Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/35/23. The contractual start date was in January 2014. The draft report began editorial review in October 2018 and was accepted for publication in February 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
T Justin Clark reports grants and personal fees from Hologic Inc. (Santa Clara, CA, USA), outside the submitted work, and membership of the Health Technology Assessment (HTA) Prioritisation Committee. John Norrie declares grants from the University of Aberdeen and the University of Edinburgh during the conduct of the study, and membership of the following National Institute for Health Research (NIHR) boards: HTA Commissioning Board (2010–16); NIHR HTA and Efficacy and Mechanism Evaluation Editorial Board (2014–19); HTA Commissioning Sub-board (Expression of Interest) (2016–present); HTA Funding Boards Policy Group (2016–present); HTA General Board (2016–present); HTA Post-board Funding Teleconference (2016–present); the Pre-exposure Prophylaxis Impact Review Panel (2018); and the NIHR Clinical Trials Unit Standing Advisory Committee (2018–present). Siladitya Bhattacharya is the Editor-in-Chief of HROpen and an Editor for Cochrane Gynaecology and Fertility.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Cooper et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Chapter 1 contains material reproduced from Cooper et al. 1 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
In 2014, the UK Government’s National Institute for Health Research Health Technology Assessment (HTA) programme funded the Hysterectomy or Endometrial AbLation Trial for Heavy menstrual bleeding (HEALTH). This report describes the research. HEALTH was a multicentre UK randomised controlled trial (RCT) investigating the clinical effectiveness (including safety) and cost-effectiveness of laparoscopic supracervical hysterectomy (LASH) compared with second-generation endometrial ablation (EA).
Background
Heavy menstrual bleeding (HMB) is a common problem, affecting approximately 1.5 million women in England and Wales. It accounts for one-fifth of all gynaecology outpatient referrals and has a major impact on women’s physical, emotional, social and material quality of life (QoL). 2 The condition is initially treated in primary care by either oral medication or insertion of the levonorgestrel-releasing intrauterine system (Mirena®; Bayer AG, Whippany, NJ, USA). If medical therapy fails or is deemed unsuitable, surgical treatment can be offered, either EA, which destroys the lining of the cavity of the uterus (endometrium), or hysterectomy (removal of the uterus). Neither medical treatment nor EA can guarantee complete resolution of symptoms and up to 59% of women on oral drugs3 and 13.5% of those using the levonorgestrel-releasing intrauterine system (Mirena®)4 ultimately undergo surgical treatment within 2 years. Over one-third (38%) of women referred to an English NHS hospital between April 2009 and March 2012 with HMB underwent a surgical procedure, with about three-quarters of these being EAs. 2 Of those treated with EA, 19% have required a subsequent hysterectomy for relief of their symptoms. 5
Scale of the problem in the UK and use of NHS resources
Hospital Episode Statistics data indicate that a total of 136,921 hysterectomies and 128,434 EAs were performed in England and Wales for HMB between April 1997 and December 2009. 6 Current types of EA that are recommended by the National Institute for Health and Care Excellence (NICE) are second-generation (non-hysteroscopic) procedures, including bipolar radiofrequency ablation (Novasure®; Hologic Inc., Marlborough, MA, USA) and thermal balloon EA. 7
Evidence explaining why this research is needed
The NICE guideline on HMB recommends both EA as well as hysterectomy as options for women with HMB resistant to medical treatment,7 but a significant minority of women treated with EA are likely to need repeat EA or hysterectomy. A recent individual patient data meta-analysis8 of results from randomised trials has shown that, despite the the greater invasiveness of conventional hysterectomy (removal of the uterus and the cervix) and associated longer hospital stay and prolonged recovery, fewer women are dissatisfied with it than with EA. Additionally, a health economic model based on these data showed that hysterectomy is more cost-effective. 9 The accompanying HTA monograph10 showed that a quarter of all women who undergo EA will require subsequent gynaecological surgery, with just under one-fifth requiring hysterectomy. These findings, which are consistent with those of a relevant Cochrane review,11 suggest that the optimal surgical treatment for HMB that is unresponsive to medical treatment may well be hysterectomy, but its effectiveness needs to be balanced against its invasive nature and increased short- and long-term morbidity. 5
Removal of the cervix as part of a conventional total hysterectomy can be technically challenging and result in injury to surrounding blood vessels, ureters and the bladder. In contrast, LASH is the use of keyhole surgery to remove the body or major part of the uterus, which is the part responsible for menstrual bleeding, but conserves the cervix. This approach reduces operating time as well as surgical morbidity, while conserving the uterosacral ligament support to the cervix and upper vagina. Routine cervical screening is required for women undergoing LASH. The procedure is quick, minimally invasive, relatively easy to learn and associated with a low risk of complications, short hospital stay (< 24 hours) and rapid recovery. 12,13
Before this technique is incorporated into routine clinical practice, it is important that it is subjected to robust evaluation. The authors of two small RCTs comparing LASH with a first-generation EA (endometrial resection13 or second-generation EA) thermal balloon12 suggest that LASH could lead to a better QoL, but emphasised the need for larger evaluative studies to confirm this.
The last decade has seen widespread use of laparoscopic techniques in gynaecology due to increased familiarity with the procedures, more sophisticated instruments, better training and greater laparoscopic surgical skill. As a result of this, LASH could be delivered by most general gynaecologists, with minimal morbidity to women who are currently being treated with EA. Advances in perioperative care mean that, unlike conventional hysterectomy, women treated by this procedure may not need to stay in hospital any longer than those receiving EA.
HEALTH is a multicentre RCT comparing the clinical effectiveness and cost-effectiveness of LASH with second-generation EA (the current first-line surgical treatment for HMB) in women with HMB seeking surgery. The trial is relevant and timely, as rigorous evaluation of this new surgical option will provide much needed high-quality evidence to underpin any decision to use it in routine NHS practice.
Description of the surgical procedures
Laparoscopic supracervical hysterectomy
Laparoscopic supracervical hysterectomy involves keyhole surgery to remove the upper part of the uterus (the body). The uterine body contains the endometrial cavity lined with tissue that undergoes cyclic growth and shedding each month, thus causing menstrual bleeding. Increased access to specialised laparoscopic equipment and training means that LASH is quick and relatively easy to learn. It is associated with low morbidity, short hospital stay (< 24 hours) and rapid recovery. Unlike conventional total hysterectomy, the cervix is not removed, thus removing the need for extended surgery around the cervix, which can lead to serious complications, such as injury to the bladder, ureters and blood vessels. 14,15 As the cervix is retained, cervical smears are still required and, although most women will cease to have periods after the procedure, light menstrual loss or cyclical spotting can occur in 5–20% of cases. 16,17
The body of the uterus is usually removed though a small 10- to 12-mm incision, often within the umbilicus or suprapubically by means of a ‘power’ morcellator, which breaks up the uterine tissue into small strips. Alternative options include removal of the uterine body through an internal incision at the top of the vagina (culdotomy) or, alternatively, morcellation within an intraperitoneal bag to prevent spread of fragmented tissue within the peritoneal cavity.
Second-generation endometrial ablation
Endometrial ablation aims to treat HMB by destroying the endometrium (lining of the womb), which is responsible for heavy periods. Historically, a number of methods have been used to achieve this. First-generation EA techniques use an operating hysteroscope under direct vision. Energy is deployed through an electric loop, laser fibre or rollerball to remove or destroy the endometrium. First-generation techniques are more complicated, require a long learning curve, are slower to perform and have a higher risk profile than second-generation techniques. They are, however, highly versatile and are the recommended approach for distorted uterine cavities or repeat ablations, and are a sensible option when the patient has had more than one caesarean section.
This century, ‘second-generation’ techniques have become the most commonly used endometrial ablative procedures, as they are quicker to learn and undertake, and have lower associated risks. These are blind global energy sources which again aim to destroy the endometrium and superficial myometrium to a depth of 5 mm (to destroy the endometrial glands). Current second-generation procedures used in the UK include two forms of thermal balloon EA and a device known as Novasure. Thermal balloon EA is undertaken by means of a silicone balloon, which is introduced through the cervix into the uterine cavity. The balloon fills and expands to conform to the inside of the uterine cavity, compressing the endometrium. Hot fluid circulating within the balloon ensures endometrial destruction and the temperature and duration of treatment are carefully controlled electronically by means of a computer attached to the device. Novasure uses bipolar radiofrequency energy delivered through an intrauterine mesh electrode that expands on insertion through the cervix to fit the shape of the uterine cavity. The energy required is calculated by the device and treatment times are < 90 seconds. All three treatments significantly reduce menstrual loss and result in complete cessation of bleeding in 40–50% of women. 7 Second-generation EA can be performed as a day-case procedure, under either general or local anaesthetic, at a NHS cost of £995 per treatment. 18 It has also been widely used in the outpatient setting. 19,20
Questions addressed by HEALTH
The aim of this study is to compare the clinical effectiveness and cost-effectiveness of LASH and second-generation EA in women with HMB.
The primary objective is to compare (1) patient-reported satisfaction, measured on a six-point Likert scale (from ‘totally satisfied’ to ‘totally dissatisfied’) and (2) condition-specific QoL, measured using the Menorrhagia Multi-Attribute Quality-of-Life Scale (MMAS), at 15 months post randomisation. The corresponding economic objective is to estimate the incremental cost per quality-adjusted life-year (QALY) gained for LASH compared with EA at 15 months post randomisation.
The hypothesis being tested is that LASH is superior to second-generation EA for the treatment of HMB in terms of patient satisfaction, QoL and costs.
Chapter 2 Methods and practical arrangements
Study design
HEALTH was a parallel-group, multicentre RCT designed to compare the clinical effectiveness and cost-effectiveness of LASH with second-generation EA in women with HMB. Further details of the study design have been described previously1 and are represented in Figure 1. All trial case report forms (CRFs) and participant-completed questionnaires are available at URL: www.journalslibrary.nihr.ac.uk/programmes/hta/123523/#/ (accessed 22 May 2019).
FIGURE 1.
HEALTH flow diagram. EQ-5D-3L, EuroQol-5 Dimensions, three-level version; NRS, Numerical Rating Scale; SF-12, Short Form questionnaire-12 items. Reproduced from Cooper et al. 1 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
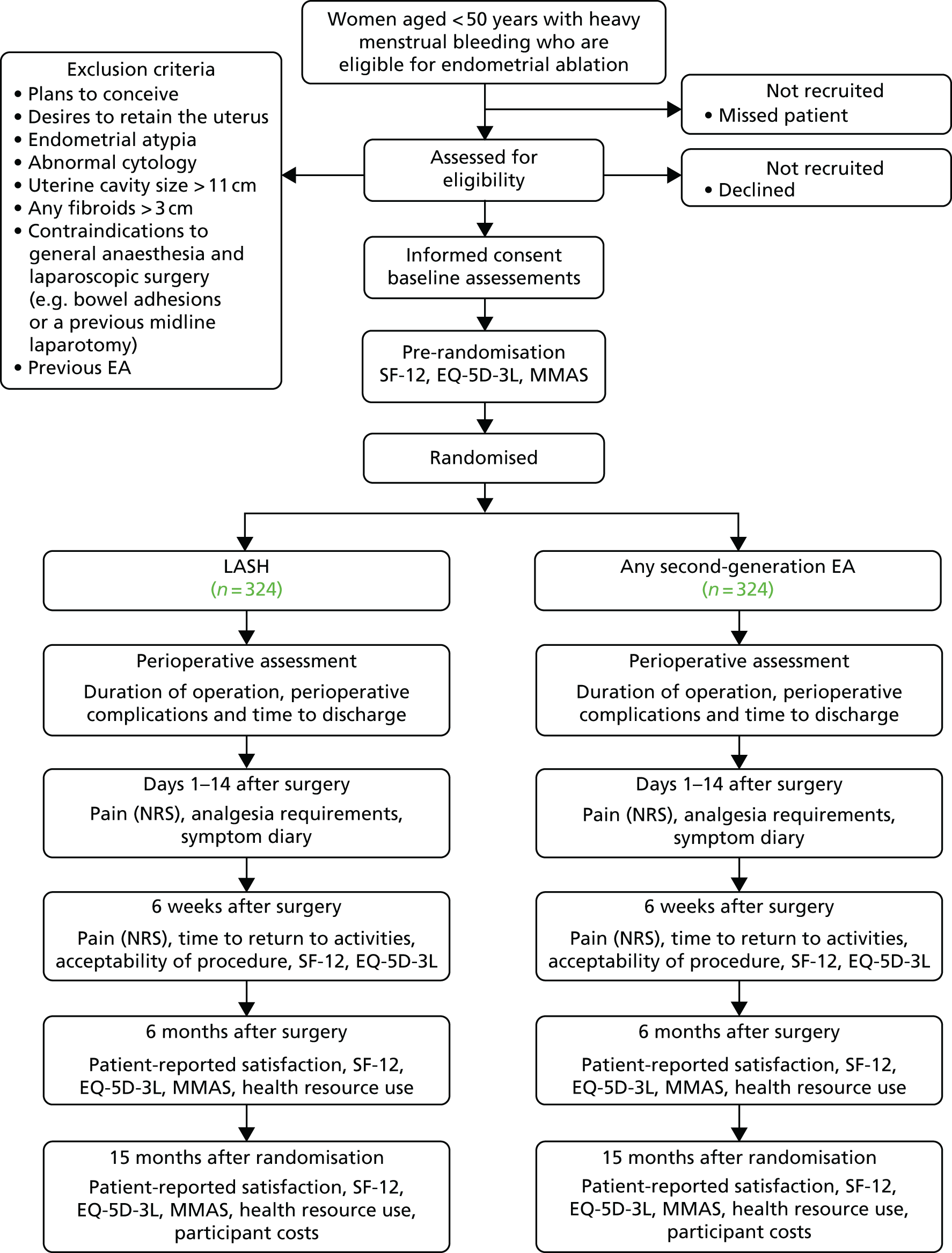
Study population
Women aged < 50 years with HMB who were eligible for EA and willing to be randomised between LASH and EA.
Women were excluded from trial entry if any of the following criteria were met: they had plans to conceive; endometrial atypia; abnormal cytology; uterine cavity size > 11 cm; any fibroids > 3 cm; contraindications to laparoscopic surgery; previous EA; and inability to give informed consent or complete trial paperwork.
Recruitment
Investigations prior to consent
Pelvic ultrasound scanning was undertaken to identify uterine or endometrial abnormality, fibroid size and number. An endometrial biopsy was taken to measure cavity length and exclude endometrial atypia.
Consent to participate
Women with HMB who fulfilled the inclusion criteria were identified at gynaecology outpatient and pre-assessment clinics. They were supplied with the patient information leaflet and given the opportunity to discuss the study with the local clinical team, family and friends, and their general practitioner (GP), as appropriate. Women could make the decision to participate during their initial consultation, during a subsequent hospital visit or after a follow-up telephone consultation at home. Written informed consent was obtained from all participants prior to trial entry.
Health technologies being compared
Women were randomised to one of two surgical treatments for HMB:
-
LASH [removal of the uterine corpus (body) by means of keyhole surgery]
-
second-generation EA [destroying the endometrium (lining of the womb) by means of a silicone balloon containing hot fluid or radiofrequency energy delivered through an intrauterine mesh electrode].
Treatment allocation
Eligible and consenting women were randomised to one of the two treatment arms in a 1 : 1 allocation ratio, using the randomisation application at the trial office at the Centre for Healthcare and Randomised Trials (CHaRT). The randomisation application was available as both an interactive voice-response telephone system and as an internet-based application, and used a minimisation algorithm based on centre and age group (< 40 vs. ≥ 40 years). 21
Blinding
Baseline data were reported by women before randomisation using self-completed questionnaires. Surgeons and participants could not be blinded to the allocated procedure.
Delivery of the intervention
Following randomisation, participants were placed on the waiting list for the appropriate treatment. As per Scottish and UK government guidelines, it was anticipated that treatment would occur within 12–18 weeks of randomisation. 22–24 Surgeons used their standard practice so that the technique they normally used was not modified for the purposes of the trial. All other aspects of care were left to the discretion of the responsible surgeon.
Data collection during follow-up
Participant-reported outcomes were assessed by self-completed questionnaires at baseline (before surgery), 6 weeks and 6 months after surgery, and 15 months following randomisation (Table 1). A self-completed 14-day diary was also collected. Up to two reminders were sent to participants by post, e-mail, telephone or text message, taking into account any preferences they had for mode of communication.
| Outcome | Pre randomisation (baseline) | Surgery | Post surgery | Post randomisation (15 months) | ||
|---|---|---|---|---|---|---|
| Days 1–14 | 6 weeks | 6 months | ||||
| CRF | ✗ | |||||
| Surgical details | ✗ | |||||
| Pain NRS symptom diary | ✗ | |||||
| Pain NRS | ✗ | |||||
| Time to return to normal activities | ✗ | |||||
| Acceptability | ✗ | |||||
| Satisfaction | ✗ | ✗ | ||||
| MMAS score | ✗ | ✗ | ✗ | |||
| Menstrual outcomes | ✗ | ✗ | ✗ | |||
| EQ-5D-3L and SF-12 scores | ✗ | ✗ | ✗ | ✗ | ||
| Health-care utilisation | ✗ | ✗ | ||||
| Participant costs | ✗ | |||||
Intraoperative and postoperative data were collected by the local research team at the time of the randomised procedure. A short CRF was also completed for any related hospital readmissions during the follow-up period.
Study outcome measures
The co-primary clinical outcome measures were:
-
patient satisfaction, measured on a six-point scale (from ‘totally satisfied’ to ‘totally dissatisfied’), at 15 months after randomisation
-
MMAS score, a condition-specific QoL outcome,25 ranging from 0 (worst possible health state) to 100 (best possible health state), based on six items, measured at 15 months after randomisation.
The primary study objective was to compare the LASH and EA groups with respect to the two co-primary outcomes. These outcomes were addressed in a hierarchy. First, the patient satisfaction outcome was considered and, if this showed a statistically significant difference at a p-value of < 0.05, then the MMAS score outcome was also considered. By specifying this hierarchy, it was not necessary to apply any adjustment for multiple statistical testing, as the overall false-positive rate is controlled at 5%.
The 15-month follow-up post-randomisation time point was chosen to accommodate the 12- to 18-week waiting time for treatment. The intention was that the primary outcome questionnaire (triggered at 15 months post randomisation) would be completed at approximately 12 months post surgery, in order to facilitate comparison with the most similar RCTs in the literature.
The primary economic outcome was the incremental cost (to the health service) per QALY gained (LASH vs. EA).
Secondary outcome measures
Other outcome measures included pain score at days 1–14 and at 6 weeks post surgery; acceptability of treatment at 6 weeks post surgery; satisfaction with treatment at 6 months post surgery; MMAS score at 6 months post surgery; menstrual outcomes at 6 months post surgery and at 15 months post randomisation; generic health-related quality of life (HRQoL) [Short Form questionnaire-12 items (SF-12) and EuroQol-5 Dimensions, three-level version (EQ-5D-3L), scores at 6 weeks and 6 months post surgery and at 15 months post randomisation]; perioperative complications, including recovery details and need for additional gynaecological surgery; cost; and cost-effectiveness.
Pathology results for all endometrial biopsies and uterine specimens were also checked.
Statistical analyses were used to compare secondary outcomes by randomised groups. These analyses, however, were regarded as hypothesis-generating and no adjustment was made for multiple statistical testing.
Safety reporting
Adverse events (AEs) were either notified to the study office by the local research team or reported by the women in their follow-up questionnaires. If an AE was suspected, it was verified by the local research team, if possible. Unrelated AEs were not recorded.
In HEALTH, ‘relatedness’ was defined as an event that occurred as a result of a procedure required by the protocol, whether or not it was either (a) the specific intervention under investigation or (b) administered outside the study as part of normal care.
The following events were also potentially expected: admission to a high-dependency unit/intensive care; emergency hysterectomy; laparotomy; port site hernia; blood transfusion; wound infection; lower urinary tract infection; endometritis; blood stained vaginal discharge; anaesthetic complications; low-grade pyrexia; blood loss; haematoma; constipation; pelvic discomfort/pain; internal bleeding or injury; deep-vein thrombosis; pulmonary embolism; injury to the wall of the uterus; bladder/bowel/ureteric injury; and voiding dysfunction.
Any serious adverse events (SAEs) related to the participants’ HMB treatment that were not further interventions (e.g. being admitted to hospital for an infection) were recorded on the SAE form. Hospital visits that were associated with further interventions due to HMB (e.g. further surgery) were recorded as an outcome measure. All deaths from any cause (related or otherwise) were recorded on the SAE form.
It was a requirement to report to the sponsor any SAEs that were deemed related and unexpected within 24 hours of receiving the signed SAE notification. Such SAEs would also be reported to the main Research Ethics Committee within 15 days of the chief investigator becoming aware of the event. All related SAEs were summarised and reported to the appropriate authorities within their regular progress reports.
Sample size
An individual participant data meta-analysis of abdominal hysterectomy compared with first-generation EA8 suggested a target difference of an odds ratio (OR) of 2.84 (95% vs. 87%) for patient satisfaction. Such an OR also equates to a medium-sized standardised effect (Cohen’s d). It was calculated that 292 participants per group would provide 90% power to detect a difference in total satisfaction rates of 8% (87% vs. 95%), using a two-sided continuity-corrected chi-squared test. This would also allow > 90% power to detect a 10-point difference in MMAS scores [assuming a standard deviation (SD) of 33 units]. Based on an expected 10% drop-out rate, the recruitment target was 648 participants in total (324 participants per group).
Owing to changes to the analysis plan during the trial, the implications for these calculations were later revisited (see Statistical analysis).
Data Monitoring Committee
The independent Data Monitoring Committee (DMC), which consisted of a methodologist, a clinician and a statistician, met on four occasions during the trial and considered interim reports of trial data by randomised groups (denoted as group 1 and group 2). On each occasion they agreed that the trial should continue as planned. At the final meeting, which took place in September 2017, after recruitment had ended, the DMC was shown the distributions of the primary outcomes using interim data.
Statistical analysis
Analyses were conducted using Stata® version 15 (StataCorp LP, College Station, TX, USA). Categorical variables were described with the number and percentage in each category. Continuous variables were described using mean and SD or median and interquartile range (IQR), depending on the distribution of data.
Analyses were based on the intention-to-treat principle, analysing women in the groups to which they were randomised. Analyses used a two-sided 5% significance level, with corresponding 95% confidence intervals (CIs) generated as appropriate [see URL: www.journalslibrary.nihr.ac.uk/programmes/hta/123523/#/ (accessed 22 May 2019)].
Analyses of the two co-primary outcomes (patient satisfaction and MMAS at 15 months after randomisation) were conducted independently.
Patient satisfaction was collected on a six-point scale: ‘totally satisfied’, ‘generally satisfied’, ‘fairly satisfied’, ‘fairly dissatisfied’, ‘generally dissatisfied’ or ‘totally dissatisfied’. The original analysis plan specified that patient satisfaction be treated as a binary variable, but after considering the distribution of interim data (September 2017), and to make better use of the data collected, the DMC requested that this outcome be treated as ordinal with four categories (‘totally satisfied’, ‘generally satisfied’, ‘fairly satisfied’, with the remaining three categories combined into a single ‘dissatisfied’ category), and analysed using an ordinal logistic regression (OLR) model with adjustment for minimisation factors: age (< 40 vs. ≥ 40 years) and centre (treated as a random effect).
We originally intended the MMAS score to be treated as a continuous outcome and analysed using a linear regression model, adjusted for baseline MMAS scores (treated as a continuous variable) and the two minimisation factors (age and centre). However, interim data presented to the DMC were highly skewed, with over half of all trial participants reporting a maximum MMAS score of 100 at 15 months post randomisation. After discussion, the DMC recommended that the analysis of this outcome be changed to an OLR model with four categories (0–50, 51–75, 76–99, 100).
The Project Management Group (PMG) accepted the recommended changes to the analysis of the primary outcomes after viewing the distribution of these outcomes with both groups combined. The decision to change the analysis method for both co-primary outcomes to an ordered categorical analysis had implications for the power of the study. It seemed appropriate to continue to use a common OR of 2.84 in the revised power calculations. Based on the formulae in a report by Whitehead,26 assuming a common OR of 2.84 and using the expected proportions in each category from interim trial data with treatment groups combined, a total of 292 participants per group would have > 90% power to detect statistically significant differences between the randomised groups for patient satisfaction. There were no accurate data on which to base our decisions regarding the MMAS outcome, so a pragmatic decision was made to use the same OR for this outcome. Both outcomes had similar distributions after classification into four categories and were addressed in a hierarchy (MMAS score was considered only if patient satisfaction showed a statistically significant difference). Therefore, it was considered unnecessary to recruit additional participants to the trial.
The MMAS total score was calculated only if the six constituent items were completed. There does not appear to be a precedent in the literature for imputing scores for MMAS items from other items as the response category weighting varies by item. In addition, to account for the nature of the treatments being offered (i.e. the vast majority of the women in the LASH arm and 40–50% of the women in the EA arm would be expected to be amenorrhoeic following treatment), the instructions for completion of the MMAS were altered slightly before the start of the study [see URL: www.journalslibrary.nihr.ac.uk/programmes/hta/123523/#/ (accessed 22 May 2019)].
It was expected that a small number of women might have to wait longer than 12–18 weeks for their operation and therefore would receive their 6 months post-surgery and 15 months post-randomisation questionnaires simultaneously. It was therefore agreed that scores for the co-primary outcomes and the main outcome used in the economic analysis (EQ-5D-3L utility score) could be used from the 6-month post-surgery questionnaire in lieu of the 15-month data, provided these were provided within 3 months of the due date of the latter.
A sensitivity analysis using a multiple imputation approach was used to explore the impact of missing data on the robustness of the results of the analyses of co-primary outcomes. Missing values for the outcome and for one covariate (MMAS score at baseline) were imputed using multiple imputation by chained equations using the mi package in Stata. 27 Twenty imputed data sets were created. There were no missing data for the other covariates (age group and centre), as these were required for the minimisation algorithm.
A further sensitivity analysis using the results of the original analysis method for MMAS score (i.e. a linear regression model treating MMAS score as a continuous outcome) was also presented.
Exploratory subgroup analyses were performed for the following groups: uterine cavity length (≤ 8 cm vs. > 8 cm), menstrual pain (dysmenorrhoea) at baseline (severe/crippling pain vs. other categories, determined using a five-point Likert scale), patient age (< 40 years vs. ≥ 40 years) and presence or absence of fibroids. These prespecified subgroup analyses were conducted by including a treatment by subgroup interaction term in the corresponding OLR model for the co-primary outcomes (patient satisfaction and MMAS score at 15 months post randomisation). The effect sizes from these subgroup analyses were displayed graphically in a forest plot, along with results for each recruiting centre and whether or not the operator was a consultant. Centres with fewer than 50 randomised participants were included in an ‘other centre’ category.
Secondary outcomes were analysed using generalised linear models (GLMs), adjusted for the minimisation factors and, when appropriate, a baseline measure. Continuous outcomes were analysed using linear regression, binary outcomes using logistic regression and ordered categorical outcomes using OLR. As many continuous outcomes had an extreme skewed distribution, an ordered categorical analysis had to be used instead. The category thresholds were decided by the PMG after viewing the distribution of the outcome of both groups combined.
Time-to-event outcomes such as time until return to normal activities (e.g. paid work for those in employment) were analysed using Cox proportional hazards regression models.
Pain data from the participant diaries (collected in the first 14 days after the operation) were analysed using a repeated-measures model.
All AEs were described using the number and percentage within each randomised group. All expected SAEs and other SAEs were recorded in detail and the number and percentage in each randomised group reported.
Economic evaluation
The economic analysis consisted of a trial-based analysis of individual patient-level cost and effect (QALY), and a decision modelling component to inform cost-effectiveness in the longer term. See Chapters 5 and 6 for a detailed description of the methods used.
Management of the study
The trial management team, based within CHaRT, University of Aberdeen, provided day-to-day support for the recruiting centres led by a local principal investigator (PI). The PIs, supported by dedicated research nurses, were responsible for all aspects of local organisation, including recruitment of participants, delivery of the interventions and notification of any problems or unexpected developments during the study period.
The study was supervised by the PMG, which consisted of representatives from the study office and grant holders. The study was further overseen by a Trial Steering Committee (TSC), which comprised four independent members and an independent DMC (see Data Monitoring Committee).
Patient and public involvement
Pre-funding application and design of the research
Prior to the initial funding application, we sought support from the Royal College of Obstetricians and Gynaecologists (RCOG) Women’s Network (URL: www.rcog.org.uk/our-profession/community/committees/rcog-womens-network, accessed 11 January 2013), a group of professional laywomen who work to advise and support the RCOG on women’s perspectives on obstetrics and gynaecology. The proposal was discussed at the Women’s Network meeting prior to the original application and the group fed back their comments to the rest of the research team. In addition, the vice chairperson of the RCOG Women’s Network was a co-applicant on the grant and gave input into the application and continued to advise the study PMG until October 2016.
Oversight of the study
One of the independent members of the TSC was a patient representative. The TSC met throughout the study and reviewed all study documentation, including patient-facing documents, newsletters and questionnaires that were sent to potential and recruited participants in HEALTH. In addition to being an integral part of the study oversight, she provided the following feedback on what she felt were the key impacts and value of her recent contributions:
In my role as a patient representative I am a member of the HEALTH TSC and attend annual meetings as scheduled. I maintain an interest in all of the various aspects of the HEALTH trial but with a particular interest in patient-related issues and the drafting of patient information, etc. In 2017 a major problem arose when the success of the trial was threatened by the low return rate of the 12-month questionnaire. Three actions were then agreed. First, I was involved with drafting a new covering letter to be sent out with the questionnaire, the main aim of which was to emphasise the importance of returning the necessary information otherwise the trial would fail, and the main constraint of which was to avoid pressurising the women. Second, approval would be sought to offer the women a monetary incentive to return the questionnaire. Third, I drafted a supporting submission from the patient perspective to the Ethics Committee seeking approval for the above two actions.
Report writing, academic paper preparation and dissemination
The patient and public involvement partner on the TSC has been actively involved in discussions of the trial results with the TSC, and has been supportive of the study in report preparation and has contributed towards the preparation of the Plain English summary. The partner will continue to be involved in dissemination activities and preparation of results dissemination to participants and academic papers.
Challenges in patient and public involvement
At the end of the study the patient and public involvement partner reflected on their input and made suggestions for possible improvements for future trials in this area:
One potential limitation might certainly be when there is only one PPI [patient and public involvement] partner on a trial. Perhaps the more important issues are the PP [patient and public] representative(s)’s (PPR’s) background, understanding and commitment, but having more than one view or interpretation should offer a wider perspective of perceived patient need and possibly areas in which greater clarity is needed to ensure patient understanding, as far as possible.
My involvement in trials has been as a member of the Project Management Group or of the Trials Steering Committee. As a member of the latter I have felt somewhat distanced from the projects because of the time lapse between meetings, normally around 12 months, and the lack of information during that interim period. I think I would have found it helpful to have had some continuity such as sight of, for instance, a copy of the PMG minutes, proposed changes to the Protocol, new developments etc. As patient representatives do not have a presence in the workplace, they obviously miss out on the various pieces of information that are discussed and circulated.
A patient representative can be used as a ‘bridge’ between the trials team and the Ethics Committee, when appropriate. For instance, my experience in some of the trials with which I have been involved has included producing a statement or proposal to assist in gaining approval from the Ethics Committee, an example of which is noted in the paragraph on ‘Oversight of the study’ above. I think this sort of direct contact can be very useful in conveying the patients’ perspective to the Ethics Committee so as to further inform the latter as to the appropriateness of certain actions required of patients to assist in, say, the successful outcome of a trial which is the major aim.
Chapter 3 Baseline results
This chapter describes how the women were identified from 31 UK hospitals (see Appendix 1, Table 26) and reports the baseline characteristics up to the point of study entry. The subsequent findings are described in Chapters 4 and 5.
The flow of women through the study is shown in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 2) in line with the CONSORT recommendations. 28
FIGURE 2.
Flow of participants to the point of randomisation.
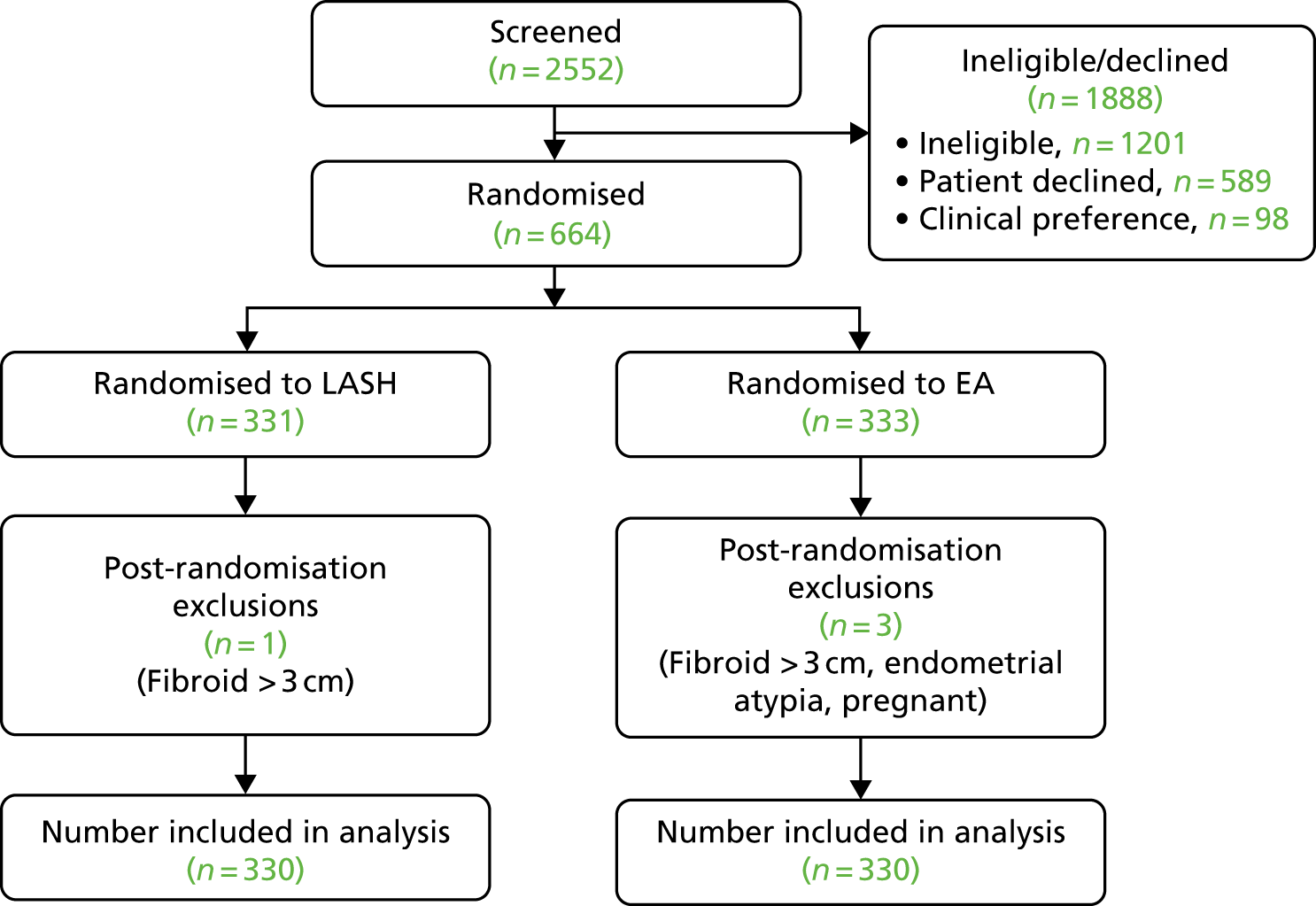
Between May 2014 and March 2017, 2552 potentially eligible patients were screened; 1351 (52.9%) were confirmed as eligible, of whom 664 (49.1%) gave their consent and were randomised, 331 to LASH and 333 to EA (see Figure 2).
After randomisation, four women were considered to be ineligible, regarded as post-randomisation exclusions and not included in any trial analyses. Therefore, 660 women (330 in each group) were included in the main trial analyses (see Figure 2).
Study recruitment
Study design and recruitment methodology have been described previously1 (see also Chapter 2). Women with HMB who attended gynaecology outpatients and pre-assessment clinics, and who were eligible for EA, were invited to participate in HEALTH. Women were asked if they would be willing to be randomised to either LASH or second-generation EA. The centres that randomised women into HEALTH, including numbers recruited by centre, are described in Appendix 1, Table 26. The recruitment rate is illustrated in Figure 3.
FIGURE 3.
Recruitment to the trial over time.
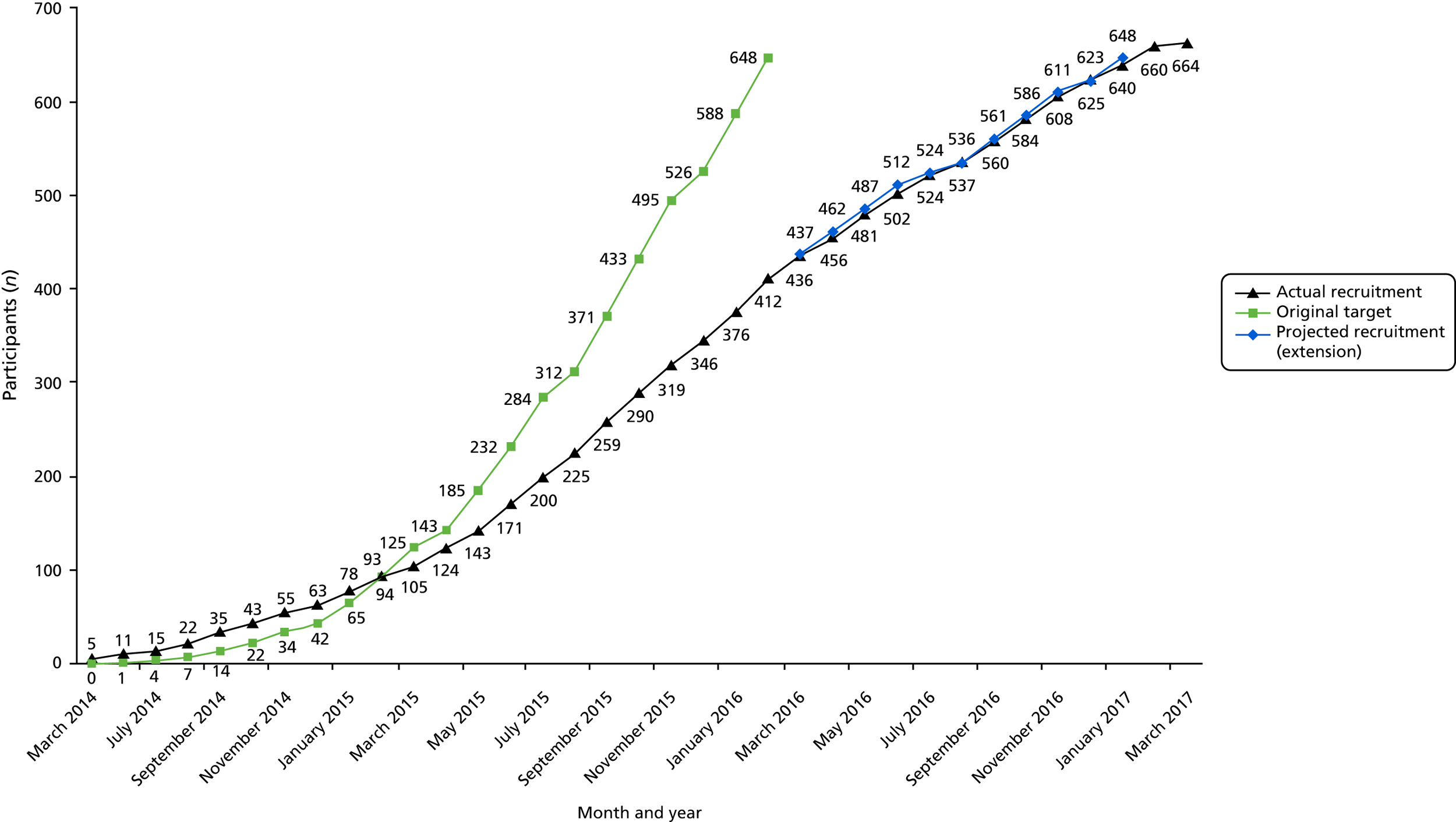
Non-recruited women
Of the 2552 women approached, 1888 (74.0%) did not participate in the trial because they were ineligible (n = 1201, 47.1%) or declined participation (n = 589, 23.1%) or because their consultant later indicated a preference for a particular treatment (n = 98, 3.8%) (see Figure 2 and Appendix 1, Table 27). The most common reasons for women declining to take part in the study were a preference for a particular treatment [preference for EA, n = 151 (25.6%); preference for hysterectomy, n = 126 (21.4%); preference for medical management, n = 89 (15.1%)] and an unwillingness to accept randomisation (n = 54, 9.2%). Ninety-eight women (16.6%) did not give a reason for declining to take part (see Appendix 1, Table 28).
Reasons for ineligibility included ‘fibroids > 3cm’ (n = 361, 30.1%), a preference to continue with medical management (n = 244, 20.3%) and age > 50 years (n = 239, 19.9%). In addition, 98 women who were deemed eligible for HEALTH were not included for other clinical reasons. In the majority of cases, this was because a treatment pathway had already been decided prior to study entry [hysterectomy, n = 25 (25.5%); EA, n = 18 (18.4%); and ‘other’ treatment, n = 16 (16.3%)] (see Appendix 1, Table 28).
Randomised participants: baseline characteristics
The baseline characteristics of the 660 women who agreed to participate in HEALTH and who were truly eligible to take part are described in Tables 2–4 and Appendix 1, Table 29.
| Baseline characteristic | LASH (N = 330) | EA (N = 330) |
|---|---|---|
| Age (years), mean (SD) [n] | 42.2 (4.89) [330] | 42.1 (4.96) [330] |
| BMI (kg/m2), mean (SD) [n] | 29.1 (5.55) [309] | 29.0 (5.34) [304] |
| Preoperative haemoglobin level (g/l), mean (SD) [n] | 131.0 (13.1) [306] | 130.1 (12.6) [282] |
| Number of vaginal deliveries, median (IQR) [n] | 2 (1–3) [326] | 2 (1–3) [330] |
| Number of caesareans, median (IQR) [n] | 0 (0–1) [326] | 0 (0–1) [327] |
| Menstrual outcome | LASH (N = 330) | EA (N = 330) |
|---|---|---|
| How long have you had trouble with your periods?, n (%) | ||
| < 1 year | 16 (4.9) | 18 (5.5) |
| 1–3 years | 84 (25.7) | 93 (28.4) |
| > 3 years | 227 (69.4) | 216 (66.1) |
| Description of period, n (%) | ||
| Light | 2 (0.6) | 0 |
| Moderate | 6 (1.8) | 7 (2.1) |
| Heavy with clots | 58 (17.7) | 61 (18.7) |
| Very heavy with clots and flooding | 261 (79.8) | 259 (79.2) |
| On average, for how many days is the bleeding heavy?, n (%) | ||
| Not heavy | 3 (0.9) | 2 (0.6) |
| 1–3 days | 50 (15.3) | 51 (15.6) |
| 4–6 days | 118 (36.1) | 125 (38.2) |
| ≥ 7 days | 156 (47.7) | 149 (45.6) |
| At any time in the last 3 months have you needed to use more than one form of sanitary protection at a time?, n (%) | ||
| No | 27 (8.3) | 25 (7.7) |
| Tampon and pad | 117 (35.9) | 118 (36.3) |
| Two pads | 88 (27.0) | 84 (25.8) |
| Tampon and two pads | 48 (14.7) | 56 (17.2) |
| More than this (e.g. bath towel) | 46 (14.1) | 42 (12.9) |
| Are your periods usually painful?, n (%) | ||
| No | 19 (5.8) | 18 (5.5) |
| Mild pain | 33 (10.1) | 38 (11.7) |
| Moderate pain | 104 (31.9) | 110 (33.7) |
| Severe/crippling pain | 170 (52.1) | 160 (49.1) |
| Bleeding (mean score of up to 10 days of period, 0 = no bleeding, 5 = worst bleeding), mean (SD) [n] | 3.59 (0.88) [322] | 3.55 (0.78) [322] |
| Pain (mean score of up to 10 days of period, 0 = no pain, 5 = worst pain), mean (SD) [n] | 2.76 (1.27) [311] | 2.70 (1.30) [313] |
| What do you want from the operation?, n (%) | ||
| No periods | 265 (82.6) | 253 (78.6) |
| Light periods | 29 (9.0) | 38 (11.8) |
| Normal periods | 27 (8.4) | 31 (9.6) |
| QoL score | LASH (N = 330) | EA (N = 330) |
|---|---|---|
| MMAS | ||
| Total score | ||
| Mean (SD) | 30.5 (19.0) | 32.3 (20.0) |
| Median (IQR) | 28.6 (14.7–43.7) | 29 (15.7–47.7) |
| n | 323 | 321 |
| EQ-5D-3L | ||
| Utility score | ||
| Mean (SD) | 0.71 (0.30) | 0.70 (0.31) |
| Median (IQR) | 0.76 (0.66–1.00) | 0.79 (0.69–1.00) |
| n | 319 | 322 |
| VAS | ||
| Mean (SD) | 65.2 (24.2) | 67.2 (23.5) |
| Median (IQR) | 70 (50–85) | 70 (52–85) |
| n | 317 | 321 |
| SF-12 | ||
| PCS | ||
| Mean (SD) | 45.0 (9.0) | 44.9 (9.7) |
| Median (IQR) | 45.8 (39.0–52.1) | 46.5 (39.0–51.9) |
| n | 318 | 321 |
| MCS | ||
| Mean (SD) | 37.2 (11.0) | 38.7 (11.6) |
| Median (IQR) | 36.6 (29.8–45.1) | 29 (15.7–47.7) |
| n | 318 | 321 |
Participant characteristics
The two randomised groups were comparable at baseline. On average, women were around 42 years of age when considering surgical treatment for their HMB symptoms. There was no difference between the randomised groups in terms of age, body mass index or preoperative haemoglobin levels (see Table 2).
Heavy menstrual bleeding symptoms at baseline
Of the women who participated in HEALTH, 67.7% (443/654) had experienced trouble with their periods for > 3 years and the majority of the women (79.5%) described their periods as very heavy with clots and flooding (see Table 3). Just under half of the women (46.6%) described heavy bleeding for ≥ 7 days and 50.6% had experienced severe/crippling pain during their periods. Almost 80% of the women hoped the operation would stop their periods completely.
The randomised groups were comparable with respect to other characteristics, including mean bleeding score and mean pain score (see Table 3).
Generic quality of life at baseline
There were no differences between the randomised groups in either the MMAS total score, EQ-5D-3L utility score, EQ-5D-3L visual analogue scale (VAS), SF-12 physical component score (PCS) or SF-12 mental component score (MCS) at baseline (see Table 4).
Chapter 4 Clinical results
This chapter describes the main clinical findings of HEALTH. Details of the trial operations are presented first. This is followed by the results for the two co-primary outcomes (patient satisfaction and MMAS score at 15 months post randomisation). Finally, the results of the secondary outcomes are provided in chronological order. All results are presented by allocated randomised group (i.e. according to intention to treat).
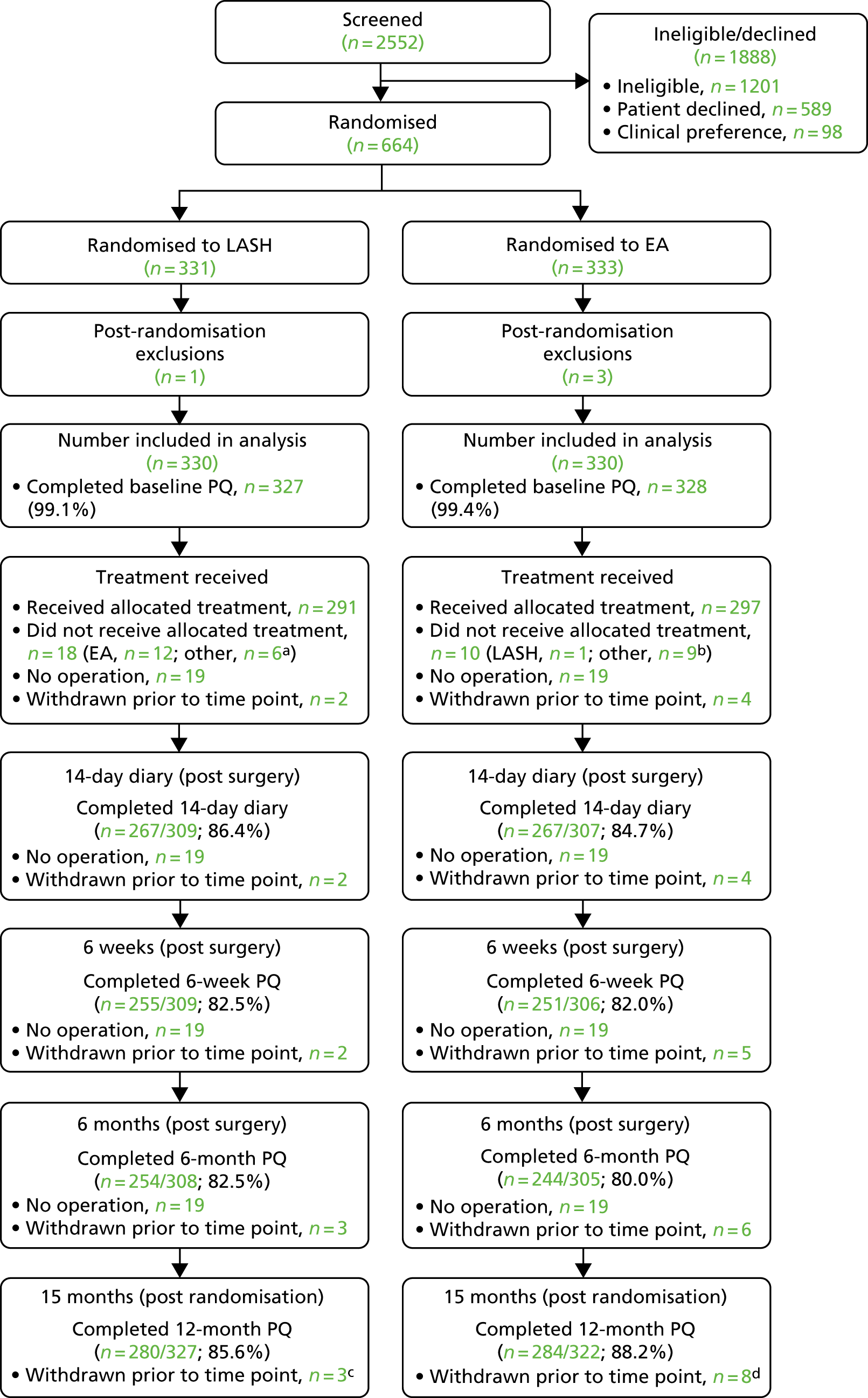
Flow of participants through the trial
The CONSORT flow diagram shows the number of participants providing data at each stage of the trial (Figure 4). Response rates are based on the number receiving an operation (14-day diary, 6-week and 6-month questionnaire) or the number randomised (15-month questionnaire), after accounting for withdrawals. Questionnaire completion rates ranged between 80% and 89% (exact return rates are reported in the CONSORT flow diagram; see Figure 4).
FIGURE 4.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. a, Other operations (LASH group): total hysterectomy (n = 5), EA (n = 1), hysteroscopy/polypectomy (n = 1); b, other operations (EA group): total hysterectomy (n = 5), hysterectomy/polypectomy (n = 4); c, reasons included: unwillingness to have surgery (n = 1), private treatment (n = 1), no reason given (n = 1); and d, reasons included: unwillingness to have surgery (n = 2), requested a different operation (n = 2), family illness (n = 1), moved abroad (n = 1), did not want to complete questionnaires (n = 2). PQ, participant questionnaire. Reproduced from Cooper et al. 29 © The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license.
Operation details and operative outcomes
Forty-four participants [21/330 (6.4%) randomised to LASH and 23/330 (7.0%) randomised to EA] did not undergo an operation. These women were not asked to complete the patient diary or questionnaires at 6 weeks and 6 months post surgery, but were sent the final questionnaire at 15 months post randomisation.
Table 5 provides details for the 616 women who received an operation. The median number of days between randomisation and treatment was higher in the LASH group [84 (IQR 57–127) days] than in the EA group [63 (IQR 41–97) days] (see Appendix 2, Figure 16). Six women across both arms waited a year or more for their operation and therefore received the 6 months post-surgery and the 15 months post-randomisation questionnaires at around the same time.
| Detail from surgical procedure | LASH (maximum N = 309) | EA (maximum N = 307) |
|---|---|---|
| Grade of surgeon, n (%) | ||
| Consultant | 239 (77.3) | 176 (57.3) |
| Specialty doctor | 8 (2.6) | 16 (5.2) |
| Nurse practitioner | 0 | 10 (3.3) |
| Registrar/junior | 62 (20.1) | 105 (34.2) |
| Supervised by consultant (if surgeon not consultant), n (%) | ||
| Yes | 66 (96) | 100 (78.1) |
| No | 3 (4) | 28 (21.9) |
| Not known | 1 | 3 |
| Type of procedure performed, n (%) | ||
| LASH | 291 (94.2) | 1 (0.3) |
| EA | 12 (3.9) | 297 (96.7) |
| Total hysterectomy | 5 (1.6) | 5 (1.6) |
| Hysteroscopy/polypectomy | 1 (0.3) | 4 (1.3) |
| Was thromboprophylaxis used?, n/N (%) | ||
| Any of below | 303/308 (98.4) | 209/302 (69.2) |
| Injectable heparinoid | 236/309 (76.4) | 23/307 (7.5) |
| TED stockings | 254/309 (82.2) | 194/307 (63.2) |
| Pneumatic anti-thrombosis boots | 69/309 (22.3) | 21/307 (6.8) |
| Type of anaesthesia, n (%) | ||
| General | 308 (100) | 291 (94.8) |
| Local | 0 | 12 (3.9) |
| Not known | 1 | 4 |
| Uterine cavity length (cm), mean (SD) [n] | 8.38 (1.63) [259] | 7.24 (1.97) [292] |
| Fibroids, n (%)a | ||
| Normal | 224 (75.7) | 275 (91.1) |
| Type 0/1 fibroids ≤ 3 cm | 11 (3.7) | 11 (3.7) |
| Type 2 fibroids ≤ 3 cm | 9 (3.0) | 6 (2.0) |
| Intramural/subserosal fibroids ≤ 3 cm | 50 (16.9) | 10 (3.3) |
| Not known | 15 | 5 |
| Time from randomisation to operation (days), median (IQR) [range], n | 84 (57–127) [0–579], 309 | 63 (41–97) [0–541], 307 |
| Time from entry to anaesthetic room to exit from operating room (minutes), mean (SD) [n] | 113.9 (38.1) [306] | 44.3 (23.3) [295] |
| Time from operating room exit to exit from recovery room (minutes), mean (SD) [n] | 75.8 (43.7) [305] | 52.4 (33.1) [297] |
| Postoperative analgesia, n/N (%) | ||
| Paracetamol/ibuprofen | 269/309 (87.1) | 226/307 (73.6) |
| Oral opiate | 136/309 (44.0) | 72/307 (23.4) |
| Opiate injection | 94/309 (30.4) | 46/307 (15.0) |
| Hours from operation to discharge, median (IQR) [n] | 21.5 (17.0–26.1) [306] | 3.2 (2.1–5.1) [303] |
| Total number of women who stayed > 24 hours, n/N (%) | 99/306 (32.4) | 16/303 (5.3) |
| Reason for stay (if stayed > 24 hours) | ||
| Pain | 30 (42) | 3 (27) |
| Nausea/vomiting | 2 (3) | 1 (9) |
| Social/geographical | 13 (18) | 2 (18) |
| Voiding problems | 14 (19) | 1 (9) |
| Other | 13 (18) | 4 (36) |
| Not known | 27 | 5 |
Of those undergoing treatment, 291 out of 309 (94.2%) randomised to LASH and 297 out of 307 (96.7%) randomised to EA received the allocated procedure. Twelve of those randomised to LASH actually received an EA, five underwent a total hysterectomy and one had a hysteroscopy/polypectomy. One woman randomised to EA received LASH, five had a total hysterectomy and four had a hysteroscopy/polypectomy (see Figure 4). In total, nine of the EA operations could not be completed during the first admission (one in the LASH group and eight in the EA group), three women were subsequently readmitted for LASH, two women were readmitted for the total hysterectomy and two women were readmitted for EA.
Compared with the EA group, the LASH group included higher proportions of women who were operated on by a consultant (77.3% vs. 57.3%), received thromboprophylaxis (98.4% vs. 69.2%) and received parenteral postoperative opiates (30.4% vs. 15.0%). Fewer women in the LASH group than in the EA group were noted to have a uterus free from fibroids during surgery (75.7% vs. 91.1%) and more (32.4% vs. 5.3%) stayed in hospital for > 24 hours (see Table 5).
Results for the co-primary outcomes
Satisfaction at 15 months post randomisation
The single question regarding satisfaction was answered by 278 out of 330 (84.2%) women randomised to the LASH group and 280 out of 330 (84.8%) women randomised to the EA group at 15 months post randomisation. This included one woman whose 6-month data were imputed for the 15-month time point (Table 6).
| Outcome | LASH (N = 330), n (%) | EA (N = 330), n (%) | Adjusted OR (95% CI), p-value |
|---|---|---|---|
| Satisfaction | |||
| Total number of women | 278 | 280 | 2.53 (1.83 to 3.48), p < 0.001 |
| Totally satisfied | 211 (75.9) | 158 (56.4) | |
| Generally satisfied | 40 (14.4) | 57 (20.4) | |
| Fairly satisfied | 19 (6.8) | 29 (10.4) | |
| Fairly/generally/totally dissatisfied | 8 (2.9) | 36 (12.9) | |
| Total MMAS score | |||
| Total number of women | 262 | 268 | 1.87 (1.31 to 2.67), p = 0.001 |
| 0–50 | 15 (5.7) | 29 (10.8) | |
| 51–75 | 17 (6.5) | 34 (12.7) | |
| 76–99 | 50 (19.1) | 59 (22.0) | |
| 100 | 180 (68.7) | 146 (54.5) | |
The proportion of women who described themselves as satisfied with their treatment was higher in the LASH group [LASH = 97.1% (270/278); EA = 87.1% (244/280); OR 4.89 (95% CI 1.91 to 12.45)]. Women in the LASH group were also more likely to choose the ‘totally satisfied’ category [LASH = 75.9% (211/278); EA = 56.4% (158/280)].
This result was statistically significant in favour of LASH. In the primary analysis, OLR adjusted for age group and centres, the odds of being in a more favourable satisfaction category were two and a half times greater for women randomised to LASH than for women randomised to EA (OR 2.53, 95% CI 1.83 to 3.48; p < 0.001). The corresponding unadjusted result was similar (OR 2.55, 95% CI 1.79 to 3.63; p < 0.001) (see Appendix 2, Table 30).
The OLR method assumes that the same underlying OR would be obtained for all three splits of the 2 × 4 table (the proportional odds assumption). We investigated this by examining the ORs obtained using binary logistic regression (adjusted for age and centre) for these three splits of the data (see Appendix 2, Table 30). The result for two of these splits was very similar to the OLR result. The results for the satisfied versus dissatisfied split used in the individual patient data meta-analysis8 had a wider CI because of the smaller cell counts involved, but the CIs were broadly consistent with the main result (OR 4.89, 95% CI 1.91 to 12.45). The Brant test was also not statistically significant (p = 0.32); therefore, there was no indication that the ordinal model was inappropriate.
There was also no difference in interpretation when considering per-protocol results (i.e. comparing those who actually received LASH with those who actually received EA), or when considering only those operated on by a consultant (see Appendix 2, Table 30).
It can be noted that the proportions who were satisfied (97% vs. 87%) were similar to those anticipated in the individual patient data meta-analysis8 (95% vs. 87%). This corresponded to an adjusted difference in proportions of 0.10 (95% CI 0.05 to 0.15) (see Appendix 2, Table 30). Although the OR from the primary analysis (2.53) was less than the 2.84 specified as an important effect size in the original calculation, it nonetheless represents a difference in satisfaction that can be regarded as clinically important.
MMAS scores at 15 months post randomisation
Total MMAS scores were available for 262 out of 330 (79.4%) women in the LASH group and 268 out of 330 (81.2%) women in the EA group at 15 months post randomisation. This included MMAS scores for two women whose 6-month data were imputed for the 15-month time point. A further 29 women completed at least one of the six items, but were excluded from the primary analysis because a total score could not be derived.
The total MMAS score ranges from 0 (worst possible health) to 100 (best possible health). Both groups reported a considerable improvement in MMAS scores after surgery. At baseline, median scores were 28.6 (IQR 14.7–43.7) in the LASH group and 29 (IQR 15.7–47.7) in the EA group, but by 15 months post randomisation the majority of women in each group had the best possible score (MMAS score = 100) (Figure 5 and Appendix 2, Table 31).
FIGURE 5.
Total MMAS scores at (a) baseline; (b) 6 months post surgery; and (c) 15 months post randomisation.
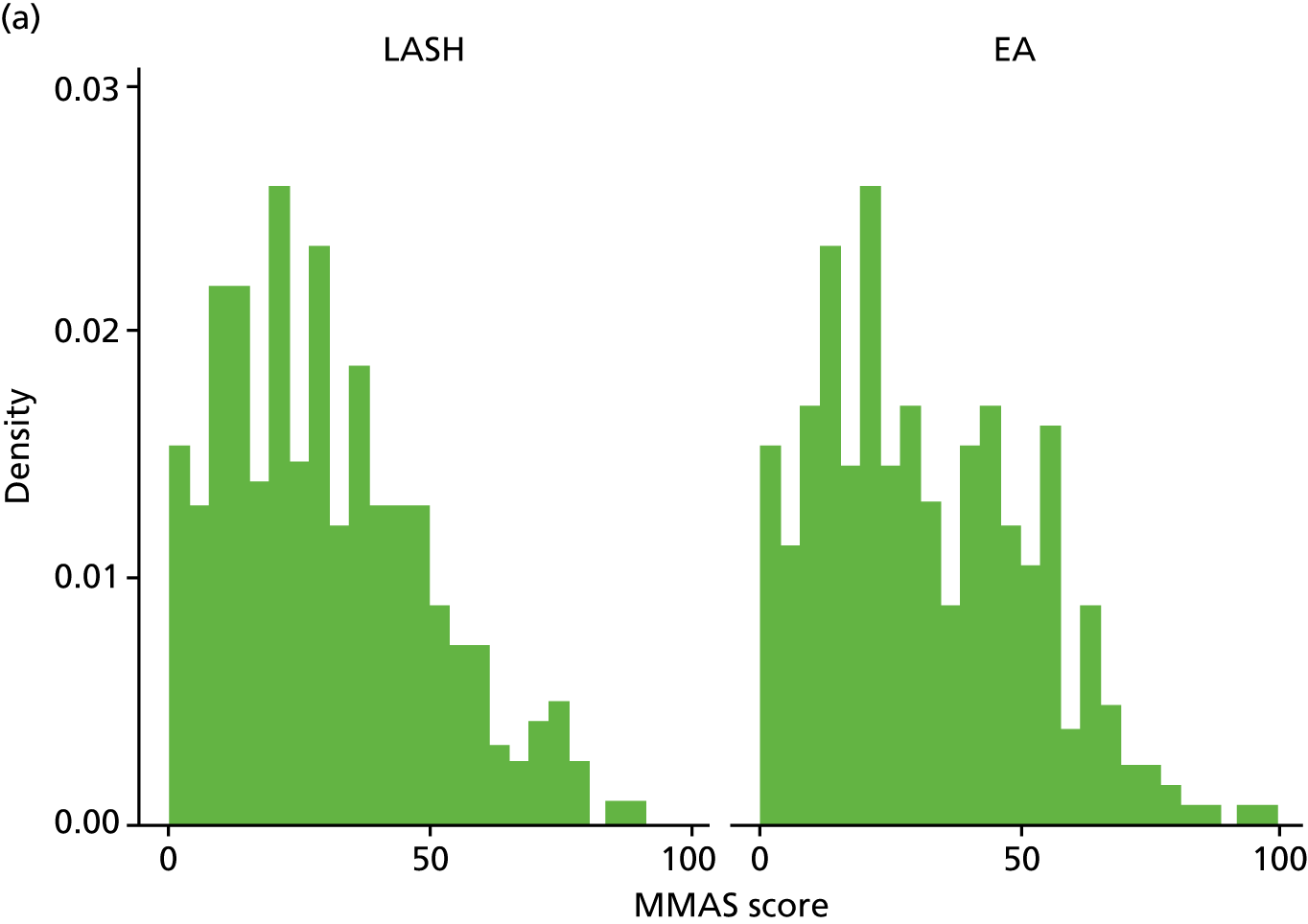
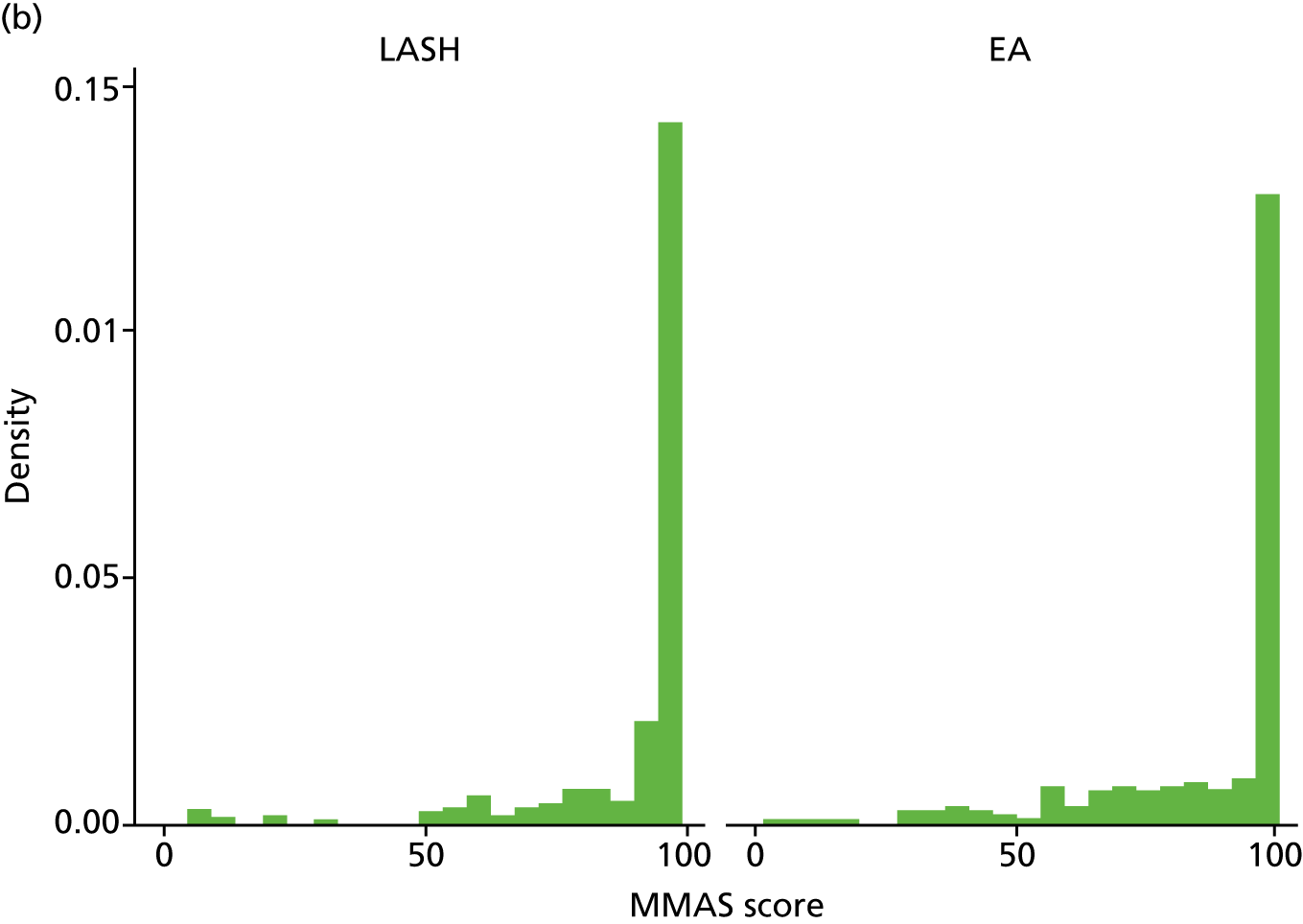
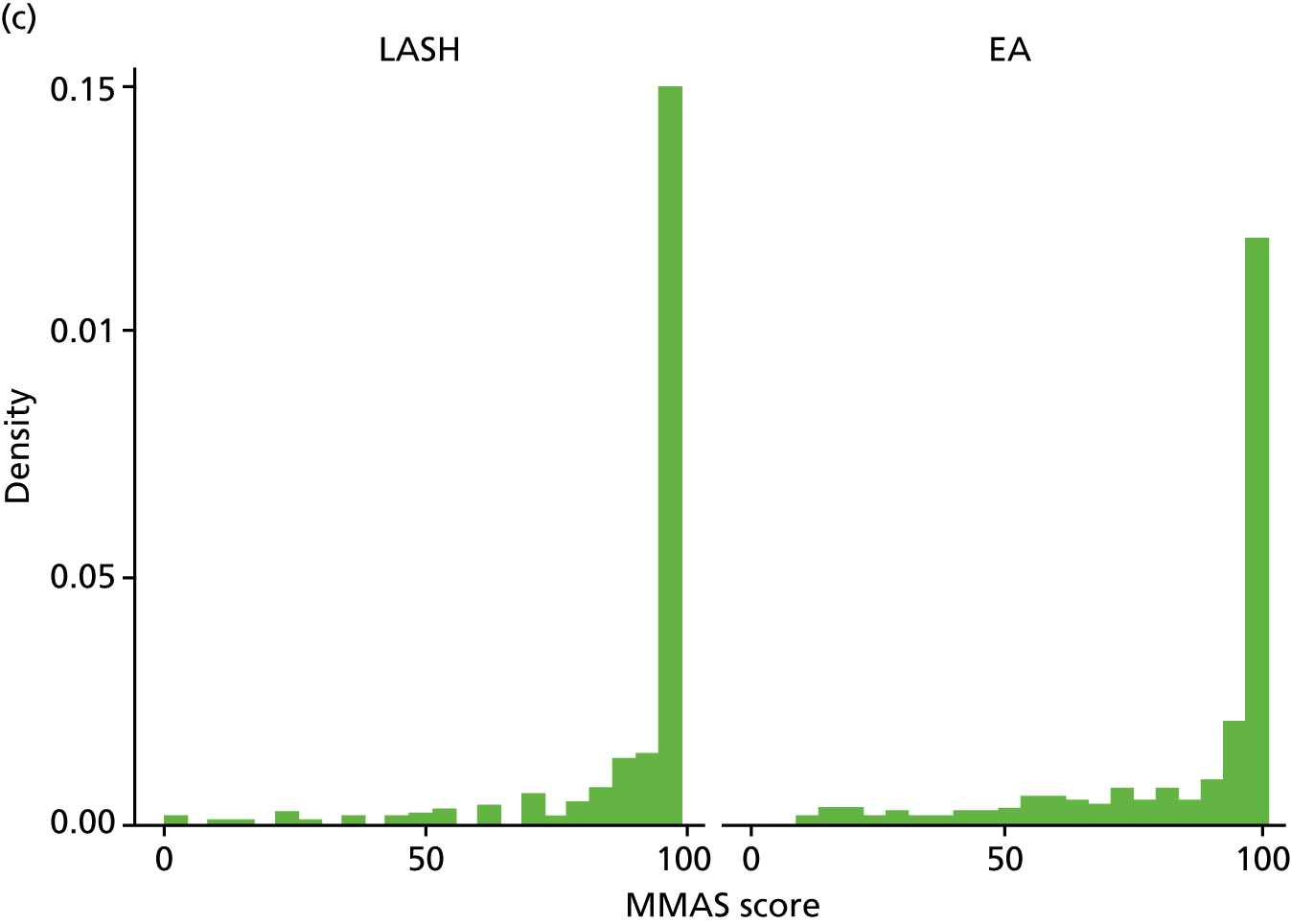
The results for the primary analysis, OLR adjusting for age, centre and baseline MMAS, are presented in Table 6. An OR of 1.87 (95% CI 1.31 to 2.67) was obtained, suggesting that women randomised to receive a LASH had almost twice the odds of being in a more favourable MMAS category than women randomised to EA (p = 0.001). The corresponding unadjusted OR was 1.90 (95% CI 1.35 to 2.68; p < 0.001) (see Appendix 2, Table 32).
The proportional odds assumption was investigated by examining binary logistic regression models using three splits of the data. All approaches yielded similar ORs that were consistent with the main result and the Brant test was not statistically significant (p = 0.07). There was also no change in interpretation using a per-protocol analysis, when restricting analyses to those operated on by a consultant or when treating the MMAS score as a continuous outcome (see Appendix 2, Table 32).
The mean between-group difference in MMAS scores of 6.3 points was lower than the difference of 10 points specified in the original sample size calculation. However, as the distribution of scores was highly skewed, the median is a more appropriate summary to use, and this was 100 in each group. The 95% CI for the effect size for the primary analysis did not include the OR of 2.84 specified in the revised sample size calculation, but an OR of 1.87 could still be considered to have a clinically important impact on patients.
Subgroup analyses
Exploratory subgroup analyses were conducted to determine if there was evidence of differential treatment effects for the co-primary outcomes by four binary factors: cavity length (< 8 cm vs. ≥ 8 cm), menstrual pain at baseline (severe/crippling period pain vs. other categories), age (< 40 years vs. ≥ 40 years) and presence or absence of fibroids.
One statistically significant interaction effect was identified. This suggested that women with fibroids who were randomised to the LASH operation had greater than expected levels of satisfaction (OR for interaction 7.27, 95% CI 2.32 to 41.8; p = 0.002). There was no evidence of any other interaction effects (see Appendix 2, Table 33). For the two primary outcomes, separate OLR results for the eight subgroups are also displayed graphically, along with results by recruiting centre and by whether or not the operator was a consultant (see Appendix 2, Figure 17). Except for the results for fibroids, each subgroup had broadly consistent results.
Sensitivity analyses
The primary analyses were limited to those with complete 15-month follow-up data. Multiple imputation techniques using chained equations were used to investigate the robustness of these findings.
For satisfaction at 15 months post randomisation, an OR of 2.15 (95% CI 1.53 to 3.02; p < 0.001) was obtained using an adjusted OLR model after combining the 20 imputed data sets (see Appendix 2, Table 30). For MMAS score at 15 months post randomisation, an OR of 1.68 (95% CI 1.16 to 2.45; p = 0.007) was obtained (see Appendix 2, Table 32). There was therefore no change in interpretation for either outcome compared with the primary analysis approach.
Results for the secondary outcomes
Serious adverse events and complications
Twenty-five women experienced a SAE. One woman randomised to LASH experienced two such events, so there were a total of 26 SAEs (15 in the LASH group and 11 in the EA group) (Table 7). There was no statistically or clinically significant difference between the randomised groups in the proportions experiencing a SAE (adjusted OR 1.30, 95% CI 0.56 to 3.02; p = 0.54).
| SAE/complication | LASH (N = 309), n (%) | EA (N = 307), n (%) |
|---|---|---|
| SAEs | ||
| Any SAEa | 14 (4.5) | 11 (3.6) |
| Infection | 5 (1.6) | 5 (1.6) |
| Pain | 3 (1.0) | 4 (1.3) |
| Catheterisation for > 72 hours | 3 (1.0) | 1 (0.3) |
| Conversion to hysterectomy | 1 (0.3) | 1 (0.3) |
| Readmitted for investigation of shortness of breath | 1 (0.3) | 0 |
| Prolonged admission for observation only | 1 (0.3) | 0 |
| Bladder injury | 1 (0.3) | 0 |
| Other complications | ||
| Voiding dysfunction | 14 (4.5) | 2 (0.7) |
| Consultation for pain | 1 (0.3) | 1 (0.3) |
| Haematoma | 1 (0.3) | 1 (0.3) |
| Blood loss > 500 ml | 1 (0.3) | 1 (0.3) |
| Uterine perforation, inactive/blunt | 1 (0.3) | 3 (1.0) |
| Pyrexia requiring antibiotics | 3 (1.0) | 2 (0.7) |
| Blood transfusion | 0 | 1 (0.3) |
In the LASH group, five women had an infection, three women were catheterised for > 72 hours, three women experienced considerable pain, one woman had a conversion to open hysterectomy, one woman was readmitted for investigation of shortness of breath and one woman had a bladder injury. A single participant, whose bowel serosa was grazed at surgery, underwent prolonged admission for observation, but did not require any treatment. One of the women in the EA group had her operation converted to hysterectomy, five women had an infection, one woman was catheterised for > 72 hours and four women experienced considerable pain.
A list of other complications from either the index operation or on subsequent hospital readmissions associated with further treatment for HMB are provided in Table 7. A total of 32 women experienced a complication following surgery. These included voiding dysfunction (LASH, n = 14; EA, n = 2); consultation for pain (LASH, n = 1; EA, n = 1); haematoma (LASH, n = 1; EA, n = 1); blood loss > 500 ml (LASH, n = 1; EA, n = 1); inactive/blunt uterine perforation (LASH, n = 1; EA, n = 3); pyrexia requiring antibiotics (LASH, n = 3; EA, n = 2); and blood transfusion (LASH, n = 0; EA, n = 1).
Further treatment for heavy menstrual bleeding
Eighteen women randomised to EA and two women randomised to LASH received further treatment for HMB during the follow-up period (Table 8). The most common reason was that the index EA procedure produced an unsatisfactory reduction in HMB (n = 12). A further seven women required unplanned further surgery because the index EA procedure could not be completed on first admission; this included one woman who was randomised to LASH but in whom an EA procedure was attempted. On five occasions, a hysterectomy was performed on the second admission.
| Further treatment | LASH (N = 309), n | EA (N = 307), n |
|---|---|---|
| Total hysterectomy for failed (unsatisfactory outcome) EA | 0 | 10 |
| Subtotal hysterectomy for failed (unsatisfactory outcome) EA | 0 | 2 |
| Removal of cervical stump for cyclical pain/bleeding | 1 | 0 |
| Readmitted to perform total hysterectomy as EA could not be performed on first admission | 0 | 2 |
| Readmitted to perform subtotal hysterectomy as EA could not be performed on first admission | 1 | 2 |
| Readmitted to perform allocated procedure which could not be performed on first admission | 0 | 2 |
| Total HMB treatmentsa | 2 | 18 |
Patient diary (1–14 days)
Table 34 in Appendix 2 reports data for the patient diary, which was completed in the first 14 days following surgery. In both groups there was a reduction in self-reported levels of pain (0 = no pain, 10 = worst imaginable pain) (Figure 6) and in the proportion of women taking paracetamol or other painkillers over these 2 weeks. In addition, fewer women were using pads for vaginal bleeding or discharge. By day 14, 177 out of 256 (69.1%) women in the EA group and 34 out of 267 (12.7%) women in the LASH group were using pads.
FIGURE 6.
Mean pain during the first 14 days post surgery (0 = no pain, 10 = worst imaginable pain).
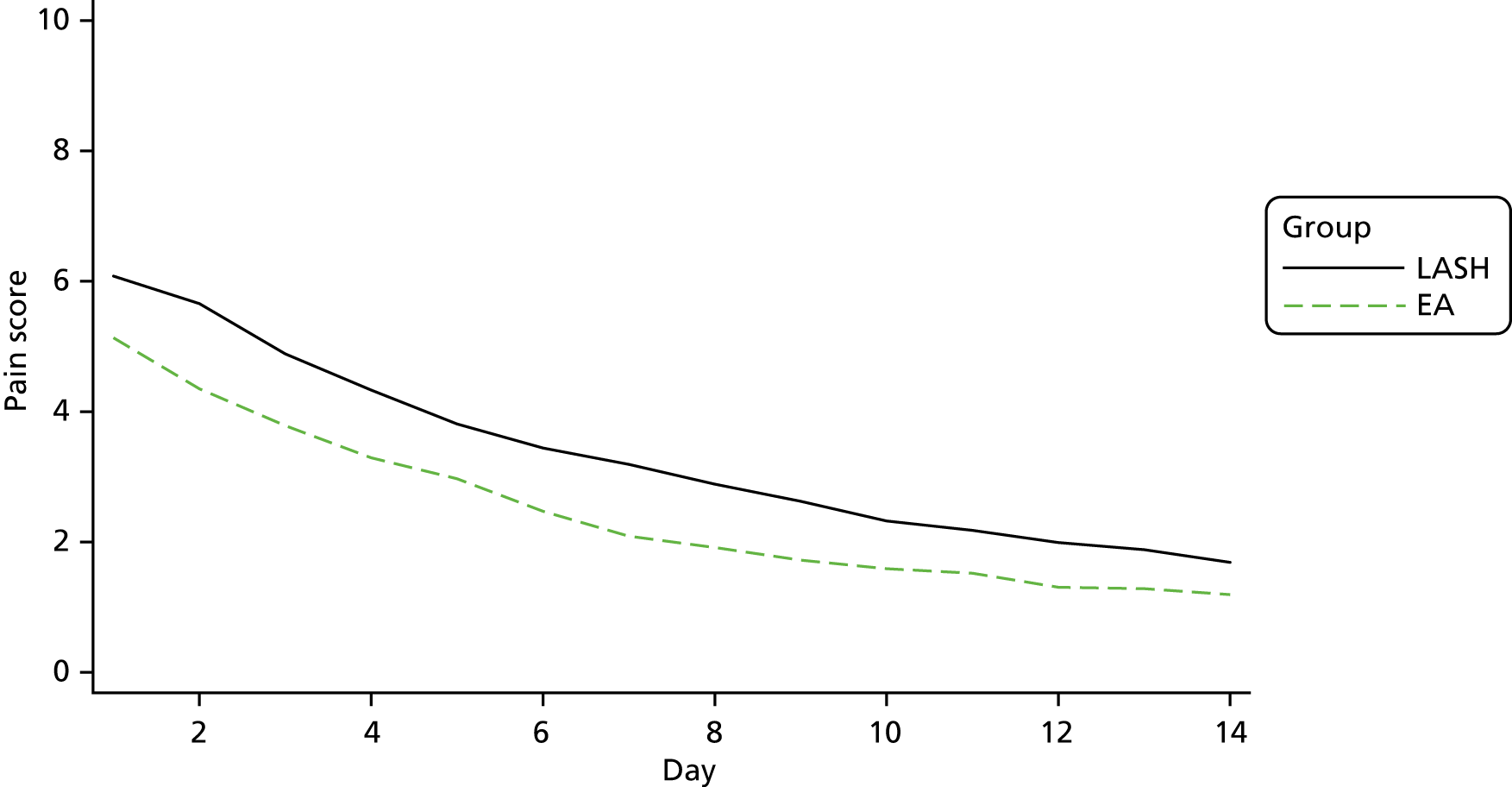
Overall, those in the LASH group had pain scores that were almost 1 point higher than those in the EA group (mean difference 0.92, 95% CI 0.59 to 1.24; p < 0.001; see Appendix 2, Table 34).
Pain and return to usual activities (6 weeks post surgery)
By 6 weeks after surgery, over half of the women in both groups reported no pain on a 10-point scale from 0, no pain, to 10, the worst pain imaginable (Table 9). After adjusting for the minimisation factors (age group and centre), an OR of 1.43 (95% CI 1.05 to 1.96; p = 0.03) was obtained, suggesting that those in the EA group had lower levels of pain at 6 weeks than those in the LASH group.
| Outcome | LASH (N = 309) | EA (N = 307) | Adjusted ORa (95% CI), p-value |
|---|---|---|---|
| Level of pain today (0 = no pain, 10 = worst imaginable), median (IQR) [range], n | 0 (0–1) [0–10], 241 | 0 (0–1) [0–10], 234 | 1.43 (1.05 to 1.96),b p = 0.03 |
| Current employment, n (%) | |||
| Full time | 104 (43.7) | 99 (42.1) | |
| Part time | 82 (34.5) | 82 (34.9) | |
| Not working | 52 (21.8) | 54 (23.0) | |
| Adjusted HRc (95% CI), p-value | |||
| Days until return to paid work, median (95% CI)d [n] | 42 (37 to 42) [186] | 10 (7 to 14) [181] | 0.23 (0.18 to 0.30),b p < 0.001 |
| Days until return to unpaid work, median (95% CI)d [n] | 21 (17 to 25) [255] | 7 (5 to 7) [251] | 0.64 (0.57 to 0.73),b p < 0.001 |
| Days until return to sporting or social activities, median (95% CI)d [n] | 42 (34 to 42) [255] | 14 (14 to 18) [250] | 0.48 (0.42 to 0.56),b p < 0.001 |
Figure 7 shows the time to return to work by randomised group for the women in full- or part-time paid employment. Those in the LASH group returned to work after a median of 42 days, whereas those in the EA group returned after a median of 10 days (see Table 9). A Cox proportional hazards regression model adjusted for age group and centre suggested a statistically significant difference in favour of EA [adjusted hazard ratio (HR) 0.23, 95% CI 0.18 to 0.30; p < 0.001].
FIGURE 7.
Time to return to (a) paid work; (b) unpaid work; and (c) sporting or social activities.
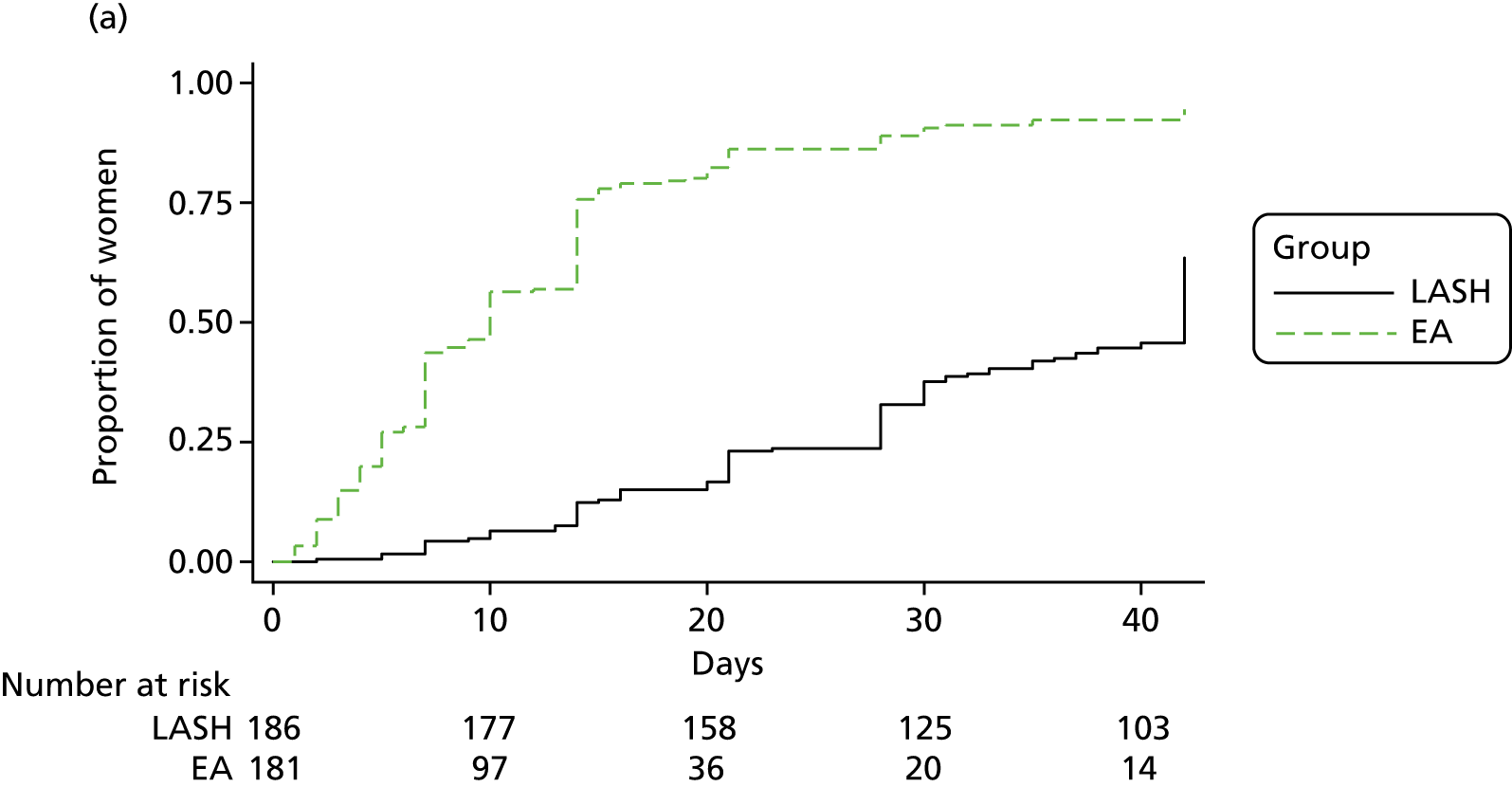
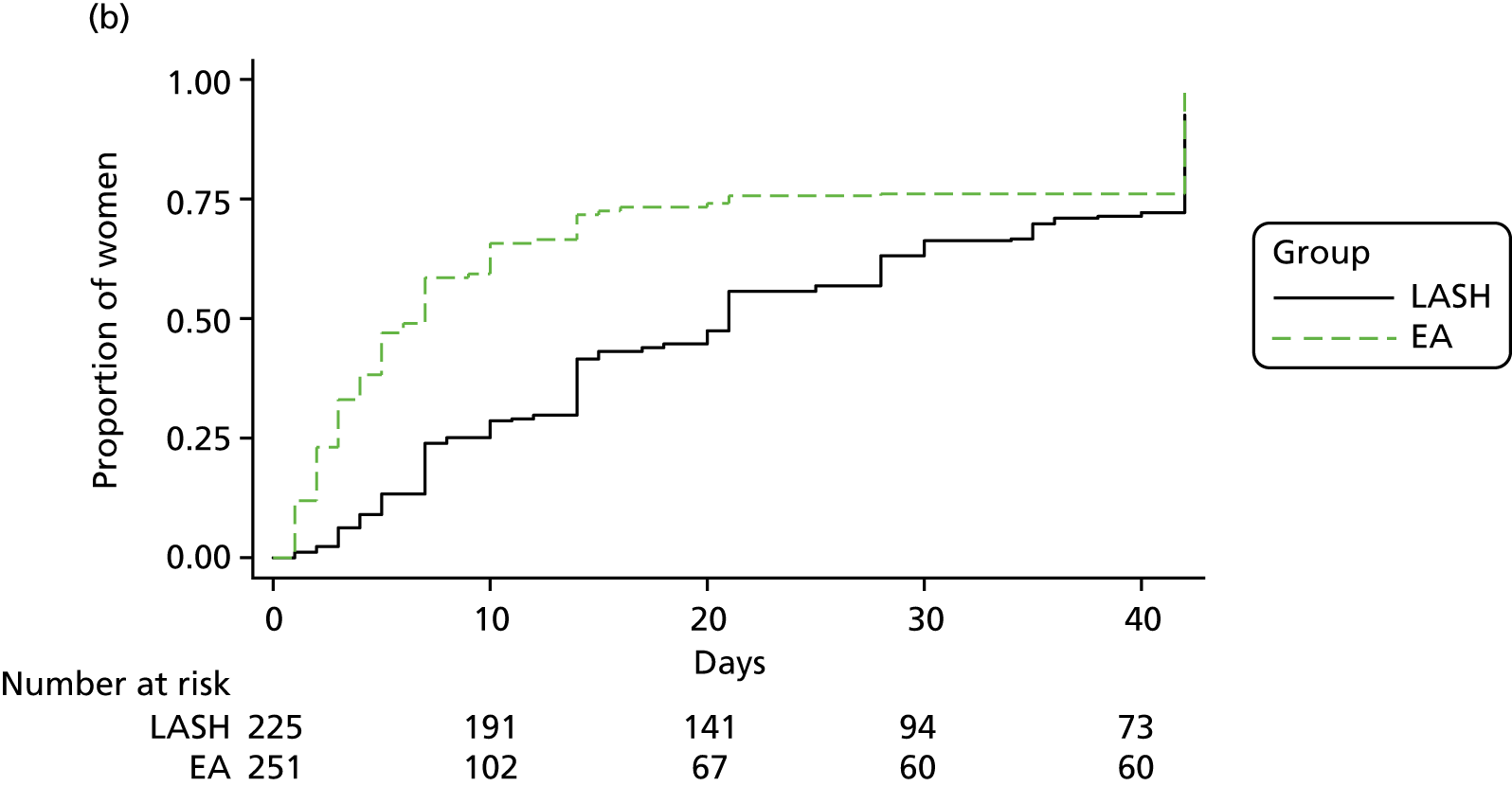
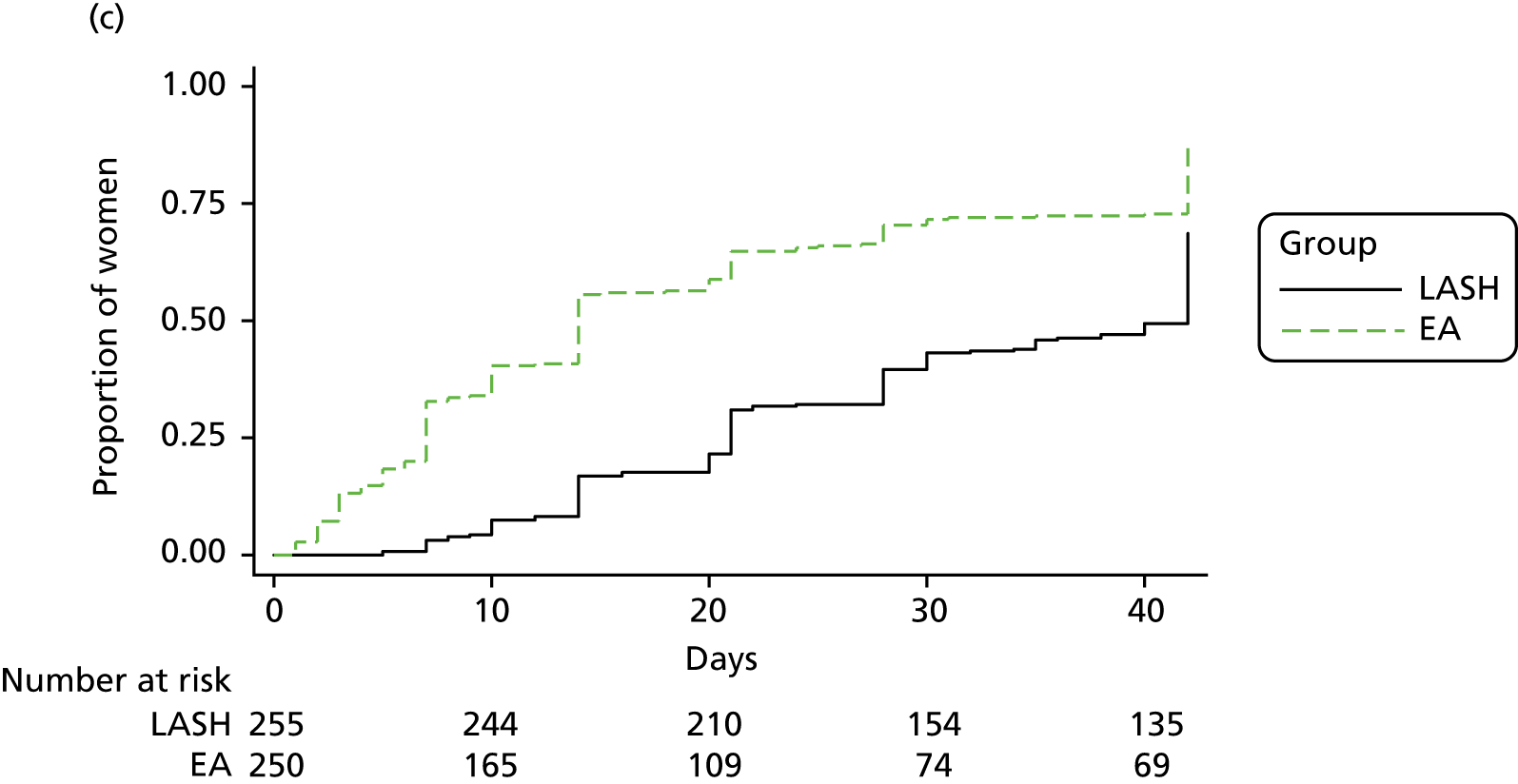
Those in the EA group returned more rapidly to both unpaid work (adjusted HR 0.64, 95% CI 0.57 to 0.73; p < 0.001) and sporting or social activities (adjusted HR 0.48, 95% CI 0.42 to 0.56; p < 0.001) (see Table 9).
Menstrual outcomes (6 months post surgery and 15 months post randomisation)
The proportion of women who continued to have periods was lower in the LASH group than in the EA group [6 months: LASH, 39/253 (15.4%), EA, 111/246 (45.7%), adjusted OR 0.22, 95% CI 0.15 to 0.32, p < 0.001; 15 months: LASH, 52/277 (18.8%), EA, 117/278 (42.1%), adjusted OR 0.32, 95% CI 0.21 to 0.48, p < 0.001] (Table 10).
| Participant outcome | LASH (N = 330)a | EA (N = 330)b | Adjusted effect size (95% CI) | p-value |
|---|---|---|---|---|
| Are you still having periods? (6 months),c n (%) | ||||
| Yes | 39 (15.4) | 111 (45.7) | 0.22 (0.15 to 0.32)d | < 0.001 |
| No | 214 (84.6) | 132 (54.3) | ||
| Are you still having periods? (15 months),c n (%) | ||||
| Yes | 52 (18.8) | 117 (42.1) | 0.32 (0.21 to 0.48)d | < 0.001 |
| No | 225 (81.2) | 161 (57.9) | ||
| MMAS total scoree | ||||
| Baseline | ||||
| Mean (SD) | 30.5 (19.0) | 32.3 (20.0) | ||
| Median (IQR) | 28.6 (14.7–43.7) | 29.0 (15.7–47.7) | ||
| n | 323 | 321 | ||
| 6 months | ||||
| Mean (SD) | 91.3 (18.1) | 86.3 (21.9) | 1.48 (1.02 to 2.14)d | 0.04 |
| Median (IQR) | 100 (91–100) | 100 (78.2–100) | ||
| n | 230 | 224 | ||
| 15 months | ||||
| Mean (SD) | 91.2 (19.0) | 84.9 (23.5) | 1.87 (1.31 to 2.67)d | 0.001 |
| Median (IQR) | 100 (93.3–100) | 100 (77.9–100) | ||
| n | 262 | 268 | ||
| EQ-5D-3L utility scoree | ||||
| Baseline | ||||
| Mean (SD) | 0.71 (0.30) | 0.70 (0.31) | ||
| Median (IQR) | 0.76 (0.66–1.00) | 0.79 (0.69–1.00) | ||
| n | 319 | 322 | ||
| 6 weeks | ||||
| Mean (SD) | 0.83 (0.22) | 0.83 (0.28) | 0.66 (0.48 to 0.90)f | 0.009 |
| Median (IQR) | 0.88 (0.74–1.00) | 1.00 (0.76–1.00) | ||
| n | 251 | 246 | ||
| 6 months | ||||
| Mean (SD) | 0.83 (0.27) | 0.83 (0.25) | 1.15 (0.84 to 1.57) | 0.38 |
| Median (IQR) | 1.00 (0.80–1.00) | 0.85 (0.76–1.00) | ||
| n | 251 | 237 | ||
| 15 months | ||||
| Mean (SD) | 0.84 (0.24) | 0.80 (0.28) | 1.21 (0.89 to 1.64) | 0.23 |
| Median (IQR) | 1.00 (0.73–1.00) | 0.85 (0.72–1.00) | ||
| n | 281 | 281 | ||
| EQ-5D-3L VASe | ||||
| Baseline | ||||
| Mean (SD) | 65.2 (24.2) | 67.2 (23.5) | ||
| Median (IQR) | 70 (50–85) | 70 (52–85) | ||
| n | 317 | 321 | ||
| 6 weeks | ||||
| Mean (SD) | 78.4 (18.6) | 76.6 (20.7) | 1.12 (0.80 to 1.58) | 0.51 |
| Median (IQR) | 80 (70–90) | 80 (70–90) | ||
| n | 248 | 245 | ||
| 6 months | ||||
| Mean (SD) | 79.9 (19.2) | 75.9 (20.5) | 1.53 (1.08 to 2.17)d | 0.02 |
| Median (IQR) | 85.5 (75–90) | 80 (69–90) | ||
| n | 246 | 235 | ||
| 15 months | ||||
| Mean (SD) | 80.1 (17.6) | 76.9 (19.5) | 1.50 (1.12 to 1.99)d | 0.006 |
| Median (IQR) | 85 (70–90) | 80 (65–90) | ||
| n | 279 | 282 | ||
| SF-12 PCSg | ||||
| Baseline | ||||
| Mean (SD) | 45.0 (9.0) | 44.9 (9.7) | ||
| Median (IQR) | 45.8 (39.0–52.1) | 46.5 (39.0–51.9) | ||
| n | 318 | 321 | ||
| 6 weeks | ||||
| Mean (SD) | 44.9 (10.1) | 49.5 (9.6) | –4.97 (–6.31 to –3.63)f | < 0.001 |
| Median (IQR) | 46.4 (38.3–52.9) | 52.2 (45.2–56.1) | ||
| n | 249 | 234 | ||
| 6 months | ||||
| Mean (SD) | 53.2 (8.7) | 52.4 (9.6) | 0.83 (–0.70 to 2.35) | 0.28 |
| Median (IQR) | 56.1 (52.1–57.8) | 55.1 (50.4–58.3) | ||
| n | 223 | 226 | ||
| 15 months | ||||
| Mean (SD) | 53.5 (8.9) | 52.4 (9.0) | 1.08 (–0.65 to 2.81) | 0.21 |
| Median (IQR) | 56.1 (52.7–57.8) | 55.1 (48.8–57.8) | ||
| n | 219 | 216 | ||
| SF-12 MCSg | ||||
| Baseline | ||||
| Mean (SD) | 37.2 (11.0) | 38.7 (11.6) | ||
| Median (IQR) | 36.6 (29.8–45.1) | 29.0 (15.7–47.7) | ||
| n | 318 | 321 | ||
| 6 weeks | ||||
| Mean (SD) | 48.0 (11.2) | 46.9 (11.8) | 1.33 (–0.78 to 3.44) | 0.21 |
| Median (IQR) | 50.7 (41.2–57.2) | 49.6 (40.7–56.0) | ||
| n | 249 | 234 | ||
| 6 months | ||||
| Mean (SD) | 48.2 (12.0) | 45.4 (12.0) | 3.36 (1.69 to 5.03)d | < 0.001 |
| Median (IQR) | 51.5 (40.2–57.2) | 48.4 (37.8–56.0) | ||
| n | 223 | 226 | ||
| 15 months | ||||
| Mean (SD) | 48.5 (11.2) | 46.6 (11.1) | 2.47 (1.07 to 3.87)d | 0.001 |
| Median (IQR) | 50.7 (43.3–57.1) | 48.8 (38.9–55.3) | ||
| n | 219 | 216 | ||
Those in the LASH group had lighter and less painful periods, fewer days with heavy bleeding and lower median bleeding scores and were less likely to require sanitary protection (see Table 10 and Appendix 2, Table 35).
Following EA, a higher proportion of all women (including those with no periods) experienced cyclical pain [6 months: LASH, 68/236 (28.8%), EA, 108/199 (54.3%); 15 months: LASH, 71/224 (31.7%), EA 118/196 (60.2%)] (see Appendix 2, Table 35). Women in the LASH group generally had less pain during intercourse. Similar proportions of women in each group had bladder problems (see Appendix 2, Table 35).
Table 36 in Appendix 2 shows results for menstrual outcomes by actual treatment received (per-protocol analysis). The results were generally similar to those of the intention-to-treat analysis.
Quality of life (6 weeks and 6 months post surgery, 15 months post randomisation)
The quality-of-life results are presented in Table 10. An alternative presentation using categories used in the OLR models is provided in Appendix 2, Table 37.
The results for the MMAS total score at 15 months have been described previously (see MMAS scores at 15 months post randomisation). The results for MMAS scores at 6 months showed more favourable scores for those in the LASH group (adjusted OR 1.48, 95% CI 1.02 to 2.14; p = 0.04).
At 6 weeks post surgery, those in the EA group had higher EQ-5D-3L utility scores than those in the LASH group (adjusted OR 0.66, 95% CI 0.48 to 0.90; p = 0.009). However, at 6 months post surgery and 15 months post randomisation, the point estimates favoured LASH, although the results were not statistically significant (6 months: adjusted OR 1.15, 95% CI 0.84 to 1.57, p = 0.38; 15 months: adjusted OR 1.21, 95% CI 0.89 to 1.64, p = 0.23).
The results for the VAS score of the EQ-5D-3L tended to favour the LASH group, and this finding was statistically significant at 6 months post surgery and 15 months post randomisation (6 weeks: adjusted OR 1.12, 95% CI 0.80 to 1.58, p = 0.51; 6 months: adjusted OR 1.53, 95% CI 1.08 to 2.17, p = 0.02; 15 months: adjusted OR 1.50, 95% CI 1.12 to 1.99, p = 0.006).
In the case of the SF-12 PCS, the EA group was favoured at 6 weeks post surgery (adjusted mean difference –4.97, 95% CI –6.31 to –3.63; p < 0.001), but there was no evidence of group differences at 6 months post surgery (adjusted mean difference 0.83, 95% CI –0.70 to 2.35; p = 0.28) or at 15 months post randomisation (adjusted mean difference 1.08, 95% CI –0.65 to 2.81; p = 0.21). There was evidence of improved SF-12 MCS in the LASH group at the final two time points only (6 weeks: adjusted mean difference 1.33, 95% CI –0.78 to 3.44, p = 0.21; 6 months: adjusted mean difference 3.36, 95% CI 1.69 to 5.03, p < 0.001; 15 months: adjusted mean difference 2.47, 95% CI 1.07 to 3.87, p = 0.001).
Satisfaction and acceptability (6 weeks and 6 months post surgery, 15 months post randomisation)
Women receiving treatment were asked questions about the acceptability of treatment (6 weeks post surgery) and satisfaction with treatment (6 months post surgery) (Table 11). Both these results favoured LASH (acceptability of treatment at 6 weeks: adjusted OR 4.73, 95% CI 2.86 to 7.81, p < 0.001; satisfaction with treatment at 6 months: adjusted OR 2.91, 95% CI 2.04 to 4.16, p < 0.001).
| Participant outcome | Analysis method (effect size) | LASH (N = 330), n (%)a | EA (N = 330), n (%)b | Adjusted effect size (95% CI) | p-value |
|---|---|---|---|---|---|
| Acceptability of treatment | |||||
| Totally acceptable | OR (OLR) | 205 (84.4) | 130 (54.9) | 4.73 (2.86 to 7.81)c | < 0.001 |
| Generally acceptable | 30 (12.3) | 53 (22.4) | |||
| Fairly acceptable | 6 (2.5) | 37 (15.6) | |||
| Fairly unacceptable | 2 (0.1) | 7 (3.0) | |||
| Generally unacceptable | 0 | 4 (1.7) | |||
| Totally unacceptable | 0 | 6 (2.5) | |||
| Satisfaction with treatment (6 months) | |||||
| Totally satisfied | OR (OLR) | 181 (73.9) | 123 (51.3) | 2.91 (2.04 to 4.16)c | < 0.001 |
| Generally satisfied | 46 (18.8) | 52 (21.7) | |||
| Fairly satisfied | 9 (3.7) | 33 (13.8) | |||
| Fairly unsatisfied | 3 (1.2) | 9 (3.8) | |||
| Generally unsatisfied | 1 (0.4) | 10 (4.2) | |||
| Totally unsatisfied | 5 (2.0) | 13 (5.4) | |||
| Satisfaction with treatment (15 months) | |||||
| Totally satisfied | OR (OLR) | 211 (75.9) | 158 (56.4) | 2.53 (1.83 to 3.48) c | < 0.001 |
| Generally satisfied | 40 (14.4) | 57 (20.4) | |||
| Fairly satisfied | 19 (6.8) | 29 (10.4) | |||
| Fairly unsatisfied | 2 (0.7) | 9 (3.2) | |||
| Generally unsatisfied | 1 (0.4) | 15 (5.4) | |||
| Totally unsatisfied | 5 (1.8) | 12 (4.3) | |||
| Recommend treatment to friend? (6 months) | |||||
| Yes | OR (Log Reg) | 245 (96.5) | 208 (85.6) | 4.49 (2.44 to 8.27)c | < 0.001 |
| No | 9 (3.5) | 35 (14.4) | |||
| Recommend treatment to friend? (15 months) | |||||
| Yes | OR (Log Reg) | 263 (97.0) | 246 (87.9) | 4.52 (2.14 to 9.53)c | < 0.001 |
| No | 8 (3.0) | 34 (12.1) | |||
Women were also asked at 6 months post surgery and 15 months post randomisation whether or not they agreed that they would recommend their treatment to a friend. Over 85% of women in each group agreed that they would, but at both time points there was strong evidence in favour of the LASH group (6 months: adjusted OR 4.49, 95% CI 2.44 to 8.27, p < 0.001; 15 months: adjusted OR 4.52, 95% CI 2.14 to 9.53, p < 0.001) (see Table 11).
Summary of the clinical effectiveness results
Table 38 in Appendix 2 provides a summary of the primary and secondary analyses, including both adjusted and unadjusted effect sizes for all outcomes as follows: mean differences for continuous outcomes; both ORs and risk differences for binary outcomes (i.e. both relative and absolute effect sizes); ORs for ordered categorical outcomes and HRs for time-to-event outcomes. Adjusted analyses include the minimisation factors, age group and centre (random effect), in the model, as well as a baseline score, if this is available.
The results of the unadjusted analyses tended to be similar to those of the adjusted analyses.
Using a threshold of a p-value of < 0.05, there was evidence that those randomised to EA had lower levels of pain in the first 6 weeks following treatment. In addition, those randomised to EA also had improved EQ-5D-3L utility scores and SF-12 PCSs at 6 weeks, but there was no evidence of a difference between groups at later time points. Women in the EA group also returned to work and usual activities sooner than those in the LASH group.
Most self-reported outcomes at the 6 months post surgery and 15 months post-randomisation time points tended to favour LASH. There was evidence that women in the LASH group had better QoL outcomes and were more satisfied with their treatment than those in the EA group.
The results of both co-primary outcomes (satisfaction and MMAS score at 15 months post randomisation), both strongly favoured those in the LASH group. The results of the secondary outcomes should be treated as exploratory because no adjustment was made for multiple statistical testing. There is, however, a pattern suggesting greater short-term benefits for EA but longer-term improvements in patient-reported outcomes for the LASH group. In particular, LASH was strongly favoured for all the questions concerning acceptability, satisfaction and recommendation to a friend.
Chapter 5 Economic evaluation: within-trial analysis
Introduction
This chapter reports on the within-trial economic evaluation of LASH compared with second-generation EA. 1 The rationale for the economic evaluation in health care is to help inform the adoption of technologies that provide good value for money in the context of constrained health service resources. The within-trial economic analysis reported in this chapter considers the 15-month post-randomisation follow-up period only. As the full impact of the alternative interventions on resource use and individuals’ HRQoL is likely to accrue over a much longer time horizon, a Markov model was also developed to extrapolate the trial-based findings. This model-based economic analysis is reported in Chapter 6 and constitutes the primary economic analysis for HEALTH.
Objectives of the economic evaluation
The primary economic objective of the within-trial cost-effectiveness analysis was to estimate the incremental cost per QALY gained for LASH compared with EA at 15 months post randomisation. Two of the three secondary economic objectives are addressed in the current chapter: (1) to compare the costs and consequences of LASH and EA at 15 months; and (2) to assess the wider societal costs associated with changes in productivity. The third secondary economic objective of modelling the longer-term cost-effectiveness of LASH compared with EA is addressed in Chapter 6.
Methods
Study design and participants
Details of the trial design are provided in the study protocol1 and in Chapter 2. The economic analysis was based on all women randomised, with the exception of four post-randomisation exclusions, and follows the same intention-to-treat principles as the statistical analysis.
Cost and outcome assessment
Costs and outcomes were assessed via the trial CRFs, patient diary of pain symptoms at days 1–14 post surgery, and postal questionnaires at 6 weeks and 6 months post surgery and 15 months post randomisation [see URL: www.journalslibrary.nihr.ac.uk/programmes/hta/123523/#/ (accessed 22 May 2019)]. Health-care utilisation questions at 6 months post surgery and 15 months post randomisation were included for the purpose of costing follow-up health-care resource use. The patient questionnaires also informed time taken to return to normal activities and time off work due to ongoing/recurrent symptoms over the follow-up period. The EQ-5D-3L and SF-12 were measured at baseline, 6 weeks and 6 months post surgery, and at 15 months post randomisation. These measures of HRQoL were used in the economic analysis for estimating QALYs out to 15 months post randomisation.
Assessment of health service costs
As the economic evaluation seeks to inform the efficient allocation of the NHS budget, the base-case analysis adopted a health service perspective. Nevertheless, the effect of incorporating patient productivity costs was also considered as a secondary analysis.
Cost of the primary interventions
The unit costs used for the valuation of health service resource use are reported in Table 12. The costs of the initial HEALTH interventions were estimated from resource use data recorded in the HEALTH operation form for each participant. In addition to the date and time of admission and operation, this CRF captured the type of procedure carried out, time in theatre, grade of surgeon, time in recovery, postoperative analgesic requirements, perioperative complications and time to discharge at the individual patient level [see URL: www.journalslibrary.nihr.ac.uk/programmes/hta/123523/#/ (accessed 22 May 2019)].
| Resource | How measured | Source of measurement | Unit cost (£) | Source of valuation |
|---|---|---|---|---|
| Time in anaesthetic room | Time in hours | HEALTH operation form | 151 per hour | Band 6 nurse (£45) + consultant anaesthetist (£106); Unit Costs of Health and Social Care 201730 |
| Time in theatre | Time in hours | HEALTH operation form | ||
| Surgeon time | Unit Costs of Health and Social Care 2017 30 | |||
| Consultant | 107 per hour | |||
| Associate specialist | 101 per hour | |||
| Registrar | 43 per hour | |||
| Foundation FY2 | 30 per hour | |||
| Foundation FY3 | 26 per hour | |||
| Nurse consultant | 62 per hour | |||
| Anaesthetist time, consultant | 106 per hour | Unit Costs of Health and Social Care 2017 30 | ||
| Theatre costs (excluding medical and nursing staff) | 596 per hour | Table R140; Information Services Division31 | ||
| Procedure consumables | ||||
| LASH (morcellator and loop package) | See Cost of the primary interventions | Clinical advice | Commercial-in-confidence information | Kebomed UK, 14 May 2018, personal communication |
| LASH haemostatic dissecting device (e.g. Enseal™, Ethicon, Johnson & Johnson, Bridgewater, NJ, USA; Ligasure™, Medtronic plc, Dublin, Ireland) | See Cost of the primary interventions | Commercial-in-confidence information | Nicki Baxter, NHS Grampian, 28 March 2018, personal communication | |
| EA (disposable ablation kit) | See Cost of the primary interventions | Clinical advice | Commercial-in-confidence information | Alan Blair, Hologic, 28 March 2018, personal communication |
| Perioperative complication costs | See Costs of perioperative complications and readmissions | HEALTH operation form | Various | Based on recorded reasons and procedures; NHS Reference Costs 2016–201732 |
| Readmissions | See Costs of perioperative complications and readmissions | Additional hospital admission CRFs; patient questionnaire | Various | Based on recorded reasons and procedures; NHS Reference Costs 2016–201732 |
| Outpatient appointments | See Costs of subsequent health-care utilisation | Patient questionnaires | ||
| Non-admitted face-to-face attendance, first appointment | 155 per attendance | NHS Reference Costs 2016–2017 32 | ||
| Non-admitted face-to-face attendance, follow-up | 130 per attendance | NHS Reference Costs 2016–2017 32 | ||
| Primary care contacts | See Costs of subsequent health-care utilisation | Patient questionnaires | ||
| GP visits | 37 per visit | Unit Costs of Health and Social Care 2017 30 | ||
| GP home visits | 45.98 per visit | Unit Costs of Health and Social Care 2017 30 | ||
| GP phone consultation | 37 per visit | Unit Costs of Health and Social Care 2017 30 | ||
| Medications | See Costs of subsequent health-care utilisation | Patient questionnaires | Various | British National Formulary 33 |
The primary costing approach assigned costs to these individual components of resource use to capture patient-level variation in costs. Time in the anaesthetic room was costed using the cost per hour (incorporating overheads) for a consultant anaesthetist and an anaesthetist nurse. 30 For time in theatre, the unit costs of the recorded grade of surgeon and a consultant anaesthetist were applied. 30 Nursing staff were costed at the requirement for gynaecology day surgery: one anaesthetic nurse, a scrub nurse and two further theatre nurses. In addition, a published unit cost was applied for time in theatre to reflect the average cost of other staff, supplies and consumables, and allocated capital charges and overheads. 31 This detailed unit cost of theatre time is available only for Scottish hospitals. However, the average cost per theatre hour in general hospitals in Scotland (£1144 including medical and nursing staff) is comparable to a previously published estimate for England (£1200 per hour). 34 Therefore, the average Scottish estimate (£596 per hour, excluding medical and nursing staff) was applied in the base-case analysis. In addition to this, we applied the unit costs of major consumable items specific to the alternative procedures. For LASH, this includes a disposable morcellator (LiNA Xcise™; LiNA Medical, Norcross, GA, USA), a disposable loop (LiNA Loop™; LiNA Medical, Norcross, GA, USA) and a disposable haemostatic dissecting device. A survey of participating centres suggested that the above consumables, or similar disposable items, were used for the majority of LASH procedures in HEALTH. However, a small number of centres reported using a reusable morcellator and/or reusable dissecting/sealing devices, so we also conducted a sensitivity analysis to assess the impact of removing the relevant consumable costs from operations carried out at these centres. For EA, the cost of a disposable NovaSure™ (Hologic Inc., Santa Clara, CA, USA) radiofrequency ablation device was used for all procedures, as the alternative Thermachoice thermal balloon (Ethicon, Inc., Johnson & Johnson, New Brunswick, NJ, USA) has been removed from the market. The cost of the NovaSure™ controller has not been included as this is loaned free of charge.
With respect to preoperative care, we assumed that the procedures would have similar workup costs. However, we did include the cost of preoperative overnight stays, which were more frequent in the LASH arm. As histopathology is a requirement before EA and following LASH (not necessarily before LASH) in routine practice, we assumed that these costs would balance out. We also assessed the impact on cost-effectiveness of including an extra cost for pathology testing in the LASH arm of the model presented in Chapter 6, to reflect the possibility that pathology costs following LASH may be higher than they are prior to EA.
Time in recovery following surgery was costed using the unit cost of a grade 6 nurse (inclusive of overheads), assuming one-to-one care. Time on the ward following recovery was costed using an estimate of the cost per excess bed-day (transformed to an hourly rate) following EA or LASH. 32
As an alternative approach to costing the initial HEALTH procedure episode, each patient record was mapped to the appropriate Healthcare Resource Group (HRG) and costed using the relevant NHS reference cost. 32 The core HRG code for second-generation EA procedures is MA12 (Resection or Ablation Procedures for Intra-Uterine Lesions). The core HRG code for LASH is MA08 (Major, Laparoscopic or Endoscopic, Upper Genital Tract Procedures). The procedures were costed by applying either the day-case reference cost (patient discharged same day) or the elective inpatient cost (stay ≥ 1 day), adjusted for length of stay using the excess bed-day cost.
Costs of perioperative complications and readmissions
For perioperative complications leading to prolonged hospital stay, the clinical management costs were based on the NHS reference cost for any additional procedures and adjustment for prolonged length of stay. The information on the type of complications experienced and any procedures undertaken were obtained from the HEALTH operation form and associated SAE forms.
Data on hospital readmissions were obtained from the ‘additional hospital admission form’ and associated SAE forms [see URL: www.journalslibrary.nihr.ac.uk/programmes/hta/123523/#/ (accessed 22 May 2019)]. Descriptions of the reason for admission and clinical procedures conducted were used to assign a HRG-based reference cost to each readmission. 32 A hospital readmission form was triggered by patients reporting readmissions in the questionnaire, but sites could also report these based on their own internal records.
Costs of subsequent health-care utilisation
Related primary and secondary outpatient care, incurred over the 15-month follow-up period, were obtained from the patient questionnaires at 6 months post surgery and 15 months post randomisation. Medications prescribed for any ongoing problems with pelvic pain, vaginal bleeding or discharge, pain at intercourse, or any new urinary problems were also recorded in these questionnaires. All the primary care contacts were costed using the Unit Costs of Health and Social Care 2017,30 and outpatient visits were costed using the NHS reference cost for a gynaecology outpatient visit (see Table 12). 32 For each participant, the number of visits reported was multiplied by the appropriate unit cost. Relevant medications and quantities prescribed at 6 months post surgery and 15 months post randomisation were costed using prices recorded in the British National Formulary. 33
Indirect costs
Indirect costs, which account for time lost from productive activities, were estimated based on time taken to return to normal activities (from the 6 weeks post-surgery questionnaire) combined with questions on work productivity delivered at 6 months post surgery and 15 months post randomisation. The time taken away from normal productive activities was estimated in hours and appropriate unit costs were used to estimate the opportunity cost of time. Gross age- and sex-specific wage rates published by the Department of Work and Pensions were used to cost time lost from paid employment. 35 Time lost from unpaid work, such as housework, was estimated using appropriate shadow prices reflecting the nature of the role. Forgone leisure time, associated with travel to and from health-care appointments, was valued at the current value of travel time savings available from the Department of Transport. 36 The productivity questions at 6 months post surgery and 15 months post randomisation related to the preceding 4 weeks.
In addition, the cost of any private health care falling on participants was estimated based on details provided by participants in the 6 months post-surgery questionnaire and the 15 months post-randomisation questionnaire.
Outcome measures
Effectiveness for the economic evaluation was measured in terms of QALYs, estimated using the EQ-5D-3L questionnaire, completed by participants at baseline and at 6 weeks and 6 months post surgery and 15 months post randomisation. Participant responses were assigned a utility score based on the UK time trade-off tariff. 37 For the base-case analysis, QALYs were estimated using the area under the curve approach, assuming a baseline utility score up to the time of the index intervention and a linear change in utility between the observed follow-up time points thereafter. The SF-12 provided an alternative source of health state utility data via the Short Form questionnaire-6 Dimensions scoring algorithm. 38 These values were used to calculate QALYs in a sensitivity analysis.
Statistical analysis of trial economic data
Aggregating costs and effects
All cost and QoL elements were summed over the follow-up period (to 15 months post randomisation), to estimate total costs and QALYs per participant. As the 6-month questionnaire was anchored on the date of surgery, and the 15-month final questionnaire on randomisation date, there was a degree of overlap or a gap between the 6-month recall periods of the questionnaires. We therefore adjusted the patient-reported use of primary care and outpatient care to avoid double counting or undercounting, by applying a multiplicative factor to the patient-reported resource use data at 15 months. The factor was equal to the duration in months between the 15-month follow-up date and the 6 months post-surgery follow-up date, divided by six. For example, if 8 months elapsed between the 15-month follow-up date and the 6 months post-surgery follow-up date, the factor was equal to 1.333 (i.e. equal to 8/6), to account for the 2-month gap between the recall periods. When < 6 months elapsed between the follow-up questionnaires, the recall periods overlapped and the multiplicative factor was < 1. Cost were expressed in 2016–17 prices.
Missing data
Economic evaluations based on participant-level trial data are likely to encounter challenges with missing data. The total estimated cost is the sum of numerous components over the observed follow-up period of the trial. Furthermore, QALYs can be computed only when participants have responded to the relevant QoL questionnaires at every follow-up point. Collected data allowed the estimation of total cost and total QALYs for 57% (53% for EA, 60% for LASH) and 63% (65% for EA, 69% for LASH) of the study sample, respectively (see Appendix 3). Reliance on complete-case data for cost-effectiveness analysis can introduce bias, unless the data are missing completely at random. It was considered more likely that data were missing at random (i.e. missing values can be predicted based on the observed data). Therefore, multiple imputation was implemented as part of the within-trial analysis, using chained equations with predicted mean matching (kth-nearest neighbour = 5) to generate 20 complete data sets with plausible fitted values assigned for missing cost and utility elements. The imputation model for each variable included all the variables incorporated in the analysis model, all the other cost and utility variables being imputed and one of the co-primary clinical outcome measures (patient satisfaction) as a further auxiliary variable (see Appendix 3). Rubin’s rules were used to pool estimates across the multiple imputation data sets. 39 Missing cost data were imputed at the level of the main cost categories at the different follow-up time points and EQ-5D data were imputed at the level of the index score. Hospital readmission costs were assumed to be zero when no readmission form was completed. This was done because these forms could be triggered by patient-reported readmission or by centres reporting readmissions independently. Given the associated uncertainty with respect to rates of readmission for further surgery in the year following surgery, the modelling reported in Chapter 6 explored the impact of applying higher rates in year 1, derived from external sources.
Incremental cost-effectiveness analysis
The trial-based economic analysis estimated the joint difference in mean costs and QALYs between LASH and EA (to 15 months post randomisation). GLMs with adjustment for minimisation factors (centre, age) and baseline EQ-5D-3L score were used. A modified Park test was implemented to select an appropriate family function. Moreover, Pearson’s correlation, Pregibon link and modified Hosmer–Lemeshow tests were used to select the link function. 40 Details of these test results are reported in Appendix 3. Based on the above, a Gaussian family with identity link function and a Poisson family with identity link were selected for the cost data and QALY data, respectively. Recycled predictions were used to recover adjusted mean values by treatment allocation group and the incremental differences between groups. 40 The incremental cost-effectiveness ratio (ICER) for LASH compared with EA was calculated as the difference in mean cost divided by difference in mean QALYs. The variance surrounding the joint incremental costs and effects was characterised using non-parametric bootstrapping (1000 iterations), with multiple imputation (m = 5) nested within the bootstrap loops. 41
Sensitivity analysis
Sensitivity analysis focused on the costing methodology for the initial interventions and the use of the SF-12 as an alternative method for deriving QALYs. In addition, predefined subgroup analyses were conducted according to uterine cavity length (≤ 8 cm vs. > 8 cm), severity of dysmenorrhoea at baseline (severe vs. non-severe), age category at baseline (< 40 years vs. ≥ 40 years) and the presence of fibroids. An indicator variable for the selected subgroup, and the interaction term between the selected subgroup indicator and the treatment group indicator, were added to the base-case analysis regression model to conduct these analyses.
Results
Resource use and costs
Table 13 summarises health service resource use and costs by intention to treat from an NHS perspective. LASH required a substantially longer time in the anaesthetic room, theatre and recovery than EA. The average time from entry into the anaesthetic room to arrival on the ward following recovery was almost 3 hours (178 minutes) for LASH, whereas the corresponding time for EA was approximately half of that (90 minutes). Hospital stay on the ward following recovery was also longer for LASH (23 hours on average, compared with 6 hours for EA). The consumed hospital resources translated into mean initial episode costs of £2757 and £1071 for LASH and EA, respectively. The corresponding costs based on alternative HRG costing methodology were £2774 and £1197 for LASH and EA, respectively.
| Variable | Number of observations | LASH (N = 330) | EA (N = 330) |
|---|---|---|---|
| Resource use | |||
| Initial procedure, mean (SD) | |||
| Time in anaesthetic room (minutes) | 643 | 16.4 (10.3) | 10.8 (8.8) |
| Time in theatre (minutes) | 651 | 90.5 (41.2) | 30 (22.1) |
| Time in recovery (minutes) | 646 | 70.9 (46.2) | 48.6 (34.6) |
| Length of hospital stay on ward (hours) | 647 | 22.9 (16.5) | 6 (10) |
| Further follow-up | |||
| Hospital readmission, n (%) | 660 | 14 (4.2) | 24 (6.4) |
| Readmission length of stay (days), mean (SD) | 38 | 1.07 (1.1) | 1.13 (1.2) |
| Number of outpatient visits, mean (SD) | 395 | 0.32 (1) | 0.29 (0.9) |
| Number of GP contacts | |||
| Face-to-face visits, mean (SD) | 391 | 0.89 (2.2) | 0.87 (1.7) |
| Telephone consultations, mean (SD) | 391 | 0.01 (0.1) | 0.01 (0.1) |
| Home visits, mean (SD) | 391 | 0.17 (0.6) | 0.22 (0.7) |
| Medication prescribed, n (%) | 391 | 5 (2.4) | 18 (9.7) |
| Costs: initial surgical episode cost, mean (SD) | |||
| Primary analysis (microcosting) | 639 | 2757 (1030) | 1071 (617) |
| HRG-based estimates | 660 | 2774 (906) | 1197 (539) |
| Readmission costs for further treatment (£) | 660 | 53 (309) | 140 (662) |
| Outpatient costs (£) | 393 | 33 (85) | 30 (79) |
| Primary care costs (£) | 391 | 40 (96) | 40 (77) |
| Medication costs (£) | 391 | 0.04 (0.3) | 1.38 (7) |
| Total NHS cost (£) | 374 | 3004 (725) | 1281 (668) |
In addition to the information on the initial procedure, follow-up data are reported in Table 13. Fourteen participants had further hospital admissions in the LASH group within 15 months post randomisation, compared with 24 participants in the EA group. Reasons for subsequent hospital admission included abdominal pain, urinary tract infections, cervical stump removal following LASH (n = 1) and hysterectomy following EA (n = 13). The duration of hospital readmissions was also slightly lower for LASH (1.07 days for LASH vs. 1.13 days for EA). Average readmission costs were therefore lower for LASH than for EA (£53 vs. £140). No major differences were observed on the average number of outpatient hospital visits or GP contacts. The number of participants reporting prescribed medications related to the condition over the follow-up period were low in both groups, but slightly lower in the LASH group than in the EA group (5 women vs. 18 women).
The combined initial treatment and follow-up resource use translated into total average NHS costs of £3004 for LASH compared with £1281 for EA, resulting in an unadjusted difference of £1722.
Utility scores and quality-adjusted life-years
Table 14 summarises the mean utility scores based on EQ-5D-3L and SF-12 responses at baseline, at 6 weeks and 6 months post surgery and at 15 months post randomisation. There was a small non-significant difference in the EQ-5D-3L score at baseline in favour of LASH. The mean EQ-5D-3L data for LASH showed an upwards trend from baseline until 15 months post randomisation, suggesting continued improvement in QoL. For EA, the increase in the EQ-5D-3L from baseline was initially greater at 6 weeks compared with LASH, but then stabilised before dropping by 15 months post randomisation. Notably, by 15 months, the EQ-5D-3L score was higher in the LASH group than in the EA group (unadjusted difference = 0.035), although not statistically significant.
| Variable | Number of observations | LASH (N = 330) | EA (N = 330) |
|---|---|---|---|
| EQ-5D-3L, mean (SD) | |||
| Baseline | 641 | 0.7065 (0.30) | 0.6983 (0.31) |
| 6 weeks post surgery | 497 | 0.8279 (0.22) | 0.8282 (0.28) |
| 6 months post surgery | 488 | 0.8315 (0.27) | 0.8269 (0.25) |
| 15 months post randomisation | 562 | 0.8357 (0.24) | 0.8005 (0.28) |
| SF-12, mean (SD) | |||
| Baseline | 568 | 0.6174 (0.12) | 0.6249 (0.14) |
| 6 weeks post surgery | 345 | 0.6762 (0.14) | 0.7506 (0.16) |
| 6 months post surgery | 412 | 0.8036 (0.14) | 0.7757 (0.15) |
| 15 months post randomisation | 405 | 0.8094 (0.14) | 0.7818 (0.14) |
Baseline utility scores obtained from the SF-12 were slightly higher for EA than for LASH. Data from both study groups show a persistent improvement in utility scores from baseline until the end of follow-up. However, the substantially lower response rate on this secondary economic outcome measure is notable.
Cost–utility analysis results
The incremental analysis was conducted using the multiple imputation data set and is presented in Table 15. The adjusted mean costs per participant were £2886 and £1282 for LASH and EA, respectively, producing an adjusted difference of £1604. The average cost difference resulting from the imputed data was slightly narrower than the unadjusted difference based on complete data (see Table 13). The mean adjusted QALYs per participant were 0.978 and 0.974 for LASH and EA, respectively, giving an adjusted QALY difference of 0.004 in favour of LASH.
| Intervention | Total cost (£) | Incremental cost (£)a | Total QALYs | Incremental QALYsa | ICER (£/QALY gained) |
|---|---|---|---|---|---|
| EA | 1282 | 0.974 | |||
| LASH | 2886 | 1604 | 0.978 | 0.004 | 458,334 |
Therefore, intention to treat with LASH resulted in significantly higher mean costs and a slight non-significant QALY gain compared with EA at 15 months post randomisation. The ICER for LASH compared with EA came to £458,334 per QALY gained over this relatively short time horizon. Although the EQ-5D-3L health state utility was higher in the LASH group by 15 months post randomisation, the mean QALYs accruing over 15 months remained very similar between groups owing to the earlier improvement in HRQoL with EA than with LASH.
Figure 8 shows the scatterplot of the difference in mean costs and difference in mean QALYs based on the 1000 bootstrapped iterations of the regression analysis with nested multiple imputation. LASH was clearly more costly, on average, than EA, as all the iterations resulted in mean cost differences that were greater than zero. However, the mean difference in QALYs was centred on 0.004 (favouring LASH), with a substantial proportion of the bootstrapped iterations favouring EA. Although Figure 8 shows that ≈60% of the bootstrapped iterations generated a QALY gain favouring LASH over EA, all these points lie above accepted cost-effectiveness thresholds,42 suggesting that LASH had little chance of being considered cost-effective based on its incremental cost-per-QALY ratio over a 15-month time horizon. This was to be expected, given the relatively short duration of follow-up combined with the higher initial treatment costs for LASH and the more delayed pattern of improvement in HRQoL described above.
FIGURE 8.
Trial-based incremental cost-effectiveness scatterplot for LASH vs. EA (base case; imputed data; 1000 bootstrap iterations).
Sensitivity, subgroup and secondary analyses
Further to the base-case analysis, several sensitivity analyses were conducted using alternative costing methodology and QoL instruments to estimate QALYs (Table 16). In addition, the results of the prespecified subgroup analyses are presented in Table 17 and indirect costs are reported in Table 18. Finally, the differences in cost are summarised in relation to the main clinical findings (cost–consequences analysis).
| Intervention | Total cost (£) | Incremental cost (£) | Total QALYs | Incremental QALYs | ICERa (£) | Probability cost-effective | ||
|---|---|---|---|---|---|---|---|---|
| £13,000 | £20,000 | £30,000 | ||||||
| Base-case analysis | ||||||||
| EA | 1282 | 0.974 | 1.000 | 1.000 | 1.000 | |||
| LASH | 2886 | 1604 | 0.978 | 0.004 | 458,334 | 0.000 | 0.000 | 0.000 |
| HRG-based reference costs to cost initial surgical episode | ||||||||
| EA | 1417 | 0.974 | 1.000 | 1.000 | 1.000 | |||
| LASH | 2905 | 1488 | 0.978 | 0.004 | 425,229 | 0.000 | 0.000 | 0.000 |
| Removal of relevant LASH consumable costs from procedures carried out using reusable equipment | ||||||||
| EA | 1280 | 0.974 | 1.000 | 1.000 | 1.000 | |||
| LASH | 2815 | 1535 | 0.978 | 0.004 | 438,709 | 0.000 | 0.000 | 0.000 |
| QALYs based on Short Form questionnaire-6 Dimensions | ||||||||
| EA | 1282 | 0.921 | 1.000 | 1.000 | 1.000 | |||
| LASH | 2886 | 1604 | 0.927 | 0.007 | 239,428 | 0.000 | 0.000 | 0.000 |
| Intervention | Total cost (£) | Incremental cost (£) | Total QALYs | Incremental QALYs | ICERa (£) | Probability cost-effective | ||
|---|---|---|---|---|---|---|---|---|
| £13,000 | £20,000 | £30,000 | ||||||
| Base-case analysis (full cohort) | ||||||||
| EA | 1282 | 0.974 | 1.000 | 1.000 | 1.000 | |||
| LASH | 2886 | 1604 | 0.978 | 0.004 | 458,334 | 0.000 | 0.000 | 0.000 |
| Uterine cavity length < 8 cm | ||||||||
| EA | 1333 | 0.969 | 1.000 | 0.993 | 0.937 | |||
| LASH | 2876 | 1543 | 0.984 | 0.015 | 102,877 | 0.000 | 0.007 | 0.063 |
| Uterine cavity length ≥ 8 cm | ||||||||
| EA | 1465 | 1.005 | 1.000 | 1.000 | 1.000 | |||
| LASH | 3189 | 1724 | 0.998 | –0.007 | Dominatedb | 0.000 | 0.000 | 0.000 |
| Severe dysmenorrhoea at baseline | ||||||||
| EA | 1295 | 0.954 | 1.000 | 0.997 | 0.969 | |||
| LASH | 2848 | 1553 | 0.976 | 0.022 | 72,225 | 0.000 | 0.003 | 0.031 |
| Non-severe dysmenorrhoea at baseline | ||||||||
| EA | 1289 | 0.995 | 1.000 | 1.000 | 1.000 | |||
| LASH | 2934 | 1645 | 0.982 | –0.013 | Dominatedb | 0.000 | 0.000 | 0.000 |
| Fibroids present | ||||||||
| EA | 1600 | 1.020 | 0.991 | 0.964 | 0.917 | |||
| LASH | 3208 | 1608 | 0.989 | –0.031 | Dominatedb | 0.009 | 0.036 | 0.083 |
| Fibroids absent | ||||||||
| EA | 1341 | 0.975 | 1.000 | 1.000 | 0.997 | |||
| LASH | 3056 | 1714 | 0.992 | 0.017 | 110,249 | 0.000 | 0.000 | 0.003 |
| Age < 40 years | ||||||||
| EA | 1310 | 0.963 | 1.000 | 0.996 | 0.937 | |||
| LASH | 2919 | 1609 | 0.982 | 0.020 | 81,277 | 0.000 | 0.004 | 0.063 |
| Age ≥ 40 years | ||||||||
| EA | 1270 | 0.979 | 1.000 | 1.000 | 0.996 | |||
| LASH | 2873 | 1603 | 0.978 | –0.001 | Dominatedb | 0.000 | 0.000 | 0.004 |
| Variable | Number of observations | LASH (N = 330) | EA (N = 330) |
|---|---|---|---|
| Time to return to usual activities (number of days) | |||
| Time to return to paid work, mean (SD) | 368 | 33.2 (11.6) | 13 (11) |
| Time to return to unpaid work, mean (SD) | 408 | 18.9 (12.8) | 7.3 (8.7) |
| Time to return to leisure/social activities, mean (SD) | 438 | 30.2 (12.3) | 16.5 (13.4) |
| Productivity costs (£) | |||
| Cost of time lost from paid work | 367 | 1886 (919) | 719 (677) |
| Cost of time lost from unpaid work | 408 | 700 (475) | 271 (321) |
Alternative costing and utility instrument
Table 16 summarises the results of the key sensitivity analyses. The analysis using HRG-based reference costs for the index procedure generates a very similar incremental cost for LASH compared with EA, and led to the same finding as the base case, with the ICER for LASH at 15 months post randomisation being above accepted cost-effectiveness thresholds. The sensitivity analysis results, using the SF-12 instrument to estimate QALYs, are also reported in Table 16. The adjusted difference in QALYs was similarly very small and in favour of LASH.
Subgroup analyses
The results of the prespecified subgroup analyses are reported in Table 17. The ICER for LASH compared with EA remained unfavourable against accepted cost-effectiveness thresholds applied in the UK NHS. In several subgroups, the estimated QALY gain favoured EA, but this may be due to the small numbers and should be treated with caution. Moreover, all the p-values for the interaction terms between the corresponding subgroup and treatment effect variables were > 0.05 (i.e. none of the subgroup indicators were found to have a statistically significant effect on incremental health service costs or incremental QALYs) (see Appendix 3, Table 45).
Indirect costs
Table 18 shows the time to return to paid work, unpaid work and leisure or social activities, reported at 6 weeks post surgery. Women in the LASH group took longer to return to all of these activities. The productivity costs associated with time away from paid and unpaid work in the LASH group came to £2586, compared with £990 following EA. Women in the LASH and EA groups reported similar amounts of time away from paid and unpaid employment and leisure activities at 6 months post surgery and 15 months post randomisation (see Appendix 3, Table 45).
Data were also collected on out-of-pocket expenses and time costs associated with travel to and from health-care appointments. There were no notable between-group differences in mean out-of-pocket expenses (£9 and £7 for the LASH and EA, respectively) or in the value of time lost to attend outpatient appointments (£26 and £22 for the LASH and EA, respectively).
Summary of costs and consequences
The results of the cost-effectiveness analysis focused on the ICER using generic QALYs as the unit of effectiveness, as prespecified in the HEALTH protocol. Although the trial-based cost-effectiveness analysis did not show any significant difference in QALYs by 15 months post randomisation, LASH was superior on both primary outcome measures for clinical effectiveness, as well as a range of secondary outcomes. To summarise, although LASH conferred significantly higher direct costs on the health service (+£1604) and indirect costs on society (+£1596) over 15 months of follow-up (post randomisation), it also provided significantly greater benefits at 15 months in terms of satisfaction with treatment, MMAS score, EQ-5D-3L VAS score, SF-12 MCS, acceptability of treatment and willingness to recommend treatment (see Tables 10 and 11 for effect sizes). These benefits were consistent with an emerging difference in the EQ-5D-3L score favouring LASH at 15 months. Although the difference in the EQ-5D-3L score did not reach statistical significance by 15 months, it pointed to the need to extrapolate over a longer time horizon to adequately inform the cost-effectiveness of LASH compared with EA.
Discussion
The within-trial cost–utility analysis reported in this chapter indicated that, over a 15-month post-randomisation follow-up period, intention to treat with LASH resulted in increased costs to the health service (mean difference £1604). This was mainly driven by the cost of the initial procedure, with LASH taking twice as long to perform and resulting in a longer hospital stay than EA. Costs to society were also increased following LASH (£1596), because women treated with LASH took longer to return to paid and unpaid productive activities than those treated with EA. There was very little difference in QALYs between the treatment allocation groups, assessed over 15 months from randomisation, resulting in the ICER for LASH being unfavourable at this time point. The 15-month ICER also remained unfavourable to LASH in all the sensitivity analyses and subgroup analyses performed.
Strengths of the trial-based economic analysis include the availability of randomised data on resource use and HRQoL profiles collected prospectively as part of HEALTH. This enables accurate and unbiased estimation of mean differences in costs and QALYs over the trial follow-up period. The pragmatic design and intention-to-treat principles also enhance the generalisability of the findings to routine practice in the UK NHS.
The key limitation of the trial-based economic analysis relates to the relatively short duration of follow-up. Although there was no notable difference in QALYs between the groups by 15 months post randomisation, this time horizon was insufficient for drawing any conclusions on cost-effectiveness. The follow-up data indicate that there were more readmissions to hospital among those randomised to EA (n = 27) than there were among those randomised to LASH (n = 15), including 13 hysterectomies following EA compared with only one surgical episode related to menstrual bleeding post LASH. This trend for an increased incidence of further gynaecological surgery following EA is likely to become more pronounced in the future,10 reducing the incremental cost of LASH and resulting in QALY gains favouring it. Furthermore, the lack of difference in QALYs by 15 months post randomisation belied the fact that the EQ-5D-3L health state utility curves crossed during follow-up. Those randomised to EA experienced a shorter waiting time and quicker rise in health state utility following surgery, but then a subsequent decline in their EQ-5D-3L score by 15 months. The LASH group, on the other hand, experienced continued improvement in their EQ-5D-3L score out to 15 months post randomisation, by which time the mean score was higher than the EA group. As QALYs were computed as the area under the health state utility curve, terminating follow-up at 15 months has truncated the incremental QALY gains that would be expected to accrue to LASH if the 15-month difference in health state utility was maintained or increased over time. It is therefore necessary to extrapolate the trial results over a longer time horizon to inform cost-effectiveness. This is the focus of Chapter 6.
Chapter 6 Economic modelling
Introduction
The purpose of this chapter is to report on the details of further modelling conducted to extrapolate the trial-based cost-effectiveness findings beyond 15 months post randomisation. Although the within-trial analysis is useful for informing differences in costs and outcomes over a relatively short time horizon, it does not capture expected differences over the medium (2–5 years) to long term (5–10 years). Eventually 20–25% of women assigned to EA are expected to require further surgical treatment,5,9,10 resulting in downstream QALY losses and higher readmission costs than with LASH. These anticipated costs and consequences should be accounted for in a full economic evaluation. 43 Furthermore, although there was no significant between-group difference in QALYs observed by 15 months post randomisation in HEALTH, the mean health state utility scores appeared to be diverging by this time point (see Chapter 5, Table 14). Although the difference was not statistically significant, the direction of the effect in favour of LASH is consistent with the reported superiority of LASH on the primary clinical outcomes and with the expectation of higher repeat surgery rates in the EA group.
To estimate longer-term economic differences, a simple Markov model was developed to extrapolate the estimated 15-month difference in utility and simulate the incidence of further gynaecological surgery over time. The key objective of the analysis was to inform the long-term cost-effectiveness of LASH compared with EA. 1 This analysis forms the primary economic analysis of HEALTH.
Methods
Model structure
The Markov model was constructed in TreeAge Pro software (TreeAge Software, Inc., Williamstown, MA, USA) and was informed by reviewing decision models identified in a recent systematic review that was undertaken to inform the NICE clinical guidelines on the assessment and management of HMB. 43 A structure similar to that of other models was developed to assess the cost-effectiveness of treatments for HMB in the UK NHS was chosen. 9,10,43 The state occupancy and estimated pay-offs are updated on a constant monthly Markov cycle.
A cohort of women with HMB enter the model in the ‘HMB’ health state and are assigned to treatment with either EA or LASH. Women are modelled to receive treatment as observed in HEALTH by intention to treat. However, for simplicity, index procedures are modelled to occur in the first cycle of the model, with the waiting time factored out.
Following the index treatment in the EA arm (Figure 9), women move to either the ‘post EA’ or ‘complication post EA’ health state. The complication health state is designed to capture the cost and utility impact of postoperative complications resulting in readmission to hospital. These events are assumed to be transitory and so the health state utility impact is modelled to last for one cycle (1 month). Following this, women transit to the ‘post EA’ health state for the subsequent model cycle unless they progress to further surgery. The transition to further surgery from the ‘post EA’ health state is modelled on a monthly basis. Although the model has the capability to include first-generation EA (rollerball) as a second-line surgical treatment, the base case assumes that hysterectomy is the treatment of choice for women who require further surgery following failed second-generation EA. Repeat EA is not explicitly recommended as a treatment option in the recently updated NICE clinical guidelines for HMB. 43 For women who transition to ‘hysterectomy post EA’, the surgical intervention occurs in the first model cycle following the transition. Following this, women either enter a temporary health state representing severe postoperative complications, or they move to ‘convalescence post total hysterectomy’. The convalescence period is modelled to last for 3 months, after which all women transition to the ‘well post total hysterectomy’ health state. Finally, ‘death’ is included as an absorbing state in the model, which can be entered from any other state based on age-specific mortality rates reported for females in UK life tables. 44
FIGURE 9.
Simplified schematic for the EA arm of the Markov model. The transition from ‘Post EA’ to ‘Well post total hysterectomy’ is included as a one off transition at month 12 to account for the small proportion of patients who had a total hysterectomy instead of EA for their index treatment.
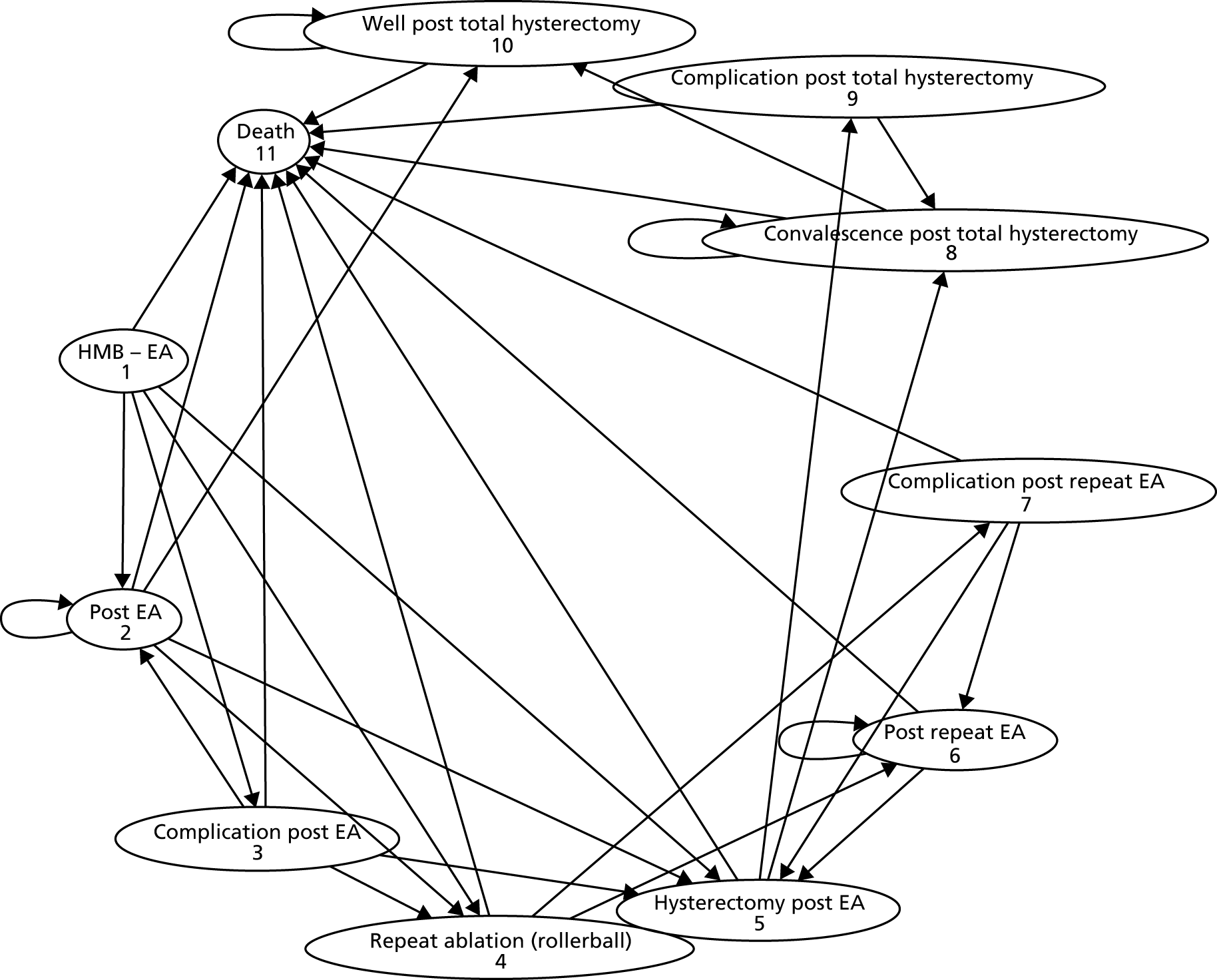
The structure of the model in the LASH arm is similar to that in the EA arm (Figure 10). However, following index treatment women either enter a temporary health state to capture postoperative complications or they transition to ‘convalescence post LASH’. The complication state serves the same purpose as it does in the EA arm. The convalesce state is included to capture the longer time to full recovery observed for LASH than for EA in HEALTH. From the post-LASH health states, a monthly probability of requiring further related gynaecological surgery is also applied. This includes further surgery related to ongoing bleeding or pain following LASH, specifically removal of the cervical stump, laparoscopy to investigate/treat pain and laparoscopic bilateral salpingo-oophorectomy (BSO). Following further surgery post LASH, women enter a post-surgical state dependent on the type of surgery received. For women who undergo cervical stump removal, a probability of severe post-surgical complications is applied and a convalescence period of 3 months is also assumed. Following convalescence, women are assumed to be well for the remainder of the modelled time horizon. For women who require laparoscopy for pain or laparoscopic BSO, no further postoperative complications or convalescence period are modelled, but women attract lower health state utility in the cycle leading up to surgery. After surgery they are also assumed to be well for the remaining time horizon.
FIGURE 10.
Simplified schematic for the LASH arm of the Markov model.
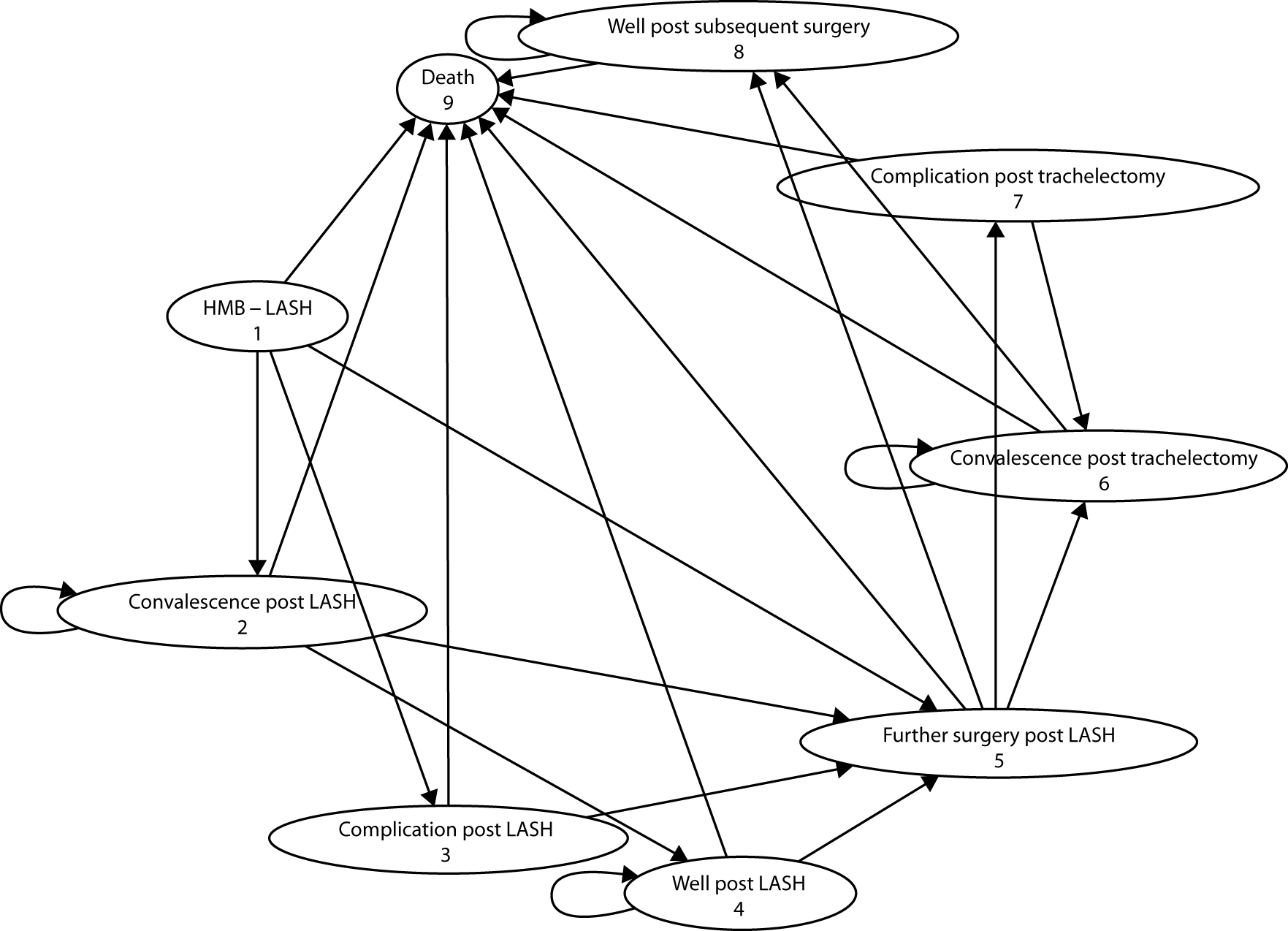
Population
The model analysis was conducted for a cohort of women with characteristics matching those of the HEALTH cohort (mean age equals 42 years at baseline). The estimation of input parameters for the economic model was based on the same intention-to-treat principles applied in the HEALTH analysis. Thus, the model reflects the fact that a number of women do not receive their intended treatment. A scenario analysis was also conducted to assess cost-effectiveness based on the restricted cohort that received their allocated treatment (per-protocol analysis).
Time horizon and discounting
In line with a previous UK HTA conducted by Bhattacharya et al. ,10 a long-term, 10-year time horizon was applied in the model base case. This is consistent with the average age at onset of menopause in the UK, and observational data which shows that the incidence of subsequent hysterectomy following EA continues to rise out to 10 years post surgery. 5 The impact of adopting a medium-term time horizon of 5 years was assessed in a sensitivity analysis. Costs and QALYs accruing beyond year 1 in the model are discounted using an annual discount rate of 3.5%. 42,45
Clinical input parameters
The key clinical input parameters in the model are tabulated in Table 19. The rates of post-surgical complications following EA and LASH were based on HEALTH data, as were the rates of subsequent gynaecological surgery for HMB out to 1 year following index surgery. Figure 11 shows the Kaplan–Meier (KM) plots for further gynaecological surgery by treatment arm in HEALTH (conditioned on receipt of index surgery); the estimated 12-month probabilities were 3% in the EA arm and 0.7% in the LASH arm. These probabilities were transformed into monthly probabilities for application over the first 12 cycles in the model.
| Variable | Point estimate | Standard error | Distributional form | Source |
|---|---|---|---|---|
| Probability of readmission for complications post EA | 0.033 | 0.01 | Beta | HEALTH |
| OR for postoperative complications (LASH vs. EA) | 1.107 | 0.420 | Lognormal | HEALTH |
| Probability of hysterectomy post EA (by 12 months)a | 0.03 | 0.0098 | Beta | HEALTH |
| Probability of further surgery for bleeding post LASH (by 12 months)a | 0.0069 | 0.0049 | Beta | HEALTH |
| Probability of hysterectomy post EA (beyond 12 months up to 10 years) | Applied and tested deterministically | |||
| Weibull rate parameter (λ) | 0.119 | Cooper et al.5 | ||
| Weibull shape parameter (γ) | 0.397 | Cooper et al.5 | ||
| Log HR for hysterectomy (second- vs. first-generation techniques)b | –0.274 | 0.0532 | Lognormal | Bansi-Matharu et al.46 |
| Inferred log HR for further surgery post LASH vs. EAc | –0.4886 | 0.122 | Lognormal | Calibrated to Lieng et al., 200817; see Extrapolation of subsequent surgery following laparoscopic supracervical hysterectomy |
| Probability of severe complications post hysterectomy | 0.0102 | 0.001 | Beta | Maresh et al.47; Bhattacharya et al.10 |
| Probability of severe complications post trachelectomy | 0.0102 | 0.001 | Beta | Assumption |
FIGURE 11.
Kaplan–Meier plot of time from index operation to further related surgery by treatment allocation.
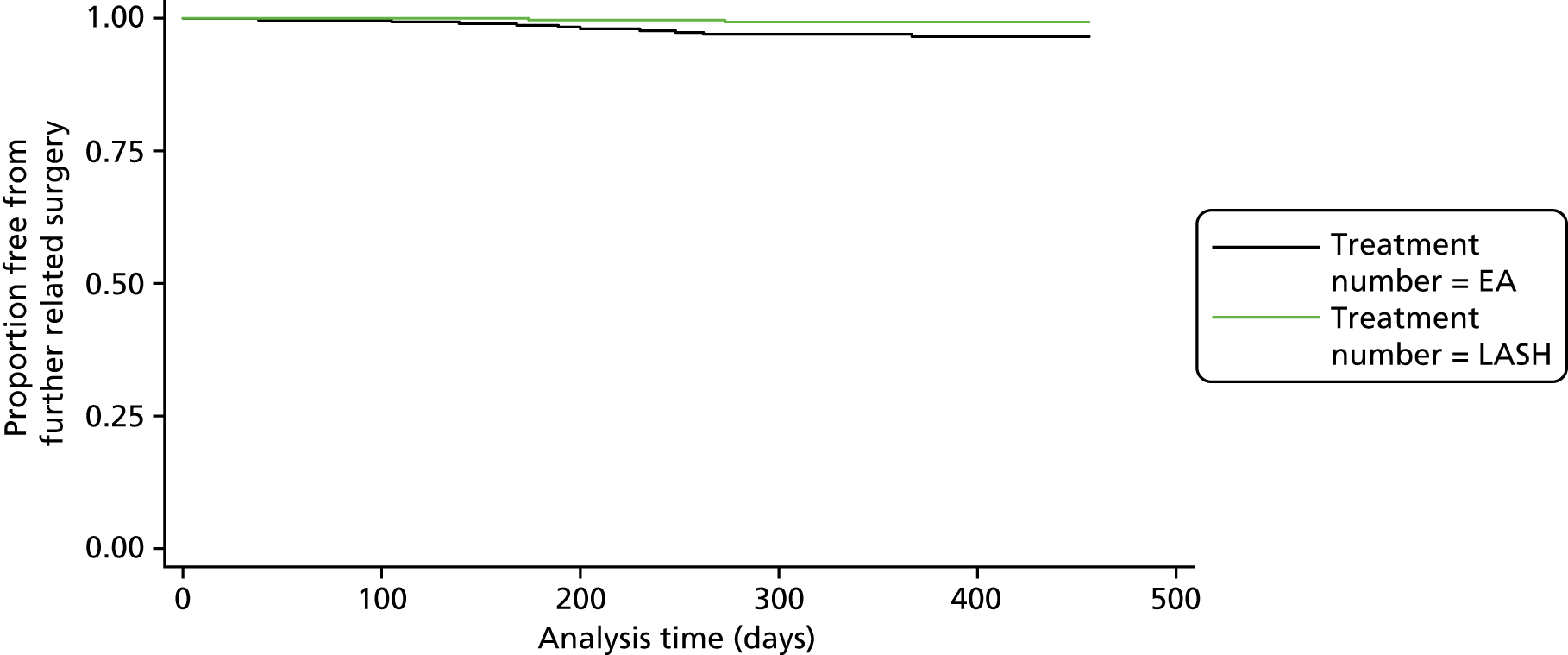
Extrapolation of subsequent hysterectomy following endometrial ablation
Given what is known about the probability of hysterectomy following EA, it is anticipated that the KM curves for time to further surgery will continue to diverge over time. In addition, as the follow-up of HEALTH is currently truncated at 15 months post randomisation and waiting times for the index surgery were 3–4 months on average, only 69% of women in the EA group and 56% in the LASH group were observed to 12 months post surgery at the time of writing. Coupled with the current waiting times for subsequent surgery among those who need it, HEALTH data do not currently give an accurate picture of further surgery rates by treatment allocation group.
Given these limitations, external data were used to inform rates of subsequent surgery beyond 12 months in the model. A focused search of MEDLINE was conducted to identify randomised trials comparing EA and LASH using the Ovid interface (see Appendix 4). This identified one small RCT which included long-term follow-up of patients. Zupi et al. 48 reported that, after a mean follow-up of 14.4 years, 20 out of 71 patients (28.1%) required a reoperation for menstrual bleeding in the EA arm of the trial, compared with none in the LASH arm (out of 82 available for analysis). They did report that 6 out of 82 women (7.3%) required further surgery in the LASH arm for indications other than menstrual bleeding (five requiring a laparoscopy to investigate pain). Although this RCT provides evidence in favour of a lower rate of subsequent related surgery following LASH, it is a small, single-centre study. Given this limitation, a further focused literature search was undertaken to identify cohort studies reporting rates of hysterectomy following EA or rates of cervical stump removal, laparoscopy and BSO following LASH (see Appendix 4). Priority was placed on identifying large population-based cohort studies reporting long-term rates. This yielded three5,46,49 population-based cohort studies reporting rates of hysterectomy following EA and eight17,50–56 smaller observational studies reporting rates of further surgery following LASH (see Appendix 4 for details).
For the probability of hysterectomy following EA, we used data from a large observational study carried out using routine Scottish health service data. Based on linked health episode data, Cooper et al. 5 reported on 14,078 women identified as having received primary EA for HMB between 1989 and 2006 in Scotland. Over a median duration of 6.8 years follow-up, 2779 (19.7%) women were observed to receive a subsequent hysterectomy. Data were extracted from the published KM curve using digitising software (WebPlotDigitizer, V4.1, 2018; URL: https://automeris.io/WebPlotDigitizer/) and regression methods were then used to fit a Weibull distribution to the observed time-to-event data (see Appendix 5 for details).
A limitation of the study by Cooper et al. 5 was an inability to discriminate between EAs carried out using second- and first-generation techniques, and a number of studies have suggested that some second-generation techniques may incur a lower risk of post-ablation hysterectomy. 46,57 However, a very similar rate of post-ablation hysterectomy has been reported following second-generation procedures carried out between 1997 and 2007 in a Finnish registry study (1086/5484, 19.8%). 49 The Weibull distribution fitted to the Scottish data was therefore used in the model to derive time-dependent probabilities of transition to hysterectomy in the EA arm (see Appendix 5 for details regarding the derivation of transition probabilities). Sensitivity analysis was used to explore the impact of adjusting the hazard rate downwards using a HR for radiofrequency ablation (HR 0.76, 95% CI 0.69 to 0.85), reported by Bansi-Matharu et al. 46 and based on a large English population-based cohort study. Further sensitivity analysis was also conducted to explore the impact of using time-dependent transition probabilities for hysterectomy following EA, derived directly from the KM curve reported by Cooper et al. 5 The model projections of post-ablation hysterectomy were compared against the 5-year rates reported by Bansi-Matharu et al. 46 as means of external validation (see Model validation).
Extrapolation of subsequent surgery following laparoscopic supracervical hysterectomy
Several studies of variable size and quality were identified to inform the risk of further surgery following LASH (see Appendix 4). The reported incidence of post-LASH cervical stump removal ranged from 0.9% to 23%. 17,50–53,58 Based on length of follow-up, consistency with the observed 12-month rate in HEALTH, and a similar rate of post-LASH menstrual bleeding (24% vs. 19%), a Norwegian cohort reported by Lieng et al. 17 was considered the most useful for informing longer-term rates in the model (see Appendix 4 for details). Lieng et al. 17 reported that, of 308 women undergoing LASH at a university hospital in Oslo during 2004 and 2005, six (1.9%) subsequently underwent laparoscopic adhesiolysis, seven (2.3%) underwent laparoscopic cervical stump removal, one (0.3%) underwent BSO and eight underwent other procedures in the 12–36 months of follow-up after LASH. The other reported procedures included laparoscopic drainage of postoperative abscess (n = 1), laparoscopy with bowel resection for postoperative peritonitis (n = 1), scar correction (n = 3), umbilical hernia repair (n = 1) and tension-free vaginal tape procedures (n = 2). These procedures were not included in the model as they were considered to be either short-term postoperative complications (included in the model based on HEALTH data) or of uncertain association with the LASH procedure. The model was therefore calibrated to yield the combined cumulative incidence of laparoscopic adhesiolysis, laparoscopic cervical stump removal and BSO (4.5%), reported by Lieng et al. 17 by month 36. This was done by applying a HR to the rate parameter of the Weibull distribution used to model time to hysterectomy following EA. Thus, it is assumed that the hazard for further surgery post LASH is proportional to the hazard of hysterectomy post EA. This approach yields a cumulative incidence of further surgery of ≈11% by 10 years post LASH in the model.
Postoperative complications following hysterectomy and removal of the cervical stump
For those women modelled to go on to receive a hysterectomy following EA, or cervical stump removal following LASH, an associated postoperative complication rate is applied. This probability is taken from the previous HTA study conducted by Bhattacharya et al. ,10 originally sourced from Maresh et al. 47 As no data were available to inform the risk of severe postoperative complications following cervical stump removal, the same probability of complications was applied to this procedure.
Health state utilities
The health state utilities applied in the model were derived primarily from the EQ-5D-3L data from HEALTH (Table 20). For the first 12 months in the model, adjusted utility estimates are applied by treatment arm. Beyond 12 months, extrapolation assumptions are applied.
| Variable | EA (mean) | LASH (mean) | Mean difference (95% CI) | Distributional form | Source |
|---|---|---|---|---|---|
| Baseline | 0.702 | 0.702 | – | Beta | HEALTH |
| 6 weeks post surgery (no complications) | 0.829 | 0.820 | –0.009 (–0.05 to 0.032) | Beta + normal for (increment) | HEALTH |
| 6 weeks post surgery (postoperative complications) | 0.786 | 0.739 | –0.047 (–0.275 to 0.182) | Beta + normal for (increment) | HEALTH |
| 6 months post surgery (from cycle 3)a | 0.815 | 0.817 | 0.0019 (–0.041 to 0.044) | Beta + normal for (increment) | HEALTH |
| 12 months post surgery (from cycle 9)a | 0.787 | 0.825 | 0.038 (–0.013 to 0.090) | Beta + normal for (increment) | HEALTH |
Post-endometrial ablation and post-laparoscopic supracervical hysterectomy health state utility (to 12 months)
The baseline utility estimate for women entering the model was taken as the mean baseline EQ-5D-3L index score observed across treatment groups. Following treatment in the first cycle of the model, the cohort is distributed between the relevant post-treatment states. In cycle 2, the cohort is dichotomised by whether or not early postoperative complications occur. Health state utility values were therefore estimated by treatment allocation group and the occurrence of postoperative complications resulting in readmission to hospital. This was done by regressing the 6-week utility data on indicators for treatment allocation, readmission for complications (yes/no) and the interaction between treatment allocation and readmission. The regression also adjusted for baseline utility and age (< 40 years vs. ≥ 40 years) as a minimisation factor used in the randomisation process. Ordinary least squares regression was used for the analysis of the utility data, given the moderately large sample size, but with cluster robust standard errors. 59 The method of recycled predictions was used to recover the adjusted mean value in the base group, and the estimated incremental effects and robust standard errors from the regression models were used to define the distributions on effect differences in the Markov model. The analyses to inform the model utility inputs were based on available complete data for the relevant variables.
Beyond cycle 2 in the model, those remaining in the post-EA or post-LASH health states were assigned the corresponding treatment arm-specific 6-month utility values derived from HEALTH data. Those remaining in the post-EA or post-LASH treatment states were then modelled to receive the relevant treatment arm-specific 15-month utility value from cycle 9 (month 9) post surgery. This assumes that, on average, the 15-month post-randomisation utility estimate from HEALTH corresponds to 12-month postoperative utility, and that the change in health state utility between 6 months and 12 months post surgery is linear. For those modelled to incur a hysterectomy following EA, or subsequent surgery post LASH, further health state utility assumptions were applied as described below under Extrapolations of post-endometrial ablation and post-laparoscopic supracervical hysterectomy health state utility.
Extrapolations of post-endometrial ablation and post-laparoscopic supracervical hysterectomy health state utility
Table 20 shows that health state utility was slightly increased following EA, relative to LASH, in the short term (6 weeks), but, by 15 months post randomisation (≈12 months post surgery), there was an emerging difference that favoured LASH. Although this difference was not significant (p = 0.14), the direction of effect is consistent with superior satisfaction and MMAS scores observed in the LASH group at 15 months (see Tables 10 and 11). A lower utility value among those remaining in the post-EA health state at 12 months is also consistent with the expected higher incidence of further surgery (hysterectomy) beyond 12 months. It may reflect an increased number of women experiencing treatment failure or recurrence by 12 months post EA, who are yet to return for further surgery.
Therefore, the model extrapolated the estimated 12-month (post-surgery) between-group difference in health state utility over an extended time horizon (Figure 12). However, it is anticipated that it will be those women who have a poorer outcome at 12 months following EA who return in the future for a hysterectomy. Thus, the average utility score among those remaining in the post-EA state would be expected to rise over time. Conversely, it is possible that the recurrence of symptoms following EA has yet to peak and that the utility curves will continue to diverge further beyond 12 months before beginning to converge. It is also uncertain to what extent the post-EA and post-LASH utility curves may converge, and how quickly. In our base-case analysis, we adopted a conservative approach (in favour of EA) and assumed that the mean difference in utility between the post-EA and post-LASH health states would diminish over time in proportion to the total expected number of post-EA hysterectomies completed. For example, from a baseline of 12 months post surgery, 50% of the further post-EA hysterectomies will have been completed after a further 3 years, by which time the extrapolated utility difference is approximately half of that observed at 12 months. By 10 years in the model, the utility difference is assumed to have diminished to zero. We explored the impact of applying alternative extrapolation assumptions in sensitivity analysis, including retention of the full 12 months post-surgery utility difference over the entire duration of the model and allowing it to diminish over a shorter time.
FIGURE 12.
Observed and extrapolated health state utility post LASH and post EA.
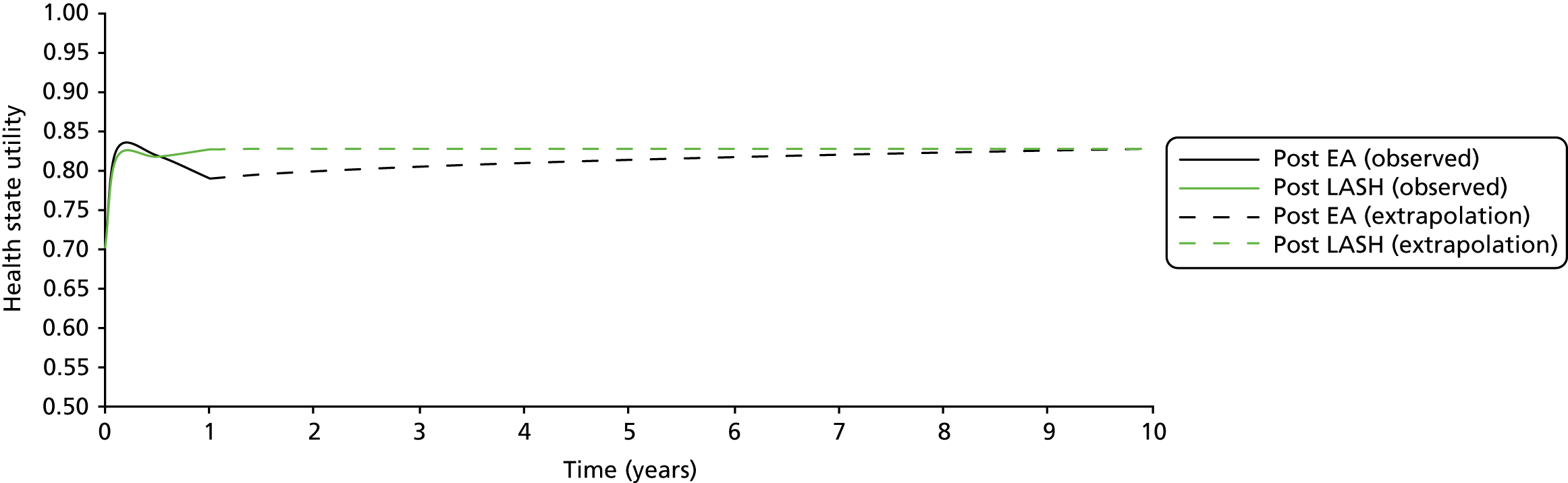
Health state utilities associated with further surgery
Regarding the heath state utility of those transitioning to hysterectomy following EA, similar assumptions to those used in the HTA by Bhattacharya et al. 10 were applied. Women were assigned the baseline utility value of 0.702 in the cycle preceding hysterectomy. Following that, a utility value appropriate to the post-hysterectomy state was applied. For severe postoperative complications following hysterectomy, a utility value of 0.49 was sourced from Bhattacharya et al. ,10 originally from Clegg et al. ,60 and applied for a single cycle. For the convalescence period following hysterectomy (3 months), a utility value of 0.74 was applied. 10,61 Finally, it was assumed that health state utility in the ‘well post hysterectomy’ health state is equivalent to that in the ‘well post LASH’ health state. Table 21 provides a summary of health state utility inputs applied for the further surgery states.
| Variable | Mean | SEM | Distributional form | Source |
|---|---|---|---|---|
| Symptomatic requiring further surgery | 0.702 | 0.012 | Beta | HEALTH |
| Severe postoperative complication following hysterectomy or removal of cervical stump | 0.49 | 0.049 | Beta | Clegg et al.60 |
| Convalescence post hysterectomy or removal of cervical stump | 0.74 | 0.05 | Beta | Sculpher61 |
| Well post hysterectomy | 0.827 | Beta | Assumption (see Health state utilities associated with further surgery) |
For women requiring cervical stump removal following LASH, the same utility assumptions pertaining to total hysterectomy were applied. For post-LASH laparoscopy or BSO, we applied baseline utility in the cycle preceding treatment and the cycle of treatment, but then assumed a return to the ‘well post LASH’ state. Thus, overall, the model base case assumes that, after 10 years, women ultimately end up at the same level of health state utility, although the pathway to that outcome varies.
Health service resource use and costs
Health service costs applied in the model were informed by the analysis of HEALTH data to 12 months post surgery. As with the analysis of health state utility data, the individual cost inputs in the economic model were informed, where possible, by ordinary least squares regression of the relevant complete trial cost variables in the first 12 cycles. Costs were regressed on indicators for treatment allocation and age group, with adjustment for clustering by centre. The method of recycled predictions was used to recover the mean adjusted costs in the base treatment group, and the estimated incremental effects and cluster robust standard errors from the regressions were used to define distributions for the cost differences applied in the model. The mean initial treatment cost was estimated by intention to treat and applied in the first cycle of the model. A two-part regression model was used to estimate the probability of further admission for postoperative complications by treatment arm and the cost of treating these complications, conditional on readmission. The first part utilised logistic regression and the second part OLS, to estimate adjusted mean readmission costs by treatment allocation, conditional on readmission. These postoperative complication costs were applied in the second cycle of the model following the initial treatment episode. Subsequent hysterectomies following EA, and cervical stump removal and further laparoscopic surgery following LASH, were costed using the appropriate HRG-based reference costs (Table 22). 32
| Variable | Mean (£) | SE | Distributional form | Source |
|---|---|---|---|---|
| Initial hospital episode cost (EA) | 1071 | 34.79 | Gamma | HEALTH |
| Incremental cost of initial episode (LASH) | 1686 | 75.32 | Normal | HEALTH |
| Postoperative complication cost (EA) | 854 | 176.81 | Gamma | HEALTH |
| Incremental cost of postoperative complications (LASH) | 194 | 149.47 | Normal | HEALTH |
| Hysterectomy post EA | 3408 | 725 | Gamma | NHS Reference Costs 2016–201732 (HRG MA08, elective inpatient) |
| Removal of cervical stump post LASH | 2776 | 704 | Gamma | NHS Reference Costs 2016–201732 (HRG MA03, elective inpatient) |
| Laparoscopic investigation for pain (post LASH) | 2482 | 628 | Gamma | NHS Reference Costs 2016–201732 (HRG MA29, average) |
| Laparoscopic BSO | 3408 | 725 | Gamma | NHS Reference Costs 2016–201732 (HRG MA08, elective inpatient) |
| Post-EA monthly health state cost (first year) | 5.96 | 0.84 | Gamma | HEALTH |
| Post-LASH monthly health state cost (first year) | 6.04 | 0.92 | Gamma | HEALTH |
| Post-EA monthly health state cost (> 1 year) | 3.49 | 0.48 | Gamma | HEALTH |
| Post-LASH monthly health state cost (> 1 year) | 3.30 | 0.56 | Gamma | HEALTH |
Finally, HEALTH follow-up data were used to estimate other costs (outpatient appointments, medications and primary care use) to the health service post LASH and post EA. These were transformed into monthly costs for application as health state costs in the model. The post EA costs were applied to the post-EA states, and the post-LASH costs were applied to the post-LASH and post-hysterectomy health states. These costs were stripped of outpatient costs during the extrapolation phase of the model and a separate outpatient referral cost was applied on transition to further surgery for those requiring it.
Values based on the analysis of HEALTH data are adjusted for baseline utility and minimisation factors using lineal regression, with adjustment for clustering within centres.
Model validation
To assess the internal validity of the model we compared the 12-month model-based cost and QALY estimates with the 15-month trial-based estimates. These data are provided in Table 23. Although the expected QALYs were lower in both arms in the model, the estimated incremental difference was similarly very small. The lower average QALY estimates are accounted for by the simplifying assumption of omitting the pre-treatment waiting period and running the model for 12 months rather than 15 months. The mean treatment arm costs were also very similar between the model and trial-based analyses, as was the incremental cost. The small differences are attributable to the fact that the model was populated using the available complete data for each input parameter, rather than the multiple imputation data set used in the trial-based analysis.
| Variable | EA | LASH | Difference |
|---|---|---|---|
| Trial-based estimates | |||
| Mean cost (£) | 1282 | 2886 | 1604 |
| Mean QALY | 0.974 | 0.978 | 0.004 |
| Model-based 12-month estimates | |||
| Mean cost (£) | 1277 | 2890 | 1612 |
| Mean QALY | 0.8119 | 0.8140 | 0.0021 |
To assess the validity of projected rates of further surgery beyond 12 months, the modelled cumulative incidence following EA and LASH was plotted over the 10-year time horizon (Figure 13). The model projected that cumulative incidence of hysterectomy following EA was 3.3%, 6.8% and 12.8% after 1, 2 and 5 years, respectively. This compares to estimates from a large population-based English study of 5.6%, 9.6% and 13.5%, respectively, at these corresponding time points. 46 The incidence was lower in the model because the lower 12-month rate from HEALTH was applied in year 1. There were few data against which to validate the projections for further surgery following LASH. As previously discussed under Extrapolation of subsequent surgery following laparoscopic supracervical hysterectomy, the projected estimates are in line with the incidence observed at 12–36 months in a Norwegian cohort,17 and reach ≈11% by 10 years post surgery when combined with the extrapolation assumptions.
FIGURE 13.
Modelled incidence of further surgery following EA and LASH.
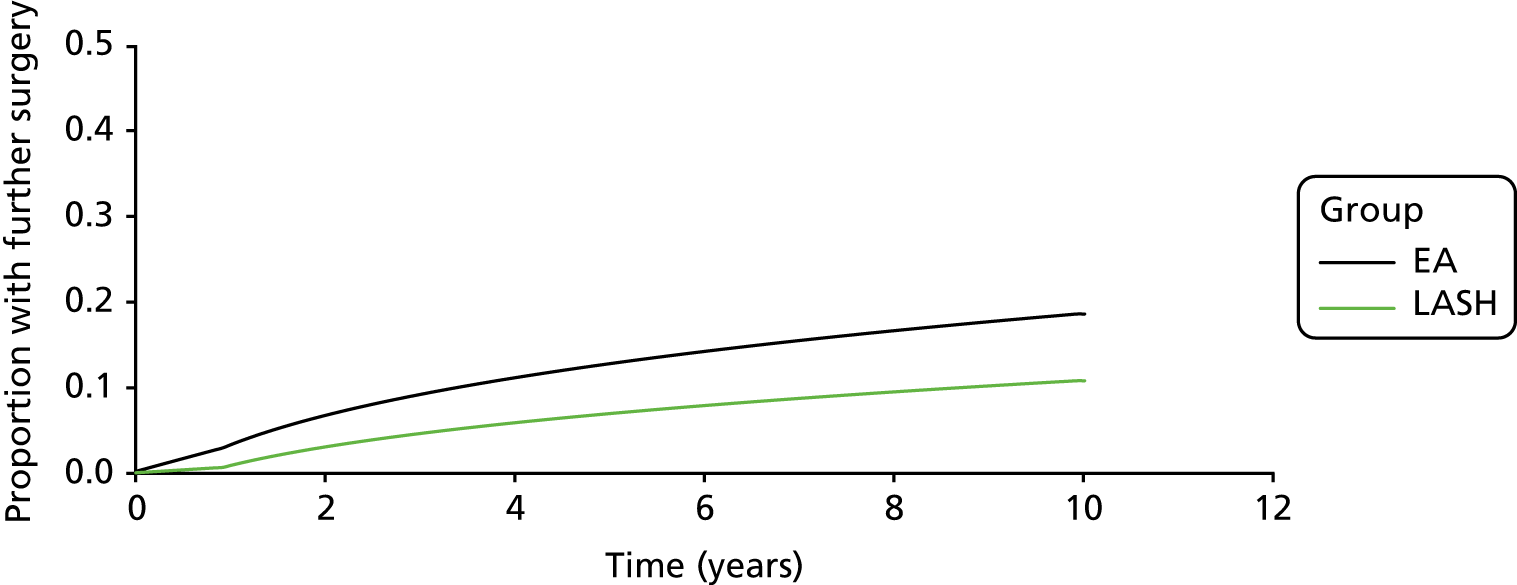
Model analysis
The model-based analysis utilised second-order Monte Carlo simulation to characterise the joint uncertainty surrounding the estimated incremental costs and effects of LASH compared with EA. 62 A probability distribution was assigned to each model input parameter, reflecting the degree of uncertainty surrounding it owing to sampling variation. The functional form and variance of each input distribution are provided in Tables 20–23. In general, gamma distributions were used to represent uncertainty surrounding cost inputs, beta distributions were applied for probabilities and utility parameters and lognormal distributions were used for HRs. The probabilistic analysis was run using 10,000 random draws from the assigned input distributions, generating 10,000 estimates of incremental costs and effects. The point estimate of the ICER was expressed as the mean incremental cost divided by the mean incremental effect across the 10,000 iterations. Tabulated results express the probability of each treatment option being preferred on grounds of cost-effectiveness at willingness-to-pay (WTP) thresholds of £13,000, £20,000 and £30,000 per QALY gained. 42,63 Cost-effectiveness scatterplots and acceptability curves are provided to further summarise the uncertainty surrounding the results. Further deterministic analysis was also conducted to assess the sensitivity of the model results to changes in key input parameters and structural assumptions.
Key assumptions
The following points summarise some of the key assumptions applied in the base-case analysis:
-
Index surgery costs associated with randomisation to EA or LASH were modelled to occur in the first cycle of the model and are estimated by intention to treat.
-
Data inputs were derived entirely from HEALTH over the first 12 cycles (months) of the model. The 15 months post-randomisation utility estimates were applied as 12 months post-surgery estimates.
-
The difference in health state utility between the post-EA and post-LASH health states is at its maximum by 12 months post surgery.
-
The difference in utility between the post-EA and post-LASH health state diminished over time in the model, in proportion with the number of post-EA hysterectomies expected to have been completed. Thus, post-EA and post-LASH health state utility converges completely by 10 years in the model.
-
Health state utility in the ‘well post total hysterectomy’ state was set equal to health state utility in the ‘well post LASH’ state of the model (i.e. this assumes that those requiring a hysterectomy following EA ultimately achieve the same level of HRQoL as those who remain well following LASH).
-
Beyond 12 months, the incidence of post-EA hysterectomy was based on Scottish data reported by Cooper et al. ,5 with the modelled cumulative incidence reaching 18.6% by 10 years.
-
Beyond 12 months, the incidence of further related surgery following LASH (removal of the stump and/or ovaries or laparoscopy investigation for pain) was calibrated to Norwegian data reported by Lieng et al. 17 The combined cumulative modelled incidence reaches 4.5% by 3 years and 11% by 10 years. It was assumed that all related surgery will have been completed by this time.
Results
Base-case analysis
Table 24 presents the results of the base-case analysis. Over the modelled 10-year time horizon, intention to treat with LASH resulted in an increased cost to the health service of £1362 per woman, for an expected QALY gain of 0.111 per woman, compared with EA. The corresponding ICER was £12,314 per QALY gained for LASH compared with EA. The chance of LASH being cost-effective ranged from 53% to 80% at WTP per QALY thresholds of £13,000 and £30,000, respectively. It can be noted that extending the time horizon of the evaluation from 1 year to 10 years reduced the incremental cost of LASH by £250 (£1362 at 10 years vs. £1612 at 1 year). This is due to the incorporation of expected costs of further surgery. The QALY gain associated with LASH resulted primarily from extrapolation of the estimated difference in 12-month post-EA and post-LASH health state utility. Further temporary reductions in utility associated with further surgery accounted for only a very small proportion of the incremental QALY.
| Strategy | Cost (£) | Δ cost (£) | QALYs | Δ QALYs | ICER (£) | Probability cost-effective | ||
|---|---|---|---|---|---|---|---|---|
| £13,000 | £20,000 | £30,000 | ||||||
| EA | 2089 | 6.938 | 0.468 | 0.291 | 0.201 | |||
| LASH | 3452 | 1362 | 7.049 | 0.111 | 12,314 | 0.532 | 0.709 | 0.799 |
Figures 14 and 15 further illustrate the uncertainty surrounding the estimated incremental costs and effects of LASH compared with EA. LASH remained the significantly more costly strategy based on extrapolation to 10 years, with all the estimated points being above zero on the y-axis of Figure 14. Most points (≈93%) also lie to the right of zero on the x-axis, indicating a 93% chance that LASH will generate more QALYs based on the simulation. The cost-effectiveness acceptability curve for LASH (see Figure 15) therefore asymptotes to 93%, as the threshold of WTP per QALY increases towards infinity. This is analogous to finding a p-value (one-sided) of 0.07 for an estimated difference in QALYs favouring LASH.
FIGURE 14.
Incremental cost-effectiveness scatterplot (LASH vs. EA).
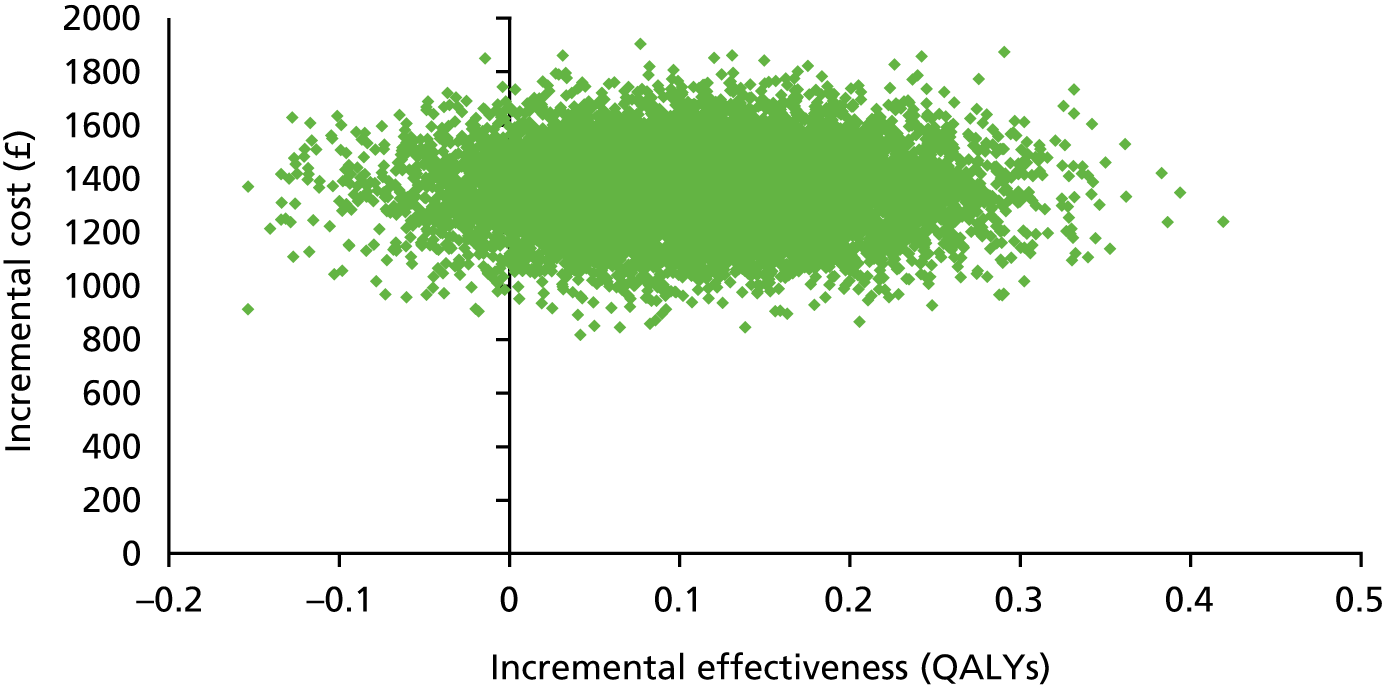
FIGURE 15.
Cost-effectiveness acceptability curve (LASH vs. EA).
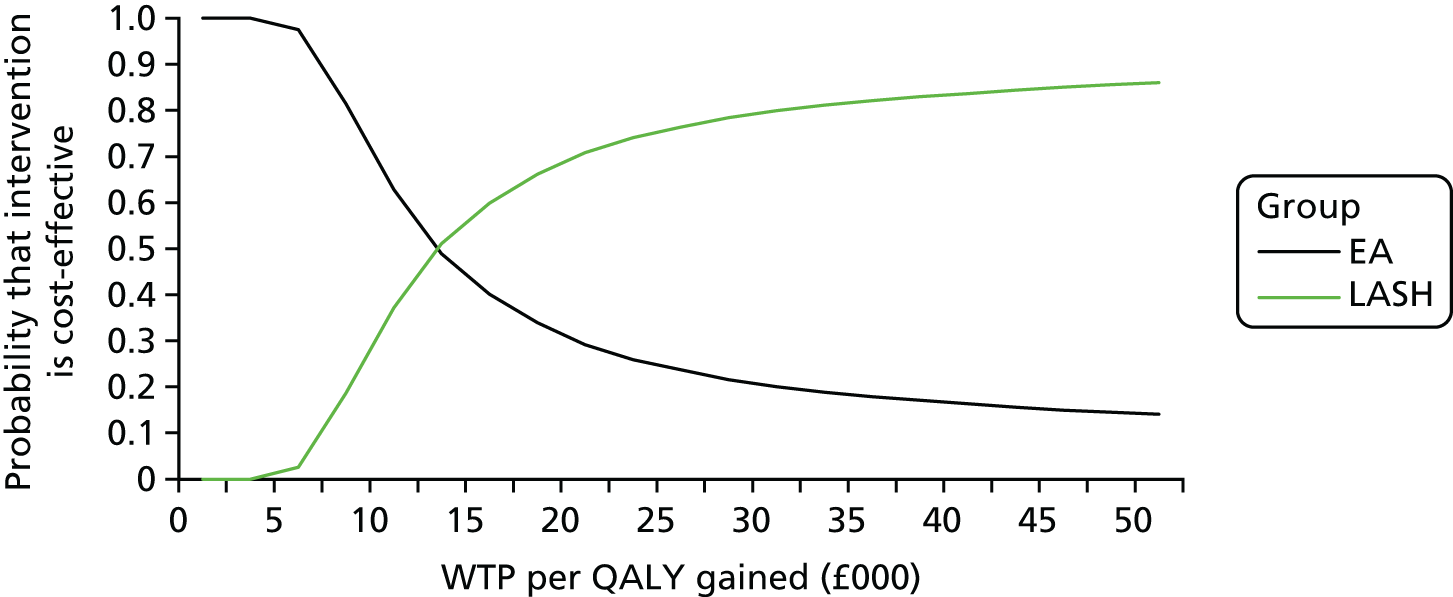
Scenario analyses
Further scenario analyses were conducted to assess the sensitivity of the model results to changes in key input parameters and assumptions. These included the following:
-
Running the model over a 5-year time horizon instead of a 10-year time horizon.
-
Lowering the rate of hysterectomy following EA, by applying a HR or 0.76 reported by Bansi-Matharu et al. 46 for radiofrequency ablation compared with first-generation techniques. The cumulative incidence of hysterectomy following EA drops to 15% by 10 years in this scenario.
-
Applying a lower rate of further surgery following LASH, by assuming that all further surgery will be complete by 5 years at a cumulative incidence of 7%.
-
Applying the estimated 12-month difference in post-EA and post-LASH utility over the entire time horizon of the model with no convergence.
-
Capping convergence of the post-EA and post-LASH health state utility at 50% of the 12-month difference.
-
Applying total convergence of the post-EA and post-LASH health state utility by 5 years post surgery, but retaining a 10-year time horizon.
-
Setting long-term health state utility following total hysterectomy (or removal of the cervical stump) 0.05 units higher than post-LASH health state utility.
-
Basing model input parameters on a per-protocol analysis of HEALTH data.
-
Inclusion of repeat ablation (first-generation rollerball) in the treatment pathway following failed second-generation ablation. Under this scenario, 2.5% of the EA cohort receive repeat ablation over the first 3 years of the model. 5
-
Inclusion of a separate pathology examination cost of £31.08 to reflect possible increased pathology costs with LASH compared with EA. 31
-
Utilisation of time-dependent transition probabilities derived directly from the KM curve reported by Cooper et al.,5 to model the incidence of hysterectomy following EA beyond month 12 in the model. In this scenario the HR for further surgery following LASH relative to EA is recalibrated to yield the 4.5% incidence at 36 months. This results in a cumulative incidence of 8% for further surgery by 10 years following LASH.
-
Utilisation of time-dependent transition probabilities derived directly from the KM curve reported by Cooper et al. 5 to model the incidence of hysterectomy over the entire time horizon of the model (i.e. from month 1). In this scenario the HR for further surgery following LASH relative to EA is recalibrated to yield the 4.5% incidence at 36 months. This results in a cumulative incidence of 8% for further surgery by 10 years following LASH.
The results of these scenario analyses are presented in Table 25. They illustrate that the ICER was most sensitive to changes in the extrapolation assumptions applied to the 12-month difference in post-EA and post-LASH health state utility (scenarios 5 to 6). When no convergence was applied in the model, the QALY gain associated with LASH increased substantially and the ICER dropped to around £5000 per additional QALY gained over EA (scenario 4). When complete convergence of the health state utility values was modelled to occur by 5 years, the QALY gain associated with LASH was approximately halved and the ICER increased substantially (scenario 6). The ICER also increased substantially when the model inputs were informed by a per-protocol analysis of HEALTH (scenario 8). This was primarily due to the cost increment being greater in this restricted population who received their allocated treatment as their index operation.
| Strategy | Cost | Incremental cost (£) | QALY | Incremental QALYs | ICER (£) | Probability cost-effective | ||
|---|---|---|---|---|---|---|---|---|
| £13,000 | £20,000 | £30,000 | ||||||
| Base case | ||||||||
| EA | 2089 | – | 6.938 | – | – | 0.468 | 0.291 | 0.201 |
| LASH | 3452 | 1362 | 7.049 | 0.111 | 12,314 | 0.532 | 0.709 | 0.799 |
| Scenario 1: 5-year time horizon | ||||||||
| EA | 1759 | – | 3.747 | – | – | 0.656 | 0.414 | 0.268 |
| LASH | 3205 | 1446 | 3.834 | 0.087 | 16,628 | 0.344 | 0.586 | 0.732 |
| Scenario 2: lower rate of hysterectomy following EA (see Scenario analyses) | ||||||||
| EA | 1979 | – | 6.936 | – | – | 0.476 | 0.296 | 0.205 |
| LASH | 3393 | 1414 | 7.050 | 0.114 | 12,429 | 0.524 | 0.704 | 0.795 |
| Scenario 3: lower rate of further related surgery following LASH (see Scenario analyses) | ||||||||
| EA | 2088 | – | 6.936 | – | – | 0.427 | 0.269 | 0.184 |
| LASH | 3362 | 1274 | 7.048 | 0.112 | 11,385 | 0.573 | 0.731 | 0.816 |
| Scenario 4: no convergence of 12-month post-EA and post-LASH health state utility | ||||||||
| EA | 2089 | – | 6.794 | – | – | 0.191 | 0.138 | 0.110 |
| LASH | 3451 | 1362 | 7.048 | 0.253 | 5377 | 0.809 | 0.862 | 0.890 |
| Scenario 5: convergence of post-EA and post-LASH health state utility capped at 50% of the 12-month difference | ||||||||
| EA | 2088 | – | 6.894 | – | – | 0.319 | 0.203 | 0.149 |
| LASH | 3450 | 1362 | 7.047 | 0.153 | 8907 | 0.681 | 0.797 | 0.851 |
| Scenario 6: total convergence of 12-month post-EA and post-LASH health state utility by 5 years post surgery | ||||||||
| EA | 2089 | – | 6.989 | – | – | 0.819 | 0.552 | 0.359 |
| LASH | 3452 | 1363 | 7.051 | 0.062 | 21,992 | 0.181 | 0.448 | 0.641 |
| Scenario 7: long-term health state utility following total hysterectomy (or removal of the cervical stump) is 0.05 higher than it is post LASH | ||||||||
| EA | 2088 | – | 6.986 | – | – | 0.597 | 0.410 | 0.300 |
| LASH | 3452 | 1364 | 7.072 | 0.086 | 15,864 | 0.403 | 0.590 | 0.700 |
| Scenario 8: model parameters based on per-protocol analysis of HEALTH data | ||||||||
| EA | 2093 | – | 6.985 | – | – | 0.636 | 0.381 | 0.241 |
| LASH | 3677 | 1584 | 7.084 | 0.099 | 15,927 | 0.364 | 0.619 | 0.759 |
| Scenario 9: include repeat ablation (first-generation rollerball) in the treatment pathway following failed second-generation ablation | ||||||||
| EA | 2137 | – | 6.934 | – | – | 0.433 | 0.273 | 0.187 |
| LASH | 3451 | 1315 | 7.048 | 0.114 | 11,544 | 0.567 | 0.727 | 0.813 |
| Scenario 10: include the cost of additional pathology testing for LASH | ||||||||
| EA | 2088 | – | 6.938 | – | – | 0.488 | 0.300 | 0.202 |
| LASH | 3480 | 1392 | 7.049 | 0.111 | 12,589 | 0.512 | 0.700 | 0.798 |
| Scenario 11: use extracted KM data from Cooper et al.5 (1–10 years) to estimate transition probabilities for hysterectomy post EA (beyond year 1)a | ||||||||
| EA | 2159 | – | 6.961 | – | – | 0.533 | 0.327 | 0.217 |
| LASH | 3386 | 1227 | 7.050 | 0.089 | 13,774 | 0.467 | 0.673 | 0.783 |
| Scenario 12: use extracted KM data from Cooper et al.5 (0–10 years) to estimate transition probabilities for hysterectomy post EA (entire time horizon)a | ||||||||
| EA | £2364 | – | 6.963 | – | – | 0.454 | 0.278 | 0.191 |
| LASH | 3386 | 1022 | 7.048 | 0.085 | 11,991 | 0.546 | 0.722 | 0.809 |
Discussion
The modelling exercise reported in this chapter indicates that intention to treat with LASH has the higher probability of being cost-effective in the longer term across a range of plausible values of WTP per QALY gained. The key sources of uncertainty in the model relate to the extrapolation of the observed difference in health state utility between the post-EA and post-LASH health states, and the impact that the incidence of further surgery will have on this parameter. The ICER for LASH ranged from as low as £5377, when the observed 15 months post-randomisation (12 months post surgery) utility difference was maintained over the entire time horizon of the model, to £21,992 when it was assumed to diminish to zero by 5 years post surgery. The further collection of patient-reported outcome data and the need for subsequent surgery at 3–5 years would help to reduce this uncertainty.
A strength of the modelling is its basis on randomised data from a large multicentre and pragmatic RCT. HEALTH utilised a detailed approach to costing the initial surgical episode and captured subsequent complications out to 15 months post randomisation (≈12 months post surgery). However, the censoring of patients for longer-term post-surgery health state utility is a limitation based on the trial design. Had a larger percentage of the cohort reached a minimum of 12 months post surgery by 15 months post randomisation, we may have observed a more marked difference in health state utility between the randomisation groups.
A further limitation relating to the duration of HEALTH was the need to rely on external non-randomised data to inform the expected longer-term incidence of further surgery by treatment allocation group. Although good data were available on the incidence of hysterectomy following EA, limited published data were available to inform the incidence of subsequent surgery post LASH. Therefore, conservative assumptions (favouring EA) were applied in the base-case analysis, with further surgery following LASH modelled to reach ≈11% by 10 years. This is substantially higher than the long-term incidence of further gynaecological surgery following total hysterectomy, reported to be 4% at a median duration of 11 years, based on Scottish population data. 5
As indicated above, the 15-month post-randomisation follow-up also necessitated the application of assumptions to extrapolate observed differences in health state utility over time. Again, conservative assumptions were applied, in that the observed 15-month (post-randomisation) difference in health state utility between the post-EA and post-LASH health states was modelled to diminish to zero by 10 years. This may underestimate the QALY gain for LASH compared with EA if the observed difference in utility at 15 months were to diverge further before converging, and/or converge less quickly over time. Nevertheless, the QALY gain for LASH compared with EA remains uncertain as it accrued primarily during the extrapolation phase of the model. A further uncertainty relates to the fact that the difference in health state utility observed at 15 months post randomisation (≈12 months post surgery) was not statistically significant. This uncertainty was propagated through the model and was reflected in the probabilistic model output. Furthermore, the direction of the effect observed for the EQ-5D-3L score at 12 months was consistent with the observed significant differences in the primary clinical outcomes.
Currently, no published studies have assessed the cost-effectiveness of LASH compared with EA, but several studies have assessed the cost-effectiveness of total hysterectomy compared with EA from a UK NHS perspective. These have generally found that hysterectomy is likely to be cost-effective. 9,10,43 These previous studies utilised a similar model structure to the one described in this chapter, but made less conservative assumptions regarding the extrapolation of the estimated difference in post-EA and post-hysterectomy utility. Thus, the above-mentioned studies reported a larger QALY gain for total hysterectomy compared with EA than we report here for LASH compared with EA. Nevertheless, when applying a cost-effectiveness threshold ratio of £20,000 per QALY gained, our results indicate a relatively high probability that LASH offers a cost-effective alternative to EA for women with HMB. It may also prove a more acceptable alternative to total hysterectomy in terms of risks of AEs. If applying the more recently suggested cost-effectiveness threshold of £13,000 per QALY,63 there is greater uncertainty surrounding the cost-effectiveness of LASH. It will therefore be important to assess the longer-term risks of subsequent surgery following LASH and to formalise comparisons with total hysterectomy through indirect treatment comparisons and decision modelling. The first of these issues will be addressed through extended follow-up of the HEALTH cohort.
In conclusion, the cost-effectiveness analysis based on HEALTH participant data indicates that LASH cannot be considered cost-effective by 15 months post randomisation (≈12 months post surgery). However, based on extrapolation over a more relevant time horizon, there is a relatively high probability that LASH offers a cost-effective alternative to EA at thresholds of WTP per QALY gained typically applied in the UK NHS (£20,000–30,000). Longer-term follow-up of HEALTH participants will be beneficial for reducing the current decision uncertainty.
Chapter 7 Discussion
Aim and overview
Heavy menstrual bleeding is a common condition that affects many women of reproductive age and significantly impairs their QoL. For those for whom medical treatment is ineffective, NICE recommends either EA or hysterectomy. 43 In comparison with EA, total hysterectomy is a more definitive procedure but is associated with higher surgical morbidity and slower postoperative recovery. 8 Two small randomised trials have suggested that a less invasive form of hysterectomy, LASH, is superior to EA but with similar morbidity and recovery. 12,13 Our large, pragmatic, randomised trial was designed to determine whether or not LASH was more effective than EA without incurring additional risks or recovery time.
Our primary clinical outcomes were satisfaction and condition-specific QoL (MMAS). Additional secondary clinical outcomes were collected along with economic end points.
Summary of findings
Primary outcomes
This large multicentre RCT showed that LASH is superior to EA in terms of the primary clinical outcomes of satisfaction and MMAS score. The levels of improvement achieved from both LASH and EA demonstrate a clear clinical benefit of both techniques, although the clinical outcomes following LASH are significantly better and are comparable with outcomes obtained following total hysterectomy. 8 The results of the health economic analyses indicate that LASH cannot be considered cost-effective compared with EA at 15 months post randomisation, but it has a 70–80% chance of being considered cost-effective at accepted cost-effectiveness thresholds (£/QALY) based on extrapolation over a 10-year time horizon.
Secondary outcomes
Both operations were associated with high rates of acceptability, but more women favoured LASH. There was a similar reduction in cyclical pain with both procedures, favouring EA at 6 weeks post surgery but LASH at 6 months post surgery and 15 months post randomisation. There was no change or difference in urinary symptoms following either procedure. Most generic QoL scores favoured the EA group at 6 weeks post surgery and LASH at 15 months post randomisation. Women who had EA were discharged more quickly and returned to work and social activities much sooner than those who had LASH. This resulted in EA imposing lower production costs on society in the short term (£990 vs. £2586).
Although the shorter recovery times following EA are to be expected, it was anticipated that the resumption of social activities and return to work following LASH would occur earlier than our data suggest. Despite making women allocated to LASH aware that there were no restrictions postoperatively, their expectations of recovery may have been coloured by more traditional views on hysterectomy and its required convalescence, as expressed by relatives, nurses and GPs. Patients are advised and know that they are entitled to up to 12 weeks off work following a hysterectomy and this information needs to be revised and based on the underlying condition, the route of procedure and other mitigating factors. It would be worth exploring this by asking patients about the factors influencing their recovery and return to activities.
Theatre time associated with EA was almost half of that for LASH. Although surgical morbidity and postoperative complications were comparable in both groups, women who had undergone EA needed less pain relief after surgery and were discharged much sooner than those treated with LASH. Improvements in immediate postoperative recovery and discharge times could probably be attained with the introduction of an enhanced recovery programme. Fourteen women in the EA group were readmitted for hysterectomy (12 for total hysterectomy and two for LASH) within 15 months of randomisation.
The results of our predetermined subgroup analyses showed that the presence of fibroids was associated with poorer outcomes following EA than following LASH. The opportunity to make a definitive diagnosis of fibroids was greater in the LASH arm, in which visual inspection of the pelvis and uterus by laparoscopy in addition to an initial screening ultrasound allowed small fibroids not identified at baseline scan to be detected. It is likely, however, that the actual numbers with fibroids up to 3 cm were similar in both arms owing to randomisation.
Strengths and limitations of the trial
To our knowledge, this was the first large randomised trial comparing LASH with EA and the first study to undertake a cost-effectiveness analysis. The pragmatic nature of the trial and inclusion of centres across the UK has generated results that are robust, reliable and generalisable.
The main weakness of our trial was a higher loss to follow-up than anticipated. As soon as we became aware of this problem, we implemented a number of strategies, including cash incentives (an unconditional £25 gift voucher issued with the first reminder) and telephone follow-up from the trial office, which enabled us to increase our response rates from 74% to 85% for the primary outcome questionnaire.
As a surgical trial recruiting within NHS hospitals with variable waiting lists, we were unable to enforce a standard time between randomisation and surgery. In view of the NHS waiting list target of 12 weeks for routine operations, we chose to collect primary outcome data at 15 months post randomisation, believing that this would give us a 12-month duration of follow-up. Although this was appropriate for most women, a minority (10%) faced delays in receiving treatment. This occurred as a result of patient unavailability through either illness or preference, theatre staff shortages in some centres and seasonal pressures on elective hospital admission. However, these were balanced across the groups, although those allocated to LASH did have to wait slightly longer for their procedures than those allocated to EA.
A strength of the economic modelling based on randomised data from a large trial was that it enabled a detailed approach to costing of the initial surgical episode and the capture of subsequent events, including complications. However, the censoring of patients for subsequent surgery and longer-term post-surgery health state utility is a limitation, as is the need to rely on external non-randomised data to inform the longer-term risk of further surgery. Although data on the incidence of hysterectomy following EA are relatively robust, this is not currently the case for the chance of subsequent surgery post LASH.
The 15-month time horizon post randomisation also required us to make assumptions about differences in health state utility over time. Again, conservative estimates were applied, in that the observed 15-month (post-randomisation) difference in health state utility between the post-EA and post-LASH health states was modelled to diminish to zero by 10 years, which may underestimate the QALY gain for LASH compared with EA if the observed difference in utility at 15 months were to diverge further before converging, and/or converge less quickly. Future longer-term follow-up of the HEALTH cohort would help to reduce the current uncertainties in the economic model. As is most commonly practised in the UK NHS, EA procedures in HEALTH were carried out in theatre as admitted patient care, the majority (95%) under general anaesthetic. Scope may exist for cost-effectiveness to move in favour of EA if it were to prove widely acceptable to deliver in an ambulatory outpatient setting in the NHS.
To our knowledge, no published studies assess the cost-effectiveness of LASH compared with EA, but several studies9,10,43 have assessed the cost-effectiveness of total hysterectomy compared with EA. These have generally found that hysterectomy is likely to be cost-effective from a UK NHS perspective. 9,10,43
Interpretation in the context of available literature
At 15 months post randomisation (approximately 12 months post surgery), our results show high levels of satisfaction in both groups, which are similar to those reported in an individual participant data meta-analysis of total hysterectomy compared with EA. 8 The key difference between the two is that total hysterectomy, but not LASH, guarantees amenorrhoea. In our trial, both types of surgery resulted in high rates of patient satisfaction, but women had 2.5 times higher odds of being in a more favourable satisfaction category following LASH without any appreciable increase in surgical or postoperative risk.
Both LASH and EA resulted in significant improvements in MMAS scores from baseline that are higher than those reported in a previous trial evaluating medical treatment for HMB. 64 Women randomised to LASH had almost twice the odds of being in a more favourable MMAS score category, and a higher proportion of women (69% vs. 54%) reported the maximum score of 100.
The amenorrhoea rate of 60% achieved in the EA arm of our trial is higher than reported in previous randomised trials, in which rates of around 40% are commonly described. 65 However, those women allocated to LASH reported an amenorrhoea rate of 80%, which is lower than the 90–95% rates quoted by a Cochrane review16 but consistent with results reported by Lieng et al. 17
Women who underwent EA spent half as much time in theatre as those who underwent LASH, required significantly less postoperative analgesia and were discharged after a median of 3 hours post procedure (compared with 22 hours following LASH). Surgical morbidity and postoperative complications were comparable in both groups.
Fourteen women in the EA arm were readmitted for hysterectomy within 15 months after randomisation. Long-term hysterectomy rates of almost 20% following EA are described in population studies. 5,49 Rates for further surgery after LASH in the longer run are less well known as follow-up of only 3 years has been reported and rates are quoted as 7% at this point. 17 An English population-based study showed that 10% of women aged > 45 years have further surgery 5 years following EA, compared with about one-third of women aged < 35 years. 46 We found no such association with age in this trial. There were poorer outcomes following EA in women with fibroids, although more fibroids were diagnosed in the LASH arm because of laparoscopy. There was no association with cavity length, which is at odds with a meta-analysis of trials on second-generation EA, which found that a uterine cavity length of > 8 cm had an adverse impact on patient satisfaction. 8
A Cochrane review of total versus subtotal hysterectomy suggests a cyclical bleeding rate of 5–10% following LASH,16 whereas a higher rate of 23% was reported by Lieng et al. 17 Despite a standardised procedure of cervical canal cautery after removal of the body of the uterus, the observed rate of cyclical bleeding in the LASH group was 19%. This is in some way explained by the fact that, of 330 women allocated to the LASH group, 21 women had no treatment and 11 women had EA. Even so, it seems that our rates of post-LASH cyclical bleeding lie between those quoted by the Cochrane database16 and the longer-term rates quoted by Lieng et al. 17
The potential risk of malignancy is always an important consideration in planning conservative surgical treatments for HMB. In EA, in which the uterus, tubes and ovaries are conserved, an endometrial biopsy is undertaken before surgery to exclude endometrial atypia. As the cervix is retained in both techniques, ongoing cervical screening is required in all women. The risk of cervical stump carcinoma in women with a previously normal Pap smear is no more than 0.3%66 and is not considered to be a justification for total over subtotal hysterectomy in countries with cervical screening programmes,16 especially where immunisation against human papillomavirus (HPV) virus is the norm.
At the time when this trial was launched, concerns were raised by the US Food and Drug Administration about the potential risk of disseminating cells from undiagnosed uterine malignancy associated with the use of morcellators during laparoscopic hysterectomy in women with fibroids. 67 This prompted us to modify our eligibility criteria to exclude women with fibroids measuring > 3 cm, as the risk of malignancy is associated with fibroid size. The results from our trial are reassuring, as specimens from the 308 women who underwent hysterectomy have not shown any evidence of histological atypia or malignancy. The initial estimate of risk of 1 in 350 for unexpected/unknown malignancy within a presumed fibroid by the Food and Drug Administration has been revised, and a recent review puts this risk figure closer to 1 in 2000. 68 Updated estimates of leiomyosarcoma (LMS) rates at hysterectomy have been published, and show that women aged > 50 years with larger presumed fibroids are at particular risk. These are exclusion criteria for participation in HEALTH. 69 Although we have been reassured by the absence of any histological abnormalities in this trial, continued vigilance is required, and morcellation avoided and total hysterectomy performed if there are concerns of possible sarcomatous change within a fibroid based on morphological appearance or rapid growth. Dissemination of morcellated material can be avoided by using containment bags, although these have not been formally assessed. An alternative is to remove unmorcellated specimens in a bag through a culdotomy, but this may be associated with different morbidities and also needs to be assessed. It must be remembered that if a woman with unknown LMS has conservative treatment of her HMB, such as EA, she continues with an unknown and untreated LMS. It is worth noting that the routine removal of fallopian tubes during LASH could halve the subsequent risk of epithelial ovarian cancer,70 without any increase in surgical risk.
Conclusions
Laparoscopic supracervical hysterectomy is superior to EA in terms of clinical effectiveness. EA is quicker, cheaper and associated with an earlier discharge and shorter recovery than LASH. It is less costly in the short term, but its higher failure rate means that LASH is more likely to be considered more cost-effective than EA by 10 years post procedure.
Acknowledgements
The authors wish to thank the women who participated in HEALTH. We also thank Angela Hyde [vice chairperson of the Royal College of Obstetricians And Gynaecologists Women’s Network (to September 2015) and co-applicant (to October 2016)] for her contribution to the design of the participant-facing documents (patient information leaflet, letter of invitation and questionnaires) and her participation in trial meetings from the perspective of a patient; Johnathan Cook [statistician and co-applicant (to April 2014)] for his contributions to the study design; and Rebecca Bruce for her secretarial support and data management; previous data co-ordinator (Fiona Cherry) and trial managers (Moira Ritchie, Dawn McRae and Jessica Wood) for their data/trial management support; Graeme MacLennan (CHaRT Director from 2017), Alison McDonald (CHaRT Senior Trials Manager) and the CHaRT programming team led by Gladys McPherson (to 2016) and Mark Forrest (2016–present); other staff in the CHaRT/Health Services Research Unit for their assistance with the trial (Lorna Aucott, Zoe Batham and Cynthia Fraser); Atefe Mashayekhi (University of Newcastle) for developing an earlier version of the economic model that was later adapted for the model-based analysis reported in Chapter 6; other staff who have contributed to the economic data collection and interpretation (Amalia Charlotte Pape); members of the PMG for their ongoing advice and support of the trial, plus the independent members of the TSC and DMC; and the staff at the recruitment sites who facilitated the recruitment, treatment and follow-up of trial participants. Finally, we would like to thank the National Institute for Health Research and the HTA programme for funding HEALTH.
Project Management Group
Siladitya Bhattacharya (co-chairperson), Kevin Cooper (co-chairperson), Suzanne Breeman, Rebecca Bruce, Justin Clark, Jed Hawe, Robert Hawthorn, Rodolfo Hernández, Graeme MacLennan, Kirsty McCormack, Alison McDonald, John Norrie, Mini Paulose, Kevin Phillips, Danielle Pirie, Graham Scotland, Neil Scott and Samantha Wileman.
Independent members of the Trial Steering Committee
Henry Kitchener (chairperson), Patrick Chien, Barbara Farrell and Isobel Montgomery.
Independent members of the Data Monitoring Committee
Jane Norman (chairperson), Peter O’Donovan and Andy Vail.
Recruitment sites
We would like to thank the staff and participants of the following sites:
Aberdeen: Kevin Cooper (PI), Premila Ashok, Stamatios Karavalos, Mini Paulose, Danielle Pirie, Lucky Saraswat, Sherif Saleh, Ruth Valentine and Sarah Wallage; Basildon: Yatin Thakur (PI), Kellie Allen, Preethi Angala, Yee Yin Chan, Mamdouh Ghobrial, Neerja Gupta, J Jadhav, Elisabeth Joseph, Syathe Kalburg, Shaneen Mannan, Claire McCormack, Anne Nicholson, Stacey Pepper, Emily Redman and Donna Southam; Basingstoke: Christian Phillips (PI), Jackie Moody, Clare Rowe Jones, Tim Sayer; Birmingham: T Justin Clark (PI), Yousri Afiffi, Fiona Beale, Andrea Galloway, Janesh Gupta, Virginia Iqbal, Shanteela McCooty, Paul Smith, Helen Stevenson and Jennifer Taylor; Cornwall: Dominic Byrne (PI), Benita Adams, Alyson Andrew, Susan Bates, Samy Bishieri, Jacqueline Dingle, Anna Fouracres, Fiona Hammonds, Rob Holmes, Farah Lone, Jonathan Lord, Tom Smith-Walker, Jessica Summer, Lisa Trembath, Liz Verity and Abigail Weeks; Chester: Jed Hawe (PI), Jonathan Ford, Elizabeth Gallimore, Nabil Haddad, Helen Jefferey, Nichola Kearsley, Sandra Leason, Michael J McCormack, Janneke Van Rij and Simon Wood; Durham: Partha Sengupta (PI), Vicki Atikinson, Remko Beukenholdt, Jonathan Bradley, Jean Dent, Jacqueline Jennings, Seema Sen, Velur Sindhu and Eileen Walton; Edinburgh: Stuart Jack (PI), Hilary Critchley, Jennifer Devlin, Paul Dewart, Catherine Hall, Andrew Horne and Lucy Whitaker; Fife: Chu Lim (PI), Julie Aitken, Laura Beveridge, Keith Boath, Isla Smith and Omar Thanoon; Forth Valley: Oliver Milling-Smith (PI), Jacqueline Gardiner, Shahzya Huda and Kara Sewnauth; Glasgow: Robert Hawthorn (PI), Hassan Ali, Carol Archibald, Steingrimur Bjornsson, Chris Hardwick, Therese McSorley, Lorna McKay and Kirsteen Paterson; Hull: Kevin Phillips (PI), Jane Allen, Frank Biervliet, Helen Bexhell, Stephen Boakye, Helen Brown, Adrian Fauvre, Melony Hayes, Jaishree Hingaroni, Piotr Lesny, Kim Mitchelson, Androniks Mumdzjans and Alexander Oboh; Kent: Mr Chappatte (PI), Elias Kovoor and Tracey Nolan; Kilmarnock: Inna Sokolova (PI), Danielle Gilmour and Margo Henry; Newcastle: Tony Chalhoub (PI), Sue Harbertson, Alison Kimber, Kayleigh Lennox, Victoria Murtha, Mark Roberts and Michelle Russell; Northampton: Clemens von Widekind (PI), Lucy Dudgeon, Kathy Hall, Rachael Hitchcock and Gillian Smith; North Tees: Somon Roy (PI), Patricia Bage, Misra Bano-Mahroo, Dolan Basu, Sarah Croft, Sharon Gowans, Anne Marie Jones, Susan Kelsey, Hany Mostafa, Sue Mullens, Atul Nalawade, Santhosh Puthuraya, Alison Samuels, Rebecca Tate, Ratan Wadia and Maggie Wilkinson; Poole: Tyrone Carpenter (PI), Anne Chalk and Daniel Webster; Plymouth: Robert Freeman (PI March 2017–present), Jonathan Frappell (PI October 2014–March 2017), Sarah Caukwell, Heidi Hollands and Abigail Patrick; Preston: Brice Rodriguez (PI), Sarah Francis, Anne Gardner, Annabel Grossmith, Sean Hughes, Catherine Langley, Lauren Murphy, Claire Noor, Katie Robinson, Katy Sanders, Mark Tattersall, William Wilson-Theaker, Robyn Wittersheim and Katey Yeowart; Sheffield: Ted Baxter (PI), Sheila Duffy; Stockport: Tarique Salman (PI August 2015–September 2018), Andrew Pickersgill (PI June 2014–August 2015), Lindsay Barber, Helen Cochrane and Julie Grindey; Southampton: Dimitrios Miligkos (PI), Agnieszka Burtt, Jane Forbes, Abby Rand, Sameer Umranikar, Fiona Walbridge and Susan Wellstead; South Tees: Pinky Khatri (PI), Hazel Alexander, Sarah Croft, Helen Cuthbert, Neil Hebblethwaite, Aethele Khunda, Padma Manda, Dayang Mohammed, Suzie Peatman, Julie Potts and Sanjay Rao; Sunderland: Nick Matthews (PI February 2016–September 2018), Jonathan Chamberlain (PI September 2014–February 2016), Amna Ahmed, Helen Cameron, Kirsten Herdman, Kim Hinshaw, Princy Mathew, Judith Ormonde, Janet Scollen, Aarti Ullal, Eileen Walton and Menem Yossry; Surrey: Saikat Banerjee (PI), Claire Atkinson, Lauren Brown, Michelle Davis, Rebecca Gilbert, Shaheen Khazali, Beth Peers and Lisa Sharpe; Whipps Cross: Funlayo Odejinmi (PI), Bashir Dawlatly, Anwen Gorry, Prudence Jones, Zandile Maseko, Anupama Shahid and Sotitis Vimplis; Winchester: Keith Louden (PI), Renee Behrens, Jane Martin, Dawn Trodd and Caroline Wrey Brown; Wirral: Thomas Aust (PI), Ash Alam, Khadija Ashraf, Mark Doyle, Michael Ellard, Julie Grindey, Stella Mwenechanya, Libby Shaw, Gillian Steele, Vasileios Minas and Jeremy Weetch; Worcester: Angus Thomson (PI), Victoria Cashmore, John Hughes, Dawn Kelly and Mamta Pathak; Worthing: Natasha Waters (PI), Vivienne Cannons, Marian Flynn-Batham, Sarah Funnell, Fani Gkrozou and Sarah House.
Contributions of authors
Kevin Cooper (Co-Chief Investigator and Professor) contributed to the conception and the design of the trial, the conduct of the trial, the interpretation of the results and the writing/editing of the report.
Suzanne Breeman (Trial Manager, Triallist) was responsible for the day-to-day management of the trial, contributed to the interpretation of the data and the writing/editing of the report.
Neil W Scott (Statistician) contributed to the design of the study, conducted the statistical analyses, contributed to the interpretation of the data and the writing/editing of the report.
Graham Scotland (Health Economist) contributed to the conception and design of the study, the analysis of the health economics data, the drafting of the health economics chapters, plus commented on other chapters of the report.
Rodolfo Hernández (Health Economist) contributed to the analysis of the health economics data, the drafting of the health economics chapters, plus commented on other chapters of the report.
T Justin Clark (Consultant Obstetrician and Gynaecologist) contributed to the conception and design of the study, the recruitment of participants, the interpretation of the data and made significant contributions to the drafting of the report.
Jed Hawe (Consultant Obstetrician and Gynaecologist) contributed to the conception and design of the study, the recruitment of participants, the interpretation of the data and made significant contributions to the drafting of the report.
Robert Hawthorn (Consultant Gynaecologist) contributed to the conception and design of the study, the recruitment of participants, the interpretation of the data and made significant contributions to the drafting of the report.
Kevin Phillips (Consultant Obstetrician and Gynaecologist) contributed to the conception and design of the study, the recruitment of participants, the interpretation of the data and made significant contributions to the drafting of the report.
Samantha Wileman (Triallist) contributed to the design of the trial, the conduct of the trial and made significant contributions to the drafting of the report.
Kirsty McCormack (Triallist) contributed to the conception and design of the study, the conduct of the trial and made significant contributions to the drafting of the report.
John Norrie (Professor, Statistician and Triallist) contributed to the conception and design of the trial, the conduct of the trial, the interpretation of results and made significant contributions to the drafting of the report.
Siladitya Bhattacharya (Co-Chief Investigator and Professor) contributed to the conception and the design of the trial, the conduct of the trial, the interpretation of the results and the writing/editing of the report.
The Health Services Research Unit and the Health Economics Research Unit are core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates.
Publications
Cooper K, McCormack K, Breeman S, Wood J, Scott NW, Clark J, et al. HEALTH: laparoscopic supracervical hysterectomy versus second-generation endometrial ablation for the treatment of heavy menstrual bleeding: study protocol for a randomised controlled trial. Trials 2018;19:63.
Cooper K, Breeman S, Scott NW, Scotland G, Clark J, Hawe J, et al. Laparoscopic supracervical hysterectomy versus endometrial ablation for women with heavy menstrual bleeding (HEALTH): a parallel-group, open-label, randomised controlled trial [published online ahead of print September 12 2019]. Lancet 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Cooper K, McCormack K, Breeman S, Wood J, Scott NW, Clark J, et al. HEALTH: laparoscopic supracervical hysterectomy versus second-generation endometrial ablation for the treatment of heavy menstrual bleeding: study protocol for a randomised controlled trial. Trials 2018;19. https://doi.org/10.1186/s13063-017-2374-9.
- Ipsos MORI . National Heavy Menstrual Bleeding Audit: Final Report. 2014. www.rcog.org.uk/globalassets/documents/guidelines/research--audit/national_hmb_audit_final_report_july_2014.pdf (accessed 18 September 2018).
- Cooper KG, Parkin DE, Garratt AM, Grant AM. Two-year follow up of women randomised to medical management or transcervical resection of the endometrium for heavy menstrual loss: clinical and quality of life outcomes. Br J Obstet Gynaecol 1999;106:258-65. https://doi.org/10.1111/j.1471-0528.1999.tb08240.x.
- Yoo HJ, Lee MA, Ko YB, Yang JB, Kang BH, Lee KH. The efficacy of the levonorgestrel-releasing intrauterine system in perimenopausal women with menorrhagia or dysmenorrhea. Arch Gynecol Obstet 2012;285:161-6. https://doi.org/10.1007/s00404-011-1937-3.
- Cooper K, Lee A, Chien P, Raja E, Timmaraju V, Bhattacharya S. Outcomes following hysterectomy or endometrial ablation for heavy menstrual bleeding: retrospective analysis of hospital episode statistics in Scotland. BJOG 2011;118:1171-9. https://doi.org/10.1111/j.1471-0528.2011.03011.x.
- Royal College of Obstetricians and Gynaecologists . National Heavy Menstrual Bleeding Audit: First Annual Report 2011. www.rcog.org.uk/files/rcog-corp/NationalHMBAudit_1stAnnualReport_May2011.pdf (accessed 5 November 2013).
- National Institute for Health and Care Excellence . Heavy Menstrual Bleeding 2007. www.nice.org.uk/guidance/ng88/evidence/full-guideline-pdf-4782291810 (accessed November 2013).
- Middleton LJ, Champaneria R, Daniels JP, Bhattacharya S, Cooper KG, Hilken NH, et al. Hysterectomy, endometrial destruction, and levonorgestrel releasing intrauterine system (Mirena) for heavy menstrual bleeding: systematic review and meta-analysis of data from individual patients. BMJ 2010;341. https://doi.org/10.1136/bmj.c3929.
- Roberts TE, Tsourapas A, Middleton LJ, Champaneria R, Daniels JP, Cooper KG, et al. Hysterectomy, endometrial ablation, and levonorgestrel releasing intrauterine system (Mirena) for treatment of heavy menstrual bleeding: cost effectiveness analysis. BMJ 2011;342. https://doi.org/10.1136/bmj.d2202.
- Bhattacharya S, Middleton LJ, Tsourapas A, Lee AJ, Champaneria R, Daniels JP, et al. Hysterectomy, endometrial ablation and Mirena® for heavy menstrual bleeding: a systematic review of clinical effectiveness and cost-effectiveness analysis. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15190.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev 2009;4. https://doi.org/10.1002/14651858.CD001501.pub3.
- Sesti F, Ruggeri V, Pietropolli A, Piancatelli R, Piccione E. Thermal balloon ablation versus laparoscopic supracervical hysterectomy for the surgical treatment of heavy menstrual bleeding: a randomized study. J Obstet Gynaecol Res 2011;37:1650-7. https://doi.org/10.1111/j.1447-0756.2011.01596.x.
- Zupi E, Zullo F, Marconi D, Sbracia M, Pellicano M, Solima E, et al. Hysteroscopic endometrial resection versus laparoscopic supracervical hysterectomy for menorrhagia: a prospective randomized trial. Am J Obstet Gynecol 2003;188:7-12. https://doi.org/10.1067/mob.2003.60.
- Garry R, Fountain J, Mason S, Hawe J, Napp V, Abbott J, et al. The eVALuate study: two parallel randomised trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ 2004;328. https://doi.org/10.1136/bmj.37984.623889.F6.
- Aarts JW, Nieboer TE, Johnson N, Tavender E, Garry R, Mol BW, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev 2015;8. https://doi.org/10.1002/14651858.CD003677.pub5.
- Lethaby A, Ivanova V, Johnson NP. Total versus subtotal hysterectomy for benign gynaecological conditions. Cochrane Database Syst Rev 2006;2. https://doi.org/10.1002/14651858.CD004993.pub2.
- Lieng M, Qvigstad E, Istre O, Langebrekke A, Ballard K. Long-term outcomes following laparoscopic supracervical hysterectomy. BJOG 2008;115:1605-10. https://doi.org/10.1111/j.1471-0528.2008.01854.x.
- Department of Health and Social Care . NHS Reference Costs: Financial Year 2011–2012 2012. www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (accessed November 2012).
- Jack SA, Cooper KG, Seymour J, Graham W, Fitzmaurice A, Perez J. A randomised controlled trial of microwave endometrial ablation without endometrial preparation in the outpatient setting: patient acceptability, treatment outcome and costs. BJOG 2005;112:1109-16. https://doi.org/10.1111/j.1471-0528.2005.00630.x.
- Clark TJ, Samuel N, Malick S, Middleton LJ, Daniels J, Gupta JK. Bipolar radiofrequency compared with thermal balloon endometrial ablation in the office: a randomized controlled trial. Obstet Gynecol 2011;117:109-18. https://doi.org/10.1097/AOG.0b013e3182020401.
- Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials: a review. Control Clin Trials 2002;23:662-74. https://doi.org/10.1016/S0197-2456(02)00242-8.
- Department of Health and Social Care . Right to Start Consultant-Led Treatment Within 18 Weeks n.d. www.gov.uk/government/publications/right-to-start-consultant-led-treatment-within-18-weeks (accessed 18 October 2018).
- Scottish Government . The Patient Rights (Treatment Time Guarantee) (Scotland) Regulations 2012 n.d. www.legislation.gov.uk/ssi/2012/110/made (accessed 18 October 2018).
- Scottish Government . Patient Rights (Scotland) Act 2011 n.d. www.gov.scot/Topics/Health/Policy/Patients-Rights (accessed 18 October 2018).
- Pattison H, Daniels JP, Kai J, Gupta JK. The measurement properties of the menorrhagia multi-attribute quality-of-life scale: a psychometric analysis. BJOG 2011;118:1528-31. https://doi.org/10.1111/j.1471-0528.2011.03057.x.
- Whitehead J. Sample size calculations for ordered categorical data. Stat Med 1993;12:2257-71. https://doi.org/10.1002/sim.4780122404.
- van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681-94. https://doi.org/10.1002/(SICI)1097-0258(19990330)18:6<681::AID-SIM71>3.0.CO;2-R.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- Cooper K, Breeman S, Scott NW, Scotland G, Clark J, Hawe J, et al. Laparoscopic supracervical hysterectomy versus endometrial ablation for women with heavy menstrual bleeding (HEALTH): a parallel-group, open-label, randomised controlled trial [published online ahead of print September 12 2019]. Lancet 2019. https://doi.org/10.1016/S0140-6736(19)31790-8.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Information Services Division . Scottish Health Services Costs 2017 2017. www.isdscotland.org/Health-Topics/Finance/Publications/2017-11-21/2017-11-21-Costs-Report.pdf (accessed 30 June 2018).
- Department of Health and Social Care . NHS Reference Costs 2016–2017 2017. https://improvement.nhs.uk/resources/reference-costs/ (accessed June 2018).
- British National Formulary n.d. https://bnf.nice.org.uk/ (accessed June 2018).
- Institution for Innovation and Improvement . Improving Quality and Efficiency in the Operating Theatre n.d. http://harmfreecare.org/wp-content/files_mf/Improving-quality-and-efficiency-in-the-operating-theatre.pdf (accessed 30 June 2018).
- Office for National Statistics . Annual Survey of Hours and Earnings. 2017 Provisional and 2016 Revised Results n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2017provisionaland2016revisedresults (accessed June 2018).
- Department for Transport . Values of Travel Time Savings and Reliability: Final Reports n.d. www.gov.uk/government/publications/values-of-travel-time-savings-and-reliability-final-reports (accessed June 2018).
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results From a UK General Population Survey n.d. www.york.ac.uk/media/che/documents/papers/discussionpapers/CHE%20Discussion%20Paper%20138.pdf (accessed 30 June 2018).
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. https://doi.org/10.1016/S0167-6296(01)00130-8.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons. Inc.; 1987.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Handbooks in Health Economic Evaluation. Oxford: Oxford University Press; 2007.
- Brand J, van Buuren S, le Cessie S, van den Hout W. Combining multiple imputation and bootstrap in the analysis of cost-effectiveness trial data. Stat Med 2019;38:210-20. https://doi.org/10.1002/sim.7956.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed September 2018).
- National Institute for Health and Care Excellence . Heavy Menstrual Bleeding: Assessment and Management n.d. www.nice.org.uk/guidance/ng88 (accessed June 2018).
- Office for National Statistics . National Life Tables: United Kingdom n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 30 June 2018).
- HM Treasury . The Green Book: Central Government Guidance on the Appraisal and Evaluation n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/685903/The_Green_Book.pdf (accessed 30 June 2018).
- Bansi-Matharu L, Gurol-Urganci I, Mahmood TA, Templeton A, van der Meulen JH, Cromwell DA. Rates of subsequent surgery following endometrial ablation among English women with menorrhagia: population-based cohort study. BJOG 2013;120:1500-7. https://doi.org/10.1111/1471-0528.12319.
- Maresh MJ, Metcalfe MA, McPherson K, Overton C, Hall V, Hargreaves J, et al. The VALUE national hysterectomy study: description of the patients and their surgery. BJOG 2002;109:302-12. https://doi.org/10.1111/j.1471-0528.2002.01282.x.
- Zupi E, Centini G, Lazzeri L, Finco A, Zullo F, Exacoustos C. Hysteroscopic endometrial resection versus laparoscopic supracervical hysterectomy for abnormal uterine bleeding: long term follow-up of a prospective randomized trial. J Minim Invasive Gynecol 2015;22. https://doi.org/10.1016/j.jmig.2015.08.108.
- Soini T, Rantanen M, Paavonen J, Grénman S, Mäenpää J, Pukkala E, et al. Long-term follow-up after endometrial ablation in Finland: cancer risks and later hysterectomies. Obstet Gynecol 2017;130:554-60. https://doi.org/10.1097/AOG.0000000000002166.
- Okaro EO, Jones KD, Sutton C. Long term outcome following laparoscopic supracervical hysterectomy. BJOG 2001;108:1017-20. https://doi.org/10.1111/j.1471-0528.2001.00252.x.
- Tsafrir Z, Aoun J, Papalekas E, Taylor A, Schiff L, Theoharis E, et al. Risk factors for trachelectomy following supracervical hysterectomy. Acta Obstet Gynecol Scand 2017;96:421-5. https://doi.org/10.1111/aogs.13099.
- van Evert JS, Smeenk JM, Dijkhuizen FP, de Kruif JH, Kluivers KB. Laparoscopic subtotal hysterectomy versus laparoscopic total hysterectomy: a decade of experience. Gynecol Surg 2010;7:9-12. https://doi.org/10.1007/s10397-009-0529-8.
- Boosz A, Lermann J, Mehlhorn G, Loehberg C, Renner SP, Thiel FC, et al. Comparison of re-operation rates and complication rates after total laparoscopic hysterectomy (TLH) and laparoscopy-assisted supracervical hysterectomy (LASH). Eur J Obstet Gynecol Reprod Biol 2011;158:269-73. https://doi.org/10.1016/j.ejogrb.2011.04.021.
- Graziano A, Lo Monte G, Hanni H, Brugger JG, Engl B, Marci R. Laparoscopic supracervical hysterectomy with transcervical morcellation: our experience. J Minim Invasive Gynecol 2015;22:212-18. https://doi.org/10.1016/j.jmig.2014.09.013.
- Schuster MW, Wheeler TL, Richter HE. Endometriosis after laparoscopic supracervical hysterectomy with uterine morcellation: a case control study. J Minim Invasive Gynecol 2012;19:183-7. https://doi.org/10.1016/j.jmig.2011.09.014.
- Wallwiener M, Taran FA, Rothmund R, Kasperkowiak A, Auwärter G, Ganz A, et al. Laparoscopic supracervical hysterectomy (LSH) versus total laparoscopic hysterectomy (TLH): an implementation study in 1,952 patients with an analysis of risk factors for conversion to laparotomy and complications, and of procedure-specific re-operations. Arch Gynecol Obstet 2013;288:1329-39. https://doi.org/10.1007/s00404-013-2921-x.
- Sambrook AM, Bain C, Parkin DE, Cooper KG. A randomised comparison of microwave endometrial ablation with transcervical resection of the endometrium: follow up at a minimum of 10 years. BJOG 2009;116:1033-7. https://doi.org/10.1111/j.1471-0528.2009.02201.x.
- Jenkins TR. Laparoscopic supracervical hysterectomy. Am J Obstet Gynecol 2004;191:1875-84. https://doi.org/10.1016/j.ajog.2004.06.096.
- Pullenayegum EM, Tarride JE, Xie F, Goeree R, Gerstein HC, O’Reilly D. Analysis of health utility data when some subjects attain the upper bound of 1: are Tobit and CLAD models appropriate?. Value Health 2010;13:487-94. https://doi.org/10.1111/j.1524-4733.2010.00695.x.
- Clegg JP, Guest JF, Hurskainen R. Cost-utility of levonorgestrel intrauterine system compared with hysterectomy and second generation endometrial ablative techniques in managing patients with menorrhagia in the UK. Curr Med Res Opin 2007;23:1637-48. https://doi.org/10.1185/030079907X199709.
- Sculpher M. A cost-utility analysis of abdominal hysterectomy versus transcervical endometrial resection for the surgical treatment of menorrhagia. Int J Technol Assess Health Care 1998;14:302-19. https://doi.org/10.1017/S0266462300012277.
- Briggs ACK, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Gupta J, Kai J, Middleton L, Pattison H, Gray R, Daniels J. ECLIPSE Trial Collaborative Group . Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med 2013;368:128-37. https://doi.org/10.1056/NEJMoa1204724.
- Bofill Rodriguez M, Lethaby A, Grigore M, Brown J, Hickey M, Farquhar C. Endometrial resection and ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev 2019;1. https://doi.org/10.1002/14651858.CD001501.pub5.
- Storm HH, Clemmensen IH, Manders T, Brinton LA. Supravaginal uterine amputation in Denmark 1978-1988 and risk of cancer. Gynecol Oncol 1992;45:198-201. https://doi.org/10.1016/0090-8258(92)90285-Q.
- Food and Drug Administration . Quantitative Assessment of the Prevalence of Unsuspected Uterine Sarcoma in Women Undergoing Treatment of Uterine Fibroids. Summary and Key Findings. n.d. www.fda.gov/downloads/medicaldevices/safety/alertsandnotices/ucm393589.pdf (accessed 1 July 2014).
- Pritts EA, Vanness DJ, Berek JS, Parker W, Feinberg R, Feinberg J, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg 2015;12:165-77. https://doi.org/10.1007/s10397-015-0894-4.
- Siedhoff MT, Doll KM, Clarke-Pearson DL, Rutstein SE. Laparoscopic hysterectomy with morcellation vs. abdominal hysterectomy for presumed fibroids: an updated decision analysis following the 2014 Food and Drug Administration safety communications. Am J Obstet Gynecol 2017;216:259.e1-6. https://doi.org/10.1016/j.ajog.2016.11.1039.
- Yoon SH, Kim SN, Shim SH, Kang SB, Lee SJ. Bilateral salpingectomy can reduce the risk of ovarian cancer in the general population: a meta-analysis. Eur J Cancer 2016;55:38-46. https://doi.org/10.1016/j.ejca.2015.12.003.
- Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. Handbooks in Health Economic Evaluation. Oxford: Oxford University Press; 2015.
- Lieng M, Lømo AB, Qvigstad E. Long-term outcomes following laparoscopic and abdominal supracervical hysterectomies. Obstet Gynecol Int 2010;2010. https://doi.org/10.1155/2010/989127.
- Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-139.
Appendix 1 Additional information for baseline results
| Centre recruiting | LASH (N = 330), n | EA (N = 330), n | Total (N) |
|---|---|---|---|
| Aberdeen | 77 | 75 | 152 |
| Birmingham | 39 | 37 | 76 |
| Glasgow | 23 | 22 | 45 |
| South Tees | 22 | 20 | 42 |
| Chester | 16 | 17 | 33 |
| Forth Valley | 15 | 16 | 31 |
| Hull | 14 | 15 | 29 |
| Poole | 14 | 14 | 28 |
| North Tees | 12 | 12 | 24 |
| Cornwall | 11 | 10 | 21 |
| Northampton | 9 | 9 | 18 |
| Kilmarnock | 8 | 9 | 17 |
| Basildon | 8 | 8 | 16 |
| Winchester | 7 | 7 | 14 |
| Newcastle | 6 | 6 | 12 |
| Basingstoke | 6 | 5 | 11 |
| Sunderland | 5 | 6 | 11 |
| Edinburgh | 4 | 5 | 9 |
| Southampton | 4 | 5 | 9 |
| Preston | 4 | 4 | 8 |
| Whipps Cross | 4 | 4 | 8 |
| Surrey | 3 | 4 | 7 |
| Durham | 3 | 4 | 7 |
| Fife | 4 | 3 | 7 |
| Wirral | 3 | 4 | 7 |
| Kent | 2 | 3 | 5 |
| Plymouth | 2 | 2 | 4 |
| Worcester | 2 | 2 | 4 |
| Stockport | 1 | 2 | 3 |
| Sheffield | 1 | 0 | 1 |
| Worthing | 1 | 0 | 1 |
| Participant status | n (%) |
|---|---|
| Screened | 2552 (100.0) |
|
664 (26.0) |
|
1888 (74.0) |
| Ineligible | 1201 (47.1) |
| Patient declined | 589 (23.1) |
| Clinical preference | 98 (3.8) |
| Reason | n (%) |
|---|---|
| Ineligible | 1201 (100.0) |
| Fibroids > 3cm | 361 (30.1) |
| Preference to continue with medical management | 244 (20.3) |
| Aged ≥ 50 years | 239 (19.9) |
| Previous EA | 145 (12.1) |
| Plans to conceive | 128 (10.7) |
| Contraindications for laparoscopic surgery | 44 (3.7) |
| Uterine cavity > 11cm | 12 (1.0) |
| Unable to understand or complete study documentation | 12 (1.0) |
| Abnormal cytology | 10 (0.8) |
| Endometrial atypia | 6 (0.5) |
| Eligible but patient declined randomisation/participation | 589 (100.0) |
| Preference: EA | 151 (25.6) |
| Preference: hysterectomy | 126 (21.4) |
| No reason given | 98 (16.6) |
| Preference: medical management | 89 (15.1) |
| Patient did not want to be randomised | 54 (9.2) |
| Unable to make contact with patient | 25 (4.2) |
| Preference: unknown | 18 (3.1) |
| Patient did not want to participate in research | 10 (1.7) |
| Preference: no treatment | 8 (1.4) |
| Personal reason | 8 (1.4) |
| Other | 2 (0.3) |
| Eligible but not recruited due to clinical preference/decision | 98 (100) |
| Clinical decision | 39 (39.8) |
| Planned hysterectomy | 25 (25.5) |
| Planned EA | 18 (18.4) |
| Other treatment planned | 16 (16.3) |
| Outcome | LASH (N = 330), n (%) | EA (N = 330), n (%) |
|---|---|---|
| Pain at intercourse? | ||
| No | 106 (35.5) | 120 (39.2) |
| Rarely | 90 (30.1) | 69 (22.5) |
| Often | 81 (27.1) | 87 (28.4) |
| Always | 22 (7.4) | 30 (9.8) |
| Not applicable | 26 | 20 |
| Severity of pain at intercourse | ||
| Mild | 78 (41.5) | 81 (42.0) |
| Moderate | 76 (40.4) | 85 (44.0) |
| Severe | 34 (18.1) | 27 (14.0) |
| Do you have problems with your bladder? | ||
| No | 135 (41.8) | 147 (45.2) |
| Yes, I need to dash to the toilet (urgency), but don’t leak | 43 (13.3) | 58 (17.8) |
| Yes, I need to dash to the toilet (urgency), but often don’t make it and leak | 26 (8.0) | 24 (7.4) |
| Yes, I regularly leak when I cough, sneeze or exercise | 69 (21.4) | 64 (19.7) |
| Yes, both. I have urgency and I also leak when I cough, sneeze or exercise | 50 (15.5) | 32 (9.8) |
Appendix 2 Additional information forclinical results
FIGURE 16.
Days from randomisation to operation by randomised group: (a) LASH; and (b) EA.
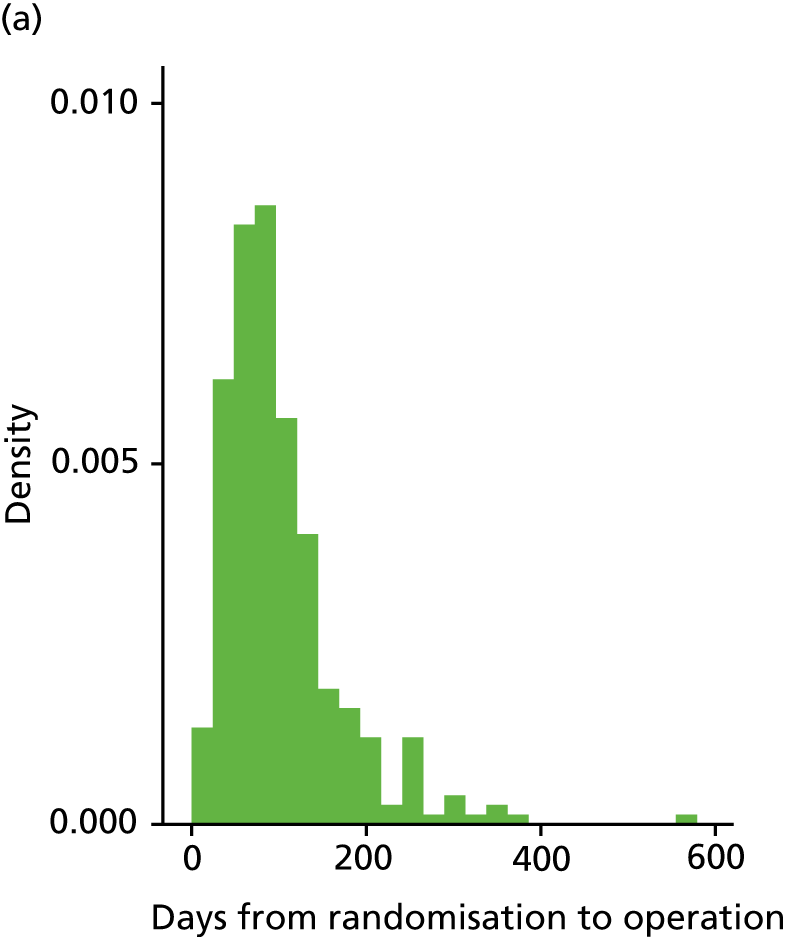
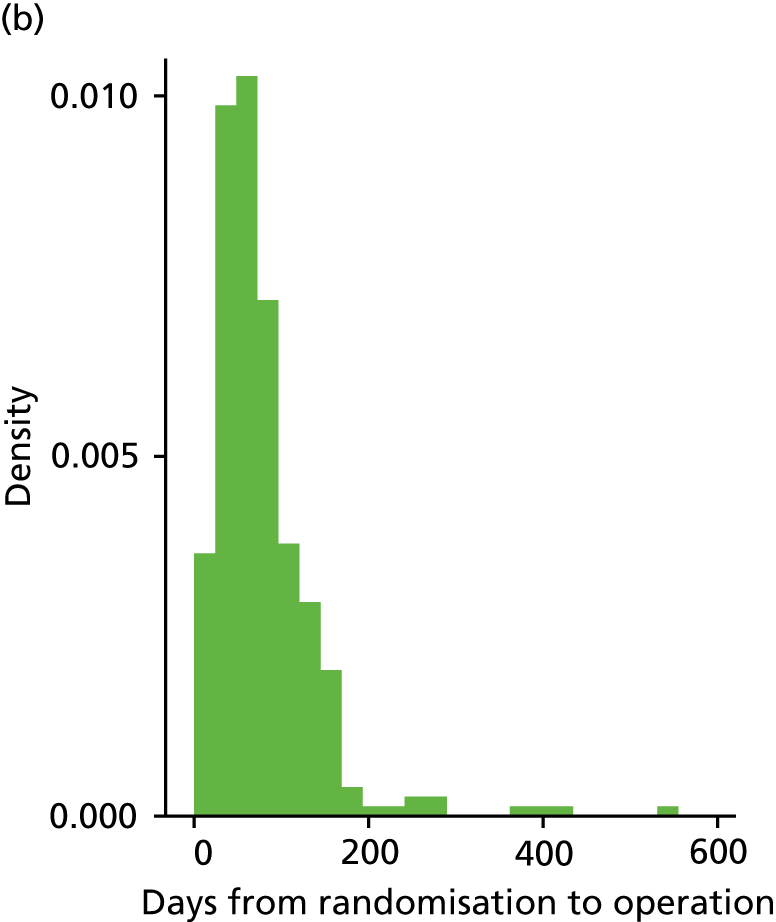
| Analysis approach | Effect size (95% CI) | p-value | |
|---|---|---|---|
| OLR (primary analysis, adjusted) | OR | 2.53 (1.83 to 3.28) | < 0.001 |
| OLR (unadjusted) | OR | 2.55 (1.79 to 3.63) | < 0.001 |
| Binary logistic regression (totally satisfied vs. other, adjusted) | OR | 2.40 (1.77 to 3.26) | < 0.001 |
| RD | 0.19 (0.13 to 0.25) | ||
| Binary logistic regression (totally/generally satisfied vs. other, adjusted) | OR | 2.78 (1.50 to 5.13) | 0.001 |
| RD | 0.13 (0.07 to 0.20) | ||
| Binary logistic regression (satisfied vs. dissatisfied, adjusted) | OR | 4.89 (1.91 to 12.45) | 0.001 |
| RD | 0.10 (0.05 to 0.15) | ||
| OLR (multiple imputation, adjusted) | OR | 2.15 (1.53 to 3.02) | < 0.001 |
| OLR (by actual treatment received, adjusted) | OR | 2.60 (1.91 to 3.55) | < 0.001 |
| OLR (including only those operated on by consultant, adjusted) | OR | 2.52 (1.74 to 3.66) | < 0.001 |
| Outcome | Item response | Baseline | 6 months post surgery | 15 months post randomisation | |||
|---|---|---|---|---|---|---|---|
| LASH (n = 330), n (%) | EA (n = 330), n (%) | LASH (n = 309), n (%) | EA (n = 307), n (%) | LASH (n = 330), n (%) | EA (n = 330), n (%) | ||
| Practical difficulties | 1 | 4 (1.2) | 5 (1.5) | 222 (93.7) | 194 (84.0) | 247 (91.1) | 226 (81.9) |
| 2 | 70 (21.5) | 65 (19.9) | 5 (2.1) | 14 (6.1) | 11 (4.1) | 30 (10.9) | |
| 3 | 109 (33.5) | 97 (29.7) | 3 (1.3) | 14 (6.1) | 6 (2.2) | 9 (3.3) | |
| 4 | 142 (43.7) | 160 (48.9) | 7 (3.0) | 9 (3.9) | 7 (2.6) | 11 (4.0) | |
| NK | 2 | 1 | 16 | 13 | 9 | 9 | |
| Social life | 1 | 12 (3.7) | 12 (3.7) | 215 (90.3) | 186 (80.2) | 249 (92.2) | 215 (77.1) |
| 2 | 93 (28.6) | 95 (29.1) | 13 (5.5) | 30 (12.9) | 13 (4.8) | 37 (13.3) | |
| 3 | 141 (43.4) | 144 (44.0) | 7 (2.9) | 8 (3.4) | 5 (1.9) | 22 (7.9) | |
| 4 | 79 (24.3) | 76 (23.2) | 3 (1.3) | 8 (3.4) | 3 (1.1) | 5 (1.8) | |
| NK | 2 | 1 | 16 | 12 | 10 | 6 | |
| Psychological health | 1 | 13 (4.0) | 24 (7.3) | 187 (78.9) | 166 (71.6) | 207 (76.4) | 192 (69.6) |
| 2 | 128 (39.4) | 124 (37.9) | 37 (15.6) | 48 (20.7) | 48 (17.7) | 50 (18.1) | |
| 3 | 120 (36.9) | 123 (37.6) | 9 (3.8) | 12 (5.2) | 10 (3.7) | 26 (9.4) | |
| 4 | 64 (19.7) | 56 (17.1) | 4 (1.7) | 6 (2.6) | 6 (2.2) | 8 (2.9) | |
| NK | 2 | 1 | 17 | 12 | 9 | 9 | |
| Physical health and well-being | 1 | 6 (1.8) | 9 (2.8) | 176 (73.9) | 155 (66.8) | 210 (77.8) | 176 (64.0) |
| 2 | 36 (11.1) | 51 (15.6) | 34 (14.3) | 39 (16.8) | 36 (13.3) | 44 (16.0) | |
| 3 | 190 (58.5) | 175 (53.7) | 23 (9.7) | 32 (13.8) | 19 (7.0) | 49 (17.8) | |
| 4 | 93 (28.6) | 91 (27.9) | 5 (2.1) | 6 (2.6) | 5 (1.9) | 6 (2.2) | |
| NK | 2 | 2 | 16 | 12 | 10 | 10 | |
| Work/daily routine | 1 | 17 (5.2) | 13 (4.0) | 207 (87.7) | 175 (76.1) | 233 (86.0) | 210 (75.8) |
| 2 | 83 (25.5) | 91 (28.2) | 21 (8.9) | 36 (15.7) | 27 (10.0) | 42 (15.2) | |
| 3 | 146 (44.9) | 134 (41.5) | 4 (1.7) | 12 (5.2) | 7 (2.6) | 20 (7.2) | |
| 4 | 79 (24.3) | 85 (26.3) | 4 (1.7) | 7 (3.0) | 4 (1.5) | 5 (1.8) | |
| NK | 2 | 5 | 18 | 14 | 9 | 8 | |
| Family life/relationships | 1 | 17 (5.2) | 30 (9.2) | 190 (80.2) | 158 (67.8) | 221 (81.5) | 200 (72.2) |
| 2 | 106 (32.4) | 111 (34.0) | 40 (16.9) | 55 (23.6) | 38 (14.0) | 54 (19.5) | |
| 3 | 131 (40.1) | 121 (37.1) | 7 (3.0) | 16 (6.9) | 7 (2.6) | 20 (7.2) | |
| 4 | 73 (22.3) | 64 (19.6) | 0 | 4 (1.7) | 5 (1.8) | 3 (1.1) | |
| NK | 0 | 2 | 17 | 11 | 9 | 8 | |
| Analysis approach (MMAS split) | Effect size (95% CI) | p-value | |
|---|---|---|---|
| OLR (primary analysis, adjusted) | OR | 1.87 (1.31 to 2.67) | 0.001 |
| OLR (unadjusted) | OR | 1.90 (1.35 to 2.68) | < 0.001 |
| Binary logistic regression (0–49.9 vs. 50–100, adjusted) | OR | 1.84 (1.04 to 3.25) | 0.04 |
| RD | 0.04 (0.00 to 0.08) | ||
| Binary logistic regression (0–74.9 satisfied vs. 75–100, adjusted) | OR | 2.10 (1.35 to 3.27) | 0.001 |
| RD | 0.10 (0.04 to 0.16) | ||
| Binary logistic regression (0–99.9 vs. 100, adjusted) | OR | 1.79 (1.18 to 2.70) | 0.006 |
| RD | 0.13 (0.04 to 0.29) | ||
| OLR (multiple imputation, adjusted) | OR | 1.68 (1.16 to 2.45) | 0.007 |
| OLR (by actual treatment received, adjusted) | OR | 1.96 (1.40 to 2.77) | 0.001 |
| OLR (including only those operated on by consultant, adjusted) | OR | 2.19 (1.33 to 3.62) | 0.002 |
| Linear regression analysis treating MMAS as continuous (adjusted) | MD | 5.59 (1.93 to 9.25) | 0.004 |
| Category | Studied category | Satisfaction at 15 months post randomisation | MMAS at 15 months post randomisation | ||
|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | ||
| Cavity length | ≥ 8 cm | 0.55 (0.25 to 1.22) | 0.14 | 0.85 (0.52 to 3.28) | 0.57 |
| Menstrual pain at baseline | Severe/crippling pain | 1.44 (0.72 to 2.89) | 0.31 | 1.15 (0.61 to 2.20) | 0.66 |
| Age (years) | ≥ 40 | 1.59 (0.77 to 3.29) | 0.21 | 1.35 (0.68 to 2.68) | 0.39 |
| Fibroids | Present | 7.27 (2.32 to 41.8) | 0.002 | 1.26 (0.39 to 4.10) | 0.70 |
FIGURE 17.
Odds ratios (95% CI) from OLR models by subgroup. For the two co-primary outcomes (satisfaction and MMAS at 15 months post randomisation) the horizontal lines show ORs and 95% CIs from eight OLR models, including particular subgroups of participants and adjusting for age and baseline score (if appropriate). The size of the square is proportional to the number of women in each subgroup. The result for fibroids for the satisfaction outcome (OR 9.85, 95% CI 3.30 to 29.34) has been truncated because of space considerations.
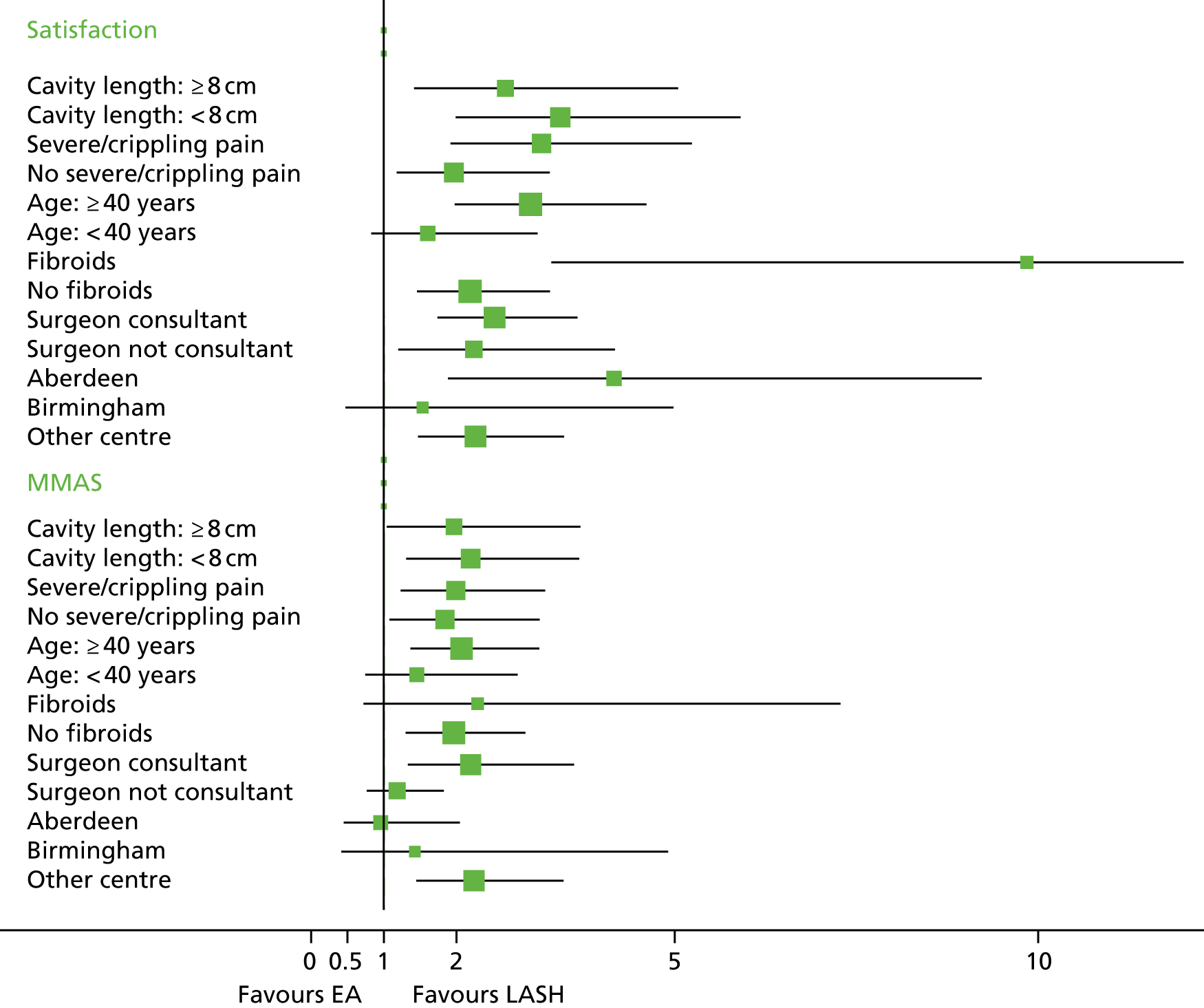
| Outcome | LASH (N = 309) | EA (N = 307) |
|---|---|---|
| Level of pain today (0 = no pain, 10 = worst imaginable), mean (SD) [n] | ||
| Day 1 | 6.08 (2.35) [265] | 5.13 (2.60) [257] |
| Day 2 | 5.66 (2.32) [265] | 4.35 (2.61) [254] |
| Day 3 | 4.89 (2.34) [264] | 3.78 (2.67) [251] |
| Day 4 | 4.33 (2.36) [263] | 3.29 (2.62) [250] |
| Day 5 | 3.81 (2.34) [264] | 2.97 (2.55) [251] |
| Day 6 | 3.44 (2.30) [265] | 2.47 (2.41) [250] |
| Day 7 | 3.19 (2.37) [262] | 2.09 (2.34) [248] |
| Day 8 | 2.89 (2.23) [259] | 1.92 (2.39) [249] |
| Day 9 | 2.63 (2.22) [256] | 1.72 (2.31) [245] |
| Day 10 | 2.32 (2.20) [254] | 1.59 (2.26) [244] |
| Day 11 | 2.18 (2.08) [257] | 1.52 (2.22) [240] |
| Day 12 | 1.99 (1.98) [257] | 1.31 (2.07) [245] |
| Day 13 | 1.88 (1.94) [255] | 1.28 (2.12) [243] |
| Day 14 | 1.69 (1.94) [256] | 1.19 (2.05) [243] |
| Used pads for vaginal bleeding or discharge?, n (%) | ||
| Day 1 | 182 (68.2) | 238 (91.9) |
| Day 2 | 134 (50.2) | 227 (87.6) |
| Day 3 | 80 (30.0) | 206 (79.8) |
| Day 4 | 54 (20.2) | 197 (77.0) |
| Day 5 | 43 (16.2) | 200 (78.1) |
| Day 6 | 29 (10.9) | 205 (79.5) |
| Day 7 | 36 (13.5) | 200 (77.8) |
| Day 8 | 33 (12.5) | 193 (75.1) |
| Day 9 | 34 (12.8) | 190 (73.9) |
| Day 10 | 34 (12.9) | 184 (71.9) |
| Day 11 | 33 (12.5) | 181 (71.0) |
| Day 12 | 29 (10.9) | 178 (69.0) |
| Day 13 | 34 (12.8) | 177 (68.9) |
| Day 14 | 34 (12.7) | 177 (69.1) |
| Took paracetamol?, n (%) | ||
| Day 1 | 223 (94.5) | 166 (86.5) |
| Day 2 | 213 (94.7) | 147 (84.0) |
| Day 3 | 195 (91.6) | 128 (81.5) |
| Day 4 | 180 (89.1) | 107 (74.8) |
| Day 5 | 164 (89.6) | 104 (73.8) |
| Day 6 | 145 (84.8) | 77 (70.6) |
| Day 7 | 138 (83.6) | 69 (65.7) |
| Day 8 | 127 (80.4) | 54 (57.4) |
| Day 9 | 112 (78.3) | 48 (54.5) |
| Day 10 | 103 (74.6) | 42 (53.8) |
| Day 11 | 92 (72.4) | 40 (51.3) |
| Day 12 | 86 (68.8) | 30 (44.8) |
| Day 13 | 77 (67.0) | 38 (49.4) |
| Day 14 | 67 (62.0) | 40 (50.6) |
| Took other painkillers?, n (%) | ||
| Day 1 | 256 (95.9) | 234 (90.7) |
| Day 2 | 253 (95.1) | 208 (81.3) |
| Day 3 | 235 (88.7) | 174 (69.3) |
| Day 4 | 220 (84.0) | 147 (59.3) |
| Day 5 | 195 (75.3) | 138 (56.3) |
| Day 6 | 180 (67.9) | 109 (44.1) |
| Day 7 | 164 (64.3) | 97 (39.6) |
| Day 8 | 159 (61.2) | 84 (34.1) |
| Day 9 | 139 (54.1) | 82 (34.0) |
| Day 10 | 127 (49.2) | 68 (27.9) |
| Day 11 | 119 (46.5) | 63 (26.4) |
| Day 12 | 111 (43.0) | 51 (20.7) |
| Day 13 | 102 (39.2) | 56 (23.3) |
| Day 14 | 88 (34.0) | 61 (25.2) |
| Outcome | Baseline | 6 months after surgery | 15 months after randomisation | |||
|---|---|---|---|---|---|---|
| LASH (N = 330) | EA (N = 330) | LASH (N = 309) | EA (N = 307) | LASH (N = 330) | EA (N = 330) | |
| Are you still having periods?, n (%) | ||||||
| Yes | nc | nc | 39 (15.4) | 111 (45.7) | 52 (18.8) | 117 (42.1) |
| No | nc | nc | 214 (84.6) | 132 (54.3) | 225 (81.2) | 161 (57.9) |
| Description of period, n (%) | ||||||
| Light | 2 (0.6) | 0 | 27 (73.0) | 56 (49.6) | 35 (76.1) | 56 (54.4) |
| Moderate | 6 (1.8) | 7 (2.1) | 7 (18.9) | 29 (25.7) | 5 (10.9) | 27 (26.2) |
| Heavy with clots | 58 (17.7) | 61 (18.7) | 3 (8.1) | 19 (16.8) | 3 (6.5) | 17 (16.5) |
| Very heavy with clots and flooding | 261 (79.8) | 259 (79.2) | 0 | 9 (8.0) | 3 (6.5) | 3 (2.9) |
| On average for how many days is the bleeding heavy?, n (%) | ||||||
| Not heavy | 3 (0.9) | 2 (0.6) | 25 (67.6) | 54 (47.8) | 26 (57.8) | 56 (54.9) |
| 1–3 days | 50 (15.3) | 51 (15.6) | 9 (24.3) | 32 (28.3) | 9 (20.0) | 28 (27.5) |
| 4–6 days | 118 (36.1) | 125 (38.2) | 2 (5.4) | 23 (20.4) | 8 (17.8) | 13 (12.7) |
| ≥ 7 days | 156 (47.7) | 149 (45.6) | 1 (2.7) | 4 (3.5) | 2 (4.4) | 5 (4.9) |
| At any time in the last 3 months have you needed to use more than one form of sanitary protection at a time?, n (%) | ||||||
| No | 27 (8.3) | 25 (7.7) | 15 (39.5) | 30 (27.0) | 19 (40.4) | 25 (23.8) |
| Tampon and pad | 117 (35.9) | 118 (36.3) | 15 (39.5) | 29 (26.1) | 14 (29.8) | 32 (30.5) |
| Two pads | 88 (27.0) | 84 (25.8) | 6 (15.8) | 35 (31.5) | 9 (19.1) | 25 (23.8) |
| Tampon and two pads | 48 (14.7) | 56 (17.2) | 2 (5.3) | 17 (15.3) | 5 (10.6) | 23 (21.9) |
| More than this (e.g. bath towel) | 46 (14.1) | 42 (12.9) | 0 | 0 | 0 | 0 |
| Are your periods usually painful?, n (%) | ||||||
| No | 19 (5.8) | 18 (5.5) | 42 (97.7) | 89 (70.2) | 41 (85.4) | 88 (82.2) |
| Mild pain | 33 (10.1) | 38 (11.7) | 1 (2.3) | 20 (17.5) | 2 (4.2) | 11 (10.3) |
| Moderate pain | 104 (31.9) | 110 (33.7) | 0 | 11 (9.6) | 5 (10.4) | 6 (5.6) |
| Severe/crippling pain | 170 (52.1) | 160 (49.1) | 0 | 3 (2.6) | 0 | 2 (1.9) |
| Bleeding score [0 = none (baseline only), 1 = mild, 5 = worst imaginable], median (IQR) [n] | 3.67 (3.1–4.2) [322] | 3.5 (3.1–4.1) [322] | 1 (1–1.7) [34] | 1.75 (1–2.6) [107] | 1.25 (1–2.25) [37] | 1.67 (1–2.35) [92] |
| Are you having cyclical (period like) pain?, n (%) | ||||||
| Yes | nc | nc | 68 (28.8) | 108 (54.3) | 71 (31.7) | 118 (60.2) |
| No | nc | nc | 168 (71.2) | 91 (45.7) | 153 (68.3) | 78 (39.8) |
| Cyclical pain score (1 = mild, 5 = worst imaginable), median (IQR) [n] | nc | nc | 1.5 (1–2.3)[58] | 2 (1.4–2.8) [107] | 1.33 (1–2.3) [62] | 2 (1.33–3) [113] |
| Pain at intercourse?, n (%) | ||||||
| No | 106 (35.5) | 120 (39.2) | 153 (71.5) | 141 (65.6) | 151 (70.9) | 124 (60.8) |
| Rarely | 90 (30.1) | 69 (22.5) | 40 (18.7) | 38 (17.7) | 42 (19.7) | 44 (21.6) |
| Often | 81 (27.1) | 87 (28.4) | 17 (7.9) | 29 (13.5) | 18 (8.5) | 27 (13.2) |
| Always | 22 (7.4) | 30 (9.8) | 4 (1.9) | 7 (3.3) | 2 (0.9) | 9 (4.4) |
| Not applicable | 26 | 20 | 29 | 20 | 21 | 20 |
| Severity of pain at intercourse, n (%) | ||||||
| Mild | 78 (41.5) | 81 (42.0) | 44 (63.8) | 42 (51.9) | 39 (65.0) | 46 (59.7) |
| Moderate | 76 (40.4) | 85 (44.0) | 22 (31.9) | 33 (40.7) | 18 (30.0) | 26 (33.8) |
| Severe | 34 (18.1) | 27 (14.0) | 3 (4.3) | 6 (7.4) | 3 (5.0) | 5 (6.5) |
| Do you have problems with your bladder?, n (%) | ||||||
| No | 135 (41.8) | 147 (45.2) | 125 (50.4) | 123 (51.3) | 116 (50.0) | 110 (49.3) |
| Yes, I need to dash to the toilet (urgency), but don’t leak | 43 (13.3) | 58 (17.8) | 29 (11.7) | 30 (12.5) | 21 (9.1) | 21 (9.4) |
| Yes, I need to dash to the toilet (urgency), but often don’t make it and leak | 26 (8.0) | 24 (7.4) | 24 (9.7) | 24 (10.0) | 18 (7.8) | 22 (9.9) |
| Yes, I regularly leak when I cough, sneeze or exercise | 69 (21.4) | 64 (19.7) | 46 (18.6) | 50 (20.8) | 45 (19.4) | 48 (21.5) |
| Yes, both. I have urgency and I also leak when I cough, sneeze or exercise | 50 (15.5) | 32 (9.8) | 24 (9.7) | 13 (5.4) | 32 (13.8) | 22 (9.9) |
| Outcome | LASH (N = 297), n (%) | EA (N = 303), n (%) |
|---|---|---|
| Are you still having periods? | ||
| Yes | 41 (16.1) | 106 (40.3) |
| No | 213 (83.9) | 157 (59.7) |
| Description of period | ||
| Light | 33 (80.5) | 58 (58.6) |
| Moderate | 4 (9.8) | 24 (24.2) |
| Heavy with clots | 2 (4.9) | 14 (14.1) |
| Very heavy with clots and flooding | 2 (4.9) | 3 (3.0) |
| On average for how many days is the bleeding heavy? | ||
| Not heavy | 25 (64.1) | 55 (56.1) |
| 1–3 days | 8 (20.5) | 26 (26.5) |
| 4–6 days | 4 (10.3) | 12 (12.2) |
| ≥ 7 days | 2 (5.1) | 5 (5.1) |
| At any time in the last 3 months have you needed to use more than one form of sanitary protection at a time? | ||
| No | 17 (41.5) | 26 (26.0) |
| Tampon and pad | 14 (34.1) | 31 (31.0) |
| Two pads | 6 (14.6) | 23 (23.0) |
| Tampon and two pads | 4 (9.8) | 20 (20.0) |
| More than this (e.g. bath towel) | 0 | 0 |
| Are your periods usually painful? | ||
| No | 38 (90.5) | 85 (82.5) |
| Mild pain | 2 (4.8) | 11 (10.7) |
| Moderate pain | 2 (4.8) | 5 (4.9) |
| Severe/crippling pain | 0 | 2 (1.9) |
| Outcome | LASH (N = 330), n (%)a | EA (N = 330), n (%)b | OR (95% CI) | p-value |
|---|---|---|---|---|
| MMAS total score (baseline) | ||||
| 0–50 | 274 (84.8) | 256 (79.8) | ||
| 51–75 | 42 (13.0) | 59 (18.4) | ||
| 76–99 | 7 (2.2) | 6 (1.9) | ||
| 100 | 0 | 0 | ||
| MMAS total score (6 months) | 1.48 (1.02 to 2.14)c | 0.04 | ||
| 0–50 | 9 (3.9) | 19 (8.5) | ||
| 51–75 | 20 (8.7) | 32 (14.3) | ||
| 76–99 | 46 (20.0) | 38 (17.0) | ||
| 100 | 155 (67.4) | 135 (60.3) | ||
| MMAS total score (15 months) | 1.87 (1.31 to 2.67) c | 0.001 | ||
| 0–50 | 15 (5.7) | 29 (10.8) | ||
| 51–75 | 17 (6.5) | 34 (12.7) | ||
| 76–99 | 50 (19.1) | 59 (22.0) | ||
| 100 | 180 (68.7) | 146 (54.5) | ||
| EQ-5D-3L utility score (baseline) | ||||
| –0.59 to 0.49 | 56 (17.6) | 63 (19.6) | ||
| 0.5–0.99 | 176 (55.2) | 170 (52.8) | ||
| 1 | 87 (27.3) | 89 (27.6) | ||
| EQ-5D-3L utility score (6 weeks) | 0.66 (0.48 to 0.90)d | 0.009 | ||
| –0.59 to 0.49 | 14 (5.6) | 23 (9.3) | ||
| 0.5–0.99 | 129 (51.4) | 88 (35.8) | ||
| 1 | 108 (43.0) | 135 (54.9) | ||
| EQ-5D-3L utility score (6 months) | 1.15 (0.84 to 1.57) | 0.38 | ||
| –0.59 to 0.49 | 27 (10.8) | 20 (8.4) | ||
| 0.5–0.99 | 87 (34.7) | 102 (43.0) | ||
| 1 | 137 (54.6) | 115 (48.5) | ||
| EQ-5D-3L utility score (15 months) | 1.21 (0.89 to 1.64) | 0.23 | ||
| –0.59 to 0.49 | 23 (8.2) | 33 (11.7) | ||
| 0.5–0.99 | 115 (40.9) | 115 (40.9) | ||
| 1 | 143 (50.9) | 133 (47.3) | ||
| EQ-5D-3L VAS (baseline) | ||||
| 0–50 | 101 (31.9) | 79 (24.6) | ||
| 51–75 | 96 (30.3) | 105 (32.7) | ||
| 76–100 | 120 (37.9) | 137 (42.7) | ||
| EQ-5D-3L VAS (6 weeks) | 1.12 (0.80 to 1.58) | 0.51 | ||
| 0–50 | 26 (10.5) | 35 (14.3) | ||
| 51–75 | 54 (21.8) | 51 (20.8) | ||
| 76–100 | 168 (67.7) | 159 (64.9) | ||
| EQ-5D-3L VAS (6 months) | 1.53 (1.08 to 2.17)c | 0.02 | ||
| 0–50 | 26 (10.6) | 29 (12.3) | ||
| 51–75 | 47 (19.1) | 66 (28.1) | ||
| 76–100 | 173 (70.3) | 140 (59.6) | ||
| EQ-5D-3L VAS (15 months) | 1.50 (1.12 to 1.99)c | 0.006 | ||
| 0–50 | 29 (10.4) | 46 (16.3) | ||
| 51–75 | 51 (18.3) | 57 (20.2) | ||
| 76–100 | 199 (71.3) | 179 (63.5) | ||
| Outcome | Analysis method (effect size) | Adjusted effect size (95% CI) | p-value | Unadjusted effect size (95% CI) | p-value |
|---|---|---|---|---|---|
| AE | |||||
| Any SAE | Log Reg (OR, RD) | 1.30 (0.56 to 3.02), 0.01 (–0.02 to 0.04) | 0.54 | 1.28 (0.57 to 2.87), 0.01 (–0.02 to 0.04) | 0.54 |
| Further treatment for HMB | Log Reg (OR, RD) | 0.11 (0.04 to 0.28),a –0.05 (–0.07 to –0.02) | < 0.001 | 0.11 (0.02 to 0.46), –0.05 (–0.07 to –0.02) | 0.003 |
| Pain | |||||
| Pain (0–10) (patient diary, 1–14 days) | RM (MD) | 0.92 (0.59 to 1.24)b | < 0.001 | 0.91 (0.58 to 1.23)b | < 0.001 |
| Level of pain (0–10) (6 weeks) | OLR (OR) | 1.43 (1.05 to 1.96)b | 0.03 | 1.40 (0.96 to 2.05) | 0.08 |
| Time-to-event outcome | |||||
| Time to return to paid work (6 weeks) | Cox Reg (HR) | 0.23 (0.18 to 0.30)b | < 0.001 | 0.24 (0.18 to 0.30)b | < 0.001 |
| Time to return to unpaid work (6 weeks) | Cox Reg (HR) | 0.64 (0.57 to 0.73)b | < 0.001 | 0.65 (0.54 to 0.78)b | < 0.001 |
| Time to return to sporting or social activities (6 weeks) | Cox Reg (HR) | 0.48 (0.42 to 0.56)b | < 0.001 | 0.49 (0.40 to 0.59)b | < 0.001 |
| Menstrual outcome | |||||
| Are you still having periods? (6 months) | Log Reg (OR, RD) | 0.22 (0.15 to 0.32),a –0.30 (–0.37 to –0.22) | < 0.001 | 0.22 (0.14 to 0.33),a –0.30 (–0.38 to –0.23) | < 0.001 |
| Are you still having periods? (15 months PR) | Log Reg (OR, RD) | 0.32 (0.21 to 0.48),a –0.23 (–0.31 to –0.15) | < 0.001 | 0.32 (0.22 to 0.47),a –0.23 (–0.31 to –0.16) | < 0.001 |
| QoL outcome | |||||
| MMAS (6 months) | OLR (OR) | 1.48 (1.02 to 2.14)a | 0.04 | 1.49 (1.03 to 2.17)a | 0.04 |
| MMAS (15 months post randomisation) | OLR (OR) | 1.87 (1.31 to 2.67) a | 0.001 | 1.90 (1.35 to 2.68) a | < 0.001 |
| EQ-5D-3L utility score (6 weeks) | OLR (OR) | 0.66 (0.48 to 0.90)b | 0.009 | 0.71 (0.50 to 1.00) | 0.05 |
| EQ-5D-3L utility score (6 months) | OLR (OR) | 1.15 (0.84 to 1.57) | 0.38 | 1.18 (0.83 to 1.66) | 0.35 |
| EQ-5D-3L utility score (15 months post randomisation) | OLR (OR) | 1.21 (0.89 to 1.64) | 0.23 | 1.20 (0.88 to 1.65) | 0.25 |
| EQ-5D-3L VAS (6 weeks) | OLR (OR) | 1.12 (0.80 to 1.58) | 0.51 | 1.18 (0.82 to 1.70) | 0.39 |
| EQ-5D-3L VAS (6 months) | OLR (OR) | 1.53 (1.08 to 2.17)a | 0.02 | 1.54 (1.06 to 2.23)a | 0.02 |
| EQ-5D-3L VAS (15 months post randomisation) | OLR (OR) | 1.50 (1.12 to 1.99)a | 0.006 | 1.47 (1.04 to 2.08)a | 0.03 |
| SF-12 PCS (6 weeks) | Lin Reg (MD) | –4.97 (–6.31 to –3.63)b | < 0.001 | –4.58 (–6.34 to –2.81)b | < 0.001 |
| SF-12 PCS (6 months) | Lin Reg (MD) | 0.83 (–0.70 to 2.35) | 0.28 | 0.83 (–0.87 to 2.53) | 0.34 |
| SF-12 PCS (15 months post randomisation) | Lin Reg (MD) | 1.08 (–0.65 to 2.81) | 0.21 | 1.16 (–0.53 to 2.85) | 0.18 |
| SF-12 MCS (6 weeks) | Lin Reg (MD) | 1.33 (–0.78 to 3.44) | 0.21 | 1.10 (–0.94 to 3.15) | 0.29 |
| SF-12 MCS (6 months) | Lin Reg (MD) | 3.36 (1.69 to 5.03)a | < 0.001 | 2.75 (0.52 to 4.98)a | 0.02 |
| SF-12 MCS (15 months post randomisation) | Lin Reg (MD) | 2.47 (1.07 to 3.87)a | 0.001 | 1.83 (–0.27 to 3.93) | 0.09 |
| Satisfaction with treatment | |||||
| Acceptability of treatment (6 weeks) | OLR (OR) | 4.73 (2.86 to 7.81)a | < 0.001 | 4.74 (3.10 to 7.27)a | < 0.001 |
| Satisfaction with treatment (6 months) | OLR (OR) | 2.91 (2.04 to 4.16)a | < 0.001 | 2.96 (2.04 to 4.30)a | < 0.001 |
| Satisfaction with treatment (15 months post randomisation) | OLR (OR) | 2.53 (1.83 to 3.48) a | < 0.001 | 2.55 (1.79 to 3.63) a | < 0.001 |
| Recommend treatment to friend (6 months) | Log Reg (OR, RD) | 4.49 (2.44 to 8.27),a 0.11 (0.07 to 0.14) | < 0.001 | 4.58 (2.15 to 9.75),a 0.11 (0.06 to 0.16) | < 0.001 |
| Recommend treatment to friend (15 months post randomisation) | Log Reg (OR, RD) | 4.52 (2.14 to 9.53),a 0.09 (0.04 to 0.14) | < 0.001 | 4.54 (2.06 to 10.01),a 0.09 (0.05 to 0.14) | < 0.001 |
Appendix 3 Additional information for the economic evaluation (within-trial analysis)
Within-trial economic evaluation: further details on the statistical analysis
Model selection
A number of regression model specifications were explored on the study raw data for total cost and total QALYs. For total cost, a GLM with a gamma family and identity link was defined initially. A modified Park test71 was conducted (Table 39), showing a Gaussian family as a better model specification. This family can be linked using identity, logarithmic or power functions and therefore alternative models were fitted using these links. Based on the p-values for the Pearson’s correlation test, the Pregibon link test and the modified Hosmer–Lemeshow test71 makes it impossible to unambiguously select one link function. Therefore, a final model specifying a Gaussian family and identity link was chosen for the base-case analysis for total costs. Alternative models fitting Gaussian family and log or power links were run showing trivial differences in the estimation of cost differences (i.e. < £1 difference in the treatment dummy coefficient between identity, log and power 2 link functions).
| Fitted model: link = identity; family = gamma | |||
|---|---|---|---|
| Coefficient | 0.172825 | ||
| Family, chi-squared and p-value in descending order of likelihood | |||
| Family | Chi-squared | p-value | |
| Gaussian | 0.3319 | 0.5645 | |
| Poisson | 7.6031 | 0.0058 | |
| Gamma | 37.0985 | 0.0000 | |
| Inverse Gaussian or Wald | 88.8181 | 0.0000 | |
| Results of tests for link; p-values | |||
| GLM, Gaussian family | Identity link | Log-link | Power 2 link |
| Pearson’s correlation test | 1.0000 | 0.9989 | 0.9952 |
| Pregibon link test | 0.6039 | 0.9376 | 0.5032 |
| Modified Hosmer–Lemeshow | 0.5487 | 0.5804 | 0.4929 |
A similar approach was followed for selecting the model for total QALYs. However, it was not possible to identify a family distribution for the raw QALY data and a simple transformation was conducted (i.e. maximum possible total QALYs at 15 months follow-up (1.25) minus observed QALYs). Table 40 reports the results for the modified Park test on the transformed QALY data, supporting a Poisson family model specification. A number of link functions were fitted (see Table 40 for selected test results), and a model defining a Poisson family and identity link was finally selected for the QALY analysis.
| Fitted model: link = identity; family = Gaussian | |||
|---|---|---|---|
| Coefficient | 0.86363 | ||
| Family, chi-squared and p-value in descending order of likelihood | |||
| Family | Chi-squared | p-value | |
| Poisson | 0.7180 | 0.3968 | |
| Gaussian | 28.80 | 0.0000 | |
| Gamma | 49.86 | 0.0000 | |
| Inverse Gaussian or Wald | 176.21 | 0.0000 | |
| Results of tests for link; p-values | |||
| GLM, Poisson family | Identity link | Log-link | Power 0.5 link |
| Pearson’s correlation test | 0.6656 | 0.1427 | 0.0723 |
| Pregibon link test | 0.4768 | 0.0000 | 0.0003 |
| Modified Hosmer–Lemeshow | 0.0000 | 0.0000 | 0.0239 |
Missing data
Table 41 reports the proportion of missing data on costs. Relying on complete data allowed us to calculate total costs for 57% of the study sample. However, total cost results and total cost differences between study groups were driven by the cost of the index interventions. It was possible to estimate index intervention costs for 97% (n = 639) of individuals. Complete data analysis would result in many of these observations being discarded (see Table 41); this issue was crucial to decide relying on multiple imputed data for the base-case analysis. In addition, the proportion of missing data differs slightly between treatment groups, dismissing the notion that data could be missing completely at random and supporting the view that data are missing at random. This missing mechanism could be explained by, for example, the treatment allocation.
| Variable | LASH | EA | ||
|---|---|---|---|---|
| Number of missing observations | Proportion (%) of missing data (over 330 possible observations) | Number of missing observations | Proportion (%) of missing data (over 330 possible observations) | |
| Index operation | 6 | 1.82 | 15 | 4.55 |
| Readmissions | 0 | 0.00 | 0 | 0.00 |
| 6-month data | ||||
| Outpatient visits | 94 | 28.48 | 99 | 30.00 |
| GP visits | 96 | 29.09 | 102 | 30.91 |
| GP home visits | 96 | 29.09 | 102 | 30.91 |
| GP telephone consultations | 96 | 29.09 | 102 | 30.91 |
| Medications | 96 | 29.09 | 102 | 30.91 |
| 15-month data | ||||
| Outpatient visits | 101 | 30.61 | 112 | 33.94 |
| GP visits | 99 | 30.00 | 115 | 34.85 |
| GP home visits | 99 | 30.00 | 115 | 34.85 |
| GP telephone consultations | 99 | 30.00 | 115 | 34.85 |
| Medications | 99 | 30.00 | 115 | 34.85 |
| Total costs | 131 | 30.00 | 155 | 46.97 |
Table 42 reports the proportion of missing data for QoL. Between 2% (baseline) and 27% (6 months post intervention) of observations were missing for EQ-5D-3L score data. Therefore, total QALY calculations were possible for 64% of the study sample. Again, the proportion of missing data differs between study groups, supporting the notion that data are missing at random.
| Variable | LASH | EA | ||
|---|---|---|---|---|
| Number of missing observations | Proportion (%) of missing data (over 330 possible observations) | Number of missing observations | Proportion (%) of missing data (over 330 possible observations) | |
| Baseline EQ-5D-3L score | 11 | 3.33 | 8 | 2.42 |
| 6-week post-surgery EQ-5D-3L score | 78 | 25.08a | 82 | 26.37a |
| 6-month post-surgery EQ-5D-3L score | 75 | 24.12a | 90 | 28.94a |
| 15-month post-randomisation EQ-5D-3L score | 49 | 14.85 | 49 | 14.85 |
| QALYs | 103 | 31.21 | 117 | 35.45 |
Imputation model
Multiple imputation was implemented as part of the within-trial analysis, using chained equations and predictive mean matching for continuous variables with imputed values drawn from the five closest observations (kth nearest neighbour = 5). The method was implemented in Stata and starts with the variable with fewer missing observations. Predictive mean matching imputes an observed value from another individual whose predicted value is close to the predicted value of the individual with the missing observation. All other variables being imputed and those included in the imputation model are used in the prediction models. This process generated 20 complete data sets, with plausible fitted values assigned for missing cost [i.e. cost of anaesthetic room, operation room, recovery room, subsequent hospital stay, operation, 6- and 15-month outpatient visits, GP visits (practice, home and telephone calls) and medications] and utility elements (i.e. EQ-5D-3L and SF-12 scores at baseline, 6 weeks and 6 months post surgery and 15 months post randomisation). The imputation model included all variables incorporated in the analysis model (centre number, age, treatment dummy), plus randomisation date and one of the co-primary clinical outcome measures (i.e. patient satisfaction as a binary variable: totally satisfied/not totally satisfied) included as auxiliary variables. Rubin’s rules were used to pool estimates across the multiple imputation data sets.
Regression model results: base case
Tables 43 and 44 report regression results for total costs and total QALYs for the base-case analysis based on the imputed data. The methods of recycled predictions71 was used to obtain the within-trial economic evaluation results reported in Chapter 5.
| GLMs; Gaussian family, identity link | ||
|---|---|---|
| Variable | Coefficient | Standard errora |
| Treatment dummy (1 = LASH) | 1604*** | 63.7 |
| Baseline EQ-5D-3L score | –9 | 142.6 |
| Age dummy (1 = ≥ 40 years) | –32 | 98.8 |
| Constant | 1312*** | 118.7 |
| Observations | 660 | |
| GLM, Poisson family, identity link | ||
|---|---|---|
| Variable | Coefficient | Standard errorb |
| Treatment dummy (1 = LASH) | –0.004 | 0.015 |
| Baseline EQ-5D-3L score | –0.669*** | 0.042 |
| Age dummy (1 = ≥ 40 years) | –0.007 | 0.015 |
| Constant | 0.752*** | 0.039 |
| Observations | 660 | |
Subgroup analyses
Subgroup analyses are shown in Table 45.
| Variable | Cost (£) | QALY |
|---|---|---|
| Uterine cavity length ≥ 8 cm | 0.19 | 0.25 |
| Severe dysmenorrhoea at baseline | 0.56 | 0.29 |
| Fibroids present | 0.57 | 0.21 |
| Age ≥ 40 years | 0.92 | 0.68 |
Productivity costs
Productivity costs are shown in Table 46.
| Variable | Number of observations | LASH | EA |
|---|---|---|---|
| 6 months post surgery | |||
| Time lost from usual activities (days within last 4 weeks) | |||
| From paid work: n (%); mean [SD] | 33 | 15 (6.3); 6.1 [8.1] | 18 (7.7); 7.2 [7.9] |
| From unpaid work: n (%); mean [SD] | 64 | 25 (11.5); 10.1 [8.4] | 39 (18.3); 5.7 [4.4] |
| From leisure/social activities: n (%); mean [SD] | 70 | 29 (14.5); 7.9 [8.5] | 41 (21.5); 6.4 [5.8] |
| Productivity costs (£) | |||
| Cost of time from paid work, mean (SD) | 470 | 26 (171) | 40 (210) |
| Cost of time from unpaid work, mean (SD) | 423 | 96 (544) | 113 (512) |
| 15 months post randomisation | |||
| Time lost from usual activities (days within last 4 weeks) | |||
| From paid work: n (%); mean [SD] | 43 | 16 (6.9); 8.1 [11] | 27 (12.2); 4.4 [4.2] |
| From unpaid work: n (%); mean [SD] | 67 | 25 (11.8); 8.9 [8.6] | 42 (20.7); 6.9 [7.3] |
| From leisure/social activities: n (%); mean [SD] | 72 | 27 (13.4); 7.7 [7.5] | 45 (23.8); 5.4 [5.3] |
| Productivity costs (£) | |||
| Cost of time from paid work, mean (SD) | 452 | 39 (260) | 54 (234) |
| Cost of time from unpaid work, mean (SD) | 401 | 81 (460) | 144 (539) |
Appendix 4 Literature searches for trials, cohort studies or case series reporting the incidence of further related surgery following endometrial ablation or laparoscopic supracervical hysterectomy
As HEALTH reported at 15 months post randomisation, too early to observe all further associated gynaecological surgery following treatment with EA or LASH, focused literature searches of MEDLINE were conducted to identify relevant studies to inform the model.
First, a search was conducted during the model development phase for RCTs of LASH. The search was broad in that it did not specify the indication or comparators of interest (Box 1).
-
exp clinical trial/ (850,485)
-
randomized controlled trial.pt. (414,266)
-
controlled clinical trial.pt. (91,931)
-
randomi?ed.ab. (404,360)
-
placebo.ab. (169,219)
-
drug therapy.fs. (1,848,006)
-
randomly.ab. (242,942)
-
trial.ab. (351,099)
-
groups.ab. (1,513,597)
-
42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 (3,886,527)
-
exp animals/not humans/ (4,132,479)
-
51 not 52 (3,369,623)
-
(laparoscop* and supracervical).tw. (240)
-
(laparoscop* and supra-cervical).tw. (5)
-
(hysterectomy and laparoscop* and subtotal).tw. (110)
-
(hysterectomy and laparoscop* and sub-total).tw. (2)
-
54 or 55 or 56 or 57 (334)
-
53 and 58 (93)
-
limit 59 to ‘review articles’ (9)
-
59 not 60 (84)
Date searched: October 2015.
No limits applied to date range searched.
This search identified nine reviews and 84 primary studies of potential relevance. The abstracts were reviewed to identify any trials comparing LASH with EA for HMB. Only two trials were identified meeting these criteria. Zupi et al. 13 compared hysteroscopic endometrial resection with LASH for abnormal uterine bleeding, and the primary results were published in 2003. A further long-term follow-up paper, including reoperations, was published in 2010. 48 The latter paper was considered potentially useful for informing the economic model and is discussed in the main body of the report in Extrapolation of subsequent hysterectomy following endometrial ablation. Sesti et al. 12 compared thermal balloon ablation with LASH for the surgical treatment of HMB, but reported only on short-term (30-day) complications resulting in readmission to hospital. Therefore, this study was not considered useful for informing the model. In addition to the trials of ablation compared with LASH, the above search identified a retrospective study53 that reported on comparative reoperation rates following total laparoscopic hysterectomy (n = 567) and laparoscopically assisted supracervical hysterectomy (n = 300). A further study72 was also identified that reported on long-term outcomes following laparoscopic (n = 315) and abdominal (n = 134) supracervical hysterectomies performed at a university hospital in Oslo, Norway. These studies were considered further to inform the economic model.
Given the limited comparative evidence on the incidence of further related surgery following LASH and EA, a broader search was developed to identify cohort studies or case series reporting on the longer-term follow-up of these procedures. The search strategy is provided in Table 47. The results were restricted to published articles in the English language from 2010 onwards, to capture follow-up of reasonably contemporary cohorts.
| # | Search | Result | Type |
|---|---|---|---|
| 1 | Menorrhagia/ | 4050 | Advanced |
| 2 | Menorrhagia.tw. | 2730 | Advanced |
| 3 | (heavy and menstrua*).tw. | 1091 | Advanced |
| 4 | abnormal uterine bleeding.tw. | 1568 | Advanced |
| 5 | 1 or 2 or 3 or 4 | 7008 | Advanced |
| 6 | (laparoscop* and supracervical).tw. | 276 | Advanced |
| 7 | (laparoscop* and supra-cervical).tw. | 5 | Advanced |
| 8 | (hysterectomy and laparoscop* and subtotal).tw. | 126 | Advanced |
| 9 | (hysterectomy and laparoscop* and sub-total).tw. | 5 | Advanced |
| 10 | Endometrial Ablation Techniques/ | 337 | Advanced |
| 11 | endometrial ablation.tw. | 1037 | Advanced |
| 12 | thermal balloon.tw. | 182 | Advanced |
| 13 | radio frequency.tw. | 5267 | Advanced |
| 14 | Novasure.tw. | 49 | Advanced |
| 15 | thermachoice.tw. | 43 | Advanced |
| 16 | cavaterm.tw. | 17 | Advanced |
| 17 | 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 | 6822 | Advanced |
| 18 | Hysterectomy/ | 27,214 | Advanced |
| 19 | Hysterectomy.tw. | 28,028 | Advanced |
| 20 | 6 or 7 or 8 or 9 | 390 | Advanced |
| 21 | (18 or 19) and 20 | 387 | Advanced |
| 22 | 17 or 21 | 6822 | Advanced |
| 23 | exp Cohort Studies/ | 1,774,326 | |
| 24 | exp case-control studies/ | 937,492 | Advanced |
| 25 | case series.ti,ab,kw. | 49,673 | Advanced |
| 26 | treatment outcome/or treatment failure/ | 885,911 | Advanced |
| 27 | 23 or 24 or 25 or 26 | 2,514,214 | |
| 28 | 22 and 27 | 1305 | Advanced |
| 29 | (surgery or surgical).ti,ab. or su.fs. | 2,469,340 | |
| 30 | 28 and 29 | 1029 | Advanced |
| 31 | limit 30 to (english language and humans and yr = ‘2010 -Current’) | 379 | Advanced |
The 379 identified abstracts were reviewed to identify studies reporting long-term rates of related gynaecological surgery following EA or LASH. When selecting studies for use in informing the rate of hysterectomy following EA, the focus was on identifying large population-based cohort studies relevant to the UK NHS. With respect to further surgery following LASH, the focus was on informing the rate of surgery considered to be directly related to the initial procedure or continued menstrual bleeding, subsequent removal of the cervical stump, laparoscopy to investigate subsequent pain and salpingo-oophorectomy. Given the paucity of published data on further relevant surgery following LASH, a less restrictive approach was applied, focusing on larger cohort studies (n > 150) reporting longer-term follow-up (> 1 year). This process yielded three large population-based cohort studies5,46,49 reporting the incidence of further surgery following EA and three smaller cohort studies reporting on rates of subsequent surgery following LASH. 54–56 The two non-randomised comparative studies identified above53,72 were added to this pool, as were three additional studies identified through the reference lists of other included studies,50–52 providing a total of eight observational studies of potential use for informing rates of post-LASH surgery in the model.
FIGURE 18.
Identification of studies.
The reported incidence of trachelectomy (removal of the cervical stump) following supracervical hysterectomy has ranged from 0.9% to 23%. 50,51 The highest reported incidence of 23% comes from an older UK series performed by a single surgeon (Okaro et al50). In this series, 16 out of 70 patients who received LASH between 1992 and 1995 were reported to require subsequent trachelectomy, with mean time to treatment of 14 months. It has been noted that 82.3% of women who required removal of the cervical stump in the Okara study had been previously treated for endometriosis (Jenkins et al. 58), and endometriosis has subsequently been identified as a significant risk factor for trachelectomy following hysterectomy (Tsafrir et al. 51). This high rate does not appear applicable to the population enrolled in HEALTH, as it suggests that the incidence of trachelectomy should already be > 10% by 12 months post LASH. Only one person is known to have required a trachelectomy following LASH by 15 months post randomisation in HEALTH.
The most recent study by Tsafrir et al. 51 reported that only 17 (0.9%) out of 1892 women who underwent supracervical hysterectomy between 2002 and 2014 at a single US medical centre subsequently underwent removal of the cervical stump. However, the duration of follow-up to which this incidence relates was not clearly reported.
From 192 laparoscopic subtotal hysterectomies carried out at three teaching hospitals in the Netherlands between 1998 and 2007 for benign and malignant indications, van Evert et al. 52 reported that four patients (2%) required further surgery to remove the cervix. The corresponding duration of follow-up was not clearly reported but the minimum was 6 months.
The study by Boosz et al. 53 turned out to include only short-term follow-up data and reported that 3.7% of 300 women receiving LASH at a university hospital in Germany (between January 2002 and December 2009) had a subsequent operation within 6 months of the initial surgery. It was reported that 2.7% required removal of the cervical stump. 53 This 6-month rate is significantly higher than the observed rate in HEALTH at 15 months post randomisation and is not useful for informing longer-term extrapolation.
Graziano et al. 54 reported on mainly perioperative and early postoperative complications occurring in 365 women undergoing LASH, but also reported one later vaginal trachelectomy and operative laparoscopies (n = 4) performed beyond 6 months. 54 However, the precise follow-up time for these further procedures was not reported.
Schuster et al. 55 reported that 5 out of 277 (1.8%) women undergoing LASH in a single Canadian centre had a repeat operative procedure for pain or bleeding. However, again, the duration of follow-up was unclear.
Wallwiener et al. 56 provided an analysis of a prospective cohort of women who underwent LASH (n = 1658) or total laparoscopic hysterectomy (n = 294) at a single centre in Germany and reported that 20 (1.2%) patients had a postoperative complication requiring surgical intervention [with adhesions being the most common reason (n = 9)]. 56 This was over mean follow-up of 2.5 years.
Finally, Lieng et al. 17 reported that 7% (22/315) of women in a Norwegian cohort had further gynaecological surgery up to 36 months following LASH. The main surgical interventions were laparoscopic adhesiolysis (1.9%) and laparoscopic extirpation of the cervical stump (2.3%). It was also noted that one woman (0.3%) underwent subsequent BSO. The other reported procedures included scar correction (1%), umbilical hernia repair (0.3%), tension-free vaginal tape procedures (0.6%), laparotomy for postoperative peritonitis (0.3%) and laparoscopic drainage of postoperative abscess (0.3%). These other surgical procedures were not included in the long-term rate of further surgery post LASH in the economic model. Some reflect short-term postoperative complications that are already informed in the model using HEALTH data, and the others are of very low incidence and of uncertain association with LASH.
Appendix 5 Extrapolation of hysterectomy post endometrial ablation
As the primary analysis of HEALTH took place at 15 months post randomisation, further modelling was conducted to extrapolate cost-effectiveness over a longer time horizon. Ten years was selected based on existing modelling studies and the availability of long-term follow-up data for a population-based cohort of similarly aged women who received primary EA on the UK NHS.
The cohort study by Cooper et al. 5 provided a published KM curve of time to subsequent hysterectomy based on 14,078 women identified as having received primary EA for HMB between 1989 and 2006 in Scotland. The data indicated that > 50% of hysterectomies were performed in the first year post ablation, but the percentage went on increasing steadily out to and beyond 10 years post ablation. The historical Scottish data also indicated that ≈10% of patients had a hysterectomy by 12 months following ablation, a substantially greater proportion than the estimated 3% by 12 months in HEALTH. Therefore, for consistency with the trial-based evaluation at 15 months post randomisation, we used the HEALTH data to inform the hysterectomy rate in the first 12 cycles (12 months) of the economic model. We then used the data reported by Cooper et al. 5 (beyond year 1) to guide extrapolation of the expected rate of further hysterectomies in the longer term. This was achieved by fitting a mathematical function to the observed KM data reported by Cooper et al. 5 and then referencing it for the estimation of cycle-specific transition probabilities from month 13 onwards in the economic model.
In order to achieve this, a sample of data points were extracted from the published KM curve using WebPlotDizitizer software [URL: https://automeris.io/WebPlotDigitizer/ (accessed 22 May 2019)]. An attempt was made to reconstruct the individual patient data behind the published KM curve using the spreadsheet developed by Hoyle and Henley. 73 This was challenging due to the large sample informing the KM plot, resolution of the published figure and lack of details on numbers at risk over time. Nevertheless, a reconstructed data set was approximated based on 15 extracted points. Alternative parametric survival functions were then fitted to the reconstructed KM data, including an exponential, Weibull, logistic, log-normal and log-logistic function. Of these alternative distributions, the Weibull provided the best statistical fit based on the Akaike information criterion and Bayesian information criterion (Table 48). However, this Weibull function did not provide a good visual fit to the extracted data points, resulting in underprediction of the event (hysterectomy) in the short term and overprediction in the longer term [Figure 19, S(t)_Weibull A]. Therefore, we utilised an alternative regression-based method to fit a Weibull function to KM data, ignoring extracted points in the first year. This involved regressing log(–log(S)) on log(t), where S represents the extracted survivor proportions and t represents the corresponding survival times. The constant term from this model gives the log of the rate parameter (lambda) for a fitted Weibull distribution, and the parameter estimate for log(t) provides the shape parameter (gamma). This fitted curve provided a better visual fit against the extracted KM data beyond year 1, the time period requiring extrapolation in the economic model (see Figure 19). Although the fitted curve is initially slightly shallower than the observed KM curve, this may not be inappropriate for extrapolation of HEALTH, in which the 1-year KM data illustrates a slower rate of progression to hysterectomy than expected based on the historical cohort (see Figure 19). The slight over prediction of hysterectomy beyond 10 years is of limited concern because the time horizon of the model is curtailed at 10 years. Therefore, this fitted Weibull curve was used to derive time-dependent monthly probabilities of hysterectomy beyond year 1 in the base case. The time-dependent monthly transition probabilities were calculated using the following equation:
in which λ (lambda) is the rate parameter of the Weibull distribution, γ (gamma) is the shape parameter (indicating a diminishing hazard over time when < 1), t0 is the time at the beginning of the interval of interest and t1 is the time at the end of the interval of interest. The parameter estimates for lambda and gamma are provided in Table 19.
| Model | Intercept | Log(scale parameter) | AIC | BIC |
|---|---|---|---|---|
| Exponential | 3.353 | 0.0190 | 25,850.18 | 25,857.59 |
| Weibull | 4.378 | 0.564 | 24,560.75 | 24,575.56 |
| Logistic | 16.256 | 1.820 | 30,952.16 | 30,966.97 |
| Log-normal | 3.459 | 0.847 | 24,648.1 | 24,662.91 |
| Log-logistic | 3.382 | 0.244 | 24,948.85 | 24,963.65 |
FIGURE 19.
Extracted data points and fitted Weibull curves for hysterectomy following EA. Survivor function (S(t)).
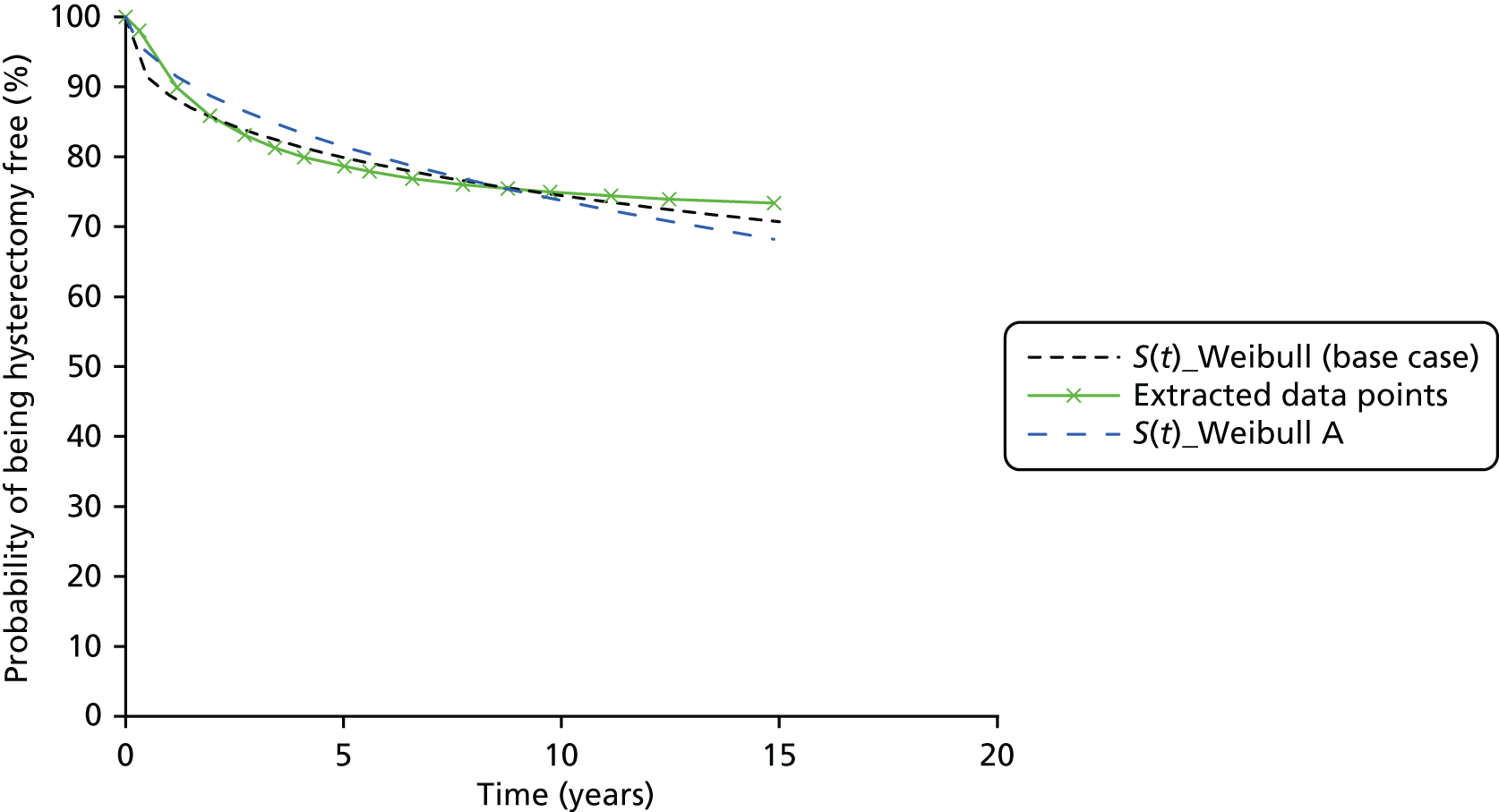
Given the uncertainty surrounding time to hysterectomy following EA, and its importance in the model with respect to extrapolation of QALYs, further sensitivity analysis was undertaken using cycle-specific transition probabilities derived directly from the published KM curve reported by Cooper et al. 5 For this analysis, further data points were extracted from the published KM curve at approximately 6-month intervals (Figure 20). A transition probability for each extracted 6-month interval was then estimated as 1 – (S(t1)/S(t0)). These were further transformed into constant monthly transition probabilities corresponding to each extracted 6-month interval for application in the model, using the following equations:
where r is a constant rate (expressed per unit of time at risk), t is the time period to which the initial probability relates (6 months) and p is the transition probability. which is rescaled to the time period of interest (1 month). In the first sensitivity analysis, the HEALTH KM data were used up to 12 months in the model, with the transition probabilities derived from year 1 onwards (see Figure 20) applied thereafter. In an alternative specification, transition probabilities derived from Figure 20 were applied over the full duration of the model. This latter scenario assessed the impact of assuming a higher probability of hysterectomy in year 1 and a long-term probability of 25% by 10 years, as indicated in the population based KM curve reported by Cooper et al. 5
FIGURE 20.
Extracted 6-monthly data points for hysterectomy following EA. Survivor function (S(t)).
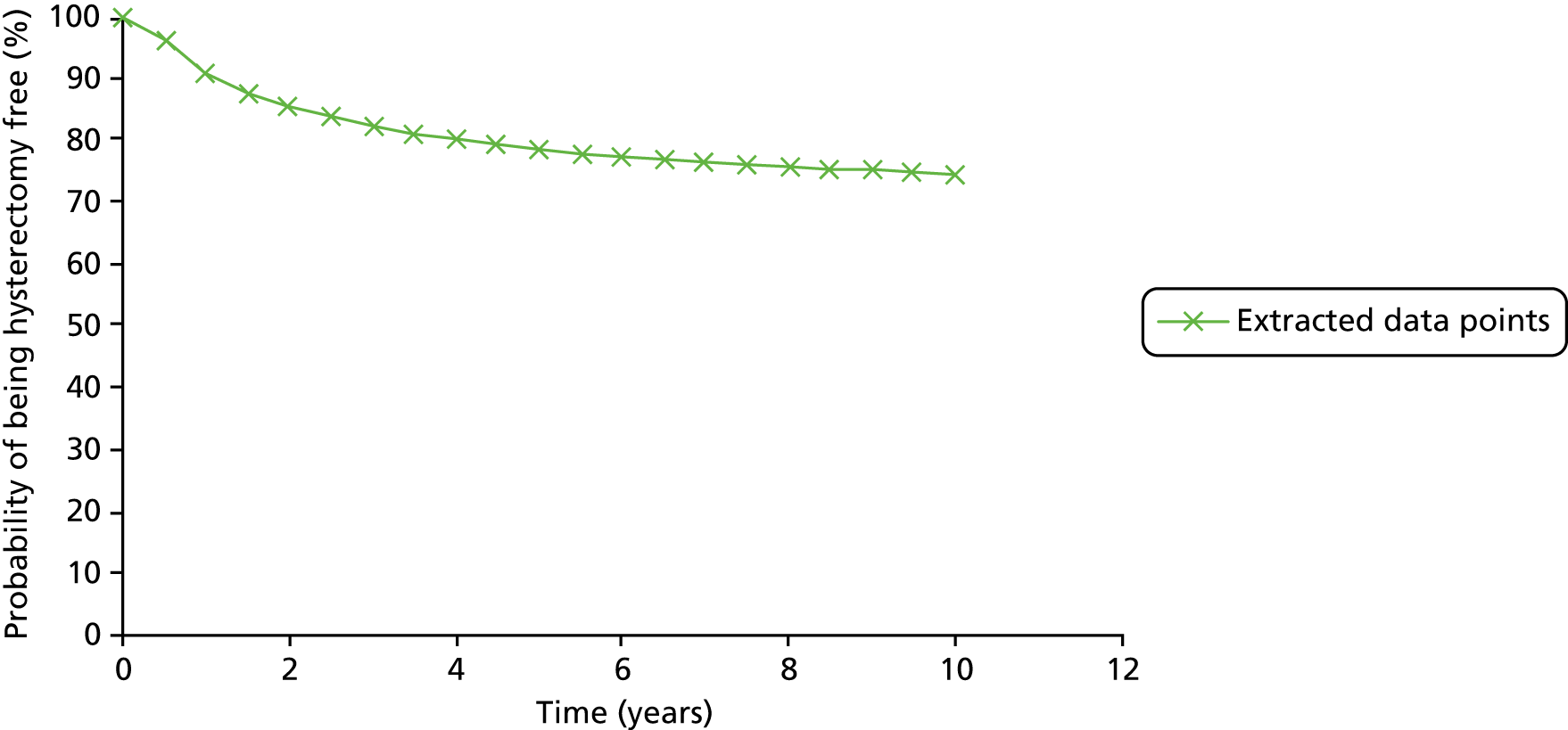
List of abbreviations
- AE
- adverse event
- BSO
- bilateral salpingo-oophorectomy
- CHaRT
- Centre for Healthcare and Randomised Trials
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DMC
- Data Monitoring Committee
- EA
- endometrial ablation
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GLM
- generalised linear model
- GP
- general practitioner
- HEALTH
- Hysterectomy or Endometrial AbLation Trial for Heavy menstrual bleeding
- HMB
- heavy menstrual bleeding
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- KM
- Kaplan–Meier
- LASH
- laparoscopic supracervical hysterectomy
- LMS
- leiomyosarcoma
- MCS
- mental component score
- MMAS
- Menorrhagia Multi-Attribute Quality-of-Life Scale
- NICE
- National Institute for Health and Care Excellence
- OLR
- ordinal logistic regression
- OR
- odds ratio
- PCS
- physical component score
- PI
- principal investigator
- PMG
- Project Management Group
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- WTP
- willingness to pay
