveries, and resultant time gaps between initial approach and actual recruitment, did not adversely affect conversion rates, and was necessary to enable patients and treating therapists to diarise these sessions to optimise attendance and ensure availability of clinical space.
Retention
Participant flow
The flow of participants through the trial is detailed in Figure 9.
FIGURE 9.
The CONSORT flow diagram. a, Only a subgroup of participants were invited to participate in the qualitative interview; b, one participant did not have complete baseline data for the EQ-5D-5L potential primary outcome.
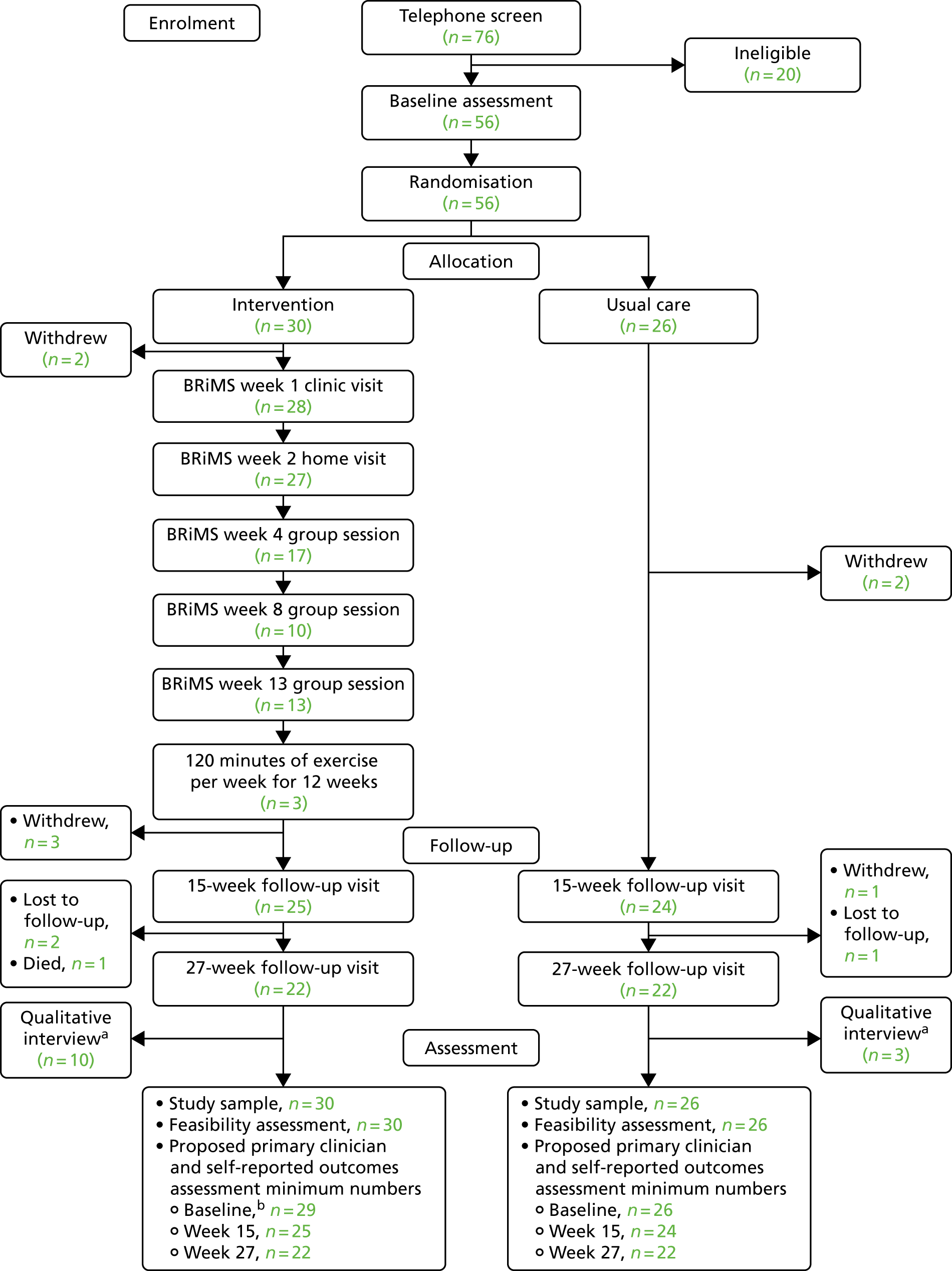
Safety and adverse events
Participants not completing the trial
Table 6 provides details of the 12 participants who did not complete the trial. Seven were from Ayrshire and five were from across the three south-west sites. These participants were split into three categories: withdrawals, losses to follow-up and deaths. Withdrawals and losses to follow-up were used to calculate the overall withdrawal rate in the trial for the purposes of the progression criteria.
| Site | Withdrawal weeka | Reason | Code |
|---|---|---|---|
| 4 | 29 | SAE – resolved with sequelae – participant in rehabilitation unit unable to follow up | Lost to follow-up |
| 4 | 3 | Family member not well, unable to commit to appointments | Withdrawn |
| 4 | 33 | Did not attend appointment week 27 × 2 | Lost to follow-up |
| 4 | 19 | Very stressed following medical intervention and stopped filling in diaries. Did not want to continue | Withdrawn |
| 4 | 3 | Bladder frequency/urgency/incontinence post Botox, and family member unwell, unable to commit to appointments | Withdrawn |
| 4 | 27 | Failed to respond to multiple requests to attend the assessment. Moving from the area. Did not want to continue | Withdrawn |
| 4 | 37 | SAE – resolved with sequelae – participant in rehabilitation unable to follow up | Lost to follow-up |
| 3 | 3 | Participant very anxious about having to write because of his MS symptoms. No answer to calls/e-mails | Withdrawn |
| 2 | 31 | Participant died | Died |
| 2 | 15 | Participant expressed lack of enthusiasm and motivation to carry on. Did not want to continue | Withdrawn |
| 2 | 5 | Anxious about using computer and about having to do the imagery exercise. Unable to continue | Withdrawn |
| 1 | 9 | Fractured pelvis, unable to continue | Withdrawn |
Eight participants (three from the usual-care group and five from the intervention group) were withdrawn. One participant was lost to follow-up because he or she was unable to attend their final research appointment, and the research therapist was unable to contact another two of the participants to conduct the final follow-up visit. One of the participants lost to follow-up was from the usual-care group and two were from the intervention group. This represents an overall withdrawal rate of 19.6%, with a rate of 11.5% in the usual-care group and 23.3% in the intervention group.
The final participant who did not complete the trial died in week 31 as a result of events unrelated to the BRiMS intervention.
Discussion
The overall withdrawal rate was within the 20% anticipated when designing this feasibility trial. There was a discrepancy between retention rates in the two arms of the trial, although, as the reasons for withdrawals are very variable, it is difficult to draw conclusions about why this might be the case. The dropout rate in the intervention group was higher than we would have hoped for. In comparison, a review of 26 exercise intervention studies reported combined dropout rates of 15% and 16% in intervention and usual-care groups, respectively. 133 We hypothesise that the higher than anticipated dropout rate in our intervention group may be associated with the feasibility issues within the BRiMS programme in its current form, which are discussed later (see Implementation of the BRiMS programme).
Safety and adverse events
Serious adverse events
In total, nine SAEs were reported to PenCTU (Table 7). One was identified from the diary review and the other eight were reported by the principal investigators. The data in the column ‘Related to trial treatment?’ were selected from the SAE form by the reporting principal investigator in response to the question ‘In the opinion of the principal investigator, what is the relationship of the event to the BRiMS programme’, to which the options were definite, probable, possible, unlikely and not related. In each case, the SAE was reported to the chief investigator (JF). In all cases, the chief investigator concurred with the principal investigator. These rates of SAEs are in line with those in other MS trials.
| Site | Allocation | Date of onset | Description | SAE definition | Outcome | Related to trial treatment? | Comment |
|---|---|---|---|---|---|---|---|
| 4 | Usual care | 26 April 2017 | Admission to hospital following macular injection | Hospitalisation | Recovered with sequelae | Not related | |
| 1 | Intervention | 30 May 2017 | Admission to hospital with broken pelvis | Hospitalisation | Recovered with sequelae | Not related | Fall in shower |
| 4 | Intervention | 30 July 2017 | Likely MS relapse | Hospitalisation | Recovered with sequelae | Not related | |
| 4 | Intervention | 4 September 2017 | Likely MS relapse | Significant disability | Recovered with sequelae | Unlikely | |
| 2 | Intervention | 8 September 2017 | MS relapse | Hospitalisation | Recovered | Not related | |
| 2 | Intervention | 1 October 2017 | MS relapse | Hospitalisation | Recovered | Not related | Possible bladder infection – recovered with antibiotics |
| 4 | Intervention | 31 October 2017 | Admission to hospital following a fractured distal fibula sustained while transferring to wheelchair | Significant disability | Recovered with sequelae | Not related | Reduced mobility |
| 4 | Usual care | 8 November 2017 | Fall caused by loss of balance, resulting in fractured neck of femur. Subsequent treatment surgery for partial hip replacement and physiotherapy | Significant disability | Recovered with sequelae | Not related | Reduced mobility |
| 2 | Intervention | 9 November 2017 | Died following serious chest infection | Died | Not related |
Adverse events
As described in Chapter 2, Participant safety and adverse events, the AEs were gathered from data self-reported by participants in the pre-formatted paper-based diaries. Analysing these data presented a number of challenges. First, a number of participants reported contradictory information in their diaries (e.g. ticking ‘no’ in the ‘any new problems’ box, but then adding free-text details suggesting that they had experienced an issue). Second, despite the inclusion of a written definition in each diary, participants’ definitions of a ‘relapse’ was highly variable. For example, a number ticked to say that they had experienced a relapse, but then described its duration as extremely short, for example suggesting that it could have lasted < 1 hour. In addition, some participants reported ongoing problems as ‘new/worsening issues’ in sequential diaries, suggesting that there may be a degree of double-counting within the data. Finally, free-text responses were extremely challenging to interpret; for example, participants reported ‘pain’, but a lack of further detail made categorising this report difficult.
The summary of reported AEs, self-reported relapses and any self-reported use of medical care is detailed in Table 8. These data are reported as actual number of reports (N), and percentages, which have been calculated based on the number of diaries returned, the expected number of 2-weekly diary returns and the number of participants randomised to each group. Details of the full method of analysis are included in Safety data.
| Problem classification | Group | Combined group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||||||||
| n | Reported (%) | Expected (%) | Randomised (%) | n | Reported (%) | Expected (%) | Randomised (%) | n | Reported (%) | Expected (%) | Randomised (%) | |
| New or worsening problems | ||||||||||||
| No | 194 | 39.7 | 28.9 | 24.7 | 135 | 27.6 | 20.1 | 17.2 | 329 | 67.3 | 49.0 | 42.0 |
| Yes | 50 | 10.2 | 7.4 | 6.4 | 64 | 13.1 | 9.5 | 8.2 | 114 | 23.3 | 17.0 | 14.5 |
| Missing | 11 | 2.2 | 1.6 | 1.4 | 35 | 7.2 | 5.2 | 4.5 | 46 | 9.4 | 6.8 | 5.9 |
| ‘Relapses’ | ||||||||||||
| No | 217 | 44.4 | 32.3 | 27.7 | 196 | 40.1 | 29.2 | 25.0 | 413 | 84.5 | 61.5 | 52.7 |
| Yes | 25 | 5.1 | 3.7 | 3.2 | 17 | 3.5 | 2.5 | 2.2 | 42 | 8.6 | 6.3 | 5.4 |
| Missing | 13 | 2.7 | 1.9 | 1.7 | 21 | 4.3 | 3.1 | 2.7 | 34 | 7.0 | 5.1 | 4.3 |
| Medical care | ||||||||||||
| No | 23 | 4.7 | 3.4 | 2.9 | 10 | 2.0 | 1.5 | 1.3 | 33 | 6.7 | 4.9 | 4.2 |
| Yes | 7 | 1.4 | 1.0 | 0.9 | 8 | 1.6 | 1.2 | 1.0 | 15 | 3.1 | 2.2 | 1.9 |
| Missing | 225 | 46.0 | 33.5 | 28.7 | 216 | 44.2 | 32.1 | 27.6 | 441 | 90.2 | 65.6 | 56.3 |
Participants made a 114 tick-box diary entries reporting ‘new/worsening problems’ and 42 reports of ‘relapses’. The free-text details of new/worsening problems are reported in Table 9, presented using the organ system classification. It should be noted that, as some participants detailed more than one problem/symptom per diary, the number of free-text entries exceeds the number of tick-box reports. Among the 42 reports of ‘relapses’, 15 participants documented accessing medical care.
| OSC | 08 Nervous system disorders (n) | 17 MSK (n) | 07 Psych (n) | 13 Resp (n) | 14 Gastro (n) | 18 Renal (n) | 10 Ear (n) | 09 Eye (n) | 24 Injury (n) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatigue | Pain/sensation | Balance | Weakness/mobility | Other | |||||||||
| ST | 42 | 13 | 3 | 8 | 0 | 12 | 3 | 0 | 0 | 0 | 1 | 1 | 2 |
| LT | 32 | 5 | 7 | 18 | 2 | 2 | 4 | 1 | 1 | 2 | 0 | 1 | 2 |
Discussion
One challenge when gathering AEs from patient self-report diary data is that there can be ambiguity in both the definition and the interpretation of a new symptom/relapse, which is a problem acknowledged in MS clinical trials. 134 A further challenge is optimising the return rate of the diaries. Overall, however, our observation is that the AEs reported were not unexpected for a sample of people with progressive MS, and are in line with those reported in other MS trials. Exercise-based interventional studies suggest that between 0% and 12% of trial participants are likely to report AEs, with minimal difference between the intervention and usual-care groups,133 which was also broadly the case in this feasibility trial.
Feasibility of trial procedures
Elapsed time between key trial time points
As participants were randomised in blocks, for each randomised batch of participants, the randomisation date was defined as the same date. Within the protocol, the time from randomisation to each follow-up was given a ± 1 week window, meaning that the week 15 follow-up should have taken place on day 105 (range 98–112 days) and the week 27 follow-up should have taken place on day 189 (range 182–196 days). As seen in Table 10, the median and IQRs at each assessment point fell within the allowed window. Overall, 30.6% of week 15 assessments and 29.5% of week 27 assessments took place outside the allowed time. This can be partly attributed to the logistical challenges of the block randomisation, wherein assessments for the whole block were due on the same date. This was a particular challenge for Ayrshire, where the research assessor was employed for only 1 day per week. Further analyses demonstrated that, despite these challenges, 96.8% of assessments were undertaken within a ± 2-week window.
| Time point | n | Median (IQR) | Minimum–maximum |
|---|---|---|---|
| Screening telephone to baseline | 56 | 21 (10–39) | 0–72 |
| Baseline to randomisation | 56 | 12 (7.5–14) | 5–37 |
| Randomisation to intervention start | 56 | 5 (5–5) | 5–5 |
| Randomisation to week 15 | 49 | 104 (98–107) | 96–119 |
| Randomisation to week 27 | 44 | 183 (181.5–189) | 174–205 |
Summary
All assessments were undertaken within a ± 2-week window of the intended assessment date, rather than the intended ± 1-week window.
Blinding
Methods to maintain blinding were largely successful, with minimal reported instances of unblinding at week 15 (n = 3, 6.1%) and no reported unblinding at week 27 (± 1 week). All three instances were due to participants telling research therapists about their group allocation during assessment sessions. However, the assessors correctly identified the allocation group for between 75% and 77% of participants at these time points. It is now acknowledged that the information that this approach provides is uncertain, and hence CONSORT guidelines135 no longer advocate this type of testing for blinding.
Participant experiences
Thirteen participants were interviewed by telephone between 2 and 6 weeks after their final assessment to explore their experience of participating in the trial/BRiMS programme. The participant characteristics are detailed in Table 11.
| Participant code | EDSS score (points) | Age (years) | Sex | Site |
|---|---|---|---|---|
| Usual-care group participants | ||||
| P1 | 6 | 52 | Male | 1 |
| P2 | 7 | 52 | Female | 1 |
| P3 | 4.5 | 59 | Female | 3 |
| Intervention group participants | ||||
| P4 | 6.5 | 65 | Female | 1 |
| P5 | 6 | 62 | Female | 1 |
| P6 | 6.5 | 64 | Female | 2 |
| P7 | 6 | 58 | Female | 2 |
| P8 | 6 | 53 | Female | 2 |
| P9 | 6 | 49 | Male | 3 |
| P10 | 6.5 | 67 | Female | 3 |
| P11 | 6 | 69 | Female | 3 |
| P12 | 6 | 46 | Male | 4 |
| P13 | 6 | 49 | Female | 4 |
Motivation for joining the trial
Participants interviewed as part of the process evaluation expressed that the topic of the trial resonated with them, with many reporting that they had been looking for extra support to address their difficulties. One participant described feeling ‘alone’, and hoped that, if she was allocated to the BRiMS programme, it would help her to find motivation. Other participants explained that they had previous experience of being involved in research and wanted to help contribute for wider benefit:
Well, it was to help me to help myself actually control my walking, control where I walked, how I walked.
P1
I was always, I am always interested in exercise for MS, I just really believe it works.
P4
Expectations of taking part
Participants’ expectations of taking part were mostly associated with a desire to improve their mobility and balance, and to exercise more:
Anything that can help mobility really, yes, that was the main reason, yeah.
P10
Well, I was hoping that they would come out with a suitable programme that we might actually get a chance of being able to try once all the study has gone through and got there sort of thing.
P2
Trial activities
Recruitment and trial information
The amount of information given to participants during the recruitment process was overwhelming for some:
I would shorten the information, the information we got initially. For me I’m cognitively impaired because of the MS and I can’t really focus a lot of the time. I then kind of lose focus and drift off. More to the point, for me personally more to the point. Less information would be better actually.
P12
However, one participant reported that the amount of information was fine for her:
I think the information was OK. I didn’t have any particular problems with it. No, it was quite explanatory, it was fine.
P13
Allocation to the intervention or usual-care group
Participants were randomised to either the intervention or the usual-care group. Not surprisingly, those randomised into the intervention group reported satisfaction and those in the usual-care group expressed disappointment. However, the vast majority remained committed to the trial:
Yes, I was really pleased because then I thought ‘oh great I will have some exercises to do’.
P4
Disappointed, because I would have liked to have had the exercises to do. So yeah, disappointed, but, you know, I carried on because in the long run hopefully it is going to help everyone. So it made sense, you know, to carry on and do it, but I was disappointed because I would have liked to have had the exercises.
P2
Despite being allocated to the usual-care group, two participants highlighted changes that they perceived to be related to taking part in the trial. It is possible that these changes resulted from these participants completing the assessments and diaries, which provided an opportunity for them to reflect on their situation and behaviour:
Yes, it definitely changed my behaviour and what I was doing and how I was doing it.
OK, in what way?
I’d say that after about the third or fourth week I’d started thinking ‘Oh yeah, that happened to me last week so let’s change it now and not do this again in the next week’ and there was a couple of weeks when I didn’t trip at all.
Trial assessments
All participants attended three research assessments at local community health facilities. Scheduling an assessment with the research therapist was reported as straightforward:
It was e-mail. When I went for one she would provisionally give me the date of the next one. So I had plenty of warning on when, well, you knew roughly what window it was going to be in anyway.
P2
The rurality of the trial sites, and the nature of MS fatigue, meant that travel time and the effort of attending sessions away from home was notable for some:
I think personally yes because the going out and the doing something like that is as far as I’m concerned is exhausting anyway. If you have got a long journey to start with you are just adding to it. You are compounding the issues, aren’t you?
P2
Participants expressed mixed feelings about the length of assessments. For some the length was not an issue, whereas others reported feeling fatigued. A number of participants recognised that the design of the assessment process enabled activities to be broken up, and felt that the opportunities for regular rests were helpful:
That was all fine. I mean she did break it up a bit . . . It was nice you weren’t all doing the physical stuff all in one go and you weren’t doing all the mental stuff all in one go. So, yeah, it was nicely paced and it was spread out that you didn’t feel too fatigued from either of them, if that makes sense.
P2
Some reported that the assessment, and in particular the cognitive demands of the questionnaires, led to significant fatigue. However, they did not necessarily feel that the assessment battery needed to be changed:
Gosh, I was really tired afterwards. It was, I mean it’s easy to, you keep going, but actually it really did sort of take it out of me . . . I think if you say that it can take up to 2 hours and there’ll be a combination of things, you sort of think as you are doing it, ‘OK I knew this’ . . . and also you were very clear any time I’d had enough just to say. And that’s all you need really. I don’t think you need to change.
P5
Summary
Overall, the qualitative feedback relating to the trial processes was positive. The interviewed participants reported that the procedures were suitable and that they were well supported during assessment sessions. The feedback suggests that the initial patient information packs need to be reviewed to ensure that the content and format are straightforward while remaining comprehensive. 136 The feedback on trial assessments supports our PPI work, which highlights the importance of interspersing cognitive and physical tasks, and incorporating rest breaks. Given that the findings from this feasibility trial have highlighted the importance of encouraging people to self-complete any questionnaires (see Table 45), it will be important to review the format of the session to ensure that people are able to do this without significant extra participant burden.
An important consideration is the feedback from our usual-care participants that they felt that simply taking part in the trial had changed their attitude to falls and falls prevention. In other areas of behaviour change research, there is recognition of the potential for research processes to affect whether or not the ‘usual-care’ group has truly received ‘usual care’. 137 Although it was not feasible in this feasibility trial, falls prevention trials of other groups have included collection of falls data prior to randomisation. 138 The publication of these data will be an important consideration in the design of future RCTs.
Trial outcome objectives (vii–ix)
Data completion and accuracy
Completion of questionnaires
Potential primary outcome measures were all self-report questionnaires. Participants could request that the questionnaires be read to them by the research assessor, who would tick the answer provided by the participant. Typically participants requested this method of completion because of issues relating to mental fatigue or manual dexterity, which are common in people with progressive MS. From an operational perspective, the research assessor was instructed not to interpret the questionnaire on behalf of the participant under any circumstances, but to simply read the question and range of potential answers and then record the participant’s response accordingly. This was the method most commonly used at each time point (baseline, 62.5%; week 15, 77.6%; week 27, 84.1%).
Completeness of the potential primary outcomes
In Tables 12 and 13 we have calculated the proportions using:
-
participants who attended the baseline (n = 56), week 15 (n = 49) and week 27 (n = 44) visits
-
participants who were randomly allocated (n = 56).
Table 12 shows the number and percentage of participants who completed all items of the questionnaire at the baseline and follow-up visits, and the number and percentage of questionnaires for which the total score was available (imputed if necessary). Only one participant was excluded from the baseline data for one measure (EQ-5D-5L), as he or she declined to complete any items.
| Outcome | Time point | Completeness | |||||
|---|---|---|---|---|---|---|---|
| Participants completed all items | Questionnaire complete | ||||||
| n | Follow-up (%) | Randomised (%) | n | Follow-up (%) | Randomised (%) | ||
| MSWS-12vs2 | Baseline | 55a | 98.2 | 98.2 | 56 | 100 | 100 |
| Week 15 | 49 | 100 | 87.5 | 49 | 100 | 87.5 | |
| Week 27 | 44 | 100 | 78.6 | 44 | 100 | 78.6 | |
| EQ-5D-3L (crosswalk) | Baseline | 55 | 98.2 | 98.2 | 55 | 98.2 | 100 |
| Week 15 | 49 | 100 | 87.5 | 49 | 100 | 87.5 | |
| Week 27 | 44 | 100 | 78.6 | 44 | 100 | 78.6 | |
| EQ-5D-5L (VAS)b | Baseline | 55c | 98.2 | 98.2 | 55 | 98.2 | 100 |
| Week 15 | 49 | 100 | 87.5 | 49 | 100 | 87.5 | |
| Week 27 | 44 | 100 | 78.6 | 44 | 100 | 78.6 | |
| MSIS-29vs2 (physical) | Baseline | 56 | 100 | 100 | 56 | 100 | 100 |
| Week 15 | 49 | 100 | 87.5 | 49 | 100 | 87.5 | |
| Week 27 | 44 | 100 | 78.6 | 44 | 100 | 78.6 | |
| MSIS-29vs2 (psychological) | Baseline | 56 | 100 | 100 | 56 | 100 | 100 |
| Week 15 | 49 | 100 | 87.5 | 49 | 100 | 87.5 | |
| Week 27 | 44 | 100 | 78.6 | 44 | 100 | 78.6 | |
Summary
In summary, completion of the potential primary outcomes was excellent and clearly met the criteria set for progressing to a definitive trial. Most participants requested for the self-report questionnaires to be read to them by the research assessor, who ticked the answers provided by the participant.
Completeness of the potential secondary outcomes
Table 13 describes the completeness of the potential secondary outcomes for the number of participants who completed all components of the test; and, if the participant attempted the test, as a percentage of the number of participants followed up and a percentage of the number of participants randomised. The completeness of the secondary outcomes was high among the proportion followed up, at > 93% for all measures. As a proportion of those randomised, it was > 73% for all measures.
| Outcome | Time point | Completeness | |||||
|---|---|---|---|---|---|---|---|
| Participants completed all test components | Assessment | ||||||
| n | Follow-up (%) | Randomised (%) | n | Follow-up (%) | Randomised (%) | ||
| Clinician-rated outcomes | |||||||
| 2MWT | Baseline | 56 | 100 | 100 | 56 | 100 | 100 |
| Week 15 | 48 | 98.0 | 85.7 | 49 | 100 | 87.5 | |
| Week 27 | 40 | 90.9 | 71.4 | 43 | 97.7 | 76.8 | |
| Mini-BEST | Baseline | 53 | 94.6 | 94.6 | 55 | 98.2 | 98.2 |
| Week 15 | 48 | 98.0 | 85.7 | 48 | 98.0 | 85.7 | |
| Week 27 | 41 | 93.2 | 73.2 | 41 | 93.2 | 73.2 | |
| Forward FRT | Baseline | 55 | 98.2 | 98.2 | 56 | 100 | 100 |
| Week 15 | 48 | 98.0 | 85.7 | 48 | 98.0 | 85.7 | |
| Week 27 | 41 | 93.2 | 73.2 | 41 | 93.2 | 73.2 | |
| Lateral FRT | Baseline | 55 | 98.2 | 98.2 | 56 | 100 | 100 |
| Week 15 | 48 | 98.0 | 85.7 | 48 | 98.0 | 85.7 | |
| Week 27 | 41 | 93.2 | 73.2 | 41 | 93.2 | 73.2 | |
| Self-reported questionnaires | |||||||
| FES-I | Baseline | 55 | 98.2 | 98.2 | 56 | 100 | 100 |
| Week 15 | 49 | 100 | 87.5 | 49 | 100 | 87.5 | |
| Week 27 | 44 | 100 | 78.6 | 44 | 100 | 78.6 | |
| CPI score 1 | Baseline | 52 | 92.9 | 92.9 | 52 | 92.9 | 92.9 |
| Week 15 | 48 | 98.0 | 85.7 | 48 | 98.0 | 85.7 | |
| Week 27 | 44 | 100 | 78.6 | 44 | 100 | 78.6 | |
| CPI score 2 | Baseline | 53 | 94.6 | 94.6 | 53 | 94.6 | 94.6 |
| Week 15 | 48 | 98.0 | 85.7 | 48 | 98.0 | 85.7 | |
| Week 27 | 44 | 100 | 78.6 | 44 | 100 | 78.6 | |
| CPI score 3 | Baseline | 52 | 92.9 | 92.9 | 52 | 92.9 | 92.9 |
| Week 15 | 48 | 98.0 | 85.7 | 48 | 98.0 | 85.7 | |
| Week 27 | 44 | 100 | 78.6 | 44 | 100 | 78.6 | |
Clinician-rated potential secondary outcome measures
There were some difficulties recording the time component of the 2MWT data in the CRF. The CRF requires that the assessor records the time walked only if this is < 2 minutes. In some cases participants reported the time even when the full 2 minutes had been walked, while in others no time was entered. Information on assistive walking devices (free text) was also occasionally missing, which could have been used for further analyses. The completion rates for the CPI were slightly lower than those for the other self-report questionnaires used in this trial. Feedback from participants during the assessments highlighted that the length and complexity of the CPI questionnaire, particularly the section that informed CPI score 1, was a challenge.
Summary
In summary, completion of the clinician-rated and self-reported secondary outcomes was excellent, and clearly met the criteria set for progressing to a definitive trial. There were some minor problems with recording the 2MWT in the CRF.
Daily diary returns
To examine the diary data, entries were converted from 2-weekly records into daily entries to identify the date that participants should have started to record diary data (i.e. the randomisation date). The BRiMS intervention always started 5 days after the randomisation date. Participants were asked to return 28 weeks of diary entries; had all 56 participants completed every diary, 784 2-week diaries would have been returned. Throughout the trial, 489 2-week diaries were returned from 48 participants (usual-care group, n =22; intervention group, n = 26), equating to a 62.3% response rate. Eight participants (four from each allocated group) failed to return any diaries. Of the diary returns, 255 were from those allocated to the usual-care group (mean of nine returns per participant) and 234 were from those allocated to the intervention group (mean of nine returns per participant).
Evaluation of the quality and completeness of the daily recording of falls in the diaries highlighted a number of issues, including duplication of data (e.g. when participants sent back two diaries with the same dates, or when there was an overlap in the dates recorded in two sequential diaries). To maximise the validity of the analyses, the decision was taken to identify and remove duplicate days; this resulted in the removal of 374 days of duplicate data from the original 7546 days of data returned, which is 5% of the available data. A further 218 (3%) entries were removed as they did not report days within the specified trial period. Allowing for non-returns and data duplications, the final data available for analysis represent 58.6% of the expected number of days’ data for falls, and 40.6% of the expected number of days’ data for injurious falls, had all diaries been completed and returned according to protocol.
Qualitative feedback indicated that diaries were quick to fill in, but were described as a little complicated:
It wasn’t very long even if I’d had a fall, it was sort of 5 minutes tops . . . That was OK but I didn’t find the forms were that easy to follow . . .
Could you explain a bit more about that?. . .
They just didn’t seem to be all that well set out . . .
OK, in terms of too much on a page, too little on a page or . . .?
Well, I mean where you fill in any falls you’d had that was fine because I mean I just literally when I had to do for the next fortnight I put it in front and then if I had fallen I just put a little one because I’d remember what the fall was sort of thing but when you turn over the pages it was a bit higgledy piggledy the way it was set out.
Participants also suggested different formats for the diaries:
It could be an online thing but then if you struggle, some aren’t technically, and they don’t do it do they, but online would have been. On an online basis you could then do it daily . . . I mean even something like a little app that would prompt you and you go and you do it, you know.
P2
Summary
The diary return rates were lower than those seen in other studies,15 and there were issues with the clarity and completeness of the data returned.
Discussion
Feedback relating to diaries suggests that the diary format, completion and mechanisms for checking returns and following up non-returns may have had an impact on user engagement and data quality. Our previous study used a similar format to record daily falls and injuries, but for this trial we added further detail about the consequences of any falls, the use of medical resources and any AEs. We also used an automated e-mail reminder system rather than personal contact details when diary returns were delayed. Despite the fact that the format of our diaries was informed by best-practice guidance,59 the findings from this trial suggest that this is an area that needs to be reviewed prior to any future definitive trial.
Accelerometry (activPAL monitors)
At each research assessment visit, participants had an accelerometer (activPAL monitor) attached to their thigh. They were provided with instructions for removing the device and a reply-paid envelope to return it to the research therapist after 1 week.
The monitors were returned according to plan by all participants who remained actively engaged in the trial (Table 14). Despite reminders, one monitor was not returned by one participant who withdrew after the baseline assessment. Participants reported that the monitors were comfortable to wear and there were no recorded issues of problems other than short-lived skin redness after removal for two participants.
| Time point | Number of assessments undertaken (n) | Number of monitors returned, n (%) | Number of data sets suitable for analysis, n (%) |
|---|---|---|---|
| Baseline | 56 | 55 (98) | 54 (96) |
| Week 15 (± 1 week) | 49 | 49 (100) | 48 (98) |
| Week 27 (± 1 week) | 44 | 43 (97) | 42 (95) |
Data checking and cleaning according to the agreed plan was undertaken for all accelerometry data sets. The cleaning and review process highlighted three data sets with unreliable data, all from the same participant. This was likely to have been due to this participant’s low level of daily activity and slow walking speed, which are known to cause issues with activity monitors of this type. 139
Summary
The use and return rate of accelerometers was high, suggesting that this is a feasible method of collecting activity data in a future trial. The data cleaning process was time-consuming.
Baseline data
Demographics and clinical characteristics
Tables 15 and 16 detail summary statistics of the participants’ demographic and clinical characteristics by allocated group and for the whole sample.
| Characteristic | Group | Total (N = 56) | |
|---|---|---|---|
| Usual care (N = 26) | Intervention (N = 30) | ||
| Age (years) | |||
| Mean (SD) | 60.0 (8.5) | 58.7 (10.8) | 59.3 (9.7) |
| Minimum–maximum | 46.0–81.0 | 34.0–80.0 | 34.0–81.0 |
| Median (IQR) | 58.5 (54.0–65.0) | 59.5 (49.0–67.0) | 59.0 (53.0–67.0) |
| Sex, n (%) | |||
| Male | 9 (34.6) | 10 (33.3) | 19 (33.9) |
| Female | 17 (65.4) | 20 (66.7) | 37 (66.1) |
| Ethnicity, n (%) | |||
| White | 26 (100) | 30 (100) | 56 (100) |
| Living arrangements,a n (%) | |||
| Alone | 9 (34.6) | 7 (23.3) | 16 (28.6) |
| With spouse/partner | 15 (57.7) | 19 (63.3) | 34 (60.7) |
| With parent(s) | 1 (3.8) | 2 (6.7) | 3 (5.4) |
| With child/children | 4 (15.4) | 4 (13.3) | 8 (14.3) |
| Other | 1 (3.3) | 1 (1.8) | |
| Place of residence, n (%) | |||
| Flat/apartment | 4 (15.4) | 5 (16.7) | 9 (16.1) |
| House/bungalow | 21 (80.8) | 25 (83.3) | 46 (82.1) |
| Other | 1 (3.8) | 0 (0) | 1 (1.8) |
| Occupation status, n (%) | |||
| Unemployed | 1 (3.8) | 2 (6.7) | 3 (5.4) |
| Part-time work | 4 (15.4) | 2 (6.7) | 6 (10.7) |
| Full-time work | 2 (7.7) | 1 (3.3) | 3 (5.4) |
| Age retired | 5 (19.2) | 5 (16.7) | 10 (17.9) |
| Medically retired | 14 (53.8) | 19 (63.3) | 33 (58.9) |
| Characteristic | Group | Total (N = 56) | |
|---|---|---|---|
| Usual care (N = 26) | Intervention (N = 30) | ||
| Age at diagnosis (years) | |||
| Mean (SD) | 41.7 (13.5) | 42.3 (13.2) | 42 (13.2) |
| Minimum–maximum | 18.0–65.0 | 21.0–68.0 | 18.0–68.0 |
| Median (IQR) | 42.0 (30.0–51.0) | 43.0 (31.0–48.0) | 43.0 (30.0–49.5) |
| Time since relapse, n (%) | |||
| At least 1 year | 18 (69.2) | 24 (80.0) | 42 (75.0) |
| 3–12 months | 5 (19.2) | 6 (20.0) | 11 (19.6) |
| Within 3 months | 2 (7.7) | 2 (3.6) | |
| EDSS (points) | |||
| Mean (SD) | 6.1 (0.7) | 6.3 (0.3) | 6.2 (0.5) |
| Minimum–maximum | 4.0–7.0 | 6.0–7.0 | 4.0–7.0 |
| Median (IQR) | 6.0 (6.0–6.5) | 6.5 (6.0–6.5) | 6.3 (6.0–6.5) |
| Cognition: SDMT | |||
| Mean (SD) | 44.5 (15.1) | 39.1 (10.9) | 41.6 (13.2) |
| Minimum–maximum | 7.0–77.0 | 20.0–60.0 | 7.0–77.0 |
| Median (IQR) | 47.0 (33.0–54.0) | 41.5 (31.0–47.0) | 44.5 (32.0–50.0) |
| Previous 4-week continence, n (%) | |||
| Not at all | 14 (53.8) | 13 (43.3) | 27 (48.2) |
| Once | 1 (3.8) | 3 (10.0) | 4 (7.1) |
| 2–4 times | 3 (11.5) | 7 (23.3) | 10 (17.9) |
| > More than weekly | 5 (19.2) | 3 (10.0) | 8 (14.3) |
| Daily | 3 (11.5) | 4 (13.3) | 7 (12.5) |
| Continence device, n (%) | |||
| No | 21 (80.8) | 27 (90.0) | 48 (85.7) |
| Yes | 5 (19.2) | 3 (10.0) | 8 (14.3) |
| Mood: depressed, n (%) | |||
| No | 15 (57.7) | 15 (50.0) | 30 (53.6) |
| Yes | 11 (42.3) | 15 (50.0) | 26 (46.4) |
| Mood: lack of pleasure, n (%) | |||
| No | 19 (73.1) | 20 (66.7) | 39 (69.6) |
| Yes | 7 (26.9) | 10 (33.3) | 17 (30.4) |
| Fear of falling, n (%) | |||
| Not at all | 7 (26.9) | 2 (6.7) | 9 (16.1) |
| Somewhat | 8 (30.8) | 11 (36.7) | 19 (33.9) |
| Fairly | 4 (15.4) | 8 (26.7) | 12 (21.4) |
| Very | 7 (26.9) | 8 (26.7) | 15 (26.8) |
| Do not know | 1 (3.3) | 1 (1.8) | |
| 3-month fall history, n (%) | |||
| Not fallena | 1 (3.3) | 1 (1.8) | |
| Twice | 7 (26.9) | 5 (16.7) | 12 (21.4) |
| 3–5 times | 11 (42.3) | 13 (43.3) | 24 (42.9) |
| More often | 8 (30.8) | 11 (36.7) | 19 (33.9) |
| Indoor walking aids,b n (%) | |||
| One stick/crutch | 9 (34.6) | 13 (43.3) | 22 (39.3) |
| Two sticks/crutches | 5 (19.2) | 4 (13.3) | 9 (16.1) |
| Walker/frame | 8 (30.8) | 12 (40.0) | 20 (35.7) |
| Wheelchair | 4 (15.4) | 4 (13.3) | 8 (14.3) |
| Outdoor walking aids,b n (%) | |||
| One stick/crutch | 17 (65.4) | 18 (60.0) | 35 (62.5) |
| Two sticks/crutches | 10 (38.5) | 7 (23.3) | 17 (30.4) |
| Walker/frame | 9 (34.6) | 14 (46.7) | 23 (41.1) |
| Wheelchair | 12 (46.2) | 15 (50.0) | 27 (48.2) |
| Assistive devices,b n (%) | |||
| AFO | 10 (38.5) | 7 (23.3) | 17 (30.4) |
| Functional electrical stimulation | 8 (30.8) | 7 (23.3) | 15 (26.8) |
| Other | 2 (7.7) | 2 (6.7) | 4 (7.1) |
| Number taking medication, n (%) | 24 (92.3) | 27 (90.0) | 51 (91.1) |
| Number of medications | |||
| Median (IQR) | 4 (2–7) | 5 (2–7) | 4 (2–7) |
| Minimum–maximum | 0–10 | 0–17 | 0–17 |
| Medication type,b n (%) | |||
| Disease modifying | 2 (7.7) | 1 (3.3) | 3 (5.4) |
| Anti-spasticity | 6 (23.1) | 11 (36.7) | 17 (30.4) |
| Tremor/ataxia | 2 (6.7) | 2 (3.6) | |
| Pain | 11 (42.3) | 13 (43.3) | 24 (42.9) |
| Fatigue | 3 (11.5) | 8 (26.7) | 11 (19.6) |
| Fampridine | 3 (11.5) | 1 (3.3) | 4 (7.1) |
| Other | 17 (65.4) | 25 (83.3) | 42 (75) |
| Missing | 3 (11.5) | 2 (6.7) | 5 (8.9) |
| Participants with historical medical conditions, n (%) | |||
| Number of participants | 21 (80.8) | 26 (86.7) | 47 (83.9) |
| Number of historical medical conditions | |||
| Median (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) |
| Minimum–maximum | 0.0–8.0 | 0.0–6.0 | 0.0–8.0 |
| Historical medical conditions,b n (%) | |||
| COPD/asthma | 4 (15.4) | 3 (10.0) | 7 (12.5) |
| Coronary heart disease/hypertension | 6 (23.1) | 3 (10.0) | 9 (16.1) |
| Depression/anxiety | 7 (26.9) | 14 (46.7) | 21 (37.5) |
| Diabetes | 2 (7.7) | 1 (3.3) | 3 (5.4) |
| Migraine | 5 (19.2) | 9 (30.0) | 14 (25.0) |
| Osteoarthritis | 5 (19.2) | 9 (30.0) | 14 (25.0) |
| Osteoporosis | 6 (23.1) | 2 (6.7) | 8 (14.3) |
| Other | 16 (61.5) | 20 (66.7) | 36 (64.3) |
| Other neurological condition | 2 (7.7) | 4 (13.3) | 6 (10.7) |
| Participants with an ongoing medical condition, n (%) | |||
| Number of participants | 16.0 (61.5) | 19 (63.3) | 35 (62.5) |
| Number of ongoing medical conditions | |||
| Median (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) |
| Minimum–maximum | 0.0–6.0 | 0.0–5.0 | 0.0–6.0 |
| Ongoing medical conditions,b n (%) | |||
| COPD/asthma | 1 (3.8) | 2 (6.7) | 3 (5.4) |
| Coronary heart disease/hypertension | 1 (3.8) | 1 (3.3) | 2 (3.6) |
| Depression/anxiety | 4 (15.4) | 7 (23.3) | 11 (19.6) |
| Diabetes | 1 (3.8) | 1 (3.3) | 2 (3.6) |
| Migraine | 3 (11.5) | 1 (3.3) | 4 (7.1) |
| Osteoarthritis | 3 (11.5) | 6 (20.0) | 9 (16.1) |
| Osteoporosis | 5 (19.2) | 2 (6.7) | 7 (12.5) |
| Other | 10 (38.5) | 11 (36.7) | 21 (37.5) |
| Other neurological condition | 1 (3.8) | 1 (3.3) | 2 (3.6) |
Overall, the sample demographic and diagnostic characteristics are similar to those of other groups of MS fallers. 15,17 However, as shown in Table 15, there are some differences in key characteristics between the two groups, notably for measures of disease severity (EDSS), cognition (Symbol Digit Modalities Test) and mood, with the intervention group scoring worse, on average, than the usual-care group. A higher proportion of participants with historical and/or ongoing depression in their medical history were in the intervention group. In addition, a higher proportion of participants in this group were taking medications, more specifically for spasticity, tremor and fatigue, as well as for ‘other’ unspecified reasons.
The usual-care group tended to use two walking aids, and had a greater use of assistive devices (e.g. ankle–foot orthoses or functional electrical stimulation). A slightly higher proportion of participants in the intervention group had fallen more than five times in the previous 3 months, and there was a slight imbalance between the groups in the proportions who reported being ‘fairly’ afraid of falling.
Summary
The sample is similar in baseline characteristics to other groups of fallers. There was an imbalance between the two allocated groups in some baseline characteristics. There was no option in the CRF for a participant to declare that they had fallen once.
Discussion
In recognition of the prevalence of comorbidity in MS,140 our recruitment criteria were specifically set to be as inclusive as possible, and the prevalence was broadly in line with published data in this field. 140 Although the distribution between groups was similar for most characteristics, the differing incidence of some (e.g. EDSS score, anxiety/depression and cognition) could potentially affect intervention outcomes, indicating that a future definitive trial may require stratification according to these variables.
Performance of potential trial outcome measures
Potential primary outcome measures
Table 17 shows the potential primary outcome measures at all trial time points. Although the sample means are similar to those of other groups of MS fallers (including for MSWS-12vs2 and MSIS-29vs2),144,145 those allocated to the intervention appear to be worse across many measures at baseline. To account for this, differences between the groups have been reported, both in their ‘raw’ format and adjusted for baseline score.
| Variable | Time point | Group | Difference between allocated groups (intervention – usual care), mean (95% CI) | Minimal clinically important difference, when available | ||||
|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||||
| n | Mean (SD) [minimum–maximum] | n | Mean (SD) [minimum–maximum] | Unadjustedc | Adjustedd | |||
| MSWS-12vs2a | Baseline | 26 | 79.6 (14.4) [52.0–100.0] | 30 | 84.2 (16.2) [45.0–100.0] | 4/6141 | ||
| Week 15 | 24 | 79.8 (13.9) [48.0–100.0] | 25 | 75.6 (19.4) [33.0–100.0] | –4.2 (–14 to 5.5) | –10.6 (–18.9 to 2.2) | ||
| Week 27 | 22 | 79.5 (21.9) [21.0–100.0] | 22 | 75.4 (16.8) [40.0–100.0] | –4 (–15.9 to 7.8) | –7.7 (–17.2 to 1.8) | ||
| EQ-5D-3Lb (crosswalk) | Baseline | 26 | 0.6 (0.2) [0.0–0.8] | 29 | 0.5 (0.2) [0.0–0.9] | 0.05–0.08142 | ||
| Week 15 | 24 | 0.6 (0.2) [0.2–0.9] | 25 | 0.6 (0.2) [0.0–0.9] | 0.0 (–0.1 to 0.1) | 0.0 (–0.1 to 0.1) | ||
| Week 27 | 22 | 0.6 (0.3) [–0.1–0.9] | 22 | 0.6 (0.1) [0.3–0.8] | 0.0 (–0.1 to 0.1) | 0.0 (–0.1 to 0.1) | ||
| EQ-5D-5L (VAS)b | Baseline | 26 | 64.6 (19.1) [20.0–95.0] | 29 | 54.6 (18.6) [15.0–95.0] | N/A | ||
| Week 15 | 24 | 61.3 (20.3) [20.0–90.0] | 25 | 54.6 (16.5) [25–97] | –6.7 (–17.4 to 3.9) | 4.7 (–4.3 to 13.6) | ||
| Week 27 | 22 | 63.3 (17.8) [25.0–95.0] | 22 | 49.9 (21.1) [15.0–95.0] | –13.4 (–25.3 to –1.5) | –2.1 (–13 to 8.8) | ||
| MSIS-29vs2a (physical) | Baseline | 26 | 64.2 (21.7) [25.0–97.0] | 30 | 64.8 (16.4) [32.0–93.0] | 8143 | ||
| Week 15 | 24 | 59.4 (23) [13.0–98] | 25 | 54.8 (19.5) [13–92] | –4.6 (–16.8 to 7.7) | –4.9 (–13.2 to 3.5) | ||
| Week 27 | 22 | 59 (24.9) [0.0–92] | 22 | 57.9 (15.2) [27–88] | –1.2 (–13.7 to 11.4) | 0.6 (–7.8 to 9) | ||
| MSIS-29vs2a (psychological) | Baseline | 26 | 45.1 (29.7) [0.0–85] | 30 | 50.4 (22.8) [4.0–96] | N/A | ||
| Week 15 | 24 | 43.3 (26.8) [0–89] | 25 | 43.7 (19) [0.0–70.0] | 0.5 (–12.8 to 13.8) | –5 (–15.5 to 5.5) | ||
| Week 27 | 22 | 40 (26.8) [0.0–93] | 22 | 43.3 (22.6) [7.0–81] | 3.3 (–11.8 to 18.4) | –0.4 (–9.9 to 9) | ||
Summary
All three of the potential primary outcome measures produced data that were comparable with those from other similar samples. There was an imbalance in the scoring between the groups at baseline, which was counteracted by reporting the raw and adjusted differences between the groups. The scores at 15 and 27 weeks (± 1 week) in the MSWS-12vs2 are in favour of the intervention group and exceed the available data for the minimal clinically important difference.
Potential secondary outcome measures
Table 18 shows the data for the potential secondary outcome measures for all time points.
| Variable | Time point | Group | Difference between allocated groups (intervention – usual care), mean (95% CI) | Minimal clinically important difference, when available | ||||
|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||||
| n | Mean (SD) [minimum–maximum] | n | Mean (SD) [minimum–maximum] | Unadjustede | Adjustedf | |||
| Clinician-rated measures | ||||||||
| 2MWTa,b | Baseline | 26 | 52.7 (27.2) [7–138.5] | 30 | 53 (32) [9–133] | 12.2 to 14.7146 or 19.21 metres147 | ||
| Week 15 | 24 | 51.9 (29.5) [8.5–120] | 24 | 53.3 (27) [13–101] | 1.4 (–15 to 17.9) | 0.3 (–9.4 to 10) | ||
| Week 27 | 21 | 56.3 (31.1) [12–126.8] | 20 | 55 (27.4) [16–103] | –1.3 (–19.9 to 17.2) | –2.2 (–14.6 to 10.2) | ||
| Mini-BESTb | Baseline | 26 | 12.2 (3.8) [6–21] | 29 | 10.7 (5.2) [2–20] | 3.594 | ||
| Week 15 | 24 | 12.5 (6.2) [1–25] | 24 | 14.2 (6.2) [4–27] | 1.7 (–1.9 to 5.3) | 2.6 (–0.1 to 5.4) | ||
| Week 27 | 21 | 13.6 (6) [3–27] | 20 | 14.1 (5.7) [3–22] | 0.5 (–3.2 to 4.2) | 1.2 (–1.2 to 3.6) | ||
| Forward FRTa | Baseline | 26 | 16.6 (6.4) [1–28.7] | 30 | 18.1 (7.9) [0–32.3] | 6.35–6.79 (vestibular disorders)148 | ||
| Week 15 | 24 | 16.1 (6.4) [0–29.3] | 24 | 20.8 (7.6) [7.7–38.7] | 4.7 (0.6 to 8.8) | 2.7 (–0.3 to 5.7) | ||
| Week 27 | 21 | 17 (7.8) [0–32] | 20 | 19.5 (5.9) [6.3–33.7] | 2.5 (–1.9 to 6.9) | 1 (–2.7 to 4.6) | ||
| Lateral FRTb | Baseline | 26 | 13.3 (6.2) [0–30] | 30 | 13.4 (6.9) [0–27.3] | N/A | ||
| Week 15 | 24 | 12.3 (5.2) [0–20.7] | 24 | 16.7 (7.6) [5–33.3] | 4.4 (0.6 to 8.2) | 4.2 (0.9 to 7.5) | ||
| Week 27 | 21 | 11.9 (5.4) [0–21.3] | 20 | 16.1 (4.9) [6–24.3] | 4.2 (0.9 to 7.5) | 4 (1.1 to 6.9) | ||
| Self-reported outcomes | ||||||||
| FES-Ic | Baseline | 26 | 43.7 (9.8) [24.0–61.0] | 30 | 44.1 (9) [27.0–60.0] | 8.2 (vestibular disorders)149 | ||
| Week 15 | 24 | 44.3 (10) [27.0–58.0] | 25 | 40.1 (8.1) [28.0–55.0] | –4.2 (–9.4 to 1) | –5.1 (–9.8 to –0.4) | ||
| Week 27 | 22 | 44.5 (12) [22.0–62.0] | 22 | 41.6 (8.4) [24.0–55.0] | –2.9 (–9.2 to 3.4) | –3.7 (–8.8 to 1.4) | ||
| CPI 1d | Baseline | 25 | 97.4 (39.2) [11.1–212.5] | 27 | 101 (55.5) [38.9–266.7] | N/A | ||
| Week 15 | 24 | 107.5 (59.1) [33.3–271.4] | 24 | 100.2 (38.7) [38.9–187.5] | –7.3 (–36.3 to 21.7) | 0.3 (–29.3 to 29.8) | ||
| Week 27 | 22 | 108.3 (47.6) [17.6–200] | 22 | 92.6 (34.6) [41.2–146.2] | –15.8 (–41.1 to 9.5) | –12.2 (–39 to 14.6) | ||
| CPI 2b | Baseline | 26 | 42.9 (8.3) [26.3–64.4] | 27 | 40.8 (7.9) [26.3–53.1] | N/A | ||
| Week 15 | 24 | 40.8 (9.3) [21.5–63.3] | 24 | 42.3 (9.9) [11.4–57.3] | 1.5 (–4.1 to 7.1) | 2 (–2 to 6.1) | ||
| Week 27 | 22 | 41.7 (11.3) [11.4–57.3] | 22 | 42.2 (9.4) [17.8–54.7] | 0.5 (–5.8 to 6.8) | 1.8 (–2.6 to 6.1) | ||
| CPI 3b | Baseline | 26 | 57.5 (10.4) [38.1–81.4] | 26 | 53.7 (9.3) [39–81.4] | N/A | ||
| Week 15 | 24 | 58.4 (14) [39–100] | 24 | 54.1 (11.1) [37.1–88.2] | –4.2 (–11.6 to 3.1) | –2.1 (–6.5 to 2.4) | ||
| Week 27 | 22 | 59.4 (15.4) [23.7–88.2] | 22 | 54.2 (10.5) [36.2–77.3] | –5.2 (–13.2 to 2.8) | –0.4 (–5.3 to 4.4) | ||
All of the potential secondary outcome measures had excellent rates of completion at all time points and produced outcomes that were broadly comparable with those of other similar samples.
With regard to the potential secondary outcomes, there are two noteworthy areas of discussion. The first relates to the measure used to evaluate participation, the CPI. When selecting this measure, we were cognisant of the fact that there is a lack of evidence on its clinical utility and measurement properties, and no normative data are available in the MS population. Nevertheless, we chose this measure based on the recommendation of the International Multiple Sclerosis Falls Prevention Network (an expert panel of MS specialists undertaking trials in the area of falls106), and a recognition that participation is a key outcome for consideration in trials of this nature. This feasibility trial provided us with the opportunity to gain first-hand experience of its use. Feedback from the research therapists highlighted that some participants found the measure complicated to interpret and complete. Our analysis of item CPI 1 (satisfaction with life activities) also highlighted ambiguity in the scoring instructions for this part of the measure.
The second area of discussion is the shorter distances walked by our participants in the 2MWT than in a number of other studies with similar sample characteristics. 89,150 For example, Gijbels et al. 147 reported a mean walking distances of 104 metres (SD 41 metres, range 40–172 metres) in a sample of 21 participants with an EDSS score of between 4.5 and 6.5 points. In line with this, our sample reported higher levels of concern (as measured with the FES-I) than other MS populations, although this is perhaps not surprising given their falls history and that they have progressive MS. 102
Summary
The inclusion of a measure of participation is important; however, there is a paucity of measures that have been evaluated for use in MS. In this feasibility study, the CPI proved burdensome to complete (particularly of part 1 of the measure), and our analysis has highlighted ambiguities in its scoring that would need to be addressed should it be used in a definitive study.
Accelerometry (activPAL) data
The activPAL results are based on the mean estimate per day for the number of days of data available for each research assessment visit when participants were asked to wear the device. Although all participants were asked to wear the device for 7 days, the number of days of data available was variable after the cleaning process undertaken to remove any incomplete days (see Potential secondary outcomes). Table 19 shows the activPAL data, highlighting the number of days of recorded data and the number of participants with at least 1 day of data that were used to estimate the mean activity levels at each time point.
| Total number of days | Time point | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Week 15 (± 1 week) | Week 27 (± 1 week) | |||||||
| n | Follow-up (%) | Randomised (%) | n | Follow-up (%) | Randomised (%) | n | Follow-up (%) | Randomised (%) | |
| 1 | – | – | – | – | – | – | – | – | – |
| 2 | – | – | – | 1 | 2.0 | 1.8 | – | – | – |
| 3 | 3 | 5.4 | 5.4 | 1 | 2.0 | 1.8 | – | – | – |
| 4 | 2 | 3.6 | 3.6 | 3 | 6.1 | 5.4 | 2 | 4.5 | 3.6 |
| 5 | 24 | 42.9 | 42.9 | 22 | 44.9 | 39.3 | 14 | 31.8 | 25.0 |
| 6 | 24 | 42.9 | 42.9 | 18 | 36.7 | 32.1 | 21 | 47.7 | 37.5 |
| 7 | 1 | 1.8 | 1.8 | 1 | 2.0 | 1.8 | 5 | 11.4 | 8.9 |
Table 20 shows the accelerometry data at each time point. Overall, among the participants randomised, at least 1 day of activPAL data were available for 54 (96.4%) at baseline, 46 (82.1%) at week 15 and 42 (75.0%) at week 27. Although the two groups of participants were similar on the majority of measures at baseline, in line with the other outcome measure results, data for the step count were fewer in the intervention group.
| Variable | Time point | Group | Difference between allocated groups | ||||
|---|---|---|---|---|---|---|---|
| Usual care | Intervention | ||||||
| n | Mean (SD) [minimum–maximum] | n | Mean (SD) [minimum–maximum] | Unadjusteda | Adjustedb | ||
| Step count | Baseline | 26 | 3286.4 (2760.7) [256.3–11,756.4] | 28 | 2654.9 (1861.1) [11.7–7543.2] | ||
| Week 15 | 23 | 3443.6 (3721.8) [283.2–13,034.5] | 23 | 2841.4 (1841) [5.2–7671.2] | –602.2 (–2347.1 to 1142.7) | –165.7 (1047.6 to 716.2) | |
| Week 27 | 22 | 2982.6 (3248) [166.7–14,739.6] | 20 | 3633.1 (2268.3) [118.3–9027.2] | 650.5 (–1113.7 to 2414.7) | 699.8 (–495.3 to 1894.8) | |
| Sitting/lying time (hours) | Baseline | 26 | 19.7 (1.7) [16.8–22.9] | 28 | 19.9 (2.1) [15.3–23.2] | ||
| Week 15 | 23 | 19.8 (2.3) [13.5–23.4] | 23 | 19.7 (1.9) [16.5–22.9] | 0 (–1.3 to 1.2) | 0.3 (–0.8 to 1.4) | |
| Week 27 | 22 | 19.7 (2.2) [13.5–23.1] | 20 | 19 (1.9) [16.1–22.7] | –0.6 (–1.9 to 0.7) | –0.2 (–1.1 to 0.8) | |
| Standing time (hours) | Baseline | 26 | 3.4 (1.4) [1–6.1] | 28 | 3.4 (1.9) [0.6–8] | ||
| Week 15 | 23 | 3.3 (1.8) [0.4–7.5] | 23 | 3.5 (1.7) [1–6.7] | 0.2 (–0.8 to 1.2) | –0.3 (–1.2 to 0.7) | |
| Week 27 | 22 | 3.5 (1.7) [0.6–7.6] | 20 | 4 (1.7) [1.2–7.2] | 0.5 (–0.6 to 1.5) | 0 (–0.8 to 0.8) | |
| Stepping time (hours) | Baseline | 26 | 0.9 (0.6) [0.1–2.9] | 28 | 0.8 (0.5) [0–2] | ||
| Week 15 | 23 | 0.9 (0.9) [0.1–3] | 23 | 0.8 (0.5) [0–1.9] | 0.5 (–0.6 to 1.5) | 0 (–0.2 to 0.2) | |
| Week 27 | 22 | 0.8 (0.7) [0.1–3] | 20 | 1 (0.5) [0.1–2.2] | 0.2 (–0.2 to 0.5) | 0.2 (–0.1 to 0.4) | |
| Sit to stand (transitions) | Baseline | 26 | 48.2 (16.9) [15.8–84.8] | 28 | 48.1 (19.8) [17–95.2] | ||
| Week 15 | 23 | 47 (20.8) [18.8–101.5] | 23 | 51.6 (21.6) [17.2–101] | 4.6 (–8 to 17.2) | 2 (–4.6 to 8.6) | |
| Week 27 | 22 | 45 (19.8) [17.3–84.8] | 20 | 56.2 (20.2) [15–101.5] | 11.1 (–1.4 to 23.6) | 4.7 (–4.1 to 13.6) | |
Summary
The recommended minimum of 5 days of accelerometry data were available for the majority of participants and time points. 85
Falls diary data
Once the data had been cleaned (see Analysis of patient-reported and clinician-rated outcome measures), one participant was identified as accounting for over half of the reported falls in the usual-care allocation group; therefore, this participant’s data were classified as an outlier and removed from all falls diary analyses.
Figures 10 and 11 represent the proportion of participants falling and sustaining injurious falls on a weekly basis. There is a significant variation over the weeks, and between the groups, although those in the BRiMS group tended to report injurious falls less frequently.
FIGURE 10.
The proportion of participants who fell at least once per week by diary week number and allocation group. The green area of the plot represents the 13-week BRiMS intervention period.
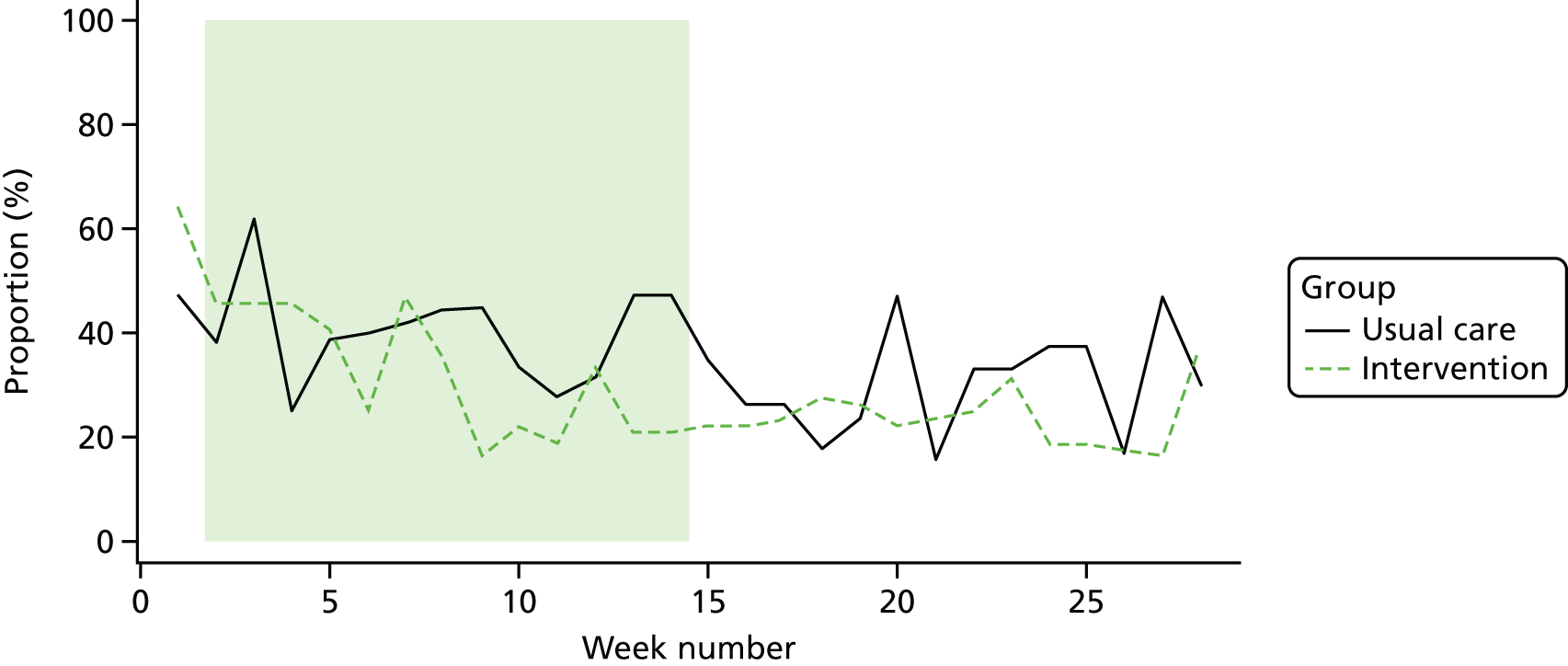
FIGURE 11.
The proportion of participants with at least one injurious fall per week by diary week number and allocation group. The green area of the plot represents the 13-week BRiMS intervention period.
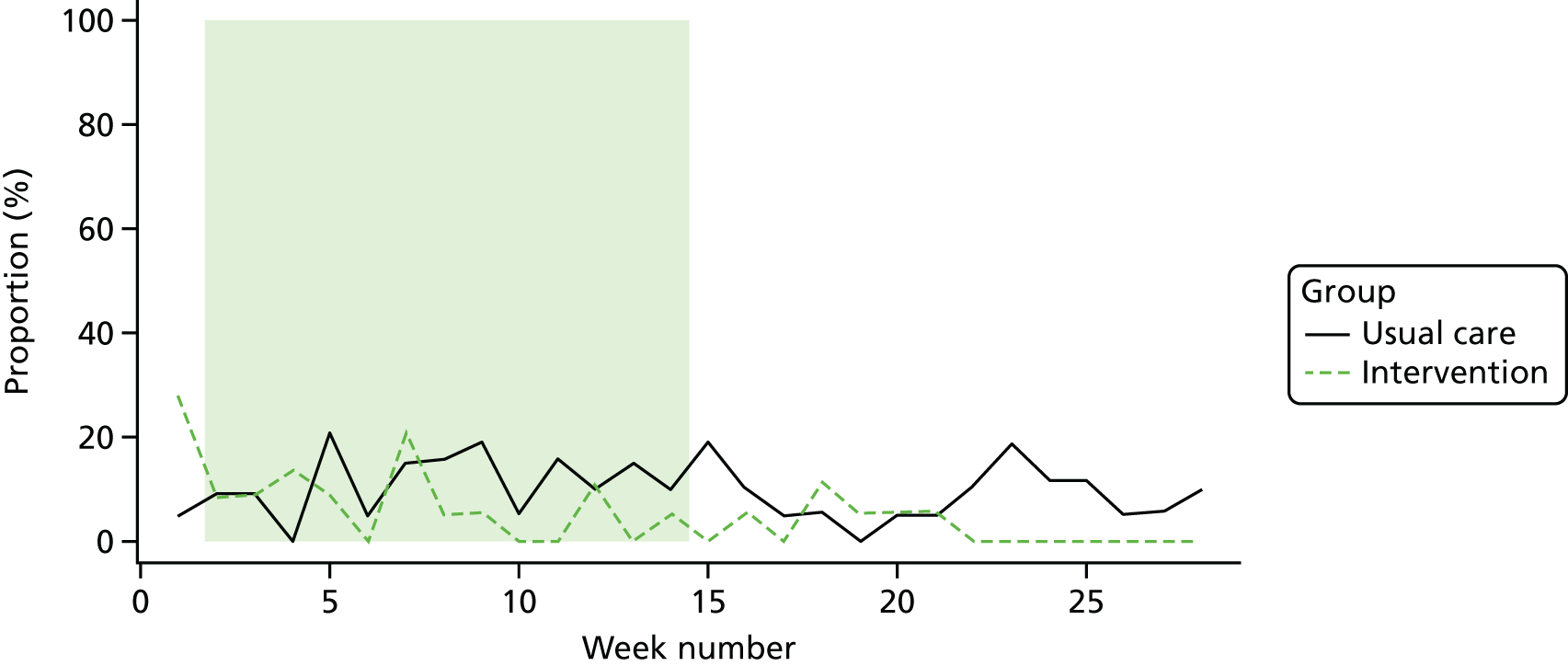
A total of 715 falls were reported across the whole sample. Of these, 362 were in the usual-care group and 353 were in the intervention group. A total of 101 injurious falls were reported: 66 in the usual-care group and 35 in the intervention group.
As described in Completeness of the potential primary outcomes, a range of issues was identified with the completion and return rate of the daily diaries. Table 21 gives the details of rates of falls and injurious falls calculated per person per year.
| Falls analyses | Group | Total | |
|---|---|---|---|
| Usual care | Intervention | ||
| Total number of falls (n) | 362 | 353 | 715 |
| Total number of injurious falls (n) | 66 | 35 | 101 |
| Falls rate (per person per year)a | |||
| Analysis 1 (reported) | |||
| Number of days with falls data (n) | 3204 | 3180 | 6384 |
| Falls rate | 41.3 | 40.5 | 40.9 |
| Analysis 1 (returned) | |||
| Number of days with injurious fall data (n) | 2478 | 1983 | 4461 |
| Injurious falls rate | 9.7 | 6.4 | 8.3 |
| Analysis 2 (expected) | |||
| Number of potential days of data (n) | 4116 | 5096 | 9212 |
| Falls rate | 32.1 | 25.3 | 28.3 |
| Injurious falls rate | 5.9 | 2.5 | 4.0 |
| Analysis 3 (randomised) | |||
| Number of potential days of data (n) | 5096 | 5880 | 10,976 |
| Falls rate | 25.9 | 21.9 | 23.8 |
| Injurious falls rate | 4.7 | 2.2 | 3.4 |
Consequences of falls
Data on the injuries sustained during falls, the use of medical services and the need for help to get up after a fall are reported in Table 22. Participants were requested to report the number of each type of injury (e.g. bruises, cuts/scrapes, fractures) that they experienced. This was not reported consistently; for example, some participants provided specific details (e.g. bruise = bottom). Therefore, these variables were converted into a binary variable representing the presence or absence of at least one of these injuries.
| Falls and injurious falls | Group | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||||||||
| n | Reported (%) | Expected (%) | Randomised (%) | n | Reported (%) | Expected (%) | Randomised (%) | N | Reported (%) | Expected (%) | Randomised (%) | |
| Any injuries during the 2-week period | ||||||||||||
| No | 52 | 10.6 | 7.7 | 6.6 | 31 | 6.3 | 4.6 | 4.0 | 83 | 17.0 | 12.4 | 10.6 |
| Yes | 87 | 17.8 | 12.9 | 11.1 | 65 | 13.3 | 9.7 | 8.3 | 152 | 31.1 | 22.6 | 19.4 |
| Not applicable (no fall) | 101 | 20.7 | 15.0 | 12.9 | 117 | 23.9 | 17.4 | 14.9 | 218 | 44.6 | 32.4 | 27.8 |
| Spoiled | 1 | 0.2 | 0.1 | 0.1 | 1 | 0.2 | 0.1 | 0.1 | 2 | 0.4 | 0.3 | 0.3 |
| Missing | 14 | 2.9 | 2.1 | 1.8 | 20 | 4.1 | 3.0 | 2.6 | 34 | 7.0 | 5.1 | 4.3 |
| Reported injuries | ||||||||||||
| Bruises | 45 | 9.2 | 6.7 | 5.7 | 28 | 5.7 | 4.2 | 3.6 | 73 | 15.7 | 10.9 | 9.3 |
| Cut(s)/scrape(s) | 25 | 5.1 | 3.7 | 3.2 | 8 | 1.6 | 1.2 | 1.0 | 33 | 0.6 | 4.9 | 4.2 |
| Strain(s)/sprain(s) | 9 | 1.8 | 1.3 | 1.1 | 5 | 1.0 | 0.7 | 0.6 | 14 | 14.0 | 2.1 | 1.8 |
| Dislocation | 0 | 0.0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | 0.0 |
| Broken bone(s) | 3 | 0.6 | 0.4 | 0.4 | 1 | 0.2 | 0.1 | 0.1 | 4 | 2.2 | 0.6 | 0.5 |
| Did you use any medical services? | ||||||||||||
| No | 123 | 25.2 | 18.3 | 15.7 | 79 | 16.2 | 11.8 | 10.1 | 202 | 41.3 | 30.1 | 25.8 |
| Yes | 5 | 1.0 | 0.7 | 0.6 | 2 | 0.4 | 0.3 | 0.3 | 7 | 1.4 | 1.0 | 0.9 |
| Not applicable (no fall) | 110 | 22.5 | 16.4 | 14.0 | 135 | 27.6 | 20.1 | 17.2 | 245 | 50.1 | 36.5 | 31.3 |
| Spoiled | 0 | 0.0 | 0.0 | 0.0 | 1 | 0.2 | 0.1 | 0.1 | 1 | 0.2 | 0.1 | 0.1 |
| Missing | 17 | 3.5 | 2.5 | 2.2 | 17 | 3.5 | 2.5 | 2.2 | 34 | 7.0 | 5.1 | 4.3 |
| Did you have help to get up after a fall? | ||||||||||||
| No | 76 | 15.5 | 11.3 | 9.7 | 54 | 11.0 | 8.0 | 6.9 | 130 | 26.6 | 19.3 | 16.6 |
| Yes | 52 | 10.6 | 7.7 | 6.6 | 32 | 6.5 | 4.8 | 4.1 | 84 | 17.2 | 12.5 | 10.7 |
| Not applicable (no fall) | 110 | 22.5 | 16.4 | 14.0 | 131 | 26.8 | 19.5 | 16.7 | 241 | 49.3 | 35.9 | 30.7 |
| Missing | 17 | 3.5 | 2.5 | 2.2 | 17 | 3.5 | 2.5 | 2.2 | 34 | 7.0 | 5.1 | 4.3 |
These data are from participant self-report pre-formatted diaries and therefore the injuries have not been corroborated by medical assessments. Both the rates of falls and the rates of injurious falls reported by our participants are high relative to published observational data. 17 This may be explained, at least in part, because all participants reported recent falls on entry to the study, and all have SPMS. Although it is known that people with progressive MS and those with an EDSS score of around 6–6.5 points have an increased risk of falls, changes in rate of falls with changing mobility status and/or disease classification has not yet been established.
Summary
Falls data, with respect to both frequency and whether or not these resulted in injury, were recorded by participants daily in a pre-formatted paper diary, which was requested to be returned on a 2-weekly basis. Issues with the quality and completeness of the recorded data, and the lower than expected diary return rate, cast some uncertainty over the confidence with which the results can be interpreted.
Associations between baseline factors most strongly associated with potential outcomes, as potential stratification factors in a definitive trial
The associations between the potential primary outcomes and the baseline demographic and clinical characteristics were evaluated as indicators of the possible need for stratification and/or whether or not the final analyses of a full definitive trial may require adjustments for associations. Estimates have not been split by allocated group and include all available data at week 27 (± 1 week) as the likely primary end point of such a trial. Data are presented in Appendix 5.
From these analyses, it appears that associations may be significant between the potential primary outcomes and disease severity (EDSS), depression and the use of mobility aid. There is evidence to suggest that those who report being depressed or lacking pleasure tend to have higher mean scores, particularly MSIS-29vs2 psychological scores, for which the 95% CIs for depression do not overlap between categories. Those with an EDSS score of ≥ 6.0 points appear to score significantly higher on all measures; however, the small numbers of participants classified at the lower end of the EDSS scale precludes definitive recommendations.
Discussion
Analysis of possible associations between baseline characteristics and potential primary outcome measures suggests that a future trial should consider stratification for mood and/or disease severity. 151–154 Small numbers in the lower EDSS groupings in our analyses limit the inferences we are able to draw; however, other studies have also highlighted this association with regard to both disease severity63 and mood. 155 Although stratification for disease severity according to the EDSS is quite common in MS physical rehabilitation trials,151–154 a literature search confirmed that this is not the case for mood.
Calculation of sample size required for a fully powered randomised controlled trial to evaluate the effectiveness of the BRiMS intervention
One of the key purposes of a feasibility trial is to obtain data to inform the sample size calculation for a full-scale trial. Pearson’s correlation coefficients were calculated between the potential primary outcome measures at baseline and at 27 weeks (± 1 week) to inform the sample size calculation for a future definitive trial; data are detailed in Appendix 5. Correlation coefficients were generally moderate, with a conservative point estimate of 0.59 for the recommended primary outcome measure for a definitive trial, the MSWS-12vs2.
The data collected as part of this feasibility trial have been used, together with previously identified relevant data, to provide potential target sample sizes under a range of assumptions in order to detect a between-group difference for the primary outcome of MSWS-12vs2 of 5.2 units156 at the primary end point of 6 months post randomisation.
It is assumed that the primary statistical analysis of such a definitive trial would be based on a multilevel modelling approach, including adjustment for the baseline MSWS-12vs2 score, to allow for the partially nested data: participants allocated to the intervention arm of the trial will be clustered within small groups (≈5 participants), whereas participants allocated to the usual-care arm will not be clustered. Sample size calculations therefore need to incorporate the estimated intracluster correlation (ICC) (‘group’ effect). 157,158 It is anticipated that BRiMS intervention groups will be small (≈5 per group; in the BRiMS feasibility trial, the intervention groups ranged from four to six participants) and, as there are only three group sessions during the intervention period, at each site there is likely to be only one treating therapist. Furthermore, the intervention is standardised/manualised, leading to the assumption that the ICC is likely to be small. The base case for the sample size calculation below assumes an ICC of 0.05, with additional calculations shown for ICCs of 0.075, 0.100 and 0.125.
Pooling the SD of MSWS-12vs2 from previous relevant studies159,160 gives a SD of 23 units. In the BRiMS feasibility trial, the point estimate of the SD of MSWS-12vs2 at 27 weeks (± 1 week) follow-up was 19.4 units (i.e. lower than the pooled estimate from the previous four studies), with a one-sided 80% upper bound of 21.5 units. The base case for the sample size calculation below conservatively assumes a SD of 23 units, with calculations also shown for SDs ranging from 19 to 25 units.
As the planned analyses would include adjustment for baseline MSWS-12vs2 scores, the effect of allowing for the correlation between baseline and 6-month MSWS-12vs2 scores has also been considered. 161 There are few relevant published estimates of the correlation between baseline and 12 month MSWS-12vs2 scores: data from the SWIMS project,55 which collects these data annually, suggested that the correlations between measures taken approximately 1 year apart are high and relatively stable (0.85 to 0.89). However, in the BRiMS feasibility trial, the point estimate of the correlation between baseline and 27 weeks (± 1 week) was 0.59, with a one-sided 80% lower bound of 0.50. The base case for the sample size calculation below assumes a correlation of 0.6, with calculations also shown for correlations of 0.5 and 0.7.
Finally, an allowance is made for the estimated follow-up rate at 6 months. Previous studies suggested follow-up of approximately 80%; however, the follow-up rate in the BRiMS feasibility trial was 78.6% (95% CI 65.6% to 88.4%) and so the target recruitment rates below assume a follow-up rate of 70%.
In summary, the base case assumes detecting a between-group difference of 5.2 units, a SD of 23 units, a cluster size (intervention group only) of five participants and an ICC of 0.05; correlation between baseline and 6-month scores of 0.6; follow-up rate of 70%; and with two-sided 5% alpha and 90% power. The sample size calculations were undertaken in Stata using the clsampsi package (based on Roberts and Roberts157) before adjustments were made for the baseline/6-month correlation and the loss to follow-up rate.
Based on Table 23, the number of participants required to be followed up at the 6-month primary end point ranges from just over 600 to nearly 1100, before allowing for the correlation between baseline and follow-up, which reduces the equivalent numbers to a range of 400 to around 700. Table 23 illustrates that the main drivers of the total sample size required are the SD and the correlation between baseline and follow-up; varying the ICC or cluster size has a smaller impact.
| Calculation | Between-group difference at 6 months | SD | Cluster size | ICC of intervention group | Unadjusted total sample size required to be followed up | Total sample size required to be recruited – adjusted for 30% LTF | Correlation between baseline and 6-month MSWS-12vs2 | Total sample size required to be followed up – adjusted for correlation | Total sample size required to be recruited – adjusted for correlation and 30% LTF |
|---|---|---|---|---|---|---|---|---|---|
| Base case | 5.2 | 23 | 5 | 0.05 | 912 | 1303 | 0.6 | 584 | 836 |
| Vary ICC | 5.2 | 23 | 5 | 0.075 | 954 | 1363 | 0.6 | 611 | 876 |
| 5.2 | 23 | 5 | 0.100 | 995 | 1421 | 0.6 | 637 | 914 | |
| 5.2 | 23 | 5 | 0.125 | 1040 | 1486 | 0.6 | 666 | 951 | |
| Vary SD | 5.2 | 19 | 5 | 0.05 | 625 | 893 | 0.6 | 400 | 576 |
| 5.2 | 21 | 5 | 0.05 | 761 | 1087 | 0.6 | 487 | 697 | |
| 5.2 | 25 | 5 | 0.05 | 1080 | 1543 | 0.6 | 691 | 987 | |
| Vary correlation | 5.2 | 23 | 5 | 0.05 | 912 | 1303 | 0.5 | 684 | 979 |
| 5.2 | 23 | 5 | 0.05 | 912 | 1303 | 0.7 | 465 | 666 | |
| Vary cluster size | 5.2 | 23 | 6 | 0.05 | 936 | 1337 | 0.6 | 599 | 856 |
| 5.2 | 23 | 7 | 0.05 | 956 | 1366 | 0.6 | 612 | 878 |
Programme feasibility: BRiMS process evaluation (objectives x–xii)
Application of process evaluation plan
Fidelity assessment
The fidelity assessment plan was devised to ensure coverage of all four sites and session types (Table 24). A greater number of assessments of sessions undertaken in site 4 were carried out as two of these were used for moderation between the three assessors (assessor 3 needed to remain blinded to the group allocation of the south-west participants). The assessors confirmed that they felt that sufficient sessions had been evaluated to enable recommendations to be made. Undertaking the assessments using audio-recordings was time-consuming, and at times challenging, because of difficulties hearing details during group interactions, and not being able to pick up on the non-verbal elements of communication that could be significant when assessing one-to-one interactions.
| Session type | Sessions undertaken, n | Assessed for fidelity, n (%) | Mapping by site, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | |||||||
| Undertaken | Assessed | Undertaken | Assessed | Undertaken | Assessed | Undertaken | Assessed | |||
| Initial assessment | 28 | 8 (29) | 5 | 1 | 9 | 2 | 4 | 2 | 10 | 3a |
| Home visit | 28 | 7 (25) | 5 | 1 | 9 | 2 | 4 | 1 | 10 | 3 |
| Group sessions | 17 | 5 (29) | 3 | 1 | 5 | 1b | 3 | 1 | 6 | 2 |
| Total face-to-face contacts | 73 | 20 (27) | 13 | 3 (23) | 23 | 5 (22) | 11 | 4 (36) | 26 | 8 (31) |
Participant interviews
A total of 13 participants were interviewed within the process evaluation; the relevant data are detailed in Participant experiences.
Therapist focus group
All four treating therapists contributed to the telephone focus group (Table 25), which was undertaken 3 weeks after the final programme delivery had been completed.
| Therapist code | Profession | Sex | Years of experience | Clinical specialty | Number of BRiMS deliveries |
|---|---|---|---|---|---|
| T1 | Physiotherapist | Female | 18 | Neurorespiratory and community rehabilitation | 1 |
| T2 | Physiotherapist | Female | 35 | Neurorehabilitation | 2 |
| T3 | Physiotherapist | Female | 21 | Community/neurorehabilitation | 1 |
| T4 | Physiotherapist | Female | 15 | Neurorehabilitation | 2 |
Summary
The fidelity assessment and qualitative aspects of the process evaluation were undertaken according to plan, and the stated aims were achieved. Listening to audio-recordings was time-consuming, but the recordings were of sufficient quality to allow an effective assessment to be undertaken, and the recorders were only minimally intrusive for both participant and therapist.
Findings
The findings are presented below using the process evaluation framework that informed the coding template used in the analysis. Issues were systematically explored regarding overall experiences, programme implementation, mechanisms of impact and context.
Overall experiences
All four therapists and most participants reported that they enjoyed being part of the programme and, although they highlighted improvements that could be made, could see value in its aims, approach and content:
. . . very positive and I have to say a very good feel-good factor about the study as well.
P8
I thought it was a wonderful concept that was really, well, it was incredibly thorough and the resources at last have gone beyond physio tool handouts and it enabled [a group of] people with MS . . . who lived in really as far apart as you could from each other, it enabled me to deliver a really high-quality content.
T1
The positive perceptions among both therapists and participants were tempered by some who expressed frustration at specific design elements of the programme, and feelings of being overwhelmed by the volume and type of content to be delivered, particularly at the beginning. For others, however, this was expressed as an issue more of pacing and presentation than of overall quantity:
There was a bit too much information.
P10
I think at the moment it lacks coherence in terms of the two web-based elements being not integrated as a whole would be one comment, that it feels a bit like bits rather than a real seamless thread.
T2
Implementation of the BRiMS programme
Here we explore the feedback from participants and treating therapists about their experiences of delivering and attending the BRiMS programme, and of using the resources available within BRiMS. Feedback about specific BRiMS sessions is also included in this section.
Attendance rates
As part of the BRiMS intervention, each participant had two individual sessions (clinic and home visit) and three group sessions, giving a total of five face-to-face sessions with a treating therapist. Therefore, there were a potential 150 face-to face sessions (five sessions × 30 participants). Summing all of the completed face-to-face session attendances gives a total of 95 (63.3%) successfully completed. Attendance rates for the one-to-one sessions were considerably higher than those for the group sessions (Table 26).
| Session | Attendees (n) | Randomised (%) |
|---|---|---|
| BRiMS visit | ||
| Clinic | 28 | 93.3 |
| Home | 27 | 90.0 |
| Group session | ||
| Week 4 | 23 | 76.7 |
| Week 8 | 10 | 33.3 |
| Week 13 | 13 | 43.3 |
Discussion
As with other programmes with occasional group sessions, attendance was variable over time and between participants. 30 The majority of the interviewees who attended the group sessions reported finding them beneficial and that the sessions supported their engagement with the more independent parts of the programme. Some feedback suggested that reduced or non-attendance did not necessarily equate to a lack of engagement; some participants who did not attend all of the group sessions still reported positive experiences with the programme. This may be because educational material was available in the paper manual provided to all participants at the first session, or because the information most critical to their personal falls management was obtained in the sessions attended. Further exploration of ‘how much is enough’ for these types of programmes is required.
The decision to run the group sessions with small cohorts was based on the need to ensure safety during the exercise components of these sessions, and to maximise opportunities for participants to interact effectively. However, the findings of this feasibility trial suggest that the current format may not be optimal, either for effective use of the therapist‘s time or to maximise the benefits reported from peer interaction. Consideration of alternative formats (e.g. a single longer workshop, rather than several sessions) is warranted.
Programme delivery
Experiences of delivering the programme
The therapists reported positive experiences of delivering the programme, including their use of the programme resources and their interactions with participants. However, they did express frustration and disappointment when they perceived that participants were not engaging fully with the programme:
I enjoyed reviewing their exercises online and the follow-up e-mails and so on, that was a really constructive way of delivering that information, so for me there was some design aspects of the actual site, but overall I really enjoyed working with them as tools.
One of the things I really enjoyed was the home visits, and to be able to set up the exercises and use what people had in their own homes.
I felt really frustrated when people, when some people weren’t giving it [online exercise programme] a chance, but it wasn’t the package per se it was the individuals not giving the internet a chance.
Fidelity to the spirit of BRiMS
Four key attributes and behaviours were defined as core to the delivery of BRiMS, and these were evaluated in every fidelity assessment. The majority of scores indicate that the items were addressed well by therapists, although there was scope for additional depth and detail.
The collated results of the assessments for these four items are detailed below. In addition to the fidelity scoring, assessors provided written qualitative feedback to support judgments where those judgements had been less than straightforward, and where they thought this was necessary.
Facilitation style
Facilitation style scores are given in Table 27.
| Score | Assessment | |||||
|---|---|---|---|---|---|---|
| Initial (n = 8) | Home visit (n = 7) | Group 1 (n = 1) | Group 2 (n = 2) | Group 3 (n = 2) | All (n = 20) | |
| Median (IQR) | 2 (2–2.25) | 2 (1.5–3) | 3 (0) | 2.5 (2.25–2.75) | 2 (1.5–2.5) | 2 (2–3) |
Therapists were described as encouraging, respectful, positive and warm in their interactions (e.g. records 21, 61 and 161), with evidence of a positive rapport being built between therapist and participants in both individual (record 121) and group (record 181) sessions. There were examples of specific techniques being used to encourage participants to engage actively with the session (e.g. records 51 and 81) and also of therapists needing to utilise high-level skills to support and facilitate participant interaction (e.g. record 141). Lack of time in sessions was felt to result in missed opportunities.
Utilisation of multiple sclerosis-specific knowledge and expertise
Utilisation of multiple sclerosis-specific knowledge and expertise scores are given in Table 28.
| Score | Assessment | |||||
|---|---|---|---|---|---|---|
| Initial (n = 8) | Home visit (n = 7) | Group 1 (n = 1) | Group 2 (n = 2) | Group 3 (n = 2) | All (n = 20) | |
| Median (IQR) | 3 (2.5–3) | 2 (2–2.75) | 2 (0) | 2.5 (2.25–2.75) | 3 (0) | 2.5 (2–3) |
Feedback suggested that MS-specific issues were recognised and discussed where required, with evidence of tailoring of exercise to address issues such as heat sensitivity and fatigue (records 132 and 172).
Developing and maintaining challenge and motivation
Developing and maintaining challenge and motivation scores are given in Table 29.
| Score | Assessment | |||||
|---|---|---|---|---|---|---|
| Initial (n = 8) | Home visit (n = 7) | Group 1 (n = 1) | Group 2 (n = 2) | Group 3 (n = 2) | All (n = 20) | |
| Median (IQR) | 2 (2–2.5) | 2 (1.5–2.5) | 3 (0) | 2 (0) | 2 (1.5–2.5) | 2 (2–3) |
Written comments identified that therapists utilised an encouraging and supportive approach (records 123, 173 and 192). However, four out of the six records that had comments relating to this topic also identified additional opportunities to support motivation and engagement.
Supporting self-management skills and autonomy
Supporting self-management skills and autonomy scores are given in Table 30.
| Score | Assessment | |||||
|---|---|---|---|---|---|---|
| Initial (n = 8) | Home visit (n = 7) | Group 1 (n = 1) | Group 2 (n = 2) | Group 3 (n = 2) | All (n = 20) | |
| Median (IQR) | 2 (1–2) | 2 (0) | 3 (0) | 2 (0) | 2.5 (2.25–2.75) | 2 (1.5–2) |
Assessors noted that peer support was used proactively to encourage participants to problem-solve during group sessions (records 154 and 164), although more time and space in sessions to enable participants to consider and develop their ideas may be valuable in future (records 24, 34 and 44).
Discussion
Feedback from the fidelity assessments and qualitative interviews suggests that the therapists needed to utilise high-level skills to establish an effective relationship with participants and to deliver the key elements of the programme. Feedback from participants was positive, but some of the evidence from the process evaluation suggests that the aim of supporting participants to take the lead in how, when and the way in which they engaged with the programme could be improved. For instance, it appears that many of the participants relied on the therapists to guide the progression of their exercise programme. Despite the reviews being scheduled regularly, the quality of information that patients provided to the therapists was often limited, potentially resulting in missed opportunities to maximise the amount of ‘highly challenging’ exercise undertaken. There is a need to explore ways of encouraging better use of exercise resources to ensure that the exercises remain challenging and that participants are able to progress without sustained face-to-face supervision from therapists. It is also important to consider how the BRiMS programme could further enhance a collaborative approach that supports self-management. Evidence from other settings and other conditions suggests that this is an ongoing challenge in clinical practice that requires a paradigm shift among many rehabilitation professionals. 162 It is acknowledged that effective training and support is essential to achieve this. 163,164
Factors affecting delivery
The therapists acknowledged that there were elements of the programme that they were less familiar with (e.g. the functional imagery training), which presented challenges. They found the peer support provided through the BRiMS website a valuable resource, and those therapists who ran two deliveries of the programme reported that familiarity did help their confidence and the ease of programme delivery. There was a recommendation from all therapists that more initial training was required than was delivered in this trial, particularly training that focused on less familiar areas:
I think that’s where my skills very quickly ran out because I didn’t have the, um, background to then be able to counsel them into a more positive attitude; I think on the whole I felt I needed more help to present the imagery.
T1
. . . by the second group, actually I felt much more comfortable with it, and so I could see that how initially in running a programme within an NHS setting you’d need the induction and the training etc., but actually fairly soon it will get embedded into practice fairly reasonably.
T2
The therapists highlighted that they consistently needed more time than was allocated to deliver the programme content and provide effective feedback to participants. The therapists also recommended that the duration of the overall programme should be reviewed to consider whether or not extending this would enable participants to experience more meaningful changes in ability:
On the home visit I consistently took one and a half hours really for that rather than the one that was allocated – I couldn’t get it any shorter than that and still be reasonably polite.
T3
Discussion
Therapists reported positive experiences of the programme, and perceived the novel format and delivery style to have many advantages. Even though the therapists we recruited were all highly experienced, there were elements for which the need for more training and support was highlighted, both in the fidelity assessment and by the therapists themselves. The therapist training session in this trial was kept short to minimise excess treatment costs and to enable all therapists to be trained at the same time (as one therapist had had to fly from Ayrshire to the south-west to attend). However, our recommendation for future trials of the programme is that a longer training session is essential. The use of a discussion board was appreciated by the therapists, which presents an opportunity for the development of further online support, such as peer-supervision sessions and debriefs.
Future deliveries of BRiMS should also be costed to reflect the actual time needed to deliver the programme, as this was underestimated in this trial. In particular, the therapists required more contact time for the one-to-one sessions than was originally allocated [see Intervention costs (objective xiii)]. A mobile version of this website should be considered, as it has the potential to simplify the logistics of some of the home visits.
Sessions and resources
BRiMS education resources (BRiMS website and paper manual)
This section analyses participant’s experiences of using the education resources associated with BRiMS, including the online education programme and its accompanying paper manual.
The therapists valued the BRiMS website resources, although some felt that the logistics of the online provision were challenging:
The BRiMS package, again it was very helpful, but the one thing that was very frustrating was not being able to jump ahead without saying that you’ve completed where you were – because I wanted to skim through quite a lot to make sure I knew what I was doing and I got a bit frustrated with having to say that I’d read it and stuff, but it was really well laid out and it contains all of the resources we needed and the fact that all the audio was uploaded on that.
T1
Participant engagement with the educational elements of BRiMS was variable, as evidenced by the website data (Table 31).
| Element | n (%) |
|---|---|
| Total number of logins | |
| Median (IQR) | 5 (4–13) |
| Minimum–maximum | 0–85 |
| Component | |
| Exercise advice | 15 (50.0) |
| Home package 1 (week 1) | 16 (53.3) |
| Home package 2 (weeks 2–4) | 12 (40.0) |
| Home package 3 (weeks 5–8) | 9 (30.0) |
| Home package 4 (weeks 9–13) | 7 (23.3) |
Participants’ feedback highlighted a number of elements that they found useful, both in the online resources and in the group sessions. However, there were mixed feelings about some of the activities, and, at times, about the approach used:
But I put down, ‘I did enjoy the online part’, and I mean the exercises and the online BRiMS. You’re working through that, I did like that and I liked the fact that you could dip in and out of it.
P13
I found the [education] exercises . . . but then I’m an ex-primary school teacher, we did them with primary school children. I did find that difficult to do exercises like that.
P5
Participants identified the exercises that encouraged self-reflection and evaluation to be particularly of value; however, the paper manual that accompanied the online resources received mixed reviews, with a general sense that the amount and format of the information provided needed to be simplified. It was identified that preferences over information format varied between participants:
The part of the manual that I found which is kind of surprising, I’ve had MS for 29 years and I think to myself ‘you are getting to know it by now’, but it’s one of those things you never know. But there were parts of the manual I really thought were important in there so for me and those were the bits that were throwing things back at you ‘what about this and how do you do this and do you feel about that’ and I thought I should know that, but actually writing it down where I had oh I did this today, kind of how was it for you.
P13
There was a big booklet the yellow pages, I think. I couldn’t begin by reading all that stuff, there were too many words and too complicated.
P9
Discussion
As with many programmes that use online delivery methods, we saw variable patterns of engagement among participants, and a drop-off over time. 44,113 The findings of our process evaluation suggest that the BRiMS education resources need to be reviewed, in particular to ensure that the amount of falls prevention content is rationalised and that the delivery style is further optimised for online delivery to a UK-based audience. As BRiMS utilised resources from an existing MS falls-prevention programme, it was not considered appropriate to omit elements of this programme without first identifying what worked and what did not in this new format. Having undertaken our feasibility trial, feedback from our participants and therapists has identified a number of areas in which content changes could be made, and adaptations to the format could make it more engaging. Simple changes, such as reducing repetition and ensuring that participants can progress easily through the online resources, could reduce the frustration and disengagement brought about by the logistical issues our participants encountered.
Exercise activities
This section looks at the exercise programme and investigates the engagement with the BRiMS online programme. Therapists and participants generally viewed the exercise component of BRiMS positively, and felt that the flexibility it offered was helpful. Participants appreciated feeling in control of their activities, compared with a more prescriptive approach. The combination of one-to-one, online and group support was seen to be helpful in maintaining engagement:
I thought the web-based exercises were wonderful, I thought they were a really brilliant resource.
T1
One of the good things is that it is very flexible, so if you wanted to do it anytime in the day you could do it . . . You put the person in the control and that’s a very good feeling as well as opposed to ‘you must show up on Monday morning at 9 o’clock to do this’. Whatever day you were having you chose the time to do it.
P8
I actually found it OK. Which almost surprised me, because you know, continuing exercises is quite difficult on your own, but I think the fact that you had something to look at or other people; it sounds a little bit weak really, but other people to do it with but when you are watching on the program I found it very helpful and without it I wouldn’t have continued for 12 weeks.
P10
The fact that the video exercises were filmed with people with MS helped participants to engage with the programme:
The people that were actually doing the exercises weren’t 18-year-old stick insects. They were the likes of you and me, they were people who you could see that it wasn’t easy necessarily for them to do it as well. So again that’s a good psychological side for us as the recipient. To think you are not being shown by some super-fit person, somebody else challenged like yourself is doing it . . .
P8
Although the exercise videos were available online and participants were requested to log their exercises online, some preferred to use the paper manual for guidance:
Interestingly most of my people favoured the paper manual over the electronic version. So they, they tended to take the paper manual with them into different rooms, and if they haven’t have had the manual . . . they didn’t really engage so well at all with the electronic versions.
T2
Documentation of exercise use began in week 2 of the programme and ended in week 13 to obtain the required 12 weeks of exercise. Some participants reported that they found the accumulative online diary a useful way of logging their exercise, while others reported challenges with the logistics of using the diary to log exercise. Quantitative data on the usage of the exercise programme were available for 27 out of the 30 participants (90.0%). The number of participants who commenced BRiMS was taken from the number who completed a clinic visit during which the participant’s online exercise programme was individualised.
Online login data (Tables 32 and 33) show that the pattern of logging exercise was variable, with five participants (17% of those randomised) entering data on only 1 of the possible 12 recording weeks. By contrast, 10 participants (32% of those randomised) entered data on ≥ 10 weeks. As with the education programme, the number of participants logging data reduced over time, although patterns of use varied among participants: some dipped into and out of the programme over time, some did not enter data after the initial weeks and some logged exercise consistently.
| Cumulative number of weeks of entered exercise data | Participants (n) | Commenced BRiMS (n = 28) (%) | Randomised (n = 30) (%) |
|---|---|---|---|
| 1 | 5 | 18.5 | 16.7 |
| 2 | 1 | 3.7 | 3.3 |
| 3 | 3 | 11.1 | 10.0 |
| 4 | 2 | 7.4 | 6.7 |
| 5 | 0 | 0 | 0 |
| 6 | 1 | 3.7 | 3.3 |
| 7 | 1 | 3.7 | 3.3 |
| 8 | 1 | 3.7 | 3.3 |
| 9 | 3 | 11.1 | 10.0 |
| 10 | 4 | 14.8 | 13.3 |
| 11 | 2 | 7.4 | 6.7 |
| 12 | 4 | 14.8 | 13.3 |
| Programme week number | Participants (n) | Commenced BRiMS (n = 28) (%) | Randomised (n = 30) (%) |
|---|---|---|---|
| 2 | 23 | 82.1 | 76.7 |
| 3 | 20 | 71.4 | 66.7 |
| 4 | 21 | 75.0 | 70.0 |
| 5 | 18 | 64.3 | 60.0 |
| 6 | 16 | 57.1 | 53.3 |
| 7 | 14 | 50.0 | 46.7 |
| 8 | 11 | 39.3 | 36.7 |
| 9 | 13 | 46.4 | 43.3 |
| 10 | 12 | 42.9 | 40.0 |
| 11 | 11 | 39.3 | 36.7 |
| 12 | 13 | 46.4 | 43.3 |
| 13 | 10 | 35.7 | 33.3 |
Therapists reported that they were confident about developing exercise prescriptions using the BRiMS exercise menu and about uploading them to the web-based physiotherapy resource, but also that this took more time than they had anticipated, and was challenging, as the two online systems did not fit together:
I think from the BRiMS exercise menu if it tied up more easily with the [online] site that would be more helpful, and if maybe the exercises, where you’ve got some progression [they’re] not always on the site – things like carrying a tray with a cup of water and things like that, so that won’t be in the video, but I did find, mine all enjoyed using the site to record their exercises.
T3
Evaluation of the online exercise programme data indicates that the participants undertook exercises from across the six BRiMS exercise categories, suggesting that the therapists utilised the prescription principles appropriately (Table 34 and Figure 12). Participants also logged exercise substitutions throughout the programme, despite these being ‘formally’ introduced only at week 8.
| Period | BRiMS exercise categories, median (IQR) | ||||||
|---|---|---|---|---|---|---|---|
| Height changes | Reaching | Sit–stand | Standing | Stepping | Substitution | Walking | |
| Programme (weeks 3–13) | |||||||
| Participants (n) | 13 (11–15) | 13 (11–15) | 11 (9–15) | 12 (11–15) | 12 (11–16) | 6 (4–8) | 10 (10–14) |
| Minutes/participant/week | 10 (6–20) | 12 (8–20) | 12 (6–18) | 16 (8–22) | 18 (9–24) | 27 (20–46) | 10 (7–17) |
| Follow-up (weeks 14–27) | |||||||
| Participants (n) | 5 (3–5) | 4 (3–5) | 4 (3–4) | 4 (3–4) | 4 (3–5) | 3 (2–3) | 4 (3–4) |
| Minutes/participant/week | 6 (4–12) | 10 (5–15) | 13 (7–19) | 11 (6–16) | 13 (8–16) | 47 (25–55) | 9 (6–14) |
FIGURE 12.
Proportion of exercise activity logged per week by category. (a) Weeks 3–13; and (b) weeks 14–27.
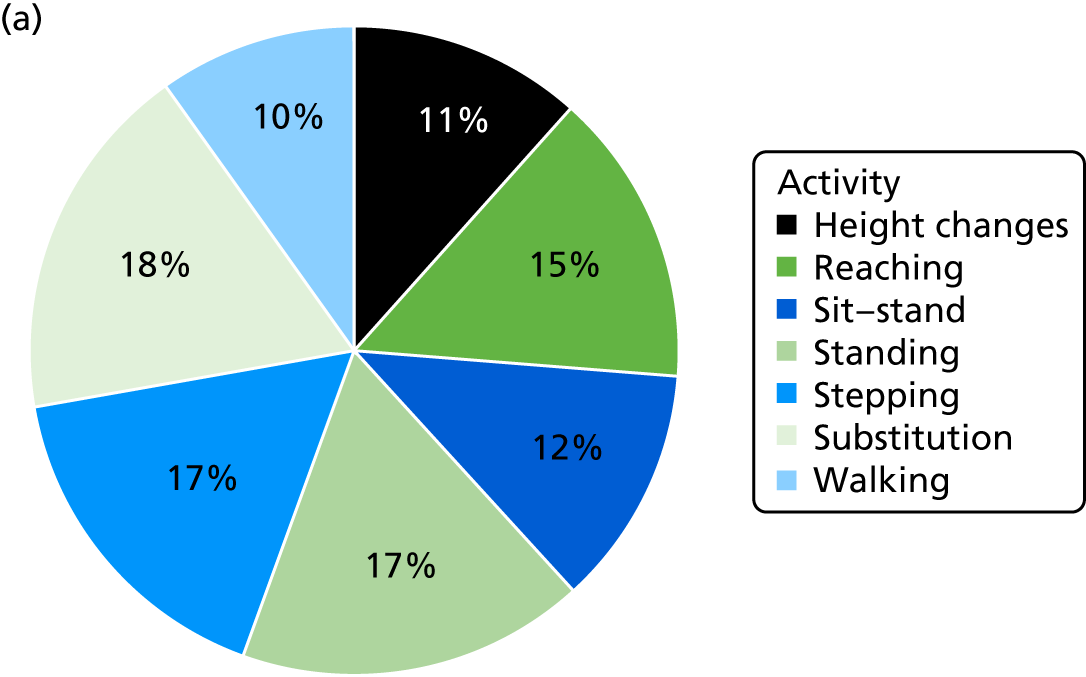
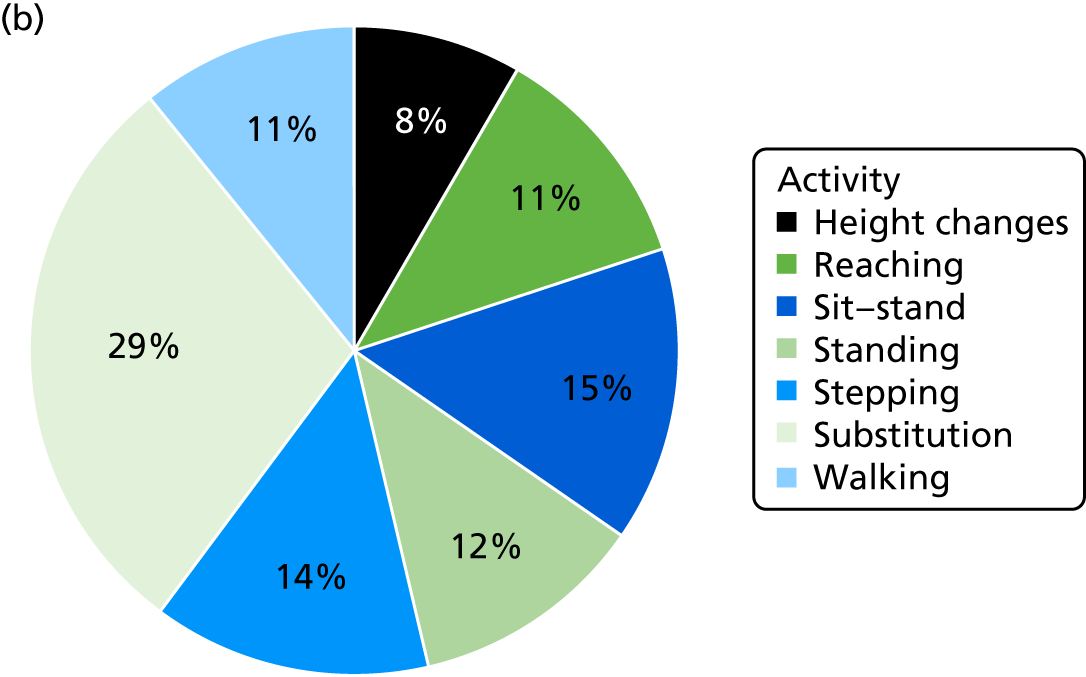
Logged minutes of exercise
Table 35 shows the time logged by participants during the 12 weeks of the intervention. Participants were asked to undertake 120 minutes of exercise per week, which some considered too demanding, particularly early in the programme:
I think a lot of my people were commenting that 120 minutes initially was a bit like wow! One person in particular felt that that was a lot and it should have been may be titrated up initially.
T3
| Exercise time per week | Weeks attempted | 12 weeks |
|---|---|---|
| Exercise time per week (minutes) | ||
| Mean (SD) | 78.2 (70.8) | 48.9 (44.7) |
| Median (IQR) | 68.4 (21.3–119.3) | 35.5 (8.4–96.3) |
| Minimum–maximum | 0–357 | 0–126.6 |
| Mean exercise per week (minutes), n (%) | ||
| ≥ 120 | 6 (22.2) | 3 (11.1) |
| 100–119 | 4 (14.8) | 3 (11.1) |
| 80–99 | N/A | 2 (7.4) |
| 60–79 | 4 (14.8) | 1 (3.7) |
| 40–59 | 3 (11.1) | 3 (11.1) |
| < 40 | 10 (37) | 15 (55.6) |
| Minimum exercise per week (minutes), n (%) | ||
| ≥ 120 | 1 (3.7) | N/A |
| 100–119 | N/A | N/A |
| 80–99 | 1 (3.7) | N/A |
| 60–79 | 1 (3.7) | N/A |
| 40–59 | 5 (18.5) | N/A |
| < 40 | 19 (70.4) | N/A |
Perceived change and achievement were significant factors influencing motivation to continue exercising; participants highlighted the importance of feeling that they were making progress by using a target/timer to record this on the exercise website, or by exercises being changed as the programme progressed:
I never got back. So I think it would have been much better to start with just a few small things, build up. My exercises were never changed so I never felt I was progressing. And that psychologically had an effect on me because I knew I wasn’t quite good enough. I hadn’t done them well enough. So psychologically I think it would be better to start small and say ‘you can ask for more and we can offer you more as we go through’, and I think that would have been a real help actually.
P5
Therapists reported that use of the online exercise resource varied among their participants. A number of logistical issues were highlighted, including the use of the timer, free-text and ‘exercise completed’ boxes and timer rollover dates. They also reported that some participants engaged with their exercises but did not use the web-based resource to log their exercise activity, and that others needed paper handouts because of logistical challenges or IT problems. Participants expressed a range of preferences for the format of the exercise resources, with some users preferring paper handouts over video for their exercises. For others, the combination of paper, video and audio contents worked well. This is important as it calls into question the validity of the online diary records of these participants:
I think in part that was also, some of them found it a bit challenging, you had to tick a box to say you had completed the exercise and if you hadn’t done that then, even though you’d filled out the number of reps, etc., it hadn’t logged that the exercise had been done and also the time, so that they might press the time and then they’d forget to stop the timer and so it looked as if they’d done like 2 hours, well maybe not 2 hours but an hour of sit to stand!
T2
[Paper exercise sheet] Absolutely fine, you know I was able to do it far better than I would have been trying to watch a video. It just doesn’t work for me, I’m sure it works for most, well lots of people, but it doesn’t, I need to have it there and be able to keep looking at it.
P4
They seemed to feel quite confident with experimenting a little bit with their exercise without having to refer back to the video, and with that came the fact that they weren’t logging in so much and because they weren’t logging in so much they then weren’t logging how much exercise they were doing. So even though they were telling me via e-mails that they were exercising, and when they came back to the class they were exercising, actually I couldn’t witness that on the web-based logins.
T2
Participants were extremely positive about the opportunities for group exercise in the programme, with some suggesting that more regular group exercise sessions would have been beneficial. Similarly, participants valued the support from the programme therapist as a source of challenge and encouragement to try exercises that they would otherwise have viewed as too difficult:
I certainly would have preferred more group exercise work, if it could have been a bit more, once a week would have been great, once a fortnight maybe so that it would be possible to just share a little bit also with the people, I know it is kind of easier to do it when someone’s you know giving you ideas of what to do and keeping you at it.
P4
Yeah, yeah it pushes you into it more you know, I thought I’ll never be able to do it but having somebody to sort of instruct you and say ‘right come on let’s do this and that’, it really, really pushes you, you know, it sort of spurs you on sort of thing you know.
P6
Therapists also viewed the group exercise elements positively; however, one fidelity assessor queried whether or not managing the group exercise with one therapist and a number of participants was difficult in terms of managing risk while maintaining challenge.
Conversely, participants reported that they had successfully integrated their exercises into their daily life, and recognised the value of being able to exercise at home:
Yes I think that being left alone in the home rather than having to go out and do the exercises is a good thing but the group sessions make you to keep going.
P11
Discussion
Feedback from treating therapists and participants suggests that the validity of the exercise logs should be viewed with caution. First, there were logistical challenges with recording exercises; for example, some participants exercised in one room and then had to move to another room, where their computer was, to record the exercises, or they had to remember to start and stop the exercise timer for each exercise. Second, preferences about resources to support exercise engagement were variable, with some participants preferring paper exercise sheets to the online system. According to participants and therapists, several participants appeared not to be engaging with the programme at all, but were, in fact, exercising regularly. This is a significant limitation to the feasibility of using this method of recording exercise engagement in both a clinical and a trial situation, and one that is a priority to address before implementing a future trial. It is likely that increasing the ease of use of the BRiMS online resources could go some way to addressing this, but the findings of the qualitative elements of the process evaluation suggest that some people may still prefer to use ‘offline’ resources.
Maintaining challenge in exercise practice
Therapists reported varied experiences with participants adapting their exercises as recommended, with some participants using the resources once or twice and then feeling confident to ‘do their own thing’ and experiment, and others relying on the therapist to lead their exercise activities:
On the site, they reckon that once you get into a particular exercise then it becomes easier, they give you hints as to how to work it harder because the whole point is making it harder so you get better.
P11
Mine tended to watch the videos once or twice but then they felt they had a handle on the exercise, so they then as they understood their exercise and as they really understand the notion that they needed to be challenging.
T2
In general it made me think about exercising little and often and every day if possible and I did something which I don’t think is particularly in the programme, but I incorporated quite a few of the exercises in daily life . . . I do it every day and every time I think of the BRiMS exercises and so I was able to, you know, and standing by the kettle waiting for the kettle to boil, I am able to stand up on my toes so I did find that very useful.
P10
BRiMS exercise programme reviews
Therapists were asked to review the participants’ progress with their online exercise programmes on six occasions, making adjustments/progressions as appropriate. Table 36 details the number of reviews that were reported by the therapists.
| Programme review | Completed (n) | Randomised (%) |
|---|---|---|
| 1 (week 3) | 26 | 86.7 |
| 2 (week 5) | 26 | 86.7 |
| 3 (week 7) | 25 | 83.3 |
| 4 (week 9) | 23 | 76.7 |
| 5 (week 11) | 21 | 70.0 |
| 6 (week 13) | 20 | 66.7 |
The extent of feedback provided by participants on the website was variable, and this had an impact on therapists’ ability to progress the exercises. Evaluation of the web-based exercise logs showed that only 2444 (35%) of the 7063 separate exercise entries recorded over the trial period included comments added in the diary section. Of these, 1599 (23% of the total entries) had comments that could enable the therapist reviewing the programme to assess the participant’s progress. The remaining 845 comments were general in nature (e.g. ‘OK’). Unsurprisingly, the majority of the comments (98%, n = 2397) were added during the intervention period, which was when programme therapists were reviewing the diary entries.
In many instances, therapists had to rely on participant feedback by other means, including e-mail. The scheduling and formatting of therapist reviews was also highlighted as a potential issue if participants logged problems using the online diary in a week when no therapist review was scheduled:
Had I not had the e-mail correspondence alongside the comments in the diary I would have struggled to progress their exercises, but because I had the e-mail where they seemed to be more willing to chat about what they were doing, then that combination worked well and I felt that I was able to progress them.
T2
It could have been just coincidence but there was the two weeks where you didn’t check their programme, and one of those weeks unfortunately was one of the weeks when my ladies really struggled and was really not well at all, so I didn’t pick that up until a week later, and I felt really bad at that.
T4
Use of imagery techniques
Therapists reported varying experiences with using functional imagery training (FIT) to improve motivation and maintain engagement, and there was a mix of feedback about participants’ experiences with using the techniques. Some participants highlighted positive experiences, particularly those who were aware of the approach or had previous experience of similar techniques:
One lady it was a very specific issue, it was getting in and out of the car to get around to the boot to be able to lift the boot to be able to get a wheelchair out, and for her the imagery worked really really well.
T4
Yes that was OK for me. That I think, because I have done the mindfulness course and I go to a mindfulness group once a month so I’m kind of used to it but I think, I know at one of the group meetings, the other participant it was new to her and she was embracing it and she was enjoying it. I’m used to it, it wasn’t a new thing for me but it was a new thing for her and it made me think that obviously it’s going to be a new concept for a lot of people and it’s not everybody’s thing either.
P13
Both therapists and participants described feeling more ‘comfortable’ with the other elements of the programme (e.g. exercise training) and appeared to place more focus on these activities. Some participants highlighted that the physical achievement of goals was a key motivator, rather than using imagery to visualise any achievements beforehand:
In terms of them practising the imagery to enhance their adherence to exercise, that came less naturally to them, but they seemed to manage that if I facilitated them quite a lot . . . but when I went back to on home visit that’s not the one they chose to practice.
T2
OK, one of the things I wanted to do was to be able to get up more gracefully, which sounds very pretentious but you know, more confidently, better all round and I do remember being in situations where I was getting up and thinking that is better than it used to be. That positive association, but not the sort of sitting down and visualising you are doing it and the benefit you are going to feel from having done it. It was more seeing the benefit of it was happening, that I was doing something.
P8
The therapists consistently expressed concern that their lack of familiarity with the technique had an impact on participant perception, and all the therapists said that more training and practice would have been valuable prior to running the programme. Fidelity assessors identified examples of good practice in delivering the imagery elements, including eliciting strong images to support goals (record 3) and encouraging participants to continue with imagery by highlighting progress (record 93). However, it was suggested that greater emphasis and reinforcement could have been used to support imagery practice in some instances (records 84, 103 and 104):
It was always something that I, perhaps didn’t fully to commit to because I perhaps underestimated its worth or perhaps didn’t understand its worth . . . I partnered up with [T2] on the [training] day and I really remember our conversation about it because I was thinking ‘this is new’ and ‘I’ve not done this before’, so I think a little bit more practical time really to play around with the language in the words and the concept.
T1
Despite the challenges, the therapists reported positive experiences of using the technique, particularly in the early sessions, when participants were guided to apply imagery to their personal goals:
So I think I’ve owned up to the fact that I probably initially in that first session didn’t spend enough time going through the nuts and bolts of practising the imagery, but what they did do was spend time really getting the feel for imagining their goals, and for me that was a really nice opportunity to open up a discussion about their expectations of what they might hope to be able to achieve really, how they envisioned themselves at the end of the programme.
T2
Some participants reported that, although they tried the imagery, they did not find it useful. One of the participants with significant previous experience acknowledged that the approach may not suit everyone, and that further support and time spent explaining and practising the technique could have been valuable:
So I wondered if it would be more useful to even have that separate because I felt it was just ‘straight into it’ and I thought even if there was maybe a half hour class or just a wee introduction, ‘here’s what it is and here’s what we hope you will get from it’, rather than just straight on in to it. It didn’t put me off because I am used to it but I can see it putting off some people because it’s like ‘hang on a minute, where did that come from?’, when there isn’t an introduction to it particularly useful . . . Just a half hour or something attached onto you know a group or whatever.
P13
Discussion
The integration of functional imagery training into the BRiMS programme is a novel technique, and one that was unfamiliar to therapists and most participants. The majority of participants in our qualitative interviews and focus group agreed that the principle of imagery was consistent with the ethos of BRiMS, and feedback from early one-to-one sessions was that the participants were able to engage with the technique when guided to do so. However, it appears that more ongoing support and facilitation to use the technique is needed, along with greater clarity on when and how to use it as part of BRiMS. The fidelity assessors suggested that therapists were trying to support participants’ motivation by using the goal-setting and imagery exercises specified in the manual, but that they sometimes struggled to do this effectively, leading to lower fidelity scores on relevant ratings.
Not surprisingly, exercise prescription was the main focus of sessions, and motivating patients to undertake the exercises sometimes seemed an optional extra, for participants as well as for therapists. This is unfortunate, because participants needed to be motivated to do their exercises, to challenge themselves appropriately, and to continue exercising after BRiMS ended. Effective motivational support could make a substantial difference to the efficacy of the programme; this is a particular priority given the finding that engagement in the BRiMS exercise programme appears to be significantly lower than that likely to be required to maximise improvements in mobility and balance.
Therefore, increasing the time to support therapists in becoming familiar with FIT and being able to use it confidently will be a priority when revising the BRiMS training session. Time is needed to develop therapists’ understanding of the difference that effective motivational support can make, for example by including some role play so that they can experience the techniques themselves, and helping them look more proactively for opportunities to use imagery and other motivational techniques during BRiMS sessions. Trainers need to recognise the considerable difference between the role of the therapist in BRiMS as a collaborator who works in partnership with patients to elicit and strengthen their motivation, and the more traditional view of the therapist as an expert providing advice and treatment.
It may also be helpful to include a specific session on motivation for participants, perhaps as a group session or as a web-based FIT session where they would independently work through some imagery-based motivational exercises. Either solution could help reduce pressure on therapists during the busy first home visit, and provide higher-quality motivational support, allowing them to get greater benefit from the other FIT exercises scheduled later in BRiMS.
Session-specific feedback
One-to-one sessions
Initial assessment
Fidelity scoring suggested that the main elements of this session were delivered appropriately, although there was scope for greater depth in places (Table 37). Assessors highlighted opportunities for a more collaborative approach to be adopted, particularly in the identification of an immediate goal (records 27 and 47) and in the choice of an exercise to practice at home (records 26 and 46).
| Session-specific items: initial assessment (n = 8) | Median score (IQR) |
|---|---|
| Provide an introduction to BRiMS, including the aims and structure of the programme | 3 (2–3) |
| Evaluate balance and mobility to inform exercise prescription | 2.5 (2–3) |
| Introduce the participant to initial exercise and imagery practice | 2.5 (1–3) |
| Help the participant identify something they would like to accomplish that could be achieved soon, or to practise imagining achieving a goal as vividly as possible | 2 (1–2.25) |
| Issue the BRiMS workbook, signposting the participant to the log-in instructions and home package 1 | 3 (0) |
| Confirm arrangements for subsequent appointments | 3 (2–3) |
Therapists reported that both they and their participants found the initial assessment session ‘overwhelming’ as a result of both the amount of content and the number of elements that were introduced. Therapists reported that explaining the session carefully, and breaking down the elements of BRiMS during the session, enabled them to support their participants to get to grips with what was expected. There were few specific comments about the one-to-one sessions from participants, possibly because of the time between the sessions taking place and the participants being interviewed. However, the general impression was that the sessions worked well and were valuable. There was no specific feedback about the amount of content in these sessions:
The initial assessment, it’s hard to remember that long ago. It was OK, there was nothing too untoward, too strenuous . . .
P9
I think people reach their capacity of what they were open to take on board relatively early in the session because we were doing lots of interviewing and starting homework early and then logins and things and I think some of mine sort of looked a bit frazzled by the end as well as me actually!
Yes, I’d agree with that, I think they got a bit saturated halfway through.
T1 and T3
I think once you spoke to them, encouraged them and broke it down then they were, quite a few of them were, then there were a few lightbulb moments and they were like ‘OK, yes, no I understand this’.
T4
Home visit
Although the average fidelity scores in this section suggest that most areas were covered appropriately, there was a greater range of scores than for the initial assessment (Table 38). Assessor comments indicate that the main area for further consideration is how the exercise plan is presented to the participant, including whether the exercise plan should be physically worked through in the session, or merely discussed with the participant (records 137 and 147). It was highlighted that, on occasion, therapists verbally suggested that they would progress the exercises, rather than handing control to participants (records 108 and 127). The comments also highlighted that participants were experiencing challenges with logging in and using the web-based resources, with varying degrees of success/engagement with the week 1 home package (records 99, 109, 129, 137 and 146).
| Session-specific item | Median score (IQR) |
|---|---|
| Deliver the exercise activity plan and ensure participant can carry out exercises safely and effectively | 3 (1.5–3) |
| Review and refine personal goals, and encourage participant to achieve these | 2 (1.5–3) |
| Explain personalised exercise prescription, including setting up online resource, and demonstrating/supervising exercises to ensure that participant is able to carry out plan safely and effectively (adjusting programme if necessary) | 2 (1.5–3) |
| Instruct participant re principles of exercise prescription, including structure of exercise practice (based on home package 1), balancing challenge and risk, and progressing the programme | 2 (1–2.5) |
| Review and problem-solve BAASE analysis and action plan (based on home package 1 scenario) | 2 (1.5–2) |
| Confirm arrangements for subsequent appointments | 2.5 (2–3) |
The home visit was very well received by therapists, who valued the opportunity to work with participants in their own homes. Therapists highlighted that being in the participant’s home appeared to encourage the participant to talk ‘honestly’ about their engagement with the programme and presented them with the opportunity to discuss specific issues and challenges in context. As with the initial assessment, there was limited specific discussion of the home visit during participant interviews; however, the value of the session for problem-solving and introducing the exercises was recognised:
In terms of a high, I think I’d really like, for me to put an emphasis on home visit, I thought as [T3] said that it was really crucial to me to be able to see the setting up of the home exercises in the home environment and things like where the computer was sited versus where they were actually going to exercise and whether they can actually get to the computer in time, for example, to start and stop the clock.
T2
Although the home visit was highlighted as taking longer than the time allocated, the activity was perceived as vital to help therapists make a judgement about what was considered ‘safe and reasonable’, which included re-evaluating the exercise plans in some situations. Given that one of the aims of BRiMS is to support participants to undertake challenging balance exercise, this aspect is likely to be particularly important to ensure that the balance between challenge and risk is optimised:
It did mean that the session took longer than was allocated.
I would agree with that because you have to move to different parts of the house – one guy had to move from the garage to his front room to find the right place to do the exercises, so it was quite time-consuming, but very worthwhile.
Yes I agree, I think it was beneficial as well . . . I think some of them found it was quite tiring to do all 12 [exercises] together, so we played that on an individual basis.
Discussion
The one-to-one sessions were viewed positively by therapists and participants and felt to be pivotal to enabling therapists to tailor the elements of BRiMS to each participant. Our evaluation suggests that we need to review the balance and timing of topics to aid clarity and reduce time pressures, particularly in the assessment session and the home visit. Key questions include whether all of the elements of BRiMS should be introduced at once or if they should be staged, and whether the feeling of sessions being ‘rushed’ relates to too much content or to therapists’ lack of familiarity with the materials. We also need to develop consensus on best practice in the prescription and explanation of the exercise programme during the home visit. Should exercises be run through during the session, or is an explanation/discussion adequate?
Group sessions
As fewer group sessions were held, the fidelity scoring and assessor feedback relating to all the group sessions are combined (Table 39).
| Session-specific item | Median score (IQR) |
|---|---|
| Group 1 (one session assessed for fidelity scoring, four participants) | |
| Introduce BRiMS group members to each other and begin to foster peer interaction | 3 (0) |
| Review of exercise programme progress and problem-solving. Group practice of core exercises | 3 (0) |
| Group activity: analysis of fall case trial and development of action plan using BAASE framework | 3 (0) |
| Group activity: review of imagery and practise of imagery as a motivational tool | 3 (0) |
| Summary and round-up | 2 (0) |
| Confirm arrangements for subsequent sessions | 1 (0) |
| Group 2 (two sessions assessed for fidelity scoring, five participants) | |
| Welcome and review of progress with exercise activity plans. Explanation of exercise activity substitution | 2.5 (2.25–2.75) |
| BAASE – environmental factors – group work | 2 (1.5–2.5) |
| Group photograph assessment activity | 2.5 (2.25–2.75) |
| Practical falls management and action planning, including getting on and off the floor (practical) | 2 (1.5–2.5) |
| Review goals: what have you achieved so far? Make new goals | 1.5 (1.25–1.75) |
| Confirm arrangements for subsequent sessions | 2.5 (2.25–2.75) |
| Group 3 (two sessions assessed for fidelity scoring, seven participants) | |
| Welcome and review of BAASE environmental factors, activities and progress | 3 (0) |
| Physical ability: review of progress and plan looking forward | 2.5 (2.25–2.75) |
| BAASE: review of progress and plan looking forward | 2 (1.5–2.5) |
| Goals: review of progress and plan looking forward | 2.5 (2.25–2.75) |
| Round-up and summary, presentation of certificates | 2 (1.5–2.5) |
Fidelity assessors highlighted that, although good discussion developed, there was a lack of resolution in several instances. For example, participants actively contributed to falls risk identification, but there was limited action-planning to avoid future issues (records 159 and 167).
In contrast to the one-to-one sessions, participants contributed a lot of feedback about the group sessions; most viewed these as a positive experience. The therapists also felt that the groups were positive, with participants describing a ‘buzz’ in the first session in particular. The group participants were described as bonding quickly, and group interaction and problem-solving were seen to be valuable and motivating. Group discussions were highlighted as an opportunity for ideas to be generated by participants rather than being therapy led, although there was recognition of the importance of therapist facilitation at times:
An independent high is the mutual support and really very powerful facilitation skills in fact that some of the group members had in terms of really supporting each other and problem-solving for each other as well.
T2
He has trouble going to the shops like I do and it was meeting him made me feel, I didn’t feel quite so alone. I think you do because you are different all the time to other people who suffer from this. But to meet somebody who has symptoms that is like your own, you know, he suffers with too many people around him because as you said to me once, that is because your body is trying to work on keeping you upright and it can’t cope with all the other things around it.
P11
When it came to falls analysis I think initially a bit of apprehension about ‘right, who’s going first here?’ and ‘how we going to play this’? I think that then, once it got going it really generated a lot of good discussion, and some of the other people rather than myself were kind of coming in with the ideas of going ‘no you can’t do that’. And they had really good tips or ‘why have you not thought about this?’ or ‘what about that?’ and that generated a lot of good discussion and I think they were really positive sessions.
T4
Taking part in the groups also encouraged participants to try activities that they would have been reluctant to do in other situations:
Yes definitely, definitely yeah. Definitely more confidence, I didn’t think I would have been able to do it you know. When I’ve come away I’ve thought ‘gosh I didn’t think I’d be able to do that’.
P6
A number of session activities invited participants to contribute their own resources, which some did, while other sessions required the use of stock resources. Regardless, discussions were described as positive and involving all participants:
The two ladies in the group had prepared very well with their manuals, so they came with all their things, and that stimulated the rest of the group into the discussion and they were all very good at including everyone else, so I think I was lucky with a mix of the group there.
T3
It was really good coming together. I had a very nice first group of people. We were quite a small group . . . but then maybe there are some people that don’t want to or wouldn’t feel as comfortable in a larger crowd of people. So that could be an aspect that they are thinking of. But it was good because you could come together and share your stories. I would say that the group sessions were very good and I met some really nice people.
P8
As intimated by participant 8, not everyone felt comfortable in a group setting, and some found contributing in a larger group challenging. Therapist support, and building familiarity with other participants, was helpful and encouraged participation. There was also recognition among participants and therapists that a group format may not suit everyone. Although for some non-attendance at the group sessions was indicative of general non-engagement in the programme, examples were highlighted of participants who had engaged actively with the programme independently, suggesting that non-attendance at the group sessions was not always an indication of non-adherence:
When it was just the other two woman that was fine, but the first time with the two women and the bloke, [T3] had to ask me directly, because I was about to talk but one of the other ones would pipe up and come in, so [T3] had to ask me what I was going to say. With that many people and if I have not known them before, I am not really that forthright myself or talkative, so if someone wants to know something they ask me directly, I don’t chip in.
P9
What really surprised me was how hard the previous [group] non-attender had worked at home because she really thought she was going to be the one that was furthest behind and really she was furthest ahead because she had committed so much to the online diary, compliance with the exercises and the booklet.
T1
Discussion
The positive experiences of participants who attended the group sessions is encouraging, particularly the utilisation of peer learning to support planning for future falls prevention. However, what needs to be considered is whether or not, and how, these discussions and plans translated into action outside the sessions. The aim of BRiMS was for the home packages and the techniques such as FIT to support the translation of plans into action; however, given the relatively low engagement of participants with these elements, it is likely that this support was suboptimal. This could be improved by changing the way in which sessions and support are delivered. Perhaps changing the programme so that a group session takes place early in the programme, and is followed by a therapist home visit, could facilitate the translation of participants’ plans into action. Additionally, given the paucity of feedback that participants provided in their exercise logs, perhaps ‘real-time’ interactive therapist reviews could provide more effective support for both the exercise and the behaviour change elements of BRiMS. For example, Learmonth et al. 165 have utilised telephone coaching to support action-planning in physical activity programmes.
Mechanisms of impact
The focus here was on exploring participants’ and treating therapists’ perceptions about the effects of engaging with the programme, and the programme-specific factors influencing engagement.
Participants and therapists reported that taking part in the programme had led to improvements in balance and mobility and a reduction in falls:
I’m just really pleased that I was on the programme. The exercises have all helped . . . Unbelievably because you know turning my head and not getting dizzy and going over, that, I mean it just amazed me that such a simple thing and doing it regularly worked . . . I was very surprised in a really good way over that.
P4
So I definitely think it has helped and the exercises that you do are tailored for you anyway, so I thought it was becoming apparent, it was visual to me when I was filling in the diaries. That I was, I was improving, my balance was improving and even a little improvement can have a huge impact you know because you are a little bit more confident and even just the exercises that I was doing and just slowing down and not [rushing] into the shower not that I [rush] that often, just taking your time and doing the step ups and all that kind of thing. So yeah it was useful for me.
P13
Participants and therapists reported a variety of other effects that they attributed to engagement with the programme, including an increased awareness of falls risk and the introduction of falls prevention strategies:
I would say that some of the falls that I had in retrospect were stupid. Right, so it was not thinking about what I was doing, so the whole study helped me to have focus and think before being stupid like leaning against the wall to put my shoes on. So now I sit down to put my shoes on . . . You look down and you think ‘oh there’s a pair of shoes in the middle of the hall’, because that’s where they got kicked off when we came in from so and so. It makes you more aware. So that’s another positive aspect of it. It makes you more responsible about what’s going on around you. I can’t just rely on [my husband] all the time. I have to look after myself as well.
P8
And I have learnt so much from it, about, towards deciding I’d do my housework on a one-day, one room basis. I’ve split it up. I’ve learnt a lot of things that I wouldn’t have done – you know when I was at work I had to do it at weekends because now I have split it up I only do what I can do.
P11
Therapists also reported positive effects associated with FIT for those participants who used it, although they were unsure about whether or not the use of FIT influenced the extent to which those participants engaged:
Mine, the ones that engaged in the programme generally, they fed back that [FIT] did, it was a positive part of it, and we kept visiting it, each time I saw them we would revisit that as well . . .
T2
The ones that found the visual imagery helpful, the ones that were able to use it, I’m not sure whether they would have not stuck to the programme anyway, I’m not sure how much of a help it was to them in sticking to the program but they certainly included it as part of the programme.
T3
However, not all participants found the programme helpful, and one participant highlighted that they found it difficult to apply some of the learning, particularly from the group sessions, as they found it hard to remember tips when they were back in the ‘real world’:
Having taken part and done the sort of the exercises and gone to the groups and so on, do you think it’s made any or you have made any changes as a result of doing that? Has it made any impact for you?
No it hasn’t.
Discussion
Qualitative feedback supports the mechanisms of impact we expected in the programme design; participants reported changes in both the physical and the behavioural aspects of falls prevention. Although the diary data has significant limitations, and it is inappropriate to draw inferences from data in a feasibility trial, it is certainly encouraging that the BRiMS participants appeared to experience fewer injurious falls. However, the process evaluation has highlighted that engagement was lower than we would have hoped, and most participants did not sustain their engagement throughout the intervention period and beyond. Given the likely need for the long-term adoption of safe mobility strategies, it is a priority for the suggested changes to the programme to be implemented before undertaking further evaluation work.
Context
This section describes the feedback given about the impact that contextual factors were perceived to have on engagement and the outcomes of the programme.
Personal circumstances
As expected, comorbidities, such as health issues that occurred alongside MS, and personal circumstances, including childcare and family commitments, had an impact on people’s ability to engage with the programme. However, the ‘portability’ of the programme was seen as useful. By contrast, some participants highlighted MS-related difficulties, such as problems with writing because of impaired upper limb dexterity, that made the paper-based elements of the programme impractical:
For me as well, at the time of year, it was holiday time. There was a couple of weeks, we were on holiday, it was just down in the Lake District, but I managed to do some exercises because we were renting a house and that was fine, so it was perfectly portable.
P13
No, no it’s me because it coincided with a really difficult time in my life anyway. I had a really rough time with somebody. So it’s kind of, that’s the other side of course, everyone’s just really getting on with their lives. We have other dramas. I think that’s probably what didn’t help as well.
P5
But the whole thing for me that put me off initially was the amount of paperwork that I was asked to complete . . . the amount of work I just couldn’t do it – I can barely lift a pencil. It was very difficult and there was an awful lot of it.
P12
Participant preference
The therapist focus groups noted variability in the patterns of engagement among participants. Although this was influenced by personal circumstances and IT-related issues, it was notable that there were few participants who engaged with all aspects of the programme. One therapist highlighted her perception that personal preference was a factor influencing patterns of engagement. Personal preference was also seen as influencing participants’ perceptions; for example, one participant suggested that they would have preferred a purely online format:
I only had one person out of my group of five that did the online and the manual and attended the groups. I found that the other candidates, they generally picked their most favourite way of interacting and communicating and then stuck with it. So I had a lady that only came to the groups and didn’t do anything online and didn’t do anything with the manual . . . the other one who didn’t come to the group, she worked really hard with the manual because she was fearful that she was missing out in the group aspect. So to get compliance across the board using the different modes of communication and interaction was quite a bonus for me!
T1
See I would really like that because I am a computer-based person. Yes, that would be good. But you wouldn’t have the advantage of taking it with you when you go to the group sessions unless you could print it off. Maybe that could be an option.
P8
Encouragement and support
The therapists highlighted the value of group interaction to encourage and support participants’ engagement. This applied to both exercise and educational activities:
One thing they absolutely loved was the exercises within the group session, so the exercises in the session was a real motivator for them and they all really liked seeing what each other was up to, and they got lots of good ideas about what each other was doing, so as a component within the group I thought the exercises and the way it was set up worked really well for the group.
T2
However, although therapists described many positive experiences from the group sessions, they also highlighted that the composition of the group could have an impact on participant experience:
In my group we had quite a frail lady who dropped out after the first group session because she broke her hip, but quite a few of the group participants came to me afterwards and said they wouldn’t come back to the group if she was there, because they were quite fearful of seeing future versions of what might possibly happen . . .
T1
I would really support that because I similarly had a chap who was really very, wheelchair dependent, pretty well, but in addition to that very very slow processing and had memory issues as well. I was lucky in that the group were an incredibly empathic group of people and he was a lovely chap, so actually that didn’t impact on my group’s experience.
T2
Alongside loss of input for the non-attenders, small group numbers were highlighted as a potential factor affecting the experience of those who did attend. Although the majority of participants seemed to still get the best from the sessions, the loss of opportunities for group interaction and shared learning when numbers dropped was recognised as a limitation by therapists and participants:
. . . that might be by design but in some cases because of circumstances there were only two of us at the meeting. So I wonder if there would have been more dialog if there had been a larger group of people.
P8
I had two guys that wouldn’t actually make it to any of the groups, and I think it does have a negative affect then on some of the others, particularly some of the more really motivated, and I had one group where there was only one lady turned up, so yeah you do miss a bit from the learning, although she was really motivated and she got a lot from the programme, I just wonder how much more she would have got from it.
T4
Technology
An important factor affecting engagement with the programme was the impact of technology-related issues. Participants reported a number of problems with access to, setting up and using the web-based elements of the programme, although these did reduce over time and with practice. For some participants, however, this initial negative experience influenced their perceptions of, and engagement with, the whole programme:
Well we couldn’t log onto it for one thing and by the time [my husband] spoke to somebody we did get on and it went down again. So that was really stressful.
P7
Two main challenges were highlighted by therapists: participants’ skills, expertise and enthusiasm for using technology, and issues relating to the BRiMS resources themselves.
Participant skills and expertise
An unexpected issue encountered by the majority of therapists was the amount of support that participants needed with basic IT tasks. Although there were issues with the programme infrastructure, the challenges encountered extended to basic skills such as using a mouse and accessing the internet. In one instance, the therapist needed to make an extra home visit just to address basic IT access issues. One of the trial inclusion criteria was ‘access to a tablet/computer and the internet’, and the need to use a computer was discussed with prospective participants. However, this issue will need careful consideration if progression to a full trial is indicated:
I had one lady drop out immediately after the home visit because she was, she really couldn’t even, she could switch on the computer but as far as actually moving the mouse and navigating she had absolutely no idea about how to do that and she chose to drop out.
T2
We had a couple of issues in that the audio would only work on new internet browsers, so there was one lady that didn’t like the internet and had an old browser and it wouldn’t work on it, and I think that sealed the deal really . . .
T1
BRiMS resource issues
Alongside issues of computer literacy, significant issues were identified with the infrastructure of the IT elements of BRiMS. The most notable problem was the need to access two separate websites (the main BRiMS site and the online exercise resource) with separate log-ins, which presented challenges for access as well as contributing to the lack of cohesion within the programme that was highlighted by participants and therapists. In addition to this, the logistics of using the online exercise resource were problematic for some participants, particularly those who relied on a desktop PC for their access. Therapists flagged that using a mobile device (e.g. a tablet) was much more successful, although some participants continued to prefer a paper resource:
Mine struggled very much with the fact that it wasn’t an integrated package, that there was a web pams [exercise] website and a BRiMS website so I think that would need to be integrated.
Most of my people had tablets and were then using the programs on tablets and were just taking it everywhere they needed to go and they were exercising with them as well . . .
Most of mine didn’t have tablets and getting back to press the start/stop button was a bit of a mission.
. . . they probably exercised as much doing the start and stop as they did . . .?
[laughs] Yes I think they did!
Discussion
The findings of our process evaluation support the contextual factors we proposed in the BRiMS logic model. However, additional factors warrant consideration. First, it appears that the way in which participants engaged with BRiMS was highly personal. In terms of programme design, allowing flexibility to suit individual needs and preferences is likely to be positive. However, in terms of gathering evidence as part of a clinical trial, this presents a number of challenges. Second, our analyses highlight the importance of ensuring that participants have a positive experience from the outset. For a number of participants, initial negative experiences, from technical glitches to not achieving the perceived ‘minimum’ 120 minutes of exercise in the first week, appear to have influenced their perceptions of the whole programme. In the words of participant 5, ‘I never got back’. This narrow window of opportunity means that ensuring that participants’ initial sessions and interaction with the BRiMS materials are as trouble-free as possible is essential. Along with revising the timing and quantity of content in each session, addressing the technical issues associated with our online materials (in particular the need to use two websites) is critical. Finally, although there was comprehensive PPI during the development of BRiMS, we need to ensure that this continues during any revisions, using as wide a variety of participants as possible to capture a range of views.
Health economics outcomes
Intervention costs (objective xiii)
Methods used in the feasibility trial have proven acceptable and appropriate for collection of resource-use data on delivery of the BRiMS intervention.
Table 40 reports the estimated staff time input for the BRiMS intervention by contact type and type of time input. Our original estimate of therapist time required was 7 hours and 15 minutes per participant (see Appendix 6). However, based on therapist contact data, we estimated an actual mean time input for each participant of 7 hours and 40 minutes. Using a unit cost of £52 per hour for staff time (scientific/professional Agenda for Change band 7; salary per annum £38,786),125 we estimated a mean cost of staff time of £400. When using a unit cost of £42 per hour for staff time (scientific/professional Agenda for Change band 6; salary per annum £31,351),125 the estimated mean cost for staff time is £323.
| Contacts | Contact time, mean (SD) | Administration time, mean (SD) | Total time per contact, mean (SD) | Contact number | Total time, mean (SD) |
|---|---|---|---|---|---|
| Clinic visit (minutes) | 93 (13) | 50 (18) | 143 (20) | 1 | 143 |
| Home visit (minutes) | 100 (18) | 36 (19) | 136 (20) | 1 | 136 |
| Online contacts (minutes) | 15 (7) | N/A | 15 (7) | 5.26 | 79 |
| Group contactsa (minutes per person) | 24 | 10 | 34 | 3 | 102 |
| Total staff time input (minutes) | 460 |
The unit cost (£ per hour/time) applied here (see above)125 includes an estimate/allowance to cover work-related travel costs (time input), so this is not included as a specific item in cost estimates. Trial data indicate a mean travel time per participant home visit/contact of between 50 and 80 minutes.
Estimates are informed by per-participant contact data for 27 participants receiving the BRiMS programme, and data reported for the time input to running of six BRiMS groups (each with three sessions). While there was variation in estimated participant contact data by centre/site, there were no strong observations/patterns across centre/site (see Appendix 7).
Data reported on the time input for group sessions were highly consistent, at 2 hours per session (contact time), plus a requirement for administration time per session. Administration time varied by session, with 16 out of the 18 sessions reporting between 30 minutes and 60 minutes (two sessions reported administration times of 75 minutes and 150 minutes). Groups consisted of relatively small numbers, with data reports showing between three and five participants for the first group session, dropping to between one and four for the final/third group session.
Face-to-face and group sessions are expected to take place on NHS property (as was the case in this feasibility trial) and are not expected to incur additional costs over and above the overhead allowances included in the unit cost for staff time input.
Based on input/advice from the trial co-ordinator, the only additional expenses that would be incurred would be the cost of printing manual copies of the BRiMS manual for participants, and the set-up cost associated with training therapists to deliver the BRiMS intervention (although not considered in detail here, we estimate this to be approximately £300 per trained person/therapist, to be distributed over an expected caseload of participants/patients in years 1–2).
Effectiveness of the proposed health economics evaluation methods (objective xiv)
Resource-use questionnaire feasibility and item completion rates
The questionnaire used to collect resource use proved acceptable, with good rates of completion (see Appendix 8). The questions themselves did not cause problems for the participants, and the ‘other’ open-text fields did not highlight any significant omissions from the resource-use questionnaire (i.e. no items appearing with notable frequency). Missing data are generally associated with participant withdrawal (loss to follow-up), as reported earlier in the main results.
Resource use and associated costs at baseline (objective xiii)
Appendix 9 summarises the resource use collected at baseline assessment (previous 6 months) and estimated costs by treatment group. The resource use and estimated total costs at baseline assessment were similar for both groups.
Resource use over the trial period (27-week follow-up)
Table 41 reports resource use over the 27-week follow-up period of the feasibility trial, for health and social care, medications, and wider resource use associated with informal care/carers. Table 42 reports the costs estimated for each of these areas associated with the resource use reported by participants.
| Resource use | Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||||
| Mean (SD) | Minimum | Maximum | n | Mean (SD) | Minimum | Maximum | n | |
| Health and social care resource use | ||||||||
| Primary care appointments/visits | ||||||||
| Continence advisor: home | 0.00 (0.00) | 0 | 0 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Community psychiatric nurse: home | 0.00 (0.00) | 0 | 0 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Chiropodist: home | 0.18 (0.66) | 0 | 3 | 22 | 0.05 (0.21) | 0 | 1 | 22 |
| Chiropodist: surgery | 0.09 (0.29) | 0 | 1 | 22 | 0.23 (0.61) | 0 | 2 | 22 |
| Counsellor | 0.00 (0.00) | 0 | 0 | 22 | 0.18 (0.85) | 0 | 4 | 22 |
| GP: home | 0.09 (0.43) | 0 | 2 | 22 | 0.36 (0.73) | 0 | 2 | 22 |
| GP: surgery | 2.41 (2.81) | 0 | 12 | 22 | 1.77 (2.65) | 0 | 11 | 22 |
| GP: telephone | 0.55 (0.86) | 0 | 3 | 22 | 1.36 (1.94) | 0 | 7 | 22 |
| MS nurse: home | 0.09 (0.43) | 0 | 2 | 22 | 0.05 (0.21) | 0 | 1 | 22 |
| MS nurse: telephone | 0.36 (0.90) | 0 | 3 | 22 | 0.23 (0.53) | 0 | 2 | 22 |
| Occupational therapist: home | 0.86 (1.78) | 0 | 8 | 22 | 0.27 (0.70) | 0 | 3 | 22 |
| Community nurse: home | 0.05 (0.21) | 0 | 1 | 22 | 0.23 (1.07) | 0 | 5 | 22 |
| Practice nurse: surgery | 1.09 (1.44) | 0 | 5 | 22 | 1.09 (1.93) | 0 | 8 | 22 |
| Physiotherapist: home | 0.50 (1.41) | 0 | 6 | 22 | 0.59 (1.30) | 0 | 5 | 22 |
| Secondary care outpatient appointments | ||||||||
| Continence advisor | 0.14 (0.35) | 0 | 1 | 22 | 0.32 (0.84) | 0 | 3 | 22 |
| Chiropodist | 0.14 (0.47) | 0 | 2 | 22 | 0.09 (0.29) | 0 | 1 | 22 |
| MS nurse | 0.59 (0.85) | 0 | 3 | 22 | 0.32 (0.48) | 0 | 1 | 22 |
| Neurologist | 0.50 (0.67) | 0 | 2 | 22 | 0.41 (0.80) | 0 | 3 | 22 |
| Occupational therapist | 0.05 (0.21) | 0 | 1 | 22 | 0.14 (0.47) | 0 | 2 | 22 |
| Ophthalmologist | 0.14 (0.35) | 0 | 1 | 22 | 0.14 (0.35) | 0 | 1 | 22 |
| Orthotist | 0.23 (0.61) | 0 | 2 | 22 | 0.23 (0.69) | 0 | 3 | 22 |
| Pain clinic | 0.14 (0.47) | 0 | 2 | 22 | 0.41 (1.92) | 0 | 9 | 22 |
| Physiotherapist | 2.45 (2.72) | 0 | 8 | 22 | 1.09 (3.71) | 0 | 17 | 22 |
| Psychiatrist | 0.00 (0.00) | 0 | 0 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Psychologist | 0.05 (0.21) | 0 | 1 | 22 | 0.09 (0.29) | 0 | 1 | 22 |
| Speech therapist | 0.00 (0.00) | 0 | 0 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Secondary care attendances/admissions | ||||||||
| Nights in hospital | 0.00 (0.00) | 0 | 0 | 22 | 2.41 (9.49) | 0 | 44 | 22 |
| A&E visits | 0.23 (0.61) | 0 | 2 | 22 | 0.09 (0.29) | 0 | 1 | 22 |
| Day admissions | 0.00 (0.00) | 0 | 0 | 22 | 0.14 (0.64) | 0 | 3 | 22 |
| Social and community care visits | ||||||||
| Social worker: home visit | 0.00 (0.00) | 0 | 0 | 22 | 0.05 (0.21) | 0 | 1 | 22 |
| Home-care visit | 9.55 (44.77) | 0 | 210 | 22 | 0.14 (0.64) | 0 | 3 | 22 |
| Day-care centre days | 0.00 (0.00) | 0 | 0 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Rehabilitation unit days | 0.14 (0.47) | 0 | 2 | 22 | 2.82 (9.88) | 0 | 44 | 22 |
| Respite care days | 0.00 (0.00) | 0 | 0 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Medication usage | ||||||||
| Disease-modifying medications | 1.55 (5.58) | 0 | 25 | 22 | 0.27 (1.28) | 0 | 6 | 22 |
| Botulinum toxin injections | 0.05 (0.21) | 0 | 1 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Intrathecal baclofen | 0.00 (0.00) | 0 | 0 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Phenol injections | 0.00 (0.00) | 0 | 0 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Intravenous steroids | 0.00 (0.00) | 0 | 0 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Steroid tablets | 0.05 (0.21) | 0 | 1 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Informal care use | ||||||||
| DIY | 1.76 (2.06) | 0 | 9 | 22 | 3.31 (4.42) | 0 | 14 | 22 |
| Gardening | 1.40 (2.23) | 0 | 7 | 22 | 0.89 (1.46) | 0 | 5 | 22 |
| Housework | 6.18 (10.66) | 0 | 44 | 22 | 5.48 (7.35) | 0 | 28 | 22 |
| Preparing meals | 5.73 (6.77) | 0 | 23 | 22 | 6.24 (8.08) | 0 | 31 | 22 |
| Personal care | 1.12 (2.64) | 0 | 9 | 22 | 0.77 (1.51) | 0 | 6 | 22 |
| Looking after pets | 2.07 (4.79) | 0 | 20 | 22 | 1.78 (2.88) | 0 | 9 | 22 |
| Shopping | 2.33 (2.25) | 0 | 7 | 22 | 2.30 (2.03) | 0 | 6 | 22 |
| Transport | 4.09 (4.97) | 0 | 13 | 22 | 3.98 (4.64) | 0 | 17 | 22 |
| Total informal care | 24.67 (24.51) | 0 | 87 | 22 | 24.74 (21.53) | 0 | 80 | 22 |
| Days off work over previous 6 months | ||||||||
| Friend/relative’s days off work | 0.59 (2.77) | 0 | 13 | 22 | 0.00 (0.00) | 0 | 0 | 22 |
| Cost | Group | |||
|---|---|---|---|---|
| Usual care | Intervention | |||
| Mean (SD) (£) | n | Mean (SD) (£) | n | |
| Summary: health and social care costs over 27 weeks | ||||
| Total primary care | 213 (224) | 22 | 190 (162) | 22 |
| Total secondary care | 349 (249) | 22 | 1451 (4335) | 22 |
| Total social and community care | 79 (309) | 22 | 282 (986) | 22 |
| Total health and social care | 640 (580) | 22 | 1922 (5340) | 22 |
| Total medications | 261 (921) | 22 | 45 (209) | 22 |
| Total all health care | 902 (1105) | 22 | 1967 (5341) | 22 |
| Summary: informal care cost for days off work over 27 weeks | ||||
| Total reported informal care (hours/week) | 24.67 (24.5) | 22 | 24.74 (21.5) | 22 |
| Total cost of weekly informal care | 444.14 (441.27) | 22 | 445.30 (387.59) | 22 |
| (Total cost × 27 weeks) | (11,992) | (12,023) | ||
| Friend/relative’s days off work (number of days) | 0.59 (2.77) | 22 | 0.00 (0.00) | 22 |
| Friend/relative’s days off work | 72.28 (339.02) | 22 | 0.00 (0.00) | 22 |
Participants mainly reported relatively modest levels of resource use, mostly focused around items of primary and secondary care (visits to general practitioner, MS nurse, occupational therapy, physiotherapy), and what appears to be an annual outpatient visit to a neurologist (approximately 0.5 visits over 6 months). Very little medication use was reported by participants, which is likely to be consistent with expectations based on the participants having progressive MS, and aligned with our inclusion/exclusion criteria. We do see consistent reporting of informal care provision across a range of activities/needs, with a similar reported mean hours per week of informal care across groups, at approximately 24–25 hours per week. When applying a unit cost to hours of informal care (a shadow cost, reflecting a scenario in which the state pays for this care), we estimate a weekly cost of approximately £445 per participant, this being a relatively huge cost component, currently provided via unpaid informal care inputs.
Table 42 provides a summary of the estimated costs for health and social care resource use, and the estimated costs associated with the items reported for informal care/carers over the feasibility trial follow-up. On health and social care we see estimated costs (27 weeks) of £640 and £1922 for those allocated to the usual-care group and the intervention group, respectively. This large difference in mean costs is almost entirely because in the usual-care group there are no (zero) reports of hospital stays (nights in hospital) versus a total of 53 days (mean of 2.51 nights) in hospital in the intervention group (three participants reported nights in hospital, with one of these reporting a stay of 44 nights/days). Furthermore, in the intervention group one participant reported a stay of 44 days in a rehabilitation unit, the same participant who reported 44 days/nights in hospital. All of these inpatient stays are unrelated to the BRiMS intervention. In addition, in terms of balance, or lack of balance in these small groups – and/or potential outliers – on reports for home-care visits (unit cost at £6.70 each), one participant in the usual-care group reported 210 home-care visits over the 27-week follow-up period.
Table 42 reports the time taken off work by friends or relatives to support the participant (as reported by the participant), and here it can be seen that there are no (zero) reports in the intervention group participants, and a mean of 0.59 days in the usual-care group participants (over 27-week follow-up). Of note, in this latter report it can be seen that one participant reports 13 days off work for a friend/relative, thereby skewing the data towards increased use in the usual-care group, when most participants reported no requirements in this area of data collection.
Discussion
These data highlight the relatively modest ‘formal’ resource use by our participants and emphasise the importance of ‘informal’ support. In addition, the data support our previous description of the likely format of ‘usual care’ for our participants as focused around primary care and involving relatively infrequent contact with rehabilitation providers.
Health state values (EuroQol-5 Dimensions, five-level version, and MSIS-8D) and quality-adjusted life-years
Table 43 summarises the estimated mean health state values and QALY estimates derived from participant reports for the EQ-5D-5L health states. We report data for the baseline assessment and the 15-week and 27-week assessments.
| Period | Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||||||
| Mean | (SD) | Minimum | Maximum | n | Mean | (SD) | Minimum | Maximum | n | |
| Baseline data | ||||||||||
| EQ-5D-3L | 0.58 | (0.16) | 0.04 | 0.77 | 26 | 0.54 | (0.17) | –0.04 | 0.88 | 29 |
| EQ-5D-5L | 0.66 | (0.20) | 0.07 | 0.89 | 26 | 0.63 | (0.17) | 0.22 | 0.95 | 29 |
| Week 15 data | ||||||||||
| EQ-5D-3L | 0.60 | (0.18) | 0.20 | 0.91 | 24 | 0.59 | (0.17) | –0.00 | 0.88 | 25 |
| EQ-5D-5L | 0.69 | (0.18) | 0.19 | 0.95 | 24 | 0.67 | (0.17) | 0.26 | 0.95 | 25 |
| Week 27 data | ||||||||||
| EQ-5D-3L | 0.59 | (0.25) | –0.13 | 0.91 | 22 | 0.57 | (0.11) | 0.30 | 0.77 | 22 |
| EQ-5D-5L | 0.67 | (0.25) | 0.05 | 0.95 | 22 | 0.65 | (0.15) | 0.38 | 0.89 | 22 |
| Estimated 27-week QALYs | ||||||||||
| EQ-5D-3L | 0.30 | (0.08) | 0.13 | 0.43 | 22 | 0.30 | (0.05) | 0.20 | 0.42 | 22 |
| EQ-5D-5L | 0.34 | (0.09) | 0.11 | 0.46 | 22 | 0.34 | (0.07) | 0.22 | 0.47 | 22 |
As with the other measures (see Data completion and accuracy), the completeness of the data to inform calculation of health state values was high within the proportion followed up and as a percentage of those randomised.
Table 44 presents the health state values and QALYs estimated using the MS-specific preference-based measure, the MSIS-8D, with estimates derived from participant reports for the MSIS-29vs2. Health state values and QALY estimates are lower when using the MSIS-8D, and further research is recommended to consider possible reasons for this (e.g. that it is linked to specific domains of health-related QoL that may not be covered fully by the EQ-5D-5L).
| Period | Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||||
| Mean (SD) | Minimum | Maximum | n | Mean (SD) | Minimum | Maximum | n | |
| MSIS-8D | ||||||||
| Baseline | 0.51 (0.21) | 0.08 | 0.80 | 26 | 0.49 (0.15) | 0.21 | 0.76 | 30 |
| Week 15 | 0.54 (0.20) | 0.13 | 0.82 | 24 | 0.56 (0.16) | 0.22 | 0.83 | 25 |
| Week 27 | 0.56 (0.19) | 0.08 | 0.88 | 22 | 0.54 (0.17) | 0.18 | 0.77 | 22 |
| Estimated 27-week QALYs | ||||||||
| MSIS-8D | 0.28 (0.10) | 0.09 | 0.42 | 22 | 0.29 (0.06) | 0.20 | 0.40 | 22 |
Summary
The feasibility research on the economic aspects of the BRiMS programme and the considerations for methods in a future full economic evaluation indicate that the methods used are acceptable, practical and feasible for use in a future full trial and economic evaluation. The methods used to collect data on resource use for intervention delivery, broader self-reported health, social care, other resource use and health outcomes are considered appropriate for a future full economic evaluation alongside a RCT of the BRiMS programme (plus usual care) versus usual care alone.
We provide good-quality data on resource use associated with delivery of the intervention, focused on therapist time input across the intervention form, and across the four research (intervention) centres. Our original estimate of the therapist time required was slightly under that reported by our treating therapists; however, we estimate that the intervention may cost in the region of £400 per participant, a relatively low cost intervention for this type of therapist-/facilitator-led self-management intervention.
We suggest that, in any future economic evaluation, the collection of data across health and social care services could be streamlined, because a number of items included in the feasibility data collection (but with very few, and often no, data reported) could be removed from a future data collection process. We suggest also that for large (high cost) items of resource use, such as hospital stay(s), a cross-check against participant records (or via SAE reporting) may be helpful.
Chapter 4 Discussion, conclusions and recommendations
This feasibility trial aimed to obtain the necessary data and operational experience to finalise the planning of an intended future definitive multicentre RCT to compare a manualised 13-week education and exercise programme (BRiMS) plus usual care with usual care alone in improving mobility, QoL and reducing falls in people with SPMS. The intention was to learn lessons to enable a definitive trial to be successfully delivered with confidence. The specific learning points related to the objectives we set are detailed below. However, there are also a number of overarching strengths and limitations associated with this trial.
First, stakeholders (including people with MS, carers, therapists and commissioners) were integral to the process of developing the BRiMS programme and trial protocol; we believe that this has contributed to the high levels of engagement with the trial and the positive feedback from treating therapists and participants. However, there were logistical issues that particularly affected those in the intervention group, and could have adversely affected retention. It is imperative that these issues are addressed before a definitive trial is undertaken.
Second, the trial was undertaken using robust methodology, with comprehensive documentation and evaluation of our processes, decisions and outcomes at each step. Despite this, there were issues with some aspects of the trial methodology. Most notably, the low return rate of the paper falls diaries means that our falls data must be viewed and interpreted with caution. In particular, this issue highlights the need to find a valid and reliable method of collecting these data before considering falls as a potential primary outcome.
This feasibility trial recruited people with SPMS. It is possible that people with other types of MS had issues that were not highlighted in this trial (e.g. relapses). In addition, the participants were followed up for only 3 months, and hence the impact of a longer follow-up period on operational issues (e.g. trial retention) and clinical outcomes is unknown. However, our experience with this and other related studies, and our ongoing strong links with service users, means that we are well placed to identify and address issues as we move forward.
Specific learning points and recommendations
The objectives were grouped into four clusters:
-
trial feasibility
-
trial outcomes
-
process evaluation
-
health economics analysis.
Many lessons have been learnt from undertaking this trial. For ease of reading and for future reference, these have been summarised in Tables 44–49, in line with the set objectives.
Trial feasibility
Trial feasibility outcomes investigated the feasibility, utility and acceptability of the trial procedures; suitability and feasibility of eligibility criteria; participant recruitment, retention and completion rates; and measures of trial safety and AEs. Overall, relatively few problems were encountered in relation to the operationalisation of the trial, and our pre-defined criteria for progressing to a definitive trial were met. Nevertheless, challenges were experienced in a number of areas, and lessons were learnt. These are described in Table 45. The recommendations we have made to improve the operationalisation of a future definitive trial should be relatively straightforward to implement.
| Activity | Lessons learnt/challenges faced | Recommendation for an anticipated definitive trial |
|---|---|---|
| 1a. Trial set-up | ||
| Governance approvals | Attempting to complete the governance approval process during a 6-month pre-funded period proved challenging as this was dependent on spare workforce capacity, which was difficult to predict | A full trial should incorporate a longer run-in period (minimum of 9 months) to ensure sufficient time to secure the appropriate approvals |
| Gaining ETC approvals |
Gaining ETC approvals was time-consuming and required considerable facilitation by the CRN deputy chief operations manager Feedback from clinical teams highlighted that there was no spare capacity to deliver the BRiMS intervention for the purposes of a research project, in addition to their usual workload |
Close communication with the local CRN is key to facilitating the ETC approval process The availability of funding for ETCs is essential to be able to deliver rehabilitation research trials |
| Embedding the BRiMS research trial within an existing NHS service | Co-ordinating the BRiMS programme was time-consuming and required pre-scheduling to enable research assessments to be undertaken within a limited time frame | Close communication with managers of local therapy services is important to ensure that the additional requirements of the project fit with existing service commitments |
| 1b. Trial procedures | ||
| Recruitment methods | Conversion rates varied according to recruitment method used; and were highest when a personal approach with clinicians and potential participants was used | Recruitment methods should prioritise the personal approach by clinicians or research staff to optimise conversion rate for recruitment. This should be further augmented by undertaking study awareness-raising activities with clinicians and people with MS prior to trial commencement and during the recruitment period |
| Inclusion criteria |
The exclusion criterion of ‘any patient who had previously had treatment with alemtuzumab’ was the most frequent reason for a patient failing the screening process. This was an issue for the recruiting sites across Devon, but not in Scotland, highlighting geographical variations in practice Exclusion criteria included being within 3 months of switching disease-modifying therapies or within 6 months of ceasing Tysabri Some participants allocated to the BRiMS group withdrew from the trial because of their lack of confidence in using a computer to access the programme resources |
On the basis of discussion with expert neurologists in the field, we would recommend that this exclusion criterion be amended to ‘people within 12 months of the second alemtuzumab cycle’. As many participants had received it in the south-west, another alternative would be to stratify for previous treatment with alemtuzumab The disease-modifying therapy exclusion criterion is appropriate; however, the move towards more frequent switching of disease-modifying therapies,132 and the potential new drugs for people in the progressive phase of MS to be licensed, should be taken into careful consideration when predicting the recruitment rate for a future trial Inclusion criteria for a definitive trial should clearly state that participants should be confident and competent in using a computer/tablet, rather than ‘have access to a computer/tablet’ |
| Recruitment blocks | Participants had to be recruited in blocks of 8–12, as group sessions (four or five per group) were integral to the BRiMS programme. Although pre-scheduling of group session dates was an advantage in that it enabled potential participants and treating therapists to diarise these sessions (should they be allocated to the intervention group), it was challenging to recruit these prespecified numbers within the available 4-week recruitment window | A future study is likely to need approximately 8 weeks to recruit each block of participants, should block randomisation be utilised |
| Elapsed time between key trial points | Within the protocol, the time from randomisation to each follow-up was given a ± 1 week window. This time frame proved too short, as the block randomisation design required assessments for the whole block to be undertaken on the same date. This was logistically challenging, particularly in one site where the research therapist was employed for only 1 day per week | In a future trial, we would recommend that a ± 2-week window be incorporated for undertaking assessments at each time point, on the basis that there is evidence to indicate that significant change in key outcomes over this time is unlikely in people with progressive disease50 |
| Methods to maintain blinding | The assessors correctly identified the allocation group for between 75% and 77% of participants at the week 15 and 27 assessments | In line with updated CONSORT guidance,135 research assessors should not be asked to guess which group the participant was allocated to at each assessment, as this method has proven unreliable. We recommend, however, that all instances of known compromises in blinding are reported |
| Recruitment and trial information provided to participants | Some of the participants interviewed felt that the initial patient information packs were too long and detailed, particularly for those with cognitive impairment | The participant information packs provided should be comprehensive, but with an appropriate level of detail to ensure that people with symptoms such as cognitive impairment and fatigue do not feel overwhelmed by the volume of information received |
| Retention | Retention rates were lower than anticipated | Given the dropout rates seen in this feasibility trial, we would recommend that a 6-month trial end point be chosen instead of the 12-month follow-up period proposed in our original plan for a future definitive trial |
| Assessment procedures |
Some of the participants interviewed felt that the assessment sessions, particularly the cognitive demands of the self-report questionnaires, were very tiring. However, overall, the sessions were considered well structured, and participants appreciated that the cognitive tasks were interspersed with physical tasks to minimise fatigue The most common method of completing the self- report questionnaires was for the research therapist to read the questionnaire to the participant and then record the participant’s response. This method increases the likelihood of assessor influence/unblinding |
The scheduling of assessments at local community health centres to reduce participant travel time is an important consideration to minimise participant burden and fatigue Assessment procedures should continue to be structured such that cognitive tasks are interspersed with physical tasks to minimise fatigue Wherever possible, to avoid any potential influence from the research assessor, the self-report questionnaires should be read and completed by the participants, and there should be consistency in the completion methods at each time point. When this is not achievable, the procedure should unambiguously state how this should be operationalised to ensure standardisation of approach across assessors A future trial should review the format of any change in assessment procedures to ensure that people can achieve this without adding significant extra burden |
| AEs reporting by pre-formatted daily diaries (in 2-week batches) |
AE data were gathered by patient self-reported daily diary data. Other trials have found that there can be ambiguity in both the definition and the interpretation of a new symptom/relapse,134 and this was reflected in our own experience in this feasibility trial on analysing the free-text diary data The injurious falls data were collected by self-report, with some participants returning their diaries at variable times (sometimes several diaries at a time). This, coupled with the timeline of normal data entry processes, occasionally caused a time lag with SAE/AE data A further challenge was optimising the return rate of the diaries |
Careful consideration should be given to the most effective and reliable system for collecting AE data in a future trial. A review of the literature should be undertaken to inform this If pre-formatted daily diaries are used to report AEs, then one option could be to allocate one line per AE, with a tick box to indicate the severity and duration of each event If pre-formatted daily diaries are used, then each diary should be clearly dated by the research team before being given to the participant |
| DMC meetings | Because of the low-risk nature of this trial, the DMC meetings were not scheduled in line with the TSC meetings. Instead, a predetermined trigger, related to injurious falls, was set | In a definitive trial, regular DMC meetings should be scheduled in line with TSC meetings |
Trial outcomes
Data on participant demographics, clinical characteristics and a range of potential primary and secondary outcomes were collected to inform a future definitive trial. The completion and performance rates of the measures were excellent, clearly meeting our pre-defined progression criteria for a definitive trial. As can be seen in Table 46, our experience suggests that few changes would be needed in relation to the primary outcomes in a future trial. A number of lessons were learnt in relation to the implementation, analysis and interpretation of some of the secondary outcomes; solutions have been identified to these and recommendations made should these outcomes be chosen for inclusion in a future trial.
| Activity | Lessons learnt/challenges faced | Recommendation for an anticipated definitive trial |
|---|---|---|
| 2a. Data completion and accuracy | ||
| Baseline data collected |
The baseline data collected were useful and none were redundant. However, there was no option in the CRF for a participant to declare that they had fallen once There was an imbalance in the groups at baseline for some characteristics (e.g. EDSS score, anxiety/depression and cognition), which could potentially affect intervention outcomes |
An option for declaring one-off falls should be included in the data collected at baseline A future definitive trial may require stratification according to key variables that may influence outcome |
| Completeness and performance of potential primary outcome measures | All three of the potential primary outcome measures had excellent rates of completion at all time points, were acceptable to participants and produced outcomes that were comparable with those of other similar samples. Criterion for progression to definitive trial was clearly met | We recommend that the primary outcome for a definitive trial, on which a sample size is calculated, is the MSWS-12vs261 |
Completeness and performance of potential secondary outcome measures:
|
All of the potential secondary outcome measures had excellent rates of completion at all time points and produced outcomes that were broadly comparable with those of other similar MS samples. Criterion for progression to definitive trial was clearly met The self-report measure of participations, the CPI, proved to be complicated to interpret and burdensome to complete. Our analysis of item CPI (part 1) highlighted ambiguity in the scoring instructions There were some minor problems with recording the 2MWT data, resulting in data discrepancies The use and return rate of accelerometers demonstrated that these were a feasible method of collecting activity data for a future trial, and the minimum recommended 5 days of data were available for the majority of participants and time points. However, a significant amount of time was required for data cleaning to ensure that 5 consecutive days of data were available for analysis Accelerometry data were unreliable for three participants who had very low levels of activity/speed of walking Evaluation of the quality and completeness of the daily recording of falls in the diaries highlighted other issues, including duplication of data (e.g. when participants sent back two diaries with the same dates, or when there was an overlap in the dates recorded in two sequential diaries) Some of the participants who were interviewed reported that the diaries were ‘a little complicated’ to fill in, which may have had an impact on user engagement and data quality Input of diary data was sometimes delayed because of reduced staff capacity A further challenge was optimising the return rate of the diaries |
A measure of participation is important and, ideally, should be included in a future definitive trial The inclusion of a measure of participation is important; however, further exploration of an alternative measure is required for use in the definitive trial. Before the inclusion of the CPI as a measure in a future trial, ambiguities in its scoring would need to be addressed with the developer of this measure If the 2MWT is included as a secondary outcome measure in a future trial, then the CRF needs to be formatted in a manner that provides a restriction on the time format, tick-box categories for assistive walking device (using the same categories as at baseline) and a requirement to record the time walked in all cases to avoid ambiguity. These amendments to the CRF could help to limit future data discrepancies Accelerometry data provided useful complementary data to the other measures, and should be considered in a future trial. However, it is recommended that the timed start/stop function on the monitor is utilised to reduce the amount of data cleaning required Further consideration of the validity of data for those with significant mobility limitations is also required if trials are to include people from across the disability spectrum Although the diary format for recording falls was that recommended by the International MS Falls Prevention Network, our recommendation would be that the diary needs further refinement and simplification to optimise clarity for participants before it is used in a definitive trial Each diary should be clearly dated by the research team before it is given to the participant to avoid participant error in diary dating Refinement of mechanisms for checking and following up on diary returns is recommended to optimise diary data completeness and accuracy. Data input within a 2-week time frame of receiving the returned diary would enhance this |
One of the key purposes of a feasibility trial is to obtain data to inform the sample size calculation for a full-scale trial. Our shortlist of potential primary outcomes was based on two key principles: the need to identify a measure with robust psychometric properties and maximum completion rates, and the need for the measure to be relevant and meaningful to the aims of the BRiMS programme and to people with MS, clinicians and service commissioners. All three of the potential primary outcome measures had excellent rates of completion at all time points, were acceptable to participants and produced outcomes that were comparable with those of other similar samples. From the outset, the MSWS-12vs2 was highlighted as the key potential primary outcome, given its direct applicability to the problem that the BRiMS programme was developed to address, namely the impact of poor balance and falls on the mobility of people with MS. The findings of the process evaluation support our hypothesis that the mechanism of impact of the BRiMS programme is likely to be through changes in walking and balance. Together, these findings inform our recommendation that the MSWS-12vs2 be used as the primary outcome for a future definitive trial, as summarised in Table 47. A potential alternative is the rate of injurious falls, which is clinically important and has been used as a primary outcome in older peoples fall prevention trials alongside falls rate. 166–168 Our hesitancy in recommending this because of the problem we experienced in terms of the self-reported falls diary data completeness and accuracy. However, if these issues can be resolved, then injurious falls should be carefully reconsidered as a primary outcome of a definitive trial.
| Activity | Results | Recommendation for an anticipated definitive trial |
|---|---|---|
| 2b. Sample size calculation and estimated number of recruiting sites | ||
| Associations between baseline characteristics and potential primary outcome measures | Associations appear to be significant between the potential primary outcomes and disease severity (EDSS), depression and use of mobility aid | These data suggest that a future trial should consider stratification for mood and/or disease severity (EDSS) |
| The sample size and number of recruiting sites required for a fully powered trial |
Sample size has been calculated to detect a between-group difference for the primary outcome of MSWS-12vs2 of 5.2 units156 at the primary end point of 6 months post randomisation. Full details regarding the assumptions used in this calculation are in Calculation of sample size required for a fully powered RCT to evaluate the effectiveness of the BRiMS intervention The recruitment rate for this trial was 14 participants per month over four sites (i.e. 3.5 participants per month per site) |
We recommend that the primary outcome for a definitive trial, on which a sample size is calculated, is the MSWS-12vs2. Based on the data from this feasibility trial, the estimated sample sizes for a definitive trial range from 575 to 900 participants We estimate that, for a definitive trial, at least 7 out of 12 sites would be required, based on the two estimates of sample size described above. This calculation assumes that each site recruits 3.5 participants per month over a 24-month recruitment period, and would be informed by ongoing evaluation and developmental activities |
Process evaluation
A process evaluation was undertaken to gain a more in-depth understanding of some of the trial procedures, which included the fidelity assessments. Table 48 highlights that only easily remediable minor changes would be needed in relation to these procedures in a future trial.
| Activity | Lessons learnt/challenges faced | Suggestions and recommendation for an anticipated definitive trial |
|---|---|---|
| 3a. Process evaluation: assessment procedures | ||
| Fidelity assessment of sessions |
The fidelity assessment was appropriate in its coverage and was feasible to implement, albeit time-consuming Assessment using audio recordings of the BRiMS face-to-face sessions was time-consuming and sometimes challenging because of difficulties hearing details during group interactions, and not being able to observe non-verbal elements of communication The qualitative feedback (in the form of annotated comments) provided by the fidelity assessors to support their scoring judgments on the Fidelity Checklist Rating Scales was undertaken ad hoc, rather than according to a prespecified method |
Fidelity assessment is essential in a definitive trial, which will invariably be multisite in nature, to ensure that all therapists deliver the intervention in line with the programme’s ethos. We recommend that this should be conducted in two stages:
|
| Qualitative interviews with participants and treating therapists | Qualitative interviews were undertaken after completion of the final trial assessment (week 27 ± 1 week), relying on participant recall of sessions undertaken up to 25 weeks previously. This may compromise the accuracy of the information collected, particularly for participants who may have memory problems | We recommend that, when logistically possible (e.g. taking into account issues such as unblinding), participant and therapist interviews should be undertaken as soon as possible after the programme is completed |
The other key purpose of the process evaluation was to gain a more in-depth understanding of the acceptability and utilisation of the BRiMS programme. The intention, outlined in the trial objectives, was to determine the optimum way of delivering the BRiMS programme and to gain a detailed understanding of its acceptability to therapists and participants, and the level of participant adherence to and engagement with the programme. This involved undertaking participant and therapist interviews, reviewing records of attendance at face-to-face sessions, and calculating online exercise diary completion and web-based programme log-in data. Together, these data provided us with a fuller understanding, from the perception of both service providers and users, of the complexities of implementing a programme of this nature in the NHS environment. Table 49 focuses on the many lessons we learnt in relation to the operationalisation of, and engagement with, this programme.
| Activity | Lessons learnt/challenges faced | Suggestions and recommendation for an anticipated definitive trial |
|---|---|---|
| 3b. Process evaluation: implementation of the BRiMS programme | ||
| Attendance rates of face-to-face sessions | Attendance rates were variable. They were considerably higher for one-to-one sessions than for group sessions, and attendance at group sessions reduced over time. At times this meant that group sessions had to be cancelled, or had a limited number of participants, which had an impact on peer interaction | Considering alternative formats (e.g. a single longer workshop, rather than several sessions) is warranted to ensure effective use of therapist time, and maximise the benefits reported from peer interaction |
| Fidelity in delivering the programme |
As anticipated, therapists needed to utilise high-level skills to establish an effective relationship with participants and to deliver the key elements of the programme Therapists experienced challenges with some aspects of the programme with which they were less familiar (e.g. the functional imagery component) The online BRiMS discussion forum was perceived as very helpful by the treating therapists, who used it throughout the trial to gain peer support and solve problems |
We recommend that band 7 level therapists be required to deliver the BRiMS programme A longer initial training session is needed for therapists, with a focus on elements such as motivational support and functional imagery training. This will need to be factored into revised estimates of costs associated with delivery of the BRiMS programme prior to a definitive RCT Identification of a therapist who can take the lead on supporting others in relation to motivational support would be helpful in a future multicentre trial wherein multiple therapists are likely to be involved in delivering the intervention An online discussion forum should remain integral as a support mechanism for therapists, but could be expanded to include peer supervision sessions and debriefs |
| Participant engagement with the programme | Engagement with the programme was suboptimal in terms of both attendance at the group sessions and online log-in of exercises and educational activities | Refinement of the BRiMS programme is necessary prior to implementation of a definitive trial. Consideration of alternative formats to increase user engagement is warranted. People with MS should be closely involved with this developmental process |
| The content and timeframe for delivering the BRiMS programme |
The time taken to deliver the BRiMS programme content was underestimated. This related to all aspects of the programme, both face-to-face sessions and online activity Logistical issues, such as problems with initial log-in to the programme and internet issues, were encountered, which proved time-consuming at the home visit |
The treating therapist time allocated for delivery of the programme (in its current format) should be increased to 8 hours, and costed accordingly There should be one website for the BRiMS programme which houses both the education and the exercise components A mobile version of this website should be considered, as it has the potential to simplify the logistics of some of the home visits |
| BRiMS educational activities |
Participant engagement with the educational elements of BRiMS was variable and dropped off over time Preferences for online/paper information format varied between participants Both participants and therapists reported that, overall, the content of the educational resources was too long and detailed, which may have been a contributing factor to levels of engagement. Some content was considered repetitive |
Rationalisation and simplification of the educational component is required, in line with suggestions made during this process evaluation. For example, the balance and timing of topics need to be revisited to aid clarity and reduce time pressures, particularly in the assessment session and home visit. People with MS should be closely involved with this developmental process |
| BRiMS exercise activities |
The combination of one-to-one, online and group support was reported to be helpful to maintain engagement Group sessions were reported to be helpful for progressing the challenge of exercise regimes People with MS liked the fact that the video exercises were filmed with people with MS, and reported that this helped them to engage with the programme Some participants reported that they found the accumulative online diary a useful way of logging their exercise, whereas others reported challenges with the logistics of using the diary to log exercise practice Participants were asked to undertake 120 minutes of exercise per week, which was considered by some to be too demanding, particularly early in the programme Many participants appeared to rely on the therapists to guide the progression of their exercise programme, rather than initiate this themselves, despite the scheduling of regular online reviews. This may have meant missed opportunities to maximise the extent of ‘highly challenging’ exercise undertaken |
Refinement of the exercise component is required, particularly with regard to the advice and support provided to help participants self-manage the progression of their exercise activities over time. People with MS should be closely involved with this developmental process Participants should be advised that they can ‘build up’ to achieving 120 minutes of exercise per week, rather than being expected to undertake this from the outset. This should be built into the timeline/analysis approach in a future definitive trial Supporting participants to take the lead in how, when and in what way they engage with the BRiMS programme needs to be further developed. Further PPI work, and an updated review of the literature, would help to inform this complex issue |
| Online logging of exercise activity |
Use of the online exercise resource varied among participants. A range of factors accounted for this variability, including participant preference for a paper-based exercise sheet; logistical difficulties, such as difficulty logging in; and participants’ confidence in using IT Online log-in data showed that the pattern of logging exercise was variable, but typically reduced over time. Logistical issues played some part in the (lack of) accuracy and completeness of these data There was a mismatch between the amount of exercise that some participants told the therapist they had undertaken, and the amount the reported online. This casts doubt on the accuracy and validity of the exercise data recorded The limited exercise data entered by most participants (including free-text comments to enable review of exercises by the therapists) restricted therapists’ ability to provide advice about progression of exercise regimes |
An alternative method of recording exercise activity is essential for valid conclusions to be drawn about adherence to unsupervised home-based exercise when the focus is on challenging balance. Wearable sensors may offer a solution, providing that they can record more subtle changes in posture and position (i.e. that they do not require any change in the axes of movement) that are a key focus of many of the exercise/balance activities utilised in the BRiMS programme. This is a priority to address before implementing a future trial Portable systems, such as response to text-based phone messages or mobile applications, may also offer a potential solution to increasing the reliability of the exercise log-in data |
| Use of imagery techniques | Functional imagery training is a novel technique that was unfamiliar both to therapists and to most participants, who felt that more ongoing support and facilitation in its use was needed, along with greater clarity on when and how to use it as part of BRiMS | When revising the BRiMS training session, more time should be allocated to educating and supporting therapists so that they can confidently include functional imagery training techniques as part of the motivational support they offer participants |
| BRiMS infrastructure | Significant issues were encountered with technological infrastructure, the most notable being the need to access two separate websites with separate log-ins (the main BRiMS site and the online exercise resource). This presented challenges for access, as well as having an impact on the cohesion of the programme | Development of an integrated online BRiMS resource, which incorporates all elements of the programme on one site, is a high priority. This should be piloted with people with MS prior to implementation of a definitive trial |
Our experience in this feasibility trial highlighted that some elements of the BRiMS programme require further developmental work prior to a definitive trial. Other issues identified have proven more straightforward and, for these, we have been able to identify solutions and make clear recommendations. It is notable that many of the issues we faced are common to MS rehabilitation trials of this nature. For example, sustaining patient engagement with unsupervised physical activities over the long term113 and monitoring patient adherence to home-based exercise programmes169 are both issues that, thus far, remain unresolved in the MS clinical and research community. Our experience of undertaking this feasibility study has provided us with invaluable learning, which moves us one step closer to addressing these very complex issues.
Health economics
The focus of the health economics evaluation was on determining the feasibility of the proposed methods for assessing health, social care and other resource use in a future definitive trial was undertaken, plus evaluation of the intervention delivery costs for the BRiMS programme. This feasibility study demonstrated that, overall, this element of the trial was feasible and few challenges were faced in relation to this. Those identified, described in Table 50, have straightforward solutions that will be easily implementable within a definitive trial.
| Activity | Lessons learnt/challenges faced | Suggestions and recommendation for an anticipated definitive trial |
|---|---|---|
| 4. Health economics: evaluation procedures | ||
| Health economics methods | The methods used were acceptable, practical and feasible for use in a future full trial and economic evaluation | A future definitive trial should use similar methods to those described in this feasibility trial |
| Data quality |
Good-quality data were collected on resource use associated with delivery of the intervention A number of items included in the resource form collection had very few, and often no, data reported suggesting redundancy of these items |
We recommend that the collection of data across health and social care services could be streamlined Items in the resource-use form that were rarely populated could be removed from a future data collection process We suggest that for large (high-cost) items of resource use, such as hospital stay(s), a cross-check against participant records (or via SAE reporting) may be helpful |
Conclusions
This trial aimed to assess the feasibility of undertaking a definitive trial to compare BRiMS plus usual care with usual care alone in a sample of people with SPMS. We achieved this. Our results suggest that our trial procedures are feasible and acceptable, and retention, programme engagement and outcome completion rates were sufficient to satisfy our a priori trial progression criteria. Challenges were experienced in some areas of data collection, such as the recording of adherence to exercise activity and the completion of daily diaries; the lessons learnt in this feasibility trial will enable these processes to be refined for a future trial. The comprehensive and robust process evaluation and input from PPI team members have proven invaluable in finding potential solutions and indicating remedial actions, which have been discussed in detail throughout this report and summarised in this chapter. Our experience has highlighted that further development of the BRiMS programme is required to address logistical issues and enhance user satisfaction and adherence, which will benefit from ongoing input from both therapists and people with MS. Following this, a definitive trial to assess the clinical effectiveness and cost-effectiveness of the BRiMS intervention is warranted.
Acknowledgements
We are grateful to all of the patients and health-care professionals who generously contributed their time and effort in the conduct of this study.
Professor Marcia Finlayson (Director, School of Rehabilitation Therapy, Queen’s University, Kingston, ON, Canada) provided materials for the BRiMS education resource based on the Safe at Home BAASE (BAASE, Behaviours and Attitudes, Activities, MS Symptoms, Environment) programme.
Members of the International MS Falls Prevention Research Network provided input on trial and programme design.
University Hospitals Plymouth NHS Trust sponsored and supported the trial, in particular Chris Rollinson and Corinna Mossop.
The Peninsula Clinical Trials Unit provided support for the day-to-day running of the trial, development of the trial database and data entry systems, data management and administrative support. Particular thanks go to Brian Wainman and Elinor Pegg.
Pauline McGlone and the CRN South West Peninsula gave support and advice on trial recruitment and excess treatment costs.
The authors acknowledge support from the MS Society UK, the MS Register and the Merlin MS centre to advertise and support recruitment for the study.
Trial Steering Committee
Professor Roshan das Nair (chairperson), Professor of Clinical Psychology and Neuropsychology, Nottingham University Hospitals NHS Trust; Rachel Phillips (independent statistician), Imperial College London; and John Kendrick (expert patient).
Data Monitoring and Ethics Committee
Dr Jeremy Chataway (chairperson), Consultant Neurologist, University College London Hospitals NHS Foundation Trust; Dr Stephanie MacNeill (statistician), University of Bristol; and Professor Lynn Rochester, Professor in Human Movement Science, Newcastle University.
Local principal investigators
Dr Katharine Stone (Cornwall Partnership Trust) and Graham Bamforth (Royal Devon and Exeter NHS Foundation Trust).
Local research and multiple sclerosis nurses
Barbara Bale, Amanda Grant, Amanda Holt, Sarah Irvine, Dr Lou Jarrett and Helen Rutherford.
Local treating and research therapists
Aleksandra Dybus, Vicki Farrell, Kathryn Smith and Alison Watson.
Contributions of authors
Hilary Gunn (Lecturer in Physiotherapy and BRiMS project co-ordinator, University of Plymouth) led the development of BRiMS, and contributed to the funding application, protocol development, ethics approval and methodological aspects, as well as the co-ordination of the trial. She led the process evaluation and the drafting of the report.
Jackie Andrade (Professor in Psychology, University of Plymouth) developed and applied the FIT elements within the BRiMS programme, contributed to the funding application, protocol development, therapist training and fidelity assessment, and contributed to the drafting of the report.
Lorna Paul (Professor of Allied Health Science, Glasgow Caledonian University) developed and applied the web-based physiotherapy resource to the BRiMS programme, contributed to the funding application, protocol development and supervision of the Ayrshire research therapist, and contributed to the drafting of the report.
Linda Miller (Consultant Physiotherapist in MS, Ayrshire Central Hospital) contributed to the funding application and protocol development, acted as principal investigator for the Ayrshire site and contributed to the fidelity assessment and drafting of the report.
Siobhan Creanor (Associate Professor in Clinical Trials and Medical Statistics and Director of PenCTU) contributed to the funding application and protocol development, provided statistical expertise and supervised the statistical analysis, and contributed to the drafting of the report.
Kara Stevens (Research Fellow, Medical Statistics, University of Plymouth) undertook the statistical analysis and contributed to the drafting of the report.
Colin Green (Professor of Health Economics, University of Exeter) constructed the health economics analysis plans, performed the health economic analysis and contributed to drafting of the report.
Paul Ewings (Honorary Visiting Professor, University of Exeter, Director of NIHR Research Design Service South West) contributed to the funding application and protocol development, provided statistical expertise and contributed to the drafting of the report.
Andrew Barton (Associate Professor in Healthcare Research Methodology, NIHR Research Design Service) contributed to the funding application, protocol development, ethics approval, methodological aspects and the management of the study.
Margie Berrow (Trial Manager, PenCTU) contributed to the protocol development and ethics approval, and co-ordinated the recruiting centres and the management of the study.
Jane Vickery (Senior Trial Manager, PenCTU) contributed to the funding application, protocol development, ethics approval, methodological aspects and the management of the study.
Ben Marshall (Expert Patient) acted as PPI representative and contributed to the management of the study and drafting of the report.
John Zajicek (Professor of Medicine, University of St Andrews) contributed to the funding application, provided clinical MS expertise and contributed to the drafting of the report.
Jennifer Freeman (Professor in Physiotherapy and Rehabilitation, University of Plymouth) led the funding application and the protocol development, ethics approval and methodological aspects, and acted as principal investigator for the Plymouth site.
All authors were involved in the interpretation of data for the work, in revising the work critically for important intellectual content and in the final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of this report are appropriately investigated and resolved.
Publications
Gunn H, Andrade J, Paul L, Miller L, Creanor S, Green C, et al. Balance Right in Multiple Sclerosis (BRiMS): a guided self-management programme to reduce falls and improve quality of life, balance and mobility in people with secondary progressive multiple sclerosis: a protocol for a feasibility randomised controlled trial. Pilot Feasibility Stud 2017;4:26.
Gunn H, Andrade J, Paul L, Miller L, Creanor S, Green C, et al. 22nd Annual RIMS Conference 2017. Balance Right in Multiple Sclerosis (BRiMS): a guided self-management programme to reduce falls and improve quality of life, balance and mobility in people with secondary progressive multiple sclerosis: a protocol for a feasibility randomised controlled trial. Mult Scler J 2017;23:878–916.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review and ethics approval.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- The National Audit of Services for People with Multiple Sclerosis 2011. London: Royal College of Physicians; 2011.
- Kobelt G, Berg J, Lindgren P, Kerrigan J, Russell N, Nixon R. Costs and quality of life of multiple sclerosis in the United Kingdom. Eur J Health Econ 2006;7:96-104. https://doi.org/10.1007/s10198-006-0380-z.
- Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J. MSCOI Study Group . New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler 2017;23:1123-36. https://doi.org/10.1177/1352458517694432.
- Rovaris M, Confavreux C, Furlan R, Kappos L, Comi G, Filippi M. Secondary progressive multiple sclerosis: current knowledge and future challenges. Lancet Neurol 2006;5:343-54. https://doi.org/10.1016/S1474-4422(06)70410-0.
- Feinstein A, Freeman J, Lo AC. Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. Lancet Neurol 2015;14:194-207. https://doi.org/10.1016/S1474-4422(14)70231-5.
- Fox RJ, Thompson A, Baker D, Baneke P, Brown D, Browne P, et al. Setting a research agenda for progressive multiple sclerosis: the International Collaborative on Progressive MS. Mult Scler 2012;18:1534-40. https://doi.org/10.1177/1352458512458169.
- Management of Multiple Sclerosis in Primary and Secondary Care. London: NICE; 2014.
- Giesser BS. Primer on Multiple Sclerosis. New York, NY: Oxford University Press; 2016.
- Jones CA, Pohar SL, Warren S, Turpin KV, Warren KG. The burden of multiple sclerosis: a community health survey. Health Qual Life Outcomes 2008;6. https://doi.org/10.1186/1477-7525-6-1.
- Heesen C, Böhm J, Reich C, Kasper J, Goebel M, Gold SM. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler 2008;14:988-91. https://doi.org/10.1177/1352458508088916.
- McCrone P, Heslin M, Knapp M, Bull P, Thompson A. Multiple sclerosis in the UK: service use, costs, quality of life and disability. PharmacoEconomics 2008;26:847-60. https://doi.org/10.2165/00019053-200826100-00005.
- Sutliff MH. Contribution of impaired mobility to patient burden in multiple sclerosis. Curr Med Res Opin 2010;26:109-19. https://doi.org/10.1185/03007990903433528.
- Cattaneo D, Jonsdottir J, Zocchi M, Regola A. Effects of balance exercises on people with multiple sclerosis: a pilot study. Clin Rehabil 2007;21:771-81. https://doi.org/10.1177/0269215507077602.
- Soyuer F, Mirza M, Erkorkmaz U. Balance performance in three forms of multiple sclerosis. Neurol Res 2006;28:555-62. https://doi.org/10.1179/016164105X49373.
- Gunn H, Creanor S, Haas B, Marsden J, Freeman J. Risk factors for falls in multiple sclerosis: an observational study. Mult Scler 2013;19:1913-22. https://doi.org/10.1177/1352458513488233.
- Gunn H, Creanor S, Haas B, Marsden J, Freeman J. Frequency, characteristics, and consequences of falls in multiple sclerosis: findings from a cohort study. Arch Phys Med Rehabil 2014;95:538-45. https://doi.org/10.1016/j.apmr.2013.08.244.
- Nilsagard Y, Gunn H, Freeman J, Hoang P, Lord S, Mazumder R, et al. Falls in people with MS-an individual data meta-analysis from studies from Australia, Sweden, United Kingdom and the United States. Mult Scler J 2014;21:1-9. https://doi.org/10.1177/1352458514538884.
- Peterson EW, Cho CC, von Koch L, Finlayson ML. Injurious falls among middle aged and older adults with multiple sclerosis. Arch Phys Med Rehabil 2008;89:1031-7. https://doi.org/10.1016/j.apmr.2007.10.043.
- Bazelier MT, van Staa TP, Uitdehaag BM, Cooper C, Leufkens HG, Vestergaard P, et al. Risk of fractures in patients with multiple sclerosis: a population-based cohort study. Neurology 2012;78:1967-73. https://doi.org/10.1212/WNL.0b013e318259e0ff.
- Peterson EW, Cho CC, Finlayson ML. Fear of falling and associated activity curtailment among middle aged and older adults with multiple sclerosis. Mult Scler 2007;13:1168-75. https://doi.org/10.1177/1352458507079260.
- Finding the Top 10 Research Priorities. Research Matters, January/February 2014. London: MS Society; n.d.
- Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology 2006;66:1696-702. https://doi.org/10.1212/01.wnl.0000218309.01322.5c.
- Mackenzie IS, Morant SV, Bloomfield GA, MacDonald TM, O’Riordan J. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry 2014;85:76-84. https://doi.org/10.1136/jnnp-2013-305450.
- Services for People with Neurological Conditions. London: National Audit Office; 2011.
- Multiple Sclerosis in Adults: Management. London: NICE; 2014.
- Campbell E, Coulter EH, Mattison PG, Miller L, McFadyen A, Paul L. Physiotherapy rehabilitation for people with progressive multiple sclerosis: a systematic review. Arch Phys Med Rehabil 2016;97:141-51.e3. https://doi.org/10.1016/j.apmr.2015.07.022.
- Gunn H, Endacott R, Haas B, Marsden J, Freeman J. Development of a balance, safe mobility and falls management programme for people with multiple sclerosis. Disabil Rehabil 2018;40:2857-66. https://doi.org/10.1080/09638288.2017.1362041.
- Batchelor F, Hill K, Mackintosh S, Said C. What works in falls prevention after stroke?: a systematic review and meta-analysis. Stroke 2010;41:1715-22. https://doi.org/10.1161/STROKEAHA.109.570390.
- Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson’s disease: a pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry 2011;82:1232-8. https://doi.org/10.1136/jnnp-2011-300919.
- Finlayson M, Peterson EW, Cho C. Pilot study of a fall risk management program for middle aged and older adults with MS. NeuroRehabilitation 2009;25:107-15. https://doi.org/10.3233/NRE-2009-0505.
- Sosnoff JJ, Finlayson M, McAuley E, Morrison S, Motl RW. Home-based exercise program and fall-risk reduction in older adults with multiple sclerosis: phase 1 randomized controlled trial. Clin Rehabil 2014;28:254-63. https://doi.org/10.1177/0269215513501092.
- High Quality Care For All: NHS Next Stage Review Final Report. London: Department of Health and Social Care; 2008.
- Five Year Forward View. London: NHS England; 2014.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455-9. https://doi.org/10.1136/bmj.39108.379965.BE.
- Sosnoff JJ, Finlayson M. International MS Falls Prevention Research Network: report from the front lines. Int J MS Care 2014;16:161-2. https://doi.org/10.7224/1537-2073.2014-061.
- Kavanagh DJ, Andrade J, May J, Connor JP. Motivational interventions may have greater sustained impact if they trained imagery-based self-management. Addiction 2014;109:1062-3. https://doi.org/10.1111/add.12507.
- Solbrig L, Whalley B, Kavanagh DJ, May J, Parkin T, Jones R, et al. Functional imagery training versus motivational interviewing for weight loss: a randomised controlled trial of brief individual interventions for overweight and obesity [published online ahead of print September 5 2018]. Int J Obes 2018. https://doi.org/10.1038/s41366-018-0122-1.
- Hale LA, Smith C, Mulligan H, Treharne GJ. ‘Tell me what you want, what you really really want….’: asking people with multiple sclerosis about enhancing their participation in physical activity. Disabil Rehabil 2012;34:1887-93. https://doi.org/10.3109/09638288.2012.670037.
- Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003;26:1-7. https://doi.org/10.1207/S15324796ABM2601_01.
- Bandura A. Health promotion by social cognitive means. Health Educ Behav 2004;31:143-64. https://doi.org/10.1177/1090198104263660.
- Rhodes J, May J, Andrade J, Kavanagh D. Enhancing grit through functional imagery training in professional soccer. Sport Psychol 2018;32:220-5.
- Gunn H, Markevics S, Haas B, Marsden J, Freeman J. Systematic review: the effectiveness of interventions to reduce falls and improve balance in adults with multiple sclerosis. Arch Phys Med Rehabil 2015;96:1898-912. https://doi.org/10.1016/j.apmr.2015.05.018.
- Paul L, Coulter EH, Miller L, McFadyen A, Dorfman J, Mattison PG. Web-based physiotherapy for people moderately affected with multiple sclerosis; quantitative and qualitative data from a randomized, controlled pilot study. Clin Rehabil 2014;28:924-35. https://doi.org/10.1177/0269215514527995.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, Close JC. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc 2008;56:2234-43. https://doi.org/10.1111/j.1532-5415.2008.02014.x.
- Gunn H, Andrade J, Paul L, Miller L, Creanor S, Green C, et al. Balance Right in Multiple Sclerosis (BRiMS): a guided self-management programme to reduce falls and improve quality of life, balance and mobility in people with secondary progressive multiple sclerosis: a protocol for a feasibility randomised controlled trial. Pilot Feasibility Stud 2017;4. https://doi.org/10.1186/s40814-017-0168-1.
- Relapsing Remitting MS (RRMS). London: MS Society; 2016.
- Billingham SA, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-104.
- Freeman J, Walters R, Ingram W, Slade A, Hobart J, Zajicek J. Evaluating change in mobility in people with multiple sclerosis: relative responsiveness of four clinical measures. Mult Scler 2013;19:1632-9. https://doi.org/10.1177/1352458513482373.
- Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002360.
- Cooper CL, Hind D, Parry GD, Isaac CL, Dimairo M, O’Cathain A, et al. Computerised cognitive behavioural therapy for the treatment of depression in people with multiple sclerosis: external pilot trial. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-259.
- Thomas S, Thomas PW, Kersten P, Jones R, Green C, Nock A, et al. A pragmatic parallel arm multi-centre randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. J Neurol Neurosurg Psychiatry 2013;84:1092-9. https://doi.org/10.1136/jnnp-2012-303816.
- Carter A, Daley A, Humphreys L, Snowdon N, Woodroofe N, Petty J, et al. Pragmatic intervention for increasing self-directed exercise behaviour and improving important health outcomes in people with multiple sclerosis: a randomised controlled trial. Mult Scler 2014;20:1112-22. https://doi.org/10.1177/1352458513519354.
- Zajicek JP, Ingram WM, Vickery J, Creanor S, Wright DE, Hobart JC. Patient-orientated longitudinal study of multiple sclerosis in south west England (The South West Impact of Multiple Sclerosis Project, SWIMS) 1: protocol and baseline characteristics of cohort. BMC Neurol 2010;10. https://doi.org/10.1186/1471-2377-10-88.
- Bowen J, Gibbons L, Gianas A, Kraft GH. Self-administered Expanded Disability Status Scale with functional system scores correlates well with a physician-administered test. Mult Scler 2001;7:201-6. https://doi.org/10.1177/135245850100700311.
- A Lottery of Treatment and Care – MS Services Across the UK. London: MS Society; 2013.
- Treml J, Husk J, Lowe D, Vasilakis N. Falling Standards, Broken Promises. Report of the National Audit of Falls and Bone Health in Older People 2010. London: Royal College of Physicians; 2010.
- Coote S, Sosnoff JJ, Gunn H. Fall incidence as the primary outcome in multiple sclerosis falls-prevention trials: recommendation from the International MS Falls Prevention Research Network. Int J MS Care 2014;16:178-84. https://doi.org/10.7224/1537-2073.2014-059.
- Lamb SE, Jørstad-Stein EC, Hauer K, Becker C. Prevention of Falls Network Europe and Outcomes Consensus Group . Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc 2005;53:1618-22. https://doi.org/10.1111/j.1532-5415.2005.53455.x.
- Hobart J, Cano S. Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13120.
- Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology 2003;60:31-6. https://doi.org/10.1212/WNL.60.1.31.
- Motl RW, Snook EM. Confirmation and extension of the validity of the Multiple Sclerosis Walking Scale-12 (MSWS-12). J Neurol Sci 2008;268:69-73. https://doi.org/10.1016/j.jns.2007.11.003.
- McGuigan C, Hutchinson M. Confirming the validity and responsiveness of the Multiple Sclerosis Walking Scale-12 (MSWS-12). Neurology 2004;62:2103-5. https://doi.org/10.1212/01.WNL.0000127604.84575.0D.
- Goodman AD, Brown TR, Krupp LB, Schapiro RT, Schwid SR, Cohen R, et al. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet 2009;373:732-8. https://doi.org/10.1016/S0140-6736(09)60442-6.
- Fox EE, Hough AD, Creanor S, Gear M, Freeman JA. Effects of pilates-based core stability training in ambulant people with multiple sclerosis: multicenter, assessor-blinded, randomized controlled trial. Phys Ther 2016;96:1170-8. https://doi.org/10.2522/ptj.20150166.
- Ball S, Vickery J, Hobart J, Wright D, Green C, Shearer J, et al. The Cannabinoid Use in Progressive Inflammatory brain Disease (CUPID) trial: a randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19120.
- Multiple Sclerosis Outcome Measures Taskforce . MS EDGE Outcome Measures Database 2011. www.neuropt.org/practice-resources/neurology-section-outcome-measures-recommendations/multiple-sclerosis (accessed 24 May 2019).
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Mulhern B, Mukuria C, Barkham M, Knapp M, Byford S, Soeteman D, et al. Using generic preference-based measures in mental health: psychometric validity of the EQ-5D and SF-6D. Br J Psychiatry 2014;205:236-43. https://doi.org/10.1192/bjp.bp.112.122283.
- Hobart JC, Jenkinson C, Peters M, Bromberg M. Quality of Life Measurement in Neurodegenerative and Related Conditions. Cambridge: Cambridge University Press; 2011.
- Hawton A, Green C, Telford C, Zajicek J, Wright D. Using the Multiple Sclerosis Impact Scale to estimate health state utility values: mapping from the MSIS-29, version 2, to the EQ-5D and the SF-6D. Value Health 2012;15:1084-91. https://doi.org/10.1016/j.jval.2012.07.007.
- Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain 2001;124:962-73. https://doi.org/10.1093/brain/124.5.962.
- Riazi A, Hobart JC, Lamping DL, Fitzpatrick R, Thompson AJ. Multiple Sclerosis Impact Scale (MSIS-29): reliability and validity in hospital based samples. J Neurol Neurosurg Psychiatry 2002;73:701-4. https://doi.org/10.1136/jnnp.73.6.701.
- Motl RW, Putzki N, Pilutti LA, Cadavid D. Longitudinal changes in self-reported walking ability in multiple sclerosis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0125002.
- Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. How responsive is the Multiple Sclerosis Impact Scale (MSIS-29)? A comparison with some other self report scales. J Neurol Neurosurg Psychiatry 2005;76:1539-43. https://doi.org/10.1136/jnnp.2005.064584.
- Goodwin E, Green C. A quality-adjusted life-year measure for multiple sclerosis: developing a patient-reported health state classification system for a multiple sclerosis-specific preference-based measure. Value Health 2015;18:1016-24. https://doi.org/10.1016/j.jval.2015.07.002.
- Goodwin E, Green C. Improving the measurement of QALYs in multiple sclerosis: estimating a preference-based index for use in deriving quality-adjusted life-years (QALYs) for multiple sclerosis. Qual Life Res 2014;23.
- Ganz DA, Higashi T, Rubenstein LZ. Monitoring falls in cohort studies of community-dwelling older people: effect of the recall interval. J Am Geriatr Soc 2005;53:2190-4. https://doi.org/10.1111/j.1532-5415.2005.00509.x.
- Dibble LE, Lopez-Lennon C, Lake W, Hoffmeister C, Gappmaier E. Utility of disease-specific measures and clinical balance tests in prediction of falls in persons with multiple sclerosis. J Neurol Phys Ther 2013;37:99-104. https://doi.org/10.1097/NPT.0b013e3182a18460.
- Nilsagård YE, von Koch LK, Nilsson M, Forsberg AS. Balance exercise program reduced falls in people with multiple sclerosis: a single-group, pretest-posttest trial. Arch Phys Med Rehabil 2014;95:2428-34. https://doi.org/10.1016/j.apmr.2014.06.016.
- Hoang PD, Cameron MH, Gandevia SC, Lord SR. Neuropsychological, balance, and mobility risk factors for falls in people with multiple sclerosis: a prospective cohort study. Arch Phys Med Rehabil 2014;95:480-6. https://doi.org/10.1016/j.apmr.2013.09.017.
- Grant PM, Ryan CG, Tigbe WW, Granat MH. The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br J Sports Med 2006;40:992-7. https://doi.org/10.1136/bjsm.2006.030262.
- Matthews CE, Hagströmer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc 2012;44:68-76. https://doi.org/10.1249/MSS.0b013e3182399e5b.
- Edwardson CL, Winkler EAH, Bodicoat DH, Yates T, Davies MJ, Dunstan DW, et al. Considerations when using the activPAL monitor in field-based research with adult populations. J Sport Health Sci 2017;6:162-78. https://doi.org/10.1016/j.jshs.2016.02.002.
- Paul L, Brewster S, Wyke S, McFadyen AK, Sattar N, Gill JM, et al. Increasing physical activity in older adults using STARFISH, an interactive smartphone application (app); a pilot study. J Rehabil Assist Technol Eng 2017;4. https://doi.org/10.1177/2055668317696236.
- Paul L, Coulter EH, Cameron S, McDonald MT, Brandon M, Cook D, et al. Web-based physiotherapy for people with axial spondyloarthritis (WEBPASS) – a study protocol. BMC Musculoskelet Disord 2016;17. https://doi.org/10.1186/s12891-016-1218-1.
- Gijbels D, Eijnde BO, Feys P. Comparison of the 2- and 6-minute walk test in multiple sclerosis. Mult Scler 2011;17:1269-72. https://doi.org/10.1177/1352458511408475.
- Gijbels D, Dalgas U, Romberg A, de Groot V, Bethoux F, Vaney C, et al. Which walking capacity tests to use in multiple sclerosis? A multicentre study providing the basis for a core set. Mult Scler 2012;18:364-71. https://doi.org/10.1177/1352458511420598.
- Baert I, Freeman J, Smedal T, Dalgas U, Romberg A, Kalron A, et al. Responsiveness and clinically meaningful improvement, according to disability level, of five walking measures after rehabilitation in multiple sclerosis: a European multicenter study. Neurorehabil Neural Repair 2014;28:621-31. https://doi.org/10.1177/1545968314521010.
- Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehabil Med 2010;46:239-48.
- Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med 2010;42:323-31. https://doi.org/10.2340/16501977-0537.
- Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A. Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys Ther 2013;93:158-67. https://doi.org/10.2522/ptj.20120171.
- Sibley KM, Howe T, Lamb SE, Lord SR, Maki BE, Rose DJ, et al. Recommendations for a core outcome set for measuring standing balance in adult populations: a consensus-based approach. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0120568.
- Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol 1990;45:M192-7. https://doi.org/10.1093/geronj/45.6.M192.
- Tyson SF, Connell LA. How to measure balance in clinical practice. A systematic review of the psychometrics and clinical utility of measures of balance activity for neurological conditions. Clin Rehabil 2009;23:824-40. https://doi.org/10.1177/0269215509335018.
- Paltamaa J, Sjögren T, Peurala SH, Heinonen A. Effects of physiotherapy interventions on balance in multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. J Rehabil Med 2012;44:811-23. https://doi.org/10.2340/16501977-1047.
- Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil 2006;87:1274-9. https://doi.org/10.1016/j.apmr.2006.06.002.
- Marck CH, Hadgkiss EJ, Weiland TJ, van der Meer DM, Pereira NG, Jelinek GA. Physical activity and associated levels of disability and quality of life in people with multiple sclerosis: a large international survey. BMC Neurol 2014;14. https://doi.org/10.1186/1471-2377-14-143.
- Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005;34:614-19. https://doi.org/10.1093/ageing/afi196.
- van Vliet R, Hoang P, Lord S, Gandevia S, Delbaere K. Falls efficacy scale-international: a cross-sectional validation in people with multiple sclerosis. Arch Phys Med Rehabil 2013;94:883-9. https://doi.org/10.1016/j.apmr.2012.10.034.
- International Classification of Functioning, Disability and Health. Geneva: World Health Organization; 2018.
- Heinemann AW, Lai JS, Magasi S, Hammel J, Corrigan JD, Bogner JA, et al. Measuring participation enfranchisement. Arch Phys Med Rehabil 2011;92:564-71. https://doi.org/10.1016/j.apmr.2010.07.220.
- Heinemann AW, Magasi S, Bode RK, Hammel J, Whiteneck GG, Bogner J, et al. Measuring enfranchisement: importance of and control over participation by people with disabilities. Arch Phys Med Rehabil 2013;94:2157-65. https://doi.org/10.1016/j.apmr.2013.05.017.
- Finlayson M, Peterson E, Patricia N. Participation as an outcome in MS fall prevention research: consensus recommendations from the International MS Fall Prevention Research Network. Int J MS Care 2014;16:171-7. https://doi.org/10.7224/1537-2073.2014-053.
- Pawson R, Tilley N. Realistic Evaluation. London: Sage; 1997.
- Onwuegbuzie A, Leech N. On becoming a pragmatic researcher: the importance of combining quantitative and qualitative research methodologies. Int J Soc Res Methodol Theory Pract 2005;8:375-87. https://doi.org/10.1080/13645570500402447.
- Driessnack M, Sousa VD, Mendes IA. An overview of research designs relevant to nursing: Part 3: mixed and multiple methods. Rev Lat Am Enfermagem 2007;15:1046-9. https://doi.org/10.1590/S0104-11692007000400025.
- Johnson RB, Onwuegbuzie AJ. Mixed methods research: a research paradigm whose time has come. Educ Res 2004;33:14-26. https://doi.org/10.3102/0013189X033007014.
- Dreyfus H, Burke J. Competency Based Education and Training. London: Falmer Press; 1989.
- Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat 2005;28:19-26. https://doi.org/10.1016/j.jsat.2004.11.001.
- Kelders SM, Kok RN, Ossebaard HC, Van Gemert-Pijnen JE. Persuasive system design does matter: a systematic review of adherence to web-based interventions. J Med Internet Res 2012;14. https://doi.org/10.2196/jmir.2104.
- Krueger RA, Casey MA. Focus Groups: A Practical Guide For Applied Research. London: Sage Publications; 2014.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud 2016;2. https://doi.org/10.1186/s40814-016-0105-8.
- Delbaere K, Close JC, Mikolaizak AS, Sachdev PS, Brodaty H, Lord SR. The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing 2010;39:210-16. https://doi.org/10.1093/ageing/afp225.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j. 2002.384.doc.x.
- Position Statement on Use of the EQ-5D-5L Valuation Set. London: NICE; 2017.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Introductory Guide MedDRA Version 15.1. Chantilly, VA: International Federation of Pharmaceutical Manufacturers and Associations; 2012.
- LeVasseur JJ. The problem of bracketing in phenomenology. Qual Health Res 2003;13:408-20. https://doi.org/10.1177/1049732302250337.
- Brooks J, McCluskey S, Turley E, King N. The utility of template analysis in qualitative psychology research. Qual Res Psychol 2015;12:202-22. https://doi.org/10.1080/14780887.2014.955224.
- NHS Reference Costs 2016–2017. London: Department of Health and Social Care; 2017.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Brazier J, Ratcliffe J, Saloman J, Tsuchiya A. Measuring and Valuing Health Benefits for Economic Evaluation. Oxford: Oxford University Press; 2017.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Walters SJ, Bonacho Dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015276.
- Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop?. World J Clin Cases 2015;3:545-55. https://doi.org/10.12998/wjcc.v3.i7.545.
- Pilutti LA, Platta ME, Motl RW, Latimer-Cheung AE. The safety of exercise training in multiple sclerosis: a systematic review. J Neurol Sci 2014;343:3-7. https://doi.org/10.1016/j.jns.2014.05.016.
- Hawkes CH, Giovannoni G. The McDonald Criteria for Multiple Sclerosis: time for clarification. Mult Scler 2010;16:566-75. https://doi.org/10.1177/1352458510362441.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c869.
- Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials 2016;17. https://doi.org/10.1186/s13063-016-1391-4.
- Brigham GS, Feaster DJ, Wakim PG, Dempsey CL. Choosing a control group in effectiveness trials of behavioral drug abuse treatments. J Subst Abuse Treat 2009;37:388-97. https://doi.org/10.1016/j.jsat.2009.05.004.
- Goodwin VA, Pickering R, Ballinger C, Roberts H, McIntosh E, Lamb S, et al. A multi-centre, randomised controlled trial of the effectiveness of PDSAFE to prevent falls among people with Parkinson’s: study protocol. BMC Neurol 2015;15. https://doi.org/10.1186/s12883-015-0332-2.
- Coulter EH, Miller L, McCorkell S, McGuire C, Algie K, Freeman J, et al. Validity of the activPAL3 activity monitor in people moderately affected by multiple sclerosis. Med Eng Phys 2017;45:78-82. https://doi.org/10.1016/j.medengphy.2017.03.008.
- Marrie RA, Cohen J, Stuve O, Trojano M, Sørensen PS, Reingold S, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler 2015;21:263-81. https://doi.org/10.1177/1352458514564491.
- Mehta L, McNeill M, Hobart J, Wyrwich KW, Poon JL, Auguste P, et al. Identifying an important change estimate for the Multiple Sclerosis Walking Scale-12 (MSWS-12v1) for interpreting clinical trial results. Mult Scler J Exp Transl Clin 2015;1. https://doi.org/10.1177/2055217315596993.
- Kohn CG, Sidovar MF, Kaur K, Zhu Y, Coleman CI. Estimating a minimal clinically important difference for the EuroQol 5-Dimension health status index in persons with multiple sclerosis. Health Qual Life Outcomes 2014;12. https://doi.org/10.1186/1477-7525-12-66.
- Costelloe L, O’Rourke K, Kearney H, McGuigan C, Gribbin L, Duggan M, et al. The patient knows best: significant change in the physical component of the Multiple Sclerosis Impact Scale (MSIS-29 physical). J Neurol Neurosurg Psychiatry 2007;78:841-4. https://doi.org/10.1136/jnnp.2006.105759.
- Nilsagård Y, Lundholm C, Denison E, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis – a longitudinal study. Clin Rehabil 2009;23:259-69. https://doi.org/10.1177/0269215508095087.
- Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler 2008;14:383-90. https://doi.org/10.1177/1352458507082607.
- Connelly DM, Thomas BK, Cliffe SJ, Perry WM, Smith RE. Clinical utility of the 2-minute walk test for older adults living in long-term care. Physiother Can 2009;61:78-87. https://doi.org/10.3138/physio.61.2.78.
- Gijbels D, Alders G, Van Hoof E, Charlier C, Roelants M, Broekmans T, et al. Predicting habitual walking performance in multiple sclerosis: relevance of capacity and self-report measures. Mult Scler 2010;16:618-26. https://doi.org/10.1177/1352458510361357.
- Mann GC, Whitney SL, Redfern MS, Borello-France DF, Furman JM. Functional reach and single leg stance in patients with peripheral vestibular disorders. J Vestib Res 1996;6:343-53. https://doi.org/10.1016/0957-4271(96)00027-4.
- Morgan MT, Friscia LA, Whitney SL, Furman JM, Sparto PJ. Reliability and validity of the Falls Efficacy Scale-International (FES-I) in individuals with dizziness and imbalance. Otol Neurotol 2013;34:1104-8. https://doi.org/10.1097/MAO.0b013e318281df5d.
- Langeskov-Christensen D, Feys P, Baert I, Riemenschneider M, Stenager E, Dalgas U. Performed and perceived walking ability in relation to the Expanded Disability Status Scale in persons with multiple sclerosis. J Neurol Sci 2017;382:131-6. https://doi.org/10.1016/j.jns.2017.09.049.
- Freeman JA, Langdon DW, Hobart JC, Thompson AJ. The impact of inpatient rehabilitation on progressive multiple sclerosis. Ann Neurol 1997;42:236-44. https://doi.org/10.1002/ana.410420216.
- Khan F, Pallant JF, Brand C, Kilpatrick TJ. Effectiveness of rehabilitation intervention in persons with multiple sclerosis: a randomised controlled trial. J Neurol Neurosurg Psychiatry 2008;79:1230-5. https://doi.org/10.1136/jnnp.2007.133777.
- Sørensen J, Lee A, Løvendahl B, Nørgaard M, Bay J, Rasmussen PV, et al. Study protocol: to investigate effects of highly specialized rehabilitation for patients with multiple sclerosis. A randomized controlled trial of a personalized, multidisciplinary intervention. BMC Health Serv Res 2012;12. https://doi.org/10.1186/1472-6963-12-306.
- Freeman JA, Hendrie W, Creanor S, Jarrett L, Barton A, Green C, et al. Standing up in multiple sclerosis (SUMS): protocol for a multi-centre randomised controlled trial evaluating the clinical and cost effectiveness of a home-based self-management standing frame programme in people with progressive multiple sclerosis. BMC Neurol 2016;16. https://doi.org/10.1186/s12883-016-0581-8.
- Lincoln NB, Yuill F, Holmes J, Drummond AE, Constantinescu CS, Armstrong S, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler J 2011;17:1250-7. https://doi.org/10.1177/1352458511408753.
- Hobart J, Blight AR, Goodman A, Lynn F, Putzki N. Timed 25-foot walk: direct evidence that improving 20% or greater is clinically meaningful in MS. Neurology 2013;80:1509-17. https://doi.org/10.1212/WNL.0b013e31828cf7f3.
- Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials 2005;2:152-62. https://doi.org/10.1191/1740774505cn076oa.
- Pals SL, Murray DM, Alfano CM, Shadish WR, Hannan PJ, Baker WL. Individually randomized group treatment trials: a critical appraisal of frequently used design and analytic approaches. Am J Public Health 2008;98:1418-24. https://doi.org/10.2105/AJPH.2007.127027.
- Motl RW, Dlugonski D, Pilutti LA, Klaren RE. Does the effect of a physical activity behavioral intervention vary by characteristics of people with multiple sclerosis?. Int J MS Care 2015;17:65-72. https://doi.org/10.7224/1537-2073.2014-016.
- Nilsagård Y, Westerdahl E, Wittrin A, Gunnarsson M. Walking distance as a predictor of falls in people with multiple sclerosis. Physiother Res Int 2016;21:102-8. https://doi.org/10.1002/pri.1625.
- Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol 2007;60:1234-8. https://doi.org/10.1016/j.jclinepi.2007.02.006.
- Norris M, Kilbride C. From dictatorship to a reluctant democracy: stroke therapists talking about self-management. Disabil Rehabil 2014;36:32-8. https://doi.org/10.3109/09638288.2013.776645.
- Jones F, Livingstone E, Hawkes L. ‘Getting the balance between encouragement and taking over’: reflections on using a new stroke self-management programme. Physiother Res Int 2013;18:91-9. https://doi.org/10.1002/pri.1531.
- Lawn S, McMillan J, Pulvirenti M. Chronic condition self-management: expectations of responsibility. Patient Educ Couns 2011;84:e5-8. https://doi.org/10.1016/j.pec.2010.07.008.
- Learmonth YC, Adamson BC, Kinnett-Hopkins D, Bohri M, Motl RW. Results of a feasibility randomised controlled study of the guidelines for exercise in multiple sclerosis project. Contemp Clin Trials 2017;54:84-97. https://doi.org/10.1016/j.cct.2016.11.012.
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;9. https://doi.org/10.1002/14651858.CD007146.pub3.
- El-Khoury F, Cassou B, Charles MA, Dargent-Molina P. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347. https://doi.org/10.1136/bmj.f6234.
- Robertson MC, Campbell AJ, Gardner MM, Devlin N. Preventing injuries in older people by preventing falls: a meta-analysis of individual-level data. J Am Geriatr Soc 2002;50:905-11. https://doi.org/10.1046/j.1532-5415.2002.50218.x.
- Frost R, Levati S, McClurg D, Brady M, Williams B. What adherence measures should be used in trials of home-based rehabilitation interventions? A systematic review of the validity, reliability, and acceptability of measures. Arch Phys Med Rehabil 2017;98:1241-56. https://doi.org/10.1016/j.apmr.2016.08.482.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Jarret L, Nandi P, Thompson AJ. Managing severe lower limb spasticity in multiple sclerosis: does intrathecal phenol have a role. J Neurol Neurosurg Psychiatry 2002;73:705-9.
Appendix 1 Daily diary example
Appendix 2 Fidelity assessment generic scoring
This scale was used to score session-specific items. The four core items were assessed using indivualised criteria, which are included in the main body of the report:
-
0: absence (or very limited/highly inappropriate coverage) of the item and lack of discussion.
-
1: some coverage of the item. Some key elements are omitted or only superficially discussed.
-
2: appropriate coverage of the item. Discussion lacks depth and/or clarity in some areas.
-
3: appropriate coverage of the item. Appropriate depth of discussion.
Appendix 3 Unit costs of BRiMS resource items
| Resource item | Unit cost (£, 2016) | Source of cost estimate | Basis of cost estimate |
|---|---|---|---|
| Primary care | |||
| GP contacts (surgery) | 27.00 | PSSRU 2016, p. 145125 | Surgery consultation, 9.22 minutes |
| GP contacts (home) | 34.20 | PSSRU 2016, p. 145;125 PSSRU 2015, p. 176126 |
Per minute of patient contact = £3 (allows for average of 12 minutes travel time per visit) Home visit, 11.4 minutes |
| GP telephone calls | 21.30 | PSSRU 2016, p. 145;125 PSSRU 2015, p. 176126 |
Per minute of patient contact = £3 Telephone call, 7.1 minutes |
| MS specialist nurse contacts (home) | 68.00 | NHS Reference Costs 2016/2017 124 | Community health services, other specialist nursing, adult, face to face |
| MS specialist nurse telephone calls | 33.00 | NHS Reference Costs 2016/2017 124 | Community health services, other specialist nursing, adult, not face to face |
| Physiotherapist contacts (home) | 53.00 | NHS Reference Costs 2016/2017 124 | Community health services, physiotherapist, adult, one to one |
| Occupational therapist contacts (home) | 77.00 | NHS Reference Costs 2016/2017 124 | Community health services, occupational therapist, adult, one to one |
| Practice nurse contacts (surgery) | 9.30 | PSSRU 2016, p. 143;125 PSSRU 2015, p. 174126 | £36 per hour, 15.5-minute consultation |
| Community nurse contacts (home) | 37.00 | NHS Reference Costs 2016/2017 124 | Community health services, district nurse, adult, face to face |
| Chiropodist/podiatrist contacts (surgery) | 41.00 | NHS Reference Costs 2016/2017 124 | Community health services, podiatrist, tier 1, general podiatry |
| Continence advisor contacts | 83.00 | NHS Reference Costs 2016/2017 124 | Community health services, specialist nursing, adult, face to face |
| Community psychiatric nurse contacts (home) | 36.00 | PSSRU 2016, p. 142125 | £36 per hour, 1-hour visit |
| Counsellor contacts | 44.00 | PSSRU 2016, p. 137125 | Band 6 scientific and professional staff; £44 per hour, 1-hour consultation |
| Secondary care | |||
| Hospital stays (nights) | 455.17 | NHS Reference Costs 2016/2017 124 | Medical care of patients with MS, non-elective stays (mean across complication and comorbidities scores by mean length of stay, £3420/7.51 days) |
| Visits to A&E | 147.80 | NHS Reference Costs 2016/2017 124 | Outpatient attendance data, A&E |
| Days in hospital | 369.00 | NHS Reference Costs 2016/2017 124 | Medical care of patients with MS, day cases (mean across complication and comorbidities scores) |
| Neurologist | 167.50 | NHS Reference Costs 2016/2017 124 | Outpatient attendance data, neurology |
| MS specialist nurse | 44.00 | PSSRU 2016, p. 188125 | Hospital-based nurses. Band 6 scientific and professional staff, £44 per hour |
| Occupational therapist | 64.99 | NHS Reference Costs 2016/2017 124 | Outpatient attendance data, occupational therapy |
| Physiotherapist | 48.81 | NHS Reference Costs 2016/2017 124 | Outpatient attendance data, physiotherapy |
| Ophthalmologist | 55.99 | NHS Reference Costs 2016/2017 124 | Outpatient attendance data, medical ophthalmology |
| Orthotist | 119.07 | NHS Reference Costs 2016/2017 124 | Outpatient attendance data, orthotics |
| Chiropodist | 46.64 | NHS Reference Costs 2016/2017 124 | Outpatient attendance data, podiatry |
| Speech therapist | 96.52 | NHS Reference Costs 2016/2017 124 | Outpatient attendance data, speech and language therapy |
| Psychologist | 168.65 | NHS Reference Costs 2016/2017 124 | Outpatient attendance data, clinical psychology |
| Psychiatrist | 142.00 | NHS Reference Costs 2016/2017 124 | Mental health, other psychiatric liaison services, adult and elderly |
| Pain clinic | 139.23 | NHS Reference Costs 2016/2017 124 | Outpatient attendance data, pain management |
| Continence advisor | 109.40 | NHS Reference Costs 2016/2017 124 | Outpatient attendance data, urology |
| New wheelchair | 191.00 | PSSRU 2016, p. 96125 | Per active user chair per year |
| New cushion | 31.99 | www.uk-wheelchairs.co.uk | This is the source used by PSSRU for wheelchair costs, based on a mid-price-range cushion |
| Medication use (per week of use) | |||
| Disease-modifying medicine | 163.50 | British National Formulary 70, p. 730170 | Multiple sclerosis, interferon beta; once-weekly injection; 12 pre-filled injections = £1962.00 |
| Botulinum toxin injections | 46.07 | British National Formulary 70, p. 324170 | Total dose of 400 units in 3-month period. Botox Allergan 200-unit powder for solution for injection vials: £276.40 |
| Phenol injections | 4.90 | British National Formulary 70, p. 81;170 Jarrett paper171 | 5% phenol in glycerol, between 1.5 and 2.5 ml. 10 × 5-ml ampoules = £47.91–50.00. Doses at least 6 weeks apart (mean of three doses) |
| Intravenous steroids | 58.00 | British National Formulary 70, p. 584170 |
Intravenous methylprednisolone: 500 mg to 1 g daily, for 3–5 days 500-mg vial of methylprednisolone powder with solvent, £9.60; 1 g vial = £17.30 Requires 3 × 500-mg vials to 5 × 1 g vials, £28.80 to £86.50 |
| Steroid tablets | 145.00 | British National Formulary 70, p. 584170 |
Oral methylprednisolone: 500 mg to 2 g daily, for 3–5 days 20 × 100-mg tablet pack, £48.32 15–100 tablets (1–5 packs), £48.32–241.60 |
| Social care | |||
| Home-care worker contacts | 6.90 | PSSRU 2016, p. 160125 | Mean hourly cost of all home care. £18 per hour, 23-minute visit |
| Social worker/care manager contacts | 55.00 | PSSRU 2016, p. 156125 | Per hour of client-related work |
| Rehabilitation unit stays (days) | 98.73 | PSSRU 2016, p. 197;125 PSSRU 2015–6, p. 40125,126 | Community rehabilitation unit, £691.13 per person, per week. 2014 cost of £671 uprated by 3% based on PSSRU’s annual percentage increases for adult service (all sectors – pay and prices including capital) |
| Respite unit/facility stays (day) | 141.00 | PSSRU 2016, p. 65125 | Local authority own-provision care homes for adults requiring physical support |
| Day-care centre (days) | 87.00 | PSSRU 2016, p. 67125 | Day care for adults requiring physical support |
| Informal care | 18.00 | Same as home-care cost | Based on replacement cost approach |
Appendix 4 Gantt chart
FIGURE 13.
The BriMS trial Gantt chart. HTA, Health Technology Assessment. The type of meeting held is referred to by ×.
Appendix 5 Correlations between outcome measures at each assessment time-point
| Variable | Time point | Correlation with baseline | |||
|---|---|---|---|---|---|
| Coefficient | 95% CI | Lower 90% one-sided CI bound | Lower 80% one-sided CI bound | ||
| Potential primary outcomes | |||||
| MSWS-12vs2 (imputed) | Week 15 | 0.54 | 0.31 to 0.71 | 0.40 | 0.45 |
| Week 27 | 0.59 | 0.36 to 0.76 | 0.45 | 0.50 | |
| EQ-5D-3L (crosswalk) | Week 15 | 0.46 | 0.21 to 0.66 | 0.30 | 0.36 |
| Week 27 | 0.50 | 0.24 to 0.69 | 0.33 | 0.39 | |
| EQ-5D-5L (VAS) | Week 15 | 0.66 | 0.46 to 0.79 | 0.54 | 0.58 |
| Week 27 | 0.60 | 0.37 to 0.76 | 0.46 | 0.51 | |
| MSIS-29vs2 (physical) | Week 15 | 0.75 | 0.59 to 0.85 | 0.65 | 0.68 |
| Week 27 | 0.77 | 0.61 to 0.87 | 0.67 | 0.71 | |
| MSIS-29vs2 (psychological) | Week 15 | 0.73 | 0.56 to 0.84 | 0.63 | 0.67 |
| Week 27 | 0.82 | 0.69 to 0.9 | 0.75 | 0.77 | |
| Potential secondary outcomes | |||||
| 2MWT | Week 15 | 0.83 | 0.72 to 0.9 | 0.76 | 0.79 |
| Week 27 | 0.83 | 0.72 to 0.9 | 0.69 | 0.72 | |
| Mini-BEST | Week 15 | 0.63 | 0.42 to 0.77 | 0.50 | 0.55 |
| Week 27 | 0.75 | 0.58 to 0.86 | 0.65 | 0.69 | |
| Forward FRT | Week 15 | 0.75 | 0.59 to 0.85 | 0.65 | 0.69 |
| Week 27 | 0.67 | 0.46 to 0.81 | 0.55 | 0.59 | |
| Lateral FRT | Week 15 | 0.62 | 0.41 to 0.77 | 0.49 | 0.54 |
| Week 27 | 0.67 | 0.46 to 0.81 | 0.54 | 0.59 | |
| FES-I | Week 15 | 0.57 | 0.34 to 0.73 | 0.43 | 0.48 |
| Week 27 | 0.63 | 0.41 to 0.78 | 0.49 | 0.54 | |
| CPI 1 | Week 15 | 0.44 | 0.17 to 0.65 | 0.27 | 0.33 |
| Week 27 | 0.49 | 0.22 to 0.69 | 0.32 | 0.38 | |
| CPI 2 | Week 15 | 0.69 | 0.51 to 0.82 | 0.58 | 0.62 |
| Week 27 | 0.73 | 0.55 to 0.84 | 0.62 | 0.66 | |
| CPI 3 | Week 15 | 0.78 | 0.64 to 0.87 | 0.70 | 0.73 |
| Week 27 | 0.82 | 0.68 to 0.9 | 0.74 | 0.77 | |
Appendix 6 Original estimate of therapist resource requirement for delivery of BRiMS
| Week | BRiMS activities (participant and therapist) | Therapist administrative and technical activities |
|---|---|---|
| 1 |
Session 1: individual assessment and introduction to the programme Takes place at local health-care establishment Expected time commitment (participant): 1 hour Expected time commitment (therapist): 1 hour per participant |
Write-up of session and development of exercise prescription (including setting up web-based exercise programme) Expected time commitment (therapist): 30 minutes per participant |
| 2 |
Session 2: home visit by BRiMS therapist to explain and set up exercise programme Expected time commitment (participant): 1.25 hours Expected time commitment (therapist): 2 hours per visit (inclusive of travel) |
Write-up of session and fidelity checklist Expected time commitment (therapist): 30 minutes per participant |
| 2–4 |
Home-based individual practice of exercise programme, plus education activities, with online support from the BRiMS therapist Expected time commitment (participant): 3 hours per week |
Online review and adjustment of web-based exercise prescription 15 minutes per review × 6 over the programme Expected time commitment (therapist): 1.5 hours over 12 weeks per participant |
| 4 |
Group session 1: group exercise and education activities Takes place at local health-care establishment Expected time commitment (participant): 2 hours Expected time commitment (therapist): 2 hours |
Group set-up and clearing away Write-up of session and fidelity checklist Expected time commitment (therapist): 45 minutes per group |
| 5–8 |
Home-based practice of exercise programme, plus education activities Expected time commitment (participant): 3 hours per week |
|
| 8 |
Group session 2: group exercise and education activities Takes place at local health-care establishment Expected time commitment (participant): 2 hours |
Group set-up and clearing away Write-up of session and fidelity checklist Expected time commitment (therapist): 45 minutes per group |
| 9–13 |
Home-based practice of exercise programme, plus education activities Expected time commitment (participant): 3 hours per week |
|
| 13 |
BRiMS group session 3: group exercise and education activities Takes place at local health-care establishment Expected time commitment (participant): 2 hours Expected time commitment (therapist): 2 hours |
Group set-up and clearing away Write-up of session and fidelity checklist Expected time commitment (therapist): 45 minutes per group |
Appendix 7 Detailed breakdown of actual contact/administration time for delivery of BRiMS one-to-one sessions
| Site | Session (participants) | ||||
|---|---|---|---|---|---|
| 1 (n = 5) | 2 (n = 9) | 3 (n = 4) | 4 (n = 9) | All | |
| Clinic visit (minutes), mean (SD) | |||||
| Contact time | 90 (0) | 101 (18) | 82 (16) | 92 (5) | 93 (13) |
| Administration time | 47 (13) | 43 (10) | 60 (40) | 55 (11) | 50 (18) |
| Total clinic visit time | 137 (13) | 144 (13) | 142 (47) | 147 (13) | 144 (20) |
| Home visit (minutes), mean (SD) | |||||
| Contact time | 93 (7) | 108 (9) | 116 (28) | 89 (15) | 100 (18) |
| Administration time | 51 (13) | 14 (4) | 39 (8) | 28 (15) | 36 (19) |
| Total home visit time | 144 (17) | 123 (16) | 155 (33) | 137 (14) | 136 (20) |
| Online contacts, n (%) | 23 (11) | 10 (2) | 16 (3) | 15 (0) | 15 (7) |
Appendix 8 Resource-use costs over the27 weeks of the trial
| Service | Group | |||
|---|---|---|---|---|
| Usual care | Intervention | |||
| Mean (SD) (£) | n | Mean (SD) (£) | n | |
| Primary care appointments/visits | ||||
| Continence advisor: home | 0.00 (0.00) | 22 | 0.00 (0.00) | 22 |
| Community psychiatric nurse: home | 0.00 (0.00) | 22 | 0.00 (0.00) | 22 |
| Chiropodist: home | 6.00 (21.93) | 22 | 1.50 (7.04) | 22 |
| Chiropodist: surgery | 3.73 (12.06) | 22 | 9.32 (25.09) | 22 |
| Counsellor | 0.00 (0.00) | 22 | 8.00 (37.52) | 22 |
| GP: home | 3.11 (14.58) | 22 | 12.44 (24.85) | 22 |
| GP: surgery | 65.05 (75.76) | 22 | 47.86 (71.64) | 22 |
| GP: telephone | 11.62 (18.27) | 22 | 29.05 (41.34) | 22 |
| MS nurse: home | 6.18 (29.00) | 22 | 3.09 (14.50) | 22 |
| MS nurse: telephone | 12.00 (29.77) | 22 | 7.50 (17.44) | 22 |
| Occupational therapist: home | 66.50 (137.12) | 22 | 21.00 (54.09) | 22 |
| Community nurse: home | 1.68 (7.89) | 22 | 8.41 (39.44) | 22 |
| Practice nurse: surgery | 10.15 (13.43) | 22 | 10.15 (17.90) | 22 |
| Physiotherapist: home | 26.50 (74.51) | 22 | 31.32 (68.73) | 22 |
| Total primary care | 212.51 (223.50) | 22 | 189.63 (162.10) | 22 |
| Secondary care outpatient appointments | ||||
| Continence advisor | 14.92 (38.43) | 22 | 34.81 (91.76) | 22 |
| Chiropodist | 6.36 (21.81) | 22 | 4.24 (13.72) | 22 |
| MS nurse | 26.00 (37.58) | 22 | 14.00 (20.98) | 22 |
| Neurologist | 83.75 (112.66) | 22 | 68.52 (133.39) | 22 |
| Occupational therapist | 2.95 (13.86) | 22 | 8.86 (30.39) | 22 |
| Ophthalmologist | 7.64 (19.67) | 22 | 7.64 (19.67) | 22 |
| Orthotist | 27.06 (72.86) | 22 | 27.06 (81.60) | 22 |
| Pain clinic | 18.99 (65.10) | 22 | 56.96 (267.16) | 22 |
| Physiotherapist | 119.81 (132.80) | 22 | 53.25 (181.33) | 22 |
| Psychiatrist | 0.00 (0.00) | 22 | 0.00 (0.00) | 22 |
| Psychologist | 7.67 (35.96) | 22 | 15.33 (49.62) | 22 |
| Speech therapist | 0.00 (0.00) | 22 | 0.00 (0.00) | 22 |
| Secondary care attendances/admissions | ||||
| Nights in hospital | 0.00 (0.00) | 22 | 1096.55 (4317.34) | 22 |
| A&E visits | 33.41 (89.95) | 22 | 13.36 (43.25) | 22 |
| Day admissions | 0.00 (0.00) | 22 | 50.32 (236.01) | 22 |
| Total secondary care | 348.55 (249.24) | 22 | 1450.89 (4334.60) | 22 |
| Social and community care visits | ||||
| Social worker: home visit | 0.00 (0.00) | 22 | 2.50 (11.73) | 22 |
| Home-care visit | 65.86 (308.93) | 22 | 0.94 (4.41) | 22 |
| Day-care centre days | 0.00 (0.00) | 22 | 0.00 (0.00) | 22 |
| Rehabilitation unit days | 13.46 (46.16) | 22 | 278.24 (975.78) | 22 |
| Respite care days | 0.00 (0.00) | 22 | 0.00 (0.00) | 22 |
| Total social and community care | 79.33 (309.37) | 22 | 281.68 (986.43) | 22 |
| Total health and social care | 640.38 (580.26) | 22 | 1922.20 (5340.09) | 22 |
| Medication | Group | |||
|---|---|---|---|---|
| Usual care | Intervention | |||
| Mean (SD) (£) | n | Mean (SD) (£) | n | |
| Disease-modifying medications | 252.68 (912.04) | 22 | 44.59 (209.15) | 22 |
| Botulinum toxin injections | 2.09 (9.82) | 22 | 0.00 (0.00) | 22 |
| Intrathecal baclofen | 0.00 (0.00) | 22 | 0.00 (0.00) | 22 |
| Phenol injections | 0.00 (0.00) | 22 | 0.00 (0.00) | 22 |
| Intravenous steroids | 0.00 (0.00) | 22 | 0.00 (0.00) | 22 |
| Steroid tablets | 6.59 (30.91) | 22 | 0.00 (0.00) | 22 |
| Task | Group | |||
|---|---|---|---|---|
| Usual care | Intervention | |||
| Mean (SD) (£) | n | Mean (SD) (£) | n | |
| DIY | 31.70 (37.07) | 22 | 59.52 (79.63) | 22 |
| Gardening | 25.16 (40.22) | 22 | 15.95 (26.19) | 22 |
| Housework | 111.27 (191.80) | 22 | 98.59 (132.26) | 22 |
| Preparing meals | 103.09 (121.79) | 22 | 112.30 (145.42) | 22 |
| Personal care | 20.11 (47.52) | 22 | 13.91 (27.14) | 22 |
| Looking after pets | 37.23 (86.25) | 22 | 32.11 (51.78) | 22 |
| Shopping | 41.93 (40.43) | 22 | 41.32 (36.50) | 22 |
| Transport | 73.64 (89.47) | 22 | 71.59 (83.48) | 22 |
| Total cost of weekly informal care | 444.14 (441.27) | 22 | 445.30 (387.59) | 22 |
| Days off work over previous 6 months | ||||
| Friend/relative’s days off work | 72.28 (339.02) | 22 | 0.00 (0.00) | 22 |
Appendix 9 Baseline (previous 6 months) resource use and costs data
Health and social care use and cost at baseline
| Resource | Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||||
| Mean (SD) | Minimum | Maximum | n | Mean (SD) | Minimum | Maximum | n | |
| Primary care appointments/visits | ||||||||
| Continence advisor: home | 0.08 (0.39) | 0 | 2 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Community psychiatric nurse: home | 0.00 (0.00) | 0 | 0 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Chiropodist: home | 0.08 (0.27) | 0 | 1 | 26 | 0.07 (0.37) | 0 | 2 | 29 |
| Chiropodist: surgery | 0.08 (0.27) | 0 | 1 | 26 | 0.17 (0.47) | 0 | 2 | 29 |
| Counsellor | 0.00 (0.00) | 0 | 0 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| GP: home | 0.04 (0.20) | 0 | 1 | 26 | 0.21 (0.49) | 0 | 2 | 29 |
| GP: surgery | 1.35 (2.40) | 0 | 12 | 26 | 1.03 (1.09) | 0 | 3 | 29 |
| GP: telephone | 0.69 (0.84) | 0 | 3 | 26 | 0.83 (1.07) | 0 | 3 | 29 |
| MS nurse: home | 0.08 (0.27) | 0 | 1 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| MS nurse: telephone | 0.35 (0.56) | 0 | 2 | 26 | 0.14 (0.35) | 0 | 1 | 29 |
| Occupational therapist: home | 0.23 (0.71) | 0 | 3 | 26 | 0.07 (0.26) | 0 | 1 | 29 |
| Community nurse: home | 0.58 (2.74) | 0 | 14 | 26 | 0.07 (0.26) | 0 | 1 | 29 |
| Practice nurse: surgery | 0.35 (0.80) | 0 | 3 | 26 | 0.31 (0.71) | 0 | 3 | 29 |
| Physiotherapist: home | 0.00 (0.00) | 0 | 0 | 26 | 0.10 (0.56) | 0 | 3 | 29 |
| Secondary care outpatient appointments | ||||||||
| Continence advisor | 0.04 (0.20) | 0 | 1 | 26 | 0.17 (0.38) | 0 | 1 | 29 |
| Chiropodist | 0.12 (0.33) | 0 | 1 | 26 | 0.07 (0.26) | 0 | 1 | 29 |
| MS nurse | 0.50 (0.65) | 0 | 2 | 26 | 0.34 (0.48) | 0 | 1 | 29 |
| Neurologist | 0.35 (0.49) | 0 | 1 | 26 | 0.17 (0.38) | 0 | 1 | 29 |
| Occupational therapist | 0.04 (0.20) | 0 | 1 | 26 | 0.10 (0.31) | 0 | 1 | 29 |
| Ophthalmologist | 0.08 (0.27) | 0 | 1 | 26 | 0.10 (0.41) | 0 | 2 | 29 |
| Orthotist | 0.15 (0.46) | 0 | 2 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Pain clinic | 0.00 (0.00) | 0 | 0 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Physiotherapist | 1.58 (3.40) | 0 | 11 | 26 | 0.31 (0.85) | 0 | 4 | 29 |
| Psychiatrist | 0.00 (0.00) | 0 | 0 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Psychologist | 0.04 (0.20) | 0 | 1 | 26 | 0.03 (0.19) | 0 | 1 | 29 |
| Speech therapist | 0.04 (0.20) | 0 | 1 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Secondary care attendances/admissions | ||||||||
| Nights in hospital | 1.54 (6.50) | 0 | 33 | 26 | 0.52 (1.94) | 0 | 10 | 29 |
| A&E visits | 0.12 (0.43) | 0 | 2 | 26 | 0.10 (0.31) | 0 | 1 | 29 |
| Day admissions | 0.00 (0.00) | 0 | 0 | 26 | 0.14 (0.58) | 0 | 3 | 29 |
| Social and community care visits | ||||||||
| Social worker: home visit | 0.00 (0.00) | 0 | 0 | 26 | 0.03 (0.19) | 0 | 1 | 29 |
| Home-care visit | 7.38 (35.29) | 0 | 180 | 26 | 0.14 (0.74) | 0 | 4 | 29 |
| Day-care centre days | 0.00 (0.00) | 0 | 0 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Rehabilitation unit days | 0.69 (2.09) | 0 | 10 | 26 | 0.28 (0.92) | 0 | 4 | 29 |
| Respite care days | 0.00 (0.00) | 0 | 0 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Costs | Group | |||
|---|---|---|---|---|
| Usual care | Intervention | |||
| Mean (SD) (£) | n | Mean (SD) (£) | n | |
| Primary care appointments/visits | ||||
| Continence advisor: home | 6.38 (32.56) | 26 | 0.00 (0.00) | 29 |
| Community psychiatric nurse: home | 0.00 (0.00) | 26 | 0.00 (0.00) | 29 |
| Chiropodist: home | 2.54 (8.97) | 26 | 2.28 (12.26) | 29 |
| Chiropodist: surgery | 3.15 (11.14) | 26 | 7.07 (19.20) | 29 |
| Counsellor | 0.00 (0.00) | 26 | 0.00 (0.00) | 29 |
| GP: home | 1.32 (6.71) | 26 | 7.08 (16.80) | 29 |
| GP: surgery | 36.35 (64.77) | 26 | 27.93 (29.30) | 29 |
| GP: telephone | 14.75 (17.84) | 26 | 17.63 (22.82) | 29 |
| MS nurse: home | 5.23 (18.48) | 26 | 0.00 (0.00) | 29 |
| MS nurse: telephone | 11.42 (18.53) | 26 | 4.55 (11.58) | 29 |
| Occupational therapist: home | 17.77 (54.70) | 26 | 5.31 (19.86) | 29 |
| Community nurse: home | 21.35 (101.56) | 26 | 2.55 (9.54) | 29 |
| Practice nurse: surgery | 3.22 (7.41) | 26 | 2.89 (6.62) | 29 |
| Physiotherapist: home | 0.00 (0.00) | 26 | 5.48 (29.53) | 29 |
| Total primary care | 123.47 (157.86) | 26 | 82.76 (74.99) | 29 |
| Secondary care outpatient appointments | ||||
| Continence advisor | 4.21 (21.46) | 26 | 18.86 (42.06) | 29 |
| Chiropodist | 5.38 (15.20) | 26 | 3.22 (12.03) | 29 |
| MS nurse | 22.00 (28.52) | 26 | 15.17 (21.28) | 29 |
| Neurologist | 57.98 (81.27) | 26 | 28.88 (64.39) | 29 |
| Occupational therapist | 2.50 (12.75) | 26 | 6.72 (20.14) | 29 |
| Ophthalmologist | 4.31 (15.22) | 26 | 5.79 (22.91) | 29 |
| Orthotist | 18.32 (55.26) | 26 | 0.00 (0.00) | 29 |
| Pain clinic | 0.00 (0.00) | 26 | 0.00 (0.00) | 29 |
| Physiotherapist | 76.97 (165.77) | 26 | 15.15 (41.46) | 29 |
| Psychiatrist | 0.00 (0.00) | 26 | 0.00 (0.00) | 29 |
| Psychologist | 6.49 (33.07) | 26 | 5.82 (31.32) | 29 |
| Speech therapist | 3.71 (18.93) | 26 | 0.00 (0.00) | 29 |
| Secondary care attendances/admissions | ||||
| Nights in hospital | 700.26 (2958.90) | 26 | 235.43 (882.45) | 29 |
| A&E visits | 16.96 (63.42) | 26 | 15.21 (45.56) | 29 |
| Day admissions | 0.00 (0.00) | 26 | 50.90 (214.35) | 29 |
| Total secondary care | 919.09 (3007.45) | 26 | 401.15 (917.84) | 29 |
| Social and community care visits | ||||
| Social worker: home visit | 0.00 (0.00) | 26 | 1.90 (10.21) | 29 |
| Home-care visit | 50.95 (243.47) | 26 | 0.95 (5.13) | 29 |
| Day-care centre days | 0.00 (0.00) | 26 | 0.00 (0.00) | 29 |
| Rehabilitation unit days | 68.35 (206.66) | 26 | 27.24 (91.01) | 29 |
| Respite care days | 0.00 (0.00) | 26 | 0.00 (0.00) | 29 |
| Total social and community care | 119.31 (309.92) | 26 | 30.08 (90.83) | 29 |
| Total health and social care | 1161.87 (3113.23) | 26 | 513.99 (984.65) | 29 |
Medication use and cost per person at baseline
| Medication | Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||||
| Mean (SD) | Minimum | Maximum | n | Mean (SD) | Minimum | Maximum | n | |
| Disease-modifying medications | 0.92 (3.26) | 0 | 12 | 26 | 0.41 (2.23) | 0 | 12 | 29 |
| Botulinum toxin injections | 0.04 (0.20) | 0 | 1 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Intrathecal baclofen | 0.00 (0.00) | 0 | 0 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Phenol injections | 0.00 (0.00) | 0 | 0 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Intravenous steroids | 0.00 (0.00) | 0 | 0 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Steroid tablets | 0.04 (0.20) | 0 | 1 | 26 | 0.00 (0.00) | 0 | 0 | 29 |
| Medication | Group | |||
|---|---|---|---|---|
| Usual care | Intervention | |||
| Mean (SD) (£) | n | Mean (SD) (£) | n | |
| Disease-modifying medications | 150.92 (533.17) | 26 | 67.66 (364.33) | 29 |
| Botulinum toxin injections | 1.77 (9.04) | 26 | 0.00 (0.00) | 29 |
| Intrathecal baclofen | 0.00 (0.00) | 26 | 0.00 (0.00) | 29 |
| Phenol injections | 0.00 (0.00) | 26 | 0.00 (0.00) | 29 |
| Intravenous steroids | 0.00 (0.00) | 26 | 0.00 (0.00) | 29 |
| Steroid tablets | 5.58 (28.44) | 26 | 0.00 (0.00) | 29 |
Informal care use and cost per person at baseline by type of task and days off work
| Care provided | Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Usual care | Intervention | |||||||
| Mean (SD) | Minimum | Maximum | n | Mean (SD) | Minimum | Maximum | n | |
| DIY | 1.08 (1.79) | 0 | 6 | 26 | 2.55 (8.84) | 0 | 48 | 29 |
| Gardening | 1.69 (4.01) | 0 | 20 | 26 | 1.13 (3.78) | 0 | 20 | 29 |
| Housework | 2.63 (3.68) | 0 | 14 | 26 | 3.76 (5.24) | 0 | 15 | 29 |
| Preparing meals | 1.94 (2.78) | 0 | 10 | 26 | 3.72 (5.93) | 0 | 25 | 29 |
| Personal care | 1.05 (2.42) | 0 | 9 | 26 | 1.06 (2.06) | 0 | 7 | 29 |
| Looking after pets | 0.92 (2.17) | 0 | 7 | 26 | 1.11 (2.62) | 0 | 10 | 29 |
| Shopping | 1.31 (1.64) | 0 | 6 | 26 | 1.62 (2.66) | 0 | 12 | 29 |
| Transport | 2.54 (4.09) | 0 | 15 | 26 | 2.84 (4.71) | 0 | 20 | 29 |
| Total informal care | 13.17 (14.56) | 0 | 63 | 26 | 17.80 (20.69) | 0 | 78 | 29 |
| Days off work over previous 6 months | ||||||||
| Friend/relative’s days off work | 1.42 (6.27) | 0 | 32 | 26 | 0.05 (0.28) | 0 | 2 | 29 |
| Care provided | Group | |||
|---|---|---|---|---|
| Usual care | Intervention | |||
| Mean (SD) (£) | n | Mean (SD) (£) | n | |
| DIY | 19.38 (32.17) | 26 | 45.93 (159.09) | 29 |
| Gardening | 30.46 (72.09) | 26 | 20.33 (67.98) | 29 |
| Housework | 47.42 (66.31) | 26 | 67.66 (94.35) | 29 |
| Preparing meals | 34.96 (50.03) | 26 | 67.03 (106.80) | 29 |
| Personal care | 18.92 (43.53) | 26 | 19.14 (37.04) | 29 |
| Looking after pets | 16.62 (39.08) | 26 | 20.02 (47.16) | 29 |
| Shopping | 23.54 (29.59) | 26 | 29.17 (47.91) | 29 |
| Transport | 45.69 (73.64) | 26 | 51.21 (84.81) | 29 |
| Total cost of weekly informal care | 237.00 (262.16) | 26 | 320.48 (372.45) | 29 |
| Days off work over previous 6 months | ||||
| Friend/relative’s days off work | 174.07 (767.54) | 26 | 6.33 (34.07) | 29 |
Appendix 10 Potential associations between potential primary outcomes and baseline characteristics at week 27
Note that the 95% CIs are not available for categories if there was only one observation. The results of any category with a small number of participants should be viewed with caution as the small sample size provides weak data.
| Characteristic | n | Potential primary outcome, mean (95% CI) | ||||
|---|---|---|---|---|---|---|
| MSWS-12vs2 | EQ-5D-3L (crosswalk) | EQ-5D-5L (VAS) | MSIS-29vs2 (physical) | MSIS-29vs2 (psychological) | ||
| Agea | 44 | 0.0 (–0.3 to 0.3) | 0.1 (0.3 to 0.1) | 0.1 (0.1 to –0.2) | –0.1 (–0.2 to 0.4) | –0.2 (0.4 to 0.1) |
| Sex | ||||||
| Male | 13 | 75.5 (62.7 to 88.3) | 0.6 (0.5 to 0.7) | 55.0 (42.7 to 67.3) | 55.8 (40.7 to 70.8) | 45.3 (28.2 to 62.4) |
| Female | 31 | 78.2 (71.3 to 85.2) | 0.6 (0.5 to 0.6) | 57.3 (49.6 to 64.9) | 59.6 (52.8 to 66.4) | 40.1 (31.6 to 48.6) |
| Living arrangements | ||||||
| Alone | 10 | 75.0 (57.7 to 92.3) | 0.6 (0.5 to 0.8) | 58.1 (44.3 to 71.9) | 51.1 (33.7 to 68.5) | 37.5 (15.3 to 59.7) |
| With spouse/partner | 29 | 79.7 (72.9 to 86.4) | 0.6 (0.5 to 0.6) | 56.1 (48.2 to 63.9) | 61.5 (54.5 to 68.5) | 43.9 (35.9 to 52) |
| With parent(s) | 3 | 61.0 (13.7 to 108.3) | 0.6 (0 to 1.1) | 56.7 (–31.5 to 144.8) | 58.7 (–16.2 to 133.5) | 43.3 (–67.3 to 153.9) |
| With children | 7 | 82.6 (72.3 to 92.8) | 0.6 (0.4 to 0.8) | 52.0 (40.6 to 63.4) | 61.7 (46.2 to 77.2) | 42.9 (27.5 to 58.2) |
| Place of residence | ||||||
| Flat/apartment | 5 | 58.0 (24.7 to 91.3) | 0.7 (0.5 to 0.8) | 64.2 (50.5 to 77.9) | 44.0 (11 to 77) | 37.0 (4.3 to 69.7) |
| House/bungalow | 39 | 79.9 (74.4 to 85.5) | 0.6 (0.5 to 0.6) | 55.6 (48.7 to 62.5) | 60.3 (54.1 to 66.5) | 42.2 (34.2 to 50.2) |
| Occupation status | ||||||
| Unemployed | 2 | 86.5 (42 to 131) | 0.3 (0 to 0.7) | 27.5 (–4.3 to 59.3) | 87.5 (81.1 to 93.9) | 78.0 (–112.6 to 268.6) |
| Part-time work | 5 | 84.8 (71.8 to 97.8) | 0.5 (0 to 0.9) | 48.6 (28.9 to 68.3) | 66.4 (54.3 to 78.5) | 60.2 (42.6 to 77.8) |
| Full-time work | 3 | 61.7 (10.4 to 113) | 0.7 (0.2 to 1.2) | 63.3 (–17.5 to 144.1) | 41.7 (–15.9 to 99.3) | 33.3 (17.6 to 49.1) |
| Age retired | 9 | 70.9 (56.6 to 85.2) | 0.7 (0.5 to 0.8) | 68.6 (56.9 to 80.2) | 46.9 (31.5 to 62.3) | 33.8 (11.2 to 56.3) |
| Medically retired | 24 | 80.7 (72.1 to 89.3) | 0.6 (0.5 to 0.6) | 53.8 (45.9 to 61.6) | 62.1 (54.3 to 69.9) | 40.1 (30.7 to 49.5) |
| Other | 1 | 50.0 | 0.7 | 95.0 | 27.0 | 7.0 |
| Age at diagnosis a (years) | 44 | 0.1 (–0.2 to 0.4) | –0.1 (0.4 to –0.1) | 0.1 (–0.1 to –0.4) | 0.0 (–0.4 to 0.2) | 0.1 (0.2 to 0.1) |
| Time since last relapse | ||||||
| At least a year | 34 | 78.2 (72 to 84.5) | 0.6 (0.5 to 0.6) | 56.1 (48.6 to 63.5) | 60.0 (53.5 to 66.5) | 44.0 (35.7 to 52.3) |
| 3–12 months | 8 | 78.3 (64.3 to 92.2) | 0.5 (0.3 to 0.8) | 59.3 (42.4 to 76.1) | 55.5 (41.9 to 69.1) | 36.6 (13.9 to 59.3) |
| With 3 months | 1 | 100.0 | 0.7 | 60.0 | 88.0 | 41.0 |
| SDMT a | 44 | 0.3 (0.1 to 0.6) | –0.2 (0.6 to –0.2) | –0.2 (–0.2 to –0.5) | 0.3 (–0.5 to 0.1) | –0.1 (0.1 to –0.2) |
| EDSS score (points) | ||||||
| 4 | 1 | 40.0 | 0.9 | 90.0 | 25.0 | 4.0 |
| 4.5 | 1 | 38.0 | 0.9 | 95.0 | 15.0 | 26.0 |
| 5 | 1 | 83.0 | 0.6 | 90.0 | 53.0 | 0.0 |
| 5.5 | 0 | |||||
| 6 | 21 | 74.0 (65.6 to 82.5) | 0.6 (0.5 to 0.6) | 50.3 (42.7 to 58) | 57.5 (49 to 66) | 45.0 (34.3 to 55.6) |
| 6.5 | 18 | 82.9 (74.8 to 91.1) | 0.6 (0.5 to 0.7) | 57.4 (46.9 to 68) | 61.2 (51.6 to 70.7) | 42.3 (29.6 to 55) |
| 7 | 2 | 99.0 (86.3 to 111.7) | 0.4 (–2.3 to 3.1) | 62.5 (30.7 to 94.3) | 85.0 (–3.9 to 173.9) | 48.0 (–91.8 to 187.8) |
| Previous 4-week continence | ||||||
| Not at all | 22 | 71.8 (62.1 to 81.5) | 0.6 (0.5 to 0.7) | 60.0 (50.9 to 69.2) | 51.5 (43 to 60.1) | 35.5 (23.6 to 47.3) |
| Once | 2 | 78.5 (46.7 to 110.3) | 0.6 (–0.4 to 1.6) | 50.0 (–204.1 to 304.1) | 51.5 (32.4 to 70.6) | 40.5 (–4 to 85) |
| 2–4 times | 6 | 84.8 (68.6 to 101) | 0.5 (0.3 to 0.7) | 51.7 (37.3 to 66) | 75.8 (61.7 to 90) | 59.8 (41.6 to 78.1) |
| More than weekly | 8 | 83.6 (67 to 100.2) | 0.6 (0.4 to 0.8) | 58.1 (36 to 80.2) | 61.0 (39 to 83) | 40.3 (18.6 to 61.9) |
| Daily | 6 | 82.0 (69.6 to 94.4) | 0.6 (0.4 to 0.7) | 49.2 (30.3 to 68.1) | 65.3 (50 to 80.7) | 48.2 (28.6 to 67.7) |
| Continence device | ||||||
| No | 39 | 77.9 (71.7 to 84.1) | 0.6 (0.5 to 0.7) | 56.2 (49.6 to 62.8) | 57.8 (51.4 to 64.1) | 39.2 (31.8 to 46.5) |
| Yes | 5 | 73.6 (44.9 to 102.3) | 0.5 (0.2 to 0.9) | 60.0 (30.6 to 89.4) | 63.8 (29.6 to 98) | 60.8 (18.6 to 103) |
| Depressed | ||||||
| No | 26 | 72.6 (64 to 81.3) | 0.6 (0.5 to 0.7) | 60.6 (51.8 to 69.4) | 51.1 (42.8 to 59.5) | 32.5 (22.9 to 42.1) |
| Yes | 18 | 84.4 (77.5 to 91.3) | 0.5 (0.5 to 0.6) | 50.8 (42.1 to 59.5) | 69.1 (61.7 to 76.4) | 54.8 (44.9 to 64.6) |
| Lack of pleasure | ||||||
| No | 32 | 75.0 (67.6 to 82.3) | 0.6 (0.5 to 0.7) | 58.5 (50.9 to 66.2) | 54.8 (46.9 to 62.6) | 36.3 (27.5 to 45) |
| Yes | 12 | 84.0 (74.3 to 93.7) | 0.6 (0.5 to 0.7) | 51.5 (39.9 to 63.1) | 68.3 (60.6 to 75.9) | 55.9 (43 to 68.8) |
| Fear of falling | ||||||
| Not at all | 8 | 73.0 (50 to 96) | 0.7 (0.6 to 0.8) | 64.4 (50 to 78.8) | 52.4 (26.4 to 78.4) | 31.5 (5.3 to 57.7) |
| Somewhat | 13 | 70.3 (59 to 81.6) | 0.6 (0.5 to 0.7) | 59.8 (45.9 to 73.8) | 55.1 (44.6 to 65.5) | 41.8 (26 to 57.6) |
| Fairly | 9 | 81.7 (74.9 to 88.4) | 0.5 (0.3 to 0.7) | 46.3 (32.3 to 60.4) | 60.7 (49.1 to 72.2) | 51.4 (35.1 to 67.8) |
| Very | 13 | 83.2 (71.5 to 94.8) | 0.6 (0.4 to 0.7) | 56.6 (44 to 69.2) | 63.7 (51.5 to 75.9) | 41.7 (28.8 to 54.6) |
| Do not know | 1 | 93.0 | 0.7 | 45.0 | 63.0 | 30.0 |
| 3-month fall history | ||||||
| Not fallen | 1 | 40.0 | 0.7 | 80.0 | 50.0 | 22.0 |
| Twice | 10 | 74.5 (61 to 88) | 0.6 (0.5 to 0.8) | 66.2 (52.1 to 80.3) | 51.2 (37.1 to 65.3) | 38.6 (18 to 59.2) |
| 3–5 times | 19 | 79.6 (69 to 90.2) | 0.6 (0.5 to 0.7) | 52.5 (43.5 to 61.5) | 59.7 (48.1 to 71.3) | 43.6 (31.8 to 55.4) |
| More often | 14 | 79.3 (71.1 to 87.5) | 0.5 (0.4 to 0.7) | 53.6 (40.9 to 66.4) | 62.6 (53.7 to 71.4) | 42.5 (28.9 to 56.1) |
| Indoor walking aids | ||||||
| One stick/crutch | 19 | 76.4 (65.5 to 87.3) | 0.5 (0.4 to 0.6) | 53.9 (44.8 to 63) | 58.5 (48.4 to 68.5) | 42.3 (30.1 to 54.5) |
| Two sticks/crutches | 6 | 79.5 (61.4 to 97.6) | 0.6 (0.5 to 0.7) | 50.5 (31.3 to 69.7) | 63.8 (43.1 to 84.5) | 48.2 (26.3 to 70) |
| Walker/frame | 16 | 86.3 (80.1 to 92.4) | 0.5 (0.4 to 0.6) | 55.9 (46.1 to 65.8) | 65.2 (56.1 to 74.3) | 43.1 (30.2 to 55.9) |
| Wheelchair | 4 | 95.8 (87.7 to 103.8) | 0.3 (–0.3 to 0.9) | 51.3 (17.3 to 85.2) | 75.0 (54.7 to 95.3) | 40.8 (12.8 to 68.7) |
| Outdoor walking aids | ||||||
| One stick/crutch | 30 | 74.3 (66.8 to 81.9) | 0.6 (0.5 to 0.7) | 55.1 (47 to 63.2) | 57.1 (49.1 to 65.1) | 41.6 (31.9 to 51.3) |
| Two sticks/crutches | 12 | 83.9 (75.2 to 92.6) | 0.6 (0.5 to 0.7) | 57.0 (46.7 to 67.3) | 65.2 (53.7 to 76.6) | 50.7 (36.6 to 64.8) |
| Walker/frame | 18 | 75.8 (64.9 to 86.8) | 0.6 (0.5 to 0.7) | 57.5 (47.4 to 67.6) | 55.8 (43.9 to 67.8) | 37.7 (26.2 to 49.1) |
| Wheelchair | 21 | 78.8 (69.3 to 88.2) | 0.6 (0.5 to 0.7) | 50.4 (41.3 to 59.4) | 61.3 (51.2 to 71.4) | 42.5 (32.7 to 52.4) |
| Assistive devices | ||||||
| AFO | 13 | 78.6 (67.6 to 89.6) | 0.7 (0.6 to 0.7) | 61.5 (47.4 to 75.5) | 58.7 (45.4 to 72) | 38.2 (22.6 to 53.7) |
| Functional electrical stimulation | 12 | 77.1 (64.3 to 89.9) | 0.6 (0.6 to 0.7) | 60.5 (52.2 to 68.8) | 56.2 (43 to 69.3) | 44.2 (29 to 59.4) |
| Other | 3 | 88.7 (75.9 to 101.4) | 0.6 (0.4 to 0.8) | 53.3 (–29.4 to 136) | 70.0 (26.3 to 113.7) | 59.0 (–21.1 to 139.1) |
List of abbreviations
- 2MWT
- Two-Minute Walk Test
- AE
- adverse event
- BRiMS
- Balance Right in MS
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CPI
- Community Participation Indicators
- CRF
- case report form
- CRN
- Clinical Research Network
- CTU
- clinical trials unit
- DMC
- Data Monitoring Committee
- EDSS
- Expanded Disability Status Scale
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ETC
- excess treatment cost
- FES-I
- Falls Efficacy Scale – International
- FIT
- functional imagery training
- ICC
- intracluster correlation
- IQR
- interquartile range
- IT
- information technology
- Mini-BEST
- Mini-Balance Evaluation Systems Test
- MS
- multiple sclerosis
- MSIS-8D
- Multiple Sclerosis Impact Scale (8-dimensions)
- MSIS-29vs2
- MS Impact Scale (29-item) version 2
- MSWS-12vs2
- MS Walking Scale (12-item) version 2
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PenCTU
- Peninsula Clinical Trials Unit
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relapsing–remitting
- RRMS
- relapsing–remitting multiple sclerosis
- SAE
- serious adverse event
- SPMS
- secondary progressive multiple sclerosis
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
