Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 17/153/01. The contractual start date was in April 2018. The draft report began editorial review in February 2019 and was accepted for publication in August 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Kirsty Le Doare has received a travel grant from Pfizer (New York, NY, USA). Paul Heath is an investigator for clinical trials (not group B streptococcus-specific) who carries out work on behalf of St George’s, University of London, and is sponsored by various vaccine manufacturers, including Novartis (Basel, Switzerland), Pfizer and GlaxoSmithKline plc (Brentford, UK). Asma Khalil has been a member of the Health Technology Assessment General Committee since November 2018, and is due to be active until 2022.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Carreras-Abad et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Group B streptococcus (GBS) is the leading cause of neonatal sepsis and meningitis in most countries. GBS is also an important cause of disease in pregnant women, immunocompromised adults and elderly people. 1 The highest incidence of invasive group B streptococcal disease (iGBS) is in the first 3 months of life and the condition is traditionally divided into early-onset disease (EOD) (i.e. occurring in infants aged < 7 days) or late-onset disease (LOD) (i.e. occurring in infants aged 7–89 days). GBS is an encapsulated bacterium and 10 serotypes are described; five serotypes (i.e. Ia, Ib, II, III, V) account for 97% of iGBS. 2
Overall, EOD accounts for 60–80% of iGBS in the first 3 months of life. Maternal colonisation with GBS in the gastrointestinal or genital tract is a prerequisite for EOD, with vertical transmission occurring during or just before birth. Around 20% of pregnant women are colonised with GBS3 and 1–2% of neonates who are born to colonised women develop invasive disease in the absence of intrapartum antibiotic prophylaxis (IAP). 4 EOD can occur rapidly, with signs evident at birth or within 12 hours in most cases, typically presenting with sepsis, pneumonia and/or meningitis. 5
Group B streptococcus is present in all regions of the world and an estimated 21.7 million pregnant women are colonised at any one time. 3 In 2015, it was estimated that annually worldwide there were at least 319,000 infants aged < 3 months with iGBS, resulting in 90,000 infant deaths and at least 10,000 children with disability related to GBS meningitis. Additionally, 33,000 maternal cases and 57,000 stillbirths are attributed to GBS disease each year. 6
The global burden of group B streptococcus is therefore high and represents an unmet public health need
Intrapartum antibiotic prophylaxis can reduce the incidence of EOD, and many high-income countries have established IAP policies. The incidence of EOD in the USA has declined significantly in the era of IAP and the incidence is also generally declining in other countries adopting a swab-based screening policy. 7,8 However, in some countries, particularly those adopting a risk-based IAP strategy, such as the Netherlands9 and the UK,10 recent increases in disease burden have been reported.
It is clear that even strict and universal implementation of IAP guidelines does not eliminate EOD,11,12 as disease can occur because of limitations to IAP administration (e.g. in precipitate labour), in infants of mothers who were negative on screening and where no risk factors are evident in labour. 13 Most significantly, IAP has no impact on GBS-related prematurity or stillbirths or LOD, where the burden of disease is substantial. The majority of GBS meningitis occurs after the first week of life, so this particular burden remains. In the USA and the UK, GBS is now the most common cause of bacterial meningitis in children aged < 5 years. 14,15
Given the very early onset of neonatal GBS disease, the shortcomings of IAP-based prevention strategies and the evidence that suggests that maternal antibodies acquired after natural exposure (when transmitted transplacentally to the fetus) may protect the young infant from invasive infection,16 the prospect of protecting mothers and their infants through vaccination in pregnancy is an attractive one. Possible candidates for an effective vaccine include one or more of the conserved surface proteins or the capsular polysaccharide (CPS). 17
Multiple studies of CPS–protein conjugate vaccines in non-pregnant and, more recently, in pregnant women have established the immunogenicity and safety of these candidates. 17 Recent estimates suggest that an effective GBS maternal vaccine (> 80% efficacy), with high (90%) global coverage, could prevent 231,000 infant and maternal GBS cases, 41,000 stillbirths and 66,000 infant deaths annually. 6
Several obstacles exist in moving the most advanced vaccines into Phase III clinical trials. The first is that, given the relative rarity of GBS disease in Europe and the USA, large numbers of infants would need to be recruited to determine vaccine efficacy. 18 Second, obstacles exist in determining what concentration of antibody is required to protect the infant for the duration of the at-risk period (i.e. the first 3 months of life), as there are currently no internationally recognised standards with which to interpret individual study results. 1 Licensure and policy decisions would be significantly accelerated if an immune marker, measured in an analytically and clinically validated assay, was established as a correlate of protection (CoP). Licensure in such a scenario would come with a commitment to establish effectiveness post licensure in a Phase IV study. This was the approach used for licensure of meningococcal C and meningococcal B vaccines. 19
Correlates of protection against invasive disease
The association between serotype-specific capsular antibody levels and iGBS in newborns was initially characterised in 1976 by Baker and Kasper. 16 In the majority of subsequent studies, levels of CPS serotype-specific antibodies in maternal delivery sera of women who had neonates with EOD caused by that serotype were low, compared with levels in sera from women delivering infants who remained healthy. 1 However, different ‘protective’ levels have been defined in different studies as well as for the different serotypes.
In a meta-analysis undertaken to compare the proportions of cases and controls with antibody levels of ≥ 2 µg/ml, the odds of contracting iGBS were 6.6 [95% confidence interval (CI) 2.1 to 20.6] and 2.4 (95% CI 1.2 to 4.7) times greater in infants whose mothers had antibody levels of < 2 µg/ml for serotypes III and Ia, respectively. 20 A threshold of 1 µg/ml has also been proposed as a CoP for serotypes Ia and III. 8 Thresholds are much higher in other studies using different case–control designs and different enzyme-linked immunosorbent assay methods,21,22 which makes direct comparisons difficult.
Interpretation of studies is confounded by the different assay methods used and the lack of standardised reference reagents for serotype-specific antibody levels. Therefore, further studies using standardised methods are warranted. 23
Defining a correlate of protection against invasive group B streptococcal disease
There is considerable evidence that serum immunoglobulin G (IgG) can protect infants against iGBS and that this IgG is maternally derived as a result of natural maternal infection (i.e. colonisation). It is essential to know precisely what level of serum IgG in women at delivery is protective so that this can be targeted through vaccination. Protective levels can be estimated by comparing IgG from babies who are exposed to GBS (through maternal colonisation) and go on to develop iGBS (cases), with IgG from babies who are exposed to GBS (through maternal colonisation) but do not develop iGBS (controls). To do this, there needs to be sufficient numbers of cases and controls to be able to define the protective level of IgG (the CoP) with sufficient precision. Although the level of IgG in women at delivery is most often proposed as the CoP, there is a predictable decline in IgG level from the mother to the fetus (transplacental transfer ratio) and, subsequently, to the infant over the first 3 months of life (reflecting the half-life of maternal IgG). Measuring IgG in the cord blood and at different time points in the infant can allow these concentrations to be compared with maternal IgG to calculate the rate of antibody decline during the at-risk period of the first 3 months of life. The level of IgG in the infant will be of particular relevance in cases of LOD where the median age at disease onset is around 21 days. 24
To generate a CoP, maternal delivery/cord sera from a cohort of mothers/babies must be collected prospectively. When an infant subsequently develops iGBS the relevant delivery samples can be retrieved for that infant and the antibody levels can be compared with those of suitable controls. The antibody levels in the infant at the time of iGBS can also be obtained and may also be used to predict the levels present at the time of delivery as it is not expected that these will change significantly between birth and the onset of iGBS, at least for EOD. 25
Study rationale
From a UK perspective, we have recently seen an increasing burden of disease, despite a national (risk-based) policy for IAP,10 and the UK National Screening Committee has recently recommended not to introduce a national screening programme. 26 The clinical effectiveness and cost-effectiveness of screening for GBS in pregnancy is the objective of another Health Technology Assessment application (17/86, GBS3). Conversely, the UK population has widely accepted the concept of maternal vaccination, with high coverage of maternal pertussis vaccination (> 70%) and the first demonstration of its effectiveness, to our knowledge. 27 The UK is therefore in an excellent position to pursue the development, licensure and implementation of a maternal vaccine against GBS.
Licensure and policy decisions for a candidate GBS vaccine would be significantly accelerated if an immune marker was established as a CoP. Regulatory bodies, including the European Medicines Agency and the Food and Drug Administration,28 have made it clear that they would now consider this approach to licensure if robust evidence can be developed. Additionally, the UK Joint Committee on Vaccination and Immunisation has indicated it would consider a recommendation for routine implementation of a vaccine licensed on the basis of CoP – as it has for other recent vaccines (e.g. meningococcal C19).
The two critical gaps that have to be filled to make decisions on the use of such vaccines (both at the regulatory and at the recommending body level) are (1) the development of a standardised immunoassay to measure antibody levels that act as the correlates of natural immunity, supported by measurement of functional antibody assays, and (2) a large biobank of sera to establish the correlate using these new standardised assays. The first of these gaps is being addressed by a consortium of groups from academia, public health and industry (led by co-applicant KLD, OPP1153630), and the second is the basis of this study.
Given the anticipated size and logistical complexities of the serocorrelates study that would be needed to address this gap in knowledge, the aim of this initial feasibility study is to test key operational aspects of the study design for the collection of a large bank of serum in the UK.
Chapter 2 Objectives
Primary objective
To test the feasibility of collecting serum at delivery (maternal, cord or both) from a large cohort of pregnant women.
Secondary objectives
To test the key operational aspects for a proposed large serocorrelates study:
-
enrolment rate [the rate (proportion) of eligible women who are willing to participate in the delivery blood collection study]
-
maternal and/or cord blood collection rate
-
key clinical exclusion data collection rate [weeks’ gestation at birth, receipt of IAP in labour (yes/no), type of IAP (list), time between administration of IAP and delivery (in hours)]
-
infant iGBS surveillance consent rate.
In a substudy of the main study above to assess:
-
rectovaginal swab consent rate
-
rectovaginal swab collection rate
-
rectovaginal GBS colonisation rate
-
rectovaginal GBS CPS serotype-specific colonisation rates.
In the substudy above, where samples of maternal/cord blood and rectovaginal swabs are all available, to assess:
-
infant blood sample consent rate
-
infant Guthrie card consent rate
-
infant Guthrie card collection rate.
To test the feasibility of collecting samples from iGBS cases:
-
maternal blood and rectovaginal swab, and the baby blood consent and collection rate from the participants of the iGBS study
-
maternal and baby blood sample consent and collection rate from national surveillance (all NHS trusts in England and Wales).
Exploratory objectives
To assess:
-
the impact of timing of processing and blood sample storage conditions on total IgG concentrations
-
the serotype-specific GBS anti-CPS IgG concentrations in maternal serum and cord blood in subjects colonised with GBS at delivery
-
the correlation between two different culture techniques for detection of GBS in rectovaginal swabs – enrichment culture medium and direct plating using selective agar.
Chapter 3 Study design and methods
Study design
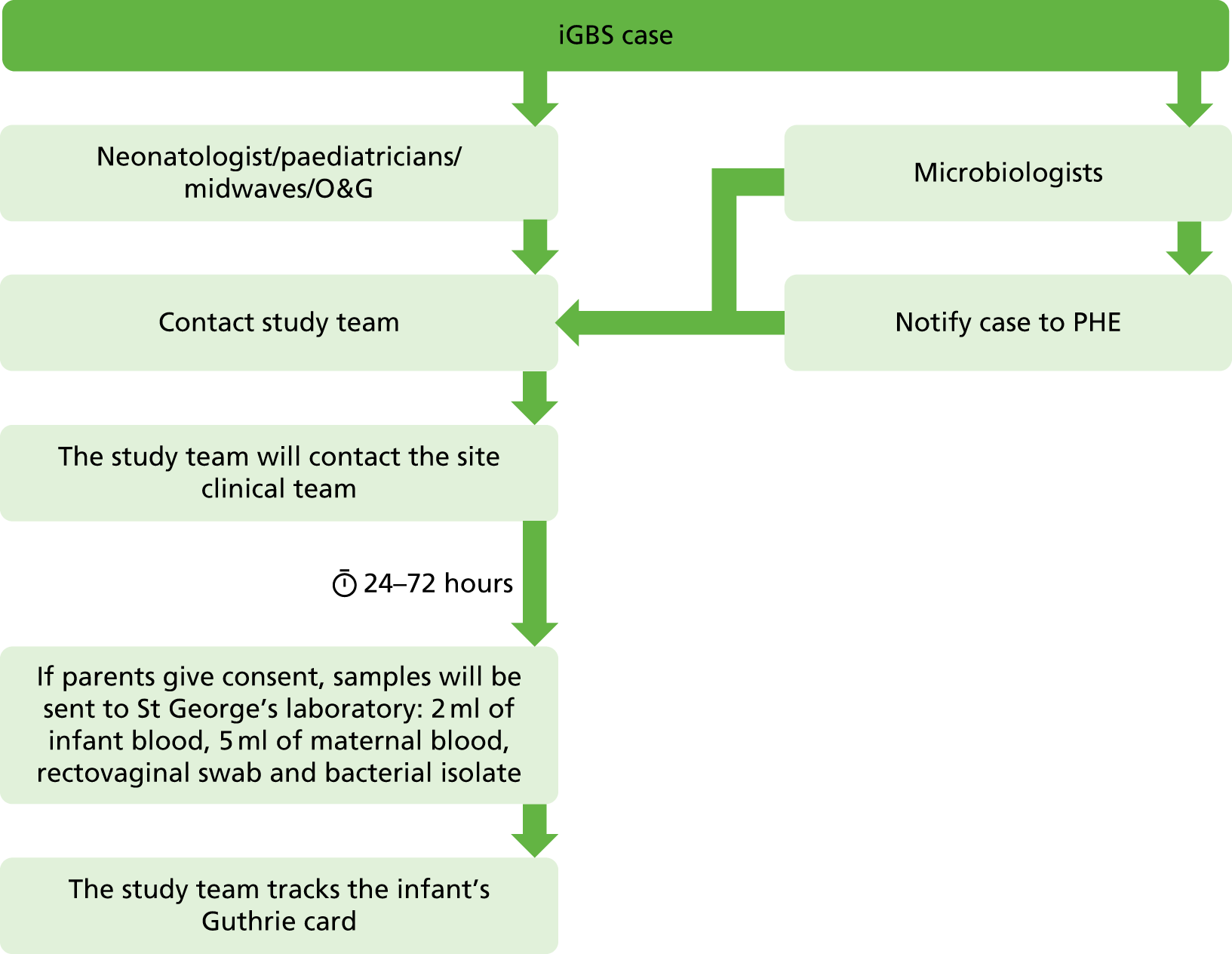
This was a prospective cohort study of pregnant women and their infants. The study design is shown in Figures 1 and 2.
FIGURE 1.
Flow chart for the feasibility study in five hospitals in London and the South East.
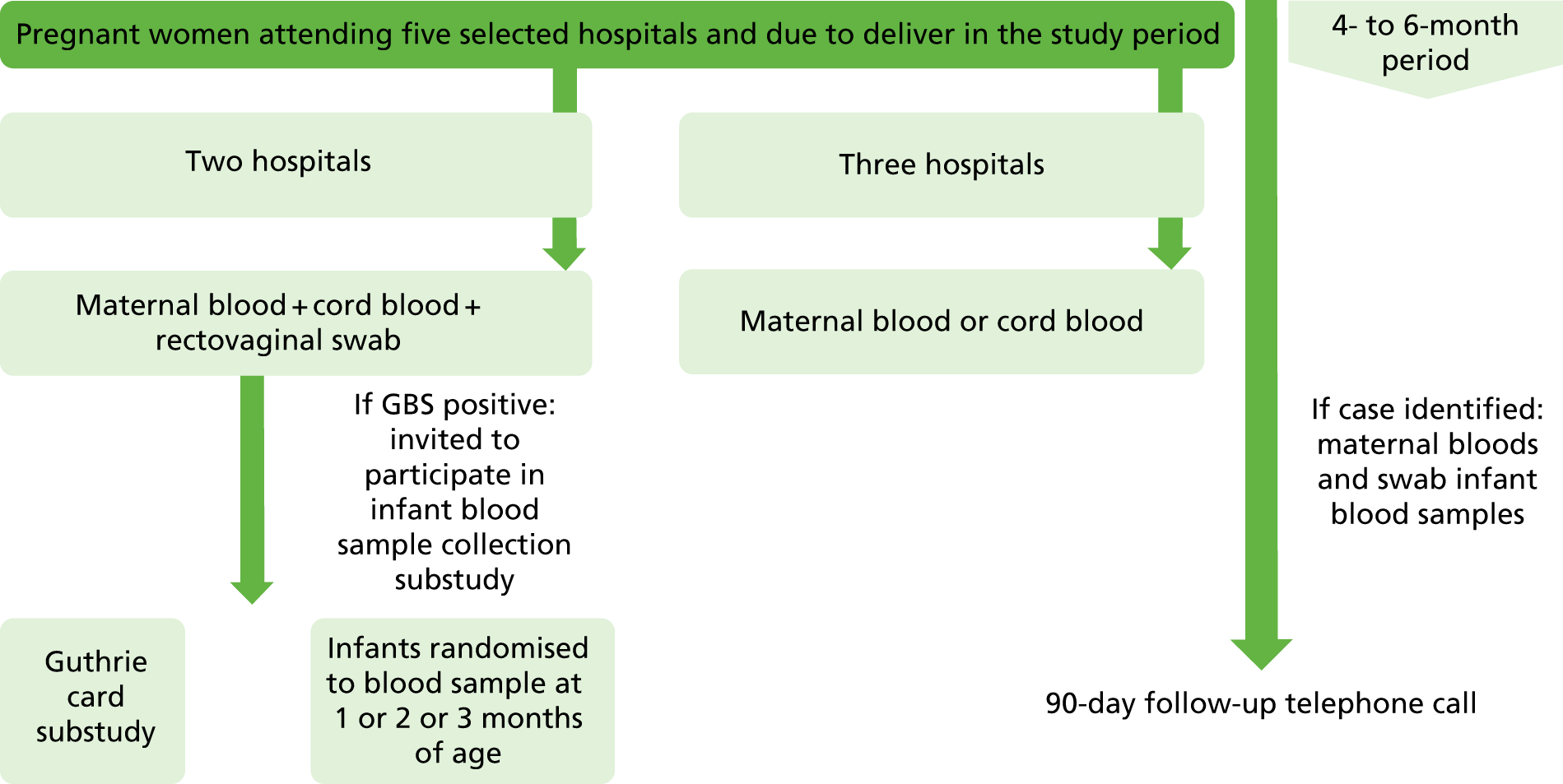
FIGURE 2.
Flow chart for the national surveillance of iGBS. O&G, obstetrics and gynaecology; PHE, Public Health England.
Participants
Inclusion criteria
-
Those who were pregnant.
-
Those who were aged ≥ 18 years.
-
Those who were delivering at one of the selected hospitals.
-
Those who consented to participate during the study period.
Exclusion criteria
As this was a feasibility study, there were no exclusion criteria other than inability to fulfil the inclusion criteria above.
Ethics approval and research governance
Ethics approval for the study was given by the West Midlands – Solihull Research Ethics Committee (REC) on 15 June 2018 with the reference number 18/WM/0147. The study was also approved by the Health Research Authority (HRA) and Care Research Wales on 15 June 2018. Site-specific capacity and capability was then given at the different research and governance departments of St George’s University Hospitals NHS Foundation Trust, Kingston Hospital NHS Foundation Trust, Poole Hospital NHS Foundation Trust, Surrey and Sussex Healthcare NHS Trust and Croydon Health Services NHS Trust.
The Antenatal and Newborn Research Advisory Committee approved the Guthrie card substudy on 3 October 2018.
During the study period, the protocol was amended three times, as follows.
Amendment 1 (5 July 2018)
-
Retrospective consent: permission was granted for retrospective written consent for sample collection in those cases where there was insufficient time to obtain this before delivery and verbal consent was previously recorded in the participant’s medical notes.
-
The GBS surveillance was extended to all the trusts of England and Wales to increase the number of neonates and infants with iGBS recruited.
Amendment 2 (25 September 2018)
-
Permission was granted for the bacterial isolate from the national iGBS surveillance to be sent to the St George’s microbiology laboratory to fully characterise the GBS strain.
Amendment 3 (9 October 2018)
-
Permission was granted to extend the study until 31 December 2018.
-
Permission was granted for consent to trace the Guthrie cards of those infants with iGBS included in the national surveillance subset through their NHS number.
Recruitment procedure
Patient recruitment at a site commenced after REC, HRA and local approvals and all subjects were screened and consented by delegates of the chief investigator.
Study information was available on social media, posters on notice boards, institutional and GBSS websites [https://gbss.org.uk/recent-research (accessed 30 January 2018)], letters, text messages or through direct approach in antenatal clinics. Women had the opportunity to ask questions of the research team by telephone or e-mail. If a woman was interested in participating, this was recorded on handheld notes to ensure that delivery staff were aware of her interest.
Collection of delivery bloods
Following confirmation of consent, blood samples were obtained from the mother at any time during labour (or within 48 hours of delivery) and/or from the cord once the placenta was delivered. At Kingston and St George’s hospitals, both maternal blood and cord blood were collected. At other sites, maternal blood was obtained if it was not possible to obtain a sample of cord blood after delivery.
Collection of swabs
At Kingston and St George’s hospitals only, pregnant women were asked to take part in the colonisation study and a single vaginorectal swab was collected.
Collection of infant blood
At Kingston and St George’s hospitals, women who were participating in both the delivery blood collection study and the colonisation study, and who were shown to be colonised with GBS, were also invited to participate in the infant antibody kinetics substudy. A home visit or hospital appointment was undertaken to collect an additional blood sample from the infant at 4, 8 or 12 weeks of age.
Collection of Guthrie cards
At Kingston and St George’s hospitals, women who were participating in both the delivery blood collection study and the colonisation study were also invited to participate in the infant Guthrie card collection substudy. Women were asked for consent to access their babies’ routine Guthrie card from the National Screening Laboratory.
Invasive group B streptococcal disease surveillance
When a case of iGBS occurred at any participating hospital in England or Wales, the relevant paediatrician was contacted by the study team and asked to recruit the mother and baby to the iGBS substudy. Women were approached in the hospital and asked to provide a maternal and an infant blood sample.
Follow-up of babies
A follow-up telephone call was made to all consenting parents 90 days after their baby was born to confirm whether or not their child had developed iGBS during this time period.
Informed consent
Informed consent from the participant, the legally authorised representative or the parents/guardians/person with legal responsibility for children was obtained following explanation of the aims, methods, benefits and potential hazards of the study.
Information about the study was given by one of the research midwives/clinical team and women had the opportunity to ask questions. There was no minimum period between receiving information and providing consent. Initially, formal written consent for participation in the study was taken at enrolment and then confirmed with the mother verbally at, during or following delivery. In some cases, there was insufficient time to obtain written informed consent from participants who were presenting and being consented in labour, although appropriately sensitised to the study. Following an amendment submitted to and approved by the ethics committee (on 5 July 2018), we amended the protocol so that we were able to obtain retrospective written consent after sample collection during delivery if verbal consent was previously recorded in the participant’s notes. Written consent could be taken retrospectively within 24 hours of birth. No data were collected until written informed consent was obtained and participants could withdraw at any time.
Parents of infants identified as having a positive culture for GBS from a normally sterile site (blood or cerebrospinal fluid) from participating hospitals in England and Wales were approached for consent to obtain blood samples from the mother and baby during the acute admission and for collection of the GBS isolate. Samples were collected when parents had had time to consider the information they had received about the study and had given full written informed consent to the local study team.
A copy of the signed informed consent form along with a copy of the most recently approved patient information sheet were given to the study participant. An original signed and dated consent form was retained in the investigator site file and a third copy was placed in the medical notes.
Sample procedures
Delivery blood samples
Blood was obtained from the mother at any time during labour (or within 48 hours of delivery) and/or from the cord once the placenta was delivered. A total of 5 ml of blood was collected into a BD® serum separator tube (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Blood samples were transported to the laboratories to be processed and the serum was collected into Eppendorf tubes (Thermo Fisher Scientific, Waltham, MA, USA) and frozen at –20 °C to –80 °C for later processing.
Relevant clinical information was extracted from the mother’s handheld or hospital notes onto the case report form. This included baby/babies gestation at birth (weeks), receipt of IAP (yes/no), type of IAP (list), time between administration of first IAP and delivery (hours), recent blood transfusion (in last 3 months), and elective caesarean section (yes/no).
Vaginal and rectal swabs
At Kingston and St George’s hospitals only, a single double-head rectovaginal swab was obtained at any time from 35 weeks’ gestation up to (and including) delivery, either by the study midwife or by the mother herself (with appropriate written guidance). The swab was inserted into the lower half of the vagina and turned slowly clockwise once before removing and inserting past the anal sphincter into the low rectum. The swabs were processed for GBS using validated methods (in an NHS laboratory) and reported using standard NHS clinical pathways. In addition, we used a second, well-established, method for identification of GBS recommended by the Centers for Disease Control and Prevention (but not currently in use in the UK): one head of the swab was inserted into a transport media tube and transported to the local clinical laboratory for processing in accordance with standard methods (direct plating onto selective agar), and the other head was inserted into Lim broth (an enrichment culture media), sent to the research laboratory, incubated for 6–24 hours and plated onto selective agar. The results (positive/negative) were communicated to the site research midwives and the mother was informed by telephone (if GBS positive) and by letter (if positive or negative). If GBS positive, women were subsequently managed in accordance with the Royal College of Obstetrics and Gynaecology (RCOG) Greentop Guideline and were offered IAP if results were available before delivery. 29 Positive results were also serotyped with a latex agglutination test (LAT) or polymerase chain reaction (PCR), if non-typeable by a LAT.
Collection of infant blood
At Kingston and St George’s hospitals only, women who were participating in both the delivery blood collection study and the colonisation study and who were shown to be colonised with GBS were also invited to participate in the infant antibody kinetics substudy. In this substudy, babies born to colonised mothers were randomised to have one blood sample obtained at 4, 8 or 12 weeks of age. Blood sampling was undertaken by doctors using venepuncture or capillary sampling (2 ml) before blood was processed and stored, as described above.
Collection of Guthrie cards
At Kingston and St George’s hospitals only, women who were participating in both the delivery blood collection study and the colonisation study and had not received IAP were invited to participate in the infant Guthrie card collection substudy. In this substudy, mothers were asked for permission to obtain a single blood spot from the Guthrie card, which is obtained routinely in all babies at around 5 days of age. This will be obtained from the National Screening Laboratory and stored for antibody analysis.
Antibody testing
Prior to the start of the study, serum samples were collected from 10 healthy non-pregnant volunteers to assess the impact of timing of processing of blood samples and storage conditions on total IgG concentrations. The effect on total IgG concentration was assessed at different times between sample collection and sample processing (6 hours/10 hours/18 hours/24 hours/48 hours/72 hours/5 days/7 days).
Antibodies against the GBS-CPS were quantified using Luminex® multiplex assays (Luminex Corporation, Austin, TX, USA) performed at St George’s, University of London, laboratories using the standardised assays that have been established as part of a Gates Foundation-funded collaboration led by Kirsty Le Doare.
Infant follow-up
A follow-up telephone call was scheduled at 90 days after the baby’s birth to establish whether or not the infant developed iGBS during this time period. This telephone call was made by the research midwives at each site in order to keep personal data locally.
National invasive group B streptococcal disease surveillance
Microbiologists and paediatricians at all NHS trusts from England and Wales were informed about the study through established research and clinical networks [British Paediatric Allergy, Immunity and Infection Group (BPAIIG), Public Health England, the Neonatal Infection Network (neonIN) and the UK Paediatric Vaccine Group]. Paediatricians and microbiologists were asked to make contact with study staff when a case of iGBS occurred at their hospital. After having set up the site, the relevant paediatrician provided study information to the parents and sought their permission for inclusion in the study. Following consent from the parent, a sample of blood was obtained from the mother and from her baby and processed as above. The participating mother was also asked to provide a single rectovaginal swab, taken either by the midwife or by mother herself, to be processed as above. Furthermore, the study team contacted the microbiology laboratory and asked for the bacterial isolate in order to culture and fully characterise the GBS strain. These samples and the bacterial isolate were sent to and stored at St Georges, University of London, in a linked anonymised fashion. In addition, parents were asked for their permission to collect the Guthrie card of the infant.
Data collection
To standardise recruitment processes across the study sites and maximise data quality, we used Research Electronic Data Capture (REDCap; 8.1.8, Vanderbilt University, Nashville, TN, USA)30 hosted at St George’s, University of London. A range of data validation checks were carried out in both REDCap and Stata® version 15 (StataCorp LP, College Station, TX, USA)27 to minimise erroneous or missing data.
Statistical design
Sample size
There is no formal sample size calculation as this is a pragmatic study conducted among a representative network of hospitals that is intended to assess the feasibility of approaching a much larger network of hospitals needed to achieve the main objective of this research. The proportions achieved against the various study end points will allow sample size calculations to be made for the main study.
Statistical analysis
For the main end point of this feasibility study, we have undertaken a descriptive analysis. All analysis is unadjusted. McNemar’s test was used to assess the exploratory objective concerning the two different GBS culture methods.
Extensive efforts were undertaken to identify missing data. All missing data were excluded from the final analyses.
Chapter 4 Results
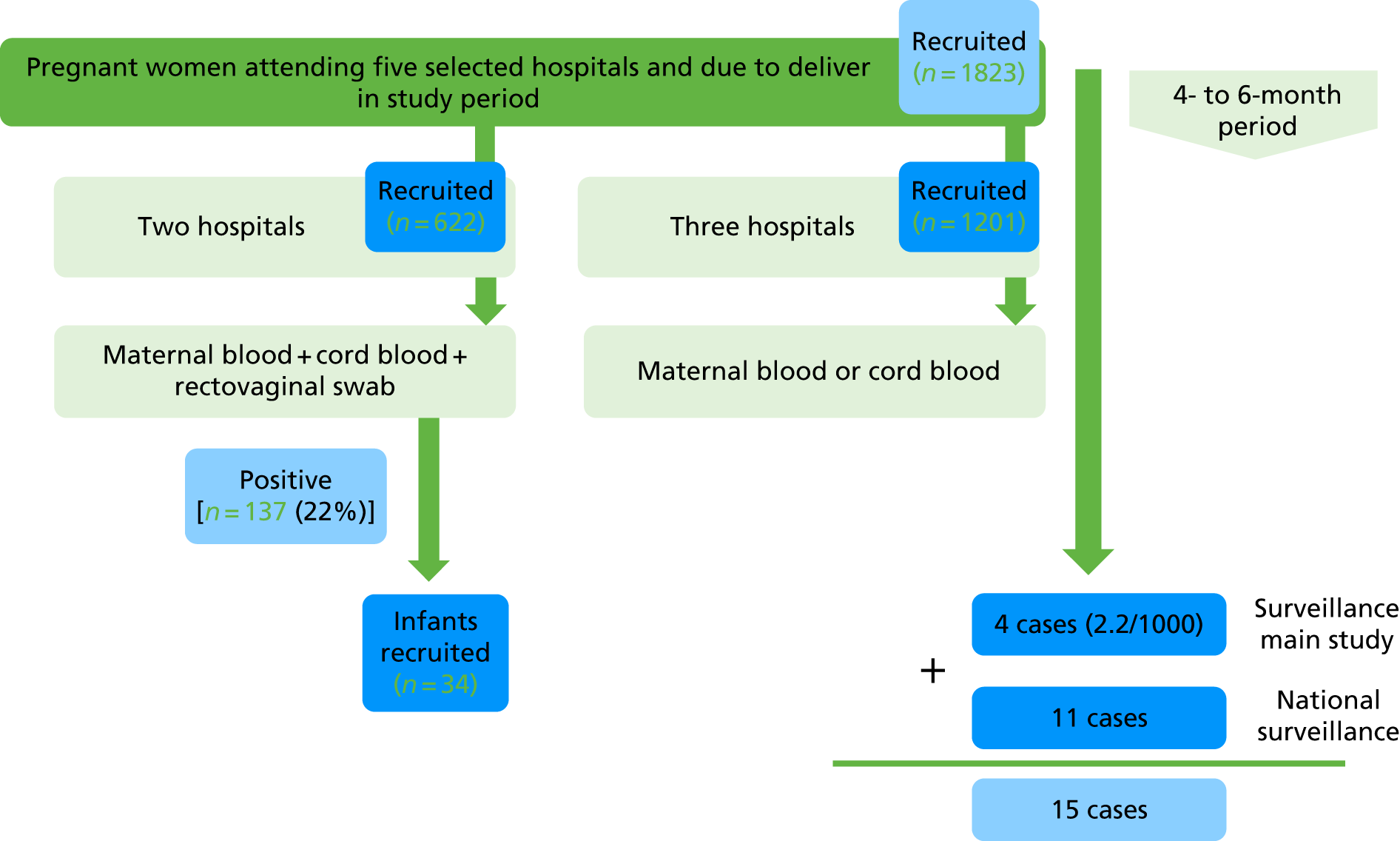
The analysis for this report is based on data collected from 2 July to 31 December 2018. The total number of participants recruited was 1823, across the five sites (Table 1). We had partial recruitment with fewer research midwives from 1 to 31 December. A summary of results is shown in Figure 3.
| Hospital | Start date | End date | Recruitment (n) |
|---|---|---|---|
| Croydon Health Services NHS Trust | 20 August 2018 | 31 December 2018 | 166 |
| Surrey and Sussex Healthcare NHS Trust | 8 August 2018 | 30 November 2018 | 414 |
| Kingston Hospital NHS Foundation Trust | 10 July 2018 | 31 December 2018 | 257 |
| Poole Hospital NHS Foundation Trust | 11 July 2018 | 31 December 2018 | 621 |
| St George’s University Hospitals NHS Foundation Trust | 2 July 2018 | 31 December 2018 | 365 |
| Total | 2 July 2018 | 31 December 2018 | 1823 |
FIGURE 3.
Summary of results.
Objective 1
To test the feasibility of collecting serum at delivery (maternal, cord or both) from a large cohort of pregnant women.
Enrolment rate
Overall, 22% (95% CI 21% to 23%) of all women delivering during the study period consented to take part in the feasibility study. However, when based on the number of women approached, the enrolment rate was 74% (95% CI 71% to 76%) (Table 2). Two sites did not collect information on the number of women approached, as the clinical teams approaching women did not accurately record when women declined to participate.
| Hospital | Consented/approached women, % (n/N) | Consented/total deliveries during study period, % (n/N) |
|---|---|---|
| Croydon Health Services NHS Trust | 49 (166/340) | 16 (166/1023) |
| Surrey and Sussex Healthcare NHS Trust | – | 30 (414/1370) |
| Kingston Hospital NHS Foundation Trust | 64 (240/377) | 10 (257/2462) |
| Poole Hospital NHS Foundation Trust | 93 (621/667) | 32 (621/1942) |
| St George’s University Hospitals NHS Foundation Trust | – | 15 (365/2458) |
| Total | 74 (1027/1384)a (95% CI 71 to 76) | 22 (1823/8340) (95% CI 21 to 23) |
Maternal and cord blood collection rates
In the two sites collecting both maternal and cord bloods, 60% and 53% of those recruited women had samples collected, respectively (Table 3). In the three hospitals collecting cord blood only, the collection rate was 85% (95% CI 83% to 87%) (Table 4).
| Hospital | Maternal blood/total recruited, % (n/N) | Cord blood/total recruited, % (n/N) |
|---|---|---|
| Kingston Hospital NHS Foundation Trust | 81 (207/257) | 79 (203/257) |
| St George’s University Hospitals NHS Foundation Trust | 45 (165/365) | 34 (125/365) |
| Total | 60 (372/622) | 53 (328/622) |
| Hospital | Cord blood/total recruited, % (n/N) | Maternal blooda/total recruited, % (n/N) | Maternal or cord blood/total recruited, % (n/N) |
|---|---|---|---|
| Croydon Health Services NHS Trust | 73 (121/166) | 0 (0/166) | 73 (121/166) |
| Surrey and Sussex Healthcare NHS Trust | 94 (389/414) | 6 (24/414) | 99 (413/414) |
| Poole Hospital NHS Foundation Trust | 76 (469/621) | 2 (14/621) | 78 (483/621) |
| Total | 82 (979/1201) | 3 (38/1201) | 85 (1017/1201) (95% CI 83 to 87) |
Key clinical data collection rate
Data on demographic and risk factors for iGBS were recorded in 90–100% of participants. Note that data regarding ethnicity and infant sex were included from 1 November 2018. Data relating to antibiotic administration were more difficult to collect retrospectively and represented a significant challenge to our teams (Table 5).
| Key clinical data | Collection rate, % (n/N) |
|---|---|
| Maternal demographic information | |
| Maternal age | 100 (1823/1823) |
| Ethnicity | 48 (878/1823a) |
| Previous pregnancy | 99 (1821/1823) |
| Previous GBS colonisation | 99 (1054/1056b) |
| Previous baby with iGBS | 99 (1050/1056b) |
| Birth information | |
| Time between membrane rupture and delivery | 92 (1676/1823) |
| Delivery type | 93 (1690/1823) |
| Term delivery | 93 (1690/1823) |
| Infant sex | 59 (1082/1823a) |
| Positive swab before birth | 91 (1666/1823) |
| Antibiotics if positive swab | 90 (166/185c) |
| Antibiotic timing | 54 (100/185c) |
| Antibiotics for other reasons | 65 (1179/1823b) |
Characteristics of participants recruited to the study are shown in Table 6.
| Demographic | Croydon Health Services NHS Trust (N = 166) | Surrey and Sussex Healthcare NHS Trust (N = 414) | Kingston Hospital NHS Foundation Trust (N = 257) | Poole Hospital NHS Foundation Trust (N = 621) | St George’s University Hospitals NHS Foundation Trust (N = 365) | Total (N = 1823) |
|---|---|---|---|---|---|---|
| Maternal age group (years), n (%) | ||||||
| < 25 | 18 (10.8) | 34 (8.2) | 12 (4.7) | 96 (15.5) | 23 (6.3) | 183 (10) |
| 25–34 | 84 (50.6) | 240 (58) | 133 (51.8) | 371 (59.7) | 195 (53.4) | 1023 (56.1) |
| 35–41 | 57 (34.3) | 125 (30.2) | 107 (41.6) | 145 (23.3) | 132 (36.2) | 566 (31) |
| > 41 | 7 (4.2) | 15 (3.6) | 5 (1.9) | 9 (1.4) | 15 (4.1) | 51 (2.8) |
| Ethnicity, n (%) | N = 120 | N = 4 | N = 31 | N = 587 | N = 136 | N = 878 |
| White British | 36 (30a) | 1 (25) | 14 (45) | 484 (82.5) | 65 (47.8) | 600 (68.3) |
| Black British | 3 (2.5) | 0 (0) | 0 (0) | 0 (0) | 8 (5.9) | 11 (1.3) |
| Asian British | 7 (5.8) | 2 (50) | 4 (12.9) | 2 (0.3) | 6 (4.4) | 21 (2.4) |
| Other white | 23 (19.2) | 0 (0) | 11 (35.5) | 42 (7.2) | 23 (16.9) | 99 (11.3) |
| Other black | 20 (16.7) | 0 (0) | 0 (0) | 3 (0.5) | 2 (1.5) | 25 (2.8) |
| Other Asian | 24 (20) | 0 (0) | 1 (3) | 12 (2) | 6 (4.4) | 43 (4.9) |
| Mixed | 2 (1.7) | 0 (0) | 1 (3) | 6 (1) | 1 (0.7) | 10 (1.1) |
| Others | 5 (4.2) | 1 (25) | 0 (0) | 38 (6.5) | 25 (18.4) | 69 (7.8) |
| Missing | 46 (27.7) | 410 (99) | 226 (87.9) | 34 (5.5) | 229 (62.7) | 945 (51.8) |
| Previous pregnancy, n (%) | ||||||
| Yes | 114 (68.7) | 253 (61.1) | 124 (48.2) | 387 (62.3) | 178 (48.8) | 1056 (57.9) |
| No | 52 (31.3) | 160 (38.6) | 133 (51.8) | 233 (37.5) | 187 (51.2) | 765 (42) |
| Missing | 0 (0) | 1 (0.2) | 0 (0) | 1 (0.2) | 0 (0) | 2 (0.1) |
| GBS colonisation in previous pregnancy, n (%) | N = 114 (68.7) | N = 253 (61.1) | N = 124 (48.2) | N = 387 (62.3) | N = 178 (48.8) | N = 1056 (57.9) |
| Yes | 4 (2.4) | 23 (5.6) | 17 (6.6) | 11 (1.8) | 13 (3.6) | 68 (3.7) |
| No | 87 (52.4) | 156 (37.7) | 99 (38.5) | 367 (59.1) | 60 (16.4) | 769 (42.2) |
| Unknown | 23 (13.9) | 73 (17.6) | 8 (3.1) | 9 (1.4) | 104 (28.5) | 217 (11.9) |
| Missing | 0 (0) | 1 (0.2) | 0 (0) | 0 (0) | 1 (0.3) | 2 (0.1) |
| Previous baby with iGBS, n (%) | N = 114 (68.7) | N = 253 (61.1) | N = 124 (48.2) | N = 387 (62.3) | N = 178 (48.8) | N = 1056 (57.9) |
| Yes | 1 (0.6) | 6 (1.4) | 1 (0.4) | 4 (0.6) | 7 (1.9) | 19 (1) |
| No | 90 (54.2) | 199 (48.1) | 119 (46.3) | 371 (59.7) | 116 (31.8) | 895 (49.1) |
| Unknown | 23 (13.9) | 47 (11.4) | 4 (1.6) | 12 (1.9) | 50 (13.7) | 136 (7.5) |
| Missing | 0 (0) | 1 (0.2) | 0 (0) | 0 (0) | 5 (1.4) | 6 (0.3) |
| Rupture of membranes, n (%) | ||||||
| < 18 hours | 131 (78.9) | 351 (84.8) | 214 (83.3) | 414 (66.7) | 260 (71.2) | 1370 (75.2) |
| ≥ 18 hours | 35 (21.1) | 57 (13.8) | 41 (16) | 102 (16.4) | 71 (19.5) | 306 (16.8) |
| Missing | 0 (0) | 6 (1.4) | 2 (0.8) | 105 (16.9) | 34 (9.3) | 147 (8.1) |
| Delivery type, n (%) | ||||||
| Vaginal | 86 (51.8) | 261 (63) | 132 (51.4) | 280 (45.1) | 214 (58.6) | 973 (53.4) |
| Caesarean section with ROM | 43 (25.9) | 75 (18.1) | 40 (15.6) | 95 (15.3) | 55 (15.1) | 308 (16.9) |
| Caesarean section without ROM | 37 (22.3) | 74 (17.9) | 83 (32.3) | 141 (22.7) | 74 (20.3) | 409 (22.4) |
| Missing | 0 (0) | 4 (1) | 2 (0.8) | 105 (16.9) | 22 (6) | 133 (7.3) |
| Gestational age, n (%) | ||||||
| < 34 weeks | 0 (0) | 3 (0.7) | 1 (0.4) | 4 (0.6) | 2 (0.5) | 10 (0.5) |
| ≥ 34 weeks | 166 (100) | 408 (98.6) | 254 (98.8) | 512 (82.4) | 340 (93.2) | 1680 (92.2) |
| Missing | 0 (0) | 3 (0.7) | 2 (0.8) | 105 (16.9) | 23 (6.3) | 133 (7.3) |
| Infant’s sex, n (%) | 144 | 79 | 120 | 509 | 230 | 1082 |
| Male | 71 (49.3a) | 40 (50.6) | 61 (50.8) | 248 (48.7) | 115 (50) | 535 (49.4) |
| Female | 73 (50.7) | 39 (49.4) | 59 (49.2) | 261 (51.3) | 115 (50) | 547 (50.6) |
| Missing | 22 (13.3) | 335 (80.9) | 137 (53.3) | 112 (18) | 135 (37) | 741 (40.6) |
| Positive swab before birth, n (%) | ||||||
| Yes | 28 (16.9) | 31 (7.5) | 28 (10.9) | 30 (4.8) | 68 (18.6) | 185 (10.1) |
| No | 138 (83.1) | 380 (91.8) | 227 (88.3) | 465 (74.9) | 271 (74.2) | 1481 (81.2) |
| Missing | 0 (0) | 3 (0.7) | 2 (0.8) | 126 (20.3) | 26 (7.1) | 157 (8.6) |
If we compare data from recruited women (see Table 6) and women delivering at the five sites during the last year (Table 7), we can assess the representativeness of our population. Overall, the sample from this study is similar to the general population of pregnant women. With regard to ethnicity, Tables 6 and 7 show that Croydon Health Services NHS Trust and St George’s University Hospitals NHS Foundation Trust have a large non-white population. Regarding delivery type, more women with elective caesarean sections were included in the study than are represented in the total study population.
| Demographic | Croydon Health Services NHS Trust | Surrey and Sussex Healthcare NHS Trust | Kingston Hospital NHS Foundation Trust | Poole Hospital NHS Foundation Trust | St George’s University Hospitals NHS Foundation Trust | Total |
|---|---|---|---|---|---|---|
| Maternal age (years) (mean) | 31 | 31.8 | 32 | 30.5 | 32.6 | 31.6 |
| Ethnicity (%) | ||||||
| White | 36 | 80 | 63 | 0 | 46 | 56 |
| Asian | 14 | 11 | 10 | 0 | 16 | 13 |
| Black | 17 | 3 | 3 | 0 | 10 | 8 |
| Mixed | 9 | 3 | 1 | 0 | 2 | 4 |
| Other ethnic groups | 24 | 2 | 7 | 0 | 10 | 11 |
| Not stated | 0 | 1 | 16 | 0 | 16 | 8 |
| Missing | 0 | 0 | 0 | 100 | 0 | 20 |
| Delivery type (%) | ||||||
| Caesarean section | 26 | 30 | 29 | 32 | 25 | 28 |
| Vaginal | 51 | 70 | 71 | 68 | 75 | 68 |
| Missing | 23 | 0 | 0 | 0 | 0 | 4 |
| Gestational age (%) | ||||||
| < 37 weeks | 4 | 9 | 6 | 0 | 9 | 7 |
| ≥ 37 weeks | 64 | 91 | 94 | 0 | 91 | 85 |
| Missing | 32 | 0 | 0 | 100 | 0 | 26 |
| Infant’s sex (%) | ||||||
| Male | 51 | 51 | 0 | 51 | 51 | 51 |
| Female | 49 | 49 | 0 | 49 | 49 | 49 |
| Missing | 0 | 0 | 100 | 0 | 0 | 20 |
The GBS-colonised women received benzylpenicillin in 62–85% of cases depending on the hospital site; the first dose was given ≥ 4 hours before delivery in 50% of women with GBS-positive swabs (60/119). The antibiotics given most frequently for reasons other than GBS colonisation were cefuroxime and cefalexin (Table 8).
| Croydon Health Services NHS Trust | Surrey and Sussex Healthcare NHS Trust | Kingston Hospital NHS Foundation Trust | Poole Hospital NHS Foundation Trust | St George’s University Hospitals NHS Foundation Trust | Total | |
|---|---|---|---|---|---|---|
| Antibiotics if positive swab (N) | 28 | 31 | 28 | 30 | 68 | 185 |
| Yes, n (%) | 21 (75) | 27 (87.1) | 22 (78.6) | 20 (66.7) | 29 (42.6) | 119 (64.3) |
| No, n (%) | 7 (25) | 4 (12.9) | 6 (21.4) | 10 (33.3) | 20 (29.4) | 47 (25.4) |
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 19 (27.9) | 19 (10.3) |
| Antibiotics type (N) | 23 | 29 | 22 | 20 | 26 | 120 |
| Benzylpenicillin, n (%) | 13 (61.9) | 23 (85.2) | 18 (81.8) | 17 (85) | 21 (72.4) | 92 (77.3) |
| Clindamycin, n (%) | 3 (14.3) | 2 (7.4) | 2 (9.1) | 1 (5) | 1 (3.4) | 9 (7.6) |
| Others, n (%) | 7 (25) | 4 (12.9) | 2 (7) | 2 (6.6) | 4 (5.9) | 19 (4.2) |
| Antibiotics timing if positive swab (N) | 21 | 27 | 22 | 20 | 29 | 119 |
| < 2 hours before birth, n (%) | 2 (9.5) | 7 (25.9) | 6 (27.3) | 4 (20) | 3 (10.3) | 22 (18.5) |
| 2–4 hours before birth, n (%) | 3 (14.3) | 3 (11.1) | 4 (18.2) | 7 (35) | 1 (3.4) | 18 (15.1) |
| > 4 hours before birth, n (%) | 16 (76.2) | 16 (59.3) | 12 (54.5) | 8 (40) | 8 (27.6) | 60 (50.4) |
| Missing, n (%) | 0 (0) | 1 (3.7) | 0 (0) | 1 (5) | 17 (58.6) | 19 (16) |
| Antibiotics for other reasons (N) | 166 | 414 | 257 | 621 | 365 | 1823 |
| Yes | 44 (26.5) | 36 (8.7) | 48 (18.7) | 232 (37.4) | 21 (5.8) | 381 (20.9) |
| No | 92 (55.4) | 219 (52.9) | 118 (45.9) | 272 (43.8) | 97 (26.6) | 798 (43.8) |
| Missing | 30 (18.1) | 159 (38.4) | 91 (35.4) | 117 (18.8) | 247 (67.7) | 644 (35.3) |
| Antibiotics type for other reasons (N) | 43 | 33 | 48 | 230 | 14 | 368 |
| Benzylpenicillin, n (%) | 1 (0.3) | 7 (1.8) | 0 (0) | 3 (0.8) | 3 (0.8) | 14 (3.7) |
| Clindamycin, n (%) | 3 (0.8) | 1 (0.3) | 0 (0) | 1 (0.3) | 0 (0) | 5 (1.3) |
| Cefalexin, n (%) | 18 (4.7) | 3 (0.8) | 0 (0) | 7 (1.8) | 7 (1.8) | 35 (9.2) |
| Ceftriaxone, n (%) | 0 (0) | 1 (0.3) | 0 (0) | 2 (0.5) | 0 (0) | 3 (0.8) |
| Cefuroxime, n (%) | 20 (5.2) | 21 (5.5) | 47 (12.3) | 217 (57) | 2 (0.5) | 307 (80.6) |
| Co-amoxiclav, n (%) | 0 (0) | 0 (0) | 1 (0.3) | 0 (0) | 2 (0.5) | 3 (0.8) |
| Vancomycin, n (%) | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.3) |
Infant invasive group B streptococcal disease surveillance
The consent rate for the 90-day follow-up telephone call to be made was 100% (1823/1823).
The telephone calls started 90 days after the first baby was born and, because of the short study period, applied only to those recruited in the first month of the study. No cases of iGBS were detected in the telephone follow-up that had not already been reported by the hospital team (Table 9).
| Croydon Health Services NHS Trust (N = 166) | Surrey and Sussex Healthcare NHS Trust (N = 414) | Kingston Hospital NHS Foundation Trust (N = 257) | Poole Hospital NHS Foundation Trust (N = 621) | St George’s University Hospitals NHS Foundation Trust (N = 365) | Total (N = 1823) | |
|---|---|---|---|---|---|---|
| Telephone call attempt 1, n (%) | ||||||
| Yes | 40 (24.1) | 51 (12.3) | 20 (7.8) | 80 (12.9) | 100 (27.4) | 291 (16) |
| No | 126 (75.9) | 363 (87.7) | 237 (92.2) | 541 (87.1) | 265 (72.6) | 1532 (84) |
| Telephone call 1: successful, n (%) | (N = 40) | (N = 51) | (N = 20) | (N = 80) | (N = 100) | (N = 291) |
| Yes | 23 (57.5) | 14 (27.5) | 6 (30) | 35 (43.8) | 40 (40) | 118 (40.5) |
| No | 17 (42.5) | 37 (72.5) | 14 (70) | 45 (56.3) | 60 (60) | 173 (59.5) |
| Telephone call attempt 2, n (%) | (N = 17) | (N = 37) | (N = 14) | (N = 45) | (N = 60) | (N = 173) |
| Yes | 17 (100) | 32 (86.5) | 5 (35.7) | 36 (80) | 32 (53.3) | 122 (70.5) |
| No | 0 (0) | 5 (13.5) | 9 (64.3) | 9 (20) | 28 (46.7) | 51 (29.5) |
| Telephone call 2: successful, n (%) | (N = 17) | (N = 32) | (N = 5) | (N = 36) | (N = 32) | (N = 122) |
| Yes | 3 (17.6) | 4 (12.5) | 0 (0) | 16 (44.4) | 11 (34.4) | 34 (27.9) |
| No | 14 (82.4) | 28 (87.5) | 5 (100) | 20 (55.6) | 21 (65.6) | 88 (72.1) |
Rectovaginal swab consent and collection rate
Of the 622 women enrolled at the two hospitals taking part in the colonisation study, rectovaginal consent was 100% and the rectovaginal collection rate was 99% (Table 10). The overall GBS colonisation rate was 22% (Table 11).
| Hospital | Rectovaginal swab study consent rate, % (n/N) | Rectovaginal swab collection rate, % (n/N) |
|---|---|---|
| Kingston Hospital NHS Foundation Trust | 100 (257/257) | 99 (255/257) |
| St George’s University Hospitals NHS Foundation Trust | 100 (365/365) | 98 (359/365) |
| Total | 100 (622/622) | 99 (614/622) |
| Hospital | GBS positive/swabs analysed, % (n/N) | Known positive swab/all swabs,a % (n/N) | GBS positive/all swabs, % (n/N) |
|---|---|---|---|
| Kingston Hospital NHS Foundation Trust | 20 (49/247) | 3 (8/255) | 22 (57/255) |
| St George’s University Hospitals NHS Foundation Trust | 20 (71/350) | 3 (9/359) | 22 (80/359) |
| Total | 20 (120/597) | 3 (17/614) | 22 (137/614) (95% CI 19% to 26%) |
Rectovaginal group B streptococcus serotype-specific colonisation rates
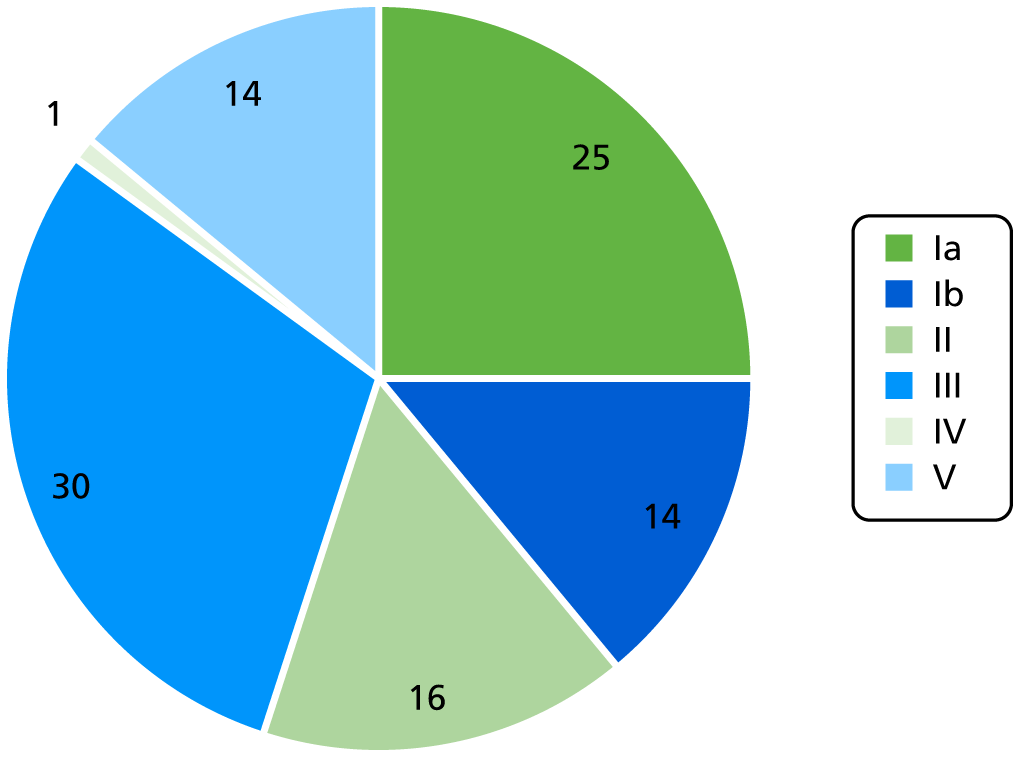
A total of 115 out of 120 positive swabs were serotyped with a rapid LAT and PCR was performed when the sample was found to be non-typeable by LAT. The most common serotype was serotype III (30%), followed by Ia (25%), II (16%), Ib (16%), V (14%) and IV (1%) (Figure 4).
FIGURE 4.
The GBS serotype distribution in colonised women.
Risk factors for group B streptococcus colonisation
No statistically significant differences were found between colonised and non-colonised women regarding demographic and risk factors for iGBS except having had a positive GBS swab in a previous pregnancy, which was more common in colonised women (Table 12).
| Demographic | Colonised, n (%) | Non-colonised, n (%) | Total, n (%) | p-value |
|---|---|---|---|---|
| 137 (22.3) | 477 (77.7) | 614 (100) | ||
| Hospital | 0.984 | |||
| Kingston Hospital NHS Foundation Trust | 57 (22.4) | 198 (77.6) | 255 (41.5) | |
| St George’s University Hospitals NHS Foundation Trust | 80 (22.3) | 279 (77.7) | 359 (58.5) | |
| Maternal age group (years) | 0.159 | |||
| < 25 | 6 (17.1) | 29 (82.9) | 35 (5.7) | |
| 25–34 | 66 (20.5) | 256 (79.5) | 322 (52.4) | |
| 35–41 | 57 (24.1) | 180 (75.9) | 237 (38.6) | |
| > 41 | 8 (40) | 12 (60) | 20 (3.3) | |
| Ethnicity | 0.124 | |||
| White British | 35 (44.9) | 43 (55.1) | 78 (12.7) | |
| Black British | 5 (62.5) | 3 (37.5) | 8 (1.3) | |
| Asian British | 3 (30) | 7 (70) | 10 (1.6) | |
| Other white | 22 (64.7) | 12 (35.3) | 34 (5.5) | |
| Other black | 1 (50) | 1 (50) | 2 (0.3) | |
| Other Asian | 5 (71.4) | 2 (28.6) | 7 (1.1) | |
| Mixed | 0 (0) | 2 (100) | 2 (0.3) | |
| Others | 16 (66.7) | 8 (33.3) | 24 (3.9) | |
| Missing | 50 (11.1) | 399 (88.9) | 449 (73.1) | |
| Previous pregnancy | 0.803 | |||
| Yes | 68 (22.7) | 231 (77.3) | 299 (48.7) | |
| No | 69 (21.9) | 246 (78.1) | 315 (51.3) | |
| GBS colonisation in previous pregnancy | 68 (22.7) | 231 (77.3) | 299 (100) | 0.041 |
| Yes | 12 (41.4) | 17 (58.6) | 29 (9.7) | |
| No | 34 (21.5) | 124 (78.5) | 158 (52.8) | |
| Unknown | 22 (19.8) | 89 (80.2) | 111 (37.1) | |
| Missing | 0 (0) | 1 (100) | 1 (0.3) | |
| Previous baby with iGBS | 68 (22.7) | 231 (77.3) | 299 (100) | 0.427 |
| Yes | 3 (42.9) | 4 (57.1) | 7 (2.3) | |
| No | 54 (23.1) | 180 (76.9) | 234 (78.3) | |
| Unknown | 11 (20.8) | 42 (79.2) | 53 (17.7) | |
| Missing | 0 (0) | 5 (100) | 5 (1.7) | |
| Rupture of membranes | 0.96 | |||
| < 18 hours | 106 (22.5) | 365 (77.5) | 471 (76.7) | |
| ≥ 18 hours | 25 (22.7) | 85 (77.3) | 110 (17.9) | |
| Missing | 6 (18.2) | 27 (81.8) | 33 (5.4) | |
| Delivery type | 0.825 | |||
| Vaginal | 74 (21.6) | 268 (78.4) | 342 (55.7) | |
| Caesarean section with ROM | 23 (24.5) | 71 (75.5) | 94 (15.3) | |
| Caesarean section without ROM | 36 (23.1) | 120 (76.9) | 156 (25.4) | |
| Missing | 4 (18.2) | 18 (81.8) | 22 (3.6) | |
| Gestation | 0.349 | |||
| < 34 weeks | 0 (0) | 3 (100) | 3 (0.5) | |
| ≥ 34 weeks | 133 (22.6) | 455 (77.4) | 588 (95) | |
| Missing | 4 (17.4) | 19 (82.6) | 23 (3.7) | |
| Infant’s sex | 0.541 | |||
| Male | 42 (25.1) | 125 (74.9) | 167 (27.2) | |
| Female | 38 (22.8) | 129 (77.2) | 167 (27.2) | |
| Missing | 57 (20.4) | 223 (79.6) | 280 (45.6) |
Infant blood sample collection rates
In the two hospitals participating in both the colonisation and the blood collection studies, consent to obtain an infant blood sample if the mother was GBS positive and consent to obtain Guthrie card samples were both 100% (Table 13). However, we were able to contact the families of 61% of eligible infants and to collect blood samples from 59% of infants whose family we contacted (Table 14). Samples were taken from seven infants at 1 month, 14 infants at 2 months and 13 infants at 3 months of life.
| Infant sample | Consent rate, % (n/N) |
|---|---|
| Blood sample | 100 (622/622) |
| Guthrie card | 100 (622/622) |
| Hospital | Infants contacted/eligible infants, % (n/N) | Infant blood sample collected/infants contacted, % (n/N) |
|---|---|---|
| Kingston Hospital NHS Foundation Trust | 56 (27/48) | 48 (13/27) |
| St George’s University Hospitals NHS Foundation Trust | 66 (31/47) | 68 (21/31) |
| Total | 61 (58/95) | 59 (34/58) |
Objective 2
To test the feasibility of collecting samples from iGBS cases.
Participants from the feasibility study
There were five notifications of iGBS within the participants of the main study, all from Poole Hospital. The clinical presentation was EOD in all cases, and three cases occurred in conjunction with cases of maternal sepsis. Samples of maternal blood, a rectovaginal swab, infant blood and the bacterial isolate were collected from four mother–infant pairs (Table 15). The fifth case was unable to be recruited as the infant was admitted to a different hospital.
| Case | Infant’s sex | Age at presentation | Weeks’ gestation | Duration of ROM | IAP | Time of first dose | Presentation | Isolate serotype |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 15 hours | 40.5 | > 18 hours | Cefuroxime + metronidazole | < 2 hours | Sepsis | II |
| 2 | Female | Birth | 39 | < 18 hours | Cefuroxime + metronidazole | > 4 hours | Sepsis | V |
| 3 | Male | Birth | 33.3 | > 18 hours | Erythromycin | > 4 hours | Sepsis | Ib |
| 4 | Female | Birth | 40.4 | < 18 hours | Cefuroxime + metronidazole | < 2 hours | Sepsis | II |
Participants from national invasive group B streptococcal disease surveillance
The protocol was amended on 6 September 2018 to include national (England and Wales) surveillance for iGBS. This resulted in 18 notifications, from which 11 cases were recruited into the study (58%). Seven cases were not recruited as there was insufficient time to set up the site before the patient was discharged. All parents approached consented to participation in the study. The clinical presentation was EOD in nine and LOD in two cases (both of which were preterm infants) (Table 16).
| Case | Infant’s sex | Age at presentation | Weeks’ gestation | Duration of ROM | IAP | Time of first dose | Presentation | Isolate serotype |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 18 hours | 40+2 | < 18 hours | No | – | Sepsis | III |
| 2 | Male | 14 hours | 42 | < 18 hours | Cefuroxime + metronidazole | < 2 hours | Sepsis | II |
| 3 | Female | 2 hours | 41 | < 18 hours | No | – | Sepsis | Ia |
| 4 | Female | 16 hours | 38+3 | > 18 hours | No | – | Sepsis | Ia |
| 5 | Female | < 24 hours | 37+4 | < 18 hours | No | – | Sepsis + meningitis | Unknown |
| 6 | Female | 6 hours | 39 | > 18 hours | No | – | Sepsis | Ia |
| 7 | Female | 7 hours | 40 | > 18 hours | No | – | Sepsis | III |
| 8 | Male | 42 days | 30+2 | > 18 hours | No | – | Sepsis | III |
| 9 | Female | 3 days | Term | < 18 hours | No | – | Sepsis | IV |
| 10 | Male | 5 hours | 40 | > 18 hours | No | – | Sepsis | III |
| 11 | Male | 53 days | 25 | < 18 hours | No | – | Sepsis | II |
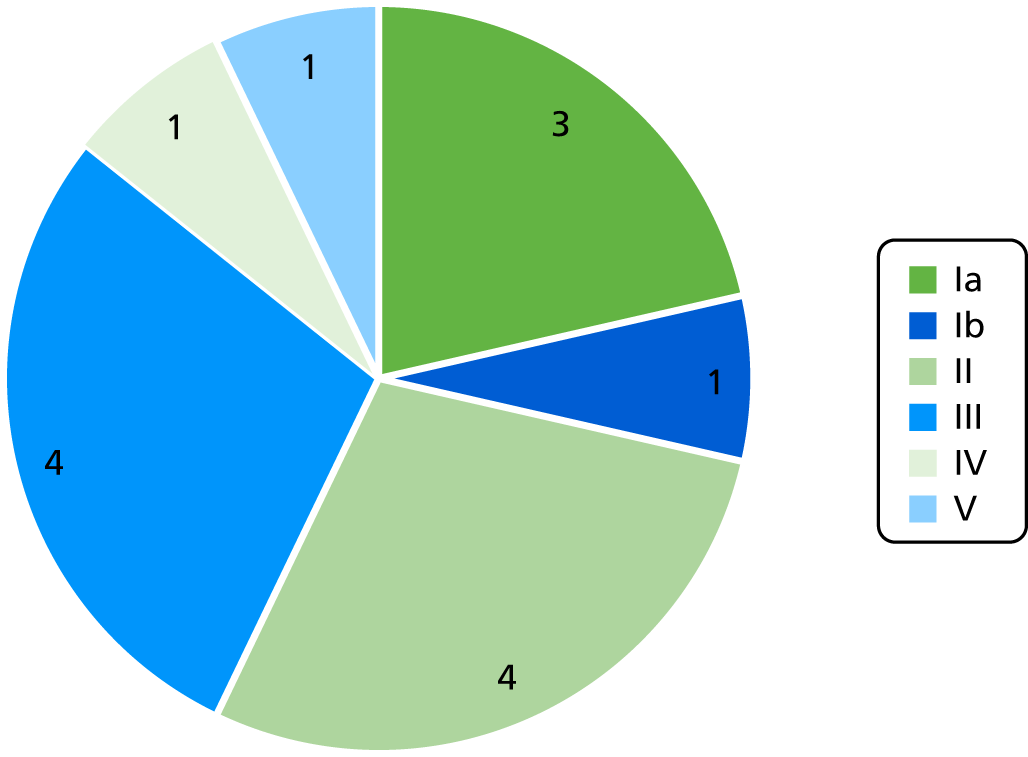
In total, serotyping data were available for 14 of the 15 iGBS cases (including the four cases from the feasibility study); the distribution is shown in Figure 5. None of the infants whose samples were collected died during the study.
FIGURE 5.
Serotype distribution in iGBS cases.
Exploratory objectives
Impact of timing of processing and blood sample storage conditions on immunoglobulin G concentrations
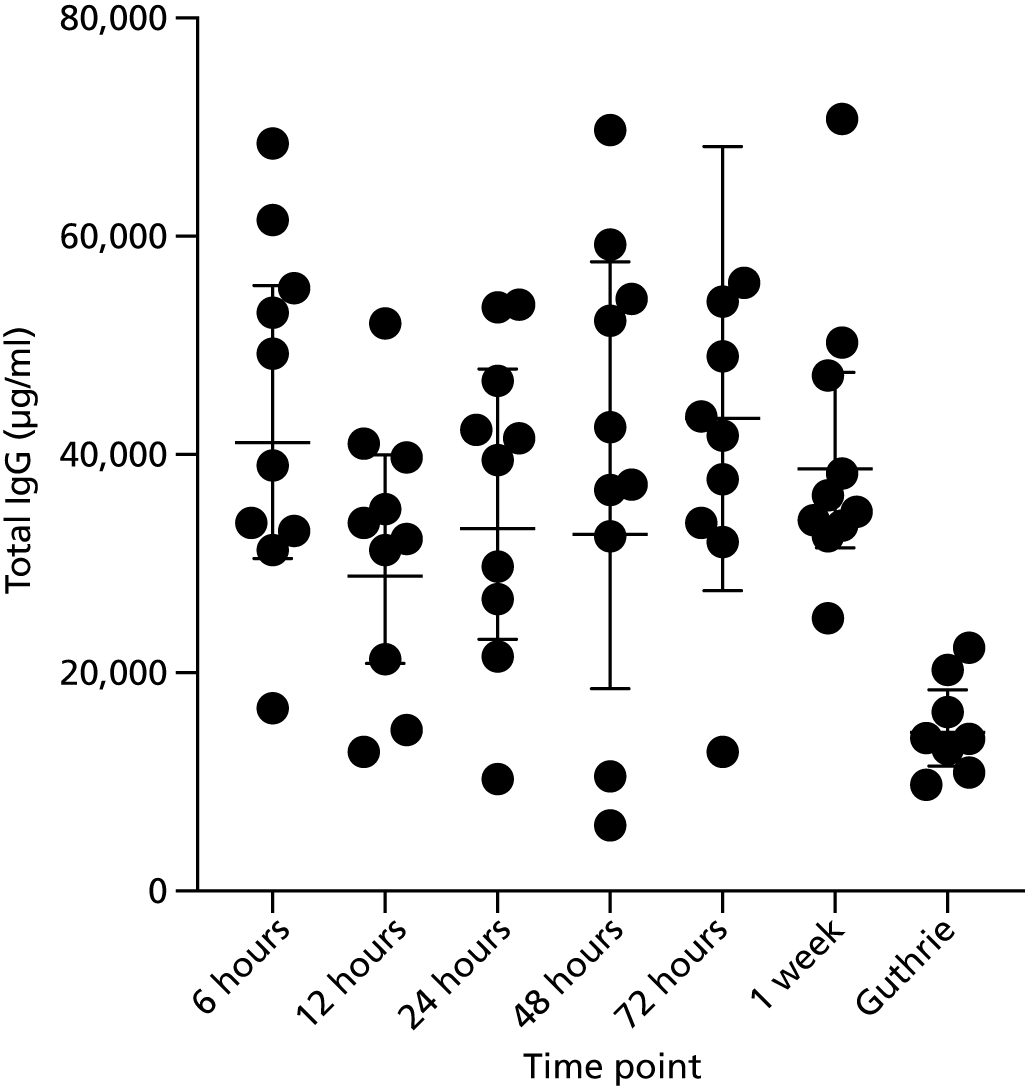
There was an acceptable impact on total IgG concentrations for periods of time from 6 hours up to 1 week between sample collection and spinning time (Figure 6). The average time of spinning and freezing samples varied between hospitals but was generally within 1 week of sample receipt (Table 17). St. George’s Hospital processed all samples from Croydon, Kingston and St. George’s Hospitals.
FIGURE 6.
Total IgG concentrations by time between sample collection and centrifuging of sample. Guthrie refers to IgG detection from Guthrie cards.
| Laboratory | Description | Number of samples | Mean (days) | Standard deviation (days) | Minimum (days) | Maximum (days) | Total (days) |
|---|---|---|---|---|---|---|---|
| Surrey and Sussex Healthcare NHS Trust | MB collection to spin | 23 | 1.4 | 1.0 | 0.0 | 4.0 | 23 |
| MB spin to freeze | 23 | 0.0 | 0.2 | 0.0 | 1.0 | ||
| CB collection to spin | 388 | 1.8 | 1.2 | 0.0 | 7.0 | 389 | |
| CB spin to freeze | 389 | 0.1 | 0.6 | 0.0 | 4.0 | ||
| St George’s University Hospitals NHS Foundation Trust | MB collection to spin | 349 | 6.5 | 6.5 | 0.0 | 44.0 | 366 |
| MB spin to freeze | 366 | 6.4 | 6.6 | 0.0 | 42.0 | ||
| CB collection to spin | 480 | 7.0 | 6.7 | 0.0 | 44.0 | 524 | |
| CB spin to freeze | 524 | 7.3 | 6.7 | 0.0 | 42.0 | ||
| Poole Hospital NHS Foundation Trust | MB collection to spin | 13 | 4.2 | 8.1 | 0.0 | 31.0 | 13 |
| MB spin to freeze | 13 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| CB collection to spin | 457 | 1.5 | 1.8 | 0.0 | 33.0 | 458 | |
| CB spin to freeze | 458 | 0.0 | 0.4 | 0.0 | 8.0 |
Serotype-specific group B streptococcus anticapsular polysaccharide immunoglobulin G concentrations in maternal serum, cord and infant blood in subjects colonised with group B streptococcus at delivery
We were able to identify serotype-specific GBS anti-CPS IgG concentrations in maternal serum and cord blood. Antibody concentrations generally declined between birth and 3 months of age.
The correlation between two different culture techniques for detection of group B streptococcus in rectovaginal swabs: enrichment culture medium versus direct plating using selective agar
The enrichment culture medium was more sensitive than direct plating at identifying GBS from rectovaginal swabs (Table 18). A total of 97% (116/120) of positive swabs were identified by enriched culture medium (ECM) plus plating onto selective agar as compared with 75% (90/120) by direct plating onto selective agar (p < 0.001, McNemar’s test).
| Direct plating onto selective agar positive | Direct plating onto selective agar negative | Number of swabs | |
|---|---|---|---|
| ECM positive | 86 | 30 | 116 |
| ECM negative | 4 | 477 | 481 |
| Total (n) | 90 | 507 | 597 |
Feedback from sites
We undertook site visits to all of the participating hospitals on two occasions during the study period. Feedback from the local teams resulted in changes to study design, including recruitment strategies and data and sample collection. Details of feedback from sites can be found in Appendix 1.
Chapter 5 Discussion
The ultimate aim of this work was to determine the serocorrelates of protection against the major GBS serotypes causing disease in young infants in order to facilitate GBS vaccine licensure. Given the anticipated size and logistical complexities of the study that would be needed to address this, the aim of this initial feasibility study was to test key operational aspects of the proposed study design.
The feasibility study recruited 1823 women from five hospitals over a period of time, ranging from 3.5 to 6 months. This represented 22% of all women delivering during this period, but 74% of those women who were approached (95% CI 71% to 76%). Once consent was obtained, there was a very high rate of blood sample collection [i.e. 85% (95% CI 83% to 87%) for the three hospitals that were collecting cord samples only]. This is very reassuring because it is cord blood collection from a large number of women nationally (180,000) that is required in the main study. The significance of this group for the large study is that it will form the cohort from which cases are captured (i.e. to determine the antibody levels in the cord blood of babies who go on to develop iGBS at < 3 months of age).
There was notable variation in recruitment across the sites, with one site (Poole Hospital NHS Foundation Trust) achieving a very high (93%) recruitment rate of those approached (32% overall). A number of factors might account for this and our observations at sites and, in particular, the feedback from sites revealed different recruitment strategies and examples of best practice. For example, by following such feedback, we sought and gained ethics approval to allow retrospective consent for cord blood collection, which led to improved recruitment and collection of samples. Engagement of the whole clinical midwife team in the study by enthusiastic research staff was found to be more effective than recruitment by the research staff alone; sending out information sheets to parents prior to approaching them was also shown to result in better recruitment at one site. Such observations (see Appendix 1) can now be implemented in a future study.
Other work undertaken during the study has also allowed us to define practical issues around sample collection that can now be applied in a future study. For example, the lack of impact on IgG concentrations for periods of time between blood sample collection and spinning time, ranging from 6 hours to 1 week (substudy), will enable sites to batch samples before spinning to maximise efficiency savings. These results differ from previous guidelines that recommended a maximum of 6 hours between collection and spinning. 31
The demographics of the recruited women suggest important differences in the populations served by the different hospitals, which is a major consideration when selecting these hospitals for the feasibility study. Croydon Health Services NHS Trust, for example, had a very high proportion of black and Asian mothers (45%) compared with Poole Hospital NHS Foundation Trust (3%). Such factors may also have influenced the success of recruitment in the different sites: Poole Hospital NHS Foundation Trust recruited 93% of those approached (32% overall) whereas Croydon Health Services NHS Trust recruited 49% of those approached (16% overall). This can be explored further by comparing the characteristics of those recruited, by site, with the characteristics of all women at the site, but does also suggest the need for additional strategies (e.g. translation of the patient information sheet into relevant local languages).
In the two hospitals undertaking the substudy (rectovaginal swab, maternal blood and cord blood), the enrolment rate was (understandably) lower: 10–15% of all women delivering during the period of the study. Again, rates of blood sample collection varied by hospital: 34–45% at St George’s University Hospitals NHS Foundation Trust versus 79–81% at Kingston Hospital NHS Foundation Trust. A key difference here was the timing of approach and sample collection, with lower rates associated with recruitment in antenatal clinics and higher rates with recruitment in the labour ward, which suggested the latter as the better model for a future study. By contrast, collection rates of vaginorectal swabs were extremely high at both sites, indicating that pregnant women are keen to participate in swabbing studies and are (probably) highly motivated to understand more regarding GBS disease. The significance of this substudy is that it defines the control group needed to address the serocorrelates of protection (i.e. the antibody levels in women who are known to be colonised at delivery and did not receive IAP but whose infants do not develop iGBS in < 3 months).
For those women who consented, collection of the key clinical information needed to interpret antibody concentrations (in controls) was also generally high, although some information, such as ethnicity and infant sex, was collected only in the final 2 months of the study.
Colonisation rates (22%, 95% CI 19% to 26%) and the serotype distribution among colonising isolates are consistent with those found in previous studies. 3 However, as these studies were conducted a number of years ago, this study provides contemporary data that are essential for planning new studies (e.g. GBS3) and for modelling new interventions (e.g. vaccination).
Parents uniformly agreed to being followed up by a telephone call once their baby was 3 months of age, indicating this to be a satisfactory method to follow up babies for later disease (and to ensure that suitable controls are chosen). However, actually telephoning these parents was very labour intensive, which suggested that alternatives, such as data linkage to national databases and networks, may be a more effective method of identifying infants who have not developed iGBS.
During the study period, parental consent and all relevant samples were collected from 15 infants with invasive GBS disease: four cases from the study cohort (of 1823; incidence 2.2/1000 live births) and 11 from national surveillance, which was instituted part-way through the study period. This provides strong reassurance that our system can successfully obtain relevant samples from cases of disease; this is critical in addressing the objectives of the serocorrelates of protection study. It will be imperative that sites are set up in advance of the main study to ensure that samples can be collected while the infant is still in the hospital.
An important substudy was to identify the best laboratory methods for detecting GBS colonisation. Of the 100 microbiology laboratories in the UK, only approximately 20% are currently equipped to undertake ECM analysis of GBS swabs. The majority of UK microbiology laboratories use direct plating onto selective agar in a semiautomated system. We compared direct plating versus incubation in ECM before plating onto selective agar. The latter method identified 97% of the positive GBS swabs compared with 75% using the standard method. These considerations are important as the UK moves towards fewer, more centralised laboratories that require automation to manage the throughput of samples within clinical microbiology. Our results have important implications for sample size calculations in future UK studies, such as the GBS3 trial.
Parents were very keen, in principle, to have blood obtained from their infants over the first 3 months of life. We enrolled 34 infants, born to colonised women, into the antibody kinetics substudy and preliminary data indicate a waning of antibodies between birth and 3 months of life. This will help us understand the relationship between the antibody at the time of iGBS with the antibody in the cord blood of that baby and of the mother. A serocorrelate of protection in the baby will be important in determining protection, but protective antibody at the time of acute disease might overestimate (or underestimate) the true protective level. Our results will therefore enable this interpretation in the main study.
Limitations and recommendations
In summary, this initial feasibility study has been able to assess key operational aspects of the large study that is needed to define the serocorrelates of protection against iGBS.
The main limitation is that we were not able to recruit the (hypothetical) target of 4000 women, mainly because of the short period of the study and insufficient staff dedicated to it. However, we believe that we have recruited a suitable number to allow us to draw conclusions that will now aid the design and co-ordination of the main trial. This includes the clinical data to be collected, how they are recorded, when to approach women in pregnancy and how the issue of cord blood collection can be managed. For example, we believe that an opt-out approach, whereby cord blood sampling would become part of normal routine practice, would help to recruit the large cohort we are aiming for in the serocorrelates study. We propose to assess this in a continuation of this study.
Chapter 6 Next steps
As described and justified earlier, we propose that to define the serocorrelates of protection against the most common serotypes of GBS causing infant disease in the UK will require a case–control study of suitable size.
Sample size calculation
Based on previous correlates of risk reduction studies,8,20,22 correlates that give risk reductions of 80–90% are feasible and acceptable. An 80% reduction in a case–control study implies an odds ratio of 0.2. The precision around 0.2 for varying case sample sizes and varying proportion of controls with IgG levels above the potential correlate levels (‘cuts’) is shown in Appendix 2.
Cases
Based on the incidence of iGBS from our 2014 national surveillance study (0.9/1000 live births up to 90 days of age),10 we anticipate being able to collect at least 150 iGBS case samples (EOD and LOD) over 2 years of collection from 180,000 women.
Controls
The number of controls is based on the need for a 3 : 1 ratio of controls to cases (see Appendix 2). Of 150 iGBS cases, we would expect approximately 100 cases of serotype III disease and, thus, 300 suitable controls are required to be matched to them (i.e. women colonised with serotype III, who do not receive IAP and whose babies do not develop iGBS). As indicated previously, and confirmed by the results of the feasibility study, with a control group of 5000 women we would expect to recruit approximately 380 healthy term infants born to GBS serotype III-colonised women who have not received IAP.
Possible strategies for completing the case–control study
On discussion with the GBS3 trial team, it is apparent that the most efficient way of recruiting the control group would be by extending the feasibility study rather than by recruiting these women within the GBS3 trial. This is because, in the context of the GBS3 trial, the women who would potentially be eligible to form the control group (i.e. women who are having a swab performed) will (essentially) all go on to receive IAP if they are found to be GBS colonised. By definition, if a woman has received IAP then she would be ineligible to be in the control group as the receipt of IAP will alter the risk of EOD in her baby. It is likely that women in the ‘standard of care’ arm in GBS3 will be less likely to receive IAP but, as they are not having a swab performed, they would not be eligible as controls. Based on the data from this feasibility study, in which 8.3% of women at the three sites that did not participate in the colonisation substudy happened to have a positive swab obtained before birth (89/1072, excluding missing data), we would need to recruit 12,000 women to identify the 1000 in the ‘standard of care’ arm who have been opportunistically swabbed and are colonised in order to capture the 380 infants born to mothers colonised with GBS serotype III.
An alternative for generating the control group would be to identify women in GBS3 who have a positive GBS swab result, but (for whatever reason) do not receive IAP. This is not our preferred method, as it seems likely that they will be an unusual (and potentially unrepresentative) group of women. However, more importantly, the GBS3 team have indicated that it will not be possible to identify (at an individual level vs. a unit level) those women who actually receive IAP. Owing to the design of the GBS3 study, it will therefore be impossible to identify these women.
The feasibility study has allowed us to identify best practice in recruiting women to have a rectovaginal swab, cord blood and key clinical information collected. We believe that the control group of 5000 women could therefore be recruited using the five sites that have participated in the feasibility study; all sites have expressed a willingness to do so, subject to sufficient funding to support recruitment.
We have recruited around 600 women with swabs in 4 months from two sites (75 per site per month). We therefore estimate that we will require an additional 12 months at five sites to complete control group recruitment (i.e. 75 women per month per site = 75 × 5 = 375 women per month; 375 women × 12 months = 4500 women), plus 3 months for site set-up (renewal of ethics and local approvals) and to ensure staff coverage within the midwifery teams (total 15 months extension). This would ensure that we achieve our target of 5000 women, of whom we expect 380 to be colonised with GBS serotype III and who would serve as our control group for iGBS III cases, recruited in the main study.
Invasive group B streptococcal disease cases from the proposed main trial (GBS3)
As indicated, cord blood will be required from a cohort of approximately 180,000 women. The feasibility study has indicated that recruitment to a study in which cord blood is to be obtained can be very high when a study midwife has the opportunity to approach a woman to obtain consent (74%, 95% CI 71% to 76%). However, as it was possible to approach only a minority of women during the study period because of the individual consent approach, the overall recruitment rate (22% of all births during the study period, 95% CI 21% to 23%) was much lower. Performing clinical trials for an intrapartum intervention has historically been challenging because of issues related to consent in an ‘emergency’ setting. Advances in experience and knowledge of delivering high-quality and high-impact research in labour have however recently emerged. A consent pathway developed by the GBS3 trial group32 now features in the RCOG guidance on intrapartum consent. 33 Recent examples of successful intrapartum emergency studies include deferred or immediate cord clamping in preterm birth,34 glyceryl trinitrate for retained placenta,35 tranexamic acid for treatment of post-partum haemorrhage,36 with further trials under way [e.g. the High Or Low Dose Syntocinon for delay in labour (HOLDS) trial ISRCTN 99841044 (Kenyon S, Taylor R, Hewston R, Johnston T, Hinshaw K, Middleton L, et al. Birmingham Women's NHS Foundation Trust, March 2016 to June 2019) and ANODE ISRCTN: 1116698437]. Feedback from the participating sites suggests that obtaining cord blood from women in labour using an opt-out strategy would be both realistic and acceptable. We propose testing this strategy more formally through patient groups and by seeking ethics committee advice.
To identify those infants in this cohort in GBS3 who develop iGBS we propose to undertake surveillance via Public Health England and specific paediatric clinical networks (BPAIIG and neonIN). We have successfully identified cases through these networks in the feasibility study and have been able to capture 15 cases of iGBS in a short period of time. This provides confidence in our approach. An additional advantage to extending the feasibility study as proposed above would be to continue to test the feasibility of national surveillance and to embed it as a routine, in preparation for the GBS3 study.
In addition, an extension would allow us to test the hypothesis that antibody concentration at the time of acute disease equates to antibody concentration from cord blood. We have already enrolled 27 babies born to healthy women into our antibody kinetics study (eight colonised with serotype III). However, to ensure that we understand the relationship between antibody in the acute disease phase and in a healthy infant exposed to GBS, we now need to undertake the kinetics study in cases of GBS disease up to 3 months of life. Collecting acute serum from 50 infants with serotype III disease will enable us to interpret antibody in health and disease up to 90 days of life. If infant antibody at the time of acute disease proves to be an accurate reflection of cord blood antibody for that infant, this would make a major difference to the design of the GBS3 trial as it would negate the need for the full 180,000 cord sera samples. This would mean that we would need blood from only 150 cases of iGBS serotype III disease rather than collecting cord blood from 180,000 women. We can resolve this during the extended feasibility study.
Acknowledgements
We extend our thanks to women and infants who participated in the study, as well as all the local research and clinical teams who helped with recruitment, sample and data collection and provided invaluable assistance throughout the study.
We would also like to thank the Steering Committee members for their advice and support: Dr Fiona Denison (The Queen’s Medical Research Institute), Mr Subhir Bedi (St George’s, University of London and St George’s University Hospitals NHS Foundation Trust), Ms Chloe Stables (Group B Strep Support), Mr Robert Pleass (Clinical Research Network South London) and Dr Farah Seedat (Public Health England).
Finally, we are grateful for the administrative assistance of the National Institute for Health Research (NIHR), the West Midlands – Solihull HRA (REC) and the HRA.
Contributions of authors
Dr Clara Carreras-Abad (https://orcid.org/0000-0002-9068-3233) was responsible for the day-to-day operation of the study, laboratory work, data analysis and drafting the final report.
Ms Madeleine Cochet (https://orcid.org/0000-0002-0436-4843) was responsible for the study management.
Mr Tom Hall (https://orcid.org/0000-0002-9001-6684) and Ms Laxmee Ramkhelawon (https://orcid.org/0000-0001-8038-8313) were responsible for the immunological analysis of serum samples at St George’s, University of London.
Dr Asma Khalil (https://orcid.org/0000-0003-2802-7670), Dr Elisabeth Peregrine (https://orcid.org/0000-0003-4504-2979), Dr Latha Vinayakarao (https://orcid.org/0000-0003-1830-7812), Dr Sharmila Sivarajan (https://orcid.org/0000-0001-8171-6358) and Dr Rosol Hamid (https://orcid.org/0000-0001-5716-9753) (co-applicants) were principal investigators at St George’s University Hospitals NHS Foundation Trust, Kingston Hospital NHS Foundation Trust, Poole Hospital NHS Foundation Trust, Surrey and Sussex Healthcare NHS Trust, and Croydon Health Services NHS Trust, respectively.
Dr Tim Planche (https://orcid.org/0000-0002-0263-0888) contributed to the microbiologic study design and was responsible for all samples analysed at St George’s University Hospitals NHS Foundation Trust.
Dr Elizabeth Sheridan (https://orcid.org/0000-0002-7804-3217) and Dr Stephen Winchester (https://orcid.org/0000-0003-3377-3256) were in charge of all samples analysed at Poole Hospital NHS Foundation Trust and Surrey and Sussex Healthcare NHS Trust, respectively.
Mrs Jane Plumb (https://orcid.org/0000-0002-0738-3695) gave invaluable support to the study design and running.
Dr Abdelmajid Djennad (https://orcid.org/0000-0002-4830-0331) and Dr Nick Andrews (https://orcid.org/0000-0003-2069-2684) were responsible for the statistical analysis.
Dr Kirsty Le Doare (https://orcid.org/0000-0002-5104-085X) and Professor Paul Heath (https://orcid.org/0000-0002-7540-7433) had overall responsibility for the study and were involved in all stages of the work: design of the study, data analysis and drafting and commenting on the final report.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review and agreement of the Steering Committee.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Heath PT, Culley FJ, Jones CE, Kampmann B, Le Doare K, Nunes MC, et al. Group B streptococcus and respiratory syncytial virus immunisation during pregnancy: a landscape analysis. Lancet Infect Dis 2017;17:e223-e234. https://doi.org/10.1016/S1473-3099(17)30232-3.
- Madrid L, Seale AC, Kohli-Lynch M, Edmond KM, Lawn JE, Heath PT, et al. Infant Group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65:S160-S172. https://doi.org/10.1093/cid/cix656.
- Russell NJ, Seale AC, O’Driscoll M, O’Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, et al. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65:S100-S111. https://doi.org/10.1093/cid/cix658.
- Russell NJ, Seale AC, O’Sullivan C, Le Doare K, Heath PT, Lawn JE, et al. Risk of early-onset neonatal Group B streptococcal disease with maternal colonization worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65:S152-S159. https://doi.org/10.1093/cid/cix655.
- Heath PT, Balfour GF, Tighe H, Verlander NQ, Lamagni TL, Efstratiou A. HPA GBS Working Group . Group B streptococcal disease in infants: a case control study. Arch Dis Child 2009;94:674-80. https://doi.org/10.1136/adc.2008.148874.
- Seale AC, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, et al. Estimates of the burden of Group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis 2017;65:S200-S219. https://doi.org/10.1093/cid/cix664.
- Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 2000;342:15-20. https://doi.org/10.1056/NEJM200001063420103.
- Le Doare K, O’Driscoll M, Turner K, Seedat F, Russell NJ, Seale AC, et al. Intrapartum antibiotic chemoprophylaxis policies for the prevention of group B streptococcal disease worldwide: systematic review. Clin Infect Dis 2017;65:S143-S151. https://doi.org/10.1093/cid/cix654.
- Bekker V, Bijlsma MW, van de Beek D, Kuijpers TW, van der Ende A. Incidence of invasive group B streptococcal disease and pathogen genotype distribution in newborn babies in the Netherlands over 25 years: a nationwide surveillance study. Lancet Infect Dis 2014;14:1083-9. https://doi.org/10.1016/S1473-3099(14)70919-3.
- O’Sullivan C, Lamagni T, Efstratiou A, . Group B Streptococcal (GBS) disease in UK and Irish infants younger than 90 days, 2014–15. Arch Dis Childhood 2016;101. https://doi.org/10.1136/archdischild-2016-310863.3.
- Hughes RG, Brocklehurst P, Steer PJ, Heath P, Stenson BM. on behalf of the Royal College of Obstetricians and Gynaecologists . Prevention of Early-onset neonatal Group B streptococcal disease: green-top Guideline No. 36. BJOG An Int J Obstet Gynaecol 2017;124:e280-305. https://doi.org/10.1111/1471-0528.14821.
- Verani JR, McGee L, Schrag SJ. Centers for Disease Control and Prevention (CDC) . Prevention of Perinatal Group B Streptococcal Disease – Revised Guidelines from CDC, 2010 2010;59:1-36.
- Verani JR, Spina NL, Lynfield R, Schaffner W, Harrison LH, Holst A, et al. Early-onset group B streptococcal disease in the United States: potential for further reduction. Obstet Gynecol 2014;123:828-37. https://doi.org/10.1097/AOG.0000000000000163.
- Okike IO, Johnson AP, Henderson KL, Blackburn RM, Muller-Pebody B, Ladhani SN, et al. Incidence, etiology, and outcome of bacterial meningitis in infants aged < 90 days in the United Kingdom and Republic of Ireland: prospective, enhanced, national population-based surveillance. Clin Infect Dis 2014;59:e150-7. https://doi.org/10.1093/cid/ciu514.
- Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, et al. Bacterial meningitis in the United States, 1998–2007. N Engl J Med 2011;364:2016-25. https://doi.org/10.1056/NEJMoa1005384.
- Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med 1976;294:753-6. https://doi.org/10.1056/NEJM197604012941404.
- Heath PT. Status of vaccine research and development of vaccines for GBS. Vaccine 2016;34:2876-9. https://doi.org/10.1016/j.vaccine.2015.12.072.
- Madhi SA, Dangor Z, Heath PT, Schrag S, Izu A, Sobanjo-Ter Meulen A, et al. Considerations for a phase-III trial to evaluate a group B Streptococcus polysaccharide-protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young-infants. Vaccine 2013;31:D52-7. https://doi.org/10.1016/j.vaccine.2013.02.029.
- Salisbury D. Introduction of a conjugate meningococcal type C vaccine programme in the UK. J Paediatr Child Health 2001;37:S34-6. https://doi.org/10.1046/j.1440-1754.2001.00738.x.
- Dangor Z, Kwatra G, Izu A, Lala SG, Madhi SA. Review on the association of Group B Streptococcus capsular antibody and protection against invasive disease in infants. Expert Rev Vaccines 2015;14:135-49. https://doi.org/10.1586/14760584.2014.953939.
- Lin FY, Philips JB, Azimi PH, Weisman LE, Clark P, Rhoads GG, et al. Level of maternal antibody required to protect neonates against early-onset disease caused by group B Streptococcus type Ia: a multicenter, seroepidemiology study. J Infect Dis 2001;184:1022-8. https://doi.org/10.1086/323350.
- Lin FY, Weisman LE, Azimi PH, Philips JB, Clark P, Regan J, et al. Level of maternal IgG anti-group B streptococcus type III antibody correlated with protection of neonates against early-onset disease caused by this pathogen. J Infect Dis 2004;190:928-34. https://doi.org/10.1086/422756.
- Le Doare K, Kampmann B, Vekemans J, Heath PT, Goldblatt D, Nahm MH, et al. Serocorrelates of protection against infant group B streptococcus disease. Lancet Infect Dis 2019;19:e162-e171. https://doi.org/10.1016/S1473-3099(18)30659-5.
- Heath PT, Balfour G, Weisner AM, Efstratiou A, Lamagni TL, Tighe H, et al. Group B streptococcal disease in UK and Irish infants younger than 90 days. Lancet 2004;363:292-4. https://doi.org/10.1016/S0140-6736(03)15389-5.
- Baker CJ, Edwards MS, Kasper DL. Role of antibody to native type III polysaccharide of group B Streptococcus in infant infection. Pediatrics 1981;68:544-9.
- GOV.UK . Screening Pregnant Women for GBS Not Recommended. n.d. www.gov.uk/government/news/screening-pregnant-women-for-gbs-not-recommended (accessed September 2019).
- Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet 2014;384:1521-8. https://doi.org/10.1016/S0140-6736(14)60686-3.
- Roberts JN, Gruber MF. Regulatory considerations in the clinical development of vaccines indicated for use during pregnancy. Vaccine 2015;33:966-72. https://doi.org/10.1016/j.vaccine.2014.12.068.
- Royal College of Obstetrics and Gynaecology . Group B Streptococcal Disease, Early-Onset (Green-Top Guideline No. 36). n.d. www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg36/ (accessed September 2019).
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
- World Health Organization . Surveillance Guidelines for Measles, Rubella and Congenital Rubella Syndrome in the WHO European Region 2012. www.ncbi.nlm.nih.gov/books/NBK143264/ (accessed 18 February 2019).
- Pushpa-Rajah A, Bradshaw L, Dorling J, Gyte G, Mitchell EJ, Thornton J, et al. Cord Pilot Trial Collaborative Group . Cord pilot trial – immediate versus deferred cord clamping for very preterm birth (before 32 weeks gestation): study protocol for a randomized controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-258.
- Kenyon S, Ewer A. Obtaining Valid Consent to Participate in Perinatal Research Where Consent is Time Critical. Clinical Governance Advice No. 6a. London: Royal College of Obstetricians and Gynaecologists; 2016.
- Duley L, Dorling J, Pushpa-Rajah A, Oddie SJ, Yoxall CW, Schoonakker B, et al. Randomised trial of cord clamping and initial stabilisation at very preterm birth. Arch Dis Child Fetal Neonatal Ed 2018;103:F6-14. https://doi.org/10.1136/archdischild-2016-312567.
- Denison FC, Norrie J, Lawton J, Norman JE, Scotland G, McPherson GC, et al. A pragmatic group sequential, placebo-controlled, randomised trial to determine the effectiveness of glyceryl trinitrate for retained placenta (GOT-IT): a study protocol. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-017134.
- WOMAN Trial Collaborators . Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 2017;389.
- Knight M, Chiocchia V, Partlett C, Rivero-Arias O, Hua X, Hinshaw K, et al. Prophylactic antibiotics in the prevention of infection after operative vaginal delivery (ANODE): a multicentre randomised controlled trial. Lancet 2019;393:2395-403. https://doi.org/10.1016/S0140-6736(19)30773-1.
Appendix 1 Feedback from sites, looking towards the ‘big study’
-
Retrospective consent has improved recruitment and collection of cord blood.
-
Having time to explain the study before consenting increases the enrolment rate.
-
Sending the information sheet prior to delivery reduces midwife face-to-face time.
-
Main refusal reasons: language barriers, taking part in research, using samples for future research, rectovaginal swabs and taking samples from babies.
-
An opt-out approach, where swabs and blood collection would be part of normal routine, would be well accepted and easier for recruitment.
-
Information of the study prior to delivery would be useful: letters, social media.
-
A patient information sheet translated to other languages would be helpful, especially Urdu.
-
REDCap, as a tool for case report form, has been well accepted and appreciated. It does not take long to fill it.
-
Data may be missing, especially data collected during labour, if research midwives are not present.
-
Collaboration of clinical midwives is essential.
-
It has been easier to recruit in labour wards in order to collect blood samples.
-
Maternal blood is easy to collect from women who need a cannula (caesarean sections, inductions).
-
Vaginorectal self-swab collection (with midwife support) helps to increase the consent rate.
-
It is essential to have a team involved on labour wards (cord blood team, midwives, obstetricians, nurses).
-
Having staff dedicated exclusively to cord blood collection would be helpful.
-
Telephone calls might be time-consuming and not so effective to detect LOD.
-
A national surveillance could be more effective to recruit iGBS cases following the logistics represented in the flow chart (Figure 7).
FIGURE 7.
The iGBS national surveillance. O&G, obstetrics and gynaecology.
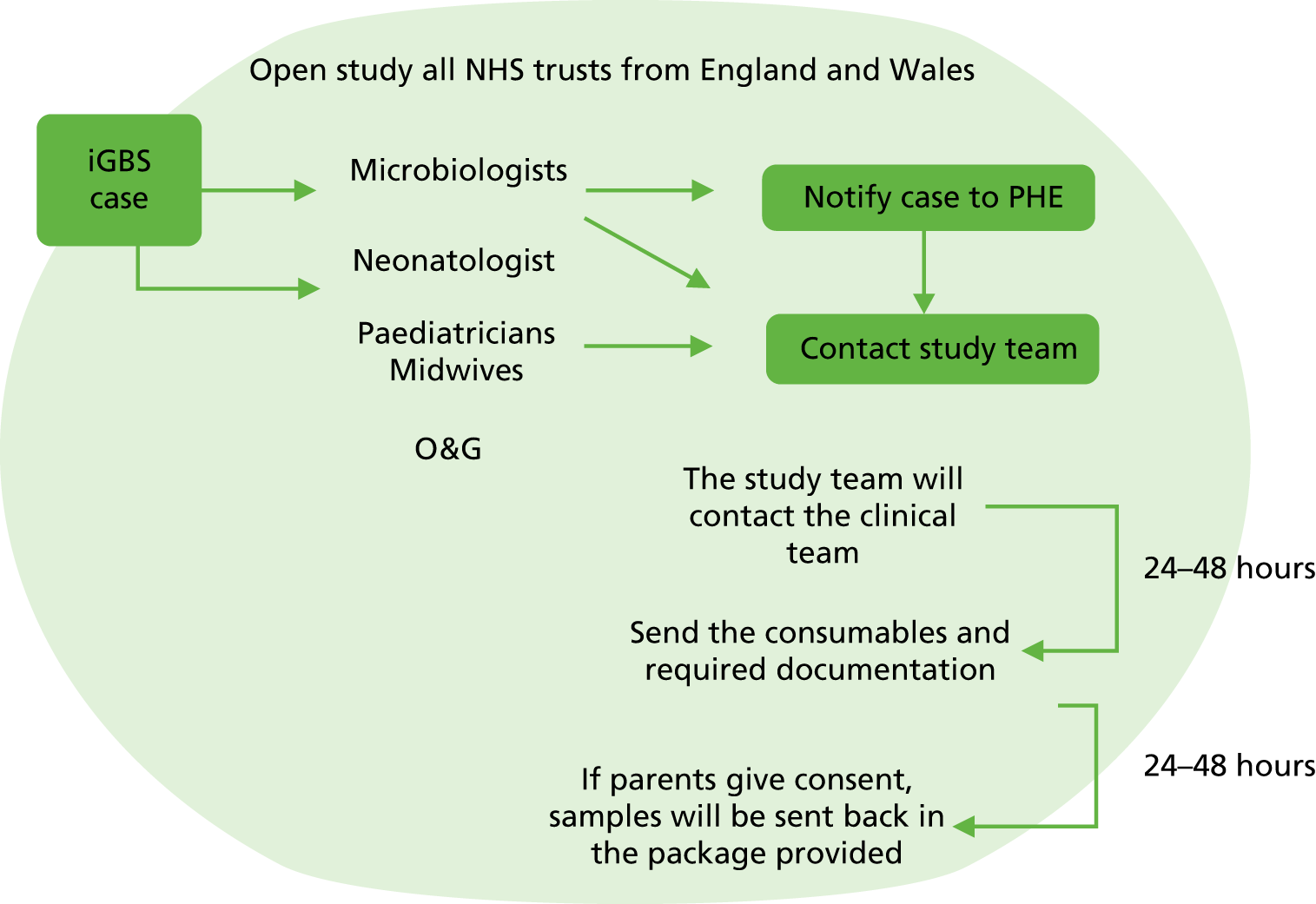
Appendix 2 Precision of correlates of risk reduction for serotype-specific group B streptococcus disease estimates according to different sample sizes
Based on previous GBS CoP studies,2–4 GBS serocorrelates that give risk reductions of 80–90% are feasible. A reduction of 80% in a case–control study implies an odds ratio of 0.2. The precision around 0.2 for varying case sample sizes and varying proportion of controls with IgG levels above the potential CoP levels (‘cuts’) is shown above with three controls per case. Thus, if 50% of controls have levels above the cut-off point, with 30 cases the 95% CI around an 80% reduction is 43% to 93%; with 90 cases it is 63% to 89% (bold values in table below).
| Controls > ‘cut’ | 95% CI | ||
|---|---|---|---|
| N = 30 cases and N = 90 controls | N = 90 cases and N = 270 controls | N = 150 cases and N = 450 controls | |
| 70% | 0.08 to 0.49 | 0.12 to 0.33 | 0.13 to 0.30 |
| 50% | 0.07 to 0.57 | 0.11 to 0.37 | 0.13 to 0.32 |
| 30% | 0.05 to 0.81 | 0.09 to 0.45 | 0.11 to 0.37 |
Glossary
- GBS3
- A multicentre prospective two-arm, parallel, cluster randomised controlled trial to test women for group B streptococcus colonisation in late pregnancy or labour compared with the current risk based strategy, with internal pilot and feasibility evaluation and parallel economic modelling for testing women.
List of abbreviations
- BPAIIG
- British Paediatric Allergy, Immunity and Infection Group
- CI
- confidence interval
- CoP
- correlate of protection
- CPS
- capsular polysaccharide
- ECM
- enriched culture medium
- EOD
- early-onset disease
- GBS
- group B streptococcus
- HRA
- Health Research Authority
- IAP
- intrapartum antibiotic prophylaxis
- iGBS
- invasive group B streptococcal disease
- IgG
- immunoglobulin G
- LAT
- latex agglutination test
- LOD
- late-onset disease
- neonIN
- Neonatal Infection Network
- NIHR
- National Institute for Health Research
- PCR
- polymerase chain reaction
- RCOG
- Royal College of Obstetrics and Gynaecology
- REC
- Research Ethics Committee
- REDCap
- Research Electronic Data Capture
