Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/134/06. The contractual start date was in January 2013. The draft report began editorial review in September 2017 and was accepted for publication in June 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Gavin D Perkins reports grants and non-financial support from the Intensive Care Foundation during the conduct of the study. Daniel McAuley reports personal fees from consultancy for GlaxoSmithKline (London, UK), SOBI (Swedish Orphan Biovitum; Stockholm, Sweden), Peptinnovate Ltd (Stevenage, UK), Boehringer Ingelheim (Ingelheim am Rhein, Germany) and Bayer AG (Leverkusen, Germany). Outside the submitted work, his institution has received funds from grants from the UK National Institute for Health Research (NIHR), the Wellcome Trust and others, and from GlaxoSmithKline for Daniel McAuley undertaking bronchoscopy as part of a clinical trial. In addition, Daniel McAuley is one of four named inventors on a patent US8962032 covering the use of sialic acid-bearing nanoparticles as anti-inflammatory agents issued to his institution, Queen’s University Belfast (www.google.com/patents/US8962032). Daniel McAuley is a member of the Health Technology Assessment (HTA) General Board. James Varley reports non-financial support from La Jolla Pharmaceutical Company (San Diego, CA, USA) and personal fees from Emas Pharma (Hitchin, UK) outside the submitted work. Nicholas Hart reports grants from Guy’s and St Thomas’ Charity during the conduct of the study, grants from Philips Respironics (Murraysville, PA, USA), non-financial support from Philips Respironics RT Meeting (Myotrace), personal fees from Fisher & Paykel Healthcare (Auckland, New Zealand), grants from ResMed (San Diego, CA, USA), grants from B & D Electromedical (Stratford-upon-Avon, UK), grants from Fisher & Paykel Healthcare. In addition, Nicholas Hart has a patent, Myotrace, pending and he is on the Pulmonary Research Advisory Board for Philips. Nicholas Hart’s Lane Fox Clinical Respiratory Physiology Research Group has received unrestricted grants (managed by Guy’s and St Thomas’ Foundation Trust) from Philips Respironics, Philips, ResMed, Fisher & Paykel Healthcare, and B & D Electromedical. Philips Respironics and Philips Research are contributing to the development of the Myotrace technology.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Perkins et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
The widespread use of positive-pressure ventilation in intensive care units (ICUs) can be traced back to the polio epidemic in Denmark in 1952. Among a cohort of 316 patients admitted to hospital with polio affecting the bulbar muscles, mortality fell from 80% to 23% following the introduction of early tracheostomy and positive-pressure ventilation. 1 The lack of mechanical ventilators at the time required teams of medical students to work around the clock in shifts to provide positive-pressure ventilation manually. The demonstrated life-saving potential of invasive positive-pressure ventilation led to an acceleration in the development of mechanical ventilators and the birth of modern-day intensive care. 2
Epidemiology of positive-pressure ventilation
Each year, an estimated 20 million people worldwide receive invasive mechanical ventilation (IMV). 3 In the UK, approximately 110,000 people require IMV annually. 4 In a large cohort study, the main reasons for requiring mechanical ventilation were acute respiratory failure (69%), coma (16%), acute exacerbation of chronic lung disease (10%) and neuromuscular disease (2%). The causes of acute respiratory failure were postoperative (20%), pneumonia (14%), congestive cardiac failure (10%), sepsis (9%), trauma (8%), acute respiratory distress syndrome (4.5%) and other (12%). 5,6 Reports from the Intensive Care National Audit and Research Centre (ICNARC) indicate that the median duration of ventilation is 5 days [interquartile range (IQR) 3–10 days] and the median length of stay on an ICU is 7 days (IQR 4–14 days). 4 Patients spend, on average, 17 days in hospital (IQR 9–31 days). Mortality during ICU admission has fallen over recent years and is currently around 28%. 5,6
Weaning from ventilation
Weaning is the process of liberating a patient from mechanical ventilation. It involves transferring the work of breathing from the ventilator to the patient. Weaning accounts for 40–50% of the time a patient requires positive-pressure ventilation. 7 Strategies to optimise weaning should find a balance between withdrawing ventilator support too early and unnecessarily prolonging ventilation. Premature withdrawal runs the risk of reintubation, which is associated with prolonged hospital stay, increased costs, increased tracheostomy use and increased mortality. 8,9 By contrast, delayed weaning is associated with increased adverse effects, such as ventilator-associated pneumonia (VAP),10,11 sinusitis,12 upper airway damage,13 respiratory muscle weakness13 and increased mortality. 14,15 The requirement for sedative and muscle relaxant drugs during mechanical ventilation may further contribute to delirium, immobility and generalised weakness. 16 The observations that 10–15%7 of patients require reintubation during the weaning process and that almost half of patients with an unplanned self-extubation during the weaning period do not require reintubation17 suggest that there is scope for improvement in current weaning approaches.
Weaning involves several stages (Figure 1). After treating the underlying illness and ensuring that there are no contraindications to weaning, a spontaneous breathing trial (SBT) is undertaken. 7 During a SBT, minimal support is provided from the ventilator and a combination of clinical and physiological measurements is used to determine whether or not the patient can breathe unaided.
FIGURE 1.
Steps involved in liberating a patient from IMV. ARF, acute respiratory failure.
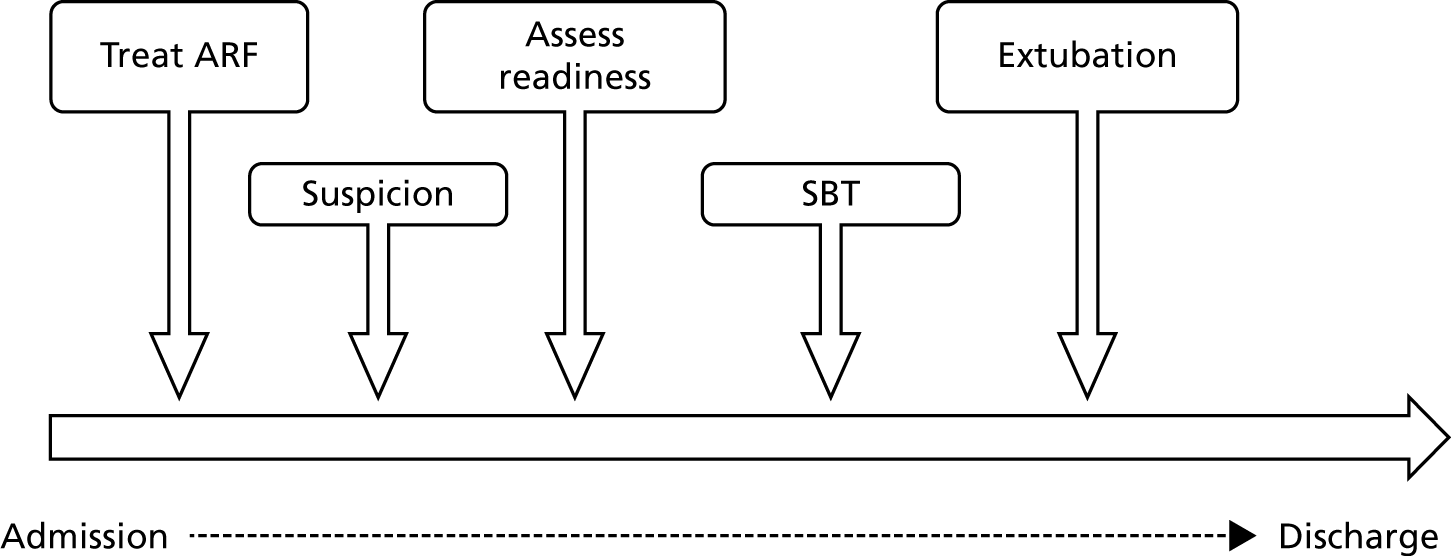
Patients who ‘pass’ the SBT and are considered otherwise ready for extubation have their tube removed. This group of patients, which represents two-thirds of mechanically ventilated patients, has a good prognosis, with an ICU mortality of approximately 5%. 7 The one-third of patients who ‘fail’ the SBT have a reported mortality of 25–30%. 7 Weaning practices after failing an initial SBT are variable. SBTs are often repeated on a daily basis until either extubation or a tracheostomy is performed.
Non-invasive ventilation as an adjunct to weaning
Non-invasive ventilation (NIV) refers to the delivery of mechanical ventilation using a mask, nasal pillows, helmet or mouthpiece interface instead of an endotracheal tube. Similar to IMV, NIV reduces the work of breathing and can improve gas exchange. 18 NIV may avoid some complications associated with prolonged endotracheal intubation, such as VAP, sinusitis and ventilator-induced lung injury. 19 In the context of weaning, NIV has been used to facilitate early extubation, to prevent respiratory failure after extubation in high-risk patients and as a rescue therapy when respiratory failure occurs following extubation. 18
Existing knowledge
A Cochrane review examined the effectiveness of NIV for weaning from IMV across 16 randomised controlled trials (RCTs) that recruited 994 patients. 20 Nine trials enrolled only patients with chronic obstructive pulmonary disease (COPD), whereas seven trials included mixed or non-COPD populations. The review found strong evidence that weaning using NIV reduced mortality [risk ratio (RR) 0.53, 95% confidence interval (CI) 0.36 to 0.80], although heterogeneity was moderate (I2 = 37%). The beneficial effect seemed limited to studies that enrolled only patients with COPD (RR 0.36, 95% CI 0.24 to 0.56) in contrast to the studies that enrolled mixed populations (RR 0.81, 95% CI 0.47 to 1.40). As shown in Table 1, NIV in this context had a number of other benefits.
| Outcome | Patient population | |
|---|---|---|
| COPD | Mixed | |
| Weaning failure, RR (95% CI) | 0.52 (0.36 to 0.74) | 0.73 (0.35 to 1.51) |
| Nosocomial pneumonia, RR (95% CI) | 0.22 (0.13 to 0.37) | 0.38 (0.15 to 0.93) |
| Hospital LOS (days), mean difference (95% CI) | –6.91 (–10.83 to –1.00) | –4.02 (–9.41 to 1.36) |
| ICU LOS (days), mean difference (95% CI) | –6.66 (–9.41 to –3.92) | –3.32 (–6.78 to 0.15) |
| Average total duration of mechanical ventilatory support (days), mean difference (95% CI) | –5.77 (–10.64 to –0.91) | 0.17 (–4.01 to 4.35) |
| Duration of endotracheal mechanical ventilation (days), mean difference (95% CI) | –7.53 (–11.47 to –3.60) | –6.85 (–10.75 to –2.95) |
| Reintubation, RR (95% CI) | 0.49 (0.35 to 0.70) | 0.82 (0.47 to 1.43) |
| Tracheostomy, RR (95% CI) | 0.04 (0.00 to 0.60) | 0.23 (0.09 to 0.57) |
| Arrhythmia, RR (95% CI) | 2.0 (0.20 to 19.78) | 0.74 (0.26 to 2.17) |
Need for a trial
The generalisability of the findings from the Cochrane review20 to current UK practice is limited. There are four main reasons for this. First, the treatment pathway for an exacerbation of COPD has changed since these early trials were conducted. Many patients who would have previously received IMV for respiratory failure now have ward- or ICU-based NIV as a strategy to prevent the need for IMV. 21 IMV is now reserved mainly for patients who fail a trial of NIV. The population of patients ventilated for COPD in contemporary UK practice therefore differs from that enrolled in trials ≥ 10 years ago. Second, none of the trials recruited patients from the UK. This research team’s collaboration in the International Survey of Weaning practices shows marked differences in weaning practices between countries. 22 Third, 3 out of the 12 studies (comprising nearly 20% of patients) are either unpublished or published as abstracts only. This limits assessment of methodological quality, and minimal information is available about the population recruited and the interventions tested. Fourth, when it was possible to assess the methodological quality of index trials, the quality of the methods was variable and eight trials had evidence of being at high risk of bias. There was also variation in the methods used to identify patients for weaning (e.g. four trials used a unique resolution of pulmonary infection criterion, which is rarely used in UK practice) and in the approaches to titration and discontinuation of ventilator support.
Although the results of the Cochrane review20 are encouraging, the size and limitations of trials conducted to date leave uncertainty as to the clinical effectiveness and cost-effectiveness of NIV as a routine tool to facilitate weaning from mechanical ventilation. This is likely to explain the limited penetration of this weaning approach into UK ICU practice. This topic is important to the intensive care community. The need for additional trials in this area was identified by the Intensive Care Society during its Research Prioritisation Exercise in 2008. 23 With these considerations it was timely to conduct a well-designed, appropriately powered randomised control trial (RCT) to examine the clinical effectiveness and cost-effectiveness of NIV-facilitated weaning in the NHS.
The UK’s National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme called for applications to examine the clinical effectiveness and cost-effectiveness of using NIV as an intermediate step in weaning patients from IMV.
The objective of the trial was defined in the commissioning brief (Table 2).
| Research question | What is the clinical effectiveness and cost-effectiveness of using NIV as an intermediate step in weaning patients off IMV? |
| Technology | NIV as an intermediate step in the protocolised weaning of patients off IMV |
| Patient group | Patients with respiratory failure requiring IMV |
| Setting | ICUs |
| Control/comparator treatment | Protocolised weaning that does not include the use of NIV |
| Design | RCT with internal pilot study. The pilot study should include clear continuation criteria, including an assessment of the likelihood of satisfactory recruitment to the full trial |
| Important outcomes | Reintubation rate, time from extubation to meeting discharge criteria, ventilator days, cost-effectiveness. Other outcomes: adverse events, ICU LOS, mortality |
| Minimum follow-up | 1 month |
The Breathe trial investigators were competitively selected to conduct a pragmatic, randomised, controlled, open, multicentre, effectiveness trial to determine if the use of NIV as an intermediate step in the protocolised weaning of patients from IMV is clinically effective and cost-effective.
Chapter 2 Methods and assessment
Trial summary
The Breathe trial was a pragmatic, randomised, controlled, open-label, multicentre, effectiveness trial to determine if protocolised weaning that includes early extubation on to NIV is clinically effective and cost-effective compared with weaning without NIV.
Patients with respiratory failure who had received IMV for > 48 hours (from the time of intubation) and failed a SBT were randomised in a 1 : 1 ratio to either the invasive or the non-invasive weaning strategy.
Data were collected on patient demographic characteristics, mechanical ventilation and other relevant variables relating to acute care. Variables required to determine health-related quality of life (HRQoL) were sought from all surviving patients at 90 and 180 days after randomisation.
The primary effectiveness outcome was time from randomisation to successful liberation from ventilation. Liberation from ventilation was defined as the time point at which the patient was free of ventilatory (invasive or non-invasive) support for > 48 hours.
Secondary clinical outcome measures were mortality at 30, 90 and 180 days; duration of IMV and total ventilator days (IMV and NIV); time to meeting ICU discharge criteria; proportion of patients receiving antimicrobials for presumed respiratory infection and total days receiving antimicrobials; reintubation rates; and the proportion of patients receiving a tracheostomy. Safety outcomes were adverse events (AEs) and serious adverse events (SAEs). HRQoL was assessed by completion of the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), and Short Form questionnaire-12 items (SF-12) at baseline (estimated retrospectively), and at 90 and 180 days.
The primary economic outcome was incremental cost per quality-adjusted life-year (QALY) gained from the perspective of the NHS and Personal Social Services (PSS). Secondary economic outcomes were cost of ICU stay (level 2 or 3 days), cost of hospital stay and utilisation of NHS and PSS resources after discharge.
A within-trial economic evaluation that covered the follow-up period of the RCT (to 180 days after randomisation) and a modelling-based economic evaluation that extrapolated cost-effectiveness over a lifetime time horizon were performed. Both were expressed in terms of incremental cost per QALY gained.
The trial was reviewed and approved by the Oxford C Research Ethics Committee (REC) (REC reference number 12/SC/0515).
Eligibility criteria for participants
Adult patients in a participating ICU who had received IMV continuously for > 48 hours (from the time of intubation) were assessed daily for their readiness to commence weaning. Readiness to wean was assessed and declared by the treating clinician/ICU clinical team. The trial eligibility criteria are presented in Box 1.
-
Aged ≥ 16 years.
-
Respiratory failure requiring IMV for > 48 hours (from the time of intubation).
-
Readiness to wean.
-
Failed a SBT.
-
Known to be pregnant.
-
Presence of tracheostomy.
-
Unable to protect airway because of neurological deficit.
-
Any absolute contraindication to NIV.
-
Home ventilation prior to ICU admission (excluding nocturnal CPAP support).
-
Decision not to reintubate or withdrawal of care anticipated.
-
Further surgery/procedure requiring sedation planned in next 48 hours.
-
Previous participation in the Breathe trial.
-
Ventilator unavailable to deliver NIV.
CPAP, continuous positive airway pressure.
Assessment of readiness to start weaning
The clinician with overall responsibility for managing the patient’s ICU treatment assessed the patient’s readiness to start weaning and to undergo a SBT.
Clinicians were provided with information about the Walsh criteria, which were suggested as guidance to indicate when the patient was ready commence weaning. 24 The Walsh criteria recommend that all the following conditions be met to indicate readiness for weaning:
-
co-operative and pain free; good cough
-
ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen (PaO2 : FiO2 ratio) of > 24 kPa
-
positive end-expiratory pressure (PEEP) of < 10 cmH2O
-
haemoglobin level of > 7 g/dl
-
axillary temperature of 36.0–38.5 °C
-
vasoactive drugs reduced or unchanged over previous 24 hours
-
spontaneous ventilatory frequency of > 6 breaths per minute.
Patients who were judged to be ready to wean were established on pressure support (Psupp) ventilation. The level of Psupp was titrated to achieve patient comfort, tidal volumes of 6–8 ml per kg of ideal body weight and respiratory rate of < 30 breaths per minute. Once the patient was stable on Psupp ventilation for at least 60 minutes, a SBT was undertaken.
Spontaneous breathing trial
A survey of practice identified that there were three main types of SBT used in the UK – a T-piece trial, use of continuous positive airway pressure (CPAP) and low-level Psupp (5–7 cmH2O). A T-piece trial involves the patient breathing spontaneously through their endotracheal tube, with the appropriate inspired oxygen concentration being maintained by a crossflow device (T-piece). CPAP involves leaving a standing pressure of 5–10 cmH2O, delivered via the ventilator, at the top of the endotracheal tube but with no assistance on inspiration. Low-level Psupp provides minimal inspiratory assistance. Clinicians were able to undertake one of these three types of SBT in accordance with local unit practices. The SBT was scheduled to last for at least 30 minutes and could be increased to up to 120 minutes in patients who were considered to be at higher risk of reintubation (e.g. prolonged ventilation, past history of COPD, heart failure).
During the SBT, patients were closely monitored for signs of distress or fatigue as described by the International Consensus Conference on Weaning (Box 2). 25 A patient was considered to pass the SBT if no signs of distress or fatigue developed. A patient who displayed any sign of distress or fatigue was judged to have failed the SBT. These patients required further weaning and were potentially eligible to be enrolled in the Breathe trial.
-
Heart rate at ≥ 20% of baseline or > 140 beats per minute.
-
Systolic blood pressure ≥ 20% of baseline or > 180 mmHg or < 90 mmHg.
-
Cardiac arrhythmias.
-
Respiratory rate of ≥ 50% of baseline value or > 35 breaths per minute.
-
Respiratory rate (minutes)/tidal volume (l) of > 105 breaths per minute per litre.
-
Arterial blood gases: PaO2 of < 8 kPa on FiO2 of ≥ 0.5 or SpO2 of < 90%.
-
PaCO2 of > 6.5 kPa or an increase of > 1 kPa.
-
-
pH of < 7.32 or a reduction in pH of > 0.07.
-
Agitation and anxiety.
-
Depressed mental status.
-
Sweating/clammy.
-
Cyanosis.
-
Increased respiratory effort (i.e. accessory muscle use, facial distress, dyspnoea).
FiO2, fraction of inspired oxygen; PaCO2, partial pressure of carbon dioxide in arterial blood; PaO2, partial pressure of oxygen in arterial blood; SpO2, saturation of oxygen in peripheral blood.
Consent
The two-stage consent process adopted for the Breathe trial maximised patient involvement in the decision-making process. It was developed with support from patient representatives and based on national laws where the trial was being conducted.
First, whenever possible, the patient’s view on enrolment was sought. Owing to the presence of an endotracheal tube, which limited communication, and likely recent exposure to sedative drugs, this process consisted of briefly imparting information about the trial and inviting the patient to give a view on participation. If the patient expressed a willingness to be involved or was unwilling to express an opinion, then we proceeded to stage two.
In stage two, a full overview of the trial was provided to the patient’s (personal or professional) consultee. The consultee was asked to express a view on what the patient would have decided if they had capacity to make a decision.
Randomisation
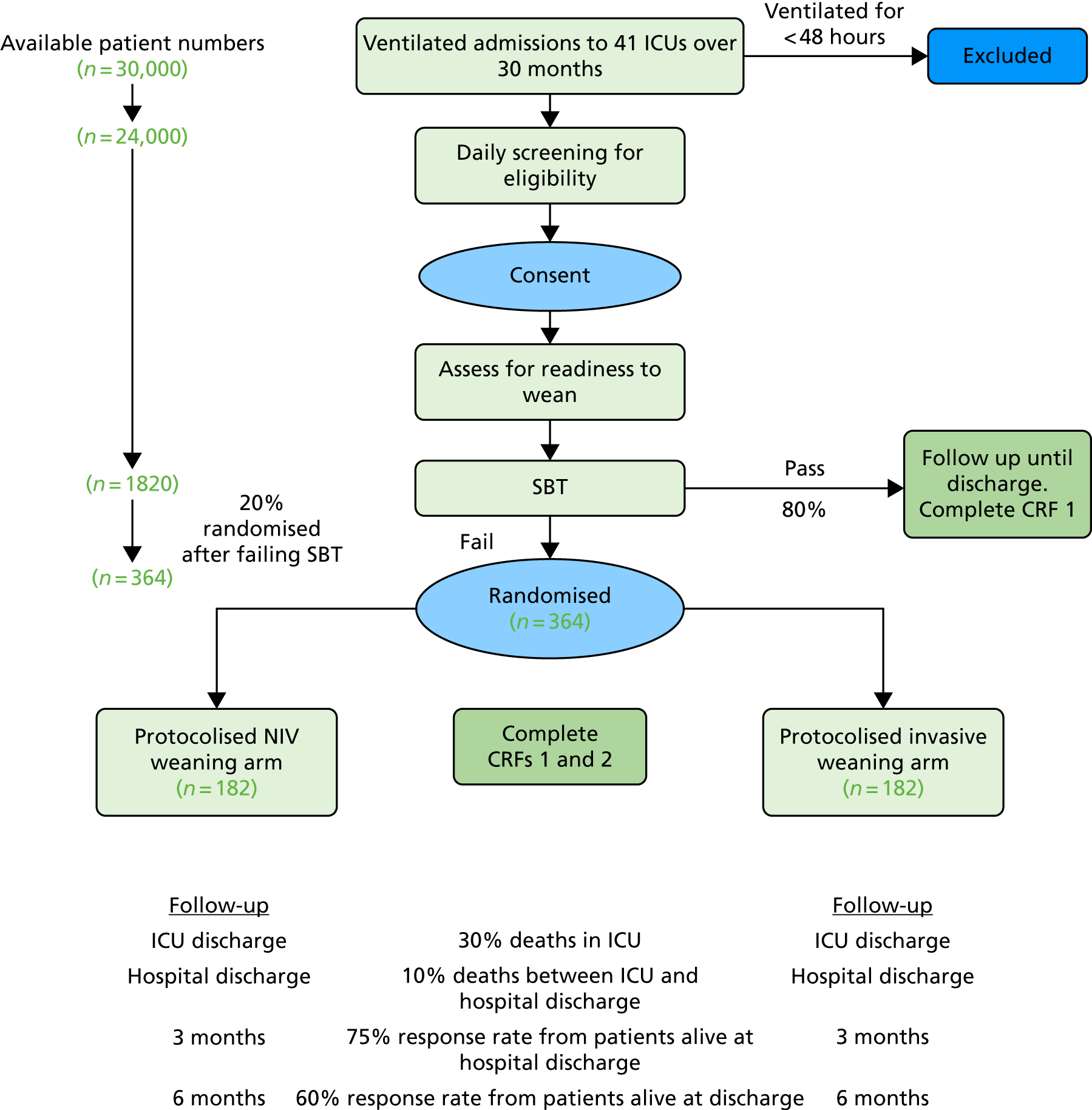
Eligible patients were randomised by a web- or telephone-based secure electronic randomisation system. Randomisation was minimised by centre, presence/absence of COPD and postoperative/non-operative reason for ICU admission to ensure that there was an equal balance between treatment groups. Patients were randomised to invasive or non-invasive weaning groups using a 1 : 1 allocation. The randomisation procedure used variable block sizes to reduce the risk of selection bias. Moreover, all allocations remained concealed prior to randomisation. The study flow diagram is shown in Figure 2.
FIGURE 2.
Flow diagram. CRF, case report form.
Trial interventions
The health technology being assessed was the use of NIV as an adjunct to protocolised weaning compared with protocolised weaning that does not include NIV, following a failed SBT.
Protocolised invasive weaning group
Participants randomised to the invasive weaning group were re-established on Psupp ventilation using the settings that had been in place prior to undertaking the SBT. If necessary, the level of Psupp was titrated to achieve patient comfort and a respiratory rate of < 30 breaths per minute. The participant was assessed for signs of distress/fatigue every 2 hours. In the absence of distress/fatigue, Psupp was reduced by 2 cmH2O. This cycle was repeated every 2 hours as tolerated. If, at any point, the participant developed signs of distress/fatigue, then clinical teams sought to identify and treat non-ventilation-associated reversible causes. When these did not lead to a reduction in distress/fatigue, the level of Psupp was increased by 2 cmH2O. The fraction of inspired oxygen (FiO2) and PEEP were titrated to maintain saturation of oxygen in peripheral blood (SpO2) of > 90%. This active weaning protocol was used between 08.00 and 22.00. Unless the participant developed signs of distress/fatigue, ventilator settings were not changed overnight. A further SBT was undertaken the next day. This cycle continued until either the participant was extubated (as a result of passing the SBT) or a tracheostomy was performed.
If a participant continued to show signs of distress/fatigue despite increases to Psupp and the treatment of any reversible causes, the weaning protocol could be temporarily discontinued and the ventilation strategy determined by the treating clinician. The participant was reassessed at least daily for readiness to wean. When the participant was ready to wean again, the weaning protocol was recommenced. The IMV weaning protocol was discontinued after the participant was extubated.
Non-invasive weaning protocol
Participants randomised to the non-invasive weaning group were re-established on Psupp using the settings that had been in place prior to undertaking the SBT. If necessary, the level of Psupp was titrated further to achieve patient comfort and a respiratory rate of < 30 breaths per minute. When the participant had recovered from the SBT (physiological parameters had returned to baseline and a clinician judged that they were ready for extubation), they were extubated and immediately provided with NIV with an appropriate level of inspiratory positive airway pressure and expiratory positive airway pressure (EPAP) to match the PEEP and Psupp prior to extubation. The level of Psupp was titrated to achieve patient comfort and a respiratory rate of < 30 breaths per minute. FiO2 and EPAP were titrated to maintain SpO2 of > 90%. The participant was assessed for signs of distress/fatigue every 2 hours. In the absence of distress/fatigue, the treating clinician either removed the NIV to allow a self-ventilation trial or reduced the level of Psupp by 2 cmH2O.
In the self-ventilation trial, supplemental oxygen (equivalent to the previous FiO2) was provided via a standard oxygen mask and titrated as necessary to maintain SpO2 of > 90%.
If no signs of distress/fatigue developed during the self-ventilation trial, the trial was continued. If the participant subsequently developed distress/fatigue, NIV was restarted.
If, at any time, the participant developed persistent signs of distress/fatigue/weaning failure, despite increases in Psupp and treating any reversible causes, the clinician could temporarily or permanently suspend the weaning protocol.
This active weaning protocol was used between 08.00 and 22.00. Ventilator settings were not changed overnight unless the participant developed signs of fatigue/distress. The NIV weaning protocol was discontinued when the participant tolerated 12 hours of unsupported spontaneous ventilation.
Outcome measures
Primary outcome
The primary outcome measure was time (in hours) from randomisation to liberation from ventilation.
Liberation from ventilation was defined based on the International Consensus Conference on Weaning’s recommendations7 as the time point following which the patient was free of ventilatory (invasive or non-invasive) support for > 48 hours. This defined the duration of weaning process (randomisation to liberation from ventilation).
Reintubation as a consequence of weaning failure generally occurs within the first 12–48 hours. 26 Defining weaning success as being free from ventilator support for 48 hours from liberation of ventilation captured weaning failures (requiring reintubation within 48 hours) but reduced confounding by late events unrelated to the weaning process, such as the need for an unrelated surgical procedure or other event requiring intubation and ventilation.
Secondary outcomes
Secondary outcome measures were:
Efficacy –
-
mortality at 30, 90 and 180 days
-
duration of IMV and total ventilator days (IMV and NIV)
-
time (days) to meeting ICU discharge criteria
-
proportion of participants receiving antibiotics for presumed respiratory infection and total of days
-
reintubation (met criteria for reintubation and whether or not they were reintubated)
-
tracheostomy.
Safety –
-
AEs
-
SAEs.
Patient-focused outcomes –
-
HRQoL: EQ-5D-3L, SF-12 at baseline (estimated), and at 3 and 6 months.
Withdrawal of consent
Participants, their consultee (personal or professional) or the ICU consultant responsible for their care could request withdrawal from the trial at any time without prejudice. In the event that the participant was withdrawn during the protocolised weaning element of the trial, the clinician responsible for their care would determine the safest and most appropriate way to continue the weaning process outside the trial protocol.
In the event of a request to withdraw from the trial, the researcher determined which elements of the trial were to be withdrawn, from the following possibilities:
-
the protocolised weaning intervention
-
ongoing data collection during hospital admission
-
confirmation of status at 30, 90 and 180 days
-
contact for follow-up questionnaires.
If the participant requested withdrawal from all four elements, only anonymised data recorded up to the point of withdrawal were included in the trial analysis.
Standardised care protocols and assessments
Daily assessment of need for critical care
Participants were assessed daily (days 0–30) by the clinical team for their need for critical care. Patients are classified as part of national data reporting arrangements in one of four categories. 27 Patients who receive level 0/1 care are classified as requiring ward-based care. Patients who receive level 2/3 care are classified as requiring critical care support.
Level 0: patients whose needs can be met through normal ward care in an acute hospital.
Level 1: patients at risk of their condition deteriorating, or those recently relocated from higher levels of care, whose needs can be met on an acute ward with additional advice and support from the critical care team.
Level 2: patients requiring more detailed observation or intervention, including support for a single failing organ system or postoperative care, and those ‘stepping down’ from higher levels of care.
Level 3: patients requiring advanced respiratory support alone or basic respiratory support together with support of at least one other organ system. This level includes all complex patients who require support for multiorgan failure.
A participant was considered to have met ICU discharge criteria when they were discharged from the ICU or they no longer required level 2 or level 3 care. This approach was chosen to overcome administrative delays to discharge because of bed capacity issues in the hospital.
Daily assessment of sedation and organ support requirements
The number of sedative drugs administered to the participant in the preceding 24 hours and the number of organs that required support were assessed daily by the clinical team. Organ support requirements were assessed based on the critical care minimum data set definitions of the need for advanced or basic cardiovascular support, advanced or basic respiratory support, and renal support. 28
Antibiotic use
Whether or not antibiotics had been administered in the previous 24 hours was recorded daily. The clinical team reported whether antibiotics were used primarily for a respiratory or non-respiratory infection. This allowed the calculation of the proportion of participants requiring antibiotics for presumed respiratory infection. In addition, total antibiotic usage (number of days) was calculated.
Criteria for reintubation
The decision to reintubate a participant was a clinical decision, made by the clinician responsible for the participant at the time of assessment. The decision to reintubate or not was complex and included factors outside the predefined reintubation criteria below (e.g. when a subsequent decision to limit treatment was taken).
The following were recorded on a case report form (CRF): when a participant met the predefined reintubation criteria and when they were actually reintubated.
Predefined reintubation criteria were any of the following:
-
cardiac or respiratory arrest
-
respiratory pauses with loss of consciousness or gasping for air
-
severe psychomotor agitation inadequately controlled by sedation
-
persistent inability to remove respiratory secretions
-
heart rate of ≤ 50 or ≥ 140 beats per minute with loss of alertness
-
haemodynamic instability, unresponsive to fluids and vasoactive drugs
-
requirement for surgery or other interventional procedure that required deep sedation or anaesthesia.
Criteria for tracheostomy
The decision about the timing of a tracheostomy rested with the treating clinician. It was suggested that a tracheostomy may be considered after at least 7 days after the time of initial intubation. Indications for tracheostomy were (1) persistent requirement for IMV, (2) inability to protect airway and (3) persistent inability to remove respiratory secretions.
Standardised ventilation bundle
The Department of Health and Social Care’s (DHSC’s) High Impact Intervention: Care Bundle to Reduce Ventilation-association Pneumonia29 mandates ICUs to have sedation protocols and protocols for VAP prevention [head-up position, oral decontamination, sedation hold, peptic ulcer prophylaxis (drug or enteral feeding)] in place. The team ensured that each site had relevant protocols in place.
Blinding
By the nature of the interventions, it was not possible to blind clinicians to whether a participant had been randomised to the invasive or non-invasive treatment group. Careful consideration was given to the strategies that were used to minimise the risk of bias as a consequence of this knowledge.
The use of secure electronic randomisation with a randomisation sequence of variable block size reduced the risk of selection bias. The use of standardised adjunctive care bundles decreased the likelihood of performance bias. The risk of detection bias was minimised by the use of protocols with clear, unambiguous criteria for the discontinuation of IMV and NIV. Intensive care clinical charts provided contemporaneous, hour-by-hour records of a participant’s physiology and current treatments. This enabled outcomes to be verified by both site staff and the co-ordinating centre. On the rare occasions that a participant or their legal representative chose to withdraw from the trial, their permission was sought to retain data collected up until that point and to continue to collect the main outcome data. These approaches minimised the risk of attrition bias. Source verification (from clinical records) and hospital computer records were used to minimise the risk of reporting bias. The main clinical and resource utilisation outcomes of this trial [e.g. ventilation status (hourly), death, level 2/3 care, AEs, antibiotic use] were recorded contemporaneously on patient clinical records and hospital information systems.
Schedule of delivery of intervention and data collection
Trial assessments are summarised in Table 3. It was anticipated that, after randomisation, most participants would be in the ICU for an average of 5–10 days, followed by a hospital stay of a similar duration. Clinical data were recorded daily during a participant’s stay in the ICU. The only daily clinical data that were collected after ICU discharge were antibiotic usage (for antibiotics started in ICU).
| Assessment | Visit | |||||
|---|---|---|---|---|---|---|
| Initial | ICU stay | Hospital stay | 30 days | 3 months | 6 months | |
| Informed consent | ✓ | |||||
| Medical history | ✓ | |||||
| Inclusion/exclusion criteria | ✓ | |||||
| Intervention | ✓ | ✓ | ||||
| Clinical variables | ✓ | ✓ | ✓ | |||
| Quality of life/health economic outcomes | ✓ | ✓ | ✓ | |||
| AEs | ✓ | ✓ | ✓ | |||
| Survival status | ✓ | ✓ | ✓ | ✓ | ✓ | |
Data collection and management
All data for an individual participant were collected by each principal investigator or their delegated nominees and recorded in the CRF. Participant identification in the CRF was through their unique participant trial number, allocated at the time of randomisation, and their initials. Data were collected from the time a participant was considered for entry into the trial through to their discharge from hospital. In the event that a participant was transferred to another hospital, the trial team liaised with the receiving hospital to ensure complete data collection.
Data were collected in duplicate using no-carbon-required forms. Once a participant had been discharged from hospital and all data were entered into the CRF, the top copy of each form was returned to the trial co-ordinating centre. The bottom copy of the CRF was retained at the recruiting centre. The trial number, name, address and other contact details of all participants who survived and agreed to follow-up were supplied to the trial co-ordinating centre at the time of hospital discharge to allow follow-up questionnaires to be posted to the participant at 3 and 6 months post intervention.
Submitted data were reviewed for completeness and entered onto a secure, backed-up, bespoke database. Due care was taken to ensure data safety and integrity, and compliance with the Data Protection Act 1998. 30
Data collection used instruments that had been optimised using data collection pilots before recruitment started. Data collection was restricted to those variables required to define patient characteristics at enrolment, to monitor the treatment received, to monitor adverse effects and to determine quality of life and the use of health-care resources.
In brief, the data set included:
-
variables describing baseline characteristics –
-
participant identifiers
-
inclusion and exclusion criteria
-
Acute Physiology and Chronic Health Evaluation version II (APACHE II) score (24 hours after admission)
-
admission diagnosis
-
presence of COPD [defined by the British Thoracic Society/National Institute for Health and Care Excellence (NICE) criteria or current treatment for COPD]
-
measured or estimated height and weight, and calculated body mass index
-
duration of ventilation prior to randomisation
-
presence of delirium, as measured by the Confusion Assessment Method-Intensive Care Unit (CAM-ICU).
-
-
variables collected daily from randomisation until discharge from ICU –
-
ventilation status (IMV, NIV, self-ventilating)
-
organ support requirements (defined by the mandatory DHSC Critical Care Minimum Data set)28
-
level of ICU support required (level 0–3, for which 0 and 1 define readiness for ICU discharge)
-
antibiotic use for respiratory and non-respiratory infections
-
tracheostomy
-
criteria met for reintubation and actual reintubation
-
AEs
-
deaths
-
sedation use.
-
-
variables collected after ICU discharge –
-
antibiotic use for respiratory and non-respiratory infections started within ICU
-
acute hospital discharge date and status (to calculate acute hospital length of stay).
-
-
variables collected after hospital discharge –
-
vital status up to 180 days post randomisation
-
EQ-5D-3L and SF-12 questionnaire at 3 and 6 months, after verification of vital status with telephone follow-up for non-responders.
-
Health-care resource use questionnaire at 3 and 6 months after verification of vital status with telephone follow-up for non-responders.
-
Participant survival after discharge from hospital was determined by the trial office contacting a participant’s general practice. This information was provided by the participant on the participant contact details form.
All survivors were followed up at 3 and 6 months after randomisation by postal questionnaire. The trial office identified any deaths after discharge from hospital by calling a participant’s general practice first to avoid sending questionnaires to participants who had died. Participants were asked to let the co-ordinating centre know if they moved house at any time after hospital discharge. The follow-up questionnaire collected data on disability and HRQoL, using the EQ-5D-3L and SF-12 questionnaires. If questionnaires were not returned, a maximum of two telephone contacts were made to a participant to check that the questionnaire had been received and that the participant was happy to complete it, followed by a second copy of the questionnaire and telephone contacts in the event of non-return. If the second questionnaire was not returned, the participant was contacted and the outcome data were collected over the telephone, when possible.
Estimating baseline health-related quality of life
Owing to the challenges of collecting baseline data from very frail patients, these data were not collected through patient questionnaires. However, assume a baseline utility score of –0.402 (the value assigned by the EQ-5D-3L tariff to an unconscious health state) was assumed and this was considered to be the same for each participant, regardless of underlying heterogeneity in health states.
Database
The database was set up by the Programming Team at Warwick Clinical Trials Unit (WCTU) and all specifications (i.e. database variables, validation checks, screens) were agreed between the programmer, statistician and trial co-ordinator. All data were entered by the data clerk and all data were periodically reviewed by the trial co-ordinator to assure accuracy and consistency. Only authorised and approved members of staff had access to the database.
Data storage
All essential documentation and trial records were stored by WCTU in conformance with the applicable regulatory requirements and access to stored information was restricted to authorised personnel. All available data can be obtained from the corresponding author.
Archiving
Trial documentation and data will be archived for 5 years after completion of the trial. Trial master files and associated data were archived by WCTU; trial data generated at trial sites were archived in accordance with local policy.
Serious adverse events
A SAE was an AE that fulfilled one or more of the following criteria:
-
resulted in death
-
was immediately life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
was a congenital abnormality or birth defect
-
any other important medical condition that, although not included in the above, required medical or surgical intervention to prevent one of the outcomes listed.
The causality (i.e. relationship to trial treatment, Table 4) and expectedness (expected or unexpected) was assessed by the investigator(s) and recorded on the SAE form.
| Relationship to trial intervention | Description |
|---|---|
| Unrelated | There was no evidence of any causal relationship |
| Unlikely to be related | There was little evidence to suggest that there was a causal relationship (e.g. the event did not occur within a reasonable time after administration of the trial medication or device). There was another reasonable explanation for the event (e.g. the participant’s clinical condition, other concomitant treatment) |
| Possible relationship | There was some evidence to suggest that there was a causal relationship (e.g. because the event occurred within a reasonable time after administration of the trial medication or device). However, the influence of other factors contributed to the event (e.g. the participant’s clinical condition, other concomitant treatments) |
| Probable relationship | There was evidence to suggest that there was a causal relationship and the influence of other factors was unlikely |
| Definitely related | There was clear evidence to suggest that there was a causal relationship and other possible contributing factors can be ruled out |
Related and unexpected SAEs that occurred between trial entry and 30 days post randomisation were reported using the mechanism described in Chapter 5, Measurement of resource use and costs.
Expected serious adverse events that did not require separate reporting
Because the Breathe trial was recruiting a population that was already in a life-threatening situation, it was expected that many of the participants would experience SAEs. Events that were expected in this population and those that were collected as outcomes of the trial were not reported as SAEs. These included:
-
death
-
organ failure
-
pneumonia
-
reintubation
-
tracheostomy.
Reporting serious adverse events
All SAEs, as defined above, were entered onto the SAE reporting form and faxed to a dedicated fax at WCTU within 24 hours of the investigator becoming aware of them. Once received, causality and expectedness were confirmed by the chief investigator. SAEs that were deemed to be unexpected and related to the trial were notified to the REC within 15 days. All such events were reported to the sponsor, Trial Steering Committee (TSC) and Data Monitoring Committee (DMC) at their subsequent meetings.
All participants who experienced SAEs were followed up as per protocol until the end of the trial.
Adverse events
Participants were screened daily for the following AEs known to be associated with the use of NIV:
-
nasal/skin/mouth sores/irritation
-
vomiting
-
gastric distension
-
barotrauma (e.g. pneumothorax)
-
non-respiratory infection
-
arrhythmia.
Statistical methods
Power and sample size
The original sample size was set at 920 participants to detect a hazard ratio (HR) of 0.8 between the intervention and control groups for the primary outcome with 80% power, allowing for time to discontinuation of ventilation to be undefined for 30% of participants because of death in the ICU, and for 2% of participants to have missing outcome data because of withdrawal from the trial. This equated to 36 fewer hours for the intervention group in the time to liberation from ventilation, based on an average of 6.4 days in the standard care group, drawn from a five-centre audit of weaning duration in the UK.
At the request of the Trial Management Group (TMG), the DMC and TSC reviewed the sample size requirements 18 months into trial recruitment in the light of slower than anticipated recruitment. Analysis of the duration of ventilation among participants in the control group revealed that the distribution of data was skewed, indicating that the median duration of weaning, which was 2.9 days, would be a better estimate for the sample size calculation.
Using a median value of 2.9 days and a minimally clinically important difference of 24 hours provided an associated HR of 1.53. However, it was anticipated that the hazards may not be constant over time (as assumed for the exponential distribution) and that the hazards were quite likely to decrease over time. For this reason, a Weibull distribution was used, as it computes a shape parameter, p, that allows for non-constant hazards. The p-value was estimated to be 0.918, thus giving a HR of 1.48.
Based on these data, a minimum sample size of 280 participants would provide 90% power to detect a clinically meaningful median difference of 24 hours between the intervention and control groups for the primary outcome at a 5% significance level. However, it was anticipated that around 23% of participants would be lost to follow-up. The sample size (n = 280) was therefore inflated by 23% to allow for loss to follow-up, resulting in a final sample size of 364 (n = 182 participants in each group).
The revised sample size was approved following review by the DMC, TSC and NIHR.
Primary analyses
Data were reported and summarised in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines for RCTs. 31 The primary analysis method for the trial was intention to treat, that is all participants were analysed as part of the group to which they were originally randomised, regardless of what treatment they actually received. Analysis of the primary outcome [time from randomisation to liberation from ventilation (hours)] and other time-to-event outcomes used survival analysis methods to estimate the HR and the associated 95% CI. Logistic regression models were used to estimate the odds ratios (ORs) and 95% CIs for differences in mortality between the two trial groups at 30, 90 and 180 days. Linear regression models were used to estimate the mean treatment difference and 95% CIs for continuous outcomes. The distribution of count data, for example the number of days on advanced respiratory support, could be either overdispersed with zero inflation, that is several participants with no days on advanced respiratory support, or non-normal. For the former, negative binomial models were used to estimate the incidence rate ratio (IRR) and the associated 95% CI; otherwise, non-parametric tests were applied. All of the analyses were adjusted for age, sex, centre, post-SBT partial pressure of carbon dioxide in arterial blood (PaCO2) and for both of the stratification variables (presence/absence of COPD and whether or not the patient was being treated after surgery).
Sensitivity analyses
A per-protocol analysis was performed as a sensitivity analysis to see how the treatment effect estimate differed when analysed by treatment received. Moreover, additional sensitivity analyses explored the effects of adjustment for any potential baseline differences between the groups.
Subgroup analyses data set access
After data lock, all members of the trial team were able to access the final data set. The chief investigator had full access to the trial data and assumed overall responsibility for the analysis.
Subgroup analyses
In the protocol, it was stated that three predefined subgroup analyses would be undertaken: (1) responsibility for weaning processes (physician led/multiprofessional), (2) presence/absence of COPD and (3) postoperative/non-operative. Of these, data on the first subgroup were not collected; thus, this subgroup analysis was not conducted. Subgroup analyses were performed, for the primary outcome, by the inclusion of interaction terms in the Cox regression models.
Chapter 3 Trial organisation and oversight
Sponsor
The Heart of England NHS Foundation Trust and the University of Warwick acted as cosponsors for the trial. Agreed responsibilities were subcontracted to the University of Warwick, as employer of the chief investigator and co-ordinating centre for the trial.
Subcontracts that delegated responsibilities to research sites were established using the University of Warwick’s standard contracting processes with NHS organisations.
Indemnity
NHS indemnity covered NHS staff, medical academic staff with honorary contracts and those conducting the trial. NHS bodies carried this risk themselves or spread it through the Clinical Negligence Scheme for Trusts,32 which provided unlimited cover for this risk.
The University of Warwick provided indemnity for any harm caused to patients by the design of the research protocol.
Trial timetable and milestones
The trial timetable is displayed in Table 5. The first site opened within 3 months of initiating the grant and all sites were open within 12 months. The internal pilot ran between 3 and 9 months from grant initiation. Following successful confirmation of recruitment rates, the internal pilot ran seamlessly into the main trial. Additional trial sites were recruited throughout the recruitment period. As most ICUs have the equipment necessary to deliver NIV, this did not present a challenge.
| Trial stage | Project activity (months) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | 36 | 39 | 42 | 45 | 48 | |
| Set up | |||||||||||||||||
| Recruit research staff | ✓ | ||||||||||||||||
| Ethics approval | ✓ | ||||||||||||||||
| Refine protocol | ✓ | ||||||||||||||||
| Pilot trial | |||||||||||||||||
| R&D approvals | ✓ | ✓ | ✓ | ||||||||||||||
| Sites open (n) | 5 | 7 | |||||||||||||||
| Participant recruitment | ✓ | ✓ | |||||||||||||||
| 3-month follow-up | ✓ | ✓ | |||||||||||||||
| 6-month follow-up | ✓ | ||||||||||||||||
| Participant accrual (pilot) (n) | 11 | 32 | |||||||||||||||
| Main trial | |||||||||||||||||
| R&D approvals | ✓ | ✓ | ✓ | ||||||||||||||
| Sites open (n) | 15 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | |||||||
| Participant recruitment | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| 3-month follow-up | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 6-month follow-up | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Trial management/reporting | |||||||||||||||||
| Data processing | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Data analysis | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| DMC meeting | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| TSC meeting | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Monitoring reports | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| HTA monograph | ✓ | ✓ | |||||||||||||||
| Other publications | ✓ (protocol) | ✓ | ✓ | ✓ | ✓ | ||||||||||||
| Participant accrual (pilot and main) (n) | 11 | 32 | 77 | 190 | 302 | 415 | 527 | 640 | 752 | 865 | 977 | 1090 | |||||
Criteria for progression to the main trial
The following criteria were used to determine progression from the pilot to the main trial:
-
recruitment > 75% of target (target 32 participants)
-
protocol compliance (> 75%)
-
daily sedation hold (yes/no)
-
compliance with allocated intervention (IMV or NIV use)
-
proportion of weaning time within relevant protocol (assessed daily)
-
adherence with ventilator care bundle (yes/no)
-
-
protocol compliance sent to the NIHR HTA programme.
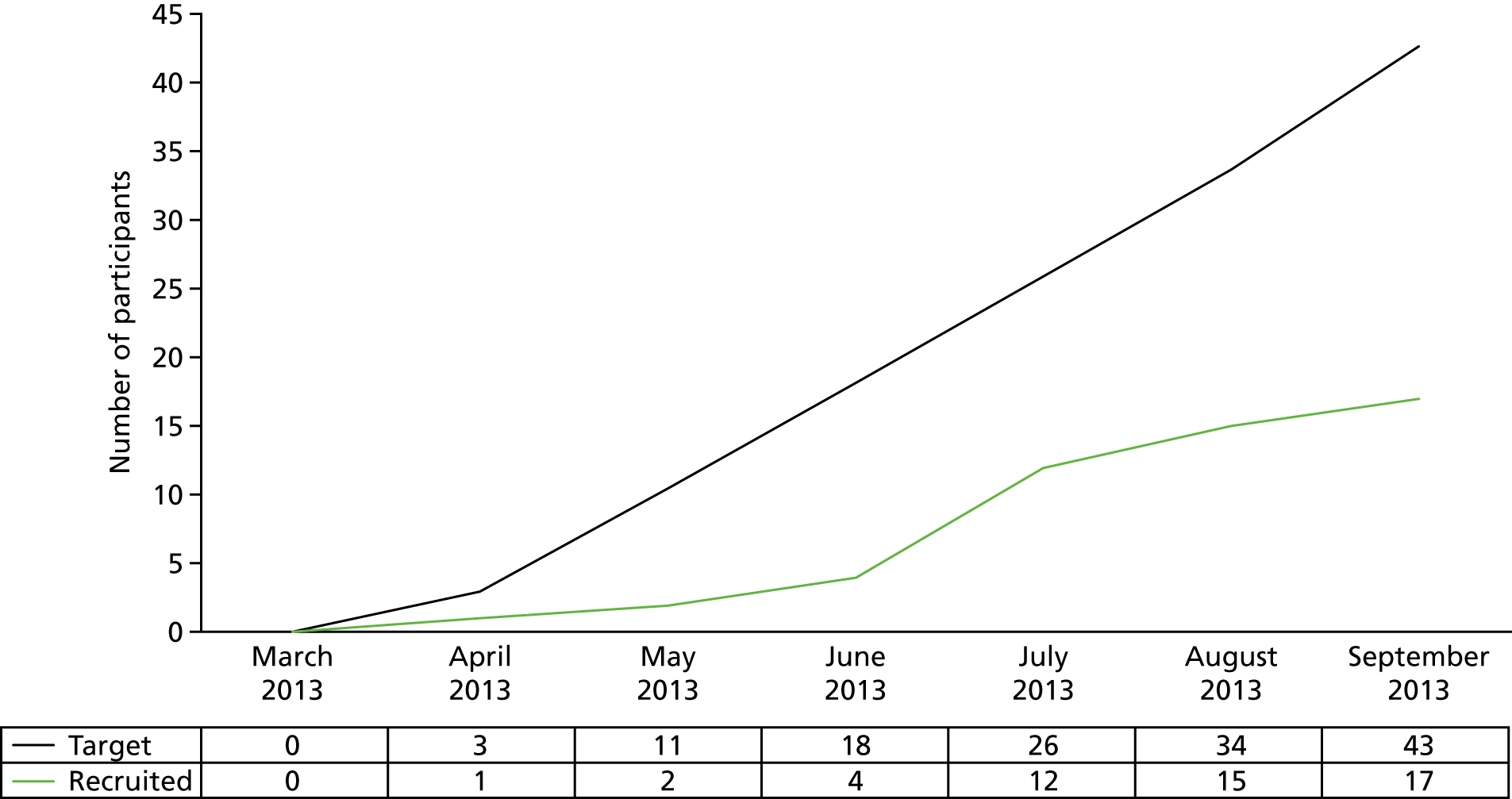
The progression criteria listed above were not met during the pilot phase. Recruitment was < 75% of the target (17/32, 53%; see pilot trial recruitment graph in Figure 3) and protocol compliance was also lower than the 75% target. However, the NIHR HTA programme acknowledged that proactive steps to boost recruitment were in place and the trial question remained important. On this basis, it was recommended that the trial progressed from the pilot phase to the main trial.
FIGURE 3.
Pilot trial recruitment graph.
Administration
The trial was co-ordinated at WCTU. All day-to-day co-ordination of the trial was the responsibility of the trial co-ordinator. All clinical co-ordination was the responsibility of the chief investigator. The trial was managed by a multidisciplinary team.
The trial office team assisted with and facilitated the setting up of centres that wished to collaborate in the trial. In addition, the trial office team:
-
distributed the standardised data collection forms to collaborators
-
organised the telephone randomisation service for formal trial entry
-
monitored the collection and processing of data, and sought missing data
-
trained local staff in recruitment processes and data collection
-
ensured the confidentiality and security of all forms and data
-
conducted extensive data-checking and cleaning
-
organised any interim and main analyses
-
organised TMG, TSC, DMC and collaborators’ meetings.
The trial office received completed data forms via the postal service. On receipt, data forms were checked for completeness and entered into a trial-specific dedicated computer program that checked the validity of the data.
Patient and public involvement
The Intensive Care Society’s Patients and Relatives Committee helped to ensure that the research question, design, conduct and interpretation were considered from the users’ perspective. Patient and public involvement in the trial was formally facilitated through the involvement of two representatives on the TSC (a former patient and a relative).
Trial Management Group
The TMG met monthly. Meetings were minuted and a list of actions recorded.
Trial Steering Committee
The role of the TSC was to provide overall supervision for a trial on behalf of the trial sponsor and trial funder and to ensure that the trial was conducted to the rigorous standards set out in the DHSC’s Research Governance Framework for Health and Social Care33 and the Guidelines for Good Clinical Practice (GCP). 34 The TSC membership comprised independent clinicians, methodologists and two patient and public involvement representatives.
The main tasks of the TSC were to:
-
provide advice, through its chairperson, to the chief investigator(s), the trial sponsor, the trial funder, the host institution and the contractor on all appropriate aspects of the trial
-
monitor the progress of the trial, adherence to the protocol and participant safety, and to consider new information of relevance to the research question
-
ensure that the rights, safety and well-being of the trial participants were the most important consideration and that they prevailed over the interests of science and society
-
ensure that appropriate ethics and other approvals were obtained in line with the project plan
-
agree proposals for substantial protocol amendments and to provide advice to the sponsor and funder regarding approvals of such amendments
-
provide advice to the investigators on all aspects of the trial.
The TSC adhered to the following guidelines:
-
A minimum of 75% were independent members. Only appointed members were entitled to vote and the chairperson had a casting vote.
-
The minimum quoracy for a meeting to conduct business was 67% of appointed members.
-
The chairperson and members signed and maintained a log of potential conflicts of interest.
-
Attendance at TSC meetings by non-members was at the discretion of the chairperson. The primary TSC reporting line was via the chairperson to the NIHR HTA programme director.
Data Monitoring and Ethics Committee
A DMC was appointed comprising two clinicians with experience in undertaking clinical trials and caring for subjects who are critically ill and a statistician who was independent of the trial.
During the period of recruitment, interim analyses of the proportion of participants alive at 28 days and analyses of deaths from all causes at 28 days were supplied, in strict confidence, to the DMC, along with any other analyses that the committee requested. The intervals for these analyses were determined by the committee.
The DMC advised the chairperson of the TSC if, in their view, the randomised comparisons had provided (1) proof beyond reasonable doubt that for all, or some, the treatment was clearly indicated or clearly contraindicated and (2) evidence that might reasonably be expected to materially influence future patient management.
Following a report from the DMC, the TSC decided what actions, if any, were required. Unless the DMC requested cessation of the trial, the TSC and the collaborators remained blinded to the interim results.
Essential documentation
A trial master file was set up according to WCTU standard operating procedures and was held securely at the co-ordinating centre.
Monitoring and quality assurance of trial procedures
Definitions
Trial protocol deviation
Deviations from clinical trial protocols and GCP occur commonly in clinical studies. The majority of these instances are technical deviations that do not result in harm to the trial subjects or significantly affect the scientific value of the reported results of the trial. These cases were documented in the protocol deviation section of the CRF for the trial and appropriate corrective and preventative actions were taken. Deviations were included and considered when the clinical study report was produced, as they may have had an impact on the analysis of the data. A clinical decision to take the patient ‘off protocol’ (for the weaning protocol) was recorded in the weaning log as opposed to a trial protocol deviation. Adherence with the weaning regime was recorded in the CRF under the adherence section in the daily data record.
Serious breach
A serious breach was defined as any protocol deviation or breach of the principles of GCP in connection with the Breathe trial that had a significant effect on the safety or physical or mental integrity of the subjects or the scientific value of the trial.
Local monitoring of protocol compliance
The following elements, related to protocol compliance, were assessed daily and recorded on the CRF by a member of the local research team:
-
daily sedation hold (yes/no)
-
compliance with allocated intervention (IMV or NIV use)
-
proportion of weaning time within relevant protocol (assessed daily)
-
adherence to ventilator care bundle (yes/no).
Monitoring
All sites were monitored by WCTU during the first few weeks after they were recruited. Monitoring sought to ensure protocol compliance, quality of data collection and storage of documentation. Monitors had access to relevant participant notes/charts and trial documentation. The primary purpose of the monitoring visit was to ensure the safety of the trial participant and the integrity of the trial data. Monitoring visits were conducted in a supportive manner with the objective of supporting centres in delivering the trial safely and in accordance with the principles of GCP.
Participating institutions permitted trial-related monitoring, audits, REC review and regulatory inspections, and provided direct access to source data/documents as required.
Reporting
Protocol deviations (and actions taken to prevent recurrence) were recorded in the CRF. Deviations from the weaning protocol were recorded in the weaning log and daily data form.
Any serious breaches of the trial protocol or GCP were immediately reported to the chief investigator. The chief investigator, in consultation with the principal investigator, took whatever immediate action was required to safeguard the well-being of the participant.
Financial support
Research costs
Research costs for this trial were funded by the NIHR HTA programme (project reference 10/124/06).
NHS service support costs
This trial was included on the NIHR portfolio and received NHS service support costs. NHS service support costs were produced through the lead comprehensive local research network (CLRN) (West Midlands South CLRN). The costing was based on their experience of similar trials in this setting and was calculated as £79.65 per participant.
End of the trial
The trial ended when 364 participants had been randomised and the last participant had completed the final follow-up (28 April 2017).
However, prior to reaching the trial target, it had been agreed that the trial would have been stopped prematurely if:
-
mandated by the REC
-
following recommendations from the DMC
-
funding for the trial ceased.
The REC that originally gave a favourable opinion of the trial would have been notified in writing if the trial had been concluded or terminated early.
Chapter 4 Results
Participant flow
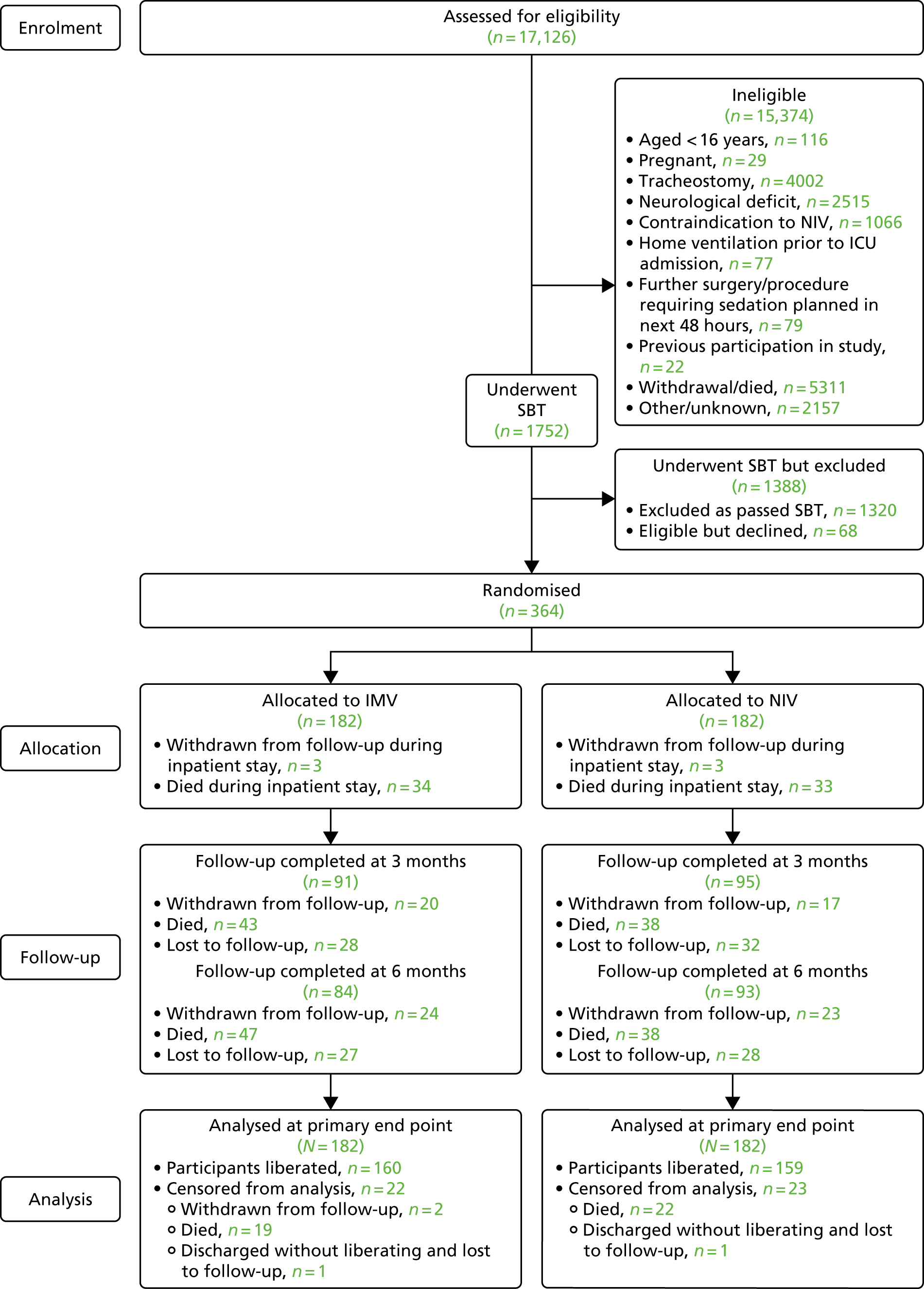
The CONSORT flow diagram in Figure 4 details the overall flow of participants through the trial.
FIGURE 4.
The CONSORT flow diagram for the Breathe trial.
Recruitment
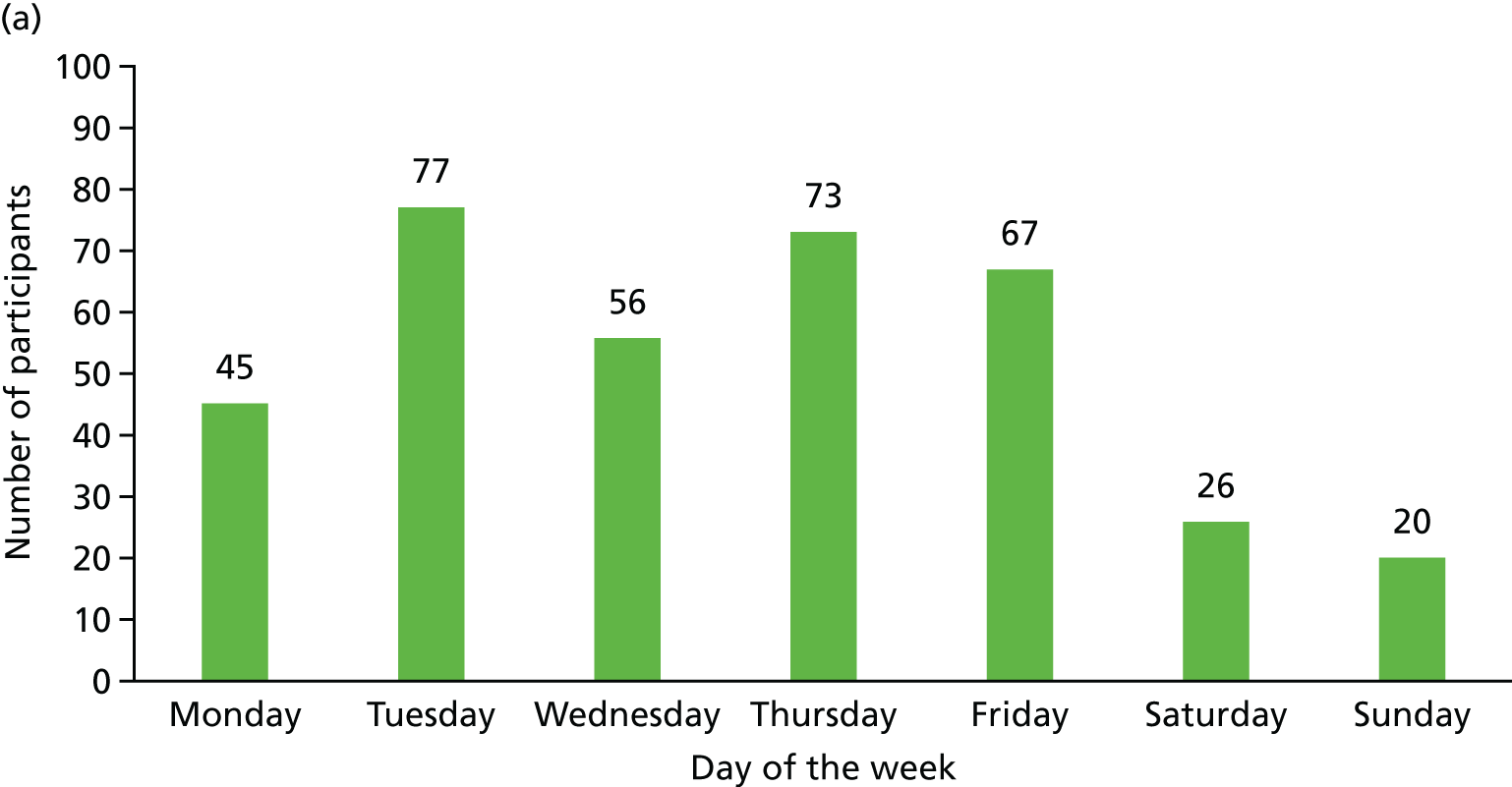
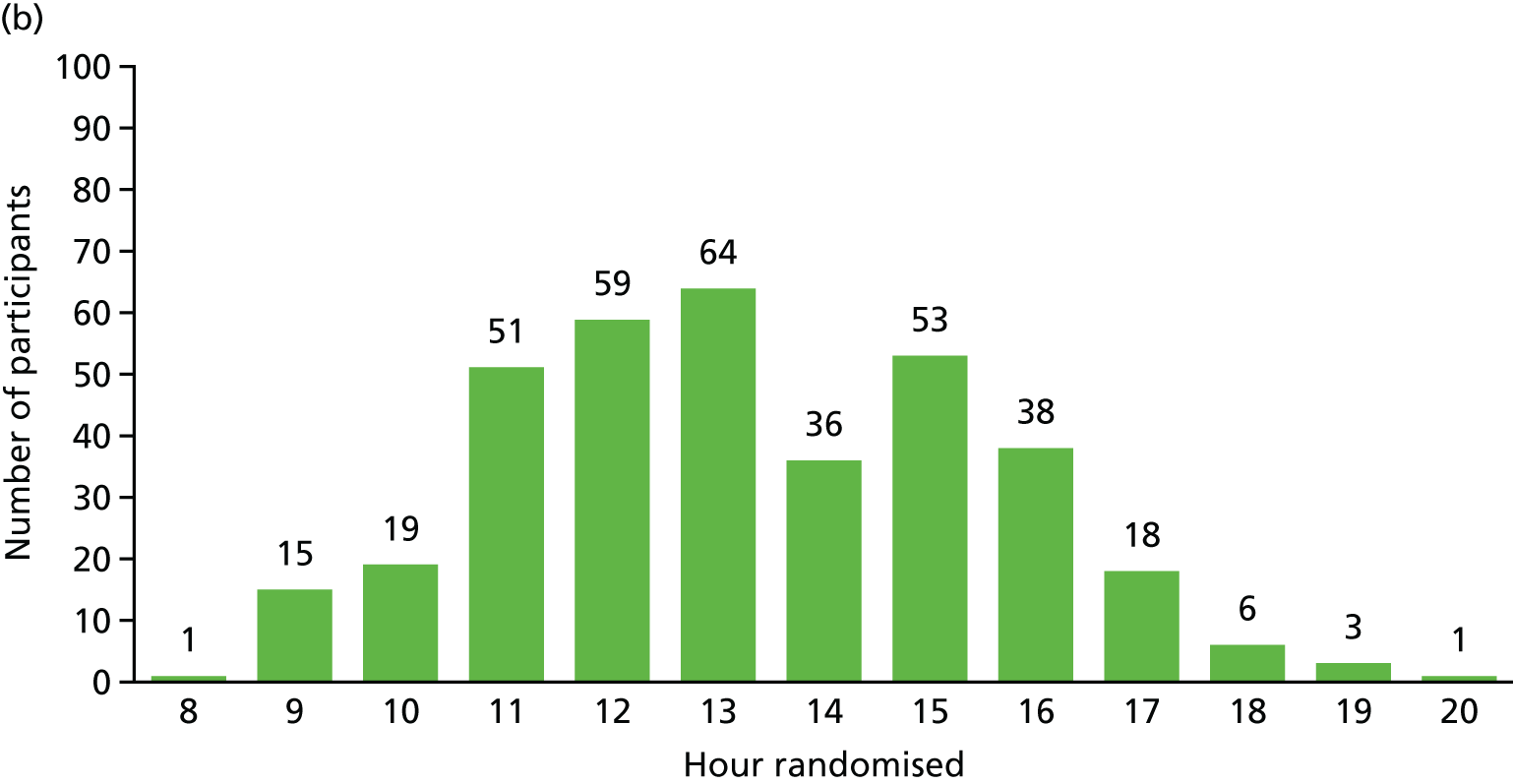
Recruitment for the overall trial (including the internal pilot trial) occurred between March 2013 and October 2016, when 364 participants were recruited from across 51 hospitals (Figures 5 and 6). A total of 17,126 potential participants were identified through the screening process and were assessed for eligibility, of whom 90% (15,374/17,126) were ineligible (see Figure 4). A breakdown of the screening process and detailed reasons for exclusion are summarised by site in Appendix 1. The remaining 1752 patients underwent a SBT, of whom 75% (1320/1752) passed the SBT and were thus excluded. Of the 432 eligible participants who failed the SBT, 16% (68/432) declined to participate and the remaining 84% (364/432) consented to trial participation and were thus randomised. There were no participants randomised in error. Most patients were randomised between 11.00 and 16.00 (Figure 7). The proportion of randomised participants in each group across all sites and also across the randomisation strata is detailed in Appendix 2. The three highest-recruiting sites [Birmingham Heartlands Hospital (BHH), Queen Elizabeth Hospital, Birmingham (QEHB) and St Thomas’ Hospital, London (GST)] were part of the pilot trial and were therefore open for recruitment for the most time. The screening logs (see Appendix 1) from these three sites had higher than average screening rates (n = 609, n = 1838 and n = 1054 for BHH, QEHB and GST, respectively) than the centre average of 335. The proportion of patients excluded after screening was lower than the 90% centre average at BHH (76%) and QEHB (88%). The proportion of patients who underwent a SBT was higher than the centre average of 9% in BHH (24%) in QEHB (12%) and in GST (10%), of whom 34%, 32% and 43%, respectively, of patients failed the SBT (centre average 23%). The proportion of patients who failed the SBT and went on to be randomised was higher than the centre average (81%) at two of these sites: BHH (100%) and GST (100%).
FIGURE 5.
Overall trial recruitment graph.
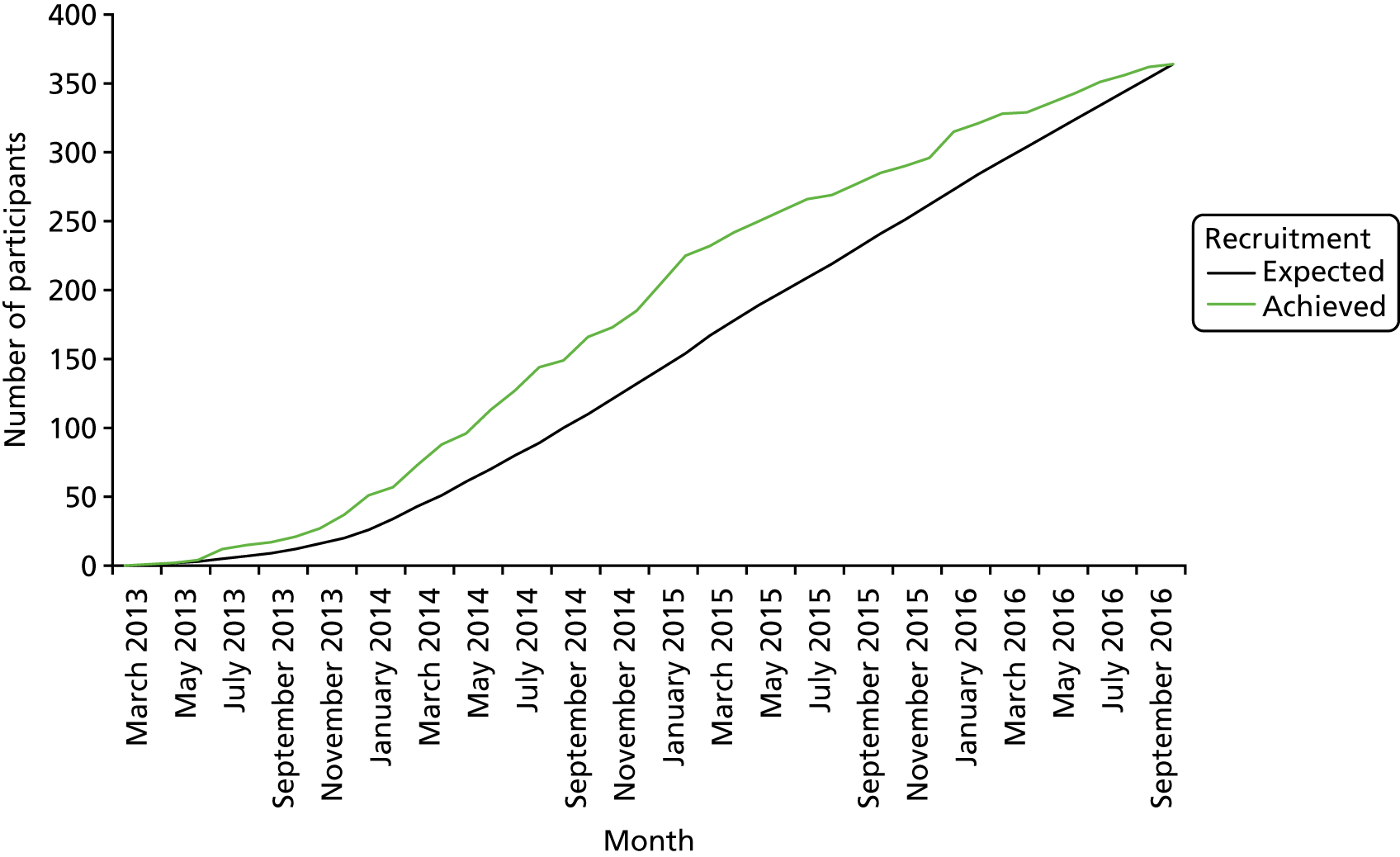
FIGURE 6.
Participant recruitment by site. BHH, Birmingham Heartlands Hospital; GST, St Thomas’ Hospital, London; QEHB, Queen Elizabeth Hospital, Birmingham; RI, Royal Infirmary; RVH, Royal Victoria Hospital; RVI, Royal Victoria Infirmary; UHCW, University Hospital Coventry and Warwickshire; UHNS, University Hospitals of North Midlands; UHOW, University Hospital of Wales; UHSM, University Hospital of South Manchester.
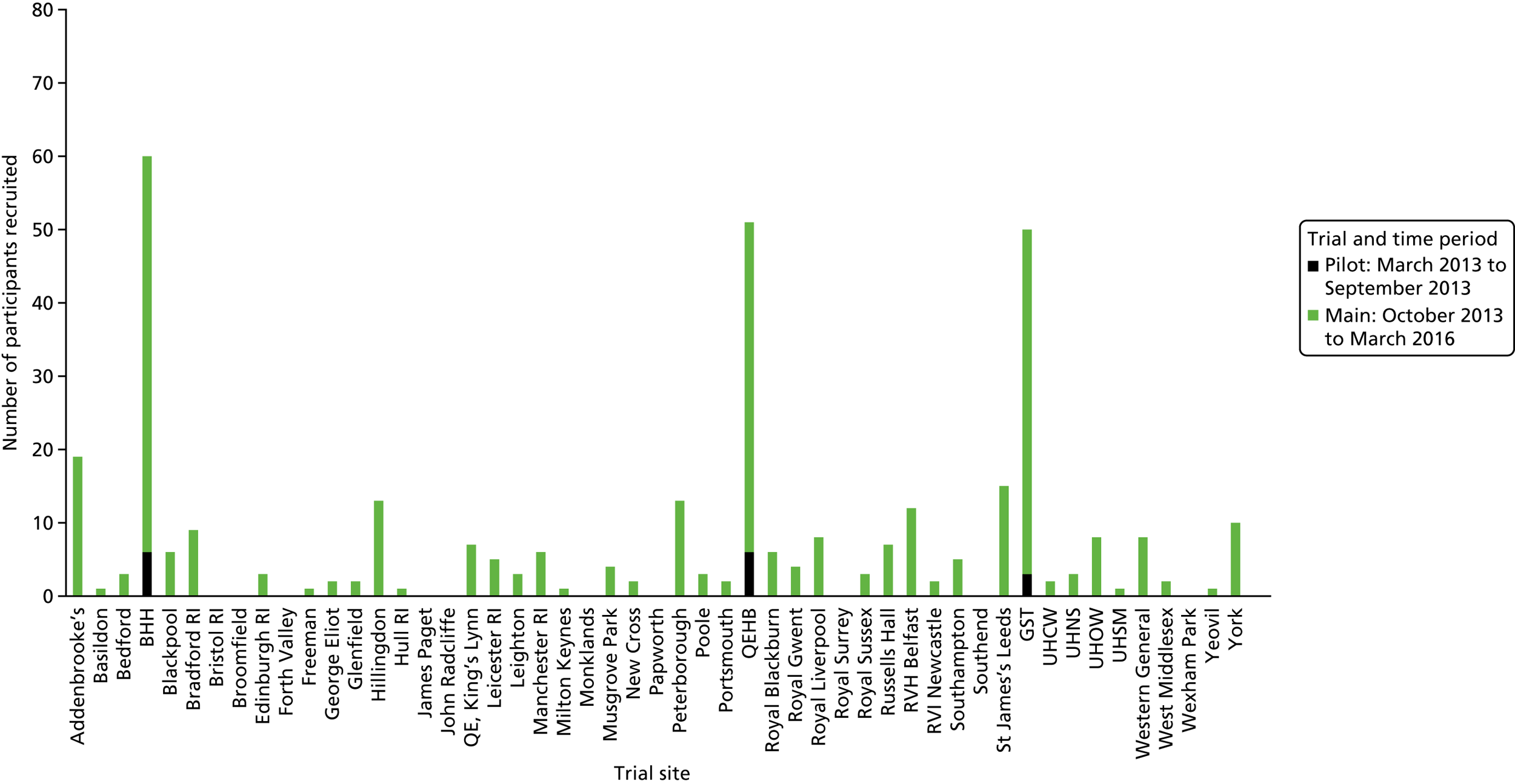
FIGURE 7.
Randomisation of participants. (a) By day of the week; and (b) by time.
Participant baseline data
Participant baseline and demographic data
The baseline and demographic data of participants are summarised in Table 6. Overall, the characteristics of the participants were well matched at baseline between the two treatment groups. Participants had a mean age of around 63 years [standard deviation (SD) 14.8 years]. The proportion of males and females was similar. Around 11% (40/364) had evidence of delirium. The risk of mortality was similar across both groups, with participants having an overall mean APACHE II score of 18.9 (SD 6.4). Most admissions were diagnosed as either pneumonia/respiratory infection (35.7%) or post-surgery respiratory failure (21.4%). The type of diagnosis was similar across both groups, apart from pneumonia/respiratory infection, for which there was around a 9% difference between the two groups (see Sensitivity analysis).
| Characteristic | Trial group | Total (N = 364) | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | ||
| Age (years), mean (SD) | 61.8 (15.8) | 64.3 (13.6) | 63.1 (14.8) |
| Sex (male), n (%) | 94 (51.6) | 90 (49.5) | 184 (50.5) |
| Evidence of delirium (CAM-ICU result), n (%) | |||
| Positive | 17 (9.3) | 23 (12.6) | 40 (11.0) |
| Negative | 132 (72.5) | 130 (71.5) | 262 (72.0) |
| Missing | 33 (18.2) | 29 (15.9) | 62 (17.0) |
| Height (cm) | |||
| Mean (SD) | 166.6 (10.7) | 167.3 (10.5) | 167.0 (10.6) |
| Missing (n) | 4 | 4 | 8 |
| Weight (kg) | |||
| Mean (SD) | 76.7 (18.8) | 78.7 (19.7) | 77.7 (19.3) |
| Missing (n) | 0 | 1 | 1 |
| BMI (kg/m2) | |||
| Mean (SD) | 27.7 (6.6) | 28.2 (6.9) | 28.0 (6.7) |
| Missing (n) | 4 | 5 | 9 |
| Type of NIV interface used, n (%) | |||
| Mask | 1 (0.5) | 174 (95.6) | 175 (48.1) |
| Helmet | 0 | 2 (1.1) | 2 (0.5) |
| Not applicable | 181 (99.5) | 6 (3.3) | 187 (51.4) |
| Duration of ventilation prior to randomisation (days), mean (SD) | 5.3 (3.0) | 6.3 (3.8) | 5.8 (3.5) |
| APACHE II score | |||
| Mean (SD) | 18.8 (6.2) | 18.9 (6.6) | 18.9 (6.4) |
| Missing (n) | 15 | 11 | 26 |
| Diagnosis, n (%) | |||
| Pneumonia/respiratory infection | 73 (40.1) | 57 (31.3) | 130 (35.7) |
| COPD/asthma exacerbation | 7 (3.9) | 7 (3.9) | 14 (3.8) |
| Non-respiratory infection | 21 (11.5) | 16 (8.8) | 37 (10.2) |
| Traumatic injuries | 5 (2.8) | 3 (1.6) | 8 (2.2) |
| Post-surgery respiratory failure | 39 (21.4) | 39 (21.4) | 78 (21.4) |
| GI bleed | 3 (1.7) | 7 (3.9) | 10 (2.8) |
| Cardiac | 18 (9.9) | 27 (14.8) | 45 (12.4) |
| Neuromuscular | 8 (4.4) | 7 (3.9) | 15 (4.1) |
| Overdose | 0 | 4 (2.2) | 4 (1.1) |
| Pancreatitis | 0 | 4 (2.2) | 4 (1.1) |
| Stroke | 1 (0.5) | 0 | 1 (0.3) |
| Other | 7 (3.8) | 11 (6.0) | 18 (4.9) |
Participant baseline physiology data
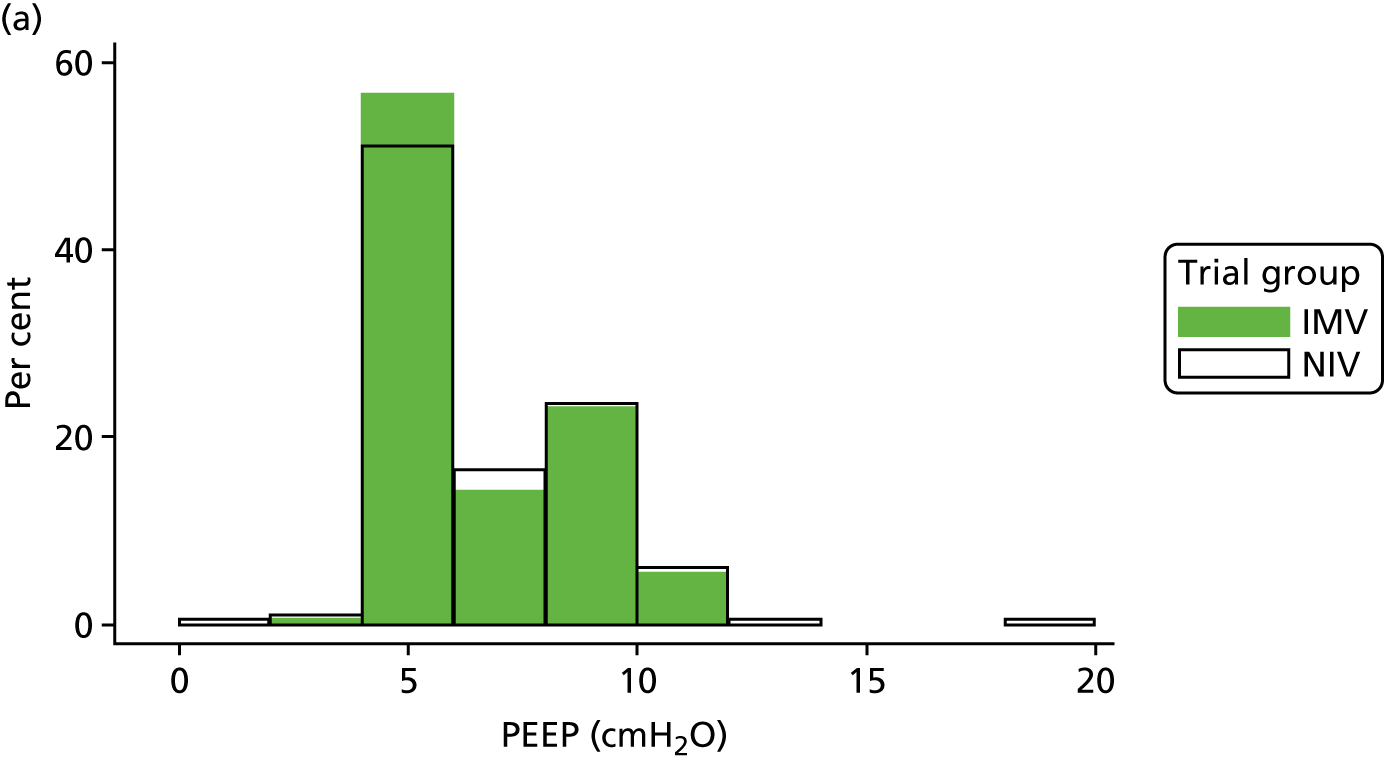
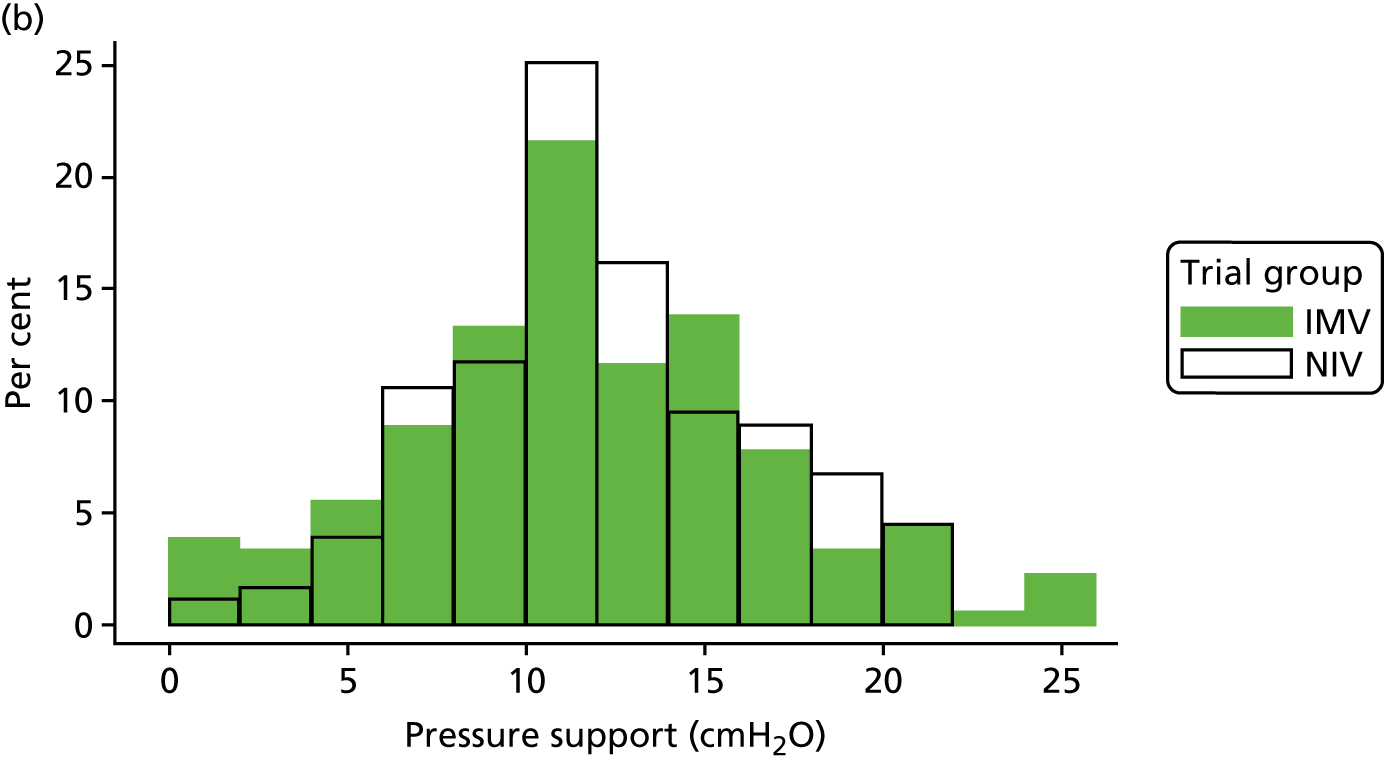
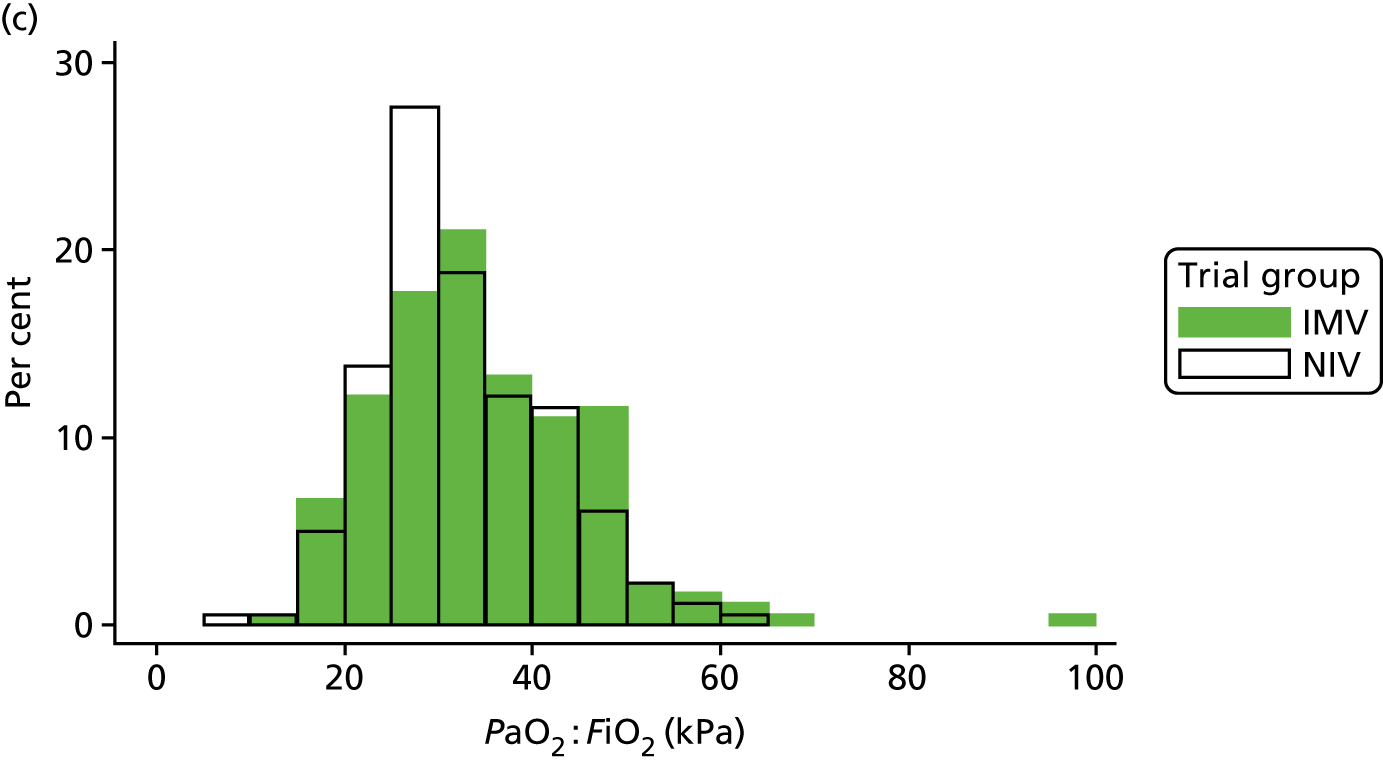
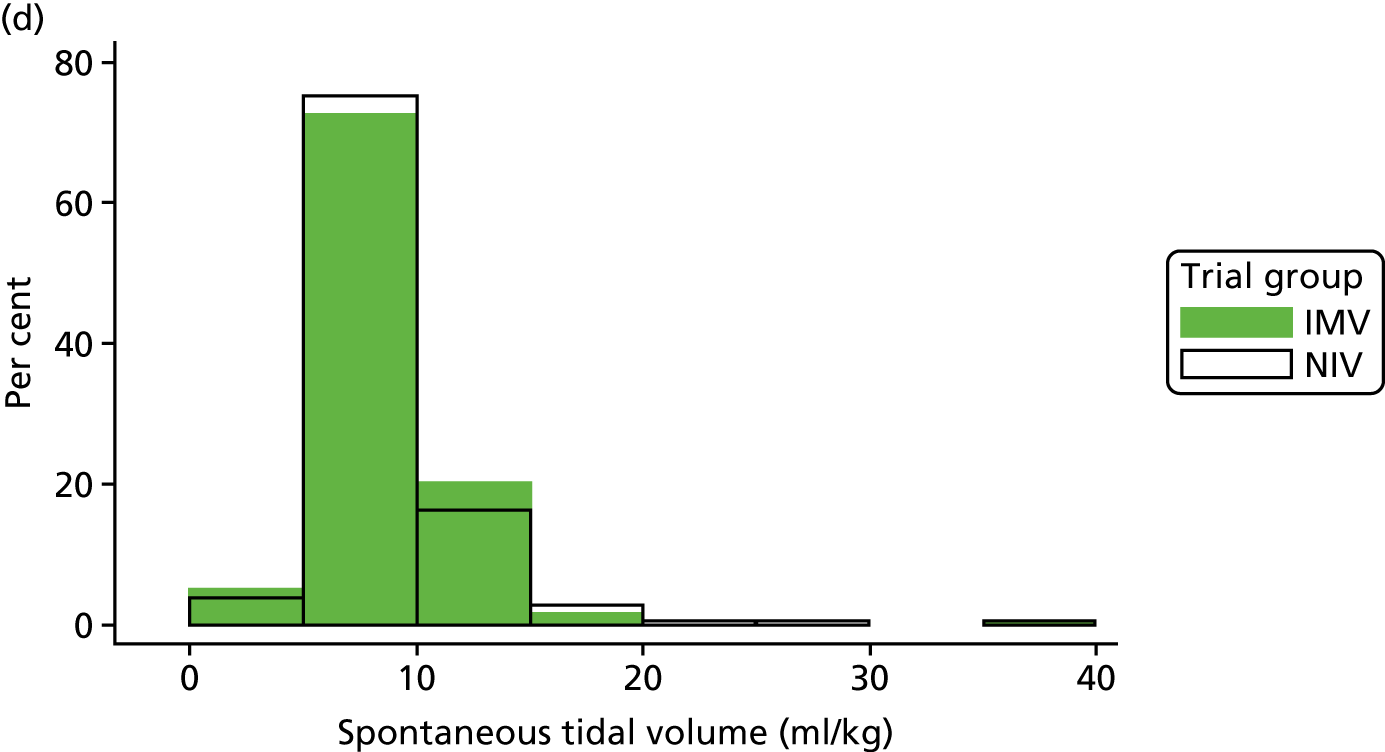
The baseline physiology data of the trial participants are summarised in Table 7. The ventilation measures, haemodynamic measures and arterial blood gas measures were similar across both treatment groups. The distribution of participants’ PEEP, Psupp, PaO2 : FiO2 ratio and spontaneous tidal volume is shown in Figure 8. Participants required high levels of ventilator support when the mean Psupp reported was 11.5 cmH2O (SD 4.8 cmH2O). The mean PaO2 : FiO2 ratio was 33.3 kPa (SD 10.4 kPa).
| SBT characteristics | Trial group | Total (n = 364) | |
|---|---|---|---|
| Invasive weaning (n = 182) | Non-invasive weaning (n = 182) | ||
| Ventilation | |||
| Exhaled minute volume (l per minute), mean (SD) | 10.9 (3.6) | 10.8 (3.9) | 10.8 (3.8) |
| Total respiratory rate (breaths per minute), mean (SD) | 22.5 (7.7) | 22.1 (7.7) | 22.3 (7.7) |
| PEEP (cmH2O), mean (SD) | 6.2 (1.6) | 6.3 (2.0) | 6.2 (1.8) |
| Plateau pressure (cmH2O) | |||
| Mean (SD) | 17.4 (5.4) | 18.0 (4.7) | 17.7 (5.1) |
| Missing (n) | 1 | 3 | 4 |
| Psupp (cmH2O) | |||
| Mean (SD) | 11.3 (5.2) | 11.7 (4.3) | 11.5 (4.8) |
| Missing (n) | 1 | 3 | 4 |
| PaO2 : FiO2 ratio (kPa) | |||
| Mean (SD) | 34.4 (11.3) | 32.2 (9.2) | 33.3 (10.4) |
| Missing (n) | 1 | 1 | 2 |
| Spontaneous tidal volume (ml/kg) | |||
| Mean (SD) | 8.6 (3.3) | 8.6 (3.7) | 8.6 (3.5) |
| Missing (n) | 4 | 4 | 8 |
| Haemodynamics | |||
| Heart rate (beats per minute), mean (SD) | 91.8 (19.8) | 89.6 (18.9) | 90.7 (19.3) |
| Systolic BP (mmHg), mean (SD) | 135.0 (25.9) | 137.7 (27.0) | 136.4 (26.4) |
| Arterial blood gas | |||
| PaO2 (kPa) | |||
| Mean (SD) | 11.2 (2.5) | 11.0 (2.7) | 11.1 (2.6) |
| Missing (n) | 1 | 1 | 2 |
| PaCO2 (kPa) | |||
| Mean (SD) | 5.7 (1.4) | 5.7 (1.2) | 5.7 (1.3) |
| Missing (n) | 1 | 2 | 3 |
| FiO2 | |||
| Mean (SD) | 0.34 (0.07) | 0.35 (0.08) | 0.35 (0.07) |
| Missing (n) | 0 | 1 | 1 |
| pH | |||
| Mean (SD) | 7.4 (0.06) | 7.4 (0.06) | 7.4 (0.06) |
| Missing (n) | 5 | 7 | 12 |
| H+ (nmol) | |||
| Mean (SD) | 37.2 (2.9) | 37.2 (2.0) | 37.2 (2.3) |
| Missing (n) | 177 | 176 | 353 |
| Haemoglobin level (g/dl) | |||
| Mean (SD) | 9.7 (1.7) | 9.6 (1.6) | 9.6 (1.7) |
| Missing (n) | 1 | 1 | 2 |
FIGURE 8.
Distribution of baseline physiology data by treatment group. (a) PEEP; (b) Psupp; (c) PaO2 : FiO2 ratio; and (d) spontaneous tidal volume.
Participant baseline outcome measures
Participants who survived to hospital discharge were asked to rate their pre-admission quality of life. The pre-admission quality-of-life measurements were similar across the two groups (Table 8).
| Baseline measure | Trial group | Total (N = 364) | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive (N = 182) weaning | ||
| EQ-5D-3L (prior to hospital admission) | |||
| n | 120 | 120 | 240 |
| Mean (SD) | 0.67 (0.37) | 0.66 (0.35) | 0.67 (0.36) |
| Median (IQR) | 0.75 (0.52–1) | 0.8 (0.52–1) | 0.78 (0.52–1) |
| EQ VAS (today) | |||
| n | 119 | 115 | 234 |
| Mean (SD) | 58.7 (21.7) | 60.8 (22.6) | 59.7 (22.1) |
| Median (IQR) | 60 (45–75) | 60 (45–80) | 60 (45–75) |
| SF-12 (mental) | |||
| n | 112 | 105 | 217 |
| Mean (SD) | 42.9 (13.4) | 44.0 (12.9) | 43.4 (13.1) |
| SF-12 (physical) | |||
| n | 112 | 105 | 217 |
| Mean (SD) | 37.3 (11.3) | 37.2 (12.4) | 37.2 (11.8) |
Participant spontaneous breathing trial characteristics
Table 9 summarises the participants’ SBT characteristics along with reasons for failure. The majority of participants had CPAP (51.1%) or Psupp (32.7%) as the type of SBT performed, with an overall mean SBT duration of 47.4 minutes (SD 36.5 minutes). Most participants (73%) failed up to three of the criteria.
| SBT charateristics | Trial group | Total (N = 364) | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | ||
| Type of SBT performed, n (%) | |||
| T-piece | 21 (11.5) | 20 (11.0) | 41 (11.3) |
| CPAP | 94 (51.6) | 92 (50.5) | 186 (51.1) |
| Psupp | 58 (31.9) | 61 (33.5) | 119 (32.7) |
| Missing | 9 (5.0) | 9 (5.0) | 18 (4.9) |
| Duration of SBT (minutes) | |||
| Mean (SD) | 46.3 (35.3) | 48.5 (37.8) | 47.4 (36.5) |
| Missing | 6 | 6 | 12 |
| Number of criteria failed, n (%) | |||
| 1 | 37 (20.3) | 32 (17.6) | 69 (19.0) |
| 2 | 42 (23.1) | 56 (30.8) | 98 (26.9) |
| 3 | 49 (26.9) | 48 (26.4) | 97 (26.7) |
| 4 | 24 (13.3) | 18 (9.9) | 42 (11.5) |
| 5 | 18 (9.9) | 15 (8.2) | 33 (9.1) |
| 6 | 6 (3.3) | 8 (4.4) | 14 (3.8) |
| 7 | 3 (1.6) | 4 (2.2) | 7 (1.9) |
| 8 | 3 (1.6) | 1 (0.5) | 4 (1.1) |
| Failed physiological assessments, n (%) | |||
| Heart rate of > 20% of baseline or > 140 beats per minute | 56 (30.8) | 34 (18.7) | 90 (24.7) |
| Systolic blood pressure of > 20% of baseline or > 180 mmHg or < 90 mmHg | 55 (30.2) | 65 (35.7) | 120 (33.0) |
| Cardiac arrhythmias | 4 (2.2) | 5 (2.8) | 9 (2.5) |
| Respiratory rate of ≥ 50% of baseline value or > 35 breaths per minute | 109 (60.0) | 106 (58.2) | 215 (59.1) |
| Respiratory rate (breaths per minute)/tidal volume (l) of > 105 breaths per minute per l | 30 (16.5) | 23 (12.6) | 53 (14.6) |
| Failed on arterial blood gases, n (%) | |||
| PaO2 of < 8 kPa on FiO2 of > 0.5 or SpO2 of < 90% | 28 (15.4) | 33 (18.1) | 61 (16.8) |
| PaCO2 of > 6.5 kPa or increase by > 1 kPa | 24 (13.2) | 22 (12.1) | 46 (12.6) |
| pH of < 7.32 or a reduction in pH of > 0.07 | 11 (6.0) | 13 (7.1) | 24 (6.6) |
| Failed clinical assessment, n (%) | |||
| Agitation and anxiety | 76 (41.8) | 79 (43.4) | 155 (42.6) |
| Depressed mental status | 12 (6.6) | 3 (1.7) | 15 (4.1) |
| Sweating/clammy | 43 (23.6) | 45 (24.7) | 88 (24.2) |
| Cyanosis | 3 (1.7) | 1 (0.6) | 4 (1.1) |
| Increased respiratory effort (accessory muscle use, facial distress, dyspnoea) | 84 (46.2) | 90 (49.5) | 174 (47.8) |
Compliance with allocated treatment
Compliance was defined as whether or not a participant received their allocated intervention up to the point of death, liberation, reintubation or tracheostomy (whichever came first). There was a high level of compliance in both groups: 86.8% (158/182) of the participants in the IMV group and 96.2% (175/182) in the NIV group received their allocated intervention after randomisation.
Participant follow-up
Mortality data were available on all participants at hospital discharge and, when possible, were collected during follow-up. During the study, 51% (186/364) and 49% (177/364) of the participants provided follow-up at 3 months and 6 months, respectively (see Figure 4). The proportion of participants providing follow-up was similar in both groups at each time point.
Withdrawals
A summary of the withdrawals during the trial is provided in Table 10. A total of 12.9% (47/364) of the participants withdrew completely, 0.6% (2/364) withdrew in the ICU/high-dependency unit (HDU) and 1.1% (4/364) withdrew while in hospital. Most participants were happy to provide data during their inpatient stay but requested to withdraw completely from follow-up post hospital discharge; hence, 11.3% (41/364) withdrew at follow-up. In addition, 1.9% (7/364) of the participants withdrew from intervention only but remained in the trial. The different components of the trial from which participants withdrew have been summarised in Table 11.
| Timing of withdrawal | Trial group, n (%) | Total, n (%) (N = 364) | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | ||
| Withdrew from intervention only during inpatient stay | 3 (1.7) | 4 (2.2) | 7 (1.9) |
| In ICU/HDU (from randomisation to ICU discharge) | 2 (1.1) | 0 | 2 (0.6) |
| In hospital (from ICU/HDU discharge to hospital discharge) | 1 (0.6) | 3 (1.7) | 4 (1.1) |
| From hospital discharge to 3 months’ follow-up | 17 (9.3) | 14 (7.7) | 31 (8.5) |
| 3–6 months’ follow-up | 4 (2.2) | 6 (3.3) | 10 (2.8) |
| Withdrawn from | Withdrawal decision made by | Trial group, n (%) | Total, n (%) (N = 364) | |
|---|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | |||
| The protocolised weaning intervention | Participant | 1 (0.5) | 0 (0.0) | 1 (0.3) |
| Personal consultee/next of kin | 3 (1.6) | 2 (1.1) | 5 (1.4) | |
| Clinical | 2 (1.1) | 2 (1.1) | 4 (1.1) | |
| Ongoing data collection during hospital admission | Participant | 1 (0.5) | 3 (1.6) | 4 (1.1) |
| Personal consultee/next of kin | 2 (1.1) | 0 (0.0) | 2 (0.5) | |
| Clinical | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Confirmation of status at 30, 90 and 180 days | Participant | 3 (1.6) | 4 (2.2) | 7 (1.9) |
| Personal consultee/next of kin | 5 (2.7) | 0 (0.0) | 5 (1.4) | |
| Clinical | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Contact for follow-up questionnaires | Participant | 3 (1.6) | 6 (3.3) | 9 (2.5) |
| Personal consultee/next of kin | 5 (2.7) | 0 (0.0) | 5 (1.4) | |
| Clinical | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Outcomes and analyses
Primary outcome: time to liberation from ventilation
Table 12 presents a summary of the primary outcome data. The proportion of participants liberated from ventilation was similar in both groups, with 87.9% (160/182) liberated in the invasive weaning group and 87.4% (159/182) liberated in the non-invasive weaning group. Moreover, there was no evidence of a statistically significant difference in the number of hours from randomisation to liberation from ventilation with an adjusted HR of 1.10 (95% CI 0.89 to 1.39). Figure 9 presents a Kaplan–Meier plot of the time to liberation from ventilation summarised by treatment group. Figure 10 presents a Kaplan–Meier plot of time to liberation from ventilation summarised by treatment group focused on days 0–30.
| Number of hours from randomisation to liberation from ventilation | Trial group | Adjusted estimate (95% CI); p-valuea | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | ||
| n b | 182 | 182 | HR 1.10 (0.89 to 1.39); 0.352 |
| Median (IQR) | 108 (57–351) | 104.3 (34.5–297) | |
FIGURE 9.
Kaplan–Meier curve of the time to liberation from ventilation by treatment group.
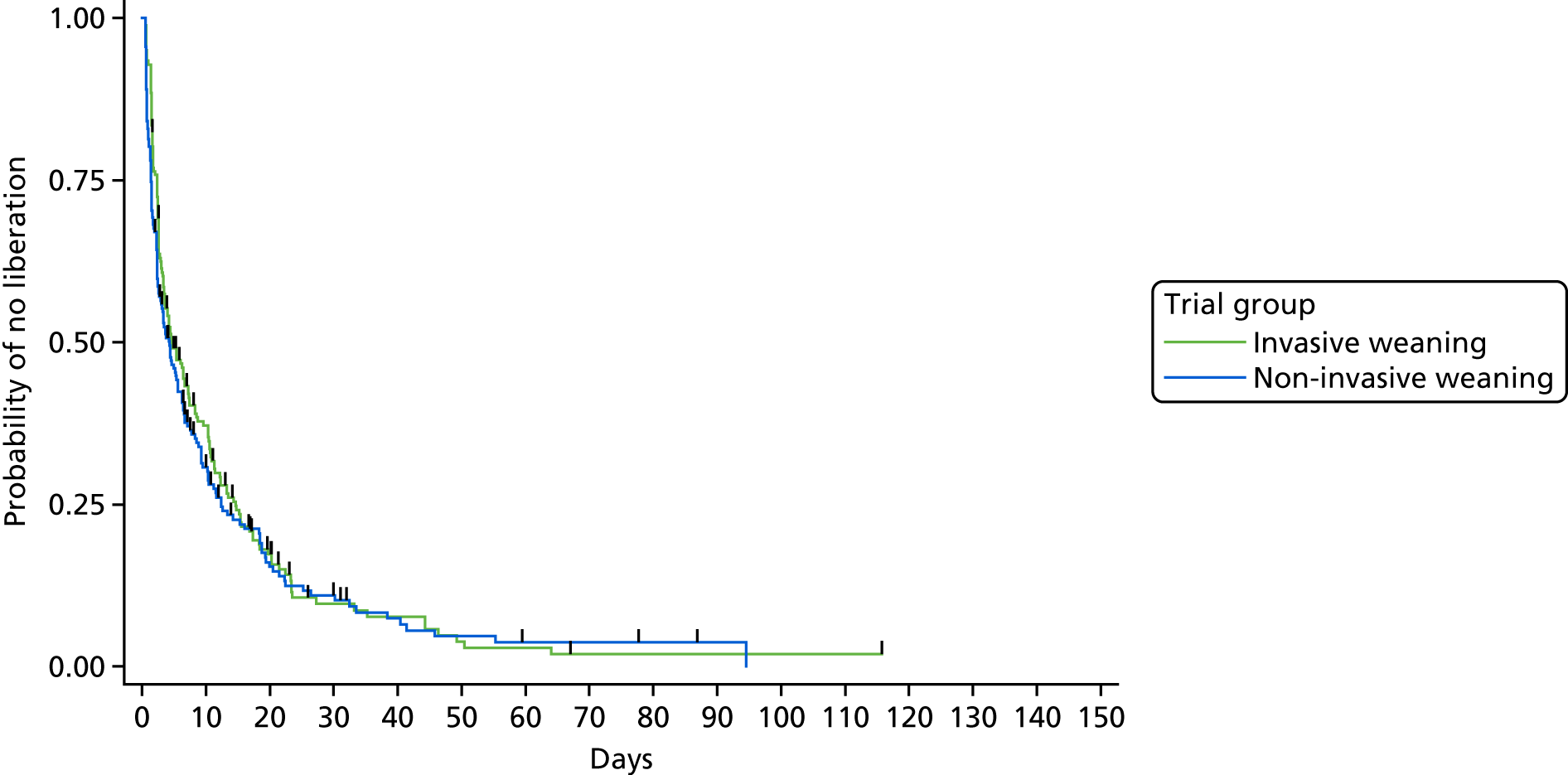
FIGURE 10.
Kaplan–Meier curve of the time to liberation from ventilation by treatment group, focused on days 0–30.
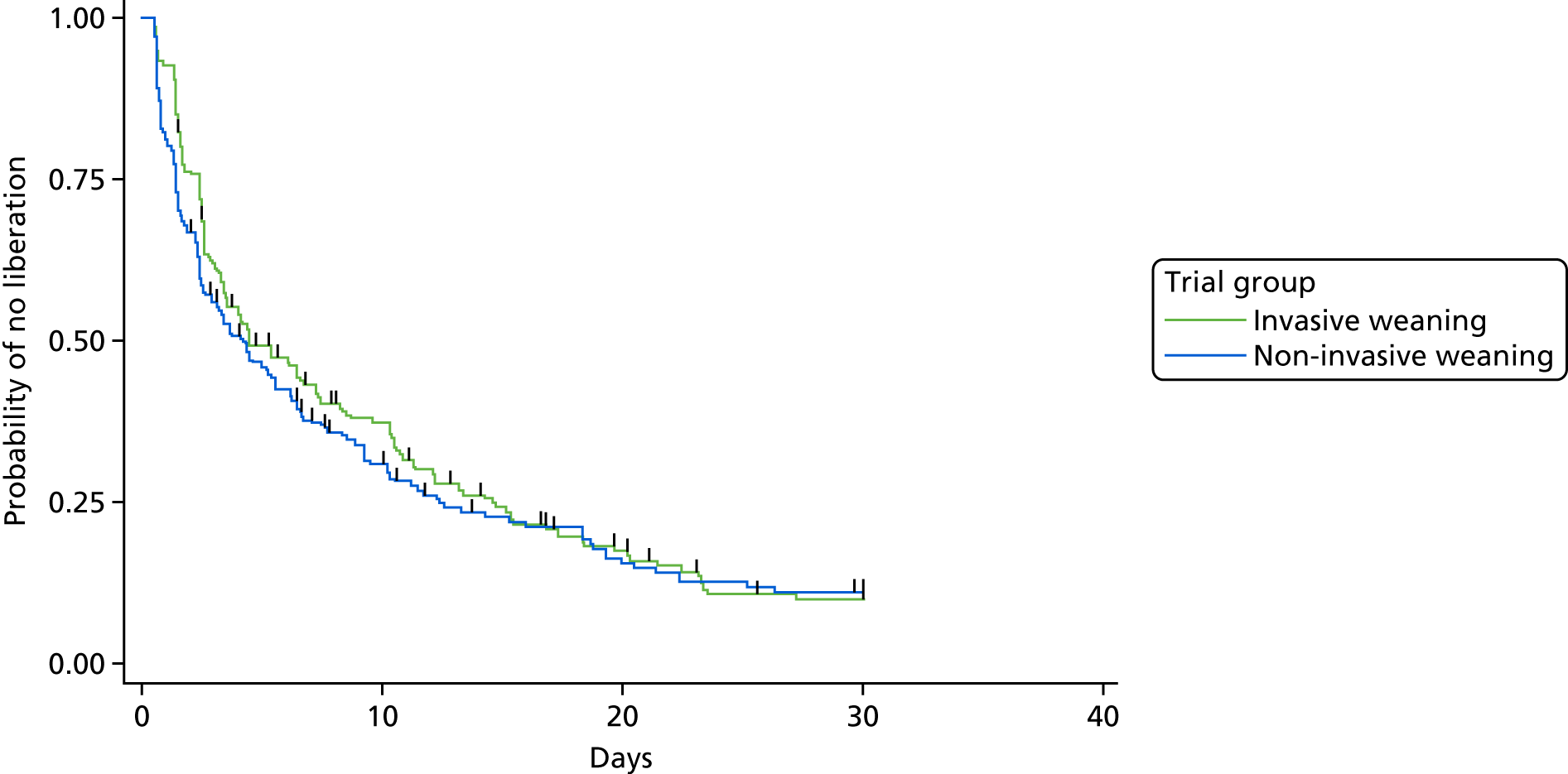
Secondary outcomes
Mortality at 30, 90 and 180 days
There was no statistically significant difference in the participants’ mortality status at 30 days, 90 days and 180 days post randomisation (Table 13). The mortality status of participants at ICU discharge and hospital discharge is also presented in Table 13.
| Mortality | Trial group, n (%) | Adjusted OR (95% CI); p-value | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | ||
| 30 days | 157 (86.3) | 158 (86.8) | 0.90 (0.51 to 1.73); 0.856 |
| 90 days | 137 (75.3) | 142 (78.0) | 0.80 (0.49 to 1.33); 0.393 |
| 180 days | 134 (73.1) | 142 (78.0) | 0.70 (0.44 to 1.18); 0.189 |
| ICU discharge | 157 (86.3) | 160 (87.9) | 0.90 (0.48 to 1.70); 0.764 |
| Hospital discharge | 146 (80.2) | 147 (80.8) | 0.90 (0.54 to 1.58); 0.776 |
Duration of invasive mechanical ventilation and total ventilation days
The duration of IMV was 3.1 days (95% CI 0.51 to 5.75 days) fewer in the non-invasive weaning group than the invasive weaning group (Table 14). Figure 11 presents a box-and-whisker plot of the IMV days and total ventilator days by treatment group.
| Outcome | Trial group | Adjusted IRR estimate (95% CI); p-valuea | Adjusted mean difference (95% CI); p-value | |
|---|---|---|---|---|
| Invasive weaning (n = 182) | Non-invasive weaning (n = 182) | |||
| Number of days on IMV | ||||
| Mean (SD) | 9.1 (13.6) | 6.0 (11.5) | 0.6 (0.47 to 0.87); 0.005 | –3.1 (–5.75 to –0.51); 0.019 |
| Median (IQR) | 4 (2–11) | 1 (0–7) | ||
| Total ventilator days (IMV and NIV) | ||||
| Mean (SD) | 9.6 (13.8) | 7.7 (11.5) | 0.8 (0.62 to 1.00); 0.049 | –2.0 (–4.61 to 0.69); 0.146 |
| Median (IQR) | 4 (2–12) | 3 (1–9) | ||
FIGURE 11.
Box-and-whisker plots of IMV days and total ventilator days by treatment group. (a) IMV days; and (b) total ventilator days.
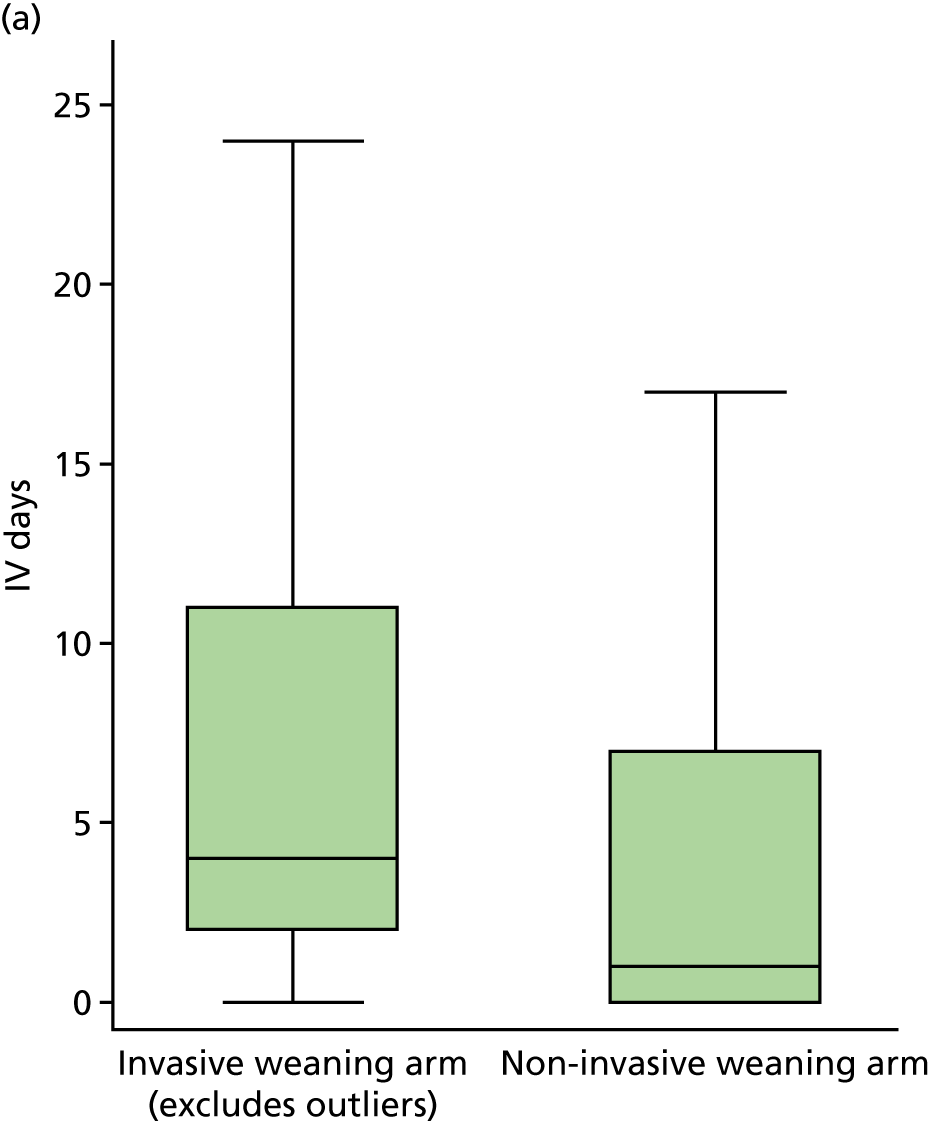
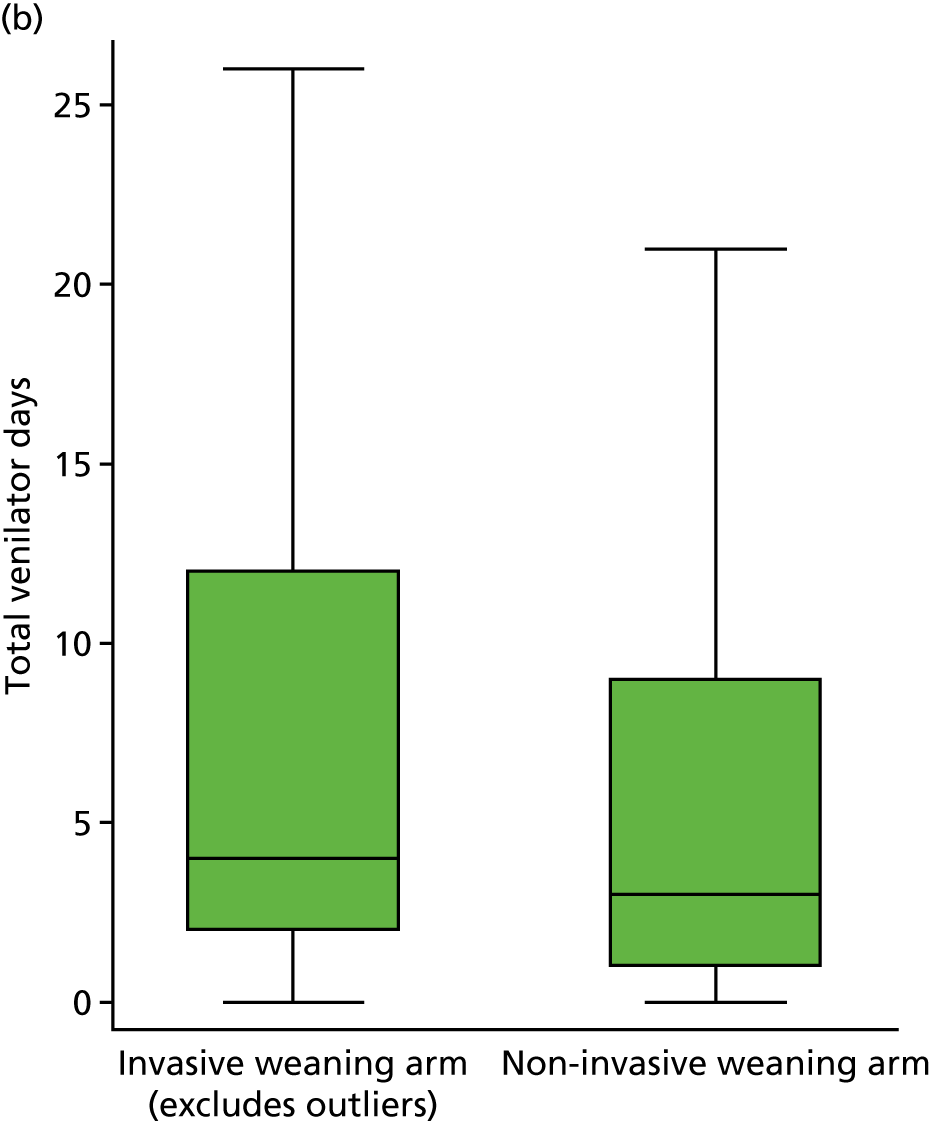
Antibiotics for respiratory infection and total antibiotic usage
Figure 12 presents a summary of the proportion of participants that required respiratory antibiotics as well as details of the total number of days of respiratory and non-respiratory antibiotic usage. A lower proportion of participants required respiratory antibiotics (OR 0.60, 95% CI 0.41 to 1.00) in the non-invasive weaning group compared with the invasive weaning group. There was no difference between the two groups in the total (respiratory and non-respiratory) number of days that antibiotics were used (IRR 0.90, 95% CI 0.68 to 1.08).
FIGURE 12.
Respiratory antibiotic and total antibiotic usage summarised by treatment group. (a) Respiratory antibiotic usage; and (b) total number of days of antibiotic usage.
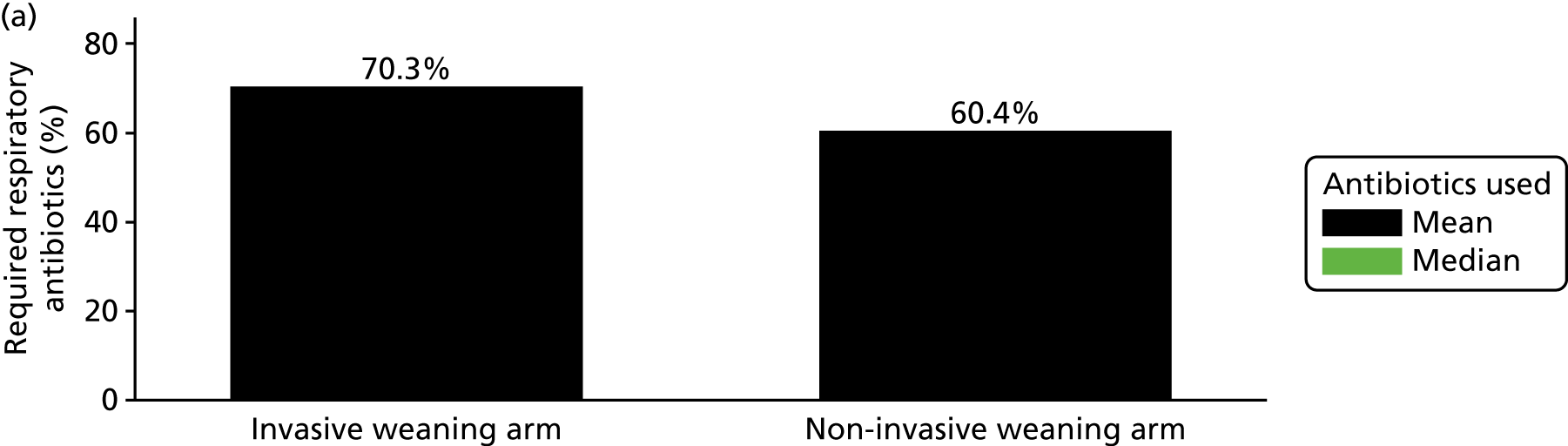
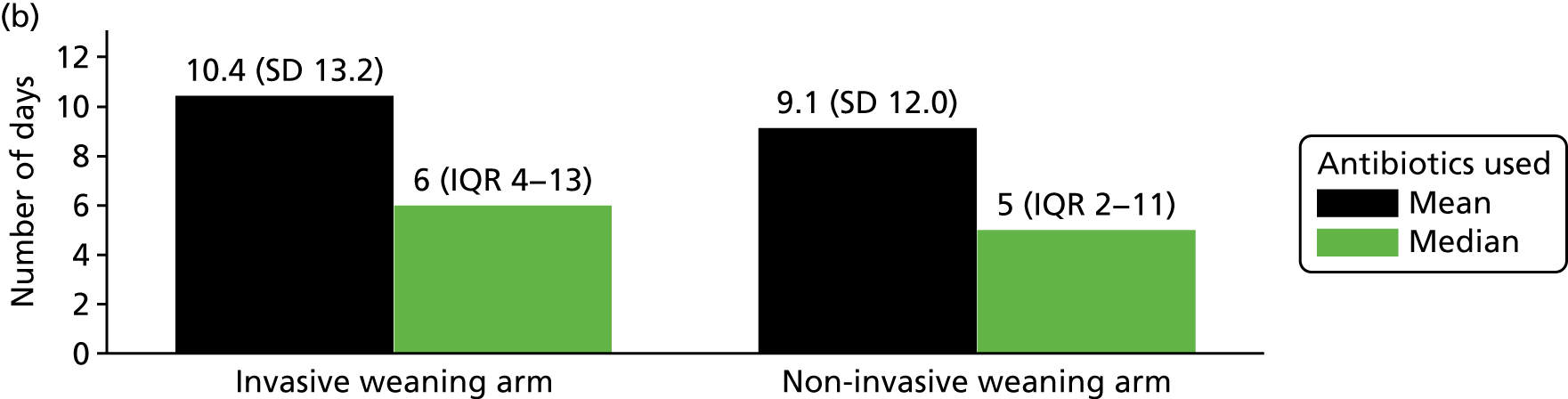
Reintubation and tracheostomy
There was a statistically significant difference in reintubation rates between the two groups (Figure 13) in which participants in the non-invasive weaning group were twice as likely to be reintubated at any time (OR 2.00, 95% CI 1.27 to 3.24). There was no difference in the tracheostomy rates between the two groups (OR 0.70, 95% CI 0.44 to 1.15).
FIGURE 13.
Reintubation and tracheostomy rates summarised by treatment group.
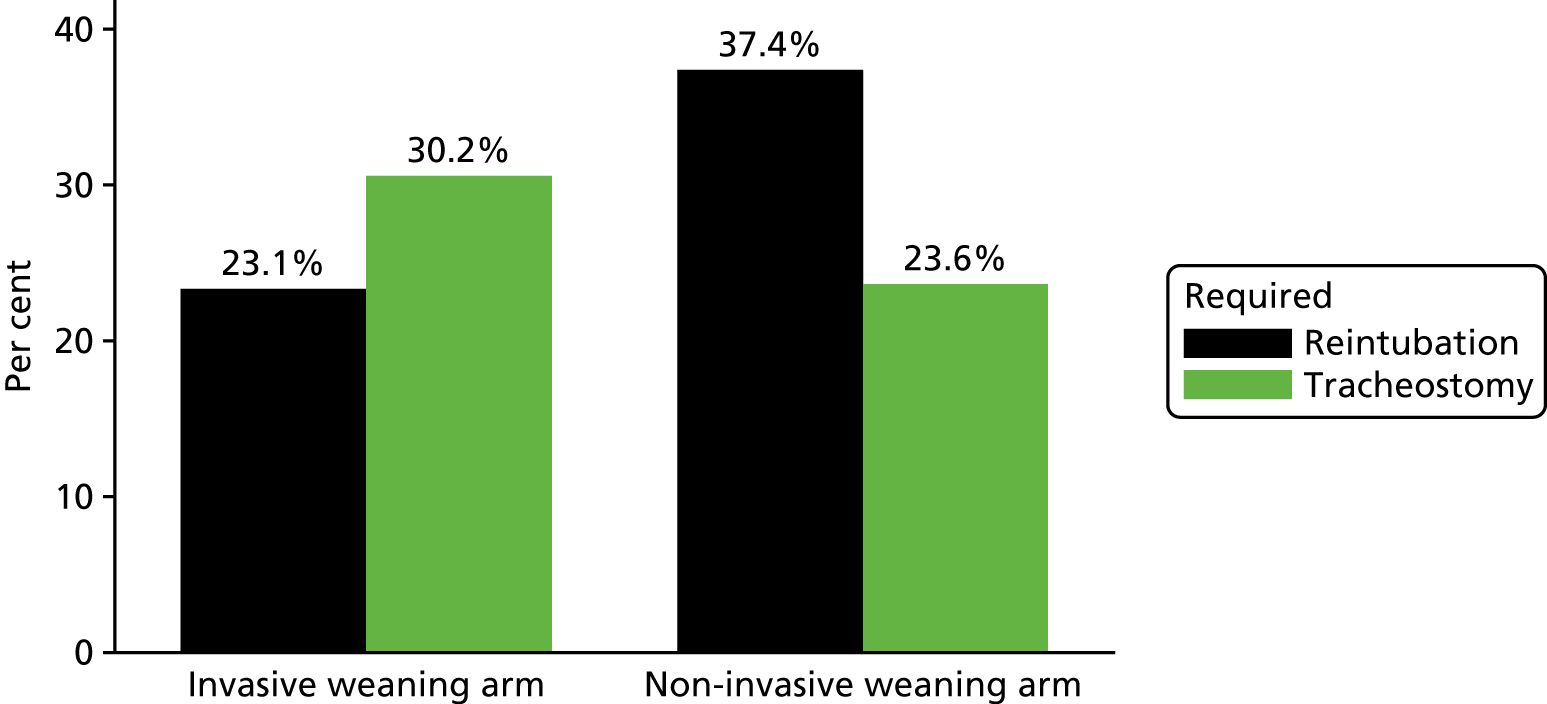
Time to meeting intensive care unit discharge criteria
The length of time (in days) until meeting ICU discharge criteria was similar between both groups (HR 1.10, 95% CI 0.91 to 1.42). Figure 14 presents a Kaplan–Meier plot of the time to meeting discharge criteria.
FIGURE 14.
Kaplan–Meier plot of the time to meeting discharge criteria.
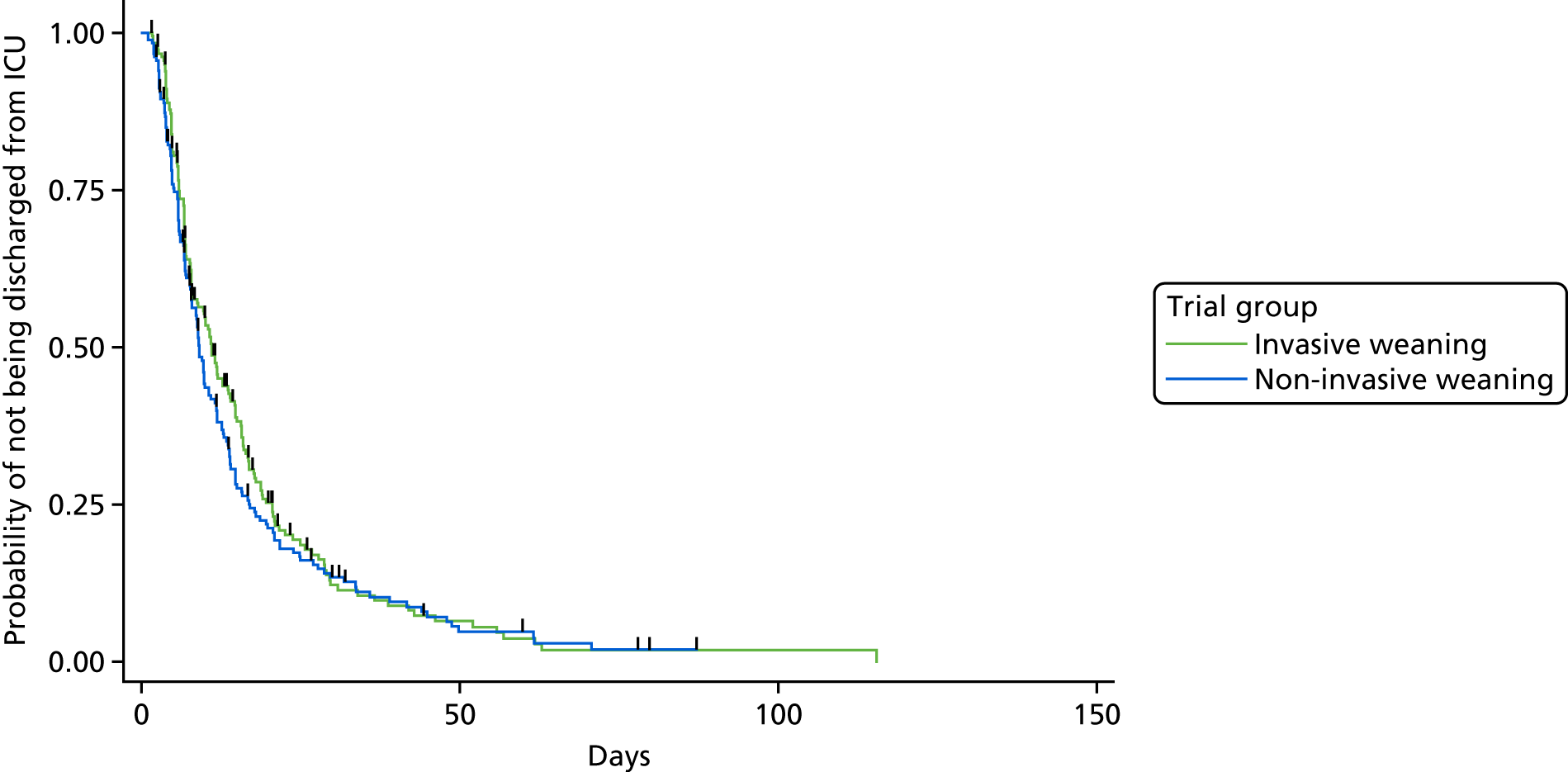
Process variables
A number of process variables were explored to provide a richer understanding of the weaning process and to enable the findings of the Breathe trial to be incorporated into future meta-analyses. This included time to extubation, sedative use, duration of organ support, duration of critical care stay and weaning failure.
Time to extubation
As expected, there is evidence of a significant difference in the time (days) from randomisation to extubation (HR 2.50, 95% CI 1.99 to 3.12): participants had a median time of 0.5 days (IQR 0.5–1 day) in the NIV group compared with a median time of 3 days (IQR 2–10 days) in the IMV group. Figure 15 presents a Kaplan–Meier plot for the time to being extubated.
FIGURE 15.
Kaplan–Meier curve for the time from randomisation to extubation.
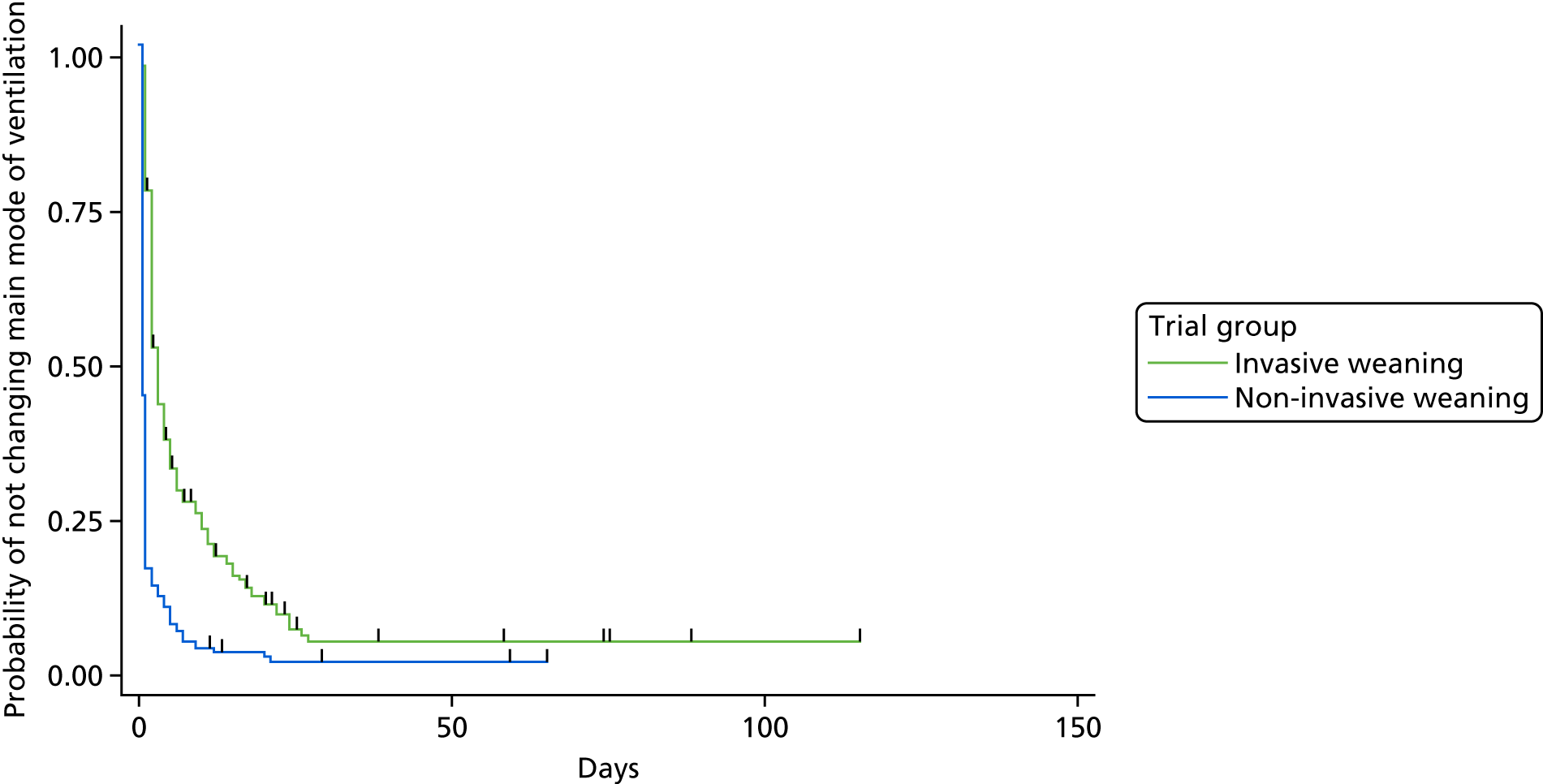
Sedative use, support/organ monitoring and care status
Table 15 presents a summary of the participants’ sedative use, support/organ monitoring and care status by treatment group. Compared with the invasive weaning group, participants in the non-invasive weaning group required fewer days on one or more class of sedative (IRR 0.7, 95% CI 0.60 to 0.90), fewer days on advanced respiratory support (IRR 0.7, 95% CI 0.51 to 0.90) and more days on basic respiratory support (IRR 1.3, 95% CI 1.04 to 1.51). There was no difference between the two groups in the number of days on advanced cardiovascular support, basic cardiovascular support or renal support. There was evidence of a significant difference in the number of days participants required level 2/3 care with participants in the non-invasive weaning group requiring fewer days of level 2/3 care than those in the invasive weaning group (p = 0.024).
| Outcomes | Trial group | Adjusted IRR estimate (95% CI); p-valuea | |
|---|---|---|---|
| Invasive weaning (n = 182) | Non-invasive weaning (n = 182) | ||
| Number of days participant on one or more class of sedative | |||
| Mean (SD) | 5.5 (5.1) | 4.1 (5.0) | 0.7 (0.60 to 0.90); 0.003 |
| Median (IQR) | 3 (2–8) | 2 (1–5) | |
| Number of days participant on advanced respiratory support | |||
| Mean (SD) | 9.4 (13.7) | 6.5 (11.7) | 0.7 (0.51 to 0.90); 0.007 |
| Median (IQR) | 4 (2–13) | 1 (0– 7) | |
| Number of days participant on basic respiratory support | |||
| Mean (SD) | 4.1 (4.5) | 5.3 (5.6) | 1.3 (1.04 to 1.51); 0.019 |
| Median (IQR) | 3 (1–6) | 4 (2–7) | |
| Number of days participant on advanced cardiovascular support | |||
| Mean (SD) | 1.1 (3.1) | 0.6 (1.6) | 0.6 (0.30 to 1.07); 0.081 |
| Median (IQR) | 0 (0–0) | 0 (0–0) | |
| Number of days participant on basic cardiovascular support | |||
| Mean (SD) | 11.3 (13.0) | 10.0 (10.8) | 0.9 (0.71 to 1.04); 0.129 |
| Median (IQR) | 7 (5–14) | 6.5 (4–13) | |
| Number of days participant on renal support | |||
| Mean (SD) | 2.1 (8.1) | 1.7 (5.2) | 1.0 (0.45 to 2.06); 0.919 |
| Median (IQR) | 0 (0–0) | 0 (0–0) | |
| Number of days participant at care level 2 or 3 | |||
| Mean (SD) | 12.2 (8.4) | 10.8 (8.8) | 0.024 |
| Median (IQR) | 10 (5–17) | 7.5 (4–14) | |
Weaning failure
Weaning failure, which was defined as reintubation within 48 hours of extubation, or as death (day 0–7), tracheostomy (day 0–7) or on IMV (on day 7), has been summarised in Table 16. There was no evidence of a difference between the two groups for either definition of weaning failure.
| Outcome | Trial group, n (%) | Adjusted OR estimate (95% CI); p-valuea | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | ||
| Weaning failure: reintubation within 48 hours of extubation | |||
| No | 174 (95.6) | 165 (90.7) | 2.30 (0.96 to 5.61); 0.063 |
| Yes | 8 (4.4) | 17 (9.3) | |
| Weaning failure: death (day 0–7), tracheostomy (day 0–7) or on IMV (on day 7) | |||
| No | 109 (89.9) | 123 (67.6) | 0.70 (0.48 to 1.13); 0.163 |
| Yes | 73 (40.1) | 59 (32.4) | |
Reintubation
The median time from randomisation to reintubation was significantly shorter (p < 0.001) in the non-invasive weaning group than in the invasive weaning group (Table 17) [invasive weaning, 3.2 days (IQR 2.3–4.7 days), vs. non-invasive weaning, 2 days (IQR 0.9–3.0 days)]. There was no difference in the final outcome of those participants who were reintubated.
| Outcome | Trial group | Adjusted estimate (95% CI); p-valuea | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | ||
| Time from randomisation to reintubation (days) | |||
| n | 42 | 68 | < 0.001 |
| Mean (SD) | 4.0 (3.0) | 2.7 (2.7) | |
| Median (IQR) | 3.2 (2.3–4.7) | 2 (0.9–3.0) | |
| Final outcome of those reintubated, n (%) | |||
| Liberated | 34 (80.9) | 51 (75.0) | OR 1.40 (0.43 to 4.26); 0.601 |
| Died | 6 (14.3) | 16 (23.5) | |
| Missing | 2 (4.8) | 1 (1.5) | |
Intensive care unit/high-dependency unit and hospital discharge data
Table 18 presents a summary of the ICU/HDU and hospital discharge data. There were no differences found in the discharge data between the two groups. Approximately 87% of the participants were discharged alive from ICU/HDU, most of whom were discharged to a ward. Approximately 80% of the participants were discharged alive from hospital, with most (74%) of the participants returning home.
| Outcomes | Trial group | p-valuea | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | ||
| Discharged alive from ICU/HDU (yes), n (%) | 157 (86.3) | 160 (87.9) | 0.842 |
| Discharged location from ICU, n (%) | |||
| Ward | 150 (95.5) | 153 (95.6) | 0.246 |
| Other critical care facility | 3 (1.9) | 6 (3.8) | |
| Other | 4 (2.6) | 1 (0.6) | |
| Discharged alive from hospital (yes), n (%) | 145 (79.7) | 146 (80.2) | 0.896 |
| Discharged location from hospital, n (%) | |||
| Home | 108 (74.5) | 108 (72.6) | 0.231 |
| Rehabilitation | 8 (5.5) | 8 (5.5) | |
| Other | 9 (6.2) | 3 (2.0) | |
| Nursing/residential home | 4 (2.8) | 10 (6.9) | |
| Another hospital | 16 (11.0) | 18 (12.3) | |
| Time from ICU admission to hospital discharge (days) | |||
| n | 145 | 146 | 0.558 |
| Mean (SD) | 41.0 (39.9) | 40.8 (28.6) | |
| Median (IQR) | 31.9 (18.9–31.9) | 32.6 (21.9–51.8) | |
Adverse events
The AE data have been detailed in Table 19. Approximately 25% of the participants in each group experienced a new AE during their ICU stay. There was no difference between the two groups in the number of days for which new AEs were reported or in the type of event reported.
| Outcomes | Trial group | Adjusted estimate (95% CI); p-valuea | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | ||
| Number of days new AEs reported | |||
| Mean (SD) | 0.5 (1.2) | 0.6 (1.4) | IRR 1.20 (0.71 to 1.92); 0.538 |
| Median (IQR) | 0 (0–1) | 0 (0–0) | |
| Participant experienced new AE (yes), n (%) | 47 (25.8) | 45 (24.7) | OR 0.90 (0.57 to 1.49); 0.739 |
| Type of new AE reported, n (%)b | |||
| Nasal/skin/mouth sores/irritation | 14 (7.7) | 19 (10.4) | OR 1.30 (0.64 to 2.82); 0.433 |
| Vomiting | 8 (4.4) | 14 (7.7) | OR 1.90 (0.76 to 4.62); 0.173 |
| Gastric distension | 6 (3.3) | 7 (3.9) | OR 1.00 (0.32 to 3.25); 0.964 |
| Barotrauma (e.g. pneumothorax) | 3 (1.7) | 3 (1.7) | OR 1.40 (0.25 to 7.43); 0.725 |
| Non-respiratory infection | 12 (6.6) | 11 (6.0) | OR 0.80 (0.35 to 2.00); 0.691 |
| Arrhythmia | 22 (12.1) | 14 (7.7) | OR 0.60 (0.29 to 1.22); 0.156 |
Serious adverse events
The SAEs reported during the trial have been detailed in Table 20. In total, 42 SAEs were reported: 16 in the invasive weaning group and 26 in the non-invasive weaning group. Most of the reported SAEs were unrelated to the trial intervention.
| Outcomes | Trial group, n (%) | |
|---|---|---|
| Invasive weaning | Non-invasive weaning | |
| Events (N = 16) | Events (N = 26) | |
| Event type | ||
| Death | 3 (18.8) | 5 (19.2) |
| Life-threatening | 8 (50.0) | 13 (50.0) |
| Hospitalisation or prolongation of existing hospitalisation | 10 (62.5) | 10 (38.5) |
| Persistent or significant disability/incapacity | 1 (6.3) | 2 (7.7) |
| Congenital anomaly/birth defect | 0 (0.0) | 0 (0.0) |
| Other reason | 2 (12.5) | 4 (15.4) |
| Event related to trial intervention (in the opinion of the reporting clinician) | ||
| Definitely | 1 (6.3) | 0 (0.0) |
| Probably | 0 (0.0) | 0 (0.0) |
| Possibly | 0 (0.0) | 4 (15.4) |
| Unlikely | 2 (12.5) | 6 (23.1) |
| Unrelated | 10 (62.5) | 16 (61.5) |
| Missing | 3 (18.7) | 0 (0.0) |
| Event expectedness | ||
| Expected | 1 (6.2) | 1 (3.9) |
| Unexpected | 0 (0.0) | 3 (11.5) |
| Not applicable | 15 (93.8) | 22 (84.6) |
| Outcome of event | ||
| Resolved – no sequelae | 6 (37.6) | 10 (38.5) |
| Resolved – with sequelae | 4 (25.0) | 3 (11.5) |
| Unresolved | 3 (18.7) | 4 (15.4) |
| Death | 3 (18.8) | 9 (34.6) |
Health-related quality of life
A summary of the HRQoL of participants at follow-up is presented in Table 21. There were no differences found between the two groups in terms of their reported quality of life.
| Outcomes | Trial group | Adjusted estimate (95% CI); p-valuea | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | ||
| EQ-5D-3L change | |||
| Baseline to 3 months | |||
| n | 81 | 81 | 0.01 (–0.12 to 0.14); 0.920 |
| Mean (SD) | 0.13 (0.40) | 0.14 (0.42) | |
| Baseline to 6 months | |||
| n | 74 | 81 | 0.08 (–0.05 to 0.21); 0.215 |
| Mean (SD) | 0.04 (0.38) | 0.14 (0.40) | |
| EQ VAS | |||
| 3 months | |||
| n | 91 | 92 | –0.5 (–6.77 to 5.84); 0.885 |
| Mean (SD) | 62.5 (20.2) | 60.9 (20.0) | |
| 6 months | |||
| n | 82 | 91 | –2.8 (–9.45 to 3.93); 0.417 |
| Mean (SD) | 65.0 (21.2) | 61.7 (22.4) | |
| SF-12 (mental) | |||
| 3 months | |||
| n | 80 | 82 | –1.0 (–4.92 to 2.83); 0.594 |
| Mean (SD) | 45.8 (10.9) | 43.8 (12.6) | |
| 6 months | |||
| n | 75 | 84 | –0.3 (–4.62 to 4.00); 0.888 |
| Mean (SD) | 45.4 (13.3) | 44.7 (12.1) | |
| SF-12 (physical) | |||
| 3 months | |||
| n | 80 | 82 | –0.4 (–3.81 to 3.01); 0.815 |
| Mean (SD) | 33.7 (9.7) | 33.4 (10.3) | |
| 6 months | |||
| n | 75 | 84 | –1.3 (–4.95 to 2.43); 0.499 |
| Mean (SD) | 37.0 (10.4) | 35.5 (11.6) | |
Subgroup analyses
The results of the prespecified (i.e. COPD status and operative status) and exploratory (i.e. site and Psupp) subgroup analyses are presented in Table 22. None of the explored subgroups showed evidence of a moderating treatment effect.
| Subgroups | Trial group, n; median (IQR) | Effect (95% CI); p-valuea | ||
|---|---|---|---|---|
| Invasive weaning | Non-invasive weaning | Within-group treatment | Interaction | |
| COPD status | ||||
| Absence of COPD | 150; 108 (57.9–322.3) | 151; 91 (34–301.7) | HR 1.10 (0.85 to 1.39); 0.517 | HR 1.20 (0.63 to 2.12); 0.640 |
| Presence of COPD | 32; 128 (38–442) | 31; 107.6 (45.7–269) | HR 1.40 (0.78 to 2.59); 0.252 | |
| Operative status | ||||
| Non-operative | 126; 156.2 (58.5–416) | 126; 111.5 (41–276) | HR 1.20 (0.94 to 1.64); 0.124 | HR 0.70 (0.45 to 1.21); 0.231 |
| Postoperative | 56; 85.5 (35.4–255.5) | 56; 57 (18.5–442) | HR 1.00 (0.63 to 1.44); 0.816 | |
| Three main sites vs. other | ||||
| Three main sites | 82; 128 (40–273) | 79; 83 (32–297) | HR 1.00 (0.73 to 1.44); 0.899 | HR 1.20 (0.76 to 1.87); 0.438 |
| Other | 100; 108 (59.7–472.6) | 103; 107 (35–301.7) | HR 1.20 (0.89 to 1.64); 0.218 | |
| Psupp (cmH2O) | ||||
| < 10 | 63; 79 (38–259) | 52; 42 (18–320) | HR 1.10 (0.73 to 1.62); 0.674 | HR 1.10 (0.67 to 1.76); 0.731 |
| ≥ 10 | 118; 174 (58–370) | 127; 125 (54–297) | HR 1.10 (0.84 to 1.47); 0.443 | |
Sensitivity analysis
Adjustment for baseline pneumonia
There was some indication of a difference between the two groups in the number of participants diagnosed with pneumonia/respiratory infection at baseline. A sensitivity analysis was undertaken but no difference was found (HR 1.10, 95% CI 0.88 to 1.38).
Per-protocol analysis
A per-protocol sensitivity analysis was undertaken to estimate the treatment effect, having excluded protocol violators, to see if the treatment effect estimates differed from the primary analysis results. The results of the sensitivity analysis were similar to those of the primary analysis results, which suggested that there was no difference between the two groups (HR 1.10, 95% CI 0.89 to 1.43).
Chapter 5 Economic evaluation
Overview of the economic evaluation
This prospective economic evaluation was conducted alongside a pragmatic, randomised, controlled, open, multicentre, effectiveness trial (Breathe RCT) with the objective of estimating the cost-effectiveness of IMV using protocolised weaning that includes NIV as an intermediate step compared with protocolised weaning without NIV. Patients with respiratory failure who received IMV for > 48 hours (from the time of intubation) and failed a SBT were randomised in a 1 : 1 ratio to either the invasive or the non-invasive weaning strategy. The economic evaluation took the form of a cost–utility analysis, expressed in terms of incremental cost per QALY gained. The primary analysis is based on both a NHS and PSS perspective as recommended by NICE, and excludes costs borne by sectors of the economy other than the health and social service sectors, and costs borne by trial participants and their families/informal carers. 35 Costs are expressed in Great British pounds (GBP) (2015–16 prices). A sensitivity analysis was conducted to recalculate cost-effectiveness from a societal perspective.
Measurement of resource use and costs
The incremental costs associated with the NIV strategy were determined through a comprehensive strategy that encompassed two strands of research: (1) estimation of costs associated with the delivery of the intervention and (2) estimation of broader health and PSS resource inputs and costs.
Resource use associated with non-invasive ventilation (intervention)
A specific focus of the economic evaluation was the assessment of hospital resource inputs associated with delivering the NIV intervention. This was based on data associated with ICU-related length of stay for organ support (advanced or basic cardiovascular or respiratory support, renal support, dermatological support, antimicrobial use, whether respiratory or non-respiratory) collected from randomisation on a daily basis for the first 30 days during a participant’s stay in ICU. In addition, daily data on the highest level of care (levels 0, 1, 2 or 3) over each 24-hour period over the first 30 days during the ICU stay and on use of antibiotics, sedatives and antiviral medications were collected. Similarly, hospital resource use data were also collected beyond the first 30 days, including the nature of respiratory support (advanced or basic), renal support, dermatological support and antimicrobial use (respiratory or non-respiratory) up to ICU discharge. Furthermore, data on the length and intensity of hospital stay, post ICU discharge until final hospital discharge, were collected; details of hospital transfers between the hospital at randomisation and other hospitals (or residence) were also included. Information on the use of high-cost medications was collected on a daily basis.
Collection of broader resource use data
Health and social service resource use data were collected at 3 and 6 months post randomisation, using self-completed postal questionnaires. Postal reminders were sent to non-responders and, when required, further telephone contacts when made to encourage the return of questionnaires or to request the completion of missing data. The postal questionnaires provided details on the frequency and duration of use of hospital inpatient care (including readmissions), hospital outpatient care, residential care services, community health and social care services, medications and any equipment or aids that were needed by participants. Medication use was categorised by drug name (or active constituent), mode of administration, dosage frequency and duration of administration. Health and social care resource inputs were subsequently converted into economic cost estimates using unit cost data. For broader societal resource impacts, the actual costs incurred by participants and their families and carers for items such as travel, child care and lost income were valued directly by trial participants and recorded on the data collection forms in monetary terms.
Valuation of resource use
Resource inputs were valued using a combination of primary research and data collated from secondary national tariff sets, using standard accounting methods. For ICU-related resource inputs, the overall costs of intensive care were derived using individual-level data on the number of days of each form of organ support, the number of days of each level of intensive care and the overall duration of the intensive care stay. These patient-level data on duration, number and type of organ support, the duration and level of intensive care and the overall duration of the intensive care stay were used as inputs to generate Healthcare Resource Group (HRG) codes that were subsequently valued using NHS Reference Costs 2014 to 2015. 36 Each ICU-related HRG code is associated with a mean length of hospital stay. For all individuals in the ICU, costs were determined over their length of stay for their derived HRG code.
The unit costs of high-cost drugs not included within ICU-specific HRG values (e.g. antifungals and antivirals) were taken from the Prescription Cost Analysis – England, 2016 database. 37 The unit costs associated with hospital readmissions, hospital day-case admissions, hospital outpatient services costs and accident and emergency attendances were extracted from NHS reference costs36 or, when necessary, the unit cost compendia published by the Personal Social Services Research Unit (PSSRU) at the University of Kent. 38–40 Residential care costs were taken from national tariffs reported in Unit Costs of Health and Social Care 2013. 38 Similarly, community health and social care costs were obtained from Unit Costs of Health and Social Care 2015. 39 NHS medication prices (per milligram) were obtained from the Prescription Cost Analysis – England, 2016 database37 and equipment and adaptation costs from the NHS Supply Chain’s National Catalogue (2014–15). 41 Unit Costs of Health and Social Care 2016 was used to inflate or deflate costs when necessary to 2015–16 prices (GBP). 40 No discounting of costs was applied because cost-effectiveness for the purposes of the within-trial economic evaluation was determined over a 6-month time horizon.
Calculation of utilities and quality-adjusted life-years
The economic evaluation estimated QALY profiles for trial participants based on patient reports of preference-based HRQoL outcomes. The HRQoL of trial participants was assessed using the EQ-5D-3L,42 measured at baseline, and at 3 and 6 months post ICU discharge as a secondary outcome in the trial. The EQ-5D-3L descriptive system consists of the following five dimensions: ‘mobility’, ‘self-care’, ‘usual activities’, ‘pain/discomfort’ and ‘anxiety/depression’. Each dimension is divided into three ordinal levels coded: (1) no problems, (2) some or moderate problems and (3) severe or extreme problems. 43,44 For the purposes of this study, the UK time trade-off tariff was applied to each set of responses to generate an EQ-5D-3L utility score (preference weight) for each participant. 42 The utility scores generated through this method range from –0.59 to 1.0, with 1.0 representing full health, zero representing death and values below 0 indicating health states that are considered to be worse than death. The second measurement component of the EQ-5D-3L is the EuroQol visual analogue scale (EQ VAS), which records the respondent’s self-reported health on the day of the survey on a 20-cm, vertical VAS; the scale ranges from 100 (best imaginable health state) to 0 (worst imaginable health state). 43 QALYs were calculated as the area under the curve, assuming a fixed baseline utility score of –0.402 (equivalent to an unconscious health state) and using linear interpolation between baseline and follow-up utility scores. 45
The SF-12 was also completed at the same time point as the EQ-5D-3L and responses were converted into Short Form questionnaire-6 Dimensions (SF-6D) utilities based on the algorithm provided by Brazier et al. 46 For the SF-6D utility scores, the fixed baseline value was set to zero when constructing QALYs for the purpose of a sensitivity analysis. No discounting of QALYs was applied for the purposes of the within-trial economic evaluation because cost-effectiveness was determined over a 6-month time horizon. Participants surviving to hospital discharge were also asked to recollect their pre-admission (pre-randomisation) health state using both the EQ-5D-3L and the SF-12; utility scores generated by these HRQoL assessments were used to inform a separate sensitivity analysis.
Missing data
Multiple imputation (MI) using the Markov chain Monte Carlo method of chained equations was used for the base-case analysis to impute missing data. This avoids potential biases associated with complete-case analysis and is consistent with good practice guidance. MI was used at the aggregate level (i.e. on missing total costs or QALYs). When data are missing at random (MAR), MI provides unbiased estimates of treatment effect. The MAR assumption was explored in the data, using logistic regression for missingness of total costs and QALYs for each time point (separately) as a function of baseline variables. A model was used to generate multiple imputed data sets for treatment groups, in which missing values were estimated conditionally on available covariates: age, sex, centre, presence/absence of COPD, non-operative/operative and post-SBT PaCO2, such that a complete data set was generated, reflecting the distributions and correlations between variables in the observed data. Mean matching using predictive methods was used to improve estimates of imputed values as normality could not be assumed. Each imputed data set was analysed independently using model-based approaches; estimates obtained were pooled to generate mean and variance estimates of costs and QALYs using Rubin’s rule in order to capture within and between variances for imputed samples. 47 Information loss from finite imputation sampling was minimised using 20 data sets, resulting in minimal loss of efficiency (< 0.5%) when compared with infinite sampling. Imputed and observed values were compared in order to establish that imputation did not introduce bias into subsequent estimations.
Analyses of resource use, costs and outcome data
Resource use items were summarised by treatment group and assessment point; differences between groups were analysed using two sample t-tests for continuous variables. The mean [standard error (SE)] of each resource type, including by resource category, was estimated by trial allocation group for each time period. Costs were estimated from both a NHS and a PSS perspective and, for the purposes of a sensitivity analysis, from a broader societal perspective. Differences between groups in terms of costs, along with their CIs, were estimated and reported. Non-parametric bootstrap estimates using 10,000 replications35 were also calculated for differences, along with their CIs. For each EQ-5D-3L dimension, the proportion of participants with suboptimal levels of function (some or moderate problems, or severe or extreme problems) at each assessment point was compared between treatment groups using chi-squared tests. EQ-5D-3L utility score differences between the groups at each follow-up point were tested using two-sample t-tests for unequal variance.
Model-based methods (seemingly unrelated regression) were used to estimate mean incremental changes in costs and QALYs and accounted for the correlation between costs and outcomes within the data while adjusting for covariates, including baseline costs (for the cost equation) and baseline utility scores (for the QALY equation) to adjust for potential baseline imbalances. Non-parametric bootstrap methods were used to generate the joint distributions of costs and outcomes to populate the cost-effectiveness plane. Bootstrapping (using the bias-corrected non-parametric approach) is a resampling method that jointly resamples costs and outcomes from the observed data while holding the sample correlation structure. From each bootstrap sample (of 10,000 samples), changes in costs and QALYs were estimated. Mean estimates are reported with 95% CIs.
Cost-effectiveness analyses
The incremental cost-effectiveness ratio (ICER) was estimated as the difference between the trial comparators in mean total costs divided by the difference in mean total QALYs. Value for money was determined by comparing the ICER value with a cost-effectiveness threshold value; the NICE cost-effectiveness threshold for British studies typically ranges between £20,000 and £30,000 per QALY. In addition, a £15,000 cost-effectiveness threshold was used to reflect more recent trends in health-care decision-making. 48 This represents society’s willingness to pay for an additional QALY; ICER values lower than the threshold could be considered to be cost-effective for use in the NHS. Base-case assumptions were explored using a range of supportive sensitivity analyses.
The incremental net monetary benefit (INMB) of using the NIV protocol was also reported as a recalculation of the ICER at a range of cost-effectiveness thresholds. The INMB succinctly describes the resource gain (or loss) when investing in a new intervention when resources can be used elsewhere at the same threshold. INMB estimates were used to generate cost-effectiveness acceptability curves (CEACs). A CEAC compares the likelihood that interventions are cost-effective as the cost-effectiveness threshold varies.
All statistical analyses and cost-effectiveness modelling were conducted in SAS® software version 9.4 (SAS Institute Inc., Cary, NC, USA) on a Microsoft Windows® (Microsoft Corporation, Redmond, WA, USA) platform.
Sensitivity and subgroup analyses
Several sensitivity analyses were undertaken to assess the impact on the base-case economic evaluation. The following sensitivity analyses were considered for the cost-effectiveness outcomes: (1) recalculation from a broader societal perspective (adopting a wider societal perspective that includes costs incurred by all sectors of the economy and by patients, families and informal carers), (2) complete-case analysis (i.e. those with complete cost and outcome data throughout the trial time horizon), (3) using the SF-6D utility scores estimated from the SF-12 for the purposes of QALY estimation and (4) additionally using the pre-randomisation EQ-5D-3L utility value (recalled at hospital discharge) as a covariate for the purpose of adjusting for QALY differences. Prespecified subgroup analyses were also conducted for the main cost-effectiveness results to explore heterogeneity in the trial population. These were conducted by (1) presence/absence of COPD and (2) operative status (non-operative vs. operative).
Long-term cost-effectiveness model
Modelling survival data
Long-term cost-effectiveness was determined over a 5-year time horizon by modelling and extrapolating survival time between randomisation and death, after examining the observed survival curves (Figure 16a). A flexible parametric model using cubic splines (Royston–Parmar approach) was used. 49 The approach adopted avoids the use of life tables to estimate projected survival rates. Extrapolation beyond 5 years is considered to be highly speculative and uncertain because most deaths occurred between randomisation and hospital discharge.
FIGURE 16.
(a) Observed Kaplan–Meier survival; and (b) extrapolated and observed survival over 5 years. R–P, Royston–Parmar three-knot; K–M, Kaplan–Meier.
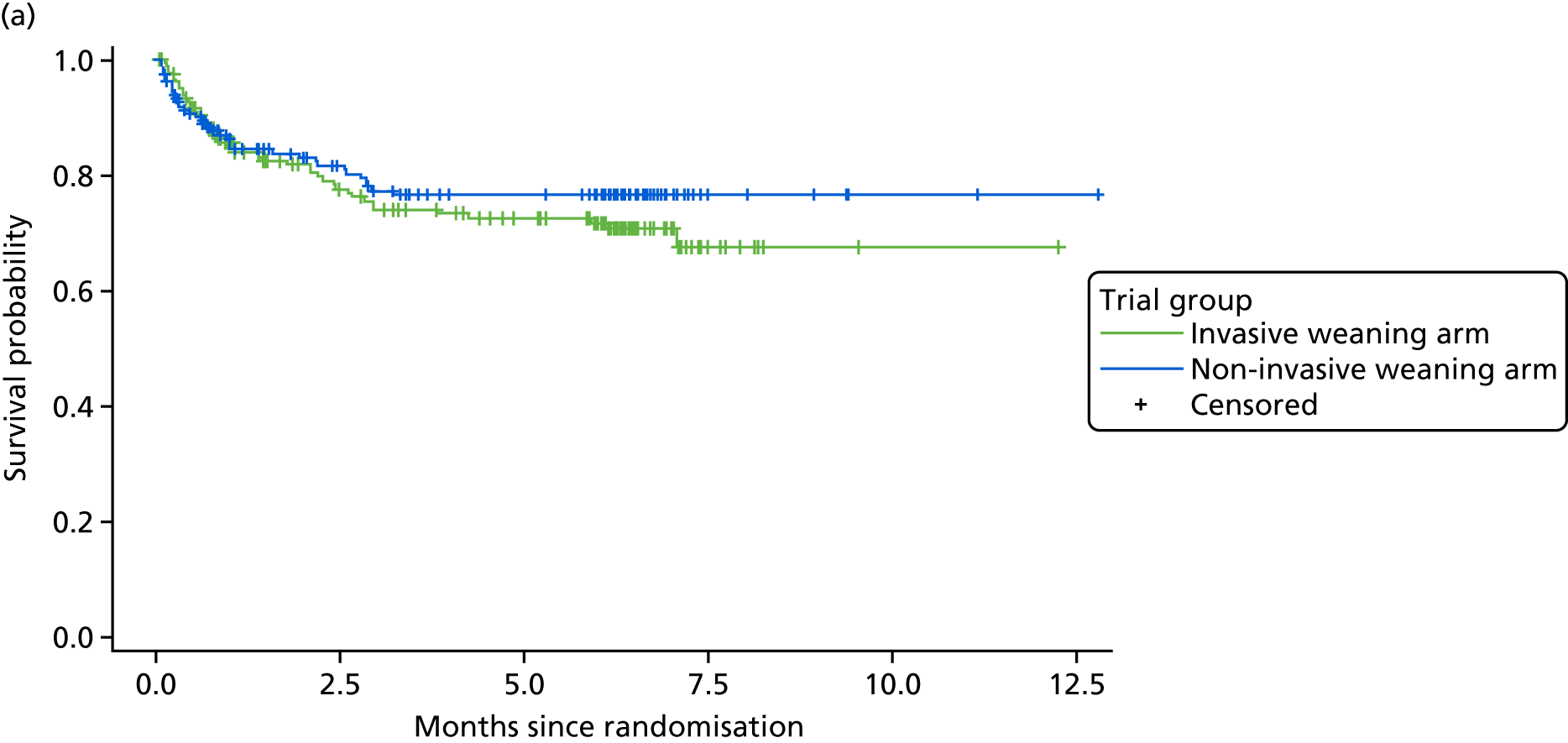
The fitted model (Royston–Parmar model) was used to predict the survival rates at each time point (Figure 16b). The survival rates at each 3-monthly interval (3, 6, 9, 12 . . . 60 months) were used to determine the expected costs and derive the QALYs (at an aggregate level).
Modelling health utilities between hospital discharge and death
Health utilities were extrapolated between 6 months post randomisation and death for each treatment group separately. The extrapolation was determined under the main assumption that utilities remained constant at 6 months post randomisation.
Adjusted health utilities and quality-adjusted life-years
The estimated health utilities at each time point between 6 months post randomisation and 60 months post randomisation were multiplied by the predicted proportion of participants alive at the corresponding time point. These values were then used to compute the 5-year QALY estimate for each group by computing the area under the curve using the linear trapezoidal rule after discounting at 3.5% per annum in each of years 2–5.
Modelling longer-term costs
Costs between 6 months post randomisation and death were estimated by using the observed 3- to 6-month post-randomisation total costs, adjusted for covariates (e.g. age, centre, COPD status), and multiplying by the predicted survival probabilities over the period extending to 5 years post randomisation (see Table 33 in Appendix 3). The primary long-term cost-effectiveness analyses was considered under the assumption that the longer-term costs and benefits (health utilities) for the NIV group are equal to those for the IMV group and only the proportion of participants alive beyond 6 months adjusts these costs and benefits (see Table 33 in Appendix 3).
Additional sensitivity analyses included:
-
assuming that, after 6 months post randomisation, costs and health utilities were the same between groups, but survival rates in the NIV group were 10% lower (justified by an examination of the plots of log-survival and hazard function) and similar to those of the IMV group
-
assuming that, after 6 months post randomisation, costs and health utilities are carried forward (unequal) from 3 to 6 months onwards until 5 years post randomisation
-
assuming that, after 6 months post randomisation, costs were the same between groups, but health utilities were carried forward from the last estimates observed.
In summary, for the long-term cost-effectiveness analyses, the analyses in Table 34 (see Appendix 3) were applied.
Results
Trial population
A total of 364 participants were randomised into the Breathe trial: 182 to the ‘non-invasive weaning’ group and 182 to the ‘invasive weaning’ group. Complete health resource information was available for all 354 participants between randomisation and ICU discharge. Consequently, the base-case economic analyses include data from all 354 participants. Approximately 52% and 50% of all health resource use data were complete at 3 months for the NIV and IMV groups, respectively; this diminished to 51% and 46%, respectively, by 6 months (Table 23). A complete QALY profile was available for 182 out of 364 (50%) participants. For about 65% of participants (see Table 35, Appendix 3), a complete QALY profile was available after deaths were taken into account. Consequently, about 35% of QALY data and between 6% and 40% of costs (at the component level) were missing (and subsequently imputed) for the primary analysis.
| Resource use item | Time point | Trial group, n (%) | |||||
|---|---|---|---|---|---|---|---|
| Non-invasive weaning group (N = 182) | Invasive weaning group (N = 182) | ||||||
| Completeda | Unavailableb | Missingc | Completeda | Unavailableb | Missingc | ||
| ICU admission length of stay by organ support and level of care | Initial hospitalisation | 182 (100) | 0 (0) | 0 (0) | 182 (100) | 0 (0) | 0 (0) |
| Post-ICU admission length of stay and procedures | Initial hospitalisation | 182 (100) | 0 (0) | 0 (0) | 182 (100) | 0 (0) | 0 (0) |
| Inpatient care (readmissions) | 3 months | 95 (52) | 87 (48) | 0 (0) | 91 (50) | 89 (49) | 2 (1) |
| 6 months | 93 (51) | 88 (48) | 1 (1) | 84 (46) | 97 (53) | 1 (1) | |
| Outpatient care (hospitals/clinics) | 3 months | 95 (52) | 87 (48) | 0 (0) | 91 (50) | 89 (49) | 2 (1) |
| 6 months | 93 (51) | 88 (48) | 1 (1) | 84 (46) | 97 (53) | 1 (1) | |
| Residential care services | 3 months | 95 (52) | 87 (48) | 0 (0) | 91 (50) | 89 (49) | 2 (1) |
| 6 months | 93 (51) | 88 (48) | 1 (1) | 84 (46) | 97 (53) | 1 (1) | |
| Community health and social care | 3 months | 95 (52) | 87 (48) | 0 (0) | 91 (50) | 89 (49) | 2 (1) |
| 6 months | 93 (51) | 88 (48) | 1 (1) | 84 (46) | 97 (53) | 1 (1) | |
| Medications | 3 months | 95 (52) | 87 (48) | 0 (0) | 91 (50) | 89 (49) | 2 (1) |
| 6 months | 93 (51) | 88 (48) | 1 (1) | 84 (46) | 97 (53) | 1 (1) | |
| Equipment or aids | 3 months | 95 (52) | 87 (48) | 0 (0) | 91 (50) | 89 (49) | 2 (1) |
| 6 months | 93 (51) | 88 (48) | 1 (1) | 84 (46) | 97 (53) | 1 (1) | |
| Broader societal resource use | 3 months | 95 (52) | 87 (48) | 0 (0) | 91 (50) | 89 (49) | 2 (1) |
| 6 months | 93 (51) | 88 (48) | 1 (1) | 84 (46) | 97 (53) | 1 (1) | |
| EQ-5D-3L index | Prior to hospital admissiond | 121 (66) | 58 (32) | 3 (2) | 120 (66) | 62 (34) | 0 (0) |
| 3 months | 91 (50) | 87 (48) | 4 (2) | 88 (48) | 91 (50) | 3 (2) | |
| 6 months | 90 (49) | 89 (49) | 3 (2) | 80 (44) | 99 (54) | 3 (2) | |
| EQ VAS | Prior to hospital admission | 121 (66) | 58 (32) | 3 (2) | 120 (66) | 62 (34) | 0 (0) |
| 3 months | 91 (50) | 87 (48) | 4 (2) | 88 (48) | 91 (50) | 3 (2) | |
| 6 months | 91 (50) | 90 (49) | 3 (2) | 82 (45) | 101 (55) | 3 (2) | |
| SF-12 | Prior to hospital admission | 120 (66) | 59 (32) | 3 (2) | 120 (66) | 62 (34) | 0 (0) |
| 3 months | 91 (50) | 87 (48) | 4 (2) | 88 (48) | 91 (50) | 3 (2) | |
| 6 months | 91 (50) | 90 (49) | 3 (2) | 82 (45) | 101 (55) | 3 (2) | |
Resource use and costs
Direct resource and cost implications of the intervention
Table 35 (see Appendix 3) summarises resource use values by trial allocation, resource category and trial period for complete cases. Table 36 (see Appendix 3) summarises the unit cost values applied to each resource input, whereas Table 24 summarises the economic cost values by trial allocation, resource category and trial period. The resource and cost components associated with the intervention are aggregated into five groups: (1) intensive care support, which includes organ support, level of care and use of sedatives; (2) tracheostomy; (3) use of high-cost antiviral and antifungal or other high-cost drugs in the ICU; (4) hospital care between ICU discharge and hospital discharge; and (5) use of any emergency transport to transfer patients between hospital sites. Total intervention costs are also presented by treatment group. These varied between £2862 and £172,543 for the non-invasive weaning group and between £5146 and £195,855 for the invasive weaning group.
There were no statistical differences in the mean costs between randomisation and initial hospital discharge, which were £29,697 and £32,052 for the non-invasive weaning and invasive weaning groups, respectively (mean cost difference –£2355, 95% CI –£7292 to £2750; p = 0.4472). There were no statistical differences in any of the component costs related to ICU stay (see Table 24), although, on average, the mean costs were lower for the non-invasive group for most cost components.
Broader resource use
Table 35 (see Appendix 3) also shows resource use values for participants with complete data by trial group allocation, resource use category and trial period. The resource values are presented for subcategories of resource use, including hospital inpatient and outpatient care, residential care, community health and social care, medications, and equipment and aids. Broader societal resource input and costs included travel, child care, income lost, housework help and laundry services costs.
In terms of specific health resource use for non-invasive weaning versus invasive weaning at 3 months, notable differences were observed for residential rehabilitation use (0.7 vs. 1.6 days; p = 0.296), home help/care work support (6.8 vs. 3.9 visits; p = 0.1868) and occupational therapist visits (0.8 vs. 0.4 visits; p = 0.3318); in addition, a smaller proportion of non-invasive weaning group participants were supported through equipment and aids (56% vs. 65%; p = 0.2113). Between 3 and 6 months, 10% fewer participants were admitted for an inpatient stay, such that the average length of stay was lower with non-invasive weaning (3.1 vs. 4.3 days); similarly, 20% fewer participants had outpatient clinic visits (53% vs. 73%; p = 0.00634). However, between randomisation and 6 months post discharge, the mean length of stay during hospital readmissions was higher for the non-invasive weaning group than for the invasive weaning group (9.5 vs. 5.2 days; p = 0.0604), suggesting the potential for later longer-term costs associated with survivors of non-invasive weaning. This feature is also noted for other health resource items in Table 35 (see Appendix 3): rehabilitation days (3.1 vs. 0.6 days; p = 0.1262); general practitioner (GP) home visits (0.8 vs. 0.4 visits; p = 0.5768); district nurse visits (8.4 vs. 6.6 visits; p = 0.4588); and purchase of aids and equipment (26% vs. 12%; p = 0.01878). For all other resource use items, there were no noticeable differences between the trial groups.
Economic costs
Economic costs for participants with complete data are presented in Table 24 by trial group, trial period and cost category. Based on complete cost data, non-invasive weaning was, on average, less costly. The total mean cost for the non-invasive weaning group was lower from a NHS/PSS perspective (£31,711 vs. £32,468), although this difference was not statistically significant (bootstrap 95% CI –£6642 to £5246; p = 0.8321). This was also true when costs were considered from a broader societal perspective: £31,934 versus £32,999 (bootstrap 95% CI –£6804 to £5056; p = 0.7981).
| Resource use category by time period | Trial group, mean (SE) | Mean difference | p-value | Bootstrap 95% CI | |
|---|---|---|---|---|---|
| Non-invasive | Invasive | ||||
| Randomisation to hospital discharge | n = 182 | n = 182 | |||
| Intensive care supporta | 20,509.7 (1762.56) | 22,018.9 (1685.17) | –1509.2 | 0.5515 | (–5533.9 to 2586.3) |
| Tracheostomy | 980.9 (131.08) | 1254.6 (141.7) | –273.7 | 0.1570 | (–584.1 to 57.2) |
| Antivirals/antifungals | 455.7 (194.91) | 758.2 (219.66) | –302.5 | 0.3036 | (–768.1 to 215.8) |
| Hospital care between ICU and hospital discharge | 7694.5 (695.69) | 7967.0 (1038.27) | –272.5 | 0.8275 | (–2511.2 to 1682.5) |
| Transfer by ambulance/NHS transport | 56.0 (7.97) | 53.1 (7.82) | 2.87 | 0.7971 | (–16.1 to 20.7) |
| Total NHS and PSS costs | 29,696.8 (2069.8) | 32,051.8 (2204.56) | –2355.0 | 0.4472 | (–7291.5 to 2750.0) |
| Hospital discharge to 3 months post randomisation | n = 130 | n = 131 | |||
| Health and social care resource use | |||||
| Hospital inpatient care | 686.8 (163.83) | 381.8 (123.63) | 305.0 | 0.1387 | (–30.0 to 642.2) |
| Hospital outpatient care | 130.7 (25.95) | 86.7 (17.41) | –4.0 | 0.1602 | (–5.2 to 97.3) |
| Residential care | 158.6 (55.55) | 43.2 (22.64) | 115.4 | 0.0560 | (20.1 to 219.7) |
| Community health and social care | 280.7 (46.03) | 301.3 (75.31) | –20.6 | 0.8157 | (–171.3 to 114.3) |
| Medications | 67.8 (21.23) | 139.5 (36.84) | –71.7 | 0.0935 | (–141.4 to -4.7) |
| Equipment and aids | 32.2 (8.79) | 13.9 (4.71) | 18.3 | 0.0679 | (2.8 to 35.5) |
| Total NHS and PSS costs | 1356.8 (196.32) | 966.4 (151.99) | 390.4 | 0.0836 | (–47.1 to 775.7) |
| Broader societal costs | |||||
| Additionalb | 108.6 (33.02) | 297.7 (129.15) | –189.1 | 0.0926 | (–324.6 to –4.2) |
| Equipment and aids (private and charity) | 29.3 (10.53) | 158.3 (120.1) | –129 | 0.2854 | (–259.3 to 131.4) |
| Total broader societal costs | 137.9 (22.47) | 456.0 (93.68) | –318.1 | 0.0012 | (–434.1 to –158.4) |
| Total societal costs | 1494.7 (179.61) | 1422.4 (202.29) | 72.3 | 0.3869 | (–219.2 to 676.5) |
| 3 months post randomisation to 6 months post randomisation | n = 129 | n = 127 | |||
| Health and social care resource use | |||||
| Hospital inpatient care | 788.4 (203.12) | 403.0 (144.18) | 385.4 | 0.1233 | (–7.0 to 1101.3) |
| Hospital outpatient care | 131.5 (20.23) | 123.0 (24.85) | 28.50 | 0.7913 | (–56.8 to 105.0) |
| Residential care | 264.4 (140.96) | 0.0 (0.00) | 264.0 | 0.0630 | (57.6 to 515.2) |
| Community health and social care | 373.2 (102.74) | 214.5 (66.54) | 158.7 | 0.1963 | (–33.2 to 362.5) |
| Medications | 176.2 (45.39) | 206.0 (46.65) | –29.8 | 0.6480 | (–135.9 to 78.5) |
| Equipment and aids | 30.9 (6.30) | 17.4 (5.25) | –13.5 | 0.1010 | (–0.27 to 26.9) |
| Total NHS and PSS costs | 1764.6 (258.47) | 963.9 (157.71) | 798.6 | 0.0031 | (308.9 to 1294.7) |
| Broader societal costs | |||||
| Additionalb | 171.8 (70.17) | 245.9 (110.38) | –74.1 | 0.3864 | (–207.6 to 52.9) |
| Equipment and aids (private and charity) | 25.9 (9.67) | 14.7 (12.5) | 11.2 | 0.4788 | (–10.8 to 33.2) |
| Total broader societal costs | 197.7 (45.3) | 260.6 (66.3) | –62.9 | 0.4336 | (–173.9 to 48.1) |
| Total societal costs | 1962.3 (265.94) | 1224.5 (178.96) | 737.8 | 0.2262 | (–432.6 to 1928.3) |
| From randomisation to 6 months post randomisation | n = 129 | n = 127 | |||
| Initial hospitalisation costs | |||||
| Intensive care supporta | 20,815.7 (2134.03) | 21,659.9 (2059.30) | –844.2 | 0.7761 | (–5699 to 4114) |
| Tracheostomy | 922.6 (149.01) | 1129.5 (159.01) | –206.9 | 0.3435 | (–570.1 to 151.2) |
| Antivirals/antifungals | 448.2 (234.98) | 968.8 (288.76) | –520.6 | 0.1635 | (–1131.2 to 89.9) |
| Hospital care between ICU and hospital discharge | 6613.3 (707.09) | 6914.7 (896.01) | –301.4 | 0.7921 | (–2229 to 1519) |
| Transfer by ambulance/NHS transport | 42.6 (8.33) | 46.1 (8.57) | –3.5 | 0.7681 | (–23.3 to 16.1) |
| Total NHS and PSS costs | 28,842.4 (2462.57) | 30,719.0 (2501.67) | –1876.6 | 0.5934 | (–7612 to 3989) |
| Post-initial-hospitalisation costs | |||||
| Health and social care resource use | |||||
| Hospital inpatient care | 1323.6 (294.88) | 698.2 (221.55) | 625.4 | 0.0913 | (28.3 to 1236.9) |
| Hospital outpatient care | 240.7 (39.10) | 188.8 (34.85) | 51.9 | 0.3222 | (–33.4 to 139.6) |
| Residential care | 405.4 (150.44) | 41.6 (21.81) | 363.8 | 0.0180 | (132.7 to 632.6) |
| Community health and social care | 613.8 (129.40) | 475.5 (124.74) | 138.3 | 0.4426 | (–153.7 to 424.5) |
| Medications | 226.3 (59.99) | 315.8 (68.56) | –89.5 | 0.3268 | (–237.9 to 60.7) |
| Equipment and aids | 59.1 (10.89) | 28.6 (8.45) | 30.5 | 0.0276 | (7.3 to 53.4) |
| Total NHS and PSS costs | 2868.9 (392.94) | 1748.5 (276.45) | 1120.4 | 0.0494 | (176.5 to 1542.9) |
| Broader societal costs | |||||
| Additionalb | 171.7 (67.65) | 374.6 (164.09) | –202.9 | 0.2542 | (–441.1 to –23.3) |
| Equipment and aids (private and charity) | 51.2 (24.45) | 157.0 (98.87) | –105.8 | 0.2498 | (–179.3 to 16.0) |
| Total broader societal costs | 222.9 (97.44) | 531.6 (120.33) | –309.7 | 0.0142 | (–489.5 to 272.6) |
| Total societal costs | 3091.8 (392.94) | 2280.1 (276.45) | 811.7 | 0.3163 | (–128.3 to 1371.6) |
| Total NHS/PSS including ICU | 31,711.3 (2498.47) | 32,467.5 (2550.99) | –756.2 | 0.8321 | (–6642.1 to 5245.7) |
| Total societal including ICU | 31,934.2 (2498.58) | 32,999.1 (2547.95) | –1064.9 | 0.7981 | (–6804.2 to 5055.9) |
Between randomisation and 6 months, initial hospitalisation costs were lower (see Table 24) for the non-invasive weaning group (£28,842 vs. £30,719; p = 0.593). Similarly, residential care costs and equipment and aids costs were higher, on average, for the non-invasive weaning group than for the invasive weaning group (residential care costs £405 vs. £42; p = 0.0180; equipment and aid costs £59 vs. £29; p = 0.0276). Moreover, the total post-initial-hospitalisation health and social care burden (in terms of costs) from the NHS/PSS perspective was higher (see Table 24) for the non-invasive weaning group than for the invasive weaning group (£2869 vs. £1749; p = 0.0494). Mean total broader societal costs were lower over the 6 months for the non-invasive weaning group than for the invasive weaning group (£223 vs. £532; p = 0.0142). There were also statistically significant differences between the groups in terms of broader societal costs at 3 months: £138 in the non-invasive weaning group versus £456 in the invasive weaning group (p = 0.0012).
Health-related quality-of-life outcomes
There were no statistically significant differences in suboptimal levels of function in HRQoL for participant-reported dimensions of the EQ-5D-3L between the intervention and control groups prior to hospital admission or at 3 months post ICU discharge (see Table 37 in Appendix 3). However, the mean EQ-5D-3L utility score among complete cases was statistically significantly lower (see Table 37 in Appendix 3) at 6 months post randomisation in the non-invasive weaning group (0.53 vs. 0.66; p = 0.0147). In contrast, the mean QALY value, which takes into account utility scores across multiple time points and sets a utility score of zero from the date of death onwards, was, on average, higher for the non-invasive weaning group than for the invasive weaning group (0.0928 vs. 0.0747; p = 0.4522), although the mean QALY difference was not statistically significant (Table 25). There were no (statistically significant) differences in the EQ VAS scores between the intervention and control groups at each of the time points of assessment.
| Parameter | QALY (EQ-5D-3L participant)a | |
|---|---|---|
| n | Mean | |
| Non-invasive (SE) | 120 | 0.0928 (0.0616) |
| Invasive (SE) | 118 | 0.0747 (0.0179) |
| Mean differenceb | – | 0.0181 |
| p-value (95% CI) | – | 0.4522 (–0.0293 to 0.0656) |
Cost-effectiveness results
Base-case analysis
The incremental cost-effectiveness of non-invasive weaning is shown in Table 26 for the participants with costs and health outcomes data subject to MI. When a NHS/PSS perspective was adopted (i.e. that which was adopted for the baseline analysis) and health outcomes were measured in terms of QALYs, the mean total cost was £36,313 in the NIV group, compared with £36,615 in the IMV group, generating a mean incremental cost of –£302. The NIV intervention was also associated with a non-statistically significant increase in QALYs (0.02 QALYs, 95% CI –0.01 to 0.05) over the entire 6-month follow-up period. In health economic terms, the intervention was dominant as average costs were lower and the net effect was higher.
| Scenario | Probability of cost-effectiveness | INMB | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (95% CI) | Incremental QALYs (95% CI) | ICERa | p-valueb | p-valuec | p-valued | INMBb | INMBc | INMBd | |
| Base case (NHS/PSS perspective) | |||||||||
| Imputed attributable costs and QALYs (fixed baseline), adjusted for covariates | –302 (–5489 to 4761) | 0.01589 (–0.01262 to 0.0465) | NIV dominant | 0.57 | 0.58 | 0.59 | 541 (–4598 to 5833) | 620 (–4545 to 5928) | 779 (–4470 to 6162) |
| Sensitivity analyses | |||||||||
| 1. Imputed societal attributable costs and QALYs (fixed baseline), adjusted for covariates | –339 (–5422 to 4645) | 0.01631 (–0.01271 to 0.04714) | NIV dominant | 0.57 | 0.58 | 0.60 | 584 (–4509 to 5733) | 665 (–4473 to 5862) | 828 (–4369 to 6075) |
| 2. Complete cases (NHS/PSS) attributable costs and QALYs (fixed baseline), adjusted for covariates | –739.5 (–7139 to 5641) | 0.01605 (–0.0211 to 0.0557) | NIV dominant | 0.59 | 0.60 | 0.62 | 980 (–5539 to 7442) | 1060 (–5505 to 7559) | 1220 (–5456 to 7833) |
| 3. Imputed attributable costs and QALYs (fixed baseline using SF-6D utility score), covariates adjusted | –330 (–540 to 4692) | 0.0295 (–0.1367 to 0.1762) | NIV dominant | 0.58 | 0.60 | 0.62 | 774 (–5033 to 6521) | 922 (–5357 to 7025) | 1218 (–6163 to 8267) |
| 4. Imputed attributable costs and QALYs (fixed baseline), adjusted for covariates and pre-admission EQ-5D-3L covariate | –445 (–5305 to 4486) | 0.0156 (–0.0151 to 0.0479) | NIV dominant | 0.59 | 0.60 | 0.61 | 625 (–4809 to 5114) | 735 (–4914 to 5044) | 894 (–5182 to 4962) |
| Subgroup analyses (COPD and operative status) | |||||||||
| COPD: presence of COPD – imputed attributable costs and QALYs (fixed baseline), covariates adjusted EQ-5D-3L utility score | –5322 (–17,899 to 6431) | 0.1169 (0.0353 to 0.215) | NIV dominant | 0.82 | 0.84 | 0.87 | 7076 (–4817 to 19,814) | 7660 (–4308 to 20,451) | 8829 (–3368 to 21,731) |
| COPD: absence of COPD – imputed attributable costs and QALYs (fixed baseline), covariates adjusted EQ-5D-3L utility score | 756 (–4768 to 6380) | –0.0050 (–0.0352 to 0.0258) | NIV dominated | 0.40 | 0.40 | 0.39 | –831 (–6588 to 4793) | –856 (–6629 to 4801) | –906 (–6745 to 4863) |
| Operative status: non-operative – imputed attributable costs and QALYs (fixed baseline), covariates adjusted EQ-5D-3L utility score | –1915 (–7955 to 3932) | 0.03743 (0.00105 to 0.0766) | NIV dominant | 0.75 | 0.76 | 0.79 | 2477 (–3491 to 8559) | 2664 (–3394 to 8806) | 3038 (–3134 to 9264) |
| Operative status: operative – imputed attributable costs and QALYs (fixed baseline), covariates adjusted EQ-5D-3L utility score | 3266 (–6808 to 13,041) | –0.0311 (–0.0747 to 0.0135) | NIV dominated | 0.27 | 0.26 | 0.25 | –3733 (–13,560 to 6415) | –3888 (–13,736 to 6284) | –4200 (–14,063 to 6042) |
Incremental net monetary benefit
The associated mean INMB of non-invasive weaning at cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY was £541, £620 and £779, respectively (see Table 25). The base-case mean INMB was > £0, suggesting that adopting the NIV protocol would result in a net economic gain of £620, on average, from a NHS/PSS perspective (INMB £541, 95% CI –£4545 to £5928), assuming a cost-effectiveness threshold of £20,000 per QALY. The cost-effectiveness plane (Figure 17) shows that the ICERs are largely spread across the north-east and south-east quadrants. These result in a probability of cost-effectiveness of between 57% and 59% (Figure 18); that is, if decision-makers are willing to pay between £15,000 and £30,000 for an additional QALY, the probability that the non-invasive weaning intervention is cost-effective falls just below 60% (see Table 25).
FIGURE 17.
Cost-effectiveness plane for base case: fixed utility baseline, imputed costs, adjustment for covariates.
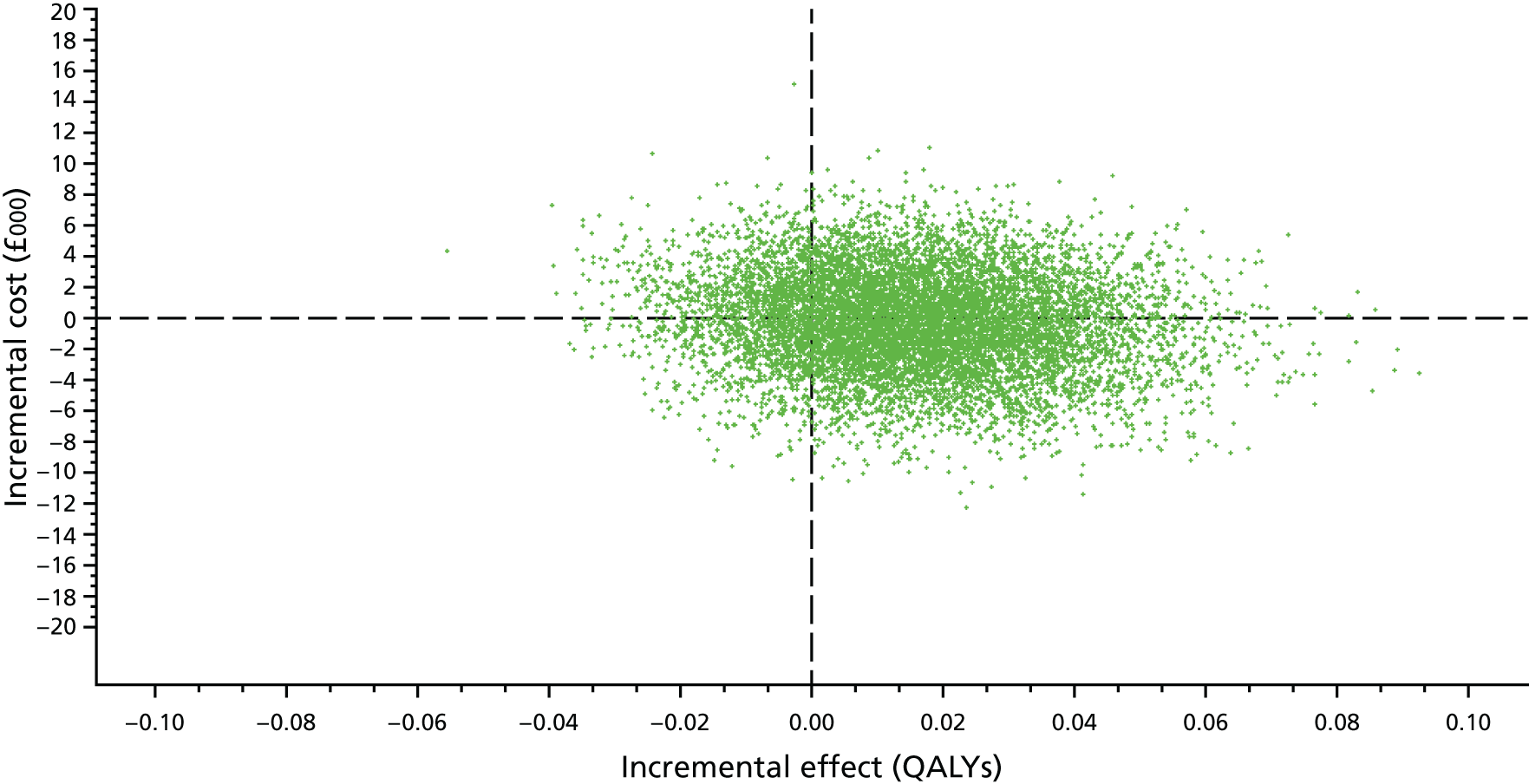
FIGURE 18.
Cost-effectiveness acceptability curves for base case: fixed utility, imputed costs, adjustment for covariates.
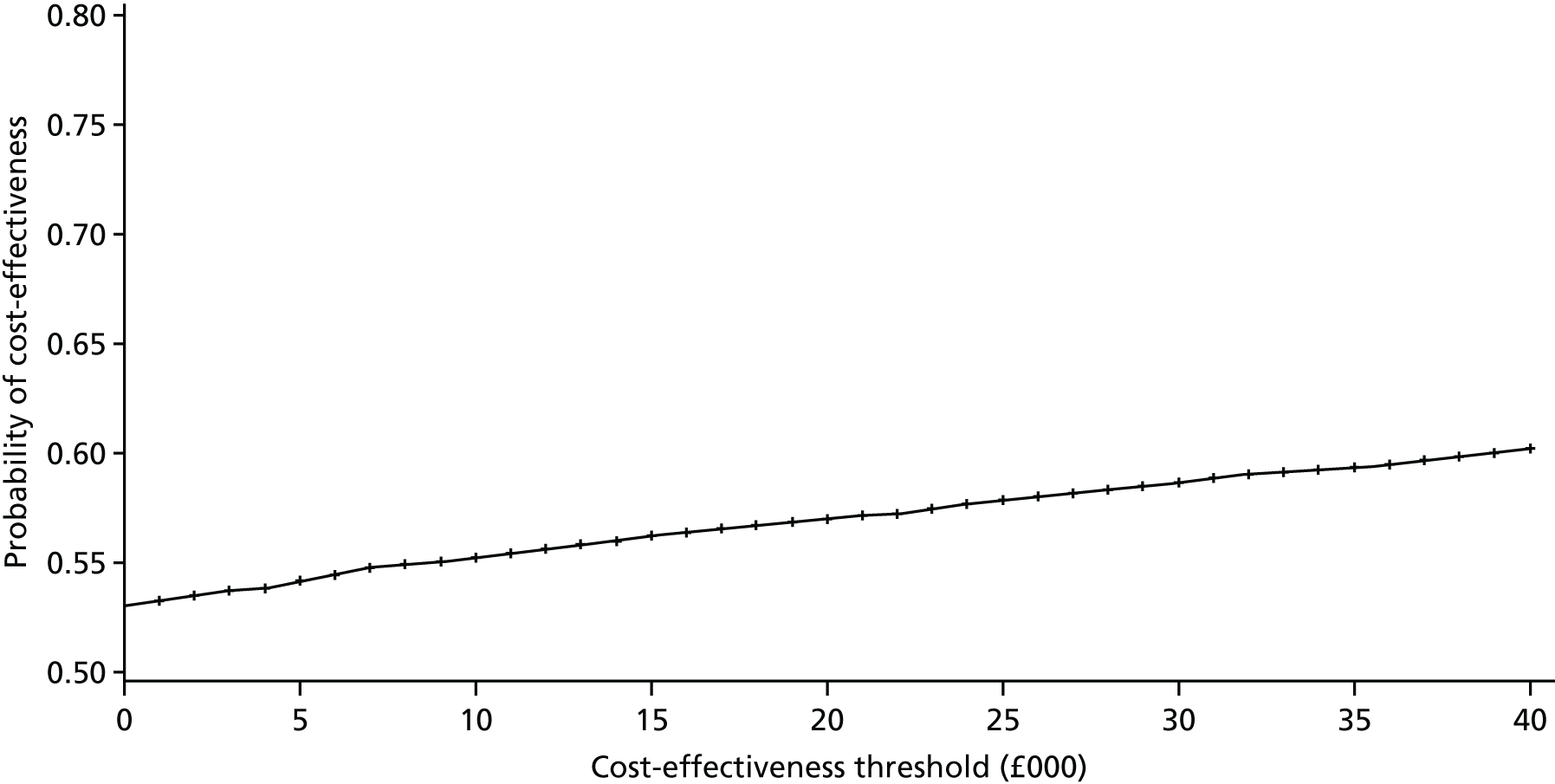
Sensitivity analyses
Several sensitivity analyses were undertaken to assess the impact of uncertainty surrounding key parameters or methodological features on the cost-effectiveness results. The probability that the non-invasive weaning intervention is cost-effective remained relatively static (at about 60%) for the majority of the sensitivity analyses (i.e. complete cases, societal costs, SF-6D-converted QALYs and using the recalled pre-randomisation EQ-5D-3L utility score as an additional covariate). All sensitivity analyses show that the average INMB is always > £0. However, because of high variability, the (bootstrap) 95% CIs cross zero and a conclusion of no difference between non-invasive weaning and invasive weaning in terms of the mean INMB cannot be completely (statistically) ruled out (see Table 26 and Figures 19–21).
FIGURE 19.
Sensitivity analyses and subgroup results (cost-effectiveness threshold of £15,000/QALY).
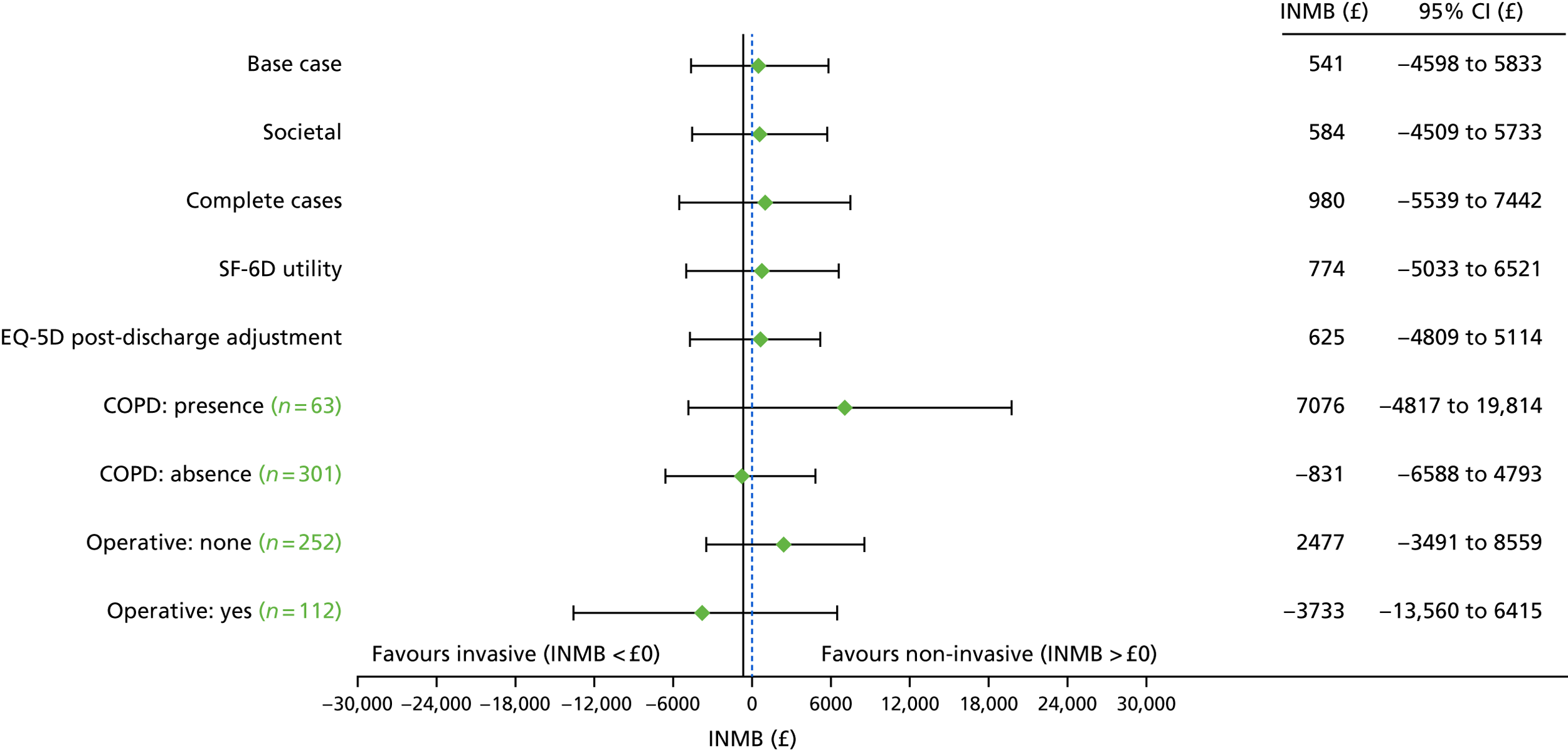
FIGURE 20.
Sensitivity analyses and subgroup results (cost-effectiveness threshold of £20,000/QALY).
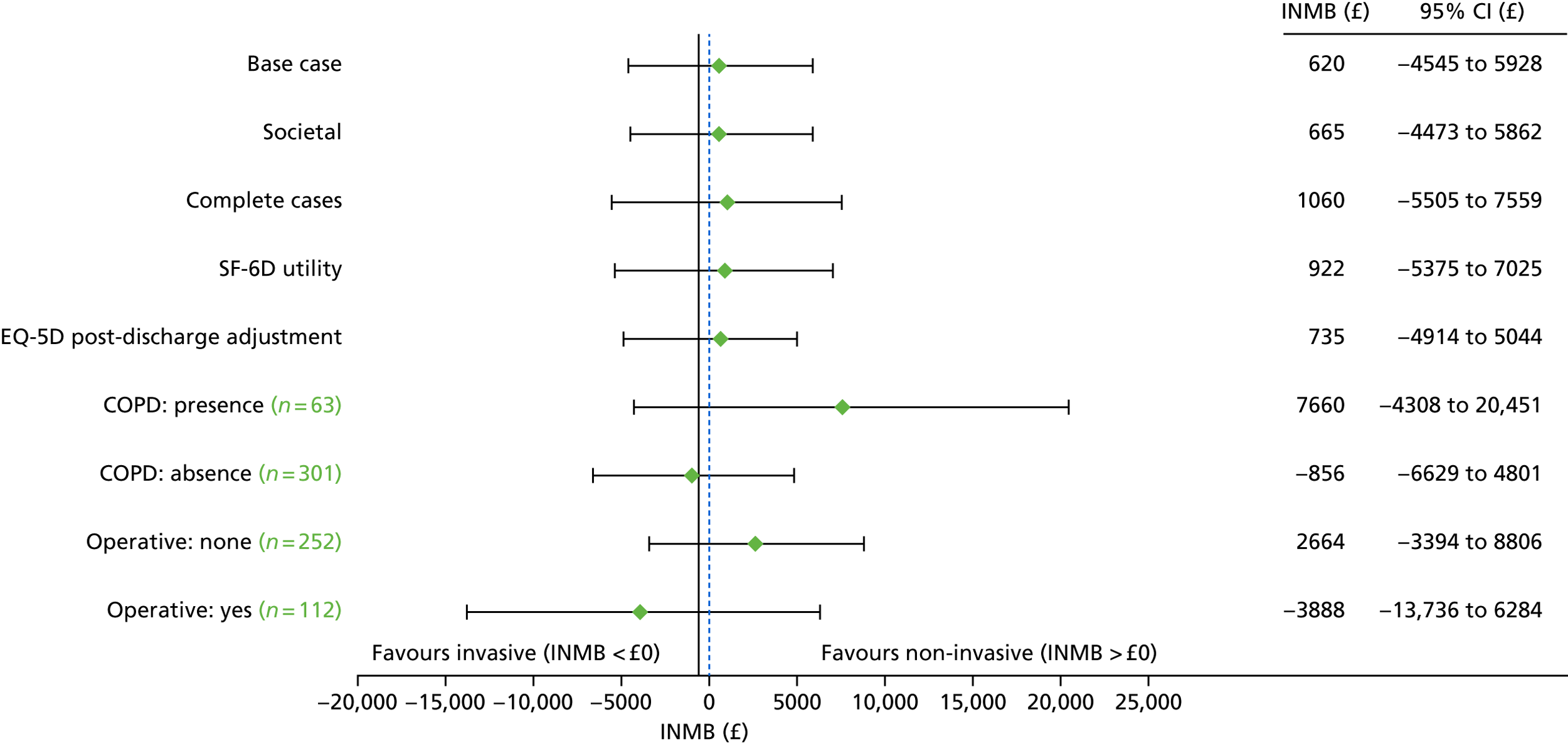
FIGURE 21.
Sensitivity analyses and subgroup results (cost-effectiveness threshold of £30,000/QALY).
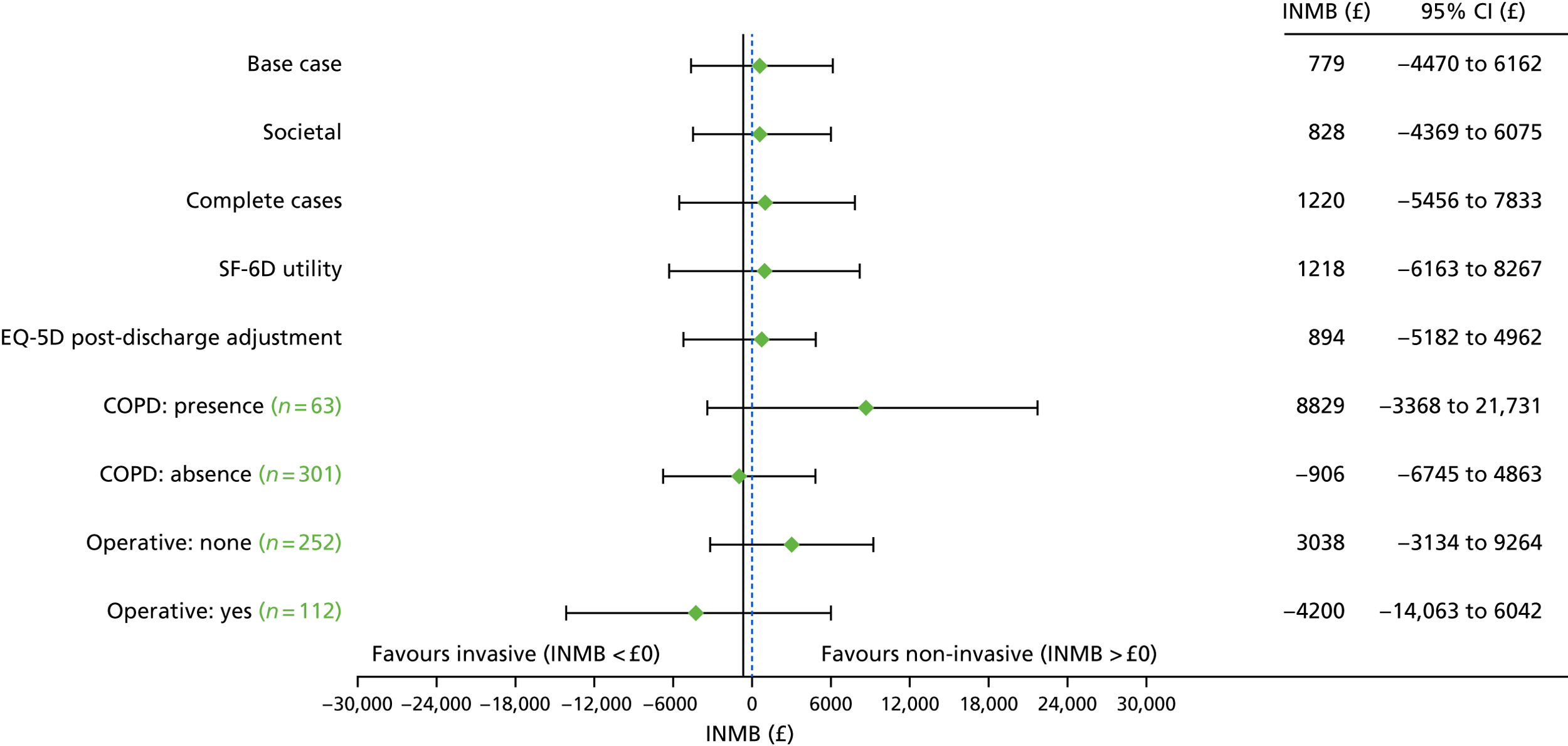
Subgroup analyses
Four subgroups were subjected to cost-effectiveness analyses to explore the heterogeneity in cost-effectiveness results: COPD (presence/absence) and operative status (operative/non-operative). All subgroup analyses were based on the patient-reported EQ-5D-3L and MI and covariate adjustments. COPD presence had a notable impact on the INMB values, with mean INMB values ranging between £7076 and £8829, depending on the value of the cost-effectiveness threshold. The probability of cost-effectiveness increased to a range of 82–87% depending on the value of the cost-effectiveness threshold in participants with COPD. Conversely, for participants with an absence of COPD, non-invasive weaning was less cost-effective than invasive weaning (see Figures 19 and 21 and Table 26), resulting in non-invasive weaning being dominated by invasive weaning in health economic terms (as it was, on average, more costly and less effective). Similarly, for operative status, non-invasive weaning was dominated by invasive weaning and was therefore less cost-effective for this subgroup: non-invasive weaning was more costly and less effective, on average, and the mean INMB was negative, ranging between –£3733 and –£4200, with probabilities of cost-effectiveness ranging between 25% and 27%, depending on the value of the cost-effectiveness threshold.
Results of the longer-term cost-effectiveness model
Survival rates
After fitting a Royston–Parmar49 three-knot model (the best model based on lowest Akaike information criterion) using published SAS macros,50 using a 5-year time horizon yielded a mean survival time of about 41.9 months versus 33.3 months for NIV versus IMV, respectively. The mean observed survival for NIV versus IMV was 4.12 months versus 4.07 months (Table 27) based on the observed Kaplan–Meier estimates from the within-trial data. By 5 years (60 months) post randomisation, 67% of participants were expected to remain alive in the NIV group, compared with 45% in the IMV group [see Figure 16b and Table 33 (see Appendix 3)]. These survival rates are lower than the national average, as reported in mortality statistics of the general population with a similar age distribution to this population. This would be 95% at 5 years from a mean age of 63 years,51 which, logically, are higher than the rates observed here as the participants in the study are patients from an ICU.
| Outcome | Trial group | Difference (NIV vs. IMV) | |
|---|---|---|---|
| NIV | IMV | ||
| Survival | |||
| Time horizon (months) | 60 | 60 | – |
| Mean observed survival (Kaplan–Meier) (months) | 4.12 | 4.07 | 0.05 |
| Royston–Parmar three-knot model, extrapolated survivala (mean) (months) | 41.9 | 33.3 | 8.6 |
| Utilities | |||
| Constant (mean)a | 0.444 | 0.361 | 0.083 (more effective) |
| QALYs | |||
| QALY (discounted)b | 2.247 | 1.824 | 0.427 (more effective) |
| Costs | |||
| NHS/PSS during trialc | £31,711 | £32,476 | £765 (less costly) |
| > 6 months to 5 yearsd | £12,048 | £9311 | £2737 (more costly) |
| Total over 5 years, including ICU | £43,759 | £41,787 | £1972 (more costly) |
| ICER | – | – | £4618 |
| INMB | |||
| Cost-effectiveness threshold of £20,000 | – | – | £6568 |
| Cost-effectiveness threshold of £30,000 | – | – | £10,838 |
Both plots (Figure 22) of the log-survival (a) and the hazard (b) functions suggest that NIV has a trend of decreasing hazards (lower risk of death over time) whereas IMV appears to have an increasing hazard trend (higher risk of death over time). This is mainly as a result of the spike in hazards after month 6 because of a later death event in the IMV group. Both hazard functions suggest that risk of death is decreasing in a similar trend until around 4 months post intervention, albeit with a more erratic hazard for the IMV group. After about 4 months, patients may die at a slightly faster rate (ignoring the spike) in the IMV group. Owing to a lack of further follow-up data, future death patterns may or may not be similar, although the curve trajectory for NIV suggests that the hazard curve may lie slightly above that of the IMV group. These plots partially support the extrapolated survival curves, suggesting that NIV patient death rates occur more slowly than death rates in the IMV group, but that survival rates over time may converge. On the basis of these results, an assumption of future costs and health utilities being similar between groups is not unreasonable.
FIGURE 22.
(a) Log-survival function; and (b) hazard function. bw, bandwidth.
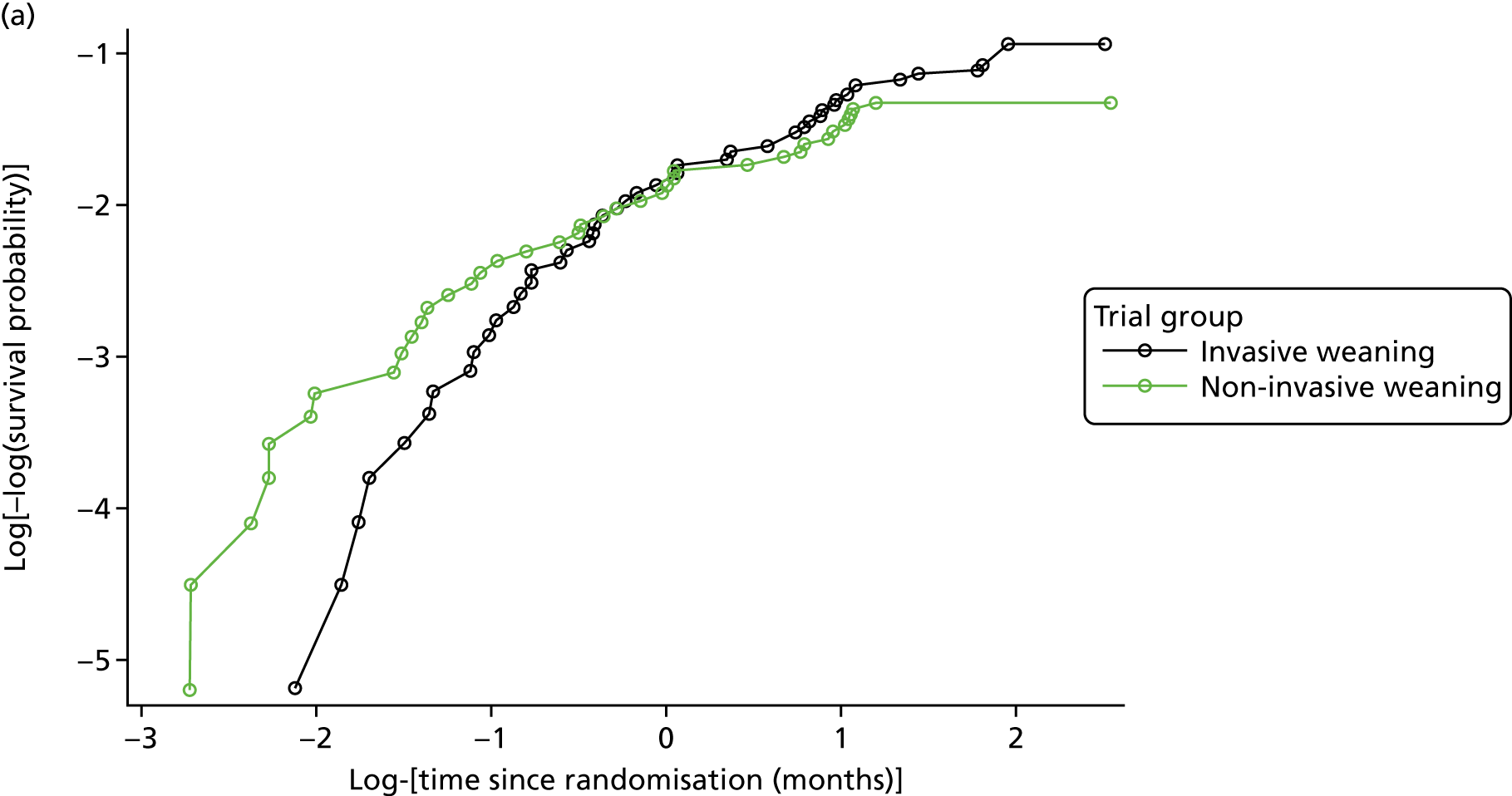
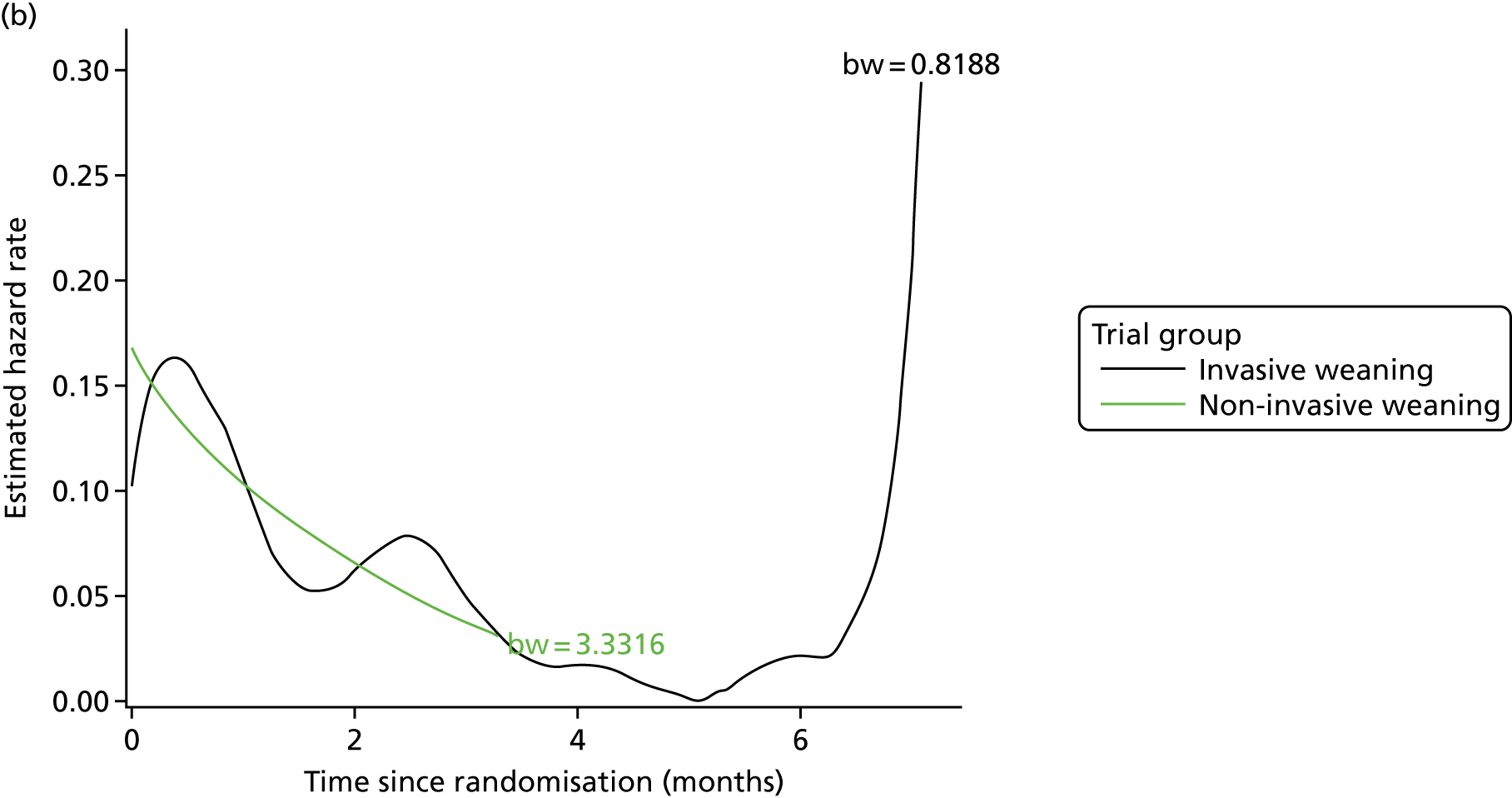
Health utilities and quality-adjusted life-years
The mean health utility scores, calculated as means across all observed and predicted estimates between randomisation and 5 years, were estimated to be 0.444 versus 0.361 (NIV vs. IMV) assuming that utilities remained constant beyond 6 months (base case for long-term cost-effectiveness). The mean discounted expected QALYs over 5 years were 2.25 for NIV versus 1.82 for NIV (see Table 27), assuming constant health utilities beyond 6 months, resulting in an incremental QALY gain of 0.427. The QALY difference appears to be driven by higher expected survival rates over time.
Costs
Using observed 3–6 months post randomisation total costs, adjusted for covariates (e.g. age, centre, COPD status), as a basis for estimating long-term costs, the total expected (discounted) costs up to 5 years post randomisation were £12,048 for NIV compared with £9311 for IMV after assuming that long-term (beyond 6 months) resource use is equal between groups.
Incremental cost-effectiveness (base case, long-term extrapolation)
The reported NHS and PSS costs over the first 6 months were £31,711 versus £32,476 for the NIV versus IMV groups during the trial (see Table 24). The total expected (mean) NHS and PSS costs over 5 years were £43,759 versus £41,787 with an incremental cost of £1972 for NIV. The 5-year ICER was therefore £4618 (see Table 27), assuming future costs and health utilities are the same in survivors in both groups. At a cost-effectiveness threshold of £30,000 per QALY, NIV was strongly cost-effective (see Table 27 and Figure 23). This is largely explained by a higher mean QALY estimate as a result of a higher survival rate in the NIV group than in the IMV group. However, the probability of cost-effectiveness of NIV is highly dependent on the assumptions about future costs and benefits beyond 6 months (see Table 27). Additional information might therefore be needed to reduce this decision uncertainty.
FIGURE 23.
Cost-effectiveness acceptability curve and cost-effectiveness plane for long-term effectiveness (base-case long-term cost-effectiveness assuming equal future costs and effects). (a) CEAC showing the probability of cost-effectiveness of NIV at varying cost-effectiveness thresholds, assuming that future costs and effects are equal between the NIV and IMV groups; and (b) cost-effectiveness plane showing the joint distribution of projected 5-year differences in costs and QALYs between the NIV and IMV groups, assuming that costs beyond the 6-month trial period and effects are equal between groups.
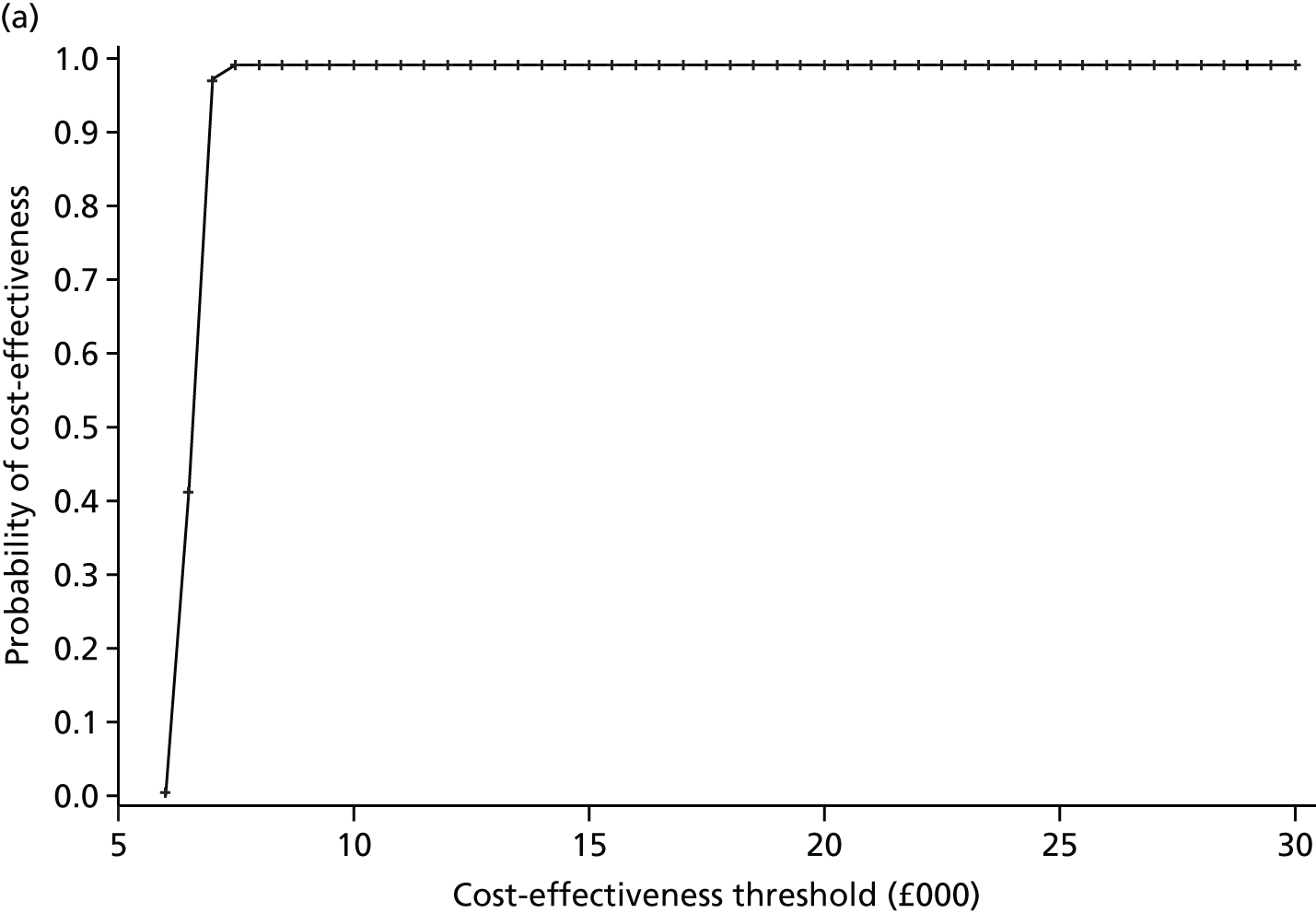
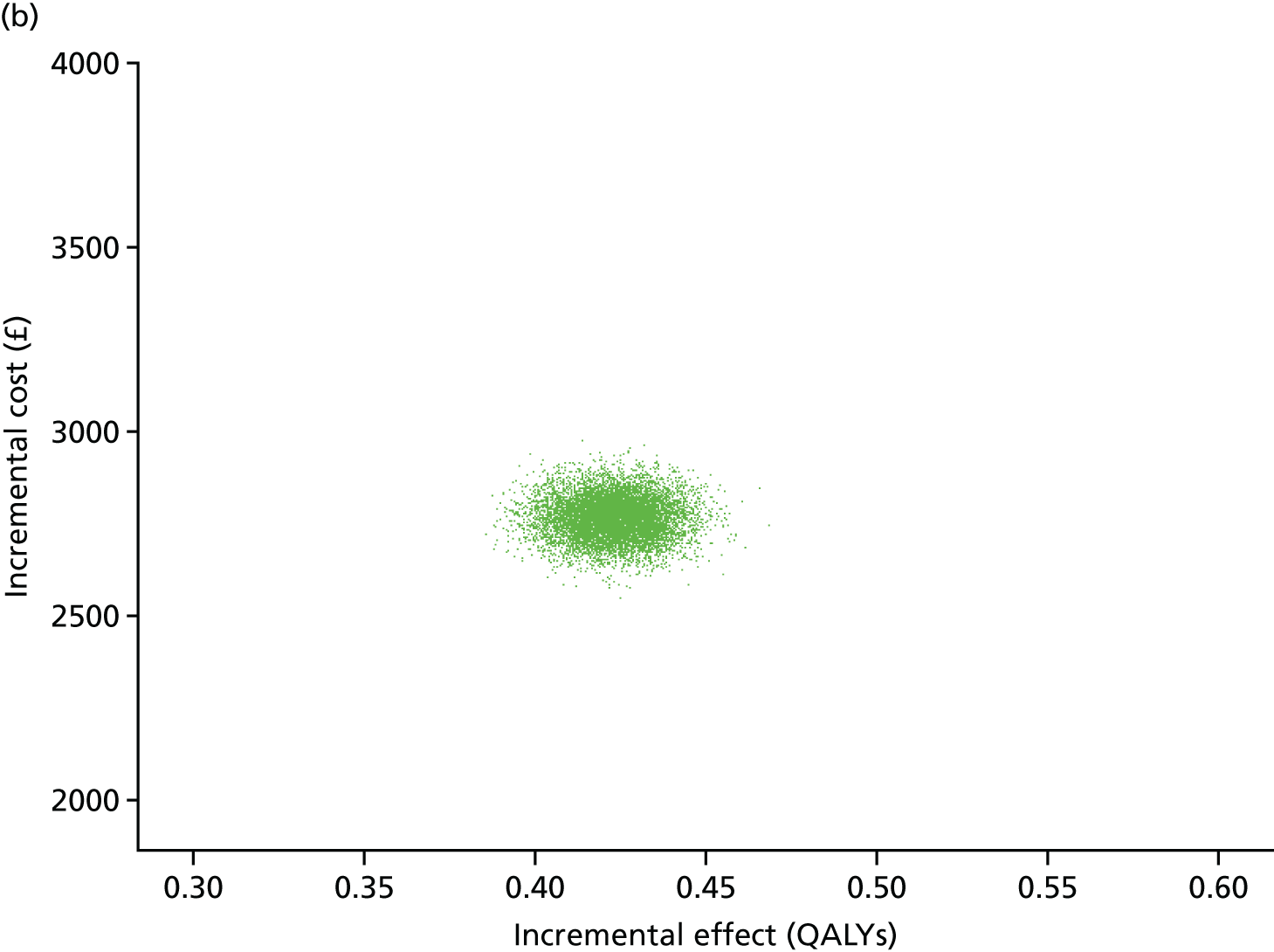
Sensitivity analyses
In the first sensitivity analyses, survival rates in the NIV group were reduced by 10% and it was assumed that costs and utilities were equal after 6 months (see Table 38; Appendix 3). This led to an incremental cost associated with NIV of £237 and a reduced incremental QALY of 0.028, with a consequent ICER of £8393 and a probability of cost-effectiveness of about 0.96 at a cost-effectiveness threshold of £30,000 per QALY (see Table 38 in Appendix 3, and Figure 24).
FIGURE 24.
Cost-effectiveness acceptability curve and cost-effectiveness plane for long-term effectiveness (sensitivity analyses assuming that survival in the NIV group is lower by 10%). (a) Cost-effectiveness plane showing the joint distribution of projected 5-year differences in costs and QALYs between the NIV and IMV groups, assuming that survival in the NIV group is lower by 10%; and (b) CEAC showing the probability of cost-effectiveness of NIV at varying cost-effectiveness thresholds, assuming that survival in non-invasive group is lower by 10%.
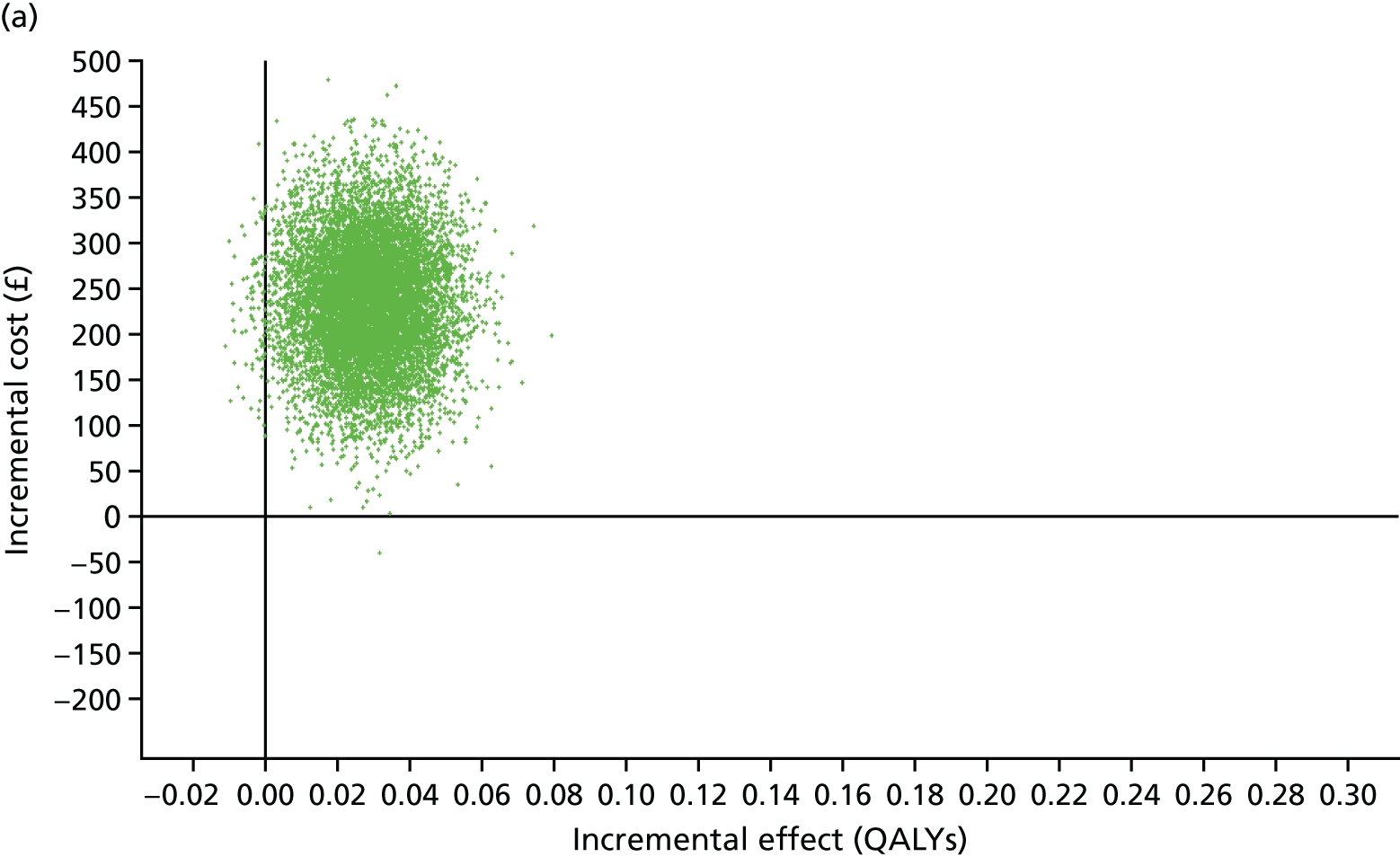
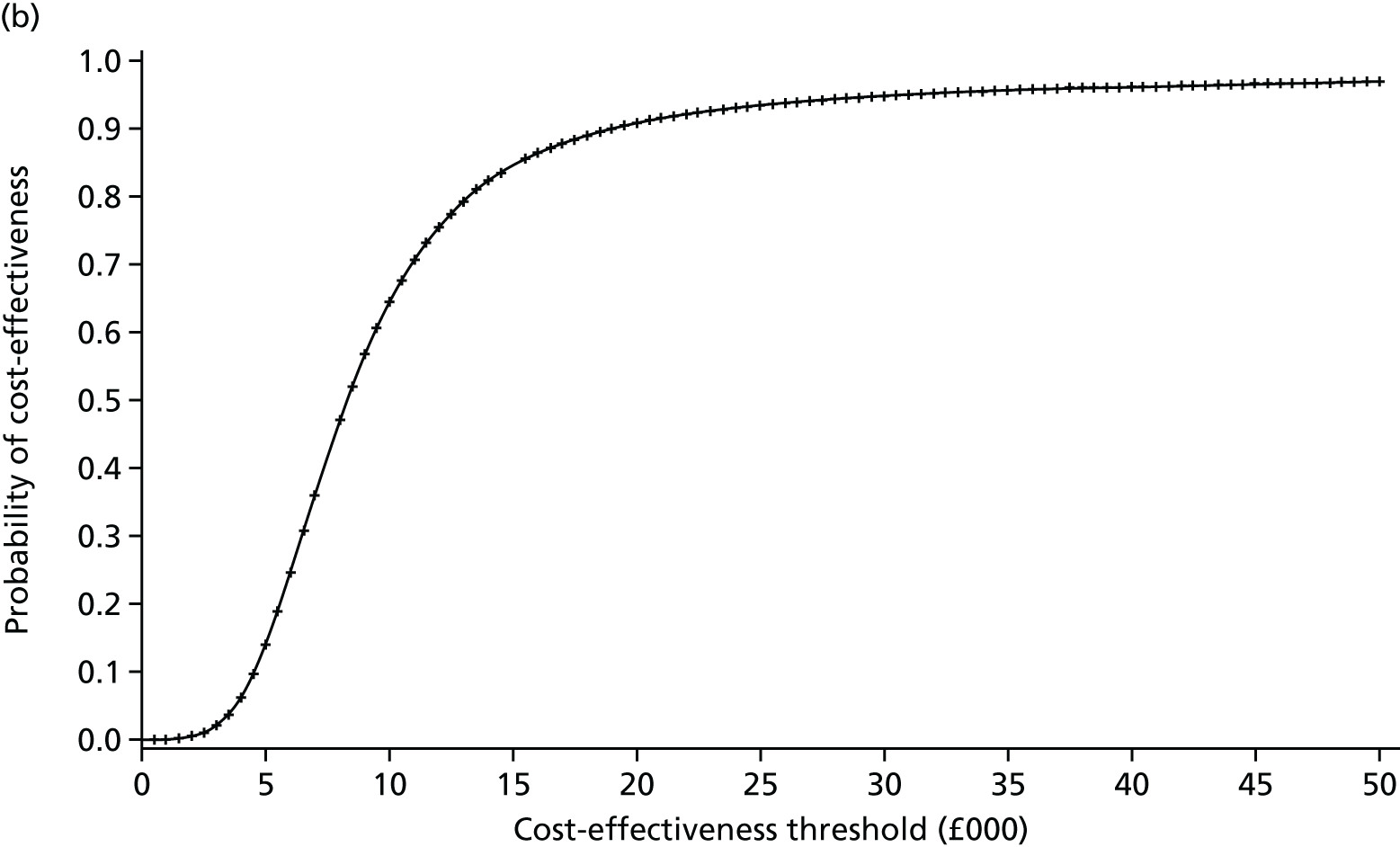
If costs and health utilities for survivors are assumed to remain constant as observed in the trial (i.e. carrying forward costs and health utility values as observed at 6 months post randomisation in their groups), NIV is no longer cost-effective (mean ICER of £349,000 per additional QALY at a cost-effectiveness threshold of £30,000) (see Table 38 in Appendix 3).
If, after 6 months, costs are assumed to be equal, but expected health utilities are carried forward (i.e. differ over time), the incremental cost associated with NIV falls to £2737 and the incremental QALY falls to 0.019, resulting in an ICER of £144,052 and a probability of cost-effectiveness of 2% (at a £30,000 cost-effectiveness threshold).
Chapter 6 Discussion
Summary of main results
The Breathe pragmatic RCT compared two protocolised weaning strategies – one that promoted early extubation to NIV and one that involved sequential reduction in Psupp with daily SBTs prior to extubation. Early extubation to NIV had no effect on the primary outcome (liberation from ventilation) or mortality, despite a higher rate of reintubation in the NIV group than in the IMV group. The study showed clinically beneficial effects for NIV as regards reduced sedation requirements and a lower proportion of patients requiring respiratory antibiotics.
Interpretation
Despite no evidence of the effectiveness of early extubation to NIV on liberation from ventilation, these findings present useful information about the role for NIV as an intermediate step in the weaning process. Furthermore, adopting the approach outlined by Pocock and Stone,52 a finding of no effect on the primary outcome should not be labelled as a ‘negative trial’. First, the primary outcome, time from liberation from all forms of ventilation, was defined, in accordance with international guidelines, as being freedom from any form of ventilation for 48 hours after extubation. The Breathe trial found a mean difference of 2.0 days total ventilation time in favour of NIV (95% CI –4.61 to 0.69 days). The upper boundary of the CI crosses the line of no effect but the lower boundary falls below the a priori-defined minimal clinically important difference of 1 day. Owing to a lower than anticipated dropout rate (25% predicted, 17% observed), a post hoc power calculation reveals that the study had 93% power to detect the a priori-defined minimal clinically important difference of 1 day in total ventilation days. The primary outcome represents an overall measure of the effectiveness of weaning but does not account for the benefits of transitioning from IMV to NIV. This suggests that the use of NIV as a weaning strategy shows some indication of potential benefit. Second, early liberation from IMV reduces exposure to the risk of ventilator-associated lung injury and VAP. Participants in the NIV group had 3 days fewer IMV (–3.1 days, 95% CI –5.75 to –0.51 days) than those in the IMV group. This conferred additional beneficial effects including less sedation, a lower proportion of participants requiring antibiotics for presumed respiratory infection and fewer days in intensive care.
The findings from the Breathe trial add new insights to evidence from a Cochrane systematic review and meta-analysis that comprised 16 RCTs and 994 participants. 20 Unlike the Breathe trial, these were mostly small (range of 20 to 264 participants), single-centre trials and included a high proportion of participants with COPD (nine of the trials enrolled exclusively COPD participants and two enrolled predominantly COPD participants). The Breathe trial differed from these previous trials as only a small proportion (3.5%) of enrolled participants had COPD as the main indication for respiratory failure. This may reflect current UK practice, with NIV being used as a tool to prevent intubation for COPD patients. Unlike the Breathe trial, the Cochrane systematic review found strong evidence that weaning using NIV reduced mortality (RR 0.52, 95% CI 0.36 to 0.80), although moderate heterogeneity was noted. 20 Subgroup analysis showed that the beneficial effects were limited to trials that exclusively enrolled participants with COPD as the main cause of acute respiratory failure (RR 0.36, 95% CI 0.24 to 0.56). This finding is consistent with the evidence in which NIV is used to supplement usual medical care during COPD exacerbation. 53 From a pathophysiological perspective, NIV reduces respiratory muscle fatigue and tachypnoea, augments tidal volume and reduces intrinsic PEEP in patients with COPD. With the increasing adoption of NIV as a tool to prevent the need for intubation, fewer COPD patients are admitted to ICU for IMV;6 thus, the population recruited to the Breathe trial probably better reflects contemporary ventilation practice. In addition, the Breathe trial reflects contemporary weaning practice by comparing NIV with a protocol using weaning of Psupp. Some of the studies included in the Cochrane report compared NIV with a weaning protocol using a mechanical ventilation mode that incorporates mandatory ventilations, which is considered a less effective weaning strategy.
The Breathe trial evaluated NIV in patients with difficult and prolonged weaning. The 2005 international consensus conference on weaning defined difficult weaning as a failure of the initial weaning attempt and a requirement for up to three SBTs or 7 days of weaning. 7 Prolonged weaning was defined as a situation in which patients require more than three SBTs or 7 days of weaning after the first SBT. 7 In the Breathe trial, the early visual separation of the Kaplan–Meier curves over the first 7–10 days after randomisation before they later come together suggests that NIV might be most beneficial in the difficult-weaning group. This has some biological rationale as the most common causes of difficult weaning (e.g. accumulation of sedative drugs, fluid overload, respiratory muscle weakness) may be resolved more quickly than the most common underlying causes of prolonged weaning (e.g. severe/chronic cardiovascular and respiratory failure, prolonged respiratory muscle weakness). 54 To test this hypothesis, future studies might seek to prospectively identify and evaluate NIV in patients predicted to face difficult rather than prolonged weaning.
Overall, the use of NIV was well tolerated. However, the proportion of participants who failed a trial of NIV and required reintubation was twice as high in the NIV group as in the IMV group (37.4% vs. 23.1%). ICU mortality among those who required reintubation was higher (20%) than among those that did not require reintubation (6%). However, as the overall mortality rates were similar at ICU discharge (12.1% in the NIV group and 13.7% in the IMV group), death after reintubation may reflect the severity of the underlying illness as opposed to reintubation causing excess mortality per se. Other than this, the rate and patterns of AEs and SAEs were similar between groups.
Strengths and limitations
The Breathe trial benefited from several design advantages compared with previous studies on IMV and NIV. First, the use of a protocolised weaning regime in both trial groups allowed the separation of the treatment intervention from the effect of protocolised weaning. 55 The inclusion of optimised best practice guidelines (i.e. ventilation bundle, daily SBTs, tracheostomy) should reduce heterogeneity between treatment groups. Second, antibiotic use was selected as a surrogate for VAP to limit the risk of detection bias arising from different approaches to obtaining respiratory samples for culture. The trial also has important limitations. By the nature of the intervention, it was not possible to blind clinicians, participants or outcome assessors. This may have led to performance and/or detection bias. Nearly half of the participants were recruited from three large centres, which might limit generalisability. However, a sensitivity analysis found no evidence of a difference in outcomes between these three centres and the other 39 participating centres. A prerequisite for centres to participate in the Breathe trial was that they were experienced in the use of NIV. Nevertheless, it is possible that performance and outcomes may have improved as centres became more experienced in the use of the NIV weaning intervention.
Health economic evaluation
This trial-based economic evaluation revealed that the NIV protocol has some potential to be cost-effective, compared with IMV, particularly for the patients who present with COPD or do not require surgery (operative status). NIV is unlikely to be cost-effective compared with IMV for patients admitted to the ICU who do not have COPD or who require surgery. The INMB estimate was positive for the NIV protocol, a finding that remained generally robust to several sensitivity and subgroup analyses. The main reason for cost-effectiveness appears to stem from lower costs associated with the experimental group and improved effectiveness based on EQ-5D-3L-derived QALYs. However, from a NHS and PSS perspective, some costs were higher, on average, for the NIV intervention group (although not always statistically significant) than for the IMV group.
A strength of the Breathe trial was that it was prospectively designed for a cost-effectiveness analysis using individual-level data. Costs and outcomes were carefully considered in the design of this trial with the purpose of reaching a robust conclusion with respect to cost-effectiveness in a large sample of individuals. There were, however, several limitations to this cost-effectiveness analysis.
First, organ support costs after the initial 30 days were collected in such a way such that it was impossible to distinguish between the costs for multiple organs or zero or just one organ. For example, when 5 additional days were required for organ support beyond the first 30 days post randomisation, it was impossible to disentangle whether the organ support costs for these 5 days were due to the cost of supporting several organs for, for instance, the first 3 days and fewer organs for the last 2 days, or vice versa.
Second, in terms of QALYs, a baseline utility score of –0.402 was assumed, the value assigned by the EQ-5D-3L tariff to an unconscious health state, and this was considered to be the same for each participant, regardless of underlying heterogeneity in health states. This may be an unrealistic assumption, but it is in keeping with broader methodological practice for trial-based economic evaluations conducted in emergency and critical care settings. 56 Moreover, we have recently demonstrated that assuming an alternative fixed baseline utility score, for example a utility score of zero representing death, would have no effect on the area under the curve within the incremental QALY calculations. 56 Furthermore, the utility assessments performed around the point of hospital discharge required participants to reflect on their health state of at least 1 month earlier, and may, therefore, suffer from some recall bias. Consequently, the QALY values generated for the purposes of the baseline cost-effectiveness analysis were based on one fixed baseline time point and utility value. The assumption of linearity of HRQoL between data collection points is open to scrutiny and more uncertain when missing data are present.
Third, the proportion of missing data and how missingness is handled is critical as this can affect the conclusions of a cost-effectiveness analysis. In this trial, approximately 35% of QALY data and between 6% and 40% of costs (at the component level) were missing by 6 months. Had the base-case cost-effectiveness analysis considered only those individuals with complete QALY and cost data, approximately 50% of participants (with possibly informative data) would have been removed from the analysis. This approach would have probably biased the results. After investigating that the data were likely to be MAR, it seemed sensible to use MI to ‘replace’ missing values in order to allow a complete-case analysis using the whole data set data.
Fourth, despite the longitudinal nature of the study, resource use was retrospectively recalled by trial participants, which is also likely to result in recall bias.
Fifth, similar pilot or Phase II trials may have been useful in identifying the critical costs that drive cost-effectiveness. Instead, a broad set of NHS and PSS costs and broader societal costs were collected, some of which had little impact on the ICER values. Some costs items did not occur (e.g. see Table 35 in Appendix 3) and a reduced form of such a type of data collection in this setting may be advisable with a focus on the largest and most relevant costs.
Finally, the potential for a more rigorous longer-term cost-effectiveness analysis may be needed because it appears that the effectiveness of NIV appears to deteriorate over time, reflected in shorter projected survival. The impact of non-linear extrapolation functions should be considered to assess the sensitivity of long-term cost-effectiveness. 57 This is because utility after hospital discharge may increase because of the use of additional therapies (including palliation) before declining (and may increase the QALY). A longer follow-up would have helped to reduce the uncertainty of extrapolated patient survival and patient utility. In conclusion, the data collected in the Breathe trial support a hypothesis that a NIV protocol intervention compared with an IMV protocol in this population of patients is likely to be cost-effective in the short term, particularly for some subgroups. However, initial longer-term cost-effectiveness modelling suggests that the benefits of NIV may not be cost-effective.
Patient and public involvement
In the formative stages of the trial, the Intensive Care Society’s Patients and Relatives Committee helped to inform the design of the study. Guidance was provided about selecting and defining the primary and secondary end points of the trial. The group assisted us to identify the optimal timing and way to approach patients and relatives for consent, both in the early phases of the trial and during follow-up. Through ongoing involvement in the TSC, two patient and public representatives (a patient and a relative of a patient) maintained oversight of the conduct of the trial and assisted with the interpretation of the trial findings. Insights from these members highlighted the physical discomfort of a tracheal tube during weaning, an aspect of patient experience that was not captured in the design of the trial. A systematic review of qualitative studies identified physical discomfort from the endotracheal tube, suctioning and inability to communicate as common adverse patient experiences. 58 It is possible that earlier liberation from IMV may have improved patient experience; although this was not formally measured in this trial, it is a relevant outcome to consider in future studies.
Chapter 7 Conclusion
The Breathe trial, a pragmatic RCT, compared two protocolised weaning strategies in patients who failed a SBT. One strategy promoted early extubation to NIV and one involved sequential reduction in Psupp with daily SBTs prior to extubation. Early extubation to NIV did not reduce the overall time to liberation from ventilation. However, participants in the early extubation/NIV group spent less time receiving IMV and required less sedation and fewer respiratory antibiotics than those in the IMV group. Although the early extubation/NIV group had a higher rate of reintubation than the IMV group, there was no overall difference in AEs, SAEs or mortality. The mean incremental cost-effectiveness of the NIV intervention was estimated at £19,006 per QALY, that is, on average, the intervention was associated with a lower net cost and a higher net effect and was dominant in health economic terms. A strategy of early extubation to NIV seems to be a reasonable alternative to continuing invasive weaning procedures.
Recommendations for research
In patients who fail a SBT, which factors predict an adverse outcome (reintubation, tracheostomy, death) if extubated and weaned using NIV?
Acknowledgements
Addenbrooke’s Hospital – John Farman ICU
-
Dr Adrian James Varley (Local PI).
-
Ms Charlotte Bone (formerly Research Nurse and Staff Nurse).
-
Ms Petra Polgarova (lead Research Nurse).
-
Ms Amy Scullion (Research Nurse and Junior Sister).
-
Dr Charlotte Summers (Consultant).
-
Ms Katarzyna Zamoscik (Research and Staff Nurse).
Basildon and Thurrock Hospitals NHS Foundation Trust
-
Dr Agilan Kaliappan (Local PI).
-
Major Mark Vertue (Research Nurse).
Bedford Hospital
-
Ms Sarah Snape (Local PI).
-
Ms Bindumal Jophy (Sister).
-
Ms Aoife McKnight (Matron).
-
Ms Catherine O’Brien (Practice Development Nurse).
Birmingham Heartlands Hospital
-
Professor Fang Gao-Smith (Local PI).
-
Ms Joanne Gresty (Critical Care Research Nurse).
-
Ms Teresa Melody (Critical Care Research Manager).
-
Mr Peter J Sutton (Critical Care Research Nurse).
Blackpool Teaching Hospitals NHS Foundation Trust
-
Dr Jason Cupitt (Local PI).
-
Dr Robert Thompson (Local PI).
-
Ms Melanie Caswell (Research Nurse).
-
Ms Emma Stoddard (Lead Research Nurse).
Bradford Royal Infirmary
-
Dr Stephen Fletcher (Local PI).
-
Dr Tom Lawton (Local PI).
-
Mr Martin Northey (Research Nurse).
Bristol Royal Infirmary
-
Dr Tim Gould (Local PI).
-
Dr Sanjoy Shah (Local PI).
Broomfield Hospital
Dr Dilshan Arawwawala (Local PI).
Royal Infirmary of Edinburgh
-
Dr Michael A. Gillies (Local PI).
-
Ms Heidi Dawson (Research Nurse).
-
Ms Jane Whitehorn (Research Nurse).
-
Ms Gosha Wojcik (Research Nurse).
Forth Valley Royal Hospital
Dr Jonathan Richards (Local PI).
Freeman Hospital – Newcastle upon Tyne
-
Sr Claire Randell (Local PI, Senior Sister).
-
Ms Verity Calder (Research Nurse).
George Eliot Hospital
-
Dr Sam George (Local PI).
-
Dr Vivek Poongavanam (Local PI).
Glenfield Hospital
-
Rakesh Vaja (Local PI).
-
Ms Dawn Hales (Research Nurse).
-
Dr Gary Lau (Consultant).
-
Ms Natalie Rich (Research Nurse).
The Hillingdon Hospitals NHS Foundation Trust
-
Dr Elisa Kam (Local PI).
-
Mr Sohan Bissoonauth (Staff Nurse).
-
Ms Lourdes Opimo (Senior Sister).
Hull Royal Infirmary
-
Dr Ian Smith (Local PI).
-
Ms Caroline Abernethy (Research Nurse).
-
Ms Victoria Martinson (Research Nurse).
-
Mr Neil Smith (Senior Research Nurse).
Intensive Care Foundation
Dr Tim Gould (Chairperson of the Intensive Care Foundation).
James Paget University Hospitals NHS Foundation Trust
-
Dr Andreas Brodbeck (Local PI).
-
Ms Lynn Everett (Research Nurse).
John Radcliffe Hospital
Dr Duncan Young (Local PI).
St James’s University Hospital, Leeds
-
Dr Elankumaran Paramasivam (Local PI).
-
Sr Elizabeth Wilby (Clinical Research Nurse).
Leicester Royal Infirmary
-
Dr Neil Flint (Local PI).
-
Ms Prem Andreou (Research Nurse).
Leighton Hospital
-
Dr Daniel Saul (Local PI).
-
Mr Philip Chilton (Charge Nurse).
-
Dr Clare Hammell (Consultant).
-
Dr Alistair Martin (Consultant).
Manchester Royal Infirmary
-
Dr Mike Sharman (Local PI).
-
Ms Katie Ball (Clinical Research Nurse).
-
Mr Andrew Brown (Clinical Research Nurse).
-
Mr Richard Clark (Clinical Research Nurse).
-
Ms Elaine Coughlan (Clinical Research Nurse Manager).
-
Ms Rachel Pearson (Clinical Research Nurse).
-
Ms Sheeba Pradeep (Clinical Research Nurse).
Milton Keynes University Hospital
-
Dr Richard Stewart (Local PI).
-
Ms Jane Adderley (Research Nurse).
-
Ms Rebecca Hinch (Research Nurse).
-
Ms Cheryl Padilla Harris (Research Nurse).
-
Ms Sara Beth Sutherland (Research Nurse).
Musgrove Park Hospital
-
Dr Richard Innes (Local PI).
-
Ms Patricia Doble (Critical Care Research Nurse).
-
Ms Moira Tait (Critical Care Research Nurse).
New Cross Hospital
Dr Shameer Gopal (Local PI).
Royal Papworth Hospital NHS Foundation Trust
-
Dr Alain Vuylsteke (Local PI).
-
Ms Fiona Bottrill (Research Nurse).
-
Mr Antonio Rubino (Consultant).
Peterborough City Hospital
-
Dr Coralie Carle (Local PI).
-
Ms Theresa Croft (Deputy Sister Critical Care).
-
Mr David Hannen (Staff Nurse Critical Care).
-
Mr Alan Pope (Research Nurse Critical Care).
Poole Hospital
-
Dr Henrik Reschreiter (Local PI).
-
Ms Helena Barcraft-Barnes (Research Nurse Critical Care).
-
Ms Julie Camsooksai (Senior Research Nurse Critical Care).
-
Ms Sarah Jenkins (Staff Nurse Critical Care).
-
Ms Sarah Patch (Research Nurse Critical Care).
Queen Alexandra Hospital, Portsmouth
-
Dr Dave Pogson (Local PI).
-
Mr Steve Rose (Charge Nurse, Research Nurse).
Queen Elizabeth Hospital Birmingham
-
Dr Catherine Snelson (Local PI).
-
Ms Toni Brunning (Anaesthetic Registrar).
-
Mr Ronald Carrera (Critical Care Research Nurse).
-
Mr Philip Pemberton (Critical Care Research Fellow).
-
Mr Martin Pope (Critical Care Clinical Trial Assistant).
-
Mr Arlo Whitehouse (Critical Care Research Nurse).
Queen Elizabeth Hospital, King’s Lynn
-
Dr Darcy Pearson (Local PI).
-
Dr Parvez Moondi (Local PI).
Royal Blackburn Hospital
-
Dr Srikanth Chukkambotla (Local PI).
-
Ms Caroline Aherne (Research Nurse).
-
Mr Martin Bland (Research Nurse).
-
Ms Lynne Bullock (Research Nurse).
-
Ms Donna Harrison-Briggs (Lead Research Nurse).
Royal Gwent Hospital
-
Dr Nicholas Mason (Local PI).
-
Ms Una Gunter (Research Nurse).
Royal Liverpool and Broadgreen University Hospitals NHS Trust
-
Dr Ingeborg Welters (Local PI).
-
Dr Peter Hampshire (Local PI).
Royal Surrey County Hospital
Dr Ben Creagh-Brown (Local PI).
Royal Sussex County Hospital
-
Dr Owen Boyd (Local PI).
-
Laura Ortiz-Ruiz de Gordoa (Critical Care Research Nurse).
Royal Victoria Hospital, Belfast
-
Dr Bronagh Blackwood (Local PI).
-
Professor Daniel McAuley (Local PI).
-
Ms Leona Bannon (Clinical Research Nurse).
-
Ms Laura Creighton (Clinical Research Nurse).
-
Ms Pauline McElhill (Research Data Manager).
-
Ms Lia McNamee (Research Physicians Associate).
-
Ms Vanessa Quinn (Clinical Research Nurse).
-
Ms Grainne White (Research Data Manager).
Royal Victoria Infirmary, Newcastle upon Tyne
-
Dr Simon Baudouin (Local PI).
-
Ms Carmen Scott (Local PI).
-
Dr Ian Clement (Consultant).
Russells Hall Hospital
-
Dr Michael Reay (Local PI).
-
Ms Clare Allcock (Research Nurse).
-
Ms Sarah Fullwood (Research Nurse).
-
Ms Ranjit Gidda (Research Nurse).
Southend University Hospital
Dr David Higgins (Local PI).
St Thomas’ Hospital, London
-
Dr Luigi Camporota (Local PI).
-
Ms Kathryn Chan (Research Nurse).
-
Ms Kate Flynn (Research Nurse).
-
Ms Katie Lei (Research Nurse).
-
Ms Nicola Purchase (Research Nurse).
-
Mr John Smith (Research Nurse).
-
Ms Samantha Smith (Research Nurse).
University Hospitals Coventry and Warwickshire NHS Trust
-
Dr Christopher Bassford (Local PI).
-
Mr Jeff Ting (Research Nurse).
-
Ms Geraldine Ward (Senior Programme Research Nurse).
University Hospital Monklands
Dr Jim Ruddy (Local PI).
University Hospitals of North Midlands NHS Foundation Trust
Dr Stephan Krueper (Local PI).
University Hospital Southampton NHS Foundation Trust
-
Dr Rebecca Cusack (Local PI).
-
Ms Clare Bolger (Senior Research Nurse).
-
Ms Karen Salmon (Senior Research Nurse).
University Hospital of South Manchester NHS Foundation Trust
Dr Andrew Bentley (Local PI).
University Hospital of Wales
Dr Matthew Wise (Local PI).
Western General Hospital, Lothian
Dr Anthony Bateman (Local PI).
West Middlesex University Hospital
-
Dr Amandeep Gupta (Local PI).
-
Ms Barbara Walczynska (ITU Clinical Data Co-ordinator).
Wexham Park Hospital
Dr Tiina Tamm (Local PI).
Yeovil District Hospital
-
Ms Agnieszka Kubisz-Pudelko (Local PI).
-
Dr Nicholas Craw (Consultant).
-
Ms Sarah Craw (Research Nurse).
-
Dr Jeremy Reid (Consultant).
York Hospital
Dr Joseph Carter (Local PI).
Trial Steering Committee
-
Chairperson – Professor Steve Goodacre [Health Services Research. School of Health and Related Research (ScHARR)].
-
Dr Simon Finney (Royal Brompton Hospital).
-
Professor Simon Gates (Warwick Medical School, University of Warwick).
-
Dr Shelia Harvey (Global Health and Development. London School of Hygiene & Tropical Medicine).
-
Dr Ranjit Lall (Warwick Clinical Trials Unit, University of Warwick).
-
Professor Gary Mills (Sheffield Teaching Hospitals, Northern General Hospital).
-
Professor Gavin Perkins (Warwick Clinical Trials Unit, University of Warwick).
-
Ms Catherine Plowright (Medway NHS Foundation Trust).
-
Mr Duncan Wells (c/o The Intensive Care Society).
-
Mr Barry Williams (c/o The Intensive Care Society).
Data Monitoring and Ethics Committee
-
Chairperson – Professor Charles Hinds (William Harvey Research Institute, Barts and The London, Queen Mary’s School of Medicine and Dentistry).
-
Dr David Harrison [Intensive Care National Audit and Research Centre (ICNARC), Napier House].
-
Dr Mark Griffiths (Royal Brompton Hospital).
-
Dr Ranjit Lall (Warwick Clinical Trials Unit, University of Warwick).
Sponsor representatives
-
Lead Sponsor – Ms Liz Adey [Heart of England Foundation Trust (HEFT)].
-
Research Manager – Ms Sarah Pountain [Heart of England Foundation Trust (HEFT)].
-
Co-Sponsor – Ms Jane Prewitt (Warwick Medical School, University of Warwick).
Warwick Clinical Trials Unit
Data Entry Clerks
-
Ms Nicola Cashin.
-
Mr Adam de Paeztron.
-
Ms Sarah Rumble.
Recruitment Facilitators
-
Ms Laura Blair.
-
Ms Julia Sampson.
Trainee Trial Co-ordinators
-
Mr Adam de Paeztron.
-
Ms Claire Jacques.
-
Ms Karoline Munro.
-
Ms Jess Smith.
-
Ms Kimberley White.
Trial Co-ordinators
-
Mr Adam de Paeztron.
-
Ms Bev Hoddell.
Contributions of authors
Gavin D Perkins (Professor of Critical Care Medicine, University of Warwick) was the Chief Investigator of the Breathe trial. He led the team of co-applicants, researchers and trial support staff named below who conceived and designed the trial and contributed to data collection, analysis and interpretation of the trial findings. He led drafting this report and revised it critically for important intellectual content.
Dipesh Mistry (Statistician, University of Warwick) contributed to the design of the work, performed the statistical analyses reported, assisted with interpretation of data and drafted the work and revised it critically for important intellectual content.
Ranjit Lall (Lead Statistician, University of Warwick) was involved in the conception and design of the work, supervised the statistical analyses reported, assisted with interpretation of data, drafted the work and revised it critically for important intellectual content.
Fang Gao-Smith (Professor of Anaesthesia, Critical Care and Pain) was Principal Investigator at Heartlands Hospital, was involved in the design of the study, acquisition, analysis and interpretation of data and revised the work critically for important intellectual content.
Catherine Snelson (Consultant, Intensive Care) was Principal Investigator at University Hospital Birmingham and was involved in the acquisition, analysis and interpretation of data and revised the work critically for important intellectual content.
Nicholas Hart (Professor of Critical Care Medicine) was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data and revised the work critically for important intellectual content.
Luigi Camporota (Reader in Intensive Care Medicine) was Principal Investigator at St Thomas’ Hospital and was involved in the design of the study, acquisition, analysis and interpretation of data, and revised the work critically for important intellectual content.
James Varley (Consultant, Intensive Care) was Principal Investigator at Addenbrooke’s Hospital, was involved in the acquisition, analysis and interpretation of data and revised the work critically for important intellectual content.
Coralie Carle (Consultant, Intensive Care) was Principal Investigator at Peterborough City Hospital and was involved in the acquisition, analysis and interpretation of data and revised the work critically for important intellectual content.
Elankumaran Paramasivam (Consultant, Intensive Care) was Principal Investigator at St James’s University Hospital, Leeds and was involved in the acquisition, analysis and interpretation of data and revised the work critically for important intellectual content.
Beverly Hoddell (Trial Manager, University of Warwick) co-ordinated the trial and was involved in the design of the study, acquisition of data and revised the work critically for important intellectual content.
Adam de Paeztron (Trial Co-ordinator, University of Warwick) co-ordinated the trial and was involved in the design of the study, acquisition of data, drafting the work and revising it critically for important intellectual content.
Sukhdeep Dosanjh (Senior Project Manager, University of Warwick) was involved in the design of the study, acquisition of data and revised the work critically for important intellectual content.
Julia Sampson (Recruitment Facilitator, University of Warwick) provided training and support to sites and was involved in the design of the study, acquisition of data and revised the work critically for important intellectual content.
Laura Blair (Recruitment Facilitator, University of Warwick) provided training and support to sites and was involved in the design of the study, acquisition of data and revised the work critically for important intellectual content.
Keith Couper (Research Nurse, Heartlands Hospital) was involved in the design of the study, acquisition of data and revised the work critically for important intellectual content.
Daniel McAuley (Professor of Critical Care Medicine, Queen’s University Belfast) was Principal Investigator at Royal Victoria Hospital, Belfast, and was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data and revised the work critically for important intellectual content.
J Duncan Young (Professor of Intensive Care Medicine, University of Oxford) was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data and revised the work critically for important intellectual content.
Tim Walsh (Professor of Intensive Care Medicine, University of Edinburgh) was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data and revised the work critically for important intellectual content.
Bronagh Blackwood (Professor, Queen’s University, Belfast) was Principal Investigator at Royal Victoria Hospital, Belfast, and was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data and revised the work critically for important intellectual content.
Louise Rose (Co-investigator) provided clinical expertise and support to the trial teams.
Sarah E Lamb (Professor, University of Oxford) was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data and revised the work critically for important intellectual content.
Melina Dritsaki (Health Economist, University of Warwick) contributed to the design of the economic research instruments and the collection of unit cost data.
Mandy Maredza (Health Economist, University of Warwick) contributed to the design of the economic research instruments and the collection of unit cost data.
Iftekhar Khan (Health Economist, University of Warwick) conducted the main body of the analyses of the health economic data.
Stavros Petrou (Professor of Health Economics, University of Warwick) provided oversight of all aspects of the economic evaluation including its design, conduct, analysis and reporting.
Simon Gates (Professor, University of Warwick) was involved in the conception and design of the work, contributed to the acquisition and interpretation of the data and revised the work critically for important intellectual content.
Publication
Perkins GD, Mistry D, Gates S, Gao F, Snelson C, Hart N, et al. Effect of protocolized weaning with early extubation to noninvasive ventilation vs. invasive weaning on time to liberation from mechanical ventilation among patients with respiratory failure: the Breathe randomized clinical trial. JAMA 2018;320:1881–8.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Lassen HC. A preliminary report on the 1952 epidemic of poliomyelitis in Copenhagen with special reference to the treatment of acute respiratory insufficiency. Lancet 1953;1:37-41. https://doi.org/10.1016/S0140-6736(53)92530-6.
- Young JD, Sykes MK. Assisted ventilation. 1. Artificial ventilation: history, equipment and techniques. Thorax 1990;45:753-8. https://doi.org/10.1136/thx.45.10.753.
- Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet 2010;376:1339-46. https://doi.org/10.1016/S0140-6736(10)60446-1.
- Patel K. Mechanically Ventilated Admissions. London: Intensive Care National Audit & Research Centre (ICNARC); 2012.
- Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 2002;287:345-55. https://doi.org/10.1001/jama.287.3.345.
- Esteban A, Frutos-Vivar F, Muriel A, Ferguson ND, Peñuelas O, Abraira V, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013;188:220-30. https://doi.org/10.1164/rccm.201212-2169OC.
- Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J 2007;29:1033-56. https://doi.org/10.1183/09031936.00010206.
- Frutos-Vivar F, Esteban A, Apezteguia C, González M, Arabi Y, Restrepo MI, et al. Outcome of reintubated patients after scheduled extubation. J Crit Care 2011;26:502-9. https://doi.org/10.1016/j.jcrc.2010.12.015.
- Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest 1997;112:186-92. https://doi.org/10.1378/chest.112.1.186.
- National Institute for Health and Care Excellence (NICE) . Technical Patient Safety Solutions for Ventilator-Associated Pneumonia in Adults 2008. www.nice.org.uk/nicemedia/live/12053/41684/41684.pdf (accessed 1 December 2014).
- Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med 1998;129:433-40. https://doi.org/10.7326/0003-4819-129-6-199809150-00002.
- Westergren V, Lundblad L, Hellquist HB, Forsum U. Ventilator-associated sinusitis: a review. Clin Infect Dis 1998;27:851-64. https://doi.org/10.1086/514932.
- Pingleton SK. Complications of acute respiratory failure. Am Rev Respir Dis 1988;137:1463-93. https://doi.org/10.1164/ajrccm/137.6.1463.
- Krinsley JS, Barone JE. The drive to survive: unplanned extubation in the ICU. Chest 2005;128:560-6. https://doi.org/10.1378/chest.128.2.560.
- de Groot RI, Dekkers OM, Herold IH, de Jonge E, Arbous MS. Risk factors and outcomes after unplanned extubations on the ICU: a case-control study. Crit Care 2011;15. https://doi.org/10.1186/cc9964.
- Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: the ‘ABCDE’ approach. Curr Opin Crit Care 2011;17:43-9. https://doi.org/10.1097/MCC.0b013e3283427243.
- Epstein SK, Nevins ML, Chung J. Effect of unplanned extubation on outcome of mechanical ventilation. Am J Respir Crit Care Med 2000;161:1912-16. https://doi.org/10.1164/ajrccm.161.6.9908068.
- Keenan SP, Sinuff T, Burns KE, Muscedere J, Kutsogiannis J, Mehta S, et al. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ 2011;183:E195-214. https://doi.org/10.1503/cmaj.100071.
- Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet 2009;374:250-9. https://doi.org/10.1016/S0140-6736(09)60496-7.
- Burns KE, Meade MO, Premji A, Adhikari NK. Noninvasive positive-pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev 2013;12. https://doi.org/10.1002/14651858.CD004127.pub3.
- Roberts CM, Brown JL, Reinhardt AK, Kaul S, Scales K, Mikelsons C, et al. Non-invasive ventilation in chronic obstructive pulmonary disease: management of acute type 2 respiratory failure. Clin Med 2008;8:517-21. https://doi.org/10.7861/clinmedicine.8-5-517.
- Burns KE, Raptis S, Bakshi J, Cook D, Jones A, Epstein S, et al. Practice variation in weaning critically ill adults from invasive mechanical ventilation: preliminary results of an international survey. Am J Respir Crit Care Med 2011;183.
- Perkins GD, McAuley D. Research prioritisation exercise 2008. J Intensive Care Soc 2008;9.
- Walsh TS, Dodds S, McArdle F. Evaluation of simple criteria to predict successful weaning from mechanical ventilation in intensive care patients. Br J Anaesth 2004;92:793-9. https://doi.org/10.1093/bja/aeh139.
- Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996;335:1864-9. https://doi.org/10.1056/NEJM199612193352502.
- Rothaar RC, Epstein SK. Extubation failure: magnitude of the problem, impact on outcomes, and prevention. Curr Opin Crit Care 2003;9:59-66. https://doi.org/10.1097/00075198-200302000-00011.
- Department of Health and Social Care . Comprehensive Critical Care. A Review of Adult Critical Care Services 2000.
- Information Standards Board for Health and Social Care . Critical Care Minimum Data Set Full Specification 2010. www.datadictionary.nhs.uk/data_dictionary/messages/supporting_data_sets/data_sets/critical_care_minimum_data_set_fr.asp?shownav=1 (accessed 16 September 2017).
- Department of Health and Social Care . High Impact Intervention: Care Bundle to Reduce Ventilation-Association Pneumonia 2011.
- Great Britain . Data Protection Act 1998 1998.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- NHS Litigation Authority . Clinical Negligence Scheme for Trusts n.d. https://resolution.nhs.uk/wp-content/uploads/2018/09/CNST-Rules.pdf (accessed February 2019).
- NHS Health Resource Authority . UK Policy Framework for Health and Social Care Research 2017. www.hra.nhs.uk/documents/1068/uk-policy-framework-health-social-care-research.pdf (accessed 1 December 2014).
- National Institute for Health Research . Good Clinical Practice (GCP) Reference Guide n.d. www.nihr.ac.uk/our-research-community/documents/GCP%20Reference%20Guide.pdf (accessed February 2019).
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- Department of Health and Social Care . NHS Reference Costs 2014 to 2015 2014.
- NHS Digital . Prescription Cost Analysis – England, 2016 2017. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/prescription-cost-analysis-england-2016 (accessed 15 May 2017).
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Curtis L. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Curtis L. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- NHS . National Catalogue 2015.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Petrou S, Morrell J, Spiby H. Assessing the empirical validity of alternative multi-attribute utility measures in the maternity context. Health Qual Life Outcomes 2009;7. https://doi.org/10.1186/1477-7525-7-40.
- Jhita T, Petrou S, Gumber A, Szczepura A, Raymond NT, Bellary S. Ethnic differences in health related quality of life for patients with type 2 diabetes. Health Qual Life Outcomes 2014;12. https://doi.org/10.1186/1477-7525-12-83.
- Kind P. UK Population Norms for the EQ-5D. York: University of York Centre for Health Economics; 1999.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 1987.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 2002;21:2175-97. https://doi.org/10.1002/sim.1203.
- Dewar R, Khan I. A new SAS macro for flexible parametric survival modeling: applications to clinical trials and surveillance data. Clin Invest 2015;5:1-11.
- Office for National Statistics . National Life Tables: UK 2017. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 21 March 2018).
- Pocock SJ, Stone GW. The primary outcome fails – what next?. N Engl J Med 2016;375:861-70. https://doi.org/10.1056/NEJMra1510064.
- Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;7. https://doi.org/10.1002/14651858.CD004104.pub4.
- Perren A, Brochard L. Managing the apparent and hidden difficulties of weaning from mechanical ventilation. Intensive Care Med 2013;39:1885-95. https://doi.org/10.1007/s00134-013-3014-9.
- Blackwood B, Burns KE, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD006904.pub3.
- Dritsaki M, Achana F, Mason J, Petrou S. Methodological issues surrounding the use of baseline health-related quality of life data to inform trial-based economic evaluations of interventions within emergency and critical care settings: a systematic literature review. PharmacoEconomics 2017;35:501-15. https://doi.org/10.1007/s40273-016-0485-x.
- Khan I. Extrapolation of Utilities Between Disease Progression and Death in Cancer Trials for Economic Evaluation n.d.
- Rose L, Dainty KN, Jordan J, Blackwood B. Weaning from mechanical ventilation: a scoping review of qualitative studies. Am J Crit Care 2014;23:e54-70. https://doi.org/10.4037/ajcc2014539.
- Department of Health and Social Care . NHS Reference Costs 2016 to 2017 2016.
- National Institute for Health and Care Excellence (NICE) . Rehabilitation After Critical Illness 2009.
- Department of Health and Social Care . NHS Reference Costs 2013 to 2014 2013.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Portsmouth Clinical Commissioning Group . Annual General Meeting 2002. www.portsmouthccg.nhs.uk/AGM%20Presentation%2002.10.13.pdf (accessed 21 March 2018).
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2011.
Appendix 1 Summary of screening
| Site name | Number screened | Excluded from trial, n (%) | Had SBT, n (%) | Failed SBT, n (%) | Randomised patients, n (%) |
|---|---|---|---|---|---|
| Addenbrooke’s | 792 | 666 (84) | 126 (16) | 35 (28) | 19 (2) |
| Basildon | 73 | 62 (85) | 11 (15) | 1 (9) | 1 (1) |
| Bedford | 308 | 258 (84) | 50 (16) | 4 (8) | 3 (1) |
| BHH | 609 | 463 (76) | 146 (24) | 60 (34) | 60 (10) |
| Blackpool | 291 | 278 (96) | 13 (4) | 6 (50) | 6 (2) |
| Bradford RI | 270 | 246 (91) | 24 (9) | 10 (42) | 9 (3) |
| Bristol RI | 199 | 174 (87) | 25 (13) | 0 (0) | 0 (0) |
| Broomfield | 90 | 82 (91) | 8 (9) | 0 (0) | 0 (0) |
| Edinburgh RI | 330 | 317 (96) | 13 (4) | 4 (31) | 3 (1) |
| Forth Valley | 132 | 100 (76) | 32 (24) | 0 (0) | 0 (0) |
| Freeman | 175 | 166 (95) | 9 (5) | 2 (22) | 1 (1) |
| George Eliot | 86 | 80 (93) | 6 (7) | 3 (50) | 2 (2) |
| Glenfield | 99 | 91 (92) | 8 (8) | 2 (25) | 2 (2) |
| Hillingdon | 85 | 56 (66) | 29 (34) | 14 (48) | 13 (15) |
| Hull RI | 393 | 375 (95) | 18 (5) | 2 (11) | 1 (0) |
| James Paget | 95 | 93 (98) | 2 (2) | 0 (0) | 0 (0) |
| John Radcliffe | 93 | 60 (65) | 33 (35) | 1 (3) | 0 (0) |
| King’s Lynn QE | 137 | 117 (85) | 20 (15) | 8 (40) | 7 (5) |
| Leicester RI | 291 | 271 (93) | 20 (7) | 6 (30) | 5 (2) |
| Leighton | 226 | 212 (94) | 14 (6) | 3 (21) | 3 (1) |
| Manchester RI | 566 | 503 (89) | 63 (11) | 9 (14) | 6 (1) |
| Milton Keynes | 154 | 148 (96) | 6 (4) | 1 (17) | 1 (1) |
| Monklands | 223 | 223 (100) | 0 (0) | 0 (0) | 0 (0) |
| Musgrove Park | 206 | 187 (91) | 19 (9) | 4 (21) | 4 (2) |
| New Cross | 256 | 211 (82) | 45 (18) | 6 (13) | 2 (1) |
| Papworth | 127 | 125 (98) | 2 (2) | 0 (0) | 0 (0) |
| Peterborough | 258 | 235 (91) | 23 (9) | 13 (57) | 13 (5) |
| Poole | 373 | 336 (90) | 37 (10) | 4 (11) | 3 (1) |
| Portsmouth | 335 | 328 (98) | 7 (2) | 2 (29) | 2 (1) |
| QEHB | 1838 | 1618 (88) | 220 (12) | 71 (32) | 51 (3) |
| Royal Blackburn | 572 | 545 (95) | 27 (5) | 3 (11) | 6 (1) |
| Royal Gwent | 187 | 173 (93) | 14 (7) | 5 (36) | 4 (2) |
| Royal Liverpool | 425 | 375 (88) | 50 (12) | 10 (20) | 8 (2) |
| Royal Surrey | 71 | 68 (96) | 3 (4) | 1 (33) | 0 (0) |
| Royal Sussex | 486 | 465 (96) | 21 (4) | 3 (14) | 3 (1) |
| Russells Hall | 105 | 73 (70) | 32 (30) | 11 (34) | 7 (7) |
| RVH Belfast | 1106 | 870 (79) | 236 (21) | 21 (9) | 12 (1) |
| RVI Newcastle | 567 | 558 (98) | 9 (2) | 2 (22) | 2 (0) |
| Southampton | 409 | 400 (98) | 9 (2) | 4 (44) | 5 (1) |
| Southend | 13 | 13 (100) | 0 (0) | 0 (0) | 0 (0) |
| St James’s Leeds | 409 | 371 (91) | 38 (9) | 14 (37) | 15 (4) |
| St Thomas’ London | 1054 | 950 (90) | 104 (10) | 45 (43) | 50 (5) |
| UHCW | 380 | 358 (94) | 22 (6) | 2 (9) | 2 (1) |
| UHNS | 427 | 417 (98) | 10 (2) | 4 (40) | 3 (1) |
| UHOW | 745 | 710 (95) | 35 (5) | 10 (29) | 8 (1) |
| UHSM | 138 | 127 (92) | 11 (8) | 1 (9) | 1 (1) |
| Western General | 452 | 399 (88) | 53 (12) | 12 (23) | 8 (2) |
| West Middlesex | 101 | 96 (95) | 5 (5) | 2 (40) | 2 (2) |
| Wexham Park | 65 | 58 (89) | 7 (11) | 2 (29) | 0 (0) |
| Yeovil | 69 | 66 (96) | 3 (4) | 1 (33) | 1 (1) |
| York | 235 | 201 (86) | 34 (14) | 18 (53) | 10 (4) |
| Total | 17,126 | 15,374 (90) | 1752 (10) | 432 (25) | 364 (2) |
| Site name | Number screened | Not meeting inclusion criteria, n (%) | Meeting exclusion criteria, n (%) | Other reasons for exclusion | SBT process, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aged > 16 years | Passed SBT | Pregnant | Tracheostomy | Neurological deficit | NIV contraindication | Home ventilation | Withdrawal/patient died | Further surgery/procedure requiring sedation planned in next 48 hours | Previous participation in the Breathe trial | Had SBT (patients who are ready for weaning) | Failed SBT | |||
| Addenbrooke’s | 792 | 0 (0) | 91 (72) | 0 (0) | 212 (27) | 115 (15) | 78 (10) | 9 (1) | 189 (24) | 4 (1) | 1 (0) | 58 (7) | 126 (16) | 35 (4) |
| Basildon | 73 | 0 (0) | 10 (91) | 0 (0) | 5 (7) | 33 (45) | 6 (8) | 0 (0) | 18 (25) | 0 (0) | 0 (0) | 0 (0) | 11 (15) | 1 (1) |
| Bedford | 308 | 21 (4) | 46 (92) | 3 (1) | 36 (12) | 21 (7) | 4 (1) | 0 (0) | 138 (45) | 2 (1) | 1 (0) | 32 (10) | 50 (16) | 4 (1) |
| BHH | 609 | 0 (0) | 96 (66) | 1 (0) | 97 (16) | 18 (3) | 41 (7) | 0 (0) | 162 (27) | 1 (0) | 3 (0) | 139 (23) | 146 (24) | 50 (8) |
| Blackpool | 291 | 2 (0) | 7 (54) | 2 (0) | 114 (39) | 14 (5) | 46 (16) | 0 (0) | 82 (28) | 0 (0) | 0 (0) | 18 (6) | 13 (4) | 6 (2) |
| Bradford RI | 270 | 0 (0) | 14 (58) | 0 (0) | 82 (30) | 21 (8) | 7 (3) | 0 (0) | 125 (46) | 0 (0) | 1 (0) | 10 (4) | 24 (9) | 10 (4) |
| Bristol RI | 199 | 0 (0) | 25 (100) | 0 (0) | 52 (26) | 13 (7) | 2 (1) | 0 (0) | 11 (6) | 6 (3) | 1 (1) | 89 (45) | 25 (13) | 0 (0) |
| Broomfield | 90 | 0 (0) | 8 (100) | 0 (0) | 33 (37) | 7 (8) | 8 (9) | 0 (0) | 18 (20) | 2 (2) | 0 (0) | 14 (16) | 8 (9) | 0 (0) |
| Edinburgh RI | 330 | 1 (0) | 9 (69) | 0 (0) | 49 (15) | 44 (13) | 22 (7) | 3 (1) | 114 (35) | 0 (0) | 0 (0) | 84 (25) | 13 (4) | 4 (1) |
| Forth Valley | 132 | 4 (2) | 32 (100) | 0 (0) | 20 (15) | 4 (3) | 7 (5) | 7 (5) | 50 (38) | 1 (1) | 0 (0) | 7 (5) | 32 (24) | 0 (0) |
| Freeman | 175 | 0 (0) | 7 (78) | 0 (0) | 54 (31) | 16 (9) | 12 (7) | 0 (0) | 57 (33) | 2 (1) | 0 (0) | 25 (14) | 9 (5) | 2 (1) |
| George Eliot | 86 | 0 (0) | 3 (50) | 0 (0) | 37 (43) | 0 (0) | 11 (13) | 0 (0) | 26 (30) | 1 (1) | 0 (0) | 5 (6) | 6 (7) | 3 (3) |
| Glenfield | 99 | 0 (0) | 6 (75) | 0 (0) | 50 (51) | 11 (11) | 5 (5) | 0 (0) | 11 (11) | 1 (1) | 0 (0) | 13 (13) | 8 (8) | 2 (2) |
| Hillingdon | 85 | 0 (0) | 15 (52) | 0 (0) | 12 (14) | 9 (11) | 8 (9) | 0 (0) | 16 (19) | 0 (0) | 0 (0) | 11 (13) | 29 (34) | 14 (16) |
| Hull RI | 393 | 11 (1) | 16 (89) | 0 (0) | 96 (24) | 63 (16) | 21 (5) | 0 (0) | 170 (43) | 0 (0) | 0 (0) | 13 (3) | 18 (5) | 2 (1) |
| James Paget | 95 | 5 (3) | 2 (100) | 2 (2) | 25 (26) | 3 (3) | 1 (1) | 0 (0) | 50 (53) | 0 (0) | 0 (0) | 7 (7) | 2 (2) | 0 (0) |
| John Radcliffe | 93 | 0 (0) | 32 (97) | 0 (0) | 11 (12) | 11 (12) | 1 (1) | 0 (0) | 8 (9) | 7 (8) | 0 (0) | 22 (24) | 33 (35) | 1 (1) |
| King’s Lynn QE | 137 | 1 (0) | 12 (60) | 0 (0) | 15 (11) | 8 (20) | 3 (2) | 0 (0) | 63 (46) | 1 (1) | 0 (0) | 26 (19) | 20 (15) | 8 (6) |
| Leicester RI | 291 | 0 (0) | 14 (70) | 0 (0) | 76 (26) | 52 (18) | 37 (13) | 1 (0) | 62 (21) | 5 (2) | 0 (0) | 38 (13) | 20 (7) | 6 (2) |
| Leighton | 226 | 1 (0) | 11 (79) | 0 (0) | 18 (8) | 15 (7) | 14 (6) | 0 (0) | 141 (62) | 0 (0) | 0 (0) | 23 (10) | 14 (6) | 3 (1) |
| Manchester RI | 566 | 0 (0) | 54 (86) | 2 (1) | 114 (20) | 170 (30) | 37 (7) | 0 (0) | 125 (22) | 4 (1) | 2 (0) | 49 (9) | 63 (11) | 9 (2) |
| Milton Keynes | 154 | 2 (1) | 5 (83) | 0 (0) | 28 (18) | 2 (1) | 0 (0) | 0 (0) | 104 (68) | 0 (0) | 0 (0) | 12 (8) | 6 (4) | 1 (1) |
| Monklands | 223 | 1 (0) | 0 (0) | 0 (0) | 48 (22) | 18 (8) | 13 (6) | 0 (0) | 103 (46) | 5 (2) | 0 (0) | 35 (16) | 0 (0) | 0 (0) |
| Musgrove Park | 206 | 10 (2) | 15 (79) | 0 (0) | 16 (8) | 17 (8) | 15 (7) | 3 (1) | 95 (46) | 1 (0) | 0 (0) | 30 (15) | 19 (9) | 4 (2) |
| New Cross | 256 | 0 (0) | 39 (87) | 0 (0) | 62 (24) | 21 (27) | 8 (3) | 0 (0) | 99 (39) | 1 (0) | 1 (0) | 19 (7) | 45 (18) | 6 (2) |
| Papworth | 127 | 0 (0) | 2 (100) | 0 (0) | 30 (24) | 1 (7) | 12 (9) | 0 (0) | 25 (20) | 2 (2) | 0 (0) | 55 (43) | 2 (2) | 0 (0) |
| Peterborough | 258 | 3 (1) | 10 (43) | 1 (0) | 62 (24) | 19 (18) | 11 (4) | 0 (0) | 94 (36) | 0 (0) | 0 (0) | 45 (17) | 23 (9) | 13 (5) |
| Poole | 373 | 1 (0) | 33 (89) | 1 (0) | 67 (18) | 67 (5) | 85 (23) | 3 (1) | 76 (20) | 0 (0) | 0 (0) | 36 (10) | 37 (10) | 4 (1) |
| Portsmouth | 335 | 21 (3) | 5 (71) | 0 (0) | 55 (16) | 16 (6) | 24 (7) | 0 (0) | 142 (42) | 0 (0) | 0 (0) | 70 (21) | 7 (2) | 2 (1) |
| QEHB | 1838 | 1 (0) | 149 (68) | 2 (0) | 633 (34) | 361 (5) | 41 (2) | 0 (0) | 264 (14) | 6 (0) | 4 (0) | 306 (17) | 220 (12) | 71 (4) |
| Royal Blackburn | 572 | 1 (0) | 24 (89) | 3 (0) | 176 (31) | 26 (20) | 12 (2) | 8 (1) | 277 (48) | 1 (0) | 0 (0) | 41 (7) | 27 (5) | 3 (1) |
| Royal Gwent | 187 | 0 (0) | 9 (64) | 0 (0) | 44 (24) | 43 (23) | 1 (1) | 0 (0) | 82 (44) | 0 (0) | 2 (1) | 1 (1) | 14 (7) | 5 (3) |
| Royal Liverpool | 425 | 1 (0) | 40 (80) | 0 (0) | 62 (15) | 83 (5) | 36 (8) | 2 (0) | 136 (32) | 1 (0) | 1 (0) | 53 (12) | 50 (12) | 10 (2) |
| Royal Surrey | 71 | 0 (0) | 2 (67) | 0 (0) | 16 (23) | 10 (20) | 18 (25) | 2 (3) | 14 (20) | 0 (0) | 0 (0) | 8 (11) | 3 (4) | 1 (1) |
| Royal Sussex | 486 | 0 (0) | 18 (86) | 0 (0) | 175 (36) | 24 (14) | 14 (3) | 1 (0) | 224 (46) | 0 (0) | 0 (0) | 27 (6) | 21 (4) | 3 (1) |
| Russells Hall | 105 | 0 (0) | 21 (66) | 0 (0) | 6 (6) | 19 (5) | 10 (10) | 1 (1) | 25 (24) | 0 (0) | 0 (0) | 12 (11) | 32 (30) | 11 (10) |
| RVH Belfast | 1106 | 16 (1) | 215 (91) | 1 (0) | 204 (18) | 98 (18) | 12 (1) | 1 (0) | 358 (32) | 3 (0) | 0 (0) | 177 (16) | 236 (21) | 21 (2) |
| Newcastle RVI | 567 | 1 (0) | 7 (78) | 0 (0) | 133 (23) | 155 (1) | 55 (10) | 5 (1) | 172 (30) | 5 (1) | 0 (0) | 32 (6) | 9 (2) | 2 (0) |
| Southampton | 409 | 1 (0) | 5 (56) | 0 (0) | 59 (14) | 62 (9) | 40 (10) | 0 (0) | 164 (40) | 8 (2) | 0 (0) | 66 (16) | 9 (2) | 4 (1) |
| Southend | 13 | 0 (0) | 0 (0) | 0 (0) | 1 (8) | 4 (15) | 1 (8) | 0 (0) | 3 (23) | 0 (0) | 0 (0) | 4 (31) | 0 (0) | 0 (0) |
| St James’s Leeds | 409 | 0 (0) | 24 (63) | 0 (0) | 105 (26) | 85 (31) | 13 (3) | 4 (1) | 134 (33) | 0 (0) | 3 (1) | 27 (7) | 38 (9) | 14 (3) |
| St Thomas’ London | 1054 | 5 (0) | 59 (57) | 10 (1) | 300 (28) | 82 (21) | 59 (6) | 9 (1) | 292 (28) | 2 (0) | 1 (0) | 190 (18) | 104 (10) | 45 (4) |
| UHCW | 380 | 0 (0) | 20 (91) | 0 (0) | 98 (26) | 53 (8) | 22 (6) | 1 (0) | 81 (21) | 0 (0) | 0 (0) | 103 (27) | 22 (6) | 2 (1) |
| UHNS | 427 | 0 (0) | 6 (60) | 0 (0) | 127 (30) | 156 (14) | 15 (4) | 0 (0) | 92 (22) | 1 (0) | 0 (0) | 26 (6) | 10 (2) | 4 (1) |
| UHOW | 745 | 1 (0) | 25 (71) | 1 (0) | 101 (14) | 168 (37) | 121 (16) | 3 (0) | 273 (37) | 0 (0) | 0 (0) | 42 (6) | 35 (5) | 10 (1) |
| UHSM | 138 | 0 (0) | 10 (91) | 0 (0) | 59 (43) | 17 (23) | 11 (8) | 2 (1) | 38 (28) | 0 (0) | 0 (0) | 0 (0) | 11 (8) | 1 (1) |
| Western General | 452 | 5 (1) | 41 (77) | 0 (0) | 57 (13) | 209 (8) | 12 (3) | 10 (2) | 83 (18) | 0 (0) | 0 (0) | 23 (5) | 53 (12) | 12 (3) |
| West Middlesex | 101 | 0 (0) | 3 (60) | 0 (0) | 25 (25) | 8 (12) | 3 (3) | 0 (0) | 52 (51) | 1 (1) | 1 (1) | 6 (6) | 5 (5) | 2 (2) |
| Wexham Park | 65 | 0 (0) | 5 (71) | 0 (0) | 16 (25) | 6 (46) | 4 (6) | 1 (2) | 28 (43) | 0 (0) | 0 (0) | 3 (5) | 7 (11) | 2 (3) |
| Yeovil | 69 | 0 (0) | 2 (67) | 0 (0) | 3 (4) | 9 (9) | 11 (16) | 0 (0) | 41 (59) | 0 (0) | 0 (0) | 2 (3) | 3 (4) | 1 (1) |
| York | 235 | 0 (0) | 16 (47) | 0 (0) | 26 (11) | 28 (13) | 16 (7) | 1 (0) | 104 (44) | 3 (1) | 0 (0) | 23 (10) | 34 (14) | 18 (8) |
| Total | 17,126 | 116 (1) | 1320 (75) | 29 (0) | 4002 (23) | 2515 (12) | 1066 (10) | 77 (1) | 5311 (24) | 79 (1) | 22 (0) | 2157 (7) | 1752 (10) | 432 (4) |
| Site name | Number screened | Other reasons for exclusion, n (%) | |||||
|---|---|---|---|---|---|---|---|
| Consultant refusal | Patient refusal | Relative refused | Other studies | Total other exclusions | Blanks, missing data | ||
| Addenbrooke’s | 792 | 5 (1) | 0 (0) | 2 (0) | 15 (2) | 28 (4) | 8 (1) |
| Basildon | 73 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Bedford | 308 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 32 (10) | 0 (0) |
| BHH | 609 | 10 (2) | 0 (0) | 5 (1) | 9 (1) | 23 (4) | 92 (15) |
| Blackpool | 291 | 1 (0) | 0 (0) | 0 (0) | 4 (1) | 13 (4) | 0 (0) |
| Bradford RI | 270 | 1 (0) | 0 (0) | 5 (2) | 0 (0) | 4 (1) | 0 (0) |
| Bristol RI | 199 | 7 (4) | 0 (0) | 0 (0) | 20 (10) | 23 (12) | 39 (20) |
| Broomfield | 90 | 2 (2) | 0 (0) | 4 (4) | 0 (0) | 4 (4) | 4 (4) |
| Edinburgh RI | 330 | 24 (7) | 0 (0) | 7 (2) | 15 (5) | 36 (11) | 2 (1) |
| Forth Valley | 132 | 0 (0) | 0 (0) | 2 (2) | 0 (0) | 5 (4) | 0 (0) |
| Freeman | 175 | 6 (3) | 0 (0) | 4 (2) | 0 (0) | 14 (8) | 1 (1) |
| George Eliot | 86 | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 4 (5) | 0 (0) |
| Glenfield | 99 | 1 (1) | 0 (0) | 4 (4) | 2 (2) | 6 (6) | 0 (0) |
| Hillingdon | 85 | 0 (0) | 1 (1) | 5 (6) | 0 (0) | 5 (6) | 0 (0) |
| Hull RI | 393 | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 11 (3) | 0 (0) |
| James Paget | 95 | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 5 (5) | 1 (1) |
| John Radcliffe | 93 | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 5 (5) | 14 (15) |
| King’s Lynn QE | 137 | 3 (2) | 0 (0) | 15 (11) | 0 (0) | 6 (4) | 2 (1) |
| Leicester RI | 291 | 13 (4) | 0 (0) | 2 (1) | 0 (0) | 23 (8) | 0 (0) |
| Leighton | 226 | 0 (0) | 4 (2) | 7 (3) | 0 (0) | 12 (5) | 0 (0) |
| Manchester RI | 566 | 24 (4) | 1 (0) | 7 (1) | 2 (0) | 14 (2) | 1 (0) |
| Milton Keynes | 154 | 3 (2) | 0 (0) | 1 (1) | 0 (0) | 8 (5) | 0 (0) |
| Monklands | 223 | 14 (6) | 2 (1) | 0 (0) | 0 (0) | 19 (9) | 0 (0) |
| Musgrove Park | 206 | 10 (5) | 0 (0) | 5 (2) | 1 (0) | 14 (7) | 0 (0) |
| New Cross | 256 | 0 (0) | 0 (0) | 1 (0) | 4 (2) | 13 (5) | 1 (0) |
| Papworth | 127 | 6 (5) | 1 (1) | 3 (2) | 5 (4) | 40 (31) | 0 (0) |
| Peterborough | 258 | 5 (2) | 1 (0) | 15 (6) | 1 (0) | 23 (9) | 0 (0) |
| Poole | 373 | 7 (2) | 1 (0) | 14 (4) | 2 (1) | 12 (3) | 0 (0) |
| Portsmouth | 335 | 3 (1) | 0 (0) | 11 (3) | 2 (1) | 47 (14) | 7 (2) |
| QEHB | 1838 | 17 (1) | 8 (0) | 5 (0) | 18 (1) | 210 (11) | 48 (3) |
| Royal Blackburn | 572 | 6 (1) | 0 (0) | 2 (0) | 2 (0) | 31 (5) | 0 (0) |
| Royal Gwent | 187 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Royal Liverpool | 425 | 4 (1) | 1 (0) | 36 (8) | 0 (0) | 12 (3) | 0 (0) |
| Royal Surrey | 71 | 5 (7) | 0 (0) | 0 (0) | 0 (0) | 3 (4) | 0 (0) |
| Royal Sussex | 486 | 0 (0) | 1 (0) | 3 (1) | 6 (1) | 17 (3) | 0 (0) |
| Russells Hall | 105 | 2 (2) | 0 (0) | 3 (3) | 0 (0) | 6 (6) | 1 (1) |
| RVH Belfast | 1106 | 2 (0) | 0 (0) | 2 (0) | 18 (2) | 125 (11) | 30 (3) |
| RVI Newcastle | 567 | 7 (1) | 2 (0) | 5 (1) | 0 (0) | 18 (3) | 0 (0) |
| Southampton | 409 | 1 (0) | 0 (0) | 4 (1) | 5 (1) | 56 (14) | 0 (0) |
| Southend | 13 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (31) | 0 (0) |
| St James’s Leeds | 409 | 3 (1) | 0 (0) | 0 (0) | 1 (0) | 8 (2) | 15 (4) |
| St Thomas’ London | 1054 | 15 (1) | 3 (0) | 15 (1) | 61 (6) | 80 (8) | 16 (2) |
| UHCW | 380 | 7 (2) | 0 (0) | 1 (0) | 14 (4) | 11 (3) | 70 (18) |
| UHNS | 427 | 3 (1) | 0 (0) | 2 (0) | 10 (2) | 11 (3) | 0 (0) |
| UHOW | 745 | 7 (1) | 1 (0) | 4 (1) | 3 (0) | 27 (4) | 0 (0) |
| UHSM | 138 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Western General | 452 | 1 (0) | 0 (0) | 2 (0) | 0 (0) | 20 (4) | 0 (0) |
| West Middlesex | 101 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (6) | 0 (0) |
| Wexham Park | 65 | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 2 (3) | 0 (0) |
| Yeovil | 69 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 0 (0) |
| York | 235 | 2 (1) | 3 (1) | 9 (4) | 0 (0) | 9 (4) | 0 (0) |
| Total | 17,126 | 234 (1) | 30 (0) | 213 (0) | 220 (2) | 1108 (4) | 352 (1) |
Appendix 2 Randomised participants by site and treatment
| Site name | Trial group, n (%) | Total (N = 364), n (%) | |
|---|---|---|---|
| Invasive weaning (N = 182) | Non-invasive weaning (N = 182) | ||
| Addenbrooke’s | 10 (5) | 9 (5) | 19 (5) |
| Basildon | 0 | 1 (1) | 1 (0) |
| Bedford | 1 (1) | 2 (1) | 3 (1) |
| BHH | 30 (16) | 30 (16) | 60 (16) |
| Blackpool | 3 (2) | 3 (2) | 6 (2) |
| Bradford RI | 4 (2) | 5 (3) | 9 (2) |
| Edinburgh RI | 1 (1) | 2 (1) | 3 (1) |
| Freeman | 0 | 1 (1) | 1 (0) |
| George Eliot | 0 | 2 (1) | 2 (1) |
| Glenfield | 1 (1) | 1 (1) | 2 (1) |
| Hillingdon | 7 (4) | 6 (3) | 13 (4) |
| Hull RI | 0 | 1 (1) | 1 (0) |
| King’s Lynn QE | 3 (2) | 4 (2) | 7 (2) |
| Leicester RI | 2 (1) | 3 (2) | 5 (1) |
| Leighton | 1 (1) | 2 (1) | 3 (1) |
| Manchester RI | 3 (2) | 3 (2) | 6 (2) |
| Milton Keynes | 1 (1) | 0 | 1 (0) |
| Musgrove Park | 2 (1) | 2 (1) | 4 (1) |
| New Cross | 1 (1) | 1 (1) | 2 (1) |
| Peterborough | 7 (4) | 6 (3) | 13 (4) |
| Poole | 1 (1) | 2 (1) | 3 (1) |
| Portsmouth | 1 (1) | 1 (1) | 2 (1) |
| QEHB | 26 (14) | 25 (14) | 51 (14) |
| Royal Blackburn | 3 (2) | 3 (2) | 6 (2) |
| Royal Gwent | 2 (1) | 2 (1) | 4 (1) |
| Royal Liverpool | 4 (2) | 4 (2) | 8 (2) |
| Royal Sussex | 2 (1) | 1 (1) | 3 (1) |
| Russells Hall | 4 (2) | 3 (2) | 7 (2) |
| RVH Belfast | 6 (3) | 6 (3) | 12 (3) |
| RVI Newcastle | 1 (1) | 1 (1) | 2 (1) |
| Southampton | 2 (1) | 3 (2) | 5 (1) |
| St James’s Leeds | 9 (5) | 6 (3) | 15 (4) |
| St Thomas’ London | 26 (14) | 24 (13) | 50 (14) |
| UHCW | 2 (1) | 0 | 2 (1) |
| UHNS | 1 (1) | 2 (1) | 3 (1) |
| UHOW | 4 (2) | 4 (2) | 8 (2) |
| UHSM | 1 (1) | 0 | 1 (0) |
| Western General | 4 (2) | 4 (2) | 8 (2) |
| West Middlesex | 0 | 2 (1) | 2 (1) |
| Yeovil | 1 (1) | 0 | 1 (0) |
| York | 5 (3) | 5 (3) | 10 (3) |
| Site name | Absence of COPD | Presence of COPD | ||||||
|---|---|---|---|---|---|---|---|---|
| Non-operative | Postoperative | Non-operative | Postoperative | |||||
| Invasive weaning | Non-invasive weaning | Invasive weaning | Non-invasive weaning | Invasive weaning | Non-invasive weaning | Invasive weaning | Non-invasive weaning | |
| Addenbrooke’s | 4 | 6 | 5 | 1 | – | – | 1 | 2 |
| Basildon | – | 1 | – | – | – | – | – | – |
| Bedford | 1 | – | – | 1 | – | 1 | – | – |
| BHH | 18 | 21 | 5 | 7 | 7 | 1 | 1 | |
| Blackpool | 2 | 3 | – | – | 1 | – | – | – |
| Bradford RI | 3 | 3 | – | 2 | – | – | 1 | – |
| Edinburgh RI | – | 1 | 1 | – | – | 1 | – | – |
| Freeman | – | 1 | – | – | – | – | – | – |
| George Eliot | – | 1 | – | – | – | 1 | – | – |
| Glenfield | – | – | – | 1 | – | – | 1 | – |
| Hillingdon | 3 | 3 | 3 | 1 | 1 | 2 | ||
| Hull RI | – | – | – | 1 | – | – | – | – |
| King’s Lynn QE | 1 | 2 | – | 1 | 2 | 1 | – | – |
| Leicester RI | 2 | 1 | – | 1 | – | 1 | – | – |
| Leighton | 1 | 2 | – | – | – | – | – | – |
| Manchester RI | 2 | 2 | – | 1 | 1 | – | – | – |
| Milton Keynes | – | – | 1 | – | – | – | – | – |
| Musgrove Park | 1 | 1 | 1 | – | – | 1 | – | – |
| New Cross | – | – | 1 | – | – | 1 | – | – |
| Peterborough | 6 | 4 | 1 | 1 | – | – | – | 1 |
| Poole | 1 | 2 | – | – | – | – | – | – |
| Portsmouth | – | 1 | – | – | 1 | – | – | – |
| QEHB | 14 | 13 | 11 | 8 | 1 | 3 | 1 | |
| Royal Blackburn | 2 | 1 | – | 1 | – | 1 | 1 | – |
| Royal Gwent | 2 | 1 | – | – | – | 1 | – | – |
| Royal Liverpool | 1 | 2 | 2 | 1 | – | 1 | 1 | – |
| Royal Sussex | – | 1 | 2 | – | – | – | – | – |
| Russells Hall | 4 | 2 | – | 1 | – | – | – | – |
| RVH Belfast | 4 | 2 | – | 3 | – | – | 2 | 1 |
| RVI Newcastle | 1 | – | – | – | – | 1 | – | – |
| Southampton | 1 | 2 | 1 | 1 | – | – | – | – |
| St James’s Leeds | 4 | 3 | 3 | 2 | 2 | 1 | – | – |
| St Thomas’ London | 15 | 11 | 7 | 11 | 3 | 1 | 1 | 1 |
| UHCW | – | – | 2 | – | – | – | – | – |
| UHNS | 1 | – | – | – | – | 2 | – | – |
| UHOW | 3 | 2 | 1 | 1 | – | 1 | – | – |
| UHSM | – | – | – | – | – | – | 1 | – |
| Western General | 4 | 1 | – | 2 | – | 1 | – | – |
| West Middlesex | – | 1 | – | – | – | 1 | – | – |
| Yeovil | – | – | – | – | 1 | – | – | – |
| York | 2 | 5 | – | – | 3 | – | – | – |
| Total | 103 | 102 | 47 | 49 | 23 | 24 | 9 | 7 |
Appendix 3 Health economics tables
| Time (months) | NIV survival rate | IMV survival rate | NIV expected costs (equal resource use)a (£) | IMV expected costs (£) | NIV expected costsb (£) |
|---|---|---|---|---|---|
| 0 | 1 | 1 | Use observed | Use observed | Use observed |
| 3 | 0.79 | 0.76 | Use observed | Use observed | Use observed |
| 6 | 0.76 | 0.70 | Use observed | Use observed | Use observed |
| 9 | 0.75 | 0.67 | 801 | 716 | 1056 |
| 12 | 0.74 | 0.64 | 790 | 684 | 1042 |
| 15 | 0.73 | 0.61 | 728 | 608 | 959 |
| 18 | 0.72 | 0.60 | 717 | 598 | 946 |
| 21 | 0.71 | 0.58 | 708 | 578 | 933 |
| 24 | 0.71 | 0.56 | 708 | 558 | 933 |
| 27 | 0.70 | 0.55 | 674 | 529 | 889 |
| 30 | 0.70 | 0.54 | 674 | 520 | 889 |
| 33 | 0.69 | 0.53 | 665 | 511 | 876 |
| 36 | 0.69 | 0.52 | 665 | 501 | 876 |
| 39 | 0.69 | 0.51 | 642 | 475 | 847 |
| 42 | 0.68 | 0.50 | 633 | 465 | 834 |
| 45 | 0.68 | 0.49 | 633 | 456 | 834 |
| 48 | 0.68 | 0.48 | 602 | 447 | 834 |
| 51 | 0.67 | 0.47 | 602 | 423 | 794 |
| 54 | 0.67 | 0.47 | 602 | 423 | 794 |
| 57 | 0.67 | 0.46 | 602 | 414 | 794 |
| 60 | 0.67 | 0.45 | 602 | 405 | 794 |
| Total | 12,048 | 9311 | 15,924 |
| Analyses | Survival | Utilities between groups | Costs between groups |
|---|---|---|---|
| 1. Primary | Different between groups | Equal between groups after 6 months (set NIV = IMV) | Equal between groups after 6 months (set NIV = IMV) |
| Sensitivity analyses | |||
| 2. | Decrease by 10% on NIV | Equal between groups after 6 months (set NIV = IMV) | Equal between groups after 6 months (set NIV = IMV) |
| 3. | Different between groups | Unequal between groups (carried forward) | Unequal between groups (carried forward) |
| 4. | Different between groups | Unequal between groups (carried forward) | Equal between groups after 6 months (set NIV = IMV) |
| Resource use category by time period | Trial group | |
|---|---|---|
| Non-invasive weaning (N = 182) | Invasive weaning (N = 182) | |
| Randomisation to hospital discharge | ||
| Number of days in ICU [mean (SE)] | 14.0 (1.078) | 15.0 (1.12) |
| Maximum number of organs supported [n (%)] | ||
| 0 | 1 (< 1) | 0 |
| 1 | 5 (3) | 6 (3) |
| 2 | 131 (72) | 139 (76) |
| 3 | 43 (24) | 34 (19) |
| 4 | 2 (1) | 3 (2) |
| Days in ICU for supporting [n (%)] | ||
| 0 organs | 7.0 (n/c)a | 0 |
| 1 organ | 12.8 (3.07) | 8.0 (2.2) |
| 2 organs | 9.7 (0.64) | 12.9 (0.7) |
| 3 organs | 18.1 (1.50) | 13.1 (1.6) |
| 4 organs | 10.5 (3.50) | 16.0 (7.4) |
| Highest level of care [n (%)] | ||
| 0/1 | 0 | 0 |
| 2 | 18 (10) | 2 (1) |
| 3 | 164 (9) | 180 (99) |
| Days in ICU with highest level of care as: [mean (SE)] | ||
| 0/1 | 10.2 (0.78) | 13.4 (1.06) |
| 2 | 10.4 (0.83) | 10.7 (0.74) |
| 3 | 19.7 (2.15) | 17.1 (1.78) |
| Days in ICU with highest level of care as: [n (%)] | ||
| Tracheostomy | 43 (24) | 55 (30) |
| Antifungal use | 10 (5) | 21 (12) |
| Antiviral use | 2 (1) | 0 |
| Emergency transport | 37 (20) | 39 (21) |
| Post discharge to 3 months (post randomisation) (3 months) | Non-invasive weaning (N = 95) | Invasive weaning (N = 91) |
| Inpatient care (hospital readmission) [mean (SE) n (%)] | ||
| Length of stay (days) | 2.8 (1.01) 19 (20) | 6.0 (1.61) 22 (24) |
| Outpatient care (hospitals/clinics) [mean (SE) n (%)] | ||
| Outpatient clinic (number of visits) | 1.3 (0.20) 51 (54) | 1.6 (0.30) 54 (59) |
| Radiology: MRI scan (number of visits) | 0.1 (0.03) 4 (4) | 0.0 (0.02) 4 (4) |
| Radiology: CT (number of visits) | 0.1 (0.05) 7 (7) | 0.1 (0.04) 10 (11) |
| Radiology: radiography (number of visits) | 0.3 (0.09) 16 (17) | 0.2 (0.05) 15 (17) |
| Radiology: ultrasonography (number of visits) | 0.0 (0.02) 4 (4) | 0.1 (0.03) 6 (7) |
| Hospital A&E (number of visits) | 0.2 (0.05) 9 (10) | 0.2 (0.06) 8 (9) |
| Other service: (number of visits) | 1.1 (0.49) 24 (25) | 0.8 (0.19) 26 (29) |
| Residential care services [mean (SE) n (%)] | ||
| Residential care home (number of days) | 0.1 (0.08) 1 (1) | 0.4 (0.24) 3 (3) |
| Rehabilitation centre (number of days) | 0.7 (0.33) 5 (5) | 1.6 (0.69) 6 (7) |
| Warden-controlled residence (number of days) | 0.0 (0.00) 0 (0) | 0.0 (0.00) 0 (0) |
| Day centre run by your local authority (number of days) | 0.0 (0.00) 0 (0) | 0.0 (0.00) 0 (0) |
| Other service (number of days) | 0.5 (0.35) 4 (4) | 0.2 (0.15) 2 (2) |
| Community health and social care [mean (SE) n (%)] | ||
| GP surgery visit (number of visits) | 2.1 (0.26) 59 (62) | 1.9 (0.29) 58 (64) |
| GP home visit (number of visits) | 0.3 (0.07) 19 (20) | 0.5 (0.15) 20 (22) |
| GP telephone consultation (number of visits) | 0.5 (0.12) 18 (19) | 0.5 (0.13) 19 (21) |
| District nurse (number of visits) | 3.5 (0.96) 33 (35) | 3.7 (1.15) 29 (32) |
| Health visitor (number of visits) | 0.0 (0.00) 0 (0) | 0.0 (0.03) 2 (2) |
| Social worker (number of visits) | 0.2 (0.11) 3 (3) | 0.2 (0.05) 9 (10) |
| Physiotherapist (number of visits) | 1.3 (0.41) 23 (24) | 1.2 (0.29) 22 (24) |
| Occupational therapist (number of visits) | 0.8 (0.29) 13 (14) | 0.4 (0.10) 15 (16) |
| Counsellor (number of visits) | 0.2 (0.14) 4 (4) | 0.0 (0.04) 1 (1) |
| Psychologist (number of visits) | 0.0 (0.02) 2 (2) | 0.1 (0.07) 4 (4) |
| Home help/care worker (number of visits) | 6.8 (2.40) 12 (13) | 3.9 (2.10) 9 (10) |
| Other (number of visits) | 2.2 (1.06) 18 (19) | 2.2 (1.05) 19 (21) |
| Medication (on prescription) | ||
| Number of medications [mean (SE) n (%)] | 5.1 (0.46) 73 (77) | 4.0 (0.39) 68 (75) |
| 0 medications [n (%)] | 17 (18) | 22 (24) |
| 1 medication [n (%)] | 8 (8) | 11 (12) |
| 2 medications [n (%)] | 9 (9) | 4 (4) |
| 3 medications [n (%)] | 5 (5) | 8 (9) |
| > 3 medications [n (%)] | 51 (54) | 45 (50) |
| Medication (over the counter) | ||
| Number of medications [mean (SE) n (%)] | 4.7 (1.11) 13 (14) | 5.9 (1.36) 12 (13) |
| 0 medications [n (%)] | 78 (82) | 81 (89) |
| 1 medication [n (%)] | 6 (6) | 2 (2) |
| 2 medications [n (%)] | 0 | 0 |
| 3 medications [n (%)] | 0 | 0 |
| > 3 medications [n (%)] | 7 (7) | 8 (9) |
| Equipment and aids | ||
| Patients who required equipment and aids [n (%)] | 53 (56) | 59 (65) |
| Provider, n (%) | ||
| Health service/NHS | 44 (46) | 46 (51) |
| Patient | 8 (8) | 16 (18) |
| Charity | 1 (1) | 4 (4) |
| Social services | 7 (7) | 4 (4) |
| Other/not stated | 1 (1) | 2 (2) |
| Additional resource use by patient or partner/relative, n (%) | ||
| Patients/partner/relative that used an additional resource | 30 (32) | 32 (35) |
| Travel cost incurred | 19 (20) | 25 (27) |
| Child care | 1 (1) | 1 (1) |
| Income lost | 7 (7) | 9 (10) |
| Housework | 8 (8) | 3 (3) |
| Laundry | 4 (4) | 3 (3) |
| Other | 1 (1) | 2 (2) |
| Post discharge 3 months to 6 months (post randomisation) (3 to 6 months) | Non-invasive weaning (N = 93) | Invasive weaning (N = 84) |
| Inpatient care (hospital readmission) [mean (SE) n (%)] | ||
| Length of stay (days)/number of hospital visits | 3.1 (1.26) 16 (17) | 4.3 (2.19) 23 (27.4) |
| Outpatient care (hospitals/clinics) (number of visits) [mean (SE) n (%)] | ||
| Outpatient clinic | 1.9 (0.35) 49 (53) | 1.5 (0.16) 61 (73) |
| Radiology: MRI scan | 0.1 (0.05) 7 (8) | 0.1 (0.04) 9 (11) |
| Radiology: CT | 0.2 (0.06) 15 (16) | 0.2 (0.04) 14 (17) |
| Radiology: radiography | 0.2 (0.05) 12 (13) | 0.2 (0.06) 12 (14) |
| Radiology: ultrasonography | 0.1 (0.03) 6 (7) | 0.1 (0.04) 11 (13) |
| Hospital A&E | 0.1 (0.05) 7 (8) | 0.2 (0.06) 10 (12) |
| Other service: | 0.8 (0.30) 16 (17) | 1.1 (0.45) 28 (33) |
| Residential care services (number of days) [mean (SE) n (%)] | ||
| Residential care home | 0.0 (0.00) 0 (0) | 1.3 (1.02) 2 (2) |
| Rehabilitation centre | 0.0 (0.00) 0 (0) | 1.8 (1.23) 3 (4) |
| Warden-controlled residence | 0.0 (0.00) 0 (0) | 0.0 (0.00) 0 (0) |
| Day centre run by local authority | 0.0 (0.00) 0 (0) | 0.0 (0.01) 1 (1) |
| Other service | 0.0 (0.00) 0 (0) | 0.0 (0.00) 0 (0) |
| Community health and social care (number of visits) [mean (SE) n (%)] | ||
| GP surgery visit | 1.9 (0.34) 48 (52) | 2.0 (0.23) 61 (73) |
| GP home visit | 0.2 (0.06) 9 (10) | 0.4 (0.14) 12 (14) |
| GP telephone consultation | 0.4 (0.09) 20 (22) | 0.2 (0.07) 13 (16) |
| District nurse | 3.9 (1.66) 18 (19) | 5.4 (2.16) 28 (33) |
| Health visitor | 0.1 (0.05) 3 (3) | 1.0 (0.98) 4 (5) |
| Social worker | 0.0 (0.04) 2 (2) | 0.2 (0.15) 4 (5) |
| Physiotherapist | 0.7 (0.24) 14 (15) | 0.9 (0.37) 14 (17) |
| Occupational therapist | 0.2 (0.15) 5 (5) | 0.3 (0.14) 9 (11) |
| Counsellor | 0.2 (0.16) 4 (4) | 0.1 (0.04) 2 (2) |
| Psychologist | 0.1 (0.04) 2 (2) | 0.1 (0.04) 5 (6.0) |
| Home help/care worker | 2.5 (1.43) 4 (4) | 3.8 (2.35) 5 (6) |
| Other | 1.1 (0.35) 13 (14) | 1.5 (0.80) 16 (19) |
| Medication (on prescription) | ||
| Number of medications [mean (SE) n (%)] | 4.8 (0.46) 67 (72) | 4.9 (0.50) 67 (80) |
| 0 medications [n (%)] | 15 (16) | 25 (30) |
| 1 medication [n (%)] | 13 (14) | 12 (14) |
| 2 medications [n (%)] | 5 (5) | 2 (2) |
| 3 medications [n (%)] | 5 (5) | 4 (5) |
| > 3 medications [n (%)] | 44 (47) | 49 (58) |
| Medication (over the counter) | ||
| Number of medications [mean (SE) n (%)] | 4.7 (1.11) 13 (14) | 5.9 (1.36) 12 (14) |
| 0 medications [n (%)] | 74 (80) | 80 (95) |
| 1 medication [n (%)] | 3 (3) | 3 (4) |
| 2 medications [n (%)] | 0 (0) | 1 (1) |
| 3 medications [n (%)] | 2 (2) | 1 (1) |
| > 3 medications [n (%)] | 3 (3) | 7 (8) |
| Equipment and aids | ||
| Patients who required equipment and aids [n (%)] | 27 (29) | 42 (50) |
| Provider, n (%) | ||
| Health service/NHS | 24 (26) | 25 (30) |
| Patient | 4 (4) | 12 (14) |
| Charity | 0 (0) | 4 (5) |
| Social services | 2 (2) | 7 (8) |
| Other/not stated | 2 (2) | 4 (5) |
| Additional resource use by patient or partner/relative, n (%) | ||
| Patients/partner/relative that used an additional resource | 23 (25) | 35 (42) |
| Travel cost incurred | 19 (20) | 23 (27) |
| Child care | 0 (0) | 0 (0) |
| Income lost | 7 (8) | 8 (10) |
| Housework | 5 (5) | 6 (7) |
| Laundry | 4 (4) | 4 (5) |
| Other | 0 (0) | 1 (1) |
| Post discharge to 6 months (post randomisation) (6 months) | Non-invasive weaning (N = 93) | Invasive weaning (N = 84) |
| Inpatient care (hospital readmission) [mean (SE) n (%)] | ||
| Length of stay (days)/number of hospital visits | 9.5 (2.45) 35 (38) | 5.2 (1.52) 28 (33) |
| Outpatient care (hospitals/clinics) (number of visits) [mean (SE) n (%)] | ||
| Outpatient clinic | 2.9 (0.35) 77 (83) | 2.8 (0.37) 71 (85) |
| Radiology: MRI scan | 0.1 (0.04) 13 (14) | 0.1 (0.05) 10 (12) |
| Radiology: CT | 0.3 (0.06) 20 (22) | 0.3 (0.08) 18 (21) |
| Radiology: radiography | 0.3 (0.07) 25 (27) | 0.4 (0.10) 23 (27) |
| Radiology: ultrasonography | 0.2 (0.05) 15 (16) | 0.1 (0.03) 10 (12) |
| Hospital A&E | 0.3 (0.09) 15 (16) | 0.2 (0.08) 14 (17) |
| Other service: | 1.7 (0.44) 48 (52) | 1.7 (0.52) 35 (42) |
| Residential care services (number of days) [mean (SE) n (%)] | ||
| Residential care home | 1.5 (0.99) 4 (4) | 0.1 (0.07) 1 (1) |
| Rehabilitation centre | 3.1 (1.27) 9 (10) | 0.6 (0.31) 5 (6) |
| Warden-controlled residence | 0.0 (0.00) 0 (0) | 0.0 (0.00) 0 (0) |
| Day centre run by your local authority | 0.0 (0.01) 1 (1) | 0.0 (0.00) 0 (0) |
| Other service | 0.2 (0.14) 2 (2) | 0.5 (0.32) 4 (5) |
| Community health and social care (number of visits) [mean (SE) n (%)] | ||
| GP surgery visit | 3.5 (0.41) 76 (82) | 3.5 (0.44) 77 (92) |
| GP home visit | 0.8 (0.24) 23 (25) | 0.4 (0.10) 24 (29) |
| GP telephone consultation | 0.6 (0.15) 27 (29) | 0.8 (0.14) 29 (35) |
| District nurse | 8.4 (2.75) 46 (49) | 6.6 (2.00) 42 (50) |
| Health visitor | 1.0 (0.89) 6 (6) | 0.1 (0.04) 3 (4) |
| Social worker | 0.3 (0.16) 11 (12) | 0.2 (0.12) 3 (4) |
| Physiotherapist | 1.9 (0.44) 30 (32) | 1.8 (0.46) 31 (37) |
| Occupational therapist | 0.6 (0.16) 21 (23) | 0.9 (0.34) 17 (20) |
| Counsellor | 0.1 (0.06) 3 (3) | 0.4 (0.25) 5 (6) |
| Psychologist | 0.2 (0.09) 8 (9) | 0.1 (0.06) 2 (2) |
| Home help/care worker | 7.1 (2.98) 13 (14) | 8.3 (2.95) 13 (15) |
| Other | 3.4 (1.21) 28 (30) | 2.9 (1.09) 24 (29) |
| Medication (on prescription) | ||
| Number of medications [mean (SE) n (%)] | 4.6 (0.849) 21 (23) | 5.8 (0.953) 24 (29) |
| 0 medications | 61 (66) | 68 (81) |
| 1 medication | 9 (10) | 5 (6) |
| 2 medications | 0 | 3 (4) |
| 3 medications | 2 (2) | 1 (1) |
| > 3 medications | 10 (11) | 15 (18) |
| Medication (over the counter) | ||
| Number of medications [mean (SE) n (%)] | 4.6 (0.849) 21 (23) | 5.8 (0.953) 24 (29) |
| 0 medications | 61 (66) | 68 (81) |
| 1 medication | 9 (10) | 5 (6) |
| 2 medications | 0 | 3 (4) |
| 3 medications | 2 (2) | 1 (1) |
| > 3 medications | 10 (11) | 15 (18) |
| Equipment and aids | ||
| Patients who required equipment and aids [n (%)] | 70 (75) | 61 (73) |
| Provider, n (%) | ||
| Health service/NHS | 54 (58) | 54 (64) |
| Patient | 24 (26) | 10 (12) |
| Charity | 6 (6) | 1 (1) |
| Social services | 10 (11) | 7 (8) |
| Other/not stated | 6 (6) | 3 (4) |
| Additional resource use by patient or partner/relative, n (%) | ||
| Patients/partner/relative that used an additional resource | 46 (49) | 41 (49) |
| Travel cost incurred | 35 (38) | 32 (38) |
| Child care | 1 (1) | 1 (1) |
| Income lost | 11 (12) | 10 (12) |
| Housework | 7 (8) | 10 (12) |
| Laundry | 6 (6) | 6 (7) |
| Other | 3 (3) | 1 (1) |
| Resource item | Unit cost (2015–16 prices)a (£) | Unit of analysis | Source | |
|---|---|---|---|---|
| Intervention related | ||||
| Number of organs supported in the ICU (includes antimicrobial and sedative costs) | ||||
| 0 | 759.00 | Per day | NHS Reference Costs 2016 to 2017, Critical Care59 | |
| 1 | 1031.00 | Per day | NHS Reference Costs 2016 to 2017, Critical Care59 | |
| 2 | 1399.00 | Per day | NHS Reference Costs 2016 to 2017, Critical Care59 | |
| 3 | 1619.00 | Per day | NHS Reference Costs 2016 to 2017, Critical Care59 | |
| 4 | 1794.00 | Per day | NHS Reference Costs 2016 to 2017, Critical Care59 | |
| 5 | 1977.00 | Per day | NHS Reference Costs 2016 to 2017, Critical Care59 | |
| ≥ 6 | 2274.00 | Per day | NHS Reference Costs 2016 to 2017, Critical Care59 | |
| Highest level of care | ||||
| Level 3 | 1641.92 | NICE – Rehabilitation After Critical Illness (CG83) 200960 | ||
| Level 2 | 1641.92 | NICE – Rehabilitation After Critical Illness (CG83) 200960 | ||
| Level 1 | 303.65 | NHS Reference Costs 2013 to 2014 61 | ||
| Tracheostomy | 4151.51 | Per procedure | NHS Reference Costs 2014 to 2015 36 | |
| High-cost drugs | ||||
| Antibiotics | ||||
| Linezolid | 0.074 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Meropenem | 0.016 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Metronidazole | 0.002 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Antifungals | ||||
| Amphotericin Liposomal | 1.644 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Caspofungin | 6.553 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Voriconazole | 0.197 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Anidulafungin | 3.000 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Micafungin | 3.41 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Antiviral | ||||
| Abacavir | 0.010 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Atazanavir | 0.034 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Didanosine | 0.013 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Efavirenz | 0.011 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Emtricitabine | 0.023 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Enfuvirtide | 10.02 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Fesamprenavir | 0.005 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Idinavir | 0.003 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Lopinavir with Ritonavir | 0.013 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Navirapine | 0.010 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Ritonavir | 0.006 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Saquinavir | 0.004 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Stavudine | 0.125 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Tenofovir disoproxil | 0.028 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Zidovudine with lamivudine and Abacavir | 0.024 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Darunavir | 0.012 | Per mg | Prescription Cost Analysis – England, 2016 37 | |
| Health resource use | ||||
| Inpatient care | ||||
| Cost per inpatient stay | 173.56 | Per bed-day | PSSRU – Unit Costs of Health and Social Care 2010,62 p. 31 | |
| General medical ward | 400.0 | Per day | DHCS Communication (2015) https://data.gov.uk/data-request/nhs-hospital-stay (Department of Health and Social Care, March 2017, personal communication) | |
| Outpatient care | ||||
| Outpatient clinic | 115.52 | Per visit | NHS Reference Costs 2014 to 2015 36 | |
| MRI scan | 170.74 | Per test | NHS Reference Costs 2013 to 2014 36 | |
| CT | 94.06 | Per test | NHS Reference Costs 2013 to 2014 36 | |
| Radiography | 27.60 | Per test | Portsmouth Clinical Commissioning Group 63 | |
| Ultrasonography | 48.05 | Per test | NHS Reference Costs 2013 to 2014 61 | |
| Emergency department | 133.76 | Per session | NHS Reference Costs 2014 to 2015 36 | |
| Emergency transport | £261.35 | Per transfer | PSSRU – Unit Costs of Health and Social Care 201164 | |
| Residential care | ||||
| Residential care home | 151.63 | Per day | PSSRU – Unit Costs of Health and Social Care 201338 | |
| Rehabilitation centre | 101.08 | Per day | PSSRU – Unit Costs of Health and Social Care 201338 | |
| Warden-controlled residence | 63.06 | Per day | PSSRU – Unit Costs of Health and Social Care 201338 | |
| Local authority day centre | 39.87 | Per day | PSSRU – Unit Costs of Health and Social Care 201338 | |
| Community health and social care | ||||
| GP surgery | 44.59 | Per contact | PSSRU – Unit Costs of Health and Social Care 2015,39 p. 177 | |
| GP home visit | 138.35 | Per home visit | PSSRU – Unit Costs of Health and Social Care 2010,62 p. 167 | |
| GP telephone call | 27.36 | Per call | PSSRU – Unit Costs of Health and Social Care 2015,39 p. 177 | |
| District nurse | 38.51 | Per contact | PSSRU – Unit Costs of Health and Social Care 2015,39 p. 169 | |
| Health visitor | 54.72 | Per hour | PSSRU – Unit Costs of Health and Social Care 2015,39 p. 169 | |
| Social worker | 57.76 | Per contact (average) | PSSRU – Unit Costs of Health and Social Care 2015,39 p. 188 | |
| Community physiotherapist | 49.02 | Per contact | PSSRU – Unit Costs of Health and Social Care 201338 | |
| Occupational therapist | 74.63 | Per contact (average) | PSSRU – Unit Costs of Health and Social Care 2013,38 p. 176 | |
| Counsellor | 49.07 | Per hour | PSSRU – Unit Costs of Health and Social Care 2013,38 p. 54 | |
| Community psychologist | 60.32 | Per hour | PSSRU – Unit Costs of Health and Social Care 201338 | |
| Home help/care worker | 24.32 | Per hour | PSSRU – Unit Costs of Health and Social Care 2015,39 p. 201 | |
| Medication | ||||
| Various medications | Range £0.01 – £271.88 | Per dose | Prescription Cost Analysis – England, 2016 37 | |
| Equipment and adaptations | ||||
| Various aidsb | Range: 4.8 – 930.0 | Per item | National Catalogue (2014–15)41 | |
| Time point | EQ-5D-3L dimension, n (%) | EQ VAS | Utility | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mobility | Self care | Usual activities | Pain/discomfort | Anxiety/depression | |||||||||||||
| Level 1 | Level 2 | Level 3 | Level 1 | Level 2 | Level 3 | Level 1 | Level 2 | Level 3 | Level 1 | Level 2 | Level 3 | Level 1 | Level 2 | Level 3 | Mean (SE) | Mean (SE) | |
| Prior to hospital admissiona | |||||||||||||||||
| NIV (N = 122) | 63 (52) | 56 (46) | 3 (2) | 93 (76) | 27 (22) | 2 (2) | 69 (57) | 39 (32) | 14 (11) | 63 (52) | 43 (35) | 16 (13) | 74 (61) | 37 (30) | 10 (8) | 58.7 (1.98) | 0.66 (0.031) |
| IMV (N = 120) | 65 (54) | 49 (41) | 6 (5) | 88 (73) | 25 (21) | 7 (6) | 76 (63) | 35 (29) | 9 (8) | 66 (55) | 39 (33) | 15 (13) | 78 (65) | 34 (28) | 8 (7) | 60.7 (2.01) | 0.67 (0.033) |
| p-valueb | 0.491 | 0.733 | |||||||||||||||
| 3 months post ICU discharge | |||||||||||||||||
| NIV (N = 91) | 25 (27) | 66 (71) | 2 (2) | 53 (58) | 36 (40) | 3 (3) | 21 (23) | 52 (57) | 19 (21) | 26 (29) | 56 (62) | 11 (12) | 39 (43) | 45 (49) | 8 (9) | 60.9 (2.03) | 0.53 (0.035) |
| IMV (N = 88) | 24 (27) | 64 (71) | 2 (2) | 52 (59) | 35 (40) | 4 (5) | 21 (24) | 54 (61) | 15 (17) | 26 (30) | 55 (63) | 9 (10) | 49 (56) | 36 (41) | 5 (6) | 62.5 (2.11) | 0.56 (0.035) |
| p-valueb | 0.593 | 0.543 | |||||||||||||||
| 6 months post ICU discharge | |||||||||||||||||
| NIV (N = 91) | 28 (31) | 59 (65) | 4 (4) | 54 (59) | 32 (35) | 5 (5) | 27 (30) | 46 (51) | 19 (21) | 30 (33) | 47 (52) | 15 (16) | 45 (49) | 40 (44) | 6 (7) | 61.7 (2.35) | 0.53 (0.032) |
| IMV (N = 82) | 31 (38) | 50 (61) | 1 (1) | 53 (65) | 26 (32) | 2 (2) | 25 (30) | 48 (59) | 7 (9) | 33 (40) | 45 (55) | 3 (4) | 50 (61) | 26 (32) | 4 (5) | 65.0 (2.34) | 0.66 (0.039) |
| p-valueb | 0.377 | 0.0147 | |||||||||||||||
| Analyses | Incremental cost (£) | Incremental QALY | ICER | INMBa (£) | Probability of cost-effectivenessa |
|---|---|---|---|---|---|
| Base case (trial) | (302) | 0.01589 | NIV dominant | 779 | 0.59 |
| 1. Assuming costs and utilities are equal between groups after 6 monthsb | 2737 | 0.424 | £4651 | 10,748 | 1.0 |
| Sensitivity analyses | |||||
| 2. Decrease in extrapolated survival rates in NIV group by 10%, equal costs and utilities carried forwardc | 237 | 0.028 | £8393 | 603 | 0.96 |
| 3. Assuming costs and utilities are unequal between groups, costs and utilities carried forwardd | 6631 | 0.019 | £349,000 | (6061) | 0.01 |
| 4. Assumes costs are equal between groups, but utilities carried forwarde | 2737 | 0.019 | £144,052 | (2167) | 0.02 |
Glossary
- PaO2 : FiO2 ratio
- Ratio of arterial oxygen pressure to fraction of inspired oxygen.
List of abbreviations
- AE
- adverse event
- APACHE II
- Acute Physiology and Chronic Health Evaluation version II
- BHH
- Birmingham Heartlands Hospital
- BMI
- body mass index
- CAM-ICU
- Confusion Assessment Method-Intensive Care Unit
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CLRN
- comprehensive local research network
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- CPAP
- continuous positive airway pressure
- CRF
- case report form
- DHSC
- Department of Health and Social Care
- DMC
- Data Monitoring Committee
- EPAP
- expiratory positive airway pressure
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ VAS
- EuroQol visual analogue scale
- FiO2
- fraction of inspired oxygen
- GBP
- Great British pounds
- GCP
- good clinical practice
- GP
- general practitioner
- GST
- St Thomas’ Hospital, London
- HDU
- high-dependency unit
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICU
- intensive care unit
- IMV
- invasive mechanical ventilation
- INMB
- incremental net monetary benefit
- IQR
- interquartile range
- IRR
- incidence rate ratio
- MAR
- missing at random
- MI
- multiple imputation
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NIV
- non-invasive ventilation
- OR
- odds ratio
- PaCO2
- partial pressure of carbon dioxide in arterial blood
- PaO2
- partial pressure of oxygen in arterial blood
- PEEP
- positive end-expiratory pressure
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- Psupp
- pressure support
- QALY
- quality-adjusted life-year
- QEHB
- Queen Elizabeth Hospital, Birmingham
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- risk ratio
- SAE
- serious adverse event
- SBT
- spontaneous breathing trial
- SD
- standard deviation
- SE
- standard error
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-12
- Short Form questionnaire-12 items
- SpO2
- saturation of oxygen in peripheral blood
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAP
- ventilator-associated pneumonia
- VAS
- visual analogue scale
- WCTU
- Warwick Clinical Trials Unit
