Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/146/06. The contractual start date was in September 2017. The draft report began editorial review in November 2018 and was accepted for publication in May 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Riley et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Objective
Our objective was to evaluate the clinical effectiveness and cost-effectiveness of oral splints for patients with temporomandibular disorder (TMD) or bruxism.
We met our aim by undertaking a comprehensive evidence synthesis, utilising Cochrane methodology, evaluating:
-
all oral splints provided by dentists or other health-care workers versus no splints for patients with TMD or bruxism
-
prefabricated splints versus custom-made splints provided by dentists or other health-care workers for patients with TMD or bruxism.
Chapter 2 Background
Parts of this chapter have been adapted from Riley et al. 1 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original text.
Description of the condition
Temporomandibular disorders are the second most common cause (after dental pain) of orofacial pain, characterised by pain in the temporomandibular joint area and in the facial muscles. Apart from pain, patients may experience other signs and symptoms, such as clicking of the joint and restricted mouth-opening. It is estimated that around 5–12% of the population have TMD symptoms to some degree, varying by age group and sex. 2 There are many ways of managing TMD (e.g. pharmacological, psychological, physiotherapy and surgical interventions); one of the most common ways that dentists, particularly in primary care, manage symptomatic TMD is the provision of oral splints. 3
Splints are also provided to help manage tooth wear caused by bruxism. Bruxism is the repetitive jaw-muscle activity characterised by clenching or grinding of the teeth and/or bracing or thrusting of the mandible. Bruxism has two distinct circadian manifestations: it can occur during sleep (indicated as sleep bruxism) or during wakefulness (indicated as awake bruxism). 4 The prevalence of bruxism ranges from 8% to 31% in the general population,5 and it is estimated that sleep bruxism affects 16%, and awake bruxism 24%, of the adult population globally. 6
In the UK it has been estimated that bruxism affects more than six million people. The severity of the symptoms and the frequency of grinding vary. Bruxism can occur in both children and adults, although it is most common in adults between the ages of 25 and 44 years. Although many patients are unaware of their bruxism habit, there can be an associated tooth wear, which can cause pathological damage and require treatment in the longer term. This is often diagnosed by the general dental practitioner when the patient is attending for a check-up or dental treatment. It is important that tooth wear alone is not taken as a sign that the patient is an active bruxist, as opposed to being a legacy of a previous bruxism habit. 7
Description of the intervention
Oral splints are removable appliances that can cover all or some of the teeth in either the maxillary or the mandibular arches. The term ‘oral splint’ is used colloquially in (UK) dentistry and is really a misnomer, as oral splints do not actually splint (i.e. immobilise) anything. Splints can also be known variously throughout the literature and the world as oral appliances, devices, orthotics or biteplates.
Oral splints can resemble a device similar to a mouthguard used in contact sports, overlaying the biting surface of the teeth with some type of material. Numerous types of oral splints are available, varying in design, material, coverage and application. Splints cover either the upper teeth (upper splints) or the lower teeth (lower splints) and can be classified by the type of material they are made from: hard (hard acrylics), soft (soft polymers or plastics), or composite amalgams of the two aforementioned materials. 8 They can then be subdivided into whether they cover all the surfaces of the teeth in one jaw (full coverage) or only some of the teeth surfaces (partial coverage, e.g. covering only the front six to eight teeth, or two to four of the anterior incisor teeth), and whether or not they provide an adjusted biting surface to equalise the way the teeth meet the splint (‘occlusally adjusted’ surface). 9,10 Finally, they may be made from impressions of a patient’s teeth (custom made) or adapted directly onto the teeth from a non-specific blank (prefabricated or non-custom made).
It should be noted that there are multiple names for different types of splints, and many variations on a design theme. For example, an upper hard stabilisation splint is also known as a Michigan splint, and a Lucia jig is similar in design to the proprietary Nociceptive Trigeminal Inhibition Tension Suppression System (NTI-tss)™ (National Dentex LLC, Palm Beach Gardens, FL, USA) splint.
Traditionally, oral splints recommended by dentists have been custom made, often in dental laboratories, sometimes requiring a number of appointments. More recently, a vast array of prefabricated splints have become available, either for provision by the dentist or health-care worker at a single appointment, or as over-the-counter purchases for patients who wish to self-manage their symptoms. 11 Prefabricated splints include soft, rubber splints (which function by separating the teeth); hydrostatic splints, which are cushioned with fluid to redistribute occlusal force; and the NTI-tss device (semi-customisable).
The aims, duration of treatment, need for adjustments, perceived mode of action and the costs of the splints vary across splint types.
How the intervention might work
There is continuing debate about the exact mechanism of action of oral splints. However, mechanisms include:
-
muscle relaxation/habit-breaking for patients with increased parafunctional or muscle-tightening habits
-
protection of teeth and jaws, particularly when teeth clenching and grinding may lead to damage of teeth, resulting in the need for restorative treatment
-
normalising periodontal ligament proprioception, by utilising a splint to spread the forces placed on individual teeth
-
repositioning of the jaws and condyles into centric relation
-
central effects that are yet to be fully understood. 12
The mode of action varies according to the type of splint used, with some splints (permissive) allowing the teeth/jaw to move or glide over the biting surfaces unimpeded (permissive splints) and others having indentations that hold the jaw in a fixed position (directive or non-permissive).
Why it was important to do this review
This systematic review arose from a National Institute for Health Research Health Technology Assessment programme call addressing the research question: ‘what is the clinical effectiveness and cost-effectiveness of prefabricated oral splints and custom-made splints for the treatment of orofacial symptoms?’. Our application was successful and we received funding to conduct this systematic review and economic evaluation, so the objectives of this review have been driven by this.
It should be noted that the original call focused on treatment for orofacial symptoms. The causes of orofacial pain are varied, but splint therapy for orofacial pain is primarily limited to pain resulting from TMD. Splint therapy is also used for non-painful TMD and bruxism. In order to reflect the use of oral splints in dental practice in the UK, the review will focus on TMD (pain related and non-pain related) and bruxism.
Although we used Cochrane methods, this was not undertaken as a Cochrane review; however, we will share all data from the screening of studies, data extraction forms and correspondence with authors of any future Cochrane reviews, or review updates that overlap with the scope of this review.
Dentists in the NHS in both primary and secondary care are currently providing oral splints for patients who have orofacial signs (such as tooth wear in patients with bruxism) or symptoms (primarily pain). In Scotland alone, the number of splints provided in NHS primary care is increasing from 1985 custom-made hard splints in 2005/06 to 3521 custom-made hard splints in 2015/16. Dentists in Scotland have also recently been allowed to provide custom-made soft splints on the NHS; 16,888 were provided in 2015/16. Oral splints are also provided privately and directly to patients, with a growing industry reported. 11
Despite the frequent use of splints for the management of orofacial sign and symptoms, their clinical effectiveness and cost-effectiveness remain uncertain. This research proposal will inform the NHS, dentists and patients as to whether or not oral splints provided by dentists or other health-care workers are effective in reducing orofacial symptoms (primarily pain) and when they are indicated to prevent tooth wear. If oral splints are found to be effective, then the effectiveness of prefabricated splints compared with custom-made splints (laboratory made, requiring more than one visit to the health-care worker to fit) will be evaluated to help inform care pathways for the target population.
If prefabricated splints are found to be at least as effective as custom-made splints, then there is the potential for a cost saving to both the NHS and directly to patients. Currently, in primary care, the provision of custom-made oral splints for these patients is a band 3 charge to the patient under the 2016 NHS dental fee scale (£256.50). Prefabricated splits are a much cheaper alternative to custom-made splints as they require only one visit for fitting rather than two, do not require laboratory costs and are a band 2 charge in the NHS (£59.10 in 2016 values). Over-the-counter splints can be purchased for < £10.
Chapter 3 Assessment of clinical effectiveness
Parts of this chapter have been adapted from Riley et al. 1 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original text.
Review methods
Types of studies
We included randomised controlled trials (RCTs) but did not include crossover studies as we do not feel that this is an appropriate design owing to the transient nature of the TMD symptoms, or bruxism in patients (which may be due to external factors such as stress).
Types of participants
Inclusion criteria: children (aged > 11 years) and adults who have either TMD or bruxism, and the dentist or other health-care worker is considering treating the patient with an oral splint, in either primary or secondary care.
Exclusion criteria: studies in which the majority of participants were undergoing fixed or removable orthodontic treatment.
Types of interventions
Two comparisons are made:
-
Splints versus no splints, which included any type of splint provided for patients, as described in Types of participants. The no-splint group also included a control splint, which is used in some trials, watchful waiting or minimal treatment. Minimal treatment included advice/counselling, education or self-performed exercises (but could not involve multiple visits/appointments).
-
Prefabricated splints versus custom-made splints. No other head-to-head comparisons were included between different splint types.
For clarity, we refer to a splint according to the jaw in which it is used (upper/lower), its material (hard/soft/composite), its degree of coverage of teeth (full/partial) and then its most generic name, unless the proprietary name is particularly pertinent.
Types of outcome measures
Primary outcomes
The primary outcome for the review was pain. This was measured in a number of ways, including changes in the pain intensity from baseline, end-score pain measures or frequency of episodes of pain. Harms were a primary outcome, which included any problems such as soreness of the oral cavity caused by the splint.
For bruxism patients, tooth wear was also considered a primary outcome.
Secondary outcomes
Secondary outcomes included clicking of the temporomandibular joint, change in restricted mouth-opening, frequency of headaches (secondary to pain-related TMD) and quality-of-life data (including physical and emotional function). Patient satisfaction and adherence to treatment were collected whenever possible. For bruxism, the index and frequency of bruxism activity were also to be recorded.
Follow-up periods for the outcome data were divided into short-term follow-up (0–3 months), medium-term follow-up (3–6 months) or long-term follow-up (6–12 months). By consensus, the clinicians in the review team decided that the 0- to 3-month follow-up was the best time point to use for primary data analysis.
Search methods for identification of studies
An information specialist developed a search strategy (see Appendix 1) and conducted the literature searches. The searches were originally undertaken on 24 August 2017, and were updated on 1 October 2018 to ensure that more recent studies were considered for inclusion prior to publication.
Electronic searches
The following databases were searched:
-
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (to issue 9, 2018, searched on 1 October 2018)
-
MEDLINE via OvidSP (from 1946 to 1 October 2018)
-
EMBASE via OvidSP (from 1980 to 1 October 2018)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost (1937 to 1 October 2018).
When appropriate, the searches of these databases were linked to study design search filters developed by Cochrane for identifying reports of randomised and controlled clinical trials. They were undertaken without restrictions on language or date of publication.
Searching other resources
Unpublished data on clinical trials was sought via searches of the US National Institutes of Health trials register (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform, which includes trials data from the European Union, the UK, Australia, China, the Netherlands, Brazil, India and Republic of Korea (South Korea). Conference proceedings were searched via EMBASE in the main literature search, and the Web of Science. Abstracts of dissertations and theses were searched via the ProQuest database. Searches of these databases were also undertaken on 1 October 2018, without any restrictions on date of publication or language.
Additional grey literature was sourced through the American Academy of Dental Sleep Medicine website. 13 The International Association of Dental Research (IADR) annual conference abstracts were searched via the IADR website14 on 1 October 2018. The protocol stated that we planned to search the conference proceedings of the American Academy of Orofacial Pain and the European Academy of Craniomandibular Disorders; however, these were not available to us.
Data collection and analysis
Selection of studies
Two review authors independently assessed the abstracts of retrieved studies. We obtained full-text copies of studies deemed to be relevant or potentially relevant, or for which there was insufficient information in the title and abstract to make a clear decision. Two review authors independently assessed the full-text papers and any disagreements on the eligibility of studies were resolved through discussion and consensus. If necessary, a third review author was consulted.
Data extraction and management
Two review authors independently extracted the following data from the included trials:
-
location/setting, type of provider, number of centres, recruitment period, trials registry identifier
-
inclusion/exclusion criteria, age and sex of participants, number randomised/analysed, any other important prognostic factors (i.e. comorbidities, concomitant prescription medicines/co-interventions)
-
population characteristics – age, sex, presenting condition [bruxism, TMD (plus subtype) or mixed] and severity, duration since presenting condition began, comorbidities
-
intervention – primary purpose of splint (e.g. pain reduction, bruxist motor activity reduction, to aid functional rehabilitation, to decrease tooth damage, jaw repositioning); type of splint in terms of jaw worn in (upper/lower), material (hard/soft/composite), teeth coverage (full/partial), design (prefabricated/custom made); duration of splint use
-
detailed description of comparator
-
details of the outcomes reported, including method of assessment and time(s) assessed
-
details of sample size calculations, funding sources, declarations/conflicts of interest.
Assessment of risk of bias in included studies
The assessment of risk of bias was done independently and in duplicate, using the Cochrane Risk of Bias tool. 15 The following domains were assessed: sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias) and other bias. We realised that it would be difficult or impossible to blind participants and personnel to whether or not a participant had been randomised to receiving a splint. This could potentially introduce performance bias, and, in the case of subjective outcomes, detection bias.
The overall risk of bias of individual studies was categorised as being low, high or unclear according to the following:
-
low risk of bias (plausible bias unlikely to seriously alter the results) if all domains had a low risk of bias
-
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more domains had an unclear risk of bias
-
high risk if one or more domains had a high risk of bias.
Measures of treatment effect
For continuous outcomes [e.g. pain on a visual analogue scale (VAS)], we used the means and standard deviations (SDs) reported in the trials to express the estimate of effect as mean difference (MD) with a 95% confidence interval (CI). In the event that different scales were used, we expressed the treatment effect as a standardised mean difference (SMD).
For dichotomous outcomes (e.g. jaw clicking/no jaw clicking), we expressed the estimate of effect as a risk ratio (RR) with a 95% CI.
Unit-of-analysis issues
The patient was the unit of analysis for all included studies.
Dealing with missing data
We attempted to contact the author(s) of all included studies, if feasible, in the event of missing data. Missing SDs were estimated according to the methods for estimating missing SDs described in section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions. 15
Assessment of heterogeneity
If a sufficient number of studies were included in any meta-analyses, we planned to assess any clinical heterogeneity by examining the following characteristics of the studies: the similarity between the types of participants [TMD, bruxism; age (< 18 and ≥ 18 years)], the type of health-care worker providing the splints, the type of splint, the control intervention and the outcomes.
We assessed heterogeneity statistically by using a chi-squared test, in which a p-value of < 0.1 indicates statistically significant heterogeneity. We quantified heterogeneity by using the I2 statistic. A guide to the interpretation of the I2 statistic, as given in the Cochrane Handbook,15 is as follows:
-
0–40% – might not be important
-
30–60% – may represent moderate heterogeneity
-
50–90% – may represent substantial heterogeneity
-
75–100% – considerable heterogeneity.
Assessment of reporting biases
If a sufficient number of studies had been included in any meta-analyses, publication bias would have been assessed in accordance with the recommendations on testing for funnel plot asymmetry,16 as described in section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions. 15 If asymmetry had been identified, other possible causes of asymmetry would have been assessed, as outlined in table 10.4.a of the Cochrane Handbook. 15 We were unable to undertake funnel plot analysis on the main primary outcome because the effect estimate was reported as SMD.
Data synthesis
We carried out meta-analyses only if there were studies of similar comparisons reporting the same outcomes. We performed meta-analyses using Cochrane’s Review Manager software (RevMan version 5.3, The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) and exported the forest plots into this document to graphically display the results. We combined MDs (or SMDs when different scales were used) for continuous data, and RRs for dichotomous data. Our general approach was to use a random-effects model. With this approach, the CIs for the average intervention effect are wider than those that would have been obtained using a fixed-effect approach, leading to a more conservative interpretation.
We used additional tables (see Appendix 2, Tables 26–29) to report the results from studies not suitable for meta-analysis.
For the meta-analysis of splints versus no splints, we planned to include prefabricated and custom-made splints as subgroups; however, there was an insufficient number of studies including prefabricated splints. Pooling across subgroups depended on the degree of heterogeneity/subgroup differences. As an additional analysis, if we had determined that there was evidence that the prefabricated splits, when placed by any health-care professional, are effective for the primary outcomes, then we planned to look at any head-to-head RCTs comparing the delivery of prefabricated splints by different types of health-care workers. There was insufficient evidence to undertake this.
We planned to consider undertaking a network meta-analysis for different splint types; however, there were insufficient data to undertake this.
Subgroup analysis and investigation of heterogeneity
For the meta-analysis of splints versus no splints, we planned to include the following subgroups:
-
prefabricated
-
hard custom-made splints that alter occlusion (jaw relationship)
-
hard custom-made splints that do not alter occlusion (jaw relationship)
-
soft custom-made splints that do not alter occlusion (jaw relationship).
There were insufficient data to undertake this.
Sensitivity analysis
For TMD patients, we undertook a sensitivity analysis restricted to trials in which the inclusion criteria were based on, or could be clearly mapped to, one of the following sets of diagnostic criteria:
-
Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) guidelines17
-
Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) guidelines18
-
American Association of Orofacial Pain (AAOP) guidelines. 19
Similarly, for bruxism patients, we planned to undertake a sensitivity analysis restricted to trials for which there was a clear diagnosis of bruxism. 4 The study should have used polysomnography to diagnose the bruxism. There were insufficient trials to do this.
We planned to test the robustness of our results by performing sensitivity analyses based on excluding studies deemed to be at high and unclear risks of bias from the analyses. However, we knew this was unlikely to be possible for the splint versus no splint comparison if we judged that there was a high risk of performance bias or detection bias or both.
If any meta-analyses had included several small studies and a single very large study, we planned to undertake sensitivity analyses comparing the effect estimates from both random-effects and fixed-effects models. If these were different, we intended to report on both analyses as part of the results section, and consider possible interpretation.
Presentation of main results
We developed a summary of findings table for each comparison and for the main outcomes of this review following Grading of Recommendations Assessment, Development and Evaluation (GRADE) methods,20 and using the GRADEPro online tool. 21 The quality of the body of evidence was assessed with reference to the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates and the risk of publication bias. We categorised the quality of the body of evidence for each of the main outcomes for each comparison as being high, moderate, low or very low.
Patient and public involvement
We established a patient advisory group during the development of the application. We asked members of the patient advisory group to help devise the final list of outcomes to be included in the review protocol. The patient advisory group worked with the Cochrane Oral Health Consumer Co-ordinator (Ruth Floate), who has experience of consulting the public and patients to ensure full and honest input into the production of systematic reviews and their relevant outputs (particularly the production of plain language summaries). At least one member of the patient advisory group attended each of the face-to-face meetings of the research team held in Manchester, and took part in most of the monthly teleconferences.
Studies included in the review
A flow chart of included studies is shown in Figure 1. Fifty-two studies were included in the review. The full details of the characteristics and reference for each study are given in Appendix 3.
FIGURE 1.
Flow of studies through the review process. Adapted from Riley et al. 1 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original text.
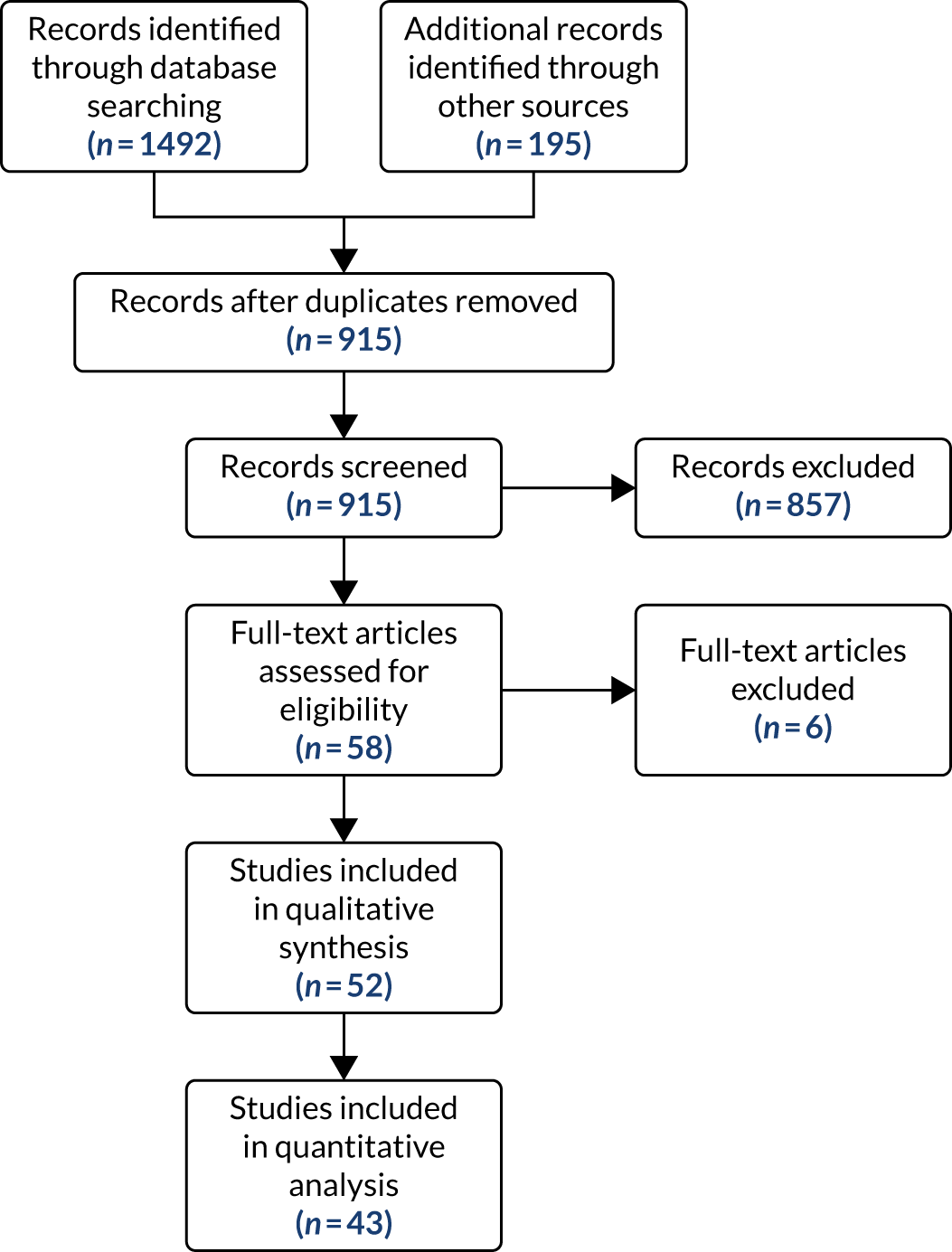
Characteristics of the studies
Study design
All included studies were of parallel design. In one of these studies, non-responders from the control group were allowed to cross over after 6 weeks, but we report data up to 6 weeks, thus treating the study as parallel (Wassell et al. 22).
Number of arms
Many studies had more than two arms as they assessed more than one splint type or more than one control type or both. Twenty-four studies had two arms, 19 had three arms, eight had four arms and one had five arms.
Setting
Fifty-one studies were conducted in universities or public hospitals/clinics. The remaining study was carried out at the Mexican Institute for Clinical Research (Tavera et al. 23).
Eleven studies were carried out in Brazil,24–34 10 in Sweden,35–44 seven in the USA,45–51 three in Turkey,52–54 two in India,55,56 two in Egypt,57,58 two in China,59,60 two in Germany,61,62 two in the UK,22,63 two in Italy,64,65 two in Japan,66,67 one in Canada,68 one in the Netherlands,69 one in Mexico,23 one in Poland70 and one in Finland. 71 The remaining two studies72,73 were carried out in both Sweden and Finland.
Forty-seven studies were conducted at a single centre. One study was conducted at 11 general dental practices in the UK (Wassell et al. 22), one study was conducted at two locations in Sweden (Lundh et al. 40), one study was conducted at two locations in the USA (DeVocht et al. 45), one study was conducted at two locations (Sweden and Finland; Nilner et al. 73) and one study was conducted at three locations (two in Sweden and one in Finland; Christidis et al. 72).
Sample size calculation
Ten studies reported sample size calculations that were met, although one of studies was not powered on a relevant outcome (Gomes et al. 32), one sample size was met at 10 weeks but not at 6 and 12 months (Nilsson et al. 43) and one stated only that a sample size calculation had been done and that it had been met (Zhang et al. 60). A further study reported a sample size calculation but it was unclear if it was met (Costa et al. 29). Four studies50,66,71,72 reported sample size calculations that were not met. Three studies reported only post hoc sample size calculations (Giannakopoulos et al. ,62 Michelotti et al. 64 and Sharma49). One study did not perform an a priori sample size calculation as it was a feasibility study, so it was not powered to detect differences between groups (DeVocht et al. 45). In the remaining 33 studies, sample size calculations were not mentioned so it was unclear whether or not they were done.
Funding and conflicts of interest
Twenty-three studies22,24–27,29,31,35,36,38,43,44,46,47,50,53,66,68–73 declared what appeared to be public funding. Five studies39–41,45,63 reported both public and industry funding. One study declared only industry funding (Ficnar et al. 61). Five studies32,52,54,55,67 declared that they received no funding. One study reported the funding source but it was unclear whether this represented public or industry funding (Yu and Qian59). The remaining 17 studies did not mention funding.
Sixteen studies27,29,32,52,54,55,57,61,62,64,65,67,70–73 declared that the authors had no conflicts of interest. However, in one of those studies, one of the authors had designed and patented the splint used in the study (Rampello et al. 65). In a further study (DeVocht et al. 45), one author declared instructing for the manufacturers of one of the interventions. However, that intervention was excluded from the review because it was ineligible. The remaining 35 studies did not mention conflicts of interest.
Characteristics of the participants
Number randomised/analysed
The studies randomised 3229 participants to the arms we included in this review (i.e. some trial arms were not eligible or were not used; therefore, those participants are not included in this number). The number of participants included in analyses varied by the time at which the outcomes were assessed, and sometimes it was unclear how many were analysed.
Age and sex
The reported age range of the participants was 10–76 years. In the majority of studies (31 studies), the participants’ mean or median age range was 30–39 years. The vast majority of participants were female.
Diagnosis
Fifty-two studies were included in this evidence synthesis. The majority of studies [47/52 (90%)] focused on people with TMD, with only four studies recruiting people with bruxism (8%). One study evaluated the use of splints in people with bruxism with comorbid TMD.
For the studies evaluating the effectiveness of splints for people with TMD, the diagnostic criteria for TMD varied. However, the predominantly used criteria were the RDC/TMD, used in 26 studies. Two studies used the DC/TMD criteria (Sharma49 and Tatli et al. 54) and an additional five studies used criteria that approximated to the RDC/TMD (either by citing the instrument and/or their description matched a similar process) (Conti et al. ,25 de Felício et al. ,30 Ekberg et al. ,35 Wassell et al. 22 and Wright et al. 51). One study used the AAOP criteria (Alencar and Becker24).
The remaining studies used criteria that we had not prespecified in our protocol (RDC/TMD, DC/TMD or AAOP)17–19 or were undefined/unclear:
-
Three had used the Helkimo index74 (Daif,57 Johansson et al. 37 and List et al. 38).
-
Two used arthrography (Lundh et al. 41 and Lundh et al. 40).
-
One used MRI (Haketa et al. 66).
-
One had defined myofascial pain dysfunction syndrome (Rubinoff et al. 48).
-
Six used diagnostic systems that it was not possible to classify (Elsharkawy and Ali,58 Leeson,63 Lundh et al. ,39 Magnusson and Syrén,42 Rampello et al. 65 and Zuim et al. 34).
If studies had not clearly used the prespecified criteria (RDC/TMD, DC/TMD or AAOP),17–19 an expert reviewer examined the information available in the paper, alongside any correspondence from authors, to identify the probable subgroup of TMD included in the study. When possible, a ‘probable’ RDC/TMD (sub)group diagnosis was assigned. If a (sub)group diagnosis was not possible, then the sample was regarded as ‘painful TMD’ (Conti et al. ,25 de Felício et al. ,30 Elsharkawy and Ali,58 Johansson et al. ,37 Katyayan et al. ,56 Leeson,63 Lundh et al. 39 and Rampello et al. 65).
Table 1 provides an overview of the number of studies, including participants for each probable RDC/TMD subgroup diagnoses.
| RDC/TMD group | RDC/TMD subgroup | Number of studies with people in specified subgroup |
|---|---|---|
| Group I: muscle disorders | Ia | 12 |
| Ib | 12 | |
| Subgroup not specifieda | 18 | |
| Total | 42 | |
| Group II: disc disorders | IIa | 10 |
| IIb | 4 | |
| IIc | 1 | |
| Subgroup not specifieda | 8 | |
| Total | 23 | |
| Group III: arthralgia and arthritides | IIIa | 10 |
| IIIb | 2 | |
| IIIc | 3 | |
| Subgroup not specifieda | 5 | |
| Total | 20 | |
| Painful TMDb | 8 | |
| Total | 93 | |
All studies that did not use the prespecified diagnostic criteria were excluded from the sensitivity analyses.
The four studies (Gomes et al. ,33 Karakis et al. ,53 Pierce and Gale46 and van der Zaag et al. 69) examining the effects of splints on bruxism all used the Lobbezoo et al. 4 criteria for likelihood of a bruxism diagnosis: ‘possible’ self-report of bruxism, ‘probable’ clinical evidence of bruxism with or without self-report, and ‘definite’ defined by polsomnography. On this basis, one study examined ‘definite’ sleep bruxism and all the other studies examined ‘probable’ sleep bruxism.
The study that examined bruxism with comorbid TMD used the Fonseca index75 for TMD and examined ‘probable’ bruxism (Gomes et al. 32). This study was classified as examining ‘painful TMD’ and excluded from the sensitivity analyses.
Characteristics of the interventions and comparisons
Most of the studies included one comparison eligible for inclusion in this review. There were four studies that included two different eligible comparisons (Ficnar et al. ,61 Giannakopoulos et al. ,62 Gomes et al. 32 and Truelove et al. 50).
Splint versus no splint for temporomandibular disorder
Comparison type
Thirty-five studies compared splints with no splints for TMD patients.
Ten of these studies used a no-treatment control group (Conti et al. ,25 Daif,57 de Felício et al. ,31 Johansson et al. ,37 List et al. ,38 Lundh et al. ,39 Lundh et al. ,41 Nitecka-Buchta et al. ,70 Rampello et al. 65 and Wright et al. 51).
Twenty had a co-intervention in each arm (e.g. splint + co-intervention vs. co-intervention alone). Of these 20 studies, 13 had a co-intervention of usual treatment, counselling, information or exercise (Conti et al. ,27 Conti et al. ,28 Costa et al. ,29 DeVocht et al. ,45 Ficnar et al. ,61 Giannakopoulos et al. ,62 Hasanoglu et al. ,52 Katyayan et al. ,56 Lundh et al. ,40 Nagata et al. ,67 Niemelä et al. ,71 Truelove et al. 50 and Wahlund et al. 44), whereas seven had a co-intervention of ‘acuhealth’, manipulative and physical therapy, massage, fluoxetine (Prozac®, Eli Lilly and Company, Indianapolis, IN, USA) microcurrent electrical nerve stimulation, physical therapy with vapocoolant spray, arthrocentesis and sodium hyaluronate {Elsharkawy and Ali,58 Gomes et al. ,32 Leeson,63 Sharma,49 Tatli et al. ,54 Yu and Qian59 [this study had four arms with which we made two separate pairwise comparisons: (1) splint + co-intervention vs. co-intervention alone and (2) splint vs. minimal treatment] and Zuim et al. 34}.
The remaining six studies had minimal treatment controls: three were self-exercises (Haketa et al. ,66 Magnusson and Syrén42 and Tavera et al. 23), and three were information-based {de Felício et al. ,30 Michelotti et al. 64 and Yu and Qian59 [this study had four arms with which we made two separate pairwise comparisons: (1) splint + co-intervention vs. co-intervention alone and (2) splint vs. minimal treatment]}.
Splint type
Seven studies compared more than one splint against no splint:
-
Conti et al. 25 – (1) stabilisation splint compared with (2) anterior repositioning splint for 3 or 4 months and then converted into stabilisation splints for the remainder of the treatment period.
-
Conti et al. 27 – (1) stabilisation splint compared with (2) nociceptive trigeminal inhibition splint.
-
Conti et al. 28 – (1) anterior repositioning splint compared with (2) NTI-tss splint.
-
Ficnar et al. 61 – (1) stabilisation splint compared with (2) prefabricated, semi-finished occlusal splint (SOLUBrux®; W3 Solutions SÀRL, Crassier Switzerland).
-
Giannakopoulos et al. 62 – (1) vacuum-formed splint compared with (2) prefabricated oral splint with water-filled elastic pads.
-
Lundh et al. 39 – (1) anterior repositioning splint compared with (2) flat occlusal splint.
-
Truelove et al. 50 – (1) flat-plane splint compared with (2) prefabricated soft thermoplastic athletic mouthguard splint.
Fifteen studies used a stabilisation splint, 12 of which were in the upper jaw (Michigan-style splints) (Costa et al. ,29 de Felício et al. ,31 Gomes et al. ,32 Haketa et al. ,66 Katyayan et al. ,56 Leeson,63 List et al. ,38 Magnusson and Syrén,42 Michelotti et al. ,64 Nagata et al. ,67 Wahlund et al. 44 and Yu and Qian59). The remaining three studies did not clearly report whether the splint was in the upper or lower jaw (Niemelä et al. ,71 Tatli et al. 54 and Tavera et al. 23).
The splint used in two studies was described as a flat-plane splint (Daif57 and Sharma49).
The splint used in two studies was described as a flat occlusal splint (Lundh et al. 40 and Lundh et al. 41).
The splint used in five studies was described only as an occlusal splint (de Felício et al. ,30 Elsharkawy and Ali,58 Johansson et al. ,37 Nitecka-Buchta et al. 70 and Zuim et al. 34).
The splint used in one study was described only as a soft splint (Wright et al. 51).
The splint used in one study was described as a reversible interocclusal splint (DeVocht et al. 45).
One study used a NTI-tss splint (Hasanoglu et al. 52).
One study used a Universal Neuromuscular Immediate Relaxing Appliance (UNIRA) splint, designed and patented by the study author (Rampello et al. 65).
Custom-made splint versus prefabricated splint for temporomandibular disorders
Six studies compared custom-made splints with prefabricated splints for TMD patients:
-
Amin et al. 55 – (1) prefabricated readily available liquid occlusal splint (Aqualizer®; Bainbridge Island, WA, USA), (2) hard occlusal splint and (3) soft occlusal splint.
-
Christidis et al. 72 – (1) prefabricated occlusal splint (Relax; Unident AB, Falkenberg, Sweden) and (2) stabilisation splint.
-
Ficnar et al. 61 – (1) prefabricated, semi-finished occlusal splint (SOLUBrux) and (2) stabilisation splint.
-
Giannakopoulos et al. 62 – (1) prefabricated oral splint with water-filled elastic pads (Aqualizer) and (2) vacuum-formed splint.
-
Nilner et al. 73 – (1) prefabricated occlusal splint (Relax) and (2) stabilisation splint.
-
Truelove et al. 50 – (1) prefabricated soft thermoplastic athletic mouthguard splint and (2) flat-plane hard splint.
Splint versus control splint for temporomandibular disorders
Ten studies compared control splints that did not alter the occlusion with active splints for TMD patients. In six of the studies, the active splint was described as a stabilisation splint (Dao et al. ,68 Ekberg et al. ,35 Ekberg et al. ,36 Rubinoff et al. ,48 Wassell et al. 22 and Zhang et al. 60). In one study it was described as a flat-plane splint (Raphael and Marbach47) and in another only as an occlusal splint (Nilsson et al. 43). The remaining studies compared two active splints against the control splint:
Splint versus no splint for bruxism
Three studies compared splints with no splints for bruxism patients:
Custom-made splint versus prefabricated splint for bruxism
One study compared custom-made stabilisation splints with prefabricated splints (Bruxogard™; Myofunctional Research Europe B.V., Waalwijk, the Netherlands) for bruxism patients (Karakis et al. 53).
Splint versus control splint for bruxism
One study compared stabilisation splints with control splints for bruxism patients (van der Zaag et al. 69).
Characteristics of the outcomes
Nine of the 52 studies did not contribute any outcome data to this review, either in the meta-analyses or the data analysis presented in the additional tables (Conti et al. ,25 Conti et al. ,26 Dao et al. ,68 Ficnar et al. ,61 Gomes et al. ,32 Karakis et al. ,53 Pierce and Gale,46 Rampello et al. 65 and Zuim et al. 34).
Primary outcomes
Pain
Only five studies did not report some form of pain outcome (Daif,57 Gomes et al. ,32 Karakis et al. ,53 Pierce and Gale46 and van der Zaag et al. 69). Four of those were bruxism studies; therefore, this was to be expected.
Table 2 demonstrates how pain was reported in the studies and that a lot of studies reported pain in multiple ways.
| Study | VAS | 50% reduction in VAS | NRS | CPI | Mod-SSI (0.035 to 1) | Pain on palpation/pressure (measured in various ways) | GCPS | Overall improvement (0–5) | Catastrophising Thoughts Subscale (0–4) | Pain (various yes/no) | Pain intensity (various ordinal scales) | Frequency (various ordinal and continuous scales) | Duration (ordinal 0–4) | Pain during mandibular movements (number of movements) | Impaired/unchanged/improved/symptom free | Pain index (VAS × frequency) | Aggregate joint tenderness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author and year | |||||||||||||||||
| Alencar 200924 | ✗ | ✗ | |||||||||||||||
| Amin 201655 | ✗ | ✗ | |||||||||||||||
| Castroflorio 201876 | |||||||||||||||||
| Christidis 201472 | ✗ | ✗ | ✗ | ||||||||||||||
| Conti 200525 | ✗ | ✗ | |||||||||||||||
| Conti 200626 | ✗ | ✗ | |||||||||||||||
| Conti 201227 | ✗ | ✗ | ✗ | ||||||||||||||
| Conti 201528 | ✗ | ✗ | |||||||||||||||
| Costa 201529 | ✗ | ||||||||||||||||
| Daif 201257 | |||||||||||||||||
| Dao 199468 | ✗ | ||||||||||||||||
| de Felício 200630 | ✗ | ✗ | |||||||||||||||
| de Felício 201031 | ? | ||||||||||||||||
| DeVocht 201345 | ✗ | ||||||||||||||||
| Ekberg 199835 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||
| Ekberg 200336 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||||
| Elsharkawy 199558 | ✗ | ✗ | ✗ | ||||||||||||||
| Ficnar 201361 | ✗ | ||||||||||||||||
| Giannakopoulos 201662 | ✗ | ||||||||||||||||
| Gomes 201432 | |||||||||||||||||
| Gomes 201533 | ✗ | ||||||||||||||||
| Haketa 201066 | ✗ | ||||||||||||||||
| Hasanoglu 201752 | ✗ | ||||||||||||||||
| Johansson 199137 | ✗ | ✗ | ✗ | ||||||||||||||
| Karakis 201453 | |||||||||||||||||
| Katyayan 201456 | ✗ | ✗ | |||||||||||||||
| Leeson 200763 | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||||
| List 199238 | ✗ | ✗ | |||||||||||||||
| Lundh 198539 | ✗ | ✗ | |||||||||||||||
| Lundh 198840 | ✗ | ✗ | |||||||||||||||
| Lundh 199241 | ✗ | ✗ | |||||||||||||||
| Magnusson 199942 | ✗ | ✗ | |||||||||||||||
| Michelotti 201264 | ✗ | ||||||||||||||||
| Nagata 201567 | ✗ | ||||||||||||||||
| Niemelä 201271 | ✗ | ✗ | |||||||||||||||
| Nilner 200873 | ✗ | ✗ | ✗ | ✗ | |||||||||||||
| Nilsson 200943 | ✗ | ✗ | ✗ | ||||||||||||||
| Nitecka-Buchta 201470 | ✗ | ||||||||||||||||
| Pierce 198846 | |||||||||||||||||
| Rampello 201365 | ✗ | ✗ | |||||||||||||||
| Raphael 200147 | ✗ | ✗ | |||||||||||||||
| Rubinoff 198748 | ✗ | ✗ | |||||||||||||||
| Sharma 201649 | ✗ | ||||||||||||||||
| Tatli 201754 | ✗ | ✗ | |||||||||||||||
| Tavera 201223 | ✗ | ||||||||||||||||
| Truelove 200650 | ✗ | ✗ | ✗ | ||||||||||||||
| van der Zaag 200569 | |||||||||||||||||
| Wahlund 200344 | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||||
| Wassell 200422 | ✗ | ✗ | ✗ | ||||||||||||||
| Wright 199551 | ✗ | ||||||||||||||||
| Yu 201659 | ✗ | ||||||||||||||||
| Zhang 201360 | ✗ | ||||||||||||||||
| Zuim 200634 | ✗ |
The most commonly used measures of pain in the included studies were VAS/numerical rating scales (NRS) and pain on palpation/pressure. In this review, we prioritised VAS/NRS for the main meta-analysis, also including Characteristic Pain Intensity (CPI) (which was reported as a composite measure encompassing current, worst and average pain over a specified period of time). Despite the majority of studies reporting one of the three pain measures, many studies did not report the data sufficiently for us to include them in the meta-analysis. Furthermore, some measured current pain intensity, whereas others measured average pain over a specified period of time or worst pain experienced. Pain at rest was favoured over pain while chewing or during any other movement.
Harms/adverse effects
Nine studies reported on harms (Christidis et al. ,72 Haketa et al. ,66 Nilner et al. ,73 Nitecka-Buchta et al. ,70 Tatli et al. ,54 Tavera et al. ,23 Truelove et al. ,50 Wahlund et al. 44 and Wright et al. 51). Eight of these were reported narratively, with one study reporting raw data for occlusal contact changes (Wright et al. 51).
Tooth wear (bruxism only)
None of the five bruxism studies reported on tooth wear.
Secondary outcomes
Temporomandibular joint clicking
Fourteen studies reported this outcome (Conti et al. ,25 Conti et al. ,26 Conti et al. ,28 de Felício et al. ,30 de Felício et al. ,31 Ekberg et al. ,35 Ekberg et al. ,36 Lundh et al. ,39 Lundh et al. ,40 Magnusson and Syrén,42 Nagata et al. ,67 Rubinoff et al. ,48 Truelove et al. 50 and Wassell et al. 22). One further study measured this outcome but did not report it (Wahlund et al. 44). Some studies reported on joint sounds and did not specify clicking. The majority of studies reported this outcome dichotomously.
Change in restricted mouth-opening
Twenty-seven studies reported on this outcome (Christidis et al. ,72 Conti et al. ,25 Conti et al. ,28 de Felício et al. ,30 de Felício et al. ,31 Ekberg et al. ,35 Ekberg et al. ,36 Ficnar et al. ,61 Giannakopoulos et al. ,62 Haketa et al. ,66 Hasanoglu et al. ,52 Katyayan et al. ,56 Leeson,63 Magnusson and Syrén,42 Michelotti et al. ,64 Nagata et al. ,67 Niemelä et al. ,71 Nilner et al. ,73 Rampello et al. ,65 Rubinoff et al. ,48 Sharma,49 Tatli et al. ,54 Truelove et al. ,50 Wahlund et al. ,44 Wassell et al. ,22 Wright et al. 51 and Yu and Qian59).
Two studies reported the incidence of participants with a mouth-opening capacity of < 40 mm (Ekberg et al. 35 and Ekberg et al. 36). One study reported this outcome as difficulty when opening the mouth (yes/no) (de Felício et al. 30). One study reported a self-assessment of functional limitation of the jaw using a 0–100 mm VAS (Hasanoglu et al. 52). One study reported only on the splint group and not on the control group, and only for those who started with restricted mouth-opening (Rampello et al. 65). The remaining studies all reported maximum mouth-opening in various ways, namely without pain/with pain/until pain, and assisted/unassisted. One of them also reported the incidence of having difficulty opening the mouth wide (yes/no) (Magnusson and Syrén42).
Frequency of headaches (secondary to pain-related temporomandibular disorder)
Four studies reported this outcome. Three were reported categorically (Costa et al. ,29 Nilner et al. 73 and Nilsson et al. 43) and one as number per week (Wassell et al. 22).
Quality of life
Thirteen studies reported on this outcome. Four used the Modified Symptom Checklist-90-Revised (SCL-90-R) (Christidis et al. ,72 Nilner et al. ,73 Nilsson et al. 43 and Raphael and Marbach47). One of those also assessed average mood using a 0–10 scale (Raphael and Marbach47). Two studies used the 14-item Oral Health Impact Profile (OHIP-14) (DeVocht et al. 45 and Niemelä et al. 71). One study used the Hospital Anxiety and Depression scale (Costa et al. 29). One study used the Short Form questionnaire-36 items (SF-36) (Gomes et al. 33). One study used the Limitation of Daily Functions for TMD Questionnaire (Haketa et al. 66). One study used the RDC/TMD Axis II biobehavioural questionnaire (Tatli et al. 54). One study used an unnamed scale (Dao et al. 68) and the remaining two studies used multiple scales: Leeson63 used the following: (1) Multidimensional Pain Inventory severity; (2) McGill Short Pain Questionnaire; (3) Kellner Illness Attitude Scale; and (4) Beck Depression Inventory scores, whereas Sharma49 used the following: (1) Patient Health Questionnaire-9 items; (2) Patient Health Questionnaire-15 items; and (3) Generalised Anxiety Disorder-7.
Patient satisfaction
Four studies reported on patient satisfaction. In one study, this was assessed using a 0–10 scale (DeVocht et al. 45); in another, it was reported dichotomously as satisfied or not (Ekberg et al. 36). The data were not usable in the remaining two studies (Conti et al. 28 and Tavera et al. 23).
Adherence to treatment
Nine studies reported on compliance (Christidis et al. ,72 Daif,57 Ekberg et al. ,36 Nilner et al. ,73 Nilsson et al. ,43 Raphael and Marbach,47 Tavera et al. ,23 Truelove et al. 50 and Wahlund et al. 44).
Risk of bias in included studies
A summary of the risk-of-bias assessments for the seven domains is given in Figure 2.
FIGURE 2.
Summary of risk-of-bias assessments for each study. Adapted from Riley et al. 1 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original text.
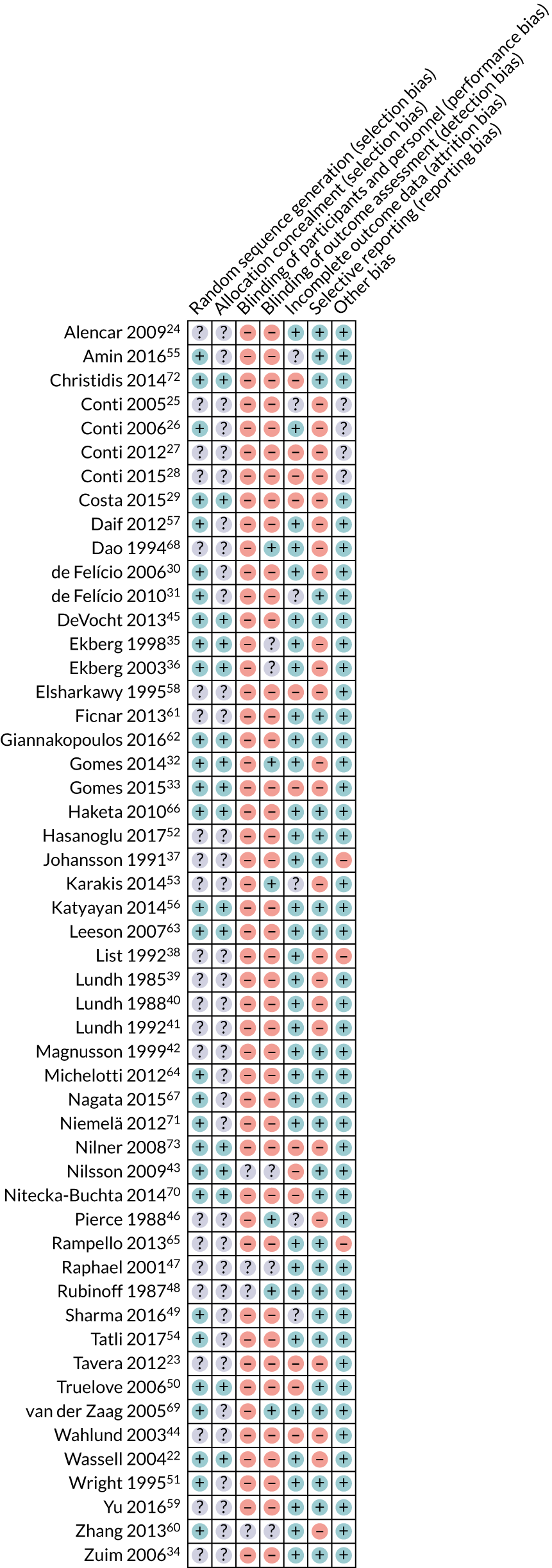
Allocation (selection bias)
Random sequence generation
Twenty-nine studies22,26,29–33,35,36,43,45,49–51,54–57,60,62–64,66,67,69–73 were judged to be at a low risk of bias for the domain of random sequence generation. The remaining 23 studies23–25,27,28,34,37–42,44,46–48,52,53,58,59,61,65,68 reported that participants were randomly allocated to interventions, but were judged to be at an unclear risk of bias owing to an inadequate description of the methods used.
Allocation concealment
Sixteen studies22,29,32,33,35,36,43,45,50,56,62,63,66,70,72,73 described an adequate method of allocation concealment and we judged them to be at a low risk of bias for this domain. The remaining 36 studies23–28,30,31,34,37–42,44,46–49,51–55,57–61,64,65,67–69,71 did not provide a description of the methods used to conceal the allocation sequence.
Overall, sixteen studies22,29,32,33,35,36,43,45,50,56,62,63,66,70,72,73 were deemed to be at a low risk of selection bias as they were rated as being at a low risk for both of the above domains. The remaining 36 studies23–28,30,31,34,37–42,44,46–49,51–55,57–61,64,65,67–69,71 had an unclear risk of selection bias as they had an unclear rating for one or both of the above domains.
Blinding of participants and personnel (performance bias)
Forty-eight studies22–42,44–46,49–59,61–73 were rated as having a high risk of performance bias because of the inability to blind patients and personnel to splint/no splint or splint type. Four studies43,47,48,60 were rated as having an unclear risk of bias. These studies all compared splints against control splints, and attempts were made to blind the personnel and/or patients; however, it was not clear if both were blinded.
Blinding of outcome assessment (detection bias)
Forty-one studies22–31,33,34,37–42,44,45,49–52,54–59,61–67,70–73 were rated as having a high risk of detection bias based on the primary outcome of pain. This was because the patients were aware of their assigned group in the studies and would then subjectively rate their own pain.
Six studies were rated as being at a low risk of detection bias. In two of these studies, comparing splints with control splints, the patients were blinded (Dao et al. 68 and Rubinoff et al. 48). Two studies used objective assessment of bruxism while the participants slept (Pierce and Gale46 and van der Zaag et al. 69). Two studies did not assess any outcomes of this review; therefore, detection bias was irrelevant (Gomes et al. 32 and Karakis et al. 53).
The remaining five studies were rated as having an unclear risk of bias. These all compared splints with control splints and it was not clear whether or not the patients were blinded (Ekberg et al. ,35 Ekberg et al. ,36 Nilsson et al. ,43 Raphael and Marbach47 and Zhang et al. 60).
Incomplete outcome data (attrition bias)
Thirty-four studies22,24,26,30,32,34–42,45,47,48,51,52,54,56,57,59–69,71 had limited or no attrition and were rated as being at a low risk of attrition bias. Twelve studies23,27–29,33,43,44,50,58,70,72,73 were rated as being at a high risk of attrition bias because of high attrition rates, substantial differences between groups in attrition rate, or both. The remaining six studies25,31,46,49,53,55 were rated as having an unclear risk of attrition bias owing to poor reporting of numbers randomised or analysed.
Selective reporting (reporting bias)
Twenty-eight studies24,31,34,37,42,43,45,47–52,54–56,59,61–67,69–72 reported outcome data adequately and were assessed as being at a low risk of reporting bias. The remaining 24 studies22,23,25–30,32,33,35,36,38–41,44,46,53,57,58,60,68,73 had problems with the way in which the data were reported and were rated as being at a high risk of reporting bias.
Other bias
For 45 studies,22–24,29–36,39–64,66–73 we did not identify any other potential source of bias and rated them as being at a low risk of bias. Three studies were rated as having a high risk of bias because outcomes were followed up at different times for the two groups (Johansson et al. ,37 List et al. 38 and Rampello et al. 65). For one of those studies, there was also a substantial sex imbalance between groups, potentially indicating that the randomisation process was inadequate or did not work (List et al. 38). The remaining four studies were rated as having an unclear risk of bias because the reporting was poor and we were unable to properly assess them (Conti et al. ,25 Conti et al. ,26 Conti et al. 27 and Conti et al. 28).
Overall risk of bias
Fifty studies22–46,49–73 were rated as having a high risk of bias overall because they received at least one high risk-of-bias rating for the above domains. The remaining two studies47,48 were rated as having an unclear risk of bias because they did not receive any high risk-of-bias ratings for the above domains, but received at least one unclear risk-of-bias rating. Therefore, no study included in this review was considered to be at a low risk of bias.
Studies excluded from the review
Six studies were excluded from the review for the following reasons: not random allocation (Al Quran and Kamal,77 Alpaslan et al. 78 and Gavish et al. 79), inappropriate study design [4-month difference in timing of outcomes between the groups (Madani and Mirmortazavi80)], the counselling group had multiple reinforcement sessions and therefore not considered minimal treatment (Manfredini et al. 81) and we were unable to obtain a full-text copy (Castroflorio et al. 76).
Results of the systematic review
The results and presented for the two comparisons specified in Chapter 1.
Comparison 1: splints versus no splints/minimal intervention/control splints
The results for the two conditions, TMD (pain-related and non-pain-related) and bruxism, are considered separately, as trials included only TMD patients or only bruxism patients because they are considered discrete groups of patients.
Patients with temporomandibular disorder
One of the main questions posed in this investigation is whether or not there is evidence that splints are effective for reducing pain when compared with no splints. We undertook two analyses. One was for the splint group compared with no/minimal intervention, such as watchful waiting or minimal treatment or self-management. A second analysis was conducted for comparisons with a placebo/control splint, which was used in some trials. There was consensus among clinicians and methodologists that 0–3 months was an appropriate time point to use for the primary analysis of the data. The primary pain outcome was any continuous scale that was sensible to combine (e.g. VAS, NRS, CPI). VAS was the most frequently reported outcome, and 0–3 months was the most frequently reported time point. Other time points, 3–6 months and 6–12 months, were also analysed and reported.
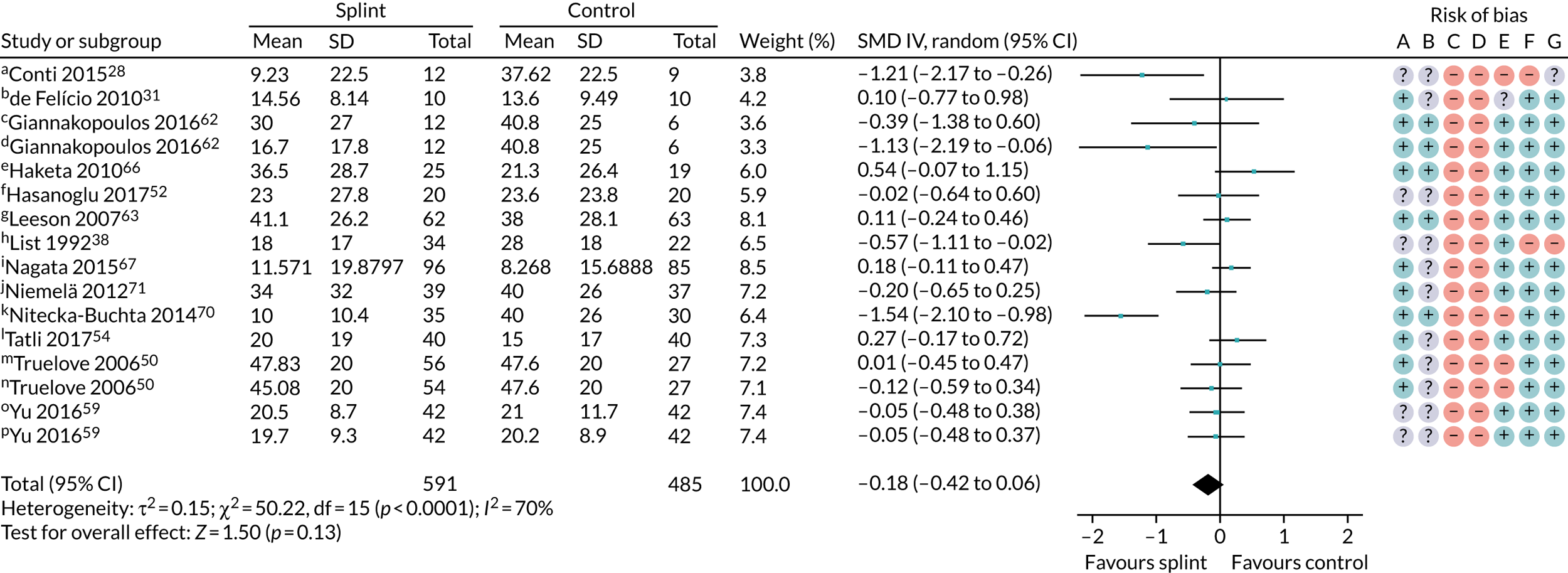
Pain (splint versus no splint/minimal intervention)
Thirteen trials of 16 pairwise comparisons (three of the studies assessed more than one type of splint), all rated as having a high risk of bias, with 1076 patients contributed to the results for the no/minimal interventions at 3 months (Figure 3). There was considerable heterogeneity and the overall SMD was –0.18 (95% CI –0.42 to 0.06). Using a rule of thumb for SMD effect estimates, 0.18 would be considered a small effect15 and, as this was not statistically significant, there is insufficient evidence, which is of very low quality,20 to show that oral splints reduce pain (Table 3). Owing to differences in splint type, the control group no/minimal interventions and different types of TMD diagnoses between the individual studies, we were unable to investigate the heterogeneity any further. There were fewer studies and patients for the other time periods (3–6 months: two trials, 160 patients; and 6–12 months: two trials, three pairwise comparisons, 246 patients), and the effect sizes were SMD –0.31 (95% CI –1.31 to 0.68) and 0.11 (95% CI –0.16 to 0.38) for the 3- to 6-month and 6- to 12-month time periods, respectively, which also fail to demonstrate that oral splints reduced pain (Table 4) (see also Appendix 4, Figures 15 and 16).
FIGURE 3.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – pain: any combinable scale (higher = more pain), 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Current pain intensity 0 to 100 mm VAS (custom anterior repositioning); b, muscle pain 0 to 10 for when (1) waking, (2) chewing, (3) speaking, (4) at rest (score summed = 0 to 40 scale); c, current pain intensity 0 to 10 NRS converted to a 0 to 100 scale (prefabricated splint); d, current pain intensity 0 to 10 NRS converted to a 0 to 100 scale (custom splint); e, current maximum daily pain intensity 0 to 100 mm VAS; f, current pain intensity 0 to 100 mm VAS; g, current pain intensity 0 to 10 cm VAS converted to 0 to 100 mm; h, 0 to 100 mm VAS, recorded three times daily with average calculated on weekly basis (appears to be reported in cm – we converted this to mm); i, current orofacial pain 0 to 10 NRS converted to a 0 to 100 scale; j, current facial pain intensity 0 to 10 cm VAS (we converted this to mm); k, current pain intensity – 0 to 10 cm VAS (we converted this to mm); l, current pain intensity – 0 to 10 cm VAS (we converted to mm); m, CPI 0 to 10 converted to 0 to 100 scale – SD is median value from range of SDs reported in the paper; n, CPI 0 to 10 converted to 0 to 100 scale – SD is median value from range of SDs reported in the paper (custom-made splint vs. control); o, current pain intensity 0 to 10 VAS – we converted to 0 to 100 (splint vs. control); p, current pain intensity 0 to 10 VAS – we converted to 0 to 100 (splint + manipulative and physical therapies vs. manipulative and physical therapies). Adapted from Riley et al. 1 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original text.
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (n studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| No splint | Oral splint | |||||
Pain SD units:
|
The pain score in the oral splint group was, on average, 0.18 SDs lower (0.06 higher to 0.42 lower) than the no/minimal intervention group | 1076 (13 RCTs; 16 pairwise comparisons) | ⊕⊝⊝⊝ very lowb |
|
||
|
The mean pain intensity in the control groups ranged from 9.23 to 41.1 mm,c median = 20 | The mean pain intensity in the splint groups was 4.48 mm lower (11.59 lower to 2.64 higher) | 874 (11 RCTs; 13 pairwise comparisons) | ⊕⊝⊝⊝ very lowb | Results similar at other time points | |
| Clicking of joint at 0–3 months (yes/no) | 500d per 1000 | 425 per 1000 (255 to 715) | RR 0.85 (0.51 to 1.43) | 252 (3 RCTs; 5 pairwise comparisons) | ⊕⊝⊝⊝ very lowb |
|
| Maximum mouth-opening (mm) at 0–3 months | The mean maximum mouth-opening in the control groups rangedc from 33.08 to 47.1 mm; median 40 mm | The mean maximum mouth-opening in the splint groups was 1.17 mm higher (0.68 lower to 3.03 higher) | 913 (13 RCTs; 16 pairwise comparisons) | ⊕⊝⊝⊝ very lowb |
|
|
| Quality of life using OHIP-14 (0 to 56, worsening scale) at 0–3 months | The meane score in the control groups was 14.84 | The mean score in the splint groups was 1.43 lower (5.11 lower to 2.24 higher) | 80 (2 RCTs) | ⊕⊝⊝⊝ very lowb |
|
|
| Adverse events | None of the studies reported any adverse events | |||||
| Outcome | Number of studies (n participants) | Effect estimate (95% CI) (random effects) | p-value for effect estimate | Heterogeneity | |
|---|---|---|---|---|---|
| χ2 p-value | I2 (%) | ||||
| Pain: any combinable scale (higher = more pain) | |||||
| 0–3 months (see Figure 3) | 13 (1076); 16 pairwise comparisons | SMD –0.18 (–0.42 to 0.06) | 0.13 | < 0.0001 | 70 |
| 3–6 months (see Appendix 4, Figure 15) | 2 (160) | SMD –0.31 (–1.31 to 0.68) | 0.54 | 0.002 | 90 |
| 6–12 months (see Appendix 4, Figure 16) | 2 (246); 3 pairwise comparisons | SMD 0.11 (–0.16 to 0.38) | 0.43 | 0.45 | 0 |
| Pain: 50% reduction in VAS pain | |||||
| 0–3 months (see Appendix 4, Figure 17) | 2 (164); 3 pairwise comparisons | RR 1.38 (0.69 to 2.73) | 0.36 | 0.19 | 39 |
| 6–12 months (see Appendix 4, Figure 18) | 1 (51) | RR 0.49 (0.26 to 0.92) | 0.03 | N/A | N/A |
| CPI (0–100 worsening scale) | |||||
| 0–3 months (see Appendix 4, Figure 19) | 2 (93) | MD –0.24 (–7.55 to 7.08) | 0.95 | 0.70 | 0 |
| 3–6 months (see Appendix 4, Figure 20) | 1 (80) | MD 5.20 (–0.62 to 11.02) | 0.08 | N/A | N/A |
The results for the other pain outcomes are shown as forest plots (see Appendix 4, Figure 17) and summarised in Table 4. There was no convincing evidence that the oral splints reduced pain (apart from a single study rated as having a high risk of bias that showed a statistically significant difference in incidence of 50% reduction in VAS pain, in favour of the control group, between 6 and 12 months), although the quality of the evidence was assessed as being very low.
Pain was also measured and reported in other ways that were not possible to meta-analyse, with mixed and inconclusive results (see Appendix 2, Table 26).
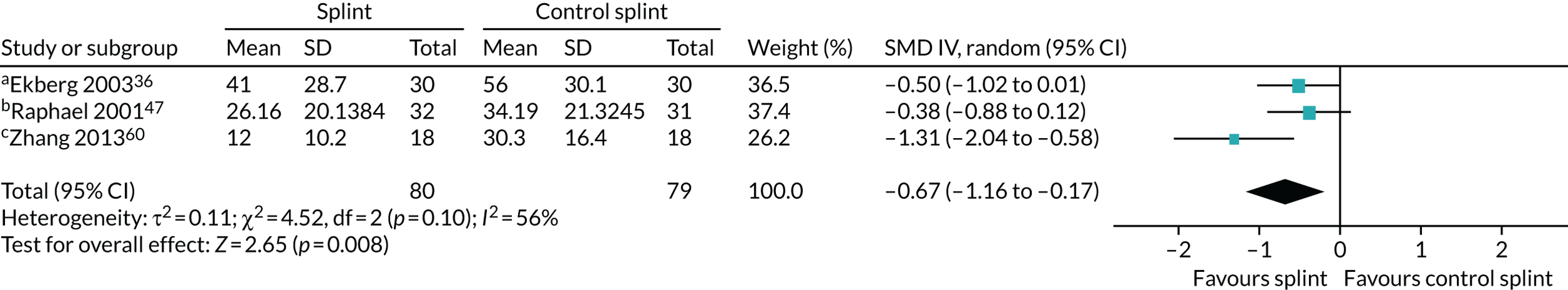
Pain (splints versus control splints)
Three trials (159 patients) were included in the comparison between splints and control splints for the 0- to 3-month time period (Figure 4). The SMD effect size was –0.67 (95% CI –1.16 to –0.17), which indicated a possible benefit for the oral splint compared with a control splint in reducing pain (very low-quality evidence). This result was not confirmed at the two longer-term time points, although the same single study was included in both (Nilsson et al. 43) (see Appendix 4, Figures 21 and 22) (Table 5).
FIGURE 4.
Forest plot of comparison: TMD, splint vs. control splint; outcome – pain: any combinable scale (higher = more pain), 0–3 months. a, Worst pain experienced 0 to 100 mm VAS; b, mean daily pain in the 2 weeks prior to follow-up – 0 to 10 scale (we converted to 0 to 100); c, current pain intensity 0 to 100 mm.
| Outcome | Number of studies (n participants) | Effect estimate (95% CI) (random effects) | p-value for effect estimate | Heterogeneity | |
|---|---|---|---|---|---|
| χ2 p-value | I2 (%) | ||||
| Pain: any combinable scale (higher = more pain) | |||||
| 0–3 months (see Figure 4) | 3 (159) | SMD –0.67 (–1.16 to -0.17) | 0.008 | 0.10 | 56 |
| 3–6 months (see Appendix 4, Figure 21) | 1 (57) | MD –12.00 (–27.76 to 3.76) | 0.14 | N/A | N/A |
| 6–12 months (see Appendix 4,Figure 22) | 1 (51) | MD 3.00 (–14.31 to 20.31) | 0.73 | N/A | N/A |
Pain was also measured and reported in other ways that were either not possible to meta-analyse or were not VAS/NRS/CPI, with mixed and inconclusive results (see Appendix 2, Table 28).
Other outcomes (splint versus no intervention/minimal intervention/control splint)
Several other outcomes were measured for these comparisons; these are summarised in Tables 6 and 7. When comparing splints with no/minimal interventions or with control splints, there was no evidence that they reduced TMD clicking or increased mouth-opening at any of the time points measured. There was no evidence that splints improved quality of life at any time point when compared with no/minimal interventions. There was also no evidence of a difference in compliance between the splints and the control splints at any time point. The quality of the evidence for all these other outcomes was assessed as being very low.
| Outcome | Number of studies (n participants) | Effect estimate (95% CI) (random effects) | p-value for effect estimate | Heterogeneity | |
|---|---|---|---|---|---|
| χ2 p-value | I2 (%) | ||||
| TMJ clicking: presence of joint noises (detected during TMJ palpation/opening/closing) | |||||
| 0–3 months (see Appendix 4, Figure 23) | 3 (252); 5 pairwise comparisons | RR 0.85 (0.51 to 1.43) | 0.55 | 0.001 | 77 |
| 3–6 months (see Appendix 4, Figure 24) | 3 (131); 4 pairwise comparisons | RR 0.90 (0.79 to 1.03) | 0.13 | 0.76 | 0 |
| 6–12 months (see Appendix 4, Figure 25) | 2 (238); 4 pairwise comparisons | RR 0.90 (0.74 to 1.10) | 0.30 | 0.15 | 43 |
| Change in restricted mouth-opening: maximum mouth-opening (mm) | |||||
| 0–3 months (see Appendix 4, Figure 26) | 13 (913); 16 pairwise comparisons | MD 1.17 (–0.68 to 3.03) | 0.22 | < 0.00001 | 83 |
| 3–6 months (see Appendix 4, Figure 27) | 3 (236) | MD 0.29 (–0.63 to 1.20) | 0.54 | 0.30 | 18 |
| Quality of life: OHIP-14 (0–56, worsening scale) | |||||
| 0–3 months (see Appendix 4, Figure 28) | 2 (80) | MD –1.43 (–5.11 to 2.24) | 0.44 | 0.62 | 0 |
| 3–6 months (see Appendix 4, Figure 29) | 2 (76) | MD 0.90 (–3.94 to 5.74) | 0.72 | 0.21 | 36 |
| 6–12 months (see Appendix 4, Figure 30) | 1 (43) | MD 1.31 (–5.11 to 7.73) | 0.69 | N/A | N/A |
| Outcome | Number of studies (n participants) | Effect estimate (95% CI) (random effects) | p-value for effect estimate | Heterogeneity | |
|---|---|---|---|---|---|
| χ2 p-value | I2 (%) | ||||
| TMJ clicking: presence of joint noises (detected during TMJ palpation/opening/closing) | |||||
| 0–3 months (see Appendix 4, Figure 31) | 4 (218) | RR 0.95 (0.68 to 1.31) | 0.74 | 0.87 | 0 |
| Change in restricted mouth-opening | |||||
| Maximum mouth-opening of < 40 mm (see Appendix 4, Figure 32) | 2 (120) | RR 0.40 (0.05 to 3.41) | 0.40 | 0.12 | 59 |
| Compliance: splint worn every night or most nights | |||||
| 0–3 months (see Appendix 4, Figure 33) | 3 (191) | RR 1.03 (0.94 to 1.12) | 0.51 | 0.53 | 0 |
| 3–6 months (see Appendix 4, Figure 34) | 1 (57) | RR 1.06 (0.68 to 1.66) | 0.80 | N/A | N/A |
| 6–12 months (see Appendix 4, Figure 35) | 1 (51) | RR 1.07 (0.58 to 1.97) | 0.83 | N/A | N/A |
Analysis of the robustness of the results (sensitivity analyses)
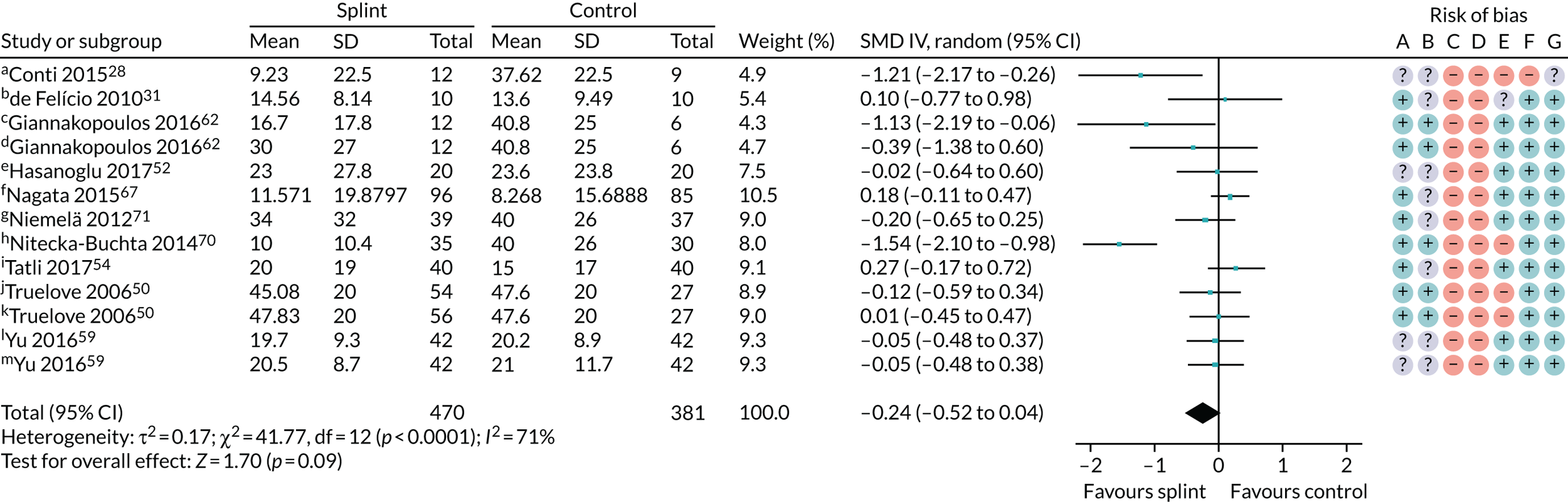
For TMD patients, we planned to undertake a sensitivity analysis restricted to trials for which the inclusion criteria were based on, or could be clearly mapped to, one of the following sets of diagnostic criteria: RDC/TMD guidelines,17 TMD (DC/TMD) guidelines18 or AAOP guidelines. 19 For the primary analysis of splints versus no/minimal intervention in the 0- to 3-month time period (see Figure 3), there was no difference in the result when removing those trials that did not use the above diagnostic criteria: SMD –0.24 (95% CI –0.52 to 0.04; p = 0.09, I2 = 71%; 851 participants) (Figure 5).
FIGURE 5.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – pain: any combinable scale (higher = more pain); sensitivity analysis of studies using the recommended diagnostic criteria, 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Current pain intensity 0 to 100 mm VAS (custom anterior repositioning); b, muscle pain 0 to 10 for when (1) waking, (2) chewing, (3) speaking, (4) at rest, score summed = 0 to 40 scale; c, current pain intensity 0 to 10 NRS converted to a 0 to 100 scale (custom splint); d, current pain intensity 0 to 10 NRS converted to a 0 to 100 scale (prefabricated splint); e, current pain intensity 0 to 100 mm VAS; f, current orofacial pain 0 to 10 NRS converted to a 0 to 100 scale; g, current facial pain intensity 0 to 10 cm VAS (we converted this to mm); h, current pain intensity – 0 to 10 cm VAS (we converted this to mm); i, current pain intensity 0 to 10 cm VAS (we converted this to mm); j, CPI 0 to 10 converted to 0 to 100 scale – SD is median value from range of SDs reported in the paper; k, CPI 0 to 10 converted to 0 to 100 scale – SD is median value from range of SDs reported in the paper (custom-made splint vs. control); l, current pain intensity 0 to 10 VAS – we converted to 0 to 100 (splint + manipulative and physical therapies vs. manipulative and physical therapies); m, current pain intensity 0 to 10 VAS – we converted to 0 to 100 (splint vs. control).
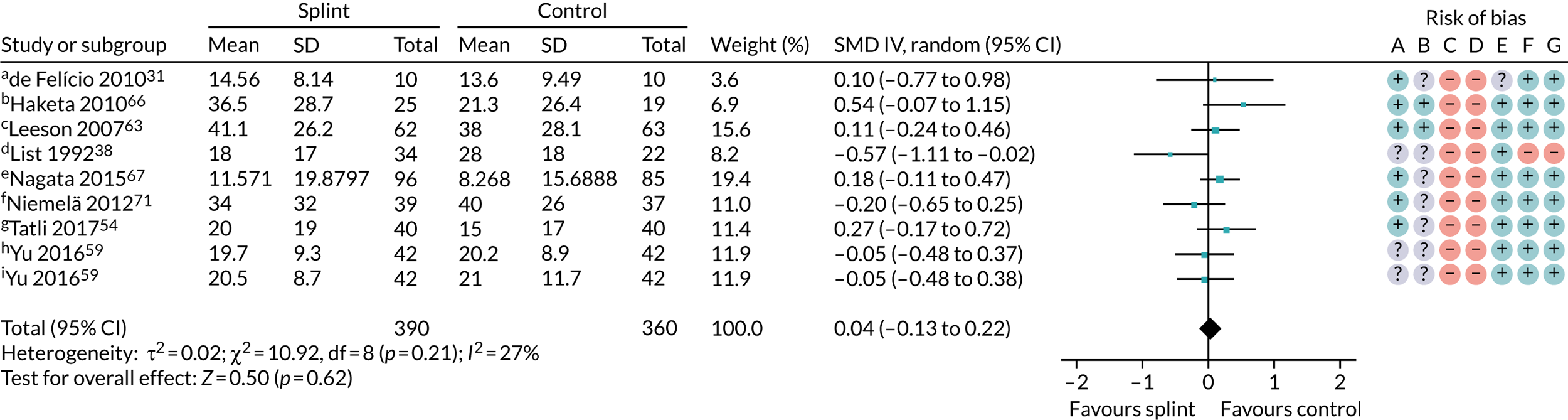
We also carried out a sensitivity analysis restricting the meta-analysis in Figure 3 to studies using stabilisation splints. Again, this did not change the result: SMD 0.04 (95% CI –0.13 to 0.22; p = 0.62, I2 = 27%; 750 participants) (Figure 6). This removed much of the heterogeneity seen in the other analyses.
FIGURE 6.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – pain: any combinable scale (higher = more pain); sensitivity analysis of studies using only stabilisation splints, 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Muscle pain 0 to 10 for when (1) waking, (2) chewing, (3) speaking, (4) at rest, score summed = 0 to 40 scale; b, current maximum daily pain intensity 0 to 100 mm VAS; c, current pain intensity 0 to 10 cm VAS converted to 0 to 100 mm; d, 0 to 100 mm VAS, recorded three times daily with average calculated on weekly basis (appears to be in cm – we converted this to mm); e, current orofacial pain 0 to 10 NRS converted to a 0 to 100 scale; f, current facial pain intensity 0 to 10 cm VAS (we converted this to mm); g, current pain intensity 0 to 10 cm VAS (we converted to mm); h, current pain intensity 0 to 10 VAS – we converted to 0 to 100 (splint + manipulative and physical therapies vs. manipulative and physical therapies); i, current pain intensity 0 to 10 VAS – we converted to 0 to 100 (splint vs. control).
We had also planned to test the robustness of the results by performing sensitivity analyses based on excluding studies deemed to be at high and unclear risks of bias from the analyses. This was not possible as all the studies in this comparison were assessed as being at a high risk of bias.
Current pain intensity on visual analogue scale/numerical rating scale
For the purposes of the economic modelling, the main pain results as SMDs needed to be presented as MDs. To do this, we undertook further sensitivity analyses including only studies that measured pain at the time of assessment (current pain), measured on a 0–100 VAS or NRS. Two studies (DeVocht et al. 45 and Michelotti et al. 64) that reported the results as change scores, and therefore were not possible to include in the main SMD analysis, were added to this analysis for the 0- to 3-month time period because they reported current pain intensity on a VAS or NRS. The results were consistent with the main SMD results, as the point estimate represented a very small, clinically unimportant, reduction in pain for splints, with imprecision in the CI that included a benefit for both using splints and not using splints: MD –4.48 (95% CI –11.59 to 2.64; p = 0.22, I2 = 94%; 874 participants) (Figure 7). However, the extremely high heterogeneity means that the results should be interpreted with caution.
FIGURE 7.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – pain: sensitivity analysis of studies reporting current pain intensity on a 0–100 VAS/NRS (higher = more pain), 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Change score reported.
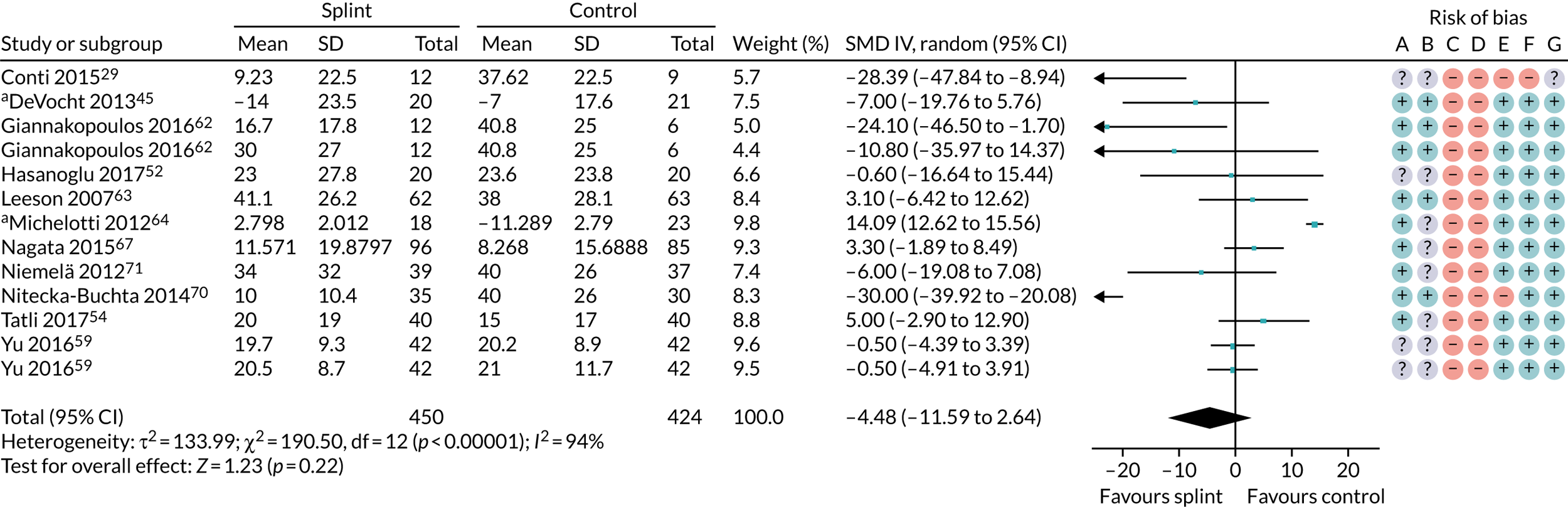
For the 3- to 6-month time period, DeVocht et al. 45 was again added to the analysis and the result was again consistent with the SMD analysis: MD –3.43 (95% CI –11.77 to 4.90; p = 0.42, I2 = 82%; 202 participants).
For the 6- to 12-month time period, when considering only studies that measured pain at the time of assessment (current pain) measured on a 0–100 VAS or NRS, this reduced the analysis to a single study. There was, again, insufficient evidence of a difference: MD 8.70 (95% CI –4.30 to 21.70; p = 0.19; 78 participants).
Patients with bruxism
An overview of the findings for bruxism is given in Table 8.
| Outcome | Illustrative comparative risks (95% CI) | Number of participants (n studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| No splint (control splint where indicated) | Oral splint | ||||
| Tooth wear | No studies reported our primary bruxism outcome of tooth wear | ||||
| Pain intensity measured on (0–10) NRS (0–3 months) | The mean pain intensity in the two control groups was 6.7 | The mean reduction in the splint groups was 2.01 (1.40 to 2.62) | 78 (1 study; 2 pairwise comparisons) | ⊕⊝⊝⊝a very low | Reduction in pain intensity for the splint group |
| Bruxism time index (% of time spent bruxing) | The mean time in the control splint group was 1.9% | The mean in the splint group was 0.18 higher (1.76 lower to 2.12 higher) | 21 (1 study) | ⊕⊝⊝⊝a very low | Insufficient evidence to determine if there is a difference in bruxism time or not |
| Episodes of bruxism per hour (0–3 months) | The mean number of episodes in the control splint group was 10.6 | The mean in the splint group was 0.54 higher (10.95 lower to 12.03 higher) | 21 (1 studies) | ⊕⊝⊝⊝a very low | Insufficient evidence to determine if there is a difference in episodes of bruxism or not |
| Quality of life | No studies reported quality of life | ||||
| Adverse events | No studies reported adverse events | ||||
Two trials that focused on patients with bruxism provided usable outcome data for the 0- to 3-month time period; however, neither study looked at the primary outcome of tooth wear. The results for the other outcomes are presented in Table 9 (compared with minimal intervention) and Table 10 (compared with control splints). There is some very low-quality evidence that splints, when compared with minimal intervention, reduced pain intensity (see Table 9); however, there was insufficient evidence to determine whether or not the splints led to shorter bruxism times, or fewer episodes than control splints (see Table 10).
| Outcome: pain | Number of studies (n participants) | Effect estimatea (95% CI) (random effects) | p-value for effect estimate |
|---|---|---|---|
| Current pain intensity [0 (no pain) to 10 (worst pain) NRS] (0–3 months) | 1 (78) | MD –2.01 (–2.62 to –1.40) favours splint | p < 0.00001 |
| Outcome: bruxism severity | Number of studies (n participants) | Effect estimate (95% CI) (random effects) | p-value for effect estimate |
|---|---|---|---|
| Bruxism time index (% of total sleep time spent bruxing) (0–3 months) | 1 (21) | MD 0.18 (–1.76 to 2.12) | 0.86 favours neither |
| Episodes per hour (0–3 months) | 1 (21) | MD 0.54 (–10.95 to 12.03) | 0.93 favours neither |
Comparison 2: prefabricated splints versus custom-made splints
Once again, we undertook separate analyses for patients with TMD and patients with bruxism.
Patients with temporomandibular disorder
Pain
Table 11 indicates that three trials (178 patients) were included in the meta-analysis comparing custom-made with prefabricated splints for pain on a combinable scale (0–100) for the 0- to 3-month time period. There was no evidence of any heterogeneity and the pooled SMD was –0.14 (95% CI –0.44 to 0.15) (Figure 8). The evidence was assessed as being of very low quality (Table 12) and there was insufficient evidence to determine whether or not there were any differences between custom-made and prefabricated splints with respect to pain measured on a combinable scale.
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Number of participants (n studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Custom-made splints | Prefabricated splints | |||||
|
The pain score in the custom-made oral splint group was, on average, 0.14 SDs lower (0.15 higher to 0.44 lower) than that of the prefabricated splint group | 178 (3 studies) | ⊕⊝⊝⊝ very lowa |
|
||
| Clicking of joint at 0–3 months (yes/no) | 500b per 1000 | 500 per 1000 (350 to 720) | RR 1.00 (0.70 to 1.44) | 110 (1 study) | ⊕⊝⊝⊝ very lowc | Insufficient evidence to determine if either splint type leads to a reduction in joint clicking, at 0–3 months and at 6–12 months |
| Maximum mouth-opening at 0–3 months (mm) | The mean maximum mouth-opening in the custom-made splint group was 41 mm | The mean maximum mouth-opening in the prefabricated splint group was 4.47 mm higher than for the custom-made splint group (6.13 lower to 15.07 higher) | 68 (2 studies) | ⊕⊝⊝⊝ very lowd | Insufficient evidence to determine if either splint type leads to an increase in maximum mouth-opening at any time point | |
| Quality of life: SCL-90-R – depression 0–4 (higher = worse) at 0–3 months | The mean quality-of-life score in the custom-made splint group was 0.743 | The mean quality-of-life score in the prefabricated splint group was 0.03 higher (0.46 lower to 0.53 higher) | 44 (1 study) | ⊕⊝⊝⊝ very lowc | Insufficient evidence to determine if either splint type leads to an increase in quality of life at any time point | |
| Quality of life: SCL-90-R – non-specific physical symptoms 0–4 (higher = worse) at 0–3 months | The mean quality-of-life score in the custom-made splint group was 0.685 | The mean quality-of-life score in the prefabricated splint group was 0.02 higher (0.46 lower to 0.5 higher) | 44 (1 study) | ⊕⊝⊝⊝ very lowc | Insufficient evidence to determine if either splint type leads to an increase in quality of life at any time point | |
| Adverse events | Two studies reported that there had been no adverse events. A further study reported on an increased overbite in one patient in the prefabricated splint group, which was treated and not present at 12 months | |||||
FIGURE 8.
Custom splint vs. prefabricated splint: pain – any combinable scale (higher = more pain), 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Assessed daily in a 1-week pain diary for the week prior to each assessment point using 0 to 100 (pain at rest); b, current pain intensity 0 to 10 NRS converted to a 0 to 100 scale; c, CPI 0 to 10 converted to 0 to 100 scale – SD is median value from range of SDs reported in the paper.
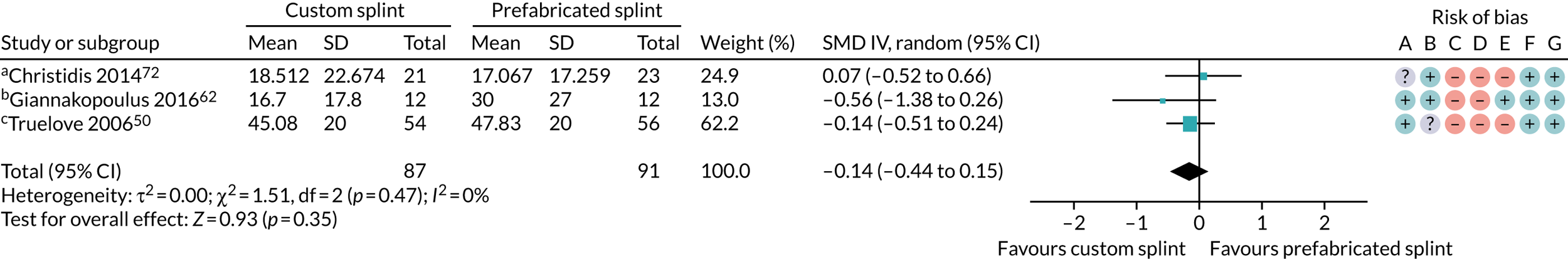
| Outcome | Number of studies (n participants) | Effect estimate (95% CI) (random effects) | p-value for effect estimate | Heterogeneity | |
|---|---|---|---|---|---|
| χ2 p-value | I2 (%) | ||||
| Pain: any combinable scale (higher = more pain) | |||||
| 0–3 months (see Figure 8) | 3 (178) | SMD –0.14 (–0.44 to 0.15) | 0.35; favours custom splint | 0.47 | 0 |
| 3–6 months (see Appendix 4, Figure 36) | 1 (37) | SMD 0.71 (–9.12 to 10.55) | 0.89; favours prefabricated | N/A | N/A |
| 6–12 months (see Appendix 4, Figure 37) | 2 (153) | SMD –0.18 (–0.50 to 0.14) | 0.26; favours custom splint | 0.43 | 0 |
| Pain: GCPS (incidence of grade III or IV) | |||||
| 0–3 months (see Appendix 4, Figure 38) | 1 (44) | RR 1.64 (0.30 to 8.89) | 0.56; favours prefabricated | N/A | N/A |
| 3–6 months (see Appendix 4, Figure 39) | 2 (85) | RR 1.48 (0.29 to 7.41) | 0.64; favours prefabricated | 0.63 | 0 |
| 6–12 months (see Appendix 4, Figure 40) | 2 (82) | RR 1.00 (0.03 to 33.30) | 1.00 | 0.09 | 65 |
Very low-quality data for pain on a combinable scale at the other time points also failed to determine whether or not there were any differences between custom-made and prefabricated splints (see Appendix 4, Figures 36 and 37).
A summary of all the pain outcome data comparing custom and prefabricated splints is shown in Table 13, with the forest plots shown in Figure 8 and in Appendix 4, Figures 36–40.
| Outcome | Number of studies (n participants) | Effect estimate (95% CI) (random effects) | p-value for effect estimate | Heterogeneity | |
|---|---|---|---|---|---|
| χ2 p-value | I2 (%) | ||||
| Change in restricted mouth-opening: maximum mouth-opening (mm) | |||||
| 0–3 months (see Appendix 4, Figure 41) | 2 (68) | MD (mm) –4.47 (–15.07 to 6.13) | 0.41 | 0.07 | 70 |
| 3–6 months (see Appendix 4, Figure 42) | 1 (37) | MD (mm) –1.00 (–6.74 to 4.74) | 0.73 | N/A | N/A |
| 6–12 months (see Appendix 4, Figure 43) | 1 (33) | MD (mm) –1.00 (–7.82 to 5.82) | 0.77 | N/A | N/A |
| Quality of life: SCL-90-R – depression, 0–4 (higher = worse) | |||||
| 0–3 months (see Appendix 4, Figure 44) | 1 (44) | MD –0.03 (–0.53 to 0.46) | 0.89 | N/A | N/A |
| 3–6 months (see Appendix 4, Figure 45) | 2 (89) | MD 0.04 (–0.31 to 0.39) | 0.83 | 0.22 | 35 |
| 6–12 months (see Appendix 4, Figure 46) | 2 (82) | MD 0.11 (–0.54 to 0.75) | 0.75 | 0.95 | 0 |
| Quality of life: SCL-90-R – non-specific physical symptoms, 0–4 (higher = worse) | |||||
| 0–3 months (see Appendix 4, Figure 47) | 1 (44) | MD –0.02 (–0.50 to 0.46) | 0.93 | N/A | N/A |
| 3–6 months (see Appendix 4, Figure 48) | 2 (89) | MD –0.07 (–0.47 to 0.33) | 0.73 | 0.17 | 48 |
| 6–12 months (see Appendix 4, Figure 49) | 2 (82) | MD 0.17 (–0.14 to 0.49) | 0.29 | 0.34 | 0 |
| Adherence to treatment: use of appliance for several nights per week or more | |||||
| 0–3 months (see Appendix 4, Figure 50) | 2 (109) | RR 0.99 (0.91 to 1.08) | 0.84 | 0.38 | 0 |
| 3–6 months (see Appendix 4, Figure 51) | 1 (37) | RR 1.02 (0.71 to 1.47) | 0.92 | N/A | N/A |
| 6–12 months (see Appendix 4,Figure 52) | 1 (33) | RR 1.09 (0.65 to 1.82) | 0.74 | N/A | N/A |
Pain was also measured and reported in other ways that were not possible to meta-analyse, with mixed and inconclusive results (see Appendix 2, Table 27).
Other outcomes
Several other outcomes were measured for this comparison; these are summarised in Table 13. When comparing custom-made splints with prefabricated splints, there was no evidence that either improved maximum mouth-opening, quality of life or adherence to treatment at any of the time points measured. Some outcomes were measured for which data were reported that were not possible to meta-analyse in the forest plots; these are reported in Appendix 2, Table 27. There was no evidence of a benefit for either type of splint for any of these additional analyses, and the quality of the evidence was assessed as being very low.
Patients with bruxism
One study including patients with bruxism compared prefabricated splints with custom-made splints, but provided no data for this review (Table 14).
| Outcomes | Illustrative comparative risks (95% CI) | Number of participants (n studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| No splint | Oral splint | ||||
| Tooth wear | No studies looked at tooth wear | ||||
| Pain | No studies looked at pain | ||||
| Bruxism time index (% of time spent bruxing) | No studies looked at bruxism time | ||||
| Episodes of bruxism per hour | No studies looked at episodes of bruxism | ||||
| Quality of life | No studies reported quality of life | ||||
| Adverse events | No studies reported adverse events | ||||
Harms
Harms/adverse events are reported for all comparisons in Appendix 2, Table 29. These were generally poorly reported and minor in nature.
Patient and public involvement
Three people (Mrs Coldrick, Mrs Lear and Mrs Palmer) who wear oral splints for TMD and/or bruxism agreed to be involved in our research to provide a patient perspective. At the stage of writing the protocol for the effectiveness review, we wanted to find out what questions and outcomes were important to them. Two patients identified pain relief as the most important outcome and found relief within 7–10 days of wearing the first splint. However, there was a discrepancy with regard to the ease of using the splint. These comments helped to assure us that the outcomes to be measured in the review and how they were measured were appropriate and that we had not missed specifying any important outcomes in the protocol.
Members of the patient advisory group provided feedback on the Plain English summary that had been written by Ruth Floate, and agreed the final version.
Chapter 4 Assessment of cost-effectiveness
Overview of the principles of economic evaluations
Highly constrained public funding for health means that health-care resources are scarce. Economic evaluation is a useful tool that compares the relative costs and benefits of different health-care interventions. It is widely used by health-care decision-makers to assess whether or not new interventions generate value for money.
The most common framework of economic evaluation is cost–utility analysis (CUA). In a CUA, health-care benefits are measured in terms of quality-adjusted life-years (QALYs). QALYs combine an individual’s length of life with the quality (utility) of those life-years. The additional costs of an intervention are compared with the additional QALYs gained to generate an incremental cost-effectiveness ratio (ICER). In the UK, an intervention is typically considered cost-effective if the ICER is < £20,000 to £30,000 per QALY gained. Interventions that are more costly and less effective than the comparator are dominated, whereas interventions that are cost-saving and also more effective are dominant. Decision modelling is often used to extrapolate trial results over the longer term to ensure that the economic evaluation captures all the costs and consequences of importance.
Systematic review of economic evaluations
This section reports the findings of cost-effectiveness studies comparing (1) splints versus no splints or (2) prefabricated splints versus custom-made splints for patients with orofacial signs or symptoms, presenting with either TMD or bruxism (tooth grinding).
Review methods
This section provides detailed methods used for the search strategy, inclusion criteria and exclusion criteria.
Search strategy
Literature searches were conducted in four databases: MEDLINE via OvidSP (including Epub Ahead Of Print, pre-indexed, etc.), EMBASE via OvidSP, NHS Economic Evaluation Database (NHS EED) and CINAHL via EBSCOhost. The initial search was conducted in October 2017. The detailed search strategy is provided in Appendix 1. Table 15 includes a summary of the studies retrieved from each database. The searches were updated on 1 October 2018, to ensure that more recent studies were considered for inclusion prior to publication. The update search did not identify any additional cost-effectiveness records.
| Database | Version/issue | Date of search | Records retrieved (n) |
|---|---|---|---|
| MEDLINE via OvidSP (including Epub Ahead Of Print, pre-indexed, etc.) | 1946 to 1 October 2018 | 1 October 2018 | 19 (with filter) |
| EMBASE via OvidSP | 1980 to 1 October 2018 | 1 October 2018 | 13 (with filter) |
| NHS EED | To issue 1, 2016 (database discontinued after this date) | 1 October 2018 | 0 |
| CINAHL via EBSCOhost | 1937 to 1 October 2018 | 1 October 2018 | 14 |
Inclusion and exclusion criteria
Study inclusion and exclusion criteria regarding types of participants and types of interventions were identical to those specified for the clinical effectiveness review (see Chapter 3, Review methods). Studies were included only if they could be classified as full economic evaluations with a comparative analysis of costs and outcomes using any of the following frameworks: cost-effectiveness, cost–utility, cost–benefit or cost minimisation. Economic evaluations of any design, including evaluations alongside single effectiveness studies and decision-analysis models, were all deemed eligible for inclusion. Partial economic evaluations (i.e. studies that did not explicitly compare costs and outcomes of two or more treatments), review articles, cost-of-illness studies and methodological studies were all excluded.
Data extraction strategy
All titles and abstracts identified from the literature search were assessed against the inclusion and exclusion criteria by one health economist (EJ). All full texts were also assessed against the inclusion and exclusion criteria, with a second health economist (DB) checking the inclusion of each study. Disagreements were addressed through mutual consensus. The plan for data extraction was for one health economist (EJ) to conduct the data extraction and quality assess the studies against standardised checklists for economic evaluations alongside trials (using the Drummond checklist82) and for economic models (using the Philips checklist83).
Review results
The number of studies identified from the database searches is provided in Figure 9.
FIGURE 9.
Flow diagram for the identification of studies (cost-effectiveness).
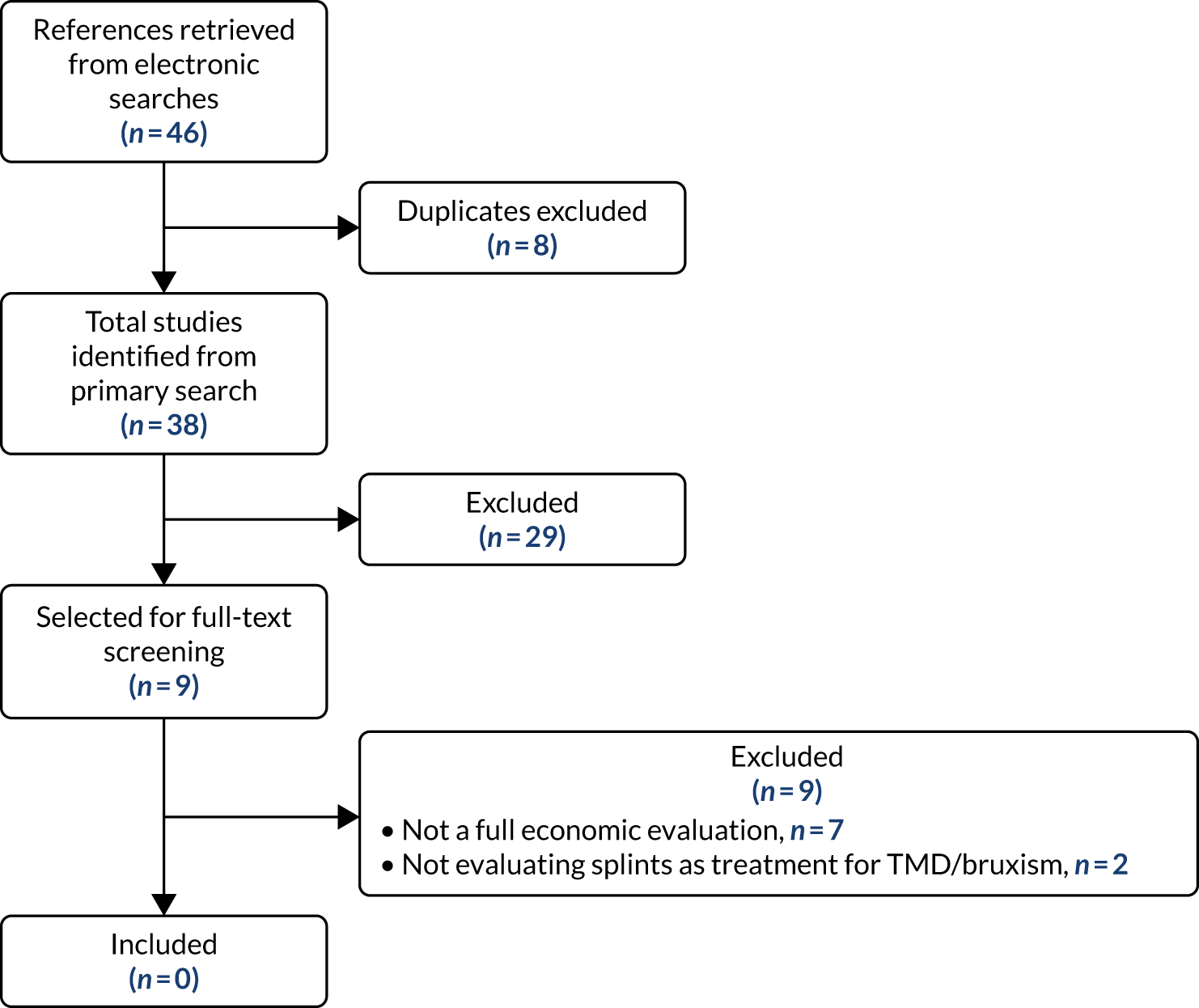
A total of 38 studies were identified from the literature searches. After screening titles and abstracts, 29 studies (76%) were excluded as they did not include an economic evaluation. Full-text versions were obtained for 9 studies (24%),11,84–91 but none were included in the review because (1) they did not conduct a formal economic evaluation (n = 7 studies) or (2) they did not evaluate splints for the treatment of orofacial signs or symptoms (n = 2).
Economic analysis methods
Introduction
As there was no evidence available from the systematic review to inform the cost-effectiveness of splints, or different types of splints for patients with orofacial signs and symptoms with TMD or bruxism, it was decided to develop a de novo decision-analysis model to answer the research question.
The overall project aim was to assess the cost-effectiveness of splints for orofacial signs and symptoms. From the outset, TMD and bruxism were considered as distinct entities that required different economic models to evaluate. However, as highlighted in Chapter 3, there is insufficient evidence regarding the effects of splints to populate a meaningfully structured model for bruxism, and the limited data that do exist cannot readily be translated into meaningful, patient-relevant health states. However, in consultation with clinical expert opinion, we propose an outline structure of a Markov cohort state-transition decision-analysis model that might be used in the future if more data become available. The suggested structure, provided in Appendix 5, might be used to guide the data collection in future research studies that could be used to help inform the cost-effectiveness of splints for treating bruxism.
Given the lack of data, this chapter focuses solely on the model developed to determine the cost-effectiveness of splints for treating TMD. The economic analysis first seeks to determine the cost-effectiveness of all splints compared with none (as defined in Chapter 3) for treating TMD and to determine if sufficient data exist to determine the most cost-effective form of splints by comparing custom-made with prefabricated splints. It is important to note from the outset that the clinical effectiveness evidence base is limited and these uncertainties inevitably translate into the economic model. The cost-effectiveness results should, therefore, be considered as exploratory in nature. The model is, however, informative in determining the key parameters that drive the cost-effectiveness results, and importantly, value-of-information (VOI) analysis is conducted to steer the future research agenda to minimise decision uncertainty.
Model structure
A Markov cohort state-transition model was developed in TreeAge Pro (TreeAge Software Inc., Williamstown, MA, USA) to evaluate the cost-effectiveness of splints in patients with TMD. The comparator was no splints. The model population was the adult population with TMD, with a starting age of 25 years, which is a common age for symptoms of TMD to start, as the 18–35 years age group are significantly more likely to experience first-onset persistent orofacial pain. 92,93 The proportion of the cohort that are male is taken from the Developing Effective and Efficient care pathways in chronic Pain (DEEP) study (19.1% male). 94 Figure 10 outlines the model structure.
FIGURE 10.
State-transition diagram and pain NRS.
The model simulated a cohort through the health states depicted in Figure 10. The health states include pain tertiles, ‘low pain’, ‘moderate pain’ and ‘high pain’, and ‘death’. The health states defined using a NRS of pain intensity (from 0 to 10) in which low-intensity pain was definied as a NRS score of 0–3, moderate pain was defined as a NRS score of 4–6 and high-intensity pain was defined as a NRS score of 7–10.
The preferred instrument to measure the impact of TMD is the Graded Chronic Pain Scale (GCPS), of which the CPI scale is a subcomponent. GCPS is preferable because it incorporates both the pain intensity and disability associated with TMD. 95 However, there was insufficient evidence comparing the use of splints with no splints in relation to the GCPS; therefore, it was not possible to parameterise the model using the preferred measure of treatment effect. In the absence of sufficient clinical effectiveness data to structure a model around GCPS, it was felt that pain tertiles provided the most feasible and practical balance between a meaningful structure to capture the potential impact of treatment on outcomes (pain) that could be populated using clinical effectiveness data, as well as cost and utility data from the DEEP cohort study. The state classification was chosen for practicality, but future research should aim to collect sufficient data to populate a TMD model structured around GCPS.
The economic model has therefore been designed to allow population of the health states (transition probabilities, effect sizes, costs and utilities) using two alternative definitions of pain (CPI and current pain intensity). CPI is a combination of three factors measuring current pain, average pain (in the previous 6 months) and worst pain intensity (in the previous 6 months) using a NRS, with the final score based on an average of the three domains. Current pain intensity asks patients to report their present pain state, on, for example, a NRS or VAS.
The proportion of the cohort entering the model in each of the pain states is determined by the corresponding proportions from the DEEP study cohort. Using a pain definition of ‘current pain’, 34%, 34% and 32% enter the cohort in low, moderate and high states, respectively. For pain defined as CPI, the corresponding proportions are 41%, 27% and 31%, respectively.
At the end of each 3-monthly model cycle, a proportion of the cohort move between pain states. A proportion of the cohort are also assumed to die in the model following age- and sex-adjusted general population all-cause mortality rates. 96 The cycle length defines the fixed period of time, at which point the cohort is introduced to a new set of transition probabilities, costs and utilities. The model estimates the accumulated costs and QALYs using a UK NHS perspective, over an 85-year time horizon in the base case, running for 340 (3-monthly) cycles in the base case up until a maximum of age 110 years to reflect all the costs and outcomes associated with pain states over a lifetime horizon. Half-cycle corrections were applied to the costs and outcomes, to reflect that, on average, events occur in the middle of a cycle rather than at the start or end of a cycle. Costs and QALYs occurring into the future are discounted at a rate of 3.5% per annum, as recommended by the National Institute for Health and Care Excellence (NICE) in England and Wales. 97
Model parameters
The model was populated using best-available data on transition probabilities, costs, utilities and clinical treatment effects. Clinical treatment effects were estimated as MDs using random-effects meta-analysis of studies included in the clinical effectiveness review (see Chapter 3) to obtain MDs between splints and no splints at 3, 6 and 12 months. Longer-term effect size estimates were unavailable, or insufficient for populating the model. The transition probabilities, costs and utilities were sourced from a reanalysis of the DEEP study data. The DEEP study was an observational study in the UK, including 35 dental and medical practices, with a total of 198 patients. 94,98,99 The cohort was followed up over a 2-year period. The reanalysis, performed and provided by the DEEP study chief investigator (Professor Durham) was tailored to provide model parameter estimates (transition probabilities, costs and utilities) applicable for a population of the desired model health states. DEEP study data are the most appropriate source of model parameter estimates, being the largest available cohort, with the longest follow-up and from a UK perspective.
Transition probabilities
Table 16 illustrates the transition probabilities used to populate the model, obtained from the DEEP study for tertiles (low, moderate and high) of both current pain and CPI. Transition probabilities are incorporated probabilistically in the model using beta distributions, with alpha and beta distribution parameters obtained using the method of moments approach. 100 Alpha is the number of individuals that transition between states in the DEEP study in a given 6-monthly time frame (0–6 months, 6–12 months, 12–18 months and 18–24 months) and beta is given as the total sample minus alpha. For example, the total number of individuals starting in the lowest tertile for the 0- to 6-month time period was 26 (and 26 individuals started with moderate pain and 24 with high pain). Six-monthly probabilities were converted to 3-monthly cycle-specific probabilities using Equation 1:101
where the time to be converted from is 6 months (0.5 years) and the time to be converted to is 3 months (0.25 years). The approach was implemented in TreeAge, using the inbuilt ‘probtoprob’ function.
| Transition probabilities | Current pain intensity | CPI | ||||
|---|---|---|---|---|---|---|
| Probability at 3 months (%) | Alpha | Beta | Probability at 3 months (%) | Alpha | Beta | |
| 0–6 months | ||||||
| Low to high | 4 | 2 | 24 | 7 | 4 | 25 |
| Low to moderate | 24 | 11 | 15 | 7 | 4 | 25 |
| Moderate to high | 10 | 5 | 21 | 15 | 5 | 17 |
| Moderate to low | 6 | 3 | 23 | 15 | 6 | 16 |
| High to moderate | 13 | 6 | 18 | 15 | 7 | 18 |
| High to low | 2 | 1 | 23 | 4 | 2 | 23 |
| 6–12 months | ||||||
| Low to high | 0 | 0 | 17 | 0 | 0 | 29 |
| Low to moderate | 31 | 9 | 8 | 19 | 10 | 19 |
| Moderate to high | 8 | 5 | 29 | 14 | 5 | 14 |
| Moderate to low | 16 | 10 | 24 | 8 | 3 | 16 |
| High to moderate | 14 | 5 | 14 | 15 | 6 | 16 |
| High to low | 3 | 1 | 18 | 2 | 1 | 21 |
| 12–18 months | ||||||
| Low to high | 3 | 1 | 15 | 0 | 0 | 20 |
| Low to moderate | 17 | 5 | 11 | 13 | 5 | 15 |
| Moderate to high | 13 | 7 | 22 | 19 | 8 | 15 |
| Moderate to low | 11 | 6 | 23 | 9 | 4 | 19 |
| High to moderate | 18 | 6 | 12 | 13 | 5 | 15 |
| High to low | 0 | 0 | 18 | 0 | 0 | 20 |
| 18–24 months | ||||||
| Low to high | 3 | 1 | 14 | 0 | 0 | 17 |
| Low to moderate | 7 | 2 | 13 | 3 | 1 | 16 |
| Moderate to high | 17 | 8 | 18 | 7 | 3 | 18 |
| Moderate to low | 17 | 8 | 18 | 18 | 7 | 14 |
| High to moderate | 14 | 5 | 14 | 23 | 9 | 13 |
| High to low | 11 | 4 | 15 | 2 | 1 | 21 |
Transition probabilities for TMD patients beyond 24 months are unknown; therefore, assumptions must be made about how the cohort will progress through pain states in the longer term. Two options were considered and discussed with clinical experts. The first assumes that, for the duration of the model, the distribution of the cohort across pain states does not change further over time, with the cohort remaining in the modelled health state at 2 years for the duration of the model until death (all-cause mortality rates). The second assumption is that the transitions observed between 18 and 24 months from the DEEP study continue until the whole cohort transits to a single pain health state or dies. Both assumptions are surrounded by considerable uncertainty, and both are considered equally plausible. To incorporate this structural uncertainty in the model-based cost-effectiveness outputs, a switch is incorporated in the model, which is sampled probabilistically from a uniform distribution, where switch = 1 means the cohort remain in their current state and switch = 2 means the cohort transition according to the DEEP study data. The switch is sampled at the start of each model stage following cycle 8 (2 years). The approach taken means that, in each model stage, either approach to estimating long-term state transitions is equally probable.
Treatment effects
Existing evidence identified from the clinical effectiveness review was used to inform the relative treatment effects of splints compared with no splints. Relative treatment effects are incorporated in the model as MDs on the VAS/NRS scale. MDs are estimated at 3 and 6 months for current pain and at 3, 6 and 12 months for CPI, using random-effects meta-analysis of studies included in the systematic review of clinical effectiveness. Further details regarding the studies included in these meta-analyses are provided in the sensitivity analysis section of the clinical effectiveness review (see Chapter 3). MDs are sampled probabilistically in the economic model from a normal distribution with standard error (SD of the sampling distribution) calculated as [(CI high – CI low) ÷ 2 × 1.96]. MD data used to populate each of the model effect sizes are summarised in Table 17.
| Time point (months) | Current pain, MD (95% CI) | CPI, MD (95% CI) |
|---|---|---|
| 3 | –0.448 (–1.159 to 0.264) | –0.074 (–1.176 to 0.672) |
| 6 | –0.343 (–1.177 to 0.490) | 0.520 (–0.062 to 1.102) |
| 12 | N/A | 0.006 (–0.664 to 0.676) |
The long-term effect size of splints versus no splints beyond 6 months is highly uncertain. A number of assumptions are thus required to populate the model over the longer term. The first assumption is that the MD in current pain at 12 months equals the MD at 6 months, namely there is no difference between the MD score at 6 months and 12 months. This assumption reflects the lack of adequate-quality data from the systematic review to populate the model. No data exist to inform the long-term impact of splints on any pain measure beyond 12 months; therefore, further assumptions are required. Two possible scenarios are considered: (1) the MD beyond 12 months is zero and (2) the MD in the longer term is the same as the MD at 6 months. These assumptions represent lower (pessimistic) and upper (optimistic) bounds on the long-term effect of splints on pain. To incorporate this structural uncertainty, the model includes a switch, sampled probabilistically at each model stage, from a uniform distribution, to allow an equal chance of either assumption being applied in the model.
Mean difference data cannot be incorporated directly in the model structure when health states are defined as pain tertiles. An algorithm was therefore developed to infer an approximated relative risk of each possible transition (low to moderate, low to high, moderate to low, moderate to high, high to low and high to moderate) based on the sampled MD data. First, transition probability data (by tertile) from the DEEP study were summarised for each possible state transition. Second, all possible MDs (ranging from –10 to 10) were converted to plausible transitions. For example, a MD of 0 had a 0% probability of changing state, whereas a MD of –10 had a 100% chance of moving to the low-pain state, regardless of the starting point. This process was repeated for the impact of each MD (ranging from –10 to 10) on all possible transitions between tertiles, accounting for the ceiling and floor effects of the scale. All possible transitions, by MD, are reported in Table 18.
| MD | p (low to low) | p (low to moderate) | p (low to high) | p (moderate to low) | p (moderate to moderate) | p (moderate to high) | p (high to low) | p (high to moderate) | p (high to high) |
|---|---|---|---|---|---|---|---|---|---|
| –10 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| –9 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| –8 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| –7 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| –6 | 1 | 0 | 0 | 1 | 0 | 0 | 0.60 | 0.40 | 0 |
| –5 | 1 | 0 | 0 | 1 | 0 | 0 | 0.33 | 0.67 | 0 |
| –4 | 1 | 0 | 0 | 1 | 0 | 0 | 0.14 | 0.86 | 0 |
| –3 | 1 | 0 | 0 | 0.75 | 0.25 | 0 | 0 | 0.75 | 0.25 |
| –2 | 1 | 0 | 0 | 0.44 | 0.56 | 0 | 0 | 0.44 | 0.56 |
| –1 | 1 | 0 | 0 | 0.20 | 0.80 | 0 | 0 | 0.2 | 0.80 |
| 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 1 | 0.80 | 0.20 | 0 | 0 | 0.80 | 0.20 | 0 | 0 | 1 |
| 2 | 0.56 | 0.44 | 0 | 0 | 0.56 | 0.44 | 0 | 0 | 1 |
| 3 | 0.25 | 0.75 | 0 | 0 | 0.25 | 0.75 | 0 | 0 | 1 |
| 4 | 0 | 0.86 | 0.14 | 0 | 0 | 1 | 0 | 0 | 1 |
| 5 | 0 | 0.67 | 0.33 | 0 | 0 | 1 | 0 | 0 | 1 |
| 6 | 0 | 0.40 | 0.60 | 0 | 0 | 1 | 0 | 0 | 1 |
| 7 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| 8 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| 9 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| 10 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
To obtain an assumption of the relative risk, these inferred transitions were divided through by the transition probabilities from the DEEP study cohort to obtain an approximation of the relative risk by MD. For example, using our approach, with a MD in pain of –3, the splints cohort is more likely to move to a better health state than the comparator group (represented by the DEEP study cohort). Those that are already in the ‘low pain’ health state will remain in that health state, accounting for the floor effects of the scale. Those in the ‘moderate pain’ health state are more likely to move to a better health state (‘low pain’) than remain in the moderate pain health state. Those in the ‘high pain’ health state are more likely to move to a better health state (‘moderate pain’) than remain in the high-pain health state.
The approach taken should be interpreted with caution, as it does not directly incorporate relative risk estimates. Future research is required to obtain effect size estimates (i.e. relative risks) that are more amenable for use in populating decision-analysis models structured around different pain states in TMD.
Mortality parameters
General population all-cause mortality risks (adjusted for age and sex) were applied in the model, obtained from UK lifetables. 96 There is no evidence to suggest an added mortality risk to those with TMD; therefore, no excess mortality is applied to either arm of the model.
Utilities and quality-adjusted life-years
Health-state utilities, by pain tertile, for both CPI and current pain definitions, were obtained from a reanalysis of the DEEP study,94 conducted specifically for this project. An average utility value for each pain tertile was calculated using the generalised estimating equation approach, using Stata® version 13.1 software (StataCorp LP, College Station, TX, USA).
Those in the low-pain state had the highest utility value, and those in the high-pain state had the lowest utility value. Utilities are incorporated in the model probabilistically, using beta distributions. The mean and SD (of the sampling distribution) of utilities by health state are listed in Table 19, rounded to the nearest two decimals.
| Health state (pain tertile) | Current pain | CPI | ||||
|---|---|---|---|---|---|---|
| Mean (SD)a | Alpha | Beta | Mean (SD)a | Alpha | Beta | |
| Low | 0.782 (0.004) | 8737 | 2439 | 0.770 (0.005) | 6241 | 1863 |
| Moderate | 0.682 (0.004) | 7591 | 3535 | 0.709 (0.004) | 8294 | 3397 |
| High | 0.596 (0.005) | 6747 | 4565 | 0.601 (0.005) | 6285 | 4181 |
The utility values obtained from the DEEP study were based on a generic EuroQol-5 Dimensions, five-level version, (EQ-5D-5L) quality-of-life measurement, with corresponding utility values obtained using an interim scoring approach to map between the EQ-5D-5L and the EuroQol-5 Dimensions, three-level version (EQ-5D-3L). 102 The EQ-5D-5L questionnaire includes five dimensions of quality of life (mobility, self-care, usual activities, anxiety/depression and pain/discomfort), each with five levels of impact (ranging from no problems to extreme problems). All utilities used in the model were age- and sex-adjusted for UK general population norms. 103
Intervention costs
All model costs are reported in 2016 Great British pounds. Intervention costs are calculated using the payment system for dentistry used in England and Wales. Every treatment in primary care dentistry is categorised into one of three treatment bands. Each treatment band is associated with a predefined number of units of dental activity (UDAs), where more UDAs reflect more complex treatments. UDAs are assigned to treatment bands as follows: band 1 (1 UDA), band 2 (3 UDAs) and band 3 (12 UDAs). Each UDA is associated with a value, with the values of each UDA varying across dental practices. Currently, the average UDA value in England is approximately £25. 104,105 An alternative estimate of the UDA value can be obtained from data published by the NHS Business Services Authority for general dental services contracts across dental practices with contracts to provide services on behalf of NHS England. Using this approach, the mean UDA value was £26.74, with a SD across practices of £18.94. 106 Using this alternative approach, it is possible to include the value of a UDA probabilistically in the model.
In the UK, patients pay a proportion of the UDA value (approximately 80% of the treatment value), unless they are exempt from payment charges (e.g. low income), in which case the full treatment value is paid for by the NHS. The cost to the NHS of each treatment band therefore depends on the proportion of patients exempt from charges. The following formula was used to calculate the average cost to the NHS of a band 1, 2 or 3 course of treatment:
Table 20 includes the inputs to the formula for the calculation of the band 1 to 3 courses of treatment.
| NHS band treatment | UDA | Treatment value (£) | Patient charge (2016) (£) | Paying adults (%) | Sources |
|---|---|---|---|---|---|
| 1 | 1 | 25 | 19.70 | 82 | NHS Dental Statistics for England – 2016–17 107 |
| 2 | 3 | 75 | 53.90 | 69 | |
| 3 | 12 | 300 | 233.70 | 49 |
Splints can be custom made or prefabricated. Custom-made splints are typically provided as a band 3 treatment charge on the NHS in England, while prefabricated splints are likely to be charged as a band 2 service because less resources are required to make the splints. The cost of splints included in the model is an average of the two types, weighted by the proportion of patients receiving each splint type in the clinical effectiveness review.
Of those studies that used current pain as their primary outcome, 91% reported using custom-made splints. Of those studies that used CPI as their primary outcome, 75% reported using custom-made splints. The weighted average approach taken ensures that the distribution of splint type used for the costing is congruent with that used to generate the treatment effect estimates used in the model. A sensitivity analysis explores the impact of alternative assumptions on the results.
The cost of replacing a splint was assumed to be the same as the cost of providing an initial splint. However, there was substantial uncertainty among the clinical expert advisors as to the most probable frequency of splint replacement, and whether or not this would differ by custom-made or prefabricated splint. Expert opinion suggested that splints would be replaced, on average, every 2–5 years, but in some cases patients may use a splint for up to 20 years. To incorporate this uncertainty in the model, three alternative values were considered based on expert opinion: every 2 years, every 5 years and every 20 years. These values were sampled from a log-normal distribution with mean = replacement every 9 years, and median = replacement every 5 years. It is clear that clinical expert opinion regarding the frequency of replacement varied widely among the project advisory group. Therefore, further deterministic analyses are considered in sensitivity analyses to explore the impact of the following alternative assumptions on the cost-effectiveness results: (1) no replacement, (2) 2-yearly replacement, (3) 5-yearly replacement and (4) 20-yearly replacement.
Health-state costs
Health-state costs were obtained from the DEEP study and include the cost of resources consumed in both the dental and general health-care budgets. Costs include contact with all health professionals (dental and general) for dental-related problems. Drug costs are also incorporated. Full details of the costing methodology are reported in the DEEP study. 98,99 For the purposes of this economic analysis, the cost of splints was excluded from the analysis to avoid a risk of double-counting. To estimate the health-care costs by health state (‘low pain’, ‘moderate pain’ and ‘high pain’), a regression analysis was conducted using Stata. As was done with the utilities, an average cost for each pain tertile was estimated using the generalised estimating equation approach separately for tertile of current pain and CPI. All costs from the DEEP study are reported in 2012 values, and have therefore been updated to 2016/17 values using the Cochrane and Campbell online tool. 108 The estimated means and SDs of the sampling distribution for health-care costs are provided in Table 21. Cost data provided reflect total costs (excluding splint provision costs) by health state of the 2-year follow-up period of the DEEP study. These costs are converted to 3-monthly cycle-specific costs and are sampled probabilistically from gamma distributions for inclusion in the model.
| Health state | Current pain | CPI | ||||
|---|---|---|---|---|---|---|
| Mean (SD)a (£) | Alpha | Lambdab | Mean (SD)a (£) | Alpha | Lambdab | |
| Low pain | 345.43 (10.10) | 1303 | 3.57 | 332.70 (11.20) | 983 | 2.80 |
| Moderate pain | 519.17 (12.27) | 1995 | 3.64 | 479.80 (11.40) | 1974 | 3.90 |
| High pain | 675.41 (13.45) | 2810 | 3.94 | 697.63 (13.19) | 3117 | 4.23 |
Assessment of cost-effectiveness
A CUA was conducted using QALYs as the measure of benefits. The results presented are over the lifetime of the simulated cohort (with a starting age of 25 years in the base case). The model is fully probabilistic, with model outputs calculated using Monte Carlo simulation with 1000 repetitions, sampling from distributions for transition probabilities, MDs, utilities and costs as described in Tables 16, 17, 19 and 21, respectively. The probabilistic analysis is advantageous because it varies all parameters simultaneously. Model results are reported as expected values of costs and QALYs over the modelled time horizon. An incremental comparison of costs and QALYs between splints and no splints is reported and ICERs are calculated as the MD in costs divided by the MD in QALYs. The ICER is then compared with a commonly used cost per QALY threshold recommended by NICE. 97 If the ICER is within the desired range (£20,000–30,000, or below), an intervention would generally be considered cost-effective. However, in all cases, determination of cost-effectiveness must also consider the variability around the point estimates of incremental costs, incremental QALYs and, hence, the ICER. Consideration of such uncertainty is important to determine if current evidence is sufficient for decision-making, and if decision-makers can be confident that the ICER is likely to fall below commonly accepted threshold values.
As all models are run probabilistically, it is possible to determine the probability of cost-effectiveness at threshold values of willingness to pay (WTP) for a QALY gained (e.g. £20,000 per QALY) for each scenario analysis. Uncertainty in the base-case results is also illustrated using scatterplots of the cost-effectiveness plan and cost-effectiveness acceptability curves (CEACs). Scatterplots and CEACs are particularly informative as they demonstrate the uncertainty arising due to the combined statistical variability in all the models’ parameter inputs. The CEAC shows the probability that splints or no splints are the most efficient use of resources at difference threshold values of society’s WTP for a QALY gain.
A range of scenario analyses were also conducted to account for structural and methodological uncertainty as well as heterogeneity. In economic models, some structural assumptions are made that come with some uncertainty. An example of this would be to vary the time horizon or vary the frequency at which individuals are assumed to replace their splints. There is also uncertainty surrounding the methods that could be used in the model. For example, there are uncertainties regarding the discount rates that should be applied to costs and benefits. The results from the analysis might be subject to heterogeneity. To account for heterogeneity, conducting the analysis in, for example, different age groups would be beneficial. All sensitivity and scenario analyses are listed in Table 22. It should be noted that all analyses, including the base-case and scenario analyses, are reported for different definitions of pain (current and CPI).
| Assumption/model parameter | Base-case assumption | Alternative assumption in scenario analysis | Justification |
|---|---|---|---|
| Definition of pain | Current pain and CPI definitions considered as a joint base-case analysis | A joint base case is provided for current pain and CPI. Although CPI is the preferred measure (as part of the GCPS), data to populate effect sizes are more complete for current pain, and based on a greater number of studies | |
| Cohort starting age | 25 years | Alternative starting ages of 40 and 56 years | Age varied to reflect alternative ages at TMD onset and presentation for treatment with splints (informed by the project advisory group) |
| Discount rate for costs and QALYs | 3.5% | Vary between 0% and 6% | Recommended variation of discount rate according to NICE methods for technology appraisal97 |
| Modelled time horizon | 85 years | 2, 10, 20 and 30 years | The base case reflects best-practice methods to incorporate all the possible costs and benefits over a lifetime. However, long-term uncertainty is extensive; therefore, shorter time horizons could be expected to yield better estimates of cost-effectiveness |
| Splint replacement | Frequency of splint replacement based on wide variation in expert opinion (0, 2, 5 and 20 years), sampled probabilistically | Scenarios include no replacement, replacement every 2, 5 and 20 years | There is likely to be substantial heterogeneity across the TMD population with regards to how often splints are replaced. This sensitivity analysis explores a range of assumptions, discussed with the project advisory board, and is based on clinical and patient expert opinion |
| Cost of splints | Weighted average of custom-made and prefabricated splints using current estimated banding | Assuming all splints are (1) band 1, (2) band 2 and (3) band 3 treatment | Reflects uncertainty in the current banding system for payment that is not necessarily based on the opportunity cost of time and resource use required for different splint treatments |
| Long-term MD (beyond 12 months) | Assumes equal likelihood of (1) long-term MD of 0 and (2) long-term MD = MD at 12 months | Varies the assumption between long-term MD = 0 and long-term MD = MD at 12 months | Long-term MD data do not exist, but are an important driver of cost-effectiveness. Clinical expert opinion considered each scenario equally probable |
| Long-term transition probabilities | Assumes equal likelihood that transition probabilities beyond the DEEP study cohort time horizon (2 years) are (1) zero and (2) equal to the transitions between 18 and 24 months | Varies the assumption between long-term probabilities equal to zero and equal to transitions between 18 and 24 months | Long-term transition probability data do not exist beyond the 2-year follow-up of the DEEP study cohort. However, they are an important driver of cost-effectiveness. Clinical expert opinion considered each scenario equally probable |
Value-of-information analysis
Value-of-information analysis is a useful tool for identifying what contributes to the decision uncertainty in the model. The expected value of perfect information (EVPI) is the difference between the expected value with perfect information and the expected value with current information, and can be used to determine whether or not future research to resolve current decision uncertainty is a worthwhile investment. The EVPI is calculated using the net monetary benefit (NMB). The NMB is calculated from the results of the probabilistic analysis. The intervention with the highest NMB is the most cost-effective intervention. The EVPI is the maximum NMB of each iteration from the Probabilistic Sensitivity Analysis, averaged:109
where λ = threshold, and
The population EVPI is the value of doing further research in the population of interest, namely those who would benefit from the intervention, for example the value of conducting further research on the relative effectiveness of splints compared with no splints in the TMD population in the UK. The expected value of perfect parameter information (EVPPI) estimates which parameters contribute to the decision uncertainty. The EVPPI is conducted by taking an iteration from the Monte Carlo simulation of a parameter in the outer loop and running the model for a defined number of simulations (e.g. 1000 runs), and repeating this exercise for all the parameter iterations from the probabilistic analysis.
To calculate the population EVPI, a number of assumptions were made both regarding the population likely to benefit from the intervention and the lifetime of the health technology evaluated. The population likely to benefit each year was assumed to be 2,311,407 (based on an annual prevalence of examiner-verified TMD of 3.5% obtained from Slade,110 and a UK-wide population of approximately 66 million111). The expected lifetime of the technology was assumed to be 10 years, in line with typical practice for the conduct of a VOI analysis, and the threshold value of WTP for a QALY gained was assumed to be £20,000, in line with typical UK decision-making processes. 97
The VOI analysis was conducted on the Sheffield Accelerated Value of Information (SAVI) application. 112 If the EVPI is positive, further research might be worthwhile. If this is the case, EVPPIs would be calculated to identify the source(s) of uncertainty in the results and the value of future research to resolve decision uncertainty surrounding the most important drivers of cost-effectiveness results.
Cost-effectiveness results
Base-case results
The base-case analyses used either current pain as the measurement of pain intensity or CPI, because the majority of included RCTs focused on current pain or CPI (see Chapter 3 for further details). Table 23 reports the base-case analysis for both current pain and CPI specifications of the model.
| Intervention | Total costs (£) | Incremental costs (£) | Total QALYs | Incremental QALYs | ICER (£) | Probability of cost-effectiveness at different WTP thresholds (%) | ||
|---|---|---|---|---|---|---|---|---|
| £0 | £20,000 | £30,000 | ||||||
| Current pain | ||||||||
| No splints | 6375 | 17.999 | 93.3 | 42.5 | 41.0 | |||
| Splints | 7463 | 1088 | 18.027 | 0.028 | 39,216 | 6.7 | 57.5 | 59.0 |
| CPI | ||||||||
| No splints | 5681 | 18.575 | 97.3 | 70.9 | 66.8 | |||
| Splints | 6660 | 980 | 18.557 | –0.018 | Dominated | 2.7 | 29.1 | 33.2 |
The point estimate of the ICER indicates that splints are not cost-effective using a model structured around current pain. Splints are less likely to be cost-effective using the CPI specification of the model, in which, on average, splints appear to be more costly and less beneficial in terms of QALYs gained. The unfavourable results for splints are driven by the point estimate of the MD for CPI at 6 months, which non-significantly favours the no-splint group. However, these results should be interpreted with caution and must be considered in the light of the very poor quality of the trials used to generate the effect size estimates used in the model. The optimal strategy is highly uncertain and estimates of the ICER should be considered as exploratory in nature.
For all analyses, considering the point estimates of the ICER in isolation may be misleading regarding the true cost-effectiveness of splints using either definition of pain. It may be more appropriate to consider the illustrations of cost-effectiveness provided by scatterplots of the cost-effectiveness plane and CEACs, which more adequately characterise the substantial uncertainty surrounding the results. Figures 11 and 12 illustrate the scatterplot of the cost-effectiveness plane and the CEACs, illustrating the probability that each strategy (splints/no splints) is the most efficient use of resources at alternative threshold values of society’s WTP for a QALY gained.
FIGURE 11.
Cost-effectiveness plane for splints vs. no splints (current pain specification).
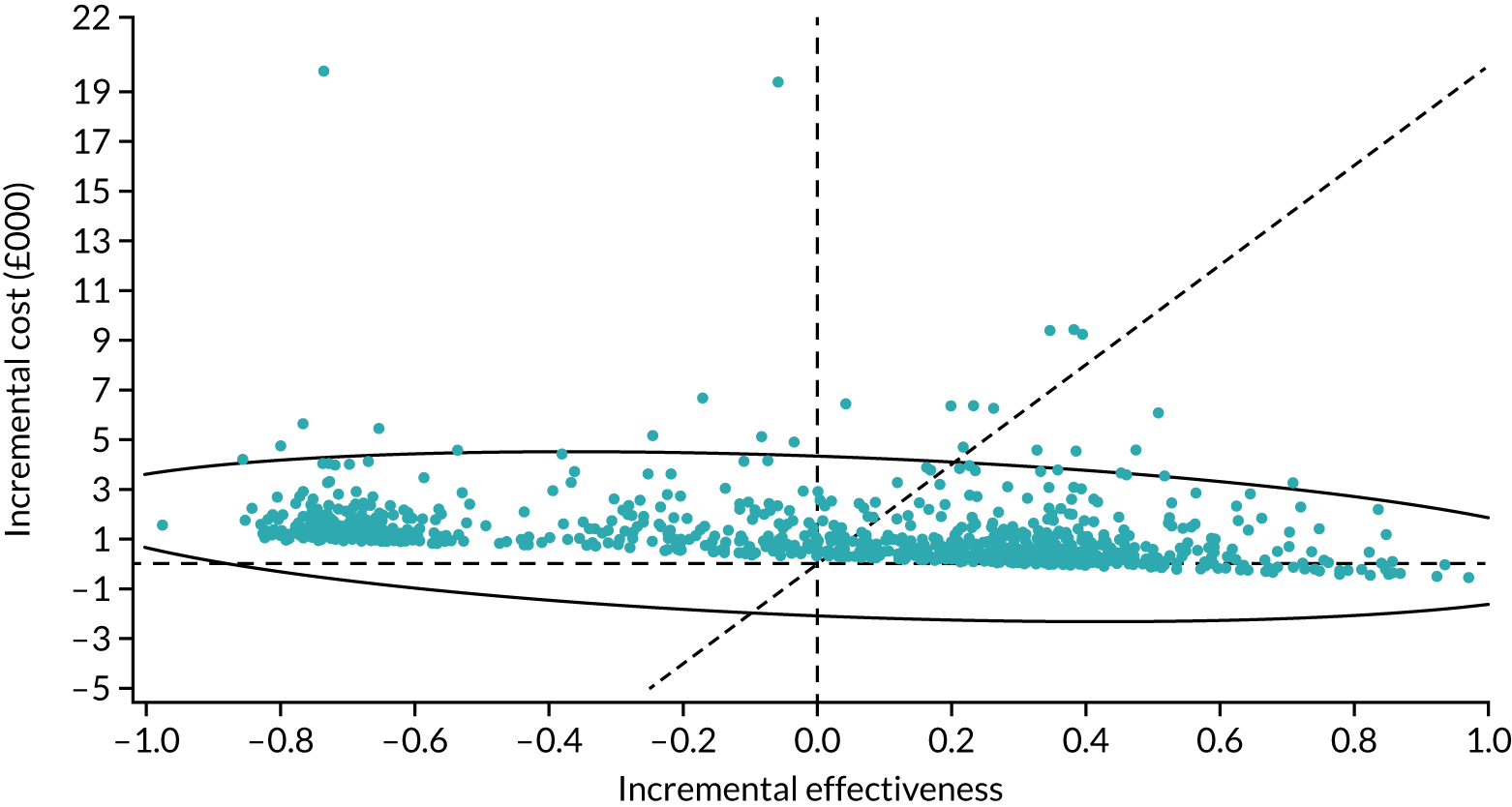
FIGURE 12.
Cost-effectiveness acceptability curve for current pain specification.
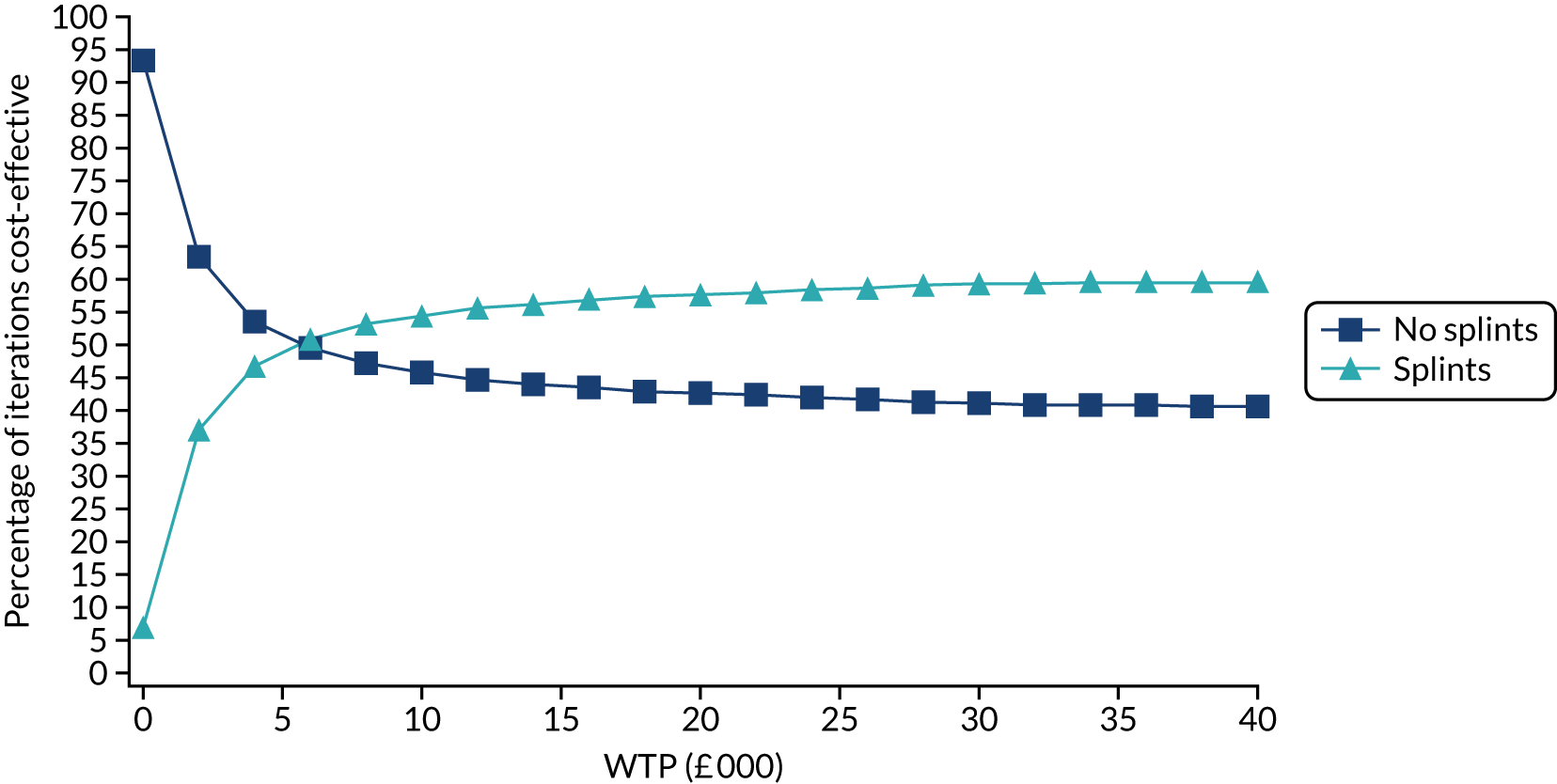
The scatterplot indicates a high level of uncertainty surrounding the incremental QALYs gained, driven by uncertainty in the effect size for pain MDs identified in the review of clinical effectiveness (see Chapter 3).
The CEAC indicates substantial uncertainty regarding the optimal strategy, with probabilities of the cost-effectiveness of splints never increasing above 60% at WTP threshold values of between £10,000 and £40,000 per QALY.
Figures 13 and 14 report similar data for the CPI specification of the model.
FIGURE 13.
Cost-effectiveness plane for splints vs. no splints (CPI specification).
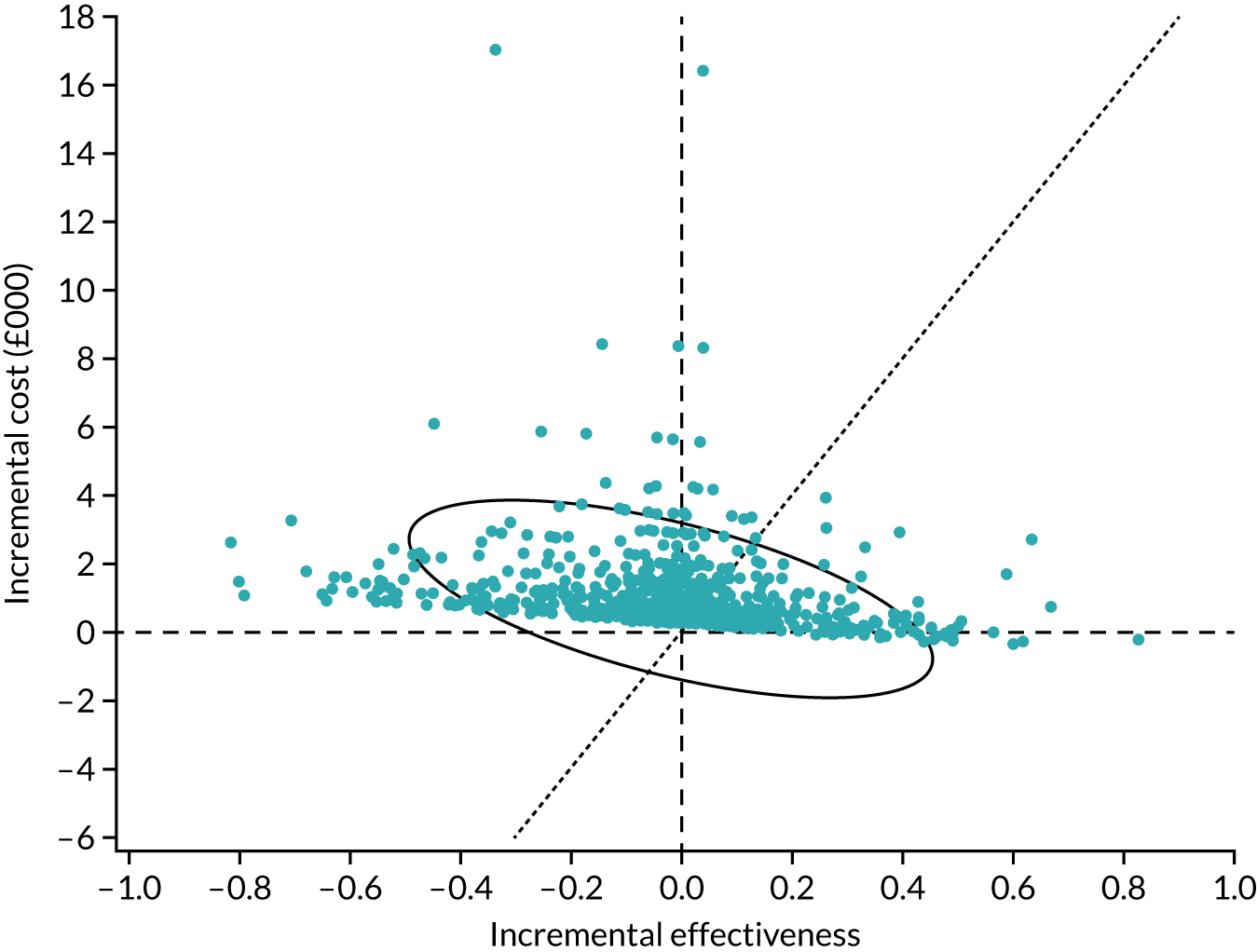
FIGURE 14.
Cost-effectiveness acceptability curve for CPI specification.
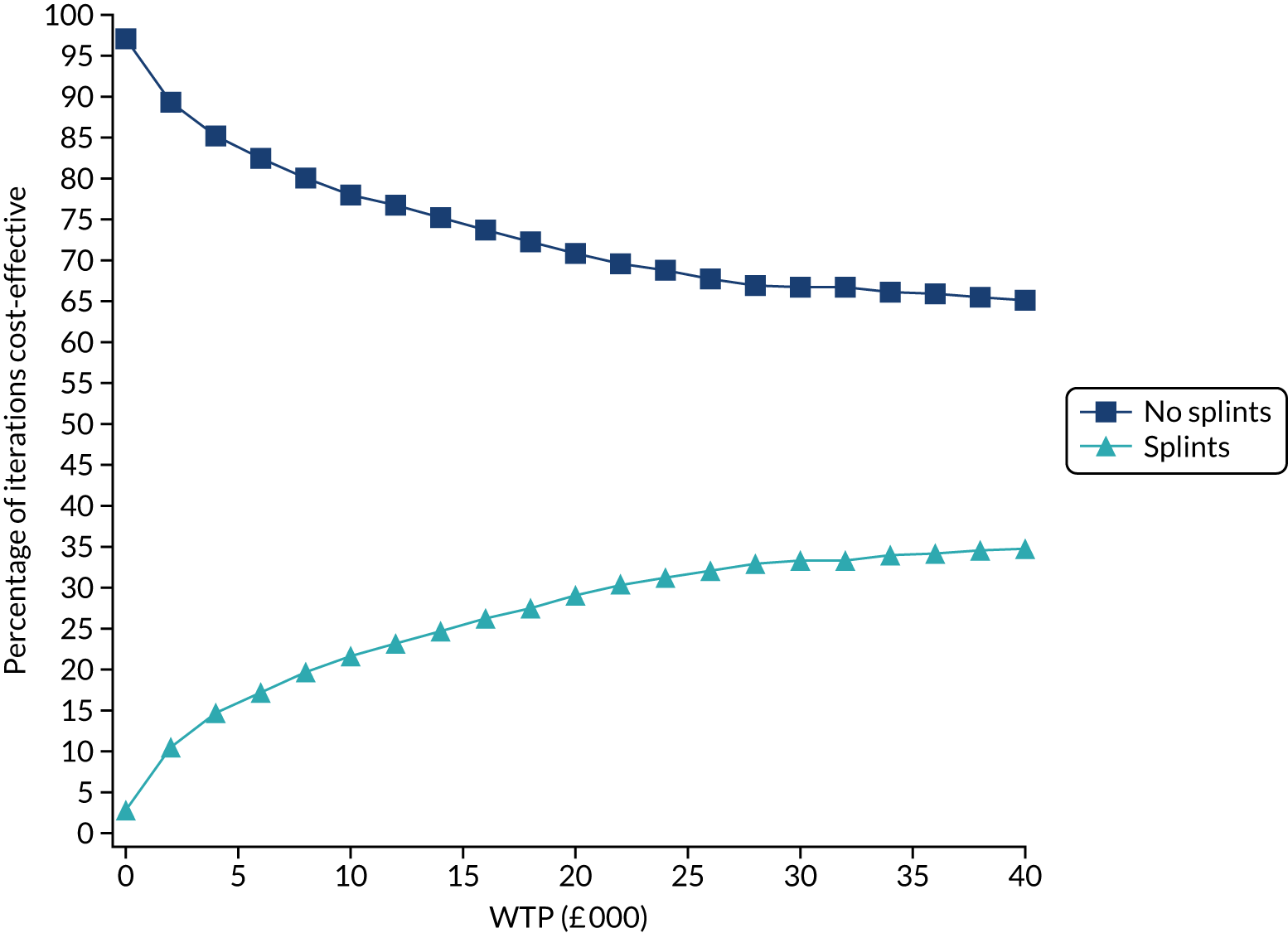
The scatterplot for CPI also indicates a high level of uncertainty surrounding the incremental QALYs gained, again driven by uncertainty in the effect size from poor-quality studies regarding the MDs in CPI between splints and no splints used to populate the model (see Chapter 3). Although the results indicate a lower likelihood of splints being cost-effective using the CPI specification, it should be noted that this is driven by the MD favouring no splints at 6 months. However, as discussed in Chapter 3, Results of the systematic review, this finding should be interpreted with caution and in the light of the poor methodological quality of the included studies in the clinical effectiveness review.
Scenario and sensitivity analyses
The base-case analysis should be interpreted with caution. A range of scenario and sensitivity analyses have also been undertaken, the results of which are presented in Table 24 and serve to illustrate the wider variation in the ICER depending on the assumptions applied in the model. All scenario and sensitivity analyses were conducted probabilistically and report results for both current pain and CPI. These analyses are informative in determining the key assumptions that drive the cost-effectiveness results.
| Intervention | Total costs (£) | Incremental costs (£) | Total QALYs | Incremental QALYs | ICER (£) | Probability of cost-effectiveness at difference WTP thresholds (%) | ||
|---|---|---|---|---|---|---|---|---|
| £0 | £20,000 | £30,000 | ||||||
| Base case | ||||||||
| Current pain | ||||||||
| No splints | 6375 | 17.999 | 93.3 | 42.5 | 41.0 | |||
| Splints | 7463 | 1088 | 18.027 | 0.028 | 39,216 | 6.7 | 57.5 | 59.0 |
| CPI | ||||||||
| No splints | 5681 | 18.575 | 97.3 | 70.9 | 66.8 | |||
| Splints | 6660 | 980 | 18.557 | –0.018 | Dominated | 2.7 | 29.1 | 33.2 |
| Assume long-term MD beyond 12 months = 0 and long-term transition probabilities continue as per DEEP study | ||||||||
| Current pain | ||||||||
| No splints | 6088 | 18.310 | 100.0 | 100.0 | 100.0 | |||
| Splints | 7849 | 1761 | 17.614 | –0.697 | Dominated | 0.0 | 0.0 | 0.0 |
| CPI | ||||||||
| No splints | 5054 | 19.115 | 100.0 | 76.1 | 70.4 | |||
| Splints | 6014 | 960 | 19.115 | 0.000 | 9,502,983 | 0.0 | 23.9 | 29.6 |
| Assume long-term MD beyond 12 months = MD at 12 months and long-term transition probabilities continue as per DEEP study | ||||||||
| Current pain | ||||||||
| No splints | 6088 | 18.310 | 93.3 | 1.1 | 0.3 | |||
| Splints | 6893 | 805 | 18.645 | 0.334 | 2407 | 6.7 | 98.9 | 99.7 |
| CPI | ||||||||
| No splints | 5054 | 19.115 | 100.0 | 85.3 | 80.2 | |||
| Splints | 6040 | 986 | 19.092 | –0.023 | Dominated | 0.0 | 14.7 | 19.8 |
| Assume long-term MD beyond 12 months = MD at 12 months and long-term transition probabilities = 0 | ||||||||
| Current pain | ||||||||
| No splints | 6626 | 17.728 | 83.4 | 17.5 | 16.1 | |||
| Splints | 7394 | 768 | 18.102 | 0.374 | 2054 | 16.6 | 82.5 | 83.9 |
| CPI | ||||||||
| No splints | 6243 | 18.092 | 95.4 | 61.8 | 59.4 | |||
| Splints | 7252 | 1009 | 18.047 | –0.045 | Dominated | 4.6 | 38.2 | 40.6 |
| Assume long-term MD beyond 12 months = 0 and long-term transition probabilities = 0 | ||||||||
| Current pain | ||||||||
| No splints | 6626 | 17.728 | 96.8 | 58.0 | 55.3 | |||
| Splints | 7730 | 1104 | 17.739 | 0.011 | 98,645 | 3.2 | 42.0 | 44.7 |
| CPI | ||||||||
| No splints | 6243 | 18.092 | 96.7 | 59.6 | 56.9 | |||
| Splints | 7214 | 971 | 18.081 | –0.011 | Dominated | 3.3 | 40.4 | 43.1 |
| Starting age of cohort set to 40 years | ||||||||
| Current pain | ||||||||
| No splints | 5670 | 15.247 | 94.4 | 42.5 | 41.0 | |||
| Splints | 6655 | 985 | 15.275 | 0.027 | 35,988 | 5.6 | 57.5 | 59.0 |
| CPI | ||||||||
| No splints | 5059 | 15.731 | 98.0 | 70.6 | 66.6 | |||
| Splints | 5948 | 889 | 15.716 | –0.016 | Dominated | 2.0 | 29.4 | 33.4 |
| Starting age of cohort set to 56 years | ||||||||
| Current pain | ||||||||
| No splints | 4524 | 11.394 | 95.5 | 42.4 | 41.4 | |||
| Splints | 5343 | 819 | 11.420 | 0.026 | 31,032 | 4.5 | 57.6 | 58.6 |
| CPI | ||||||||
| No splints | 4049 | 11.749 | 98.4 | 70.4 | 67.1 | |||
| Splints | 4792 | 743 | 11.737 | –0.012 | Dominated | 1.6 | 29.6 | 32.9 |
| Discount rate of 0% | ||||||||
| Current pain | ||||||||
| No splints | 14,658 | 39.733 | 86.4 | 42.8 | 41.6 | |||
| Splints | 16,953 | 2296 | 39.754 | 0.021 | 107,798 | 13.6 | 57.2 | 58.4 |
| CPI | ||||||||
| No splints | 12,982 | 41.048 | 96.0 | 72.4 | 68.4 | |||
| Splints | 15,023 | 2040 | 41.010 | –0.038 | Dominated | 4.0 | 27.6 | 31.6 |
| Discount rate of 6% | ||||||||
| Current pain | ||||||||
| No splints | 4289 | 12.291 | 96.0 | 42.3 | 40.9 | |||
| Splints | 5077 | 788 | 12.320 | 0.029 | 27,437 | 4.0 | 57.7 | 59.1 |
| CPI | ||||||||
| No splints | 3840 | 12.675 | 98.7 | 69.4 | 66.5 | |||
| Splints | 4555 | 715 | 12.662 | –0.013 | Dominated | 1.3 | 30.6 | 33.5 |
| Time horizon of 2 years | ||||||||
| Current pain | ||||||||
| No splints | 539 | 1.459 | 100.0 | 38.2 | 31.4 | |||
| Splints | 825 | 285 | 1.482 | 0.022 | 12,787 | 0.0 | 61.8 | 68.6 |
| CPI | ||||||||
| No splints | 513 | 1.490 | 100.0 | 74.1 | 67.7 | |||
| Splints | 778 | 264 | 1.489 | –0.001 | Dominated | 0.0 | 25.9 | 32.3 |
| Time horizon of 10 years | ||||||||
| Current pain | ||||||||
| No splints | 2222 | 6.482 | 98.7 | 44.2 | 42.5 | |||
| Splints | 2708 | 486 | 6.509 | 0.027 | 18,275 | 1.3 | 55.8 | 57.5 |
| CPI | ||||||||
| No splints | 2021 | 6.665 | 100.0 | 64.7 | 61.3 | |||
| Splints | 2463 | 442 | 6.662 | –0.003 | Dominated | 0.0 | 35.3 | 38.7 |
| Time horizon of 20 years | ||||||||
| Current pain | ||||||||
| No splints | 3748 | 10.963 | 95.7 | 43.4 | 41.5 | |||
| Splints | 4451 | 703 | 10.989 | 0.026 | 26,700 | 4.3 | 56.6 | 58.5 |
| CPI | ||||||||
| No splints | 3366 | 11.299 | 99.3 | 69.3 | 65.4 | |||
| Splints | 4005 | 640 | 11.288 | –0.011 | Dominated | 0.7 | 30.7 | 34.6 |
| Time horizon of 30 years | ||||||||
| Current pain | ||||||||
| No splints | 4813 | 13.979 | 95.0 | 43.3 | 41.9 | |||
| Splints | 5674 | 861 | 14.004 | 0.026 | 33,212 | 5.0 | 56.7 | 58.1 |
| CPI | ||||||||
| No splints | 4304 | 14.418 | 98.5 | 67.2 | 63.2 | |||
| Splints | 5078 | 774 | 14.407 | –0.011 | Dominated | 1.5 | 32.8 | 36.8 |
| Cost of splints and replacement set at band 3 charge | ||||||||
| Current pain | ||||||||
| No splints | 6375 | 17.999 | 94.8 | 42.7 | 41.2 | |||
| Splints | 7548 | 1173 | 18.027 | 0.028 | 42,289 | 5.2 | 57.3 | 58.8 |
| CPI | ||||||||
| No splints | 5681 | 18.575 | 98.5 | 73.9 | 68.9 | |||
| Splints | 6897 | 1216 | 18.557 | –0.018 | Dominated | 1.5 | 26.1 | 31.1 |
| Cost of splints and replacement set at band 2 charge | ||||||||
| Current pain | ||||||||
| No splints | 6375 | 17.999 | 56.9 | 40.1 | 40.0 | |||
| Splints | 6601 | 226 | 18.027 | 0.028 | 8139 | 43.1 | 59.9 | 60.0 |
| CPI | ||||||||
| No splints | 5681 | 18.575 | 87.1 | 61.4 | 60.3 | |||
| Splints | 5950 | 269 | 18.557 | –0.018 | Dominated | 12.9 | 38.6 | 39.7 |
| Cost of splints and replacement set at band 1 charge | ||||||||
| Current pain | ||||||||
| No splints | 6375 | 17.999 | 43.4 | 39.6 | 39.6 | |||
| Splints | 6413 | 38 | 18.027 | 0.028 | 1352 | 56.6 | 60.4 | 60.4 |
| CPI | ||||||||
| No splints | 5681 | 18.575 | 73.4 | 57.9 | 57.4 | |||
| Splints | 5761 | 81 | 18.557 | –0.018 | Dominated | 26.6 | 42.1 | 42.6 |
| Replacing splints every 2 years | ||||||||
| Current pain | ||||||||
| No splints | 6375 | 17.999 | 100.0 | 45.7 | 43.4 | |||
| Splints | 8903 | 2528 | 18.027 | 0.028 | 91,119 | 0.0 | 54.3 | 56.6 |
| CPI | ||||||||
| No splints | 5681 | 18.575 | 100.0 | 83.0 | 78.1 | |||
| Splints | 7904 | 2223 | 18.557 | –0.018 | Dominated | 0.0 | 17.0 | 21.9 |
| Replacing splints every 5 years | ||||||||
| Current pain | ||||||||
| No splints | 6375 | 17.999 | 99.5 | 42.0 | 41.3 | |||
| Splints | 7489 | 1114 | 18.027 | 0.028 | 40,156 | 0.5 | 58.0 | 58.7 |
| CPI | ||||||||
| No splints | 5681 | 18.575 | 100.0 | 74.5 | 69.1 | |||
| Splints | 6683 | 1002 | 18.557 | –0.018 | Dominated | 0.0 | 25.5 | 30.9 |
| Replacing splints every 20 years | ||||||||
| Current pain | ||||||||
| No splints | 6375 | 17.999 | 88.6 | 40.5 | 40.0 | |||
| Splints | 6790 | 415 | 18.027 | 0.028 | 14,951 | 11.4 | 59.5 | 60.0 |
| CPI | ||||||||
| No splints | 5681 | 18.575 | 96.0 | 63.8 | 61.6 | |||
| Splints | 6079 | 398 | 18.557 | –0.018 | Dominated | 4.0 | 36.2 | 38.4 |
| Cost of replacing splints set to £0 | ||||||||
| Current pain | ||||||||
| No splints | 6375 | 17.999 | 51.0 | 39.8 | 39.7 | |||
| Splints | 6543 | 168 | 18.027 | 0.028 | 6041 | 49.0 | 60.2 | 60.3 |
| CPI | ||||||||
| No splints | 5681 | 18.575 | 87.2 | 59.9 | 59.0 | |||
| Splints | 5865 | 185 | 18.557 | –0.018 | Dominated | 12.8 | 40.1 | 41.0 |
The model results for the current pain configuration were most sensitive to varying structural assumptions about the long-term MD and long-term transition probabilities. Assuming a long-term MD of zero, namely that splints have no additional effectiveness beyond 1 year of usage, generates additional costs to the NHS with mean QALY losses, and thus a very low probability of cost-effectiveness. However, assuming that the MD observed at 6 months continues over a patient’s lifetime, splints would be a highly cost-effective use of resources, with an ICER of just over £2000 per QALY gained. These analyses serve to illustrate the sensitivity of the model to long-term assumptions about differences in pain, and highlight the need for further research to adequately determine the appropriate long-term trajectory of the effectiveness of splints. The CPI-configured model is also sensitive to this assumption, but less so given that the distribution of the MD in CPI at 12 months is centred closer to zero. It should also be noted that extrapolation of MD data to the longer term are based on poor-quality shorter-term MDs (at 3, 6 or 12 months), adding a further layer of uncertainty to the results. It is imperative that high-quality data are obtained for MD in pain related to TMD to generate more robust estimates of cost-effectiveness from the modelling analysis.
The model is also somewhat sensitive to assumptions around the costs of splints and the frequency of splint replacement. There is substantial uncertainty over the actual banded change that might be applied to splints, particularly for prefabricated splints, which could feasibly incur a band 1 or 2 charge, with custom-made splints more likely to incur a band 2 or 3 charge. As anticipated, lower banding improves the cost-effectiveness case for splints. Similarly, there was much uncertainty among the project’s clinical advisors regarding the most probable frequency of splint replacement. This is an important driver of incremental costs in the model, with more frequent replacement generating lower likelihoods of cost-effectiveness. For the current pain configuration, the probability of cost-effectiveness (at £20,000 per QALY) drops from 60% when never replaced to 54% when replaced every 2 years. The CPI results are more sensitive to this assumption, decreasing from 40% to only 17% for the same replacement frequencies.
The scenario indicates that the model results were not particularly sensitive to the discount rate or time horizon chosen.
Value-of-information results
This section reports the results of the VOI analysis. Given the high level of uncertainty described in the previous section, it is important to determine the value of additional research to determine the cost-effectiveness of splints. The EVPI results in Table 25 indicate that future research is worthwhile. The EVPPI results build on this to identify the parameters in the model for which further research is most valuable to reduce uncertainty regarding the optimal treatment decision (splints or no splints).
| VOI | Current pain (£) | CPI (£) |
|---|---|---|
| EVPI | ||
| Per person | 3961 | 867 |
| Population (10 years) | 91.57B | 20.04B |
| EVPPI | ||
| Transition probabilities | ||
| Per person | 2318 | 404 |
| Population (10 years) | 53.59B | 9.34B |
| Mean difference | ||
| Per person | 2847 | 15 |
| Population (10 years) | 65.80B | 0.35B |
| Costs | ||
| Per person | 201 | 26 |
| Population (10 years) | 4.64B | 0.59B |
| Utilities | ||
| Per person | 40 | 2 |
| Population (10 years) | 0.93B | 0.06B |
The VOI analysis further emphasises the decision uncertainty, and identified the key drivers of the cost-effectiveness results. The large positive values of EVPI indicate that further research would be a good investment. EVPPI helps to indicate the parameters in the model that contribute most to decision uncertainty. Large EVPPI values (for current pain in particular) indicate that further research should be prioritised to resolve decision uncertainty about the impact of splints on pain (i.e. the MDs used in the model). This should also encompass an assessment of the long-term effectiveness to more accurately guide extrapolation in the model. In addition, further research regarding the long-run trajectory of disease would be worthwhile, to determine transition probabilities over the full life course. Research on the costs of splints and, in particular, the replacement cost of splints over time is also worthy of further research, although EVPPIs are lower than for the clinical effectiveness data. Although generating positive values of EVPPI, further research with regard to health-state utilities is less valuable, and should be considered only after decision uncertainty in clinical effectiveness and costs of splints has been resolved.
It is noted that parameter EVPPI is positive, but remains consistently lower in models parameterised around CPI than in those parameterised around current pain. This finding must be considered in the light of the other findings in the report, and, in particular, the poor methodological quality of the underlying effect size studies. VOI is a useful tool in prioritising future research, but is driven solely by sampling uncertainty around the input parameters. It does not consider the methodological quality of the clinical effectiveness studies. Details of the economic evaluation results, including VOI results, comparing custom-made and prefabricated splints with no splints can be found online. 113
Chapter 5 Discussion
Parts of this chapter have been adapted from Riley et al. 1 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original text.
Summary of main results
Despite the inclusion of 35 studies comparing oral splints with no splints or a minimal intervention in patients with TMD, the body of evidence was assessed as being of very low quality. There was no evidence that oral splints reduced pain, reduced clicking of the temporomandibular joint or increased mouth-opening, when TMD is considered as a group of conditions. When comparing oral splints with control splints, there was some very low-quality evidence from three studies36,47,60 that oral splints reduced pain when compared with control splints for a time period of 0–3 months; however, this was not supported at the other time periods (3–6 months and 6–12 months). In the light of the absence of any evidence showing that splints reduce pain against no/a minimal control, the benefit for splints, when compared with control splints, seen at 3 months may indicate that such control splints are actually detrimental. It is therefore unclear if the provision of control splints is an appropriate control to use in RCTs of this type.
The economic analysis was configured to report cost-effectiveness based on differences in pain measured as current pain or CPI. The modelling for TMD is based on poor-quality clinical effectiveness data and estimates of cost-effectiveness should be considered exploratory in nature. The base-case results showed that there was substantial uncertainty regarding the most cost-effective treatment strategy. The scatterplots of the cost-effectiveness plane depict the high uncertainty in the results. For the current pain configuration, about half the point estimates of the ICER favour splints and the other half favour the no-splint group, meaning that there is an equal chance that either strategy may be cost-effective. The results were slightly less uncertain for the CPI configuration, with a ≈29% chance that splints are cost-effective and a 71% chance that no splints offers the best value for money. However, as described by the scenario analyses undertaken, substantial variability exists in incremental costs and incremental QALYs, depending on the assumptions applied, meaning that the most cost-effective treatment strategy cannot be determined. The estimates of cost-effectiveness should be interpreted with caution. It is probably more informative to consider the substantial impact of plausible variations in important assumptions on the ICER and to thus use the modelling results to identify the key parameters that drive the cost-effectiveness results, on which further research would be informative.
For patients with bruxism, there was insufficient evidence to conclude whether or not the provision of oral splints reduced tooth wear, as no studies reported this. Although a small number of studies reported pain and other outcomes, there was also insufficient evidence to conclude whether or not oral splints were beneficial. With regard to cost-effectiveness, there was no evidence to support or refute the use of splints for bruxism and there was no evidence available to populate a decision model.
Six studies50,55,61,62,72,73 compared prefabricated splints with custom-made splints in patients with varying subtypes of TMD. There was insufficient evidence to determine whether or not there was a difference between the splint types for any outcomes included in this review. This evidence was assessed as being of very low quality, and insufficient to draw any robust conclusions regarding either clinical effectiveness or cost-effectiveness. It should be noted that many types of prefabricated splints exist, some of which are readily available to patients via the internet, without the need for dental consultation/fitting. Such splints were not evaluated in the trials identified for this review.
Overall completeness and applicability of evidence
For TMD, we undertook a sensitivity analysis restricted to trials for which the inclusion criteria were based on, or could be clearly mapped to, one of the following sets of diagnostic criteria: RDC/TMD guidelines,17 TMD (DC/TMD) guidelines18 or AAOP guidelines. 19 There was no difference in the results when removing studies that did not use these diagnostic criteria.
Similarly, for bruxism patients, we planned to undertake a sensitivity analysis restricted to trials for which there was a clear diagnosis of bruxism. 4 We were unable to do this for the five bruxism trials included in the systematic review owing to the lack of outcome data reported in these studies.
For both patients with TMD and with bruxism, owing to differences in the diagnoses of the included trial participants and differences in the types of splints and control groups used, the applicability of the evidence is questionable, and certainly incomplete for patients with bruxism. We suspect that this same variability and lack of diagnostic criteria is mirrored in primary care when splints are being prescribed.
Pain was reported in numerous different ways, at different times, and this reduced the number of studies that could be combined in a meta-analysis to produce a pooled estimate. The use of an agreed measure for pain and how and when this is measured would enable the pain data from all the studies to contribute to one single pooled estimate. It is also important to consider what would be a clinically important reduction in pain. It is suggested that around a 20% reduction represents a minimally important decrease, 30% a moderately important decrease and 50% a substantial decrease. 114
Several of the studies reported study outcomes but we were unable to use the data. The main reason for this was missing SDs. Once again, this compromised the completeness of the results of the meta-analyses.
Numerous studies reported on some of our outcomes but did not report the data in a suitable format for inclusion in our meta-analyses. This can mean that meta-analyses are biased by missing information. However, the Cochrane Risk of Bias tool15 and meta-analyses do not currently address this issue adequately. A study may be assessed as having a high risk of selective outcome reporting, but if that study is not included in a meta-analysis because it has no data, then this is not reflected or accounted for. This highlights the need for standardisation in both ‘what to measure’ and ‘how to measure it’ in clinical trials in this area of research. Otherwise, there will continue to be research waste, with data that are not able to be pooled in data syntheses. There are initiatives such as Initiative on Methods, Measurement and Pain Assessment in Clinical Trials (IMMPACT),114,115 Core Outcome Measures in Effectiveness Trials (COMET)116 and COnsensus-based Standards for the selection of health Measurement Instruments (COSMIN)117 that can help with these issues, and future research in these areas would be beneficial.
The use of a control/placebo splint is also questionable in trials conducted on patients with TMD or bruxism. It is unclear what effect the control splint may have on the outcomes measured, and this may explain why the comparisons of splints versus minimal interventions, and splints versus control splints, led to different findings.
Meta-analysis is the key tool for facilitating progress in science by quantifying what is known and identifying what is not known. 118 The most consequential effect of introducing formal research synthesis methodology has been a profound change in the way scientists think about the outcomes of scientific research. An individual primary study may now be seen as a contribution towards the accumulation of evidence, rather than revealing the conclusive answer to a scientific problem. 118,119 In the field of TMD, it could be considered that each new trial be designed with the current evidence in mind (as part of a funding application). This could help to ensure that the trial is asking an important question, on the right population using the right methodology, especially the measurement of pain using consistent methods. Unless research in this area does not address these issues, then there may continue to be a mismatch of poor-quality trials on different interventions, in different groups of patients, with different diagnoses, and using different ways of measuring pain or other TMD outcomes. So, rather than there being a call for a reduction in evidence synthesis in this area,120 it is vital that there is a methodologically sound evidence base that is kept up to date in order for the science in this area to progress.
Quality of the evidence
The quality of the evidence for comparing splints with no splints/minimal interventions/control splints in patients with all subtypes of TMD was downgraded to ‘very low’ owing to the studies being at a high risk of bias, heterogeneity and a lack of precision in the estimates. Most studies were assessed as being at a high risk of bias because of the inability of researchers to blind patients to wearing a splint or not, or wearing different types of splint. As the primary outcome for the TMD patients was pain assessed by the patients, this meant that this outcome measurement was also assessed as being at a high risk of bias. It is difficult to design trials to overcome this problem. We were unable to investigate the heterogeneity of the effect estimates because of the different splint types used, different patient diagnoses and the different minimal interventions being used as control groups.
There were no studies looking at tooth wear, and very few studies and a lack of useable other data for patients with bruxism, so we were unable to determine whether or not splints are effective in these patients. The quality of the evidence was therefore deemed to be very low for the reported outcomes.
The risk of bias of 5022–46,49–73 of the 52 studies was high. Although patient blinding is not possible when comparing oral splints with no splints or a minimal intervention, there were also problems with selective reporting bias and incomplete outcome data. The majority of the studies were assessed as having an unclear risk for selection bias, with researchers not reporting the trial methodology and data according to the Consolidated Standards of Reporting Trials (CONSORT). 121
Patient and public involvement
The project benefited from the establishment of a patient advisory group during the development of the application. At least one member of the patient advisory group attended each of the face-to-face meetings of the research team held in Manchester, and took part in most of the monthly teleconferences. On reflection, involving patients in discussions about the questions and outcomes for the protocol, and in the readability of the Plain English summary was helpful. It is more difficult to involve patients in the more detailed work of the effectiveness review, as this is a specialist methodological exercise with clinical involvement.
Economic modelling
A decision-analysis model was developed to fill a gap in the literature regarding the cost-effectiveness of splints. Although the project intended to evaluate cost-effectiveness separately for bruxism and TMD, it was possible to build a model for TMD only. There were insufficient data available to populate a model focused around degrees of tooth wear resulting from bruxism activity. A suggested model structure is provided in Appendix 5, and further research is required to refine the structure and populate the model. Further research will be required to determine the long-term care pathway for bruxism patients, the long-term impact of bruxing on tooth wear and treatment need, the effectiveness of splints, and the costs and utilities of different bruxism health states.
The TMD model was populated using the best-available evidence from a UK decision-making perspective. A systematic approach was taken to search for the clinical effectiveness data. Costs, utilities and transition probabilities were based on detailed cohort data from the DEEP study, a UK cohort study on people with TMD.
There was a lack of available long-term data to inform the economic model, particularly with regards to the long-run transition probabilities beyond 2 years. The effects of splints on pain are a particular area of uncertainty, especially over longer time periods beyond 3 months. This uncertainty is evident in the model results, with VOI analysis being used to inform further research priorities
One important limitation of the economic analysis is that there was no RR data available from the clinical effectiveness review to inform the economic model. To populate the model, structured around tertiles of pain, it was necessary to make assumptions about the probable RR obtained from a range of plausible MDs observed in the clinical effectiveness review. The approach taken adds substantial additional uncertainty to the results. Although the resulting data behave in a manner that is encouraging for face validity, further work is required to determine the true RR of health-state occupation. The results provided by the economic analysis are subject to the same limitations of the clinical effectiveness data described previously.
This project initially set out to also compare custom-made with prefabricated splints. However, the majority of studies in the clinical effectiveness review used custom-made splints in their comparison, meaning that a robust assessment of the cost-effectiveness of custom-made versus prefabricated splints was not possible.
Chapter 6 Conclusions
Parts of this chapter have been adapted from Riley et al. 1 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original text.
Implications for health care
From this systematic review, there is no clear evidence to support the provision of splints for the various subtypes of TMD or bruxism. However, the body of evidence that this conclusion is based on is of very low quality. The studies included in this review differed in three important factors: (1) diagnoses, (2) splint type and (3) outcome measurement/reporting. This made it difficult to draw clear and definitive conclusions. We addressed these problems by performing sensitivity analyses to investigate the effects of the three factors on the results, but this resulted in a decrease in the numbers of studies and patients available with which to perform further analyses. We were still unable to demonstrate that splints reduce pain in the study participants.
With regard to cost-effectiveness, there was no published evidence to determine the most efficient allocation of resources. Our decision-analysis model identifies important drivers of cost-effectiveness and highlights the need for future research, but definitive statements regarding cost-effectiveness cannot be made as a result of the limited evidence on clinical effectiveness, identified in Chapter 3, Results of the systematic review.
Recommendations for future research
Further research is urgently needed to determine whether or not the use of splints is clinically effective, generates meaningful patient benefit and whether or not splints offer an efficient use of scarce NHS resources for both bruxism and TMD. There is a need for well-conducted RCTs involving both TMD and bruxism patients. These trials should compare oral splints with an agreed minimal intervention such as advice/counselling, education or self-performed exercises (applied to both the intervention and control groups). Multiple trials will be required to answer questions about patients with different subtypes of TMD. The selection of patients for inclusion in these studies should, ideally, conform to the DC/TMD diagnostic guidelines to ensure that patients have well-defined conditions and are a homogeneous group. Trials should be conducted in those settings that reflect the current provision of splints provided in the NHS. Triallists should carefully report the data for the patients included in each TMD subgroup separately, to ensure that the data can be pooled for each subgroup in future meta-analyses.
The results of the EVPI analysis indicate that there is substantial decision uncertainty and that future research is worthwhile. The EVPPI analysis identified two important areas of future research to reduce decision uncertainty with regards to cost-effectiveness, as well as two areas of research in which further research is unlikely to reduce decision uncertainty. The priority areas for further research are:
-
The treatment effectiveness of splints and, consequently, that of custom-made versus prefabricated splints. Future economic evaluations should also include provision for extended follow-up to resolve longer-term decision uncertainty in pain trajectory and clinical effectiveness.
-
Further data are required to determine the long-term costs to the NHS of different types of splint provision. This includes generating evidence regarding the appropriate banding for different splint types on the NHS in England, as well as determining the frequency at which splints will require replacement if rolled out to TMD patients.
The VOI analysis indicated that further research to determine the costs or utilities associated with pain states would not be worthwhile, as current evidence obtained from the DEEP study94 is sufficient to aid decision-making.
Acknowledgements
We would like to thank Anne Littlewood for undertaking the comprehensive searches for the systematic review. We would like to acknowledge the help from the patient and public involvement members, Mrs Lear, Mrs Coldrick and Mrs Palmer, and for the support of Ruth Floate in writing the Plain English summary. Our thanks go to Sarah Faulkner for her clarifications on splint types and materials. The authors would also like to thank Janet Lear for her invaluable assistance in providing administrative support and for preparing and formatting the report. We are very grateful to Matthew Byrne and James Darcey for their clinically focused feedback on the first draft of the review.
Contributions of authors
Philip Riley (https://orcid.org/0000-0003-3902-6112) (Research Fellow) contributed to the published protocol, conducted the effectiveness review and contributed to writing the report.
Anne-Marie Glenny (https://orcid.org/0000-0003-4037-2899) (Professor of Health Science Research) contributed to the published protocol, provided methodological advice and undertook data extraction for the effectiveness review, and contributed to the report.
Helen V Worthington (https://orcid.org/0000-0002-4851-7469) (Professor of Evidence-based Care) led the project, contributed to the published protocol, provided methodological advice and undertook data extraction for the effectiveness review, and drafted the report.
Elisabet Jacobsen (https://orcid.org/0000-0002-3211-936X) (Health Economist) conducted the cost-effectiveness evaluation and drafted the cost-effectiveness section of the report.
Clare Robertson (https://orcid.org/0000-0001-6019-6795) (Research Fellow) contributed to the published protocol, provided methodological advice and undertook data extraction for the effectiveness review, and contributed to the report.
Justin Durham (https://orcid.org/0000-0002-5968-1969) (Professor of Orofacial Pain) contributed to the published protocol, provided clinical advice throughout the project, examined the diagnostic criteria for the studies relating to TMD, undertook extra analysis of his DEEP study data to inform the economic modelling and contributed to the report.
Stephen Davies (https://orcid.org/0000-0001-9821-6798) (Lead Clinician, Temporomandibular Clinic) contributed to the published protocol and provided clinical advice throughout the project.
Helen Petersen (https://orcid.org/0000-0003-1845-8573) (Consultant and Honorary Senior Lecturer in Oral Surgery) provided clinical advice throughout the project and undertook some data extraction for the effectiveness review.
Dwayne Boyers (https://orcid.org/0000-0002-9786-8118) (Health Economist) contributed to the published protocol, led the cost-effectiveness evaluation and contributed to writing the report.
Publication
Riley P, Glenny AM, Worthington HV, Jacobsen E, Robertson C, Durham J, et al. Oral splints for temporomandibular disorder or bruxism: a systematic review. Br Dent J 2020;228:191–7.
Data-sharing statement
Further data from the systematic review can be obtained from the corresponding author on request.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Riley P, Glenny AM, Worthington HV, Jacobsen E, Robertson C, Durham J, et al. Oral splints for temporomandibular disorder or bruxism: a systematic review. Br Dent J 2020;228:191-7.
- National Institute of Dental and Craniofacial Research . Prevalence of TMJD and Its Signs and Symptoms n.d. www.nidcr.nih.gov/datastatistics/finddatabytopic/facialpain/prevalencetmjd.htm (accessed March 2019).
- Aggarwal VR, Joughin A, Zakrzewska J, Appelbe P, Tickle M. Dentists’ preferences for diagnosis, management and referral of chronic oro-facial pain: results from a national survey. Health Educ J 2012;71:662-9. https://doi.org/10.1177/0017896911419350.
- Lobbezoo F, Ahlberg J, Glaros AG, Kato T, Koyano K, Lavigne GJ, et al. Bruxism defined and graded: an international consensus. J Oral Rehabil 2013;40:2-4. https://doi.org/10.1111/joor.12011.
- Manfredini D, Winocur E, Guarda-Nardini L, Paesani D, Lobbezoo F. Epidemiology of bruxism in adults: a systematic review of the literature. J Orofac Pain 2013;27:99-110. https://doi.org/10.11607/jop.921.
- Lobbezoo F, Ahlberg J, Manfredini D, Winocur E. Are bruxism and the bite causally related?. J Oral Rehabil 2012;39:489-501. https://doi.org/10.1111/j.1365-2842.2012.02298.x.
- Marbach JJ, Raphael KG, Janal MN, Hirschkorn-Roth R. Reliability of clinician judgements of bruxism. J Oral Rehabil 2003;30:113-18. https://doi.org/10.1046/j.1365-2842.2003.01146.x.
- Klasser GD, Greene CS. Oral appliances in the management of temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;107:212-23. https://doi.org/10.1016/j.tripleo.2008.10.007.
- Clark GT, Minakuchi H, Laskin DM, Greene CS, Hylander WL. Temporomandibular Disorders: An Evidence-based Approach to Diagnosis and Treatment. Hanover Park, IL: Quintessence Publishing Co. Inc.; 2006.
- Wright EF, Wright EF. Manual of Temporomandibular Disorders. Ames, IA: Wiley-Blackwell; 2014.
- Wassell RW, Verhees L, Lawrence K, Davies S, Lobbezoo F. Over-the-counter (OTC) bruxism splints available on the Internet. Br Dent J 2014;216. https://doi.org/10.1038/sj.bdj.2014.452.
- Lickteig R, Lotze M, Kordass B. Successful therapy for temporomandibular pain alters anterior insula and cerebellar representations of occlusion. Cephalalgia 2013;33:1248-57. https://doi.org/10.1177/0333102413491028.
- American Academy of Dental Sleep Medicine . About Dental Sleep Medicine n.d. https://aadsm.org (accessed 9 October 2019).
- Abstract Archives . Welcome to the IADR Abstract Archive n.d. https://iadr.abstractarchives.com/home (accessed 9 October 2019).
- The Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions n.d. https://training.cochrane.org/handbook (accessed 24 October 2019).
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. https://doi.org/10.1136/bmj.315.7109.629.
- Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 1992;6:301-55.
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. J Oral Facial Pain Headache 2014;28:6-27. https://doi.org/10.11607/jop.1151.
- De Leeuw R, Klasser GD. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management. Hanover Park, IL: Quintessence Publishing Co. Inc.; 2013.
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328. https://doi.org/10.1136/bmj.328.7454.1490.
- GRADEPro . GRADE Your Evidence and Improve Your Guideline Development in Health Care n.d. www.guidelinedevelopment.org (accessed 9 October 2019).
- Wassell RW, Adams N, Kelly PJ. Treatment of temporomandibular disorders by stabilising splints in general dental practice: results after initial treatment. Br Dent J 2004;197:35-41. https://doi.org/10.1038/sj.bdj.4811420.
- Tavera AT, Montoya MC, Calderón EF, Gorodezky G, Wixtrom RN. Approaching temporomandibular disorders from a new direction: a randomized controlled clinical trial of the TMDes ear system. Cranio 2012;30:172-82. https://doi.org/10.1179/crn.2012.027.
- Alencar F, Becker A. Evaluation of different occlusal splints and counselling in the management of myofascial pain dysfunction. J Oral Rehabil 2009;36:79-85. https://doi.org/10.1111/j.1365-2842.2008.01913.x.
- Conti PC, Miranda JE, Conti AC, Pegoraro LF, Araújo Cdos R. Partial time use of anterior repositioning splints in the management of TMJ pain and dysfunction: a one-year controlled study. J Appl Oral Sci 2005;13:345-50. https://doi.org/10.1590/S1678-77572005000400006.
- Conti PC, dos Santos CN, Kogawa EM, de Castro Ferreira Conti AC, de Araujo Cdos R. The treatment of painful temporomandibular joint clicking with oral splints: a randomized clinical trial. J Am Dent Assoc 2006;137:1108-14. https://doi.org/10.14219/jada.archive.2006.0349.
- Conti PC, de Alencar EN, da Mota Corrêa AS, Lauris JR, Porporatti AL, Costa YM. Behavioural changes and occlusal splints are effective in the management of masticatory myofascial pain: a short-term evaluation. J Oral Rehabil 2012;39:754-60. https://doi.org/10.1111/j.1365-2842.2012.02327.x.
- Conti PC, Corrêa AS, Lauris JR, Stuginski-Barbosa J. Management of painful temporomandibular joint clicking with different intraoral devices and counseling: a controlled study. J Appl Oral Sci 2015;23:529-35. https://doi.org/10.1590/1678-775720140438.
- Costa YM, Porporatti AL, Stuginski-Barbosa J, Bonjardim LR, Conti PC. Additional effect of occlusal splints on the improvement of psychological aspects in temporomandibular disorder subjects: a randomized controlled trial. Arch Oral Biol 2015;60:738-44. https://doi.org/10.1016/j.archoralbio.2015.02.005.
- de Felício CM, Mazzetto MO, de Silva MA, Bataglion C, Hotta TH. A preliminary protocol for multi-professional centers for the determination of signs and symptoms of temporomandibular disorders. Cranio 2006;24:258-64. https://doi.org/10.1179/crn.2006.041.
- de Felício CM, de Oliveira MM, da Silva MA. Effects of orofacial myofunctional therapy on temporomandibular disorders. Cranio 2010;28:249-59. https://doi.org/10.1179/crn.2010.033.
- Gomes CA, El Hage Y, Amaral AP, Politti F, Biasotto-Gonzalez DA. Effects of massage therapy and occlusal splint therapy on electromyographic activity and the intensity of signs and symptoms in individuals with temporomandibular disorder and sleep bruxism: a randomized clinical trial. Chiropr Man Therap 2014;22. https://doi.org/10.1186/s12998-014-0043-6.
- Gomes CA, El-Hage Y, Amaral AP, Herpich CM, Politti F, Kalil-Bussadori S, et al. Effects of massage therapy and occlusal splint usage on quality of life and pain in individuals with sleep bruxism: a randomized controlled trial. J Jpn Phys Ther Assoc 2015;18:1-6. https://doi.org/10.1298/jjpta.18.1.
- Zuim PR, Garcia AR, Turcio KH, Hamata MM. Evaluation of microcurrent electrical nerve stimulation (MENS) effectiveness on muscle pain in temporomandibular disorders patients. J Appl Oral Sci 2006;14:61-6. https://doi.org/10.1590/S1678-77572006000100012.
- Ekberg EC, Vallon D, Nilner M. Occlusal appliance therapy in patients with temporomandibular disorders. A double-blind controlled study in a short-term perspective. Acta Odontol Scand 1998;56:122-8. https://doi.org/10.1080/00016359850136102.
- Ekberg E, Vallon D, Nilner M. The efficacy of appliance therapy in patients with temporomandibular disorders of mainly myogenous origin. A randomized, controlled, short-term trial. J Orofac Pain 2003;17:133-9.
- Johansson A, Wenneberg B, Wagersten C, Haraldson T. Acupuncture in treatment of facial muscular pain. Acta Odontol Scand 1991;49:153-8. https://doi.org/10.3109/00016359109005900.
- List T, Helkimo M, Andersson S, Carlsson GE. Acupuncture and occlusal splint therapy in the treatment of craniomandibular disorders. Part I. A comparative study. Swed Dent J 1992;16:125-41.
- Lundh H, Westesson PL, Kopp S, Tillström B. Anterior repositioning splint in the treatment of temporomandibular joints with reciprocal clicking: comparison with a flat occlusal splint and an untreated control group. Oral Surg Oral Med Oral Pathol 1985;60:131-6. https://doi.org/10.1016/0030-4220(85)90280-4.
- Lundh H, Westesson PL, Jisander S, Eriksson L. Disk-repositioning onlays in the treatment of temporomandibular joint disk displacement: comparison with a flat occlusal splint and with no treatment. Oral Surg Oral Med Oral Pathol 1988;66:155-62. https://doi.org/10.1016/0030-4220(88)90084-9.
- Lundh H, Westesson PL, Eriksson L, Brooks SL. Temporomandibular joint disk displacement without reduction. Treatment with flat occlusal splint versus no treatment. Oral Surg Oral Med Oral Pathol 1992;73:655-8. https://doi.org/10.1016/0030-4220(92)90003-9.
- Magnusson T, Syrén M. Therapeutic jaw exercises and interocclusal appliance therapy. A comparison between two common treatments of temporomandibular disorders. Swed Dent J 1999;23:27-3.
- Nilsson H, Limchaichana N, Nilner M, Ekberg EC. Short-term treatment of a resilient appliance in TMD pain patients: a randomized controlled trial. J Oral Rehabil 2009;36:547-55. https://doi.org/10.1111/j.1365-2842.2009.01973.x.
- Wahlund K, List T, Larsson B. Treatment of temporomandibular disorders among adolescents: a comparison between occlusal appliance, relaxation training, and brief information. Acta Odontol Scand 2003;61:203-11. https://doi.org/10.1080/00016350310003891.
- DeVocht JW, Goertz CM, Hondras MA, Long CR, Schaeffer W, Thomann L, et al. A pilot study of a chiropractic intervention for management of chronic myofascial temporomandibular disorder. J Am Dent Assoc 2013;144:1154-63. https://doi.org/10.14219/jada.archive.2013.0034.
- Pierce CJ, Gale EN. A comparison of different treatments for nocturnal bruxism. J Dent Res 1988;67:597-601. https://doi.org/10.1177/00220345880670031501.
- Raphael KG, Marbach JJ. Widespread pain and the effectiveness of oral splints in myofascial face pain. J Am Dent Assoc 2001;132:305-16. https://doi.org/10.14219/jada.archive.2001.0173.
- Rubinoff MS, Gross A, McCall WD. Conventional and nonoccluding splint therapy compared for patients with myofascial pain dysfunction syndrome. Gen Dent 1987;35:502-6.
- Sharma AR. Short Term Efficacy of Appliance and Physical Therapy for the Treatment of Bilateral Masseter Myalgia: A Double Blinded Randomized Clinical Trial 2016.
- Truelove E, Huggins KH, Mancl L, Dworkin SF. The efficacy of traditional, low-cost and nonsplint therapies for temporomandibular disorder: a randomized controlled trial. J Am Dent Assoc 2006;137:1099-107. https://doi.org/10.14219/jada.archive.2006.0348.
- Wright E, Anderson G, Schulte J. A randomized clinical trial of intraoral soft splints and palliative treatment for masticatory muscle pain. J Orofac Pain 1995;9:192-9.
- Hasanoglu Erbasar GN, Alpaslan C, Eroglu Inan G. Can an NTI-tss device be effective as a first-line therapy in patients with TMD myofascial pain?. J Oral Rehabil 2017;44:589-93. https://doi.org/10.1111/joor.12524.
- Karakis D, Dogan A, Bek B. Evaluation of the effect of two different occlusal splints on maximum occlusal force in patients with sleep bruxism: a pilot study. J Adv Prosthodont 2014;6:103-8. https://doi.org/10.4047/jap.2014.6.2.103.
- Tatli U, Benlidayi ME, Ekren O, Salimov F. Comparison of the effectiveness of three different treatment methods for temporomandibular joint disc displacement without reduction. Int J Oral Maxillofac Surg 2017;46:603-9. https://doi.org/10.1016/j.ijom.2017.01.018.
- Amin A, Meshramkar R, Lekha K. Comparative evaluation of clinical performance of different kind of occlusal splint in management of myofascial pain. J Indian Prosthodont Soc 2016;16:176-81. https://doi.org/10.4103/0972-4052.176521.
- Katyayan PA, Katyayan MK, Shah RJ, Patel G. Efficacy of appliance therapy on temporomandibular disorder related facial pain and mandibular mobility: a randomized controlled study. J Indian Prosthodont Soc 2014;14:251-61. https://doi.org/10.1007/s13191-013-0320-4.
- Daif ET. Correlation of splint therapy outcome with the electromyography of masticatory muscles in temporomandibular disorder with myofascial pain. Acta Odontol Scand 2012;70:72-7. https://doi.org/10.3109/00016357.2011.597776.
- Elsharkawy TM, Ali NM. Evaluation of acupuncture and occlusal splint therapy in the treatment of temporomandibular joint disorders. Egypt Dent J 1995;41:1227-32.
- Yu CH, Qian HX. Evaluation of short term efficacy of the stabilized splint and the combination of manipulative and physical therapies for temporomandibular joint disc displacement without reduction. [Chinese. J Shanghai Jiaotong Univ (Sci) 2016;36:850-5.
- Zhang FY, Wang XG, Dong J, Zhang JF, Lü YL. Effect of occlusal splints for the management of patients with myofascial pain: a randomized, controlled, double-blind study. Chin Med J 2013;126:2270-5.
- Ficnar T, Middelberg C, Rademacher B, Hessling S, Koch R, Figgener L. Evaluation of the effectiveness of a semi-finished occlusal appliance – a randomized, controlled clinical trial. Head Face Med 2013;9. https://doi.org/10.1186/1746-160X-9-5.
- Giannakopoulos NN, Katsikogianni EN, Hellmann D, Eberhard L, Leckel M, Schindler HJ, et al. Comparison of three different options for immediate treatment of painful temporomandibular disorders: a randomized, controlled pilot trial. Acta Odontol Scand 2016;74:480-6. https://doi.org/10.1080/00016357.2016.1204558.
- Leeson RMA. A Comparison of Medical and Physical Therapies in the Management of Facial Arthromyalgia (Temporomandibular Joint Dysfunction) 2007.
- Michelotti A, Iodice G, Vollaro S, Steenks MH, Farella M. Evaluation of the short-term effectiveness of education versus an occlusal splint for the treatment of myofascial pain of the jaw muscles. J Am Dent Assoc 2012;143:47-53. https://doi.org/10.14219/jada.archive.2012.0018.
- Rampello A, Saccucci M, Falisi G, Panti F, Polimeni A, Di Paolo C. A new aid in temporomandibular joint disorders’ therapy: the universal neuromuscular immediate relaxing appliance. J Biol Regul Homeost Agents 2013;27:1011-19.
- Haketa T, Kino K, Sugisaki M, Takaoka M, Ohta T. Randomized clinical trial of treatment for TMJ disc displacement. J Dent Res 2010;89:1259-63. https://doi.org/10.1177/0022034510378424.
- Nagata K, Maruyama H, Mizuhashi R, Morita S, Hori S, Yokoe T, et al. Efficacy of stabilisation splint therapy combined with non-splint multimodal therapy for treating RDC/TMD axis I patients: a randomised controlled trial. J Oral Rehabil 2015;42:890-9. https://doi.org/10.1111/joor.12332.
- Dao TT, Lavigne GJ, Charbonneau A, Feine JS, Lund JP. The efficacy of oral splints in the treatment of myofascial pain of the jaw muscles: a controlled clinical trial. Pain 1994;56:85-94. https://doi.org/10.1016/0304-3959(94)90153-8.
- van der Zaag J, Lobbezoo F, Wicks DJ, Visscher CM, Hamburger HL, Naeije M. Controlled assessment of the efficacy of occlusal stabilization splints on sleep bruxism. J Orofac Pain 2005;19:151-8.
- Nitecka-Buchta A, Marek B, Baron S. CGRP plasma level changes in patients with temporomandibular disorders treated with occlusal splints – a randomised clinical trial. Endokrynol Pol 2014;65:217-23. https://doi.org/10.5603/EP.2014.0030.
- Niemelä K, Korpela M, Raustia A, Ylöstalo P, Sipilä K. Efficacy of stabilisation splint treatment on temporomandibular disorders. J Oral Rehabil 2012;39:799-804. https://doi.org/10.1111/j.1365-2842.2012.02335.x.
- Christidis N, Doepel M, Ekberg E, Ernberg M, Le Bell Y, Nilner M. Effectiveness of a prefabricated occlusal appliance in patients with temporomandibular joint pain: a randomized controlled multicenter study. J Oral Facial Pain Headache 2014;28:128-37. https://doi.org/10.11607/ofph.1216.
- Nilner M, Ekberg E, Doepel M, Andersson J, Selovuo K, Le Bell Y. Short-term effectiveness of a prefabricated occlusal appliance in patients with myofascial pain. J Orofac Pain 2008;22:209-18.
- Helkimo M. Studies on function and dysfunction of the masticatory system. II. Index for anamnestic and clinical dysfunction and occlusal state. Sven Tandlak Tidskr 1974;67:101-21.
- Fonseca DM, Bonfante G, Valle AL, de Freitas SFT. Diagnóstico pela anamnese da disfunção craniomandibular. Rev Gauch De Odontol 1994;4:23-32.
- Castroflorio T, Bargellini A, Lucchese A, Manuelli M, Casasco F, Cugliari G, et al. Effects of clear aligners on sleep bruxism: randomized controlled trial. J Biol Regul Homeost Agents 2018;32:21-9.
- Al Quran FA, Kamal MS. Anterior midline point stop device (AMPS) in the treatment of myogenous TMDs: comparison with the stabilization splint and control group. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:741-7. https://doi.org/10.1016/j.tripleo.2005.04.021.
- Alpaslan C, Kahraman S, Guner B, Cula S. Does the use of soft or hard splints affect the short-term outcome of temporomandibular joint arthrocentesis?. Int J Oral Maxillofac Surg 2008;37:424-7. https://doi.org/10.1016/j.ijom.2008.01.022.
- Gavish A, Winocur E, Ventura YS, Halachmi M, Gazit E. Effect of stabilization splint therapy on pain during chewing in patients suffering from myofascial pain. J Oral Rehabil 2002;29:1181-6. https://doi.org/10.1046/j.1365-2842.2002.00994.x.
- Madani AS, Mirmortazavi A. Comparison of three treatment options for painful temporomandibular joint clicking. J Oral Sci 2011;53:349-54. https://doi.org/10.2334/josnusd.53.349.
- Manfredini D, Favero L, Cocilovo F, Monici M, Guarda-Nardini L. A comparison trial between three treatment modalities for the management of myofascial pain of jaw muscles: a preliminary study. Cranio 2018;36:327-31. https://doi.org/10.1080/08869634.2017.1349571.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- Lincoff NS. Treatment of chronic facial pain. Curr Treat Options Neurol 2006;8:69-7. https://doi.org/10.1007/s11940-996-0025-7.
- Patel K, Hemmings KW, Vaughan S. The provision of occlusal splints in primary dental care. Prim Dent Care 2000;7:109-13. https://doi.org/10.1308/135576100322694196.
- Banhiran W, Kittiphumwong P, Assanasen P, Chongkolwatana C, Metheetrairut C. Adjustable thermoplastic mandibular advancement device for obstructive sleep apnea: outcomes and practicability. Laryngoscope 2014;124:2427-32. https://doi.org/10.1002/lary.24607.
- Wiedel AP. Fixed or removable appliance for early orthodontic treatment of functional anterior crossbite. Swed Dent J Suppl 2015;238:10-72.
- Yekkalam N, Wänman A. Factors associated with clinical decision-making in relation to treatment need for temporomandibular disorders. Acta Odontol Scand 2016;74:134-41. https://doi.org/10.3109/00016357.2015.1063159.
- Vos LM, Stant AD, Quik EH, Huddleston Slater JJR, Stegenga B. Cost effectiveness of arthrocentesis as initial treatment for temporomandibular joint arthralgia: a randomized controlled trial. Int J Oral Maxillofac Surg 2013;42. https://doi.org/10.1016/j.ijom.2013.07.710.
- Koyano K, Tsukiyama Y, Ichiki R, Kuwata T. Assessment of bruxism in the clinic. J Oral Rehabil 2008;35:495-508. https://doi.org/10.1111/j.1365-2842.2008.01880.x.
- Korthals-de Bos IB, Hoving JL, van Tulder MW, Rutten-van Mölken MP, Adèr HJ, de Vet HC, et al. Cost effectiveness of physiotherapy, manual therapy, and general practitioner care for neck pain: economic evaluation alongside a randomised controlled trial. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7395.911.
- Aggarwal VR, Macfarlane GJ, Farragher TM, McBeth J. Risk factors for onset of chronic oro-facial pain – results of the North Cheshire oro-facial pain prospective population study. Pain 2010;149:354-9. https://doi.org/10.1016/j.pain.2010.02.040.
- Macfarlane TV, Glenny AM, Worthington HV. Systematic review of population-based epidemiological studies of oro-facial pain. J Dent 2001;29:451-67. https://doi.org/10.1016/S0300-5712(01)00041-0.
- Durham J, Steele JG, Breckons M, Story W, Vale L. DEEP Study: does EQ-5D-5L measure the impacts of persistent oro-facial pain?. J Oral Rehabil 2015;42:643-50. https://doi.org/10.1111/joor.12296.
- Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain 1992;50:133-49. https://doi.org/10.1016/0304-3959(92)90154-4.
- Office for National Statistics . National Life Tables, UK: 2013 to 2015 n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/20132015 (accessed 10 October 2018).
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Durham J, Breckons M, Araujo-Soares V, Exley C, Steele J, Vale L. Developing Effective and Efficient care pathways in chronic Pain: DEEP study protocol. BMC Oral Health 2014;14. https://doi.org/10.1186/1472-6831-14-6.
- Durham J, Shen J, Breckons M, Steele JG, Araujo-Soares V, Exley C, et al. Healthcare cost and impact of persistent orofacial pain: the DEEP study cohort. J Dent Res 2016;95:1147-54. https://doi.org/10.1177/0022034516648088.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Welton NJ, Ades AE. Estimation of Markov chain transition probabilities and rates from fully and partially observed data: uncertainty propagation, evidence synthesis, and model calibration. Med Decis Making 2005;25:633-45. https://doi.org/10.1177/0272989X05282637.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.
- Steele J. NHS Dental Services in England: An Independent Review Led by Professor Jimmy Steele 2009. www.sigwales.org/wp-content/uploads/dh_101180.pdf (accessed October 2018).
- Lord J, Longworth L, Singh J, Onyimadu O, Fricke J, Bayliss S, et al. Oral Health Guidance – Economic Analysis of Oral Health Promotion Approaches for Dental Teams. Birmingham &Amp; Brunel Consortium External Assessment Centre (BBC EAC) 2015. www.nice.org.uk/guidance/ng30/documents/oral-health-promotion-approaches-for-dental-teams-health-economic-analysis2 (accessed October 2018).
- NHS Business Services Authority . NHS Payments to General Practice, England, 2015–16 n.d. https://www.nhsbsa.nhs.uk/search?aggregated_field=contract+payment (accessed October 2018).
- NHS Digital . NHS Dental Statistics for England – 2016–17 2017. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-dental-statistics/nhs-dental-statistics-for-england-2016-17 (accessed October 2018).
- Campbell Collaboration, EPPI Centre . CCEMG – EPPI-Centre Cost Converter 2016. http://eppi.ioe.ac.uk/costconversion/default.aspx (accessed April 2018).
- Tuffaha HW, Roberts S, Chaboyer W, Gordon LG, Scuffham PA. Cost-effectiveness and value of information analysis of nutritional support for preventing pressure ulcers in high-risk patients: implement now, research later. Appl Health Econ Health Policy 2015;13:167-79. https://doi.org/10.1007/s40258-015-0152-y.
- Slade GD. Epidemiology of temporomandibular joint disorders and related painful conditions. Molecular Pain 2014;10. https://doi.org/10.1186/1744-8069-10-S1-O16.
- Office for National Statistics . Population Estimates n.d. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates (accessed October 2018).
- Strong M, Brennan A, Oakley J. How to calculate value of information in seconds using ‘SAVI’, the Sheffield Accelerated Value of Information web app. Value Health 2015;18:A725-6. https://doi.org/10.1016/j.jval.2015.09.2759.
- Health Economics Research Unit, University of Aberdeen . Oral Splints for Orofacial Symptoms: An Evidence Synthesis n.d. www.abdn.ac.uk/heru/research/assessment-of-technologies/inuse-tech/oral-splints-for-orofacial-symptoms--an-/ (accessed 23 October 2019).
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105-21. https://doi.org/10.1016/j.jpain.2007.09.005.
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9-19. https://doi.org/10.1016/j.pain.2004.09.012.
- Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: version 1.0. Trials 2017;18. https://doi.org/10.1186/s13063-017-1978-4.
- Prinsen CA, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, et al. How to select outcome measurement instruments for outcomes included in a ‘Core Outcome Set’ – a practical guideline. Trials 2016;17. https://doi.org/10.1186/s13063-016-1555-2.
- Gurevitch J, Koricheva J, Nakagawa S, Stewart G. Meta-analysis and the science of research synthesis. Nature 2018;555:175-82. https://doi.org/10.1038/nature25753.
- Murad MH, Montori VM. Synthesizing evidence: shifting the focus from individual studies to the body of evidence. JAMA 2013;309:2217-18. https://doi.org/10.1001/jama.2013.5616.
- Manfredini D, Greene CS, Ahlberg J, De Laat A, Lobbezoo F, Klasser GD. Evidence-based dentistry or meta-analysis illness? A commentary on current publishing trends in the field of temporomandibular disorders and bruxism. J Oral Rehabil 2019;46:1-4. https://doi.org/10.1111/joor.12707.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- The Cochrane Library . How CENTRAL Is Created n.d. www.cochranelibrary.com/help/central-creation-details.html (accessed 22 October 2019).
- Healthcare Improvement Scotland, Scottish Intercollegiate Guidelines Network . Search Filters n.d. www.sign.ac.uk/search-filters.html (accessed 22 October 2019).
- Doepel M, Nilner M, Ekberg E, Vahlberg T, Bell Yl. Headache: short- and long-term effectiveness of a prefabricated appliance compared to a stabilization appliance. Acta Odontol Scand 2011;69:129-36. https://doi.org/10.3109/00016357.2010.538719.
- NHS Digital . 2: Disease and Related Disorders – A Report from the Adult and Dental Health Survey 2009 n.d. https://files.digital.nhs.uk/publicationimport/pub01xxx/pub01086/adul-dent-heal-surv-summ-them-the2-2009-rep4.pdf (accessed March 2019).
Appendix 1 Literature search strategies
Reproduced from Riley et al. 1 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original text.
Clinical effectiveness
Cochrane Central Register of Controlled Trials
Date range searched: inception to issue 9 2018.
Date searched: 1 October 2018.
Search strategy
#1 [mh ^’Occlusal adjustment’]
#2 [mh ^’Occlusal splints’]
#3 [mh ^’Orthodontic appliances’]
#4 ((occlusal or oral or temporomandibular or jaw* or mandib* or mouth* or bite* or TMJ or dental) near/5 splint*)
#5 ((dental or mouth or gum) next (guard* or shield*))
#6 (mouthguard* or gumguard* or nightguard* or gumshield* or ‘bite plane*’ or toothprotector*or ‘tooth protector*’)
#7 ‘splint therapy’
#8 ((oral or TMJ or orofacial) next appliance*)
#9 {or #1-#8}
#10 [mh ‘craniomandibular disorders’]
#11 [mh ^’facial pain’]
#12 [mh ^’facial neuralgia’]
#13 [mh ^’trigeminal neuralgia’]
#14 [mh ^arthralgia]
#15 [mh ^’temporomandibular joint’]
#16 #14 and #15
#17 [mh bruxism]
#18 (bruxism or (teeth near/5 grind*) or (teeth near/5 clench) or (jaw* near/5 clench) or (jaw* near/5 grind*))
#19 ((craniofacial or myofacial or myofascial or facial or orofacial) near/5 (pain* or syndrome*))
#20 (‘trigeminal neuralgia’ or ‘sphenopalatine neuralgia’ or ‘Costen* syndrome*’)
#21 ((‘temporomandibular joint’ or craniomandibular or jaw* or mandib*) near/5 (pain* or disorder* or dysfunction* or arthralgia or syndrome*))
#22 (TMD or TMJD or (TMJ near/3 (disorder* or dysfunction* or syndrome* or pain*))):ti,ab
#23 ((temporomandibular or jaw* or mandib*) near/5 (disk or disc) next displac*)
#24 #10 or #11 or #12 or #13 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#25 #9 and #24
MEDLINE (via OvidSP)
Date range searched: 1946 to 1 October 2018.
Date searched: 1 October 2018.
Search strategy
-
Occlusal adjustment/
-
Occlusal splints/
-
Orthodontic appliances/
-
((occlusal or oral or temporomandibular or jaw$ or mandib$ or mouth$ or bite$ or TMJ or dental) adj5 splint$).mp.
-
((dental or mouth or gum) adj (guard$ or shield$)).mp.
-
(mouthguard$ or gumguard$ or nightguard$ or gumshield$ or ‘bite plane$’ or toothprotector$ or ‘tooth protector$’).mp.
-
‘splint therapy’.mp.
-
((oral or TMJ or orofacial) adj appliance$).mp.
-
or/1-8
-
exp Craniomandibular disorders/
-
Facial pain/
-
Facial neuralgia/
-
Trigeminal neuralgia/
-
Arthralgia/ and temporomandibular joint/
-
exp bruxism/
-
(bruxism or (teeth adj5 grind$) or (teeth adj5 clench) or (jaw$ adj5 clench) or (jaw$ adj5 grind$)).mp.
-
((craniofacial or myofacial or myofascial or facial or orofacial) adj5 (pain$ or syndrome$)).mp.
-
(‘trigeminal neuralgia’ or ‘sphenopalatine neuralgia’ or ‘Costen$ syndrome$’).mp.
-
((‘temporomandibular joint’ or craniomandibular or jaw$ or mandib$) adj5 (pain$ or disorder$ or dysfunction$ or arthralgia or syndrome$)).mp.
-
(TMD or TMJD or (TMJ adj3 (disorder$ or dysfunction$ or syndrome$ or pain$))).ti,ab.
-
((temporomandibular or jaw$ or mandib$) adj5 (disk or disc) adj displac$).mp. 22. or/10-21
-
9 and 22
Cochrane search filter for MEDLINE Ovid
Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
or/1-8
-
exp animals/ not humans.sh.
-
9 not 10
EMBASE
Date range searched: 1980 to 1 October 2018.
Date searched: 1 October 2018.
Search strategy
-
Occlusal splint/
-
Orthodontic device/
-
((occlusal or oral or temporomandibular or jaw$ or mandib$ or mouth$ or bite$ or TMJ or dental) adj5 splint$).mp.
-
((dental or mouth or gum) adj (guard$ or shield$)).mp.
-
(mouthguard$ or gumguard$ or nightguard$ or gumshield$ or ‘bite plane$’ or toothprotector$ or ‘tooth protector$’).mp.
-
‘splint therapy’.mp.
-
((oral or TMJ or orofacial) adj appliance$).mp.
-
or/1-7
-
Temporomandibular joint disorder/
-
Face pain/
-
Trigeminus neuralgia/
-
Arthralgia/ and temporomandibular joint/
-
exp bruxism/
-
(bruxism or (teeth adj5 grind$) or (teeth adj5 clench) or (jaw$ adj5 clench) or (jaw$ adj5 grind$)).mp.
-
((craniofacial or myofacial or myofascial or facial or orofacial) adj5 (pain$ or syndrome$)).mp.
-
(‘trigeminal neuralgia’ or ‘sphenopalatine neuralgia’ or ‘Costen$ syndrome$’).mp.
-
((‘temporomandibular joint’ or craniomandibular or jaw$ or mandib$) adj5 (pain$ or disorder$ or dysfunction$ or arthralgia or syndrome$)).mp.
-
(TMD or TMJD or (TMJ adj3 (disorder$ or dysfunction$ or syndrome$ or pain$))).ti,ab.
-
((temporomandibular or jaw$ or mandib$) adj5 (disk or disc) adj displac$).mp. or/9-19
-
8 and 20
The above subject search was linked to adapted version of the Cochrane EMBASE Project filter for identifying RCTs in EMBASE Ovid (see online122 for information):
-
Randomized controlled trial/
-
Controlled clinical study/
-
Random$.ti,ab.
-
randomization/
-
intermethod comparison/
-
placebo.ti,ab.
-
(compare or compared or comparison).ti.
-
((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
-
(open adj label).ti,ab.
-
((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
-
double blind procedure/
-
parallel group$1.ti,ab.
-
(crossover or cross over).ti,ab.
-
((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 orpatient$1 or subject$1 or participant$1)).ti,ab.
-
(assigned or allocated).ti,ab.
-
(controlled adj7 (study or design or trial)).ti,ab.
-
(volunteer or volunteers).ti,ab.
-
trial.ti.
-
or/1-18
-
(exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
-
19 not 20
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost)
Date range searched: 1937 to 1 October 2018.
Date searched: 1 October 2018.
Search strategy
S22 S8 and S21
S21 S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20
S20 ((temporomandibular or jaw* or mandib*) N5 (disk or disc))
S19 (TMD or TMJD or (TMJ N3 (disorder* or dysfunction* or syndrome* or pain*)))
S18 ((‘temporomandibular joint’ or craniomandibular or jaw* or mandib*) N5 (pain* or disorder* or dysfunction* or arthralgia or syndrome*))
S17 (‘trigeminal neuralgia’ or ‘sphenopalatine neuralgia’ or ‘Costen* syndrome*’)
S16 ((craniofacial or myofacial or myofascial or facial or orofacial) N5 (pain* or syndrome*))
S15 (bruxism or (teeth N5 grind*) or (teeth N5 clench) or (jaw* N5 clench) or (jaw* N5 grind*))
S14 (MH bruxism+)
S13 (MH arthralgia) AND (MH ‘temporomandibular joint’)
S12 (MH ‘trigeminal neuralgia’)
S11 (MH ‘facial neuralgia’)
S10 (MH ‘facial pain’)
S9 (MH ‘craniomandibular disorders+’)
S8 S1 or S2 or S3 or S4 or S5 or S6 or S7
S7 ((oral or TMJ or orofacial) N1 appliance*)
S6 ‘splint therapy’
S5 ((dental or mouth or gum) N1 (mouthguard* or gumguard* or nightguard* or gumshield* or ‘bite plane*’ or toothprotector* or ‘tooth protector*’) guard* or shield*))
S4 ((dental or mouth or gum) N1 (guard* or shield*))
S3 ((occlusal or oral or temporomandibular or jaw* or mandib* or mouth* or bite* or TMJ or dental) N5 splint*)
S2 (MH ‘Orthodontic appliances’)
S1 (MH ‘Splints’)
The above subject search was linked to Cochrane Oral Health’s filter for identifying RCTs in CINAHL EBSCOhost:
S1 MH Random Assignment or MH Single-blind Studies or MH Double-blind Studies or MH Triple-blind Studies or MH Crossover design or MH Factorial Design
S2 TI (‘multicentre study’ or ‘multicenter study’ or ‘multi-centre study’ or ‘multi-center study’) or AB (‘multicentre study’ or ‘multicenter study’ or ‘multi-centre study’ or ‘multi-center study’) or SU (‘multicentre study’ or ‘multicenter study’ or ‘multi-centre study’ or ‘multi-center study’)
S3 TI random* or AB random*
S4 AB ‘latin square’ or TI ‘latin square’
S5 TI (crossover or cross-over) or AB (crossover or cross-over) or SU (crossover or cross-over)
S6 MH Placebos
S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*)
S8 TI blind* or AB mask* or AB blind* or TI mask*
S9 S7 and S8
S10 TI Placebo* or AB Placebo* or SU Placebo*
S11 MH Clinical Trials
S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial)
S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
ProQuest Dissertations & Theses
Date range searched: no restriction.
Date searched: 1 October 2018.
Search strategy
all((splint or guard or shield or mouthguard or gumguard or gumshield or mouthshield or ‘tooth protector’ or orthodontic)) AND all((‘temporomandibular joint’ or TMD or TMJD or ‘facial pain’ or (face and pain) or bruxism))
Conference Proceedings Citation Index (via Web of Science)
Date range searched: no restriction.
Date searched: 1 October 2018.
Search strategy
# 15 #6 and #14
# 14 #7 or #8 or #9 or #10 or #11 or #12 or #13
# 13 TS=((temporomandibular or jaw* or mandib*) AND (disk or disc))
# 12 TS=(TMJ AND (disorder* or dysfunction* or syndrome* or pain*))
# 11 TS=(TMD or TMJD)
# 10 TS=((‘temporomandibular joint’ or craniomandibular or jaw* or mandib*) AND (pain* or disorder* or dysfunction* or arthralgia or syndrome*))
# 9 TS=(‘trigeminal neuralgia’ or ‘sphenopalatine neuralgia’ or ‘Costen* syndrome*’)
# 8 TS=((craniofacial or myofacial or myofascial or facial or orofacial) AND (pain* or syndrome*))
# 7 TS=(bruxism or (teeth and grind*) or (teeth and clench) or (jaw* and clench) or (jaw* and grind*))
# 6 #1 or #2 or #3 or #4 or #5
# 5 TS=((oral or TMJ or orofacial) AND appliance*)
# 4 TS=‘splint therapy’
# 3 TS=((dental or mouth or gum) and (guard* or shield*))
# 2 TS=(mouthguard* or gumguard* or nightguard* or gumshield* or ‘bite plane*’ or toothprotector or ‘tooth protector*)
# 1 TS=((occlusal or oral or temporomandibular or jaw* or mandib* or mouth* or bite* or TMJ or dental) AND splint*)
US National Institutes of Health trials registry (ClinicalTrials.gov)
Date range searched: no restriction.
Date searched: 1 October 2018.
Search strategy
Condition: temporomandibular joint disorder
Other terms: splint*
Condition: Facial pain
Other terms: splint*
World Health Organization International Clinical Trials Registry Platform
Date range searched: no restriction.
Date searched: 1 October 2018.
Search strategy
Condition: temporomandibular joint disorder
Intervention: splint*
Condition: face AND pain
Intervention: splint*
American Academy of Dental Sleep Medicine website
Date range searched: no restriction.
Date searched: 1 October 2018.
Search strategy
temporomandibular and splint
International Association of Dental Research conference abstracts
Date range searched: no restriction.
Date searched: 1 October 2018.
Search strategy
occlusal splint and temporomandibular
occlusal splint and pain
occlusal splint and bruxism
Cost-effectiveness
MEDLINE (via OvidSP)
Date range searched: 1946 to 1 October 2018.
Date searched: 1 October 2018.
Search strategy
-
Occlusal adjustment/
-
Occlusal splints/
-
Orthodontic appliances/
-
((occlusal or oral or temporomandibular or jaw$ or mandib$ or mouth$ or bite$ or TMJ or dental or ‘vacuum form$’) adj5 splint$).mp.
-
((dental or mouth or gum) adj (guard$ or shield$)).mp.
-
(mouthguard$ or gumguard$ or nightguard$ or gumshield$ or ‘bite plane$’ or toothprotector$ or ‘tooth protector$’).mp.
-
‘splint therapy’.mp.
-
((oral or TMJ or orofacial) adj appliance$).mp.
-
(‘bite rais$’ adj appliance$).mp.
-
or/1-9
-
exp Craniomandibular disorders/
-
Facial pain/
-
Facial neuralgia/
-
Trigeminal neuralgia/
-
Arthralgia/ and temporomandibular joint/
-
exp bruxism/
-
(bruxism or (teeth adj5 grind$) or (teeth adj5 clench) or (jaw$ adj5 clench) or (jaw$ adj5 grind$)).mp.
-
((craniofacial or myofacial or myofascial or facial or orofacial) adj5 (pain$ or syndrome$)).mp.
-
(‘trigeminal neuralgia’ or ‘sphenopalatine neuralgia’ or ‘Costen$ syndrome$’).mp.
-
((‘temporomandibular joint’ or craniomandibular or jaw$ or mandib$) adj5 (pain$ or disorder$ or dysfunction$ or arthralgia or syndrome$)).mp.
-
(TMD or TMJD or (TMJ adj3 (disorder$ or dysfunction$ or syndrome$ or pain$))).ti,ab.
-
((temporomandibular or jaw$ or mandib$) adj5 (disk or disc) adj displac$).mp.
-
or/11-22
-
10 and 23
The above search was linked to the economic studies filter used by the Scottish Intercollegiate Guidelines Network123 (an adaptation of the strategy designed by the NHS Centre for Reviews and Dissemination at the University of York).
-
Economics/
-
‘costs and cost analysis’/
-
Cost allocation/
-
Cost-benefit analysis/
-
Cost control/
-
Cost savings/
-
Cost of illness/
-
Cost sharing/
-
‘deductibles and coinsurance’/
-
Medical savings accounts/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
Exp economics, hospital/
-
Exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
Exp ‘fees and charges’/
-
Exp budgets/
-
(low adj cost).mp.
-
(high adj cost).mp.
-
(health?care adj cost$).mp.
-
(fiscal or funding or financial or finance).tw.
-
(cost adj estimate$).mp.
-
(cost adj variable).mp.
-
(unit adj cost$).mp.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
Or/1-32
EMBASE (via OvidSP)
Date range searched: 1980 to 1 October 2018.
Date searched: 1 October 2018.
Search strategy
-
Occlusal splint/Orthodontic device/((occlusal or oral or temporomandibular or jaw$ or mandib$ or mouth$ or bite$ or TMJ or dental or ‘vacuum form$’) adj5 splint$).mp.
-
((dental or mouth or gum) adj (guard$ or shield$)).mp.
-
(mouthguard$ or gumguard$ or nightguard$ or gumshield$ or ‘bite plane$’ or toothprotector$ or ‘tooth protector$’).mp.
-
‘splint therapy’.mp.
-
((oral or TMJ or orofacial) adj appliance$).mp.
-
(‘bite rais$’ adj appliance$).mp.
-
or/1-8
-
Temporomandibular joint disorder/
-
Face pain/
-
Trigeminus neuralgia/
-
Arthralgia/ and temporomandibular joint/
-
exp bruxism/
-
(bruxism or (teeth adj5 grind$) or (teeth adj5 clench) or (jaw$ adj5 clench) or (jaw$ adj5 grind$)).mp.
-
((craniofacial or myofacial or myofascial or facial or orofacial) adj5 (pain$ or syndrome$)).mp.
-
(‘trigeminal neuralgia’ or ‘sphenopalatine neuralgia’ or ‘Costen$ syndrome$’).mp.
-
((‘temporomandibular joint’ or craniomandibular or jaw$ or mandib$) adj5 (pain$ or disorder$ or dysfunction$ or arthralgia or syndrome$)).mp.
-
(TMD or TMJD or (TMJ adj3 (disorder$ or dysfunction$ or syndrome$ or pain$))).ti,ab.
-
((temporomandibular or jaw$ or mandib$) adj5 (disk or disc) adj displac$).mp
-
or/10-20
-
9 and 21
The above search was linked to the economic studies filter used by the Scottish Intercollegiate Guidelines Network123 (an adaptation of the strategy designed by the NHS Centre for Reviews and Dissemination at the University of York).
-
Socioeconomics/
-
Cost benefit analysis/
-
Cost effectiveness analysis/
-
Cost of illness/
-
Cost control/
-
Economic aspect/
-
Financial management/
-
Health care cost/
-
Health care financing/
-
Health economics/
-
Hospital cost/
-
(fiscal or financial or finance or funding).tw.
-
Cost minimization analysis/
-
(cost adj estimate$).mp.
-
(cost adj variable$).mp.
-
(unit adj cost$).mp.
-
Or/1-16
NHS EED Database (via OvidSP)
Date range searched: inception to issue 1 2016.
Date searched: 1 October 2018.
Search strategy
-
Splints/
-
(splint$ or guard$ or shield$ or mouthguard$ or gumguard$ or nightguard$ or gumshield$ or plane$ or ‘tooth protector$’ or toothprotector$ or ‘oral appliance$’ or ‘orofacial appliance$’ or ‘bite rais$ appliance$’).mp.
-
1 or 2
-
Temporomandibular Joint Disorders/
-
Facial pain/
-
(TMD or TMJD).mp.
-
(bruxism or (teeth and grind$) or (teeth and clench$) or (jaw and clench$) or (jaw and grind$)).mp.
-
((temporomandibular$ or (craniofacial or myofacial or myofascial or facial or orofacial or face)) adj5 (pain$ or syndrome$)).mp.
-
or/4-8
-
3 and 9
Cumulative Index to Nursing and Allied Health Literature (via EBSCOhost)
Date range searched: 1937 to 1 October 2018.
Date searched: 1 October 2018.
Search strategy
S22 S8 and S21
S21 S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20
S20 ((temporomandibular or jaw* or mandib*) N5 (disk or disc))
S19 (TMD or TMJD or (TMJ N3 (disorder* or dysfunction* or syndrome* or pain*)))
S18 ((‘temporomandibular joint’ or craniomandibular or jaw* or mandib*) N5 (pain* or disorder* or dysfunction* or arthralgia or syndrome*))
S17 (‘trigeminal neuralgia’ or ‘sphenopalatine neuralgia’ or ‘Costen* syndrome*’)
S16 ((craniofacial or myofacial or myofascial or facial or orofacial) N5 (pain* or syndrome*))
S15 (bruxism or (teeth N5 grind*) or (teeth N5 clench) or (jaw* N5 clench) or (jaw* N5 grind*))
S14 (MH bruxism+)
S13 (MH arthralgia) AND (MH ‘temporomandibular joint’)
S12 (MH ‘trigeminal neuralgia’)
S11 (MH ‘facial neuralgia’)
S10 (MH ‘facial pain’)
S9 (MH ‘craniomandibular disorders+’)
S8 S1 or S2 or S3 or S4 or S5 or S6 or S7
S7 ((oral or TMJ or orofacial or ‘bite rais*’) N1 appliance*)
S6 ‘splint therapy’
S5 ((dental or mouth or gum) N1 (mouthguard* or gumguard* or nightguard* or gumshield* or ‘bite plane*’ or toothprotector* or ‘tooth protector*’) guard* or shield*))
S4 ((dental or mouth or gum) N1 (guard* or shield*))
S3 ((occlusal or oral or temporomandibular or jaw* or mandib* or mouth* or bite* or TMJ or dental or ‘vacuum form’) N5 splint*)
S2 (MH ‘Orthodontic appliances’)
S1 (MH ‘Splints’)
The above search was linked to the economic studies filter used by the Scottish Intercollegiate Guidelines Network123 (an adaptation of the strategy designed by the NHS Centre for Reviews and Dissemination at the University of York).
S23 S20 NOT (S21 or S22)
S22 AU Anonymous
S21 SO Cochrane
S20 S18 NOT S19
S19 (MH ‘Animal Studies’)
S18 S13 NOT S17
S17 S14 or S15 or S16
S16 PT News
S15 PT Letter
S14 PT Editorial
S13 S11 or S12
S12 (cost or costs or economic* or pharmacoeconomic* or price* or pricing*)
S11 S7 or S10
S10 S8 OR S9
S9 SU health resource utilization
S8 SU health resource allocation
S7 S1 NOT S6
S6 S2 or S3 or S4 or S5
S5 (MH ‘Business+’)
S4 (MH ‘Financing, Organized+’)
S3 (MH ‘Financial Support+’)
S2 (MH ‘Financial Management+’)
S1 (MH ‘Economics+’)
Appendix 2 Additional data tables
| Outcome: pain | Assessment point (months) | Study | Splint, mean (SD); number of events, or number of events/total number analysed | No/minimal treatment, mean (SD); number of events | Result: MD/RR (95% CI) |
|---|---|---|---|---|---|
| Pain | |||||
| Catastrophizing Thoughts subscale of the Pain Related Self-Statement Scale. Self-reported questionnaire consisting of nine statements related to catastrophising thoughts involved in pain perception. Respondent asked to answer each statement indicating the frequency of thinking about pain during a pain crisis, on a 0–4 scale. The sum of all frequencies was divided by the total number of questions. Higher values demonstrate higher levels of pain catastrophising | 3–6 | Costa 201529 | 0.76 (0.82); n = 24 | 1.14 (1.28); n = 17 | MD –0.38 (–1.07 to 0.31); p = 0.28; favours splint |
| Presence of muscular pain (yes/no) | 0–3 | de Felício 200630 | 22/42 | 41/42 | RR 0.54 (0.40 to 0.72); p < 0.0001; favours splint |
| Current pain intensity using a 0 (no pain) to 10 (worst pain) NRS; reported as change from baseline score (unable to combine change score in primary meta-analysis using SMD; used in sensitivity analyses of studies reporting current pain intensity on VAS/NRS at 0–3 months and at 3–6 months) | 0–3 | DeVocht 201345 | 1.4 (2.3504); n = 20 | 0.7 (1.7575); n = 21 | MD 0.70 (–0.58 to 1.98); p = 0.28; favours splint |
| 3–6 | DeVocht 201345 | 2 (1.923); n = 20 | 1.7 (1.7575); n = 21 | MD 0.30 (–0.83 to 1.43); p = 0.6; favours splint | |
| Changes in pain assessed according to the following scale: impaired, unchanged, improved, symptom free (we dichotomised this as incidence of improved and symptom free) | 0–3 | Elsharkawy 199558 | 21/23 | 20/23 | RR 1.05 (0.86 to 1.29); p = 0.64; favours splint |
| Changes in facial pain and headache: reported as incidence of impaired, unchanged, improved, symptom free (we dichotomised the data to report the incidence of improved and symptom free) | 0–3 | Johansson 199137 | 13/15 | 2/15 | RR 6.50 (1.76 to 23.98); p = 0.005; favours splint |
| Number of painful muscle sites on palpation (out of 20 sites); 2 lb of pressure for extraoral muscles, 1 lb of pressure on the joints and intraoral muscles | 3–6 | Katyayan 201456 | 8.725 (3.088); n = 40 | 7.375 (3.2); n = 40 | MD 1.35 (–0.03 to 2.73); p = 0.05; favours control |
| TMJ pain as part of clinical dysfunction index Di (Helkimo74) categorised as none, mild and severe – we dichotomised as incidence of being pain free | 0–3 | Magnusson 199942 | 7/9 | 9/9 | RR 0.79 (0.54 to 1.16); p = 0.23; favours control |
| 3–6 | Magnusson 199942 | 8/9 | 9/9 | RR 0.89 (0.67 to 1.20); p = 0.46; favours control | |
| Muscle pain as part of clinical dysfunction index Di (Helkimo74) categorised as none, mild and severe – we dichotomised as incidence of being pain free | 0–3 | Magnusson 199942 | 4/9 | 5/9 | RR 0.80 (0.31 to 2.04); p = 0.64; favours control |
| 3–6 | Magnusson 199942 | 6/9 | 9/9 | RR 0.68 (0.42 to 1.10); p = 0.12; favours control | |
| Pain on movement as part of clinical dysfunction index Di (Helkimo74) categorised as none, mild and severe – we dichotomised as incidence of being pain free | 0–3 | Magnusson 199942 | 8/9 | 8/9 | RR 1.00 (0.72 to 1.39); p = 1; favours neither |
| 3–6 | Magnusson 199942 | 9/9 | 9/9 | RR 1.00 (0.82 to 1.22); p = 1; favours neither | |
| Spontaneous muscle pain and pain on chewing (chew bilaterally for 60 seconds on a stick of chewing gum) assessed separately using 0 mm (no pain) to 100 mm (worst pain) VAS; reported as change from baseline score (unable to combine change score in primary meta-analysis using SMD; used in sensitivity analyses of studies reporting current pain intensity on VAS/NRS at 0–3 months) | 0–3 | Michelotti 201264 | 2.798 (2.012); n = 18 | –11.289 (2.79); n = 23 | MD 14.09 (12.62 to 15.56); p = < 0.00001; favours control |
| Pain on palpation: number of extraoral muscle sites (0–16) | 0–3 | Truelove 200650 | Arm 1: custom splint
|
4.3 (4); n = 54 | MD 0.80 (–0.59 to 2.18); p = 0.26; favours control |
| 6–12 | Truelove 200650 | Arm 1: custom splint
|
4.5 (4.5); n = 48 | MD –0.66 (–2.14 to 0.82); p = 0.38; favours splint | |
| Pain on palpation: number of intraoral muscle sites (0–4) | 0–3 | Truelove 200650 | Arm 1: custom splint
|
2.5 (1.6); n = 54 | MD 0.40 (–0.11 to 0.91); p = 0.12; favours control |
| 6–12 | Truelove 200650 | Arm 1: custom splint
|
2.6 (1.4); n = 48 | MD 0.20 (–0.28 to 0.68); p = 0.41; favours control | |
| Pain on palpation: number of TMJ sites (0–4) | 0–3 | Truelove 200650 | Arm 1: custom splint
|
1.1 (1.3); n = 54 | MD 0.10 (–0.34 to 0.54); p = 0.67; favours control |
| 6–12 | Truelove 200650 | Arm 1: custom splint
|
0.8 (0.9); n = 48 | MD 0.15 (–0.17 to 0.47); p = 0.35; favours control | |
| Pressure pain threshold measured using a pressure algometer that applied pressure on the skin surface (scale/units of measurement not stated but higher score = better outcome): TMJ | 0–3 | Wahlund 200344 | 164.7 (51.5); n = 37 | 148.3 (53.8); n = 39 | MD 16.40 (–7.27 to 40.07); p = 0.17; favours splint |
| 3–6 | Wahlund 200344 | 158.4 (39.3); n = 37 | 161.3 (61.1); n = 39 | MD –2.90 (–25.88 to 20.08); p = 0.8; favours control | |
| Pressure pain threshold measured using a pressure algometer that applied pressure on the skin surface (scale/units of measurement not stated but higher score = better outcome): masticatory muscles | 0–3 | Wahlund 200344 | 334 (100.3); n = 37 | 295 (99.3); n = 39 | MD 39.00 (–5.90 to 83.90); p = 0.09; favours splint |
| 3–6 | Wahlund 200344 | 344.5 (100.7); n = 37 | 335 (94.3); n = 39 | MD 9.50 (–34.42 to 53.42); p = 0.67; favours splint | |
| Muscle pain threshold assessed with a pressure algometer on the anterior temporal muscle and on the superior and inferior areas of the masseter muscle (psi: higher score = better outcome) | 0–3 | Wright 199551 | 45.3 (12.7); n = 10 | 38.1 (22.8); n = 10 | MD 7.20 (–8.98 to 23.38); p = 0.38; favours splint |
| TMJ clicking | |||||
| Articular noise (yes/no) – ‘The predominant type of articular noise was a click (83.33% of cases)’ | 0–3 | de Felício 200630 | 25/42 | 37/42 | RR 0.68 (0.51 to 0.89); p = 0.005; favours splint |
| Assessed using printed 0 (absence of symptom) to 10 (worst severity) for the following four situations: (1) when waking up, (2) during chewing, (3) when speaking and (4) at rest. The score was then summed and is therefore a 0–40 scale | 0–3 | de Felício 201031 | 10.22 (8.11); n = 10 | 10.8 (6.68); n = 10 | MD –0.58 (–7.09 to 5.93); p = 0.86; favours splint |
| Measured using a 0–10 worsening NRS | 0–3 | Nagata 201567 | 1.856 (2.211); n = 96 | 1.5 (1.9565); n = 85 | 0.36 (–0.25 to 0.96); p = 0.25; favours control |
| Change in restricted mouth-opening | |||||
| Difficulty opening mouth (yes/no) | 0–3 | de Felício 200630 | 17/42 | 31/42 | RR 0.55 (0.36 to 0.83); p = 0.004; favours splint |
| Self-assessment of functional limitation of jaw using 0 (no limitation) to 100 (severe limitation) mm VAS | 0–3 | Hasanoglu 201752 | 12.1 (18); n = 20 | 19.2 (19.2); n = 20 | MD –7.10 (–18.63 to 4.43); p = 0.23; favours splint |
| Incidence of having difficulty in opening the mouth wide | 0–3 | Magnusson 199942 | 1/9 | 1/9 | RR 1.00 (0.07 to 13.64); p = 1; favours neither |
| 3–6 | Magnusson 199942 | 0/9 | 0/9 | Not estimable | |
| Frequency of headaches (secondary to pain-related TMD) | |||||
| Categorised as number having either infrequent/absent headache (< 1 day/month), frequent headache (1–14 days/month) or chronic headache (> 14 days/month) – we dichotomised the data as incidence of frequent or chronic headache | 3–6 | Costa 201529 | 18/24 | 11/17 | RR 1.16 (0.76 to 1.76); p = 0.49; favours control |
| Quality of life (including physical and emotional function) | |||||
| Pain-related disability (0–100 worsening scale) assessed using RDC/TMD Axis II biobehavioural questionnaire | 0–3 | Tatli 201754 | 14.9 (12.7); n = 40 | 17.7 (9.3); n = 40 | MD –2.80 (–7.68 to 2.08); p = 0.26; favours splint |
| 3–6 | Tatli 201754 | 4.9 (6.4); n = 40 | 4.1 (6.6); n = 40 | MD 0.80 (–2.05 to 3.65); p = 0.58; favours control | |
| Psychological status (0–4 worsening scale) assessed using RDC/TMD Axis II biobehavioural questionnaire | 0–3 | Tatli 201754 | 0.7 (0.4); n = 40 | 0.8 (0.4); n = 40 | MD –0.10 (–0.28 to 0.08); p = 0.26; favours splint |
| 3–6 | Tatli 201754 | 0.5 (0.3); n = 40 | 0.6 (0.3); n = 40 | MD –0.10 (–0.23 to 0.03); p = 0.26; favours splint | |
| Patient satisfaction | |||||
| 0 (not at all satisfied) to 10 (extremely satisfied) NRS | 0–3 | DeVocht 201345 | 7.2 (1.4); n = 20 | 4.9 (2.3); n = 21 | 2.30 (1.14 to 3.46); p = 0.0001; favours splint |
| Adherence to treatment | |||||
| Incidence of those not totally complying with postoperative instructions | 3–6 | Daif 201257 | 5/20 | Not applicable | Not applicable |
| Outcome | Assessment point (months) | Study | Custom splint: mean (SD); number of events | Prefabricated splint: mean (SD); number of events | Result: MD/RR (95% CI) |
|---|---|---|---|---|---|
| Pain | |||||
| Modified Symptom Severity Index (Mod-SSI) – 28 characters for each of three variables: intensity, frequency and pain duration. Average of the three variables obtained and final scores ranged from 0.035 to 1 (higher = worse) | 0–3 | Amin 201655 | Arm 1: hard splint
|
0.02 (0.13); n = 15 | MD 0.00 (–0.08 to 0.09); p = 0.91; favours neither |
| Muscular palpation (masseter, temporalis and pterygoid muscles) performed bilaterally with tight and constant pressure of 1500 g, classified on 0–3 scale (0, no pain; 1, verbally reported pain; 2, pain or discomfort followed by facial musculature contraction and 3, patient backed away or showed lacrimation) | 0–3 | Amin 201655 | Arm 1: hard splint
|
0 (0); n = 15 | Not estimable |
| Overall improvement in TMJ pain assessed by the patient on a six-point rating scale: 0 = symptom free; 1 = much better; 2 = better; 3 = unchanged; 4 = worse; 5 = much worse (we dichotomised as incidence of unchanged or worse/much worse) | 0–3 | Christidis 201472 | 4/21 | 9/23 | RR 0.49 (0.18 to 1.35); p = 0.17; favours custom splint |
| 3–6 | Christidis 201472 | 2/17 | 6/20 | RR 0.39 (0.09 to 1.70); p = 0.21; favours custom splint | |
| 6–12 | Christidis 201472 | 1/15 | 2/18 | RR 0.60 (0.06 to 5.99); p = 0.66; favours custom splint | |
| Incidence of 30% reduction in worst reported VAS pain | 0–3 | Nilner 200873 | 22/33 | 23/32 | RR 0.93 (0.67 to 1.28); p = 0.65; favours prefabricated splint |
| 3–6 | Nilner 200873 | 22/24 | 23/28 | RR 1.12 (0.90 to 1.38); p = 0.31; favours custom splint | |
| 6–12 | Nilner 200873 | 21/22 | 26/27 | RR 0.99 (0.88 to 1.11); p = 0.31; favours neither | |
| Incidence of 50% reduction in worst reported VAS pain | 0–3 | Nilner 200873 | 18/33 | 21/32 | RR 0.83 (0.56 to 1.24); p = 0.36; favours prefabricated splint |
| > 3–6 | Nilner 200873 | 21/24 | 22/28 | RR 1.11 (0.87 to 1.42); p = 0.39; favours custom splint | |
| > 6–12 | Nilner 200873 | 17/22 | 24/27 | RR 0.87 (0.67 to 1.13); p = 0.3; favours prefabricated splint | |
| Pain on palpation: number of extraoral muscle sites (0–16) | 0–3 | Truelove 200650 | 5.6 (5.4); n = 54 | 4.7 (4.1); n = 56 | MD 0.90 (–0.90 to 2.70); p = 0.33; favours prefabricated splint |
| > 6–12 | Truelove 200650 | 3.6 (4.1); n = 65 | 4.1 (4.4); n = 55 | MD –0.50 (–2.03 to 1.03); p = 0.52; favours custom splint | |
| Pain on palpation: number of intraoral muscle sites (0–4) | 0–3 | Truelove 200650 | 3 (1.4); n = 54 | 2.8 (1.6); n = 56 | MD 0.20 (–0.36 to 0.76); p = 0.48; favours prefabricated splint |
| > 6–12 | Truelove 200650 | 2.6 (1.6); n = 65 | 3 (1.4); n = 55 | MD –0.40 (–0.94 to 0.14); p = 0.14; favours custom splint | |
| Pain on palpation: number of TMJ sites (0–4) | 0–3 | Truelove 200650 | 1.3 (1.5); n = 54 | 1.1 (1.4); n = 56 | MD 0.20 (–0.34 to 0.74); p = 0.47; favours prefabricated splint |
| > 6–12 | Truelove 200650 | 0.9 (1.1); n = 65 | 1 (1); n = 55 | MD –0.10 (–0.48 to 0.28); p = 0.6; favours custom splint | |
| TMJ clicking | |||||
| On opening, closing or both | 0–3 | Truelove 200650 | 28/54 | 29/56 | RR 1.00 (0.70 to 1.44); p = 0.99; favours neither |
| 6–12 | Truelove 200650 | 37/65 | 25/55 | RR 1.25 (0.87 to 1.79); p = 0.22; favours prefabricated splint | |
| Frequency of headaches (secondary to pain-related TMD) | |||||
| During the preceding 6 months on a verbal scale, as follows: no headache; rarely; once a month; once a week; at least 15 times a month; continuous (we dichotomised this as once per week or more) | 0–3 | Nilner 200873 | 7/32 | 12/32 | RR 0.58 (0.26 to 1.29); p = 0.18; favours custom splint |
| > 3–6 | Nilner 200873 | 3/24 | 7/28 | RR 0.50 (0.15 to 1.72); p = 0.27; favours custom splint | |
| > 6–12 | Nilner 200873 | 2/22 | 7/27 | RR 0.35 (0.08 to 1.52); p = 0.16; favours custom splint | |
| Adherence to treatment | |||||
| Not clear what level of compliance (e.g. using splint all the time/majority of the time, etc.) | 0–3 | Truelove 200650 | 48/54 | 42/56 | RR 1.19 (0.99 to 1.42); p = 0.06; favours custom splint |
| 6–12 | Truelove 200650 | 47/65 | 17/55 | RR 2.34 (1.53 to 3.57); p < 0.0001; favours custom splint | |
| Outcome | Assessment point (months) | Study | Splint: mean (SD)/number of events | Control splint: mean (SD)/number of events | Result: MD/RR (95% CI) |
|---|---|---|---|---|---|
| Pain | |||||
| Modified Symptom Severity Index (Mod-SSI) – 28 characters for each of three variables: intensity, frequency and pain duration. Average of the three variables obtained and final scores ranged from 0.035 to 1 (higher = worse) | 0–3 | Alencar 200924 | Arm 1: hard splint
|
0.24 (0.24); n = 14 | MD 0.07 (–0.09 to 0.22); p = 0.4; favours control splint |
| Muscular palpation (masseter, temporalis and pterygoid muscles) performed bilaterally with tight and constant pressure of 1500 g, classified on 0–3 scale (0, no pain; 1, verbally reported pain; 2, pain or discomfort followed by facial musculature contraction and 3, patient backed away or showed lacrimation) | 0–3 | Alencar 200924 | Arm 1: hard splint
|
0.3 (0.7); n = 14 | MD 0.00 (–0.42 to 0.42); p = 1; favours neither |
| Incidence of > 30% reduction in current pain intensity on VAS | 0–3 | Dao 199468 | 13/18 | 15/19 | RR 0.91 (0.63 to 1.32); p = 0.64; favours control splint |
| Incidence of 50% reduction of worst pain on VAS | 0–3 | Ekberg 199835 | 11/30 | 6/30 | RR 1.83 (0.78 to 4.32); p = 0.17; favours splint |
| Current pain intensity assessed using a five-point verbal scale: 0 = no pain, 1 = slight pain, 2 = moderate pain, 3 = severe pain, 4 = very severe pain (we dichotomised as incidence of moderate to very severe pain) | 0–3 | Ekberg 199835 | 19/30 | 26/30 | RR 0.68 (0.53 to 0.88); p = 0.004; favours splint |
| Ekberg 200336 | 13/30 | 22/30 | |||
| Pain frequency assessed using a nine-point verbal scale: 0 = never, 1 = rarely, 2 = once a month, 3 = once every second week, 4 = once a week, 5 = twice a week, 6 = 3 or 4 times a week, 7 = daily, 8 = constantly (we dichotomised as incidence of once a week or more) | 0–3 | Ekberg 199835 | 19/30 | 25/30 | RR 0.68 (0.46 to 1.01); p = 0.06; favours splint |
| Ekberg 200336 | 8/30 | 16/30 | |||
| Decrease in pain at rest (yes/no) | 0–3 | Ekberg 199835 | 29/30 | 22/30 | RR 1.32 (1.05 to 1.65); p = 0.02; favours splint |
| Pain at rest (yes/no) | 0–3 | Ekberg 200336 | 7/30 | 13/30 | RR 0.54 (0.25 to 1.16); p = 0.11; favours splint |
| Decrease in pain during mandibular movements (yes/no) | 0–3 | Ekberg 199835 | 15/30 | 22/30 | RR 0.68 (0.45 to 1.04); p = 0.07; favours control splint |
| Pain during mandibular movements (yes/no) | 0–3 | Ekberg 200336 | 12/30 | 21/30 | RR 0.57 (0.35 to 0.94); p = 0.03; favours splint |
| Pain during non-guided mandibular movements (we dichotomised as incidence of pain during 2–4 movements) | 0–3 | Ekberg 199835 | 12/30 | 24/30 | RR 0.52 (0.34 to 0.80); p = 0.003; favours splint |
| Ekberg 200336 | 5/30 | 8/30 | |||
| Number of painful masticatory muscles on palpation (we dichotomised as incidence of ≥ 4 sites) | 0–3 | Ekberg 199835 | 20/30 | 24/30 | RR 0.73 (0.52 to 1.03); p = 0.07; favours splint |
| Ekberg 200336 | 13/30 | 22/30 | |||
| Degree of tenderness of masticatory muscles on palpation assessed using a four-point scale: 0 = no tenderness, 1 = tenderness reported by the patient, 2 = tenderness with a palpebral reflex, 3 = tenderness with a defence reaction (we dichotomised as incidence of scores 2 or 3) | 0–3 | Ekberg 199835 | 26/30 | 28/30 | RR 0.93 (0.82 to 1.05); p = 0.23; favours splint |
| Ekberg 200336 | 26/30 | 28/30 | |||
| Incidence of 30% reduction in worst pain VAS score | 0–3 | Nilsson 200943 | 22/40 | 17/40 | RR 1.29 (0.82 to 2.04); p = 0.27; favours splint |
| 6–12 | Nilsson 200943 | 19/40 | 14/40 | RR 1.36 (0.80 to 2.31); p = 0.26; favours splint | |
| Characteristic pain intensity (0 to 100 worsening scale: mean of three scales – current pain, worst and average in previous 6 months) | 3–6 | Nilsson 200943 | 19 (18); n = 32 | 29 (23); n = 25 | MD –10.00 (–20.96 to 0.96); p = 0.07; favours splint |
| 6–12 | Nilsson 200943 | 23 (18); n = 28 | 24 (21); n = 23 | MD –1.00 (–11.87 to 9.87); p = 0.86; favours splint | |
| Incidence of 30% reduction in characteristic pain intensity (described above) | 0–3 | Nilsson 200943 | 24/35 | 20/33 | RR 1.13 (0.79 to 1.61); p = 0.5; favours splint |
| 6–12 | Nilsson 200943 | 20/40 | 17/40 | RR 1.18 (0.73 to 1.89); p = 0.5; favours splint | |
| Frequency of pain reported categorically: we dichotomised the data as incidence of recurrent or persistent (vs. never/one-time experience) | 0–3 | Nilsson 200943 | 19/35 | 18/33 | RR 1.00 (0.64 to 1.54); p = 0.98; favours neither |
| 3–6 | Nilsson 200943 | 10/32 | 11/25 | RR 0.71 (0.36 to 1.40); p = 0.32; favours splint | |
| 6–12 | Nilsson 200943 | 7/28 | 10/23 | RR 0.57 (0.26 to 1.27); p = 0.17; favours splint | |
| Mean value of worst pain 0 (no pain) to 10 (worst pain) NRS (author informed us that pain was probably recorded daily for 2 weeks prior to the follow-up) | 0–3 | Raphael 200147 | 5.16 (2.5456); n = 32 | 6.13 (2.4498); n = 31 | MD –0.97 (–2.20 to 0.26); p = 0.12; favours splint |
| Pain on palpation using 2 lb of pressure: mean number of painful facial muscles (unclear how many muscles were palpated) | 0–3 | Raphael 200147 | 9.97 (5.4871); n = 32 | 10.94 (5.5678); n = 31 | MD –0.97 (–3.70 to 1.76); p = 0.49; favours splint |
| Tenderness on palpation scored 0 (no response) to 3 (retreat of head in anticipation and report of considerable pain on contact) on multiple regions and scores summed to obtain a palpation score (length of scale unclear) – change scores reported (mean decrease in score) | 0–3 | Rubinoff 198748 | 4.1 (4.4); n = 15 | 1.82 (4.51); n = 11 | MD 2.28 (–1.19 to 5.75); p = 0.2; favours splint |
| Change in restricted mouth-opening | |||||
| Maximal interincisal distance in mm – change scores reported (mean increase in score) | 0–3 | Rubinoff 198748 | 2.0 (4.8); n = 15 | 0.55 (2.98); n = 11 | MD 1.45 (–1.55 to 4.45); p = 0.34; favours splint |
| Frequency of headaches (secondary to pain-related TMD) | |||||
| Reported categorically at 6 and 12 months: we dichotomised the data as incidence of recurrent or persistent (vs. never/one-time experience) | 3–6 | Nilsson 200943 | 12/32 | 11/25 | RR 0.85 (0.45 to 1.60); p = 0.62; favours splint |
| 6–12 | Nilsson 200943 | 10/28 | 9/23 | RR 0.91 (0.45 to 1.86); p = 0.8; favours splint | |
| Quality of life (including physical and emotional function) | |||||
| Depression (20 questions) from subscales of the SCL-90-R – reported as incidence of normal, moderate or severe (we dichotmised as incidence of moderate to severe) | 3–6 | Nilsson 200943 | 11/32 | 7/25 | RR 1.23 (0.56 to 2.71); p = 0.61; favours control splint |
| 6–12 | Nilsson 200943 | 10/28 | 8/23 | RR 1.03 (0.49 to 2.17); p = 0.94; favours neither | |
| Somatisation (12 questions) from subscales of the SCL-90-R – reported as incidence of normal, moderate or severe (we dichotmised as incidence of moderate to severe) | 3–6 | Nilsson 200943 | 13/32 | 13/25 | RR 0.78 (0.44 to 1.37); p = 0.39; favours splint |
| 6–12 | Nilsson 200943 | 10/28 | 11/23 | RR 0.75 (0.39 to 1.44); p = 0.38; favours splint | |
| Average mood assessed using a 0 (best possible mood) to 10 (worst possible mood) scale | 0–3 | Raphael 200147 | 3.44 (2.2062); n = 32 | 4.23 (2.3941); n = 31 | MD –0.79 (–1.93 to 0.35); p = 0.17; favours splint |
| Depression (13 items) from subscales of the SCL-90-R (higher score = worse depression) (length of scale not reported but is 0–4 in other included studies) | 0–3 | Raphael 200147 | 1.5 (0.6223); n = 32 | 1.66 (0.6681); n = 31 | MD –0.16 (–0.48 to 0.16); p = 0.33; favours splint |
| Patient satisfaction | |||||
| Satisfied with treatment (yes/no) | 0–3 | Ekberg 200336 | 26/30 | 13/30 | RR 2.00 (1.30 to 3.08); p = 0.002; favours splint |
| Study | Results |
|---|---|
| Custom splint vs. prefabricated splint | |
| Christidis 201472 | There were no reported adverse events |
| Nilner 200873 | At the 6-month follow-up, one patient in the R group with a vertical overbite of –1 mm at baseline had an overbite of –3 mm. The patient was given a stabilisation appliance, and no further increase in vertical overbite could be registered at the 12-month follow-up |
| Truelove 200650 | No subjects reported an adverse effect with any of the treatments |
| Splint vs. no splint | |
| Haketa 201066 | No significant adverse effect was reported resulting from either treatment |
| Nitecka-Buchta 201470 | No complications or any unintended effects in either group |
| Tatli 201754 |
There were no adverse effects reported that were due to splint use. Both groups had arthrocentesis and sodium hyaluronate, and the adverse events, which were mild transient swelling of the TMJ area and transient hemifacial paralysis, were related to this Group 1 = arthrocentesis plus sodium hyaluronate (control); Group 2 = splint + arthrocentesis plus sodium hyaluronate Comment: there were no adverse effects reported that were due to splint use |
| Tavera 201223 | Treatment-related adverse events:
|
| Truelove 200650 | No subjects reported an adverse effect with any of the treatments |
| Wahlund 200344 | None of the patients in any of the treatment modes reported any major adverse effects |
| Wright 199551 |
There was insufficient evidence of a difference in occlusal contact changes: MD –0.60 (95% CI –1.48 to 0.28; p = 0.18) Splint: mean 1.3 (SD 1.1); n = 10 Control splint: mean 1.9 (SD 0.9); n = 10 |
Appendix 3 Characteristics and risk of bias of included studies
Parts of this appendix have been adapted from Riley et al. 1 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. This includes minor additions and formatting changes to the original text.
| Attribute | Study details |
|---|---|
| Alencar 200924 | |
| Characteristics | |
| Study details |
|
| Participants |
|
| Interventions |
Comparison: splint vs. control splint for TMD Group AGroup BGroup CDuration of treatment: 90 days |
| Outcomes |
Assessed at 7, 30, 60 and 90 days; we used the 90-day data for our 0–3 month analysis Primary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients to type of splint used |
Blinding of outcome assessment (detection bias):
|
Patient-assessed pain is subjective; unclear if objective assessment is really objective (muscular palpation) |
Incomplete outcome data (attrition bias):
|
|
Selective reporting (reporting bias):
|
Outcomes fully reported |
Other bias:
|
No apparent other bias |
| Amin 201655 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: Department of Prosthodontics and Department of Oral Medicine and Radiology, Sri Dharmasthala Manjunatheshwara (SDM) College of Dental Sciences and Hospital, Karnataka, India Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: ‘Nil’ Declarations/conflicts of interest: ‘There are no conflicts of interest’ |
| Participants |
Diagnosis: diagnosed with myofascial pain based on a standardised and complete clinical examination based on the RDC/TMD Duration since presenting condition began: not reported Age at baseline (years): not reported (inclusion was 18–65) Sex: not reported Number randomised: 45 (group A: 15; group B: 15; group C: 15) Number evaluated: 45 (group A: 15; group B: 15; group C: 15) – assuming no attrition but not clearly reported |
| Interventions |
Comparison: custom-made splint vs. prefabricated splint for TMD Group AGroup BGroup CDuration of treatment: 90 days |
| Outcomes |
Assessed at 7, 30, 60 and 90 days: we used the 90-day data for our 0–3 months’ analysis Primary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
‘Randomly assigned using randomization table’ Comment: appropriate method |
Allocation concealment (selection bias):
|
Not mentioned |
Blinding of participants and personnel (performance bias):
|
Unable to blind patients to type of splint used |
Blinding of outcome assessment (detection bias):
|
Patient-assessed pain is subjective; unclear if objective assessment is really objective (muscular palpation) |
Incomplete outcome data (attrition bias):
|
Assuming no attrition but not entirely clear |
Selective reporting (reporting bias):
|
Outcomes fully reported |
Other bias:
|
No apparent other bias |
| Christidis 201472 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Orofacial Pain and Jaw Function, Malmö University, Sweden; Section of Orofacial Pain and Jaw Function, Karolinska Institutet, Sweden; Department of Stomatognathic Physiology, University of Turku, Finland Number of centres: three Recruitment period: not reported, but conducted from October 2008 to December 2011 Sample size calculation: yes (not met) Funding: public (supported by Finska Lakaresallskapet – scientific organisation of Swedish-speaking physicians in Finland) Declarations/conflicts of interest: ‘no conflicts of interest. None of the authors have any commercial affiliation or financial interest in any of the appliances used in this study’ Authors provided unpublished data |
| Participants |
Diagnosis: a diagnosis of arthralgia or osteoarthritis of the TMJ according to RDC/TMD; self-assessed worst TMJ pain during the previous 6 months of at least 4 on a 0–10 (higher = worse) graded NRS; and duration of pain ≥ 3 months Duration since presenting condition began: group A: mean 57 weeks; group B: mean 40 weeks Age at baseline (years): group A (stabilisation appliance) – mean 41 (range 19–73); group B (prefabricated) – mean 40 (range 21–71) Sex: group A: 8% male; group B: 4% male Number randomised: 48 (group A: 24; group B: 24) Number evaluated: 10 weeks – 44 (group A: 21; group B: 23); 6 months – 37 (group A: 17; group B: 20); 12 months – 33 (group A: 15; group B: 18) |
| Interventions |
Comparison: custom-made splint vs. prefabricated splint for TMD Group AGroup BDuration of treatment: 12 months |
| Outcomes |
Assessed at 10 weeks, 6 months and 12 months: we used these in our 0–3 months’, 3–6 months’ and 6–12 months’ analyses Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
‘For each center, 16 consecutively numbered, opaque, sealed envelopes containing a note with the treatment (8 for each treatment) were made and placed in a larger envelope. For each patient, an independent person at each center randomly drew an envelope and handed it to Dentist B. This was repeated until 16 patients at each center were included’ Comment: adequate method (lottery) |
Allocation concealment (selection bias):
|
‘For each center, 16 consecutively numbered, opaque, sealed envelopes containing a note with the treatment (8 for each treatment) were made and placed in a larger envelope. For each patient, an independent person at each center randomly drew an envelope and handed it to Dentist B. This was repeated until 16 patients at each center were included’ Comment: the next assignment was adequately concealed from the person randomising patients |
Blinding of participants and personnel (performance bias):
|
Unable to blind patients to type of splint used |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘change in restricted mouth-opening’ which may be considered objective and was measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
31% attrition at 12 months (group A: 38%; group B: 25%). This could potentially bias the results |
Selective reporting (reporting bias):
|
No evidence of selective reporting |
Other bias:
|
No apparent other bias |
| Conti 200525 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: Orofacial Pain Clinic at Bauru Dental School, University of São Paulo, Brazil Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: public (CAPES – Brazilian Government) Declarations/conflicts of interest: not reported We emailed authors for data but none provided so far |
| Participants |
Diagnosis: presence of TMJ disc displacement with reduction and chief complaint of pain in the joint followed by positive TMJ tenderness to manual palpation, accompanied or not by muscle symptoms. The presence of at least a clicking joint during opening, eliminated on opening in protrusion, was also an inclusion criterion Duration since presenting condition began: not reported Age at baseline (years): group A (stabilisation splint) – mean 32.7; group B (repositioning splint) – mean 31.4; group C (no treatment) – mean 31.1 Sex: not reported Number randomised: 60 Number evaluated: 52 |
| Interventions |
Comparison: splint vs. no splint for TMD Group AGroup BGroup CDuration of treatment: 12 months |
| Outcomes |
Assessed at 1 and 2 weeks and at 1, 3, 6 and 12 months: we would have used the the 3-, 6- and 12-month data in our 0–3 months’, 3–6 months’ and 6–12 months’ analyses, respectively Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
‘Subjects were randomly located into one of the following groups’ Comment: insufficient information |
Allocation concealment (selection bias):
|
‘Subjects were randomly located into one of the following groups’ Comment: insufficient information |
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘TMJ clicking’ and ‘change in restricted mouth-opening’, which may be considered objective and were measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
Numbers per group at randomisation and assessment points were not reported |
Selective reporting (reporting bias):
|
Results very poorly reported with very limited data for all outcomes |
Other bias:
|
Level of reporting extremely poor so unable to assess this |
| Conti 200626 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: Orofacial Pain Centre, Bauru Dental School, University of São Paulo, Brazil Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: public [supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq BRAZIL) grant number 14164312000–5] Declarations/conflicts of interest: not reported We emailed authors for data but none provided so far |
| Participants |
Diagnosis: RDC/TMD – subjects who met the diagnosis criteria for group II (disk displacement) and group IIIa (arthralgia) Duration since presenting condition began: TMJ pain for at least 3 months Age at baseline (years): group A – mean 28.9; group B – mean 31.3; group C – mean 29.5 Sex: 8% male (not reported by group) Number randomised: 60 (not reported by group) Number evaluated: 57 (not reported by group) |
| Interventions |
Comparison: splint versus control splint for TMD Group AGroup BGroup CDuration of treatment: 6 months |
| Outcomes |
Assessed at 1 and 2 weeks and at 1, 3 and 6 months: we would have used the 3- and 6-month data in our 0–3 months’ and 3–6 months’ analyses, respectively Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
‘We used a table generated by a computer to perform the randomization’ Comment: appropriate method |
Allocation concealment (selection bias):
|
‘We used a table generated by a computer to perform the randomization’ Comment: insufficient information |
Blinding of participants and personnel (performance bias):
|
Unable to blind patients to type of splint used |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘TMJ clicking’, which may be considered objective and was measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
Only three patients dropped out so unlikely to affect the results |
Selective reporting (reporting bias):
|
Data not reported in full for any outcome |
Other bias:
|
Poorly reported so difficult to assess |
| Conti 201227 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: Bauru School of Dentistry, University of São Paulo, Brazil Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: public [supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil] Declarations/conflicts of interest: ‘The authors declare that they have no conflicts of interest’ We emailed authors for data but none provided so far |
| Participants |
Diagnosis: RDC/TMD – myofascial pain with or without jaw opening limitation (Ia and Ib) Duration since presenting condition began: not reported Age at baseline (years): group A – mean 38.1; group B – mean 35.3; group C – mean 38.1 Sex: group A – 19% male; group B – 12% male; group C – 0% male Number randomised: 51 (group A: 21; group B: 16; group C: 14) Number evaluated: at 3 months = 39 (group A: 17; group B: 13; group C: 9) |
| Interventions |
Comparison: splint vs. no splint for TMD All patients received counselling for habits and behavioural changes (reinforced at each visit): instructed about beneficial behavioural changes and received a printed version of the instructions, containing information about relaxation techniques, sleep hygiene, diet modification, thermotherapy and massage in the painful area, as well as avoidance of caffeine and daytime clenching Group AGroup BGroup CDuration of treatment: 3 months |
| Outcomes |
Assessed at 2 and 6 weeks and at 3 months: we used the 3-month data for our 0–3 months’ analysis Primary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
‘The patients were randomly allocated into one of the following three groups’ Comment: insufficient information |
Allocation concealment (selection bias):
|
‘The patients were randomly allocated into one of the following three groups’ Comment: insufficient information |
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients |
Incomplete outcome data (attrition bias):
|
Very high overall attrition (24%) and especially high in the control group (group A: 19%; group B: 19%; group C: 36%) |
Selective reporting (reporting bias):
|
Data not adequately reported for pain on 0–100 VAS |
Other bias:
|
Lacking in detail so unable to assess |
| Conti 201528 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: Orofacial Pain Clinic at Bauru Dental School, University of São Paulo, Brazil Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported We emailed authors for data but none provided so far |
| Participants |
Diagnosis: disc displacement with reduction (IIa) and arthralgia (IIIa) according to RDC/TMD (myofascial pain, disc displacement without reduction and osteoarthritis according to RDC/TMD were all excluded) Duration since presenting condition began: not reported Age at baseline (years): group A – mean 38.4; group B – mean 38.4; group C – mean 46 Sex: 3% male (not reported by group) Number randomised: 60 (group A: 20; group B: 20; group C: 20) Number evaluated: 3 months – 33 (group A: 12; group B: 12; group C: 9 |
| Interventions |
Comparison: splint vs. no splint for TMD All patients received counselling: instructions containing information about relaxation techniques, sleep hygiene, diet modification and hot thermotherapy, as well as avoidance of caffeine and awaking clenching Group AGroup BGroup CDuration of treatment: 3 months |
| Outcomes |
Assessed at 2 and 6 weeks and at 3 months: we used the 3-month data for our 0–3 months’ analysis PrimarySecondary |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘TMJ clicking’ and ‘change in restricted mouth-opening’, which may be considered objective and were measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
Overall attrition: 32% at 6 weeks and 45% at 3 months |
Selective reporting (reporting bias):
|
Data not adequately reported (e.g. for VAS pain, no SD reported and p-value reported only for comparison between groups A and C) |
Other bias:
|
Lacking in detail so unable to assess |
| Costa 201529 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Orofacial Pain Clinic at Bauru Dental School, University of São Paulo, Brazil Number of centres: one Recruitment period: August 2011 to November 2012 Sample size calculation: reported incompletely (unclear if met) Funding: public (grant number 2011/04441–6 from FAPESP – São Paulo Research Foundation) Declarations/conflicts of interest: ‘The authors declare no conflicts of interest’ We emailed authors for data but none provided so far |
| Participants |
Diagnosis: RDC/TMD – myofascial pain Duration since presenting condition began: pain duration at least 3 months Age at baseline (years) (inclusion was 18–50): group A – mean 27.7 (SD 6.7); group B – mean 36 (SD 6.6) Sex: group A: 10% male; group B: 10% male Number randomised: 60 (group A: 30; group B: 30) Number evaluated: 5 months – 41 (group A: 24; group B: 17); unclear how many participants were evaluated at 2 months |
| Interventions |
Comparison: splint vs. no splint for TMD All patients received counselling: verbal and written information about TMD aetiology and prognostics, diet modification in the sense of avoiding hard foods, use of reminders to avoid parafunctional habits, relaxation techniques of the jaw, application of a heating pad on painful muscles followed by stretching and self-massage, as well as sleep hygiene and encouragement to practise social and aerobic activities Group AGroup BDuration of treatment: 5 months |
| Outcomes |
Assessed at 2 and 5 months: we used the 5-month data for our 3–6 months’ analysis (we were unable to use the 2-month data as the numbers analysed were not reported) PrimarySecondary |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients |
Incomplete outcome data (attrition bias):
|
Overall attrition at 5 months was 32% and also differed by group (group A: 20%; group B: 43%). This could potentially bias the results |
Selective reporting (reporting bias):
|
We would have expected the authors to also report a more simple pain intensity outcome in line with other RCTs in this field (e.g. 0–100 mm VAS) |
Other bias:
|
No apparent other bias |
| Daif 201257 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Oral and Maxillofacial, Faculty of Oral & Dental Medicine, Cairo University, Egypt Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: ‘The authors report no conflicts of interest’ |
| Participants |
Diagnosis: TMD with myofascial pain by the presence of a non-teeth-related chronic orofacial pain with localised areas of tenderness in the masticatory muscles. Signs and symptoms were recorded according to the clinical dysfunction index of Helkimo74 Duration since presenting condition began: not reported Age at baseline (years): overall – mean 32 (range 22–46) Sex: overall – 42.5% male Number randomised: 40 (group A: 20; group B: 20) Number evaluated: 40 (group A: 20; group B: 20) |
| Interventions |
Comparison: splint vs. no splint for TMD Group AGroup BDuration of treatment: 6 months |
| Outcomes |
Assessed at 6 months: grouped under 3–6 months’ analysis Secondary |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Patients were not blinded but self reported compliance |
Incomplete outcome data (attrition bias):
|
All randomised participants were included in analysis |
Selective reporting (reporting bias):
|
The study focused on TMD with pain; therefore, we would have expected pain to have been measured separately |
Other bias:
|
No apparent other bias |
| Dao 199468 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: Faculty of Dentistry and Neuroscience Research Centre, University of Montreal, Montreal, QC, Canada Number of centres: one Recruitment period: not reported Sample size calculation: yes (met) Funding: public (Medical Research Council of Canada and the Fonds de la Recherche en Sante du Quebec) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: chief complaint of frequent pain (at least four times per week) in the jaw muscles of at least 12 weeks duration; positive report of tenderness to palpation of at least three sites in the masticatory muscles Duration since presenting condition began: history of myofascial pain varied from 3 months to 12 years, with a mean of 43.4 months Age at baseline (years): group A – mean 29 (range 16–40); group B – mean 28 (range 16–42) Sex: group A – 18% male; group B – 20% male Number randomised: 43 (group A: 22; group B: 21) Number evaluated: 42 (group A: 22; group B: 20) |
| Interventions |
Comparison: splint vs. control splint for TMD Group AGroup BGroup CDuration of treatment: 8 weeks |
| Outcomes |
Assessed at 1, 3, 5 and 8 weeks: we would have used the 8-week data for our 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
‘Patients were randomly allocated to 1 of the 3 experimental groups’ Comment: insufficient information |
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
|
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessed by blinded patients |
Incomplete outcome data (attrition bias):
|
Only one participant was missing from the control group |
Selective reporting (reporting bias):
|
It was not possible to use any VAS pain or quality-of-life data |
Other bias:
|
No apparent other bias |
| de Felício 200630 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Dental School of Ribeirão Preto of the University of São Paulo, Brazil Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: presence of signs and symptoms characteristic of TMD: pain in the masticatory muscles and/or in the TMJ during mandibular function and palpation of the structures, limitation or deviation of mandibular movements, noises in the TMJ, and abnormal static or dynamic occlusal relation Duration since presenting condition began: not reported Age at baseline (years): not reported Sex: not reported Number randomised: 84 (group A: 42; group B: 42) Number evaluated: 84 (group A: 42; group B: 42) |
| Interventions |
Comparison: splint vs. minimal treatment for TMD Group AGroup B Continued to attend occlusion outpatient clinic, receiving information about TMD Duration of treatment: 50 days |
| Outcomes |
Assessed at 50 days: grouped under 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
Not mentioned |
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessed by the patients |
Incomplete outcome data (attrition bias):
|
All randomised patients were included in the analyses |
Selective reporting (reporting bias):
|
Poor reporting of NRS severity scores |
Other bias:
|
No apparent other bias |
| de Felício 201031 | |
| Characteristics | |
| Study details |
Trial design: parallel (four arms) Location: Faculty of Medicine of Ribeirão Preto of the University of São Paulo, Brazil Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: public (supported by Fundação de Amparo à Pesquisa do Estado de São Paulo -FAPESP, Process N. 2004/08478–8 and Conselho Nacional de Pesquisa – CNPq, Process N. 300950/2007–1) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: long-lasting associated articular and muscular TMD based on RDC/TMD Duration since presenting condition began: mean duration of TMD was 74.4 months (range 6–300 months) Age at baseline (years): group A – mean 29 (range 17–64); group B – mean 34 (range 14–63) Sex: not reported Number randomised: 20 (group A: 10; group B: 10) Number evaluated: 20 (group A: 10; group B: 10) – this is assumed as attrition was not mentioned |
| Interventions | Comparison: splint vs. no splint for TMD
|
| Outcomes |
Assessed at 45 days: grouped under 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘change in restricted mouth-opening’ and ‘TMJ clicking’ which were objective but blinded assessor not mentioned) |
Incomplete outcome data (attrition bias):
|
Assuming no attrition but not entirely clear |
Selective reporting (reporting bias):
|
Outcomes fully reported |
Other bias:
|
No other apparent bias |
| DeVocht 201345 | |
| Characteristics | |
| Study details |
Trial design: parallel (four arms) Location: Craniofacial Clinical Research Centre, University of Iowa, Iowa City, IA, USA; Palmer College of Chiropractic, Davenport, IA, USA Number of centres: two Recruitment period: over 18 months, ending in July 2011 Sample size calculation: no (‘We chose the sample size to determine feasibility and, therefore, the study was not powered to detect differences between groups’) Funding: public and industry (grants from National Institutes of Health; ineligible interventions mentioned above were provided by the manufacturer) Declarations/conflicts of interest: one author declared instructing for Activator Methods International, Phoenix (manufacturers of the ineligible interventions mentioned above). None of the other authors reported any disclosures |
| Participants |
Diagnosis: myofascial pain (RDC/TMD Axis I) with TMD pain over the previous week of at least a 3 on a 0–10 NRS Duration since presenting condition began: (inclusion criteria required participants having had TMD symptoms for at least 6 months) – group A: median 10 years (IQR 12.5); group B: median 10 years (IQR 11) Age at baseline (years): group A – mean 31 (range 13–76); group B: mean 30 (range 15–72) Sex: group A – 15% male; group B – 24% male Number randomised: 41 (group A: 20; group B: 21) Number evaluated: 41 (group A: 20; group B: 21) – ITT used (multiple imputation for the missing outcomes) |
| Interventions |
Comparison: Splint versus no splint for TMD All patients received TMD self-care programme: similar to usual recommendations given to patients with TMD. Conservative and reversible strategies requiring the dentist or dental care co-ordinator to review TMD with the participant; explain to them the current understanding of prognosis; and provide standardised treatment checklist identifying recommendations for care (e.g. jaw relaxation, reduction of parafunctional behaviours, use of thermal packs, use of over-the-counter pain medications, passive jaw-opening stretches and suggestions about stress reduction)Duration of treatment: 2 months |
| Outcomes |
Assessed at 2 and 6 months: we used these data in our 0–3 month and 3–6 month analyses, respectively Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessed by the patients |
Incomplete outcome data (attrition bias):
|
ITT used (multiple imputation for the missing outcomes) |
Selective reporting (reporting bias):
|
No evidence of selective reporting |
Other bias:
|
No other apparent bias |
| Ekberg 199835 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Stomatognathic Physiology, Lund University, Sweden Number of centres: one Recruitment period: over 3 years Sample size calculation: yes (met) Funding: public (supported by the Faculty of Odontology at Lund University and the Swedish Dental Society) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: TMD of arthrogeneous origin – pain localised to the TMJ region and lateral and/or posterior tenderness to palpation of the TMJ combined with self-assessed TMJ pain of ≥ 40 mm on a 100 mm VAS Duration since presenting condition began: TMJ pain (months) – group A: median 24 (range 3–360); group B: median 14 (range 2–120) Age at baseline (years): group A – mean 31 (range 13–76); group B – mean 30 (range 15–72) Sex: group A – 13% male; group B – 3% male Number randomised: 60 (group A: 30; group B: 30) Number evaluated: 60 (group A: 30; group B: 30) |
| Interventions |
Comparison: splint vs. control splint for TMD Group AGroup BDuration of treatment: 10 weeks |
| Outcomes |
Assessed at 10 weeks: grouped under 0–3 month analysis Primary:Secondary |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
The person delivering and adjusting the splints was not blinded |
Blinding of outcome assessment (detection bias):
|
Examiner was blinded. Many of the outcomes were patient-reported and it was not clear if the patients were aware of their group assignment |
Incomplete outcome data (attrition bias):
|
All randomised participants were included in analysis |
Selective reporting (reporting bias):
|
Some outcomes measured but not reported fully (current pain VAS and maximum mouth-opening mean and SD) |
Other bias:
|
No other apparent bias |
| Ekberg 200336 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Stomatognathic Physiology, Malmö University, Sweden Number of centres: one Recruitment period: over approximately 2 years Sample size calculation: not reported Funding: public (grants from the Faculty of Odontology at Lund University and the Swedish Dental Society) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: myofascial pain with or without limited opening according to RDC/TMD; self-assessed myofascial pain of at least 40 mm on a 100 mm VAS Duration since presenting condition began: myofascial pain (months) – group A: median 36 (range 4–420); group B: median 24 (range 1–120) Age at baseline (years): group A – mean 31 (range 14–54); group B: mean 28 (range 14–56) Sex: group A – 17% male; group B – 10% male Number randomised: 60 (group A: 30; group B: 30) Number evaluated: 60 (group A: 30; group B: 30) |
| Interventions |
Comparison: splint vs. control splint for TMD Group AGroup BDuration of treatment: 10 weeks |
| Outcomes |
Assessed at 10 weeks: grouped under 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
The person delivering and adjusting the splints was not blinded |
Blinding of outcome assessment (detection bias):
|
Examiner was blinded. Many of the outcomes were patient-reported and it was not clear if the patients were aware of their group assignment |
Incomplete outcome data (attrition bias):
|
All randomised participants were included in analysis |
Selective reporting (reporting bias):
|
Some outcomes measured but not reported fully (current pain VAS and maximum mouth-opening mean and SD) |
Other bias:
|
No other apparent bias |
| Elsharkawy 199558 | |
| Characteristics | |
| Study details |
Trial design: parallel (four arms) Location: Oral Surgery Department, Cairo University, Egypt Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: based on presence of two or more of TMJ pain and tenderness when palpated both laterally in the preauricular area and via the external auditory meatus, masticatory muscle tenderness, clicking and jaw locking, and trismus (patients with disc displacement were excluded) Duration since presenting condition began: not reported Age at baseline (years): not reported Sex: not reported Number randomised: 50 (group A: 25; group B: 25) Number evaluated: 46 (group A: 23; group B: 23) |
| Interventions |
Comparison: splint vs. no splint for TMD All patients in groups A and B received acuhealth therapy: acuhealth unit detects energy acupucture points and performs stimulation/treatment without penetrating the skin; weekly sessions for 8 weeks*Groups C and D are excluded from this review as it was not possible to make any eligible pairwise comparisons using them Duration of treatment: 8 weeks |
| Outcomes |
Assessed at 3 months: grouped under 0–3 month analysis (also assessed at 6 and 12 months but patients had crossed over and were no longer analysed according to the group they were originally randomised to, so the data were not eligible for inclusion) Primary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective pain outcomes assessment by patients |
Incomplete outcome data (attrition bias):
|
We were unable to use data at 6 and 12 months as some patients were no longer analysed according to the group they were originally randomised to |
Selective reporting (reporting bias):
|
Incomplete reporting of pain data |
Other bias:
|
No other apparent bias |
| Ficnar 201361 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: Department of Prosthetic Dentistry and Biomaterials and the Department of Orthodontics of the Center for Dental, Oral and Maxillofacial Diseases of Münster University Hospital, Germany Number of centres: one Recruitment period: 2009–10 Sample size calculation: not reported Funding: industry (‘The expenses for this study were payed by Jaxeurope [Taunusstein, Germany]’) Declarations/conflicts of interest: ‘The authors declare that they have no competing interests’ |
| Participants |
Diagnosis: RDC/TMD Ia or Ib (myofascial pain) also in combination with arthralgia (IIIa) and/or disk displacement with reduction (IIa) and a maximum ‘von Korff’ pain grade of I (functional pain with low levels of intensity) to II (functional pain with high levels of intensity) Duration since presenting condition began: not reported Age at baseline (years): median 35 (not reported by group) Sex: 21% male (not reported by group) Number randomised: 63 (group A: 21; group B: 21; group C: 21) Number evaluated: 58 (group A: 18; group B: 21; group C: 19) |
| Interventions |
Comparison: splint vs. no splint for TMD – prefabricated splint vs. custom-made splint for TMD All patients received conservative therapy: self-exercises (muscle exercise form according to Professor Schulte, self-massage techniques, mouth-opening exercises), medication-based therapy using NSAID, muscle relaxants as well as manual therapy Group AGroup BGroup CDuration of treatment: 2.5 months |
| Outcomes |
Assessed at 2 weeks and 2.5 months: we would have used the 2.5 month data for our 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective pain outcome (‘change in restricted mouth-opening’ was more objective but unclear whether or not it was measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
Low (8%) overall attrition and fairly equally distributed |
Selective reporting (reporting bias):
|
No evidence of selective reporting |
Other bias:
|
No other apparent bias |
| Giannakopoulos 201662 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: University clinic, Heidelberg, Germany Number of centres: one Recruitment period: 2009–11 Sample size calculation: no (only post hoc to estimate sample size required for future trials) Funding: not reported Declarations/conflicts of interest: ‘The authors report no conflicts of interest’ Authors provided unpublished data |
| Participants |
Diagnosis: painful non-chronic (i.e. non-dysfunctional) TMD-related pain, diagnosed by use of the RDC/TMD – patients with a GCPS value of 3 or 4, indicative of disabling chronic pain, were not eligible for the study Duration since presenting condition began: pain duration mean 42.98 weeks (SD 51.33 weeks) Age at baseline (years): overall mean 41.58 (SD 16.68) – not reported by group Sex: group A – 50% male; group B – 33.3% male; group C – 8.3% male Number randomised: 36 (group A: 12; group B: 12; group C: 12) Number evaluated: 36 (group A: 12; group B: 12; group C: 12) |
| Interventions |
Comparison: (1) splint vs. no splint for TMD; (2) custom-made splint vs. prefabricated splint for TMD All patients received counselling: their disease and its multifactorial aetiology were explained, and they were given advice on how to reduce stress on their masticatory system by avoiding extreme movements of the jaw (e.g. yawning) and by avoiding chewing hard food or chewing gum. All patients in extreme pain were allowed to use common over-the-counter analgesics, the type, amount and frequency of which were to be reported on recall Group AGroup BGroup CDuration of treatment: 2 weeks |
| Outcomes |
Assessed at 2 weeks: grouped under 0 to 3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective pain outcome assessment by patients (but ‘change in restricted mouth-opening’ was objective and measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
All randomised patients were included in the analyses |
Selective reporting (reporting bias):
|
Pain outcome fully reported and author provided mean and SD for each group for the outcome of maximum mouth-opening |
Other bias:
|
No apparent other bias |
| Gomes 201432 | |
| Characteristics | |
| Study details |
Trial design: parallel (four arms) Location: Nove de Julho University, São Paulo, Brazil Number of centres: one Recruitment period: June 2011 to December 2012 Sample size calculation: yes (met – not powered on any of the relevant outcomes from our review) Funding: ‘This study had no financial support’ Declarations/conflicts of interest: ‘The authors declare that they have no competing interests’ |
| Participants |
Diagnosis: severe TMD and sleep bruxism – (1) the Fonseca Patient History Index was used to diagnose the presence and intensity of TMD; (2) those with incisal and/or occlusal tooth wear and clinical signs in the buccal mucosa and tongue of clenching or grinding were diagnosed with bruxism based on the criteria of the American Academy of Sleep Medicine and a positive self-report of awake bruxism Duration since presenting condition began: at least 1 year Age at baseline (years): (inclusion was 18–40 years) group A – mean 26 (SD 3); group B – mean 29 (SD 4) Sex: group A – 7% male; group B – 13% male Number randomised: 30 (group A: 15; group B: 15) Number evaluated: 30 (group A: 15; group B: 15) |
| Interventions |
Comparison: splint vs. no splint for TMD and bruxism All patients in groups A and B received massage: three weekly 30-minute sessions of massage therapy performed by a physiotherapist who had undergone a training exercise for the administration of sliding and kneading manoeuvres of the masseter and anterior temporal muscles, bilaterally, over 4 consecutive weeks (total: 12 sessions)*Groups C and D are excluded from this review as it was not possible to make any eligible pairwise comparisons using them Duration of treatment: 4 weeks |
| Outcomes | The outcomes measured at 4 weeks (electromyographic analysis of the masseter and anterior temporal muscles, reported as median frequency, and the Fonseca Patient History Index) were not outcomes of this review and therefore there were no usable data in this study |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Irrelevant as there are no outcomes of use for this review |
Incomplete outcome data (attrition bias):
|
All randomised patients appear to have been included in the analyses (from correspondence with authors) |
Selective reporting (reporting bias):
|
We would expect to see pain reported in the assessment of TMD patients |
Other bias:
|
No apparent other bias |
| Gomes 201533 | |
| Characteristics | |
| Study details |
Trial design: parallel (four arms) Location: Nove de Julho University, São Paulo, Brazil Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: sleep bruxism diagnosed by experienced dentist based on criteria of the International Classification for Sleep Disorders of the American Academy of Sleep Medicine, self-reported awake bruxism, and a minimum pain intensity score of 3 on an 11-point NRS Duration since presenting condition began (months): group A – mean 18.16 (SD 9.33); group B – mean 23.19 (SD 4.84); group C – mean 27.55 (SD 9.41); group D – mean 22.94 (SD 5.02) Age at baseline (years): (inclusion was 18–40 years) group A – mean 24.40 (SD 4.10); group B – mean 25.72 (SD 6.20); group C – mean 28.60 (SD 4.20); group D – mean 24.40 (SD 4.10) Sex: all female Number randomised: 100 (group A: 25; group B: 25; group C: 25; group D: 25) Number evaluated: 78 (group A: 19; group B: 19; group C: 23; group D: 17) |
| Interventions |
Comparison: splint vs. no splint for bruxism We split the four groups/arms into two pairwise comparisons of A vs. B and C vs. D Group AGroup B: no treatment Group C: combined (splint + massage) – as groups A and D Group D: massage – three weekly 30-minute sessions of massage of the muscles of mastication over 4 consecutive weeks (total: 12 sessions). Massage therapy performed by a physiotherapist who had undergone a training exercise for the administration of the protocol, involving sliding and kneading manoeuvres on the masseter and temporal muscles Duration of treatment: 4 weeks |
| Outcomes |
Assessed at 4 weeks Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients |
Incomplete outcome data (attrition bias):
|
Overall attrition was 22% and also differed by group (group A: 24%; group B: 24%; group C: 8%; group D: 32%). High attrition for such a short-term study |
Selective reporting (reporting bias):
|
No typical bruxism outcomes measured or reported |
Other bias:
|
No apparent other bias |
| Haketa 201066 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: TMJ Clinic of the Tokyo Medical and Dental University, Japan Number of centres: one Recruitment period: January to December 2006 Trials registry ID: NCT00936338 Sample size calculation: yes (not met) Funding: public (supported by the Dental Hospital and the Department of Temporomandibular joint and Occlusion of Tokyo Medical and Dental University) Declarations/conflicts of interest: not reported We emailed authors for data but none provided so far |
| Participants |
Diagnosis: anterior disc displacement without reduction – confirmed by MRI; must have mouth-opening pain on TMJ-affected side and maximum mouth-opening of < 40 mm Duration since presenting condition began: > 2 weeks Age at baseline (years): group A – mean 38.6 (SD 13.8); group B – mean 38.8 (SD 15.2) Sex: group A – 16% male; group B – 0% male Number randomised: 52 (group A: 28; group B: 24) Number evaluated: 44 (group A: 25; group B: 19) |
| Interventions |
Comparison: splint vs. minimal treatment (exercise) for TMD Instructions to all participants in both groups: all participants received a verbal explanation of the pathological conditions based on X-ray and MRI findings, and a general self-care protocol such as good posture, soft diet, teeth apart, etc. All participants were prescribed a NSAID three times every dayDuration of treatment: 8 weeks |
| Outcomes |
Assessed at 4 and 8 weeks: we used the 8-week data for our 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘change in restricted mouth-opening’ which was objective and measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
Overall attrition was 22% (group A: 11%; group B: 21%) – probably not sufficient to cause serious bias |
Selective reporting (reporting bias):
|
No evidence of selective reporting |
Other bias:
|
No apparent other bias |
| Hasanoglu 201752 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Oral Surgery, Gazi University, Turkey Number of centres: one Recruitment period: January to June 2014 Sample size calculation: yes (met) Funding: ‘The authors have no support or funding to report’ Declarations/conflicts of interest: ‘The authors have stated explicitly that there are no conflict of interests in connection with this article’ |
| Participants |
Diagnosis: myofascial pain (RDC/TMD Group I: pain or ache in the jaw, temples, face, pre-auricular area or inside the ear at rest or during function and pain in response to palpation of ≥ 3 of the specified 20 muscle sites. In addition, at least one site must be ipsilateral to the site of pain complaint) Duration since presenting condition began: group A – mean 3.49 years (SD 2.75 years); group B – mean 1.16 years (SD 1.36 years) Age at baseline (years): (inclusion was ≥ 18 years) group A – mean 24.6 years (SD 9.2 years); group B – mean 32.25 years (SD 11.97 years) Sex: group A – 20% male; group B – 15% male Number randomised: 40 (group A: 20; group B: 20) Number evaluated: 40 (group A: 20; group B: 20) |
| Interventions |
Comparison: splint vs. no splint for TMD Both groups received first line therapy for facial pain: guidance, assurance, counselling and behavioural changes (no further description given)Duration of treatment: 6 weeks |
| Outcomes |
Assessed at 3 and 6 weeks: we used the 6-week data for our 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients |
Incomplete outcome data (attrition bias):
|
All randomised patients were included in the analyses |
Selective reporting (reporting bias):
|
No evidence of selective reporting |
Other bias:
|
No apparent other bias |
| Johansson 199137 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: Department of Stomatognathic Physiology, University of Gothenberg, Sweden Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: CMD – a history including signs and symptoms of CMD; complaints of headache and/or facial pain; clinical examination demonstrating tenderness to palpation in the masticatory muscles; exclusion of individuals with psychologic/psychogenic factors, trauma, surgery, or systemic joint, muscle, or skin diseases influencing the symptoms; exclusion of pathologic conditions in TMJs, facial skeleton, or teeth using radiographs Duration since presenting condition began: not reported Age at baseline (years): not reported Sex: not reported Number randomised: 30 (group A: 15; group B: 15) Number evaluated: 30 (group A: 15; group B: 15) |
| Interventions | Comparison: splint vs. no splint for TMD
|
| Outcomes |
Group A assessed at 3 months, group B assessed at 2 months Primary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients |
Incomplete outcome data (attrition bias):
|
All randomised patients were included in the analyses |
Selective reporting (reporting bias):
|
Poor reporting but probably not done selectively |
Other bias:
|
Outcomes were assessed at 3 months for the splint group but at 2 months for the control group |
| Karakis 201453 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Prosthodontics, Gazi University, Ankara, Turkey Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: public (Cumhuriyet University, Foundation of Scientific Research Projection) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: sleep bruxism according to the following criteria – history of frequent tooth grinding occurring at least 3 nights per week for the preceding 6 months, as confirmed by a sleep partner; clinical presence of tooth wear; masseter muscle hypertrophy; report of jaw muscle fatigue or tenderness in the morning Duration since presenting condition began: at least 6 months Age at baseline (years): ranging from 18 to 27 (not reported by treatment group) Sex: not reported Number randomised: 12 (group A: 6; group B: 6) Number evaluated: not reported |
| Interventions |
Comparison: custom-made splint vs. prefabricated splint for bruxism Group AGroup BDuration of treatment: 6 weeks |
| Outcomes | The outcomes assessed at 3 and 6 weeks (Craniomandibular Index and occlusal force) were not outcomes of this review; therefore, there were no usable data in this study |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Irrelevant as there are no outcomes of use for this review |
Incomplete outcome data (attrition bias):
|
Numbers of participants analysed is not reported |
Selective reporting (reporting bias):
|
There are no useful bruxism outcomes |
Other bias:
|
No apparent other bias |
| Katyayan 201456 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Prosthetic Dentistry, Government Dental College and Hospital, Ahmedabad, India Number of centres: one Recruitment period: not reported (‘over a period of one year’) Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: TMD (RDC/TMD axis I) Duration since presenting condition began: at least 6 months Age at baseline (years): mean 34.4 (range 20–56) – not reported by group Sex: 22.5% male – not reported by group Number randomised: 80 (group A: 40; group B: 40) Number evaluated: 80 (group A: 40; group B: 40) |
| Interventions |
Comparison: splint vs. no splint for TMD All patients received counselling and masticatory muscle exercises: mandible held in the maximal position for a few seconds on each movement (laterotrusive and protrusive), then with resistance from the patient’s fingers. After jaw exercised, the patients were suggested to open the jaw wide stretching it with their fingers a few times for 10–20 seconds. Movements were repeated 7–10 times per training session and sessions were performed two or three times per day. Patients received written instructions and the movements were demonstrated by the dentist before treatment and after, if necessaryDuration of treatment: 6 months |
| Outcomes |
Assessed at 6 months: grouped under 3 to 6 months analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘change in restricted mouth-opening’, which was objective and measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
All randomised patients were included in the analyses |
Selective reporting (reporting bias):
|
No evidence of selective reporting |
Other bias:
|
No apparent other bias |
| Leeson 200763 | |
| Characteristics | |
| Study details |
Trial design: parallel (four arms) Location: Eastman Dental Hospital, London, UK Number of centres: one Recruitment period: unclear but appears to be 1995–97 Sample size calculation: yes (met) Funding: public and pharmaceutical (medication donated by Lilly Pharmaceutical Company and the project was funded by a Department of Health and Social Care grant and locally organised research funding) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: chronic TMD of recent onset (of > 3 months duration, hence exposed to minimal treatment intervention) – pain in one or both TMJs with or without (1) clicking, (2) limited mouth-opening, (3) muscle tenderness Duration since presenting condition began: at least 3 months Age at baseline (years): group A – mean 34.1 (SD 9.99), range 16–55; group B – mean 29.8 (SD 7.99), range 16–55 Sex: group A – 21.0% male; group B – 23.8% male Number randomised: 125 (group A: 62; group B: 63) Number evaluated: 125 (group A: 62; group B: 63) imputational analysis used (last score brought forward) |
| Interventions |
Comparison: splint vs. no splint for TMD All patients in groups A and B received medication: SSRI fluoxetine, Prozac. Initial 20 mg daily, then doubled to 40 mg at the 2-month review. After 3 months, patients who improved on medical therapy and wished to continue on treatment, remained on medication, usually at the 40 mg dosage. If pain had failed to respond, or worsened, patients were reassessed and, in some cases, withdrawn from continuation in the study. Further data were collected from these patients to include in the ITT analysis. All patients requested to only embark on minimal essential dental treatment and refrain from alternative pain therapies during treatment*Groups C and D are excluded from this review as it was not possible to make any eligible pairwise comparisons using them Duration of treatment: 3 months |
| Outcomes |
Assessed at 1, 2 and 3 months: we used the 3-month data for our 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘change in restricted mouth-opening’, which was objective but unclear whether or not it was measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
lnputational analysis used (last score brought forward) so that all randomised patients were included in the analyses |
Selective reporting (reporting bias):
|
No evidence of selective reporting |
Other bias:
|
No apparent other bias |
| List 199238 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: Department of Stomatognathic Physiology, University of Gothenberg, Sweden Number of centres: one Recruitment period: April 1987 to March 1989 Sample size calculation: not reported Funding: public (Jonkoping County Council and Swedish Medical Research Council, project 55) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: craniomandibular disorder (CMD): signs and symptoms of CMD of primarily muscular origin; pain for > 6 months; clinical dysfunction index of Di II or more according to Helkimo74 Duration since presenting condition began: pain for > 6 months – median duration in years (range) – group A: 3.0 (14.5); group B: 4.3 (24.5) Age at baseline (years): group A – mean 39 (SD 11); group B – mean 48 (SD 13) Sex: group A – 35% male; group B – 3% male Number randomised: 70 (group A: 40; group B: 30) Number evaluated: 56 (group A: 34; group B: 22) |
| Interventions |
Comparison: splint vs. no splint for TMDGroup B: no treatment (3-month wait list) Group C: acupuncture (not eligible for this review) Duration of treatment: group A – 6–8 weeks (but preceded by 1-month pre-treatment period); group B – on waiting list for 3 months |
| Outcomes |
Group A assessed at 2 months, group B assessed at 3 months: grouped under 0–3 months’ analysis There were also 6-month and 12-month assessments but they are not reported Primary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unable to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients |
Incomplete outcome data (attrition bias):
|
Overall attrition 20% (group A: 15%; group B: 27%). There were no dropouts in the study but only pain diaries in which > 70% of the required recordings had been completed were included in the analysis. Unlikely to change the results much |
Selective reporting (reporting bias):
|
The assessments at 6 and 12 months are reported in a separate study report, but only for groups A and C |
Other bias:
|
|
| Lundh 198539 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: Department of Stomatology, University of Lund, Sweden Number of centres: one Recruitment period: January 1982 to March 1984 Sample size calculation: not reported Funding: public and industry, that is private health-care company (financial support from University of Lund, and Praktikertjanst AB, Sweden; study supported by Magnus Bergvalls Foundation, Torsten and Ragnar Soderbergs Foundations, and Swedish Medical Research Council) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: ‘1704 patients referred for pain and dysfunction of the masticatory system’, every third patient given an appointment (n = 568). These were then subdivided into those with reciprocal clicking (clicking on opening and closing) (n = 88) these were then subdivided again into those that could eliminate clicking by beginning mandibular movements in a position anterior to intercuspal position (centric occlusion), but not as far as edge to edge incisal potion and only these added to the trial (n = 78). Those that could not eliminate clicking unless mandibular movements were started from edge to edge incisal position, these were excluded from the trial (n = 10) Duration since presenting condition began: not reported Age at baseline (years): median 30, range 10–69 (not reported by group) Sex: 31% male (not reported by group) Number randomised: 70 (group A: 24; group B: 23; group C: 23) Number evaluated: 70 (group A: 24; group B: 23; group C: 23) |
| Interventions |
Comparison: splint vs. no splint for TMD Group AGroup BGroup CDuration of treatment: 6 weeks (but followed by 2 weeks of reduction in use and unclear thereafter) |
| Outcomes |
Assessed at 6, 17 and 52 weeks: we used these in our 0–3 month, 3–6 month and 6–12 month analyses, respectively Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessed by patient (except for clicking – but blinding was not mentioned) |
Incomplete outcome data (attrition bias):
|
There did not appear to be any dropouts |
Selective reporting (reporting bias):
|
No data reported for the VAS pain outcomes |
Other bias:
|
No other bias apparent |
| Lundh 198840 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: (1) Department of Stomatology, School of Dentistry, Malmö, Sweden and (2) Department of Oral Surgery, University Hospital, Lund, Sweden Number of centres: two Recruitment period: not reported Sample size calculation: not reported Funding: public and industry, that is both private health-care company and pharmaceutical company (supported by Magnus Bergvalls Foundation, University of Lund, Praktikertjanst AB, Sweden, Swedish Medical Research Council, Torsten and Ragnar Soderbergs Foundations, and the Ake Wiberg Foundation; Nycomed AB, Sweden provided contrast medium used for arthrography) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: disk displacement with reduction:– confirmed by arthrography Duration since presenting condition began: not reported Age at baseline (years): median 24, range 13–74 (not reported by group) Sex: 14% male (not reported by group) Number randomised: 43 (group A: 21; group B: 22) Number evaluated: 43 (group A: 21; group B: 22) |
| Interventions |
Comparison: splint vs. no splint for TMD All patients were informed about basic anatomy and function of the TMJ, the mechanisms of clicking and locking, and the possible caused of painDuration of treatment: 6 months |
| Outcomes |
Assessed at 6 months: grouped under 3–6 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessed by patient (except for clicking – but blinding was only done for around half of the assessments) |
Incomplete outcome data (attrition bias):
|
There did not appear to be any dropouts |
Selective reporting (reporting bias):
|
Pain at rest was not reported |
Other bias:
|
No other bias apparent |
| Lundh 199241 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Stomatology, University of Lund, Malmö, Sweden Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: public and industry, that is private health-care company (supported by grants from Praktikertjanst AB, Sweden and by the Torsten and Ragnar Soderbergs Foundations) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: pain on chewing (> 50 on a 0–100 mm VAS) with arthographically documented disc displacement without reduction in one or both TMJs Duration since presenting condition began: not reported Age at baseline (years): mean 29, range 14–61 (not reported by group) Sex: 10% male (not reported by group) Number randomised: 51 (group A: 25; group B: 26) Number evaluated: 51 (group A: 25; group B: 26) |
| Interventions | Comparison: splint vs. no splint for TMD
|
| Outcomes |
Assessed at 12 months: grouped under 6–12 month analysis Primary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessed by patient |
Incomplete outcome data (attrition bias):
|
There did not appear to be any dropouts |
Selective reporting (reporting bias):
|
Only outcomes with statistically significant differences were reported |
Other bias:
|
No other bias apparent |
| Magnusson 199942 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Stomatognathic Physiology, The Institute for Postgraduate Dental Education, Jonkoping, Sweden Number of centres: one Recruitment period: November 1993 to September 1996 Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: TMD of mainly muscular origin: patients referred to specialist clinic with main subjective symptom of tension-type headache and/orofacial pain of non-neurogenic or non-dental origin Duration since presenting condition began: pain history of at least 1 year Age at baseline (years): group A – mean 32 (range 17–49); group B – mean 37 (range 16–67) Sex: not reported Number randomised: 26 (group A: 14; group B: 12) Number evaluated: 18 (group A: 9; group B: 9) |
| Interventions |
Comparison: splint vs. minimal treatment for TMD Group AGroup BPatients with significant symptoms after 3 months of treatment were offered complementary treatment with the other treatment modality. Those receiving combined treatment were analysed separately (group not included in this review) Duration of treatment: 6 months |
| Outcomes |
Assessed at 3 and 6 months: we used these in our 0–3 month and 3–6 month analyses, respectively Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessed by patient (except for clicking – but the outcome assessor was not blinded) |
Incomplete outcome data (attrition bias):
|
Overall attrition 31% (group A: 36%; group B: 25%) – reasons mostly the same |
Selective reporting (reporting bias):
|
No evidence of selective reporting |
Other bias:
|
No other bias apparent |
| Michelotti 201264 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Clinic for Temporomandibular Disorders and Orofacial Pain, University of Naples Federico II, Italy Number of centres: one Recruitment period: 9 months (dates not reported) Sample size calculation: no (post hoc only) Funding: not reported Declarations/conflicts of interest: ‘None of the authors reported any disclosures’ |
| Participants |
Diagnosis: myogenous pain according to RDC/TMD categories Ia and Ib; also objective evidence of joint pathology or dysfunction; spontaneous muscle pain > 30 mm on 100 mm VAS Duration since presenting condition began: recurrent or constant myogenous pain for > 3 months Age at baseline (years): group A – mean 30 (range 20–53); group B – mean 30 (range 18–49) Sex: group A – 29% male; group B – 17% male Number randomised: 44 (group A: 21; group B: 23) Number evaluated: 41 (group A: 18; group B: 23) |
| Interventions |
Comparison: splint vs. minimal treatment for TMD Group AGroup BDuration of treatment: 3 months |
| Outcomes |
Assessed at 3 months: grouped under 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘change in restricted mouth-opening’ which was objective and measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
Overall attrition 7% (group A: 14%; group B: 0%) – only 3 participants dropped out in group A so probably not enough to bias the results in a meaningful way |
Selective reporting (reporting bias):
|
Outcomes fully reported |
Other bias:
|
No other bias apparent |
| Nagata 201567 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Nippon Dental University, Niigata Hospital, Niigata, Japan Number of centres: one Recruitment period: June 2009 to July 2013 Sample size calculation: yes (met) Funding: none Declarations/conflicts of interest: ‘None of the authors received support from a corporation or any funding for this study’ |
| Participants |
Diagnosis: TMD (RDC/TMD axis I); RDC/TMD axis II was excluded Duration since presenting condition began (months): group A – median 24 (range 3–360); group B – median 24 (range 4–72) Age at baseline (years): group A – mean 41 (SD 19); group B – mean 43 (SD 18) Sex: group A – 31% male; group B – 39% male Number randomised: 201 (group A: 103; group B: 98) Number evaluated: 181 (group A: 96; group B: 85) |
| Interventions |
Comparison: splint vs. no treatment for TMD All patients in both groups received multimodal therapy: self-exercise of the jaw (pulled down on bilateral lower last molars with secondary fingers while opening jaw to the greatest possible extent – performed with 20 repetitions three times per day), CBT (guidance about clenching control during waking hours and coping with pain and stress) and received education about TMD self-management (i.e. a diet of soft foods, avoiding gum chewing and correcting bad posture). Participants with mouth-opening of < 35 mm also underwent jaw manipulationDuration of treatment: 10 weeks |
| Outcomes |
Assessed at 2, 4, 6, 8 and 10 weeks: we used the 10-week data for our 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective pain outcome assessment by patients (except for ‘change in restricted mouth-opening’ and clicking which were objective – described as single blind so probably the assessors for these outcomes) |
Incomplete outcome data (attrition bias):
|
Overall attrition 10% (group A: 7%; group B: 13%) – low attrition and similar reasons stated |
Selective reporting (reporting bias):
|
No evidence of selective reporting |
Other bias:
|
No other bias apparent |
| Niemelä 201271 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Oral and Maxillofacial Department, Oulu University Hospital, Finland Number of centres: one Recruitment period: March 2008 to September 2009 Sample size calculation: yes (not met) Funding: public (supported by the Finnish Dental Society, Apollonia and the Academy of Finland) Declarations/conflicts of interest: ‘No conflict of interests are declared’ |
| Participants |
Diagnosis: TMD (RDC/TMD) – the patients were referred to the Oral and Maxillofacial Department, Oulu University Hospital, for treatment of TMD and had thus been suffering from relatively chronic and severe TMD Duration since presenting condition began: not reported Age at baseline (years): (inclusion = at least 20) group A – mean 43 (SD 13); group B – mean 44 (SD 13) Sex: group A – 18% male; group B – 27% male Number randomised: 80 (group A: 39; group B: 41) Number evaluated: 1 month: 76 (group A: 39; group B: 37); 1 year: 78 (group A: 37; group B: 41) – ITT |
| Interventions |
Comparison: splint vs. no treatment for TMD All patients in both groups received counselling and instructions for masticatory muscle exercises – at the beginning of the training programme, active mouth-openings, laterotrusive movements and protrusive movements were performed. The mandible was held in the maximal positions for a few seconds on each movement. Thereafter, these movements were made towards resistance (using patient’s own fingers). After jaw exercises, the patients were suggested to open the jaw wide, stretching it with fingers a few times for 10–20 seconds. These movements were repeated 7–10 times per training sessions, and the sessions were performed two or three times per day. The patients received written instructions, and the movements were also demonstrated by the dentist before the treatment and reprised if necessaryDuration of treatment: 1 year |
| Outcomes |
Assessed at 1, 3, 6 months and 1 year (mouth-opening only assessed at 1 month) VAS pain only reported as median at 3 and 6 months so data not used Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective pain outcomes assessed by patients (except for ‘change in restricted mouth-opening’, which was objective and measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
Low attrition and ITT was used at 1 year for pain on VAS (but quality-of-life data have very high attrition at all assessment points and should be considered at high risk of bias) |
Selective reporting (reporting bias):
|
Outcomes fully reported |
Other bias:
|
No other apparent bias |
| Nilner 200873 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: (1) Department of Stomatognathic Physiology, Malmö University, Sweden; (2) Department of Stomatognathic Physiology, Turku University, Finland Number of centres: two Recruitment period: study performed from February 2005 to August 2007 Sample size calculation: yes (met) – equivalence Funding: public (supported by the Finnish Dental Society, Apollonia and Finska Lakaresallskapet) Declarations/conflicts of interest: ‘The authors report no conflicts of interest’ E-mailed authors for data but none provided so far |
| Participants |
Diagnosis: pain of muscular origin with or without limited opening, according to the RDC/TMD; myofascial pain of at least 4 on a 0–10 NRS Duration since presenting condition began (months): myofascial pain – group A: median 36 (range 6–240); group B: median 36 (range 3–480) Age at baseline (years): (inclusion = at least 18) group A – mean 36 (range 18–71); group B – mean 37 (range 20–63) Sex: group A – 6% male; group B – 16% male Number randomised: 65 (group A: 33; group B: 32) Number evaluated: 10 weeks: 65 (group A: 33; group B: 32); 6 months: 52 (group A: 24; group B: 28); 12 months: 49 (group A: 22; group B: 27) |
| Interventions |
Comparison: custom-made splint vs. prefabricated splint for TMD All patients in both groups were informed about the lack of a clear-cut cause of their myofascial pain and about contributing factors. They were reassured and informed about the nature of TMD and the relationship between muscle fatigue, muscle pain, the psychophysiologic aspects of stress and how to self-monitor TMD symptoms Group AGroup BDuration of treatment: 12 months |
| Outcomes |
Assessed at 6 and 10 weeks, 6 months and 12 months: we used the 10-week, 6-month and 12-month data for our 0–3 month, 3–6 month, and 6–12 month analyses, respectively Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘harms/adverse effects’ – vertical overbite) |
Incomplete outcome data (attrition bias):
|
20% attrition (group A: 27%; group B: 13%) at 6 months; this rose to 25% (group A: 33%; group B: 16%) at 6 months and 36% (group A: 30%; group B: 43%) at 12 months; unequal between groups |
Selective reporting (reporting bias):
|
Some outcomes measured but not reported fully (i.e. with SDs or p-values for between-group differences), or not reported at all at some time points (e.g. VAS pain) |
Other bias:
|
No other apparent bias |
| Nilsson 200943 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Stomatognathic Physiology, University of Malmö, Sweden Number of centres: one Recruitment period: April 2000 to April 2003 Sample size calculation: yes (met at 10 weeks but not 6 or 12 months) Funding: public (study was supported by the Swedish Dental Society and Malmö University) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: mixed TMDs according to RDC/TMD; worst self-assessed TMD pain at least 40 mm on 100 mm VAS Duration since presenting condition began (months): group A – median 24 (range 3–360); group B – median 24 (range 4–72) Age at baseline (years): group A – mean 35 (range 14–67); group B – mean 33 (range 13–68) Sex: group A – 25% male; group B – 11% male Number randomised: 80 (group A: 40; group B: 40) Number evaluated: 68 (group A: 35; group B: 33) at 10 weeks but ITT results reported for outcome of 30% reduction in worst pain; 57 (group A: 32; group B: 25) at 6 months; 51 (group A: 28; group B: 23) at 12 months |
| Interventions |
Comparison: splint vs. control splint for TMD Group A:Group B:Duration of treatment: 12 months |
| Outcomes |
Assessed at 6 and 10 weeks, 6 and 12 months: we used the 10-week, 6-month and 12-month data for our 0–3 month, 3–6 month, and 6–12 month analyses, respectively Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
A TMD specialist not involved in the study fitted and made all adjustments. However, not clear if the patients were aware of their group assignment. If they were aware, this might affect behaviours and introduce performance bias |
Blinding of outcome assessment (detection bias):
|
|
Incomplete outcome data (attrition bias):
|
15% attrition (group A: 13%; group B: 18%) at 10 weeks but this rose to 29% (group A: 20%; group B: 38%) at 6 months and 36% (group A: 30%; group B: 43%) at 12 months |
Selective reporting (reporting bias):
|
TMD pain VAS at 10 weeks reported with no SD or a p-value for the difference between the groups. However, this was reported fully for the longer-term outcomes at 6 and 12 months |
Other bias:
|
No other apparent bias |
| Nitecka-Buchta 201470 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Orthodontics and TMJ Dysfunction, Medical University of Silesia Katowice, Zabrze, Poland Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: public (study was funded by the Medical University of Silesia Katowice, Poland) Declarations/conflicts of interest: ‘The authors have no conflict of interest regarding this commentary’ |
| Participants |
Diagnosis: RDC/TMD examination for group Ia (myofascial pain) and Ib (myofascial pain with limited opening) Duration since presenting condition began: not reported Age at baseline (years): overall mean 47 (range 44–70) Sex: group A – 29% male; group B – 30% male Number randomised: 72 (group A: 36; group B: 36) Number evaluated: 65 (group A: 35; group B: 30) |
| Interventions | Comparison: splint vs. no splint for TMD
|
| Outcomes |
Assessed at 30 days: grouped under 0–3 month analysis Primary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective pain outcome assessment by patients |
Incomplete outcome data (attrition bias):
|
10% attrition (group A: 3%; group B: 17%), which differed by group and may feasibly have biased results |
Selective reporting (reporting bias):
|
Pain reported clearly |
Other bias:
|
No other apparent bias |
| Pierce 198846 | |
| Characteristics | |
| Study details |
Trial design: parallel (five arms) Location: School of Dental Medicine, State University of New York, Buffalo, USA Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: public (study was supported in part by research grants DE-05344 and DE-04358 from the National Institutes of Health, USA) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: (1) self-reported history of bruxism; or (2) currently bruxing and someone else had heard them bruxing; or (3) tooth wear indicating bruxism. This was then confirmed by EMG activity and patients were included only if they had a baseline of mean bruxing episodes per hour of > 1.0 Duration since presenting condition began: not reported Age at baseline (years): overall mean 38 (range 18–72) Sex: 35% male Number randomised: 40 (group A: 20; group B: 20) Number evaluated: not reported |
| Interventions |
Comparison: splint vs. no splint for bruxismEMG monitoring of all patients while sleeping (at their home i.e. not in a sleep clinic); use of EMG monitored at regular appointments Duration of treatment: 2 weeks |
| Outcomes |
Outcomes assessed at 2 weeks (for 2-week treatment phase) and at 6 months (EMG monitoring carried out for a 2-week period and to calculate the means for the bruxism outcomes) Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Not possible to blind |
Blinding of outcome assessment (detection bias):
|
Objective assessment using EMG monitoring while participants were asleep |
Incomplete outcome data (attrition bias):
|
The numbers analysed per group at each assessment were not reported |
Selective reporting (reporting bias):
|
Poor reporting of outcomes |
Other bias:
|
No other apparent bias |
| Rampello 201365 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Clinical Gnathology Service, Umberto I Polyclinic, Sapienza University, Rome, Italy Number of centres: one Recruitment period: January to May 2011 Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: ‘all authors report no conflict of interest relevant to this article’ – however, one of the authors designed and patented the splint [Universal Neuromuscular Immediate Relaxing Appliance (UNIRA)] used in the study E-mailed authors for information and data but none provided so far |
| Participants |
Diagnosis: muscular, articular and headache/migraine VAS scores all > 30; non-reducing dislocations of the articular disc in acute cases of miocene; parafunctions associated with muscular and/or articular pain; limited mouth-opening of muscular origin; abstract mentions ‘according to the RDC-TMD (SPEC) criteria’ Duration since presenting condition began: not reported Age at baseline (years): group A – mean 30.9, SD 7.9 (range 20–46); group B – mean 30.2, SD 7.3 (range 20–45) Sex: group A – 20% male; group B – 12% male Number randomised: 50 (group A: 25; group B: 25) Number evaluated: 50 (group A: 25; group B: 25) |
| Interventions |
Comparison: splint vs. no splint for TMD Group AGroup BDuration of treatment: maximum of 3 months |
| Outcomes |
Assessed at 3 months for splint group but 4 months for control: we would have grouped under 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective pain outcome assessment by patients |
Incomplete outcome data (attrition bias):
|
Does not appear to have been any attrition |
Selective reporting (reporting bias):
|
Although there are no usable data, this is not related to selective reporting |
Other bias:
|
The splint group outcomes were assessed at 3 months (end of treatment) whereas the ‘no treatment’ control group were assessed at 4 months |
| Raphael 200147 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Oral Medicine Clinic, University of Medicine and Dentistry of New Jersey, USA Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: public (study supported by National Institute of Dental and Craniofacial Research grants DE11714 and DE13486) Declarations/conflicts of interest: not reported Author provided some clarification regarding measurement of pain and quality of life |
| Participants |
Diagnosis: myofascial face pain according to RDC/TMD (facial pain complaint was associated with localised tenderness in response to palpation at 3 or more of 20 muscle sites); patients meeting criteria for other TMDs (e.g. TMJ osteoarthritis) also included but only if primary complaint was pain (rather than clicking or restricted mouth-opening) Duration since presenting condition began: mean duration of pain = 5 years (30% reported duration of ≤ 1 year; 19% reported duration of ≥ 10 years) Age at baseline (years): mean 33.7 (SD 10.9) Sex: all female Number randomised: 68 (group A: 35; group B: 33) Number evaluated: 63 (group A: 32; group B: 31) |
| Interventions |
Comparison: splint vs. control splint for TMD Group AGroup BDuration of treatment: 6 weeks |
| Outcomes |
Some outcomes measured at 2, 4 and 6 weeks – in all cases, we used only the 6-week data, which were grouped under 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Unclear if the personnel fitting and adjusting the splints were involved in the study. Also unclear if the patients were aware of their group assignment. If they were aware, this might affect behaviours and introduce performance bias |
Blinding of outcome assessment (detection bias):
|
Personnel carrying out the RDC examinations were blinded. However, other outcomes were patient-reported and it was not clear if the patients were aware of their group assignment |
Incomplete outcome data (attrition bias):
|
7% attrition (group A: 9%; group B: 6%): low attrition, similar between groups and reasons stated |
Selective reporting (reporting bias):
|
Although details around some outcomes are poorly reported, there is no evidence of selective reporting |
Other bias:
|
No other apparent bias |
| Rubinoff 198748 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: School of Dental Medicine, State University of New York, Buffalo, NY, USA Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: myofascial pain dysfunction based on complaint of facial pain plus one or more of the following: limited opening, joint sounds, deviation on opening, tenderness to muscle palpation. Also required to have absence of organic pathologic condition of the TMJ assessed clinically and radiographically Duration since presenting condition began: not reported Age at baseline (years): mean 33.7 (range 18–62) Sex: group A – 20% male; group B – 8% male Number randomised: 28 (group A: 15; group B: 13) Number evaluated: 25 (group A: 14; group B: 11) for patient-reported pain; 26 (group A: 15; group B: 11) for all other outcomes |
| Interventions |
Comparison: splint vs. control splint for TMD Group AGroup BDuration of treatment: 6 weeks |
| Outcomes |
Assessed at 6 weeks: grouped under 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
‘Two dentists participated in this study, one as an examiner and one as a therapist. Neither the examiner nor the patient knew which type of appliance the patient wore. Moreover, the therapist was unaware of the data collected by the examiner during the six weeks of active treatment’ Comment: unclear if therapist was aware of the purpose of the study. Patients did not know which type of splint they had |
Blinding of outcome assessment (detection bias):
|
See above quote Comment: patients and outcome assessors did not know which type of splint they had |
Incomplete outcome data (attrition bias):
|
Only one patient missing from group A and two from group B for patient-reported pain |
Selective reporting (reporting bias):
|
Individual patient data all reported |
Other bias:
|
No other bias apparent |
| Sharma 201649 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: School of Dental Medicine, State University of New York, Buffalo, USA Number of centres: one Recruitment period: not reported Sample size calculation: no (post hoc only) Funding: not reported Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: bilateral masseter myalgia according Diagnostic Criteria for TMDs (DC/TMD); pain intensity of 5 or more on a 0 (no pain) to 10 (worst pain) scale; morning symptoms of jaw pain and stiffness Duration since presenting condition began: not reported Age at baseline (years): (overall range 24 to 62) group A: mean 42.6 (SD 9.6); group B: mean 35 (SD 9.5) Sex: group A – 0% male; group B – 17% male Number randomised: 13 (group A: 7; group B: 6) Number evaluated: 13 (group A: 7; group B: 6) – two dropouts but not reported by group A, B or C, and not clear if ITT used |
| Interventions |
Comparison: splint vs. no splint for TMD In groups A and B, if indicated, ethyl chloride vapocoolant spray was used during spray and stretch physical therapy sessions once per week for a total of four treatment sessionsDuration of treatment: 5 weeks |
| Outcomes |
Assessed at 5 weeks: grouped under 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
No mention of allocation concealment |
Blinding of participants and personnel (performance bias):
|
Not possible to blind patients |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘change in restricted mouth-opening’ which was objective and measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
Two dropouts but not reported which group and not clear if ITT used in analyses |
Selective reporting (reporting bias):
|
Outcomes fully reported |
Other bias:
|
No other bias apparent |
| Tatli 201754 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: TMD clinic, Cukurova University Dental Hospital, Adana, Turkey Number of centres: one Recruitment period: not reported Sample size calculation: yes (achieved) Funding: none Declarations/conflicts of interest: ‘nothing to declare’ |
| Participants |
Diagnosis: unilateral TMJ disc displacement without reduction diagnosis based on clinical DC/TMD (history of reduction in mouth-opening, TMJ pain during palpation and/or function, TMJ clicking) and MRI Duration since presenting condition began: not reported Age at baseline (years): group A – mean 38.9 (SD 11.3); group B – mean 35.2 (SD 9.4) Sex: group A – 2.5% male; group B – 12.5% male Number randomised: 80 (group A: 40; group B: 40) Number evaluated: 80 (group A: 40; group B: 40) |
| Interventions |
Comparison: splint vs. no splint for TMD All patients in groups A and B were treated with arthrocentesis plus sodium hyaluronate at the start of the studyDuration of treatment: 6 months |
| Outcomes |
Assessed as 1, 3 and 6 months: we used the 3- and 6-month data in our 0–3 month and 3–6 month analyses, respectively Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘change in restricted mouth-opening’, which was objective and measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
All randomised patients were included in the analyses |
Selective reporting (reporting bias):
|
Outcomes fully reported |
Other bias:
|
No other bias apparent |
| Tavera 201223 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: Mexican Institute for Clinical Research, Mexico Number of centres: one Recruitment period: May to September 2008 Trials registry ID: NCT00815776 Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported We e-mailed authors for data but none provided so far |
| Participants |
Diagnosis: RDC/TMD diagnosis of myofascial pain, arthralgia, and/or disc displacement with reduction, and a VAS pain score of > 4 (0–10 worsening scale) Duration since presenting condition began: not reported Age at baseline (years): group A – mean 38 (SD 11); group B – mean 36.3 (SD 13) Sex: group A – 17% male; group B – 11% male Number randomised: 108 (group A: 71; group B: 37) Number evaluated: 78 (group A: 56; group B: 22) |
| Interventions |
Comparison: splint vs. minimal treatment for TMD Group A:Group BGroup CDuration of treatment: 3 months |
| Outcomes |
Assessed at 1, 2 and 3 months: we would have used the 3-month data in our 0–3 month analyses Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding was not possible |
Blinding of outcome assessment (detection bias):
|
Neither patients nor study personnel were blinded |
Incomplete outcome data (attrition bias):
|
Overall attrition was 28% (group A: 20%; group B: 43% at 2 months; very similar at 3 months). Attrition was notably higher in group B |
Selective reporting (reporting bias):
|
Very poor reporting of outcomes – focuses on TMDes group (group C) |
Other bias:
|
No other bias apparent |
| Truelove 200650 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Orofacial Pain Clinic, Department of Oral Medicine, University of Washington, Seattle, USA Number of centres: one Recruitment period: not reported Sample size calculation: yes (not met) Funding: public (study supported by National Institute of Dental and Craniofacial Research grant P01 DE-08773) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: RDC/TMD Axis I diagnosis of myofascial pain (group Ia or Ib) with or without a concurrent diagnosis of arthralgia (Group IIIa) or disk displacement with reduction (Group IIa), as well as a RDC/TMD Axis II Graded Chronic Pain score of grade I (low pain) or grade II (high pain), both of which had no or minimal pain-related psychosocial interference. Any other RDC/TMD Axis I diagnosis (e.g. arthritis, disk displacement without reduction) was excluded Duration since presenting condition began: years with facial pain: group A – mean 6 (SD 9); group B – mean 5 (SD 6); group C – mean 5 (SD 5) Age at baseline (years): group A – mean 36 (SD 11); group B – mean 35 (SD 12); group C – mean 36 (SD 11) Sex: group A – 13% male; group B – 10% male; group C – 19% male Number randomised: 200 (group A: 68; group B: 68; group C: 64) Number evaluated: 3 months: 164 (group A: 54; group B: 56) |
| Interventions |
Comparison: (1) splint vs. no splint for TMD; (2) custom-made splint vs. prefabricated splint for TMD All groups received usual treatment: dentist-prescribed, conservative and reversible self-care strategies that required the dentist to follow a standardised treatment checklist that identifies all treatment recommendations (jaw relaxation, reduction of parafunction, thermal packs, NSAIDs, passive opening stretches and suggestions about stress reduction); treatments such as narcotic analgesics, antidepressant medications and use of a non-study prescribed splint were discouraged Group AGroup B:Group CDuration of treatment: 12 months |
| Outcomes |
Assessments at 3, 6* and 12 months: we used the 3- and 12-month data in our 0–3 month and 6–12 months analyses, respectively *Data at 6 months not reported because ‘we typically found six-month data to be intermediate or equivalent to 12-month data’ Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding was not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘change in restricted mouth-opening’ and ‘TMJ clicking’, which were objective and measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
Overall attrition 18% (group A: 21%; group B: 18%; group C: 16%) at 3 months; overall attrition 16% (group A: 4%; group B: 19%; group C: 25%) at 12 months. There was a large difference between group A and the other groups at 12 months |
Selective reporting (reporting bias):
|
Although we were unable to use some of the data, this does not appear to be because of selective reporting |
Other bias:
|
No other bias apparent |
| van der Zaag 200569 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Clinic for Oral Kinesiology at Academic Centre for Dentistry Amsterdam (ACTA), the Netherlands Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: public (study supported by The Netherlands Institute of Dental Sciences) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: recent history of tooth-grinding sounds for at least 3 nights per week during the previous 6 months, confirmed by patient or their partner; tooth wear to at least the degree of exposed dentine (grade 2); presence of bruxism was clinically established by means of an inspection of the soft and hard intraoral tissues; patients with a TMD pain diagnosis were excluded (this was examined using the RDC/TMD); if the participant was eligible to enrol in the study protocol, they underwent a first polysomnographic recording at the hospital’s sleep laboratory (i.e. does not seem to be part of eligibility/diagnosis) Duration since presenting condition began: at least 6 months Age at baseline (years): (had to be aged ≥ 18 years as part of eligibility) group A – mean 34.2 (SD 13.1; range 21–68); group B – mean 34.9 (SD 11.2; range 18–55) Sex: group A – 36% male; group B – 10% male Number randomised: 27 (not reported by group) Number evaluated: 21 (group A: 11; group B: 10) |
| Interventions |
Comparison: splint vs. control splint for bruxism Group AGroup BDuration of treatment: 4 weeks |
| Outcomes |
Assessed at 4 weeks: grouped under 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
|
Blinding of outcome assessment (detection bias):
|
Objective measurements carried out by a machine. Furthermore, the data were analysed by an investigator blinded to the patients’ allocated splint |
Incomplete outcome data (attrition bias):
|
Although overall attrition was 22% (not reported by group), none of the reasons were linked to treatment allocation or outcomes |
Selective reporting (reporting bias):
|
Data fully reported |
Other bias:
|
No other apparent bias |
| Wahlund 200344 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: TMD Unit, Specialist Centre for Oral Rehabilitation, Linkoping, Sweden Number of centres: one Recruitment period: 1996–2000 Sample size calculation: not reported Funding: public (study was supported by the Public Dental Service of Ostergotland – County Council) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: TMD pain according to RDC/TMD Duration since presenting condition began: at least 3 months Age at baseline (years): overall range 12–18; group A – mean 15.7 (SD 2.1); group B – mean 14.8 (SD 1.9) Sex: group A – 26% male; group B – 31% male Number randomised: 81 (group A: 42; group B: 39) Number evaluated: 76 (group A: 37; group B: 39) |
| Interventions |
Comparison: splint vs. no splint for TMD All patients received an individual 30-minute session in which TMD-related anatomy, pain epidemiology, parafunction and stress were discussedDuration of treatment: not clear from the text of the study report. There was a treatment period that seems to have been 2 or 4 weeks long, but then there was follow-up at 6 months. From the end of the treatment period to the 6-month follow-up, patients were instructed to wear their splint whenever needed |
| Outcomes |
All outcomes are reported at the end of treatment period (unclear how many weeks), which we included in our 0–3 month analysis, and at 6 months’ follow-up, which we included in our 3–6 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Subjective outcomes assessment by patients (except for ‘change in restricted mouth-opening’, which was objective and measured by a blinded assessor) |
Incomplete outcome data (attrition bias):
|
Overall attrition 6% (group A: 12; group B: 0%); ‘subjects who dropped out had lower pain scores and less motivation to participate in treatment’ – this may have biased the results |
Selective reporting (reporting bias):
|
Outcomes poorly reported and mostly unusable |
Other bias:
|
No other bias apparent |
| Wassell 200422 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) – non-responders from the control group were allowed to cross over after 6 weeks; therefore, we report data up to 6 weeks Location: general dental practices in and around Newcastle, UK Number of centres: 11 (two of these centres did not return their data) Recruitment period: February 1994 to end of July 1996 Sample size calculation: not reported Funding: public (sponsored by British Dental Association Research Foundation through the Shirley Glasstone Hughes Memorial Prize) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: pain in TMJ or muscles or both plus one or more of the following: joint sounds; history of jaw locking or limited opening; TMJ or muscle tenderness on palpation Subdiagnosis later assigned using criteria developed in collaboration between the International Headache Society and the American Academy of Craniomandibular Disorders Duration since presenting condition began: > 4 weeks Age at baseline (years): (had to be aged ≥ 18 years) group A – mean 37.9 (SD 12.6); group B – mean 35.9 (SD 10.3) Sex: 12% male (of 78 patients who started treatment – not reported by group) Number randomised: 93 (not reported by group) Number evaluated: 72 (group A: 38; group B: 34) |
| Interventions |
Comparison: splint vs. control splint for TMD Group AGroup BDuration of treatment: 6 weeks (treatment continued for longer but non–responders crossed over after 6 weeks) |
| Outcomes |
Assessed at 3 and 6 weeks: we used the 6-week data for our 0–3 month analysis (TMJ-clicking only) Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
The authors state that it was not a blinded study |
Blinding of outcome assessment (detection bias):
|
Outcome assessment was not blinded |
Incomplete outcome data (attrition bias):
|
Overall attrition 23%, but mostly due to general dental practice withdrawal |
Selective reporting (reporting bias):
|
Very poorly reported with no measures of variability around the means |
Other bias:
|
No other bias apparent |
| Wright 199551 | |
| Characteristics | |
| Study details |
Trial design: parallel (three arms) Location: TMJ and Craniofacial Pain Clinic, University of Minnesota, MN, USA Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: orofacial pain with clinical evidence of a masticatory muscle origin (medical history and clinical examination used to rule out other sources of pain such as dental, metabolic and neurological disorders); inclusion criteria included (1) patient’s pain aggravated by jaw function (e.g. talking/eating) or parafunctional habits (e.g. clenching or grinding teeth) – based on patient history and (2) pain aggravated/duplicated by palpation of the muscles of mastication – based on clinical examination; TMJ intra-articular sources of pain ruled out by exclusion criteria: (1) pain aggravated by clinical loading of TMJ – based on clinical examination, (2) pain aggravated by TMJ clicking or catching or both – based on patient history and clinical examination Duration since presenting condition began: not reported Age at baseline (years): (overall range 19–51): group A – mean 34; group B – mean 31 Sex: not reported Number randomised: 20 (group A: 10; group B: 10) Number evaluated: 20 (group A: 10; group B: 10) |
| Interventions | Comparison: splint vs. no splint for TMD
|
| Outcomes |
Assessed at end of treatment (roughly 6 weeks): grouped under 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
|
Incomplete outcome data (attrition bias):
|
Two dropouts but they were replaced (see above) |
Selective reporting (reporting bias):
|
Outcomes fully reported |
Other bias:
|
No other apparent bias |
| Yu 201659 | |
| Characteristics | |
| Study details |
Trial design: parallel (four arms) Location: Department of Prosthodontics, Shanghai Ninth People’s Hospital, Shanghai, China Number of centres: one Recruitment period: February 2013 to March 2015 Sample size calculation: not reported Funding: unclear if public or other (Fund of Construction of Shanghai Key Subject, T0202) Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: TMJ disc displacement without reduction (RDC/TMD) Duration since presenting condition began: unclear Age at baseline (years): mean 32.5 (SD 9.8) (only overall data available) Sex: 11.3% male (only overall data available) Number randomised: 168 (group A: 42; group B: 42; group C: 42; group D: 42) Number evaluated: 168 (group A: 42; group B: 42; group C: 42; group D: 42) |
| Interventions |
Comparison: splint vs. no/minimal treatment for TMD We split the four groups/arms into two pairwise comparisons of A vs. D and C vs. BDuration of treatment: 3 months |
| Outcomes |
Assessed at 3 months: grouped under 0–3 month analysis Primary:Secondary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Pain assessed by patients, who were not blinded |
Incomplete outcome data (attrition bias):
|
No dropouts |
Selective reporting (reporting bias):
|
Outcomes fully reported |
Other bias:
|
No other apparent bias |
| Zhang 201360 | |
| Characteristics | |
| Study details |
Trial design: parallel (two arms) Location: Department of Stomatology, AnZhen Hospital, Capital Medical University, Beijing, China Number of centres: one Recruitment period: not reported Sample size calculation: ‘The sample size for this study was calculated’ – no further details (apparently met) Funding: not reported Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: RDC/TMD Axis 1 (myofascial pain) Duration since presenting condition began: as part of eligibility criteria, patients had to have chronic TMD pain for > 6 months (longest reported duration was 3 years): group A – mean 8.3 months (SD 6.4); group B – mean 6.5 months (SD 6.4) Age at baseline (years): (overall range 16–57) group A – mean 31.4 (SD 9); group B – mean 31.3 (SD 8.3) Sex: 33% male (not reported by group) Number randomised: 36 (group A: 18; group B: 18) Number evaluated: 36 (group A: 18; group B: 18) |
| Interventions |
Comparison: splint vs. control splint for TMD Group AGroup BDuration of treatment: 1 month |
| Outcomes |
Assessed at 1 month: grouped under 0–3 month analysis Primary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
‘double-blind’ Comment: only blinding of the examiner was specified |
Blinding of outcome assessment (detection bias):
|
‘double-blind’ and ‘The second examiner . . . performed the clinical examination . . . This examiner was blinded to the type of treatment the patient received’ Comment: blinding of the examiner is not relevant to the outcome of pain, and it is not clear if the patients were properly blinded to their treatment |
Incomplete outcome data (attrition bias):
|
All randomised patients were included in the analyses |
Selective reporting (reporting bias):
|
Very poorly reported outcomes. Pain outcome not mentioned in the methods section and the description of the results is vague and does not appear to match the results reported in the table |
Other bias:
|
No other apparent bias |
| Zuim 200634 | |
| Characteristics | |
| Study details |
Trial design: parallel (four arms) Location: Temporomandibular Disorders Diagnostic and Treatment Centre, Aracatuba Dental School, São Paulo State University, Brazil Number of centres: one Recruitment period: not reported Sample size calculation: not reported Funding: not reported Declarations/conflicts of interest: not reported |
| Participants |
Diagnosis: TMD patients with chronic pain, muscle pain on palpation Duration since presenting condition began: at least 6 months Age at baseline (years): 13–47 (not reported by group) Sex: 10% male (not reported by group) Number randomised: 20 (group A: 5; group B: 5; group C: 5; group D: 5) Number evaluated: 20 (group A: 5; group B: 5; group C: 5; group D: 5) |
| Interventions |
Comparison: splint vs. no splint for TMD We split the four groups/arms into two pairwise comparisons of A vs. B and C vs. D:Group AGroup BGroup CGroup DDuration of treatment: 1 month |
| Outcomes |
Assessed at 1 month: we would have grouped under 0–3 month analysis Primary: |
| Risk of bias | |
Random sequence generation (selection bias):
|
|
Allocation concealment (selection bias):
|
|
Blinding of participants and personnel (performance bias):
|
Blinding not possible |
Blinding of outcome assessment (detection bias):
|
Pain assessed by patients, who were not blinded |
Incomplete outcome data (attrition bias):
|
All randomised patients were included in the analyses |
Selective reporting (reporting bias):
|
Individual patient data reported |
Other bias:
|
No other apparent bias |
Appendix 4 Forest plots of comparisons for the systematic review
FIGURE 15.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – pain: any combinable scale (higher = more pain), 3–6 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Current pain intensity 0 to 100 mm VAS (authors confirmed that scores were accidentally reported in cm – we converted them to mm); b, current pain intensity 0 to 10 cm VAS (we converted this to mm).
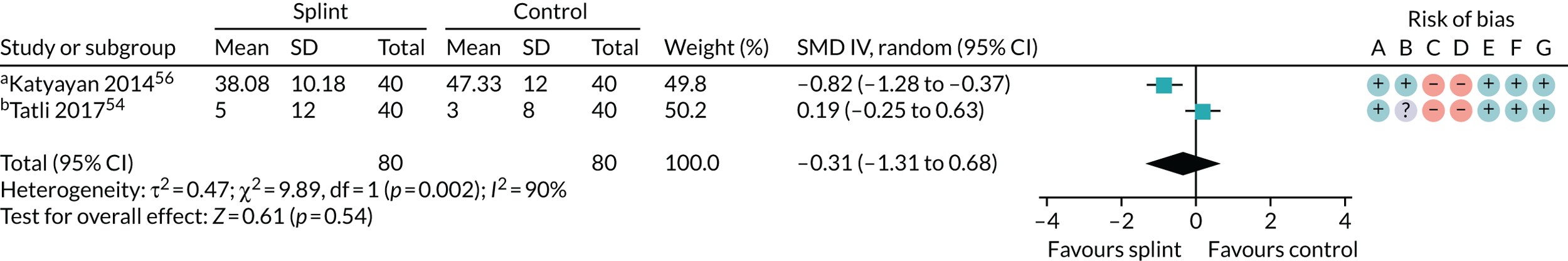
FIGURE 16.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – pain: any combinable scale (higher = more pain), 6–12 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Current facial pain intensity 0 to 10 cm VAS (we converted this to mm); b, CPI 0 to 10 converted to 0 to 100 scale – SD is median value from range of SDs reported in paper; c, CPI 0 to 10 converted to 0 to 100 scale – SD is median value from range of SDs reported in paper (custom-made splint vs. control).
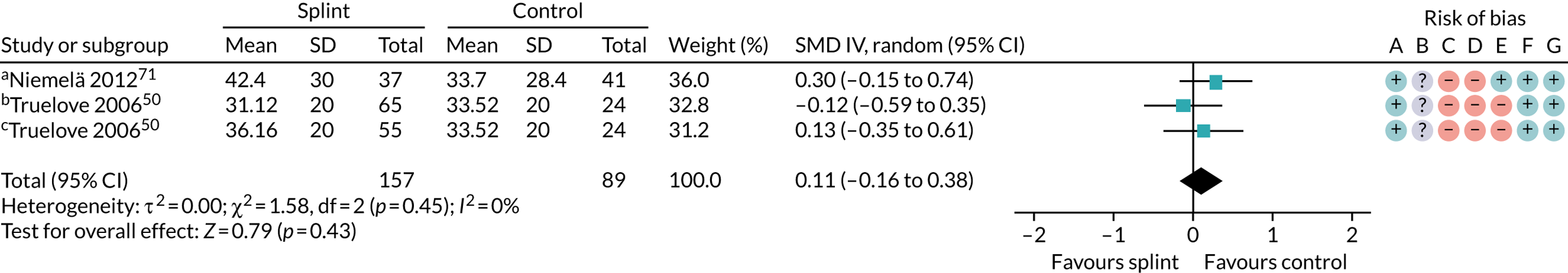
FIGURE 17.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – pain: 50% reduction in VAS pain, 0–3 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Custom stabilisation; b, custom NTI.
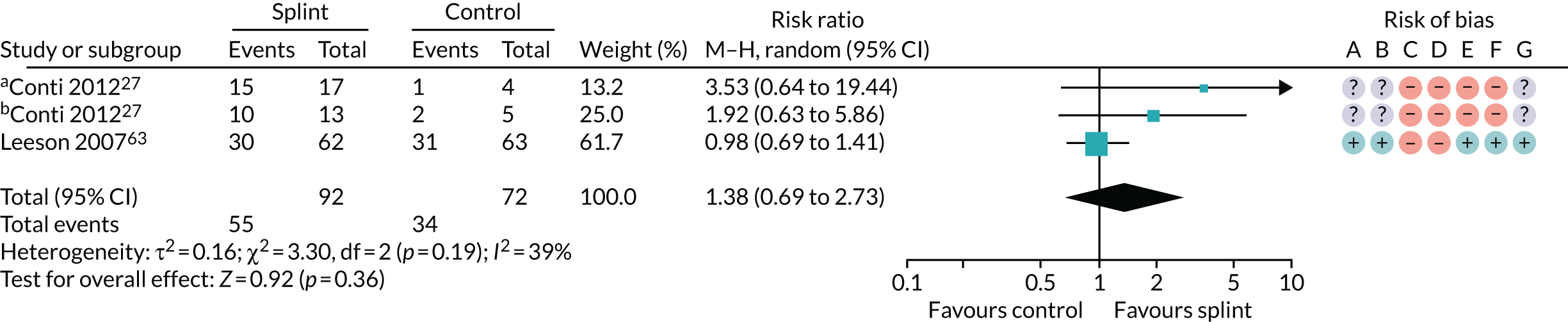
FIGURE 18.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – pain: 50% reduction in VAS pain, 6–12 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
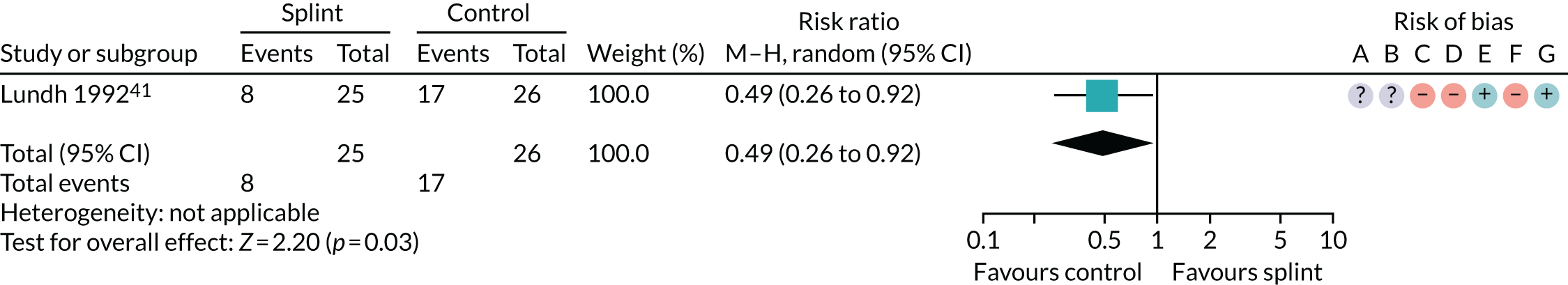
FIGURE 19.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – CPI 0–100 worsening scale, 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Reported as change score (mean decrease in score).
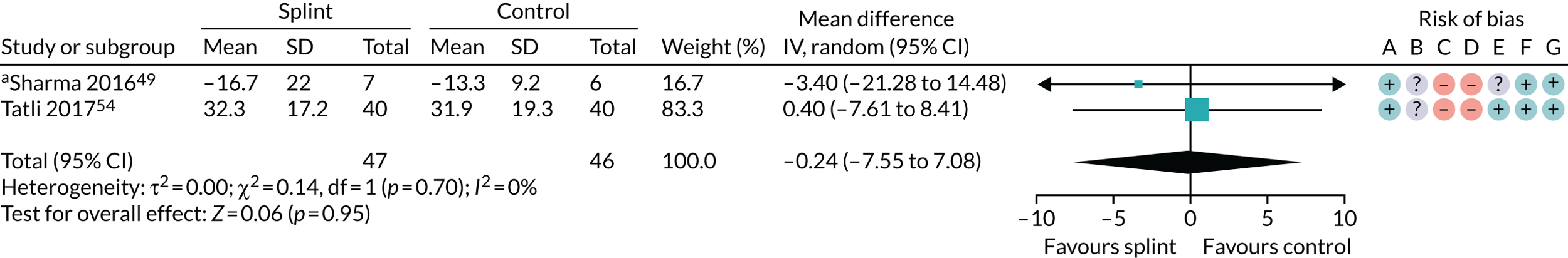
FIGURE 20.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – CPI 0–100 worsening scale, 3–6 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
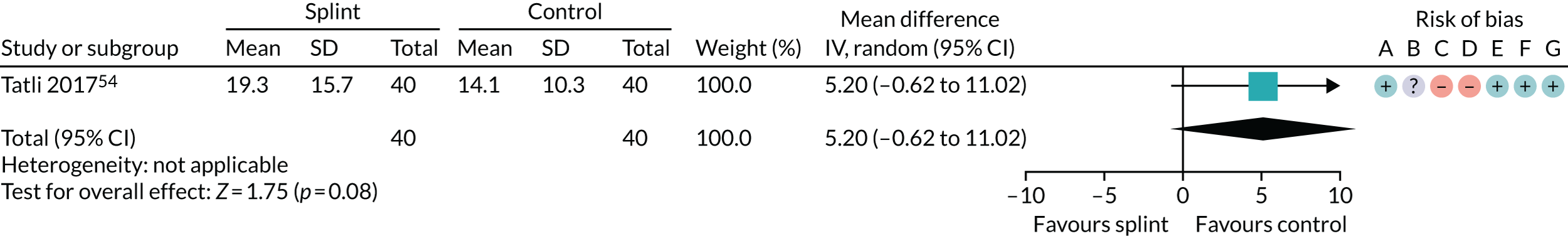
FIGURE 21.
Forest plot of comparison: TMD, splint vs. control splint; outcome – pain: any combinable scale (higher = more pain), 3–6 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
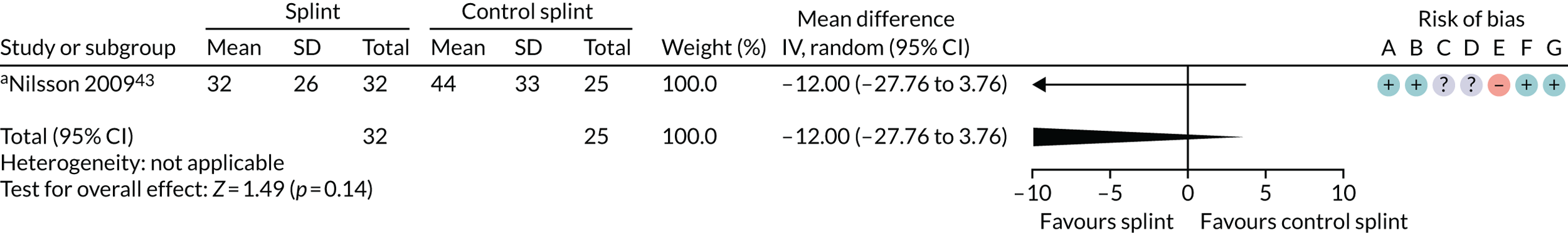
FIGURE 22.
Forest plot of comparison: TMD, splint vs. control splint; outcome – pain: any combinable scale (higher = more pain), 6–12 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Worst pain on 0 to 100 VAS.

FIGURE 23.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – TMJ clicking: presence of joint noises (detected during TMJ palpation/opening/closing), 0–3 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Anterior repositioning splint; b, flat occlusal splint; c, custom; d, prefabricated.
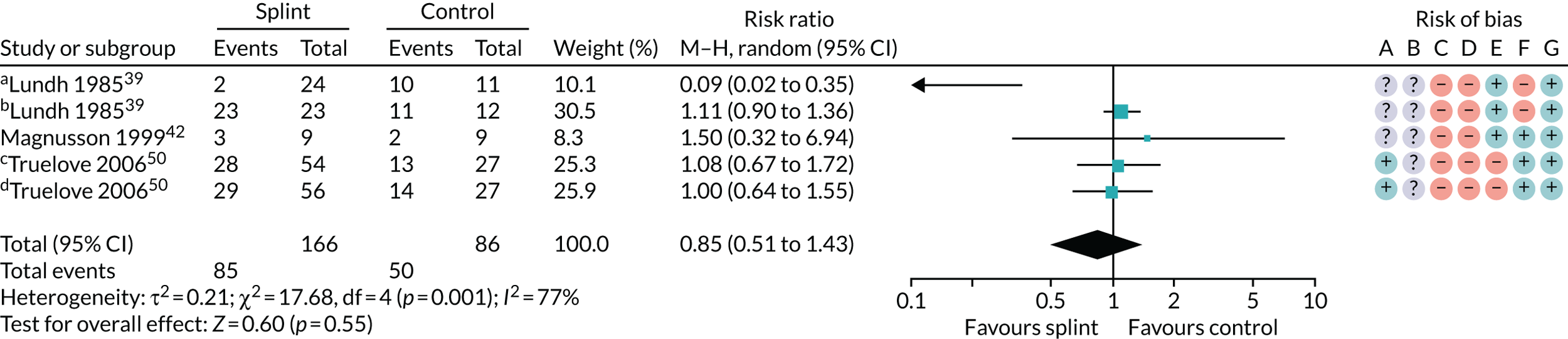
FIGURE 24.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – TMJ clicking: presence of joint noises (detected during TMJ palpation/opening/closing), 3–6 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Flat occlusal splint; b, anterior repositioning splint.
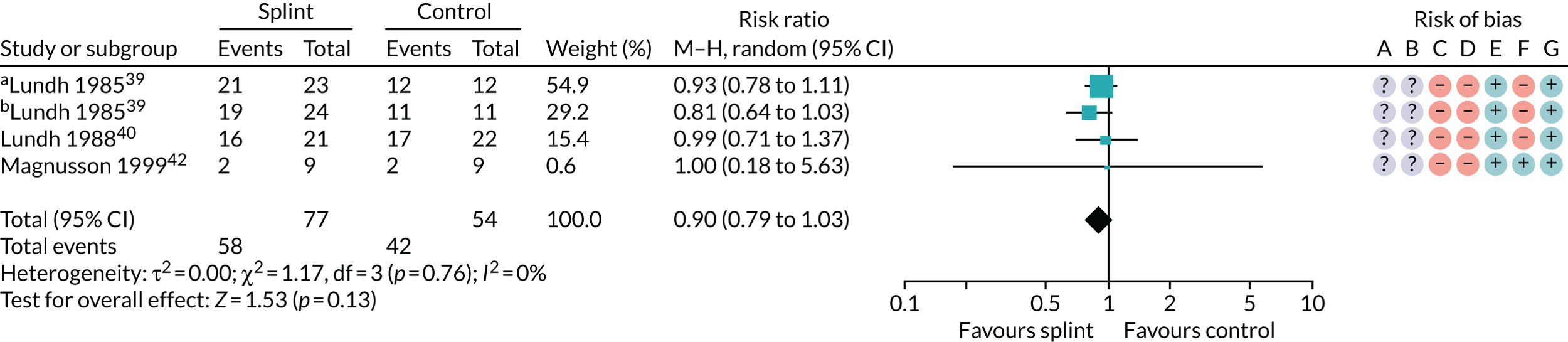
FIGURE 25.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – TMJ clicking: presence of joint noises (detected during TMJ palpation/opening/closing), 6–12 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Flat occlusal splint; b, anterior repositioning splint; c, custom; d, prefabricated.
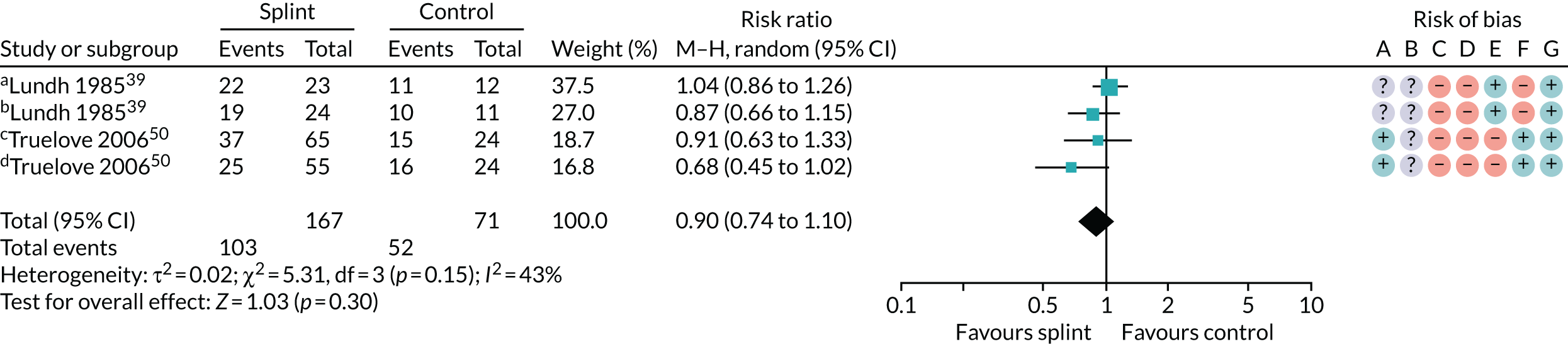
FIGURE 26.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – change in restricted mouth-opening: maximum mouth-opening (mm), 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Custom NTI-tss (unassisted opening until pain felt); b, custom anterior repositioning (unassisted opening until pain felt); c, unclear if with/without/until pain or assisted/unassisted; d, custom splint (unassisted opening without pain); e, prefabricated splint (unassisted opening without pain); f, opening without pain; g, unassisted pain-free opening; h, unassisted pain-free opening (reported as change score); i, asked to open mouth as wide as possible, even if they felt pain; j, unassisted (with/without/until pain not reported); k, pain-free opening (reported as change score); l, unclear if with/without/until pain or assisted/unassisted; m, assisted opening without pain; n, pain-free opening; o, pain-free unassisted opening (splint vs. control); p, pain-free unassisted opening (splint + manipulative and physical therapies vs. manipulative and physical therapies).
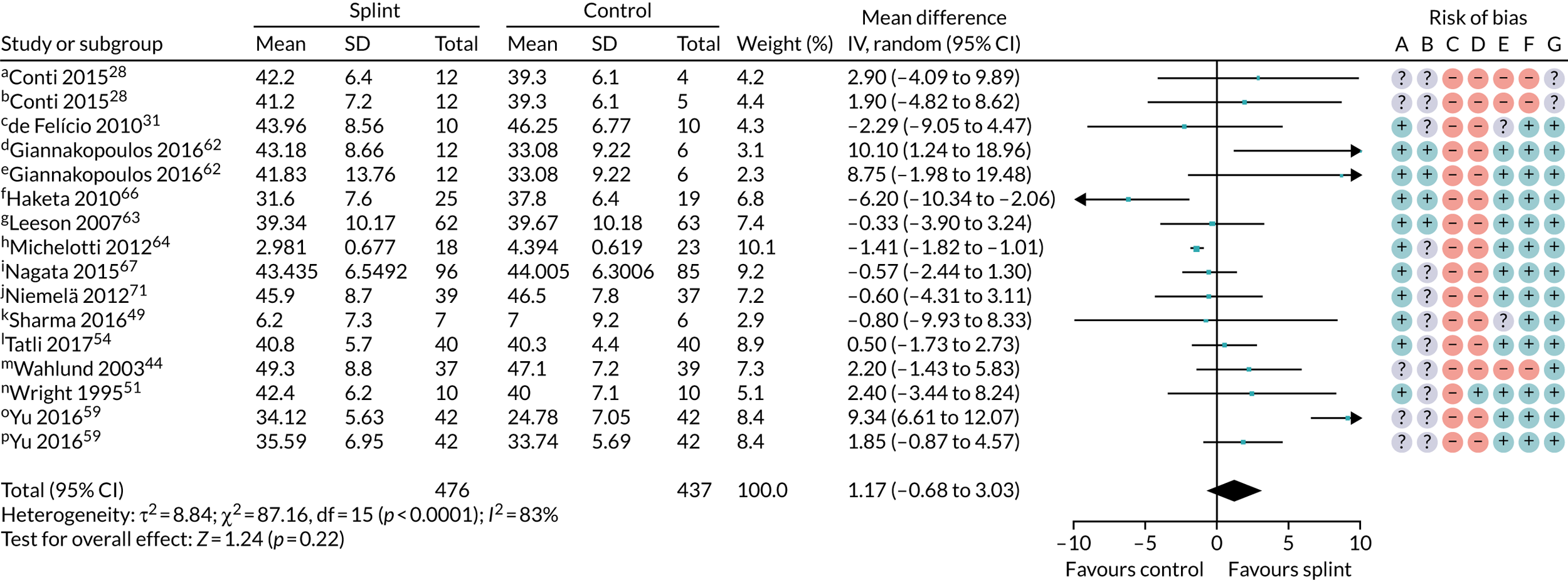
FIGURE 27.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – change in restricted mouth-opening: maximum mouth-opening (mm), 3–6 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, The sum of unassisted maximal interincisal opening and the vertical incisal overlap; b, unclear if with/without pain or assisted/unassisted; c, assisted opening without pain.
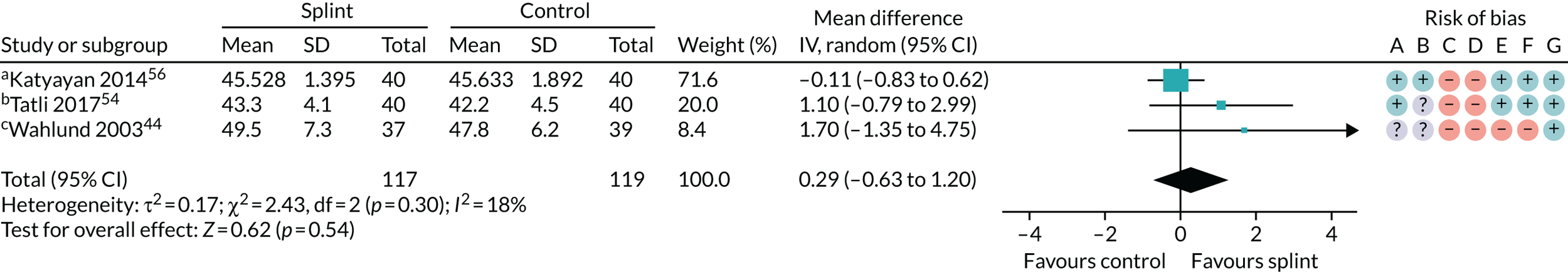
FIGURE 28.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – quality of life: OHIP-14, 0–56 worsening scale, 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Change score reported; b, end score reported.
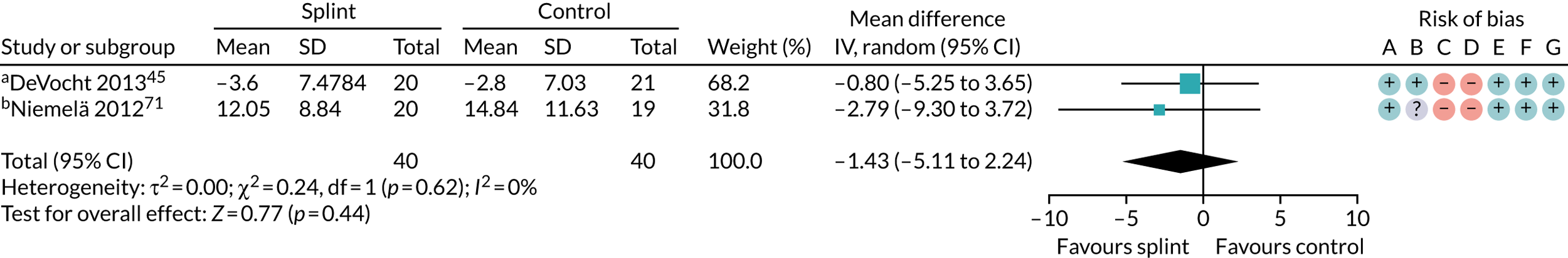
FIGURE 29.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – quality of life: OHIP-14, 0–56 worsening scale, 3–6 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Change score reported; b, end score reported.
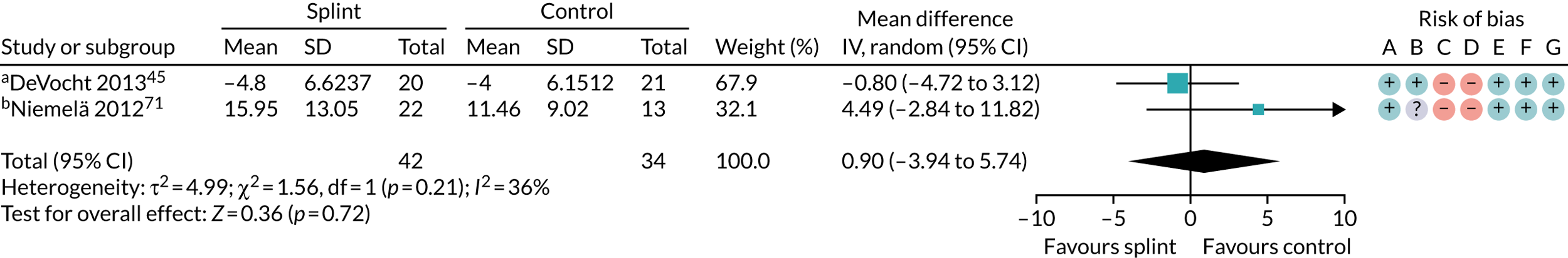
FIGURE 30.
Forest plot of comparison: TMD, splint vs. no/minimal treatment; outcome – quality of life: OHIP-14, 0–56 worsening scale, 6–12 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
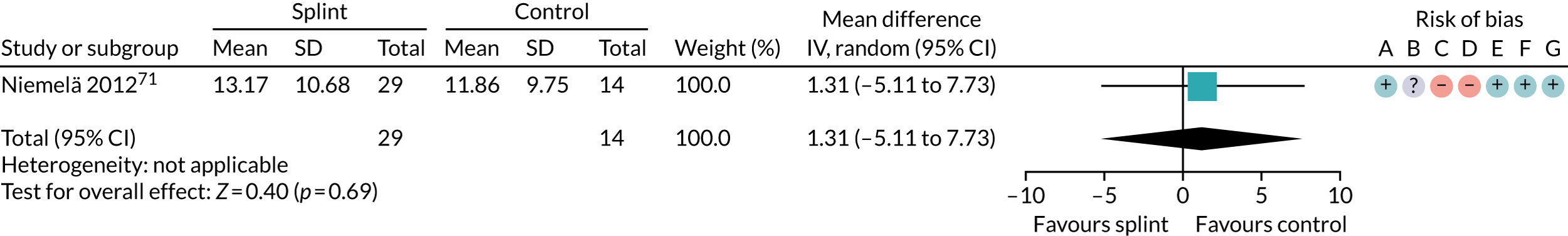
FIGURE 31.
Forest plot of comparison: TMD, splint vs. control splint; outcome – TMJ clicking: presence of joint sounds during palpation, 0–3 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
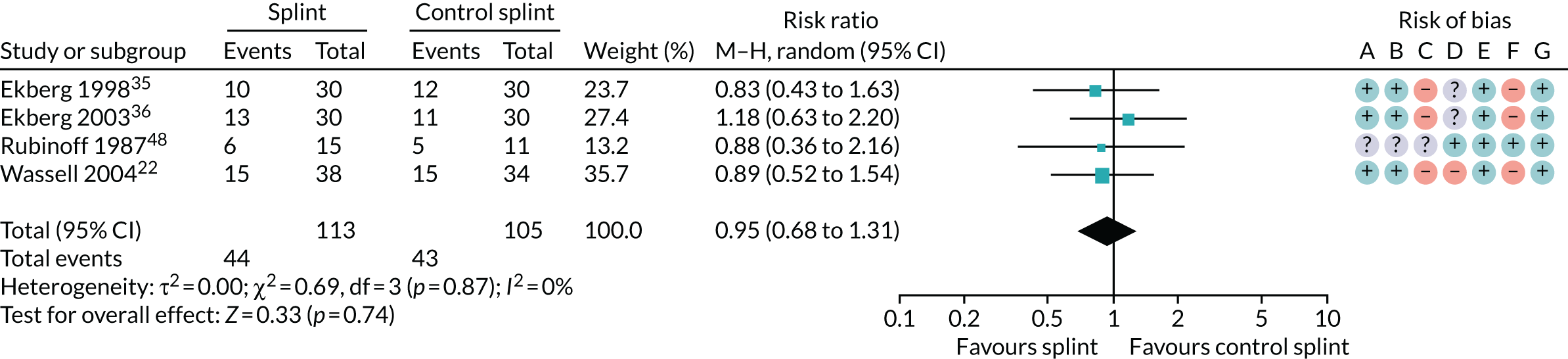
FIGURE 32.
Forest plot of comparison: TMD, splint vs. control splint; outcome – change in restricted mouth-opening: opening < 40 mm. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
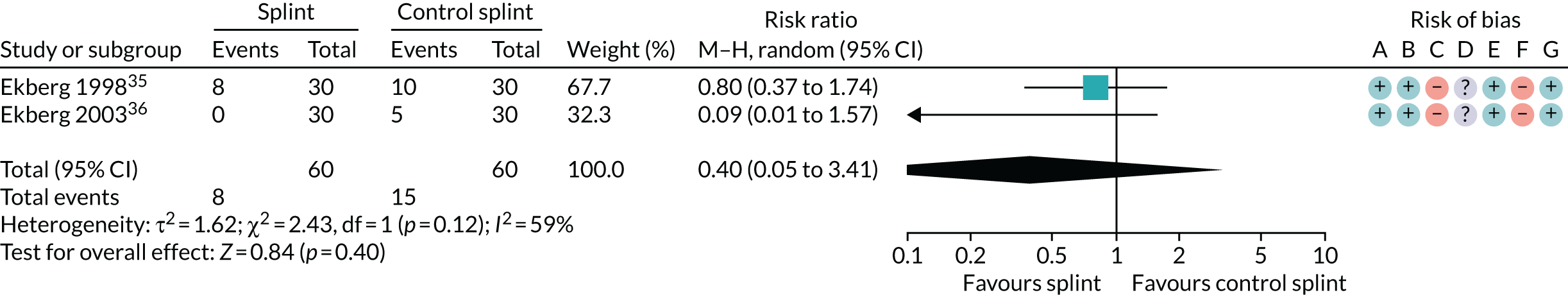
FIGURE 33.
Forest plot of comparison: TMD, splint vs. control splint; outcome – compliance: splint worn every night or most nights, 0–3 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
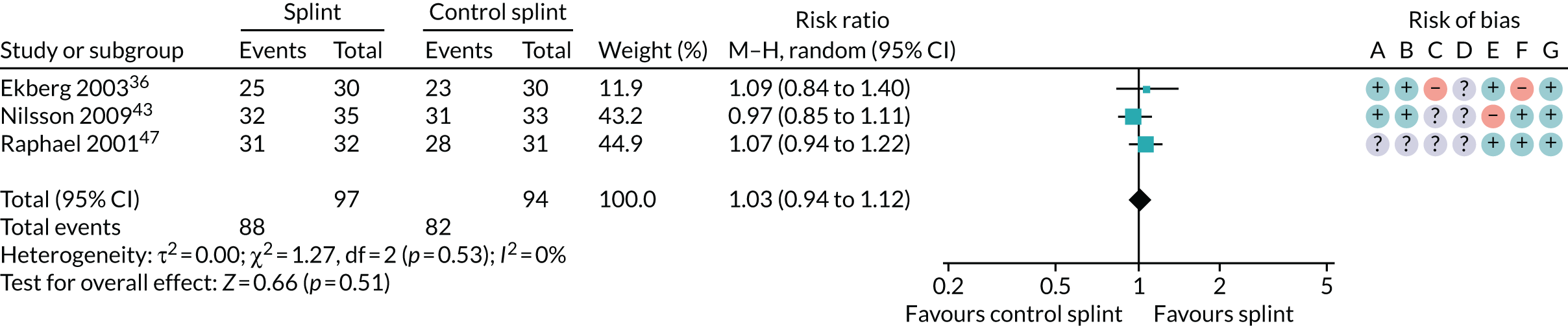
FIGURE 34.
Forest plot of comparison: TMD, splint vs. control splint; outcome – compliance: splint worn every night or most nights, 3–6 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
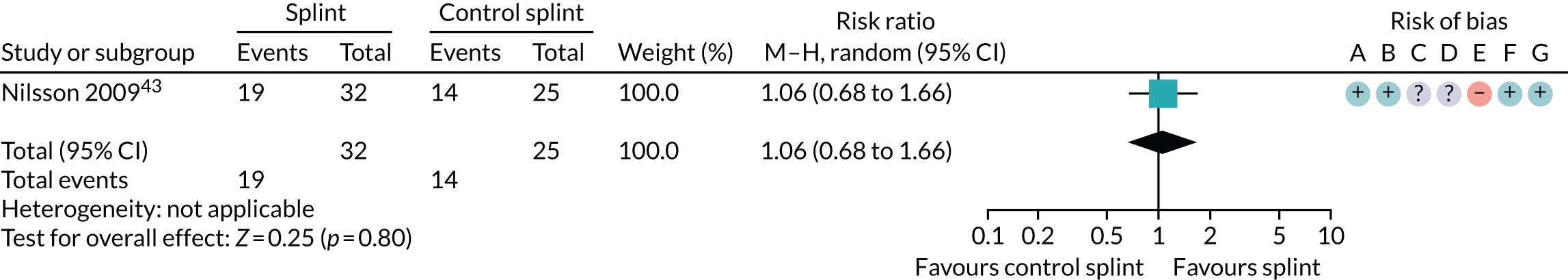
FIGURE 35.
Forest plot of comparison: TMD, splint vs. control splint; outcome – compliance: splint worn every night or most nights, 6–12 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
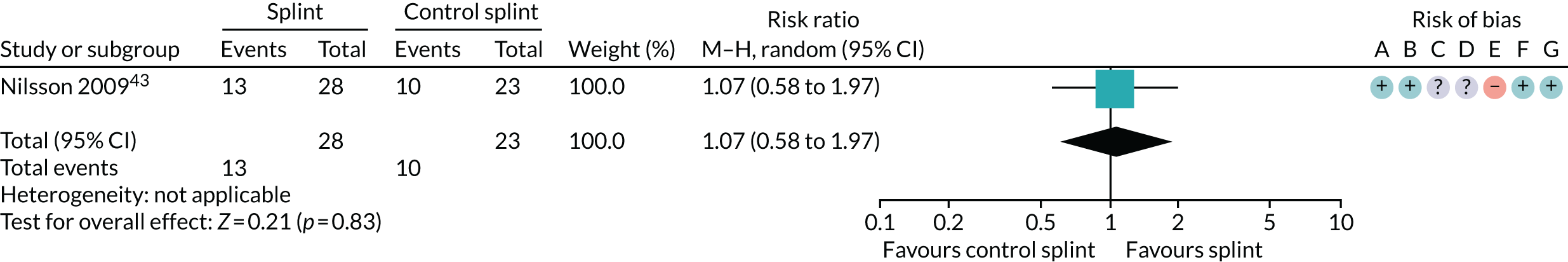
FIGURE 36.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – pain: any combinable scale (higher = more pain), 3–6 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Assessed daily in a 1-week pain diary for the week prior to each assessment point using 0 to 100 VAS (pain at rest).
FIGURE 37.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – pain: any combinable scale (higher = more pain), 6–12 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Assessed daily in a 1-week pain diary for the week prior to each assessment point using 0 to 100 VAS (pain at rest); b, CPI 0 to 10 converted to 0 to 100 scale – SD is median value from range of SDs reported in the paper.
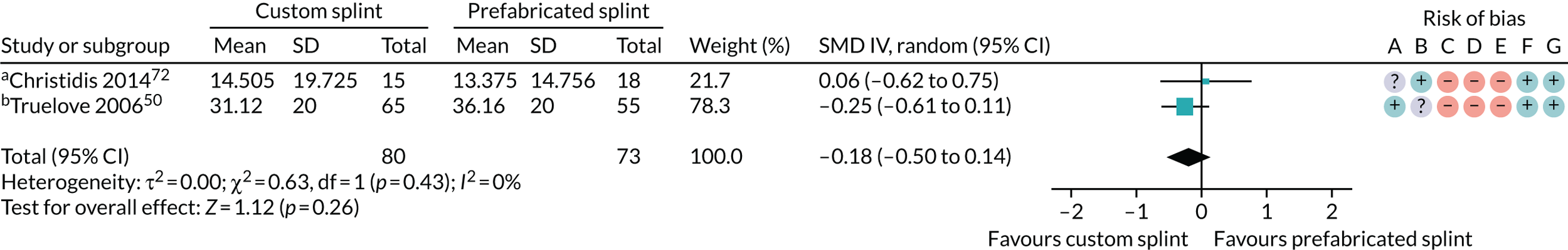
FIGURE 38.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – pain: GCPS (incidence of grade III or IV), 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
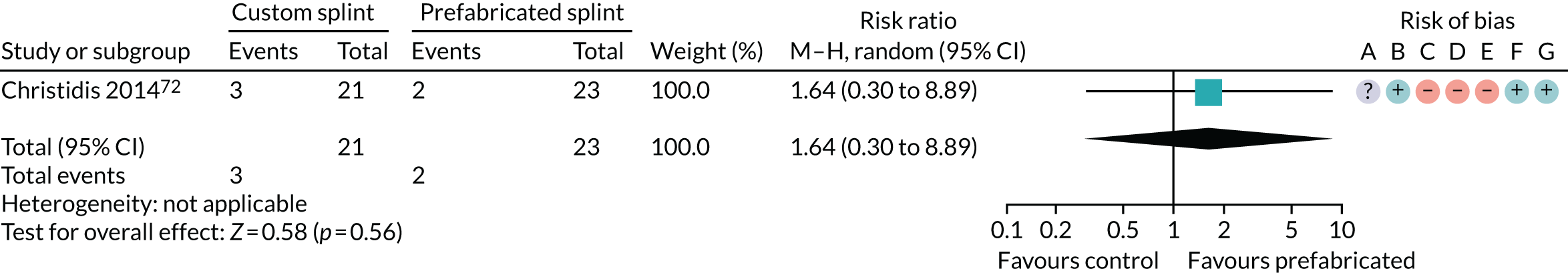
FIGURE 39.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – pain: GCPS (incidence of grade III or IV), 3–6 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
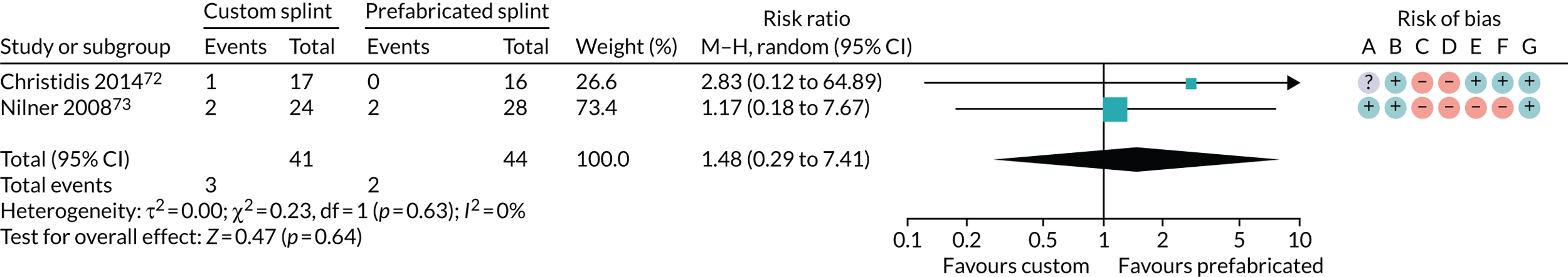
FIGURE 40.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – pain: GCPS (incidence of grade III or IV), 6–12 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
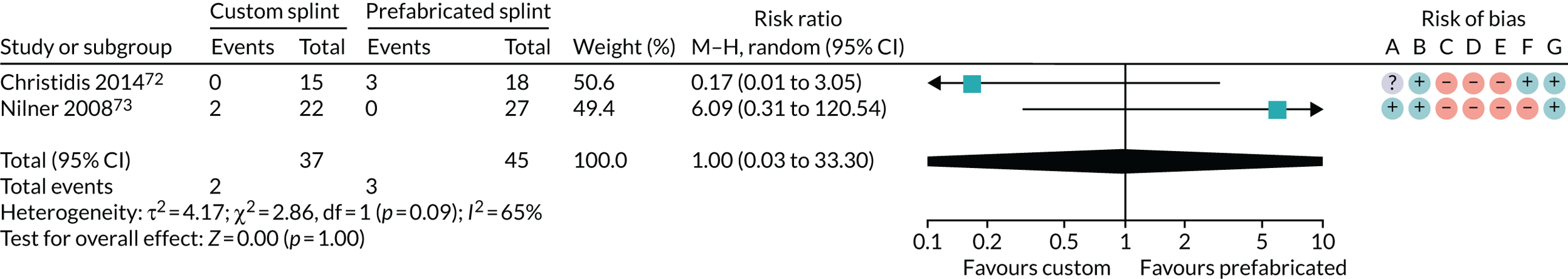
FIGURE 41.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – change in restricted mouth-opening: maximum opening (mm), 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Maximum voluntary mouth-opening; b, unassisted opening without pain.
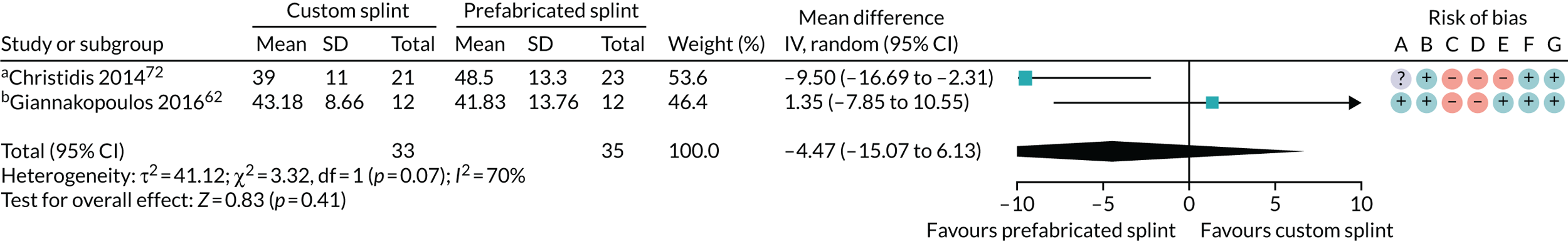
FIGURE 42.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – change in restricted mouth-opening: maximum opening (mm), 3–6 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias. a, Maximum voluntary mouth-opening.
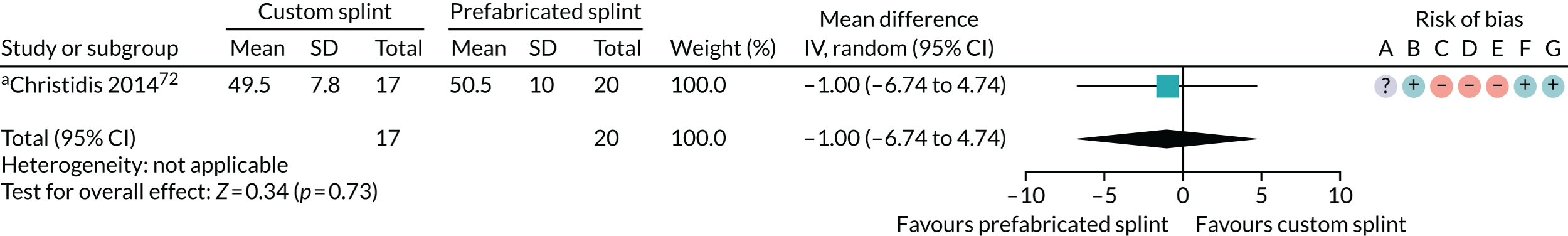
FIGURE 43.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – change in restricted mouth-opening: maximum opening (mm), 6–12 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
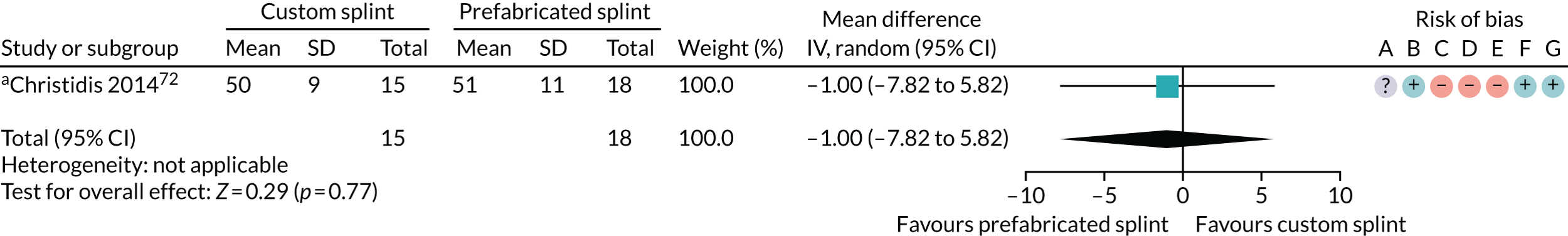
FIGURE 44.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – quality of life: SCL-90-R – depression 0–4 (higher = worse), 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
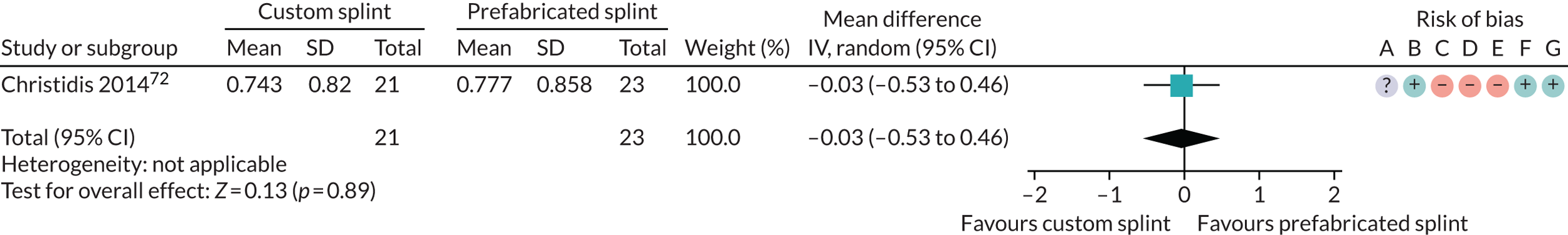
FIGURE 45.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – quality of life: SCL-90-R – depression 0–4 (higher = worse), 3–6 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.

FIGURE 46.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – quality of life: SCL-90-R – depression 0–4 (higher = worse), 6–12 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
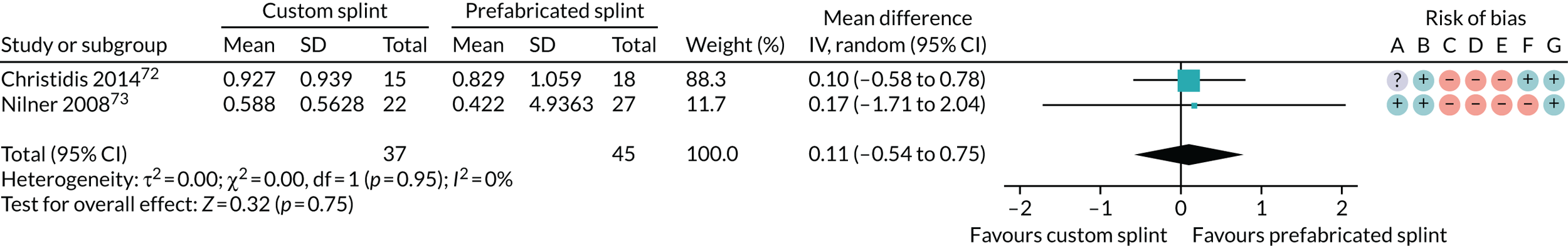
FIGURE 47.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – quality of life: SCL-90-R – non-specific physical symptoms 0–4 (higher = worse), 0–3 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
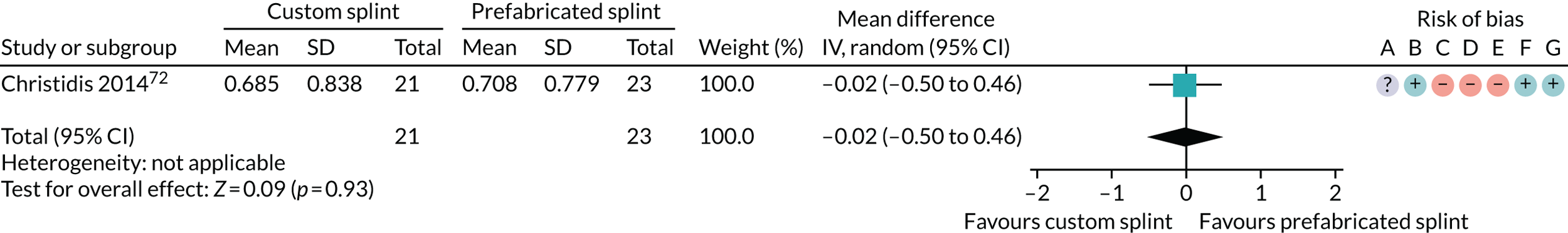
FIGURE 48.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – quality of life: SCL-90-R – non-specific physical symptoms 0–4 (higher = worse), 3–6 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
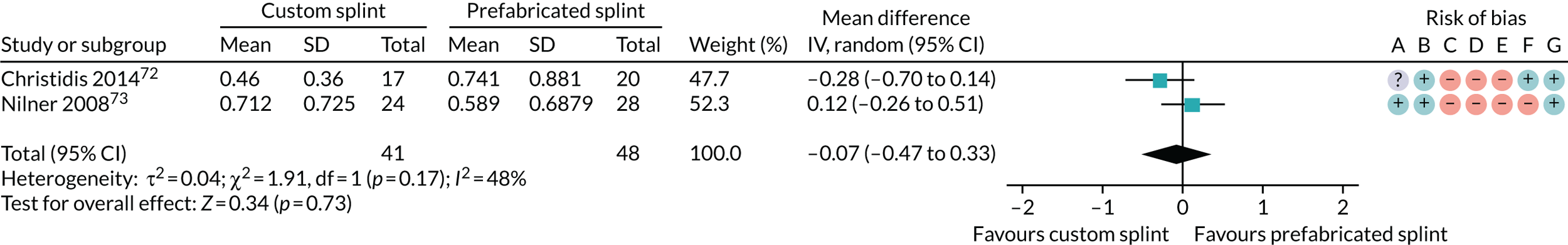
FIGURE 49.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – quality of life: SCL-90-R – non-specific physical symptoms 0–4 (higher = worse), 6–12 months. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
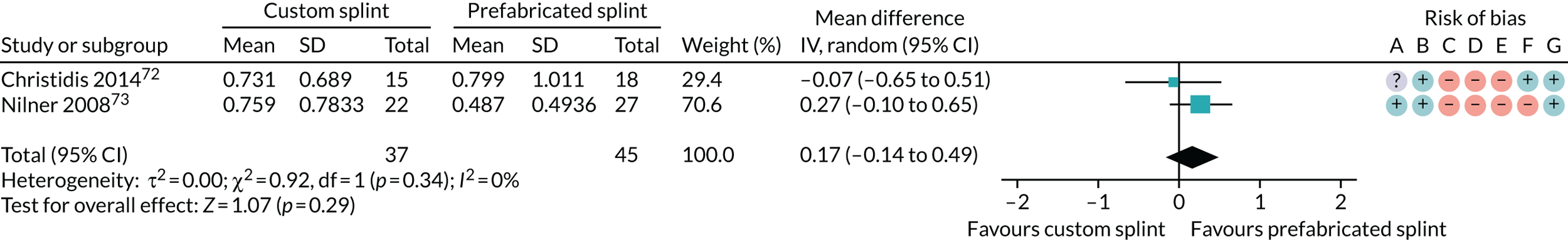
FIGURE 50.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – adherence to treatment: use of appliance for several nights per week or more, 0–3 months. M–H, Mantel–Haenszel.
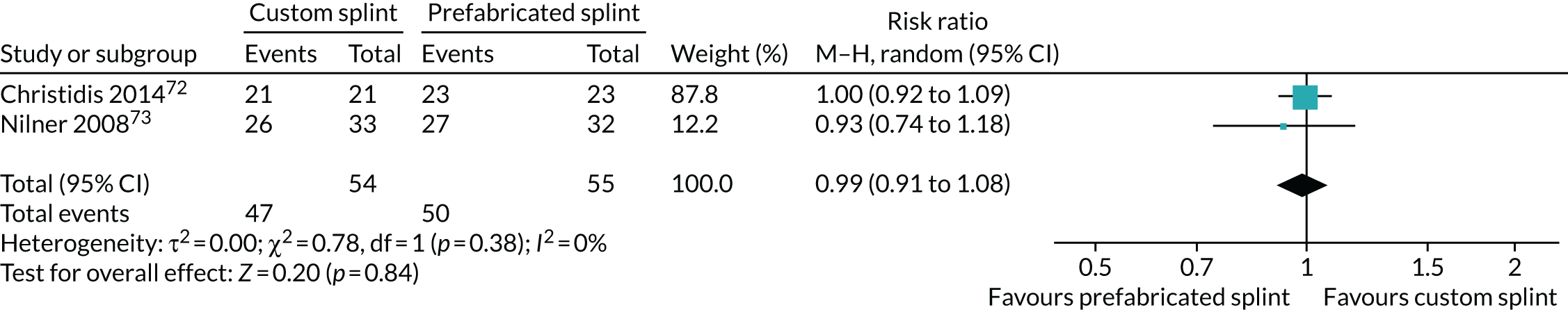
FIGURE 51.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – adherence to treatment: use of appliance for several nights per week or more, 3–6 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
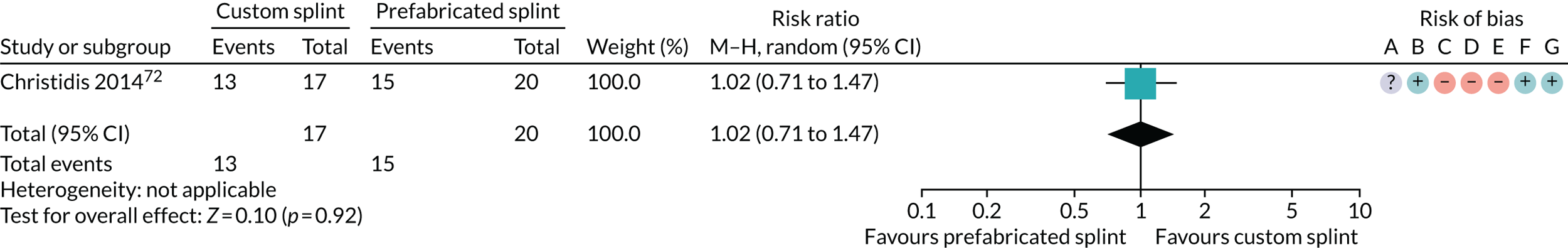
FIGURE 52.
Forest plot of comparison: TMD, custom splint vs. prefabricated splint; outcome – adherence to treatment: use of appliance for several nights per week or more, 6–12 months. M–H, Mantel–Haenszel. Risk of bias: A, random sequence generation (selection bias); B, allocation concealment (selection bias); C, blinding of participants and personnel (performance bias); D, blinding of outcome assessment (detection bias); E, incomplete outcome data (attrition bias); F, selective reporting (reporting bias); and G, other bias.
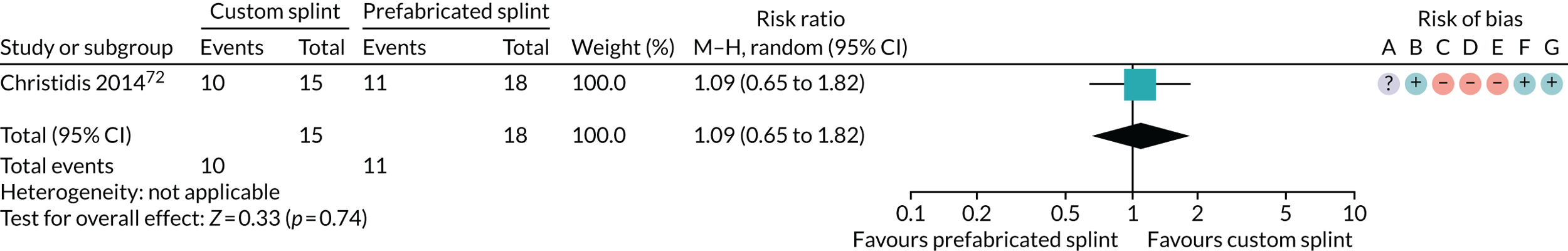
Appendix 5 Draft structure of bruxism model
The proposed model structure in Figure 53 is based on the severity of tooth wear (defined in Adult and Dental Health Survey Report 2009125). Tooth wear was identified as the primary outcome for bruxism patients according to clinical expert opinion (Stephen Davies, School of Dentistry, Manchester, 2018, personal communication).
FIGURE 53.
Transition state diagram for bruxism.
List of abbreviations
- AAOP
- American Association of Orofacial Pain
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CPI
- Characteristic Pain Intensity
- CUA
- cost–utility analysis
- DC/TMD
- Diagnostic Criteria for Temporomandibular Disorders
- DEEP
- Developing Effective and Efficient care pathways in chronic Pain
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EVPI
- expected value of perfect information
- EVPPI
- expected value of perfect parameter information
- GCPS
- Graded Chronic Pain Scale
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- IADR
- International Association of Dental Research
- ICER
- incremental cost-effectiveness ratio
- MD
- mean difference
- NHS EED
- NHS Economic Evaluation Database
- NMB
- net monetary benefit
- NICE
- National Institute for Health and Care Excellence
- NRS
- Numerical Rating Scale
- NTI-tss
- Nociceptive Trigeminal Inhibition Tension Suppression System
- OHIP-14
- 14-item Oral Health Impact Profile
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RDC/TMD
- Research Diagnostic Criteria for Temporomandibular Disorders
- RR
- risk ratio
- SCL-90-R
- Modified Symptom Checklist-90-Revised
- SD
- standard deviation
- SMD
- standardised mean difference
- TMD
- temporomandibular disorder
- UDA
- unit of dental activity
- VAS
- visual analogue scale
- VOI
- value of information
- WTP
- willingness to pay
