Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/104/30. The contractual start date was in December 2012. The draft report began editorial review in April 2019 and was accepted for publication in November 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Michael J Griffiths has a patent 068347A1 pending for a novel method of detection of bacterial infection. Tom Solomon reports grants from the National Institute for Health Research (NIHR) outside the submitted work and other support from the Data Safety and Monitoring Committee of the GlaxoSmithKline plc (London, UK) study to evaluate the safety and immunogenicity of a candidate ebola vaccine in children (GSK3390107A) (ChAd3 EBO-Z), outside the submitted work. He also chairs the Siemens Healthineers (Munich, Germany) Clinical Advisory Board. Dyfrig Hughes was member of the Health Technology Assessment (HTA) programme Pharmaceuticals Panel (2008–12) and the HTA programme Clinical Evaluation and Trials board (2010–16). Carrol Gamble reports grants from NIHR outside the submitted work and is a member of the NIHR Efficacy and Mechanism Evaluation programme committee (January 2015–present).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Mallucci et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter have been reproduced from Jenkinson et al. 1 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter have been reproduced from Mallucci et al. 2 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Current practice
Hydrocephalus affects one in every 500 births,3 and is thus one of the most common developmental disabilities in children. The condition also affects older children and adults of all ages, and can be secondary to a variety of causes, including intracranial tumours, haemorrhage and infection. In the late 1950s, the development of a treatment with cerebral shunts revolutionised the management of these patients.
Standard treatment for hydrocephalus remains the ventriculoperitoneal shunt (VPS) catheter. A VPS comprises silicone tubing with the addition of an in-line valve that is designed to control the rate of cerebrospinal fluid (CSF) flow. The tubing passes from the brain fluid cavities (ventricles) under the skin to the peritoneum (abdominal cavity). The shunt drains CSF from the ventricles to the peritoneal cavity.
Insertion of a VPS to treat hydrocephalus is now one of the most common procedures performed in neurosurgical units, and between 3000 and 3500 shunt operations are carried out per year in the UK in adults and children. 4 Once inserted, a shunt is generally required for life; it will inevitably be susceptible to failure, in terms of both infection (as it remains an implanted foreign body) and mechanical failure, usually due to blockage of tubing or valve failure. Thus, patients with shunts will need lifelong follow up and usually require multiple surgeries. Therefore, VPS treatment for hydrocephalus is a major health burden to the NHS.
Industry produces a number of different VPS types, and costs associated with these can vary. The market comprises a wide variety of different valves and, more recently, different types of shunt catheter. The treating surgeon and hospital often chooses the type of valve and shunt tubing based on personal preference and/or associated costs.
There are three types of VPS catheter available (standard, antibiotic impregnated and silver impregnated). There is no standard practice or guidance in the UK as to which shunt catheter is the most effective at reducing infection. Practice is variable across the UK and the world. There are no current National Institute for Health and Care Excellence (NICE) guidelines, nor is there a position statement from the Society of British Neurological Surgeons regarding the use of any type of VPS.
As an infection in a newly implanted shunt can have such devastating consequences for the patient, with far-reaching health economic sequelae,5 the industry has led the way in trying to develop types of shunt catheters that will reduce infection. It is incumbent on clinical researchers to assess the effectiveness of these developments; this study attempts to answer this question.
Rationale
Shunt failure due to infection has plagued this neurosurgical advance ever since it was developed. The reported incidence of shunt infection varies markedly in the literature from 3% to 27%6–10 and is higher in certain groups, for example neonates and children aged < 1 year, and patients treated with a previous temporary external ventricular drain (EVD). Episodes of shunt infection have a major impact on both patients and the NHS and require prolonged inpatient hospitalisation, additional surgery to remove the infected hardware, placement of a temporary EVD, intravenous and intrathecal antibiotics and further surgery to place a new shunt once the infection has been treated. Other clinical consequences of infection, including epilepsy, reduced intelligence quotient (IQ) and loculation, have often been reported8 but never formally studied in the context of a prospective clinical trial. The number of shunt infections is an independent predictor of death in patients requiring CSF shunts [hazard ratio (HR) 1.66, 95% confidence interval (CI) 1.02 to 2.72]. 11
The most common pathogens detected were staphylococcus species, but, in a proportion of patients with suspected infection, the organism is never determined, especially if the patient has already received antimicrobial treatment or if there was a delay in culturing the organism, both of which hamper microbiological treatment. 5 However, newer molecular approaches are being developed,12 including the substudy within this trial.
Data from the UK shunt registry (to which most neurosurgical units contribute) report that 15% of shunt revisions are for infection. 13 In the largest randomised controlled shunt trial worldwide, the infection rate was 8.4% for primary VPSs. 14
Impregnated VPS catheters have been introduced as a means to reduce VPS infection, in addition to the usual surgical site infection prevention care bundles that are not standardised across neurosurgery clinical practice.
There are three types of VPS catheters available, and there are cost implications associated with impregnated shunt catheters that, typically, are more than double the cost of the standard non-impregnated VPS catheters:
-
standard VPSs are made of silicone and are available and supplied by a number of different companies
-
antibiotic-impregnated VPSs are made of silicone and are impregnated with antibiotics (0.15% clindamycin and 0.054% rifampicin) [available as Bactiseal® (Codman®; Integra LifeSciences Holdings Corporation, Plainsboro, NJ, USA) and Ares™ (Medtronic plc, Dublin, Ireland)]
-
silver-impregnated VPSs are made of silicone and impregnated with silver [available as Silverline® (Spiegelberg GmbH & Co. KG, Hamburg, Germany)].
Despite a large number of publications15–22 prior to our study, there has been limited evidence to date indicating the clinical effectiveness of these impregnated shunt catheters. Prior to our study, a systematic review and meta-analysis23 of the Bactiseal VPS identified one randomised controlled trial (RCT)15 and 11 observational studies. The RCT,15 conducted in a single centre in South Africa, demonstrated a trend favouring impregnated VPSs, but did not show a statistically significant difference between the two trial groups [relative risk (RR) 0.38, 95% CI 0.11 to 1.30; p = 0.12]; however, meta-analysis of the 11 observational studies showed a statistically significant difference favouring the Bactiseal VPS (RR 0.37, 95% CI 0.23 to 0.60; p < 0.01). 23 Research on the Bactiseal VPS conducted in Liverpool, UK, has shown that, over a 2-year period, the infection rate reduced among paediatric patients who were given the Bactiseal VPS compared with historical controls. 16 However, continued data collection over 3.5 years, published as part of a Liverpool-led multicentre observational study in collaboration with two other UK paediatric neurosurgical units, showed no significant reduction in infection. 17 Indeed, the reduction in infection achieved by the Bactiseal VPS in the multicentre observational study17 was seen only in neonates and was heavily weighted by the results from one unit. This study17 was not part of the published systematic review. 23
Silver-impregnated shunts were launched in the UK in March 2011. There is little doubt that silver ions have antimicrobial effects and they elute from Silverline shunt catheters. However, the efficacy of Silverline shunt catheters at preventing VPS infections is not proven. In vitro models have shown varying results and clinical studies are limited. 18,19 There is one observational study of the Silverline VPS,20 in which the Silverline VPS was used to successfully treat seven patients with active CSF infection. There are no observational studies comparing Silverline VPS infection rates with those of either standard or Bactiseal VPSs. However, in a RCT of EVDs (an EVD is a temporary tube placed in the ventricles that is prone to infection) in children and adults, Silverline EVDs have been shown to reduce infection from 21.4% (30/140) with standard shunt catheters to 12.3% (17/138) (p = 0.043) for silver shunt catheters. 22 Two further observational studies comparing standard with Silverline EVDs also show a reduction in infection rates. 21,24
Risks and benefits
The potential beneficial effect on health status of these impregnated shunt catheters is reduced shunt infection and its negative sequelae. Prior to this study, approximately 70%4,13 of shunt operations in the UK were with Bactiseal shunts (verified by feasibility screening logs) and it was felt that, just like Bactiseal, there was likely to be a significant uptake of Silverline shunts by neurosurgeons, despite the lack of evidence of clinical or cost benefit.
The potential adverse effects of impregnated shunt catheters has never been studied prospectively, to our knowledge. One of the potential concerns of antibiotic-impregnated shunt catheters is the potential for selecting out resistant organisms or missing potential infections owing to an inability to culture them.
Thus, before the wide adoption of these impregnated shunt catheters, an adequately powered RCT is needed to assess their effectiveness at reducing infection and to determine their safety (including the type of organisms cultured), antibiotic sensitivities and antibiotic resistances.
Aims and objectives
Primary objective
The primary objective was to determine whether or not antibiotic- or silver-impregnated VPSs reduce infection compared with standard VPSs in patients with hydrocephalus, following insertion of a de novo VPS.
Secondary objectives
-
To determine the proportion of first VPS infections occurring > 6 months after insertion of a de novo VPS.
-
To determine whether or not antibiotic- or silver-impregnated VPSs reduce shunt failure due to any cause compared with standard VPSs in patients with hydrocephalus following insertion of a de novo VPS.
-
To assess whether or not the reason for shunt failure is different across the three different types of VPS.
-
To determine which organisms and their resistances/sensitivities subsequently infect three alternative VPSs.
-
To determine whether or not antibiotic- or silver-impregnated VPSs reduce infection following first (non-infected) clean VPS revision for mechanical failure, compared with standard VPSs in patients with hydrocephalus, following insertion of a de novo VPS.
-
To assess the impact of VPS infection on patients in terms of quality of life.
-
To assess the cost-effectiveness of antibiotic- and silver-impregnated VPSs compared with standard VPSs.
Chapter 2 Trial design and methods
Parts of this chapter have been reproduced from Jenkinson et al. 1 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Parts of this chapter have been reproduced from Mallucci et al. 2 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Trial design
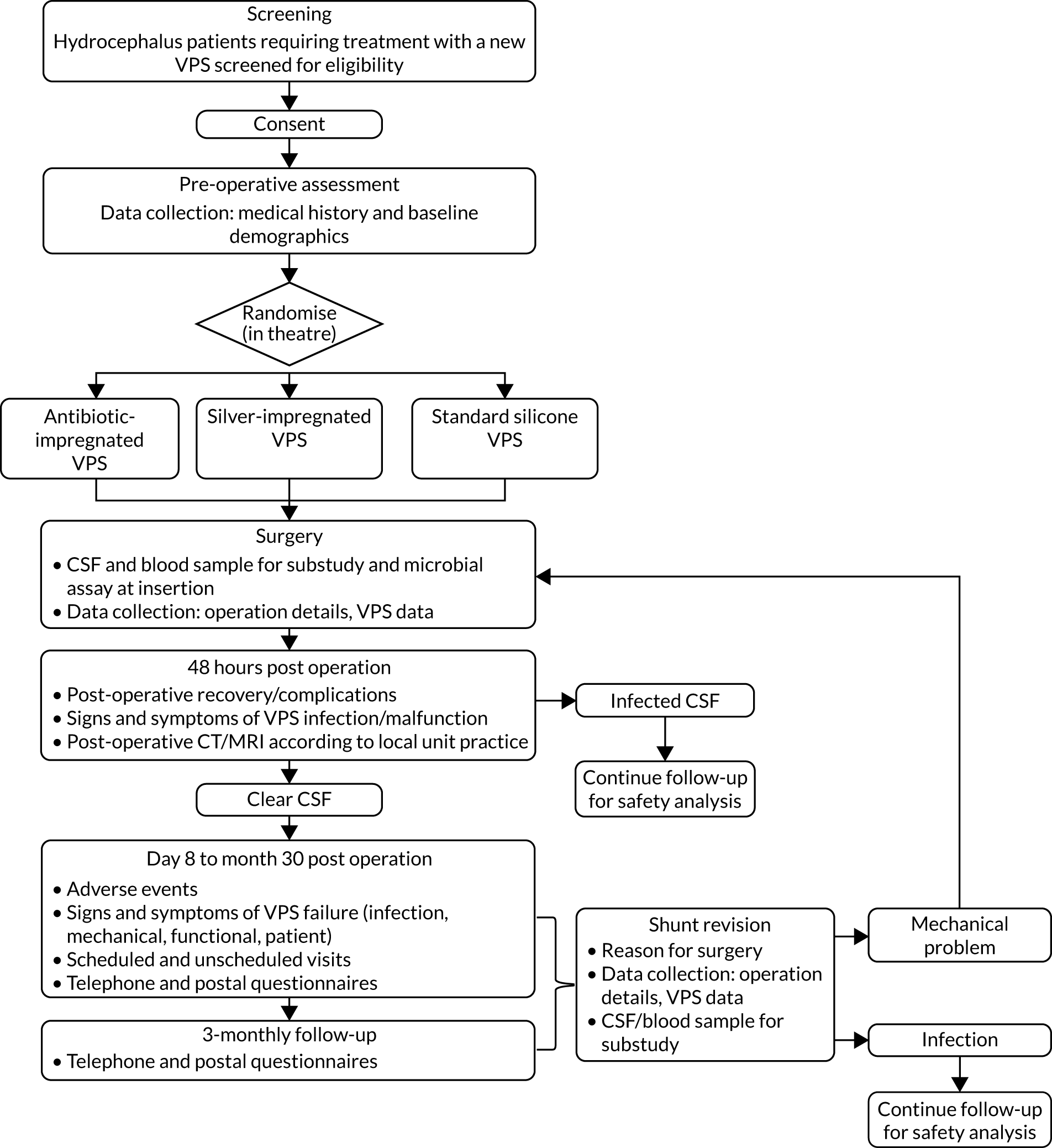
A schematic representation of the trial design is given in Figure 1.
FIGURE 1.
Trial design. CT, computerised tomography; MRI, magnetic resonance imaging. Reproduced from Jenkinson et al. 1 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The figure includes minor additions and formatting changes to the original.
Ethics approval and research governance
The protocol was approved by the Greater Manchester South Research Ethics Committee (reference number 12/NW/0790). The trial was funded by the National Institute for Health Research Health Technology Assessment programme (number 10/104/30) and included on the International Standard Randomised Controlled Trial Number registry (ISRCTN49474281). Centre-specific approval was obtained at all of the recruiting centres.
The protocol has been published previously. 1 The trial opened on protocol version 3.0, and the final approved version of the protocol was version 13.0, which contains a complete list of protocol changes [see www.fundingawards.nihr.ac.uk/award/10/104/30 (accessed 22 January 2020)]. A summary of substantial protocol amendments are provided in Table 1.
| Protocol version and date | Key amendments |
|---|---|
| 2.0 (21 November 2012) |
|
| 3.0 (22 March 2013) |
|
| 4.0 (25 July 2013) |
|
| 5.0 (20 December 2013) |
|
|
|
| 6.0 (1 April 2014) |
|
| 8.0 (10 August 2015) |
|
| 9.0 (10 August 2016) |
|
| 10.0 (11 August 2017) |
|
|
|
| 11.0 (5 April 2018) | Section added to the protocol to access HES data for patients with a Welsh postcode |
| 13.0 (25 September 2018) |
|
Selection of trial centres
Participants were recruited from 21 regional adult and paediatric neurosurgery centres in the UK and the Republic of Ireland. To be eligible to participate in the trial, centres had to meet the British Antibiotic and Silver Impregnated Catheters for ventriculoperitoneal Shunts (BASICS) trial centre suitability assessment criteria:
-
minimum of three patients per month
-
neurosurgical unit treating adults or paediatrics
-
evidence of a team to undertake trial activities
-
principal investigator (PI) had previous experience of RCTs or a significant role
-
no local issues to prevent trial set-up
-
completion of prospective screening log.
Participants
The trial was open to all patients (children and adults) who had hydrocephalus requiring treatment with a first permanent VPS who met the eligibility criteria.
Inclusion criterion
Patients were considered for inclusion in the trial if they met the following criterion:
-
Hydrocephalus of any aetiology [including idiopathic intracranial hypertension (IIH)] requiring a first VPS.
Note that failed primary endoscopic third ventriculostomy was allowed, indwelling ventricular access devices (e.g. Ommaya or Rickham reservoir or ventriculosubgaleal shunt or similar) were allowed and indwelling EVDs were allowed.
Exclusion criteria
Patients with the following characteristics were excluded from the trial:
-
previous indwelling ventricular or lumbar peritoneal or atrial shunt
-
active and ongoing CSF or peritoneal infection (previously infected cases were allowed once they were clear of infection)
-
multiloculated hydrocephalus requiring multiple VPS or neuroendoscopy
-
ventriculoatrial or ventriculopleural shunt planned
-
allergy to antibiotics associated with the antibiotic shunt
-
allergy to silver.
Recruitment procedure
Screening
Screening was performed daily by clinical staff or the designated research nurse (throughout this report, ‘research nurse’ means either the research nurse or someone who has been delegated that duty) to identify potentially eligible patients. This was carried out on the daily ward rounds or at an appropriate time point, depending on the clinical setting.
All patients having a first VPS for hydrocephalus of any aetiology (including IIH) were screened for eligibility and recorded on the screening log. Reasons for non-recruitment were documented (e.g. not eligible, declined consent) and the information was used for monitoring purposes.
Informed consent
Eligible patients were provided with patient information sheets. In the case of children or adults who lacked mental capacity to consent, the parents, consultee or legal representative were approached to discuss participation. When feasible, this was at a clinic visit prior to admission. The research nurse gave the family sufficient time to discuss the trial and to decide whether or not to consent to trial entry.
Patients were eligible to be randomised to the trial if written consent was provided by the patient, parent, legal representative or consultee.
Randomisation, concealment and blinding
Patients were randomised to standard silicone or antibiotic- or silver-impregnated VPS catheters in a ratio of 1 : 1 : 1 in random permuted blocks of three and six. The randomisation sequence was generated by an independent statistician and was stratified by neurosurgical unit, age group (adult or paediatrics was defined according to unit practice) and envelope storage room within the neurosurgical unit. Randomisation was undertaken in the operating theatre at the time when the VPS was required. Pressure-sealed envelopes were opaque and tamper-proof: they were opened by tearing perforated edges. Patients and a central review panel, but not surgeons or operating staff, were blinded to the type of VPS inserted. VPS type was not recorded in the operating record and was not disclosed outside the operating room. Training on non-disclosure of VPS type was provided to all investigators. All VPS types were medical devices used in accordance with the manufacturer’s instructions for their intended purpose.
Trial assessments
Table 2 provides the schedule of trial assessments. Participants were followed up for a minimum of 6 months and a maximum of 2 years, dependent on their randomisation date.
| Time point | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Screening | Baselinea (pre-operative assessment) | Randomisation (first surgery) | Early post-operative assessment | First routine post-operative assessmentb | 12-weekly follow up assessment | Subsequent routine post-operative assessment(s) | End-of-trial telephone call | Unscheduled visit/admission | Shunt revision/removal | |
| Informed consentc | ✗ | |||||||||
| Assessment of eligibility criteria | ✗ | ✗ | ✗ | |||||||
| Review of relevant medical history | ✗ | ✗ | ||||||||
| Collect demographic data | ✗ | ✗ | ||||||||
| Review of concomitant medications | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Weight | ✗ | |||||||||
| Heart rate | ✗ | ✗ | (✗) | |||||||
| Head circumference | (✗) | (✗) | (✗) | (✗) | (✗) | |||||
| Neurological assessment (Glasgow Coma Scale) | ✗ | ✗ | (✗) | |||||||
| Temperature | ✗ | (✗) | ||||||||
| Randomisation | ✗ | |||||||||
| Trial intervention | ✗ | ✗ | ||||||||
| Wound check | ✗ | (✗) | (✗) | (✗) | ||||||
| CSF sample taken | ✗d | (✗e) | ✗d | |||||||
| Additional CSF and blood taken for substudy | (✗) | (✗) | ||||||||
| CSF results reviewed | ✗f | (✗) | ||||||||
| Health economics questionnaire | ✗ | ✗ | ✗g | ✗ | ||||||
| Health service diary | ✗ | ✗ | ✗ | |||||||
| Post-operative CT/MRI | (✗) | (✗) | (✗) | (✗) | ||||||
| Assessment of AEs | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
Data collection
Data were collected on paper-based case report forms (CRFs) completed by centre staff who were authorised to do so and returned to the Liverpool Clinical Trials Centre (LCTC) Clinical Trials Unit (CTU). Participants were issued with diaries to record their health-care utilisation every 12 weeks and administered questionnaires to measure quality of life. A planned analysis of data from NHS Digital could not be achieved as the sponsor was unable to meet NHS Digital requirements for obtaining Hospital Episode Statistics data within the project timeline. Electronic health-care data were therefore obtained from Patient-Level Information and Costing Systems (PLICS).
Data were collected at baseline, randomisation, the peri-operative assessment, the early post-operative assessment, the first routine post-operative assessment and the subsequent post-operative assessment, and at the time points described in the remainder of this section.
The 12-weekly follow-up assessment
The 12-weekly follow-up was conducted face to face if there was a routine appointment scheduled at the same time point; if not, the follow-up was conducted by telephone. The following data were recorded:
-
related adverse events (AEs)
-
concomitant medications
-
pregnancy.
At the first 12-week assessment, the research nurse completed the relevant quality-of-life questionnaire (Table 3) with the participant over the telephone.
| Age (years) | Completed by | |
|---|---|---|
| Participant | Parent/carer | |
| < 5 | None administered | None administered |
| 5 to < 8 | None administered | HOQ (parent version) |
| EQ-5D-3L (proxy 1)a | ||
| 8 to < 18 | HOQ (child version) | EQ-5D-3L (proxy 1)a (including EQ-VAS) |
| EQ-5D-Y (including EQ-VAS) | ||
| ≥ 18 years | EQ-5D-3L (including EQ-VAS) | EQ-5D-3L (proxy 1)b (including EQ-VAS) |
Unscheduled visit/admission assessment
The ‘unscheduled visit/admission’ CRF was completed for any non-routine attendance at the treating neurosurgical centre and the following data were recorded:
-
source of unscheduled visit
-
reason for return
-
physical examination
-
microbiology
-
blood samples
-
imaging
-
wound check
-
CSF leak
-
related AEs
-
concomitant medications
-
pregnancy
-
outcome of visit.
Shunt revision/removal
If a patient was admitted for a clean VPS revision (for mechanical shunt failure, functional shunt failure or failure due to the patient) or removal (for suspected infection), the following data were recorded:
-
surgery details (separate sections for revision/removal)
-
surgeon details
-
CSF sample details (including samples for substudies, if patient is taking part)
-
related AEs
-
concomitant medications.
In addition, the shunt surgery log was completed for all surgeries that took place after the initial surgery when the randomised shunt was inserted.
For instances in which the shunt was removed for suspected infection, concomitant medications were reported up until 14 days after removal and the patient was reviewed for 48 hours for AEs and serious adverse events (SAEs).
Quality of life and health service diaries
Questionnaires
The EuroQol-5 Dimensions, three-level version (EQ-5D-3L), the EQ-5D-3L Proxy, the EuroQol-5 Dimensions Youth (EQ-5D-Y) (youth version), the EuroQol Visual Analogue Scale (EQ-VAS) or the Hydrocephalus Outcome Questionnaire (HOQ)25 were administered to participants, or their parent or carer, according to age (see Table 3) to measure participants’ health outcome and quality of life.
Resource use questionnaires were given/posted out to participants every 12 weeks for participants to complete and return to the centres 12 weeks later. Participants were reminded by the research nurse to return diaries during the 12-weekly assessments if they had not done so.
Measures
Primary outcome
The primary outcome was time to VPS infection as assessed by the central review panel, which comprised the chief investigator (or delegate for participants treated at the centre of the chief investigator) and a microbiologist, who were masked to participant allocation. Each VPS revision was classified as infection or no infection. Infections were further classified as definite (culture positive), probable (culture uncertain), probable (culture negative), possible (culture uncertain) or VPS deep incisional infection according to the microbiological samples sent and the criteria in the trial protocol.
Secondary outcomes
Secondary outcomes were as follows:
-
time to removal of the first VPS due to suspected infection, as defined by the treating surgeon at the time of revision
-
time to VPS failure of any cause (infection, mechanical, patient or functional)
-
reason for failure (infection, mechanical, patient, functional) as classified by the treating surgeon
-
types of bacterial VPS infection (organism, antibiotic resistances)
-
time to VPS infection following first clean (non-infected) revision, as classified by the central review panel
-
quality of life measured using the HOQ25
-
health economics outcomes – incremental cost per VPS failure averted and quality-adjusted life-year (QALY) gained, measured using the EQ-5D-3L, EQ-5D-3L Proxy and EQ-5D-Y questionnaires.
Data on complications and SAEs were also collected.
Sample size
The sample size for the primary outcome was calculated using the Pintilie26 method with the following assumptions: (1) failure for infection was the event of interest, with all other reasons for failure a competing risk, (2) the rate of infection was 8% in the standard silicone arm14 and 4% in the impregnated shunt catheter arms, (3) the competing risk event rate was 30% and (4) there was a 5% loss to follow-up. A total sample size of 1200 participants with 119 events demonstrated good statistical power (88%), with leverage for a lower event rate if required (Table 4). A feasibility study conducted in trial centres for 1 month indicated an annual eligible participant figure of 1200; a conservative estimate of consent of 50% suggested that the sample size would be achievable within a 2-year recruitment period, with participants followed up for a minimum of 6 months.
| Infection rate | HR | Power (%) | Total sample size (across the three trial arms) (n) | |
|---|---|---|---|---|
| Control arm | Treated arms | |||
| 0.1 | 0.05 | 0.48 | 94 | 1140 |
| 0.08 | 0.04 | 0.49 | 80 | 942 |
| 0.08 | 0.04 | 0.49 | 88 | 1157 |
| 0.05 | 0.025 | 0.49 | 67 | 1144 |
An interim analysis was planned after 50% of the total events had been observed, using the Haybittle–Peto method. 27
Monitoring of the infection rate during the trial demonstrated that the majority of events occur within 1 month of VPS insertion (i.e. they are not exponentially distributed), and that the rates of infection, competing risk and loss to follow-up were lower than expected. In January 2016, the Independent Data and Safety Monitoring Committee (IDSMC) reviewed the sample size calculations and recommended increasing recruitment to a target of 1606 participants with 101 events, to provide 80% power; the Trial Steering Committee (TSC) agreed and approved this change. The early occurrence of events and assumption of exponential risk were managed in the Pintilie26 method assumptions by reducing the accrual and follow-up rates to 1 month.
Statistical methods
The main features of the analysis plan were specified in the protocol; the final analyses were undertaken according to a more detailed and prespecified statistical analysis plan (see Report Supplementary Material 1), consistent with the protocol.
Efficacy outcomes were analysed according to the intention-to-treat principle as far as practically possible; AEs and SAEs were reported according to the type of VPS in situ. A Bonferroni adjustment28 was made to allow for multiple comparisons (antibiotic-impregnated vs. standard VPS, and silver-impregnated vs. standard VPS) and a 2.5% level of statistical significance and 97.5% CIs were used throughout.
Outcomes with infection as the event of interest used Fine and Gray29 survival regression models with cause-specific hazard ratios (csHRs) and subdistribution hazard ratios (sHRs) presented. 30,31 Cox regression models were used to analyse time to VPS failure due to any cause. Reason for VPS failure is presented descriptively (see Chapter 3, Secondary outcome 3: reason for shunt failure) and with a chi-squared test. Types of organisms and their resistances and sensitivities are presented descriptively in Chapter 3, Secondary outcome 4: types of bacterial infection. Quality-of-life outcomes were analysed using mixed models. All survival models were adjusted for the age category of the recruiting centre (paediatric or adult), with adult centres further categorised by age > 65 years. A post hoc analysis was conducted that explored revision rates, and reason for revision, by aetiology of the hydrocephalus, type of valve and operative approach. Results of the post hoc analysis are presented descriptively in Chapter 3, Post hoc analyses.
Primary outcome and safety analyses were validated by independent programming from the point of raw data extraction. All analyses were carried out with SAS® software version 9.4 with SAS/STAT package 14.3 (SAS Institute Inc, Cary, NC, USA).
Patient and public involvement
The trial team collaborated with young people and parent contributors throughout the trial:
-
Advice was sought from the Medicines for Children Research Network Young Person’s Group on the content and presentation of patient information leaflets and consent forms. The Medicine for Children’s Research Network is a division of the LCTC, part of the Liverpool Clinical Trials Collaborative.
-
Three lay members were invited at the trial outset to join the Trial Management Group (TMG) and TSC; one was recruited to be a member of the TMG.
-
Members of the TMG, including the lay member, met via teleconference with the patient and public involvement co-ordinator for the LCTC early in the trial to establish the timings for return of the patient-completed diaries and to identify ways to maximise the return rate of these diaries. They decided that it would be appropriate for the centre team to contact the participant or representative via telephone every 3 months as a reminder to complete and return the diaries.
-
The charity Shine (Spina bifida Hydrocephalus Information Networking Equality) was continually supportive of the trial. A Shine representative was a member of the TSC.
Trial oversight and role of funders
The TMG, comprising the chief investigator, other lead investigators (clinical and non-clinical) and members of the LCTC CTU, was responsible for the day-to-day running and management of the trial. The membership of the oversight committees was suggested by members of the TMG to the trial funders and appointed by the funders with their constitution following funder requirements.
The TSC consisted of an independent chairperson, an independent microbiologist, a lay representative from the Shine charity and an independent statistician. The chief investigator was a non-independent member of the TSC. The role of the TSC was as the executive decision-making committee, considering the recommendations of the IDSMC. Monitoring reports viewed by the TSC were not split by treatment group.
The IDSMC consisted of an independent chairperson, plus two independent members: an expert in the field of microbiology and an expert in medical statistics. The IDSMC was responsible for reviewing and assessing recruitment, interim monitoring of safety and effectiveness, trial conduct and external data. The IDSMC provided recommendations to the TSC concerning the continuation of the trial and viewed accumulating data split by treatment group.
All protocol amendments were approved by the funder prior to ethics submission.
Chapter 3 Results
Parts of this chapter have been reproduced from Mallucci et al. 2 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Recruitment and screening
The trial opened to recruitment on 26 June 2013 and closed on 9 October 2017.
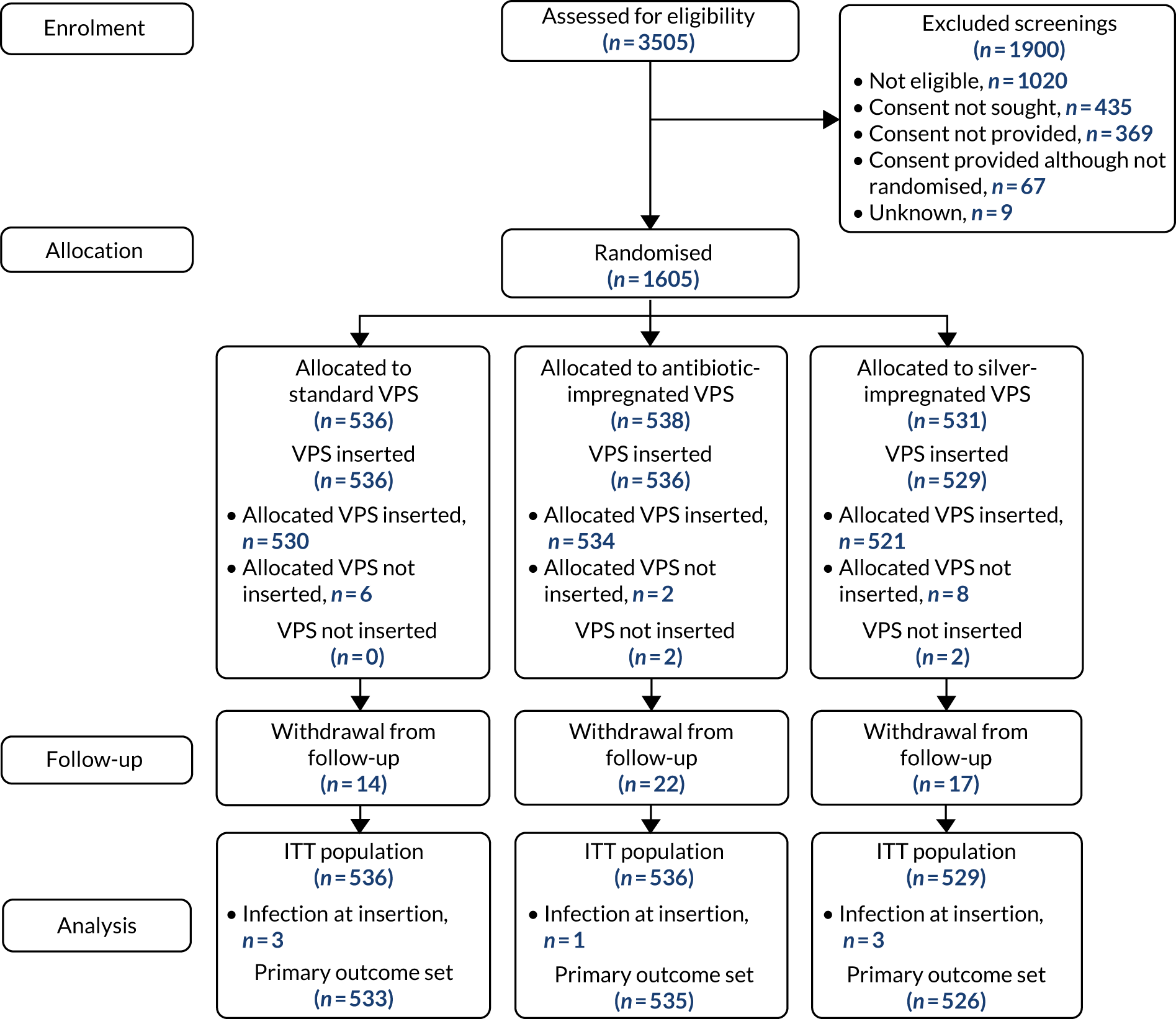
During this period, 3505 patients were screened for eligibility, of whom 1605 patients were randomised from 21 centres. One patient was randomised twice and their data contributed from the first randomisation only. See Appendix 2, Figure 8 and Tables 32 and 33, for screening and recruitment data.
Screened patients who were not randomised fell into four categories: the patient did not meet eligibility criteria (n = 1020); the patient was eligible but consent was not sought (n = 435); consent was sought but the patient declined (n = 369); and the patient was not randomised for another reason (n = 67). The overall consent rate in patients who were approached for participation was 82%.
A Consolidated Standards of Reporting Trials (CONSORT) flow diagram illustrating the pathway of patients from screening to consent and randomisation is provided in Figure 2.
FIGURE 2.
The CONSORT flow chart. ITT, intention to treat.
Baseline comparability
The three groups were similar in their baseline characteristics (Table 5) and baseline risk assessment (Table 6). Approximately 40% of all participants were paediatric patients, with one-quarter of all participants being aged < 1 year at the time of randomisation. The factors recorded on the baseline risk assessment were those regarded within the literature as being associated with a high risk of infection.
| Characteristic | Trial group | Total | ||
|---|---|---|---|---|
| Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | ||
| Number randomised | 536 | 538 | 531 | 1605 |
| Age (years) | ||||
| n (n missing) | 536 (0) | 538 (0) | 531 (0) | 1605 (0) |
| Median (IQR) | 42.5 (0.8–69.7) | 43.9 (1.1–70.8) | 41.1 (0.5–68.8) | 42.5 (0.8–69.6) |
| Minimum, maximum | 0.0, 90.3 | 0.0, 88.9 | 0.0, 91.1 | 0.0, 91.1 |
| Age category | ||||
| n (n missing) | 536 (0) | 538 (0) | 531 (0) | 1605 (0) |
| Paediatric, n (%) | 200 (37.3) | 201 (37.4) | 198 (37.3) | 599 (37.3) |
| Adult (≤ 65 years), n (%) | 174 (32.5) | 156 (29.0) | 172 (32.4) | 502 (31.3) |
| Adult (> 65 years), n (%) | 162 (30.2) | 181 (33.6) | 161 (30.3) | 504 (31.4) |
| Sex | ||||
| n (n missing) | 535 (1) | 538 (0) | 531 (0) | 1605 (0) |
| Female, n (%) | 246 (46.0) | 260 (48.3) | 282 (53.1) | 788 (49.1) |
| Male, n (%) | 289 (54.0) | 278 (51.7) | 249 (46.9) | 816 (50.9) |
| Weight (kg) | ||||
| n (n missing) | 523 (13) | 523 (15) | 515 (16) | 1561 (44) |
| Median (IQR) | 64.0 (8.8–82.7) | 63.0 (9.6–82.0) | 63.0 (7.3–80.0) | 63.1 (8.7–81.5) |
| Minimum, maximum | 1.1, 161.0 | 0.8, 163.0 | 1.3, 145.0 | 0.8, 163.0 |
| Heart rate (BPM) | ||||
| n (n missing) | 530 (6) | 532 (6) | 521 (10) | 1583 (22) |
| Median (IQR) | 84 (72–120) | 85 (70–116.5) | 84 (70–124) | 84 (70–121) |
| Minimum, maximum | 48, 190 | 44, 185 | 43, 185 | 43, 190 |
| Risk indicator | Trial group | Total | ||
|---|---|---|---|---|
| Standard VPS (N = 536) | Antibiotic-impregnated VPS (N = 538) | Silver-impregnated VPS (N = 531) | ||
| Previous Staphylococcus aureus infection (requiring treatment in the previous 6 months) | ||||
| n (n missing) | 534 (2) | 538 (0) | 531 (0) | 1603 (2) |
| Yes, n (%) | 18 (3.4) | 15 (2.8) | 16 (3.0) | 49 (3.1) |
| No, n (%) | 516 (96.6) | 523 (97.2) | 515 (97.0) | 1554 (96.9) |
| Active skin/wound infection | ||||
| n (n missing) | 534 (2) | 538 (0) | 530 (1) | 1602 (3) |
| Yes, n (%) | 7 (1.3) | 8 (1.5) | 5 (0.9) | 20 (1.2) |
| No, n (%) | 527 (98.7) | 530 (98.5) | 525 (99.1) | 1582 (98.8) |
| MRSA infection in the previous 6 months | ||||
| n (n missing) | 535 (1) | 537 (1) | 529 (2) | 1601 (4) |
| Yes, n (%) | 6 (1.1) | 4 (0.7) | 5 (0.9) | 15 (0.9) |
| No, n (%) | 529 (98.9) | 533 (99.3) | 524 (99.1) | 1586 (99.1) |
| Pre-term at birth | ||||
| n (n missing) | 513 (23) | 522 (16) | 505 (26) | 1540 (65) |
| Yes, n (%) | 78 (15.2) | 82 (15.7) | 76 (15.0) | 236 (15.3) |
| No, n (%) | 435 (84.8) | 440 (84.3) | 429 (85.0) | 1304 (84.7) |
| Abdominal surgery in the previous month | ||||
| n (n missing) | 533 (3) | 538 (0) | 531 (0) | 1602 (3) |
| Yes, n (%) | 3 (0.6) | 3 (0.6) | 8 (1.5) | 14 (0.9) |
| No, n (%) | 530 (99.4) | 535 (99.4) | 523 (98.5) | 1588 (99.1) |
| Tracheotomy | ||||
| n (n missing) | 534 (2) | 538 (0) | 531 (0) | 1603 (2) |
| Yes, n (%) | 32 (6.0) | 13 (2.4) | 21 (4.0) | 66 (4.1) |
| No, n (%) | 502 (94.0) | 525 (97.6) | 510 (96.0) | 1537 (95.9) |
| Percutaneous endoscopic gastrostomy | ||||
| n (n missing) | 534 (2) | 538 (0) | 531 (0) | 1603 (2) |
| Yes, n (%) | 14 (2.6) | 7 (1.3) | 15 (2.8) | 36 (2.2) |
| No, n (%) | 520 (97.4) | 531 (98.7) | 516 (97.2) | 1567 (97.8) |
| CSF leak in the previous month | ||||
| n (n missing) | 534 (2) | 538 (0) | 531 (0) | 1603 (2) |
| Yes, n (%) | 57 (10.7) | 51 (9.5) | 35 (6.6) | 143 (8.9) |
| No, n (%) | 477 (89.3) | 487 (90.5) | 496 (93.4) | 1460 (91.1) |
| EVD in previous 3 months | ||||
| n (n missing) | 532 (4) | 538 (0) | 531 (0) | 1601 (4) |
| Yes, n (%) | 105 (19.7) | 95 (17.7) | 90 (16.9) | 290 (18.1) |
| No, n (%) | 427 (80.3) | 443 (82.3) | 441 (83.1) | 1311 (81.9) |
Retention and adherence
Table 7 summarises compliance with the randomly allocated shunt. Of the 1605 participants randomised, four (0.2%) had no VPS inserted and 16 (1%) received a different VPS to the one that was randomly allocated. Reasons for participants having an alternative trial VPS inserted or no VPS are provided in Appendix 2, Tables 35 and 36, respectively. Participants receiving no VPS were excluded from the intention-to-treat population; for the safety analysis, participants were analysed according to the VPS received. The analysis sets are summarised in Table 8.
| Randomised VPS | Number randomised | Allocated VPS inserted, n (%) | Other VPS inserted, n (%) | No VPS inserted, n (%) |
|---|---|---|---|---|
| Standard | 536 | 530 (98.9) | 6 (1.1) | 0 (0.0) |
| Antibiotic impregnated | 538 | 534 (99.3) | 2 (0.4) | 2 (0.4) |
| Silver impregnated | 531 | 521 (98.1) | 8 (1.5) | 2 (0.4) |
| Total | 1605 | 1585 (98.8) | 16 (1.0) | 4 (0.2) |
| Population | Trial group, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| Standard VPS | Antibiotic-impregnated VPS | Silver impregnated-VPS | ||
| Randomised | 536 (33.4) | 538 (33.5) | 531 (33.1) | 1605 (100) |
| Intention to treat | 536 (33.5) | 536 (33.5) | 529 (33.0) | 1601 (99.8) |
| Safety | 531 (33.2) | 545 (34.0) | 525 (32.8) | 1601 (99.8) |
In total, 53 (3.3%) randomised participants withdrew from the trial. No participants withdrew consent to use collected data. Table 9 summarises the level of and reasons for withdrawal.
| Withdrawal summary | Trial group, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | ||
| Randomised | 536 (33.4) | 538 (33.5) | 531 (33.1) | 1605 (100) |
| Withdrawals | 14 (2.6) | 22 (4.1) | 17 (3.2) | 53 (3.3) |
| Level of withdrawal | ||||
| Withdrawal of dataa | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Consent revoked to use data collected | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Withdrawal from follow-upb | 14 (2.6) | 22 (4.1) | 17 (3.2) | 53 (3.3) |
| Consent revoked for trial-specific data to be collected | 6 (1.1) | 9 (1.7) | 9 (1.7) | 24 (1.5) |
| Consent revoked for any trial data to be collected | 8 (1.5) | 13 (2.4) | 8 (1.5) | 29 (1.8) |
| Consent revoked for additional substudy samples to be taken | 3 (0.6) | 1 (0.2) | 1 (0.2) | 5 (0.3) |
| Reasons for withdrawal | ||||
| Unexpected related AE or SAE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Burden of additional trial data collection | 4 (0.7) | 9 (1.7) | 5 (0.9) | 18 (1.1) |
| Other | 10 (1.9) | 16 (3) | 13 (2.4) | 39 (2.4) |
| Decision for withdrawal made by | ||||
| Participant (aged ≥ 16 years) | 1 (0.2) | 5 (0.9) | 5 (0.9) | 11 (0.7) |
| Parent/guardian/consultee | 8 (1.5) | 12 (2.2) | 7 (1.3) | 27 (1.7) |
| Clinical | 4 (0.7) | 5 (0.9) | 5 (0.9) | 14 (0.9) |
| None listed | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
Unblinding
A total of 32 participants from 10 centres were unblinded during the course of the trial.
Unblinding could be accidental or intentional. Accidental unblinding was defined as an unplanned occurrence; for example, the allocation was incorrectly recorded in the participant notes. Intentional unblinding occurred when the unblinding envelope was opened; for example, if a patient was transferred to another hospital and staff needed to be aware of their allocation. Twenty-five participants from eight centres were accidentally unblinded and seven participants from four centres were intentionally unblinded. Table 10 summarises unblinding events, both overall and by randomised VPS.
| Type of unblinding | Level | Trial group (n) | Total (n) | ||
|---|---|---|---|---|---|
| Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | |||
| Accidental | Patient | 10 | 5 | 10 | 25 |
| Centre | 6 | 3 | 7 | 8 | |
| Intentional | Patient | 1 | 1 | 5 | 7 |
| Centre | 1 | 1 | 4 | 4 | |
| Total | Patient | 11 | 6 | 15 | 32 |
| Centre | 7 | 4 | 4 | 10 | |
Protocol deviations
Prespecified protocol deviations are summarised in Appendix 2, Table 37. The most common major protocol deviation was that shunt components were not taken for culture at shunt revision/removal (n = 320, 19.9%). This was not routine practice in many units, and the impact of this deviation on the identification of infections for the primary outcome was mitigated by the low numbers of missing CSF at revision (n = 14), as CSF analysis and culture was the primary factor used for defining shunt infection (not culture of the shunt tubing or components).
Antibiotic sensitivity data were not returned on many isolates.
Primary outcome: time to ventriculoperitoneal infection as assessed by the central review panel
The primary outcome was time to VPS infection, as assessed by the central review panel.
Four participants received no shunt and seven participants had an infection at insertion; these participants were excluded from the primary analysis set (see Figure 2).
A summary of the first VPS revisions and infections among participants in the primary intention-to-treat analysis set is provided in Table 11. The overall revision rate of first VPS was 25% (398/1594), and was approximately equal between each of the three VPS groups.
| Primary VPS revisions | Trial group, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| Standard VPS (N = 536) | Antibiotic-impregnated VPS (N = 538) | Silver-impregnated VPS (N = 531) | ||
| Summary of surgeries | ||||
| Eligible for primary outcomea | 533 (99.4) | 535 (99.8) | 526 (99.4) | 1594 (99.6) |
| No VPS removal/revision | 403 (75.6) | 403 (75.3) | 390 (74.1) | 1196 (75.0) |
| VPS removal/revision (for any cause) | 130 (24.4) | 132 (24.7) | 136 (25.9) | 398 (25.0) |
| Reason for revision as classified by central review | ||||
| Reason for revision | ||||
| Revision for infection | 32 (6.0) | 12 (2.2) | 31 (5.9) | 75 (4.7) |
| Revision for other reason (no infection) | 98 (18.4) | 120 (22.4) | 105 (20.0) | 323 (20.3) |
| Type of infection | ||||
| VPS CSF or peritoneal infection | ||||
| Definite: culture positive | 22 (68.8) | 6 (50.0) | 25 (80.6) | 53 (70.7) |
| Probable: culture uncertain | 1 (3.1) | 0 (0.0) | 2 (6.5) | 3 (4.0) |
| Probable: culture negative | 3 (9.4) | 3 (25.0) | 1 (3.2) | 7 (9.3) |
| Possible: culture uncertain | 1 (3.1) | 0 (0.0) | 1 (3.2) | 2 (2.7) |
| Clinically classified infectionb | 1 (3.1) | 0 (0.0) | 0 (0.0) | 1 (1.3) |
| VPS deep incisional infection | 4 (12.5) | 3 (25.0) | 2 (6.5) | 9 (12.0) |
All first revisions were classified as to whether or not the revision was for suspected infection by the central review panel. If there was insufficient information for the central review panel to classify an infection (n = 1/398; see Table 11), the clinical classification, as recorded on the CRFs by the treating surgeon, was used.
Of the total number of first revisions, 75 were classified infections (4.7%). The infection rate was approximately equal in the standard and silver-impregnated VPS arms (6.0% and 5.9%, respectively) and lowest in the antibiotic-impregnated VPS arm (2.2%). The time to infection was similar across all treatment arms {standard VPS arm: median 1 month [lower quartile (LQ)–upper quartile (UQ) 0–1.5 months]; antibiotic-impregnated VPS arm: median 1 month [LQ–UQ 0–2 months]; silver-impregnated VPS arm: median 1 months [LQ–UQ 0–1 months]}.
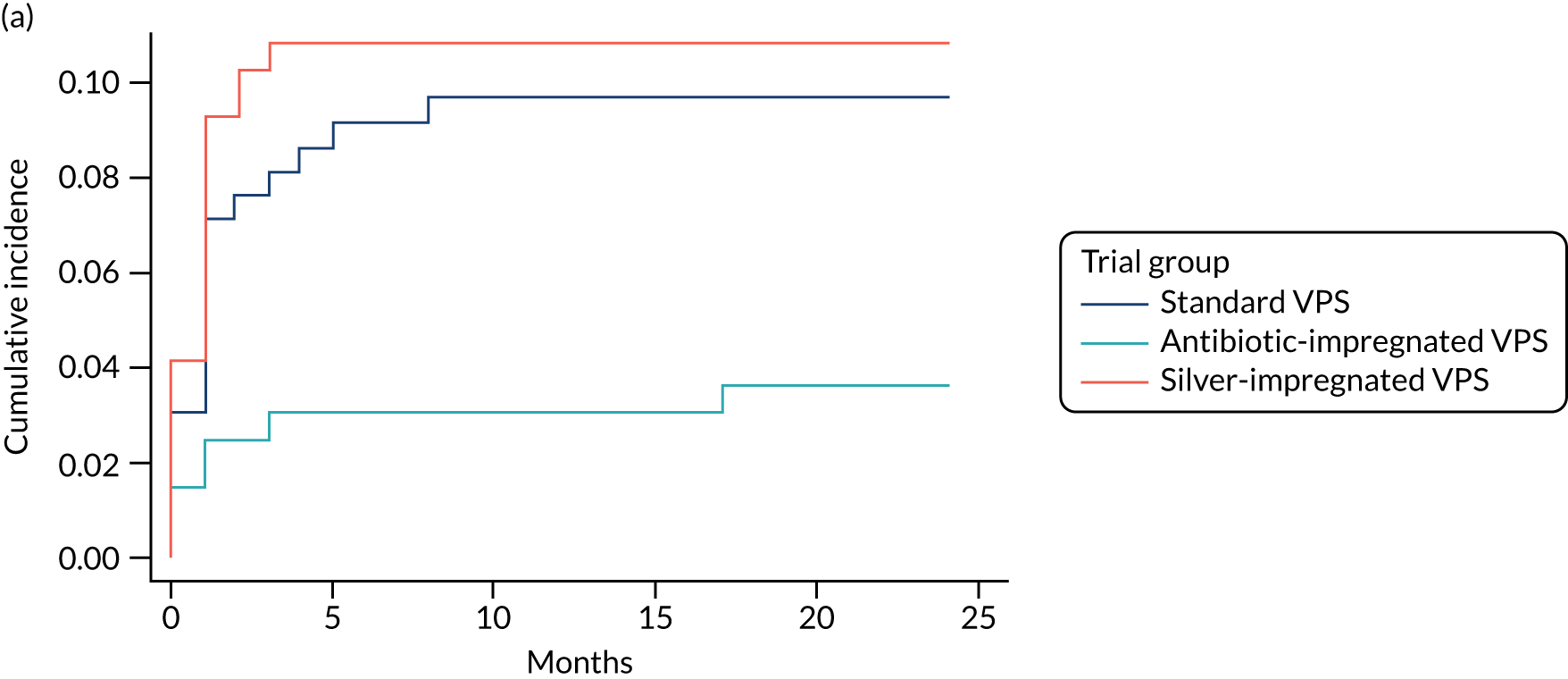
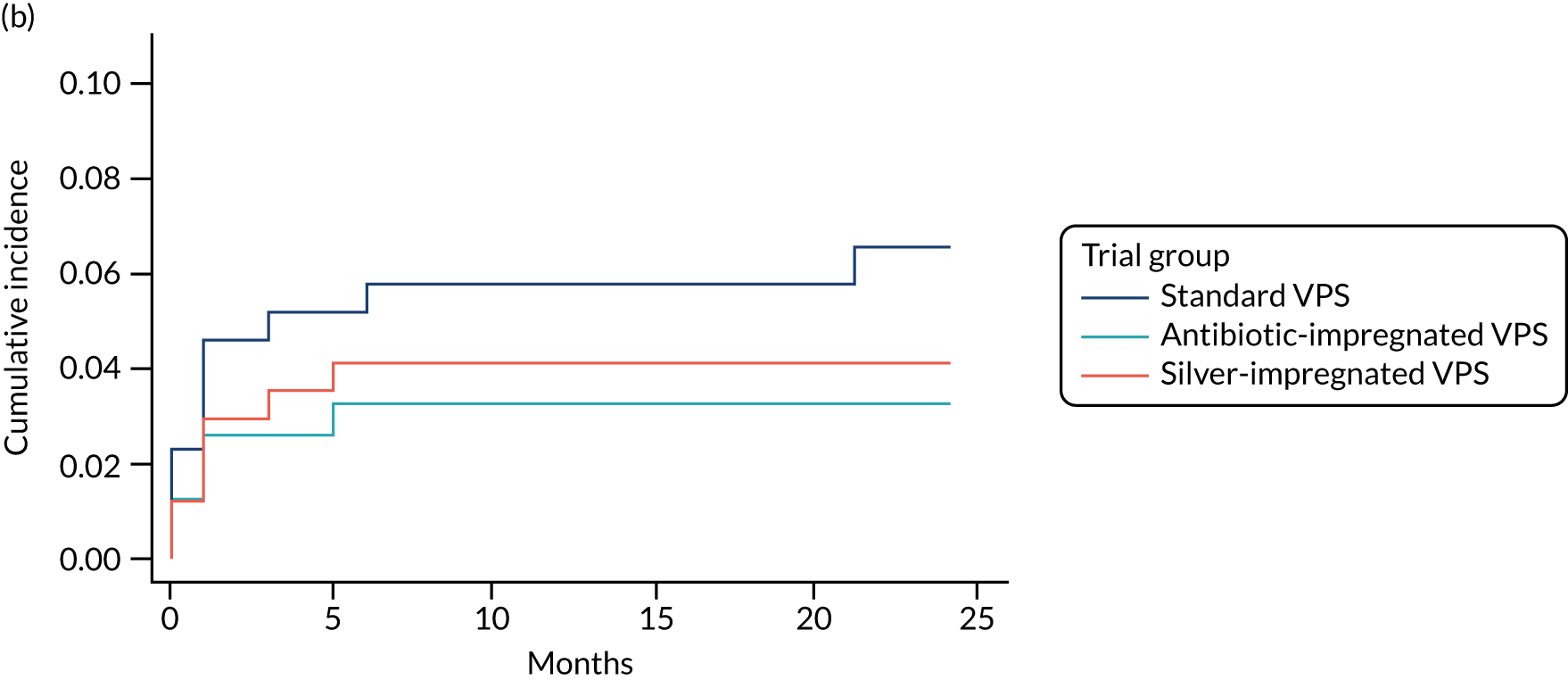
When compared with the standard VPS, antibiotic-impregnated VPSs decreased the risk of infection (csHR 0.38, 97.5% CI 0.18 to 0.80; p < 0.01) (Table 12). Silver-impregnated VPSs were comparable to standard VPSs (csHR 0.99, 97.5% CI 0.56 to 1.74; p = 0.96) (see Table 12). Figure 3 displays the cumulative incidences of infection and no infection by VPS and age group. Figure 4 displays the cumulative incidence plots of infection or no infection by VPS group, stratified by age group.
| Covariate | Infection, HR (97.5% CI); p-value | Competing risk, HR (97.5% CI); p-value |
|---|---|---|
| Cox: csHR | ||
| VPS | ||
| Standard | – | – |
| Antibiotic-impregnated | 0.38a (0.18 to 0.80); < 0.01 | 1.22b (0.90 to 1.65); 0.15 |
| Silver-impregnated | 0.99a (0.56 to 1.74); 0.96 | 1.11b (0.81 to 1.51); 0.47 |
| Age group | ||
| Paediatric | – | – |
| Adult (≤ 65 years) | 0.55a (0.31 to 0.97); 0.02 | 0.58b (0.44 to 0.77); < 0.01 |
| Adult (> 65 years) | 0.12a (0.04 to 0.34); < 0.01 | 0.28b (0.20 to 0.40); < 0.01 |
| Fine–Gray: sHR | ||
| VPS | ||
| Standard | – | – |
| Antibiotic-impregnated | 0.38c (0.18 to 0.80); < 0.01 | 1.26d (0.93 to 1.70); 0.08 |
| Silver-impregnated | 0.99c (0.56 to 1.72); 0.95 | 1.10d (0.81 to 1.50); 0.50 |
| Age group | ||
| Paediatric | – | – |
| Adult (≤ 65 years) | 0.56c (0.32 to 0.99); 0.02 | 0.60d (0.45 to 0.80); < 0.01 |
| Adult (> 65 years) | 0.12c (0.04 to 0.35); < 0.01 | 0.30d (0.21 to 0.43); < 0.01 |
FIGURE 3.
Cumulative incidence plots of revisions for infection and competing risk by VPS group and age group. (a) Infection by VPS group; (b) competing risk by VPS group; (c) infection by age group; and (d) competing risk by age group.
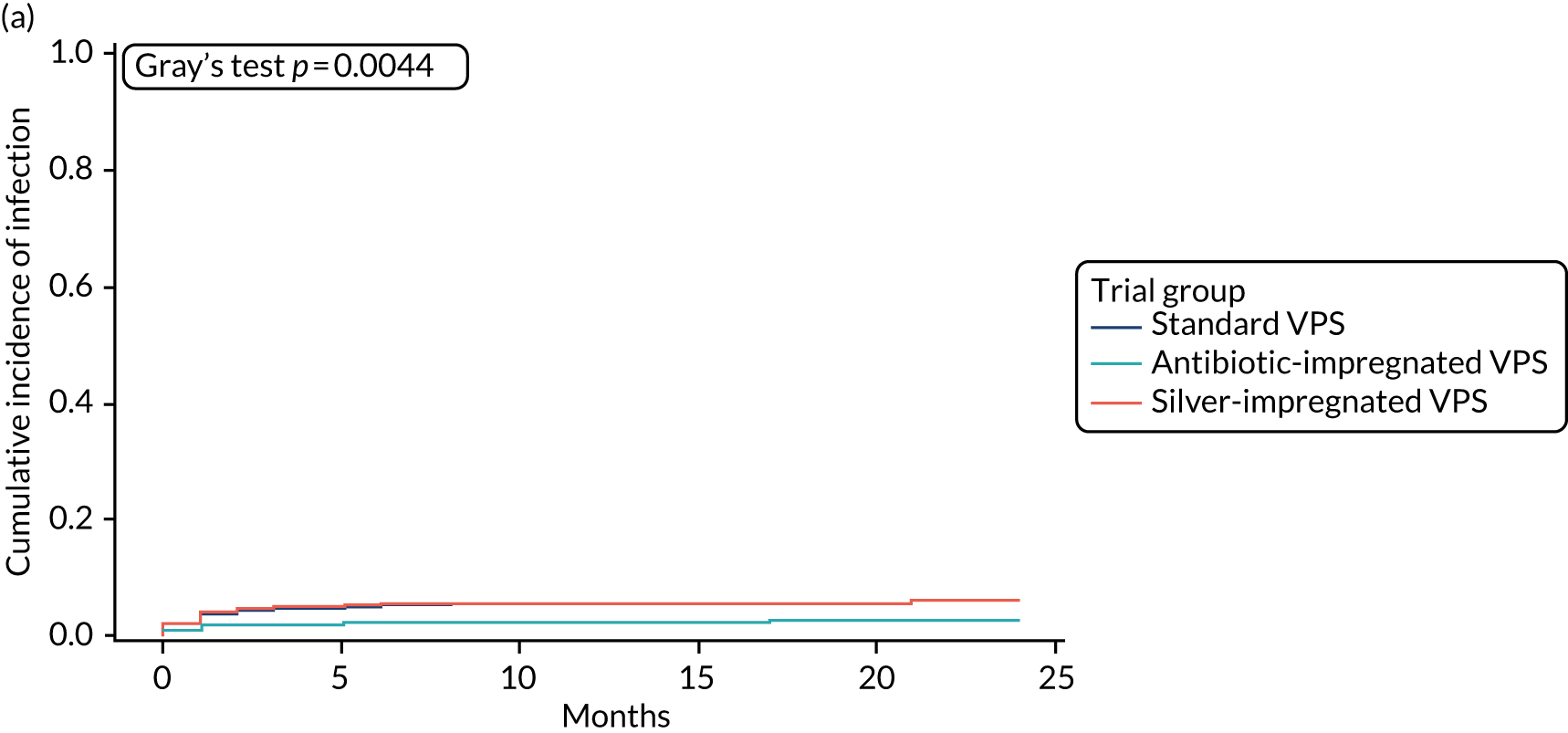
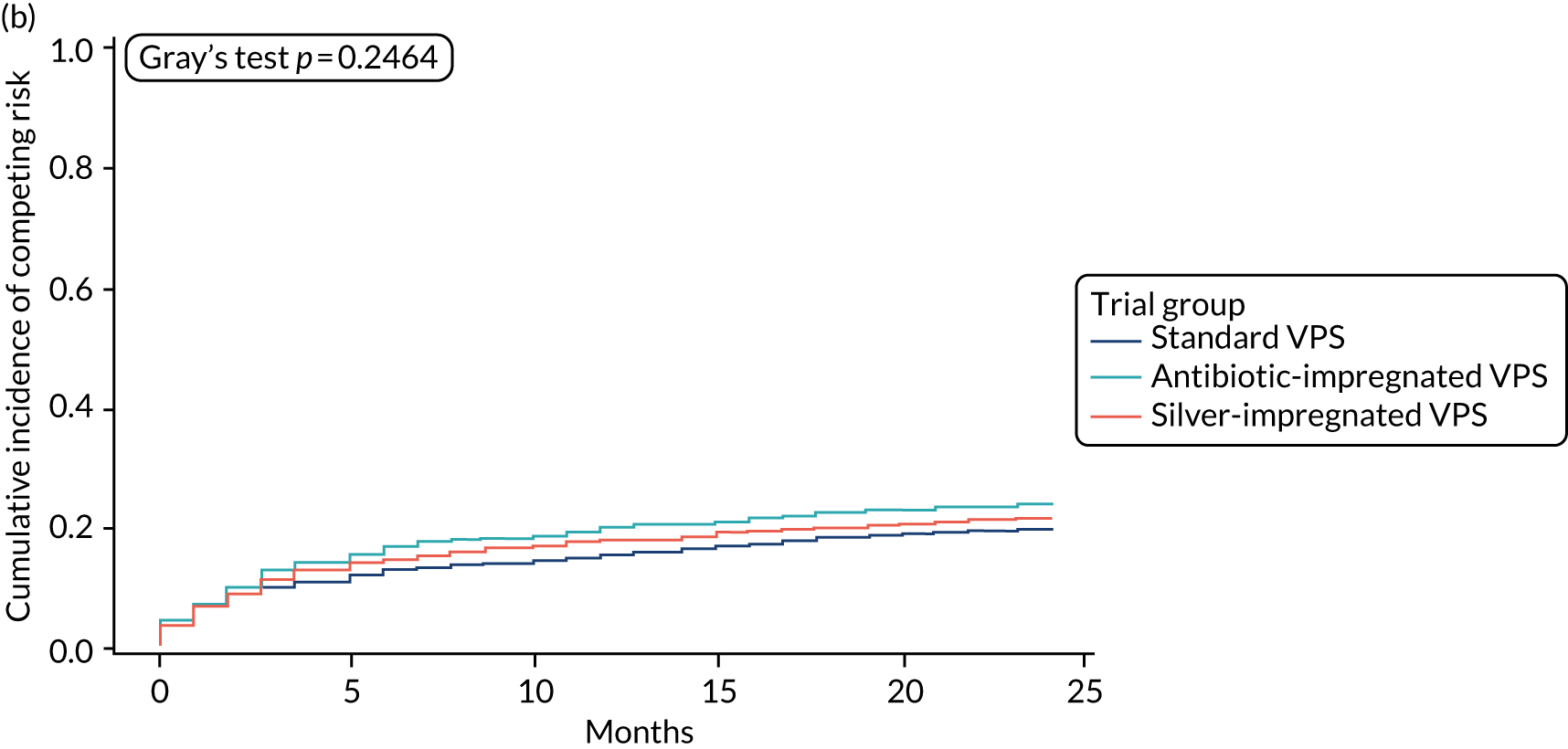
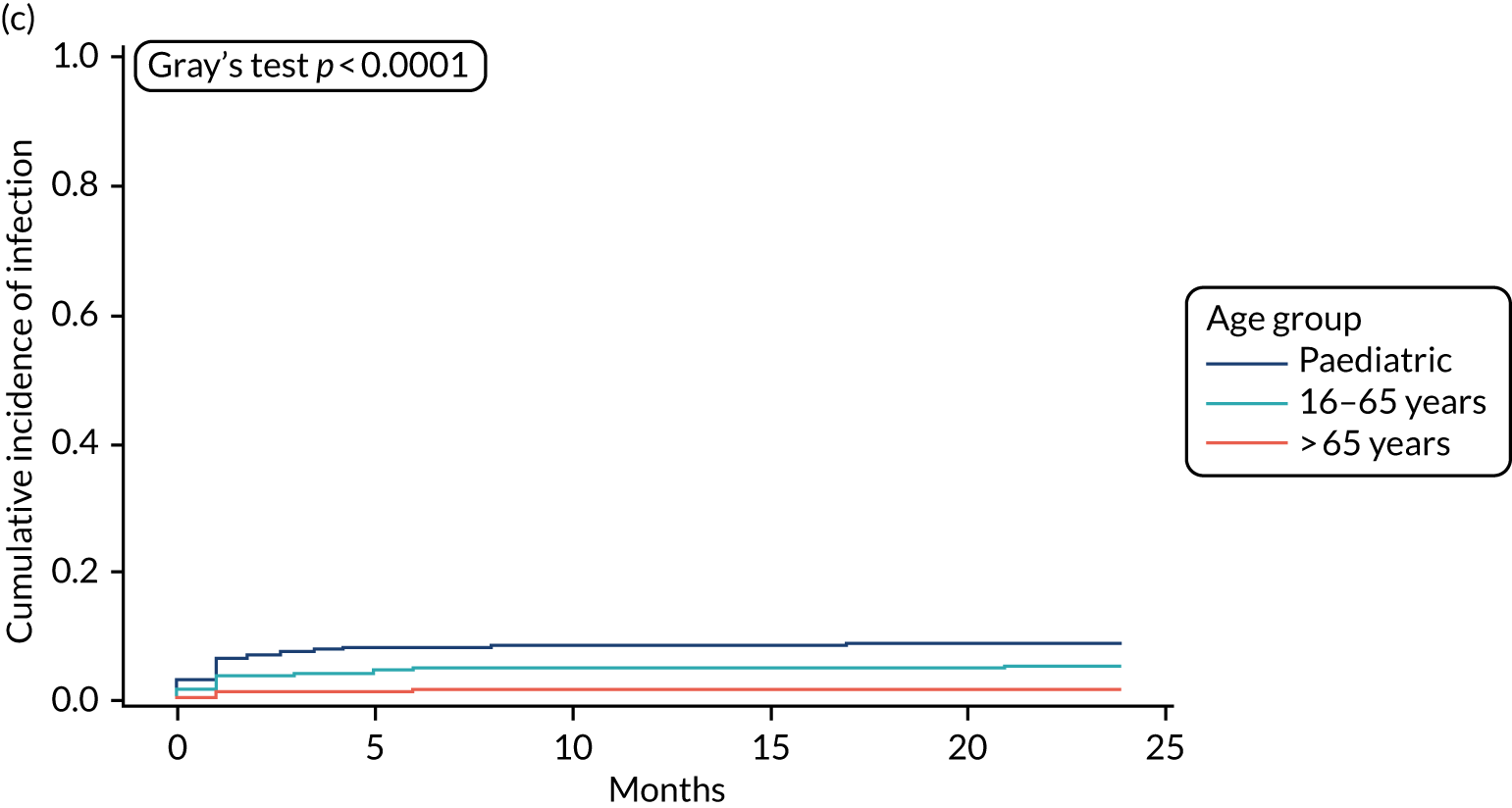
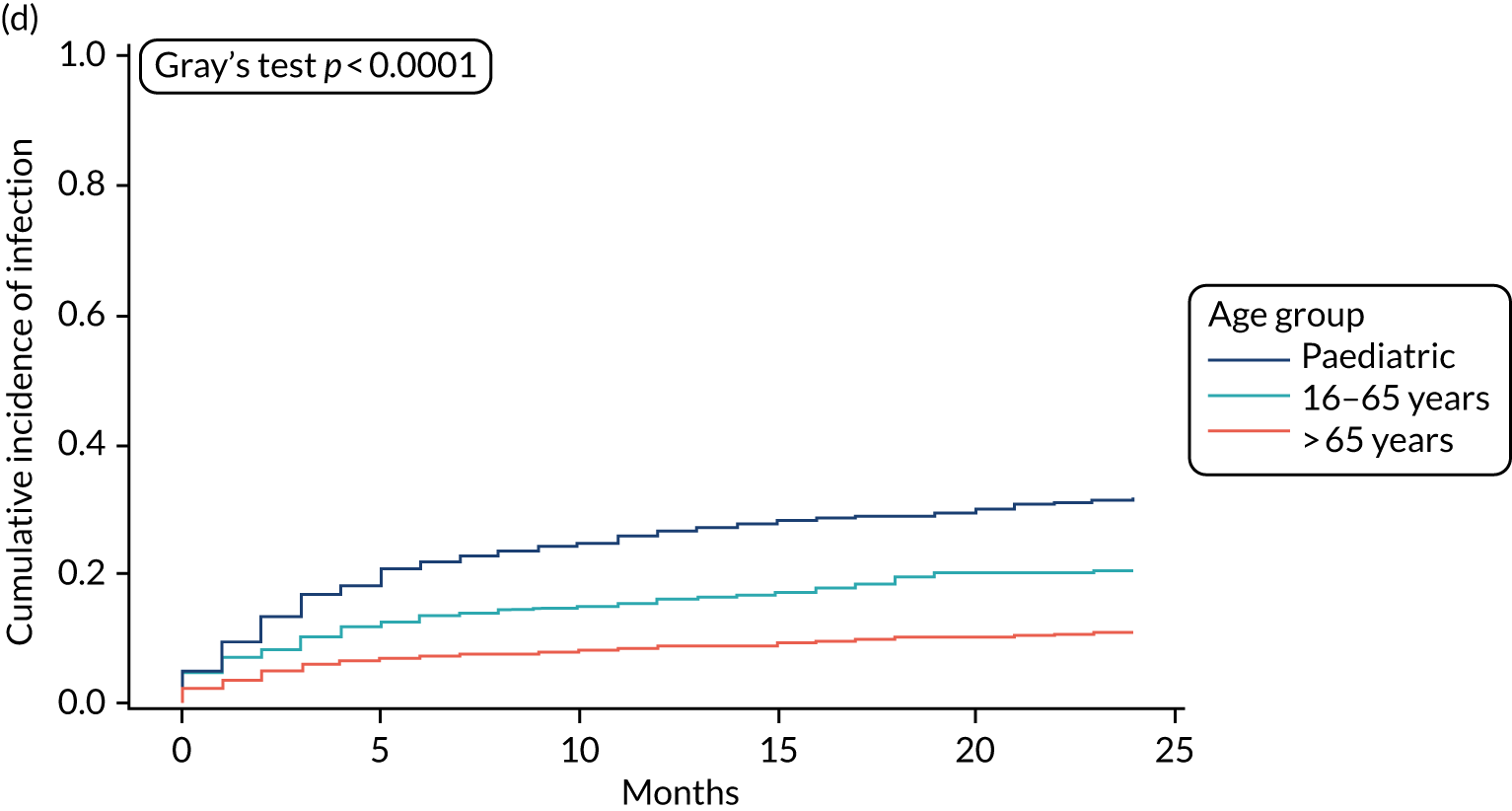
FIGURE 4.
Cumulative incidence plots of revisions for infection and competing risk by VPS group, stratified by age group. (a) Infection in the paediatric group; (b) infection in those aged ≤ 65 years; (c) infection in those aged > 65 years; (d) competing risk in the paediatric group; (e) competing risk in those aged ≤ 65 years; and (f) competing risk in those aged > 65 years.
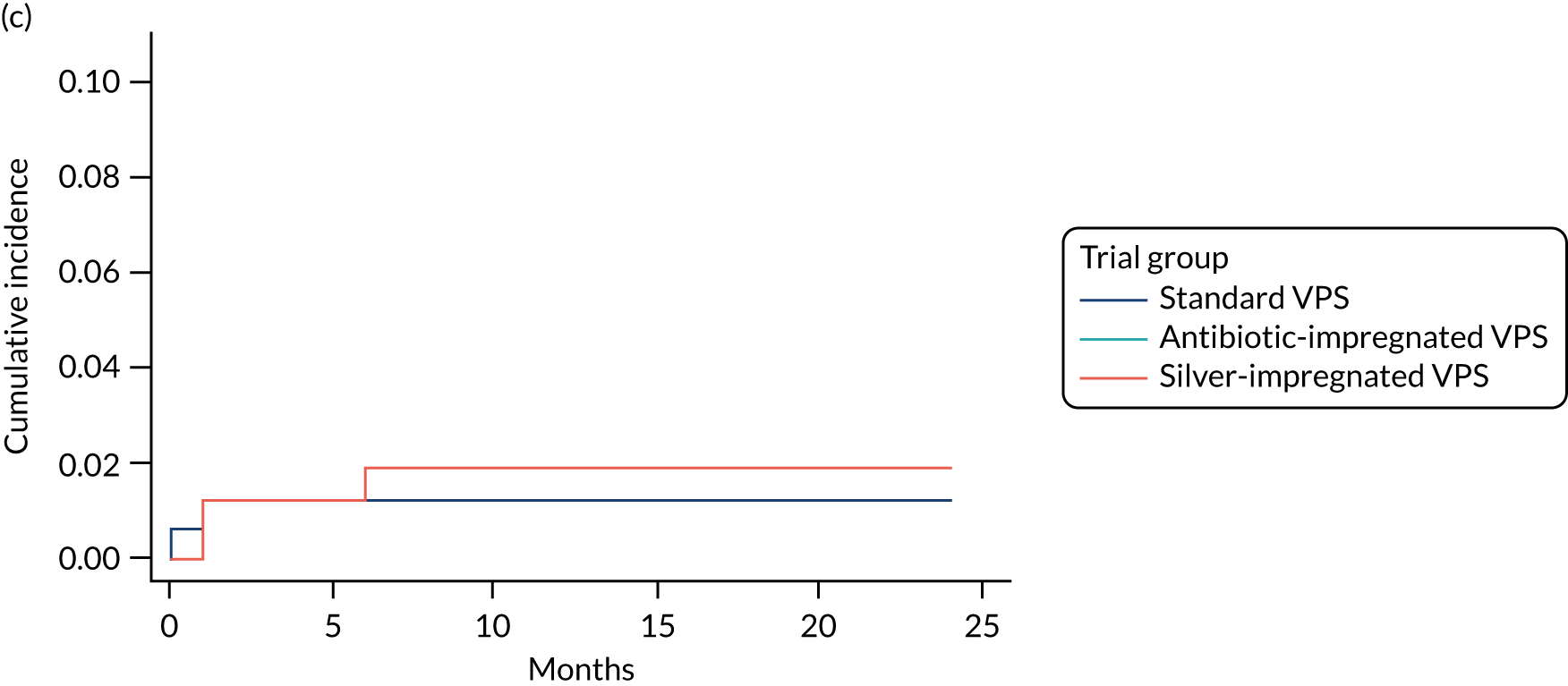
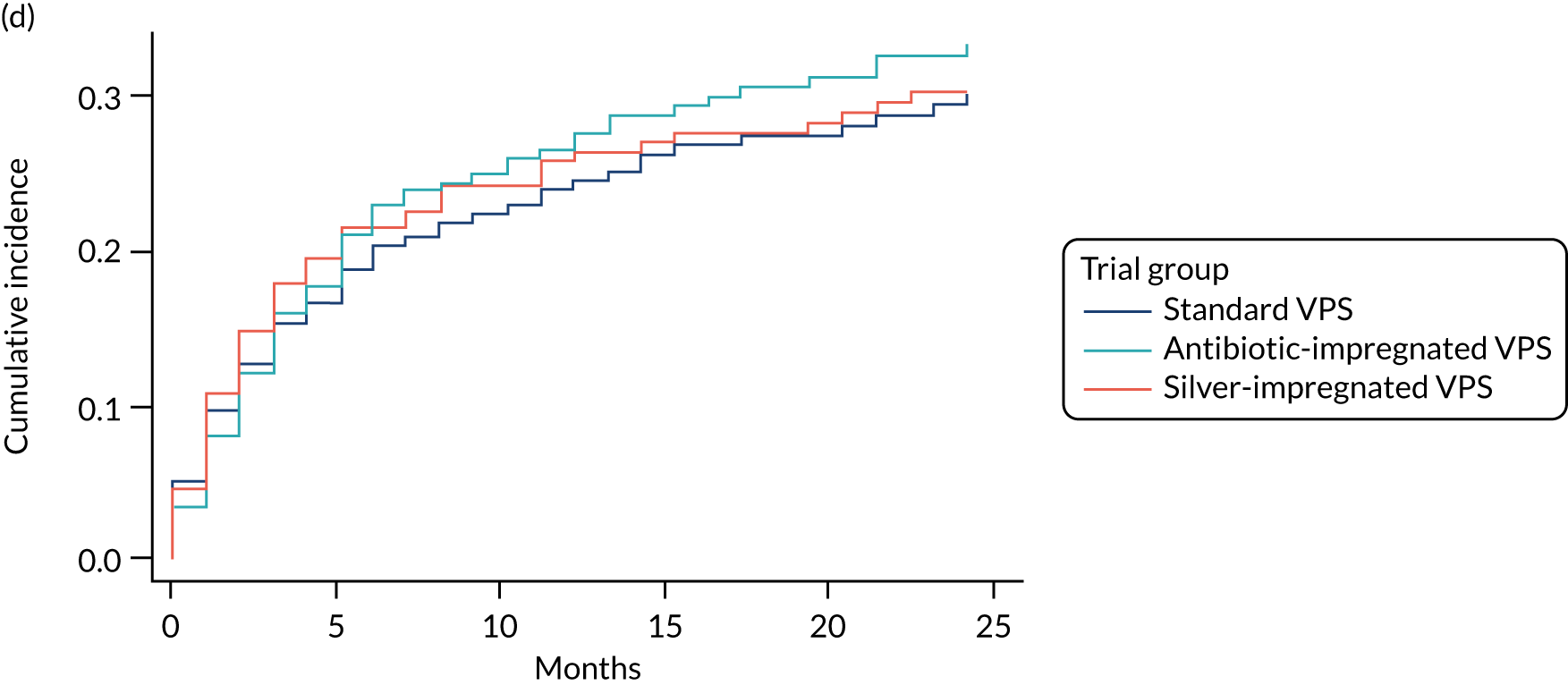
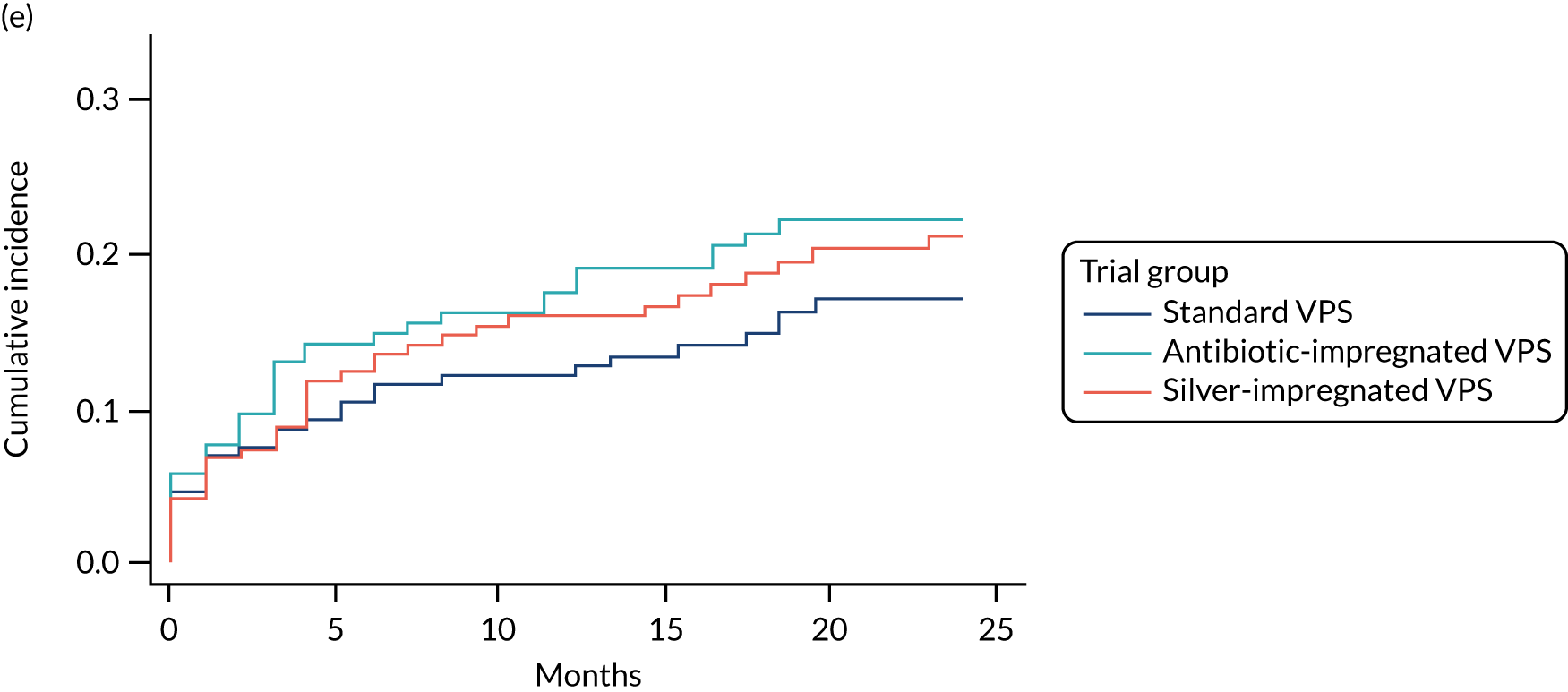
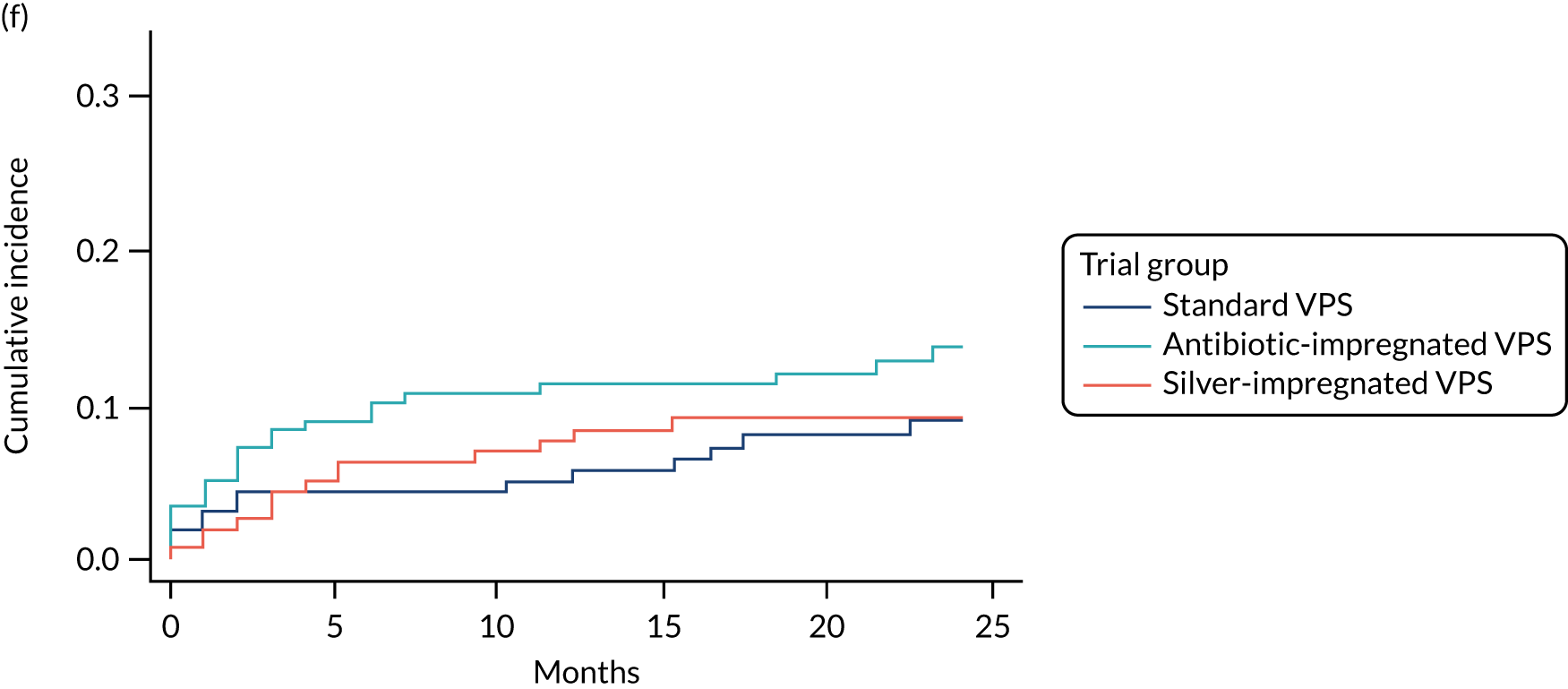
All revisions for infection were further classified by type, with the majority being classified as ‘definite: culture positive’ across all arms (standard VPS arm: 22/32, 68.8%; antibiotic-impregnated VPS arm: 6/12, 50%; silver-impregnated VPS arm: 25/31, 80.6%).
Secondary outcomes
Secondary outcome 1: time to removal of first ventriculoperitoneal shunt due to suspected infection as assessed by treating surgeon
This secondary outcome, time to removal of first VPS due to suspected infection, complemented the primary outcome of revision for infection classified by central review by defining revision for infection according to the treating surgeon, as reported on the CRFs.
Of the total number of revisions, 78 (4.9%) were classified as infections by the treating surgeon. As with the primary outcome, when revisions were centrally classified, the infection rate was approximately equal in the standard and silver-impregnated VPS arms (6.2% and 5.7%, respectively), and was lowest in the antibiotic-impregnated arm (2.8%) (Table 13).
| Reason for revision | Trial group, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | ||
| VPS removal/revision (for any cause) | 130 (100) | 132 (100) | 136 (100) | 398 (100) |
| Suspected infection | 33 (6.2) | 15 (2.8) | 30 (5.7) | 78 (4.9) |
| Revision for other reason (no infection) | 97 (18.2) | 117 (21.9) | 106 (20.2) | 320 (20.1) |
When compared with the standard VPS, antibiotic-impregnated VPSs decreased the risk of infection (csHR 0.45, 97.5% CI 0.23 to 0.91; p = 0.01) (Table 14). Silver-impregnated VPSs were comparable to standard VPSs (csHR 0.93, 97.5% CI 0.53 to 1.64; p = 0.77) (see Table 14). Appendix 2, Figure 9, displays the cumulative incidences of infection and no infection by VPS and age group.
| Covariate | Infection, HR (97.5% CI); p-value | Competing risk, HR (97.5% CI); p-value |
|---|---|---|
| Cox: csHR | ||
| VPS | ||
| Standard | – | – |
| Antibiotic impregnated | 0.45a (0.23 to 0.91); 0.01 | 1.20b (0.88 to 1.63); 0.19 |
| Silver impregnated | 0.93a (0.53 to 1.64); 0.77 | 1.13b (0.82 to 1.54); 0.39 |
| Age group | ||
| Paediatric | – | – |
| Adult (≤ 65 years) | 0.51a (0.29 to 0.91); < 0.01 | 0.59b (0.44 to 0.79); < 0.01 |
| Adult (> 65 years) | 0.11a (0.04 to 0.31); < 0.01 | 0.29b (0.20 to 0.41); < 0.01 |
| Fine–Gray: sHR | ||
| VPS | ||
| Standard | – | – |
| Antibiotic-impregnated | 0.45c (0.23 to 0.91); 0.01 | 1.24d (0.92 to 1.68); 0.11 |
| Silver-impregnated | 0.92c (0.53 to 1.61); 0.74 | 1.13d (0.83 to 1.54); 0.38 |
| Age group | ||
| Paediatric | – | – |
| Adult (≤ 65 years) | 0.53c (0.30 to 0.93); 0.01 | 0.61d (0.46 to 0.81); < 0.01 |
| Adult (> 65 years) | 0.12c (0.04 to 0.33); < 0.01 | 0.31d (0.21 to 0.44); < 0.01 |
Secondary outcome 2: time to ventriculoperitoneal shunt failure due to any cause
The overall revision rate was 25.0% (398/1594), which was approximately equal between each of the three VPS groups (standard VPS arm: 130/533, 24.4%; antibiotic-impregnated VPS arm: 132/535, 24.7%; silver-impregnated VPS arm: 136/526, 25.9%) (see Table 11).
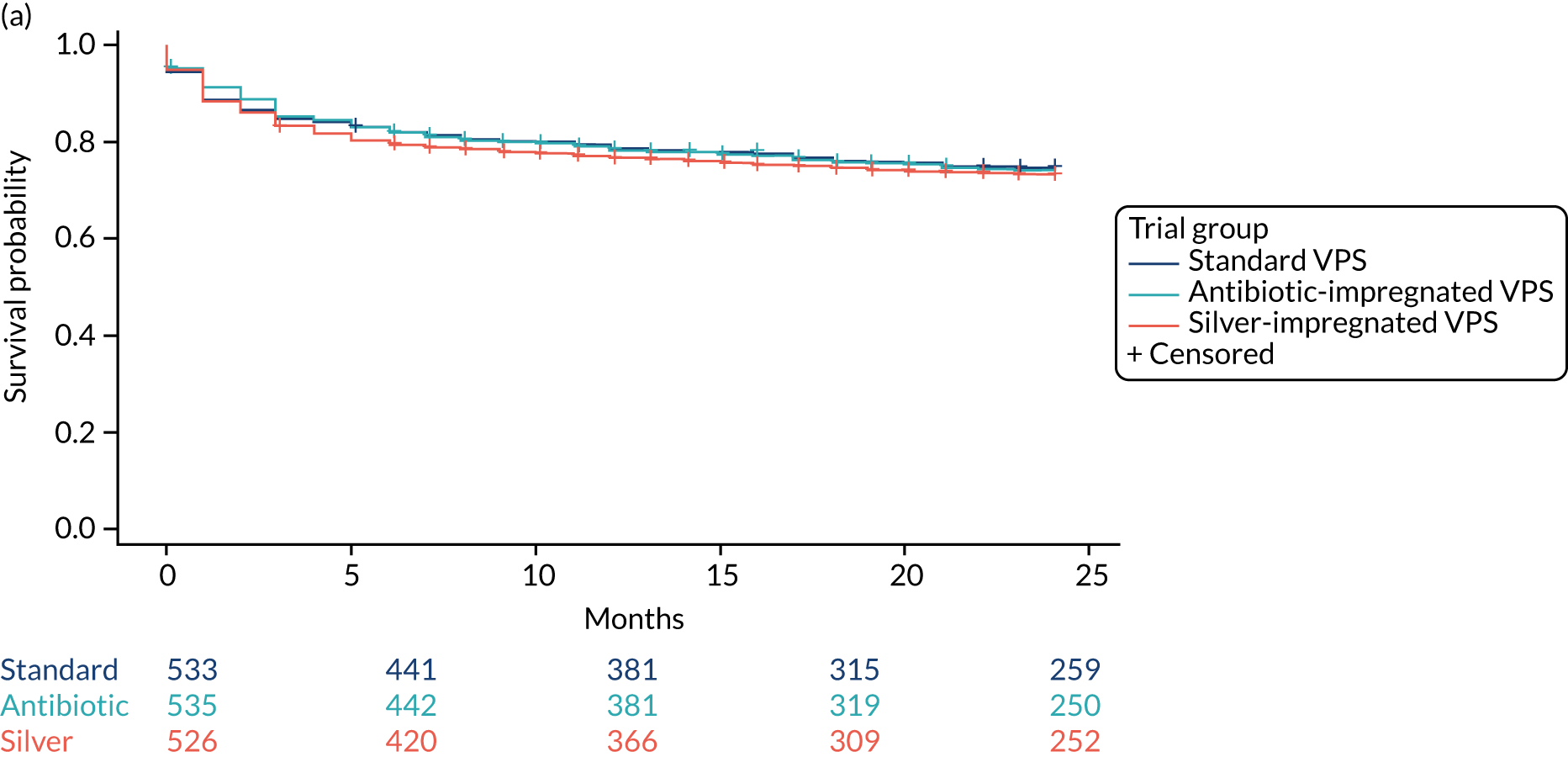
There was no significant difference for time to failure between the antibiotic-impregnated or silver-impregnated VPS arms when compared with the standard VPS arm (Table 15). Figure 5 shows the Kaplan-Meier curve for time to VPS failure for any cause, split by VPS and age group.
| Covariate | Revision, HR (97.5% CI); p-value |
|---|---|
| VPS | |
| Standard | – |
| Antibiotic impregnated | 1.01 (0.77 to 1.33); 0.94 |
| Silver impregnated | 1.08 (0.82 to 1.42); 0.54 |
| Age group | |
| Paediatric | – |
| Adult (≤ 65 years) | 0.57 (0.44 to 0.74); < 0.01 |
| Adult (> 65 years) | 0.25 (0.18 to 0.35); < 0.01 |
FIGURE 5.
Kaplan–Meier curves for time to VPS failure for any cause, split by (a) VPS group and (b) age group.
Secondary outcome 3: reason for shunt failure
At the time of revision, the treating surgeon recorded the reason for shunt failure on the CRFs. The reason for shunt failure could fall into one of four categories: suspected shunt infection, mechanical shunt failure, functional shunt failure and failure due to the patient. The outcome explored the reasons for shunt failure in the antibiotic-impregnated and silver-impregnated VPS arms, compared with the standard VPS arm.
The reasons for shunt failure, according to VPS type, are summarised in Table 16. Although the number of revisions across the VPS groups is similar, comparing the reasons within group indicates:
-
Failures due to suspected shunt infections are lower in the antibiotic-impregnated group (n = 15/132, 11.4%) than in the standard (n = 33/130, 25.4%) or the silver-impregnated VPS groups (n = 30/136, 22.1%).
-
Mechanical shunt failures are higher in the antibiotic-impregnated VPS group (n = 69/132, 52.3%) than in the standard (n = 52/130, 43.0%) or the silver-impregnated VPS groups (n = 64/136, 47.1%).
| Comparator | Reason for VPS failure, n observed failures (row %,a column %b) | Total (n) | Chi-squared test results | ||||
|---|---|---|---|---|---|---|---|
| Suspected infection | Mechanical shunt failure | Functional shunt failure | Failure due to patient | Test | Result | ||
| Antibiotic-impregnated vs. standard VPS | |||||||
| Standard | 33 (25.4, 68.8) | 52 (40.0, 43.0) | 40 (30.8, 47.6) | 5 (3.8, 55.6) | 130 | Value | 9.4 |
| Antibiotic-impregnated | 15 (11.4, 31.3) | 69 (52.3, 57.0) | 44 (33.3, 52.4) | 4 (3.1, 44.4) | 132 | Degrees of freedom | 3 |
| Total (n) | 48 | 121 | 84 | 9 | 262 | p-value | 0.02 |
| Silver-impregnated vs. standard VPS | |||||||
| Standard | 33 (25.4, 52.4) | 52 (40.0, 44.8) | 40 (30.8, 51.9) | 5 (3.8, 50.0) | 130 | Value | 1.4 |
| Silver-impregnated | 30 (22.1, 47.6) | 64 (47.1, 55.2) | 37 (27.2, 48.1) | 5 (3.7, 50.0) | 136 | Degrees of freedom | 3 |
| Total (n) | 63 | 116 | 77 | 10 | 266 | p-value | 0.71 |
These results indicate that, although the number of VPS failures is similar between the three groups, the underlying reason for failure differs when comparing standard with antibiotic-impregnated VPSs (p = 0.02), with fewer infections with antibiotic-impregnated VPSs, but a higher frequency of failure for other causes.
Secondary outcome 4: types of bacterial infection
The proportion of ‘definite – culture positive’ infections was 68.8% in the standard VPS group, 50.0% in the antibiotic-impregnated VPS group and 80.6% in the silver-impregnated VPS group. The central review panel classified all shunt infections that were ‘definite – culture positive’ and ‘probable – culture uncertain’ (n = 56/75; see Table 11) by the organism that was cultured.
The organisms cultured are summarised by species in Tables 17 and 18. Coagulase-negative staphylococci (37.5%) and Staphylococcus aureus (30%) accounted for the majority of cultured organisms in ‘all VPS infection’ but not in the antibiotic-impregnated VPS group. Culture results show a reduction in staphylococcal/Gram-positive infections for the antibiotic-impregnated VPS group, compared with the standard and the silver-impregnated VPS groups. All three VPS types have a similar number of Gram-negative infections.
| Summary of Gram-positive organisms cultured | Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | Total |
|---|---|---|---|---|
| Total number of infectionsa | 23b | 6 | 27c | 56 |
| Gram-positive organisms isolated (n) | 20 | 2 | 23 | 45 |
| Gram-positive organism cultured, n (%)d | ||||
| Staphylococcus aureus | 6 (26.1) | 0 (0.0) | 11 (40.7) | 17 (30.4) |
| Coagulase-negative staphylococci | ||||
| Coagulase-negative staphylococci, species not given | 5 (21.7) | 1 (16.7) | 3 (11.1) | 9 (16.1) |
| Staphylococcus epidermidis | 4 (17.4) | 0 (0.0) | 3 (11.1) | 7 (12.5) |
| Staphylococcus capitas | 3 (13.0) | 0 (0.0) | 1 (3.7) | 4 (7.1) |
| Staphylococcus hominis | 1 (4.3) | 0 (0.0) | 0 (0.0) | 1 (1.8) |
| Other Gram-positive organisms | ||||
| Enterococcus faecalis | 0 (0.0) | 0 (0.0) | 2 (7.4) | 2 (3.6) |
| Propionibacterium acnes | 0 (0.0) | 0 (0.0) | 2 (7.4) | 2 (3.6) |
| Propionibacterium species | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (1.8) |
| Streptococcus mitis | 0 (0.0) | 0 (0.0) | 1 (3.7) | 1 (1.8) |
| Streptococcus salivaris | 1 (4.3) | 0 (0.0) | 0 (0.0) | 1 (1.8) |
| Summary of Gram-negative organisms cultured | Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | Total |
|---|---|---|---|---|
| Total number of infectionsa | 23b | 6 | 27c | 56 |
| Gram-negative organisms isolated (n) | 6 | 4 | 5 | 15 |
| Gram-negative organisms cultured, n (%)d | ||||
| Enterobacteriaceae | ||||
| Enterobacter cloacae | 0 (0.0) | 1 (16.7) | 2 (7.4) | 3 (5.4) |
| Escherichia coli | 0 (0.0) | 1 (16.7) | 2 (7.4) | 3 (5.4) |
| Klebsiella pneumoniae | 3 (13.0) | 0 (0.0) | 0 (0.0) | 3 (5.4) |
| Citrobacter species | 0 (0.0) | 0 (0.0) | 1 (3.7) | 1 (1.8) |
| Serratia marcescens | 1 (4.3) | 0 (0.0) | 0 (0.0) | 1 (1.8) |
| Serratia species | 1 (4.3) | 0 (0.0) | 0 (0.0) | 1 (1.8) |
| Proteus mirabilis | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (1.8) |
| Pseudomonas aeruginosa | 1 (4.3) | 1 (16.7) | 0 (0.0) | 2 (3.6) |
Line listings of details of each infection against their organism cultured and antibiotic sensitivities are provided in Appendix 2, Table 38. Antibiotic sensitivity data were not consistently returned, so displayed data are limited.
Secondary outcome 5: time to removal of ventriculoperitoneal shunt because of suspected infection
Following first clean revision
This outcome explored revisions for infections in patients who had their first VPS revised for a reason other than infection (clean revision), that is those with a competing risk in the primary outcome set (n = 323; see Table 11). Participants in this group, who subsequently had a second revision, had their data centrally assessed by the panel and had the reason for revision classified as infection, or no infection, based on the data available. As with the primary outcome, for which there was insufficient information for the central panel to classify, the clinical classification was used (n = 4/128; Table 19).
| Second VPS revisions | Standard VPS, n (%) | Antibiotic-impregnated VPS, n (%) | Silver-impregnated VPS, n (%) | Total, n (%) |
|---|---|---|---|---|
| Summary of revisions | ||||
| Eligible for primary outcomea | 98 (100) | 120 (100) | 105 (100) | 323 (100) |
| No VPS removal/revision | 61 (62.2) | 69 (57.5) | 65 (61.9) | 195 (60.4) |
| VPS removal/revision (for any cause) | 37 (37.8) | 51 (42.5) | 40 (38.1) | 128 (39.6) |
| Reason for revisions as classified by central review | ||||
| Revision for infection | 9 (9.2) | 6 (5.0) | 5 (4.8) | 20 (6.2) |
| Revision for other reason (no infection) | 28 (28.6) | 45 (37.5) | 35 (33.3) | 108 (33.4) |
| VPS CSF or peritoneal infection | ||||
| Definite – culture positive | 7 (18.9) | 3 (5.9) | 5 (12.5) | 15 (11.7) |
| Probable – culture uncertain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Probable – culture negative | 1 (2.7) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Possible – culture uncertain | 1 (2.7) | 2 (3.9) | 0 (0.0) | 3 (2.3) |
| Clinically classified infectionb | 0 (0.0) | 1 (2.0) | 0 (0.0) | 1 (0.8) |
| VPS deep incisional infection | ||||
| VPS deep incisional infection | 0 (0.0) | 0 (0.0) | 1 (2.5) | 1 (0.8) |
There were 128 secondary revisions following a first clean revision, of which 20 were classified as an infection (6.2%; see Table 19). The infection rate was approximately equal in the antibiotic-impregnated and silver-impregnated VPS groups (5.0% and 4.8%, respectively), and was higher in the standard VPS group (9.2%). However, these differences were not statistically significant when survival models were applied to the data (Table 20).
| Covariate | Infection, HR (97.5% CI); p-value | Competing risk, HR (97.5% CI); p-value |
|---|---|---|
| Cox: csHR | ||
| VPS | ||
| Standard | – | – |
| Antibiotic-impregnated | 0.55a (0.17 to 1.81); 0.26 | 1.38b (0.80 to 2.36); 0.19 |
| Silver-impregnated | 0.47a (0.13 to 1.63); 0.17 | 1.11b (0.63 to 1.97); 0.67 |
| Age group | ||
| Paediatric | – | – |
| Adult (≤ 65 years) | 1.64a (0.58 to 4.61); 0.28 | 0.80b (0.49 to 1.30); 0.30 |
| Adult (> 65 years) | 0.34a (0.03 to 3.64); 0.14 | 0.43b (0.19 to 0.95); 0.10 |
| Fine-Gray: sHR | ||
| VPS | ||
| Standard | – | – |
| Antibiotic-impregnated | 0.55c (0.17 to 1.75); 0.25 | 1.40d (0.83 to 2.37); 0.16 |
| Silver-impregnated | 0.48c (0.14 to 1.67); 0.19 | 1.14d (0.65 to 1.99); 0.61 |
| Age group | ||
| Paediatric | – | – |
| Adult (≤ 65 years) | 1.72c (0.62 to 4.81); 0.24 | 0.80d (0.50 to 1.28); 0.29 |
| Adult (> 65 years) | 0.38c (0.04 to 3.91); 0.14 | 0.44d (0.20 to 0.97); 0.11 |
Seventy-five per cent of the secondary revisions for infection were classified by the committee as ‘definite – culture positive’ (n = 15/20).
Additional analysis
Comparing the identification of infections between assessors
The reason for revision (infection or no infection), as classified by the central panel, was the primary outcome (see Primary outcome: time to ventriculoperitoneal infection as assessed by the central review panel), and the reason for revision, as classified by the treating surgeon, was a complementary secondary outcome (see Secondary outcome 1: time to removal of first ventriculoperitoneal shunt due to suspected infection, as assessed by the treating surgeon). The classification made by these two independent assessors was the same in 95.7% (381/398) of revisions. Appendix 2, Table 39, provides further detail.
Revision and infection rates by age group
The proportion of revisions of first VPS for any cause ranged from 38.0% (n = 225/592) for paediatrics to 10.9% (n = 55/503) for those aged > 65 years. The proportion of infections, as classified by the central review panel, was also higher for paediatrics than for older participants. Table 21 provides more detail.
| Summary of revisions | Age group | Total | ||
|---|---|---|---|---|
| Paediatric | ≤ 65 years | > 65 years | ||
| Eligible for primary outcome | 592 (100) | 499 (100) | 503 (100) | 1594 (100) |
| No VPS removal/revision | 367 (62.0) | 381 (76.4) | 448 (89.1) | 1196 (74.5) |
| Revision for other reason (no infection) | 178 (30.1) | 95 (19.0) | 50 (9.9) | 323 (20.3) |
| Revision for infection | 47 (7.9) | 23 (4.6) | 5 (1.0) | 75 (4.7) |
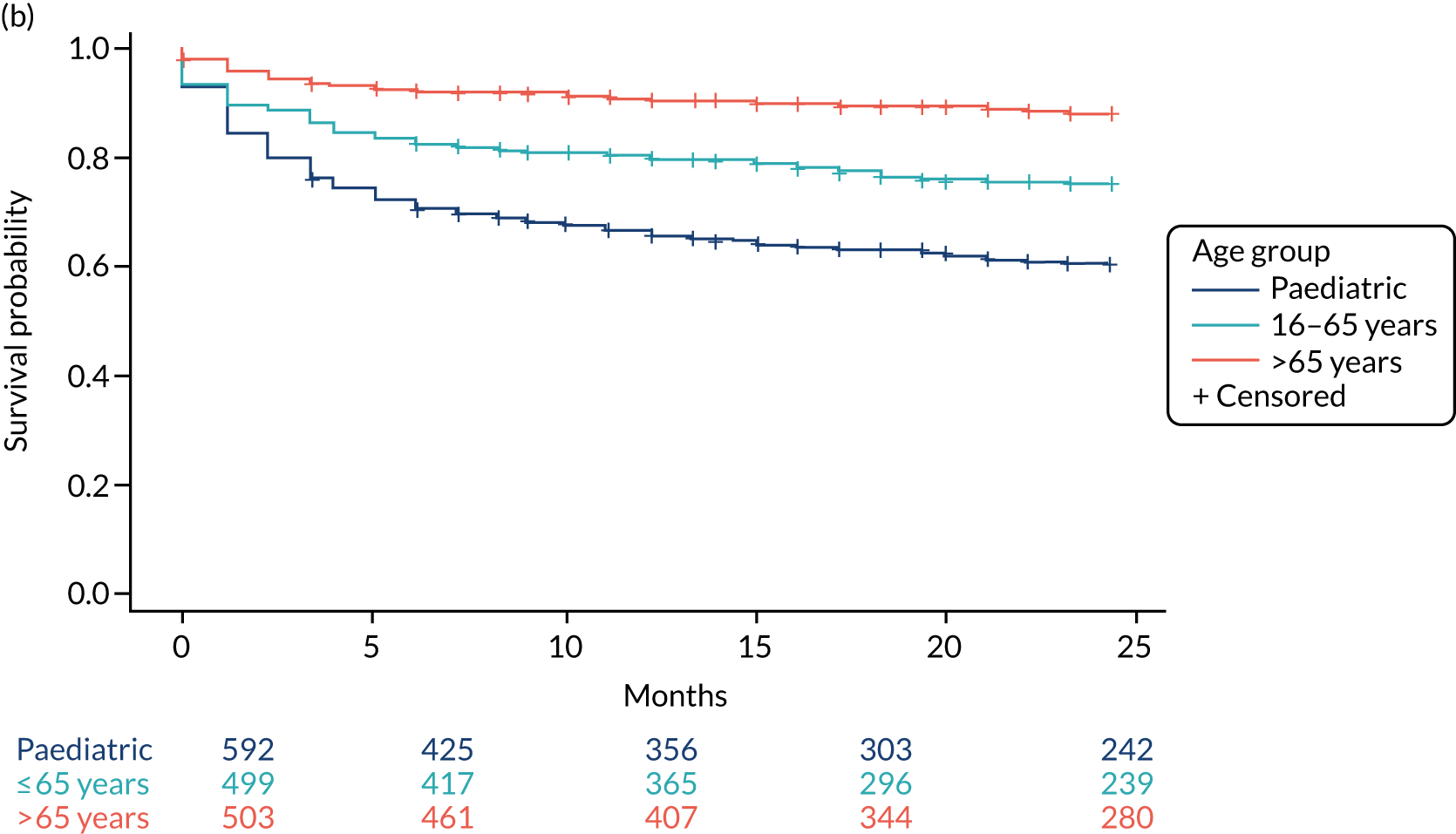
All survival models were adjusted for age category of the recruiting centre (paediatric or adult), with adult centre being further categorised by age > 65 years. The risk of infection was significantly lower for participants aged 16–65 years (csHR 0.56, 97.5% CI 0.32 to 0.99; p = 0.02) and for those aged > 65 years (csHR 0.12, 97.5% CI 0.04 to 0.35; p < 0.01) than for paediatric participants (see Table 12). See Figure 3 for the cumulative incidence of infection by age category; see Figure 4 for the cumulative incidence of infection by VPS type, stratified by age group.
Revision and infection rates by centre
Heterogeneity between centres in revision rates and infection rates was explored by summary statistics.
Revision rates varied from a minimum of 4.8% (97.5% CI 0.0% to 15.2%, adult-only centre) to a maximum of 75.0% (97.5% CI 40.7% to 100.0%, paediatric-only centre), as presented in Appendix 2, Table 41. Infection rates, presented in Appendix 2, Table 41, varied from 0.0% (97.5% CI 0.0% to 0.0%, adult-only centre) to 25.0% (97.5% CI 0.0% to 59.3%, paediatric-only centre). Paediatric-only centres generally had higher rates of revisions and infections than adult-only centres and centres that treated both adults and paediatrics.
Post hoc analyses
A post hoc analysis explored revision rates, and reason for revision, by aetiology of the hydrocephalus, type of valve, operative approach and component replaced at first revision.
Revision and infection rates by aetiology
Table 22 and Appendix 2, Table 42, summarise aetiologies of the hydrocephalus, overall and split by treatment group, respectively. The rates of revision for infection and mechanical failure varied within certain aetiologies. For example, the revision and infection rates for participants with congenital malformations was 38.4% and 9.2%, respectively, both higher than the equivalent overall rates of 25.0% and 4.7% (see Table 11). Similarly, participants with idiopathic normal pressure hydrocephalus had much lower revision and infection rates of 10.0% and 1.1%, respectively, than the rates for patients with other aetiologies of hydrocephalus.
| Summary of aetiology | Clean insertion,a n (%) | Revision required | Reason for revision | |||||
|---|---|---|---|---|---|---|---|---|
| No revision, n (%) | Revision,b n (%) | Failure due to patient, n (%) | Functional shunt failure, n (%) | Mechanical shunt failure, n (%) | Failure due to infection, n (%) | Failure – no infection,c n (%) | ||
| Total number of patients | 1594 | 1196 | 398 | 14 | 121 | 185 | 78 | 320 |
| Congenital malformations | ||||||||
| Patients with congenital malformations | 294 (18.4) | 181 (61.6) | 113 (38.4) | 2 (0.7) | 36 (12.2) | 48 (16.3) | 27 (9.2) | 86 (29.3) |
| Type of congenital malformation | ||||||||
| Aqueduct stenosis | 68 (23.1) | 46 (67.6) | 22 (32.4) | 0 (0.0) | 8 (11.8) | 7 (10.3) | 7 (10.3) | 15 (22.1) |
| Dandy–Walker | 7 (2.4) | 6 (85.7) | 1 (14.3) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 1 (14.3) |
| Chiari | 62 (21.1) | 43 (69.4) | 19 (30.6) | 0 (0.0) | 7 (11.3) | 7 (11.3) | 5 (8.1) | 14 (22.6) |
| Spina bifida | 111 (37.8) | 62 (55.9) | 49 (44.1) | 1 (0.9) | 11 (9.9) | 25 (22.5) | 12 (10.8) | 37 (33.3) |
| Other | 72 (24.5) | 38 (52.8) | 34 (47.2) | 1 (1.4) | 12 (16.7) | 13 (18.1) | 8 (11.1) | 26 (36.1) |
| Not known | 1 (0.3) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Acquired hydrocephalus | ||||||||
| Patients with acquired hydrocephalus | 819 (51.4) | 615 (75.1) | 204 (24.9) | 9 (1.1) | 54 (6.6) | 102 (12.5) | 39 (4.8) | 165 (20.1) |
| Type(s) of acquired hydrocephalus | ||||||||
| Cysts (colloid or arachoid) | 32 (3.9) | 24 (75.0) | 8 (25.0) | 0 (0.0) | 4 (12.5) | 3 (9.4) | 1 (3.1) | 7 (21.9) |
| Trauma | 30 (3.7) | 25 (83.3) | 5 (16.7) | 1 (3.3) | 1 (3.3) | 3 (10.0) | 0 (0.0) | 5 (16.7) |
| Tumour: benign | 124 (15.1) | 96 (77.4) | 28 (22.6) | 2 (1.6) | 9 (7.3) | 14 (11.3) | 3 (2.4) | 25 (20.2) |
| Tumour: malignant | 133 (16.2) | 105 (78.9) | 28 (21.1) | 2 (1.5) | 6 (4.5) | 14 (10.5) | 6 (4.5) | 22 (16.5) |
| Post haemorrhagic/intracranial haemorrhage | 337 (41.1) | 244 (72.4) | 93 (27.6) | 2 (0.6) | 21 (6.2) | 45 (13.4) | 25 (7.4) | 68 (20.2) |
| Infection: meningitis | 32 (3.9) | 23 (71.9) | 9 (28.1) | 0 (0.0) | 2 (6.3) | 5 (15.6) | 2 (6.3) | 7 (21.9) |
| Infection: cerebral abscess | 8 (1.0) | 7 (87.5) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (12.5) |
| Infection: other | 21 (2.6) | 17 (81.0) | 4 (19.0) | 0 (0.0) | 0 (0.0) | 4 (19.0) | 0 (0.0) | 4 (19.0) |
| Other factors | 140 (17.1) | 99 (70.7) | 41 (29.3) | 3 (2.1) | 16 (11.4) | 17 (12.1) | 5 (3.6) | 36 (25.7) |
| Idiopathic condition | ||||||||
| Patients with idiopathic condition | 496 (31.1) | 408 (82.3) | 88 (17.7) | 4 (0.8) | 32 (6.5) | 38 (7.7) | 14 (2.8) | 74 (14.9) |
| Type(s) of idiopathic condition | ||||||||
| Idiopathic normal pressure hydrocephalus of the elderly | 361 (72.8) | 325 (90.0) | 36 (10.0) | 1 (0.3) | 15 (4.2) | 16 (4.4) | 4 (1.1) | 32 (8.9) |
| IIH | 98 (19.8) | 63 (64.3) | 35 (35.7) | 2 (2.0) | 10 (10.2) | 16 (16.3) | 7 (7.1) | 28 (28.6) |
| Other | 38 (7.7) | 20 (52.6) | 18 (47.4) | 1 (2.6) | 7 (18.4) | 7 (18.4) | 3 (7.9) | 15 (39.5) |
Rates between VPS groups also differed according to aetiology. For example, the infection rate in participants with spina bifida in the antibiotic-impregnated VPS group was 2.9%, compared with 14.3% each for the standard and silver-impregnated VPS groups. The infection rate in participants with idiopathic normal pressure hydrocephalus was 13.6% in the standard VPS group and 2.9% in the antibiotic-impregnated VPS group.
Revision and infection rates by operative approach
Table 23 and Appendix 2, Table 43, summarise valve type and operative approach at insertion, both overall and split by treatment group.
| Summary of operative approach/valve type | Clean insertion,a n (%) | Revision required | Reason for revision | |||||
|---|---|---|---|---|---|---|---|---|
| No revision, n (%) | Revision,b n (%) | Failure due to patient, n (%) | Functional shunt failure, n (%) | Mechanical shunt failure, n (%) | Failure for infection, n (%) | Failure – no infection,c n (%) | ||
| Total number of patients | 1594 | 1196 | 398 | 14 | 121 | 185 | 78 | 320 |
| Use of guidance system | ||||||||
| Patients for whom guidance system was used for VPS placement | 653 (41.0) | 489 (74.9) | 164 (25.1) | 7 (1.1) | 45 (6.9) | 78 (11.9) | 34 (5.2) | 130 (19.9) |
| Type of guidance system | ||||||||
| Electromagnetic | 413 (63.2) | 309 (74.8) | 104 (25.2) | 4 (1.0) | 26 (6.3) | 50 (12.1) | 24 (5.8) | 80 (19.4) |
| Ultrasonography | 128 (19.6) | 88 (68.8) | 40 (31.3) | 3 (2.3) | 16 (12.5) | 14 (10.9) | 7 (5.5) | 33 (25.8) |
| Optical | 46 (7.0) | 37 (80.4) | 9 (19.6) | 0 (0.0) | 2 (4.3) | 6 (13.0) | 1 (2.2) | 8 (17.4) |
| Stereotactic frame | 63 (9.6) | 52 (82.5) | 11 (17.5) | 0 (0.0) | 1 (1.6) | 8 (12.7) | 2 (3.2) | 9 (14.3) |
| Not known | 3 (0.5) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Placement of proximal shunt catheter | ||||||||
| Frontal | 134 (8.4) | 93 (69.4) | 41 (30.6) | 0 (0.0) | 13 (9.7) | 15 (11.2) | 13 (9.7) | 28 (20.9) |
| Parietal, occipital or parietal/occipital | 1453 (91.2) | 1100 (75.7) | 353 (24.3) | 14 (1.0) | 107 (7.4) | 167 (11.5) | 65 (4.5) | 288 (19.8) |
| Combination | 3 (0.2) | 2 (66.7) | 1 (33.3) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 1 (33.3) |
| Not known | 4 (0.3) | 1 (25.0) | 3 (75.0) | 0 (0.0) | 0 (0.0) | 3 (75.0) | 0 (0.0) | 3 (75.0) |
| Type of valve | ||||||||
| Fixed | 935 (58.7) | 652 (69.7) | 283 (30.3) | 10 (1.1) | 91 (9.7) | 126 (13.5) | 56 (6.0) | 227 (24.3) |
| Programmable | 627 (39.3) | 525 (83.7) | 102 (16.3) | 4 (0.6) | 26 (4.1) | 52 (8.3) | 20 (3.2) | 82 (13.1) |
| Not known | 32 (2.0) | 19 (59.4) | 13 (40.6) | 0 (0.0) | 4 (12.5) | 7 (21.9) | 2 (6.3) | 11 (34.4) |
The rate of revisions when there was no evidence of infection (the competing risk in the primary outcome analysis) was > 10% lower among participants with a programmable valve (n = 82/627, 13.1%) than among those with a fixed valve (n = 227/935, 24.3%). On the other hand, revision rates for no infection were equivalent when comparing frontal placement of proximal shunt catheter with parietal and/or occipital placement: 20.9% and 19.8%, respectively.
Revision and infection rates by component replaced at first revision
Table 24 and Appendix 2, Table 44, summarise the component replaced in participants who had their VPS revised for reason other than infection, as classified by the treating surgeon.
| Summary of shunt components replaced | Failure – no infection,a n (%) | Reason for revision for no infection | ||
|---|---|---|---|---|
| Failure due to patient, n (%) | Functional shunt failure, n (%) | Mechanical shunt failure, n (%) | ||
| Total number of patients | 320 | 14 | 121 | 185 |
| Was a complete new shunt inserted? | ||||
| No | 268 (83.8) | 12 (4.5) | 99 (36.9) | 157 (58.6) |
| If no, which component was replaced? | ||||
| Ventricular shunt catheter only | 72 (26.9) | 0 (0.0) | 22 (30.6) | 50 (69.4) |
| Peritoneal shunt catheter only | 31 (11.6) | 3 (9.7) | 5 (16.1) | 23 (74.2) |
| Valve only | 76 (28.4) | 3 (3.9) | 35 (46.1) | 38 (50.0) |
| Combination | 38 (14.2) | 1 (2.6) | 19 (50.0) | 18 (47.4) |
| Not known | 51 (19.0) | 5 (9.8) | 18 (35.3) | 28 (54.9) |
Most commonly, when a shunt is revised for a reason other than infection, the component replaced is the ventricular shunt catheter (n = 72/268, 26.9%) or the valve (n = 76/268, 28.4%). The component replaced was similar between VPS groups, although valve changes were more common in the antibiotic-impregnated VPS group than in the standard or silver-impregnated VPS groups.
Safety analysis
Efficacy outcomes were analysed by the intention-to-treat analysis population as much as possible. For AEs and SAEs, participants are reported according to the type of VPS in situ at the time of the event. The shunt in situ was known for all patients up to the first revision. However, events occurring following a revision whereby the shunt was not replaced like for like are reported in under ‘other VPS’ group.
The total number of AEs experienced and the number of participants experiencing at least one AE are provided, both overall and split according to the VPS in situ at the time of the event. Of the 1601 participants who received a trial shunt, 413 (25.8%) experienced 654 events. Summarising these events, split by VPS in situ at the time of event, indicates the following:
-
Standard VPS – 135 out of 531 participants (25.4%) experienced 201 events.
-
Antibiotic-impregnated VPS – 136 out of 545 participants (25.0%) experienced 210 events.
-
Silver-impregnated VPS – 140 out of 525 participants (26.7%) experienced 191 events.
-
Other VPS – when the initial trial shunt was removed and not replaced like for like, 35 out of 136 participants (25.7%) experienced 52 events.
Common events were ventricular shunt catheter obstruction (96 events in 79 participants), shunt valve obstruction (65 events in 52 participants) and valve change for symptomatic overdrainage (54 events in 50 participants).
Appendix 2, Table 45, provides the summary of related AEs.
No participant experienced a SAE.
Chapter 4 Economic evaluation
Parts of this chapter have been reproduced from Mallucci et al. 2 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Introduction
A number of studies32–36 (although none from the UK) have estimated the costs of managing patients with VPS infections. These often combine cost estimates with observed differences in infection rates, between antibiotic-impregnated and standard VPS catheters, in rudimentary cost-effectiveness analyses, to calculate the incremental cost (saving) per VPS infection avoided. This is relevant to inform the best use of health-care resources, given that impregnated VPS catheters are about twice as expensive as standard shunt catheters, but are limited in not assessing all-cause VPS failure or impacts on patients’ health-related quality of life.
Adopting the perspective of the German health-care setting, Eymann et al. 32 estimated the costs of VPS infections by considering the lengths, and per diem costs, of adult and paediatric intensive care and ward stays. Based on 48 cases of VPS infections among a cohort of 197 hydrocephalus patients with antibiotic-impregnated VPS catheters and 98 patients with standard VPS catheters, the estimated mean cost per infection was US$16,933 in children and US$14,886 in adults. Considering the benefits and additional costs of antibiotic-impregnated shunt catheters, the authors estimated a net saving of US$51,651 for the 197 patients.
Farber et al. 33 conducted a retrospective cohort study of 500 shunt surgeries performed in adult patients with hydrocephalus in a US hospital: 250 received antibiotic-impregnated VPS catheters and 250 received standard VPS catheters. The hospital billing records of all patients who were treated were analysed to estimate the costs associated with hospital stays for infections. The overall mean cost per VPS infection was US$40,371 and, based on an absolute reduction in the risk of shunt infections of 2.8% with antibiotic-impregnated shunt catheters, savings of US$47,193 in infection-related direct costs were estimated per 100 shunt surgeries performed.
Attenello et al. 34 analysed the hospital billing records of 406 paediatric patients who underwent 608 shunt placement procedures (400 antibiotic-impregnated and 208 standard shunt catheters) in a single US hospital. The majority of procedures (93%) were for VPSs. The hospital resources used to treat the 38 patients in whom a shunt infection developed were estimated to cost an average of US$48,454. Based on an observed decreased incidence of shunt infection in the antibiotic-impregnated shunt catheter cohort, the estimated infection-related cost savings per 100 patients was $442,133 per 100 patients with shunts.
Parker et al. 35,36 analysed patient discharge and billing records from 287 US hospitals to estimate the shunt infection rates and costs of antibiotic-impregnated shunt catheters compared with standard shunt catheters. The unadjusted difference in the incidence of shunt infection was 1.4% and 4.5% in adult and paediatric populations, respectively, and the costs of managing infections was US$45,714 and US$56,104, respectively. The authors estimated that the use of antibiotic-impregnated shunt catheters in adults was associated with infection-related cost savings of US$42,125 per 100 de novo shunts placed (US$230,390 per 100 in paediatrics), which corresponds to US$30,854 and US$51,181 per shunt infection avoided, respectively (calculated from data presented in the paper).
Each of the above studies relied on retrospective cohorts of patients, mainly from single centres, but result in estimates for the costs of managing VPS infections in the order of US$50,000 in the US health-care setting. A key limitation, however, is that, in each of these analyses, infection rates were determined from observational data, and were therefore potentially subject to bias, reducing the reliability of the cost-effectiveness estimates. Analyses that considered the RR of VPS infection associated with antibiotic-impregnated shunt catheters, determined from clinical trial data, are limited to three studies. 37–39
Root et al. 37 conducted a meta-analysis of clinical trials and observational studies, and estimated that the number needed to treat with antibiotic-impregnated (compared with standard) EVDs to prevent a shunt catheter-associated infection was 19 (95% CI 15 to 36). Based on assumed costs of US$100 per antibiotic-impregnated shunt catheter and US$30,000 per episode of infection, they estimated that antibiotic-impregnated shunt catheters could result in overall savings, from the perspective of a US neurosurgical unit, of US$28,100 (95% CI US$26,400 to US$28,500) for each shunt catheter-associated infection prevented.
Klimo et al. 38 similarly reviewed the literature for clinical trials and observational studies of antibiotic-impregnated shunts, and conducted a meta-analysis and a simple cost calculation. Assuming a cost of US$50,000 to treat a shunt infection, the cost savings per shunt infection prevented ranged from just under US$90,000 to > US$1.3M per 200 shunts performed. No data were presented on the incremental cost (saving) of avoiding a shunt infection.
Edwards et al. 39 developed a decision-analytic model of the clinical and economic consequences of using antibiotic-impregnated shunts from the perspective of a US hospital. Using trial and observational data, they estimated that, for every 100 patients requiring shunts, antibiotic-impregnated shunt catheters may be associated with 0.5 fewer deaths, 71 fewer hospital days, 11 fewer surgeries and a net saving of US$128,228 owing to decreased infection. The cost of a shunt infection was US$46,394, derived from earlier estimates of Attenello et al. 34 and Farber et al. 33
All analyses have many limitations, not least the assumption that potential avoidance of the cost of managing VPS infections can be equated to a cost saving. Moreover, the analyses lacked any consideration of health outcomes associated with shunt catheter-associated infections or other potential causes of VPS failure.
Aim
The aim of the economic evaluation was to assess the cost-effectiveness of antibiotic-impregnated, silver-impregnated and standard VPS catheters in children and adults with hydrocephalus who were recruited in the BASICS trial.
Methods
The economic analysis adopted the perspective of the NHS and Personal Social Services providers in the UK. The analytical approach for the primary analysis was a cost-effectiveness analysis, based on the incremental cost per first shunt failure (due to any cause) averted for impregnated and standard VPSs. A cost–utility analysis was conducted to estimate the incremental cost per QALY gained in a restricted sample of trial participants.
Resource use and costs
Within-trial costs were estimated by measuring the health-care resource use associated with each of the trial interventions during the trial period. These included (1) hospital inpatient procedures; (2) hospital outpatient and accident and emergency (A&E) visits; (3) concomitant medications; and (4) contact with other health-care professionals, including general practitioners (GPs) and school nurses.
Estimation of resource use was based on complementary approaches using data collected as part of the trial and as part of routine care. These were as follows:
-
Patient-Level Information and Costing System (PLICS). PLICS data contain details of admission and discharges, Healthcare Resource Group (HRG) codes relating to the type of care patients received, and the point of delivery (A&E, inpatient, outpatient). PLICS data were requested for all participants from 3 months prior to randomisation to the final follow-up of the last participant (April 2018).
-
Resource use questionnaires completed by trial participants, their guardian or their parents. These were designed to collect information on participants’ use of primary care services, Personal Social Services and non-scheduled clinic attendances. 40,41 Resource use questionnaires were administered early post operatively, and then posted to participants by research nurses every 12 weeks until the end of the trial. Participants completed these and returned them to the trial centre.
-
Dedicated sections in the CRF. These were used to record trial participants’ use of concomitant medications at each clinic visit and for the duration of their participation in the trial, or up until 14 days following shunt removal in cases of confirmed infection.
-
Interventions. The type of initial VPS catheter was according to a participant’s treatment allocation. Costs associated with any subsequent revisions were included in participants’ PLICS data.
Unit costs
All resource use was valued in monetary terms using appropriate UK unit costs for the 2016–17 cost year. When necessary, for any costs from an earlier period, adjustments were made for inflation using the pay cost index and the health service cost index. 42
The unit costs of shunt catheters were sourced from the manufacturers. A silver antimicrobial shunt catheter set (Silverline), consisting of ventricular and peritoneal shunt catheters, costs £361.62. A Bactiseal shunt catheter kit (ventricular and peritoneal) costs £384.00; and standard, plain Codman® Hakim® (Integra LifeSciences Holdings Corporation) ventricular or peritoneal shunt catheters each cost £172.00.
Health resource groups were used as the main currency of the economic analysis43 for inpatient stays (see Appendix 3, Table 46) and outpatient contacts (see Appendix 3, Table 47), with cost codes allocated based on the latest available national schedule of reference costs44 or, when not available, based on the national tariff. 45 Reference costs are the average unit costs of providing services to NHS patients in England, and are collected each financial year. National tariff costs relate to bundled care packages reimbursed at a national level, based on the NHS payment-by-results scheme. National average unit costs were based on the hospital spell, and incorporated excess ward days and whether the case was elective or emergency. National tariff codes were obtained primarily from PLICS data, but, if unavailable, appropriate HRG codes were assigned based on the reason for admission and a patient’s condition, extracted from the patient resource use questionnaires, which asked about participants’ contacts with health-care professionals during their time in the trial.
The compendium of Unit Costs of Health and Social Care42 was the source of unit costs of all items of primary health-care resource use and outpatient contacts (see Appendix 3, Table 48). The number of health-care professional contacts recorded in the resource use questionnaires and baseline forms were multiplied by their respective unit costs.
The unit costs of medicines were based on drug tariff prices, as referenced in the British National Formulary46 and the Prescription Costs Analysis Data47 for NHS England. The cost of each medicine was calculated by multiplying the unit price by the daily quantity of prescribed medication (e.g. number of vials, ampoules, prefilled syringes, capsules or tablets) and by the number of days of treatment. If the dispensed medication was an oral suspension, the total quantity for the prescribed period was calculated and rounded up to the nearest whole bottle.
Health outcomes
The primary health outcome for the economic analysis was the first VPS failure (due to any cause) averted. A sensitivity analysis considered the first VPS failure (due to confirmed infection) averted, consistent with the primary clinical outcome.
The secondary economic health outcome measure was the QALY, calculated from responses to EuroQol-5 Dimensions (EQ-5D) questionnaires. The EQ-5D-3L-Proxy (parent or guardian) questionnaire was used for participants aged from 5 years to just under 18 years, and for participants aged > 18 years who lacked capacity to consent for themselves. The EQ-5D-Y was administered to participants aged from 8 years to just under 18 years. Adults were asked to complete the EQ-5D-3L questionnaire, and all participants aged ≥ 8 years were administered the EQ-VAS.
The EQ-5D-3L descriptive system includes five dimensions (mobility, self-care, usual activities, pain and anxiety); each dimension has three levels of morbidity (no problems, some problems and extreme problems), which are scored 1, 2 and 3, respectively. UK tariff48 scores for the EQ-5D-3L questionnaire were applied to responses to the EQ-5D-3L, EQ-5D-Y and EQ-5D-3L-Proxy questionnaires, as no separate scoring systems are yet available for the youth and proxy versions.
Utility scores from each version of the EQ-5D questionnaires were combined to achieve the most complete data set by taking scores from trial participants, when available, and incorporating proxy responses.
In addition, the child version of the HOQ was administered to participants aged 8–18 years, and the parent proxy version was administered for participants aged from 5 years to just under 8 years. The HOQ is a Canadian 51-item outcome questionnaire designed specifically for use in paediatric hydrocephalus. 25,49 Responses to each item are given a score from 0 (worse health status) to 4 (better health status). A set combination of items make up three health dimensions: physical, socioemotional and cognitive. A final score is obtained by summing each item score and then dividing it by the highest possible summed score, which gives a utility value anchored at 0 (worse health state) or 1 (best health state).
Health outcome questionnaires were completed during clinic visits, or over the telephone at baseline (pre-operative assessment visit), at the early post-operative assessment, 12 weeks after randomisation and at the end of the trial.
Economic analyses
Analytic approach
Analyses included all randomised participants, consistent with the ‘intention to treat’ principle. All statistical tests were two-sided; the statistical significance level was set at 2.5% and CIs were calculated at 97.5% to adjust for multiplicity.
Data were examined for missingness. The appropriate method for dealing with missing cost data was dependent on the number of missing data and the likely mechanism of missingness. 50 Costs relating to hospitalisations were primarily sourced from PLICS data. If PLICS data were not available or missing, the use of hospital services was based on entries in CRFs, or otherwise from participants’ resource use questionnaires. 51 In the base-case analysis, any remaining missing data were multiply imputed using the method of chained equations. 52 The number of imputed data sets was based on the fraction of missing information (FMI) value to limit the loss in power to no more than 1%, and to maximise model convergence. Imputed data sets were generated using predictive mean matching, from a set of imputation models that were constructed from all potential prognostic factors: sex, age (paediatrics aged from 0 to just under 16 years, adults aged 16–65 years and adults aged > 65 years), site, time spent in the trial, whether or not a first treatment failure had occurred and intervention group.
In the base-case analysis, costs and outcomes incurred in the second year were discounted at a rate of 3.5%, in accordance with the NICE Guide to the Methods of Technology Appraisal 2013. 53
Cost analysis
Hospitalisations were costed from baseline to 24 months. Hospital admissions were included if the hospital episode start date commenced within the 0- to 24-month time horizon. Adjustments were made to apportion the costs of hospital stays that crossed baseline or that continued after the 24-month time horizon. Similarly, adjustments were made to courses of drug treatment that spanned the period preceding baseline or beyond the 24-month time horizon, to apportion costs to those administered during the 0- to 24-month time horizon only.
Participants’ use of health care and Personal Social Services between randomised groups was described and tabulated, reporting mean resource use items for each intervention and differences between the intervention groups. The 97.5% CIs for differences in mean costs were calculated using bias-corrected and accelerated non-parametric bootstrap with 10,000 replications.
Total costs were analysed using a regression model to account for any imbalance in participants’ characteristics between intervention groups, and to estimate the mean cost of VPS failure. Owing to the large sample, the near-normality of sample means was assumed and ordinary least squares regression was applied in the base case. 54 The regression was specified with total (discounted) per-patient costs as the dependent variable, and the stratifying variables [randomisation group, site (discrete), age (three categories), time in the trial (continuous, in days) and treatment failure] as predictors:
Similarly, the mean outcome by intervention group was also calculated by ordinary least squares regression, specified with treatment failure (discounted) as the dependent variable, and total cost (discounted), site (discrete), age (three categories), time in the trial (continuous) and intervention group as predictors:
Cost-effectiveness analysis
In the base-case cost-effectiveness analysis, the outcome of interest was the incremental cost per (first) VPS failure (due to any cause) averted. Interventions were ranked according to their effectiveness (reverse order for interventions in the south-west quadrant of the cost-effectiveness plane). Dominated and extendedly dominated interventions were removed, and the incremental cost-effectiveness ratios (ICERs) were calculated for the remaining shunt catheters.
Sensitivity and scenario analyses
A number of sensitivity analyses were performed to assess the robustness of the base-case ICER to key assumptions and analytic approaches. These were (1) bivariate sensitivity analyses to vary the unit costs of antibiotic-impregnated and silver-impregnated VPSs, (2) applying different discount rates (0%, 1.5% and 6% per annum for both costs and outcomes), (3) using observed data for costs (no multiple imputation) and (4) using a different analytic approach for analysing costs [generalised linear models (GLMs), acknowledging the skewness in the underlying data]. The GLM regression was specified using a combination of families (gamma, Gaussian and Poisson) and links (log and square root). Appropriate link function was determined using the Akaike information criterion (AIC) and Bayesian information criterion (BIC) and the modified Park test to determine the distribution family. 55
In addition, a stratified cost-effectiveness analysis was undertaken for the three age categories of paediatrics, adults aged 16–65 years and adults aged ≥ 65 years.
Alternative cost-effectiveness and utility analysis
Additional cost-effectiveness analyses were conducted based on the incremental cost per averted case of first shunt failure due to (1) confirmed infection, (2) a mechanical cause, (3) a functional reason and (4) patient factors. A cost–utility analysis was performed to estimate the incremental cost per QALY gained; this analysis was restricted to participants aged ≥ 5 years, as no utility data were collected for children aged < 5 years. Uncertainty in the incremental cost–utility ratio was considered using non-parametric bootstrap analysis, using 1000 replicates, and depicted in cost-effectiveness acceptability curves, which present the probability of each VPS catheter being cost-effective for given ceiling thresholds of costs per QALY. 56 The cost–utility analysis considered the reference threshold range of between £20,000 and £30,000 per QALY. 53
All analyses were conducted using Stata® version 13 (StataCorp LP, College Station, TX, USA) and reported according to the Consolidated Health Economic Evaluation Reporting Standards. 57
Results
Data completeness
The PLICS data were provided by 10 out of the 21 neurosurgical units. These related to 199 out of 536 participants allocated to the standard VPS, 207 out of 538 participants allocated to the antibiotic-impregnated VPS and 210 out of 531 participants allocated to the silver-impregnated VPS (Table 25). The reasons given by the 11 centres for not providing PLICS data included:
-
They were short staffed and were unable to assign resources to pull the data together.
-
One centre was procuring a new PLICS system and were unable to supply any patient-level information.
-
Finance officers were unwilling to provide data; others agreed to provide data but they did not deliver by the deadline.
-
Some trusts did not want to share the data or asked for data-sharing agreements, but these could not be arranged within the project timelines.
| Trial group | Variable | Participants (n) | |||||
|---|---|---|---|---|---|---|---|
| Aged ≥ 5 years (N = 1098) | All trial (N = 1594) | ||||||
| Complete | Incomplete (imputed) | Total | Complete | Incomplete (imputed) | Total | ||
| Standard VPS | Utility at baseline | 240 | 129 | 369 | N/A | N/A | N/A |
| Utility early post operatively | 233 | 136 | 369 | N/A | N/A | N/A | |
| Utility at 12 weeks | 190 | 179 | 369 | N/A | N/A | N/A | |
| Utility at the end of the trial | 189 | 180 | 369 | N/A | N/A | N/A | |
| PLICS (total) | 140 | 229 | 369 | 199 | 334 | 533 | |
| Diaries (total) | 91 | 278 | 369 | 145 | 388 | 533 | |
| Concomitant medicines (total) | 314 | 55 | 369 | 466 | 67 | 533 | |
| Antibiotic-impregnated VPS | Utility at baseline | 244 | 125 | 369 | N/A | N/A | N/A |
| Utility early post operatively | 231 | 138 | 369 | N/A | N/A | N/A | |
| Utility at 12 weeks | 174 | 195 | 369 | N/A | N/A | N/A | |
| Utility at the end of the trial | 179 | 190 | 369 | N/A | N/A | N/A | |
| PLICS (total) | 129 | 240 | 369 | 208 | 327 | 535 | |
| Diaries (total) | 98 | 271 | 369 | 146 | 389 | 535 | |
| Concomitant medicines (total) | 309 | 60 | 369 | 463 | 72 | 535 | |
| Silver-impregnated VPS | Utility at baseline | 224 | 136 | 360 | N/A | N/A | N/A |
| Utility early post operatively | 220 | 140 | 360 | N/A | N/A | N/A | |
| Utility at 12 weeks | 177 | 183 | 360 | N/A | N/A | N/A | |
| Utility at the end of the trial | 191 | 169 | 360 | N/A | N/A | N/A | |
| PLICS (total) | 130 | 230 | 360 | 210 | 316 | 526 | |
| Diaries (total) | 87 | 273 | 360 | 132 | 394 | 526 | |
| Concomitant medicines (total) | 310 | 50 | 360 | 467 | 59 | 526 | |
| Overall | Utility at baseline | 708 | 390 | 1098 | N/A | N/A | N/A |
| Utility early post operatively | 684 | 414 | 1098 | N/A | N/A | N/A | |
| Utility at 12 weeks | 541 | 557 | 1098 | N/A | N/A | N/A | |
| Utility at the end of the trial | 559 | 539 | 1098 | N/A | N/A | N/A | |
| PLICS (total) | 399 | 699 | 1098 | 617 | 977 | 1594 | |
| Diaries (total) | 276 | 822 | 1098 | 423 | 1171 | 1594 | |
| Concomitant medicines (total) | 933 | 165 | 1098 | 1396 | 198 | 1594 | |
Resource use questionnaires were completed by 423 (27%) participants: 145 participants allocated to standard VPSs, 146 allocated to antibiotic-impregnated VPSs and 132 allocated to silver-impregnated VPSs. The costs of concomitant medications were available for 88% of trial participants: 466, 463 and 467 participants allocated to standard, antibiotic-impregnated and silver-impregnated VPSs, respectively. In relation to self-reported hospital stays, respondents rarely provided information that was amenable to being costed. For example, most respondents who answered ‘yes’ to the question ‘Have you attended any hospital as an outpatient because of your hydrocephalus since your last BASICS study visit? (Please include reason and details of health-care professional seen)’ provided no information on the reason for attending or the health-care professional seen, which limited our ability to cost episodes of hospital care.
Overall, however, the numbers of missing hospital cost data, resource use diaries and concomitant medication were balanced across the three intervention groups, and by age and sex (Table 26).
| Group or characteristic | PLICS data, n (%) | Concomitant medications, n (%) | Diaries, n (%) |
|---|---|---|---|
| Standard | 199 (37.3) | 466 (87.4) | 145 (27.2) |
| Antibiotic impregnated | 208 (38.9) | 463 (86.5) | 146 (27.3) |
| Silver impregnated | 210 (39.9) | 467 (88.8) | 132 (25.1) |
| Paediatric | 261 (42.3) | 550 (39.4) | 167 (39.5) |
| Adult aged 16–65 years | 200 (32.5) | 461 (33.0) | 116 (27.4) |
| Adult aged ≥ 65 years | 156 (25.3) | 385 (27.6) | 140 (33.1) |
| Male | 303 (49.1) | 698 (50.0) | 205 (48.5) |
| Female | 314 (51.0) | 698 (50.0) | 218 (51.5) |
For the multiple imputation, and based on the variable with the highest FMI value (FMI 0.580), 50 data sets were imputed. 58
Resource use and cost analysis
Table 27 presents observed, mean disaggregated health-care resource use from randomisation up to 24 months, by intervention group. There were no discernible differences between intervention groups with respect to participants’ use of primary or secondary health care.
| Item of resource use | Trial group, mean count (range), n participants | ||
|---|---|---|---|
| Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | |
| GP visits | 2.7 (0–25), 140 | 1.9 (0–10), 112 | 2.0 (0–9), 110 |
| Nurse visits | 2.8 (0–18), 37 | 2.5 (0–18), 44 | 1.4 (0–5), 29 |
| Health visitor | 3.0 (0–10), 27 | 5.3 (0–25), 20 | 3.4 (0–15), 26 |
| Physiotherapy | 4.0 (0–30), 32 | 4.5 (0–21), 34 | 3.9 (0–12), 38 |
| Occupational therapist | 3.7 (0–35), 20 | 3.4 (0–15), 21 | 2.0 (0–6), 25 |
| Inpatient HRG | |||
| AA13A | 1.0 (0–1), 27 | 1.0 (0–1), 36 | 1.0 (0–2), 439 |
| AA19A | 1.4 (0–3), 7 | 1.5 (0–4), 8 | 1.5 (0–3), 10 |
| AA25A | 0.6 (0–3), 12 | 0.72 (0–3), 11 | 0.85 (0–2), 7 |
| AA52C | 1 (0–1), 12 | 1 (0–1), 9 | 0.83 (0–2), 12 |
| PA44Z | 2.3 (0–4), 3 | 3.0 (0–5), 4 | 4.0 (0–7), 3 |
| PA42Z | 6.7 (0–19), 4 | 6.7 (0–15), 4 | 3.0 (0–6), 4 |
| AA52G | 1.5 (0–3), 6 | 1.0 (0–1), 8 | 1.2 (0–3), 10 |
| PM44Z | 0.4 (0–4), 10 | 1 (0–1), 5 | 0.46 (0–6), 13 |
| Outpatient HRG | |||
| WF01A | 5.7 (0–36), 63 | 5.7 (0–72), 67 | 5.5 (0–28), 60 |
| WF01B | 2.4 (0–16), 38 | 2.0 (0–9), 38 | 1.7 (0–5), 38 |
| VB05Z | 0.44 (0–5), 18 | 0.28 (0–11), 25 | 0.25 (0–22), 35 |
| VB02Z | 1.3 (0–3), 7 | 1.8 (0–4), 4 | 2.0 (0–3), 3 |
| VB03Z | 1.0 (0–1), 2 | 1.5 (0–4), 6 | 1.7 (0–2), 3 |
| VB09Z | 6.0 (0–6), 1 | 1.0 (0–1), 3 | 1.0 (0–1), 3 |
| WF01C | 1 (0–1), 5 | 0.77 (0–2), 9 | 0.62 (0–4), 8 |
| BZ | 1 (0–1), 8 | 0.5 (0–4), 6 | 1 (0–1), 1 |
Based on the observed data, the majority of costs related to hospital inpatient procedures, followed by outpatient clinic visits and contacts with health-care professionals in primary care (Table 28). With the exception of GP costs, there were no significant differences in costs between the antibiotic- or silver-impregnated VPSs and the standard VPS catheter groups.
| Costs relating to | Trial group, mean (97.5% CI) (£) | Difference between antibiotic-impregnated and standard VPS, mean (97.5% CI) (£) | Difference between silver-impregnated and standard VPS, mean (97.5% CI) (£) | ||
|---|---|---|---|---|---|
| Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | |||
| Inpatient visits | 14,181 (10,618 to 20,557) | 11,738 (9730 to 14,401) | 14,481 (11,868 to 17,744) | –2442 (–8954 to 1933) | 300 (–6242 to 4965) |
| Outpatient visits | 2336 (1542 to 3651) | 2117 (1499 to 2989) | 2220 (1562 to 3442) | –219 (–1649 to 940) | –116 (–1559 to 1295) |
| GP visits | 188 (108 to 373) | 91 (71 to 121) | 91 (66 to 129) | –97 (–280 to –11) | –97 (–277 to –7) |
| Nurse visits | 133 (57 to 314) | 97 (49 to 169) | 60 (28 to 119) | –36 (–224 to 67) | –73 (–254 to 22) |
| Health visitor | 303 (77 to 784) | 131 (76 to 205) | 287 (157 to 378) | –171 (–663 to 64) | –15 (–490 to 245) |
| Physiotherapy | 500 (242 to 1110) | 190 (121 to 279) | 655 (356 to 1084) | –310 (–921 to –35) | 155 (–472 to 666) |
| Occupational therapist | 81 (18 to 184) | 139 (60 to 243) | 73 (15 to 175) | 58 (–69 to 173) | –8 (–123 to 119) |
| Other health-care professionals | 392 (211 to 817) | 367 (189 to 682) | 246 (190 to 328) | 24 (–465 to 329) | –145 (–592 to 57) |
| Concomitant medications | 203 (126 to 342) | 125 (80 to 189) | 272 (138 to 513) | –78 (–219 to 20) | 68 (–110 to 313) |
The adjusted base-case analysis yielded a total cost of £18,707 (97.5% CI £13,888 to £26,966) in the standard VPS group, £14,192 (97.5% CI £12,450 to £17,786) in the antibiotic-impregnated VPS group, and £17,385 (97.5% CI £14,649 to £22,355) in the silver-impregnated VPS group. Based on incremental analysis, the difference in 2-year costs between the silver-impregnated and the standard VPS groups was –£1322 (97.5% CI –£9295 to £5592), and was –£3192 (97.5% CI –£8382 to £1227) between the antibiotic-impregnated and the silver-impregnated VPSs (see Appendix 3, Table 49).
Overall, the cost of VPS failures was £8604 (97.5% CI £4696 to £12,511) due to any cause; £10,844 (97.5% CI £4267 to £17,436) due to confirmed infection; £5479 (97.5% CI £882 to £10,076) due to mechanical failure; £5149 (97.5% CI –£542 to £10,840) due to functional failure; and £7028 (97.5% CI –£5803 to £19,859) due to patient factors.
Economic health outcomes
The proportions of participants who experienced a first VPS failure (due to any cause) within 2 years were 130 out of 533, 132 out of 535 and 136 out of 526 in the standard, antibiotic-impregnated and silver-impregnated VPS groups, respectively. In the base-case analysis, with a 3.5% annual discount rate, the VPS failure rate was 23.3% (97.5% CI 19.1% to 27.3%) in the standard VPS group, 25.9% (97.5% CI 21.8% to 30.3%) in the antibiotic-impregnated VPS group and 25.4% (97.5% CI 20.9% to 29.6%) in the silver-impregnated VPS group.
The distribution of participants (or their parents’ or guardians’) responses to the EQ-5D questionnaires are presented in Figure 6. There was a low return rate of the EQ-5D questionnaire, with combined (EQ-5D-Y, EQ-5D-3L-Proxy and EQ-5D-3L) data available for only about half of the participants. Their responses suggested that there was a general improvement across all dimensions from baseline to the end of the trial, with no clear differences between intervention groups for any given dimension. Similarly, the response rates of participants, their parents or their guardians to the EQ-VAS, which are presented in Appendix 3, Table 50, were also low, but indicate a general trend for improvement from baseline to the end of the trial.
FIGURE 6.
Distribution of participants’ responses to each EQ-5D attribute, by treatment allocated and time. Levels range from 1 to 3, with 3 representing the most severe problem. The numbers of completed responses (n) are reported by intervention group. (a) Mobility; (b) self-care; (c) usual activities; (d) pain or discomfort; (e) anxiety or depression.
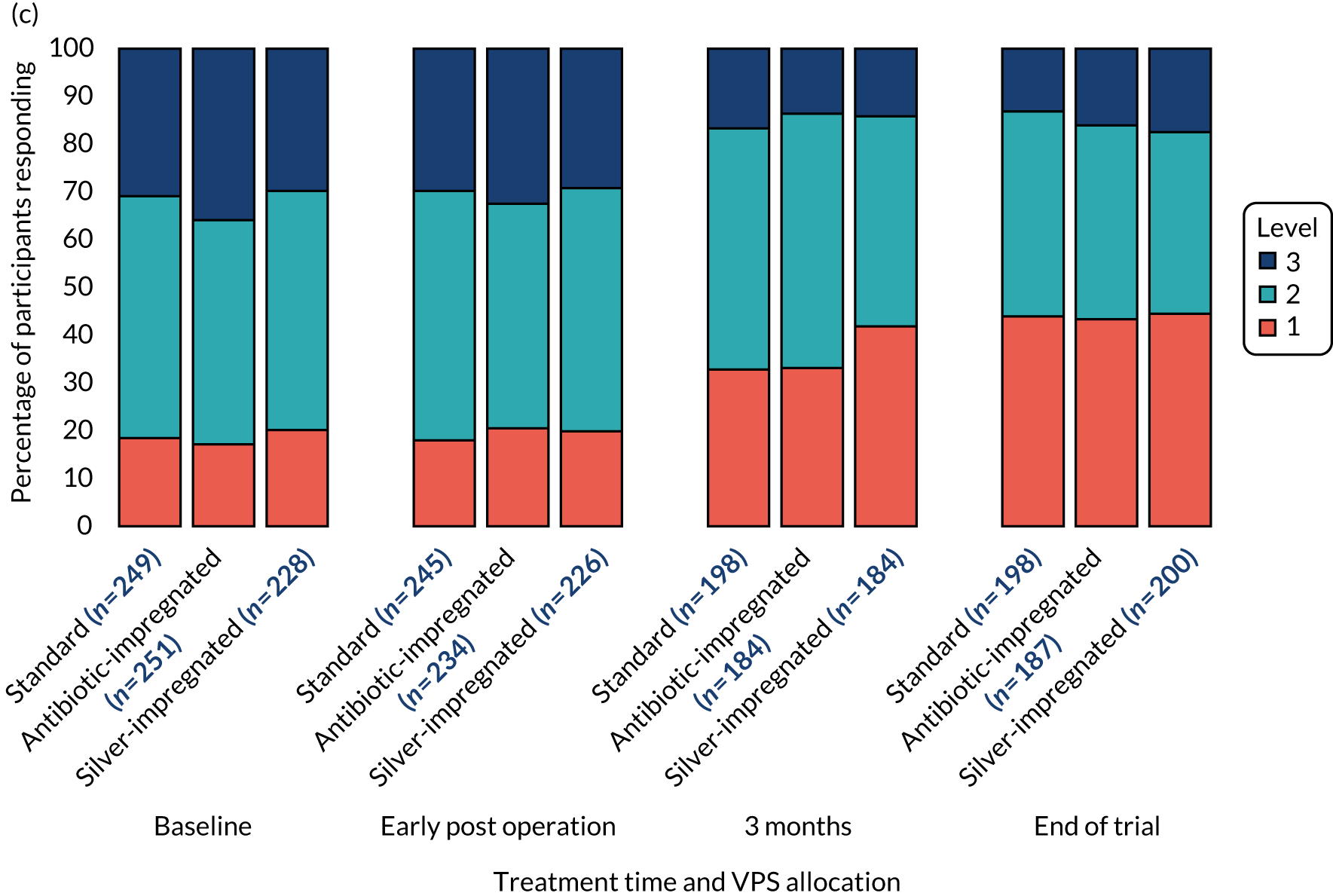
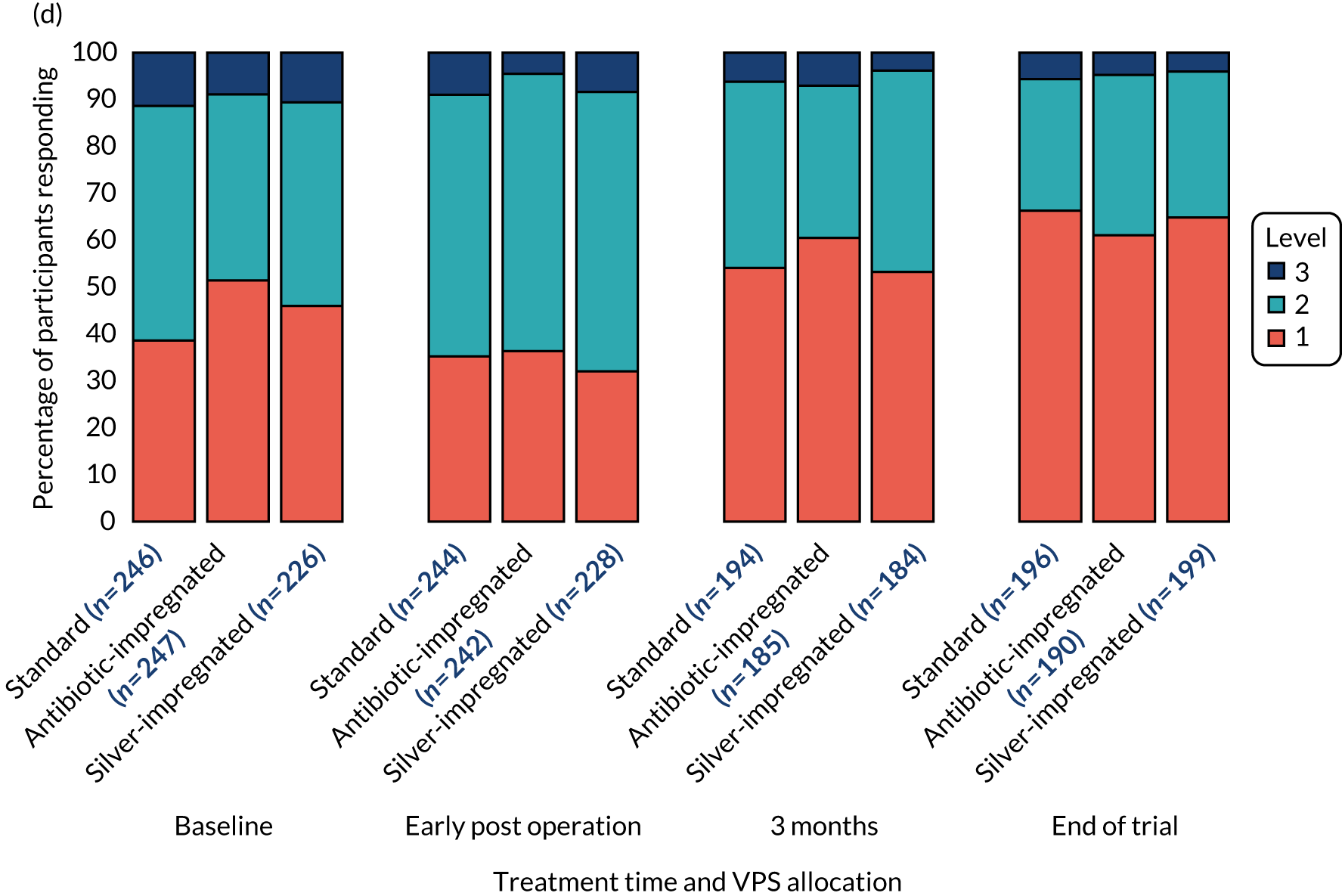
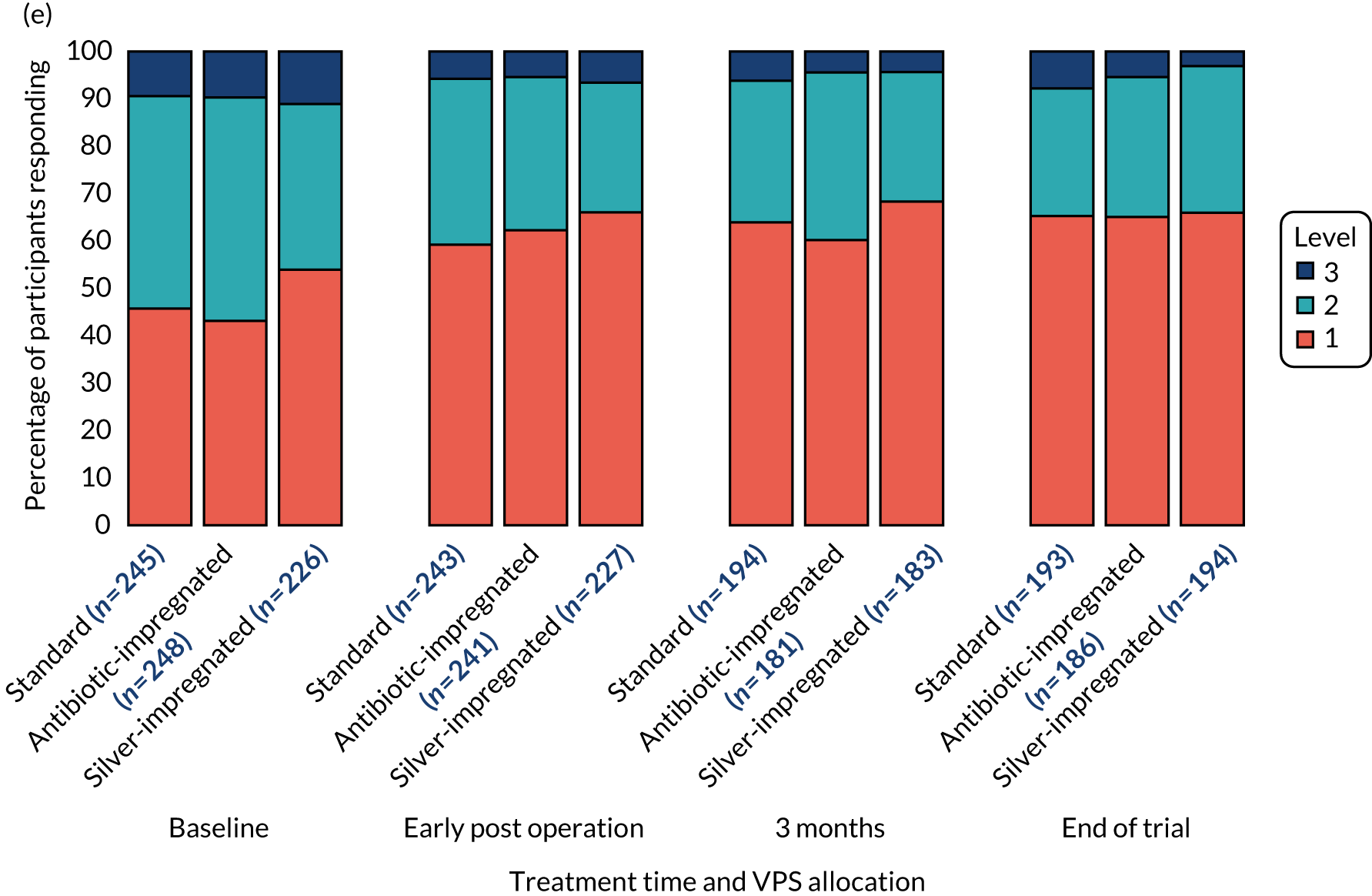
Quality of life, assessed using the Hydrocephalus Outcome Questionnaire
The return rate for the HOQ was low, and is summarised in Appendix 3, Table 51. Because of the low return rate, there were insufficient data to formally analyse the HOQ by mixed models, with the patient model not converging and the parent model output containing warnings that the final Hessian matrix not positive definite. For this reason, the patient questionnaire and the parent questionnaire are presented descriptively in Appendix 3, Tables 52 and 53.
Incremental analysis: base case
In the base-case analysis, both antibiotic-impregnated and silver-impregnated VPSs were located in the south-west quadrant of the cost-effectiveness plane; in relation to the standard VPS, they were less effective (associated with higher rates of first VPS failure due to any reason), but also less expensive, overall. The interpretation in the south-west quadrant is that interventions are more cost-effective with increasingly negative ICERs (larger savings associated with small health losses result in increasingly negative ICERs). Incrementally, silver-impregnated VPSs save £62,358 for each additional failure, compared with standard VPSs, and antibiotic-impregnated VPS catheters save £638,600 per additional failure, compared with silver-impregnated VPSs.
Sensitivity analyses
The ICERs were sensitive to changes in the unit costs of antibiotic-impregnated VPSs (Table 29). Compared with silver-impregnated VPSs, the incremental cost per first VPS failure became positive (north-east quadrant of the cost-effectiveness plane) when the cost of an antibiotic-impregnated VPS exceeded 10 times its current list price. The ICER (of antibiotic- vs. silver-impregnated VPSs) was insensitive to a change in the cost of silver-impregnated VPSs, even at 10% of the list price. However, a 35% reduction in cost, combined with a ninefold increase in the cost of antibiotic-impregnated VPSs, resulted in a positive ICER. In the comparison of silver-impregnated with standard VPSs, a fivefold increase in the cost of a silver-impregnated VPS resulted in a positive ICER (north-east quadrant of the cost-effectiveness plane), whereas no degree of reduction in the price of standard VPSs had a meaningful influence on the ICER.
| ICER vs. silver-impregnated VPS | x-fold decrease in the price of a silver-impregnated VPS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x-fold increase in the price of an antibiotic-impregnated VPS | 1 | 0.9 | 0.8 | 0.7 | 0.6 | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 | |
| 1 | –638,600 | –631,368 | –624,135 | –616,903 | –609,670 | –602,438 | –595,206 | –587,973 | –580,741 | –573,508 | |
| 2 | –561,800 | –554,568 | –547,335 | –540,103 | –532,870 | –525,638 | –518,406 | –511,173 | –503,941 | –496,708 | |
| 3 | –485,000 | –477,768 | –470,535 | –463,303 | –456,070 | –448,838 | –441,606 | –434,373 | –427,141 | –419,908 | |
| 4 | –408,200 | –400,968 | –393,735 | –386,503 | –379,270 | –372,038 | –364,806 | –357,573 | –350,341 | –343,108 | |
| 5 | –331,400 | –324,168 | –316,935 | –309,703 | –302,470 | –295,238 | –288,006 | –280,773 | –273,541 | –266,308 | |
| 6 | –254,600 | –247,368 | –240,135 | –232,903 | –225,670 | –218,438 | –211,206 | –203,973 | –196,741 | –189,508 | |
| 7 | –177,800 | –170,568 | –163,335 | –156,103 | –148,870 | –141,638 | –134,406 | –127,173 | –119,941 | –112,708 | |
| 8 | –101,000 | –93,768 | –86,535 | –79,303 | –72,070 | –64,838 | –57,606 | –50,373 | –43,141 | –35,908 | |
| 9 | –24,200 | –16,968 | –9735 | –2503 | 4730 | 11,962 | 19,194 | 26,427 | 33,659 | 40,892 | |
| 10 | 52,600 | 59,832 | 67,065 | 74,297 | 81,530 | 88,762 | 95,994 | 103,227 | 110,459 | 117,692 | |
| ICER vs. standard VPS | x–fold decrease in the price of a standard VPS | ||||||||||
| x-fold increase in the price of a silver-impregnated VPS | 1 | 0.9 | 0.8 | 0.7 | 0.6 | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 | |
| 1 | –62,358 | –61,547 | –60,736 | –59,925 | –59,113 | –58,302 | –57,491 | –56,679 | –55,868 | –55,057 | |
| 2 | –45,301 | –44,490 | –43,678 | –42,867 | –42,056 | –41,244 | –40,433 | –39,622 | –38,810 | –37,999 | |
| 3 | –28,243 | –27,432 | –26,621 | –25,809 | –24,998 | –24,187 | –23,375 | –22,564 | –21,753 | –20,942 | |
| 4 | –11,186 | –10,375 | –9563 | –8752 | –7941 | –7129 | –6318 | –5507 | –4695 | –3884 | |
| 5 | 5872 | 6683 | 7494 | 8306 | 9117 | 9928 | 10,740 | 11,551 | 12,362 | 13,174 | |
| 6 | 22,929 | 23,741 | 24,552 | 25,363 | 26,175 | 26,986 | 27,797 | 28,608 | 29,420 | 30,231 | |
| 7 | 39,987 | 40,798 | 41,609 | 42,421 | 43,232 | 44,043 | 44,855 | 45,666 | 46,477 | 47,289 | |
| 8 | 57,044 | 57,856 | 58,667 | 59,478 | 60,290 | 61,101 | 61,912 | 62,724 | 63,535 | 64,346 | |
| 9 | 74,102 | 74,913 | 75,725 | 76,536 | 77,347 | 78,158 | 78,970 | 79,781 | 80,592 | 81,404 | |
| 10 | 91,159 | 91,971 | 92,782 | 93,593 | 94,405 | 95,216 | 96,027 | 96,839 | 97,650 | 98,461 | |
The ICERs were stable to changes in discount rate (ranging from undiscounted to a discount rate of 6% per annum) and choice of regression modelling (Table 30). However, there were differences in cost-effectiveness when limiting the analysis to observed data, without multiple imputation. In this analysis, antibiotic-impregnated VPS dominated silver-impregnated VPS catheters, and saved £56,771 for each additional failure, compared with standard VPS catheters.
| VPS | Mean (97.5% CI) | ICER (£ per QALY) | |||
|---|---|---|---|---|---|
| Total cost (£) | Proportion failure | Incremental cost | Incremental failure | ||
| Base case | |||||
| Antibiotic-impregnated | 14,192 (12,450 to 17,786) | 0.259 (0.218 to 0.303) | –3192 (–8382 to 12,272) | 0.005 (–0.046 to 0.063) | –638,600 |
| Silver-impregnated | 17,385 (14,649 to 22,355) | 0.254 (0.209 to 0.296) | –1322 (–9295 to 5592) | 0.021 (–0.035 to 0.078) | –62,358 |
| Standard | 18,707 (13,888 to 26,966) | 0.233 (0.191 to 0.273) | – | – | – |
| 0% discount rate | |||||
| Antibiotic-impregnated | 14,331 (12,621 to 18,064) | 0.260 (0.219 to 0.302) | –3212 (–8619 to 1534) | –0.006 (–0.048 to 0.061) | –535,333 |
| Silver-impregnated | 17,542 (14,768 to 22,523) | 0.254 (0.209 to 0.298) | –1340 (–9454 to 5782) | –0.021 (–0.036 to 0.078) | –63,810 |
| Standard | 18,882 (14,015 to 27,224) | 0.234 (0.192 to 0.275) | – | – | – |
| 1.5% discount rate | |||||
| Antibiotic-impregnated | 14,269 (12,515 to 17,989) | 0.260 (0.219 to 0.301) | –3023 (–8575 to 1527) | –0.006 (–0.048 to 0.060) | –539,821 |
| Silver-impregnated | 17,473 (14,570 to 22,449) | 0.254 (0.209 to 0.297) | –1332 (–9386 to 5764) | –0.021 (–0.035 to 0.078) | –63,429 |
| Standard | 18,805 (13,959 to 27,070) | 0.233 (0.191 to 0.273) | – | – | – |
| 6% discount rate | |||||
| Antibiotic-impregnated | 14,099 (12,378 to 17,776) | 0.258 (0.217 to 0.301) | –3179 (–8364 to 1224) | –0.005 (–0.046 to 0.062) | –635,800 |
| Silver-impregnated | 17,278 (14,551 to 22,242) | 0.253 (0.208 to 0.295) | –1310 (–9184 to 5715) | –0.021 (–0.035 to 0.078) | –62,381 |
| Standard | 18,589 (13,802 to 26,721) | 0.231 (0.190 to 0.271) | – | – | – |
| Observed data (without imputation) | |||||
| Silver-impregnated | 6186 (5842 to 6530) | 0.255 (0.247 to 0.258) | – | – | Dominated |
| Antibiotic-impregnated | 5296 (4952 to 5640) | 0.250 (0.243 to 0.258) | –545 (–1128 to 2215) | 0.010 (–0.046 to 0.065) | –56,771 |
| Standard | 5841 (5497 to 6185) | 0.241 (0.233 to 0.248) | – | – | – |
| Generalised linear modelling for costs | |||||
| Antibiotic-impregnated | 15,012 (12,893 to 18,955) | 0.259 (0.218 to 0.303) | –1680 (–8333 to 3033) | –0.005 (–0.046 to 0.063) | –336,000 |
| Silver-impregnated | 16,693 (14,397 to 20,888) | 0.254 (0.209 to 0.296) | –1819 (–12813 to 4506) | –0.021 (–0.035 to 0.078) | –85,802 |
| Standard | 18,512 (13,766 to 26,178) | 0.233 (0.191 to 0.273) | – | – | – |
Based on the GLM, whereby the gamma family and log-link performed best (lowest AIC and BIC values and a coefficient close to 2 in the modified Park test), the ICERs were consistent with the base case, with a saving of £336,000 per additional VPS failure (due to any cause) with antibiotic-impregnated shunt catheters (compared with silver-impregnated VPSs), and a saving of £85,802 per additional VPS failure (due to any cause) with silver-impregnated VPS catheters (compared with standard VPSs).
Subgroup analyses
A stratified cost-effectiveness analysis indicated that cost-effectiveness was dependent on age (Table 31). In paediatrics, antibiotic-impregnated VPS catheters were dominant (south-east quadrant of the cost-effectiveness plane), with mean savings of £5312 and additional benefits of 0.004 VPS failures (due to any reason) averted. Put another way, for every 250 patients first receiving an antibiotic-impregnated instead of a standard VPS catheter, there would be one fewer case of VPS failure (due to any reason), and a cost-saving of £1,328,000.
| VPS | Mean (97.5% CI) | ICER (£ per QALY) | |||
|---|---|---|---|---|---|
| Total cost (£) | Proportion of VPS failure | Incremental cost (£) | Incremental VPS failure | ||
| Base case | |||||
| Antibiotic-impregnated | 14,192 (12,450 to 17,786) | 0.259 (0.218 to 0.303) | –3192 (–8382 to 12,272) | 0.005 (–0.046 to 0.063) | –638,600 |
| Silver-impregnated | 17,385 (14,649 to 22,355) | 0.254 (0.209 to 0.296) | –1322 (–9295 to 5592) | 0.021 (–0.035 to 0.078) | –62,358 |
| Standard | 18,707 (13,888 to 26,966) | 0.233 (0.191 to 0.273) | – | – | – |
| Paediatrics aged < 16 years | |||||
| Antibiotic-impregnated | 14,859 (11,650 to 22,381) | 0.362 (0.248 to 0.469) | –5312 (–16,289 to 2271) | 0.004 (–0.107 to 0.102) | Dominant |
| Standard | 20,171 (14,632 to 33,160) | 0.365 (0.242 to 0.484) | – | – | – |
| Silver-impregnated | 19,518 (15,338 to 28,372) | 0.384 (0.256 to 0.493) | – | – | Dominated |
| Adults aged 16–65 years | |||||
| Antibiotic-impregnated | 13,940 (9748 to 18,489) | 0.306 (0.173 to 0.453) | –2651 (–8841 to 2058) | 0.039 (–0.063 to 0.149) | Extendedly dominated |
| Silver-impregnated | 16,591 (11,992 to 22,565) | 0.266 (0.131 to 0.420) | –2845 (–10,188 to 4751) | 0.027 (–0.076 to 0.140) | –105,370 |
| Standard | 19,437 (13,109 to 28,306) | 0.239 (0.113 to 0.384) | – | – | – |
| Adults aged > 65 years | |||||
| Antibiotic-impregnated | 14,730 (11,676 to 21,353) | 0.123 (0.069 to 0.179) | –1881 (–8011 to 4666) | 0.024 (–0.052 to 0.106) | –78,375 |
| Silver-impregnated | 16,611 (12,693 to 23,830) | 0.099 (0.043 to 0.157) | –329 (–9205 to 6657) | 0.011 (–0.059 to 0.089) | –29,375 |
| Standard | 16,941 (12,374 to 27,346) | 0.088 (0.036 to 0.138) | – | – | – |
For adults aged 16–65 years, silver-impregnated VPSs were the most cost-effective, with antibiotic-impregnated VPS catheters being extendedly dominated. In adults aged > 65 years, silver-impregnated VPSs save £29,375 for each additional failure, compared with standard VPSs, and antibiotic-impregnated VPSs save £786,375 per additional failure, compared with silver-impregnated VPSs.
Alternative cost-effectiveness and cost–utility analyses
A cost-effectiveness analysis based on the incremental cost per confirmed infection averted indicated that silver-impregnated VPS catheters were dominated by standard VPSs, whereas antibiotic-impregnated VPS catheters were dominant, saving £4059 per 0.030 fewer infection-related VPS failures. Compared with standard VPSs, antibiotic-impregnated VPS catheters save £135,753 per VPS infection avoided (Table 32).
| VPS | Mean (97.5% CI) | ICER (£ per QALY) | |||
|---|---|---|---|---|---|
| Total cost (£) | Outcome | Incremental cost (£) | Incremental outcome | ||
| Confirmed infections | |||||
| Antibiotic-impregnated | 14,446 (12,660 to 18,054) | 0.027 (0.013 to 0.043) | –4059 (–12,567 to 1422) | –0.030 (–0.058 to –0.002) | Dominant |
| Standard | 18,505 (13,872 to 27,274) | 0.057 (0.035 to 0.083) | – | – | – |
| Silver-impregnated | 17,331 (14,584 to 22,136) | 0.057 (0.038 to 0.080) | – | – | Dominated |
| Mechanical failures | |||||
| Standard | 14,110 (14,021 to 27,648) | 0.092 (0.066 to 0.120) | – | – | – |
| Silver-impregnated | 17,426 (14,682 to 22,445) | 0.119 (0.088 to 0.154) | – | – | Dominated |
| Antibiotic-impregnated | 18,749 (12,303 to 17,564) | 0.134 (0.103 to 0.167) | – | – | Dominated |
| Functional failures | |||||
| Silver-impregnated | 17,483 (14,767 to 22,396) | 0.069 (0.047 to 0.092) | –1163 (–9349 to 5815) | –0.003 (–0.040 to 0.030) | 387,667 |
| Standard | 18,646 (13,837 to 27,066) | 0.072 (0.048 to 0.101) | – | – | – |
| Antibiotic-impregnated | 14,157 (12,397 to 17,576) | 0.084 (0.057 to 0.108) | –4488 (–12,919 to 960) | 0.011 (–0.027 to 0.049) | –374,000 |
| Patient factors | |||||
| Antibiotic-impregnated | 14,196 (12,438 to 17,648) | 0.008 (0.001 to 0.018) | –4441 (–12,825 to 987) | –0.0006 (–0.015 to 0.012) | 7,401,667 |
| Standard | 18,638 (13,983 to 27,464) | 0.009 (0.001 to 0.018) | – | – | – |
| Silver-impregnated | 17,451 (14,712 to 22,543) | 0.009 (0.001 to 0.019) | 1186 (–9255 to 5694) | –0.0002 (–0.011 to 0.010) | –3,953,333 |
| Cost–utility analysis based on imputed data | |||||
| Silver-impregnated | 9115 (7596 to 12,682) | 1.319 (1.207 to 1.365) | 183 (–3035 to 3854) | 0.096 (–0.488 to 0.188) | 1904 |
| Standard | 8932 (7301 to 11,980) | 1.223 (1.136 to 1.298) | – | – | – |
| Antibiotic-impregnated | 9643 (7545 to 11,736) | 1.250 (1.163 to 1.336) | – | – | Dominated |
For the cost-effectiveness measure of incremental cost per mechanical failure averted, both silver-impregnated and antibiotic-impregnated VPS catheters were dominated by standard VPSs, as they were associated with higher rates of mechanical failures and higher costs than standard VPSs. With regards to functional VPS failures, antibiotic-impregnated VPS catheters are both less effective and less expensive than standard VPSs, whereas silver-impregnated shunt catheters cost an additional £387,667 per additional functional failure averted. The opposite was observed when considering the incremental cost per VPS failure due to patient-related factors, although failure rates due to patient influences are much lower, and the reporting of this outcome was less reliable. Antibiotic-impregnated VPS catheters cost an additional £7.4M per failure averted, whereas silver-impregnated VPS catheters save £3.9M per additional failure, each in comparison with standard shunt catheters.
In the cost–utility analysis of trial participants aged ≥ 5 years, and based on multiple imputation to account for missing data, antibiotic-impregnated VPS catheters were dominated by silver-impregnated VPS. Compared with standard VPSs, silver-impregnated VPSs are £183 more costly, and yield 0.096 additional QALYs overall, resulting in an incremental cost of £1904 per QALY gained. The cost-effectiveness acceptability curve showing the probability of each VPS catheter being cost-effective, by a range of cost-per-QALY thresholds, is depicted in Figure 7.
FIGURE 7.
Cost-effectiveness acceptability curves indicating the probability of each VPS catheter being cost-effective (based on the incremental cost per QALY gained) for a range of threshold (willingness-to-pay) values. Vertical lines indicate the usual NICE threshold range53 of £20,000–30,000 per QALY.
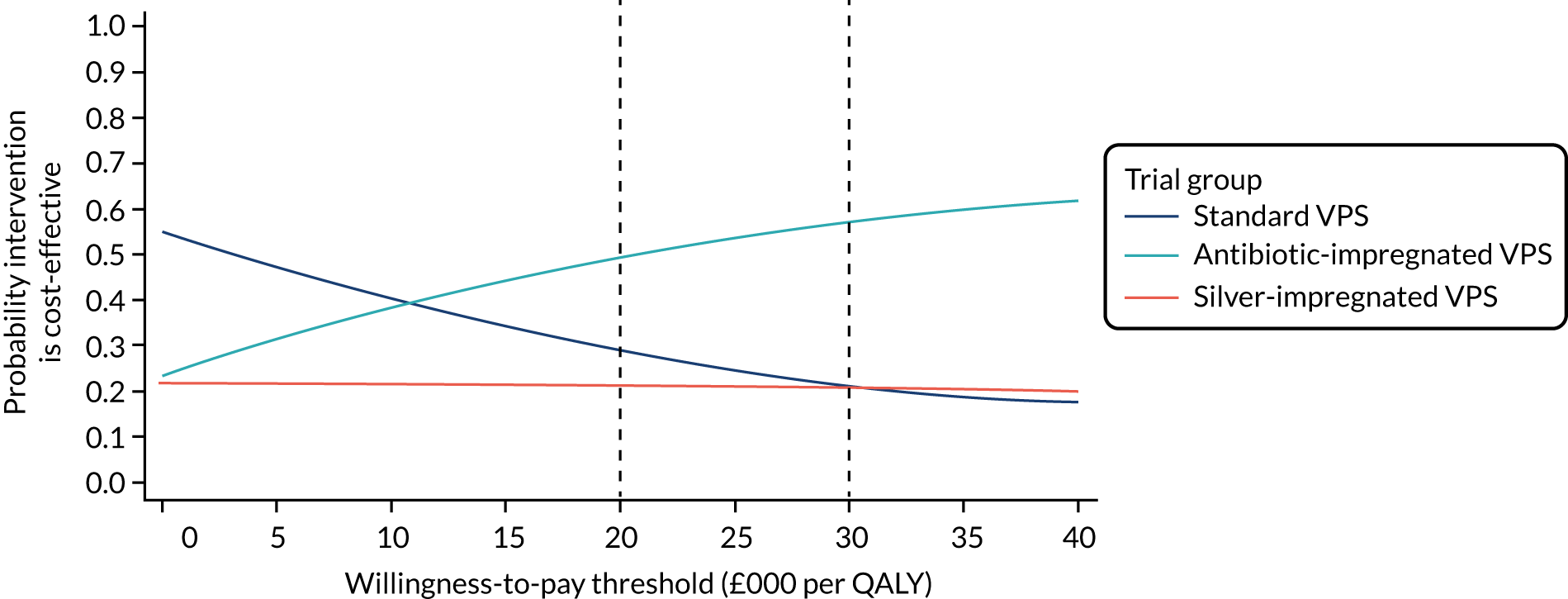
Chapter 5 Discussion
Parts of this chapter have been reproduced from Mallucci et al. 2 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Summary of findings
In this trial of patients with hydrocephalus who were undergoing insertion of their first permanent VPS, the infection rates were 6.0% in the standard VPS group, 2.2% in the antibiotic-impregnated VPS group and 5.9% in the silver-impregnated VPS group. Compared with standard VPSs, antibiotic-impregnated VPSs were associated with a significantly lower rate of infection, whereas silver-impregnated VPSs were not. This effect was present across all age categories. There is significant economic benefit for every shunt infection averted. There were notable differences in infection rates for differing aetiologies of hydrocephalus and between different age groups.
Clinical effectiveness
The BASICS trial provides definitive evidence favouring the use of antibiotic-impregnated VPSs to reduce infection. A previously reported underpowered randomised trial15 demonstrated a trend favouring antibiotic-impregnated over standard VPSs, but did not show a statistically significant difference between the two groups in the risk of infection (RR 0.38, 95% CI 0.11 to 1.30). Silver-impregnated shunt catheters have been evaluated only in a randomised trial of EVDs,22 which found a reduction in infection from 21.4% (30/140) with standard shunt catheters to 12.3% (17/138) (p = 0.043) with silver-impregnated shunt catheters, although this is much higher than the UK national reported infection rate (9.3%) for EVDs. 59 The BASICS trial was conceived to evaluate both antibiotic-impregnated and silver-impregnated VPSs before the widespread introduction of these products into routine practice, despite a lack of evidence. The results of this trial will inform neurosurgery practice and choice of VPS for insertion, to the benefit of patients.
In this trial, the classification of VPS infection was determined by the central review committee. The proportion of culture-positive infections was 68.8% in the standard VPS group, 50.0% in antibiotic-impregnated VPS group and 80.6% in silver-impregnated VPS group; there was a lower rate of culture-positive infection with antibiotic-impregnated VPSs than with either the standard-impregnated or the silver-impregnated VPS. The analysis allowed for culture-negative infections to be included when there was sufficient supporting clinical evidence of VPS infection. We postulate that the presence of antibiotic-impregnated shunt catheters reduced the ability to culture organisms in infected VPSs. This trial would have shown an even greater effect in favour of antibiotic-impregnated VPSs if we had included culture-positive infections only.
The reduction of types of infections seen is consistent with the expected microbiological spectrum of the antibiotic-impregnated VPSs, which are especially active against Gram-positive organisms, and were incorporated to prevent staphylococcus species infections. The culture results show a large reduction in staphylococcal infection with antibiotic-impregnated VPSs, compared with the standard and silver-impregnated VPSs, which accounts for the majority of the infections prevented and supports the biological plausibility as clindamycin and rifampicin are preferentially active against Gram-positive organisms. All three VPS types have a similar number of Gram-negative infections, which the antibiotics were not expected to reduce.
It should be noted that the overall VPS failure rate was the same for all groups, although infection was reduced in the antibiotic-impregnated VPS arm. When infection is removed as a cause, the clean non-infected revision rates were slightly higher in the antibiotic-impregnated VPS group. In the post hoc analysis, the only pattern that could be found was a higher rate of valve change in the antibiotic-impregnated VPS group than in the standard and silver-impregnated VPS groups when analysing the components of the shunt that were changed and/or blocked at surgery, as reported by the operating surgeon on the CRF. Interestingly, the valve itself is not impregnated and is common to all shunt types. Unfortunately, in most cases, the valves were not sent for culture as it was not part of most units’ protocol at clean shunt revision. Of note, there were no increased revisions for any group for peritoneal shunt catheters. One of the concerns voiced by some of the surgeons was that silver-impregnated peritoneal shunt catheters were more rigid than the other types, and that this might lead to more peritoneal catheter revisions. This was not borne out in the results.
The reasons for the higher non-infective blockages in the antibiotic-impregnated VPS group are not known. One hypothesis is that the antibiotic-impregnated shunt catheters convert an ‘infected’ shunt revision to an apparently ‘clean’ shunt revision in the following ways:
-
Low virulent pathogens are restricted to a biofilm in the valve, not causing a CSF change as there is no ventriculitis and not isolated from the revision CSF as the planktonic bacteria are low in number or not able to grow in the presence of the eluted antibiotics.
-
A sufficient change in CSF composition and flow (such as debris or high protein) to promote a blockage in the system such as the valve, which is probably the most vulnerable to content of CSF in terms of blockage in view of the intricate mechanical valve mechanisms. This study has not been powered or designed to answer this question directly, but, in future research, this question will be important.
Of note, from a patient well-being and health economic standpoint, a mechanical clean shunt revision, such as a valve change, will have a minor effect on the patient and a minor cost to the NHS. A clean revision for valve change typically involves an overnight stay only, no brain operation (as the ventricular shunt catheter is not changed) and none of the health implications of suffering from clinical meningitis. In contrast, a shunt infection will involve two brain operations: a first operation to remove the whole shunt system and to insert a temporary ventricular shunt catheter (an average of 2 weeks in a hospital bed for both intravenous and intrathecal antibiotics, and the health implications of clinical meningitis) and a second operation to re-insert a whole new shunt system including a ventricular shunt catheter.
In addition to the above, if a patient has ventricular shunt catheter change at shunt revision [as opposed to valve change or peritoneal shunt catheter change, (not a brain operation)], they will lose their driving licence for 6 months because of the increased risk of epilepsy after ventricular shunt catheter revision (as it is a brain operation).
Cost effectiveness
Complications associated with VPS failures are expensive to manage. 32–38 Consequently, the use of VPS catheters, which result in fewer complications, even if more expensive to purchase, could be cost-effective or yield cost savings to the NHS. The economic analysis within the BASICS trial estimated that, although antibiotic-impregnated shunt catheters are about twice the price of standard silicone shunt catheters, this upfront cost could be justified by the reduced infection rate and associated cost savings of further surgery and prolonged hospital care. Based on the primary economic outcome of incremental cost per VPS failure (due to any cause) averted, there appeared to be large potential savings for additional cases of VPS failures with silver- and antibiotic-impregnated VPSs compared with standard VPSs. This conservative estimate does not assume equivalence, despite no difference between groups in VPS failure rate. Had a cost-minimisation analysis been considered appropriate, the saving per patient having an antibiotic-impregnated rather than a standard VPS would be £4515 (97.5% CI £140 to £9170).
In this context, the secondary outcome based on the incremental cost per confirmed infection averted is more relevant, as well as for comparison with previous such analyses, which use the same outcome measure. Notably, antibiotic-impregnated VPS catheters were dominant, saving £4059 per 0.030 fewer infection-related VPS failures. Compared with standard VPSs, antibiotic-impregnated VPS catheters save £135,753 per VPS infection avoided. In terms of determining technical efficiency, no cost-effectiveness threshold is necessary, given the dominance of antibiotic-impregnated VPS catheters.
For the purposes of informing decisions on allocative efficiency, the cost–utility analysis of participants aged ≥ 5 years indicated that antibiotic-impregnated VPSs were dominant, whereas the incremental cost per QALY gained with silver-impregnated VPSs was £1904, which is within the threshold applied by NICE for the determination of cost-effectiveness. However, the cost–utility analysis was limited, with respect to missing data and the exclusion of participants who were at the highest risk of VPS infections.
The analyses are robust to a range of assumptions and analytic approaches. Important subgroup analyses indicate differences in cost-effectiveness by age. The risk of infection in the BASICS trial was highest in paediatrics; it was lower for adults and was particularly low for the elderly. Some patterns also emerged with the aetiology of hydrocephalus; for example, congenital causes and post-haemorrhagic hydrocephalus (both prevalent in the paediatric age group) resulted in much higher infection rates than those observed for other aetiologies seen in older patients. Although the primary economic analysis was based on all-cause VPS failures, subgroup analyses demonstrated higher cost-effectiveness in paediatrics than in adult populations.
Generalisability and cost impact
In the context of the NHS, there should be a major health benefit and cost-saving impact by routinely adopting antibiotic-impregnated VPSs for all first-time VPS insertions for hydrocephalus.
Clearly, in paediatrics, for whom the effect of reduction in shunt infections is greatest, the economic benefit will also be the most significant. For example, for every 100 paediatric new VPS insertions, using antibiotic-impregnated rather than standard shunt catheters should avert up to six or seven shunt infections, which translates to a potential cost saving of between £814,000 and £950,000. Even in those aged > 65 years, averting one infection per 100 shunts could potentially save £135,753.
Such savings may well translate to equivalent health economies, such as the in the USA, but clearly the potential value and savings will vary in different health models internationally.
Strengths and limitations
The strengths of this trial are that (1) infections were centrally classified by a panel blinded to treatment allocation, thereby removing the risk of treating-surgeon bias; (2) participant retention was very high owing to the nature of the intervention and the primary outcome (patients with infected VPSs are always re-admitted to hospital); (3) patient withdrawal was low (n = 53, 3.3%) so it is unlikely that events were missed; (4) participants were recruited across the whole of the UK and the Republic of Ireland to encompass all ages and socioeconomic classes; and (5) the trial sample size was large.
Some limitations of the trial should be noted. First, it was not possible to blind the treating neurosurgeon to the VPS type, because the physical appearance of each shunt catheter is distinctive. Participants were blinded to the shunt catheter type, and shunt catheter type was not recorded in patient records. The majority of VPS revisions and removals for infection happen as emergencies and are managed by the emergency neurosurgery team. Therefore, the likelihood of the same neurosurgeon who inserted the VPS being involved in the decision to remove the VPS was low, given the work rotas of neurosurgical staff. Furthermore, there was high agreement (95.7%) in the classification of VPS infection between treating surgeons and the central review panel, suggesting that any bias that the treating surgeon may have had did not affect the study conclusion. Second, ventriculoatrial and ventriculopleural shunts were excluded, although we postulate that the results are translatable to patients undergoing these procedures. Finally, the return rate for patient-reported outcomes was low, thereby limiting the analysis of the impact of VPS infection.
Limitations to the economic evaluation include a lack of detailed costing for revisions, and failure to obtain Hospital Episode Statistics data from NHS Digital, as per protocol. Reliance on PLICS data mitigated these limitations, but, as with other data on costs, there was a high degree of missingness due to some centres not being able to share electronic data and patient questionnaires not being returned. This was addressed using robust methods (multiple imputation), but still may have introduced bias to the analysis.
Safety
The data suggest that there is no increased health risk in using impregnated shunt catheters. There were no serious AEs. All of the AEs that were seen were expected events predominantly related to expected shunt revisions and/or due to re-admission for expected known complications associated with VPS surgery; no differences were seen between catheter types.
Implications for practice and health care
From a patient perspective and that of the treating clinician, the hospital and the health service, every effort to reduce shunt infection should be made, and health technologies such as antibiotic-impregnated shunt catheters, with their potential to reduce VPS infections, deserve proper evaluation through appropriately planned and powered trials.
Having demonstrated a marked reduction in such infections, with all of the potentially catastrophic and life-changing health sequalae that result from each infection, the BASICS trial has provided evidence to support that the adoption of antibiotic-impregnated shunt catheters is justified in all patients receiving their first VPS in the UK. The increased upfront cost of the impregnated shunt catheters is offset by the added health economic benefit demonstrated in the health economic analysis and in a previous publication. 39 The benefits and implications, both from an efficacy and health economic point of view, are most pronounced in younger patients.
A wider discussion and analysis, particularly from a health economics point of view, is required when considering recommendations and implications globally.
Conclusions
In conclusion, antibiotic-impregnated shunt catheters significantly reduce the VPS infection rate and the probability of infection, compared with standard silicone shunt catheters in all age groups, whereas silver-impregnated shunt catheters do not. The routine use of these shunt catheters would result in a significant cost saving to the NHS.
These results support the use of antibiotic-impregnated shunt catheters in the treatment of patients undergoing insertion of a first VPS for hydrocephalus.
Implications for future research
To our knowledge, the BASICS trial is the largest-ever prospective randomised trial for VPSs in hydrocephalus worldwide.
A critical value-added aspect of the BASICS trial was the development of systematic clinical sample collection (of CSF and blood) from participants undergoing VPS insertion. We have established a unique neurosurgical patient sample collection, which will enable us to identify surrogate markers of VPS infection in CSF or blood using molecular techniques (transcriptomics/proteomics); to assess whether or not infection may be associated with shunt failure cases (by detection of pathogen nucleic acid via next-generation sequencing); and to begin to explore whether or not host differences contribute to the different rates of infection in children, compared with adults. Proteomic analysis of CSF has already been undertaken to identify a series of candidate surrogate markers of neurosurgical infection. These candidate markers are now being re-tested among the BASICS trial samples to assess their accuracy in identifying confirmed infection. The BASICS trial sample collection, stored at the University of Liverpool, is a valuable resource to help answer the ongoing questions around VPS infection identified in the BASICS trial (as outlined in this paragraph) and to support future additional studies.
A large number of data was collected regarding aetiology, surgical techniques, types of valves and the technology used, which can guide future management and trials. We plan to undertake further analysis exploring patterns related to mechanical shunt failure. This is led by the results and will, therefore, be post hoc. We also plan to create a detailed shunt failure model and predictive score. Failure and infection rates across different aetiologies of hydrocephalus will be analysed further.
Surgical techniques and factors that potentially affect failure rates will be further analysed, such as the use of neuronavigation and the placement of the ventricular shunt catheter, surgical site infection information collected at the time of surgery and seniority of the surgeon. This may feed further questions and prospective randomised studies.
From a methodological perspective, the economic evaluation was significantly affected by missing data. However, organisational familiarity with the procedures and requirements for accessing routine data, post General Data Protection Regulation,60 should facilitate Hospital Episode Statistics data access from NHS Digital in future trials. Acute trusts are now mandated to produce PLICS data, and early engagement with hospital finance offices should resolve many of the difficulties that were faced in the BASICS trial. Poor response rates to questionnaires is a common problem in self-reported resource use. Further research into methods to mitigate this ought to focus not just on technical solutions, but also on operational solutions, including a broader appreciation that achieving complete data on economic outcomes should be regarded as having equal importance to achieving complete data on primary clinical end points. This may be especially relevant for time-to-event analyses, in which costs and disutilities are most likely to accrue after such events.
Acknowledgements
Alder Hey Children’s NHS Foundation Trust, The Walton Centre NHS Foundation Trust, and the University of Liverpool are members of Liverpool Health Partners.
We would like to thank the external members of the TSC for their advice and support on the project: Professor Deborah Stocken (TSC chairperson, University of Leeds), Professor John RW Kestle (Pediatric Neurosurgery Primary Children’s Medical Center, Salt Lake City, UT, USA), Craig Williams (Yorkhill NHS Trust), Abhaya Kulkani (University of Toronto) and Gill Yaz (lay member, Shine).
Our thanks go also to the IDSMC: Peter Hutchinson (Chairperson, University of Cambridge and Addenbrooke’s Hospital), Andy Vail (University of Manchester) and Carmel Curtis (University College London Hospitals NHS Foundation Trust). We would also like to thank Dianne Jones (lay member), who was a member of TMG.
We would like to thank the BASICS trial co-ordinators: Helen Hickey (Head of Trial Management), Helen Gillard (Senior Trials Manager) and Heather Granville. We also thank Lynsey Finnetty, data manager; Laura Sutton, who undertook the quality-of-life analysis; Ashley Best, who undertook quality assurance checks from the LCTC; and Catrin Plumpton and Yankier Pijeira Perez, who assisted with the economic evaluation.
We are grateful to all of the patients and their families for their commitment to the trial. We would like to thank the PIs and research nurses who recruited patients and supported them during the trial:
-
Alder Hey Children’s Hospital, Liverpool – Conor Mallucci (PI), Benedetta Pettorini, Christopher Parks, Ajay Sinha, Libby van Tonder and Mitchel T Foster
-
Birmingham Children’s Hospital, Birmingham – Guirish Solanki (PI) and Desiderio Rodrigues
-
Frenchay Hospital, Bristol – Richard Edwards (PI) and Adam Williams (Co-PI)
-
Addenbrooke’s Hospital, Cambridge – Matthew Garnett (co-PI), Angelos Kolias (co-PI), Karen Caldwell and Silvia Tarantino
-
University Hospital of Wales, Cardiff – Paul Leach (PI), Malik Zaben, Gulam Zilani, Dmitri Shastin, Joseph Merola, Rahim Hussain, Ravindra Vemaraju, Liudmila Selezneva, Georgina Radford and Nadine Lloyd
-
Temple Street Children’s University Hospital, Dublin – Darach Crimmins (PI), John Caird (co-PI), Maria Nunez Sayar and Noelle O’Mahoney
-
Great Ormond Street Hospital, London – Dominic Thompson (PI), Kristian Aquilina and Gregory James
-
The James Cook University Hospital, Middlesbrough – Roger Strachan (PI), Nitin Mukerji and Jonathan Pesic-Smith
-
King’s College Hospital, London – Bassel Zebian (PI), Bhaskar Thakur (Co-PI), Holly Dickson, Eniola Nsirim and Adedamola Adebayo
-
Leeds General Infirmary, Leeds – John Goodden (PI), Kenan Deniz, Janet Clarke, Mary Kambafwile, Ian Anderson, Rebecca Chave-Cox, Asim Sheik, Ryan Mathew, Oliver Richards, Soumya Mukherjee, Paul Chumas, Atul Tyagi and Gnanamurthy Sivakumar
-
National Hospital for Neurology and Neurosurgery, London – Ahmed Toma (PI), Linda D’Antona, Laurence Watkins, Lewis Thorne, Claudia Carven and Vanessa Bassen
-
Newcastle General Hospital, Newcastle upon Tyne – Damian Holliman (PI) and Ian Coulter (co-PI)
-
Queen’s Medical Centre, Nottingham – Donald Macarthur (PI), Maria Cartmill, Simon Howarth, Stuart Smith and Shazia Javed
-
Royal Manchester Children’s Hospital, Manchester – Ian Kamaly (PI) and Roberto Ramirez
-
Salford Royal Hospital, Salford – Andrew King (PI), Ardash Nadig (Co-PI) and John Thorne
-
Sheffield Children’s Hospital and Royal Hallamshire Hospital, Sheffield – Shungu Ushewokunze (PI), Saurabh Sinha (co-PI), Hesham Zaki and John McMullan
-
Southampton General Hospital, Southampton – Diederik Bulters (PI), Ryan Waters (Co-PI), George Zilidis, Joy Roach, Ahmed Sadek, Patrick Holton, Ardalan Zolnourian and Aabir Chakraborty
-
The Walton Centre, Liverpool – Michael D Jenkinson (PI), Catherine McMahon, Neil Buxton, Emmanuel Chavredakis, Andrew R Brodbelt, David DA Lawson, Paul Eldridge, Jibril Farah, Rasheed Zakaria, Geraint Sunderland and Tom Solomon
-
Western General Hospital and Royal Hospital for Sick Children, Edinburgh – Jothy Kandasamy (PI), Mark Hughes (Co-PI) and Paul Brennan.
We wish to acknowledge the charity Shine for its continued support.
Contributions of authors
Conor L Mallucci (https://orcid.org/0000-0002-5509-0547) (Chief Investigator and Consultant Neurosurgeon) developed the trial protocol in collaboration with co-investigators. He oversaw the delivery of the trial, prepared trial update reports and oversaw clinical aspects of the statistical analysis plan and clinical interpretation of the trial data. He co-led the preparation of the final report (drafting, reviewing and editing). He was chairperson of the TMG and a member of the central review panel.
Michael D Jenkinson (https://orcid.org/0000-0003-4587-2139) (Co-Chief Investigator and Consultant Neurosurgeon) developed the funding application and trial protocol in collaboration with co-investigators. He contributed to clinical interpretation of the trial data and to the preparation of the final report (drafting, reviewing and editing).
Elizabeth J Conroy (https://orcid.org/0000-0003-4858-727X) (Trial Statistician) contributed to protocol development and data capture methods, wrote the statistical analysis plan, undertook the final statistical analysis under the supervision of Carrol Gamble, prepared data for reports throughout the trial, prepared data tables and figures for final report and co-led the preparation of the final report.
John C Hartley (https://orcid.org/0000-0003-0503-5985) (Trial Microbiologist) contributed to protocol development, data capture methods, central classification of infections, preparation and review of progress and final reports.
Michaela Brown (https://orcid.org/0000-0002-7772-271X) (Senior Statistician) contributed to protocol development and data capture methods, proposed statistical analysis methods, approved the statistical analysis plan and oversaw trial monitoring activities.
Tracy Moitt (https://orcid.org/0000-0002-5579-996X) (Senior Trials Manager) contributed to protocol development, gave guidance and support on all aspects of governance and trial delivery and supported the preparation of progress reports. She also contributed to the final report (drafting and reviewing).
Joanne Dalton (https://orcid.org/0000-0003-1199-3047) (Data Manager) supported the central review panel in its assessment.
Tom Kearns (https://orcid.org/0000-0001-5416-8376) (Trial Co-ordinator) contributed to protocol development, all aspects of governance and study delivery and preparation of progress reports. He also contributed to the final report (reviewing).
Michael J Griffiths (https://orcid.org/0000-0003-1851-6155) (Senior Lecturer in Paediatic Neurology) co-ordinated and developed the sample collection substudy; contributed to protocol development; oversaw sample recruitment, collection and storage; and contributed to the preparation of the final report (reviewing, editing).
Giovanna Culeddu (https://orcid.org/0000-0001-5032-4255) (Study Health Economist) contributed to protocol development and data capture methods, undertook the economic analysis under the supervision of Dyfrig Hughes and contributed to the final report (drafting).
Tom Solomon (https://orcid.org/0000-0001-7266-6547) (Professor of Neurology) helped devise the trial, assisted with obtaining grant funding and reviewed the final report.
Dyfrig Hughes (https://orcid.org/0000-0001-8247-7459) (Senior Health Economist) led the economic evaluation, contributed to protocol development and data capture methods and contributed to the writing of the report (drafting, reviewing and editing).
Carrol Gamble (https://orcid.org/0000-0002-3021-1955) (Statistical Lead) led trial design and developed the funding application, trial protocol and data capture methods in collaboration with co-investigators. She led blind review of the data and supervised the final analysis. She also contributed to the preparation of the final report (drafting, reviewing and editing).
All authors were members of the TMG.
Publications
Jenkinson MD, Gamble C, Hartley JC, Hickey H, Hughes D, Blundell M, et al. The British antibiotic and silver-impregnated catheters for ventriculoperitoneal shunts multi-centre randomised controlled trial (the BASICS trial): study protocol. Trials 2014;15:4.
Mallucci CL, Jenkinson MD, Conroy EJ, Hartley JC, Brown M, Dalton J, et al. Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): a multicentre, single-blinded, randomised trial and economic evaluation. Lancet 2019;394:1530–9.
Data-sharing statement
All requests for data should be sent to the corresponding author. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Jenkinson MD, Gamble C, Hartley JC, Hickey H, Hughes D, Blundell M, et al. The British antibiotic and silver-impregnated catheters for ventriculoperitoneal shunts multi-centre randomised controlled trial (the BASICS trial): study protocol. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-4.
- Mallucci CL, Jenkinson MD, Conroy EJ, Hartley JC, Brown M, Dalton J, et al. Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): a multicentre, single-blinded, randomised trial and economic evaluation. Lancet 2019;394:1530-9. https://doi.org/10.1016/S0140-6736(19)31603-4.
- Dewan MC, Rattani A, Mekary R, Glancz LJ, Yunusa I, Baticulon RE, et al. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg 2018;130:1065-79. https://doi.org/10.3171/2017.10.JNS17439.
- Richards HK, Seeley HM, Pickard JD. Efficacy of antibiotic-impregnated shunt catheters in reducing shunt infection: data from the United Kingdom Shunt Registry. J Neurosurg Pediatr 2009;4:389-93. https://doi.org/10.3171/2009.4.PEDS09210.
- Vinchon M, Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst 2006;22:692-7. https://doi.org/10.1007/s00381-005-0037-8.
- Borgbjerg BM, Gjerris F, Albeck MJ, Børgesen SE. Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first-time shunts. Acta Neurochir 1995;136:1-7. https://doi.org/10.1007/bf01411427.
- Choux M, Genitori L, Lang D, Lena G. Shunt implantation: reducing the incidence of shunt infection. J Neurosurg 1992;77:875-80. https://doi.org/10.3171/jns.1992.77.6.0875.
- Enger PØ, Svendsen F, Wester K. CSF shunt infections in children: experiences from a population-based study. Acta Neurochir 2003;145:243-8. https://doi.org/10.1007/s00701-002-1068-5.
- Pople IK, Bayston R, Hayward RD. Infection of cerebrospinal fluid shunts in infants: a study of etiological factors. J Neurosurg 1992;77:29-36. https://doi.org/10.3171/jns.1992.77.1.0029.
- Ronan A, Hogg GG, Klug GL. Cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J 1995;14:782-6. https://doi.org/10.1097/00006454-199509000-00010.
- Schreffler RT, Schreffler AJ, Wittler RR. Treatment of cerebrospinal fluid shunt infections: a decision analysis. Pediatr Infect Dis J 2002;21:632-6. https://doi.org/10.1097/00006454-200207000-00006.
- van Tonder L, Griffiths M, Mallucci C, Jenkinson M, Kamali I, Griffiths M, et al. PO250 study of the feasibility and accuracy of next generation sequencing, proteomics, transcriptomics and cytokine measurement for improving the diagnosis of neurosurgical cerebrospinal fluid infection. J Neurol Neurosurg Psychiatry 2017;88. https://doi.org/10.1136/jnnp-2017-ABN.271.
- Richards H, Mallucci CL, Sgouros S. Cerebrospinal Fluid Disorders. New York, NY: Informa Healthcare; 2010.
- Drake JM, Kestle JR, Milner R, Cinalli G, Boop F, Piatt J, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery 1998;43:294-303. https://doi.org/10.1097/00006123-199808000-00068.
- Govender ST, Nathoo N, van Dellen JR. Evaluation of an antibiotic-impregnated shunt system for the treatment of hydrocephalus. J Neurosurg 2003;99:831-9. https://doi.org/10.3171/jns.2003.99.5.0831.
- Hayhurst C, Cooke R, Williams D, Kandasamy J, O’Brien DF, Mallucci CL. The impact of antibiotic-impregnated catheters on shunt infection in children and neonates. Childs Nerv Syst 2008;24:557-62. https://doi.org/10.1007/s00381-007-0521-4.
- Kandasamy J, Dwan K, Hartley JC, Jenkinson MD, Hayhurst C, Gatscher S, et al. Antibiotic-impregnated ventriculoperitoneal shunts – a multi-centre British paediatric neurosurgery group (BPNG) study using historical controls. Childs Nerv Syst 2011;27:575-81. https://doi.org/10.1007/s00381-010-1290-z.
- Bayston R, Vera L, Mills A, Ashraf W, Stevenson O, Howdle SM. In vitro antimicrobial activity of silver-processed catheters for neurosurgery. J Antimicrob Chemother 2010;65:258-65. https://doi.org/10.1093/jac/dkp420.
- Secer HI, Kural C, Kaplan M, Kilic A, Duz B, Gonul E, et al. Comparison of the efficacies of antibiotic-impregnated and silver-impregnated ventricular catheters on the prevention of infections. An in vitro laboratory study. Pediatr Neurosurg 2008;44:444-7. https://doi.org/10.1159/000172966.
- Izci Y, Secer H, Akay C, Gonul E. Initial experience with silver-impregnated polyurethane ventricular catheter for shunting of cerebrospinal fluid in patients with infected hydrocephalus. Neurol Res 2009;31:234-7. https://doi.org/10.1179/174313209X380973.
- Fichtner J, Güresir E, Seifert V, Raabe A. Efficacy of silver-bearing external ventricular drainage catheters: a retrospective analysis. J Neurosurg 2010;112:840-6. https://doi.org/10.3171/2009.8.JNS091297.
- Keong NC, Bulters DO, Richards HK, Farrington M, Sparrow OC, Pickard JD, et al. The SILVER (Silver Impregnated Line Versus EVD Randomized trial): a double-blind, prospective, randomized, controlled trial of an intervention to reduce the rate of external ventricular drain infection. Neurosurgery 2012;71:394-403. https://doi.org/10.1227/NEU.0b013e318257bebb.
- Thomas R, Lee S, Patole S, Rao S. Antibiotic-impregnated catheters for the prevention of CSF shunt infections: a systematic review and meta-analysis. Br J Neurosurg 2012;26:175-84. https://doi.org/10.3109/02688697.2011.603856.
- Lackner P, Beer R, Broessner G, Helbok R, Galiano K, Pleifer C, et al. Efficacy of silver nanoparticles-impregnated external ventricular drain catheters in patients with acute occlusive hydrocephalus. Neurocrit Care 2008;8:360-5. https://doi.org/10.1007/s12028-008-9071-1.
- Kulkarni AV, Rabin D, Drake JM. An instrument to measure the health status in children with hydrocephalus: the Hydrocephalus Outcome Questionnaire. J Neurosurg 2004;101:134-40. https://doi.org/10.3171/ped.2004.101.2.0134.
- Pintilie M. Dealing with competing risks: testing covariates and calculating sample size. Stat Med 2002;21:3317-24. https://doi.org/10.1002/sim.1271.
- Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol 1971;44:793-7. https://doi.org/10.1259/0007-1285-44-526-793.
- Miller RG. Simultaneous Statistical Inference. New York, NY: McGraw-Hill; 1966.
- Fine PF, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. https://doi.org/10.1080/01621459.1999.10474144.
- Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol 2013;66:648-53. https://doi.org/10.1016/j.jclinepi.2012.09.017.
- Austin PC, Fine JP. Practical recommendations for reporting Fine–Gray model analyses for competing risk data. Stat Med 2017;36:4391-400. https://doi.org/10.1002/sim.7501.
- Eymann R, Chehab S, Strowitzki M, Steudel WI, Kiefer M. Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. J Neurosurg Pediatr 2008;1:444-50. https://doi.org/10.3171/PED/2008/1/6/444.
- Farber SH, Parker SL, Adogwa O, Rigamonti D, McGirt MJ. Cost analysis of antibiotic-impregnated catheters in the treatment of hydrocephalus in adult patients. World Neurosurg 2010;74:528-31. https://doi.org/10.1016/j.wneu.2010.07.014.
- Attenello FJ, Garces-Ambrossi GL, Zaidi HA, Sciubba DM, Jallo GI. Hospital costs associated with shunt infections in patients receiving antibiotic-impregnated shunt catheters versus standard shunt catheters. Neurosurgery 2010;66:284-9. https://doi.org/10.1227/01.NEU.0000363405.12584.4D.
- Parker SL, Farber SH, Adogwa O, Rigamonti D, McGirt MJ. Comparison of hospital cost and resource use associated with antibiotic-impregnated versus standard shunt catheters. Clin Neurosurg 2011;58:122-5. https://doi.org/10.1227/neu.0b013e318226ffe5.
- Parker SL, McGirt MJ, Murphy JA, Megerian JT, Stout M, Engelhart L. Cost savings associated with antibiotic-impregnated shunt catheters in the treatment of adult and pediatric hydrocephalus. World Neurosurg 2015;83:382-6. https://doi.org/10.1016/j.wneu.2014.06.010.
- Root BK, Barrena BG, Mackenzie TA, Bauer DF. Antibiotic impregnated external ventricular drains: meta and cost analysis. World Neurosurg 2016;86:306-15. https://doi.org/10.1016/j.wneu.2015.09.032.
- Klimo P, Thompson CJ, Ragel BT, Boop FA. Antibiotic-impregnated shunt systems versus standard shunt systems: a meta- and cost-savings analysis. J Neurosurg Pediatr 2011;8:600-12. https://doi.org/10.3171/2011.8.PEDS11346.
- Edwards NC, Engelhart L, Casamento EM, McGirt MJ. Cost–consequence analysis of antibiotic-impregnated shunts and external ventricular drains in hydrocephalus. J Neurosurg 2015;122:139-47. https://doi.org/10.3171/2014.9.JNS131277.
- Ridyard CH, Hughes DA. Methods for the collection of resource use data within clinical trials: a systematic review of studies funded by the UK Health Technology Assessment program. Value Health 2010;13:867-72. https://doi.org/10.1111/j.1524-4733.2010.00788.x.
- Database of Instruments for Resource Use Measurement . BASICS Health Service Diary n.d. www.dirum.org/instruments/details/112 (accessed 15 March 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Geue C, Lewsey J, Lorgelly P, Govan L, Hart C, Briggs A. Spoilt for choice: implications of using alternative methods of costing hospital episode statistics. Health Econ 2012;21:1201-16. https://doi.org/10.1002/hec.1785.
- Department of Health and Social Care . NHS Reference Costs 2015 to 2016 n.d. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 10 March 2019).
- NHS England . NHS National Tariff Payment System 2016 17 n.d. www.gov.uk/government/publications/nhs-national-tariff-payment-system-201617 (accessed 10 March 2019).
- Joint Formulary Committee . British National Formulary 2017.
- NHS Business Services Authority . Prescription Cost Analysis Data n.d. www.nhsbsa.nhs.uk/prescription-data/dispensing-data/prescription-cost-analysispca-data (accessed 10 March 2019).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Kulkarni AV, Drake JM, Rabin D, Dirks PB, Humphreys RP, Rutka JT. Measuring the health status of children with hydrocephalus by using a new outcome measure. J Neurosurg 2004;101:141-6. https://doi.org/10.3171/ped.2004.101.2.0141.
- Gabrio A, Mason AJ, Baio G. Handling missing data in within-trial cost-effectiveness analysis: a review with future recommendations. Pharmacoecon Open 2017;1:79-97. https://doi.org/10.1007/s41669-017-0015-6.
- Franklin M, Lomas J, Walker S, Young T. An educational review about using cost data for the purpose of cost-effectiveness analysis. PharmacoEconomics 2019;37:631-43. https://doi.org/10.1007/s40273-019-00771-y.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/process/pmg9/ (accessed 10 March 2019).
- Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ 2011;20:897-916. https://doi.org/10.1002/hec.1653.
- Polsky D, Glick H. Costing and cost analysis in randomized controlled trials: caveat emptor. PharmacoEconomics 2009;27:179-88. https://doi.org/10.2165/00019053-200927030-00001.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci 2007;8:206-13. https://doi.org/10.1007/s11121-007-0070-9.
- Jamjoom AAB, Joannides AJ, Poon MT, Chari A, Zaben M, Abdulla MAH, et al. Prospective, multicentre study of external ventricular drainage-related infections in the UK and Ireland. J Neurol Neurosurg Psychiatry 2018;89:120-6. https://doi.org/10.1136/jnnp-2017-316415.
- Council of the European Union . Regulation (EU) 2016 679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons With Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95 46 EC (General Data Protection Regulation) n.d. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32016R0679&from=EN (accessed 23 January 2020).
Appendix 1 The BASICS study collaborators: principal investigators
Conor L Mallucci (Principal Investigator), Alder Hey Children’s NHS Foundation Trust, Liverpool, UK.
Guirish Solanki (Principal Investigator), Birmingham Children’s Hospital, Birmingham, UK.
Richard Edwards (Principal Investigator), Frenchay Hospital, Bristol, UK.
Adam Williams (Co-principal Investigator), Frenchay Hospital, Bristol, UK.
Matthew Garnett (Co-principal Investigator), Addenbrooke’s Hospital, Cambridge, UK.
Angelos Kolias (Co-principal Investigator), Addenbrooke’s Hospital, Cambridge, UK.
Paul Leach (Principal Investigator), University Hospital of Wales, Cardiff, UK.
Darach Crimmins (Principal Investigator), Temple Street Children’s University Hospital, Dublin, Republic of Ireland.
John Caird (Co-principal Investigator), Temple Street Children’s University Hospital, Dublin, Republic of Ireland.
Dominic Thompson (Principal Investigator), Great Ormond Street Hospital, London, UK.
Roger Strachan (Principal Investigator), James Cook University Hospital, Middleborough, UK.
Bassel Zebian (Principal Investigator), King’s College Hospital, London, UK.
Bhaskar Thakur (Co-principal Investigator), King’s College Hospital, London, UK.
John Goodden (Principal Investigator), Leeds General Infirmary, Leeds, UK.
Ahmed Toma (Principal Investigator), National Hospital for Neurology and Neurosurgery, London, UK.
Damian Holliman (Principal Investigator), Newcastle General Hospital, Newcastle upon Tyne, UK.
Ian Coulter (Co-principal Investigator), Newcastle General Hospital, Newcastle upon Tyne, UK.
Donald Macarthur (Principal Investigator), Queen’s Medical Centre, Nottingham, UK.
Ian Kamaly (Principal Investigator), Royal Manchester Children’s Hospital, Manchester, UK.
Andrew King (Principal Investigator), Salford Royal Hospital, Salford, UK.
Ardash Nadig (Co-principal Investigator), Salford Royal Hospital, Salford, UK.
Shungu Ushewokunze (Principal Investigator), Sheffield Children’s Hospital and Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK.
Saurabh Sinha (Co-principal Investigator), Sheffield Children’s Hospital and Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK.
Diederik Bulters (Principal Investigator), Southampton General Hospital, Southampton, UK.
Ryan Waters (Co-principal Investigator), Southampton General Hospital, Southampton, UK.
Michael D Jenkinson (Principal Investigator), Walton Centre, Liverpool, UK.
Jothy Kandasamy (Principal Investigator), Western General Hospital, Edinburgh, UK.
Mark Hughes (Co-principal Investigator), Western General Hospital, Edinburgh, UK.
Appendix 2 Clinical effectiveness study: additional data
Parts of this appendix have been reproduced from Mallucci et al. 2 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
| Centre code | Date of centre openinga | Date of centre closure | Initial planned total recruitment (n) | Date of first randomisation | Date of last randomisation | Number randomised |
|---|---|---|---|---|---|---|
| 0243 | 26 June 2013 | 9 October 2017 | 70 | 26 June 2013 | 12 September 2017 | 119 |
| 0114 | 3 July 2013 | 30 September 2017 | 115 | 5 July 2013 | 15 September 2017 | 175 |
| 0578 | 24 July 2013 | 9 October 2017 | 100 | 25 July 2013 | 9 October 2017 | 155 |
| 0232 | 1 October 2013 | 28 August 2015 | 50 | 29 December 2013 | 12 August 2015 | 22 |
| 0249 | 7 October 2013 | 31 August 2017 | 100 | 16 October 2013 | 4 August 2017 | 71 |
| 0400 | 8 October 2013 | 30 September 2017 | 80 | 23 October 2013 | 4 July 2017 | 82 |
| 0213 | 1 November 2013 | 30 September 2017 | 90 | 27 November 2013 | 13 September 2017 | 141 |
| 0248 | 21 November 2013 | 30 September 2017 | 50 | 16 December 2013 | 10 May 2017 | 41 |
| 0133 | 10 December 2013 | 31 August 2016 | 40 | 28 January 2014 | 24 June 2016 | 30 |
| 0007 | 21 January 2014 | 30 September 2017 | 60 | 7 February 2014 | 13 September 2017 | 85 |
| 0246 | 12 March 2014 | 30 September 2017 | 65 | 29 April 2014 | 22 September 2017 | 48 |
| 0352 | 1 April 2014 | 30 September 2017 | 65 | 7 April 2014 | 20 September 2017 | 129 |
| 0030 | 29 April 2014 | 30 September 2017 | 115 | 17 June 2014 | 22 September 2017 | 92 |
| 0185 | 17 June 2014 | 30 September 2017 | 65 | 26 June 2014 | 10 July 2017 | 188 |
| 0161 | 25 September 2014 | 30 September 2017 | 60 | 25 September 2014 | 15 December 2016 | 36 |
| 0361 | 1 October 2014 | 31 August 2016 | 20 | 31 October 2014 | 3 September 2015 | 5 |
| 0393 | 1 October 2014 | 31 August 2016 | 20 | 4 March 2015 | 14 January 2016 | 8 |
| 0006 | 7 January 2015 | 30 September 2017 | 0 | 30 January 2015 | 19 May 2017 | 22 |
| 9999 | 23 February 2015 | 30 September 2017 | 35 | 23 February 2015 | 5 June 2017 | 69 |
| 0672 | 1 April 2015 | 30 September 2017 | 0 | 16 April 2015 | 4 September 2017 | 73 |
| 0004 | 20 October 2015 | 30 September 2017 | 0 | 6 November 2015 | 9 September 2016 | 14 |
| All centres | 26 June 2013 | 9 October 2017 | 1200 | 26 June 2013 | 9 October 2017 | 1605 |
| Centre codea | Number of patients screenedb (n) | Ineligible,c n (%) | Consent not sought,d n (%) | Consent not provided (a), n (%) | Consented but not randomised (b), n (%) | Randomised (c), n (%) | Consent rate (%) b+ca+b+c |
|---|---|---|---|---|---|---|---|
| 0243 | 194 | 47 (24.2) | 5 (2.6) | 18 (9.3) | 5 (2.6) | 119 (61.3) | 87.3 |
| 0114 | 508 | 197 (38.8) | 68 (13.4) | 55 (10.8) | 13 (2.6) | 175 (34.4) | 77.4 |
| 0578 | 223 | 33 (14.8) | 14 (6.3) | 14 (6.3) | 7 (3.1) | 155 (69.5) | 92.0 |
| 0232 | 70 | 32 (45.7) | 9 (12.9) | 4 (5.7) | 3 (4.3) | 22 (31.4) | 86.2 |
| 0249 | 164 | 26 (15.9) | 28 (17.1) | 35 (21.3) | 3 (1.8) | 71 (43.3) | 67.9 |
| 0400 | 266 | 87 (32.7) | 62 (23.3) | 26 (9.8) | 8 (3.0) | 82 (30.8) | 77.6 |
| 0213 | 327 | 105 (32.1) | 52 (15.9) | 26 (8.0) | 3 (0.9) | 141 (43.1) | 84.7 |
| 0248 | 130 | 54 (41.5) | 11 (8.5) | 21 (16.2) | 2 (1.5) | 41 (31.5) | 67.2 |
| 0133 | 105 | 26 (24.8) | 28 (26.7) | 21 (20.0) | 0 (0.0) | 30 (28.6) | 58.8 |
| 0007 | 184 | 12 (6.5) | 59 (32.1) | 22 (12.0) | 6 (3.3) | 85 (46.2) | 80.5 |
| 0246 | 200 | 129 (64.5) | 12 (6.0) | 9 (4.5) | 2 (1.0) | 48 (24.0) | 84.7 |
| 0352 | 238 | 65 (27.3) | 18 (7.6) | 20 (8.4) | 1 (0.4) | 129 (54.2) | 86.7 |
| 0030 | 199 | 71 (35.7) | 11 (5.5) | 21 (10.6) | 3 (1.5) | 92 (46.2) | 81.9 |
| 0185 | 225 | 3 (1.3) | 17 (7.6) | 10 (4.4) | 7 (3.1) | 188 (83.6) | 95.1 |
| 0161 | 53 | 1 (1.9) | 1 (1.9) | 15 (28.3) | 0 (0.0) | 36 (67.9) | 70.6 |
| 0361 | 8 | 1 (12.5) | 2 (25.0) | 0 (0.0) | 0 (0.0) | 5 (62.5) | 100.0 |
| 0393 | 11 | 0 (0.0) | 0 (0.0) | 2 (18.2) | 1 (9.1) | 8 (72.7) | 81.8 |
| 0006 | 36 | 10 (27.8) | 3 (8.3) | 1 (2.8) | 0 (0.0) | 22 (61.1) | 95.7 |
| 9999 | 144 | 34 (23.6) | 13 (9.0) | 26 (18.1) | 2 (1.4) | 69 (47.9) | 73.2 |
| 0672 | 176 | 62 (35.2) | 19 (10.8) | 21 (11.9) | 1 (0.6) | 73 (41.5) | 77.9 |
| 0004 | 44 | 25 (56.8) | 3 (6.8) | 2 (4.5) | 0 (0.0) | 14 (31.8) | 87.5 |
FIGURE 8.
Recruitment graph.
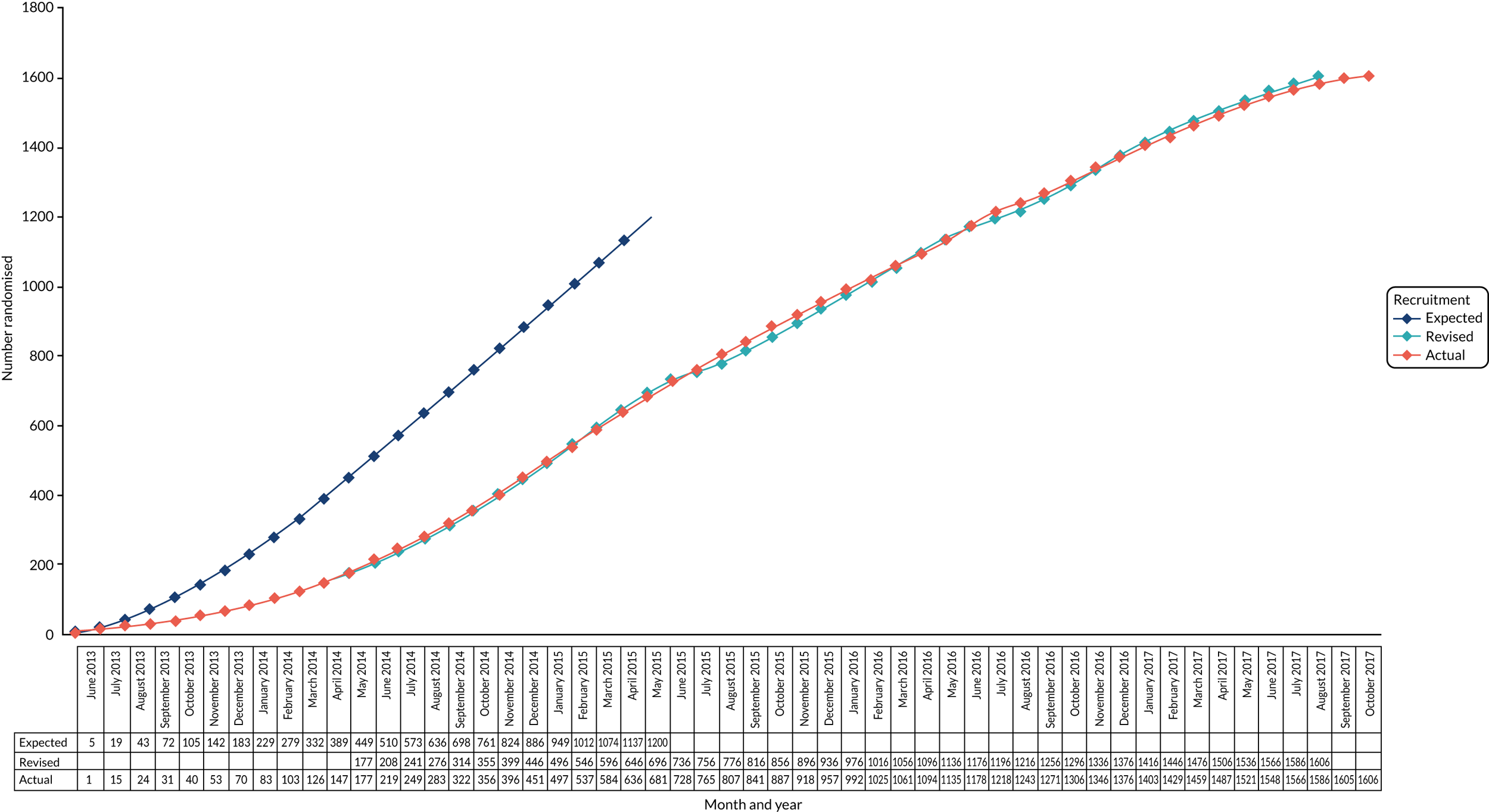
| Reason | Trial group, n (%) | Total (N = 16), n (%) | ||
|---|---|---|---|---|
| Standard VPS (N = 6) | Antibiotic-impregnated VPS (N = 7) | Silver-impregnated VPS (N = 3) | ||
| Allocated VPS not available | 1 (16.7) | 1 (14.3) | 0 (0.0) | 2 (12.5) |
| Allocated catheter not available | 3 (50.0) | 1 (14.3) | 0 (0.0) | 4 (25.0) |
| Allocated shunt not available | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (6.3) |
| Allocated shunt too short | 0 (0.0) | 1 (14.3) | 1 (33.3) | 2 (12.5) |
| Allocated tubing not available | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (6.3) |
| Catheter too firm; difficult to remove if required | 0 (0.0) | 0 (0.0) | 1 (33.3) | 1 (6.3) |
| Consultant felt that allocated shunt would affect quality of future scans | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (6.3) |
| Miscommunication within the trial team | 0 (0.0) | 1 (14.3) | 1 (33.3) | 2 (12.5) |
| No long catheter available for allocated VPS | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (6.3) |
| Technical difficulties | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (6.3) |
| Reason | Trial group, n (%) | Total (N = 4), n (%) | ||
|---|---|---|---|---|
| Standard VPS (N = 0) | Antibiotic-impregnated VPS (N = 2) | Silver-impregnated VPS (N = 2) | ||
| MRI brain reviewed (done pre surgery); decision made to cancel surgery as ventricle size had reduced | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (25.0) |
| Unsuccessful ventricular shunt catheter insertion | 0 (0.0) | 1 (50.0) | 0 (0.0) | 1 (25.0) |
| Abnormal ECG; surgery abandoned | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (25.0) |
| Pus found intraoperatively; EVD inserted | 0 (0.0) | 1 (50.0) | 0 (0.0) | 1 (25.0) |
| Deviation | Trial group, n (%) | Total (N = 1605), n (%) | ||
|---|---|---|---|---|
| Standard VPS (N = 536) | Antibiotic-impregnated VPS (N = 538) | Silver-impregnated VPS (N = 531) | ||
| Any protocol deviation | 134 (25.0) | 146 (27.1) | 159 (29.9) | 439 (27.4) |
| Major deviations | ||||
| PD1: consent not obtained | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PD2: patient has had at least one previous indwelling VPS | 2 (0.4) | 2 (0.4) | 1 (0.2) | 5 (0.3) |
| PD3: patient had an active CSF infection at the time of VPS insertion | 3 (0.6) | 1 (0.2) | 3 (0.6) | 7 (0.4) |
| PD4: patient had an allergy to the antibiotics with which one of the randomised shunts is impregnated | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PD5: patient could have an allergy to the silver with which one of the randomised shunts is impregnated | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PD6: patient did not receive allocated intervention | 0 (0.0) | 2 (0.4) | 2 (0.4) | 4 (0.2) |
| PD7A: CSF samples not taken or sent for culture at time of VPS insertion | 16 (3.0) | 12 (2.2) | 19 (3.6) | 47 (2.9) |
| PD7B: CSF samples not taken or sent for culture at time shunt revision/removal | 6 (1.1) | 3 (0.6) | 5 (0.9) | 14 (0.9) |
| PD7C: shunt components not taken for culture at shunt revision/removal | 99 (18.5) | 108 (20.1) | 113 (21.3) | 320 (19.9) |
| At least one major deviation | 112 (20.9) | 120 (22.3) | 131 (24.7) | 363 (22.6) |
| Minor deviations | ||||
| PD8: patient had multiloculated hydrocephalus, necessitating multiple VPSs or neuroendoscopy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PD9: patient had ventriculoatrial or ventriculopleural shunt planned | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| PD10: patient prematurely withdrew from follow-up | 14 (2.6) | 22 (4.1) | 17 (3.2) | 53 (3.3) |
| PD11: patient missed scheduled assessments (early post-operative assessment) | 1 (0.2) | 2 (0.4) | 6 (1.1) | 9 (0.6) |
| PD12: randomisation envelope opened out of sequence | 12 (2.2) | 9 (1.7) | 10 (1.9) | 31 (1.9) |
| PD13: unblinding occurred | 11 (2.1) | 6 (1.1) | 15 (2.8) | 32 (2.0) |
| At least one minor deviation | 38 (7.1) | 37 (6.9) | 45 (8.5) | 120 (7.5) |
FIGURE 9.
Cumulative incidence plots of revisions for infection and competing risk as classified by treating surgeon, by VPS group and age group. (a) Infection by VPS group; (b) competing risk by VPS group; (c) infection by age group; (d) competing risk by age group.
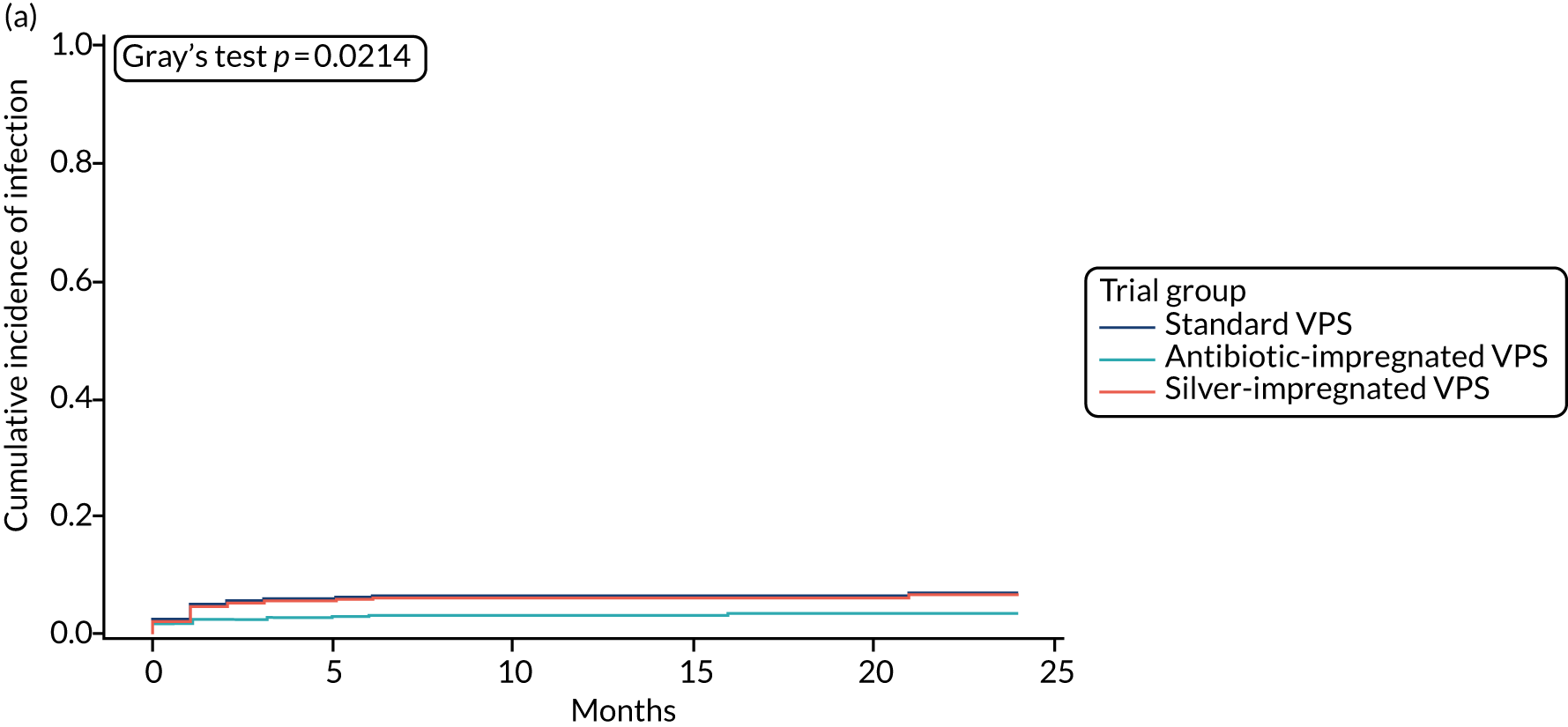
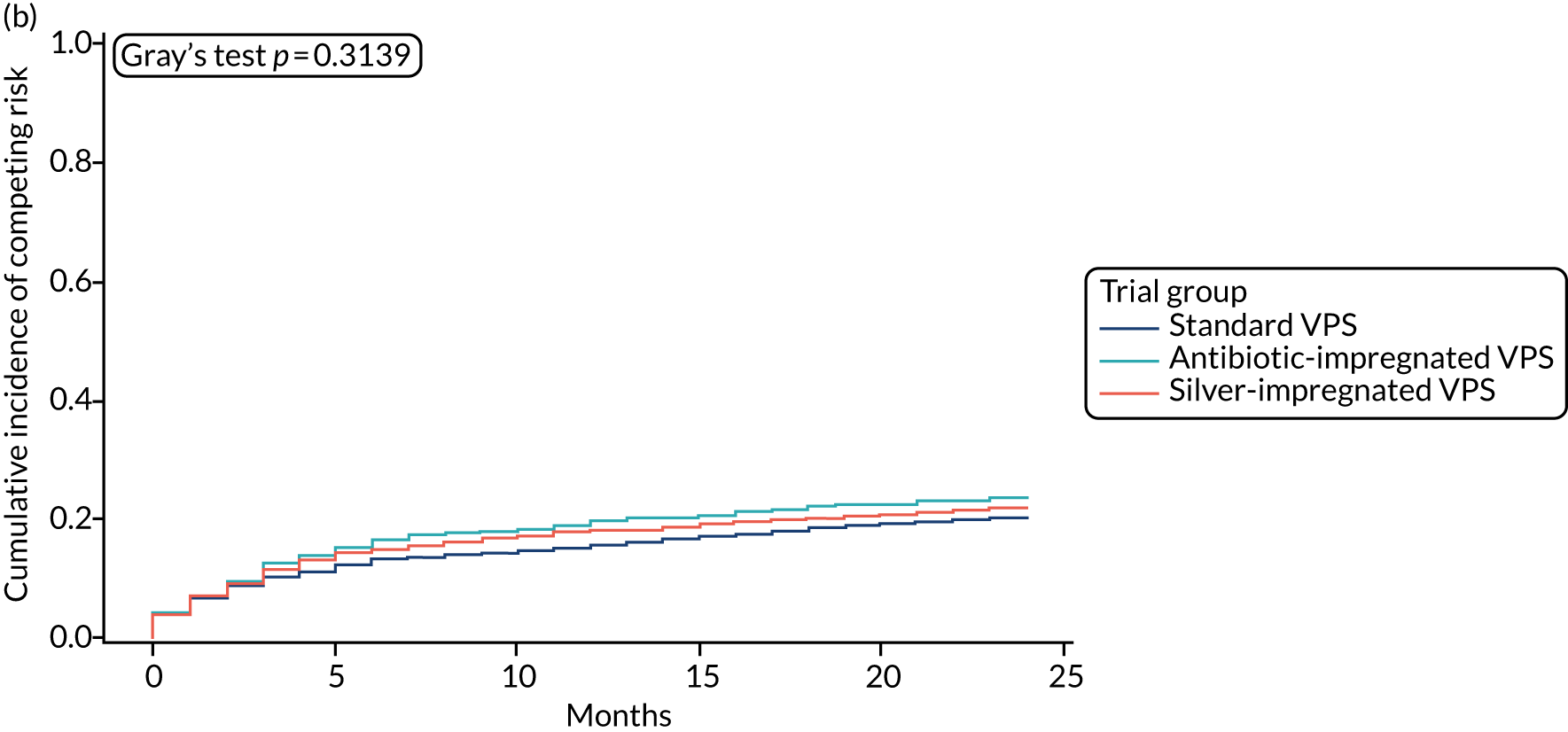
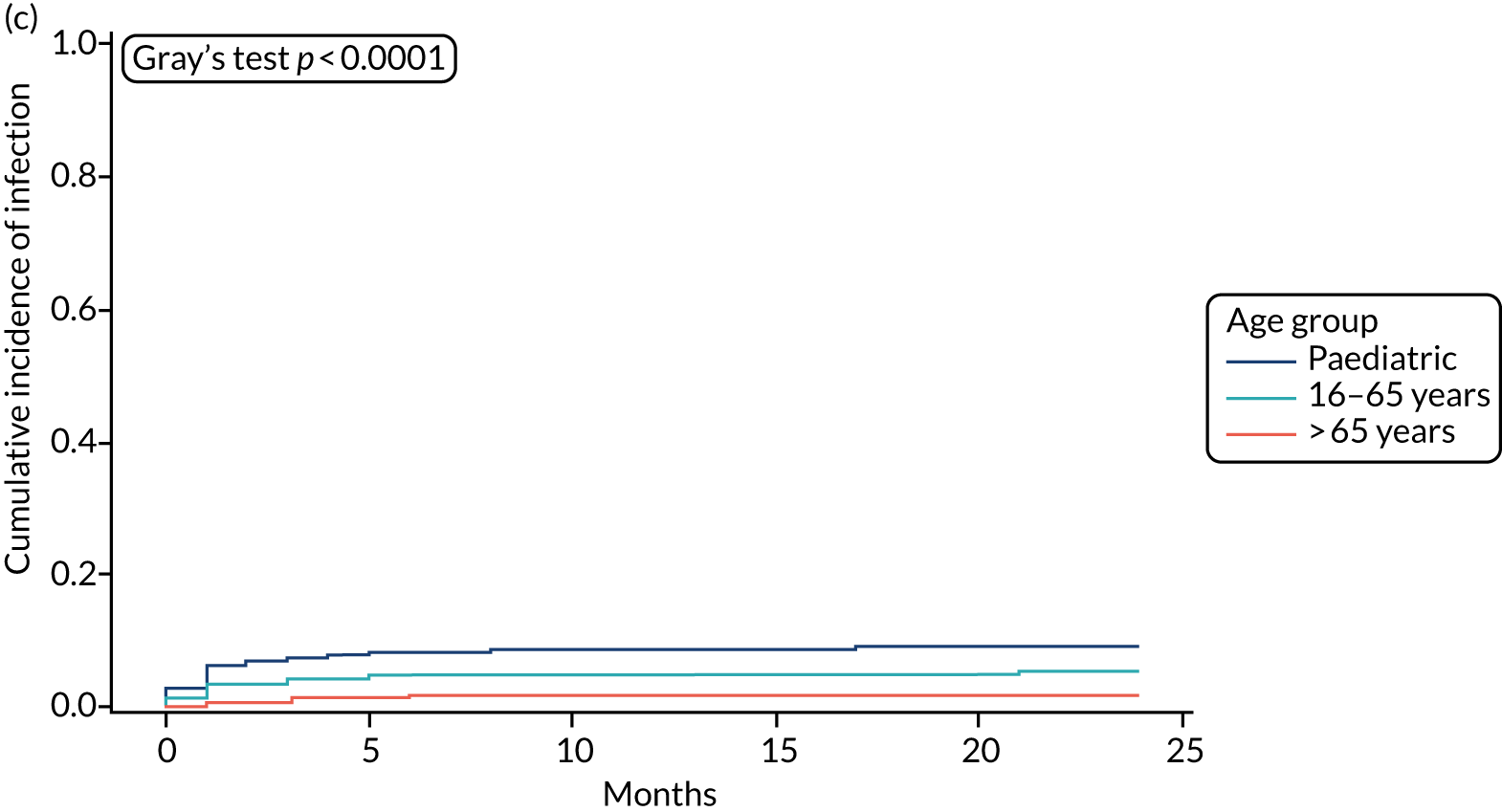
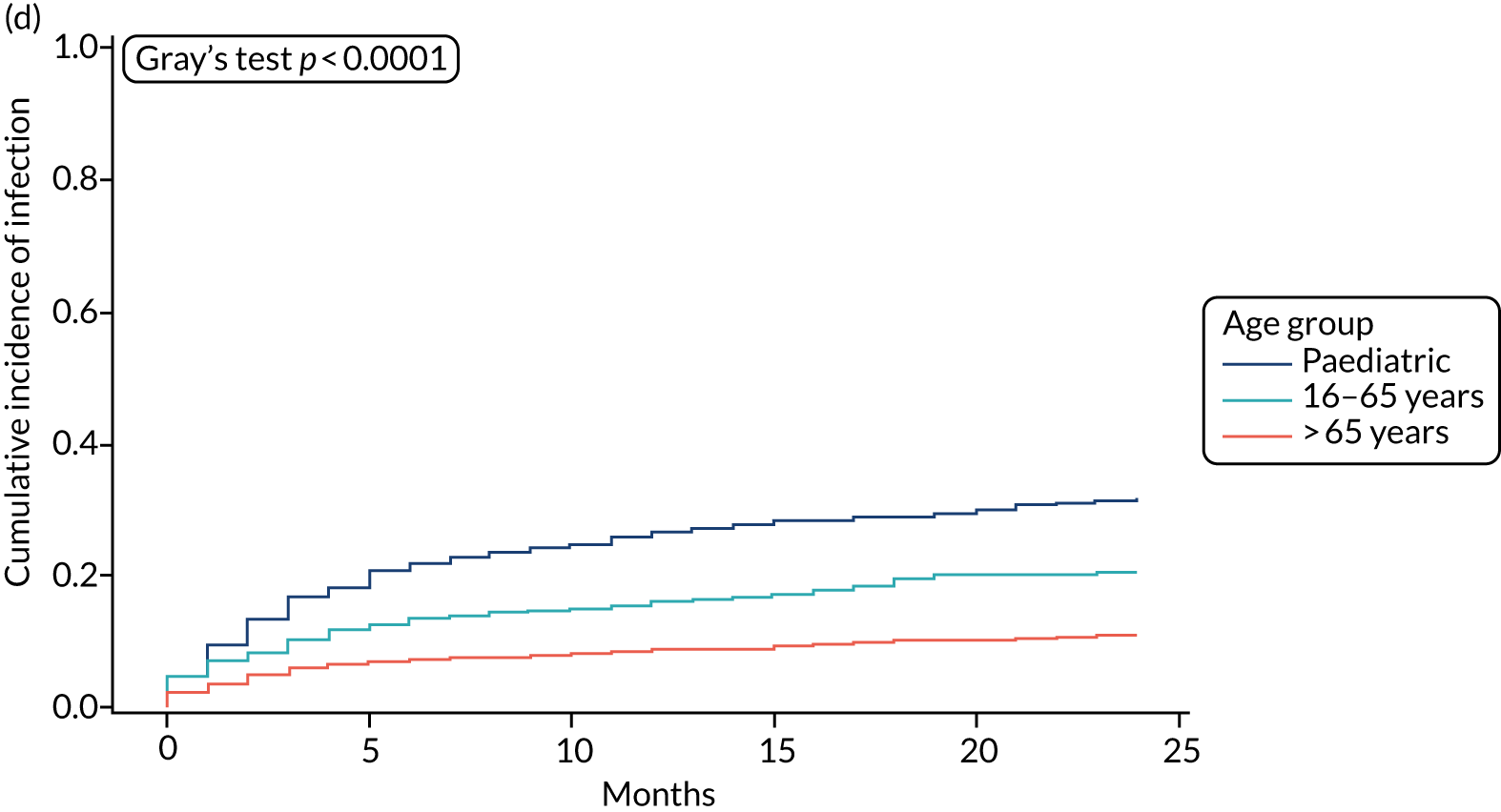
| Infection | Type of infection | Organism cultured | Sample (n) | Antibiotic sensitivities | ||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Resistance | Partial sensitivity | Sensitivities unknown | Antibiotics unknown | ||||
| (A) Line listings of infections associated with the standard VPS (n = 23) | ||||||||
| 1 | A ‘definite – culture positive’ infection | Staphylococcus capitas | 1 | Vancomycin | ||||
| 2 | A ‘definite – culture positive’ infection | Coagulase-negative staphylococcus | 1 | Yes | ||||
| Serratia species | a | Yes | ||||||
| Staphylococcus capitas | 1 | Clarithromycin, doxycycline, erythromycin, flucloxacillin, Fucidin® (Leo Pharma A/S, Ballerup, Denmark) | ||||||
| 3 | A ‘probable – culture uncertain’ infection | Staphylococcus epidermidis | 1 | Yes | ||||
| 4 | A ‘definite – culture positive’ infection | Pseudomonas aeruginosa | 1 | Meropenem | ||||
| 2 | Meropenem | |||||||
| 3 | Meropenem | |||||||
| 5 | A ‘definite – culture positive’ infection | Staphylococcus epidermidis | 1 | Vancomycin | Ciprofloxacin, clindamycin, flucloxacillin, gentamicin, rifampicin, trimethoprim | |||
| 2 | Fusidic acid; vancomycin | Erythromycin, flucloxacillin, penicillin | ||||||
| 6 | A ‘definite – culture positive’ infection | Klebsiella pneumoniae | 1 | Co-amoxiclav (Augmentin®; GlaxoSmithKline plc, London, UK) | Amoxicillin, ampicillin | |||
| 7 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Cefuroxime, flucloxacillin | ||||
| 8 | A ‘definite – culture positive’ infection | Coagulase-negative staphylococcus | 1 | Flucloxacillin | Vancomycin | |||
| 9 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Flucloxacillin, vancomycin | ||||
| 2 | Flucloxacillin | |||||||
| 3 | Flucloxacillin, vancomycin | |||||||
| 10 | A ‘definite – culture positive’ infection | Coagulase-negative staphylococcus | 1 | Vancomycin | ||||
| 11 | A ‘definite – culture positive’ infection | Klebsiella pneumoniae | 1 | Cefotaxime, gentamicin | ||||
| 12 | A ‘definite – culture positive’ infection | Coagulase-negative staphylococcus | 1 | Vancomycin | ||||
| 2 | Vancomycin | |||||||
| 3 | Vancomycin | |||||||
| 13 | A ‘definite – culture positive’ infection | Staphylococcus hominis | 1 | Penicillin | ||||
| 2 | Gentamicin, linezolid, rifampicin, vancomycin | Erythromycin, Fucidin, penicillin | ||||||
| Staphylococcus aureus | 1 | Amikacin, flucloxacillin, gentamicin, rifampicin, teicoplanin, vancomycin | ||||||
| 2 | Erythromycin, flucloxacillin, gentamicin, linezolid, penicillin, rifampicin | |||||||
| 3 | Erythromycin, flucloxacillin, Fucidin, gentamicin, linezolid, penicillin, rifampicin, vancomycin | |||||||
| 14 | A ‘definite – culture positive’ infection | Staphylococcus epidermidis | 1 | Amikacin, vancomycin | Flucloxacillin | |||
| 15 | A ‘definite – culture positive’ infection | Coagulase-negative staphylococcus | 1 | Yes | ||||
| 16 | A ‘definite – culture positive’ infection | Staphylococcus species mixed | 1 | Yes | ||||
| 2 | Yes | |||||||
| 17 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Flucloxacillin | ||||
| 2 | Flucloxacillin | |||||||
| 18 | A ‘definite – culture positive’ infection | Klebsiella pneumoniae | 1 | Meropenem | ||||
| 2 | Meropenem | |||||||
| 19 | A ‘definite – culture positive’ infection | Staphylococcus epidermidis | 1 | Vancomycin | ||||
| 2 | Vancomycin | |||||||
| 3 | Vancomycin | |||||||
| 20 | A ‘definite – culture positive’ infection | Serratia marcescens | 1 | Gentamicin, meropenem | ||||
| 21 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Chloramphenicol | ||||
| 2 | Erythromycin, flucloxacillin | |||||||
| 3 | Chloramphenicol, teicoplanin, vancomycin | |||||||
| 4 | Chloramphenicol, teicoplanin, vancomycin | |||||||
| 5 | Chloramphenicol, teicoplanin, vancomycin | |||||||
| 22 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Vancomycin | ||||
| 3 | Vancomycin | |||||||
| Staphylococcus capitas | 2 | Vancomycin | ||||||
| 23 | A ‘definite – culture positive’ infection | Streptococcus salivaris | 1 | Vancomycin | Cefotaxime, penicillin | |||
| (B) Line listings of infections associated with the antibiotic-impregnated VPSs (n = 6) | ||||||||
| 1 | A ‘definite – culture positive’ infection | Pseudomonas aeruginosa | 1 | Ciprofloxacin, gentamicin, piperacillin/tazobactam | ||||
| 2 | A ‘definite – culture positive’ infection | Propionibacterium species | 1 | Penicillin | ||||
| 3 | A ‘definite – culture positive’ infection | Coagulase-negative staphylococcus | 1 | Vancomycin | ||||
| 4 | A ‘definite – culture positive’ infection | Escherichia coli | 1 | Cefotaxime | ||||
| 5 | A ‘definite – culture positive’ infection | Enterobacter cloacae | 1 | Cefotaxime, ciprofloxacin | ||||
| 6 | A ‘definite – culture positive’ infection | Proteus mirabilis | 1 | Cefuroxime | ||||
| (C) Line listings of infections associated with silver-impregnated VPSs (n = 27) | ||||||||
| 1 | A ‘definite – culture positive’ infection | Staphylococcus epidermidis | 1 | |||||
| 2 | A ‘definite – culture positive’ infection | Staphylococcus capitas | 1 | |||||
| 3 | A ‘definite – culture positive’ infection | Streptococcus mitis | 1 | Penicillin, vancomycin | ||||
| 2 | Penicillin, vancomycin | |||||||
| 4 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Erythromycin, flucloxacillin, vancomycin | ||||
| 5 | A ‘probable – culture uncertain’ infection | Staphylococcus aureus | 1 | Flucloxacillin, Vancomycin | Trimethoprim | |||
| 6 | A ‘definite – culture positive’ infection | Staphylococcus epidermidis | 1 | Erythromycin, rifampicin, vancomycin | Flucloxacillin, penicillin | |||
| 2 | Erythromycin, rifampicin, vancomycin | Flucloxacillin, penicillin | ||||||
| 3 | Rifampicin, vancomycin | Flucloxacillin | ||||||
| 7 | A ‘definite – culture positive’ infection | Escherichia coli | 1 | Colomycin® (Teva Pharmaceutical Industries Ltd, Petah Tikva, Israel) (colistin), meropenem | Amoxicillin, ceftazidime, cefuroxime, ciprofloxacin, co-amoxiclav (Augmentin), co-trimoxazole, gentamicin | |||
| 8 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Clarithromycin, erythromycin, flucloxacillin, rifampicin | ||||
| 2 | Yes | |||||||
| 3 | Flucloxacillin, rifampicin | |||||||
| 4 | Clarithromycin, erythromycin, rifampicin | |||||||
| 9 | A ‘definite – culture positive’ infection | Enterobacter cloacae | 1 | |||||
| 2 | ||||||||
| 3 | ||||||||
| 4 | ||||||||
| 5 | ||||||||
| 10 | A ‘definite – culture positive’ infection | Enterobacter cloacae | 1 | |||||
| 11 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Flucloxacillin | ||||
| 12 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Flucloxacillin, vancomycin | ||||
| 2 | Flucloxacillin, vancomycin | |||||||
| 13 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Vancomycin | Flucloxacillin | |||
| 14 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Vancomycin | ||||
| 15 | A ‘definite – culture positive’ infection | Escherichia coli | 1 | Cefotaxime, gentamicin, meropenem | ||||
| 2 | Cefotaxime, gentamicin, meropenem | |||||||
| 16 | A ‘probable – culture uncertain’ infection | Enterococcus faecalis | 1 | Amoxicillin, teicoplanin, vancomycin | ||||
| 17 | A ‘definite – culture positive’ infection | Coagulase-negative staphylococcus | 1 | Vancomycin | ||||
| 2 | Vancomycin | |||||||
| 18 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Flucloxacillin | ||||
| 19 | A ‘definite – culture positive’ infection | Enterococcus faecalis | 1 | Ampicillin, gentamicin, vancomycin | ||||
| 20 | A ‘definite – culture positive’ infection | Coagulase-negative staphylococcus | 1 | Amikacin, erythromycin, flucloxacillin, linezolid | ||||
| 21 | A ‘definite – culture positive’ infection | Coagulase-negative staphylococcus | 1 | Amikacin, cefotaxime, linezolid, rifampicin | ||||
| 22 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Flucloxacillin, rifampicin, teicoplanin, vancomycin | ||||
| 23 | A ‘definite – culture positive’ infection | Propionibacterium acnes | 1 | Yes | ||||
| 2 | Yes | |||||||
| 24 | A ‘definite – culture positive’ infection | Propionibacterium acnes | 1 | Ciprofloxacin, penicillin, vancomycin | ||||
| 25 | A ‘definite – culture positive’ infection | Citrobacter species | 1 | Flucloxacillin | ||||
| Staphylococcus aureus | 1 | Flucloxacillin | ||||||
| 2 | Vancomycin | |||||||
| 26 | A ‘definite – culture positive’ infection | Staphylococcus aureus | 1 | Ciprofloxacin, flucloxacillin, gentamicin, rifampicin, vancomycin | ||||
| 2 | Ceftriaxone | |||||||
| 27 | A ‘definite – culture positive’ infection | Staphylococcus epidermidis | 1 | Chloramphenicol, teicoplanin, vancomycin | Penicillin | |||
| 2 | Clindamycin, daptomycin, flucloxacillin, Fucidin, gentamicin, tetracycline | |||||||
| 3 | Yes | |||||||
| 4 | Yes | |||||||
| 5 | Yes | |||||||
| 6 | Chloramphenicol, vancomycin | |||||||
| 7 | Chloramphenicol, vancomycin | |||||||
| Reason for revision (central panel) | Reason for revision (treating surgeon), n (%) | |
|---|---|---|
| Infection | No infection | |
| Infection | 68 (17.1) | 7 (1.8) |
| No infection | 10 (2.5) | 313 (78.6) |
| Centre code | Centre demographic | Primary outcome set (N) | Revisions | |
|---|---|---|---|---|
| n | % (97.5% CI) | |||
| 0393 | Paediatric only | 8 | 6 | 75.0 (40.7 to 100.0) |
| 9999 | Paediatric only | 68 | 34 | 50.0 (36.4 to 63.6) |
| 0243 | Paediatric only | 118 | 56 | 47.5 (37.2 to 57.8) |
| 0248 | Paediatric only | 40 | 19 | 47.5 (29.8 to 65.2) |
| 0246 | Paediatric only | 47 | 20 | 42.6 (26.4 to 58.7) |
| 0006 | Adults only | 22 | 9 | 40.9 (17.4 to 64.4) |
| 0361 | Adults only | 5 | 2 | 40.0 (0.0 to 89.1) |
| 0249 | Paediatric only | 71 | 23 | 32.4 (20.0 to 44.8) |
| 0400 | Adults only | 81 | 25 | 30.9 (19.4 to 42.4) |
| 0133 | Paediatric only | 30 | 8 | 26.7 (8.6 to 44.8) |
| 0578 | Adults only | 154 | 40 | 26.0 (18.1 to 33.9) |
| 0352 | Both adults and paediatrics | 128 | 30 | 23.4 (15.1 to 31.8) |
| 0030 | Both adults and paediatrics | 91 | 21 | 23.1 (13.2 to 33.3) |
| 0114 | Both adults and paediatrics | 175 | 35 | 20.0 (13.2 to 26.8) |
| 0161 | Both adults and paediatrics | 36 | 6 | 16.7 (2.8 to 30.6) |
| 0213 | Both adults and paediatrics | 140 | 23 | 16.4 (9.4 to 23.4) |
| 0004 | Adults only | 14 | 2 | 14.3 (0.0 to 35.2) |
| 0007 | Both adults and paediatrics | 84 | 12 | 14.3 (5.7 to 22.8) |
| 0672 | Adults only | 73 | 8 | 11.0 (2.8 to 19.1) |
| 0185 | Both adults and paediatrics | 188 | 18 | 9.6 (4.8 to 14.4) |
| 0232 | Adults only | 21 | 1 | 4.8 (0.0 to 15.2) |
| Centre code | Centre demographic | Primary outcome set (N) | Infection | |
|---|---|---|---|---|
| n | % (97.5% CI) | |||
| 0393 | Paediatric only | 8 | 2 | 25.0 (0.0 to 59.3) |
| 0361 | Adults only | 5 | 1 | 20.0 (0.0 to 60.1) |
| 0243 | Paediatric only | 118 | 13 | 11.0 (4.6 to 17.5) |
| 0248 | Paediatric only | 40 | 4 | 10.0 (0.0 to 20.6) |
| 0006 | Adults only | 22 | 2 | 9.1 (0.0 to 22.8) |
| 0249 | Paediatric only | 71 | 6 | 8.5 (1.1 to 15.8) |
| 9999 | Paediatric only | 68 | 5 | 7.4 (0.3 to 14.4) |
| 0161 | Both adults and paediatrics | 36 | 2 | 5.6 (0.0 to 14.1) |
| 0578 | Adults only | 154 | 8 | 5.2 (1.2 to 9.2) |
| 0400 | Adults only | 81 | 4 | 4.9 (0.0 to 10.3) |
| 0030 | Both adults and paediatrics | 91 | 4 | 4.4 (0.0 to 9.2) |
| 0246 | Paediatric only | 47 | 2 | 4.3 (0.0 to 10.9) |
| 0672 | Adults only | 73 | 3 | 4.1 (0.0 to 9.3) |
| 0007 | Both adults and paediatrics | 84 | 3 | 3.6 (0.0 to 8.1) |
| 0213 | Both adults and paediatrics | 140 | 5 | 3.6 (0.1 to 7.1) |
| 0133 | Paediatric only | 30 | 1 | 3.3 (0.0 to 10.7) |
| 0352 | Both adults and paediatrics | 128 | 4 | 3.1 (0.0 to 6.6) |
| 0114 | Both adults and paediatrics | 175 | 3 | 1.7 (0.0 to 3.9) |
| 0185 | Both adults and paediatrics | 188 | 3 | 1.6 (0.0 to 3.6) |
| 0004 | Adults only | 14 | 0 | 0.0 (0.0 to 0.0) |
| 0232 | Adults only | 21 | 0 | 0.0 (0.0 to 0.0) |
| Summary of aetiology by VPS group | Clean insertion,a n (%) | Revision required | Reason for revision | |||||
|---|---|---|---|---|---|---|---|---|
| No revision, n (%) | Revision,b n (%) | Failure due to patient, n (%) | Functional shunt failure, n (%) | Mechanical shunt failure, n (%) | Failure due to infection, n (%) | Failure – no infection,c n (%) | ||
| Total number of patients | 1594 | 1196 | 398 | 14 | 121 | 185 | 78 | 320 |
| Congenital malformations | ||||||||
| Patients with congenital malformations | ||||||||
| Overall | 294 (18.4) | 181 (61.6) | 113 (38.4) | 2 (0.7) | 36 (12.2) | 48 (16.3) | 27 (9.2) | 86 (29.3) |
| Standard VPS | 95 (17.8) | 62 (65.3) | 33 (34.7) | 1 (1.1) | 13 (13.7) | 11 (11.6) | 8 (8.4) | 25 (26.3) |
| Antibiotic-impregnated VPS | 93 (17.4) | 57 (61.3) | 36 (38.7) | 0 (0.0) | 13 (14.0) | 17 (18.3) | 6 (6.5) | 30 (32.3) |
| Silver-impregnated VPS | 106 (20.2) | 62 (58.5) | 44 (41.5) | 1 (0.9) | 10 (9.4) | 20 (18.9) | 13 (12.3) | 31 (29.2) |
| Type(s) of congenital malformation | ||||||||
| Aqueduct stenosis | ||||||||
| Overall | 68 (23.1) | 46 (67.6) | 22 (32.4) | 0 (0.0) | 8 (11.8) | 7 (10.3) | 7 (10.3) | 15 (22.1) |
| Standard VPS | 21 (22.1) | 15 (71.4) | 6 (28.6) | 0 (0.0) | 4 (19.0) | 1 (4.8) | 1 (4.8) | 5 (23.8) |
| Antibiotic-impregnated VPS | 15 (16.1) | 9 (60.0) | 6 (40.0) | 0 (0.0) | 2 (13.3) | 3 (20.0) | 1 (6.7) | 5 (33.3) |
| Silver-impregnated VPS | 32 (30.2) | 22 (68.8) | 10 (31.3) | 0 (0.0) | 2 (6.3) | 3 (9.4) | 5 (15.6) | 5 (15.6) |
| Dandy–Walker | ||||||||
| Overall | 7 (2.4) | 6 (85.7) | 1 (14.3) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 1 (14.3) |
| Standard VPS | 2 (2.1) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) |
| Antibiotic-impregnated VPS | 3 (3.2) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Silver-impregnated VPS | 2 (1.9) | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Chiari | ||||||||
| Overall | 62 (21.1) | 43 (69.4) | 19 (30.6) | 0 (0.0) | 7 (11.3) | 7 (11.3) | 5 (8.1) | 14 (22.6) |
| Standard VPS | 23 (24.2) | 17 (73.9) | 6 (26.1) | 0 (0.0) | 4 (17.4) | 0 (0.0) | 2 (8.7) | 4 (17.4) |
| Antibiotic-impregnated VPS | 22 (23.7) | 15 (68.2) | 7 (31.8) | 0 (0.0) | 3 (13.6) | 4 (18.2) | 0 (0.0) | 7 (31.8) |
| Silver-impregnated VPS | 17 (16.0) | 11 (64.7) | 6 (35.3) | 0 (0.0) | 0 (0.0) | 3 (17.6) | 3 (17.6) | 3 (17.6) |
| Spina bifida | ||||||||
| Overall | 111 (37.8) | 62 (55.9) | 49 (44.1) | 1 (0.9) | 11 (9.9) | 25 (22.5) | 12 (10.8) | 37 (33.3) |
| Standard VPS | 35 (36.8) | 19 (54.3) | 16 (45.7) | 1 (2.9) | 3 (8.6) | 7 (20.0) | 5 (14.3) | 11 (31.4) |
| Antibiotic-impregnated VPS | 34 (36.6) | 22 (64.7) | 12 (35.3) | 0 (0.0) | 4 (11.8) | 7 (20.6) | 1 (2.9) | 11 (32.4) |
| Silver-impregnated VPS | 42 (39.6) | 21 (50.0) | 21 (50.0) | 0 (0.0) | 4 (9.5) | 11 (26.2) | 6 (14.3) | 15 (35.7) |
| Other | ||||||||
| Overall | 72 (24.5) | 38 (52.8) | 34 (47.2) | 1 (1.4) | 12 (16.7) | 13 (18.1) | 8 (11.1) | 26 (36.1) |
| Standard VPS | 27 (28.4) | 18 (66.7) | 9 (33.3) | 0 (0.0) | 4 (14.8) | 4 (14.8) | 1 (3.7) | 8 (29.6) |
| Antibiotic-impregnated VPS | 23 (24.7) | 11 (47.8) | 12 (52.2) | 0 (0.0) | 4 (17.4) | 4 (17.4) | 4 (17.4) | 8 (34.8) |
| Silver-impregnated VPS | 22 (20.8) | 9 (40.9) | 13 (59.1) | 1 (4.5) | 4 (18.2) | 5 (22.7) | 3 (13.6) | 10 (45.5) |
| Not known | ||||||||
| Overall | 1 (0.3) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Standard VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antibiotic-impregnated VPS | 1 (1.1) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Silver-impregnated VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Number of types per patient with congenital malformations | ||||||||
| 0 | ||||||||
| Overall | 1 (0.3) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Standard VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antibiotic-impregnated VPS | 1 (1.1) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Silver-impregnated VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1 | ||||||||
| Overall | 268 (91.2) | 166 (61.9) | 102 (38.1) | 2 (0.7) | 33 (12.3) | 44 (16.4) | 23 (8.6) | 79 (29.5) |
| Standard VPS | 83 (87.4) | 55 (66.3) | 28 (33.7) | 1 (1.2) | 10 (12.0) | 10 (12.0) | 7 (8.4) | 21 (25.3) |
| Antibiotic-impregnated VPS | 87 (93.5) | 52 (59.8) | 35 (40.2) | 0 (0.0) | 13 (14.9) | 16 (18.4) | 6 (6.9) | 29 (33.3) |
| Silver-impregnated VPS | 98 (92.5) | 59 (60.2) | 39 (39.8) | 1 (1.0) | 10 (10.2) | 18 (18.4) | 10 (10.2) | 29 (29.6) |
| 2 | ||||||||
| Overall | 23 (7.8) | 13 (56.5) | 10 (43.5) | 0 (0.0) | 3 (13.0) | 4 (17.4) | 3 (13.0) | 7 (30.4) |
| Standard VPS | 11 (11.6) | 6 (54.5) | 5 (45.5) | 0 (0.0) | 3 (27.3) | 1 (9.1) | 1 (9.1) | 4 (36.4) |
| Antibiotic-impregnated VPS | 5 (5.4) | 4 (80.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 1 (20.0) |
| Silver-impregnated VPS | 7 (6.6) | 3 (42.9) | 4 (57.1) | 0 (0.0) | 0 (0.0) | 2 (28.6) | 2 (28.6) | 2 (28.6) |
| 3 | ||||||||
| Overall | 2 (0.7) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) |
| Standard VPS | 1 (1.1) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antibiotic-impregnated VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Silver-impregnated VPS | 1 (0.9) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) |
| Acquired hydrocephalus | ||||||||
| Patients with acquired hydrocephalus | ||||||||
| Overall | 819 (51.4) | 615 (75.1) | 204 (24.9) | 9 (1.1) | 54 (6.6) | 102 (12.5) | 39 (4.8) | 165 (20.1) |
| Standard VPS | 274 (51.4) | 201 (73.4) | 73 (26.6) | 3 (1.1) | 22 (8.0) | 27 (9.9) | 21 (7.7) | 52 (19.0) |
| Antibiotic-impregnated VPS | 266 (49.7) | 207 (77.8) | 59 (22.2) | 2 (0.8) | 15 (5.6) | 37 (13.9) | 5 (1.9) | 54 (20.3) |
| Silver-impregnated VPS | 279 (53.0) | 207 (74.2) | 72 (25.8) | 4 (1.4) | 17 (6.1) | 38 (13.6) | 13 (4.7) | 59 (21.1) |
| Type(s) of acquired hydrocephalus | ||||||||
| Cysts (colloid or arachoid) | ||||||||
| Overall | 32 (3.9) | 24 (75.0) | 8 (25.0) | 0 (0.0) | 4 (12.5) | 3 (9.4) | 1 (3.1) | 7 (21.9) |
| Standard VPS | 11 (4.0) | 8 (72.7) | 3 (27.3) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 1 (9.1) | 2 (18.2) |
| Antibiotic-impregnated VPS | 13 (4.9) | 10 (76.9) | 3 (23.1) | 0 (0.0) | 2 (15.4) | 1 (7.7) | 0 (0.0) | 3 (23.1) |
| Silver-impregnated VPS | 8 (2.9) | 6 (75.0) | 2 (25.0) | 0 (0.0) | 0 (0.0) | 2 (25.0) | 0 (0.0) | 2 (25.0) |
| Trauma | ||||||||
| Overall | 30 (3.7) | 25 (83.3) | 5 (16.7) | 1 (3.3) | 1 (3.3) | 3 (10.0) | 0 (0.0) | 5 (16.7) |
| Standard VPS | 12 (4.4) | 10 (83.3) | 2 (16.7) | 1 (8.3) | 1 (8.3) | 0 (0.0) | 0 (0.0) | 2 (16.7) |
| Antibiotic-impregnated VPS | 7 (2.6) | 6 (85.7) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 1 (14.3) |
| Silver-impregnated VPS | 11 (3.9) | 9 (81.8) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 2 (18.2) |
| Tumour: benign | ||||||||
| Overall | 124 (15.1) | 96 (77.4) | 28 (22.6) | 2 (1.6) | 9 (7.3) | 14 (11.3) | 3 (2.4) | 25 (20.2) |
| Standard VPS | 40 (14.6) | 33 (82.5) | 7 (17.5) | 0 (0.0) | 3 (7.5) | 4 (10.0) | 0 (0.0) | 7 (17.5) |
| Antibiotic-impregnated VPS | 35 (13.2) | 26 (74.3) | 9 (25.7) | 1 (2.9) | 3 (8.6) | 5 (14.3) | 0 (0.0) | 9 (25.7) |
| Silver-impregnated VPS | 49 (17.6) | 37 (75.5) | 12 (24.5) | 1 (2.0) | 3 (6.1) | 5 (10.2) | 3 (6.1) | 9 (18.4) |
| Tumour: malignant | ||||||||
| Overall | 133 (16.2) | 105 (78.9) | 28 (21.1) | 2 (1.5) | 6 (4.5) | 14 (10.5) | 6 (4.5) | 22 (16.5) |
| Standard VPS | 38 (13.9) | 29 (76.3) | 9 (23.7) | 1 (2.6) | 1 (2.6) | 5 (13.2) | 2 (5.3) | 7 (18.4) |
| Antibiotic-impregnated VPS | 54 (20.3) | 45 (83.3) | 9 (16.7) | 1 (1.9) | 2 (3.7) | 4 (7.4) | 2 (3.7) | 7 (13.0) |
| Silver-impregnated VPS | 41 (14.7) | 31 (75.6) | 10 (24.4) | 0 (0.0) | 3 (7.3) | 5 (12.2) | 2 (4.9) | 8 (19.5) |
| Post haemorrhagic/intracranial haemorrhage | ||||||||
| Overall | 337 (41.1) | 244 (72.4) | 93 (27.6) | 2 (0.6) | 21 (6.2) | 45 (13.4) | 25 (7.4) | 68 (20.2) |
| Standard VPS | 118 (43.1) | 81 (68.6) | 37 (31.4) | 0 (0.0) | 9 (7.6) | 12 (10.2) | 16 (13.6) | 21 (17.8) |
| Antibiotic-impregnated VPS | 102 (38.3) | 76 (74.5) | 26 (25.5) | 0 (0.0) | 5 (4.9) | 18 (17.6) | 3 (2.9) | 23 (22.5) |
| Silver-impregnated VPS | 117 (41.9) | 87 (74.4) | 30 (25.6) | 2 (1.7) | 7 (6.0) | 15 (12.8) | 6 (5.1) | 24 (20.5) |
| Infection: meningitis | ||||||||
| Overall | 32 (3.9) | 23 (71.9) | 9 (28.1) | 0 (0.0) | 2 (6.3) | 5 (15.6) | 2 (6.3) | 7 (21.9) |
| Standard VPS | 13 (4.7) | 9 (69.2) | 4 (30.8) | 0 (0.0) | 1 (7.7) | 2 (15.4) | 1 (7.7) | 3 (23.1) |
| Antibiotic-impregnated VPS | 9 (3.4) | 7 (77.8) | 2 (22.2) | 0 (0.0) | 1 (11.1) | 1 (11.1) | 0 (0.0) | 2 (22.2) |
| Silver-impregnated VPS | 10 (3.6) | 7 (70.0) | 3 (30.0) | 0 (0.0) | 0 (0.0) | 2 (20.0) | 1 (10.0) | 2 (20.0) |
| Infection: cerebral abscess | ||||||||
| Overall | 8 (1.0) | 7 (87.5) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (12.5) |
| Standard VPS | 6 (2.2) | 5 (83.3) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (16.7) |
| Antibiotic-impregnated VPS | 1 (0.4) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Silver-impregnated VPS | 1 (0.4) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Infection: other | ||||||||
| Overall | 21 (2.6) | 17 (81.0) | 4 (19.0) | 0 (0.0) | 0 (0.0) | 4 (19.0) | 0 (0.0) | 4 (19.0) |
| Standard VPS | 8 (2.9) | 5 (62.5) | 3 (37.5) | 0 (0.0) | 0 (0.0) | 3 (37.5) | 0 (0.0) | 3 (37.5) |
| Antibiotic-impregnated VPS | 6 (2.3) | 5 (83.3) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (16.7) |
| Silver-impregnated VPS | 7 (2.5) | 7 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other factors | ||||||||
| Overall | 140 (17.1) | 99 (70.7) | 41 (29.3) | 3 (2.1) | 16 (11.4) | 17 (12.1) | 5 (3.6) | 36 (25.7) |
| Standard VPS | 47 (17.2) | 34 (72.3) | 13 (27.7) | 1 (2.1) | 8 (17.0) | 1 (2.1) | 3 (6.4) | 10 (21.3) |
| Antibiotic-impregnated VPS | 49 (18.4) | 37 (75.5) | 12 (24.5) | 1 (2.0) | 3 (6.1) | 8 (16.3) | 0 (0.0) | 12 (24.5) |
| Silver-impregnated VPS | 44 (15.8) | 28 (63.6) | 16 (36.4) | 1 (2.3) | 5 (11.4) | 8 (18.2) | 2 (4.5) | 14 (31.8) |
| Number of types per patient with acquired hydrocephalus | ||||||||
| 0 | ||||||||
| Overall | 1 (0.1) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Standard VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antibiotic-impregnated VPS | 1 (0.4) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Silver-impregnated VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1 | ||||||||
| Overall | 782 (95.5) | 590 (75.4) | 192 (24.6) | 8 (1.0) | 50 (6.4) | 98 (12.5) | 36 (4.6) | 156 (19.9) |
| Standard VPS | 257 (93.8) | 189 (73.5) | 68 (26.5) | 3 (1.2) | 20 (7.8) | 26 (10.1) | 19 (7.4) | 49 (19.1) |
| Antibiotic-impregnated VPS | 254 (95.5) | 199 (78.3) | 55 (21.7) | 1 (0.4) | 14 (5.5) | 35 (13.8) | 5 (2.0) | 50 (19.7) |
| Silver-impregnated VPS | 271 (97.1) | 202 (74.5) | 69 (25.5) | 4 (1.5) | 16 (5.9) | 37 (13.7) | 12 (4.4) | 57 (21.0) |
| 2 | ||||||||
| Overall | 33 (4.0) | 22 (66.7) | 11 (33.3) | 1 (3.0) | 3 (9.1) | 4 (12.1) | 3 (9.1) | 8 (24.2) |
| Standard VPS | 15 (5.5) | 11 (73.3) | 4 (26.7) | 0 (0.0) | 1 (6.7) | 1 (6.7) | 2 (13.3) | 2 (13.3) |
| Antibiotic-impregnated VPS | 11 (4.1) | 7 (63.6) | 4 (36.4) | 1 (9.1) | 1 (9.1) | 2 (18.2) | 0 (0.0) | 4 (36.4) |
| Silver-impregnated VPS | 7 (2.5) | 4 (57.1) | 3 (42.9) | 0 (0.0) | 1 (14.3) | 1 (14.3) | 1 (14.3) | 2 (28.6) |
| 3 | ||||||||
| Overall | 3 (0.4) | 2 (66.7) | 1 (33.3) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 1 (33.3) |
| Standard VPS | 2 (0.7) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (50.0) |
| Antibiotic-impregnated VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Silver-impregnated VPS | 1 (0.4) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Idiopathic condition | ||||||||
| Patients with idiopathic condition | ||||||||
| Overall | 496 (31.1) | 408 (82.3) | 88 (17.7) | 4 (0.8) | 32 (6.5) | 38 (7.7) | 14 (2.8) | 74 (14.9) |
| Standard VPS | 169 (31.7) | 145 (85.8) | 24 (14.2) | 3 (1.8) | 5 (3.0) | 11 (6.5) | 5 (3.0) | 19 (11.2) |
| Antibiotic-impregnated VPS | 177 (33.1) | 142 (80.2) | 35 (19.8) | 0 (0.0) | 15 (8.5) | 17 (9.6) | 3 (1.7) | 32 (18.1) |
| Silver-impregnated VPS | 150 (28.5) | 121 (80.7) | 29 (19.3) | 1 (0.7) | 12 (8.0) | 10 (6.7) | 6 (4.0) | 23 (15.3) |
| Type(s) of idiopathic condition | ||||||||
| Idiopathic ‘normal pressure’ hydrocephalus of the elderly | ||||||||
| Overall | 361 (72.8) | 325 (90.0) | 36 (10.0) | 1 (0.3) | 15 (4.2) | 16 (4.4) | 4 (1.1) | 32 (8.9) |
| Standard VPS | 119 (70.4) | 110 (92.4) | 9 (7.6) | 1 (0.8) | 3 (2.5) | 4 (3.4) | 1 (0.8) | 8 (6.7) |
| Antibiotic-impregnated VPS | 135 (76.3) | 118 (87.4) | 17 (12.6) | 0 (0.0) | 8 (5.9) | 8 (5.9) | 1 (0.7) | 16 (11.9) |
| Silver-impregnated VPS | 107 (71.3) | 97 (90.7) | 10 (9.3) | 0 (0.0) | 4 (3.7) | 4 (3.7) | 2 (1.9) | 8 (7.5) |
| IIH | ||||||||
| Overall | 98 (19.8) | 63 (64.3) | 35 (35.7) | 2 (2.0) | 10 (10.2) | 16 (16.3) | 7 (7.1) | 28 (28.6) |
| Standard VPS | 38 (22.5) | 27 (71.1) | 11 (28.9) | 1 (2.6) | 1 (2.6) | 5 (13.2) | 4 (10.5) | 7 (18.4) |
| Antibiotic-impregnated VPS | 32 (18.1) | 19 (59.4) | 13 (40.6) | 0 (0.0) | 5 (15.6) | 7 (21.9) | 1 (3.1) | 12 (37.5) |
| Silver-impregnated VPS | 28 (18.7) | 17 (60.7) | 11 (39.3) | 1 (3.6) | 4 (14.3) | 4 (14.3) | 2 (7.1) | 9 (32.1) |
| Other | ||||||||
| Overall | 38 (7.7) | 20 (52.6) | 18 (47.4) | 1 (2.6) | 7 (18.4) | 7 (18.4) | 3 (7.9) | 15 (39.5) |
| Standard VPS | 13 (7.7) | 8 (61.5) | 5 (38.5) | 1 (7.7) | 1 (7.7) | 3 (23.1) | 0 (0.0) | 5 (38.5) |
| Antibiotic-impregnated VPS | 10 (5.6) | 5 (50.0) | 5 (50.0) | 0 (0.0) | 2 (20.0) | 2 (20.0) | 1 (10.0) | 4 (40.0) |
| Silver-impregnated VPS | 15 (10.0) | 7 (46.7) | 8 (53.3) | 0 (0.0) | 4 (26.7) | 2 (13.3) | 2 (13.3) | 6 (40.0) |
| Number of types per patient with idiopathic condition | ||||||||
| 0 | ||||||||
| Overall | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Standard VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antibiotic-impregnated VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Silver-impregnated VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1 | ||||||||
| Overall | 495 (99.8) | 408 (82.4) | 87 (17.6) | 4 (0.8) | 32 (6.5) | 37 (7.5) | 14 (2.8) | 73 (14.7) |
| Standard VPS | 168 (99.4) | 145 (86.3) | 23 (13.7) | 3 (1.8) | 5 (3.0) | 10 (6.0) | 5 (3.0) | 18 (10.7) |
| Antibiotic-impregnated VPS | 177 (100.0) | 142 (80.2) | 35 (19.8) | 0 (0.0) | 15 (8.5) | 17 (9.6) | 3 (1.7) | 32 (18.1) |
| Silver-impregnated VPS | 150 (100.0) | 121 (80.7) | 29 (19.3) | 1 (0.7) | 12 (8.0) | 10 (6.7) | 6 (4.0) | 23 (15.3) |
| 2 | ||||||||
| Overall | 1 (0.2) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| Standard VPS | 1 (0.6) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| Antibiotic-impregnated VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Silver-impregnated VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Summary of operative approach/valve type | Clean insertion,a n (%) | Revision required | Reason for revision | |||||
|---|---|---|---|---|---|---|---|---|
| No revision, n (%) | Revision,b n (%) | Failure due to patient, n (%) | Functional shunt failure, n (%) | Mechanical shunt failure, n (%) | Failure due to infection, n (%) | Failure – no infection,c n (%) | ||
| Total number of patients | 1594 | 1196 | 398 | 14 | 121 | 185 | 78 | 320 |
| Use of guidance system | ||||||||
| Patients for whom guidance system used for ventricular shunt catheter placement | ||||||||
| Overall | 653 (41.0) | 489 (74.9) | 164 (25.1) | 7 (1.1) | 45 (6.9) | 78 (11.9) | 34 (5.2) | 130 (19.9) |
| Standard VPS | 225 (42.2) | 174 (77.3) | 51 (22.7) | 2 (0.9) | 9 (4.0) | 24 (10.7) | 16 (7.1) | 35 (15.6) |
| Antibiotic-impregnated VPS | 218 (40.7) | 164 (75.2) | 54 (24.8) | 3 (1.4) | 17 (7.8) | 29 (13.3) | 5 (2.3) | 49 (22.5) |
| Silver-impregnated VPS | 210 (39.9) | 151 (71.9) | 59 (28.1) | 2 (1.0) | 19 (9.0) | 25 (11.9) | 13 (6.2) | 46 (21.9) |
| Type of guidance system | ||||||||
| Electromagnetic | ||||||||
| Overall | 413 (63.2) | 309 (74.8) | 104 (25.2) | 4 (1.0) | 26 (6.3) | 50 (12.1) | 24 (5.8) | 80 (19.4) |
| Standard VPS | 147 (65.3) | 120 (81.6) | 27 (18.4) | 1 (0.7) | 5 (3.4) | 12 (8.2) | 9 (6.1) | 18 (12.2) |
| Antibiotic-impregnated VPS | 138 (63.3) | 99 (71.7) | 39 (28.3) | 1 (0.7) | 12 (8.7) | 22 (15.9) | 4 (2.9) | 35 (25.4) |
| Silver-impregnated VPS | 128 (61.0) | 90 (70.3) | 38 (29.7) | 2 (1.6) | 9 (7.0) | 16 (12.5) | 11 (8.6) | 27 (21.1) |
| Ultrasonography | ||||||||
| Overall | 128 (19.6) | 88 (68.8) | 40 (31.3) | 3 (2.3) | 16 (12.5) | 14 (10.9) | 7 (5.5) | 33 (25.8) |
| Standard VPS | 38 (16.9) | 22 (57.9) | 16 (42.1) | 1 (2.6) | 4 (10.5) | 5 (13.2) | 6 (15.8) | 10 (26.3) |
| Antibiotic-impregnated VPS | 40 (18.3) | 33 (82.5) | 7 (17.5) | 2 (5.0) | 2 (5.0) | 3 (7.5) | 0 (0.0) | 7 (17.5) |
| Silver-impregnated VPS | 50 (23.8) | 33 (66.0) | 17 (34.0) | 0 (0.0) | 10 (20.0) | 6 (12.0) | 1 (2.0) | 16 (32.0) |
| Optical | ||||||||
| Overall | 46 (7.0) | 37 (80.4) | 9 (19.6) | 0 (0.0) | 2 (4.3) | 6 (13.0) | 1 (2.2) | 8 (17.4) |
| Standard VPS | 16 (7.1) | 13 (81.3) | 3 (18.8) | 0 (0.0) | 0 (0.0) | 2 (12.5) | 1 (6.3) | 2 (12.5) |
| Antibiotic-impregnated VPS | 19 (8.7) | 15 (78.9) | 4 (21.1) | 0 (0.0) | 2 (10.5) | 2 (10.5) | 0 (0.0) | 4 (21.1) |
| Silver-impregnated VPS | 11 (5.2) | 9 (81.8) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 2 (18.2) |
| Stereotactic frame | ||||||||
| Overall | 63 (9.6) | 52 (82.5) | 11 (17.5) | 0 (0.0) | 1 (1.6) | 8 (12.7) | 2 (3.2) | 9 (14.3) |
| Standard VPS | 22 (9.8) | 17 (77.3) | 5 (22.7) | 0 (0.0) | 0 (0.0) | 5 (22.7) | 0 (0.0) | 5 (22.7) |
| Antibiotic-impregnated VPS | 20 (9.2) | 16 (80.0) | 4 (20.0) | 0 (0.0) | 1 (5.0) | 2 (10.0) | 1 (5.0) | 3 (15.0) |
| Silver-impregnated VPS | 21 (10.0) | 19 (90.5) | 2 (9.5) | 0 (0.0) | 0 (0.0) | 1 (4.8) | 1 (4.8) | 1 (4.8) |
| Not known | ||||||||
| Overall | 3 (0.5) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Standard VPS | 2 (0.9) | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antibiotic-impregnated VPS | 1 (0.5) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Silver-impregnated VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Placement of proximal shunt catheter | ||||||||
| Frontal | ||||||||
| Overall | 134 (8.4) | 93 (69.4) | 41 (30.6) | 0 (0.0) | 13 (9.7) | 15 (11.2) | 13 (9.7) | 28 (20.9) |
| Standard VPS | 49 (9.2) | 33 (67.3) | 16 (32.7) | 0 (0.0) | 5 (10.2) | 4 (8.2) | 7 (14.3) | 9 (18.4) |
| Antibiotic-impregnated VPS | 48 (9.0) | 35 (72.9) | 13 (27.1) | 0 (0.0) | 4 (8.3) | 7 (14.6) | 2 (4.2) | 11 (22.9) |
| Silver-impregnated VPS | 37 (7.0) | 25 (67.6) | 12 (32.4) | 0 (0.0) | 4 (10.8) | 4 (10.8) | 4 (10.8) | 8 (21.6) |
| Parietal, occipital or parietal/occipital | ||||||||
| Overall | 1453 (91.2) | 1100 (75.7) | 353 (24.3) | 14 (1.0) | 107 (7.4) | 167 (11.5) | 65 (4.5) | 288 (19.8) |
| Standard VPS | 481 (90.2) | 369 (76.7) | 112 (23.3) | 5 (1.0) | 35 (7.3) | 46 (9.6) | 26 (5.4) | 86 (17.9) |
| Antibiotic-impregnated VPS | 486 (90.8) | 368 (75.7) | 118 (24.3) | 4 (0.8) | 39 (8.0) | 62 (12.8) | 13 (2.7) | 105 (21.6) |
| Silver-impregnated VPS | 486 (92.4) | 363 (74.7) | 123 (25.3) | 5 (1.0) | 33 (6.8) | 59 (12.1) | 26 (5.3) | 97 (20.0) |
| Combination | ||||||||
| Overall | 3 (0.2) | 2 (66.7) | 1 (33.3) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 1 (33.3) |
| Standard VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antibiotic-impregnated VPS | 1 (0.2) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Silver-impregnated VPS | 2 (0.4) | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not known | ||||||||
| Overall | 4 (0.3) | 1 (25.0) | 3 (75.0) | 0 (0.0) | 0 (0.0) | 3 (75.0) | 0 (0.0) | 3 (75.0) |
| Standard VPS | 3 (0.6) | 1 (33.3) | 2 (66.7) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 0 (0.0) | 2 (66.7) |
| Antibiotic-impregnated VPS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Silver-impregnated VPS | 1 (0.2) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| Type of valve | ||||||||
| Fixed | ||||||||
| Overall | 935 (58.7) | 652 (69.7) | 283 (30.3) | 10 (1.1) | 91 (9.7) | 126 (13.5) | 56 (6.0) | 227 (24.3) |
| Standard VPS | 320 (60.0) | 228 (71.3) | 92 (28.8) | 3 (0.9) | 28 (8.8) | 37 (11.6) | 24 (7.5) | 68 (21.3) |
| Antibiotic-impregnated VPS | 304 (56.8) | 212 (69.7) | 92 (30.3) | 3 (1.0) | 32 (10.5) | 47 (15.5) | 10 (3.3) | 82 (27.0) |
| Silver-impregnated VPS | 311 (59.1) | 212 (68.2) | 99 (31.8) | 4 (1.3) | 31 (10.0) | 42 (13.5) | 22 (7.1) | 77 (24.8) |
| Programmable | ||||||||
| Overall | 627 (39.3) | 525 (83.7) | 102 (16.3) | 4 (0.6) | 26 (4.1) | 52 (8.3) | 20 (3.2) | 82 (13.1) |
| Standard VPS | 202 (37.9) | 172 (85.1) | 30 (14.9) | 2 (1.0) | 8 (4.0) | 13 (6.4) | 7 (3.5) | 23 (11.4) |
| Antibiotic-impregnated VPS | 219 (40.9) | 182 (83.1) | 37 (16.9) | 1 (0.5) | 12 (5.5) | 19 (8.7) | 5 (2.3) | 32 (14.6) |
| Silver-impregnated VPS | 206 (39.2) | 171 (83.0) | 35 (17.0) | 1 (0.5) | 6 (2.9) | 20 (9.7) | 8 (3.9) | 27 (13.1) |
| Not known | ||||||||
| Overall | 32 (2.0) | 19 (59.4) | 13 (40.6) | 0 (0.0) | 4 (12.5) | 7 (21.9) | 2 (6.3) | 11 (34.4) |
| Standard VPS | 11 (2.1) | 3 (27.3) | 8 (72.7) | 0 (0.0) | 4 (36.4) | 2 (18.2) | 2 (18.2) | 6 (54.5) |
| Antibiotic-impregnated VPS | 12 (2.2) | 9 (75.0) | 3 (25.0) | 0 (0.0) | 0 (0.0) | 3 (25.0) | 0 (0.0) | 3 (25.0) |
| Silver-impregnated VPS | 9 (1.7) | 7 (77.8) | 2 (22.2) | 0 (0.0) | 0 (0.0) | 2 (22.2) | 0 (0.0) | 2 (22.2) |
| Summary of shunt components replaced | Failure – no infection,a n (%) | Reason for revision for no infection | ||
|---|---|---|---|---|
| Failure due to patient, n (%) | Functional shunt failure n (%) | Mechanical shunt failure, n (%) | ||
| Total number of patients | 320 | 14 | 121 | 185 |
| Was a complete new shunt inserted? | ||||
| No | ||||
| Overall | 268 (83.8) | 12 (4.5) | 99 (36.9) | 157 (58.6) |
| Standard VPS | 84 (86.6) | 5 (6.0) | 35 (41.7) | 44 (52.4) |
| Antibiotic-impregnated VPS | 97 (82.9) | 3 (3.1) | 33 (34.0) | 61 (62.9) |
| Silver-impregnated VPS | 87 (82.1) | 4 (4.6) | 31 (35.6) | 52 (59.8) |
| If no, which component was replaced? | ||||
| Ventricular shunt catheter only | ||||
| Overall | 72 (26.9) | 0 (0.0) | 22 (30.6) | 50 (69.4) |
| Standard VPS | 21 (25.0) | 0 (0.0) | 7 (33.3) | 14 (66.7) |
| Antibiotic-impregnated VPS | 27 (27.8) | 0 (0.0) | 11 (40.7) | 16 (59.3) |
| Silver-impregnated VPS | 24 (27.6) | 0 (0.0) | 4 (16.7) | 20 (83.3) |
| Peritoneal shunt catheter only | ||||
| Overall | 31 (11.6) | 3 (9.7) | 5 (16.1) | 23 (74.2) |
| Standard VPS | 12 (14.3) | 1 (8.3) | 4 (33.3) | 7 (58.3) |
| Antibiotic-impregnated VPS | 12 (12.4) | 0 (0.0) | 1 (8.3) | 11 (91.7) |
| Silver-impregnated VPS | 7 (8.0) | 2 (28.6) | 0 (0.0) | 5 (71.4) |
| Valve only | ||||
| Overall | 76 (28.4) | 3 (3.9) | 35 (46.1) | 38 (50.0) |
| Standard VPS | 20 (23.8) | 0 (0.0) | 10 (50.0) | 10 (50.0) |
| Antibiotic-impregnated VPS | 33 (34.0) | 2 (6.1) | 13 (39.4) | 18 (54.5) |
| Silver-impregnated VPS | 23 (26.4) | 1 (4.3) | 12 (52.2) | 10 (43.5) |
| Combination | ||||
| Overall | 38 (14.2) | 1 (2.6) | 19 (50.0) | 18 (47.4) |
| Standard VPS | 15 (17.9) | 1 (6.7) | 8 (53.3) | 6 (40.0) |
| Antibiotic-impregnated VPS | 11 (11.3) | 0 (0.0) | 5 (45.5) | 6 (54.5) |
| Silver-impregnated VPS | 12 (13.8) | 0 (0.0) | 6 (50.0) | 6 (50.0) |
| Not known | ||||
| Overall | 51 (19.0) | 5 (9.8) | 18 (35.3) | 28 (54.9) |
| Standard VPS | 16 (19.0) | 3 (18.8) | 6 (37.5) | 7 (43.8) |
| Antibiotic-impregnated VPS | 14 (14.4) | 1 (7.1) | 3 (21.4) | 10 (71.4) |
| Silver-impregnated VPS | 21 (24.1) | 1 (4.8) | 9 (42.9) | 11 (52.4) |
| Summary of AEs | VPS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Standard VPS (531 patients) | Antibiotic-impregnated VPS (545 patients) | Silver-impregnated VPS (525 patients) | Other VPS (136 patients) | Totala (1601 patients) | ||||||
| Events (n) | Patients experiencing an AE, n (%) | Events (n) | Patients experiencing an AE, n (%) | Events (n) | Patients experiencing an AE, n (%) | Events (n) | Patients experiencing an AE, n (%) | Events (n) | Patients experiencing an AE, n (%) | |
| Total | 201 | 135 (25.4) | 210 | 136 (25.0) | 191 | 134 (36.4) | 52 | 35 (25.7) | 654 | 413 (25.8) |
| Expected AEs related to the VPS | ||||||||||
| Ventricular shunt catheter obstruction | 21 | 20 (3.8) | 39 | 31 (5.7) | 29 | 26 (5.0) | 7 | 7 (5.1) | 96 | 79 (4.9) |
| Shunt infectionb | 40 | 39 (7.3) | 17 | 16 (2.9) | 24 | 24 (4.6) | 9 | 9 (6.6) | 90 | 88 (5.5) |
| Shunt valve obstructionc | 15 | 12 (2.3) | 25 | 22 (4.0) | 18 | 17 (3.2) | 7 | 7 (5.1) | 65 | 52 (3.2) |
| Valve change for symptomatic over/underdrainage | 13 | 12 (2.3) | 19 | 19 (3.5) | 16 | 15 (2.9) | 6 | 5 (3.7) | 54 | 50 (3.1) |
| CSF leak | 16 | 16 (3.0) | 17 | 14 (2.6) | 16 | 12 (2.3) | 4 | 3 (2.2) | 53 | 45 (2.8) |
| Wound infectionb,c | 13 | 10 (1.9) | 11 | 11 (2.0) | 16 | 14 (2.7) | 3 | 2 (1.5) | 43 | 37 (2.3) |
| Distal shunt catheter obstruction | 16 | 15 (2.8) | 10 | 9 (1.7) | 12 | 10 (1.9) | 3 | 3 (2.2) | 41 | 36 (2.2) |
| Seizures (early, post operatively, delayed) | 13 | 12 (2.3) | 7 | 7 (1.3) | 9 | 9 (1.7) | 1 | 1 (0.7) | 30 | 29 (1.8) |
| Migration of shunt | 10 | 7 (1.3) | 6 | 5 (0.9) | 7 | 6 (1.1) | 1 | 1 (0.7) | 24 | 18 (1.1) |
| Subdural haematoma from excessive CSF drainage | 4 | 4 (0.8) | 10 | 10 (1.8) | 6 | 6 (1.1) | 0 | 0 (0.0) | 20 | 20 (1.2) |
| Misplacement of distal shunt catheter | 4 | 3 (0.6) | 7 | 6 (1.1) | 5 | 5 (1.0) | 0 | 0 (0.0) | 16 | 14 (0.9) |
| Misplacement of ventricular shunt catheter | 3 | 3 (0.6) | 5 | 5 (0.9) | 4 | 4 (0.8) | 1 | 1 (0.7) | 13 | 13 (0.8) |
| Disconnection of shunt | 1 | 1 (0.2) | 3 | 3 (0.6) | 3 | 3 (0.6) | 2 | 2 (1.5) | 9 | 9 (0.6) |
| Wound dehiscence | 1 | 1 (0.2) | 4 | 4 (0.7) | 3 | 3 (0.6) | 0 | 0 (0.0) | 8 | 8 (0.5) |
| Independent abdominal infections | 5 | 4 (0.8) | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 6 | 5 (0.3) |
| Intracranial haemorrhage | 1 | 1 (0.2) | 4 | 3 (0.6) | 1 | 1 (0.2) | 0 | 0 (0.0) | 6 | 5 (0.3) |
| Brain injury related to procedure with new neurologic deficit | 0 | 0 (0.0) | 0 | 0 (0.0) | 5 | 4 (0.8) | 0 | 0 (0.0) | 5 | 4 (0.2) |
| Fracture of shunt | 2 | 2 (0.4) | 1 | 1 (0.2) | 2 | 2 (0.4) | 0 | 0 (0.0) | 5 | 5 (0.3) |
| Bowel perforation as a result of shunt surgery | 0 | 0 (0.0) | 2 | 2 (0.4) | 2 | 2 (0.4) | 0 | 0 (0.0) | 4 | 4 (0.2) |
| Extra-axial fluid collections | 3 | 3 (0.6) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 3 | 3 (0.2) |
| Tunnelling injury (organ, viscus, lung, vascular) | 1 | 1 (0.2) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 2 | 2 (0.1) |
| Abdominal hernia | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Loculation of ventricles | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Malabsorption | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Pseudocysts | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Sunken fontanelle | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Vascular injury to brain pseudoaneurysm | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Unexpected AEs related to the VPS | ||||||||||
| Vomiting | 4 | 3 (0.6) | 1 | 1 (0.2) | 1 | 1 (0.2) | 2 | 2 (1.5) | 8 | 6 (0.4) |
| Headaches | 1 | 1 (0.2) | 3 | 3 (0.6) | 0 | 0 (0.0) | 1 | 1 (0.7) | 5 | 4 (0.2) |
| Abdominal pain | 0 | 0 (0.0) | 2 | 2 (0.4) | 2 | 2 (0.4) | 0 | 0 (0.0) | 4 | 4 (0.2) |
| Distended abdomen | 2 | 2 (0.4) | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 3 | 3 (0.2) |
| Lethargy | 1 | 1 (0.2) | 0 | 0 (0.0) | 1 | 1 (0.2) | 1 | 1 (0.7) | 3 | 3 (0.2) |
| Swelling at shunt site | 0 | 0 (0.0) | 2 | 1 (0.2) | 1 | 1 (0.2) | 0 | 0 (0.0) | 3 | 2 (0.1) |
| Functional valve problems | 1 | 1 (0.2) | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 2 | 2 (0.1) |
| Irritability | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.7) | 2 | 2 (0.1) |
| Blank spells | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Blurred vision | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Difficult to cannulate, catheter too short | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Distal shunt catheter extra peritoneal | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Externalisation of VPS | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Fluid leaking from ears | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Foreign body removed from shunt surgery site | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Intracranial pressure bolt insertion | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Intra-abdonimal collection | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Itching at shunt site | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Losing balance | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Memory loss | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Nausea and vomiting | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Nausea | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Numbness | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Pneumocephalus | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Poor feeding | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.7) | 1 | 1 (0.1) |
| Pseudomeningocoele | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Pyrexia | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.7) | 1 | 1 (0.1) |
| Seizure | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Shutting-down episodes | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Skull fracture associated with VPS migration | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Tremor in hand | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.7) | 1 | 1 (0.1) |
| Ventriculer shunt catheter coiled and kinked | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
| Vision bubbles | 0 | 0 (0.0) | 1 | 1 (0.2) | 0 | 0 (0.0) | 0 | 0 (0.0) | 1 | 1 (0.1) |
Appendix 3 Health economics study: additional data
Parts of this chapter have been reproduced from Mallucci et al. 2 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nd/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
| HRG code | HRG name | Attendance | Unit cost (£) | Source |
|---|---|---|---|---|
| AA13A | Intermediate intracranial procedures except trauma with cerebral degenerations or miscellaneous disorders of nervous system, with CCs | Elective/day case | 4888.00 | NHS National Tariff Payment System 45 |
| PA42Z | Brain tumours with length of stay of ≥ 1 day | Elective/day case | 3052.00 | NHS National Tariff Payment System 45 |
| AA19A | Minor intracranial procedures except trauma with cerebral degenerations or miscellaneous disorders of nervous system, with CCs | Elective/day case | 2041.00 | NHS National Tariff Payment System 45 |
| AA52G | Very major intracranial procedures, aged ≤ 18 years an, with a CC score of 0–3 | Elective/day case | 6210.00 | NHS Reference Costs 2015 to 2016 44 |
| PA44Z | Neoplasm diagnoses with length of stay of 0 days | Elective/day case | 533.00 | NHS National Tariff Payment System 45 |
| AA25A | Cerebral Degenerations or miscellaneous disorders of nervous system, with CCs | Elective/day case | 1269.00 | NHS National Tariff Payment System 45 |
| AA52C | Very major intracranial procedures, aged ≤ 18 years, with a CC score of 0–3 | Elective/day case | 6210.00 | NHS Reference Costs 2015 to 2016 44 |
| PM44Z | Paediatric neoplasm diagnoses with length of stay of 0 days | Elective/day case | 1373.00 | NHS Reference Costs 2015 to 2016 44 |
| AA13B | Intermediate intracranial procedures except trauma with cerebral degenerations or miscellaneous disorders of nervous system without CCs | Elective/day case | 4409.00 | NHS National Tariff Payment System 45 |
| PA01A | Nervous system disorders with CCs | Elective/day case | 1056.00 | NHS National Tariff Payment System 45 |
| AA21A | Minor intracranial procedures except trauma with other diagnoses with CCs | Elective/day case | 1489.00 | NHS National Tariff Payment System 45 |
| AA52D | Very major intracranial procedures, aged ≥ 19 years, with a CC score of 0–3 | Elective/day case | 7907.00 | NHS Reference Costs 2015 to 2016 44 |
| PR01C | Paediatric nervous system disorders with a CC score of 2–4 | Elective/day case | 2417.00 | NHS Reference Costs 2015 to 2016 44 |
| PA28A | Feeding difficulties and vomiting, without CCs | Elective/day case | 2190.00 | NHS National Tariff Payment System 45 |
| AA54A | Intermediate intracranial procedures, aged ≥ 19 years, with a CC score of ≥ 4 | Elective/day case | 5787.00 | NHS Reference Costs 2015 to 2016 44 |
| HRG | Treatment function code | HRG name | Unit cost (£) | Source |
|---|---|---|---|---|
| WF01A | 150 | Neurosurgery | 188.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 218 | Paediatric neurosurgery | 179.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 300 | General medicine | 164.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 400 | Neurology | 161.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 216 | Paediatric ophthalmology | 115.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 420 | Paediatrics | 180.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 252 | Paediatric endocrinology | 229.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 260 | Paediatric medical oncology | 243.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 218 | Paediatric neurosurgery | 179.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 251 | Paediatric gastroenterology | 195.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 100 | General surgery | 123.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 258 | Paediatric respiratory medicine | 204.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01A | 290 | Community paediatrics | 265.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 150 | Neurosurgery | 236.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 400 | Neurology | 217.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 290 | Community paediatrics | 376.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 216 | Paediatric ophthalmology | 119.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 320 | Cardiology | 156.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 252 | Paediatric endocrinology | 330.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 218 | Paediatric neurosurgery | 255.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 303 | Clinical haematology | 223.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 214 | Paediatric trauma and orthopaedics | 136.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 314 | Rehabilitation service | 248.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 130 | Ophthalmology | 110.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 171 | Paediatric surgery | 185.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 180 | A&E | 157.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 713 | Psychotherapy | 158.00 | NHS Reference Costs 2015 to 2016 44 |
| WF01B | 191 | Pain management | 177.00 | NHS Reference Costs 2015 to 2016 44 |
| WF02A | 216 | Paediatric ophthalmology | 102.00 | NHS Reference Costs 2015 to 2016 44 |
| WF02A | 214 | Paediatric trauma and orthopaedics | 142.00 | NHS Reference Costs 2015 to 2016 44 |
| WF02A | 260 | Paediatric medical oncology | 258.00 | NHS Reference Costs 2015 to 2016 44 |
| WF02A | 421 | Paediatric neurology | 375.00 | NHS Reference Costs 2015 to 2016 44 |
| WF02A | 218 | Paediatric neurosurgery | 170.00 | NHS Reference Costs 2015 to 2016 44 |
| WF02A | 258 | Paediatric respiratory medicine | 176.00 | NHS Reference Costs 2015 to 2016 44 |
| WF02A | 251 | Paediatric gastroenterology | 251.00 | NHS Reference Costs 2015 to 2016 44 |
| WF02A | 256 | Paediatric infectious diseases | 269.00 | NHS Reference Costs 2015 to 2016 44 |
| WF02A | 252 | Paediatric endocrinology | 230.00 | NHS Reference Costs 2015 to 2016 44 |
| WF02A | 253 | Paediatric clinical haematology | 328.00 | NHS Reference Costs 2015 to 2016 44 |
| WF02A | 219 | Paediatric plastic surgery | 145.00 | NHS Reference Costs 2015 to 2016 44 |
| Consultation | Unit cost (£) | Source |
|---|---|---|
| GP surgery visit (per 9.22-minute consultation) | 38.00 | PSSRU 201742 |
| Nurse at surgery (per 9-minute consultation) | 5.40 | PSSRU 201742 |
| Telephone triage: GP led (per call) | 14.75 | PSSRU 201742 |
| Telephone triage: nurse led (per call) | 7.90 | PSSRU 201742 |
| Prescription | 29.20 | PSSRU 201742 |
| Paediatric consult (per consultation) | 196.00 | PSSRU 201742 |
| Physiotherapy (per consultation) | 86.00 | PSSRU 201742 |
| Continence nurse (per consultation) | 80.00 | NHS Reference Costs 2015 to 2016 44 |
| Specialist nurse, adult, face to face (per consultation) | 77.00 | NHS Reference Costs 2015 to 2016 44 |
| District nurse | 38.00 | NHS Reference Costs 2015 to 2016 44 |
| Doctor: home visit (per visit) | 87.46 | PSSRU 201742 |
| Consultant psychiatrist (per consultation) | 108.00 | PSSRU 201742 |
| Health visitor (per consultation) | 53.00 | NHS Reference Costs 2015 to 2016 44 |
| School nurse (per consultation) | 54.00 | NHS Reference Costs 2015 to 2016 44 |
| Occupational therapist (per consultation) | 79.00 | NHS Reference Costs 2015 to 2016 44 |
| Speech therapist, adult (per consultation) | 88.00 | NHS Reference Costs 2015 to 2016 44 |
| Dietitian (per consultation) | 81.00 | NHS Reference Costs 2015 to 2016 44 |
| Speech therapist, child (per consultation) | 94.00 | NHS Reference Costs 2015 to 2016 44 |
| Clinical psychologist (per consultation) | 144.70 | NHS Reference Costs 2015 to 2016 44 |
| Care work and social care (per intervention) | 54.00 | PSSRU 201742 |
| Social worker (per intervention) | 54.00 | PSSRU 201742 |
| Community nurse (per consultation) | 89.00 | NHS Reference Costs 2015 to 2016 44 |
| Shunt nurse specialist (per consultation) | 77.00 | NHS Reference Costs 2015 to 2016 44 |
| Variable | Coefficient (£) | p-value | 97.5% CI (£) |
|---|---|---|---|
| Intercept | 28,796.83 | 0.000 | 10,845.34 to 46,748.32 |
| Antibiotic-impregnated VPS | –4514.67 | 0.030 | –9169.53 to 140.19 |
| Silver-impregnated VPS | –1322.34 | 0.557 | –6456.95 to 3812.27 |
| Treatment failure | 8603.91 | 0.000 | 4696.00 to 12,511.82 |
| Age: 16–65 years | –3670.40 | 0.113 | –8886.24 to 1545.44 |
| Age: > 65 years | –2872.09 | 0.227 | –8233.51 to 2489.33 |
| Time in trial (days) | –7.09 | 0.129 | –17.61 to 3.43 |
| Centre | |||
| A | 33.59 | 0.997 | –23,137.15 to 23,204.33 |
| B | –901.01 | 0.906 | –18,118.44 to 16,316.41 |
| C | 732.23 | 0.922 | –16,116.42 to 17,580.88 |
| D | –8262.26 | 0.289 | –25,868.11 to 9343.59 |
| E | –1615.54 | 0.856 | –21,698.98 to 18,467.90 |
| F | –8657.07 | 0.282 | –26,785.97 to 9471.82 |
| G | –11,152.65 | 0.147 | –28,493.21 to 6187.91 |
| H | –5695.04 | 0.477 | –23,805.53 to 12,415.46 |
| I | 638.42 | 0.943 | –19,533.70 to 20,810.55 |
| J | –1701.73 | 0.825 | –19,070.15 to 15,666.69 |
| K | –4921.65 | 0.543 | –23,203.33 to 13,360.04 |
| L | –4898.48 | 0.561 | –23,919.14 to 14,122.18 |
| M | –6878.41 | 0.374 | –24,346.42 to 10,589.60 |
| N | –7992.50 | 0.295 | –25,226.53 to 9241.53 |
| O | 1158.21 | 0.940 | –33,654.62 to 35,971.04 |
| P | –2290.29 | 0.846 | –28,875.46 to 24,294.88 |
| Q | –5408.04 | 0.485 | –22,913.22 to 12,097.13 |
| R | –7347.52 | 0.336 | –24,590.30 to 9895.26 |
| S | –1171.56 | 0.878 | –18,447.40 to 16,104.27 |
| T | –5911.51 | 0.460 | –23,978.95 to 12,155.93 |
| EQ-VAS | Trial group | |||||
|---|---|---|---|---|---|---|
| Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | ||||
| n | Mean (97.5% CI) | n | Mean (97.5% CI) | n | Mean (97.5% CI) | |
| Youth version (8–18 years) | ||||||
| Baseline | 8 | 43.25 (13.73 to 72.75) | 10 | 58.00 (40.18 to 75.81) | 4 | 72.75 (46.59 to 98.90) |
| Early post operatively | 12 | 65.33 (50.33 to 80.32) | 10 | 68.90 (49.42 to 88.37) | 8 | 65.75 (45.56 to 85.93) |
| 12 weeks | 9 | 80.77 (64.42 to 97.13) | 8 | 79.25 (63.31 to 95.18) | 8 | 81.37 (66.28 to 96.46) |
| End of trial | 7 | 70.14 (45.79 to 94.48) | 6 | 80.00 (55.49 to 104.50) | 6 | 84.00 (50.87 to 117.12) |
| Adult version | ||||||
| Baseline | 182 | 54.12 (50.70 to 57.54) | 171 | 56.79 (53.34 to 60.24) | 162 | 55.79 (51.94 to 59.64) |
| Early post operatively | 173 | 61.15 (57.99 to 64.30) | 168 | 61.49 (58.33 to 64.65) | 157 | 60.29 (56.50 to 64.08) |
| 12 weeks | 145 | 67.34 (63.68 to 71.00) | 137 | 67.09 (63.27 to 70.91) | 133 | 69.20 (64.94 to 73.45) |
| End of trial | 155 | 68.15 (64.71 to 71.59) | 159 | 67.53 (63.84 to 71.22) | 155 | 71.71 (68.20 to 75.23) |
| Proxy version | ||||||
| Baseline | 57 | 36.75 (29.46 to 44.04) | 63 | 38.55 (31.56 to 45.54) | 55 | 43.43 (36.25 to 50.61) |
| Early post operatively | 62 | 46.38 (39.35 to 53.41) | 61 | 50.22 (43.32 to 57.13) | 59 | 54.15 (47.53 to 60.76) |
| 12 weeks | 42 | 61.45 (52.86 to 70.03) | 39 | 63.00 (54.47 to 71.52) | 38 | 65.10 (56.11 to 74.09) |
| End of trial | 34 | 64.61 (57.12 to 72.10) | 22 | 57.27 (43.28 to 71.25) | 39 | 58.87 (50.16 to 67.18) |
| Combined | ||||||
| Baseline | 247 | 50.32 (47.11 to 53.53) | 246 | 52.92 (49.76 to 56.08) | 224 | 52.98 (49.66 to 56.30) |
| Early post operatively | 246 | 57.22 (54.23 to 60.20) | 240 | 58.90 (55.95 to 61.84) | 225 | 58.00 (54.75 to 61.24) |
| 12 weeks | 194 | 65.94 (62.58 to 69.30) | 187 | 65.95 (62.59 to 69.31) | 183 | 67.86 (64.14 to 71.58) |
| End of trial | 196 | 67.00 (63.85 to 70.14) | 187 | 66.43 (62.94 to 69.93) | 200 | 68.90 (65.53 to 72.27) |
| Questionnaire | Trial group | Total | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | ||||||||||||||||||
| N returned form expected | Baseline, n (%) | Early post operatively, n (%) | 12 weeks, n (%) | End of trial, n (%) | N returned form expected | Baseline, n (%) | Early post operatively, n (%) | 12 weeks, n (%) | End of trial, n (%) | N returned form expected | Baseline, n (%) | Early post operatively, n (%) | 12 weeks, n (%) | End of trial, n (%) | N returned form expected | Baseline, n (%) | Early post operatively, n (%) | 12 weeks, n (%) | End of trial, n (%) | |
| HOQ patient | 25 | 7 (28.0) | 14 (56.0) | 8 (32.0) | 5 (20.0) | 27 | 7 (25.9) | 7 (25.9) | 4 (14.8) | 5 (18.5) | 18 | 5 (27.8) | 8 (44.4) | 8 (44.4) | 7 (38.9) | 70 | 19 (27.1) | 29 (41.4) | 20 (28.6) | 17 (24.3) |
| HOQ parent | 11 | 4 (36.4) | 5 (45.5) | 6 (54.5) | 2 (18.2) | 12 | 8 (66.7) | 8 (66.7) | 7 (58.3) | 2 (16.7) | 11 | 6 (54.5) | 8 (72.7) | 6 (54.5) | 3 (27.3) | 34 | 18 (52.9) | 21 (61.8) | 19 (55.9) | 7 (20.6) |
| Scale | Trial group | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | ||||||||||||||
| Baseline | Early post operatively | 12 weeks | End of trial | Baseline | Early post operatively | 12 weeks | End of trial | Baseline | Early post operatively | 12 weeks | End of trial | Baseline | Early post operatively | 12 weeks | End of trial | |
| Physical health | ||||||||||||||||
| Completed item, n (% completed) | 7 (100.0) | 14 (100.0) | 8 (100.0) | 5 (100.0) | 7 (100.0) | 7 (100.0) | 4 (100.0) | 5 (100.0) | 5 (100.0) | 8 (100.0) | 8 (100.0) | 7 (100.0) | 19 (100.0) | 29 (100.0) | 20 (100.0) | 17 (100.0) |
| Median (IQR) | 0.6 (0.4–0.8) | 0.7 (0.6–0.9) | 0.8 (0.7–1.0) | 0.7 (0.7–0.8) | 0.8 (0.3–0.8) | 0.8 (0.6–0.9) | 0.8 (0.7–0.9) | 0.7 (0.7–1.0) | 0.6 (0.2–0.8) | 0.7 (0.5–0.9) | 0.9 (0.5–1.0) | 0.9 (0.9–1.0) | 0.6 (0.3–0.8) | 0.8 (0.6–0.9) | 0.9 (0.7–1.0) | 0.9 (0.7–1.0) |
| Socioemotional health | ||||||||||||||||
| Completed item, n (% completed) | 6 (85.7) | 14 (100.0) | 8 (100.0) | 5 (100.0) | 7 (100.0) | 6 (85.7) | 3 (75.0) | 5 (100.0) | 5 (100.0) | 8 (100.0) | 8 (100.0) | 7 (100.0) | 18 (94.7) | 28 (96.6) | 19 (95.0) | 17 (100.0) |
| Median (IQR) | 0.8 (0.7–0.9) | 0.8 (0.8–0.9) | 0.8 (0.8–0.9) | 0.8 (0.7–0.9) | 0.7 (0.4–0.9) | 0.8 (0.3–0.9) | 0.8 (0.7–0.8) | 0.9 (0.7–0.9) | 0.4 (0.4–0.9) | 0.8 (0.6–1.0) | 0.8 (0.6–0.9) | 0.9 (0.7–1.0) | 0.8 (0.4–0.9) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.9 (0.7–0.9) |
| Cognitive health | ||||||||||||||||
| Completed item, n (% completed) | 5 (71.4) | 14 (100.0) | 8 (100.0) | 5 (100.0) | 7 (100.0) | 6 (85.7) | 3 (75.0) | 5 (100.0) | 5 (100.0) | 8 (100.0) | 8 (100.0) | 6 (85.7) | 17 (89.5) | 28 (96.6) | 19 (95.0) | 16 (94.1) |
| Median (IQR) | 0.8 (0.7–0.8) | 0.8 (0.7–0.9) | 0.9 (0.6–0.9) | 0.7 (0.6–0.9) | 0.8 (0.4–0.9) | 0.8 (0.6–0.9) | 0.8 (0.4–0.8) | 0.8 (0.7–0.8) | 0.3 (0.2–1.0) | 0.8 (0.4–1.0) | 0.8 (0.6–0.9) | 0.9 (0.8–0.9) | 0.8 (0.3–0.9) | 0.8 (0.6–0.9) | 0.8 (0.6–0.9) | 0.8 (0.7–0.9) |
| Total health | ||||||||||||||||
| Completed item, n (% completed) | 6 (85.7) | 14 (100.0) | 8 (100.0) | 5 (100.0) | 7 (100.0) | 6 (85.7) | 4 (100.0) | 5 (100.0) | 5 (100.0) | 8 (100.0) | 8 (100.0) | 7 (100.0) | 18 (94.7) | 28 (96.6) | 20 (100.0) | 17 (100.0) |
| Median (IQR) | 0.8 (0.7–0.8) | 0.8 (0.7–0.8) | 0.8 (0.8–0.9) | 0.7 (0.7–0.8) | 0.7 (0.4–0.9) | 0.7 (0.6–0.9) | 0.7 (0.7–0.8) | 0.8 (0.6–0.9) | 0.4 (0.3–0.9) | 0.8 (0.5–0.9) | 0.8 (0.6–0.9) | 0.9 (0.8–1.0) | 0.7 (0.4–0.8) | 0.8 (0.6–0.9) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) |
| Scale | Trial group | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard VPS | Antibiotic-impregnated VPS | Silver-impregnated VPS | ||||||||||||||
| Baseline | Early post operatively | 12 weeks | End of trial | Baseline | Early post operatively | 12 weeks | End of trial | Baseline | Early post operatively | 12 weeks | End of trial | Baseline | Early post operatively | 12 weeks | End of trial | |
| Physical health | ||||||||||||||||
| Completed item, n (% completed) | 4 (100.0) | 5 (100.0) | 6 (100.0) | 2 (100.0) | 8 (100.0) | 8 (100.0) | 7 (100.0) | 2 (100.0) | 6 (100.0) | 8 (100.0) | 6 (100.0) | 3 (100.0) | 18 (100.0) | 21 (100.0) | 19 (100.0) | 7 (100.0) |
| Median (IQR) | 0.3 (0.1–0.5) | 0.1 (0.0–0.4) | 0.5 (0.3–0.8) | 0.7 (0.6–0.8) | 0.5 (0.3–0.8) | 0.5 (0.3–0.7) | 0.6 (0.3–0.9) | 0.6 (0.6–0.6) | 0.6 (0.4–0.7) | 0.5 (0.5–0.8) | 0.7 (0.4–0.9) | 0.6 (0.5–1.0) | 0.5 (0.3–0.7) | 0.5 (0.3–0.6) | 0.6 (0.3–0.9) | 0.6 (0.6–0.8) |
| Socioemotional health | ||||||||||||||||
| Completed item, n (% completed) | 2 (50.0) | 3 (60.0) | 6 (100.0) | 2 (100.0) | 7 (87.5) | 7 (87.5) | 6 (85.7) | 2 (100.0) | 6 (100.0) | 8 (100.0) | 6 (100.0) | 3 (100.0) | 15 (83.3) | 18 (85.7) | 18 (94.7) | 7 (100.0) |
| Median (IQR) | 0.7 (0.5–0.8) | 0.5 (0.1–0.8) | 0.6 (0.5–0.8) | 0.8 (0.8–0.8) | 0.8 (0.7–0.9) | 0.7 (0.6–0.9) | 0.6 (0.6–0.7) | 0.7 (0.6–0.8) | 0.8 (0.7–0.9) | 0.8 (0.6–0.9) | 0.8 (0.7–0.9) | 0.9 (0.6–0.9) | 0.8 (0.7–0.9) | 0.7 (0.5–0.9) | 0.7 (0.5–0.8) | 0.8 (0.6–0.9) |
| Cognitive health | ||||||||||||||||
| Completed item, n (% completed) | 3 (75.0) | 2 (40.0) | 5 (83.3) | 2 (100.0) | 7 (87.5) | 6 (75.0) | 4 (57.1) | 2 (100.0) | 6 (100.0) | 8 (100.0) | 6 (100.0) | 3 (100.0) | 16 (88.9) | 16 (76.2) | 15 (78.9) | 7 (100.0) |
| Median (IQR) | 0.2 (0.0–0.6) | 0.4 (0.2–0.6) | 0.4 (0.2–0.4) | 0.2 (0.1–0.2) | 0.6 (0.5–0.9) | 0.6 (0.4–0.7) | 0.4 (0.3–0.7) | 0.6 (0.2–1.0) | 0.7 (0.3–0.8) | 0.7 (0.2–0.9) | 0.7 (0.0–0.9) | 0.3 (0.1–1.0) | 0.6 (0.3–0.8) | 0.6 (0.2–0.9) | 0.4 (0.2–0.9) | 0.2 (0.1–1.0) |
| Total health | ||||||||||||||||
| Completed item, n (% completed) | 3 (75.0) | 3 (60.0) | 6 (100.0) | 2 (100.0) | 7 (87.5) | 6 (75.0) | 6 (85.7) | 2 (100.0) | 6 (100.0) | 8 (100.0) | 6 (100.0) | 3 (100.0) | 16 (88.9) | 17 (81.0) | 18 (94.7) | 7 (100.0) |
| Median (IQR) | 0.5 (0.1–0.6) | 0.5 (0.1–0.6) | 0.5 (0.4–0.7) | 0.6 (0.6–0.6) | 0.7 (0.6–0.8) | 0.7 (0.5–0.8) | 0.6 (0.5–0.7) | 0.7 (0.5–0.8) | 0.7 (0.5–0.8) | 0.6 (0.5–0.9) | 0.7 (0.5–0.9) | 0.7 (0.5–0.9) | 0.7 (0.5–0.8) | 0.6 (0.5–0.8) | 0.6 (0.5–0.8) | 0.6 (0.5–0.8) |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AIC
- Akaike information criterion
- BASICS
- British Antibiotic and Silver Impregnated Catheters for ventriculoperitoneal Shunts
- BIC
- Bayesian information criterion
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CSF
- cerebrospinal fluid
- csHR
- cause-specific hazard ratio
- CTU
- Clinical Trials Unit
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-Y
- EuroQol-5 Dimensions Youth
- EQ-VAS
- EuroQol Visual Analogue Scale
- EVD
- external ventricular drain
- FMI
- fraction of missing information
- GLM
- generalised linear model
- GP
- general practitioner
- HOQ
- Hydrocephalus Outcome Questionnaire
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- IDSMC
- Independent Data and Safety Monitoring Committee
- IIH
- idiopathic intracranial hypertension
- LCTC
- Liverpool Clinical Trials Centre
- LQ
- lower quartile
- NICE
- National Institute for Health and Care Excellence
- PI
- principal investigator
- PLICS
- Patient-Level Information and Costing Systems
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- Shine
- Spina bifida Hydrocephalus Information Networking Equality
- sHR
- subdistribution hazard ratio
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UQ
- upper quartile
- VPS
- ventriculoperitoneal shunt
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta24170).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
