Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/200/04. The contractual start date was in June 2014. The final report began editorial review in January 2018 and was accepted for publication in July 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The EME editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Chris A Rogers reports grants from the British Heart Foundation to April 2017, outside the submitted work. Chris A Rogers is a member of Clinical Trials Units funded by the National Institute for Health Research (NIHR) and the Health Technology Assessment (HTA) Commissioning Board. Melanie J Calvert reports personal fees from Ferring Pharmaceuticals (Saint-Prex, Switzerland), outside the submitted work. Rhiannon C Macefield has a patent Wound Healing Questionnaire pending to the University of Bristol. Stephen O’Brien reports grants from Saving Lives at Birth Partners, outside the submitted work. Tim Draycott reports personal fees from Ferring Pharmaceuticals, outside the submitted work. Barnaby C Reeves reports membership of the HTA Commissioning Board (up to 31 March 2016), the Systematic Reviews Programme Advisory Group (up to 5 July 2017) and Interventional Procedures Panel Methods Group, the HTA Efficient Study Designs Board, SRP – Cochrane Programme Grant Funding Meeting and Systematic Reviews NIHR Cochrane Incentive Awards (all current).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Reeves et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background and definition of the clinical problem
Each year there are > 4.5 million hospital admissions for surgery in England. 1 At the end of each procedure the skin edges of the wound are approximated using sutures or clips. Closing a surgical incision in this way creates what is called ‘a closed primary wound’. Following most surgery in adults, it is standard practice to cover closed primary wounds with a dressing.
Up to 25% of closed primary wounds may be complicated by a surgical site infection (SSI). 2 A SSI requires treatment with antibiotics and dressings and it may require further investigation and treatment. Interventions for SSI may lead to complications, which can delay recovery and reduce quality of life. SSIs therefore have a significant impact on patients and the health service. 3–7
The risk of developing a SSI is multifactorial. Major factors include the contamination level of the wound and whether surgery is performed in the elective or the emergency setting. 8–10 The site of the operation plays an important part in the likelihood of developing a SSI. Abdominal surgery carries one of the highest rates of SSI, particularly if the operation involves the colon or rectum. 11,12 Caesarean section is another procedure that carries a high rate of SSI. Another factor associated with an increased risk of SSI is a compromised immune system (e.g. diabetes mellitus, malnutrition, immunosuppressive therapy or human immunodeficiency virus). 13
Possible ways for reducing SSI include modification of preoperative, perioperative and postoperative factors. The role of the method of wound dressing in minimising SSI is of major interest. 14
The health technology being assessed: dressings for primary surgical wounds
Dressings are widely used in the postoperative care of wounds, theoretically to promote healing and prevent infection and for convenience to absorb blood or tissue fluid. Several attributes of an ideal wound dressing have been described and include the absorbency of wound exudates without leakage or strike-through, the absence of particulate contaminant, the suitability of the dressing for use with different skin closures, minimal wound trauma on removal of the dressing, minimal requirements for dressing changes and optimal cosmesis. 15 More recently, interactive dressings have been developed that, in addition to these passive roles, deliberately interact with the wound-bed components to potentially further enhance wound healing. These interactive dressings are often called ‘complex’ or ‘advanced’. (In this report, we follow the terminology of the brief, describing such dressings as complex.) They tend to be more expensive than their simple counterparts, but their use is increasing, despite the absence of robust evidence supporting their efficacy in preventing SSI.
Although it is standard to use a dressing in adult surgery, with the dressing type varying substantially by organisation and practitioner, it is standard practice in paediatric surgery not to use dressings following surgery. 14,16 Discussions with paediatric staff suggest that reasons for not doing so include a belief that children may be afraid of what is underneath a dressing and that the removal is painful. A systematic review [including randomised controlled trials (RCTs) in adult and paediatric practice] available when this study was planned summarised evidence for the effects of simple and interactive wound dressings, as well as no dressing, on the risk of SSI. 17 It found no differences between types of dressings, or no dressing at all. The review highlighted the need for better studies addressing this important and common issue and it recommended in-depth exploratory work to examine the culture and practices associated with the use of wound dressings.
Rationale for the study
The burden placed on health-care systems and patients by SSI means that all possible interventions to reduce the incidence or severity of SSI warrant detailed exploration. A particular advantage of optimising postoperative dressings is the potential for a major impact on clinical outcomes.
Surgical site infection has been shown to be an independent predictor of mortality. 18 In 2002 there were 8205 deaths in the USA attributable to SSI, accounting for 8% of all deaths caused by a nosocomial infection. 19 SSI has been shown to increase the duration and cost of patient hospitalisation, predominantly as a result of reoperation, additional nursing care and drug treatment costs. 20 In a case–controll US study4 of 255 patient pairs, it was found that, in those patients with SSI, hospital discharge was delayed by a mean of 6.5 days [95% confidence interval (CI) 5 to 8 days], with an additional direct cost of US$3089 per patient. In a later Swiss study7 of 6283 surgical procedures, the researchers found that the mean additional hospital cost in patients with SSI was 19,638 Swiss francs (95% CI 8492 to 30,784 Swiss francs) and the mean additional days in hospital was 16.8 days (95% CI 13 to 20.6 days). In the UK, length of stay is typically doubled, with additional costs of £814–10,523 incurred in patients with SSI. 3,5,6 The indirect costs of SSI as a result of loss of productivity, patient dissatisfaction and litigation, and reduced quality of life are very important but have been studied less extensively.
There are at least 30 different types of wound dressings, with variable unit costs and requirements for frequency of replacement. 13 Manufacturers are keen to extol the relative benefits of their products but high-quality evidence to support these claims is lacking. No recommendations were made in the last national UK guidelines13 on the treatment and prevention of SSI, which reviewed five RCTs available up to September 2007 and concluded that no differences in SSI rates were evident. As described above, a Cochrane review17 examined data from 16 RCTs and found no evidence to suggest either that one dressing type was better than another or that covering wounds with dressings was better at preventing SSI than not covering wounds. The authors noted that many trials were of poor quality. Other recent publications have reviewed evidence about risk factors for SSI. 21,22
We aimed to carry out initial feasibility and pilot work across several NHS sites, to maximise generalisability of the findings and to demonstrate practical issues that researchers would be likely to have to address in a definitive trial. We did not stipulate specific dressings within dressing types described in the brief in order to avoid constraining the dressing types in common use. We made these decisions to allow the trial to evaluate the strategy of using one or other of these various broad types of dressing (simple, complex or no dressing) rather than the relative efficacy of specific products.
Chapter 2 Aims and objectives
We conceived this feasibility study with reference to a future definitive trial to establish the effectiveness, acceptability and cost-effectiveness of complex, simple and/or no dressings in elective and unplanned abdominal surgery. In this feasibility study, we aimed to establish the methods and infrastructure required for the main trial, including the development of new methods where we found them to be necessary. The study involved two programmes of work: the preliminary work (Phase A), which we considered to be a necessary precursor of the pilot RCT, and the pilot RCT (Phase B).
Phase A objectives
The aims of Phase A of the Bluebelle study were to assess the scope for comparisons of simple, complex and/or no dressings in a pilot trial and to develop and pre-test a comprehensive patient-centred measure to assess SSI. Phase A also included work with professional groups and the literature to define and categorise dressing types into three groups (complex, simple and no dressing).
The specific objectives of Phase A were as follows:
-
understand the reasons underpinning the current choice and use of dressings (e.g. simple vs. complex) across elective abdominal, obstetric and paediatric surgery, including perspectives on the clinical effectiveness and cost-effectiveness of dressings, patients’ expectations and experience of wound care and dressing use and how these issues vary by patient, clinical and procedure-related factors
-
explore patients’ and health-care professionals’ (HCPs’) attitudes towards a trial of dressing type, including the range of procedures and comparisons (simple, complex, no dressing) that would be deemed acceptable for inclusion in a trial, perspectives on random allocation to dressing type and views on the important outcomes to measure in a trial of dressing use
-
identify dressings commonly used in the NHS
-
develop the method for SSI assessment to use in the main trial
-
develop and test a patient-centred measure of practical wound measurement
-
define and categorise dressing into pragmatic groups with agreed boundaries: (1) complex, (2) simple and (3) no dressing; agree protocols for the application and removal of the dressings; identify their costs and contextual information
-
investigate the feasibility of photographing wounds in theatre and assessing the quality of wound closure
-
analyse the value of information (VoI) to the NHS that would be provided by a definitive trial
-
bring together the results of the above objectives to design Phase B of the study (i.e. a randomised external pilot trial).
Phase B objectives
The overall aim of Phase B of the Bluebelle feasibility study was to establish whether or not it is possible to carry out a large definitive RCT to compare the effectiveness and cost-effectiveness of simple dressings, tissue adhesive used as a dressing (glue-as-a-dressing) and no dressing to reduce the risk of a SSI following elective and unplanned surgery, to improve aspects of wound management and to improve participants’ experiences of the care of their surgical wounds. The specific objectives of Phase B were as follows:
-
establish the numbers of potential participants at different hospitals who are likely to be eligible and who can be approached about the trial, and the proportions confirmed as eligible, recruited and randomised
-
pilot the randomisation process and investigate reasons for any difficulties that affect recruitment (through conducting a ‘qualitative recruitment investigation’), so that issues can be tackled before progressing to a main trial
-
assess the acceptability of trial interventions and processes to participants and clinical staff using qualitative research methods
-
assess adherence to dressing type allocation and the follow-up protocol through detailed reports, qualitative research methods and follow-up
-
assess the appropriateness and feasibility of collecting a range of secondary outcomes and resource use data
-
establish the validity and reliability of the developed tools for assessing wounds for SSI, practical wound management and participants’ experience of wound care
-
explore the feasibility of obtaining digital photographs of wounds in theatre after wound closure by theatre personnel and after discharge by participants
-
work with the patient and public involvement (PPI) group to inform the conduct of Phase B and the design of a future main trial
-
design a large, definitive RCT based on information from the pilot trial and from integrated and interactive meetings with nurses/midwives, surgeons, methodologists and patient partners.
Additional objectives
Objectives A3, A7 and A8 were not in the protocol for Phase A. The need for these additional objectives emerged during the conduct of Phase A and they were included as objectives of a funded extension to Phase A. As part of this extension, we also collaborated with the Cochrane Wounds Group to update its 2011 review of dressings for the prevention of SSI, primarily with respect to the inclusion of tissue adhesive as a dressing. The review did not identify any eligible trials that studied tissue glue. It has been recently published and no further details are included in this report. 17 Findings from the survey to address objective A3 led to the inclusion of unplanned surgery, as well as elective surgery, as part of our general aim and in the pilot RCT.
Chapter 3 Study methods
Objectives A1 and A2: understand practice and views in relation to dressings
Study design
Qualitative research methods, using semistructured interviews with HCPs and patients, were used to address objectives A1 and A2:
-
understand the reasons underpinning the current choice and use of dressings (e.g. simple vs. complex) across elective abdominal, obstetric and paediatric surgery
-
explore patients’ and HCPs’ attitudes towards a trial of dressing type, including the range of procedures and comparisons (e.g. simple, complex, no dressing) that will be deemed acceptable for inclusion in a trial, perspectives on random allocation to dressing type, and views on the important outcomes to measure in a trial of dressing use.
Scope: clinical specialties
The qualitative research integrated throughout the Bluebelle study (i.e. Phases A and B) focused on abdominal [upper and lower gastrointestinal (GI)] and obstetric (caesarean section) surgery, as these were surgical specialties specified in the description of the study population in the funding application. Interviews were also conducted in paediatric surgery as a means of comparing dressing practices with those in adult surgery. This was because informal preliminary enquiries and the literature14,16 had indicated that dressings are not routinely used in paediatric surgery, thus providing the potential to further the team’s understanding of clinical practice related to dressing use.
Setting
Interviews were conducted across six university and district NHS hospitals in the South West and West Midlands regions of England.
Sampling strategy
Health-care professionals
Sampling was purposeful, based on a prior intention to interview a wide range of health-care professionals with expertise in abdominal, paediatric and obstetric surgery. Prior discussion with clinical members of the Study Management Group (SMG) established that dressing practice would be relevant to a wide range of professionals who provided care at various stages of the patient pathway for surgery. Relevant informants were identified by clinical members of the SMG based on the above criteria. Further potential interviewees were identified partly on a snowball sampling basis. Sampling was also guided by intentions to explore new lines of enquiry; for instance, ‘tissue viability experts/nurses’ were identified as a key group to target, based on emerging findings from prior interviews. Sampling proceeded using a hybrid of the above approaches until the point of saturation.
Patients
Patients eligible for interview were aged > 18 years and had undergone or were scheduled to undergo an abdominal surgical procedure within 3 months of the interview date. Eligible patients were identified by research nurses and surgical trainee collaboratives. Initially, recruitment was guided by these criteria alone. As interviews progressed, sampling became increasingly purposeful to achieve maximum variation according to age, sex and type of surgery.
Data collection
Interviews were conducted by Leila Rooshenas, Christel M McMullan, Daisy Elliott and Jonathan M Mathers over a 7-month period (July 2014–January 2015), either via telephone or in person. Face-to-face interviews took place on NHS premises or in informants’ homes. The qualitative researchers obtained written consent to conduct and audio-record all interviews. Separate topic guides were used for HCPs and patients to ensure that broad topics were consistently covered across each set of interviews. The topic guides evolved as data collection proceeded, either through rewording of questions to elicit more detailed responses or through the addition of new topics based on emerging lines of enquiry from earlier interviews (see Results, Objectives A1 and A2: Understand practice and views in relation to dressings). The initial and final topic guides used are shown in Report Supplementary Material 1. All interviews were transcribed ‘smart verbatim’ [i.e. a full transcript of the recording with some amendments to spoken content to ease readability (e.g. removal of filler utterances such as ‘um’)].
Approach to analysis
Interview transcripts were analysed thematically using the constant comparison method adopted from grounded theory methodology. 23 This involved line-by-line coding of text, whereby descriptive words or phrases were attached to lines of transcript and arranged into themes. Themes were subsequently arranged into hierarchies (with overarching themes and subthemes). Data collection and analysis proceeded in tandem, with the coding frame evolving as new data were collected and analysed. Previously coded interviews were reread in the light of the evolving coding frame to ensure that this comprehensively captured data across the full set of interviews. HCP interviews and patient interviews were analysed separately (i.e. with distinct coding frames).
The analysis of Phase A interviews was conducted primarily by the researchers who led each interview. A subset of interviews (10%) were double-coded by two members of the qualitative team in the early stages of analysis. This helped to inform the earlier versions of the coding frame. The coding frame was regularly discussed among the team as it evolved during the study. Given the wider study aims, the team agreed that themes that had implications for the pilot RCT protocol would be prioritised and coded in greater detail.
Descriptive accounts summarising emerging themes and overall study findings were prepared for the SMG throughout the analytical process. One researcher took responsibility for collating and synthesising findings from all sites for the purpose of producing final report(s) of Phase A findings. These tasks involved consulting descriptive accounts from individual sites and referring to coding frames and raw data (transcripts/recordings) where needed. There was an attempt to search for ‘negative cases’ in relation to particular themes or theories; where present, these are fully reported in the findings. All final reports were scrutinised by all members of the qualitative team.
Presentation of findings
The key Phase A findings that had implications for the pilot RCT design are summarised in Chapter 4, Objectives A1 and A2: Understand practice and views in relation to dressings, and cover HCPs’ accounts of current wound-dressing practice, HCPs’ perspectives on the proposed pilot RCT and patients’ perceived acceptability of the proposed pilot RCT.
Objective A3: identify dressings commonly used in the NHS
Initial findings for objectives A1 and A2 revealed that key words used in the commissioning brief to describe the intervention dressing of interest (i.e. ‘interactive’ and ‘complex’) were not recognised by professionals in the context of closed primary surgical wounds. There was also evidence to suggest that informants had variable interpretations of what constituted a ‘dressing’, and in some cases used products (e.g. tissue glue) that were not marketed as ‘dressings’ to cover primary wounds. After discussion, the SMG decided to carry out a survey on dressings used for primary surgical wounds, in collaboration with the Severn and Peninsula Audit and Research Collaborative for Surgeons (SPARCS)24,25 and the West Midlands Research Collaborative (WMRC).
Survey design
A prospective multicentre study was undertaken. All hospitals located in the two trainee-led research collaborative networks were invited to participate by e-mail and personal communication. A surgical trainee-level principal investigator, responsible for local co-ordination of data collection and entry, was identified within each participating hospital. The study was registered with the clinical audit department in each hospital.
Survey population
Abdominal wounds created during elective or unplanned abdominal surgery, and closed wounds primarily, were surveyed during a 2-week interval in January 2015. We considered a wound to be a closed primary wound if the edges of the incised skin were apposed (using suture material, tissue adhesive or clips) at the end of the procedure. Vascular, gynaecological, urological and paediatric procedures were excluded. Cases were included only if trainees were present (and therefore able to collect the data prospectively).
Data collected
Trainees completed anonymised data collection forms at the end of each surgical procedure, recording information about skin closure and dressings. Dressings were categorised as complex (those with advanced practical and/or therapeutic properties, including amorphous material, silicone, hydrocolloid, foam, antimicrobials or negative pressure) or basic (those without therapeutic properties that are adherent around the perimeter or entire surface, with or without a pad to absorb exudate). ‘No dressing’ was documented when an already closed wound was left without a covering at the end of the operation. The use of tissue adhesive to cover an already closed wound (i.e. when it was used as a dressing rather than wound closure technique) was categorised separately.
Operative and patient-related risk factors that might influence dressing selection were recorded. Operative risk factors included the type of procedure performed and access (open, laparoscopic or laparoscopically assisted), whether or not a stoma was formed and the degree of wound contamination (clean, clean-contaminated, contaminated and dirty). 26,27 Procedures were classified as planned (elective) or unplanned (emergency). The following patient-related risk factors were recorded: age, sex, body mass index (BMI), diabetic status and American Society of Anesthesiologists (ASA) fitness grade.
The reason for dressing selection (by the surgeon responsible for closing the wound) was recorded in the following three categories: (1) personal preference, (2) selected for specific wound characteristics or (3) the dressing was simply handed to the surgeon at the end of the procedure, without discussion. Dressings could be selected for multiple reasons and space was provided for free-text answers. To supplement this, procurement officers from each hospital were contacted to obtain information about local policies for purchasing dressings.
Data analysis
Data were entered into a password-protected online database held on a server [developed and maintained by the Bristol Clinical Trials and Evaluation Unit (CTEU)] in one of the participating hospitals. Analyses summarised the frequency of different dressing types using descriptive statistics. Descriptive statistics were also used to examine whether patient characteristics or the type and urgency of surgery were associated with particular dressing strategies. Analyses were performed in Stata® version 13 (StataCorp, College Station, TX, USA).
Objective A4: develop a patient-centred comprehensive measure of surgical site infection
This objective sought to develop a questionnaire to capture information on wound healing after a patient has been discharged from hospital. The questionnaire was intended for patient use (self-assessment of wounds) and/or HCP completion (observer assessment).
Study design
A mixed-methods study was conducted in three steps:
-
analysing existing tools and in-depth interviews with patients and HCPs to establish the content of the questionnaire
-
developing questionnaire items and designing the tool
-
pre-testing the questionnaire for content validity, acceptability and understanding using cognitive interviews with patients and HCPs.
Step 1: generation of questionnaire content
Analysis of existing tools
A previous systematic review28 identified the US Centers for Disease Control and Prevention (CDC) criteria and the ASEPSIS (Additional treatment, the presence of Serous discharge, Erythema, Purulent exudate, and Separation of the deep tissues, the Isolation of bacteria, and the duration of inpatient Stay) grading scale as the most commonly used definitions and grading scales for assessing SSI. 29,30 In the UK, the Public Health England (PHE) SSI surveillance programme has designed a checklist based on the CDC criteria (including signs, symptoms and wound care interventions) to collect information to determine incidence of SSI. 31 The surveillance programme also uses a questionnaire for patients to complete to collect information to ascertain post-discharge SSI. 31
The ASEPSIS grading scale is a clinical tool designed for use in hospital to aid HCPs to identify and grade the severity of SSI. 30 ASEPSIS also has an associated questionnaire for patients to collect information post discharge. 32
We undertook a detailed content analysis of the PHE clinical data sheet and post-discharge patient questionnaire, and the ASEPSIS grading scale and associated patient questionnaire. The purpose was to ascertain the important signs, symptoms and criteria for diagnosing SSI. First, individual criteria or items were extracted from the tools and recorded verbatim. Next, criteria and items referring to the same issue or underlying construct were grouped together into SSI ‘domains’ based on the sign, the symptom or the intervention carried out to manage infection.
In-depth interviews
General abdominal surgery patients identified as having had a SSI, and staff involved in post-surgical wound care, were interviewed. The purpose of interviews was to elicit information on the signs, symptoms and interventions relevant to SSI occurrence and to identify any new domains relevant to SSI diagnosis that were not covered in the CDC and ASEPSIS clinical tools and patient questionnaires.
Patient participants were identified and approached by research nurses and surgical trainees at one UK NHS trust. Contact details of interested participants were given to the SSI study team so that they could arrange an interview. HCP participants were identified and approached directly by the SSI study team. Written consent was taken at the time of interview. Sampling and eligibility criteria were similar to those reported previously (see Objectives A1 and A2: Understand practice and views in relation to dressings).
Semistructured interviews were conducted to explore SSI experiences and opinion on relevant aspects for SSI diagnosis. Views on the PHE and ASEPSIS clinical tools and associated questionnaires were also sought, asking participants to comment on their suitability, relevance and practicality for completion. Pre-designed topic guides were used (see Report Supplementary Material 2). Interviews were audio-recorded and transcribed in full.
Interview data were analysed using an inductive approach. Data were coded and grouped into themes (thematic content analysis). A descriptive account of the common identified themes was generated as an ongoing iterative document and updated with subsequent interview data. Accounts concluded with a summary of points to consider when designing the new questionnaire.
Themes identified from the analysis of interviews were mapped to SSI domains derived from the analysis of the existing tools. Any new themes emerging from the interview data that were not found in the analysis of the existing tool were listed separately.
Step 2: designing the questionnaire
A comprehensive list of all possible domains identified from step 1 was considered for inclusion in the new questionnaire by the study team, surgeons and methodological experts in trial design and outcome data collection. Domains unsuitable for a patient-reported questionnaire were dropped and those retained were constructed into items for a questionnaire. Domains were ‘operationalised’ into items for a questionnaire. Items were designed to be clear and unambiguous, written using plain language targeted at a lay audience without technical or medical terms. At the end of the item, where possible, the medical term for the underlying domain that the item was intended to measure was included in parentheses. Response categories took the format of either a ‘yes/no’ option or an ordinal scale (initially ‘not at all’, ‘a little’, ‘moderately’, ‘quite a bit’, ‘very much’), depending on what was most appropriate for the individual item. Options for ‘do not know’ were available for some signs and symptoms and wound care interventions anticipated to be potentially unclear to patients. Instructions for questionnaire completion were written, and adapted for patient and professional use. The only other difference between the questionnaires for patients and professionals was use of the first- or third-person pronoun in the phrasing of the items.
Step 3: pre-testing the questionnaire
The provisional questionnaire was pre-tested in cognitive interviews.
Participants
General abdominal surgery patients and women who had had a caesarean section were identified from two UK NHS trusts. Research nurses, midwives and surgical trainees identified and approached patients for participation. Contact details were passed on to the SSI study team so that they could follow up and arrange an interview. HCP participants were identified and approached directly by the SSI study team. Participants were purposively sampled to include a range of surgeries and specialties.
Data collection
Cognitive interviews were conducted by two members of the research team. Participants were asked to complete the provisional questionnaire using a ‘think aloud’ technique. Interviewers used probes to explore issues further such as ‘What does that word mean to you?’. Detailed memoranda were written up for each interview with summary points of key issues and suggested improvements to the questionnaire.
Analysis
Interviews and revisions to the questionnaire were made as an iterative process so that new versions of the questionnaire could be tested in subsequent interviews. Suggested improvements were considered; substantial or recurring problems were addressed by revisions to the questionnaire. The study team met at regular intervals throughout steps 1–3 to discuss findings and revise the questionnaire.
Objective A5: develop and test a patient-centred measure of practical wound management
This objective was included to address an observation of the 2011 Cochrane review,33 namely that dressings may need to be evaluated on the basis of their wound management attributes because trials to evaluate the effect of dressings on the risk of SSI would have to be very large. The intention was that an initial measure would be developed in time for inclusion the pilot RCT, while recognising that further development and validation would be likely to be required.
Study design
We planned to develop a measure based on an existing framework for developing patient-reported outcome measures,34,35 incorporating guidance on eliciting health domain concepts using qualitative methods. 36,37 Step 1 aimed to produce a comprehensive list of potential issues relating to wound and dressing experience and practical management issues. Step 2 developed issues identified from step 1 into questionnaire items. Step 3 evaluated the measures for acceptability and relevance using cognitive interviews with patients and HCPs. Steps 1–3 were overseen by a working group (DE, JB, LR, RM and CMM) that was part of the wider SMG. The SMG met before and between each step to discuss progress and make decisions about how the measures should be adapted.
Step 1: generating relevant issues
Interviews
Eligible patients who had undergone abdominal general surgery or caesarean section were identified and approached by research nurses and surgical trainees. Contact details of interested participants were passed on to members of the working group so that they could arrange an interview. HCP participants were identified and approached directly by the SSI study team. A purposeful sampling strategy ensured that perspectives were captured from a range of participants. 37 Within this sampling approach, maximum variation was sought in relation to age, sex, ethnicity, type of surgery, dressing type and location.
Interviews were conducted by Leila Rooshenas, Daisy Elliott and Christel M McMullan and explored and characterised participants’ experiences of wounds and dressings. A topic guide was developed (based on literature and views of HCPs in the Bluebelle study team) to ensure that discussions covered the same core issues but with sufficient flexibility to allow new issues of importance to the informants to emerge (see Report Supplementary Material 3).
Interviews were audio-recorded and transcribed in full. Transcripts were imported into NVivo (version 10; QSR International, Melbourne, VIC, Australia). All data relating to outcomes and issues of importance to patients that were relevant to dressing use and the practical management of the wound in the initial period after surgery were assigned labels (coded) by two experienced qualitative researchers. Data were analysed using techniques of constant comparison derived from grounded theory methodology, and emerging codes across the data set were then compared to look for shared or disparate views among participants. 38 A subset of approximately half of the interviews (n = 19) was double coded by a third researcher to highlight any differences in the interpretation of codes. 36 Data collection and analysis continued until the team were confident that saturation had been reached (i.e. no more patterns or themes emerged from the data).
Extraction of information from three systematic reviews
Three systematic reviews39–41 were used to identify RCTs that included outcomes relating to wounds and dressings. Papers were scrutinised for outcomes relating to practical wound management or symptoms and patient experience. Relevant data from the RCT reports were then extracted on the outcome (as described by the authors), the verbatim wording to measure the outcome, who reported the outcome, the measurement scale and the assessment time point. Attempts were made to contact the authors for more information.
Synthesis of literature and qualitative data
A list of issues from the analysis of the interviews and literature search was collated into an item-tracking matrix (see Report Supplementary Material 4). 42 The working group agreed on a set of words or phrases to reflect each issue and also noted additional phrasing used by participants in a subsequent column. 36 Issues that were conceptually similar were organised into categories. For instance, issues such as ‘itchiness/irritation’, ‘presence of pulling sensation’ and ‘tightness of wound’ were mapped into a ‘wound comfort’ category.
Step 2: designing the questionnaire
The working group used the item-tracking matrix to agree on those issues that should be written into questionnaire items. Items featured words and phrases used by patients in the interviews to enhance content validity. 37,43
Step 3: pre-testing the questionnaire
Cognitive interviews, with a new sample of patients who had undergone surgery, were conducted. Cognitive interviews are used widely in questionnaire development36 and involve asking respondents to verbalise their thoughts while answering questions. This methodology enabled us to explore the acceptability of the measure and coverage of patients’ and HCPs’ concerns (in terms of language, accuracy and relevance, as well as layout).
Patients who had undergone abdominal general surgery or caesarean section at one of five hospitals in two cities in the UK were identified and approached by research nurses and surgical trainees. Contact details were passed on to members of the working group to follow up and arrange an interview. HCP participants were identified and approached directly by the working group. As in step 1, sampling was purposeful to achieve maximum variation in relation to clinical role, age, sex and geographic location (for HCPs) and age, sex, ethnicity, type of surgery, dressing type and location (for patients).
Interviews explored the acceptability of the measure and coverage of patients’ and HCPs’ concerns (in terms of language, accuracy and relevance, as well as layout). 37 During each interview, participants were asked to complete the measure by reading each item aloud and commenting on their understanding. Interviews were guided by a series of probes (e.g. ‘What does this item mean to you?’, ‘Are there other ways you would describe it?’). 44 Participants’ body language was also observed and prompted further discussion about specific items (such as the participant nodding in agreement or frowning). 36 A copy of the topic guide is available.
Operationalisation and modification of the measures was an iterative process. Findings from cognitive interviews and suggestions for amendments were regularly disseminated to the Bluebelle SMG. Each stage of feedback informed amendments to modify and reword items to improve understanding, which was repeated following efforts to revise questions and eliminate problems. 44 This process continued until no new issues were identified and no further refinements were believed to be necessary.
Objective A6: use the literature and views of experts to define and categorise commonly used dressings into three pragmatic groups
The primary literature source for this objective was the 2011 Cochrane review and the ongoing collaboration to update the review,17,33 and the British National Formulary (BNF). 45 A summary of the dressing types that were evaluated in previous trials was compiled and draft definitions were circulated to the SMG before a meeting on 21 October 2014. Findings from the literature were supplemented by emerging findings from qualitative interviews carried out during Phase A. The draft definitions were discussed in detail at that meeting and at the subsequent one (24 February 2015), at which definitions for the pilot RCT were finalised.
Objective A7: investigate the feasibility of photographing wounds in theatre and assessing the quality of wound closure
Feasibility of photographing wounds in theatre
We attempted to collect wound photographs in theatre at three of the participating hospitals. This required obtaining approvals from the hospitals, separately from the research ethics approval for doing this. Special safeguards were in place at NHS trust level in all three hospitals. We were ultimately successful in gaining approval to take photographs in theatre but the process was time-consuming and cumbersome. The challenges encountered are described under the results for this objective.
Feasibility of assessing the quality of wound closure
Poor wound closure may be a contributory factor to SSI development that has not previously been investigated. The reasons why poor wound closure may increase the risk of SSI include the presence of gaps in the wound allowing either exudate to leak out or contamination to enter, subcutaneous suture material visible on the skin acting as a nidus for infection, tethering or puckering of the skin edges that may invite bacterial colonisation and even tension and poor vascularisation increasing wound ischaemia. These issues have not been intensively studied but it is notable that cosmetic and plastic surgeons (wound experts) take great care to ensure that primary surgical wounds are closed well and heal well to optimise cosmesis and it is plausible that the quality of wound closure also affects the risk of SSI.
Therefore, we sought to investigate markers that constitute good-quality wound closure, in the context of such markers providing the basis for the development of a metric to measure the quality of wound closure and its relevance to the development of a SSI.
We were also interested in measuring the quality of wound closure in the Phase B pilot RCT because of concern about the feasibility of randomising after wound closure and the risk of performance bias. Randomising after wound closure was preferred to avoid knowledge of dressing allocation influencing how a surgeon closed the wound. For example, if a surgeon is aware that the patient has been allocated to ‘no dressing’, then the surgeon may take extra care to ensure that the wound edges are well approximated and that there is no puckering and tethering. Conversely, if the surgeon is aware that the patient has been allocated to a simple dressing, then the surgeon may take less care, knowing that the dressing will cover the wound.
Study design
We used a mixed-methods design to address this objective, comprising literature reviews, qualitative interviews with surgeons and observation of wound closure in the operating theatre. Mixed methods were selected to enable an in-depth exploration of a complex phenomenon (closure of surgical wounds) within its original setting (the operating theatre).
Literature review
We searched for literature pertaining to wound closure, with the aim of identifying theories about ‘what makes a good wound closure’, and how wound closure might influence wound healing and the development of SSI. Relevant articles were identified using snowballing techniques, and by reviewing the grey literature.
Snowballing
Preliminary searches in PubMed identified a RCT in which the quality of wound closure was assessed. Starting with this citation, forwards and backwards snowballing was used to identify further relevant articles. ‘Forwards snowballing’ refers to the identification of relevant articles based on papers citing the paper being examined. ‘Backwards snowballing’ involved searching reference lists of included papers to identify new papers of relevance to the review. The snowballing process ended when no new information about wound closure emerged from the papers.
Grey literature
Sources such as training videos and surgical textbooks were searched alongside the snowballing process.
Interviews and observations
Sampling and recruitment
For the observations, surgical procedures performed at three centres were purposively sampled to include a variety of approaches (laparoscopic and open surgery), disease types (cancer and non-cancer) and anatomical areas (upper and lower GI surgery). Surgeons who were observed were also invited to take part in an interview. Additional surgeons (from the same three centres) were purposefully selected for interview, with the intention of including a range of clinical experience (e.g. consultant and trainee level) and subspecialty interests (e.g. GI and plastic surgery). Observations and interviews continued up to the point of data saturation (i.e. the point at which additional data were not adding anything new to the analytical framework).
Data collection
Non-participant observation of the operation was undertaken to enable documentation of the operative steps of wound closure, as well as contextual factors that were thought to potentially influence wound closure. As the project progressed, visits to the operating theatre were also used as an opportunity to establish the feasibility of capturing the characteristics of a well-closed wound (which were identified within the literature work and during surgeon interviews). Observations were recorded by hand on an observation schedule, which was developed during preparatory visits to the operating theatre. Handwritten notes were later transferred into an electronic Microsoft Word (Microsoft Corporation, Redmond, WA, USA) document.
All surgeons whose operations were observed were invited to participate in an interview immediately after the operation to discuss whether or not the wound closure had progressed smoothly and to identify and explore reasons behind any unusual events or deviations from the usual procedure. Interviews that took place without a preceding observation were arranged at the surgeon’s convenience and were conducted by a second interviewer. Interviews were semistructured and topics were based on existing literature and clinical knowledge. The topics were adapted as interviews and analyses progressed to explore emergent findings. Interviews were guided by a list of open-ended questions to ensure that all topics were covered in each interview but were sufficiently flexible to enable unanticipated emerging topics to be explored (e.g. issues important to the participants). Questions encouraged surgeons to reflect on their wound closure (and incision) practices, their rationale for these, and whether or not, in their opinion, these may affect wound healing. Surgeons were also asked for their interpretations of what constitutes a well-closed wound and whether or not, and how, this may be assessed in a photograph of a freshly closed wound.
Interviews were conducted face to face by two medically qualified trainees with limited direct surgical experience of wound closure, between 2014 and 2015. The interviewers were not familiar with the research participants or the operating theatre environments in which the research was conducted.
Data analysis
Data collection and analysis ran in parallel. NVivo version 10, Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) and Microsoft Word were used to aid the storage and analyses of all types of data. Notes from the observations were written up as soon as possible afterwards. Patterns noted across series of observations were developed into themes, which in turn were refined through further observations and exploration through interviews. Interview data were used to confirm, challenge and clarify findings from the observations. For example, if an unexpected event or step was identified, this was explored with the surgeon in the postoperative interview. The term ‘unexpected’ was defined in relation to patterns across observed surgeons, and the medical knowledge of the observer.
Interviews were transcribed and analysed as previously described (see Objective A4: Develop a patient-centred comprehensive measure of surgical site infection), generating themes and forming an early coding framework. The coding framework was added to, and coded material regrouped, with further data collection and analysis. Further analysis involved scrutinising the textual data for differences and similarities within themes and relating findings back to the observational analyses.
Objective A8: analyse the value of information to the NHS that would be provided by a definitive trial
By carrying out a VoI analysis,46 this objective sought to establish whether or not a main trial of alternative surgical wound-dressing methods would be a worthwhile NHS investment.
The VoI analysis was based on a simple decision model, which depended on SSI rates and costs and dressing costs. Network meta-analysis (NMA) was used to combine the available RCT evidence on relative effects identified in the updated Cochrane review17 and other studies identified. Expected value of partial perfect information (EVPPI) was computed to give an upper bound on the value of research on particular model inputs. Expected value of sample information (EVSI) was computed for various sample sizes to identify (1) if a trial is worthwhile and (2) the relative benefit of trial designs, which differ in the included intervention groups and samples sizes. We first calculated results based on existing evidence, and then updated the results incorporating the results from the Bluebelle Phase B study.
Decision question
Patient population
The population of interest was patients having elective or unplanned general surgery. This population includes patients having surgical procedures involving the abdomen, oesophagus, stomach, small bowel, colon, liver, pancreas, gall bladder, bile ducts, thyroid, head and neck, breast and chest. These procedures are primarily conducted on adults.
Interventions
Simple, complex and no dressing categories were defined in accordance with the Bluebelle definitions (see Chapter 4, Objective A6: use the literature and views of experts to define and categorise commonly used dressings into three pragmatic groups). Tissue adhesive (glue-as-a-dressing; see Objective B1: establish the numbers of potential participants at different hospitals who are considered likely to be eligible and who can be approached about the trial, and the proportions confirmed as eligible, recruited and randomised) was considered as a fourth category.
Because the category of complex dressing was not included in the Phase B pilot RCT and may not be relevant in the context of closed primary wounds, we present results for two scenarios:
-
where all four dressing categories (no dressing, simple dressing, glue-as-a-dressing, complex dressing) are decision options
-
where only the non-complex dressing categories (no dressing, simple dressing, glue-as-a-dressing) are decision options.
In Phase B, glue-as-a-dressing replaced the ‘complex’ dressing specified in the commissioning brief. Technically, it does not satisfy the definition of a complex dressing adopted in Phase B (see Chapter 4, Objective A6: use the literature and views of experts to define and categorise commonly used dressings into three pragmatic groups) because it does not have an ‘intended therapeutic property’. Nevertheless, it has a similar cost to complex dressings and has complex properties, in that it is adhesive in a different way from simple dressings and does not require removal.
In both cases, evidence on relative effectiveness comes from a NMA of all four dressing categories because this allows as much evidence as possible to be incorporated; the evidence on the complex dressing category can indirectly strengthen the estimates of the other dressing categories.
Outcomes
The main economic analysis included the costs of dressings, nurse time, SSI treatment and management, and the quality-of-life impact of a SSI. We did not include any impact of dressings on quality of life as a result of wound management, for example reducing exudates, making it easier to shower and dress, ease of dressing removal and scarring. This is because it is expected that any effects of wound management on quality of life will be small in comparison with the costs and disutility associated with a SSI.
We conducted a probabilistic analysis, whereby probability distributions are used to represent the uncertainty in model inputs. We report the expected net benefit, which is the mean quality-adjusted life-years (QALYs), monetarised by multiplying by willingness to pay per QALY, minus the mean total costs. Because the key outcome is SSI risk, which is both costly and detrimental to quality of life, we anticipate that the expected net benefit will be negative, representing the overall health costs for a given dressing type. We prefer dressing types that minimise these costs (i.e. the least negative net benefits). We display uncertainty in the decision by plotting the joint distribution of incremental costs and effects (relative to the reference intervention, simple dressing) in the cost-effectiveness plane. We also present the probability that each intervention is the most cost-effective. All results are shown for a willingness to pay per QALY threshold of £20,000. 47
In the VoI analyses we report:
-
expected value of perfect information, which measures the maximum amount a decision-maker would be willing to pay to eliminate uncertainty in all the inputs to the economic model
-
EVPPI, which measures the maximum amount a decision-maker would be willing to pay to eliminate uncertainty in a subset of the model input parameters (e.g. the relative effects of the different interventions on SSI risk, or the cost of a SSI)
-
EVSI, which measures the value to a decision-maker of reducing uncertainty in a subset of parameter by collecting data in a given study design (e.g. a RCT with a balanced three-group design with a given sample size per group).
Details on computational methods used in the VoI analyses are given in Report Supplementary Material 5.
Economic model
The total cost of dressing k, costk, is the sum of the cost of dressing k and the probability of a SSI (pSSI) using dressing k multiplied by the cost of a SSI. We also include a utility decrement associated with a SSI, so that the decision model is to identify the dressing k that maximises net benefit:
where NB is net benefit, SSIQALYloss is the QALY decrement resulting from a SSI and WTP is the willingness to pay per QALY threshold.
This model is shown as a decision tree in Appendix 1 (see Figure 13).
Model inputs
Prevalence of selective and emergency and general surgery procedures and wounds
The Royal College of Surgeons of England quotes 1.2 million general surgery procedures in 2013/14. 48 Data from our survey (see Objective A3: identify dressings commonly used in the NHS, and Chapter 4, Objective A3: identify dressings commonly used in the NHS) found that patients who would have been eligible for the pilot RCT had a mean of 1.84 wounds per procedure. 49 Assuming that this rate of wounds per procedure can be generalised to the whole population of England and Wales, we estimate a total of 1.84 × 1.2 million = 2.208 million wounds resulting from general surgery per year.
Appendix 1 (see Table 39) shows the number of elective operations in a single large hospital in 2 years50 and the number of procedures in the PHE surveillance data in 2014/15. 51 The PHE data include both elective and unplanned procedures, but it is optional for hospitals to report these figures to PHE; approximately 70 hospitals contributed data. Assuming that the proportion of operations described as missing in the PHE report is the same as those seen in Jenks et al. ,50 we have predicted the proportions of operations in all categories using the PHE data.
Surgical site infection risk with simple dressings (standard practice)
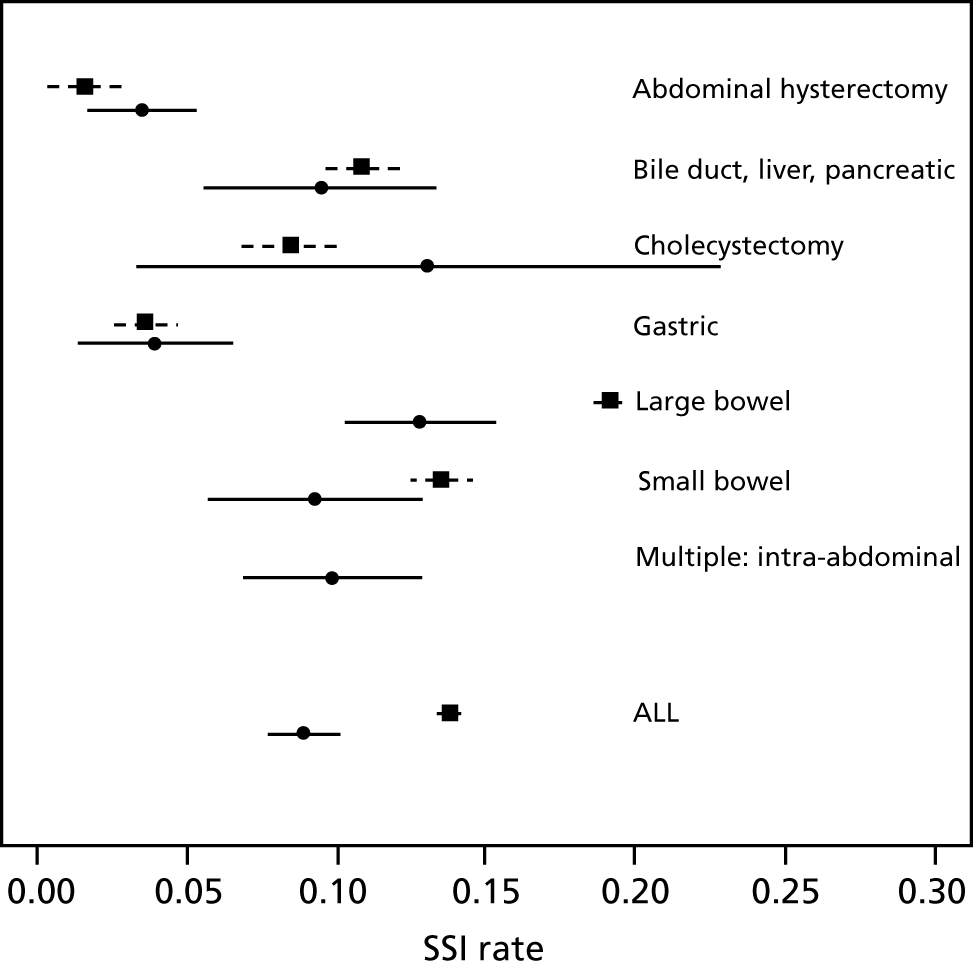
Surgical site infection risk is variable across surgery types, and Jenks et al. 50 and the PHE SSI surveillance programme51 report this risk by surgery type (Figure 1). The two different data sources are broadly comparable, although the risk of SSI following large bowel surgery was higher in PHE surveillance survey than in Jenks et al. 50 The PHE survey is optional and the data may not be representative of all hospitals, and it is not clear how the selective inclusion of data may have had an effect; hospitals may have wished to report good results, but managers may also have wanted to report poorer results to encourage improvements. The data in the Jenks et al. 50 study may be unrepresentative (i.e. expected to be better than average owing to awareness of SSI risk in participating hospitals and to the restriction to elective surgery only). PHE includes more hospitals and regions than the Jenks et al. 50 study (multiple hospitals in the University Hospitals Plymouth NHS Trust) and PHE covers a broader and more recent time period. We therefore prefer to use the SSI risk estimate from the PHE survey but, because PHE does not give information for the ‘multiple: intra-abdominal’ surgery type included in Jenks et al. ,50 we have used the Jenks et al. 50 data on the proportion of procedures and SSI risk for this category. The overall SSI risk under this assumption across all surgery types of interest was estimated to be 13.8% (95% CI 13.45% to 14.15%). We use this estimate in our base case but use the estimate from Jenks et al. 50 of 8.94% (95% CI 7.75% to 10.13%) in a sensitivity analysis.
The Jenks et al. 50 study is based on data from April 2010 to March 2012, whereas the PHE survey covers the period from April 2010 to March 2015 (between 50 and 70 hospitals per year). The estimated risk across all these surgery types is labelled ‘ALL’ for Jenks et al. ;50 this is the overall SSI risk, but for PHE, this is calculated on the basis of the above assumption for the surgery type multiple: intra-abdominal.
Odds ratios of surgical site infection comparing different dressing types
To estimate the odds ratios of SSI comparing different dressing types, we use results from a recent update of the Cochrane review of dressings for the prevention of SSI. 17 Appendix 1 (see Table 40) shows the included studies that report the SSI outcome, surgery type and interventions (I) with their classifications according to the Cochrane review classes (C) and according to the Bluebelle definition.
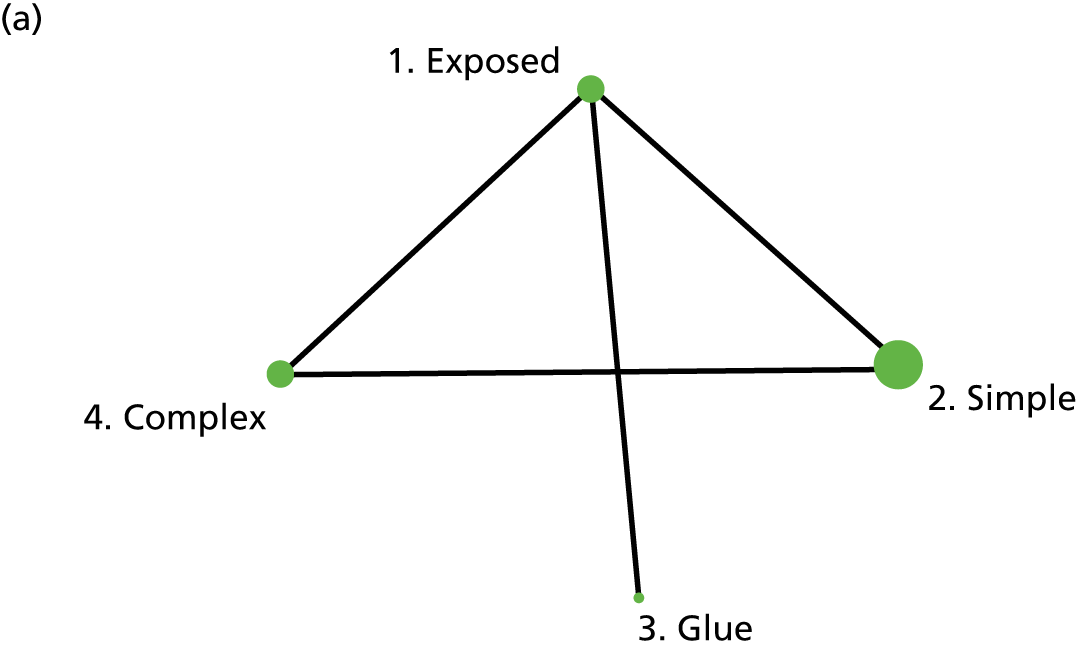
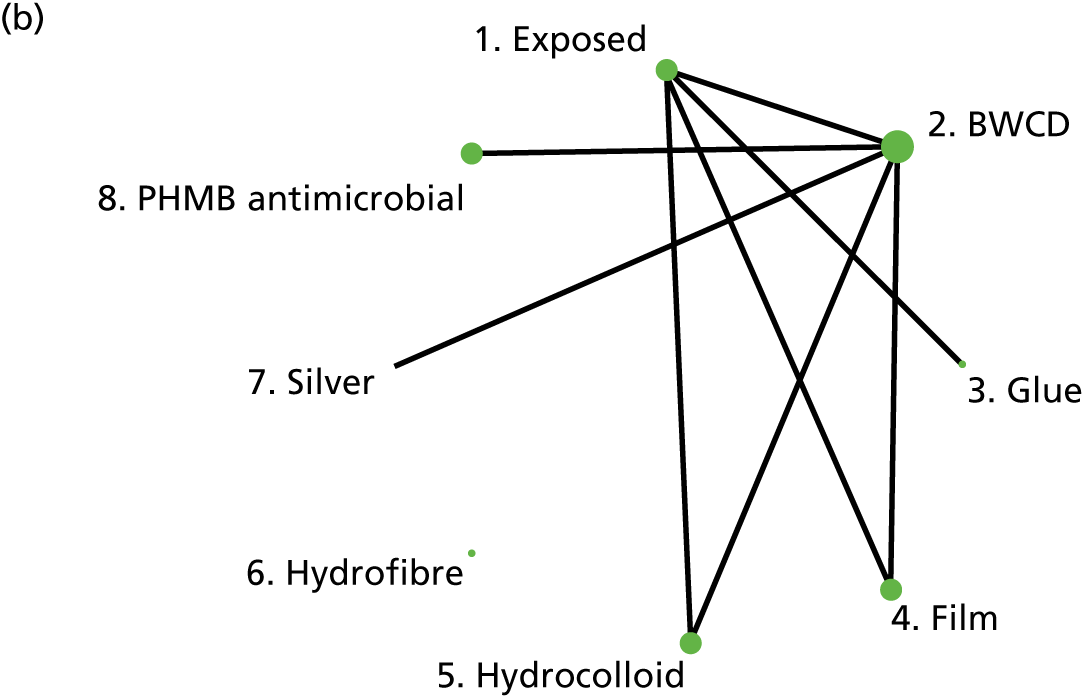
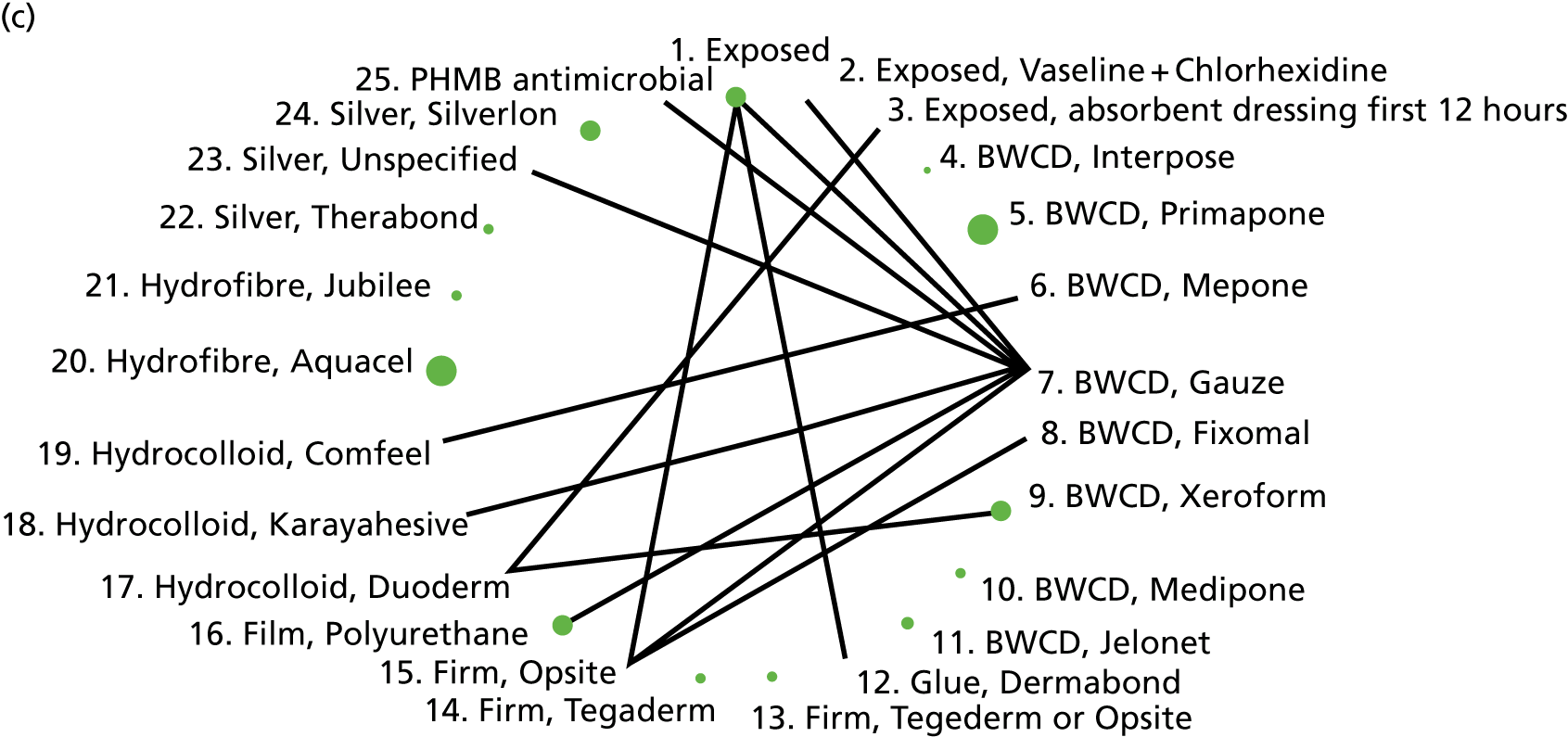
The intervention comparisons made in the included studies can be represented pictorially in a network plot, in which interventions are joined by lines if there is a RCT comparing those two interventions, and the thickness of the lines indicates the number of RCTs making that comparison. We display these plots for the surgery types representative of the Bluebelle population (Figure 2) and for all surgery types (see Appendix 1, Figure 14). Each figure shows network plots at three different classification levels of the interventions: Bluebelle project classification, intervention level and Cochrane review classification (see Appendix 1, Table 40). It is apparent that some level of grouping of the interventions is necessary because the networks are ‘unconnected’ when using the most detailed level of intervention definition; comparisons cannot be made between interventions when there is no connecting path.
FIGURE 2.
Network plots showing comparisons that have been made between interventions (for three different classification schemes) in RCTs included in the Cochrane update review17 where surgery type was representative of the Bluebelle population of interest. The term ‘exposed’ in the figure presents leaving the wound uncovered, described as ‘no dressing’ in the pilot Bluebelle trial. BWCD, basic wound contact dressing; PHMB, polyhexamethylene biguanide.
We considered two different NMA models to estimate the relative efficacy of wound dressings according to the Bluebelle classification: (1) a random-effects model with intervention effects defined by the Bluebelle classification and (2) a hierarchical model with three levels: studies nested within interventions defined using the Cochrane classification, which in turn are nested within the coarser Bluebelle classification. The fit of these two models was practically identical, and heterogeneity was not decreased by including the additional level of hierarchy. Therefore, we present only results from the simpler random-effects model using the Bluebelle classification. To put credible bounds on the estimates of intervention efficacy, we used informative priors on relative effects, chosen to give a 99% prior credible interval that the odds ratios lie between a factor of 3 either way (i.e. between 0.33 and 3, which represent extremes of effect). This corresponds to a 95% prior credible interval of 0.43 to 2.3. Any posterior intervals similar to this indicate comparisons where evidence is lacking, so that results are dominated by the prior.
Table 1 shows results from the NMA for each dressing type compared with simple dressings (Bluebelle definitions). Owing to the low SSI event rate and relatively small sample sizes in the included studies, all estimates have wide credible intervals, and all include 1 (no effect). The results for the ‘all surgery’ population are comparable to those for Bluebelle population surgery types. There is very little improvement in model fit by allowing for surgery type and heterogeneity increases. We therefore use the estimates from all surgery types because they are more precisely estimated.
| Surgery type | Odds ratio (95% credible interval) | ||
|---|---|---|---|
| Exposed vs. simple | Glue vs. simple | Complex vs. simple | |
| All surgery types | |||
| All wound types | 0.979 (0.561 to 1.546) | 1.049 (0.371 to 2.413) | 0.858 (0.535 to 1.263) |
| Clean wounds | 0.787 (0.403 to 1.388) | 0.847 (0.278 to 2.001) | 0.740 (0.397 to 1.277) |
| Mixed/unclear/contaminated | 1.153 (0.608 to 1.92) | 1.263 (0.392 to 3.029) | 1.068 (0.534 to 1.865) |
| Only surgery types representative of Bluebelle population | |||
| All wound types | 0.889 (0.465 to 1.497) | 0.956 (0.315 to 2.214) | 0.726 (0.329 to 1.382) |
| Clean wounds | 0.805 (0.348 to 1.629) | 0.860 (0.245 to 2.232) | 0.753 (0.224 to 1.908) |
| Mixed/unclear/contaminated | 1.064 (0.532 to 1.841) | 1.172 (0.353 to 2.887) | 0.902 (0.394 to 1.757) |
Results are shown for all surgery types included in Dumville et al. 17 and also restricting to studies with surgery types representative of the Bluebelle population of interest. Results are also shown on the basis of whether or not there were clean wounds only. Some of the estimates are associated with considerable uncertainty (posterior approximately equal to the prior), reported in grey font.
Most of the evidence is for complex versus simple dressings and shows a trend for reduced SSI rates with complex dressings, although the credible interval crosses 1 (no effect). Exposed wounds, that is wounds left uncovered with no dressing, show similar effectiveness to simple dressings and a trend towards a reduced SSI rate when used on clean wound types, although credible intervals are wide and cross 1 (no effect). There is no direct evidence for glue versus simple wound dressings, and, although an indirect comparison can be formed, this estimate is extremely imprecise, so there is effectively no evidence for this comparison. Similarly, there is effectively no evidence for wounds that are non-clean.
In the economic model we use the results for ‘all wound types’ and ‘all surgery types’ in the base case, but note in interpreting the results that these results apply mainly to clean wounds. We use the results for ‘all wound types’ and ‘Bluebelle population surgery types’ in a sensitivity analysis.
The probabilities of each dressing being ranked first, second, third or fourth in terms of effectiveness in preventing SSIs are shown in Appendix 1 (see Figure 15). Complex dressings have the highest probability of being most effective for SSI outcomes, but this probability is < 0.5 for ‘all surgery’ and only just over 0.5 for the Bluebelle population surgery types, suggesting a high level of uncertainty. Appendix 1 (see Figure 16) also presents these probabilities cumulatively (i.e. the probability that each dressing is ranked in each position or better, so all dressings have a probability 1 of being fourth or better). The ideal curve would go straight to 1 and stay there, and we prefer dressings with curves above the others. Complex dressings are preferred according to the cumulative ranking curves, but the curves are all close to each other. Simple dressings perform least well. Table 2 gives the mean rank (where rank 1 is best and 4 is worst). Complex dressings have the best mean rank and simple dressings have the worst; however, these are very similar and 95% credible intervals span all ranks from 1 to 4 for each dressing type, reflecting the high degree of uncertainty.
| Dressing type | Mean rank (95% credible interval) | |
|---|---|---|
| All surgery types | Bluebelle population surgery types | |
| Complex | 1.93 (1 to 4) | 1.71 (1 to 4) |
| Glue | 2.52 (1 to 4) | 2.53 (1 to 4) |
| Exposed | 2.62 (1 to 4) | 2.56 (1 to 4) |
| Simple | 2.94 (1 to 4) | 3.20 (1 to 4) |
Surgical site infection cost
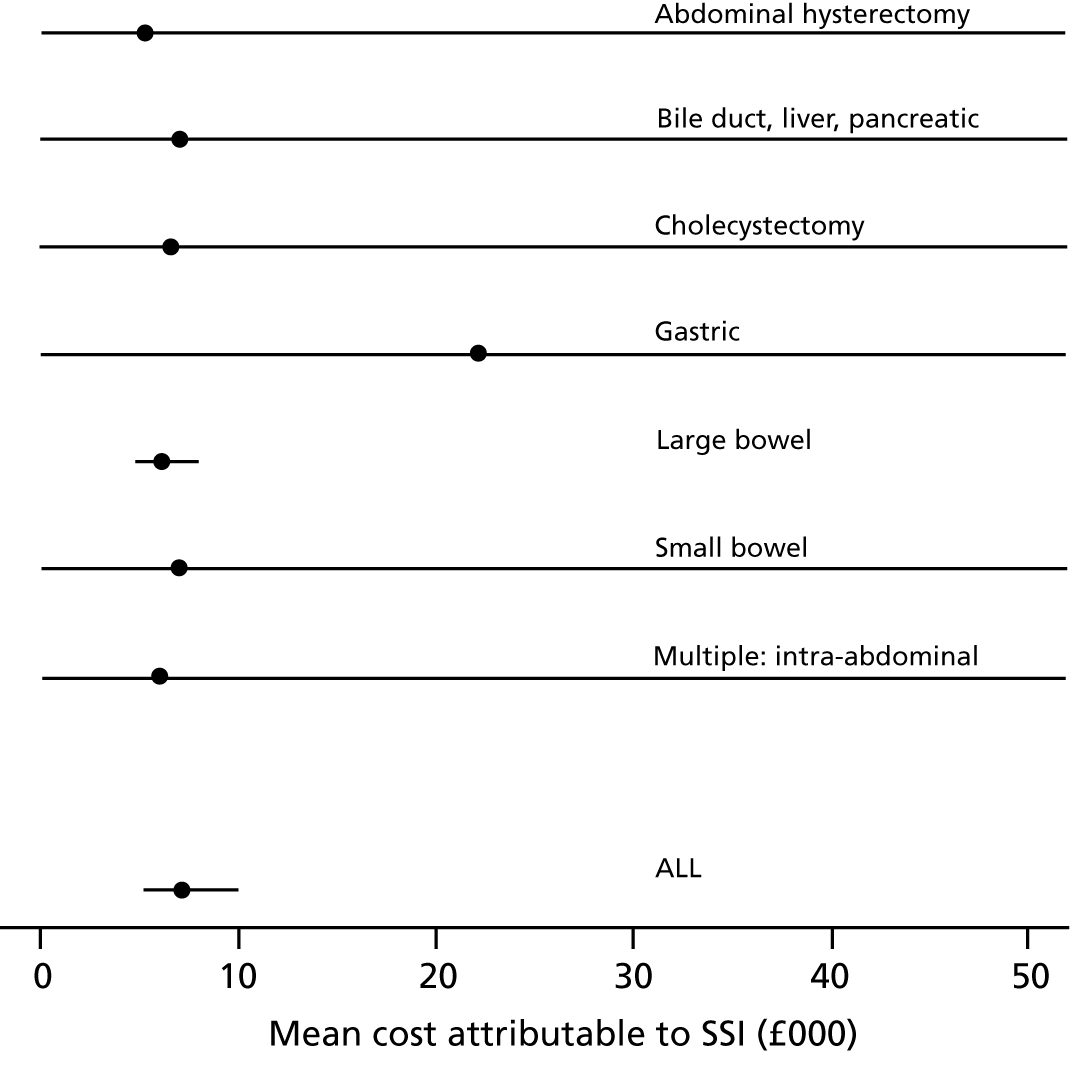
Jenks et al. 50 provide estimates of mean costs attributable to SSI, shown in Figure 3. Some of these estimates are very uncertain (very wide or incalculable CIs). SSI costs associated with gastric surgery are estimated to be much higher than those associated with other surgery types, although this estimate is based on only six patients. It is possible that gastric surgery included bariatric surgery (not our population of interest), which involves prosthetic ‘bands’ and may have led to higher costs. With the exception of gastric surgery, mean costs are quite similar across surgical sites. The estimated mean cost over all surgery types is £7179.79 with a 95% CI of £5225.01 to £9865.88. Assuming that these costs were correct for 2012, we inflate them to 2015/16 prices using an inflation factor of 1.097694841. 52 This gives a mean cost of £7881.22 with a 95% CI of £5724.25 to £10,850.95 in our model, described by a log-normal distribution with a mean of 8.972 and a standard error of 0.1631 on the log-scale.
FIGURE 3.
Mean cost (GBP) attributable to SSI by surgery type based on University Hospitals Plymouth NHS Trust data April 2010–March 2012 (Jenks et al. 50), assumed 2012 prices. CIs for the mean cost are obtained from reported confidence limits on the median cost, assuming that costs can be described by a log-normal distribution.
Dressing costs
Table 3 summarises the computation of dressing costs, based on the BNF15 and Personal Social Services Research Unit unit costs, 2015 prices. 53 We assume that a wound requires a large dressing (10 × 20 cm or 15 × 15 cm) or two vials of tissue adhesive; that simple or complex dressings may require changing (once), so that two dressings are required in total; and that the nurse time required to perform the change of dressing is as advised (clinical opinion). Dressing types on the BNF typical of each Bluebelle definition were chosen, and the product with the lowest cost within those types was chosen.
| Cost component | Exposed | Simple | Glue | Complex |
|---|---|---|---|---|
| Unit cost (BNF) (£) | 0 | 0.29 [10 × 20 cm absorbent perforated dressing with adhesive border (Cutiplast® Steril, Smith & Nephew, Zaventem, Belgium)] | 6.93 [1 × 500 mg vial Histoacryl® (B. Braun Melsungen AG, Hessen, Germany)] | 8.36 [15 × 15 cm hydrocolloid with silver antimicrobial (Aquacel® Ag Extra™, ConvaTec, Deeside, UK)] |
| Number of dressings per wound (including changes) | N/A | 2 | 2 vials | 2 |
| Total dressing cost (£) | 0 | 0.58 | 13.86 | 16.72 |
| Nurse cost to change dressing (£) | 0 | 4.67 | 0 | 4.67 |
| Total cost (£) | 0 | 5.25 | 13.86 | 21.39 |
Total cost is equal to the dressing cost plus the cost of nurse time to administer dressings. Dressing costs are computed as the number of dressings multiplied by the dressing unit cost. Nurse time is computed as £4.67 for 5 minutes based on a general practice-based nurse with qualifications patient contact time. 53
Quality-adjusted life-year decrement attributable to surgical site infection
Gheorghe et al. 54 systematically reviewed SSI utility values reported in patient-level and decision modelling studies. Only 6 out of the 28 studies identified related to the Bluebelle population surgery types. Most modelling studies used utility decrements based on the authors’ assumptions or on assumptions made in previous modelling studies. Only one study was conducted in a population relevant to Bluebelle (UK patients undergoing upper abdominal surgery laparotomy) and reported utility decrements based on patient-level EuroQol-5 Dimensions, three-level version, data attributable to superficial SSI. 2 Estimated SSI decrements were 0.05 and 0.12 at 7 days and 30 days, respectively. 2,54 The 30-day estimate is consistent with estimates from studies in other surgical areas reporting at 1 year55 and postoperatively. 56 We therefore assume a fixed utility decrement of 0.12 QALYs resulting from a SSI in our base case (SSIQALYloss = 0.12). We were concerned, however, that this may be an overestimate due to confounding (those with worse outcomes in general are more likely to get a SSI, and so reduction in quality of life may be due to other factors as well as SSI). We therefore used values SSIQALYloss = 0.06 and SSIQALYloss = 0 in sensitivity analyses.
Quality-adjusted life-year gain as a result of wound management compared with ‘no dressing’
We assume that there is no quality-of-life benefit of dressing a wound compared with leaving a wound exposed (which allows a gauze covering to be applied as long as it is not sealed around the perimeter). The model inputs are summarised in Table 4.
| Parameter | Base-case valuea | Source (base case) | Sensitivity analysis | Source (sensitivity analysis) |
|---|---|---|---|---|
| Prevalence of general surgery wounds | 2.208 million | Royal College of Surgeons,48 SPARCS49 | ||
| Proportion of surgery types | See Table 39 |
Jenks et al. 50 PHE51 |
||
| SSI risk with simple dressings | 0.1380 (0.1345 to 0.1415) |
Jenks et al. 50 PHE51 |
0.0894 (0.0775 to 0.1013) | Jenks et al.50 |
| Mean SSI cost | £7881.22 (£5724.25, £10,850.95) (log-normal mean = 8.972, SE = 0.1631 on log-scale) | Jenks et al.,50 inflated to 2015 prices | ||
| OR exposed vs. simple | 0.979 (0.561 to 1.546) | NMA (see Table 40), all surgery types | 0.889 (0.465 to 1.497) | NMA (see Table 40), Bluebelle population |
| OR glue vs. simple | 1.049 (0.371 to 2.413) | NMA (see Table 40), all surgery types | 0.956 (0.315 to 2.214) | NMA (see Table 40), Bluebelle population |
| OR complex vs. simple | 0.858 (0.535 to 1.263) | NMA (see Table 40), all surgery types | 0.726 (0.329 to 1.382) | NMA (see Table 40), Bluebelle population |
| Utility decrement resulting from a SSI | SSIQALYloss = 0.12 |
Pinkney et al. 20132 Gheorghe et al. 201554 |
0.06 and 0 | Assumption |
| Cost exposed (£) | 0.00 | Assumption | ||
| Cost simple (£) | 10.13 | BNF,15 PSSRU53 | ||
| Cost glue (£) | 13.86 | BNF,15 PSSRU53 | ||
| Cost complex (£) | 26.05 | BNF,15 PSSRU53 |
Time horizon and discounting
We assume a 1-year time horizon as adequate to capture all the cost and quality-of-life benefits attributable to the choice of wound dressing following a surgical procedure. It is, therefore, unnecessary to discount costs and QALYs in the economic model.
In the VoI analysis, we multiply per patient-year results by the population prevalence of general surgery wounds (2.208 million per annum) and by the ‘lifetime’ of the dressing intervention (assumed to be 5 years discounted at a rate of 3.5% to give a multiplier of 4.673) to obtain the population VoI. The ‘lifetime’ of the dressing can be thought of as the length of time until it is superseded by new evidence/innovations, or as a proxy for other uncertainties about the future. The VoI will increase as the ‘lifetime’ of the intervention increases.
Incorporating results from Bluebelle Phase B Study
We updated the NMA model (all wound types, all surgery types) incorporating the Bluebelle Phase B results using denominators based on either (1) intention to treat (ITT) or (2) per protocol (PP). We also updated the base-case EVPPI and EVSI results incorporating the Bluebelle results.
Computation
The WinBUGS code used to compute the NMAs is described in Report Supplementary Material 6. The methods used to compute the VoI, and the R-code used to compute all cost-effectiveness and VoI estimates, are also described in Report Supplementary Material 5 and 7. It was necessary to make a multivariate normal approximation to the NMA estimates used in the computation of EVSI. Making this approximation did not much alter any of the other model outputs and we therefore present all results under the multivariate normal approximation.
Objective A9: bring together the results of the above objectives to design Phase B of the study
The results of the research to address objectives A1–8 were presented to the SMG as Phase A progressed. The results were discussed and informed the design of the pilot trial, as described below (see the next section).
Objective B1: establish the numbers of potential participants at different hospitals who are considered likely to be eligible and who can be approached about the trial, and the proportions confirmed as eligible, recruited and randomised
Study design
Phase B was a pragmatic feasibility and pilot parallel group RCT, using mixed methods. The trial used a factorial design, allocating participants to one of three dressing types and to disclosure of the dressing allocation before or after wound closure (Figure 4). If a participant had multiple closed primary wounds, all wounds were intended to be managed on the basis of the allocation.
FIGURE 4.
Trial schema for the Bluebelle Phase B external pilot trial, showing the double randomisation.
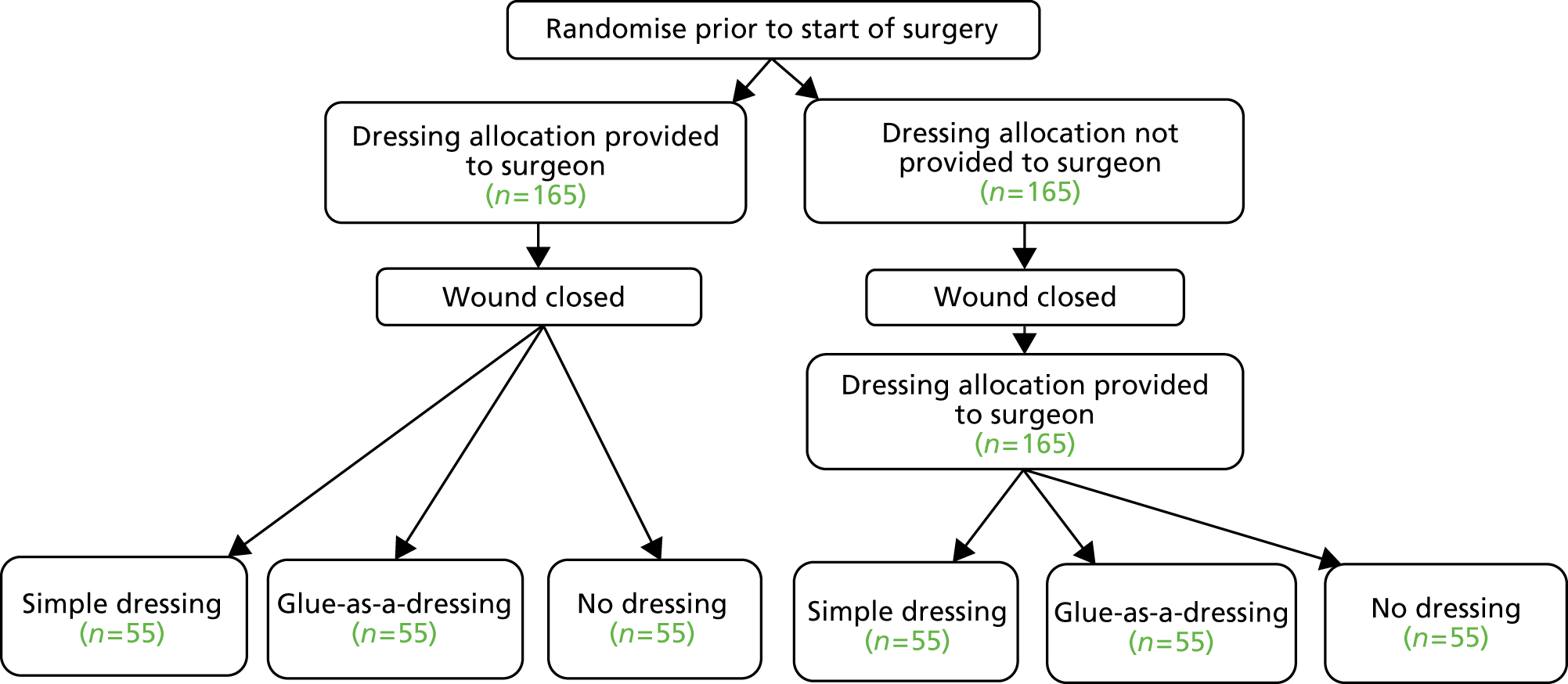
The time-of-disclosure factor was included because disclosing allocation after wound closure was preferred, to prevent surgeons closing the wound in different ways depending on the allocation, but was considered likely to be problematic by surgeons. This factor allowed the trial to test (1) the feasibility of randomising after wound closure and if photographs of the closed wound could be obtained in the operating theatre and subsequently assessed for the quality of wound closure and (2) the effect of timing of disclosure of dressing allocation on wound closure. This effect was potentially important as an indicator of the risk of performance bias if randomisation after wound closure proved impossible.
Study setting and population
The trial was set in secondary care (i.e. acute and maternity NHS hospitals). Patients aged ≥ 16 years undergoing primary elective or unplanned open or laparoscopic abdominal general surgery [including, but not limited to, gastrectomy for benign or malignant disease, cholecystectomy, anti-reflux procedures, hepatic resection, small or large bowel resection for benign or malignant conditions, abdominal wall hernia surgery (inguinal, femoral, incisional, epigastric and para-umbilical)] or elective or unplanned obstetric surgery (caesarean section) were eligible. Patients undergoing simultaneous abdominal and chest surgery were eligible but only those with abdominal wounds were allocated to one of the study interventions. At the time of recruitment, research nurses emphasised the need to attend a follow-up clinic 4–8 weeks after surgery and did not recruit patients who said that they would be unable to do so.
Patients with any of the following characteristics were ineligible:
-
abdominal or other major surgery < 3 months before the index operation
-
those for whom the surgeon intends to ‘close’ the wound with tissue adhesive (glue)
-
any contraindication to one of the dressing allocations, including allergy to dressings
-
undergoing surgery where no skin incision occurs
-
lacking capacity to consent
-
an inability to complete patient-reported outcome questionnaires
-
detained in the prison service.
Reasons for ineligibility were recorded.
Eligible patients were provided with a participant information leaflet (PIL), either at a clinic visit before the operation or sent in advance of admission. They were given as long as possible to consider the study before being approached for consent (usually > 4 hours for elective surgery and usually > 1 hour for unplanned surgery). Consent was not requested if a patient asked for longer thinking time or appeared visibly distressed.
Participants were also asked to consent to four optional aspects of the trial, designed to explore feasibility:
-
willing to be interviewed about the acceptability of, and adherence to, the allocated dressing type (objective B3)
-
willing for a member of the research team to take a photograph of the wound(s) in the operating theatre
-
willing to take a photograph of their wound(s) themselves 4–8 weeks after the operation and to send it to the research team (described to participants as a ‘wound selfie’, a term that was readily understood; objective B7)
-
willing for a skin transfer to be applied after the operation to remind staff that the participant was in the study.
Surgery was carried out in accordance with local protocols. Apposition of wound edges and the method of closure of the skin were at the discretion of the surgeon and could include sutures, clips, wound closure strips or combinations of these wound closure methods.
Randomisation
The factorial design created six groups (see Figure 4). Both factors were randomised in blocks of varying size and stratified by hospital trust and specialty (abdominal surgery/obstetric surgery). The random allocation sequences were generated by computer in advance of starting to recruit. Local research team members accessed a participant’s allocation via the internet. The allocation was concealed until a participant’s eligibility and consent had been documented and information to identify the participant uniquely had been entered.
A member of the local research team (trainee or consultant surgeon/research midwife or nurse) accessed a secure password-protected computer system (within the study database) and entered the information needed to proceed to randomise the participant, usually at the beginning of the operation. Depending on the randomisation result, the dressing group allocation was disclosed immediately, or the user was advised to log into the system after the wound had been closed and to enter the time of wound closure, after which the allocation was disclosed.
Blinding
It was not possible to blind surgeons, participants or staff caring for participants to the dressing allocation. However, we planned to blind research staff assessing outcomes 4–8 weeks after randomisation. Methods to achieve blinding were piloted to test their feasibility and acceptability for the main trial. These included requiring the reference SSI assessment and the Wound Healing Questionnaire (WHQ) to be completed by HCPs who had not been involved in a participant’s care during the index admission. (The study also required these assessments to be carried out by different people, in order to validate the WHQ.) The success of blinding among assessors of SSI 4–8 weeks after randomisation (HCPs completing the reference SSI assessment or the WHQ) was assessed by asking them which study group they thought the participant was allocated to.
Trial interventions
The three dressing groups were simple dressing, glue-as-a-dressing and no dressing. The interventions for the first two groups were defined as described in Objective A6: use the literature and views of experts to define and categorise commonly used dressings into three pragmatic groups. Tissue adhesives are topical skin adhesives. In this trial, the glue-as-a-dressing intervention was defined as tissue adhesive applied in accordance with the manufacturers’ instructions and only to the surface of an already closed primary wound, acting as a dressing (i.e. not to close the wound, which would involve applying the tissue adhesive below skin level).
Bioclusive® (Systagenix, Gatwick, UK), C-View® (Aspen Medical, Ashby-de-la-Zouch, UK), Hydrofilm® (Hartmann, Heywood, UK), Opsite® (Smith & Nephew, Zaventem, Belgium), Mepore® (Mölnlycke, Oldham, UK) and Tegaderm® (3M, Bracknell, UK) are examples of commonly available simple dressings. 49 Hospital trusts could have stocked other types and were advised to use the dressing that represented usual care. Dermabond ProPen® (Ethicon, Wokingham, UK), Epiglu® (Meyer-Haake, Ober-Moerlen, Germany) and Histoacryl® (Braun, Sheffield, UK) are examples of commonly available tissue adhesives that could be used. 49 In the no dressing group, no simple dressing or tissue adhesive was applied to the wound at the end of the operation.
The following aspects of wound care applied to all interventions:
-
A participant’s wounds should be dressed on the basis of the participant’s treatment allocation throughout their hospital stay.
-
When a participant had more than one wound (e.g. multiple port sites for laparoscopic surgery), all eligible wounds should be dressed on the basis of the randomised allocation.
-
A wound might manifest slow discharge or ongoing seepage of fluid (‘ooze’) from the wound in the first 24 hours. We allowed a simple gauze swab to be applied to the area that was oozing in all groups without compromising adherence; nursing staff were instructed to tape the swab in place temporarily and not around its entire perimeter. Gauze swabs (filmated or not), non-woven fabric swabs (filmated or not), knitted viscose and paraffin gauze dressings are examples of swabs that could be used. If oozing continued, or if a SSI occurred (i.e. after the outcome of interest had been ascertained), the clinical team could apply any dressing or resuture the wound if necessary; when this represented a deviation from the allocated dressing, the action was documented.
-
Cointerventions that might have influenced SSI rates (e.g. the use of prophylactic antibiotics and other aspects of pre-, peri- or postoperative care) were allowed at the discretion of the team and hospital looking after the participants. Their distribution by allocated group was monitored, because the differential implementation of cointerventions arising because the usual care team was not blinded to the allocated dressing could introduce bias. This information informed decisions about the need to standardise care when designing the main trial.
-
To encourage adherence to the treatment allocation, colour-coded skin transfers showing the study logo and the dressing allocation were applied on the participant’s skin, near the surgical wound(s), as a reminder to HCPs looking after the patient (see Appendix 2, Figure 17). The skin transfer was not visible by the time of the 4- to 8-week assessment.
Study centres and surgeons
Four NHS trusts took part: University Hospitals Bristol NHS Foundation Trust (Bristol Royal Infirmary), North Bristol NHS Trust (Southmead Hospital), University Hospitals Birmingham NHS Foundation Trust (Queen Elizabeth Hospital) and Worcestershire Acute Hospitals NHS Trust. One recruited participants having either abdominal surgery or caesarean section. One trust joined in month 6 of recruitment. All general surgical teams carried out a wide range of operations. The participating obstetric unit carried out approximately 1800 caesarean sections each year. Because all operations were being carried out as part of the usual care of participants, there were no restrictions on operating personnel or ward care.
Primary feasibility outcome
The primary outcome was successful screening of a participant, and determination of the participant’s eligibility and consent to be randomised in the pilot trial. This information, together with the denominator describing the total number of participants approached, established whether or not recruitment into the main trial was possible.
Secondary feasibility outcomes
These comprised:
-
adherence to disclosure of dressing category allocation at the designated time
-
adherence to the allocated dressing type by the usual care team during the index hospital admission and, if applicable, the reason for non-adherence
-
quality and completeness of the data for different outcomes anticipated to be measured in the main trial (see below; assessed at different times), including component assessments contributing to an overall judgement about the occurrence of a SSI
-
adherence to the follow-up schedule
-
documentation of cointerventions (e.g. details of hair removal, use of skin cleansing agents, type of wound closure methods, prescription of prophylactic antibiotics) and classification of surgery as ‘clean’, ‘contaminated’ or ‘dirty’ at the time of the operation
-
completion of the reference SSI assessment at 4–8 weeks by a blinded observer
-
completion of a WHQ57 at 4–8 weeks by a blinded observer and by the participant
-
completion of a Wound Management Questionnaire (WMQ) (developed during Phase A) up to 4 days after randomisation by an observer or the participant (if discharged early, e.g. day case surgery)
-
completion of a Wound Experience Questionnaire (WEQ) (developed during Phase A) up to 4 days after randomisation by the participant
-
assessment of wounds from digital photographs at 4–8 weeks, if submitted.
Some of these secondary outcomes were developed in Phase A and piloted in Phase B, and detailed scoring methods were not necessarily available. This report focuses on the questionnaire response rates, the number of fully completed questionnaires and rates of missing items in this pilot RCT as part of the evaluation of the acceptability of the new questionnaires to patients. The data from the pilot trial were also used to validate some of the questionnaires.
Anticipated outcomes in a subsequent main trial
The following outcomes were expected to be assessed in a main trial. Therefore, we documented the collection of these outcomes carefully:
-
occurrence of a SSI up to 4–8 weeks after randomisation (primary)
-
wound management – WMQ
-
patient-reported outcomes (WEQ) and generic health status, assessed by the EuroQol-5 Dimensions, five-level version (EQ-5D-5L)58
-
wound complications arising up to 4–8 weeks after randomisation
-
resource use up to 4–8 weeks, including length of postoperative hospital stay, rates of readmission and duration.
Trial procedures and data collection up to 4 days after randomisation
The schedule of data collection is described in the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) diagram (Table 5). Surgery and closure of wounds took place as per usual practice. A member of theatre personnel, or a member of the research team, recorded (1) the time taken to close the wound, (2) the use of laparoscopic or open surgical methods and (3) the method of wound closure. A member of theatre personnel or the research team could also take one or more digital photographs of the wound(s). If a participant was randomised to disclosure of allocation after wound closure, the time of completing wound closure had to be entered into the database to obtain the allocation. A range of methods were used to promote adherence to the randomised dressing allocations in hospital, adapted to the circumstances of participating hospitals. These included simple labels on medical notes or placed by the bedside, as well as skin transfers (see Appendix 2, Figure 17).
| SPIRIT item | Study period | ||||||
|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post allocation | Close-out | ||||
| In hospital | Post discharge | ||||||
| Pre randomisation | Before surgery | After wound closure | Up to 4 days after surgery | Any time if wound problem | Day 15 post surgery | 4–8 weeks post surgery | |
| Time point (days) | 0 | 0 | +4 | 1–56 | +15 | 28–56 | |
| Enrolment | |||||||
| Eligibility screen | ✗ | ||||||
| Informed consent | ✗ | ||||||
| Randomisation to dressing allocation | ✗ | ||||||
| Randomisation to time of disclosure of dressing allocation | ✗ | ||||||
| Disclosure of dressing allocation | ✗a | ✗a | |||||
| Interventions | |||||||
| Simple dressing | ✗ | ||||||
| Glue-as-a-dressing | ✗ | ||||||
| No dressing | ✗ | ||||||
| Assessments | |||||||
| Demographics | ✗ | ||||||
| EQ-5D-5L | ✗ | ✗ | ✗ | ✗ | |||
| Adherence to allocation | ✗ | ✗ | ✗ | ✗ | |||
| Type(s) of wound dressing and frequency of use | ✗ | ✗ | ✗ | ||||
| Resource use | ✗ | ✗ | ✗ | ||||
| WMQ (observer or participant) | ✗ | ||||||
| WEQ (participant) | ✗ | ||||||
| WHQ (participant) | ✗ | ||||||
| WHQ (observer) | ✗ | ||||||
| Wound photograph by participant (optional) | ✗ (for a subgroup) | ||||||
| Reference SSI assessment | ✗ | ||||||
| SSI-specific resource use | ✗ | ||||||
| Qualitative | |||||||
| Qualitative interview (telephone or face to face) (optional) | ✗ | ✗ | ✗ | ||||
| Audio-recording of consultation about study participation (optional) | ✗ | ||||||
Up to 4 days after surgery, after any early wound care that was required, a HCP completed the WMQ. This questionnaire captures information about aspects of the participant’s wound management. If discharged early (e.g. after day case surgery or the day after surgery), the research team gave the participant the WMQ to complete by day 4 and asked the participant to return the completed WMQ in a prepaid envelope.
The local research team gave each participant the WEQ to complete up to 4 days after surgery. For participants who were in hospital at this time, the WEQ was collected by the research team. If discharged early (e.g. after day case surgery or the day after surgery), the participant completed the WEQ at home by day 4 and returned it in a prepaid envelope.
Data collection up to 4–8 weeks after randomisation
Local research teams gave each participant a copy of the EQ-5D-5L and a prepaid envelope to take home and instructed the participant to complete and return it if the wound became infected or problematic. The reason for asking for the EQ-5D-5L to be completed if there was a problem with a wound was to try to document the peak impact of a wound problem on health status to inform a future health economic evaluation.
Several follow-up assessments were required 4–8 weeks after randomisation. First, the participant was asked to complete a copy of the EQ-5D-5L and the WHQ; these questionnaires were given to participants on discharge from hospital or posted shortly before they were due. Around this time, participants who had agreed to send a photograph might also have been contacted about this. A blinded health professional completed the WHQ; this could be done face to face, typically at the same clinic attendance but before the reference SSI assessment, or administered by telephone. The face-to-face reference SSI assessment was completed by a blinded health professional, who had to be different from the professional completing the WHQ. This assessment included eliciting information about potential wound-related complications. Whenever possible, the face-to-face clinic visit was arranged to coincide with a usual care clinic appointment. When this was not possible, travel expenses were offered to the participant. For women who had had a caesarean section, the WHQ questionnaire was administered by telephone and research midwives then carried out the reference SSI assessment during a home visit.
To promote retention, reminders were sent to participants who failed to return postal questionnaires promptly. Participants who failed to attend the face-to-face SSI assessment were offered further appointments.
Assessment and analysis of resource use
Introduction and aims
From an economic point of view, the pilot trial had two aims: (1) to explore the feasibility of collecting key health-care resource use data that were deemed important in determining the cost-effectiveness of the assessed dressing options and (2) to identify resource use elements that make a significant contribution to the total per-patient cost of care provided under each of the assessed options. In the context of the assessed dressing options, relevant resource use included initial dressings and dressing changes while in hospital and after discharge, care provided in response to wound healing complications at the primary and secondary care settings, and use of medications.
With these aims in mind, we (1) assessed the completion rates for each of the above key categories, to explore the feasibility of collecting relevant resource use data through the utilised sources, and (2) carried out a cost analysis to translate resource use into costs, with a view to identifying key cost drivers. In addition, the study offered an opportunity to obtain information that will be useful in future analyses as part of a definitive trial, including unit costs of different dressings and services and frequency of dressing changes.
Methods
Elements of resource use relevant to the assessed options were grouped in the following categories:
-
use of dressing and redressings while in hospital
-
use of care as a result of postoperative complications in hospital
-
use of dressings after initial discharge
-
hospital and primary care appointments after initial discharge
-
initiation of new medications.
Data on resource use under each of these categories were made available from two main sources: case report forms (CRFs) collecting clinical information and use of medical services related to the wound(s), and a participant dressing log. Initial wound dressings and dressing changes in hospital were collected through CRFs designed to capture randomisation, surgery, and dressing allocation and use details. Care provided in response to wound healing was available through CRFs on postoperative complications. Use of dressings and dressing changes after hospital discharge was taken from the participants’ self-reported dressing logs. Hospital care (outpatient appointments, accident and emergency visits, overnight admissions) and use of primary care services [e.g. general practitioner (GP) appointments] after the initial hospital discharge were available from CRFs collecting data on the use of medical services related to wound healing. Details on use of new medications (e.g. steroids, antibiotics) and on the type and frequency of antibiotics use were collected from a CRF form collecting information about infective complications.
Data cleaning was carried out to correct spelling mistakes (e.g. histoaycrl was changed to histoacryl), as well as to ‘impute’ answers as required.
A cost analysis was carried out to convert resource use into cost estimates. Collected data were combined with unit cost information to give estimates of the total per-patient cost associated with (1) no wound dressing, (2) simple wound dressing or (3) glue-as-a-dressing. Unit cost information was obtained from various sources, including the NHS Reference Costs Schedules,59 the BNF60 and the Unit Costs of Health and Social Care report by Curtis and Burns. 61 Costs are reported in 2015/16 prices. In line with recommendations, the analysis adopted the perspective of the NHS and Personal Social Services. 47 Costs incurred by the patients (e.g. out-of-pocket expenses for self-bought dressings) are also presented.
The analysis was conducted in accordance with the ITT principle, with data being analysed on the basis of participants’ allocated intervention. 62 Mean differences in the total cost per patient associated with each wound-dressing group were analysed using regression methods. As the distribution of costs is typically positively skewed by the existence of a small number participants with very high cost, CIs of differences were obtained through non-parametric bootstrap methods (bias corrected and accelerated, 1000 replications). 63,64 Given the aims of this pilot study, the time horizon of the analysis corresponds to the trial follow-up period (4–8 weeks). As a result, undiscounted costs are presented.
Both a complete-case analysis (CCA) and an available-case analysis (ACA) were undertaken. 65,66 In CCA, participants were included in the cost calculations only if they had complete data for all cost categories. In ACA, all available data contributed to calculations for each of the cost categories, irrespective of whether of not data for the same participant were missing from other categories.
Detailed information on data availability and calculation of costs for each resource use category is available in Report Supplementary Material 8.
Trial duration
The trial was timetabled to take up to 11 months, randomising the target number of 330 participants in 9 months and then following up the last participants for 4–8 weeks.
Sample size
A target sample size of 920 eligible participants was set, allowing a recruitment rate of 36% (corresponding to the target number randomised of 330) to be estimated with a 95% CI of 32% to 39% and a recruitment rate of 60% (95% CI 56% to 64%). For the simple dressings group, we anticipated an adherence rate of 90%. Assuming a 36% recruitment rate and 110 randomised participants per group, a 90% adherence rate would be estimated with a 95% CI of 82% to 95%. We had no information on which to base any estimate of adherence in the no dressing and glue-as-a-dressing groups. However, if adherence were < 70% in either group, we stated in the protocol that we would conclude that randomisation to the group in question in the main trial was not feasible.
Statistical analyses
Summary descriptive statistics to inform plans for the main trial were compiled, including:
-
the number of potentially eligible participants per month per centre
-
the percentage of potential participants confirmed as eligible
-
the percentage of participants consenting to the pilot RCT
-
the percentage of randomised participants receiving the allocated treatment and completing outcome measurements at the 4- to 8-week assessment
-
the rate of, and reasons for, non-adherence to allocation at both a wound and a participant level
-
the mean number of wounds per participant
-
the mean (or median, if skewed) time from wound closure to randomisation
-
the mean (or median, if skewed) time to complete the randomisation process
-
the completeness of data items and reasons for missing data
-
the rate of unblinding of outcome assessors and reasons for unblinding.
Only the statisticians (CAR and RAH) had access to the data. The analysis population comprised all randomised participants. Results were described by centre and by specialty as well as overall. If the data allowed, we planned to carry out subgroup analyses of the secondary outcome measures relating to wound closure, estimating the interaction of timing of randomisation by dressing group.
The primary analysis took place when follow-up was complete for all recruited participants. During the pilot trial, we monitored recruitment and adherence periodically. The Study Steering Committee (SSC) reviewed safety data. In any interim reports, for example about withdrawals after randomisation, the data were presented by group, with uninformative labels used to keep the allocation masked.
Objectives B2 and B3: use qualitative research methods to investigate reasons for any difficulties that affect recruitment and assess acceptability of trial interventions and processes to participants and clinical staff
As stated in the funding proposal, we planned to integrate the QuinteT Recruitment Intervention (QRI)67 into the pilot RCT if recruitment proved problematic. As recruitment to the pilot started (and proceeded) well, the QRI was not implemented, although we did explore the barriers to and facilitators of recruitment as part of a wider qualitative study integrated throughout Phase B. The main aim of this qualitative study was to understand issues relating to adherence and acceptability in the pilot RCT. Specific objectives were to investigate:
-
HCPs’ and patients’ perspectives on adherence to the trial allocations (including possible actions or behaviours that could have unwittingly threatened adherence)
-
HCPs’ and patients’ perceived acceptability of the dressing strategies under comparison.
Study design
We conducted a qualitative study, consisting of semistructured face-to-face or telephone interviews with Bluebelle participants (patients) and HCPs involved in delivering the pilot RCT.
Sampling and recruitment
Patients
Patients participating in the pilot RCT were eligible to take part in interviews. Research nurses provided all patients who consented to the pilot RCT with a PIL about the integrated qualitative research and asked if they would be willing for their contact details to be sent to the qualitative research team for a potential interview. Those who agreed provided written consent for their contact details to be shared, forming the sampling frame for the qualitative interviews. Potential interviewees were then purposefully selected, with the intention of including a range of patients based on the following criteria:
-
surgical specialty (upper GI, lower GI or obstetric)
-
surgical approach (laparoscopic or open procedures)
-
nature of hospital admission (elective or unplanned)
-
Bluebelle study allocation (simple dressing, no dressing or glue-as-a-dressing)
We intentionally weighted the sample towards patients receiving no dressing and glue-as-a-dressing, as we anticipated that patients’ experiences of these dressing strategies would have greater implications for the feasibility of a future RCT. We considered ‘simple dressings’ to be aligned with ‘usual care’. The sampling of patients continued until data saturation had been achieved (i.e. the point at which further analysis did not add any meaningful insights in relation to the study objectives).
Members of the qualitative research team contacted patients by telephone and invited them to take part in an interview. Interviews were arranged at a mutually convenient time/date. The date of the initial telephone call was logged along with the outcome so that non-responders could be contacted again. No further contact was made after the second attempt.
Health-care professionals
Health-care or research professionals who were considered ‘key informants’ were purposefully selected for potential interview. ‘Key informants’ were defined as any individuals with responsibility for caring for Bluebelle participants or delivering aspects of the pilot RCT (e.g. recruitment, randomisation or follow-up). Given the somewhat limited pool of relevant staff to sample from, data collection continued until all key informants had been approached for interview. All staff members were invited to interview via e-mail, with a final follow-up e-mail sent in the event of non-response.
Data collection
Interviews were conducted face to face or by telephone up to 30 days post randomisation, between February 2016 and August 2016. Interviews were conducted by Leila Rooshenas or Christel M McMullan and audio-recorded following receipt of written consent. Field notes were recorded to note any contextual information that could have influenced the interview (or have a bearing on analysis). Face-to-face interviews took place in participants’ homes or on NHS premises.
Analysis
Audio-recorded interviews were transcribed verbatim and analysed thematically using the constant comparison method, as described previously (see Objectives A1 and A2: understand practice and views in relation to dressings). Transcripts were coded line by line in NVivo version 10. HCP and patient interviews were analysed separately. Data collection and analysis proceeded iteratively, enabling the qualitative team to refine topic guides to explore new lines of inquiry that emerged from the ongoing analysis. The initial and final topic guides for HCP and patient interviews are shown in Report Supplementary Material 9.
Interviews were analysed by Leila Rooshenas, with Christel M McMullan analysing a subset in the early stages of analysis to enhance coding reliability. This helped to establish an early version of the coding frame, which subsequently evolved over time. There was an attempt to search for ‘negative cases’ in relation to particular themes from both patient and HCP interviews; where present, these are fully reported. Some of the key themes relating to wound healing and wound management from the patient interviews were presented in a matrix (as columns), set out against patient interviewees (rows) grouped on the basis of their allocated dressing strategy. This facilitated comparison of patients’ accounts from the three trial comparison groups.
Objective B4: assess adherence to dressing type allocation and the follow-up protocol
This objective was assessed both quantitatively and qualitatively. Quantitative information included the proportions of participants whose wound care adhered to different dressing types at different stages of the patient’s pathway through the pilot trial and the proportions of participants with data for the data items that we attempted to collect, both in hospital and after discharge through questionnaires. The methods for these aspects of the trial have been described previously (see Objective B1: establish the numbers of potential participants at different hospitals who are considered likely to be eligible and who can be approached about the trial, and the proportions confirmed as eligible, recruited and randomised). Similarly, the qualitative methods used to study adherence and follow-up have been described in Objectives B2 and B3: use qualitative research methods to investigate reasons for any difficulties that affect recruitment and assess acceptability of trial interventions and processes to participants and clinical staff.
Objective B5: assess the appropriateness and feasibility of collecting a range of secondary outcomes and resource use data
This objective was assessed quantitatively through the proportions of participants with data for the secondary outcomes that we attempted to collect, both in hospital and after discharge, through questionnaires. The methods for collecting secondary outcomes have been described previously (see Objective B1: establish the numbers of potential participants at different hospitals who are considered likely to be eligible and who can be approached about the trial, and the proportions confirmed as eligible, recruited and randomised).
Objective B6: establish the validity and reliability of the developed tool for assessing wounds for surgical site infection
Study design
Evaluation of the WHQ used data from a specifically designed cohort study (Phase A) and the pilot RCT.
Study setting and population
Centres and participants in the pilot RCT are described elsewhere (see Objective B1: establish the numbers of potential participants at different hospitals who are considered likely to be eligible and who can be approached about the trial, and the proportions confirmed as eligible, recruited and randomised). Three NHS trusts participated in the cohort study. Participants with similar inclusion and exclusion criteria to those of participants in the pilot RCT were identified, approached and recruited by research nurses and surgical trainees.
Data collection
The collection of questionnaire data in the Phase B pilot RCT has been described elsewhere (see Objective B1: establish the numbers of potential participants at different hospitals who are considered likely to be eligible and who can be approached about the trial, and the proportions confirmed as eligible, recruited and randomised).
In the cohort study, participants were recruited either prior to or within a few days of surgery. Sociodemographic and operative details were collected at the time of recruitment. Data were collected on study data collection forms and recorded and managed using purpose-designed electronic data capture tools developed using Research Electronic Data Capture (REDCap) software. 68
Participants were asked to complete the EQ-5D-5L (baseline assessment). Approximately 3 weeks after surgery, participants were posted the WHQ (self-assessment). Included was a ‘debriefing’ questionnaire asking questions on the feasibility and practicality of completing the WHQ (e.g. time taken to complete the questionnaire and whether any items were confusing or difficult to answer) and the EQ-5D-5L (follow-up assessment). A stamped addressed envelope was provided to return the questionnaires.
A subset of participants (n = 50) within the Phase A cohort were invited to complete a second self-assessment to evaluate the test–retest reliability of the WHQ. Sampling was carried out by convenience during 1 month of the study. Participants were posted a second copy of the WHQ within a few days of receipt of the first questionnaire. This was done to provide data.
Approximately 30–35 days after surgery, staff attempted to contact participants and complete the WHQ (observer assessment) by telephone. This assessment took place face to face if the patient was still in hospital or had been readmitted. During the telephone assessment, a number of participants were invited to a face-to-face follow-up appointment in clinic. Convenience sampling methods were applied, inviting participants based on staff and clinic availability, and participant willingness and proximity to the clinic. At the face-to-face appointment, a clinical member of the research team (blinded to the wound self-assessment and observer assessment) made a SSI diagnosis using the CDC criteria (reference SSI assessment).
Statistical analyses
Combined cohort and RCT data were used to examine the adherence to and acceptability of the WHQ (response rates and missing data), inter-rater patient and observer agreement in responses and ability of the WHQ to discriminate between SSI/no SSI [receiver operating characteristic (ROC) curve analyses]. Phase A cohort data alone were used to examine the feasibility and practicability of completing the WHQ (debriefing questions) and intrapatient test–retest reliability. Possible scale structures of the WHQ were initially explored in Phase A cohort data and subsequently tested (as a method of internal validation) with data from the Phase B RCT.
Preparing the data
Core items 1–16 and subitems 3a, 3b and 3c and 4a and 4b, which sought to assess wound healing for SSI, were retained for WHQ validation. Responses to other subitems (9a–d, 10a–e, 12a–b) were excluded because their purpose was to collect health economic data for the pilot RCT and they were not directly relevant to assessing wound healing and SSI. Data on a total of 16 core items and five subitems were therefore included for further analysis. Responses to ordinal items were assigned values of ‘not at all’ = 0, ‘a little’ = 1, ‘quite a bit’ = 2 and ‘a lot’ = 3. Binary items were assigned values of ‘no’ = 0 and ‘yes’ = 1. Additional responses of ‘do not know’ (items 12–16) were recoded as missing data for parts of the analysis that examined item correlations (see below).
Analyses were performed using Stata version 14.0.
Analyses addressed the following aspects.
Wound Healing Questionnaire adherence and acceptability
The total number of completed WHQ self-assessments and observer assessments were examined as a measure of the questionnaire’s overall adherence and acceptability. Participants’ adherence to completing and returning the WHQ by post was examined by the number of questionnaires that were sent, returned and completed by patients. Demographics of patients who did not respond to the postal questionnaire and did not have an observer assessment (i.e. did not respond to telephone or face-to-face follow-up) were examined.
Feasibility and practicality
Answers to the debriefing questions on feasibility and practicality of completing the WHQ (cohort participants only) were examined using descriptive statistics and free-text responses.
Missing data
The frequency of missing data for items and subitems in the WHQ was examined to determine whether or not missing data occurred more often for specific items. Free text and any notes made by the participants or study staff were examined to explore reasons for any missing data.
Distribution of responses
Descriptive statistics of the distribution of responses to each ordinal (not at all, a little, a lot, quite a bit) variable for symptom items and binary (yes, no) variable for wound care interventions items were examined. This allowed for an assessment of whether or not items were relevant and informative, and the extent to which the different response categories were used.
Participant and observer agreement: inter-rater reliability
A direct comparison of responses in WHQ self-assessments and observer assessments was made for participants with both sets of data. This allowed for an examination of whether or not the patient self-assessment differed from the observer assessment, where further probing about symptoms or interventions, professional judgement or other unknown influences may have led to a different classification of response. Cross-tabulations of responses and the extent of the agreement between patient responses and observer assessments were examined for each item. Where possible, the level of agreement was calculated using weighted kappa statistics for ordinal data. Differences between response category options (‘not at all’, ‘a little’, ‘quite a bit’, ‘a lot’) were assumed to be equal, and weights of 0, 0.333, 0.667 and 1 were used. Kappa values of between 0.4 and 0.75 were considered as indicating fair to good agreement. 69
In cases in which the calculation of kappa values was not possible (owing to few observations in any one of the binary response categories), the proportion of agreement for ‘not at all’ or ‘no’ responses was considered. For items assessing the occurrence of wound care interventions, the number of observer assessments that agreed with the patient’s self-assessment ‘yes’ response (i.e. that the intervention had occurred) were examined. The number of observer assessments that contradicted the patient’s ‘no’ response (i.e. that the intervention had not occurred) was also examined.
Test–retest agreement: intrapatient reliability
The stability of individuals’ responses to each item was explored for the subset of participants completing first and second test–retest assessments. Cross-tabulations of responses and the extent of agreement between the first and retest assessments were compared for each item. Where possible, the level of agreement was calculated using weighted kappa statistics (as above).
Scale structure of the Wound Healing Questionnaire and dimensionality of the data
Possible scale structures were initially explored using cohort study data.
First, an inter-item correlation matrix was examined to determine whether or not any items showed a very high correlation (> 0.9, indicating similarity or overlap of items) and could be deleted before conducting factor analysis. 69 Exploratory factor analyses were performed to examine the suitability of a single-, two- and three-factor model, separately for patient and observer data. Models were specified with a maximum of one, two or three factors to be retained. Eigenvalues, item factor loadings and unique variances were examined. Maximum likelihood was used to assess the model fit; this is recommended as the preferred method for the estimation of factor structure in quality-of-life assessment. 70 No rotation methods were applied. Finally, the most suitable model derived from cohort data was applied to data from the RCT.
Internal consistency reliability for scales was examined using Cronbach’s alpha coefficients. Values of > 0.7 were considered to have good internal consistency.
Multitrait scaling analyses
These analyses were also applied as a comparative statistical approach (see Report Supplementary Material 10).
Item reduction and Wound Healing Questionnaire modification
Evidence on how the items were performing was evaluated on an item-by-item basis. An examination of the distribution of responses, missing data, test–retest reliability, patient and observer agreement and scaling structure and data from the debriefing questionnaire were all considered to inform the decision on whether items should be modified or dropped from the questionnaire completely. The clinical relevance of items was paramount to any decisions on whether or not items should be dropped from the questionnaire.
Sensitivity and specificity of the Wound Healing Questionnaire for surgical site infection/no surgical site infection discrimination
A WHQ total score was calculated for participant self-assessments. A simplistic approach, summing the raw scores without any weightings, was used. Reference assessment diagnosis of wound infection (CDC classifications: SSI of any type or no SSI) from the 4- to 8-week face-to-face follow-up assessment was used to provide a binary reference variable of SSI/no SSI.
A cross-tabulation of participants’ WHQ total score against the reference SSI diagnosis was examined. Sensitivity and specificity were calculated from these frequencies and used to plot a ROC curve. The area under the curve and 95% CIs were examined to assess how well the WHQ correctly classified individuals as having had a SSI and not having had a SSI against the reference assessment.
Objective B7: explore the feasibility of photographing wounds after wound closure in theatre by theatre personnel, and by participants after discharge
Photographing wounds after wound closure
We encountered major information governance challenges when seeking permission to take photographs of wounds in theatre after wound closure (see Objective A7: investigate the feasibility of photographing wounds in theatre and assessing the quality of wound closure). Consequently, we did not attempt to implement photographs after wound closure in theatre during Phase B.
Photographing wounds after discharge
If the occurrence of a SSI can be assessed from a photograph after a dressing has been removed, the assessment of the photograph could be blinded. For this reason, the trial tested the feasibility of participants submitting ‘wound selfies’ securely to the trial database.
There were challenges in developing the information technology to allow participants to submit a photograph confidentially, ensuring that the submitted photograph was linked with the rest of a participant’s data and stored securely. A system was successfully put in place that relied on sending a secure web link in an e-mail but we achieved this only towards the end of the recruitment period. The text of the e-mail is in Report Supplementary Material 11. We implemented the system during the last 4 weeks of recruitment to pilot participants’ willingness to take and submit a photograph.
Consent for this part of the study was obtained at recruitment. This pilot was limited to a subgroup of participants who had consented to take and submit a photograph and who offered an e-mail address for correspondence. A letter providing instructions on taking a photograph of the wound, along with a disposable ruler, was posted in advance of the due date for the completing the 4- to 8-week WHQ questionnaire. The success of the pilot was assessed by the proportion of participants in the subgroup who successfully submitted a photograph. The submitted photographs were not assessed for quality of the photograph or wound healing.
Objective B8: work with the patient and public involvement group to inform the conduct of Phase B and the design of a future main trial
Two PPI meetings were held during Bluebelle Phase A and Phase B.
Meeting 1
The aim of the first meeting was to explore PPI members’ thoughts about and reactions to the protocol and materials for the Bluebelle pilot RCT (Phase B). Key objectives were to elicit views about three main topics: (1) the presentation, content and delivery of the PIL for the pilot RCT, (2) the design and delivery of the WEQ and (3) strategies for improving adherence to trial allocation in the pilot RCT.
All invitees had participated in qualitative interviews in Phase A of the feasibility study. The meeting took place at the School of Social and Community Medicine, University of Bristol. Two members of the research team co-ordinated this meeting, and a third member of the research team attended part of the meeting to discuss the WEQ.
Meeting 2
The aim of the second PPI meeting was to update the group on the progress of the Bluebelle study and discuss thoughts and ideas for a possible large-scale RCT, with a focus on findings from the feasibility study. Key objectives were to discuss and elicit views about two main topics: (1) ideas for encouraging participants to stay in a study and be followed up by the study team (retention) and (2) methods for patients or their carers to take and send digital photographs of their wounds to the study team (to allow remote, and potentially blinded, wound assessment).
Setting and participants
Some invitees had taken part in the first PPI meeting and had participated in Phase A interviews. Others were new members who had taken part in the Phase B pilot RCT or were their partners (carers during surgery and recovery). The meeting took place at the same venue and was co-ordinated by the same two members of the research team as the first PPI meeting.
Objective B9: design a large, definitive randomised controlled trial based on information from the pilot trial and from integrated and interactive meetings with nurses/midwives, surgeons, methodologists and patient partners
This objective was addressed through discussions among the SMG and SSC. The discussions drew together information from Phase A, in particular the VoI analysis, and emerging information from Phase B.
Research governance approvals
The University Hospitals Bristol NHS Foundation Trust was the sponsor for the entire study. Separate applications to NHS Research Ethics Committees (RECs) were submitted for Phase A and Phase B. We decided on separate submissions because the details of Phase B could not be specified without the findings from Phase A.
Phase A
Phase A was registered with Current Controlled Trials as ISRCTN06792113 (registration assigned on 20 March 2014). Research ethics approval was granted by the NHS Health Research Authority (HRA) National Research Ethics Service (NRES) Committee London – Camden & Kings Cross (reference number 14/LO/0640). All participants gave written informed consent. One substantial amendment was approved (18 November 2014, implementing v3.0), allowing patients having unplanned abdominal surgery or an unplanned caesarean to be recruited for the SSI interviews.
Phase B
The pilot trial was registered with Current Controlled Trials as ISRCTN49328913 (registration assigned on 20 October 2015). Research ethics approval was granted by the South West–Frenchay REC (reference number 15/SW/0008) in February 2015 and subsequently by the HRA NRES (24 August 2016). All participants gave written informed consent. Two substantial amendments were approved. The first (8 December 2015, implementing v3.0) added patients having unplanned surgery to the population, substituted glue as-a-dressing for complex dressing as one of the groups, and included the use of the WMQ and the WEQ. The second (6 September 2016, implementing v5.0) reduced the time required between giving the PIL and seeking consent, allowed observer-completed WHQs to be completed over the telephone, clarified that an exclusion criterion applied to contraindications to dressing allocation, not just to dressings, and recruited a fourth NHS trust (Worcester Acute Hospitals NHS Trust). This ethics approval covered all participating sites.
Chapter 4 Results
Objectives A1 and A2: understand practice and views in relation to dressings
The key Phase A findings that had implications for the pilot RCT design are summarised in this section. Detailed findings are reported in Report Supplementary Material 12, and have been published elsewhere. 71
The pre-pilot qualitative research provided initial, in-depth insights into current wound-dressing practices and explored HCPs’ and patients’ perspectives on the proposed RCT. Dressing application was found to be ingrained in surgical specialties, although HCPs acknowledged a need for evidence. HCPs and patients engaged with the underlying question of a future RCT (i.e. whether dressings, or particular dressing types, prevent SSI). Hypothetically speaking, most patients felt that they would participate in such a trial, but many expressed concerns about the practical aspects of managing wounds without a dressing. Similar practical considerations were also raised by HCPs, who appeared particularly concerned about managing wound exudate and bleeding.
Although there was general support for a trial of dressing type (including ‘no dressing’), HCPs’ accounts raised a number of methodological issues about the pilot RCT design. First, interviews indicated that the trial comparison groups specified in the funder’s commissioned call may not be relevant to front-line HCPs, as complex dressings did not appear to feature in current practice. Second, HCPs’ descriptions of what constituted a ‘dressed’ and ‘non-dressed’ wound were inconsistent, pointing to the need for a clear definition of what qualifies as a ‘dressing’ in the pilot RCT. Particular products, such as tissue glue and wound closure strips, were not traditionally viewed as dressings but were reportedly being applied over the top of wounds. This obfuscated their classification as ‘dressings’ or ‘non-dressings’, reinforcing the need for a pragmatic definition of what constitutes a ‘dressing’ in a future RCT. Finally, both patients and HCPs appeared to link dressings with practical considerations, such as the physical protection of the wound and the absorption of exudate. HCPs in particular felt that these issues could have an impact on patients’ satisfaction, and, as such, felt that it was important to measure patient comfort and acceptability as outcomes in a future trial.
In addition to reconsidering the relevance of the trial comparison groups and outcomes, some clinicians noted the importance of performance bias, hypothesising that a ‘no dressing’ scenario may influence the quality of wound closure and post-surgical care.
Objective A3: identify dressings commonly used in the NHS
Centres and patients
In total, 25 hospitals within the SPARCS and WMRC networks were approached, and 20 (80%) participated. Data were included from 727 patients (1794 wounds), of whom 193 (27%) underwent upper GI surgery (Table 6). The number of wounds per patient varied from 1 to 7. The proportions of patients with each number of wounds was as follows: one (n = 299, 41%), two (n = 51, 7%), three (n = 155, 21%), four (n = 190, 26%), five (n = 25, 4%) and more than five (n = 7, 1%). Complete data sets were submitted for 675 (93%) patients. There was one missing data item for 36 (5%) patients and 16 (2%) patients had more than one missing item.
| Characteristic | Category | n (%) (N = 727) |
|---|---|---|
| Patients | ||
| Sexa | Male | 348 (48) |
| Female | 375 (52) | |
| Age (years)b | < 30 | 119 (16) |
| 30–40 | 90 (12) | |
| 41–50 | 104 (14) | |
| 51–60 | 109 (15) | |
| 61–70 | 144 (20) | |
| ≥ 71 | 157 (22) | |
| ASA gradec | 1 | 224 (31) |
| 2 | 342 (47) | |
| 3 | 140 (19) | |
| 4 | 15 (2) | |
| Diabetic statusd | Non-diabetic | 659 (91) |
| NIDDM | 51 (7) | |
| IDDM | 12 (2) | |
| BMI (kg/m2)e | < 20 | 50 (7) |
| 20–25 | 276 (39) | |
| 26–30 | 237 (34) | |
| > 30 | 142 (20) | |
| Procedures | ||
| Upper GI surgery | Oesophagogastric resection | 8 (1) |
| Pancreaticobiliary resection | 11 (2) | |
| Antireflux surgery | 10 (1) | |
| Bariatric surgery | 11 (2) | |
| Cholecystectomy | 153 (21) | |
| Lower GI surgery | Colectomy | 82 (11) |
| Hartmann’s procedure | 10 (1) | |
| Rectal resection | 40 (6) | |
| Stoma formation | 24 (3) | |
| Stoma closure | 24 (3) | |
| General surgery | Groin hernia repair | 90 (12) |
| Abdominal wall hernia repair | 38 (5) | |
| Appendectomy | 109 (15) | |
| Laparoscopy/laparotomy | 81 (11) | |
| Small bowel resection | 9 (1) | |
| Adhesiolysis | 8 (1) | |
| Other | 19 (3) | |
Wounds and dressings
Sutures were most commonly used to achieve skin closure (n = 1531/1770, 87%), with clips (n = 158, 9%) and wound closure strips (n = 48, 3%) less commonly used.
Of the 1794 wounds, dressing type was recorded for 1769, with 1706 out of 1769 (96%) covered and 63 out of 1769 (4%) not covered by a dressing. The majority of dressings were classified as simple (n/N = 1203/1769, 68%); just 18 out of 1769 (1%) were classified as complex. Tissue adhesive was applied over closed skin to 485 out of 1769 (27%) of wounds.
Use of dressings on the basis of operative and patient risk factors
Variation in the types of dressing depending on the category, urgency and modality of surgery is described in Tables 7 and 8. Dressing types were similar across different types of procedure and between elective and unplanned surgery. There was no apparent association between the type of dressing used and patient risk factors such as diabetes mellitus, stoma formation, BMI and ASA grade.
| Operative factor | Dressing type, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Basic | Complex | Tissue adhesive | No dressing | |||||
| Patients (N = 512) | Wounds (N = 1203) | Patients (N = 17) | Wounds (N = 18) | Patients (N = 186) | Wounds (N = 485) | Patients (N = 31) | Wounds (N = 63) | |
| Operation category | ||||||||
| Clean | 199 (39) | 449 (37) | 2 (12) | 2 (11) | 58 (31) | 128 (26) | 11 (35) | 24 (38) |
| Clean contaminated | 242 (47) | 606 (50)b | 12 (71) | 13 (72) | 106 (57) | 305 (63) | 14 (45) | 33 (52) |
| Contaminated | 50 (10) | 115 (10) | 2 (12) | 2 (11) | 12 (6) | 32 (7) | 5 (16) | 5 (8) |
| Dirty | 21 (4) | 33 (3) | 1 (6) | 1 (6) | 10 (5) | 20 (4) | 1 (3) | 1 (2) |
| Urgency of surgeryc | ||||||||
| Elective | 320 (63) | 809 (67) | 10 (59) | 11 (61) | 132 (71) | 371 (76) | 22 (71) | 51 (81) |
| Emergency | 191 (37) | 393 (33) | 7 (41) | 7 (39) | 54 (29) | 114 (24) | 9 (29) | 12 (19) |
| Modality of surgery | ||||||||
| Open | 245 (48) | 296 (25) | 9 (53) | 10 (56) | 75 (40) | 96 (20) | 12 (39) | 15 (24) |
| Laparoscopic | 264 (52) | 907 (75) | 8 (47) | 8 (44) | 111 (60) | 389 (80) | 19 (61) | 48 (76) |
| Type of operation | ||||||||
| Upper GI | 132 (26) | 465 (39) | 1 (6) | 1 (6) | 55 (30) | 211 (44) | 7 (23) | 22 (35) |
| Lower GI | 119 (23) | 256 (21) | 11 (65) | 12 (67) | 54 (29) | 122 (25) | 7 (23) | 17 (27) |
| General | 261 (51) | 482 (40) | 5 (29) | 5 (28) | 77 (41) | 152 (31) | 17 (55) | 24 (38) |
| Risk factor | Dressing type, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Basic | Complex | Tissue adhesive | No dressing | |||||
| Patients (N = 512) | Wounds (N = 1203) | Patients (N = 17) | Wounds (N = 18) | Patients (N = 186) | Wounds (N = 485) | Patients (N = 31) | Wounds (N = 63) | |
| Stoma formation | 56 (11) | 96 (8) | 5 (29) | 5 (28) | 32 (17) | 70 (14) | 6 (19) | 9 (14) |
| Diabetes mellitusb | 43 (8) | 85 (7) | 2 (12) | 2 (14) | 17 (9) | 51 (11) | 3 (10) | 6 (10) |
| ASA gradec | ||||||||
| 1 | 163 (32) | 403 (34) | 5 (29) | 6 (33) | 55 (30) | 148 (31) | 8 (27) | 20 (32) |
| 2 | 238 (47) | 584 (49) | 7 (41) | 7 (39) | 92 (50) | 231 (48) | 16 (53) | 31 (50) |
| 3 | 98 (19) | 198 (17) | 5 (29) | 5 (28) | 36 (19) | 96 (20) | 6 (20) | 11 (18) |
| 4 | 10 (2) | 11 (1) | 0 (0) | 0 (0) | 2 (1) | 6 (1) | 0 (0) | 0 (0) |
| BMI (kg/m2)d | ||||||||
| < 20 | 36 (7) | 81 (7) | 1 (6) | 1 (6) | 12 (6) | 19 (4) | 3 (11) | 9 (15) |
| 20–25 | 196 (40) | 426 (37) | 5 (31) | 5 (29) | 74 (40) | 175 (36) | 13 (46) | 23 (39) |
| 26–30 | 163 (33) | 401 (35) | 6 (38) | 7 (41) | 63 (34) | 165 (34) | 8 (29) | 19 (32) |
| > 30 | 101 (20) | 246 (21) | 4 (25) | 4 (24) | 36 (19) | 122 (25) | 4 (14) | 8 (14) |
Reasons for selection of dressings
Most (n = 925, 75%) surgeons used the dressings that were handed to them by the nursing staff at the end of the operation (Table 9). Information from procurement staff (n = 29) revealed that cost was the overwhelming factor when selecting which dressings to purchase, enabling bulk ordering and keeping the range of available dressings to a minimum.
| Reasons for dressing selection | Dressing type, n (%) | |||
|---|---|---|---|---|
| Basic | Complex | |||
| Patients (N = 512) | Wounds (N = 1203) | Patients (N = 17) | Wounds (N = 18) | |
| Handed by nursing staffc | 380 (75) | 909 (76) | 15 (88) | 16 (89) |
| Personal preferenced | 170 (34) | 371 (31) | 1 (6) | 1 (6) |
| Wound characteristicse | 53 (10) | 120 (10) | 5 (29) | 5 (28) |
| Otherf,g | 4 (1) | 10 (1) | 0 (0) | 0 (0) |
Objective A4: develop and validate a patient-centred comprehensive measure of surgical site infection
Phase 1: generating questionnaire content
Analysis of existing tools
A list of 42 criteria or items were extracted from the existing tools. These were grouped into 18 domains relating to eight signs/symptoms [wound healing, wound heat, wound redness, wound discharge, layer separating (spontaneous), wound swelling, wound pain and fever] and 10 wound management interventions [contact with HCP, dressings needed, antibiotics needed, layer separating (deliberate), hospital admission, drainage needed, wound cleaning, abscess, microbiology and prolonged hospital stay].
In-depth interviews
Interviews were conducted with nine patients and 10 HCPs. 72 Identified themes reflected the findings from the analysis of existing tools. In addition, one new domain (smell) emerged from both patient and HCP interviews. A total of 19 domains were therefore considered for inclusion in the new questionnaire (Table 10).
| SSI domain | Criterion/item from existing tools | Source (existing tool) |
|---|---|---|
| Wound healing | Have all of these wounds healed without any problem at all? | ASEPSIS PQ |
| Have you had any problems with the healing of your wound? | PHE PQ | |
| Wound heat | The area around the wound felt warmer/hotter than the surrounding skin | PHE PQ |
| Heat | ASEPSIS PQ | |
| Wound redness | Has the wound been red? | ASEPSIS PQ |
| Redness or inflammation spreading from the edges of the wound | PHE PQ | |
| Erythema | ASEPSIS CDS | |
| Redness | PHE CDS | |
| Wound discharge | Has the wound discharged clear yellow fluid? | ASEPSIS PQ |
| Has the wound discharged pus? | ||
| Purulent drainage | PHE CDS | |
| Was there any discharge or leakage of fluid from any part of the wound? If yes, was it clear or blood stained? If yes, was it yellow/green (pus)? | PHE PQ | |
| Serous discharge/exudate | ASEPSIS CDS | |
| Purulent exudate | ||
| Layers separating – spontaneous | Has the wound broken open? | ASEPSIS PQ |
| Have the edges of any part of the wound separated or gaped open? | PHE PQ | |
| Separation of deep tissues | ASEPSIS CDS | |
| Incision spontaneously dehisces (/opened by surgeon) | PHE CDS | |
| Wound swelling | The area around the wound became swollen | PHE PQ |
| Localised swelling | PHE CDS | |
| Wound pain | Pain or soreness in addition to the discomfort experienced following the operation | PHE PQ |
| Localised pain and tenderness | PHE CDS | |
| Fever | Fever (temperature ≥ 38°C) | PHE CDS |
| Contact with HCP | If you saw a health-care worker because of these symptoms, please indicate who you saw from the list (GP/district nurse/midwife/doctor or nurse at the hospital/other – please specify) | PHE PQ |
| Dressing needed | Has a district nurse had to dress the wound? | ASEPSIS PQ |
| Antibiotics needed | Have you been given antibiotics for wound infection? | ASEPSIS PQ |
| Have you been prescribed antibiotics for an infection in the wound? If yes, who prescribed them?________ | PHE PQ | |
| Antibiotics prescribed | ASEPSIS | |
| Antibiotics prescribed by GP for SSI (patient reported only) | PHE CDS | |
| Layers separating – deliberate | Incision opened by surgeon (/spontaneously dehisces) | PHE CDS |
| Hospital admission | Have you been admitted to hospital elsewhere? | ASEPSIS PQ |
| Have you been readmitted to hospital with an infection of the surgical wound? To the hospital at which the operation was carried out? To another hospital? | PHE PQ | |
| Drainage needed | Drainage of pus under local anaesthesia (including vac therapy) | ASEPSIS CDS |
| Purulent drainage | PHE CDS | |
| Wound cleaning | Has the wound been opened and cleaned under general anaesthetic in hospital? | ASEPSIS PQ |
| Debridement of wound (general anaesthesia) | ASEPSIS CDS | |
| Purulent drainage | PHE CDS | |
| Abscess | Has a doctor opened/drained an abscess? | ASEPSIS PQ |
| Abscess or other evidence of infection found during a reoperation, by radiology or histopath examination | PHE CDS | |
| Microbiology | Did any health-care worker take a sample from your wound to send to the laboratory? | PHE PQ |
| Aspirated fluid/swab of surgical site yields organisms and pus cells are present | PHE CDS | |
| Isolation of bacteria | ASEPSIS CDS | |
| SSI causative micro-organisms | PHE CDS | |
| Prolonged hospital stay | Stay as inpatient prolonged > 14 days | ASEPSIS CDS |
| Smell | – | – |
Phase 2: designing the questionnaire
Two domains (microbiology and prolonged hospital stay) were excluded as they were considered inappropriate for patient self-report after hospital discharge as well as not being specific to all SSIs. Retained domains were constructed (‘operationalised’) into items for the first version of the questionnaire. Initially, eight medical terms were included in items in parentheses after plain language descriptions.
Phase 3: pre-testing the questionnaire
A total of 42 cognitive interviews were conducted (with 28 patients and 14 HCPs) (Table 11). Interviews lasted a median time of 27 minutes (range 13–52 minutes). The mean time between patients’ surgery and Interview was 46 days.
| Characteristic | Category | Professionals (n = 14) | Patients (n = 28) |
|---|---|---|---|
| Sex | Female | 10 | 11 |
| Male | 4 | 17 | |
| Age at time of interview (years) | 21–30 | 0 | 1 |
| 31–40 | 7 | 2 | |
| 41–50 | 3 | 2 | |
| 51–60 | 3 | 6 | |
| > 60 | 1 | 17 | |
| Role | Midwife | 3 | – |
| Hospital/research nurse | 3 | – | |
| Practice/community nurse | 1 | – | |
| Surgical trainee | 4 | – | |
| GP | 3 | – | |
| Specialty | General practice/community | 4 | – |
| Obstetrics | 3 | – | |
| Upper/lower GI surgery | 6 | – | |
| Intensive care | 1 | – | |
| Length of time qualified (years) | < 10 | 1 | – |
| 10–20 | 7 | – | |
| > 20 | 6 | – | |
| Time since surgery (weeks) | < 1 | – | 1 |
| 1–2 | – | 2 | |
| 2–4 | – | 9 | |
| > 4 | – | 16 | |
| Type of surgery | Upper GI | – | 9 |
| Lower GI | – | 10 | |
| Caesarean section | – | 3 | |
| Hernia repair | – | 6 |
During pre-testing, interviews highlighted that the initial plain language description for some items required modification to ensure a more accurate interpretation of the intended sign/symptom or intervention. 72 Throughout the cyclical pre-testing phase of interviews and revisions, a total of eight versions of the WHQ were tested. Modifications included changes to the wording and structure of items, layout, instructions and response categories.
The final version of the WHQ after pre-testing included 16 core items (Table 12). Items 1–8 related to patient-reported signs or symptoms that are potentially indicative of SSI. Items 9–16 related to wound care management and clinical interventions for treating SSI. Two core items assessing SSI signs and symptoms (items 3 and 4) had further subitems, to be completed if the response to the core item was anything other than ‘not at all’. Other core items (numbered 9, 10 and 12) had subitems that were included specifically to collect health economic data for the pilot trial.
| Item | Item description | Response categories |
|---|---|---|
| 1 | Was there redness spreading away from the wound? (erythema/cellulitis) | Not at all/a little/quite a bit/a lot |
| 2 | Was the area around the wound warmer than the surrounding skin? | Not at all/a little/quite a bit/a lot |
| 3 | Was any part of the wound leaking fluid? | Not at all/a little/quite a bit/a lot |
| (a) Was it clear fluid? (serous exudate) | Not at all/a little/quite a bit/a lot | |
| (b) Was it blood-stained fluid? (haemoserous exudate) | Not at all/a little/quite a bit/a lot | |
| (c) Was it thick and yellow/green fluid? (pus/purulent exudate) | Not at all/a little/quite a bit/a lot | |
| (d) I do not know | ||
| 4 | Have the edges of any part of the wound separated/gaped open on their own accord? (spontaneous dehiscence) | Not at all/a little/quite a bit/a lot |
| (a) Did the skin separate? | Not at all/a little/quite a bit/a lot | |
| (b) Did the deeper tissue separate? | Not at all/a little/quite a bit/a lot | |
| (c) I do not know | ||
| 5 | Has the area around the wound become swollen? | Not at all/a little/quite a bit/a lot |
| 6 | Has the wound been smelly? | Not at all/a little/quite a bit/a lot |
| 7 | Has the wound been painful to touch? | Not at all/a little/quite a bit/a lot |
| 8 | Have you had, or felt like you have had, a raised temperature or fever? (fever ≥ 38 °C) | Not at all/a little/quite a bit/a lot |
| 9 | Have you sought advice because of a problem with your wound, other than at a routine planned follow-up appointment? | Yes/no |
| If yes, please tell us who you sought advice from: | ||
| (a) A doctor or nurse at the GP surgery/medical centre/walk-in centre | Yes/no | |
| (b) A doctor or nurse at the hospital | Yes/no | |
| (c) A midwife or health visitor | Yes/no | |
| (d) Another health advisor | Yes/no | |
| Please describe who the other health advisor was __________________ | ||
| 10 | Has anything been put on the skin to cover the wound? (dressing) | Yes/no |
| If yes, | ||
| (a) Was this done by a doctor or nurse at the GP surgery/medical centre/walk-in centre? | Yes/no | |
| (b) Was this done by a nurse/midwife/health visitor at home? | Yes/no | |
| (c) Was this done by you/your partner/friend/family member? | Yes/no | |
| (d) Was this done by a doctor/nurse/midwife at the hospital? | Yes/no | |
| (e) Please describe what was put on to cover the wound __________________ | ||
| 11 | Have you been back into hospital for treatment with a problem with your wound? | Yes/no |
| 12 | Have you been given antibiotics for a problem with your wound? | Yes/no/do not know |
| If yes, | ||
| (a) Were the antibiotics given as tablets/liquid? | Yes/no/do not know | |
| (b) Were the antibiotics given via drip? | Yes/no/do not know | |
| If you know the name of the antibiotic(s) you have taken, please write it here __________________ | ||
| 13 | Have the edges of your wound been deliberately separated by a doctor or nurse? | Yes/no/do not know |
| 14 | Has your wound been scraped or cut to remove any unwanted tissue? (debridement of wound) | Yes/no/do not know |
| 15 | Has your wound been drained? (drainage of pus/abscess) | Yes/no/do not know |
| 16 | Have you had an operation under general anaesthetic for treatment of a problem with your wound? | Yes/no/do not know |
Medical terms were included in parentheses at the end of nine items (six core items and three subitems) in the final version of the questionnaire after pre-testing.
Response categories for signs and symptom items were changed from a five-point option to a four-point option, removing the middle ‘moderately’ option. ‘Very much’ was change to ‘a lot’.
Written instructions informed responders to complete the questionnaire in relation to just one wound only: either the main wound or another wound if there had been any concerns about how it had been healing. Instructions asked responders to answer items based on what had happened since leaving hospital after having surgery.
A full report on the development of the WHQ has been published. 72
Objective A5: develop and test a patient-centred measure of practical wound management
Phase 1: generating questionnaire content
Interviews
A total of 39 interviews were conducted between July 2014 and July 2015. Interviews were conducted in person (n = 10), unless patients preferred to be interviewed by telephone (n = 29). Interviews lasted for about 25 minutes (range 15–50 minutes). The sample consisted of 27 women and 12 men, most of whom described themselves as white British (90%). They had a mean age of 56 years (range 22–88 years). Thirty-seven of the 39 participants had undergone either abdominal general surgery (85%) or a caesarean section (15%) and were interviewed about 18 days after their surgery (range 6–40 days). Two of the 39 patients were scheduled to undergo abdominal general surgery and discussed issues that they anticipated would be important to them. Demographic characteristics of participants who were interviewed for Phase 1 are shown in Table 13.
| Patient characteristic | Phase 1: generation of relevant issues | Phase 3: pre-testing including cognitive interviews | |
|---|---|---|---|
| Qualitative interviews (n = 79) | 39 patients | 25 patients | 15 HCPs |
| Age (years) | |||
| Range | 22–88 | 19–76 | 23–60 |
| Mean | 56 | 54 | 41 |
| Sex | |||
| Female | 27 | 12 | 13 |
| Male | 12 | 13 | 2 |
| Ethnicity | |||
| White | 35 | 22 | 14 |
| Asian | 1 | 1 | 0 |
| African | 2 | 1 | 1 |
| Indian | 1 | 0 | 0 |
| Filipino | 0 | 1 | 0 |
| Type of surgery | |||
| Abdominal | 33 | 25 | 15 |
| Obstetric | 6 | 0 | 0 |
| Dressing type | |||
| Tissue adhesive | 7 | 5 | – |
| Adhesive | 32 | 18 | – |
| No dressing | 0 | 2 | – |
| Location | |||
| Bristol | 28 | 15 | 9 |
| Birmingham | 11 | 10 | 6 |
Extraction of information from three systematic reviews
Published papers for 26 studies that included outcomes relating to patient experience and management of wound healing were identified from the three systematic reviews. 73–98 Only two studies included a validated instrument, or modification of a validated instrument, to assess outcomes. 75,89 These were for long-term scarring and cosmesis. 99,100 However, no studies reported using validated measures relating to issues associated with practical wound management and patient experiences in the early postoperative period. Descriptions of outcomes were heterogeneous and often poorly defined. The most commonly reported outcomes related broadly to cosmetic result (reported in 15/26 studies), dressing changes (e.g. frequency, comfort, ease of application and removal; reported in 11/26 studies) and skin reactions (e.g. itching, blistering; reported in 10/26 studies). Data extracted from the 26 studies are described in Report Supplementary Material 13.
Synthesis of findings from interviews and data extraction
When describing experiences in the interviews, patients commented on several factors that affected perceptions of how well their wound was healing, including how it felt (tightness, pain and itchiness) and whether or not any fluid had leaked from the wound. An analysis of existing RCT outcomes showed that these issues had been captured in some previous (unvalidated) outcomes.
All patients had at least one dressing applied after surgery, although this varied between adhesive coverings (absorptive or non-absorptive) and tissue adhesive as a dressing. Both the interviews and the analysis of existing RCT outcomes highlighted multiple practical advantages of dressing use (including the ability to contain exudate and ease of removal). The interviews also demonstrated that there were psychological factors that affected dressing experience and satisfaction (e.g. anxiety about the cleanliness of the wound). Patients with tissue adhesive as a dressing commented that they had been surprised that their wounds had been dressed this way (rather than with adhesive dressings, which they had had in the past for other wounds). However, these patients stated that, compared with past experiences of adhesive dressings, they liked how glue was transparent, waterproof, did not require multiple applications and came off naturally.
The interviews and the analysis of existing RCT outcomes produced a total of 69 issues. These were grouped into 10 broad categories: (1) wound comfort, (2) exudate and its impact, (3) allergic reactions to the dressing, (4) dressing removal, (5) dressings to protect the wound, (6) impact on daily activities, (7) ease of movement, (8) anxiety about the wound, (9) satisfaction with dressing and (10) wound appearance.
Phase 2: designing the questionnaire
A provisional measure was designed based on the findings from Phase 1. Nine key categories were included: (1) wound comfort, (2) exudate and its impact, (3) allergic reactions to the dressing, (4) dressing removal, (5) dressings to protect the wound, (6) impact on daily activities, (7) ease of movement, (8) anxiety about the wound and (9) satisfaction with dressing. Issues relating to the appearance of the wound were not included as they were relevant only to longer-term outcomes of wound healing (not within first days after surgery). In addition, because most patients reported having an adhesive dressing, many had not seen their wound within this time frame. The first version of the measure included 16 items and was provisionally called the Practical WMQ.
Phase 3: pre-testing the questionnaire
Cognitive interviews (n = 40) were conducted between July 2015 and March 2016. All interviews were conducted face to face. These consisted of interviews with 25 patients who were in hospital and had undergone abdominal general surgery and 15 HCPs involved in surgical wound care. Demographic characteristics for participants who had cognitive interviews are shown in Table 13.
Interviews highlighted issues with content in the initial measure. For example, items regarding the colour of the wound exudate were removed. Questions were rephrased to focus on the experience of having a dressing rather than general recovery after surgery [i.e. ‘Have you been able to perform everyday tasks? (i.e. showering/bathing)’ was changed to ‘Has your dressing prevented you from showering/washing?’]. In addition, because four patients commented that a question on the smell of their wound was missing from the measure, an item was added to capture this.
The measure had intended to be administered 2 days after surgery, although feedback suggested that this needed to be completed up to day 4 as the patient may be disorientated from surgery in the first few days. However, because there were clear differences in recovery between caesarean section and abdominal surgery patients, a time frame of within 4 days of surgery was set, and the measures recorded the date of surgery and the date completed to determine context of responses.
Feedback from patients suggested that it was difficult to respond to questions about exudate, because a HCP cared for their wound while they were in hospital. If their dressing had been changed, they were also uncertain about the reason why (i.e. if it was simply as part of standard practice or for other reasons). Therefore, the study team decided to separate the measure into two. The first measure related to the practical aspects of wound management and the second related to the patient’s experience of the wound/dressing and the psychological aspects (anxiety, satisfaction, etc.). The two measures were named the WMQ and the WEQ.
Seven versions of measures were modified throughout the pre-testing phase. Pre-testing continued until no new issues were identified and no further refinements were believed to be necessary. The final version of the WMQ contains four items, whereas the WEQ contains 10 items (see Appendix 3). Overall, the final versions of the measures were well received. In addition, 96% of participants stated that each measure took < 5 minutes to complete. Further details of the development of these outcome measures have been published elsewhere. 101
Objective A6: use the literature and views of experts to define and categorise commonly used dressings into three pragmatic groups
The draft definitions circulated prior to the SSC meeting were discussed. The SSC noted that all definitions needed to include the phrase ‘on an already closed wound’ to make it clear that the definitions referred to the dressing of a wound, not to wound closure. Additional characteristics were proposed to define the three groups.
-
A simple dressing should have the following properties:
-
provide a barrier applied to an already closed wound
-
adhere to the skin.
-
-
A complex dressing should have the following properties:
-
in addition to the properties of a simple dressing, must have an intended therapeutic property (e.g. an anti-microbial property).
-
-
No dressing should have the following properties:
-
not provide a barrier applied to an already closed wound
-
not adhere to the skin
-
have no intended therapeutic property.
-
The discussion noted additional complexities:
-
Circumference and covering were added to the definition of a simple dressing, after the team agreed that a dressing should cover the whole wound.
-
A ‘vacuum’ dressing device would be considered a complex dressing based on the above properties. However, the SSC decided that it should be not be considered in the study (1) because members were not aware of examples of a vacuum dressing being applied to a primary closed wound and (2) because a vacuum dressing has a very high cost and involves a different care pathway from that of other complex dressings.
-
Application of a gauze swab to one part of an oozing wound after the end of an operation would be allowed within the no dressing definition.
-
Internal wounds, arising for example from intravaginal surgery, should be excluded.
The final definitions are described in Table 14.
| Type of dressing | Definition |
|---|---|
| Simple dressing | A covering (opaque or transparent) that is directly applied to an already closed wound, over the entire wound, adherent around its entire circumference or surface, in contact with the skin |
| Complex dressing |
A covering (opaque or transparent) with an intended therapeutic property that is directly applied to an already closed wound, over the entire wound, adherent around its entire circumference or surface, in contact with the skin ‘Vacuum’ dressings are excluded from this definition in the study |
| No dressing |
Nothing is applied to the wound after wound closure If there is subsequent oozing from the wound, a simple gauze swab may be applied to just the area that is oozing (not the entire wound). This swab can be taped in place but not around its entire circumference. It must not have therapeutic properties |
Objective A7: investigate the feasibility of photographing wounds in theatre and assessing the quality of wound closure
Feasibility of photographing wounds in theatre
We attempted to obtain permission to take photographs in theatre in three trusts. Although we had already obtained research ethics approval to take photographs in theatre as a substudy, all three trusts required additional approvals.
At one trust, anyone taking a photograph had to:
-
read the trust’s policies for taking photographs of patients
-
obtain ‘level 3’ consent for photography from the patient, separately from study consent for the photograph substudy
-
register as a Camera User
-
register with the trust’s Information Management and Technology (IM&T) department to be able to upload a digital photograph to the study website.
Level 3 consent required participants to give:
consent for these photographs/video recordings to be used for publication in clinical journals and textbooks etc. including the Internet. (Personal details will not accompany the images. The general public as well as medical professionals can view the images). Please Note images consented for publication can only be removed from our system and cannot be called back once published in the public domain.
Lower levels of consent only applied to ‘treatment, diagnosis and medical records’ or ‘the teaching of healthcare professionals’. 102
The other trusts required that similar approvals and processes be followed. An additional requirement for one trust (not the sponsor) was to retain an electronic copy of the digital photograph at the site because they regarded the photographs as the property of the trust. After a lengthy process, the trust agreed that the information governance and our IM&T processes (storage of the photographs on the sponsor’s NHS IM&T servers, with access on request) were satisfactory.
These challenges had not been anticipated and it was time-consuming to address them. Given the short duration of the pilot RCT (only 9 months), we decided not to try to implement wound photography in theatre as part of data collection for the trial. Nevertheless, at one trust we piloted how to obtain a good digital photograph of abdominal wound(s) after wound closure, which resulted in draft instructions for wound photography. Photographs obtained during piloting were used to inform our investigation of the feasibility of assessing the quality of wound closure.
Feasibility of assessing the quality of wound closure
Literature reviews
There were few high-level publications (i.e. systematic reviews of RCTs) about the characteristics of ‘good wound closure’. Exceptions to this were two recent reviews summarising World Health Organization recommendations about the pre- and perioperative prevention of SSI. 21,22 Several (non-systematic) review articles were identified, each containing hypotheses about how the quality of wound closure might influence wound healing and the development of SSI.
Semistructured interviews
Seventeen interviews were undertaken at two NHS trusts. Interview duration ranged from 5 to 21 minutes. Interviews were conducted face to face either immediately after the wound closure observation (n = 6) or independently (n = 11).
Non-participant observations
Twelve observations (six with interviews) involving general surgery and obstetric teams were undertaken. Types of surgical procedures observed included laparoscopic cholecystectomy, inguinal and incisional hernia repair, caesarean section and staging laparoscopies for assessment of cancer.
In these procedures, most wounds were closed using subcuticular suturing. However, some surgeons closed smaller laparoscopic wounds using wound closure strips without sutures, some used tissue adhesive and, in one obstetric case, interrupted sutures were used owing to the perceived increased SSI risk in an obese patient. Dermal (i.e. below the surface in deeper tissue) suture layers were seldom used.
The observations provided context for understanding surgeons’ interview accounts in which they described their ideas of what constituted ‘good wound closure’ and how they went about achieving this. The key issues they discussed fell into three categories: the process of making an incision, closing the wound, and extenuating factors that could influence the quality of wound healing independent of wound closure technique. A detailed account of these factors is presented in Report Supplementary Material 14.
Summary of findings
Information from literature reviews, observations in the operating theatre and surgeon interviews led to the development of content for a tool to assess the quality of primary wound closure. The tool comprises metrics of wound closure (which are possible to visualise in a closed wound) and mediators, which comprise factors influencing wound healing.
Integration of literature, observation and interview data
Findings from each data source were compared and a ‘long list’ of items relating to wound closure was compiled. The ‘long list’ (n = 38) is provided in Table 15, together with the source of each item. Overlapping data items were removed and the remaining items were categorised into (1) factors relating to the appearance of a wound at the end of an operation and (2) factors that might influence this appearance (Table 16). Factors relating to the appearance of the wound formed the basis of the wound ‘metric’ items (and were possible to visualise in a newly closed wound, either in real life or in a photograph). Factors influencing the appearance of a wound at the end of an operation were considered to represent ‘mediators’ that would not be visible at the end of an operation but would be important to consider when assessing the quality of wound closure.
| Age | Method of skin incision | Urinary/tracheal catheter, nasogastric tube |
| Alcohol | MRSA carrier status of patient | Use of drains (skin layers or cavity) |
| Alignment of the linea nigra | Oozing/bleeding wound | Use of a dressing |
| Antibiotic-impregnated suture material | Operative time | Use of a knot (or not) to secure the suture at the end of wound closure |
| Antimicrobial skin sealants | Osteoporosis | Use of a wound protector device |
| Apposition/approximation of wound edges | Overlapping | Use of incise drapes |
| BMI | Perioperative control of blood glucose | Use of negative pressure therapy |
| Category of surgery (clean/contaminated) | Perioperative euvolaemia | Use of perioperative prophylactic antibiotics |
| Closure of the dermal layer | Perioperative normothermia | Use of postoperative prophylactic antibiotics |
| COPD | Perioperative nutritional support | Use of sterile drapes |
| Coronary heart disease, acute myocardial infarction or heart failure | Playing music in theatre | Use of sterile gowns |
| Decolonisation with body wash | Pre-existing infection | Use of wound irrigation with saline/iodine/antibiotics |
| Decolonisation with ointment | Preoperative bathing | Use of wound closure strips to aid closure |
| Diabetes mellitus | Presence of a scrubbed observer | Use of a wound protector device |
| Equipment used to close the wound | Presence of a unscrubbed observer | Use of incise drapes |
| Evenness | Presence of drains | Use of negative pressure therapy |
| Eversion of wound edges | Previous chemotherapy/radiotherapy | Use of perioperative prophylactic antibiotics |
| Flatness | Prolonged preoperative stay | Use of postoperative prophylactic antibiotics |
| Gaping | Puckering | Use of sterile drapes |
| Grade of surgeon closing the wound | Renal failure | Use of sterile gowns |
| Hair removal over incision area | Size of suture | Use of wound irrigation with saline/iodine/antibiotics |
| Immunosuppressants | Spacing of the sutures | Use of wound closure strips to aid closure |
| Incision through existing scar tissue | Step-offs | Visible subcuticular tissues below the sutures |
| Infection at a remote site | Straightness | Visible suture material, including knot |
| Insertion of device | Surgical hand preparation | Wound length |
| Intraoperative blood loss | Surgical site preparation | |
| Jaundice | Suture length | |
| Laminar airflow in the operating theatre | Suture material | |
| Location of the wound | Tension | |
| Loose suture material | Time pressures | |
| Malignancy | Time taken to close the wound | |
| Mechanical bowel preparation | Type of surgery (elective/emergency) |
| Metric | Mediators | |
|---|---|---|
| Technical factors | Non-technical factors | |
Approximation of wound edges
|
Incision
|
Expertise
|
| Horizontal alignment (including linea nigra for caesarean section) | Bite size | Type of surgery
|
Vertical alignment
|
Overall length of suture used | Patient factors
|
Straightness
|
Type of suture used
|
Cointerventions
|
| Eversion of wound edges | Tension across the wound | Time pressures |
| Visible subcuticular sutures or knots | Subdermal layer
|
|
Signs of devitalised tissues
|
Time taken to close wound | |
| Use of wound closure strips | Overall operative time | |
| Bleeding or oozing | ||
Practicalities of collecting data in the operating theatre
Observations provided the opportunity to establish whether or not factors identified in the interviews would be practicable to measure in the operating theatre. For example, surgeons had mentioned that the length of time taken to close a wound may be important in terms of influencing its quality and SSI development. However, this was found to be very difficult to measure or define, for several reasons. If there were multiple wounds, sometimes more than one surgeon performed the closure, meaning that more suture material was required and that there was a delay. Estimating the time taken to close a wound was difficult in view of these complexities.
Another variable that appeared difficult to measure in the observations was ‘supervision’ of trainees tasked with closing the wound. Some surgeons suggested that the grade of the surgeon and/or provision of ‘supervision’ (in cases in which trainees closed wounds) could influence the quality of wound closure. However, observations showed that it was difficult to demarcate what ‘supervision’ might constitute; although the senior surgeon often left the operating table before the end of the procedure, they were usually still in the room, although not directly watching the placement of each suture bite. Indeed, the senior surgeon did not watch the entire process of skin closure in any of the observed cases. Other variables found to be difficult to quantify were issues such as time pressures, delays or measuring the total procedural time.
The feasibility of wound photography was found to be influenced by the height of the operating table, levels of overhead lighting, distance from the patient and the angle at which it was possible to take the photograph. In general, despite these challenges, photographs were found to reflect the in situ findings (except for one case where a tiny amount of suture material was visible in the wound but was not visible on the corresponding photograph).
Practicalities of assessing the quality of wound closure
Surgeons anticipated various challenges to using a photograph to assess the quality of wound closure. They were unsure if a photograph could adequately convey three-dimensional detail (e.g. uneven skin). Other areas they were sceptical about included the visibility of the tissue deep to the skin, and whether or not it would be possible to visually assess how the closure/non-closure of this layer might affect wound closure. The amount of tension across the wound closure was also seen as a problematic area for assessment with a photograph:
I think photographs could show the wound looked very well closed but you can’t really assess hmm the soft tissue tension just by looking at a clinical picture, uh just my thoughts.
Surgeon 1
Some surgeons were mindful of the personnel who would be tasked with assessing the photos. They thought that a certain number of observations of the procedure, or a certain level of training, might be required:
I don’t think they’d have to be an expert but I think they would have to have seen it done a few times. I would have thought they’d have to have seen it done by someone who is appropriately good at it, perhaps about five times to know what is standard.
Surgeon 2
In conclusion, there is potential for using photographs to assess a wound closure but further thought is required in terms of, for example, how to properly capture uneven areas and gradients of tension. Further consideration must also be given to factors affecting wound healing that cannot be seen in a photograph, such as patient demographics and tissue handling, as well as training for the photograph assessors.
Objective A8: analyse the value of information to the NHS that would be provided by a definitive trial
Cost-effectiveness analysis
Table 17 shows the results of the cost-effectiveness analysis. All dressing types give a negative expected net-benefit, indicating that they are associated with costly outcomes (i.e. SSI). Costs should be minimised, and so the intervention with the highest net benefit (smallest costs) is preferred. Based on the evidence available currently, complex dressings have the lowest mean health-related cost (£1256/patient), followed by exposed dressing (£1411/patient). Complex dressings are the optimal dressing based on current information when they are included in the decision options; otherwise, exposed dressings are the optimal dressing.
| Willingness to pay per QALY of £20,000 | Dressing type | |||
|---|---|---|---|---|
| Exposed | Simple | Glue | Complex | |
| Expected net benefit (£) | –1411 | –1444 | –1455 | –1256 |
| Probability most cost-effective (including complex) | 0.14 | 0.08 | 0.34 | 0.45 |
| Probability most cost-effective (excluding complex) | 0.28 | 0.28 | 0.44 | |
There is a high level of uncertainty in the optimal dressing type. Glue has a probability of being most cost-effective (almost as high as complex) and glue has the highest probability of being cost-effective when complex dressings are excluded (even though exposed dressings have the highest expected net benefit). However, this is an artefact of the extreme levels of uncertainty in the effectiveness of glue; it also has the highest probability of being the least cost-effective intervention. This uncertainty can also be seen in the cost-effective plane (Figure 5), with each dressing type demonstrating uncertainty whether it is more costly and less effective than simple dressings, or vice versa.
FIGURE 5.
Cost-effectiveness planes showing the joint distribution of incremental costs and effects for each dressing type compared with simple dressings. Base-case model. (a) Exposed vs. simple; (b) glue vs. simple; and (c) complex vs. simple.
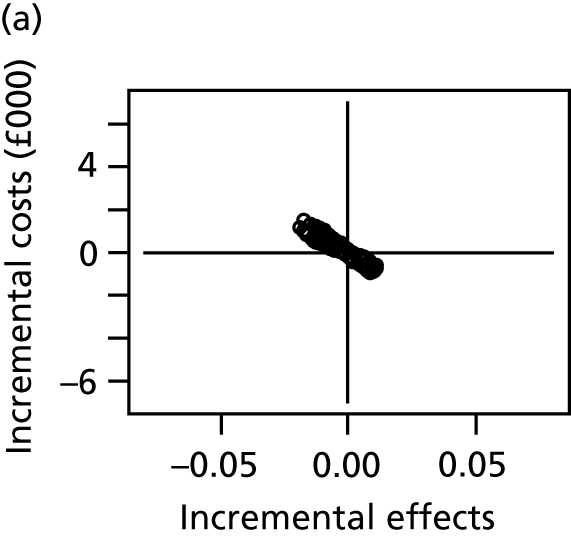
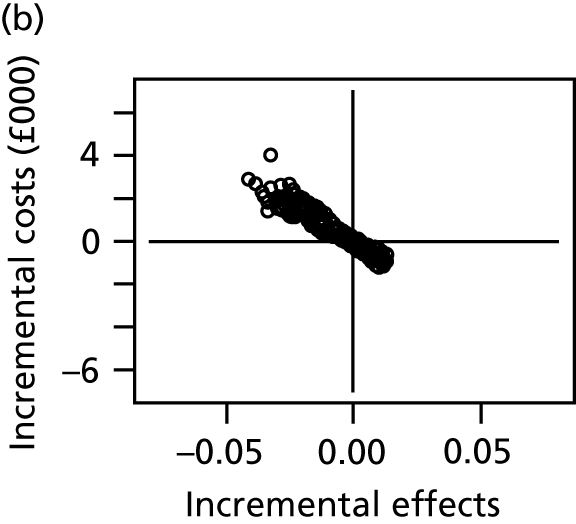
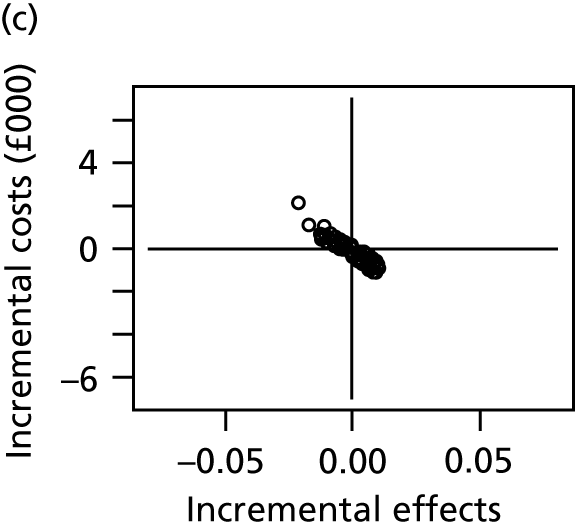
Expected value of partial perfect information
The EVPPI (Table 18) can be interpreted as the amount a decision-maker would be willing pay to eliminate all uncertainty in a subset of the model inputs. It can be seen that there is no value in eliminating uncertainty in the cost of a SSI or in the SSI risk on the reference intervention (simple dressings). There is, however, considerable value in eliminating uncertainty in the relative effectiveness of the different interventions, with an EVPPI of £171 per patient when complex dressing is included as a decision option. This corresponds to a population EVPPI of £1760M, which is substantial. The increase in EVPPI is higher when complex dressing is excluded as a decision option (£222 per patient wound, £2295M per population over 5 years) owing to the greater uncertainty about the most effective dressing.
| Willingness to pay per QALY of £20,000 | EVPPI for SSI costs (per wound) (£) | EVPPI for SSI risk on reference (per wound) (£) | EVPPI for relative effects (per wound) (£) | Population EVPPI for relative effects (£) |
|---|---|---|---|---|
| Decision options: exposed, simple, glue, complex | 0 | 0 | 171 | 1760M |
| Decision options: exposed, simple, glue | 0 | 0 | 222 | 2295M |
The EVPPI analysis therefore suggests that there is, potentially, value in conducting new research to obtain better estimates of the relative effectiveness of the different types of dressings. To explore the value of particular trial designs, we compute EVSI.
Value of Bluebelle randomised controlled trial: expected value of sample information
Scenario A: decision options – exposed (E), simple (S), glue (G), complex (A)
Below we present results for EVSI when complex dressings are included as a decision option for Bluebelle population surgery types.
The EVSI for balanced four-group designs (exposed vs. simple vs. glue vs. complex) for different total sample size (summed over groups) is shown in Appendix 4 (see Table 42 and Figure 18). There is value in even a small study (50 randomised patients), although the benefits of the trial increase steeply as the sample size increases until the EVSI levels off for total sample sizes of more than approximately 3000 patients.
Appendix 4 (Figure 19) shows the per-patient-wound EVSI for balanced designs with varying numbers of groups and included interventions, all of which include the reference intervention (simple dressings). It can be seen that there is greatest value in trials that include glue. Adding a complex dressing third group brings more benefit than adding an exposed third group. The additional benefits of adding an exposed group are seen only for larger sample sizes, suggesting that the decision to include exposed wounds will require a much higher sample size.
Appendix 4 (Figure 20) shows the per-patient-wound EVSI for three-group trials of exposed versus simple versus glue, comparing a balanced design with an unbalanced 2 : 2 : 1 design (one option for a main trial). There is little to choose between these two designs, although the balanced design has a little higher expected benefit.
The EVSI needs to be interpreted together with the cost of the trial for a given sample size. However, note that the population EVSI is very large and would easily exceed the cost of such a trial.
Scenario B: decision options – exposed (E), simple (S), glue (G)
Below we present results for EVSI when complex dressings are excluded, so that exposed, simple and glue are the only decision options for Bluebelle population surgery types. As for scenario A, in scenario B (decision options: E, S and G) even a small study (50 patients randomised) has value, although the benefits of a trial increase steeply as sample size increases, until the EVSI levels off for total sample size of more than approximately 3000 patients. The EVSI is higher for scenario B (decision options: E, S and G) (Figure 6; see also Appendix 4, Table 43) than for scenario A (decision options: E, S, G and A; see Appendix 4, Table 42 and Figure 18), owing to the increased uncertainty in the optimal dressing.
FIGURE 6.
Scenario B: decision options – exposed, simple, glue. EVSI per patient for balanced three-group designs plotted against total sample size, at a willingness to pay per QALY of £20,000.
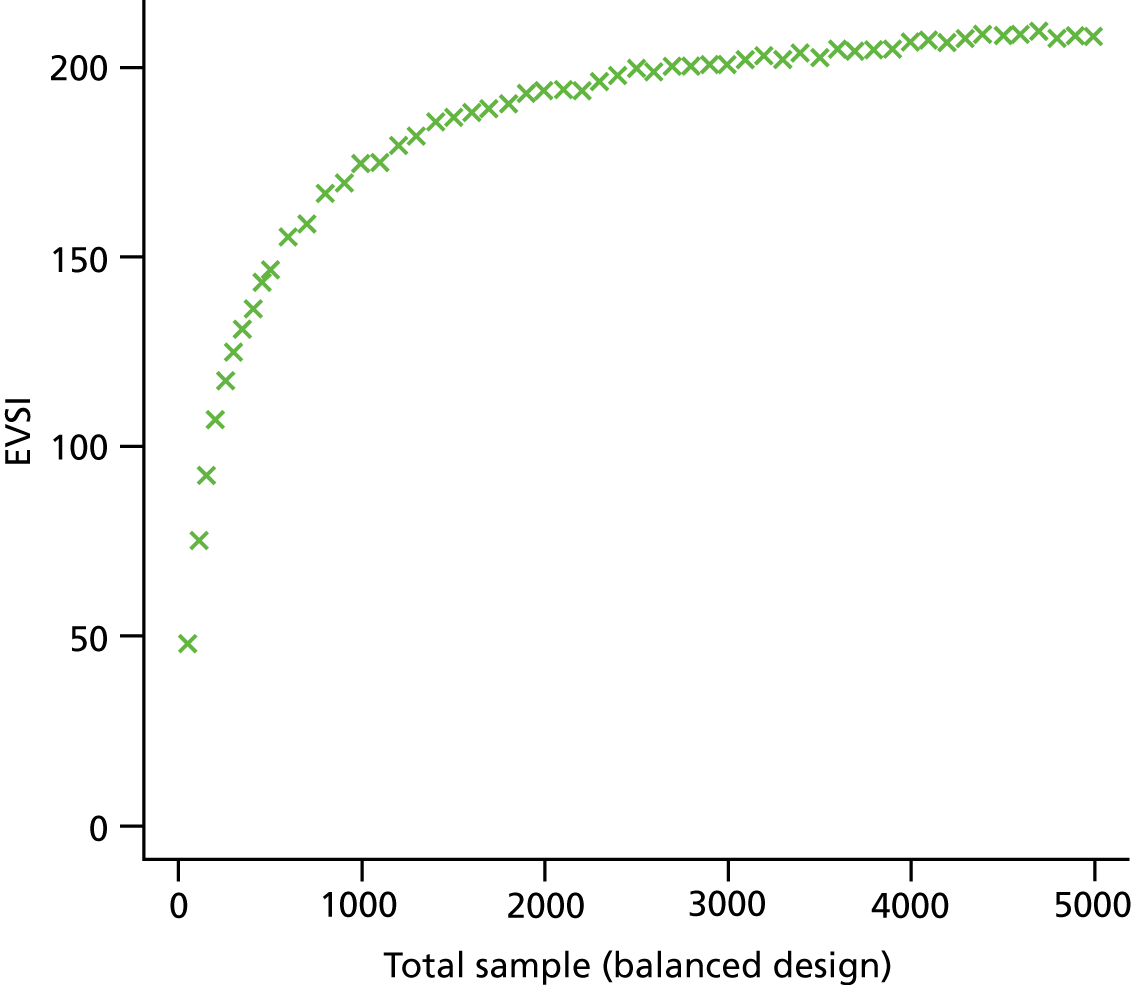
Figure 7 shows the per-patient-wound EVSI for balanced designs with two or three groups. There is greatest value in a three-group trial of exposed versus simple versus glue, but the added value of including an exposed group to a two-group trial of simple versus glue is apparent only for sample sizes of greater than around 750. Of the two-group trials, there is greatest benefit in a simple versus glue trial, followed by exposed versus glue, and least value in a two-group trial of exposed versus glue. In general, there is greater value in trials that include a glue group, reflecting the lack of existing evidence on this dressing type.
FIGURE 7.
Scenario B: decision options – exposed, simple, glue. EVSI per patient for balanced designs plotted against total sample size, at a willingness to pay per QALY of £20,000. Results are shown for designs with different numbers of groups and different included interventions. E, exposed, S, simple, G, glue. EvSvG, three-group trial of exposed vs. simple vs. glue.
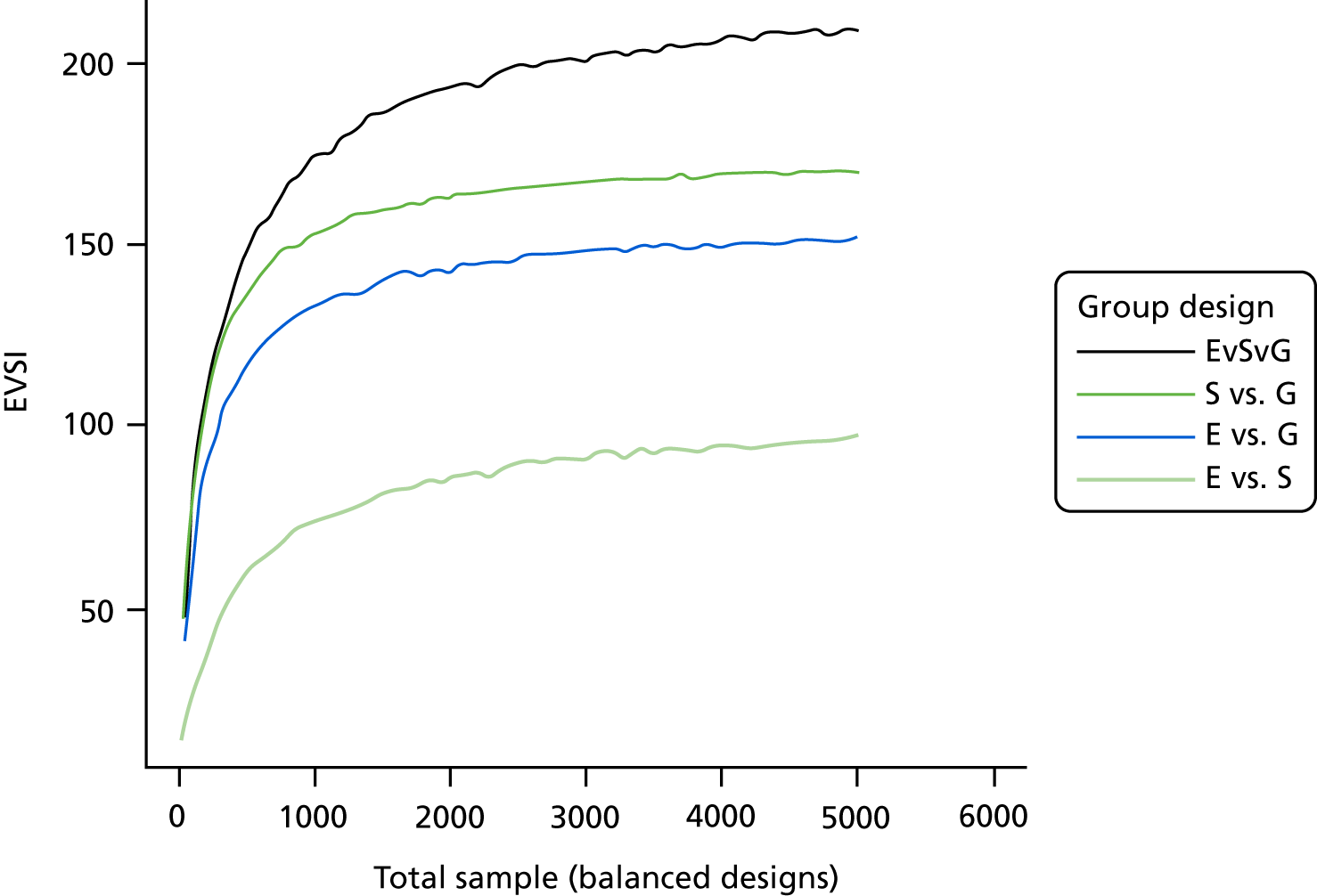
On the basis of EVSI, there is little to choose between a 1 : 1 : 1 or a 2 : 2 : 1 (as proposed for Bluebelle) allocation ratio for a three-group trial of exposed versus simple versus glue, given the same total sample size (see Appendix 4, Figure 21).
Sensitivity analyses
Tables 19 and 20 show results from sensitivity analyses for scenario A:
A – using the NMA results from Bluebelle population surgery types only
B – using Jenks et al. 50 alone to estimate SSI risk on reference (simple) dressing
C – assuming SSIQALYloss = 0.06
D – assuming SSIQALYloss = 0.
| Expected net benefit [P (most cost-effective)] | Model inputs | |||
|---|---|---|---|---|
| Exposed | Simple | Glue | Complex | |
| Base case | –1411 (0.14) | –1444 (0.08) | –1455 (0.34) | –1256 (0.45) |
| A: relative effects from Bluebelle population surgery types only | –1301 (0.10) | –1445 (0.06) | –1362 (0.27) | –1085 (0.57) |
| B: SSI risk on simple dressings from Jenks et al.50 only | –919 (0.14) | –939 (0.08) | –957 (0.34) | –813 (0.45) |
| C: SSIQALYloss = 0.06 | –1249 (0.14) | –1277 (0.08) | –1288 (0.34) | –1112 (0.45) |
| D: SSIQALYloss = 0 | –1088 (0.14) | –1114 (0.08) | –1123 (0.34) | –969 (0.45) |
| Per patient | Scenario | ||
|---|---|---|---|
| EVPPI for SSI costs (£) | EVPPI for SSI risk on reference (£) | EVPPI for relative effects (£) | |
| Base case | 0 | 0 | 171 |
| A: relative effects from Bluebelle population surgery types only | 0 | 0 | 139 |
| B: SSI risk on simple dressings from Jenks et al.50 only | 0 | 0 | 117 |
| C: SSIQALYloss = 0.06 | 0 | 0 | 152 |
| D: SSIQALYloss = 0 | 0 | 0 | 133 |
Using a lower SSI risk on the reference (simple) dressing (from Jenks et al. 50) reduces the overall value of the expected net benefit and, consequently, reduces the VoI. However, it does not change the probability that each dressing is the most cost-effective, nor the optimal dressing (complex). Similarly, reducing the value of QALYs lost as a result of a SSI reduces the overall value of expected net benefit and VoI but does not change the optimal decision or uncertainty in that decision.
Cost-effectiveness results depend on the evidence used to inform the NMA. If the relative effectiveness of the different dressing types is based on studies with Bluebelle population surgery types only, then the probability that a dressing type is most cost-effective increases, although the optimal decision is unchanged and, hence, our uncertainty in the decision is reduced, as is reflected in the lower estimate of VoI.
Incorporating the Bluebelle Phase B study results
We reran the NMA (‘all surgery types’ and ‘all wound types’) including the Bluebelle Phase B study results. Table 21 presents the results from an analysis using the ITT denominator and the PP denominator, alongside the results from the analysis omitting Bluebelle (taken from Table 22). The results from the ITT and PP analyses are very similar, suggesting that participants who do not complete the full assessments are not associated with the treatment allocation. The results are very similar to those obtained without the inclusion of Bluebelle Phase B. However, uncertainty in the estimates has been reduced by the inclusion of Bluebelle Phase B. Because the results are so similar, we present the cost-effectiveness and VoI results only for the ITT analysis (as reported by other studies included in the NMA).
| Results | Odds ratio (95% credible interval) | ||
|---|---|---|---|
| Exposed vs. simple | Glue vs. simple | Complex vs. simple | |
| Including Bluebelle: ITT | 1.019 (0.641 to 1.494) | 0.983 (0.480 to 1.752) | 0.868 (0.563 to 1.228) |
| Including Bluebelle: PP | 1.003 (0.627 to 1.478) | 0.999 (0.482 to 1.828) | 0.869 (0.566 to 1.240) |
| Omitting Bluebelle | 0.979 (0.561 to 1.546) | 1.049 (0.371 to 2.413) | 0.858 (0.535 to 1.263) |
Table 22 shows the cost-effectiveness results with and without the Bluebelle Phase B results. Expected net benefit is still highest for complex dressings and is very similar between exposed, simple and glue (although glue now has the highest expected net benefit of these three decision options). There is still a high degree of uncertainty as to which is the most cost-effective option, especially if complex dressing is removed from the decision options. Table 23 shows the EVPPI for subsets of parameters. Adding the Bluebelle Phase B results leads to a reduction in EVPPI, but the results still suggest that the relative effects are the key areas of decision uncertainty and that there is likely to be value in conducting a trial to reduce uncertainty in these parameters.
| Results | Dressing type | |||
|---|---|---|---|---|
| Exposed | Simple | Glue | Complex | |
| Base case (omitting Bluebelle) | ||||
| Expected net benefit | –1411 | –1444 | –1455 | –1256 |
| Scenario A: P(CE) | 0.14 | 0.08 | 0.34 | 0.45 |
| Scenario B: P(CE) | 0.28 | 0.28 | 0.44 | |
| Including Bluebelle: ITT | ||||
| Expected net benefit | –1463 | –1443 | –1407 | –1272 |
| Scenario A: P(CE) | 0.10 | 0.08 | 0.34 | 0.48 |
| Scenario B: P(CE) | 0.23 | 0.28 | 0.48 | |
| Per-patient EVPPI | Scenario (£) | |||
|---|---|---|---|---|
| EVPPI for SSI costs | EVPPI for SSI risk on reference | EVPPI for relative effects | Population EVPPI for relative effects | |
| Scenario A: decision options – exposed, simple, glue, complex | ||||
| Base case (omitting Bluebelle) | 0 | 0 | 171 | 1760M |
| Including Bluebelle: ITT | 0 | 0 | 138 | 1419M |
| Scenario B: decision options – exposed, simple, glue | ||||
| Base case (omitting Bluebelle) | 0 | 0 | 222 | 2295M |
| Including Bluebelle: ITT | 0 | 0 | 177 | 1823M |
Appendix 4 (see Figures 22 and 23) shows per-patient EVSI for a range of different, balanced study designs (depending on the intervention groups included and overall sample size). Figure 22 shows results when complex dressings are included as a decision option (scenario A) and Figure 23 shows results when complex dressings are excluded as a decision option (scenario B). We see that, although EVSI is reduced when including the Bluebelle Phase B study results, the overall patterns and conclusions are similar to those seen when the Bluebelle Phase B study results were omitted from the analysis (see Figure 7 and Appendix 4, Figure 19). There is highest benefit in studies that include glue-as-a-dressing group (reflecting the high level of uncertainty as regards the effectiveness of this intervention). The added benefit of including an exposed group is lower when the Bluebelle Phase B results are included in the NMA. This is because the results obtained for exposed and simple dressings in Bluebelle Phase B are similar, reducing our uncertainty in this comparison. If complex dressings are considered an option, then there is value in including this in a new trial.
Multiplying results to give population EVSI gives very large figures, suggesting that a trial comparing dressing types is likely to be a good use of resources. The benefits increase with sample size, but at a decreasing rate, with a high proportion of the benefits accrued after 3000 patients are randomised.
Discussion
Summary and implications of findings
We found that the existing evidence base for the relative effectiveness of different wound-dressing types was limited in quality and lacked precision. A cost-effectiveness analysis based on this rather limited evidence base suggests that, if complex dressings are considered appropriate for the Bluebelle population, then these are most likely to be cost-effective, but that this decision is very uncertain. If complex dressings are not considered appropriate, then simple dressings, exposed dressings and glue-as-a-dressing have similar cost-effectiveness on average, but these results are extremely uncertain. The conclusions were not altered by the inclusion of the results from the Bluebelle Phase B study, and there was a modest reduction in uncertainty in the results.
The VoI analysis indicated that there is substantial value of a trial to reduce our uncertainty in the relative efficacy of the different dressing types and that, when considering the benefit to the population undergoing general surgery, this benefit easily outweighs the costs. A large proportion of the benefit is accrued by the time that 3000 patients are randomised, but the benefit continues beyond this sample size. Assuming that a future study would include simple dressings as a control group, studies that also include a glue-as-a-dressing group have the greatest value. If complex dressings are considered relevant to the decision problem, then a third group including complex dressings is of value. Inclusion of an exposed intervention group could also be of value, as long as a sufficiently large sample size is used to have the required power to estimate the comparison between exposed and simple dressings. These conclusions were unaltered with the inclusion of the results from the Bluebelle Phase B study.
The Bluebelle steering group and study team felt that complex dressings were not a relevant comparator for the Bluebelle population. On the basis of our VoI analyses that excluded complex dressings, a three-group trial comparing simple dressings with glue-as-a-dressing with exposed wounds with 3000 patients randomised would have a population EVSI of £2069M, much more than the cost of such a trial. A two-group trial comparing simple dressings and glue-as-a-dressing with 3000 patients randomised would have a population EVSI of £1731M, which, again, is much more than the cost of such a trial, but the benefits from additionally including an exposed group would be lost. Inclusion of the Bluebelle Phase B study results gives a population EVSI of £1556M for a three-group trial comparing simple dressings with glue-as-a-dressing with exposed with 3000 patients randomised, and a population EVSI of £1360M for a two-group trial comparing simple dressings with glue-as-a-dressing (3000 patients randomised).
Limitations
The RCT evidence on which the cost-effectiveness and VoI modelling was based was limited and at potential risk of bias (all studies except one17 were rated as being at unclear or high risk of bias). Limitations in the existing evidence base, however, support the generation of more robust evidence via a future well-designed methodologically rigorous trial.
Under the Bluebelle definition, an exposed wound is one to which a gauze may be applied as needed to soak up exudate. Some of the included RCTs classified as ‘simple’ have applied a simple gauze, and one could argue that these could have been classified as ‘exposed’ under the Bluebelle definition. It is assumed that in a RCT the dressing would be always applied, rather than ‘as needed’, and for this reason the simple classification has been retained in these cases. Without more information than is reported in the including studies, this assumption is a potential a limitation of our analyses.
We pooled evidence from all wound types (clean, mixed and contaminated), owing to limited evidence. Although 9 of the 21 studies did not restrict to clean wounds, these studies randomised relatively small numbers of patients, and the results for those study populations were very uncertain. The results that we used in our cost-effectiveness analysis were driven primarily by the studies that restricted analysis to clean wounds only. Generalising the results to non-clean wounds should be done very cautiously, and this remains a question for further research.
To estimate SSI rate for the reference intervention (simple dressings), we were required to make some assumptions:
-
The proportion of surgery types missing in PHE51 is the same proportion of all surgery types seen in Jenks et al. 50
-
SSI rate in surgery types missing in PHE51 is the same as that seen in Jenks et al. 50
-
The split between surgery types seen in PHE51 (and where missing in Jenks et al. 50) is representative of the population of the whole of England and Wales.
The VoI analysis found that there was no value in reducing uncertainty in the SSI rate on the reference dressing, suggesting that our decision is unlikely to be sensitive to these assumptions. However, the overall value of a new study and the optimal sample size may depend on the SSI rate on the reference treatment. This is because we would expect more events in a population with a higher SSI rate, and, therefore, more precise results can be obtained for smaller numbers of patients randomised.
We have presented EVSI, which measures the benefits of a new study of a given design. But these figures should be considered together with the costs of such a trial. This can be done formally using the expected net benefit of sampling, which is the difference between the population-level EVSI and the cost of the study for a given study design. We prefer study designs that give a larger expected net benefit of sampling. However, given our results, it is clear that the population-level EVSI will be substantially higher than the cost of a trial for the range of sample sizes that we explored, suggesting that such a trial is likely to represent an efficient use of resources.
Objective A9: bring together the results of the above objectives to design Phase B of the study
The results of the research to address objectives A1–8 were presented to the SMG as Phase A progressed. The results were discussed and informed the design of the pilot trial, as described below (see the next section).
Objective B1: establish the numbers of potential participants at different hospitals who are considered likely to be eligible and who can be approached about the trial, and the proportions confirmed as eligible, recruited and randomised
Four NHS trusts took part in the pilot RCT; in one trust, both general surgery and obstetric departments recruited trial participants (considered as two sites). The flow of participants is shown in Figure 8. Recruitment over the 9-month duration of the pilot trial is shown in Appendix 5 (see Figure 24). Initially, when only two sites were recruiting, the trial recruited more slowly than projected. As additional sites started to recruit, recruitment increased and exceeded the projection; the total number randomised (n = 394) was larger than the target (n = 330).
FIGURE 8.
Flow of participants. a, Patients may be ineligible for more than one reason. Reason for withdrawal pre surgery: surgery cancelled (glue-as-a-dressing). Reasons for withdrawals post surgery: participant changed their mind (one simple dressing, two glue-as-a-dressing, two no dressing), personal reasons (two simple dressing, one glue-as-a-dressing), participant died (one simple dressing, one glue-as-a-dressing), randomisation failed in theatre (two glue-as-a-dressing), clinician withdrew as it was in participant’s best interest (simple dressing), participant rushed back for emergency operation (simple dressing), participant did not want treatment allocation (no dressing), clinician did not want to use treatment allocation (no dressing). EQ-5D, EuroQoL-5 Dimensions.
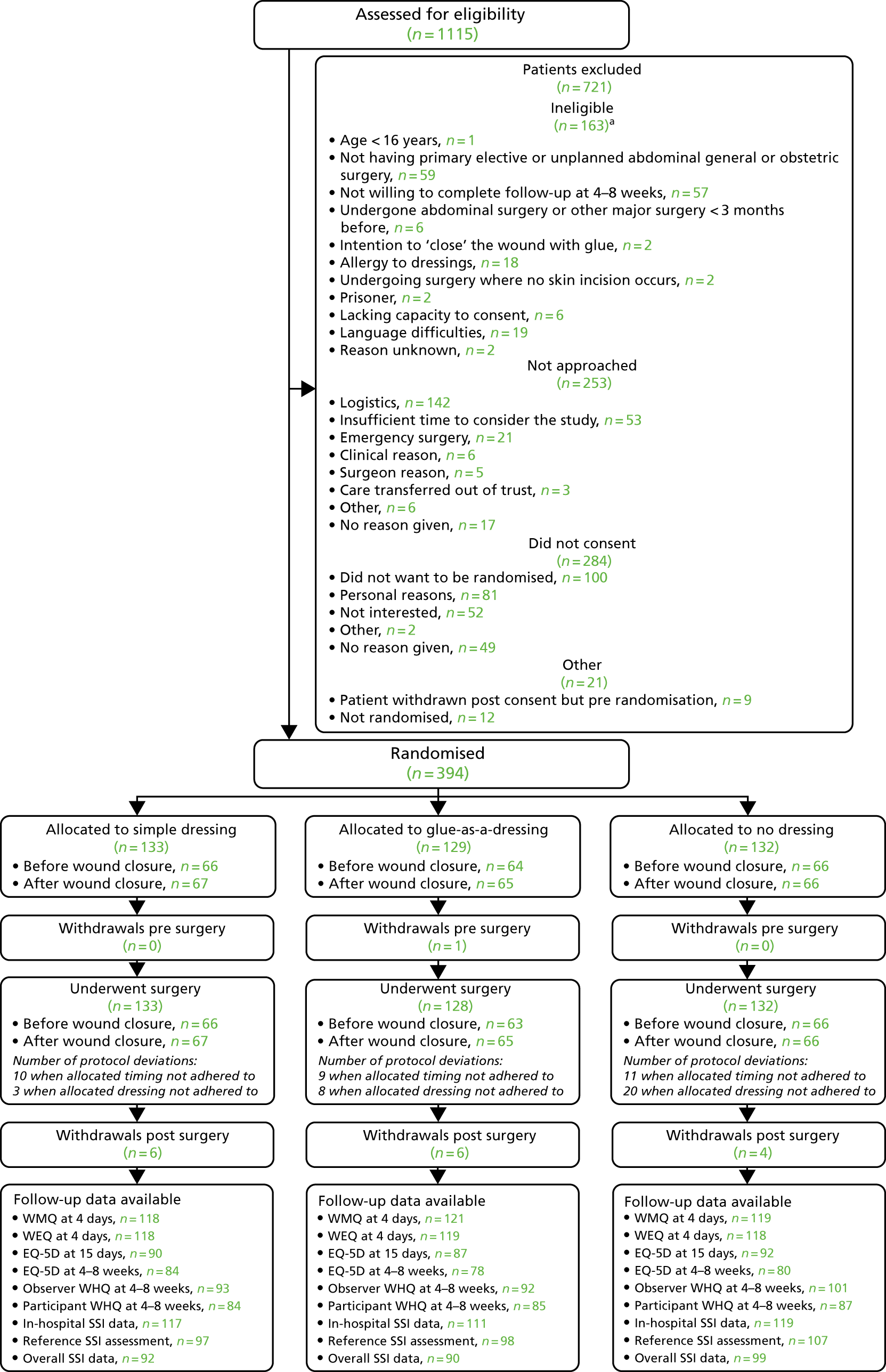
Numbers analysed
The analysis population consisted of 388 participants (i.e. the 394 randomised participants, excluding three participants who withdrew and were unhappy for their data to be used, two participants who were allocated to disclosure of dressing allocation after wound closure and whose randomisation in theatre was not completed, and one participant whose surgery was cancelled).
Participant demographics and operative characteristics
The demographic characteristics and past medical histories of randomised participants are shown in Table 24, and intraoperative characteristics are shown in Table 25, by allocated group. (The same information by centre is described in Report Supplementary Material 15.)
| Characteristic | Group | Overall (N = 388) | ||
|---|---|---|---|---|
| Randomised to simple dressing (N = 131) | Randomised to glue-as-a-dressing (N = 126) | Randomised to no dressing (N = 131) | ||
| Demography | ||||
| Age (years), median (IQR) | 55 (35.9–65.3) | 48 (32.3–66.2) | 53 (36.4–68.2) | 52 (34.7–66.9) |
| Male, n/N (%) | 51/131 (38.9) | 51/126 (40.5) | 59/131 (45.0) | 161/388 (41.5) |
| BMI (kg/m2), median (IQR)a | 28 (24.5–31.8) | 27 (24.2–32.0) | 28 (24.6–31.0) | 28 (24.3–31.6) |
| Ethnicity, n/N (%) | ||||
| White | 120/128 (93.8) | 105/119 (88.2) | 116/127 (91.3) | 341/374 (91.2) |
| Mixed | 3/128 (2.3) | 2/119 (1.7) | 4/127 (3.1) | 9/374 (2.4) |
| Asian | 3/128 (2.3) | 4/119 (3.4) | 5/127 (3.9) | 12/374 (3.2) |
| Black | 2/128 (1.6) | 7/119 (5.9) | 2/127 (1.6) | 11/374 (2.9) |
| Chinese | 0/128 (0.0) | 1/119 (0.8) | 0/127 (0.0) | 1/374 (0.3) |
| Medical history | ||||
| Smoking, n/N (%) | ||||
| Yes | 16/131 (12.2) | 22/125 (17.6) | 22/130 (16.9) | 60/386 (15.5) |
| Ex (> 1 month) | 53/131 (40.5) | 36/125 (28.8) | 47/130 (36.2) | 136/386 (35.2) |
| No | 62/131 (47.3) | 67/125 (53.6) | 61/130 (46.9) | 190/386 (49.2) |
| Current therapeutic oral/IV/IM steroids | 15/131 (11.5) | 4/126 (3.2) | 6/131 (4.6) | 25/388 (6.4) |
| Diabetes mellitus (any type) | 11/130 (8.5) | 10/126 (7.9) | 8/130 (6.2) | 29/386 (7.5) |
| ASA class, n/N (%) | ||||
| I: healthy, no medical problems | 43/128 (33.6) | 51/125 (40.8) | 40/131 (30.5) | 134/384 (34.9) |
| II: mild systemic disease | 72/128 (56.3) | 58/125 (46.4) | 73/131 (55.7) | 203/384 (52.9) |
| III: severe systemic disease, not incapacitating | 13/128 (10.2) | 16/125 (12.8) | 16/131 (12.2) | 45/384 (11.7) |
| IV: severe systemic disease, constant threat to life | 0/128 (0.0) | 0/125 (0.0) | 2/131 (1.5) | 2/384 (0.5) |
| Characteristic | Group | Overall (N = 388) | ||
|---|---|---|---|---|
| Randomised to simple dressing (N = 131) | Randomised to glue-as-a-dressing (N = 126) | Randomised to no dressing (N = 131) | ||
| Operation duration (hours), median (IQR)a | 1 (0.7–2.7) | 1 (0.6–2.4) | 1 (0.7–2.5) | 1 (0.7–2.5) |
| Grade of surgeon performing skin closure, n/N (%) | ||||
| Foundation doctor | 1/126 (0.8) | 1/125 (0.8) | 1/127 (0.8) | 3/378 (0.8) |
| Core trainee | 16/126 (12.7) | 19/125 (15.2) | 18/127 (14.2) | 53/378 (14.0) |
| Specialty trainee | 74/126 (58.7) | 67/125 (53.6) | 75/127 (59.1) | 216/378 (57.1) |
| Consultant | 35/126 (27.8) | 38/125 (30.4) | 33/127 (26.0) | 106/378 (28.0) |
| Number of wounds (excluding drain sites), median (IQR)a,b | 2 (1.0–4.0) | 2 (1.0–4.0) | 2 (1.0–4.0) | 2 (1.0–4.0) |
| Stoma formed, n/N (%) | 14/102 (13.7) | 14/100 (14.0) | 18/103 (17.5) | 46/305 (15.1) |
| Time from randomisation to wound closure (hours), median (IQR)a,c | 2 (1.0–3.4) | 2 (0.9–3.7) | 2 (0.9–3.8) | 2 (1.0–3.6) |
| Delay between wound closure and logging wound closure in randomisation database (minutes), median (IQR)a | 0 (0.0–1.0) | 0 (0.0–1.0) | 0 (0.0–1.0) | 0 (0.0–1.0) |
| Day case, n/N (%) | 28/130 (21.5) | 27/125 (21.6) | 27/130 (20.8) | 82/385 (21.3) |
Primary feasibility outcome
With respect to the primary feasibility outcome, a total of 862 patients were approached and had their eligibility determined; 163 were found to be ineligible (see Figure 8). Of the remaining 699 eligible patients, 284 declined to take part (41%), 21 consented but were not randomised (3%) and 394 (56%) were randomised. (The same information by centre is described in Report Supplementary Material 15.) Table 26 shows the primary feasibility outcomes by centre.
| Outcome | Centre | Total screened participants (N = 1115) | ||||
|---|---|---|---|---|---|---|
| NBTG (N = 96) | NBTO (N = 230) | QEHB (N = 558) | UHBR (N = 196) | WORC (N = 35) | ||
| Number of potentially eligible participants per month, median (IQR) | 14 (3.0–25.0) | 27 (25.0–48.0) | 71 (57.0–80.0) | 21 (13.0–25.0) | 10 (4.5–13.0) | 142 (57.0–152.0) |
| Proportion of potential participants eligible for the trial, n/N (%) | 90/96 (93.8) | 205/230 (89.1) | 469/558 (84.1) | 154/196 (78.6) | 34/35 (97.1) | 952/1115 (85.4) |
| Proportion of eligible patients approached, n/N (%) | 87/90 (96.7) | 126/205 (61.5) | 317/469 (67.6) | 136/154 (88.3) | 33/34 (97.1) | 699/952 (73.4) |
| Proportion of approached patients who consent to randomisation, n/N (%) | 65/87 (74.7) | 81/126 (64.3) | 120/317 (37.9) | 127/137 (92.7) | 22/33 (66.7) | 415/700 (59.3) |
Objectives B2 and B3: use qualitative research methods to investigate reasons for any difficulties that affect recruitment and assess acceptability of trial interventions and processes to participants and clinical staff
Sample characteristics
Interviews were conducted with a total of 55 participants during the pilot RCT (professionals, n = 18; patients, n = 37). None of the individuals who agreed to be contacted refused participation or withdrew. Three out of the 18 professional interviewees took part in a group interview (from site 3). Table 27 shows the breakdown of professionals interviewed by site and by their principal role.
| Professional role | Surgical specialty and site (n) | Total, n | ||
|---|---|---|---|---|
| General | ||||
| Site 1 (abdominal surgery) | Site 2 (obstetric surgery) | Site 3 (abdominal surgery) | ||
| Ward nurses | 3 | 0 | 1 | 4 |
| Theatre staff | 4 | 0 | 0 | 4 |
| Surgeons | 1 | 0 | 0 | 1 |
| Surgical registrars | 2 | 0 | 0 | 2 |
| Research nurses | 1 | 3 | 3 | 7 |
| Total | 11 | 0 | 4 | 18 |
Of the 37 patient interviewees, five had been allocated to receive a ‘simple dressing’, 18 had been allocated to receive ‘no dressing’ and 14 had been allocated to receive ‘glue-as-a-dressing’. Each of these three groups comprised a mix of patients who had undergone general or obstetric surgery and laparoscopic or open procedures (Table 28). Seven patients took part in two interviews (one in the early stages of post-surgical recovery and one in the later stages).
| Pilot RCT dressing strategy allocation | Surgical specialty (general or obstetric) and mode of surgical access (laparoscopic or open) (n) | Total | |||||
|---|---|---|---|---|---|---|---|
| General | Obstetric | ||||||
| Laparoscopic | Open | Not recorded | Laparoscopic | Open | Not recorded | ||
| Simple dressing | 2 | 1 | 1 | 0 | 1 | 0 | 5 |
| No dressing | 8 | 5 | 2 | 0 | 3 | 0 | 18 |
| Glue-as-a-dressing | 8 | 3 | 0 | 0 | 3 | 0 | 14 |
| Total | 18 | 9 | 3 | 0 | 7 | 0 | 37 |
Except for two, all patients had been admitted as elective cases. The two ‘unplanned’ cases were both abdominal surgery patients recruited from site 1.
Presentation of findings
Findings have been presented in two parts, each mapping on to one of the objectives for the integrated qualitative study. Part 1 focuses on issues of adherence, drawing on HCP and patient interviews; and part 2 presents HCPs’ and patients’ perceived acceptability of the study processes and trial comparison groups. Findings have been supported by illustrative quotations, some of which have been edited for ease of comprehension (without altering the meaning). The following identifiers have been used for quotations: I, interviewer; S, surgeon; SR, surgical registrar; N, nurse; RN, research nurse; TS, theatre staff; and P, patient.
Part 1: adherence
Health-care professionals’ perspectives on adherence
Perceived levels of adherence
Health-care professionals appeared to have a full understanding of what each of the Bluebelle dressing strategies entailed. The only exceptions to this were informants who had just started working on Bluebelle but who had not yet become familiar with the protocol (n = 2). Professionals’ understanding of the Bluebelle definition of ‘dressing’ was a particular area of concern that arose in Phase A interviews, but staff involved in the pilot appeared well informed. This was illustrated through explanations of how wound exudate/blood was managed in theatre, in recovery rooms and on the wards. All staff reported placing gauze or attaching pads to the weeping wound but were clear that these products should not fully adhere to the wound (i.e. around their entire perimeter):
I think I remember one incident [of placing gauze on the wound], but not securing it down on four sides to make it a dressing.
Research/ward nurse in obstetrics 003H
It depends on the severity. Occasionally I think there might have been some sterile gauze and tape applied then removed once it seems to have settled down again.
[. . .] how is the tape being applied over the gauze?
Just laterally really, so straight across the middle.
So it’s not been all the way around?
No.
Senior theatre practitioner in abdominal surgery 005H
Interviews with HCPs on the whole suggested an absence of notable issues, problems or extraordinary behaviours when it came to caring for Bluebelle participants. The interviewers used a variety of approaches to elicit examples of non-adherence, including open questions about how professionals managed patients with ‘no dressing’ and ‘glue-as-a-dressing’, and more directive questions asking if any products had been placed over glued or non-dressed wounds. None of the informants recalled any such examples. Collectively, HCPs’ accounts suggested that adherence to dressing allocation had not been problematic. The quantitative measures of adherence are concordant with these qualitative data, as there were very few recorded examples of non-adherence to dressing allocation (see Objective B4: assess adherence to allocation and the follow-up protocol). These findings suggest that the strategies used to promote adherence in Bluebelle were successful. Interviews provided an opportunity to explore professionals’ perspectives on how useful the protocolised strategies had been (e.g. use of skin transfers), in addition to any other strategies that staff had employed themselves.
Strategies to promote adherence
Staff from all sites discussed various communication-based strategies to ensure that patients’ wound-dressing allocations were adhered to. These included introducing and explaining the Bluebelle study to peers within teams, using Bluebelle transfers and specifically recording of dressing strategy allocation on hospital documentation that ‘travelled with’ the patient (i.e. from the theatre room to the recovery room, and on to the wards). Staff from two wards in one site reported incorporating information about the patients’ Bluebelle status into routine handover documentation, referred to in the example below as the ‘handover sheet’:
I mean we put it [Bluebelle allocation] on the handover sheet and we always mention it on handover, unless someone doesn’t know what it is, you can always just quickly explain. And I’ve not come across any problems at handover and things like that.
So it is routinely mentioned on handover or . . .?
Yeah yeah. And if it’s not mentioned it’s always on the handover sheet anyway.
What is the handover sheet?
It is a list of all the patients on the ward, why they’ve come in, who their consultant is, their medical history, any social issues like if they’re independent living alone, or if they have help at home [. . .] And it’s got the nursing plan, so (things) like can they eat and drink, are they on regular medication, antibiotics. And then they’ve got jobs we need to do like removing drains and things like that.
I see. And so the Bluebelle patients, is it like written or is there [. . .]
Yeah yeah, it’s written, so in [. . .] kind of, the nursing plan – it’s written on there. Or sometimes in the reason why they’re in (hospital) it will say what surgery they’ve had, and then we’ll put what study they’re part of, so like we’ll put Bluebelle next to their operation, if that makes sense.
Ward nurse in abdominal surgery 10H
Although professionals were not aware of any protocol deviations, most acknowledged the possibility that these could occur. In particular, informants contemplated that dressings could easily be applied by ward staff who might be less familiar with the Bluebelle study protocol (e.g. bank staff and agency staff). Some also discussed how relying on documentation to promote adherence was not sufficient, as handover sheets and patient notes were not necessarily read at the beginning of shifts:
The last 2 days I’ve worked has been so busy. All my documentation has been put to one side until the end of the day, so I stayed on for an hour the other day just to get my written work done at the end of my shift. That could mean a dressing (has) been on a wound for 6 hours that shouldn’t have been there.
Ward nurse in abdominal surgery 10H
The Bluebelle skin transfers were pre-emptively introduced as a means of preventing the issues discussed above. Not all informants were actively using the Bluebelle skin transfers at the time of interview, and some were not aware that these were available. One registrar suggested that this might have been due to the delayed introduction of the transfers, rather than any aversion to their use:
It kind of crept in, and people are still learning how to do it. It’s just one of the many moving parts of the study, because it wasn’t all one package upfront. When you add things in after things have already started to get going then it takes time for people to then know about it.
Registrar in abdominal surgery 11H
Others reported that they did not know where the transfers were stored or were unsure about who was responsible for applying them to the patient. There was nonetheless general support for their uptake and/or continued use:
Because sometimes if a dressing’s leaking or whatever we quickly grab some stuff and we don’t have time to look through all the paperwork to check if they’re on Bluebelle. Obviously if they’ve got their little stamp (transfer) then we can see straight away, so we know if we’re about to move a simple dressing we can just replace it and whatnot.
Ward nurse in abdominal surgery 09H
Most professionals from the obstetrics site also reported that the transfers were useful for alerting busy midwives about the patient’s allocation. Both of the informants below also discussed how the transfers had created a sense of joviality in their interactions with patients. Unlike most abdominal surgery patients, women undergoing caesarean section would be awake during the surgical procedure and, thus, experienced the in-theatre processes (such as randomisation and the application of transfers). Skin transfers were reportedly discussed in a light-hearted manner, which was thought to help some patients relax:
One of the ladies I had today said ‘Ooh, that was the reason I consented to the study!’ [Laughter]. She wanted the tattoo [. . .] [transfer] I think she was joking, but it is a kind of novelty – it’s a fun idea. As adults, we don’t really get to play with things like that [. . .] So, it’s just a bit of fun, and we find generally in theatre a kind of bit of light-hearted humour goes a long way to helping the patients relax.
Research/ward nurse in obstetrics 02H
In contrast to most informants, one research nurse from the obstetrics site did not feel that additional adherence aids – such as the skin transfers – were particularly useful. This individual was confident that their efforts to raise awareness about the study were sufficient, particularly given the close-knit nature of the midwifery team:
I think if you were in a big surgical hospital as in [name of hospital], that might be a different scenario, but I think the way that we have sold, promoted, and worked together on this in maternity [. . .] maternity is quite a specialised area [. . .] you know, I’ve approached the postnatal managers, went to the meeting, they all know so much, they all know about it now so they’re all quite happy with it. Whereas, if you were in a much bigger unit and you’ve got four different surgical wards, you would then have an agency nurse that’s working that day go ‘Oh my goodness that person hasn’t got a dressing on, let me go and get a dressing’ [. . .] We work together as a team so well, but everybody knows a lot. We get regular e-mails and everything, but I don’t know how that would work on a bigger population.
Research/ward nurse in obstetrics 01H
Patients’ accounts of adherence
Perceived levels of adherence in hospital
Overall, patients corroborated HCPs’ accounts of adherence being non-problematic. Patients’ accounts of how wound exudate had been managed on the wards indicated adherence to the trial protocol. Most patients who received glue-as-a-dressing or no dressing reported that no other products had been applied over their wound in hospital. In most cases, participants reported that staff had visually inspected the wound at various times but did not physically interact with it. However, a considerable number of patients acknowledged that they could not be confident in what may or may not have happened to their wound in the immediate postoperative period, as they were recovering from the effects of general anaesthesia or receiving heavy pain medication.
Some participants from the ‘no dressing’ group reported that products had been applied to their wound in response to exudate/bleeding, although this had been in accordance with the Bluebelle study protocol. Strategies to manage exudate included the temporary application of gauze and the placement of non-adhesive dressings (e.g. pads) that had been secured with tape. A few of these participants made unprompted reference to these strategies adhering to the Bluebelle study protocol, based on discussions that they had overheard or taken part in with ward staff, or their own knowledge of the Bluebelle study definition of a ‘dressing’:
They just gauzed it up and patched it up and that’s been happening in [sic] almost every time I’ve either got into bed or out of bed or one of the other, so that’s fine.
So have they put a dressing on your wound?
Not a proper dressing, no.
OK, what have they put on?
Just a gauze and tape, only three sides, because you can’t have the four.
Abdominal surgery patient 037, ‘no dressing’
Two participants’ accounts of wound management differed markedly from others. One informant – an abdominal surgery patient allocated to ‘glue-as-a-dressing’ – reported having a piece of gauze placed over the glue. This was reportedly applied in hospital and remained on the wound when the patient was discharged. This was the only report of a product being applied over glue. The patient did not recall having any particular symptoms that might have prompted the use of gauze:
Yeah I had the glue, and then a piece of gauze put on top of it, didn’t I?
Oh I see, OK. And why was the gauze put on? Do you remember?
No I don’t. I just presumed that’s what they put on top of a glue dressing.
Obstetric patient 002, ‘glue-as-a-dressing’
According to the patient, the gauze remained on the wound for several days (throughout the hospital stay) and came off gradually as she showered at home.
The second example came from a patient allocated to the ‘no dressing’ group, who reported receiving several dressings to manage what was described as significant exudate. This individual’s case would not be considered a breach of protocol, based on the patient’s account of the symptoms experienced. As shown in the quotation below, the wound was deemed to be ‘infected’ and had reportedly broken down in places. It was not clear whether the products applied to the wound breached the ‘no dressing’ allocation, as the patient reported products being applied over areas of the wound (thus not covering it in its entirety). Regardless, it appears that the decision to cover the wound had been based on the clinician’s discretion:
Um it’s uh, it was a clear dressing . . .
Right yes, yes
[. . .] with a white band going through the middle [. . .] Allevyn was it? [. . .] Yes I think that’s what it was – little pink ones [laugh]. I was like a little patchwork quilt on me [sic] tummy I’ll be honest with you [laugh].
And was this over the whole wound that they put it, or just over part of the wound?
Just in parts, just in parts because I had um [. . .] an infection in the wound, so they took a swab and apparently they said no antibiotics were needed because it’s one of those germs that lives on the skin anyway.
I see, yes.
And uh, so they didn’t worry about that, they just sort of like kept putting a dressing on it because it was weeping.
Yes, yes, but it wasn’t covering the whole of the incision, the whole of the wound was it . . .?
Oh no no no no no, no it was just in the parts that have split.
Abdominal surgery patient 030, ‘no dressing’
The patient also reported that a stoma bag had been used to collect exudate while in hospital. It was clear that s(he) received a dressing when discharged and continued to receive dressing changes from a district nurse at home:
When I came home they put on a dressing and it leaked [. . .] I looked down, I could see it was all discoloured on my pyjamas, and I knew then it was coming from the wound [. . .] so three times it happened. And when I told the district nurse about it she said ‘Oh [gives own name]’, she said, ‘You don’t have to put up with that.’ She said ‘This is a 24-hour call-out’, you know [. . .] so she said if it should happen again [. . .] ring and somebody will come and they’ll do the dressing for you [. . .] So they just used to come in and dress every – well, not every day – twice a week.
[Later]
Oh so what kind of dressing did they put on then was it . . .?
Um it’s uh, it was a clear dressing with a white band going through the middle.
Abdominal surgery patient 030, ‘no dressing’
This patient experienced exudate with and without a dressing and was thus able to compare the relative benefits and disadvantages of both approaches. This patient’s perspectives on these matters are covered later (see Exudate).
Adherence aids and awareness of Bluebelle
Most patients were not aware of skin transfers, despite these having been introduced to the clinical teams at the time of interviews. This suggests that transfers had not been used for all patients (although recall issues may have influenced responses). Patients who reported having received a transfer (n = 7) did not express any particular concerns, although two did not recall having been informed that this was a component of the Bluebelle study. Furthermore, one of these individuals indicated that she would have found more information about transfer removal helpful:
Maybe mention that it only needs to stay on until you’ve seen the midwife and then after that you just, you do need to give it a bit of a scrub to get it off maybe. If you mentioned that then that might sort of um [. . .] make it a bit clearer to people but I don’t [. . .] I mean, I can’t imagine that it’s going to be an issue for anybody to be honest with you.
Obstetric patient 071, ‘no dressing’
Although skin transfers were not necessarily used, most patients sensed that clinical staff were aware of the Bluebelle study. Not all patients were able to expand on why they held this impression, but some specifically recalled discussion of the study among (or with) clinical staff. By contrast, two participants noted that not all staff seemed to be familiar with Bluebelle. In these cases, the patients recalled explaining the study to nurses. The skin transfer appeared to prompt this in the first patient’s case:
They [saw] my wound, like tattoo, and they were like, ‘What’s that?’. I was like, ’That’s a Bluebelle research stamp’, so obviously they didn’t know that.
Obstetric patient 056, ‘no dressing’
The nurses didn’t really seem to be aware of what research was going on, and so I felt like I had to keep on repeating myself and telling them about it all.
Abdominal surgery patient 007, ‘no dressing’
Other patients also took a proactive approach to mentioning their participation in Bluebelle to ensure that staff were aware of the implications for their wound care:
I think I remember them just putting over a piece of gauze – you know, when you sort of put something on top of a wound just to maybe soak a bit of excessive fluid or blood up. Like I said, I don’t know for sure but I seem to remember that being done just whilst I was in recovery but then it was taken off [. . .] because I think I asked at the time, ‘That’s not a dressing is it?’ [They said] ‘No, no no, it’s not’ [. . .]
Obstetric patient 071, ‘no dressing’
Adherence post discharge
Overall, patients’ accounts of wound management after discharge suggested that there were very few – if any – examples of behaviours that might have breached their allocated dressing strategy. When asked if they had covered their wounds with a dressing or any other product, patients in the ‘glue-as-a-dressing’ and ‘no dressing’ groups were clear that they had not taken any such steps.
Interviews also explored patients’ day-to-day activities, with a view to understanding any examples of behaviours that might have unwittingly breached the Bluebelle study protocol. In general, patients reported a tendency to have little interaction with their wounds, which may have linked to the lack of notable symptoms or practical issues encountered. Patients could not recall any noteworthy issues that were specific to their wounds or the products covering their wounds. Only a few individuals reported particular symptoms or events that prompted them to actively think about their wound (e.g. exudate/bleeding, bruising and the wound coming apart in areas). The actions (or lack thereof) taken in response to these symptoms reinforced the impression that patients generally adhered to their allocation. This tended to be underpinned by perceptions that the symptoms were not sufficiently serious or bothersome to warrant action:
Yeah, yeah [I] just took a tissue in the bathroom and just dabbed it, looked at it, it didn’t run any more. I didn’t see it [blood] had come back, looked at it again, and just carried on really.
Abdominal surgery patient 067, ‘glue-as-a-dressing’
I think it was perfectly dry for the rest of the time but I don’t [. . .] maybe 5 or 6 days ago I think I knocked it and I had a little bit more blood but I didn’t dress it – but again, it was not a bleed, it was just a few spots had come through onto my t-shirt.
Abdominal surgery patient 106, ‘no dressing’
Only one participant reported covering his/her glued wound after discharge. This individual had been uncertain whether or not (s)he needed to keep the wound dry. Having consulted various sources (the internet and a family member with nursing expertise), (s)he opted to cover the torso with cling film in an attempt to protect the wound from getting wet while showering:
My only little problem has been that I can’t recall the [nurses] in the hospital actually telling me regarding the shower. I’ve basically looked online and spoke to one of my cousins who’s a nurse because I wasn’t too sure how soon I could have a shower, if I could get the glue wet [. . .] uh, that’s been the only problem I’ve had.
OK so you would have liked to have more information about how to look after your wound after your discharge, OK. And regarding the shower – did you take a shower soon after . . .?
Yeah what I did, what I did was I wrapped my stomach in cling wrap before I got into the shower. It just really got a little wet but it wasn’t saturated.
Abdominal surgery patient 033, ‘glue-as-a-dressing’
Although not specified in the protocol, two patients randomised to ‘glue-as-a-dressing’ recalled actively refraining from picking at their glue, based on advice from ward nurses:
The only thing is you did want to pick it [both laugh] [. . .] because it was just this hard layer and you just kind of sit there thinking ‘Ah I’d really love to just pull that bit off and see what’s going on underneath’. But I didn’t to be honest, but um it was hard not to.
Abdominal surgery patient 033, ‘glue-as-a-dressing’
Part 2: acceptability of dressing strategies
Health-care professionals’ perspectives on delivering care
Changes to routine care
Health-care professionals involved in delivering Bluebelle were asked to reflect on the impact the study might have had on their routine practices, with particular reference to how ‘glue-as-a-dressing’ and ‘no dressing’ compared with typical practice (‘simple dressing’). On the whole, professionals reported that their practice in theatre and on wards remained the same, regardless of the dressing strategy employed. On further probing, some described aspects of care that differed for patients who received ‘no dressing’ or ‘glue-as-a dressing’, although none of these was presented as a concern. Ward staff discussed these issues in relation to (1) the application of gauze (rather than a dressing) to manage wound exudate and (2) the possibility that patients in the ‘no dressing’ group may have received more clinical time/attention through incidental observation (while performing other tasks). These two issues were inter-related.
Although the application of gauze was not deemed problematic, one nurse alluded to this being a little more burdensome than the usual practice of applying a dressing. This was based on the idea that gauze needed to be changed more frequently:
We do probably have to get involved more frequently if we have got an oozy wound that’s not to be dressed, because inevitably gauze doesn’t stay put very long.
Oh I see. So it requires more frequent gauze changes I suppose?
Yeah so more, probably more frequent gauze changes on those. No [. . .] and no complaints about glue. I’m sure there have been instances using gauze, where I’ve thought ‘Why can’t we just put a dressing on this?’, but that’s just logic in my head [laughs] [. . .] you know, you have to stick to what the plan is [. . .] but you do think sometimes, ‘Oh it would be easier if we just stick a dressing on it’.
Ward nurse in abdominal surgery 09H
The other potential change to practice, identified by one informant, was the possibility that patients who did not have a simple dressing would receive more incidental observations of the wound. This was thought to be a by-product of the wound simply being visible. The informant theorised that this might indirectly be beneficial by allowing issues to be identified sooner:
I suppose to some degree if you’re washing a patient [. . .] without a dressing on, you can see it [the wound] immediately [. . .] so they’re seen probably more often when they’re glued and not got a dressing on, because you can visualise that site a lot more. To some degree I think it’s better because you can see if there are issues a lot sooner than perhaps you would do. So if we have got things like oozing or redness or something that doesn’t look quite right, we are spotting it earlier, maybe.
Ward nurse in abdominal surgery 02H
Like ward staff, theatre staff did not perceive any notable differences to their routine practice, although, on further discussion, some noted that they tended not to open dressing packaging in advance. Dressings were opened only if the patient was allocated ‘simple dressing’ after randomisation. The only other difference, noted by a single theatre nurse, was the perception that the final cleaning of the wound area took longer in cases in which the patient was allocated ‘no dressing’. In these instances, the nurse felt that (s)he needed to take more care to avoid directing exudate/blood to the wound:
Because usually when you clean you hold like this [demonstrates], with a dressing.
Yes, you just push it down?
Push, yeah, not really down, down – it’s like, you hold it, and then clean the sides, but without the dressing and the skin glue I’m more slow cleaning, because [. . .] just in case um [. . .] because I can’t obviously [. . .] I don’t want to touch it, you see.
Theatre recovery nurse in abdominal surgery 08H
Health-care professionals’ impressions of patient acceptance
Ward nurses were able to reflect on their general interpretations of patients’ reactions to the three dressing strategies, based on their experience of delivering care. All thought that patients found all three dressing strategies acceptable; there had been no notable clinical or practical issues arising. One ward nurse suggested that patients had fed back the practical merits of ‘glue-as-a-dressing’ and ‘no dressing’, such as the avoidance of dressing changes and being able to shower sooner. However, the only exception to these positive accounts was provided by this same informant, who pointed out the practical issues around not having a dressing to cover staples; this had been reported as painful for some patients:
I think initially the patients were a bit ‘Oh I haven’t got a dressing’, and once it’s explained I think they’re happy because then they can go and they can have a shower and just keep that dry. So I think [. . .] so far the feedback from patients is they feel a bit better not having plasters everywhere and dressing changes, and we can see the wounds to review them regularly, so if there are any issues we can pick them up. [Later] There haven’t really been any problems. Initially when they started first trying to glue [the wounds], they were putting clips in and glue – that meant we had a few issues with getting the clips out, but that has since stopped, so we’ve had no problems since.
Ward nurse in abdominal surgery 02H
Acceptability to patients of the pilot randomised controlled trial of dressing strategies
In general, patients’ accounts of their recovery process suggested that all three dressing strategies were acceptable on clinical, practical and psychological fronts. Patients allocated to all three groups experienced varying degrees of exudate, practical issues and wound sensations, but only one interviewee expressed adverse views about the dressing strategy that they had received. The general acceptability of all three strategies was often implicit in patients’ accounts of having paid little attention to their wounds, having not encountered any problems.
Exudate
Only three (out of 14) patients interviewed who received glue-as-a-dressing recalled noticing blood or exudate from their wounds. By contrast, approximately half of the patient interviewees who had been allocated to ‘no dressing’ and a similar proportion who had a ‘simple dressing’ experienced at least some exudate. In most cases, exudate was easily managed. Some patients who had received ‘glue-as-a-dressing’ or ‘no dressing’ reported taking no action on account of the small volume of exudate:
There was a little bit of serous ooze for a bit which stained my t-shirt but I didn’t put any other dressing on top of it and it just dried up.
OK. OK, so the nurses didn’t, did the nurses spot it or . . .?
No no, they didn’t do anything [. . .] It was a tiny, tiny amount of stuff on the first postoperative day that wasn’t worth bothering with.
Abdominal surgery patient 019, ‘glue-as-a-dressing’
I think it was the following day, it [the wound area] looked a little bit damp, but they weren’t concerned – the midwives at the hospital weren’t concerned.
Obstetric surgery patient 173, ‘no dressing’
One patient in the ‘glue-as-a-dressing’ group reported using tissue to dab away the exudate, whereas patients in the ‘no dressing’ group reported that gauze or pads had been applied. Patients who experienced exudate in the ‘simple dressing’ group recalled some staining on their dressings, although no additional dressings were used.
Although exudate/bleeding did not appear to have caused any concern for most patients, two in the ‘no dressing’ group had exceptional experiences, marked by relatively more severe discharge and application of dressings. One of these patients (037) had this exudate managed with frequent gauze changes, whereas the other (030) received a dressing (as discussed earlier). Neither of these patients attributed their experience to the initial absence of a dressing, with both assuming that their experiences of exudate would have been similar regardless of the trial group to which they had been allocated:
The first time I got out of bed it did all weep from the bottom, but the surgeon said that’s ‘cos I’ve got a lot of water and that’s [. . .] it would have happened whether I’d got a dressing on or not. So they just gauzed it up and patched it up and that’s been happening almost every time I’ve either got into bed or out of bed.
Abdominal surgery patient 037, ‘no dressing’
I think the problems I had would have still occurred. If I had a dressing I’m sure it would have still leaked, but [. . .] so [. . .] you know, it doesn’t matter I don’t think – not really.
Abdominal surgery patient 037, ‘no dressing’
Both of the above patients were able to compare the ‘no dressing’ and ‘dressing’ strategies, having experienced an initial period during which they had not received a dressing. Both suggested that leaving the wound exposed might have been preferable for comfort or hygiene reasons:
I think all wounds should have air get to it anyway so that is a good idea and me having bowel surgery [. . .] we always leak – we never heal properly straightaway so it’s nothing [. . .] so I would have expected it anyway, do you know what I mean? To be honest if you put a dressing on, because I’ve got my stoma right next to it, one is going to dirty the other or I’m going to have to keep changing one or the other a lot because it’s so close.
Abdominal surgery patient 037, ‘no dressing’
That really did weep and it was quite profusely at times, and they had to put – well they didn’t have to – but they put on a stoma bag to catch it rather than keep putting on the dressings and pulling the dressings off because of the sensitivity of the area [. . .] To be honest, to me that seemed a lot cleaner because it was weeping quite badly, and I just used to feel wet all the time.
Abdominal surgery patient 030, ‘no dressing’
Practical issues
Health-care professionals and patients who took part in Phase A interviews frequently expressed concern that forgoing a dressing may result in the wound area catching on clothing or bedding. Contrary to these expectations, this did not appear to be a widespread or serious concern among patients who participated in the pilot. Most patients reported no issues when asked about the practicalities of not having a simple dressing, even when probed specifically about difficulties with their wound catching on clothing or objects. A handful of patients in the ‘glue-as-a-dressing’ and ‘no dressing’ groups reported some discomfort but had managed to adapt their choice of clothing to address this:
Because it’s a stomach wound it’s just trying to (–) keep elasticated things off it.
Abdominal surgery patient 041, ‘glue-as-a-dressing’
I was braless for a couple of weeks [. . .] because of that wound [. . .] Yeah, because it was virtually on my bra line and I didn’t want anything touching it.
Abdominal surgery patient 042, ‘no dressing’
Some patients from both the ‘no dressing’ and ‘glue-as-a-dressing’ groups also discussed opting for looser garments or specially adapted clothing (maternity wear) to enhance their overall comfort following surgery. However, these decisions were related to the post-surgical recovery process in general, rather than to the wound itself:
[The wound] might have [caught on clothing] but I think where the actual wounds are [. . .] I think probably I would have worn a dress anyway, whether I had a dressing on or not, because it is on the waistband area of my trousers.
Abdominal surgery patient 101, ‘no dressing’
Two patients who had received glue-as-a-dressing reported catching the glue or exposed suture on their hands, although this did not detract from their overall satisfaction with this dressing strategy. One of these patients (039) also reported that glue had stuck to his/her stoma bag, a comment that was offered in response to the interviewer asking if there had been any disadvantages to receiving glue-as-a-dressing:
As for improvement [. . .] hmm there wasn’t really much I could think of, improvement-wise. Um [. . .] yeah, as I said, the only problem I had was, as it [glue] was coming off, the edges were kind of catching on the bag, or I’d catch it with my finger as the edges were starting to peel off.
Abdominal surgery patient 039, ‘glue-as-a-dressing’
Finally, one patient from the ‘no dressing’ group had a somewhat different perspective. This individual was concerned about his/her hospital gown making contact with the wound. Their more general comments about needing ‘something’ to cover or protect the wound were indicative that they were not comfortable with being allocated to the ‘no dressing’ group. However, the comments about the process of recruitment (below) indicated that they may not have entered the trial with a fully informed mindset:
It was explained to me – unfortunately I was in a bit of a rush because I was just taken down to theatre at the time but I didn’t really think it through that much [. . .] didn’t have time to think it through [. . .] um, still, I would have done the same.
Abdominal surgery patient 043, ‘glue-as-a-dressing’
Patients often discussed showering without prompting, resulting in this being added as a prompt on the topic guide. There was a tendency for patients in the ‘no dressing’ and ‘glue-as-a-dressing’ groups to assume that they were able to shower sooner (or with greater ease) than would have been the case with a ‘simple dressing’. The perceived ability to shower without restriction was often discussed as a particular benefit of ‘no dressing’ and ‘glue-as-a-dressing’:
I think one of the main advantages [. . .] was the fact that I could actually have a shower, whereas my friend [who had a dressing] had to wait a few days before she was allowed to get the wound wet.
Abdominal surgery patient 002, ‘no dressing’
Although some patients held these views, others – particularly women who had undergone caesarean sections – reported that showering soon after surgery was no different from past experiences with a dressing, as they had previously been encouraged to remove the dressing and shower within the first 2 days of surgery:
I mean with my first [caesarean] section they told me to take the dressing off during my shower anyway, so it was no different to what I was used to anyway.
Obstetric surgery patient 067, ‘no dressing’
Patients who had been allocated ‘simple dressing’ had this removed prior to discharge and thus had no dressing at the time of interview. These patients reported being able to shower without any problems.
Wound sensations
Interviewees did not indicate any serious concerns about wound sensations that they specifically associated with any of the dressing strategies. Patients described an array of sensations, using terms associated with pain and discomfort (e.g. ‘soreness’), tightness and itchiness. However, wound sensations were usually mentioned in the context of their broader post-surgical recovery. Patients tended to associate sensations with the incision itself, rather than with the dressing strategy:
Yeah, yeah. You know, she [nurse] said ‘How are you feeling’, I said ‘Well fine, except I still feel a little bit sore’, but there again it was like a major operation I suppose, so, you know.
Abdominal surgery patient 076, ‘simple dressing’
I’ve had no problems with it. The only problem I had was sort of deep inside [. . .] a just achy pain from the operation, but nothing to do with the wound.
Abdominal surgery patient 002, ‘no dressing’
Um I did find it quite painful but I don’t know if that [. . .] I don’t think that’s got anything to do with the glue, I think it was just the incision and being a second C-section. I don’t know if it caused any more pain because of the scar tissue.
Obstetric surgery patient 100, ‘glue-as-a-dressing’
I mean it was obviously [. . .] I would say it’s [. . .] being a wound, you could feel it anyway, but it got slightly itchy sometimes, but I mean that could be anything – just the fact that I’ve got the wound there, so it might not have been because of the actual glue.
Abdominal surgery patient 010, ‘glue-as-a-dressing’
As an exception, some patients who received glue-as-a-dressing reported sensations of tightness, pulling, pushing and itchiness. These were presented as observations rather than issues that affected patients’ recovery in any meaningful way:
It’s a bit ridged – so when you sit down, the big thick one [wound] [. . .] it does tend to push at your skin a little bit and it sometimes just feels like a little bit of a sharp pull – and then you realise it’s just the glue on your skin.
Abdominal surgery patient 119, ‘glue-as-a-dressing’
Advantages and disadvantages of specific dressing strategies
The sections above collectively show how patients’ accounts of their post-surgical recovery experiences were broadly comparable across the three dressing strategies, each of which appeared to have similar merits and drawbacks. However, interviews with patients also highlighted specific advantages and disadvantages of each dressing strategy, as summarised below.
Patients who had received ‘no dressing’ and ‘glue-as-a-dressing’ often commented on the low-maintenance nature of these, as they could avoid the burden of dressing changes and dressing removal:
Well you’re not going to pull them off accidentally if there’s nothing there to pull off. It feels more natural not to have anything there.
Abdominal surgery patient 011, ‘no dressing’
I suppose it’s quite neat and tidy, so no dressings to change or anything like that. So, yeah that was the advantage.
Obstetric surgery patient 100, ‘glue-as-a-dressing’
Two patients presumed that glue had an advantage over ‘no dressing’ in terms of its ‘maintenance free’ nature, as it was thought to provide confidence that the wound was protected from the external environment. This was thought to reduce the need to be as ‘careful’ as they assumed they would have needed to be had they been allocated to ‘no dressing’:
[. . .] in fact if anything I think the glue is less restrictive. I would definitely have felt more unsafe if I’d had nothing on. I didn’t feel I had to be particularly careful of the wound.
Abdominal surgery patient 119, ‘glue-as-a-dressing’
It should be noted that the above account was based on the patient’s assumption of how they would react to ‘no dressing’ (i.e. a hypothetical account). In the pilot, patients who actually received ‘no dressing’ and some who received ‘glue-as-a-dressing’ described being more careful with their wounds.
Patients who received ‘glue-as-a-dressing’ and ‘no dressing’ talked about the advantage of allowing the wound to ‘breathe’ – a perceived positive attribute of not having a dressing, in terms of comfort/sensation:
I would say it felt as if there was nothing there but in a sense it was a good feeling because when you have a dressing on, you know the dressing is there, because you can feel the dressing and you know it feels as if the wound is not breathing [. . .] but with the glue I never had that feeling – it was just as if nothing was there.
Abdominal surgery patient 12, ‘glue-as-a-dressing’
One patient in the ‘no dressing’ group also felt that exposure to air facilitated healing:
I think it dried quicker because the air was going to it, it probably scabbed over. It’s been a bit itchy at times but I think that’s just the scab healing, isn’t it?
Abdominal surgery patient 002, ‘no dressing’
Feasibility lessons for a future main trial
The in-depth accounts of patients and HCPs reported in the previous sections provide insight into the ‘feasibility’ of a main RCT. The key points that can be derived from patients’ and HCPs’ experiences of adherence and their perceptions of acceptability will be summarised in the final section of this chapter. In addition to these lessons that can be implicitly derived from the data, patients and HCPs were directly asked to offer suggestions on how trial processes could be improved if repeated in the context of a main RCT. HCPs tended to focus on trial processes, specifically issues of engagement, capacity and communication among clinical teams. Patients had a tendency to repeat their satisfaction with the dressing strategy they had been allocated in response to this question, although issues of informed consent and information provision indirectly emerged as areas for potential refinement.
Staff engagement and capacity
All HCPs identified staff engagement and communication as important factors in a future trial’s success, with some stressing these as imperative. These concepts tended to be discussed either as a triumph of the feasibility study that needed to continue in a main trial or as a deficiency of the pilot RCT that needed to be addressed. Professionals clearly believed in the scientific question underpinning Bluebelle and had no suggestions for changing the trial comparison groups. However, the process of delivering the trial, from recruitment to randomisation and follow-up, was deemed to require large, engaged teams.
Research nurses in particular emphasised the importance of having sufficient engagement and capacity to deliver a future trial. Although feasibility targets were met, research nurses from a number of sites spoke candidly about the personal resources they had expended to deliver the pilot. Examples of these efforts included working beyond their usual hours and carrying out tasks that they had not anticipated would be required in their role (e.g. helping with the in-theatre randomisation):
So when I was drafted in, I was trying to set up a surgical service that didn’t exist, and trying to recruit to the study largely by myself or with [name of another nurse] and, you know, it was [. . .] we struggled because we’d come in at 7 [a.m.] to see the patients because that’s what time they arrive [. . .] and then we’d stay later and we’d have patients that were going to the theatre at 4, 5 [p.m.], and then of course we’d have to stay to randomise them. So we were doing extremely long days.
Research nurse 031H
All research nurses emphasised that Bluebelle could be easily delivered if tasks were shared among engaged clinical teams. For instance, surgeons were thought to be ideally placed to assist with recruitment and in-theatre randomisation. Several research nurses across different sites suggested that the pilot might have been easier to deliver had clinical staff taken more ownership of the trial:
If you get [. . .] a confederate within the certain key branches [. . .] so what would make it work very well is if you had somebody who was on your side in pre-assessment, in the theatre, so one of the theatre team and some of the extra surgeons that just felt an element of ownership for the trial. They wouldn’t necessarily need to do a lot, but you would just sort of say ‘Look, I’ve got a patient coming through on the emergency list at 9 o’clock at night, could you just fill these [forms] in and leave them in a secure place for me?’
Research nurse 032H
One research nurse emphasised that galvanising support and engagement was crucial at the outset of the study, alongside upfront, comprehensive training covering all aspects of the trial:
So they need training on how to randomise – formal training – not me standing in pre-op showing them a bit of paper with some instructions on [. . .] The new registrars started in September – I e-mailed them all, and not one of them replied to me. It can’t be done, this word of mouth [. . .] So, if anything can be learned from this, you don’t start until you’ve got sufficient members of staff trained and engaged and signed up. I think that’s key to the whole thing.
Research nurse 011H
Although professionals discussed ‘in house’ Bluebelle processes that they had adopted to publicise Bluebelle, there was also a suggestion that knowledge about the study had gradually diffused across their teams over time (i.e. somewhat passively). For example, professionals from one site mentioned that they had only just started to notice Bluebelle participants coming on to their wards, despite the study having been open for some time (at the time of interview). The importance of engaging staff through upfront training was highlighted by nurses from the obstetrics site, who discussed this as a strategy that they deemed had been successful. In contrast to other sites, this team had actively planned how they would integrate the trial into their centre, through discussions that took place before recruitment opened:
Even before we’d started randomising we made sure to go around, to show our face [. . .] well to all of the staff actually, to let them know that the study was starting, to explain the study to people, because a lot of staff didn’t have any idea, because they’re not working in theatre, they don’t really know anything about the different options in theatre [. . .] Obviously the patients go down with their tattoo on and there’s also a little sign on the end of the bed saying that they’re in the Bluebelle study. And to my knowledge we haven’t had anyone stick a dressing on because they thought we’d forgotten.
Research nurse 02H
It was really beneficial to have the first few weeks just setting it up rather than [. . .] you know, whilst we were obviously in anticipation of approval of whether we could actually consent to it, we could get the administrative set-up up and running and meetings to discuss how we were going to get the Bluebelle project going.
Research nurse 01H
In addition to upfront planning and engaging ward staff, the team in the above site was able to deploy at least one research nurse to oversee each of the Bluebelle study processes, allowing for a ‘study champion’ to take ownership and be present at various stages of the patient pathway:
Often when I’m on [recruitment] [. . .] [another research nurse] [. . .] she’s already in theatre scrubbed, and then [another research nurse] is probably, maybe ... she’s on [the ward] at the moment, so we’re able to really keep that continuity going.
Research nurse 01H
This, again, contrasted with the accounts of research nurses from other sites, most of whom discussed the need for more support to deliver the study, suggesting that there had been insufficient capacity:
Recruitment is the easy bit – it’s the rest that’s the challenge. The randomising, and getting the paper completed, and the discharge paperwork [. . .] If I’m not there, it won’t happen.
Research nurse 011H
Information provision to patients
Although patients were generally satisfied with their allocated treatment and the care that they had received, there were some recurring examples of misunderstandings about the study. These examples highlighted opportunities for refining information provision to patients in a main trial, particularly in relation to what the different dressing strategies entailed, and the implications for wound care after discharge.
Some patient interviewees appeared to hold misconceptions about the dressing strategies under comparison. In particular, a few individuals appeared to confuse wound dressings with wound closure materials, in that they assumed Bluebelle could result in a wound being left open:
[Gives name] I think it was the surgeon [. . .] when we first saw him he explained that it would be one of three and options for the scar, and um then I think [. . .] I got the impression that you’re particularly interested because the [. . .] uh [. . .] the children’s hospital they don’t stitch [. . .] they don’t do anything, they leave an open wound, is that right?
Abdominal surgery patient 129, ‘no dressing’
You know this was such a minor operation that I assumed it would be either the dissolvable stitches or the one that I actually had, which was the glue which I hadn’t heard of before.
Abdominal surgery patient 039, ‘glue-as-a-dressing’
Patient interviewees also highlighted strategies that appeared to facilitate recruitment. Reassuring patients that their clinical needs would be prioritised above the study emerged as a key ‘feasibility lesson’. Patients’ recollections of how they reached a decision to participate in Bluebelle varied. Altruism was at the heart of many informants’ accounts, but some recalled deliberating more than others. Most suggested that they were not fazed by how their wound would be managed, associating this with an underlying trust in clinical professionals overseeing their care, and/or a suggestion that they had bigger priorities to consider at the time of surgery:
I thought ‘Well this is just such minor stuff compared with what I’m actually going to have to go through, but I’m quite happy to help in anything you know, anything that I can do to help in this situation, that’s a bonus really’.
Abdominal surgery patient 119, ‘glue-as-a-dressing’
By contrast, around one-third of the interviewees recalled experiencing some level of concern about the prospect of not receiving a dressing when first approached. Some of the practical issues that arose in Phase A interviews re-emerged here, such as concerns about leakage and the perception that forgoing a dressing could increase the risk of the wound coming apart:
There is a lot of information to swallow and I think there’s a lot of anticipation if you’ve had dressings before [. . .] feeling like it’s a little bit alien to not have a dressing. I think you feel a little bit anxious that, I don’t know, maybe the dressing will stop your wound from separating or something like that. I think it can worry you a little bit, but the staff were great [. . .] they reassured [. . .] well, me personally, they really reassured that everything would be fine, and should anything happen then they would easily pop a dressing on – it’s not a problem.
Obstetric surgery patient 173, ‘no dressing’
All patients who described initial concerns discussed how these had been alleviated by the recruiters. In particular, patients appeared reassured by the explanation that clinicians’ discretion would supersede the needs of the trial, and that they would still receive a dressing if this was deemed necessary by clinicians (e.g. if their wound developed a problem):
Oh yeah I was happy [. . .] to be honest, for me the biggest concern was if I was start to bleed or something bad [was] happening with the wound, then [would] I get like proper treatment or am I going to be tied up to the study? So, when they tell me that I can pull out and everything then my mind was absolutely clear, and I was happy to participate.
Obstetric surgery patient 037, ‘glue-as-a-dressing’
Some patients in the ‘no dressing’ and ‘glue-as-a-dressing’ groups commented on a desire for more information about how to care for their wound after discharge. Patients were particularly concerned about whether or not they could have a shower, how long glue should stay on for and what they should do if the glue came off earlier than the ‘minimum required time’ (if this was relevant):
If my wound was to open or anything [and I had to] go to my doctor to redress it, I said to myself ‘Considering it’s glue, how are they going to redress the glue because they normally give normal dressings and this is a new procedure . . .’ So that’s the only thing I was wondering – how they [would] have redressed the glue.
Abdominal surgery patient 119, ‘glue-as-a-dressing’
Summary
This integrated qualitative study indicated that there had been no major adherence or acceptability issues in the pilot RCT. The variable awareness and uptake of skin transfers to promote adherence limited the possibilities for fully exploring stakeholders’ perspectives on these adherence aids; however, those who had used the transfers were generally positive about their application and utility, and those who had not encountered them envisaged that they could be helpful. Relying on written documentation to flag patients’ participation in Bluebelle was thought to be unreliable, as this was not necessarily consistently reviewed at the start of shifts. Notably, patients’ understanding and awareness of the Bluebelle study and their allocated dressing strategy appeared to be key in promoting adherence.
In general, there was no indication that the Bluebelle study processes had any major impact on HCPs’ usual practice. Wound management for patients with ‘no dressing’ and ‘glue-as-a dressing’ was not perceived to be any different from that for patients who received simple dressings (i.e. routine care). The only exceptions to this were accounts of being more careful with cleaning undressed wounds, and the possibility of more incidental observation of wounds that were visible (i.e. the ‘no dressing’ and ‘glue-as-a-dressing’ groups).
Interviews with patients and HCPs were typically characterised by an absence of notable issues or concerns about any aspect of the Bluebelle study or its impact on routine care. Although a few patients who received ‘no dressing’ experienced issues with exudate, they did not attribute this to their allocated dressing strategy, believing that these issues would have still arisen had they received a dressing after surgery. Although some patients and HCPs talked about specific advantages of ‘no dressing’ or ‘glue-as-a-dressing’, it was the absence of issues (rather than notable positive experiences) that underpinned the sense that a future RCT would be acceptable to key stakeholders.
On a logistical note, research nurses from all sites indicated that a future trial’s success would be contingent on upfront training to optimise staff engagement and co-ordination across clinical teams. Although they had successfully delivered the pilot RCT, most felt the current model of having research nurses lead most components of the trial would not be feasible on a larger scale.
Objective B4: assess adherence to allocation and the follow-up protocol
Adherence to allocation
Adherence to the timing of disclosure of the dressing allocation and to the dressing allocation itself by group is shown in Table 29. (The same information by centre is described in Report Supplementary Material 15.) Overall, adherence was good, with 99% adherence to the timing of disclosure when allocated to disclosure before wound closure and 86% when allocated to disclosure after wound closure. Adherence to the allocated dressing was > 97% for the initial dressing and > 86% for patients requiring one or more wounds to be redressed. Redressing wounds in the glue-as-a-dressing group was rare but, when required, glue was not used to redress the wound.
| Adherence | Group, n/N (%) | Overall (N = 388), n/N (%) | ||
|---|---|---|---|---|
| Simple dressing (N = 131) | Glue-as-a-dressing (N = 126) | No dressing (N = 131) | ||
| Adherence to disclosure of dressing category allocation at appropriate time | ||||
| Allocated to disclosure before wound closure | 62/63 (98.4) | 60/60 (100.0) | 64/65 (98.5) | 186/188 (98.9) |
| Allocated to disclosure after wound closure | 56/65 (86.2) | 54/63 (85.7) | 55/65 (84.6) | 165/193 (85.5) |
| Adherence to dressing allocation (patient level) | ||||
| Adherence to initial dressing | 125/127 (98.4) | 121/123 (98.4) | 123/129 (95.3) | 369/379 (97.4) |
| Adherence to redressings (if applicable) | 17/18 (94.4) | 0/6 (0.0) | 117/131 (89.3) | 134/155 (86.5) |
| Overall adherencea | 124/127 (97.6) | 115/123 (93.5) | 109/129 (84.5) | 348/379 (91.8) |
| Wound adherence to dressing allocation (wounds/patients) | ||||
| Adherence to initial dressingb | 251/126 (99.2) | 252/122 (99.2) | 243/123 (95.3) | 746/371 (97.9) |
| Adherence to redressings (if applicable)c | 21/18 (94.7) | 0/0 (0.0) | 379/131 (100.0) | 400/149 (95.5) |
| Overall adherencea | 272/126 (99.2) | 252/122 (99.2) | 622/131 (100.0) | 1146/379 (99.5) |
| Observer completing face-to-face SSI assessment at 4–8 weeks unblinded | 11/96 (11.5) | 31/100 (31.0) | 16/106 (15.1) | 58/302 (19.2) |
| Observer completing 4- to 8-week WHQ unblinded | 9/45 (20.0) | 7/36 (19.4) | 12/46 (26.1) | 28/127 (22.0) |
Co-interventions
Co-interventions by group are shown in Table 30. (The same information is described by centre in Report Supplementary Material 15.) Most wounds were closed with sutures and approximately three-quarters of participants were prescribed prophylactic antibiotics. There is no indication that these co-interventions were used differentially by group.
| Outcome | Group, n/N (%) | Overall (N = 388), n/N (%) | ||
|---|---|---|---|---|
| Simple dressing (N = 131) | Glue-as-a-dressing (N = 126) | No dressing (N = 131) | ||
| Type of wound closure methoda | ||||
| Sutures (wounds/patients) | 240/121 (95.3) | 240/117 (95.1) | 229/117 (90.7) | 709/355 (93.7) |
| Clips (wounds/patients) | 14/10 (9.9) | 13/6 (6.1) | 16/12 (11.5) | 43/28 (9.2) |
| Wound closure strips (wounds/patients) | 20/9 (7.1) | 1/1 (0.8) | 7/5 (3.8) | 28/15 (4.0) |
| Glue (unplanned) (wounds/patients) | 4/2 (2.0) | 2/2 (2.0) | 4/2 (1.9) | 10/6 (2.0) |
| Prescription of prophylactic antibiotics | 101/129 (78.3) | 99/126 (78.6) | 96/130 (73.8) | 296/385 (76.9) |
| Classification of surgeryb | ||||
| Clean | 46/131 (35.1) | 49/126 (38.9) | 44/131 (33.6) | 139/388 (35.8) |
| Clean contaminated | 81/131 (61.8) | 72/126 (57.1) | 81/131 (61.8) | 234/388 (60.3) |
| Contaminated | 4/131 (3.1) | 5/126 (4.0) | 4/131 (3.1) | 13/388 (3.4) |
| Dirty infected | 0/131 (0.0) | 0/126 (0.0) | 2/131 (1.5) | 2/388 (0.5) |
Adherence to the follow-up protocol
Participation in follow-up is described by group in Appendix 5 (see Table 44). (The same information is described by centre in Report Supplementary Material 15.) The WMQ and WEQ at 4 days were completed for > 90% of participants (355/385) and completion rates were similar in the three groups. Completion of the EQ-5D-5L was excellent at recruitment (385/388, > 99%) but fell to 269 out of 382 (70%) at 15 days and 242 out of 377 (64%) at 4–8 weeks. Again, response rates were similar across the three treatment groups. The response rate for the participant-completed WHQ was similar, at 254 out of 378 (67%). Face-to-face assessments were higher: 303 out of 377 (80%) had a face-to-face SSI reference assessment and 281 out of 378 (74%) had an observer-completed WHQ assessment. The participation rate in the reference assessment was highest for patients allocated to no dressing (107/128, 84%) and lowest for those who had a simple dressing (97/127, 76%) but this trend was not observed for the observer-completed WHQ assessment.
The SSI reference assessor was unblinded in 58 out of 302 (19%) assessments, most often for participants who had their wounds dressed with glue (31/100, 31%) and least often for those who had a simple dressing group (11/96, 11.5%). The overall unblinding percentage for observer-completed WHQ assessments was 22% (28/177) and was similar across the groups.
Completeness of data for outcomes anticipated to be measured in the main trial
Completeness of key data items by group is shown in Appendix 5 (see Table 45). (The same information is described by centre in Report Supplementary Material 15.) The reference SSI assessment, when done, was well completed. Wound complication data were completed for 326 out of 388 (84%) participants during the postoperative hospital stay and for 315 out of 378 (83%) participants at 4–8 weeks, with similar completion rates for the three wound-dressing groups.
Resource use data were well completed (> 97%), with the exception of data relating to the level of care that participants received during their postoperative stay, particularly the time of admission to a particular level of care, which was complete for approximately three-quarters of participants.
Objective B5: assess the appropriateness and feasibility of collecting a range of secondary outcomes and resource use data
Potential outcomes of a main trial are summarised by group in Table 31. (The same information is described by centre in Report Supplementary Material 15.) There were 51 out of 281 (18%) incident SSIs, four of which were reported in the period before discharge. The SSI rate to 4–8 weeks was similar in the three groups. EuroQol-5 Dimensions (EQ-5D) utility scores suggested that, on average, quality of life at 15 days was lower than before surgery, but that it then improved to close to pre-surgery levels by 4–8 weeks.
| Summary | Group | Overall (N = 388) | ||
|---|---|---|---|---|
| Simple dressing (N = 131) | Glue-as-a-dressing (N = 126) | No dressing (N = 131) | ||
| EQ-5D score, median (IQR) | ||||
| Baselinea | 0.92 (0.83–0.98) | 0.92 (0.80–1.00) | 0.94 (0.87–1.00) | 0.92 (0.83–1.00) |
| 15 daysb | 0.84 (0.74–0.89) | 0.84 (0.76–0.89) | 0.87 (0.75–0.94) | 0.84 (0.75–0.90) |
| 4–8 weeksc | 0.89 (0.79–0.95) | 0.90 (0.84–1.00) | 0.94 (0.86–1.00) | 0.89 (0.82–1.00) |
| SSI in-hospital, n/N (%) | 1/117 (0.9) | 1/111 (0.9) | 2/119 (1.7) | 4/347 (1.2) |
| SSI at 4- to 8-week reference assessment, n/N (%) | ||||
| None | 80/97 (82.5) | 83/98 (84.7) | 90/107 (84.1) | 253/302 (83.8) |
| Superficial | 14/97 (14.4) | 14/98 (14.3) | 17/107 (15.9) | 45/302 (14.9) |
| Deep | 3/97 (3.1) | 0/98 (0.0) | 0/107 (0.0) | 3/302 (1.0) |
| Organ/space | 0/97 (0.0) | 1/98 (1.0) | 0/107 (0.0) | 1/302 (0.3) |
| Any SSI (overall), n/N (%) | 17/92 (18.5) | 16/90 (17.8) | 18/99 (18.2) | 51/281 (18.1) |
Overall, 28 wound-related complications in 16 participants were reported during the postoperative stay. Of these, nine occurred in the simple dressing group (n = 9 participants), two were in the glue-as-a-dressing group (n = 1 participant) and 17 occurred in the no dressing group (n = 6 participants) (see Appendix 5, Table 46). Six out of the 28 were classified as serious events (one each of leakage requiring vacuum dressing, sepsis, pyrexia and necrotising fasciitis and two reoperations for wound problems). All serious wound-related events were in the no dressing group. In addition, there were two deaths, one in the simple dressing group and one in the glue-as-a-dressing group. Wound-related adverse events (AEs) were also captured at the 4- to 8-week follow-up. A total of 138 events were reported in 73 participants. Twenty-one events in eight patients were classified as serious (see Appendix 5, Table 47).
Health economics
The numbers of available observations for the five cost categories are described in Appendix 6 (see Table 48). The percentage of participants who contributed data for each of these categories ranged from 81% (for primary care appointments and use of medicines) to 98% (for dressings taking place during the follow-up period) of the total number of participants. The CCA is reported below and the ACA in Appendix 6 (see Tables 47–49 and Figure 26).
Complete-case analysis
Complete-case analysis comprises patients for whom all data are available. Effectively, this involves excluding patients for whom data on one or more cost categories are missing.
Overall, the mean per-patient cost was higher in the ‘no dressing’ group (approximately £147) than in the ‘simple’ and ‘glue-as-a-dressing’ groups. Participants allocated to simple dressing were associated with a mean per-patient cost of about £146, whereas those who were randomised to receive ‘glue-as-a-dressing’ presented the lowest mean cost (£118) (Table 32).
| Allocated intervention | Cost (£) | ||
|---|---|---|---|
| Mean | SEa | 95% CI | |
| No dressing | 147.13 | 68.05 | 13.76 to 280.50 |
| Simple dressing | 145.69 | 32.87 | 81.26 to 210.11 |
| Glue | 118.19 | 16.58 | 85.70 to 150.68 |
The cost associated with each resource use category by treatment group is detailed in Table 33 and depicted in Figure 9. In the ‘no-dressing’ group, the greatest share of the total per-patient cost is attributed to hospital visits after discharge, followed closely by the cost of new medicines. In the ‘simple dressing’ group, the main contributor to the total cost is the cost of new medicines, followed by the cost of in-hospital dressings and redressings, and the cost of hospital appointments after the initial discharge. In the ‘glue-as-a-dressing’ group, the largest contribution to the total cost is the cost of dressings and redressings in the hospital, followed by the costs associated with primary care appointments and new medicines.
| Cost category | Number of observations | Percentage of all patients (n = 388) | Total cost per patient (£) | Total cost by allocated intervention (£) | |||||
|---|---|---|---|---|---|---|---|---|---|
| No dressing | n | Simple dressing | n | Glue-as-a dressing | n | ||||
| Cost of dressings and redressings in hospital | 268 | 69 | 33.54 | 6.86 | 96 | 42.72 | 84 | 53.89 | 88 |
| Cost of hospital appointments after initial discharge | 268 | 69 | 34.36 | 59.99 | 96 | 23.59 | 84 | 16.66 | 88 |
| Cost of primary care appointments after initial discharge | 268 | 69 | 16.54 | 14.99 | 96 | 15.29 | 84 | 19.45 | 88 |
| Cost of redressings after initial discharge incurred by NHS | 268 | 69 | 8.21 | 4.66 | 96 | 11.17 | 84 | 9.25 | 88 |
| Cost of redressings after initial discharge incurred by patients | 268 | 69 | 0.38 | 0.04 | 96 | 0.59 | 84 | 0.56 | 88 |
| Cost of complications arising while in hospital | 268 | 69 | 1.15 | 3.22 | 96 | 0.00 | 84 | 0.00 | 88 |
| Cost of new medicines | 268 | 69 | 42.99 | 57.38 | 96 | 52.33 | 84 | 18.39 | 88 |
| Total cost (CCA) | 268 | 137.18 | 147.13 | 96 | 145.69 | 84 | 118.19 | 88 | |
FIGURE 9.
Total cost by cost category and allocated intervention (CCA).
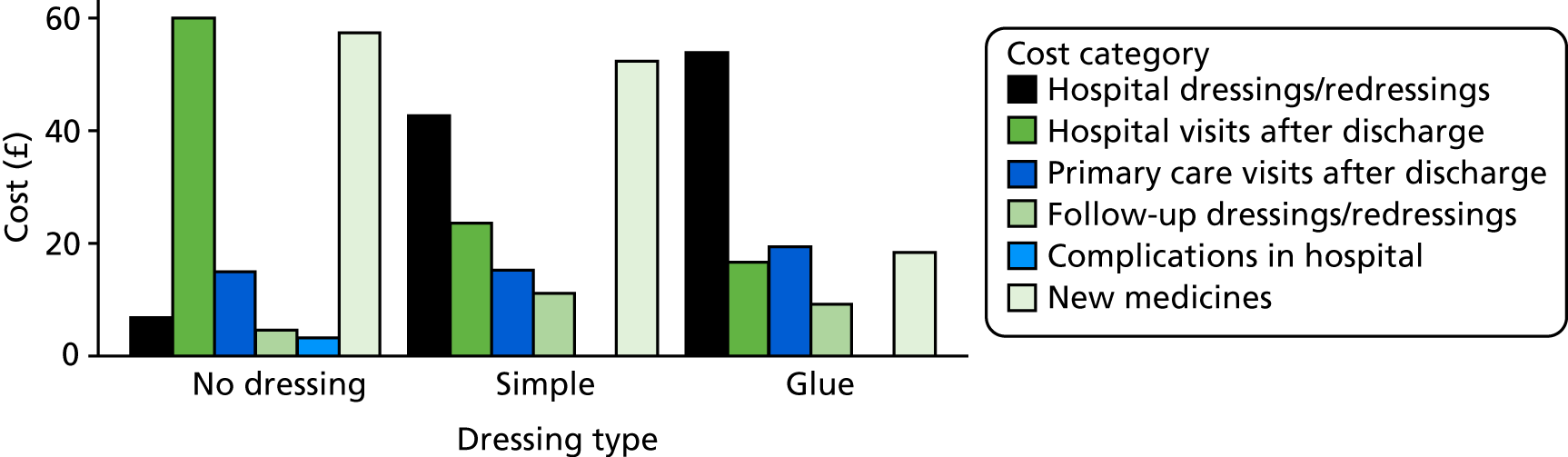
Differences in mean per-patient cost between trial groups are presented in Table 34. Although the mean cost differences range from approximately £20 (for no dressing vs. simple dressing) to > £50 (for no dressing vs. glue-as-a-dressing), none of these differences reached statistical significance.
| Allocated intervention | Cost difference (£) | p-value | 95% CI (£)a |
|---|---|---|---|
| No dressing vs. simple dressing | 1.45 | 0.985 | –146.87 to 149.76 |
| No dressing vs. glue | 28.94 | 0.673 | –105.56 to 163.44 |
| Simple dressing vs. glue | 27.49 | 0.459 | –45.30 to 100.28 |
Discussion
The findings of this pilot study showed that it is feasible to obtain detailed information on relevant resource use categories, including the type of dressings used, the frequency of dressing changes and use of health-care resources in response to wound-related problems. The proportion of available data ranged from slightly over 80% to 98% for all categories of resource use; a future full trial could use imputation methods to ameliorate problems of missing data.
The fact that much of the information for the cost analysis was sourced from CRFs meant that participants were called to provide information only in cases in which this was not available through routine sources, which avoids placing a great burden on participants. 103 This, and the short time period within which this information was collected, helped to alleviate the issue of recall bias, that is, bias arising from the fact that respondents are asked to recall all instances of health-care use based on memory. 104
The analysis showed that some additional information would be useful to collect in a definitive trial. In particular, more detailed information is needed around the place where dressing changes were performed during the follow-up period (e.g. at a patient’s home, during a follow-up appointment), as well as detailed information on the person who carried out (or assisted with performing) the dressing change in the hospital (e.g. grade of hospital-based nurse) or at a patient’s home (e.g. participant, family member, community nurse). It would also have been useful to have details on additional care provision for non-serious SSIs. More details around patients’ travels to hospitals would allow a better appreciation of the costs associated with such trips.
Translating the use of resources into costs showed that ‘glue-as-a-dressing’ was associated with the lowest cost, followed by ‘simple dressing’ and ‘no dressing’, in this small-scale feasibility study. Cost drivers varied across dressing options, although it is evident that key cost drivers were hospital appointments, dressings and redressings, use of new medicines and primary care appointments. As expected, the cost of dressings and redressings made a sizeable contribution to the total cost associated with ‘simple dressing’ and ‘glue-as-a-dressing’, although, unsurprisingly, it did not have a notable influence in the total cost for the ‘no-dressing’ group. In general, instances of care that are particularly costly were rare.
Overall, the collection of economic data for this trial proved feasible and the major cost categories that should be focused on in a future trial have been established.
Objective B6: establish the validity and reliability of the developed tools for assessing wounds for surgical site infection
Data sets
In total, data from 792 and 791 participants from the cohort study and pilot RCT contributed to the WHQ self-assessment and observer-assessment validation analyses, respectively (Figure 10).
FIGURE 10.
Phase A and Phase B participant data contributing to validation of the WHQ.
Analyses
Wound Healing Questionnaire adherence and acceptability
Data were available for 561 out of 792 (70.8%) participant self-assessments and 597 out of 791 (74.4%) observer assessments (see Appendix 7, Figure 25). A self-assessment and an observer assessment were available for 470 out of 791 (59.4%) participants. A self-assessment or an observer assessment was available for 688 out of 791 (87.0%) participants, meaning that 104 out of 791 (13.1%) participants did not have a self-assessment or an observer assessment available at all. SSI diagnoses (reference assessments) were available for 417 out of 791 (52.7%) participants. Patient self-assessments were a median of 29 days [interquartile range (IQR) 24–33 days] after surgery. Observer assessments were a median of 37 days (IQR 32–48 days) after surgery.
Demographics for participants contributing to the WHQ validation (combined cohort and RCT participants) are described in Appendix 7 (see Table 50). Demographics of participant responders (patients who completed a WHQ self-assessment) are reported in Appendix 7 (see Table 51). Participants who did not complete a self-assessment and for whom no observer assessment was obtained (i.e. it was not possible to conduct a telephone or face-to-face follow-up assessment) are also described, demonstrating characteristics of complete non-responders to the WHQ.
Feasibility and practicality
Completion of the WHQ took < 10 minutes for 91% of participants. Less than 6% of participants required help to complete any of the items or found any of the items difficult or confusing to answer (see Appendix 7, Table 52). Reasons for missing data included, for example, not being able to see the wound.
Missing data
Missing data are described separately for core items and subitems.
Core items (1–16)
Missing data for core items 1–16 within completed WHQs were few (Table 35). Patient self-assessments had < 2.7% missing data for 10 out of 16 core items and < 4% for all core items. Observer assessments had < 2.0% missing data for all core items, with the exception of one for which the proportion of missing data was 3.7% (item 11 on hospital readmission. The relatively high numbers of missing data for this item compared with for other items may be explained by error as a result of the questionnaire layout; item 11 was the last item on the second page.
| Item/description | Response, n (%) | ||||
|---|---|---|---|---|---|
| Not at all | A little | Quite a bit | A lot | Missinga | |
| 1. Was there redness spreading away from the wound? (erythema/cellulitis) | |||||
| Participant self-assessments | 314 (56.78) | 192 (34.72) | 36 (6.51) | 11 (1.99) | 8 (1.43) |
| Observer assessments | 416 (69.80) | 130 (21.81) | 33 (5.54) | 17 (2.85) | 1 (0.17) |
| 2. Was the area around the wound warmer than the surrounding skin? | |||||
| Participant self-assessments | 312 (57.56) | 189 (34.87) | 32 (5.90) | 9 (1.66) | 19 (3.39) |
| Observer assessments | 444 (74.50) | 108 (18.12) | 34 (5.70) | 10 (1.68) | 1 (0.17) |
| 3. Was any part of the wound leaking fluid? | |||||
| Participant self-assessments | 351 (63.93) | 125 (22.77) | 45 (8.20) | 28 (5.10) | 12 (2.14) |
| Observer assessments | 419 (71.02) | 116 (19.66) | 26 (4.41) | 29 (4.92) | 7 (1.17) |
| 3a. Was it clear fluid? (serous exudate) | |||||
| Participant self-assessments | 48 (40.34) | 51 (42.86) | 17 (14.29) | 3 (2.52) | 86 (43.43) |
| Observer assessments | 80 (51.61) | 58 (37.42) | 10 (6.45) | 7 (4.52) | 23 (13.45) |
| 3b. Was it blood-stained fluid? (haemoserous exudate) | |||||
| Participant self-assessments | 45 (28.48) | 77 (48.73) | 24 (15.19) | 12 (7.59) | 47 (23.74) |
| Observer assessments | 77 (46.67) | 56 (33.94) | 18 (10.91) | 14 (8.48) | 14 (8.19) |
| 3c. Was it thick and yellow/green fluid? (pus/purulent exudate) | |||||
| Participant self-assessments | 71 (57.26) | 27 (21.77) | 18 (14.52) | 8 (6.45) | 81 (40.61) |
| Observer assessments | 96 (61.94) | 30 (19.35) | 13 (8.39) | 16 (10.32) | 23 (13.45) |
| 4. Have the edges of any part of the wound separated/gaped open of their own accord? (spontaneous dehiscence) | |||||
| Participant self-assessments | 423 (78.04) | 93 (17.16) | 17 (3.14) | 9 (1.66) | 19 (3.39) |
| Observer assessments | 489 (83.59) | 76 (12.99) | 11 (1.88) | 9 (1.54) | 12 (2.01) |
| 4a. Did the skin separate? | |||||
| Participant self-assessments | 41 (28.52) | 78 (52.35) | 20 (13.42) | 10 (6.71) | 6 (5.04) |
| Observer assessments | 17 (15.60) | 71 (65.14) | 11 (10.09) | 10 (9.17) | 6 (6.25) |
| 4b. Did the deeper tissue separate? | |||||
| Participant self-assessments | 93 (75.61) | 15 (12.20) | 11 (8.94) | 4 (3.25) | 27 (22.69) |
| Observer assessments | 77 (79.38) | 10 (10.31) | 4 (4.12) | 6 (6.19) | 16 (16.67) |
| 5. Has the area around the wound become swollen? | |||||
| Participant self-assessments | 345 (63.07) | 160 (29.25) | 35 (6.40) | 7 (1.28) | 14 (2.50) |
| Observer assessments | 481 (80.70) | 96 (16.11) | 12 (2.01) | 7 (1.17) | 1 (1.17) |
| 6. Has the wound been smelly? | |||||
| Participant self-assessments | 488 (90.54) | 36 (6.68) | 9 (1.67) | 6 (1.11) | 22 (3.92) |
| Observer assessments | 547 (91.78) | 31 (5.20) | 13 (2.18) | 5 (0.84) | 1 (0.17) |
| 7. Has the wound been painful to touch? | |||||
| Participant self-assessments | 207 (37.77) | 274 (50.00) | 50 (9.12) | 17 (3.10) | 13 (2.32) |
| Observer assessments | 351 (58.79) | 180 (30.15) | 51 (8.54) | 15 (2.51) | 0 (0) |
| 8. Have you had, or felt like you have had, a raised temperature or fever? (fever ≥ 38 °C) | |||||
| Participant self-assessments | 462 (85.40) | 57 (10.54) | 11 (2.03) | 11 (2.03) | 20 (3.57) |
| Observer assessments | 524 (87.92) | 37 (6.21) | 15 (2.52) | 20 (3.36) | 1 (0.17) |
| No | Yes | Missing | |||
| 9. Have you sought advice because of a problem with your wound, other than at a routine planned follow-up appointment? | |||||
| Participant self-assessments | 396 (71.10) | 161 (28.90) | N/A (N/A) | N/A (N/A) | 4 (0.71) |
| Observer assessments | 442 (75.04) | 147 (24.96) | N/A (N/A) | N/A (N/A) | 8 (1.34) |
| 10. Has anything been put on the skin to cover the wound? (dressing) | |||||
| Participant self-assessments | 333 (60.00) | 222 (40.00) | N/A (N/A) | N/A (N/A) | 6 (1.07) |
| Observer assessments | 396 (66.78) | 197 (33.22) | N/A (N/A) | N/A (N/A) | 4 (0.67) |
| 11. Have you been back into hospital for treatment with a problem with your wound? | |||||
| Participant self-assessments | 514 (94.49) | 30 (5.51) | N/A (N/A) | N/A (N/A) | 17 (3.03) |
| Observer assessments | 548 (95.30) | 27 (4.70) | N/A (N/A) | N/A (N/A) | 22 (3.69) |
| No | Yes | Don’t know | Missing | ||
| 12. Have you been given antibiotics for a problem with your wound? | |||||
| Participant self-assessments | 463 (83.88) | 82 (14.86) | 7 (1.27) | N/A (N/A) | 9 (1.60) |
| Observer assessments | 511 (86.32) | 81 (13.68) | 0 (0) | N/A (N/A) | 5 (0.84) |
| 13. Have the edges of your wound been deliberately separated by a doctor or nurse? | |||||
| Participant self-assessments | 532 (96.03) | 16 (2.89) | 6 (1.08) | N/A (N/A) | 7 (1.25) |
| Observer assessments | 572 (96.46) | 21 (3.54) | 0 (0) | N/A (N/A) | 4 (0.67) |
| 14. Has your wound been scraped or cut to remove any unwanted tissue? (debridement of wound) | |||||
| Participant self-assessments | 539 (98.36) | 6 (1.09) | 3 (0.55) | N/A (N/A) | 13 (2.32) |
| Observer assessments | 588 (98.99) | 6 (1.01) | 0 (0) | N/A (N/A) | 3 (0.50) |
| 15. Has your wound been drained? (drainage of pus/abscess) | |||||
| Participant self-assessments | 518 (95.40) | 21 (3.87) | 4 (0.74) | N/A (N/A) | 18 (3.21) |
| Observer assessments | 580 (97.97) | 11 (1.86) | 1 (0) | N/A (N/A) | 5 (0.84) |
| 16. Have you had an operation under general anaesthetic for treatment of a problem with your wound? | |||||
| Participant self-assessments | 542 (99.27) | 2 (0.37) | 2 (0.37) | N/A (N/A) | 15 (2.67) |
| Observer assessments | 590 (100.0) | 0 (0) | 0 (0) | N/A (N/A) | 7 (1.17) |
Subitems (3a–c and 4a and b)
Subitems followed core items 3 and 4, asking further information on signs/symptoms of leaking fluid and wound dehiscence. Data showed that completion of these subitems did not always occur as intended and the degree of missing data was markedly higher than for any of the core items. As many as 43% of participants missed a subitem in self-assessments for which a response would have been expected (see Table 35). In some cases, responders appeared to select only the relevant subitems (e.g. ‘Was it clear fluid?’) and responses to other subitems (e.g. ‘Was it blood-stained fluid?’ and ’Was it thick and yellow/green fluid?’) were left missing rather than marked ‘not at all’.
The number of missing data and frequency of incorrectly completed subitems highlighted a need to reconsider the use of subitems and/or the layout of the questionnaire in order to minimise missing data.
Distribution of responses
Most patients reported no or little experience of signs or symptoms, and the spread of responses across the categories was skewed (Table 35). The most ‘severe’ wound care interventions, for example debridement of the wound and reoperation under general anaesthetic (items 14 and 16), were very rare within the study sample. Only two patients (in the cohort study) reported that they had had an operation under general anaesthetic for treatment with a problem with their wound. Observer assessments verified neither of these cases.
Participant and observer agreement (inter-rater reliability)
Data from a self-assessment and an observer assessment were available for 470 out of 791 (59.4%) participants. The median time between self-assessments and observer assessments was 8 days (IQR 2–16 days).
Agreement between self-assessments and observer assessments for signs and symptoms (items 1–8) was generally high, although patients showed a trend to report signs and symptoms slightly more severely than observers. This is demonstrated in Figure 11.
FIGURE 11.
Bar charts comparing responses to the participant self-assessment and the observer assessment, for participants with data from both assessments (n = 470). (a) Item 4b: Did the deeper tissue separate?; (b) Item 5: Has the area around the wound become swollen?; (c) Item 6: Has the wound been smelly?; (d) Item 7: Has the wound been painful to touch?; (e) Item 8: Have you had, or felt like you have had, a raised temperature or fever (fever ≥ 38 °C)?; (f) Item 9: Have you sought advice because of a problem with your wound?; (g) Item 10: Has anything been put on the skin to cover the wound? (dressing); (h) Item 11: Have you been back into hospital for treatment of a problem with your wound?; (i) Item 12: Have you been given antibiotics for a problem with your wound?; (j) Item 13: Have the edges of your wound been deliberately separated by a doctor or nurse?; (k) Item 14: Has your wound been scraped or cut to remove any unwanted tissue (debridement of wound)?; (l) Item 15: Has your wound been drained (drainage of pus/abscess)?; and (m) Item 16: Have you had an operation under general anaesthetic for treatment of a problem with your wound?
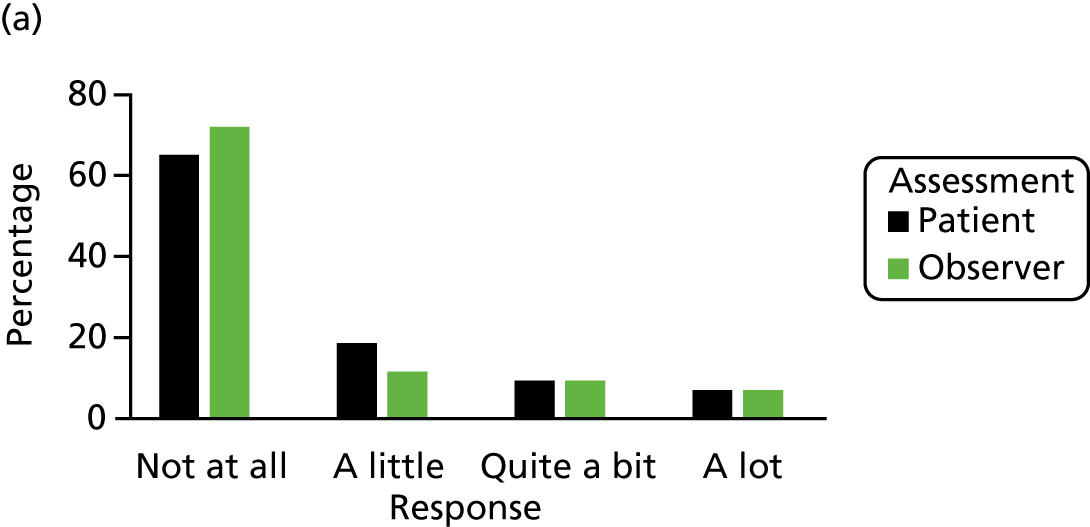
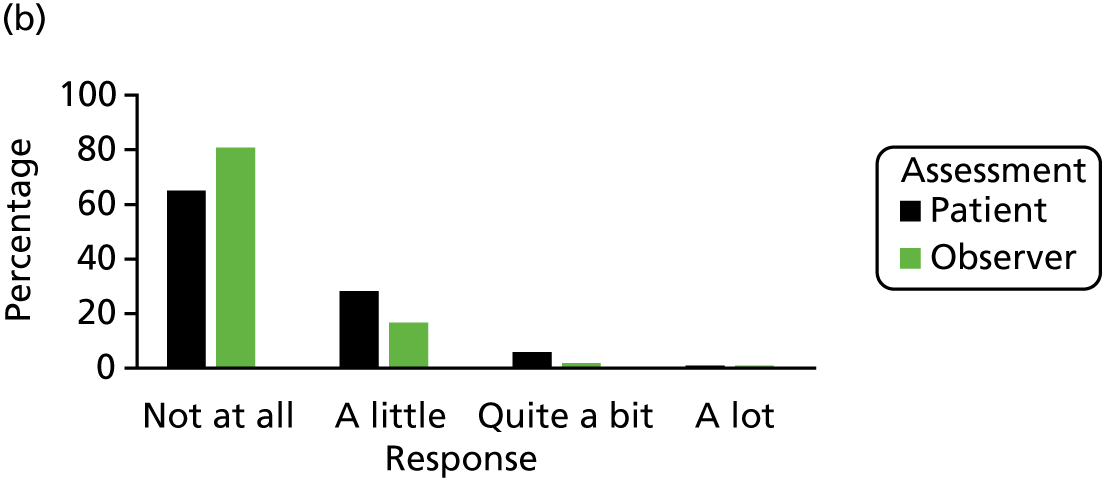
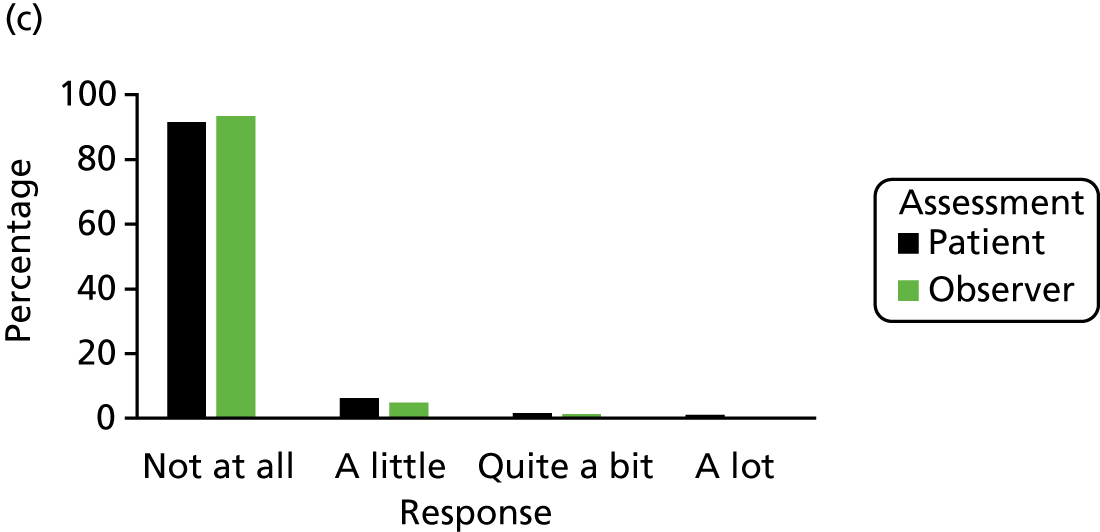
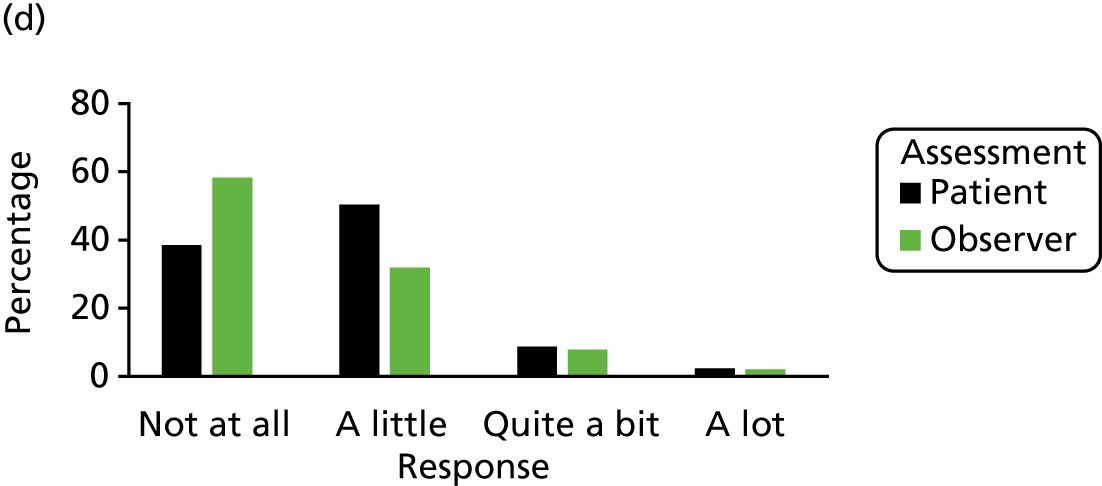
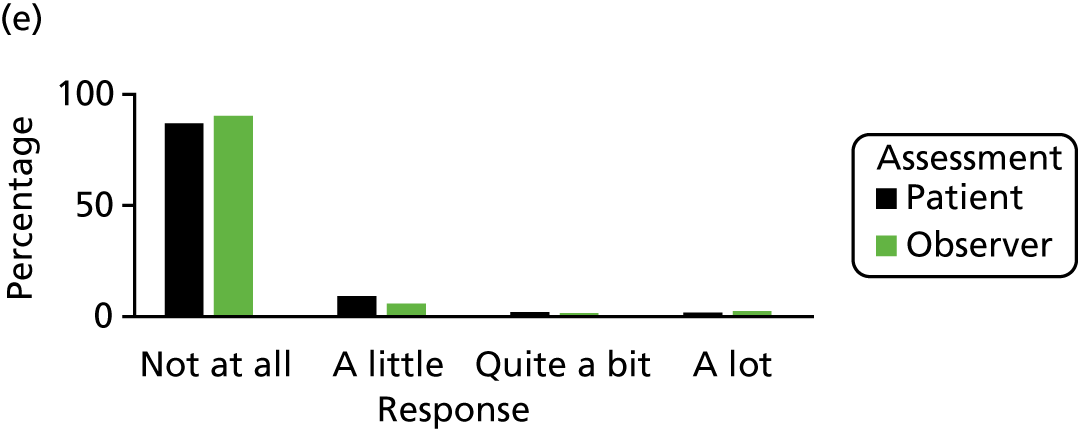
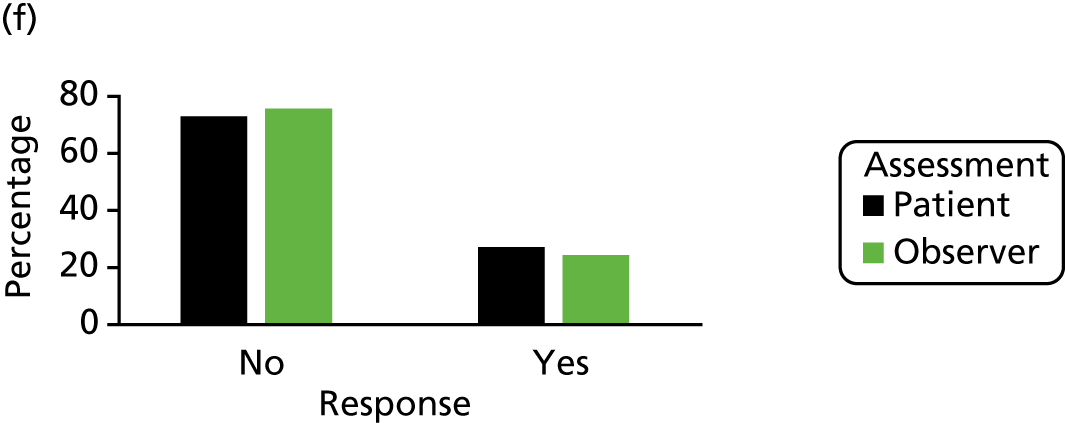
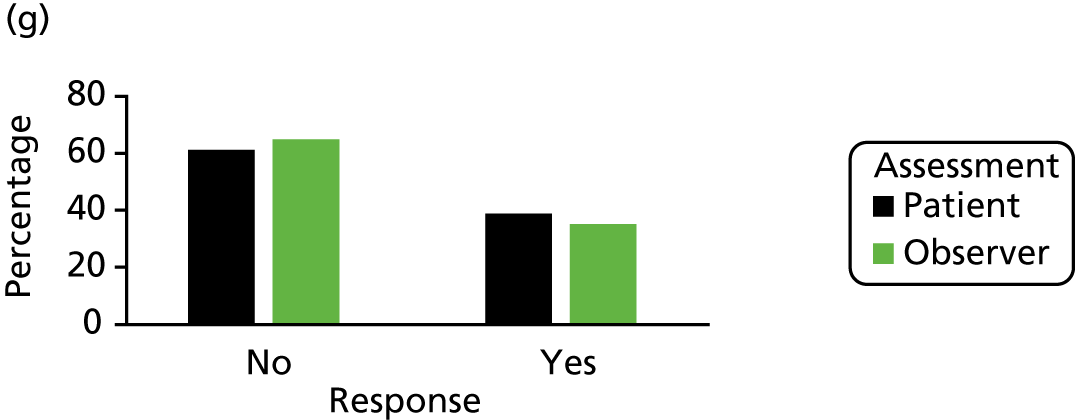
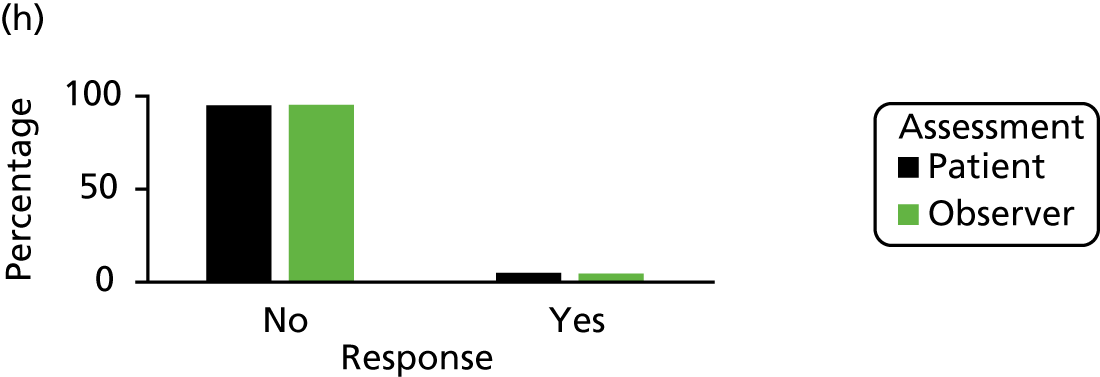
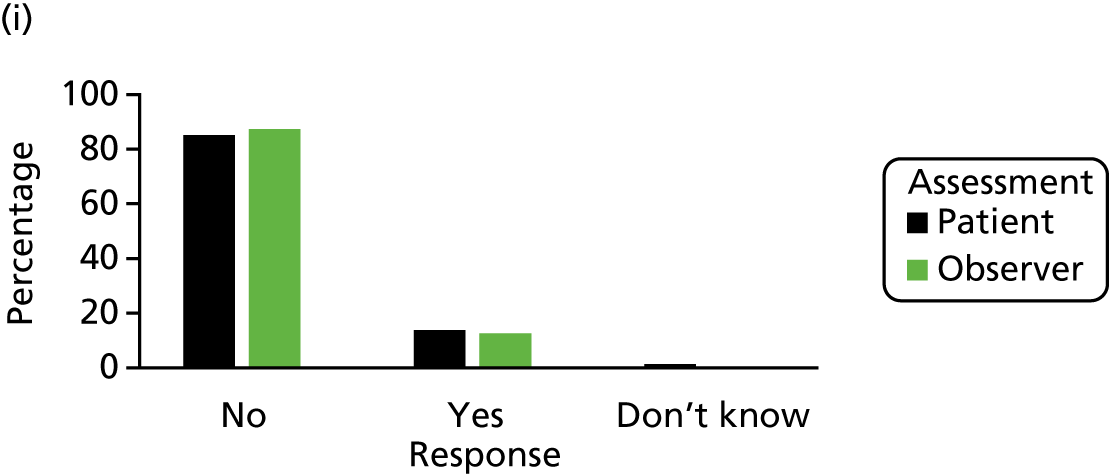
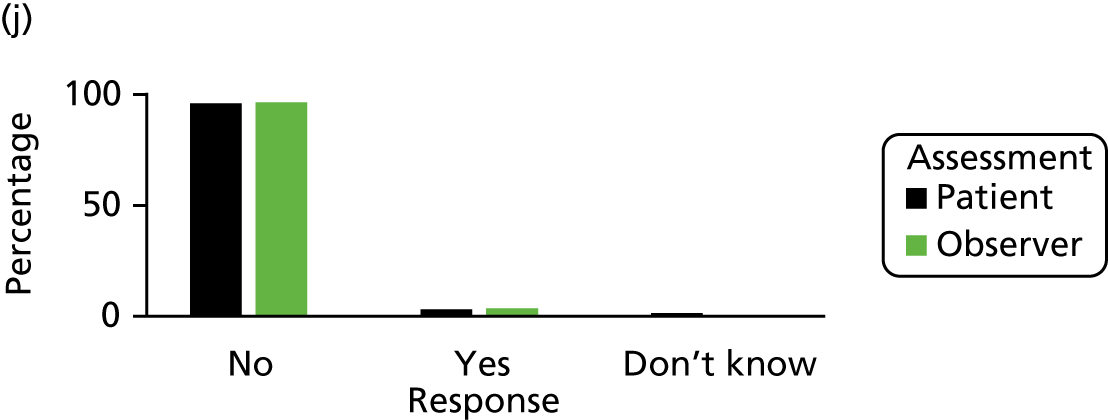
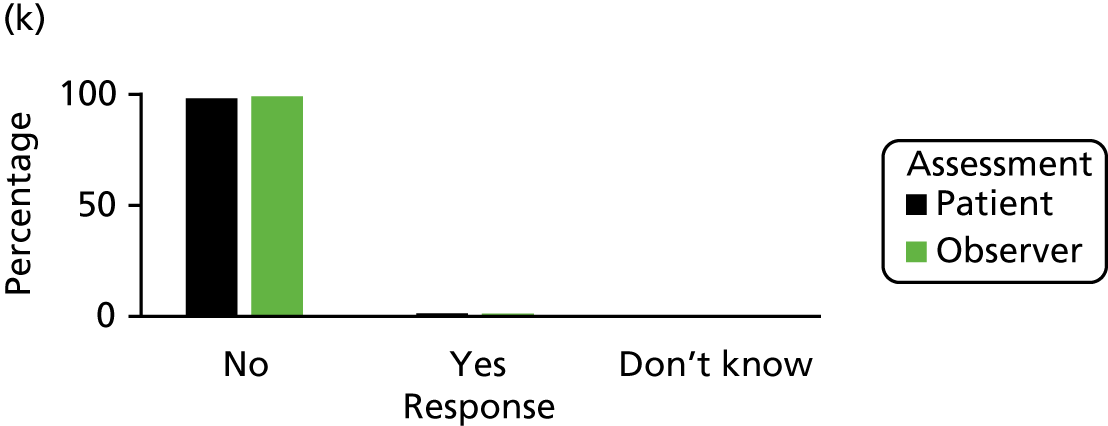
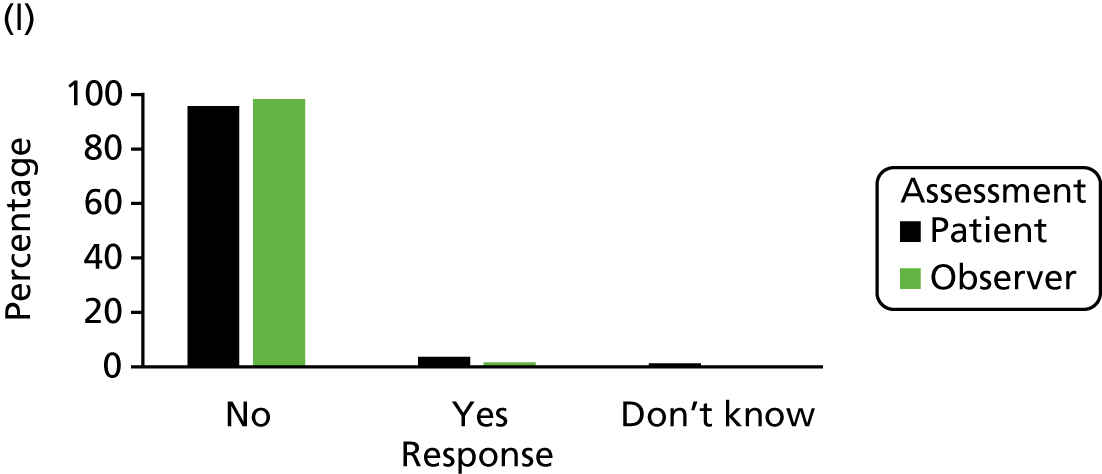
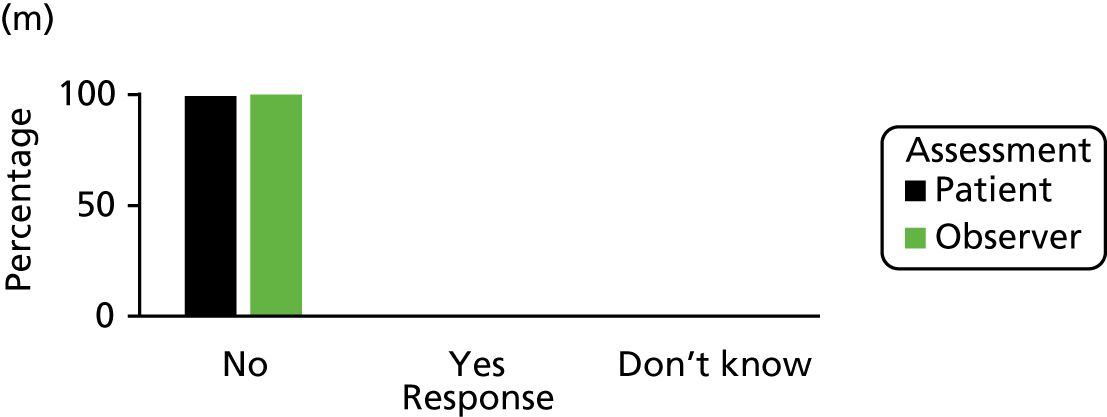
Although cases were rare, examination of the raw data showed that there was slight discrepancy in agreement between self-assessments and observer assessments for some of the intervention items, with patient reports of having had that intervention not being supported by the observer assessment (see Report Supplementary Material 16). For example, six patients responded that their wound had been scraped or cut to remove unwanted tissue (item 14). Observer data, however, agreed with only two of these cases. Furthermore, observer assessments implied that another two patients had had debridement of their wound, which was not indicated by the patients themselves.
Test–retest agreement: intrapatient reliability
In total, 44 out of 50 (88.0%) participants included in the test–retest sample completed and returned a second WHQ. Median time between assessments was 5 days (IQR 4–7 days). No participants were excluded based on the checks for stability in health. Observed agreement between assessments was high, with > 86% agreement in responses for all items. When numbers were sufficient to produce kappa statistics, values were > 0.6 for 9 out of 13 core items (Table 36).
| Item | N | % observed agreement | % expected agreement | Weighted kappa | % agreed responses that were ‘not at all’/’no’ | Missing | |
|---|---|---|---|---|---|---|---|
| 1. | Was there redness spreading away from the wound? (erythema/cellulitis) | 41 | 86.18 | 80.11 | 0.3051 | 39.02 |
1 (first test) 2 (retest) |
| 2. | Was the area around the wound warmer than the surrounding skin? | 38 | 88.60 | 82.46 | 0.3500 | 47.37 |
2 (first test) 5 (retest) |
| 3. | Was any part of the wound leaking fluid? | 43 | 92.25 | 73.79 | 0.7043 | 55.81 | 1 (retest) |
| 3a. | Was it clear fluid? (serous exudate) | 4 | 100.00 | 87.50 | 1.000 | 75.00 |
6 (first test) 9 (retest) |
| 3b. | Was it blood-stained fluid? (haemoserous exudate) | 11 | 93.94 | 74.10 | 0.7660 | 14.29 |
2 (first test) 3 (retest) |
| 3c. | Was it thick and yellow/green fluid (pus/purulent exudate) | 5 | 86.67 | 54.67 | 0.7059 | 14.29 |
5 (first test) 6 (retest) |
| 4. | Have the edges of any part of the wound separated/gaped open on their own accord? (spontaneous dehiscence) | 43 | 97.67 | 84.41 | 0.8509 | 74.42 | 1 (retest) |
| 4a. | Did the skin separate? | 8 | 100.00 | 87.50 | 1.00 | 75.00 | 1 (first test) |
| 4b. | Did the deeper tissue separate? | 6 | 100.00 | 87.50 | 1.00 | 83.33 |
3 (first test) 3 (retest) |
| 5. | Has the area around the wound become swollen? | 44 | 93.18 | 81.82 | 0.6250 | 59.09 | 0 |
| 6. | Has the wound been smelly? | 43 | 97.67 | 96.34 | 0.3645 | 90.70 |
1 (first test) 1 (retest) |
| 7. | Has the wound been painful to touch? | 43 | 91.47 | 79.43 | 0.5854 | 37.21 | 1 (retest) |
| 8. | Have you had, or felt like you have had, a raised temperature or fever? (fever ≥ 38 °C) | 43 | 96.12 | 93.74 | 0.3804 | 83.72 | 1 (retest) |
| 9. | Have you sought advice because of a problem with your wound, other than at a routine planned follow-up appointment? | 43 | 86.05 | 55.98 | 0.6830 | 60.47 | 1 (first test) |
| 10. | Has anything been put on the skin to cover the wound? (dressing) | 44 | 97.73 | 50.62 | 0.9540 | 43.18 | 0 |
| 11. | Have you been back into hospital for treatment with a problem with your wound? | 44 | 100.00 | 83.47 | 1.000 | 90.91 | 0 |
| 12. | Have you been given antibiotics for a problem with your wound? | 43 | 95.35 | 75.88 | 0.8072 | 83.72 | 1 (retest) |
| 13. | Have the edges of your wound been deliberately separated by a doctor or nurse? | 43 | 97.67 | 97.67 | 0.0000 | 95.45 | 1 do not know (retest) |
| 14. | Has your wound been scraped or cut to remove any unwanted tissue? (debridement of wound) | 39 | 94.87 | 95.00 | –0.0263 | 92.50 |
1 (first test) 3 (retest) 1 do not know (retest) |
| 15. | Has your wound been drained? (drainage of pus/abscess) | 41 | 100.00 | 95.24 | 1.000 | 97.56 |
1 (first test) 2 (retest) |
| 16. | Have you had an operation under general anaesthetic for treatment of a problem with your wound?a | 40 | 100.00 | – | – | 100.00 |
2 (first test) 2 (retest) |
Scale structure of the Wound Healing Questionnaire
Responses for item 4 and subitem 4a were very highly correlated (> 0.9 in both patient and observer data), indicating that these items were similar or overlapping. A cross-tabulation of the data supported this, showing that responses to item 4, ‘Have the edges of any part of the wound separated/gaped open on their own accord? (spontaneous dehiscence)’, corresponded to responses to item 4a, ‘Did the skin separate?’. Subitem 4a was therefore deemed redundant and excluded from further analysis of the scale structure.
Factor analysis results from a single-factor model (item 4a removed) are shown in Table 37. Data from both participant self-assessments and observer assessments fitted a single scale structure satisfactorily. All factor loadings of item variables were positive. Rarely reported items 14 (‘Has your wound been scraped or cut to remove any unwanted tissue?’) and 16 (‘Have you had an operation under general anaesthetic for treatment with a problem with your wound?’) did not fit the model well or were dropped by the software because these interventions occurred at very low frequency in the data set. There was little evidence in support of a better fit for a two- or three-factor model, and item factor loadings were not substantially larger on a second or third factor when these models were explored. Parameters from a three-factor model are shown for comparison in Report Supplementary Material 17.
| Item | Patient self-assessment (n = 201) | Observer assessment (n = 299) | |
|---|---|---|---|
| Factor 1 | Factor 1 | ||
| Eigenvalue | 4.67 | 5.09 | |
| 1. | Was there redness spreading away from the wound? (erythema/cellulitis) | 0.4178 | 0.6425 |
| 2. | Was the area around the wound warmer than the surrounding skin? | 0.2234 | 0.5743 |
| 3. | Was any part of the wound leaking fluid? | 0.8685 | 0.8110 |
| 3a. | Was it clear fluid? (serous exudate) | 0.5127 | 0.3917 |
| 3b. | Was it blood-stained fluid? (haemoserous exudate) | 0.7467 | 0.6262 |
| 3c. | Was it thick and yellow/green fluid (pus/purulent exudate) | 0.4642 | 0.6368 |
| 4. | Have the edges of any part of the wound separated/gaped open on their own accord? (spontaneous dehiscence) | 0.5620 | 0.6980 |
| 4b. | Did the deeper tissue separate? | 0.4837 | 0.5110 |
| 5. | Has the area around the wound become swollen? | 0.1724 | 0.3231 |
| 6. | Has the wound been smelly? | 0.4456 | 0.3524 |
| 7. | Has the wound been painful to touch? | 0.2701 | 0.3063 |
| 8. | Have you had, or felt like you have had, a raised temperature or fever? (fever ≥ 38 °C) | 0.2962 | 0.4221 |
| 9. | Have you sought advice because of a problem with your wound, other than at a routine planned follow-up appointment? | 0.5934 | 0.5963 |
| 10. | Has anything been put on the skin to cover the wound? (dressing) | 0.4072 | 0.5158 |
| 11. | Have you been back into hospital for treatment with a problem with your wound? | 0.5028 | 0.3712 |
| 12. | Have you been given antibiotics for a problem with your wound? | 0.6599 | 0.6845 |
| 13. | Have the edges of your wound been deliberately separated by a doctor or nurse? | 0.4134 | 0.3891 |
| 14. | Has your wound been scraped or cut to remove any unwanted tissue? (debridement of wound) | 0.2894 | 0.0426 |
| 15. | Has your wound been drained? (drainage of pus/abscess) | 0.5106 | 0.3289 |
| 16.a | Have you had an operation under general anaesthetic for treatment of a problem with your wound? | – | – |
A single scale structure (without item 4a) was further tested with data from the RCT. The single structure was supported with higher factor loadings of items than for Phase A data (Table 38).
| Item | Patient self-assessment (n = 161) | Observer assessment (n = 211) | |
|---|---|---|---|
| Factor 1 | Factor 1 | ||
| Eigenvalue | 6.43 | 5.07 | |
| 1. | Was there redness spreading away from the wound? (erythema/cellulitis) | 0.4811 | 0.6664 |
| 2. | Was the area around the wound warmer than the surrounding skin? | 0.4117 | 0.5708 |
| 3. | Was any part of the wound leaking fluid? | 0.8636 | 0.9299 |
| 3a. | Was it clear fluid? (serous exudate) | 0.6070 | 0.5172 |
| 3b. | Was it blood-stained fluid? (haemoserous exudate) | 0.6816 | 0.3994 |
| 3c. | Was it thick and yellow/green fluid (pus/purulent exudate) | 0.7096 | 0.7118 |
| 4. | Have the edges of any part of the wound separated/gaped open on their own accord? (spontaneous dehiscence) | 0.8425 | 0.4112 |
| 4b.a | Did the deeper tissue separate? | 0.7517 | – |
| 5. | Has the area around the wound become swollen? | 0.5067 | 0.4525 |
| 6. | Has the wound been smelly? | 0.5662 | 0.5885 |
| 7. | Has the wound been painful to touch? | 0.4448 | 0.4765 |
| 8. | Have you had, or felt like you have had, a raised temperature or fever? (fever ≥ 38 °C) | 0.5566 | 0.3571 |
| 9. | Have you sought advice because of a problem with your wound, other than at a routine planned follow-up appointment? | 0.6060 | 0.5042 |
| 10. | Has anything been put on the skin to cover the wound? (dressing) | 0.4545 | 0.5238 |
| 11. | Have you been back into hospital for treatment with a problem with your wound? | 0.4044 | 0.2865 |
| 12. | Have you been given antibiotics for a problem with your wound? | 0.6696 | 0.6648 |
| 13. | Have the edges of your wound been deliberately separated by a doctor or nurse? | 0.4334 | 0.4798 |
| 14. | Has your wound been scraped or cut to remove any unwanted tissue? (debridement of wound) | 0.4578 | –0.0036 |
| 15. | Has your wound been drained? (drainage of pus/abscess) | 0.0856 | 0.3835 |
| 16.b | Have you had an operation under general anaesthetic for treatment of a problem with your wound? | – | – |
Cronbach’s alpha coefficients for a single scale were 0.8 for patient data and 0.9 for observer data from the cohort data set. Cronbach’s alpha coefficients were 0.88 for both patient and observer data in the RCT data set.
Comparative multitrait scaling analyses are reported in Report Supplementary Material 18 and 19. Findings also supported a single scale structure.
Item reduction and modification
Evidence supported that subitem 4a was overlapping with core item 4 and therefore could be dropped from the WHQ. Large numbers of missing data for subitems 3a, 3b and 3c, where a response would have been expected, suggested that these items and the current layout of the questionnaire required modification.
Items 14 (referring to debridement) and 16 (referring to reoperation under general anaesthetic) were shown to be very rare interventions in this data set, showing poor fit in the underlying scale model and some discrepancy in the patient and observer reports. However, these items were considered to be highly relevant for SSI diagnosis and may occur more frequently in different surgical populations, justifying the decision to retain them in the WHQ.
Similarly, few (< 3%) participants reported that their wound had been smelly (item 6), and 4% of participants reported that they had had, or felt like they had had, a raised temperature or fever (item 8). Item-scale correlations and factors loadings for these items in a single scale structure were moderate. However, a conservative approach was taken to retain them in the questionnaire to allow for variability in future use in other patient populations.
Sensitivity and specificity of the Wound Healing Questionnaire for surgical site infection/no surgical site infection discrimination
A simple summated scoring system without weighted scores was used, supported by findings from the scaling analyses, which meant that the WHQ total score had a possible minimum and maximum range of 0–44. Actual scores in the data from self-assessments ranged between 0 and 30 (median 3; IQR 1–5).
A contingency table of raw data showing the number of patients scoring all levels of WHQ total score against the reference assessment SSI diagnosis is provided in Report Supplementary Material 19. Sensitivity and specificity for selected WHQ ‘cut-off’ scores are shown. Analyses showed that the sensitivity and specificity of the WHQ patient self-assessment for discriminating SSI/no SSI was high, with an area under the ROC curve of 0.9056 (95% CI 0.8271 to 0.9841) (Figure 12).
FIGURE 12.
Receiver operating characteristic curve for overall WHQ score (after item reduction) for discriminating SSI diagnosis compared with reference assessment (CDC SSI classification). Area under ROC curve = 0.9056.
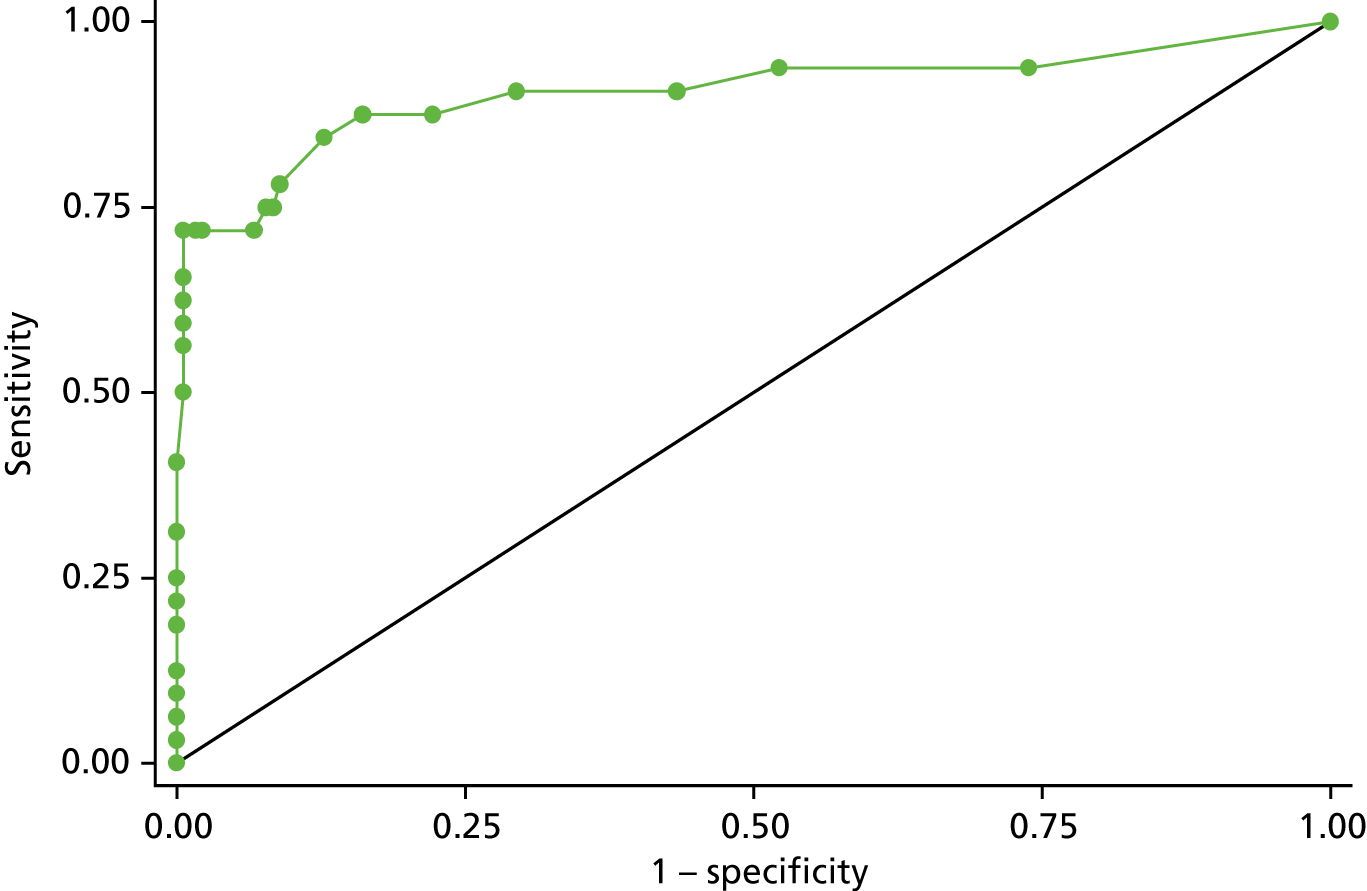
Revised Wound Healing Questionnaire
The findings from the validation study were considered and a revised version of items for the WHQ was produced (see Appendix 7).
Revisions were made as follows:
-
Subitems that were specific to collecting health economic data for the purpose of the pilot RCT (10a–d, 11a–e and 12a and b) were removed.
-
Subitem 4a (‘Did the skin separate?’) was removed owing to the overlap with core item 4, ‘Have the edges of any part of the wound separated/gaped open on their own accord?’ (spontaneous dehiscence).
-
Core item 3 (‘Was any part of the wound leaking fluid?’) and the associated subitems 3a–c were combined and restructured to form three separate items to minimise missing data and avoid overlap in responses.
-
Items were renumbered to accommodate these changes.
-
Any ‘do not know’ response categories were dropped.
Further testing and validation of the WHQ is now needed in the light of these minor revisions.
Objective B7: explore the feasibility of obtaining digital photographs of wounds in theatre after wound closure by theatre personnel, and after discharge by participants
Digital photographs after wound closure in theatre
As described above (see Objective A7: investigate the feasibility of photographing wounds in theatre and assessing the quality of wound closure), we did not attempt to implement obtaining digital wound photographs after wound closure in theatres for logistical reasons.
Digital photographs by participants after discharge
During the last month of recruitment, we piloted the submission of wound photographs taken by participants after discharge from hospital in three of the four participating trusts. Those participants who had consented to this substudy and who had e-mail addresses were sent instructions by post and then were e-mailed a secure web link, which invited them to upload a photograph securely to the study database; those who did not submit a photograph were subsequently sent a reminder. The flow of participants during this pilot is shown in Appendix 8 (see Figure 27). Of the 26 participants who consented to the substudy and provided an e-mail address, nine (35%) submitted a photograph.
Objective B8: work with the patient and public involvement group to inform the conduct of Phase B and the design of a future main trial
Meeting 1: informing the design of the pilot randomised controlled trial (Phase B)
Three key issues were discussed.
Presentation, content, and delivery of the participant information leaflet
A brief overview of the function of PILs and the type of information that these tend to cover was presented to the group. PPI members were offered the opportunity to provide immediate thoughts on the presentation and layout of the PIL and invited to return more detailed comments about the language/content via a prepaid postage envelope (after the meeting). The PILs were generally well received, although there were some recommendations for improving the visual appearance and content of the document. Specifically, members felt that the size of the font needed to be increased to ease readability, along with the size of the study logo. The latter was thought to make the leaflet more eye-catching, setting it apart from other hospital documentation that patients receive around the time of surgery. Some members commented that PILs in research generally tend to be difficult to digest and lack impact. One member suggested that a brief summary of the Bluebelle study, in bold, at the beginning of the PIL might help to sustain the reader’s attention.
Much of the discussion about the PIL related to its distribution. All PPI members agreed that they were most likely to consider the PIL if a member of the clinical team had already explained the study in advance (rather than receiving the PIL in the post, without prior discussion). All agreed that the PIL would ideally be distributed in hospital and handed over by a clinician/member of the research team following a brief verbal explanation of the study.
In summary, PPI member recommendations relating to the PIL were to (1) increase the font size of the main body text, (2) increase the size of the Bluebelle study logo, (3) add a short summary of the study, in bold, at the beginning of the PIL and (4) consider opportunities for HCPs to explain the study in advance of distributing the PIL.
The design and delivery of the Wound Experience Questionnaire
A provisional draft of the WEQ was distributed at the meeting to ascertain members’ thoughts about its content, ease of completion, presentation and administration. PPI members attempted to fill out the questionnaire to inform their feedback. The following key issues arose from the discussion.
Questionnaire content
Some individuals questioned some of the terminology used in the questionnaire items, particularly those that asked about sensations arising from the wound. For instance, words such as ‘pulling’ and ‘tightness’ were deemed difficult to distinguish, prompting questions around why they were separate questionnaire items. Members also queried what the term ‘protected’ meant in a scenario in which a patient had not received a dressing. Members appreciated that the questionnaire design and content had been informed by qualitative interview data. Thus, there was consensus that the research team should revisit the qualitative data with a view to ensuring that a clear empirical rationale for the terminology and items was included.
Two questionnaire items (one asking about pain levels and the other asking about ability to shower) were deemed to be ambiguous. Members felt that respondents may find it difficult to attribute the above issues to their wounds, rather than to the more general effects of recovering from surgery.
Ease of completion
Some PPI members were unsure about which wound/dressing to think about when completing the questionnaire, drawing on their own experiences of receiving multiple wounds after surgery. Although written instructions provided clarification around this issue, this was not noted by all members. The group therefore felt that the instructions for completion should be made more prominent (by increasing the font size).
Presentation
One member suggested that it might be helpful to have a space for comments at the bottom of the questionnaire, as she felt the need to explain her responses in more detail.
Administration
There was consensus that the 4-day cut-off point for completion was too short for some patients (e.g. one member of the PPI group had still been on morphine at this point). The group suggested offering more flexibility in the timing of questionnaire completion, given the heterogeneity of operative procedures and recovery experiences.
In summary, PPI member recommendations relating to the WEQ were to (1) make the instructions for completing the WEQ more prominent, (2) refer back to the qualitative data informing questionnaire items to ensure that there is an empirical rationale for their inclusion and (3) provide more flexibility around the timing of questionnaire completion.
Strategies for improving adherence to trial allocation in the pilot randomised controlled trial
Thoughts on potential strategies to ensure that patients adhered to their allocated dressing strategy in the forthcoming pilot RCT (especially in the case of a ‘no dressing’ strategy) were sought. A number of strategies were proposed by the group. These included (1) the use of stickers to indicate patients’ statuses as ‘Bluebelle participants’ in patient notes, (2) indicating Bluebelle participation on any ‘ward-facing’ information (e.g. whiteboards, often used to indicate patients’ treating clinician, bed number, etc.), (3) placing images of the Bluebelle logo at the end of patients’ beds and (4) encouraging nurses to discuss Bluebelle participation on handover between shifts.
Members’ thoughts on the use of a temporary skin transfer to indicate the dressing strategy to which the patient had been allocated were sought. The group did not have any strong views for or against this proposal but questioned the durability of a skin transfer if this came into contact with sweat.
In summary, PPI members recommended highlighting Bluebelle participation through a number of approaches, most of which entailed placing stickers on patient documentation/information. This was anticipated to be sufficient for enhancing adherence, although there were no objections or major concerns in relation to using skin transfers.
Meeting 2: informing plans for a possible randomised controlled trial
Two key issues were discussed.
Participants’ views on retention
Members were provided with a summary of questionnaire return rates and numbers returning for face-to-face follow-up assessments in the pilot RCT. Participants suggested several reasons why people may not return to hospital for follow-up assessment:
-
Participants may not come back if everything has gone well; the prospect of returning to hospital is not high on their priority list, or they may not want to revisit their hospital experience.
-
Travel may be difficult; poorly or elderly participants or new mothers may need help or a chaperone to come with them.
-
People are working and cannot afford to take the time off.
Members suggested that financial incentives may improve retention rates in the main trial. They also suggested that continued contact with the study team was important to build rapport. A common theme was ‘choice’ at the recruitment appointment, for example asking participants how they would prefer to complete questionnaires (electronic or paper copy).
Participants’ views on methods for own-wound digital photography
Members were provided with an overview of the proposed methods for patients or their carers to take and send digital photographs of their wounds to the study team in a large RCT. The group showed general positivity and encouragement for such a method. Members were asked views on what would be important to include in a study to explore and test this method before including it in a large RCT, and what should be included in PILs. An important issue was confidentiality. Members commented that it should be clear who will see the photograph and how and where it will be stored. Participant information should give full explanation on terminology, for example ‘study database’.
Other comments arising from the meeting
The next steps and future plans for the study were discussed, as were opportunities for PPI meetings as part of the proposed large RCT. All members were willing to read and provide feedback on a plain English summary for this Health Technology Assessment (HTA) programme report. All members expressed interest in attending future PPI meetings.
Impact of patient and public involvement
Members said that they had enjoyed being involved in a PPI group and attending the meetings. Written feedback on the PPI meeting was rated as excellent (4/7 response) or very good (3/7 responses). Comments from members included that PPI was regarded as very important and that it had been valuable to meet others and share experiences.
One PPI member read and commented on the lay summary for this HTA report. All members wanted to be kept informed about the study, for example in terms of whether or not a large trial will go ahead and any findings and results.
Key issues and suggestions will be taken forward and incorporated into plans for the design and conduct of a future large RCT and will inform the development of PILs and interview topic guides for a study to test methods for own-wound photography.
Objective B9: design a large, definitive randomised controlled trial based on information from the pilot trial and from integrated and interactive meetings with nurses/midwives, surgeons, methodologists and patient partners
The VoI analysis confirmed that a main trial would, in principle, be valuable to the NHS. The results of the Phase B external pilot trial confirmed that a RCT of the three dressing strategies trialled would be feasible. Therefore, the SMG discussed in detail how a main trial should be designed.
The SMG discounted a trial including a complex dressing group, given the information obtained during Phase A. The SMG formulated the following two hypotheses for comparisons between the three dressing strategies (glue-as-a-dressing, no dressing, simple dressing):
-
Hypothesis 1 (H1): glue-as-a-dressing is superior to a simple dressing.
-
Hypothesis 2 (H2): no dressing is non-inferior to a simple dressing.
The main components of the trial were considered to be as follows:
-
Population: primary closed surgical wounds in patients having abdominal surgery.
-
Interventions: glue-as-a-dressing (intervention 1, defined as in Bluebelle Phase B); no dressing (intervention 2, defined as in Bluebelle Phase B).
-
Comparator: simple dressing (as defined in Bluebelle Phase B).
-
Primary outcome: SSI, defined as in-hospital SSI (as ascertained in Bluebelle Phase B) or SSI ascertained by WHQ (cut-off point to be decided).
-
Secondary outcomes: WMQ (after further development/validation); WEQ (after further development/validation); wound complications; readmission for SSI; and resource use (hospital stay; antibiotics, dressings and other treatments for SSI).
As considered by the VoI analysis, both hypotheses can be addressed in a three-group trial and only H1 can be addressed in a two-group trial comparing glue-as-a-dressing and simple dressing groups. A test of H1 would be most valuable to the NHS but a test of H2 would also be valuable and there would be efficiency in testing the two hypotheses in one trial.
The SMG discussed target margins for the two hypotheses, resulting in the following proposed randomisation ratios and sample size justifications. Superiority and non-inferiority margins were chosen in a conventional way (i.e. through discussion between statistician, methodologist and clinicians). The SMG set a superiority margin of ≥ 2% for H1 and a non-inferiority margin of ≤ 1.5% for H2, assuming a probability of SSI of 10% when using a simple dressing.
For a three-group trial, it was proposed to randomise 1 : 2 : 2 owing to the different margins. For illustration, a trial with a total sample size of 25,000 participants (5000 : 10,000 : 10,000) would have 90% power to detect a decrease in the absolute risk of SSI of ≥ 1.7% (two-tailed alpha p ≤ 0.05) with glue-as-a-dressing compared with a simple dressing, and a difference in the absolute risk of SSI of ≤ 1.4% (one-tailed alpha p ≤ 0.05) with no dressing being non-inferior to a simple dressing.
For a two-group trial, it was proposed to randomise 1 : 1. For illustration, a trial with a total sample size of 10,000 participants (5000 : 5000) would have 90% power to detect a decrease in the absolute risk of SSI of ≥ 2% (two-tailed alpha p ≤ 0.05) with glue-as-a-dressing compared with a simple dressing.
The SMG made a number of additional recommendations and comments about a main trial:
-
It should include an internal pilot to test the triallists’ ability to obtain the self-reported WHQ data at 4–8 weeks from a credible percentage of patients.
-
The trial should not include EQ-5D-5L (or a similar questionnaire) as a secondary outcome for the following reasons. For H1, if glue-as-a-dressing were shown to be superior to a simple dressing with respect to SSI (given the superiority target margin), it would be cost-effective simply in terms of SSI avoided, irrespective of QALY difference; if glue-as-a-dressing were shown not to be superior to a simple dressing, glue-as-a-dressing could not be cost-effective for a plausible QALY difference. For H2, if no dressing were to be shown to be non-inferior (given the non-inferiority margin), no dressing would be dominant (cheaper); if no dressing were to be shown to be inferior, the cost consequences could not be offset by a plausible QALY difference.
-
The superiority margin for H1 is slightly smaller in the three-group design than in the two-group design because the former would be tested with a total sample size of 15,000 (glue-as-a-dressing = 5000 vs. simple dressing 10,000). The larger number of participants allocated to the simple dressing group in the three-group trial would be necessary to test H2 with adequate power. The SMG did not consider that the narrower superiority margin with a 5000 : 10,000 comparison in the two-group trial would be worth the additional cost.
Chapter 5 Discussion
Main findings: study conduct
Phase A
The study comprised multiple elements that investigated the feasibility of a main trial. These were found to be valuable to inform the pilot RCT design. However, the complexity of the work, combined with the requirement to design and set up the pilot RCT within the time frame, was challenging.
The qualitative research showed that additional work was needed. This was achieved by increasing collaborations and gaining an extension to Phase A. Collaborations with the trainee research collaboratives, the Medical Research Council (MRC) ConDuCT-II Hub (NJW for objective A7 and LR and DE for objective A4), and with the Cochrane wounds group (JCD) were established. The funded extension allowed the additional work to be completed to inform the pilot RCT.
The process of applying for funding for a commissioned topic is the same, regardless of whether the topic is requesting a feasibility, pilot or substantive study. The Bluebelle study demonstrates the difficulty of defining the scope of initial feasibility research in advance of doing the research. The research expanded to address additional uncertainties that were identified from the planned feasibility work. These included a survey of current practice, a VoI analysis, literature reviews and the development of secondary outcome measures.
The terminology used in the commissioning brief illustrates this issue. The qualitative case studies carried out to address objectives A1 and A2 found that the concept of a ‘complex’ dressing for a primary closed surgical wound was not recognised by staff. Informants reported examples of using products not traditionally marketed as dressings (e.g. tissue glue) to cover primary wounds. This led to the survey of dressings use in the NHS (objective A3). A second example relates to our original objective to develop a patient-centred measure of practical wound management (A4). The case studies and literature showed that two new measures, one of wound management and one of patient experience, were needed rather than a combined measure (as conceived by the Cochrane systematic review). 33 Therefore, more research was required than had been anticipated in Phase A.
On reflection, feasibility research shares some features with an adaptive trial design. 105,106 When feasibility research is commissioned, it is likely that there will be considerable uncertainty about the precise nature of the research. If the research is going to address fully both the uncertainties identified in a commissioned brief and the emerging uncertainties, both the researchers and the funders may need to be flexible in their responses to new and emerging findings. The Bluebelle study demonstrates this flexibility, to the extent that the HTA programme agreed contract extensions and additional funding. However, the ability to draw on wider collaborations (above) was vital in ensuring that the study generated the maximum value.
The need for flexibility when undertaking feasibility research potentially creates risks; researchers may seek extensions, justifying these as demonstrating the required flexibility to adapt to emerging findings, when the objective of the extended research is not sufficiently aligned with the original commissioned topic. Arguably, this is no different from any request for a contract variation to extend a study. However, in a ‘standard’ adaptive trial, a proposal to add or drop a group is similar to prioritising any effectiveness research question, so it is likely to be familiar to panels and commissioners. In the case of feasibility research, it would be important to ensure that the emerging findings and request for extensions truly did align with the NHS agreed research priorities.
Phase B
It was planned that recruitment to the pilot RCT would start 3 months after completion of Phase A. REC approval for the pilot RCT was applied for in advance of completion of Phase A. It was envisaged that approval for the main elements of the RCT could be obtained and an amendment to update the approval for the latest findings from Phase A could subsequently be submitted.
-
The approach with respect to the REC was successful but research to address some Phase A objectives extended up to and beyond the start of Phase B.
-
Given that the primary objective of Phase B was to test whether or not recruitment would be satisfactory, starting to recruit to the pilot trial was prioritised over the need to have all of the trial management features in place. For example, local research teams were able to consent and randomise participants but CRFs for later stages of follow-up had not been finalised and the database for data entry was not ready. Not having all of the trial management features in place at the outset made trial conduct inefficient and frustrating for all involved.
-
Other consequences were that processes for following up participants were largely manual, which may have influenced the questionnaire response rates and led to a backlog of data entry.
-
It became apparent as the study progressed that the CRFs contained redundant items. We had intended to pilot the CRFs and optimise them during the study (as is customary in a pilot). This was not possible with the resources available; once recruitment started, all of the resources were invested in this.
-
Submission of the substantial amendment coincided with the introduction of the new NRES/HRA systems. This led to a 3-month delay in implementing a revised and improved consent process for patients having emergency surgery and obtaining approval to open a fifth centre.
Main findings: study results
Phase A
The main Phase A findings were as follows.
It was found that the terminology for dressing types used in the HTA commissioning brief was not recognised by health professionals and did not appear to be relevant to the management of primary wounds (objectives A1 and A2). Dressings were referred to by their trade names. The selection of dressing type for use on primary abdominal wounds was reported to be dependent on hospital policy rather than clinician preference and did not vary between emergency and elective settings. Complex dressings as envisaged in the commissioning brief (e.g. antibiotic-impregnated dressing) were rarely used, and primary wounds were generally covered. Some interviewees described the use of tissue adhesive as a dressing on a closed wound, which was one reason for surveying current practice.
The survey (objective A3) found that simple dressings were used most frequently on primary abdominal wounds and that tissue adhesive was also used as a dressing (27%) despite a lack of evidence for this practice. Patients and staff reported that it was acceptable to leave a closed wound uncovered in the context of a RCT, acknowledging that there were uncertainties around the role of dressings and SSI outcomes. They postulated there might be practical and psychological advantages and disadvantages around dressing use, indicating the importance of a future RCT.
It was planned to develop and test a questionnaire to ascertain SSI after discharge to be completed at 30 days, potentially supplemented by a wound photograph taken by patients themselves (objective A4). The intention was to devise a way to reduce the research costs of collecting outcome data in a main trial (i.e. avoiding the need for a participant to have a SSI assessment by a trained researcher/clinician, whether in hospital or at home), while at the same time developing a validated instrument that could be used for SSI surveillance. The questionnaire, for completion by a patient or an observer, was successfully developed. After refinement through cognitive interviews, this WHQ included 16 items; the WHQ was acceptable and quick to complete, and was completed with few missing data. Two new measures of wound management and patient experience (WMQ and WEQ) were developed (objective A5), based on the literature and interviews to identify domains.
Working definitions of ‘simple’ and ‘complex’ dressings were established and the parameters of ‘no dressing’ were defined (objective A6). It was found to be feasible to photograph wounds in theatre but challenging to obtain the necessary approvals to do so (over and above the research approvals). Surgeons’ perceptions of what constituted ‘good quality’ wound closure were investigated, as were methods to assess these (objective A7). It was shown that a definitive trial, comparing three groups (simple vs. complex dressing vs. no dressing) or only two groups (simple vs. complex dressing), would be valuable to the NHS (objective A8). All of these findings were used to design the pilot RCT (objective A9) carried out in Phase B.
Phase B
The main Phase B findings were as follows.
Participants were successfully recruited and randomised, exceeding the target sample size within the scheduled recruitment period of 9 months, and 59% of approached eligible patients consented to take part (objective B1). The consent rate might be higher in a future main RCT because we experienced constraints on recruitment in the pilot RCT (see Strengths and limitations of the study). The qualitative research supported the conclusion that randomisation to the different dressing strategies was acceptable to patients and professionals (objective B2).
There were similarly consistent qualitative and quantitative findings about adherence to the allocated dressing strategies (objectives B3 and B4); it was found that adherence was not problematic and that the adherence aids that were used (notably the skin transfers) were acceptable. Adherence to the allocated dressing was > 97% for the initial dressing and > 86% for patients who needed to have a wound redressed. Adherence to the timing of disclosure of allocation was 99% when disclosure was before wound closure and 86% when disclosure was after wound closure.
Adherence to the follow-up protocol (objectives B4 and B5) varied depending on when outcomes were collected. More than 90% of participants completed the WMQ and WEQ at 4 days; the EQ-5D-5L was well completed at recruitment, but less so at 15 days (70%) and 4–8 weeks (64%). The response rate for the participant-completed WHQ at 4–8 weeks was 67%. The reference assessment was carried out for 80% of participants, with blinding of this assessment being compromised in 19% of participants.
Considering resource use as a secondary outcome, there were no obstacles to collecting the data required (available for > 80% of participants in all categories identified and > 95% of participants in relation to dressings and redressings, both before and after discharge). The costs analysis showed that some additional information would be useful, including information about dressing changes after discharge (by whom and where), additional care provided for non-serious SSIs, and how patients who need additional wound care travel to hospital. Cost drivers varied across dressing options, although it is evident that key cost drivers were hospital appointments, dressings and redressings, use of new medicines, and primary care appointments. In general, instances of care that were particularly costly were rare and, therefore, did not have a major prominent effect on the mean cost per patient.
The WHQ was implemented in a cohort study in Phase A and in the pilot RCT, and data contributed to validation of the questionnaire (objective B6). The response rate at 4–8 weeks (67%) would probably be considered insufficient for the primary outcome of a main trial. However, the low response rate may be due, at least in part, to the limitations of the pilot RCT (see Strengths and limitations of the study), which could be addressed. With respect to a main trial, the authors recommend that researchers implement measures to enhance the response rate in an internal pilot phase and specify an adequate response rate as a progression criterion.
The WHQ was found to have good psychometric attributes: when the questionnaire was completed, few data were missing, and responses were distributed as expected and were consistent with the frequencies of observed wound complications and SSI. Agreement between participant and observer, and within participants, was good, although, compared with observers, patients tended to report signs and symptoms as being slightly more severe. All items in the WHQ fitted a single plausible scale structure; factor analyses of Phase A data supported a single factor model, which was replicated using data from the pilot RCT. Cronbach’s alpha coefficients for a single scale were between 0.8 and 0.9 in both data sets, whether the WHQ was patient completed or observer completed. The new measure demonstrated good sensitivity and specificity compared with the CDC clinical assessment.
The short duration of the pilot trial meant that we were able to test the taking and submitting of wound photographs (objective B7) in only a small subgroup of individuals. We showed that it was possible to implement secure uploading of photographs, linking the photographs to other trial data, but only one-third of participants who consented to this substudy uploaded a photograph. The impact of involving patients and the public in the design of Phase B (objective B8) is covered below (see Patient and public involvement and engagement). Our proposals for a main trial (objective B9) are discussed in Future research recommendations.
Patient and public involvement and engagement
Patient and public involvement comprised patient representation on the Study Steering Committee; a meeting of a group of patients to discuss details of the pilot RCT (e.g. ideas for adherence aids, and refining the wording/presentation of patient-facing documents); and a second meeting to discuss how some of the limitations of the pilot RCT could be addressed in a possible main trial.
The first discussion led us to review the need for at least 4 hours’ thinking time after giving a potential participant the PIL and seeking written informed consent. Members of the group said that they would be more likely to consider the PIL if a member of the clinical team had discussed the study before giving out the PIL. Members considered the design and content of the WEQ and the time for completion. Finally, they considered the skin transfers that were proposed for use to promote adherence to dressing allocation without raising any major concerns.
The second discussion, held when we had become aware of the unsatisfactory participant attendance rate at the reference SSI assessment at 4–8 weeks in the pilot RCT, focused on ways to improve this and on patients’ views about taking photographs of their wounds. Members voiced a range of reasons why trial participants might be unwilling to return. They suggested that a financial incentive might improve retention and endorsed the proposal to allow participants to choose a method of completing questionnaires (i.e. electronically or by post). Members were generally positive about the proposals for trial participants to take wound photographs but stressed the importance of confidentiality. Subsequently, members of the group commented on a paper explaining the uncertainties about dressings that led to the study14 and the plain English summary included in this report.
With respect to wider public engagement, findings from Bluebelle have been presented at many conferences, including some that involved delegates outside conventional academic circles or generic audiences (e.g. a Bristol showcase of research and innovation in health care). We used social media [Twitter (Twitter, Inc., San Francisco, CA, USA), using both the account of the CTEU and personal accounts of team members], to promote Bluebelle activities (presentations, publications, PPI meetings, recruitment milestones, etc.).
Strengths and limitations of the study
Strengths
The collaborations within the Bluebelle group made it feasible to conduct multiple preliminary studies to inform a pilot RCT. This was a major strength because the multidisciplinary inputs were critical to the pilot design. Without the MRC ConDuCT-II Hub and close working with the surgical trainee research collaboratives this may have not been feasible because of the range of skills required. These collaborations enabled us to complete and publish within the extension period for Phase A, and to recruit into the pilot trial in Phase B on time and target. Participants engaged extremely well with the whole study.
We included a new centre, a district general hospital that was keen to participate, towards the end of the pilot RCT (month 7 of 9, delayed by the HRA moratorium). We included this centre to test the feasibility of delivering the study in a less experienced setting; it was the first time that the general surgical team had participated in a National Institute for Health Research (NIHR) portfolio study. The hospital recruited successfully for 3 months, confirming the acceptability of the design to patients and staff.
There were some teams that had not used tissue adhesive as a dressing prior to participation in this study and they requested training. This was provided by a principal investigator from one trust, who visited the team to demonstrate the intervention. Application of tissue adhesive on a closed wound is a straightforward process and one training session was sufficient to build the team’s confidence. It was also necessary to reassure the teams about the safety of leaving wounds exposed. None had routinely left wounds exposed without a dressing prior to the pilot study. We consider the successful recruitment and adherence rates as an important strength of the pilot trial, given the unfamiliarity of some local surgical teams with the interventions.
We used skin transfers close to the wound(s) to provide an immediate reminder of the dressing allocation. Although there were some concerns from nursing staff about the skin transfers, the qualitative research and PPI group confirmed that these were acceptable to patients and were a useful method to promote adherence. We recommend that this simple aid to adherence be employed in a main trial.
The outstanding research required to inform a main trial is described below (see Future research recommendations).
Limitations
The multiple activities required for the study, largely carried out in parallel with one another, were challenging to co-ordinate and caused some limitations. Research nurses and the trainee collaboratives were involved with recruitment, which was initially found complex to organise. Centres were required to set up additional processes to streamline communication between the teams. Trainees often identified patients and gave out information sheets. The nurses were then required to recruit and follow them up. A main trial would require additional administrative support and formal processes to co-ordinate the efforts of the different teams and trainees and track the large numbers of people involved.
A participant in the Phase B qualitative interviews described the Bluebelle study as having ‘many moving parts’. This sense of too many things going on at once, which were not always co-ordinated as well as we had hoped, was reflected in qualitative interviews with staff. Research nurses from all centres emphasised that the success of a future main trial would depend on staff engagement and co-ordination. The pilot RCT would not have succeeded without commitment from the research nurses, who went beyond the call of duty (the time of local research nurses was not costed in the budget, except in the case of a research midwife); the research nurses felt that the model of having research nurses lead most components of the pilot trial at a centre level would not be feasible on a larger scale.
A separate cohort study was added to Phase A to inform the validation of the WHQ, providing a larger sample for characterisation of some attributes of the WHQ and the opportunity to explore the scale structure in one sample and replicate it in the second. However, the Phase A cohort study captured fewer operative data than the pilot RCT and only a relatively small proportion of participants had a reference SSI assessment. The Phase A cohort study was resourced mainly through wider collaborations established for the project but, nevertheless, may not have represented the best use of these resources.
The pilot RCT could have recruited more quickly had it not been for the HRA moratorium on approving amendments, which delayed implementation of a discretionary time window between giving the PIL and requesting consent. This limited recruitment of patients having emergency operations and delayed the opening of the fifth centre.
The numeric description of adherence to timing of disclosure of dressing allocations disguises the fact that some centres devised ‘workarounds’ to make disclosure after wound closure more practicable – avoiding, in practice, the need for teams to log into the database twice. The short time taken to close a wound (< 5 minutes), and allowing for small discrepancies between theatre clocks (the time of wound closure had to be entered into the database manually in order to disclose the dressing allocation) and the server clock automatically recording time in the database (when the wound closure time was entered), makes these adherence percentages somewhat uncertain, but not to an extent that would undermine our conclusion about the feasibility of disclosing the dressing allocation after wound closure. Randomisation in theatre did work but was challenging for some surgical teams/hospitals, hence the need for workarounds. These included telephoning a research nurse (outside theatre) to ask him or her to enter the wound closure time and disclose the dressing allocation.
The logistics of obtaining the patient-completed WHQ, an observer-completed WHQ and an independent (blinded) reference SSI assessment at 4–8 weeks were challenging, because this required two members of staff. Some centres were not able to do this. Others co-ordinated the work with research nurses, academic surgeons and members of trainee research collaboratives. The low proportion of participants who had a reference SSI assessment might have introduced attrition bias (i.e. owing to participants being more or less willing to have a reference assessment depending on how the wound had healed). We cannot rule out this limitation but note that the percentages of participants who had a SSI were similar in each group, and that these percentages are consistent with published SSI percentages in similar clinical settings. It is important to remember that potential bias with respect to the SSI percentages was less important here because of the feasibility and pilot objectives. A main trial would be collecting data for a single measure of SSI at 4–8 weeks, not three measures.
Although the study recruited well, the response rates for the patient-completed outcomes at 4–8 weeks were 64% (EQ-5D-5L) to 67% (WHQ), which are considered inadequate for the primary outcome in a main trial. Owing to the manual processes for follow-up, we did not have up-to-date reports on response rates. We believe that it is possible to improve the response rate substantially and recommend that researchers be required to demonstrate a high response rate in an internal pilot phase of a main trial. Bluebelle hospitals achieve response rates of > 85% for periodic SSI surveillance in usual care for PHE.
We were unsuccessful in implementing the capture of digital photographs in theatre after wound closure, which prevented us from attempting to quantify the quality of wound closure and assessing potential performance bias from knowledge of the dressing allocation in advance of wound closure. We faced several challenges in capturing and transmitting photographs of wounds at the end of the procedure. Although we had obtained ethics approval and had consented patients to take photographs and use them, there was resistance from theatre staff. Each trust required us to apply additional local governance processes for digital data capture and transfer. These procedures in each of the trusts were burdensome, and it was not possible to implement them in all centres. With more time, we think that some of these obstacles could be surmounted. Jane M Blazeby has recently appointed a research photographer through the MRC ConDuCT-II Hub and her NIHR Senior Investigator award to investigate these issues.
The challenges in participants taking photographs of their own wounds and submitting them securely were different. We devised a way to send a single-use weblink to participants by e-mail that allowed a participant to upload a photograph securely and directly into the study database. We implemented this system during the last month of recruitment, sending instructions by post about how to take a photograph before e-mailing the weblink. We showed that the method worked, but only one-third of participants who agreed to do so uploaded a photograph (with one reminder). We did not have time to investigate further whether participants understood the instructions or experienced a problem with taking a photograph or following the weblink. We expect that this can be improved, and Rhiannon C Macefield is continuing this work as part of her Doctor of Philosophy project.
In the pilot RCT, data collection forms were completed on paper and entered into the online study database by the local research teams. This conventional method of data capture provides a clear audit trail but many of the surgeons involved had worked in other trials for which data had been directly entered online. We recommend that direct data entry be used in a main trial.
Lessons for the future: implications for clinicians and policy-makers
We have described findings about study conduct above (see Main findings: study conduct). We believe that these findings lead to the following lessons for the future:
-
When a feasibility study is commissioned, either the brief needs to be more focused or the commissioning process needs to be responsive to requests for flexibility in the research, to allow the research to answer the brief fully and to address emerging relevant uncertainties.
-
When researching future commissioning briefs, extreme care needs to be taken with respect to the information provided and the terminology used. We do not know how the 12/200 brief came to specify a comparison between a complex dressing and a simple dressing (as well as inviting a three-group comparison involving no dressing). What became clear to us very quickly when interviewing surgeons and nurses was that complex dressings were simply not used for ‘clean surgical wounds’ in usual care. Did the question in the brief arise from a misunderstanding about context (e.g. primary vs. secondary closed wounds or specific operations)? Or did this arise from opportunistic marketing by medical device manufacturers to promote the use of expensive dressings? What evidence was there at the outset that complex dressings were beginning to be used for primary closed wounds and that this question was therefore a priority for the NHS?
-
In relation to the use of glue-as-a-dressing, we were struck by the ‘cultural’ spread of technology. The survey suggested that there were apparent foci of adoption. We think that this is an important aspect of ‘technology creep’ in surgery. Indeed, even within the Bluebelle study itself, the investigators began to use glue as-a-dressing in patients who were not participating, knowing full well that it was not an evidence-based practice. In view of recent events in surgery and the use of unproven and untested invasive procedures and devices (e.g. vaginal mesh), there may be a case for commissioning such interventions only in the context of a trial [e.g. as in the principle of a National Institute for Health and Care Excellence ‘only in research’ recommendation, used in the context of verteporfin photodynamic therapy to treat predominantly classic subfoveal choroidal neovascularisation some years ago (see www.nice.org.uk/guidance/TA68; accessed 25 April 2018); we are uncertain whether or not this option is still available]. However, unless practitioners themselves accept such a ruling, it is very difficult to police, particularly when a technology has a legitimate indication. It is notable that surgeons never refer to carrying out an operation ‘off label’.
-
The SMG noted that the sample sizes for the proposed three-group or two-group main trials, calculated by the conventional method of seeking a consensus among surgeons about relevant target differences, are substantially larger than the sample size that the VoI analysis judged would provide valuable information to the NHS. The VoI analyst and the triallists did not resolve this discrepancy. However, this may be explained by the fact that the estimated cost of trials with different sample sizes was not an input to the VoI analysis. We plan to include this information and revise the VoI shortly.
-
We recommend that, where feasibility studies include qualitative research methods to explore participants’ views of recruitment, adherence and retention, there is clarity about the role of PPI work, to make best use of PPI panel members’ and researchers’ time. The qualitative research and PPI served very different purposes in Bluebelle, and we found it helpful to clarify this distinction from the outset. Patients’ perspectives were empirically sought through case studies to inform the design of the pilot RCT and a possible future main RCT, and PPI panel members contributed on how best to engage with future patient participants and the public (e.g. producing lay summaries).
Future research recommendations
Most future research recommendations relate to the main trial, in addition to our proposals described above (see Objective B9: design a large, definitive RCT based on information from the pilot trial and from integrated and interactive meetings with nurses/midwives, surgeons, methodologists and patient partners). Other research recommendations represent ‘unfinished’ business arising from the study, for example in relation to wound photography and further validation of patient-reported outcomes.
Further considerations in relation to a main trial
This study has shown that a main trial is feasible (see The health technology being assessed: dressings for primary surgical wounds) and the VoI analysis has established that the evidence generated by a main trial would be valuable to the NHS (see Objective A8: analyse the value of information to the NHS that would be provided by a definitive trial). We have outlined the main research questions for a three-group (as in the pilot RCT) or a two-group trial (simple dressing vs. glue-as-a-dressing) and proposed sample sizes in Objective B9: design a large, definitive RCT based on information from the pilot trial and from integrated and interactive meetings with nurses/midwives, surgeons, methodologists and patient partners. In addition to the choice between a three-group or two-group trial, some other aspects have not been resolved, namely ensuring an adequate response rate for the primary outcome, establishing a threshold for defining SSI when using the WHQ and choosing the time of randomisation.
Our preference is for a three-group trial, despite the daunting size of the proposed trial. The reason for this preference is to do with the potential interaction between the allocated dressing strategy and the quality of wound closure (which also affects the choice of time of randomisation). A key decision for a commissioner of a main trial is whether the question of interest is specifically about the effect of different dressing strategies or about the combined effect of different dressing strategies and their – potential – effect on the quality of wound closure. For example, one might hypothesise that glue-as-a-dressing reduces the risk of SSI because surgeons close the wound more carefully; this effect would not be evident if any effect of dressing strategy on wound closure was excluded by randomising after wound closure. We were unsuccessful in the pilot trial in answering the question of whether or not an interaction exists because we were unable to implement the capture of wound photographs in theatre on a routine basis. Nevertheless, we believe that the two-group trial comparing a simple dressing and glue-as-a-dressing would also be very important to the NHS because, if glue-as-a-dressing does not have benefits, the NHS needs to try to stop the spread of this practice as soon as possible given the relatively high cost of tissue adhesive.
We propose that the primary outcome should be a combination of information about SSI collected at discharge (as in this study) and SSI ascertained by the patient-reported WHQ. Using this primary outcome would require (1) a much better response rate for the WHQ than we achieved in the pilot RCT and (2) a threshold score for the WHQ to define SSI after discharge. We do not believe that a conventional ‘reference’ SSI assessment would be practicable to use as the primary outcome in a main trial because of the high cost, and the validation achieved in the Bluebelle study demonstrates that the WHQ identifies a high proportion of SSIs.
As described above, we believe that the response rate achieved in the pilot RCT could be greatly improved, primarily by implementing existing evidence-based methods107,108 and by using information technology solutions such as text messaging. We recommend that, if the HTA programme decides to commission a main trial, applicants should be required to include an internal pilot, progressing to a main trial only if the WHQ response rate at 4–8 weeks can be shown to exceed 90%.
Choosing an optimum WHQ threshold score to define SSI after discharge is not straightforward. The point on the ROC that lies further from the leading diagonal minimises total misclassifications but assumes that the clinical and economic consequences of false-negative and false-positive misclassifications are the same. This is unlikely to be the case: the consequences of an undiagnosed SSI are likely to outweigh those of treating a suspected SSI that, in fact, was not a SSI. We intend to research an optimum threshold using quantitative information collected in the study and consensus methods with the Bluebelle co-investigators. It is also important to explore whether or not subthreshold signs of SSI collected at discharge, combined with information from the WHQ, might improve diagnostic accuracy. The ASEPSIS instrument combined information collected in hospital and after discharge in an additive manner,32,109 whereas we have assumed that SSI should be defined by a Boolean ‘or’ operator (SSI ascertained in hospital or SSI ascertained after discharge, applying a WHQ threshold score). We propose to continue to research the WHQ to address these issues. We have incorporated the WHQ in the HTA-funded CIPHER cohort study (reference number 14/166/01), which aims to recruit 4000 participants having abdominal surgery. The data for the WHQ collected in CIPHER will complement those already collected.
Team members could not agree on the best time to disclose dressing allocation in a main trial, primarily because of differing opinions about the question of interest (see above), but also for logistical reasons. Although the surgical teams had worked hard to achieve good adherence to the timing of wound disclosure in the pilot RCT, surgeon members of the team felt that disclosing dressing allocation after wound closure would be very difficult to deliver consistently in a main trial, given its proposed magnitude. The triallists, however, were confident that, without the factorial aspect of the pilot RCT, it would be possible to disclose allocation after wound closure in a convenient manner. Both groups agreed that it would be very difficult to achieve a factorial design in a main trial and, therefore, the timing of disclosure of allocation needs to be decided by clearer specification by the HTA prioritisation panel of the research question of interest.
With respect to the timing of a future main trial, we recommend that the HTA programme waits for 12 months until it becomes clearer (1) how well the NHS can support the large wound trials that have recently been funded and that are starting to recruit (e.g. see www.birmingham.ac.uk/research/activity/mds/trials/BiSTC/trials/ROSSINI-2.aspx; accessed 25 April 2018) and (2) whether or not the large investment required for a main trial (on top of the existing research investment on SSI) fits with other NIHR priorities.
Research about wound photographs taken by patients
We remain attracted by the idea of patients taking and submitting a photograph of their wound(s) at 4–8 weeks, primarily because such a photograph could be assessed without knowledge of the way the wound was initially dressed (or, indeed, treated in other ways). We have shown that this is possible and we suspect that, by working with patients of different ages and aptitudes for smartphones, both the response rate and the quality of photographs could be enhanced.
Research about facilitating the capture of wound photographs for research in theatre
We were frustrated by the data protection challenges that we experienced in trying to implement wound photography in theatre, despite having ethics approval and consent to do so. All trusts in which we tried to obtain permission for wound photography in theatre insisted on obtaining additional permissions and imposed restrictions on storing digital images; the specific permissions required varied by trust. Photography and video imaging in theatre may create many research opportunities that extend beyond wound-related research (e.g. defining interventions, monitoring intervention fidelity and assessing outcomes). We believe that it is important to facilitate the collection of images by establishing a policy that would apply to all hospitals.
Assess the quality of wound closure, including from photographs
We made considerable progress in characterising aspects of wound appearance that could constitute a metric of quality of wound closure. Such a metric is needed to answer the question of whether or not there is an interaction between dressing strategy and quality of wound closure. Quality of wound closure could also be useful as a future outcome in surgical research (e.g. in assessing cosmesis). A further aspect of this research is to compare measurement of the metric ‘in vivo’ with measurement from digital images.
Carry out further validation of the Wound Management Questionnaire and Wound Experience Questionnaire
Similarly, we made considerable progress in developing patient- and practitioner-reported outcomes of wound experience and wound management to fill the gap for such tools identified in the Cochrane review of wound dressings. 17 We showed that these instruments are acceptable to patients and practitioners and are well completed. However, we recommend that both measures are validated so that these tools can be deployed in future trials.
Research whether patients can recall wound problems in hospital at 4–8 weeks after discharge
One of the challenges in designing the WHQ was to develop a tool that was appropriate for procedures with varying lengths of stay after surgery or carried out as day-cases. This was important so that the WHQ could be used for patients having a wide range of operations and because hospital stays after surgery continue to shorten. For patients having day-case surgery, the concept of SSI at discharge was irrelevant and SSI was ascertained only from the WHQ at 4–8 weeks. This raises the question about whether it is necessary to collect data about SSI signs and symptoms at discharge in patients who do stay in hospital (i.e. could the timeframe for the WHQ be extended to include time in hospital?). We recommend that this question be researched because, if the answer is yes, then data collection for trials of interventions to prevent SSI could be reduced. Recall of signs and symptoms of SSI in hospital may vary according to the nature of the surgical procedure undertaken so it is likely to be important to include a wide range of operations with varying average hospital stay in such research.
Chapter 6 Conclusion
We have shown that a main trial of different dressing strategies, including no dressing, is feasible and would be valuable to the NHS. Patients and practitioners supported the premise of a future trial of dressing types and engaged with the idea that there is equipoise in this area. This engagement was also reflected through the excellent participation in both the qualitative and the quantitative studies, the high consent rate and good adherence to allocation.
We have also developed, validated and tested a new tool, the WHQ, for assessing SSI, which can be used by patients after hospital discharge. This tool has immense potential to improve the efficiency of studies evaluating interventions to reduce SSI.
The NHS now needs to decide whether the research question in a main trial should be specifically about the effect of different dressing strategies, or about the combined effect of different dressing strategies and their effect on wound closure and whether a two-group or three-group trial is needed. There is also the need to consider whether this evaluation should be a standalone study or whether an existing study should be adapted to include glue-as-a-dressing and no dressing groups.
Acknowledgements
The Bluebelle team would like to acknowledge the contributions of the following to the study: Laura Magill (University of Birmingham Clinical Trials Unit, Birmingham); Katrina Hurley (Bristol Dental Hospital, Bristol); Trudie Young (University of Glamorgan, Glamorgan); Cathy Winter (North Bristol NHS Trust, Bristol); Jo Chambers (University Hospitals Bristol NHS Foundation Trust, Bristol); Katy Chalmers (Department of Population Health Sciences, Bristol Medical School, Bristol); David Hutton (CTEU, Department of Translational Health Sciences, Bristol Medical School, Bristol); Barry Main (Department of Population Health Sciences, Bristol Medical School, Bristol); and Helen Talbot (CTEU, Department of Translational Health Sciences, Bristol Medical School, Bristol). We are grateful to the research nurses who supported the study at our participating centres; University Hospitals Bristol NHS Foundation Trust (Rebecca Houlihan, Joanna Nicklin, Louise Flintoff, Jo Chambers and Karen Bobruk), North Bristol NHS Trust (Helen Cheshire, Suriya Kirkpatrick, Louise Solomon, Alice Jarvie, Cathy Winter, Clementine Skilton, Susan Hughes, Michelle Mayer, Jade Knowlden, Mary Alvarez and Sherrie Villis), University Hospitals Birmingham NHS Foundation Trust (Kelly Hollier, Natalie Jackson, Victoria Hardy, David Tyrell, Sharon Garner and Arlo Whitehouse), and Worcestershire Acute Hospitals NHS Trust (Caroline Alton, Jessica Thrush and Julie Wollaston). Simon Davey identified and recruited patients and contributed to study delivery in his local trust.
The authors would like to thank the patient and public contributors for sharing their experiences of their care and contributing their opinions in relation to interpretation of the findings.
Contributions of authors
Barnaby C Reeves was co-investigator with responsibility for the pilot trial design and protocol; he devised strategies to minimise bias and drafted the manuscript.
Leila Rooshenas was co-investigator with responsibility for design and delivery of the qualitative studies in Phase A and the pilot trial; she drafted the qualitative sections of the manuscript and co-led the PPI work.
Rhiannon C Macefield led the development and validation of the WHQ under supervision from Jane M Blazeby and Barnaby C Reeves, drafted the manuscript reporting the development and validation of the WHQ and co-led the PPI work.
Mark Woodward was a co-investigator, contributing experience of not using dressings on surgical wounds in children who have had abdominal surgery.
Nicky J Welton was a co-investigator responsible for conducting a VoI analysis about a full-scale trial.
Benjamin R Waterhouse identified and recruited patients and contributed to study delivery in his local trust.
Andrew D Torrance was a co-investigator. He was also a member of the SMG and a co-applicant.
Sean Strong was responsible for the survey of wound dressings and data collection in Phase B.
Dimitrios Siassakos was responsible for the implementation of the study protocol in one centre.
William Seligman identified and recruited patients and contributed to study delivery in his local trust.
Chris A Rogers was a co-investigator with responsibility for estimating the target sample size and planning the quantitative analyses.
Lloyd Rickard identified and recruited patients and contributed to study delivery in his local trust.
Anne Pullyblank was a co-investigator with responsibility for set-up and delivery in one participating centre of the study in general surgery.
Caroline Pope assisted with setting up and managing the trial, including site initiation visits and first and second protocol amendments.
Thomas D Pinkney was a co-investigator with responsibility for one participating centre recruiting patients having abdominal surgery and overall study design.
Samir Pathak identified and recruited patients and contributed to study delivery in his local trust.
Anwar Owais identified and recruited patients and contributed to study delivery in his local trust.
Jamie O’Callaghan identified and recruited patients and contributed to study delivery in his local trust.
Stephen O’Brien identified and recruited patients and contributed to study delivery in his local trust.
Dmitri Nepogodiev identified and recruited patients and contributed to study delivery in his local trust.
Khaldoun Nadi identified and recruited patients and contributed to study delivery in his local trust.
Charlotte E Murkin identified and recruited patients and contributed to study delivery in her local trust.
Tonia Munder conducted patient follow-up and contributed to study delivery in her local trust.
Tom Milne researched definitions of simple, complex and no dressings under supervision.
David Messenger identified and recruited patients and contributed to study delivery in his local trust.
Christel M McMullan was responsible for qualitative data collection/analysis during Phase A and the pilot trial.
Jonathan M Mathers was a co-investigator with responsibility for the co-design and delivery of the qualitative studies in Phase A and the pilot trial.
Matthew Mason identified and recruited patients and contributed to study delivery in his local trust.
Morwena Marshall identified and recruited patients and contributed to study delivery in her local trust.
Richard Lovegrove was a co-investigator with responsibility for one participating centre recruiting patients having abdominal surgery.
Robert J Longman was a co-investigator with responsibility for one participating centre recruiting patients having abdominal surgery.
Jessica Lloyd Identified and recruited patients and contributed to study delivery in her local trust.
Jeffrey Lim identified and recruited patients and contributed to study delivery in his local trust.
Kathryn Lee identified and recruited patients and contributed to study delivery in her local trust.
Vijay Korwar identified and recruited patients and contributed to study delivery in his local trust.
Daniel Hughes identified and recruited patients and contributed to study delivery in his local trust.
George Hill identified and recruited patients and contributed to study delivery in his local trust.
Rosie Harris was responsible for preparing the statistical analysis plan, with advice from Chris Rogers, and for producing tables and graphs.
Mohammed Hamdan identified and recruited patients and contributed to study delivery in his local trust.
Hannah Gould Brown was responsible for the development of wound metric measures under supervision.
Rachael Gooberman-Hill was a co-investigator with responsibility for PPI.
James Glasbey identified and recruited patients and contributed to study delivery in his local trust.
Caroline Fryer identified and recruited patients and contributed to study delivery in her local trust.
Lucy Ellis prepared the first protocol for the pilot trial.
Daisy Elliott led the development of the WEQ and WMQ under supervision from Jane M Blazeby.
Jo C Dumville updated the Cochrane review of wound dressings to consider the use of tissue adhesives as a dressing.
Tim Draycott was a co-investigator with responsibility for one participating centre recruiting women having caesarean sections.
Jenny L Donovan was a co-investigator with responsibility for supervising qualitative research.
David Cotton identified and recruited patients and contributed to study delivery in his local trust.
Joanna Coast was a co-investigator with responsibility for supervising the health economic aspects of the pilot trial.
Madeleine Clout assisted Kate Ashton in managing the pilot trial and helped to prepare the final manuscript.
Melanie J Calvert was a co-investigator advising and supporting development of SSI measures, the feasibility study and pilot trial design.
Benjamin E Byrne helped to set up one participating centre including designing training materials and improving study design and processes.
Oliver D Brown identified and recruited patients and contributed to study delivery in his local trust.
Natalie S Blencowe led the survey of wound dressings and involved with interpreting Phase A findings and the way in which they should inform the design of the pilot trial. She also contributed to the development of the WEQ, WMQ, WHQ, wound metric measures and the Cochrane review work.
Katarzyna D Bera contributed to the development of wound metric measures under supervision of Jane M Blazeby.
Joanne Bennett identified and recruited patients and contributed to study delivery in her local trust.
Richard Bamford identified and recruited patients and contributed to study delivery in his local trust.
Danya Bakhbakhi identified and recruited patients and contributed to study delivery in her local trust.
Muhammad Atif identified and recruited patients and contributed to study delivery in his local trust.
Kate Ashton managed the pilot trial, including site initiation visits and the second protocol amendment.
Elizabeth Armstrong identified and recruited patients and contributed to study delivery in her local trust.
Lazaros Andronis was a co-investigator with responsibility for carrying out the health economic aspects of the pilot trial under supervision. He conducted the cost analysis and drafted the economic analysis part of the manuscript.
Piriyankan Ananthavarathan identified and recruited patients and contributed to study delivery in local trust.
Jane M Blazeby was the chief investigator responsible for the overall conception of Bluebelle study and the design of Phase A and Phase B pilot trial. She supervised the development of the WHQ, WEQ and WMQ and wound metric measures. She led the work on defining pragmatic definitions of wound dressing and led the work to develop wound metric measures. She contributed to the Cochrane review work and oversaw the whole project, contributed to SMG meetings and team meetings and drafted sections of the report as well as being responsible for its final text.
Publications
Blazeby J. Do dressing prevent infection of closed primary wounds after surgery? BMJ 2016;353:i2270.
Severn and Peninsula Audit and Research Collaborative for Surgeons (SPARCS) and West Midlands Research Collaborative (WMRC) on behalf of the Bluebelle Study Group. Feasibility work to inform the design of a randomised clinical trial of wound dressings in elective and unplanned abdominal surgery. Br J Surg 2016;103:1738–44.
The Bluebelle Study Group, the Severn and Peninsula Audit and Research Collaborative for Surgeons, and the West Midlands Research Collaborative. Bluebelle study (phase A): a mixed-methods feasibility study to inform a RCT of surgical wound dressing strategies. BMJ Open 2016;6:e012635.
Macefield RC, Reeves BC, Milne TK, Nicholson A, Blencowe NS, Calvert M, et al. Development of a single, practical measure of surgical site infection (SSI) for patient report or observer completion. J Infect Prev 2017;18:170–9.
Reeves BC, Andronis L, Blazeby JM, Blencowe NS, Calvert M, Coast J, et al. A mixed-methods feasibility and external pilot randomised controlled trial to inform a large pragmatic randomised controlled trial of the effects of surgical wound dressing strategies on surgical site infections. Trials 2017;18:401.
Data-sharing statement
All enquiries and requests should be submitted to the corresponding author for consideration in the first instance. Data requests should include a prespecified protocol describing the purpose, methods and analysis of the planned research and analysis (e.g. a protocol for a Cochrane systematic review). Access to anonymised data may be granted following review and assurances being in place.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Royal College of Surgeons . From Theory to Theatre: Overcoming Barriers to Innovation in Surgery 2011.
- Pinkney T, Calvert M, Bartlett D, Gheorghe A, Redman V, Dowswell G, et al. Impact of wound edge protection devices on surgical site infection after laparotomy: multi-centre randomised controlled trial (ROSSINI trial). BMJ 2013;347. https://doi.org/10.1136/bmj.f4305.
- Coello R, Charlett A, Wilson J, Ward V, Pearson A, Borriello P. Adverse impact of surgical site infections in English hospitals. J Hosp Infect 2005;60:93-103. https://doi.org/10.1016/j.jhin.2004.10.019.
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 1999;20:725-30. https://doi.org/10.1086/501572.
- Plowman R, Graves N, Griffin MA, Roberts JA, Swan AV, Cookson B, et al. The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J Hosp Infect 2001;47:198-209. https://doi.org/10.1053/jhin.2000.0881.
- Tanner J, Khan D, Aplin C, Ball J, Thomas M, Bankart J. Post-discharge surveillance to identify colorectal surgical site infection rates and related costs. J Hosp Infect 2009;72:243-50. https://doi.org/10.1016/j.jhin.2009.03.021.
- Weber WP, Zwahlen M, Reck S, Feder-Mengus C, Misteli H, Rosenthal R, et al. Economic burden of surgical site infections at a European university hospital. Infect Control Hosp Epidemiol 2008;29:623-9. https://doi.org/10.1086/589331.
- Berard F, Gandon J. Postoperative wound infections: the influence of ultraviolet irradiation of the operating room and of various other factors. Ann Surg 1964;160:1-192.
- A report from the NNIS System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32:470-85. https://doi.org/10.1016/j.ajic.2004.10.001.
- Health Protection Agency . Surveillance of Surgical Site Infections in NHS Hospitals in England, 2011 2012 n.d. www.gov.uk/government/publications/surgical-site-infections-ssi-surveillance-nhs-hospitals-in-england (accessed 16 July 2018).
- Blumetti J, Luu M, Sarosi G, Hartless K, McFarlin J, Parker B, et al. Surgical site infections after colorectal surgery: do risk factors vary depending on the type of infection considered?. Surgery 2007;142:704-11. https://doi.org/10.1016/j.surg.2007.05.012.
- Smyth ET, McIlvenny G, Enstone JE, Emmerson AM, Humphreys H, Fitzpatrick F, et al. Four country healthcare associated infection prevalence survey 2006: overview of the results. J Hosp Infect 2008;69:230-48. https://doi.org/10.1016/j.jhin.2008.04.020.
- National Institute for Health and Care Excellence (NICE) . Surgical Site Infection – Prevention and Treatment of Surgical Site Infection 2008.
- Blazeby J. Bluebelle Study Group . Do dressings prevent infection of closed primary wounds after surgery?. BMJ 2016;353. https://doi.org/10.1136/bmj.i2270.
- British National Formulary . Appendix 5: Wound Management Products and Elasticated Garments 2016 n.d. https://bnf.nice.org.uk/wound-management (accessed 25 April 2018).
- Merei JM. Pediatric clean surgical wounds: is dressing necessary?. J Pediatr Surg 2004;39:1871-3. https://doi.org/10.1016/j.jpedsurg.2004.08.017.
- Dumville JC, Gray TA, Walter CJ, Sharp CA, Page T, Macefield R, et al. Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev 2016;12. https://doi.org/10.1002/14651858.CD003091.pub4.
- Astagneau P, Rioux C, Golliot F, Brücker G. INCISO Network Study Group . Morbidity and mortality associated with surgical site infections: results from the 1997–1999 INCISO surveillance. J Hosp Infect 2001;48:267-74. https://doi.org/10.1053/jhin.2001.1003.
- Klevens RM, Edwards JR, Richards CL, Horan TC, Gaynes RP, Pollock DA, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007;122:160-6. https://doi.org/10.1177/003335490712200205.
- Wilson AP, Gibbons C, Reeves BC, Hodgson B, Liu M, Plummer D, et al. Surgical wound infection as a performance indicator: agreement of common definitions of wound infection in 4773 patients. BMJ 2004;329. https://doi.org/10.1136/bmj.38232.646227.DE.
- Allegranzi B, Bischoff P, de Jonge S, Kubilay NZ, Zayed B, Gomes SM, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016;16:e276-87. https://doi.org/10.1016/S1473-3099(16)30398-X.
- Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, de Jonge S, de Vries F, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016;16:e288-303. https://doi.org/10.1016/S1473-3099(16)30402-9.
- Glaser B, Strauss A. Grounded theory: the discovery of grounded theory. Sociology 1967;12:27-49.
- Health Education England . Severn and Peninsula Audit and Research Collaborative for Surgeons n.d. http://surgery.severndeanery.nhs.uk/about-us/trainee-groups-and-other-organisations/sparcs/ (accessed 1 September 2015).
- UK National Surgical Research Collaborative . Multicentre observational study of adherence to Sepsis Six guidelines in emergency general surgery. Br J Surg 2017;104:e165-e71. https://doi.org/10.1002/bjs.10432.
- Garner JS. CDC guideline for prevention of surgical wound infections, 1985. Supersedes guideline for prevention of surgical wound infections published in 1982. (Originally published in November 1985). Revised. Infect Control 1986;7:193-200. https://doi.org/10.1017/S0195941700064080.
- Cruse PJ, Foord R. The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds. Surg Clin North Am 1980;60:27-40. https://doi.org/10.1016/S0039-6109(16)42031-1.
- Bruce J, Russell EM, Mollison J, Krukowski ZH. The measurement and monitoring of surgical adverse events. Health Technol Assess 2001;5. https://doi.org/10.3310/hta5220.
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606-8. https://doi.org/10.1017/S0195941700015241.
- Wilson AP, Treasure T, Sturridge MF, Grüneberg RN. A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet 1986;1:311-13. https://doi.org/10.1016/S0140-6736(86)90838-X.
- PHE . Protocol for the Surveillance of Surgical Site Infection – Surgical Site Infection Surveillance Service 2013.
- Gibbons C, Bruce J, Carpenter J, Wilson AP, Wilson J, Pearson A, et al. Identification of risk factors by systematic review and development of risk-adjusted models for surgical site infection. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15300.
- Dumville JC, Walter CJ, Sharp CA, Page T. Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev 2011;7. https://doi.org/10.1002/14651858.CD003091.pub2.
- Lasch KE, Marquis P, Vigneux M, Abetz L, Arnould B, Bayliss M, et al. PRO development: rigorous qualitative research as the crucial foundation. Qual Life Res 2010;19:1087-96. https://doi.org/10.1007/s11136-010-9677-6.
- Sprangers MA, Cull A, Bjordal K, Groenvold M, Aaronson NK. The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: guidelines for developing questionnaire modules. EORTC Study Group on Quality of Life. Qual Life Res 1993;2:287-95. https://doi.org/10.1007/BF00434800.
- Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. Content validity – establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1 – eliciting concepts for a new PRO instrument. Value Health 2011;14:967-77. https://doi.org/10.1016/j.jval.2011.06.014.
- Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. Content validity – establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2 – assessing respondent understanding. Value Health 2011;14:978-88. https://doi.org/10.1016/j.jval.2011.06.013.
- Strauss ACJ, Ly DN. Handbook of Qualitative Research. Thousand Oaks, CA: Sage Publications; 1994.
- Dumville JC, Gray TA, Walter CJ, Sharp CA, Page T. Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev 2014;9. https://doi.org/10.1002/14651858.CD003091.pub3.
- Dumville JC, Coulthard P, Worthington HV, Riley P, Patel N, Darcey J, et al. Tissue adhesives for closure of surgical incisions. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD004287.pub4.
- Chow A, Marshall H, Zacharakis E, Paraskeva P, Purkayastha S. Use of tissue glue for surgical incision closure: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Surg 2010;211:114-25. https://doi.org/10.1016/j.jamcollsurg.2010.03.013.
- US Department of Health and Human Services Food and Drug Administration (FDA) . Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims 2009.
- Leidy NK, Vernon M. Perspectives on patient-reported outcomes: content validity and qualitative research in a changing clinical trial environment. PharmacoEconomics 2008;26:363-70. https://doi.org/10.2165/00019053-200826050-00002.
- Beatty PC, Willis GB. Research synthesis: the practice of cognitive interviewing. Public Opin Q 2007;71:287-311. https://doi.org/10.1093/poq/nfm006.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 25 April 2018).
- Pratt JW, Raiffa H, Schlaiffer R. Statistical Decision Theory. Cambridge, MA: Massachusetts Institute of Technology; 2001.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Royal College of Surgeons . Surgery and the NHS in Numbers 2013 14 n.d. www.rcseng.ac.uk/news-and-events/media-centre/media-background-briefings-and-statistics/surgery-and-the-nhs-in-numbers/ (accessed 25 April 2018).
- SPARCS and WMRC on behalf of the Bluebelle Study Group . Feasibility work to inform the design of a randomized clinical trial of wound dressings in elective and unplanned abdominal surgery. Br J Surg 2016;103:1738-44. https://doi.org/10.1002/bjs.10274.
- Jenks PJ, Laurent M, McQuarry S, Watkins R. Clinical and economic burden of surgical site infection (SSI) and predicted financial consequences of elimination of SSI from an English hospital. J Hosp Infect 2014;86:24-33. https://doi.org/10.1016/j.jhin.2013.09.012.
- PHE . Surveillance of Surgical Site Infections in NHS Hospitals in England 2014–15 2015. www.gov.uk/government/uploads/system/uploads/attachment_data/file/484874/Surveillance_of_Surgical_Site_Infections_in_NHS_Hospitals_in_England_report_2014-15.pdf (accessed 25 April 2018).
- Office for National Statistics . ONS Consumer Price Index: Medical Services 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterburry: Personal Social Services Research Unit, University of Kent; 2015.
- Gheorghe A, Moran G, Duffy H, Roberts T, Pinkney T, Calvert M. Health utility values associated with surgical site infection: a systematic review. Value Health 2015;18:1126-37. https://doi.org/10.1016/j.jval.2015.08.004.
- Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol 2002;23:183-9. https://doi.org/10.1086/502033.
- Falavigna A, Righesso O, Traynelis VC, Teles AR, da Silva PG. Effect of deep wound infection following lumbar arthrodesis for degenerative disc disease on long-term outcome: a prospective study: clinical article. J Neurosurg Spine 2011;15:399-403. https://doi.org/10.3171/2011.5.SPINE10825.
- Macefield R, Nicholson A, Milne TT, Pinkney TT, Calvert M, Avery K, et al. Development of a single, practical measure of surgical site infection (SSI) for patient self-report or observer completion. J Infect Prev 2015;16.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. https://doi.org/10.3109/07853890109002087.
- Department of Health and Social Care . National Schedules of Reference Costs: The Main Schedule 2016.
- Joint Formulary Committee . British National Formulary 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670-4. https://doi.org/10.1136/bmj.319.7211.670.
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. https://doi.org/10.1002/1097-0258(20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Glick HA, Jalpa DA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing. presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. https://doi.org/10.1002/hec.766.
- Little R, Rubin D. Statistical Analysis with Missing Data. New York, NY: John Wiley & Sons; 2002.
- Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials 2016;17. https://doi.org/10.1186/s13063-016-1391-4.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
- de Vet HCW, Terwee CB, Mokkink LB, Knol DL. Measurement in Medicine: A Practical Guide. Cambridge: Cambridge University Press; 2011.
- Fayers PM, Machin D. Quality of Life: Assessment, Analysis and Interpretation. Hoboken, NJ: John Wiley & Sons; n.d.
- The Bluebelle Study Group, the Severn and Peninsula Audit and Research Collaborative for Surgeons, and the West Midlands Research Collaborative . Bluebelle study (phase A): a mixed-methods feasibility study to inform an RCT of surgical wound dressing strategies. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012635.
- Macefield RC, Reeves BC, Milne TK, Nicholson A, Blencowe NS, Calvert M, et al. Development of a single, practical measure of surgical site infection (SSI) for patient report or observer completion. J Infect Prev 2017;18:170-9. https://doi.org/10.1177/1757177416689724.
- Amin M, Glynn F, Timon C. Randomized trial of tissue adhesive vs staples in thyroidectomy integrating patient satisfaction and Manchester score. Otolaryngol Head Neck Surg 2009;140:703-8. https://doi.org/10.1016/j.otohns.2009.01.003.
- Avşar AF, Üstüner I, Keskin HL, Öztürk Ö, Taş EE. 2-octylcyanoacrylate tissue adhesive versus polypropylene suture for skin closure of Pfannenstiel incision. J Turk Soc Obstet Gynecol 2009;6:117-22.
- Blondeel PN, Murphy JW, Debrosse D, Nix JC, Puls LE, Theodore N, et al. Closure of long surgical incisions with a new formulation of 2-octylcyanoacrylate tissue adhesive versus commercially available methods. Am J Surg 2004;188:307-13. https://doi.org/10.1016/j.amjsurg.2004.04.006.
- Burke NG, Green C, McHugh G, McGolderick N, Kilcoyne C, Kenny P. A prospective randomised study comparing the jubilee dressing method to a standard adhesive dressing for total hip and knee replacements. J Tissue Viability 2012;21:84-7. https://doi.org/10.1016/j.jtv.2012.04.002.
- Chibbaro S, Tacconi L. Use of skin glue versus traditional wound closure methods in brain surgery: a prospective, randomized, controlled study. J Clin Neurosci 2009;16:535-9. https://doi.org/10.1016/j.jocn.2008.08.004.
- Cosker T, Elsayed S, Gupta S, Mendonca AD, Tayton KJ. Choice of dressing has a major impact on blistering and healing outcomes in orthopaedic patients. J Wound Care 2005;14:27-9. https://doi.org/10.12968/jowc.2005.14.1.26722.
- Dowson CC, Gilliam AD, Speake WJ, Lobo DN, Beckingham IJ. A prospective, randomized controlled trial comparing n-butyl cyanoacrylate tissue adhesive (LiquiBand) with sutures for skin closure after laparoscopic general surgical procedures. Surg Laparosc Endosc Percutan Tech 2006;16:146-50. https://doi.org/10.1097/00129689-200606000-00005.
- Gennari R, Rotmensz N, Ballardini B, Scevola S, Perego E, Zanini V, et al. A prospective, randomized, controlled clinical trial of tissue adhesive (2-octylcyanoacrylate) versus standard wound closure in breast surgery. Surgery 2004;136:593-9. https://doi.org/10.1016/j.surg.2004.02.015.
- Greene D, Koch RJ, Goode RL. Efficacy of octyl-2-cyanoacrylate tissue glue in blepharoplasty. A prospective controlled study of wound-healing characteristics. Arch Facial Plast Surg 1999;1:292-6. https://doi.org/10.1001/archfaci.1.4.292.
- Holm C, Petersen JS, Grønboek F, Gottrup F. Effects of occlusive and conventional gauze dressings on incisional healing after abdominal operations. Eur J Surg 1998;164:179-83. https://doi.org/10.1080/110241598750004616.
- Kent A, Liversedge N, Dobbins B, McWhinnie D, Jan H. A prospective, randomized, controlled, double-masked, multi-center clinical trial of medical adhesives for the closure of laparoscopic incisions. J Minim Invasive Gynecol 2014;21:252-8. https://doi.org/10.1016/j.jmig.2013.10.003.
- Keng TM, Bucknall TE. A clinical trial of tissue adhesive (histoacryl) in skin closure of groin wounds. Med J Malaysia 1989;44:122-8.
- Khan RJ, Fick D, Yao F, Tang K, Hurworth M, Nivbrant B, et al. A comparison of three methods of wound closure following arthroplasty: a prospective, randomised, controlled trial. J Bone Joint Surg Br 2006;88:238-42. https://doi.org/10.1302/0301-620X.88B2.16923.
- Law NW, Ellis H. Exposure of the wound – a safe economy in the NHS. Postgrad Med J 1987;63:27-8. https://doi.org/10.1136/pgmj.63.735.27.
- Lawrentschuk N, Falkenberg MP, Pirpiris M. Wound blisters post hip surgery: a prospective trial comparing dressings. ANZ J Surg 2002;72:716-19. https://doi.org/10.1046/j.1445-2197.2002.02529.x.
- Livesey C, Wylde V, Descamps S, Estela CM, Bannister GC, Learmonth ID, et al. Skin closure after total hip replacement. J Bone Joint Surg 2009;91-B. https://doi.org/10.1302/0301-620X.91B6.21831.
- Michie DD, Hugill JV. Influence of occlusive and impregnated gauze dressings on incisional healing: a prospective, randomized, controlled study. Ann Plast Surg 1994;32:57-64. https://doi.org/10.1097/00000637-199401000-00011.
- Ong CC, Jacobsen AS, Joseph VT. Comparing wound closure using tissue glue versus subcuticular suture for pediatric surgical incisions: a prospective, randomised trial. Pediatr Surg Int 2002;18:553-5. https://doi.org/10.1007/s00383-002-0728-0.
- Pronio A, Di Filippo A, Narilli P, Caporillli D, Vestri A, Ciamberlano B, et al. Closure of cutaneous incision after thyroid surgery: a comparison between metal clips and cutaneous octyl-2-cyanoacrylate adhesive. A prospective randomized clinical trial. Eur J Plast Surg 2011;34:103-10. https://doi.org/10.1007/s00238-010-0477-6.
- Ravnskog FA, Espehaug B, Indrekvam K. Randomised clinical trial comparing Hydrofiber and alginate dressings post-hip replacement. J Wound Care 2011;20:136-42. https://doi.org/10.12968/jowc.2011.20.3.136.
- Romero P, Frongia G, Wingerter S, Holland-Cunz S. Prospective, randomized, controlled trial comparing a tissue adhesive (Dermabond) with adhesive strips (Steri-Strips) for the closure of laparoscopic trocar wounds in children. Eur J Ped Surg 2011;21:159-62. https://doi.org/10.1055/s-0030-1270458.
- Shamiyeh A, Schrenk P, Stelzer T, Wayand WU. Prospective randomized blind controlled trial comparing sutures, tape, and octylcyanoacrylate tissue adhesive for skin closure after phlebectomy. Dermatol Surg 2001;27:877-80.
- Sniezek PJ, Walling HW, DeBloom JR, Messingham MJ, VanBeek MJ, Kreiter CD, et al. A randomized controlled trial of high-viscosity 2-octyl cyanoacrylate tissue adhesive versus sutures in repairing facial wounds following Mohs micrographic surgery. Dermatol Surg 2007;33:966-71.
- Vogt KC, Uhlyarik M, Schroeder TV. Moist wound healing compared with standard care of treatment of primary closed vascular surgical wounds: a prospective randomized controlled study. Wound Repair Regen 2007;15:624-7. https://doi.org/10.1111/j.1524-475X.2007.00294.x.
- Wikblad K, Anderson B. A comparison of three wound dressings in patients undergoing heart surgery. Nurs Res 1995;44:312-16. https://doi.org/10.1097/00006199-199509000-00009.
- Wynne R, Botti M, Stedman H, Holsworth L, Harinos M, Flavell O, et al. Effect of three wound dressings on infection, healing comfort, and cost in patients with sternotomy wounds: a randomized trial. Chest 2004;125:43-9. https://doi.org/10.1378/chest.125.1.43.
- Sullivan T, Smith J, Kermode J, McIver E, Courtemanche DJ. Rating the burn scar. J Burn Care Rehabil 1990;11:256-60. https://doi.org/10.1097/00004630-199005000-00014.
- Hollander JE, Singer AJ, Valentine S, Henry MC. Wound registry: development and validation. Ann Emerg Med 1995;25:675-85. https://doi.org/10.1016/S0196-0644(95)70183-4.
- Elliott D. Bluebelle Study Group . Developing outcome measures assessing wound management and patient experience: a mixed methods study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-016155.
- University Hospitals Bristol NHS Foundation Trust . Consent and Request Form for Medical Photographs Video Recordings n.d.
- Johnston K, Buxton MJ, Jones DR, Fitzpatrick R. Assessing the costs of healthcare technologies in clinical trials. Health Technol Assess 1999;3.
- Petrou S, Murray L, Cooper P, Davidson LL. The accuracy of self-reported healthcare resource utilization in health economic studies. Int J Technol Assess Health Care 2002;18:705-10. https://doi.org/10.1017/S026646230200051X.
- Stallard N, Friede T. A group-sequential design for clinical trials with treatment selection. Stat Med 2008;27:6209-27. https://doi.org/10.1002/sim.3436.
- Sydes MR, Parmar MK, James ND, Clarke NW, Dearnaley DP, Mason MD, et al. Issues in applying multi-arm multi-stage methodology to a clinical trial in prostate cancer: the MRC STAMPEDE trial. Trials 2009;10. https://doi.org/10.1186/1745-6215-10-39.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Increasing response rates to postal questionnaires: systematic review. BMJ 2002;324. https://doi.org/10.1136/bmj.324.7347.1183.
- Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, Nazareth I, et al. Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-003821.
- Wilson AP, Helder N, Theminimulle SK, Scott GM. Comparison of wound scoring methods for use in audit. J Hosp Infect 1998;39:119-26. https://doi.org/10.1016/S0195-6701(98)90325-5.
- Parvizi D, Friedl H, Schintler MV, Rappl T, Laback C, Wiedner M, et al. Use of 2-octyl cyanoacrylate together with a self-adhering mesh (Dermabond TM Prineo TM) for skin closure following abdominoplasty: an open, prospective, controlled, randomized, clinical study. Aesthet Plast Surg 2013;37:529-37. https://doi.org/10.1007/s00266-013-0123-3.
- Gardezi SA, Chaudhary AM, Sial AK, Ahmad I, Rashid M. Role of ’polyurethane membrane’ in post operative wound management. J Pak Med Assoc 1983;33:219-22.
- Rohde H, Thon K, Stoltzing H, Schirren J. The transparent adhesive drape as post-operative dressing – a randomised clinical study for comparison with a conventional dressing. Der Chirurg 1981;52:46-50.
- Shinohara T, Yamashita Y, Satoh K, Mikami K, Yamauchi Y, Hoshino S, et al. Prospective evaluation of occlusive hydrocolloid dressing regarding the healing effect after abdominal operations: randomised controlled trial. As J Surg 2008;31:1-5. https://doi.org/10.1016/S1015-9584(08)60046-9.
- Persson M, Svenberg T, Poppen B. To dress or not to dress surgical wounds? Patients’ attitudes to wound care after major abdominal operations. Eur J Surg 1995;161:791-3.
- Ruiz-Tovar J, Llavero C, Morales V, Gamallo C. Total occlusive ionic silver-containing dressing vs mupirocin ointment application vs conventional dressing in elective colorectal surgery: effect on incisional surgical site infection. J Am Coll Surg 2015;221:424-9. https://doi.org/10.1016/j.jamcollsurg.2015.04.019.
- Phan M, Van der Auwera P, Andry G, Aoun M, Chantrain G, Deramaecker R, et al. Wound dressing in major head and neck cancer surgery: a prospective randomised study of gauze dressing vs sterile vaseline ointment. Eur J Surg Oncol 1993;19:10-6.
- Martin-Trapero C, Martin-Torrijos M, Fernandez-Conde L, Torrijos-Torrijos M, Manzano-Martin E, Pacheco-del Cerro JL, et al. Surgical site infections. Effectiveness of polyhexamethylene biguanide wound dressings. Enferm Clin 2013;23:56-61.
- Bennett K, Kellett W, Braun S, Spetalnick B, Huff B, Slaughter J, et al. Silver ion-eluting dressings for prevention of post cesarean wound infection: a randomized, controlled trial. Am J Obstet Gynecol 2013;208. https://doi.org/10.1016/j.ajog.2012.10.141.
- De Win M, De Blaere X, Scheers R. The Effect of Choice of Surgical Wound Dressing on the Direct Cost of Healing n.d.
- Politano A, Tracci M, Strider D, Sawyer R, Kern J, Upchurch G, et al. A Randomized, Prospective Study of Surgical Site Infections Following Vascular Reconstructive Surgery: Untreated Vs. Silver-Impregnated Dressings n.d.
- Ozaki CK, Hamdan AD, Barshes NR, Wyers M, Hevelone ND, Belkin M, et al. Prospective, randomized, multi-institutional clinical trial of a silver alginate dressing to reduce lower extremity vascular surgery wound complications. J Vasc Surg 2015;61:419-27. https://doi.org/10.1016/j.jvs.2014.07.034.
- Devlin N, Shah K, Feng Y, Mulhern B, Van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2016;27:7-22. https://doi.org/10.1002/hec.3564.
Appendix 1 Additional details of methods for value-of-information analysis
FIGURE 13.
Net-benefit model incorporating quality-of-life decrements resulting from a SSI, used in a sensitivity analysis to compare different wound dressings.
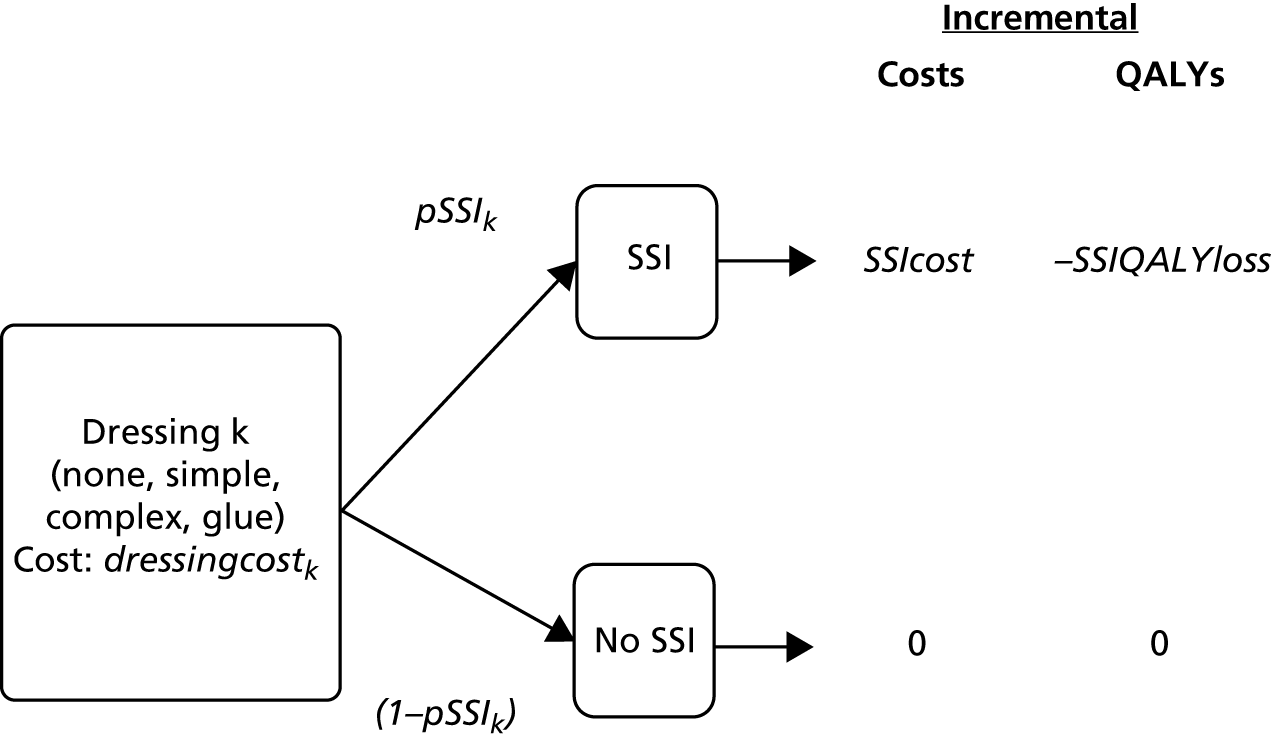
| Surgery type | Jenks et al.50 1 April 2010–31 March 2012, n (%) | PHE 1 April 2014–31 March 2015, n (%)a |
|---|---|---|
| Abdominal hysterectomy | 402 (18.1) | 3882 (10.3) |
| Bile duct, liver, pancreatic | 222 (10.0) | 2572 (6.8) |
| Cholecystectomy | 46 (2.1) | 999 (2.6) |
| Gastric | 228 (10.3) | 1239 (3.3) |
| Large bowel | 673 (30.4) | 18,500 (48.8) |
| Small bowel | 259 (11.7) | 4097 (10.8) |
| Multiple: intra-abdominal | 385 (17.4) | – (17.4) |
| All reported | 2215 (100) | 31,289 (82.6) |
| Study | Procedure | Wound contamination | Intervention group | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Surgery types representative of the Bluebelle population | |||||
| Law and Ellis 198786 | Inguinal hernia repair or high saphenous ligation | Clean |
I: Exposed C: Exposed BB: Exposed |
I: Gauze C: BWCD BB: Simple |
I: Opsite (5d) C: Film BB: Simple |
| Parvizi et al. 2013110 | Abdominoplasty | Clean |
I: Exposed C: Exposed BB: Exposed |
I: Prineo = dermabond + mesh C: Glue BB: Glue |
|
| Romero et al. 201193 | Laparoscopic appendicectomy in children | Clean |
I: Exposed C: Exposed BB: Exposed |
I: Dermabond C: Glue BB: Glue |
|
| Gardezi et al. 1983111 | General surgery | Mixed + unclear |
I: Gauze C: BWCD BB: Simple |
I: Polyurethane membrane C: Film BB: Simple |
|
| Rohde et al. 1981112 | Abdominal | Unclear |
I: fixomull-stretch C: BWCD BB: Simple |
I: Opsite C: Film BB: Simple |
|
| Michie and Hugill 199489 | Plastic and reconstructive surgery incision of < 200 mm, not exceeding 200 mm | Clean |
I: Xeroform (Medtronic, Dublin, Republic of Ireland) C: BWCD BB: Simple |
I: DuoDerm ExtraThin CGF (ConvaTec Ltd, Deeside, UK) C: Hydrocolloid BB: Complex |
|
| Shinohara et al. 2008113 | GI surgery | Clean + mixed + contaminated |
I: Gauze C: BWCD BB: Simple |
I: Karayahesive (Alcare, Tokyo, Japan) C: Hydrocolloid BB: Complex |
|
| Holm et al. 199882 | Abdominal incision of > 5 cm | Clean + mixed + contaminated |
I: Mepore (2d) C: BWCD BB: Simple |
I: Comfeel plus transparent dressing (10d) (Coloplast, Peterborough, UK) C: Hydrocolloid BB: Complex |
|
| Persson et al. 1995114 | Benign GI disease | Clean + contaminated |
I: Exposed, absorbent dressing (first 12 hours only) C: Exposed BB: Exposed |
I: DuoDerm E C: Hydrocolloid BB: Complex |
|
| Ruiz-Tovar et al. 2015115 | Colorectal | Mixed |
I: Gauze (5d) C: BWCD BB: Simple |
I: Silver (5d) C: Silver BB: Complex |
|
| Phan et al. 1993116 | H&N cancer | Clean + mixed |
I: Vaseline (Unilever, Leatherhead, UK) + chlorhexidine C: Exposed BB: Exposed |
I: Gauze + chlorhexidine C: BWCD BB: Simple |
|
| Martin-Trapero et al. 2013117 | Laparoscopic cholecystectomy | Clean |
I: Gauze C: BWCD BB: Simple |
I: PHMB antimicrobial C: PHMB antimicrobial BB: Complex |
|
| Other surgery types (not representative of Bluebelle population) | |||||
| Bennett et al. 2013118 | Caesarean | Clean + mixed |
I: Medipore C: BWCD BB: Simple |
I: SilverIon (Argentum Medical, Geneva, IL, USA) C: Silver BB: Complex |
|
| Lawrentschuk et al. 200287 | Hip surgery | Clean |
I: Interpose (Multigate, Villawood, NSW, Australia) C: BWCD BB: Simple |
I: Paraffin tulle [Jelonet (smith&nephew, Zaventem, Belgium)] C: BWCD BB: Simple |
|
| Cosker et al. 200578 | Hip and knee | Clean |
I: Primapore C: BWCD BB: Simple |
I: Tegaderm + pad or Opsite C: Film BB: Simple |
|
| De Win et al. 1998119 | Neurovascular or cardiovascular | Clean |
I: Mepore C: BWCD BB: Simple |
I: Tegaderm + pad C: Film BB: Simple |
|
| Wynne et al. 200498 | Cardiac surgery that required a median sternotomy incision | Clean |
I: Primapore (2d) C: BWCD BB: Simple |
I: Opsite (5d) C: Film BB: Simple |
I: DuoDerm Thin ConvaTec (5d) (ConvaTec Ltd, Deeside, UK) C: Hydrocolloid BB: Complex |
| Vogt et al. 200796 | Elective vascular | Clean |
I: Mepore (4d) C: BWCD BB: Simple |
I: Aquacel (4d) C: Hydrofibre BB: Complex |
|
| Burke et al. 201276 | Hip/knee surgery | Clean |
I: Mepore C: BWCD BB: Simple |
I: Jubilee (Aquacell + Duoderm) C: Hydrofibre BB: Complex |
|
| Politano et al. 2014120 | Vascular reconstructions | Clean |
I: Primapore C: BWCD BB: Simple |
I: Therabond C: Silver BB: Complex |
|
| Ozaki et al. 2015121 | Peripheral vascular disease | Clean + mixed |
I: Gauze (3d) C: BWCD BB: Simple |
I: Silver (3d) C: Silver BB: Complex |
|
Details are given on surgery type (grouped according to whether or not it matches the population of interest to the Bluebelle project), contamination status of the wounds in the included studies, and interventions in the groups of the included studies, classified by I, intervention; C, classification used in the Cochrane review; and BB, classification used in the Bluebelle project. See the Cochrane update review17 for full citations and details of included studies.
FIGURE 14.
Network plots showing comparisons that have been made between interventions (for three different classification schemes) in RCTs included in the Cochrane update review,17 for all surgery types. BB, Bluebelle project classification; BWCD, basic wound contact dressing; C, Cochrane review classification; I, intervention level; PHMB, polyhexamethylene biguanide.
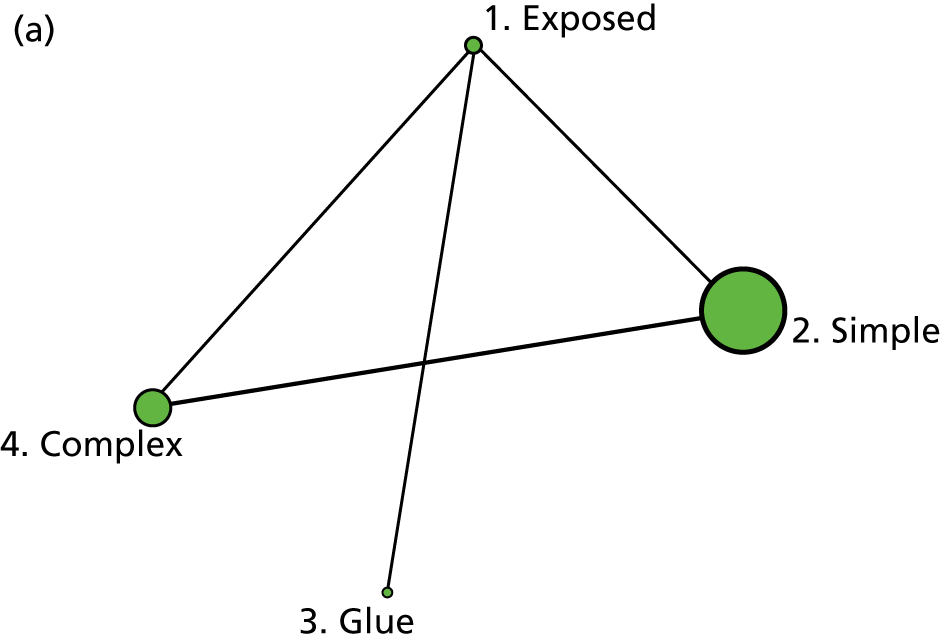
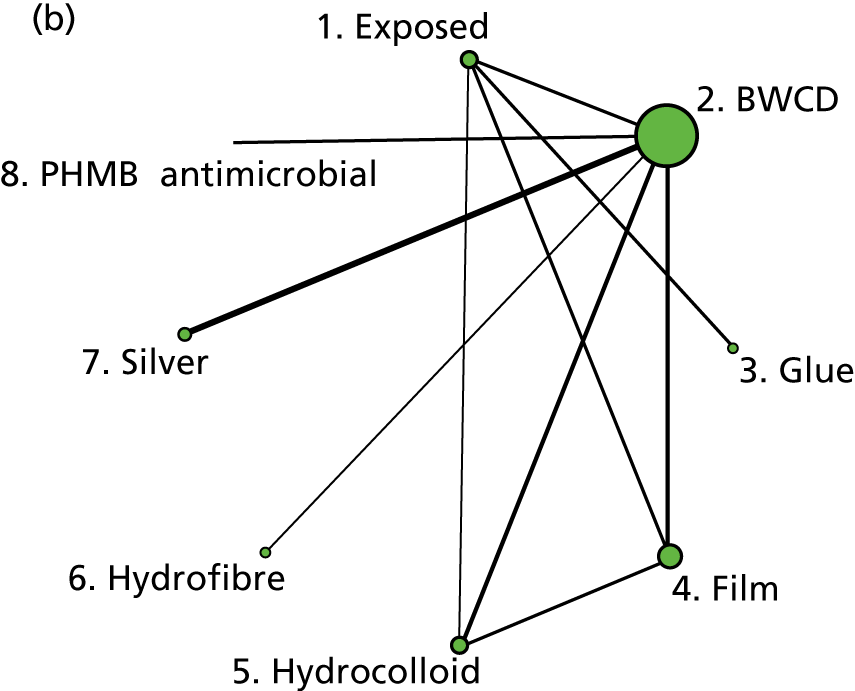
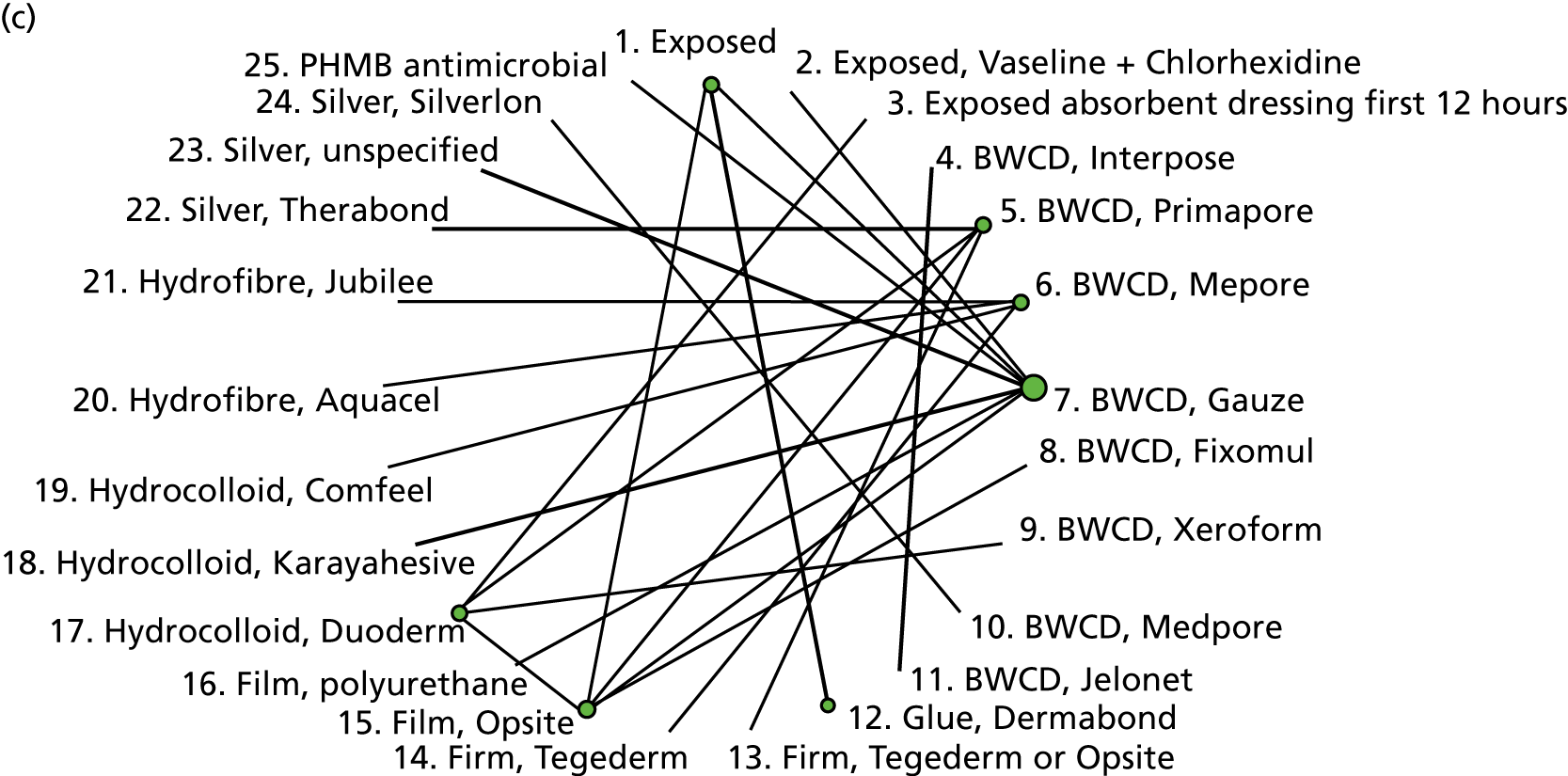
FIGURE 15.
The probability that each dressing type is ranked first, second, third or last for SSI outcome, plotted separately for (a) all surgery types and (b) Bluebelle population surgery types. Note that rank 1 is the best and rank 4 is the worst.
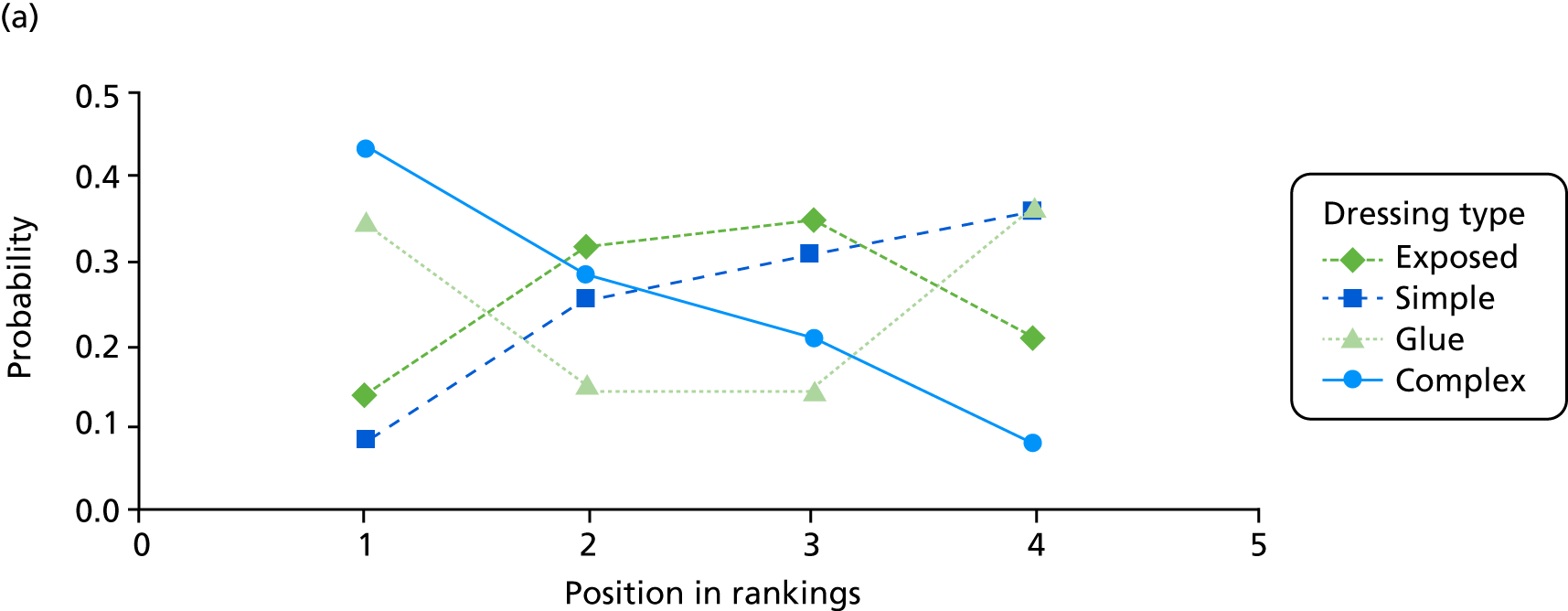
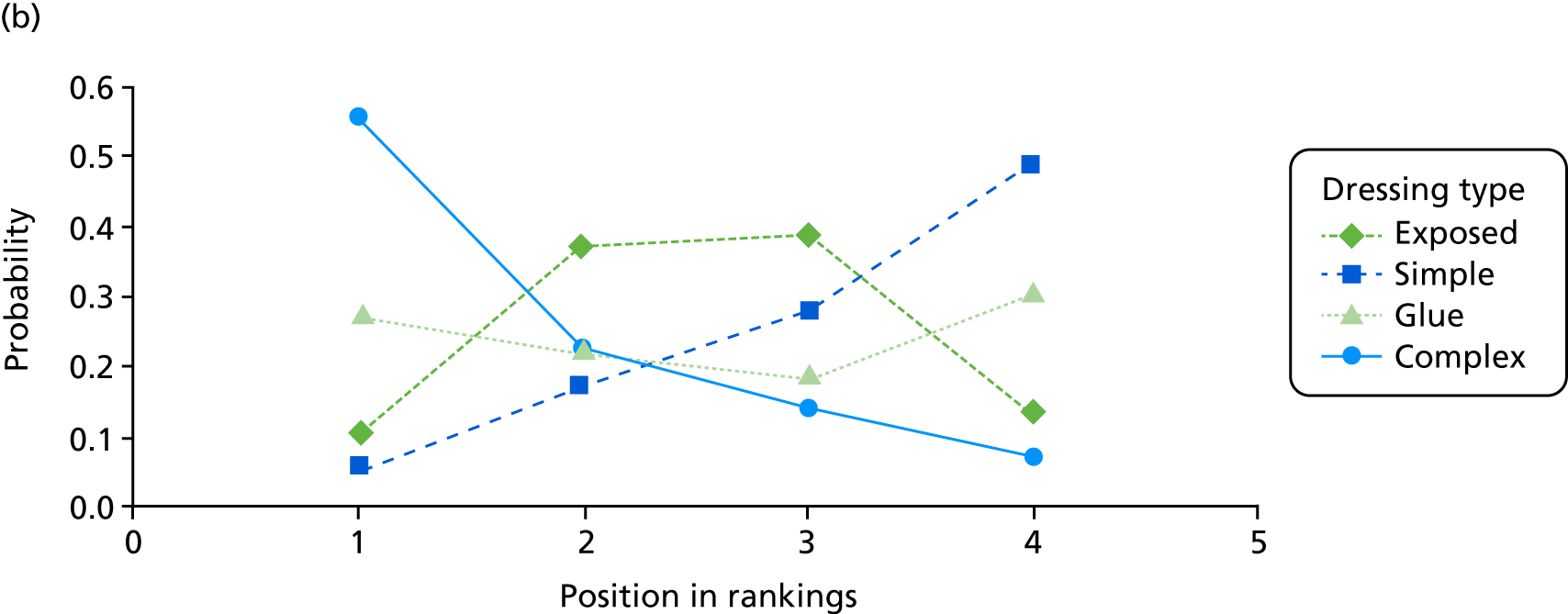
FIGURE 16.
The cumulative probability that each dressing type is ranked in that position or better for SSI outcome, plotted separately for (a) all surgery types and (b) Bluebelle population surgery types. Note that rank 1 is the best and rank 4 is the worst.
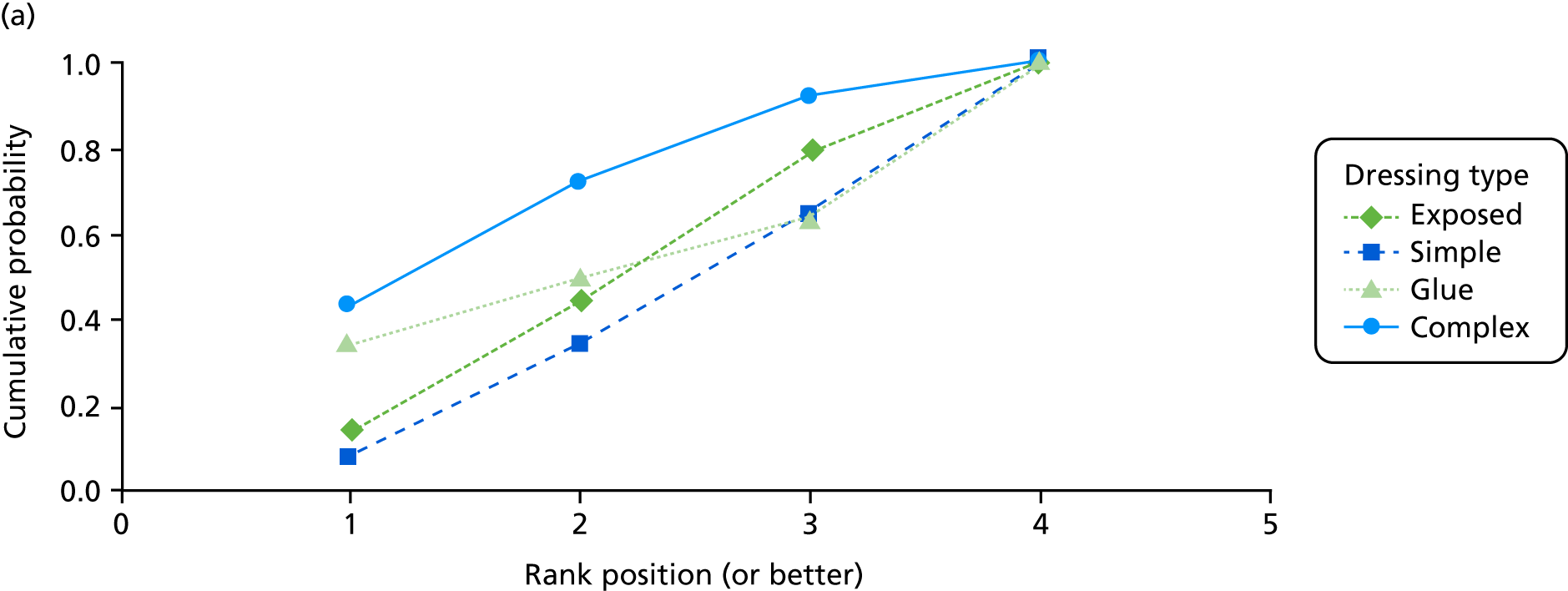
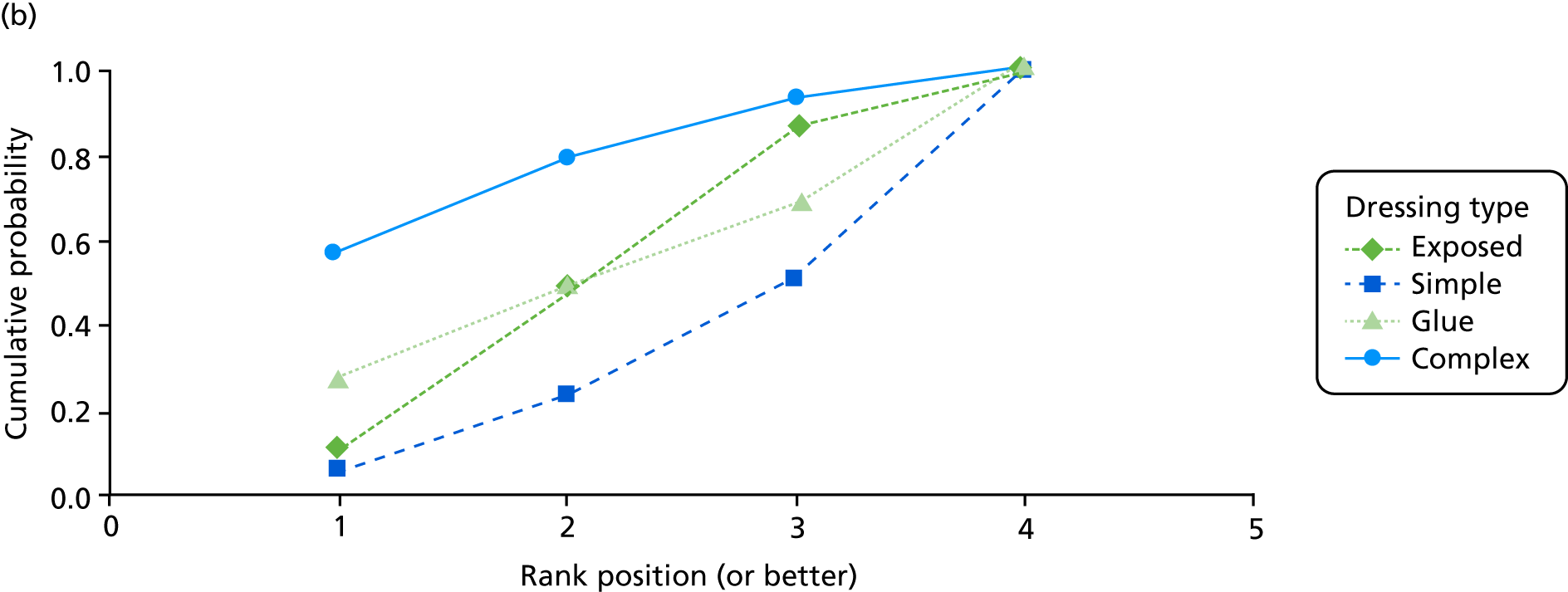
Appendix 2 Skin transfer to promote adherence
FIGURE 17.
Example of a skin transfer (modelled by a volunteer) that was applied near the wound(s) to promote adherence to the dressing allocation.

Appendix 3 Final versions of wound experience and wound management questionnaires
Appendix 4 Additional results of the value-of-information analysis
| Total sample size for balanced exposed vs. simple vs. glue vs. complex trial | EVSI per patient (£) | EVSI per 1.208 million wounds for 5 years (£) |
|---|---|---|
| 50 | 14 | 140M |
| 100 | 31 | 319M |
| 150 | 44 | 454M |
| 200 | 54 | 559M |
| 250 | 63 | 647M |
| 300 | 68 | 707M |
| 350 | 76 | 781M |
| 400 | 82 | 848M |
| 450 | 84 | 871M |
| 500 | 90 | 928M |
| 1000 | 116 | 1201M |
| 1500 | 128 | 1316M |
| 2000 | 135 | 1397M |
| 2500 | 142 | 1461M |
| 3000 | 145 | 1494M |
| 3500 | 148 | 1527M |
| 4000 | 151 | 1554M |
| 4500 | 154 | 1584M |
| 5000 | 154 | 1588M |
FIGURE 18.
Scenario A: decision options – exposed, simple, glue, complex. EVSI per patient for balanced four-group designs plotted against total sample size, at a willingness to pay per QALY of £20,000.
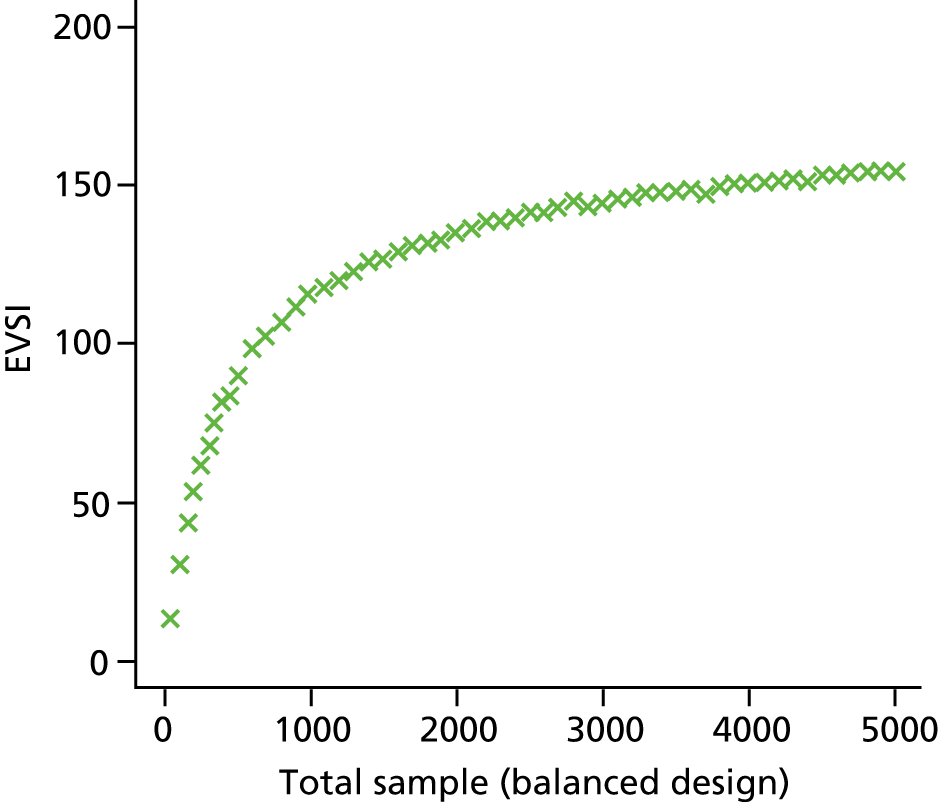
FIGURE 19.
Scenario A: decision options – exposed, simple, glue, complex. EVSI per patient for balanced designs plotted against total sample size, at a willingness to pay per QALY of £20,000. Results shown for designs with different numbers of groups and different included interventions. C, complex; E, exposed; G, glue; S, simple. EvSvC is a three-group trial of exposed vs. simple vs. complex.
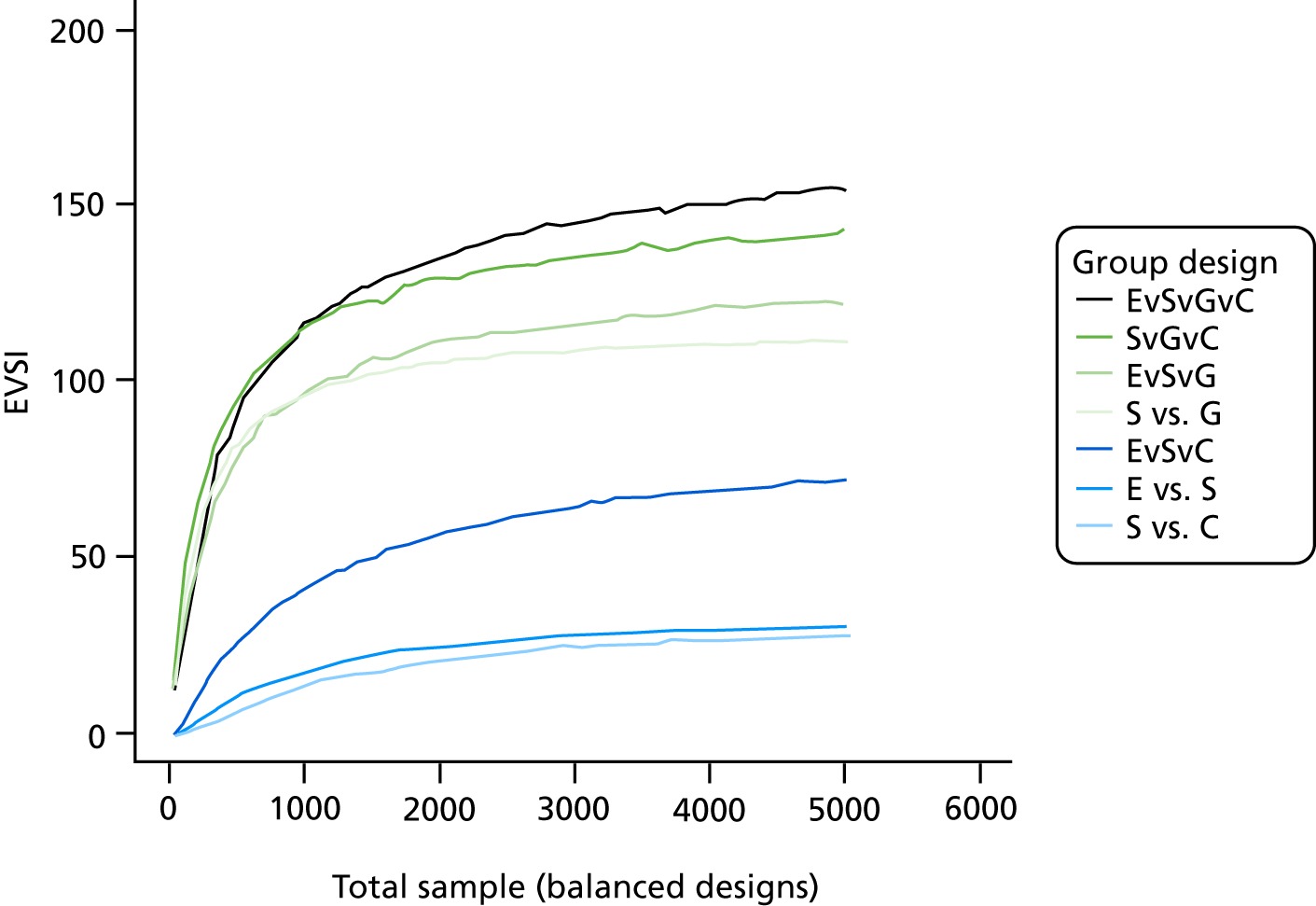
FIGURE 20.
Scenario A: decision options – exposed, simple, glue, complex. EVSI per patient for a three-group trial of exposed vs. simple vs. glue, comparing a balanced design (EvSvG) with a 2 : 2 : 1 allocation [EvSvG (2 : 2 : 1)], plotted against total sample size, at a willingness to pay per QALY of £20,000.
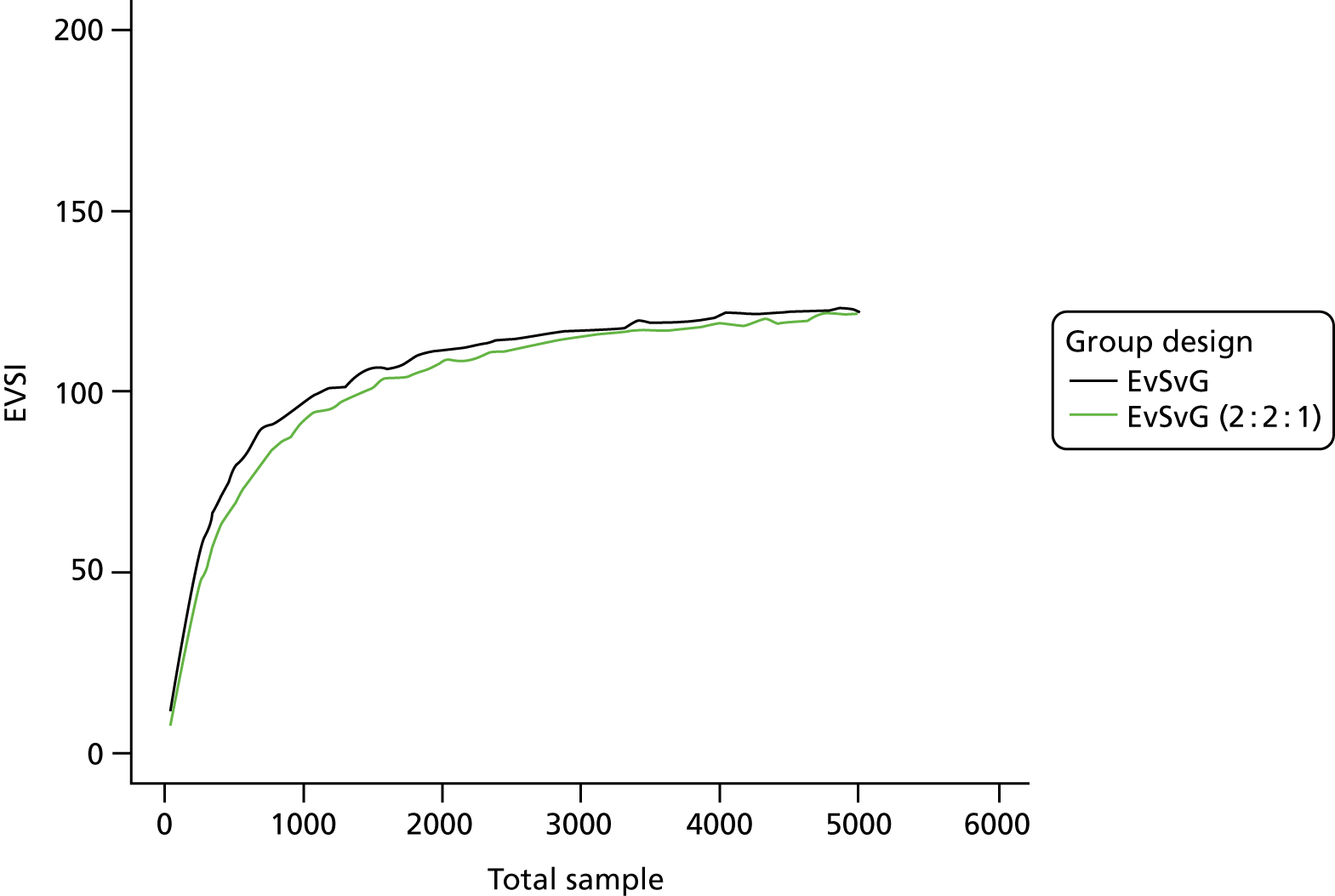
| Total sample size for balanced exposed vs. simple vs. glue trial | EVSI per patient (£) | EVSI per 2.208 million wounds for 5 years (£) |
|---|---|---|
| 50 | 48 | 496M |
| 100 | 75 | 775M |
| 150 | 93 | 9575M |
| 200 | 107 | 1107M |
| 250 | 118 | 1217M |
| 300 | 125 | 1292M |
| 350 | 131 | 1352M |
| 400 | 136 | 1407M |
| 450 | 143 | 1476M |
| 500 | 147 | 1515M |
| 1000 | 175 | 1802M |
| 1500 | 187 | 1926M |
| 2000 | 194 | 1997M |
| 2500 | 199 | 2057M |
| 3000 | 201 | 2069M |
| 3500 | 203 | 2090M |
| 4000 | 207 | 2131M |
| 4500 | 208 | 2147M |
| 5000 | 208 | 2150M |
FIGURE 21.
Scenario B: decision options – exposed, simple, glue. EVSI per patient for a three-group trial of exposed vs. simple vs. glue, comparing a balanced design (EvSvG) with a 2 : 2 : 1 allocation [EvSvG (2 : 2 : 1)], plotted against total sample size, at a willingness to pay per QALY of £20,000. E, exposed; G, glue; S, simple.
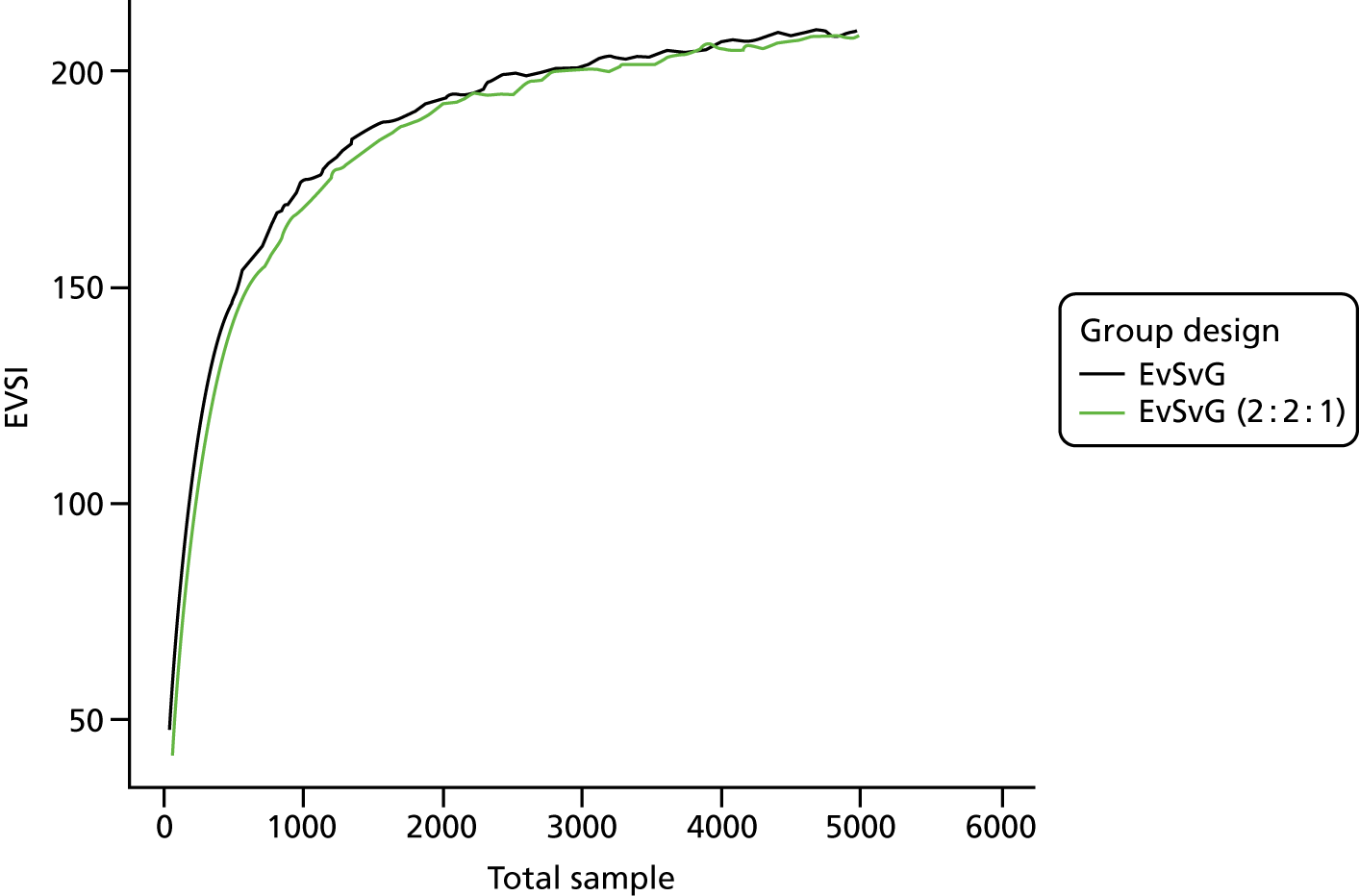
FIGURE 22.
Including Bluebelle Phase B ITT results. Scenario A: decision options – exposed, simple, glue, complex. EVSI per patient for balanced designs plotted against total sample size, at a willingness to pay per QALY of £20,000. Results shown for designs with different numbers of groups and different included interventions. C, complex; E, exposed; G, glue; S, simple. EvSvC is a three-group trial of exposed vs. simple vs. complex.
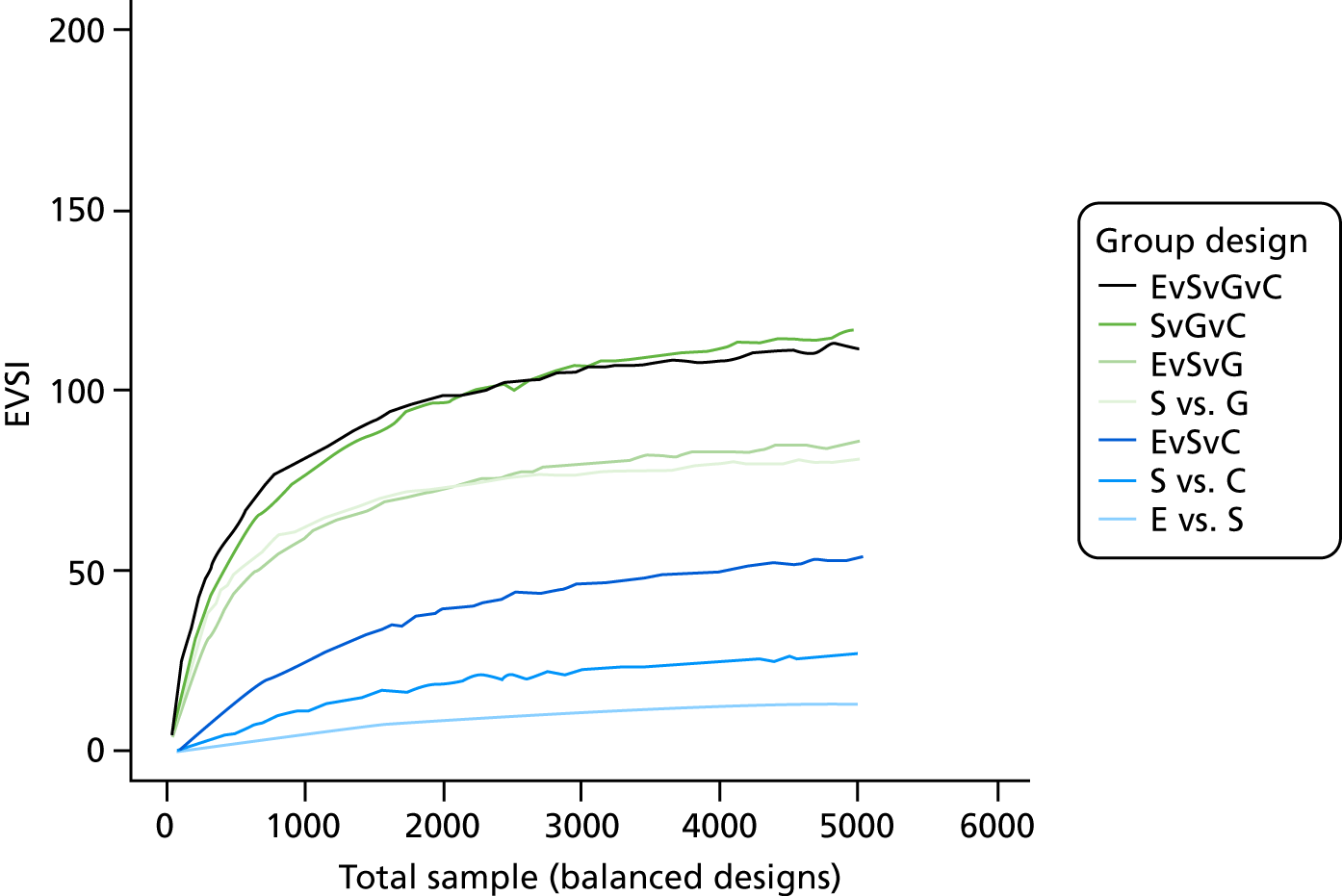
FIGURE 23.
Including Bluebelle Phase B ITT results. Scenario B: decision options – exposed, simple, glue. EVSI per patient for balanced designs plotted against total sample size, at a willingness to pay per QALY of £20,000. Results shown for designs with different numbers of groups and different included interventions. E, exposed; G, glue; S, simple. EvSvG is a three-group trial of exposed vs. simple vs. glue.
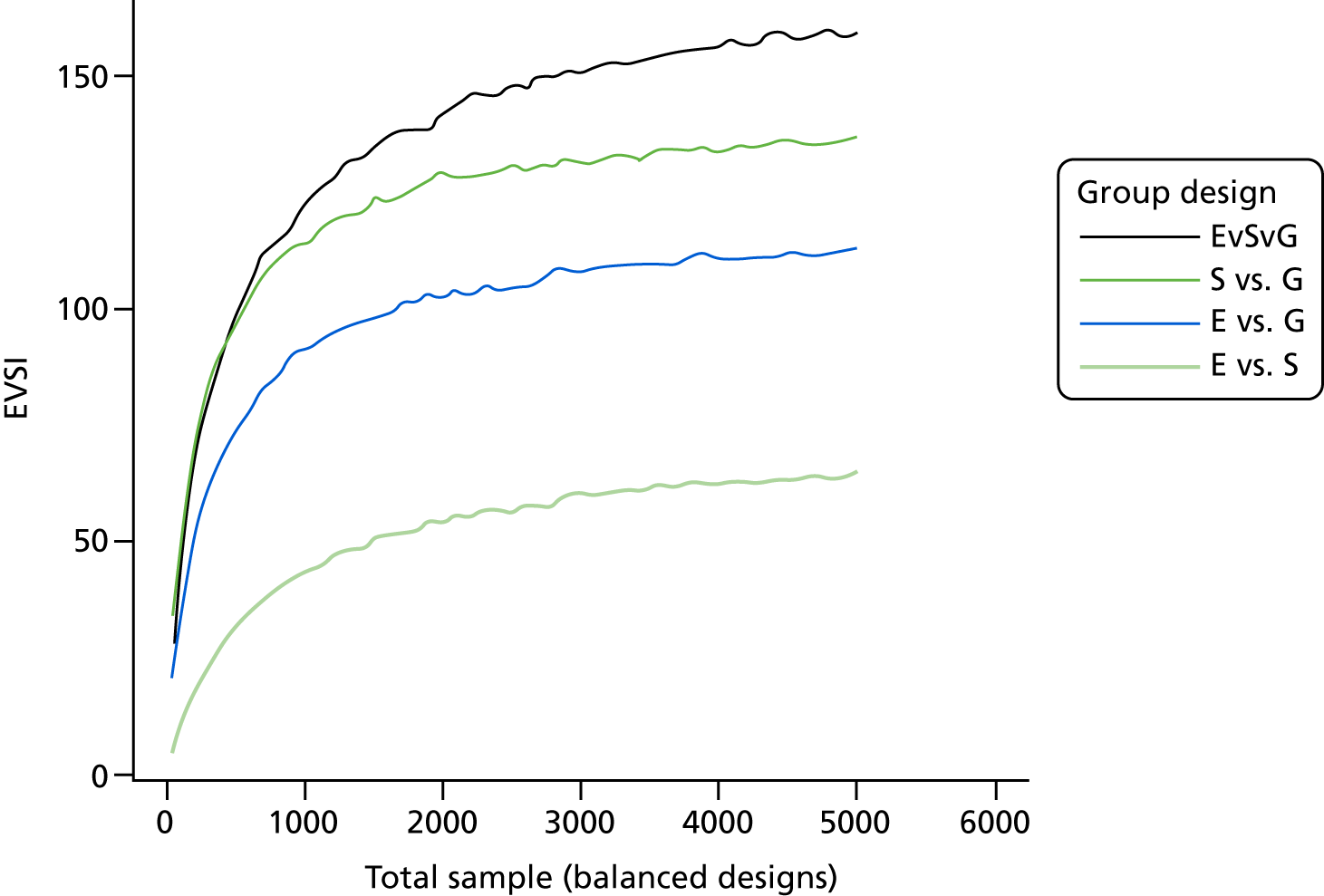
Appendix 5 Additional quantitative results of the Phase B pilot randomised controlled trial
FIGURE 24.
Recruitment over time.
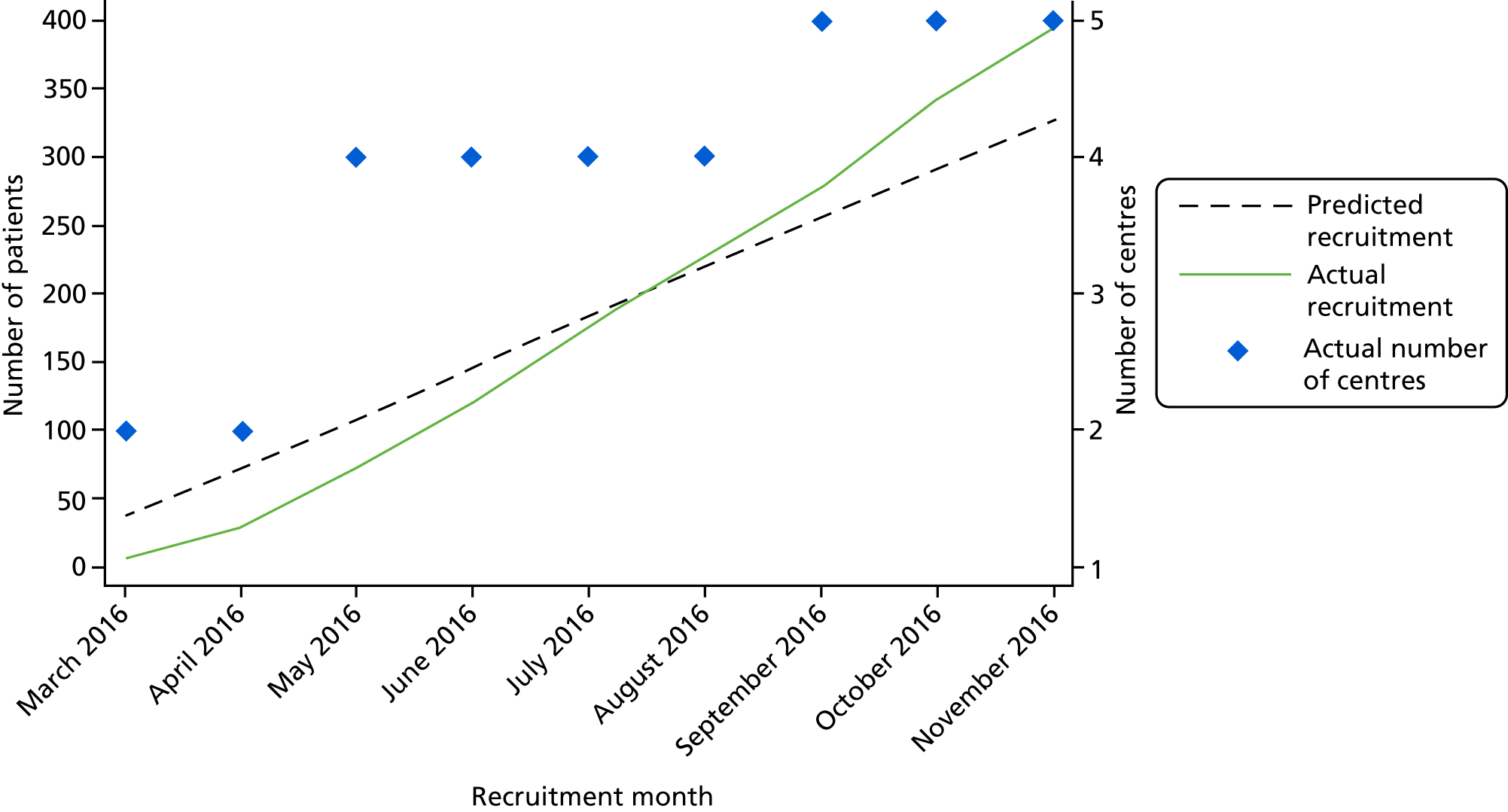
| Retention | Group, n/N (%) | Overall (n = 388), n/N (%) | ||
|---|---|---|---|---|
| Randomised to simple dressing (n = 131) | Randomised to glue-as-a-dressing (n = 126) | Randomised to no dressing (n = 131) | ||
| EQ-5D | ||||
| Baseline | 128/131 (97.7) | 126/126 (100.0) | 131/131 (100.0) | 385/388 (99.2) |
| 15 days | 90/128 (70.3) | 87/125 (69.6) | 92/129 (71.3) | 269/382 (70.4) |
| 4–8 weeks | 84/127 (66.1) | 78/122 (63.9) | 80/128 (62.5) | 242/377 (64.2) |
| WMQ at 4 days | 118/131 (90.1) | 121/125 (96.8) | 119/129 (92.2) | 358/385 (93.0) |
| WEQ at 4 days | 118/131 (90.1) | 119/125 (95.2) | 118/129 (91.5) | 355/385 (92.2) |
| Face-to-face reference SSI assessment at 4–8 weeksa | 97/127 (76.4) | 98/122 (80.3) | 107/128 (83.6) | 302/377 (80.1) |
| Observer WHQ at 4–8 weeksa | 93/127 (73.2) | 92/122 (75.4) | 101/128 (78.9) | 286/377 (75.9) |
| Participant WHQ at 4–8 weeks | 84/127 (66.1) | 85/122 (69.7) | 87/129 (67.4) | 256/378 (67.7) |
| Event type | Randomised to simple dressing (n = 131) | Randomised to glue-as-a-dressing (n = 126) | Randomised to no dressing (n = 131) | Overall (n = 388) | ||||
|---|---|---|---|---|---|---|---|---|
| Expected, n | Complete, n (%) | Expected, n | Complete, n (%) | Expected, n | Complete, n (%) | Expected, n | Complete, n (%) | |
| Occurrence of a SSI 4–8 weeks after randomisation | ||||||||
| Reference SSI assessment at 4–8 weeks | 127 | 97 (76.4) | 122 | 99 (81.1) | 128 | 107 (83.6) | 377 | 303 (80.4) |
| Abscess or other evidence of infection found during reoperation | 97 | 96 (99.0) | 99 | 99 (100.0) | 107 | 107 (100.0) | 303 | 302 (99.7) |
| Aspirated fluid/swab of surgical site yields organisms and pus cells present | 97 | 97 (100.0) | 99 | 99 (100.0) | 107 | 107 (100.0) | 303 | 303 (100.0) |
| Clinician’s diagnosis | 97 | 97 (100.0) | 99 | 98 (99.0) | 107 | 107 (100.0) | 303 | 302 (99.7) |
| Fever | 97 | 97 (100.0) | 99 | 99 (100.0) | 107 | 107 (100.0) | 303 | 303 (100.0) |
| Heat | 97 | 97 (100.0) | 99 | 98 (99.0) | 107 | 107 (100.0) | 303 | 302 (99.7) |
| Incision spontaneously dehisces or opened by surgeon | 97 | 97 (100.0) | 99 | 98 (99.0) | 107 | 107 (100.0) | 303 | 302 (99.7) |
| Localised pain and tenderness | 97 | 97 (100.0) | 99 | 98 (99.0) | 107 | 107 (100.0) | 303 | 302 (99.7) |
| Localised swelling | 97 | 97 (100.0) | 99 | 98 (99.0) | 107 | 107 (100.0) | 303 | 302 (99.7) |
| Purulent drainage | 97 | 97 (100.0) | 99 | 98 (99.0) | 107 | 107 (100.0) | 303 | 302 (99.7) |
| Redness | 97 | 97 (100.0) | 99 | 97 (98.0) | 107 | 107 (100.0) | 303 | 301 (99.3) |
| Type of SSI | 97 | 97 (100.0) | 99 | 98 (99.0) | 107 | 107 (100.0) | 303 | 302 (99.7) |
| Wound complications arising in hospital and up to 4–8 weeks post randomisation | ||||||||
| Postoperative wound complications (CRF D2) | 131 | 108 (82.4) | 126 | 103 (81.7) | 131 | 115 (87.8) | 388 | 326 (84.0) |
| Wound complications [4–8 weeks] (CRF F7) | 128 | 102 (79.7) | 122 | 103 (84.4) | 128 | 110 (85.9) | 378 | 315 (83.3) |
| Measures of resource use | ||||||||
| Surgery date | 131 | 131 (100.0) | 126 | 126 (100.0) | 131 | 131 (100.0) | 388 | 388 (100.0) |
| Time of knife-to-skin | 131 | 129 (98.5) | 126 | 124 (98.4) | 131 | 129 (98.5) | 388 | 382 (98.5) |
| Time of skin closure | 131 | 128 (97.7) | 126 | 123 (97.6) | 131 | 130 (99.2) | 388 | 381 (98.2) |
| Level 3: patient admitted to area? | 130 | 120 (92.3) | 126 | 118 (93.7) | 131 | 124 (94.7) | 387 | 362 (93.5) |
| Date of initial admission | 2 | 2 (100.0) | 0 | – | 3 | 3 (100.0) | 5 | 5 (100.0) |
| Time of initial admission | 2 | 1 (50.0) | 0 | – | 3 | 3 (100.0) | 5 | 4 (80.0) |
| Readmitted to this area? | 2 | 2 (100.0) | 0 | – | 3 | 3 (100.0) | 5 | 5 (100.0) |
| Date of readmission | 2 | 1 (50.0) | 0 | – | 0 | – | 2 | 1 (50.0) |
| Time of readmission | 2 | 0 (0.0) | 0 | – | 0 | – | 2 | 0 (0.0) |
| Level 2: patient admitted to area? | 130 | 120 (92.3) | 126 | 118 (93.7) | 131 | 124 (94.7) | 387 | 362 (93.5) |
| Date of initial admission | 13 | 13 (100.0) | 11 | 11 (100.0) | 12 | 12 (100.0) | 36 | 36 (100.0) |
| Time of initial admission | 13 | 9 (69.2) | 11 | 8 (72.7) | 12 | 10 (83.3) | 36 | 27 (75.0) |
| Readmitted to this area? | 13 | 13 (100.0) | 11 | 11 (100.0) | 12 | 11 (91.7) | 36 | 35 (97.2) |
| Date of readmission | 1 | 1 (100.0) | 1 | 1 (100.0) | 0 | – | 2 | 2 (100.0) |
| Time of readmission | 1 | 0 (0.0) | 1 | 1 (100.0) | 0 | – | 2 | 1 (50.0) |
| Level 1: patient admitted to area? | 130 | 124 (95.4) | 126 | 121 (96.0) | 131 | 126 (96.2) | 387 | 371 (95.9) |
| Date of initial admission | 103 | 103 (100.0) | 103 | 103 (100.0) | 101 | 100 (99.0) | 307 | 306 (99.7) |
| Time of initial admission | 103 | 71 (68.9) | 103 | 83 (80.6) | 101 | 72 (71.3) | 307 | 226 (73.6) |
| Readmitted to this area? | 103 | 100 (97.1) | 103 | 102 (99.0) | 101 | 99 (98.0) | 307 | 301 (98.0) |
| Date of readmission | 4 | 3 (75.0) | 4 | 3 (75.0) | 4 | 2 (50.0) | 12 | 8 (66.7) |
| Time of readmission | 4 | 3 (75.0) | 4 | 1 (25.0) | 4 | 2 (50.0) | 12 | 6 (50.0) |
| Date of discharge/death | 130 | 130 (100.0) | 126 | 125 (99.2) | 131 | 130 (99.2) | 387 | 385 (99.5) |
| Complication | Group, events/patients (%) | |||||
|---|---|---|---|---|---|---|
| Randomised to simple dressing | Randomised to glue-as-a-dressing | Randomised to no dressing | ||||
| Non-adherence to dressings (n = 3) | Adherence to dressings (n = 124) | Non-adherence to dressings (n = 8) | Adherence to dressings (n = 115) | Non-adherence to dressings (n = 20) | Adherence to dressings (n = 109) | |
| Dehiscence | 0/0 (0.0) | 2/2 (1.9) | 1/1 (20.0) | 0/0 (0.0) | 2/2 (11.1) | 1/1 (1.1) |
| Seroma | 0/0 (0.0) | 6/6 (5.7) | 0/0 (0.0) | 0/0 (0.0) | 1/1 (5.6) | 1/1 (1.1) |
| Haematoma | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 1/1 (5.6) | 0/0 (0.0) |
| Allergic response | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) |
| Wound infection (all types) | 0/0 (0.0) | 1/1 (1.0) | 1/1 (20.0) | 0/0 (0.0) | 1/1 (5.6) | 1/1 (1.1) |
| Skin loss | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) |
| Prolonged leakage and need for vacuum dressings | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 1/1 (5.6) | 0/0 (0.0) |
| Sepsis | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 3/2 (11.1) | 0/0 (0.0) |
| Pyrexia | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 1/1 (5.6) | 1/1 (1.1) |
| Reoperation for wound problems (all types)a | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 2/1 (5.6) | 0/0 (0.0) |
| Necrotising fasciitis | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 1/1 (5.6) | 0/0 (0.0) |
| Death | 0/0 (0.0) | 1/1 (1.0) | 1/1 (16.7) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) |
| Overall | 0/0 (0.0) | 10/10 (9.5) | 3/2 (33.3) | 0/0 (0.0) | 13/4 (22.2) | 4/2 (2.1) |
| Event type | Randomised to simple dressing (n = 102), events/patients (%) | Randomised to glue-as-a-dressing (n = 103), events/patients (%) | Randomised to no dressing (n = 110), events/patients (%) | Total participants with follow-up AE data (n = 315), events/patients (%) | ||||
|---|---|---|---|---|---|---|---|---|
| AE | SAE | AE | SAE | AE | SAE | AE | SAE | |
| Any event | 48/26 (25.5) | 10/3 (2.9) | 50/24 (23.3) | 5/2 (1.9) | 40/23 (20.9) | 6/3 (2.7) | 138/73 (23.2) | 21/8 (2.5) |
| Any wound dehiscence | 18/17 (16.7) | 3/3 (3.0) | 10/10 (9.7) | 0/0 (0.0) | 9/9 (8.2) | 1/1 (0.9) | 37/36 (11.4) | 4/4 (1.3) |
| Superficial | 16/15 (14.7) | 2/2 (2.0) | 10/10 (9.7) | 0/0 (0.0) | 8/8 (7.3) | 1/1 (0.9) | 34/33 (10.5) | 3/3 (1.0) |
| Deep | 2/2 (2.0) | 1/1 (1.0) | 0/0 (0.0) | 0/0 (0.0) | 1/1 (0.9) | 0/0 (0.0) | 3/3 (1.0) | 1/1 (0.3) |
| Wound seroma | 3/3 (2.9) | 1/1 (1.0) | 10/10 (9.7) | 0/0 (0.0) | 5/5 (4.5) | 0/0 (0.0) | 18/18 (5.7) | 1/1 (0.3) |
| Wound haematoma | 1/1 (1.0) | 0/0 (0.0) | 2/2 (1.9) | 0/0 (0.0) | 2/2 (1.8) | 0/0 (0.0) | 5/5 (1.6) | 0/0 (0.0) |
| Allergic response to wound dressing | 3/3 (2.9) | 0/0 (0.0) | 1/1 (1.0) | 0/0 (0.0) | 1/1 (0.9) | 0/0 (0.0) | 5/5 (1.6) | 0/0 (0.0) |
| Wound infection (all types) | 17/16 (15.7) | 3/3 (3.0) | 16/16 (15.7) | 1/1 (1.0) | 15/15 (13.6) | 3/3 (2.7) | 48/47 (15.0) | 7/7 (2.2) |
| Skin loss | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) |
| Prolonged leakage and need for vacuum dressings | 2/2 (2.0) | 2/2 (2.0) | 1/1 (1.0) | 0/0 (0.0) | 0/0 (0.0) | 0/0 (0.0) | 3/3 (1.0) | 2/2 (0.6) |
| Sepsis as a consequence of a SSI | 0/0 (0.0) | 0/0 (0.0) | 2/2 (1.9) | 1/1 (1.0) | 1/1 (0.9) | 1/1 (0.9) | 3/3 (1.0) | 2/2 (0.6) |
| Pyrexia (> 38 °C) as a consequence of a SSI | 4/4 (3.9) | 1/1 (1.0) | 5/5 (4.9) | 1/1 (1.0) | 6/6 (5.5) | 1/1 (0.9) | 15/15 (4.8) | 3/3 (1.0) |
| Reoperation for wound problems (all types)a | 0/0 (0.0) | 0/0 (0.0) | 3/1 (1.0) | 2/1 (1.0) | 1/1 (0.9) | 0/0 (0.0) | 4/2 (0.6) | 2/1 (0.3) |
Appendix 6 Additional health economic information from the Phase B pilot randomised controlled trial
| Category | Number of participants contributing data | Percentage |
|---|---|---|
| Dressings and redressings in hospital | 378 | 97 |
| Hospital appointments after initial discharge | 319 | 82 |
| Primary care appointments after initial discharge | 314 | 81 |
| Redressings after initial discharge (cost incurred by the NHS) | 379 | 98 |
| Redressings after initial discharge (cost incurred by patients) | 379 | 98 |
| New medicines | 316 | 81 |
FIGURE 25.
Participant self-assessment and observer assessments available for analysis. ACA took into account all available observations. Although this analysis uses a larger sample (i.e. it is not restricted to cases where all observations were complete), it is based on different subsets of the data.
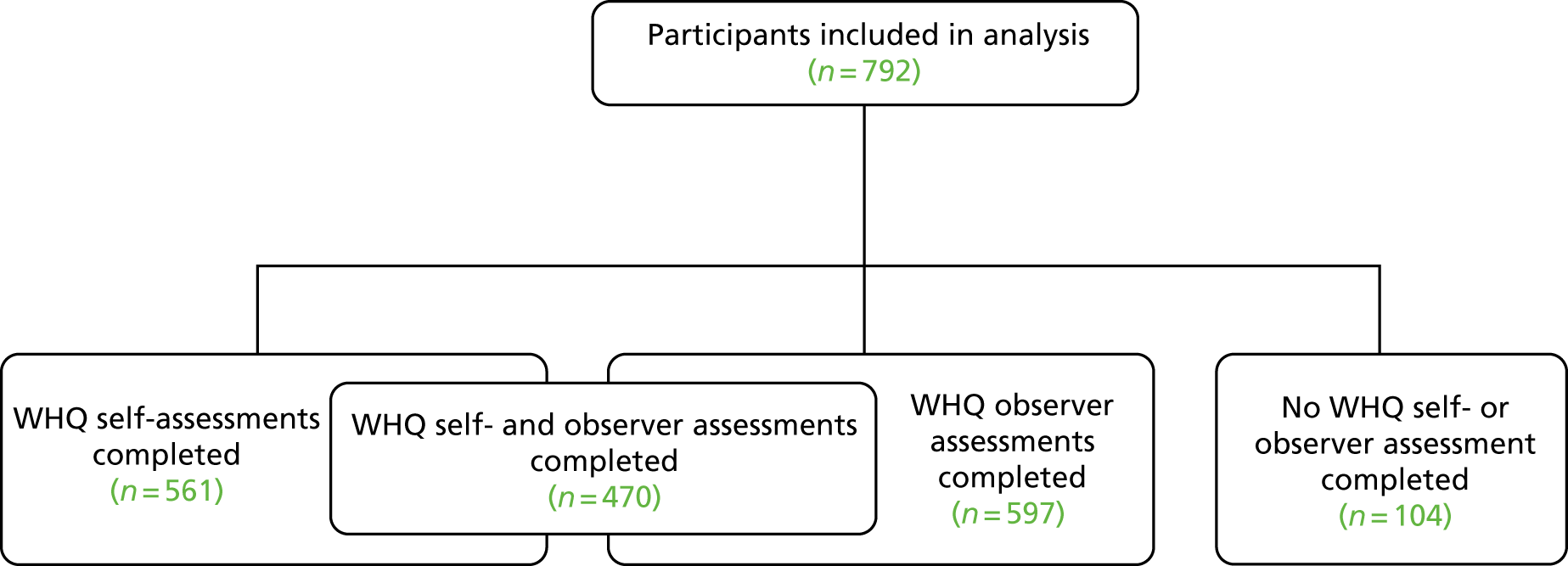
Available-case analysis showed that the highest mean per-patient cost was seen in the ‘simple dressing’ group (£119), followed by the ‘no dressing’ group (£109) and the ‘glue-as-a-dressing’ group (£106) (see Table 48 and Figure 26, which show the mean per-patient cost for resource use category by treatment arm). As in the CCA above, the greatest proportion of the total per-patient cost in the ‘no-dressing arm’ is attributed to hospital visits after discharge, followed by the cost of new medicines. In the ‘simple dressing’ arm, the total cost is largely made up the cost of new medicines, the cost of in-hospital dressings and redressings, and, to a lesser extent, the cost of hospital appointments after initial discharge. For patients in the ‘glue-as-a-dressing’ arm, the largest proportion of the total cost is attributable to the cost of dressings and redressings in the hospital, followed by the cost of new medicines and services received in the primary care.
| Allocated intervention | Mean cost (£) | SE (£)a | 95% CI (£)a |
|---|---|---|---|
| No dressing | 109.04 | 50.01 | 11.02 to 207.07 |
| Simple dressing | 119.07 | 23.27 | 73.47 to 164.67 |
| Glue-as-a-dressing | 106.73 | 13.14 | 80.97 to 132.49 |
FIGURE 26.
Total cost by cost category and allocated intervention (ACA).
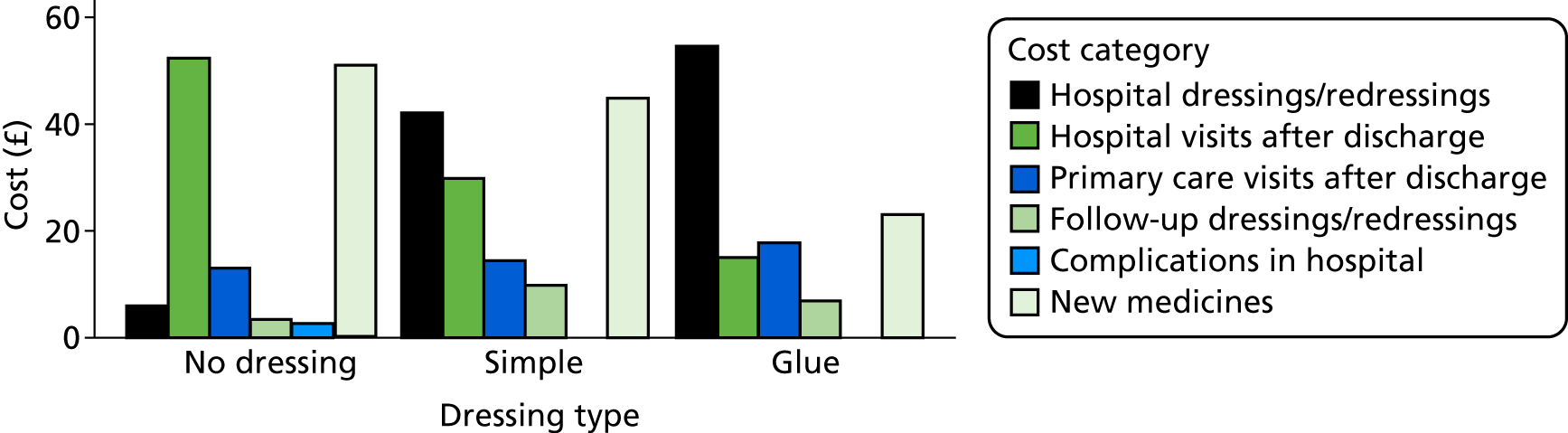
Differences in mean per-patient cost between trial groups are presented in Table 48. Here the mean cost differences range from just under £10 (for no dressing vs. simple dressing) to just under £30 (for no dressing vs. glue-as-a-dressing) and, as with the CCA, none of these differences reached statistical significance.
| Cost category | Number of observations | Percentage of all patients (n = 388) | Total cost per patient (£) | Total cost (£) by allocated intervention (n) | ||
|---|---|---|---|---|---|---|
| No dressing | Simple dressing | Glue-as-a-dressing | ||||
| Cost of dressings and redressings in hospital | 378 | 97 | 33.93 | 6.00 (128) | 42.07 (127) | 54.59 (123) |
| Cost of hospital appointments after initial discharge | 319 | 82 | 32.63 | 52.36 (110) | 29.82 (102) | 15.02 (107) |
| Cost of primary care appointments after initial discharge | 314 | 81 | 15.08 | 13.08 (110) | 14.40 (100) | 17.84 (104) |
| Cost of redressings after initial discharge incurred by NHS | 379 | 98 | 6.71 | 3.47 (129) | 9.81 (127) | 6.92 (123) |
| Cost of redressings after initial discharge incurred by patients | 379 | 98 | 0.34 | 0.05 (129) | 0.57 (127) | 0.40 (123) |
| Cost of complications arising while in hospital | 327 | 84 | 0.94 | 2.69 (115) | 0.00 (108) | 0.00 (104) |
| Cost of new medicines | 316 | 81 | 39.59 | 50.57 (110) | 44.82 (101) | 23.05 (105) |
| Total cost (ACA) (£) | 388 | 100 | 111.68 | 109.04 (131) | 119.07 (131) | 106.73 (126) |
Appendix 7 Additional information from the Wound Healing Questionnaire validation study
| Characteristic | Phase | |
|---|---|---|
| A (n = 414) | B (n = 378) | |
| Male, n/N (%) | 209/414 (50.5) | 155/378 (41.0) |
| Mean age (years) (SD) | 54.9 (17.2) | 51.4 (17.6) |
| Ethnicity, n/N (% white) | 380/398 (95.5) | 333/377 (88.3) |
| Mean BMI (kg/m2) (SD)a | 27.6 (6.09) | 28.4 (6.01) |
| ASA grade, n/N (%) | ||
| I | 98/357 (27.5) | 134/374 (35.8) |
| II | 178/357 (49.9) | 195/374 (52.1) |
| III | 75/357 (21.0) | 43/374 (11.5) |
| IV | 6/357 (1.7) | 2/374 (0.5) |
| Smoker, n/N (%) | ||
| Yes | 56/400 (14.0) | 58/376 (15.4) |
| Ex (> 1 month) | 103/400 (25.8) | 133/376 (35.4) |
| No | 241/400 (60.3) | 185/376 (49.2) |
| Diabetes mellitus, n/N (%) | ||
| Any type | 34/399 (8.5) | 26/376 (6.9) |
| None | 339/399 (85.0) | 350/376 (93.1) |
| Unsure | 26/399 (6.5) | (Option not provided) |
| Surgery type, n/N (%) | ||
| Elective | 289/391(73.9) | 317/354 (89.6) |
| Unplanned | 102/391 (26.1) | 37/354 (10.5) |
| Operation time (hours), n/N (%) | ||
| < 1 | 52/402 (12.9) | 161/369 (43.6) |
| 1–2 | 95/402 (23.6) | 87/369 (23.6) |
| 2–3 | 80/402 (19.9) | 59/369 (16.0) |
| > 3 | 156/402 (38.8) | 62/369 (16.8) |
| Unsure | 19/402 (4.7) | (Option not provided) |
| Number of wounds, n/N (%) | ||
| 1 | 187/398 (47.0) | 159/370 (43.0) |
| 2–4 | 180/398 (45.2) | 177/370 (47.8) |
| > 5 | 31/398 (7.8) | 34/370 (9.2) |
| Characteristic | Completed WHQ self-assessments (n = 561) | No WHQ self- or observer assessment (n = 104) |
|---|---|---|
| Male, n/N (%) | 278/561 (49.6) | 43/104 (41.4) |
| Mean age (years) (SD) | 56.3 (16.5) | 47.0 (18.4) |
| ASA grade, n/N (%) | ||
| I | 146/510 (28.6) | 37/97 (38.1) |
| II | 277/510 (54.3) | 39/97 (40.2) |
| III | 81/510 (15.9) | 20/97 (20.6) |
| IV | 6/510 (1.2) | 1/97 (1.0) |
| Surgery, n/N (%) | ||
| Elective | 453/529 (85.6) | 65/99 (65.7) |
| Unplanned | 76/529 (14.4) | 34/99 (34.3) |
| Mean EQ-5D-5L index score (SD)a | 0.79 (0.20) | 0.75 (0.22) |
| Debriefing item | n (%) |
|---|---|
| Time taken to complete questionnaire (minutes) | |
| < 5 | 167 (55.3) |
| 6–10 | 109 (36.1) |
| > 10 | 26 (8.6) |
| Help needed for any items | 15 (5.0) |
| Confusion/difficulty answering any items | 18 (6.0) |
| Item | Response categories | |
|---|---|---|
| 1 | Was there redness spreading away from the wound? (erythema/cellulitis) | Not at all/a little/quite a bit/a lot |
| 2 | Was the area around the wound warmer than the surrounding skin? | Not at all/a little/quite a bit/a lot |
| 3 | Has any part of the wound leaked clear fluid? (serous exudate) | Not at all/a little/quite a bit/a lot |
| 4 | Has any part of the wound leaked blood-stained fluid? (haemoserous exudate) | Not at all/a little/quite a bit/a lot |
| 5 | Has any part of the wound leaked thick and yellow/green fluid (pus/purulent exudate) | Not at all/a little/quite a bit/a lot |
| 6 | (i) Have the edges of any part of the wound separated/gaped open on their own accord? (spontaneous dehiscence) | Not at all/a little/quite a bit/a lot |
| (ii) Did the deeper tissue separate? | Not at all/a little/quite a bit/a lot | |
| 7 | Has the area around the wound become swollen? | Not at all/a little/quite a bit/a lot |
| 8 | Has the wound been smelly? | Not at all/a little/quite a bit/a lot |
| 9 | Has the wound been painful to touch? | Not at all/a little/quite a bit/a lot |
| 10 | Have you had, or felt like you have had, a raised temperature or fever? (fever ≥ 38 °C) | Not at all/a little/quite a bit/a lot |
| 11 | Have you sought advice because of a problem with your wound, other than at a routine planned follow-up appointment? | Yes/no |
| 12 | Has anything been put on the skin to cover the wound? (dressing) | Yes/no |
| 13 | Have you been back into hospital for treatment with a problem with your wound? | Yes/no |
| 14 | Have you been given antibiotics for a problem with you wound? | Yes/no |
| 15 | Have the edges of your wound been deliberately separated by a doctor or nurse? | Yes/no |
| 16 | Has your wound been scraped or cut to remove any unwanted tissue? (debridement of wound) | Yes/no |
| 17 | Has your wound been drained? (drainage of pus/abscess) | Yes/no |
| 18 | Have you had an operation under general anaesthetic for treatment of a problem with your wound? | Yes/no |
| Total score cut-off threshold | Sensitivity (%) | Specificity (%) |
|---|---|---|
| ≥ 0 | 100.00 | 0.00 |
| ≥ 1 | 93.75 | 24.43 |
| ≥ 2 | 93.75 | 48.86 |
| ≥ 3 | 90.63 | 60.23 |
| ≥ 4 | 90.63 | 77.84 |
| ≥ 5 | 87.50 | 85.80 |
| ≥ 6 | 78.13 | 89.20 |
| ≥ 7 | 78.13 | 92.61 |
| ≥ 8 | 71.88 | 93.75 |
| ≥ 9 | 68.75 | 97.73 |
| ≥ 10 | 65.63 | 98.86 |
| ≥ 11 | 59.38 | 98.86 |
| ≥ 12 | 53.13 | 99.43 |
| ≥ 13 | 46.88 | 99.43 |
| ≥ 15 | 40.63 | 100.00 |
| ≥ 17 | 28.13 | 100.00 |
| ≥ 18 | 18.75 | 100.00 |
| ≥ 19 | 15.63 | 100.00 |
| ≥ 20 | 12.50 | 100.00 |
| ≥ 26 | 9.38 | 100.00 |
| ≥ 28 | 6.25 | 100.00 |
| ≥ 30 | 3.13 | 100.00 |
| > 30 | 0.00 | 100.00 |
Appendix 8 Pilot of participants taking photographs of their wounds after discharge
FIGURE 27.
Flow of participants in pilot of participants taking wound photographs after discharge.
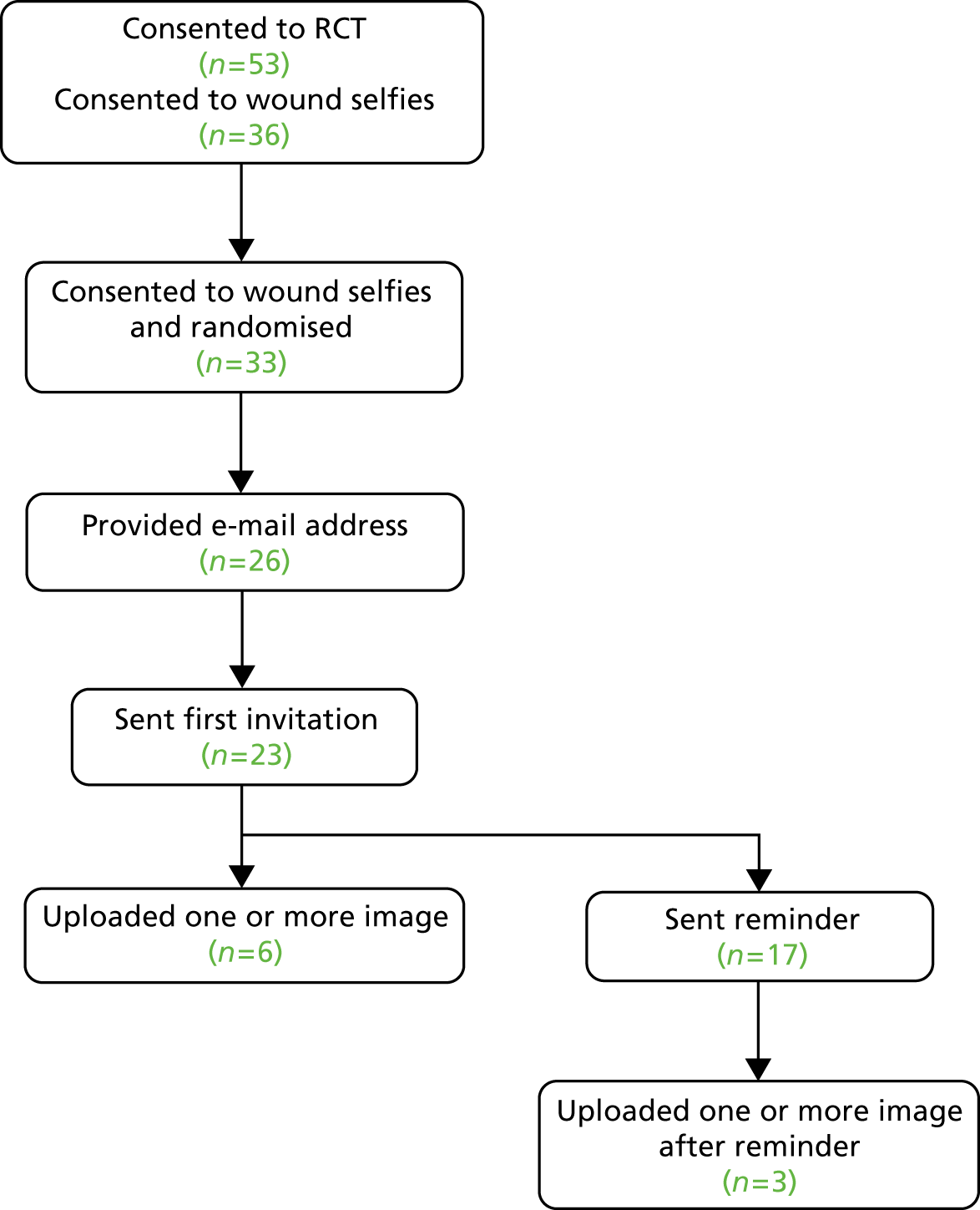
List of abbreviations
- ACA
- available-case analysis
- AE
- adverse event
- ASA
- American Society of Anesthesiologists
- ASEPSIS
- Additional treatment, the presence of Serous discharge, Erythema, Purulent exudate, and Separation of the deep tissues, the Isolation of bacteria, and the duration of inpatient Stay
- BMI
- body mass index
- BNF
- British National Formulary
- CCA
- complete-case analysis
- CDC
- Centers for Disease Control and Prevention
- CI
- confidence interval
- CRF
- case report form
- CTEU
- Clinical Trials and Evaluation Unit
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EVPPI
- expected value of partial perfect information
- EVSI
- expected value of sample information
- GI
- gastrointestinal
- GP
- general practitioner
- H1
- hypothesis 1
- H2
- hypothesis 2
- HCP
- health-care professional
- HRA
- Health Research Authority
- HTA
- Health Technology Assessment
- IM&T
- Information Management and Technology
- IQR
- interquartile range
- ITT
- intention to treat
- MRC
- Medical Research Council
- NIHR
- National Institute for Health Research
- NMA
- network meta-analysis
- NRES
- National Research Ethics Service
- PHE
- Public Health England
- PIL
- participant information leaflet
- PP
- per protocol
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QRI
- QuinteT Recruitment Intervention
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- ROC
- receiver operating characteristic
- SMG
- Study Management Group
- SPARCS
- Severn and Peninsula Audit and Research Collaborative for Surgeons
- SPIRIT
- Standard Protocol Items: Recommendations for Interventional Trials
- SSC
- Study Steering Committee
- SSI
- surgical site infection
- VoI
- value of information
- WEQ
- Wound Experience Questionnaire
- WHQ
- Wound Healing Questionnaire
- WMQ
- Wound Management Questionnaire
- WMRC
- West Midlands Research Collaborative