Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/172/01. The contractual start date was in February 2016. The draft report began editorial review in August 2018 and was accepted for publication in January 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Catherine Hewitt reports being a member of the National Institute for Health Research Health Technology Assessment Commissioning Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Hughes et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction and background
This report presents the findings from a Health Technology Assessment programme commissioned study that aimed to establish the feasibility of a novel sexual health promotion intervention for people with serious mental illness (SMI). This study had two main aims:
-
to develop a novel evidence-informed and co-produced sexual health promotion intervention that could be routinely delivered in community mental health services
-
to assess the feasibility and acceptability of both the intervention and the randomised controlled trial (RCT).
Secondary aims were to gain a better understanding of the sexual health needs of people with SMI who use mental health services, and to explore the cost-effectiveness of the intervention in preparation for a future large-scale fully powered trial.
This chapter provides the background and rationale for developing a sexual health intervention for people with SMI, and describes the research objectives. The remainder of the report is divided into the following seven chapters, each representing specific phases of the study:
-
Chapter 2 describes the process undertaken to develop the intervention manual and the selection and training of mental health workers to deliver the intervention in the NHS sites. The content and format of the intervention is described.
-
Chapter 3 describes the method and procedure of both the trial and the nested qualitative study.
-
Chapter 4 outlines changes to the protocol, which were implemented after the study had commenced.
-
Chapter 5 presents the main feasibility trial results and reports on the numbers screened and eligible, and the numbers recruited and retained. It reports on data completeness. It also presents descriptive data on demographics, participants and the outcome data by gender and study arm (intervention arm and control arm).
-
Chapter 6 presents the qualitative analysis and, following this, the data obtained from the recruitment stage feedback:
-
recruitment stage questionnaire and study exit questionnaire
-
Clinical Research Network (CRN) staff feedback.
-
-
Chapter 7 presents details of the dissemination events and engagement activities in which the research team have been involved.
-
Chapter 8 presents the overall discussion and conclusion.
Background
People with SMI (e.g. psychosis, schizophrenia and bipolar affective disorders) account for approximately 1% of the population. 1 SMI includes mental disorders such as schizophrenia-spectrum disorders, bipolar disorders and severe major depression where there is significant functional impairment and limitation of major life activities. 1 In the UK, people with these needs typically require the care of secondary mental health services. Some people with SMI may require the services of secondary mental health care over an extended period of time because of the impact that these disorders have on functioning and the activities of daily life. In addition to their mental health needs, this population experiences significant inequalities in physical health compared with the general population, with their life expectancy approximately 15–20 years less than the general population. 2 To address this disparity, the physical health and well-being of those with SMI has been recently prioritised in mental health services3 and there is some evidence that the sexual health of this group has not been addressed as effectively as other aspects of health. 4
The nature of the problem
The World Health Organization’s (WHO’s) definition of sexual health5 includes not just freedom from sexually acquired infections; it defines it as experiencing one’s own sexuality that is satisfying, positive and respectful, and free from exploitation and violence.
Most people with SMI live in the community and many are sexually active throughout their adult life. It is hard to estimate the level of sexual activity of people with SMI in the UK as there have been no studies undertaken; however, in a systematic review of 52 studies (predominantly conducted in the USA, with a few in Australia, India and Canada),6 it was estimated that just under half (44%) of people with SMI were sexually active in the preceding 12 months. In a reanalysis of data from a previous study, Bonfils et al. 7 found that 30% of people with SMI had been sexually active in the last 3 months prior to data collection.
Although sexual activity is generally less frequent than levels reported in data for the general adult population6 for a range of reasons (impact of mental illness, medication and/or lack of sexual partner), sexual practices seem to involve a greater proportion of ‘high-risk behaviour’ such as condomless vaginal and anal sex. 8
Human immunodeficiency virus
People with SMI have an elevated risk of infection with human immunodeficiency virus (HIV), hepatitis B and hepatitis C. A systematic review and meta-analysis of 91 prevalence studies of blood-borne viruses9 (BBVs) (hepatitis B and C, and HIV) found that pooled HIV prevalence estimates were elevated in every area of the world for people with SMI, and much higher than expected in that area’s general population. Pooled data for the prevalence of HIV infection in people with SMI in the USA were 10 times higher than for the general population (6% vs. 0.6%). 10
Sexually transmitted infections
There is less research attention paid to sexually transmitted infection (STI) prevalence in people with SMI than in people with HIV. However, studies from the USA and Brazil have indicated that STI rates are also elevated for people with SMI. In the USA, Vanable et al. 11 found that 38% of 464 psychiatric outpatients reported having had STI. In a study of sexual health interviews for 2475 psychiatric patients in Brazil, Dutra et al. 12 found that 26% had a lifetime history of STIs. In the questionnaires, participants were asked about STI symptoms as well as whether or not they had any medical diagnosis of a STI; the majority of participants reported symptoms as opposed to medically diagnosed STIs (although 10% reported having had a diagnosis of gonorrhoea).
Reproductive health
Pregnancy rates are lower in women with SMI, yet the rates of terminations of pregnancy are higher than in the general population. 13 Simoila et al. 14 undertook a comparison of termination of pregnancy rates in Finland for women with schizophrenia and schizoaffective disorder, and matched healthy women using large health data sets. They found that the numbers of terminations of pregnancy were similar between the two groups; however, as pregnancy was a rarer event in the women with schizophrenia, the risk of abortion was twofold. Terminations in the group with SMI were associated with being younger, being single and having a lack of contraception. Therefore, women with SMI should have access to advice about a range of contraception and the planning of pregnancies in order to have control over their fertility, as well as having support around decisions regarding whether or not to continue with a pregnancy.
People with SMI experience higher levels of exploitation and violence in sexual relationships. 15 Elkington et al. 16 have suggested that one factor that mediates exploitative relationships is stigma (from self as well as others) regarding mental illness. People who feel that they are less attractive because of their mental health diagnosis may be more likely to be vulnerable to exploitative and abusive partners, as they perceive that they have limited choices of partners. High scores on the Mental Illness Stigma Scale (MISS-Q) have been associated with risky sexual behaviour. 17
In addition, people with SMI are more likely to have a history of childhood sexual abuse. Abuse histories are associated with sexual risk-taking as adults such as condomless anal and vaginal sex with multiple partners, sex working and sex trading. 8,12 Another factor that could be mediating risky sexual behaviour is that many people with SMI have co-occurring drug and/or alcohol use and drug and/or alcohol problems. This can lead to unsafe sex as a result of intoxication (poor decision-making, being unprepared) as well as trading sex for drugs and/or alcohol. During an acute phase of illness, some people are more likely to engage in unsafe sex as a result of hyper-sexuality, sexual disinhibition and poor judgement and planning. 8
Informed about sexual health
Several studies indicate that there are gaps in information and awareness related to sexual health issues for people with SMI (Table 1).
| Author and year of study | Location | Type of participants | Method | Findings |
|---|---|---|---|---|
| Grassi 200118 | Italy | Inpatient psychiatric units and two mental health outpatient clinics in Ferrara, in north-eastern Italy, and a control group | AIDS risk behaviour knowledge test | Knowledge was lower in the SMI group than in the control group:
|
| Shield 200519 | Early intervention psychosis, Sydney, NSW | Young people using the service, n = 62 | Paper survey: mail and in person |
School was the main source of sex information Gender difference in the knowledge of transmission of infection – males had poorer knowledge Risks – in terms of rating rate sexual practices as low, medium or high – responses were reasonably accurate (but some rated oral sex as ‘high’ risk and a small percentage rated anal sex moderate to low (15%) 31% reported a concern about unsafe sex in the past 12 months Sexually active women in this sample reported more than three sexual partners in last 12 months Safer sex attitudes: women were more concerned than men regarding STIs: |
| McCann 201020 | Community mental health team, London | Schizophrenia and schizotypal, n = 30: 15 men, 15 women | Qualitative interviews |
Anxiety about HIV No past sex education – basic education at school Rarely talk about issues with health-care staff, knowledge is patchy |
| Ngwena 201121 | Acute psychiatric hospital, London, UK | Patients on acute wards, able to give consent, aged 18–65 years, n = 30 | Questionnaire survey |
Knowledge – ‘from TV’ Doctors and nurses were least likely to be sources of info Risk knowledge: |
Mental health service response to sexual health
Sexuality and sexual health issues are rarely discussed with service users in mental health settings. 22–24 Service users themselves value positive sexual relationships,20 yet because of ‘self-stigma’ and other vulnerabilities they feel limited in their choices of sexual partners and, therefore, more vulnerable to sexual exploitation and abuse in relationships. 16
In focus group discussions held in two different NHS services in England,4 mental health clinicians reported awareness of a range of sexual health needs of the people in their care, but some reported that they would usually avoid raising the topic of sexuality, sexual health and abuse. The reasons for this ranged from fear of offending the person, concerns about destabilising their mental state, feeling that they lacked the knowledge to address the sexual health issues that may be raised, and lack of knowledge about local sexual health services. Despite this, the participants recognised that sexual health is an important aspect of people’s lives. They were able to describe a number of issues that they had become aware of related to the topic and they saw promotion of sexual health, as well as facilitating access to appropriate family planning and sexual health clinics, as part of their clinical role. In addition, they recognised that they could play an advocacy role in terms of assisting people to get access to appropriate family planning and sexual health clinics. However, they also acknowledged that their knowledge regarding sexual health and sexual health services was limited. These findings were echoed in a survey of mental health staff in England and Australia about sexual health provision. 25 Participants from both countries reported that very limited sexual health work was being undertaken in routine practice but they did see it as part of their role. Therefore, in order to address this issue, mental health staff require training and guidance in relation to sexual health issues in SMI and in how to engage people in conversations about sexual behaviour, and offer advice about where to access help for contraception and sexual health concerns.
Despite experiencing significant health disparities, people with SMI struggle to get their wider physical needs assessed and treated. 2 Therefore, promoting the sexual health of people with SMI could fall into routine practice in mental health services that can often be the only health service that some people with SMI are engaged with.
Current sexual health concerns in England
Sexual health is an important public health concern in England26 and there are key areas that require attention for the promotion of sexual health in the general population. Overall, the incidence of new diagnoses of many STIs has remained stable in recent years but there are some disproportionate rises in STIs in specific populations. Chlamydia is the most common STI (almost half of all STIs diagnosed are chlamydia) and the impact of STIs remains greatest in young people aged under 25 years. There has been a 21% rise in gonorrhoea and a 19% increase in syphilis in men who have sex with men (MSM), and this is possibly as a result of an increase in condomless sex. 27
HIV rates are declining for the first time since it was first identified 30 years ago. 28 The mortality of someone diagnosed promptly (shortly after infection) with HIV is comparable with the general population for the first time. This is because of access to effective treatments to suppress the virus. However, this is not the case for late diagnosis and those diagnosed late have a greater risk of death within the first 12 months of diagnosis. Therefore, people engaging in condomless sex with new or casual partners of unknown HIV status should be tested for HIV every 3 months, and gay/bisexual men and other MSM should be tested for HIV every year.
In summary, in the general population, STIs are most prevalent in young people and in specific groups, such as MSM (who may be more likely to engage in high-risk sexual behaviours). Early detection and treatment of STIs is crucial, not only to improve the prognosis and prevent further harms (e.g. untreated chlamydia leading to fertility problems) but, in some cases, to prevent mortality (e.g. late diagnosis of HIV is associated with death from AIDS within 12 months of diagnosis). In addition, the treatment of STIs (and HIV) can also prevent onward transmission, hence the term ‘testing as treatment’. Therefore, it is essential that all health professionals are aware of the current issues in sexual health, able to ask questions about sex and sexuality as part of routine care, able to offer advice about testing and treatment, and able to offer access to condoms in the local area.
Policy context
There is no specific mention of people with mental illness in the recent Public Health England sexual health policy. 29 However, there is some emerging awareness of this issue in mental health policy, specifically within the ‘improving physical health for people with mental illness’ agenda. A recent document from the Department of Health3 (Improving the physical health of people with mental health problems. Actions for mental health nurses) includes a chapter on sexual health, outlining sexual health needs, and care responses to those needs, by mental health nurses. In addition, sexual health is mentioned (albeit very briefly) in a recent joint report by the Academy of Medical Royal Colleges and the Royal Colleges of General Practitioners, Nursing, Pathologists, Psychiatrists, Physicians, the Royal Pharmaceutical Society and Public Health. 30 Both of these documents support the role of mental health nurses and psychiatrists in promoting sexual health in mental health care settings.
Justification for sexual health promotion in mental health
Sexual health promotion activities such as regular check-ups, increasing knowledge and awareness of risky sexual behaviour and how to keep oneself and sexual partners protected are essential in terms of prevention of STIs, as well as in detection. Untreated STIs can lead to significant health problems (e.g. the human papilloma virus can lead to cervical cancer, and other STIs such as chlamydia can result in infertility). BBV such as hepatitis B and hepatitis C can result in premature death through the development of cirrhosis and liver cancer in the long term. Comorbidity of HIV and SMI, such as schizophrenia, poses significant challenges for both the person themselves as well as in the provision of treatment. 31 The treatment and management of HIV requires early detection and adherence to a complex medicine regime to suppress the virus. This requires engagement with services and treatment adherence. Early diagnosis and treatment has resulted in people living well with HIV and has the potential to reduce onward transmission (treatment as prevention) by suppressing the virus to the extent that it is not present in sufficient quantities in blood and body fluids. However, many people are receiving a late diagnosis of HIV and are starting treatment after the point of maximum benefit. 28 This latest data suggests that 13% of HIV diagnoses occur at a later stage of the disease and the prognosis is likely to be poorer. 28
Evidence to support sexual health promotion interventions
Current evidence around improving sexual health for people with SMI has been conducted in the USA and Brazil, where a different set of cultural, organisational and socioeconomic factors exist from those in the UK.
Simply providing information (e.g. leaflets) alone is insufficient to bring about health behavioural change, and behavioural interventions that address knowledge, confidence, attitudes/motivations and behavioural skills are recommended. 19 There have been two recent literature reviews that have examined the evidence for sexual health promotion interventions and found that the evidence is currently equivocal. 32,33 All studies were conducted in the USA and most were group-based interventions. Some studies34,35 demonstrated a significant reduction in condomless sex, when compared with a control group, and some studies showed no overall effect. 36,37 Both reviews32,33 recommended that further research should be undertaken in the UK to develop and evaluate an intervention, as well as the feasibility of undertaking a study that addresses sexual health needs in SMI.
Rationale
The overall aim of the project was to design a sexual health intervention for people with SMI and to establish the feasibility and acceptability of undertaking a RCT in order to establish key parameters to inform a future trial of effectiveness.
Research objectives
The main objectives of the Randomised Evaluation of Sexual health Promotion Effectiveness informing Care and Treatment (RESPECT) study were as follows.
Stage 1: intervention development
-
To undertake a stakeholder consultation to inform the development of an intervention.
-
To use intervention mapping to develop an evidence-informed and co-produced manualised sexual health promotion intervention.
Stage 2: feasibility randomised controlled trial
Objective 1 was to assess the feasibility and acceptability of undertaking a trial by:
-
quantitatively assessing the numbers screened, numbers eligible and those agreeing to participate
-
qualitatively assessing the feasibility and acceptability of the randomisation process, as well as the intervention
-
quantitatively evaluating the acceptability of the intervention by assessing retention in treatment (number of sessions attended)
-
quantitatively evaluating the acceptability of the proposed method of data collection and data collection tools by assessing overall questionnaire response rates and for each data collection tool.
Objective 2 was to identify the key parameters to inform the sample size calculation for the main trial: the standard deviation (SD) of the primary outcome measure, quantify the average caseload per therapist and tentatively explore clustering within therapist using intracluster correlation coefficients (ICCs).
In addition, a number of secondary aims were anticipated to be met by this study, especially as this was the first UK study to our knowledge to collect data specifically about sexual health and service use of people with SMI:
-
to develop an understanding of the sexual health needs of people with SMI who use NHS mental health services
-
to establish the use and uptake of sexual health services by people with SMI
-
to establish the barriers to accessing information and service provision
-
to establish workforce capacity to undertake such an intervention in mental health services
-
to explore cost-effectiveness in preparation for a future large trial
-
to develop recommendations for care pathways between mental health and sexual health service.
Chapter 2 Stage 1: intervention development
The overall aim of the RESPECT study was to undertake a feasibility study of an intervention designed to promote sexual health for people who have SMI. This chapter will outline the theoretical underpinning and the process that was adopted to develop the manualised behavioural intervention for the RESPECT study.
Background and theoretical basis
It was important that all stakeholders (including service users and clinicians) were involved in the development of an intervention in order to ensure that it addressed specific challenges faced by this population and that it was feasible and acceptable to participants. 38,39
The Medical Research Council (MRC) framework for the development of complex interventions38 is an iterative process to guide the development of interventions in the real-world health-care setting (Figure 1).
FIGURE 1.
Key elements of the development and evaluation process. 38 Reproduced from Developing and evaluating complex interventions: the new Medical Research Council guidance, Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, Medical Research Council Guidance, 337, a1655, 2008, with permission from BMJ Publishing Group Ltd.
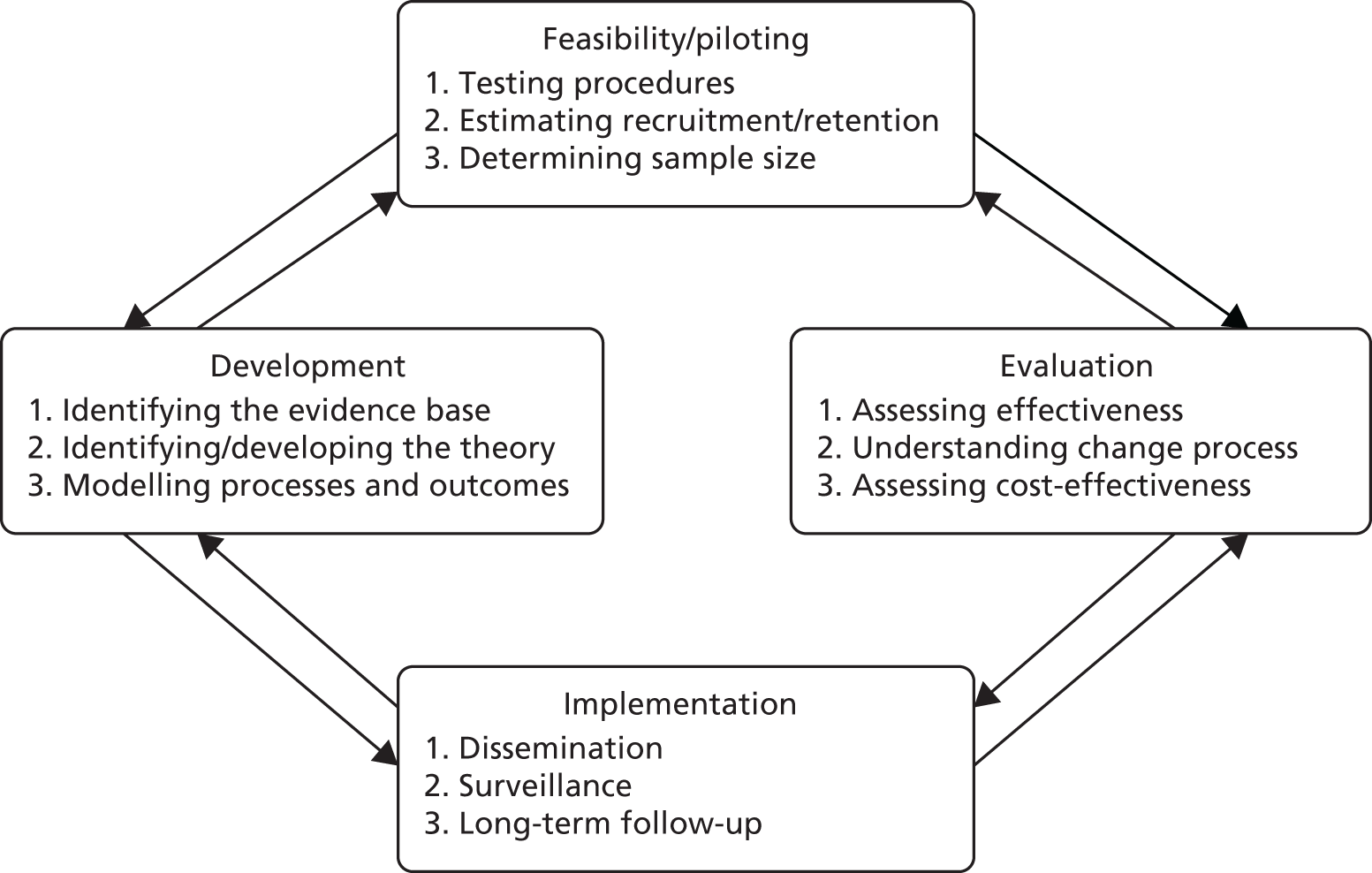
Interventions should be developed based on the best available evidence (previous studies, stakeholder consultation and expert opinion) and based on an appropriate theory. The rationale for the intervention (i.e. what changes are expected and how that change will be achieved) should be based on that theory but may be refined by development work such as stakeholder consultations. Once an intervention is developed, feasibility work should be undertaken to obtain vital information about recruitment and retention, barriers and practical challenges, as well as further feedback on the intervention. Once feasibility is established, a full-scale definitive trial (or other appropriate design) can be undertaken. It is always important to keep in mind the implementation of the intervention at every stage of the process and consider how it could be integrated into wider health care should it prove effective. This is why it is crucial to involve key stakeholders in the development and evaluation process.
For the purposes of the RESPECT study, the MRC Framework38 stages that will be focused on are the development and feasibility/piloting stages. This chapter will describe the stage 1 development process including the theory underpinning the intervention and the process undertaken to combine evidence from previous studies as well as stakeholder consultations to finalise the content and mode of delivery (techniques). The feasibility study itself will address the second stage of the MRC framework,38 that is, assessing recruitment, retention and the acceptability of the study in order to prepare for a fully powered trial, which would assess clinical effectiveness and cost-effectiveness (stage 3).
To develop an intervention, it is important to consider what end point (behaviour change) it hopes to have an impact on and how intervention components can be designed with that end point in mind. It is useful at this point to reflect on what is meant by sexual health and how people assess if their sexual health is good or if they have any unmet needs in this area. The promotion of sexual health is by definition a complex intervention as it covers a range of activities and topics as well as requiring a range of intended outcomes.
Sexual health is defined by the WHO5 as not just simply the absence of disease (e.g. HIV or STIs); it is more broadly about the right to freely express one’s own sexuality and to have safe, satisfying sexual relationships, free from exploitation or discrimination:
. . . a state of physical, emotional, mental and social well-being in relation to sexuality; it is not merely the absence of disease, dysfunction or infirmity. Sexual health requires a positive and respectful approach to sexuality and sexual relationships, as well as the possibility of having pleasurable and safe sexual experiences, free of coercion, discrimination and violence. For sexual health to be attained and maintained, the sexual rights of all persons must be respected, protected and fulfilled.
WHO, 2006. 5
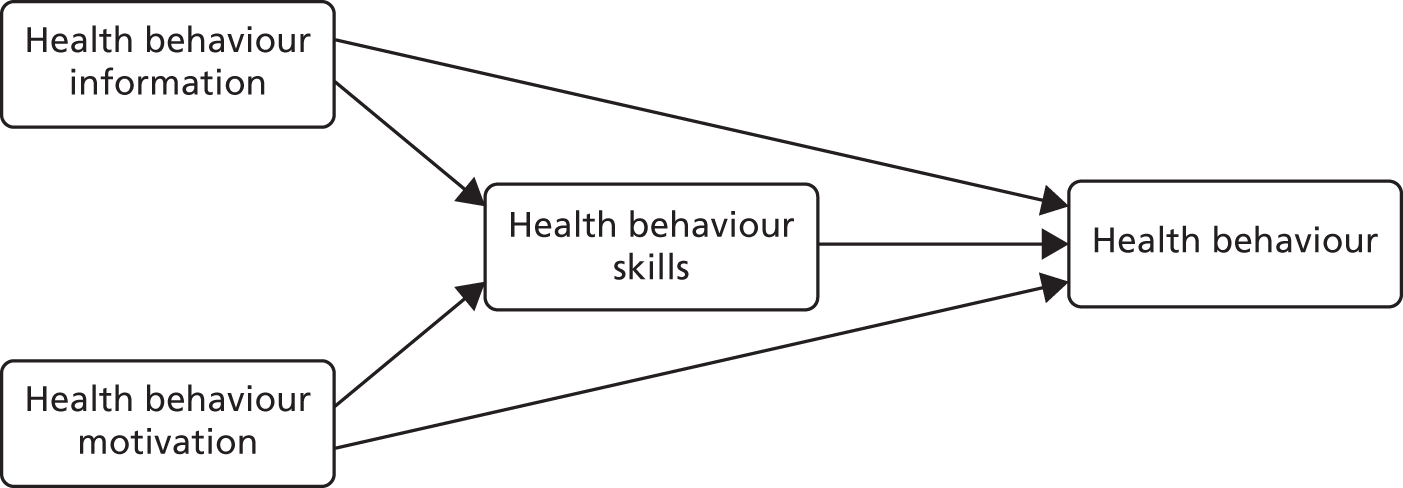
Theoretical model: the information–motivation–behavioural skills model of health behaviour change
The theoretical model adopted was the information–motivation–behavioural skills (IMB) model. 39 This model has been used to inform the development of HIV prevention (and other sexual health interventions) and has demonstrated good predictive qualities with a wide range of participants including young people, MSM and people with SMI. 35
The IMB model is a social cognitive model of health behaviour change. It proposes that although information (about the impact of a particular behaviour) is important, it is not sufficient on its own to influence behaviour change. In addition, it is important that individuals can personalise the information about health risk to their own situation and adjust their cognitions to be concerned about the consequences of their behaviour for their own well-being and the well-being of others (perceptions of risk). However, there is a third and crucial step in the process of behaviour change: putting the intention into action. This requires significant behaviour and cognitive skills such as self-efficacy, communication skills (e.g. asserting oneself in negotiating for safer sex with sexual partners), belief in success and practical skills (e.g. in the case of sexual health, being able to use a condom correctly). A theoretical model should guide the developer in considering the components of an intervention and the choice of outcome measures that relate to those components.
In this model, three constructs (i.e. information, motivation and behavioural skills) are needed to engage in a specific health behaviour (Figure 2). Motivation is comprised of personal motivation (which includes beliefs about the outcome and attitudes) as well as social motivation (which is the perceived social support for engaging in a particular behaviour). According to the IMB model, behaviour change is facilitated by an increase in self-efficacy (confidence in one’s own ability to do something) through an enhancement of practical skills.
Process of intervention development
To develop the intervention, a process called intervention mapping was used. 40 This methodology identifies barriers to, facilitators of and motivators for adoption of a specific intervention derived from an analysis of the literature, as well as input from key stakeholders drawn from the given ‘at risk’ group and service providers. It provides a framework on which to base development that includes theory and stakeholder input in decision-making.
Intervention mapping consists of six stages:
-
needs assessment
-
mapping
-
selecting techniques
-
selecting intervention components
-
planning for intervention adoption
-
evaluation.
Note that as the RESPECT study is a feasibility study stages 5 and 6 will not be covered, as it was not the intention to be able to undertake widespread adoption or evaluation of effectiveness in this particular study.
Step 1: needs assessment
Chapter 1 presented the background literature related to sexual health issues for people with SMI. A needs assessment sets out the context and the target population and defines their specific needs in order to design a targeted and relevant intervention.
Context
Setting: community mental health services.
Population: people who experience SMI such as psychosis, bipolar affective disorder, schizophrenia or severe depressive disorders.
Needs: people with SMI have needs in relation to the following –
-
information about sexual health, STIs and how to prevent transmission
-
information about where to access help and advice in relation to sexual health
-
perceptions of self as at risk for STIs, HIV, unplanned pregnancy, etc.
-
behavioural skills to be able to implement intentions to engage in safer sex.
Step 2: mapping
The first step involved mapping the key elements from manualised interventions that were identified in the literature review33 in terms of behaviour change objectives and underpinning mechanisms of change and corresponding change techniques and implementation strategies. Walsh et al. 33 undertook a systematic review of interventions to promote sexual health for people with SMI. Eleven studies were identified and synthesised. All were group interventions with people who were defined as living with SMI and all took place in mental health provider settings.
-
State the expected outcomes for behaviour and environment:
-
increased use of sexual health services
-
reduced unprotected sex (increased use of contraception including condoms).
-
-
Specify performance objectives for behavioural and environmental outcomes:
-
planning skills
-
problem-solving
-
assertive communication in negotiating safer sex.
-
-
Select determinants for behavioural and environmental outcomes:
-
increased knowledge of sexual health issues
-
increased motivation to adopt safer sexual practices
-
more positive attitudes to using condoms.
-
Steps 3 and 4: selecting techniques and intervention components
Manuals from previous trials were obtained (note that an adapted version of the Susser et al. Sex, Games and Video-tapes34 manual was also used in the Berkman et al. 36 and Berkman et al. 41 RCTs) and the content was coded according to the IMB model in terms of anticipated impact on information, motivation and behavioural skills (Table 2).
| Components of the intervention | Information | Motivation | Behavioural skills | Susser et al.34 (1998) | Carey et al.35 (2004) | Rosenberg et al.42 (2010) | Wainberg et al.17 (2016) |
|---|---|---|---|---|---|---|---|
| Warm-up/introduction | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Ground rules | ✗ | ✗ | ✗ | ||||
| Knowledge of HIV | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Dispelling myths about HIV | ✗ | ✗ | ✗ | ||||
| Knowledge of STIs | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Knowledge of risk behaviours | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Rationale for condom use to keep safe | ✗ | ✗ | ✗ | ✗ | |||
| Pros and cons of using condoms | ✗ | ✗ | |||||
| Pros and cons of risk taking | ✗ | ||||||
| Pros and cons of HIV testing | ✗ | ✗ | |||||
| Behavioural skill: male condom | ✗ | ✗ | ✗ | ✗ | |||
| Behavioural skill: female condom | ✗ | ✗ | |||||
| Negotiating skills: use of condom | ✗ | ✗ | ✗ | ✗ | |||
| Assertive communication | ✗ | ✗ | ✗ | ||||
| How to eroticise using condoms | ✗ | ✗ | |||||
| Risk perception exercise (high, low, no risk) | ✗ | ✗ | ✗ | ✗ | |||
| Problem-solving skills | ✗ | ✗ | ✗ | ||||
| Avoiding/dealing with high-risk situations | ✗ | ✗ | |||||
| Goal setting and planning skills | ✗ | ✗ | ✗ | ✗ |
Selecting intervention components
The components of the intervention were chosen based on components from previous manuals. This involved a small group of the RESPECT study researchers and the people with lived experience (PWLE) representatives. Pragmatic decisions were made to choose the ones that were directly related to the change objectives and were then included in a prototype manual.
After identifying the core common components of the previous interventions, a prototype outline of the suggested intervention was presented to stakeholders to aid in the discussion of what the RESPECT intervention should contain as well as what methods should be used in the intervention.
Stakeholder consultation
Several consultation meetings were held during the stage 1 phase of the RESPECT study. The aim of these events was to obtain views from a range of stakeholders in order to refine the content and mode of delivery to meet the needs of people with SMI (Table 3). In these meetings, the prototype of the intervention was provided and the discussion was then focused on how best to adapt these components to suit both the service users’ needs as well as the service setting, feasibility and the likely uptake of intervention components. Modifications based on these discussions were then made. The range of participants included:
-
people with lived experience of SMI, people who cared for people with SMI, mental health nurses, sexual health workers, drug and alcohol workers, and support workers.
| Themes | Feedback | How feedback influenced manual development |
|---|---|---|
| Who should be included? | Discussion about who the manual would be suitable for. Consensus that all people interested in taking part will be included regardless of current relationship status | Eligibility criteria were wide to include as many people as possible |
| Number and length of sessions | Original plan suggested was 6 × 30-minute sessions. Some variation in numbers suggested, and suggestion to reduce number of sessions but increase the time of them | The manual was designed to be delivered in 3 × 1-hour sessions. However, it was divided into components within the session so that there could be flexibility in delivery |
| Frequency | Frequency should be weekly to space out the intervention enough for people to absorb information and to not be too intense | The intervention was recommended to be delivered weekly but also allowed some flexibility (holidays, work commitments, etc.). We also allowed interventionists to offer less sessions but more content per session to cover the material |
| Mode of delivery |
We asked whether or not it should be over telephone or face to face: consensus was face to face Be flexible in the delivery so that people can opt-out of some sessions if felt irrelevant or if they just did not want to do them |
We changed the plan to only being delivered face to face |
| Recording intervention | Be able to record important or interesting observations from the sessions in a more systematic way so that participants’ progress can be tracked. For example, might it be useful to rate how well people do in the condom demonstration? This could be in a column next to each section in the session guide, in addition to the notes section at the end | We decided not to assess people’s skill as this was felt too threatening and stressful |
| Location of delivery |
Location? Appropriate to deliver in people’s homes? Need for privacy Factors in who can be present (e.g. family or a partner) |
We offered the choice of location dependent on participant preference. We advised the interventionists to check out the privacy or otherwise of the home visit (being mindful of other people being present, or children, etc. Advised to use a clinic room if that was an issue) |
| Modifications for the SMI population | Provide intervention sequentially and offer a recap at the start of the next session and at the end; promote cohesiveness between the sessions to help learning and retention | Incorporated introduction and recap into each session |
| Training for interventionists | Sexual health services involved to deliver awareness training | We included a sexual health practitioner in the development of the manual and also attendance at the initial intervention training event |
| Qualities of interventionists | It was deemed important that the person delivering the intervention should be aware of all types of sexual behaviour and sexual relationship and to be non-judgemental, be able to build rapport at the start of the intervention and avoid jargon | We incorporated this into the interventionist training |
| Safety and confidentiality | There was a need for the boundaries of confidentiality in the intervention to be clarified and also for the interventionists to understand what to do regarding disclosures that related to safeguarding | This was attended to in the training and the interventionists were advised that in the first instance as employees of the NHS service that they follow NHS trust processes first (especially if the issue was urgent) and then inform their local research and development department or the RESPECT study researcher |
| Information on STIs and HIV | There would need to be some content related to increasing the awareness of and knowledge about various STIs and HIV | The decision was made to include quizzes on the transmission of both STIs and HIV |
| Contraception |
Make sure that there is discussion of other forms of contraception The nurses delivering the intervention should be aware of how certain forms of contraception can affect those with diabetes and are contraindicated for people on some antipsychotic medication because of the associated risk It was felt that use of female condoms should be demonstrated Need for demonstration of female condoms, preferably with model vagina |
A section on the whole range of contraceptives was included: ‘what’s in the bag’ We did not include any input on sexual dysfunction as the HTA commissioning brief was specifically to exclude sexual dysfunction We did not include a specific component on female condom use (because they are not commonly used and they are expensive). However, we did advise people to seek advice regarding female condom use from local family planning services |
| Condoms |
There was a consensus that the person demonstrating how to put on a condom should not hold the condom demonstrator between their legs There should be more emphasis on the importance of getting the condom on the right way round. Discuss more explicitly about what it should look like. If it’s on right it looks like a Mexican hat or bobble hat. Discuss what should be done if you put it on the wrong way round (i.e. throw it away). If you notice it is difficult to roll down it is probably on the wrong way Using other forms of contraception other than condoms. Without acknowledging this the intervention could be taken as suggesting that you will have to use condoms for the rest of your life. This is both demoralising and could stigmatise mental health service users by denying them the usual progression of a relationship that others experience Gel charging should be included in the condom use section. This is when a pea-sized amount of lubricant is placed in the tip of the condom before putting it on. Not too much though as this will make it slip off. This is helpful for men who say they do not like the feel of condoms Warnings about fingernails and rings ripping the condoms should be included in the intervention |
Condom demonstrator not to be held between legs Specific advice regarding getting the condom the right way round was included in a script and the interventionists all practised putting a condom on and off as part of the training We incorporated gel charging and also gave out some condoms and lubricant gel sachets to participants in the intervention We covered all forms of contraception |
| Assertiveness skills | Generally in this assertiveness section there needs to be some flexibility over the thing being negotiated depending on the individual participant’s circumstances. Suggest role play within sessions as a good way of building confidence | We used discussion exercises and a role play if the person was comfortable doing so |
| Relationships |
There was a discussion over the use of the word ‘friend’ in the relationship game and it was suggested to change it to ‘friend or partner’ There should be a section dedicated to how relationships progress |
We used ‘friend’ at the request of our patient and public involvement group We did not include a section on ‘how relationships progress’ as it was felt that this could be drifting away from a sexual health focus |
| Range of sexual practices | There should be more information about the alternatives to penetrative sex. This is helpful for the times when a condom is not available and also for building confidence to explore sexuality | There is an exercise (traffic lights) in which alternatives to penetrative sex are discussed |
| Local information and signposting | Information be supplied regarding sexual health clinics as an external resource | All participants received a localised advice sheet regarding services for sexual health, intimate partner violence and family planning |
At each event, notes were taken of the discussion and passed to the chief investigator. A simple thematic framework was developed based on the content of the discussions.
Table 3 summarises the range of issues discussed in the stakeholder events and the response to these issues in the intervention development. The overall responses were positive and feedback from each discussion was used to modify the intervention procedures and information packs.
In summary, there was significant overlap in the content identified from a thematic analysis of the previous study intervention manuals (see Table 2) and the content suggested in the stakeholder and PWLE discussions. The addition was the suggestion of the inclusion of contraception more broadly than just a focus on condoms. In addition, people generally felt that the tone of the intervention should be positive and focusing on ‘health’, ‘being safer’ and having ‘positive intimate relationships’.
Two main behavioural changes were anticipated for the intervention: a reduction in condomless sex and an increase in access to sexual health and family planning services. Stakeholder feedback had been clear that they wanted a broader view of sexual health that included contraception within a monogamous relationship and that they wanted the content to reflect the positive aspects of intimate relationships.
The intervention content has been aligned with the IMB model (Figure 3). To achieve those behavioural outcomes, it is postulated that the person requires knowledge of HIV and STIs, as well as the range of contraception available (and what services are available locally). As well as having that information, increasing perceptions of self as potentially at risk is important. In the IMB model, information and motivation to adopt safer sexual practices is mediated by behavioural skills. In the intervention, key skills were included and were demonstrated by the interventionist and then the person was invited to try it out for themselves. There were also exercises in developing problem-solving skills and planning skills, which are important for supporting self-efficacy for new behavioural strategies.
FIGURE 3.
Content of the intervention mapped on to the IMB model.
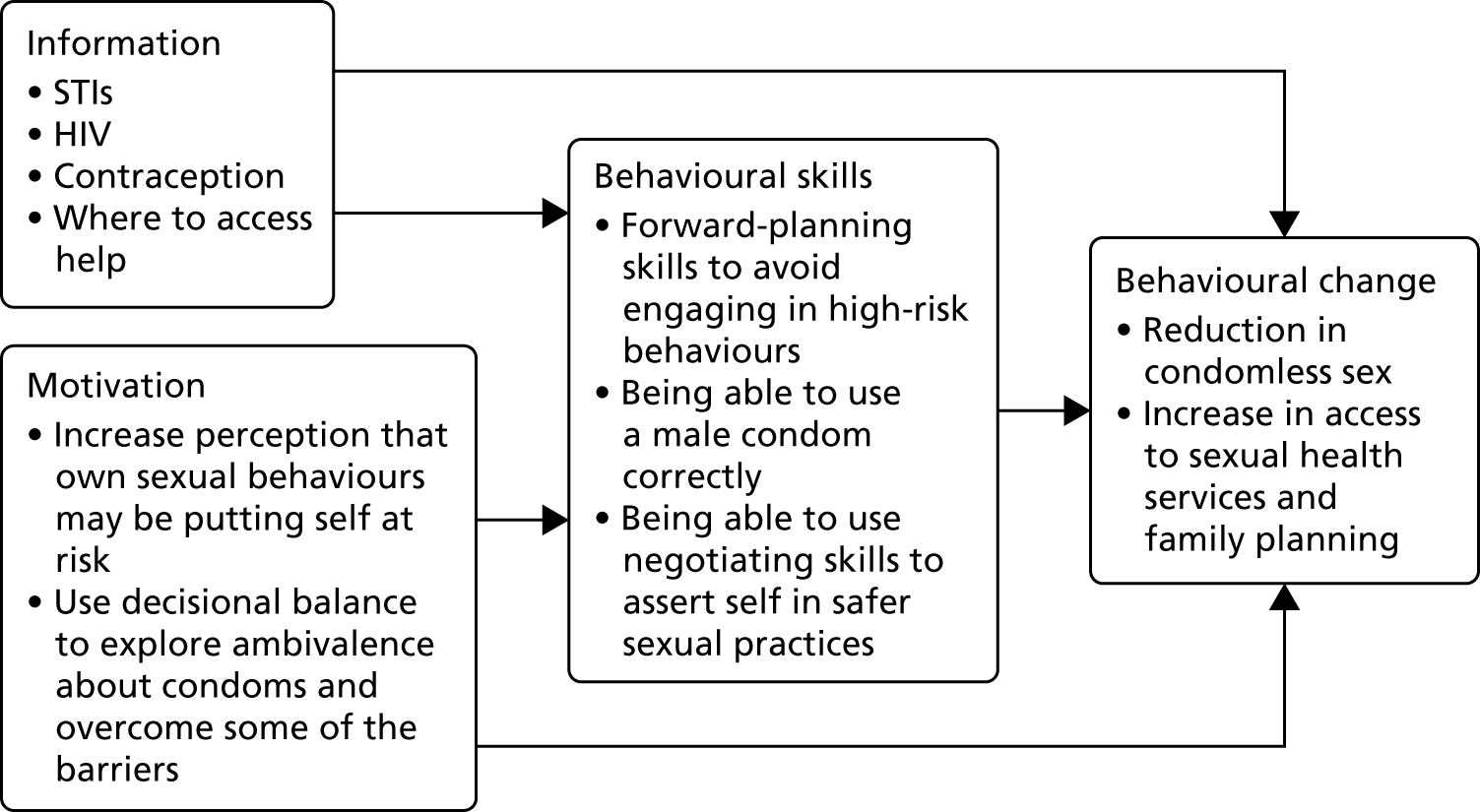
The outcome measures chosen for the study related to the components of the model:
-
information – measured with the knowledge about HIV questionnaire (HIV-KQ)
-
motivation – measured with the ‘motivations to engage in safer sex’ scale
-
behaviour – measured with the Sexual Risk Behaviour Assessment Schedule (SERBAS), self-reported condomless sex acts, the Behavioural Intentions for Safer Sex measure and the Condom Use Self-Efficacy Scale (see Chapter 3 for more information about the measures).
Training for intervention delivery
At each site, mental health staff were identified to deliver the intervention and were invited to attend a 2-day training event. The criteria for inclusion in the training were:
-
experience of delivering clinical care to people with SMI
-
availability to be able to deliver the intervention during the specified study period.
The training took place over 2 consecutive working days. It covered the rationale, aims and design of the study eligibility and data collection, the background literature regarding sexual health in mental health and the evidence from previous studies. The rest of the training was focused on familiarising the staff with the content of the intervention and providing an opportunity to practise the delivery. Each component was explained in terms of why it was there and what purpose it served, and there was an accompanying script within the manual to promote consistency of delivery. In the training the interventionists were able to complete each exercise as a participant would and then discuss the outcomes. The trainees were also informed of the intervention monitoring paperwork that would be required to be completed by them.
Intervention: frequency and length of session
Timing: there were three intervention sessions each lasting 60 minutes or these could be delivered in two (longer) sessions.
Location: the intervention was delivered at the local clinical service where the person usually attended or at home.
Interventionist: the intervention was delivered by a mental health worker from the same service (as the service user) who had received the bespoke RESPECT intervention training.
Monitoring: a checklist was completed at every session by the interventionist for each component of that session. All interventionists were able to access advice at any time from the RESPECT intervention team during the delivery phase by telephone or e-mail.
There are three main areas to focus on in the intervention based on the needs assessment: (1) knowledge and perception of risk (session 1); (2) ways to keep safe, focusing on safe condom use as well as a whole range of contraceptive choices and where to seek advice and help (session 2); and (3) negotiating safer sex in relationships including communication skills and focus on ‘mutual respect’ (session 3). The person will be encouraged to develop their own ‘action plan’ based on the needs and goals identified, which (with permission) will be shared with the care co-ordinator to incorporate into their overall care plan.
Outline of intervention
The intervention manual can be seen on the National Institute for Health Research journals library website.
Session 1: knowledge of human immunodeficiency virus, sexually transmitted infections and safer sex
The first part of session 1 involved introducing the intervention and building a rapport. This is always a requirement for any new intervention, but especially so when discussing a potentially sensitive topic. Interventionists were advised to spend a bit of time on this, getting to know the person first; a suggested ice-breaker was to ask why the person had been interested in taking part in the study. In addition to this, some ground rules were discussed including:
The interventionist promises that –
-
I will be non-judgemental
-
I will listen
-
if I do not know something, I will find out and let you know
and
-
there is no such thing as a ‘stupid question’
-
if anything at all concerns you, flag it up and we can stop and discuss
-
none of the activities are compulsory
-
talk about the boundaries of confidentiality – everything discussed during the intervention is completely confidential (except the disclosure of risk to self/others).
Information giving about sexual health
In some of the previous group interventions,34,35 the information had been imparted using didactic methods as well as interactive exercises. It was felt by PWLE that a didactic approach would be too intensive for an individually delivered intervention, so a set of quizzes was developed to be used to help establish what is already known and where any knowledge gaps exist. The interventionist allowed time to complete the quizzes and then went through the responses, and addressed the items that were incorrect. In terms of introducing the quiz it was really important to make it clear that most people in the general population have things to learn about sexual health and that it is OK not to get it all correct.
Correct use of the male condom
After the knowledge quizzes, the interventionist picked up on a specific item from one of the quizzes (‘using condoms always prevents pregnancy’) and linked this to the fact that condoms can fail if used incorrectly. The next section focused on the correct use of condoms and (if the person agrees) the interventionist runs through a (scripted) description and physical demonstration of how to correctly put on and off a condom using a plastic condom demonstrator. Session 2 went into condom use in more detail, but it was felt that if there was poor attendance at the subsequent sessions then having the condom demonstration in session 1 would ensure that all participants received this.
Types of contraception
The final part of session 1 was a general discussion of contraception using ‘what’s in the bag’, which was a bag containing information and photos of all the variants of contraception available. They were grouped as barrier, non-hormonal and hormonal. At the end of the session the next appointment was arranged.
Session 2: keeping safe – focus on the role of condoms and contraception choices
Session 2 started with a recap of session 1, and the participants were asked to recall anything they had remembered or had been thought-provoking from session 1. After that was discussed, the session 2 content was introduced. The aim of session 2 was to increase the motivation to adopt safer sexual practices by becoming aware of different levels of risk posed by a range of sexual practices. This was delivered by an exercise that involved three coloured circles: red was high risk, yellow was some (low) risk and green was no risk. The participant was given a set of sexual acts and they had to sort them by placing them on the correct circle. Any errors were then discussed. This exercise was designed to reinforce understanding of the behaviours that are high risk and that the person should consider using protection (e.g. a condom) if their or their partner’s sexual health status is unknown. In addition, the lists of behaviours that carry no risk were then reinforced as options for sexual expression if there are no condoms available. The second aim of session 2 was to explore motivation to use condoms by addressing some of the perceived barriers to use of and some of the myths about condoms in the hope that the participant would become more motivated to use them. This was undertaken by a discussion of the less good things about condoms followed by the good things about using condoms. Following this, there was an exercise on identifying ‘triggers for unsafe sex’ using two scenarios. The participant then brainstormed possible options and solutions for each scenario about how to adopt safer sex in the future. This was followed by a practical exercise in which the person was offered the chance to demonstrate the correct use of a condom using a plastic demonstrator.
Session 3: the RESPECT intervention – relationships and communication
The final session focused on relationships: feeling safe in those relationships and having control and choice in those relationships. The first exercise was for participants to sort a set of cards that had statements on them about feelings, behaviours and experiences in relationships and whether they were compatible with a ‘good relationship’ or a ‘bad relationship’. This aimed to increase the awareness of power dynamics in relationships and link it to autonomy and choice about sex in that relationship. This exercise then links to sexual behaviour and being able to be assertive in a relationship. Assertive communication was discussed and then practised with the interventionist. There was an exercise using scenarios and the participant was asked to think about assertive responses to the situation when a partner does not want to use a condom. Finally, there was an exercise that aims to bring the strands of the intervention together in a planning and goal-setting exercise. At the end of the session the participant and the interventionist recap on the three sessions and, if the person is in agreement, design an action plan for any outstanding needs that they would like to address (such as access to contraception). This plan was shared with their care co-ordinator but only with signed consent.
Chapter 3 Feasibility trial
Trial design
We conducted a pragmatic, multicentred, open feasibility RCT. Participants meeting the eligibility criteria were individually randomised (1 : 1) to receive one of the following:
-
The control arm – treatment as usual (TAU), which consisted of usual mental health care. All participants were free to pursue reproductive health and sexual health services via general services in their local area.
-
The intervention arm – in addition to TAU, participants were invited to participate in a manualised intervention designed to promote sexual health, which was delivered in the NHS service where the person usually attended (or at their home) in three sessions of 1 hour, on a one-to-one basis, by a suitably experienced mental health worker employed by the NHS trust and who had received the specific training.
All participants received written information on local sexual health services, contraceptive services and national helplines at the baseline appointment irrespective of the arm to which they were allocated.
The study summary can be seen in Figure 10 (see Appendix 1).
Sample size
The sample size calculations were based on estimating the attrition rates and SD of the primary outcome. Assuming that 30% of participants were lost to follow-up (as in the SCIMITAR trial43) with a sample size of 100, then the 95% confidence interval for this level of attrition would be the observed difference ± 9 percentage points (i.e. between 21% and 39%44). Hence, an external pilot trial of 100 participants would ensure robust estimates of follow-up in this population. Furthermore, an external feasibility study of at least 70 measured subjects provides robust estimates of the SD of the outcome measure to inform the sample size calculation for the subsequent larger definitive fully powered trial. 45
Approvals obtained
This study was approved by the NHS East Midlands – Derby Research Ethics Committee (REC) on 30 September 2016 (reference number 16/EM/0334) and Health Research Authority (HRA) approval was obtained on 10 November 2016. Confirmation of capacity and capability was sought for each trial centre thereafter. This trial was assigned the number ISRCTN15747739.
Trial centres
Initially four (later five) NHS trusts were selected as trial sites in four UK cities across England: Leeds and York Partnership NHS Foundation Trust (LYPFT); Community Links – aspire service for Early Intervention Psychosis in Leeds [a third-sector provider of the NHS contract and part of LYPFT research and development (R&D) provision]; South West Yorkshire Partnership NHS Foundation Trust (SWYFT) (Barnsley only); Sussex Partnership NHS Foundation Trust (SPFT) (Brighton only); Camden and Islington NHS Foundation Trust (C&IFT); and North East London NHS Foundation Trust (NELFT).
Patient and public involvement in the feasibility trial
People with lived experience were integral to all stages of this study (design, development and training for the intervention, decisions about outcome measures, recruitment, analysis and dissemination). There was PWLE representation in the Trial Management Group. Four experienced researchers and trainers with ‘lived experience’ of mental health service use acted as the patient and public involvement advisory group. Specific meetings were convened to review the battery of outcome measures and how to collect the data with participant comfort in mind. The PWLE and researchers have delivered presentations on the study at the Leeds R&D conference. There were also meetings to discuss the methods of engaging potential participants in the study. The information sheet was written by one of the PWLE. The PWLE were also involved in undertaking the qualitative interviews for participants to discuss the experience of being in the study, focusing on acceptability and feasibility aspects. They have been involved in the analysis as well as the report.
Additionally, the research team engaged the NHS service users from each site in focus groups alongside other stakeholders, such as clinicians for the development of the intervention.
Recruitment
Participant eligibility
Inclusion criteria
The following people were eligible for inclusion in the trial:
-
those on the caseload of selected community mental health services in each NHS site
-
those diagnosed with a ‘severe mental illness’ (defined as schizophrenia, other psychosis, bipolar affective disorder, schizoaffective disorder or major depressive disorder)
-
those aged ≥ 18 years
-
those willing to provide written informed consent
-
those able to provide written informed consent.
Exclusion criteria
Potential participants were excluded if they were identified as:
-
having an acute exacerbation of their mental illness that precludes them from active participation (as indicated by hospitalisation and/or being under the crisis/home treatment team at the time of consenting)
-
having a case note diagnosis that did not meet the criteria of SMI (see Inclusion criteria)
-
having a severe physical illness that precludes them from active participation
-
having a significant cognitive impairment (e.g. an organic brain disorder) as determined by case notes
-
being a non-English speaker (adapting the intervention is currently beyond the scope of this study)
-
lacking capacity to consent (as guided by the Mental Capacity Act 200546)
-
being unable or unwilling to give written informed consent
-
being on the Sex Offenders Register, or having a history of inappropriate sexual behaviour.
All case managers in the selected community mental health teams (CMHTs) were informed of the inclusion and exclusion criteria, and were contacted regarding potential participants to check that there were no areas for concern regarding researcher safety (e.g. on home visits) prior to entry into the study.
Recruitment into the trial
Potentially eligible participants were identified using three main methods: screening of caseloads for potentially eligible people, direct approach by research staff in clinic waiting rooms and self-referral (via study e-mail, telephone or via an online form on the study website). The details for self-referral were provided on all participant-facing materials such as the posters and leaflets.
Caseload screening and eligibility assessment process
Local researchers [known as Clinical Studies Officers (CSOs)] employed by the CRN based in each site’s R&D office worked with the trust’s clinical staff to promote the study and undertake caseload screening for potentially eligible participants using the eligibility criteria. Screening was undertaken in two main ways. The initial approach was to visit CMHTs and talk through each individual case manager’s caseload with them, creating a list of people for the case manager to informally approach. They were given one study information pack for every person identified. The second method was the CSO staff screening caseloads via the electronic records system and then verifying the list with case managers. Once a member of the team had verified that it was appropriate to make an approach, the CSO sent out a study information pack with a letter signed from their case manager or the team manager.
Participant-facing information and study information pack
A simple leaflet and poster introducing the study were developed and distributed widely in each study site. In addition, potentially eligible people received a ‘pack’ (either face to face or by post). The information pack included an invitation letter, a participant information sheet (PIS), a consent to contact (CtoC) form and a baseline feedback questionnaire.
Completed CtoC forms were returned (by fax scanned, by hard copy or verbally) to the CSO research team at each NHS site. Once a CtoC form was received by post at the York Trials Unit, the details were passed to the relevant RESPECT study researcher: either one in the north of England (based at the University of Huddersfield) or one in the south of England (based at University College London).
Self-referral
The participant-facing materials were all designed to offer an option of self-referral to the study. The study posters and leaflets about the study contained a Quick Response code (QR code) and website address to the project website. This website was designed to provide information about the study for staff and potential participants, as well as other interested parties. The posters and leaflets provided a brief description of the study and methods for contacting the RESPECT study research team directly. Local CRN staff and RESPECT study researchers attended various service user groups/events (e.g. recovery colleges, creative groups and clinics) to give out leaflets. At those events, CtoC (by a researcher) was obtained verbally or with the CtoC form. This method of recruitment was utilised to ensure that all potentially eligible participants had the opportunity to take part. The method of recruitment was recorded to inform the most effective recruitment strategies for the main trial. A study e-mail address was set up specifically to manage enquiries regarding the study that was managed by the RESPECT study research team. By contacting the RESPECT study research team directly, it was assumed that the potential participant had implicitly agreed to contact by the research team and, therefore, a CtoC form was not required. However, a record of the contact and any contact information was recorded by the research team.
Once a self-referral had been received, one of the research team made contact by e-mail or telephone to determine interest and eligibility, and to answer any initial queries and concerns that the potential participant had about participating. If they were interested in pursuing involvement, they were sent a study information pack (information sheet and consent form). They were also informed verbally that the researcher would be in touch with their local NHS service R&D team to inform them of the person’s interest, and that their case manager would also be contacted by the CSO in the R&D team to check the person’s eligibility for the study. The RESPECT researchers confirmed that no information would be shared other than their expression of interest in taking part, and the fact that they had self-referred to the research team. In addition, no clinical information would be shared from the NHS back to the RESPECT study team other than whether or not the potential participant met the eligibility criteria.
Flow of participants from identification to entry into study
The numbers of people who were screened, eligible and had consented to participate were recorded when possible. Eligible patients who did not wish to take part (i.e. were unwilling to give consent) and those found to be ineligible went on to receive usual care from the service without prejudice.
As the primary objective of this trial was feasibility, reasons for participation and non-participation were collected by various means to inform future studies. Clinicians handed out feedback forms to those with whom they discussed the study. Service users who received a study information pack by post were contacted by CSOs by telephone 2 weeks later to discuss the information and gauge if there was any interest in participating or not. The feedback form was completed over the telephone for both potential participants and for those who declined to pursue this.
Informed consent and baseline assessment
After eligibility was confirmed by the NHS Trust, a RESPECT study researcher arranged a convenient time and venue to meet with the potential participant. At this meeting, the RESPECT study researcher explained the study in full and gave the potential participant an opportunity to ask any questions. Participants were assured of confidentiality regarding the information that they provided as part of the research, advised about the boundaries of confidentiality (i.e. under what circumstances that would have to be breached), told what to expect after the study ceases, and given contact details in the event of a complaint or the need for further information. They were also given a localised information sheet with local sexual health services and contact details. They were informed that participation is not compulsory and that they could withdraw from the intervention and/or data collection at any time without affecting their care. Written informed consent was then obtained, and (if convenient for the participant) baseline data were collected at this appointment (see Report Supplementary Material 1).
When the questionnaires had been completed, the RESPECT study researcher then contacted the independent randomisation service at York Trials Unit by telephone, and the person was randomised independently to either intervention as an adjunct to TAU or TAU. The participant was then informed of their allocation face to face. If it was not possible to access the randomisation line at that time, randomisation was completed later and the participant was then informed by telephone.
Participant follow-up
The original plan was that all participants in the RESPECT study would be followed up with a repeat of the questionnaires/interview at 3 months and 6 months post randomisation (see Report Supplementary Material 2). However, as a result of requiring an extension to the recruitment period, and balancing the need to complete the study within a new time frame, an agreement was reached between the research team and the funder to only collect 3-month data on any participants who entered the trial after October 2017 and for all recruitment to be completed by 31 December 2017. This allowed for all the follow-up data collection to be completed by 31 March 2018.
Outcomes
The main outcome of the RESPECT study was to establish the feasibility and acceptability of an evidence-based intervention to promote sexual health, and to establish key parameters to inform a future main trial. In conjunction with the qualitative study, this was to be established by measuring recruitment rates, retention rates and follow-up completion rates.
Secondary outcome assessment
The following outcome measures were collected at baseline, 3 months and (for some participants) 6 months post randomisation:
-
SERBAS34 – a validated HIV risk behaviour measure that was developed in the USA and has been validated for use with populations who have SMI. It gathers information on sexual activity in the last 3 months and records frequency of high-risk behaviours (for HIV infection), such as intercourse without a condom, sexual activity under the influence of drugs and alcohol, and sex work/sex trading. It takes into account sexuality and gender in the schedule.
-
The National Survey of Sexual Attitudes and Lifestyle (Natsal) – we have included specific items that cover broader aspects of sexual health including contraception use, STI and HIV tests, and knowledge on family planning advice.
-
HIV-KQ35 – a 17-item measure that assesses knowledge about HIV. (This originally comprised 18 items but we removed one question about lamb-skin condoms as this is now outdated.)
-
Motivations to Engage in Safer Sex35 – a four-item scale to assess people’s own perception of their risk of infection with a STI.
-
Condom Use Self-Efficacy Scale35 – an 18-item Likert scale to assess attitudes towards the use of condoms as well as questions on self-efficacy in the use and negotiation of use.
-
Behavioural Intentions for Safer Sex35 – a six-item measure in which patients are presented with a scenario describing a possible sexual encounter and asked to rate how likely it was that they would engage in six risky or protective behaviours (e.g. ‘I will tell the person I don’t want to have sex without a condom’). Patients responded to each behaviour using a six-point scale (ranging from 0 ‘definitely will not do’ to 5 ‘definitely will do’).
-
MISS-Q17 – a 32-item tool that has been developed and validated to measure a person’s perceived stigma as a result of their mental health problem and its impact on perceptions of attractiveness and opportunities for intimate relationships.
-
EQ-5D-5L (EuroQol-5 Dimensions, five-level version) – a standardised instrument for use as a measure of health outcome that is applicable to a wide range of health conditions and treatments (https://euroqol.org; accessed 5 September 2019).
-
The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST)47 – developed for the WHO by an international group of substance abuse researchers to detect and manage substance use and related problems in primary and general medical care settings.
-
Recovering Quality of Life (ReQoL)48 – a new 20-item patient-reported outcome measure that has been developed to assess the quality of life for people with different mental health conditions.
-
Cost assessment – commonly used generic instruments to measure health-related quality of life (e.g. EQ-5D-5L) will be used and assessed for completion rates at various time points and for patterns of missing data. Sensitivity of generic instruments will be evaluated against sexual health-specific clinical outcomes. A bespoke resource use questionnaire has been designed and piloted in the target population and responses will be evaluated to identify the key cost drivers (incorporated into participant-completed questionnaires; see Report Supplementary Material 1).
Randomisation
Randomisation was performed by a secure, remote, telephone randomisation service based at York Trials Unit. An independent statistician at the University of York undertook the generation of the randomisation sequence. Randomisation was on a 1 : 1 basis using stratified block randomisation with stratification by centre and variable block sizes. Periodic checks were made on the computerised randomisation system during the trial following standard operating procedures. Baseline data were collected prior to randomisation; therefore, treatment allocation was concealed at this point. Owing to the nature of the intervention, it was not possible to conceal treatment allocation from the participant or the professional delivering the intervention.
Trial interventions
Participants were randomised to receive either:
-
TAU or
-
the RESPECT intervention, as an adjunct to TAU.
Once randomised, participants were informed of their allocation at the face-to-face baseline interview, or by telephone shortly afterwards (the researchers did not always have access to a private telephone line at the time of data collection).
Intervention arm
In addition to usual care, people who were randomised to receive the intervention were offered three 1-hour sessions of a manualised intervention. This was delivered by a specifically trained mental health worker based in the NHS sites (specific training was provided by the RESPECT study team) and was supported by a specifically devised manual and intervention pack. The sessions were delivered in a private room at the local clinical service or at their home. The intervention was delivered as soon as possible following randomisation and before the 3-month follow-up point. The intervention is described in more detail in Chapter 2.
Control arm
Participants randomised to receive TAU continued to receive their usual care. The TAU for sexual health (including contraception) included their local primary care and/or specialist sexual health services. All participants, irrespective of allocation, received a leaflet listing the local sexual health, family planning and domestic abuse services relevant to their local area, and some condoms at the point of baseline data collection.
The participants’ general practitioners (GPs) were sent a letter informing them that the named person was taking part in the trial and also notifying them of the arm of the trial to which they had been allocated. The RESPECT study information leaflets and sheets contained a link to the study website that also contained links to national helplines and resources related to sexual health and relationships (www.respectstudy.co.uk; accessed 5 September 2019).
Trial completion and exit
Participants were considered to have exited the trial when they:
-
withdrew consent
-
had been withdrawn by an interventionist/researcher for reasons of risk or harm to self and/or others
-
had reached the end of the trial
-
died.
Withdrawals
Withdrawals were possible at any point during the study at the request of the participant. When a participant expressed that he or she wished to withdraw from the study, a researcher would speak to them to clarify their level of withdrawal (i.e. to confirm withdrawal from the intervention only, from follow-up only, or from all aspects of the study). If the participant requested to be withdrawn from the intervention only, follow-up data continued to be collected. All data were retained for all participants until the date of withdrawal unless a participant specifically requested that these be destroyed.
A participant could also be withdrawn without their consent from the intervention and/or the trial for reasons of risk or harm to self and/or others. This was actioned only when there was evidence of serious and significant risk. In these instances, the risk protocol (see Report Supplementary Material 3) guided the interventionist and/or researcher to the appropriate action to be taken in conjunction with the lead research clinician and the duty worker in the organisation.
Adverse events
General clinical decisions remained the responsibility of the participant’s care team, and participation in the study had no bearing on this process. When participants sought an opinion on their care/medication from a member of the RESPECT study team, they were strongly advised to seek advice from a member of their clinical team.
During the study, adverse events (AEs) were monitored and, at each data collection point, the participants were asked if anything significant had happened to them with regards to their mental well-being (such as psychiatric admission or an escalation of the level of care needing input from the crisis team). A study-specific AE protocol described the process by which a potential AE would be notified and assessed before being passed to the Data Monitoring and Ethics Committee (DMEC).
Adverse events were monitored by an independent DMEC and the Trial Steering Committee (TSC). The DMEC/TSC would be immediately notified and asked to review any reported serious adverse events (SAEs) that were deemed to be study and/or intervention related.
Statistical analysis
The flow of participants through the trial is detailed in the CONSORT flow diagram (see Figure 5). The number of people screened, randomly assigned, receiving the intervention, completing the study protocol and providing outcome data are summarised overall and by trial arm. The number of individuals withdrawing from the intervention and/or the trial and any reasons for withdrawal are summarised by trial arm. To quantify the acceptability and feasibility of the intervention the number of sessions attended is also summarised. All data are presented descriptively with no formal statistical analyses undertaken. For each data collection point and outcome measure, the numbers of non-responders were calculated and completion rates were compared. The average caseload per therapist is detailed.
Primary outcomes: feasibility parameters
To establish feasibility, the key parameters were whether or not there were eligible people in the mental health services, and whether or not they could be recruited to the study and give informed consent to participate. Therefore, the recruiting sites were required to complete logs of screening and eligibility numbers, as well as how many CtoC forms (or other forms of CtoC, e.g. telephone calls, verbal requests) were received. This information was to be recorded by each research site on a Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) spreadsheet and reported to the trial team via e-mail on a monthly basis.
In addition to recruitment, retention in the trial at subsequent time points was of interest.
To quantitatively assess feasibility of data collection as well as the completeness of the data within the questionnaires, each outcome measure was reported in terms of completeness as a percentage for the baseline and the 3- and 6-month follow-up time points.
Retention in intervention was quantitatively assessed as a percentage of the content completed and the number of sessions attended. There was no established a priori parameter for what would be an acceptable ‘dose’ of intervention; instead, any attendance would be counted as a favourable outcome given the novelty of the topic, and the challenges faced by people with SMI in engaging with additional interventions in addition to routine care. Observing the pattern of attendance would also inform the design of future intervention studies.
Economic analysis methods
An economic analysis was conducted with the aim of evaluating the feasibility of collecting data on costs and the health-related quality-of-life outcomes to conduct a full within-trial economic evaluation from the UK health services perspective. Data were collected at baseline and at the 3-month and 6-month time points. Resource use data were collected to estimate (1) the cost of delivering the intervention and (2) the individual-level cost of health service resource use by trial participants over the trial follow-up period of 6 months (this particularly focused on services related to sexual health). Resource use was multiplied by unit costs to arrive at the total cost in each trial arm.
The cost of delivering the intervention was based on the time spent by the health professional delivering the intervention. Participants were offered up to three sessions to cover the training material with the health professional and were also allowed the option to cover the material in fewer but longer sessions. Sessions were delivered at individual level and the length of each session was recorded. The time spent by the health professional delivering the session was multiplied by their hourly rate.
Individual-level service use data were based on self-reported use of health care using a bespoke resource utilisation questionnaire. The questionnaire collected data on service utilisation over the previous 3 months for general health and community service use (i.e. appointments with GPs and nurses and pharmacy visits), A&E (accident and emergency) visits, and sexual health-related service use (i.e. consultation at a contraception clinic, sexual health clinic and sexual health assault centre). Unit costs of health service use were obtained from the UK national database Reference Costs 2016–1749 and the Unit Costs of Health and Social Care report50 produced by the Personal and Social Services Resource Unit.
Health-related quality of life was measured using the EQ-5D-5L questionnaire. 51 Individual-level responses on the EQ-5D-5L were used to estimate health-related quality of life based on a UK population valuation set. 52 The EQ-5D-5L, originally developed by the EuroQoL group,53 is a widely used measure of health-related quality of life. Using this measure, respondents are able to classify their own health on a 5-point scale: 1 = no problems, 2 = slight problems, 3 = moderate problems, 4 = severe problems and 5 = extreme problems. Health-related quality of life is measured over five dimensions: mobility, self-care, usual activities, pain and/or discomfort, and anxiety and/or depression. All questions refer to the participant’s health state ‘today’.
Finally, analysis of the cost and health-related quality-of-life data were conducted in terms of the overall response rate for each questionnaire, rate of missing items in each questionnaire as well as changes from baseline to follow-up in health service resource use as well as quality of life by treatment arm.
Qualitative study
A nested qualitative study was undertaken to qualitatively assess the feasibility and acceptability of the RESPECT study from the perspective of the participants themselves. At the consenting process for the RESPECT study, they were able to give consent to a 30-minute telephone interview about their experience of taking part, which would be scheduled after they completed the last data collection appointment. At the last appointment, it was clarified if they were still interested in taking part in this further telephone interview and, if they still wished to take part, the RESPECT study team got in touch to arrange the telephone interview at a convenient date and time.
Development of the interview schedule
The interview topic guide was developed in conjunction with the lived experience researchers and reflected the research questions for the study. A teleconference was held prior to the start of the qualitative study with the four people who would be conducting the interviews and the chief investigator in order to plan the telephone interviews, and to ensure consistency of approach. The two RESPECT study researchers already had permission from the NHS sites to undertake the research and have access to participants as part of the wider ethics and governance approvals. For the lived experience researchers, they each obtained ‘letters of access’ from the relevant NHS sites.
The interview topic guide was employed to standardise the procedure across all interviews. Interview questions focused on the participant’s reasons for taking part, their recall of the recruitment strategies, their views on the presentation of study information (PIS and leaflet), the randomisation process and their experience of meeting the researcher and the interventionist (when applicable). The telephone interviews lasted approximately 30 minutes and were audio recorded with the participant’s permission.
Procedure
The researcher at Huddersfield was informed by e-mail that a participant had confirmed verbally that they were still interested in taking part in the qualitative interview.
The researcher undertaking the telephone interview was not the same person who had been involved in the recruitment and data collection during trial participation to ensure some level of independence, and to minimise social desirability in responses to questions about how they had found the experience of being a participant.
Data protection
The participants’ contact details were stored in the secure trial database that could only be accessed by the RESPECT study team. To pass these contact details to the two lived experience researchers for them to be able to conduct the interview, the lead researcher sent the name and preferred contact details in a password-protected document in an e-mail. They were asked to destroy these documents after use, and not to store personal details such as names and telephone numbers in their own telephones or anywhere else. To ensure privacy and to protect data, the telephone interviews were conducted in private (i.e. where no-one could overhear), recorded by using a password or PIN (personal identification number) protected digital recorder, and the recordings were transferred securely to the University of Huddersfield as soon as possible following the interview and then deleted from the device. The data were transcribed, stored and subsequently analysed at the University of Huddersfield. Anonymity was ensured by assigning a unique identification code specific to the study to both the electronic sound files and transcripts of individual interviews. This study identification list was retained at Huddersfield. The researchers were informed of the participant’s name and contact details for the purposes of arranging the telephone interview and this information was sent in a password-protected word document via e-mail. The researchers destroyed this document once the interview had taken place.
Ethics approval
The qualitative study was approved by East Midlands – Derby REC (reference number 16/EM/0334).
Analysis
The analysis was thematic54 and analyst driven (deductive) and a framework approach55 was employed to organise and synthesise the data. This (deductive) approach to qualitative analysis allowed for a more structured approach to data collection based on the pre-determined aims (to assess the acceptability of the intervention and the design and trial processes). Phases of analysis included familiarisation with the data, generating initial codes, searching for predetermined themes, reviewing the themes and generating a thematic map. Software (NVivo version 11; QSR International, Warrington, UK) was used to code and organise the data within a thematic matrix. The matrix was populated with the pre-determined themes as column headings and relevant data from each case in the corresponding rows. Finally, a thematic map of the factors influencing acceptability and suggestions for improvement was generated. Initial analysis was undertaken by the lead qualitative researcher (AE) and further refined through presentations of the analysis/discussion with the other interviewers [Harminder Kaur, CD and the chief investigator (EH)] for feedback/critique.
Chapter 4 Protocol changes
We monitored progress on recruitment carefully and as a result of poor initial take-up and to promote better engagement we made some minor amendments to the protocol. Minor amendments were also submitted for minor changes to documentation. Table 4 shows the list of amendments and when they were submitted.
| Amendment | Date of submission | Details |
|---|---|---|
| Minor amendment 1 (HRA) | 31 January 2017 | Minor revisions to baseline questionnaire booklets, participant documents and protocol. Notification of the change of local principal investigator at C&IFT |
| Substantial amendment 1 (REC) | 3 April 2017 |
Remove the inclusion criteria of people under Care Programme Approach as we have found this to be too restrictive when screening for potentially eligible participants CRN and RESPECT study research staff attend depot/clozapine clinics, service user groups and recovery colleges to hand out leaflets, answer any questions potential participants may have about the study and obtain CtoC where interest is shown. This has been included to expand capacity at these events and because of several service users being unable to leave their contact details with CRN staff despite being interested The baseline feedback questionnaire has also been shortened to reduce the burden on participants but to ensure that we capture the main reasons for participation/non-participation A further amendment made to allow CRN staff to make follow-up calls approximately 2 weeks after people had received the pack by post or face to face. This was to see if this extra contact would lead to increased recruitment |
| Minor amendment 2 (HRA) | 7 June 2017 | Minor revisions to follow-up (3- and 6-month) questionnaire booklets. The RESPECT study advert text for posting on local/national Facebook (Facebook, Inc., Menlo Park, CA, USA) support groups |
| Minor amendment 3 (HRA) | 27 July 2017 | Minor revisions to the protocol, PIS and participant leaflet |
| Minor amendment 4 (HRA) | 8 August 2017 | Addition of a new site (NELFT) |
The main substantial amendments were made after it became apparent that using the inclusion criteria of being in receipt of the Care Programme Approach (as indicated on the electronic records system) was excluding people who would otherwise fit the eligibility criteria, so the decision was made that this was not useful or reliable to use as an inclusion criterion. In addition to this, permission was sought for the RESPECT study researchers to make a direct approach to people who attended day centres and outpatient clinics to informally discuss the study and hand out leaflets. The requirement for eligibility screening and checking with the case manager remained a requirement. The reason for this change was that it became apparent that many of the packs given out at CMHTs were not being passed on to the potentially eligible service users and, therefore, adding to the low recruitment rates. In addition, permission was granted to CSOs to make follow-up contact with those who had received an information pack to discuss the study and see if they wanted to give consent to speak to a RESPECT study researcher.
The study recruitment period was extended after an extraordinary independent TSC meeting (July 2017). Recruitment was not at the level of the targets set prior to the trial but, after making adjustments to the eligibility and recruitment strategy, recruitment numbers had picked up by July 2017. Therefore, the recommendation from the TSC was to request a no-cost extension for a further 6 months. However, this would not give time to collect the 6-month follow-up points for anyone recruited after September 2017 as all data collection had to be completed by the end of March 2018 in order to be able to process the data and produce the final report by August 2018.
Chapter 5 Results
Statistical analysis
Baseline characteristics
Participant characteristics at baseline are shown in Table 5. The average age was 44.8 years, ranging from 22 years to 66.1 years. There was almost an equal split of men (48.6%) and women (47.2%); three participants stated that they were ‘other’, describing themselves as intersex, unable to select one answer, or not providing information. The majority of the participants classed themselves as heterosexual (81.9%), with four stating they were gay or lesbian (5.6%), six saying bisexual (8.3%), one participant preferring not to say and two responding ‘other’. Of the two that responded ‘other’ regarding their sexuality, one was unsure and one stated they were pansexual. Forty-six (63.9%) of the participants in the RESPECT study were white British; however, the other 36.1% of participants were from a wide range of ethnic backgrounds (see Table 5).
| Characteristics | Intervention (N = 36) | Control (N = 36) | Overall (N = 72) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 44.2 (12.1) | 45.3 (11.5) | 44.8 (11.8) |
| Median (minimum, maximum) | 47.1 (22.9, 66.1) | 46.9 (22.0, 65.1) | 46.9 (22.0, 66.1) |
| Gender, n (%) | |||
| Male | 18 (50.0) | 17 (47.2) | 35 (48.6) |
| Female | 17 (47.2) | 17 (47.2) | 34 (47.2) |
| Other | 1 (2.8) | 2 (5.6) | 3 (4.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sexuality, n (%) | |||
| Heterosexual | 29 (80.6) | 30 (83.3) | 59 (81.9) |
| Gay or lesbian | 3 (8.3) | 1 (2.8) | 4 (5.6) |
| Bisexual | 3 (8.3) | 3 (8.3) | 6 (8.3) |
| Prefer not to say | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Other | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| Ethnicity, n (%) | |||
| White British | 23 (63.9) | 23 (63.9) | 46 (63.9) |
| White Irish | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Black African | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| Black Caribbean | 2 (5.6) | 0 (0.0) | 2 (2.8) |
| Black other | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Asian Indian | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| Asian Pakistani | 1 (2.8) | 2 (5.6) | 3 (4.2) |
| Asian Bangladeshi | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Asian other | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| White and Black Caribbean | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| White and Asian | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Other mixed background | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Prefer not to say | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| Other | 4 (11.1) | 4 (11.1) | 8 (11.1) |
| Religion, n (%) | |||
| No religion | 15 (41.7) | 14 (38.9) | 29 (40.3) |
| Muslim | 3 (8.3) | 3 (8.3) | 6 (8.3) |
| Christian | 14 (38.9) | 13 (16.1) | 27 (37.5) |
| Sikh | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Buddhist | 0 (0.0) | 3 (0.0) | 3 (4.2) |
| Hindu | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Jewish | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prefer not to say | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Other | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| Missing | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| Highest qualification, n (%) | |||
| None | 3 (8.3) | 3 (8.3) | 6 (8.3) |
| GCSEs/GCEs/CSEs | 9 (25.0) | 2 (5.6) | 11 (15.3) |
| AS/A levels | 6 (16.7) | 6 (16.7) | 12 (16.7) |
| Diploma | 1 (2.8) | 3 (8.3) | 4 (4.6) |
| Higher degree | 7 (19.4) | 5 (13.9) | 12 (16.7) |
| Further higher degree | 2 (5.6) | 5 (13.9) | 7 (9.7) |
| Vocational education | 4 (11.1) | 6 (16.7) | 10 (13.9) |
| Other | 3 (8.3) | 5 (13.9) | 8 (11.1) |
| Missing | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| Employment, n (%) | |||
| Full time | 1 (2.8) | 5 (13.9) | 6 (8.3) |
| Part time | 4 (11.1) | 3 (8.3) | 7 (9.7) |
| Unable to work because of poor health | 17 (47.2) | 21 (58.3) | 38 (52.8) |
| Unemployed | 8 (22.2) | 5 (13.9) | 13 (18.1) |
| Retired | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| Student | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| Other | 2 (5.6) | 0 (0.0) | 2 (2.8) |
| Living arrangements, n (%) | |||
| Live with parent/carer | 4 (11.1) | 7 (19.4) | 11 (15.3) |
| Live alone | 24 (66.7) | 18 (50.0) | 42 (58.3) |
| Live with relative | 1 (2.8) | 3 (8.3) | 4 (5.6) |
| Live in a hostel | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| Live with a friend | 2 (5.6) | 2 (5.6) | 4 (5.6) |
| With partner/spouse | 2 (5.6) | 4 (11.1) | 6 (8.3) |
| Other | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| Relationship status, n (%) | |||
| Single, not married | 26 (72.2) | 22 (61.1) | 48 (66.7) |
| Married | 2 (5.6) | 5 (13.9) | 7 (9.7) |
| Civil partnership | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cohabiting | 2 (5.6) | 0 (0.0) | 2 (2.8) |
| In a relationship, not living together | 4 (11.1) | 6 (16.7) | 10 (13.9) |
| Separated | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Divorced | 2 (5.6) | 2 (5.6) | 4 (5.6) |
| Widowed | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Similarly, there was a wide range of religious beliefs represented in the trial participants. The most common was Christian (37.5%), or no religion (40.3%). However, the trial participants also included six Muslims (8.3%), one Sikh (1.4%), three Buddhists (4.2%), one participant preferring not to say (1.4%), and two (2.8%) stating that they had other beliefs.
The level of qualifications varied from six participants having no qualifications (8.3%), to seven participants having further higher degrees (9.7%). The most common level of education was AS (Advanced Subsidiary) level/A (Advanced) level and higher degrees, both having been completed by 12 participants (16.7%), with GCSE (General Certificate of Secondary Education)/GCE (General Certificate of Education)/CSE (Certificate of Secondary Education) having been obtained by 11 participants (15.3%). Over half of the participants in the trial were currently unable to work because of poor health (52.8%) and 13 were unemployed (18.1%). Of those that were currently in work, six were working full time and seven were working part time. Three participants (4.2%) were retired and three (4.2%) were students.
The majority of participants stated that they were living alone (58.3%), others were living with their parent/carers (15.3%), other relatives (5.6%), friends (4.6%) or their partner/spouse (8.3%). Three participants were living in hostels, and two had other living arrangements (one living in a house of multiple occupancy and the other in a supported living facility).
Primary outcomes
Recruitment rates
Over the course of 12 months, 138 people were screened. (This is based on data from the screening log. There is a larger proportion of people identified informally as eligible and packs were given to care co-ordinators or posted out; however, we were unable to get accurate information regarding how many of those potentially eligible people actually received the packs.) Of these 138 people, 117 people met the defined eligibility criteria for inclusion in the trial. This figure of 84.8% eligible people is much higher than the 50–60% anticipated. 35,41 Of these, a total of 72 participants were randomised into the trial (March 2017 to January 2018), giving a recruitment rate of 61.5% (from which 52.2% of screened participants then went on to enter the study), again higher than the 40% which was predicted (Figure 4). The flow of participants can be seen in Figure 5.
FIGURE 4.
Recruitment graph for the RESPECT trial from March to December 2017 (by centre).
FIGURE 5.
The CONSORT flow diagram. a, Reasons for not meeting inclusion criteria are not mutually exclusive; b, this eligibility criterion was dropped after 27 April 2017.
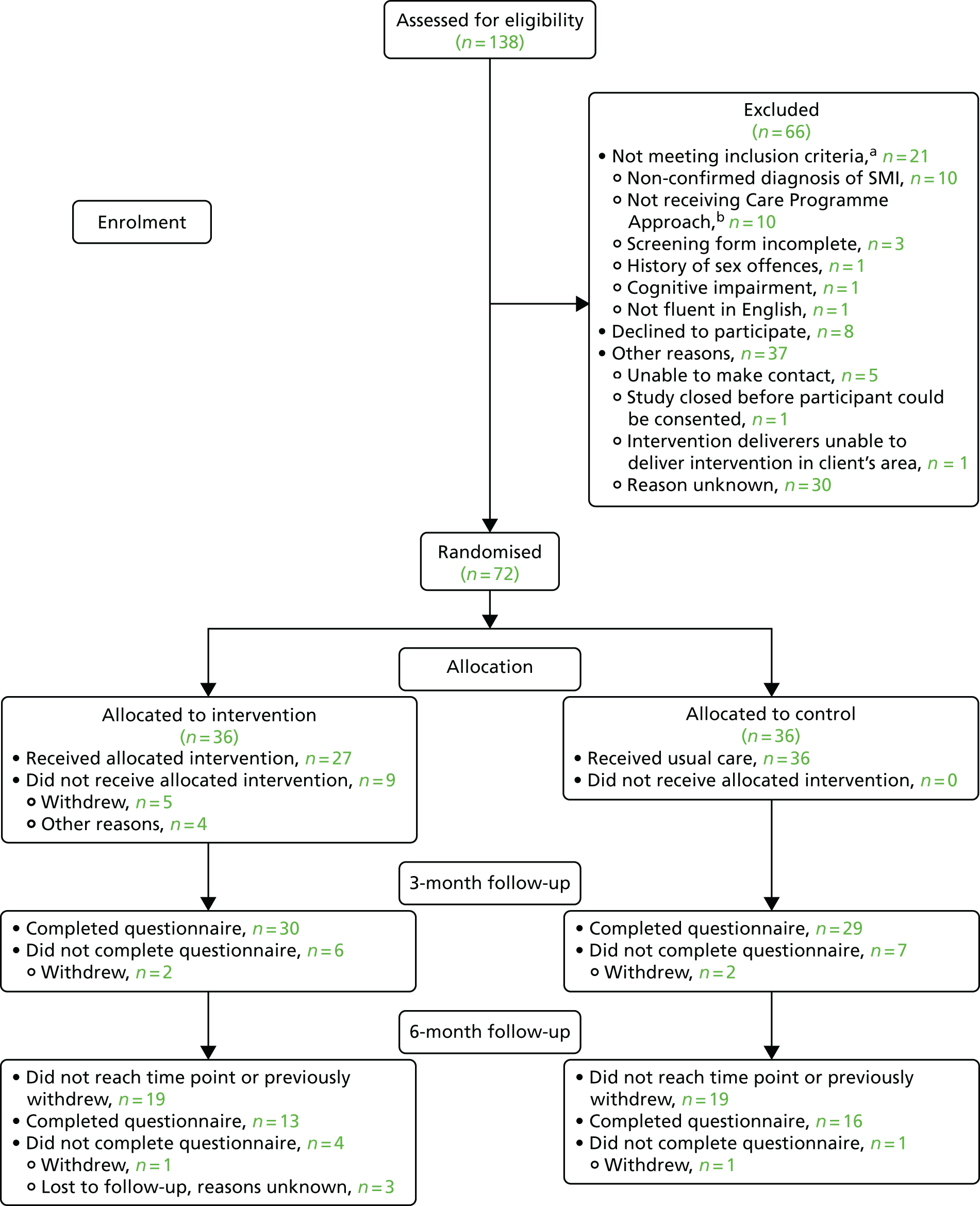
These 72 participants were recruited from the six sites, with LYPFT and C&IFT recruiting the majority (70.8%) of the participants, 24 and 27, respectively. The remaining 21 participants were recruited from SWYFT (n = 8), NELFT (n = 7), SPFT (n = 4) and the Community Links – aspire service (n = 2).
Retention rate
Intervention
Thirty-six participants (50%) were randomised to receive the intervention. The intervention consisted of three 1-hour sessions, each covering different material.
The first session was attended by 25 participants (69.4%), the second by 19 (52.8%) and the third by 18 (50%). In addition to this, five participants had combined sessions, in which they covered the material from multiple sessions at one time. In total, 17 participants attended all three sessions (47.2%), with 22 covering all the material from the three sessions (61.1%).
Twenty-five per cent (n = 9) of the participants never received any of the intervention sessions for various reasons; five had withdrawn from the intervention (see Withdrawals for more details) and reasons for the other four not attending included wanting a specific gender of interventionist (n = 1), logistical problems arranging appointments (n = 1) and other reasons (not known) (n = 2).
Completion of data
Questionnaires
The 3-month questionnaires were completed by 59 participants (81.9%); this was split equally across the two arms, with 30 from the intervention arm and 29 from the control arm. Owing to an extension to the recruitment period (during which there was only time to do the 3-month follow-up) not all participants reached the 6-month time point before the trial ended. Of the 72 that were randomised, 38 reached the 6-month time point (52.8%). Of these 38 participants, 29 completed the 6-month questionnaire (76.3%); these were split almost equally, 13 from the intervention arm and 16 from the control arm. Further details can be found in the CONSORT flow diagram (see Figure 5).
Completion of standardised measures
The details of the completion of the standardised measures can be found in Table 6. The percentages are given out of those who completed the relevant time point interview. These completion rates do not account for the attrition rates of the trial and, as such, represent the completion of the returned questionnaires. This is so that the standardised measures can be assessed for suitability for use as a future primary outcome, rather than assessing the level of attrition in the trial. For those measures in which there was no fixed scoring mechanism, or when not all questions were applicable to all, the completion rate is taken as a response to any element of the questionnaire.
| Measure | Interventiona (n = 36, 30, 13) | Controlb (n = 36, 29, 16) | Overallc (n = 72, 59, 29) |
|---|---|---|---|
| SERBAS,d n (%) | |||
| Baseline | 36 (100.0) | 36 (100.0) | 72 (100.0) |
| 3 months | 25 (83.3) | 23 (79.3) | 48 (81.4) |
| 6 months | 13 (100.0) | 16 (100.0) | 29 (100.0) |
| Natsal, n (%) | |||
| Baseline | 27 (75.0) | 21 (86.1) | 58 (100.0) |
| 3 months | 26 (86.7) | 27 (93.1) | 53 (89.8) |
| 6 months | 12 (92.3) | 11 (68.8) | 23 (79.3) |
| HIV-KQ, n (%) | |||
| Baseline | 36 (100.0) | 36 (100.0) | 72 (100.0) |
| 3 months | 30 (100.0) | 28 (96.6) | 58 (98.3) |
| 6 months | 11 (84.6) | 16 (100.0) | 27 (93.1) |
| Motivation to engage in safer sex, n (%) | |||
| Baseline | 35 (97.2) | 34 (94.4) | 69 (95.8) |
| 3 months | 30 (100.0) | 27 (93.1) | 57 (96.6) |
| 6 months | 13 (100.0) | 16 (100.0) | 29 (100.0) |
| Condom Use Self-Efficacy Scale, n (%) | |||
| Baseline | 35 (97.2) | 34 (94.4) | 69 (95.8) |
| 3 months | 26 (86.7) | 25 (86.2) | 51 (86.4) |
| 6 months | 11 (84.6) | 16 (100.0) | 27 (93.1) |
| Behavioural intention for safer sex, n (%) | |||
| Baseline | 36 (100.0) | 36 (100.0) | 72 (100.0) |
| 3 months | 30 (100.0) | 28 (93.3) | 58 (98.3) |
| 6 months | 13 (100.0) | 15 (93.8) | 28 (96.6) |
| ASSIST,d n (%) | |||
| Baseline | 36 (100.0) | 36 (100.0) | 72 (100.0) |
| 3 months | 30 (100.0) | 28 (93.3) | 58 (98.3) |
| 6 months | 13 (100.0) | 15 (93.8) | 28 (96.6) |
| ReQoL, n (%) | |||
| Baseline | 36 (100.0) | 36 (100.0) | 72 (100.0) |
| 3 months | 30 (100.0) | 28 (96.6) | 58 (98.3) |
| 6 months | 13 (100.0) | 15 (93.8) | 28 (96.6) |
Overall, the standardised measures were all completed, with minimal missing data. There was also no visual difference in the completion rates between the treatment arms.
Identifying a possible future primary outcome
Although the completion rates of the various outcome measures that were implemented in the RESPECT trial were high, it was determined that none of these would be a suitable primary outcome. If a large-scale RCT were to be undertaken after the completion of this trial, the main focus would be around the proportion of unprotected sexual acts performed, which the intervention aims to reduce, and as such this would be the primary outcome. In the RESPECT trial, this was measured using the SERBAS; however, this measure also provided a lot of extra information that would not be deemed necessary in future and, as such, aspects of this measure may be taken or altered to be incorporated into a future trial, but the SERBAS as a whole would not be used.
In the RESPECT trial, a large proportion of the participants were not sexually active throughout the trial (n = 36; 50% reported no acts at any time point), which has reduced the sample on which the reduction of unprotected acts can be calculated. As such, there were only 22 (30.6%) participants who had been sexually active in the 3 months prior to baseline and at the 3-month follow-up: 11 intervention and 11 control participants (Table 7).
| Number of sexual acts | Intervention | Control | Overall |
|---|---|---|---|
| Number of sexual acts unprotected | |||
| Baseline | n = 15 | n = 15 | N = 30 |
| Mean (SD) | 30.8 (48.9) | 12.7 (13.9) | 21.8 (36.5) |
| Month 3 | n = 13 | n = 14 | N = 27 |
| Mean (SD) | 21 (31.6) | 17.1 (19.1) | 19.0 (25.5) |
| Reduction in number from baseline to month 3 | n = 11 | n = 11 | N = 22 |
| Mean (SD) | 15.9 (39.7) | –2.3 (17.3) | 6.8 (31.3) |
| Percentage of sexual acts unprotected | |||
| Baseline | n = 15 | n = 15 | N = 30 |
| Mean (SD) | 62.7 (42.8) | 77.4 (31.9) | 70.1 (37.8) |
| Month 3 | n = 13 | n = 14 | N = 27 |
| Mean (SD) | 54.6 (40.7) | 79.7 (27.2) | 67.6 (36.0) |
| Reduction in percentage from baseline to month 3 | n = 11 | n = 11 | N = 22 |
| Mean (SD) | 4.0 (22.0) | –9.7 (32.2) | –2.9 (27.8) |
On average, the participants in the trial saw a reduction of 6.8 unprotected sexual acts over the 3 months between baseline and the 3-month follow-up, with a SD of 31.3 unprotected sexual acts. The control arm had an average increase of 2.3 in the number of unprotected sexual acts (SD 17.3) and the intervention arm had an average reduction of 15.9 unprotected sexual acts (SD 39.7).
On average, this equated to a 2.9% increase in the percentage of unprotected sexual acts; a 4.0% decrease for the intervention arm (SD 22.0) and a 9.7% increase (SD 32.2) for the control arm. It should be noted that of the 22 participants included, seven (31.8%) had no change in the proportion of unprotected sexual acts they undertook between baseline and 3 months. The 3-month time point was chosen as the point at which to measure this as all participants were still involved, whereas the 6-month follow-up was only reached by around 50% of the participants.
However, when looking at the 6-month time point, only nine participants have been sexually active both at baseline and at 6 months, and eight participants were in the control arm and one participant was in the intervention arm. There was an average 20.1% increase in the proportion of unprotected sex, with a SD of 33.5; however, it should be noted that this will be skewed by the intervention participant having a 100% increase in unprotected sexual acts, as the control arm had only a 10.1% increase.
For an external feasibility study, such as the RESPECT trial, to provide a robust estimate of the SD of an outcome measure to inform a sample size calculation, a minimum of 70 measured subjects is needed. 45 Unfortunately, because of the recruitment problems and the large proportion of participants not participating in unprotected sexual acts in this instance, this had not been reached here.
Exploring possible clustering effects by therapist
As can be seen in Table 8, there were 11 different therapists involved in delivering the intervention to participants in the RESPECT trial. Of these 11, one therapist delivered 41.8% of the sessions, to 39.3% (n = 11) of the participants in the intervention arm. Five of the therapists delivered the intervention to only one participant each. As a result, the calculation of an ICC would be inappropriate at this time, as the data would not provide a reliable estimate.
| Therapist | Number of sessions conducted | Number of participants treated |
|---|---|---|
| A | 28 | 11 |
| B | 4 | 2 |
| C | 1 | 1 |
| D | 7 | 3 |
| E | 5 | 2 |
| F | 3 | 1 |
| G | 6 | 2 |
| H | 3 | 1 |
| I | 3 | 1 |
| J | 5 | 2 |
| K | 1 | 1 |
Possible future sample size
At baseline in the RESPECT trial, the participants undertaking any sexual acts were on average undertaking 70.1% of these without protection (SD 37.8). Using the percentage of unprotected sexual acts per person as the primary outcome for a future trial, a sample of 202 participants would be needed to power the trial to show a mean reduction of 15% in unprotected sexual acts from 70% in the control arm to 55% in the intervention arm, at 3 months after randomisation, with 80% power and 5% two-sided significance. Allowing for 25% attrition, as seen at the 6-month time point in the RESPECT trial, this increases this sample size to 270, with 135 participants per arm.
However, this number would need to be inflated further to account for those participants who may not be undertaking any sexual acts at 3 months but who were at baseline; in the RESPECT trial this was 6.8% of those who completed the 3-month assessment. Conservatively adjusting for this, using 10% loss, would increase the final sample size to 300 participants who currently engage in sexual activities.
Given that it took 12 months to recruit 72 participants into the RESPECT trial, from six sites, we would need to increase both the length of recruitment and the number of sites to reach a target of 300 participants. The six sites were open to recruitment for a combined total of approximately 40 months, and only 30 of the 72 recruited participants were sexually active when recruited; therefore, the recruitment in the RESPECT trial can be thought of as equivalent to 0.75 sexually active participants per site per month. In a future trial, allowing 18 months for the recruitment period would require 23 sites to be opened. Alternatively, 17 sites could be open for 24 months.
Alternatively, to detect a 10% reduction in unprotected sexual acts, with the same assumptions and conditions as above, a final sample size of 670 participants would be required. This would require 50 sites to be open for 18 months, or 38 sites for 24 months.
Secondary outcomes
As the RESPECT study is a feasibility trial and, as such, had a small sample size, no formal statistical comparisons have been conducted, and all comparisons are instead considered as purely indicative.
Sexual and risk behaviour measures
The SERBAS is a measure that is split into two separate questionnaires, one to be completed by males and one by females. Of the three participants who classified themselves as another gender, two completed the female version and one completed the male version. It is not known if the participant or the researcher selected which version to complete.
The main focus of the SERBAS in the RESPECT trial was quantifying the proportion of sex acts that were undertaken without any form of protection, with the hope that the intervention may influence this. Table 9 details the proportion of all sex acts (vaginal, oral and anal) that were undertaken without protection, within the last 3 months at each time point, overall, by treatment arm and by gender. Full details on the results from the SERBAS can be found in Tables 24 and 25 in Appendix 2.
| Time point | Intervention, n (%) | Control, n (%) | Overall, N (%) |
|---|---|---|---|
| Baseline | |||
| Male | 71 (97.2) | 95 (87.4) | 166 (91.6) |
| Female | 532 (73.9) | 159 (67.9) | 691 (72.5) |
| 3 months | |||
| Male | 66 (84.8) | 154 (77.9) | 220 (80.0) |
| Female | 367 (59.1) | 159 (74.8) | 526 (63.9) |
| 6 months | |||
| Male | 2 (50.0) | 58 (79.3) | 60 (78.3) |
| Female | 38 (52.6) | 64 (96.9) | 102 (80.4) |
It can be seen in Table 9 that there is a high level of unprotected sexual acts occurring in the participants in the RESPECT study. However, it should be noted that there were participants in the study that were not sexually active over the course of the study. At baseline, only 20 participants who completed the female SERBAS (55.6%) and only 10 participants (27.8%) who completed the male SERBAS had undertaken any sexual act within the previous 3 months.
Within the RESPECT study there was almost no sex trading reported. At baseline, two participants reported having paid for sex once and that they had not used condoms on these occasions. At 3 months, a different participant reported having paid for sex once and did use a condom, and at 6 months one participant, who had also done so at baseline, reported that they had paid for sex three times and did not use a condom on any occasion. All of the participants who did not use a condom were in the control arm, and the participant who used a condom was in the intervention arm. Only one participant reported an episode of forced sex, which had occurred once. At the point of disclosure during the administration of the SERBAS (as per protocol) the researcher asked the participant if they had someone to discuss this with and they replied that they were discussing the incident with their care co-ordinator and it was being dealt with, and that no action or further support was required from the RESPECT study team. The chief investigator was informed of this and approved no further action.
From Tables 24 and 25 in Appendix 2, it can be noted that the female participants in the study reported having more vaginal sex than the men at baseline: an average of 37.3 times in the last 3 months for women, with 58.4% of that not involving condom use, compared with an average of five times for men, with 81.4% of that being unprotected. This gender difference continued at 3- and 6-month follow-up. It can also be seen that very few participants in the study reported having anal sex, but that almost all of the acts were undertaken without condoms. Similarly the majority of the oral sex (both participant on partner and vice versa) was reported as being undertaken without protection, despite a substantial amount of oral sex being reported. In this instance, protection could be either condoms or dental dams dependent on the gender of the participating people.
Participants were asked to select up to three reasons for not participating in sexual activity with male and with female partners in the last 3 months; not all participants utilised the possible six reasons. The majority of participants were not interested in having sex with a same-sex partner (n = 55) but the other reasons given (as frequencies) are as follows:
-
no current partner (n = 27)
-
participant not interested in sex (n = 10)
-
participant’s mental illness/medication side effects (n = 9)
-
not interested with opposite sex partner (n = 4)
-
participant’s illness or fatigue (n = 3)
-
lack of privacy (n = 3)
-
partner not interested in sex (n = 3)
-
partner died (n = 2)
-
partner’s mental illness/medication side effects (n = 1)
-
participant’s drug use (n = 1)
-
other reasons included: no opportunity (n = 2), does not trust women (n = 1), having to ‘fend off’ harassers (n = 1), religious reasons (n = 1), last partner cheated (n = 1), not looking for partner (n = 1), currently separated from wife (n = 1) and one participant gave no additional information.
Natsal-3
Elements of the Natsal-3 questionnaire were used within the RESPECT study. The results were similar across all three time points and the full details can be found in Table 26 in Appendix 2. One of the main areas covered by questions from the Natsal-3 questionnaire included contraception use. The male condom was found to be the most popular contraception method currently used, with 74.3% of men and 97.1% of women reporting using it as one of their most usual methods at baseline. Contraception was mainly obtained from health-care professionals (including doctors, or staff in pharmacies and sexual health clinics) and this was also found to be the favoured way to obtain contraception if participants had the option. Over half of the participants had visited a sexual health clinic, but most had not visited in the last year. Most participants had never been told that they had had a STI, and of those who had, chlamydia and genital warts were the most commonly reported STIs. Forty-two of the participants (58.3%) had been tested for HIV at some point, mainly as part of a sexual health check-up or because of concern for their own safety. For most of the participants this test was over 5 years ago, but eight participants (19.1%) had had a HIV test in the last year.
Knowledge about human immunodeficiency virus
The HIV-KQ consists of 18 questions, with a ‘yes’, ‘no’ and ‘don’t know’ option, meaning an average correct score can be calculated. Within the RESPECT study, only 17 questions were asked as it was felt that one of the questions, ‘A natural skin condom works better against HIV than does a latex condom’, was outdated and possibly confusing. The results are given in Table 10.
| Time point | Intervention (N = 36) | Control (N = 36) | Overall (N = 72) |
|---|---|---|---|
| Baseline | n = 32 | n = 34 | n = 66 |
| Mean (SD) | 68.8 (23.1) | 65.9 (21.5) | 67.3 (22.2) |
| Median (minimum, maximum) | 76.5 (0, 94.1) | 0.71 (5.9, 94.1) | 76.5 (0, 94.1) |
| 3 months | n = 30 | n = 28 | n = 58 |
| Mean (SD) | 75.7 (18.8) | 65.3 (25.7) | 70.1 (22.8) |
| Median (minimum, maximum) | 76.5 (5.9, 100) | 70.6 (0, 100) | 76.5 (0, 100) |
| 6 monthsa | n = 11 | n = 16 | n = 27 |
| Mean (SD) | 74.3 (23.0) | 68.8 (22.4) | 71.0 (22.4) |
| Median (minimum, maximum) | 82.4 (29.4, 100) | 73.5 (23.5, 100) | 76.5 (23.5, 100) |
At baseline, the average score was 67.3% correct; this equates to between five and six incorrect answers on average. No one knew the answers to all of the questions and one person did not get any of the questions correct. The average score was similar in the two arms: 68.8% for the intervention arm and 65.9% for the control arm.
At 3 months, the average score had slightly increased (to 70.7%), equating to an average of five questions answered incorrectly. The average score was slightly different between arms: 75.7% for the intervention arm and 65.3% for the control arm. However, three participants now answered all questions correctly (two in the intervention arm and one in the control arm) with two participants answering all questions incorrectly, both from the control arm.
Despite the reduced number of participants who had data collected at 6 months, the results seen at 3 months still hold. The average score was 71.6% (74.3% for the intervention arm and 68.8% for the control arm). However, at this time point no participant received a score of 0.0%.
The most commonly incorrectly, or unknown, answered question was ‘All pregnant women infected with HIV will have babies born with AIDS’.
Motivations to engage in safer sex scale
The motivations to engage in safer sex scale consists of four questions asking how much risk the participant believes they are at of getting a STI, the chance of ever getting a STI, how big a problem STIs are in their community and whether or not they are worried about getting a STI. The majority of participants answered all questions in the ‘Somewhat at risk’ to ‘No risk at all’, with proportions staying constant across time points. One exception to this was the final question at month 3, which had an unexplainably large number of missing data (n = 33 of 59, 55.9%). Full details of the results can be found in Table 27 in Appendix 2.
Condom Use Self-Efficacy Scale
The Condom Use Self-Efficacy Scale is detailed in Table 11, and the results for sections one and two independently can be found in Table 28 in Appendix 2. The results between baseline and month 3 do indicate a slight rise in condom efficacy that drops slightly at month 6, but this may be explained by the reduced sample size. The Condom Use Self-Efficacy Scale was one of the least completed measures in the RESPECT trial (see Table 6 for completion rates); however, this is most likely to be because of the missing data mechanism used within this measure, in which only one item from each section could be missing and a score still calculated.
| Time point | Intervention (N = 36) | Control (N = 36) | Overall (N = 72) |
|---|---|---|---|
| Baseline | n = 35 | n = 34 | n = 69 |
| Mean (SD) | 46.8 (8.5) | 45.7 (5.0) | 46.2 (6.9) |
| Median (minimum, maximum) | 48 (22, 63) | 46 (35, 57) | 47 (22, 63) |
| 3 months | n = 26 | n = 25 | n = 51 |
| Mean (SD) | 49.7 (7.4) | 45.4 (7.0) | 47.5 (7.5) |
| Median (minimum, maximum) | 49 (30, 66) | 47 (28, 62) | 48 (28, 66) |
| 6 monthsa | n = 11 | n = 16 | n = 27 |
| Mean (SD) | 49.4 (6.3) | 44.3 (7.8) | 46.4 (7.5) |
| Median (minimum, maximum) | 48 (42, 60) | 46 (25, 56) | 48 (25, 60) |
Behavioural Intentions for Safer Sex
Scored from 0 to 40, the Behavioural Intentions for Safer Sex measure indicates the level of intention a participant has to have safe sex, with higher scores indicating more intention. Table 12 details the scores by arm and overall, at each time point. In Table 12 it can be seen that there is minimal change in the responses across the time points, but that it increases slightly for the intervention arm and that there is a slight decrease in the control arm.
| Time point | Intervention (N = 36) | Control (N = 36) | Overall (N = 72) |
|---|---|---|---|
| Baseline | n = 36 | n = 36 | n = 72 |
| Mean (SD) | 24.3 (8.1) | 23.1 (7.6) | 23.7 (7.8) |
| Median (minimum, maximum) | 25 (4, 36) | 24 (5, 34) | 24 (4, 36) |
| 3 months | n = 30 | n = 27 | n = 57 |
| Mean (SD) | 26.7 (6.9) | 21.5 (7.5) | 24.2 (7.6) |
| Median (minimum, maximum) | 27 (4, 36) | 21 (3, 37) | 25 (3, 37) |
| 6 monthsa | n = 13 | n = 15 | n = 28 |
| Mean (SD) | 27.1 (5.9) | 20.1 (10.0) | 23.3 (9.0) |
| Median (minimum, maximum) | 28 (10, 34) | 22 (0, 34) | 27 (0, 34) |
The Mental Illness Stigma Scale
Details of the results of the questions of the MISS-Q at each of the three time points can be found in Table 29 in Appendix 2. These results are given with the count and proportions who agreed with the statements, those who did not answer the question, and those who said that it was not applicable to them. One of the questions, which asked about protecting oneself or one’s partner from getting pregnant, has a larger than normal proportion of ‘N/A’ (not applicable) responses, which is because the question was printed incorrectly; it asked about protecting oneself from pregnancy and thus was not answered by most male respondents. At baseline, the statement with most agreement was ‘You are the one who chooses the course of your sexual life’ with 76.4% agreement overall. The statements with the least agreement were ‘Often a mental health provider has said you should not have a romantic or sexual relationship with people who do not have a mental illness’ and ‘Often a mental health provider has said you should have a hysterectomy, etc. as a form of birth control’, both with 0% agreement.
Alcohol, Smoking and Substance Involvement Screening Test
The ASSIST calculates risk scores for a set list of substances. The majority of the participants in the RESPECT trial were in the ‘Low risk’ category for all substances except tobacco, for which the majority of participants were categorised as at ‘Moderate risk’. These results were consistence across all three time points in the study. Table 13 details the total substance involvement scores, where the lower the score the less involvement the participant has had with any substance. The overall levels stay similar across the three time points. It can be seen that there are some participants who had no involvement with any substances (a score of zero) and some who had extensive involvement.
| Time point | Intervention (N = 36) | Control (N = 36) | Overall (N = 72) |
|---|---|---|---|
| Baseline | n = 36 | n = 36 | n = 72 |
| Mean (SD) | 38.9 (24.9) | 34.7 (18.2) | 36.8 (21.7) |
| Median (minimum, maximum) | 33.5 (2, 89) | 36 (0, 60) | 35.5 (0, 89) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Mean (SD) | 40.7 (25.1) | 28.6 (20.1) | 34.7 (23.4) |
| Median (minimum, maximum) | 38 (2, 88) | 35 (0, 65) | 36 (0, 88) |
| 6 monthsa | n = 13 | n = 16 | n = 29 |
| Mean (SD) | 37.8 (19.6) | 29.9 (18.8) | 33.4 (19.2) |
| Median (minimum, maximum) | 46 (6, 73) | 30.5 (0, 58) | 35 (0, 73) |
Full details of the results of the ASSIST, including the breakdown of individual substances scores, can be found in Table 30 in Appendix 2.
Recovering Quality of Life
The ReQoL is a measure that looks at the quality of life of the participants, scored from 0 to 80, with a higher score indicating a higher quality of life. A score of < 50 is defined as characteristic of a clinical population. At each time point, the mean and median scores for the ReQoL were both < 50 (as detailed in Table 14), inferring that our population is typical of mental health service users. At baseline there were 42 participants (58.3%) with a score of < 50 (19 in the intervention arm and 23 in the control arm), at 3 months this was 36 participants (61.0%) (16 participants in the intervention arm and 20 participants in the control arm) and at 6 months it was 17 participants (58.6%) (eight participants in the intervention arm and nine in the control arm).
| Time point | Intervention (N = 36) | Control (N = 36) | Overall (N = 72) |
|---|---|---|---|
| Baseline | n = 35 | n = 35 | n = 70 |
| Mean (SD) | 44.5 (19.3) | 43.6 (16.9) | 44.1 (18.0) |
| Median (minimum, maximum) | 44 (12, 80) | 41 (13, 78) | 42.5 (12, 80) |
| 3 months | n = 29 | n = 28 | n = 57 |
| Mean (SD) | 46.6 (18.9) | 43.9 (15.3) | 45.3 (17.1) |
| Median (minimum, maximum) | 46 (17, 75) | 44.5 (16, 75) | 46 (16, 75) |
| 6 months | n = 13 | n = 15 | n = 28 |
| Mean (SD) | 48.1 (11.2) | 46.5 (20.6) | 47.2 (16.6) |
| Median (minimum, maximum) | 47.9 (28, 70) | 45 (9, 80) | 45.5 (9, 80) |
| Please describe your physical health over the last week: n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| No problems | 2 (5.6) | 3 (8.3) | 5 (6.9) |
| Slight problems | 6 (16.7) | 5 (13.9) | 11 (15.3) |
| Moderate problems | 10 (27.8) | 6 (16.7) | 16 (22.2) |
| Severe problems | 4 (11.1) | 7 (19.4) | 11 (15.3) |
| Very severe problems | 13 (36.1) | 12 (33.3) | 25 (34.7) |
| Missing | 1 (2.8) | 3 (8.3) | 4 (5.6) |
| 3 months | n = 30 | n = 29 | n = 59 |
| No problems | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| Slight problems | 5 (16.7) | 4 (13.8) | 9 (15.3) |
| Moderate problems | 9 (30.0) | 5 (17.2) | 14 (23.7) |
| Severe problems | 4 (13.3) | 10 (34.5) | 14 (23.7) |
| Very severe problems | 11 (36.7) | 7 (24.1) | 18 (30.5) |
| Missing | 0 (0.0) | 2 (6.9) | 2 (3.4) |
| 6 months | n = 13 | n = 16 | n = 29 |
| No problems | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Slight problems | 1 (7.7) | 2 (12.5) | 3 (10.3) |
| Moderate problems | 7 (53.9) | 3 (18.8) | 10 (34.5) |
| Severe problems | 2 (15.4) | 4 (25.0) | 6 (20.7) |
| Very severe problems | 3 (23.1) | 6 (37.5) | 9 (31.0) |
| Missing | 0 (0.0) | 1 (6.3) | 1(3.5) |
Withdrawals
Through the course of the RESPECT study, 10 participants withdrew. Five participants withdrew from the intervention: one could no longer commit to the sessions as there had been a delay in receiving the intervention; one did not want to receive the intervention from a male and there was no other interventionist to deliver it; one withdrew after multiple cancelled sessions; and the other two gave no reason. Two participants were withdrawn from follow-up only, one after discussion with the chief investigator and clinician, and the other gave no reason. One of these participants was in the intervention arm and one was in the control arm. Full withdrawal from the trial was requested by four participants, with two in each treatment arm; one made a complaint to their local R&D office and three gave no reason. The complaint was made about the study focus (i.e. that it was about sex) after completion of the baseline interview, although the individual had read the information sheet and signed the consent form. The nature of the complaint was that we should not be undertaking research on the topic of sex. Our response was that we had NHS ethics approval to undertake the study and that the individual had had full information about the research topic during the recruitment process and was free to completely withdraw from the study, which they did. One of these full withdrawals had previously withdrawn from treatment; thus, there were a total of 11 instances of withdrawal.
Adverse events
No AEs were reported during the trial.
Health economics analyses
The objective of the economic analysis was to explore the feasibility of collecting cost-effectiveness data for a full RCT. As such, the economic analysis evaluated response rate, item completion rate and any emerging trends in the level and changes in health services resource use and health-related quality of life.
Health services resource use
Data completion
The questionnaire response rate was based on completion of at least one resource use item (including zeros). Table 15 shows that the number of respondents who completed at least one item of the resource use questionnaire was 36 (100%), 30 (83%) and 13 (36%) at baseline, 3 months and 6 months, respectively in the intervention arm. The response rate was comparable in the control arm with 36 (100%), 26 (72%) and 15 (42%) respondents completing the questionnaire at baseline, 3 months and 6 months, respectively. Moreover, this was similar to the overall questionnaire completion rate in the study.
| Service use | Intervention | Usual care | ||||
|---|---|---|---|---|---|---|
| Baseline (n = 36) | 3 months (n = 30) | 6 months (n = 13) | Baseline (n = 36) | 3 months (n = 26) | 6 months (n = 15) | |
| GP consultations | 1 | 0 | 0 | 0 | 0 | 0 |
| Nurse consultations | 0 | 0 | 0 | 0 | 1 | 0 |
| A&E or urgent care centre visits | 1 | 0 | 0 | 0 | 0 | 0 |
| Pharmacy visits/appointments | 1 | 0 | 0 | 3 | 0 | 0 |
| Contraception clinic consultations | 0 | 0 | 0 | 0 | 0 | 0 |
| Sexual health clinic consultations | 0 | 0 | 0 | 0 | 1 | 0 |
| Sexual Assault Referral Centre (SARC) consultations | 0 | 0 | 0 | 0 | 0 | 0 |
Table 15 also shows that the rate of missing items in the resource use questionnaire was very low. Response to an item was considered missing if response to the binary (yes/no) question about a service use item (such as GP consultations) was missing or the number of visits conditional on a ‘yes’ response was missing. In the intervention arm, one response was missing at baseline for GP consultations, A&E or urgent care visits and pharmacy visits. In the control arm, three responses were missing for pharmacy visits and one each for nurse and sexual health clinic consultations. Overall, there is a slightly higher number of missing values for pharmacy visits; however, overall the number of missing responses remained low.
Frequency and reasons for health service use
Table 16 shows the frequency of use of each type of health service in the last 3 months. Given the small number of respondents (particularly during the follow-up), the aim of this section is to summarise the results rather than draw a statistical inference of a difference between groups or patterns of service use over time.
| Service use | Intervention | Control | ||||
|---|---|---|---|---|---|---|
| Baseline (N = 36) | 3 months (N = 30) | 6 months (N = 13) | Baseline (N = 36) | 3 months (N = 26) | 6 months (N = 15) | |
| GP consultations | ||||||
| Patients with at least one consultation, n (%) | 28 (77.8) | 20 (66.7) | 7 (53.8) | 29 (80.6) | 17 (60.7) | 8 (53.3) |
| Mean number of consultations for those who consulted at least once | 3.2 | 2.4 | 2.9 | 2.6 | 2.3 | 2.4 |
| Nurse consultations | ||||||
| Patients with at least one consultation, n (%) | 23 (63.9) | 19 (63.3) | 9 (69.2) | 24 (66.7) | 17 (60.7) | 12 (80) |
| Mean number of consultations for those who consulted at least once | 3.1 | 3.6 | 3.2 | 3.6 | 3.0 | 2.5 |
| A&E department or urgent care centre visits | ||||||
| Patients with at least one visit, n (%) | 4 (11.1) | 0 (0) | 1 (7.7) | 9 (25) | 5 (17.9) | 1 (6.7) |
| Mean number of visits for those who visited at least once | 2.7 | 0.0 | 1.0 | 2.0 | 1.2 | 1.0 |
| Pharmacy visits or appointments | ||||||
| Patients with at least one visit, n (%) | 1 (2.8) | 0 (0) | 0 (0) | 4 (11.1) | 0 (0) | 0 (0) |
| Mean number of visits for those who visited at least once | 2.0 | 0.0 | 0.0 | 2.0 | 0.0 | 0.0 |
| Contraception clinic consultations | ||||||
| Patients with at least one consultation, n (%) | 3 (8.3) | 1 (3.3) | 0 (0) | 0 (0) | 0 (0) | 1 (6.7) |
| Mean number of consultations for those who consulted at least once | 1.3 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 |
| Sexual health clinic consultations | ||||||
| Patients with at least one consultation, n (%) | 6 (16.7) | 4 (13.3) | 2 (15.4) | 1 (2.8) | 2 (7.1) | 0 (0) |
| Mean number of consultations for those who consulted at least once | 1.2 | 1.0 | 1.0 | 2.0 | 1.0 | 0.0 |
| Sexual Assault Referral Centre (SARC) consultations | ||||||
| Patients with at least one consultation, n (%) | 1 (2.8) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 0 (0) |
| Mean number of consultations for those who consulted at least once | 1.0 | 0.0 | 0.0 | 4.0 | 0.0 | 0.0 |
At baseline, GP and nurse consultations were most common in both the intervention arm and the control arm, with 77.8% and 63.9% of respondents reporting at least one consultation with a GP or a nurse, respectively, in the intervention arm. The figures were similar in the control arm at 80.6% and 66.7% of respondents with a GP or nurse consultation, respectively. Moreover, the mean number of consultations for those who consulted was also similar in the two groups at baseline. During the follow-up period, the proportion of patients with GP or nurse consultations reduced over time at a similar rate in both the intervention and control groups, except at the 6-month time point when a higher proportion of respondents in the control arm (80%) had at least one nurse consultation than in the intervention arm (69%). However, given the small number of respondents at the 6-month time point, any statistically meaningful conclusion cannot be drawn.
Table 17 presents a summary of sexual health-related reasons for consultations for those who had at least one consultation (note: this may include more than one consultation by the same patient). For GP consultations, the two most common reasons for consultations at baseline were ‘to get advice for discharge through vagina, penis or anus; or sores in genital area; or pain when passing urine’ and ‘to get the morning-after pill or to discuss contraception or family planning’. For nurse consultations, a small number of visits were related to sexual health, with the two most common reasons being ‘to get tested for HIV, hepatitis or other STIs’ and ‘to discuss other sexual health issues not listed, including sexual assault’.
| Reason for consultation (number of consultations) | Intervention | Control | ||||
|---|---|---|---|---|---|---|
| Baseline (n = 36) | 3 months (n = 30) | 6 months (n = 13) | Baseline (n = 36) | 3 months (n = 26) | 6 months (n = 15) | |
| GP consultations | ||||||
| To get advice for discharge through vagina, penis or anus; or sores in genital area; or pain when passing urine | 6 | 1 | 0 | 3 | 0 | 0 |
| To get tested for HIV, hepatitis or other STIs | 1 | 2 | 1 | 2 | 0 | 0 |
| To get vaccinated to prevent sexually-transmitted infections or hepatitis or human papilloma virus | 0 | 0 | 0 | 0 | 0 | 0 |
| To get the morning-after pill or to discuss contraception or family planning | 2 | 0 | 0 | 4 | 1 | 0 |
| To discuss termination of pregnancy | 0 | 0 | 0 | 1 | 0 | 0 |
| To discuss other sexual health issues not listed above, including sexual assault | 2 | 0 | 0 | 0 | 1 | 1 |
| Nurse consultations | ||||||
| To get advice for discharge through vagina, penis or anus; or sores in genital area; or pain when passing urine | 1 | 0 | 0 | 1 | 0 | 0 |
| To get tested for HIV, hepatitis or other STIs | 0 | 1 | 1 | 1 | 0 | 0 |
| To get vaccinated to prevent STIs or hepatitis or human papilloma virus | 0 | 0 | 0 | 0 | 0 | 0 |
| To get the morning-after pill or to discuss contraception or family planning | 0 | 1 | 0 | 0 | 0 | 0 |
| To discuss termination of pregnancy | 0 | 0 | 0 | 0 | 0 | 0 |
| To discuss other sexual health issues not listed above, including sexual assault | 1 | 1 | 0 | 0 | 1 | 1 |
| A&E department or urgent care centre visits | ||||||
| To get help for a STI | 0 | 0 | 0 | 0 | 0 | 0 |
| To get the morning-after pill | 0 | 0 | 0 | 0 | 0 | 0 |
| To get support after a sexual assault | 0 | 0 | 0 | 0 | 0 | 0 |
| To ask for treatment to prevent HIV infection after sexual intercourse (post exposure prophylaxis following sexual exposure) | 0 | 0 | 0 | 0 | 0 | 0 |
| Sexual health clinic consultations | ||||||
| To get advice for discharge through vagina, penis or anus; or sores in genital area; or pain when passing urine | 1 | 1 | 0 | 1 | 0 | 0 |
| To get tested for HIV, hepatitis or other STIs | 3 | 3 | 2 | 1 | 1 | 0 |
| To get vaccinated to prevent STIs or hepatitis or human papilloma virus | 1 | 0 | 1 | 0 | 0 | 0 |
| To get the morning-after pill or to discuss contraception or family planning | 2 | 1 | 0 | 1 | 2 | 0 |
| To discuss termination of pregnancy | 0 | 0 | 0 | 1 | 0 | 0 |
| To discuss other sexual health issues not listed above, including sexual assault | 0 | 0 | 0 | 0 | 0 | 0 |
| To discuss treatment to prevent (HIV pre exposure prophylaxis or post exposure prophylaxis) | 0 | 0 | 0 | 0 | 0 | 0 |
Table 16 shows that, at baseline, a higher number (and proportion) of usual-care respondents (9 respondents, 25%) had at least one A&E or urgent care centre visit which was higher than the figure in the treatment arm (4 respondents, 11%). During the follow-up, the number of A&E (and urgent care) visits reduced over time in both arms. However, more importantly, none of these visits at baseline or at follow-up time points were related to a sexual health reason (see Table 17).
Pharmacy visits or appointments were few in both groups at baseline as well as at follow-up time points. The use of contraception clinics was low in both groups, with only three respondents using them in the intervention group at baseline, reducing to one and none at 3 and 6 months, respectively. Only one respondent in the control group used the service at 6 months. This, together with other responses related to accessing contraception, suggests that respondents were more likely to get contraception/family planning advice or the morning-after pill from a sexual health clinic or their GP than from a contraception clinic.
Sexual health consultations were relatively high at baseline in the intervention group (6 respondents, 16.7%) compared with the control arm (one respondent, 2.8%). These consultations reduced over time in the intervention group and remained low in the control arm. The most common reasons for consultations at a sexual health clinic were ‘to get tested for HIV, hepatitis or other sexually-transmitted infections’ and ‘to get the morning-after pill or to discuss contraception or family planning’. The use of the Sexual Assault Referral Centre (SARC) was also very low, with only one respondent each in the intervention and control arms using the service at baseline and no respondents using it at follow-up.
Unit costs of health service use
Unit costs were obtained from national databases, when available, and otherwise from other published sources. Sources of and assumptions for unit costs are presented in Table 18.
| Service use | Unit cost (£) | Source |
|---|---|---|
| GP (per consultation) | 38.00 | PSSRU 201750 (p. 162) |
| Nurse (per consultation) | 10.50 | PSSRU 201750 (p. 160, assumed 15 minutes) |
| A&E visit | 147.80 | NHS Reference Costs 2016–17 (outpatient)49 |
| Pharmacy visit | 8.80 | NHS prescription cost |
| Contraception clinic consultation | 20.00 | Oral contraception in Pharmacy report (2012)56 |
| Sexual health clinic consultation | 25.60 | Crawford et al.57 |
| Mental health worker (per hour) | 39.00 | PSSRU 2017 (p. 186)50 |
Intervention cost
The intervention consisted of up to three 1-hour sessions of sexual health promotion delivered by a trained mental health worker. These sessions delivered specifically designed training material. A total of 25 participants attended at least one session, 19 attended two sessions and 17 attended all three sessions (see Table 18). However, in some cases (n = 5) based on participants’ preference, a ‘combo’ session was delivered that covered material for more than one session, which reduced the number of sessions required/attended.
The duration of the three sessions was similar, with the mean being 59.2 minutes for the first session and 57.1 and 57.8 minutes for sessions 2 and 3, respectively (Table 19). This is in line with the time allocated for these sessions (i.e. up to 60 minutes per session). The duration of the combo sessions was longer with the mean being 93.8 minutes. There was some variability in the length of sessions, which is clear from the minimum and maximum values.
| Training session | Participants, (n) | Mean | Minimum | Maximum | |||
|---|---|---|---|---|---|---|---|
| Minutes | Cost (£) | Minutes | Cost (£) | Minutes | Cost (£) | ||
| Session 1 | 24 | 59.2 | 38.50 | 35 | 22.80 | 115 | 74.80 |
| Session 2 | 19 | 57.1 | 37.10 | 33 | 21.50 | 90 | 58.50 |
| Session 3 | 17 | 57.8 | 37.60 | 40 | 26.00 | 75 | 48.80 |
| Combo sessions (i.e. material for two sessions covered in one) | 4a | 93.8 | 60.90 | 60 | 39.00 | 120 | 78.00 |
| Total of all sessions/material | 25 | 170.0 | 110.50 | 101 | 65.70 | 307.5 | 199.90 |
Data were also collected on the proportion of the training material covered across all sessions by each participant who attended at least one session. This was used to estimate the total time required to deliver the full material to each participant; for example, if two-thirds of the material was covered in two sessions lasting 55 minutes each, then the estimated time to deliver the full material for this particular participant would be 165 minutes. Based on this approach, the estimated mean time to deliver the full training material was 170 minutes (range 101–307.5 minutes) (see Table 19). This was then multiplied by the hourly rate of a mental health worker (who delivered these sessions) which is £39 per hour (see Table 19). Based on this, the estimated mean cost of delivering the training material was £110.50 (range £65.70–199.90). However, it should be noted that the maximum value represents one outlier participant with one long (non-combo) session and excluding this participant changes the maximum cost to £146.
In addition to the cost of delivering the intervention, we considered the cost of training the interventionists (mostly, mental health workers). This included two components: (1) the time cost of the interventionist and (2) the time cost of the trainer. The training log (see Table 22) shows that the training was delivered over 1–2 days and each training session had between one and four participants. Overall, 21 people working in the mental health services received training to deliver the intervention. Initially it was delivered over 2 days but in the later stages it was condensed into 1 day. Overall, 16 training days were delivered by the study team. Assuming a full day equals 8 hours, and using the hourly wage of £39 for mental health workers, the estimated time cost for the interventionist to receive the training was £237.70. Regarding the second component of the training cost, we assumed that in practice the training will be delivered by a mental health worker (although during the project the training was delivered by the principal investigator of the research project). As a result, the total cost of delivering the training, including the time cost of the interventionist as well as the trainer, was equal to £475.20. Because this is a one-off cost, it was not added to the intervention delivery cost. Finally, although all the professionals providing the training were offered the option of using supervision support, none of them used this option.
Total cost of health service resource use
Figure 6 presents the total health services resource use cost in the last 3 months, by intervention arm and time point. This does not include the intervention cost. At baseline, resource use cost was slightly higher in the control arm than in the intervention arm: this is partly because of a higher number of A&E visits in the control arm than in the intervention arm. Health services costs reduced over time in both the intervention arm and the control arm, with costs in the control arm being higher than in the intervention arm at the 3-month time point and lower at the 6-month time point. These figures should be interpreted with caution because of the small number of respondents, particularly at the 6-month follow-up (at 6 months, n = 13 in the intervention arm and n = 15 in the control arm).
FIGURE 6.
Health service resource use cost (excluding the intervention cost), by time point.
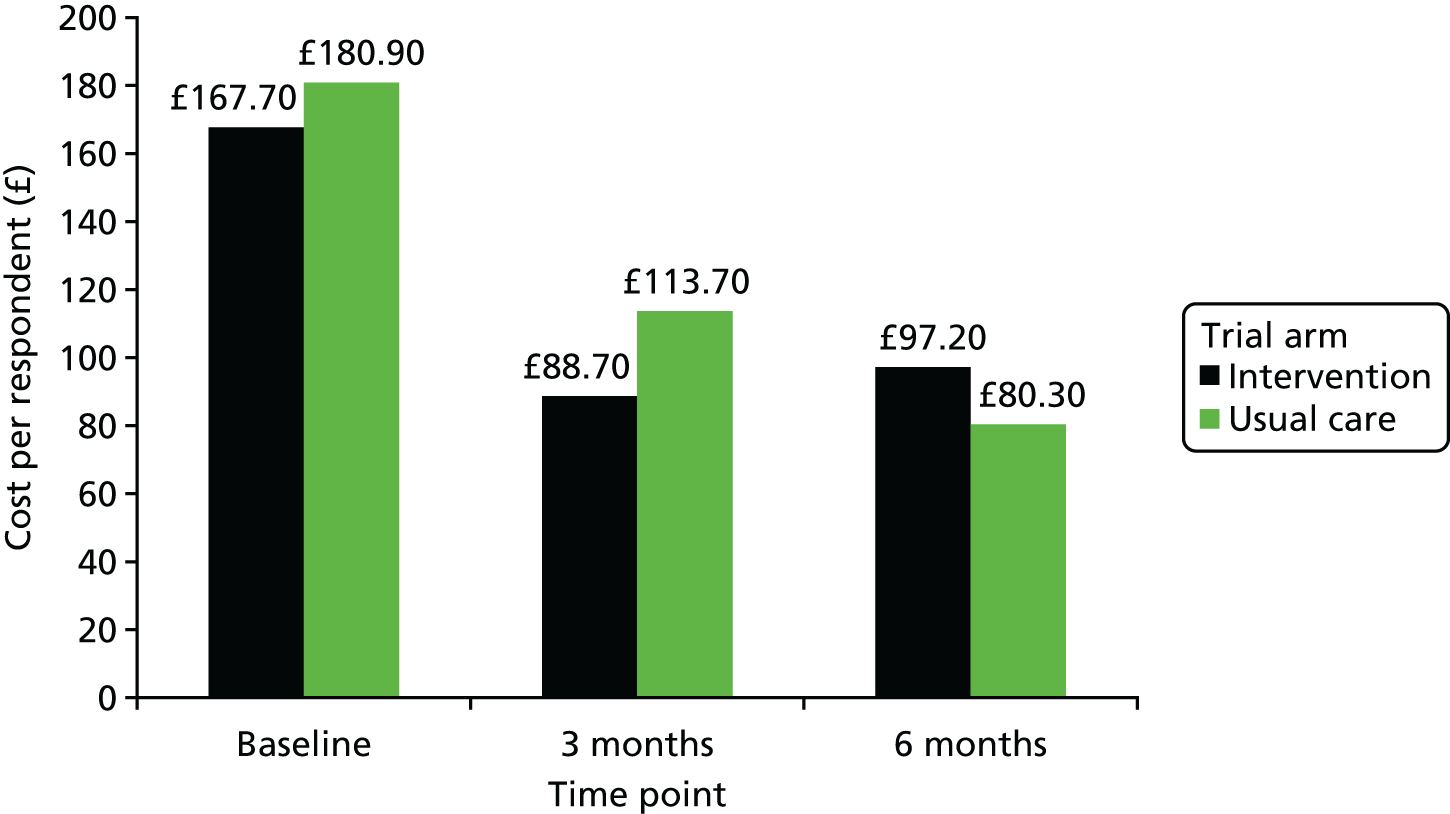
Health-related quality of life
Data completion
Table 20 shows the response rate for the EQ-5D questionnaire at baseline and at the 3-month and the 6-month follow-up. At baseline, all 36 respondents in the control arm completed all items in the questionnaire whereas one participant in the intervention arm did not complete the questionnaire. At the 3-month and the 6-month time points, EQ-5D completion rate was in line with the resource use questionnaire completion rate and the overall response rate (i.e. 30 and 13 responses at 3 months and 6 months, respectively, in the intervention arm, and 28 and 15 responses, respectively, in the control arm). Moreover, it is clear that, apart from those lost to follow-up, there were no missing data in EQ-5D items.
| Time point | Mobility (n) | Self care (n) | Usual activities (n) | Pain/discomfort (n) | Anxiety/depression (n) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Usual care | Treatment | Usual care | Treatment | Usual care | Treatment | Usual care | Treatment | Usual care | |
| Baseline | 35 | 36 | 35 | 36 | 35 | 36 | 35 | 36 | 35 | 36 |
| 3 months | 30 | 28 | 30 | 28 | 30 | 28 | 30 | 28 | 30 | 28 |
| 6 months | 13 | 15 | 13 | 15 | 13 | 15 | 13 | 15 | 13 | 15 |
Frequency and pattern of EQ-5D item responses
Figure 7 presents the distribution of completed responses to each domain or item of the EQ-5D questionnaire at baseline and at follow-up in the intervention arm. For mobility and self-care domains, over 70% of respondents in the intervention arm had no problem or slight problems at baseline and follow-up. However, there was a small shift in mobility from moderate problems at baseline and 3 months to improvement (slight problems) or worsening (severe problems) at 6 months. For the self-care domain, there was a small improvement over the follow-up period in the intervention arm, which was observed as a reduction in severe problems. For the usual activities domain, just over 50% of respondents in the intervention arm reported having some level of problems. There was a small improvement in usual activities at 6 months with no respondent reporting severe or extreme problems. For the pain and/or discomfort item, just under half of all respondents in the intervention arm reported having some level of problems at baseline. Over the follow-up period, the proportion of respondents with severe problems reduced but at the same time there were fewer respondents with no problems. Finally, for the anxiety and/or depression domain, most respondents in the intervention arm reported having some level of problems; however, there was a small improvement during the follow-up in terms of a reduction in respondents with severe or extreme problems.
FIGURE 7.
The EQ-5D responses at baseline (BL), 3 months (3M) and 6 months (6M) for the intervention group.
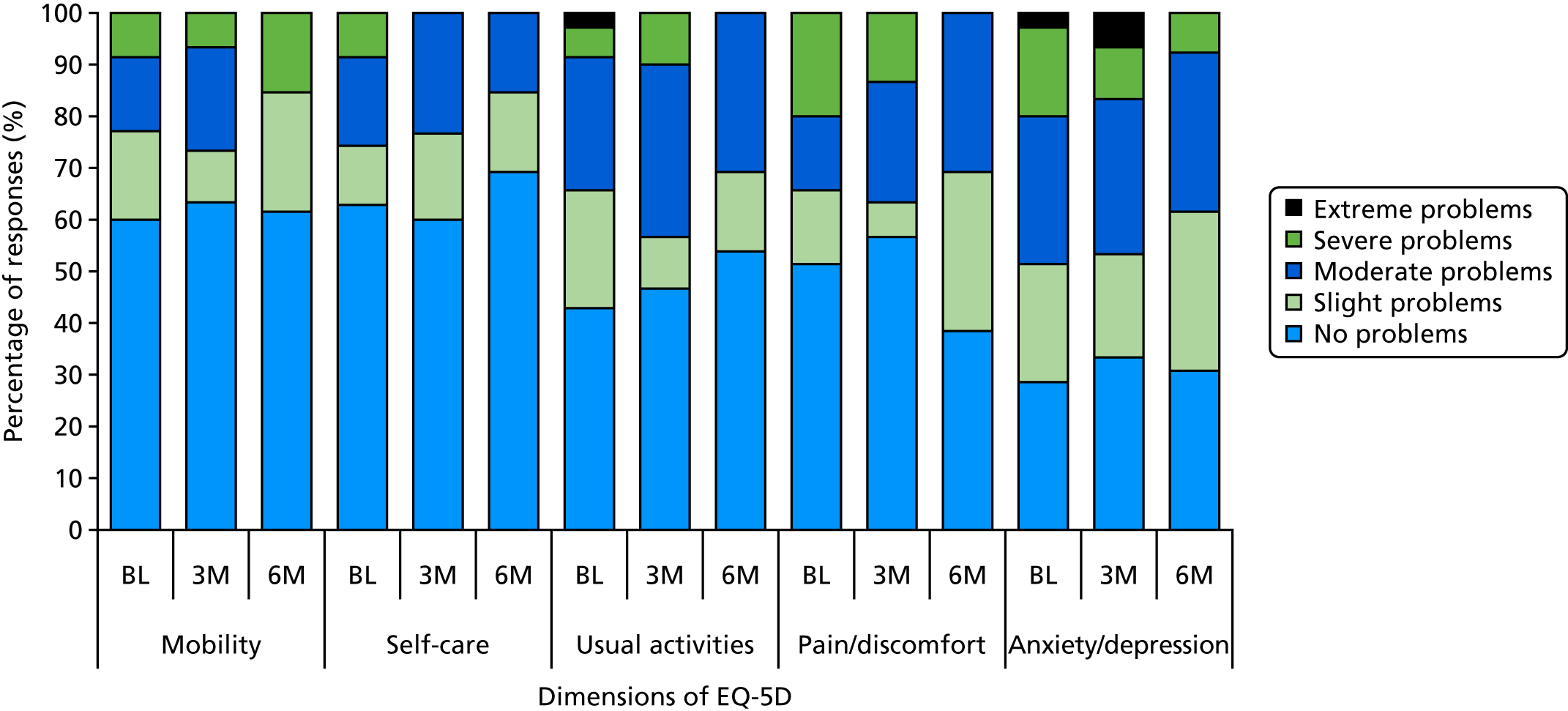
Figure 8 presents the distribution of completed responses to EQ-5D domains at baseline and follow-up in the control arm. Approximately 90% and 80% respondents in the control arm had no problem or slight problems at baseline on the mobility and self-care domains, respectively. This suggests that the control arm was slightly healthier at baseline than the intervention arm. The same was true for the usual activities, pain/discomfort and anxiety/depression domains at baseline. During the follow-up period, the mobility, self-care and pain/discomfort domains had minimal change but there was an increase in slight problems in the usual-care activities domain at 6 months compared with baseline. Finally, there was an increase in the percentage of respondents with no problems as well as those with moderate problems in terms of anxiety and/or depression.
FIGURE 8.
The EQ-5D responses at baseline (BL), 3 months (3M) and 6 months (6M) for the usual-care group.
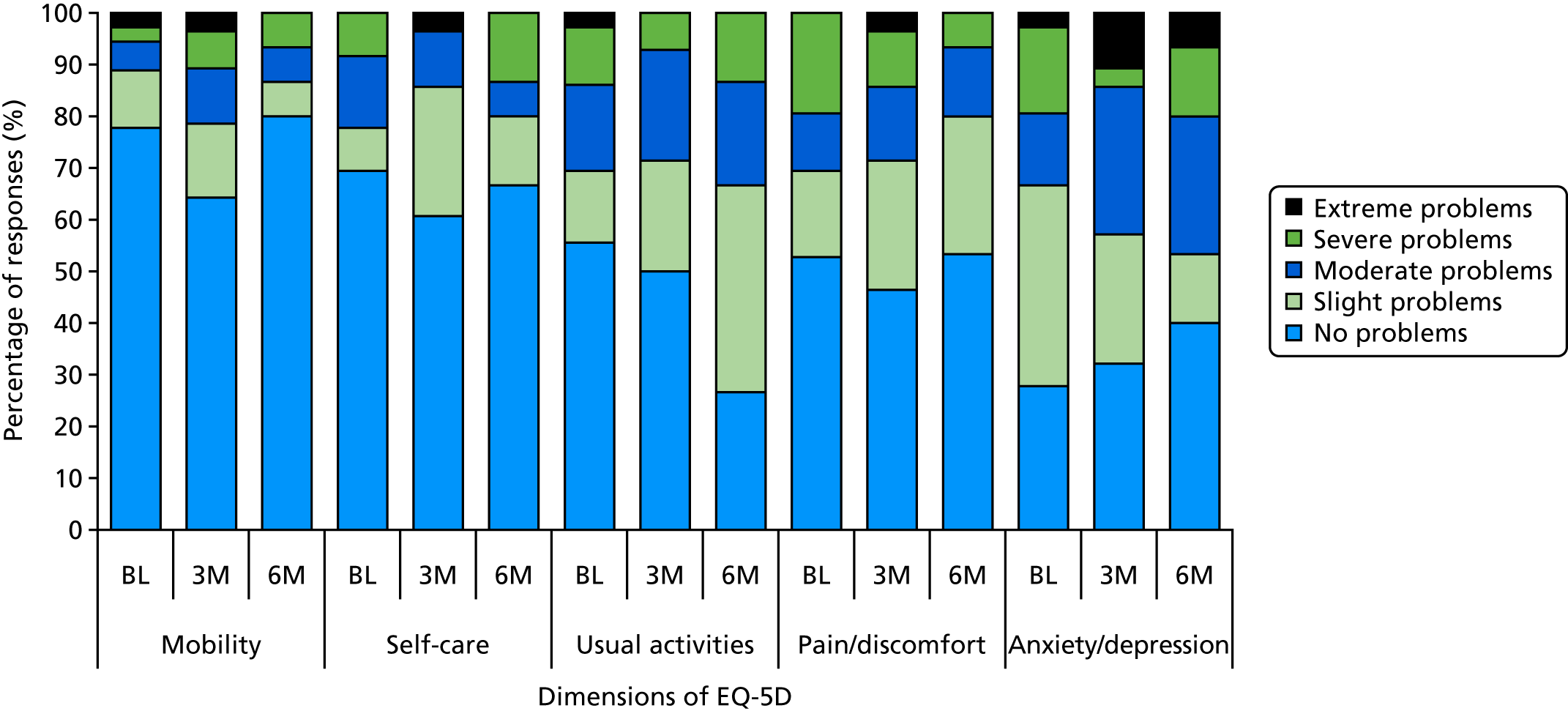
Health-related quality of life (utility values)
Figure 9 compares utility values for the 22 patients who completed three sessions of the intervention with the control arm. The figure shows greater improvement in utility value in the intervention arm than for usual care, and also than for the overall intervention group. However, as with the cost data, these results should be interpreted with caution as they represent only the complete cases and a small sample size. Moreover, the intervention group in Figure 9 represents a selected group who underwent the maximum number of sessions and may not represent the overall target population. This is in line with Figures 7 and 8, which also showed a slightly bigger improvement in quality-of-life domains in the intervention group than in the control group.
FIGURE 9.
Utility values based on EQ-5D-5L responses at baseline, 3 months and 6 months for the treatment (those who completed three sessions) and usual-care groups.
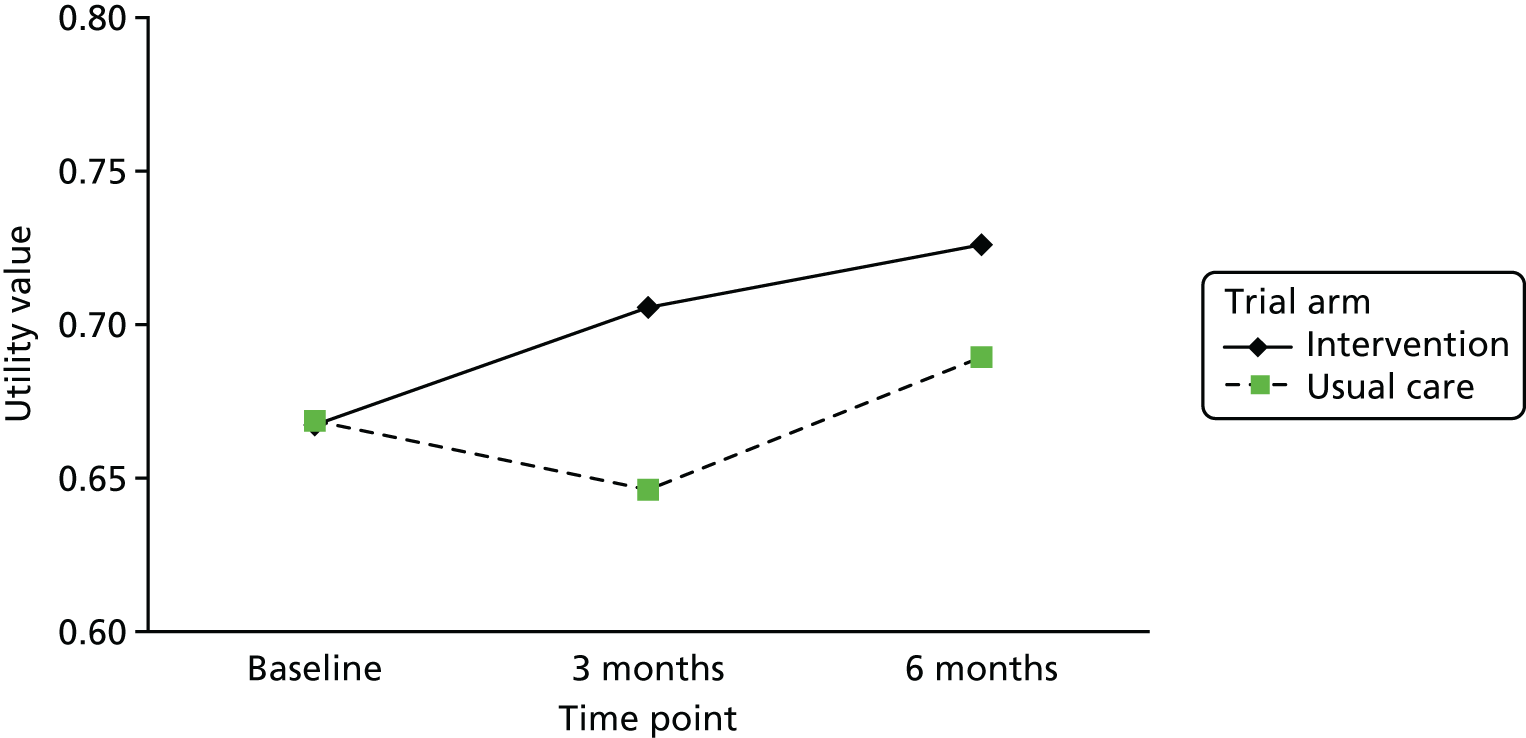
Chapter 6 Feedback
Recruitment: feedback from the NHS sites
The CRN staff at each site were asked to reflect on what worked well and less well in terms of implementing the study in their organisation and in meeting the recruitment targets. They were also asked for suggestions for improvements to the recruitment strategy.
South West Yorkshire Partnership NHS Foundation Trust
In the SWYFT, the study was only opened to recruitment in one locality (Barnsley), as this was the location of the staff who had been trained to deliver the study intervention. Participants were recruited from three core teams and the early intervention team. At the start of the study period, all the teams were using the care model of Assertive Outreach and Community (AOT) mental health teams. An initial approach was made to the AOT staff and 51 potentially eligible service users were identified. Study packs were given to staff to pass on to those service users at routine appointments. This only yielded one service user, who expressed a tentative interest but later declined to participate. However, it was difficult to assess how many of the packs had actually been given out to service users, and the monitoring form that was given to the CMHTs was poorly complied with. The remainder of those service users identified as eligible (n = 149) had information packs mailed to their home address. In total in SWYFT, eight people consented to participate and the vast majority were recruited via the mail-out method. One person at this site was recruited via a clinic visit by research staff.
Overall, giving study information to clinical staff for them to pass on to service users was unreliable and yielded no recruits at this site. The optimal approach at the SWYFT was posting the information and then doing a callback after a week or so. The research staff also recommend taking advantage of all available communication resources, for example using the trust service directory, asking team leaders to circulate information and promoting research on the trust’s electronic weekly newsletter. It has been useful for CSOs to base some of their working day at the CMHT hubs as those more informal conversations can be really useful in keeping research studies on the agenda. These are also places where service users attend appointments, so it was useful to maintain contact, have conversations and disseminate new study information.
Leeds and York Partnership NHS Foundation Trust
The LYPFT was the second best recruiting site and recruited 24 participants to the study. In terms of what went well, attending multidisciplinary team (MDT) meetings to talk about the study was identified as useful and it was felt even more useful if the study researchers were also able to attend those meetings. Attendance at these meetings allowed for preparatory work and conversations to occur prior to recruitment. As the recruitment period progressed, it was also useful to maintain a regular presence at the MDT monthly meetings during the recruitment phase. In addition to targeting the MDT community teams, LYPFT engaged with the psychiatrists. Although screening psychiatrists’ caseloads could be time-consuming, it ensured that all eligible service users received an invitation pack. The mail-out recruitment method seemed to work best for those people on psychiatrists’ caseloads as they see service users less frequently. As well as attending the MDT meetings, having face-to-face meetings with specific care co-ordinators was useful in terms of being able to properly discuss the aims of the study and eligibility criteria. Attending service user groups and mental health day centres, and talking to service users directly, also proved useful as they had the opportunity to ask questions, to express concerns and for discussion of those concerns. Staff mentioned that they felt that the recruitment packs contained too much information and that this was off-putting for both staff and service users. Because staff do not usually have e-mail contact with service users, sharing social media links or videos (YouTube videos about the study) (YouTube, LLC, San Bruno, CA, USA; www.youtube.com) was difficult through this medium, as was the use of social media to promote the study.
Camden and Islington NHS Foundation Trust
The C&IFT was the top recruiting site, with a total of 27 participants. The R&D team initially presented to teams (mainly rehabilitation and recovery teams) and obtained a couple of referrals from clinicians after these presentations. In addition, the team used the mail-out recruitment method and conducted some follow-up calls, resulting in a couple of CtoC forms. However, the majority of participants were recruited through making a direct and personal approach. This included the RESPECT study researcher attending the waiting rooms of clozapine and depot clinics (this resulted in 14 CtoCs being completed, which resulted in the recruitment of seven participants) and in the waiting room of one outpatient mental health service (which resulted in 12 CtoCs being completed, from which six service users agreed to participate). Ringing patients on the caseloads of psychiatrists from one locality resulted in 17 CtoCs being completed and 12 participants. The psychiatrists in the local recovery team have large caseloads of outpatients (in some cases > 200), so this proved a quick and effective method of reaching people.
Sussex Partnership NHS Foundation Trust
The SPFT recruited four participants. In this trust, when patients are first registered they have the option to opt-in to research; therefore, mail-outs and follow-up calls were made to participants who had opted-in and were potentially eligible. Attempts were made to engage with community mental health groups but the trust closed as a recruiting site for the RESPECT study early (because of a lack of staff resource to deliver the intervention) before these links could be fully established.
North East London NHS Foundation Trust
This site began recruiting in the autumn of 2017 and, despite a late start and being open for only about 8 weeks, it was able to recruit seven participants. Like SPFT, NEFLT also operates an opt-in to its research scheme, which means that all service users are asked to give permission to be contacted about studies that they may be eligible for by the R&D team as part of routine care. Using this opt-in list, NELFT was able to mail out study information to those on the list, targeting people who use the early intervention services and community recovery teams, and then make follow-up calls 2 weeks later. This method yielded all seven recruits within a few weeks of opening.
Feedback questionnaires
The recruitment study pack contained an initial feedback questionnaire that was designed to obtain data about the reasons why people chose (or declined) to participate in the RESPECT study. In addition, at the final appointment, participants were given an exit questionnaire.
Recruitment stage feedback questionnaire
A total of 158 completed feedback questionnaires were received. Most respondents felt that they understood what the RESPECT study was about (62%, n = 98) and they agreed that others with similar needs would benefit from the study results (59.5%, n = 94). Regarding the question on randomisation, only 15.8% (n = 25) agreed that they felt ‘worried’ about this aspect of the study, although the majority of people who answered this question disagreed with the statement (41.7%, n = 66). Overall, respondents did not feel that participation would be difficult because of practical reasons, with only 10.1% (n = 16) agreeing that this was an issue.
Almost one-third (27.2%, n = 43) of participants identified things that had helped them make their decision about whether or not to participate, including their desire to learn more about sexual health and previous concerns about sexual health (having had a STI in the past). Some mentioned that the PIS and other study materials had helped them to make the decision to participate. Some people stated that they wanted to take part in research as they wanted to contribute to mental health research. Other reasons to participate included flexibility in how appointments could be offered, the positive manner of the researchers and being able to talk through the study with family and/or staff.
Reasons some people declined to take part
Of those who completed the questionnaire, 42.4% decided not to take part (n = 67). The most frequent reason for declining was feeling that the topic was of no relevance to them. The specific reasons for it not being relevant included not being sexually active (n = 15), being in a stable relationship/not having casual sex (n = 10), being ‘too old’ (n = 9) and feeling that they had enough knowledge already (n = 4). Other reasons for declining included not wishing to discuss sexual health (n = 15) and not feeling comfortable with discussing the topic of sexual health (n = 9).
Only two responses related specifically to the study design/process: being put off by the time commitment for the data collection (the PIS stated that it could take up to 2 hours). Finally, six responses related to time pressures and commitment to the study as a whole.
Approximately one-third of respondents (30.4%, n = 38) provided further comments about the study. Generally there was support for the study, and even some people who had decided not to take part expressed the view that the study was a ‘good idea’ and that they were pleased that the ‘elephant in the room of psychiatry’ was finally being discussed.
Exit feedback questionnaire
A total of 45 participants (out of 72; 62.5%) completed the exit feedback questionnaire with 43 of the questionnaires being fully completed. All those who responded felt that it had been a good thing to participate in the trial. Only two expressed some minor concerns and anxieties, but even their responses were tempered with a positive overall view of the study process:
Fear of what the questions would be and revealing myself to someone but everyone was really excellent. The researcher went through it all with me.
I didn’t like some of the questions. Some of them were personal but I suppose it’s essential.
Anonymous participant quotations
Forty-three respondents (95.56%) said that they did not wish they had known anything else about the study prior to taking part and that they would recommend the study to others.
Thirty-three respondents (73.33%) provided reasons why they would (or would not) recommend the study to others. The most commonly reported reason was the value of gaining information about sexual health and relationships, and how they had enjoyed the experience, in particular of opening up a taboo conversation. Other reasons included how it felt good to give back and contribute to NHS mental health services and how they found the researchers and interventionists to be non-judgemental and friendly.
On the basis of the fully completed questionnaires, overall respondents felt that the RESPECT study had been helpful (93%, n = 40), they understood what the RESPECT study was about (95.3%, n = 41), they understood what it meant to be randomised (83.7%, n = 36), they felt that the researcher set up appointments at a convenient time (100%, n = 43), that the questionnaires did not take too long to complete (93%, n = 40) and that the questionnaires were easy to complete (88.4%, n = 38).
Logistics of intervention delivery
In the protocol, participants were expected to commence the intervention as soon as feasible after the baseline and allocation. Prior to the start of the study, staff were identified and received training regarding the study and the RESPECT study manual.
A total of 20 staff were trained to deliver the study intervention for the duration of the study. The first wave of training occurred in October 2016 and took place in two locations: Yorkshire and Brighton. In this wave sufficient staff in each site received the training to deliver the manual. However, the study did not receive HRA approval until the end of November 2016 and, consequently, the trial did not open to recruitment until January 2017. Recruitment was very slow initially and by the end of March only one participant had been recruited. This meant that there was a considerable delay between the training and delivery of the intervention. During this time some of the interventionists subsequently moved on to other jobs and were unable to be available to deliver the intervention (n = 2 in Sussex; n = 1 in Leeds). Therefore, to maintain a critical mass, further staff were identified at each site for delivery of the intervention and further training sessions were provided in Yorkshire (one session) and London (two sessions) to ensure capacity to deliver the study.
During the summer of 2017, it was decided to expand the number of sites involved after the Sussex site withdrew. Two sites were added later on in the recruitment period: NELFT and Community Links – aspire (a third-sector service for Early Intervention in Psychosis in Leeds). It took a few weeks to organise these additions, as the sites needed to be added formally as a HRA amendment, and then the sites needed to identify interventionists, book the RESPECT study training and confirm capacity and capability. However, the addition of these two sites did yield more participants. The NELFT recruited seven participants to the study and all were seen within the time frame. In addition, Community Links – aspire was able to recruit two people to the study and the participant allocated to intervention was able to receive the intervention according to the protocol timelines.
In the end, 11 different interventionists were involved in the actual delivery of the intervention in this trial and a total of 67 sessions were conducted (Table 21). This gives an average of 6.1 sessions per interventionist; however, it should be noted that one interventionist undertook 41.8% of the sessions (28 sessions) and one interventionist undertook only one session. On average, the sessions were 58 minutes long (excluding the combined sessions).
| Therapist | Session 1 | Session 2 | Session 3 | Combined sessions | Overall |
|---|---|---|---|---|---|
| A | 11 | 8 | 7 | 2 | 28 |
| B | 1 | 0 | 1 | 2 | 4 |
| C | 1 | 0 | 0 | 0 | 1 |
| D | 3 | 2 | 2 | 0 | 7 |
| E | 2 | 2 | 1 | 0 | 5 |
| F | 1 | 1 | 1 | 0 | 3 |
| G | 2 | 2 | 2 | 0 | 6 |
| H | 1 | 1 | 1 | 0 | 3 |
| I | 1 | 1 | 1 | 0 | 3 |
| J | 2 | 2 | 2 | 0 | 5 |
| K | 0 | 0 | 0 | 1 | 1 |
Overall, the intervention was delivered as planned and in the protocol time frame, but there were a few periods when this was not the case.
Challenges in delivery of intervention
Despite recruiting the most participants to the study, C&IFT experienced the most significant problems in the actual intervention delivery. The details of the various training waves can be seen in Table 22. The original group trained in wave 1 had consisted of three research nurses who also worked in the R&D office. It was only after they had received training in Brighton that it became apparent that they would not be able to deliver the intervention in practice because it was a conflict with their role in the CRN. So, after discussion with the local principal investigator, a mental health nurse (who worked as a bank nurse at C&IFT) was identified as a suitable person, and had the flexibility to be able to deliver the intervention. They were trained in the intervention in early February 2017 (shortly after the study recruitment had opened) but the first recruit to the study at C&IFT did not occur until 25 May 2017 (allocated to usual care) and the first person to be allocated to intervention did not happen until 13 July 2017 (almost 5 months after the person had received the training to deliver the intervention). The interventionist was able to deliver the intervention to this participant but, after this point, the interventionist had a change of circumstance meaning that they could no longer continue to deliver the intervention (in August 2017). Owing to the lack of an interventionist, recruitment at C&IFT had to be paused (despite having received many CtoC forms) as there was no-one available to deliver the intervention. The local principal investigator identified two people who had experience of working with people with SMI who would be available to deliver the intervention as a ‘bank contract’, but they were required to obtain a contract with the NHS site to do this. They were able to receive the intervention training in early September but there was a significant delay in getting the contract processed with the NHS trust. This delay meant a significant backlog in recruits and also explains the spike in recruitment in November and December. Unfortunately, during this delay, one of the trained interventionists changed jobs and could no longer deliver the intervention. Hence, only one person remained active at C&IFT and this also explains why the site delivered 40% of the intervention (C&IFT was the best recruitment site).
| Site | Numbers trained | Date of training | Outcome |
|---|---|---|---|
| LYPFT | 7 |
Wave 1 (three staff members) – 3 October 2017 Wave 2 (four staff members) – 17/18 July |
One person (ID1) moved jobs and was no longer able to deliver the intervention and another (ID2) was allocated two participants but did not deliver the intervention – reasons being that the participant did not attend any sessions and a significant delay in booking the intervention owing to the interventionist’s own circumstances led to the participant cancelling the sessions Three of the four staff trained in the second wave did not go on to deliver the intervention (confusion about expectations to deliver it following training) One of the three staff delivered one session only One staff member delivered the intervention as planned |
| Community Links – aspire | 1 | One staff member – 17 and 18 July | Delivered as planned |
| SWYFT | 2 | Two team members – 3 October 2017 | Delivered as planned |
| C&IFT | 6 |
Wave 1 (three research nurses) 5–7 October Wave 2 (community mental health nurse on the bank) – 6/7 February 2017 Wave 3 (mental health workers on bank contract) – 5 September 2017 |
Research nurses were unable to deliver any sessions because of conflict with their CRN role Bank nurse #1 delivered only one set of interventions and then changed role – moved jobs One person moved jobs before bank contract in place One person delivered as planned |
| NELFT | 2 | Clinical psychologists – 11 October 2017 | Delivered as planned |
| Sussex | 3 |
Wave 1 – two staff members 5 October 2016 Wave 2 – one staff member – 6/7 February 2017 |
One person moved jobs and did not deliver any intervention sessions (both done by EH) |
| Total | 21 |
Of the 21 people who received the training to deliver the intervention, almost half (n = 10) were not able to deliver it at all as a result of changes in their circumstances (changed job or moved away from area).
Qualitative interview results
A subsample of participants (n = 22) agreed to take part in telephone interviews to examine their views and experiences in relation to being a participant and, if allocated to it, their views on the intervention. A set of a priori questions were developed and a framework analysis method was used to analyse the responses.
Assessment of recruitment strategies used
The opening question asked participants how they found out about the study and the responses indicated that the participants had been recruited via a range of recruitment strategies. These included through face-to-face conversations with their keyworker, receiving a letter through the post from their care co-ordinator, being handed an information leaflet during a visit to a clinic (by the research team), seeing the trial poster/leaflet in a clinical area and attending a day centre presentation by the research team.
Although none of the participants expressed disapproval about particular methods, there was a clear theme of preference for a conversation as well as receiving information. For example, one participant (who had received study information by post) talked about the difficulty she has in opening her mail, as well as being able to read detailed information and then responding to the mail:
I think probably inform people’s care co-ordinators, just in general, just say OK, you know, we have a research study, just inform your clients you know, because a lot of people don’t reply to post and a lot of mental health service users, I know for me, sometimes I don’t want to open my mail.
Participant 22, female, intervention
Another participant, who also received information by post, felt that he would have liked a prior conversation with a member of staff:
. . . my co-ordinator . . . they could have let me know beforehand . . .
Participant 3, male, usual care
A participant who had found out about the study from their care co-ordinator suggested that this method was suitable and a good way of approaching others. Other participants described it as a useful approach because they saw their nurse often and it offered an opportunity to ask questions.
Other suggested ways to promote the study included promotion via the radio, social media postings from mental health charities and sexual health services, leaflets via their GP and through inpatient hospital settings (specifically approaching service users).
There were also some comments about the volume of information received (study pack). It was suggested that initial written information should be brief (leaflet, social media posting) or the study pack should be handed out with a verbal discussion:
Tell them verbally, I mean obviously you can’t go around to everybody individually and say can you take part in our study, but telling departments in mental health services, telling people at [facility name redacted], you know, pass these along to your clients, because like I said, a lot of people don’t open their mail, or they’re not well enough to be able to, you know, read or want to understand what’s going on and sometimes, I know for myself, I’ve missed opportunities like that. When I think of it now, I kind of think well I could have actually gone for it if I did probably sit down, but then someone would still need to have been able to reiterate that to me and explain it to me.
Participant 22, female, intervention
In thinking about how a future study could optimise recruitment, participant 22 suggested a more graduated approach to information giving during the recruitment phase.
The face-to-face (and/or telephone) conversations about the study were really valued and helped people to understand the study better and make informed decisions to take part or not.
Acceptability of the participant information sheet
Participants were asked to recall and express their opinion of the PIS used in study. Participants were prompted to think about the amount of information and the readability.
Most could recall the information sheet and described it as easy to understand, with enough information. One participant described it as:
Informative without being boring.
Participant 17, female, intervention
However, five of the participants described the PIS as being ‘too much’: too much information, reading and paperwork. Some participants suggested that we simplify the language for future use. One participant expressed the view:
I was really struggling to be honest, getting to read past the first two lines [Interviewer: Right]. I think it’s probably my mental health that’s affecting me.
Participant 11, female, usual care
Acceptability and understanding of randomisation
During the interview, participants were asked to recall the process of randomisation (a prompt was offered) and offer their view:
You might recall the randomisation process – where all participants were put into one of two groups: ‘sexual health intervention’ or ‘usual care’. A computer chose which of the two groups people went into. What did you think about that?
Interviewer
Most of the participants (n = 17) could recall the process of randomisation and described the process as ‘good’ and ‘fair’:
I think that’s a fair way of doing it, yep, that was, you know, randomisation, is, it’s the only way really to do something like that, so that was fine with me.
Participant 13, female, usual care
I don’t mind it randomised, as long as it helps you guys choose the best option for me.
Participant 20, male, usual care
Four participants were unable to recall the randomisation process at all and four expressed some confusion regarding the purpose and the process of randomisation:
I’ve got to be honest, I didn’t really understand it and I’m quite bright but it was, it kind of, it’s either yeah, it was convoluted.
Participant 6, female, intervention
I felt a little bit anxious not knowing which group I’ll end up in.
Participant 11, female, usual care
However, overall, most had found the process of randomisation an acceptable feature of the trial.
Acceptability of the data collection procedure
Participants were asked to give their view on several features of the data collection procedure, which included their experience of meeting the researcher, the location, the duration, the research questions, and the payment for their time (£10 voucher). Each of these will be discussed separately.
Meeting the researcher
Arranging meetings with the RESPECT study researchers was generally extremely positive. Participants described it as ‘good’, ‘very easy’, ‘simple’, ‘convenient’ and ‘flexible’. Researchers were described as ‘pleasant’, ‘approachable’; ‘easy to talk to’, ‘professional’ and ‘friendly’. Participants reported feeling ‘comfortable’ and ‘reassured’. Two participants reported having some difficulty in arranging the appointment. On both occasions it was as a result of logistical issues (i.e. the participant had limited availability because of their own work commitments and limited room availability in a local clinic).
They had a couple of cancellations, but we managed to make the arrangement, we were just trying to find an hour out of my time, with me working and stuff.
Participant 1, male, intervention
The location
Participants were offered the choice of meeting the researcher at home or at a local clinic. Participant preference was fairly evenly split with similar (yet contrasting) reasons offered; for example, feeling ‘safe’ was associated with being in the home and/or clinic, being out of the house was described as both something difficult and something favourable. Twelve participants opted for a home appointment. Their reasons varied:
I’m a bit of a recluse, so it was nice that she could come out to me. . . . I felt that it was more private in my home than it would be in an office. . . . I don’t like actually going to the mental health centre at all.
Participant 6, female, intervention
Obviously it’s more convenient for her to come [to my home] and she was very prepared and it wasn’t very intrusive, which is good, so I could just sit on my couch and do it, which was good.
Participant 17, female, intervention
Similarly, nine participants opted to see the researcher in a local clinic as it felt like a safe environment for appointments. One participant expressed a strong preference:
How would you have felt if they had taken place at home?
I really wouldn’t have liked that very much actually, I must admit, I wouldn’t have liked that as much at all.
Finally, one participant chose to meet the researcher at a day centre (the location where they first met the researcher and heard about the study). The research team endeavoured to offer a consistent approach to the data collection procedure (i.e. the same researcher, location, time of day, etc.). Seeing the same researcher throughout the study was described as helpful:
It was the same person that came to see me, so that helped as well.
Participant 4, male, intervention
Duration of the visit
Most of the participants recalled the duration of the (researcher) visit taking between 1 and 2 hours, and for most this was described as ‘OK’, ‘just about the right amount’ or ‘perfect’. Two participants commented on the duration being OK for them but maybe not for other service users. One participant felt that the visit could have been longer and another expressed how the visit provided him with company:
It were alright, because I mean I don’t get nobody who comes out anyway like, so it was a bit of company.
Participant 3, male, usual care
Six of the participants found the duration of the visit too long. One participant felt that the visit could be separated into two parts.
The research questions (the measures)
Information on sexual risk behaviour (in the last 3 months), attitudes towards condoms and condom use, knowledge about STIs, contraception and family planning, quality of life, substance use and sexual stigma was collected using the various measures listed (see Chapter 3, Secondary outcome assessment, for further details). The participants expressed very mixed responses to the questions. Some participants described the questions as ‘fine’, ‘interesting’ and ‘funny’, others described them as ‘intense’, ‘intrusive’, ‘difficult’ and ‘repetitive’. Issues raised included feelings that the questions elicited, the focus and relevance of the questions, the amount and type of questions and the gender of the person asking the questions (all researchers were female).
Two male participants described feeling ‘awkward’ (see excerpts below). Participant 2 described the feeling as generally related to talking about ‘sexual stuff’ and acknowledged feeling reassured by the researcher:
You just feel awkward don’t you, I think, when you’re talking about like sexual stuff and that.
So you felt a little bit awkward in that data collection. Was there anything they could have done to help?
No, she were quite good at like saying, you know, it doesn’t have to feel daft or owt because, you know, she was good at that, you know.
. . . so she reassured you.
Yeah.
One participant (participant 3, male) expressed some surprise as to the nature of the questions (despite having conversations with the CRN staff and the RESPECT study researcher about the study). He described feeling ‘gobsmacked’ that there was a study about sexual health. He also felt ‘disgusted’ by the questions about ever having had a STI but overall was happy to participate.
Other participant responses captured views about the quantity, content and other factors (i.e. relationship status):
There were lots and lots and lots of questions. There were a lot more than what I anticipated, a bit tense, a bit in-depth, some very personal questions as well, which I never anticipated them to be so deep.
[then, talking about follow-up interviews] . . . having to repeat the questions again, you know, about asking your knowledge on say, for example, HIV and AIDS and all that. But I understand where they were coming from, asking me again, because things might have changed since the last time you saw them.
Participant 5, female, usual care
It’s a lot to take in on the first visit, because like I say, it’s near enough an hour long, so and you’re being passed bits of paper and being told different things. So it maybe, maybe it could take two visits, just to go over it, because it’s a lot to face in one go.
Participant 4, male, intervention
Other responses focused on the types of questions asked. Questions were described as ‘monotonous’, ‘bipolar’ (unable to select a neutral response) and ‘repetitive’:
. . . [the questions] kept asking me for my sexuality, am I straight and I kept being asked about this, that and the other and I just thought maybe I shouldn’t have to repeat that every time . . . sometimes I probably didn’t answer the questions the right way that I did the first time, because I just wanted to hurry up and get through the interview.
Participant 5, female, usual care
One participant expressed disappointment in there being too much emphasis on the physical aspects of sex and a lack of questions relating to relationships and self-esteem, etc.:
I was a bit disappointed that the emphasis was so much on the physical aspects of sex – how often, who with, when, did you take drugs or alcohol with it, rather than how has your illness affected your ability to see yourself as a sexual person, your self-esteem, the way that you think your partner sees you, has your medication interfered with your sexual performance at all?
Participant 9, male, intervention
In response to being asked whether or not more information about the types of questions would have helped, one participant confirmed that the information provided offered a ‘fair idea’:
I think I had enough information, I had a fair idea of what it was going to be, what sort of questions there were going to be, from the information that was provided.
Participant 12, male, usual care
Positive responses to the questions included feeling reassured about confidentiality, openness (of the participant) and appropriateness of the questions:
It was all confidential and it was between me and the researcher and the other person who came to my house, so it was fine to talk about that really and then, yeah, it was OK.
Participant 15, female, intervention
I’m quite open about stuff like that, so I didn’t mind it . . .
Participant 17, female, intervention
It was quite interesting, it made me think that I needed to brush up on a few things, for example sexual health knowledge, for example how like things are transmitted and stuff because I’d clearly to read up on really. I think the questions were appropriate, suitable, well scaled, in terms of the scoring scale and stuff. So I thought it was good.
Participant 18, male, usual care
I found most of them fine and fairly easy to answer.
Participant 6, female, intervention
Payment
Overall, participants responded positively to the offering of a £10 Love2shop voucher (www.love2shopbusiness.co.uk) each time they met with a researcher. The amount was described as ‘right’ and most participants responded positively to the type of voucher. One participant expressed a preference for the voucher, as opposed to cash:
I’m tempted to say the voucher is better than cash because it means you have to spend it on, you know, something from one of the stores that you can spend it in. You couldn’t just, you know, go and buy a loaf of bread and whatever with it.
Participant 14, male, usual care
Acceptability of the intervention content and delivery
Most of the participants (n = 12, intervention) positively recalled the content and delivery (including location and duration) of the intervention sessions. Participants’ responses captured how the intervention not only covered ‘helpful’ content, but it also provided a ‘human face’ and a space/service to talk about sex and relationships and the associated affective experiences:
Kind of more natural, it wasn’t just a questionnaire, you know, we had practising putting on a condom and I actually showed him how to put one on, but, you know, it was nice, you know, it was sort of the human face of it really.
Participant 6, female, intervention
I actually need to talk about this [a previous sexual relationship] because it’s to do with sex and there wasn’t any sort of provision for like, you know.
Participant 6, female, intervention
It was quite good though, to talk about, you know, relationships and how they affect you.
Participant 21, male, intervention
It was very good, it was good to express myself.
Participant 19, female, intervention
The different features of delivery (i.e. arranging the sessions, the interactive and practical elements, the location and duration) were all acceptable. Participants experienced the intervention at their home or in a local clinic and both locations were described as ‘good’ or ‘comfortable’.
The sessions were described as ‘relaxed’, ‘helpful’, ‘interactive’, ‘informative’ and ‘an education’:
The quizzes were, were good, some things were kind of obvious, but some things weren’t, you know. I didn’t know stuff about AIDS that I really should know and things like that . . .
Participant 6, female, intervention
They were quite informative and they used these little sort of laminated sort of pieces of paper and moved those around a lot and so it, it’s a good technique to talk about things and you get to sort of think about things.
Participant 15, female, intervention
They [the sessions] were quite relaxed, just took place in like a room, he, it was like quite interactive, which is good . . . it was really educational and good. I felt quite comfortable doing it.
Participant 17, female, intervention
All of the participants felt that the length of session (1 hour) was acceptable and allowed enough time to cover the planned materials. Only one participant suggested that an extra visit(s) might be useful:
They’re [the sessions] not a bad length, because there’s enough time to cover quite a lot. I found that we kind of covered, for the first two or three, we kind of covered everything a little bit quicker, because like I said, its, I had some knowledge of it myself anyway. But then obviously when we started getting into things like the STIs and the stuff like that, then it possibly could be, not a bit longer, say an extra one or two visits, just to cover them and go into a bit more depth.
Participant 4, male, intervention
Other participants suggested improvements focused on content, for example more information on how to use (and where to find) the female condom and spending more time discussing assertiveness in relationships:
There were a lot of questions and you know, testing your knowledge and that and it wasn’t so much that I were lacking, its more assertiveness in relationships that I lack, you know what I mean.
Participant 2, male, intervention
I asked these things, can you show me how to use a female condom and she answered well we don’t have any, it’s not something that was catered for very, very well.
Participant 22, female, intervention
Other comments about the trial and suggested improvements
Given the sensitive nature of the trial, we were very interested to find out the reasons participants chose to take part and whether or not they had any initial (or ongoing) concerns.
Reasons for participation
The most common reason related to helping those services that had helped them, through participation in research. Others referred to their interest in the (novel) research topic or research generally and some were clearly motivated by a wish to challenge the associated stigma:
It’s one of them subjects that not a lot of people talk about. Quite a lot of people shy away and are very prude. With me, you know what I mean, if I can do anything that helps, then yeah, I’m all for it.
Participant 4, male, intervention
Anything to help and I felt that I wanted to help young people with mental health problems understand the importance of using protection.
Participant 6, female, intervention
I wanted to find out more.
Participant 7, female, usual care
For me, it was taking part because I believe it’s a very important topic, sexuality.
Participant 8, female, intervention
Because it’s probably an area I needed to address for my own personal well-being.
Participant 5, female, usual care
I’m going through a time in my life when I’m trying to make use of all available opportunities to me and take part in everything that I can take part in.
Participant 14, male, usual care
Some participants either spoke of their own benefit, relating to the topic area, or in a general sense (i.e. they wished to use their time in a helpful way). Three participants mentioned the financial incentive (the voucher).
Some participants expressed some initial concerns at the start of the study that related to confidentiality, specifically related to the ‘action plan’ developed through the intervention sessions and whether or not this information would be shared with the participants’ community psychiatric nurse (note that it was only shared with the participants’ written permission). There was some anxiety about the topic and the questions they might be asked, arranging interviews at times that were convenient and meeting someone new. However, all felt that they had been reassured about these initial concerns during the study. Consistent approaches such as seeing the same researcher every time and using the same locations had helped ease concerns about meeting someone new:
I think for me personally, it was meeting someone I didn’t know. But taking part, other than that, was, I was fine with it. What helped was it was in my own environment. Other than that, I spoke to them prior, until they visited and it was the same person that came to see me, so that helped as well.
Participant 4, male, intervention
Two participants reported initial concerns about the topic and one participant acknowledged the topic as ‘sensitive’ but not a concern:
I think obviously knowing that it was about sexual health, was a little bit, made it a little bit more sensitive, perhaps, than taking part in another study. But I wasn’t daunted by the idea of that.
Participant 13, female, usual care
One participant expressed relief that the study did not ask about particular experiences that he was not willing to disclose:
There were some sexual experiences I’ve had that I didn’t want to talk about and I tried to not think about, let alone talk about and I was slightly worried that there may be questions that touched on those experiences. But in the end, there weren’t at all, I didn’t even have to mention that I didn’t want to talk about these experiences, it just didn’t touch on them really.
Participant 14, male, usual care
Five participants in the intervention arm mentioned that they had never discussed sexual health with a mental health clinician previously. Three participants did report having discussed sexual health in their mental health care.
One participant reported going on to have a conversation about sexual health with their care co-ordinator following the intervention, and they felt the study has served as an ‘ice breaker’. She described a change in her perception of the role of the care co-ordinator and feeling less ‘bad talking about it’:
In terms of maybe safety, I think I actually have since, actually since, afterwards, I’ve now discussed it more with my like care co-ordinator.
And was it due to the intervention, or not?
I think so, I think it was a good like ice breaker and now I don’t feel so bad talking about it and I realise that it is something that I can talk to them about, whereas before I wouldn’t necessarily have thought about.
Two male participants went on to describe similar reasons why they do not discuss issues of sexual nature with certain mental health clinicians involved in their care:
I think it’s still a bit taboo, you know, I think with mental health professionals, you don’t, because they’re your prescribing doctors as well. If you talk about it with them, it’s not necessarily confidential, a lot of it would be, you know, it would go as part of your psychiatric test. Whereas if it’s a third party, like yourselves, or a psychologist, you know, it doesn’t get written down in the same sort of way.
Participant 21, male, intervention
It’s quite funny who you talk to about this, because if I was to talk like you’re talking to me now, if I was to talk to a doctor like you’re talking to me now, I’d probably end up on a section. I don’t think the psychiatrist would, but it moves in different circles. In my first, I think my first ever section that I went on was because of whatever was going on sexually with myself and they found that offensive, or inappropriate, so you’ve got to be careful really, you haven’t, but I have got to be careful how I talk about these things to whoever I’m talking to.
Participant 10, male, usual care
Almost all of the participants stated that they would positively recommend the trial to others. Two participants acknowledged issues with future participation, relating to a person’s circumstances at the time and their well-being, and the other participant offered a cautionary note about the questions but went on to state the benefits of taking part:
I wouldn’t say it would be something that everyone would want to take part in. I mean it depends where people are in their lives, I think, as to whether or not they’d want to take part. But someone who’s having a crisis wouldn’t want to be dealing with it.
Participant 12, male, usual care
Just be prepared for some unexpected questions, personal ones, and answer it the best you can, that’s all you can do, just be open and honest, because it does actually, on reflection, it makes you, you know, as I say, look at your own behaviour. Like for me, searching for love, making me think about, I need to stop doing it. So it does, it makes you think about your own behaviour and what you’re doing and your sexuality as well, I guess.
Participant 5, female, usual care
Other participants also talked about the ways in which the trial was helpful to them. They referred to changes in their perceptions of risk, behavioural changes (both current and future), increased knowledge, including the opportunity to learn about STIs and to be offered condoms, and the therapeutic benefit of talking about sex and relationships:
It was kind of a little bit of, you know, therapy for me, you know, you know, talking about it really.
Participant 6, female, intervention
OK you might not be able to get pregnant, but you know, you can get diseases.
Participant 6, female, intervention
As far as I’m concerned, the next opportunity I have in that manner, I’m going to make sure that I am well protected, thank you.
Participant 8, female, intervention
The study, yes, definitely, it’s like educational, so like increase and improve my knowledge, because I haven’t really studied it or looked at it in that detail since school and there’s been developments, so yeah, it was definitely helpful.
Participant 17, female, intervention
Suggested improvements
The participants suggested that a number of areas of the study and the intervention that could be improved. There were some suggestions that the researchers should be the same gender as the participant (or at least have the choice). They also suggested that recruitment could be targeted towards people who are ready to be discharged from hospital. In terms of recruitment, they recommended keeping a range of methods but also having a face-to-face conversation or telephone call to assist in understanding the study information:
The one thing I thought was quite odd was, or what could be improved could maybe to be able to choose the gender of the researcher. Not because I had any problem with her being a female kind of interviewer, but I just, if it had to be something that I felt odd about, it would be the, yeah, automatically female interviewer, was one area where you could maybe have a choice.
Participant 14, male, usual care
In terms of the questions, more clarity in relation to the number, type, content (and purpose) of the questions is recommended:
If I was doing the survey myself, I probably wouldn’t have asked as many questions.
Participant 10, male, usual care
Some of the questions were also considered a little ‘outdated’. For example, the addition of a ‘friends with benefits’ category (in the demographic information section) and legal highs [in substance use questionnaire(s)] was also suggested:
The family has changed in the last decade and also women have as well and we, you know, just completely different roles isn’t it. Yeah, I did find myself in a situation, I wouldn’t say it’s something that I, but yeah, it should be put in a box, do you have a friend with benefits, rather than saying are you in a relationship.
Participant 8, female, intervention
I think just include kind of legal highs or so-called research chemicals, I think you kind of stuck mostly to, you know, the traditional drugs that most people know about [I: Right OK]. But actually a lot of people nowadays in particular as well, young people are ordering drugs from the internet or may have taken legal highs or drugs that were legal highs at one point but are now illegal.
Participant 14, male, usual care
Suggested improvements to the intervention included more of a focus on assertiveness and more information about female condoms (how to use them/where to access them), as mentioned previously in Acceptability of the intervention content and delivery.
Chapter 7 Impact
The study team utilised multiple opportunities to raise the profile of the topic and the study using local national and international forums throughout the study period. Table 23 summarises these activities up to the point of submission of the report in August 2018.
| Dissemination event/engagement activities | Date |
|---|---|
| Presentation at an invitation-only meeting of results to co-investigators and other involved research staff | 2018 |
| Presentation: CLAHRC National Multimorbidity Research Event | 2018 |
| Chief Investigator (EH) engaged in a Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com) chat led by Cochrane on sex and health care | 2017 |
| Presentation: CRN event, Yorkshire and Humber. Engaging community mental health staff in recruiting people with severe mental illness to a feasibility trial to improve sexual health | 2017 |
| Newsletter to CMHTs across participating NHS sites | 2017/18 |
| Presentation: Engage Research Festival, University of Huddersfield | 2017 |
| Participation in workshop as part of Wakefield Recovery College Programme – Sexual Health in Mental Health | 2018 |
| Participation in workshop, psychiatry CPD event, Manchester. Sexual Health in Serious Mental Health: It’s Time to Talk about Sex | 2018 |
| Formal working group (two stakeholder consultation events): development of a sexual health intervention for the RESPECT study | 2016 |
| NHS Trust communications bulletin (all recruitment sites) to share knowledge about the study and to promote recruitment | 2017 |
| The RESPECT study poster at CLAHRC research event | 2017 |
| Engagement focused website | 2017 |
| Stakeholder meeting – London and Brighton | 2017 |
| Participation in workshop – Give a little respect. Patient and public involvement in research | 2016 |
Chapter 8 Discussion and conclusions
Main findings
A bespoke intervention was developed specifically for the RESPECT study based on the IMB model of health behaviour change. 39 The content was informed by both previous trial manuals and consultation with key stakeholders including sexual health practitioners, mental health workers and PWLE of mental health.
The thematic analysis of the manuals (those that were shared by the authors) revealed that, with the exception of one, all were delivered as group sessions. The number of sessions offered and completed by participants varied widely. However, there were common themes across the content. All included information about HIV and STIs: what they are and how they are transmitted. They all included input on increasing people’s own motivation to engage in safer sex and all involved role-playing social-communication skills (e.g. negotiating condom use). In terms of the consultation feedback, the inclusion of content around positive relationships and inclusion of contraception more broadly (not just condoms) was felt to be really important, so that it was relevant to people in monogamous relationships as well as people who may be engaging in more risky sexual behaviour. It was also felt that people who were not currently in a sexual relationship (and not sexually active) should not be excluded as the information could be useful in the future for their next sexual relationship.
Therefore, the RESPECT intervention aimed to improve people’s knowledge and understanding of sexual health including safer sex and access to contraception (not just condoms). It was designed as a one-to-one intervention. In addition to imparting knowledge, the intervention was designed to address risk perception and motivation to adopt safer sex behaviours. To do this in practice, there was an emphasis on experiential learning through role play of social communication skills and practical skills such as planning, problem-solving and being able to correctly use a male condom. We also included a third session that focused on positive relationships and communication skills within those relationships (assertiveness, negotiating skills). The manual was created and was accompanied by a resource pack of games and exercises corresponding to the components. The intervention was intended to be delivered by mental health workers in NHS mental health care settings. Members of staff with appropriate experience of working with people with SMI were recruited as interventionists across the study NHS sites and received training on how to deliver the intervention as part of the study.
Four NHS mental health care providers were initially engaged as sites for the study: LYPFT, SWYFT, SPFT and C&IFT. The NELFT and Community Links – aspire (Early Intervention Psychosis Team) in Leeds were added in the latter months of the study to support the recruitment after the loss of SPFT as well as low recruitment in Barnsley (SWYFT). Over a period of 12 months (including a 6-month extension to the recruitment period), 72 people with SMI were recruited from the caseloads of CMHTs in the NHS sites and 36 were randomised to receive the RESPECT intervention as an adjunct to usual care (intervention arm) and 26 were randomised to usual care (control arm). There were equal numbers of women to men in each arm. There were no discernible differences in baseline characteristics between the control and intervention arms (apart from slightly more were single in the intervention arm (n = 26) than in the control arm (n = 22).
Of the 36 participants who were allocated to the intervention, 27 received at least one session. In terms of retention in the study, there were four full withdrawals from the entire study and five withdrawals from intervention alone. At 3 months, data were collected on 59 participants (81.9%). The first 38 people recruited in the earlier phase of the study were also followed up to the 6-month point and data were obtained from 29 (76.9%) of them. Attrition at both time points of follow-up was less than the predicted 30%. The rest of the participants could not be followed up at the 6-month time point as data collection had to be completed by the end of March 2018 in order to meet the deadline for the report in August 2018. The latter recruited group (n = 34) were followed up at the 3-month time point only (they could not be followed up at the 6-month time point). Data completeness was excellent, with most measures 100% completed and none less than 79% at each data collection time point. There was no difference in attrition or data completeness between control and intervention groups.
Qualitative interviews were conducted with a sample of 22 participants at the end of the study to assess acceptability of both the intervention and the trial design. In addition, participants were asked to complete an exit questionnaire at the end of the study. The results of these two methods of feedback confirmed that participants found the study process acceptable and comfortable, and, for those who had had the intervention, the content was useful and acceptable. The attendance rates for the intervention also support the acceptability of the intervention.
There were some significant challenges to the implementation of the study. The first was a delay in obtaining all of the required regulatory approvals. Approvals were sought during the first few months of the new HRA approval process and there was a significant delay in receiving HRA approval following the favourable NHS ethics opinion. The second significant factor was a very slow start to recruitment. After 3 months of being open to recruitment, only one participant had been recruited. It was recognised that there was some resistance and concerns about the topic of the study by care co-ordinators in the CMHTs who may have inadvertently added in additional exclusion criteria on top of our very broad inclusion criteria. We noticed that when we approached potential participants directly, our recruitment greatly improved.
Although we did not recruit the target of 100 participants, the trajectory of recruitment suggests that, with an additional 2–3 months, this would have been possible. The intervention and the study process appear acceptable and feasible; the learning from this feasibility study will be of great benefit to developing a protocol for a large full-powered trial of effectiveness. Future studies should account for engaging more with clinical teams and providing reassurance regarding anxieties about studies on the topic of sex. The fact that we now have evidence in the UK that this study is deemed acceptable by participants and that the topic did not cause distress should alleviate some of these previously expressed concerns for both staff and potential participants. In addition, the projected recruitment period should reflect the reality of engaging participants in such a study and allow sufficient time to reach the sample target based on the learning from the feasibility study.
Feasibility parameters and decision to progress to a definitive randomised controlled trial
Progression criteria for a definitive RCT were not predefined, but the aims of the study were to establish the following:
-
whether or not sufficient numbers of eligible people consent to participate (recruitment)
-
whether or not sufficient numbers of participants attend follow-up appointments and complete the questionnaires (retention)
-
the completeness of the outcome measures at each time point
-
whether or not the intervention, study processes and design were acceptable.
A future trial would expect to recruit at a revised rate based on the recruitment information that we have (see Chapter 5, Possible future sample size) and retain at least 70% of participants at follow-up time points.
Numbers recruited
In the RESPECT study, the target sample size was 100 as this has been deemed an optimal number required for the calculation of future sample size;45 this was accounting for an estimated 30% to give an analysable sample of 70. Unfortunately, the target of 100 participants was not met, with only 72 participants being randomised into the trial. Owing to this, and the extension to recruitment, only a subsample of participants (n = 29) completed the 6-month follow-up, making the robustness of a SD, and thus a sample size, questionable.
This was a feasibility study and there was limited information from which to plan a realistic recruitment target, and, therefore, one of the aims was to establish this. The proposed recruitment period was 6 months with a monthly target of 16 participants. However, recruitment across all sites was initially very poor. After adjusting the recruitment strategy (to a direct approach by face-to-face conversations at clinics or by mail-out of study packs), recruitment rates picked up significantly. However, this was at the ending point of the recruitment phase so an emergency TSC was convened and the study progress was discussed. The chairperson of the TSC supported the proposal to extend recruitment and add on a no-cost extension. This would be for a maximum of 6 months only. This was agreed with the funders but it also meant that anyone recruited after October 2017 would only be followed up for 6 months. At the end of (the extended) recruitment period, a total of 72 participants had been recruited and randomised into the study. At the closure of recruitment on 31 December 2017, a few CtoC forms were still received from interested patients. It could have been possible to reach the target with an extra 3 months of the study being open, and at the close of study there were more CtoCs forms received that we were unable to process.
The recruitment chart by month demonstrates an exponential increase in recruitment over time, as can been seen in Figure 4, and the trajectory suggests that it would have been possible to recruit to target. It is also worth noting that only two of the sites recruited to their own targets of 25 participants (LYPFT and C&IFT). The other two sites experienced problems in being able to recruit and support the study. We did add additional sites to compensate; by the time they opened to recruitment there were only 2–3 months left of the recruitment period. Future studies should ensure that there is sufficient capacity in each NHS site for the duration of the study and that there are no planned service transformations that will greatly affect engagement and recruitment.
Recruitment methods
A large proportion of people in the care of CMHTs were potentially eligible as a result of the broad eligibility criteria. A range of strategies were adopted to enable those people to learn more about the study and consider participation. These strategies included giving their case managers a study pack to pass on, handing out leaflets at clinics and service users groups, posting out the packs to participants’ homes and adding in a ‘callback’. All of the strategies that we adopted yielded CtoCs. Clearly the biggest challenge for the recruitment was in the initial marketing to both care co-ordinators and participants themselves. The RESPECT study is the first study on the topic of the sexual health of people with SMI in the UK (and Europe). It has already been established that this topic is one that is routinely avoided in mental health care4 and, knowing this, the study team engaged with each service area prior to recruitment by visiting team meetings, providing written information, as well as providing regular e-newsletters on the progress of the study. However, it became apparent that there were some significant issues in terms of clinical staff understanding of research in general and their role in the promotion of the study (including a lack of awareness of patients’ rights in the NHS constitution to be informed of studies that they may be eligible for). In addition, it seems that the topic itself created some anxiety on the part of the care co-ordinators. All people who received a recruitment pack were given a feedback questionnaire to complete whether or not they entered the study. We received 158 feedback questionnaires and 42% of those had declined to take part. The main reason for declining was ‘topic not relevant’. Only two responses related to being put off by the topic itself or the design of the study.
A future trial can use the learning from the RESPECT study to offer reassurance to both service users and mental health staff that this is an acceptable and feasible study, and does not lead to adverse effects. We should be clearer in the information leaflets and other marketing material regarding the nature of the intervention, as a significant number of people saw it as not relevant to them. The involvement of those PWLE will also be important in ensuring that staff understand the purpose of the study and support recruitment from their perspective. It will be important to harness the voices of the service user participants who overwhelmingly found the study to be comfortable, interesting, thought-provoking and novel.
Participant characteristics
One of the research aims was to see if people under the care of community mental health services could be recruited to and retained in a sexual health promotion study. Most of those identified were eligible and (of those who expressed an interest) agreed to take part in the study. The average age was 44.8 years (range 22–66.1 years). Many participants were not in a relationship, lived alone or with friends/family, and many were not sexually active within the 3-month windows of assessment at baseline, 3-month and 6-month follow-up points. Most of the participants identified as heterosexual; 15.2% identified as other sexualities (lesbian, gay bisexual, transsexual, pansexual). This is higher than estimated in the general population as the Natsal-358 reported 97.1% of a representative sample of adults in Britain identified as heterosexual. The sample was equally split between males and females, although 3% preferred not to state their gender. The quality of life of participants was measured as having a mean of 44 on the ReQoL, which indicates that we recruited a clinical population, as scores of > 50 are defined as falling into the range of general population scores. In terms of drug and alcohol use, on average this was scored as low risk across the cohort using the ASSIST. A future study should consider the fact that there is a large range of, and fluctuation in, sexual activity when determining sample size. However, in summary, as planned we were able to recruit people who were under the care of the community mental health services.
Participant safety
Over the whole study, no AEs related to the study were recorded. Robust processes were in place to monitor for SAEs and researchers prompted participants for any AEs at each data collection point. The researchers also checked with the CSOs at each site regarding the well-being of the participants prior to data collection points. The interventionists were also asked to inform the research team if they became aware of any potential AEs. One potential AE was reported, but following investigation at the NHS sites and review by the research team (and Independent Steering Group chairperson and DMEC chairperson) it was ruled that this was unrelated to participation in the study. There was only one other issue that was reported by a care co-ordinator, and that was that one participant had become ‘pre-occupied’ with sexual health concerns following the baseline data collection. The study researcher was only informed of this when the 3-month follow-up appointment was due and, after further enquiry with the case manager, it was discovered that the participant had not deteriorated to such an extent that they required an escalation of care, such as an inpatient admission. Therefore, it was agreed that, according to the risk protocol, no AE had occurred. However, in discussion with the participant, it was agreed not to collect follow-up data.
Retention
At the 3-month follow-up point, data were collected on 81.9% of the participants (n = 59), and for those who reached the 6-month follow-up (which was a subsample of 38 participants), 76.3% (n = 29) completed the interview.
The retention rates are acceptable and on a par with other health intervention studies with SMI populations conducted in the UK43 and with previous sexual health promotion studies conducted in the USA. 33
Completeness of the outcome measures
Overall, the participants found the data collection process to be acceptable. In the qualitative study, they did report that it was quite long, and feedback from the researchers also indicated that the length (approximately 1 hour 30 minutes) might be a bit too long for the baseline. There was some feedback that there was some repetition between the outcome measures, and that the case report form (CRF) could be streamlined to reduce the length and the repetition. The £10 shopping voucher that was given at each data collection point was helpful in encouraging attendance and was deemed ‘about right’ in terms of incentive. Recommendations for a future study included more clarity on the exact nature of the questions (one participant reported feeling a bit shocked that he was asked about sex with men, when he had already stated that he was heterosexual) and that it would be useful to schedule some breaks to be added into the data collection appointment (or over a couple of sessions). The researchers had been advised to offer breaks to participants and to be flexible regarding the data collection. If the CRF was reduced for a future study then this would hopefully resolve the issue of needing breaks. However, it is always good practice to make the data collection appointments as comfortable as possible, so the option for breaks will remain (as will support to read and answer the questionnaires).
The study was underpowered to detect statistically significant differences in outcomes between intervention and control at follow-up. There were some trends in favour of the intervention but these cannot be considered as robust with such small numbers. Only a fully powered study would be able to confirm effectiveness. These are summarised as:
-
HIV-KQ knowledge – there was a trend towards a slight increase in knowledge score in the intervention arm compared with the control arm at 3 months.
-
Use of sexual health services – the use of sexual health and family planning services was low in this population; the intervention group had a higher use of sexual health services at baseline. However, as the numbers are so small (in terms of use), no conclusions can be drawn regarding the intervention. However, this does indicate that some of the people we recruited did experience current sexual health needs.
-
Quality of life – there were no discernible changes to ReQoL scores (a reliable change is defined as at least 10 points on average).
-
Sexual risk behaviour – there was some reduction in reports of condomless sex at 3 months in the intervention arm, but not in the control arm. It is important to note that all participants were given condoms at baseline (as well as during the intervention) and yet the only reduction was seen in those who had accessed the intervention. Not all participants were sexually active during the period of the study; yet, of those who were, none reported increased condomless sex as a result of taking part, suggesting that participation does not encourage unsafe sex.
-
Many of the participants described themselves as single and most were not having sex during the 3-month follow-up periods. However, among the people who were having sex, most of this was unprotected (condomless) at baseline. More women than men reported actively engaging in sex. There was a clear reduction in unprotected vaginal sex acts in the intervention group at 3 months and 6 months. This was a particularly obvious reduction for women. There were very few anal sex acts so it is difficult to comment on these data. There were no changes in unprotected oral sex but this is a relatively low-risk activity (compared with condomless anal and vaginal sex). 59
-
Motivations to adopt safer sex – most of the participants did not perceive themselves at high risk for STIs based on previous behaviour or future behaviour. There was a slight shift in proportions reporting higher risk perceptions at 3 months.
-
Condom self-efficacy – the mean score in the intervention arm was higher at 3 months than in the control arm.
-
Behavioural intentions to adopt safer sex – there was an increase in scores at 3 months for the intervention arm, and the control arm scores were slightly lowered.
Specific lessons from the feasibility study
The learning from the RESPECT study provides unique insight into how a successful definitive trial should be conducted.
Trial implementation
Two of the original four sites experienced difficulties that affected the capacity to deliver on the targets for the study. In SPFT, delays to the start of the recruitment period affected the capacity to deliver the intervention and, therefore, recruitment was ceased at the point they could no longer provide the intervention. In SWYFT, the community mental health services in the designated locality of Barnsley underwent a complete service transformation. Had the study recruitment start date not been delayed, we could have continued to engage with the existing services up to the point of transformation. However, recruitment was greatly affected by this as team managers changed and also individual care co-ordinator’s caseloads changed so it was very difficult to undertake screening and recruitment during this period. We did engage other sites during the summer of 2017, but by the time they were open to recruitment there was only a few weeks of the recruiting period left. Future studies should factor in engaging with more sites than are actually required at the start of the study as ‘contingency sites’ that would be ready to open to recruitment should the need arise.
Some participants who took part in the qualitative study had not understood the process of randomisation, and this is not an uncommon phenomenon. 60 A future trial should attend to the explanation of the purpose of randomisation and build in a check of understanding to ensure that all participants understand why randomisation is used in intervention studies.
Delivery of intervention
It is not uncommon for intervention studies to experience logistical problems. The intervention is provided by the NHS and is an ‘excess treatment cost’ as opposed to a research cost. Although much work was undertaken to engage with the NHS sites regarding the provision of staff who would be able to deliver a trial intervention, the reality of providing that intervention from the clinical services at a time of increased demand is one of the main challenges of conducting intervention research in real-world settings. Owing to service demands and natural staff attrition, the interventionists are likely to change within the study period. However, the significant initial delays in recruitment added to the challenges as those originally identified had moved on by the time the first participants came through (in some cases several months after the first wave of intervention training had occurred).
In the RESPECT study, interventionist attrition was mostly resolved by training new people to deliver at each site but in the case of C&IFT, the loss of anyone to provide the intervention for some weeks meant that recruitment for some of the participants had to be stalled. A future study should aim to have a rolling programme of training for staff to be able to deliver the intervention throughout the study period to account for role changes, new jobs, maternity and other leave that naturally occurs in the workforce. This is especially pertinent as a definitive trial is likely to be open to recruitment for a longer period than the RESPECT study and is, therefore, at risk from interventionist attrition.
There were no significant issues reported from the interventionists regarding the logistics of arranging appointments, and the intervention sessions were conducted in a range of settings, such as private rooms at a local NHS site, or in the person’s own home.
Recruitment rates
No single method of recruitment seemed more effective than the others and so the recommendation would be to keep a range of strategies for recruitment in a subsequent study. In terms of feedback from participants, there were comments suggesting that having someone to talk through the study with was really valued and this could be the care co-ordinator or one of the researchers. It is also recommended that initial information and marketing is kept brief so a future study should consider a layered approach to information giving, using a brief easy-to-read leaflet first before receiving the full PIS. Recommendations for improving recruitment are as follows:
-
More intensive engagement with CMHTs – a future study should ensure that the research team is given adequate time to spend with the CMHT teams prior to the commencement of recruitment. In the qualitative studies, participants stated that they preferred to be provided with information from their case manager rather than receive a leaflet or mail-out. It is important that this feedback is communicated to the team in order to encourage them to mention the study face to face. In preparation for recruitment, it will be important that the concerns regarding the study topic are addressed in the information that is given out (for both the staff and the service users). From the qualitative study and the exit questionnaires there are many positive reasons for taking part and positive experiences of the study, which can be communicated in the next phase.
-
The length of data collection appointments – in the feedback questionnaires and anecdotally, some potential participants may have been deterred from taking part because of the burden of data collection. Although this did not seem to be a major issue for the actual participants (the information sheet clearly stated a completion time of up to 2 hours). After reviewing the data and the fact that participants found some aspects repetitive, it will be important to streamline the data that are collected and reduce the data collection appointment to a target of 1 hour. Despite the SERBAS being the ‘gold standard’ of sexual risk behaviour assessment, it is time-consuming and collects a large number of data, which, although really useful in terms of profiling risk behaviour, is limited by the burden of length of time to complete. It is recommended that a future study should simply focus on collecting data on the number of partners over the past 3 months, the total number of episodes of condomless anal, vaginal and oral sex (across all partners) and episodes of sex trading (condomless). Reducing the questions used from the SERBAS would significantly reduce the time of the interview.
-
Using the positive experience of the participants in future information and promotional materials – the overwhelming feedback of those who took part was that it was interesting, thought-provoking and something novel. They also felt comfortable with the data collection process and, for those who received it, with the intervention itself. Using this positive feedback may assist more potentially eligible people to take part and allay some concerns people might have.
Retention rates
Retention rates in the study were good and one of the things that may have helped this was the £10 voucher, but also the consistency of seeing the same researcher and at a location and time to suit the participant. We were able to offer appointments after working hours for some of the participants who worked or who had other daytime commitments.
Resource use and health-related quality of life
The economic analysis evaluated the feasibility of collecting data on resource use and health-related quality of life. Data were collected on intervention-related costs, self-reported health service visits/consultations and quality of life based on EQ-5D-5L. The intervention cost per patient was estimated to be £110.50 (range £65.70–146.00); in addition, a one-off cost of training the interventionists was estimated to be £475.20 per health professional trained. Questionnaire response rate, item completion rate and changes in resource use and quality of life over the trial follow-up period were also evaluated. Health service resource use data had a very high completion rate among respondents who continued the trial. The pharmacy visits question had the most missing values at baseline but the overall rate of missing items was very low. Visits to the GP and nurse were the most common types of resource use. It was noted that visits to A&E were low and unrelated to sexual health problems, and given their cost it may be useful to reconsider whether or not they should be included in a full trial. Regarding the EQ-5D-5L questionnaire, there was no missing data among respondents who stayed in the study. During the study follow-up, the intervention arm showed more improvement in quality of life than the control arm; however, given the sample size, no statistically significant conclusions can be drawn. Overall, the economic analysis suggested a high level of questionnaire completion rate and a low level of item missingness in participants who stayed in the study; moreover, we did not find any unreasonable or out-of-range responses.
The summary of recommendations for the design and implementation of a future trial is as follows:
-
More in-depth engagement at sites to ensure that CMHT staff are fully conversant with the aims of the trial and reassured regarding risk and safety for participants.
-
Reduction in the length of time required for data collection by streamlining the CRF.
-
Recruitment period to be set to reflect the actual recruitment rates identified in this feasibility study.
-
Engage more NHS sites and intervention staff at the start of the study and have contingencies in place to provide a critical mass of people at each site to deliver intervention.
Summary of implications for practice
Previous studies have suggested that sexual health is a topic that is often avoided in mental health. However, the findings from the RESPECT study do indicate that this topic is of interest to people with SMI and that, for some, it is a priority. Although the sample may not be representative of people with SMI (as participants self-selected to participate), the data indicate that some people have poor knowledge about sexual health risks, have low perceptions of risk and motivation to engage in safer sex, and, by self-report, are engaging in condomless vaginal sex. While acknowledging that this is not a representative sample, these findings do confirm the findings from studies undertaken in the USA and support the view that sexual health and relationships are important aspects of health for people with SMI (as for the general population). Around half of the sample did not report sex in the 3 months prior to data collection and they were asked to tick up to three reasons for this. The most common answers were ‘not having a partner’ followed by ‘not interested in sex’ and ‘psychiatric illness/medication-related problems’. It is possible that having a mental illness has an impact on people’s sex life by impeding their ability to find and maintain intimate relationships; even if they do find a relationship, illness and medication factors could affect their sexual desire and functioning. A study by McCann20 about relationships of people with psychosis suggests that sexual relationships are something that people aspire to have and that loneliness and isolation,61 as well as stigma17 around having a mental illness, could have an impact on people’s ability to form and maintain intimate partner relationships.
In the RESPECT study, all participants were given male condoms and sachets of water-based lubrication, as well as information about local sexual health services. These are relatively straightforward interventions, yet we know from surveys conducted in the UK that condoms are not routinely provided in mental health services. In addition, mental health staff appear to lack knowledge about local services. There is a need for mental health services to consider providing standard health promotion information to all service users.
Recommendations for further research
The RESPECT study was designed to assess the feasibility and acceptability of a sexual health intervention. A fully powered RCT would be required to establish effectiveness. There is a need for a further, more robust, examination of the factors that are associated with sexual risk behaviour for people with SMI and what factors mediate risk (e.g. hypersexuality). Meade and Sikkema6 reviewed the literature on the link between having a mental illness and sexual behaviour, and none of the studies had been conducted in the UK (mostly in the USA). One of the key concerns raised by the implementation of the study was resistance to the topic by mental health staff. Further research should examine how to enable staff to engage with service users regarding sexual issues in the context of routine care as well as for the support of future research. An area that was beyond the scope of this study was to examine the needs of sexual health and family planning practitioners in the delivery of sexual and reproductive health for people with SMI, and what the care pathways should be in order for people with SMI to effectively navigate them.
Conclusions
The RESPECT study is the first study related to sexual health promotion in people with SMI in the UK. The overall aim was to establish the acceptability and feasibility of an intervention to promote sexual health in people with SMI (as defined by having psychosis, bipolar, or schizoaffective disorder and being on the caseload of a CMHT). Despite a very slow start to recruitment, adequate numbers (for the needs of a feasibility study) of eligible participants with SMI within community mental health services were recruited in five locations in England. In addition, there were good rates of retention at 3- and 6-month time points, and good completeness of data across the outcome measures. The qualitative study conducted with a subsample of participants confirmed that they had found the study to be acceptable in terms of both the overall design and the implementation, and also that the intervention had been acceptable. Some minor suggestions for changes to process and intervention were given and will be taken into account for a future study.
The outcome measure results at follow-up time points suggest a positive direction in favour of the intervention; however, the study was underpowered to detect statistically significant differences and a larger fully powered study would be able to evaluate the effectiveness of the intervention with more confidence. Successful strategies for recruitment have been identified.
Although there were no predetermined criteria regarding the feasibility parameters required for progression to a future definitive RCT of the RESPECT intervention, this feasibility study has demonstrated that it is both acceptable and feasible to undertake a RCT of sexual health promotion for people with SMI, and participants valued the experience and the importance of the topic to their lives. Given that this is an area of unmet clinical need, more research is justified, despite the limitations of this study.
Acknowledgements
We would first like to acknowledge the service users who agreed to participate in this study and who not only participated in the study but also gave really insightful and critical feedback about the experience of being in the study, as well as the intervention itself.
We would like to acknowledge the NHS trusts that took part, particularly the R&D teams who worked tirelessly on the promotion of and recruitment for the study. In addition, thanks to the NHS site local principal investigators who worked hard to manage the study recruitment and also helped in identifying the interventionists. Furthermore, the study could not have happened without the support of the CMHT managers who agreed for the RESPECT study to recruit from their caseloads. We also thank the case managers who helped identify potentially eligible people and pass on information about the study to people on their caseloads. We also wish to acknowledge the staff and service users who took part in stakeholder consultations that helped shape and inform the intervention content and style of delivery.
We would also like to acknowledge members of our lived experience group who worked with us throughout the process from application to completion of the report: Isaac Samuels, Harminder Kaur and Sheena Foster.
In addition, we would like to acknowledge members of the DMEC and TSC, especially Professor Karina Lovell, who was the independent TSC chairperson, and was a huge support to the success of the study, as well as Professor Stephen Prymajchuk, Dr Dawn Teare, Professor Patrick Callaghan, Professor John Baker, Dr Leslie Hoggart, Dr John McSorley and Dr Cassandra Brookes.
Finally, we would like to acknowledge the support of Professor Francine Cournos, Dr Karen Mckinnon and Dr Milton Wainberg of Columbia University, USA, who generously shared their outcome measures and intervention manuals and offered advice and support during the whole research cycle. We especially thank Dr Karen McKinnon for providing the training for the SERBAS to the study team.
Contributions of authors
Elizabeth Hughes (Chief Investigator) was the lead applicant and was responsible for the overall management of the study, intervention design and writing of the report.
Natasha Mitchell (Trial Manager) developed the protocol and CRFs, and managed the day-to-day running of the study (April 2016 to January 2017).
Samantha Gascoyne (Trial Manager) managed the day-to-day running of the study (February 2017 to December 2017) and contributed to the report.
Thirimon Moe-Byrne (Trial Manager) assisted with the day-to-day running of the study (April 2017–January 2018) and contributed to the report.
Amanda Edmondson (Research Fellow) supported the development of the intervention, the NHS ethics and HRA applications, led on the qualitative study, collated data on intervention delivery and contributed to the report.
Elizabeth Coleman (Statistician) was responsible for the preparation of the statistical analysis plan, analysis of data and contributed to writing the report.
Lottie Millett (Research Fellow) was responsible for recruitment and data collection, and contributed to the report.
Shehzad Ali (Health Economist) was a co-applicant, and was responsible for the health economics component of the feasibility trial and conducted the health economics analysis presented in Chapter 5.
Ceri Dare (Expert by Experience) provided input from a lived experience on the intervention content and delivery as well as the outcome measures, undertook qualitative interviews with participants and assisted in the analysis of the qualitative data, and reviewed data and feasibility data and the report.
Catherine Hewitt (Deputy Director of York Trials Unit) was a co-applicant and lead statistician, contributed to the overall study design and implementation, and supervised the statistical analyses.
Sonia Johnson (Professor of Social and Community Psychiatry) was a co-applicant, contributed to the overall design and protocol development, acted as local principal investigator for Camden and Islington, supervised the southern sites researcher, and was involved in writing the report.
Carrie Llewellyn (Professor of Applied Behavioural Medicine) was a co-applicant, informed the methodology for the intervention development and contributed to the report.
Catherine Mercer (Reader in Applied Statistics) was a co-applicant and an advisor on sexual health data collection and contributed to the report.
Fiona Nolan (Professor of Mental Health Nursing) was a co-applicant, undertook stakeholder consultations, was principal investigator for southern sites until December 2017, and was involved in reviewing data and the report.
Charlotte Walker (Expert by Experience) was involved in the application and design of the study, helped to devise the PIS and leaflet, and reviewed data and feasibility data and the report.
Judith Watson (Senior Research Fellow) was a co-applicant, contributed to the overall study design and implementation, responsible for oversight of the study, led on (and supervised) York Trials Unit tasks, and co-wrote the report with the Chief Investigator.
All authors were involved in the design of all phases of the research, intervention development, data interpretation and drafting of the report.
Data-sharing statement
All available data can be obtained from the corresponding author. We shall make data available to the scientific community with as few restrictions as feasible, while retaining exclusive use of the data until the publication of major outputs.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Ruggeri M, Leese M, Thornicroft G, Bisoffi G, Tansella M. Definition and prevalence of severe and persistent mental illness. Br J Psychiatry 2000;177:149-55. https://doi.org/10.1192/bjp.177.2.149.
- British Medical Association (BMA) . Recognising the Importance of Physical Health in Mental Health and Intellectual Disability: Achieving Parity of Outcomes 2014.
- Department of Health and Social Care . Improving the Physical Health of People With Mental Health Problems. Actions for Mental Health Nurses 2016.
- Hughes E, Edmondson AJ, Onyekwe I, Quinn C, Nolan F. Identifying and addressing sexual health in serious mental illness: views of mental health staff working in two NHS organizations in England. Int J Ment Health Nurs. 2018;27:966-74. https://doi.org/10.1111/inm.12402.
- World Health Organization (WHO) . Sexual and Reproductive Health: Defining Sexual Health 2006. www.who.int/reproductivehealth/topics/sexual_health/sh_definitions/en/ (accessed 27 July 2018).
- Meade CS, Sikkema KJ. HIV risk behavior among adults with severe mental illness: a systematic review. Clin Psychol Rev 2005;25:433-57. https://doi.org/10.1016/j.cpr.2005.02.001.
- Bonfils KA, Firmin RL, Salyers MP, Wright ER. Sexuality and intimacy among people living with serious mental illnesses: factors contributing to sexual activity. Psychiatr Rehabil J 2015;38:249-55. https://doi.org/10.1037/prj0000117.
- Meade CS, Sikkema KJ. Psychiatric and psychosocial correlates of sexual risk behavior among adults with severe mental illness. Community Ment Health J 2007;43:153-69. https://doi.org/10.1007/s10597-006-9071-6.
- Hughes E, Bassi S, Gilbody S, Bland M, Martin F. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness: a systematic review and meta-analysis. Lancet Psychiatry 2016;3:40-8. https://doi.org/10.1016/S2215-0366(15)00357-0.
- UNAIDS . UNAIDS Report on the Global AIDS Epidemic 2012 2012.
- Vanable PA, Carey MP, Carey KB, Maisto SA. Differences in HIV-related knowledge, attitudes, and behavior among psychiatric outpatients with and without a history of a sexually transmitted infection. J Prev Interv Community 2007;33:79-94. https://doi.org/10.1300/J005v33n01_07.
- Dutra MR, Campos LN, Guimarães MD. Sexually transmitted diseases among psychiatric patients in Brazil. Braz J Infect Dis 2014;18:13-20. https://doi.org/10.1016/j.bjid.2013.04.004.
- Coverdale JH, Turbott SH, Roberts H. Family planning needs and STD risk behaviours of female psychiatric out-patients. Br J Psychiatry 1997;171:69-72. https://doi.org/10.1192/bjp.171.1.69.
- Simoila L, Isometsä E, Gissler M, Suvisaari J, Sailas E, Halmesmäki E, et al. Schizophrenia and induced abortions: a national register-based follow-up study among Finnish women born between 1965-1980 with schizophrenia or schizoaffective disorder. Schizophr Res 2018;192:142-7. https://doi.org/10.1016/j.schres.2017.05.039.
- Khalifeh H, Oram S, Osborn D, Howard LM, Johnson S. Recent physical and sexual violence against adults with severe mental illness: a systematic review and meta-analysis. Int Rev Psychiatry 2016;28:433-51. https://doi.org/10.1080/09540261.2016.1223608.
- Elkington KS, Hackler D, Walsh TA, Latack JA, McKinnon K, Borges C, et al. Perceived mental illness stigma, intimate relationships and sexual risk behavior in youth with mental illness. J Adolesc Res 2013;28:378-404. https://doi.org/10.1177/0743558412467686.
- Wainberg ML, Cournos F, Wall MM, Norcini Pala A, Mann CG, Pinto D, et al. Mental illness sexual stigma: implications for health and recovery. Psychiatr Rehabil J 2016;39:90-6. https://doi.org/10.1037/prj0000168.
- Grassi L, Biancosino B, Righi R, Finotti L, Peron L. Knowledge about HIV transmission and prevention among Italian patients with psychiatric disorders. Psychiatr Serv 2001;52:679-81. https://doi.org/10.1176/appi.ps.52.5.679.
- Shield H, Fairbrother G, Obmann H. Sexual health knowledge and risk behaviour in young people with first episode psychosis. Int J Ment Health Nurs 2005;14:149-54. https://doi.org/10.1111/j.1440-0979.2005.00372.x.
- McCann E. The sexual and relationship needs of people who experience psychosis: quantitative findings of a UK study. J Psychiatr Ment Health Nurs 2010;17:295-303. https://doi.org/10.1111/j.1365-2850.2009.01522.x.
- Ngwena J. HIV/AIDS awareness in those diagnosed with mental illness. J Psychiatr Ment Health Nurs 2011;18:213-20. https://doi.org/10.1111/j.1365-2850.2010.01657.x.
- Hughes E, Gray R. HIV prevention for people with serious mental illness: a survey of mental health workers’ attitudes, knowledge and practice. J Clin Nurs 2009;18:591-600. https://doi.org/10.1111/j.1365-2702.2007.02227.x.
- McCann E. Investigating mental health service user views regarding sexual and relationship issues. J Psychiatr Ment Health Nurs 2010;17:251-9. https://doi.org/10.1111/j.1365-2850.2009.01509.x.
- Quinn C, Happell B, Browne G. Talking or avoiding? Mental health nurses’ views about discussing sexual health with consumers. Int J Ment Health Nurs 2011;20:21-8. https://doi.org/10.1111/j.1447-0349.2010.00705.x.
- Quinn C, Platania-Phung C, Bale C, Happell B, Hughes E. Understanding the current sexual health service provision for mental health consumers by nurses in mental health settings: findings from a survey in Australia and England. Int J Ment Health Nurs 2018;27:1522-34. https://doi.org/10.1111/inm.12452.
- Department of Health and Social Care . Framework for Sexual Health Improvement in England 2013.
- Public Health England . Sexually Transmitted Infections and Screening for Chlamydia in England, 2018. Health Protection Report Volume 13 Number 19 2019.
- Public Health England . HIV in the United Kingdom: Decline in New HIV Diagnoses in Gay and Bisexual Men in London, 2017 Report 2018.
- Public Health England . A Framework for Sexual Health Improvement in England 2013.
- Working Group for Improving the Physical Health of People with SMI . Improving the Physical Health of Adults With Severe Mental Illness: Essential Actions 2016.
- Angelino AF, Treisman GJ. Issues in co-morbid severe mental illnesses in HIV infected individuals. Int Rev Psychiatry 2008;20:95-101. https://doi.org/10.1080/09540260701861989.
- Kaltenthaler E, Pandor A, Wong R. The effectiveness of sexual health interventions for people with severe mental illness: a systematic review. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18010.
- Walsh C, McCann E, Gilbody S, Hughes E. Promoting HIV and sexual safety behaviour in people with severe mental illness: a systematic review of behavioural interventions. Int J Ment Health Nurs 2014;23:344-54. https://doi.org/10.1111/inm.12065.
- Susser E, Valencia E, Berkman A, Sohler N, Conover S, Torres J, et al. Human immunodeficiency virus sexual risk reduction in homeless men with mental illness. Arch Gen Psychiatry 1998;55:266-72. https://doi.org/10.1001/archpsyc.55.3.266.
- Carey MP, Carey KB, Maisto SA, Gordon CM, Schroder KE, Vanable PA. Reducing HIV-risk behavior among adults receiving outpatient psychiatric treatment: results from a randomized controlled trial. J Consult Clin Psychol 2004;72:252-68. https://doi.org/10.1037/0022-006X.72.2.252.
- Berkman A, Cerwonka E, Sohler N, Susser E. A randomized trial of a brief HIV risk reduction intervention for men with severe mental illness. Psychiatr Serv 2006;57:407-9. https://doi.org/10.1176/appi.ps.57.3.407.
- Collins PY, Geller PA, Miller S, Toro P, Susser ES. Ourselves, our bodies, our realities: an HIV prevention intervention for women with severe mental illness. J Urban Health 2001;78:162-75. https://doi.org/10.1093/jurban/78.1.162.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Fisher EB, Fitzgibbon ML, Glasgow RE, Haire-Joshu D, Hayman LL, Kaplan RM, et al. Behavior matters. Am J Prev Med 2011;40. https://doi.org/10.1016/j.amepre.2010.12.031.
- Bartholomew LK, Mullen PD. Five roles for using theory and evidence in the design and testing of behavior change interventions. J Public Health Dent 2011;71:20-33. https://doi.org/10.1111/j.1752-7325.2011.00223.x.
- Berkman A, Pilowsky DJ, Zybert PA, Herman DB, Conover S, Lemelle S, et al. HIV prevention with severely mentally ill men: a randomised controlled trial. AIDS Care 2007;19:579-88. https://doi.org/10.1080/09540120701213989.
- Rosenberg SD, Goldberg RW, Dixon LB, Wolford GL, Slade EP, Himelhoch S, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv 2010;61:885-91. https://doi.org/10.1176/ps.2010.61.9.885.
- Gilbody S, Peckham E, Man MS, Mitchell N, Li J, Becque T, et al. Bespoke smoking cessation for people with severe mental ill health (SCIMITAR): a pilot randomised controlled trial. Lancet Psychiatry 2015;2:395-402. https://doi.org/10.1016/S2215-0366(15)00091-7.
- Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health 2008;31:180-91. https://doi.org/10.1002/nur.20247.
- Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-264.
- Great Britain . Mental Capacity Act 2005 2005. www.legislation.gov.uk/ukpga/2005/9/contents.
- World Health Organization (WHO) . The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) Manual for Use in Primary Care 2010.
- Keetharuth AD, Brazier J, Connell J, Bjorner JB, Carlton J, Taylor Buck E, et al. Recovering Quality of Life (ReQoL): a new generic self-reported outcome measure for use with people experiencing mental health difficulties. Br J Psychiatry 2018;212:42-9. https://doi.org/10.1192/bjp.2017.10.
- NHS Improvement . Reference Costs 2016–17 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 10 January 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Oral Contraception in Community Pharmacy . Evaluation of Oral Contraception in Community Pharmacy Pilot in Southwark and Lambeth, Final Evaluation Report, January 2012 2012.
- Crawford MJ, Sanatinia R, Barrett B, Byford S, Dean M, Green J, et al. The clinical and cost-effectiveness of brief advice for excessive alcohol consumption among people attending sexual health clinics: a randomised controlled trial. Sex Transm Infect 2015;91:37-43. https://doi.org/10.1136/sextrans-2014-051561.
- Geary RS, Tanton C, Erens B, Clifton S, Prah P, Wellings K, et al. Sexual identity, attraction and behaviour in Britain: The implications of using different dimensions of sexual orientation to estimate the size of sexual minority populations and inform public health interventions. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0189607.
- Family Planning Association . Oral Sex and Sexually Transmitted Infections 2014. www.fpa.org.uk/sexually-transmitted-infections-stis-help/oral-sex-and-sexually-transmitted-infections (accessed 27 July 2018).
- Jenkins V, Fallowfield L, Cox A. The preferences of 600 patients for different descriptions of randomisation. Br J Cancer 2005;92:807-10. https://doi.org/10.1038/sj.bjc.6602445.
- Mushtaq R, Shoib S, Shah T, Mushtaq S. Relationship between loneliness, psychiatric disorders and physical health ? A review on the psychological aspects of loneliness. J Clin Diagn Res 2014;8. https://doi.org/10.7860/JCDR/2014/10077.4828.
Appendix 1 Study summary
FIGURE 10.
Study summary.
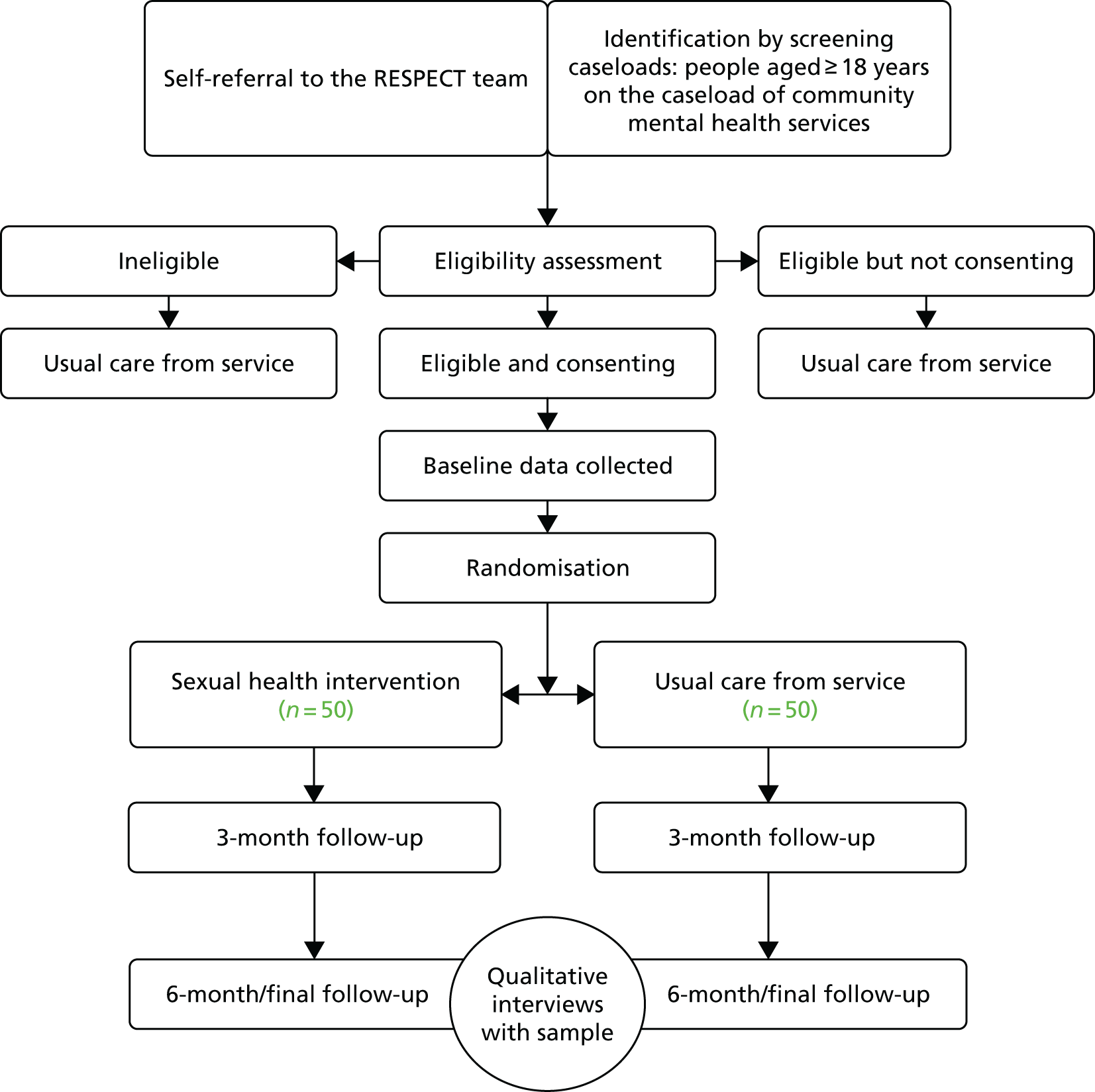
Appendix 2 Additional statistical results tables
| SERBAS items | Intervention (N = 17) | Control (N = 19) | Overall (N = 36) |
|---|---|---|---|
| Type of relationship with main partner | |||
| Baseline | n = 17 | n = 19 | n = 36 |
| Steady relationship | 5 | 4 | 9 |
| Casual relationship | 5 | 7 | 12 |
| Missing | 7 | 8 | 15 |
| Month 3 | n = 14 | n = 15 | n = 29 |
| Steady relationship | 6 | 6 | 12 |
| Casual relationship | 2 | 2 | 4 |
| Missing | 6 | 7 | 13 |
| Month 6 | n = 5 | n = 9 | n = 14 |
| Steady relationship | 1 | 3 | 4 |
| Casual relationship | 0 | 2 | 2 |
| Missing | 4 | 4 | 8 |
| Vaginal sex | |||
| Baseline | n = 9 | n = 9 | n = 18 |
| Mean (SD) | 35.9 (49.9) | 9 (7.5) | 22.4 (37.3) |
| Median (min., max.) | 4 (1, 120) | 9 (1, 21) | 6.5 (1, 120) |
| Proportion of acts unprotected (%) | 58.5 | 58.0 | 58.4 |
| Month 3 | n = 8 | n = 7 | n = 15 |
| Mean (SD) | 24.3 (32.5) | 18.9 (22.0) | 21.7 (27.3) |
| Median (min., max.) | 8 (3, 90) | 7 (1, 60) | 7 (1, 90) |
| Proportion of acts unprotected (%) | 24.7 | 70.4 | 43.3 |
| Month 6 | n = 1 | n = 4 | n = 5 |
| Mean (SD) | 19 (–) | 11.5 (16.3) | 13 (14.5) |
| Median (min., max.) | 19 (19, 19) | 3.5 (3, 36) | 4 (3, 36) |
| Proportion of acts unprotected (%) | 5.3 | 95.7 | 69.2 |
| Anal sex, n | n = 0 | n = 2 | n = 2 |
| Mean (SD) | – (–) | 1.5 (0.7) | 1.5 (0.7) |
| Median (min., max.) | – (–, –) | 1.5 (1, 2) | 1.5 (1, 2) |
| Proportion of acts unprotected (%) | – | 100 | 100 |
| Month 3 | n = 2 | n = 1 | n = 3 |
| Mean (SD) | 1 (0) | 2 (–) | 1.3 (0.6) |
| Median (min., max.) | 1 (1, 1) | 2 (2, 2) | 1 (1, 2) |
| Proportion of acts unprotected (%) | 100 | 100 | 100 |
| Month 6 | n = 0 | n = 0 | n = 0 |
| Mean (SD) | – (–) | – (–) | – (–) |
| Median (min., max.) | – (–, –) | – (–, –) | – (–, –) |
| Proportion of acts unprotected (%) | – | – | – |
| Oral sex, n | n = 7 | n = 10 | n = 17 |
| Mean (SD) | 29.9 (41.0) | 7.5 (8.1) | 16.7 (28.2) |
| Median (min., max.) | 12 (1, 113) | 3.5 (1, 20) | 5 (1, 113) |
| Proportion of acts unprotected (%) | 97.6 | 77.3 | 92.3 |
| Month 3 | n = 7 | n = 7 | n = 14 |
| Mean (SD) | 24.4 (28.8) | 3.6 (2.0) | 14 (22.4) |
| Median (min., max.) | 10 (3, 72) | 3 (1, 7) | 4.5 (1, 72) |
| Proportion of acts unprotected (%) | 97.7 | 96.0 | 97.4 |
| Month 6 | n = 1 | n = 5 | n = 6 |
| Mean (SD) | 19 (–) | 3.6 (3.8) | 6.2 (7.1) |
| Median (min., max.) | 19 (19, 19) | 2 (1, 10) | 3 (1, 19) |
| Proportion of acts unprotected (%) | 100 | 100 | 100 |
| Total number of sex acts | n = 10 | n = 10 | n = 20 |
| Mean (SD) | 53.2 (80.4) | 15.9 (14.1) | 34.6 (59.4) |
| Median (min., max.) | 9.5 (2, 233) | 11 (3, 43) | 9.5 (2, 233) |
| Proportion of acts unprotected (%) | 73.9 | 67.9 | 72.5 |
| Month 3 | n = 9 | n = 8 | n = 17 |
| Mean (SD) | 40.8 (58.3) | 19.9 (22.7) | 30.9 (45.2) |
| Median (min., max.) | 15 (5, 163) | 10 (3, 163) | 14 (3, 163) |
| Proportion of acts unprotected (%) | 59.1 | 74.8 | 63.9 |
| Month 6 | n = 1 | n = 5 | n = 6 |
| Mean (SD) | 38 (–) | 12.8 (18.7) | 17 (19.6) |
| Median (min., max.) | 38 (38, 38) | 5 (1, 46) | 6 (1, 46) |
| Proportion of acts unprotected (%) | 52.6 | 96.9 | 80.4 |
| Number of sex acts performed as part of sex trading | |||
| Baseline | 0 | 1 | 1 |
| Month 3 | 0 | 0 | 0 |
| Month 6 | 0 | 0 | 0 |
| Proportion of acts unprotected (%) | |||
| Baseline | – | 100 | 100 |
| Month 3 | – | – | – |
| Month 6 | – | – | – |
| SERBAS items | Intervention (N = 19) | Control (N = 17) | Overall (N = 36) |
|---|---|---|---|
| Type of relationship with main partner | |||
| Baseline | n = 19 | n = 17 | n = 36 |
| Steady relationship | 2 | 3 | 5 |
| Casual relationship | 3 | 2 | 5 |
| Missing | 14 | 12 | 26 |
| Month 3 | n = 16 | n = 13 | n = 29 |
| Steady relationship | 1 | 4 | 5 |
| Casual relationship | 3 | 2 | 5 |
| Missing | 12 | 7 | 19 |
| Month 6 | n = 8 | n = 7 | n = 15 |
| Steady relationship | 1 | 3 | 4 |
| Casual relationship | 1 | 1 | 2 |
| Missing | 6 | 3 | 9 |
| Vaginal sex | |||
| Baseline | n = 4 | n = 4 | n = 8 |
| Mean (SD) | 7 (9.5) | 10.5 (10.1) | 8.8 (9.3) |
| Median (min., max.) | 3 (1, 21) | 8.5 (1, 24) | 5 (1, 24) |
| Proportion of acts unprotected (%) | 92.9 | 73.8 | 81.4 |
| Month 3 | n = 3 | n = 6 | n = 9 |
| Mean (SD) | 10 (13.9) | 13.5 (9.5) | 12.3 (10.4) |
| Median (min., max.) | 3 (1, 26) | 16 (1, 26) | 15 (1, 26) |
| Proportion of acts unprotected (%) | 70.0 | 63.0 | 64.9 |
| Month 6 | n = 1 | n = 4 | n = 5 |
| Mean (SD) | 1 (–) | 7.8 (8.3) | 6.4 (7.8) |
| Median (min., max.) | 1 (1, 1) | 4.5 (2, 20) | 3 (1, 20) |
| Proportion of acts unprotected (%) | 0 | 61.3 | 59.4 |
| Anal sex, n | |||
| Baseline | n = 1 | n = 1 | n = 2 |
| Mean (SD) | 5 (–) | 2 (–) | 3.5 (2.1) |
| Median (min., max.) | 5 (5, 5) | 2 (2, 2) | 3.5 (2, 5) |
| Proportion of acts unprotected (%) | 100 | 100 | 100 |
| Month 3 | n = 1 | n = 1 | n = 2 |
| Mean (SD) | 4 (–) | 6 (–) | 5 (1.4) |
| Median (min., max.) | 4 (4, 4) | 6 (6, 6) | 5 (4, 6) |
| Proportion of acts unprotected (%) | 75 | 66.7 | 70 |
| Month 6 | n = 0 | n = 0 | n = 0 |
| Mean (SD) | – (–) | – (–) | – (–) |
| Median (min., max.) | – (–, –) | – (–, –) | – (–, –) |
| Proportion of acts unprotected (%) | – | – | – |
| Oral sex | |||
| Baseline | n = 4 | n = 5 | n = 9 |
| Mean (SD) | 9.5 (10.6) | 10.2 (11.7) | 9.9 (10.6) |
| Median (min., max.) | 6 (1, 25) | 3 (1, 24) | 5 (1, 25) |
| Proportion of acts unprotected (%) | 100 | 98.0 | 98.9 |
| Month 3 | n = 2 | n = 5 | n = 7 |
| Mean (SD) | 16 (17.0) | 13.4 (11.2) | 14.1 (11.5) |
| Median (min., max.) | 16 (4, 28) | 17 (1, 24) | 17 (1, 28) |
| Proportion of acts unprotected (%) | 100 | 97.0 | 98.0 |
| Month 6 | n = 1 | n = 3 | n = 4 |
| Mean (SD) | 1 (–) | 9 (10.4) | 7 (9.4) |
| Median (min., max.) | 1 (1, 1) | 4 (2, 21) | 3 (1, 21) |
| Proportion of acts unprotected (%) | 100 | 100 | 100 |
| Total number of sex acts | |||
| Baseline | n = 5 | n = 5 | n = 10 |
| Mean (SD) | 14.2 (21.0) | 19 (21.9) | 16.6 (20.4) |
| Median (min., max.) | 8 (1, 51) | 8 (1, 50) | 8 (1, 51) |
| Proportion of acts unprotected (%) | 97.2 | 87.4 | 91.6 |
| Month 3 | n = 4 | n = 6 | n = 10 |
| Mean (SD) | 16.5 (27.7) | 25.7 (19.1) | 22 (21.9) |
| Median (min., max.) | 3.5 (1, 58) | 30.5 (2, 45) | 15.5 (1, 58) |
| Proportion of acts unprotected (%) | 84.8 | 77.9 | 80.0 |
| Month 6 | n = 2 | n = 4 | n = 6 |
| Mean (SD) | 1 (0) | 14.5 (17.7) | 10 (15.4) |
| Median (min., max.) | 1 (1, 1) | 6 (5, 41) | 5.5 (1, 41) |
| Proportion of acts unprotected (%) | 50.0 | 79.3 | 78.3 |
| Number of sex acts performed as part of sex trading | |||
| Baseline | 0 | 1 | 1 |
| Month 3 | 1 | 0 | 1 |
| Month 6 | 0 | 3 | 3 |
| Proportion of acts unprotected (%) | |||
| Baseline | – | 100 | 100 |
| Month 3 | 0 | – | 0 |
| Month 6 | – | 100 | 100 |
| Intervention (N = 36) | Control (N = 36) | Overall (N = 72) | |
|---|---|---|---|
| Contraception used ever, n (%)a | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| No method used | 9 (25.0) | 5 (13.9) | 14 (19.4) |
| Been sterilised | 1 (2.8) | 2 (5.6) | 3 (4.2) |
| Partner sterilised | 3 (8.3) | 4 (11.1) | 7 (9.7) |
| The pill | 24 (66.7) | 24 (66.7) | 48 (66.7) |
| Male condom | 29 (80.6) | 33 (91.7) | 62 (86.1) |
| Female condom | 4 (11.1) | 2 (5.6) | 6 (8.3) |
| Morning-after pill | 14 (38.9) | 13 (36.1) | 27 (37.5) |
| Coil/IUD | 6 (16.7) | 10 (27.8) | 16 (22.2) |
| Hormonal IUD (Mirena®) (Bayer Healthcare Pharmaceuticals Inc., Whippany, NJ) | 3 (8.3) | 2 (5.6) | 5 (6.9) |
| Cap/diaphragm | 4 (11.1) | 1 (2.8) | 5 (6.9) |
| Injection | 5 (13.9) | 6 (16.7) | 11 (15.3) |
| Spermicides | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| Natural family planning | 4 (11.1) | 6 (16.7) | 10 (13.9) |
| Withdrawal | 16 (44.4) | 12 (33.3) | 28 (38.9) |
| Implants | 5 (13.9) | 3 (8.3) | 8 (11.1) |
| N/A (same-sex relationships) | 4 (11.1) | 6 (16.7) | 10 (13.9) |
| Other | 0 (0.0) | 2 (5.6) | 2 (2.8) |
| Month 3 | n = 30 | n = 29 | n = 59 |
| No method used | 18 (60.0) | 17 (58.6) | 35 (59.3) |
| Been sterilised | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Partner sterilised | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| The pill | 1 (3.3) | 4 (13.8) | 5 (8.5) |
| Male condom | 10 (33.3) | 6 (20.7) | 16 (27.1) |
| Female condom | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| Morning-after pill | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Emergency IUD | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Coil/IUD | 1 (3.3) | 2 (6.9) | 3 (5.1) |
| Hormonal IUD (Mirena®) | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| Cap/diaphragm | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Spermicides | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Natural family planning | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| Withdrawal | 3 (10.0) | 2 (6.9) | 5 (8.5) |
| Implants | 1 (3.3) | 2 (6.9) | 3 (5.1) |
| N/A (same-sex relationships) | 2 (6.7) | 1 (3.5) | 3 (5.1) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Month 6 | n = 13 | n = 16 | n = 29 |
| No method used | 9 (69.2) | 8 (50.0) | 17 (58.6) |
| Been sterilised | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Partner sterilised | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| The pill | 0 (0.0) | 2 (12.5) | 2 (6.9) |
| Male condom | 2 (15.4) | 4 (25.0) | 6 (20.7) |
| Female condom | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Morning-after pill | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Emergency IUD | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Coil/IUD | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Hormonal IUD (Mirena®) | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Cap/diaphragm | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Spermicides | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Natural family planning | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Withdrawal | 0 (0.0) | 1 (6.3) | 0 (0.0) |
| Implants | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A (same-sex relationships) | 1 (7.7) | 2 (12.5) | 3 (10.3) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Which have you used in the last year? n (%)a | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| No method used | 17 (47.2) | 17 (47.2) | 34 (47.2) |
| Partner sterilised | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| The pill | 5 (13.9) | 5 (13.9) | 10 (13.9) |
| Male condom | 13 (36.1) | 14 (38.9) | 27 (37.5) |
| Female condom | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Morning-after pill | 3 (8.3) | 2 (5.6) | 5 (6.9) |
| Coil/IUD | 1 (2.8) | 4 (11.1) | 5 (6.9) |
| Hormonal IUD (Mirena®) | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| Injection | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| Natural family planning | 1 (2.8) | 2 (5.6) | 3 (4.2) |
| Withdrawal | 7 (19.4) | 3 (8.3) | 10 (13.9) |
| Implants | 2 (5.6) | 0 (0.0) | 2 (2.8) |
| Other | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Month 3 | n = 30 | n = 29 | n = 59 |
| No method used | 17 (56.7) | 18 (62.1) | 35 (59.3) |
| Participant sterilised | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Partner sterilised | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| The pill | 0 (0.0) | 4 (13.8) | 4 (6.8) |
| Male condom | 11 (36.7) | 7 (24.1) | 18 (30.5) |
| Female condom | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| Morning-after pill | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Emergency IUD | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Coil/IUD | 1 (3.3) | 2 (6.9) | 3 (5.1) |
| Hormonal IUD (Mirena®) | 2 (6.7) | 0 (0.0) | 2 (3.4) |
| Cap/Diaphragm | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Spermicides | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Natural family planning | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| Withdrawal | 4 (13.3) | 2 (6.9) | 6 (10.2) |
| Implants | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Month 6 | n = 13 | n = 16 | n = 29 |
| No method used | 9 (69.2) | 10 (62.5) | 19 (65.5) |
| Participant sterilised | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Partner sterilised | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| The pill | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Male condom | 3 (23.1) | 4 (25.0) | 7 (24.1) |
| Female condom | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Morning-after pill | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Emergency IUD | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Coil/IUD | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Hormonal IUD (Mirena®) | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Cap/Diaphragm | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Spermicides | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Natural family planning | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Withdrawal | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Implants | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Which is the most usual these days? n (%)a | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| No method used | 20 (55.6) | 17 (47.2) | 37 (51.4) |
| Been sterilised | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Partner sterilised | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| The pill | 3 (8.3) | 5 (13.9) | 8 (11.1) |
| Male condom | 16 (44.4) | 12 (33.3) | 28 (38.9) |
| Female condom | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Morning-after pill | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Coil/IUD | 0 (0.0) | 2 (5.6) | 2 (2.8) |
| Hormonal IUD (Mirena®) | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| Injection | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Withdrawal | 2 (5.6) | 0 (0.0) | 2 (2.8) |
| Month 3 | n = 30 | n = 29 | n = 59 |
| No method used | 20 (66.7) | 18 (62.1) | 38 (64.4) |
| Been sterilised | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Partner sterilised | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| The pill | 0 (0.0) | 3 (10.3) | 3 (5.1) |
| Male condom | 8 (26.7) | 4 (13.8) | 12 (20.3) |
| Female condom | 1 (3.5) | 0 (0.0) | 1 (1.7) |
| Morning-after pill | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Coil/IUD | 0 (0.0) | 2 (6.9) | 2 (3.4) |
| Hormonal IUD (Mirena®) | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| Cap/diaphragm | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Spermicides | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Natural family planning | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| Withdrawal | 3 (10.0) | 0 (0.0) | 3 (5.1) |
| Implants | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Month 6 | n = 13 | n = 16 | n = 29 |
| No method used | 9 (69.2) | 8 (50.0) | 17 (58.6) |
| Been sterilised | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Partner sterilised | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| The pill | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Male condom | 3 (23.1) | 3 (18.8) | 6 (20.7) |
| Female condom | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Morning-after pill | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Emergency IUD | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Coil/IUD | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Hormonal IUD (Mirena®) | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Cap/diaphragm | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Spermicides | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Natural family planning | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Withdrawal | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Implants | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| If condoms were used in the last year, for what purpose? n (%)a | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| To prevent pregnancy | 11 (30.6) | 10 (27.8) | 21 (29.2) |
| To protect against HIV | 7 (19.4) | 7 (19.4) | 14 (19.4) |
| To protect against other STIs | 9 (25.0) | 8 (22.2) | 17 (23.6) |
| N/A | 16 (44.4) | 20 (55.6) | 36 (50.0) |
| Month 3 | n = 30 | n = 29 | n = 59 |
| To prevent pregnancy | 10 (33.3) | 6 (20.7) | 16 (27.1) |
| To protect against HIV | 8 (26.7) | 3 (10.3) | 11 (18.6) |
| To protect against other STIs | 10 (33.3) | 5 (17.2) | 15 (25.4) |
| N/A | 13 (43.3) | 17 (58.6) | 30 (50.9) |
| Month 6 | n = 13 | n = 16 | n = 29 |
| To prevent pregnancy | 2 (15.4) | 4 (25.0) | 6 (20.7) |
| To protect against HIV | 2 (15.4) | 1 (6.3) | 3 (10.3) |
| To protect against other STIs | 2 (15.4) | 3 (18.8) | 5 (17.2) |
| N/A | 7 (53.9) | 7 (43.8) | 14 (48.3) |
| Have you got contraception from any of these sources in the last year? n (%)a | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Doctor or nurse at GP surgery | 3 (8.3) | 9 (25.0) | 12 (16.7) |
| Sexual health clinic | 9 (25.0) | 5 (13.9) | 14 (19.4) |
| Family planning clinic | 2 (5.6) | 2 (5.6) | 4 (5.6) |
| Private doctor/clinic | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Pharmacy/chemist | 5 (13.9) | 8 (22.2) | 13 (18.1) |
| Internet website | 2 (5.6) | 0 (0.0) | 2 (2.8) |
| School/college/university services | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Over the counter at petrol station/supermarket | 5 (13.9) | 5 (13.9) | 10 (13.9) |
| Mail order | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Hospital A&E | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Other | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| N/A (not in the last year) | 15 (41.7) | 16 (44.4) | 31 (43.1) |
| Month 3 | n = 30 | n = 29 | n = 59 |
| Doctor or nurse at GP surgery | 2 (6.7) | 4 (13.8) | 6 (10.2) |
| Sexual health clinic | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| Family planning clinic | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| Private doctor/clinic | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pharmacy/chemist | 2 (6.7) | 3 (10.3) | 5 (8.5) |
| Internet website | 1 (3.3) | 0 (0.0) | 0 (0.0) |
| School/college/university services | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Over the counter at petrol station/supermarket | 3 (10.0) | 0 (0.0) | 3 (5.1) |
| Vending machine | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| Mail order | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hospital A&E | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 13 (43.3) | 0 (0.0) | 13 (22.0) |
| N/A (not in the last year) | 11 (36.7) | 19 (65.5) | 30 (50.9) |
| Month 6 | n = 13 | n = 16 | n = 29 |
| Doctor or nurse at GP surgery | 0 (0.0) | 2 (12.5) | 2 (6.9) |
| Sexual health clinic | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Family planning clinic | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Private doctor/clinic | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pharmacy/chemist | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Internet website | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| School/college/university services | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Over the counter at petrol station/supermarket | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vending machine | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mail order | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hospital A&E | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| N/A (not in the last year) | 11 (84.6) | 10 (62.5) | 21 (72.4) |
| If all were available, where would you prefer to get contraception from? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Doctor or nurse at GP surgery | 6 (16.7) | 13 (36.1) | 19 (26.4) |
| Sexual health clinic | 7 (19.4) | 4 (11.1) | 11 (15.3) |
| Family planning clinic | 2 (5.6) | 0 (0.0) | 2 (2.8) |
| Pharmacy/chemist | 14 (38.9) | 15 (41.7) | 29 (40.3) |
| None of these | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| Not needed | 3 (8.3) | 3 (8.3) | 6 (8.3) |
| Missing | 2 (5.6) | 0 (0.0) | 2 (2.8) |
| Month 3 | n = 30 | n = 29 | n = 59 |
| Doctor or nurse at GP surgery | 7 (23.3) | 9 (31.0) | 16 (27.1) |
| Sexual health clinic | 1 (3.3) | 4 (13.8) | 5 (8.5) |
| Family planning clinic | 6 (20.0) | 0 (0.0) | 6 (10.2) |
| Youth advisory clinic | 10 (33.3) | 13 (44.8) | 23 (39.0) |
| Pharmacy/chemist | 2 (6.7) | 0 (0.0) | 2 (2.4) |
| None of these | 2 (6.7) | 1 (3.5) | 3 (5.1) |
| Not needed | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Missing | 2 (6.7) | 1 (3.5) | 3 (5.1) |
| Month 6 | n = 13 | n = 16 | n = 29 |
| Doctor or nurse at GP surgery | 1 (7.7) | 6 (37.5) | 7 (24.1) |
| Sexual health clinic | 3 (23.1) | 0 (0.0) | 3 (10.3) |
| Family planning clinic | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| Youth advisory clinic | 4 (30.8) | 4 (25.0) | 8 (27.6) |
| Pharmacy/chemist | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| None of these | 0 (0.0) | 3 (18.8) | 3 (10.3) |
| Not needed | 4 (30.8) | 1 (6.3) | 5 (17.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| If you thought you had a STI, where would you go first? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| GP surgery | 18 (50.0) | 23 (63.9) | 41 (56.9) |
| Sexual health clinic | 16 (14.4) | 11 (30.6) | 27 (37.5) |
| Pharmacy/chemist | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Somewhere else | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Missing | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| Month 3 | n = 30 | n = 29 | n = 59 |
| GP surgery | 15 (50.0) | 21 (72.4) | 36 (61.0) |
| Sexual health clinic | 13 (43.3) | 6 (20.7) | 19 (32.2) |
| NHS Family planning clinic | 2 (6.7) | 0 (0.0) | 2 (3.4) |
| Pharmacy/chemist | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Missing | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Month 6 | n = 13 | n = 16 | n = 29 |
| GP surgery | 5 (38.5) | 11 (68.8) | 16 (55.2) |
| Sexual health clinic | 7 (53.9) | 3 (18.8) | 10 (34.5) |
| Pharmacy/chemist | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| NHS website | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Missing | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| Have you ever attended a sexual health clinic? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Yes | 19 (52.8) | 20 (55.6) | 39 (54.2) |
| No | 13 (36.1) | 12 (33.3) | 25 (34.7) |
| Missing | 4 (11.1) | 4 (11.1) | 8 (11.1) |
| If yes, when was it? | n = 19 | n = 20 | n = 39 |
| < 1 year ago | 6 (31.6) | 3 (15.0) | 9 (23.1) |
| Between 1 and 5 years ago | 7 (36.8) | 7 (35.0) | 14 (35.9) |
| Between 5 and 10 years ago | 1 (5.3) | 2 (10.0) | 3 (7.7) |
| > 10 years ago | 5 (26.3) | 7 (35.0) | 12 (30.8) |
| Missing | 0 (0.0) | 1 (5.0) | 1 (2.6) |
| In the last 3 months | |||
| Month 3 | n = 30 | n = 29 | n = 59 |
| Yes | 3 (10.0) | 3 (10.3) | 6 (10.2) |
| No | 27 (90.0) | 25 (86.2) | 52 (88.1) |
| Missing | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| In the last 3 months | |||
| Month 6 | n = 13 | n = 16 | n = 29 |
| Yes | 2 (15.4) | 0 (0.0) | 2 (6.9) |
| No | 11 (84.6) | 15 (93.8) | 26 (89.7) |
| Missing | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Have you ever been told by a doctor or health-care professional that you have any of the following? n (%)a | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Chlamydia | 7 (19.4) | 2 (5.6) | 9 (12.5) |
| Gonorrhoea | 3 (8.3) | 4 (11.1) | 7 (9.7) |
| Genital warts | 6 (16.7) | 3 (8.3) | 9 (12.5) |
| Syphilis | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| Trichomonas vaginalis | 1 (2.8) | 2 (5.6) | 3 (4.2) |
| Herpes | 2 (5.6) | 4 (11.1) | 6 (8.3) |
| Pubic lice/carbs | 3 (8.3) | 2 (5.6) | 5 (6.9) |
| Hepatitis B | 3 (8.3) | 0 (0.0) | 3 (4.2) |
| None of these | 14 (38.9) | 16 (44.4) | 30 (41.7) |
| Other | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Men only | n = 18 | n = 17 | n = 35 |
| NSU or NGU | 2 (11.1) | 0 (0.0) | 2 (5.7) |
| Epididymitis | 1 (5.6) | 0 (0.0) | 1 (2.8) |
| Women only | n = 17 | n = 17 | n = 34 |
| Pelvic inflammatory disease | 1 (5.9) | 1 (5.9) | 2 (5.9) |
| Vaginal thrush | 11 (64.7) | 10 (58.8) | 21 (61.8) |
| Bacterial vaginosis | 5 (29.4) | 3 (17.7) | 8 (23.5) |
| In the last 3 months | |||
| Month 3 | n = 30 | n = 29 | n = 59 |
| Chlamydia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gonorrhoea | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Genital warts | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Syphilis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Trichomonas vaginalis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Herpes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pubic lice/carbs | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hepatitis B | 0 (0.0) | 1 (3.3) | 1 (1.7) |
| None of these | 28 (93.3) | 24 (82.8) | 52 (88.1) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Men only | n = 16 | n = 13 | n = 29 |
| NSU or NGU | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Epididymitis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Women only | n = 14 | n = 14 | n = 28 |
| Pelvic inflammatory disease | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vaginal thrush | 1 (7.1) | 1 (7.1) | 2 (7.1) |
| Bacterial vaginosis | 1 (7.1) | 0 (0.0) | 1 (3.6) |
| In the last 3 months | |||
| Month 6 | n = 13 | n = 16 | n = 29 |
| Chlamydia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gonorrhoea | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Genital warts | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Syphilis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Trichomonas vaginalis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Herpes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pubic lice/carbs | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hepatitis B | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| None of these | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Men only | n = 8 | n = 7 | n = 15 |
| NSU or NGU | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Epididymitis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Women only | n = 5 | n = 9 | n = 14 |
| Pelvic inflammatory disease | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vaginal thrush | 0 (0.0) | 1 (11.1) | 1 (7.1) |
| Bacterial vaginosis | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Have you ever been tested for HIV? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Yes | 24 (66.7) | 18 (50.0) | 42 (58.3) |
| No | 11 (30.6) | 16 (44.4) | 27 (37.5) |
| Not sure/maybe | 1 (2.8) | 2 (5.6) | 3 (4.2) |
| If yes, why were you tested? n (%)a | n = 24 | n = 18 | n = 42 |
| I/my partner was pregnant | 2 (8.3) | 1 (5.6) | 3 (7.1) |
| As part of a sexual health check-up | 9 (37.5) | 8 (44.4) | 17 (40.5) |
| As part of a general health check-up | 1 (4.2) | 3 (16.7) | 4 (9.5) |
| I wanted to stop using condoms | 2 (8.3) | 1 (5.6) | 3 (7.2) |
| I was concerned about personal risks to myself or partner | 11 (45.8) | 10 (55.6) | 21 (50.0) |
| A doctor advised me to | 2 (8.3) | 1 (5.6) | 3 (7.1) |
| Other | 3 (12.5) | 2 (11.1) | 5 (11.9) |
| When was it? n (%) | n = 24 | n = 18 | n = 42 |
| In the last year | 5 (20.8) | 3 (16.7) | 8 (19.1) |
| Between 1 and 2 years ago | 3 (12.5) | 4 (22.2) | 7 (16.7) |
| Between 2 and 5 years ago | 5 (20.8) | 4 (22.2) | 9 (21.4) |
| < 5 years ago | 11 (45.8) | 7 (38.9) | 18 (42.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Where were you tested? n (%) | n = 24 | n = 18 | n = 42 |
| GP surgery | 1 (4.2) | 1 (5.6) | 2 (4.8) |
| Sexual health clinic | 13 (54.2) | 13 (72.2) | 26 (61.9) |
| NHS family planning clinic | 2 (8.3) | 0 (0.0) | 2 (4.8) |
| NHS antenatal clinic/midwife | 1 (4.2) | 0 (0.0) | 1 (2.4) |
| Hospital A&E | 1 (4.2) | 1 (5.6) | 2 (4.8) |
| Somewhere else | 5 (20.8) | 3 (16.7) | 8 (19.1) |
| Missing | 1 (4.2) | 0 (0.0) | 2 (2.4) |
| Were you given the results? n (%) | n = 24 | n = 18 | n = 42 |
| Yes | 22 (91.7) | 18 (100.0) | 40 (95.2) |
| No | 1 (4.2) | 0 (0.0) | 1 (2.4) |
| Missing | 1 (4.2) | 0 (0.0) | 1 (2.4) |
| Month 3 | |||
| In the last 3 months were you tested for HIV? n (%) | n = 30 | n = 29 | n = 59 |
| Yes | 4 (13.3) | 1 (3.5) | 5 (8.5) |
| No | 26 (86.7) | 27 (93.1) | 53 (89.8) |
| Not sure/maybe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| If yes, why were you tested? n (%)a | n = 4 | n = 1 | n = 5 |
| I/my partner was pregnant | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| As part of a sexual health check up | 2 (50.0) | 1 (100.0) | 3 (60.0) |
| As part of a general health check up | 1 (25.0) | 0 (0.0) | 1 (20.0) |
| I wanted to stop using condoms | 0 (0.0) | 1 (100.0) | 1 (20.0) |
| I was concerned about personal risks to myself or partner | 2 (50.0) | 1 (100.0) | 3 (60.0) |
| A doctor advised me to | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Where were you tested? n (%) | |||
| GP surgery | 1 (25.0) | 0 (0.0) | 1 (20.0) |
| Sexual health clinic | 3 (75.0) | 1 (100.0) | 4 (80.0) |
| NHS family planning clinic | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| NHS antenatal clinic/midwife | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hospital A&E | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Somewhere else | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Were you given the results? n (%) | |||
| Yes | 4 (100.0) | 1 (100.0) | 5 (100.0) |
| No | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Month 6 | |||
| In the last 3 months were you tested for HIV? n (%) | n = 13 | n = 16 | n = 29 |
| Yes | 3 (23.1) | 0 (0.0) | 3 (10.3) |
| No | 10 (76.9) | 15 (93.8) | 25 (86.2) |
| Not sure/maybe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| If yes, why were you tested? n (%)a | n = 3 | n = 0 | n = 3 |
| I/my partner was pregnant | 0 (0.0) | – (–) | 0 (0.0) |
| As part of a sexual health check up | 0 (0.0) | – (–) | 0 (0.0) |
| As part of a general health check up | 2 (66.7) | – (–) | 2 (66.7) |
| I wanted to stop using condoms | 1 (33.3) | – (–) | 1 (33.3) |
| I was concerned about personal risks to myself or partner | 0 (0.0) | – (–) | 0 (0.0) |
| A doctor advised me to | 1 (33.3) | – (–) | 1 (33.3) |
| Other | 0 (0.0) | – (–) | 0 (0.0) |
| Where were you tested? n (%) | 0 (0.0) | – (–) | 0 (0.0) |
| GP surgery | 1 (33.3) | – (–) | 1 (33.3) |
| Sexual health clinic | 2 (66.7) | – (–) | 2 (66.7) |
| NHS family planning clinic | 0 (0.0) | – (–) | 0 (0.0) |
| NHS antenatal clinic/midwife | 0 (0.0) | – (–) | 0 (0.0) |
| Hospital A&E | 0 (0.0) | – (–) | 0 (0.0) |
| Somewhere else | 0 (0.0) | – (–) | 0 (0.0) |
| Missing | 0 (0.0) | – (–) | 0 (0.0) |
| Were you given the results? n (%) | |||
| Yes | 3 (100.0) | – (–) | 3 (100.0) |
| No | 0 (0.0) | – (–) | 0 (0.0) |
| Missing | 0 (0.0) | – (–) | 0 (0.0) |
| Have you or your partner been pregnant in the last 12 months? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Yes | 0 (0.0) | 2 (5.6) | 2 (2.8) |
| No | 35 (97.2) | 33 (91.7) | 68 (94.4) |
| Missing | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| In the last 3 months: | |||
| Month 3 | n = 30 | n = 29 | n = 59 |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No | 28 (93.3) | 28 (96.6) | 56 (94.9) |
| Missing | 2 (6.7) | 1 (3.5) | 3 (5.1) |
| Month 6 | n = 13 | n = 16 | n = 29 |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No | 13 (100.0) | 14 (87.5) | 27 (93.1) |
| Missing | 0 (0.0) | 2 (12.5) | 2 (6.9) |
| If you were planning a family do you know who could offer advice/support? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Yes | 20 (55.6) | 21 (58.3) | 41 (56.9) |
| No | 12 (33.3) | 14 (38.9) | 26 (36.1) |
| Missing | 4 (11.1) | 1 (2.8) | 5 (6.9) |
| If yes, where?a | n = 20 | n = 21 | n = 41 |
| GP | 12 (60.0) | 11 (52.4) | 23 (56.1) |
| Family and friends | 3 (15.0) | 5 (23.8) | 8 (19.5) |
| Family planning clinic | 4 (20.0) | 2 (9.5) | 6 (14.6) |
| Online | 2 (10.0) | 1 (4.8) | 3 (7.3) |
| Sexual health clinic | 1 (5.0) | 3 (14.3) | 4 (9.8) |
| Pharmacy | 1 (5.0) | 0 (0.0) | 1 (2.4) |
| Hospital | 1 (5.0) | 0 (0.0) | 1 (2.4) |
| Other | 1 (5.0) | 1 (4.8) | 2 (4.9) |
| Month 3 | n = 30 | n = 29 | n = 59 |
| Yes | 20 (66.7) | 17 (58.6) | 37 (62.7) |
| No | 9 (30.0) | 10 (34.5) | 19 (32.2) |
| Missing | 1 (3.3) | 2 (6.9) | 3 (5.1) |
| Month 6 | n = 13 | n = 16 | n = 29 |
| Yes | 8 (61.5) | 8 (50.0) | 16 (55.2) |
| No | 5 (38.6) | 6 (37.5) | 11 (37.9) |
| Missing | 0 (0.0) | 2 (12.5) | 2 (6.9) |
| Intervention (N = 36) | Control (N = 36) | Overall (N = 72) | |
|---|---|---|---|
| Based on your sexual behaviour over the past year, how much do you think you are at risk of getting a STI? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| No risk at all | 21 (58.3) | 22 (61.1) | 43 (59.7) |
| Slightly at risk | 5 (13.9) | 12 (33.3) | 17 (23.6) |
| Somewhat at risk | 2 (5.6) | 0 (0.0) | 2 (2.8) |
| Good deal at risk | 7 (19.4) | 2 (2.6) | 9 (12.5) |
| Great deal at risk | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Extremely at risk | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| No risk at all | 21 (70.0) | 18 (62.1) | 39 (66.1) |
| Slightly at risk | 7 (23.3) | 8 (27.6) | 15 (25.4) |
| Somewhat at risk | 2 (6.7) | 3 (10.3) | 5 (8.5) |
| Good deal at risk | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Great deal at risk | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Extremely at risk | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| No risk at all | 9 (69.2) | 9 (56.3) | 18 (62.1) |
| Slightly at risk | 4 (30.8) | 6 (37.5) | 10 (34.5) |
| Somewhat at risk | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Good deal at risk | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Great deal at risk | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Extremely at risk | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| What is the chance that you will someday get a STI? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| No risk at all | 14 (38.9) | 9 (25.0) | 23 (31.9) |
| Slightly at risk | 8 (22.2) | 18 (50.0) | 26 (36.1) |
| Somewhat at risk | 9 (25.0) | 5 (13.9) | 14 (19.4) |
| Good deal at risk | 1 (2.8) | 2 (5.6) | 3 (4.2) |
| Great deal at risk | 3 (8.3) | 0 (0.0) | 3 (4.2) |
| Extremely at risk | 1 (2.8) | 2 (5.6) | 3 (4.2) |
| 3 months | n = 30 | n = 29 | n = 59 |
| No risk at all | 12 (40.0) | 6 (20.7) | 18 (30.5) |
| Slightly at risk | 12 (40.0) | 16 (55.2) | 28 (47.5) |
| Somewhat at risk | 3 (10.0) | 5 (17.2) | 8 (13.6) |
| Good deal at risk | 2 (6.7) | 0 (0.0) | 2 (3.4) |
| Great deal at risk | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Extremely at risk | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| Missing | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| No risk at all | 7 (53.9) | 6 (37.5) | 13 (44.8) |
| Slightly at risk | 3 (23.1) | 8 (50.0) | 11 (37.9) |
| Somewhat at risk | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| Good deal at risk | 2 (15.4) | 1 (6.3) | 3 (10.3) |
| Great deal at risk | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Extremely at risk | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I think STIs are a serious problem in my community, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| No risk at all | 4 (11.1) | 8 (22.2) | 12 (16.7) |
| Slightly at risk | 4 (11.1) | 8 (22.2) | 12 (16.7) |
| Somewhat at risk | 10 (27.8) | 11 (30.6) | 21 (29.2) |
| Good deal at risk | 8 (22.2) | 2 (5.6) | 10 (13.9) |
| Great deal at risk | 2 (5.6) | 3 (8.3) | 5 (6.9) |
| Extremely at risk | 7 (19.4) | 3 (8.3) | 10 (13.9) |
| Missing | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| 3 months | n = 30 | n = 29 | n = 59 |
| No risk at all | 5 (16.7) | 6 (20.7) | 11 (18.6) |
| Slightly at risk | 2 (6.7) | 7 (24.1) | 9 (15.3) |
| Somewhat at risk | 8 (26.7) | 7 (24.1) | 15 (25.4) |
| Good deal at risk | 5 (16.7) | 3 (10.3) | 8 (13.6) |
| Great deal at risk | 2 (6.7) | 2 (6.9) | 4 (6.8) |
| Extremely at risk | 8 (26.7) | 2 (6.9) | 10 (17.0) |
| Missing | 0 (0.0) | 2 (6.9) | 2 (3.4) |
| 6 months | n = 13 | n = 16 | n = 29 |
| No risk at all | 3 (23.1) | 3 (18.8) | 6 (20.7) |
| Slightly at risk | 2 (15.4) | 3 (18.8) | 5 (17.2) |
| Somewhat at risk | 3 (23.1) | 3 (18.8) | 6 (20.7) |
| Good deal at risk | 2 (15.4) | 5 (31.3) | 7 (24.1) |
| Great deal at risk | 1 (7.7) | 2 (12.5) | 3 (10.3) |
| Extremely at risk | 2 (15.4) | 0 (0.0) | 2 (6.9) |
| I worry about getting a STI, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| No risk at all | 17 (47.2) | 16 (44.4) | 33 (45.8) |
| Slightly at risk | 7 (19.4) | 12 (33.3) | 19 (26.4) |
| Somewhat at risk | 3 (8.3) | 4 (11.1) | 7 (9.7) |
| Good deal at risk | 4 (11.1) | 1 (2.8) | 5 (6.9) |
| Great deal at risk | 4 (11.1) | 0 (0.0) | 4 (5.6) |
| Extremely at risk | 1 (2.8) | 2 (5.6) | 3 (4.2) |
| Missing | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| No risk at all | 6 (20.0) | 4 (13.8) | 10 (17.0) |
| Slightly at risk | 1 (3.3) | 6 (20.7) | 7 (11.9) |
| Somewhat at risk | 2 (6.7) | 4 (13.8) | 6 (10.2) |
| Good deal at risk | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Great deal at risk | 2 (6.7) | 0 (0.0) | 2 (3.4) |
| Extremely at risk | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| Missing | 18 (60.0) | 15 (51.7) | 33 (55.9) |
| 6 months | n = 13 | n = 16 | n = 29 |
| No risk at all | 6 (46.2) | 5 (31.3) | 11 (37.9) |
| Slightly at risk | 1 (7.7) | 7 (43.8) | 8 (27.6) |
| Somewhat at risk | 2 (15.4) | 4 (25.0) | 6 (20.7) |
| Good deal at risk | 1 (7.7) | 0 (0.0) | 1 (3.5) |
| Great deal at risk | 2 (15.4) | 0 (0.0) | 2 (6.9) |
| Extremely at risk | 1 (7.7) | 0 (0.0) | 1 (3.5) |
| Intervention (N = 36) | Control (N = 36) | Overall (N = 72) | |
|---|---|---|---|
| Section 1 | |||
| Baseline | n = 35 | n = 34 | n = 69 |
| Mean (SD) | 35.5 (5.3) | 35.6 (4.0) | 35.5 (4.7) |
| Median (min., max.) | 35 (22, 47) | 35 (28, 45) | 35 (22, 47) |
| 3 months | n = 26 | n = 25 | n = 51 |
| Mean (SD) | 35.6 (4.4) | 35.7 (5.4) | 35.7 (4.9) |
| Median (min., max.) | 35.5 (28, 45) | 35 (23, 48) | 35 (23, 48) |
| 6 months | n = 11a | n = 16a | n = 27a |
| Mean (SD) | 36.1 (5.4) | 36.3 (5.7) | 36.2 (5.5) |
| Median (min., max.) | 37 (28, 45) | 37 (22, 45) | 37 (22, 45) |
| Section 2 | |||
| Baseline | n = 35 | n = 34 | n = 69 |
| Mean (SD) | 11.3 (5.6) | 10.1 (5.0) | 10.7 (5.3) |
| Median (min., max.) | 12 (0, 24) | 10 (2, 24) | 11 (0, 24) |
| 3 months | n = 26 | n = 25 | n = 51 |
| Mean (SD) | 14.0 (5.0) | 9.6 (3.9) | 11.9 (5.0) |
| Median (min., max.) | 14.5 (2, 24) | 10 (2, 17) | 13 (2, 24) |
| 6 months | n = 11a | n = 16a | n = 27a |
| Mean (SD) | 13.3 (4.5) | 8.1 (5.5) | 10.2 (5.7) |
| Median (min., max.) | 14 (6, 21) | 7 (0, 17) | 7 (0, 17) |
| Overall | |||
| Baseline | n = 35 | n = 34 | n = 69 |
| Mean (SD) | 46.8 (8.5) | 45.7 (5.0) | 46.2 (6.9) |
| Median (min., max.) | 48 (22, 63) | 46 (35, 57) | 47 (22, 63) |
| 3 months | n = 26 | n = 25 | n = 51 |
| Mean (SD) | 49.7 (7.4) | 45.4 (7.0) | 47.5 (7.5) |
| Median (min., max.) | 49 (30, 66) | 47 (28, 62) | 48 (28, 66) |
| 6 months | n = 11 | n = 16 | n = 27 |
| Mean (SD) | 49.4 (6.3) | 44.3 (7.8) | 46.4 (7.5) |
| Median (min., max.) | 48 (42, 60) | 46 (25, 56) | 48 (25, 60) |
| Intervention (N = 36) | Control (N = 36) | Overall (N = 72) | |
|---|---|---|---|
| Your beliefs about what staff think about mental illness and sexuality | |||
| Staff make patients feel comfortable to talk about sexuality and sex issues, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 21 (58.3) | 26 (72.2) | 47 (65.3) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 2 (5.6) | 3 (8.3) | 5 (6.9) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 21 (70.0) | 16 (55.2) | 37 (62.7) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 3 (10.0) | 3 (10.3) | 6 (10.2) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 7 (53.9) | 10 (62.5) | 17 (58.6) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 2 (15.4) | 1 (6.3) | 3 (10.3) |
| Staff talk to patients about how psychiatric medication can interfere in sexual functioning, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 15 (41.7) | 14 (38.9) | 29 (40.3) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 3 (8.3) | 2 (5.6) | 5 (6.9) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 12 (40.0) | 13 (44.8) | 25 (42.4) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 3 (10.3) | 3 (5.1) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 6 (46.2) | 8 (50.0) | 14 (48.3) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Staff are supportive when patients express interest in having a romantic or sexual relationship, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 21 (58.3) | 21 (58.3) | 42 (58.3) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 7 (19.4) | 6 (16.7) | 13 (18.1) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 19 (63.3) | 18 (62.1) | 37 (62.7) |
| Missing/refused to answer | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| N/A | 4 (13.3) | 4 (13.8) | 8 (13.6) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 8 (61.5) | 12 (75.0) | 20 (69.0) |
| Missing/refused to answer | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| N/A | 2 (15.4) | 1 (6.3) | 3 (10.3) |
| Staff are not supportive when patients talk about sexual issues, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 11 (30.6) | 10 (27.8) | 21 (29.2) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 6 (16.7) | 4 (11.1) | 10 (13.9) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 3 (10.0) | 6 (20.7) | 9 (15.3) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 7 (23.3) | 2 (6.9) | 9 (15.3) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 4 (30.8) | 4 (25.0) | 8 (27.6) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 2 (15.4) | 2 (12.5) | 4 (13.8) |
| Your beliefs about how most people think about mental illness and sexuality | |||
| Most people do not show interest in having a sexual or romantic relationship with someone who has a mental illness, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 25 (69.4) | 22 (61.1) | 47 (65.3) |
| Missing/refused to answer | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| N/A | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 21 (70.0) | 17 (58.6) | 38 (64.4) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 11 (84.6) | 7 (43.8) | 18 (62.1) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Most people think that a person with mental illness does not get to be a good partner for someone who does not have a mental illness, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 29 (80.6) | 28 (77.8) | 57 (79.2) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 20 (66.7) | 20 (69.0) | 40 (67.8) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 11 (84.6) | 8 (50.0) | 19 (65.5) |
| Missing/refused to answer | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Most people when they find out someone is a user of mental health services, they do not think that person is sexually desirable, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 19 (52.8) | 18 (50.0) | 37 (51.4) |
| Missing/refused to answer | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| N/A | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 19 (63.3) | 15 (51.7) | 34 (57.6) |
| Missing/refused to answer | 0 (0.0) | 2 (6.9) | 2 (3.4) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 7 (53.9) | 8 (50.0) | 15 (51.7) |
| Missing/refused to answer | 2 (15.4) | 2 (12.5) | 4 (13.8) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Most people think users of mental health services should not have sexual or romantic relationships, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 9 (25.0) | 13 (36.1) | 22 (30.6) |
| Missing/refused to answer | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 5 (16.7) | 8 (27.6) | 13 (22.0) |
| Missing/refused to answer | 0 (0.0) | 2 (6.9) | 2 (3.4) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 6 (46.2) | 4 (25.0) | 10 (34.5) |
| Missing/refused to answer | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Your own beliefs about mental illness and sexuality | |||
| Having a mental Illness has a negative impact on your opportunities for sexual relationships, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 24 (66.7) | 23 (63.9) | 47 (65.3) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 19 (63.3) | 22 (75.9) | 41 (69.5) |
| Missing/refused to answer | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| N/A | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 11 (84.6) | 10 (62.5) | 21 (72.4) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| You are the one who chooses the course of your sexual life, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 25 (69.4) | 30 (83.3) | 55 (76.4) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 26 (86.7) | 19 (65.5) | 45 (76.3) |
| Missing/refused to answer | 1 (1.3) | 2 (6.9) | 3 (5.1) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 12 (92.3) | 13 (81.3) | 25 (86.2) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| You hide the fact that you have been diagnosed with a mental illness from people you are interested in having a romantic or sexual relationship with, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 20 (55.6) | 20 (55.6) | 40 (55.6) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 2 (5.6) | 2 (5.6) | 4 (5.6) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 21 (70.0) | 12 (41.4) | 33 (55.9) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 1 (3.3) | 2 (6.9) | 3 (5.1) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 11 (84.6) | 3 (18.8) | 14 (48.3) |
| Missing/refused to answer | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| N/A | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Having a mental illness makes me you feel less attractive than other women/men, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 24 (66.7) | 24 (66.7) | 48 (66.7) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 16 (53.3) | 19 (65.5) | 35 (59.3) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 10 (76.9) | 9 (56.3) | 19 (65.5) |
| Missing/refused to answer | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| N/A | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| You feel more comfortable having a romantic or sexual relationship with people who have also used mental health services, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 13 (36.1) | 13 (36.1) | 26 (36.1) |
| Missing/refused to answer | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| N/A | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 11 (36.7) | 13 (44.8) | 24 (40.7) |
| Missing/refused to answer | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| N/A | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Agree or strongly agree | 6 (46.2) | 5 (31.3) | 11 (37.9) |
| Missing/refused to answer | 1 (7.7) | 2 (12.5) | 3 (10.3) |
| N/A | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| In order to be sexually active, you always do what other people ask of you, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 8 (22.2) | 10 (27.8) | 18 (25.0) |
| Missing/refused to answer | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| N/A | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 9 (30.0) | 6 (20.7) | 15 (25.4) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 1 (7.7) | 0 (0.0) | 1 (3.5) |
| Missing/refused to answer | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| You avoid approaching someone you are interested in having a sexual or romantic relationship with if you think s/he has negative attitudes about users of mental health services, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 25 (69.4) | 22 (61.1) | 47 (65.3) |
| Missing/refused to answer | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| N/A | 3 (8.3) | 1 (2.8) | 4 (5.6) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 21 (70.0) | 21 (72.4) | 42 (71.2) |
| Missing/refused to answer | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| N/A | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 8 (61.5) | 11 (68.8) | 19 (65.5) |
| Missing/refused to answer | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| N/A | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| You explain what mental illness is to those you are interested in having a sexual or romantic relationship with, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Agree or strongly agree | 21 (58.3) | 25 (69.4) | 46 (63.9) |
| Missing/refused to answer | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| N/A | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Agree or strongly agree | 19 (63.3) | 18 (62.1) | 37 (62.7) |
| Missing/refused to answer | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| N/A | 2 (6.7) | 1 (3.5) | 3 (5.1) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 6 (46.2) | 9 (56.3) | 15 (51.7) |
| Missing/refused to answer | 1 (7.7) | 2 (12.5) | 3 (10.3) |
| N/A | 2 (15.4) | 1 (6.3) | 3 (10.3) |
| Family and staff where you are currently receiving services | |||
| How often has someone in your family said that because you are a user of mental health services you should not have sex? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 2 (5.6) | 3 (8.3) | 5 (6.9) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 0 (0.0) | 4 (13.8) | 4 (6.8) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 4 (30.8) | 1 (6.3) | 5 (17.2) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| And how often has a mental health care provider said that to you? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 1 (7.7) | 0 (0.0) | 1 (3.5) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| How often has someone in your family said that because you are a user of mental health services you should not have children or get pregnant? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 4 (11.1) | 5 (13.9) | 9 (12.5) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 4 (13.3) | 6 (20.7) | 10 (17.0) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 2 (15.4) | 4 (25.0) | 6 (20.7) |
| Missing/refused to answer | 0 (0.0) | 2 (12.5) | 2 (6.9) |
| N/A | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| And how often has a mental health care provider said that to you? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 3 (8.3) | 1 (2.8) | 4 (5.6) |
| Missing/refused to answer | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| N/A | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 2 (6.7) | 1 (3.5) | 3 (5.1) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 1 (7.7) | 0 (0.0) | 1 (3.5) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| How often has someone in your family said that because you are a user of mental health services you should not have a romantic or sexual relationship with other patients? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 5 (13.9) | 7 (19.4) | 12 (16.7) |
| Missing/refused to answer | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| N/A | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 4 (13.3) | 6 (20.7) | 10 (17.0) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 1 (7.7) | 2 (12.5) | 3 (10.3) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| And how often has a mental health care provider said that to you? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 5 (13.9) | 4 (11.1) | 9 (12.5) |
| Missing/refused to answer | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| N/A | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 2 (6.7) | 3 (10.3) | 5 (8.5) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| How often has someone in your family said that because you are a user of mental health services you should not have a romantic or sexual relationship with people who do not have a mental illness? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 2 (5.6) | 3 (8.3) | 5 (6.9) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 1 (3.3) | 2 (6.9) | 3 (5.1) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 0 (0.0) | 2 (12.5) | 2 (6.9) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| And how often has a mental health care provider said that to you? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| How often has someone in your family said that because you are a user of mental health services you should have a hysterectomy, tubal ligation or abortion as a form of birth control? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 1 (2.8) | 3 (8.3) | 4 (5.6) |
| Missing/refused to answer | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| N/A | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 0 (0.0) | 2 (6.9) | 2 (2.4) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| All of most of them | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 1 (7.7) | 2 (12.5) | 3 (10.3) |
| And how often has a mental health care provider said that to you? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing/refused to answer | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| N/A | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 1 (1.35) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 2 (12.5) | 2 (6.9) |
| Your personal experience about certain areas of your life | |||
| How often has someone made fun of you because you have a mental illness? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 17 (47.2) | 16 (44.4) | 33 (45.8) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 11 (36.7) | 15 (51.7) | 26 (44.1) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 8 (61.5) | 5 (31.3) | 13 (44.8) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| How often has someone called you crazy or nuts? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 19 (52.8) | 20 (55.6) | 39 (54.2) |
| Missing/refused to answer | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 16 (53.3) | 14 (48.3) | 30 (50.9) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 8 (61.5) | 8 (50.0) | 16 (55.2) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| How often has someone ignored you not taken seriously what you had to say because you have a mental illness? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 19 (52.8) | 22 (61.1) | 41 (56.9) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 18 (60.0) | 18 (62.1) | 36 (61.0) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 11 (84.6) | 10 (62.5) | 21 (72.4) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| How often have you been treated differently from others because they learnt you have a mental illness? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 19 (52.8) | 25 (61.1) | 41 (56.9) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 18 (60.0) | 19 (65.5) | 37 (62.7) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 9 (69.2) | 10 (62.5) | 19 (65.5) |
| Missing/refused to answer | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| How often have you experienced people trying to take advantage of you because they know you have a mental illness? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 20 (55.6) | 18 (50.0) | 38 (52.8) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 13 (43.3) | 14 (48.3) | 27 (45.8) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 8 (61.5) | 5 (31.3) | 13 (44.8) |
| Missing/refused to answer | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| How often have you found yourself having sex with people you do not like? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Often or sometimes | 9 (25.0) | 6 (16.7) | 15 (20.8) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Often or sometimes | 8 (26.7) | 9 (31.0) | 17 (28.8) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Often or sometimes | 3 (23.1) | 0 (0.0) | 3 (10.3) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| People you have wanted to have a romantic or sexual relationship with | |||
| How many of them said they did not want to be involved with you because you were a user of mental health services? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| All of most of them | 3 (8.3) | 2 (5.6) | 5 (6.9) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 2 (5.6) | 1 (2.8) | 3 (4.2) |
| 3 months | n = 30 | n = 29 | n = 59 |
| All of most of them | 2 (6.7) | 1 (3.5) | 3 (5.1) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| 6 months | n = 13 | n = 16 | n = 29 |
| All of most of them | 1 (7.7) | 0 (0.0) | 1 (3.5) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 2 (15.4) | 0 (0.0) | 2 (6.9) |
| How good or successful you are when it comes to relationships | |||
| Having a sexual or romantic relationship with someone you are attracted to or interested in getting to know better? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Very good or good | 22 (61.1) | 18 (50.0) | 40 (55.6) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 0 (0.0) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Very good or good | 16 (53.3) | 18 (62.1) | 34 (57.6) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 2 (6.7) | 0 (0.0) | 2 (3.4) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Very good or good | 8 (61.5) | 6 (37.5) | 14 (48.3) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 2 (15.4) | 1 (6.3) | 3 (10.3) |
| Being attractive to the person you are having a relationship with? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Very good or good | 23 (63.9) | 20 (55.6) | 43 (59.7) |
| Missing/refused to answer | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| N/A | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Very good or good | 23 (76.7) | 19 (65.5) | 42 (71.2) |
| Missing/refused | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Very good or good | 6 (46.2) | 7 (43.8) | 13 (44.8) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 1 (7.7) | 0 (0.0) | 1 (3.5) |
| Negotiating with your partner to get your sexual needs met? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Very good or good | 17 (47.2) | 18 (50.0) | 35 (48.6) |
| Missing/refused to answer | 2 (5.6) | 0 (0.0) | 2 (2.8) |
| N/A | 0 (0.0) | 2 (5.60) | 2 (2.8) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Very good or good | 20 (66.7) | 13 (44.8) | 33 (55.9) |
| Missing/refused | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 2 (6.7) | 1 (3.5) | 3 (5.1) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Very good or good | 5 (38.5) | 5 (31.3) | 10 (34.5) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 1 (7.7) | 0 (0.0) | 1 (3.5) |
| Helping your partner meet his/her sexual needs? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Very good or good | 25 (69.4) | 23 (63.9) | 48 (66.7) |
| Missing/refused to answer | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| N/A | 0 (0.0) | 2 (5.6) | 2 (2.8) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Very good or good | 24 (80.0) | 17 (58.6) | 41 (69.5) |
| Missing/refused | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 2 (6.7) | 2 (3.5) | 3 (5.1) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Very good or good | 9 (69.2) | 8 (50.0) | 17 (58.6) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 1 (7.7) | 0 (0.0) | 1 (3.5) |
| Protecting yourself/your partner from getting pregnant?a n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Very good or good | 22 (61.1) | 17 (47.2) | 39 (54.2) |
| Missing/refused to answer | 3 (8.3) | 0 (0.0) | 3 (4.1) |
| N/A | 6 (16.7) | 13 (36.1) | 19 (26.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Very good or good | 17 (56.7) | 17 (58.6) | 34 (57.6) |
| Missing/refused | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 9 (30.0) | 7 (24.1) | 16 (27.1) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Very good or good | 7 (53.9) | 8 (50.0) | 15 (51.7) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 4 (30.8) | 5 (31.3) | 9 (31.0) |
| Protecting yourself from getting STIs, including HIV? n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Very good or good | 25 (69.4) | 24 (66.7) | 49 (68.1) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 2 (5.6) | 2 (2.8) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Very good or good | 24 (80.0) | 19 (65.5) | 43 (72.9) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Very good or good | 11 (84.6) | 9 (56.3) | 20 (69.0) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 1 (7.7) | 0 (0.0) | 1 (3.5) |
| How you feel about your sexuality | |||
| You feel discouraged about your sex life, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Not at all or a little | 17 (47.2) | 26 (72.2) | 43 (59.7) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Not at all or a little | 21 (70.0) | 23 (79.3) | 44 (74.6) |
| Missing/refused to answer | 0 (0.0) | 1 (3.5) | 1 (1.7) |
| N/A | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Not at all or a little | 9 (69.2) | 10 (62.5) | 19 (65.5) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| You are not satisfied with your sexual experiences, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Not at all or a little | 20 (55.6) | 27 (75.0) | 47 (65.3) |
| Missing/refused to answer | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| N/A | 1 (2.8) | 0 (0.0) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Not at all or a little | 23 (76.7) | 22 (75.9) | 45 (76.3) |
| Missing/refused to answer | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| N/A | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Not at all or a little | 10 (76.9) | 12 (75.0) | 22 (75.9) |
| Missing/refused to answer | 0 (0.0) | 1 (6.3) | 1 (3.5) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| You feel good about the way you express your own sexual needs and desires, n (%) | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Not at all or a little | 21 (58.3) | 20 (55.6) | 41 (56.9) |
| Missing/refused to answer | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| N/A | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| 3 months | n = 30 | n = 29 | n = 59 |
| Not at all or a little | 11 (36.6) | 15 (51.7) | 26 (44.1) |
| Missing/refused to answer | 1 (3.3) | 1 (3.5) | 2 (3.4) |
| N/A | 1 (3.3) | 0 (0.0) | 1 (1.7) |
| 6 months | n = 13 | n = 16 | n = 29 |
| Not at all or a little | 4 (30.8) | 7 (43.8) | 11 (37.9) |
| Missing/refused to answer | 1 (7.7) | 1 (6.3) | 2 (6.9) |
| N/A | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Intervention (N = 36) | Control (N = 36) | Overall (N = 72) | |
|---|---|---|---|
| Total substance involvement | |||
| Baseline | n = 36 | n = 36 | n = 72 |
| Mean (SD) | 38.9 (24.9) | 34.7 (18.2) | 36.8 (21.7) |
| Median (min., max.) | 33.5 (2, 89) | 36 (0, 60) | 35.5 (0, 89) |
| Month 3 | n = 30 | n = 29 | n = 59 |
| Mean (SD) | 40.7 (25.1) | 28.6 (20.1) | 34.7 (23.4) |
| Median (min., max.) | 38 (2, 88) | 35 (0, 65) | 36 (0, 88) |
| Month 6 | n = 13 | n = 16 | n = 29 |
| Mean (SD) | 37.8 (19.6) | 29.9 (18.8) | 33.4 (19.2) |
| Median (min., max.) | 46 (6, 73) | 30.5 (0, 58) | 35 (0, 73) |
| Tobacco, n (%) | |||
| Baseline | 32 (88.9) | 31 (86.1) | 63 (87.5) |
| Low | 9 (28.1) | 8 (25.8) | 17 (27.0) |
| Moderate | 21 (65.6) | 18 (58.1) | 39 (61.9) |
| High | 2 (6.3) | 5 (16.1) | 7 (11.1) |
| Mean (SD) | 14.3 (10.2) | 14.1 (10.8) | 14.2 (10.4) |
| Median (min., max.) | 18 (0, 30) | 15 (0, 30) | 17 (0, 30) |
| 3 months | 26 (89.7) | 24 (85.7) | 50 (87.7) |
| Low | 7 (26.9) | 9 (37.5) | 16 (32.0) |
| Moderate | 15 (57.7) | 13 (54.2) | 28 (56.0) |
| High | 4 (15.4) | 2 (8.3) | 6 (12.0) |
| Mean (SD) | 15.6 (10.2) | 10.7 (10.8) | 13.2 (10.7) |
| Median (min., max.) | 18 (0, 32) | 6 (0, 34) | 13.5 (0, 34) |
| 6 months | 10 (76.9) | 13 (86.7) | 23 (82.1) |
| Low | 3 (30.0) | 4 (30.8) | 7 (30.4) |
| Moderate | 7 (70.0) | 6 (46.2) | 13 (56.5) |
| High | 0 (0.0) | 3 (23.1) | 3 (13.0) |
| Mean (SD) | 12.3 (9.6) | 16.2 (13.5) | 14.5 (11.9) |
| Median (min., max.) | 14.5 (0, 26) | 24 (0, 38) | 15 (0, 38) |
| Alcohol, n (%) | |||
| Baseline | 33 (91.7) | 34 (94.4) | 67 (93.1) |
| Low | 26 (78.8) | 26 (76.5) | 52 (77.6) |
| Moderate | 7 (21.2) | 7 (20.6) | 14 (20.9) |
| High | 0 (0.0) | 1 (2.9) | 1 (1.5) |
| Mean (SD) | 6.8 (6.8) | 7.6 (7.3) | 7.2 (7.0) |
| Median (min., max.) | 4 (0, 23) | 5.5 (0, 32) | 5 (0, 32) |
| 3 months | 29 (96.7) | 25 (89.3) | 54 (93.1) |
| Low | 23 (79.3) | 18 (72.0) | 41 (75.9) |
| Moderate | 5 (17.2) | 6 (24.0) | 11 (20.4) |
| High | 1 (3.5) | 1 (4.0) | 2 (3.7) |
| Mean (SD) | 6.9 (6.9) | 7.6 (6.4) | 7.2 (6.6) |
| Median (min., max.) | 6 (0, 29) | 6 (0, 27) | 6 (0, 29) |
| 6 months | 12 (92.3) | 14 (93.3) | 26 (15.4) |
| Low | 11 (91.7) | 11 (78.6) | 22 (84.6) |
| Moderate | 1 (8.3) | 3 (21.4) | 4 (15.4) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 5.1 (6.6) | 5.9 (6.7) | 5.5 (6.5) |
| Median (min., max.) | 3.5 (0, 23) | 3.5 (0, 22) | 3.5 (0, 23) |
| Cannabis, n (%) | |||
| Baseline | 27 (75.0) | 25 (69.4) | 52 (72.2) |
| Low | 18 (66.7) | 18 (72.0) | 36 (69.2) |
| Moderate | 7 (25.9) | 6 (24.0) | 13 (25.0) |
| High | 2 (7.4) | 1 (4.0) | 3 (5.8) |
| Mean (SD) | 6.1 (9.7) | 3.4 (6.4) | 4.8 (8.3) |
| Median (min., max.) | 3 (0, 37) | 0 (0, 27) | 2 (0, 37) |
| 3 months | 19 (65.5) | 19 (70.4) | 38 (6.9) |
| Low | 9 (47.4) | 15 (79.0) | 24 (63.2) |
| Moderate | 9 (47.4) | 4 (21.1) | 13 (34.2) |
| High | 1 (5.3) | 0 (0.0) | 1 (2.6) |
| Mean (SD) | 6.9 (8.5) | 2.9 (5.2) | 4.9 (7.3) |
| Median (min., max.) | 4 (0, 27) | 0 (0, 22) | 3 (0, 27) |
| 6 months | 9 (69.2) | 11 (73.3) | 20 (71.4) |
| Low | 4 (44.4) | 8 (72.7) | 12 (60.0) |
| Moderate | 5 (55.6) | 3 (27.3) | 8 (40.0) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 5.4 (6.5) | 1.9 (2.8) | 3.5 (5.0) |
| Median (min., max.) | 4 (0, 18) | 0 (0, 6) | 0 (0, 18) |
| Cocaine, n (%) | |||
| Baseline | 12 (33.3) | 10 (27.8) | 22 (30.6) |
| Low | 10 (83.3) | 10 (100.0) | 20 (90.9) |
| Moderate | 2 (16.7) | 0 (0.0) | 2 (9.1) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 1.7 (2.8) | 0.3 (0.9) | 1.0 (2.2) |
| Median (min., max.) | 0 (0, 8) | 0 (0, 3) | 0 (0, 8) |
| 3 months | 10 (35.7) | 9 (34.6) | 19 (35.2) |
| Low | 8 (80.0) | 9 (100.0) | 17 (89.5) |
| Moderate | 2 (20.0) | 0 (0.0) | 2 (10.5) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 2.3 (2.2) | 1 (1.5) | 1.7 (2.0) |
| Median (min., max.) | 3 (0, 6) | 0 (0, 3) | 0 (0, 6) |
| 6 months | 7 (58.3) | 5 (35.7) | 12 (46.2) |
| Low | 5 (71.4) | 5 (100.0) | 10 (83.3) |
| Moderate | 2 (28.6) | 0 (0.0) | 2 (16.7) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 2.3 (3.9) | 0 (0.0) | 1.3 (3.1) |
| Median (min., max.) | 0 (0, 9) | 0 (0, 0) | 0 (0, 9) |
| Amphetamine, n (%) | |||
| Baseline | 13 (36.1) | 11 (30.6) | 24 (33.3) |
| Low | 10 (76.9) | 10 (90.9) | 20 (83.3) |
| Moderate | 3 (23.1) | 1 (9.1) | 4 (16.7) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 2.1 (2.6) | 1.6 (2.5) | 1.9 (2.5) |
| Median (min., max.) | 0 (0, 6) | 0 (0, 8) | 0 (0, 8) |
| 3 months | 15 (51.7) | 8 (30.8) | 23 (41.8) |
| Low | 11 (73.3) | 8 (100.0) | 19 (82.6) |
| Moderate | 4 (26.7) | 0 (0.0) | 4 (17.4) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 2 (2.7) | 0.3 (0.7) | 1.4 (2.3) |
| Median (min., max.) | 0 (0, 6) | 0 (0, 2) | 0 (0, 6) |
| 6 months | 8 (61.5) | 4 (28.6) | 12 (44.4) |
| Low | 7 (87.5) | 4 (100.0) | 11 (91.7) |
| Moderate | 1 (12.5) | 0 (0.0) | 1 (8.3) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 1.1 (3.2) | 0 (0.0) | 0.8 (2.6) |
| Median (min., max.) | 0 (0, 9) | 0 (0, 0) | 0 (0, 9) |
| Inhalants, n (%) | |||
| Baseline | 5 (13.9) | 7 (19.4) | 12 (16.7) |
| Low | 4 (80.0) | 7 (100.0) | 11 (91.7) |
| Moderate | 1 (20.0) | 0 (0.0) | 1 (8.3) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 1.8 (4.0) | 0 (0.0) | 0.8 (2.6) |
| Median (min., max.) | 0 (0, 9) | 0 (0, 0) | 0 (0, 9) |
| 3 months | 7 (25.0) | 2 (8.3) | 9 (17.3) |
| Low | 6 (85.7) | 2 (100.0) | 8 (88.9) |
| Moderate | 1 (14.3) | 0 (0.0) | 1 (11.1) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 1.6 (2.1) | 0 (0.0) | 1.2 (1.9) |
| Median (min., max.) | 0 (0, 5) | 0 (0, 0) | 0 (0, 5) |
| 6 months | 3 (27.3) | 1 (7.1) | 4 (16.0) |
| Low | 3 (100.0) | 1 (100.0) | 4 (100.0) |
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 0.7 (1.2) | 0 (–) | 0.5 (1) |
| Median (min., max.) | 0 (0, 2) | 0 (0, 0) | 0 (0, 2) |
| Sedatives, n (%) | |||
| Baseline | 8 (22.2) | 4 (11.1) | 12 (16.7) |
| Low | 4 (50.0) | 4 (100.0) | 8 (66.7) |
| Moderate | 3 (37.5) | 0 (0.0) | 3 (25.0) |
| High | 1 (12.5) | 0 (0.0) | 1 (8.3) |
| Mean (SD) | 7.4 (10.2) | 0 (0.0) | 4.9 (8.9) |
| Median (min., max.) | 4 (0, 30) | 0 (0, 0) | 0 (0, 30) |
| 3 months | 4 (14.3) | 3 (12.5) | 7 (13.5) |
| Low | 1 (25.0) | 2 (66.7) | 3 (42.9) |
| Moderate | 3 (75.0) | 1 (33.3) | 4 (57.1) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 6 (4.2) | 2 (3.5) | 4.3 (4.2) |
| Median (min., max.) | 7.5 (0, 9) | 0 (0, 6) | 6 (0, 9) |
| 6 months | 2 (16.7) | 1 (7.1) | 3 (11.5) |
| Low | 2 (100.0) | 1 (100.0) | 3 (100.0) |
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 0 (0.0) | 0 (–) | 0 (0.0) |
| Median (min., max.) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Hallucinogens, n (%) | |||
| Baseline | 12 (33.3) | 10 (27.8) | 22 (30.6) |
| Low | 11 (91.7) | 9 (90.0) | 20 (90.9) |
| Moderate | 1 (8.3) | 1 (10.0) | 2 (9.1) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 1 (3.0) | 0.9 (2.0) | 1.0 (1.9) |
| Median (min., max.) | 0 (0, 6) | 0 (0, 6) | 0 (0, 6) |
| 3 months | 9 (32.1) | 8 (32.0) | 17 (32.1) |
| Low | 8 (88.9) | 7 (87.5) | 15 (88.2) |
| Moderate | 1 (11.1) | 1 (12.5) | 2 (11.8) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 1 (2.1) | 1.1 (2.2) | 1.1 (2.1) |
| Median (min., max.) | 0 (0, 6) | 0 (0, 6) | 0 (0, 6) |
| 6 months | 5 (41.7) | 3 (21.4) | 8 (30.8) |
| Low | 5 (100.0) | 2 (66.7) | 7 (87.5) |
| Moderate | 0 (0.0) | 1 (33.3) | 1 (12.5) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 0 (0.0) | 2 (3.5) | 0.8 (2.1) |
| Median (min., max.) | 0 (0, 0) | 0 (0, 6) | 0 (0, 6) |
| Opioids, n (%) | |||
| Baseline | 4 (11.1) | 5 (13.9) | 9 (12.5) |
| Low | 3 (75.0) | 5 (100.0) | 8 (88.9) |
| Moderate | 1 (25.0) | 0 (0.0) | 1 (11.1) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 2.3 (4.5) | 1 (1.4) | 1.6 (3.0) |
| Median (min., max.) | 0 (0, 9) | 0 (0, 3) | 0 (0, 9) |
| 3 months | 5 (17.9) | 4 (17.4) | 9 (17.7) |
| Low | 4 (80.0) | 2 (50.0) | 6 (66.7) |
| Moderate | 2 (20.0) | 2 (50.0) | 3 (33.3) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 1.2 (2.7) | 3 (3.5) | 2 (3.0) |
| Median (min., max.) | 0 (0, 6) | 3 (0, 6) | 0 (0, 6) |
| 6 months | 3 (25.0) | 0 (0.0) | 3 (11.5) |
| Low | 3 (100.0) | – | 3 (100.0) |
| Moderate | 0 (0.0) | – | 0 (0.0) |
| High | 0 (0.0) | – | 0 (0.0) |
| Mean (SD) | 3 (0.0) | – (–) | 3 (0.0) |
| Median (min., max.) | 0 (0, 0) | – (–, –) | 0 (0, 0) |
| Others (M-CAT and homeopathy remedies), n (%) | |||
| Baseline | 1 (2.8) | 1 (2.8) | 2 (2.8) |
| Low | 1 (100.0) | 0 (0.0) | 1 (50.0) |
| Moderate | 0 (0.0) | 1 (100.0) | 1 (50.0) |
| High | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean (SD) | 0 (–) | 6 (–) | 3 (4.2) |
| Median (min., max.) | 0 (0, 0) | 6 (6, 6) | 3 (0, 6) |
| 3 months (methoxamine) | 0 (0.0) | 1 (4.8) | 1 (2.4) |
| Low | – | 0 (0.0) | 0 (0.0) |
| Moderate | – | 1 (100.0) | 1 (100.0) |
| High | – | 0 (0.0) | 0 (0.0) |
| Mean (SD) | – (–) | 6 (–) | 6 (–) |
| Median (min., max.) | – (–, –) | 6 (6, 6) | 6 (6, 6) |
| 6 months (ecstasy, methadone) | 0 (0.0) | 1 (11.1) | 1 (6.3) |
| Low | – | 0 (0.0) | 0 (0.0) |
| Moderate | – | 1 (100.0) | 1 (100.0) |
| High | – | 0 (0.0) | 0 (0.0) |
| Mean (SD) | – (–) | 6 (–) | 6 (–) |
| Median (min., max.) | – (–, –) | 6 (6, 6) | 6 (6, 6) |
| Have you ever used any drug by injection? n (%) | |||
| Baseline | |||
| No, never | 33 (91.7) | 34 (94.4) | 67 (93.1) |
| Yes, but not in the past 3 months | 2 (5.6) | 0 (0.0) | 2 (2.8) |
| Missing | 1 (2.8) | 2 (5.6) | 3 (4.2) |
| Month 3 | |||
| No, never | 26 (86.7) | 24 (82.8) | 50 (84.8) |
| Yes, but not in the past 3 months | 3 (10.0) | 0 (0.0) | 3 (5.1) |
| Missing | 1 (3.3) | 5 (17.2) | 6 (10.2) |
| Month 6 | |||
| No, never | 11 (84.6) | 15 (93.8) | 26 (89.7) |
| Yes, but not in the past 3 months | 1 (7.7) | 0 (0.0) | 1 (3.5) |
| Missing | 1 (7.7) | 1 (6.3) | 2 (6.9) |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AOT
- Assertive Outreach and Community
- ASSIST
- Alcohol, Smoking and Substance Involvement Screening Test
- BBV
- blood-borne virus
- C&IFT
- Camden and Islington NHS Foundation Trust
- CMHT
- community mental health team
- CRF
- case report form
- CRN
- Clinical Research Network
- CSO
- Clinical Studies Officer
- CtoC
- consent to contact
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HIV
- human immunodeficiency virus
- HIV-KQ
- knowledge about HIV questionnaire
- HRA
- Health Research Authority
- ICC
- intracluster correlation coefficient
- IMB
- information–motivation–behavioural skills model
- LYPFT
- Leeds and York Partnership NHS Foundation Trust
- MDT
- multidisciplinary team
- MISS-Q
- Mental Illness Stigma Scale
- MRC
- Medical Research Council
- MSM
- men who have sex with men
- Natsal
- National Survey of Sexual Attitudes and Lifestyle
- NELFT
- North East London NHS Foundation Trust
- PIS
- participant information sheet
- PWLE
- people with lived experience
- RCT
- randomised controlled trial
- R&D
- research and development
- REC
- Research Ethics Committee
- ReQoL
- recovering quality of life
- RESPECT
- Randomised Evaluation of Sexual health Promotion Effectiveness informing Care and Treatment
- SAE
- serious adverse event
- SD
- standard deviation
- SERBAS
- Sexual Risk Behaviour Assessment Schedule
- SMI
- serious mental illness
- SPFT
- Sussex Partnership NHS Foundation Trust
- STI
- sexually transmitted infection
- SWYFT
- South West Yorkshire Partnership NHS Foundation Trust
- TAU
- treatment as usual
- TSC
- Trial Steering Committee
- WHO
- World Health Organization
Notes
-
Baseline data collection questionnaires
-
The 3- and 6-month follow-up data collection questionnaires
-
Risk protocol and letter of notification
Supplementary material can be found on the NIHR Journals Library report project page (www.journalslibrary.nihr.ac.uk/programmes/hta/1417201/#/documentation).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.