Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/115/62. The contractual start date was in April 2016. The draft report began editorial review in June 2019 and was accepted for publication in October 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Matthew L Costa is a National Institute for Health Research (NIHR) Senior Investigator and a member of the NIHR Health Technology Assessment (HTA) General Board (1 November 2016 to present). Rebecca S Kearney is a member of the NIHR HTA Clinical Evaluation and Trials Board (8 November 2018–present) and the NIHR Integrated Clinical Academic Doctoral Panel (29 November 2017–present) and was a member of NIHR Research for Patient Benefit Board (28 January 2016–24 January 2019). Sarah E Lamb reports that she was a member of the following boards: HTA Additional Capacity Funding Board (2012–15); HTA Clinical Trials Board (2010–15); HTA End of Life Care and Add on Studies (2015); HTA Funding Boards Policy Group (formerly Clinical Specialty Group) (2010–15); HTA Maternal, Neonatal, Child Health Methods Group (2013–15); HTA Post-board funding teleconference (2010–15); HTA Primary Care Themed Call Board (2013–14); HTA Prioritisation Group (2012–15); and the NIHR Clinical Trials Unit Standing Advisory Committee (2012–16). Stavros Petrou is a NIHR Senior Investigator.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Costa et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
The Achilles tendon is the largest tendon in the human body and transmits the powerful contractions of the calf muscles that are required for walking and running. A rupture of this tendon is painful and has an immediate and serious detrimental impact on daily activities of living. 1 In the longer term, tendon rupture results in prolonged periods off work and time away from sporting activity (average time away from work is between 4 and 8 weeks and time away from sport is between 26 and 39 weeks). 1 This results in lost income and restricted daily activities in the early phase and reduced physical activity, with associated negative health and social consequences, in the long term. For high-level sportsmen it is frequently a ‘career-ending’ injury.
Achilles tendon rupture affects > 11,000 people each year in the UK, and the incidence is increasing as the population remains more active into older age. 2 It affects all age groups in a bimodal distribution, with the first peak in patients aged 30–40 years and the second in patients aged 60–80 years. 2 The first peak in incidence is often associated with participation in sport, such as football and racquet sports, whereas the second peak often occurs during normal daily activities, such as climbing stairs. 2,3 However, all Achilles tendon ruptures are associated with a pre-existing ‘tendinopathy’, which is attributed to failures in the protective/regenerative functions that respond to repeated microscopic injury. 4,5
Historically, the main question in relation to the management of patients with rupture of the Achilles tendon has been whether or not to perform a surgical repair of the tendon. In 1981, Nistor6 designed and published the first randomised controlled trial (RCT) to address this clinical question. This study was followed by a series of RCTs that were pooled in a meta-analysis by the Cochrane review group in 2004. 7 The results suggested that surgical repair reduced the risk of re-rupture, but this came with an increased cost and a greatly increased risk of other complications, most of which were associated with infection and wound healing. There were few data on functional outcome at the time of this review. More recent trials comparing surgical repair and non-operative treatment have found no difference in functional outcome. 8,9 As surgery carries considerable costs, and carries considerable risks to the patient in terms of complications,7 there is an increasing trend towards non-operative treatment. However, some surgeons have been reluctant to advocate non-operative treatment because of concerns about the lack of evidence to guide early rehabilitation for this group of patients,10 specifically whether or not functional bracing is safe and effective if the tendon has not been not surgically repaired.
Traditionally, patients have been treated in plaster casts after rupture of the Achilles tendon, with the cast immobilising the foot and ankle while the tendon heals. 11 However, there are potential problems with this approach. First, there is the immediate impact on mobility for a period of around 8 weeks, affecting activities of daily life. Second, there are the complications and risks associated with prolonged immobilisation: muscle atrophy, deep-vein thrombosis (DVT) and joint stiffness. 12,13 Finally, there are the potential long-term consequences, which include prolonged gait abnormalities, persistent calf muscle weakness and an inability to return to previous activity levels. 14 Functional bracing, involving immediate, protected weight-bearing in a brace, was designed to address these issues.
In patients having a surgical repair, seven RCTs,1,15–20 directly comparing plaster casts with early movement and/or weight-bearing in a ‘functional brace’, had been conducted at the time that the protocol was developed for the UK Study of Tendo Achilles Rehabilitation (UKSTAR). The results favoured functional bracing in terms of re-rupture rate, functional outcome and quality-of-life (QoL) measures. Therefore, in the first guideline produced on this topic in 2009,21 the American Academy of Orthopaedic Surgeons recommended functional bracing for patients undergoing surgical repair of their tendon.
What about patients managed non-operatively?
Although there are clear guidelines for rehabilitation of patients who have a surgical repair, there is no clarity about the use of functional bracing in non-operatively managed patients. Does functional bracing provide improved function and QoL if the tendon is not surgically repaired? Or, in the context of a tendon that has not been stitched together, does a plaster cast provide greater protection and therefore improved healing? Does functional bracing facilitate faster return to work and is this cost-effective? Or, is the tendon more vulnerable to re-rupture in a brace, with the subsequent risk and cost of reconstructive surgery?
At the time that UKSTAR was developed, we supplemented the 2004 Cochrane review7 with an updated literature search and found that in total only two additional studies22,23 had been performed that compared functional bracing with plaster cast for patients managed non-operatively following rupture of the Achilles tendon. Both studies suggested potential benefits from bracing. However, the data from the studies should be interpreted with caution because patient numbers were small (90 in total), patients received different functional bracing regimes and the reporting of outcomes was minimal.
This gap in the evidence was recognised by the American Academy of Orthopaedic Surgeons in their 2009 guideline,21 which stated that they were unable to make a recommendation with regards the use of immediate functional bracing. With the incidence of Achilles tendon rupture on the rise, and in the light of the high associated personal and societal cost, this evidence gap is a clear priority. A Versus Arthritis (formerly known as Arthritis Research UK) multidisciplinary ‘Think Tank’ (Arthritis Research UK, Birmingham, 2013) on tendon injuries reported that rehabilitation following non-operative treatment of acute Achilles tendon rupture was ‘the top research priority’ in this area.
Since the start of the UKSTAR, a number of small randomised trials have investigated both the mechanistic and the functional effects of early weight-bearing in a brace compared with cast immobilisation. A trial of 56 patients indicated that tendon healing at a molecular level may be enhanced by early mobilisation, but, given the small number of participants, there was no difference in objective functional outcome (heel raise testing in this study). 24 A second trial investigated the biomechanical properties of the healing tendon in patients randomised to early weight-bearing or delayed weight-bearing. 25 The investigators noted that there was less tendon stiffness in the group treated with early weight-bearing. However, in terms of functional outcomes, the authors reported no evidence of a difference in Achilles Tendon Rupture Score (ATRS), although they did report a statistically significant improvement in health-related quality of life (HRQoL) at 1 year in the group treated with early weight-bearing. 25 Another trial included 47 patients treated non-operatively for an acute Achilles tendon rupture. Half of the patients were treated with partial weight-bearing beginning on the first day of treatment and the other half were treated with non-weight-bearing for the first 4 weeks. 26 The authors concluded that early weight-bearing was ‘safe’ in terms of the incidence of re-rupture, but there was no evidence of a difference in functional outcome (ATRS or Physical Activity Scale) in the first 12 months after the rupture. Finally, another trial compared two types of cast immobilisation of Achilles tendon rupture. 27 Half of the patients wore a traditional cast, which restricted weight-bearing, whereas the other group wore a modified cast, which included a heel ‘iron’ to facilitate weight-bearing. The authors found no evidence of a difference in functional outcome (Leppilahti Score), but there were only 84 patients in the trial. One further study, published very recently, randomised patients to cast immobilisation or ‘early controlled motion’ and involved 130 patients at a single centre. The authors found no evidence that early controlled motion was of benefit compared with immobilisation in any of the investigated outcomes. 28
Pre-pilot data
Before UKSTAR, we completed four phases of pilot and preparatory work to establish the following.
External pilot study
We randomised 48 patients receiving non-operative treatment for acute rupture of the Achilles tendon to either functional bracing or plaster cast. This trial1 showed that patients and clinicians had equipoise for this question and were happy to take part. However, the trial identified that although plaster casting was a mature intervention, the important facets of the complex intervention, namely functional bracing, were inadequately defined, and that this needed to be addressed before a larger trial was undertaken.
Defining the functional brace intervention
In keeping with the Medical Research Council framework for developing complex interventions, our group and collaborators performed a UK survey of current practice, a systematic review of published rehabilitation methods, gait analysis experiments using different functional brace and heel wedge combinations, and qualitative interviews to define the optimal functional bracing regime and refine the trial design. 29,30 The rehabilitation strategy proposed in UKSTAR was the summation of that work that identified the optimal type of orthosis (brace), the optimal foot position within the orthosis and the duration of application of the orthosis.
To investigate the number of patients potentially eligible for UKSTAR, we carried out a UK-wide survey of orthopaedic trauma clinicians. 10 This clearly showed that clinicians were enthusiastic about the study and that the number of eligible patients was large enough for a full trial.
Research objectives
The primary objective was to quantify and draw inferences about observed differences in ATRS between the trial treatment groups at 9 months post injury.
The secondary objectives were to:
-
quantify and draw inferences about observed differences in ATRS between the trial treatment groups at 8 weeks and 3 and 6 months post injury
-
identify any differences in HRQoL between the trial treatment groups in the first 9 months post injury
-
determine the complication rate of the trial treatment groups in the first 9 months post injury
-
investigate, using appropriate statistical and economic analytical methods, the resource use, costs and comparative cost-effectiveness of the trial treatment groups in the first 9 months post injury.
Patient and public involvement
We have been working with and listening to the views of patients with Achilles tendon injuries for many years. However, as well as this informal contribution, a series of formal qualitative interviews with patients and clinicians were carried out in the development of the UKSTAR (ISRCTN68273773). 11 The views of patients were used to inform and refine the trial interventions and processes, in particular the development of the trial information and materials. The patient perspective was key during the development of the trail protocol to ensure that the interventions, and participation in the trial, would be acceptable.
Two of the patients who contributed to our development work agreed to act as lay representatives on the Trial Management Group (TMG) and as co-applicants on the research grant award. Mrs Richmond later had to leave the research team for personal reasons, but Mr Grant attended TMG meetings throughout the trial and contributed to all trial process and paperwork, with particular input on patient information leaflets. Mr Grant will be crucially involved in the dissemination of the study findings to the wider public. He will lead the development of any materials, leaflets and website information to be used for this purpose. Mr Grant has reviewed the Plain English summary of this report.
Mr Grant was supported by the chief investigator and the trial co-ordination team. He had peer support from the UK musculoskeletal trauma patient and public involvement (PPI) group, hosted in Oxford. He also had access to and support from the University/User Teaching and Research Action Partnership network through University of Warwick, an organisation that promotes the engagement and involvement of service users and carers from the local community in research and teaching in health and social care.
Chapter 2 Clinical trial methods
Summary of study design
UKSTAR was a multicentre, randomised, pragmatic, two-group superiority trial. Patients presenting at 39 NHS hospitals in England and Scotland with an acute primary Achilles tendon rupture for non-surgical treatment were randomised 1 : 1 to receive either functional brace or plaster cast.
Settings and locations
The 39 NHS hospital orthopaedic or trauma clinics in England and Scotland that screened and recruited participants for this trial were:
-
King’s College Hospital NHS Foundation Trust
-
Nottingham University Hospitals NHS Trust
-
Royal Berkshire Hospital, Royal Berkshire NHS Foundation Trust
-
Aberdeen Royal Infirmary, NHS Grampian
-
Ninewells Hospital and Medical School, NHS Tayside
-
Glasgow Royal Infirmary, NHS Greater Glasgow and Clyde
-
Pilgrim Hospital, United Lincolnshire Hospitals NHS Trust
-
University Hospital of North Tees, North Tees and Hartlepool Hospitals NHS Foundation Trust
-
Airedale NHS Foundation Trust
-
Salisbury District Hospital, Salisbury NHS Foundation Trust
-
The Rotherham NHS Foundation Trust
-
George Eliot Hospital NHS Trust
-
James Paget University Hospitals NHS Foundation Trust, Great Yarmouth
-
Southampton General Hospital, University Hospital Southampton NHS Foundation Trust
-
Lister Hospital, East and North Hertfordshire NHS Trust
-
Royal Cornwall Hospital, Royal Cornwall Hospitals NHS Trust
-
Tunbridge Wells Hospital, Maidstone and Tunbridge Wells NHS Trust
-
Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust
-
Derriford Hospital, University Hospitals Plymouth NHS Trust
-
Hull Royal Infirmary, Hull University Teaching Hospitals NHS Trust
-
Luton and Dunstable University Hospital NHS Foundation Trust
-
Salford Royal NHS Foundation Trust
-
Scunthorpe General Hospital, Northern Lincolnshire and Goole NHS Foundation Trust
-
Pinderfields Hospital, The Mid Yorkshire Hospitals NHS Trust
-
Leeds General Infirmary, Leeds Teaching Hospitals NHS Trust
-
Worcestershire Royal Hospital, Worcestershire Acute Hospitals NHS Trust
-
Doncaster Royal Infirmary, Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust
-
St Helier Hospital, Epsom and St Helier University Hospitals NHS Trust
-
St Mary’s Hospital, Imperial College Healthcare NHS Trust
-
Raigmore Hospital, NHS Highland
-
Whiston Hospital, St Helens and Knowsley Hospitals NHS Trust
-
Milton Keynes University Hospital NHS Foundation Trust
-
Warwick Hospital, South Warwickshire NHS Foundation Trust
-
Queen’s Hospital, Burton Hospitals NHS Foundation Trust
-
Hereford County Hospital, Wye Valley NHS Trust
-
Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust
-
John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust
-
University Hospital of South Manchester NHS Foundation Trust
-
Musgrove Park Hospital, Taunton and Somerset NHS Foundation Trust.
Participants
Participant screening
All adult patients presenting at a trial centre with a primary (first-time) rupture of the Achilles tendon were screened. The patient, in conjunction with their surgeon, decided whether or not non-surgical treatment was appropriate, as per normal clinical practice. If they decided not to have surgery, they were potentially eligible to take part in the trial.
Participant eligibility
In order that the trial findings would be generalisable to a UK-wide population, the eligibility criteria were broad. Patients with acute rupture of the Achilles tendon were eligible if they met all of the inclusion criteria and none of the exclusion criteria.
The inclusion criteria were:
-
being aged ≥ 16 years
-
having a primary rupture of the Achilles tendon
-
having decided to have non-operative treatment.
The exclusion criteria were:
-
presenting to the treating hospital > 14 days after injury
-
likely to be unable to adhere to trial procedures or complete questionnaires
-
having had a previous rupture of the Achilles tendon.
The first exclusion criterion related to patients with late presentation, which is not uncommon after this injury. Patients who present late may have problems with chronic tendon lengthening, irrespective of treatment, and are frequently offered surgical intervention. The limit of 14 days since injury has been widely used to define ‘acute’ rupture.
If a patient taking part in the study sustained a contralateral rupture during the trial period, the second rupture was not included in the study because the result of an intervention for the second injury would not be independent from that of the first injury. However, the patient remained in the trial, with both previous and future data related to the initial rupture included in the final analysis.
Screening logs were completed at recruiting centres and collected by the UKSTAR office throughout the trial to assess the main reasons for patient exclusion at each recruitment centre and the number of patients who were unwilling to participate.
Members of the local research team informed the patient of the study and carried out the informed consent process, baseline data collection and randomisation.
Baseline assessment
Potential participants were allowed as much time as they needed to consider the trial information and had the opportunity to ask questions of the attending clinical team and a member of the research team. The trial information was delivered verbally and in writing, detailing the exact nature of the study, the implications and constraints of the protocol, what to expect as a participant and any risks involved in taking part. It was stated clearly that the participant was free to withdraw from the study at any time, for any reason, without prejudice to future care and with no obligation to give the reason for withdrawal. If the patient was happy to participate, they were asked to sign and date a consent form, which was also signed and dated by the person who obtained consent. Consent was obtained by an appropriately trained member of the research team who had been delegated to do so by the local principal investigator.
A copy of the signed consent form was given to the participant and another copy was sent to the study co-ordinating team in Oxford to facilitate central monitoring. The original signed consent form was retained in the medical notes and a copy was held in the investigator site file. Consent forms were held in a secure location separately from study data. Permission was obtained to inform the participant’s general practitioner (GP) about study participation.
Participants were asked for their consent for their name and contact details (including address, telephone numbers and e-mail address) to be collected to facilitate follow-up, data collection and reporting of results, and for a copy of their contact details to be sent to the UKSTAR central office team in Oxford. The study team used these details to contact participants for follow-up at the 3-, 6- and 9-month time points, to resolve queries and to send a thank-you letter when a participant’s involvement in the trial ended.
Permission was sought to allow members of the University of Oxford or the NHS trust who were responsible for monitoring or audit of the study to access participant data, to ensure compliance with regulations.
Following consent, baseline data were collected and the participant was randomised. The treatment took place at the same visit. A Good Clinical Practice-trained member of the local research team oversaw the participant’s completion of the paper baseline questionnaire, which included:
-
date, mechanism and side of injury
-
baseline demographics – height, weight, smoking and alcohol status, employment status
-
current medication
-
medical history – diabetes mellitus, rheumatoid arthritis, lower limb fracture, ligament, tendon or nerve injury to lower limb in last 12 months, arthritis, Achilles tendinopathy or other relevant conditions.
Randomisation
Participants were randomly allocated (1 : 1) to either functional bracing or plaster cast using a computer-generated allocation sequence, stratified by recruitment centre, via a secure, centralised web-based randomisation service provided by the Oxford Clinical Trials Research Unit (OCTRU). The research associate informed the treating clinical team of the allocated treatment.
Stratification by recruitment centre helped to ensure that any cluster effect related to the recruitment centre itself was equally distributed between the trial groups. The catchment area was similar for all of the recruitment centres (each recruitment centre was a trauma unit dealing with these injuries on a daily basis). All of the recruitment centres were familiar with both techniques (i.e. the clinical staff used both plaster casts and functional bracing as part of their routine clinical practice).
Post-randomisation withdrawals
Participants were free to decline to take part in, consent to take part in or withdraw from the trial at any time without prejudice and without affecting the standard of care that they received. Participants had two options for withdrawal:
-
to withdraw from completing further questionnaires, but allow the trial team to view and record de-identified data are recorded as part of the normal standard of care
-
to withdraw wholly from the study and permit data obtained only up to the point of withdrawal to be included in the final analysis.
Withdrawn participants were not replaced, as the target sample size allowed for losses to follow-up.
Interventions
Participants received their allocated treatment (plaster cast or functional brace) following randomisation.
Although the principles of application of both plaster casts and functional brace are inherent in the technique, there are different types of plaster cast material and functional brace design. Each patient underwent the allocated intervention as specified below (see Table 9), but the details of application and materials used for the plaster and brace were left to the discretion of the treating clinician, as per their usual practice. This was intended to ensure that the results could be generalised across the NHS.
Plaster cast
Participants randomised to plaster cast received a cast in the ‘gravity equinus’ position (i.e. the position that the foot naturally adopts when unsupported). In this position, with the toes pointing down towards the floor, the ends of the ruptured tendon are roughly approximated. Ultrasonography to assess the approximation of the tendon ends is not routine in the NHS10 and this was left to the discretion of the treating clinician. The participant was permitted to mobilise with crutches immediately, using their toes for balance (toe-touch), but was advised not to bear weight on the injured hindfoot. Over the first 8 weeks, as the tendon was healing, the position of the plaster cast was changed until the foot achieved plantigrade (i.e. the foot flat to the floor). At this point the patient was permitted to start to bear weight in the plaster cast. The number of changes of plaster cast and the time to weight-bearing were left to the discretion of the treating clinician, as per their usual practice. The cast was removed at 8 weeks.
The plaster cast provided maximum protection for the healing tendon, specifically restricting upwards movement (dorsiflexion) of the ankle, which may stretch the healing tendon, but it did not allow the patient to bear weight on the foot immediately or to move the ankle.
Functional brace
Participants randomised to functional brace received a rigid brace, as opposed to a flexible brace. 29 Initially, two solid heel wedges (or equivalent) were inserted into the brace to replicate the ‘gravity equinus’ position of the foot. 29 The patient was able to mobilise with immediate full weight-bearing within the functional brace. The brace also permitted some movement at the ankle joint. The number of wedges and foot position were reduced over 8 weeks until the patient reached plantigrade. Again, the timing of the removal of wedges and change in foot position were left to the discretion of the treating clinician, as per their usual practice. The brace was removed at 8 weeks, as per routine clinical care.
Monitoring intervention delivery and compliance
Clinic staff recorded the participant’s treatment in clinic records, as per usual practice. At the 8-week follow-up visit, research staff recorded on the 8-week trial case report form (CRF):
-
the intervention to which the patient was randomised
-
the intervention that they received
-
the date of the 8-week follow-up appointment
-
for participants treated with a functional brace, irrespective of their randomisation allocation, the:
-
number of heel wedges inserted into the heel of the functional brace at baseline (date of treatment), and at 2, 4, 6 and 8 weeks after treatment
-
number of weeks after treatment when the patient was allowed to fully weight bear
-
number of weeks after treatment when the functional brace was removed
-
brand of functional bracing
-
-
for participants treated with a plaster cast, irrespective of their randomisation allocation, the number of:
-
plaster cast changes over the 8 weeks since treatment
-
weeks after treatment when the patient was allowed to fully weight bear
-
weeks after treatment when the plaster cast was removed
-
-
whether or not the patient switched to another intervention during the 8 weeks after treatment, the date of switching and the reason for switching
-
whether or not the participant received treatment with venous thromboembolism (VTE) prophylaxis and, if so, the type and duration.
Rehabilitation
At the patient’s 8-week clinic appointment, the plaster cast or functional brace was removed unless the clinical team directed otherwise. All participants were provided with the same standardised written physiotherapy advice, detailing the exercises that they needed to perform for rehabilitation. This advice was based on a published systematic review of current rehabilitation protocols. 30 All of the participants were advised to move their toes, ankle and knee joints fully, within the limits of their comfort, and walking was encouraged. In this pragmatic trial, any other rehabilitation input beyond the written physiotherapy advice (including a formal referral to physiotherapy) was left to the discretion of the treating clinician. A record of any rehabilitation input (type and number of additional appointments), as well as other investigations or interventions, was collected as part of the 8-week and 3-, 6- and 9-month follow-up questionnaires.
Outcome measures
Primary outcome measure
The primary outcome measure for this study was the ATRS31 at 9 months post injury. The ATRS is a validated questionnaire32 that is completed by the participant. It has 10 items, assessing symptoms and physical activity specifically related to the Achilles tendon. It measures strength, fatigue, stiffness, pain, activities of daily living, walking on uneven surfaces, walking upstairs or uphill, running, jumping and physical labour. Each ATRS item is rated on an 11-point scale from 0 (major limitations/symptoms) to 10 (no limitations/symptoms). The final ATRS is derived from the sum of the 10 questions, with a total possible score ranging between 0 and 100 (100 being the best possible score).
Secondary outcome measures
The secondary outcome measures were:
-
The ATRS, collected at 8 weeks and 3 and 6 months post injury.
-
The EuroQol-5 Dimensions (EQ-5D)/EuroQol-5 Dimensions, five-level version (EQ-5D-5L), which is a validated, generic HRQoL measure consisting of five dimensions, each with a five-level answer possibility, and a visual analogue scale (VAS). 33 The EQ-5D can be used to report HRQoL in each of the five dimensions and each combination of answers can be converted into a health utility score, with 1 representing perfect health and 0 indicating death. The EQ-5D VAS takes values between 0 and 100, with 0 representing worst imaginable health and 100 representing best imaginable health. It has good test–retest reliability, is simple for patients to use and gives a single preference-based index value for health status that can be used for broader cost-effectiveness comparative purposes.
-
Complications were recorded from medical notes at the 8-week review and were patient reported at the 3-, 6- and 9-month follow-ups. The predefined complication categories were tendon re-rupture, DVT, pulmonary embolism (PE), non-injurious falls, injurious falls, pain under the heel, numbness around the foot and pressure sores. In addition, three categories were created based on the recorded text and included skin condition requiring medication, surgery related to Achilles rupture and fractured toe.
Adverse events
An adverse event (AE) is defined as any untoward medical occurrence in a clinical trial subject and does not necessarily have a causal relationship with the treatment. All AEs were listed on the CRF for routine return to the UKSTAR central office.
A serious adverse event (SAE) is defined as any untoward and unexpected medical occurrence that:
-
results in death
-
is life-threatening
-
requires hospitalisation or prolongation of existing hospitalisation
-
results in persistent or significant disability or incapacity
-
is a congenital anomaly or birth defect
-
is any other important medical condition that, although not included in the above, may require medical or surgical intervention to prevent one of the outcomes listed.
All SAEs were recorded by recruitment centre staff on the trial SAE reporting form and e-mailed to a secure NHS.net account, which was accessed only by the research team within 24 hours of the investigator becoming aware of the SAE. Once the information was received, causality and expectedness were confirmed by the chief investigator. SAEs deemed unexpected and related to the trial were notified to the Research Ethics Committee within 15 days. All such events were reported to the Trial Steering Committee (TSC) and Data and Safety Monitoring Committee (DSMC) at their next meetings.
Some AEs were foreseeable as part of the proposed treatment – including those that met the definition of ‘serious’ as described above – and did not need to be reported immediately to the UKSTAR central office, provided that they were recorded in the ‘complications’ section of the CRF or participant questionnaire. These events were re-rupture, blood clots/emboli, pressure areas/hindfoot pain, falls and neurological symptoms in the foot.
All participants experiencing a SAE were followed up as per protocol until the end of the trial.
All unexpected SAEs or suspected unexpected SAEs that occurred between the date of consent and the date of the 9-month follow-up time point were reported.
Blinding
As the type of rehabilitation used was clearly visible, participants could not be blinded to their treatment. In addition, the treating clinician was not blinded to the treatment but took no part in the post-injury assessment of the participants. The outcome data were collected and entered onto the trial central database via questionnaire by a research assistant or a data entry clerk in the trial central office, which reduced the risk of assessment bias.
Follow-up
The UKSTAR office staff contacted the participants directly for follow-up at 3, 6 and 9 months using the contact details that the participant had supplied. Participants were contacted by post, by e-mail or by short message service (SMS), according to their preference; if no response was received, they were telephoned. All follow-up contacts and attempted contacts were logged without personal identifying details.
Participants who had supplied an e-mail address were sent a link to an online questionnaire. Participants who had supplied a mobile phone number were sent the same link by SMS. Participants who had supplied both an e-mail address and a mobile phone number were sent the link via both mechanisms. If a participant did not respond to any of these initial approaches, they were sent a reminder 1 week later. If there was still no response after another week, the participant was sent a paper questionnaire. If the paper questionnaire was not returned within 2 weeks, UKSTAR office staff telephoned the participant. If the participant was uncontactable during working hours, attempts were made to telephone them during the evening, as many participants were of working age.
Participants who had specified that they preferred to be contacted by post, or who had not supplied an e-mail address or mobile phone number, were sent a questionnaire in the post and sent a second postal questionnaire if no response was received within 2 weeks. UKSTAR office staff attempted to telephone the participant for follow-up if the second postal questionnaire was not returned within 2 weeks.
Deep-vein thrombosis, PE and re-rupture were reported by participants through completing a questionnaire or directly to the study office, or by recruitment centre staff after participants had returned to the centre for further treatment. These reports underwent validation, as follows. In the case of a patient-reported DVT or PE, recruitment centres were requested to complete a DVT/PE form, which detailed symptoms, results of any ultrasonography, results of any computerised tomography pulmonary angiogram imaging, treatment received and treatment duration. In the case of a patient-reported re-rupture, recruitment centres were requested to provide details of diagnosis and treatment. If the patient underwent surgery for a re-rupture, an operation note was requested. All information submitted in connection with a re-rupture was reviewed by the chief investigator, blinded to the treatment allocation, to confirm the diagnosis.
Sample size
The minimum clinically important difference (MCID) for the primary outcome ATRS was 8 points. At an individual patient level, a difference of 8 points represents the ability to walk upstairs or run with ‘some difficulty’ compared with ‘great difficulty’. At a population level, 8 points represents the difference between a ‘healthy patient’ and a ‘patient with a minor disability’. 32
In previous work, the standard deviation (SD) of the ATRS at 9 months post injury was 20 points. 34 Assuming a likely population variability of 20, MCID value of 8 and 90% power to detect the selected MCID, 264 participants needed to be randomised. Allowing a margin of 20% loss of primary outcome data to include patients who would cross over between interventions and those lost to follow-up led to a requirement of 330 participants. We intended to recruit a minimum of 330 patients from at least 22 centres over 16 months. The trial reached its primary recruitment target of 330 participants before the end of the proposed recruitment window and therefore the sample size was recalculated based on a larger population variability equivalent to a SD of 25 points, following a blinded review of the variability by the DSMC. As per Table 1 calculations for a SD of 25, MCID value of 8, 5% two-sided tests and 20% loss to follow-up, 516 participants were required. The maximum number of participants to be recruited for the trial was set at 550.
| MCID/SD | 80% power | 90% power | ||||
|---|---|---|---|---|---|---|
| MCID | 6 | 8 | 10 | 6 | 8 | 10 |
| SD | ||||||
| 15 | 198 | 112 | 72 | 264 | 150 | 96 |
| 20 | 350 | 198 | 128 | 468 | 264 | 170 |
| 25 | 548 | 308 | 198 | 732 | 412 | 264 |
Statistical analysis
Software used
All analyses outlined here were undertaken using Stata® version 15.0 (StataCorp LP, College Station, TX, USA).
Blinded analysis
The distribution of variables, missing data distributions and outliers were assessed as part of a blinded analysis of data (not separated by treatment group) prior to the final data lock. This analysis was also used to help confirm the key prognostic variables to be included in the adjusted analysis. The treatment code was added to the database after the data cleaning had been completed and all subsequent analyses described were conducted on an unblinded data set. The statistical analysis plan was updated to incorporate necessary changes.
Data validation
To ensure consistency, validation checks of the data were conducted. These included checking for duplicate records, checking the range of variable values or missing items and validating potential outliers by comparing with CRFs and referring back to recruitment centres, when necessary. Calculations for derived variables, such as the ATRS, were checked by hand calculations on 20 randomly selected participants from the data set. These checks confirmed that the data had been imported into the statistical software correctly, calculation of derived variables had been performed correctly and merging of different data to form an analysis data set had been verified.
Study populations
Two populations were considered for analysis: the intention-to-treat (ITT) population and the complier-average causal effect (CACE) population. 35 The ITT population comprised all participants in their randomised groups and the CACE population comprised all randomised participants compliant with treatment. Participants were considered compliant with the intervention if they wore their allocated treatment for a period of ≥ 6 weeks without any change of treatment during this period.
Descriptive analysis
All available data from both treatment groups (functional brace and plaster cast) were used in a descriptive analysis. The flow of participants through each stage of the trial, including numbers of participants eligible for randomisation, those randomised, those receiving intended treatment, those completing the study protocol and those analysed for the primary outcome, was assessed. Reporting of the results was in accordance with the Consolidated Standards of Reporting Trials (CONSORT) for patient-reported outcomes (PROs) statement using the extension for non-pharmacologic treatment interventions and PROs. 36 Any protocol deviations and violations were investigated.
Participant baseline characteristics were reported by treatment group and overall, and included recruitment centre stratification, demographic variables (age, sex, site of injury and mechanism of injury, body mass index, smoking status, alcohol consumption, medication, diagnoses, employment status) and baseline values for ATRS and EQ-5D-5L before and after the injury. Numbers (with percentages) for categorical variables and means (and SDs) or medians [and interquartile ranges (IQRs)] for continuous variables were presented for each treatment group and overall. There were no tests of statistical significance or confidence intervals (CIs) for differences between randomised groups on any baseline variable.
Data collected at the 8-week and 3-, 6- and 9-month post-injury follow-ups were summarised and the proportion of missing items from completed questionnaires was examined. The patterns of data availability for primary and secondary outcomes from baseline to end of follow-up were summarised for the two treatment groups, as well as reasons for missingness, when known. The nature and pattern of missing data [missing completely at random, missing at random (MAR) or missing not at random (MNAR)] were explored. Differentiation was made between partially completed and fully missing outcome data. Validation rules for the primary outcome ATRS ensured that data were entered in the correct format, within valid ranges, minimising the chance of missing data. When ATRS item responses were missing and at least half of the items were present, a pro rata estimation of the final ATRS score was imputed based on the average of the available ATRS item responses. No pre-injury ATRS values were imputed, two ATRS scores were imputed at 8 weeks, five were imputed at 3 months, four were imputed at 6 months and eight were imputed at 9 months.
Withdrawals and losses to follow-up were compared between the groups at each time point and the reasons were reported when known. Absolute risk differences (with 95% CIs) between the groups were calculated, and the importance of differences was determined using chi-squared tests or Fisher’s exact test, if appropriate. When participants were identified as having tendon re-ruptures followed by surgery, the participant was not treated as lost to follow-up. Deaths and their causes were reported separately.
Quality assurance and compliance with treatment was assessed. The treatment received was reported by group and summarised, with reasons for not receiving the assigned treatment given when possible.
For all analyses, tests were two sided and considered to provide evidence of a significant statistical difference if p-values to three decimal places were < 0.05 (5% significance level). In addition, any reported treatment estimates will be presented with their associated 95% CI.
Analysis of primary outcome
The primary outcome ATRS at 9 months post injury was reported for each of the treatment groups. The main findings of the trial show the difference in the ATRS between the two treatment groups, estimated with a linear mixed-effects regression model, including outcome information from all follow-up points and adjusting for age, sex and baseline ATRS as fixed effects, and centre and observations within participants as random effects.
An additional fully adjusted model included age, sex, baseline ATRS, smoking status and diabetic condition as prognostic variables, with random effects for centre and repeated measures within participants. Important clinician-specific effects were not expected as each clinician treated only a small number of patients, but recruitment centre was included in the model as a random-effect factor to adjust for potential cluster differences. Estimates of treatment effects were presented with 95% CIs. Histograms and residual checks were used to assess an approximate normal distribution of the ATRS and, when relevant, the medians and IQRs reported for each treatment group.
An unadjusted analysis was also undertaken to assess the differences between treatment groups using Student’s t-test, based on a normal approximation of the ATRS score. Estimates of treatment effects were presented with 95% CIs for both unadjusted and adjusted analyses. The ITT adjusted analysis of the primary outcome ATRS was used to determine the success, or otherwise, of the trial.
Sensitivity analyses to examine the robustness of conclusions to different assumptions were conducted for the CACE population. Compliance was defined as using the allocated intervention for a minimum of 6 weeks, and further sensitivity analysis was undertaken using different definitions of compliance, namely minimum of 4 weeks and minimum of 2 weeks. Adherence to the allocated treatment can affect the interpretation of the impact of what was offered to patients. This may be a particular issue in an ITT analysis, which includes all patients as they were expected to be treated and does not take into account if patients received or adhered to the intervention allocated to them.
Supplementary analysis
To explore recovery in the two treatment groups over time, a further analysis of the ATRS was conducted. This summarised longitudinal data collected at all four time points to a single value, the area under the curve (AUC),37 to facilitate a comparison of the ATRS between the treatment groups over time. Parameter estimates from the mixed-effects models were used to calculate AUCs for each treatment group from baseline to the 9-month post-injury follow-up. This provided an overall estimate of recovery over time in each group. Higher ATRS scores were associated with fewer limitations and difficulties related to the injured Achilles tendon, and therefore larger AUCs were suggestive of improved function. The AUC for each treatment group and their differences, calculated using a t-test, were presented, together with their associated 95% CIs. The lincom command in Stata was used to calculate the AUC for each group. This analysis was also conducted for the EQ-5D utility score and the EQ-5D VAS.
Analysis of secondary outcomes
The continuous secondary outcomes ATRS at 8-week and 3- and 6-month post-injury follow-up and EQ-5D-5L were evaluated and analysed for the ITT population using the methodology described for the primary outcome (see Analysis of primary outcome). Histograms and residual checks were used to assess whether or not these variables were approximately normally distributed. Means and SDs were reported at the 8-week and 3-, 6- and 9-month post-injury follow-up time points, and medians and IQRs were reported when appropriate. A linear mixed-effects regression model with random effects for recruitment centre and participant outcome information from all time points, and fixed effects for age, sex and baseline pre-injury outcome values, was used to examine the difference between the treatment groups.
Complications in each of the treatment groups were reported as numbers (with percentages) and compared over the 9-month study period using chi-squared tests or Fisher’s exact test. The results were reported with their associated 95% CI and p-values for comparison between the two treatment groups. The population for this analysis was ITT. Complications were further grouped to identify the number of patients with one or more complications at each time point.
Sensitivity analyses were also conducted for the secondary outcome EQ-5D-5L analysis using the CACE population.
Health economics methods
Overview
The main objective of the health economic evaluation was to assess the comparative cost-effectiveness of the two non-surgical treatment options (plaster cast and functional brace) for patients with a primary (first-time) rupture of the Achilles tendon. To achieve this, a systematic comparison of the cost of resource inputs used by participants in the two groups of the trial and the consequences associated with the interventions was conducted. The primary analysis adopted an NHS and Personal Social Services (PSS) perspective, in accordance with National Institute for Health and Care Excellence (NICE) recommendations. 38 A societal perspective for costs was adopted for the sensitivity analysis, and this included private costs incurred by trial participants and their families, as well as productivity losses and loss of earnings as a result of work absences.
The economic evaluation took the form of a cost–utility analysis, expressed in terms of incremental cost per quality-adjusted life-year (QALY) gained. The time horizon covered the period from randomisation to end of follow-up at 9 months post injury. Costs and outcomes were not discounted because of the short (i.e. 9 months) time horizon adopted for this within-trial evaluation.
Measuring resource use and costs
Data were collected on:
-
resource use and costs associated with delivery of the interventions (direct intervention costs)
-
broader health and social care service use during the 9 months of follow-up
-
broader societal resource use and costs (this encompassed private medical costs and lost productivity costs, such as lost income over the 9 months of follow-up).
All costs were expressed in Great British pounds and valued in 2017–18 prices. When appropriate, costs were inflated or deflated to 2017–18 prices using the Hospital and Community Health Services Pay and Prices Inflation Index. 39
Direct intervention costs
Direct intervention costs were costs associated with the application of the two interventions. These included cost of the walking boot and wedges, materials used for plaster cast, the cost associated with fitting the interventions to patients (hospital staff time) and the costs associated with any changes required to either plaster cast or functional bracing (Table 2). Information on how long it took to deliver each intervention and the type and volume of materials used was collected at each recruitment centre using a questionnaire completed by recruitment centre staff in consultation with the staff responsible for fitting the functional brace or applying the plaster cast. Unit costs for staff were obtained from the Personal Social Services Research Unit (PSSRU) Unit Costs of Health and Social Care 2018 compendium39 and were multiplied by the median time it takes to deliver each intervention. The median time to fit a functional brace was 10, 11 and 17.5 minutes for a plaster technician, nurse and other staff (including physiotherapists, orthotists and occupational therapists), respectively. The median time to change wedges was 5 minutes for a plaster technician and a nurse and 10 minutes for other staff. The median time to change a plaster cast was 15 minutes for a plaster technician and 17.5 minutes for a nurse. The base-case analysis assumed the costs of a plaster technician. Unit costs of plaster cast materials, walking boots and wedges were obtained from the 2018 NHS Supply Chain catalogue. 40 The total direct intervention cost for each patient was calculated by combining the resource inputs with their unit cost values.
| Direct intervention costs: resource item | Unit cost (£) | Unit of analysis | Source of unit cost |
|---|---|---|---|
| Functional brace: walking boota cost by brand | |||
| Samson walking boot (AliMed Inc., Dedham, MA, USA) | 15.00 | Per walking boot | John Radcliffe Hospital, Oxford (Claire Granville, Outpatients Manager, Trauma Unit, 2017, personal communication); NHS Supply Chain Catalogue 201840 |
| Donjoy walking boot (DJO UK, Guildford, UK) | 19.24 | ||
| Airstep walking boot (DJO UK) | 68.66 | ||
| Plaster cast materialsb | |||
| Poly rolls: 2 × 7.5 cm | 2.83 | Per roll | NHS Supply Chain catalogue 201840 |
| Poly rolls: 2 × 10 cm | 6.69 | ||
| Fibreglass casting tape: 5 inches × 3.6 m | 11.48 | Per roll | NHS Supply Chain catalogue 201840 |
| 1-m stockinette | 3.23 | Per roll | NHS Supply Chain catalogue 201840 |
| Wool bandage: 2 × rolls of 5 inches | 3.00 | Per roll | NHS Supply Chain catalogue 201840 |
Measuring broader resource use
Broader resource use data were collected using follow-up questionnaires completed by trial participants at the four follow-up assessment points: 8 weeks and 3, 6 and 9 months post injury. The questionnaires captured details of inpatient and day-case admissions, outpatient and emergency care attendances, contacts with primary or community health and social care services, medication use and walking aids provided/self-purchased, as well adaptations to home environments. In addition, the questionnaires captured the direct non-medical costs (including travel expenses) incurred by patients and their carers, as well as the number of days off work and gross loss of earnings attributable to the participant’s health state or contacts with care providers.
Valuing of resource use
Resource inputs were valued by attaching unit costs derived from national compendia in accordance with NICE’s Guide to the Methods of Technology Appraisal. 38 The key databases for deriving unit cost data included the Department of Health and Social Care’s Reference Costs 2017–18 schedules,41 the PSSRU’s Unit Costs of Health and Social Care 2018 compendium,39 the 2018 NHS Prescription Cost Analysis database for England,42 the 2016 volume of the British National Formulary43 and the NHS Supply Chain catalogue 2018. 40 Table 24 (see Appendix 1) gives a summary of the unit costs values and data sources for broader resource use categories identified within the follow-up questionnaires.
Per diem costs for hospital inpatient admissions during the follow-up period were calculated individually as a weighted average of Healthcare Resource Group (HRG) codes of related procedures and/or clinical diagnoses. For example, the average cost per day of an inpatient stay in a medical ward to treat a PE was calculated as the sum total of weighted average HRG codes (DZ09J–DZ09Q; PE with or without interventions), divided by the average length of stay across elective and non-elective inpatient services. The individual HRG codes were derived using the NHS HRG4 2017/18 Reference Cost Grouper software version RC1718 (NHS Digital, Leeds, UK). The Department of Health and Social Care’s Reference Costs 2017–1841 schedule was used to assign the costs of each of the derived HRG codes.
Costs of community-based health and social care services were calculated by applying unit costs extracted from national tariffs, primarily the PSSRU Unit Costs of Health and Social Care 2018 compendium,39 to resource volumes. Costs of medications for individual participants were estimated based on their reported doses and frequencies, when these were available, or based on assumed daily doses using British National Formulary43 recommendations. When a dose range was reported as ‘as required’, or when the quantities were not recorded, we assumed a mean cost of that medication item based on the prescription cost analysis values (net ingredient cost per item). When medication dosages were missing, we conservatively assumed that the patient received the same dosage as other trial participants who reported taking the same medication.
The costs of walking aids and adaptations (equipment participants receive to manage their injury and make daily lives easier) were derived by combining data on the number and type of items received with their unit cost values. Unit cost values were derived from the NHS Supply Chain catalogue40 if equipment was provided by a health provider during the trial follow-up period. When aids and adaptations were self-financed, the costs were provided by participants.
We used data on sex- and employment status-specific median earnings from the UK national annual survey of hours and earnings44 to derive the costs of time taken off work. The employment status of trial participants was derived from self-reported information. Broader societal costs were calculated by combining the productivity losses and the income losses attributable to work absences.
Summary statistics were generated for resource use variables by treatment allocation and assessment point. Between-group differences in resource use and costs at each assessment point were compared using the two-sample t-test. Statistical significance was assessed at the 5% significance level. Standard errors are reported for treatment group means and bootstrap 95% CIs for the between-group differences in mean resource use and cost estimates.
Measuring outcomes
In accordance with NICE guidelines, the primary health outcome of the health economic evaluation was the QALY metric. 38 The QALY is a measure that combines quantity of life and preference-based HRQoL into a single metric. To calculate QALYs, it is imperative to obtain health state values for participants within the trial. The HRQoL of trial participants was assessed at baseline (both pre and post injury) and at 8 weeks and 3, 6 and 9 months post injury using the EQ-5D-5L. 38 The EQ-5D-5L defines HRQoL in terms of five dimensions: (1) mobility, (2) self-care, (3) usual activities, (4) pain/discomfort and (5) anxiety/depression. Responses in each dimension are divided into five ordinal levels, coded (1) no problems, (2) slight problems, (3) moderate problems, (4) severe problems and (5) extreme problems. Responses to each health dimension were categorised as optimal or suboptimal with respect to function, where optimal function indicates no impairment (e.g. ‘no problems in walking about’ for the mobility dimension) and suboptimal function refers to any functional (below level 1) impairment. Between-group differences in optimal compared with suboptimal function for each health dimension were compared at each time point using chi-squared tests.
Responses to the EQ-5D-5L instrument were converted into health utility scores using the EQ-5D-5L Crosswalk Index Value Calculator currently recommended by NICE,42 which maps the EQ-5D-5L descriptive system data onto the EQ-5D-3L valuation set. A detailed description of the mapping methodology is described elsewhere. 42 QALYs were generated for each patient using the area under the baseline-adjusted utility curve, assuming linear interpolation between health utility measurements across assessment points.
The health utility values and QALYs accrued over the 9-month follow-up period were summarised by treatment group and assessment point, and presented as means and associated standard errors. Between-group differences were compared using the two-sample t-test, in a similar way to the descriptive analyses of resource inputs and costs.
Cost-effectiveness analysis methods
Missing data
Missing data are common in RCTs: participants may be lost to follow-up, questionnaires may be unreturned or responses to individual questionnaire items may be missing. 45 As costs and outcomes of individuals with missing data may differ systematically from those of individuals with fully observed data, it is important to handle missing data using a principled approach that is justified by, among other factors, the missing data mechanism. Missing costs and health utility data were imputed at each time point using fully conditional multiple imputation by chain equations, implemented through the MICE package (run within Stata version 15.0; http://fmwww.bc.edu/RePEc/bocode/m) under the MAR assumption. The appropriateness of the MAR assumption was assessed by (1) investigating the missing data patterns (monotonic vs. non-monotonic) and (2) comparing the attributes of participants with and those of participants without missing costs and HRQoL data at each follow-up time point.
Regression models were used to generate multiple imputed data sets, in which missing values were predicted drawing on predictive covariates (age, sex and baseline pre-injury HRQoL scores). Costs and EQ-5D utility scores at each time point contributed as both predictors and imputed variables. Imputations were generated separately by treatment group using predictive mean matching drawn from the five knh-nearest-neighbours (knn = 5); predictive mean matching preserves distribution of the data and is more robust to violations of the normality assumption. The multiple imputation was run 50 times, generating 50 complete data sets that reflected the distributions of and correlations between variables.
Bivariate regressions using a seemingly unrelated regression model (Sureg) were used to independently analyse the multiply imputed data sets so as to estimate the costs and QALYs in each treatment group over the 9-month trial horizon. Joint distributions of costs and outcomes from the original data set were generated through non-parametric bootstrapping and changes in costs and QALYs were calculated for each sample. A total of 1000 bootstrap samples were drawn and means for both incremental costs and incremental QALYs (with associated 95% CIs) were calculated. Estimates from each imputed data set were combined using Rubin’s rules38 to generate overall mean estimates of costs and QALYs and their standard errors. The standard errors reflect the variability within and across imputations. The imputation model was validated by assessing the distributions of imputed and observed values. A mixed model with adjustment made for baseline pre-injury EQ-5D health utility scores is also presented for comparison.
Presentation of cost-effectiveness results
The cost-effectiveness results are expressed in terms of the incremental cost-effectiveness ratio (ICER) and calculated as the difference between treatments in mean total costs divided by mean total QALYs. Given the pattern of results, plaster cast has been selected as the referent and functional brace as the comparator (i.e. functional brace minus plaster cast) for the estimation of ICER values. The bootstrap replicates generated by the non-parametric bootstrapping, described in Missing data, were used to populate cost-effectiveness scatterplots. Cost-effectiveness acceptability curves, which showed the probability that functional brace is cost-effective relative to plaster cast across a range of cost-effectiveness thresholds, were also generated based on the proportion of bootstrap replicates with positive incremental net benefits. The net monetary benefit (NMB) of using functional brace compared with plaster cast was also calculated across three prespecified cost-effectiveness thresholds, namely £15,000 per QALY,44 £20,000 per QALY and £30,000 per QALY. 46 A positive incremental NMB indicates that functional brace is cost-effective compared with plaster cast at the given cost-effectiveness threshold. For the secondary analysis that adopted the ATRS as the health outcome measure of interest, the NMB was estimated at cost-effectiveness thresholds of £100–500 per unit change in ATRS score. We failed to identify any external evidence on economic values for changes in ATRS score and therefore a range of arbitrary threshold values had to be selected for this analysis.
Sensitivity and secondary outcomes analyses
Several sensitivity analyses were conducted to test the robustness of the cost-effectiveness estimates. These involved re-estimating the main cost-effectiveness outcomes under the following scenarios: (1) restricting the analyses to complete cases (i.e. those participants with complete cost and outcome data over the 9-month follow-up period); (2) adopting a wider societal perspective that included private costs incurred by trial participants and their families, as well as economic losses attributable to work absences; (3) estimating incremental cost-effectiveness using a CACE population; and (4) evaluating the impact on cost-effectiveness results of assuming that data may be MNAR, rather than MAR, as the tests for exploring missing data mechanisms described above cannot rule out MNAR. Data are MNAR when the probability of missingness is directly linked to the unobserved value itself. To explore this assumption in sensitivity analyses, we used pattern mixture models with multiple imputation, following the published tutorial by Leurent et al. 47 Using this approach, missing values were first imputed using multiple imputation under a MAR assumption. Second, the MAR-imputed data were modified by a scale parameter (c) to reflect that HRQoL and cost data may be MNAR under a range of plausible scenarios. Specifically, we assumed that participants with missing HRQoL were likely to be in poorer health, whereas those with missing cost data were likely to have used more resources. In the absence of expert data on what the likely reduction could be, we assumed conservatively that a participant with missing HRQoL values would, on average, have a 10% lower HRQoL than a trial participant with similar characteristics who had available data. We applied the same reasoning for costs, but this time assuming a 10% higher cost. The combination of scenarios is shown in Table 3. The resulting multiply imputed data set was analysed as explained above for multiple imputation under MAR, combining the results using Rubin’s rules. Results are presented as cost-effectiveness scatterplots and cost-effectiveness acceptability curves for the different scenarios (see Figures 15 and 16). Furthermore, we present a graph to show NMB over a range of MNAR parameter values, specifically 0–50% reduction in imputed HRQoL values (see Figure 17) in order to show when a tipping point (change in cost-effectiveness decision) might occur.
| Scenario description | MNAR rescaling parameter | |||
|---|---|---|---|---|
| HRQoL in plaster cast group | HRQoL in functional brace group | Cost in plaster cast group | Cost in functional brace group | |
| 1. MAR | ||||
| Same parameters in both treatment groups | ||||
| 2. 10% reduction in HRQoL in both groups | –10% | –10% | 1 | 1 |
| 3. 10% increase in costs in both groups | 1 | 1 | +10% | +10% |
| 4. 10% increase in costs and 10% reduction in HRQoL | –10% | –10% | +10% | +10% |
| Different parameters by treatment group | ||||
| 5. 10% reduction in HRQoL in functional brace group | 1 | –10% | 1 | 1 |
| 6. 10% reduction in HRQoL in plaster cast group | –10% | 1 | 1 | 1 |
| 7. 10% increase in cost in functional brace group | 1 | 1 | 1 | +10% |
| 8. 10% increase in cost in plaster cast group | 1 | 1 | +10% | 1 |
In addition, as this was a secondary analysis, cost-effectiveness was estimated using the ATRS, rather than the QALY, as the health outcome measure of interest.
Longer-term economic modelling
The study protocol also allowed for decision-analytic modelling to estimate longer-term cost-effectiveness of functional brace or plaster cast, provided that the costs and health outcomes did not converge at the end of the 9-month post injury follow-up period.
Data management
In accordance with the standard operating procedures of the OCTRU, data management procedures were defined in a data management plan. This covered trial databases and data handling, definition of critical data fields, forms and questionnaires used, data collection, how protocol deviations were recorded, data rulings, handling data deviations, data security and confidentiality, data set closure, archiving and data sharing. Each data management plan version was signed off by the chief investigator and the trial statistician.
The monitoring plan determined the need for central and on-site data monitoring. All recruitment centres were monitored centrally. The monitoring plan specified that on-site monitoring was not required for this trial and no monitoring visits were conducted.
Statistics on data collection, data entry and query management were presented at each TMG meeting for oversight.
UK legislation requires data to be anonymised as soon as it is practical to do so. Participants were identified only by their initials and a participant number on UKSTAR questionnaires and in the study database. All documents were stored securely and accessible only by study staff and authorised personnel. Personal data and sensitive information required for the study were collected directly from trial participants and hospital notes. All personal information received in paper format for the trial was held securely and treated as strictly confidential. Personal data were stored separately from study outcomes in lockable cabinets in secure keycard-accessed rooms in the Kadoorie Centre in the John Radcliffe Hospital and in the Botnar Research Centre, University of Oxford. All paper and electronic data will be retained for at least 5 years after completion of the trial.
Patient and public involvement
The UKSTAR TSC and TMG both included a patient representative as a PPI member. Mrs S Webb was TSC PPI representative and attended meetings from the initial meeting and Mr R Grant was PPI representative at TMG meetings from September 2017.
Ethics approval and monitoring
Ethics approval
The study received favourable opinion from the South Central – Oxford B Research Ethics Committee on 7 April 2016 (reference 16/SC/0109) and each recruitment centre was granted site-specific approval from its NHS trust research and development department before the trial commenced.
Data and Safety Monitoring Committee
The DSMC was a group of independent experts external to the trial who assessed the progress, conduct, participant safety and critical end points of the trial. The UKSTAR DSMC adopted a DAMOCLES (DAta MOnitoring Committees: Lessons, Ethics, Statistics) charter,48 which defined its terms of reference and operation in relation to oversight of the trial. It reviewed copies of data accrued to date, including information on allocation balance, data quality and participant safety summarised by treatment group, and assessed the screening algorithm against the eligibility criteria. No formal interim analysis of the outcome data was requested for review by the DSMC. During the period of recruitment to the trial, all information was supplied to the DSMC members in strict confidence. The DSMC also considered emerging evidence from other related trials or research and reviewed related SAEs that have been reported. It was able to advise the chairperson of the TSC at any time if, in its view, the trial should be stopped for ethical reasons, including concerns about participant safety. DSMC meetings were held at least annually during the recruitment phase of the study.
Trial Steering Committee
The TSC, which included independent members and had an independent chairperson, provided overall supervision of the trial on behalf of the funder. Its terms of reference were defined in a TSC charter, agreed with the Health Technology Assessment programme, which also approved the appointment of TSC members. The TSC’s remit was to:
-
monitor and supervise the progress of the trial towards its interim and overall objectives
-
review, at regular intervals, relevant information from other sources
-
consider the recommendations of the DSMC
-
inform the funding body of the progress of the trial.
Trial Steering Committee meetings were held at least annually during the recruitment phase of the study.
Trial Management Group
The TMG was made up of the study investigators and staff working on the project. This group oversaw the day-to-day running of the trial and met regularly throughout the study.
Summary of changes to the trial protocol
All protocol versions can be found on the NIHR Journals Library website at www.journalslibrary.nihr.ac.uk/programmes/hta/1311562/#/ (accessed 11 November 2019).
The changes to the project protocol are summarised in Table 4.
| Protocol version number | Date | Details of changes made |
|---|---|---|
| 1 | 27 January 2016 | The first version |
| 2 | 18 August 2016 |
References to fax removed; replaced with description of sending confidential documents to a secure nhs.net e-mail address Addition of resource use questionnaire at 8 weeks Clarification of data collection roles of recruitment centre staff and UKSTAR office staff Update to the statistical analysis section of the protocol so that it reflects the statistical analysis plan for the trial Clarification of the consent process Correction of typographical errors and clarifications |
| 3 | 10 July 2017 | Clarification that questionnaires at the 3-, 6- or 9-month time points may be sent electronically to patients via e-mail or SMS, as an alternative to by post |
| 4 | 19 September 2017 (not issued) | Update of sample size to a maximum of 550 patients |
| 5 | 23 October 2017 | Correction of protocol version number from 4.1 to 5.0 |
| 6 | 16 May 2018 |
Addition of ‘study within a trial’ to assess the effect of thank-you e-mails on follow-up rates Updates to study personnel, TSC membership and sponsor address details Correction of minor typographical errors |
| 7 | 13 November 2018 |
Removal of ‘study within a trial’ Addition of thank-you letter to participants after final follow-up |
Chapter 3 Clinical trial results
Study participants
Patients with an Achilles tendon rupture typically attend the emergency department at their local hospital and, following their diagnosis, are referred to the next available fracture/trauma clinic to discuss the management of their injury.
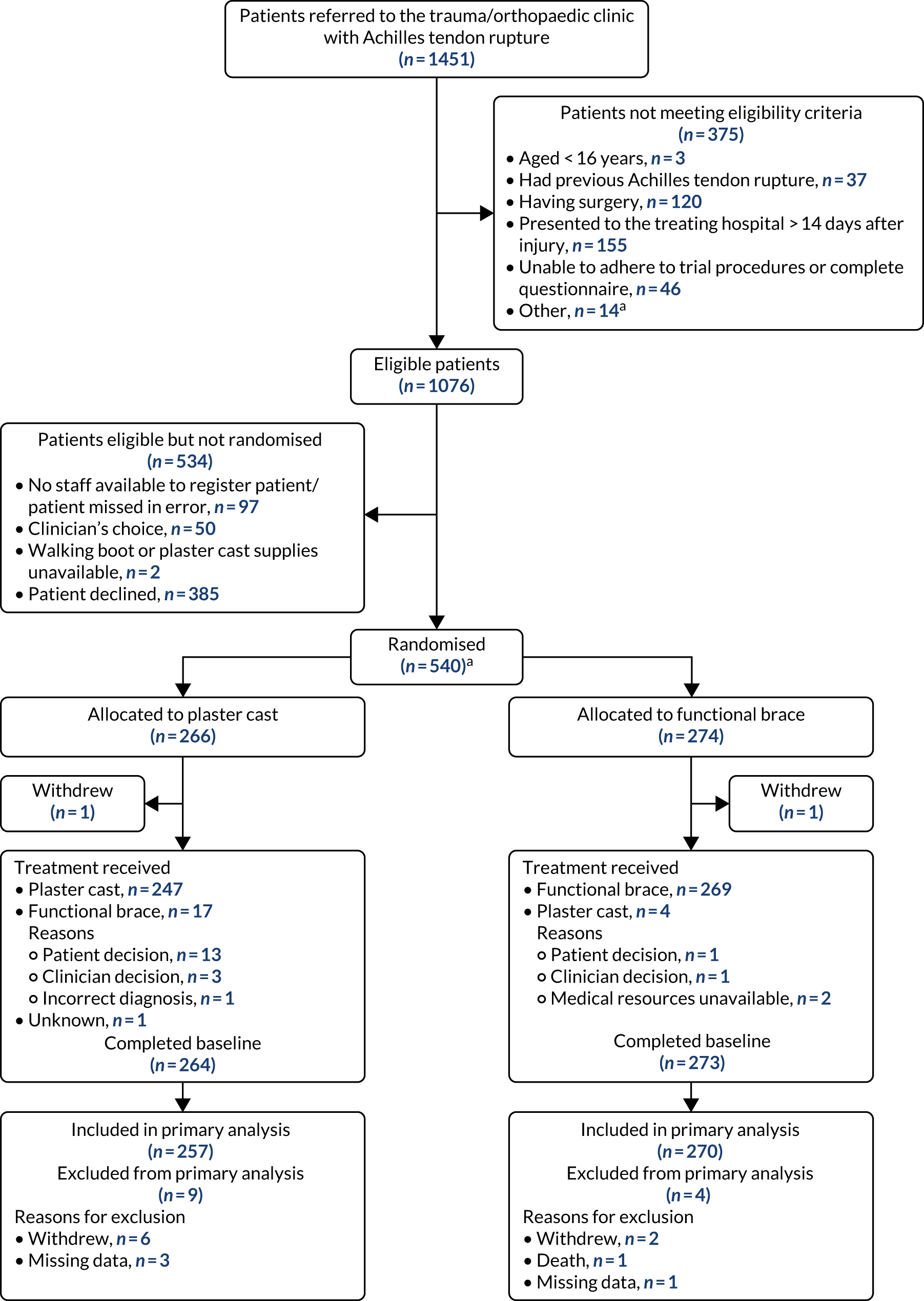
The flow of participants through the study is summarised in Figure 1. This includes details on the total number of patients referred to the trauma clinic with an Achilles tendon rupture and those randomised. The availability of the primary outcome for analysis is also reported by treatment group, as is the total number of patients excluded from the primary outcome analysis.
FIGURE 1.
The UKSTAR CONSORT flow diagram. a, Two additional patients were randomised in error without giving consent to be in the study. These participants were excluded from all data analyses. Reproduced from Costa et al. 49 © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Recruitment
A total of 1076 eligible participants were screened from July 2016 to May 2018 from 39 NHS hospitals across England and Scotland (see Figure 1). Of these, 540 participants consented to take part in the trial. Reasons why patients were not included in the trial are presented. Participants attended clinic visits at the time of randomisation (baseline) and at the 8-week follow-up. Participants were also contacted by the trial team by post, e-mail or telephone to complete follow-up questionnaires at 3, 6 and 9 months post injury. Two participants were randomised in error before consenting and are therefore not included in the numbers allocated to each treatment group.
Baseline characteristics
The randomisation was stratified by centre, and the allocation of participants to the treatment groups in each centre and the overall numbers is given in Table 5. The descriptive characteristics of the participants included in the ITT population are summarised by treatment group and overall in Table 6. These values are presented as numbers and percentages for categorical factors and as means and SD or medians and IQR, as appropriate, for continuous variables. These variables all appear well balanced between the two treatment groups. The distribution of participant ages by sex at enrolment is shown in Figure 2. This distribution has a peak in male patients aged 30–40 years and in female patients aged 40–60 years. Baseline values of patient-reported outcome measures (PROMs), ATRS and EQ-5D-5L are summarised by group in Table 7. ATRS values range from 0 to 100, with lower scores indicating more functional limitations; EQ-5D utility scores range from –0.511 to 1, with higher scores indicating better QoL and 0 being equivalent to death; and EQ-5D VAS scores range from 0 to 100, with higher scores indicating better QoL. The values reported are similar in the two treatment groups.
| Trial centrea | Plaster cast group (N = 266) | Functional brace group (N = 274) | Overall (N = 540) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| ABD | 31 | 11.7 | 33 | 12.0 | 64 | 11.9 |
| AIR | 11 | 4.1 | 12 | 4.4 | 23 | 4.3 |
| BRT | 4 | 1.5 | 4 | 1.5 | 8 | 1.5 |
| CHX | 1 | 0.4 | 1 | 0.4 | 2 | 0.4 |
| CUH | 12 | 4.5 | 14 | 5.1 | 26 | 4.8 |
| DBH | 7 | 2.6 | 6 | 2.2 | 13 | 2.4 |
| DUN | 9 | 3.4 | 10 | 3.6 | 19 | 3.5 |
| ENH | 17 | 6.4 | 18 | 6.6 | 35 | 6.5 |
| GEH | 11 | 4.1 | 12 | 4.4 | 23 | 4.3 |
| GLA | 3 | 1.1 | 5 | 1.8 | 8 | 1.5 |
| HCH | 2 | 0.8 | 3 | 1.1 | 5 | 0.9 |
| HEY | 3 | 1.1 | 2 | 0.7 | 5 | 0.9 |
| INV | 6 | 2.3 | 5 | 1.8 | 11 | 2.0 |
| KCH | 1 | 0.4 | 0 | 0.0 | 1 | 0.2 |
| LDH | 4 | 1.5 | 4 | 1.5 | 8 | 1.5 |
| LDS | 13 | 4.9 | 14 | 5.1 | 27 | 5.0 |
| MKN | 12 | 4.5 | 12 | 4.4 | 24 | 4.4 |
| MPH | 6 | 2.3 | 6 | 2.2 | 12 | 2.2 |
| MTW | 6 | 2.3 | 7 | 2.6 | 13 | 2.4 |
| MYH | 2 | 0.8 | 1 | 0.4 | 3 | 0.6 |
| NLG | 5 | 1.9 | 6 | 2.2 | 11 | 2.0 |
| NTE | 1 | 0.4 | 3 | 1.1 | 4 | 0.7 |
| NUH | 9 | 3.4 | 8 | 2.9 | 17 | 3.1 |
| OUH | 3 | 1.1 | 3 | 1.1 | 6 | 1.1 |
| PLY | 10 | 3.8 | 9 | 3.3 | 19 | 3.5 |
| QEH | 13 | 4.9 | 12 | 4.4 | 25 | 4.6 |
| RBK | 4 | 1.5 | 3 | 1.1 | 7 | 1.3 |
| RCH | 6 | 2.3 | 6 | 2.2 | 12 | 2.2 |
| RED | 5 | 1.9 | 5 | 1.8 | 10 | 1.9 |
| RTH | 5 | 1.9 | 5 | 1.8 | 10 | 1.9 |
| SAL | 6 | 2.3 | 7 | 2.6 | 13 | 2.4 |
| SHC | 5 | 1.9 | 4 | 1.5 | 9 | 1.7 |
| SLF | 8 | 3.0 | 7 | 2.6 | 15 | 2.8 |
| UHS | 6 | 2.3 | 6 | 2.2 | 12 | 2.2 |
| ULH | 11 | 4.1 | 10 | 3.6 | 21 | 3.9 |
| WAR | 1 | 0.4 | 3 | 1.1 | 4 | 0.7 |
| WHI | 6 | 2.3 | 6 | 2.2 | 12 | 2.2 |
| WYT | 1 | 0.4 | 2 | 0.7 | 3 | 0.6 |
| Characteristic | Plaster cast group (N = 264) | Functional brace group (N = 274) | Overall (N = 538) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 213 (80.7) | 213 (77.7) | 426 (79.2) |
| Female | 51 (19.3) | 61 (22.3) | 112 (20.8) |
| Age (years), mean (SD) | 49.0 (13.9) | 48.3 (13.8) | 48.7 (13.8) |
| Body mass index (kg/m2), mean (SD), n | 27.5 (4.5), 255 | 27.8 (5), 265 | 27.7 (4.8), 520 |
| Days since injury, median (IQR) | 5.0 (2.5–8) | 5.0 (2–8) | 5.0 (2–8) |
| Mechanism of injury, n (%) | |||
| Fall from height | 3 (1.2) | 8 (3) | 11 (2) |
| Fall on steps/stairs | 22 (8.4) | 14 (5.1) | 36 (6.7) |
| Fall/trip from standing height | 6 (2.4) | 11 (3.9) | 17 (3.2) |
| Sports | 187 (70.8) | 192 (70.2) | 379 (70.4) |
| Walking | 14 (5.4) | 28 (10.2) | 42 (7.8) |
| Other | 14 (5.4) | 6 (2.1) | 20 (3.7) |
| Side of injury, n (%) | |||
| Right | 122 (46.2) | 138 (50.4) | 260 (48.3) |
| Left | 142 (53.7) | 136 (49.5) | 278 (51.7) |
| Regular smoker, n (%) | |||
| No | 225 (85.2) | 234 (85.5) | 459 (85.2) |
| Yes | 39 (14.7) | 39 (14.1) | 78 (14.4) |
| Missing | 0 (0) | 1 (0.3) | 1 (0.2) |
| Cigarettes (per day), median (IQR), n | 10.0 (5–15), 39 | 10.0 (5–15), 39 | 10.0 (5–15), 78 |
| Smoking duration (years), median (IQR), n | 20.0 (10–25), 38 | 20.5 (13–30), 38 | 20.0 (10–30), 76 |
| Alcohol units (per week), n (%) | |||
| 0–7 | 162 (61.5) | 161 (58.8) | 323 (60) |
| 8–14 | 49 (18.6) | 65 (23.7) | 114 (21.3) |
| 15–21 | 40 (15.3) | 35 (12.9) | 75 (13.8) |
| > 21 | 12 (4.5) | 10 (3.6) | 22 (4.2) |
| Missing | 1 (0.3) | 3 (1.2) | 4 (0.6) |
| Taking the following medication, n (%) | |||
| Fluoroquinolone antibiotics | 5 (1.9) | 4 (1.5) | 9 (1.7) |
| Steroids | 7 (2.7) | 14 (5.1) | 21 (3.9) |
| DMARDs | 2 (0.8) | 1 (0.4) | 3 (0.6) |
| Diabetic medication | 5 (1.9) | 14 (5.1) | 19 (3.5) |
| Regular analgesia | 23 (8.7) | 14 (5.1) | 37 (6.9) |
| Anticoagulant medication | 66 (25) | 78 (28.5) | 144 (26.8) |
| Diagnosis prior to injury, n (%) | |||
| Diabetes mellitus | 5 (1.9) | 18 (6.6) | 23 (4.3) |
| Rheumatoid arthritis | 0 (0) | 3 (1.1) | 3 (0.6) |
| Lower limb fracture (last 12 months) | 1 (0.4) | 4 (1.5) | 5 (0.9) |
| Ligament, tendon or nerve injury to lower limb (last 12 months) | 5 (1.9) | 8 (2.9) | 13 (2.4) |
| Arthritis | 21 (8) | 21 (7.7) | 42 (7.8) |
| Achilles tendinopathy | 10 (3.8) | 10 (3.6) | 20 (3.7) |
| Employment status, n (%) | |||
| Full-time employed | 160 (60.6) | 168 (61.3) | 328 (61) |
| Part-time employed | 18 (6.8) | 15 (5.5) | 33 (6.1) |
| Self-employed | 39 (14.8) | 29 (10.6) | 68 (12.6) |
| Retired/looking after home/inactive | 35 (13.3) | 41 (15) | 76 (14.1) |
| Unpaid work | 1 (0.4) | 2 (0.7) | 3 (0.6) |
| Unemployed | 8 (3) | 8 (2.9) | 16 (3) |
| Full-time student | 3 (1.1) | 9 (3.3) | 12 (2.2) |
| Missing | 0.0 (0) | 2.0 (0.7) | 2.0 (0.4) |
| Employment category, n (%) | |||
| Unskilled manual | 11 (4.2) | 11 (4) | 22 (4.1) |
| Skilled manual | 62 (23.5) | 64 (23.4) | 126 (23.4) |
| Unskilled non-manual | 6 (2.3) | 7 (2.6) | 13 (2.4) |
| Skilled non-manual | 29 (11) | 21 (7.7) | 50 (9.3) |
| Professional | 109 (41.3) | 108 (39.4) | 217 (40.3) |
| Missing | 0.0 (0) | 3.0 (1) | 3.0 (0.6) |
FIGURE 2.
Participant age (years) at randomisation by sex. (a) Male; and (b) female.
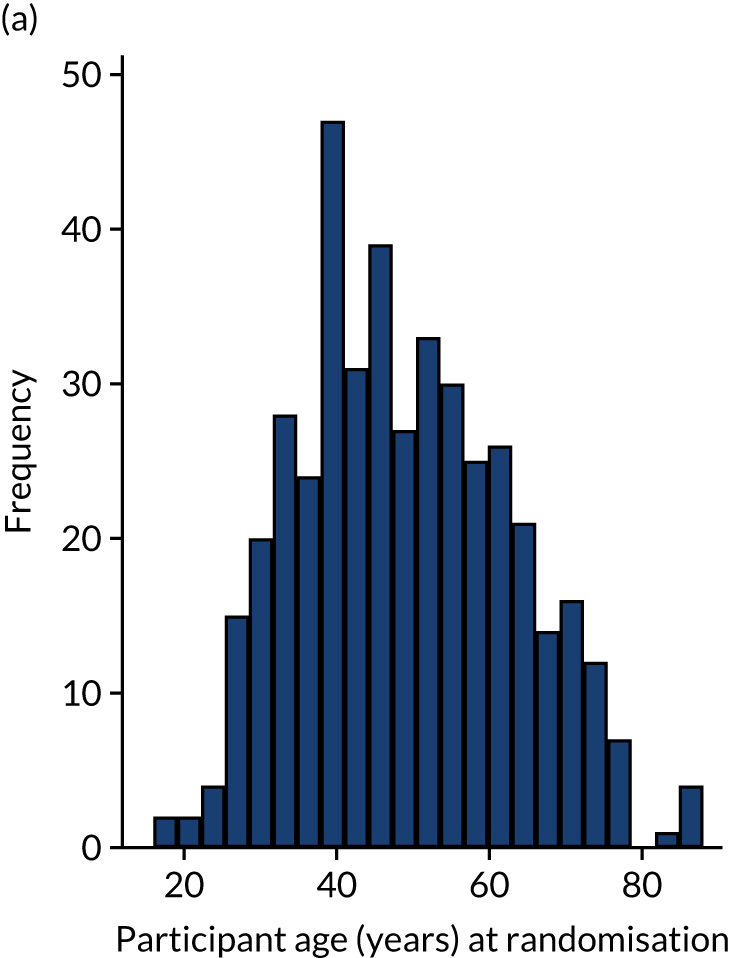
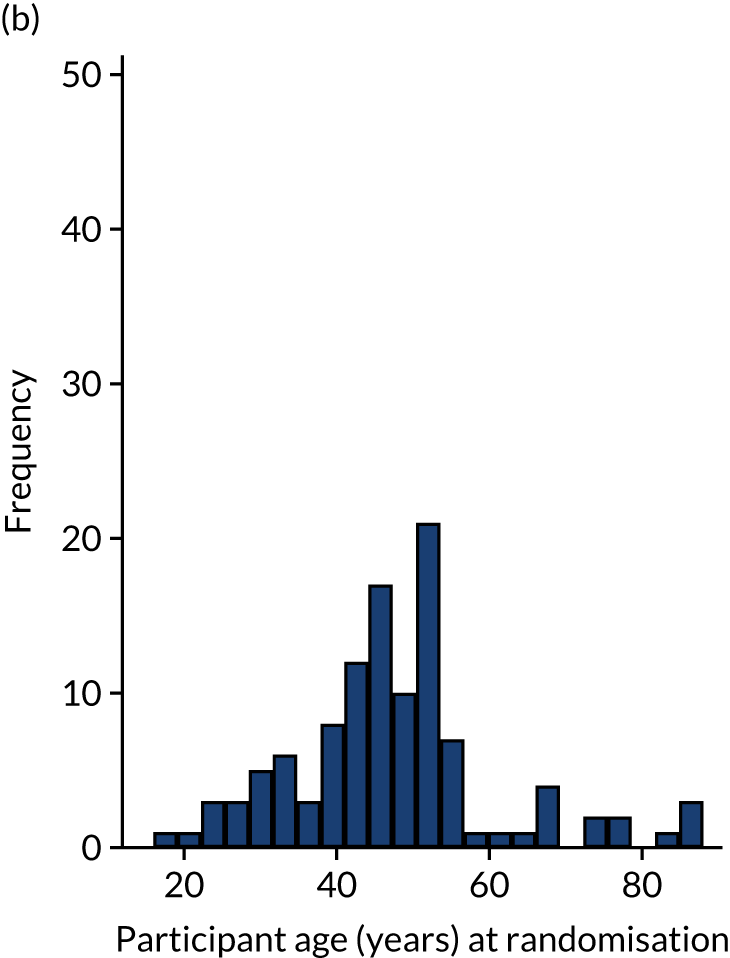
| PROMa | Plaster cast group (N = 264) | Functional brace group (N = 274) | Overall (N = 538) |
|---|---|---|---|
| ATRS pre injury, median (IQR), n | 100 (96.5–100), 264 | 100 (94–100), 273 | 100 (96–100), 537 |
| EQ-5D VAS pre injury, median (IQR), n | 90 (80–95), 263 | 90 (80–95), 273 | 90 (80–95), 536 |
| EQ-5D VAS post injury, mean (SD), n | 57.6 (21.1), 262 | 58.3 (21.5), 273 | 58.0 (21.3), 535 |
| EQ-5D utility pre injury, median (IQR), n | 1 (1–1), 262 | 1 (1–1), 273 | 1 (1–1), 535 |
| EQ-5D utility post injury, mean (SD), n | 0.2 (0.3), 262 | 0.3 (0.3), 273 | 0.3 (0.3), 535 |
Compliance
Participant compliance with treatment is presented in Table 8. The population compliant with treatment for ≥ 6 weeks was made up of 477 (88.2%) participants overall, 212 (79.7%) of whom were in the plaster cast group and 265 (96.7%) of whom were in the functional brace group. The numbers of patients compliant with treatment for ≥ 4 weeks and ≥ 2 weeks are also listed in Table 8.
| Compliance with treatmenta | Plaster cast group (N = 266) | Functional brace group (N = 274) | ||
|---|---|---|---|---|
| n | % | n | % | |
| ≥ 6 weeks | 212 | 79.7 | 265 | 96.7 |
| ≥ 4 weeks | 223 | 83.8 | 268 | 97.8 |
| ≥ 2 weeks | 240 | 90.2 | 268 | 97.8 |
Details of the treatment received following randomisation are listed in Table 9. There were 247 (92.9%) participants in the plaster cast group and 269 (98.2%) who received their allocated treatment immediately at baseline. Those who did not receive the allocated treatment at baseline received the other treatment instead or withdrew. A further 35 participants (13.2%) in the plaster cast group and 4 (1.5%) in the functional brace group changed from the treatment to which they were randomised within the first 6 weeks and received the other treatment or surgery instead. The reasons why participants changed from their allocated treatment are listed in Table 10 and include patient decision, clinician decision, medical resources unavailable, incorrect Achilles tendon rupture diagnosis, surgery and withdrawal.
| Intervention | Plaster cast group (N = 266) | Functional brace group (N = 274) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Received allocated treatment at baseline | 247 | 92.9 | 269 | 98.2 |
| Changed treatment at baseline | 19 | 7.1 | 5 | 1.8 |
| Received the other treatment | 17 | 6.4 | 4 | 1.5 |
| Withdrew | 1 | 0.4 | 1 | 0.4 |
| Unknown | 1 | 0.4 | 0 | 0.0 |
| Changed treatment within 6 weeks, excluding changes at baseline | 35 | 13.2 | 4 | 1.5 |
| Received the other treatment | 30 | 11.3 | 1 | 0.4 |
| Received surgery | 5 | 1.9 | 3 | 1.1 |
| Reason | Plaster cast group (N = 54) | Functional brace group (N = 9) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Reason for treatment change at baseline | ||||
| Patient requested | 13 | 24.1 | 1 | 11.1 |
| Clinician decision | 3 | 5.6 | 1 | 11.1 |
| Medical resource unavailable | 0 | 0.0 | 2 | 22.2 |
| Incorrect Achilles tendon rupture diagnosis | 1 | 1.9 | 0 | 0.0 |
| Withdrew | 1 | 1.9 | 1 | 11.1 |
| Unknown | 1 | 1.9 | 0 | 0.0 |
| Reason for treatment change within 6 weeks after baseline | ||||
| Patient requested | 18 | 33.3 | 1 | 11.1 |
| Clinician decision | 12 | 22.2 | 0 | 0.0 |
| Surgery | 5 | 9.3 | 3 | 33.3 |
Details of the treatment received in each group, including time point when the patient was allowed to fully bear weight, time point when cast/brace was removed, number of cast changes, type of brace used, number of heel wedges and VTE prophylaxis are listed in Table 11. The data collected show that the number of participants allowed to bear weight early was larger in the functional brace group than in the plaster cast group. Proportions of these patients are listed for each treatment group and are restricted to patients who did not change from their allocated treatment in the first 6 weeks following randomisation.
| Treatment | Plaster cast group (N = 266) | Functional brace group (N = 274) |
|---|---|---|
| Time point when patient is allowed to fully bear weighta | ||
| Baseline | 6 (2.8) | 121 (45.7) |
| 2 weeks | 7 (3.3) | 24 (9.1) |
| 4 weeks | 15 (7.1) | 26 (9.8) |
| 6 weeks | 99 (46.7) | 30 (11.3) |
| ≥ 8 weeks | 81 (38.2) | 57 (21.5) |
| Unknown | 0 (0.0) | 1 (0.4) |
| Missing | 4 (1.9) | 6 (2.3) |
| Time point when cast/brace is removed, n (%) | ||
| Before 2 weeks | 1 (0.4) | 1 (0.4) |
| 2 weeks | 1 (0.4) | 2 (0.7) |
| 4 weeks | 4 (1.5) | 1 (0.4) |
| 6 weeks | 25 (9.4) | 10 (3.6) |
| 8 weeks | 164 (61.7) | 167 (60.9) |
| Still not removed | 12 (4.5) | 76 (27.7) |
| Number of plaster cast changes over 8 weeks, median (range), n | 3.0 (1–6), 241 | N/A |
| Functional brace make at baseline, n (%) | ||
| Donjoy | N/A | 15 (5.5) |
| Samson | N/A | 10 (3.6) |
| Aircast | N/A | 122 (44.5) |
| Ossur (Ossur UK Ltd, Stockport, UK) | N/A | 64 (23.4) |
| VACOped (Oped UK Ltd, Bowerhill Melksham, UK) | N/A | 25 (9.1) |
| Promedics (Promedics Orthopaedic Ltd, Port Glasgow, UK) | N/A | 23 (8.4) |
| Not known | N/A | 5 (1.8) |
| Number of heel wedges in functional brace, median (range), n | ||
| Baseline | N/A | 3.0 (0–5), 263 |
| 2 weeks | N/A | 3.0 (0–4), 258 |
| 4 weeks | N/A | 2.0 (0–4), 258 |
| 6 weeks | N/A | 1.0 (0–4), 260 |
| 8 weeks | N/A | 0.0 (0–4), 258 |
| VTE prophylaxis, n (%) | 187 (70.3) | 158 (57.7) |
| VTE treatment, n (%) | ||
| Low-molecular-weight heparin | 148 (79.1) | 120 (75.9) |
| Oral anticoagulant | 39 (20.9) | 38 (24.1) |
| VTE treatment duration (weeks), median (range), n | 8.0 (0–12), 181 | 8.0 (1–12), 156 |
There were no differences between the two treatment groups in the time point when the plaster cast or functional brace was removed. On average, patients in the plaster cast group changed their plaster cast three times during the treatment period. The most frequently used functional brace make was Aircast (44.5%) and the median number of heel wedges was three, although the number varied from zero to five, depending on time point. VTE prophylaxis was offered to more patients in the plaster cast group (n = 187, 70.3%) than in the functional brace group (n = 158, 57.7%). The VTE treatments used were low-molecular-weight heparin and oral anticoagulant in similar proportions in the two groups.
Numbers analysed
The ITT population comprised all patients who were randomised and gave consent to participate in the trial. These patients were analysed in the group to which they were allocated, including in the analysis of PROMs. Data for the two randomised participants who did not give their consent were excluded from the analysis. The ITT population was made up of 540 participants overall, 266 of whom were in the plaster cast group and 274 of whom were in the functional brace group. Analyses of all primary and secondary outcomes were performed for this population.
The CACE population35 comprised all randomised participants who were compliant with treatment for ≥ 6 weeks.
Withdrawals
Table 12 provides the available data at each follow-up time point, including the number of CRFs and PROs and the number of participants who withdrew or died, according to treatment group. There was a good completion rate of CRFs and PROs across both treatment groups throughout the study period. In total, 10 patients withdrew (seven from the plaster cast group and three from the functional brace group). There were two deaths, both in the functional brace group. Reasons for withdrawal are listed in Table 13; these include clinician decision, patient decision and private treatment.
| Time point | Plaster cast group (N = 266) | Functional brace group (N = 274) | Total (N = 540) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| 8 weeks | ||||||
| CRF completed | 264 | 99.2 | 273 | 99.6 | 537 | 99.4 |
| PRO completed | 234 | 88.0 | 241 | 88.0 | 475 | 88.0 |
| Withdrawna | 6 | 2.3 | 2 | 0.7 | 8 | 1.5 |
| Died | 0 | 0.0 | 1 | 0.4 | 1 | 0.2 |
| CRF not completed | 1 | 0.4 | 0 | 0.0 | 1 | 0.2 |
| PRO not completed | 26 | 9.8 | 30 | 10.9 | 56 | 10.4 |
| 3 months | ||||||
| PRO completed | 229 | 86.1 | 245 | 89.4 | 474 | 87.8 |
| Withdrawna | 7 | 2.6 | 3 | 1.1 | 10 | 1.9 |
| Died | 0 | 0.0 | 1 | 0.4 | 1 | 0.2 |
| PRO not completed | 30 | 11.3 | 25 | 9.1 | 55 | 10.2 |
| 6 months | ||||||
| PRO completed | 225 | 84.6 | 238 | 86.9 | 463 | 85.7 |
| Withdrawna | 7 | 2.6 | 3 | 1.1 | 10 | 1.9 |
| Died | 0 | 0.0 | 2 | 0.7 | 2 | 0.4 |
| PRO not completed | 34 | 12.8 | 31 | 11.3 | 65 | 12.0 |
| 9 months | ||||||
| PRO completed | 244 | 91.7 | 260 | 94.9 | 504 | 93.3 |
| Withdrawna | 7 | 2.6 | 3 | 1.1 | 10 | 1.9 |
| Died | 0 | 0.0 | 2 | 0.7 | 2 | 0.4 |
| PRO not completed | 15 | 5.6 | 9 | 3.3 | 24 | 4.4 |
| Time point | Plaster cast group (N = 266) | Functional brace group (N = 274) | Total (N = 540) | |||
|---|---|---|---|---|---|---|
| n | %a | n | %a | n | %a | |
| 8 weeks | ||||||
| Withdrawn | 6 | 2.3 | 2 | 0.7 | 8 | 1.5 |
| Withdrawal reason | ||||||
| Clinician decision | 1 | 0.4 | 0 | 0.0 | 1 | 0.2 |
| Patient decision | 4 | 1.5 | 1 | 0.4 | 5 | 0.9 |
| Private treatment | 1 | 0.4 | 1 | 0.4 | 2 | 0.4 |
| 3 months | ||||||
| Withdrawn | 1 | 0.4 | 1 | 0.4 | 2 | 0.4 |
| Withdrawal reason | ||||||
| Patient decision | 0 | 0.0 | 1 | 0.4 | 1 | 0.2 |
| Private treatment | 1 | 0.4 | 0 | 0.0 | 1 | 0.2 |
Analyses to address primary outcome
The primary outcome in this study is the ATRS measured at 9 months post injury, as described in the statistical analysis plan.
Achilles Tendon Rupture Score was assessed at baseline (pre injury), 8 weeks and 3, 6 and 9 months after the tendon rupture. The mean ATRS score and SD for each treatment group at each time point are provided in Table 14. The mean ATRS differences between the treatment groups was estimated based on a linear mixed-effects regression model both unadjusted and adjusted for the stratification factors age, sex and baseline ATRS as fixed effects, and recruitment centre and observations within participants as random effects. The adjusted analysis was prespecified as the principal analysis of the trial results.
| Time point | Plaster cast group | Functional brace group | Between-group difference (95% CI) | p-value | |
|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||
| Baseline, median (IQR), n | 100.0 (96.5–100), 264 | 100.0 (94–100), 273 | |||
| 8 weeks, mean (SD), n | 35.3 (20.1), 234 | 40.3 (17.8), 240 | 4.98 (1.3 to 8.7) | 5.53 (2 to 9.1) | 0.002 |
| 3 months, mean (SD), n | 44.4 (21.1), 229 | 45.6 (20.4), 244 | 1.23 (–2.5 to 4.9) | 1.76 (–1.8 to 5.3) | 0.335 |
| 6 months, mean (SD), n | 63.9 (21.4), 224 | 63.5 (23), 235 | –0.44 (–4.2 to 3.3) | 0.35 (–3.3 to 4) | 0.850 |
| 9 months, mean (SD), n | 74.4 (19.8), 244 | 72.8 (20.4), 259 | –1.65 (–5.2 to 1.9) | –1.38 (–4.9 to 2.1) | 0.439 |
The adjusted analysis showed no statistically significant difference in ATRS between the treatment groups at 9 months (–1.38, 95% CI –4.9 to 2.1). The 8-week follow-up results show a statistically significant difference in the ATRS in favour of the functional brace group (5.53, 95% CI 2.0 to 9.1); however, this effect fades during the 9-month follow-up. The marginal mean ATRS values from the mixed-effects model are presented from the 8-week follow-up to the 9-month follow-up in Figure 3.
FIGURE 3.
Marginal mean ATRS values from the mixed-effects model and associated 95% CIs for the two treatment groups pre injury to 9 months post injury. ATRS values range from 0 to 100, with higher scores indicating better outcome.
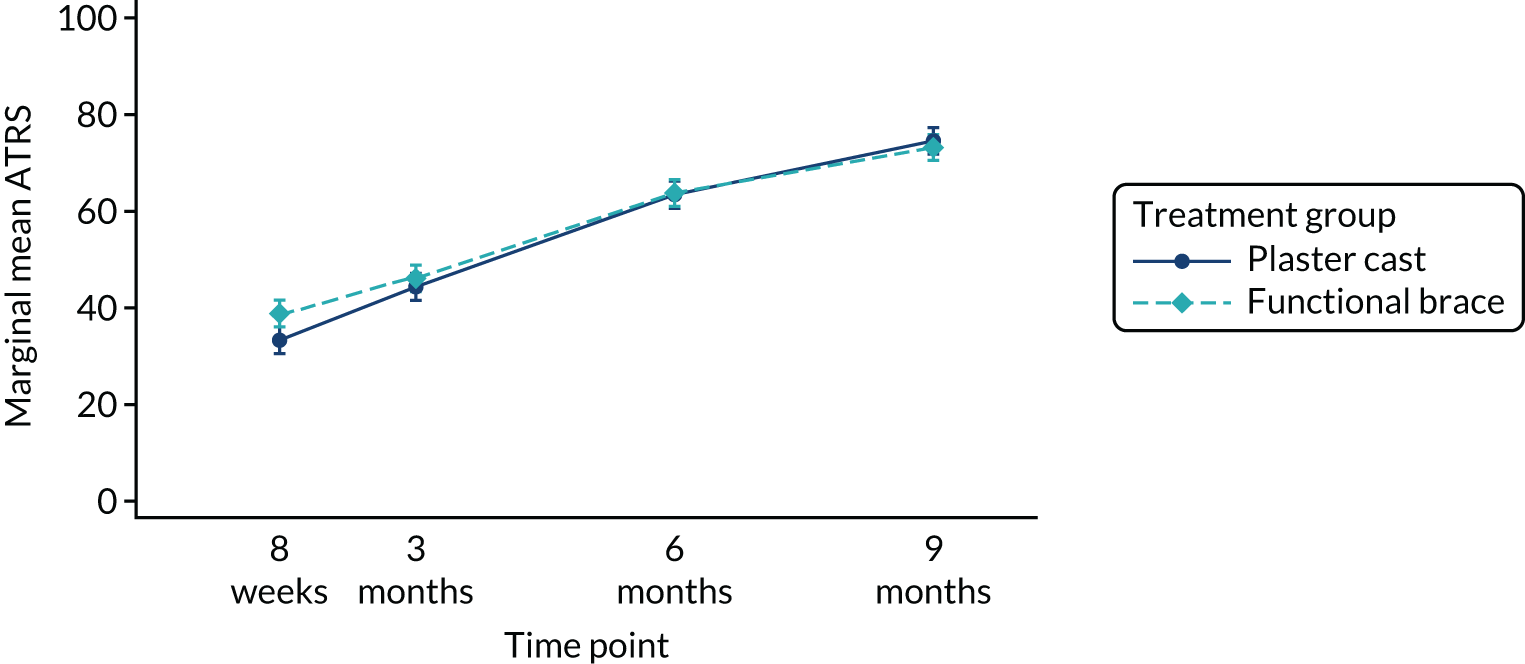
Supplementary fully adjusted analyses were carried out, accounting for further predefined prognostic variables, and included age, sex, baseline ATRS, diabetes mellitus and smoking status as fixed effects, with random effects for centre and observations within participant (Table 15). These results showed a similar between-group difference to those results of the primary analysis at 9 months post injury (–1.15, 95% CI –4.7 to 2.4).
| Analysis (population) | Time point | Between-group difference (95% CI) | p-value |
|---|---|---|---|
| Unadjusted (ITT)a | 9 months | –1.65 (–5.2 to 1.9) | 0.367 |
| Adjusted (ITT)b | 9 months | –1.38 (–4.9 to 2.1) | 0.439 |
| Fully adjusted (ITT)c | 9 months | –1.15 (–4.7 to 2.4) | 0.520 |
| Unadjusted (CACE)a | 9 months | –1.70 (–5.3 to 1.9) | 0.349 |
| Adjusted (CACE)b | 9 months | –1.18 (–4.5 to 2.1) | 0.486 |
| AUC adjusted (ITT) | 8 weeks to 9 months | –5.26 (–24.66 to 14.14) | 0.595 |
Sensitivity analyses
Complier-average causal effect analysis
The number of participants compliant with the allocated treatment from ≥ 6 weeks, ≥ 4 weeks and ≥ 2 weeks is shown in Table 8. A CACE analysis was conducted to estimate the mean effect of treatment in compliers with treatment for ≥ 6 weeks (n = 265, 96.7% in the functional brace group). The numbers of participants compliant with treatment for ≥ 4 weeks and for ≥ 2 weeks were identical in the functional brace group (n = 268, 97.8%) and hence a CACE analysis was conducted including only patients compliant for ≥ 4 weeks.
We estimated the CACE using the xtivreg and xtset commands in Stata. The unadjusted and the adjusted analyses estimating the ITT effect and the CACE analysis effect are shown in Table 14. The unadjusted CACE estimate was marginally greater in modulus than the ITT (–1.70, 95% CI –5.3 to 1.9), but this difference was small given the ATRS scoring scale. The adjusted CACE analysis showed similar results to the ITT population analysis (–1.17, 95% CI –4.5 to 2.1).
Area under the curve
Parameter estimates from the mixed-effects models were used to calculate the AUC from the 8-week follow-up to the 9-month follow-up for a male participant of a mean age of 48.65 years. Results showing an overall estimate of recovery over time and a t-test comparison of the two treatment groups are presented in Table 15. Higher AUCs indicate better overall functionality. The functional brace group showed a better overall functionality than the plaster cast group; however, the difference (–5.26, 95% CI –24.66 to 14.14) was not statistically significant.
Prespecified subgroup analysis
There were no prespecified subgroups and therefore no subgroup analyses were conducted.
Analyses to address secondary outcomes
The secondary outcomes collected and analysed in the UKSTAR were EQ-5D-5L and complications evaluated at 8 weeks and 3, 6 and 9 months after the injury.
EuroQol-5 Dimensions, five-level version
The EQ-5D-5L was analysed as a continuous outcome using the utility score values from the five-level questions and based on the reported EQ-5D VAS. Summary results for the EQ-5D utility score and EQ-5D VAS are reported for each treatment group at each time point in Table 16, together with the unadjusted and adjusted mixed-effects model estimates. The analyses for both EQ-5D utility score and EQ-5D VAS are presented graphically in Figures 4 and 5. Both EQ-5D scores show a trend of improvement over time. The EQ-5D utility score analysis showed a statistically significant difference at the 8-week follow-up in favour of the functional brace group (0.069, 95% CI 0.03 to 0.1), but this difference was no longer present by the 9-month post injury follow-up.
| EQ-5D | Plaster cast group (N = 266) | Functional brace group (N = 274) | Between-group difference (95% CI) | p-value | |
|---|---|---|---|---|---|
| Unadjusted | Adjusteda | ||||
| EQ-5D utility | |||||
| Baseline post injury, median (IQR), n | 0.242 (0.02–0.47), 264 | 0.282 (0.03–0.52), 273 | 0.042 (0.01 to 0.08) | 0.041 (0.01 to 0.07) | 0.017 |
| 8 weeks, mean (SD), n | 0.588 (0.23), 234 | 0.7 (0.18), 241 | 0.066 (0.03 to 0.1) | 0.069 (0.03 to 0.1) | 0.000 |
| 3 months, mean (SD), n | 0.638 (0.22), 229 | 0.669 (0.19), 245 | 0.031 (–0.01 to 0.07) | 0.035 (0.0 to 0.07) | 0.056 |
| 6 months, mean (SD), n | 0.766 (0.15), 224 | 0.757 (0.18), 237 | –0.009 (–0.05 to 0.03) | –0.002 (–0.04 to 0.03) | 0.916 |
| 9 months, median (IQR), n | 0.829 (0.72–0.91), 244 | 0.795 (0.72–0.88), 259 | –0.010 (–0.05 to 0.03) | –0.009 (–0.04 to 0.03) | 0.623 |
| EQ-5D VAS, median (IQR), n | |||||
| Baseline post injury | 90.0 (80–95), 263 | 90.0 (80–95), 273 | 0.77 (–2.18 to 3.72) | 1.28 (–1.4 to 3.97) | 0.349 |
| 8 weeks | 75.0 (60–85), 234 | 75.0 (65–85), 240 | 1.08 (–2.05 to 4.2) | 1.61 (–1.21 to 4.43) | 0.264 |
| 3 months | 80.0 (65–85), 229 | 80.0 (65–90), 245 | 1.29 (–1.84 to 4.42) | 1.66 (–1.16 to 4.48) | 0.249 |
| 6 months | 81.5 (70–90), 224 | 80.0 (70–90), 236 | 0.49 (–2.69 to 3.66) | 1.08 (–1.77 to 3.93) | 0.458 |
| 9 months | 86.0 (80–92), 242 | 85.0 (75–91), 259 | –0.76 (–3.8 to 2.28) | –0.56 (–3.32 to 2.2) | 0.693 |
FIGURE 4.
Marginal mean EQ-5D utility values from the mixed-effects model and associated 95% CIs for the two treatment groups from baseline (post injury) to 9 months.
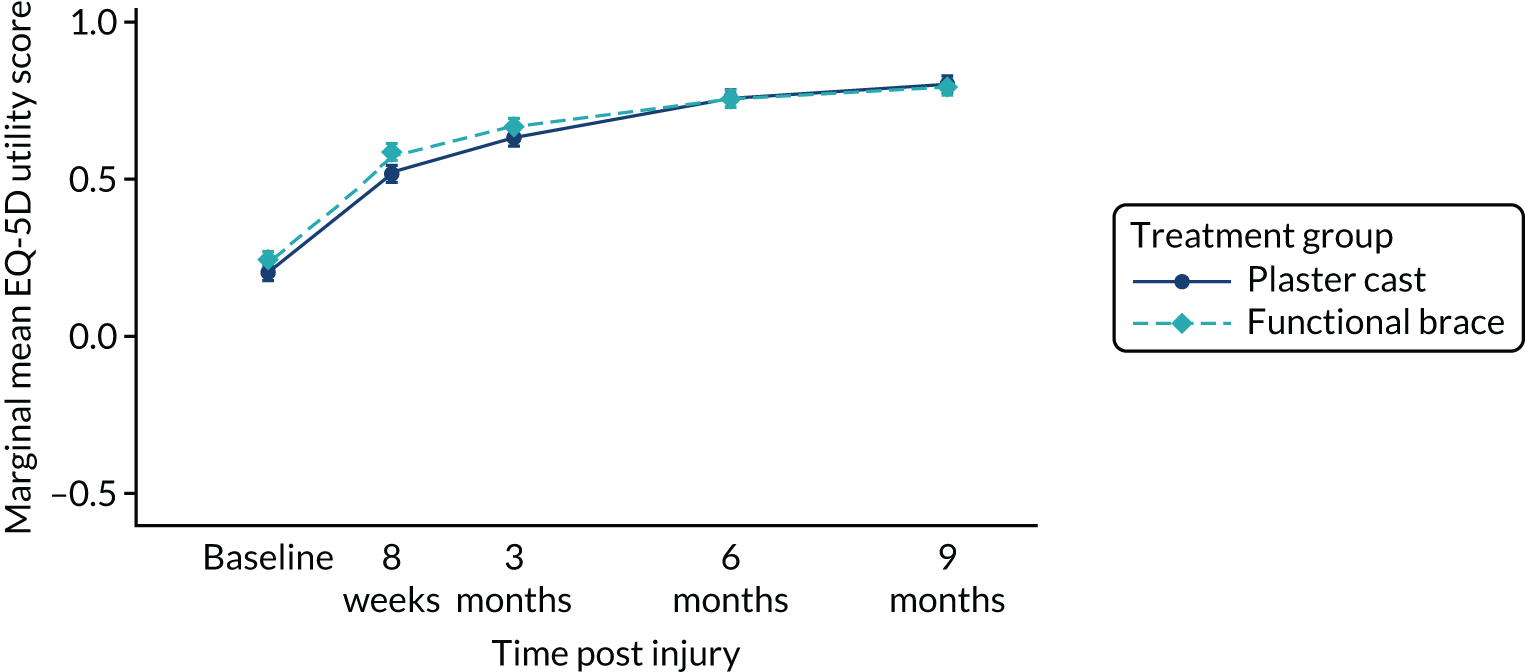
FIGURE 5.
Marginal mean EQ-5D VAS values from the mixed-effects model and associated 95% CIs for the two treatment groups from baseline (post injury) to 9 months.
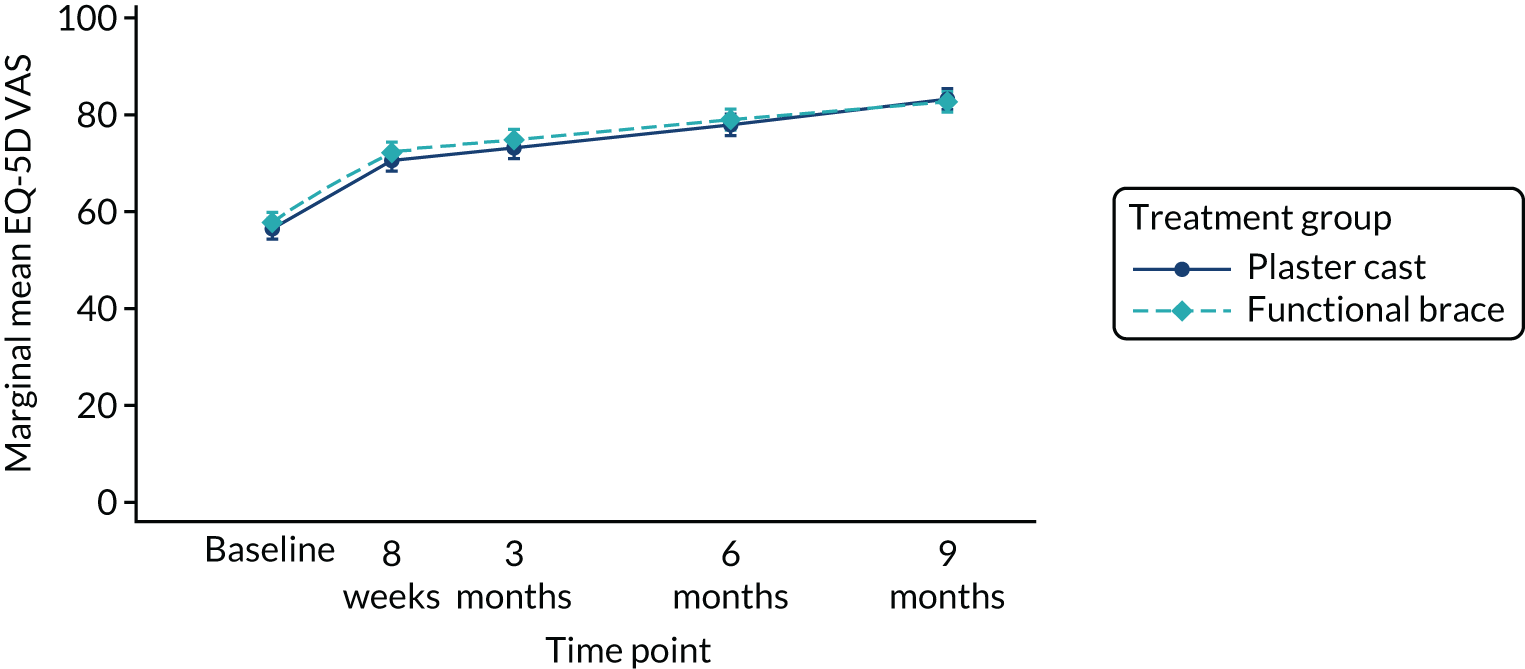
Sensitivity analyses
Sensitivity analyses were performed for the EQ-5D utility and VAS outcomes using CACE analysis and the AUC (Table 17). The CACE analysis was conducted using a similar approach as for the primary outcome ATRS and showed similar results to the EQ-5D ITT population analysis. The AUC summary statistics were estimated for a male participant of a mean age of 48.65 years, calculated from baseline post injury to 9 months post injury, with higher AUCs indicating better QoL.
| Analysis (population) | Time point | Between-group difference (95% CI) | p-value |
|---|---|---|---|
| EQ-5D utility | |||
| Adjusted (ITT)a | 9 months | –0.009 (–0.04 to 0.03) | 0.623 |
| Adjusted (CACE)a | 9 months | –0.008 (–0.03 to 0.02) | 0.502 |
| AUC adjusted (ITT)a,b | 8 weeks to 9 months | –0.20 (–0.4 to 0.01) | 0.056 |
| EQ-5D VAS | |||
| Adjusted (ITT)a | 9 months | –0.56 (–3.3 to 2.2) | 0.693 |
| Adjusted (CACE)a | 9 months | –0.53 (–2.7 to 1.7) | 0.637 |
| AUC adjusted (ITT)a,b | 8 weeks to 9 months | –9.42 (–26.9 to 8.1) | 0.292 |
Complications
The number of participants with one or more complications in each treatment group is presented overall from baseline to 9 months in Table 18 and at every time point for the ITT population in Table 19. Fisher’s exact and chi-squared tests showed no statistically significant results when testing for associations between the treatment groups and each type of complication across time.
| Complication | Plaster cast group (N = 266) | Functional brace group (N = 274) | p-value | ||
|---|---|---|---|---|---|
| n a | % | n a | % | ||
| Tendon re-rupture | 17 | 6.4 | 13 | 4.7 | 0.404 |
| DVT | 3 | 1.1 | 6 | 2.2 | 0.505 |
| PE | 0 | 0.0 | 2 | 0.7 | 0.499 |
| Fall: no injury | 60 | 22.6 | 53 | 19.3 | 0.359 |
| Fall: injury sustained | 21 | 7.9 | 24 | 8.8 | 0.716 |
| Pain under the heel | 158 | 59.4 | 180 | 65.7 | 0.131 |
| Numbness around the foot | 108 | 40.6 | 130 | 47.4 | 0.109 |
| Pressure sores | 39 | 14.0 | 51 | 18.6 | 0.218 |
| Complication | Plaster cast group (N = 266) | Functional brace group (N = 274) | ||
|---|---|---|---|---|
| n | % | n | % | |
| 8 weeks | ||||
| Tendon re-rupture | 3 | 1.1 | 3 | 1.1 |
| DVT | 2 | 0.8 | 6 | 2.2 |
| PE | 0 | 0.0 | 2 | 0.7 |
| Fall: no injury | 26 | 9.8 | 12 | 4.4 |
| Fall: injury | 3 | 1.1 | 6 | 2.2 |
| Pain under the heel | 33 | 12.4 | 48 | 17.5 |
| Numbness around the foot | 24 | 9.0 | 32 | 11.7 |
| Pressure sores | 9 | 3.4 | 9 | 3.3 |
| Skin condition requiring medication | 0 | 0.0 | 4 | 1.5 |
| Surgery related to Achilles rupture | 0 | 0.0 | 3 | 1.1 |
| Fractured toe | 1 | 0.4 | 0 | 0.0 |
| 3 months | ||||
| Tendon re-rupture | 8 | 3.0 | 4 | 1.5 |
| DVT | 2 | 0.8 | 1 | 0.4 |
| PE | 0 | 0.0 | 2 | 0.7 |
| Fall | 20 | 7.5 | 15 | 5.5 |
| Fall: injury | 7 | 2.6 | 9 | 3.3 |
| Pain under the heel | 125 | 47.0 | 137 | 50.0 |
| Numbness around the foot | 59 | 22.2 | 79 | 28.8 |
| Pressure sores | 23 | 8.6 | 35 | 12.8 |
| 6 months | ||||
| Tendon re-rupture | 6 | 2.3 | 6 | 2.2 |
| DVT | 0 | 0.0 | 0 | 0.0 |
| PE | 0 | 0.0 | 2 | 0.7 |
| Fall: no injury | 19 | 7.1 | 23 | 8.4 |
| Fall: injury | 6 | 2.3 | 11 | 4.0 |
| Pain under the heel | 78 | 29.3 | 82 | 29.9 |
| Numbness around the foot | 54 | 20.3 | 66 | 24.1 |
| Pressure sores | 9 | 3.4 | 15 | 5.5 |
| 9 months | ||||
| Tendon re-rupture | 0 | 0.0 | 0 | 0.0 |
| DVT | 0 | 0.0 | 0 | 0.0 |
| PE | 0 | 0.0 | 0 | 0.0 |
| Fall: no injury | 16 | 6.0 | 11 | 4.0 |
| Fall: injury | 10 | 3.8 | 5 | 1.8 |
| Pain under the heel | 48 | 18.0 | 66 | 24.1 |
| Numbness around the foot | 51 | 19.2 | 50 | 18.2 |
| Pressure sores | 2 | 0.8 | 8 | 2.9 |
Ancillary analyses
Following a presentation of the preliminary results, the TSC wanted to explore where the apparent differences at 8 weeks in EQ-5D utility score came from. The individual domains of the EQ-5D were explored using box plots in Figures 18–21 (see Appendix 3). The median score is marked with a triangle, whiskers are IQRs and the individual dots and crosses mark the outliers. The differences at 8 weeks appear to lie in the ability to self-care and usual activities only. Furthermore, in keeping with the health economics analysis plan, the distribution of the responses to the EQ-5D-5L questionnaires by treatment group and assessment point is presented in Appendix 4. That analysis showed no significant differences in the proportions of individuals reporting suboptimal health [i.e. any functional (below level 1) impairment] within dimensions between the two treatment groups at each time point.
Adverse events
Foreseeable AEs were reported as complications in Analyses to address secondary outcomes.
Two deaths were reported in this study, one of which was a SAE. This was due to a known lung cancer condition and was unrelated to the Achilles injury. The second death was due to a cardiac arrest following a bilateral PE and, as judged by the investigators, was potentially related but not unexpected. Both deaths were in the functional brace group.
Chapter 4 Health economics
This section presents the results of the health economic analyses comparing plaster cast with functional brace. We compare (1) missing data by treatment group; (2) resource use and economic costs for different health and social care categories; (3) distribution of the responses to the EQ-5D-5L questionnaires and the EQ-5D-5L utility scores; and (4) cost-effectiveness results of the base-case and sensitivity analyses.
Results of economic analysis
Table 20 shows the degree of missing health economic data by treatment group and follow-up time point. The missing data pattern is non-monotonic, as individuals with missing data at one follow-up time point could return to the trial subsequently. For example, there are more missing EQ-5D data at 6 months than at 9 months post injury. A similar pattern can be observed for economic costs. It is worth noting that the smaller number of participants with complete data for the entire duration of follow-up (baseline to 9 months post injury) was because of a strict application of the term missing (i.e. we considered a participant as having incomplete data if, for example, they responded positively to vising a GP surgery at 3 months but did not specify the number of consultations, despite all other resource use items being completed). However, for the cost-effectiveness analysis, imputation was not at the aggregate level, such that most of the data used for the analysis were based on actual participant responses.
| Variable | Description | Treatment group, missing values, n (%) | Total, missing values, n (%) | |
|---|---|---|---|---|
| Plaster cast (N = 266) | Functional brace (N = 274) | |||
| eq5db | EQ-5D index score pre injury | 2 (0.75) | 2 (0.73) | 4 (0.74) |
| eq5d0 | EQ-5D index score post injury | 2 (0.75) | 1 (0.36) | 3 (0.56) |
| eq5d1 | EQ-5D at 8 weeks | 32 (12.06) | 33 (12.04) | 65 (12.04) |
| eq5d2 | EQ-5D at 3 months | 37 (13.91) | 29 (10.58) | 66 (12.22) |
| eq5d3 | EQ-5D at 6 months | 42 (15.79) | 37 (13.5) | 79 (14.63) |
| eq5d4 | EQ-5D at 9 months | 22 (26) | 15 (5.47) | 37 (8.27) |
| QALY | QALYs generated from EQ-5D utility scores | 76 (28.57) | 74 (27.01) | 149 (27.78) |
| c0 | Total resource use between baseline and 8 weeks post injury | 66 (24.8) | 59 (21.53) | 125 (23.15) |
| c1 | Total resource use between 8 weeks and 3 months post injury | 59 (22.18) | 47 (17.15) | 106 (19.63) |
| c2 | Total resource use between 3 and 6 months post injury | 56 (21.05) | 48 (8.89) | 104 (19.26) |
| c3 | Total resource use between 6 and 9 months post injury | 31 (11.65) | 18 (6.57) | 49 (9.07) |
| c4 | Total resource use between baseline and 9 months post injury | 132 (49.62) | 116 (42.34) | 248 (45.93) |
Health and social care resource use
Table 25 (see Appendix 1) shows the resource use values for participants by treatment group, resource use category and follow-up period for complete cases. The values are presented for subcategories of resource use, including hospital inpatient and outpatient care, community health and social care, prescribed medications, equipment and aids, and productivity losses.
In terms of specific resource use at the 8-week follow-up (see Appendix 1, Table 25), notable differences were observed between the groups for the proportion prescribed anticoagulant as VTE prophylaxis treatment (0.72 vs. 0.59; p = 0.003), the mean number of NHS outpatient orthopaedic visits (2.63 vs. 1.80; p < 0.001), the mean number of NHS outpatient physiotherapy visits (0.23 vs. 0.46; p = 0.003), the mean number of GP surgery visits (0.10 vs. 0.19; p = 0.028) and the mean number of grab rail installations (0.05 vs. 0; p = 0.019). For all other resource use items, there were no noticeable differences between the groups.
Between 8 weeks and 3 months post injury (see Appendix 1, Table 25), there were differences in resource use between the groups for the proportion of participants prescribed analgesics (0.11 vs. 0.05; p = 0.015) and the proportion of participants prescribed other medications (0.02 vs. 0; p = 0.038). For all other resource use items, there were no noticeable differences between the groups.
There were no significant differences in resource use between the plaster cast and the functional brace groups at 6 or 9 months post injury.
Economic costs
Table 21 summarises the total NHS and PSS costs associated with resource use during the trial period among complete cases by cost category and by follow-up period. The mean direct intervention cost was £35.71 for the plaster cast group compared with £108.64 for the functional brace group; the mean difference of £72.93 was statistically significant at the 5% level. The mean total NHS and PSS costs were significantly lower in the functional brace group between randomisation and 8 weeks post injury and between 8 weeks and 3 months post injury, with mean between-group cost differences of £107.73 and £92.95, respectively. The mean total NHS and PSS cost during the entire follow-up period was £1182.64 for the plaster cast group and £1018.26 for the functional brace group; the mean between-group cost difference of £164.39 was not statistically significant at the 5% level. Figure 14 (see Appendix 1) shows the distribution of total NHS and PSS costs during the entire follow-up period and indicates that differences in measures of central tendency (i.e. mean, median) for total economic costs between the treatment groups were not driven by high costs generated by a small number of participants in the trial.
| Cost category by period | Treatment group, mean (SE) cost (£) | Mean difference | p-valuea | Bootstrap 95% CIb | |
|---|---|---|---|---|---|
| Plaster cast | Functional brace | ||||
| Baseline to 8 weeks post injury: direct intervention costsc (total, N = 497: plaster cast, n = 241; functional brace, n = 256) | |||||
| Total direct intervention costs | 35.71 (0.492) | 108.64 (3.114) | –72.93 | < 0.0001 | –79.22 to –66.64 |
| Baseline to 8 weeks post injury: NHS PSS resource use (total, N = 432: plaster cast, n = 210; functional brace, n = 222) | |||||
| Inpatient care | 55.8 (28.382) | 39.3 (22.163) | 16.51 | 0.647 | –53.48 to 86.49 |
| Outpatient care | 370.2 (15.114) | 282.6 (15.078) | 87.59 | < 0.0001 | 45.97 to 129.21 |
| Community care | 9.66 (2.521) | 28.94 (14.493) | –19.28 | 0.191 | –47.64 to 9.07 |
| Medications | 151.35 (9.334) | 106.45 (8.701) | 44.9 | < 0.001 | 20.34 to 69 |
| Aids and adaptations | 9.51 (0.842) | 7.32 (0.568) | 2.19 | 0.032 | 0.20 to 4.19 |
| PSS | 0.15 (0.151) | 0 (0) | 0.15 | 0.318 | –0.14 to 0.45 |
| Total NHS and PSS cost | 596.67 (36.596) | 464.61 (32.946) | 132.06 | 0.008 | 33.35 to 230.78 |
| Total costs during first 8 weeks (including direct intervention costs)d | 647.88 (37.99) | 540.15 (26.10) | 107.73 | 0.02 | 16.15 to 199.31 |
| 8 weeks to 3 months post injury (total, N = 434: plaster cast, n = 207; functional brace, n = 227) | |||||
| Inpatient care | 61.69 (27.278) | 4.74 (4.74) | 56.95 | 0.041 | 4.33 to 109.57 |
| Outpatient care | 118.74 (9.948) | 98.76 (7.608) | 19.98 | 0.111 | –5.07 to 45.03 |
| Community care | 31.96 (15.86) | 19.89 (4.77) | 12.07 | 0.339 | 20.69 to 44.83 |
| Medications | 5.95 (3.359) | 2.86 (2.133) | 3.1 | 0.437 | –4.56 to 10.75 |
| Aids and adaptations | 1.65 (0.359) | 0.75 (0.261) | 0.9 | 0.044 | 0.014 to 1.78 |
| PSS | 0 (0) | 0.04 (0.04) | –0.04 | 0.318 | –0.12 to 0.039 |
| Total NHS and PSS cost | 220.00 (36.662) | 127.04 (12.333) | 92.95 | 0.017 | 14.80 to 171.11 |
| 3–6 months post injury (total, N = 436: plaster cast, n = 210; functional brace, n = 226) | |||||
| Inpatient care | 21.08 (16.736) | 43.94 (22.392) | –22.86 | 0.414 | –80.61 to 34.90 |
| Outpatient care | 128.56 (11.731) | 142.56 (13.215) | –14 | 0.429 | –47.66 to 19.67 |
| Community care | 33.56 (2.617) | 27.75 (5.679) | 5.812 | 0.376 | –19.98 to 31.60 |
| Medications | 0 (0) | 1.51 (1.015) | –1.51 | 0.138 | –3.56 to 0.54 |
| Aids and adaptations | 1.02 (0.472) | 1.03 (0.534) | –0.01 | 0.989 | –1.38 to 1.36 |
| PSS | 0.49 (0.491) | 0 (0) | 0.49 | 0.318 | –0.42 to 1.40 |
| Total NHS and PSS cost | 184.70 (26.350) | 216.78 (29.988) | –32.07 | 0.422 | –108.74 to 44.58 |
| 6–9 months post injury (total, N = 491: plaster cast, n = 235; functional brace, n = 256) | |||||
| Inpatient care | 5.94 (4.771) | 45.45 (45.45) | –39.51 | 0.388 | –133.06 to 54.05 |
| Outpatient care | 76.44 (22.253) | 65.98 (9.884) | 10.46 | 0.668 | –39.34 to 60.26 |
| Community care | 14.03 (4.022) | 17.302 (7.197) | –3.27 | 0.691 | –19.78 to 13.24 |
| Medications | 0.33 (0.228) | 0.13 (0.058) | 0.2 | 0.396 | –0.26 to 0.66 |
| Aids and adaptations | 0.36 (0.217) | 0.08 (0.052) | 0.28 | 0.214 | –0.15 to 0.71 |
| PSS | 0.11 (0.11) | 0 (0) | 0.11 | 0.318 | –0.10 to 0.32 |
| Total NHS and PSS cost | 97.21 (23.666) | 128.94 (46.80) | –31.73 | 0.543 | –127.40 to 63.94 |
| 0–9 months post injury (total, N = 292: plaster cast, n = 134; functional brace, n = 158) | |||||
| Total direct intervention costs | 35.96 (0.646) | 106.46 (4.08) | –70.5 | –78.30 to –62.69 | |
| Inpatient care | 162.29 (85.042) | 45.43 (24.373) | 116.86 | 0.188 | –62.66 to 296.38 |
| Outpatient care | 722.78 (39.326) | 653.42 (40.288) | 69.36 | 0.219 | –38.61 to 177.33 |
| Community care | 103.22 (33.32) | 91.18 (22.809) | 12.04 | 0.766 | –67.72 to 91.80 |
| Medications | 146.41 (11.364) | 112.61 (10.884) | 33.8 | 0.033 | –2.66 to 64.94 |
| Aids and adaptations | 11.75 (1.331) | 9.16 (1.127) | 2.59 | 0.139 | –0.71 to 5.88 |
| PSS | 0.24 (0.236) | 0 (0) | 0.24 | 0.319 | –0.22 to 0.70 |
| Total NHS and PSS costs during first 9 months | 1182.64 (114.696) | 1018.26 (58.143) | 164.39 | 0.203 | –95.75 to 424.52 |
Health outcomes
Summary results of the EQ-5D-5L analysis are presented in detail Chapter 3, Analyses to address secondary outcomes and Ancillary analyses. Below we provide the cost-effectiveness results.
Cost-effectiveness results
The cost-effectiveness results are presented in Table 22, with plaster cast as the referent and functional brace as the comparator (i.e. functional brace minus plaster cast) for the estimation of ICER values. The analytic time horizon is the entire 9-month post-injury follow-up period of the trial. The joint distribution of costs and outcomes for the base-case analysis and sensitivity analyses is represented in Figures 6–13.
| Scenario | Treatment group, mean (SE) cost (£) | Incremental cost (95% CI) | Treatment group, mean (SE) QALY | Incremental QALYs (95% CI) | ICERa (£) | Probability that functional brace is cost-effective | NMBs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Functional brace | Plaster cast | Functional brace | Plaster cast | p-valueb | p-valuec | p-valued | NMBe (95% CI) | NMBf (95% CI) | NMBg (95% CI) | ||||
| Base-case analysis | |||||||||||||
| Imputed attributable costs and QALYs; covariate adjusted | 1078.16 (83.42) | 1180.72 (89.63) | –102.56 (–289.28 to 84.16) | 0.506 (0.0064) | 0.492 (0.0066) | 0.015 (–0.0013 to 0.030) | Dominant | 0.963 | 0.965 | 0.966 | 312.28 (–31.26 to 655) | 383.82 (–32.67 to 793.80) | 526.90 (–42.50 to 1076.87) |
| Sensitivity analyses | |||||||||||||
| Complete-case attributable costs and QALYs; covariate adjusted | 948.77 (53.91) | 1117.28 (110.66) | –168.51 (–458.01 to 32.88) | 0.513 (0.00642) | 0.497 (0.0064) | 0.017 (–0.0035 to 0.037) | Dominant | 0.976 | 0.976 | 0.972 | 443.54 (19.83 to 933.22) | 527.26 (9.11 to 1094.07) | 694.70 (–17.56 to 1406.23) |
| Societal perspective | 4362.15 (348.71) | 4114.54 (292.18) | 247.61 (–476.44 to 971.66) | 0.506 (0.0063) | 0.502 (0.007) | 0.015 (–0.0042 to 0.031) | 16,510 | 0.501 | 0.576 | 0.688 | –29.65 (–991.50 to 874.93) | 44.36 (–964.19 to 991.46) | 192.39 (–926.97 to 1244.53) |
| CACE population | 1038.6 (62.89) | 1169.44 (78.48) | –130.84 (–335.38 to 90.36) | 0.510 (0.00609) | 0.488 (0.00688) | 0.022 (0.0051 to 0.038) | Dominant | 0.992 | 0.993 | 0.994 | 44.52 (89.86 to 852.63) | 57.36 (127.50 to 1030.39) | 818.02 (199.26 to 1434.03) |
| Secondary cost-effectiveness analysis using ATRSh as outcome measure | 1057.22 (71.91) | 1149.44 (79.25) | –92.21 (–273.86 to 89.44) | 45.09 (0.72) | 44.30 (0.73) | 0.78 (–1.12 to 2.69) | Dominant | 0.875 | 0.839 | 0.822 | 174.03 (–117.37 to 463.44) | 328.84 (–306 to 970.91) | 406.25 (–403.75 to 1218.76) |
FIGURE 6.
Cost-effectiveness scatterplot at 9 months for base-case analysis (NHS and PSS perspective, imputed, additionally controlled for pre-injury utility, ITT analysis). Reproduced from Costa et al. 49 © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
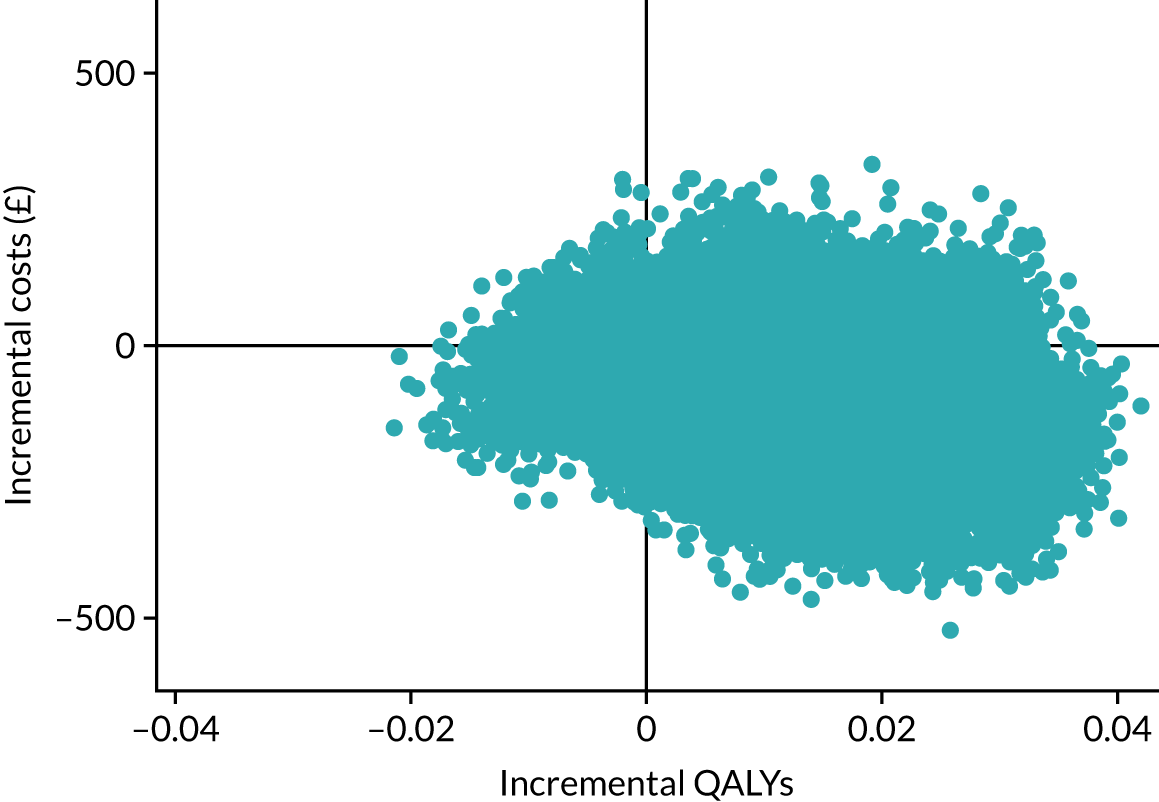
FIGURE 7.
Cost-effectiveness acceptability curve at 9 months for base-case analysis (NHS and PSS perspective, imputed, additionally controlled for pre-injury utility, ITT analysis). Reproduced from Costa et al. 49 © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
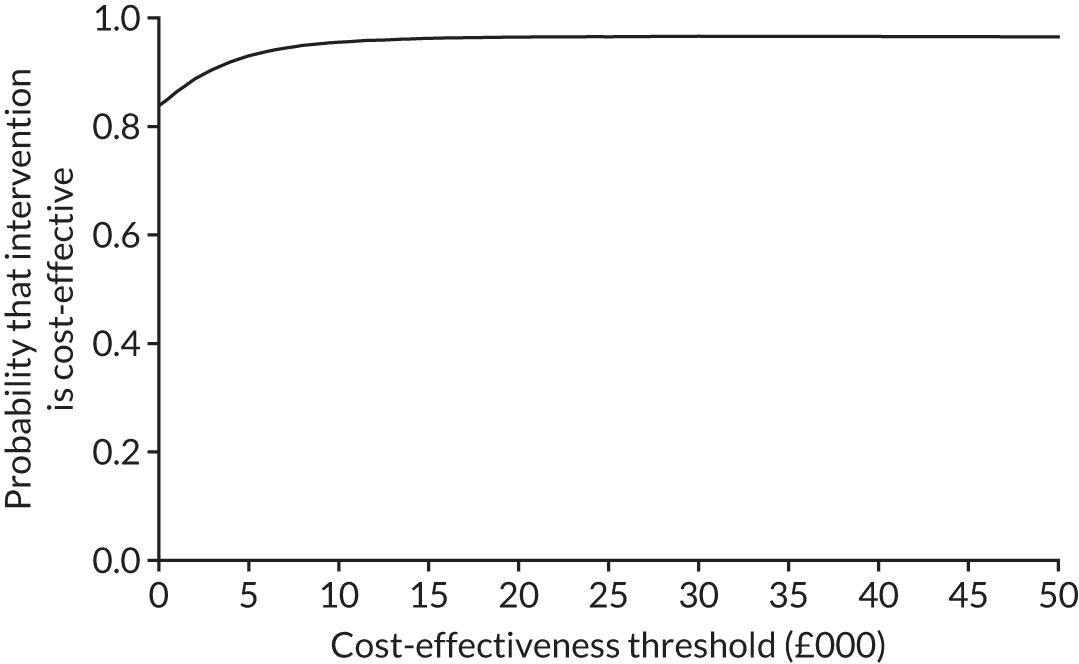
FIGURE 8.
Cost-effectiveness scatterplot at 9 months for complete cases (NHS and PSS perspective, ITT analysis).
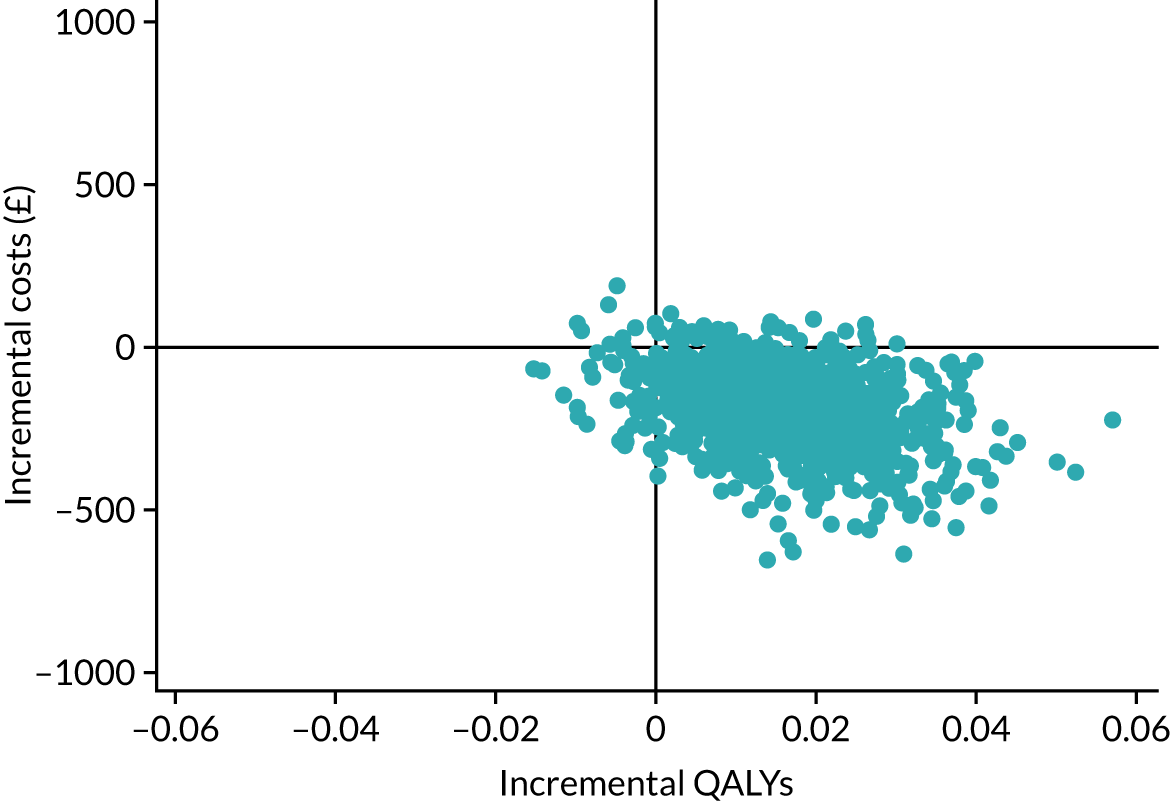
FIGURE 9.
Cost-effectiveness acceptability curve for complete cases (NHS and PSS perspective, ITT analysis).
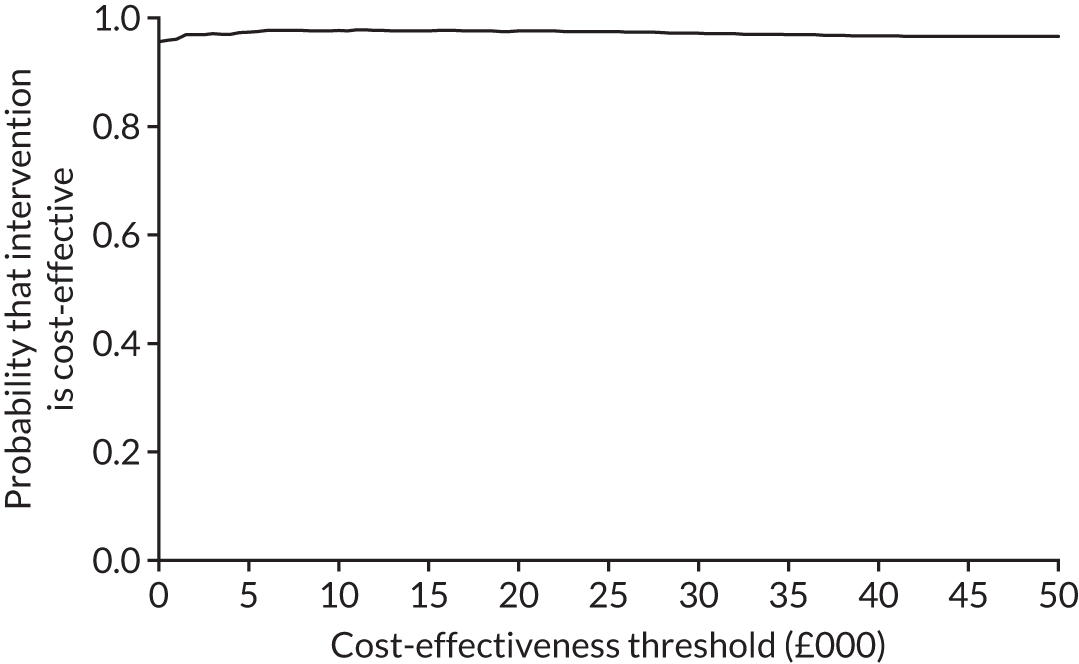
FIGURE 10.
Cost-effectiveness scatterplot for societal perspective (imputed, ITT analysis).
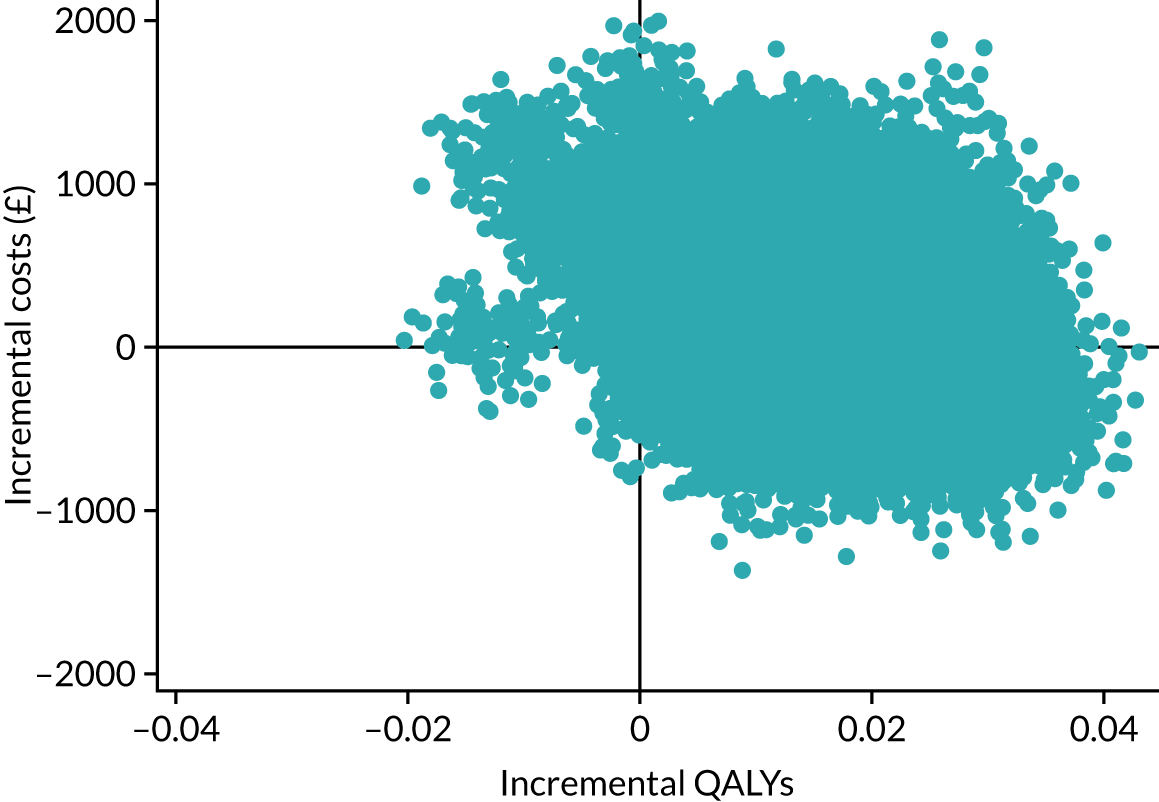
FIGURE 11.
Cost-effectiveness acceptability curve for societal perspective (imputed, ITT analysis).
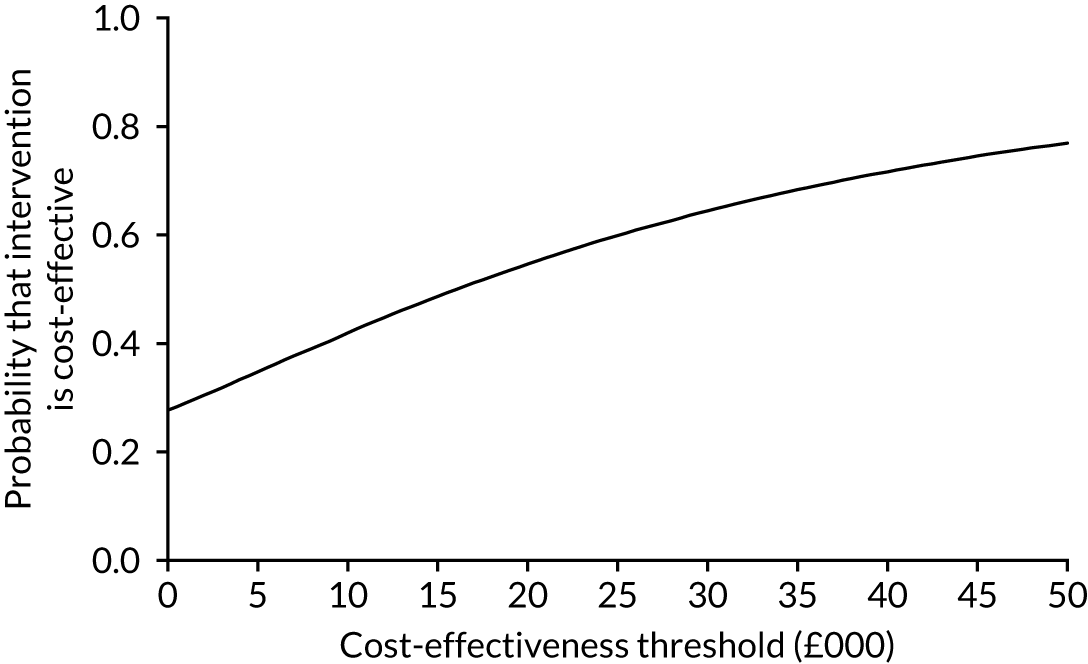
FIGURE 12.
Cost-effectiveness scatterplot for CACE population (imputed, NHS and PSS perspective).
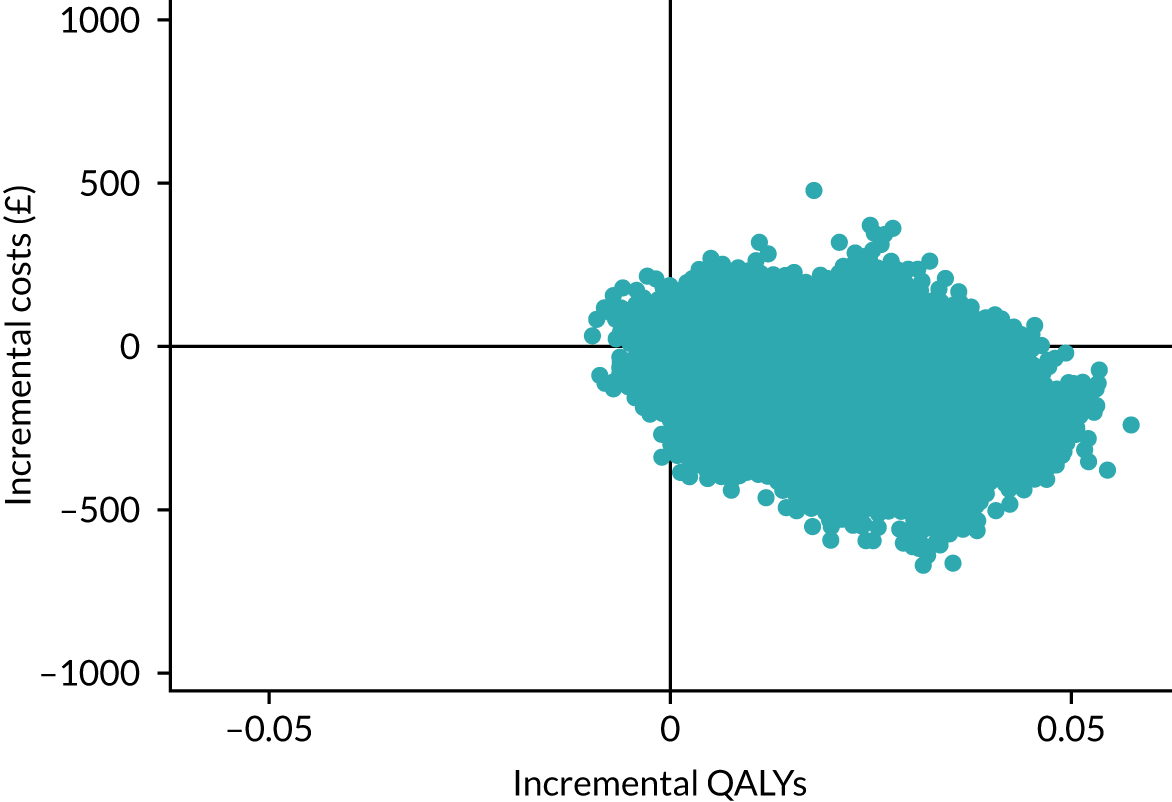
FIGURE 13.
Cost-effectiveness acceptability curve for CACE population (imputed, NHS and PSS perspective, covariate adjusted).
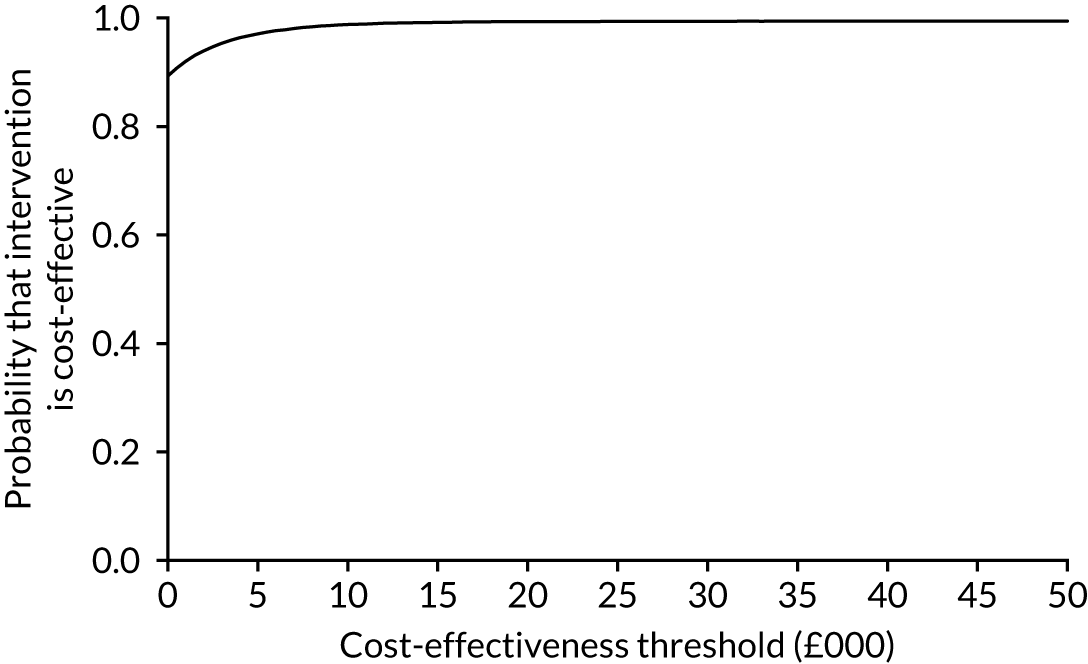
Base-case analysis
Patients in the functional brace group experienced a non-statistically significant increase in QALYs in the base case (0.015 QALYs, 95% CI –0.0013 to 0.030 QALYs) over the 9-month follow-up period. In addition, mean NHS and PSS costs were lower in the functional brace group (mean cost difference –£103, 95% CI –£289 to £84). The ICER for the base-case analysis indicates that functional brace is the dominant procedure, as average costs for this intervention were lower and average benefits were greater than those for plaster cast.
Assuming cost-effectiveness thresholds of £15,000 per QALY, £20,000 per QALY and £30,000 per QALY, respectively, the probability that functional brace was cost-effective ranged from 0.96 to 0.97, and the NMB associated with functional brace was positive (see Table 22).
Sensitivity analyses
Comparing mean costs and QALY estimates using different analytical scenarios (complete case, societal perspective and CACE population) revealed that the cost-effectiveness results generally supported the base-case finding, with the exception of the sensitivity analysis that adopted a societal perspective. From the societal perspective, mean costs were higher in the functional brace group (£248, 94% CI –£476 to £972). However, the QALY results followed the same pattern as that for the base-case analysis and indicated that participants in the functional brace group experienced a non-statistically significant increase in QALYs over the 9-month follow-up period (0.015 QALYs, 95% CI –0.0042 to 0.031 QALYs). The probability that functional brace was cost-effective declined to a range of 0.50–0.69 at cost-effectiveness thresholds of £15,000 per QALY, £20,000 per QALY and £30,000 per QALY. The results of the mixed-effects model followed a similar pattern to those of the base-case (imputed) model: patients in the functional brace group experienced a non-statistically significant increase in QALYs (0.014 QALYs, 95% CI –0.0018 to 0.031 QALYs) over the 9-month follow-up period. In addition, mean NHS and PSS costs were lower in the functional brace group (mean cost difference –£135, 95% CI –£342 to £71).
The results of the sensitivity analyses to the MAR assumption, presented in Appendix 1, indicate that the cost-effectiveness of functional brace remained stable across all of the MAR departure scenarios. In addition, Figure 17 shows that a change in cost-effectiveness decision is likely to occur if participants with missing HRQoL data have up to 50% lower HRQoL than trial participants with similar characteristics whose HRQoL data are available.
Long-term economic modelling
The protocol allowed for decision-analytic modelling to estimate the longer-term cost-effectiveness of functional brace or plaster cast. However, we note that cost and health utility values started to converge from the 3-month follow-up time point and converged at subsequent time points, even though functional brace was cost-effective over the entire follow-up period. It was therefore concluded that longer-term extrapolation of cost-effectiveness of functional brace is highly unlikely to be meaningful. Furthermore, we did not identify external studies that compared differences in economic costs, functional outcomes or HRQoL beyond 9 months post injury between non-surgical patients treated with plaster cast and those treated with functional brace. This lack of the data needed to parameterise a model further challenged any efforts to conduct longer-term decision modelling.
Chapter 5 Discussion
Recruitment
A total of 375 patients screened were found to be ineligible for the trial. The most common reason for being ineligible (n = 155) was presenting > 14 days after the Achilles tendon injury. Late presentation after an Achilles tendon rupture is not uncommon. Although a patient may have severe pain when the tendon ruptures, this acute pain usually settles quickly and the patient can often put weight through their leg, albeit without being able to walk normally. Some patients feel that they would not be able to walk at all if they had suffered a ‘serious’ injury and therefore continue to try to mobilise on their leg before eventually seeking treatment some time later, when their ability has not improved. The cut-off point of 14 days used to define an ‘acute’ rupture is somewhat arbitrary but in keeping with definitions used in previous research into this injury. Patients presenting at later times may have partial or complete healing of the tendon but often with tendon lengthening, which restricts their function. As the treatment of late presentation injuries is not straightforward and often requires surgical intervention, these patients were excluded from this trial of acute non-operative management.
The second common reason why patients were ineligible (n = 120) was that they chose to have surgery. This number is perhaps smaller than had been anticipated when UKSTAR was designed and, to some degree, accounts for the faster than expected rate of recruitment. However, it is in keeping with the worldwide trend towards non-operative treatment of acute rupture of the Achilles tendon, as per the recent evidence base, which suggests little functional advantage of surgery. 8,9
Only 46 patients were excluded because they were unable to adhere to trial procedures or complete questionnaires, most commonly because they could not read written English, which was used in the follow-up questionnaires. Thirty-seven patients were excluded because they had suffered a previous Achilles tendon injury, which was likely to have affected their baseline, pre-injury function. Achilles tendon rupture is very rare in children and hence it is not surprising that only three patients were excluded because they were aged < 16 years. The remaining 14 patients were excluded by recruitment centres under the heading ‘other’; we did not record details of these individual cases.
Of the 1076 potentially eligible patients screened across the 39 recruitment centres, 540 consented to enter the trial. Ninety-nine patients were not approached about the trial because no research associate was available to discuss the trial (n = 97), usually because the patient had presented at the weekend or because no functional brace was available at the time of presentation (n = 2). A further 50 patients were not offered the opportunity to take part in the trial because of a clinician decision. In some cases, specific reasons were given for this decision, for example ‘active treatment of a local skin lesions precluding the use of a cast’, but in other cases the clinician did not provide a reason. Therefore, a total of 149 potentially eligible patients were never offered the opportunity to take part in the trial. This reduced the number of participants but is unlikely to have caused selection bias.
Of the 927 patients who had the opportunity to take part, 385 declined. Patients may decline to take part in a trial for various reasons. Those who do not want to be part of any research – often because of the perception, or indeed the reality, of filling out extra, onerous questionnaires – are unlikely to adversely affect the trial in terms of the difference between treatment groups. However, those who decline because they have a preference for one treatment over another do create selection bias. In this trial, it is reassuring that 540 out of the 927 patients who were offered the opportunity to take part in the trial (58%) agreed to participate.
Participants and interventions
The two groups of participants were well balanced in terms of baseline pre-injury characteristics.
In keeping with the epidemiology of Achilles tendon rupture, more men were aged 30–40 years, whereas women were a little older at the time of their injury, with the age range of 40–60 years being most common. Sports accounted for the great majority of ruptures (70.4%) and relatively few patients declared predisposing risk factors; only 4.3% of participants had diabetes mellitus and 3.9% were taking steroids, these being associated with an increased risk of tendon rupture. Only 3.7% declared a pre-existing Achilles tendinopathy, despite the fact that histological studies indicate that degenerative changes are almost always present on biopsies taken from tendons immediately after an injury. 4,5
The median pre-injury ATRS score was 100 in both groups, indicating normal Achilles function. Similarly, the median EQ-5D utility score was 1, indicting perfect health. This, and the fact that the large majority of participants were employed or self-employed, suggests that most Achilles ruptures affect working-age people with good pre-injury health. In studies of acute injury, it is necessary to collect functional and QoL data by recall, in this case as part of the baseline post-injury clinical reporting form. Although some recall bias/response shift is inevitable, there is no alternative in this sort of trial. In clinical areas in which pre-injury QoL is much more variable than for patients with Achilles tendon rupture, we have demonstrated that recall estimates of QoL still agree with age- and sex-matched controls. 50 However, the characteristics of the participants reflect that Achilles rupture affects all age groups, with both men and women in their 80s represented in the trial. Overall, the participants in the trial are representative in terms of demographics of previously reported patients with this injury.
We anticipated some crossover between the treatment groups following the random allocation, but in fact this was relatively uncommon. Only one participant decided to have a cast after having been allocated a functional brace. Thirteen participants decided to change to a functional brace after having been allocated a cast, which may reflect the perception that the brace made mobilisation easier. However, the numbers were small and we did not formally investigate the qualitative aspects of the decision to change treatment. Two further participants withdrew from the trial immediately after randomisation and seven others crossed over treatment groups for what were described as clinical or unknown reasons. Given the small number of crossovers at baseline, these are very unlikely to have influenced the results of the trial.
In terms of compliance with treatment once implemented, the trial protocol stipulated that patients would be deemed compliant if they maintained their allocated treatment for a minimum of 6 weeks. The choice of 6 weeks reflects the fact that this was the time at which weight-bearing would usually be permitted for those patients in a cast, those in a functional brace generally being fully weight-bearing from the outset. Overall, 88% of participants were fully compliant. However, compliance was higher in the functional brace group (97%) than in the plaster cast group (80%). This may reflect participants’ desire to have the cast removed as soon as possible (most of these participants used a functional brace for a further 2 weeks or longer), but we did not interview participants about the reasons why they changed treatment after 6 weeks.
Some patients did, of course, change treatment before 6 weeks, having initially accepted their allocated intervention. This was more common in the plaster cast group; 11.3% changed to a functional brace, compared with the 0.4% allocated a functional brace who changed to a plaster cast. This may also suggest that functional brace was preferred but, although we asked these participants if they or the clinician treating them chose or recommended changing treatment, we were not able to formally explore the reasons behind decisions to change treatment. An additional 3% of participants chose to have surgery before 6 weeks (1.9% in the plaster cast group and 1.1% in the functional brace group). In some cases, these participants described another fall or injury to their tendon. However, we have not reported these as ‘re-ruptures’ of the tendon on the basis that the tendon was unlikely to have healed before 6 weeks.
One other notable element of participants’ treatment beyond the treatment allocation was the use of VTE prophylaxis. Patients with Achilles tendon rupture are at increased risk of VTE, as the injury defunctions the triceps surae muscles, which are an important part of the calf muscle pump that helps to return venous blood to the heart. In the plaster cast group, 70% of patients had VTE prophylaxis, most commonly self-administered low-molecular-weight heparin injections. Fewer patients (58%) had VTE prophylaxis in the functional brace group. This difference may reflect the belief that patients who are able to fully weight bear in a functional brace are at lower risk of VTE than those with restricted weight-bearing in a cast, but this trial was not designed to address questions related to the management of VTE.
Results
In total, 93.3% of participants completed the primary outcome measure 9 months after their Achilles tendon rupture: 91.7% in the plaster cast group and 94.9% in the functional brace group. Therefore, loss to follow-up was considerably lower than the 20% accounted for in the trial design, which, alongside the fact that the trial was able to recruit more patients than the minimum of 330 required by the sample size calculation, ensures that the trial had considerably more than 90% power.
Follow-up was also good at other time points, with 88%, 88% and 86% of participants completing questionnaires at 8 weeks, 3 months and 6 months, respectively.
Primary outcome
The adjusted ITT analysis showed no statistically significant difference in ATRS between the two treatment groups at the primary end point of 9 months post injury (–1.38, 95% CI –4.9 to 2.1).
There was a statistically significant difference in ATRS at 8 weeks in favour of the functional brace group (5.53, 95% CI 2.0 to 9.1), although this is of borderline clinical importance. However, any benefit of functional brace was not evident later in the participants’ recovery, with very similar ATRS scores at 3 and 6 months, as well as at 9 months.
As expected, given the relatively small number of patients who were non-compliant with treatment, the secondary sensitivity analysis, using adjusted CACE, showed the same pattern. There was no evidence of a difference at 9 months post injury (–1.17, 95% CI –4.5 to 2.1), nor was there any evidence of a difference on the other prespecified analysis of overall ATRS scores (AUC) over the full period of follow-up.
Mean ATRS was imputed pro rata when no more than 50% of the items were missing (at least five questions answered). Although this method may underestimate the variance, the number of ATRS items imputed was small (see Chapter 2, Descriptive analysis).
Secondary outcomes
The analysis of patient-reported HRQoL (EQ-5D utility score) provides powerful corroborating evidence in support of the findings using ATRS. There was a statistically significant and clinically relevant difference in favour of functional brace at 8 weeks (0.069, 95% CI 0.03 to 0.1). A breakdown of the EQ-5D by domain of health showed that this difference at 8 weeks lies in ‘self-care’ ability and ‘usual activities’. This difference in EQ-5D utility scores was of borderline statistical significance (0.035, 95% CI 0 to 0.07) at 3 months, but there was no evidence of a difference at any subsequent time point. There was no evidence of a difference in EQ-5D VAS scores.
The trial was designed to compare patient-centred outcomes between participants randomly allocated to a plaster cast and those allocated to a functional brace. However, the safety profile of the functional brace was another important consideration. Specifically, if the risk of re-rupture of the tendon was higher in those patients allowed to fully weight-bear in a functional brace, this would influence decision-making in this area, even when PROs were similar. Interestingly, the risk of re-rupture was generally lower than that reported in the literature, with a total of 17 (6.4%) cases in the plaster cast group and 13 (4.7%) cases in the functional brace group. None of the re-ruptures occurred > 6 months after the injury.
There was no evidence of an association between the treatment group and any other type of complication, with the exception of non-injurious falls, which were more common in the plaster cast group (p = 0.015).
Health economics evaluation
The mean direct intervention costs were £36 for the plaster cast group and £109 for the functional brace group. The higher upfront cost of the functional brace (mean difference £73) was statistically significant. However, by 8 weeks this difference had reversed, such that the mean total NHS and PSS costs were significantly lower in the functional brace group. The difference was driven mostly by the greater number of outpatient appointments required in the plaster cast group.
This is an important finding, as it will reassure the finance teams in trauma and orthopaedic departments that, despite the extra initial cost of a functional brace, they will reduce their overall costs when treating patients with an Achilles tendon rupture.
The mean total NHS and PSS costs during the entire follow-up period were £1183 for the plaster cast group and £1018 for the functional brace group. Although functional brace was marginally cheaper, the mean between-group cost difference of £164 was not statistically significant.
In terms of HRQoL, the mean QALY value was, on average, marginally higher for the functional brace group among complete cases and in the sensitivity analyses, although this mean QALY difference was not statistically significant.
Therefore, as the functional brace group incurred slightly lower costs and achieved slightly better QoL over the course of the study, in health economic terms, functional brace is the dominant intervention.
In summary, the health economic evaluation indicates that functional brace is very likely to be cost-effective.
Limitations
A concern at the start of the study was that patients would not be willing to take part in a trial comparing two interventions that needed to be worn for a prolonged period of time. Some of the 385 patients who declined did so because they did not want to be part of a research project. These patients, although undoubtedly affecting the external validity of the trial, are unlikely to create selection bias when comparing the two interventions. By contrast, those who declined because they had a preference for one treatment over another do create selection bias. However, in total, 540 of the 927 patients who were offered the opportunity to take part in the trial (58%) agreed to participate, so we can be confident that the participants in the trial are broadly representative of the population of patients having non-operative treatment for acute rupture of the Achilles tendon.
A further anticipated limitation was crossover from the allocated treatment and, indeed, 14 patients did not receive their allocated intervention after being randomised. There were also some cases of incomplete compliance with treatment. The ability to bear weight immediately using a functional brace may have triggered a desire to change treatment, given that the majority changed from the plaster cast group to the functional brace group. However, the overall number is small for a trial of this size and the CACE analysis, that is the analysis adjusted for incomplete compliance, confirmed the result of the primary analysis (i.e. there was no evidence of a difference between the two groups of participants at 9 months post injury).
Loss to follow-up is another potential limitation. However, > 93% of participants provided primary outcome data at 9 months, which is considerably higher than the 80% assumed in the trial design. Therefore, given that the trial also exceeded the minimum sample size by some margin, we can be confident that the conclusions are robust and the risk of type II error is very low.
Chapter 6 Conclusions
This trial provides strong evidence that early weight-bearing in a functional brace provides similar outcomes to traditional plaster casting and is safe for patients having non-operative treatment of an Achilles tendon rupture. The probability that functional bracing is cost-effective exceeds 95% for the base-case imputed analysis, assuming a cost-effectiveness threshold of £20,000 per QALY. On average, functional brace is associated with lower costs (–£103, 95% CI –£290 to £84) and more QALYs (0.015, 95% CI –0.0013 to 0.030) than plaster cast.
Although UKSTAR provides guidance on the early management of patients, rehabilitation following an Achilles tendon rupture is prolonged and further research is required to define the optimal mode of rehabilitation after the initial cast or brace is removed.
Acknowledgements
UKSTAR study team
Chief investigator: Matthew Costa.
Co-investigators: Matthew Costa, Juul Achten, Rebecca Kearney, Sarah Lamb, Nick Parsons, Stavros Petrou, Ben Ollivere, Malvenia Richmond and Richard Grant.
Study lead: Juul Achten.
Trial managers: Susan Wagland and Anna Liew.
Senior trial managers: Juul Achten and Damian Haywood.
Study co-ordinators and administrators: Catherine Thompsett, Ramona Barbu, Kylea Draper, Hugo Strachwitz, Hasina Mangal and Alice Brealy.
Health economists: Stavros Petrou and Mandy Maredza.
Study statisticians: Michael Maia Schlüssel, Ioana Marian and Susan Dutton.
Recruitment centres
| NHS trust | Principal investigator |
|---|---|
| King’s College Hospital NHS Foundation Trust | Ines Reichert |
| Nottingham University Hospitals NHS Foundation Trust | Ben Ollivere |
| Royal Berkshire NHS Foundation Trust | Andrew McAndrew |
| NHS Grampian – Aberdeen Royal Infirmary | Alan Johnstone |
| NHS Tayside – Dundee | Fraser Harrold |
| NHS Greater Glasgow and Clyde – Glasgow Royal Infirmary | Jane Madeley |
| United Lincolnshire Hospitals NHS Trust | Harish Kurup |
| North Tees and Hartlepool Hospitals NHS Foundation Trust – University Hospital of North Tees | Rajesh Nanda |
| Airedale NHS Foundation Trust | Avijeet Ghosh |
| Salisbury NHS Foundation Trust | Sridhar Sampalli |
| Rotherham NHS Foundation Trust | Sandeep Kapoor |
| George Eliot Hospital NHS Trust | Asterios Dramis |
| James Paget University Hospitals NHS Foundation Trust | Nitin Modi |
| University Hospital Southampton NHS Foundation Trust | Nicholas Hancock |
| East and North Hertsfordshire NHS Trust | Rupe Deol |
| Royal Cornwall Hospitals NHS Trust | Richard Walter |
| Maidstone and Tunbridge Wells NHS Trust | Justin Forder |
| Cambridge University Hospitals NHS Foundation Trust | Peter Hull |
| University Hospitals Plymouth NHS Trust | Mark Westwood |
| Hull University Teaching Hospitals NHS Trust | Viren Mishra |
| Luton and Dunstable University Hospital NHS Foundation Trust | Simon Burtt |
| Salford Royal NHS Foundation Trust | Victoria Lyle |
| Northern Lincolnshire and Goole NHS Foundation Trust | Nikos Reissis |
| The Mid Yorkshire Hospitals NHS Trust | Jason Eyre |
| Leeds Teaching Hospitals NHS Trust | Paul Harwood |
| Worcestershire Acute Hospitals NHS Trust | Abhijit Guha |
| Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust | Sanjeev Madan |
| Epsom and St Helier University Hospitals NHS Trust | Andrea Sott |
| Imperial College Healthcare NHS Trust | Rajarshi Bhattacharya |
| NHS Highland – Raigmore Hospital | James Beastall |
| St Helens and Knowsley Hospitals NHS Trust | Jordi Ballester |
| Milton Keynes University Hospital NHS Foundation Trust | Atif Malik |
| South Warwickshire NHS Foundation Trust | Sameh El-Kawy |
| Burton Hospitals NHS Foundation Trust | Babis Karagkevrekis |
| Wye Valley NHS Trust | Amr Abdallah |
| University Hospitals Birmingham NHS Foundation Trust | Ansar Mahmood |
| Oxford University Hospitals NHS Foundation Trust | Mark Deakin |
| University Hospital of South Manchester NHS Foundation Trust | Moez Ballal, Nasser Kurdy |
| Taunton and Somerset NHS Foundation Trust | Andrew Kelly |
Study Steering Committees
The TSC comprised:
-
Independent members: Mr Paul Baker (Orthopaedic Surgeon), chairperson; Mrs Sarah Webb (Patient Representative); Dr Dylan Morrissey (Consultant Physiotherapist); and Dr Anne-Marie Hutchison (Consultant Physiotherapist).
-
Non-independent member: Professor Matthew Costa (Chief Investigator).
The DSMC comprised:
-
Independent members: Professor Lee Shepstone (Statistician), chairperson; Professor Simon Donell (Orthopaedic Surgeon); and Dr Jean Craig (Reseach Advisor).
-
Non-independent members: Ioana Marian (Trial Statistician) and Susan Dutton (OCTRU lead statistician).
Contributions of authors
Matthew L Costa (https://orcid.org/0000-0003-3644-1388) (Professor of Trauma Surgery, Oxford Trauma) was chief investigator. He led the funding application, study conception and design, development of interventions, provided overall study supervision, and wrote and reviewed the report.
Juul Achten (https://orcid.org/0000-0002-8857-5743) (Research Manager, Oxford Trauma) prepared the funding application, provided overall study supervision, developed the study, developed the intervention, designed the study, and wrote and reviewed the report.
Susan Wagland (https://orcid.org/0000-0002-5566-0925) (Clinical Trials Manager, Oxford Trauma) managed and supervised the study, and wrote and reviewed the report.
Ioana R Marian (https://orcid.org/0000-0002-0692-8112) (Medical Statistician) was trial statistician, conducted the statistical analysis and wrote the report.
Mandy Maredza (https://orcid.org/0000-0002-2030-3338) (Research Fellow, Health Economics) was trial health economist. She designed the study, conducted the health economics analysis and wrote the report.
Michael Maia Schlüssel (https://orcid.org/0000-0002-1711-9310) (Medical Statistician) was trial statistician. He conducted the statistical analysis and wrote the report.
Anna S Liew (https://orcid.org/0000-0002-6568-1631) (Clinical Trials Manager, Oxford Trauma) managed and supervised the study, developed the study, developed the intervention, designed the study and reviewed the report.
Nick R Parsons (https://orcid.org/0000-0001-9975-888X) (Associate Professor of Medical Statistics) developed the study, developed the intervention, designed the study and reviewed the report.
Susan J Dutton (https://orcid.org/0000-0003-4573-5257) (OCTRU Lead Statistician) was lead statistician. She designed the study, conducted the statistical analysis, and wrote and reviewed the report.
Rebecca S Kearney (https://orcid.org/0000-0002-8010-164X) [Associate (Clinical) Professor and Associate Director of Warwick Clinical Trials Unit] developed the study, developed the intervention, designed the study and reviewed the report.
Sarah E Lamb (https://orcid.org/0000-0003-4349-7195) (Professor and Director of Centre for Statistics in Medicine) developed study conception, designed the study and reviewed the report.
Benjamin Ollivere (https://orcid.org/0000-0002-1410-1756) (Clinical Associate Professor and Honorary Consultant Orthopaedic and Major Trauma Surgeon) developed the study, developed the intervention, designed the study and reviewed the report.
Stavros Petrou (https://orcid.org/0000-0003-3121-6050) (Professor of Health Economics) oversaw the health economics analysis, and wrote and reviewed the report.
Publications
Achten J, Parsons NR, Kearney RL, Maia Schlüssel M, Liew AS, Dutton S, et al. Cast versus functional brace in the rehabilitation of patients treated non-operatively for a rupture of the Achilles tendon: protocol for the UK study of tendo achilles rehabilitation (UK STAR) multi-centre randomised trial. BMJ Open 2017;7:e019628.
Marian I, Costa ML, Dutton S. Cast versus functional brace in the rehabilitation of patients with a rupture of the Achilles tendon: statistical analysis plan for the UK study of tendo Achilles rehabilitation (UK STAR) multi-centre randomised controlled trial. Trials 2019;20:311.
Costa ML, Achten J, Marian IR, Dutton SJ, Lamb SE, Ollivere B, et al. Plaster cast versus functional brace for non-surgical treatment of Achilles tendon rupture (UKSTAR): a multicentre randomised controlled trial and economic evaluation. Lancet 2020;395:441–8.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Costa ML, MacMillan K, Halliday D, Chester R, Shepstone L, Robinson AH, et al. Randomised controlled trials of immediate weight-bearing mobilisation for rupture of the tendo Achillis. J Bone Joint Surg Br 2006;88:69-77. https://doi.org/10.1302/0301-620X.88B1.16549.
- Maffulli N, Waterston SW, Squair J, Reaper J, Douglas AS. Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clin J Sport Med 1999;9:157-60. https://doi.org/10.1097/00042752-199907000-00007.
- Kearney RS, Costa ML. Current concepts in the rehabilitation of an acute rupture of the tendo Achillis. J Bone Joint Surg Br 2012;94:28-31. https://doi.org/10.1302/0301-620X.94B1.28008.
- Graham R. Tendinopathy – from basic science to treatment. Nat Clin Pract Rheumatol 2008;4. https://doi.org/10.1038/ncprheum0700.
- Tallon C, Maffulli N, Ewen SW. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med Sci Sports Exerc 2001;33:1983-90. https://doi.org/10.1097/00005768-200112000-00002.
- Nistor L. Surgical and non-surgical treatment of Achilles tendon rupture. A prospective randomized study. J Bone Joint Surg Am 1981;63:394-9. https://doi.org/10.2106/00004623-198163030-00012.
- Khan RJ, Carey Smith RL. Surgical interventions for treating acute Achilles tendon ruptures. Cochrane Database Syst Rev 2010;9. https://doi.org/10.1002/14651858.CD003674.pub4.
- Metz R, Verleisdonk EJ, van der Heijden GJ, Clevers GJ, Hammacher ER, Verhofstad MH, et al. Acute Achilles tendon rupture: minimally invasive surgery versus nonoperative treatment with immediate full weightbearing – a randomized controlled trial. Am J Sports Med 2008;36:1688-94. https://doi.org/10.1177/0363546508319312.
- Willits K, Amendola A, Bryant D, Mohtadi NG, Giffin JR, Fowler P, et al. Operative versus nonoperative treatment of acute Achilles tendon ruptures: a multicenter randomized trial using accelerated functional rehabilitation. J Bone Joint Surg Am 2010;92:2767-75. https://doi.org/10.2106/JBJS.I.01401.
- Kearney RS, Costa ML. UK National Survey – Rehabilitation for Achilles Tendon Rupture. Coventry: University of Warwick; 2013.
- Kearney RS, Parsons N, Underwood M, Costa ML. Achilles tendon rupture rehabilitation: a mixed methods investigation of current practice among orthopaedic surgeons in the United Kingdom. Bone Joint Res 2015;4:65-9. https://doi.org/10.1302/2046-3758.44.2000400.
- Healy B, Beasley R, Weatherall M. Venous thromboembolism following prolonged cast immobilisation for injury to the tendo Achillis. J Bone Joint Surg Br 2010;92:646-50. https://doi.org/10.1302/0301-620X.92B5.23241.
- Suchak AA, Spooner C, Reid DC, Jomha NM. Postoperative rehabilitation protocols for Achilles tendon ruptures: a meta-analysis. Clin Orthop Relat Res 2006;445:216-21. https://doi.org/10.1097/01.blo.0000203458.05135.74.
- Costa ML, Kay D, Donell ST. Gait abnormalities following rupture of the tendo Achillis: a pedobarographic assessment. J Bone Joint Surg Br 2005;87:1085-8. https://doi.org/10.1302/0301-620X.87B8.16540.
- Cetti R, Henriksen LO, Jacobsen KS. A new treatment of ruptured Achilles tendons. A prospective randomized study. Clin Orthop Relat Res 1994;308:155-65. https://doi.org/10.1097/00003086-199411000-00022.
- Kangas J, Pajala A, Siira P, Hämäläinen M, Leppilahti J. Early functional treatment versus early immobilization in tension of the musculotendinous unit after Achilles rupture repair: a prospective, randomized, clinical study. J Trauma 2003;54:1171-80. https://doi.org/10.1097/01.TA.0000047945.20863.A2.
- Kerkhoffs GM, Struijs PA, Raaymakers EL, Marti RK. Functional treatment after surgical repair of acute Achilles tendon rupture: wrap vs walking cast. Arch Orthop Trauma Surg 2002;122:102-5. https://doi.org/10.1007/s004020100312.
- Maffulli N, Tallon C, Wong J, Lim KP, Bleakney R. Early weightbearing and ankle mobilization after open repair of acute midsubstance tears of the achilles tendon. Am J Sports Med 2003;31:692-700. https://doi.org/10.1177/03635465030310051001.
- Mortensen HM, Skov O, Jensen PE. Early motion of the ankle after operative treatment of a rupture of the Achilles tendon. A prospective, randomized clinical and radiographic study. J Bone Joint Surg Am 1999;81:983-90. https://doi.org/10.2106/00004623-199907000-00011.
- Suchak AA, Bostick GP, Beaupré LA, Durand DC, Jomha NM. The influence of early weight-bearing compared with non-weight-bearing after surgical repair of the Achilles tendon. J Bone Joint Surg Am 2008;90:1876-83. https://doi.org/10.2106/JBJS.G.01242.
- American Academy of Orthopaedic Surgeons (AAOS) . The Diagnosis and Treatment of Acute Achilles Tendon Rupture: Guideline and Evidence Report 2009.
- Saleh M, Marshall PD, Senior R, MacFarlane A. The Sheffield splint for controlled early mobilisation after rupture of the calcaneal tendon. A prospective, randomised comparison with plaster treatment. J Bone Joint Surg Br 1992;74:206-9. https://doi.org/10.1302/0301-620X.74B2.1544953.
- Petersen OF, Nielsen MB, Jensen KH, Solgaard S. Randomized comparison of CAM walker and light-weight plaster cast in the treatment of first-time Achilles tendon rupture. Ugeskr Laeg 2002;164:3852-5.
- Valkering KP, Aufwerber S, Ranuccio F, Lunini E, Edman G, Ackermann PW. Functional weight-bearing mobilization after Achilles tendon rupture enhances early healing response: a single-blinded randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2017;25:1807-16. https://doi.org/10.1007/s00167-016-4270-3.
- Barfod KW, Bencke J, Lauridsen HB, Dippmann C, Ebskov L, Troelsen A. Nonoperative, dynamic treatment of acute achilles tendon rupture: influence of early weightbearing on biomechanical properties of the plantar flexor muscle-tendon complex-a blinded, randomized, controlled trial. J Foot Ankle Surg 2015;54:220-6. https://doi.org/10.1053/j.jfas.2014.11.018.
- Korkmaz M, Erkoc MF, Yolcu S, Balbaloglu O, Oztemur Z, Karaaslan F. Weight bearing the same day versus non-weight bearing for 4 weeks in Achilles tendon rupture. J Orthop Sci 2015;20:513-16. https://doi.org/10.1007/s00776-015-0710-z.
- Young SW, Patel A, Zhu M, van Dijck S, McNair P, Bevan WP, et al. Weight-bearing in the nonoperative treatment of acute achilles tendon ruptures: a randomized controlled trial. J Bone Joint Surg Am 2014;96:1073-9. https://doi.org/10.2106/JBJS.M.00248.
- Barfod KW, Hansen MS, Hölmich P, Kristensen MT, Troelsen A. Efficacy of early controlled motion of the ankle compared with immobilisation in non-operative treatment of patients with an acute Achilles tendon rupture: an assessor-blinded, randomised controlled trial [published online ahead of print October 9 2019]. Br J Sports Med 2019. https://doi.org/10.1136/bjsports-2019-100709.
- Kearney RS, Lamb SE, Achten J, Parsons NR, Costa ML. In-shoe plantar pressures within ankle-foot orthoses: implications for the management of achilles tendon ruptures. Am J Sports Med 2011;39:2679-85. https://doi.org/10.1177/0363546511420809.
- Kearney RS, McGuinness KR, Achten J, Costa ML. A systematic review of early rehabilitation methods following a rupture of the Achilles tendon. Physiotherapy 2012;98:24-32. https://doi.org/10.1016/j.physio.2011.04.349.
- Nilsson-Helander K, Thomeé R, Silbernagel KG, Grävare-Silbernagel K, Thomeé P, Faxén E, et al. The Achilles tendon Total Rupture Score (ATRS): development and validation. Am J Sports Med 2007;35:421-6. https://doi.org/10.1177/0363546506294856.
- Kearney RS, Achten J, Lamb SE, Parsons N, Costa ML. The Achilles tendon total rupture score: a study of responsiveness, internal consistency and convergent validity on patients with acute Achilles tendon ruptures. Health Qual Life Outcomes 2012;10. https://doi.org/10.1186/1477-7525-10-24.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Kearney RS, Achten J, Parsons NR, Costa ML. The comprehensive cohort model in a pilot trial in orthopaedic trauma. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-39.
- Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res 2005;14:369-95. https://doi.org/10.1191/0962280205sm403oa.
- Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. CONSORT PRO Group . Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013;309:814-22. https://doi.org/10.1001/jama.2013.879.
- Bell ML, King MT, Fairclough DL. Bias in area under the curve for longitudinal clinical trials with missing patient reported outcome data: summary measures versus summary statistics. Sage Open 2014;4. https://doi.org/10.1177/2158244014534858.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- NHS . NHS Supply Chain 2018. https://my.supplychain.nhs.uk/catalogue (accessed 18 July 2018).
- NHS Improvement . Reference Costs 2017 18 2018.
- NHS Digital . Prescription Cost Analysis – England, 2018 2019.
- Joint Formulary Committee . British National Formulary n.d. www.evidence.nhs.uk/formulary/bnf/current (accessed 6 May 2019).
- Office for National Statistics . Employee Earnings in the UK: 2018 2018.
- NHS Improvement . NHS Reference Costs 2016–17 2018.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Leurent B, Gomes M, Faria R, Morris S, Grieve R, Carpenter JR. Sensitivity analysis for not-at-random missing data in trial-based cost-effectiveness analysis: a tutorial. PharmacoEconomics 2018;36:889-901. https://doi.org/10.1007/s40273-018-0650-5.
- DAMOCLES Study Group . A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)17965-3.
- Costa ML, Achten J, Marian IR, Dutton SJ, Lamb SE, Ollivere B, et al. Plaster cast versus functional brace for non-surgical treatment of Achilles tendon rupture (UKSTAR): a multicentre randomised controlled trial and economic evaluation. Lancet 2020;395:441-8. https://doi.org/10.1016/S0140-6736(19)32942-3.
- Parsons N, Griffin XL, Achten J, Costa ML. Outcome assessment after hip fracture: is EQ-5D the answer?. Bone Joint Res 2014;3:69-75. https://doi.org/10.1302/2046-3758.33.2000250.
- Capital Physio . Physiotherapy Prices 2019. www.capitalphysio.com/fees-and-payment/ (accessed 30 September 2019).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Turner J, O’Cathain A, Knowles E, Nichol J, Sampson F, Coleman P, et al. Evaluation of NHS 111 Pilot Sites. Sheffield: University of Sheffield; 2012.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- National Association of Care Catering . Meals on Wheels Survey 2018 2018. http://costsectorcatering.co.uk/sites/default/files/attachment/nacc_-_meals_on_wheels_report_2018.pdf (accessed 5 June 2019).
- Keene DJ, Alsousou J, Harrison P, Hulley P, Wagland S, Parsons SR, et al. Platelet rich plasma injection for acute Achilles tendon rupture: PATH-2 randomised, placebo controlled, superiority trial. BMJ 2019;367. https://doi.org/10.1136/bmj.l6132.
Appendix 1 Health economics
| Resource item | Unit cost (£)a | Unit of analysis | Source of unit cost |
|---|---|---|---|
| Outpatient care | |||
| Specialty | |||
| Orthopaedics | 121.30 | Per visit | NHS Reference Costs 2016–17 45 |
| Pathology | 114.03 | Per visit | NHS Reference Costs 2016–17 45 |
| Radiology | 47.78 | Per visit | NHS Reference Costs 2016–17 45 |
| Physiotherapy (NHS) | 48.81 | Per visit | NHS Reference Costs 2016–17 45 |
| Physiotherapy (private) | 82.00 | Per visit | Capital Physio physiotherapy prices51 |
| Emergency department | 147.80 | Per visit | NHS Reference Costs 2016–17 45 |
| Primary and community care | |||
| GP consultations in surgery | 4.00 | Per minute contact | PSSRU 201839 |
| GP home visits | 4.00 | Per minute contact | PSSRU 201839 |
| GP telephone contacts | 3.80 | Per 1 minute of a telephone consultation lasting 7.1 minutes | PSSRU 201552 |
| Practice nurse contacts | 0.53 | Per call | PSSRU 201839 |
| District nurse contacts | 0.97 | Per minute of patient-related work | PSSRU 201839 |
| Community physiotherapy contacts | 0.87 | PER minute of patient-related work | PSSRU 201839 |
| Calls to NHS Direct/111 | 8.00 | Per call | Turner et al.53 |
| Calls for an ambulance or paramedic | 7.21 | Per call | |
| Occupational therapy contacts | 0.70 | Per minute | PSSRU 201754 |
| PSS | |||
| Meals on wheels (frozen, daily) | 3.60 | Per meal | Meals On Wheels Survey 2018 55 |
| Meals on wheels (hot, daily) | 3.60 | Per meal | Meals On Wheels Survey 2018 55 |
| Laundry services | 4.52 | Per load | North Yorkshire social care (www.northyorks.gov.uk/paying-care-home; accessed 3 June 2019) |
| Social worker contacts | 48.00 | Per visit | PSSRU 201754 |
| Care worker contacts, including help at home | 0.43 | Per minute | PSSRU 201754 |
| Other | |||
| Aids and adaptations | |||
| Crutches | 5.61 | Per item | NHS Supply Chain 201840 |
| Stick | 3.98 | Per item | NHS Supply Chain 201840 |
| Zimmer frame | 37.70 | Per item | NHS Supply Chain 201840 |
| Grab rail | 5.03 | Per item | NHS Supply Chain 201840 |
| Dressing aids | 4.42 | Per item | NHS Supply Chain 201840 |
| Long-handle shoe horn | 1.78 | Per item | NHS Supply Chain 201840 |
| Productivity losses | |||
| Days off work | 90.90 | Per day | Office for National Statistics44 |
| Resource | Plaster cast group (N = 266) | Functional brace group (N = 274) | Mean difference (bootstrapped 95% CI) |
|---|---|---|---|
| 8-week follow-up | |||
| Inpatient care: hospital stay (days), mean (SE) | 0.035 (0.018) | 0.071 (0.032) | –0.036 (–0.110 to 0.028) |
| VTE prophylaxis: proportion of participants prescribed anticoagulant as VTE prophylaxis treatment (SE) | |||
| Anticoagulant treatment | 0.716 (0.028) | 0.594 (0.030) | 0.122 (0.032 to 0.200) |
| Outpatient care: number of visits, mean (SE) | |||
| Orthopaedics | 2.627 (0.107) | 1.800 (0.097) | 0.827 (0.574 to 1.119) |
| Pathology | 0.041 (0.014) | 0.068 (0.023) | –0.027 (–0.084 to 0.025) |
| Radiology | 0.150 (0.023) | 0.146 (0.029) | 0.004 (–0.070 to 0.074) |
| Physiotherapy (NHS) | 0.228 (0.042) | 0.460 (0.064) | –0.232 (–0.397 to –0.095) |
| Physiotherapy (private) | 0.091 (0.037) | 0.184 (0.160) | –0.093 (–0.575 to 0.117) |
| Emergency department (injury related) | 0.104 (0.023) | 0.096 (0.021) | 0.008 (–0.051 to 0.070) |
| Emergency department (other reasons) | 0.029 (0.012) | 0.016 (0.008) | 0.013 (–0.012 to 0.044) |
| Other | 0.111 (0.037) | 0.168 (0.045) | –0.058 (–0.162 to 0.062) |
| Community health care: number of contacts, mean (SE) | |||
| GP visits (surgery) | 0.100 (0.024) | 0.188 (0.032) | –0.088 (–0.176 to –0.012) |
| GP (home visits) | 0.008 (0.006) | 0 (0) | 0.008 (0 to 0.024) |
| GP (telephone contacts) | 0.084 (0.025) | 0.108 (0.031) | –0.024 (–0.103 to 0.049) |
| Practice nurse contacts | 0.008 (0.006) | 0.008 (0.006) | 0 (–0.015 to 0.018) |
| District nurse contacts | 0.151 (0.146) | 0 (0) | 0.151 (0 to 0.553) |
| Community physiotherapy contacts | 0.021 (0.013) | 0.040 (0.020) | –0.019 (–0.074 to 0.020) |
| Calls to NHS Direct | 0.017 (0.010) | 0.008 (0.008) | 0.009 (–0.012 to 0.039) |
| Calls for an ambulance or paramedic | 0.004 (0.004) | 0 (0) | 0.004 (0 to 0.017) |
| Occupational therapy contacts | 0.013 (0.009) | 0.008 (0.006) | 0.005 (–0.012 to 0.034) |
| Other | 0.216 (0.146) | 0.034 (0.015) | 0.183 (–0.010 to 0.580) |
| Medicines: proportion of participants prescribed each class of drug (SE) | |||
| Analgesics | 0.388 (0.055) | 0.330 (0.050) | 0.058 (–0.083 to 0.213) |
| Anti-inflammatories | 0.042 (0.013) | 0.076 (0.017) | –0.034 (–0.081 to 0.004) |
| Anticoagulant | 0.151 (0.023) | 0.112 (0.020) | 0.039 (–0.026 to 0.093) |
| Other | 0.017 (0.008) | 0.048 (0.014) | –0.031 (–0.064 to –0.001) |
| Aids and adaptations: mean count (SE) | |||
| Crutches | 1.290 (0.059) | 1.124 (0.062) | 0.166 (0.012 to 0.341) |
| Stick | 0.017 (0.010) | 0.024 (0.010) | –0.007 (–0.033 to 0.024) |
| Zimmer frame | 0.054 (0.018) | 0.028 (0.010) | 0.026 (–0.014 to 0.068) |
| Grab rail | 0.046 (0.020) | 0 (0) | 0.046 (0.013 to 0.090) |
| Dressing aids | 0.008 (0.008) | 0.008 (0.006) | 0 (–0.016 to 0.024) |
| Long-handle shoe horn | 0.004 (0.004) | 0 (0) | 0.004 (0 to 0.016) |
| Other | 0.387 (0.045) | 0.220 (0.043) | 0.166 (0.040 to 0.277) |
| PSS: number of contacts (SE) | |||
| Frozen meals on wheels | 0 | 0 | |
| Hot meals on wheels | 0 | 0 | |
| Laundry services | 0.029 (0.029) | 0 (0) | 0.029 (0 to 0.095) |
| Social worker contacts | 0 | 0 | |
| Care worker/home help | 0.668 (0.542) | 0 (0) | 0.668 (0 to 2.165) |
| Other | 0 | 0 | |
| Productivity losses: days off work, mean (SE) | 21.227 (1.682) | 20.786 (1.637) | 0.441 (–3.947 to 5.176) |
| 3-month follow-up | |||
| Inpatient care: hospital stay (days), mean (SE) | 0.009 (0.009) | 0 (0) | 0.009 (0 to 0.034) |
| Outpatient care: number of visits, mean (SE) | |||
| Orthopaedics | 0.428 (0.055) | 0.318 (0.045) | 0.110 (–0.035 to 0.256) |
| Pathology | 0.017 (0.011) | 0.024 (0.014) | –0.007 (–0.044 to 0.026) |
| Radiology | 0.057 (0.020) | 0.045 (0.017) | 0.012 (–0.038 to 0.062) |
| Physiotherapy (NHS) | 0.978 (0.070) | 0.959 (0.067) | 0.019 (–0.175 to 0.180) |
| Physiotherapy (private) | 0.271 (0.073) | 0.180 (0.045) | 0.091 (–0.069 to 0.279) |
| Emergency department (injury related) | 0.061 (0.022) | 0.033 (0.011) | 0.028 (–0.013 to 0.085) |
| Emergency department (other reasons) | 0.009 (0.006) | 0.004 (0.004) | 0.005 (–0.008 to 0.023) |
| Other | 0.057 (0.018) | 0.050 (0.024) | 0.007 (–0.057 to 0.062) |
| Community health care: number of contacts, mean (SE) | |||
| GP visits (surgery) | 0.088 (0.022) | 0.107 (0.029) | –0.019 (–0.099 to 0.057) |
| GP (home visits) | 0 (0) | (0) | |
| GP (telephone contacts) | 0.044 (0.017) | 0.029 (0.013) | 0.015 (–0.026 to 0.057) |
| Practice nurse contacts | 0.004 (0.004) | 0.008 (0.008) | –0.004 (–0.026 to 0.009) |
| District nurse contacts | 0.004 (0.004) | 0.004 (0.004) | 0 (–0.011 to 0.013) |
| Community physiotherapy contacts | 0.253 (0.065) | 0.201 (0.044) | 0.052 (–0.085 to 0.223) |
| Calls to NHS Direct | 0.004 (0.004) | 0.004 (0.004) | 0 (–0.009 to 0.013) |
| Calls for an ambulance or paramedic | 0.013 (0.010) | 0.004 (0.004) | 0.009 (–0.008 to 0.036) |
| Occupational therapy contacts | 0.022 (0.014) | 0.049 (0.027) | –0.027 (–0.096 to 0.022) |
| Other | 0.061 (0.032) | 0.021 (0.012) | 0.041 (–0.017 to 0.122) |
| Medicines: proportion of participants prescribed each class of drug (SE) | |||
| Analgesics | 0.109 (0.021) | 0.049 (0.014) | 0.060 (0.014 to 0.111) |
| Anti-inflammatories | 0.008 (0.006) | 0.008 (0.006) | 0.001 (–0.015 to 0.019) |
| Anticoagulant | 0.022 (0.010) | 0.016 (0.008) | 0.005 (–0.019 to 0.031) |
| Other | 0.017 (0.009) | 0 (0) | 0.017 (0.004 to 0.039) |
| Aids and adaptations: mean count (SE) | |||
| Crutches | 0.118 (0.030) | 0.106 (0.029) | 0.012 (–0.071 to 0.100) |
| Stick | 0.070 (0.20) | 0.033 (0.014) | 0.037 (–0.009 to 0.086) |
| Zimmer frame | 0 (0) | 0.004 (0.004) | 0.004 (–0.016 to 0) |
| Grab rail | 0.022 (0.013) | 0 (0) | 0.022 (0 to 0.055) |
| Dressing aids | 0.031 (0.020) | 0 (0) | 0.031 (0.004 to 0.083) |
| Long-handle shoe horn | 0.013 (0.008) | 0 (0) | 0.013 (0 to 0.032) |
| Other | 0.227 (0.064) | 0.155 (0.038) | 0.072 (–0.056 to 0.244) |
| PSS: number of contacts (SE) | |||
| Frozen meals on wheels | 0 (0) | 0 (0) | |
| Hot meals on wheels | 0 (0) | 0 (0) | |
| Laundry services | 0 (0) | 0.008 (0.008) | –0.008 (–0.033 to 0) |
| Social worker contacts | 0 (0) | 0 (0) | |
| Care worker/home help | 0 (0) | 0 (0) | |
| Other | 0.009 (0.009) | 0 (0) | 0.009 (0 to 0.029) |
| Productivity losses: days off work, mean (SE) | 4.511 (0.820) | 5.44 (0.880) | –0.930 (–3.342 to 1.494) |
| 6-month follow-up | |||
| Subsequent inpatient care: hospital stay (days), mean (SE) | 0 (0) | 0 (0) | |
| Outpatient care: number of visits, mean (SE) | |||
| Orthopaedics | 0.224 (0.043) | 0.289 (0.059) | –0.065 (–0.230 to 0.071) |
| Pathology | 0.018 (0.011) | 0.030 (0.015) | –0.012 (–0.048 to 0.024) |
| Radiology | 0.044 (0.015) | 0.033 (0.012) | 0.011 (–0.027 to 0.050) |
| Physiotherapy (NHS) | 1.946 (0.257) | 1.915 (0.182) | 0.031 (–0.550 to 0.674) |
| Physiotherapy (private) | 0.417 (0.103) | 0.366 (0.091) | 0.051 (–0.218 to 0.316) |
| Emergency department (injury related) | 0.013 (0.008) | 0.026 (0.010) | –0.012 (–0.039 to 0.014) |
| Emergency department (other reasons) | 0.013 (0.008) | 0.017 (0.008) | –0.004 (–0.026 to 0.018) |
| Other | 0.093 (0.031) | 0.067 (0.031) | 0.026 (–0.065 to 0.103) |
| Community health care: number of contacts, mean (SE) | |||
| GP visits (surgery) | 0.094 (0.037) | 0.060 (0.018) | 0.035 (–0.034 to 0.122) |
| GP (home visits) | 0 (0) | 0.009 (0.009) | –0.009 (–0.028 to 0) |
| GP (telephone contacts) | 0.018 (0.011) | 0.021 (0.015) | –0.003 (–0.046 to 0.031) |
| Practice nurse contacts | 0 (0) | 0.004 (0.004) | –0.004 (–0.017 to 0) |
| District nurse contacts | 0 (0) | 0 (0) | |
| Community physiotherapy contacts | 0.605 (0.187) | 0.557 (0.101) | 0.048 (–0.311 to 0.547) |
| Calls to NHS Direct | 0 (0) | 0 (0) | |
| Calls for an ambulance or paramedic | 0 (0) | 0 (0) | |
| Occupational therapy contacts | 0.067 (0.033) | 0.043 (0.023) | 0.025 (–0.054 to 0.115) |
| Other | 0.058 (0.043) | 0.106 (0.077) | –0.048 (–0.264 to 0.085) |
| Medicines: proportion of participants prescribed each class of drug (SE) | |||
| Analgesics | 0.103 (0.020) | 0.064 (0.016) | 0.040 (–0.008 to 0.090) |
| Anti-inflammatories | 0.009 (0.006) | 0.021 (0.009) | –0.012 (–0.036 to 0.009) |
| Anticoagulant | 0.004 (0.004) | 0.013 (0.007) | –0.008 (–0.025 to 0.009) |
| Other | 0.009 (0.006) | 0.008 (0.006) | 0 (–0.013 to 0.019) |
| Aids and adaptations: mean count (SE) | |||
| Crutches | 0.054 (0.021) | 0.051 (0.020) | 0.003 (–0.051 to 0.066) |
| Stick | 0.031 (0.012) | 0.030 (0.015) | 0.002 (–0.041 to 0.035) |
| Zimmer frame | 0.009 (0.009) | 0.013 (0.007) | –0.004 (–0.022) |
| Grab rail | 0.018 (0.011) | 0.008 (0.008) | 0.009 (–0.013 to 0.038) |
| Dressing aids | 0 (0) | 0 (0) | |
| Long-handle shoe horn | 0.018 (0.009) | 0.008 (0.006) | 0.009 (–0.009 to 0.032) |
| Other | 0.144 (0.047) | 0.091 (0.030) | 0.054 (–0.031 to 0.188) |
| PSS: number of contacts (SE) | |||
| Frozen meals on wheels | 0 (0) | 0 (0) | |
| Hot meals on wheels | 0 (0) | 0 (0) | |
| Laundry services | 0 (0) | 0 (0) | |
| Social worker contacts | 0 (0) | 0 (0) | |
| Care worker/home help | 0.036 (0.036) | 0 (0) | 0.036 (0 to 0.138) |
| Other | 0 (0) | 0 (0) | |
| Productivity losses: days off work, mean (SE) | 1.894 (0.743) | 4.301 (1.172) | –2.407 (–5.642 to –0.110) |
| 9-month follow-up | |||
| Subsequent inpatient care: hospital stay (days), mean (SE) | 0 (0) | 0 (0) | |
| Outpatient care: number of visits, mean (SE) | |||
| Orthopaedics | 0.090 (0.024) | 0.077 (0.030) | 0.013 (–0.060 to 0.081) |
| Pathology | 0.016 (0.008) | 0.073 (0.027) | –0.057 (–0.120 to –0.012) |
| Radiology | 0.029 (0.011) | 0.012 (0.009) | 0.017 (–0.005 to 0.047) |
| Physiotherapy (NHS) | 0.709 (0.108) | 0.857 (0.147) | –0.148 (–0.540 to 0.178) |
| Physiotherapy (private) | 0.234 (0.073) | 0.174 (0.058) | 0.060 (–0.103 to 0.260) |
| Emergency department (injury related) | 0.004 (0.004) | 0.008 (0.005) | –0.004 (–0.016 to 0.012) |
| Emergency department (other reasons) | 0.020 (0.011) | 0.030 (0.014) | –0.010 (–0.051 to 0.019) |
| Other | 0.140 (0.058) | 0.089 (0.045) | 0.051 (–0.090 to 0.206) |
| Community health care: number of contacts, mean (SE) | |||
| GP visits (surgery) | 0.058 (0.024) | 0.046 (0.017) | 0.011 (–0.046 to 0.072) |
| GP (home visits) | 0 (0) | 0 (0) | |
| GP (telephone contacts) | 0.008 (0.006) | 0.004 (0.004) | 0.004 (–0.007 to 0.021) |
| Practice nurse contacts | 0 (0) | 0.004 (0.004) | –0.004 (–0.016 to 0) |
| District nurse contacts | 0 (0) | 0 (0) | |
| Community physiotherapy contacts | 0.169 (0.052) | 0.255 (0.066) | –0.085 (–0.258 to 0.071) |
| Calls to NHS Direct | 0 (0) | 0 (0) | |
| Calls for an ambulance or paramedic | 0 (0) | 0 (0) | |
| Occupational therapy contacts | 0.074 (0.038) | 0.031 (0.017) | 0.043 (–0.033 to 0.128) |
| Other | 0.136 (0.070) | 0.131 (0.100) | 0.005 (–0.307 to 0.214) |
| Medicines: proportion of participants prescribed each class of drug (SE) | |||
| Analgesics | 0.037 (0.012) | 0.031 (0.011) | 0.006 (–0.027 to 0.037) |
| Anti-inflammatories | 0.012 (0.007) | 0 (0) | 0.012 (0 to 0.029) |
| Anticoagulant | 0.004 (0.004) | 0 (0) | 0.004 (0 to 0.016) |
| Other | 0.004 (0.004) | 0.004 (0.004) | 0 (–0.008 to 0.016) |
| Aids and adaptations: mean count (SE) | |||
| Crutches | 0 (0) | 0.008 (0.008) | –0.008 (–0.029 to 0) |
| Stick | 0.012 (0.009) | 0.004 (0.004) | 0.009 (–0.008 to 0.036) |
| Zimmer frame | 0 (0) | 0 (0) | |
| Grab rail | 0.008 (0.008) | 0 (0) | 0.008 (0 to 0.031) |
| Dressing aids | 0 (0) | 0 (0) | |
| Long-handle shoe horn | 0.004 (0.004) | 0 (0) | 0.004 (0 to 0.017) |
| Other | 0.062 (0.025) | 0.093 (0.026) | –0.031 (–0.097 to 0.046) |
| PSS: number of contacts (SE) | |||
| Frozen meals on wheels | 0.045 (0.045) | 0 (0) | 0.045 (0 to 0.182) |
| Hot meals on wheels | 0 (0) | 0 (0) | |
| Laundry services | 0.045 (0.045) | 0 (0) | 0.045 (0 to 0.182) |
| Social worker contacts | 0 (0) | 0 (0) | |
| Care worker/home help | 0.008 (0.008) | 0 (0) | 0.008 (0 to 0.037) |
| Other | 0 (0) | 0 (0) | |
| Productivity losses: days off work, mean (SE) | 0.340 (0.340) | 1.952 (0.758) | –1.613 (–3.357 to 0.019) |
FIGURE 14.
Dot plot showing distribution of total NHS and PSS costs for participants with complete data for the entire follow-up period by treatment group. Dots represent individuals; blue line with plusses represents the median; and one observation for the plaster cast group with total costs of £13,000 is not shown for figure clarity.
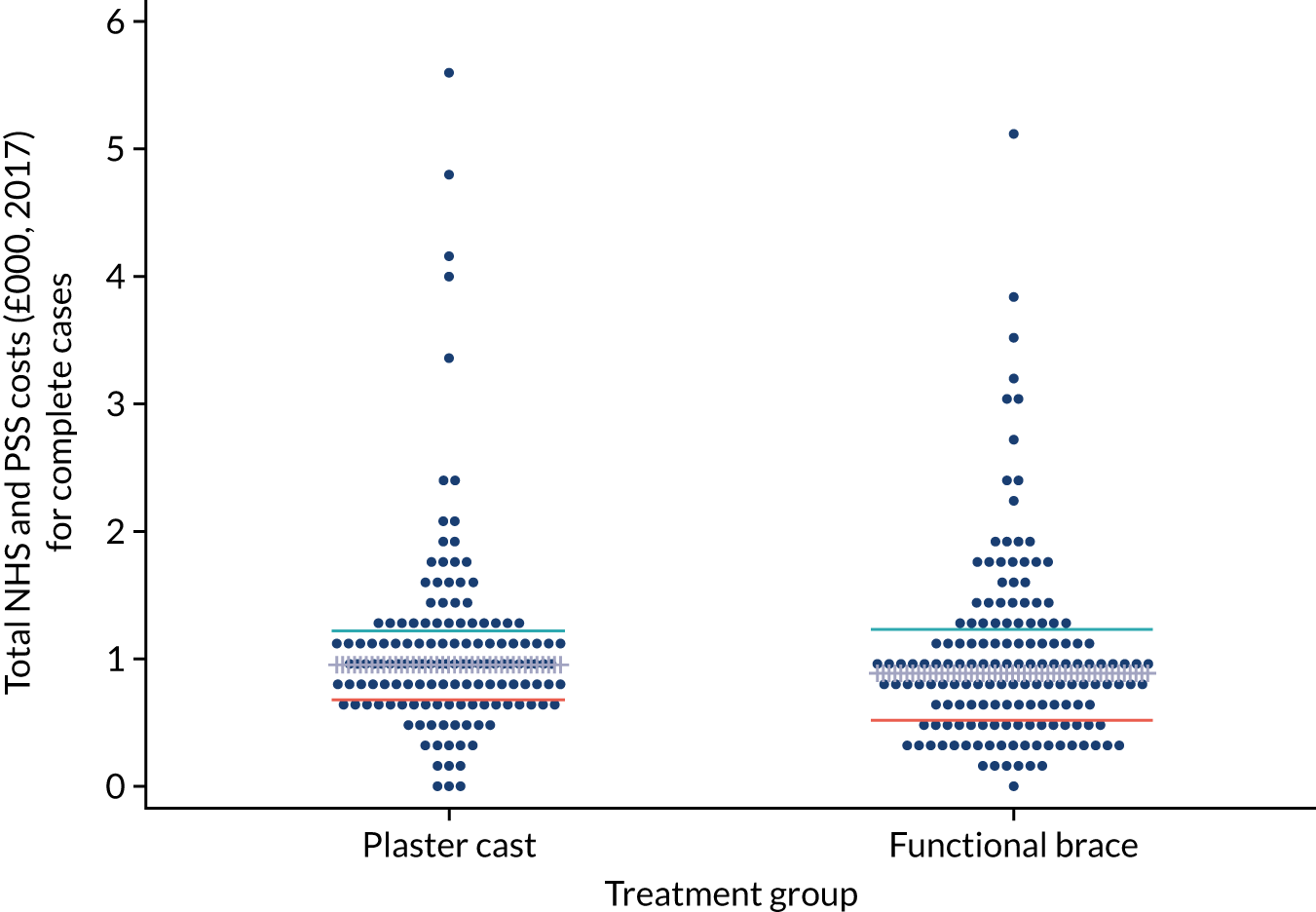
FIGURE 15.
Cost-effectiveness planes under different MNAR assumptions. (a) 1 (1,1,1,1); (b) 2 (0.9,0.9,1,1); (c) 3 (1,1,1.1,1.1); (d) 4 (0.9,0.9,1.1,1.1); (e) 5 (1,0.9,1,1); (f) 6 (0.9,1,1,1); (g) 7 (1,1,1,1.1); and (h) 8 (1,1,1.1,1). These headings indicate the scenario number and the MNAR rescaling parameters (c plaster cast, c functional brace). For example, in scenario 2 (0.9, 0.9, 1,1) imputed QoL values have been reduced by 10% in both treatment groups, but no adjustment has been made for costs, whereas in scenario 7 (1, 1,1.1,1) imputed costs increased by 10% in the plaster cast group only and no other adjustment was made for imputed QALYs in either group or on imputed costs in the functional brace group.
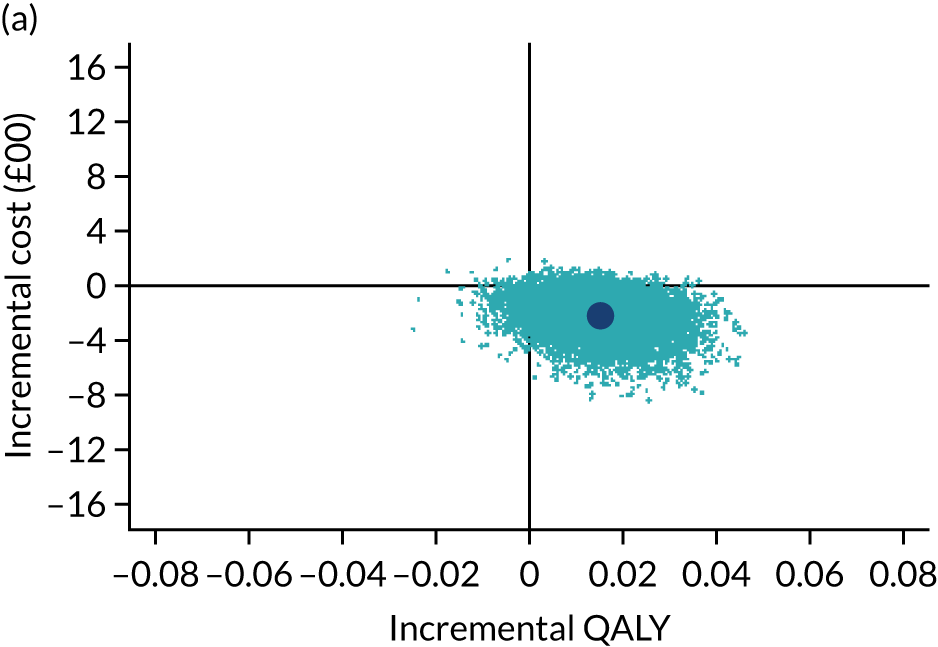
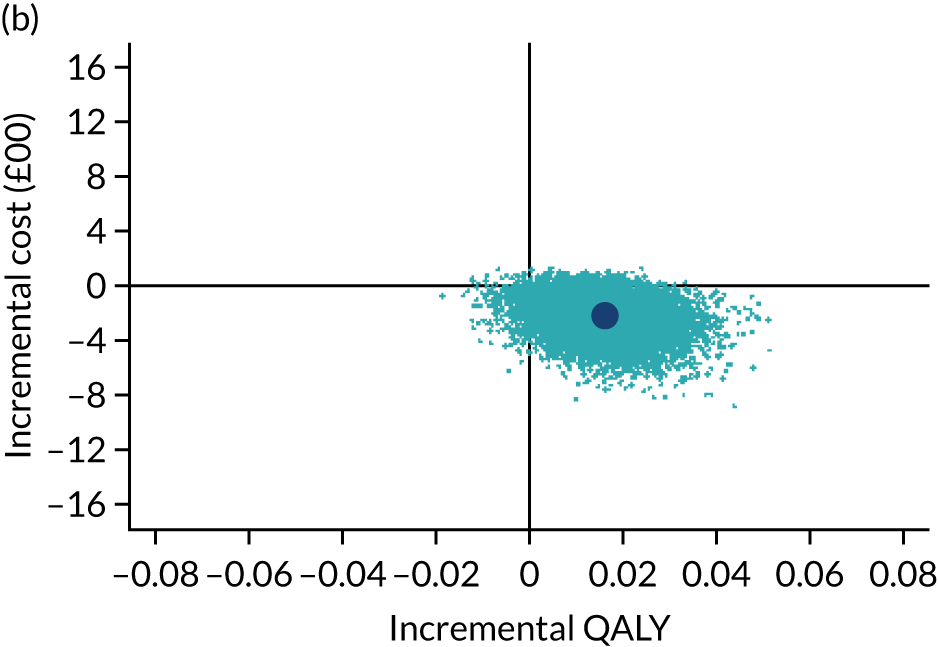
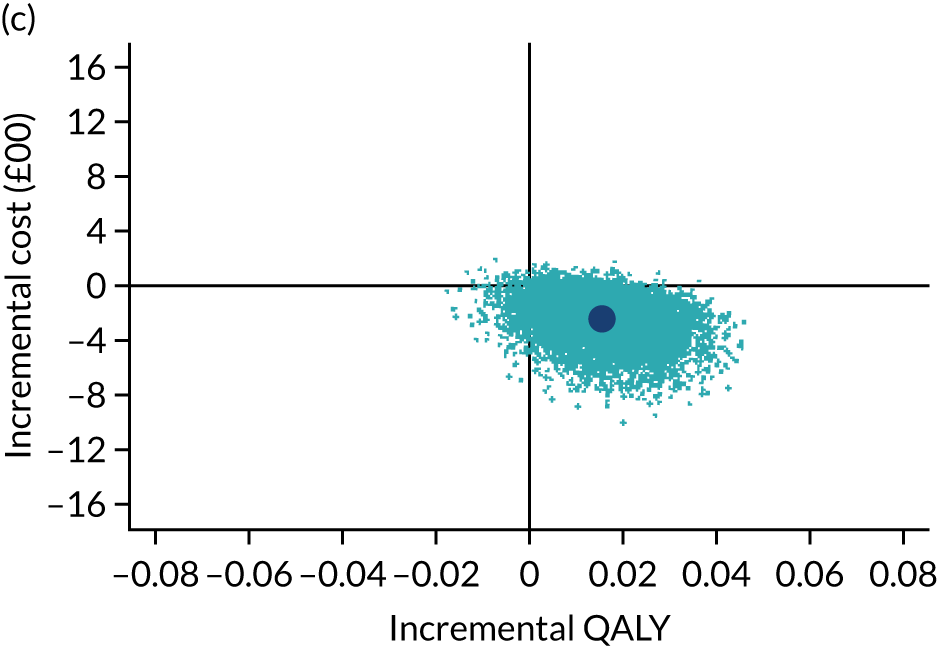
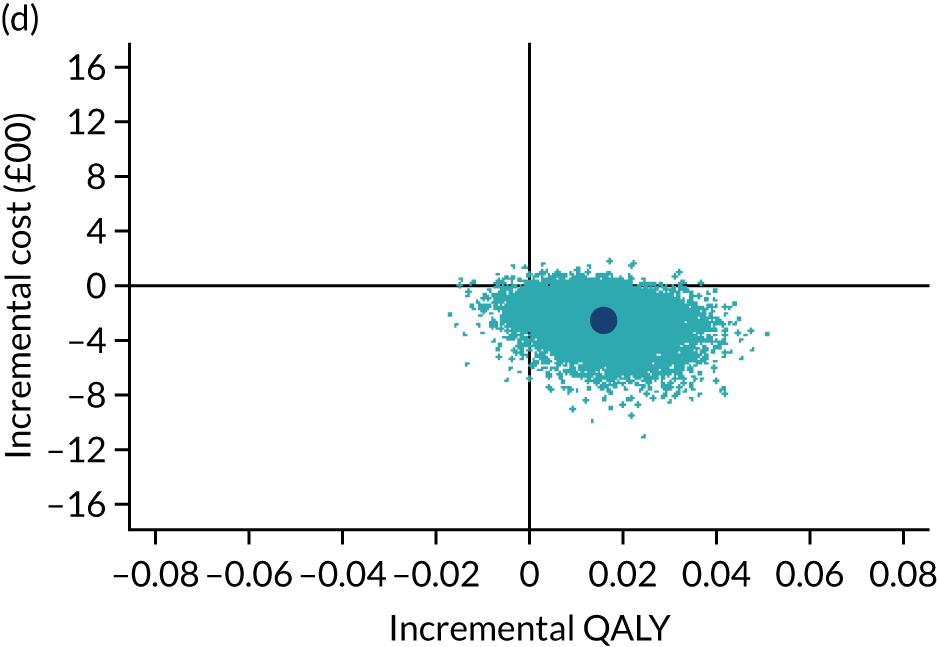
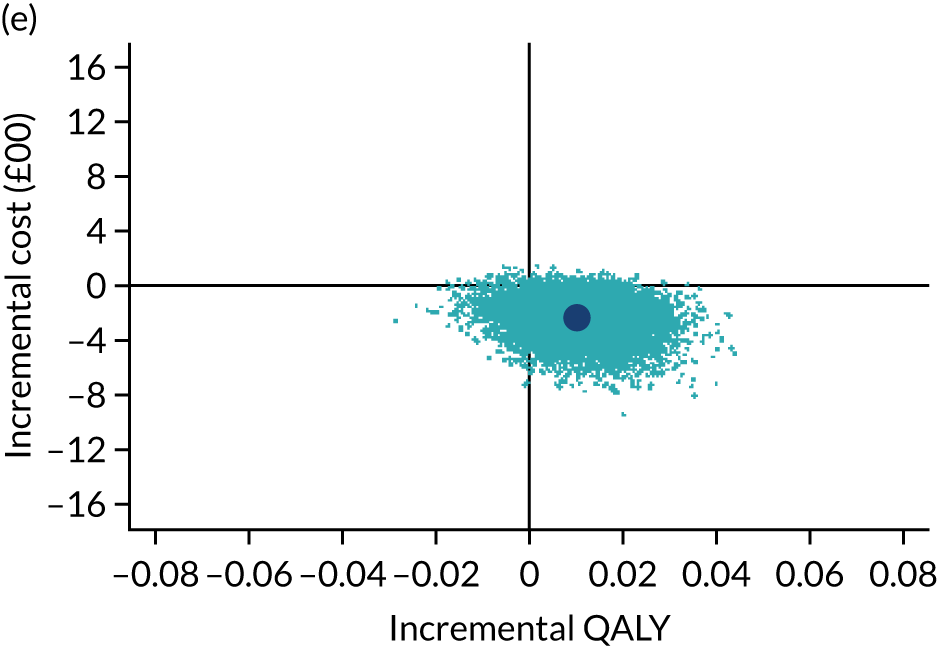
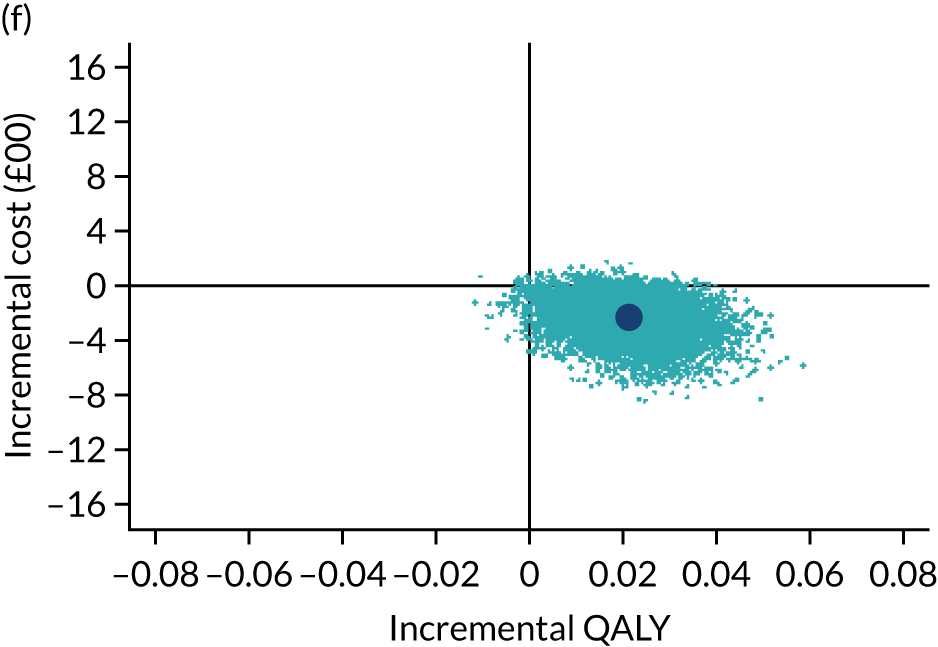
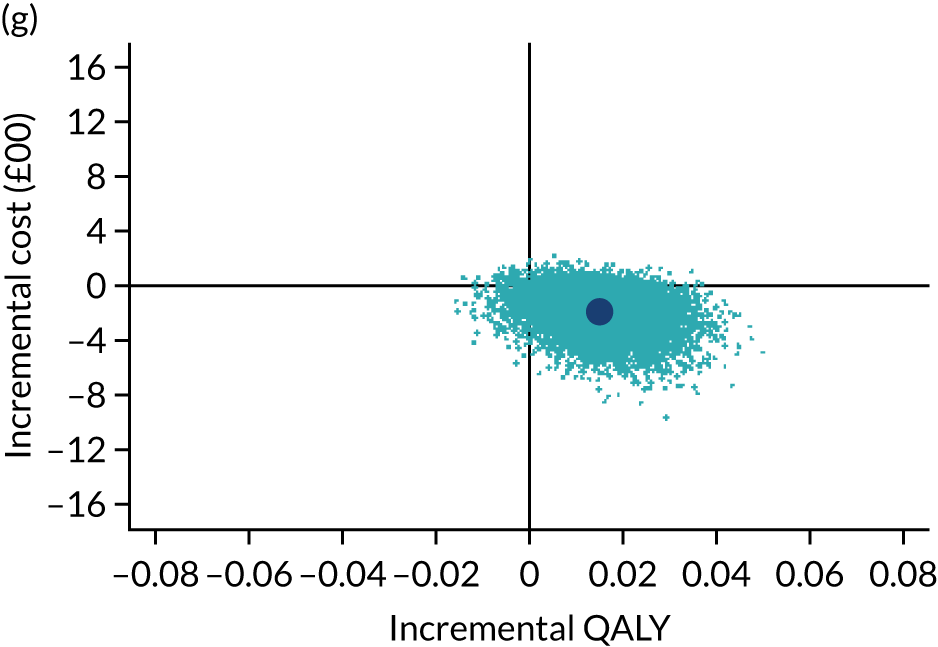
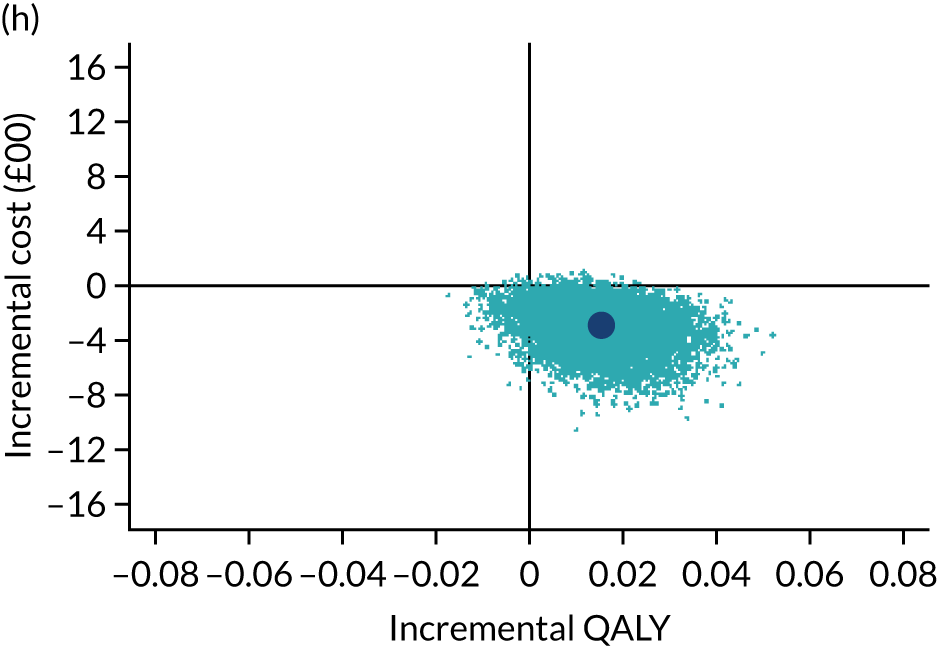
FIGURE 16.
Cost-effectiveness acceptability curves under different MNAR assumptions. Key indicates the scenario number and the MNAR rescaling parameters. Note that (0.9, 0.9): imputed QoL values have been reduced by 10% in both treatment groups, whereas (1.1, 1.1) imputed costs have been increased by 10% in both groups.
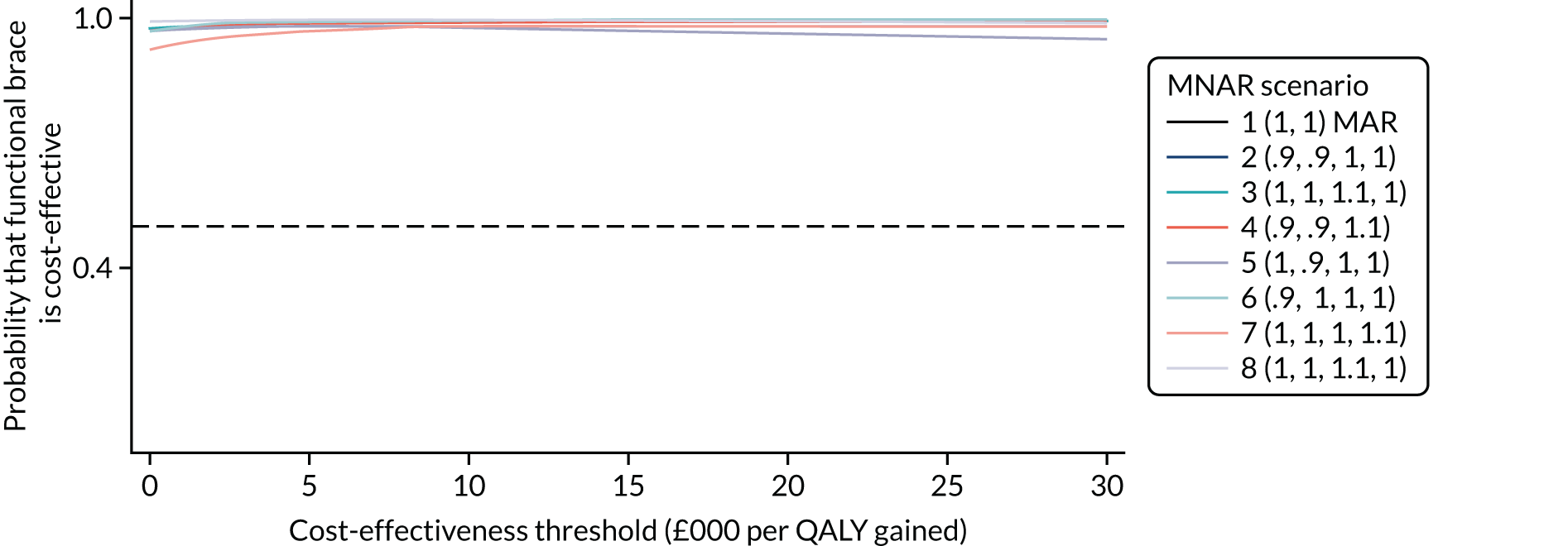
FIGURE 17.
Incremental NMB of functional brace compared with plaster cast (at £20,000/QALY) for different values of the MNAR rescaling parameter.
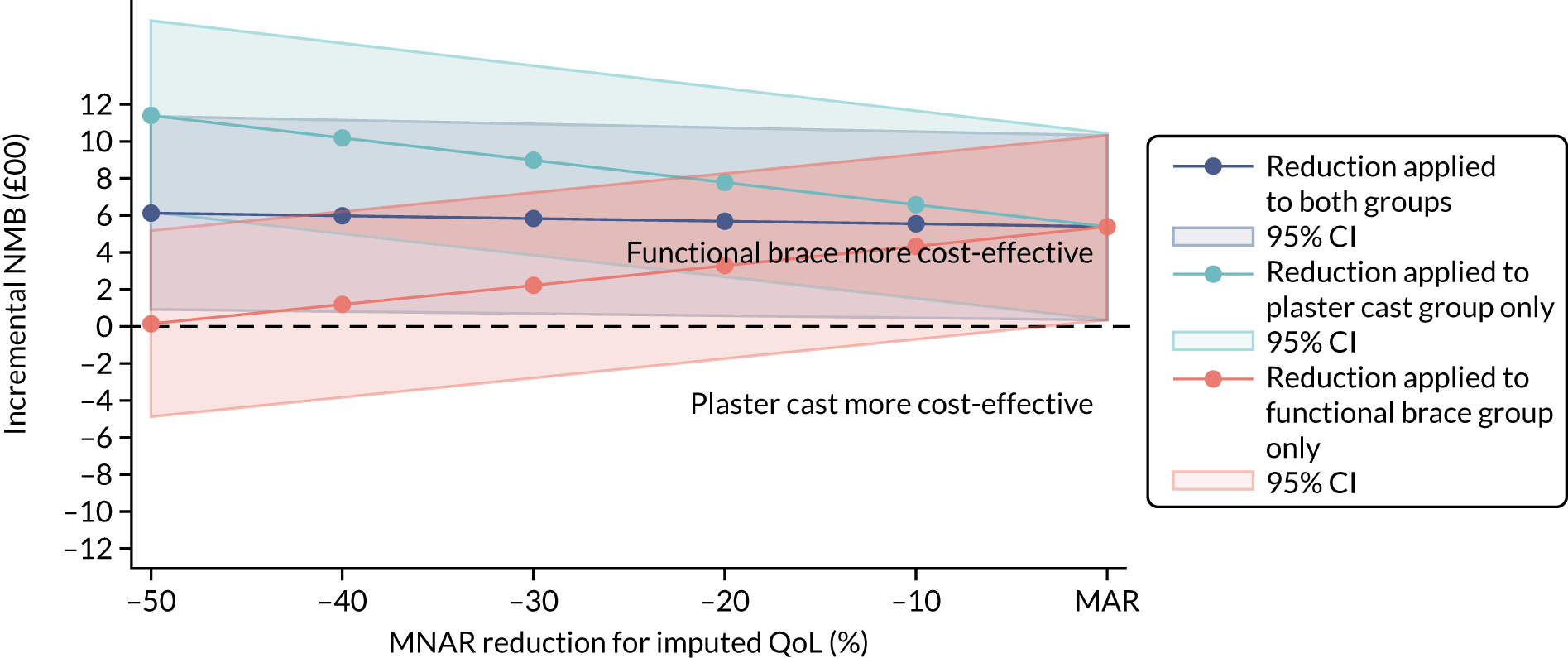
Appendix 2 Recruitment centre names by NHS trust
| Recruitment centre | Trust name |
|---|---|
| ABD | Aberdeen Royal Infirmary, NHS Grampian |
| AIR | Airedale NHS Foundation Trust |
| BRT | Queen’s Hospital, Burton Hospitals NHS Foundation Trust |
| CHX | St Mary’s Hospital, Imperial College Healthcare NHS Trust London |
| CUH | Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust |
| DBH | Doncaster Royal Infirmary, Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust |
| DUN | Ninewells Hospital and Medical School, NHS Tayside |
| ENH | Lister Hospital, East and North Hertfordshire NHS Trust |
| GEH | George Eliot Hospital NHS Trust, Nuneaton |
| GLA | Glasgow Royal Infirmary, NHS Greater Glasgow and Clyde |
| HCH | Hereford County Hospital, Wye Valley NHS Trust |
| HEY | Hull Royal Infirmary, Hull University Teaching Hospitals NHS Trust |
| INV | Raigmore Hospital, NHS Highland |
| JPN | James Paget University Hospitals NHS Foundation Trust |
| KCH | King’s College Hospital NHS Foundation Trust |
| LDH | Luton and Dunstable University Hospital NHS Foundation Trust |
| LDS | Leeds General Infirmary, Leeds Teaching Hospitals NHS Trust |
| MKN | Milton Keynes University Hospital NHS Foundation Trust |
| MPH | Musgrove Park Hospital, Taunton and Somerset NHS Foundation Trust |
| MTW | Tunbridge Wells Hospital, Maidstone and Tunbridge Wells NHS Trust |
| MYH | Pinderfields Hospital, The Mid Yorkshire Hospitals NHS Trust |
| NLG | Scunthorpe General Hospital, Northern Lincolnshire and Goole NHS Foundation Trust |
| NTE | University Hospital of North Tees, North Tees and Hartlepool Hospitals NHS Foundation Trust |
| NUH | Nottingham University Hospitals NHS Trust |
| OUH | John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust |
| PLY | Derriford Hospital, University Hospitals Plymouth NHS Trust |
| QEH | Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust |
| RBK | Royal Berkshire Hospital, Royal Berkshire NHS Foundation Trust |
| RCH | Royal Cornwall Hospital, Royal Cornwall Hospitals NHS Trust |
| RED | Worcestershire Royal Hospital, Worcester Acute Hospitals NHS Trust |
| RTH | The Rotherham NHS Foundation Trust |
| SAL | Salisbury District Hospital, Salisbury NHS Foundation Trust |
| SHC | St Helier Hospital, Epsom and St Helier University Hospitals NHS Trust |
| SLF | Salford Royal NHS Foundation Trust |
| UHS | Southampton General Hospital, University Hospital Southampton NHS Foundation Trust |
| ULH | Pilgrim Hospital, United Lincolnshire Hospitals NHS Trust |
| WAR | Warwick Hospital, South Warwickshire NHS Foundation Trust |
| WHI | Whiston Hospital, St Helens and Knowsley Hospitals NHS Trust |
| WYT | University Hospital of South Manchester NHS Foundation Trust |
Appendix 3 EuroQol-5 Dimensions individual-level items
In Figures 18–22, the median score is marked as a triangle, whiskers are IQRs, and the individual dots and crosses mark the outliers. Numbers and percentages for each EQ-5D-5L dimension and level are further presented by treatment group and time point in Appendix 4.
FIGURE 18.
EuroQol-5 Dimensions: mobility from baseline pre injury to 9 months. EQ-5D mobility values range from 1 to 5, with 1 indicating ‘no problems’.
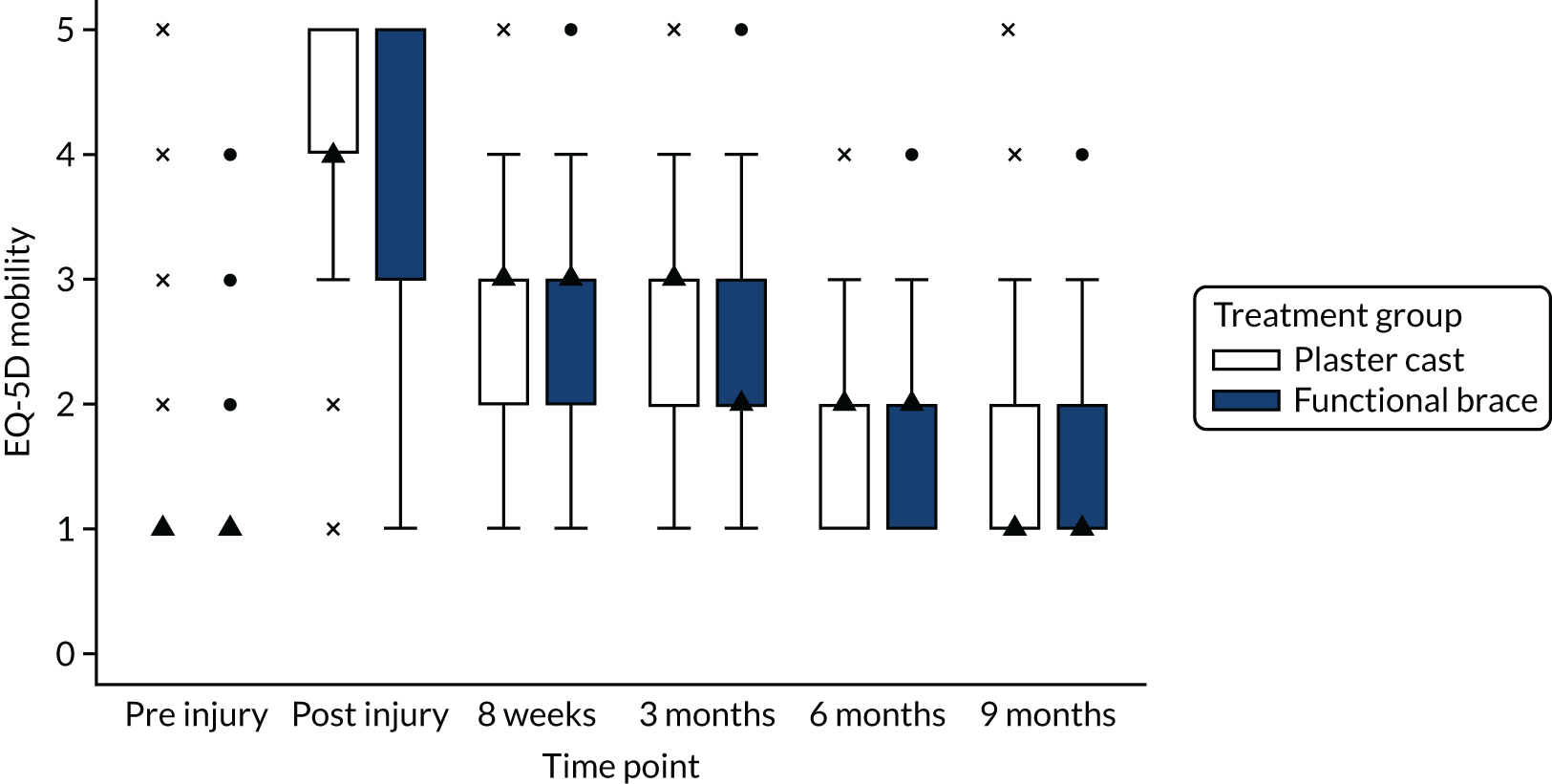
FIGURE 19.
EuroQol-5 Dimensions: self-care from baseline pre injury to 9 months. EQ-5D self-care values range from 1 to 5, with 1 indicating ‘no problems’.
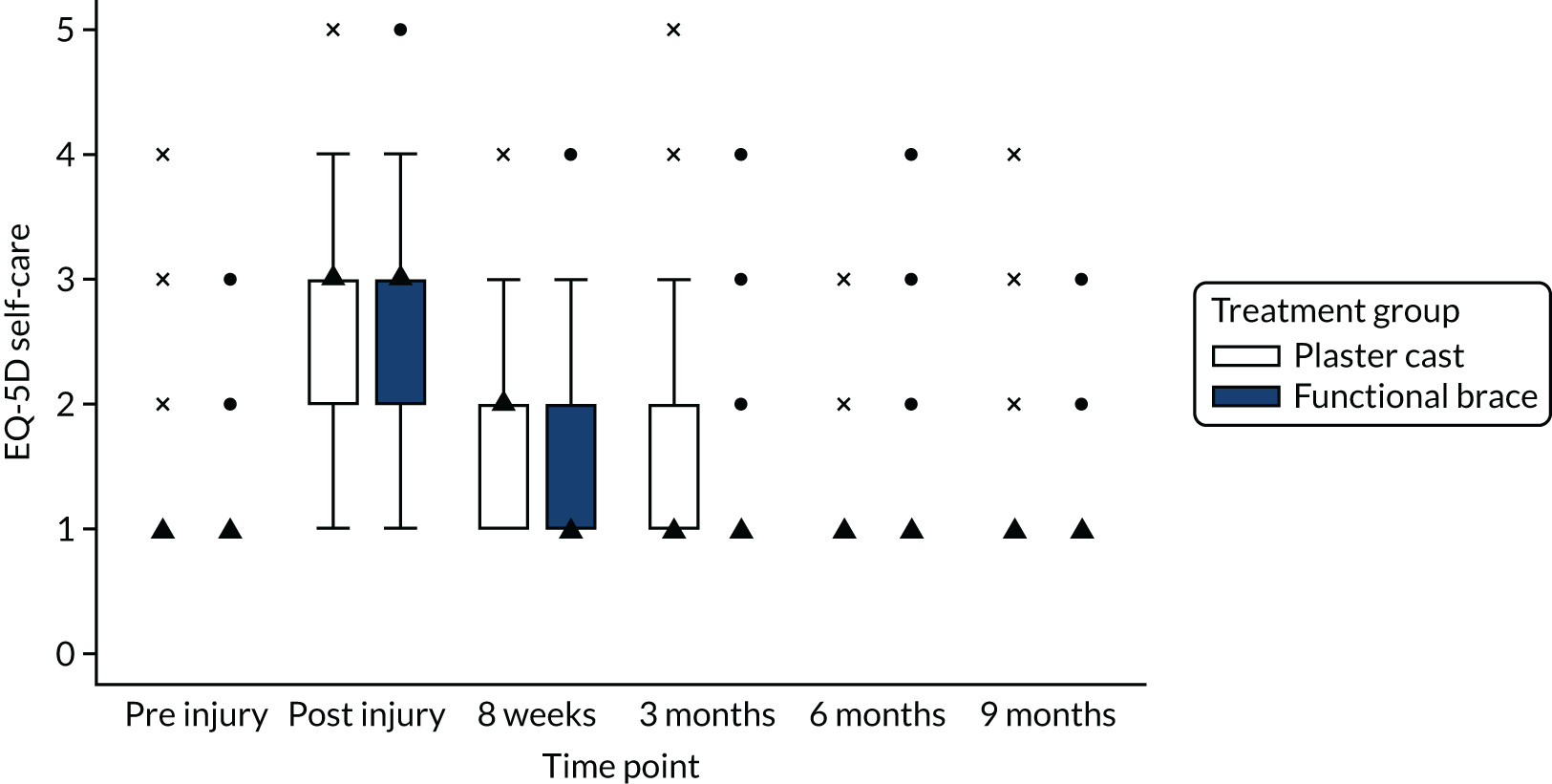
FIGURE 20.
EuroQol-5 Dimensions: usual activities from baseline pre injury to 9 months. EQ-5D usual activities values range from 1 to 5, with 1 indicating ‘no problems’.
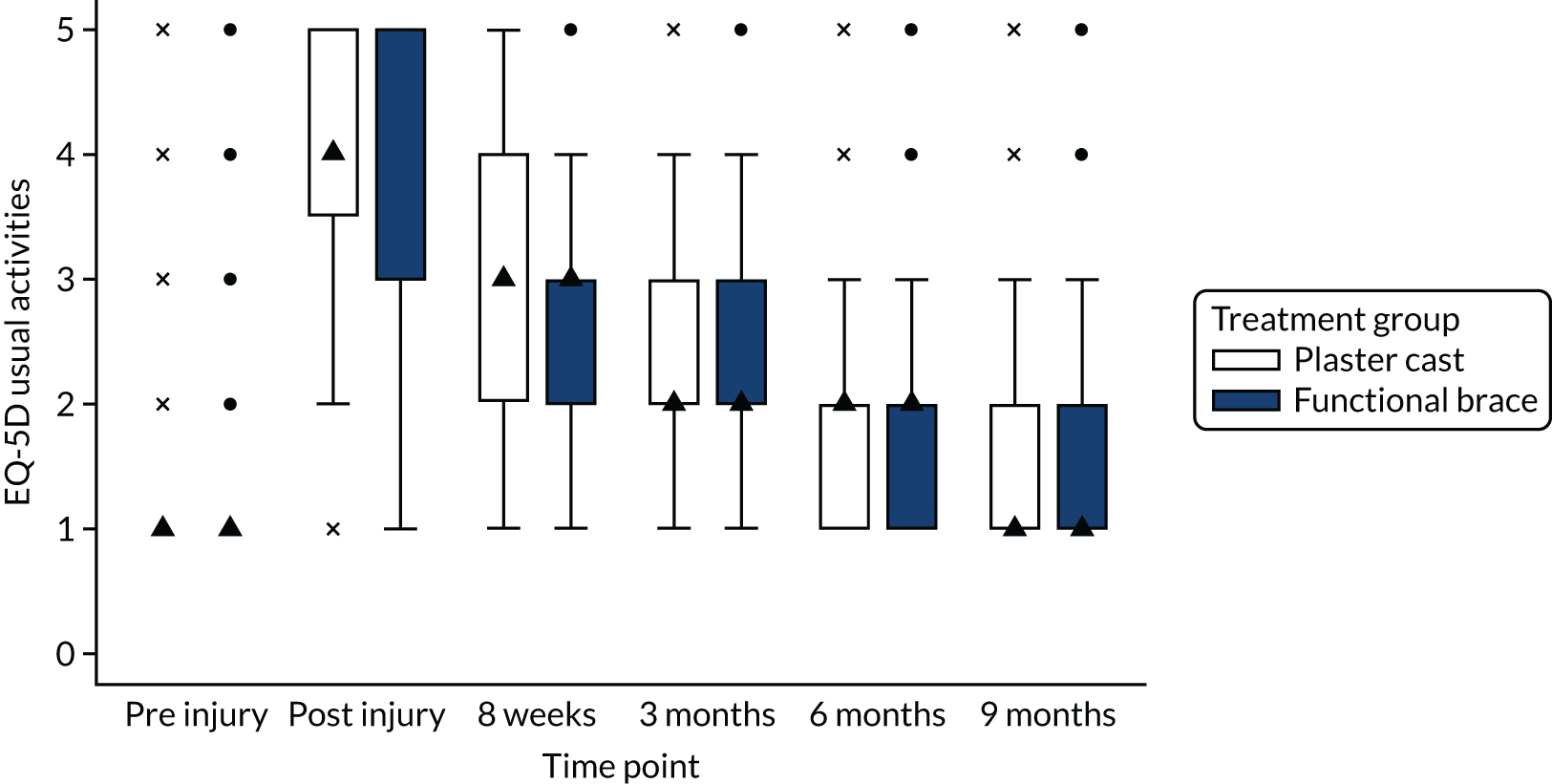
FIGURE 21.
EuroQol-5 Dimensions: pain and discomfort from baseline pre injury to 9 months. EQ-5D pain and discomfort values range from 1 to 5, with 1 indicating ‘no problems’.
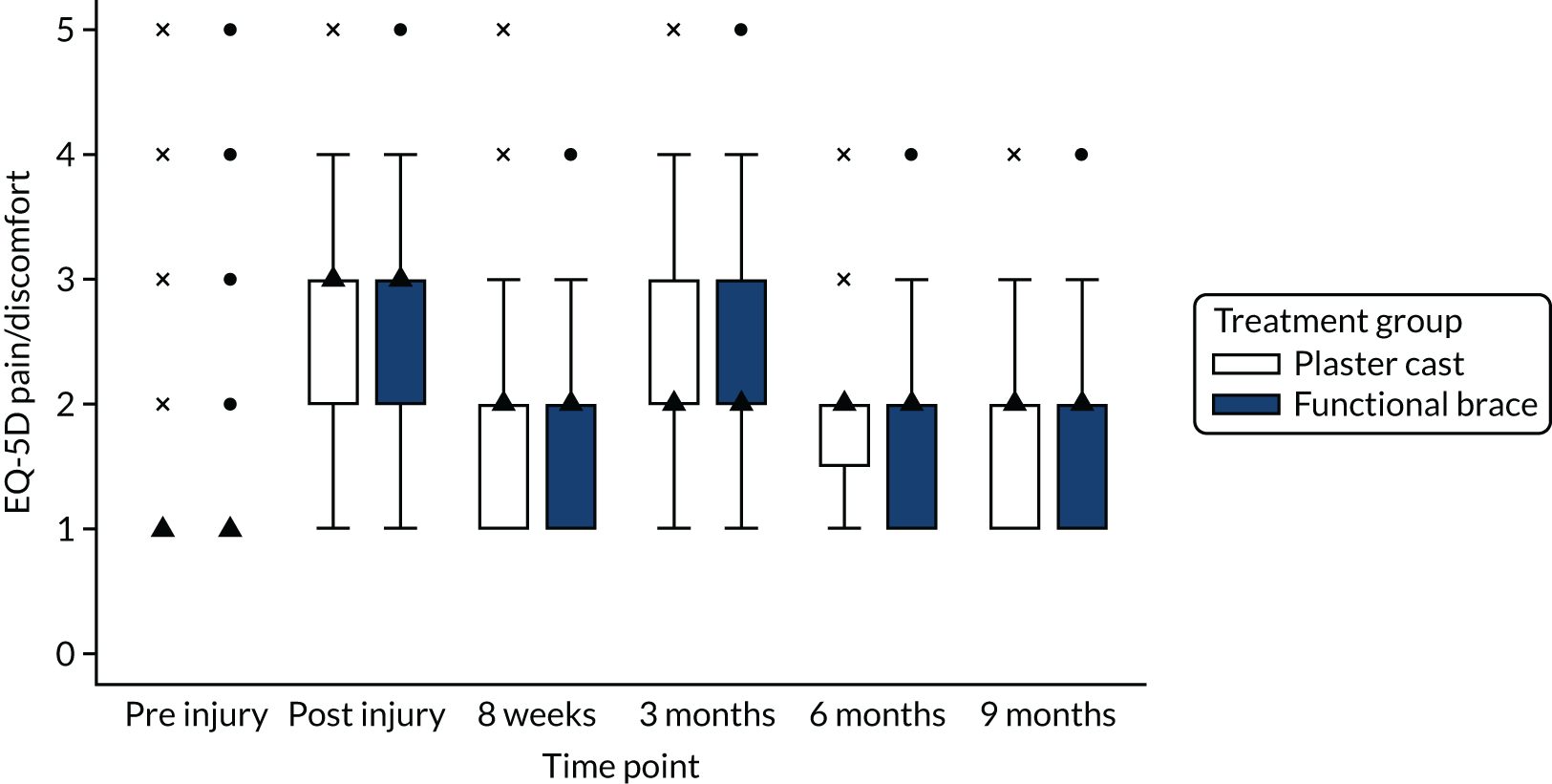
FIGURE 22.
EuroQol-5 Dimensions: anxiety and depression from baseline pre injury to 9 months. EQ-5D anxiety and depression values range from 1 to 5, with 1 indicating ‘no problems’.
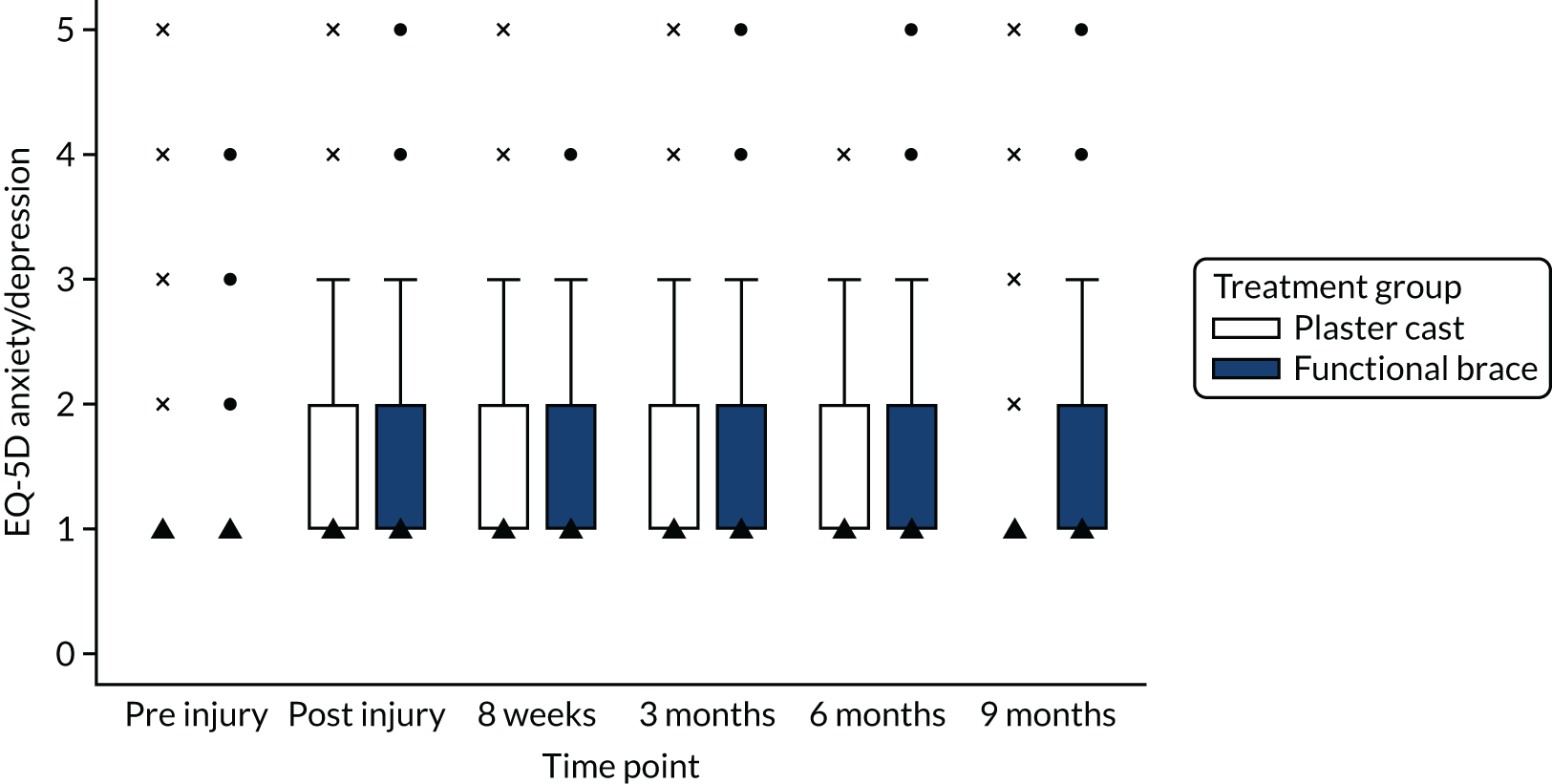
Appendix 4 Distribution of EuroQol-5 Dimensions, five-level version, responses by treatment group
| EQ-5D-5L dimension | EQ-5D-5L dimension level, n (%) | Pre-injury baseline | Post-injury baseline | 8 weeks post injury | 3 months post injury | 6 months post injury | 9 months post injury | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plaster cast group (N = 266) | Functional brace group (N = 274) | Plaster cast group (N = 266) | Functional brace group (N = 274) | Plaster cast group (N = 266) | Functional brace group (N = 274) | Plaster cast group (N = 266) | Functional brace group (N = 274) | Plaster cast group (N = 266) | Functional brace group (N = 274) | Plaster cast group (N = 266) | Functional brace group (N = 274) | ||
| Mobility | Level 1 | 243 (91.4) | 247 (90.2) | 1 (0.4) | 1 (0.4) | 24 (9.0) | 34 (12.4) | 24 (9.0) | 34 (12.4) | 94 (35.3) | 103 (37.6) | 143 (53.8) | 148 (54.0) |
| Level 2 | 10 (3.8) | 14 (5.1) | 10 (3.8) | 11 (4.0) | 63 (23.7) | 88 (32.1) | 90 (33.8) | 101 (36.9) | 89 (33.5) | 85 (31.0) | 74 (27.8) | 80 (29.2) | |
| Level 3 | 6 (2.3) | 8 (2.9) | 51 (19.2) | 64 (23.4) | 92 (34.6) | 100 (36.5) | 89 (33.5) | 90 (32.9) | 40 (15.0) | 40 (14.6) | 22 (8.3) | 28 (10.2) | |
| Level 4 | 3 (1.1) | 3 (1.1) | 103 (38.7) | 108 (39.4) | 41 (15.4) | 22 (8.0) | 19 (7.1) | 17 (6.2) | 1 (0.4) | 9 (3.3) | 4 (1.5) | 3 (1.1) | |
| Level 5 | 2 (0.8) | 0 | 99 (37.2) | 88 (32.1) | 14 (5.3) | 1 (0.4) | 7 (2.6) | 3 (1.1) | 0 | 1 (0.4) | 0 | ||
| Missing | 2 (0.8) | 2 (0.7) | 2 (0.8) | 1 (0.4) | 32 (12.0) | 33 (12.0) | 37 (13.9) | 29 (10.6) | 42 (15.8) | 37 (13.5) | 22 (8.3) | 15 (5.5) | |
| Suboptimal | 21 (7.9) | 25 (9.1) | 264 (99.25) | 273 (99.6) | 210 (78.9) | 207 (75.5) | 205 (77.1) | 211 (77.0) | 130 (48.9) | 134 (48.9) | 101 (38.0) | 111 (40.5) | |
| p-valuea | 0.647 | 0.682 | 0.357 | 0.266 | 0.778 | 0.787 | |||||||
| Self-care | Level 1 | 254 (95.5) | 265 (96.7) | 35 (13.2) | 45 (16.4) | 114 (42.9) | 131 (47.8) | 169 (63.5) | 192 (70.1) | 204 (76.7) | 207 (75.6) | 230 (86.5) | 242 (88.3) |
| Level 2 | 7 (2.6) | 6 (2.2) | 68 (25.6) | 51 (18.6) | 74 (27.8) | 81 (29.6) | 38 (14.3) | 36 (13.1) | 18 (6.8) | 23 (8.4) | 10 (3.8) | 17 (6.2) | |
| Level 3 | 1 (0.4) | 1 (0.4) | 107 (40.2) | 117 (42.7) | 41 (15.4) | 26 (9.5) | 19 (7.1) | 15 (5.5) | 2 (0.8) | 4 (1.5) | 3 (1.1) | 1 (0.4) | |
| Level 4 | 2 (0.8) | 0 | 49 (18.4) | 55 (20.1) | 5 (1.9) | 3 (1.1) | 2 (0.8) | 2 (0.7) | 0 | 3 (1.1) | 1 (0.4) | 0 | |
| Level 5 | 0 | 0 | 5 (1.9) | 5 (1.8) | 0 | 0 | 1 (0.4) | 0 | 0 | 0 | 0 | 0 | |
| Missing | 2 (0.8) | 2 (0.7) | 2 (0.8) | 1 (0.4) | 32 (12.0) | 33 (12.0) | 37 (13.9) | 29 (10.6) | 42 (15.8) | 37 (13.5) | 22 (8.3) | 14 (5.1) | |
| Suboptimal | 10 (3.8) | 7 (2.6) | 229 (86.1) | 227 (82.9) | 120 (45.1) | 110 (40.1) | 60 (22.6) | 53 (19.3) | 20 (7.5) | 30 (11.0) | 14 (5.3) | 18 (6.6) | |
| p-valuea | 0.468 | 0.404 | 0.258 | 0.281 | 0.232 | 0.715 | |||||||
| Usual activities | Level 1 | 245 (92.1) | 253 (92.3) | 3 (1.1) | 1 (0.4) | 18 (6.8) | 19 (6.9) | 26 (9.8) | 40 (14.6) | 80 (30.1) | 96 (35.0) | 141 (53.0) | 134 (48.9) |
| Level 2 | 10 (3.8) | 10 (3.7) | 13 (4.9) | 13 (4.7) | 59 (22.2) | 89 (32.5) | 92 (34.6) | 100 (36.5) | 132 (38.4) | 91 (33.2) | 80 (30.1) | 85 (31.0) | |
| Level 3 | 5 (1.9) | 6 (2.2) | 50 (18.8) | 73 (26.6) | 83 (31.2) | 85 (31.0) | 74 (27.8) | 76 (27.7) | 38 (14.3) | 34 (12.4) | 19 (7.1) | 33 (12.0) | |
| Level 4 | 2 (0.8) | 2 (0.7) | 100 (37.6) | 102 (37.2) | 51 (19.2) | 30 (10.9) | 25 (9.4) | 17 (6.2) | 3 (1.1) | 13 (4.7) | 2 (0.8) | 3 (1.1) | |
| Level 5 | 2 (0.8) | 1 (0.4) | 98 (36.8) | 84 (30.7) | 23 (8.6) | 18 (6.6) | 12 (4.5) | 12 (4.4) | 1 (0.4) | 3 (1.1) | 2 (0.8) | 5 (1.8) | |
| Missing | 2 (0.8) | 2 (0.7) | 2 (0.8) | 1 (0.7) | 32 (12.0) | 33 (12.0) | 37 (13.9) | 29 (10.6) | 42 (15.8) | 37 (13.5) | 22 (8.3) | 14 (5.1) | |
| Suboptimal | 19 (7.1) | 19 (7.1) | 261 (98.1) | 273 (99.3) | 216 (81.2) | 222 (81.0) | 203 (76.3) | 205 (74.8) | 144 (54.1) | 141 (51.5) | 103 (38.7) | 126 (46.0) | |
| p-valuea | 1.0 | 0.279 | 1.0 | 0.144 | 0.294 | 0.179 | |||||||
| Pain/discomfort | Level 1 | 226 (85.0) | 226 (82.5) | 18 (6.8) | 13 (4.7) | 63 (23.7) | 74 (27.0) | 23 (8.7) | 33 (12.0) | 56 (21.1) | 61 (22.3) | 87 (32.7) | 86 (31.3) |
| Level 2 | 24 (9.0) | 33 (12.0) | 86 (32.3) | 98 (35.8) | 125 (47.0) | 112 (40.9) | 139 (52.3) | 139 (50.7) | 126 (47.4) | 134 (48.9) | 124 (46.6) | 140 (51.1) | |
| Level 3 | 7 (2.6) | 10 (3.7) | 102 (38.4) | 113 (41.2) | 38 (14.3) | 52 (19.0) | 61 (22.9) | 68 (24.8) | 38 (14.3) | 35 (12.8) | 29 (10.9) | 29 (10.6) | |
| Level 4 | 4 (1.5) | 1 (0.4) | 46 (17.3) | 40 (14.6) | 6 (2.3) | 3 (1.1) | 3 (1.1) | 4 (1.5) | 4 (1.5) | 7 (2.6) | 4 (1.5) | 5 (1.8) | |
| Level 5 | 3 (1.1) | 2 (0.7) | 12 (4.5) | 9 (3.3) | 2 (0.8) | 0 | 3 (1.1) | 1 (0.4) | 0 | 0 | 0 | 0 | |
| Missing | 2 (0.8) | 2 (0.7) | 2 (0.8) | 1 (0.4) | 32 (12.0) | 33 (12.0) | 37 (13.9) | 29 (10.6) | 42 (15.8) | 37 (13.5) | 22 (8.3) | 14 (5.1) | |
| Suboptimal | 38 (14.3) | 46 (16.8) | 246 (92.5) | 260 (94.9) | 171 (64.3) | 167 (60.9) | 206 (77.4) | 212 (77.4) | 168 (63.2) | 176 (64.2) | 157 (59.0) | 174 (63.5) | |
| p-valuea | 0.487 | 0.289 | 0.425 | 0.258 | 0.832 | 0.574 | |||||||
| Anxiety/depression | Level 1 | 231 (86.8) | 240 (87.6) | 145 (54.5) | 158 (57.7) | 141 (53.0) | 158 (57.7) | 138 (51.9) | 149 (54.4) | 155 (58.3) | 174 (63.5) | 190 (71.4) | 190 (69.3) |
| Level 2 | 20 (7.5) | 20 (7.3) | 69 (25.9) | 70 (25.5) | 57 (21.4) | 59 (21.5) | 59 (22.2) | 69 (25.2) | 49 (18.4) | 38 (13.9) | 41 (15.4) | 55 (20.1) | |
| Level 3 | 9 (3.4) | 11 (4.0) | 39 (14.7) | 38 (13.9) | 32 (12.0) | 22 (8.0) | 26 (9.8) | 23 (8.4) | 17 (6.4) | 21 (7.7) | 7 (2.6) | 13 (4.7) | |
| Level 4 | 2 (0.8) | 1 (0.4) | 9 (3.4) | 5 (1.8) | 3 (1.1) | 2 (0.7) | 3 (1.1) | 3 (1.1) | 3 (1.1) | 3 (1.1) | 5 (1.9) | 1 (0.4) | |
| Level 5 | 2 (0.8) | 0 | 2 (0.8) | 2 (0.7) | 1 (0.4) | 0 | 3 (1.1) | 1 (0.4) | 0 | 1 (0.4) | 1 (0.4) | 1 (0.4) | |
| Missing | 2 (0.8) | 2 (0.7) | 2 (0.8) | 2 (0.7) | 32 (12.0) | 33 (12.0) | 37 (13.9) | 29 (10.6) | 42 (15.8) | 37 (13.5) | 22 (8.3) | 14 (5.1) | |
| Suboptimal | 33 (12.4) | 32 (11.7) | 119 (44.7) | 115 (42.0) | 91 (34.2) | 96 (35.0) | 69 (25.9) | 63 (23.0) | 54 (20.3) | 70 (25.6) | |||
| p-valuea | 1.0 | 0.544 | 0.271 | 0.925 | 0.354 | 0.216 | |||||||
Appendix 5 Trial management
Introduction
UKSTAR completed recruitment on schedule (Figure 23), with 540 participants recruited.
FIGURE 23.
UKSTAR actual and predicted recruitment.
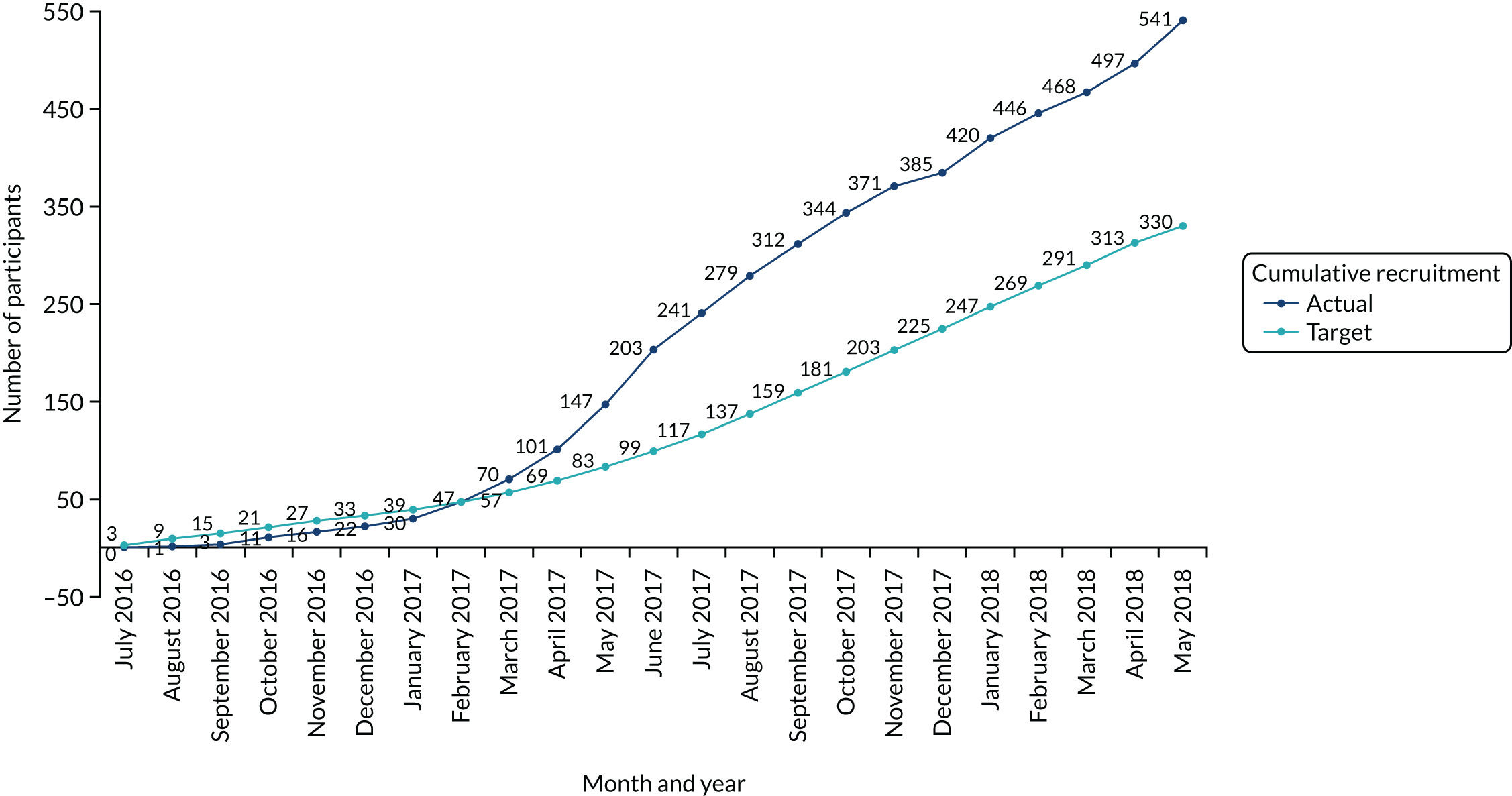
Completeness of baseline and 8-week data and rates of follow-up at all time points were excellent, thanks to the dedication of clinicians and researchers at the recruitment centres and an experienced, dedicated, central trial management team.
Management milestones
Table 28 shows the dates when milestones in the project management plan were reached.
| Event | Planned date | Actual date |
|---|---|---|
| Grant activation | 1 April 2016 | 1 April 2016 |
| Trial open | 16 August 2016 | 16 August 2016 |
| First DSMC/TSC meeting | 6 July 2016 | 6 July 2016 |
| Expected end of recruitment | 31 May 2018 | 31 May 2018 |
| Expected end of follow-up | 31 March 2019 | 12 March 2019 |
| Expected start of data cleaning | January 2019 | January 2019 |
| Expected start of final analysis | March 2019 | March 2019 |
| End of grant | 31 May 2019 | 31 May 2019 |
Recruitment target and recruitment centre selection
Principal investigators at potential recruitment centres were approached through the NIHR/Orthopaedic Trauma Society Research Network and British Orthopaedic Foot and Ankle Society. We included sites of all sizes (from major trauma centres to local emergency hospitals) from regions across England and Scotland.
Each potential recruitment centre completed a site feasibility questionnaire, which was reviewed by the chief investigator. Sites were asked to declare how many patients presented at their site with Achilles tendon rupture and were treated non-operatively, and their expected recruitment rate, which needed to be at least one participant per month. Some sites declined on the grounds that all of their patients had already been put into a functional brace, or were all treated with a plaster cast. Sites that were actively recruiting for another Achilles trial by our research group, the Platelet Rich Plasma in Achilles Tendon Healing (PATH-2) study,56 were not opened to UKSTAR until recruitment for PATH-2 was complete to avoid competition between trials for the same patients and potential recruitment bias.
UKSTAR opened to recruitment on 16 August 2016, 1 month later than planned as a result of contractual delays at recruitment centres. The original application predicted a recruitment rate of one participant per month per centre, which led to the conclusion that a minimum of 22 centres was required. However, during the first 6 months, although more recruitment centres were opened than planned (nine as opposed to six), recruitment in most centres was slower than expected (27 participants in total) and weighted towards a single, high-recruiting centre (Aberdeen, with 15 participants). We decided, therefore, to expand the number of recruitment centres and were successful in opening 39 recruitment centres, including some whose trauma departments were new to participating in trials.
One recruitment centre (James Paget University Hospitals NHS Foundation Trust, Norfolk) closed to recruitment early because of lack of research staff, having recruited no participants. All other recruitment centres recruited at least one participant.
The trial reached its original recruitment target of 330 participants in October 2017, 7 months ahead of schedule. The required sample had been estimated a priori to achieve 90% power in the primary outcome (ATRS at 9 months) and allowing for 20% loss to follow-up and crossovers. Crossover data, protocol deviations and outcome completion information were assessed prior to reaching the initial target. To avoid the power of the trial being compromised by potentially large numbers of crossovers and loss to follow-up at 9 months, we submitted, with the support of the DSMC and TSC, a substantial amendment to increase the sample size to a maximum of 550, and to recruit up to the original recruitment end date (31 May 2018). The trial continued recruiting until its original planned end date, recruiting 540 participants (see Figure 23).
Monitoring of trial recruitment
Recruitment centres were trained in recruitment procedures during site initiation visits, which were in person or by conference call. It was not necessary for trial staff to travel to distant recruitment centres if staff at those centres were experienced in trial recruitment. Staff at recruitment centres completed a monthly screening log, declaring all patients who had presented to the emergency department, specialist fracture clinic or foot and ankle clinic with Achilles tendon rupture. We monitored the reasons for non-recruitment, looking for trends at particular recruitment centres, and addressed them on a centre-by-centre basis. Recruitment centres were informed of how their recruitment rate compared with that of other recruitment centres in a monthly newsletter.
Data management
The data management plan defined the trial’s data management procedures in accordance with the trials unit’s standard operating procedures. The data management plan identified databases and information technology systems used by the trial, defined data types, data sharing and access, the critical data items, questionnaires and events, and the location of the trial data matrix; and described confidentiality, how protocol deviations were defined and what action to take, how source data were collected and entered at each time point, and how follow-up was managed. It recorded decisions on follow-up time windows made by the TMG, data rulings made by the chief investigator, statistician, health economist or trial manager, how data queries were handled and how data were to be processed at the end of the trial.
The trial monitoring plan described procedures for central monitoring and stated that no site monitoring would take place unless triggered by concerns. All recruitment centres were monitored centrally and none generated concerns sufficient to merit a monitoring visit.
Trial promotion
Promotion to patients and the public
A publicly available web page hosted on the OCTRU trials unit website provided trial information, current recruitment figures and news.
Promotion within the trauma and scientific communities
UKSTAR was featured on a poster at the 7th NIHR OTS Musculoskeletal Trauma Trials Annual Meeting on 9 January 2019. The Kadoorie Centre newsletter, sent to recruitment centres participating in Oxford trauma trials, highlighted the end of follow-up and excellent retention rate in its March 2019 issue. A monthly newsletter was sent to recruitment centres during recruitment to help maintain engagement and to acknowledge and thank centres that recruited well.
Appendix 6 Statistical analysis model
This appendix provides the mixed-effects model reporting the primary outcome results ATRS at 9 months and the post-estimation commands output. ‘Treatment’ is functional brace compared with plaster cast.
List of abbreviations
- AE
- adverse event
- ATRS
- Achilles Tendon Rupture Score
- AUC
- area under the curve
- CACE
- complier-average causal effect
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DSMC
- Data and Safety Monitoring Committee
- DVT
- deep-vein thrombosis
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- MAR
- missing at random
- MCID
- minimum clinically important difference
- MNAR
- missing not at random
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- OCTRU
- Oxford Clinical Trials Research Unit
- PE
- pulmonary embolism
- PPI
- patient and public involvement
- PRO
- patient-reported outcome
- PROM
- patient-reported outcome measure
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SMS
- short message service
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UKSTAR
- UK Study of Tendo Achilles Rehabilitation
- VAS
- visual analogue scale
- VTE
- venous thromboembolism
