Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/190/05. The contractual start date was in September 2014. The draft report began editorial review in September 2018 and was accepted for publication in February 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Alan A Montgomery reports grants from the National Institute for Health Research (NIHR) and membership of the NIHR Health Technology Assessment (HTA) Clinical Evaluation and Trials Funding Board during the conduct of the study. Roshan das Nair reports membership of the NIHR Health Services and Delivery Research Board, the HTA End of Life Care and Add-on Studies Board and the NIHR Research for Patient Benefit (East Midlands), and personal fees from Biogen Inc. (Cambridge, MA, USA). Avril ER Drummond reports membership of the NIHR Clinical Lectureships panel. Cris S Constantinescu reports grants, personal fees and other from Bayer AG (Leverkusen, Germany); Biogen Inc.; Merck, Sharp & Dohme (Kenilworth, NJ, USA); Novartis International AG (Basel, Switzerland), Sanofi Genzyme (Cambridge, MA, USA) and Teva Pharmaceuticals Industries Ltd (Petah Tikva, Israel). He also reports grants and personal fees from GW Pharmaceuticals (Cambridge, UK), Morphosys (Planegg, Germany), Roche (Basel, Switzerland); and grants from Sanofi-Pasteur-MSD (Lyon, France), outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Lincoln et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Multiple sclerosis (MS) is a progressive neurological condition that affects ≈127,000 people in the UK. 1 It is an incurable, progressive disease usually diagnosed in young adulthood or early middle age, and is a leading cause of neurological disability in young adults. 2 Cognitive problems are common in people with MS, affecting about 70% of people with MS, and include impairments of attention, information processing, executive function and memory. 3–6 Impairment of memory and impairment of information processing speed are the most common cognitive deficits in people with MS. 7–10
Cognitive impairment has a detrimental effect on daily life activities and quality of life. People with MS with cognitive problems are less independent in activities of daily living than those with MS without cognitive problems. 11–14 In particular, cognitive problems have a detrimental effect on employment. 15–18 There is also some evidence that they also negatively affect overall quality of life. 16,19–21
People with cognitive problems may be offered cognitive rehabilitation to retrain cognitive skills or to improve their ability to cope with cognitive problems in daily life. Cognitive rehabilitation is a structured set of therapeutic activities designed to improve cognitive function and to reduce the impact of cognitive impairment on daily life. There are recommendations for the provision of rehabilitation for specific symptoms for people with MS in The National Service Framework for Long-term Conditions. 22 The National Institute for Health and Care Excellence (NICE) clinical guidelines for the management of adults with MS23 mention the need to refer people with MS and persisting memory or cognitive problems to both an occupational therapist and a neuropsychologist to assess and manage these symptoms. They also identify the need for research on the clinical effectiveness and cost-effectiveness of cognitive rehabilitation for people with MS. 23
In clinical practice in the UK, people with MS may receive an assessment of their cognitive abilities and general advice on strategies to cope with their cognitive problems. In addition, information is available on the web pages of MS charities, such as the MS Society and MS Trust. Very few people with MS receive a systematic retraining of cognitive skills or structured training in strategies to cope with their cognitive difficulties. A recent survey of professionals and clinical staff in the UK24 found that ≈58% of those surveyed used some cognitive assessment to screen for cognitive problems and ≈49% of those surveyed provided some form of cognitive rehabilitation when problems were identified, but only about 3% used a manualised rehabilitation approach.
Research evidence
An early systematic review by O’Brien et al. 25 considered a range of evidence from randomised trials, non-randomised trials and case series studies. The authors found low-level evidence for positive effects of neuropsychological rehabilitation in people with MS, but suggested that more high-quality randomised trials were needed. More recent reviews (in the years 2013–18)3,10,26–28 have reached similar conclusions. Amato et al. 3 considered a broad range of evidence for cognitive rehabilitation, including non-randomised trials, and concluded that some interventions showed promise, but that further evaluations were needed. D’Amico et al. 27 highlighted that targeted training in specific cognitive domains improved the trained function in studies with people with mixed types of MS and in people with relapsing–remitting MS. Mitolo et al. 28 reviewed 33 studies and considered controlled clinical trials and before-and-after studies, in addition to randomised controlled trials (RCTs). They highlighted that recent studies, which have focused on cognitive domains other than memory (i.e. attention, speed of processing and executive abilities), seem to provide the best evidence for beneficial effects of intervention. Goverover et al. 26 identified 16 RCTs published between 2007 and 2016, but did not include a statistical analysis of results.
Systematic reviews restricted to RCTs of cognitive rehabilitation have generally concluded that, overall, there is insufficient evidence to support the effectiveness of cognitive rehabilitation for people with multiple sclerosis. 29–31 Both Cochrane reviews29,31 reported that there was some evidence of benefit on measures of cognitive function. Das Nair et al. 29 also found some evidence of benefit on measures of quality of life. However, neither review29,31 found any evidence of benefit on emotional outcomes. These conclusions were not supported by a more selective review that was confined to RCTs with a low risk of bias,30 but this review included only five studies. Hämäläinen and Rosti-Otajärvi10 also suggested that the weak evidence emerging from the Cochrane reviews29,31 may stem from the strict analysis methods used, and that such methods may not be best suited for the evaluation of rehabilitation studies. They advocate more consideration for a more qualitative analysis of best evidence.
Research has also examined the evidence for effectiveness according to the type of intervention. Some controlled trials32–43 have demonstrated the effectiveness of computerised cognitive rehabilitation to retrain cognitive skills in people with MS and some have also shown changes in brain activation on imaging outcomes. 44–48 However, these studies have rarely included any long-term follow-up to assess whether or not the observed benefits persist. A few studies have evaluated whether or not the effects of computerised cognitive rehabilitation continue for up to 1 year. Some have shown persisting effects on cognitive abilities and quality of life41,49,50 but some have not. 32,45 In addition, some non-computerised strategies have been used to retrain cognitive skills, such as the Story Memory Technique,51,52 self-generated learning53 and paper-and-pencil exercises. 54 These studies have demonstrated the short-term effects of retraining cognitive skills and provide limited evidence that the benefits persist. However, it has been questioned whether or not interventions that involve retraining of cognitive skills produce clinically meaningful effects. 55
An alternative approach in cognitive rehabilitation is to teach people skills to cope with the cognitive impairment and provide aids to enable them to compensate for the loss of cognitive abilities. These skills may be taught early in cognitive decline to prevent the functional consequences of cognitive decline affecting daily life, or later on to enable people to cope better with their cognitive impairments.
One study evaluated using an electronic memory aid as a compensatory strategy for people with MS. 56 It found that participants receiving reminder text messages encountered fewer problems in daily life as recorded in a daily diary and were less distressed than those receiving non-specific text messages. Some studies have combined this compensatory approach with either computerised cognitive training57,58 or non-computerised practice on cognitive tasks. 59,60
The studies that combined computerised cognitive rehabilitation with strategy training showed a few benefits on cognitive outcomes but also reported differences in the number of perceived cognitive deficits58 and in the use of memory strategies. 57 However, neither study57,58 found any evidence of effects on mood or quality of life.
The two trials59,60 that combined non-computerised activities with compensatory strategy training both used a similar cognitive rehabilitation programme. The Rehabilitation of Memory in Neurological Disabilities (ReMiND) trial59 was conducted with people with a range of neurological disabilities, including many who had MS. This trial evaluated the effectiveness of group memory rehabilitation programmes in participants with memory problems by comparing compensation strategy training, restitution strategy training and a self-help control. Both quantitative and qualitative data from the study indicated that the interventions were worthy of further evaluation. 59,61,62 The ReMIND-MS trial60 was a modified version of the group intervention, combining restitution and compensation strategies, compared with a control group receiving usual care (total participants, n = 48); all participants had MS. The results showed a significant effect on mood, favouring the intervention.
Thus, overall, despite the suggestion that compensatory strategy training may be the most appropriate intervention for people who have not benefited from retraining,63 the evidence to support the provision of such an intervention is weak. Therefore, the present trial was designed to assess the clinical effectiveness and cost-effectiveness of a group cognitive rehabilitation programme, which combined training in strategies to restore cognitive function with compensatory strategies for people with MS.
Rationale
Currently, people with MS with attention or memory problems do not routinely receive cognitive rehabilitation. This is in part because of the current lack of evidence for the clinical effectiveness and cost-effectiveness of cognitive rehabilitation, and also because of resource limitations.
Research question
What is the clinical effectiveness and cost-effectiveness of cognitive rehabilitation for people with MS with attention and memory problems?
Objectives
Primary objective
The primary objective was to determine whether or not attending a cognitive rehabilitation programme (the intervention) in addition to usual care was associated with reduced psychological impact of MS on quality of life, as measured on the Multiple Sclerosis Impact Scale – Psychological Subscale64 (MSIS-Psy) compared with usual care alone (control).
Secondary objectives
The secondary objectives were to assess cost-effectiveness of the intervention, and whether or not the intervention was associated with improvements in participants’ attention and memory abilities or in self-reported memory problems in daily life, mood, fatigue, employment status and carer strain.
Chapter 2 Methods
Parts of this chapter have been reproduced from the Cognitive Rehabilitation for Attention and Memory in people with Multiple Sclerosis (CRAMMS) trial protocol. 65 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Design
This was a multicentre, parallel-group, pragmatic RCT of a cognitive rehabilitation programme, provided in addition to usual care and compared with usual care alone.
An economic analysis was conducted to determine the costs and cost-effectiveness of cognitive rehabilitation compared with usual care (see Chapter 6). Treatment fidelity was assessed (see Chapter 4) and a qualitative study was conducted to explore participants’ experiences of the cognitive rehabilitation and usual care (see Chapter 5).
Trial setting and participants
Sites
The trial was conducted in five sites in England (see Appendix 1, Table 25). Each site was an NHS trust providing neurology services for people with MS.
Three sites were opened to recruitment between March 2015 and May 2015. A fourth site opened in February 2016 and a fifth site in September 2016. All sites were open to recruitment until March 2017.
Identification of participants
Participants were identified through NHS hospitals and participant identification centres (PICs) near to sites. An invitation pack – which included a letter of invitation, a participant information sheet, a consent form, a contact details slip and a prepaid reply envelope – was sent to individuals with MS on hospital neurology and neuropsychology databases. Those who were interested in taking part were asked to return the contact slip to the assistant psychologist (AP) at their nearest site. In addition, people with MS attending neurology and rehabilitation clinics were invited to take part by members of the clinical teams. The clinician asked for permission to pass on their contact details to the AP at the site, who then sent them the invitation pack.
Posters about the CRAMMS trial, giving contact details of the AP at the nearest site, were displayed in MS therapy centres and at MS Society branch offices in areas covered by the recruiting sites. There was information about the trial on the MS Society and MS Trust websites. Members of the research team also attended MS Society branch meetings to talk about the study and invite those who were interested to take part. Self-referral was possible for those who accessed public-facing information on the study website, newsletters and posters. Those who contacted the sites were then sent the information pack.
An invitation to complete the Multiple Sclerosis Neuropsychological Screening Questionnaire (MSNQ)66 was sent to those who were enrolled on the UK MS Register who were in the study age range and had a registered postcode in the areas covered by the sites. The replies were reviewed anonymously and those who scored > 27 on the MSNQ were sent an invitation pack through the MS Register.
Informed consent
Written informed consent was obtained by the AP, and participants were given a copy for their records. Participant information sheets and consent forms were based on those developed for the pilot study and had been checked for clarity and readability by a service user representative. Potential participants had the opportunity to read and discuss the study with other clinical staff, family and friends, and the research team before they decided to take part. They were given at least 24 hours to do this.
Inclusion and exclusion criteria
The inclusion and exclusion criteria were based on those used in the pilot studies. 59,60
Inclusion criteria
People with MS were eligible for the trial if they met the following inclusion criteria:
-
Aged 18–69 years. The upper age limit was to ensure that memory problems were likely to be because of MS rather than age-related memory impairments.
-
Reported having relapsing–remitting or progressive MS.
-
Diagnosed with MS at least 3 months prior to the screening assessment.
-
Reported having cognitive problems as determined by a cut-off score of > 27 on the patient version of the MSNQ. 66 The cut-off score was based on the original validation study by Benedict et al. 66 with 50 participants with MS. The cut-off score was used to identify those with cognitive impairment on a neuropsychological test battery.
-
Had cognitive deficits, defined as performance more than one standard deviation (SD) below the mean of healthy control participants corrected for age and education67 on at least one of the Brief Repeatable Battery of Neuropsychological Tests (BRBN). 68
-
Able to travel to one of the centres to attend group sessions. Participants had to live in the geographical area covered by the sites and be able to get to the sites independently. Travel expenses were offered to those who needed them.
-
Willing to receive treatment in a group if allocated to intervention.
-
Able to speak English sufficiently to complete the cognitive assessments and take part in group sessions.
-
Gave informed consent.
Exclusion criteria
Potential participants were excluded if they:
-
had vision or hearing problems, such that they were unable to complete the cognitive assessments
-
had concurrent severe medical or psychiatric conditions that prevented them from engaging in treatment
-
were involved in other psychological intervention trials.
Initial screening assessment
The AP conducted an initial telephone screening with those who expressed an interest in taking part in the trial. The screening included confirmation of the diagnosis of MS and a participant’s age. The MSNQ was administered to those who were willing to complete the questionnaire over the telephone. If it was not administered over the telephone, it was administered at the screening visit. For those recruited through the UK MS Register, the MSNQ was included in the online form to identify potential participants, but repeated at the screening visit, as scores were not available for specific individuals from the online version. The scale comprises 15 questions about the frequency of cognitive failures in daily life. Each question is rated on a four-point scale from ‘never’ to ‘very often’. Scores range from 0 to 60 and higher scores indicate more cognitive complaints. There is some evidence to support the reliability and validity of the scale. 57,69–72
Initial screening visit
At the screening visit, the AP explained the trial and obtained written consent.
Assessments were completed in the participant’s home, unless they preferred to be seen elsewhere. A quiet room was used whenever possible and an attempt was made to keep distractions to a minimum. The AP explained that the initial assessments were required to check that the participant met the inclusion criteria, and to obtain demographic and clinical data.
Demographic information included gender, date of birth, ethnicity, marital status, living arrangements and years of education. Clinical information included type of MS and number of relapses in the previous 6 months.
All assessment measures were selected on the basis of their clinical utility, relevant psychometric properties and ease of use for participants. Furthermore, the measures reflect the three levels of the International Classification of Function domains: impairment, activity limitations and participation restrictions. 73
The following assessments were completed:
-
The BRBN. 68 This is a cognitive screening battery that mainly assesses attention and memory and takes motor problems into consideration. It consists of the Selective Reminding, 10/36 Spatial Recall, Symbol Digit Modalities, Paced Auditory Serial Addition and Controlled Oral Word Association tests. The BRBN is a brief cognitive screening measure for people with MS, which is sensitive to detect cognitive impairment more so than other similar batteries and has been widely used in trials of medical interventions with people with MS. Higher scores indicate better cognitive function.
-
Guy’s Neurological Disability Scale (GNDS). 74 The self-report postal version of this measure75 was used to document the symptoms of MS. It comprises questions on 11 domains, including cognition and mood; the optional sexual function domain was excluded. Higher scores indicate greater disability. There is evidence to support its reliability and validity,76,77 responsiveness to change78 and to support the use of a total score. 79 It was chosen in preference to the more commonly used Expanded Disability Status Scale (EDSS)80 because it covers a wider range of activities and is less focused on mobility. The EDSS has also been criticised for being insensitive to cognitive dysfunction. 81 The GNDS has been compared directly with the EDSS and demonstrated good validity. 75,82
The results from the MSNQ and BRBN were used to assess the inclusion criteria. Participants who met the inclusion criteria were given the following questionnaires to complete in their own time, to be collected at the baseline assessment visit:
-
Multiple Sclerosis Impact Scale (MSIS). 64 This is a MS-specific quality-of-life scale that encompasses both the physical and psychological effects of MS on everyday life. It comprises 29 questions: 20 on the physical subscale and 9 on the psychological subscale. Each item is rated in four categories: ‘not at all’, ‘a little’, ‘moderately’ and ‘extremely’. Scores on the physical impact scale range from 20 to 80 and scores on the psychological impact scale range from 9 to 36, with higher scores indicating greater negative impact of MS. The scale has good psychometric properties83–88 and has been used as an outcome measure in rehabilitation trials. 89–92 The psychological and physical subscales of version two93 were both used.
-
Everyday Memory Questionnaire – patient version (EMQ-p). 94 This assesses the frequency of participants’ everyday attention and memory problems in daily life. It has good ecological and face validity and has been previously used as an outcome measure in cognitive rehabilitation studies. 59,60,95 The EMQ-p consists of 28 items asking about the frequency of memory failures in everyday life over the previous month. Each item is rated on a five-point Likert scale (from ‘once or less in the last month/never’ to ‘once or more a day’). Total scores range from 0 to 112, with higher scores indicating more frequent memory problems.
-
Fatigue Severity Scale (FSS). 96 The five-item, Rasch-analysed version of the FSS97 was used to document the severity of fatigue. This assesses patient-perceived fatigue over the previous week. Each item was scored from 0 to 7, with higher scores indicating more severe fatigue. The sum of the five items was then converted to the Rasch person location using the table provided by Mills et al. ,97 and ranged from –3.4 to 3.4. Several studies have supported the reliability and validity of the FSS in people with MS. 98,99
-
The 30-Item General Health Questionnaire (GHQ-30). 100 This questionnaire was designed to detect psychological distress in the general population. It assesses a participant’s mood over the previous few weeks compared with their usual mood. The GHQ-30 was chosen as it is suitable for postal administration and is easy to complete. The GHQ (12-, 28- or 30-item versions) has also been shown to be responsive to the effects of psychological interventions in people with neurological conditions. 60,89,101 Likert scoring was used for the clinical outcome, with scores ranging from 0 to 90; higher scores indicate more psychological distress.
Participants who did not meet the eligibility criteria following the initial screening visit were notified by letter to thank them for their interest in the trial; a brief report of their test results was also provided, if requested. Those who met the inclusion criteria were invited to complete the baseline assessment visit.
Participants were asked to nominate a relative or friend who knew about their cognitive problems in daily life. A questionnaire booklet for the relative or friend was sent to participants following the initial screening visit and they were asked to pass this on to their friend or relative. The questionnaire booklet consisted of the Everyday Memory Questionnaire – relative version (EMQ-r). The EMQ-r is identical to the patient version, but is completed by a relative or friend who knows the participant and their cognitive problems. This version tends to correlate more highly with the presence of ‘objectively’ identified cognitive problems than participant self-report, which can be influenced by mood102 or fatigue. 103 The EMQ-r was included to identify any effect of treatment on daily life problems as observed by another person, which might not have been detected by participants themselves. EMQ-r scores range from 0 to 112, with higher scores indicating more frequent memory problems. Participants were asked to return completed relative/friend questionnaires at the baseline assessment visit or they could be returned by post.
Baseline assessment visit
The participant questionnaires that had been completed were collected at the baseline visit, which was conducted within 2 weeks of the screening visit. If they had not been completed, they were administered at the visit if time allowed, or the participant was asked to complete them after the visit and return them by post.
In addition, the following assessments were completed:
-
Doors and People. 104 This is a measure of memory function in four domains: verbal, visual, immediate and delayed. This was used to determine the type of memory problems and was used when planning treatment sessions to ensure that the treatment was adjusted according to the nature of each participant’s memory problems. Higher scores indicate better memory ability.
-
Trail Making Test from the Delis–Kaplan Executive Function System. 105 This was administered as a measure of attention and executive abilities. Conditions two (letter sequencing) and four (letter–number sequencing) were used. The difference in the time taken between the two conditions was used. A smaller difference indicates less interference effect, which indicates better executive functioning.
-
EuroQol-5 Dimensions, five-level version (EQ-5D-5L). 106 This is a generic health-related quality-of-life measure, used for health economic evaluations. Utility scores were derived from the EQ-5D-5L descriptive system and used to estimate quality-adjusted life-years (QALYs) and incremental cost-effectiveness ratios (ICERs).
-
Use of Health and Social Services Questionnaire (UHSSQ). This bespoke self-report questionnaire was used to assess NHS health-care use. It included questions on the frequency with which participants accessed NHS services, such as visits to general practitioners (GPs), neurologists and MS nurses, and services provided by charities, such as MS Society groups. Use of hospital services, including outpatient appointments, rehabilitation services and hospitalisation, were recorded. Current medication and medications prescribed over the previous 3 months were recorded. The period covered by the questionnaire was the previous 3 months; this time period was chosen so that people would have received a range of services, and they would still remember what they had received. The UHSSQ was adapted from previous studies. 107
Current employment status and the number of relapses in the previous 3 months were also documented.
If a participant was randomised to receive cognitive rehabilitation, the AP checked whether or not that participant could attend groups on certain days. Participants were randomised only if they were able to attend on the days that groups were due to take place. Those who were unable to attend on the planned days were held in reserve until such time that a new group, matching their availability, was formed. While participants were waiting for sufficient participants to form a group, the AP remained in regular contact to keep individuals aware of probable timescales.
The AP also checked whether participants would prefer to receive the outcome questionnaires by post or to complete them online.
Randomisation
Clusters of between 9 and 11 participants were formed by the AP at each site; in case they would be allocated to the cognitive rehabilitation group, cluster formation was based on participants’ availability to attend for treatment at the same time and same venue. Randomisation took place once there were 9–11 individuals in a cluster.
Participants were individually randomised to the intervention or control group on a 6 : 5 ratio, to allow for clustering in the intervention group. Allocation was stratified by recruitment site, and minimised by MS type (relapsing–remitting or progressive) and gender. Those who were allocated to the intervention group were offered cognitive rehabilitation. Those who were allocated to the control condition were not offered cognitive rehabilitation and received only their usual care.
The allocation algorithm was created by the Nottingham Clinical Trials Unit (NCTU), in accordance with their standard operating procedure, and held on a secure server. A remote, internet-based randomisation system was used by APs to obtain the group allocation for each participant. Access to the sequence was confined to the NCTU information technology (IT) manager. The sequence of group allocations was concealed from the trial statistician until all participants had been allocated, and recruitment, data collection and all other trial-related assessments were complete.
Participants who were waiting to be randomised at the time their site closed to recruitment were sent a letter informing them that the AP had not been able to recruit enough people to create a group at a time and place that was convenient for that participant, and that their participation in the trial was at an end.
Interventions
Usual care
All participants received their usual clinical care. In the standard NHS care pathway, people with MS with cognitive problems may get general advice from MS specialist nurses and occupational therapists on how to manage any cognitive difficulties. There are information sheets available on web pages of MS charities, which include suggestions for coping with cognitive problems. However, usual care does not normally include any specific intervention for cognitive problems or cognitive rehabilitation.
Other clinical services available to participants included employment rehabilitation services, self-help groups and support from specialist charities, such as the MS Society. Any input, including psychological or medical interventions, that participants received during the trial was recorded on the UHSSQ.
Cognitive rehabilitation
Cognitive rehabilitation is a structured set of therapeutic activities designed to improve cognitive function and to reduce the impact of cognitive impairment on daily life. 65 The emphasis of the intervention was on identifying the most appropriate strategies to help individuals overcome their cognitive problems and in providing participants with a range of techniques that they could use and adapt in accordance with their own needs. Although the intervention was delivered in a group format, every effort was made to ‘personalise’ or ‘individualise’ the intervention to meet the needs of each participant. For instance, if a participant found it particularly difficult and stressful to remember faces, the strategies taught were focused on this problem.
Each cognitive rehabilitation group consisted of four to six participants. Sessions were held at NHS sites or community venues. Participants received 10 sessions, each of 1.5 hours’ duration, which included a break mid-way through. Sessions were held once a week for 10 weeks. The content of each session was defined in a treatment manual. 60 The intervention included restitution strategies to retrain attention and memory functions., and methods to improve encoding and retrieval. In addition, participants were taught compensation strategies, such as internal mnemonics (e.g. chunking, first letter cues and rhymes), the use of external memory aids (e.g. diaries, notebooks and mobile phones) and methods of coping with attention and memory problems in daily life. The programme was adjusted according to each participant’s cognitive abilities, as determined during the baseline assessment, yet it also provided a systematic framework for addressing problems with attention and memory. Homework assignments were set at the end of each session to help participants practise the strategies learnt in their everyday life. These were reviewed each week at the following session. Carers and family members were invited to attend the final session, if participants agreed, and this session was used to summarise the previous sessions.
The AP recorded whether or not participants attended each of the intervention sessions and the reasons why any sessions were not attended. Participants who missed sessions were offered a half-hour catch-up session prior to the next session. If a participant was no longer able to attend their allocated group for the remaining number of treatment sessions, they could attend another group, provided the sessions were completed by the 6-month outcome assessment and the same AP was delivering the sessions. Participants’ travel expenses were reimbursed.
Treatment fidelity
The fidelity of the cognitive rehabilitation programme was assured in two ways:
-
Manualised treatment – the cognitive rehabilitation programme followed a manual that was developed and tested in a pilot study. 60 A detailed description of the manual has been published elsewhere108 and in the Template for Intervention Description and Replication (TIDieR)109 checklist (see Appendix 2, Table 26).
-
Training and supervision – staff delivering the intervention (i.e. APs) were psychology graduates with clinical experience. They received supervision from a clinical psychologist based at their site. A clinical psychologist provided training on the delivery of the intervention. Monthly teleconferences were conducted to provide peer group supervision. In addition, individual telephone supervision was provided monthly with a clinical psychologist for the discussion of specific challenges relating to treatment or assessment. When staff changes occurred, former staff completed a ‘handover’ document for new staff, to ensure continuity. The new staff were trained by the same trainers to ensure consistency.
Formal assessment of the fidelity of the cognitive rehabilitation was undertaken through analysis of video-recordings of treatment sessions. This is described in detail in Chapter 4.
Outcome assessment
Outcomes were assessed at 6 and 12 months after randomisation to determine the immediate and longer-term effects of the intervention. The primary outcome was 12 months after randomisation, in order to determine whether or not any treatment gains had been maintained over time. The 6-month assessment was to determine the immediate effects of the intervention, allowing time for completing 10 sessions, including allowing for sessions to be rescheduled if they had to be cancelled through illness or holidays.
Outcomes were assessed by questionnaire and at an outcome assessment visit.
Questionnaire outcomes
A questionnaire pack was posted to each participant 2 weeks before the 6- and 12-month appointments were due. They were asked to complete this questionnaire pack at home and hand it to the research assistant (RA) during the follow-up visit. Those who requested to complete questionnaires online were sent an e-mail with a link to open the questionnaires. Prior to the follow-up visit, the RAs checked if the questionnaires had been returned or competed online.
The pack included the following:
-
the MSIS
-
the EMQ-p
-
the GHQ-30
-
the FSS
-
GNDS
-
a question about the number of relapses in the previous 6 months.
In addition, the EMQ-r and Modified Carer Strain Index (MCSI) were posted for completion by the participant’s nominated relative/friend.
The MCSI110 is a measure of burden on carers and family members. It comprises 13 items from the Carer Strain Index111 that, in the modified version, are rated according to the frequency of their occurrence as ‘yes, on a regular basis’ (score 2), ‘yes, sometimes’ (score 1) and ‘no’ (score 0). Scores range from 0 to 26; the higher the score, the higher the level of caregiver strain. The internal reliability and test–retest reliability are high. 110
Returned questionnaires were checked for completeness at the visit so that if items were missing or clarification was needed about responses (e.g. unclear marking on questionnaires), these could be corrected. The RA also took a copy of the questionnaires to the visit so that these could be completed during the visit if they had not already been completed. If there was not sufficient time to complete the questionnaires during the visit, and they had not been done previously, the RA left a copy of the questionnaires and a prepaid envelope so that these could be completed and returned to the co-ordinating centre after the visit.
Outcome assessment visits
Research assistants were responsible for conducting outcome visits 6 and 12 months after randomisation. The RAs were not involved in recruitment to the trial of or delivery of the intervention to any of the participants that they followed up, and were blind to treatment allocation. At the start of the appointment, the RA reminded the participants of the importance of them remaining blind to group allocation and asked participants not to discuss any aspects of their involvement in the trial. Before conducting the outcome visit assessments, the RA recorded whether or not they had been unblinded and their opinion as to which group the participant had been allocated to, using the categories definitely usual care, probably usual care, probably intervention or definitely intervention. At the end of the visit, the RA recorded whether or not they had been unblinded during the visit and also what treatment they thought the participant had received.
The following assessments were completed at the follow-up visits:
-
the EQ-5D-5L
-
the UHSSQ
-
the BRBN
-
Doors and People
-
Trail Making.
The schedule of outcome assessments is summarised in Appendix 3, Table 27.
All participants were contacted in order to complete all assessments. Participants were initially contacted by telephone when follow-up visits were due. If telephone contact failed, then a letter was sent to the participant’s last known address, asking the participant to contact the outcome assessor. Any changes in contact details were recorded.
Feedback interviews
A feedback interview was conducted between the 6- and 12-month appointments, with 36 purposefully selected and willing participants: this qualitative component of the trial is described in detail in Chapter 5.
End of the study
Participants completed the trial when the 12-month follow-up was completed. The end of the trial was defined as being the last 12-month follow-up appointment. However, questionnaires returned after completion of the final visit were accepted to allow for any delays in the post.
If participants discontinued the intervention or withdrew from follow-up, this was reported and the reasons were documented, if they were given. Outcome data collection was completed if a participant discontinued treatment but agreed to remain in the trial. Participants were informed at the start of the trial that data collected up to the point of withdrawal would be retained and used in the final analysis. Participants who withdrew from the trial were not replaced with other participants.
Assessment of safety and adverse events
The risks of taking part in the trial were assessed as being low. However, non-specific risks for participants involved travelling to the research sites. In addition, participants may have experienced some distress if they found that they were not performing as well as they expected on cognitive assessments. However, such distress was considered improbable and likely to be mild. Any distress was managed by the APs, who were trained to deal with such situations. They discussed any concerns with the supervising clinical psychologists and made referrals to a participant’s GP, if needed. Distress during the course of the intervention was managed similarly. As the risk, overall, was assessed as being low, no adverse events or serious adverse events were reported. However, as a safety outcome, the number of participants who showed an increase in scores on the GHQ-30 of > 30 points between baseline and the 6-month assessment were monitored by the independent Data Monitoring Committee (DMC), and any concerns were discussed with the clinical teams. In addition, adverse outcomes, such as hospitalisation, distress and death, were recorded.
‘Notable events’ occurring during assessments or the intervention were recorded by the APs or RAs throughout the trial. Notable events were any events that were considered to be out of the ordinary, such as problems arising during cognitive rehabilitation sessions or any events that may pose a risk to either the participants or the researchers. These were reviewed during supervision.
Research governance
The trial was conducted in accordance with the recommendations for clinicians involved in research on human subjects adopted by the 18th World Medical Association General Assembly, Helsinki, 1964,112 and later revisions, the UK Policy Framework for Health and Social Care Research113 and the principles of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice (ICH-GCP) guidelines. 114
Trial registration
The trial was prospectively registered as ISRCTN09697576 (International Standard Randomised Controlled Trial Number) on 14 August 2014.
Ethics
The National Research Ethics Service West Midlands, South Birmingham Committee gave ethics approval for the study (reference number 14/WM/1083).
Site initiation and training
Prior to the commencement of the trial, a meeting was held between members of the central research team (i.e. the chief investigator, the co-chief investigator and NCTU staff) and trial collaborators from the initial three sites to discuss implementation and training issues to ensure that all members were familiar with all aspects of the trial. For sites that were involved after this initial meeting, training was carried out on an individual basis. NCTU staff provided trial-specific training on the trial documentation and database, a clinical neuropsychologist provided training on conducting baseline assessments and, as part of ensuring treatment fidelity, a clinical psychologist provided training on the delivery of the intervention (as detailed in Treatment fidelity).
Protocol deviations
A protocol deviation was defined as an unanticipated or unintentional divergence or departure from the expected conduct of the trial that was inconsistent with the protocol, consent document or other trial procedures This was based on standard practice within NCTU.
The APs and RAs recorded protocol deviations on the electronic case report form. Protocol violations were reviewed by the Trial Management Group (TMG) and defined as those deviations that affected participant eligibility or outcome measures.
Oversight
A number of oversight groups monitored the progress and conduct of the trial. The roles and responsibilities of these groups were described in the protocol,65 and specific charters were developed for the independent Trial Steering Committee (TSC) and DMC.
Trial Management Group
The TMG comprised the co-chief investigators, members of NCTU responsible for the running of the trial and the health economists. They met regularly to monitor the day-to-day running of the trial.
Trial Steering Committee
The TSC was responsible for the overall conduct of the trial. The TSC had an independent chairperson and four independent members. The independent members were rehabilitation professionals and service user representatives who were not otherwise involved in the trial. Members of the trial team, including the chief investigator, co-chief investigator and trial manager, were also part of the TSC. The TSC advised on recruitment strategies, monitored progress with recruitment and checked adherence to the trial protocol. Representatives of the sponsor were also invited to TSC meetings.
Data Monitoring Committee
The DMC was an independent group, the members of which had no other involvement with the trial. Members of the DMC included two rehabilitation professionals and a professor of health service research with expertise in statistics. The role of the DMC was to safeguard the interests of trial participants, with particular reference to the safety of the intervention; monitor the overall progress and conduct of the trial; and protect the validity and credibility of the trial.
The TSC and the DMC met independently of each other, with the DMC providing reports to the TSC.
Patient and public involvement
During the planning of the trial, four service users were identified through the MS Society research network and one service user co-applicant had been involved in the pilot study for the trial. All commented on the trial design and documentation, particularly the participant information sheet and consent form. Two service user representatives were directly involved in the trial as members of the TSC. In addition, one of the service user representatives assisted with interviewing participants as part of the qualitative component of the trial. This service user representative was also involved in preparing a video to disseminate the results to participants and to people with MS. Three participants also contributed to the dissemination of results by taking part in a video to illustrate the cognitive rehabilitation sessions. All service users were invited to comment on the lay summary.
Statistical methods
The primary outcome was the psychological impact of MS on everyday life at 12 months, as a reflection of health-related quality of life (HRQoL), as measured using the MSIS-Psy.
Sample size
The sample size was estimated using the primary outcome of the MSIS-Psy scores at 12 months post randomisation. A clinically meaningful effect using this outcome is probably in the range of 3.0–3.5 (using version 1 of the MSIS-Psy, scored 9–45). Based on a two-sample test, 143 participants per group were required for analysis in order to detect a difference of 3 points on the MSIS-Psy, assuming a SD of 9 points, with 80% power and 5% two-sided alpha. However, a clustering effect may be expected to occur in the intervention group as a result of the cognitive rehabilitation being delivered in groups. Based on an average cluster size of five evaluable participants (those providing primary outcome data at 12 months after randomisation), an intracluster correlation (ICC) of 0.1 in the intervention group and an optimal allocation ratio of 6 : 5 in favour of the intervention group, a total of 336 evaluable patients would provide 80% power to detect such a difference (182 to the intervention and 154 to usual care). A sample size of 400 randomised participants was set (216 to the intervention and 184 to usual care) to allow for non-collection of primary outcome data for 15% of participants.
Version 2 of the MSIS-Psy was used in this trial, with scores ranging from 9 to 36. The SD of the MSIS-Psy version 2 in the UK South West Impact of Multiple Sclerosis (SWIMS) cohort was 6.4 points. 115 Differences of between 2 and 3 points on version 2 of the MSIS-Psy are detectable based on the effect size specified above (assuming a SD of between 6 and 9), with assumed similar clinical importance as for version 1.
Statistical analysis
The planned analyses were described in the statistical analysis plan (SAP), which was finalised prior to database lock and release of the treatment allocation codes for analysis. All analyses were carried out using Stata®/SE version 15.1 (StataCorp LP, College Station, TX, USA) and R studio version 3.4.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Preliminary analysis
Descriptive statistics were used to examine balance between the two groups on demographic and clinical measures.
Analysis populations
The main approach for the analysis was to analyse participants as randomised, regardless of the number of cognitive rehabilitation sessions attended for all primary and secondary outcomes.
The data used at each time point were as follows:
-
questionnaires/visits completed within 9 months of randomisation (i.e. within 275 nights of randomisation) for the outcomes at 6 months
-
questionnaires/visits completed within 15 months of randomisation (i.e. within 456 nights of randomisation) for the outcomes at 12 months.
Outcomes completed outside these time periods were used only in a sensitivity analysis for the primary outcome and for the safety outcome. The main analyses were conducted on data from participants with available data; no imputation was undertaken for participants who had missing outcomes.
Descriptive analyses
The adherence to the intervention was described by tabulating the attendance at each session and summarising the number of sessions that each participant attended (with and without catch-up sessions). The reasons for non-attendance at sessions were also described and summarised.
The number of participants returning the questionnaire booklet/completing the follow-up visit at 6 and 12 months was summarised in the two groups, along with the number of days between randomisation and completion. The pattern of missing outcome data was explored, overall and in the two groups, and the baseline characteristics were compared between participants with and participants without primary outcome data.
Unblinding of RAs at a follow-up visits was reported descriptively. The kappa statistic was used to assess the agreement between a participant’s actual treatment allocation and the RA’s opinion of treatment allocation, collapsing probably and definitely into one category.
Missing data in questionnaires
Missing items in questionnaires were imputed with the mean of the completed items if ≥ 8 of the 9 items on the MSIS-Psy were completed, ≥ 17 of the 20 items on the Multiple Sclerosis Impact Scale – Physical Subscale (MSIS-Phy) were completed, ≥ 25 of the 28 items on the Everyday Memory Questionnaire (EMQ) were completed, ≥ 27 of the 30 items on the GHQ-30 were completed and ≥ 12 of the 13 items on the MCSI were completed. Otherwise, scores from questionnaires were treated as missing.
To be able to include all participants in the regression analysis of the outcome score, if scores from the questionnaires remained missing at baseline after the process outlined above, baseline data were imputed for the analysis using the mean score at each centre. These simple imputation methods are better than more complicated imputation methods in which baseline variables are included in an adjusted analysis to improve the precision of the treatment effect. 116 This imputation was done only for the regression analyses and not for summarising the baseline scores.
Primary outcome
The primary analysis estimated the difference in the mean MSIS-Psy score at 12 months between the two groups using a multilevel linear model, with baseline MSIS-Psy score, gender, MS type and centre as covariates. Participants in the usual-care group had no contact with each other; therefore, outcomes in this group were assumed to be independent. However, participants in the intervention group received cognitive rehabilitation sessions together, which needed to be taken into account in the analysis. A fully heteroscedastic model was used, which estimates group-level residual variance in the intervention group and also permits individual-level residual variance to differ between intervention and control groups. 117,118 The assumptions for the multilevel linear model were checked using diagnostic plots. The ICC coefficient in the intervention group was estimated using the estimates of the group-level residual variance and individual-level residual variance in the intervention group.
Sensitivity analyses for the primary outcome
The following sensitivity analyses were conducted:
-
Including all 12-month questionnaires – the analysis was repeated including participants whose 12-month questionnaires were returned after the 15-month post-randomisation window.
-
Using different methods for missing baseline MSIS-Psy score – the analysis was repeated restricting the analysis to participants with the MSIS-Psy completed at baseline and using the missing indicator method for missing baseline MSIS-Psy values.
-
Multiple imputation of missing primary outcome data – multiple imputation was performed using multivariate multilevel linear regression using the pan procedure in R, under the assumption that missing data were missing at random. Variables included in the imputation model were centre, age, gender, MS type, MSIS-Psy score at baseline and at 6 months, MSNQ score, Doors and People overall age-scaled score at baseline, Symbol Digit Modalities Test from the BRBN, whether or not the participant had reported a MS relapse at 6 and 12 months, the number of cognitive rehabilitation sessions attended and the following scores from baseline, 6 months and 12 months: GHQ-30 total score, EMQ-p score and EuroQol-5 Dimensions visual analogue scale (EQ-5D VAS) score. Thirty data sets were imputed and the results of the analyses on the imputed data sets were combined using Rubin’s rules.
-
The complier average causal effect was estimated in a secondary analysis of the primary outcome. Instrumental variable regression was used to estimate the effect of the intervention for participants who would comply with the allocated treatment for whichever group they were randomised to. 119,120 Participants in the intervention group were classified as adherent if they attended at least three cognitive rehabilitation sessions. The instrumental variable regression model included baseline MSIS-Psy score, gender, MS type and centre. The complier average causal effect was estimated using both the observed data and the multiply imputed data.
Earlier effects on the primary outcome were investigated in a secondary analysis by comparing the MSIS-Psy at 6 months after randomisation in the two groups using the same analysis model as for the primary outcome.
Subgroup analyses for the primary outcome
Four exploratory subgroup analyses for the primary outcome were performed by including an interaction term in the model for the primary analysis between allocated group and the following variables:
-
MS type (progressive or relapsing–remitting)
-
MSNQ score (i.e. according to cognitive problems at baseline)
-
Doors and People score (overall score, i.e. according to memory function at baseline)
-
Symbol Digit Modalities Test score from the BRBN (i.e. according to speed of processing of information).
The MSNQ score, the overall Doors and People score and Symbol Digit Modalities Test score were split into three categories, according to the tertiles at baseline.
Secondary outcomes at 6 and 12 months
The difference in means between the two groups at 6 and 12 months was estimated, using the same analysis model described for the primary outcome, for the following outcomes:
-
memory problems in everyday life, measured using the EMQ-p and EMQ-r
-
mood, measured using the GHQ-30
-
fatigue, measured using the Rasch-transformed FSS
-
carer strain, measured using the MCSI
-
quality of life, measured using the EQ-5D VAS (from the EQ-5D-5L), which asks participants to rate their current health status
-
attention and memory abilities, using the Selective Reminding total and delayed recall scores; 10/36 Spatial Recall Test total correct and delayed recall scores; the Symbol Digit Modalities total score; the Paced Auditory Serial Addition Test, Easy and Hard total scores; and the Word Fluency total score from the BRBN
-
overall age-scaled score, verbal total score, non-verbal total score and forgetting score from the Doors and People test
-
the difference between the number of seconds to complete parts A and B of the Trail Making test (derived as B – A)
-
physical impact of MS on quality of life, measured using the MSIS-Phy.
The number of participants in any form of employment (full or part-time) was used for the analysis for employment status. The odds ratio for any form of employment at 6 and 12 months in the two groups was estimated using a generalised estimating equation with a logistic link function and robust standard errors to account for potential clustering in the intervention group. 121
The secondary outcomes for disability (as measured using GNDS) and the number of reported relapses in the previous 6 months are reported descriptively.
The safety outcome, defined as an increase of ≥ 30 points on the GHQ-30 (using Likert scoring) between baseline and 6 months, is also reported descriptively. All participants completing the 6-month questionnaire were included in the analysis population for the safety outcome.
Chapter 3 Results
Recruitment
Participants were recruited between April 2015 and March 2017 (see Appendix 4, Figure 10). Of the 3556 people with MS who were invited to participate or expressed an interest in the trial, 818 had an initial telephone eligibility screening and, of these, 579 consented. The majority of the patients screened initially who did not consent were not eligible (n = 194; 81%). The breakdown of reasons for exclusion is shown in Appendix 5, Table 28.
Of the 579 participants who gave consent, 449 were randomised (78%). Non-randomisation after consent was mainly as a result of not being eligible (n = 68; 52%) or the trial being closed to recruitment (n = 40; 31%); the Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 1 and reasons participants were not eligible are shown in Appendix 5, Table 29. The randomisation target was exceeded because there was a pool of eligible participants who had been screened, consented and were awaiting randomisation at the time the target was reached, as a result of the requirement to form groups of 9–11 participants who could all attend for cognitive rehabilitation sessions at the same time and place (if allocated).
FIGURE 1.
The CONSORT flow diagram. Reproduced with permission from Lincoln et al. 122 This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 License (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
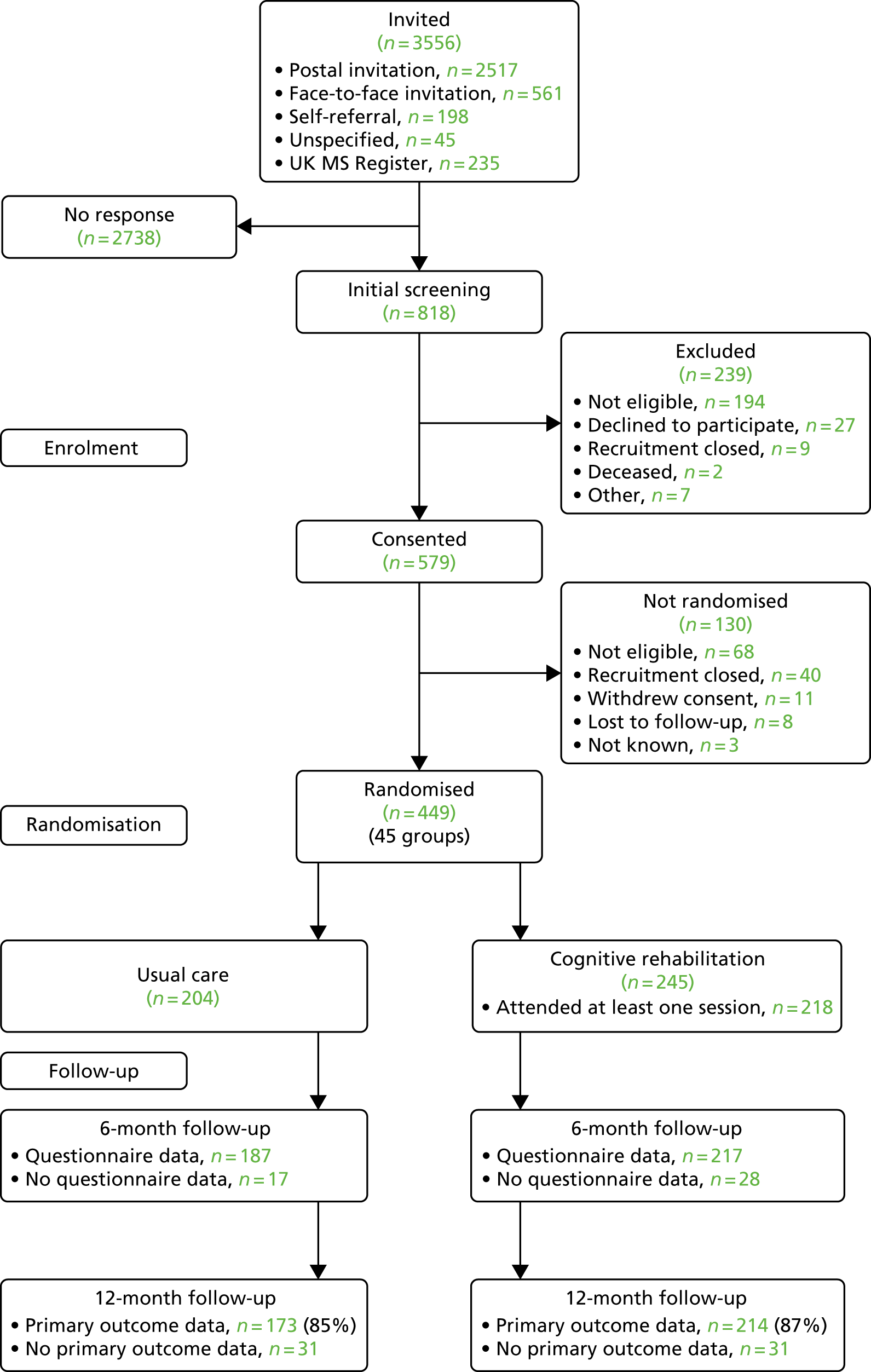
Of the 449 participants randomised, 204 were randomised to usual care and 245 to cognitive rehabilitation in addition to usual care (allocation ratio of 6 : 5 to the cognitive rehabilitation group, as planned). There were 45 cognitive rehabilitation groups: two groups of four participants (4%), 21 groups of five participants (47%) and 22 groups of six participants (49%).
Participants waited a median of 42 days between the baseline assessment and randomisation. However, a few participants (n = 25; 6%) waited for ≥ 6 months to be randomised as a result of waiting for other participants who could attend the intervention sessions at the same time or awaiting the availability of an AP to deliver the intervention.
Baseline data
The mean age of participants was 49 years (SD 9.9 years), 326 (73%) were women and almost all were white (96%) (Table 1). Approximately two-thirds of participants reported that they had relapsing–remitting MS with a mean time since diagnosis of 11.7 years (SD 8.4 years). One-third of participants reported being in employment, one-third reported being retired and one-third reported not being in either employment or education.
| Characteristic | Usual care (N = 204) | Cognitive rehabilitation (N = 245) |
|---|---|---|
| Age at randomisation (years) | ||
| Mean (SD) | 48.9 (10.0) | 49.9 (9.8) |
| Minimum, maximum | 25, 69 | 18, 69 |
| Gender, n (%) | ||
| Men | 56 (27) | 67 (27) |
| Women | 148 (73) | 178 (73) |
| Ethnicity, n (%) | ||
| White | 195 (96) | 237 (97) |
| Black | 3 (1) | 3 (1) |
| Asian | 2 (1) | 3 (1) |
| Mixed | 4 (2) | 2 (1) |
| Marital status, n (%) | ||
| Single/divorced/widowed | 66 (32) | 81 (33) |
| Married/with partner | 138 (68) | 164 (67) |
| Participant-reported time since MS diagnosis (years) | ||
| Mean (SD) | 11.1 (8.7) | 12.1 (8.0) |
| Minimum, maximum | 0, 40 | 0, 36 |
| Type of MS (participant reported), n (%) | ||
| Relapsing–remitting | 132 (65) | 159 (65) |
| Primary progressive | 24 (12) | 22 (9) |
| Secondary progressive | 48 (24) | 64 (26) |
| Number of relapses in the previous 6 months, as assessed at screening (participant reported), n (%) | ||
| 0 | 155 (76) | 186 (76) |
| 1 | 31 (15) | 47 (19) |
| 2 | 13 (6) | 5 (2) |
| ≥ 3 | 5 (2) | 7 (3) |
| Relapse between baseline and randomisation (participant reported), n (%) | ||
| No | 190 (93) | 220 (90) |
| Yes | 12 (6) | 24 (10) |
| Not known | 2 (1) | 1 (< 0.5) |
| Years of education | ||
| Mean (SD) | 13.9 (2.9) | 14.2 (3.4) |
| Minimum, maximum | 10, 30 | 10, 35 |
| Employment status, n (%) | ||
| Retired | 64 (31) | 80 (33) |
| Not employed or in education | 70 (34) | 83 (34) |
| Employed part time | 31 (15) | 44 (18) |
| Employed full time | 38 (19) | 31 (13) |
| In education full time | 0 (0) | 1 (< 0.5) |
| In education part time | 0 (0) | 6 (2) |
| Not knowna | 1 (< 0.5) | 0 (0) |
| Any employment (full or part time) | 69 (34) | 75 (31) |
| Living arrangements, n (%) | ||
| Lives alone | 38 (19) | 49 (20) |
| Lives with others | 166 (81) | 196 (80) |
| Living in care home | 0 (0) | 0 (0) |
The characteristics assessed at baseline were well balanced between groups, including the minimisation stratification variables of gender and MS type. However, a slightly higher percentage of participants in the cognitive rehabilitation group than in the usual-care group reported a relapse between baseline and randomisation (10% vs. 6%, respectively). Attention and memory abilities, psychological and physical impact of MS on quality of life, frequency of memory problems, and mood and fatigue were similar in the two groups at baseline (Table 2).
| Assessment | Usual care, mean [SD] or n (%) (N = 204) | Cognitive rehabilitation, mean [SD] or n (%) (N = 245) |
|---|---|---|
| MSNQa | 39.0 (7.4) | 38.9 (7.1) |
| BRBNb | ||
| Selective Remindingc | ||
| Total recall | 40.2 [10.5] | 40.6 [11.0] |
| Long-term storage | 30.9 [14.5] | 31.2 [15.7] |
| Consistent long-term retrieval | 18.7 [13.6] | 19.0 [14.2] |
| Delayed recall | 5.7 [2.8] | 5.8 [2.8] |
| 10/36 Spatial Recalld | ||
| Total correct | 18.3 [4.9] | 18.1 [4.5] |
| Total confabulations | 11.3 [5.0] | 11.5 [4.6] |
| Delayed recall | 6.3 [2.1] | 6 [2.2] |
| Symbol Digit Modalitiese | 37.8 [12.1] | 36.3 [11.5] |
| Paced Auditory Serial Additionf | n = 199 | n = 239 |
| Easy total correct | 31.1 [16.4] | 31.6 [16.2] |
| Hard total correct | 15.9 [15.8] | 17.3 [16.5] |
| Word fluencyg | n = 203 | n = 244 |
| Total score | 25.1 [8.9] | 24.8 [8.8] |
| GNDSh | 20.0 [6.7] | 19.9 [7.1] |
| Doors and Peoplei | n = 203 | n = 245 |
| Overall age-scaled score | 7 [3.9] | 7 [3.7] |
| Verbal total score | 7.7 [3.9] | 7.8 [3.7] |
| Non-verbal total score | 7.7 [3.5] | 7.5 [3.4] |
| Total forgetting score | 8.8 [3.0] | 8.8 [3.0] |
| Trail Makingj | n = 200 | n = 244 |
| Part B – part A | 69.6 [41.4] | 71.7 [41.0] |
| EQ-5D VAS scorek | 59.6 [20.3] | 59.9 [21.2] |
| Baseline questionnaire booklet returned | 198 (97) | 233 (95) |
| MSIS-Psyl | 24.7 [6.0] (n = 197) | 23.3 [5.8] (n = 233) |
| MSIS-Phym | 53.4 [13.1] (n = 197) | 52 [13.6] (n = 232) |
| EMQ-pn | 47.1 [23.2] (n = 194) | 45.0 [22.8] (n = 229) |
| FSSo | 1.3 [1.3] (n = 197) | 1.4 [1.4] (n = 230) |
| GHQ-30p | ||
| Total score (Likert scoring) | 39.7 [15.8] (n = 197) | 36.5 [14.2] (n = 230) |
| GHQ score of ≥ 5 (GHQ scoring) | 136 (67) | 150 (61) |
| EMQ-rn | 38.2 [25.9] (n = 185) | 34.7 [23.4] (n = 213) |
The EMQ-r questionnaire was completed for 398 participants (89%) at baseline: 185 (91%) in the usual-care group and 213 (87%) in the cognitive rehabilitation group.
Baseline questionnaires should have been completed prior to randomisation; however, six participants (3%) in the usual-care group and 12 (5%) in the cognitive rehabilitation group were randomised without the questionnaires being completed.
Two randomised participants (one in each group) were found to not be eligible for the trial during checks of the BRBN scoring as they did not have any test scores more than one SD below the mean of healthy controls, corrected for age and years of education. These participants are included in all analyses.
Cognitive rehabilitation sessions
Attendance at sessions
Participants attended a mean of 7 sessions (SD 3.4 sessions) not including catch-up sessions, and a mean of 7.7 sessions (SD 3.5) when catch-up sessions were included (Table 3). Three or more sessions (including catch-up sessions) were attended by 208 participants (85%). The reasons that participants did not attend sessions are shown in Table 4. Twenty-four participants (10%) missed sessions as they did not want to continue and six participants (2%) stopped attending as they withdrew from the trial (not mutually exclusive); otherwise, sessions tended to be missed because of illness or other commitments.
| Sessions attended | (N = 245) |
|---|---|
| Including catch-up sessions | |
| Mean (SD) | 7.7 (3.5) |
| Median (25th, 75th centile) | 10 (7, 10) |
| Number of sessions attended, n (%) | |
| 0–2 | 37 (15) |
| 3–7 | 32 (13) |
| 8–10 | 176 (72) |
| Excluding catch-up sessions | |
| Mean (SD) | 7 (3.4) |
| Median (25th, 75th centile) | 8 (6, 10) |
| Reason scheduled session missed | Total number of sessions | Total number of participants |
|---|---|---|
| Did not want to continue | 162 | 24 |
| Withdrew from trial | 40 | 6 |
| Unable to contact | 92 | 26 |
| Forgot to attend | 14 | 13 |
| Unwell – MS relapse | 20 | 11 |
| Unwell – other | 119 | 66 |
| Holiday | 65 | 49 |
| Work commitments | 49 | 25 |
| Travel problems | 12 | 11 |
| Othera | 157 | 69 |
| Not known | 11 | 10 |
Follow-up
Participant follow-up was between November 2015 and April 2018. At the 6-month follow-up, 412 (92%) participants completed the assessment visit and 405 (90%) completed the questionnaire booklet (Table 5). Completion rates were similar in the two groups.
| Follow-up completion | 6-month follow-up | 12-month follow-up | ||
|---|---|---|---|---|
| Usual care (N = 204) | Cognitive rehabilitation (N = 245) | Usual care (N = 204) | Cognitive rehabilitation (N = 245) | |
| Face-to-face visit | ||||
| Attended, n (%) | 187 (92) | 225 (92) | 175 (86) | 212 (87) |
| Not done,a n (%) | 5 (2) | 5 (2) | 3 (1) | 7 (3) |
| Discontinued with reasons, n (%) | 12 (6) | 15 (6) | 26 (13) | 26 (11) |
| Death (n) | 0 | 0 | 0 | 0 |
| Withdrawal of consent (n) | 9 | 10 | 16 | 13 |
| Lost to follow-up (n) | 3 | 4 | 10 | 12 |
| Other – not known (n) | 0 | 1 | 0 | 1 |
| Days to assessment visit from randomisation | ||||
| Median (25th, 75th centile) | 182 (178, 188) | 184 (180, 188) | 365 (359, 370) | 364 (359.5, 370) |
| Minimum, maximum | 166, 261 | 167, 288 | 343, 546 | 334, 479 |
| Visit completed within 3 months of due date, n (%) | ||||
| No | – | 1 (< 0.5) | 2 (1) | 3 (1) |
| Yes | 187 (92) | 224 (91) | 173 (85) | 209 (85) |
| Questionnaire booklet, n (%) | ||||
| Returned | 187 (92) | 218 (89) | 176 (86) | 216 (88) |
| Not donea | 5 (2) | 12 (5) | 2 (1) | 3 (1) |
| Discontinued | 12 (6) | 15 (6) | 26 (13) | 26 (11) |
| Days to completion from randomisation | ||||
| Median (25th, 75th centile) | 177 (170, 187) | 178 (170, 184) | 357 (351, 366.5) | 358 (350, 366) |
| Minimum, maximum | 151, 260 | 152, 288 | 327, 532 | 313, 478 |
| Questionnaire completed within 3 months of due date, n (%) | ||||
| No | – | 1 (< 0.5) | 3 (1) | 1 (< 0.5) |
| Yesb | 187 (92) | 217 (89) | 173 (85) | 215 (88) |
At the 12-month follow-up, 387 (86%) participants completed the assessment visit and 392 (87%) completed the questionnaire booklet; completion rates were similar in the two groups (see Table 5). A total of 80% of questionnaire booklets at both time points were completed on paper rather than electronically.
Almost all of the visits and questionnaires were completed within 3 months of the scheduled time point (see Table 5).
Fifty-two participants did not complete the 12-month follow-up (12%; 26 participants in each group) because they either withdrew consent or were lost to follow-up (see Table 5). The main reasons for withdrawal (when given) were health problems or having too many other commitments.
Inclusion in primary analysis of the primary outcome
A total of 173 participants (85%) in the usual-care group and 214 (87%) participants in the cognitive rehabilitation group were included in the primary analysis of the primary outcome at 12 months. Three participants in the usual-care group and two participants in the cognitive rehabilitation group who completed questionnaires at 12 months were not included in the primary analysis of the MSIS-Psy because either they completed the questionnaire > 15 months after randomisation (n = 4) or there was more than one item missing on the MSIS-Psy (n = 1).
Participants in both groups with no primary outcome were slightly younger, more likely to report having relapsing–remitting MS and had poorer scores on cognitive assessments and questionnaires at baseline (see Appendix 6, Tables 30 and 31).
Relative/friend questionnaire follow-up
At 6 months relative/friend questionnaires were completed for 158 participants (77%) in the usual-care group and 185 participants (76%) in the cognitive rehabilitation group. All of the 6-month relative/friend questionnaires were completed within 9 months of a participant’s randomisation.
At 12 months, relative/friend questionnaires were completed for 147 participants (72%) in the usual-care group and 171 participants (70%) in the cognitive rehabilitation group. One relative/friend questionnaire in the usual-care group and three relative/friend questionnaires in the cognitive rehabilitation group were completed > 15 months after the participant’s randomisation.
Unblinding at follow-up visits
Research assistants reported being unblinded prior to the follow-up for 17 participants at 6 months (4% of completed visits) and for six participants at 12 months (2% of completed visits). The percentage of RAs who reported being unblinded prior to the visits were similar in the two groups at each follow-up time point.
Research assistants reported being unblinded during the visit more often in the cognitive rehabilitation group than in the usual-care group. At 6 months, RAs reported being unblinded during the visit for 36 participants (16%) in the cognitive rehabilitation group and for 16 participants (9%) in the usual-care group. At 12 months, RAs reported being unblinded during the visit for 17 participants (8%) in the cognitive rehabilitation group and for six participants in the usual-care group (3%).
Overall, there was little agreement between the RA opinion of treatment allocation and the actual treatment allocation as assessed using the kappa statistic. The kappa values were 0 at the start of the 6-month visit, 0.17 at the end of the 6-month visit, 0.02 at the start of the 12-month visit and 0.07 at the end of the 12-month visit. Details of the RA opinion of treatment allocation at the start and end of the visit are shown in Appendix 7, Table 32.
Primary outcome: MSIS-Psy at 12 months
Primary analysis
A total of 387 participants were included in the primary analysis at 12 months (86%); 376 of these completed the MSIS-Psy at baseline. There was a mean of 4.8 participants per cognitive rehabilitation group who had MSIS-Psy data at the 12-month follow-up.
There was no evidence of a difference between the two groups in the MSIS-Psy score at 12 months (Table 6). The adjusted difference in means was –0.6, with 95% confidence interval (CI) –1.5 to 0.3, which does not include the 2-point difference assumed to be clinically important on version 2.0 of the MSIS-Psy.
| Trial group | Time point, mean (SD); n | Adjusted difference in means (95% CI) | p-value | |
|---|---|---|---|---|
| Baselinea | 12 months | |||
| Usual care | 24.2 (5.9); 170 | 23.4 (6.0); 173 | ||
| Cognitive rehabilitation | 23.0 (5.7); 206 | 22.2 (6.1); 214 | –0.6 (–1.5 to 0.3) | 0.20 |
There was no evidence that the assumptions for the model were not met. The estimated ICC coefficient for the MSIS-Psy score from the model was 0 in the cognitive rehabilitation group.
Sensitivity analyses
The sensitivity analyses for the primary analysis were all consistent with the primary analysis, showing no clinically important difference between the groups in MSIS-Psy score at 12 months (Table 7).
| Sensitivity analysis | n | Adjusted difference in means (95% CI) |
|---|---|---|
| Including participants completing the 12-month questionnaire booklet > 15 months after randomisation | 391 | –0.5 (–1.4 to 0.4) |
| According to method used for missing baseline MSIS-Psy score | ||
| Participants with observed baseline MSIS-Psy score | 376 | –0.7 (–1.6 to 0.2) |
| Using missing indicator method | 387 | –0.7 (–1.6 to 0.2) |
| Multiple imputation for missing MSIS-Psy score at 12 monthsa | 449 | –0.5 (–1.5 to 0.5) |
Secondary analysis
The complier average causal effect estimate of the adjusted difference in means using the observed data was –0.7 (95% CI –1.7 to 0.4, n = 387), and using the multiply imputed data it was –0.6 (95% CI –1.7 to 0.4, n = 449), based on attending three or more cognitive rehabilitation sessions.
Subgroup analysis for the primary outcome
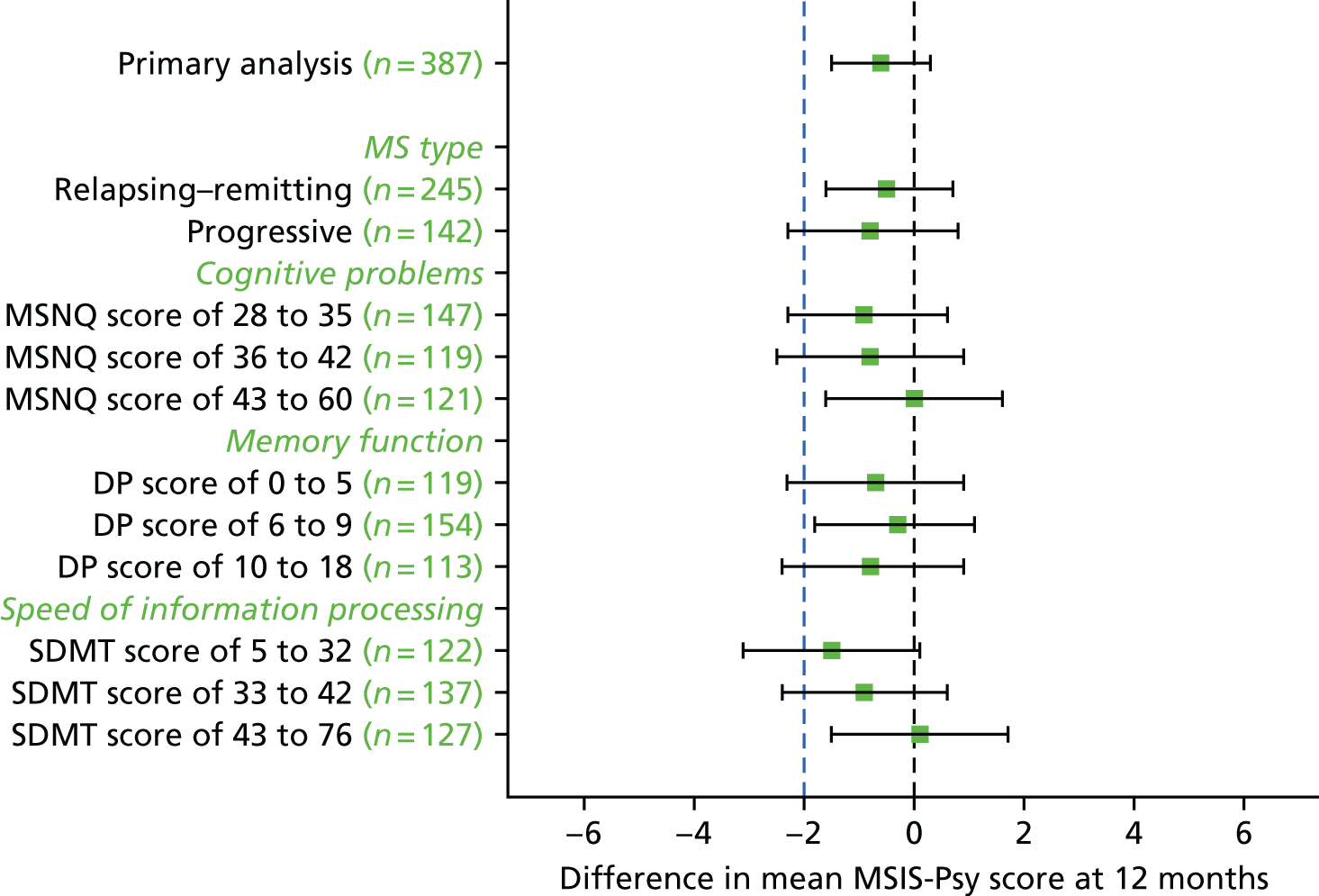
There was no evidence of a difference in the effect of cognitive rehabilitation according to MS type (p-value for interaction effect, 0.79), cognitive problems at baseline assessed on the MSNQ (p-value for interaction effect, 0.71), memory function at baseline using the Doors and People overall age-scaled score (p-value for interaction effect, 0.92) or speed of information processing at baseline using the Symbol Digit Modalities Test from the BRBN (p-value for interaction effect, 0.38) (Figure 2; see also Appendix 8, Table 33).
FIGURE 2.
Adjusted difference in mean MSIS-Psy score according to subgroup. DP, Doors and People; SDMT, Symbol Digit Modalities Test.
The MSIS-Psy score at 6 months
At 6 months, the adjusted difference in mean MSIS-Psy score and 95% CI favoured the cognitive rehabilitation group (Table 8). However, the 95% CI does not include the 2-point difference that is assumed to be clinically important.
| Trial group | Time point, mean (SD); n | Adjusted difference in means (95% CI) | p-value | |
|---|---|---|---|---|
| Baselinea | 6 months | |||
| Usual care | 24.5 (6.0); 181 | 24.1 (5.9); 187 | ||
| Cognitive rehabilitation | 23.2 (5.8); 210 | 22.3 (6.2); 217 | –0.9 (–1.7 to –0.1) | 0.03 |
Secondary outcomes
Secondary outcomes were considered to be supportive to the primary analysis. No formal adjustment for multiple significance testing was applied so the number of tests conducted should be taken into consideration when interpreting the results.
Secondary outcomes assessed on questionnaires
At 6 and 12 months, the differences between the two groups favouring the cognitive rehabilitation group were observed for participant-reported frequency of memory problems assessed using the EMQ-p and mood using the GHQ-30 (Table 9). The EMQ-r scores (relative/friend report of a participant’s frequency of memory problems) were lower for the cognitive rehabilitation group, with an effect size for the difference in means between the two groups of a similar magnitude to the participant report (see Table 9).
| Questionnaire | Time point, mean (SD); n | Adjusted difference in means at 6 months (95% CI); n | 12 months, mean (SD); n | Adjusted difference in means at 12 months (95% CI); n | |
|---|---|---|---|---|---|
| Baseline | 6 months | ||||
| EMQ-pa | |||||
| Usual care | 47.1 (23.2); 194 | 44.5 (23.5); 181 | 43.1 (24.0); 168 | ||
| Cognitive rehabilitation | 45.0 (22.8); 229 | 37.6 (23.4); 214 | –5.3 (–8.7 to –1.9); 395 | 37.9 (22.7); 210 | –4.4 (–7.8 to –0.9); 378 |
| EMQ-ra | |||||
| Usual care | 38.2 (25.9); 185 | 38.6 (25.7); 152 | 38.5 (26.4); 142 | ||
| Cognitive rehabilitation | 34.7 (23.4); 213 | 31.3 (22.9); 184 | –5.4 (–9.1 to –1.7); 336 | 30.5 (23.3); 164 | –5.5 (–9.6 to –1.5); 306 |
| GHQ-30b (Likert scoring) | |||||
| Usual care | 39.7 (15.8); 197 | 37.8 (14.8); 183 | 38.3 (16.2); 167 | ||
| Cognitive rehabilitation | 36.5 (14.2); 230 | 32.9 (15.1); 212 | –3.4 (–5.9 to –0.8); 395 | 33.9 (16.1); 209 | –3.4 (–6.2 to –0.6); 376 |
| FSSc (Rasch-transformed score) | |||||
| Usual care | 1.3 (1.3); 197 | 1.1 (1.4); 185 | 1.2 (1.4); 168 | ||
| Cognitive rehabilitation | 1.4 (1.4); 230 | 1.1 (1.4); 214 | –0.1 (–0.3 to 0.2); 399 | 1.0 (1.4); 210 | –0.3 (–0.5 to 0.0); 378 |
| EQ-5D VASd score | |||||
| Usual care | 59.6 (20.3); 203 | 59.2 (20.2); 187 | 59.7 (20.0); 173 | ||
| Cognitive rehabilitation | 59.9 (21.2); 245 | 61.7 (19.5); 224 | 2.6 (–0.9 to 6.0); 411 | 61.6 (19.3); 209 | 2.6 (–0.9 to 6.0); 382 |
| MSIS-Phye | |||||
| Usual care | 53.4 (13.1); 197 | 53.0 (13.9); 187 | 52.5 (13.6); 173 | ||
| Cognitive rehabilitation | 52.0 (13.6); 232 | 51.4 (13.3); 215 | –0.6 (–2.2 to 0.9); 402 | 51.8 (14.0); 214 | –0.1 (–1.8 to 1.5); 387 |
| MCSIf score (relative/friend) | |||||
| Usual care | – | 6.8 (6.2); 154 | 6.2 (6.0); 141 | ||
| Cognitive rehabilitation | – | 5.9 (5.6); 173 | –0.9 (–2.2 to 0.4); 327 | 5.8 (5.2); 159 | –0.4 (–1.6 to 0.8); 300 |
| GNDSg | |||||
| Usual care | 20.0 (6.7); 204 | 20.5 (7.3); 183 | – | 20.5 (7.4); 166 | – |
| Cognitive rehabilitation | 19.9 (7.1); 245 | 20.6 (7.2); 211 | – | 20.1 (7.4); 202 | – |
There was no evidence of a difference between the groups in fatigue, health status (based on the EQ-5D VAS score), physical impact of MS on quality of life, or carer strain at 6 or 12 months (see Table 9).
The percentage of participants in employment (full or part time) was ≈30% at baseline and at 6 and 12 months in each group (Table 10). Further details on employment status can be seen in Appendix 9, Tables 34 and 35.
| Employment and relapse | Screening/baseline, n (%); N | 6 months, n (%); N | Adjusted odds ratio (95% CI); n | 12 months, n (%); N | Adjusted odds ratio (95% CI); n |
|---|---|---|---|---|---|
| Any employment (full or part time) | |||||
| Usual care | 69 (34); 203 | 57 (30); 187 | 50 (29); 173 | ||
| Cognitive rehabilitation | 75 (31); 245 | 62 (28); 224 | 0.88 (0.55 to 1.39); 411 | 60 (29); 209 | 0.99 (0.60 to 1.63); 382 |
| Relapse in the previous 6 months | |||||
| Usual care | 49 (24); 204 | 39 (21); 187 | 34 (20); 173 | ||
| Cognitive rehabilitation | 59 (24); 245 | 37 (17); 217 | – | 41 (19); 215 | – |
The mean scores on the GNDS, to assess disability due to MS, at 6 and 12 months were similar to baseline in both groups (see Table 9). The percentage of participants reporting that they had had a relapse in the previous 6 months was also similar in the two groups at follow-up (see Table 10). Details on the number of reported relapses can be seen in Appendix 10, Table 36.
Cognitive abilities
Scores from the BRBN, Doors and People, and Trail Making tests are shown in Table 11. There was no evidence of a difference between groups at 6 or 12 months for six of the eights tests compared on the BRBN. On the selective reminding test, there was evidence of higher total recall scores at 6 months and higher delayed recall scores at 12 months in the cognitive rehabilitation group. However, there was no evidence of a difference between groups in the selective reminding test total recall score at 12 months or the selective reminding test delayed recall scores at 6 months.
| Test | Baseline, mean (SD); n | 6 months, mean (SD); n | Adjusted difference in means at 6 months (95% CI); n | 12 months, mean (SD); n | Adjusted difference in means at 12 months (95% CI); n |
|---|---|---|---|---|---|
| BRBNa | |||||
| Selective reminding testb | |||||
| Total recall | |||||
| Usual care | 40.2 (10.5); 204 | 43.5 (10.4); 182 | 46.5 (11.3); 170 | ||
| Cognitive rehabilitation | 40.6 (11.0); 245 | 45.6 (10.5); 220 | 1.6 (0.1 to 3.0); 402 | 47.5 (10.9); 206 | 0.6 (–0.9 to 2.1); 376 |
| Delayed recall | |||||
| Usual care | 5.7 (2.8); 204 | 6.5 (2.9); 182 | 7.1 (2.9); 170 | ||
| Cognitive rehabilitation | 5.8 (2.8); 245 | 6.7 (2.9); 220 | 0.2 (–0.2 to 0.6); 402 | 7.5 (2.8); 206 | 0.4 (0.1 to 0.8); 376 |
| 10/36 Spatial recallc | |||||
| Total recall | |||||
| Usual care | 18.3 (4.9); 204 | 19.8 (5.4); 182 | 20.4 (5.4); 170 | ||
| Cognitive rehabilitation | 18.1 (4.5); 245 | 19.1 (5.3); 217 | –0.6 (–1.5 to 0.3); 399 | 20.1 (4.9); 206 | –0.1 (–1.0 to 0.8); 376 |
| Delayed recall | |||||
| Usual care | 6.3 (2.1); 204 | 6.6 (2.3); 182 | 7.0 (2.3); 170 | ||
| Cognitive rehabilitation | 6.0 (2.2); 245 | 6.6 (2.3); 217 | 0.0 (–0.4 to 0.4); 399 | 6.8 (2.2); 206 | –0.1 (–0.5 to 0.2); 376 |
| Symbol Digit Modalitiesd | |||||
| Usual care | 37.8 (12.1); 204 | 40.7 (12.7); 181 | 39.9 (12.8); 170 | ||
| Cognitive rehabilitation | 36.3 (11.5); 244 | 41.4 (12.1); 220 | 1.3 (–0.6 to 3.2); 401 | 39.9 (11.9); 205 | 0.4 (–1.7 to 2.5); 375 |
| Paced Auditory Serial Additione | |||||
| Easy total | |||||
| Usual care | 31.1 (16.4); 199 | 35.7 (17.6); 178 | 36.5 (17.7); 169 | ||
| Cognitive rehabilitation | 31.6 (16.2); 239 | 36.6 (16.1); 217 | 0.0 (–2.4 to 2.5); 395 | 36.4 (17.8); 205 | –0.6 (–3.1 to 1.9); 374 |
| Hard total | |||||
| Usual care | 15.9 (15.8); 199 | 19.3 (17.7); 178 | 19.2 (18.9); 169 | ||
| Cognitive rehabilitation | 17.3 (16.5); 239 | 20.7 (17.5); 217 | –0.3 (–2.9 to 2.2); 395 | 18.5 (19.2); 205 | –1.9 (–4.8 to 1.0); 374 |
| Word fluencyf total score | |||||
| Usual care | 25.1 (8.9); 203 | 27.2 (9.3); 182 | 28.3 (10.2); 169 | ||
| Cognitive rehabilitation | 24.8 (8.8); 244 | 27.4 (9.4); 219 | 0.0 (–1.3 to 1.3); 401 | 28.0 (10.3); 206 | –0.2 (–1.5 to 1.2); 375 |
| Doors and Peopleg | |||||
| Overall age-scaled score | |||||
| Usual care | 7.0 (3.9); 203 | 9.1 (4.4); 181 | 9.9 (4.4); 168 | ||
| Cognitive rehabilitation | 7.0 (3.7); 245 | 9.5 (4.2); 221 | 0.4 (–0.1 to 0.9); 402 | 10.5 (4.1); 206 | 0.6 (0.0 to 1.1); 374 |
| Combined visual score | |||||
| Usual care | 7.7 (3.5); 203 | 9.1 (3.7); 181 | 9.4 (3.8); 169 | ||
| Cognitive rehabilitation | 7.5 (3.4); 245 | 9.2 (3.4); 221 | 0.2 (–0.2 to 0.7); 402 | 9.7 (3.6); 207 | 0.4 (–0.1 to 1.0); 376 |
| Combined verbal score | |||||
| Usual care | 7.7 (3.9); 203 | 9.5 (3.9); 183 | 10.5 (4.0); 168 | ||
| Cognitive rehabilitation | 7.8 (3.7); 245 | 10.1 (4.2); 221 | 0.6 (0.0 to 1.2); 404 | 11.5 (4.0); 206 | 0.8 (0.2 to 1.5); 374 |
| Total forgetting score | |||||
| Usual care | 8.8 (3.0); 203 | 9.2 (2.9); 181 | 9.9 (2.7); 168 | ||
| Cognitive rehabilitation | 8.8 (3.0); 245 | 9.5 (2.8); 221 | 0.2 (–0.3 to 0.8); 402 | 10.0 (2.6); 207 | 0.1 (–0.4 to 0.6); 375 |
| Trail Makingh (part B – part A) | |||||
| Usual care | 69.6 (41.4); 200 | 62.3 (38.3); 179 | 63.0 (40.3); 165 | ||
| Cognitive rehabilitation | 71.7 (41.0); 244 | 63.0 (39.1); 218 | –0.3 (–6.8 to 6.2); 397 | 61.3 (39.7); 205 | –3.2 (–10.0 to 3.6); 370 |
On the Doors and People test, there was evidence that the combined verbal scores were better for the cognitive rehabilitation group than for the usual-care group at 6 and 12 months. However, there was no evidence of a difference between the groups for the combined visual score or total forgetting score at either time point.
There was no evidence of a difference between the two groups in the Trail Making Test.
Safety outcome
At 6 months, four participants (2%) in the usual care group and three participants (1%) in the cognitive rehabilitation group had an increase of ≥ 30 points in GHQ-30 score from baseline (see Appendix 11, Table 37).
Chapter 4 Assessing intervention fidelity in the CRAMMS trial
Background
In evaluations of complex interventions, it is important to know the content of the treatment that was delivered in a trial. This is so that the results obtained can be attributed to what was actually delivered, and to allow replication of the interventions across studies. An accurate description of what was delivered may also help in implementing the intervention in health-care settings. Treatment fidelity is the degree to which a treatment is implemented as intended (treatment integrity) and whether or not the treatment being evaluated differs from other treatments or the control condition (treatment differentiation). 123 Dumas et al. 124 distinguished between content fidelity, what was done, and process fidelity, the way it was done. Treatment fidelity requires not only that the treatment is delivered, but also that participants engage with the treatment. 125 This requires participants to understand the intervention, be able to perform the skills required by the intervention (‘intervention receipt’), and to use these skills in daily life (‘intervention enactment’). 126
There are useful recommendations on methods to ensure the integrity of the intervention in the design and conduct of the study. Bellg et al. 127 produced best-practice guidelines for ensuring the fidelity of intervention in randomised trials. The recommendations at the design stage included documenting the ‘dose’ of therapy to be delivered and identifying methods to ensure that the intervention is the same for all participants, documenting the delivery of both intervention and control conditions and planning for setbacks in the provision of treatment. They also recommended standardisation of training for treatment providers, followed by observation of treatment sessions, conducting booster sessions to ensure that there is no ‘drift’ in treatment delivery and monitoring drop-outs from treatment across those providing treatment. Strategies to maintain fidelity during the conduct of the trial included having treatment manuals and monitoring the delivery of treatment using audio- or video-recordings.
There are also methods to check the fidelity of the intervention after the trial has been completed. Walton et al. 125 conducted a review of measures of fidelity of treatment delivery and treatment engagement. They identified that, in 66 studies, the most common single method of recording fidelity of delivery was observation, used by 39% of those studies reviewed, although 25% of studies used multiple methods including observation, self-report and interviews. The main methods to assess fidelity of engagement was self-report (18%), although some studies (17%) also used multiple methods. The authors highlighted a lack of information on the psychometric and implementation qualities of the measures used. 125
In the CRAMMS trial, the aims of the treatment integrity evaluation were to:
-
report the training received by AP delivering the intervention
-
describe the therapists’ reported dose of therapy delivered across sites
-
check whether or not the content of the delivery corresponded with the treatment manual using video-recordings of treatment sessions
-
check the consistency in the content of therapy delivered across sites.
Methods
The initial three AP (from sites 1, 2 and 3) delivering the intervention attended a 1-day workshop, facilitated by a qualified clinical psychologist, to introduce them to the trial and the content of the intervention in the manual. Subsequent APs who joined the trial later (sites 4 and 5) and additional APs in sites 1, 2 and 3 covered this in individual 2-hour meetings about the intervention as described in the manual, delivered by a qualified clinical psychologist.
Once an AP had started delivering the intervention, they received weekly supervision from a clinical psychologist, based at their site, on issues related to dealing with participants. These tended to focus on site-specific issues that affected intervention delivery. Monthly individual telephone supervision with a clinical psychologist co-applicant allowed for discussion of specific challenges relating to the delivery of the intervention. This person offered supervision for all the APs. In addition, monthly teleconferences provided an opportunity for peer group supervision, with all APs participating, and to facilitate consistency in the delivery of intervention across sites. Furthermore, when staff changes occurred, former staff completed a handover document for new staff.
Treatment sessions were video-recorded by the AP, unless the session was the first run by an AP or a participant in the group did not consent to being recorded. Practices for video-recording drew on guidance on minimising intrusiveness of the recording. 128,129 The camera was set up at the beginning of the session to show the AP delivering the intervention, and then was left to run for the entire session.
A coding schedule, as shown in Appendix 12, Tables 38 and 39, was based on the components of treatment described in the manual and previous work on the content of rehabilitation contexts. 130,131 The schedule listed activities that were expected in each session for both the APs (e.g. facilitating discussion or providing feedback) and participants (e.g. asking for information about the intervention content or describing problems).
A distinction was made between content that was applicable in all sessions (e.g. describing strategies, discussing take-home activities and reflecting on personal experiences) and content that was specific to a session (e.g. learning about rhyming mnemonics in session 6 and discussing the use of diaries in session 9). Non-rehabilitation activities (e.g. social chat, information about sessions, and preparing tasks or materials) were coded as ‘other’ content. The primary activity of the therapist or participant was also recorded at each time point.
An independent researcher, not involved in the delivery of the intervention, coded the videos using a time-sampling procedure. Coding took place on the minute, every minute, throughout the video-recording. On each observation, the content of the treatment and the activity of the AP or participant were given the appropriate activity codes. A 10% sample of sessions was randomly selected by group number, using an online random number generator. These sessions were checked by another observer. The inter-rater reliability was determined using Cohen’s kappa. The discrepancies were then resolved by discussion and the coding for all sessions amended on the basis of this discussion. Data from the coding sheets were entered into IBM SPSS Statistics version 23 (IBM Corporation, Armonk, NY, USA) for analysis. The frequencies of each code were reported. Discrepancies of > 5% between the frequency of a specific observation at a site and the overall average across all sites were described.
After each session, the AP recorded the number of people who had attended and reasons for non-attendance.
Results
The breakdown of attendance according to site is shown in Table 12.
| Attendance | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Total |
|---|---|---|---|---|---|---|
| Including catch-up sessions | ||||||
| Mean | 8.0 | 7.6 | 7.1 | 7.8 | 8.2 | 7.7 |
| SD | 2.9 | 3.7 | 3.8 | 3.8 | 3.5 | 3.5 |
| Excluding catch-up sessions | ||||||
| Mean | 7.1 | 7.1 | 6.3 | 7.3 | 7.5 | 7.0 |
| SD | 2.9 | 3.5 | 3.7 | 3.7 | 3.4 | 3.4 |
The results show that the mean number of sessions attended at all sites was between 7.1 and 8.2 when catch-up sessions were included and between 6.3 and 7.5 when catch-up sessions were excluded. The pattern of attendance was similar between sites. The main reasons for non-attendance were that the participant did not want to continue with treatment (24 participants, 162 sessions), being unable to contact the participants (26 participants, 92 sessions), illness (66 participants, 119 sessions) and holidays (48 participants, 64 sessions).
There were 45 treatment groups delivered by 10 APs. Of these, 35 treatment groups (78%) had at least two sessions recorded. The reason for non-recording of a treatment group was that for four groups (9%) it was the first conducted by an AP and for two groups (4%) a participant did not consent. Both these reasons were part of the exclusion criteria for recording. Of the remaining four groups, for two (4%), the group was not recorded in error and for two (4%) the reason was unknown. Of those groups recorded, all sessions were recorded for two (6%) groups. The main reasons for some sessions not being recorded were as follows: there were camera problems for 26 groups (74%), there was a misunderstanding of the requirements for recording for 3 groups (9%), there was resistance from participants even though they had initially agreed to the recordings for 3 groups (9%) and the reason is unknown for 1 group (3%).
The number of recordings per session is shown in Appendix 12, Table 40. Of the 450 sessions held, 252 (56%) were fully recorded and 39 (9%) were partially recorded. The inter-rater reliabilities of the coding categories were very good according to criteria reported by Altman132 (manual content applicable to all sessions, κ = 0.98; session-specific manual content, κ = 0.99; other content not related to cognitive rehabilitation, κ = 0.89; AP activities, κ = 0.83; participant activities, κ = 0.91).
The distribution of manual content applicable to all sessions and according to site is shown in Table 13. This showed that about one-quarter of the time was spent on discussing strategies to help people remember and pay attention. Just under one-fifth of the time was spent reviewing and explaining take-home activities. Less than 10% of time was spent on content that was not related directly to cognitive rehabilitation, such as social chat and making arrangements for future sessions. Therefore, the focus of the intervention was on content directly related to delivering cognitive rehabilitation. This pattern of delivery was consistent between sites. The only notable variation was that the sessions at site 2 included less learning about attention and memory than the other four sites.
| Content | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | N | % | |
| Manual content applicable to all sessions | ||||||||||||
| Summarise previous session | 416 | 5.3 | 18 | 3.3 | 103 | 2.1 | 107 | 3.6 | 72 | 4 | 716 | 4 |
| Reflect on personal experience | 879 | 11.2 | 70 | 12.9 | 593 | 12.3 | 280 | 9.3 | 295 | 16.4 | 2117 | 11.7 |
| Discuss take-home activities | 1354 | 17.2 | 85 | 15.7 | 920 | 19 | 482 | 16.1 | 319 | 17.7 | 3160 | 17.5 |
| Learn about attention and memory | 634 | 8.1 | 24 | 4.4 | 365 | 7.5 | 394 | 13.1 | 189 | 10.5 | 1606 | 8.9 |
| Learn about strategies to address problems | 1949 | 24.8 | 159 | 29.3 | 1108 | 22.9 | 699 | 23.3 | 390 | 21.6 | 4305 | 23.9 |
| Other content | ||||||||||||
| Social chat | 367 | 4.7 | 28 | 5.2 | 278 | 5..7 | 82 | 2.7 | 42 | 2.3 | 797 | 4.4 |
| Discussing the CRAMMS trial research activities | 72 | 0.9 | 0 | 0 | 21 | 0.4 | 31 | 1 | 8 | 0.4 | 132 | 0.7 |
| Discuss living with MS | 158 | 2.0 | 2 | 0.4 | 130 | 2.7 | 29 | 1 | 36 | 2 | 355 | 2 |
| Administration, for example reimbursing travel expenses | 28 | 0.4 | 2 | 0.4 | 15 | 0.3 | 4 | 0.1 | 3 | 0.2 | 52 | 0.3 |
| Hospital visits, and other MS services | 20 | 0.3 | 0 | 0 | 9 | 0.2 | 2 | 0.1 | 1 | 0.1 | 32 | 0.2 |
| Providing information about sessions; housekeeping | 158 | 2.0 | 1 | 0.2 | 83 | 1.7 | 48 | 1.6 | 14 | 0.8 | 305 | 1.7 |
| Missing values (session specific) | 1819 | 23.2 | 152 | 28 | 1214 | 25.1 | 843 | 28.1 | 433 | 24 | 4461 | 24.7 |
| Total | 18,038 | 100 | ||||||||||
The distribution of manual content applicable to all sessions in each session is shown in Table 14. The distribution of time was consistent with expectations based on the manual. The discussion of take-home activities occurred a similar proportion of times in all sessions apart from session 1, which was lower as no take-home activity would have been completed. Learning about attention and memory occurred predominantly in session 2, in which the focus was on explaining memory and the processes involved. The summary of previous sessions gradually increased over time, from 3% in session 2 to 6% in session 8. This indicates that, as more material was covered, there was more review of previous material. Reflections on personal experiences occurred mainly in the final few sessions.
| Content | Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | Session 6 | Session 7 | Session 8 | Session 9 | Session 10 | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | N | % | |
| Manual content applicable to all sessions | ||||||||||||||||||||||
| Summarise previous session | 0 | 0 | 49 | 2.8 | 70 | 3.7 | 89 | 3.8 | 73 | 4 | 57 | 3.2 | 75 | 4.7 | 111 | 6.2 | 73 | 4.3 | 119 | 6 | 716 | 4.0 |
| Reflect on personal experience | 150 | 11 | 117 | 6.6 | 218 | 11.6 | 200 | 8.6 | 167 | 9.1 | 195 | 10.9 | 121 | 7.6 | 354 | 19.9 | 266 | 15.5 | 329 | 16.6 | 2117 | 11.7 |
| Discuss take-home activities | 53 | 8.9 | 389 | 22 | 433 | 23.1 | 428 | 18.3 | 358 | 19.4 | 243 | 13.6 | 346 | 21.7 | 242 | 13.6 | 328 | 19.1 | 340 | 17.2 | 3160 | 17.5 |
| Learn about attention and memory | 49 | 3.6 | 809 | 45.8 | 368 | 19.6 | 64 | 2.7 | 35 | 1.9 | 115 | 6.5 | 75 | 4.7 | 3 | 0.2 | 3 | 0.2 | 85 | 4.3 | 1606 | 8.9 |
| Learn about strategies to address problems | 119 | 8.8 | 56 | 3.2 | 231 | 12.3 | 814 | 34.8 | 735 | 39.9 | 692 | 38.9 | 519 | 32.5 | 461 | 25.9 | 432 | 25.3 | 246 | 12.4 | 4305 | 23.9 |
| Other content | ||||||||||||||||||||||
| Social chat | 78 | 5.7 | 72 | 4.1 | 88 | 4.7 | 89 | 3.8 | 73 | 4 | 67 | 3.8 | 54 | 3.4 | 92 | 5.2 | 83 | 4.8 | 101 | 5.1 | 769 | 4.3 |
| Discussing the CRAMMS trial research activities | 17 | 1.3 | 4 | 0.2 | 0 | 0 | 1 | 0 | 2 | 0.1 | 1 | 0.1 | 6 | 0.4 | 2 | 0.1 | 1 | 0.1 | 98 | 4.9 | 132 | 0.7 |
| Discuss living with MS | 67 | 4.9 | 50 | 2.8 | 35 | 1.9 | 19 | 0.8 | 23 | 1.2 | 13 | 0.7 | 20 | 1.3 | 38 | 2.1 | 30 | 1.8 | 60 | 3 | 355 | 2 |
| Administration, for example travel expenses | 10 | 0.7 | 6 | 0.3 | 5 | 0.3 | 7 | 0.3 | 2 | 0.1 | 9 | 0.5 | 3 | 0.3 | 4 | 0.2 | 2 | 0.1 | 4 | 0.2 | 39 | 0.2 |
| Hospital visits, and other MS services | 8 | 0.6 | 2 | 0.1 | 1 | 0.1 | 0 | 0 | 2 | 0.1 | 1 | 0.1 | 2 | 0.1 | 3 | 0.2 | 6 | 0.4 | 7 | 0.4 | 32 | 0.2 |
| Providing information about sessions, venue, group, etc.; housekeeping | 100 | 7.4 | 47 | 2.7 | 20 | 1.1 | 28 | 1.2 | 19 | 1.0 | 16 | 0.9 | 19 | 1.2 | 20 | 1.1 | 19 | 1.1 | 17 | 0.9 | 197 | 1.1 |
| Missing values (session specific) | 709 | 52.1 | 166 | 9.4 | 404 | 21.6 | 600 | 25.7 | 355 | 19.3 | 372 | 20.9 | 357 | 22.4 | 451 | 25.3 | 471 | 27.5 | 576 | 29.1 | 4461 | 24.7 |
| Total | 18,038 | 100 | ||||||||||||||||||||
The distribution of content that was specific to individual sessions, as described in the manual, is summarised in Table 15. This showed that all expected components of the manual occurred and no components of the manual were missed. The distribution of time reflects the main theme of the session.
| Session-specific activity | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | N | % | |
| Session 1 | ||||||||||||
| Introduction to others | 66 | 11.3 | 0 | 0 | 45 | 9.0 | 19 | 7.0 | 0 | 0 | 130 | 9.6 |
| Discuss format of programme | 64 | 10.9 | 0 | 0 | 44 | 8.8 | 28 | 10.3 | 0 | 0 | 136 | 10.0 |
| Establish group rules | 21 | 3.6 | 0 | 0 | 18 | 3.6 | 19 | 7.0 | 0 | 0 | 58 | 4.3 |
| Explore attention and memory | 14 | 2.4 | 0 | 0 | 16 | 3.2 | 8 | 0.2.9 | 0 | 0 | 38 | 2.8 |
| Things that we forget | 102 | 17.4 | 0 | 0 | 102 | 20.3 | 50 | 18.4 | 0 | 0 | 254 | 18.7 |
| How do you feel? | 32 | 5.5 | 0 | 0 | 21 | 4.2 | 26 | 9.6 | 0 | 0 | 79 | 5.8 |
| Own techniques to help | 110 | 18.8 | 0 | 0 | 100 | 19.8 | 78 | 28.7 | 0 | 0 | 288 | 21.2 |
| What else do you want to know? | 11 | 0.1 | 0 | 0 | 3 | 0.1 | 6 | 0.2 | 0 | 0 | 20 | 1.5 |
| Session 2 | ||||||||||||
| What is memory? | 92 | 12.7 | 0 | 0 | 47 | 10.3 | 50 | 13.9 | 29 | 12.6 | 218 | 12.3 |
| Processes involved in memory | 87 | 12.0 | 0 | 0 | 50 | 11.0 | 72 | 20.1 | 36 | 15.7 | 245 | 13.9 |
| Memory storage | 126 | 17.4 | 0 | 0 | 44 | 9.7 | 49 | 13.6 | 26 | 11.3 | 245 | 13.9 |
| Memory retrieval | 35 | 4.8 | 0 | 0 | 42 | 9.2 | 57 | 15.9 | 29 | 12.6 | 163 | 9.2 |
| Memory systems | 57 | 7.9 | 0 | 0 | 40 | 8.8 | 21 | 5.8 | 18 | 7.8 | 136 | 7.7 |
| Session 3 | ||||||||||||
| Different types of attention | 118 | 14.8 | 6 | 8.6 | 74 | 15.1 | 84 | 32.2 | 42 | 16.3 | 324 | 17.3 |
| Different types of distractors | 66 | 8.3 | 4 | 5.7 | 47 | 9.6 | 21 | 8.0 | 18 | 7.0 | 156 | 8.3 |
| Attention exercise | 160 | 20.1 | 15 | 21.4 | 104 | 21.3 | 55 | 21.1 | 47 | 18.2 | 381 | 20.3 |
| Techniques to improve attention | 144 | 18.1 | 21 | 30.0 | 78 | 16.0 | 21 | 8.0 | 66 | 25.6 | 330 | 17.6 |
| Session 4 | ||||||||||||
| Story-recall exercise | 118 | 12.6 | 22 | 15.2 | 78 | 12.8 | 61 | 15.3 | 40 | 16.3 | 319 | 13.6 |
| 5Ws and the H | 74 | 7.9 | 7 | 4.8 | 30 | 4.9 | 33 | 8.3 | 13 | 5.3 | 157 | 6.7 |
| Story-recall with 5Ws and the H | 134 | 14.3 | 32 | 22.1 | 115 | 18.8 | 54 | 13.5 | 38 | 15.4 | 373 | 15.9 |
| When difficult to attend | 58 | 6.2 | 8 | 5.5 | 41 | 06.7 | 20 | 5.0 | 18 | 7.3 | 145 | 6.2 |
| How attention improved | 55 | 5.9 | 9 | 6.2 | 21 | 3.4 | 20 | 5.0 | 14 | 5.7 | 119 | 5.1 |
| Strategies to remember | 59 | 6.3 | 6 | 4.1 | 57 | 9.3 | 2 | 0.5 | 2 | 0.8 | 126 | 5.4 |
| Case study handout | 99 | 10.6 | 25 | 17.2 | 90 | 14.7 | 66 | 21.6 | 34 | 22.8 | 356 | 15.2 |
| Session 5 | ||||||||||||
| Use of internal memory aids | 81 | 9.6 | 0 | 0 | 28 | 5.7 | 36 | 12.9 | 27 | 12.0 | 172 | 9.3 |
| Rehearsal | 66 | 7.8 | 0 | 0 | 39 | 7.9 | 23 | 8.2 | 21 | 9.3 | 149 | 8.1 |
| Chunking/telephone number exercise | 131 | 15.5 | 0 | 0 | 102 | 20.6 | 41 | 14.6 | 40 | 17.8 | 314 | 17.0 |
| Categorisation | 240 | 28.4 | 0 | 0 | 140 | 28.3 | 91 | 32.5 | 77 | 34.2 | 548 | 29.7 |
| Session 6 content (associations and visual imagery) | 0 | 0 | 0 | 0 | 15 | 3.0 | 0 | 0 | 0 | 0 | 15 | 0.8 |
| Session 6 | ||||||||||||
| Levels of processing | 242 | 32.1 | 29 | 33.7 | 91 | 20.1 | 99 | 27.6 | 36 | 27.7 | 497 | 27.9 |
| Visual imagery | 139 | 18.5 | 17 | 19.8 | 72 | 15.9 | 65 | 23.7 | 20 | 15.4 | 333 | 18.7 |
| Associations | 58 | 7.7 | 9 | 10.5 | 22 | 4.9 | 25 | 7.0 | 16 | 12.3 | 130 | 7.3 |
| Story method | 76 | 10.1 | 9 | 10.5 | 79 | 17.4 | 54 | 15.0 | 29 | 22.3 | 247 | 13.9 |
| First-letter cues | 17 | 2.3 | 1 | 1.2 | 15 | 3.3 | 11 | 3.1 | 3 | 2.3 | 47 | 2.6 |
| Rhymes | 15 | 2.0 | 1 | 1.2 | 11 | 2.4 | 6 | 1.7 | 1 | 0.8 | 34 | 1.9 |
| Session 7 | ||||||||||||
| Little-and-often strategy | 124 | 14.9 | 16 | 19.3 | 97 | 19.4 | 21 | 17.9 | 9 | 14.1 | 267 | 16.7 |
| PQRST method | 217 | 26.1 | 18 | 21.7 | 151 | 30.2 | 41 | 35.0 | 21 | 32.8 | 448 | 28.1 |
| Learning strategies | 124 | 14.9 | 11 | 13.3 | 35 | 7.0 | 5 | 4.3 | 15 | 23.4 | 190 | 11.9 |
| Relaxation | 53 | 6.4 | 12 | 14.5 | 35 | 7.0 | 7 | 6.0 | 2 | 3.1 | 109 | 6.8 |
| Session 8 | ||||||||||||
| Memory aids | 56 | 7.0 | 8 | 10.0 | 43 | 10.0 | 38 | 15.3 | 11 | 5.0 | 156 | 8.8 |
| Diary use | 328 | 40.8 | 30 | 37.5 | 179 | 41.7 | 91 | 36.5 | 83 | 37.9 | 711 | 39.9 |
| Pacing | 102 | 12.7 | 18 | 22.5 | 68 | 15.9 | 54 | 21.7 | 54 | 24.7 | 296 | 16.6 |
| Positive attitude | 46 | 5.7 | 7 | 8.8 | 16 | 3.7 | 5 | 2.0 | 5 | 2.3 | 79 | 4.4 |
| Session 9 | ||||||||||||
| Problems with external aids | 287 | 38.3 | 36 | 46.2 | 165 | 39.8 | 143 | 53.4 | 77 | 39.3 | 711 | 41.5 |
| Memory aids use in future | 30 | 4.0 | 2 | 2.6 | 12 | 2.8 | 15 | 5.6 | 6 | 3.1 | 65 | 03.8 |
| Case studies: Making Life Easier | 93 | 12.4 | 20 | 25.6 | 79 | 18.7 | 38 | 14.2 | 49 | 25.0 | 279 | 16.3 |
| Useful tips | 13 | 1.7 | 1 | 1.3 | 11 | 2.6 | 2 | 0.7 | 9 | 4.6 | 36 | 2.1 |
| Session 10 | ||||||||||||
| Overview of sessions | 170 | 20.5 | 0 | 0 | 126 | 26.1 | 113 | 25.9 | 43 | 18.4 | 452 | 22.8 |
| Favourite strategies | 78 | 9.4 | 0 | 0 | 56 | 11.6 | 64 | 14.6 | 35 | 15.0 | 233 | 11.7 |
| Reflecting on memory problems | 132 | 15.9 | 0 | 0 | 91 | 18.8 | 71 | 16.2 | 50 | 21.4 | 344 | 17.4 |
| Group feedback | 92 | 11.1 | 0 | 0 | 36 | 7.5 | 33 | 7.6 | 49 | 20.9 | 210 | 10.6 |
Session 1 covered things people forgot (e.g. where they parked their car) and the strategies they used to overcome this. Site 4 spent a relatively high proportion of time discussing strategies that people used to overcome problems (28.7% compared with just less than 20% in the other sites). In session 2, the focus was on memory storage and the processes involved in memory. Site 1 spent a relatively high proportion of time on memory storage and site 4 spent time on the processes involved in memory. In session 3, the session-specific content was distributed approximately equally between different types of attention, the attention exercise and techniques to improve attention. In site 2, more time was spent on techniques to improve attention and less on discussing types of attention and types of distractors, compared to the other four sites. Most session-specific time in session 4 was spent on story recall using the five Ws and the H (who, what, when, where, why and how). In session 5, one-third of session-specific content was on categorisation. Session 5 also included material from session 6 from one group at site 3. In session 6, just under one-third of time was spent on the levels of processing exercise, with about one-fifth of time spent on visual imagery. Little time was spent on first-letter cues or rhyming. The main focus of session 7 was on the PQRST (preview, question, read, state, test) technique and then the ‘little-and-often’ strategy. Session 8 was mainly focused on diary use, which accounted for almost 40% of the time. Site 4 included more discussion of memory aids in general and site 5 included more on pacing, relative to the other sites. The problems with the use of external memory aids were the most frequent component of session 9. Site 4 included a relatively high proportion of time on problems with external memory aids and sites 2 and 4 both included a relatively high proportion of time on the case study on making life easier by using external memory aids. Session 10 was mainly an overview of the material covered in the manual and reflecting back on problems discussed in session1. Site 4 spent a relatively high proportion of time on group feedback. Overall, some material from earlier sessions was repeated in subsequent sessions, but only one group included material that was designated for later sessions.
The distribution of AP and participant activities is shown in Table 16. The APs were engaged in activities on 49% of observations and participants were engaged in activities on 51% of observations. The main activity for APs was presenting and discussing strategies (19%); this was consistent across sites. The second most frequent activity was presenting and discussing educational material (12%), which was also consistent across sites. The only difference between sites was that APs at site 2 had a lower proportion of providing an explanation of attention and memory (0.9%) than at other sites (overall average, 7%). The main activities observed in participants were discussing and filling in educational material (17%), followed by discussing strategies (16%). These were consistent across sites. The distribution of AP and participant activities across sessions is shown in Appendix 12, Table 41.
| Activity | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | N | % | |
| AP activity | ||||||||||||
| Discussing/conducting administrative aspects | 179 | 2.3 | 5 | 0.9 | 70 | 1.4 | 58 | 1.9 | 19 | 1.1 | 331 | 1.8 |
| Presenting/discussing educational material | 967 | 12.3 | 63 | 11.6 | 557 | 11.5 | 330 | 11.0 | 157 | 8.7 | 2074 | 11.5 |
| Presenting/discussing strategies | 1483 | 18.9 | 114 | 21.0 | 713 | 14.7 | 513 | 17.1 | 231 | 12.8 | 3054 | 16.9 |
| Providing explanation | 521 | 6.6 | 5 | 0.9 | 311 | 6.4 | 317 | 10.6 | 110 | 6.1 | 1264 | 7.0 |
| Facilitating discussion | 360 | 4.6 | 21 | 3.9 | 251 | 5.2 | 190 | 6.3 | 64 | 3.6 | 886 | 4.9 |
| Providing feedback | 239 | 3.0 | 15 | 2.8 | 185 | 3.8 | 146 | 4.9 | 54 | 3.0 | 639 | 3.5 |
| Summarising | 134 | 1.7 | 4 | 0.7 | 48 | 1.0 | 30 | 1.0 | 14 | 0.8 | 230 | 1.3 |
| Paraphrasing | 83 | 1.1 | 12 | 2.2 | 66 | 1.4 | 56 | 1.9 | 10 | 0.6 | 227 | 1.3 |
| Discussing the CRAMMS trial research process | 58 | 0.7 | 0 | 0 | 8 | 0.2 | 19 | 0.6 | 6 | 0.3 | 91 | 0.5 |
| Discussing diagnosis/living with MS | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | 3 | 0 |
| Social chat | 39 | 0.5 | 2 | 0.4 | 20 | 0.4 | 10 | 0.3 | 1 | 0.1 | 72 | 0.4 |
| Participant activities | ||||||||||||
| Discussing/conducting administrative aspects | 13 | 0.2 | 0 | 0 | 11 | 0.2 | 8 | 0.3 | 1 | 0.1 | 33 | 0.2 |
| Discussing/filling in educational material | 1294 | 16.5 | 143 | 26.4 | 897 | 18.5 | 521 | 17.4 | 512 | 28.4 | 3367 | 18.7 |
| Discussing strategies | 1230 | 15.7 | 86 | 15.9 | 759 | 15.7 | 454 | 15.1 | 310 | 17.2 | 2839 | 15.7 |
| Asking for information related to intervention content | 41 | 0.5 | 1 | 0.2 | 33 | 0.7 | 30 | 1 | 8 | 0.4 | 113 | 0.6 |
| Asking for information not related to intervention content | 6 | 0.1 | 0 | 0 | 4 | 0.1 | 3 | 0.1 | 0 | 0 | 13 | 0.1 |
| Describing problems | 577 | 7.3 | 40 | 7.4 | 426 | 8.8 | 167 | 5.6 | 167 | 9.3 | 1377 | 7.6 |
| Describing emotions and coping strategies | 93 | 1.2 | 5 | 0.9 | 45 | 0.9 | 16 | 0.5 | 22 | 1.2 | 181 | 1.0 |
| Discussing MS diagnosis/hospital visit | 196 | 2.5 | 4 | 0.7 | 165 | 3.4 | 45 | 1.5 | 68 | 3.8 | 478 | 2.6 |
| Discussing the CRAMMS trial research process | 6 | 0.1 | 0 | 0 | 8 | 0.2 | 6 | 0.1 | 6 | 0.3 | 13 | 0.1 |
| Social chat | 328 | 4.2 | 21 | 3.9 | 262 | 5.4 | 80 | 2.7 | 43 | 2.4 | 734 | 4.1 |
| Missing | 5 | 0.1 | 1 | 0.2 | 8 | 0.2 | 4 | 0.1 | 1 | 0.1 | 19 | 0.1 |
Discussion
The APs were trained to deliver the intervention and received supervision from clinical psychologists and peer supervision to ensure the integrity of the intervention. Most participants received the treatment as intended and the number of sessions delivered was consistent between sites. The reasons for non-attendance related to unavoidable constraints on time, such as conflicting appointments, and were similar across sites.
The video-recordings indicated that, generally, most time was spent on the content of the manual; the distribution of time was consistent with what was expected. All components of treatment were observed. There was also overall consistency between sites in the content of the intervention that was delivered. The slight differences in proportions observed in site 2 recordings may be because fewer sessions were recorded here. This was partly because three APs covered the site and so there were three first groups to be run by an AP and partly because of a misunderstanding about the recording requirements. Other variations between sites were minor and are likely to reflect issues raised by participants and a response to the requirements of the group.
The limitation of the video analysis was that not all sites recorded all the sessions that were expected to be recorded, especially site 2. This may have masked minor discrepancies between sites. However, based on feedback obtained during monthly peer and supervisor meetings with the APs, there was no indication that the unrecorded sessions would be markedly different from the recorded ones. Some sessions were incomplete due to the camera battery running out or having no charging point at the group venue. These incomplete sessions could not be analysed as the end of the session was missing in each case; therefore, the proportions of time allocated to content could not be ascertained. However, these partial recordings occurred at all sites and across sessions so it is improbable that their omission has influenced the results.
There is a possibility that the presence of the camera may have influenced the way the APs delivered the intervention, but, as most sessions were recorded, this effect was minimised. The coding was done by one observer, but supported by good evidence of inter-rater reliability. In addition, in one of the pilot studies, there was good inter-rater reliability of a similar coding system (97% agreement). 130 In addition, using only one observer may have increased the consistency in the interpretation of the codes used. All analyses were completed after all treatments were completed. This meant that omissions in recording were not identified until it was too late to rectify the problem. In addition, had any major discrepancies occurred between sites, it was too late to conduct further training with the APs to ensure greater consistency between sites. Observing the videos of the intervention at each site, as each group was completed, would have helped to ensure that recordings were more complete and could have minimised any discrepancies between sites.
Attendance at groups was comparable to the pilot study60 (7.9 sessions) and slightly better than a comparable study for people with traumatic brain injury95 (6.3 sessions). The higher attendance rates for people with MS may reflect that fewer had had any previous cognitive rehabilitation compared with those with traumatic brain injury, who may also have had behavioural problems that may lead to worse attendance.
A similar assessment of treatment fidelity using video-recordings was conducted for the ReMemBrin (Rehabilitation of Memory following traumatic Brain Injury) trial95 and the pilot study,131 but different coding systems were used. Therefore, direct comparisons are not possible. However, the pilot study seemed to have less emphasis on take-home activities than in the CRAMMS trial (3% vs. 17% of time). Overall, these studies also established that APs were able to deliver the intervention according to the manual.
The results support confidence that the cognitive rehabilitation delivered in the CRAMMS trial was consistent with that described in the treatment manual. Therefore, the results reflect the effectiveness of treatment as described in the treatment manual.
Chapter 5 Qualitative feedback interviews
Rationale
The CRAMMS trial was a mixed-methods RCT, so that, although the primary outcomes of the trial were quantitative, there was a nested qualitative study to unpack some of the subtle experiential and phenomenological aspects of participating in a cognitive rehabilitation trial. The use of such qualitative studies in RCT designs is becoming more commonplace,133 particularly when the RCT is designed to evaluate the effectiveness of a complex intervention.
Aims
The aims were to examine and compare feedback from participants who took part in the CRAMMS trial. Participants’ perceptions of the RCT and overall outcomes were examined and compared between those who were in the intervention group and those who were in the control group. Specifically, from both groups, we wanted to:
-
understand the care participants received in relation to their cognitive problems prior to enrolling in the trial
-
assess whether or not they perceived any changes to their cognitive problems after joining the trial.
For those in the intervention group, we also wanted to:
-
explore participants’ views on the perceived mechanism of change (if any) of their cognitive problems.
Methods
Epistemological position
Participant perspectives provide us with unique access to understand their thoughts, feelings and experiences about the services they received and the impact this had on their daily life. With respect to the provision of cognitive rehabilitation services (or lack thereof), we assumed that a ‘truth’ about its effectiveness could be approximated through the investigation of patient feedback. This is in line with a critical realist epistemological position. This position allows for the acceptance of the existence of a reality independent of human conception; human experience can know aspects of this reality, but it is fallible. 134 Thus, we acknowledge that the self-reports collected as part of this qualitative study are fallible; however, they can be viewed as an approximation of reality until better information is available.
Sampling
We aimed to recruit participants from all participating sites to obtain a wide range of views and experiences. We recruited eight participants (four from the intervention group and four from the control group) each from four of the sites to interview, as planned. However, as an additional site was added part way through recruitment, we included four interviews from this site: two from the intervention and two from the control group.
Participants were selected based on the maximum variation sampling technique135 to recruit individuals with different self-reported cognitive impairment levels (based on the EMQ) and self-reported demographic characteristics. Once an initial group of participants was recruited, subsequent participants were selected on the basis of how ‘different’ they were from the initial group, in order to have a diverse sample.
Sample size
There is no agreed sample size calculation for qualitative studies, so we adopted a pragmatic approach to interview as many participants as we could over a defined time frame, and based on our available resources. However, in deciding what was ‘pragmatic’, we took account of the sample sizes reported in other similar studies. For a similar trial of memory rehabilitation in people with traumatic brain injuries,95 we interviewed 32 participants (16 from the control group and 16 from the intervention group), and this provided us with sufficient data to answer our research questions (which were similar to our current research questions). We also sought information from two meta-syntheses: (1) of cognitive rehabilitation for people with MS,136 which had sample sizes of between 31 and 98 participants (it must be noted that some of the larger studies used focus groups and content analyses of written texts) and (2) of group-based memory rehabilitation,137 which had sample sizes of 10 to 38 participants. Therefore, we were confident that with 36 participants we would have rich data to allow us to explore with sufficient depth the areas we were interested in. We did have the facility to recruit a few more participants if we were not satisfied with the richness of the data from the 36 interviews. We did not look for data saturation, but our conceptual depth criterion was one of sufficiency. 138 We operationalised conceptual depth based on work by Nelson138 in terms of (1) the range of evidence (or quotations from different respondents), (2) a complex network of connection between themes, (3) subtlety in concepts, (4) resonance with extant literature and (5) external validity. 138 We aimed to obtain sufficient data within each cell of our framework matrix that related to each of our domains (or ‘conceptual categories’).
Procedure
Participants who had consented to be contacted about the qualitative study from the CRAMMS trial were considered for inclusion. We conducted one-to-one feedback interviews with participants enrolled in the trial within 2 months of completion of the 6-month follow-up assessment. To reduce social desirability bias, the feedback interviews were conducted by two individuals who were not involved with the assessments or intervention aspects of the trial.
The interviewers were a PhD student (Olga Klein) who was researching cognitive rehabilitation implementation in MS, and a carer of a person with MS. We decided to include a patient and public involvement (PPI) member as an interviewer based on our PPI group’s feedback to consider more ambitious ways to include PPI in our research. We also wanted to determine how the interviews conducted by both parties differed. Both the PhD student and the PPI interviewer were trained to conduct the interviews and use the interview schedule by one of the co-applicants (RdN).
Two semistructured interview schedules were developed based on extant literature and in relation to the content of the groups that participants attended. The interview schedules focused on the following domains:
-
general experienced cognitive problems
-
advice received about managing cognitive problems (before and during the trial, but not as part of the trial)
-
treatments received to manage cognitive problems from the point participants were recruited to the point of the interview
-
strategy use before the trial
-
changes of cognitive abilities in the past 6 months (if any)
-
what would have made the participants’ experience of being involved in the trial better.
The interview schedule for the control group included two additional specific aspects:
-
whether or not the participants had received any form of cognitive rehabilitation during the trial
-
participants’ experience and thoughts on being part of the control group.
The interview schedule for the intervention group focused on some specific aspects:
-
most and least useful aspects of the cognitive rehabilitation programme
-
strategy use after the trial, and impact on daily life activities
-
participants’ experience and thoughts on being part of the intervention group
-
overall experience of the cognitive rehabilitation programme.
The interviews were conducted over the telephone. Before the interview began, the interviewers reminded the participants of the purpose of the interviews, reassured them about confidentiality and told them that they could pause the interview or choose not to answer questions if they did not wish to. They were also told that the interview was going to be audio-recorded and transcribed verbatim. Transcription was conducted by a professional transcribing service. Transcripts were checked against the original audio-recording and any errors were corrected.
Analysis
Framework analysis, an atheoretical form of thematic analysis, was used to analyse the data. This method has become an increasingly popular approach in medical and health research. 139 In framework analysis, the data from the transcripts are mapped onto a predefined framework. To establish the analytic framework, four transcripts were chosen, two from the intervention and two from the control group, for initial coding by two independent researchers. They read and re-read the transcripts a few times to form initial impressions of the data. The left-hand margin was used to describe each segment of data (which was underlined) with a code or codes, and the right-hand margin was used to record more detailed notes and ideas about that segment of data. We underlined and annotated key words, parts of sentences or whole paragraphs. We followed Gale et al. ’s139 guide to framework analysis.
Following the initial coding of the first four transcripts, the PhD student met with one of the co-applicants (RdN) to discuss the codes and to check whether or not the coding was consistent and comprehensive. Disagreements were resolved through discussion. The remainder of the interview transcripts were then analysed in the same vein.
Once all the coding was completed, Olga Klein and Roshan das Nair met again to confirm the thematic structuring of the data. Again, any disagreements were resolved through re-examining the data and its fit in the framework matrix and through discussion. Finally, Olga Klein and Roshan das Nair met with our PPI co-applicant (and co-interviewer) to share the coding and thematic structure and to have a general ‘sense check’ of our interpretation and structuring of the data. Some minor modifications were made as a result of this consultation.
Quality considerations
We followed published criteria in relation to ensuring rigour in our analysis and the quality of our presentation. 140 We have fully described our approach and methods for replicability, we have considered all aspects of every transcript and included ‘negative’ cases where we have found them, and we used multiple coders and checks to ensure that the coding was consistent and did not privilege any one point of view.
Results
Details of interview participants are given in Table 17.
| Interview ID | Interviewer | Site | Group | Gender | Ethnicity | Years of education (n) | Year diagnosed | Type of MS |
|---|---|---|---|---|---|---|---|---|
| 2 | SE | 1 | Intervention | Female | White | 11 | 2007 | Secondary progressive |
| 3 | OK | 1 | Intervention | Male | White | 18 | 1992 | Relapsing–remitting |
| 4 | OK | 1 | Intervention | Female | White | 14 | 2015 | Relapsing–remitting |
| 5 | OK | 1 | Intervention | Female | White | 11 | 2008 | Relapsing–remitting |
| 6 | SE | 2 | Intervention | Female | White | 13 | 2003 | Relapsing–remitting |
| 7 | SE | 2 | Intervention | Female | White | 13 | 1986 | Secondary progressive |
| 8 | OK | 2 | Intervention | Female | White | 14 | 2003 | Relapsing–remitting |
| 9 | SE | 2 | Intervention | Female | White | 13 | 2007 | Relapsing–remitting |
| 10 | SE | 3 | Intervention | Female | White | 17 | 2003 | Secondary progressive |
| 11 | SE | 3 | Intervention | Female | White | 17 | 1987 | Relapsing–remitting |
| 12 | SE | 3 | Intervention | Female | White | 18 | 1996 | Relapsing–remitting |
| 13 | SE | 3 | Intervention | Female | White | 35 | 2010 | Relapsing–remitting |
| 14 | OK | 4 | Intervention | Female | White | 10 | 1987 | Secondary progressive |
| 15 | OK | 4 | Intervention | Male | White | 20 | 1990 | Relapsing–remitting |
| 16 | OK | 4 | Intervention | Male | White | 16 | 2013 | Relapsing–remitting |
| 17 | OK | 4 | Intervention | Female | White | 16 | 2010 | Relapsing–remitting |
| 18 | OK | 5 | Intervention | Female | White | 11 | 1998 | Secondary progressive |
| 19 | OK | 5 | Intervention | Male | White | 12 | 2000 | Relapsing–remitting |
| 21 | SE | 1 | Control | Male | White | 11 | 2012 | Primary progressive |
| 22 | SE | 1 | Control | Female | White | 12 | 2009 | Secondary progressive |
| 23 | SE | 1 | Control | Female | White | 16 | 2011 | Relapsing–remitting |
| 24 | OK | 1 | Control | Male | White | 17 | 2015 | Relapsing–remitting |
| 25 | SE | 2 | Control | Male | White | 12 | 2012 | Relapsing–remitting |
| 26 | SE | 2 | Control | Male | White | 12 | 1985 | Relapsing–remitting |
| 27 | OK | 2 | Control | Female | White | 15 | 1992 | Secondary progressive |
| 28 | SE | 2 | Control | Female | White | 17 | 2013 | Relapsing–remitting |
| 29 | SE | 3 | Control | Female | White | 10 | 1986 | Relapsing–remitting |
| 30 | OK | 3 | Control | Female | White | 11 | 2014 | Primary progressive |
| 31 | OK | 3 | Control | Male | White | 11 | 2009 | Relapsing–remitting |
| 32 | OK | 3 | Control | Female | White | 13 | 2011 | Relapsing–remitting |
| 33 | OK | 4 | Control | Female | White | 13 | 2011 | Secondary progressive |
| 34 | OK | 4 | Control | Male | White | 16 | 2004 | Relapsing–remitting |
| 35 | OK | 4 | Control | Female | white | 11 | 1999 | Relapsing–remitting |
| 36 | OK | 4 | Control | Female | white | 11 | 2008 | Secondary progressive |
| 37 | OK | 5 | Control | Male | white | 12 | 2011 | Relapsing–remitting |
| 38 | OK | 5 | Control | Female | white | 12 | 2012 | Secondary progressive |
Thirty-six participants, 24 women and 12 men (18 from the intervention and 18 from the control groups), were interviewed. All self-identified as being of white British ethnicity. The mean age of the participants was 50 years (SD 9.13 years, range 26–70 years). They had an average of 14 years of education (SD 4.55 years, range 10–35 years). Two-thirds of participants had relapsing–remitting MS [n = 23 (64%)], just under one-third had secondary progressive MS [n = 10 (28%)] and three (8%) had primary progressive MS. In terms of time since MS diagnosis, this varied from 3 to 32 years. Interviews lasted between 6 and 59 minutes, with most interviews lasting ≈30 minutes.
In the following sections, we outline the various themes elicited from the data, and offer illustrative quotations from the interviews. Each quotation is followed by a unique participant identification (ID) number. When relevant, we have provided a segment of the conversation between the participant (P) and the interviewer (I). As per our methods, the themes map onto the initial framework that relate to the specific foci of our research, but additional themes were added when we felt that there were substantial data to support these themes. Each theme has a number of subthemes. These subthemes relate to various aspects of the theme, and some subthemes are broken down further into subtheme categories (when data were available) to provide further nuance to the subtheme. Table 18 lists these themes, subthemes and subtheme categories.
| Theme | Subtheme | Subtheme categories |
|---|---|---|
| 1. Management of cognition before the CRAMMS trial | 1.1 Advice received | 1.1.1 None |
| 1.1.2 External memory aids | ||
| 1.1.3 Information about MS on websites/leaflets/booklets | ||
| 1.2 No cognitive rehabilitation | ||
| 1.3 Self-generated strategy use | ||
| 2. Perceived cognitive changes | 2.1 Improved | |
| 2.2 Stayed the same | ||
| 2.3 Worsened | ||
| 3. Perceived mechanism of change of cognitive function for intervention group | 3.1 Increased strategy use | 3.1.1 Repetition and habit formation |
| 3.1.2 Improved confidence and mood | ||
| 3.1.3 Selective and adapted strategy use | ||
| 3.1.4 Increased activity in daily life | ||
| 3.2 Psychoeducation | 3.2.1 Acceptance of cognitive problems | |
| 3.2.2 Theoretical understanding of cognition | ||
| 3.3 Effect of group format | 3.3.1 Being understood | |
| 3.3.2 Sharing of experiences and ideas | ||
| 3.3.3 Social aspect | ||
| 3.3.4 Altering perspectives | ||
| 3.4 Workbook | 3.4.1 Enabling homework activities | |
| 3.4.2 Useful in combination with group sessions | ||
| 3.4.3 Useful for notes and reminder of contents | ||
| 3.4.4 Used as reference after intervention | ||
| 4. Additional reasons for adherence to intervention and trial | 4.1 Improvements seen in daily life activities | |
| 4.2 Positive appraisal of therapist skills | ||
| 4.3 Suitability of venues | ||
| 4.4 Having cognitive assessments | ||
| 4.5 Possibility of receiving cognitive rehabilitation | ||
| 4.6 Altruism | ||
| 5. Possible improvements | 5.1 Refresher/booster sessions | |
| 5.2 Option to download workbook | ||
| 5.3 Adding mindfulness to help focus? |
Management of cognition before the CRAMMS trial
Advice received
None
The majority of participants had not received any advice about managing attention and memory problems:
No I haven’t really only – the only time I’ve ever talked to anybody about it was when I went to the meetings.
ID 02
No, not really. I’ve just been carrying on with my day-to-day way I deal with things.
ID 03
One interviewee mentioned that they had seen a neuropsychologist about 10 years ago for cognitive tests, but not rehabilitation:
Just a test, yep, to say you’ve not imagined it.
ID 10
Participants believed that they did not receive cognitive rehabilitation for a few reasons. One reason was that they did not initially see cognitive problems as being directly associated with MS; therefore, they did not bring it up in consultation with health-care practitioners:
I just always thought I had a bad memory. From when I was younger I just always thought I had a bad memory.
ID 36
Another reason was that other symptoms, such as fatigue and mobility limitations, were the focus of annual reviews and these symptoms were managed first, whereas cognitive problems were ignored.
Mh, I am going to the hospital quite regularly, but it’s looking at my lung functioning not my memory.
ID 23
External memory aids
Some participants who brought up the issue of having cognitive problems at annual clinical reviews or hospital visits did receive advice on managing their cognitive problems, such as keeping a diary/calendar and using Post-it® notes (3M, Cynthiana, KY, USA). This advice was mostly focused on external memory aids rather than internal memory aids:
. . . very, very little [advice on cognitive rehabilitation]. I know that I have been given advice, that was all, erm just advice that says keep a diary, a to-do list and take notes from your fridge [tape notes to your fridge]. It was just, you know, er A4 side of what things might help with memory.
ID 10
One participant stated that she had received advice from a MS nurse specialist and an information pack from day 1 when she was diagnosed (ID 09).
Information about cognitive problems in multiple sclerosis on websites/leaflets/booklets
Some participants said that they were signposted to relevant information in the form of leaflets, booklets or online resources:
Erm, just a booklet really, erm, from the MS nurse on memory and, erm, concentration. One of the m – m – is it MS society?
Yeah.
Like leaflet things.
Yes, yes and, erm, have you – have you managed to look through that and follow any of it? Has any of it been useful?
Erm, it is when I can read properly ‘cause [. . .] You know when I’m reading to you [. . .] I tend to either read the same lines or soon as I’ve read it, it’s like, ‘what’ve I just read?’
Yeah, yeah.
So you – you’re constantly going back on yourself. So things don’t tend to sink in.
However, as this interaction suggests, merely providing workbooks or information about memory aids without additional support is unlikely to be helpful for people with MS, particularly because of their cognitive problems.
No cognitive rehabilitation
Besides the information given about external memory aids, no participants received any other form of cognitive rehabilitation:
Well no, b– but, er, apart from that, my – my usual care is just my annual review . . . Where the doctor sort of talks to me about how I’m feeling and sort of has a bit of a look at my sort of, um, motor function, if you like. Watch me walk, look you know . . . Checks my reflexes and what have you. And – and that, that’s the only sort of usual care apart from – from going to the – on the CRAMMS [. . .] that I received.
ID 02
No, not a single thing.
ID 16
Self-generated strategy use
Generally, the majority of participants used external memory aids before they were recruited to the trial. These strategies included writing lists, keeping a calendar with appointments, and asking family members or friends to remind them of appointments or tasks they needed to complete. These tasks were self-generated, in that participants decided to try them out themselves:
Well, as I said, I did already, um, have – use lists for everything [. . .] And, erm, just to not get upset by it really [. . .] (and look after) myself.
ID 02
You know, erm, yeah. I write on my hand, if there’s some emergency, if an appointment I have to keep physically.
ID 10
Participants changed tack when they found that some of the strategies they were accustomed to were no longer providing them with the results they expected:
So, ‘cause that [writing lists] was sort of starting to not work, erm, I’ll say to my friend I’m like, ‘Can you remind me?’
ID 22
Perceived cognitive changes
Improved
All six interviewees who stated that their cognitive abilities had improved over the previous 6 months were from the intervention group:
I think I’ve improved.
ID 12
I say that the CRAMMS has helped.
ID 07
I think that actually since I’ve been on – on the CRAMMS sort of meetings, they’ve [cognitive problems] improved.
ID 02
Stayed the same
Some participants from both the control and intervention groups stated that their cognitive abilities had stayed the same over the previous 6 months. However, the numbers of those in the intervention and control groups endorsing this view differed: 11 participants from the intervention group and six participants from the control group. Furthermore, although participants in the intervention group reported that their cognitive abilities had stayed the same, they did feel that they managed better (using strategies) as a result of the intervention:
No, I don’t think so. I don’t think they’ve [cognitive problems] got any better or any worse, about the same. It’s just that I’ve put strategies in place to help me with those things.
ID 04
No. I don’t know. I don’t know [whether cognitive problems have got better or worse]. Since I’ve been able to do, since being on the course thing, . . . I’m able to remember . . . that I just could not before . . . so that’s really good.
ID 14
I think I’m remembering a bit more than I was before. But a lot of it is just writing things down, doing different things.
ID 16
What is interesting about these quotations, all from participants from the intervention group, is that although they reported not knowing or not thinking that their cognitive problems had reduced, they do report improvements in daily life (‘I’m able to remember’, ‘I’m remembering a bit more than I was before’). However, they minimise the impact of these changes because they are using cognitive strategies to compensate for their deficits (‘It’s just that I’ve put strategies in place’, ‘But a lot of it is just writing things down’). Thus, they do not see this as improvement.
However, by contrast, those in the control group only reported not seeing any change over time:
I don’t think so, no. It might be slightly worse but nothing major. I’ve not noticed anything major.
ID 32
No, about the same.
ID 35
Actually, they probably stayed the same, to be honest.
ID 28
Worsened
Thirteen participants stated that they thought that their attention and memory had worsened; of these, all but one were in the control group:
. . . I’m forgetting more and more.
ID 31
Yes, it’s definitely got worse, yeah.
ID 36
Er, yeah, erm, my memory is getting worse definitely. I forget things within, within moments really.
ID 25
Many of these participants used words like ‘definitely’ to indicate that they had no doubt that their cognitive abilities had deteriorated. One person from the intervention group reported a worsening of their cognitive abilities:
I think they [cognitive problems] have got worse. [. . .] I am struggling with things, like I said, at work. And I am being now – I’m being performance managed because I am struggling because of my memory.
ID 08
Perceived mechanism of change of cognitive function for intervention group
For those who felt that there was an improvement in their cognitive ability or reduction in their cognitive problems, we were interested to know what could have caused these changes. All these participants were in the intervention group. Participants referred to increased strategy use; improved awareness of their cognitive problems (psychoeducation) and, thereby, improved acceptance; the positive effects of the group; and the workbook as potential causes of the positive changes they observed. We outline each of these perceived mechanisms.
Increased strategy use
All participants in the intervention group reported that they were using significantly more strategies as a result of the cognitive rehabilitation programme. Although several were using external memory aids prior to joining the programme, the majority reported that they were using considerably more internal memory aids after the intervention, to help them to memorise things and forget less. Some of the strategies that participants adopted were putting things in the same place (e.g. keys, mobile phone, wallet), using descriptions when they could not recall the name of an object, using pictures to recall events or things, grouping items on a grocery list (vegetables, fruits) and making little stories about things they wanted to remember.
We investigated further why they were using more strategies after having attended the intervention groups.
Repetition and habit formation
Participants highlighted that the repetition of using different strategies helped them to keep the strategies ‘in their head’, resulting in a behaviour change:
To try and help myself ‘cause they’re sort of in your head, you don’t forget them. Because we did – we did them for so long – we did them week after week for 10 weeks. And – and once you’ve done things so often you sort of use the same – or –or similar technique and – and new ones. You add to them and – and then you go back and review them. But – but by the time you’re done with it – it – it’s set into your long-term memory so you don’t forget it so it – you – it – it’s just there in your head; well, this is the way my mind works [. . .].
ID 02
I think it’s some of the techniques, repeating things over or going over things over and over again and making stories. I just tend to go over it in my head so after a while it tends to sink in a lot more. It’s just through techniques I am using a lot more. That’s helping a lot.
ID 16
Behaviour change was also attributed to habit formation:
Yeah, I try and put things in the same place when I, so when I go to bed at night, I put my phone in one place, my wallet in a different place. But they’re always in the same place so in the morning I know where I’m supposed to be looking for them and picking them up. Things like that I think are helping me.
ID 15
Improved confidence and mood
Some participants attributed their increase in strategy use to improved confidence and mood. Participants reported having an increase in confidence to tackle different tasks in daily life. One participant stated that she was nervous when she found out that she was assigned to the intervention group because she was never a ‘very confident person’. However, when she felt that she was among the best in the intervention group at performing certain tests (as part of the intervention) and she felt that she was improving, her self-confidence developed:
Yeah, it did boost my self-esteem so much and it made me feel a lot more positive about; it did help me. Yeah, I’ve got memory problems, but that does not mean, you know, yeah I’m (almost forget) people, yes I’m useless. It just means, yeah, erm, I can say, er, I have psoriasis on my scalp [. . .] You know, it’s just another little hiccup in my life. My confidence has increased so much.
ID 10
Yes, definitely. That’s [memory exercises and using different strategies] helped me a lot, yeah. I feel a lot more confident in a lot of things now trying to solve things and stuff like this [participant talking about feeling more confident to approach other problems he comes across in daily life].
ID 16
For some participants, the improved confidence was not directly attributed to attending the intervention, but because of changes they had made to their lifestyle, such as exercising:
I feel I am more confident because I didn’t used to be very active before. But I do go to the gym once a week. Even though I’m not very mobile, they’ve set me up with a session because I’ve improved in certain areas in my session at the gym. I’ve actually asked them if there’s something else I can try out. So I’m trying to better myself all the time going to the gym. Now that the weather is better, I do tend to just have a little walk round . . . but I tend to use my walker and just walk round once a day, just a bit of exercise, and that helps me gain confidence as well.
ID 05
Participants not only stated that they felt more confident, but they also experienced fewer negative feelings as a result of forgetting, as the strategies they learned helped them to remember more:
Yeah, I found it [the intervention] really, really useful. To make me feel less worried about th– I’m – I’m not saying that I worried unduly, but I did have some concerns. I think it really eased my concerns. [. . .] I’ve been better really because I’ve been less sort of anxious, if you like.
ID 02
Selective and adapted strategy use
Participants noted that they adapted some of the strategies they learnt to make these strategies work for them. In addition, some participants noted that some of the strategies were not as useful as others, and they would only use the strategies that suited them:
And I have taught myself, different la– erm, language strategies of remembering words and when I don’t have the precise words, huge vocabulary, I can carry on talking and come up with some of it that I can in moderate enough to be and . . .
ID 10
[. . .] It’s about putting things in boxes and trying to remember them in a list. So, which is about learning the different techniques – or the best techniques that work for yourself. I mean not everybody, I don’t suppose everybody using the same techniques when we have left the class. I just used the ones that I have found that were really good for me like, you know, chunking and the diary and you know, putting stuff on my phone and then doing the same things with the keys and the bags and then if I have got something, you know when the phone rings and I have got to remember to give someone a message then I write it down and obviously pass the message on.
ID 18
Some strategies were not considered useful for some participants:
Some of the techniques that we were talking about for me didn’t work. So I put those on the side burner.
ID 03
No, there was nothing I didn’t like about them [group sessions], they were all fine and, although I may not do some of the things, it’s probably I discarded them at the time because I didn’t think they would help me or I didn’t see them being useful for me.
ID 17
Psychoeducation
Participants commented that the intervention provided them and their families with information and support to accept, better understand and cope with their cognitive problems.
Acceptance of cognitive problems
Some participants reported feeling that they were ‘going mad’ because they noticed their cognitive problems but were not certain what caused them, and others reported that their family did not take them seriously when they shared their concerns about their cognitive problems:
Erm, because I have accepted it [cognitive problems] so I don’t get frustrated, so I don’t use the energy for frustration. And to be able to accept it, I think you need to be able to go through a process which we know. But if I know what’s wrong, I can then accept, I can treat and then I can sort of move on with my life; where[as], other people, they don’t want to know; out of sight, out of mind. I don’t really want to know, but I need to know so I can address my issues and problems.
ID 13
This participant highlighted the importance of knowing what the problems were so that she could accept them, and then take the necessary action.
One participant reported that only his wife knew about his cognitive problems and that he had tried to hide these problems from his family and friends, prior to receiving the intervention:
No, it’s not the case no more. I’m quite open with it [cognitive problems] now and my wife said I’m getting better anyway with remembering things.
ID 16
Another participant talked about how her husband’s behaviour had changed after he found out that she really did have cognitive problems:
Erm, the action if you like, he [husband] was sort of like more, ‘mm, yeah we do have a problem, do you?’ . . . So they [family] think you’re either making it up or you’re going over the top or to, to be invited to CRAMMS was very significant for me because then that showed my family I had a problem.
ID 7
Theoretical understanding of cognition
Participants stated that the theoretical session of the intervention was useful to them because it helped them to understand and visualise what was happening:
. . . I understand a bit more now why it’s [cognitive problems] happening and where it’s happening and stuff, yeah. It’s great to learn.
ID 16
[. . .] and also having theoretical background for the workbook. So, you know, for somebody, I think that’s what I’s trying to say, so the theoretical background was really interesting to me. Look at how we are actually, erm, are made and why we forget, why, erm, you know, why we need to practice, erm, and just reinforcing [. . .]
ID 13
Effect of group format
All participants reported that the group format was a very useful aspect of the intervention. Participants commented that sharing experiences and giving advice in a group setting was very helpful to them. In addition, learning about other people’s issues made participants feel relieved that they were not the only ones who experienced these problems, and they felt comfortable to share their experiences because they felt understood by the others in the group.
Sharing of experiences and ideas
Participants reported that the most valued aspect of being part of a group was that of being able to share experiences with others who were in a similar situation. Some such sharing related to the general experience of having cognitive problems, but, in some instances, participants also spoke about the value of sharing ‘ideas’ and offering and receiving ‘advice’ from the others:
The most useful thing to me was the opportunity to actually share experiences with other people. And other people, they would give a little bit of advice, you know, what they’ve come across themselves and share that experience with other people. And I think it was good for other people to be able to share their experiences with us. So it was just communicating with other people in the group, which I thought was really good. But that was one of the best things, being able to speak with other people.
ID 05
And it’s just nice to have people who have got the same disability just talking about their issues, talking about some things they have to go through. Often it was very humorous, very funny.
ID 03
Can’t tell you how helpful it was [being in a group], just to meet other people going through similar things, that we, we’ve all been there and we could all say to each other, ‘yeah I felt stupid but I found this way’, and, and, just sharing our ideas and stories was wonderful.
ID 10
And, um. . .but it was just sort of, you felt as though you’d got somebody there that you could talk to anything about, you know, somebody to call on and um [. . .] and help really. [. . .] Because I yeah – I – I would recommend it to anybody, I really would. I thought it [joining the group] was absolutely the best thing I could’ve done.
ID 02
Being understood
Being understood by others was a very important aspect for the participants, who sometimes felt that others who did not share their condition or problems did not understand how it felt to forget and not remember in specific situations:
As – as I say so that you don’t feel like you’re all on your own and you’ve got these problems and nobody understands.
ID 02
I think, in terms of the sessions themselves, it’s being with other people who are suffering from the same condition and being able to talk to them about the problem you have, particularly on the memory side, and the likeness that we all had in terms of our ability to remember things was quite marked.
ID 03
And hearing other people’s problems as well, it wasn’t just me.
ID 16
Social aspect
For some participants, the groups offered them a social space to connect with people and make new friends:
Yeah, I’ve met one of the ladies from the group. We’ve met for lunch and we’re trying to get the others [in the group] together as well, but I feel like we all know each other, to a limited extent, and it would be nice now to get to know the others better.
ID 04
And you can sort of relate to them and even pick up a phone now and we made good friends, erm [. . .] Also that has been good.
ID 06
Altering perspectives
A few participants voiced initial concerns over being with other people with MS who had more severe visible symptoms, because it worried them about their own future:
It was [useful to be with other people with MS], but it was distressing because they were different various, erm [. . .] So seeing other people which might have got tremors, it was, it’s new sort of experience to see people and thinking ‘oh, gosh, is that something that might come to myself?’ and people with more walk, err, difficult – walking difficulties.
ID 13
However, this participant also found a positive aspect to being with a more disabled group of people:
On the other side of the coin, you can look and think, ‘well, I am fortunate’ and so I suppose it’s double-edged really [. . .] So . . . it’s not a disadvantage, it’s, it’s, – it’s an eye opener and it, – it’s also, erm, don’t know, it’s, it’s rationalising and sort of thinking ‘well, you know that doesn’t necessarily have to be yourself’, and just so I suppose, do I isolate myself from the symptoms that could be, erm, but it’s useful to see how people manage when they have got worse symptoms, so it is, it is a positive really, in a way.
ID 13
Another participant also found this shift in perspective by recognising how well they were in comparison to others, but also recognising that, even with severe disabilities, people had learnt to cope:
I suppose there were a couple of people who were more disabled than I was (and so I hadn’t realised there was one gentleman who wasn’t able to write very well. But he was able to use his smartphone to remind himself about things, appointments he had to go to, so that really worked well for him.) Yeah, so it made me appreciate how well I am compared to a lot of people.
ID 04
Workbook
We enquired about how participants made use of the workbooks they were provided with as part of the intervention. Participants made use of the workbook in different ways.
Enabling homework activities
Overall, most participants found that the workbook enabled them to complete their homework activities:
It [workbook] was really helpful and obviously I came away with my little booklet which we did the little tests [homework activities] every week.
ID 02
[. . .] it was the workbook, it was ex, – sort of experimenting with workbooks, coming back with the homework, and also having theoretical background for the workbook.
ID 13
Useful in combination with group sessions
Although participants stated that the workbook was useful for the take-home activities, they felt that the workbook alone, in the absence of the group sessions, may not have been as useful. During the intervention, the workbook was useful as a reference point and served as a reminder of the content of the sessions:
It was good with the combination of the intervention as well, because you can see what you’re talking about.
ID 17
I think you need both the workbook and the intervention.
ID 04
Useful for notes and reminder of contents
While still in the intervention programme, participants also stated that they used the workbook to make notes about the session, and to refer back to what they had learned in each session, or to use it to prepare for upcoming sessions. They appreciated its simplicity:
[. . .] and, you know, sort of our answers and notes and what have you, and I’ve got that, you know, that I can to refer back to and – and I just, you know, even though it was quite simple things, I mean you need, you need simple things, you don’t want it to be too complex do you otherwise . . . You know, some people might, er, not understand it or they might find it difficult. But it was just really – I thought it was just really useful. Really, really useful.
ID 02
Yeah, at the time, I found it [workbook] particularly useful. Yes, because after the weekly session, I could get back and think about what we’d done over a couple of days and try and remember what we’ve been doing.
ID 15
So it was really good for something to refer to and know what you’re going to be talking about.
ID 05
Used as reference after intervention
Some participants reported that they had used the workbook as a refresher following completion of the intervention. Others had not used the booklet since the intervention ended, but reported that it felt good knowing that they had it with them in case they needed to refer to it:
[. . .] And since then [after the intervention], I’ve had another look over it [workbook] a few times just to remind me of what we were doing. So, yeah, it was good.
ID 15
Yes, I still refer to it [workbook] now and look over things and stuff now, so yeah, it was very helpful.
ID 16
Yes, and, – and I can refer back to that [workbook], err, and oh and that really, erm, so I think that’s really useful [. . .].
ID 13
Additional reasons for adherence to intervention and trial
Participants in the intervention group felt that they benefited from the cognitive rehabilitation sessions; therefore, they adhered to the intervention. This was particularly in terms of improvements seen in daily life activities. However, participants in both the intervention and control groups mentioned additional reasons for adherence to the intervention and the trial. For those in the intervention group, participants appraised the therapists’ skills as well as the venues where the cognitive rehabilitation sessions took place.
Improvements seen in daily life activities
Participants in the intervention group reported that they had increased the level and types of activities they were involved in:
Yes, I think, remembering, erm, much more detail about things, and attacking, – attacking things, if that makes sense.
ID 10
Rather than me just sitting and thinking about things, it’s [the intervention] made me more active. Rather than thinking about something, it’s a case of rather than thinking I’ll do that later, I now think I’ll do that now. [. . .] So it’s made me get up and go really.
ID 05
I’ve started doing things myself more [. . .] I play games. It tends to helps me, certain games, trying to remember things and I’m writing things down more and I’ve got diaries and tend to use my phone a lot more now. My social life, I tend to spend a lot more time going to places now than my wife. I’ve just joined up as a member of the Man[chester] United fan club so I tend to go to a lot of football matches every other week. I tend to go to Liverpool to see my family and different things like that. And just spending time with the kids really, doing things with the kids.
ID 16
Positive appraisal of therapist skills
Many participants commented on the skill with which the therapists facilitated the group:
But I think the ladies [therapists], there were two of them over the 10 weeks who did it, who work for the CRAMMS units, were very good. They really were good at what they did, and they put the whole idea of the process to us very clearly and very succinctly, so I was very happy with that.
ID 03
No, I mean, no, the whole – as I said to you – the whole experience, I don’t think you could have done it any better. [. . .] And it was made really interesting [by the therapists] by having flip charts and you know things – there were little exercises that we could do that really were interesting. So, I think the whole experience was quite enjoyable.
ID 18
Suitability of venues
Although the venue was dependent on the site the participant was recruited from, most participants stated that the venue was easy to get to and that it was appropriate for them:
Lovely premises, oh thank you very much. For the rented room, oh yeah. They’ve got a balls room and oh, it was just beautiful. Erm, we were all these professionals, sat round this big posh table with these lovely chairs.
ID 10
Although some participants who used wheelchairs found that some venues were not easy to access, most participants found them easy to find and get to by public transport:
. . . there’s a taxi company that I use all the time. And they took me all the time and picked me up. Because I’ve got quite a good rapport with the taxi company, they’re always there to support me anyway. So I didn’t have any problems getting there [venue] at all.
ID 05
Having cognitive assessments
Participants in both the intervention and control groups mentioned that it was good to have the cognitive assessments completed both at baseline and at follow-up assessments. This allowed them to get an accurate idea of their cognitive abilities and profile:
I think it [cognitive assessment] highlighted certain issues I was not quite happy with in the first place, so it was good to actually do that, to do the study [. . .] With neurological things, you need to test yourself and see what your capabilities are with certain things. So it’s always good to test you. I quite enjoyed that, not enjoyed as such, because certain things I think I don’t like doing that but you have to do it. It was good to try them.
ID 34
It was just me and one other – she came to my house and did tests on me. Yeah that, yeah, and that took about – she came twice. [. . .] Yes, they [cognitive assessments] were very helpful, yes.
ID 38, talking about being in the control group
Possibility of receiving cognitive rehabilitation
Participants joined the trial with the expectation that, if they were allocated to the intervention group, this might be of benefit to them:
I was rather pleased I was in that [intervention group], because I thought, if you’re in a control group, there’s nothing going to make you improve. If you’re in the intervention group, there’s a chance that you’ll improve through the intervention.
ID 15
Actually, I was really looking forward to it, because I felt it was a chance to meet up with other people and for us to actually share our experiences together. So it was one of those times where you could actually speak to other people as well. So I was really looking forward to it. And I was really pleased when I was nominated to be on it.
ID 05
I was happy that I was going to get help [. . .] talking to different people that have got the problems I have. I think being there [in the intervention] helped a lot. I learned quite a lot from there so it’s not like, even though I wasn’t expecting much from it, but it helped in a way that I knew people out there were trying to do stuff and it, like, encouraged me to try and do more there as well. It made me feel a lot better.
ID 16
Altruism
Participants reported that, although they preferred to be part of the intervention group, they recognised the need for some people to be in the control group. For such participants, it was a sense of altruism that motivated them to join the trial and remain in it even when they were allocated to the control group:
I understand that [there had to be a control group]. That [being part of the intervention group] was the only thing I would say that would have helped me. I’m fine being in either group just to try and help and everything, but, yeah that [not being part of the intervention group] was the only thing really. That [finding out that he was part of the control group] was fine. I don’t mind because as long as I can help in some way, that’s why I do these trials, to help further with MS and help whatever way I can.
ID 24
Possible improvements
Although most participants did not feel that the intervention needed further improvements, a few participants did suggest some improvements that could be made.
Refresher/booster sessions
Some participants felt that a refresher/booster session would have been useful at a later point because they felt that the 10 weeks went by quite quickly. A few such sessions, they felt, would enable them to sustain what they had learnt:
But I’m just trying to think whether there could be anything else just to keep it going for a short, not another 10-week course or anything like that, just something that could have followed it up. Perhaps another meeting or another two meetings just to go through it again and even think of anything new that might come up in terms of techniques.
ID 03
Option to download workbook
Some participants also suggested that it would be useful to have access to the workbook online, but also reiterated the importance of having someone to go through the workbook with them:
I think it would [be a good idea to have a downloadable workbook], but I think it would have made a lot more sense for someone to explain it when you couldn’t understand certain things.
ID 16
But we ask, erm, that if there is a workbook that could be printed on the web, on the NHS, you know on the website, that will be able to help people, the research programme. It will be useful for people to dip in and look at memory issues and how they can be addressed and how they can probably manage them, yeah so that would be useful [. . .] Yes, I don’t know whether its licensed or not, but it would be really useful, erm, yeah. No I think it, I think, – I think it would be a shame to lose that effort of compiling it and the knowledge that was used to do it, and the fact that I felt that, for me personally, I really benefited from it and, – and use it on a daily basis, and now its hardwired with myself and equally for any others, erm, you can. ‘Cause, er, lots of people have memory problems, you don’t just have to have MS.
ID 13
Adding mindfulness to help focus
Two participants highlighted the importance of mindfulness and how it could help participants to be more in the ‘here and now’ and focus on the things they are doing. They felt that it would help to incorporate this aspect into the intervention programme:
Having mindfulness to call is really useful to keep focus on what your aim is to do, so that’s where I come with that really . . . ‘cause, I think, I think that if we all, well I don’t mean that I’m like a Buddha, but if we [are] all calm and serene and the, we not on edge or we not all frustrated, we, we just accept, erm where we are, a bit like acceptance and commitment therapy really, where we accepting what is happening to us, err, or, yeah, we accept what’s happening to us and then the different stage down the line, we know how to accept that second stage, we know and it continues, and so I think that, errm, part of that is having all the tools if you want to use in your tool bag.
ID 13
I don’t really utilise them [strategies to compensate for cognitive problems] as I should do really. I know deep down I should literally pause and think about it and just clear my mind and use mindfulness and stuff like that, but in the grand scheme of things I don’t as such. I’m fully aware I can do more.
ID 34
Discussion
Cognitive rehabilitation does not appear to be provided as part of routine care for people with cognitive problems associated with MS. Although some participants received some ‘advice’ to help them with their cognitive problems, this was limited to providing them with some suggestions of external memory aids to use and signposting to other resources. This cannot be considered cognitive rehabilitation. Indeed, none of the participants had received any cognitive rehabilitation and, for all of those who received the intervention, it was the first time that they were systematically taught about cognition, why they were experiencing problems and what they could do to address and manage the cognitive problems.
Some participants had developed their own strategies for coping with the cognitive problems they faced; this was limited to using a few external memory aids, such as lists, using a calendar and requesting others to remind them to do things. There was no suggestion that participants spontaneously used any internal strategies or ways to improve their concentration or reduce distractions.
Participants in the control group did not report any reductions to their cognitive problems, and, in fact, were certain (using words such as ‘definitely’) that their cognitive problems had got worse. Most participants in the intervention group, however, reported benefiting from having attended the cognitive rehabilitation groups. Even when participants felt that their cognitive abilities had remained the same since they were enrolled in the trial, they did acknowledge that their cognitive problems in daily life had reduced. The significance of this, however, was somewhat minimised in participants’ accounts because they were using strategies to compensate for these cognitive deficits. This was clearly highlighted in their talk, in which they justified their talk using phrases such as ‘it’s just that I’ve put strategies in place’ or ‘it is just writing things down’.
The majority of participants who had received the intervention did feel that their cognitive problems had reduced. Their attributions for this change are similar to those found in extant cognitive rehabilitation literature in MS136 and other acquired brain injuries. 61,137 Participants had a sense that one reason that they saw this change was because of an increase in their use of strategies to compensate for their cognitive deficits. Increased use of these strategies was as a result of these strategies having become habitual to individuals, participants’ improved confidence and mood and the selective use of the strategies with necessary adaptations to suit individuals’ daily lives.
Participants recognised the role of psychoeducation in providing them with better knowledge and understanding of their problems, and associated with it an acceptance of their problems and a desire to act or change things. They therefore saw strategy use as a way to make these necessary changes.
Most participants also commented on the role the group format of the intervention had to play, in terms of the positive changes that they had observed in themselves. The group served as an arena where they could share their experiences of having MS and cognitive problems, and share ideas about how to manage these problems. Participants felt that the group understood them, when others, including family members and friends, could not. The groups also offered them an opportunity to connect socially with others and make new friends. Some people were initially concerned about being part of a group of people with different stages of MS and severity of MS symptoms, where they felt that they would be distressed by what their own futures may look like. However, these concerns abated over time; in fact, the diversity in the group allowed those who had less severe problems to reconsider the impact of their symptoms, recognising that they were better off than some others. For this group of people, it was also beneficial for them to see that people, despite their severe symptoms, were coping well.
Most participants found the workbook useful. It helped them during the cognitive rehabilitation sessions because they could annotate it with additional notes; it helped them to complete their homework activities and to review and prepare for the next session. The workbook was also found to be beneficial after the rehabilitation programme had ended, because it served as a useful reference for people to return to at a later date. However, all participants were certain that the workbook was useful only if used or if provided as part of the whole package of cognitive rehabilitation, including the group work.
Participants in the intervention group remained in the group sessions and the trial because they felt that they benefited from the intervention. However, another reason why this group remained in the trial was because of the therapists who facilitated the group sessions, who were seen to be highly skilled to deliver the cognitive rehabilitation. Most also found the venues easy to get to and accessible; however, this was not the case with some wheelchair users.
For both participants in the intervention and control groups, having the cognitive assessments at baseline and follow-up was a motivator to remain in the trial. They felt that such assessments allowed them to understand and monitor their cognitive deficits better.
Finally, although most participants did not find anything that they would like to change about the intervention, some participants had some suggestions to improve the intervention. They felt that refresher or booster sessions would be useful to help them maintain the gains they made from the cognitive rehabilitation. Some participants wanted the option of downloading or viewing the working book online. They felt that this would reach a wider audience. Two participants also felt that adding a ‘mindfulness’ component to the intervention would help people to focus on the ‘here and now’.
The interviews were conducted by two people: a PhD student and a PPI partner, who is the mother of a person with MS. Although there were some differences between the interviews and how participants responded to the interviewers, there were no major differences in how much participants disclosed with either interviewer. We also did not feel that there was any difference in participants’ willingness to share information with either interviewer. Some participants disclosed more than others, but this was the same for both interviewers. The interviews were of similar length. There were no differences in the quality of the data that were generated. Participants did ask different types of questions depending on who the interviewer was. The PPI partner was asked about her daughter by a few participants and how she was doing, whereas participants interviewed by the PhD student asked more research and treatment-related questions towards the end of the interview, when participants were asked whether or not they had any additional comments. The themes elicited were, therefore, consistent across interviewers.
The findings from this trial must be viewed in light of its methodological limitations. It is probable that the overwhelmingly positive response participants had to the trial and the intervention could be attributed mainly to a sampling bias, in that, only those who had a positive experience agreed to be interviewed. However, the data do suggest that participants were willing and able to be open about their experiences, with some clearly mentioning that their memory problems had stayed the same or got worse. Participants also made comments on potential improvements that could be made to the trial and the intervention.
All participants were white British; therefore, the sample was not heterogenous in terms of gender. We were limited in terms of seeking out non-white participants, because the sample for the interview study was drawn from the wider RCT in which only 4% of participants were non-white, reflecting the low incidence of MS in those from some non-white communities. 141 We did, however, attempt to vary the selection as much as we could by seeking individuals with different self-reported cognitive impairment levels and other demographic characteristics (such as gender, age, time since diagnosis and type of MS).
Participant accounts of their experiences were retrospective, so some details may have been forgotten or misremembered. This is particularly relevant for this patient group who have memory problems. However, the depth of the interview data and the lack of inconsistencies in the talk (even when probed) would suggest that participants were able to recall their experiences relatively well.
The interviews were conducted over the telephone, as opposed to being face to face. Therefore, the visual nuances associated with participants’ talk were not available to the interviewers. These cues may have been useful in exploring aspects of the talk in which a discrepancy was seen between the words and the manner in which they were articulated. However, the analysts did pay attention to intonation and phrasing of the interviewees’ talk, and made note of this, when relevant, to aid interpretations. Furthermore, telephone interviews also have their advantages over face-to-face interviews, over and above the practical aspects of conducting such interviews (e.g. reduced costs, increased geographical reach and ability to make notes unobtrusively). For instance, a review of the use of telephone interviews in qualitative research142 noted other benefits including allowing participants to be in their own space (which is particularly important for people with MS who may have mobility issues), decreased social pressure and allowing greater anonymity.
Given that cognitive rehabilitation is a complex intervention and includes several components with various interconnecting parts,143 it is difficult to define with precision the active ingredients and how these relate to each other. 144 However, the outcomes of the feedback interviews indicate that it is likely to be an interplay between the factors that were identified.
Chapter 6 Economic evaluation
Parts of this chapter have been reproduced from the CRAMMS trial protocol. 65 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Study question
The aim of the economic evaluation was to assess the cost-effectiveness of cognitive rehabilitation for attention and memory problems in addition to usual care when compared with usual care alone in people with MS.
Selection of alternatives
The economic evaluation used the cognitive rehabilitation intervention in addition to usual care versus usual care alone as the competing alternatives. These are described in Chapter 2.
Evaluation methods
An economic evaluation was undertaken alongside the clinical trial. A cost–utility analysis (incremental cost per QALY gained) was conducted using the EQ-5D-5L results at 12 months as the primary outcome for the economic analysis. A cost-effectiveness analysis was undertaken using the primary clinical outcome (incremental cost per point improvement in the MSIS-Psy score) at 12 months. Decision-analytic modelling was not undertaken to extrapolate the trial results to a longer-term horizon to explore longer-term cost-effectiveness (see Results of model-based analysis for longer-term cost-effectiveness).
The trial methodology is described in detail in Chapter 2; only the methods and results specific to the economic analysis are described in this chapter. A health economic analysis plan was produced alongside the SAP and finalised with the trial team before completion of data collection. Table 19 summarises the methods used for the base-case analysis and subsequent sensitivity analyses, which are discussed in more detail in the following sections.
| Aspect of methodology | Strategy for base-case analysis | Alternative strategy for sensitivity analysis |
|---|---|---|
| Data set | All randomised participants, analysed on an intention-to-treat basis | ACA |
| Health outcome measurement | EQ-5D-5L (administered at baseline, 6 months and 12 months), mapping to three-level valuation set |
|
| Clinical outcome measurement |
|
|
| Costs included in analysis |
|
|
| Missing data | Multiple imputation | ACA |
| Adjustment for baseline covariates | Regression used to adjust outcomes for differences at baseline. Adjusting for baseline covariates should also improve the precision of the between group estimate | No adjustment for baseline covariates |
| Base-case analysis |
|
|
Effectiveness data
The economic analysis used the primary clinical outcome (MSIS-Psy score), as summarised in Chapter 2, in a cost-effectiveness analysis. The EQ-5D-5L and MSIS-8D (Multiple Sclerosis Impact Scale – 8 Dimensions; derived from the MSIS, as described in Benefit measurement and evaluation) were used for cost–utility analyses. Cost-effectiveness was evaluated at the primary end point of 12 months and at 6 months to evaluate any short-term effects of cognitive rehabilitation.
Benefit measurement and evaluation
Quality-adjusted life-years were generated using utility scores derived from the EQ-5D-5L index. The EuroQol-5 Dimensions (EQ-5D) is a standardised instrument for use as a measure of HRQoL. 106 The study employed the five-level version of the questionnaire (EQ-5D-5L). Individual-level utility scores were obtained at each assessment point using the EQ-5D-5L questionnaire. In August 2017, NICE issued a position statement advising that the five-level valuation set for England is not recommended for use in deriving utility values and instead advise that the validated mapping function to the three-level version be used for reference-case analysis. 145 The utility scores derived from the direct valuation set for the five-level version were used in a sensitivity analysis. Utilities were summarised and QALYs for each participant in the intervention and control groups were calculated over 6 and 12 months using the area under the curve approach (assuming linear interpolation). The mean difference per participant in QALY gain/loss between the cognitive rehabilitation and usual-care groups is presented over 6 and 12 months.
The Multiple Sclerosis Impact Scale (MSIS-29) was used to derive the MSIS-8D using published methods. 146 MSIS-8D is an 8-dimensional (general physical function, mobility, employment, social function, fatigue, cognition, depression and general emotional well-being) HRQoL measure used to produce a MSIS-8D utility score. Each item of the MSIS-8D has four levels, describing 65,536 health states. A descriptive analysis of MSIS-8D responses was undertaken; the responses were summarised and used to generate QALYs at 6 and 12 months.
The primary outcome for the CRAMMS trial was the psychological impact of MS on everyday life, as a reflection of HRQoL measured using the MSIS-Psy, version 2.0. As higher scores indicate greater psychological impact of MS on everyday life; an improvement would be represented by a negative difference in the MSIS-Psy score.
Costing
The perspectives taken were the UK NHS and Personal Social Services. Individual-level resource use was collected from the UHSSQ, summarised in Chapter 2.
The data collected were the costs of:
-
the CRAMMS trial intervention (including training, implementation and delivery costs)
-
health-care resource use (primary and secondary care) and personal social services
-
medications.
All resource use data were valued in Great British pounds using published unit costs147–149 at 2017 prices. When current unit costs were unavailable, the most recently available costs were used and inflated to 2017 prices using the Hospital and Community Health Service index. 149
Intervention costs
Resource use resulting from the implementation of the CRAMMS trial intervention (including materials, consumables, staff time and training) was estimated through interviews and direct communications with clinical staff involved in the trial and the trial team, and by using data collected during the trial (e.g. supervision records). No travel costs were included for training or delivery of the CRAMMS trial intervention because, in routine clinical practice, cognitive rehabilitation training would be delivered at the base site of the clinical psychology team.
The resource use and costs associated with the delivery of the CRAMMS trial intervention were estimated from published sources and discussion with the trial team. Unit costs of materials and consumables were obtained directly from financial records collected by the trial team. Staff costs were obtained from published unit costs. 147 The costs of additional catch-up sessions delivered to participants who missed one or more previous sessions were based on the assumption that these required 30 minutes and were delivered as a group, rather than on an individual basis.
The assumptions were made from consulting with clinical experts on the trial team and the impact on the results was tested as part of the sensitivity analysis. For the base-case analysis, the costs of the cognitive assessment to determine eligibility for the CRAMMS trial were excluded as these were assumed to be part of usual care. The impact of including the cognitive assessment as part of the CRAMMS trial intervention was tested in a sensitivity analysis.
Costs of health-care and Personal Social Services resource use
Health-care resource use [including primary care consultations, accident and emergency (A&E) visits, outpatient appointments and inpatient stays] and Personal Social Services use (e.g. home adaptations) were collected using data from a health and social services questionnaire that had been developed and used in a previous MS trial. 107 Information was collected at baseline, and at 6 and 12 months’ follow-up to assess the difference in profile of health-care resource use in the intervention group compared with the usual-care group.
Costs were assigned using published unit costs. 147–149 Outpatient visits and inpatient stays were costed individually according to the reasons for health-care contact, length of stay and specialty/department visited recorded in the UHSSQ.
A description of unit costs associated with health and personal and social care resource use are presented in Appendix 13, Table 42.
Costs of medications
Participants were asked to recall the medications (including drug name, dosage and frequency) they had used over the previous 3 months at baseline and at follow-up assessments. The UHSSQ did not specify when medication was started or stopped, or how long the medication had been taken for. It was assumed that the medication was taken or used for the whole 3-month period [or, when applicable, the prescribed length from the British National Formulary (BNF)149]. This may have overestimated the cost of medications for some participants, but accounts for non-compliance to the length of prescription in both the usual care and the cognitive rehabilitation groups. For medications with multiple indications from the BNF,149 an assumption was made regarding the most probable indication applicable to the patient group or most common indication for the general population. Clinical members of the trial team checked all medication data and assumptions made before analysis.
The health-care costs in both the intervention and control groups were summated and presented for each of the time points with differences in resource utilisation between the CRAMMS trial intervention at 6 and 12 months calculated, using methods consistent with the statistical analysis of outcomes (as described in Chapter 2). The difference per participant in costs (including 95% CIs) was calculated, using non-parametric bootstrapping.
Missing data
Issues concerning missing data are particularly prevalent to health economic analyses. Missing items relating to health-care resource use may undervalue costs, whereas missing outcome data may be intrinsically linked to effects. Missing data may relate to item non-response, where a questionnaire is partially incomplete, or unit non-response, where all information is missing. 150
Missing questionnaire items in outcome measures
Partially completed questionnaires for the health outcomes used in the economic analysis were handled and the total score was calculated using the same approach as in the statistical analysis (see Chapter 2). If the number of missing items exceeded that for which total scores were calculable, the questionnaire was treated in accordance with unit non-response procedures. Any missing items in the EQ-5D-5L descriptive questionnaire were not individually imputed; utility values were instead imputed in accordance with unit non-response procedures.
Missing baseline data
If baseline scores for the outcome measures remained missing (e.g. the questionnaire was not usable, even taking into account the above rule for missing data), data were imputed using the mean baseline score at each study site. This enabled all participants to be included in the regression analyses of the outcome score, ensuring consistency with the statistical analysis. These simple imputation methods are superior to more complicated imputation methods when baseline variables are included in an adjusted analysis to improve the precision of the treatment effect. 116
Missing outcome data at follow-up
For the base-case health economic analysis, data were assumed to be missing at random (MAR). Multiple imputation using chained equations was undertaken to impute missing items. Imputations were combined following Rubin’s rules. 151 Owing to the predefined range of outcome scores, including utility derived from the EQ-5D-5L, truncated regression methods were used to ensure that imputed values were consistent with the valid range. Predictive mean matching (PMM) was used to impute missing cost data, ensuring that imputed values were consistent with observed data.
An available-case analysis (ACA) was conducted on both outcomes and costs as part of a sensitivity analysis. ACA is generally biased under MAR or missing not at random assumptions, with the ACA mean unbiased only if missingness in the parameter of interest is independent of its value. ACA examined the robustness of conclusions from the imputed data compared with those from the observed data, after conducting the procedures outlined in handling missing questionnaire items.
Missing service use data
The UHSSQ was used to estimate the resources used and costs of health service usage by participants because of MS. Participants were asked to complete the relevant boxes with the number of contacts received over the previous 3 months for each item, or to place a ‘00’ in the relevant box should no usage of this item have occurred. Item non-response may, however, represent either the individual failing to record the non-zero number of contacts or no contact. Missing UHSSQ data were thus treated according to the following rules:
-
If one or more items were completed (values of ‘0’ or greater) on the UHSSQ, then the questionnaire was assumed to be fully completed and any missing items were imputed with zeros.
-
If the UHSSQ was marked as ‘not done’ or otherwise fully incomplete, appropriate imputation methods were used. At baseline, data were imputed using the site mean akin to the process outlined above. At the 6-month and 12-month follow-ups, data were imputed using multiple imputation or ACA methods, with imputation conducted on the total for each contact type (e.g. general practice and community services, social services, medications).
Because of the typically skewed distribution of cost data, in which the UHSSQ was marked ‘not done’, PMM was used to impute missing costs under multiple imputation using the methodology outlined in Missing service use data.
Adjustments for timing of costs and benefits
As the trial period did not exceed 12 months, discounting was not applied to the within-trial analysis of either costs or outcomes.
Cost-effectiveness analyses
Cost–utility and cost-effectiveness analyses were undertaken to estimate the incremental cost per QALY gained and the incremental cost per improvement in MSIS-Psy score based on the primary clinical end point at 12 months. The multiple imputation approach to missing data for costs and QALYs was used for the primary cost–utility analysis. For the cost-effectiveness analysis, a similar methodological approach to the statistical analysis outlined in Chapter 3 was conducted to ensure consistency with the analysis and reporting of the primary outcome.
Results of the comparative analysis of incremental costs and effects can be summarised in terms of ICERs. An ICER can be represented as:
where C1 and E1 are the costs and effects of the intervention arm, C0 and E0 are the cost and effects of the control arm and ΔC and ΔE are the incremental costs and effects of the intervention compared with the control. For the cost–utility analysis using QALYs, ΔE > 0 would represent an improvement favouring the intervention group. For the cost-effectiveness analysis on the MSIS-Psy, higher scores represent a greater psychological impact of MS on everyday life; therefore, ΔE < 0 would represent an improvement favouring the intervention group.
If the intervention is less costly and more effective than usual care, then the intervention is classified as dominant and no ICER would be calculated. Conversely, if the intervention is more expensive and less effective than usual care, the intervention is considered to be dominated by usual care. ICERs are calculated when a trade-off between cost and effect is evident, for example when the intervention is more costly but generates an improved health effect (QALY gain) compared with usual care.
The ICER is reported to determine the cost-effectiveness of cognitive rehabilitation compared with usual care and to aid decision-making. NICE reports a base cost-effectiveness threshold of £20,000 per QALY, but cost-effectiveness is a spectrum rather than a dichotomy, with the threshold changing according to the circumstances. The ICERs are presented to assist with the decision-making process and are not an absolute statement on whether or not the intervention can be deemed cost-effective. No established willingness-to-pay (WTP) threshold for the MSIS-Psy or other secondary outcomes is available.
Net monetary benefit (NMB) was also calculated. NMB is a summary statistic that represents the value of the intervention in monetary terms when a WTP threshold for a unit of benefit is known. 152 NMB for the cognitive rehabilitation and control groups is based on the following equation:
where ΔE and ΔC represent the change in effects and costs and λ represents the WTP threshold. Incremental NMB was calculated as the difference in NMB between the intervention and usual care groups. If the incremental NMB is positive, the intervention can be identified as cost-effective at the given WTP threshold relative to usual care.
Allowance for uncertainty
Sensitivity analyses were undertaken to account for the uncertainty in the parameters used in the cost-effectiveness analyses. Deterministic one-way sensitivity analyses (OWSAs) were undertaken to examine the impact of changes in key parameters on ICERs by modifying the value of one parameter at a time within a plausible range (e.g. upper/lower 95% CIs, ±30% to key parameters). A threshold analysis was conducted to determine the required changes to affect base-case findings (e.g. changes in QALY gains to provide a cost-effective result).
Bootstrap resampling was undertaken to analyse the joint uncertainty of parameter estimates. A minimum of 1000 resamples was used. The results of the probabilistic sensitivity analysis are illustrated using cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs). The probability that the intervention is cost-effective at various WTP thresholds was examined and expressed as a percentage. Where dominance is observed, the probability of dominance is presented.
Subgroup analysis
In the health economics plan, subgroup analysis was to be conducted only with sufficient evidence obtained from the exploratory analyses of interaction for the primary outcome (see Chapter 3) to justify further investigation on the cost-effectiveness findings. The trial team concluded that no subgroup analysis was required.
Longer-term cost-effectiveness
It was originally proposed to construct a decision-analytical model to extrapolate the findings from the trial to estimate the cost-effectiveness of cognitive rehabilitation compared with usual care beyond the trial horizon of 12 months. The modelling exercise was based on extrapolating the results from the within-trial analysis to longer-term cost per QALY estimates supplemented with data sources from the literature and, when necessary, clinical opinion from the trial team. A model plan is presented in Appendix 14, Figure 11.
Results
The costs for the economic evaluation consisted of two broad categories:
-
the implementation and delivery of the group-based cognitive rehabilitation programme
-
health and personal and social care resource use.
Intervention costs
The resource use and associated costs of delivering the intervention are summarised in Table 20; a detailed account of each component is presented in Appendix 15, Table 43. The primary analysis was conducted excluding costs associated with cognitive assessment, which the trial team agreed to be incurred by both usual care and cognitive rehabilitation groups. The total cost of cognitive rehabilitation was calculated at £209.
| Resource area | Resource | Intervention total (£) |
|---|---|---|
| Training | Training in cognitive rehabilitation provided by clinical psychologist to AP | 1900.00 |
| Delivery of cognitive rehabilitation | Sessions delivered by AP | 39,385.50a |
| Supervision conducted by clinical psychologist | 2375.00 | |
| Administration | 5625.00 | |
| Intervention manual | 500.00 | |
| Stationery | 245.00 | |
| Refreshments | 1225.00 | |
| Total cost of cognitive rehabilitation (excluding cognitive assessment) | 51,255.50 | |
| Total cost of cognitive rehabilitation per participant (excluding cognitive assessment) | 209.21 |
The main cost drivers of the intervention cost were training, the delivery of the sessions, supervision and administration. One clinical psychologist (band 8a, £62 per hour) delivered the training across all sites to APs (band 5, £33 per hour, one per site) on a one-to-one basis. Training was assumed to require 4 hours. No provision was included for travel costs and room hire associated with training as, in standard practice, this would take place on NHS premises. As detailed in Chapter 2, an AP delivered 10 cognitive rehabilitation sessions to each group individually. Each session lasted for approximately 1 hour and 30 minutes. An additional 30 minutes was required for both set-up and close-down; provision for 30-minute catch-up sessions was included for 137 of 450 sessions for those participants who missed one or more sessions previously.
To ensure that cognitive rehabilitation was delivered appropriately, provision for supervision is included in the cost of the intervention. Each AP routinely received supervision sessions with a clinical psychologist lasting 1 hour on a weekly basis, of which it was assumed that 30 minutes was attributable to the delivery of cognitive rehabilitation.
The delivery of cognitive rehabilitation required one administrator to set up each group, send invitations to participants and send reminders of upcoming sessions. It was assumed that an administrator (band 3, £25 per hour) would be required for 30 minutes per session. Additional costs of stationery, refreshments and providing an intervention manual to each participant were included.
Cognitive assessment is used to determine patient eligibility for cognitive rehabilitation and was a key cost driver in providing the intervention. From discussions with the TMG and clinicians, it was determined that cognitive assessment would form part of usual care and was not exclusive to the intervention group. This may differ on a site-by-site basis. It was decided that cognitive assessment would not be included in the primary analyses, but would be included in the sensitivity analyses.
Resource use and costs
The resource use for available cases is presented in Appendix 16, Tables 44–46. Resource use costs were summarised by:
-
general practice and community nursing services
-
hospital and community services
-
therapy services
-
social services
-
A&E services
-
other hospital services
-
medications.
The key cost drivers for this group of participants were medications, and social services; however, for each component of the resource use questionnaire, outliers had a substantial impact on the mean value, with a few high-cost users increasing the average cost, resulting in high SDs. t-tests on differences between groups for each component of resource use were typically not statistically significant.
At 12 months, the cognitive rehabilitation group had medication costs that were £320.45 cheaper (95% CI –£928.61 to £287.71) than the medication costs of the usual-care group; however, the usual-care group had a much larger SD and a maximum medication cost of £34,929, compared with a maximum of £7395 for the cognitive rehabilitation group. Similarly, at 6 months, the cognitive rehabilitation group had medication costs that were £313.55 cheaper (95% CI –£860.86 to £233.77) than those of the usual-care group. However, the usual-care group had a much larger SD and a maximum medication cost of £26,516, which was over three times larger than the maximum medication cost for the cognitive rehabilitation group.
Excluding the costs of disease-modifying therapies resulted in a small increase in the difference in medication costs to –£332.33 (95% CI –£812.80 to £148.14) at 12 months, and a reduction at 6 months to –£181.13 (95% CI –£522.38 to £160.12); at both follow-ups the difference was not statistically significant.
Multiple imputation was used to impute missing costs. Imputation was performed on total costs for unit non-response after the procedures outlined in Chapter 2 regarding item non-response were performed.
The multiple imputation results in Table 21 show that, at 6 months, cognitive rehabilitation had total costs of £3144 and was about £20 cheaper than usual care. However, the difference was not statistically significant, with wide CIs observed. At 12 months, the mean total costs associated with cognitive rehabilitation were £5623; the difference between the two groups increased at 12 months, with cognitive rehabilitation about £575 cheaper than usual care. This indicates that cognitive rehabilitation was cost-saving compared with usual care; however, the differences in costs were not statistically significant at either 6 months or 12 months, with wide CIs at both follow-ups.
| Trial group | Baseline, mean (SD), n | 12 months, mean (SD), n | Adjusted difference in means (95% CI)a | p-value |
|---|---|---|---|---|
| Panel A: total cumulative costs at 12 months | ||||
| Usual care | 3068.32 (224.48), 204 | 6092.01 (630.07), 204 | –574.93 (–1878.93 to 729.07) | 0.39 |
| Cognitive rehabilitation | 3271.68 (327.76), 245 | 5623.67 (391.38), 245) | ||
| Panel B: total cumulative costs at 6 months | ||||
| 6 months, mean (SD), n | ||||
| Usual care | 3068.32 (224.48), 204 | 3115.49 (313.36), 204 | –19.91 (–777.13 to 737.31) | 0.96 |
| Cognitive rehabilitation | 3271.68 (327.76), 245 | 3144.40 (251.12), 245 | ||
Further analysis was conducted to explore the drivers of costs in the two groups. Figure 3 presents a histogram of cumulative total costs for available cases at 12 months by intervention group.
FIGURE 3.
Histogram of cumulative total costs at 12 months. (a) Usual care; and (b) cognitive rehabilitation.
Figure 3 shows that at 12 months approximately 40% of participants in both the cognitive rehabilitation and usual care groups incurred total costs of ≤ £2500. Similarly, > 75% of participants in both groups incurred costs of ≤ £10,000. A few participants incurred costs in excess of £20,000, of whom nine were in the usual-care group and five were in the cognitive rehabilitation group. No participants in the cognitive rehabilitation group had costs in excess of £30,000, whereas there were two notable outliers in the usual-care group, with costs of £53,784, and £81,074. These outliers were investigated further. The participant with total costs of £53,784 incurred £24,809 in medication costs at both 6 months and 12 months, attributable to daily administration of teriparatide (Forsteo®; Eli Lilly and Company, Indianapolis, IN, USA). The participant with total costs of £81,074 incurred £77,250 of costs due to 30 nights spent on a hospital ward as a planned admission (based on NHS reference costs148 for elective inpatient medical care of patients with MS). Two further participants in the usual-care group had total costs at 12 months in excess of £30,000. Excluding these two outliers from the ACA (see Appendix 17, Table 47) reduces mean total costs for the usual-care group to £5849.49 (SD 6190.98) and the adjusted difference in total costs is –£38.85 (95% CI –£1162.14 to £1084.45) (i.e. cognitive rehabilitation was slightly cheaper than usual care).
Outcomes
Utility values derived from EQ-5D-5L and MSIS-8D scores were converted into QALYs using linear interpolation and the area-under-the-curve method, with adjustment for baseline covariates including baseline utility, gender, MS type and site. In accordance with the methods for total costs, multiple imputation was used to impute missing outcome data for utility scores derived from both the EQ-5D-5L and the MSIS-8D. Multiple imputation was performed on items for which utility scores could not be calculated after the item non-response procedures detailed in Chapter 2 were performed. Results are presented in Table 22.
| Trial group | Baseline, mean (SD), n | 12 months, mean (SD), n | Adjusted difference in means (95% CI)a | p-value |
|---|---|---|---|---|
| Panel A: EQ-5D-5L QALYs at 12 months | ||||
| Usual care | 0.57 (0.02), 204 | 0.58 (0.02), 204 | ||
| Cognitive rehabilitation | 0.60 (0.02), 245 | 0.60 (0.02), 245 | 0.00 (–0.04 to 0.05) | 0.91 |
| QALY gain | 0.00 (–0.02 to 0.02) | 0.91 | ||
| Panel B: EQ-5D QALYs at 6 months | ||||
| 6 months, mean (SD), n | ||||
| Usual care | 0.57 (0.02), 204 | 0.57 (0.02), 204 | ||
| Cognitive rehabilitation | 0.60 (0.02), 245 | 0.60 (0.02), 245 | 0.01 (–0.03 to 0.05) | 0.59 |
| QALY gain | 0.00 (–0.01 to 0.01) | 0.59 | ||
Table 22 shows the EQ-5D-5L scores at baseline and at 12 months. The mean utility value was slightly higher in both groups at 12 months than at baseline; however, there was no evidence of an observable QALY gain as a result of cognitive rehabilitation compared with usual care. The CIs ranged from –0.02 to 0.02, with no statistically significant difference between the two groups. Similar results were observed for EQ-5D-5L scores at 6 months.
We also examined HRQoL derived from the MS-specific MSIS-8D instrument using the modelling methodology presented by Goodwin et al. 146 Results are presented in Table 23.
| Trial group | Baseline, mean (SD), n | 12 months, mean (SD), n | Adjusted difference in means (95% CI)a | p-value |
|---|---|---|---|---|
| Panel A: MSIS-8D QALYs at 12 months | ||||
| Usual care | 0.51 (0.01), 204 | 0.52 (0.01), 204 | ||
| Cognitive rehabilitation | 0.53 (0.01), 245 | 0.56 (0.01), 245 | 0.02 (–0.01 to 0.05) | 0.19 |
| QALY gain | 0.01 (–0.01 to 0.03) | 0.19 | ||
| Panel B: MSIS-8D QALYs at 6 months | ||||
| 6 months, mean (SD), n | ||||
| Usual care | 0.51 (0.01), 204 | 0.54 (0.01), 204 | ||
| Cognitive rehabilitation | 0.53 (0.01), 245 | 0.57 (0.01), 245 | 0.03 (–0.00 to 0.06) | 0.06 |
| QALY gain | 0.01 (–0.00 to 0.01) | 0.06 | ||
Table 23 shows the MSIS-8D scores at baseline and at 12 months. The mean utility value was slightly higher in both groups at 12 months than at baseline. There was a numerically small QALY gain of 0.01 (95% CI –0.01 to 0.03) in the cognitive rehabilitation group compared with the usual-care group. A small baseline imbalance in MSIS-8D scores was noted, with the cognitive rehabilitation group having a slightly higher utility score than the usual-care group, and was included as a covariate in the analysis model. There was no statistical difference in QALY gains between the two groups. At 6 months, utility values in both groups were higher than at baseline, with a larger increase observed for the cognitive rehabilitation group than for the usual-care group. The adjusted difference in means for MSIS-8D and QALY gains, favouring the intervention group, was nearly statistically significant. This corresponds with the results for the MSIS-Psy in Chapter 3, with statistical significance observed at 6 months but not at 12 months.
The cost–utility analysis required the calculation of ICERs using the total costs and outcomes presented in Tables 21–23. The results for the cost–utility analysis are presented in Table 24.
| Outcome measure | Incremental cost (95% CI) (£) | Incremental effect (95% CI) | ICER |
|---|---|---|---|
| EQ-5D-5L QALY | |||
| 6 months | –19.91 (–777.13 to 737.31) | 0.00 (–0.01 to 0.01) | Intervention dominant. No ICER calculated |
| 12 months | –574.93 (–1878.93 to 729.07) | 0.00 (–0.02 to 0.02) | Intervention dominant. No ICER calculated |
| MSIS-8D QALY | |||
| 6 months | –19.91 (–777.13 to 737.31) | 0.01 (–0.00 to 0.01) | Intervention dominant. No ICER calculated |
| 12 months | –574.93 (–1878.93 to 729.07) | 0.01 (–0.01 to 0.03) | Intervention dominant. No ICER calculated |
| MSIS-Psy | |||
| 12 months | –574.93 (–1878.93 to 729.07) | –0.5 (–1.5 to 0.5) | Intervention dominant. No ICER calculated |
Table 24 requires careful explanation. Cognitive rehabilitation shows numerical differences in costs at 6 and 12 months compared with usual care. With no evidence of any incremental effect on QALY gain between the groups (when the EQ-5D-5L is used), cognitive rehabilitation could be considered to be less expensive against no difference in effect (QALY gain) than usual care. When the MISIS-8D was used to generate QALYs, there was a numerically small QALY gain in cognitive rehabilitation compared with usual care, which (technically) presents cognitive rehabilitation as dominant over usual care (i.e. cognitive rehabilitation is less expensive and has a greater effect). A similar picture is presented when available cases are used to generate QALYs (EQ-5D-5L or MSIS-8D; see Appendix 17). For the MSIS-Psy, the negative effect is representative of an improvement for the cognitive rehabilitation group. This would, again, present cognitive rehabilitation as dominant over usual care for the primary outcome. However, across all scenarios, the CIs for both incremental costs and incremental effects span zero, and for the costs, CIs are wide. Given this, caution should be applied in interpreting these results.
Cost-effectiveness planes and associated CEACs were constructed using non-parametric bootstrapping (5000 replications with replacement) for the primary and secondary cost–utility analyses (cost per QALY) derived from the EQ-5D-5L and MSIS-8D, respectively.
Cost-effectiveness planes and CEACs were also constructed for the multiple imputation analysis on EQ-5D-5L and MSIS-8D QALY gains, and for the change in the MSIS-Psy. These are presented in Figures 4–6.
FIGURE 4.
Total costs and EQ-5D-5L QALYs at 12 months (multiply imputed analysis). (a) Cost-effectiveness plane; and (b) CEAC. Reproduced with permission from Lincoln et al. 122 This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 License (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
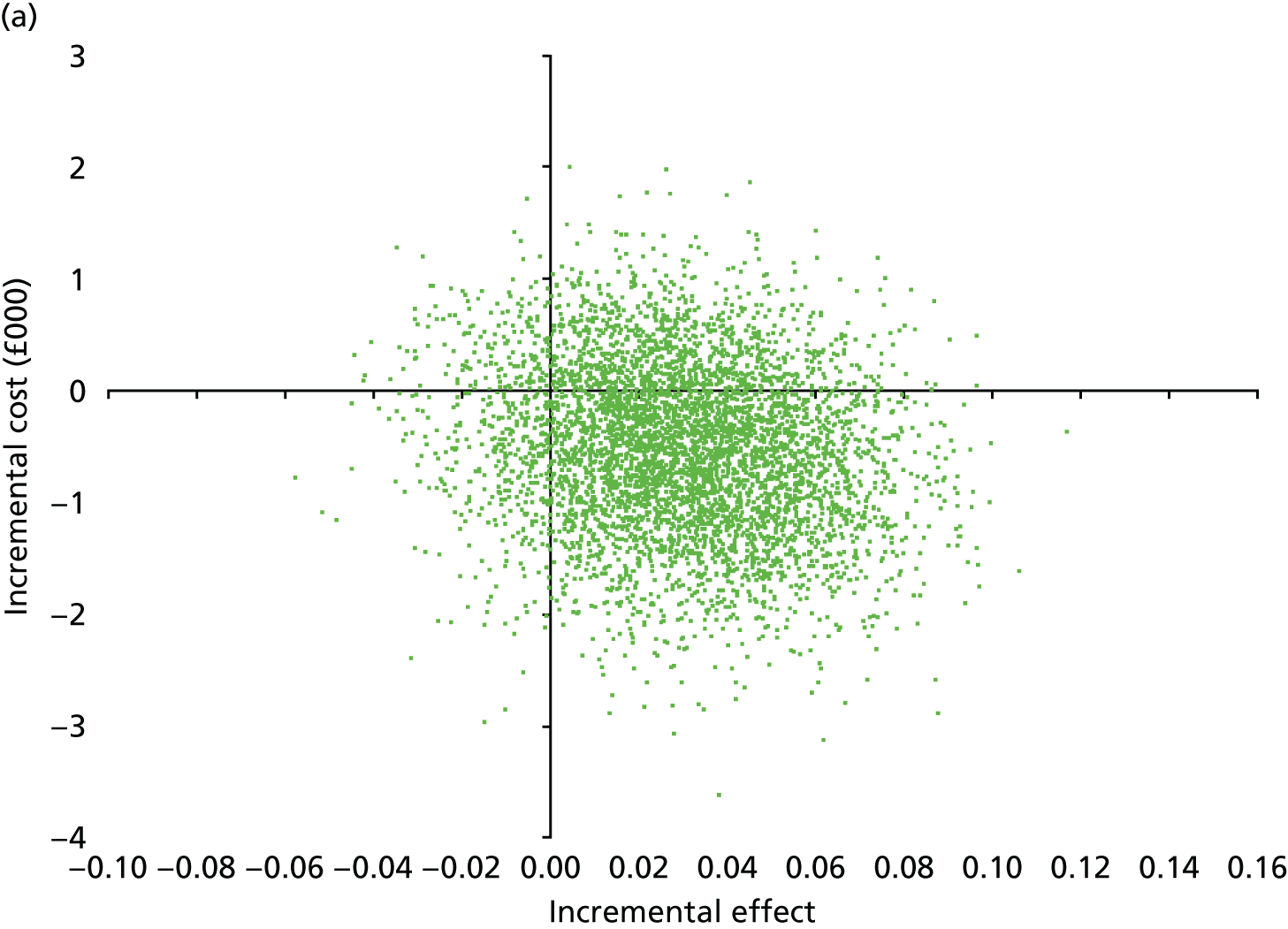
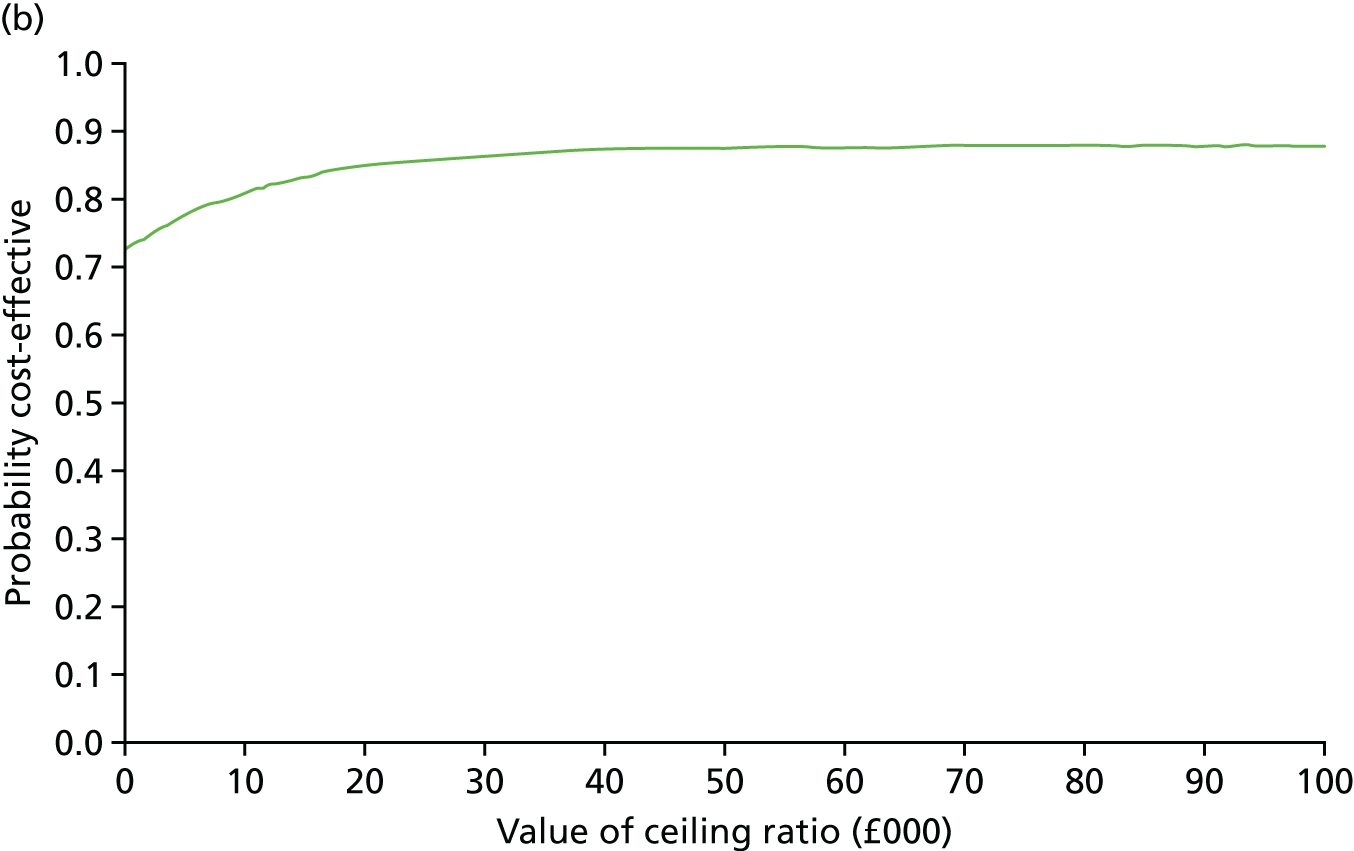
FIGURE 5.
Total costs and MSIS-8D QALYs at 12 months (multiply imputed analysis). (a) Cost-effectiveness plane; and (b) CEAC.
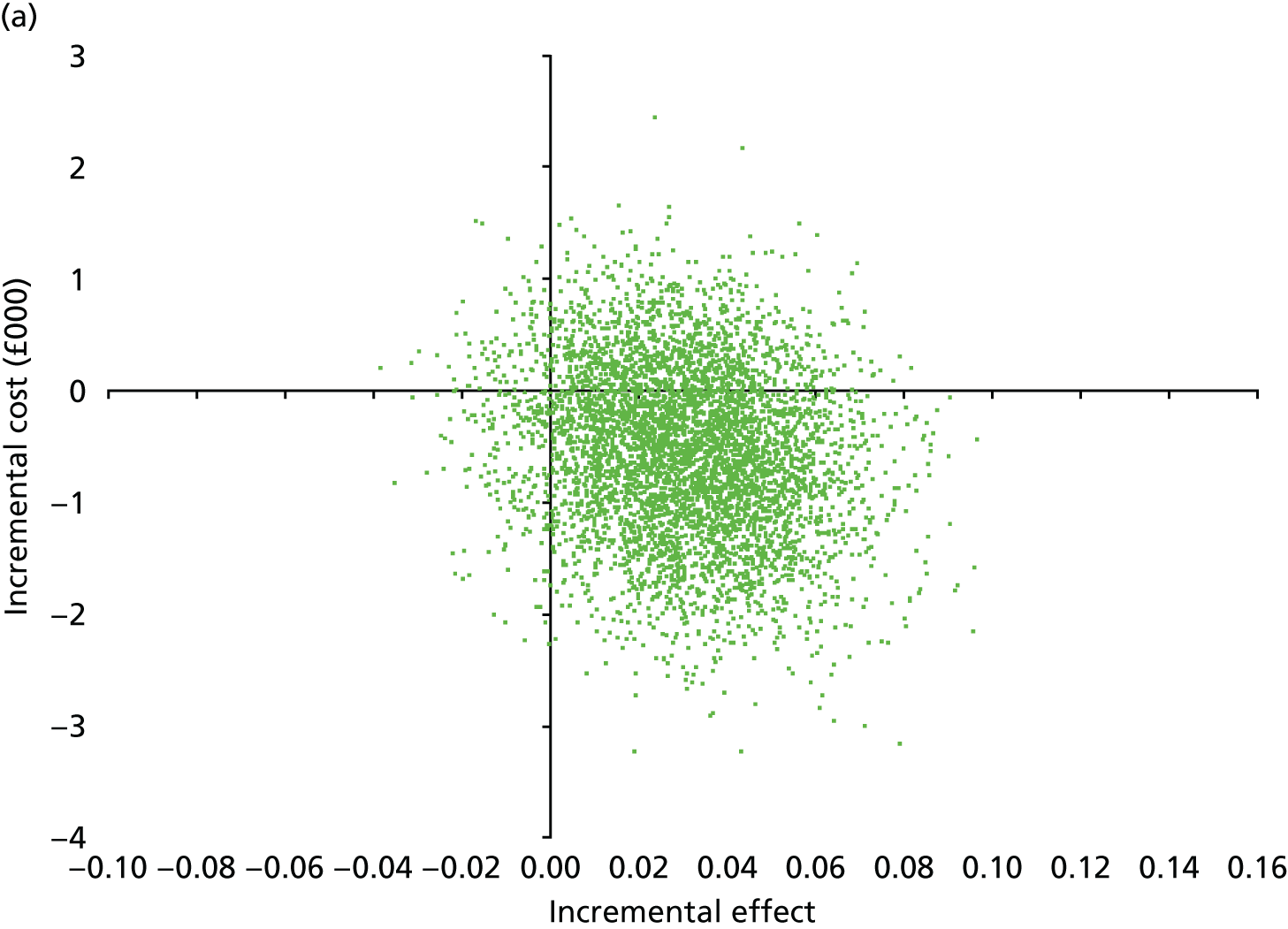
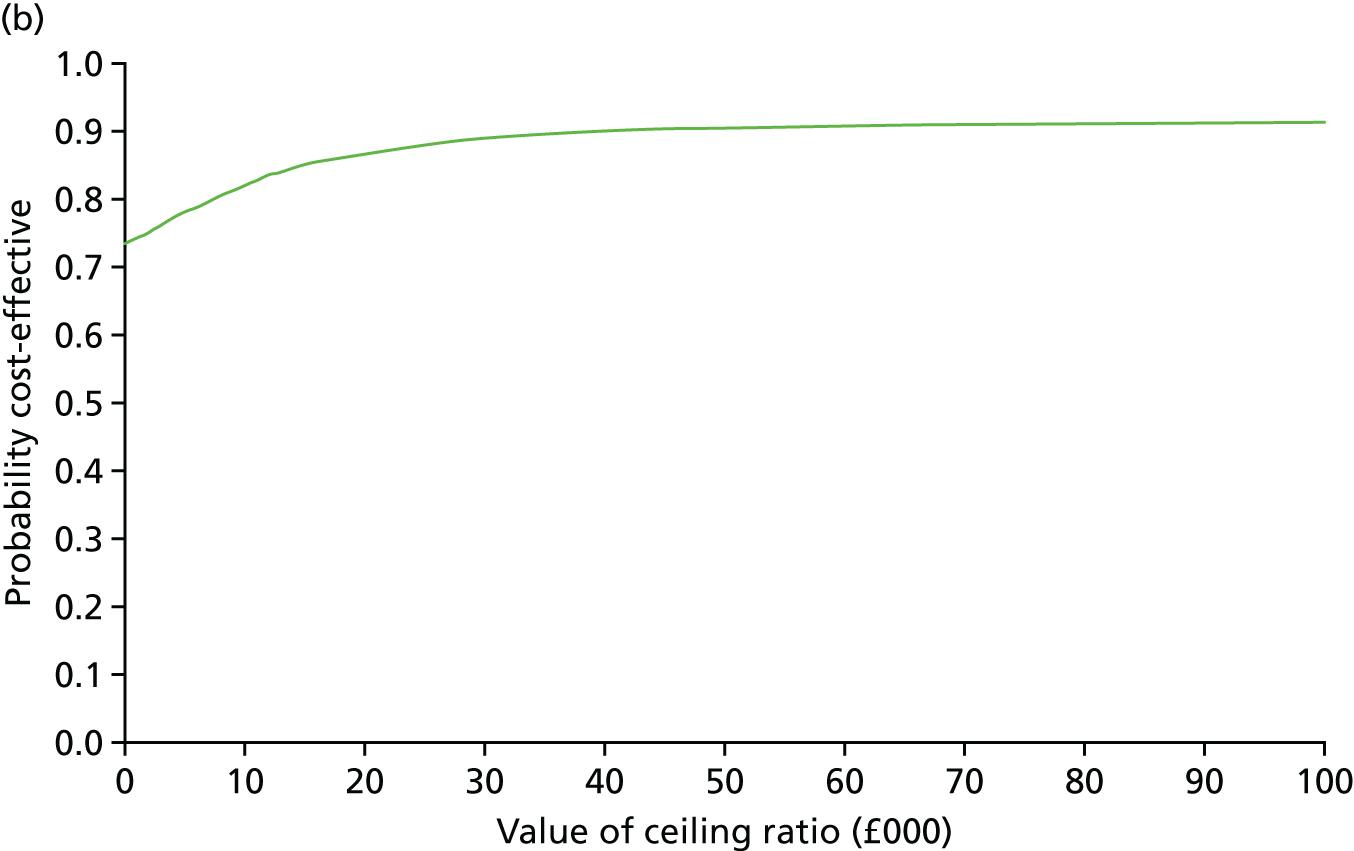
FIGURE 6.
Total costs and change in MSIS-Psy at 12 months (multiply imputed analysis). (a) Cost-effectiveness plane; and (b) CEAC.
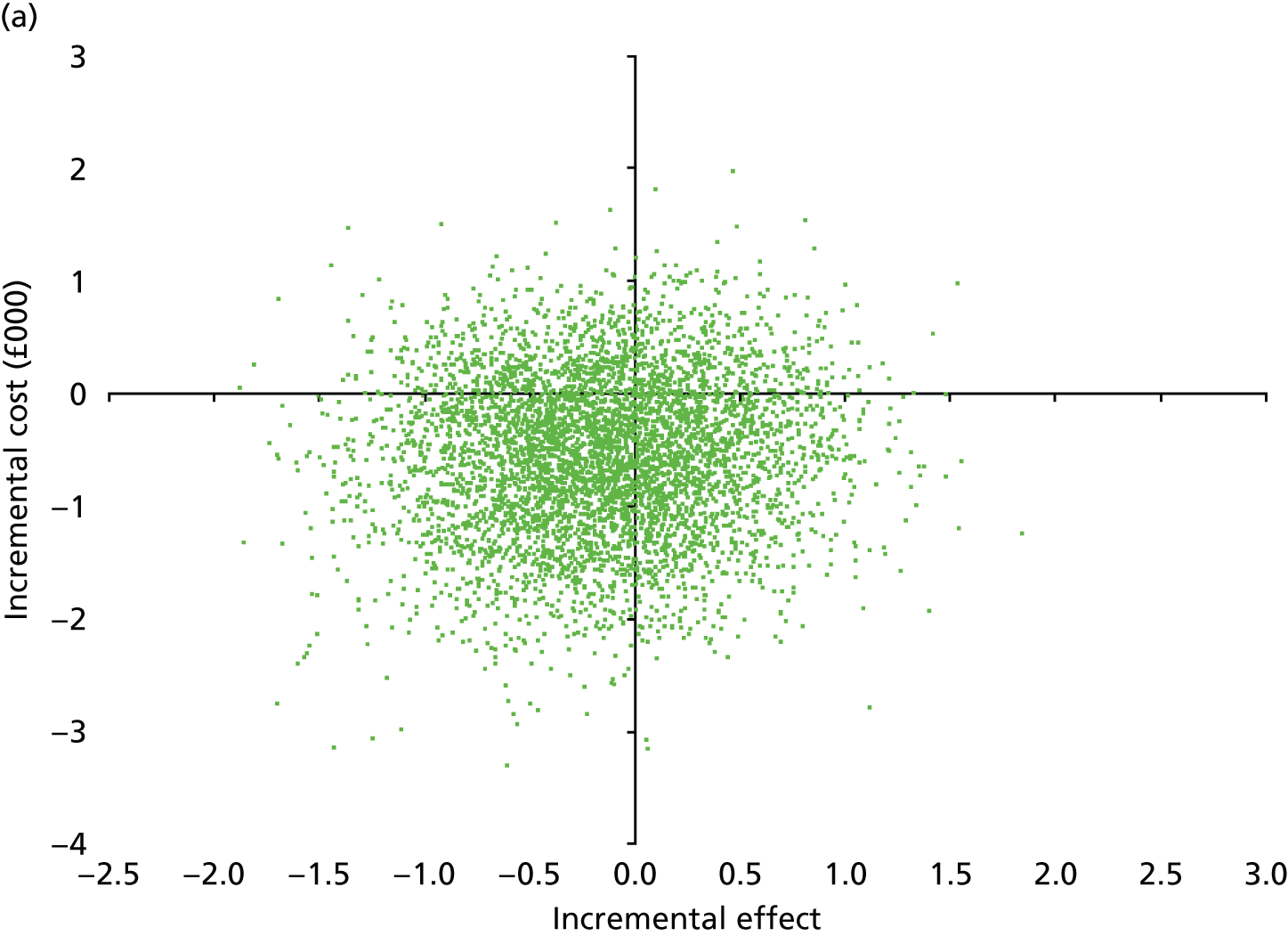
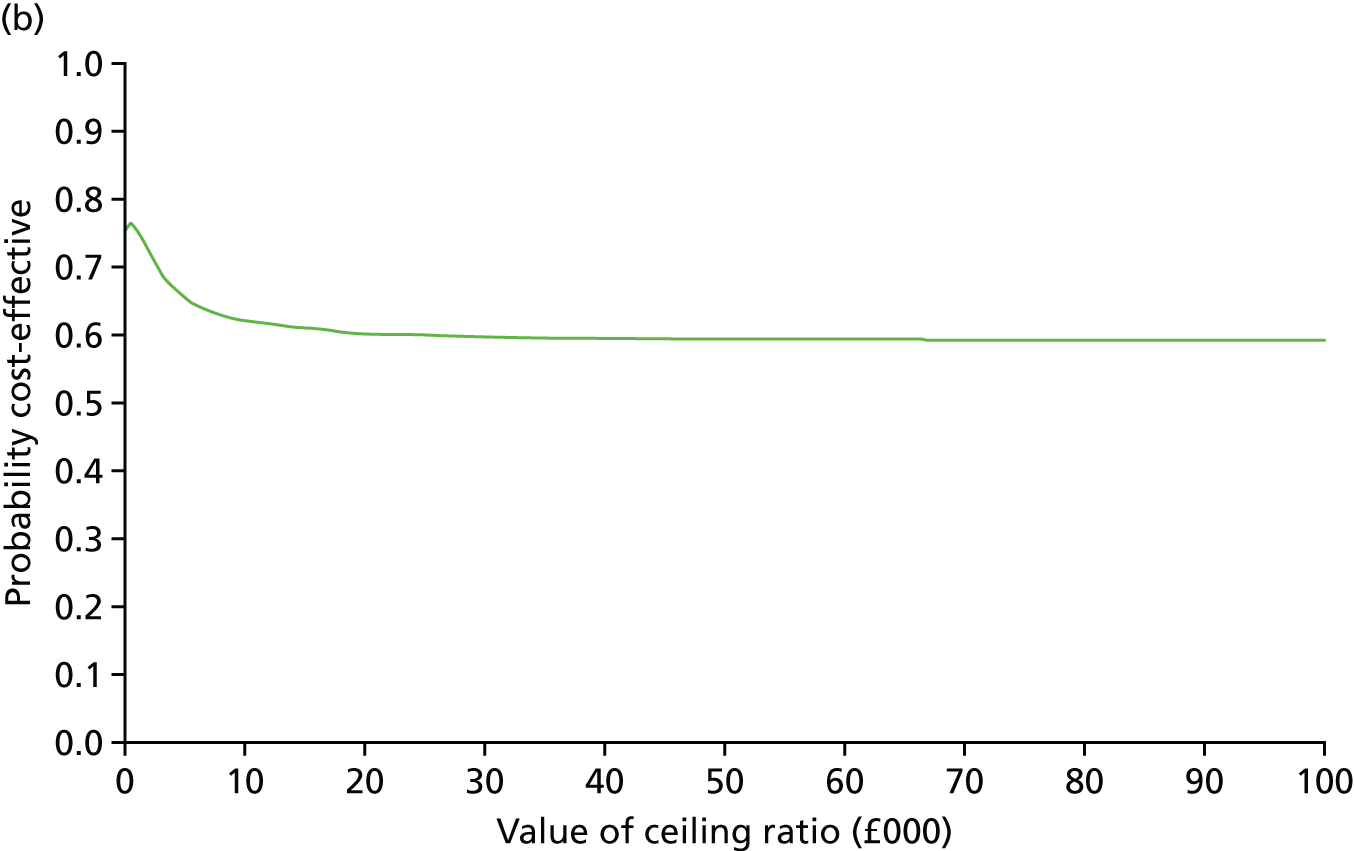
At 12 months, the cost-effectiveness planes (see Figures 4 and 5) show that cognitive rehabilitation was less costly and more effective than usual care; the distribution of bootstrapped estimates are centred in the south-east quadrant, which is consistent with the intervention dominating usual care. The estimates for MSIS-8D are centred further eastwards than those for the EQ-5D-5L. As higher scores on the MSIS-Psy indicate a greater psychological impact of MS on everyday life, a negative incremental effect represents an improvement in favour of cognitive rehabilitation, with the interpretation of the cost-effectiveness plane reversed on the x-axis compared with the analysis of QALY gains. The cost-effectiveness plane in Figure 6 has the largest proportion of estimates in the south-west quadrant; this is, again, consistent with the intervention being more effective and less costly than usual care.
The CEACs in Figures 4–6 show a high probability that cognitive rehabilitation was cost-effective compared with usual care at WTP thresholds of between £0 and £100,000 per QALY gain. For QALYs derived from the EQ-5D-5L, cognitive rehabilitation has an 84.8% probability of being cost-effective at a WTP threshold of £20,000 per QALY gain, and an 85.7% probability of being cost-effective at a WTP threshold of £30,000 per QALY gain. In comparison, for QALYs derived from the MSIS-8D, cognitive rehabilitation has an 86.7% probability of being cost-effective at a WTP threshold of £20,000 per QALY gain, and an 88.8% probability of being cost-effective at a WTP threshold of £30,000 per QALY gain. For both HRQoL measures, the probability that cognitive rehabilitation was cost-effective is very high at all WTP thresholds. For the MSIS-Psy, at a WTP threshold of £20,000 per point improvement, cognitive rehabilitation had a 60.6% probability of being cost-effective, and a 59.8% probability of being cost-effective at a WTP threshold of £30,000. However, caution should be given to interpreting these thresholds for the cost-effectiveness analysis as no established threshold exists for improvement in MSIS-Psy score.
Cost-effectiveness planes and CEACs were also constructed on multiply imputed data at 6 months to investigate earlier effects of the intervention on HRQoL; results are presented in Figures 7–9.
FIGURE 7.
Total costs and EQ-5D-5L QALYs at 6 months (multiply imputed analysis). (a) Cost-effectiveness plane; and (b) CEAC.
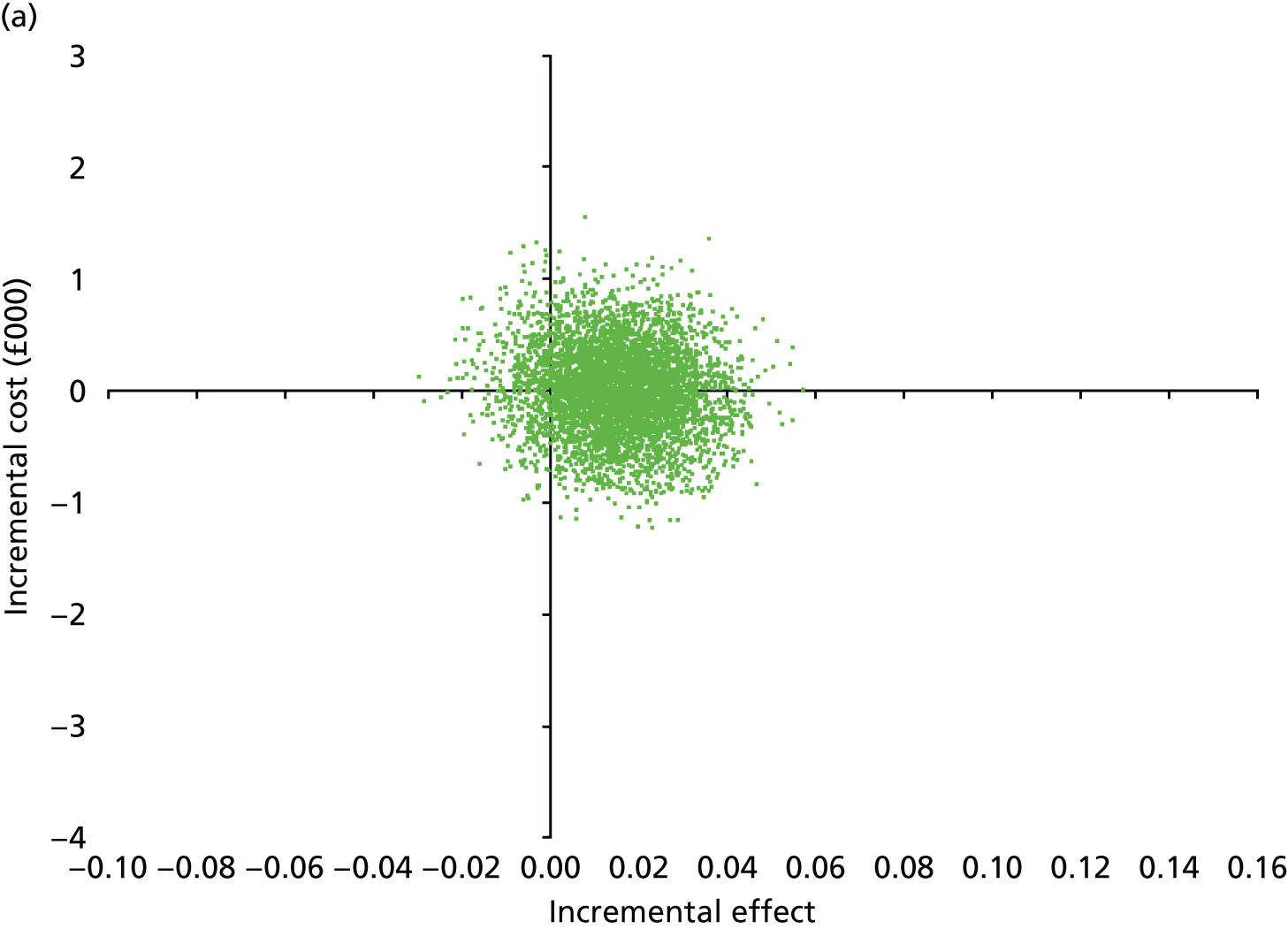
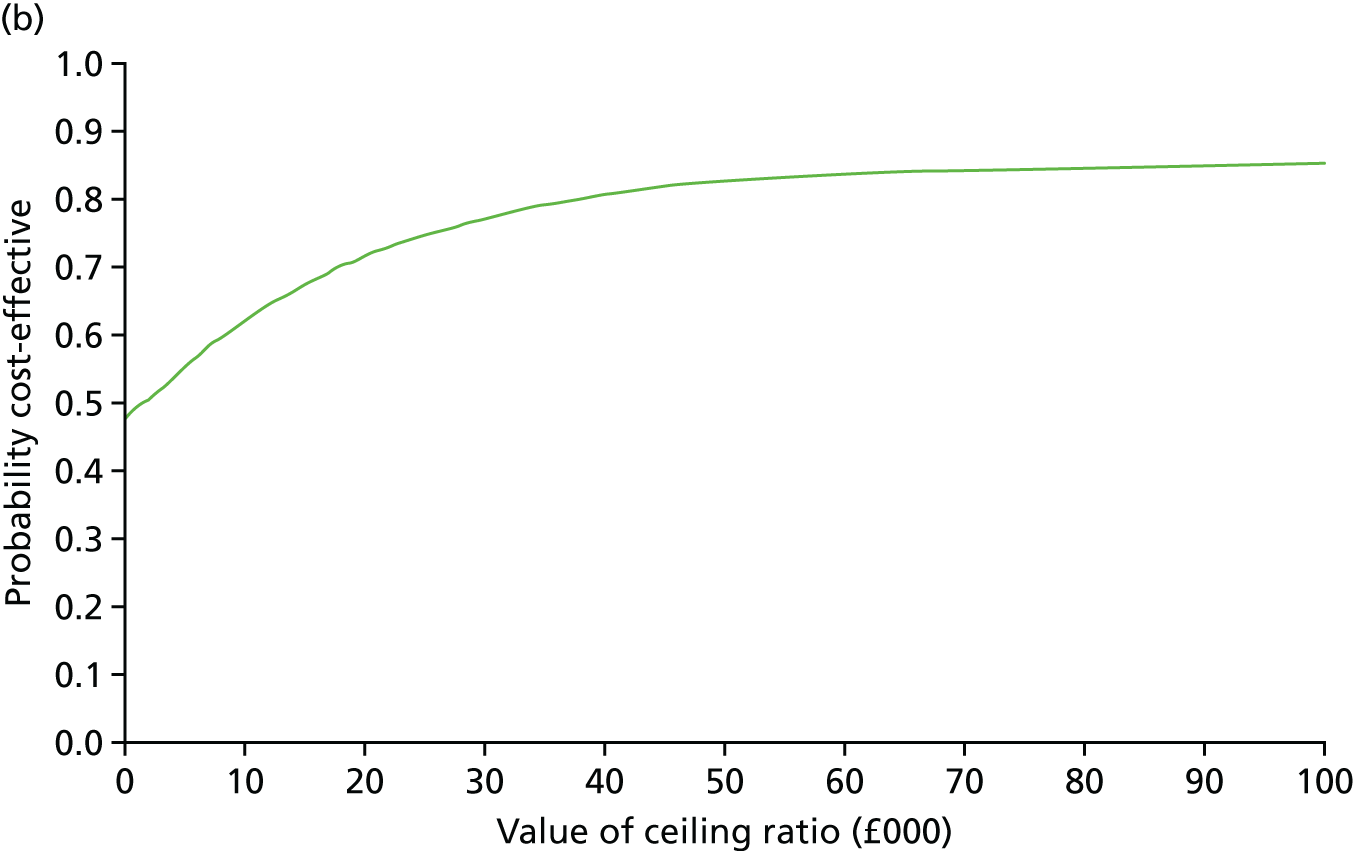
FIGURE 8.
Total costs and MSIS-8D QALYs at 6 months (multiply imputed analysis). (a) Cost-effectiveness plane; and (b) CEAC.
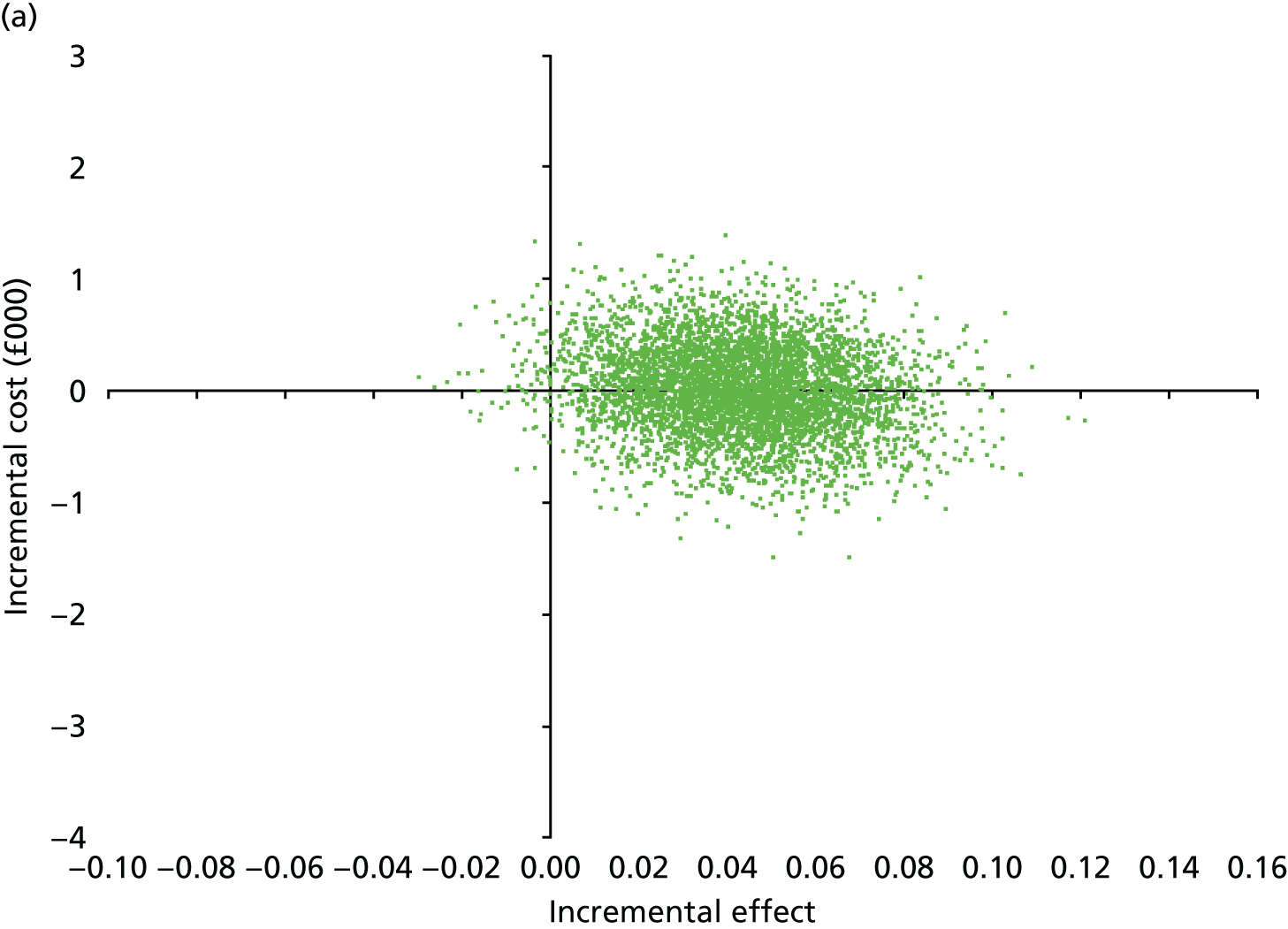
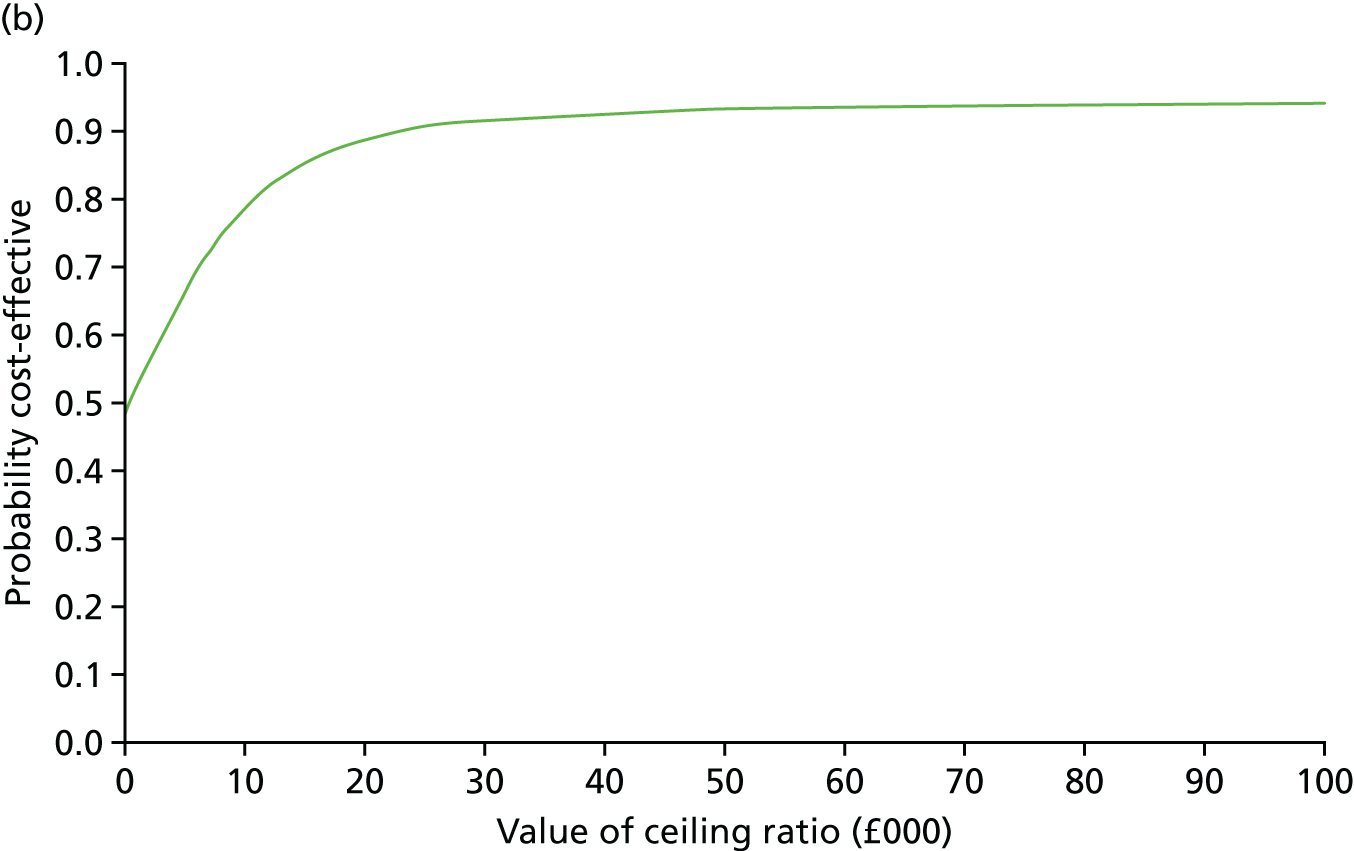
FIGURE 9.
Total costs and MSIS-Psy at 6 months (multiply imputed analysis). (a) Cost-effectiveness plane; and (b) CEAC.
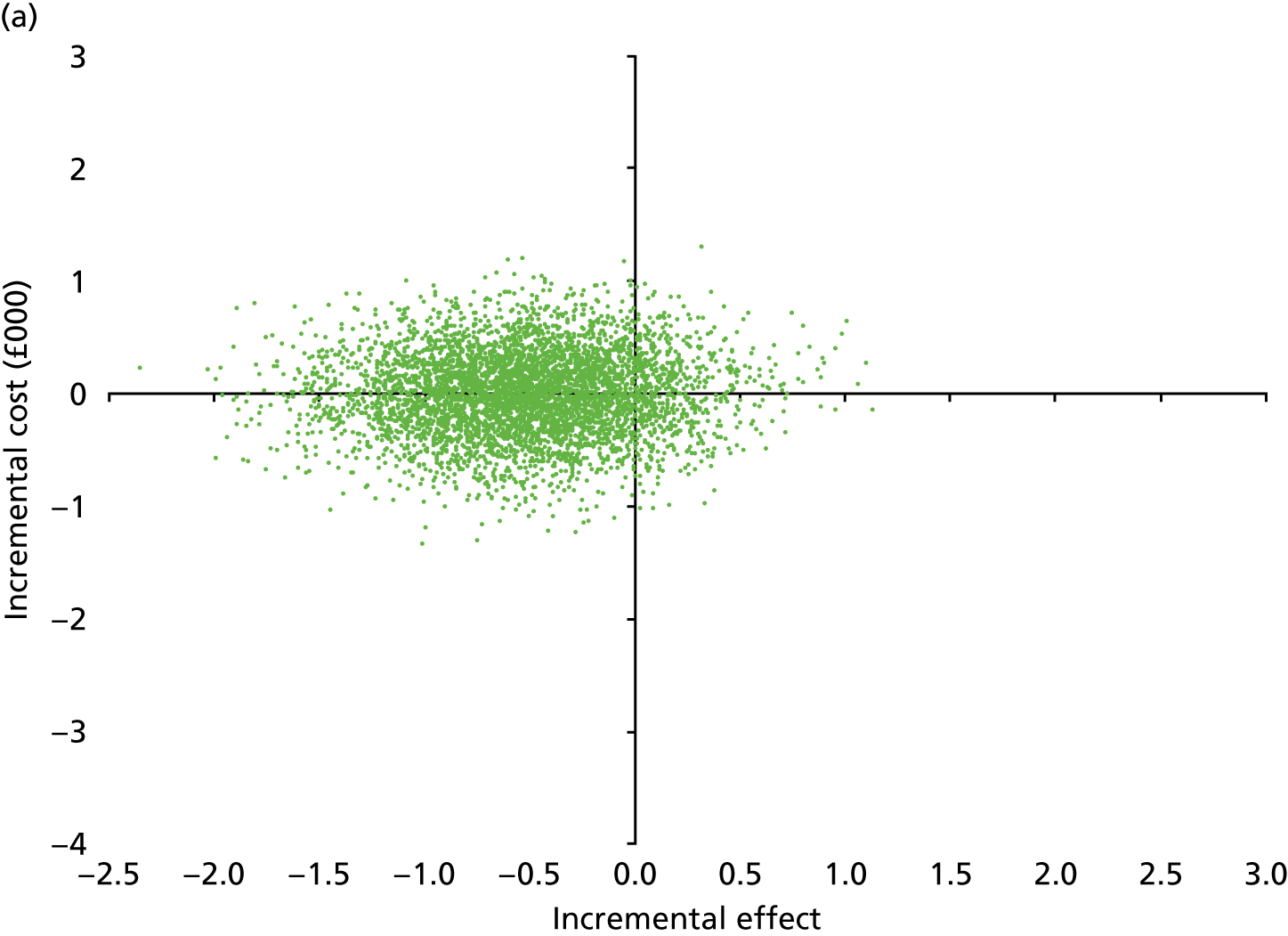
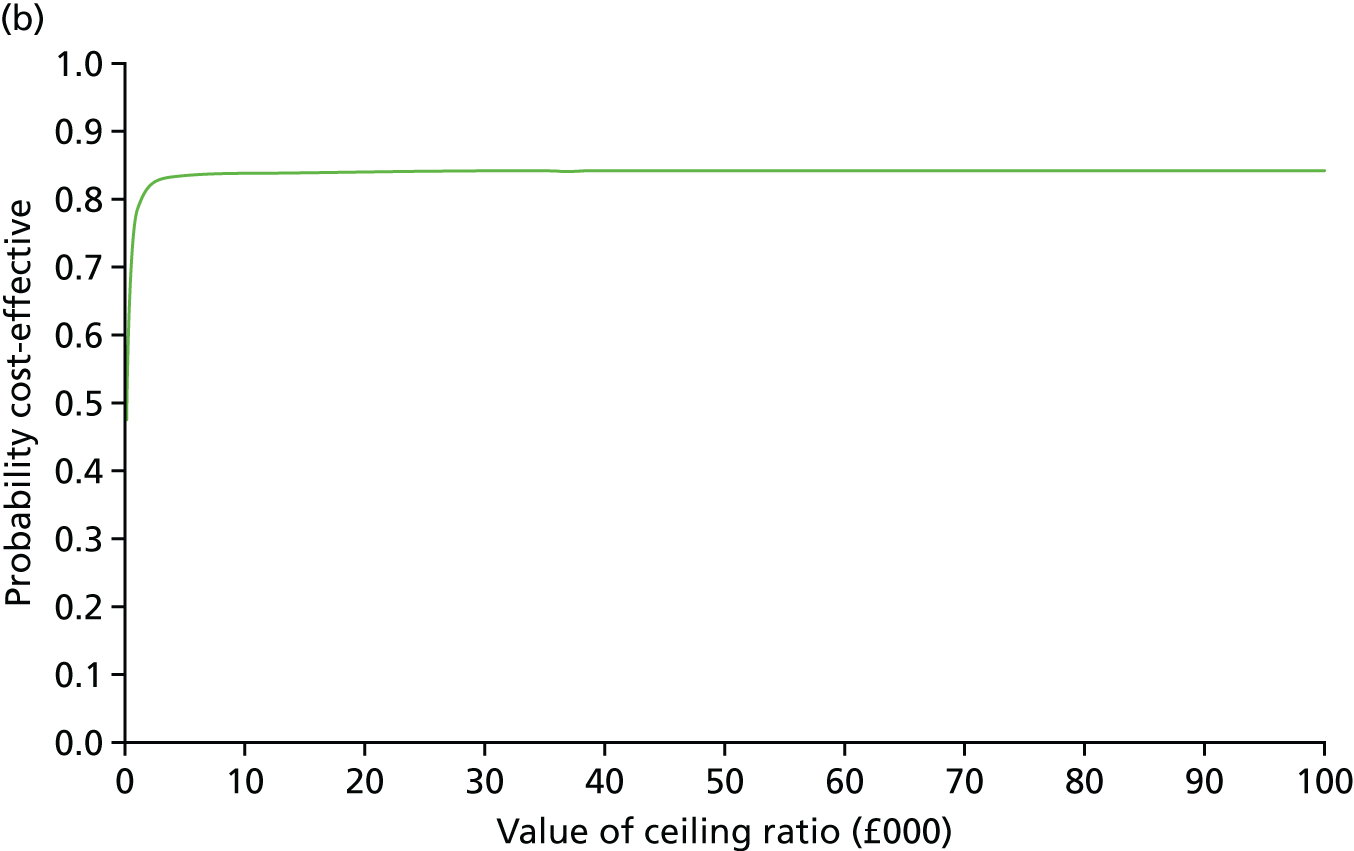
At 6 months, the cost-effectiveness plane estimates in Figures 7 and 8 for the EQ-5D-5L and MSIS-8D are, again, primarily clustered on the x-axis, and to the east of the y-axis. The distribution of bootstrapped estimates for the MSIS-8D was slightly further east of the y-axis than for the EQ-5D-5L, consistent with the greater level of effectiveness observed for the disease-specific measure of HRQoL. The CEACs show that, at WTP thresholds of ≥ £20,000, a high probability of cost-effectiveness of cognitive rehabilitation is observed. For the MSIS-Psy, (see Figure 9) the distribution of bootstrapped estimates is located mainly to the west of the x-axis, consistent with the intervention being more effective than usual care. At a WTP threshold of £20,000, the intervention has an 83.9% probability of being cost-effective, which rises to 84.1% at a WTP threshold of £30,000.
Sensitivity analyses
To assess the impact of the base-case findings to both methodological and parameter uncertainty, a series of sensitivity analyses were conducted, including an ACA and a multitude of OWSAs.
The base-case analysis used multiple imputation to address missing data for both costs and outcomes. To examine the impact that imputation had on the findings, an ACA was conducted on the data directly observable from the questionnaire responses. Tables summarising cumulative costs, and QALYs derived from the EQ-5D-5L and MSIS-8D are presented in Appendix 17, Tables 47–49.
For available cases, Appendix 17, Table 47, shows that, at 12 months, the mean total costs associated with cognitive rehabilitation [£5885 (n = 208)] were over £800 lower than those of usual care [£6574 (n = 170)]. Consistent with the primary multiple imputation analysis (see Table 21), this indicates that cognitive rehabilitation was less costly than usual care. Again, the difference was not statistically significant, with wide CIs observed.
The incremental cost-effectiveness analysis for the ACA is presented in Appendix 17, Table 50. For the primary cost–utility analysis (incremental cost per QALY on EQ-5D-5L at 12 months) and all secondary cost–utility analyses, negative incremental costs and positive incremental QALYs are observed. This is consistent with the multiple imputation analysis and represents dominance of the intervention, with cognitive rehabilitation being less costly and more effective than usual care. As CIs for both costs and QALYs span zero and are not statistically significant, the interpretation of cognitive rehabilitation as cost-effective requires some caution. For the MSIS-Psy, negative incremental effects observed at 6 and 12 months are representative of an improvement in favour of the intervention group. The intervention can therefore be considered dominant at both 6 months and 12 months; however, as noted in Chapter 3, the difference at both follow-ups is not considered to be of clinical significance.
Cost-effectiveness planes and CEACs were also constructed for the ACA on EQ-5D-5L and MSIS-8D QALY gains. These are presented in Appendix 17, Figures 12–15. As observed for the multiple imputation analysis, at 12 months, the cost-effectiveness planes (Figures 12 and 13) show that the distribution of bootstrapped estimates were centred in the south-east quadrant, which is consistent with the intervention dominating usual care. The estimates for MSIS-8D are centred further eastwards than those for the EQ-5D-5L. Figures 12 and 13 show a high probability that cognitive rehabilitation was cost-effective compared with usual care at WTP thresholds of between £0 and £100,000 per QALY gain.
Cost-effectiveness planes and CEACs were also constructed at 6 months to investigate earlier effects of the intervention on HRQoL; the results are presented in Appendix 17, Figures 14 and 15. At 6 months, the cost-effectiveness plane estimates in Figures 14 and 15 for the EQ-5D-5L and MSIS-8D are, again, primarily centred in the south-east quadrant. Points are clustered closer to zero, with smaller incremental costs and QALY gains than in the 12-month analysis. In addition, all four quadrants of Figures 14 and 15 are populated, indicating greater uncertainty regarding the cost-effectiveness of cognitive rehabilitation at 6 months. In accordance with the CEACs at 12 months, Figures 14 and 15 show a high probability that cognitive rehabilitation was cost-effective at all WTP thresholds up to £100,000.
A series of OWSAs were undertaken to assess the impact of parameter uncertainty on the estimations of cost-effectiveness. These sensitivity analyses were conducted on the multiple imputation data set, providing an objective approach in handling uncertainty regarding missing data. The results are presented in Appendix 18, Tables 51 and 52.
As the 95% CIs for both incremental costs and incremental effects span zero, the OWSAs for the EQ-5D-5L QALY gains using a combination of these bounds provide four separate conclusions regarding the cost-effectiveness of cognitive rehabilitation. At 12 months, using the lower bound for cost and the upper bound for effect, intervention dominance is observed to be consistent with the base-case analysis, whereas when using the upper bound for cost and the lower bound for effects, usual care is dominant. Using the upper bound for both incremental costs and incremental effects produces an ICER of £31,055 per QALY gained, whereas using the lower bound for both incremental costs and incremental effects produces an ICER of £89,306 per QALY lost. Similar results are observed at 6 months and for the MSIS-8D at both 6 and 12 months. These sensitivity analyses emphasise that, despite the base-case analysis suggesting dominance of the intervention, these results are subject to uncertainty and are not conclusive.
Including the cost of cognitive assessment increased the intervention cost from £209, used in the primary analyses, to £333 (see Appendix 19, Table 53). At 12 months, the calculated ICERs for the QALYs derived from both the EQ-5D-5L and the MSIS-8D continued to be representative of intervention dominance. At 6 months, the inclusion of cognitive assessment resulted in an incremental cost of £104.09 (95% CI –£653.13 to £861.31). When cognitive assessment was included as a component of the intervention and used to estimate the incremental cost per QALY gain at 12 months using the EQ-5D-5L, an ICER of £39,826 was calculated. The ICER for the QALYs derived from the MSIS-8D would be £14,559.
As noted in Resource use and costs, two notable outliers with total costs in excess of £40,000 at 12 months were identified. Removing these two outliers from the ACA at 12 months reduced the incremental cost from –£808.33 to –£38.85 (95% CI –£1162.14 to £1084.45). This illustrates that the two outliers have a large effect on mean costs, despite not resulting in statistical significance. As cognitive rehabilitation continued to be less costly than usual care, albeit this cost difference was considerably reduced, there is no change in the interpretation in the ICER of intervention dominance.
Chapter 2 detailed the use of upper and lower 95% CIs for incremental costs and effects; we assessed the impact of changes in key parameters to the ICERs.
Results of model-based analysis for longer-term cost-effectiveness
The original trial protocol65 and health economics plan proposed a model-based analysis to evaluate the longer-term cost-effectiveness of cognitive rehabilitation at horizons > 1 year. In the proposal, criteria were determined to ensure that any model-based analysis would be rigorously undertaken and produce reliable and informative estimates of the longer-term cost-effectiveness of cognitive rehabilitation. Given the results of the within-trial analysis the conclusions are as follows:
-
Cognitive rehabilitation does not show sufficient evidence of clinical effectiveness. Neither a statistically significant nor clinically important difference was observed between the cognitive rehabilitation and usual-care groups in the MSIS-Psy score at 12 months.
-
Although the primary cost–utility analysis indicated that cognitive rehabilitation was dominant compared with usual care, differences in costs and QALY gains were not statistically significant.
-
There was a lack of available evidence for the longer-term effects of cognitive rehabilitation. Clinical opinion deemed that, in the absence of any observed effect in the short term, no effect would be observed in the long term, during which patients no longer received the intervention; therefore, the decision was taken not to proceed with the model-based analysis.
-
Although a robust mathematical model could be constructed, the data inputs would yield an extrapolation of the within-trial results and not produce plausible, reliable or informative estimates of the longer-term costs and consequences of cognitive rehabilitation.
One potential area of future consideration would be to re-examine the evidence in the light of extending the meta-analysis reported in the Cochrane Review29,31 to include the CRAMMS trial findings. This would provide more robust and reliable data to inform a model-based analysis in the future.
Discussion
A comprehensive economic evaluation was undertaken of cognitive rehabilitation for attention and memory problems in people with MS. The analysis was designed and reported following recognised standards. 153 The economic evaluation was conducted alongside the main trial, which had high methodological quality and rigour. This allowed for the economic analysis to benefit from the collection of a range of resource use, clinical effectiveness and health utility information to inform future economic evaluations in this area. A particular strength of the design is the use of the EQ-5D-5L and MSIS-8D to assess the impact of using a generic-based HRQoL compared with deriving utilities from a condition-specific measure for people with MS.
The results of the economic analysis are complex. Overall, cognitive rehabilitation was found to have non-significantly lower costs at 12 months (–£575) than usual care, with no gain in QALYs (based on the EQ-5D-5L) as a result of cognitive rehabilitation compared with usual care, that is the treatment groups did not differ significantly in either costs or benefits. When the MSIS-8D was used as an alternative method for deriving utilities, there was a numerically small, significant incremental QALY gain. In this scenario, this small QALY gain (0.01) was not statistically significant. These results indicate that the intervention dominates usual care; however, although cognitive rehabilitation is technically cost-effective, QALY gains were very small and differences in incremental costs and incremental effects are not statistically significant. These results are consistent with the analysis of the primary outcome, which also failed to observe a clinically significant impact in the MSIS-Psy.
The probability of cognitive rehabilitation being cost-effective at 12 months at a societal WTP threshold of < £20,000 per QALY was 84.8% and 85.7% (utilities derived from the EQ-5D-5L and MSIS-8D, respectively). In the light of the results, further scrutiny is needed to ensure that such findings are appropriately interpreted in the context of the main trial findings. Sensitivity analyses were undertaken to assess the impact of varying the methods and parameters used in the evaluation. As the 95% CIs used to calculate the OWSAs span zero, the incremental costs and effects resulted in varying interpretation of the cost-effectiveness of the intervention, including scenarios of intervention dominance and usual care dominance. This further highlights the uncertainty in the health economic results and the inability to draw decisive conclusions regarding the cost-effectiveness of cognitive rehabilitation.
As previously reported, the MSIS-8D produced a slight, but statistically non-significant, QALY gain at 12 months (0.01, 95% CI 0.01 to 0.03; p = 0.19) for cognitive rehabilitation compared with usual care, whereas the EQ-5D-5L produced no numerical change in QALYs at 12 months for cognitive rehabilitation compared with usual care (0.00, 95% CI –0.02 to 0.02; p = 0.91). For both measures, the 95% CIs include zero, indicating no difference in QALY gain between cognitive rehabilitation and usual care at 12 months. A similar picture was shown for the multiply imputed and available-cases scenarios, suggesting that the analysis was relatively robust to the methodological uncertainty utilised to derive utilities in the economic analysis, with regard to QALY outcomes. Overall, cognitive rehabilitation made little or no impact on QALYs gained as a result of the intervention compared with usual care. This is commensurate with the main clinical findings, which found that cognitive rehabilitation did not confer a clinical benefit in improving HRQoL for this group of people with MS.
The impact of cost presents a mixed picture, particularly in accounting for the impact of key cost drivers in the overall results. As reported in the results, the largest health resource use and costs were incurred in the last 6 months of follow-up. In the first 6 months of follow-up, cognitive rehabilitation was £20 (95% CI –£777.13 to 737.31; p = 0.96) cheaper than usual care, whereas, at 12 months, cognitive rehabilitation was £575 (95% CI –1878.93 to 729.07; p = 0.39) cheaper than usual care. When available cases were examined, these differences in costs at follow-up showed a similar but greater pattern, with cognitive rehabilitation £13 cheaper at 6 months, increasing to £808 cheaper at 12 months, than usual care. It is important that further post hoc analysis does not violate or undermine the principles applied to the health economic methods, particularly with respect to missing data. However, when the available cases were examined in more detail to explore potential factors driving this cost difference, the impact of two extreme outliers in the usual-care group was identified: one due to medication use to treat osteoporosis and one due to an extended hospital stay. When these two outliers were removed from the analysis, the cost difference was £39 (95% CI –£1162 to £1084) in favour of cognitive rehabilitation over usual care. Although the finding that cognitive rehabilitation was less expensive than usual care remained robust, there was uncertainty, introduced by wide variances in the figures, ranging from cognitive rehabilitation being £39 cheaper than usual care on removal of two outliers in usual care who incurred large health-care costs, to cognitive rehabilitation being £808 cheaper than usual care when all available cases were examined. The variation in findings could result in different interpretations of the impact and magnitude of the conclusion on any potential for cost savings as a result of cognitive rehabilitation compared with usual care. The plausibility of cognitive rehabilitation having an impact on reducing resource use and costs over the longer term should also be placed in the context that, although there was some evidence from the trial that the programme improved HRQoL over the short term, these effects were not maintained over time.
In the light of the clinical findings, the lack of evidence of whether or not such a programme of cognitive rehabilitation would result in longer-term clinical benefits and the conclusion from the trial team and TSC that any longer-term modelling would not produce plausible, reliable or informative estimates of the longer-term costs, further modelling would not add value to the analysis. This is a departure from the trial protocol and was endorsed by the Health Technology Assessment (HTA) programme following a detailed explanation.
There are important limitations that are acknowledged. A health and Personal Social Services perspective was employed that excluded wider costs, such as costs borne by other sectors, patients and family and employers. Although employment status was captured in the trial, this was not further considered in the economic analysis. However, changes in employment status and the MCSI score were not statistically significant between groups (see Tables 9 and 10).
Although the resource use measure had been developed and used in a trial of a similar population,107 a number of caveats must be recognised. The costing methodology considered service use over the previous 3 months. As follow-up visits took place at 6 months and 12 months, any differences in costs arising between 0–3 months and 6–9 months are unlikely to be adequately captured. A notable issue, which has affected the explanation of the findings, is that the questionnaire did not consider medication costs specific to the management of MS, other than being able to extract the impact of disease-modifying therapies on health-care costs. Although the questionnaire tailored other health-care use to ‘as a result of your MS’, data were not collected on the reasons for hospital stay, which, as shown in the analysis, was high; thus, a unit cost specific to MS was applied. Although the resource use measure provided a good basis given its use in a previous trial,95 further work to enable the collection of as accurate and comprehensive resource use as possible balanced against the costs and burden of doing so should be undertaken for similar trials in the future. 154
Chapter 7 Discussion
Context
This pragmatic trial was designed and conducted in response to a commissioned call for proposals concerning cognitive rehabilitation for people with MS from the HTA programme (HTA number 12/190). This call highlighted both the lack of research evidence to support the provision of cognitive rehabilitation for people with MS and the potential to provide evidence to improve clinical practice in rehabilitation for people with MS. The important outcomes to be addressed were the impact of cognitive rehabilitation on functional performance and HRQoL. Additional outcomes suggested by the commissioning brief included objective measures of cognitive function, subjective scores of cognitive functions, adherence to the intervention, depression scores, fatigue, employment status and impact on family members and carers. The minimum period of follow-up was stipulated as 1 year.
Summary of findings
Clinical effectiveness
The results indicate that there was no benefit of this cognitive rehabilitation programme for this group of people with MS on their quality of life, with no clinically important difference in MSIS-Psy score (i.e. the primary outcome) between the two groups at the 12-month follow-up. However, there was a small difference in MSIS-Psy score at 6 months follow-up, favouring the intervention group. There was a difference between the cognitive rehabilitation and the usual-care groups in terms of subjective participant and relative reports of memory problems, as assessed on the EMQ, favouring the cognitive rehabilitation group at both 6 and 12 months. A similar finding was observed on mood outcomes, indicating that the cognitive rehabilitation group showed less psychological distress than the usual-care group at both 6 and 12 months. There were, however, no important differences between the groups on objectively assessed cognitive abilities, fatigue, employment status or carer strain at the 6- or 12-month follow-ups. No safety concerns were raised and no deaths were reported. The evaluation of treatment fidelity indicated that cognitive rehabilitation was delivered as intended. Interviews with a subset of participants found that the treatment was well received and was different from usual care. During interviews, the majority of participants reported that they found the intervention helpful in reducing cognitive problems and improving confidence in daily life.
Cost-effectiveness
The health economic analysis illustrates that cognitive rehabilitation had lower costs and higher QALY gain than usual care; however, incremental differences in both these measures were small. Although this represents dominance of the intervention, at 12 months differences were not statistically significant and sensitivity analyses demonstrated uncertainty concerning the base-case analysis. Therefore, definitive conclusions cannot be drawn regarding the cost-effectiveness of cognitive rehabilitation compared with usual care.
Interpretation
This cognitive rehabilitation programme did not reduce the psychological impact of MS at 12 months, but it may have reduced the psychological impact of MS at 6 months, reduced the frequency of subjective complaints of cognitive problems and improved mood at the 6- and 12-month follow-ups. In the following sections, the possible reasons for this pattern of results are considered.
Choice of primary outcome measure
The commissioning brief indicated that quality of life was an important outcome; therefore, a quality-of-life measure was used as the primary outcome. The MSIS was chosen as it was developed specifically for people with MS, provides a patient’s perspective on the impact of their disease on daily life and Rasch Analysis has confirmed the validity of two separate subscales: physical and psychological. 84,85 The MSIS-Psy subscale includes items on mood, and coping and cognition, has good psychometric properties and has been shown to be sensitive to the effects of rehabilitation interventions (see Chapter 2). Most of the MS-specific quality-of-life scales include many items that reflect the MS disease process, such as balance, stiffness and spasms. However, cognitive rehabilitation does not alter the disease process; therefore, the physical symptoms of MS are not expected to change. The aim is to enable people to cope with their symptoms better. Although the MSIS-Psy subscale does include items related to cognition, such as problems concentrating, it also includes items not likely to be influenced by cognitive rehabilitation, such as feeling unwell and having problems sleeping.
Another option was one of two modified versions of the Short Form questionnaire-36 items (SF-36), which has been amended to use as outcomes for pharmacological trials in people with MS: the Multiple Sclerosis Quality of Life-54 (MSQOL-54),155 and the Multiple Sclerosis Quality of Life Inventory (MSQLI). 156 However, both are very long157 and include a large proportion of items that are unlikely to be influenced by cognitive rehabilitation. A shorter version, the Multiple Sclerosis International Quality of Life questionnaire (MusiQoL),158 may have been appropriate but, as noted by Bandari et al. ,157 there is a lack of information on the responsiveness of the instrument compared with other clinical end points. In addition, Baumstarck et al. 159 found that the MusiQoL was not responsive to changes in disability as recorded on other measures.
There are two outcome measures that have been developed to assess the outcome of cognitive rehabilitation. The Adaptation to Memory Difficulties Outcome questionnaire (AMEDO)160 was developed to assess the effects of memory rehabilitation, and the validation studies included people with MS. Patchick et al. 161 developed an outcome measure for cognitive rehabilitation after stroke. Although these measures seem appropriate, their responsiveness to the effects of intervention and their psychometric properties had not been fully evaluated at the time of starting the trial. However, they might be considered in future evaluations of cognitive rehabilitation.
Therefore, the MSIS-Psy subscale was probably the most appropriate quality-of-life measure. However, even on the MSIS-Psy subscale, many of the items are unlikely to be directly influenced by cognitive rehabilitation. Quality of life is, of necessity, a multidomain construct, and changing quality of life, although desirable, may be unrealistic for interventions that focus on specific symptoms of MS. Future triallists may also consider a more direct measure of cognitive abilities in daily life as a co-primary outcome for cognitive rehabilitation trials. Co-primary outcomes are useful to provide a more ‘comprehensive picture of the intervention’s effects’,162 but have implications for the trial design.
Long-term versus short-term effects of the intervention
The aim of the cognitive rehabilitation programme was to change the long-term effects of cognitive impairment and, therefore, the primary outcome at the 12-month follow-up. Although this time frame is useful to determine the longevity of treatment effects, not having interim assessment time points more proximal to the end of the intervention risks missing the immediate impact of the intervention. As with most other behaviour change interventions, it is probable that the impact of the intervention is greatest immediately after the intervention, with gains diminishing over time. There was some evidence in this trial to suggest that the programme improved quality of life in the short term (i.e. at the 6-month follow-up), but the effects were not maintained over time. This is consistent with previous memory rehabilitation research that shows that there are short-term benefits in quality of life, which are not maintained. 29 Therefore, future research should consider having more outcome time points. However, the addition of further assessment time points has the potential to add to participant burden, risking lower numbers consenting to participate in the trial and increasing the likelihood of practice effects on some measures. Future research should also focus on ways in which the effects of cognitive rehabilitation can be maintained. This might include booster sessions, telephone calls to review progress or online support; an evaluation of booster sessions in a previous trial of improving learning in people with MS52 indicated little benefit from these, but the sample sizes were small.
Subjective cognitive impairment and mood
There were effects of cognitive rehabilitation on the reported frequency of everyday memory problems and mood. This could be because cognitive rehabilitation improved participants’ everyday memory, which had a beneficial effect on mood, or it could be that the intervention improved mood, which led to a decreased frequency of reporting cognitive problems.
Previous research has shown that there is a strong relationship between subjective cognitive problems and mood in people with MS. 102,103,163–166 Therefore, some of those recruited may have reported cognitive problems associated with low mood, and this is consistent with the improvement in mood that also occurred.
These effects may be partly attributable to the social contact afforded by attending for cognitive rehabilitation rather than the cognitive strategy training aspects of the intervention per se. The group component was reported to be a very useful aspect of the intervention by all participants in the intervention group who were interviewed. Future research on this type of cognitive rehabilitation programme should control for the therapeutic effect of the group interaction itself. However, creating an acceptable attention placebo control for such programmes is difficult:59 they run the risk of disproportionate attrition from the control group and there is no established gold standard for implementing an attention placebo control in psychosocial intervention trials. 167
It seems more probable that the change in mood was in response to changes in everyday memory, rather than the cause of the changes in everyday memory. The reason is that relatives’ reports of the frequency of everyday memory problems also showed a difference between groups, and relatives’ reports would be unlikely to be influenced by the participants’ mood. 102
One of the reasons that the EMQ was included as an outcome measure was to provide comparison with other studies of the same intervention. 59,60,95 The difference in EMQ scores at 6 and 12 months was about 5 points. The differences in the participant-reported EMQ are slightly smaller than those observed in previous studies of the intervention in people with MS,60,62 but consistent with the difference observed in people with traumatic brain injury. 95 The smaller difference in relation to previous studies of people with MS may be due to the broader recruitment strategies used and the difference may be comparable to the study of people with traumatic brain injury because similar recruitment strategies were used.
It is, therefore, also probable that teaching people with MS the cognitive strategies improved their confidence to use these in daily life. Improved confidence was also reported during the interviews by those who had received the intervention. Increased strategy use, the benefits of the group environment and improved confidence have all been reported in other qualitative studies of perceived effects of cognitive rehabilitation among people with MS. 136
Effect on cognitive impairment
The results, overall, indicated that the intervention had no effect on cognitive impairment. Scores in both the intervention and the usual-care groups tended to improve over time, which is probably as a result of practice effects and familiarity with the tasks. This finding is consistent with some studies of interventions to improve strategy use,58,168 whereas change in cognitive abilities tends to be observed in studies that provide specific training on cognitive tasks through computerised activities that are closely linked to the cognitive outcomes. 32,35,36,43,45,169
Other outcomes
Fatigue was assessed to determine whether or not the intervention exacerbated fatigue-related problems, but there was no indication of any negative effect on fatigue. Several previous trials of cognitive rehabilitation have included fatigue as an outcome measure and none has shown any effects of cognitive rehabilitation on general levels of fatigue. 35,38,40,42,46,58,170 Two studies have shown positive effects on cognitive fatigue,35,170 but in both these studies the intervention was computerised retraining of cognitive abilities. Therefore, it is improbable that cognitive rehabilitation has any effect on levels of general fatigue.
Guy’s Neurological Disability Scale and the number of relapses were recorded to determine whether or not any differences between the two groups could be attributed to relapses or increasing disability. There were no differences in the MSIS-Phy subscale score or the GNDS between the groups, so there is no suggestion that the findings were due to differences in the physical effects of MS.
High levels of unemployment are common among adults with MS. 171 In this trial, employment status was no different between the groups at baseline or at the follow-ups. The majority (97%) of those who were not employed at baseline were not employed at follow-up. Only a few participants gained employment during the trial and this was equally balanced between the groups. Therefore, the intervention did not facilitate return to work. This finding is likely to be partly due to the low rate of employment at baseline, and because the sample had relatively severe cognitive problems at the time of recruitment. There was also no evidence that the intervention enabled people to continue to work. This finding, however, needs to be considered with caution. The questions around employment and education did not consider whether or not participants had taken up voluntary positions. Although, to our knowledge, there is no research on the effect of volunteering on people with MS, there is good evidence that volunteering has a positive effect on psychological well-being and mood and slows self-reported decline in health and functioning levels among older adults. 172,173 Furthermore, absenteeism or presenteeism were not investigated. Research has demonstrated that there is a link between cognitive impairment and levels of absenteeism. 174 Future research could consider more nuanced ways of collecting information about employment status.
There was also no indication of an effect of the intervention on carers and relatives. Previous studies of people with MS have not examined the effects of cognitive rehabilitation on carers. However, one study with patients with acquired brain injuries found that holistic neuropsychological rehabilitation did reduce carer strain. 175 This study, however, used a pre–post repeated-measures design, and is therefore susceptible to bias.
Consistency with previous research
There are relatively few trials that have been conducted using a similar intervention with people with MS. The results support the pilot study,60 which demonstrated a beneficial effect on mood. A similar finding was observed in the ReMIND trial,62 which used a similar treatment approach, whereby people with MS who were taught the use of compensation strategies had better mood outcomes than those who had only restitution approaches. The finding that perceived cognitive deficits improved in response to group treatment is consistent with two studies with people with MS. 58,168 However, comparison of the present trial with the ReMemBrIn trial,95 which evaluated the same intervention in people with traumatic brain injury, suggested that those with MS responded better than those with traumatic brain injury. The most probable reason for this discrepancy is that usual care for people with traumatic brain injury often includes cognitive rehabilitation whereas it does not for people with MS. Those with traumatic brain injury may have been taught many of the strategies in the intervention before they were recruited and so they responded less well to the intervention.
The cost of providing cognitive rehabilitation in this trial was slightly higher than the costs of providing the intervention to people with traumatic brain injury (£209 vs. £167). 95 This was due to higher administration costs and the inclusion of the manual, stationery and refreshments in the cost of the intervention in the CRAMMS trial. If these are excluded, then the cost of providing the intervention is very similar in the two trials. However, the overall costs were higher for those with MS than for those with traumatic brain injury, largely because of the much higher medication costs for those with MS. The cost of providing cognitive rehabilitation was also similar to other group psychological interventions for people with multiple sclerosis. 90,107 Overall, the cost of providing cognitive rehabilitation is very low in the context of the overall costs of the disease. 176 The finding that there was little evidence to support the cost-effectiveness of cognitive rehabilitation was also consistent with the ReMemBrIn trial. 95
Strengths and limitations
The findings from this trial should be viewed in the light of its strengths and limitations.
Trial design
The strength of the trial lies in its methodological quality and rigour. The trial was prospectively registered and the protocol was published. 65 There was allocation concealment, meaning that those recruiting participants had no control over the random allocation or, therefore, who would receive treatment. Randomisation was conducted by a web-based system prepared in advance of the trial. The clinically relevant end points were defined a priori. The primary outcome was a questionnaire, so it was completed independently. The face-to-face outcome assessments were conducted mainly by a researcher who was blind to group allocation. Treatment sessions were video-recorded in order to assess the fidelity of the intervention. The SAP was agreed in advance of the data lock stage. The trial was reported in line with the CONSORT guidelines. 177 The content, format and delivery of the intervention was reported in line with TIDieR and other relevant guidelines. 109,178 The trial was adequately powered and recruited the required sample, and the attrition level was as predicted in the sample size calculation. There were independent trial steering and data monitoring committees to ensure that the trial was conducted as planned and that participant safety issues were considered.
One possibility for the small differences in subjective reports of cognitive problems in daily life and mood is that the effects observed were not due to the strategies taught as part of cognitive rehabilitation, but were due to additional attention and meeting others with MS. The ideal design would have been to also have a control condition in which participants met as a group for the same frequency and duration of sessions as the cognitive rehabilitation group but did not receive the active component of cognitive rehabilitation. In one of the pilot studies,59 there was an attention placebo self-help group as the control condition. In this condition, participants met to discuss their health and memory problems and were taught relaxation exercises. The facilitator did not initiate any memory talk. The fidelity analysis of these sessions130 confirmed that the self-help control group had significantly less discussion of memory than the active treatment groups. However, the self-help group was difficult to run, as participants wanted to discuss their memory problems and they felt that the problems they faced were not being addressed in the sessions. This may have had a negative effect, rather than controlling for the positive effect of being in a group.
In NHS clinical practice, very few people with MS receive treatment for their cognitive problems. They may receive some advice from MS nurses and occupational therapists and may be directed to information on MS charity websites. Therefore, it was decided to use usual care as the control condition rather than attempting to devise an attention placebo condition, which would be difficult to deliver. This means that the results are valuable for informing NHS practice, but the mechanism of the changes observed remains unclear.
Recruitment
Recruitment took place in five sites across England; thus, the participants are probably representative of those attending clinical neurology services in England. The recruitment strategy was to invite as many people as possible from each site to consider taking part in the CRAMMS trial. This was to ensure that all those who thought that they had cognitive problems were aware of the trial. As a result, the letters of invitation were sent to many who were not eligible. There were also a high proportion of people who did not respond to the invitation. The most probable reason is that they did not consider that they had cognitive problems, but there is no way of verifying whether or not this is the case.
Multiple recruitment sources and strategies were used to identify potential participants, including self-referrals. This is a strength, as it is inclusive, but is also a limitation. Some people may have received information from more than one source. As there is no demographic data available on all those who were approached and invited, it is not possible to ascertain the exact proportion of those who were invited who were eligible for the study and whether or not those recruited differ in any way from those who did not take part. Also, because some people were recruited through posters and publicity on websites, such as the MS Society and MS Trust web pages, it is not known how many people viewed the information and, therefore, what proportion of people chose to self-refer as a result.
The lack of information on the demographic characteristics of those who did not wish to take part in the CRAMMS trial limits the ability to determine the proportion of people who would be eligible for the treatment if it were available in the NHS.
Selection of participants
The inclusion criteria were broad in order to involve all those who might be treated in clinical practice, thereby increasing the generalisability of the findings. The most common reason for exclusion of those who were screened for eligibility was that they did not meet the criterion score on the MSNQ. Almost half of those excluded (116/283, 49%) were excluded for not meeting this criterion, yet they wanted to take part in a trial of cognitive rehabilitation, and so believed that they had cognitive difficulties.
In the pilot study,60 participants were recruited if they reported cognitive problems in daily life, and no standardised assessment of subjective cognitive impairment was used. In order to maintain consistency across sites, the MSNQ was used in the CRAMMS trial with a predefined cut-off score. As a result, some people who reported cognitive problems in daily life were excluded from the CRAMMS trial because they did not meet the cut-off score on the MSNQ. This may have led to a sample with relatively severe self-reported cognitive problems. This is supported by the difference in EMQ scores at baseline, which were lower in the pilot study (intervention, mean score 27; control, mean score 30)60 than in the CRAMMS trial (mean score 46).
A score of > 27 on the MSNQ was used as an inclusion criterion to identify those with cognitive problems in daily life, based on the original validation study. 66 Benedict et al. 66 included 50 participants with MS and used the informant version of the questionnaire rather than the patient-reported version. This cut-off score was also used by Vanotti et al. ,72 who found high sensitivity and specificity for the informant version. However, lower cut-off scores have been recommended subsequently in other studies. 69,179,180 Benedict et al. 69 found that the optimum cut-off score was > 23 on the patient-reported version and > 22 on the informant-reported version to identify those with cognitive impairment. O’Brien et al. 179 evaluated these cut-off scores in a sample of 48 people with MS, but found that the optimum cut-off score was > 8 to indicate impairment, using the same criterion for cognitive impairment as both studies by Benedict et al. 66,69 Comparison of cut-off scores for an internet version of the MSNQ181 showed that the MSNQ had a sensitivity of 69% using 27 as a cut-off score, in contrast to 79% using the lower cut-off score of > 22. In comparison, the specificity reduced from 42% to 17%. Therefore, the criterion used in the CRAMMS trial required a high level of reporting of problems in daily life; consequently, some people with fewer problems in daily life, who may have benefited from the intervention, were excluded.
It is possible that those with very high frequency of cognitive problems in daily life may not benefit from the intervention. It may be that > 10 sessions are required to address all their difficulties and to ensure that they are able to retain the information. The subgroup analysis of the MSNQ score at baseline in relation to 1-year outcomes on the MSIS-Psy, although not providing evidence of an interaction effect, suggested that the more severe the self-reported cognitive problems, the lower the response to intervention. Future trials should consider whether or not those with fewer problems in daily life who are less severely cognitively impaired may benefit more.
The second most frequent reason for exclusion was that participants were not able to travel to the treatment site (33/238, 14%). Travel expenses were offered but funding was not sufficient to provide taxis for all those who could not manage on public transport and who lived a long distance from where the treatment sessions were held. It is improbable that these people would have attended this treatment if it were offered in NHS clinical services, as transport would probably not be provided. Treatment sessions were held at several centres surrounding each site, so those unable to get to these centres would probably not have been able to get to NHS rehabilitation settings either.
Only a few people were excluded on the basis of being aged > 69 years (11/238, 5%), but in clinical practice these people probably would have been offered the treatment. In this respect, the group is slightly more homogeneous that those who would have been offered treatment in NHS clinical practice, but as the proportion is low it is unlikely to have had any real effect on the results.
Participants were recruited who failed one subtest of the BRBN. As a result, participants with problems in attention, memory or executive abilities were included. The BRBN was chosen as it is a relatively short screening test with appropriate normative data. The Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS)182 is similar but takes longer to administer. A shorter alternative was the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS),183 which has the advantage of being brief, but at the time of designing the study there was limited information of the psychometric properties of this measure. 184 More information is now available,185–189 and a direct comparison between the two190 suggested that the BICAMS is an acceptable alternative, but is less sensitive to cognitive problems, than the BRBN. The BICAMS may be a suitable alternative in future studies as a brief measure to detect cognitive impairment.
The use of a criterion of impairment relative to a healthy control population meant that some people were excluded who were impaired relative to their own pre-morbid level, but within the average range of the general population. This problem, highlighted by Sumowski et al. ,191 means that not all those with cognitive impairment from their MS would have been included, even though they may have reported problems on the subjective measure.
Participant characteristics
Participant demographic characteristics were largely consistent with what would be expected in a sample of people with MS. The average age was 50 years, with the majority in their late 40s and early 50s. This reflects the likelihood that they were not recruited very soon after diagnosis, when the average age is more likely to be < 40 years,192,193 and that some recruitment was through charities, such as the MS Society, whose meetings tend to be attended by a higher proportion of people with secondary progressive MS, than those attending neurology clinics.
The proportion of women in the trial was 73%. This is consistent with the expectation that women are more likely to have MS than men. 192,193
Most participants were of white ethnicity, consistent with the general UK population,194 which partly reflects the communities where participants were recruited. 195 The south-west and north-east of England have relatively low numbers of people from ethnic minority groups. 194 However, even in the East Midlands there were very few participants from an ethnic minority background. Some ethnic minorities may have been excluded because we required people to have a sufficient level of English proficiency to allow them to independently complete the standardised tests and questionnaires and because the intervention was delivered in English; however, the under-representation of ethnic minority groups in health-care research in Western countries has been observed more widely. 196 In addition, the incidence of MS is recognised to be lower in some non-white ethnic groups than in those who are white. 141
Participants were, on average, recruited 11 years after their MS diagnosis; most were recruited > 5 years after diagnosis. Therefore, they had established problems and were not adjusting to the effect of diagnosis at the same time as engaging in the intervention. For some people, their cognitive problems may have been so long standing that they had established coping strategies and found it difficult to adapt to new ones. Feedback in the interviews suggested that some people thought that they should have received cognitive rehabilitation sooner after the onset of their problems, so that they could learn to use strategies to prevent their cognitive problems affecting their daily life.
Most participants (65%) reported having relapsing–remitting MS. However, this may be an overestimate, as some commented that this was the diagnosis they had been given and now they thought that they were probably secondary progressive. As many participants were recruited through community settings, they may not have received an updated diagnosis from a neurologist. This is consistent with the majority (76%) reporting no relapses in the previous 6 months at the time of recruitment. Although it would have been desirable to have the type of MS confirmed by a neurologist at the time of recruitment, there was no indication of differential effects of cognitive rehabilitation according to reported type of MS.
The cognitive rehabilitation programme was intended to teach participants strategies to cope with both attention and memory problems. However, the content of the intervention was much more focused on coping with memory problems and only two of the 10 sessions were devoted to problems of attention. It is possible that those who primarily had problems with attention did not have enough intervention for their specific problems to enable them to benefit. It is also possible that attention problems respond to restitution strategies rather than compensation strategies. Most of the studies of computerised cognitive rehabilitation have targeted the attention deficit in people with MS, rather than the memory impairment. Mitolo et al. 28 reported that most recent studies have focused on retraining attention, speed of processing and executive functions, rather than memory, and these have mainly involved computerised practice. It may be that those with attention problems benefit more from a restitution strategy and that compensation strategies should be reserved for those with memory problems. This is supported by the finding that there was a trend for those with more severe attention problems, as assessed on the Symbol Digit Modalities Test, to respond less well to the intervention than those with milder problems. Future studies of this rehabilitation programme could select those with memory problems, rather than also including those with exclusively attention problems, even though the programme does include consideration of strategies to improve attention.
Intervention
Attendance at sessions was relatively good; therefore, most people received most of the intervention. The ability to provide catch-up sessions for those who could not attend some sessions also ensured that people received most of the intervention and reflected procedures used in clinical practice. The attendance rate [mean 7.7 (SD 3.5) sessions] was consistent with the pilot study60 [mean 7.9 (SD 0.23) sessions] and higher than in a similar trial of the cognitive rehabilitation programme for people with traumatic brain injury64 [mean 6.3 (SD 3.5) sessions]. However, many studies do not report attendance rates,197 so direct comparisons of attendance rates across trials are limited.
Many of the reasons for non-attendance reflect aspects of daily life unrelated to cognitive problems, such as illness and holidays, rather than withdrawing or not wanting to continue in the group. The optimum ‘dose’ of the intervention remains unknown, and so it is not clear whether or not more sessions would have improved the outcome or fewer sessions would have maintained the outcome. More Phase II trials are needed to establish the optimum dose of the intervention.
The focus of the intervention was on teaching strategies to cope with cognitive problems, rather than addressing the underlying cognitive deficit. The strength of this approach is that the effects of rehabilitation are more likely to persist over time as cognitive abilities deteriorate.
This cognitive rehabilitation programme may not be appropriate to all those with cognitive problems and some clinicians may select treatments according to participant characteristics or to the nature of their cognitive problems. However, there is little information available on who might benefit the most from specific types of cognitive rehabilitation.
The cognitive rehabilitation was conducted in groups. An advantage of this is that people may benefit from the experiences of others in a similar situation. Some of the qualitative feedback in previous studies suggested that this is an important element of the intervention. 136 Indeed, the qualitative data from the interviews did suggest that participants highly valued the group format. However, the groups included people with a range of cognitive impairments. Those with severe problems may have found that too much material was covered too quickly; those with mild problems may have found the pace too slow and not sufficiently tailored to their specific problems. However, there was nothing in the qualitative data that supported this assumption. Furthermore, in clinical practice, it is unusual to be able to create groups based on level of ability and so the results reflect the way the intervention would be delivered in clinical practice. Individual treatment has the advantage that the intervention can be specifically tailored to an individual’s pattern of cognitive deficits, and may also improve attendance compared with group formats. 29 However, evaluation of such individualised treatment becomes problematic as it is difficult to describe the content of the intervention in such a way that it can be replicated.
Some people may have declined to take part because they did not wish to be treated in a group. Providing a few individual sessions before participants are allocated to a group may allow their specific concerns to be addressed and they can be prepared for sharing their problems with others. A combination of individual and group treatment may maximise the benefits to be gained from cognitive rehabilitation. This strategy has been used previously,57,168,170 but there is no clear indication that outcomes are any better using this format.
The intervention was conducted over 10 sessions, which were held approximately once a week. There may be advantages in spreading the intervention over a longer period of time so that participants can practise between session tasks for longer, and to get more familiar with one strategy before learning about another. However, this frequency of sessions is similar to a previous study of cognitive rehabilitation delivered in groups,57 which provided eight sessions over 13 weeks. In addition, the provision of refresher sessions after the end of the structured programme may facilitate the maintenance of any gains obtained from the intervention. There was support for this suggestion in the participant feedback interview data.
Fidelity of the intervention
There were several mechanisms in place to ensure the fidelity of the intervention. The treatment was standardised by having a manual outlining the content of each session and facilitator notes were provided to guide the AP. The APs were all trained to deliver the intervention by one of the co-applicants. The co-applicant also provided one-to-one monthly supervision in addition to the monthly AP peer supervision. This enabled the APs, who delivered the intervention, to share experiences and to clarify any uncertainties relating to the manual and facilitator notes.
The video analysis suggested that the cognitive rehabilitation programme was delivered as planned and as described in the manual. However, the main limitation of the video analysis was that recordings were incomplete and did not include all sessions at all sites. Some were only partially recorded, as a result of the camera battery running low or the camera not being turned on again after the break. This limited the recordings that could be included in the analysis. Therefore, it is not possible to ascertain whether or not all sessions were delivered consistently in accordance with the manual at all sites. However, based on feedback obtained during monthly peer and supervisor meetings with the APs, it is improbable that the unrecorded sessions were different from the recorded ones.
It was not possible to check for drift over time as not enough sessions were recorded from each site. It would also have been better to observe the videos over the course of the trial, rather than in the final analysis, so that any minor discrepancies in the delivery of the intervention could have been addressed as the trial progressed. The video analysis focused on the content of treatment, but there was no assessment of the skills of the therapists. Important aspects of the group sessions, such as group cohesiveness and the skill of the therapist in managing group dynamics, were not monitored. However, most participants were complimentary about the therapists’ skills at facilitating the group.
Outcome assessment
The outcomes included a broad range of assessments. The primary outcome was a measure of quality of life, so that it would detect the general effects of receiving cognitive rehabilitation. The more specific effects of cognitive rehabilitation were assessed using subjective measures, which reflect the effect of cognitive problems in daily life, and objective tests of cognitive abilities. The potential negative consequences of the intervention were assessed through measures of fatigue and monitoring the safety outcome. The effect on relatives/friends was assessed through the MCSI.
Having patient-reported outcomes that rely on recall of information over time can be a challenge for people with cognitive problems. However, given that the primary outcome had to be a quality-of-life measure, it was inevitable that this was a patient-reported outcome measure.
The primary outcome questionnaire was completed and returned by post or online, and so was mainly conducted independently of an outcome assessor. However, when participants failed to return their questionnaires, they were reminded at the home visit and some opted to complete the questionnaire with the outcome assessor at the visit. The outcome assessments were conducted by researchers not involved in delivering treatment so that they were blind to group allocation. However, some unblinding of outcome assessors occurred. This was largely due to participants telling the assessor about their treatment, despite having been prompted not to. The cognitive tests were completed by outcome assessors at home visits and some unblinding occurred. However, the rate of unblinding was low. Comparison between the guessed group and the allocated group showed very weak correspondence and, in some instances, those who thought that they were unblinded guessed the allocation incorrectly. However, as noted by Bang,198 it is impossible to eliminate bias even when perfect blinding is achieved.
The follow-up completion rates were good and consistent with planned loss to follow-up. The main reasons for non-completion of follow-ups were health problems, non-response to postal assessments and failure to contact participants to arrange assessment visits. The rate of completion of cognitive tests was slightly lower than the rate for completion of questionnaires. Some participants refused to do some of the cognitive tests. This was most marked on the Paced Auditory Serial Addition Test (PASAT). It was also not clear from some records whether the PASAT was missing because it was not offered as a result of time constraints or technical problems or whether it was refused. If participants were offered a cognitive test but refused to answer or were unable to provide any responses, they were scored the minimum, consistent with standard administration procedures, but the outcome assessors did not always make this distinction clear in their records and some missing data may have occurred when participants were offered the test but were unable to complete the task. These were recorded as protocol deviations.
There is an inherent problem with using attention and memory tests as outcomes of cognitive rehabilitation, in that although the rehabilitation focuses on teaching people to effectively use attention and memory aids to reduce their cognitive problems, these standardised tests themselves do not allow the use of such aids. Therefore, it is improbable that there will be changes observed on these tests if participants are not allowed to use any aids, but equally, if aids are permitted, the tests would not have been administered as per the test manual.
The cognitive assessments were lengthy to administer and, as a result, could not always be completed in one session. If participants ran out of time or were too tired to continue, they were offered the opportunity to complete the cognitive assessments at a second visit; however, this sometimes proved difficult to arrange and did not always occur.
Questionnaires could be completed online or on paper versions that were returned by post. Only 86 (19%) participants chose to complete outcome questionnaires online, but the completion rate was higher (6 months: 98% online, 92% postal; 12 months: 92% online, 83% postal) than for the postal questionnaires.
Outcomes were assessed at both the 6- and 12-month follow-ups. Therefore, the long-term effects of cognitive rehabilitation could be determined, although it would be desirable to monitor whether or not the benefits persisted for longer.
Sample size
Most previous studies of cognitive rehabilitation have been small and underpowered. The median total sample size for MS memory rehabilitation studies is 42 (range 19–240). 29 The target sample size of 400 was exceeded, with a total of 449 randomised. The loss to follow-up for the primary outcome of 15% was consistent with that predicted in the calculation of sample size needed. The baseline characteristics of participants included in the primary analysis were similar in the two groups, as were the characteristics of the participants not included.
Economic analysis
Few studies have considered the cost-effectiveness of cognitive rehabilitation. Overall, providing cognitive rehabilitation cost less than usual care, due to a reduction in the use of other health and social services while receiving cognitive rehabilitation and differences in medication costs. This occurred mainly in non-disease-modifying medications, and so could not be attributed to differences in the disease progression between the two groups. Costs varied widely across participants in both groups, and lower costs for the intervention group were not robust to sensitivity analysis.
One limitation of the calculation of the costs of the intervention is that it relied on self-report of services used and medication. There is evidence from people with stroke, who are also likely to have cognitive impairments, that reporting of health and social services received is not accurate when compared against formal records. 199 Although the inaccuracies would have occurred in both groups, it may have reduced the ability to detect differences between the groups. In addition, participants were asked to self-report on medications used and many opted to show the RA their prescription list. However, some disease-modifying medications would not be listed on prescription lists, as they were issued directly from hospitals. Although this under-reporting would have affected both groups, it may have also diluted the ability to detect differences.
The cost-effectiveness analysis relied on the EQ-5D-5L to calculate QALYs. However, as highlighted by others,90,200 this is relatively insensitive to changes in HRQoL in people with MS and no difference in QALY gain was observed from the EQ-5D-5L. This is supported by the greater effects observed on the MSIS-8D, which was designed to assess QALYs in those with MS. However, even when the MSIS-8D was used as an alternative method for deriving utilities, there was a numerically small, significant, incremental QALY gain. However, only two of the eight items of the MSIS-8D would be expected to change with cognitive rehabilitation, and so any overall changes are likely to be small.
In addition, the cost-effectiveness analysis was considered in relation to the primary clinical outcome, another quality-of-life measure, the MSIS-Psy. This may not be sensitive to the effects of cognitive rehabilitation, as discussed above; therefore, cognitive rehabilitation was deemed unlikely to be cost-effective as incremental costs and effects are not statistically significant. Consideration of the incremental cost per point gained on the EMQ or GHQ-30 may be useful to inform the cost of provision of cognitive rehabilitation in clinical practice.
Patient and public involvement
The trial was actively supported by service user representatives. They provided suggestions to make the trial documents and lay summary clearer and more easily understood by service users. They made suggestions for the recruitment of participants, particularly from MS charities and local MS Society branches. One service user contributed to the qualitative study by interviewing participants and was involved in discussions of the analysis of themes. This service user also assisted with the dissemination of results by contributing to a video for service users. The contribution helped to ensure that the recruitment target was met. It also demonstrated that service users can be actively involved in data collection. The limitation was that some service users were not able to maintain their input over the full 4 years of the study. Two had relapses that affected their ability to attend meetings. Although six people originally agreed to take part in the video to disseminate the findings, three dropped out and could not be replaced in time for filming to take place before the end of the trial.
Conclusions
Implications for health care
-
People with MS have problems with attention and memory and are seeking help for these problems.
-
This trial has not shown any long-term effect of this cognitive rehabilitation programme on quality of life for people with MS, measured using the MSIS-Psy subscale, but there was an effect in the short term.
-
There was some evidence that cognitive rehabilitation improved both memory problems in daily life and mood.
-
Participant feedback was positive, with some participants reporting daily life benefits of attending the cognitive rehabilitation programme.
-
Providing cognitive rehabilitation cost no more than usual care.
-
It may be appropriate to offer this intervention in clinical practice given the clinical effects and lack of alternative effective treatments, until there is evidence for alternative effective treatments.
-
Further evaluation of those with MS who may benefit most from cognitive rehabilitation is needed.
Recommendations for research
-
There needs to be more small-scale efficacy studies to establish the appropriate selection criteria for cognitive rehabilitation programmes, so that the intervention is tailored to those who may benefit most.
-
Future studies should attempt to control for the effects of the group environment, in order to ascertain whether it is the contact with others with similar problems or the content of the programme that is most important.
-
Further research is needed to explore how short-term benefits of cognitive rehabilitation can be maintained in the long term.
Acknowledgements
We thank all those who took part in the trial and the clinical staff at the participating sites and PICs for their support.
The CRAMMS Trial Collaborative Group
Miriam Morgan (PPI Representative, Co-applicant).
Sites
-
Nottingham University Hospitals NHS Trust: Cris Constantinescu (Principal Investigator), Holly Chappell (AP), Sara Clarke (AP), Kara Crossley (AP), Cara Knight (AP) and Kristy Martin (AP).
-
The Walton Centre NHS Foundation Trust: Perry Moore (Principal Investigator), Carolyn Young (Neurology Adviser), Alexandra Cunliffe (AP), Catherine Pollard (AP) and John Wilson (AP).
-
Sheffield Teaching Hospitals NHS Foundation Trust: Basil Sharrack (Principal Investigator), Claire Isaac (Clinical Neuropsychology Adviser), David Griffiths (Clinical Neuropsychology Adviser), Emma Trigg (AP) and Serena Vanzan (AP).
-
North Bristol NHS Trust: Vera Fixter (Principal Investigator), Laura Hanley (Principal Investigator), Joanna Dalton (AP) and Stephanie Pegnall (AP).
-
South Tees Hospitals NHS Foundation Trust: Stephen Evans (Principal Investigator) and Miranda Wheeler (AP).
Participant Identification Centre leads
Lena Palmer, Tracy Tyrell, Shannon Gaughan, Gemma Elliot, Sophie Keogh, Helen Oldknow, Catherine Edwards and Nigel Schofield.
Outcome assessors
Hannah Carpenter, Heather Cogger, Rachel Harnell, Jacqueline Mhizha-Murira, Katie Powers and Luke Squires.
Qualitative interviewers
Susan Evans and Olga Klein.
Fidelity analysis
Jacqueline Mhizha-Murira.
Trial and data management
Kirsty Sprange (Senior Trial Manager), Amy Evans (Trial Manager from 2015 to 2016), Florence Day (Trial Manager from 2016), Jo Hobbs (Trial Co-ordinator from 2015), Natalie Wakefield (Trial Administrator/Co-ordinator from 2016), Brian Barnes (Data Manager), Monica Crone (Data Co-ordinator), Matthew Foster (Data Administrator), Chris Rumsey (IT Programmer) and Alexandra Erven (Research Facilitator).
Trial Steering Committee (independent members)
Rona Moss-Morris (chairperson), Audrey Bowen, Rory O’Connor, Julia Scott, Susan Evans and Shirlee McKeown (until September 2015).
Data Monitoring Committee (independent members)
Jenny Freeman, Rod Taylor and Anita Rose.
Contributions of authors
Nadina B Lincoln (https://orcid.org/0000-0001-5604-2339) (Professor of Clinical Psychology) was the Chief Investigator and co-authored the final report.
Lucy E Bradshaw (https://orcid.org/0000-0001-8382-6040) (Medical Statistician) analysed the clinical effectiveness data and prepared the results for publication.
Cris S Constantinescu (https://orcid.org/0000-0003-2066-7585) (Professor of Neurology) contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Florence Day (https://orcid.org/0000-0003-0306-5558) (Clinical Trial Manager) was the Trial Manager from July 2016 and contributed to the preparation of the final report.
Avril ER Drummond (https://orcid.org/0000-0003-1220-8354) (Professor of Healthcare Research, Director of Research and Occupational Therapist) contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Deborah Fitzsimmons (https://orcid.org/0000-0002-7286-8410) (Professor of Health Outcome Research) oversaw the health economics analysis and contributed important intellectual content to the report.
Shaun Harris (https://orcid.org/0000-0001-7724-6621) (Health Economist) analysed the health economics data and contributed important intellectual content to the report.
Alan A Montgomery (https://orcid.org/0000-0003-0450-1606) (Professor of Medical Statistics and Clinical Trials) contributed to the development of the grant application and trial protocol, oversaw the clinical effectiveness analysis and contributed important intellectual content to the report.
Roshan das Nair (https://orcid.org/0000-0001-8143-7893) (Professor of Clinical Psychology and Neuropsychology) was the Co-chief Investigator and co-authored the final report.
Publications
Lincoln NB, das Nair R, Bradshaw L, Constantinescu CS, Drummond AE, Erven A, et al. Cognitive Rehabilitation for Attention and Memory in people with Multiple Sclerosis: study protocol for a randomised controlled trial (CRAMMS). Trials 2015;16:556.
Lincoln NB, Bradshaw LE, Constantinescu CS, Day F, Drummond AE, Fitzsimmons D, et al. Cognitive rehabilitation for attention and memory in people with multiple sclerosis: a randomized controlled trial (CRAMMS) [published online ahead of print November 26 2019]. Clin Rehabil 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Mackenzie IS, Morant SV, Bloomfield GA, MacDonald TM, O’Riordan J. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry 2014;85:76-84. https://doi.org/10.1136/jnnp-2013-305450.
- Dutta R, Trapp BD. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol 2011;93:1-12. https://doi.org/10.1016/j.pneurobio.2010.09.005.
- Amato MP, Langdon D, Montalban X, Benedict RH, DeLuca J, Krupp LB, et al. Treatment of cognitive impairment in multiple sclerosis: position paper. J Neurol 2013;260:1452-68. https://doi.org/10.1007/s00415-012-6678-0.
- Winkelmann A, Engel C, Apel A, Zettl UK. Cognitive impairment in multiple sclerosis. J Neurol 2007;254:II35-42. https://doi.org/10.1007/s00415-007-2010-9.
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008;7:1139-51. https://doi.org/10.1016/S1474-4422(08)70259-X.
- Fischer M, Kunkel A, Bublak P, Faiss JH, Hoffmann F, Sailer M, et al. How reliable is the classification of cognitive impairment across different criteria in early and late stages of multiple sclerosis?. J Neurol Sci 2014;343:91-9. https://doi.org/10.1016/j.jns.2014.05.042.
- Calabrese P. Neuropsychology of multiple sclerosis – an overview. J Neurol 2006;253:I10-5. https://doi.org/10.1007/s00415-006-1103-1.
- Costa SL, Genova HM, DeLuca J, Chiaravalloti ND. Information processing speed in multiple sclerosis: past, present, and future. Mult Scler 2017;23:772-89. https://doi.org/10.1177/1352458516645869.
- Rouleau I, Dagenais E, Tremblay A, Demers M, Roger É, Jobin C, Duquette P. Prospective memory impairment in multiple sclerosis: a review. Clin Neuropsychol 2018;32:922-36. https://doi.org/10.1080/13854046.2017.1361473.
- Hämäläinen P, Rosti-Otajärvi E. Cognitive impairment in MS: rehabilitation approaches. Acta Neurol Scand 2016;134:8-13. https://doi.org/10.1111/ane.12650.
- Kalmar JH, Gaudino EA, Moore NB, Halper J, Deluca J. The relationship between cognitive deficits and everyday functional activities in multiple sclerosis. Neuropsychology 2008;22:442-9. https://doi.org/10.1037/0894-4105.22.4.442.
- Goverover Y, Genova HM, Hillary FG, DeLuca J. The relationship between neuropsychological measures and the Timed Instrumental Activities of Daily Living task in multiple sclerosis. Mult Scler 2007;13:636-44. https://doi.org/10.1177/1352458506072984.
- Goverover Y, Chiaravalloti N, DeLuca J. Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) and performance of everyday life tasks: actual reality. Mult Scler 2016;22:544-50. https://doi.org/10.1177/1352458515593637.
- Shevil E, Finlayson M. Pilot study of a cognitive intervention program for persons with multiple sclerosis. Health Educ Res 2010;25:41-53. https://doi.org/10.1093/her/cyp037.
- Morrow SA, Drake A, Zivadinov R, Munschauer F, Weinstock-Guttman B, Benedict RH. Predicting loss of employment over three years in multiple sclerosis: clinically meaningful cognitive decline. Clin Neuropsychol 2010;24:1131-45. https://doi.org/10.1080/13854046.2010.511272.
- Campbell J, Rashid W, Cercignani M, Langdon D. Cognitive impairment among patients with multiple sclerosis: associations with employment and quality of life. Postgrad Med J 2016;93:143-7. https://doi.org/10.1136/postgradmedj-2016-134071.
- Honan CA, Brown RF, Batchelor J. Perceived cognitive difficulties and cognitive test performance as predictors of employment outcomes in people with multiple sclerosis. J Int Neuropsychol Soc 2015;21:156-68. https://doi.org/10.1017/S1355617715000053.
- Raggi A, Covelli V, Schiavolin S, Scaratti C, Leonardi M, Willems M. Work-related problems in multiple sclerosis: a literature review on its associates and determinants. Disabil Rehabil 2016;38:936-44. https://doi.org/10.3109/09638288.2015.1070295.
- Benito-León J, Morales JM, Rivera-Navarro J. Health-related quality of life and its relationship to cognitive and emotional functioning in multiple sclerosis patients. Eur J Neurol 2002;9:497-502. https://doi.org/10.1046/j.1468-1331.2002.00450.x.
- Samartzis L, Gavala E, Zoukos Y, Aspiotis A, Thomaides T. Perceived cognitive decline in multiple sclerosis impacts quality of life independently of depression. Rehabil Res Pract 2014;2014. https://doi.org/10.1155/2014/128751.
- Van Schependom J, D’hooghe MB, De Schepper M, Cleynhens K, D’hooge M, Haelewyck MC, et al. Relative contribution of cognitive and physical disability components to quality of life in MS. J Neurol Sci 2014;336:116-21. https://doi.org/10.1016/j.jns.2013.10.020.
- Department of Health and Social Care . The National Service Framework for Long-Term Conditions 2005.
- National Institute for Health and Care Excellence (NICE) . Multiple Sclerosis in Adults: Management. Clinical Guideline 186 [CG186] 2014.
- Klein OA, das Nair R, Ablewhite J, Drummond A. Assessment and management of cognitive problems in people with multiple sclerosis: a national survey of clinical practice. Int J Clin Pract 2019;73.
- O’Brien AR, Chiaravalloti N, Goverover Y, Deluca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: a review of the literature. Arch Phys Med Rehabil 2008;89:761-9. https://doi.org/10.1016/j.apmr.2007.10.019.
- Goverover Y, Chiaravalloti ND, O’Brien AR, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil 2018;99:390-407. https://doi.org/10.1016/j.apmr.2017.07.021.
- D’Amico E, Leone C, Hayrettin T, Patti F. Can we define a rehabilitation strategy for cognitive impairment in progressive multiple sclerosis? A critical appraisal. Mult Scler 2016;22:581-9. https://doi.org/10.1177/1352458516632066.
- Mitolo M, Venneri A, Wilkinson ID, Sharrack B. Cognitive rehabilitation in multiple sclerosis: a systematic review. J Neurol Sci 2015;354:1-9. https://doi.org/10.1016/j.jns.2015.05.004.
- das Nair R, Martin KJ, Lincoln NB. Memory rehabilitation for people with multiple sclerosis. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD008754.pub3.
- Magalhaes R, Alves J, Thomas RE, Chiaravalloti N, Goncalves OF, Petrosyan A, et al. Are cognitive interventions for multiple sclerosis effective and feasible?. Restor Neurol Neurosci 2014;32:623-38. https://doi.org/10.3233/rnn-140388.
- Rosti-Otajärvi EM, Hämäläinen PI. Neuropsychological rehabilitation for multiple sclerosis. Cochrane Database Syst Rev 2014;2. https://doi.org/10.1002/14651858.CD009131.pub3.
- Amato MP, Goretti B, Viterbo RG, Portaccio E, Niccolai C, Hakiki B, et al. Computer-assisted rehabilitation of attention in patients with multiple sclerosis: results of a randomized, double-blind trial. Mult Scler 2014;20:91-8. https://doi.org/10.1177/1352458513501571.
- Brissart H, Leroy M, Morele E, Baumann C, Spitz E, Debouverie M. Cognitive rehabilitation in multiple sclerosis. Neurocase 2013;19:553-65. https://doi.org/10.1080/13554794.2012.701644.
- Charvet LE, Yang J, Shaw MT, Sherman K, Haider L, Xu J, et al. Cognitive function in multiple sclerosis improves with telerehabilitation: results from a randomized controlled trial. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0177177.
- De Giglio L, De Luca F, Prosperini L, Borriello G, Bianchi V, Pantano P, et al. A low-cost cognitive rehabilitation with a commercial video game improves sustained attention and executive functions in multiple sclerosis: a pilot study. Neurorehabil Neural Repair 2015;29:453-61. https://doi.org/10.1177/1545968314554623.
- Gich J, Freixanet J, García R, Vilanova JC, Genís D, Silva Y, et al. A randomized, controlled, single-blind, 6-month pilot study to evaluate the efficacy of MS-Line!: a cognitive rehabilitation programme for patients with multiple sclerosis. Mult Scler 2015;21:1332-43. https://doi.org/10.1177/1352458515572405.
- Pérez-Martín MY, González-Platas M, Eguía-Del Río P, Croissier-Elías C, Jiménez Sosa A. Efficacy of a short cognitive training program in patients with multiple sclerosis. Neuropsychiatr Dis Treat 2017;13:245-52. https://doi.org/10.2147/NDT.S124448.
- Shatil E, Metzer A, Horvitz O, Miller A. Home-based personalized cognitive training in MS patients: a study of adherence and cognitive performance. NeuroRehabilitation 2010;26:143-53. https://doi.org/10.3233/NRE-2010-0546.
- Solari A, Motta A, Mendozzi L, Pucci E, Forni M, Mancardi G, et al. CRIMS Trial . Computer-aided retraining of memory and attention in people with multiple sclerosis: a randomized, double-blind controlled trial. J Neurol Sci 2004;222:99-104. https://doi.org/10.1016/j.jns.2004.04.027.
- Tesar N, Bandion K, Baumhackl U. Efficacy of a neuropsychological training programme for patients with multiple sclerosis – a randomised controlled trial. Wien Klin Wochenschr 2005;117:747-54. https://doi.org/10.1007/s00508-005-0470-4.
- Fink F, Rischkau E, Butt M, Klein J, Eling P, Hildebrandt H. Efficacy of an executive function intervention programme in MS: a placebo-controlled and pseudo-randomized trial. Mult Scler 2010;16:1148-51. https://doi.org/10.1177/1352458510375440.
- Hildebrandt H, Lanz M, Hahn HK, Hoffmann E, Schwarze B, Schwendemann G, et al. Cognitive training in MS: effects and relation to brain atrophy. Restor Neurol Neurosci 2007;25:33-4.
- Mattioli F, Flavia M, Stampatori C, Zanotti D, Parrinello G, Capra R. Efficacy and specificity of intensive cognitive rehabilitation of attention and executive functions in multiple sclerosis. J Neurol Sci 2010;288:101-5. https://doi.org/10.1016/j.jns.2009.09.024.
- Bonavita S, Sacco R, Della Corte M, Esposito S, Sparaco M, d’Ambrosio A, et al. Computer-aided cognitive rehabilitation improves cognitive performances and induces brain functional connectivity changes in relapsing remitting multiple sclerosis patients: an exploratory study. J Neurol 2015;262:91-100. https://doi.org/10.1007/s00415-014-7528-z.
- Campbell J, Langdon D, Cercignani M, Rashid W. A randomised controlled trial of efficacy of cognitive rehabilitation in multiple sclerosis: a cognitive, behavioural, and MRI study. Neural Plast 2016;2016. https://doi.org/10.1155/2016/4292585.
- Cerasa A, Gioia MC, Valentino P, Nisticò R, Chiriaco C, Pirritano D, et al. Computer-assisted cognitive rehabilitation of attention deficits for multiple sclerosis: a randomized trial with fMRI correlates. Neurorehabil Neural Repair 2013;27:284-95. https://doi.org/10.1177/1545968312465194.
- Filippi M, Riccitelli G, Mattioli F, Capra R, Stampatori C, Pagani E, et al. Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures – an explorative study. Radiology 2012;262:932-40. https://doi.org/10.1148/radiol.11111299.
- Parisi L, Rocca MA, Valsasina P, Panicari L, Mattioli F, Filippi M. Cognitive rehabilitation correlates with the functional connectivity of the anterior cingulate cortex in patients with multiple sclerosis. Brain Imaging Behav 2014;8:387-93. https://doi.org/10.1007/s11682-012-9160-9.
- Parisi L, Rocca MA, Mattioli F, Copetti M, Capra R, Valsasina P, et al. Changes of brain resting state functional connectivity predict the persistence of cognitive rehabilitation effects in patients with multiple sclerosis. Mult Scler 2014;20:686-94. https://doi.org/10.1177/1352458513505692.
- Mattioli F, Stampatori C, Scarpazza C, Parrinello G, Capra R. Persistence of the effects of attention and executive functions intensive rehabilitation in relapsing remitting multiple sclerosis. Mult Scler Relat Disord 2012;1:168-73. https://doi.org/10.1016/j.msard.2012.06.004.
- Chiaravalloti ND, DeLuca J, Moore NB, Ricker JH. Treating learning impairments improves memory performance in multiple sclerosis: a randomized clinical trial. Mult Scler 2005;11:58-6. https://doi.org/10.1191/1352458505ms1118oa.
- Chiaravalloti ND, Moore NB, Nikelshpur OM, DeLuca J. An RCT to treat learning impairment in multiple sclerosis: the MEMREHAB trial. Neurology 2013;81:2066-72. https://doi.org/10.1212/01.wnl.0000437295.97946.a8.
- Goverover Y, Chiaravalloti N, Genova H, DeLuca J. A randomized controlled trial to treat impaired learning and memory in multiple sclerosis: the self-GEN trial. Mult Scler 2018;24:1096-104. https://doi.org/10.1177/1352458517709955.
- Rilo O, Peña J, Ojeda N, Rodríguez-Antigüedad A, Mendibe-Bilbao M, Gómez-Gastiasoro A, et al. Integrative group-based cognitive rehabilitation efficacy in multiple sclerosis: a randomized clinical trial. Disabil Rehabil 2018;40:208-16. https://doi.org/10.1080/09638288.2016.1250168.
- Brochet B. Functional training is a senseless strategy in MS cognitive rehabilitation: strategy training is the only useful approach – commentary. Mult Scler 2017;23:932-3. https://doi.org/10.1177/1352458517699877.
- Goodwin RA, Lincoln NB, das Nair R, Bateman A. Evaluation of NeuroPage as a memory aid for people with multiple sclerosis: a randomised controlled trial [published online ahead of print March 20 2018]. Neuropsychol Rehabil 2018. https://doi.org/10.1080/09602011.2018.1447973.
- Stuifbergen AK, Becker H, Perez F, Morison J, Kullberg V, Todd A. A randomized controlled trial of a cognitive rehabilitation intervention for persons with multiple sclerosis. Clin Rehabil 2012;26:882-93. https://doi.org/10.1177/0269215511434997.
- Mantynen A, Rosti-Otajarvi E, Koivisto K, Lilja A, Huhtala H, Hamalainen P. Neuropsychological rehabilitation does not improve cognitive performance but reduces perceived cognitive deficits in patients with multiple sclerosis: a randomised, controlled, multi-centre trial. Mult Scler 2014;20:99-107. https://doi.org/10.1177/1352458513494487.
- das Nair R, Lincoln NB. Evaluation of Rehabilitation of Memory in Neurological Disabilities (ReMiND): a randomized controlled trial. Clin Rehabil 2012;26:894-903. https://doi.org/10.1177/0269215511435424.
- Carr SE, das Nair R, Schwartz AF, Lincoln NB. Group memory rehabilitation for people with multiple sclerosis: a feasibility randomized controlled trial. Clin Rehabil 2014;28:552-61. https://doi.org/10.1177/0269215513512336.
- das Nair R, Lincoln NB. The effectiveness of memory rehabilitation following neurological disabilities: a qualitative inquiry of patient perspectives. Neuropsychol Rehabil 2013;23:528-45. https://doi.org/10.1080/09602011.2013.792290.
- Martin K-J, Lincoln N, das Nair R, Kneebone I. Group-based memory rehabilitation for people with multiple sclerosis: subgroup analysis of the ReMiND trial. Int J Ther Rehabil 2014;21:590-6. https://doi.org/10.12968/ijtr.2014.21.12.590.
- Hulst HE, Langdon DW. Functional training is a senseless strategy in MS cognitive rehabilitation: strategy training is the only useful approach – NO. Mult Scler 2017;23:930-2. https://doi.org/10.1177/1352458517692422.
- Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29) – a new patient-based outcome measure. Brain 2001;124:962-73. https://doi.org/10.1093/brain/124.5.962.
- Lincoln NB, das Nair R, Bradshaw L, Constantinescu CS, Drummond AE, Erven A, et al. Cognitive Rehabilitation for Attention and Memory in people with Multiple Sclerosis: study protocol for a randomised controlled trial (CRAMMS). Trials 2015;16. https://doi.org/10.1186/s13063-015-1016-3.
- Benedict RH, Munschauer F, Linn R, Miller C, Murphy E, Foley F, et al. Screening for multiple sclerosis cognitive impairment using a self-administered 15-item questionnaire. Mult Scler 2003;9:95-101. https://doi.org/10.1191/1352458503ms861oa.
- Sepulcre J, Vanotti S, Hernández R, Sandoval G, Cáceres F, Garcea O, et al. Cognitive impairment in patients with multiple sclerosis using the Brief Repeatable Battery-Neuropsychology test. Mult Scler 2006;12:187-95. https://doi.org/10.1191/1352458506ms1258oa.
- Rao SM. A Manual for the Brief Repeatable Battery of Neuropsychological Tests in Multiple Sclerosis. Milwaukee, WI: Medical College of Wisconsin; 1990.
- Benedict RH, Cox D, Thompson LL, Foley F, Weinstock-Guttman B, Munschauer F. Reliable screening for neuropsychological impairment in multiple sclerosis. Mult Scler 2004;10:675-8. https://doi.org/10.1191/1352458504ms1098oa.
- Benedict RH, Zivadinov R. Reliability and validity of neuropsychological screening and assessment strategies in MS. J Neurol 2007;254:II22-II25. https://doi.org/10.1007/s00415-007-2007-4.
- Benedict RH, Duquin JA, Jurgensen S, Rudick RA, Feitcher J, Munschauer FE, et al. Repeated assessment of neuropsychological deficits in multiple sclerosis using the Symbol Digit Modalities Test and the MS Neuropsychological Screening Questionnaire. Mult Scler 2008;14:940-6. https://doi.org/10.1177/1352458508090923.
- Vanotti S, Benedict RH, Acion L, Cáceres F. VANEM Workgroup . Validation of the Multiple Sclerosis Neuropsychological Screening Questionnaire in Argentina. Mult Scler 2009;15:244-50. https://doi.org/10.1177/1352458508097924.
- World Health Organization . International Classification of Functioning, Disability and Health 2001.
- Sharrack B, Hughes RA. The Guy’s Neurological Disability Scale (GNDS): a new disability measure for multiple sclerosis. Mult Scler 1999;5:223-33. https://doi.org/10.1177/135245859900500406.
- Rossier P, Wade DT. The Guy’s Neurological Disability Scale in patients with multiple sclerosis: a clinical evaluation of its reliability and validity. Clin Rehabil 2002;16:75-9. https://doi.org/10.1191/0269215502cr447oa.
- Pappalardo A, Patti F. Psychometric properties of the Italian version of the Guy’s Neurological Disability Scale. Funct Neurol 2010;25:223-33.
- Fraser C, McGurl J. Psychometric testing of the Americanized version of the Guy’s Neurological Disability Scale. J Neurosci Nurs 2007;39:13-9. https://doi.org/10.1097/01376517-200702000-00004.
- Bosma LV, Sonder JM, Kragt JJ, Polman CH, Uitdehaag BM. Detecting clinically-relevant changes in progressive multiple sclerosis. Mult Scler 2015;21:171-9. https://doi.org/10.1177/1352458514540969.
- Mokkink LB, Knol DL, Uitdehaag BM. Factor structure of Guy’s Neurological Disability Scale in a sample of Dutch patients with multiple sclerosis. Mult Scler 2011;17:1498-503. https://doi.org/10.1177/1352458511413098.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-52. https://doi.org/10.1212/WNL.33.11.1444.
- Novakovic AM, Krekels EH, Munafo A, Ueckert S, Karlsson MO. Application of item response theory to modeling of expanded disability status scale in multiple sclerosis. AAPS J 2017;19:172-9. https://doi.org/10.1208/s12248-016-9977-z.
- Hoogervorst EL, van Winsen LM, Eikelenboom MJ, Kalkers NF, Uitdehaag BM, Polman CH. Comparisons of patient self-report, neurologic examination, and functional impairment in MS. Neurology 2001;56:934-7. https://doi.org/10.1212/WNL.56.7.934.
- Riazi A, Hobart JC, Lamping DL, Fitzpatrick R, Thompson AJ. Multiple Sclerosis Impact Scale (MSIS-29): reliability and validity in hospital based samples. J Neurol Neurosurg Psychiatry 2002;73:701-4. https://doi.org/10.1136/jnnp.73.6.701.
- Ramp M, Khan F, Misajon RA, Pallant JF. Rasch analysis of the Multiple Sclerosis Impact Scale MSIS-29. Health Qual Life Outcomes 2009;7. https://doi.org/10.1186/1477-7525-7-58.
- Bacci ED, Wyrwich KW, Phillips GA, Vollmer T, Guo S. Analysis of the psychometric properties of the Multiple Sclerosis Impact Scale-29 (MSIS-29) in relapsing-remitting multiple sclerosis using classical and modern test theory. Mult Scler J Exp Transl Clin 2016;2. https://doi.org/10.1177/2055217316673235.
- McGuigan C, Hutchinson M. The multiple sclerosis impact scale (MSIS-29) is a reliable and sensitive measure. J Neurol Neurosurg Psychiatry 2004;75:266-9.
- Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. How responsive is the Multiple Sclerosis Impact Scale (MSIS-29)? A comparison with some other self report scales. J Neurol Neurosurg Psychiatry 2005;76:1539-43. https://doi.org/10.1136/jnnp.2005.064584.
- Cleanthous S, Cano S, Kinter E, Marquis P, Petrillo J, You X, et al. Measuring the impact of multiple sclerosis: Enhancing the measurement performance of the Multiple Sclerosis Impact Scale (MSIS-29) using Rasch Measurement Theory (RMT). Mult Scler J Exp Transl Clin 2017;3. https://doi.org/10.1177/2055217317725917.
- Lincoln NB, Yuill F, Holmes J, Drummond AE, Constantinescu CS, Armstrong S, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler 2011;17:1250-7. https://doi.org/10.1177/1352458511408753.
- Thomas S, Thomas PW, Kersten P, Jones R, Green C, Nock A, et al. A pragmatic parallel arm multi-centre randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. J Neurol Neurosurg Psychiatry 2013;84:1092-9. https://doi.org/10.1136/jnnp-2012-303816.
- Bogosian A, Chadwick P, Windgassen S, Norton S, McCrone P, Mosweu I, et al. Distress improves after mindfulness training for progressive MS: a pilot randomised trial. Mult Scler 2015;21:1184-94. https://doi.org/10.1177/1352458515576261.
- das Nair R, Kontou E, Smale K, Barker A, Lincoln NB. Comparing individual and group intervention for psychological adjustment in people with multiple sclerosis: a feasibility randomised controlled trial. Clin Rehabil 2016;30:1156-64. https://doi.org/10.1177/0269215515616446.
- Hobart J, Cano S. Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13120.
- Sunderland A, Harris JE, Baddeley AD. Do laboratory tests predict everyday memory? A neuropsychological study. J Verbal Learn Verbal Behav 1983;22:341-57. https://doi.org/10.1016/S0022-5371(83)90229-3.
- das Nair R, Bradshaw LE, Carpenter H, Clarke S, Day F, Drummond A, et al. A group memory rehabilitation programme for people with traumatic brain injuries: the ReMemBrIn RCT. Health Technol Assess 2019;23. https://doi.org/10.3310/hta23160.
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121-3. https://doi.org/10.1001/archneur.1989.00520460115022.
- Mills R, Young C, Nicholas R, Pallant J, Tennant A. Rasch analysis of the Fatigue Severity Scale in multiple sclerosis. Mult Scler 2009;15:81-7. https://doi.org/10.1177/1352458508096215.
- Rosti-Otajärvi E, Hämäläinen P, Wiksten A, Hakkarainen T, Ruutiainen J. Validity and reliability of the Fatigue Severity Scale in Finnish multiple sclerosis patients. Brain Behav 2017;7. https://doi.org/10.1002/brb3.743.
- Ottonello M, Pellicciari L, Giordano A, Foti C. Rasch analysis of the Fatigue Severity Scale in Italian subjects with multiple sclerosis. J Rehabil Med 2016;48:597-603. https://doi.org/10.2340/16501977-2116.
- Goldberg DP, Williams P. A User’s Guide to the General Health Questionnaire. Windsor: NFER-Nelson; 1988.
- Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work?. J Consult Clin Psychol 2013;81:251-62. https://doi.org/10.1037/a0029132.
- Rosti-Otajärvi E, Ruutiainen J, Huhtala H, Hämäläinen P. Relationship between subjective and objective cognitive performance in multiple sclerosis. Acta Neurol Scand 2014;130:319-27. https://doi.org/10.1111/ane.12238.
- Kinsinger SW, Lattie E, Mohr DC. Relationship between depression, fatigue, subjective cognitive impairment, and objective neuropsychological functioning in patients with multiple sclerosis. Neuropsychology 2010;24:573-80. https://doi.org/10.1037/a0019222.
- Baddeley AD, Emslie H, Nimmo-Smith I. Doors and People: a test of visual and verbal recall and recognition. Bury St Edmunds: Thames Valley Test Co.; 1994.
- Delis D, Kaplan E, Kramer J. Delis–Kaplan Executive Function System (D-KEFS). San Antonio, TX: The Psychological Corporation; 2001.
- EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990;3:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Humphreys I, Drummond AE, Phillips C, Lincoln NB. Cost-effectiveness of an adjustment group for people with multiple sclerosis and low mood: a randomized trial. Clin Rehabil 2013;27:963-71. https://doi.org/10.1177/0269215513488608.
- University of Nottingham . Long Term Conditions: Cognitive Rehabilitation for Attention and Memory in Multiple Sclerosis (CRAMMS) Trial n.d. www.nottingham.ac.uk/research/groups/longtermconditions/cognitive-impact/cramms/index.aspx (accessed 12 August 2019).
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Thornton M, Travis SS. Analysis of the reliability of the modified caregiver strain index. J Gerontol B Psychol Sci Soc Sci 2003;58:S127-32. https://doi.org/10.1093/geronb/58.2.S127.
- Robinson BC. Validation of a Caregiver Strain Index. J Gerontol 1983;38:344-8. https://doi.org/10.1093/geronj/38.3.344.
- World Medical Association . WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects n.d. www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed 13 September 2018).
- Health Research Authority . UK Policy Framework for Health and Social Care Research 2017.
- European Medicines Agency . ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice n.d. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf (accessed 13 September 2018).
- Hawton A, Green C, Telford C, Zajicek J, Wright D. Using the Multiple Sclerosis Impact Scale to estimate health state utility values: mapping from the MSIS-29, version 2, to the EQ-5D and the SF-6D. Value Health 2012;15:1084-91. https://doi.org/10.1016/j.jval.2012.07.007.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials 2005;2:152-62. https://doi.org/10.1191/1740774505cn076oa.
- Baldwin SA, Bauer DJ, Stice E, Rohde P. Evaluating models for partially clustered designs. Psychol Methods 2011;16:149-65. https://doi.org/10.1037/a0023464.
- White IR. Uses and limitations of randomization-based efficacy estimators. Stat Methods Med Res 2005;14:327-47. https://doi.org/10.1191/0962280205sm406oa.
- Shrier I, Steele RJ, Verhagen E, Herbert R, Riddell CA, Kaufman JS. Beyond intention to treat: what is the right question?. Clin Trials 2014;11:28-37. https://doi.org/10.1177/1740774513504151.
- Roberts C, Batistatou E, Roberts SA. Design and analysis of trials with a partially nested design and a binary outcome measure. Stat Med 2016;35:1616-36. https://doi.org/10.1002/sim.6828.
- Lincoln NB, Bradshaw LE, Constantinescu CS, Day F, Drummond AE, Fitzsimmons D, et al. Cognitive rehabilitation for attention and memory in people with multiple sclerosis: a randomized controlled trial (CRAMMS). Clin Rehabil 2019. https://doi.org/10.1177/0269215519890378.
- Moncher FJ, Prinz RJ. Treatment fidelity in outcome studies. Clin Psychol Rev 1991;11:247-66. https://doi.org/10.1016/0272-7358(91)90103-2.
- Dumas JE, Lynch AM, Laughlin JE, Phillips Smith E, Prinz RJ. Promoting intervention fidelity. Conceptual issues, methods, and preliminary results from the EARLY ALLIANCE prevention trial. Am J Prev Med 2001;20:38-47. https://doi.org/10.1016/S0749-3797(00)00272-5.
- Walton H, Spector A, Tombor I, Michie S. Measures of fidelity of delivery of, and engagement with, complex, face-to-face health behaviour change interventions: a systematic review of measure quality. Br J Health Psychol 2017;22:872-903. https://doi.org/10.1111/bjhp.12260.
- Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent 2011;71:S52-S63. https://doi.org/10.1111/j.1752-7325.2011.00233.x.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- Jordan B, Henderson A. Interaction analysis: foundations and practice. J Learn Sci 1995;4:39-103. https://doi.org/10.1207/s15327809jls0401_2.
- Heath C, Hindmarsh J, May T. Qualitative Research in Action. London: Sage; 1997.
- O’Brien MC, das Nair R, Lincoln NB. A comparison of the content of memory rehabilitation groups for patients with neurological disabilities. Neuropsychol Rehabil 2013;23:321-32. https://doi.org/10.1080/09602011.2012.753920.
- Smale KJ, Carr SE, Schwartz AF, das Nair R, Lincoln NB. An evaluation of treatment integrity in a randomised controlled trial of memory rehabilitation for people with multiple sclerosis. Clin Rehabil 2015;29:493-9. https://doi.org/10.1177/0269215514548733.
- Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991.
- Johnson RB, Onwuegbuzie AJ. Mixed methods research: a research paradigm whose time has come. Educational Researcher 2004;33:14-26. https://doi.org/10.3102/0013189X033007014.
- Bhaskar R. A Realist Theory of Science. Hemel Hempstead: Harvester; 1978.
- Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health 2015;42:533-44. https://doi.org/10.1007/s10488-013-0528-y.
- Klein OA, Drummond A, Mhizha-Murira JR, Mansford L, dasNair R. Effectiveness of cognitive rehabilitation for people with multiple sclerosis: a meta-synthesis of patient perspectives. Neuropsychol Rehabil 2019;29:491-512. https://doi.org/10.1080/09602011.2017.1309323.
- das Nair R, Martin KJ, Sinclair EJ. A meta-synthesis of qualitative research on perceptions of people with long-term neurological conditions about group-based memory rehabilitation. Neuropsychol Rehabil 2015;25:479-502. https://doi.org/10.1080/09602011.2014.971820.
- Nelson J. Using conceptual depth criteria: addressing the challenge of reaching saturation in qualitative research. Qual Res 2017;17:554-70. https://doi.org/10.1177/1468794116679873.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ 2000;320:50-2. https://doi.org/10.1136/bmj.320.7226.50.
- Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology 2013;80:1734-9. https://doi.org/10.1212/WNL.0b013e3182918cc2.
- Novick G. Is there a bias against telephone interviews in qualitative research?. Res Nurs Health 2008;31:391-8. https://doi.org/10.1002/nur.20259.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. https://doi.org/10.1136/bmj.321.7262.694.
- Hawe P, Shiell A, Riley T. Complex interventions: how ‘out of control’ can a randomised controlled trial be?. BMJ 2004;328:1561-3. https://doi.org/10.1136/bmj.328.7455.1561.
- National Institute for Health and Care Excellence . Position Statement on the Use of the EQ-5D-5L Valuation Set 2017. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisal-guidance/eq5d5l_nice_position_statement.pdf (accessed 10 May 2018).
- Goodwin E, Green C, Spencer A. Estimating a preference-based index for an eight-dimensional health state classification system for multiple sclerosis. Value Health 2015;18:1025-36. https://doi.org/10.1016/j.jval.2015.10.004.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Department of Health and Social Care . NHS Reference Costs 2015 to 2016 2017. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 18 April 2018).
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 18 April 2017).
- Carpenter JR, Kenward MG. Multiple Imputation and its Application. Chichester: John Wiley & Sons; 2013.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 1987.
- York Health Economics Consortium . Net Monetary Benefit 2016. www.yhec.co.uk/glossary/net-monetary-benefit/ (accessed 10 May 2018).
- Husereau D, Drummon M, Petrou S, Greenberg D, Augustovski F, Briggs AH, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- Thorn JC, Brookes ST, Ridyard C, Riley R, Hughes DA, Wordsworth S, et al. Core items for a standardized resource use measure: expert Delphi consensus survey. Value Health 2018;21:640-9. https://doi.org/10.1016/j.jval.2017.06.011.
- Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res 1995;4:187-206. https://doi.org/10.1007/BF02260859.
- Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Mult Scler 1999;5:251-9. https://doi.org/10.1177/135245859900500410.
- Bandari DS, Vollmer TL, Khatri BO, Tyry T. Assessing quality of life in patients with multiple sclerosis. Int J MS Care 2010;12:34-41. https://doi.org/10.7224/1537-2073-12.1.34.
- Simeoni M, Auquier P, Fernandez O, Flachenecker P, Stecchi S, Constantinescu C, et al. Validation of the Multiple Sclerosis International Quality of Life questionnaire. Mult Scler 2008;14:219-30. https://doi.org/10.1177/1352458507080733.
- Baumstarck K, Butzkueven H, Fernández O, Flachenecker P, Stecchi S, Idiman E, et al. Responsiveness of the Multiple Sclerosis International Quality of Life questionnaire to disability change: a longitudinal study. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-127.
- Chouliara N, Lincoln NB. Developing a questionnaire to assess the outcome of memory rehabilitation for people with neurological disabilities. Int J Ther Rehabil 2015;22:470-7. https://doi.org/10.12968/ijtr.2015.22.10.470.
- Patchick E, Vail A, Wood A, Bowen A. PRECiS (Patient Reported Evaluation of Cognitive State): psychometric evaluation of a new patient reported outcome measure of the impact of stroke. Clin Rehabil 2016;30:1229-41. https://doi.org/10.1177/0269215515624480.
- Hamasaki T, Sugimoto T, Evans S, Sozu T. Sample size determination for clinical trials with co-primary outcomes: exponential event times. Pharm Stat 2013;12:28-34. https://doi.org/10.1002/pst.1545.
- Maor Y, Olmer L, Mozes B. The relation between objective and subjective impairment in cognitive function among multiple sclerosis patients – the role of depression. Mult Scler 2001;7:131-5. https://doi.org/10.1177/135245850100700209.
- Julian L, Merluzzi NM, Mohr DC. The relationship among depression, subjective cognitive impairment, and neuropsychological performance in multiple sclerosis. Mult Scler 2007;13:81-6. https://doi.org/10.1177/1352458506070255.
- Bruce JM, Hancock LM, Arnett P, Lynch S. Treatment adherence in multiple sclerosis: association with emotional status, personality, and cognition. J Behav Med 2010;33:219-27. https://doi.org/10.1007/s10865-010-9247-y.
- Henneghan A, Stuifbergen A, Becker H, Kullberg V, Gloris N. Perceived cognitive deficits in a sample of persons living with multiple sclerosis. J Neurosci Nurs 2017;49:274-9. https://doi.org/10.1097/JNN.0000000000000314.
- Popp L, Schneider S. Attention placebo control in randomized controlled trials of psychosocial interventions: theory and practice. Trials 2015;16. https://doi.org/10.1186/s13063-015-0679-0.
- Hanssen KT, Beiske AG, Landrø NI, Hofoss D, Hessen E. Cognitive rehabilitation in multiple sclerosis: a randomized controlled trial. Acta Neurol Scand 2016;133:30-4. https://doi.org/10.1111/ane.12420.
- Hancock LM, Bruce JM, Bruce AS, Lynch SG. Processing speed and working memory training in multiple sclerosis: a double-blind randomized controlled pilot study. J Clin Exp Neuropsychol 2015;37:113-27. https://doi.org/10.1080/13803395.2014.989818.
- Pusswald G, Mildner C, Zebenholzer K, Auff E, Lehrner J. A neuropsychological rehabilitation program for patients with multiple sclerosis based on the model of the ICF. Neuro Rehabilitation 2014;35:519-27. https://doi.org/10.3233/NRE-141145.
- Julian LJ, Vella L, Vollmer T, Hadjimichael O, Mohr DC. Employment in multiple sclerosis. Exiting and re-entering the work force. J Neurol 2008;255:1354-60. https://doi.org/10.1007/s00415-008-0910-y.
- Piliavin JA, Siegl E. Health benefits of volunteering in the Wisconsin longitudinal study. J Health Soc Behav 2007;48:450-64. https://doi.org/10.1177/002214650704800408.
- Lum TY, Lightfoot E. The effects of volunteering on the physical and mental health of older people. Res Aging 2005;27:31-55. https://doi.org/10.1177/0164027504271349.
- Salter A, Thomas N, Tyry T, Cutter G, Marrie RA. Employment and absenteeism in working-age persons with multiple sclerosis. J Med Econ 2017;20:493-502. https://doi.org/10.1080/13696998.2016.1277229.
- Goodwin RA, Lincoln NB, Bateman A. Dysexecutive symptoms and carer strain following acquired brain injury: changes measured before and after holistic neuropsychological rehabilitation. Neuro Rehabilitation 2016;39:53-64. https://doi.org/10.3233/NRE-161338.
- Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J. MSCOI Study Group . New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler 2017;23:1123-36. https://doi.org/10.1177/1352458517694432.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-32.
- Martin KJ, Sinclair EJ, dasNair R. Descriptions of memory rehabilitation group interventions for neurological conditions: a systematic review. Clin Rehabil 2016;30:705-13. https://doi.org/10.1177/0269215515595273.
- O’Brien A, Gaudino-Goering E, Shawaryn M, Komaroff E, Moore NB, DeLuca J. Relationship of the Multiple Sclerosis Neuropsychological Questionnaire (MSNQ) to functional, emotional, and neuropsychological outcomes. Arch Clin Neuropsychol 2007;22:933-48. https://doi.org/10.1016/j.acn.2007.07.002.
- Nauta IM, Balk LJ, Sonder JM, Hulst HE, Uitdehaag BM, Fasotti L, et al. The clinical value of the patient-reported multiple sclerosis neuropsychological screening questionnaire. Mult Scler 2019;25:1543-6. https://doi.org/10.1177/1352458518777295.
- Akbar N, Honarmand K, Kou N, Levine B, Rector N, Feinstein A. Validity of an internet version of the Multiple Sclerosis Neuropsychological Questionnaire. Mult Scler 2010;16:1500-6. https://doi.org/10.1177/1352458510379615.
- Benedict RH, Cookfair D, Gavett R, Gunther M, Munschauer F, Garg N, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 2006;12:549-58. https://doi.org/10.1017/S1355617706060723.
- Langdon DW, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult Scler 2012;18:891-8. https://doi.org/10.1177/1352458511431076.
- Dusankova JB, Kalincik T, Havrdova E, Benedict RH. Cross cultural validation of the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS) and the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Clin Neuropsychol 2012;26:1186-200. https://doi.org/10.1080/13854046.2012.725101.
- Costers L, Gielen J, Eelen PL, Schependom JV, Laton J, Remoortel AV, et al. Does including the full CVLT-II and BVMT-R improve BICAMS? Evidence from a Belgian (Dutch) validation study. Mult Scler Relat Disord 2017;18:33-40. https://doi.org/10.1016/j.msard.2017.08.018.
- Goretti B, Niccolai C, Hakiki B, Sturchio A, Falautano M, Minacapelli E, et al. The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS): normative values with gender, age and education corrections in the Italian population. BMC Neurol 2014;14. https://doi.org/10.1186/s12883-014-0171-6.
- Walker LA, Osman L, Berard JA, Rees LM, Freedman MS, MacLean H, et al. Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS): Canadian contribution to the international validation project. J Neurol Sci 2016;362:147-52. https://doi.org/10.1016/j.jns.2016.01.040.
- Spedo CT, Frndak SE, Marques VD, Foss MP, Pereira DA, Carvalho Lde F, et al. Cross-cultural adaptation, reliability, and validity of the BICAMS in Brazil. Clin Neuropsychol 2015;29:836-46. https://doi.org/10.1080/13854046.2015.1093173.
- O’Connell K, Langdon D, Tubridy N, Hutchinson M, McGuigan C. A preliminary validation of the brief international cognitive assessment for multiple sclerosis (BICAMS) tool in an Irish population with multiple sclerosis (MS). Mult Scler Relat Disord 2015;4:521-5. https://doi.org/10.1016/j.msard.2015.07.012.
- Niccolai C, Portaccio E, Goretti B, Hakiki B, Giannini M, Pastò L, et al. A comparison of the brief international cognitive assessment for multiple sclerosis and the brief repeatable battery in multiple sclerosis patients. BMC Neurol 2015;15. https://doi.org/10.1186/s12883-015-0460-8.
- Sumowski JF, Benedict R, Enzinger C, Filippi M, Geurts JJ, Hamalainen P, et al. Cognition in multiple sclerosis. Neurology 2018;90:278-88. https://doi.org/10.1212/WNL.0000000000004977.
- Wallin MT, Culpepper WJ, Coffman P, Pulaski S, Maloni H, Mahan CM, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain 2012;135:1778-85. https://doi.org/10.1093/brain/aws099.
- Compston A, Coles A. Multiple sclerosis. Lancet 2002;359:1221-31. https://doi.org/10.1016/S0140-6736(02)08220-X.
- Office for National Statistics . Ethnicity and National Identity in England and Wales: 2011 2012. www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicityandnationalidentityinenglandandwales/2012-12-11 (accessed 14 September 2018).
- Office for National Statistics . Ethnic Group 2011. http://www.nomisweb.co.uk/census/2011/ks201ew (accessed 14 September 2018).
- National Institutes of Health . National Institutes of Health (NIH) Guidelines on the Inclusion of Women and Minorities As Subjects in Clinical Research 1994.
- Mhizha-Murira JR, Drummond A, Klein OA, dasNair R. Reporting interventions in trials evaluating cognitive rehabilitation in people with multiple sclerosis: a systematic review. Clin Rehabil 2018;32:243-54. https://doi.org/10.1177/0269215517722583.
- Bang H. Random guess and wishful thinking are the best blinding scenarios. Contemp Clin Trials Commun 2016;3:117-21. https://doi.org/10.1016/j.conctc.2016.05.003.
- Luther A, Lincoln NB, Grant F. Reliability of stroke patients’ reports on rehabilitation services received. Clin Rehabil 1998;12:238-44. https://doi.org/10.1191/026921598671668617.
- Phillips CJ, Humphreys I. Assessing cost-effectiveness in the management of multiple sclerosis. Clinicoecon Outcomes Res 2009;1:61-78. https://doi.org/10.2147/CEOR.S4225.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- National Institute for Health and Care Excellence . Routine Preoperative Tests for Elective Surgery (Appendix M: Economic Considerations for Delphi) 2015 n.d. www.nice.org.uk/guidance/NG45/documents/guideline-appendices-13 (accessed September 2019).
- Turner J, O’Cathain A, Knowles E, Nicholl J, Tosh J, Sampson F, et al. Evaluation of NHS 111 Pilot Sites. Sheffield: University of Sheffield, Medical Care Research Unit; 2012.
- National Institute of Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 20 April 2017).
- Office for National Statistics . National Life Tables: England and Wales n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandandwalesreferencetables (accessed 20 August 2019).
- Manouchehrinia A, Tanasescu R, Tench CR, Constantinescu CS. Mortality in multiple sclerosis: meta-analysis of standardised mortality ratios. J Neurol Neurosurg Psychiatry 2016;87:324-31. https://doi.org/10.1136/jnnp-2015-310361.
- Briggs A, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
Appendix 1 Sites
| Lead NHS trust | Date opened to recruitment | Months open |
|---|---|---|
| Nottingham University Hospitals NHS Trust | 13 March 2015 | 24.6 |
| The Walton Centre NHS Foundation Trust | 1 April 2015 | 24.0 |
| Sheffield Teaching Hospitals NHS Foundation Trust | 21 May 2015 | 22.3 |
| North Bristol NHS Trust | 24 February 2016 | 13.2 |
| South Tees Hospitals NHS Foundation Trust | 26 September 2016 | 6.2 |
Appendix 2 The CRAMMS trial intervention details
| 1 |
Name Attention and memory rehabilitation or just cognitive rehabilitation |
| 2 |
Why Cognitive rehabilitation is a structured set of therapeutic activities designed to retrain an individual’s attention and memory and help people compensate for these deficits |
| 3 |
What (materials) Cognitive rehabilitation sessions followed a treatment manual, which was provided to participants at the start of the programme. The manual was accompanied by facilitator notes to guide delivery of the sessions |
| 4 |
What (procedures) Strategies taught included restitution (including attention retraining) and strategies to improve encoding and retrieval (such as deep-level processing). Compensation strategies taught included mnemonics (chunking, use of first letter cues, rhymes), use of external devices (diaries, mobile phones, calendars) and ways of coping with attention and memory problems. This was a ‘mixed’ approach, because research has found merits for both approaches. Practical day-to-day problems, such as forgetting people’s names, improving concentration by avoiding distractions, ways to remember where the car was parked, etc., were discussed as a way to demonstrate how the memory aids could be used. Examples were drawn from participants’ daily lives, so as to personalise the intervention. Each session began with a review of the previous session, followed by the teaching of a new strategy and setting of homework. Homework exercises were prescribed to enable generalisation of what was taught in the sessions to daily life |
| 5 |
Who provided Facilitators delivering the intervention were psychology graduates with clinical experience. A clinical psychologist provided trial-specific training on the delivery of the intervention and monthly teleconferences provided an opportunity for peer group supervision. Additional monthly one-to-one supervision with a clinical psychologist allowed for discussion of specific challenges relating to treatment or assessment. Ad hoc supervision for specific queries was also provided by clinical psychologists at each site |
| 6 |
How Face-to-face sessions were held in groups comprising four to six participants, led by a single facilitator at each site |
| 7 |
Where Sessions were held at NHS hospitals or community venues |
| 8 |
When and how much Participants were offered 10 weekly sessions, lasting approximately 1.5 hours each, with a 15-minute break mid-session |
| 9 |
Tailoring The aim was to identify the most appropriate strategies to help individuals overcome their memory problems, and to provide participants with techniques that they could adapt and use according to the nature of their problems. Participants were able to review strategies they had previously learned and to discuss the ways in which the strategies could be applied in a community setting. Homework assignments were set following each session, which encouraged the participants to try the strategies learnt in the session in their home or work environment |
| 10 |
Modifications There were no changes to the intervention during the course of the trial |
| 11 |
How well (planned) Formal fidelity assessment was undertaken through analysis of video-recordings of treatment sessions against a coding schedule based on the activities and skills described in the manual |
| 12 |
How well (actual) The results of the fidelity analysis indicate that the components of therapy described in the manual were delivered to participants |
Appendix 3 Schedule of assessments
| Assessment | Telephone screen | Screening assessment | Baseline assessment | Intervention period | 6- and 12-month follow-ups | ||
|---|---|---|---|---|---|---|---|
| Postal | Face to face | Postal or online | Face to face | ||||
| Initial eligibility screening | ✗ | ✗a |
|
||||
| Informed consent | ✗ | ||||||
| Demographic information | ✗ | ||||||
| MSNQ | ✗ | ✗a | |||||
| BRBN | ✗ | ✗ | |||||
| GNDS | ✗ | ✗ | |||||
| MSIS | ✗ | ✗ | |||||
| EMQ-p | ✗ | ✗ | |||||
| FSS | ✗ | ✗ | |||||
| GHQ-30 | ✗ | ✗ | |||||
| Doors and People test | ✗ | ✗ | |||||
| Trail Making Test | ✗ | ✗ | |||||
| EQ-5D-5L | ✗ | ✗ | |||||
| Service use questionnaire | ✗ | ✗ | |||||
| Check availability for treatment group | ✗ | ||||||
| EMQ-r | ✗ | ✗ | |||||
| MCSI | ✗ | ||||||
| Feedback interviews | ✗b | ||||||
Appendix 4 Recruitment
FIGURE 10.
Cumulative participant recruitment against target.
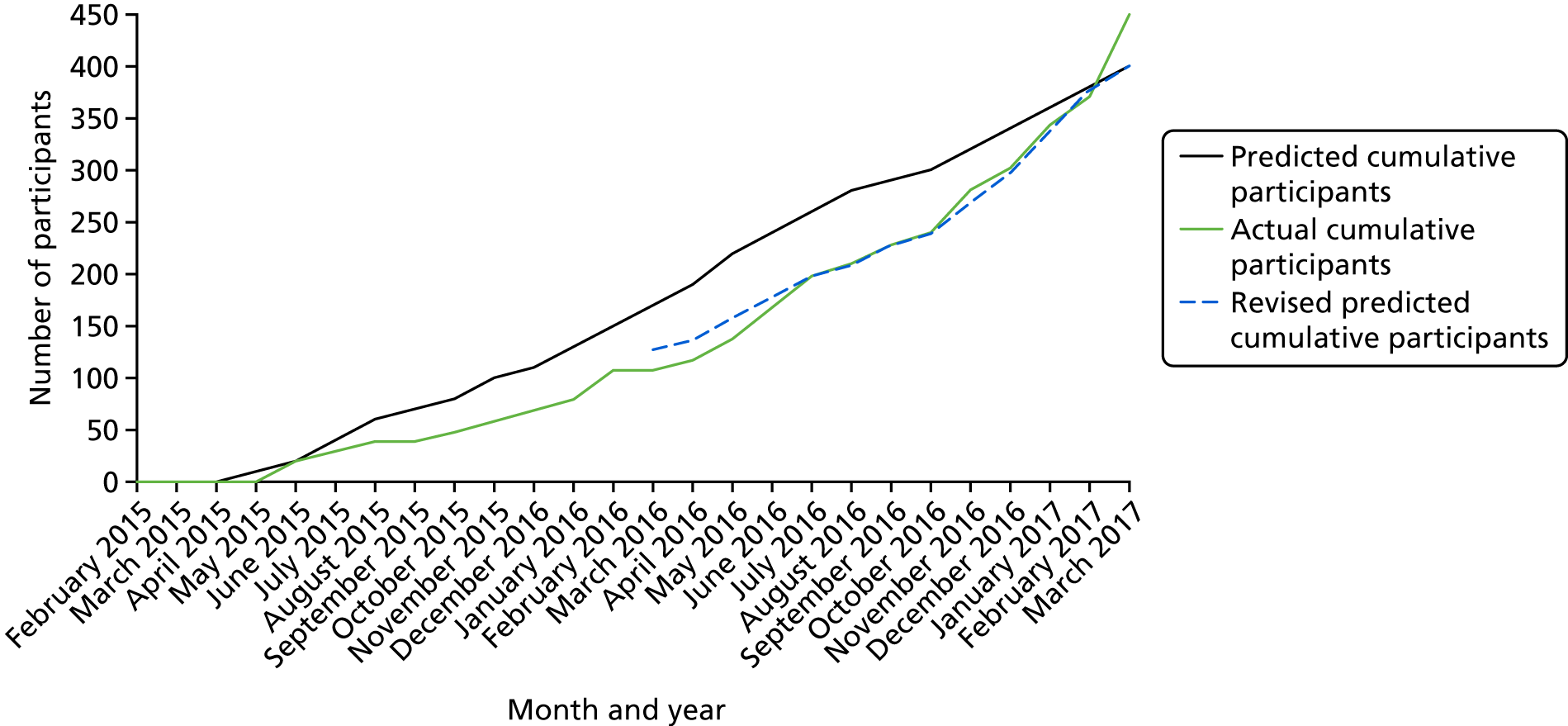
Appendix 5 Reasons for exclusion
| Reason | Total (N = 194) (n) |
|---|---|
| Did not report cognitive problems (scored < 27 points on the MSNQ) | 109 |
| Unable to travel to a trial centre and attend group sessions | 38 |
| Unable/unsuitable to engage in group treatment if allocated | 15 |
| Aged > 69 years | 13 |
| No MS diagnosis | 10 |
| Have impaired vision or hearing such that unable to complete the cognitive assessments, as judged by the assessor | 8 |
| Involved in other psychological intervention studies | 1 |
| Reason | Total (N = 68) (n) |
|---|---|
| Did not have cognitive deficits (none of the tests on BRBN > 1 SD below age- and education-corrected norms) | 38 |
| Did not report cognitive problems (scored < 27 points on the MSNQ) | 22 |
| Have impaired vision or hearing such that unable to complete the cognitive assessments, as judged by the assessor | 4 |
| Did not have relapsing or progressive MS diagnosed at least 3 months prior to the baseline assessment | 1 |
| Concurrent severe medical or psychiatric conditions that would prevent participants from engaging in treatment, if allocated | 1 |
| Unable to travel to one of the centres and attend group sessions | 1 |
| Unable to speak English sufficiently to complete the cognitive assessments and take part in group sessions | 1 |
Appendix 6 Baseline characteristics and assessment according to primary outcome completion and allocated group
| Characteristic | Usual care | Cognitive rehabilitation | ||
|---|---|---|---|---|
| No primary outcome (N = 31) | Primary outcome (N = 173) | No primary outcome (N = 31) | Primary outcome (N = 214) | |
| Age at randomisation (years) | ||||
| Mean (SD) | 45.3 (11.3) | 49.5 (9.6) | 47.6 (12.2) | 50.3 (9.4) |
| Minimum, maximum | 27, 67 | 25, 69 | 27, 69 | 18, 69 |
| Gender, n (%) | ||||
| Men | 9 (29) | 47 (27) | 11 (35) | 56 (26) |
| Women | 22 (71) | 126 (73) | 20 (65) | 158 (74) |
| Ethnicity, n (%) | ||||
| White | 29 (94) | 166 (96) | 29 (94) | 208 (97) |
| Black | 0 (0) | 3 (2) | 2 (6) | 1 (0) |
| Asian | 1 (3) | 1 (1) | 0 (0) | 3 (1) |
| Mixed | 1 (3) | 3 (2) | 0 (0) | 2 (1) |
| Marital status, n (%) | ||||
| Single/widowed/divorced | 10 (32) | 56 (32) | 12 (39) | 69 (32) |
| Married/with partner | 21 (68) | 117 (68) | 19 (61) | 145 (68) |
| Participant-reported time since MS diagnosis (years) | ||||
| Mean (SD) | 10.2 (9.3) | 11.3 (8.6) | 11 (9) | 12.3 (7.9) |
| Minimum, maximum | 1, 37 | 0, 40 | 2, 36 | 0, 34 |
| Type of MS (participant reported), n (%) | ||||
| Relapsing–remitting | 24 (77) | 108 (62) | 22 (71) | 137 (64) |
| Primary progressive | 1 (3) | 23 (13) | 3 (10) | 19 (9) |
| Secondary progressive | 6 (19) | 42 (24) | 6 (19) | 58 (27) |
| Relapses in the previous 6 months, as assessed at screening (participant reported), n (%) | ||||
| 0 | 23 (74) | 132 (76) | 24 (77) | 162 (76) |
| 1 | 4 (13) | 27 (16) | 6 (19) | 41 (19) |
| 2 | 2 (6) | 11 (6) | 1 (3) | 4 (2) |
| ≥ 3 | 2 (6) | 3 (2) | – | 7 (3) |
| Relapse between baseline and randomisation (participant reported), n (%) | ||||
| No | 29 (94) | 161 (93) | 25 (81) | 195 (91) |
| Yes | 2 (6) | 10 (6) | 6 (19) | 18 (8) |
| Not known | – | 2 (1) | – | 1 (< 0.5) |
| Years of education | ||||
| Mean (SD) | 13.1 (2.3) | 14.1 (3) | 14.1 (3.2) | 14.2 (3.5) |
| Minimum, maximum | 11, 18 | 10, 30 | 10, 22 | 10, 35 |
| Employment status, n (%) | ||||
| Retired | 7 (23) | 57 (33) | 8 (26) | 72 (34) |
| Not employed | 14 (45) | 56 (32) | 12 (39) | 71 (33) |
| Employed part time | 3 (10) | 28 (16) | 5 (16) | 39 (18) |
| Employed full time | 7 (23) | 31 (18) | 4 (13) | 27 (13) |
| In education full time | – | – | – | 1 (< 0.5) |
| In education part time | – | – | 2 (6) | 4 (2) |
| Not known | – | 1 (1) | – | – |
| Any employment (full or part time) | 10 (32) | 59 (34) | 9 (29) | 66 (31) |
| Living arrangements, n (%) | ||||
| Lives alone | 6 (19) | 32 (18) | 7 (23) | 42 (20) |
| Lives with others | 25 (81) | 141 (82) | 24 (77) | 172 (80) |
| Assessment | Usual care, mean (SD) | Cognitive rehabilitation, mean (SD) | ||
|---|---|---|---|---|
| No primary outcome (N = 31) | Primary outcome (N = 173) | No primary outcome (N = 31) | Primary outcome (N = 214) | |
| MSNQ | 40.5 (8.4) | 38.8 (7.3) | 40.6 (7.7) | 38.7 (7) |
| BRBN | ||||
| Selective Reminding | ||||
| Total recall | 38.8 (9.2) | 40.4 (10.7) | 36.8 (14.2) | 41.2 (10.4) |
| Long-term storage | 28.8 (13) | 31.2 (14.8) | 27 (19.2) | 31.8 (15.1) |
| Consistent long-term retrieval | 16.3 (11.6) | 19.2 (13.9) | 16.3 (17.9) | 19.4 (13.5) |
| Delayed recall | 4.7 (3) | 5.9 (2.8) | 4.8 (3.3) | 5.9 (2.7) |
| 10/36 Spatial Recall | ||||
| Total correct | 18.1 (4.9) | 18.4 (5) | 17.5 (5.5) | 18.2 (4.4) |
| Total confabulations | 11 (4.7) | 11.3 (5) | 12.1 (5.5) | 11.4 (4.5) |
| Delayed recall | 6.3 (1.8) | 6.3 (2.1) | 5.4 (2.2) | 6.1 (2.2) |
| Symbol Digit Modalities | 36.2 (10.7) | 38.1 (12.3) | 31.9 (12.1) | 36.9 (11.3) (n = 213) |
| Paced Auditory Serial Addition | ||||
| Easy total correct | 25.3 (18) | 32.2 (15.9) (n = 168) | 25.2 (15.8) (n = 29) | 32.5 (16.1) (n = 210) |
| Hard total correct | 10.7 (13.1) | 16.8 (16.1) (n = 168) | 10.6 (15.3) (n = 29) | 18.2 (16.5) (n = 210) |
| Word fluency | 23.3 (7.3) | 25.4 (9.1) (n = 172) | 21.5 (7.8) | 25.2 (8.8) |
| GNDS total score | 21.2 (6.3) | 19.8 (6.8) | 21.4 (7.9) | 19.7 (7) |
| Doors and People | n = 31 | n = 172 | n = 31 | n = 214 |
| Overall age-scaled score | 6.1 (4.1) | 7.2 (3.9) | 4.8 (3.8) | 7.4 (3.6) |
| Verbal total score | 7.2 (4.1) | 7.8 (3.8) | 6 (3.4) | 8 (3.7) |
| Non-verbal total score | 7 (4.2) | 7.8 (3.4) | 5.6 (3.2) | 7.8 (3.4) |
| Total forgetting score | 9 (2.9) | 8.7 (3) | 8.4 (4.1) | 8.9 (2.9) |
| Trail Making Score | n = 30 | n = 170 | n = 31 | n = 213 |
| Part B – part A | 75.2 (41.4) | 68.6 (41.4) | 80.1 (47.5) | 70.5 (40) |
| EQ-5D-5L health status VAS score | 52.2 (24.4) | 60.9 (19.3), n = 172 | 62.1 (21.8) | 59.6 (21.1) |
| MSIS-Psy subscale | 27.6 (6.2), n = 27 | 24.2 (5.9), n = 170 | 25.6 (6.2), n = 27 | 23 (5.7), n = 206 |
| MSIS-Phy subscale | 58.1 (12.3), n = 27 | 52.6 (13), n = 170 | 55.6 (16.3), n = 26 | 51.6 (13.2), n = 206 |
| EMQ-p | 54.9 (25.2), n = 27 | 45.8 (22.7), n = 167 | 53.1 (26.9), n = 25 | 44 (22.2), n = 204 |
| FSS | 1.5 (1.3), n = 27 | 1.3 (1.3), n = 170 | 1.6 (1.4), n = 27 | 1.3 (1.4), n = 203 |
| GHQ-30 | 48.1 (16.4), n = 27 | 38.3 (15.4), n = 170 | 40.5 (16.5), n = 26 | 35.9 (13.8), n = 204 |
| EMQ-r | 43.8 (24.3), n = 24 | 37.4 (26.1), n = 161 | 40.6 (23.7), n = 24 | 34 (23.4), n = 189 |
Appendix 7 Unblinding
| Opinion at each follow-up time point | Trial group, n (%) | |
|---|---|---|
| Usual care | Cognitive rehabilitation | |
| 6-month assessment | n = 187 | n = 225 |
| Opinion of treatment allocation at the start of the visit | ||
| Definitely usual care | 8 (4) | 1 (< 0.5) |
| Probably usual care | 173 (93) | 216 (96) |
| Probably intervention | 4 (2) | 3 (1) |
| Definitely intervention | 2 (1) | 5 (2) |
| Opinion of treatment allocation at the end of the visit | ||
| Definitely usual care | 13 (7) | 4 (2) |
| Probably usual care | 125 (67) | 123 (55) |
| Probably intervention | 44 (24) | 67 (30) |
| Definitely intervention | 5 (3) | 31 (14) |
| 12-month assessment | n = 175 | n = 212 |
| Opinion of treatment allocation at the start of the visit | ||
| Definitely usual care | 1 (1) | – |
| Probably usual care | 171 (98) | 204 (96) |
| Probably intervention | 2 (1) | 4 (2) |
| Definitely intervention | 1 (1) | 4 (2) |
| Opinion of treatment allocation at the end of the visit | ||
| Definitely usual care | 3 (2) | 2 (1) |
| Probably usual care | 121 (69) | 133 (63) |
| Probably intervention | 47 (27) | 62 (29) |
| Definitely intervention | 4 (2) | 15 (7) |
Appendix 8 Subgroup analysis
| Time point, mean (SD), n | Adjusted difference in means (95% CI) | Adjusted interaction effect (95% CI) | ||
|---|---|---|---|---|
| Baseline | 12 months | |||
| MS type | ||||
| Relapsing–remitting | ||||
| Usual care | 24.8 (5.6), 107 | 23.5 (5.9), 108 | ||
| Cognitive rehabilitation | 23.0 (5.9), 133 | 21.9 (6.1), 137 | –0.5 (–1.6 to 0.7) | |
| Progressive | ||||
| Usual care | 23.2 (6.1), 63 | 23.3 (6.2), 65 | ||
| Cognitive rehabilitation | 23.1 (5.4), 73 | 22.6 (6.3), 77 | –0.8 (–2.3 to 0.8) | –0.3 (–2.2 to 1.6) |
| Cognitive problems using MSNQ | ||||
| MSNQ tertile 1: 28 to 35 | ||||
| Usual care | 22.2 (5.3), 70 | 21.5 (5.3), 70 | ||
| Cognitive rehabilitation | 21.4 (5.2), 76 | 20.4 (5.5), 77 | –0.9 (–2.3 to 0.6) | |
| MSNQ tertile 2: 36 to 42 | ||||
| Usual care | 24.6 (5.3), 43 | 23.4 (5.3), 44 | ||
| Cognitive rehabilitation | 22.9 (5.3), 71 | 21.6 (5.8), 75 | –0.8 (–2.5 to 0.9) | 0.1 (–2.1 to 2.3) |
| MSNQ tertile 3: 43 to 60 | ||||
| Usual care | 26.3 (6.2), 57 | 25.8 (6.5), 59 | ||
| Cognitive rehabilitation | 25.3 (6.0), 59 | 25.1 (6.3), 62 | 0.0 (–1.6 to 1.6) | 0.8 (–1.3 to 3.0) |
| Memory function using the Doors and People test overall age-scaled score | ||||
| Tertile 1: 0 to 5 | ||||
| Usual care | 25.2 (5.7), 56 | 25.0 (6.0), 58 | ||
| Cognitive rehabilitation | 24.9 (6.1), 55 | 24.2 (6.2), 61 | –0.7 (–2.3 to 0.9) | |
| Tertile 2: 6 to 9 | ||||
| Usual care | 24.9 (5.5), 64 | 23.4 (5.7), 65 | ||
| Cognitive rehabilitation | 23.4 (5.1), 89 | 22.1 (5.8), 89 | –0.3 (–1.8 to 1.1) | 0.3 (–1.8 to 2.5) |
| Tertile 3: 10 to 18 | ||||
| Usual care | 22.1 (6.1), 49 | 21.7 (6.1), 49 | ||
| Cognitive rehabilitation | 20.9 (5.5), 62 | 20.3 (6.0), 64 | –0.8 (–2.4 to 0.9) | –0.1 (–2.4 to 2.3) |
| Speed of information processing using Symbol Digit Modalities from the BRBN | ||||
| Tertile 1: 5 to 32 | ||||
| Usual care | 25.1 (5.7), 51 | 24.7 (5.7), 51 | ||
| Cognitive rehabilitation | 23.8 (6.0), 68 | 22.3 (6.1), 71 | –1.5 (–3.1 to 0.1) | |
| Tertile 2: 33 to 42 | ||||
| Usual care | 24.6 (5.1), 54 | 24.7 (5.7), 56 | ||
| Cognitive rehabilitation | 23.2 (5.4), 78 | 23.0 (6.3), 81 | –0.9 (–2.4 to 0.6) | 0.6 (–1.7 to 2.8) |
| Tertile 3: 43 to 76 | ||||
| Usual care | 23.1 (6.5), 65 | 21.4 (6.1), 66 | ||
| Cognitive rehabilitation | 21.8 (5.4), 59 | 20.7 (5.6), 61 | 0.1 (–1.5 to 1.7) | 1.6 (–0.7 to 3.8) |
Appendix 9 Employment status at 6 and 12 months
| Follow-up time point | Trial group, n (%) | |
|---|---|---|
| Usual care | Cognitive rehabilitation | |
| 6 months | ||
| Visit completed within 9 months of randomisation | n = 187 | n = 224 |
| Retired | 68 (36) | 75 (33) |
| Not employed or in education | 59 (32) | 81 (36) |
| Employed part time | 27 (14) | 35 (16) |
| Employed full time | 30 (16) | 27 (12) |
| In education full time | – | 1 (< 0.5) |
| In education part time | 3 (2) | 5 (2) |
| 12 months | ||
| Visit completed within 15 months of randomisation | n = 173 | n = 209 |
| Retired | 71 (41) | 72 (34) |
| Not employed or in education | 49 (28) | 74 (35) |
| Employed part time | 27 (16) | 40 (19) |
| Employed full time | 23 (13) | 20 (10) |
| In education full time | – | 2 (1) |
| In education part time | 3 (2) | 1 (< 0.5) |
| Time point and group | Baseline, n (%) | Total (N) | |
|---|---|---|---|
| No employment | Any employment | ||
| 6 months | |||
| Usual-care group | |||
| No employment | 119 (97) | 10 (16) | 129 |
| Any employment | 4 (3) | 53 (84) | 57 |
| Total | 123 (100) | 63 (100) | 186 |
| Cognitive rehabilitation group | |||
| No employment | 153 (98) | 9 (13) | 162 |
| Any employment | 3 (2) | 59 (87) | 62 |
| Total | 156 (100) | 68 (100) | 224 |
| 12 months | |||
| Usual-care group | |||
| No employment | 111 (97) | 11 (19) | 122 |
| Any employment | 3 (3) | 47 (81) | 50 |
| Total | 114 (100) | 58 (100) | 172 |
| Cognitive rehabilitation group | |||
| No employment | 140 (97) | 9 (14) | 149 |
| Any employment | 5 (3) | 55 (86) | 60 |
| Total | 145 (100) | 64 (100) | 209 |
Appendix 10 Relapse
| Time point and relapse | Usual care | Cognitive rehabilitation |
|---|---|---|
| 6 months | ||
| Questionnaire completed within 9 months of randomisation (n) | 187 | 217 |
| Relapse in the previous 6 months, n (%) | ||
| No | 138 (74) | 174 (80) |
| Yes | 39 (21) | 37 (17) |
| Not answered | 10 (5) | 6 (3) |
| Number of relapses, n (%) | ||
| 1 | 23 (12) | 25 (12) |
| 2 | 13 (7) | 9 (4) |
| ≥ 3 | – | 1 (< 0.5) |
| Not given | 3 (2) | 2 (1) |
| 12 months | ||
| Questionnaire completed within 15 months of randomisation (n) | 173 | 215 |
| Relapse in the previous 6 months, n (%) | ||
| No | 132 (76) | 157 (73) |
| Yes | 34 (20) | 41 (19) |
| Not answered | 7 (4) | 17 (8) |
| Number of relapses,a n (%) | ||
| 1 | 23 (13) | 34 (16) |
| 2 | 3 (2) | 6 (3) |
| ≥ 3 | 4 (2) | – |
| Not given | 4 (2) | 2 (1) |
Appendix 11 Safety
| Safety outcome | Trial group, n (%) | |
|---|---|---|
| Usual care (n = 187) | Cognitive rehabilitation (n = 218) | |
| Increase of ≥ 30 points on the GHQ-30 between baseline and the 6-month assessment, n (%) | ||
| No | 174 (93) | 202 (93) |
| Yes | 4 (2) | 3 (1) |
| Change in score not able to be calculateda | 9 (5) | 13 (6) |
Appendix 12 Fidelity analysis: additional tables
| Activity | Code |
|---|---|
| Therapist activities | |
| Discussing administrative aspects | DAA |
| Discussing CRAMMS trial research activities/research process, for example outcome visits | TFRA |
| Discussing MS, for example diagnosis, living with MS, impact on family and/or caregivers | TLM |
| Facilitating discussion (non-specific prompts) and providing encouragement/reassurance | FD |
| Paraphrasing | PP |
| Presenting/discussing educational material | PE |
| Presenting/discussing strategies | PS |
| Providing explanation of memory and attention and/or strategies | EP |
| Providing feedback | PF |
| Summarising | SU |
| Therapist social chat | TSC |
| Participant activities | |
| Asking for information related to intervention content | AI |
| Asking questions not related to intervention content | AQ |
| Describing emotions and coping strategies | DE |
| Discussing CRAMMS trial research activities/research process, for example outcome visits | PFRA |
| Describing problems related to memory and attention | DP |
| Discussing MS, for example diagnosis, living with MS, impact on family and/or caregivers and hospital visit discussion | PLM |
| Discussing strategies | DS |
| Discussing/filling in educational material | PEM |
| Discussing/conducting administrative aspects | PDAA |
| Social chat | PSC |
| Treatment manual content: all sessions | |
| Summarise previous session | P |
| Reflect on personal experience | R |
| Discuss take-home activities | D |
| Learn about attention and memory | L |
| Learn about strategies to address attention and memory problems | ST |
| Other content (not cognitive rehabilitation, not related to treatment) | |
| Administration, for example reimburse participant travel and travel arrangements | AD |
| Discussing CRAMMS trial research activities/research process, for example outcome visits | FRA |
| Discussing living with/general information MS, for example symptoms such as fatigue | GI |
| Hospital visits, accessing other MS services | HV |
| Providing information on organisation of sessions, venue, date, group, etc. and housekeeping (fire exits, no smoking policy, toilets, drinks, breaks) | ISP |
| Social chat | SC |
| Activity | Description | Code |
|---|---|---|
| Session 1: introduction to the programme | ||
| Introduction to others | Reintroduce self and then encourage people to introduce themselves – briefly state why they are part of the study (wear name badges) | IO |
| Discuss format of session and memory programme | Present the aims of the programme and the structure – use to motivate people | FS |
| Establish group rules | Discuss group rules | GR |
| Explore attention and memory | Discussing what attention and memory are | AM |
| Things that participant forgets | Can compare memory before MS diagnosis | TF |
| How do you feel when you are having difficulty with memory? | Emphasise that it’s difficult to evaluate own memory against other peoples. Instil confidence in the group that there are techniques/strategies that can be done to deal with the problems better | FD |
| Own techniques to help | Have you come up with any techniques to reduce these problems or help you cope better? | OT |
| What else do you want to know? | Making each session work – want to know more about the programme – can illustrate will be looking at that in session . . . | WK |
| Session 2: memory and memory problems | ||
| What is memory | Can you discuss what you think memory is and why is it important? | M |
| Processes involved in memory | Attention, encoding, storage, consolidation, retrieval. Where do people’s problems lie? | PI |
| Memory storage |
|
MS |
| Memory retrieval | Immediate recall/delayed recall/distractors | MR |
| Memory systems | Aim to illustrate how memory works/how complicated it is | MSY |
| Session 3: attention I | ||
| Different types of attention | What is attention? Why is it important? What makes us pay attention? | DA |
| Different types of distractors | What is sustained/divided attention? Distracters? – Sustained, for example staying attentive in a meeting, watching a television programme, reading a book. Divided, for example talking on the telephone while cooking, cleaning while listening to music, singing while washing in the shower | DD |
| Attention exercise: newspaper exercise |
|
AE |
| Techniques to improve attention |
|
TI |
| Session 4: attention II | ||
| Story recall exercise |
|
SR |
| 5Ws and the H: who, what, where, when, why and how? | Introduce the 5Ws and the H strategy and how it can help. This technique teaches you to focus and pick out the important information – filtering out irrelevant background information (distractors). The information is also simplified making recollection easier | WH |
| Story recall with the 5Ws and the H |
|
SRW |
| When difficult | When is it difficult to pay attention? | WD |
| How attention improved | How can our attention be improved? | AI |
| Strategies to remember | STR | |
| Exercise 3: case study handout | Based on the 5Ws and the H, and the information about attention that we know of, what are the best strategies to improve attention in the following case studies? | EX |
| Session 5: introducing internal memory aids | ||
| Use of internal memory aids |
|
IA |
| Rehearsal | Rehearsal and repetition? Think of a script for a presentation that you have had to remember in the past. How did you remember it? | REH |
| Chunking |
|
CH |
| Categorisation |
|
CT |
| Session 6: internal memory aids II | ||
| Levels of processing |
|
LP |
| Visual imagery |
|
VI |
| Associations | What do we mean by associations? For example, remembering to carry out a new activity by linking it to an existing routine, taking medication with mealtimes, learning the name of someone you have just met by thinking of somebody you already know with the same name (friend/movie star), leaving your walking boots by the door to remember you are going out walking with a friend | AS |
| Story method | Spend 5 minutes devising a way of linking and remembering the information using the story method and share with the group | SM |
| First-letter cues | First-letter cues: practise on additional lists | FL |
| Rhymes | RH | |
| Session 7: internal memory aids III | ||
| Little-and-often strategy |
|
LO |
| PQRST method |
|
PQ |
| Learning strategies |
|
CTR |
| Relaxation |
|
REL |
| Session 8: introduction to external memory aids and diaries | ||
| Memory aids – internal and external | Complete the table reflecting on internal and external memory aids | MA |
| Diary use – using a diary effectively, useful tips when using a diary |
|
DU |
| Pacing: making the most of your energy – pacing activity (3Ps) | For example, avoid rushing; pre plan and organise activities; eliminate unnecessary tasks; use good posture and body mechanics; avoid unnecessary motion or energy expenditure; use assistive devices or appliances to decrease work; ensure work heights, lighting, ventilation, noise levels are appropriate; delegate/ask for help; allow adequate time for sleep, meals and exercise; spread out tasks throughout the day/week; set time limits for tasks | PC |
| Positive attitude: developing a positive attitude to memory aids | Everyone uses them; experiment; recognise a purpose for the strategy; be flexible – use different strategies for different situations; try to use 3–6 aids for better memory | PA |
| Session 9: external memory aids II | ||
| Problems with external aids | Discuss external memory aids already use. Potential problems and solutions with various memory aids | PA |
| Memory aids use in future? |
|
MF |
| Case studies: making life a bit easier | Which external memory aids can each individual use to help him/her remember better? | CST |
| Useful tips when using external memory aids | For example, incorporate things into daily routine, keep list in prominent position, writing things down as soon as you remember, use different colours, marker pens, sticky notes, etc. – NEW | UT |
| Session 10: review and fine-tuning | ||
| Overview of sessions | Look back through workbook | OS |
| Favourite strategies | List favourite and most effective strategies – attention, internal, external | FAS |
| Reflecting on memory problems identified in session 1 | Reflect on the memory problems you recorded in session 1 (take-home activity) and state how often each occurs now, how much they affect you now and the techniques used to deal with each strategy | PS |
| Group feedback – changes since the programme started |
|
GF |
| Site | Group | Sessions recorded | Total complete sessions (n) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| 01 | 01 | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | 3 |
| 01 | 03 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 0 |
| 01 | 04 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 0 |
| 01 | 08 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 01 | 09 | ✓ | ✓ | ✓ | ✓ | ○ | ✗ | ✓ | ✗ | ✓ | ✓ | 7 |
| 01 | 12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 10 |
| 01 | 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ○ | ✓ | 8 |
| 01 | 18 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| 01 | 19 | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| 01 | 27 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | 9 |
| 01 | 31 | ○ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 01 | 32 | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 01 | 34 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 10 |
| 01 | 36 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 0 |
| 01 | 38 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ○ | ✗ | 8 |
| 01 | 42 | ✓ | ✓ | ✓ | ✓ | ✓ | ○ | ✓ | ✓ | ✓ | ✓ | 9 |
| 02 | 02 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 0 |
| 02 | 06 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 0 |
| 02 | 10 | ✗ | ✗ | ✓ | ✓ | ○ | ✗ | ✗ | ✗ | ✗ | ✗ | 2 |
| 02 | 15 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 0 |
| 02 | 23 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 0 |
| 02 | 43 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 0 |
| 02 | 45 | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| 03 | 05 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | 4 |
| 03 | 07 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ○ | ✓ | ✓ | 9 |
| 03 | 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | 9 |
| 03 | 13 | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | 8 |
| 03 | 21 | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | 7 |
| 03 | 24 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ○ | ✓ | 9 |
| 03 | 28 | ✗ | ✓ | ○ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ○ | 7 |
| 03 | 29 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | 8 |
| 03 | 35 | ✓ | ✓ | ✓ | ○ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 03 | 37 | ✓ | ✓ | ✓ | ✓ | ○ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 03 | 39 | ○ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ○ | ✓ | ○ | 5 |
| 04 | 16 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 0 |
| 04 | 17 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | 0 |
| 04 | 20 | ✓ | ✓ | ✓ | ○ | ✓ | ✓ | ✗ | ○ | ✓ | ✓ | 7 |
| 04 | 22 | ○ | ✓ | ✓ | ✓ | ✓ | ○ | ✗ | ✓ | ○ | ✓ | 6 |
| 04 | 30 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | 8 |
| 04 | 33 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ○ | ✓ | ✓ | 9 |
| 04 | 41 | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ? | ✓ | 7 |
| 04 | 44 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ | 9 |
| 05 | 25 | ✗ | ✓ | ✓ | ✓ | ✓ | ○ | ✓ | ✓ | ✓ | ✓ | 8 |
| 05 | 26 | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| 05 | 40 | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| Category | Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | Session 6 | Session 7 | Session 8 | Session 9 | Session 10 | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Therapist activity | ||||||||||||||||||||||
| Discussing/conducting administrative aspects | 80 | 5.9 | 40 | 2.3 | 36 | 1.9 | 32 | 1.4 | 24 | 1.3 | 29 | 1.6 | 20 | 1.3 | 24 | 1.3 | 14 | 0.8 | 32 | 1.6 | 331 | 1.8 |
| Providing explanation | 180 | 13.2 | 513 | 29 | 219 | 11.7 | 66 | 2.8 | 35 | 1.9 | 77 | 4.3 | 69 | 4.3 | 23 | 13 | 10 | 0.6 | 72 | 3.6 | 1264 | 7 |
| Facilitating discussion | 179 | 13.2 | 125 | 7.1 | 99 | 9.3 | 49 | 2.1 | 42 | 2.3 | 59 | 3.3 | 66 | 4.1 | 105 | 5.9 | 87 | 5.1 | 75 | 3.8 | 886 | 4.9 |
| Providing feedback | 21 | 1.5 | 64 | 3.6 | 90 | 4.8 | 58 | 2.5 | 43 | 2.3 | 55 | 3.1 | 57 | 3.6 | 100 | 5.6 | 57 | 3.3 | 94 | 4.7 | 639 | 3.5 |
| Summarising | 13 | 1 | 38 | 2.2 | 28 | 1.5 | 35 | 1.5 | 10 | 0.5 | 6 | 0.3 | 10 | 0.6 | 14 | 0.8 | 10 | 0.6 | 66 | 3.3 | 230 | 1.3 |
| Paraphrasing | 27 | 2 | 51 | 2.9 | 23 | 1.2 | 21 | 0.9 | 9 | 0.5 | 18 | 1 | 9 | 0.6 | 33 | 1.9 | 15 | 0.9 | 21 | 1.1 | 227 | 1.3 |
| Presenting/discussing educational material | 129 | 9.5 | 240 | 13.6 | 278 | 14.8 | 267 | 11.4 | 165 | 8.9 | 203 | 11.4 | 199 | 12.5 | 157 | 8.8 | 226 | 13.2 | 210 | 10.6 | 2074 | 11.5 |
| Presenting/discussing strategies | 54 | 4 | 58 | 3.3 | 133 | 7.1 | 517 | 22.1 | 483 | 26.2 | 410 | 23 | 323 | 20.2 | 12 | 0.7 | 359 | 20.5 | 238 | 12 | 3054 | 16.9 |
| Discussing CRAMMS trial research process | 11 | 0.8 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.1 | 1 | 0.1 | 1 | 0.1 | 0 | 0 | 0 | 0 | 76 | 3.8 | 91 | 0.5 |
| Discussing diagnosis/living with MS | 2 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.1 | 0 | 0 | 0 | 0 | 3 | 0 |
| Therapist social chat | 14 | 1 | 8 | 0.5 | 3 | 0.2 | 10 | 0.4 | 3 | 0.2 | 5 | 0.3 | 2 | 0.1 | 7 | 0.4 | 6 | 0.4 | 14 | 0.7 | 72 | 0.4 |
| Participant activities | ||||||||||||||||||||||
| Social chat | 64 | 4.7 | 69 | 3.9 | 85 | 4.5 | 81 | 3.5 | 72 | 3.9 | 61 | 3.4 | 53 | 3.3 | 85 | 4.8 | 69 | 4 | 95 | 4.8 | 734 | 4.1 |
| Discussing/filling in educational material | 38 | 2.8 | 125 | 7.1 | 425 | 22.7 | 669 | 28.6 | 437 | 23.7 | 429 | 24.1 | 466 | 29.2 | 92 | 5.2 | 143 | 8.3 | 319 | 16.1 | 3143 | 17.4 |
| Discussing strategies | 146 | 10.7 | 86 | 4.9 | 108 | 5.8 | 318 | 13.6 | 362 | 19.6 | 293 | 16.5 | 214 | 13.4 | 422 | 23.7 | 602 | 35.1 | 271 | 13.7 | 2822 | 15.6 |
| Asking for information related to intervention content | 19 | 1.4 | 39 | 2.2 | 20 | 1.1 | 10 | 0.4 | 3 | 0.2 | 9 | 0.5 | 5 | 0.3 | 0 | 0 | 1 | 0.1 | 7 | 0.4 | 113 | 0.6 |
| Asking for information not related to intervention content | 2 | 0.1 | 3 | 0.2 | 1 | 0.1 | 2 | 0.1 | 1 | 0.1 | 3 | 0.2 | 0 | 0 | 0 | 0 | 1 | 0.1 | 0 | 0 | 13 | 0.1 |
| Describing problems | 225 | 16.6 | 225 | 12.7 | 277 | 14.8 | 170 | 7.3 | 115 | 6.2 | 91 | 5.1 | 61 | 3.8 | 77 | 4.3 | 54 | 3.2 | 82 | 4.1 | 1377 | 7.6 |
| Describing emotions and coping strategies | 57 | 4.2 | 28 | 1.6 | 11 | 0.6 | 6 | 0.3 | 12 | 0.7 | 9 | 0.5 | 13 | 0.8 | 12 | 0.6 | 7 | 0.4 | 26 | 1.3 | 181 | 1 |
| Discussing MS diagnosis/hospital visit | 80 | 5.9 | 43 | 2.4 | 33 | 1.8 | 22 | 0.9 | 25 | 1.4 | 14 | 0.8 | 25 | 1.6 | 126 | 7.1 | 38 | 2.2 | 72 | 3.6 | 478 | 2.6 |
| Discussing/conducting administrative aspects | 8 | 0.6 | 5 | 0.3 | 1 | 0.1 | 1 | 0 | 1 | 0.1 | 4 | 0.2 | 2 | 0.1 | 2 | 0.1 | 4 | 0.2 | 5 | 0.3 | 33 | 0.2 |
| Missing | 3 | 0.2 | 1 | 0.1 | 1 | 0.1 | 0 | 0 | 0 | 0 | 3 | 0.2 | 1 | 0.1 | 6 | 0.3 | 2 | 0.1 | 2 | 0.1 | 19 | 0.1 |
Appendix 13 Unit costs
| Resource | Cost (£) per contact | Average consultation time | Source (service code/currency code) | Assumptions |
|---|---|---|---|---|
| General practice and community nursing | ||||
| GP (surgery) | 36 | 9.22 minutes | aPSSRU 2016,201 page 145 | Based on consultation lasting 9.22 minutes (PSSRU 2016201) |
| GP (home) | 120 | 23.4 minutes | aPSSRU 2013,202 page 191 | Based on consultation time of 23.4 minutes |
| GP (telephone) | 28 | 7.1 minutes | aPSSRU 2015,203 page 171 | |
| Nurse (general practice) | 11.11 | 15.5 minutes | aPSSRU 2015,203 page 192 | Based on consultation time of 15.5 minutes (PSSRU 2015203) |
| Nurse (home) | 16.77 | 23.4 minutes | Assume same consultation time | |
| Nurse (telephone) | 4.70 | 6.56 minutes | aPSSRU 2016,201 pages 143 and 147 | |
| Counsellor (surgery) | 42 per hour | |||
| Other | ||||
| Midwife | 44 per hour | aPSSRU 2016,201 pages 188 and 198 | Based on band 6 hospital-based nurses | |
| Chiropodist | 32 per hour | aPSSRU 2016,201 pages 200 and 136 | Costed at band 5 community professional staff | |
| Dietitian | 32 per hour | aPSSRU 2016,201 pages 200 and 136 | Costed at band 5 community professional staff | |
| District nurse | 44 per hours | aPSSRU 2016,201 page 142 | Costed at band 6 community-based nurses | |
| Mental health practitioner | 38 per hour | aPSSRU 2016,201 page 168 | Community mental health team for adults with mental health problems | |
| Phlebotomist | 24 per hour | |||
| Urine test (using urinalysis analyser) | 4.25 | aPSSRU 2017,147 page 216; NICE 2015, page 7204 | Inflated to 2016/17 prices using HCHS index | |
| Hospital and community services | ||||
| Neurologist | 217 | NHS Reference Costs 2015 to 2016 148 |
|
|
| MS nurse (hospital) | 53 per hour | 30 minutes | aPSSRU 2016,201 page 188; MS Trust [www.mstrust.org.uk (last accessed 9 September 2019)] | Based on band 7. Costed per working hour |
| MS nurse (home) | 52 per hour | aPSSRU 2016,201 page 142; MS Trust (www.mstrust.org.uk) | Based on band 7. Costed per working hour | |
| Therapy services | ||||
| Physiotherapist (hospital) | 34 per hour | aPSSRU 2016,201 pages 183–5 | Based on band 5 | |
| Physiotherapist (community) | 32 per hour | aPSSRU 2016,201 pages 135–7 | Based on band 5 | |
| Occupational therapist (hospital) | 34 per hour | aPSSRU 2016,201 pages 183–5 | Based on band 5 | |
| Occupational therapist (community) | 44 per hour | aPSSRU 2016,201 page 159 | ||
| Psychologist | 55 per hour | aPSSRU 2016,201 pages 183–5 | Based on band 7 clinical psychologist | |
| Pharmacist | 42 per hour | aPSSRU 2016,201 pages 135–7 | Based on band 6 | |
| NHS 111 service | 13.29 per call | Turner et al.205 | Inflated from £12.26 to 2016 prices | |
| Social services | ||||
| Social worker | 79 per hour | 30 minutes | aPSSRU 2016,201 page 156 | |
| Counsellor | 43 per hour | 30 minutes | aPSSRU 2017,147 pages 153–5 | Based on band 6 community-based health-care staff, scientific and professional staff |
| Home help | 25 per hour | 30 minutes | aPSSRU 2016,201 pages 198 and 135–7 | Based on band 3 community-based health-care staff |
| Care assistant | 25 per hour | 30 minutes | aPSSRU 2016,201 pages 198 and 135–7 | Based on band 3 community-based health-care staff |
| Day centre | 41 per day | aPSSRU 2013,202 page 128 (inflated to 2016 prices) | Based on community care for older people: high cost | |
| Other | ||||
| Crisis resolution team | 195 per team contact | 5 team-member hours | aPSSRU 2016,201 page 169 | Based on assumption that each team contact requires a total of 5 hours from team members |
| Care assistant (telephone) | 25 per hour | 6.56 minutes | aPSSRU 2016,201 pages 198 and 135–7 | Assumes same consultation time as general practice nurse (telephone) |
| Clinical support worker | 23.50 per hour | 30 minutes | aPSSRU 2017,147 pages 153–5 | Based on band 3 community-based health-care staff, scientific and professional staff |
| Social worker (home visit) | 79 per hour | 23.4 minutes | aPSSRU 2016,201 page 156 | Assumes same consultation time as GP home visit |
| District nurse (telephone) | 44 per hour | 6.56 minutes | aPSSRU 2016,201 page 142 | Costed at band 6 community-based nurses. Assumes same consultation time as general practice nurse (telephone) |
| Occupational therapist (telephone) | 44 per hour | 6.56 minutes | aPSSRU 2016,201 page 159 | Uses community occupational therapist costs. Assumes same consultation time as general practice nurse (telephone) |
| Home improvement agency administrator (visit) | 23 per hour | 1 hour | ||
| Home improvement agency administrator (telephone) | 23 per hour | 6.56 minutes | ||
| Home adaptations | ||||
| Bath board | 28.99 | Mobility Smart (Preston, UK) | Product: Marina bath board (Inracare Ltd, Bridgend, UK) | |
| Bath seat | 268.69 | Mobility Smart | Product: width-adjustable swivelling bath seat | |
| Bath stool | 26.29 | Mobility Smart | Product: bath stool with rotating seat | |
| Commode | 41.66 | Mobility Smart | Product: Mobility Smart Super Light Folding Commode | |
| Electric bed | 535.69 | Mobility Smart | Product: Mobility Smart Devon Electric Adjustable Bed, 3 ft | |
| Fit handrail (external) | 42 | – | aPSSRU 2016,201 page 98 | Equipment and adaptations – minor adaptations |
| Fit handrail (internal) | 28 | – | aPSSRU 2016,201 page 98 | Equipment and adaptations – minor adaptations |
| Fit handrail to bath | 18 | – | aPSSRU 2016,201 page 98 | Equipment and adaptations – minor adaptations |
| Perching stool | 71.29 | Mobility Smart | Product: bariatric perching stool | |
| Profiling bed | 899.00 | Mobility Smart | Product: Mobility Smart Profiling Standard Bed | |
| Raised toilet seat | 16.49 | Mobility Smart | Product Savanah Raised Toilet Seat (Homecraft, Patterson Medical, Sutton-in-Ashfield, UK) | |
| Ramp | 316 | – | aPSSRU 2016,201 page 98 | Equipment and adaptations – minor adaptations |
| Rollator | 100.89 | Mobility Smart | Product: Genesis Rollator (Mobility Smart) | |
| Seat assist | 152.59 | Mobility Smart | Product: UpEasy Seat Assist (Patterson Medical, Sutton-in-Ashfield, UK) | |
| Shower chair | 108.19 | Mobility Smart | Product: folding shower chair – polyurethane seat | |
| Slide sheet | 19.19 | Mobility Smart | Product: Mobility Smart Slide Sheets (150 × 70 cm) | |
| Stair lift (straight) | 1927 | – | aPSSRU 2016,201 page 97 | Equipment and adaptations – major adaptations |
| Step | 481 | – | aPSSRU 2016,201 page 98 | Equipment and adaptations – major adaptations |
| Tap turner | 8.98 | Mobility Smart | Product: Universal Tap Turners (Drive DeVilbiss Healthcare Ltd, Birkenshaw, UK) | |
| Toilet frame | 41.99 | Mobility Smart | Product: Stamford Combi Scandia Toilet Frame (Drive DeVilbiss Healthcare Ltd) | |
| Bed transfer aid | 28.19 | Mobility Smart | Product: Solo Fixed Height Bed Transfer Mobility Smart Aid | |
| Trolley walker | 93.79 | Mobility Smart | Product: adjustable-height trolley walker | |
| Walker | 61.99 | Mobility Smart | Product: Days Lightweight Tri-Wheel Walker [Days Healthcare (Patterson Medical), Sutton-in-Ashfield, UK] | |
| Crutches | 22.79 | Mobility Smart | Product: Coopers Elbow Double Adjustable Crutches (Sunrise Medical Limited, West Midlands, UK) | |
| Level-access shower | 5,160 | aPSSRU 2017,147 page 112 | ||
| Walking frame/Zimmer frame (Zimmer Biomet Holdings, Inc. Warsaw, IN, USA) | 37.59 | Mobility Smart | Product: folding walking/Zimmer frame with wheels | |
| Walking stick/cane | 10.99 | Mobility Smart | Product: folding walking stick | |
| Magnifying glass | 3.79 | Mobility Smart | Product: Mobility Smart Magnifying Glass | |
| Bed rail | 103.49 | Mobility Smart | Product: 30-inch safety bed rail | |
| Bed lever | 37.39 | Mobility Smart | Product: bed lever, double-ended | |
| Bed raiser | 82.69 | Mobility Smart | Product: Langham Multi-Purpose Furniture Raiser (Langham, Castle Donington, UK) | |
| Kettle tipper | 14.19 | Mobility Smart | Product: cordless kettle tipper | |
| Hoist | 823.69 | Mobility Smart | Product: Samsoft 150 Patient Hoist (Drive DeVilbiss Healthcare Ltd) | |
| Hoist sling | 58.99 | Mobility Smart | Product: Economy Universal Sling (Mobility Smart) | |
| Bath lift | 818.89 | Mobility Smart | Product: Relaxa Belt Bath Lift (CR Manufacturing Ltd, Aylesbury, UK) | |
| Foot supports/orthotics | 38.59 | Mobility Smart | Product: foot supports metatarsal raise | |
| Air pressure mattress | 892.49 | Mobility Smart | Product: UTS Air Pressure Mattress System (Mobility Smart) | |
| Electric door opener | 6000 | UHS/SQ response | Social Service partial contribution | |
| Widen front door | 578 | aPSSRU 2017147 | ||
| Transfer board | 14.69 | Mobility Smart | Product: short transfer board | |
| Hospital bed | 899.98 | Mobility Smart | Product: Invacare Medley Ergo Bed (Invacare Corporation, Elyria, OH, USA) | |
| Central heating | 0.00b | |||
| A&E services | ||||
| Visit to A&E by ambulance | 236 | |||
| A&E attendance | 137.82 | NHS Reference Costs 2015 to 2016 148 | Weighted average of emergency medicine (all investigation/treatment categories) | |
| Other hospital services | ||||
| Day hospital | 477 | Day cases | NHS Reference Costs 2015 to 2016148 AA30C/D/E/F | Weighted average of medical care of patients with MS |
| Nights on hospital ward for planned admission | 2575 | Elective inpatient | NHS Reference Costs 2015 to 2016148 (AA30C/D/E/F) | Weighted average of medical care of patients with MS |
| Contact | Unit cost (£) | Area | ||
| Number of contacts with anyone else from the hospital | ||||
| Acupuncture | 111 | Outpatient procedures | NHS Reference Costs 2015 to 2016148 (191-AB23Z) | Pain management: acupuncture for pain management |
| Clinical haematology | 136 | Consultant led | NHS Reference Costs 2015 to 2016148 (WF01D) | Non-admitted face-to-face attendance, first; national average unit cost |
| Consultant (no specific details) | 64 | Hospital-based scientific and professional staff | aPSSRU 2016,201 pages 183–85 | Cost per working hour: band 8a |
| Radiology | 182 | Consultant led | NHS Reference Costs 2015 to 2016148 (WF01B) | Non-admitted face-to-face attendance, first; national average unit cost |
| Walk-in clinic | 44 per hour | Community-based health-care staff | aPSSRU 2017,147 page 160; PSSRU 2015203 | Nurses – assumed at band 6 with an average consultation time of 15.5 minutes |
| Wheelchair assessment | 247 | Community health services | NHS Reference Costs 2015 to 2016148 (WCA-WC02) | Wheelchair services adults – assessment, medium need |
Appendix 14 Plan for longer-term cost-effectiveness modelling
Modelling approach
A Markov model was developed, given the chronic properties of MS. The decision-analytic model was developed using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) and Visual Basic for Applications (Microsoft Corporation). The model simulated costs and QALYs over a lifetime horizon. For horizons exceeding 12 months, future costs and QALYs were discounted at 3.5% per year, as recommended by NICE. 206
Model structure
Figure 11 illustrates the outline structure of the Markov model used to evaluate the longer-term cost-effectiveness of cognitive rehabilitation.
FIGURE 11.
Markov model structure.
The psychological impact of MS was categorised into three mutually exclusive states of mild impact, moderate impact and severe impact, as follows:
-
For participants with MSIS-Psy scores of 9–17, the psychological impact of their MS was classified as mild.
-
For participants with MSIS-Psy scores of 18–26, the psychological impact of their MS was classified as moderate.
-
For participants with MSIS-Psy scores of ≥ 27, the psychological impact of their MS was classified as severe.
The probabilistic structure of the model, uncertainty of the input parameters and repeated simulation requires assumptions to be made regarding the distributions to be applied.
Transition probabilities are constrained to the unit interval between 0 and 1. To satisfy this property, the beta distribution was used to estimate transition probability. The probability of remaining in a state was calculated as 1 minus the sum of the other transition probabilities.
Utilities were estimated from the EQ-5D-5L trial data over the interval –0.594 to 1.000. Despite estimating over the unit interval, the beta distribution was employed, as the proportion of trial participants with HRQoL worse than death is low.
Cost data are bounded in the 0 – infinity interval, but are often skewed. Although the Poisson distribution would be a suitable candidate for these counts, we used the gamma distribution (conjugate to the Poisson distribution) as this was considered better able to account for the skewness in cost data.
Transitions are permitted between any two live health states, or between any live state and the absorbing death state. Aside from death, it was assumed that there were no intervention-related adverse events that affected an individual’s HRQoL or the psychological impact of MS.
Model Inputs
We assumed that the population adhered to the trial protocol, with participants randomly allocated to either cognitive rehabilitation or usual care. The base-case analysis was to be estimated using a cohort of 10,000 patients aged 49 years, corresponding with the average age of participants in the CRAMMS trial.
The CRAMMS trial did not report any deaths. Therefore, we assumed that patients would not be at increased risk of death from the intervention. Nevertheless, individuals with MS are recognised to face a higher all-cause mortality rate and shorter life expectancy than those of the general population,154 with risk factors dependent on a range of factors including MS type, disability status (as defined by the EDSS), age at MS diagnosis and comorbidities. All-cause, age-related mortality rates were based on the 2014–16 Office for National Statistics National Life Tables: England and Wales,207 with adjustment for the gender-specific MS standardised mortality ratio presented in a meta-analysis. 208
To convert the standardised mortality ratio-adjusted mortality probability for a cycle length of 6 months, the formulae below were used, in which pn and rn are the n-month probability and rate of death, respectively:209
Namely, the 12-month mortality probability was transformed to the monthly death rate, which was subsequently transformed back to a 6-month probability.
All costs in the model were reported as Great British pounds and inflated to 2016 prices, based on the within-trial costs reported. Costs associated with each health state defined by the model concern the costs relating to the delivery of the intervention and any health-care service usage recorded by participants over the preceding interval.
For the deterministic analyses, mean costs per participant obtained from the within-trial analysis were used. For the probabilistic analyses, the gamma distribution utilising mean costs and associated standard errors obtained from the trial were used, as recommended by Briggs et al. 209 Similarly, mean utilities observed in the within-trial analysis were used for the deterministic analysis, whereas the beta distribution utilising mean utility and associated standard errors were used, as recommended by Briggs et al. 209
Appendix 15 Intervention costs
| Resource area | Resource | Explanation/details | Resource use | Unit cost | Source | Intervention total (£) |
|---|---|---|---|---|---|---|
| Training | Training in CRAMMS trial provided by clinical psychologist to AP | 1× clinical psychologist (band 8a) to deliver a half-day training session to 1× AP. Training is delivered once per site | 4 hours per site | Clinical psychologist: £62 per hour (£248 per site) |
|
1240.00 |
| Training provided to 1× AP (band 5) in order to appropriately conduct CRAMMS trial sessions | 4 hours per trainee at each site | AP: £33 per hour (£132 per site) |
|
660.00 | ||
| Cognitive assessment | Assessment conducted by clinical psychologist | Following referral of a patient by a neurologist to clinical psychology, an assessment is conducted by a clinical psychologist (band 8a) to determine patient eligibility for the CRAMMS trial | 2 hours per participant | Clinical psychologist: £62 per hour (£124 per participant) |
|
30,380.00 |
| Delivery of cognitive rehabilitation | Sessions delivered by AP | AP (band 5) delivers 10 sessions individually to each group. Each session lasts for 1 hour and 30 minutes. An additional 30 minutes is required for both set-up and close-down. Provision for a 30-minute catch-up session has been included for 137 of 450 sessions, for those participants missing the previous session | 2 hours 45 minutes per session | AP: £33 per hour (£90.75 per session) |
|
39,385.50 |
| Supervision conducted by clinical psychologist | 1× clinical psychologist (band 8a) conducts supervision of AP. Supervision sessions of 1 hour occur on a weekly basis, of which, it is assumed, 30 minutes is attributable to the CRAMMS trial | 30 minutes per site |
|
|
2375.00 | |
| Administration | 1× administrator (band 3) required for 30 minutes per session to send reminders to patients of upcoming intervention session | 30 minutes per session | Administration: £25 per hour (£12.50 per session) |
|
5625.00 | |
| Intervention manual | One intervention manual per participant for the full length of the intervention plus one set of facilitator notes for the AP required. No provision for spare copies has been included | 1× manual per participant | £2.00 per participant manual/facilitator notes | Trial team | 500.00 | |
| Stationery | Stationery required for participants to complete various session tasks and the intervention manual | Estimated on per-participant basis | £1.00 per participant | Trial team | 245.00 | |
| Refreshments | Offered as part of the rehabilitation session, with participants encouraged to share experiences with one another | Estimated on per-participant, per-session basis | £0.50 per participant per session | Trial team | 1225.00 | |
| Total cost (£) of cognitive rehabilitation (including cognitive assessment) | 81,635.50 | |||||
| Total cost (£) of cognitive rehabilitation per participant (including cognitive assessment) | 333.21 | |||||
| Total cost (£) of cognitive rehabilitation (excluding cognitive assessment) | 51,255.50 | |||||
| Total cost (£) of cognitive rehabilitation per participant (excluding cognitive assessment) | 209.21 | |||||
Appendix 16 Resource use for available cases
| Resource | Trial group | Number of appointments | Mean (SD) | Minimum | Maximum | Differencea (95% CI) |
|---|---|---|---|---|---|---|
| General practice and community nursing services | ||||||
| Number of times you saw a GP at the surgery | Usual care (n = 203) | 270 | 1.33 (4.17) | 0 | 50 | –0.06 (–0.68 to 0.55) |
| Cognitive rehabilitation (n = 245) | 311 | 1.27 (2.34) | 0 | 20 | ||
| Number of times you saw a GP at your home | Usual care (n = 203) | 7 | 0.03 (0.21) | 0 | 2 | 0.01 (–0.04 to –0.07) |
| Cognitive rehabilitation (n = 245) | 12 | 0.05 (0.37) | 0 | 5 | ||
| Number of times you spoke to a GP on the telephone | Usual care (n = 203) | 141 | 0.70 (3.30) | 0 | 45 | –0.25 (–0.69 to 0.19) |
| Cognitive rehabilitation (n = 245) | 108 | 0.44 (1.06) | 0 | 10 | ||
| Number of times you saw a nurse at the surgery | Usual care (n = 203) | 93 | 0.46 (1.16) | 0 | 12 | –0.02 (–0.22 to 0.19) |
| Cognitive rehabilitation (n = 245) | 108 | 0.44 (1.05) | 0 | 8 | ||
| Number of times you saw a nurse at your home | Usual care (n = 203) | 38 | 0.19 (1.46) | 0 | 20 | –0.05 (–0.29 to 0.19) |
| Cognitive rehabilitation (n = 245) | 33 | 0.13 (1.11) | 0 | 12 | ||
| Number of times you saw a counsellor at the surgery | Usual care (n = 203) | 25 | 0.12 (0.94) | 0 | 10 | –0.03 (–0.20 to 0.015) |
| Cognitive rehabilitation (n = 245) | 24 | 0.10 (0.90) | 0 | 12 | ||
| Number of contacts with anyone else from the surgery | Usual care (n = 203) | 33 | 0.16 (0.96) | 0 | 10 | –0.01 (–0.19 to 0.016) |
| Cognitive rehabilitation (n = 245) | 37 | 0.15 (0.92) | 0 | 12 | ||
| Number of times you saw a GP outside usual surgery hours (e.g. out-of-hours GP service) | Usual care (n = 203) | 0 | – | 0 | 0 | – |
| Cognitive rehabilitation (n = 245) | 0 | – | 0 | 0 | ||
| Total cost (£) of general practice and community nursing services | Usual care (n = 203) | 88.77 (267.78) | 0 | 3430 | –11.35 (–49.29 to 26.59) | |
| Cognitive rehabilitation (n = 245) | 77.42 (127.90) | 0 | 790.22 | |||
| Hospital and community services | ||||||
| Number of times you saw a neurologist at the hospital | Usual care (n = 203) | 89 | 0.43 (0.63) | 0 | 3 | 0.01 (–0.11 to 0.13) |
| Cognitive rehabilitation (n = 245) | 110 | 0.45 (0.65) | 0 | 5 | ||
| Number of times you saw a MS nurse at the hospital | Usual care (n = 203) | 140 | 0.69 (1.20) | 0 | 12 | –0.05 (–0.26 to 0.15) |
| Cognitive rehabilitation (n = 245) | 156 | 0.64 (1.03) | 0 | 8 | ||
| Number of times you saw a MS nurse at your home | Usual care (n = 203) | 17 | 0.08 (0.48) | 0 | 5 | –0.01 (–0.10 to 0.07) |
| Cognitive rehabilitation (n = 245) | 17 | 0.07 (0.042) | 0 | 5 | ||
| Total cost (£) of hospital and community services | Usual care (n = 203) | 115.02 (145.63) | 0 | 730.50 | 1.08 (–26.60 to –28.77) | |
| Cognitive rehabilitation (n = 245) | 116.11 (151.07) | 0 | 1164.50 | |||
| A&E services | ||||||
| Number of times you went to an A&E unit | Usual care (n = 55) | 5 | 0.09 (0.29) | 0 | 1 | –0.01 (–0.12 to 0.09) |
| Cognitive rehabilitation (n = 79) | 6 | 0.08 (0.31) | 0 | 2 | ||
| Of these, how many were by ambulance? | Usual care (n = 55) | 3 | 0.05 (0.23) | 0 | 1 | –0.02 (–0.10 to 0.07) |
| Cognitive rehabilitation (n = 79) | 3 | 0.04 (0.25) | 0 | 2 | ||
| Did your visit to A&E result in an overnight stay in hospital? | Usual care (n = 55) | 0 | 0.03 (–0.01 to 0.06) | |||
| Cognitive rehabilitation (n = 79) | 2 | |||||
| Total number of nights spent in night as a result of A&E visits | Usual care (n = 55) | 0 | 0 (0) | 0 | 0 | 0.06 (–0.04 to 0.17) |
| Cognitive rehabilitation (n = 79) | 5 | 0.06 (0.05) | 0 | 3 | ||
| Total cost (£) of A&E services | Usual care (n = 55) | 6.85 (46.96) | 0 | 373.82 | 29.26 (–22.18 to 80.70) | |
| Cognitive rehabilitation (n = 79) | 36.11 (371.33) | 4403.64 | ||||
| Other hospital services | ||||||
| Number of times you attended a day hospital | Usual care (n = 203) | 35 | 0.17 (0.63) | 0 | 4 | 0.17 (–0.02 to 0.36) |
| Cognitive rehabilitation (n = 245) | 84 | 0.34 (1.26) | 0 | 12 | ||
| Number of times you went to a hospital outpatient clinic | Usual care (n = 203) | 103 | 0.51 (0.84) | 0 | 4 | –0.05 (–0.21 to 0.11) |
| Cognitive rehabilitation (n = 245) | 111 | 0.45 (0.87) | 0 | 5 | ||
| Number of nights you spent on a hospital ward as a result of a planned admission | Usual care (n = 203) | 4 | 0.02 (0.17) | 0 | 2 | 0.17 (–0.04 to 0.38) |
| Cognitive rehabilitation (n = 245) | 47 | 0.19 (1.53) | 0 | 22 | ||
| Number of contacts with anyone else from the hospital | Usual care (n = 203) | 40 | 0.20 (0.71) | 0 | 6 | 0.04 (–0.11 to 0.20) |
| Cognitive rehabilitation (n = 245) | 59 | 0.24 (0.92) | 0 | 10 | ||
| Total cost (£) of other hospital services | Usual care (n = 203) | 246.44 (553.37) | 0 | 5150.00 | 498.12 (–54.53 to 1050.77) | |
| Cognitive rehabilitation (n = 245) | 744.56 (3983.82) | 56,832.50 | ||||
| Therapy services | ||||||
| Number of contacts with a hospital-based physiotherapist | Usual care (n = 203) | 112 | 0.55 (1.60) | 0 | 12 | 0.15 (–0.18 to 0.48) |
| Cognitive rehabilitation (n = 245) | 172 | 0.70 (1.92) | 0 | 12 | ||
| Number of contacts with a community-based physiotherapist | Usual care (n = 203) | 65 | 0.32 (1.27) | 0 | 12 | 0.09 (–0.19 to 0.37) |
| Cognitive rehabilitation (n = 245) | 100 | 0.41 (1.66) | 0 | 12 | ||
| Number of contacts with a hospital-based occupational therapist | Usual care (n = 203) | 29 | 0.14 (0.67) | 0 | 5 | –0.04 (–0.14 to 0.06) |
| Cognitive rehabilitation (n = 245) | 25 | 0.10 (0.43) | 0 | 4 | ||
| Number of contacts with a community-based occupational therapist | Usual care (n = 203) | 32 | 0.16 (0.58) | 0 | 5 | 0.06 (–0.07 to 0.18) |
| Cognitive rehabilitation (n = 245) | 53 | 0.22 (0.74) | 0 | 7 | ||
| Number of contacts with a psychologist | Usual care (n = 203) | 52 | 0.26 (0.91) | 0 | 6 | –0.04 (–0.20 to 0.12) |
| Cognitive rehabilitation (n = 245) | 53 | 0.22 (0.83) | 0 | 6 | ||
| Number of contacts with a pharmacist | Usual care (n = 203) | 365 | 1.80 (2.69) | 0 | 12 | –0.61 (–1.05 to -0.18) |
| Cognitive rehabilitation (n = 245) | 290 | 1.18 (2.03) | 0 | 12 | ||
| Number of times you contacted the NHS 111 service | Usual care (n = 203) | 15 | 0.07 (0.30) | 0 | 2 | –0.00 (–0.7 to 0.07) |
| Cognitive rehabilitation (n = 245) | 18 | 0.07 (0.41) | 0 | 5 | ||
| Total cost (£) of therapy services | Usual care (n = 203) | 130.74 (163.29) | 0 | 834.00 | –18.23 (–47.45 to 10.98) | |
| Cognitive rehabilitation (n = 245) | 112.51 (151.28) | 0 | 846.00 | |||
| Social services | ||||||
| Number of times you saw a social worker | Usual care (n = 203) | 17 | 0.08 (0.41) | 0 | 4 | 0.00 (–0.09 to 0.09) |
| Cognitive rehabilitation (n = 245) | 21 | 0.09 (0.52) | 0 | 5 | ||
| Number of times you saw a home help | Usual care (n = 203) | 520 | 2.56 (15.05) | 0 | 168 | –0.05 (–2.93 to 2.83) |
| Cognitive rehabilitation (n = 245) | 616 | 2.51 (15.74) | 0 | 180 | ||
| Number of times you saw a care assistant | Usual care (n = 203) | 1140 | 5.62 (28.77) | 0 | 275 | –1.34 (–6.34 to 3.66) |
| Cognitive rehabilitation (n = 245) | 1048 | 4.28 (25.08) | 0 | 336 | ||
| Number of times you visited a day centre | Usual care (n = 203) | 65 | 0.32 (1.86) | 0 | 15 | 0.18 (–0.26 to 0.62) |
| Cognitive rehabilitation (n = 245) | 123 | 0.50 (2.70) | 0 | 20 | ||
| Number of contacts with anyone else from social services | Usual care (n = 203) | 48 | 0.24 (2.00) | 0 | 24 | –0.19 (–0.45 to 0.07) |
| Cognitive rehabilitation (n = 245) | 11 | 0.04 (0.43) | 0 | 6 | ||
| Total cost (£) of social services | Usual care (n = 203) | 715.55 (2414.36) | 0 | 12,684.00 | –179.38 (–610.23 to 251.47) | |
| Cognitive rehabilitation (n = 245) | 536.17 (2225.14) | 20,776.63 | ||||
| Medications | ||||||
| Number of medications taken | Usual care (n = 203) | 4.86 (3.22) | 0 | 18 | –0.34 (–0.95 to 0.26) | |
| Cognitive rehabilitation (n = 245) | 4.51 (3.25) | 0 | 17 | |||
| Cost (£) of medications taken | Usual care (n = 203) | 1758.24 (2232.84) | 0 | 15,173.91 | –109.43 (–527.73 to 308.87) | |
| Cognitive rehabilitation (n = 245) | 1648.81 (2250.62) | 0 | 18,551.35 | |||
| Number of medications taken (excluding disease-modifying therapies) | Usual care (n = 203) | 4.44 (3.29) | 0 | 18 | –0.32 (–0.93 to 0.30) | |
| Cognitive rehabilitation (n = 245) | 4.12 (3.26) | 0 | 16 | |||
| Cost (£) of medications taken (excluding disease-modifying therapies) | Usual care (n = 203) | 261.06 (1232.32) | 0 | 15,173.91 | –120.03 (–289.28 to 49.22) | |
| Cognitive rehabilitation (n = 245) | 141.03 (497.77) | 0 | 6582.07 | |||
| Resource | Group | Number of appointments | Mean (SD) | Minimum | Maximum | Differencea (95% CI) | p-valuea |
|---|---|---|---|---|---|---|---|
| General practice and community nursing services | |||||||
| Number of times you saw a GP at the surgery | Usual care (n = 187) | 165 | 0.88 (1.55) | 0 | 12 | –0.03 (–0.35 to 0.30) | 0.880 |
| Cognitive rehabilitation (n = 224) | 192 | 0.86 (1.78) | 0 | 20 | |||
| Number of times you saw a GP at your home | Usual care (n = 187) | 3 | 0.02 (0.22) | 0 | 3 | 0.01 (–0.03 to 0.05) | 0.593 |
| Cognitive rehabilitation (n = 224) | 6 | 0.03 (0.01) | 0 | 2 | |||
| Number of times you spoke to a GP on the telephone | Usual care (n = 187) | 93 | 0.50 (1.39) | 0 | 12 | –0.11 (–0.33 to 0.12) | 0.342 |
| Cognitive rehabilitation (n = 224) | 87 | 0.39 (0.92) | 0 | 7 | |||
| Number of times you saw a nurse at the surgery | Usual care (n = 187) | 64 | 0.34 (0.78) | 0 | 7 | 0.01 (–0.14 to 0.17) | 0.852 |
| Cognitive rehabilitation (n = 224) | 80 | 0.36 (0.83) | 0 | 5 | |||
| Number of times you saw a nurse at your home | Usual care (n = 187) | 12 | 0.06 (0.47) | 0 | 6 | 0.16 (–0.14 to 0.45) | 0.289 |
| Cognitive rehabilitation (n = 224) | 50 | 0.22 (2.00) | 0 | 26 | |||
| Number of times you saw a counsellor at the surgery | Usual care (n = 187) | 27 | 0.14 (0.98) | 0 | 10 | 0.02 (–0.18 to 0.21) | 0.869 |
| Cognitive rehabilitation (n = 224) | 36 | 0.16 (1.02) | 0 | 12 | |||
| Number of contacts with anyone else from the surgery | Usual care (n = 187) | 30 | 0.16 (0.79) | 0 | 6 | –0.09 (–0.21 to 0.02) | 0.121 |
| Cognitive rehabilitation (n = 224) | 15 | 0.07 (0.39) | 0 | 4 | |||
| Number of times you saw a GP outside usual surgery hours (e.g. out-of-hours GP service) | Usual care (n = 187) | 0 | – | 0 | 0 | – | – |
| Cognitive rehabilitation (n = 224) | 0 | – | 0 | 0 | |||
| Total cost (£) of general practice and community nursing services | Usual care (n = 187) | 61.42 (106.77) | 0 | 792.00 | –1.32 (–22.52 to 19.88) | 0.903 | |
| Cognitive rehabilitation (n = 224) | 60.10 (110.76) | 0 | 957.76 | ||||
| Hospital and community services | |||||||
| Number of times you saw a neurologist at the hospital | Usual care (n = 187) | 78 | 0.42 (0.69) | 0 | 3 | –0.00 (–0.14 to 0.14) | 0.979 |
| Cognitive rehabilitation (n = 224) | 93 | 0.42 (0.76) | 0 | 6 | |||
| Number of times you saw a MS nurse at the hospital | Usual care (n = 187) | 101 | 0.54 (0.99) | 0 | 6 | 0.01 (–0.17 to 0.20) | 0.887 |
| Cognitive rehabilitation (n = 224) | 124 | 0.55 (0.93) | 0 | 5 | |||
| Number of times you saw a MS nurse at your home | Usual care (n = 187) | 12 | 0.06 (0.42) | 0 | 5 | –0.00 (–0.07 to 0.07) | 0.964 |
| Cognitive rehabilitation (n = 224) | 14 | 0.06 (0.32) | 0 | 3 | |||
| Total cost (£) of hospital and community services | Usual care (n = 187) | 106.49 (163.28) | 0 | 810.00 | –0.58 (–33.93 to 32.77) | 0.973 | |
| Cognitive rehabilitation (n = 224) | 105.92 (177.94) | 0 | 1408.00 | ||||
| Therapy services | |||||||
| Number of contacts with a hospital-based physiotherapist | Usual care (n = 187) | 133 | 0.71 (2.12) | 0 | 16 | –0.13 (–0.52 to 0.26) | 0.50 |
| Cognitive rehabilitation (n = 225) | 130 | 0.58 (1.90) | 0 | 15 | |||
| Number of contacts with community-based physiotherapist | Usual care (n = 187) | 209 | 1.12 (3.49) | 0 | 26 | –0.34 (–0.94 to 0.26) | 0.27 |
| Cognitive rehabilitation (n = 225) | 175 | 0.78 (2.72) | 0 | 26 | |||
| Number of contacts with a hospital-based occupational therapist | Usual care (n = 187) | 51 | 0.27 (1.24) | 0 | 10 | –0.16 (–0.35 to 0.03) | 0.10 |
| Cognitive rehabilitation (n = 225) | 25 | 0.11 (0.68) | 0 | 6 | |||
| Number of contacts with a community-based occupational therapist | Usual care (n = 187) | 31 | 0.17 (0.53) | 0 | 3 | 0.09 (–0.07 to 0.24) | 0.27 |
| Cognitive rehabilitation (n = 225) | 57 | 0.25 (0.97) | 0 | 7 | |||
| Number of contacts with a psychologist | Usual care (n = 187) | 44 | 0.24 (1.18) | 0 | 12 | –0.03 (–0.24 to 0.18) | 0.77 |
| Cognitive rehabilitation (n = 225) | 46 | 0.20 (1.01) | 0 | 12 | |||
| Number of contacts with a pharmacist | Usual care (n = 187) | 329 | 1.76 (3.27) | 0 | 24 | –0.45 (–0.98 to 0.08) | 0.10 |
| Cognitive rehabilitation (n = 225) | 295 | 1.31 (2.21) | 0 | 12 | |||
| Number of times you contacted the NHS 111 service | Usual care (n = 187) | 10 | 0.05 (0.36) | 0 | 4 | 0.02 (–0.05 to 0.09) | 0.62 |
| Cognitive rehabilitation (n = 225) | 16 | 0.07 (0.36) | 0 | 3 | |||
| Total cost (£) of therapy services | Usual care (n = 187) | 164.06 (211.92) | 0 | 1084.00 | –37.34 (–74.45 to 0.24) | 0.05* | |
| Cognitive rehabilitation (n = 225) | 127.71 (171.19) | 0 | 930.00 | ||||
| Social services | |||||||
| Number of times you saw a social worker | Usual care (n = 187) | 11 | 0.06 (0.33) | 0 | 3 | 0.08 (–0.06 to 0.21) | 0.25 |
| Cognitive rehabilitation (n = 225) | 31 | 0.14 (0.88) | 0 | 10 | |||
| Number of times you saw a home help | Usual care (n = 187) | 149 | 0.80 (3.89) | 0 | 24 | 1.71 (–0.50 to 3.91) | 0.13 |
| Cognitive rehabilitation (n = 225) | 563 | 2.50 (14.94) | 0 | 180 | |||
| Number of times you saw a care assistant | Usual care (n = 187) | 2483 | 13.28 (51.12) | 0 | 364 | –9.15 (–16.51 to –1.80) | 0.01** |
| Cognitive rehabilitation (n = 225) | 928 | 4.12 (21.14) | 0 | 182 | |||
| Number of times you visited a day centre | Usual care (n = 187) | 135 | 0.72 (3.69) | 0 | 36 | 0.07 (–0.60 to 0.74) | 0.84 |
| Cognitive rehabilitation (n = 225) | 178 | 0.79 (3.25) | 0 | 24 | |||
| Number of contacts with anyone else from social services | Usual care (n = 187) | 37 | 0.20 (1.49) | 0 | 16 | –0.12 (–0.32 to 0.09) | 0.26 |
| Cognitive rehabilitation (n = 225) | 18 | 0.08 (0.41) | 0 | 4 | |||
| Total cost (£) of social services | Usual care (n = 187) | 510.40 (1664.66) | 0 | 10,544.00 | –66.26 (–392.55 to 260.02) | 0.69 | |
| Cognitive rehabilitation (n = 225) | 444.14 (1687.85) | 0 | 11,170.19 | ||||
| A&E services | |||||||
| Number of times you went to an A&E unit | Usual care (n = 132) | 7 | 0.05 (0.26) | 0 | 2 | 0.05 (–0.02 to 0.12) | 0.19 |
| Cognitive rehabilitation (n = 167) | 17 | 0.10 (0.36) | 0 | 2 | |||
| Of these, how many were by ambulance? | Usual care (n = 132) | 2 | 0.02 (0.12) | 0 | 1 | 0.01 (–0.02 to 0.05) | 0.46 |
| Cognitive rehabilitation (n = 167) | 5 | 0.03 (0.20) | 0 | 2 | |||
| Did your visit to A&E result in an overnight stay in hospital? | Usual care (n = 132) | 1 | 0.02 (–0.01 to 0.04) | 0.27 | |||
| Cognitive rehabilitation (n = 167) | 4 | ||||||
| Total number of nights spent in night as a result of A&E visits | Usual care (n = 132) | 1 | 0.01 (0.32) | 0 | 1 | 0.18 (–0.05 to 0.41) | 0.12 |
| Cognitive rehabilitation (n = 167) | 5 | 0.19 (1.32) | 0 | 12 | |||
| Total cost (£) of A&E services | Usual care (n = 132) | 27.23 (280.63) | 0 | 3793.82 | 94.54 (–37.74 to 226.82) | 0.16 | |
| Cognitive rehabilitation (n = 167) | 121.77 (58.92) | 0 | 8059.64 | ||||
| Other hospital services | |||||||
| Number of times you attended a day hospital | Usual care (n = 187) | 23 | 0.12 (0.53) | 0 | 4 | 0.13 (–0.04 to 0.30) | 0.14 |
| Cognitive rehabilitation (n = 225) | 57 | 0.25 (1.11) | 0 | 12 | |||
| Number of times you went to a hospital outpatient clinic | Usual care (n = 187) | 119 | 0.64 (1.27) | 0 | 7 | –0.04 (–0.28 to 0.21) | 0.77 |
| Cognitive rehabilitation (n = 225) | 135 | 0.60 (1.21) | 0 | 9 | |||
| Number of nights you spent on a hospital ward as a result of a planned admission | Usual care (n = 187) | 1 | 0.01 (0.07) | 0 | 1 | 0.03 (–0.01 to 0.07) | 0.18 |
| Cognitive rehabilitation (n = 225) | 8 | 0.04 (0.30) | 0 | 3 | |||
| Number of contacts with anyone else from the hospital | Usual care (n = 187) | 95 | 0.51 (1.33) | 0 | 9 | 0.11 (–0.25 to 0.48) | 0.54 |
| Cognitive rehabilitation (n = 225) | 140 | 0.62 (2.23) | 0 | 24 | |||
| Total cost (£) of other hospital services | Usual care (n = 187) | 173.01 (398.26) | 0 | 3217.00 | 136.50 (–36.97 to 309.97) | 0.12 | |
| Cognitive rehabilitation (n = 225) | 309.51 (1150.64) | 13 to 568.00 | |||||
| Medications | |||||||
| Number of medications taken | Usual care (n = 187) | 5.29 (4.10) | 0 | 20 | –0.33 (–1.10 to 0.45) | 0.41 | |
| Cognitive rehabilitation (n = 225) | 4.97 (3.89) | 0 | 26 | ||||
| Cost of medications taken | Usual care (n = 187) | 1987.31 (3478.53) | 0 | 26,516.08 | –313.55 (–860.86 to 233.77) | 0.26 | |
| Cognitive rehabilitation (n = 225) | 1673.76 (2107.78) | 0 | 8557.67 | ||||
| Number of medications taken (excluding disease-modifying therapies) | Usual care (n = 187) | 4.89 (4.12) | 0 | 20 | –0.29 (–1.08 to 0.49) | 0.46 | |
| Cognitive rehabilitation (n = 225) | 4.59 (3.94) | 0 | 26 | ||||
| Cost (£) of medications taken (excluding disease-modifying therapies) | Usual care (n = 187) | 428.61 (2390.56) | 0 | 24,841.15 | –181.13 (–522.38 to 160.12) | 0.30 | |
| Cognitive rehabilitation (n = 225) | 247.48 (942.13) | 0 | 8557.67 | ||||
| Resource | Group | Number of appointments | Mean (SD) | Minimum | Maximum | Differencea (95% CI) | p-valuea |
|---|---|---|---|---|---|---|---|
| General practice and community nursing services | |||||||
| Number of times you saw a GP at the surgery | Usual care (n = 172) | 106 | 0.62 (1.22) | 0 | 10 | 0.31 (–0.09 to 0.72) | 0.130 |
| Cognitive rehabilitation (n = 211) | 196 | 0.93 (2.46) | 0 | 30 | |||
| Number of times you saw a GP at your home | Usual care (n = 172) | 10 | 0.06 (0.43) | 0 | 5 | –0.02 (–0.09 to 0.05) | 0.576 |
| Cognitive rehabilitation (n = 211) | 8 | 0.04 (0.27) | 0 | 3 | |||
| Number of times you spoke to a GP on the telephone | Usual care (n = 172) | 66 | 0.38 (1.07) | 0 | 10 | –0.02 (–0.22 to 0.18) | 0.856 |
| Cognitive rehabilitation (n = 211) | 77 | 0.36 (0.96) | 0 | 7 | |||
| Number of times you saw a nurse at the surgery | Usual care (n = 172) | 52 | 0.30 (0.62) | 0 | 3 | 0.04 (–0.11 to 0.19) | 0.603 |
| Cognitive rehabilitation (n = 211) | 72 | 0.34 (0.80) | 0 | 6 | |||
| Number of times you saw a nurse at your home | Usual care (n = 172) | 11 | 0.06 (0.57) | 0 | 7 | 0.04 (–0.12 to 0.20) | 0.623 |
| Cognitive rehabilitation (n = 211) | 22 | 0.10 (0.94) | 0 | 13 | |||
| Number of times you saw a counsellor at the surgery | Usual care (n = 172) | 12 | 0.07 (0.53) | 0 | 6 | 0.17 (–0.04 to 0.38) | 0.109 |
| Cognitive rehabilitation (n = 211) | 51 | 0.24 (0.09) | 0 | 13 | |||
| Number of contacts with anyone else from the surgery | Usual care (n = 172) | 12 | 0.07 (0.33) | 0 | 3 | 0.15 (–0.01 to 0.32) | 0.069 |
| Cognitive rehabilitation (n = 211) | 47 | 0.22 (1.06) | 0 | 10 | |||
| Number of times you saw a GP outside usual surgery hours (e.g. out-of-hours GP service) | Usual care (n = 172) | 0 | – | 0 | 0 | – | – |
| Cognitive rehabilitation (n = 211) | 0 | – | 0 | 0 | |||
| Total cost (£) of general practice and community services | Usual care (n = 172) | 48.07 (86.43) | 0 | 600.00 | 17.07 (–6.93 to 41.07) | 0.163 | |
| Cognitive rehabilitation (n = 211) | 65.15 (141.08) | 0 | 1488.43 | ||||
| Hospital and community services | |||||||
| Number of times you saw a neurologist at the hospital | Usual care (n = 172) | 65 | 0.38 (0.62) | 0 | 3 | 0.01 (–0.11 to 0.13) | 0.863 |
| Cognitive rehabilitation (n = 211) | 82 | 0.39 (0.59) | 0 | 3 | |||
| Number of times you saw a MS nurse at the hospital | Usual care (n = 172) | 82 | 0.48 (0.83) | 0 | 4 | 0.01 (–0.15 to 0.17) | 0.935 |
| Cognitive rehabilitation (n = 211) | 102 | 0.48 (0.76) | 0 | 3 | |||
| Number of times you saw a MS nurse at your home | Usual care (n = 172) | 8 | 0.05 (0.26) | 0 | 2 | –0.00 (–0.06 to 0.07) | 0.979 |
| Cognitive rehabilitation (n = 211) | 10 | 0.05 (0.36) | 0 | 4 | |||
| Total cost (£) of hospital and community services | Usual care (n = 172) | 95.75 (144.23) | 0 | 730.50 | 2.16 (–25.72 to 30.04) | 0.879 | |
| Cognitive rehabilitation (n = 211) | 97.91 (134.23) | 0 | 677.50 | ||||
| Therapy services | |||||||
| Number of contacts with a hospital-based physiotherapist | Usual care (n = 175) | 72 | 0.41 (1.40) | 0 | 11 | 0.30 (–0.06 to 0.65) | 0.11 |
| Cognitive rehabilitation (n = 212) | 150 | 0.71 (2.05) | 0 | 13 | |||
| Number of contacts with community-based physiotherapist | Usual care (n = 175) | 156 | 0.89 (7.05) | 0 | 90 | –0.26 (–1.32 to 0.80) | 0.63 |
| Cognitive rehabilitation (n = 212) | 134 | 0.63 (3.14) | 0 | 39 | |||
| Number of contacts with a hospital-based occupational therapist | Usual care (n = 175) | 27 | 0.15 (0.71) | 0 | 7 | –0.10 (–0.21 to 0.02) | 0.09 |
| Cognitive rehabilitation (n = 212) | 12 | 0.06 (0.41) | 0 | 5 | |||
| Number of contacts with a community-based occupational therapist | Usual care (n = 175) | 26 | 0.15 (0.54) | 0 | 4 | –0.12 (–0.06 to 0.30) | 0.19 |
| Cognitive rehabilitation (n = 212) | 57 | 0.27 (1.12) | 0 | 12 | |||
| Number of contacts with a psychologist | Usual care (n = 175) | 51 | 0.29 (1.54) | 0 | 13 | –0.09 (–0.34 to 0.15) | 0.45 |
| Cognitive rehabilitation (n = 212) | 42 | 0.20 (0.86) | 0 | 8 | |||
| Number of contacts with a pharmacist | Usual care (n = 175) | 204 | 1.17 (2.24) | 0 | 12 | –0.04 (–0.48 to 0.39) | 0.85 |
| Cognitive rehabilitation (n = 212) | 238 | 1.12 (2.10) | 0 | 20 | |||
| Number of times you contacted the NHS 111 service | Usual care (n = 175) | 16 | 0.09 (0.38) | 0 | 3 | 0.02 (–0.11 to 0.08) | 0.73 |
| Cognitive rehabilitation (n = 212) | 16 | 0.08 (0.52) | 0 | 6 | |||
| Total cost (£) of therapy services | Usual care (n = 175) | 120.50 (293.59) | 0 | 3054.00 | –3.41 (–51.83 to 45.01) | 0.89 | |
| Cognitive rehabilitation (n = 212) | 117.09 (187.12) | 0 | 1457.00 | ||||
| Social services | |||||||
| Number of times you saw a social worker | Usual care (n = 175) | 27 | 0.15 (0.88) | 0 | 10 | 0.09 (–0.21 to 0.39) | 0.57 |
| Cognitive rehabilitation (n = 212) | 51 | 0.24 (1.85) | 0 | 26 | |||
| Number of times you saw a home help | Usual care (n = 175) | 250 | 1.43 (12.81) | 0 | 156 | 0.46 (–1.90 to 2.81) | 0.70 |
| Cognitive rehabilitation (n = 212) | 400 | 1.89 (10.77) | 0 | 90 | |||
| Number of times you saw a care assistant | Usual care (n = 175) | 2249 | 12.93 (55.10) | 0 | 364 | –6.75 (–15.99 to 2.50) | 0.15 |
| Cognitive rehabilitation (n = 212) | 1310 | 6.18 (2.53) | 0 | 364 | |||
| Number of times you visited a day centre | Usual care (n = 175) | 47 | 0.27 (1.99) | 0 | 22 | 0.03 (–0.33 to 0.40) | 0.86 |
| Cognitive rehabilitation (n = 212) | 64 | 0.30 (1.65) | 0 | 13 | |||
| Number of contacts with anyone else from social services | Usual care (n = 175) | 124 | 0.71 (4.85) | 0 | 45 | –0.53 (–1.21 to 0.15) | 0.12 |
| Cognitive rehabilitation (n = 212) | 38 | 0.18 (1.16) | 0 | 13 | |||
| Total cost (£) of social services | Usual care (n = 175) | 568.80 (2194.07) | 0 | 19,607.40 | –112.61 (–502.44 to 277.23) | 0.570 | |
| Cognitive rehabilitation (n = 212) | 456.19 (1704.96) | 0 | 10,134.00 | ||||
| A&E services | |||||||
| Number of times you went to an A&E unit | Usual care (n = 148) | 10 | 0.07 (0.32) | 0 | 3 | –0.05 (–0.10 to 0.01) | 0.09 |
| Cognitive rehabilitation (n = 183) | 4 | 0.02 (0.15) | 0 | 1 | |||
| Of these, how many were by ambulance? | Usual care (n = 148) | 6 | 0.04 (0.28) | 0 | 3 | –0.04 (–0.08 to 0.01) | 0.11 |
| Cognitive rehabilitation (n = 183) | 1 | 0.01 (0.07) | 0 | 1 | |||
| Did your visit to A&E result in an overnight stay in hospital? | Usual care (n = 148) | 3 | –0.02 (–0.04 to 0.00) | 0.05 | |||
| Cognitive rehabilitation (n = 183) | 0 | ||||||
| Total number of nights spent in night as a result of A&E visits | Usual care (n = 148) | 13 | 0.09 (0.06) | 0 | 6 | –0.09 (–0.18 to 0.01) | 0.07 |
| Cognitive rehabilitation (n = 183) | 0 | 0.00 (0.00) | 0 | 0 | |||
| Total cost (£) of A&E services | Usual care (n = 148) | 78.64 (511.47) | 0 | 4029.82 | –74.93 (–144.12 to –5.73) | 0.03* | |
| Cognitive rehabilitation (n = 183) | 3.71 (30.31) | 0 | 373.82 | ||||
| Other hospital services | |||||||
| Number of times you attended a day hospital | Usual care (n = 175) | 28 | 0.16 (0.64) | 0 | 3 | –0.00 (–0.14 to 0.13) | 0.95 |
| Cognitive rehabilitation (n = 212) | 33 | 0.16 (0.71) | 0 | 6 | |||
| Number of times you went to a hospital outpatient clinic | Usual care (n = 175) | 77 | 0.44 (0.93) | 0 | 6 | 0.10 (–0.14 to 0.34) | 0.43 |
| Cognitive rehabilitation (n = 212) | 114 | 0.54 (1.38) | 0 | 13 | |||
| Number of nights you spent on a hospital ward as a result of a planned admission | Usual care (n = 175) | 38 | 0.22 (2.33) | 0 | 30 | –0.16 (–0.48 to 0.17) | 0.35 |
| Cognitive rehabilitation (n = 212) | 13 | 0.06 (0.55) | 0 | 6 | |||
| Number of contacts with anyone else from the hospital | Usual care (n = 175) | 100 | 0.57 (1.57) | 0 | 11 | –0.12 (–0.42 to 0.18) | 0.43 |
| Cognitive rehabilitation (n = 212) | 96 | 0.45 (1.41) | 0 | 12 | |||
| Total cost (£) of other hospital services | Usual care (n = 175) | 730.47 (6036.85) | 0 | 77,727.00 | –422.64 (–1267.77 to 422.49) | 0.33 | |
| Cognitive rehabilitation (n = 212) | 307.83 (1505.38) | 0 | 16,103.00 | ||||
| Medications | |||||||
| Number of medications taken | Cognitive rehabilitation (n = 212) | 5.23 (3.55) | 0 | 20 | –0.08 (–0.834 to 0.68) | 0.84 | |
| Usual care (n = 175) | 5.15 (3.93) | 0 | 25 | ||||
| Cost (£) of medications taken | Cognitive rehabilitation (n = 212) | 2040.97 (3847.59) | 0 | 34,929.11 | –320.45 (–928.61 to 287.71) | 0.30 | |
| Usual care (n = 175) | 1720.52 (2127.86) | 0 | 7394.58 | ||||
| Number of medications taken (excluding disease-modifying therapies) | Cognitive rehabilitation (n = 212) | 4.82 (3.58) | 0 | 20 | –0.04 (–0.80 to 0.72) | 0.91 | |
| Usual care (n = 175) | 4.77 (3.95) | 0 | 25 | ||||
| Cost (£) of medications taken (excluding disease-modifying therapies) | Cognitive rehabilitation (n = 212) | 581.48 (3408.86) | 0 | 34,929.11 | –332.33 (–812.80 to 148.14) | 0.17 | |
| Usual care (n = 175) | 249.15 (929.10) | 0 | 7037.20 | ||||
Appendix 17 Available-case analyses
| Trial group | Baseline, mean (SD), n | 12 months, mean (SD), n | Adjusted difference in means (95% CI)a | p-value |
|---|---|---|---|---|
| Panel A: total cumulative costs at 12 months | ||||
| Usual care | 3067.70 (3214.14), 203 | 6573.95 (9188.35), 170 | –808.33 (–2248.22 to 631.56) | 0.27 |
| Cognitive rehabilitation | 3271.68 (5130.30), 245 | 5885.49 (5640.75), 208 | ||
| Panel B: total cumulative costs at 6 months | ||||
| 6 months, mean (SD), n | ||||
| Usual care | 3067.70 (3214.14), 203 | 3029.93 (3877.71), 187 | –12.51 (–682.88 to 657.86) | 0.97 |
| Cognitive rehabilitation | 3271.68 (5130.30), 245 | 2841.92 (3438.15), 225 | ||
| Trial group and QALY gain | Baseline, mean (SD), n | 12 months, mean (SD), n | Adjusted difference in means (95% CI)a | p-value |
|---|---|---|---|---|
| Panel A: EQ-5D-5L QALYs at 12 months (available cases) | ||||
| Usual care | 0.57 (0.25), 204 | 0.57 (0.27), 172 | ||
| Cognitive rehabilitation | 0.60 (0.26), 245 | 0.60 (0.25), 211 | 0.01 (–0.03 to 0.05) | 0.52 |
| QALY gain | 0.00 (–0.01 to 0.02) | 0.52 | ||
| Panel B: EQ-5D-5L QALYs at 6 months (available cases) | ||||
| 6 months, mean (SD), n | ||||
| Usual care | 0.57 (0.25), 204 | 0.57 (0.27), 187 | ||
| Cognitive rehabilitation | 0.60 (0.26), 245 | 0.60 (0.25), 224 | 0.01 (–0.03 to 0.05) | 0.54 |
| QALY gain | 0.00 (–0.01 to 0.01) | 0.54 | ||
| Trial group and QALY gain | Baseline, mean (SD), n | 12 months, mean (SD), n | Adjusted difference in means (95% CI)a | p-value |
|---|---|---|---|---|
| Panel A: MSIS-8D QALYs at 12 months (available cases) | ||||
| Usual care | 0.51 (0.18), 204 | 0.55 (0.19), 176 | ||
| Cognitive rehabilitation | 0.53 (0.17), 245 | 0.57 (0.18), 215 | 0.02 (–0.01 to 0.04) | 0.23 |
| QALY gain | 0.01 (–0.01 to 0.02) | 0.23 | ||
| Panel B: MSIS-8D QALYs at 6 months (available cases) | ||||
| 6 months, mean (SD), n | ||||
| Usual care | 0.51 (0.18), 204 | 0.52 (0.20), 187 | ||
| Cognitive rehabilitation | 0.53 (0.17), 245 | 0.57 (0.18), 216 | 0.03 (0.00 to 0.06) | 0.03 |
| QALY gain | 0.01 (0.00 to 0.01) | 0.03 | ||
| Outcome measure | Incremental cost (95% CI) | Incremental effect (95% CI) | ICER |
|---|---|---|---|
| EQ-5D-5L QALY 6 months | –12.51 (–682.88 to 657.86) | 0.00 (–0.01 to 0.01) | NA |
| EQ-5D-5L QALY 12 months | –808.33 (–2248.22 to 631.56) | 0.00 (–0.01 to 0.02) | NA |
| MSIS-8D QALY 6 months | –12.51 (–682.88 to 657.86) | 0.01 (0.00 to 0.01) | NA |
| MSIS-8D QALY 12 months | –808.33 (–2248.22 to 631.56) | 0.01 (–0.01 to 0.02) | NA |
| MSIS-Psy 6 months | –12.51 (–682.88 to 657.86) | –0.9 (–1.7 to –0.1) | NA |
| MSIS-Psy 12 months | –808.33 (–2248.22 to 631.56) | –0.6 (–1.5 to 0.3) | NA |
FIGURE 12.
Total costs and EQ-5D-5L QALYs at 12 months (available cases). (a) Cost-effectiveness plane; and (b) CEAC.
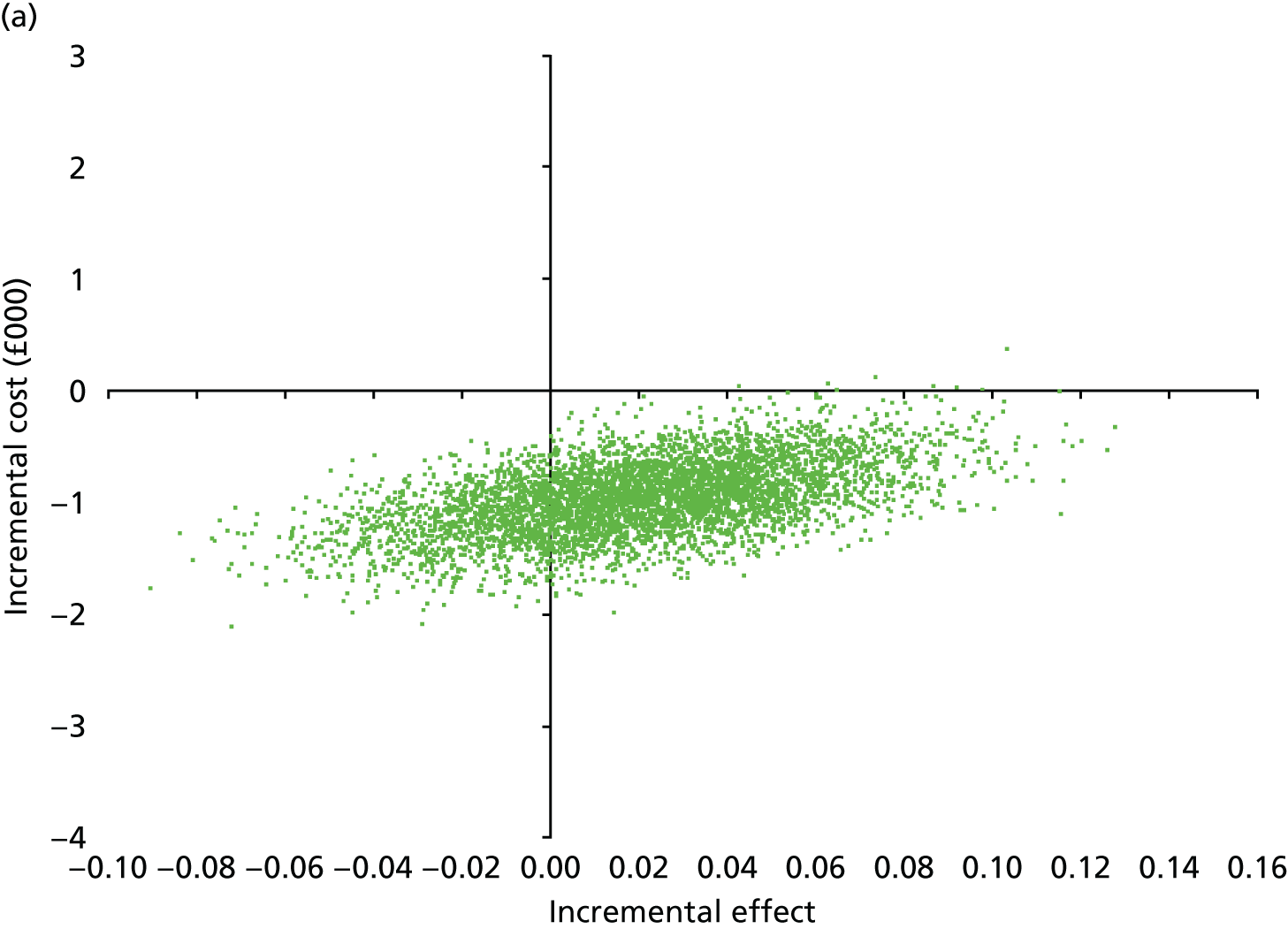
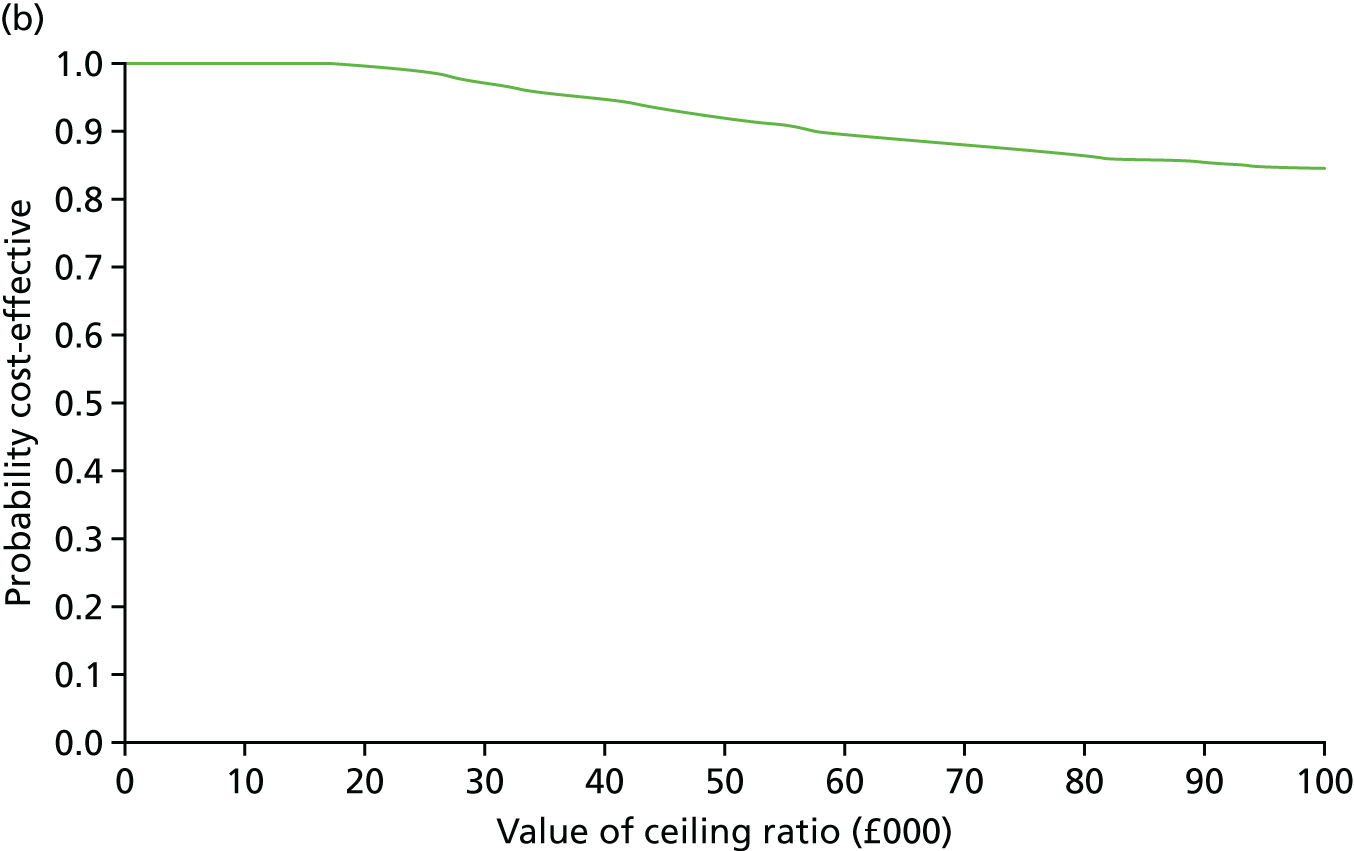
FIGURE 13.
Total costs and MSIS-8D QALYs at 12 months (available cases). (a) Cost-effectiveness plane; and (b) CEAC.
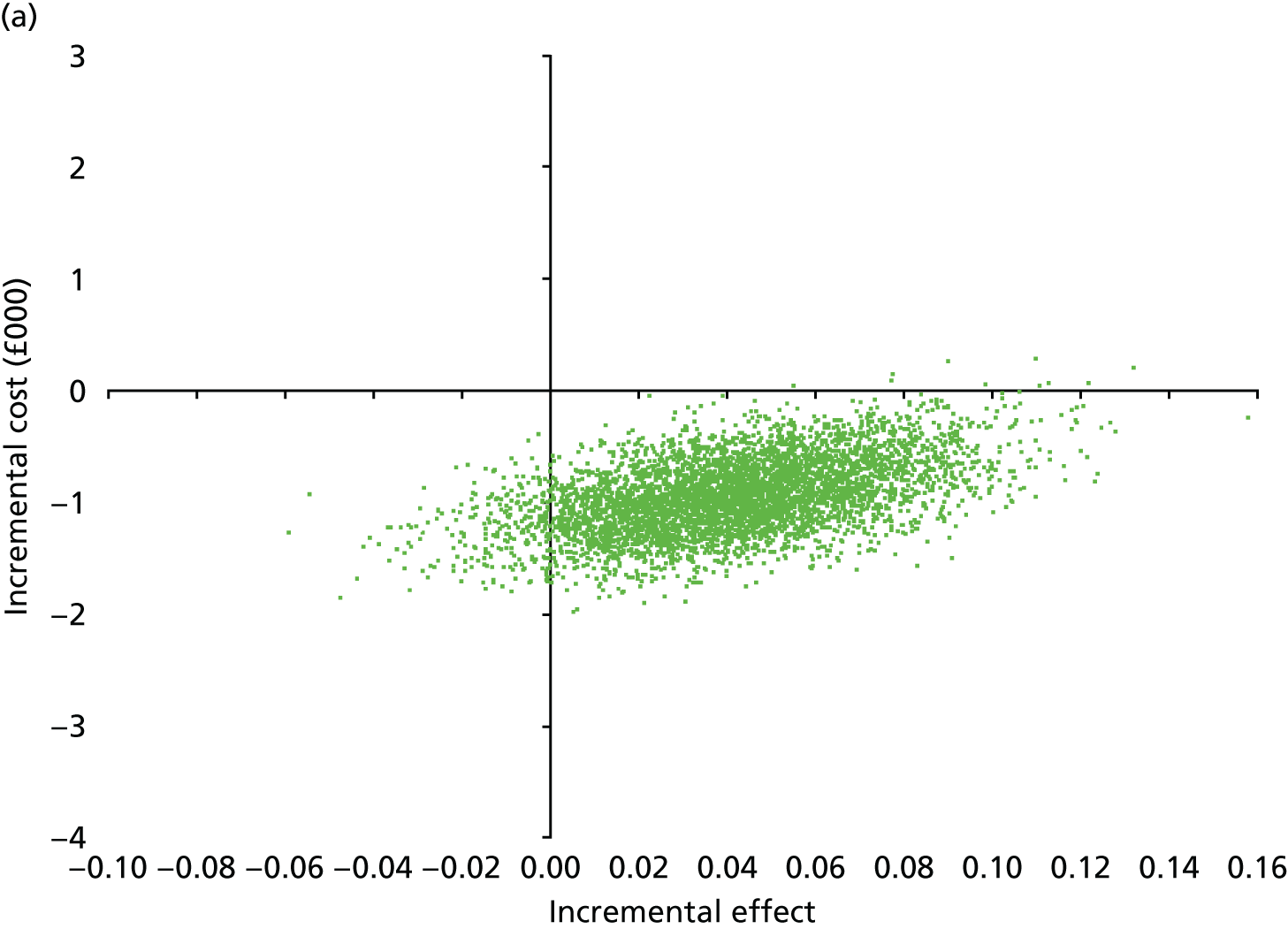
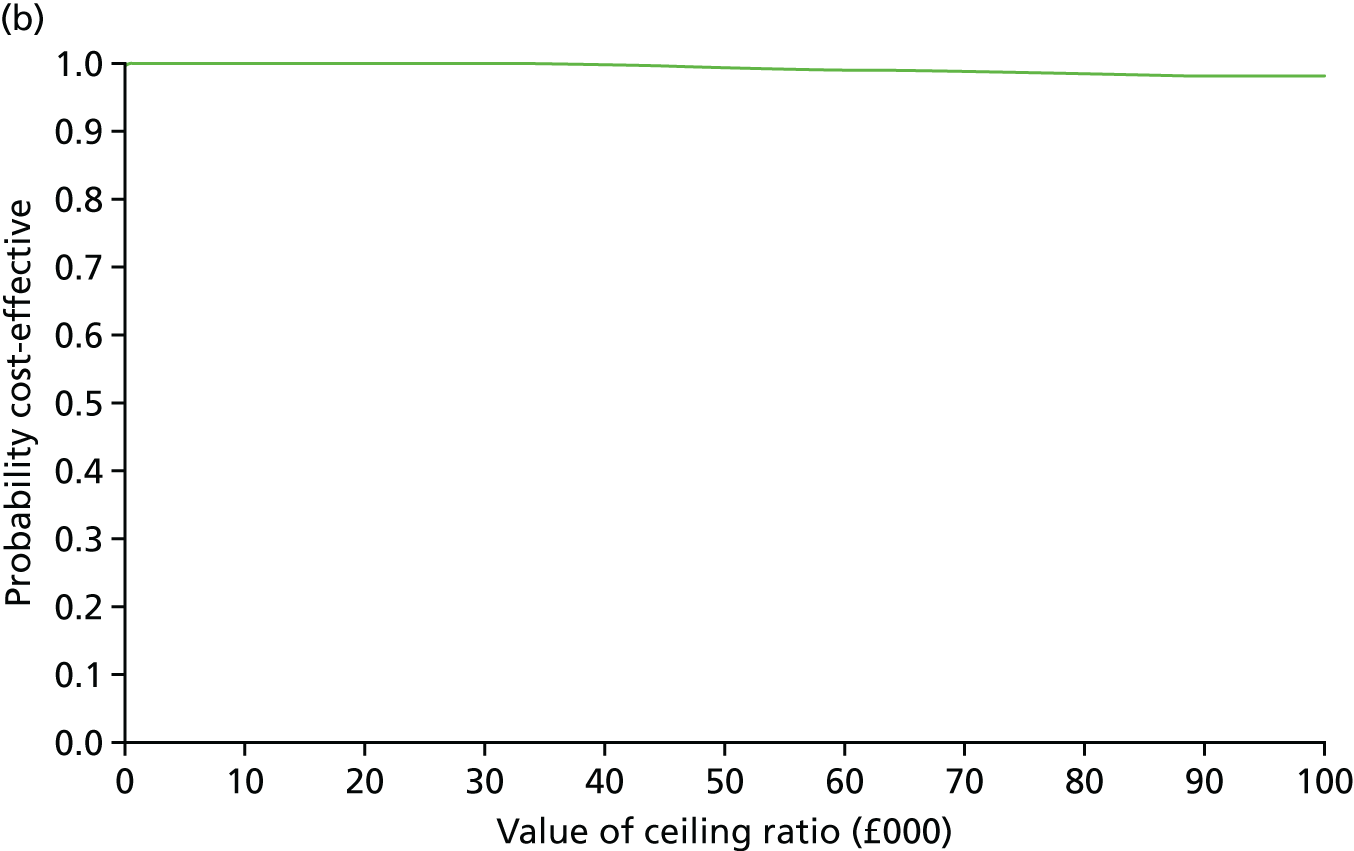
FIGURE 14.
Total costs and EQ-5D-5L QALYs at 6 months (available cases). (a) Cost-effectiveness plane; and (b) CEAC.
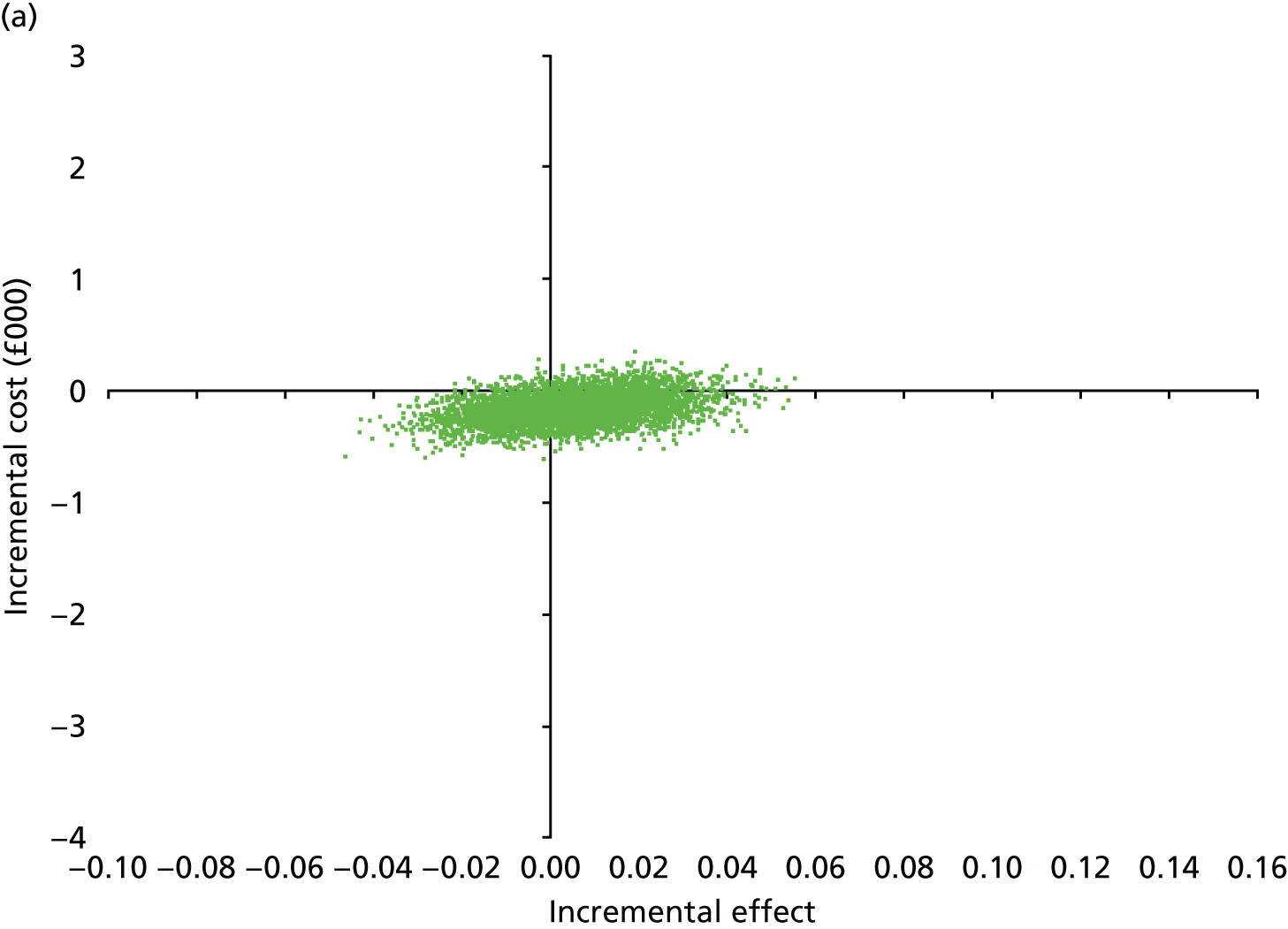
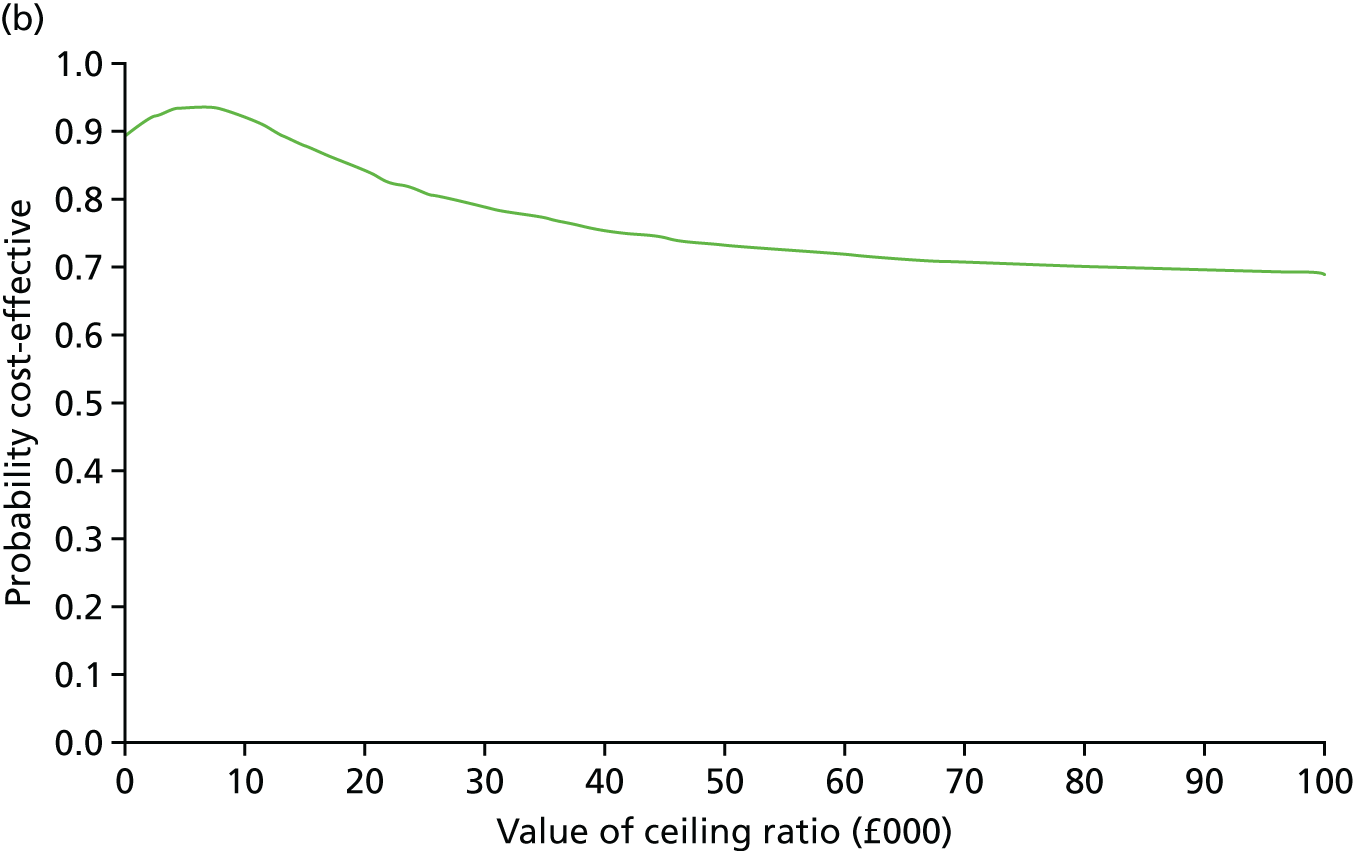
FIGURE 15.
Total costs and MSIS-8D QALYs at 6 months (available cases). (a) Cost-effectiveness plane; and (b) CEAC.
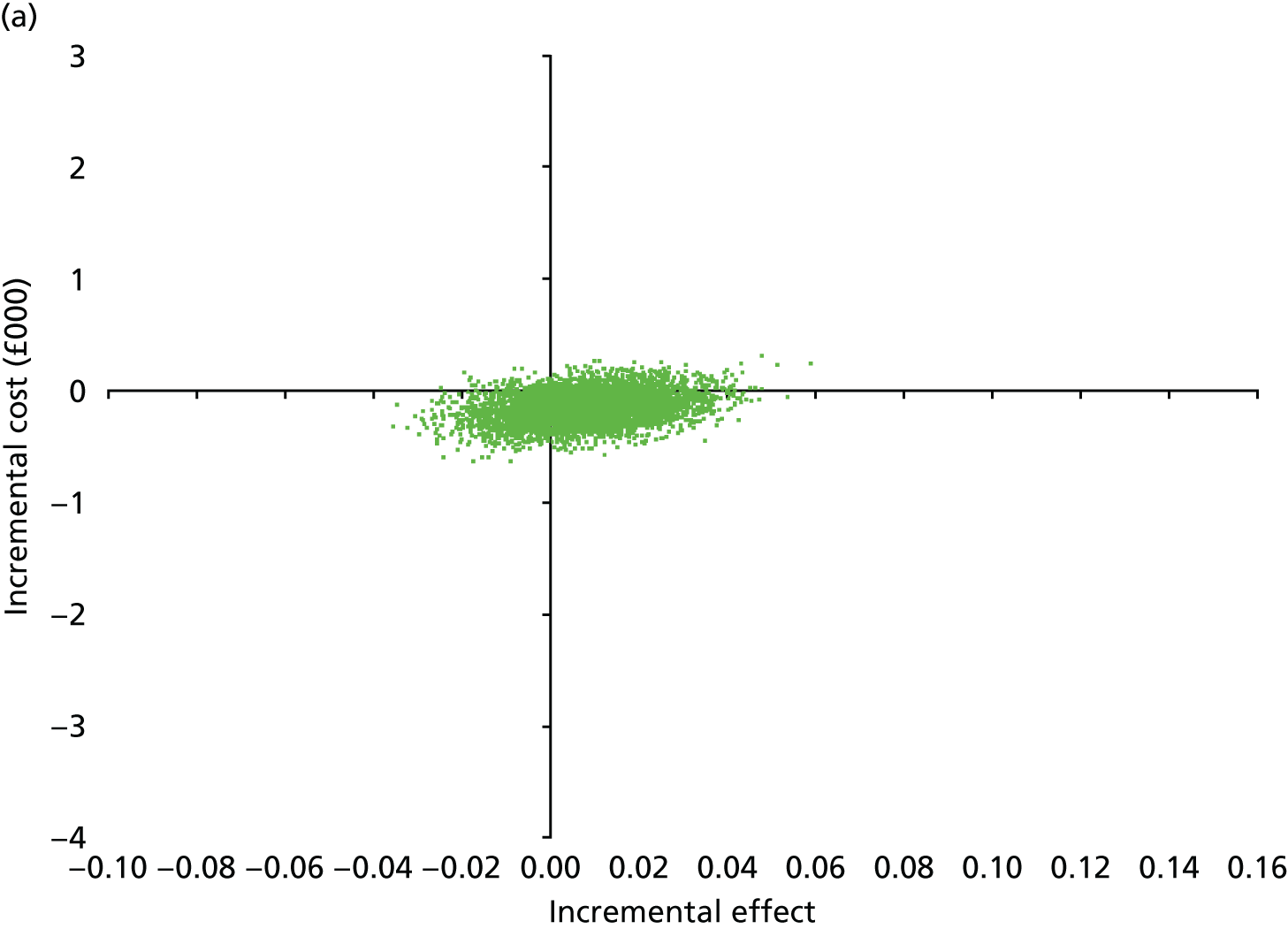
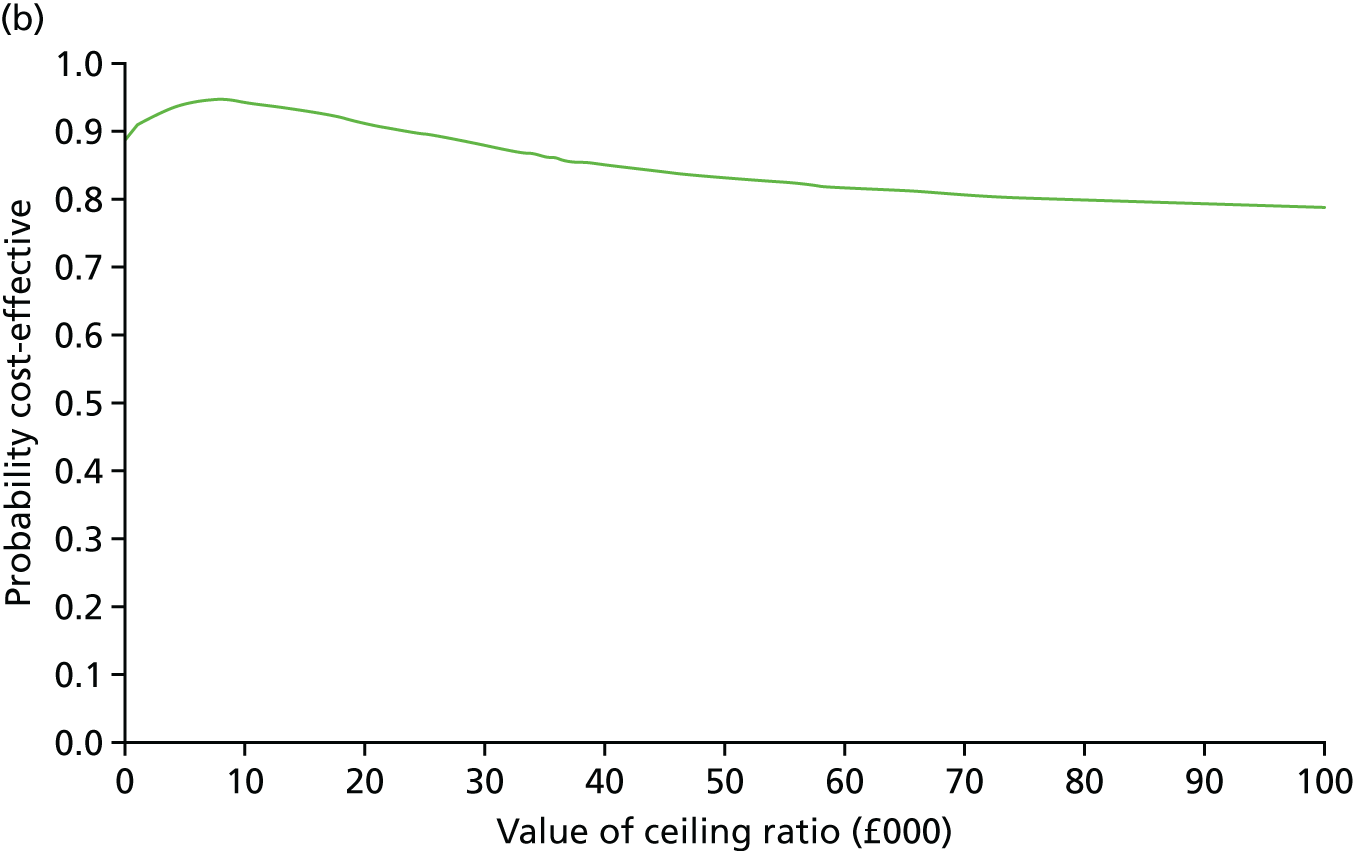
Appendix 18 One-way sensitivity analyses
| Scenario | 12 months | 6 months | ||||
|---|---|---|---|---|---|---|
| Incremental cost (95% CI) | Incremental effect (95% CI) | ICER | Incremental cost (95% CI) | Incremental effect (95% CI) | ICER | |
| Base case | –574.93 (–1878.93 to 729.07) | 0.00 (–0.02 to 0.02) | NA | –19.91 (–777.13 to 737.31) | 0.00 (–0.01 to 0.01) | NA |
|
U95% Cost U95% Effect |
729.07 | 0.02 | 31,055 | 737.31 | 0.01 | 60,269 |
|
U95% Cost L95% Effect |
729.07 | –0.02 | NA | 737.31 | –0.01 | NA |
|
L95% Cost U95% Effect |
–1878.93 | 0.02 | NA | –777.13 | 0.01 | NA |
|
L95% Cost L95% Effect |
–1878.93 | –0.02 | 89,306 | –777.13 | –0.01 | 110,917 |
| Intervention cost, including cognitive assessment | –450.93 (–1754.93 to 853.07) | 0.00 | NA | 104.09 (–653.13 to 861.31) | 0.00 | 39,826 |
| Outlier removala | –38.85 (–1162.14 to 1084.45) | 0.00 | NA | – | – | – |
| Scenario | 12 months | 6 months | ||||
|---|---|---|---|---|---|---|
| Incremental cost (95% CI) | Incremental effect (95% CI) | ICER | Incremental cost (95% CI) | Incremental effect (95% CI) | ICER | |
| Base case | –575.93 (–1878.93 to 729.07) | 0.01 (–0.00 to 0.01) | NA | –19.91 (–777.13 to 737.31) | 0.01 (–0.01 to 0.03) | NA |
|
U95% Cost U95% Effect |
729.07 | 0.01 | 27,529 | 737.31 | 0.03 | 50,902 |
|
U95% Cost L95% Effect |
729.07 | –0.00 | NA | 737.31 | –0.01 | NA |
|
L95% Cost U95% Effect |
–1878.93 | 0.01 | NA | –777.13 | 0.03 | NA |
|
L95% Cost L95% Effect |
–1878.93 | –0.00 | 348,007 | –777.13 | –0.01 | 418,261 |
| Intervention Cost inc. Cognitive Assessment | –450.93 | 0.01 | NA | 104.09 | 0.01 | 14,559 |
| Outlier removala | –38.85 (–1162.14 to 1084.45) | 0.00 | NA | – | – | – |
Appendix 19 Sensitivity analysis: intervention costs
| Trial group | Baseline, mean (SD), n | 12 months, mean (SD), n | Adjusted difference in means (95% CI)a | p-value |
|---|---|---|---|---|
| Panel A: total cumulative costs at 12 months | ||||
| Usual care | 3067.70 (3214.14), 203 | 6773.03 (9573.36), 170 | –886.25 (–2369.55 to 597.06) | 0.24 |
| Cognitive rehabilitation | 3271.68 (5130.30), 245 | 6009.49 (5640.75), 208 | ||
| Panel B: total cumulative costs at 6 months | ||||
| 6 months, mean (SD), n | ||||
| Usual care | 3067.70 (3214.14), 203 | 3029.93 (3877.71), 187 | 111.49 (–558.88 to 781.86) | 0.74 |
| Cognitive rehabilitation | 3271.68 (5130.30), 245 | 3175.12 (3438.15), 225 | ||
List of abbreviations
- ACA
- available-case analysis
- A&E
- accident and emergency
- AP
- assistant psychologist
- BICAMS
- Brief International Cognitive Assessment for Multiple Sclerosis
- BNF
- British National Formulary
- BRBN
- Brief Repeatable Battery of Neuropsychological Tests
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRAMMS
- Cognitive Rehabilitation for Attention and Memory in people with Multiple Sclerosis
- DMC
- Data Monitoring Committee
- EDSS
- Expanded Disability Status Scale
- EMQ
- Everyday Memory Questionnaire
- EMQ-p
- Everyday Memory Questionnaire – patient version
- EMQ-r
- Everyday Memory Questionnaire – relative version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EQ-5D VAS
- EuroQol-5 Dimensions visual analogue scale
- FSS
- Fatigue Severity Scale
- GHQ-30
- 30-Item General Health Questionnaire
- GNDS
- Guy’s Neurological Disability Scale
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICC
- intracluster correlation
- ICER
- incremental cost-effectiveness ratio
- IT
- information technology
- MAR
- missing at random
- MCSI
- Modified Carer Strain Index
- MS
- multiple sclerosis
- MSIS
- Multiple Sclerosis Impact Scale
- MSIS-8D
- Multiple Sclerosis Impact Scale – 8 Dimensions
- MSIS-Phy
- Multiple Sclerosis Impact Scale – Physical subscale
- MSIS-Psy
- Multiple Sclerosis Impact Scale – Psychological subscale
- MSNQ
- Multiple Sclerosis Neuropsychological Screening Questionnaire
- MusiQoL
- Multiple Sclerosis International Quality of Life questionnaire
- NCTU
- Nottingham Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- OWSA
- one-way sensitivity analysis
- PASAT
- Paced Auditory Serial Addition Test
- PIC
- participant identification centre
- PMM
- predictive mean matching
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RA
- research assistant
- RCT
- randomised controlled trial
- ReMemBrIn
- Rehabilitation of Memory following traumatic Brain Injury
- ReMiND
- Rehabilitation of Memory in Neurological Disabilities
- SAP
- statistical analysis plan
- SD
- standard deviation
- TIDieR
- Template for Intervention Description and Replication
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UHSSQ
- Use of Health and Social Services Questionnaire
- WTP
- willingness to pay
