Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/14/08. The contractual start date was in January 2010. The draft report began editorial review in February 2019 and was accepted for publication in October 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jonathan A Cook reports grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme during the conduct of the study and was a member of the NIHR HTA Efficient Trial Designs Board between 2014 and 2016. He was also a member of the NIHR HTA End of Life Care and Add-on Studies Board during these years and a member of a NIHR Research for Patient Benefit programme regional advisory committee (South Central/South East & Central) between 2015 and 2019. Graeme MacLennan reports grants from the NIHR HTA programme during the conduct of the study. Ray Fitzpatrick reports membership of the HTA Prioritisation Group and the HTA National Stakeholder Advisory Group during the conduct of the study (October 2015 to present). Nigel Arden reports grants from Merck & Co. (Kenilworth, NJ, USA), personal fees from Flexion Therapeutics (Burlington, MA, USA), Freshfields Bruckhaus Deringer (London, UK), Merck & Co., Regeneron Pharmaceuticals (Tarrytown, NY, USA) and Eli Lilly and Company (Indianapolis, IN, USA)/Pfizer Inc. (New York, NY, USA) outside the submitted work. Andrew Price reports personal fees from Zimmer Biomet (Warsaw, IN, USA), DePuy (Warsaw, IN, USA) and Smith & Nephew (Watford, UK); he also reports grants from NIHR and Arthritis Research UK outside the submitted work. David Murray reports grants and personal fees from Zimmer Biomet outside the submitted work; in addition, he has various patents relating to knee replacement with royalties paid. Marion K Campbell reports grants from NIHR during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Beard et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter have been adapted from Beard et al. 1 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Background
Osteoarthritis (OA) of the knee is a common condition that produces pain, swelling and stiffness in the knee, which, in many cases, can result in a reduction in patient function. 2,3 The pattern of arthritic changes affects people differently. Epidemiological studies have shown that the predominant pattern of OA change is found in the medial compartment of the knee joint, with lesser changes occurring on the lateral side or patellofemoral joint. 4 Patients can be managed conservatively or surgically, with treatment aimed at relieving pain and discomfort, reducing stiffness and minimising further damage to the joint. When patients develop joint symptoms (e.g. pain, stiffness and reduced function) that have a substantial impact on their quality of life and have not been controlled by non-surgical treatment, surgical intervention may be required. 5
Current treatment/management options
Knee replacement for OA of the knee is an effective and common procedure, with 303,960 procedures performed in the UK between 2015 and 2017. 6 There are two main surgical options to replace the diseased areas of late-stage medial compartment OA. The more commonly used procedure, total knee replacement (TKR), is a highly effective treatment strategy that is associated with significant improvement in pain, function and quality of life. 7,8 Some surgeons consider TKR to be the treatment of choice for this pathology, whereas others prefer to replace only the damaged component of the knee, partial (or unicompartmental) knee replacement (PKR), preserving the cruciate ligaments, meniscus and tibial plateau of the healthy compartment. Such variation in the decision-making for patients who have similar pathology is well recognised, with high levels of disagreement between surgeons regarding implant choice. 9 Fewer than 9% of knee replacements in the UK are unicompartmental,6 although it is estimated that up to 47% of patients who require knee replacement have unicompartmental disease that would be suitable for a PKR. 4
There are arguments for both approaches. Both interventions are established and well-documented procedures and each intervention is considered standard care. However, little evidence exists to prove the clinical effectiveness and cost-effectiveness of either management option. Proponents of the TKR procedure believe that the operation is less complex than PKR and, thus, in the short term, TKRs are less susceptible to early problems and failure. Proponents of the TKR procedure also believe that in the long term the joint disease will progress to the other, normal, compartments of the knee. 10,11 It is felt that a PKR would eventually fail and necessitate revision surgery, which involves a TKR procedure. 11,12 In contrast, proponents of the PKR procedure believe that PKR results in faster recovery,13,14 fewer complications15 and superior function. 16 Proponents of the PKR procedure also believe that it is more cost-effective than TKR4 and is associated with long-term survival of the joint. 17,18
Rationale for TOPKAT
At the outset of the study, the evidence available to select the best treatment option for medial compartment OA was limited. A literature search conducted before study submission was performed with no language restrictions for reports published between 1990 and 2008. We searched NLH, EMBASE, MEDLINE, PEDro, MetaRegister of Controlled Trials (active and archived registers), ClinicalTrials.gov, Centre for Reviews and Dissemination, Cochrane Database of Systematic Reviews and The Cochrane Central Register of Controlled Studies databases, with the search terms ‘total’, ‘partial’, ‘unicompartmental’, ‘knee replacement’, ‘knee arthroplasty’, ‘trial’ and ‘outcome’. There was no systematic review of the clinical effectiveness of PKR at the time owing to a lack of published evidence. There was one small randomised controlled trial (RCT)17,18 as well as individual cohort studies, indirect comparisons and retrospective studies. These had been undertaken to address specific, and often focused, aspects of OA treatment and knee replacement and many involved short-term assessments only. These studies included a comparison between TKR and PKR of the kinematics,19 proprioception,20 ability to kneel,16 ease of revision,21–23 success of revision after various procedures,24–27 appropriateness for specific pathology,28 accuracy of implantation29–31 and complications. 32,33 No large, well-powered, multicentre RCT had been undertaken to directly compare PKR with TKR.
The only other previous attempt at comparing these operations on a large scale was one of the arms of the Knee Arthroplasty Trial (KAT). 34 However, this arm of the trial failed because of a lack of equipoise and confidence towards PKR among surgeons. This led to such low levels of patient recruitment that this arm of the KAT was stopped.
Other previous studies35–38 that showed a trend towards PKR being the more effective management option are characterised by low-level evidence, consensus and peer influence. To test the validity of these results and to examine the clinical effectiveness and cost-effectiveness of both treatment options, further investigation was required using an appropriate patient base and long-term assessments.
Evidence update since study submission
During the course of the study, further studies have been completed. An update of the initial literature search was conducted to inform this report and set the results in context.
Three RCTs39–41 and five ongoing trials42–46 were identified (Table 1). The three completed RCTs were relatively small single-centre studies, ranging from 56 to 72 participants per trial, with a mean participant age of 60–73 years. Two of the RCTs39,40 included patients who underwent a TKR in one knee and a PKR in the contralateral leg and the other41 was of patients who received simultaneous bilateral TKR or PKR. Overall, no RCT showed a statistically significant difference between the two types of procedure being compared. 47 Of the ongoing RCTs, three trials are currently recruiting,43,44,46 one is a feasibility study (not yet recruiting)42 and the other has a recruitment status reported as ‘unknown’. 45
| First author, year of publication | Study design | Blinding | Sample size | Participants | Interventions | Primary outcome | Results |
|---|---|---|---|---|---|---|---|
| Completed studies | |||||||
| Kulshrestha, 201741 | RCT, single centre | Blinding of outcome assessment (pain) | 72 knees (36 patients) | Bilateral isolated medial compartment knee arthritis | Unicompartmental knee arthroplasty | KOS-ADLS and HAAS at 2 years | No statistically significant difference (p = 0.2143 and p = 0.2010) |
| Total knee arthroplasty | |||||||
| Sun, 201240 | RCT, single centre | Blinding of outcome assessment (pain) | 56 knees (28 patients) | Unicompartmental OA of the knee | Unicompartmental knee arthroplasty | KSS and range of motion at 4 years | No statistically significant difference |
| Total knee arthroplasty | Mean KSS 80.5 (range 70–100) for UKR and 78.9 (range 70–87) for TKR | ||||||
| Costa, 201139 | RCT, single centre | Not reported | 68 knees (34 patients) | Bilateral unicompartmental knee OA: mean age 73 years | Unicompartmental knee arthroplasty | KSS at 5 years | Similar for both groups |
| Total knee arthroplasty | |||||||
| Newman, 199817,18 | RCT, single centre | Not reported | 102 knees (94 patients) | Unicompartmental OA of the knee | Unicompartmental knee arthroplasty | BKS at 5 years | Mean BKS 91.1 for UKR and 86.7 for TKR |
| Weale, 199933 | Total knee arthroplasty | Patella tendon length at 5 years33 | |||||
| Ongoing studies | |||||||
| Unicompartmental Knee Arthroplasty (UKA) Versus Total Knee Arthroplasty (TKA) of Medial Osteoarthritis (Canada)42 | RCT feasibility study, single centre | Not blinded | 54 | OA: knee, considered candidates for UKA, aged 50–80 years | Unicompartmental knee arthroplasty | WOMAC index at 36 months | Not yet recruiting |
| Total knee arthroplasty | |||||||
| Finnish Unicompartmental and Total Knee Arthroplasty Investigation43 | RCT, multicentre | Participant, care provider, investigator, outcomes assessor | 140 | Symptomatic medial knee OA: aged 45–79 years | Unicondylar knee replacement | KOOS at 10 years | Recruiting |
| TKR | |||||||
| Medial Unicondylar Knee Arthroplasty vs. Total Knee Arthroplasty (Copenhagen)44 | RCT, multicentre | Participant, care provider, investigator, outcomes assessor | 350 | Anteromedial OA of the knee: aged 18–110 years | Unicondylar knee arthroplasty | OKS at 20 years | Recruiting |
| Total knee arthroplasty | |||||||
| Unicondylar Knee Arthroplasty Versus Total Knee Arthroplasty in Patients with Anteromedial Osteoarthritis of the Knee45 (Canada) | RCT, single centre | Not blinded | 38 | Anteromedial compartment OA: aged 40–80 years | Unicompartmental knee arthroplasty | WOMAC and OKS at 2 years | Recruitment status unknown |
| Total knee arthroplasty | |||||||
| Unicondylar- or Total Knee Replacement? Patient Satisfaction, Function and Muscle Mass46 (Sweden) | RCT, single centre | Not blinded | 80 | Knee OA medial gonarthritis: aged 50–100 years | Unicompartmental knee replacement | Muscle mass at 2 years | Recruiting |
| TKR | |||||||
Potential advantages of undergoing a PKR over a TKR for patients with medial compartment OA have been discussed in several registry and cohort studies. For example, studies have demonstrated reduced blood loss and risk of transfusion at the time of operation;48,49 shorter length of stay and a lower 30-day re-admission rate;50–52 more cost-effective,53,54 better restoration of physiological gait pattern;55 and better patient-reported pain and function scores and forgotten joint scores56–60 for PKR compared with TKR. In addition, PKR has been reported to be more cost-effectiveness,53,54 have lower incidence of medical complications, such as thromboembolism, infection, stroke and myocardial infarction, and lower mortality rates than TKR. 61
However, systematic reviews and unadjusted data from national registries show a significantly higher revision rate for PKR than for TKR, with the chance of revision for PKR being more than double that of TKR. 6,62,63 Higher revision rate was also identified when comparing matched patients who were undergoing PKR with those undergoing TKR: PKR had worse implant survival both for revision [subhazard ratio 2.12, 95% confidence interval (CI) 1.99 to 2.26] and for revision/reoperation (subhazard ratio 1.38, 95% CI 1.31 to 1.44) than TKR at 8 years post treatment. 61
Despite large sample sizes and best efforts of propensity matching, retrospective cohort data to assess clinical effectiveness and cost-effectiveness remain at risk of bias because of inherent selection bias for individual patients between the two operations.
Research objectives
-
The primary objective for Total or Partial Knee Arthroplasty Trial (TOPKAT) was to assess the clinical effectiveness of PKR compared with that of TKR in patients with medial compartment OA of the knee.
-
Secondary objectives included investigation of the complications, patient satisfaction and cost implications of the PKR and TKR replacements for patients, surgeons and health-care providers.
Chapter 2 Methods
Parts of this chapter have been adapted from Beard et al. 1 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Trial design
TOPKAT was designed as a multicentre parallel-group superiority RCT to evaluate the clinical effectiveness and cost-effectiveness of TKR compared with PKR (unicompartmental) for medial compartmental OA. The trial used a novel combined equipoise/expertise approach (hybrid expertise-based design). The expertise-based design component was included to help address some of the known challenges of conducting a RCT of skilled-based interventions, such as clinician preference for one intervention over another. 64 Use of this design helped to maximise surgeon participation in the study and negate known obstacles arising from clinician preference, a feature that was observed in an earlier attempt to evaluate the differences between PKR and TKR. 34
The hybrid equipoise/expertise design allowed surgeons who were in equipoise (‘equipoise surgeon’) and had sufficient expertise to perform both TKR and PKR to participate in the trial, as per a conventional surgical trial design. The expertise-based randomisation component also enabled surgeons not in equipoise to participate in the trial by working in pairs as a treatment allocation or delivery unit, one surgeon providing the TKR expertise and one providing the PKR expertise, each providing only the operation type that they felt was appropriate (as ‘experts’); patients were randomised to the TKR or PKR surgeon in the pair. For this ‘expertise’ approach to succeed, there had to be a surgeon with expertise in TKR and a surgeon with expertise in PKR in the same centre who could participate together as the ‘treatment delivery unit’. Patients recruited to the study who were under the care of such a surgeon (‘expertise surgeon’) were randomised to one of the two groups and were treated by the appropriate surgeon. In cases where, following randomisation, allocation was to a surgeon different from that at the initial consultation, a patient was internally referred to the other surgeon’s operating list.
No restriction was made on the number of ‘treatment delivery units’ within a participating site. However, for practical and statistical reasons, a surgeon was able to be in one delivery unit only, that is they were classified as either an ‘equipoise surgeon’ or an ‘expertise surgeon’.
Blinding patients to treatment allocation was considered for the study design, but was thought not to be practicable for such a long-term study. However, clinical evaluators were masked from treatment allocation where practical. Surgeons implanting the device could not be blinded from allocation.
The trial was designed as pragmatic in terms of both device choice (see Interventions) and delivery of care. No formal restrictions were placed on clinical pathways and rehabilitation approach/content for either group, but advice and minimal quality levels were shared with local site personnel at set-up visits.
The study protocol was published in 2013. 1 The main changes to the protocol since 2013 included the addition of two secondary outcomes measures: the High Activity Arthroplasty Score (HAAS) and the Oxford Knee Score-Activity and Participation Questionnaire (OKS-APQ) (years 4 and 5 follow-up questionnaire only). An additional Oxford Knee Score (OKS) questionnaire at 1 year post surgery for participants with a time > 12 weeks of between randomisation and surgery was also included (see Appendix 1, Table 26).
Interventions
TOPKAT compared PKR with TKR. Surgeons were free to use any implant of their own or their institution’s choice (i.e. brand and model), providing the device was in common clinical use and had no previous safety issues recognised by the National Joint Registry. The implant type, model and manufacturer were recorded on the primary procedure hospital form.
Total knee replacement
A TKR involves all surfaces of the knee being replaced. The procedure involves excising both diseased and normal femoral condyles, the tibial plateau and often the patella. This is carried out through a large skin incision that provides easy access to the knee joint. Each component is replaced with an artificial implant that is secured in position with either a cemented or a cementless fixation. No stipulation was provided for patella replacement or cement use, although these variables were recorded.
Partial knee replacement
A PKR or unicompartmental knee replacement involves replacing the diseased area of the joint only. The healthy (or healthier) compartment of the knee is retained and artificial implants are inserted in place of the diseased dysfunctional area. The implants are secured in position with either a cemented or a cementless fixation. For this study, the medial compartment was replaced for medial compartment OA, which was usually achieved using a minimally invasive surgical technique.
Participating surgeons required a ‘minimum level of expertise’ for the types of surgery undertaken. A paper-based simple audit of participating surgeons’ routine practice was undertaken prior to commissioning each participating centre. Levels of experience were set to allow participation. PKR surgeons must have had appropriate training, been practising the technique for ≥ 1 year and had to have performed the operation ≥ 10 times in the previous year. The implants used by PKR surgeons in the study required evidence of good clinical results and had to be a commonly used knee system that did not require patella dislocation. TKR surgeons had to satisfy similar criteria and use a conventional approach with patella dislocation. ‘Equipoise surgeons’, who delivered both operations, were required to satisfy the criteria for both operations, that is they had appropriate training in both operations and had performed a minimum of 10 PKR and 10 TKR procedures in the previous year.
Participants
Patients with medial compartmental OA who satisfied general surgical requirements for a medial PKR (see Inclusion criteria) were eligible for inclusion. If patients met the inclusion criteria with both of their knees, only one knee (designated the ‘study’ knee) was operated on according to the random allocation assigned to the patient as they entered the study. Patients requiring simultaneous bilateral knee replacement were excluded. Previous knee replacement on the non-study knee was not an exclusion criterion. Subsequent knee replacement on the other, non-study knee was recorded, but the subsequent operation did not lead to a second random allocation.
Inclusion criteria
-
Medial compartment OA with exposed bone on both the femur and the tibia.
-
Functionally intact anterior cruciate ligament. Superficial damage or splitting of the ligament was acceptable.
-
Full-thickness and good-quality lateral cartilage present.
-
Correctable intra-articular varus deformity (suggestive of adequate medial collateral ligament function).
-
Medically fit with an American Society of Anesthesiologists (ASA) grade of 1 or 2.
Exclusion criteria
-
Required revision knee replacement surgery.
-
Had rheumatoid arthritis or other inflammatory disorders.
-
Were unlikely to be able to perform the required clinical assessment tasks.
-
Had concurrent symptomatic foot, hip or spinal pathology.
-
Had previous knee surgery other than diagnostic arthroscopy and medial meniscectomy.
-
Previously had septic arthritis.
-
Had significant damage to the patellofemoral joint, especially on the lateral facet.
Setting and locations
Participants were recruited between January 2010 and September 2013 from 27 NHS secondary care hospitals from across the UK. Each site is listed below:
-
Chesterfield Royal Hospital, Chesterfield Royal Hospital NHS Foundation Trust.
-
Cumberland Infirmary, North Cumbria University Hospitals NHS Trust.
-
Great Western Hospital, Great Western Hospitals NHS Foundation Trust.
-
Harrogate District Hospital, Harrogate and District NHS Foundation Trust.
-
Hull Royal Infirmary, Hull and East Yorkshire Hospitals NHS Trust.
-
Ipswich Hospital, The Ipswich Hospital NHS Trust.
-
King’s Mill Hospital, Sherwood Forest Hospitals NHS Foundation Trust.
-
Leicester Royal Infirmary, University Hospitals of Leicester NHS Trust.
-
Lincoln County Hospital, United Lincolnshire Hospitals NHS Trust.
-
Medway Maritime Hospital, Maidstone and Tunbridge Wells NHS Trust.
-
Milton Keynes General Hospital, Milton Keynes University Hospital NHS Foundation Trust.
-
Musgrave Park Hospital, Belfast Health and Social Care Trust.
-
Nuffield Orthopaedic Centre, Oxford University Hospitals NHS Foundation Trust.
-
Pilgrim Hospital, United Lincolnshire Hospitals NHS Trust.
-
Pinderfields Hospital, Mid Yorkshire Hospital NHS Trust.
-
Royal Blackburn Hospital, East Lancashire Hospitals NHS Trust.
-
Royal Gwent Hospital, Aneurin Bevan University Health Board.
-
Royal Stoke University Hospital, University Hospitals of North Midlands NHS Trust.
-
Royal United Hospital, Royal United Hospital Bath NHS Trust.
-
Southampton General Hospital, University Hospital Southampton NHS Foundation Trust.
-
Southmead Hospital, North Bristol NHS Trust.
-
Stepping Hill Hospital, Stockport NHS Foundation Trust.
-
Torbay Hospital, South Devon Healthcare NHS Foundation Trust.
-
University Hospital of North Durham, County Durham and Darlington NHS Foundation Trust.
-
University Hospital of North Tees, North Tees and Hartlepool NHS Foundation Trust.
-
Woodend Hospital, NHS Grampian.
-
Yeovil District Hospital, Yeovil District Hospital NHS Foundation Trust.
Recruitment and consent
Potential patients were identified and approached in routine orthopaedic outpatient and pre-assessment clinics by the participating surgeon [or their late-stage trainee (registrar)].
Eligible patients who were interested in participating were provided with a letter of invitation and an information sheet by the surgeon or a member of the research team. This information explained why they had been approached and further details about the study. An ‘opt in’ form was also provided to the patient that was used to indicate if they were willing to be contacted again by the research team. This form was either completed at this initial visit or returned to the research team in a prepaid return envelope. Patients who indicated ‘yes’ were then contacted by local study staff to arrange a screening visit.
At some participating sites, potential patients were also identified from local hospital databases. These patients were sent a letter, information sheet and the ‘opt-in’ form with a prepaid envelope for its return, which documented whether or not they were willing to be contacted subsequently by the research team.
During the screening visit, all eligible patients who agreed to participate in the trial signed the TOPKAT consent form. Following consent and prior to randomisation a baseline assessment was undertaken by the research team. The research nurse/practitioner then enrolled the patient details onto the automated web-randomisation system (see Randomisation) to activate the randomisation and initiated appropriate arrangements in line with the allocation.
Baseline assessment
The baseline preoperative assessment included a patient-reported questionnaire that examined pain and function (OKS), activity level [the University of California, Los Angeles (UCLA), activity measure and the HAAS measure] and health-care resource use (in the preceding 12-month period). In addition, the American Knee Society Score (AKSS), a clinical assessment of the range of motion and function of the knee, was carried out. Full details of these measures are provided in Outcomes. Routine preoperative X-rays (anteroposterior and lateral views) were also collected.
Randomisation
Randomisation was performed by computer allocation (thus ensuring concealment of sequence generation) using the web-based automated randomisation service provided by the Centre for Healthcare Randomised Trials (CHaRT), Health Services Research Unit, University of Aberdeen. Random allocation to TKR or PKR was in a 1 : 1 ratio and was minimised using sex, age (< 50, 50–70 or > 70 years), baseline OKS (≤ 14, 15–21 or ≥ 22) and ‘treatment delivery unit’. A treatment delivery unit was either an ‘equipoise surgeon’ or a pair of ‘expertise surgeons’ with complementary expertise (i.e. one TKR-focused surgeon and one PKR-focused surgeon). The treatment delivery unit was included as a minimisation factor to ensure that a balance was maintained for individual equipoise surgeons and more generally by centre. Randomisation and allocation could not be conducted intraoperatively because of the expertise aspect to the design and the requirement to set up the theatre and lists appropriately for each implant type. Randomisation took place following the baseline assessment visit, which occurred either at the time of the patient’s outpatient preoperative assessment visit or at a ‘separate research visit’ around these routine appointments, depending on the local hospital set-up.
Participating surgeons were discouraged from changing their routine clinical practice during the course of the trial.
There was no blinding of trial participants or care providers.
Outcomes
The primary outcome measure for the trial was the OKS completed at 5 years after randomisation. The OKS is a 12-item patient-reported outcome questionnaire that is specifically designed and developed to assess function and pain after TKR surgery. Each item on the OKS is scored from 0 to 4, with 4 representing the best outcome. When the 12 items are summed, an overall score ranging from 0 to 48 is produced, with 48 representing the best outcome. The OKS has been demonstrated to be a validated and effective measure of change over time for knee replacement patients, with appropriate psychometric properties. 65,66
Secondary outcome measures were used to further assess functional outcome, patient health-related quality of life, frequency of complications and failure of operation. These were:
-
The AKSS67 at 2 months and 1 year post surgery and 5 years post randomisation. This score is divided into two separate components: (1) clinical assessment (Clinical AKSS – ‘Knee Score’) and (2) an assessment of an individual’s functionality (Functional AKSS – ‘Function Score’). The Clinical AKSS evaluates pain (50 points), stability (25 points) and range of motion (25 points). The maximum score of 100 points is reached when there is no pain, with good alignment of the knee in extension and at least 125° of range of motion, without any anteroposterior or mediolateral instability. Deductions are made for flexion contracture, loss of extension and poor alignment. The AKSS Function Score evaluates walking distance (50 points) and stair climbing ability (50 points). The maximum score of 100 points is attributed to an individual capable of walking unlimited distances without walking aids and of climbing and descending stairs normally. Deductions are made for the use of crutches or a walking frame/sticks. 68
-
The UCLA Activity Score69 at 2 months post surgery and 1–5 years post randomisation. This is a measurement of activity level in arthroplasty patients who have mid-/lower-level activity. The score has 10 descriptive activity levels ranging from wholly inactive and dependent on others (level 1), to moderate activities such as unlimited housework and shopping (level 6), to regular participation in impact sports such as jogging or tennis (level 10).
-
The HAAS70 at 2 months post surgery and 1–5 years post randomisation. This is a measurement of patient activity and accounts for patients with potentially higher levels of activity. The score is a four-item self-assessment measure covering four domains of walking, running, stair climbing and general activities, with a possible score ranging from 0 to 18 points (maximum score 18 points).
-
The EuroQol-5 Dimensions, three-level version (EQ-5D-3L),71 at 2 months post surgery and 1–5 years post randomisation. The EQ-5D-3L is a validated, generic, self-reported outcome measure of health-related quality of life consisting of a five-dimension health status classification system and a separate visual analogue scale (VAS). Responses to the health status classification system are used to facilitate the calculation of quality-adjusted life-years (QALYs) in health economic evaluations. It consists of five items on mobility, self-care, pain, usual activities and psychological status, with three possible answers for each item (i.e. 1 = no problem, 2 = moderate problems and 3 = severe problems). Health utilities or scores are generated from these responses using a validated algorithm. 72 They summarise overall health-related quality of life between 0 (death) and 1 (full health). In addition, health status was also assessed using the EQ-5D-3L VAS, which requires participants to assess their own health from the worst imaginable (0) to the best imaginable (100).
-
Three self-reported anchor-type questions were also recorded at 2 months post surgery and 1–5 years post randomisation. These asked about satisfaction (the ‘Lund score’, a measure of patient satisfaction),73 transition in relation to problems compared with an earlier time point, overall health, transition in relation to overall health compared with the previous year and whether or not the patient would have the operation again. Anchor-type questions are used in psychometrics and evaluate the same type of area/domain as the primary outcome variable but have a different style or format. They are useful to check concordance and, as a question, are often more meaningful to the patient. The responses are outlined below and were dichotomised (in a prespecified manner) for the analysis as follows:
-
How satisfied are you with your knee? (Dissatisfied, uncertain, satisfied, very satisfied.) This was analysed as a binary outcome of satisfied/very satisfied versus dissatisfied/uncertain.
-
How are the problems related to your knee now, compared with before your knee surgery? (No problems at all now, much better, slightly better, no change, slightly worse, much worse.) This was analysed as a binary outcome of better (no problems at all now, much better, slightly better) versus not better (no change, slightly worse, much worse).
-
If you could go back in time, would you still choose to have the knee operation? (Yes, no, not sure.) This was analysed as a binary outcome of yes (yes) versus no/unsure (no, not sure).
-
-
Surgical complications – any intraoperative and postoperative complications associated with the knee replacement (study knee) throughout the study period were collected.
-
Hospital length of stay.
-
Other health care and patient resource use (e.g. length of hospital stay at time of operation, 2 months post surgery and 1–5 years post randomisation).
-
Reoperation rate following knee replacement surgery, including revision.
-
Composite outcome assessment: failure of intervention was assessed using a prespecified composite outcome assessment. Failure was defined as the patient having any reoperation (including revision) and/or poor outcome indicated on the OKS. Poor outcome using the OKS was defined as an improvement in score of < 4 points. The anchor-based minimally important change of the OKS was used to identify poor outcome (‘lack of success’) for functional outcome. 66
-
The OKS-APQ adjunct score to the OKS was collected at years 4 and 5 post randomisation. This eight-item patient-reported outcome measure was developed and validated74 to complement the OKS as an additional scale for assessing higher levels of activity and (social) participation in patients following knee replacement. Used in combination with the primary outcome measure, this would capture additional data regarding patients’ activity and participation levels.
-
Radiographic imaging of the knee to assess positioning and radiolucency was carried out preoperative, immediately postoperatively and at 5 years post randomisation.
Data collection and management
Intraoperative and post-operative data on surgery, knee component used, length of stay, operation time and complications were collected on a primary procedure hospital form. Postoperative X-rays were collected immediately postoperatively and at 5 years post randomisation.
Follow-up
Follow-up outcome data were collected via postal questionnaires and clinical assessment visits. The postal questionnaire [containing OKS, UCLA, HAAS, EuroQol-5 Dimensions (EQ-5D), Lund, health care and patient resource use) was sent out to participants at 2 months post surgery and 1–5 years post randomisation. The OKS-APQ was collected at 4 and 5 years post randomisation. The clinical assessment visit (AKSS, health-care use and X-rays) was carried out at 2 months and at 1 year post surgery and 5 years post randomisation. Outcomes and time points are outlined in Table 2.
| Outcome measure | Time point | ||||||
|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative (months) | Post randomisation (years) | |||||
| Baseline | 2 | 1 | 2 | 3 | 4 | 5 | |
| OKS (self-report function) | ▲ | ○ | ○ | ○ | ○ | ○ | ○ |
| OKS-APQ (self-report function) | ○ | ○ | |||||
| AKSS (clinical exam) | ▲ | ▲ | ▲ | ▲ | |||
| UCLA (self-report activity) | ▲ | ○ | ○ | ○ | ○ | ○ | ○ |
| HAAS (self-report activity) | ▲ | ○ | ○ | ○ | ○ | ○ | ○ |
| Imaging (X-rays) | ▲ | ▲a | ▲ | ||||
| EQ-5D (global heath) | ▲ | ○ | ○ | ○ | ○ | ○ | ○ |
| Lund (patient satisfaction) | ○ | ○ | ○ | ○ | ○ | ○ | |
| Complications | ▲○ | ▲○ | ○ | ○ | ○ | ▲○ | |
| Health care (hospital length of stay and further operation) and resource use | ▲ | ○ | ○ | ○ | ○ | ○ | ○ |
In cases where there were > 12 weeks between randomisation and the operation date (as a result of hospital waiting lists), an additional OKS was administered at the clinical assessment 1 year post surgery. This additional assessment was included because of the variation in waiting times for surgery at participating sites. It was possible that some patients would have their 1-year assessment too early in their recovery for the results to be valid, and the potential variation in follow-up time could cause problems with interpretation. This was more meaningful when assessing early results (1 year) but is less significant for the primary outcome at 5 years.
Participants whose questionnaires had not been returned were contacted by the central study teams based at the Health Services Research Unit, University of Aberdeen, and the Surgical Intervention Trials Unit (SITU), University of Oxford. In the first instance this was through a reminder letter by post. If a questionnaire had still not been returned within a specified time frame, participants were offered the option of completing the questionnaire over the telephone. A number of other initiatives were taken to promote ongoing interest in, and commitment to, the trial, including participant newsletters and annual Christmas cards.
Post-surgical complications and adverse events
The trial involved routine knee replacement surgery for OA. Knee replacement surgery was carried out as per standard management regime and participants were informed of the standard risks associated with anaesthetic and knee replacement operations.
Adverse events that may have been expected as a potential recognised complication or harm related to the study treatments were recorded as complications. Adverse events attributed to medical comorbidities or anaesthesia (part of normal care) were only recorded as complications and were not reported as serious adverse events. Medical problems or surgery not associated with the study interventions were not systematically collected or reported. A serious adverse event for TOPKAT was defined as any untoward medical occurrence that was both unexpected and related to the study treatments that (1) resulted in death, (2) was life-threatening, (3) required inpatient hospitalisation or prolongation of existing hospitalisation or (4) resulted in persistent or significant disability/incapacity. Participating sites were provided with adverse event (reporting) forms to record adverse events and to guide them when determining whether an event was a serious/non-serious event or should be recorded as a complication.
Data on complications and reoperation were collected from various sources for cross-referencing and to ensure completeness. Complication data were collected from participants in their follow-up questionnaires and clinical assessment visits. Annual postal self-report questionnaires asked participants if they had been admitted to hospital at any point over the last 12 months for any reason related to their study knee. Any re-admissions were followed up by the trial manager in Oxford, who contacted the research team at the patient’s hospital to check details on the local hospital system. Once confirmed, the trial manager would record details on a re-admission operation form.
In addition, at the follow-up clinical visits, participants were asked if they had experienced any complications related to their study knee since their last visit that had resulted in them visiting a health-care practitioner.
A final check by local research teams on hospital records was undertaken at 5 years post randomisation to ensure that complication data were collected from all participants (i.e. those who had not returned a questionnaire or attended a follow-up visit). Data from any identified re-admission events were recorded on a re-admission operation form.
Statistical methods and study analysis
The methods outlined here are primarily for clinical effectiveness. The methods for the cost-effectiveness analysis are included in Chapter 5.
Ground rules for statistical analysis
The trial analysis followed the statistical analysis plan that was agreed in advance by the Trial Steering Committee (TSC). The main analysis was based on the intention-to-treat (ITT) basis (i.e. analysed as randomised irrespective of non-compliance). Baseline and follow-up data were summarised using appropriate descriptive statistics. The analysis was based on all available data and no attempt was made to impute missing follow-up data; however, for baseline data for continuous variables, data were imputed using the mean of that variable. Owing to the level of non-compliance with treatment allocation, complications and further operations were also analysed on a treatment-received basis. This was not carried out for any other outcomes. The principal set of analyses used data from baseline to 5-year follow-up. An earlier more restricted in scope set of analyses used 1-year follow-up data. Statistical significance was at the two-sided 5% level, with corresponding CIs and p-values reported for 1- and 5-year follow-up time points only where applicable (e.g. OKS).
Sample size
The sample size was calculated on both the OKS and the reoperation rate. Detection of change in reoperation rate of 7% between groups (from 5% to 12%) at 80% power and using a significance level of p < 0.05 (two-sided) required a sample size of 250 patients per group. A total of 250 patients per group also provided 90% power to detect a reoperation increase to 14% (difference of 9%). For the primary end point at 5 years, a sample size of 500 patients (250 in each group) was also required to identify clinically significant differences in OKS. This sample size allowed detection of a 2.0-point minimally important difference in OKS with 80% power at 5% (two-sided) significance level using a standard deviation (SD) of 10.0 points (or equivalently 90% power to detect a 3.0-point change). 75 (Note that, at the time of planning, a 2-point difference in OKS was considered the minimally important difference to detect; however, subsequent research conducted and published after the trial had commenced now suggests that a 5-point difference in OKS should become the appropriate minimally important difference in OKS.) This difference of 3.0 points in the OKS is equivalent to a typical category change in the AKSS. 67 The analysis also adjusted for the baseline values (and for the surgical treatment delivery unit), which would also have increased precision.
Primary/secondary outcome analysis
The primary outcome, OKS for PKR and TKR, was compared at the principal assessment point alone using linear regression analysis adjusted for minimisation variables (age and sex as continuous and binary variables, respectively), baseline OKS (as a continuous variable) and cluster robust variance to account for surgery delivery unit. A secondary analysis using an independent t-test was also calculated. Under a further planned secondary analysis, a marginal estimate of treatment effect over the whole 5-year period is also presented.
To explore the timing of follow-up for participants who received surgery > 12 weeks post randomisation, their 1-year post-randomisation OKS was replaced with the 1-year post-surgery OKS in a sensitivity analysis. A within-person difference was also analysed for participants who had both follow-up scores to assess the impact of data collection timing.
Any effect of expertise versus equipoise on the treatments was explored using treatment-by-delivery unit interaction in a planned analysis.
To explore the impact of surgeon experience on the analysis of OKS, the model was extended by including the number of procedures previously performed by the surgeon in a further analysis (also adding an additional factor and a treatment-by-experience interaction). 76 For surgeons whose baseline experience data were missing, the median number of procedures by intervention (TKR or PKR) was imputed.
To assess the impact of compliance (the operation being delivered as intended), instrumental variable methods were used to estimate the complier-average causal effect (CACE) for the 1 year and 5 years post-randomisation OKS.
There was concern that the planned analyses may not have been sensitive to a small but consistent difference over the 5-year period. Therefore, a post hoc analysis of OKS using area under the curve (AUC), generated for each participant using the trapezoidal rule, was conducted given the findings of the planned principal and secondary analysis. It was also analysed using linear regression.
For the secondary outcomes, AKSS, UCLA, HAAS, EQ-5D-3L index and VAS, OKS-APQ and overall health were analysed using linear regression that was adjusted for minimisation variables and baseline score, and the surgery delivery unit was accounted for using cluster robust variance. The self-reported anchor questions, hospital length of stay (days in hospital postoperatively), complications and reoperations were analysed using Poisson regression adjusted for minimisation variables and a cluster robust variance to account for surgery delivery unit.
Planned subgroup analysis
Subgroup analyses (defined a priori) explored the possible effect modification through the use of treatment-by-subgroup interactions for the following:
-
age (< 55 years, 55–70 years and > 70 years)
-
baseline OKS (0–14, 15–21 and 22–48)
-
sex.
Independent interpretation of results
Trials are susceptible to interpretation bias, especially if differences are small and investigators have prior preference for a particular intervention. A summary set of blinded results was sent to a group of entirely independent and unconnected assessors (trial experts and surgeons) to help interpret the results and the impact of the trial in an unbiased manner.
The results summary was masked for group identifier (i.e. group 1 and group 2, rather than implant type) and was sent to six separate reviewers entirely independent of the study. The independent experts were all familiar with orthopaedics and trial interpretation. They were selected, largely at random, from the trials network that David J Beard is involved in (Royal College of Surgeons, Versus Arthritis, National Institute for Health Research, British Orthopaedic Association). These members were as follows:
-
York – Professor David Torgerson – UK Professor of statistics and surgical clinical trials unit director with substantial orthopaedic trial experience.
-
Sydney – Professor Chris Maher – Australian Public Health Professor and Director of the Institute for Musculoskeletal Health with substantial orthopaedic and musculoskeletal clinical trials experience.
-
Liverpool – Mr Peter Brownson – UK academic shoulder surgeon with an experience and interest in clinical trials of efficacy.
-
Norwich – Professor Simon Donell – UK academic knee surgeon (TSC chairperson) with experience of both TKR and PKR.
-
Swansea – Mr Mark Mullins – UK academic knee surgeon (teaching hospital) with experience of both TKR and PKR.
-
Bristol – Professor Jane Blazeby – UK academic upper gastrointestinal surgeon and surgical clinical trials unit director with substantial trial interpretation experience.
The chosen members were each sent an e-mail (Box 1) and were asked to comment on the findings without awareness of grouping.
I write to request your help and (hopefully) a quick favour, in confidence.
We have now completed the 5 year follow up of the NIHR HTA TOPKAT study comparing Total versus Partial Knee Arthroplasty. A brief summary of the project is enclosed before the results section.
To ensure appropriate interpretation of the results we have sent a summary of the data/results to a selection of independent surgical trials or orthopaedic qualified individuals for them to make their own assessment and interpretation of the data. In this way we hope to reduce or remove bias from any interpretation.
We would be really grateful if you could spend 15–20 minutes reviewing the data and write in a few short sentences (like the conclusion of an abstract) what you think the study shows. The main points involve the primary outcome measure, any important secondary, re-operation and complications, and the health economic plot at 5 years. Other data is shown but do not feel you have to examine it all.
The groups are anonymised and so the labels for your conclusions will have to refer to Group 1 and Group 2 rather than PKR or TKR. The received interpretations will help us provide an unbiased opinion of the results.
We have included some extra data in appendices but feel free to ignore this if short of time.
We would most appreciate it if you did not share or communicate the results, or even discuss your role in this exercise. If able to do this task you will be acknowledged on the manuscript when published.
Your time is valuable and I really thank you for your assistance.
Please destroy the PDF when complete.
HTA, Health Technology Assessment; PDF, portable document format.
A consolidated opinion of these individuals, alongside that of the chief investigator (DB), senior author (MC) and the statistical team at Aberdeen, was utilised to achieve an unbiased interpretation of the results and any clinical implications. A summary of the responses from this group of experts is available on request.
Any investigation team members with strong personal opinion or conflicts of interest, although very helpful in the setting up of the study in 2008, had little or no part in the conduct of the study or the interpretation of the results.
Patient and public involvement
A patient representative was an active member of the TSC and, as part of this role, contributed to the monitoring and supervision of the trial progress.
Ethics approval and monitoring
Favourable ethics opinion for TOPKAT was given by the Oxfordshire Research Ethics Committee (REC) on 4 September 2009 (REC reference number 09/H0606/88).
Project Management Group
The Project Management Group (PMG) was responsible for the day-to-day management of the trial. This group was a collaboration between the Oxford and Aberdeen teams, consisting of the chief investigator, co-investigators, statisticians, trial managers and data co-ordinators.
Trial Steering Committee
The TSC was responsible for monitoring and supervising the progress of TOPKAT. The committee met 10 times between April 2010 and November 2016, at time points agreed by the committee. The TSC consisted of four independent experts, a lay member, the chief investigator and key members of the PMG. Membership of the TSC is given in the Acknowledgements section.
Data Monitoring Committee
The Data Monitoring Committee (DMC) was independent of the trial and was tasked with monitoring ethics, safety and data integrity. The committee met four times between January 2011 and September 2013, at approximately yearly intervals. The trial statistician provided the data and reports requested by the DMC at each of the meetings. No formal interim analyses were requested by the DMC; interim data summarised were reviewed by the DMC. Membership of the DMC is given in the Acknowledgements section.
Chapter 3 Description of the study population
Trial recruitment
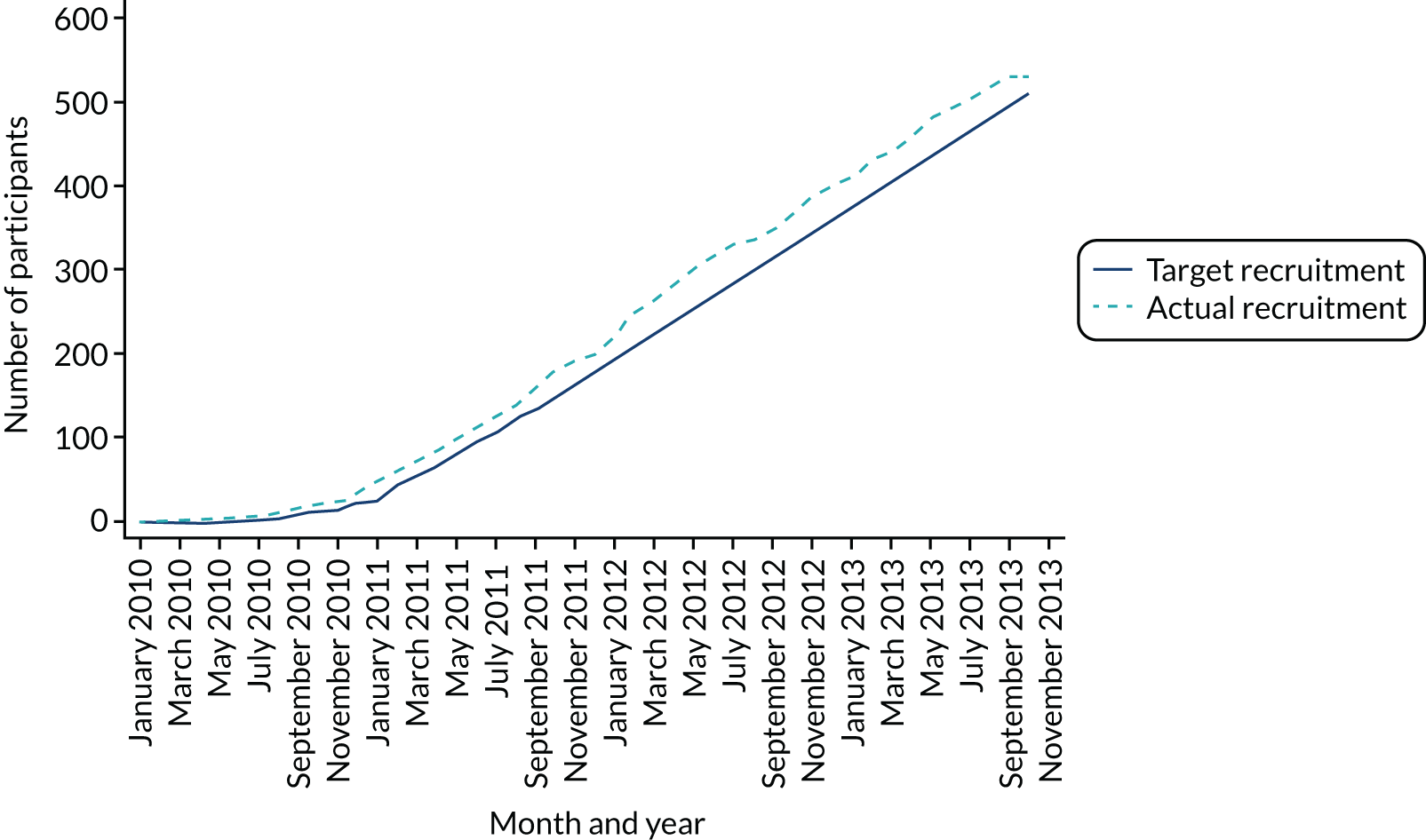
In total, 27 sites across the UK recruited 528 participants from 18 January 2010 to 30 September 2013 (Table 3), with the 5-year follow-up ending in September 2018. Three individuals were randomised twice in error, making a total of 531 randomisations. There were 264 participants randomised to the PKR group and 264 to the TKR group. Figure 1 shows the trajectory of the number of participants randomised over the recruitment period.
| Site | PKR (N = 264), n (%) | TKR (N = 264), n (%) | Randomised (N = 528), n (%) |
|---|---|---|---|
| Musgrave Park Hospital | 53 (20.1) | 53 (20.1) | 106 (20.1) |
| Great Western Hospital | 29 (11.0) | 28 (10.6) | 57 (10.8) |
| University Hospital of North Tees | 28 (10.6) | 26 (9.8) | 54 (10.2) |
| Royal Gwent Hospital | 20 (7.6) | 22 (8.3) | 42 (8.0) |
| Nuffield Orthopaedic Centre | 20 (7.6) | 20 (7.6) | 40 (7.6) |
| King’s Mill Hospital | 13 (4.9) | 13 (4.9) | 26 (4.9) |
| Leicester Royal Infirmarya | 12 (4.5) | 14 (5.3) | 26 (4.9) |
| Pilgrim Hospitala,b | 12 (4.5) | 13 (4.9) | 25 (4.7) |
| University Hospital of North Staffordshire | 11 (4.2) | 13 (4.9) | 24 (4.5) |
| Yeovil District Hospitala | 8 (3.0) | 8 (3.0) | 16 (3.0) |
| Ipswich Hospitala,b | 7 (2.7) | 7 (2.7) | 14 (2.7) |
| Chesterfield Royal Hospitala | 7 (2.7) | 6 (2.3) | 13 (2.5) |
| Lincoln County Hospitala | 6 (2.3) | 6 (2.3) | 12 (2.3) |
| Royal United Hospital Bath NHS Trust | 5 (1.9) | 5 (1.9) | 10 (1.9) |
| Harrogate District Hospital | 5 (1.9) | 5 (1.9) | 10 (1.9) |
| Stepping Hill Hospital | 4 (1.5) | 5 (1.9) | 9 (1.7) |
| Aberdeen Royal Infirmary | 5 (1.9) | 4 (1.5) | 9 (1.7) |
| North Bristol NHS Trust | 3 (1.1) | 4 (1.5) | 7 (1.3) |
| Royal Blackburn Hospital | 3 (1.1) | 3 (1.1) | 6 (1.1) |
| University Hospital of North Durham | 4 (1.5) | 2 (0.8) | 6 (1.1) |
| Medway Hospital | 3 (1.1) | 2 (0.8) | 5 (0.9) |
| Milton Keynes General Hospitala | 2 (0.8) | 1 (0.4) | 3 (0.6) |
| Southampton General Hospital | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Cumberland Infirmary | – | 2 (0.8) | 2 (0.4) |
| Pindefields Hospitala | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Torbay Hospital | 1 (0.4) | – | 1 (0.2) |
| Hull and East Yorkshire Hospitals NHS Trust | 1 (0.4) | – | 1 (0.2) |
FIGURE 1.
Recruitment graph: January 2010 to October 2013.
Equipoise versus expertise-based randomisation
A total of 68 surgeons were involved with recruitment, of whom 50 surgeons randomised a patient to the study. Thirty-six surgeons at 23 sites participated as ‘equipoise surgeons’ and randomised 454 participants (227 in each group). Eighteen surgeons at eight sites participated as ‘expertise surgeons’ and randomised 74 participants (37 in each group) (one surgeon in an ‘expertise pair’ did not randomise a patient). Of these, three surgeons changed from participating as an ‘equipoise’ to an ‘expertise’ surgeon (or the alternative) shortly after starting recruitment because of local decisions regarding participation. For this reason, these surgeons randomised participants as both ‘equipoise’ and ‘expertise’ surgeons at different time points during the study.
Participant flow
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 2. There were 962 participants assessed for eligibility, of whom 528 were randomised. Three participants were randomised twice in error (resulting in 531 randomisations). The reasons for exclusion were that 121 individuals did not meet the inclusion criteria and 310 individuals declined to participate.
FIGURE 2.
The CONSORT flow diagram.
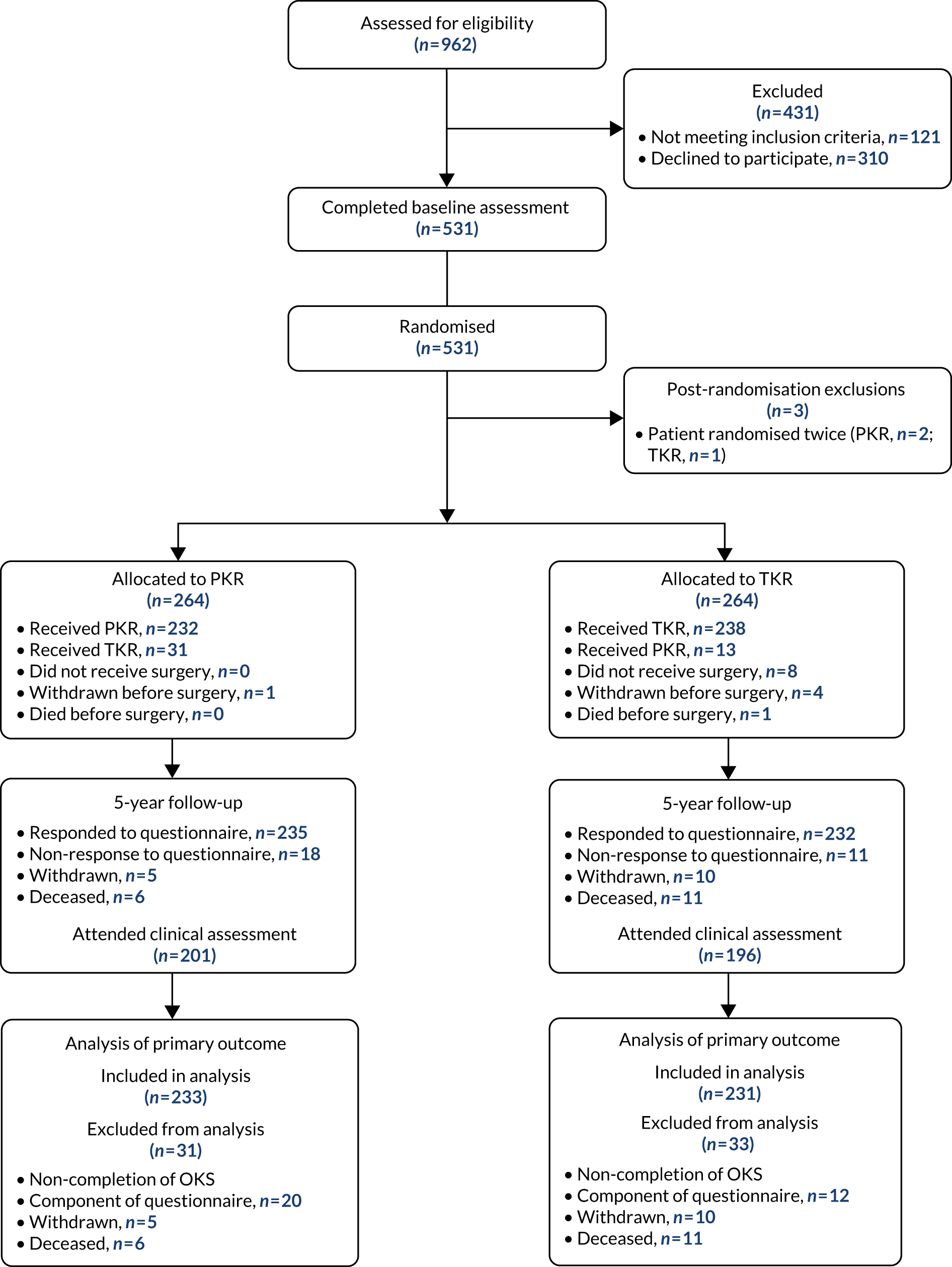
In the PKR group, all participants completed the baseline assessment, 263 participants received surgery and one withdrew before surgery. In the TKR group, 251 received surgery, four withdrew before surgery, one died before surgery and the remaining eight did not receive surgery (further details of treatment received are provided in Figure 2). At the 5-year follow-up, a total of 467 participants responded to the questionnaire and 397 attended the clinical assessments. Fifteen (PKR, n = 5; TKR, n = 10) participants withdrew (i.e. the participant no longer wanted to be in the study from this point forward) and there were 17 (PKR, n = 6; TKR, n = 11) deaths. For the other follow-up time points see Appendix 2, Table 27.
Baseline characteristics
Table 4 shows that the baseline characteristics in the two groups were, in general, equally balanced. The mean age was 65 years, 58% of participants were male and the mean body mass index (BMI) was 31 kg/m2 in both groups. The time since onset of OA in the study knee was < 3 years for 28% of participants in both groups, and > 10 years for 14% of participants in the PKR group and for 11% in the TKR group. The frequency of a previous TKR in the non-study leg was 9% in the PKR group and 5% in the TKR group, whereas 6% of participants in both groups had previous PKR replacement in the non-study leg. The mean OKS was 19 points for both groups, and the EQ-5D-3L was 0.428 in the PKR group and 0.381 in the TKR group.
| Characteristic | PKR (N = 264) | TKR (N = 264) |
|---|---|---|
| Age (years), mean (SD); n | 65.2 (8.8); 264 | 64.7 (8.5); 264 |
| Sex, n (%) | ||
| Male | 153 (58.0) | 153 (58.0) |
| Female | 111 (42.0) | 111 (42.0) |
| Study knee, n (%) | ||
| Left | 140 (53.0) | 141 (53.4) |
| Right | 124 (47.0) | 123 (46.6) |
| Time of OA, n (%) | ||
| < 3 years | 75 (28.4) | 73 (27.7) |
| 3–5 years | 82 (31.1) | 72 (27.3) |
| 6–10 years | 59 (22.3) | 73 (27.7) |
| > 10 years | 36 (13.6) | 30 (11.4) |
| Missing | 12 (4.5) | 16 (6.1) |
| Medical history, n (%) | ||
| Other joint problems | 106 (40.2) | 96 (36.4) |
| Cardiovascular | 80 (30.3) | 86 (32.6) |
| Diabetes | 27 (10.2) | 26 (9.8) |
| Gastrointestinal | 17 (6.4) | 18 (6.8) |
| Respiratory | 19 (7.2) | 12 (4.5) |
| Cancer | 6 (2.3) | 8 (3.0) |
| Renal/urological | 8 (3.0) | 8 (3.0) |
| Neurological | 7 (2.7) | 6 (2.3) |
| Mental health | 7 (2.7) | 6 (2.3) |
| Thyroid problems | 3 (1.1) | 2 (0.8) |
| Othera | 5 (1.9) | 4 (1.5) |
| Employment status, n (%) | ||
| Retired | 159 (60.2) | 162 (61.4) |
| Not in employment | 15 (5.7) | 21 (8.0) |
| In paid employment | 82 (31.1) | 73 (27.7) |
| Missing | 8 (3.0) | 8 (3.0) |
| BMI (kg/m2), mean (SD); n | 31.0 (4.6); 210 | 31.1 (4.8); 221 |
| Extent of knee arthritis affecting mobility (Charnley ABC), n (%) | ||
| Single | 99 (37.5) | 119 (45.1) |
| Both | 142 (53.8) | 121 (45.8) |
| Multiple | 6 (2.3) | 11 (4.2) |
| Missing | 17 (6.4) | 13 (4.9) |
| General health, mean (SD); n | 2.6 (0.9); 259 | 2.8 (0.9); 260 |
| General health compared with 1 year ago, mean (SD); n | 3.3 (0.8); 259 | 3.3 (0.8); 260 |
| Previous treatment on study knee, n (%) | ||
| Analgesia | 207 (78.4) | 184 (69.7) |
| Arthroscopy | 44 (16.7) | 47 (17.8) |
| Arthroscopic investigative washout/debridement | 44 (16.7) | 36 (13.6) |
| Open/arthroscopic meniscus | 33 (12.5) | 30 (11.4) |
| Knee injection: steroid | 19 (7.2) | 21 (8.0) |
| Knee injection: viscosupplementation | 2 (0.8) | 3 (1.1) |
| Knee injection not stated | 4 (1.5) | 6 (2.3) |
| Acupuncture | 4 (1.5) | 5 (1.9) |
| Chiropractor/osteopath | 1 (0.4) | 3 (1.1) |
| Cartilage implantation | 1 (0.4) | 2 (0.8) |
| Anterior cruciate ligament repair | 1 (0.4) | – |
| Otherb | – | 3 (1.2) |
| None | 13 (4.9) | 26 (9.8) |
| Problems with the other knee, n (%) | ||
| None | 86 (32.6) | 99 (37.5) |
| Mild | 93 (35.2) | 74 (28.0) |
| Moderate | 63 (23.9) | 52 (19.7) |
| Severe | 18 (6.8) | 30 (11.4) |
| Missing | 4 (1.5) | 9 (3.4) |
| Contralateral knee, n (%) | ||
| TKR | 24 (9.1) | 14 (5.3) |
| PKR | 16 (6.1) | 16 (6.1) |
| Unsure | 1 (0.4) | 1 (0.4) |
| None | 208 (78.8) | 217 (82.2) |
| Missing | 15 (5.7) | 16 (6.1) |
| OKS, mean (SD); n | 18.8 (7.0); 264 | 19.0 (7.2); 264 |
| HAAS, mean (SD); n | 4.8 (2.3); 258 | 4.6 (2.3); 256 |
| UCLA activity score, mean (SD); n | 3.6 (1.5); 260 | 3.7 (1.5); 260 |
| AKSS: objective, mean (SD); n | 41.0 (16.1); 260 | 42.3 (16.0); 259 |
| AKSS: functional, mean (SD); n | 59.3 (15.6); 262 | 58.7 (15.5); 259 |
| EQ-5D-3L, mean (SD); n | 0.428 (0.301); 257 | 0.381 (0.324); 252 |
| EQ-5D VAS, mean (SD); n | 62.8 (27.0); 249 | 60.7 (28.7); 257 |
Treatment received
Of the 263 participants who had surgery in the PKR group (Table 5), 232 (88%) received their allocated treatment and 31 (12%) received TKR. For the participants who had a non-allocated trial operation (‘crossed over’), 25 were intraoperative decisions [pattern of OA not suitable for PKR (n = 20) and anterior cruciate ligament absent/deterioration (n = 5)] and six were preoperative decisions [pattern of OA not suitable for PKR (n = 2), patient decision (n = 1), inflammatory arthropathy (not suitable for PKR) (n = 1), diagnosis (post randomisation) patient not suitable for PKR (although randomised, later found not to have suitable inclusion criteria, i.e. incorrect diagnosis of medial compartment OA) (n = 1) and error in communication of randomisation allocation (n = 1)].
| Treatment details | PKR (N = 264) | TKR (N = 264) |
|---|---|---|
| Received surgery, n (%) | ||
| Yes | 263 (99.6) | 251 (95.1) |
| No | 1 (0.4) | 13 (4.9) |
| Received allocated knee replacement surgery, n (%) | ||
| Yes | 232 (88.2) | 238 (94.8) |
| No | 31 (11.8) | 13 (5.2) |
| n = 263 | n = 251 | |
| Surgical technique (and implant used), n (%) | ||
| PKR | 232 (88.2) | 13 (5.2) |
| Oxford® Partial Knee (Zimmer Biomet, Warsaw, IN, USA) | 150 (64.7) | 7 (53.8) |
| Zimmer (Zimmer Biomet) | 36 (15.5) | 4 (30.8) |
| M/G® Unicompartmental Knee System (Zimmer Biomet) | 22 (9.5) | 1 (7.7) |
| Uniglide™ (Corin Group, Cirencester, UK) | 9 (3.9) | – |
| AMC (Corin Group) | 5 (2.2) | – |
| DePuy (DePuy Orthopaedics Inc., Warsaw, IN, USA) | 4 (1.7) | – |
| Mathys (Mathys Ltd, Bettlach, Switzerland) | 4 (1.7) | – |
| Medacta (Medacta International, Castel San Pietro, Switzerland) | 1 (0.4) | – |
| Sigma (DePuy Orthopaedics Inc.) | 1 (0.4) | – |
| Vanguard® (Zimmer Biomet) | – | 1 (7.7) |
| TKR | 31 (11.8) | 238 (94.8) |
| Low Contact Stress (DePuy Orthopaedics Inc.) | 10 (32.3) | 61 (25.6) |
| PFC/Sigma (DePuy Orthopaedics Inc.) | 3 (9.7) | 54 (22.7) |
| Vanguard (Zimmer Biomet) | 3 (9.7) | 41 (17.2) |
| NexGen® (Zimmer Biomet) | 8 (25.8) | 29 (12.2) |
| Triathlon® Knee System (Stryker, Mahwah, NJ, USA) | 4 (12.9) | 27 (11.3) |
| Genesis (Smith & Nephew, Memphis, TN, USA) | 2 (6.5) | 7 (2.9) |
| Scorpio/Kinemax (Stryker) | 1 (3.2) | 7 (2.9) |
| ACS® (Implantcast, Buxtehude, Germany) | – | 6 (2.5) |
| EUROS (Euros SAS, La Ciotat, France) | – | 2 (0.8) |
| AGC (Zimmer Biomet) | – | 1 (0.4) |
| AllPoly (Zimmer Biomet) | – | 1 (0.4) |
| Oxinium (Smith & Nephew) | – | 1 (0.4) |
| Unknown | – | 1 (0.4) |
Of the 251 participants who received surgery in the TKR group, 238 (95%) received their allocated treatment and 13 (5%) received PKR. For the 13 participants who crossed over, five were intraoperative decisions (independent surgeon decision, i.e. all of the eligibility criteria were met but decision made for PKR) and eight were preoperative decisions [patient decision (n = 5) and error in communication of randomisation allocation at site (n = 3)]. Ten different PKR implants were used, with the majority receiving the Oxford mobile bearing implant. For the TKR group, 12 (one was missing) different implants were used, none of which was used more often than any other (see Table 5).
The median waiting time to surgery was 6 weeks in the PKR group [interquartile range (IQR) 2–14 weeks], with 85 participants waiting > 12 weeks. For TKR the median waiting time was 4 weeks (IQR 1–11 weeks), with 53 participants waiting > 12 weeks.
Details of the operation for those who received surgery are shown in Table 6. Three-quarters of the participants (PKR 73%, TKR 75%) who were operated on were classified as ASA grade 2. The ASA grade of 17 participants in both groups was classified as grade 3 at the time of operation. Over 90% of participants in both groups had a straightforward replacement. A mobile bearing device was used in 63% of the participants in the PKR group compared with 34% of participants in the TKR group. A fixed bearing was used in 37% of the PKR patients compared with 64% of the TKR patients. For the remaining participants (PKR 1%, TKR 2%), the implant information was missing. The median operation time was 68 minutes in the PKR group and 65 minutes in TKR group.
| Measure | PKR (N = 264) | TKR (N = 264) |
|---|---|---|
| Received surgery | n = 263 | n = 251 |
| ASA grade, n (%) | ||
| 1 | 46 (17.5) | 39 (15.5) |
| 2 | 192 (73.0) | 188 (74.9) |
| 3 | 17 (6.5) | 17 (6.8) |
| Missing | 8 (3.0) | 7 (2.8) |
| Ease of replacement, n (%) | ||
| Straightforward | 245 (93.2) | 236 (94.0) |
| Difficult | 16 (6.1) | 14 (5.6) |
| Missing | 2 (0.8) | 1 (0.4) |
| Patella replaced, n (%) | ||
| Yes | 2 (0.8) | 20 (8.0) |
| No | 258 (98.1) | 229 (91.2) |
| Missing | 3 (1.1) | 2 (0.8) |
| Bearing, n (%) | ||
| Mobile | 165 (62.7) | 84 (33.5) |
| Fixed | 96 (36.5) | 161 (64.1) |
| Missing | 2 (0.8) | 6 (2.4) |
| Size of bearing, mean (SD); n | 6.3 (2.9); 250 | 10.0 (1.7); 235 |
| Cement, n (%) | ||
| Palacos | 145 (55.1) | 137 (54.6) |
| CMW I | 11 (4.2) | 18 (7.2) |
| Optipac | 13 (4.9) | 11 (4.4) |
| Smart ser | 15 (5.7) | 7 (2.8) |
| Simplex | 1 (0.4) | 2 (0.8) |
| None | 77 (29.3) | 74 (29.5) |
| Missing | 1 (0.4) | 2 (0.8) |
| X-ray performed, n (%) | ||
| Yes | 2 (0.8) | 1 (0.4) |
| No | 253 (96.2) | 240 (95.6) |
| Missing | 8 (3.0) | 10 (4.0) |
| Type of anaesthetic, n (%) | ||
| Spinal | 201 (76.4) | 198 (78.9) |
| Periarticular LA | 125 (47.5) | 92 (36.7) |
| Femoral block | 68 (25.9) | 104 (41.4) |
| GA | 61 (23.2) | 66 (26.3) |
| Sciatic block | 15 (5.7) | 12 (4.8) |
| Epidural | 7 (2.7) | – |
| Received surgery | n = 262 | n = 250 |
| Knee structure (ACL), n (%) | ||
| Normal | 221 (84.0) | 197 (78.5) |
| Mild damage | 34 (12.9) | 39 (15.5) |
| Severe damage | 2 (0.8) | 6 (2.4) |
| Absent | 3 (1.1) | 1 (0.4) |
| Missing | 3 (1.1) | 8 (3.2) |
| Knee structure (PFJ), n (%) | ||
| Lateral | ||
| Normal | 214 (81.4) | 184 (73.3) |
| Partial thickness | 35 (13.3) | 53 (21.2) |
| Exposed bone | 6 (2.3) | 6 (2.4) |
| Missing | 8 (3.0) | 8 (3.2) |
| Medial | ||
| Normal | 146 (55.5) | 120 (47.8) |
| Partial thickness | 84 (31.9) | 78 (31.1) |
| Exposed bone | 24 (9.1) | 46 (18.3) |
| Missing | 9 (3.4) | 7 (2.8) |
| Trochlear | ||
| Normal | 110 (41.8) | 118 (47.0) |
| Partial thickness | 123 (46.8) | 105 (41.8) |
| Exposed bone | 22 (8.4) | 18 (7.2) |
| Missing | 8 (3.0) | 10 (4.0) |
| Operation time (minutes), median (IQR); n | 68.0 (55.0–80.0); 261 | 65.0 (55.0–80.0); 249 |
| Theatre time (minutes), median (IQR); n | 113.0 (95.0–129.0); 260 | 110.0 (90.0–128.0); 249 |
Chapter 4 Clinical results
Primary outcome: Oxford Knee Score
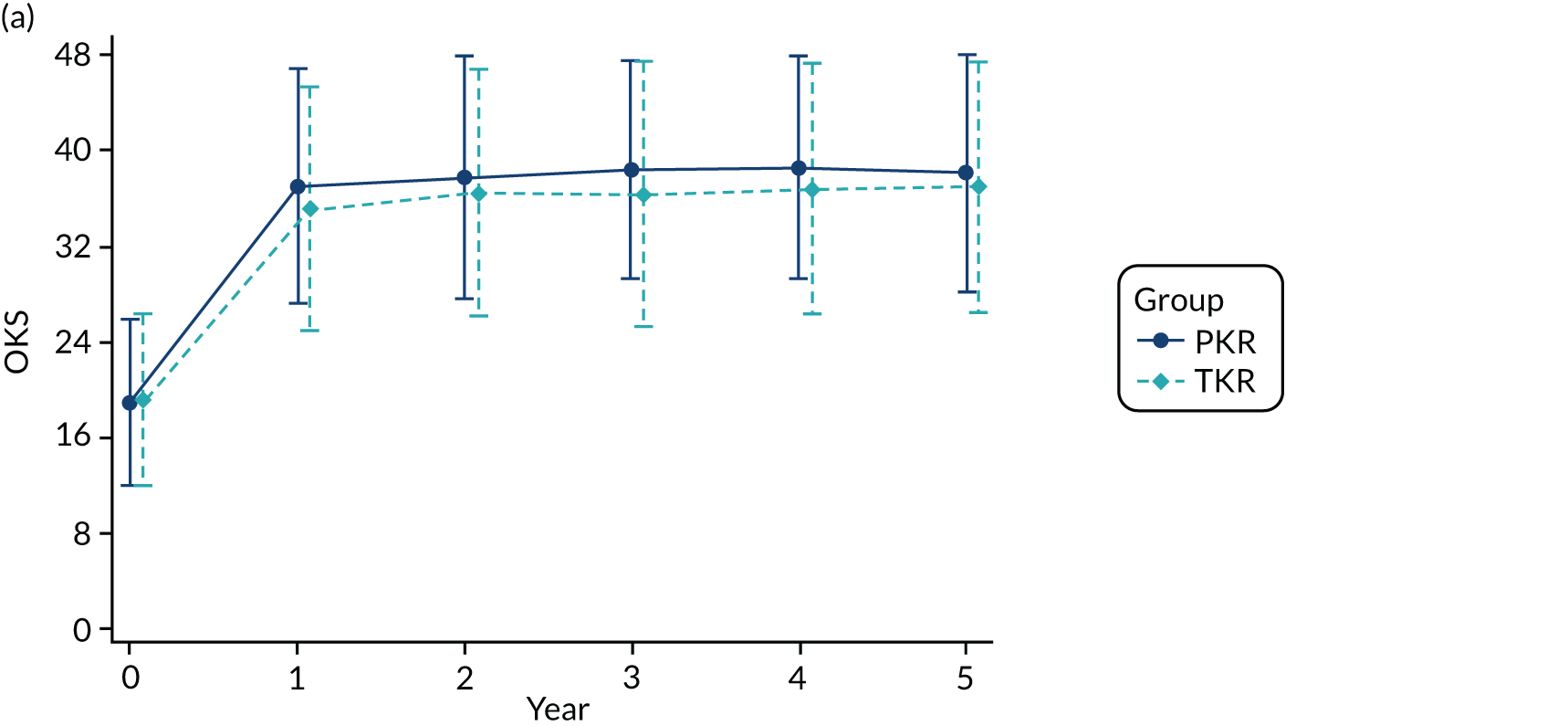
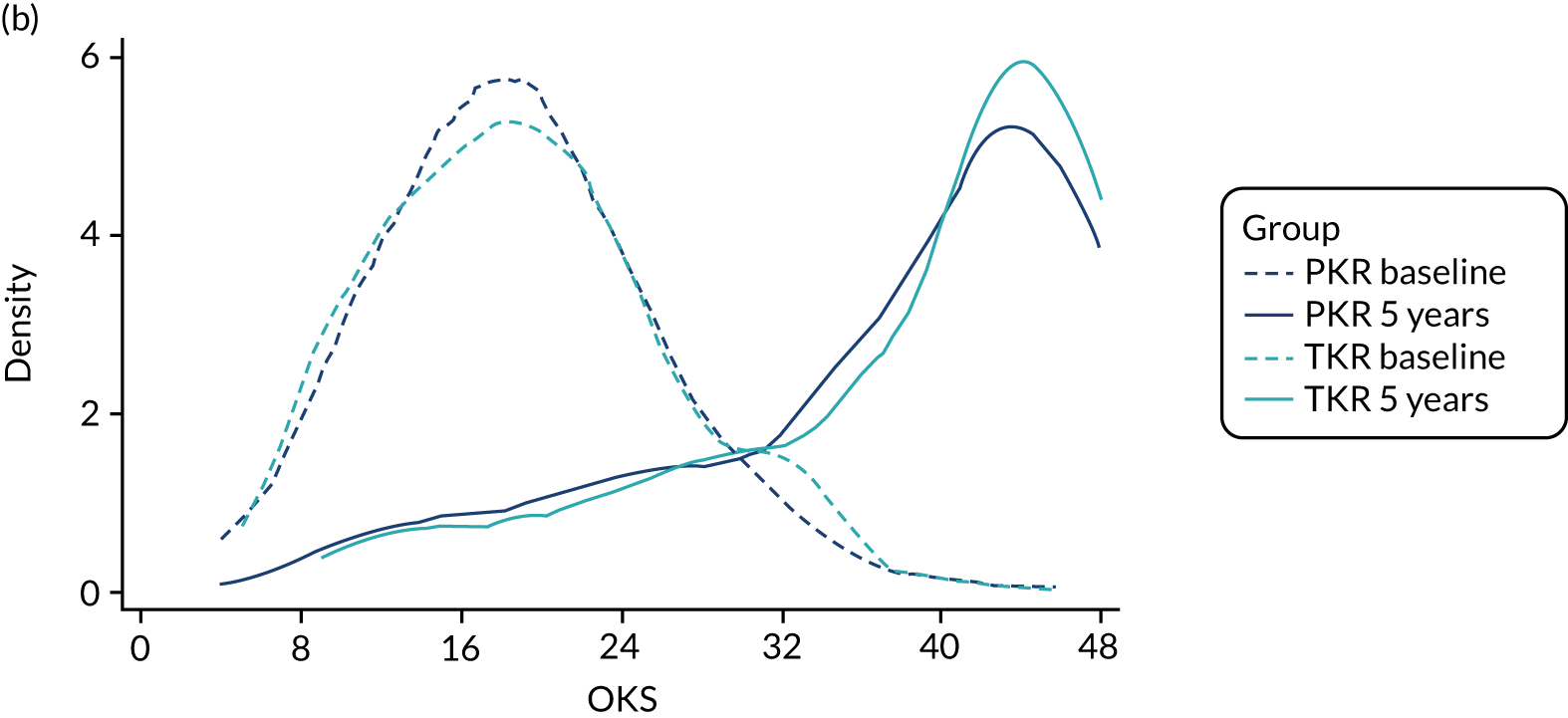
The mean OKS for patients who were allocated to PKR and TKR at baseline was 18.8 and 19.0 points, respectively (Table 7). Figures 3a and 3b show the mean (SD) OKS for the post-randomisation time points, as well as the kernel density plot for the OKS at baseline and 5-year follow-up, respectively. The OKS was available for 491 participants (93%) at 1 year and for 464 participants (88%) at 5 years. At 1 year post randomisation, the mean OKS increased to 36.9 in the PKR group and 35.1 in the TKR group, with evidence of a difference in favour of PKR [mean difference (MD) 1.91, 95% CI 0.20 to 3.62; p = 0.029]. At the further follow-up time points, the mean OKS was higher in the PKR group than in the TKR group (see Figure 3a) but there was no evidence of a difference for the principal analysis, where the 5-year estimate was MD 1.04 (95% CI –0.42 to 2.50; p = 0.159). Figure 3c shows the estimated treatment effect (95% CI) at each follow-up time point. Sensitivity analyses exploring imputation of the worse case value did not substantially alter the 1- and 5-year OKS findings, except for extreme one-way assumptions. A planned secondary analysis, an unadjusted analysis using an independent t-test, was carried out and showed similar results (MD 1.79, 95% CI < 0.01 to 3.58, p = 0.0497 and MD 1.02, 95% CI –0.86 to 2.91 for 1 and 5 years, respectively), as did the marginal estimate of the treatment effect over the whole 5-year follow-up from the mixed model, which was 1.39 (95% CI –0.12 to 2.90; p = 0.071). A post hoc analysis of AUC for participants who had a 5-year follow-up was statistically significant: for the 233 participants in the PKR group the mean was 36.6 (SD 8.3) and for the 231 participants in the TKR group the mean was 35.1 (SD 9.1) (MD 1.54, 95% CI 0.07 to 3.01; p = 0.040).
| Time point | PKR (N = 264), mean (SD); n | TKR (N = 264), mean (SD); n | MD | 95% CI |
|---|---|---|---|---|
| Baseline | 18.8 (7.0); 264 | 19.0 (7.2); 264 | ||
| 2 months post surgery | 31.1 (9.6); 247 | 29.4 (9.0); 239 | 1.81 | –0.52 to 4.15 |
| 1 year | 36.9 (9.9); 247 | 35.1 (10.3); 244 | 1.91 | 0.20 to 3.62 |
| 2 years | 37.7 (10.3); 240 | 36.4 (10.4); 238 | 1.43 | –0.23 to 3.10 |
| 3 years | 38.3 (9.2); 218 | 36.2 (11.2); 226 | 2.15 | –0.01 to 4.31 |
| 4 years | 38.5 (9.4); 205 | 36.7 (10.6); 219 | 1.78 | –0.23 to 3.79 |
| 5 years | 38.0 (10.1); 233 | 37.0 (10.6); 231 | 1.04 | –0.42 to 2.50 |
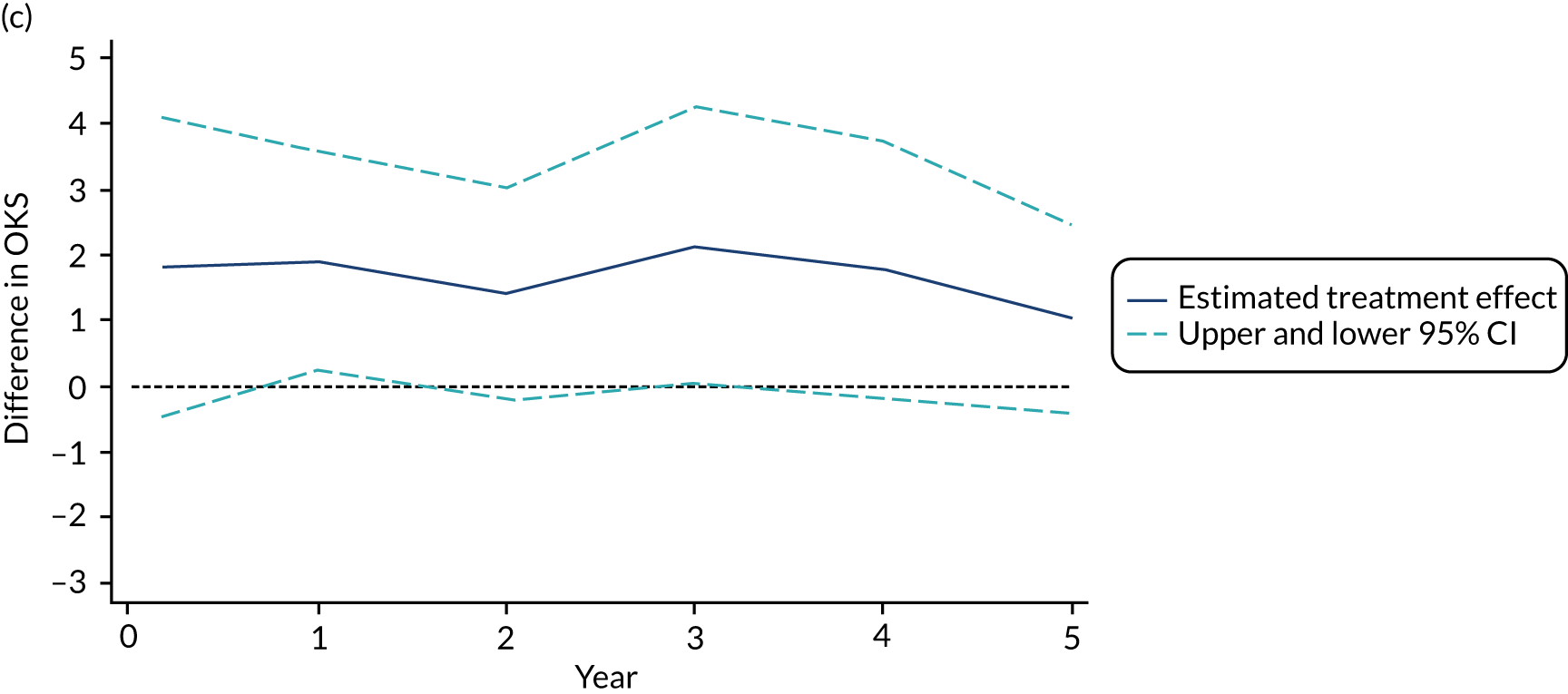
FIGURE 3.
(a) Mean (SD) OKS by group at each follow-up time point post randomisation; (b) kernel density plot for OKS at baseline and 5-year follow-up by treatment group; and (c) estimated treatment effect on OKS (95% CI) at each follow-up time point.
Impact of timing of follow-up
As expected in elective surgery in the NHS, there were delays to intervention (surgery) in several patients. This may have had some impact on the results and was, therefore, explored.
Of the 528 participants randomised, 514 (97.3%) received surgery. In total, 138 out of 514 participants (26.8%) waited > 12 weeks from randomisation to surgery.
These 138 participants were distributed across the two groups as follows: 85 out of 263 (32.3%) participants in the PKR group waited more than 12 weeks and 53 out of 251 (21.1%) in the TKR group waited more than 12 weeks. Figure 4 shows the mean (SD) OKS at baseline, 1 year post randomisation and post surgery.
FIGURE 4.
Mean (SD) OKS by group at baseline and 1 year post randomisation and post surgery, and effect estimates. Post-randomisation difference 1.91 (95% CI 0.20 to 3.62; p = 0.029). Post-surgery difference 1.62 (95% CI –0.17 to 3.41; p = 0.074). Within-person difference –1.61 (95% CI –3.47 to –0.24; p = 0.084).
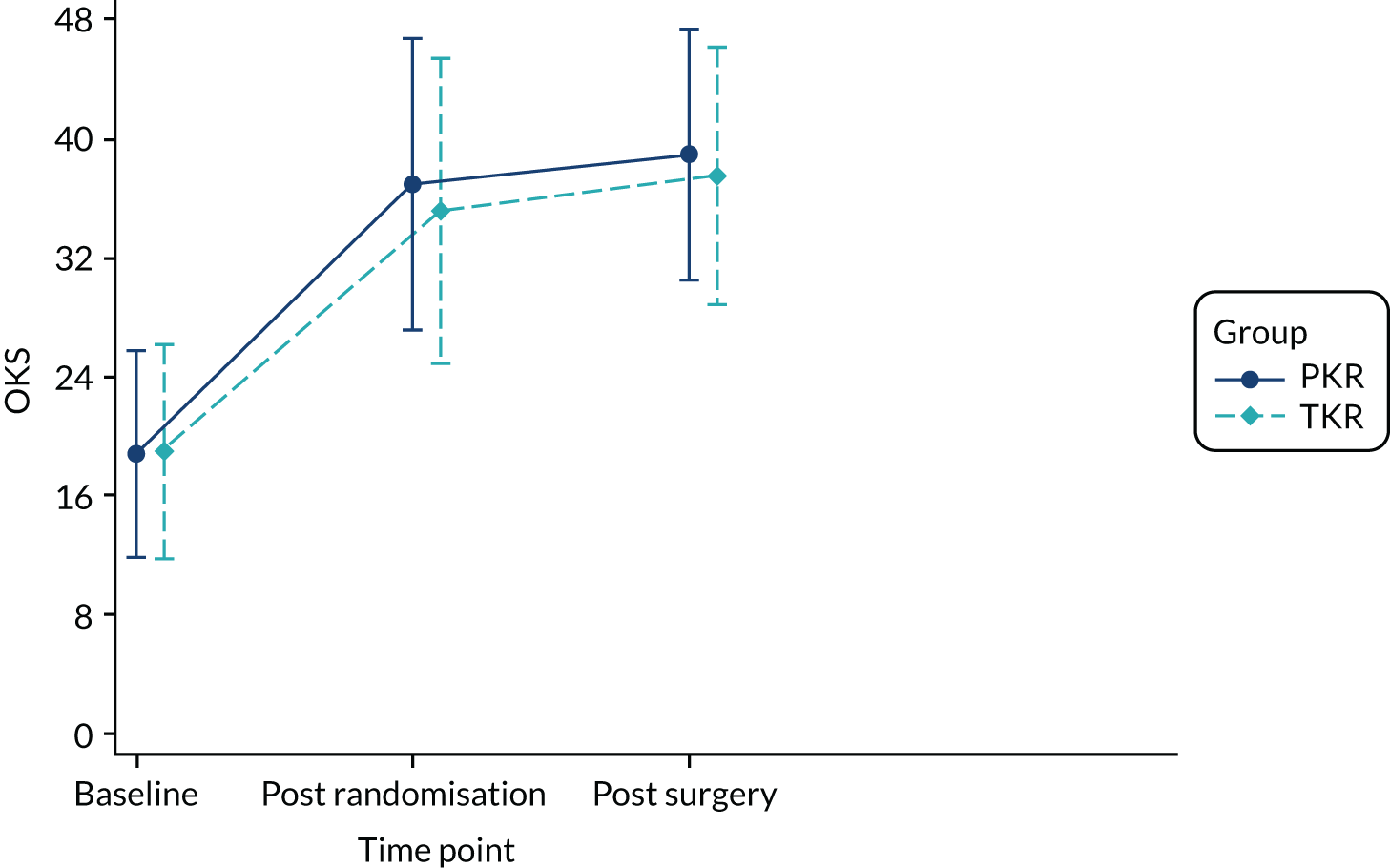
The MD was 1.91 favouring PKR (95% CI 0.20 to 3.62). Using data from 1 year post surgery for participants who had a 12-week delay, the MD was slightly lower but still favouring PKR (1.62, 95% CI –0.17 to 3.41). In the 91 participants with both a 1-year post-randomisation and a 1-year post-surgery follow-up (53 PKR, 38 TKR), the within-participant difference was –1.61 (95% CI –3.47 to 0.24), showing an improvement at the later time point but with uncertainty.
Any delay to surgery was thus considered to have minimal impact on the results.
Surgeon experience
The experience of the surgeon (as defined by the number of procedures of each operation type performed prior to the study) may have had an effect on the results and was, therefore, explored. The median (IQR) number of procedures completed by surgeons for participants undergoing PKR at baseline was 100 procedures (50–200 procedures), and for those undergoing TKR was 300 procedures (260–400 procedures). Table 8 shows the standard OKS model that was used previously and also an extended model that adjusts for the number of procedures performed by each surgeon (as a proxy of experience, including within-trial experience). There was statistical evidence for an improvement in OKS outcome with an increased number of procedures performed at 1 year, which is indicated by the non-zero ‘learning effect’. This was not the case for the equivalent 5-year post-randomisation OKS analysis. There was, however, evidence of a differential learning effect by treatment group (as shown by the significant interaction terms) for the analysis of the 1-year OKS, although the impact that this had on the predicted level and the treatment difference was small.
| Model | Treatment difference in OKS (95% CI) | Learning effect (95% CI) | Learning by treatment interaction (95% CI) |
|---|---|---|---|
| 1 year | |||
| Standard OKS model | 1.91 (0.20 to 3.62) | ||
| Model extended to adjust for surgeon experience | 3.10 (1.34 to 4.86) | 0.0004 (0.0002 to 00006) | –0.0030 (–0.0056 to –0.0004) |
| 5 years | |||
| Standard OKS model | 1.04 (–0.42 to 2.50) | ||
| Model extended to adjust for surgeon experience | 1.84 (0.02 to 3.65) | 0.0001 (–0.0003 to 0.0005) | –0.0032 (–0.0054 to –0.0010) |
Surgeon expertise
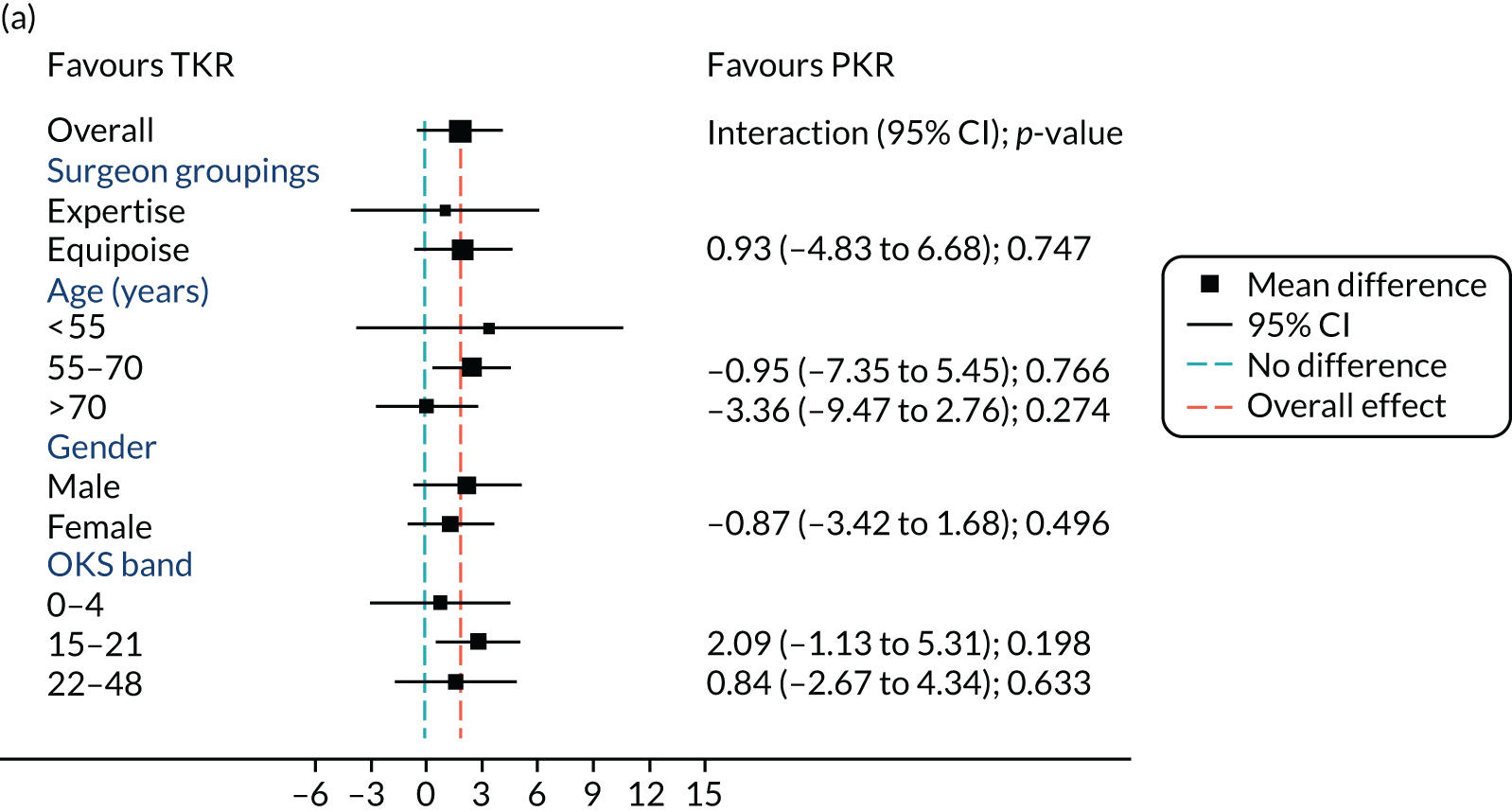
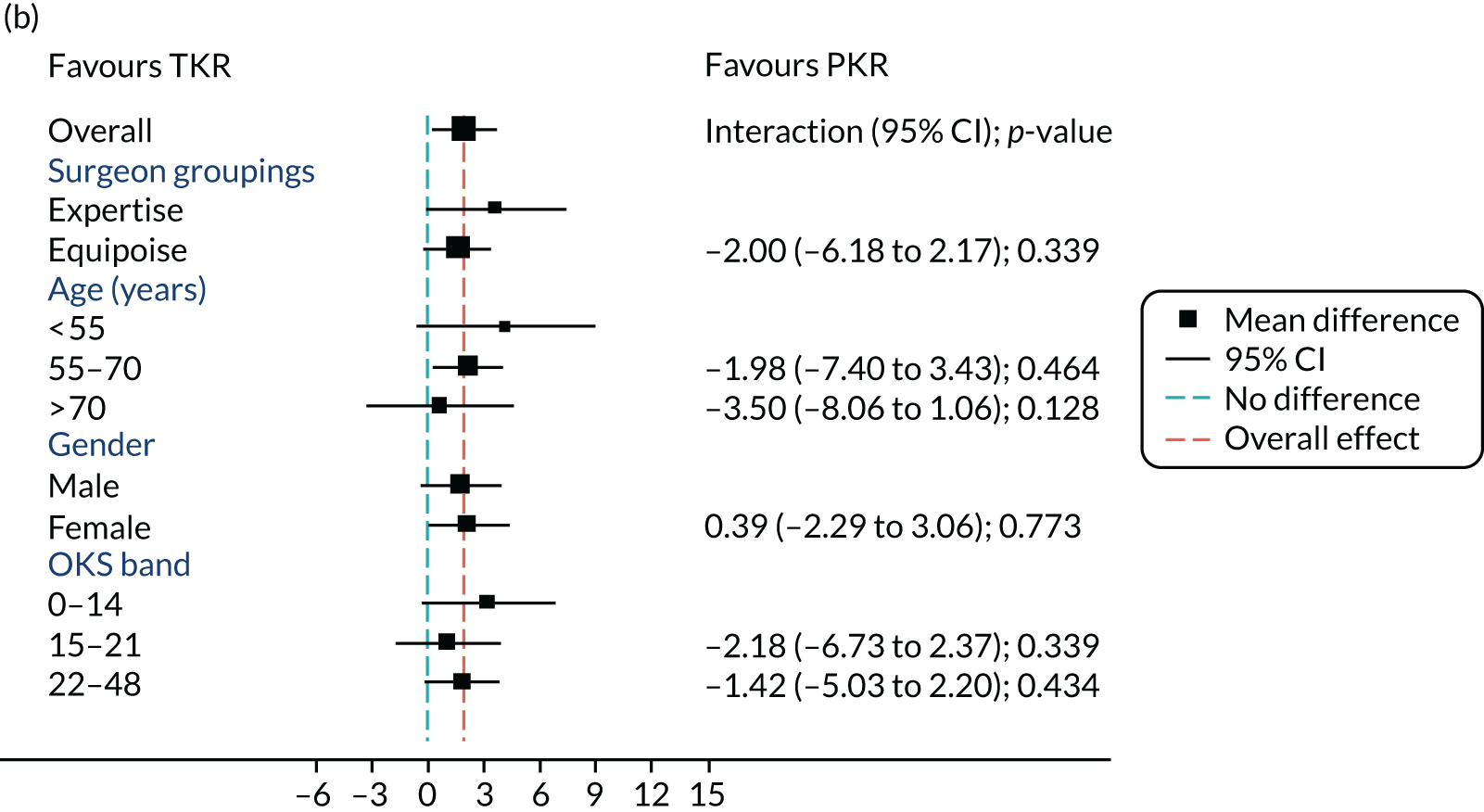
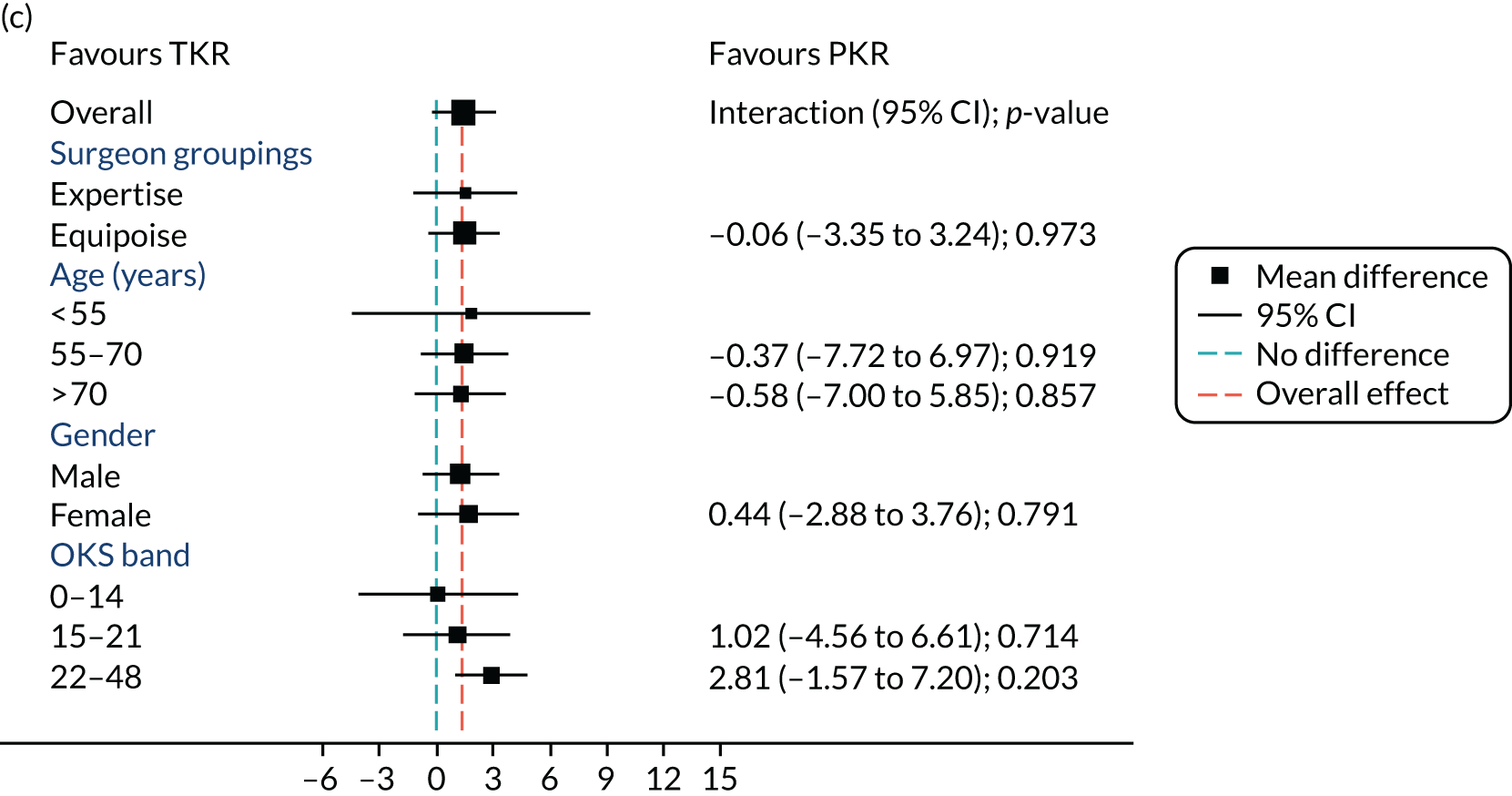
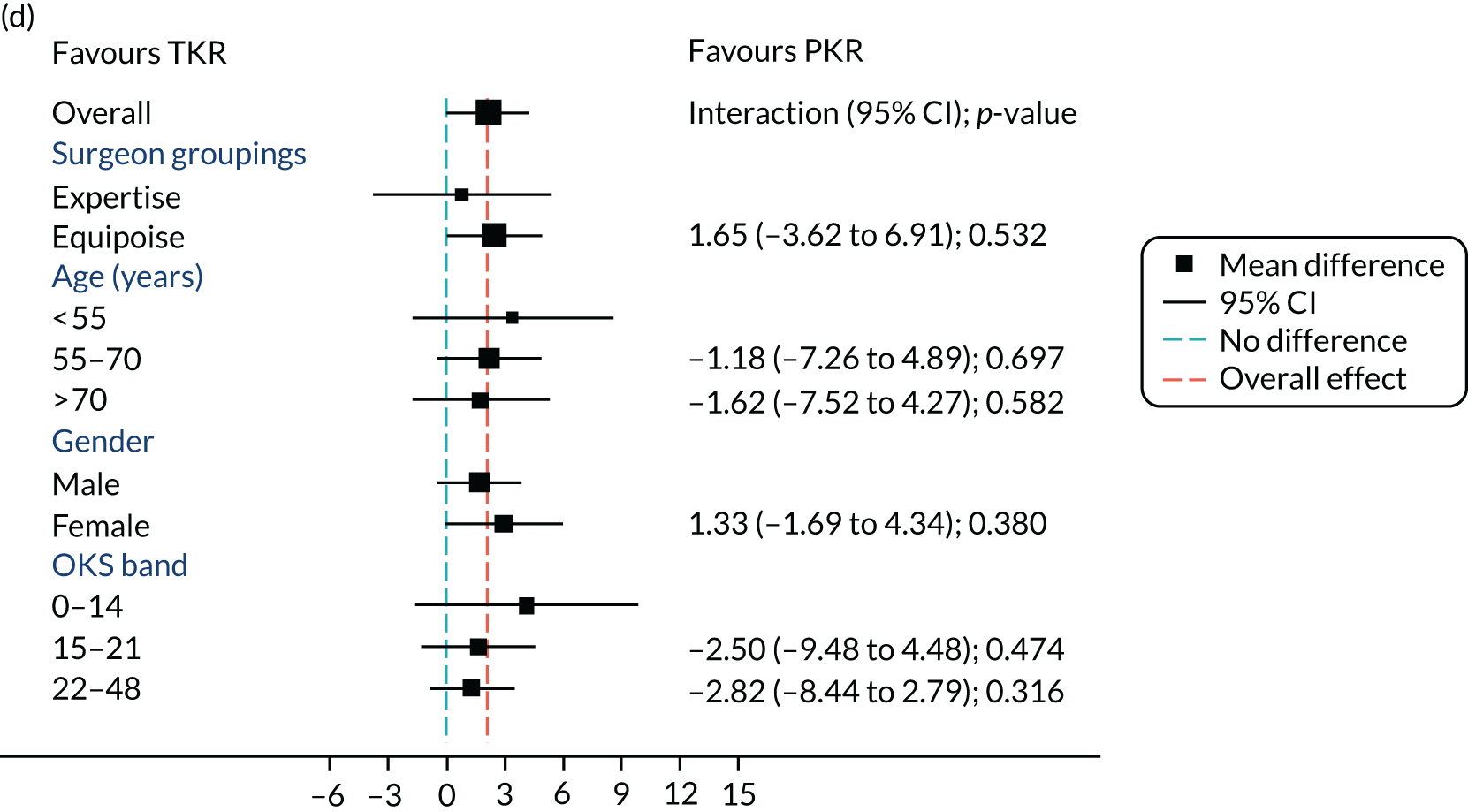
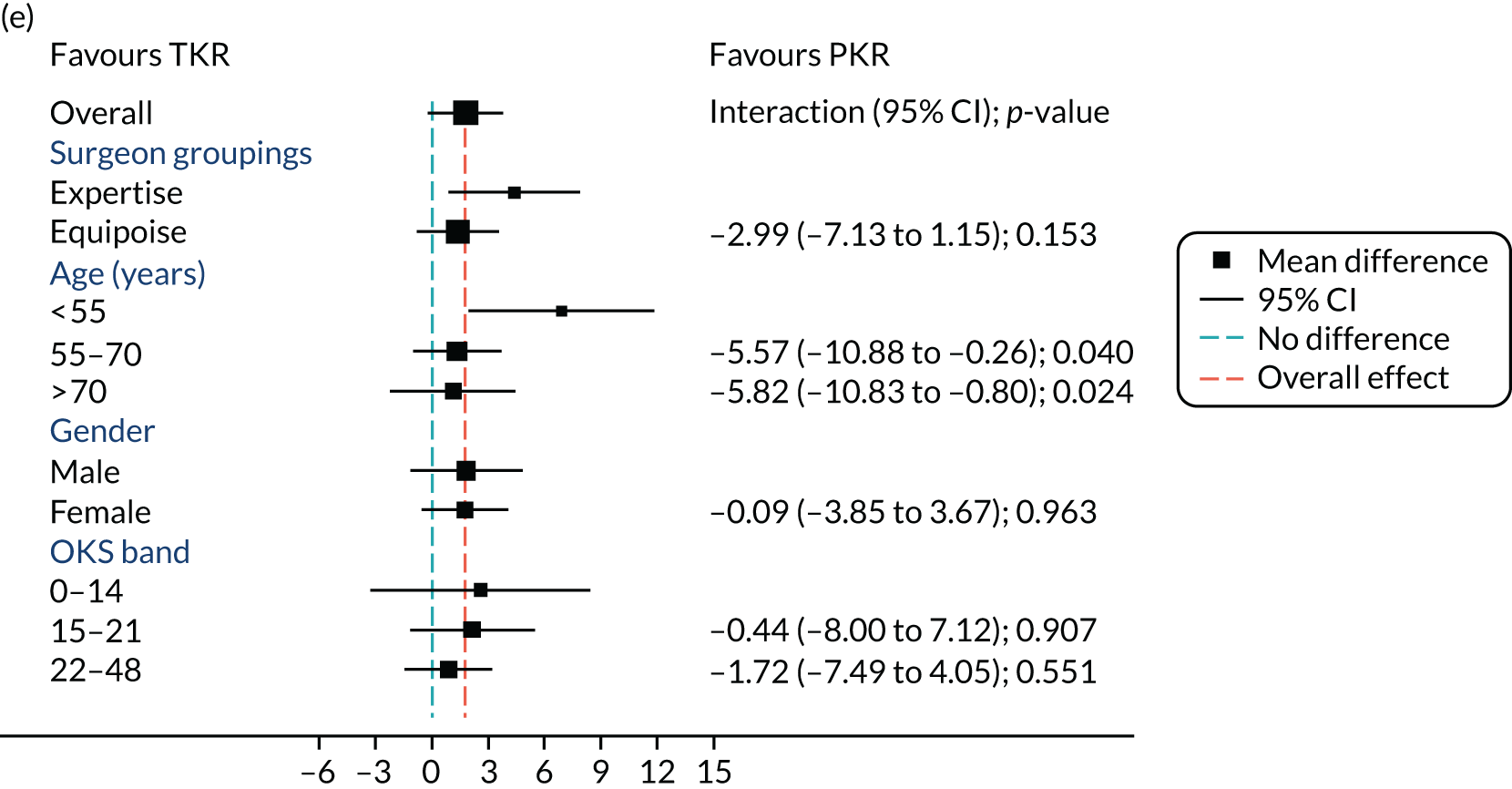
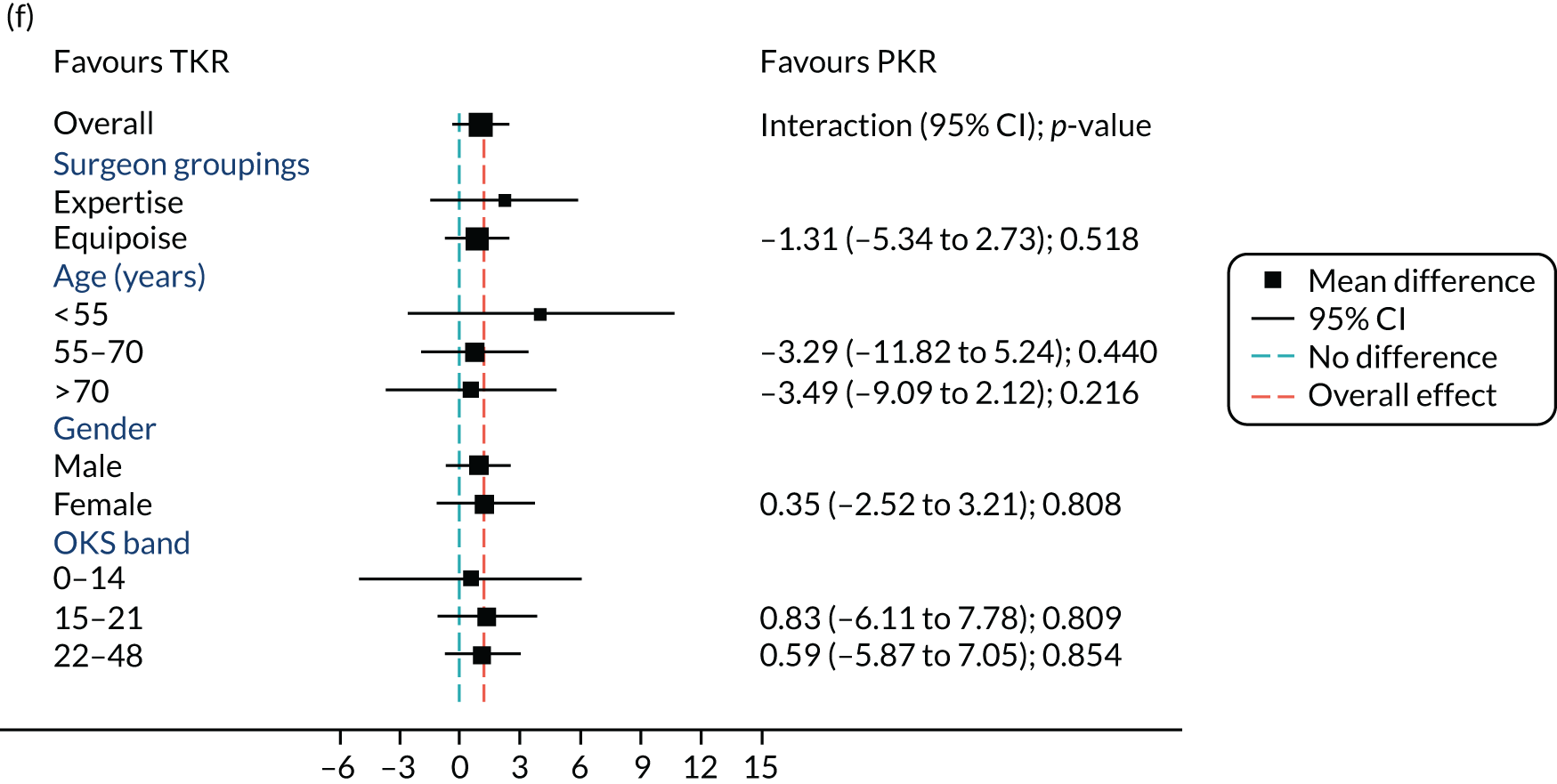
Figure 5 shows the differences between ‘expertise pair’ and ‘equipoise surgeons’ at the different follow-up time points. At the 5-year analysis, there was no evidence of a difference between the surgeon groupings (interaction effect –1.31, 95% CI –5.34 to 2.73; p = 0.518).
FIGURE 5.
Summary plots to compare expertise with equipoise randomisation (surgeon groups) and other subgroup analysis (age, sex and baseline OKS) in primary outcome OKS for PKR versus TKR (a) 2 months post surgery; (b) 1 year post randomisation; (c) 2 years post randomisation; (d) 3 years post randomisation; (e) 4 years post randomisation; and (f) 5 years post randomisation.
Subgroup analysis
Age, baseline OKS and sex were all considered a priori to have some potential effect on the final outcome. Figure 5 also shows the prespecified subgroup analyses for OKS, age (< 55, 55–70 or > 70 years), baseline OKS (0–14, 15–21 or 22–48) and sex (male or female). There was no clear evidence that sex, baseline OKS or age modified the treatment effect. The only apparent moderating effect of age at 4-year follow-up is most likely a statistical artefact, given the number of tests and the pattern of age by treatment interactions at other time points.
Compliance with treatment allocation
Not all patients allocated to the specific treatment type underwent their allocated treatment. Among the 262 participants who had been allocated to the PKR group and underwent surgery, 232 received a PKR and 31 received a TKR. In the TKR group, 251 participants were allocated to TKR and underwent surgery and, of these, 238 received a TKR and 13 received a PKR. Treatment effects from a CACE analysis for OKS at 1 year showed evidence of a difference in favour of PKR (MD 1.97, 95% CI 0.11 to 3.83; p = 0.038). At 5 years there was no evidence of a difference between groups (MD 0.86, 95% CI –0.62 to 2.34; p = 0.254).
Secondary outcomes
All of the secondary outcomes are shown in Table 9.
| Measure | PKR (N = 264) | TKR (N = 264) | Estimatea | 95% CI |
|---|---|---|---|---|
| AKSS Knee Score, mean (SD); n | ||||
| Baseline | 41.0 (16.1); 260 | 42.3 (16.0); 259 | ||
| 2 months | 78.2 (16.8); 249 | 74.4 (18.1); 237 | 3.66 | 0.44 to 6.87 |
| 1 year | 86.0 (16.1); 228 | 83.0 (16.5); 229 | 3.07 | 0.57 to 5.56 |
| 5 years | 85.8 (16.6); 191 | 86.6 (16.4); 185 | –0.89 | –5.18 to 3.41 |
| AKSS Function Score, mean (SD); n | ||||
| Baseline | 59.3 (15.6); 262 | 58.7 (15.5); 259 | ||
| 2 months | 73.7 (18.0); 251 | 69.9 (17.8); 241 | 3.47 | 1.02 to 5.93 |
| 1 year | 84.2 (18.0); 232 | 82.1 (18.2); 237 | 1.79 | –0.85 to 4.43 |
| 5 years | 82.6 (18.5); 195 | 81.7 (19.0); 192 | 0.37 | –3.81 to 4.55 |
| UCLA, mean (SD); n | ||||
| Baseline | 3.6 (1.5); 260 | 3.7 (1.5); 260 | ||
| 2 months | 4.3 (1.6); 239 | 4.0 (1.3); 235 | 0.29 | 0.07 to 0.51 |
| 1 year | 5.1 (1.8); 238 | 4.8 (1.8); 232 | 0.26 | –0.09 to 0.61 |
| 2 years | 5.1 (1.9); 235 | 4.8 (1.8); 232 | 0.23 | –0.03 to 0.50 |
| 3 years | 5.0 (2.0); 215 | 4.8 (1.9); 221 | 0.19 | –0.12 to 0.50 |
| 4 years | 5.2 (1.8); 204 | 5.0 (1.9); 211 | 0.21 | –0.13 to 0.56 |
| 5 years | 5.0 (1.9); 221 | 4.9 (2.0); 215 | 0.17 | –0.09 to 0.43 |
| HAAS, mean (SD); n | ||||
| Baseline | 4.8 (2.3); 258 | 4.6 (2.3); 256 | ||
| 2 months | 6.4 (2.9); 233 | 5.7 (2.4); 223 | 0.60 | 0.10 to 1.10 |
| 1 year | 8.0 (3.2); 228 | 7.6 (3.1); 228 | 0.35 | –0.15 to 0.84 |
| 2 years | 8.3 (3.3); 227 | 7.5 (3.4); 226 | 0.71 | 0.29 to 1.14 |
| 3 years | 8.1 (3.4); 209 | 7.8 (3.5); 216 | 0.20 | –0.40 to 0.79 |
| 4 years | 7.9 (3.4); 199 | 7.8 (3.5); 203 | 0.09 | –0.52 to 0.70 |
| 5 years | 7.9 (3.5); 218 | 7.6 (3.4); 207 | 0.22 | –0.24 to 0.67 |
| EQ-5D-3L, mean (SD); n | ||||
| Baseline | 0.428 (0.301); 257 | 0.381 (0.324); 252 | ||
| 2 months | 0.689 (0.243); 238 | 0.664 (0.226); 235 | 0.022 | –0.019 to 0.063 |
| 1 year | 0.766 (0.241); 240 | 0.723 (0.260); 232 | 0.038 | –0.003 to 0.079 |
| 2 years | 0.775 (0.261); 234 | 0.719 (0.282); 238 | 0.051 | –0.007 to 0.110 |
| 3 years | 0.771 (0.249); 214 | 0.729 (0.289); 220 | 0.034 | –0.026 to 0.094 |
| 4 years | 0.770 (0.251); 205 | 0.719 (0.301); 214 | 0.043 | 0.003 to 0.084 |
| 5 years | 0.744 (0.293); 224 | 0.717 (0.318); 212 | 0.018 | –0.033 to 0.069 |
| EQ-5D VAS, mean (SD); n | ||||
| Baseline | 62.8 (27.0); 249 | 60.7 (28.7); 257 | ||
| 2 months | 75.2 (16.1); 241 | 74.1 (16.2); 236 | 0.91 | –3.16 to 4.98 |
| 1 year | 76.4 (17.0); 239 | 75.5 (16.0); 236 | 0.69 | –1.71 to 3.09 |
| 2 years | 76.4 (16.4); 233 | 74.3 (16.6); 233 | 1.81 | –0.91 to 4.53 |
| 3 years | 76.1 (16.1); 217 | 73.2 (16.9); 222 | 2.37 | –0.55 to 5.29 |
| 4 years | 75.1 (16.3); 208 | 72.6 (18.0); 216 | 2.12 | –0.11 to 4.35 |
| 5 years | 75.4 (16.5); 228 | 71.1 (19.7); 217 | 4.02 | 1.36 to 6.67 |
| Composite outcome: failure, n (%) | ||||
| 1 year | 26 (9.9) | 37 (14.0) | 0.70 | 0.45 to 1.08 |
| 5 years | 28 (10.6) | 38 (14.4) | 0.74 | 0.51 to 1.08 |
| OKS-APQ | ||||
| 4 years | 58.5 (31.7); 205 | 53.2 (33.2); 211 | 5.52 | –1.34 to 12.38 |
| 5 years | 56.7 (31.8); 221 | 55.5 (33.5); 216 | 1.00 | –3.50 to 5.50 |
| Overall health now | ||||
| Baseline | 2.6 (0.9); 259 | 2.8 (0.9); 260 | ||
| 2 months | 2.7 (0.8); 243 | 2.7 (0.8); 235 | –0.01 | –0.15 to 0.13 |
| 1 year | 2.8 (0.9); 244 | 2.7 (0.9); 238 | 0.12 | 0.02 to 0.22 |
| 2 years | 2.8 (0.9); 237 | 2.8 (1.0); 235 | 0.06 | –0.11 to 0.24 |
| 3 years | 2.7 (0.9); 217 | 2.8 (1.0); 226 | 0.02 | –0.16 to 0.19 |
| 4 years | 2.8 (0.9); 208 | 2.9 (1.0); 217 | 0.03 | –0.11 to 0.16 |
| 5 years | 2.9 (0.9); 226 | 2.9 (1.0); 220 | 0.01 | –0.21 to 0.24 |
| Overall health now compared with 1 year ago | ||||
| Baseline | 3.3 (0.8); 259 | 3.3 (0.8); 260 | ||
| 2 months | 2.4 (0.9); 243 | 2.6 (0.9); 235 | –0.13 | –0.32 to 0.06 |
| 1 year | 2.2 (1.1); 244 | 2.3 (1.0); 238 | –0.13 | –0.33 to 0.07 |
| 2 years | 2.6 (1.0); 237 | 2.6 (0.9); 234 | –0.04 | –0.19 to 0.10 |
| 3 years | 2.8 (0.8); 217 | 3.0 (0.8); 225 | –0.16 | –0.29 to -0.0 |
| 4 years | 2.9 (0.8); 209 | 2.9 (0.8); 216 | –0.00 | –0.12 to 0.12 |
| 5 years | 3.0 (0.8); 226 | 3.0 (0.7); 219 | –0.02 | –0.19 to 0.16 |
| Satisfied with knee, n/N (%) | ||||
| 2 months | 184/245 (75.1) | 169/236 (71.6) | 1.05 | 0.93 to 1.18 |
| 1 year | 195/243 (80.2) | 173/237 (73.0) | 1.10 | 1.00 to 1.22 |
| 2 years | 197/236 (83.5) | 173/229 (75.5) | 1.11 | 1.02 to 1.20 |
| 3 years | 182/217 (83.9) | 172/222 (77.5) | 1.08 | 0.99 to 1.18 |
| 4 years | 169/204 (82.8) | 164/213 (77.0) | 1.08 | 0.99 to 1.17 |
| 5 years | 190/233 (81.5) | 173/225 (76.9) | 1.06 | 0.99 to 1.13 |
| Received surgery | n = 262 | n = 250 | ||
| Problems with knee better now compared with before surgery, n/N (%) | ||||
| 2 months | 217/245 (88.6) | 202/235 (86.0) | 1.03 | 0.95 to 1.12 |
| 1 year | 225/244 (92.2) | 207/236 (87.7) | 1.05 | 1.01 to 1.10 |
| 2 years | 224/236 (94.9) | 200/226 (88.5) | 1.07 | 1.03 to 1.12 |
| 3 years | 206/218 (94.5) | 192/222 (86.5) | 1.09 | 1.03 to 1.16 |
| 4 years | 195/207 (94.2) | 191/210 (91.0) | 1.04 | 0.99 to 1.09 |
| 5 years | 219/230 (95.2) | 200/222 (90.1) | 1.06 | 1.01 to 1.11 |
| Still would choose to have a knee operation, n/N (%) | ||||
| 2 months | 206/245 (84.1) | 185/236 (78.4) | 1.07 | 0.97 to 1.19 |
| 1 year | 213/240 (88.8) | 177/230 (77.0) | 1.16 | 1.06 to 1.26 |
| 2 years | 207/236 (87.7) | 181/225 (80.4) | 1.09 | 1.04 to 1.16 |
| 3 years | 195/213 (91.5) | 177/219 (80.8) | 1.13 | 1.05 to 1.22 |
| 4 years | 190/206 (92.2) | 177/210 (84.3) | 1.09 | 1.02 to 1.18 |
| 5 years | 208/228 (91.2) | 183/217 (84.3) | 1.08 | 1.02 to 1.15 |
| Length of hospital stay (days) | ||||
| Mean (SD); n | 3.3 (1.5); 263 | 4.3 (3.7); 249 | ||
| Median (IQR) | 3.0 (2.0–4.0) | 4.0 (3.0–5.0) | 0.77 | 0.66 to 0.90 |
American Knee Society Score
There was no difference in the baseline mean clinical AKSS (PKR 41.0 vs. TKR 42.3) or functional AKSS (PKR 59.3 vs. TKR 58.7). At 1 year, there was evidence of a difference in favour of PKR for clinical AKSS (1 year: 3.07, 95% CI 0.57 to 5.56; p = 0.027). There was no difference at 5 years (MD –0.89, 95% CI –5.18 to 3.41; p = 0.678). For functional AKSS, there was no evidence of a difference at 1 year (MD 1.79, 95% CI –0.85 to 4.43; p = 0.179) or at 5 years (MD 0.37, 95% CI –3.81 to 4.55; p = 0.859).
University of California, Los Angeles, score
The baseline mean UCLA score for PKR and TKR was 3.6 and 3.7, respectively. At the later follow-up time points this increased but there was no evidence of a difference between groups at 1 year (MD 0.26, 95% CI –0.09 to 0.61; p = 0.140) or at 5 years (MD 0.17, 95% CI –0.009 to 0.43; p = 0.188).
High Activity Arthroplasty Score
The baseline mean HAAS for PKR and TKR was 4.8 and 4.6, respectively. At 1 year the HAAS MD was 0.35 (95% CI –0.15 to 0.84; p = 0.163) and at 5 years the MD was 0.22 (95% CI –0.24 to 0.67; p = 0.334). The scores at both time points show that there is no evidence of a difference between groups.
EuroQol-5 Dimensions, three-level version
The mean baseline score for EQ-5D-3L was 0.428 in the PKR group and 0.381 in the TKR group. At both 1 year (MD 0.038, 95% CI –0.003 to 0.079; p = 0.066) and 5 years (MD 0.018, 95% CI –0.033 to 0.069; p = 0.478) there was no evidence of a difference between PKR and TKR. Sensitivity analyses assuming worst case findings did not alter the results.
EuroQol-5 Dimensions Visual Analogue Scale
The mean baseline score for EQ-5D VAS was 62.8 in the PKR group and 60.7 in the TKR group. At 1 year the MD was 0.69 (95% CI –1.71 to 3.09; p = 0.56), but at the 5-year follow-up there was evidence of a difference in favour of PKR (MD 4.02, 95% CI 1.36 to 6.67; p = 0.004).
Failure
Failure is a composite outcome and reflects both an incidence of reoperation or revision surgery and a low level (or no) post-operation improvement, as defined by OKS (< 4-point improvement from baseline). At 1 year there were 26 (9.9%) failures in the PKR group and 37 (14.0%) in the TKR group, but there was no evidence of a difference [rate ratio (RR) 0.70, 95% CI 0.45 to 1.08; p = 0.104]; the results were similar at 5 years (RR 0.74, 95% CI 0.51 to 1.08; p = 0.118).
Oxford Knee Score-Activity Participation Questionnaire
At 5 years, the mean OKS-APQ score was 56.7 in the PKR group and 55.5 in the TKR group, with no evidence of a difference (MD 1.00, 95% CI –3.50 to 5.50; p = 0.656).
Overall health now
The mean baseline overall health now was 2.6 in the PKR group and 2.8 in the TKR group. These figures slightly increased and there was evidence of a difference at only 1 year in favour of PKR (MD 0.12, 95% CI 0.02 to 0.22; p = 0.023). At 5 years, the MD was 0.01 (95% CI –0.21 to 0.24; p = 0.895).
Overall health now compared with 1 year ago
The mean baseline score was the same in both groups (3.3 points). These figures decreased at the follow-up time points and there was no evidence of a difference at any time point. At 1 year the MD was –0.13 (95% CI –0.33 to 0.07; p = 0.190) and at 5 years the MD was –0.02 (95% CI –0.19 to 0.16; p = 0.833).
Self-reported anchor questions
The Lund Score73 was used to assess patient satisfaction. For ‘How satisfied are you with your knee?’, there was no evidence of a difference at 1 year (RR 1.10, 95% CI 1.00 to 1.22; p = 0.059) or at 5 years (RR 1.06, 95% CI 0.99 to 1.13; p = 0.097).
There were differences in how the two groups answered the question ‘How are the problems related to your knee now, compared with before your knee surgery?’. Among participants who received surgery, 92.2% in the PKR group and 87.7% in the TKR group reported that the problems related to their knee were better now than before surgery at 1 year, with evidence of a difference in favour of PKR (RR 1.05, 95% CI 1.01 to 1.10; p = 0.021). At 5 years the results were similar (RR 1.06, 95% CI 1.01 to 1.11; p = 0.016).
Similarly, differences existed for the question ‘If you could go back in time, would you still choose to have the knee operation?’. At 1 year, 88.8% of participants who had a PKR would choose to have the operation again, whereas only 77.0% of participants who had a TKR would have the operation again. Therefore, at 1 year there is evidence of a difference in favour of PKR and there was a similar result at 5 years (RR 1.08, 95% CI 1.02 to 1.15; p = 0.010).
Although these patient-reported outcome findings are important, they should not be overemphasised as they could be chance findings and are susceptible to bias from lack of blinding.
Length of hospital stay
The mean number of days spent in hospital by treatment allocation was 3.3 days for the PKR group and 4.3 days for the TKR group, with evidence of a difference in favour of PKR [incidence rate ratio (IRR) 0.77, 95% CI 0.66 to 0.90; p = 0.001]. For treatment received, the results were similar (IRR 0.74, 95% CI 0.63 to 0.87; p = < 0.001).
Complications, reoperation and revision
Complications, reoperations and revisions are reported both per allocation and per treatment received (for completeness). Per treatment received analyses are considered more meaningful from a surgical perspective for these particular data. For the low frequency (but critical) complication and revision data, it would be highly misleading to state that a TKR had failed or has been revised when the primary implant was actually a PKR, and vice versa.
Complications
By allocation
During the 5-year follow-up, 19% (50/263) of the PKR group and 28.3% (71/251) of the TKR group had a complication (Table 10; RR 0.67, 95% CI 0.51 to 0.88; p = 0.004). Four participants (and complications) (1.5%) in the PKR group and eight participants (3.2%) in the TKR group were re-admissions requiring medical treatment only. There were 14 participants in the PKR group (5.3%) who required re-admission and further surgery (21 separate events). There were 22 participants in the TKR group (8.7%) who required re-admission and further surgery (32 separate events). The main reasons for the complication were unexplained pain (PKR, n = 6; TKR, n = 11), knee stiffness (PKR, n = 1; TKR, n = 9) and unexplained pain as well as knee stiffness (PKR, n = 1; TKR, n = 6).
| Measure | PKR (N = 263),a number of events (%) | TKR (N = 251),a number of events (%) |
|---|---|---|
| Number of participants with a complication (RR 0.67, 95% CI 0.51 to 0.88) | 50 (19.0) | 71 (28.3) |
| Total number of complications | 79 | 111 |
| Details of complications, related to primary operation | ||
| Intra-operative | ||
| Number of participants (and complications) | – | 3 (1.2) |
| Blood transfusion | – | 2 |
| Medical reasons | – | 1 |
| Postoperative | ||
| Number of participants (and complications) | 10 (3.8) | 19 (7.6) |
| Blood transfusions | – | 6 |
| Respiratory problems | 2 | 4 |
| Renal and urological problems | 3 | 2 |
| Miscellaneous | 2 | 3 |
| Treated DVT or PE | 2 | 1 |
| Cardiac problems | – | 2 |
| Treated DVT or PE/cardiac problems | – | 1 |
| Anaesthetic problems | 1 | – |
| Required re-admission | ||
| Required medical treatment only | ||
| Number of participants (and complications) | 4 (1.5) | 8 (3.2) |
| Unexplained pain | 1 | 3 |
| Bearing dislocation | 1 | – |
| Wound breakdown | – | 2 |
| Bronchopneumonia | – | 1 |
| Cardiac problems | – | 1 |
| Cellulitis | 1 | – |
| Treated DVT or PE | – | 1 |
| Superficial infection | 1 | – |
| Required surgery | ||
| Number of participants | 14 (5.3) | 22 (8.7) |
| Number of complications | 21 | 32 |
| Unexplained pain | 6 | 11 |
| Knee stiffness | 1 | 9 |
| Bearing dislocation | 4 | – |
| Device loosening (tibia) | 1 | 1 |
| Infection | 1 | 1 |
| Ligamentous instability | 1 | 1 |
| Pain from trauma | – | 1 |
| Periprosthetic fracture | 1 | – |
| Unexplained pain and knee stiffness | 1 | 6 |
| Mechanical failure and infection | – | 1 |
| Unexplained pain and swelling | 1 | – |
| Unexplained pain and skin complication | 1 | – |
| Unexplained pain and bearing dislocation | 1 | – |
| Device loosening (tibia) and renal/urological problems | 1 | – |
| Bearing dislocation and renal/urological problems | 1 | – |
| Unknown | – | 1 |
| Re-admission further surgery intra-operative | ||
| Number of participants (and complications) | – | 1 (0.4) |
| Medical reasons | – | 1 |
| Re-admission further surgery postoperative | ||
| Number of participants | 3 (1.1) | – |
| Number of events | 4 | – |
| Blood transfusion | 1 | – |
| Renal and urological problems | 1 | – |
| Skin complications | 1 | – |
| Bearing dislocation, renal/urological problems and blood transfusion | 1 | – |
| Did not require re-admission | ||
| 2-month follow-up | ||
| Number of participants | 7 (2.7) | 13 (5.2) |
| Number of events | 9 | 14 |
| Wound infection | 3 | 6 |
| Unexplained pain | 2 | 1 |
| Wound breakdown | 1 | 2 |
| Swelling | 1 | 2 |
| Miscellaneous | – | 2 |
| Knee stiffness | 1 | 1 |
| Skin complication | 1 | – |
| 1-year follow-up | ||
| Number of participants | 14 (5.3) | 17 (6.8) |
| Number of events | 16 | 20 |
| Unexplained pain | 9 | 11 |
| Knee stiffness | 1 | 5 |
| Swelling | 3 | 1 |
| Instability | 1 | 1 |
| Wound infection | 1 | – |
| Periprosthetic fracture | – | 1 |
| Miscellaneous | 1 | – |
| Skin complication | – | 1 |
| 5-year follow-up | ||
| Number of participants | 14 (5.3) | 14 (5.6) |
| Number of events | 15 | 14 |
| Unexplained pain | 12 | 8 |
| Knee stiffness | 1 | 1 |
| Medical reasons | – | 2 |
| Miscellaneous | 1 | 1 |
| Wound infection | – | 1 |
| Wound infection | – | 1 |
| Ligamentous instability | 1 | – |
By treatment received
Table 11 shows the complications by the treatment received. During the 5-year follow-up, 19.6% (48/245) of the PKR group and 27.1% (73/269) of the TKR group had a complication (RR 0.72, 95% CI 0.53 to 0.98; p = 0.036). The number of participants (and complications) who were re-admissions requiring medical treatment only was three (1.2%) in the PKR group and nine (3.3%) in the TKR group. There were 15 (6.1%) PKR participants who required re-admission and further surgery (25 separate events). There were 21 (7.8%) participants in the TKR group who required re-admission and further surgery (28 separate events). The main reasons for the complications were unexplained pain (PKR, n = 9; TKR, n = 8), knee stiffness (PKR, n = 0; TKR, n = 10) and unexplained pain as well as knee stiffness (PKR, n = 1; TKR, n = 6).
| Measure | PKR (N = 245),a number of events (%) | TKR (N = 269),a number of events (%) |
|---|---|---|
| Number of participants with a complication (RR 0.72, 95% CI 0.53 to 0.98) | 48 (19.6) | 73 (27.1) |
| Total number of complications | 76 | 114 |
| Details of complications, related to primary operation | ||
| Intra-operative | ||
| Number of participants (and complications) | 1 (0.4) | 2 (0.7) |
| Blood transfusion | – | 2 |
| Medical reasons | 1 | – |
| Postoperative | ||
| Number of participants (and complications) | 10 (4.1) | 19 (7.1) |
| Blood transfusion | – | 6 |
| Respiratory problems | 3 | 3 |
| Renal and urological problems | 2 | 3 |
| Miscellaneous | 2 | 3 |
| Treated DVT or PE | 2 | 1 |
| Cardiac problems | – | 2 |
| Treated DVT or PE/cardiac problems | – | 1 |
| Anaesthetic problems | 1 | – |
| Required re-admission | ||
| Required medical treatment only | ||
| Number of participants (and complications) | 3 (1.2) | 9 (3.3) |
| Unexplained pain | 1 | 3 |
| Bearing dislocation | 1 | – |
| Wound breakdown | – | 2 |
| Bronchopneumonia | – | 1 |
| Cardiac problems | – | 1 |
| Cellulitis | – | 1 |
| Treated DVT or PE | – | 1 |
| Superficial infection | 1 | – |
| Required surgery | ||
| Number of participants | 15 (6.1) | 21 (7.8) |
| Number of complications | 25 | 28 |
| Unexplained pain | 9 | 8 |
| Knee stiffness | – | 10 |
| Bearing dislocation | 4 | – |
| Device loosening (tibia) | 2 | – |
| Infection | 1 | 1 |
| Ligamentous instability | 1 | 1 |
| Pain from trauma | 1 | – |
| Periprosthetic fracture | 1 | – |
| Unexplained pain and knee stiffness | 1 | 6 |
| Mechanical failure and infection | – | 1 |
| Unexplained pain and swelling | 1 | – |
| Unexplained pain and skin complication | 1 | – |
| Unexplained pain and bearing dislocation | 1 | – |
| Device loosening (tibia) and renal/urological problems | 1 | – |
| Bearing dislocation and renal/urological problems | 1 | – |
| Unknown | – | 1 |
| Re-admission further surgery intra-operative | ||
| Number of participants (and complications) | – | 1 |
| Medical reasons | – | 1 |
| Re-admission further surgery postoperative | ||
| Number of participants | 3 (1.2) | – |
| Number of events | 4 | – |
| Blood transfusion | 1 | – |
| Renal and urological problems | 1 | – |
| Skin complications | 1 | – |
| Bearing dislocation, renal/urological problems and blood transfusion | 1 | – |
| Did not require re-admission | ||
| 2-month follow-up | ||
| Number of participants | 5 (2.0) | 15 (5.6) |
| Number of events | 5 | 18 |
| Wound infection | 3 | 6 |
| Unexplained pain | 1 | 2 |
| Wound breakdown | – | 3 |
| Swelling | – | 3 |
| Miscellaneous | – | 2 |
| Knee stiffness | – | 2 |
| Skin complication | 1 | – |
| 1-year follow-up | ||
| Number of participants | 13 (5.3) | 18 (6.7) |
| Number of events | 15 | 21 |
| Unexplained pain | 8 | 12 |
| Knee stiffness | 1 | 5 |
| Swelling | 3 | 1 |
| Instability | 1 | 1 |
| Wound infection | 1 | – |
| Periprosthetic fracture | – | 1 |
| Miscellaneous | 1 | – |
| Skin complication | – | 1 |
| 5-year follow-up | ||
| Number of participants | 12 (4.9) | 16 (5.9) |
| Number of events | 13 | 16 |
| Unexplained pain | 10 | 10 |
| Knee stiffness | 1 | 1 |
| Medical reasons | – | 2 |
| Miscellaneous | 1 | 1 |
| Wound infection | – | 1 |
| Wound infection | – | 1 |
| Ligamentous instability | 1 | – |
Suspected complications (i.e. adverse medical events) that were thought to be related to the study but were found to be false positives were also documented. These are listed in Appendix 3, Table 28.
Reoperations including revision
By allocation
Among those who had surgery, 5.3% (14/263) had a reoperation in the PKR group and 8.8% (22/251) had a reoperation in the TKR group (RR 0.60, 95% CI 0.30 to 1.19; p = 0.143) (Table 12). One additional participant had a reoperation, but this event was a result of trauma and, therefore, was not related to the trial, so is not included in the table. The number of revisions was eight (3.0%) in the PKR group and 12 (4.8%) in the TKR group, with the reason mainly being unexplained pain (PKR, n = 1; TKR, n = 6) or bearing dislocation (PKR, n = 3; TKR, n = 0).
| Measure | PKR (N = 263),a number of events (%) | TKR (N = 251),a number of events (%) |
|---|---|---|
| Number of participants having a reoperation (RR 0.60, 95% CI 0.30 to 1.19) | 14 (5.3) | 22 (8.8) |
| Total number of reoperations | 18 | 32 |
| Number of participants who had a revision, n (%) | 8 (3.0) | 12 (4.8) |
| Reasons for revisions | ||
| Unexplained pain | 1 | 6 |
| Bearing dislocation | 3 | – |
| Device loosening (tibia) | 1 | 1 |
| Bearing dislocation and unexplained pain | 1 | – |
| Ligamentous instability | 1 | – |
| Infection | 1 | – |
| Infection and mechanical failure | – | 1 |
| Unknown | – | 2 |
| Knee stiffness and unexplained pain | – | 1 |
| Ligamentous instability and malalignment | – | 1 |
| Number of other procedures | 10 | 20 |
| Details of other procedures | ||
| MUA | 1 | 11 |
| Aspiration | 3 | 2 |
| Arthroscopy | 3 | – |
| Arthroscopy and debridement/exploration/washout | 1 | 1 |
| Debridement/exploration/washout | – | 2 |
| Open reduction and internal fixation of avulsion fracture (tibial tuberosity) | 1 | – |
| MUA and marcaine injection | 1 | – |
| Marcaine injection, MUA and ROM achieved, without manipulation | – | 1 |
| Arthroscopy and MUA | – | 1 |
| Arthroscopy and biopsy | – | 1 |
| Arthroscopy and partial medial meniscectomy | – | 1 |
By treatment received
For those that had surgery, 6.1% (15/245) of participants who underwent PKR had a reoperation and 7.8% (21/269) of participants who underwent TKR had a reoperation (RR 0.75, 95% CI 0.37, 1.53; p = 0.432) (see Table 13). The number of revision operations after PKR was 10 (4.1%) and after TKR was eight (3.0%), with the reason for revision mainly being a result of unexplained pain (PKR, n = 2; TKR, n = 5) or bearing dislocation (PKR, n = 3; TKR, n = 0). Table 13 shows the reoperations by treatment received.
| Measure | PKR (N = 245),a number of events (%) | TKR (N = 269),a number of events (%) |
|---|---|---|
| Number of participants having a reoperation (RR 0.75, 95% CI 0.37 to 1.53) | 15 (6.1) | 21 (7.8) |
| Total number of reoperations | 22 | 28 |
| Number of participants who had a revision, n (%) | 10 (4.1) | 10 (4.0) |
| Reasons for revisions | ||
| Unexplained pain | 2 | 5 |
| Bearing dislocation | 3 | – |
| Device loosening (tibia) | 2 | – |
| Bearing dislocation and unexplained pain | 1 | – |
| Ligamentous instability | 1 | – |
| Infection | 1 | – |
| Infection and mechanical failure | – | 1 |
| Unknown | – | 2 |
| Knee stiffness and unexplained pain | – | 1 |
| Ligamentous instability and malalignment | – | 1 |
| Number of other procedures | 12 | 18 |
| Details of other procedures | ||
| MUA | – | 12 |
| Aspiration | 3 | 2 |
| Arthroscopy | 3 | – |
| Arthroscopy and debridement/exploration/washout | 1 | 1 |
| Debridement/exploration/washout | 2 | – |
| Open reduction and internal fixation of avulsion fracture (tibial tuberosity) | 1 | – |
| Marcaine injection, MUA and ROM achieved without manipulation | 1 | – |
| MUA and marcaine injection | 1 | – |
| Arthroscopy and MUA | – | 1 |
| Arthroscopy and biopsy | – | 1 |
| Arthroscopy and partial medial meniscectomy | – | 1 |
Mortality
During the 5-year follow-up there were a total of 17 deaths [PKR, n = 6 (2.3%); TKR, n = 11 (4.2%)] (Table 14). The main cause of death was cancer (PKR, n = 4; TKR, n = 6) and there were two deaths in each group the cause of which was unknown.
| Measure | PKR (N = 264) | TKR (N = 264) |
|---|---|---|
| Number of deaths, n (%) | 6 (2.3) | 11 (4.2) |
| Reason for death, n | ||
| Cancer | 4 | 6 |
| Suicide | – | 1 |
| Sepsis | – | 1 |
| Multi-organ failure/sepsis | – | 1 |
| Unknown | 2 | 2 |
Radiographic imaging of the knee
Although at the start of the trial we had anticipated that routine 5-year follow-up radiological data might provide insight into the failure mechanism, the differences in clinical practice across sites resulted in these data being insufficiently complete and reliable. The low rate of implant failure observed in both groups also contributes to the limitation of interpreting these data. This issue is also discussed in Chapter 6, Limitations.
Summary
To our knowledge, TOPKAT is by far the largest RCT comparing TKR with PKR. In addition, we had very good retention up to 5 years post randomisation. A hybrid design was used to allow surgeons to deliver either both procedures or only the surgery of their stated preference and expertise. Minimum criteria were applied to participating surgeons for both procedures to ensure generalisability, and the data collected suggest that they are an experienced group of surgeons (although more experienced in TKR than PKR given that the latter is a less common operation).
We found no difference in the primary outcome of OKS at 5 years, despite finding a statistically significant, albeit small, difference at 1 year. Secondary and sensitivity analyses provided similar findings, but one post hoc AUC analysis was statistically significant for 5 years. All analyses are consistent with a small difference in OKS (around 1 point) in favour of PKR. This difference is of uncertain clinical value. Other pain and function knee outcomes and quality-of-life outcomes have a similar pattern of some small statistically significant differences up to 1 year, but by 5 years there is little evidence of a difference.
A more compelling pattern in favour of PKR was observed for a range of other outcomes, including EQ-5D VAS (but not EQ-5D-3L), patient satisfaction, willingness to choose the same operation again and perception of their knee problem. Hospital length of stay was also favourable for PKR.
The level of non-compliance is worthy of comment. Some non-compliance was anticipated and clinically understandable (conversion from PKR to TKR from operative findings), other occurrences stem from a variety of factors. We used a CACE analysis to assess potential impact of non-compliance on effect and it did not appear to indicate this was an issue. The pattern of observed complications and reoperation reflected this and for this reason we analysed it as both ITT and treatment received. Statistical findings were similar in terms of overall level of complications and reoperations, favouring PKR for both. This is contrary to some previous work,6 which has suggested that PKR has a higher level of both complications and reoperation. The finding that PKR had lower levels of complications and reoperations than TKR may reflect the more experienced group of surgeons in this trial and the more robust nature of this comparison in terms of controlling for selection bias than previous observational research studies.
Overall, the findings show some modest clinical benefit for PKR, although minimal in terms of knee pain and function. Other benefits appeared to be in terms of lower complication rate and reoperation and recovery. Notably, not one observed outcome provided evidence in favour of TKR over PKR. The cost-effectiveness of the two procedures is considered in Chapter 5.
Chapter 5 Cost-effectiveness analysis
Introduction
This chapter reports the methods and results of a within-trial cost-effectiveness analysis of TOPKAT. Between January 2010 and October 2013, TOPKAT randomly allocated 528 adults with OA of the medial compartment of the knee to receive either PKR (n = 264), in which only the diseased area of the knee is replaced, or TKR (n = 264), in which all surfaces of the knee are replaced. Participants have been followed up to 5 years following randomisation. Information on recruitment, including inclusion and exclusion criteria, is presented in more detail in Chapter 2. Participant characteristics at recruitment and clinical results are presented in Chapters 3 and 4.
In this chapter, we compared PKR with TKR in terms of QALYs gained and health-care costs and calculated incremental cost-effectiveness ratios (ICERs) that give the additional spending required to generate one additional QALY.
Methods
Health economic data collection
Information was collected for each participant in the trial on the resources consumed during initial surgery, including the type of implant, time in theatre, days in hospital and any intraoperative or postoperative complications. At baseline, data were reported during a clinic visit about participants’ use of health-care services related to the study knee [inpatient stays, outpatient appointments, consultations with a general practitioner (GP) or practice nurse and visits to a physiotherapist or occupational therapist] in the preceding year and their current health status including the EQ-5D-3L questionnaire. In the EQ-5D-3L questionnaire, participants are asked to report whether they have no problems, some problems or extreme problems in the following domains: mobility, self-care, usual activities, pain and discomfort and anxiety/depression.
At 2 months, 1 year and annually thereafter to 5 years, patients were asked via postal questionnaire to report their health-care use related to the study knee since the last follow-up and their current health status. Hospital admissions potentially related to the study knee were identified from data reported in the postal questionnaires; clinic visits at 2 months, 1 year and 5 years post randomisation; and the assessment of hospital records for all participants by local research teams. Where potentially relevant admissions were identified, a data extraction sheet was completed providing details of the admission.
Health-care costs
Unit costs were derived from national databases77–79 or published studies,80 and are reported in Appendix 4. All unit costs were inflated, where necessary, to 2016–17 prices using the health care and community health services inflation index. 78
The initial admission was costed according to the type of implant used (if any), time in theatre, number of days in hospital, days in an intensive care unit or high-dependency unit (from here on, critical care) and any intra- or post-operative complications. We did not separately cost the anaesthetic used or the staff present during the operation, as this is all included within the cost per minute of theatre time. We did not include an additional cost for interoperative conversion from PKR to TKR, and the cost did not depend on whether or not cement was used.
Appendix 4, Table 29, presents the number of implants and the mean cost by implant type and treatment allocation. Fourteen participants received an implant type for which there were no costs (data on implant type was imputed using mean imputation by treatment allocation). Theatre time was reported in minutes and the costs were attached per minute of theatre time. Data were collected on the total length of hospital stay and the hours spent in critical care. Hours in critical care were converted to days and subtracted from total length of stay to estimate the days spent in other wards (see Appendix 4, Table 30, for unit cost data).
Intraoperative and postoperative complications that required blood transfusion were costed per unit of blood (see Appendix 4, Table 30). Other post-operative complications were costed using a Healthcare Resource Group (HRG) approach. This involved attaching the World Health Organization’s International Classification of Diseases, Tenth Revision (ICD-10), codes81 to each complication (see Appendix 4, Table 30), and using the Costing – HRG4+ 2016/17 Reference Costs Grouper82 from NHS Digital to attach a HRG. Mean costs for elective patients for each HRG were derived from the NHS Reference Costs Schedule 2016–1783 (see Appendix 4, Table 31) using a recommended approach. 84 We assumed that the absence of data on complications is indicative of the absence of complications.
Unit costs were attached to self-reported data on consultations with GPs or practice nurses, outpatient appointments and visits to a physiotherapist or occupational therapist (see Appendix 4, Table 30). ICD-10 diagnostic and Office of Population, Censuses and Surveys Classification of Surgical Operations and Procedures, Fourth Revision (OPCS-4), procedural codes85 were attached to each hospital admission (see Appendix 4, Tables 32 and 33) and costing followed a HRG approach, as described above for post operative complications (see Appendix 4, Table 34). Because of the multifaceted identification process (questionnaire, clinic visits and hospital records), data on inpatient care were considered complete.
Quality-adjusted life-years
Responses to EQ-5D-3L questionnaires were converted into utility scores using the standard UK tariff. 72 QALYs are calculated using the AUC approach, which involves estimating the average EQ-5D-3L utility between each follow-up time point, and weighting by survival time.
Methods for dealing with missing data
We followed best practice methods for addressing missing data in cost-effectiveness studies. 86 Missing data on characteristics of participants at baseline were imputed using mean imputation, and missing data on components of the index surgery (e.g. implant type) were imputed using mean imputation by treatment allocation. Data on deaths and admitted patient care costs were considered to be complete; therefore, no imputation was performed. For non-admitted patient care health-care costs, we imputed missing values as zero if any components of resource use were reported.
We used multiple imputation by chained equations to impute missing data on EQ-5D-3L utility scores, total health-care costs (except inpatient care, which is complete) and OKS at each follow-up time point. Each missing value was imputed as a function of follow-up period, sex and BMI at randomisation, and OKS and age at follow-up. Total non-admitted patient care health-care costs were also imputed as a function of EQ-5D-3L score and inpatient care costs at each follow-up point, and EQ-5D-3L score was also imputed as a function of total health-care costs at each follow-up point in addition to the above stated covariates. We used predictive mean matching to create 10 imputed data sets. We imputed annual costs and EQ-5D-3L utility score in each period; in periods when death was observed, these were adjusted. For costs, we assumed that they were incurred linearly over time, such that, if an individual died 6 months into an annual period, they incurred half of the predicted costs. For EQ-5D-3L utility, we assumed that the imputed utility score prevailed until the time of death. Imputation was performed separately in subgroups by treatment allocation.
Analysis
We report descriptive statistics (mean, SD) for resource use, costs and EQ-5D utilities at each follow-up time point using complete data only. Differences between groups were estimated using linear regression, controlling for treatment allocation, age group (< 50, 50–70 or > 70 years), sex and baseline OKS band (0–14, 15–21 or 22–48). For EQ-5D, we additionally controlled for baseline EQ-5D utility, and for health-care costs (except relating to the index admission), we controlled for health-care costs in the year preceding randomisation. Standard errors were adjusted to reflect clustering by the delivery unit.
Following multiple imputation, we estimated total costs and QALYs for all 528 participants in TOPKAT from the date of study recruitment to the earliest of death, withdrawal from the study or the end of follow-up at 5 years. Both costs and QALYs were discounted at 3.5% per year following guidelines from the National Institute for Health and Care Excellence. 87 Our analysis used ITT principles, with health-care resource use, costs and EQ-5D-3L scores analysed according to treatment allocation, regardless of the treatment that the participant actually received.
On each imputed data set we estimated mean total health-care costs (and by cost type) and QALYs using separate linear regression models with the same covariate patterns as described above for descriptive analyses, and cluster-robust standard errors. Estimates derived from each imputed data set were combined using Rubin’s rule to estimate the adjusted MD and standard error for each outcome.
We calculated the ICER by dividing the mean cost difference between PKR and TKR by the mean QALY difference. The ICER reports the amount that must be spent to generate one additional QALY. We also present results within participant subgroups defined by age at recruitment (< 55, 55–70 or > 70 years), sex (male or female) and OKS band (0–14, 15–21 or 22–48) at recruitment.
We estimated the joint uncertainty around incremental total costs and QALYs (i.e. the difference between PKR and TKR) by bootstrapping 500 times from each of our 10 imputed data sets, running the estimation model on each bootstrapped dataset and extracting the estimated treatment effects. We presented these data graphically in a cost-effectiveness scatterplot. From these bootstrapped results, we also calculated the probability that PKR is more cost-effective than TKR for different threshold values per QALY gained. 88 These are calculated by estimating the proportion of bootstrap replicates with a net monetary benefit (NMB) above 0 for each threshold value, where the NMB is given by the product of the MD in QALYs and the threshold value minus the MD in costs.
As a sensitivity analyses, we explored using a complete-case analysis including only individuals who provided complete data over 5 years. In those periods in which an individual died, we used the last observation carried forward for EQ-5D and non-admitted patient care health-care costs. As with the main analysis, we adjusted these estimates for death by assuming that costs were incurred linearly over time, and that the EQ-5D utility prevailed until death. We re-estimated the base-case analysis assuming equal costs for the PKR and TKR implant devices. In a preliminary analysis, we also considered the use of different imputation models using linear regression rather than predictive mean matching. Finally, we repeated the main analysis but compared groups by treatment received rather than treatment allocated.
All analyses were conducted using R version 3.4 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Study participant follow-up time
Of the 528 study participants, 17 died and 15 withdrew from the study.
Table 15 presents the percentage of missing data observations for EQ-5D utility and the total non-admitted patient care health-care costs at each follow-up point. Patterns of missing data are very similar for these two outcomes and in both treatment groups. We found no strong evidence that the probability that data were missing depended on baseline participant characteristics, or on lagged outcomes. Across all time periods, 13% of questionnaires on health-care costs and EQ-5D were missing.
| Follow-up time | Health-care resource use, number of missing questionnaires (%) | EQ-5D utility, number of missing questionnaires (%) | ||
|---|---|---|---|---|
| PKR | TKR | PKR | TKR | |
| Baseline | 6 (2.3) | 7 (2.7) | 7 (2.7) | 12 (4.5) |
| 2 months | 20 (7.6) | 28 (10.6) | 26 (9.8) | 29 (11.0) |
| 1 year | 24 (9.1) | 27 (10.3) | 24 (9.1) | 29 (11.1) |
| 2 years | 31 (11.8) | 29 (11.2) | 29 (11.0) | 20 (7.8) |
| 3 years | 47 (18.0) | 36 (14.0) | 47 (18.0) | 37 (14.4) |
| 4 years | 57 (22.0) | 44 (17.3) | 54 (20.8) | 41 (16.1) |
| 5 years | 34 (13.2) | 43 (17.0) | 33 (12.8) | 41 (16.2) |
Health-care costs
Index admission
Table 16 presents costs and cost differences by treatment allocation for total costs associated with the allocated procedure and each major component of these costs. Overall, the index admission cost an additional £470 (95% CI £211 to £729) in those allocated to the TKR group compared with those allocated to the PKR group, and this was due, in roughly equal parts, to the additional costs of the implants and time in hospital (mean 0.77 additional days). Assuming equal costs for the device, the cost difference reduced to £179 (95% CI –£67 to £425) and still favoured PKR, but was no longer statistically significant.
| Type of cost | PKR (£), mean (SD) | TKR (£), mean (SD) | Difference (£) (PKR vs. TKR), mean (95% CI) |
|---|---|---|---|
| Total admission costs | 3991 (889) | 4463 (1709) | –470 (–729 to –211) |
| Implant device | 915 (199) | 1205 (340) | –292 (–408 to –176) |
| Time in theatre | 1932 (479) | 1820 (639) | 112 (18 to 207) |
| Hospital stay | 1101 (536) | 1358 (1212) | –256 (–463 to –50) |
| Complications | 44 (278) | 79 (382) | –35 (–91 to 21) |
Follow-up health-care use
Table 17 presents self-reported use of different health-care services in the year preceding recruitment and between subsequent follow-up periods. On average, participants allocated to TKR had substantially more outpatient appointments in the first 2 years following randomisation, in addition to somewhat elevated rates of GP consultations and visits to physiotherapists or occupational therapists. Towards the end of the 5-year follow-up, differences in resource use between groups were minimal.
| Follow-up period | PKR, mean (SD) | TKR, mean (SD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | GP | Inpatienta | Nurse | Physiotherapist | Outpatient | n | GP | Inpatienta | Nurse | Physiotherapist | Outpatient | |
| Year before baseline | 258 | 2.70 (2.10) | 0.11 (0.96) | 0.17 (0.63) | 1.96 (1.55) | 1.17 (2.30) | 257 | 2.77 (2.68) | 0.02 (0.14) | 0.15 (0.64) | 2.04 (1.46) | 1.37 (4.30) |
| Baseline–2 months | 244 | 0.66 (1.09) | 0.00 (0.06) | 0.83 (1.21) | 1.03 (1.66) | 2.26 (5.05) | 236 | 0.97 (1.30) | 0.02 (0.17) | 0.99 (1.77) | 1.11 (1.16) | 2.70 (3.07) |
| 2 months–1 year | 240 | 0.81 (1.52) | 0.03 (0.17) | 0.40 (0.87) | 0.95 (1.20) | 1.20 (2.50) | 234 | 1.04 (1.79) | 0.04 (0.19) | 0.26 (0.63) | 1.39 (2.07) | 3.17 (6.29) |
| 1–2 years | 232 | 0.44 (1.28) | 0.02 (0.15) | 0.11 (0.47) | 0.34 (0.71) | 0.35 (1.20) | 229 | 0.48 (1.19) | 0.02 (0.17) | 0.11 (0.57) | 0.62 (1.22) | 0.66 (3.19) |
| 2–3 years | 214 | 0.33 (0.89) | 0.00 (0.06) | 0.09 (0.54) | 0.21 (0.61) | 0.13 (0.68) | 221 | 0.47 (1.57) | 0.02 (0.15) | 0.04 (0.41) | 0.31 (1.09) | 0.33 (1.83) |
| 3–4 years | 202 | 0.29 (0.94) | 0.03 (0.32) | 0.05 (0.26) | 0.22 (0.77) | 0.20 (1.09) | 211 | 0.44 (1.44) | 0.03 (0.16) | 0.02 (0.14) | 0.29 (0.94) | 0.38 (1.84) |
| 4–5 years | 223 | 0.27 (0.91) | 0.00 (0.00) | 0.06 (0.34) | 0.31 (1.69) | 0.30 (1.25) | 210 | 0.27 (0.77) | 0.01 (0.11) | 0.05 (0.27) | 0.60 (1.97) | 0.53 (2.16) |
Table 18 presents health-care costs (excluding inpatient care) for each follow-up period. In line with observed differences in resource use by treatment allocation, average costs were lower among those allocated to PKR than to TKR, with differences especially large in the first year of follow-up.
| Follow-up period | PKR | TKR | Difference (PKR vs. TKR)a | ||
|---|---|---|---|---|---|
| n | Cost (£), mean (SD) | n | Cost (£), mean (SD) | Cost (£), mean (95% CI) | |
| Baseline–2 months | 244 | 286 (371) | 236 | 332 (232) | –46 (–111 to 20) |
| 2 months–1 year | 240 | 222 (254) | 234 | 388 (511) | –172 (–258 to –85) |
| 1–2 years | 232 | 80 (146) | 229 | 136 (284) | –56 (–98 to –15) |
| 2–3 years | 214 | 49 (123) | 221 | 77 (230) | –25 (–56 to 5) |
| 3–4 years | 202 | 52 (164) | 211 | 75 (223) | –21 (–67 to 24) |
| 4–5 years | 164 | 68 (248) | 163 | 118 (325) | –50 (–112 to 13) |
Table 19 presents inpatient care costs at each time point. There was no clear pattern by treatment allocation, with PKR having higher average costs in some years and lower costs in others.
| Follow-up period | PKR | TKR | Difference (PKR vs. TKR)a | ||
|---|---|---|---|---|---|
| n | Cost (£), mean (SD) | n | Cost (£), mean (SD) | Cost (£), mean (95% CI) | |
| Baseline–2 months | 264 | 6 (99) | 264 | 28 (214) | –22 (–47 to 3) |
| 2 months–1 year | 264 | 128 (1002) | 261 | 49 (246) | 80 (–78 to 238) |
| 1–2 years | 263 | 124 (1008) | 258 | 166 (1864) | –47 (–299 to 206) |
| 2–3 years | 261 | 3 (51) | 257 | 124 (1013) | –121 (–245 to 3) |
| 3–4 years | 259 | 163 (1955) | 255 | 99 (843) | 70 (–215 to 356) |
| 4–5 years | 257 | 0 (0) | 253 | 82 (837) | –83 (–187 to 22) |
EuroQol-5 Dimensions utility
Table 20 presents EQ-5D-3L utility scores and differences by treatment allocation at each time point. EQ-5D-3L score was higher in the PKR group than the TKR group, albeit largely not statistically significant. The distribution of responses to each EQ-5D domain at each follow-up time point is presented by treatment allocation in Appendix 5, Table 35. Differences in EQ-5D scores cannot be explained by differences in any single dimension of the EQ-5D.
| Follow-up period | PKR | TKR | Difference (PKR vs. TKR),a mean (95% CI) | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Baseline | 257 | 0.428 (0.301) | 252 | 0.381 (0.324) | – |
| 2 months | 238 | 0.689 (0.243) | 235 | 0.664 (0.226) | 0.022 (–0.019 to 0.063) |
| 1 year | 240 | 0.766 (0.241) | 232 | 0.723 (0.260) | 0.038 (–0.003 to 0.079) |
| 2 years | 234 | 0.775 (0.261) | 238 | 0.719 (0.282) | 0.051 (–0.007 to 0.110) |
| 3 years | 214 | 0.771 (0.249) | 220 | 0.729 (0.289) | 0.034 (–0.026 to 0.094) |
| 4 years | 205 | 0.770 (0.251) | 214 | 0.719 (0.301) | 0.043 (0.003 to 0.084) |
| 5 years | 224 | 0.744 (0.293) | 212 | 0.717 (0.318) | 0.018 (–0.033 to 0.069) |
Main analysis
Table 21 shows the main analysis results at 5 years (see Appendix 5, Table 34, for descriptive statistics on health-care costs and EQ-5D utility at each follow-up time point following multiple imputation). There was a small difference in life-years lived favouring PKR (MD 0.085 years, 95% CI –0.032 to 0.202 years). Differences in QALYs were larger and significantly favoured PKR (MD 0.240 QALYs, 95% CI 0.046 to 0.434 QALYs). Total costs were, on average, lower among those allocated to PKR (MD £910, 95% CI £317 to £1503), reflecting, in equal part, the lower costs of the index admission (MD £471, 95% CI £214 to £729) and the lower health-care costs during follow-up (MD £433, 95% CI –£114 to £979). PKR, therefore, dominated TKR with lower costs and higher QALYs. Assuming equal costs of PKR and TKR the implant device, the MD in total costs fell to £618 (95% CI –£7 to £1243) and PKR remained dominant (cheaper and more effective than TKR). When comparing outcomes by treatment received rather than treatment allocated at 5 years (Table 22), the difference in costs was greater, at £1033 (95% CI £368 to £1697), mostly because of higher costs of the surgery, whereas differences in QALYs were similar, at 0.209 QALYs (95% CI 0.044 to 0.373 QALYs).
| Outcomes | PKR (n = 264), mean (SE) | TKR (n = 264), mean (SE) | Difference (PKR vs. TKR), mean (95% CI) |
|---|---|---|---|
| Life-years | 4.917 (0.709) | 4.831 (0.888) | 0.085 (–0.032 to 0.202) |
| QALYs | 3.448 (0.970) | 3.193 (1.060) | 0.240 (0.046 to 0.434) |
| Total costs (£) | 5149 (56) | 6048 (60) | –910 (–1503 to –317) |
| Initial admission | 3991 (30) | 4463 (41) | –471 (–729 to –214) |
| Follow-up | 1158 (54) | 1585 (55) | –433 (–979 to 114) |
| ICER (£) | – | – | –3792 |
| Outcomes | PKR (n = 245), mean (SE) | TKR (n = 269), mean (SE) | Difference (PKR vs. TKR), mean (95% CI) |
|---|---|---|---|
| Life-years | 4.931 (0.666) | 4.918 (0.681) | –0.038 (–0.101 to 0.026) |
| QALYs | 3.456 (0.965) | 3.280 (1.015) | –0.209 (–0.373 to –0.044) |
| Total costs (£) | 5167 (55) | 6323 (59) | 1033 (368 to 1697) |
| Initial admission | 4007 (29) | 4694 (38) | 802 (545 to 1059) |
| Follow-up | 1160 (53) | 1629 (56) | 230 (–288 to 748) |
| ICER (£) | – | – | –4943 |
Figure 6 presents the cost-effectiveness scatterplot giving differences in mean total costs and QALYs for PKR versus TKR, using observed (blue) and assumed equal (orange) device costs. In both scenarios, most bootstrap replicates remained largely in the south-east quadrant of the cost-effectiveness scatterplot, indicating that PKR dominated TKR with higher QALYs and lower costs. The probability of PKR being the most cost-effective option was > 99.9% for all reasonable threshold values.
FIGURE 6.
Cost-effectiveness scatterplot for the base-case analysis and assuming equal implant device costs (the larger black points present mean values for each modelled scenario).
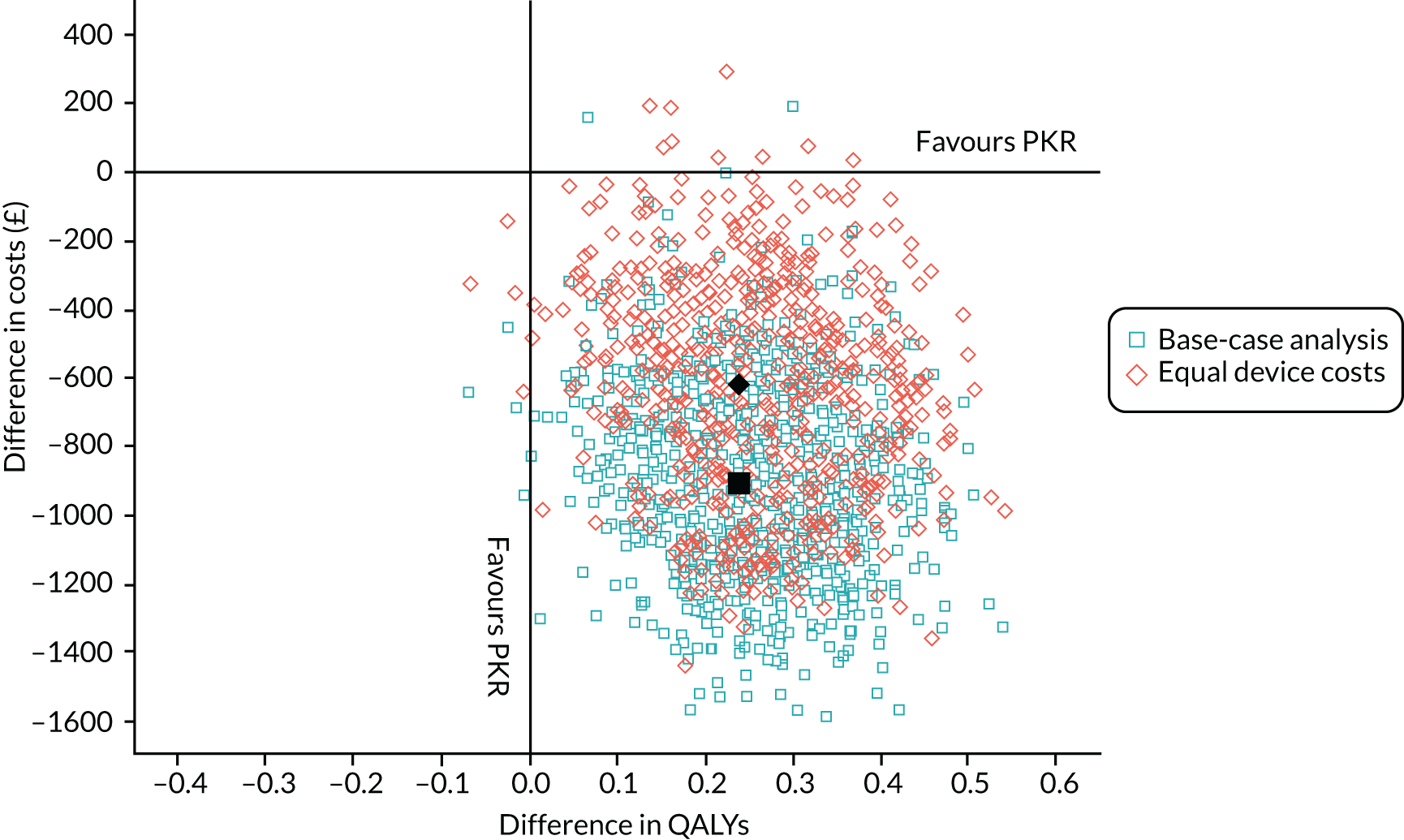
Table 23 reports the cumulative costs and QALYs at each follow-up time point. Mean costs are higher for TKR than for PKR at each time point, and the difference increases with time. For QALYs, there was a negligible difference in QALYs at 2 months, but QALY differences between PKR and TKR increase steadily with follow-up time.
| Follow-up time point | Costs (£) | QALYs | ||||
|---|---|---|---|---|---|---|
| PKR, mean (SE) | TKR, mean (SE) | Difference, mean (95% CI) | PKR, mean (SE) | TKR, mean (SE) | Difference, mean (95% CI) | |
| 2 months | 4290 (32) | 4828 (43) | –545 (–814 to –275) | 0.092 (0.189) | 0.086 (0.189) | 0.002 (–0.001 to 0.006) |
| 1 year | 4638 (39) | 5250 (46) | –619 (–975 to –264) | 0.691 (0.455) | 0.647 (0.463) | 0.037 (–0.001 to 0.076) |
| 2 years | 4846 (47) | 5542 (53) | –711 (–1173 to –248) | 1.422 (0.640) | 1.320 (0.667) | 0.093 (0.011 to 0.175) |
| 3 years | 4903 (47) | 5728 (55) | –841 (–1307 to –374) | 2.126 (0.769) | 1.971 (0.820) | 0.144 (0.018 to 0.270) |
| 4 years | 5095 (55) | 5886 (58) | –804 (–1369 to –238) | 2.805 (0.873) | 2.598 (0.947) | 0.193 (0.031 to 0.356) |
| 5 years | 5149 (56) | 6048 (60) | –910 (–1503 to –317) | 3.448 (0.970) | 3.193 (1.060) | 0.240 (0.046 to 0.435) |
Table 24 presents cost-effectiveness results at 5 years by subgroups of participants. There is no clear evidence that cost-effectiveness differs between participant subgroups.
| Follow-up time point | n | Costs (£) | QALYs | ICER (£) | |||||
|---|---|---|---|---|---|---|---|---|---|
| PKR | TKR | PKR, mean (SE) | TKR, mean (SE) | Difference, mean (95% CI) | PKR, mean (SE) | TKR, mean (SE) | Difference, mean (95% CI) | ||
| All participants | 264 | 264 | 5149 (56) | 6048 (60) | –910 (–1503 to –317) | 3.448 (0.970) | 3.193 (1.060) | 0.240 (0.046 to 0.434) | –3792 |
| Age (years) | |||||||||
| < 55 | 33 | 31 | 5602 (135) | 8077 (112) | –3725 (–7786 to 335) | 3.207 (1.043) | 2.682 (1.196) | 0.426 (–0.376 to 1.227) | –8755 |
| 55–70 | 161 | 162 | 5128 (52) | 5900 (55) | –779 (–1288 to –269) | 3.472 (0.961) | 3.171 (1.050) | 0.258 (0.077 to 0.440) | –3012 |
| > 70 | 70 | 71 | 5127 (63) | 6053 (66) | –869 (–2226 to 489) | 3.426 (0.983) | 3.341 (1.048) | 0.065 (–0.199 to 0.329) | –13,318 |
| Sex | |||||||||
| Male | 153 | 153 | 5167 (59) | 5972 (65) | –846 (–1628 to –65) | 3.475 (0.974) | 3.206 (1.083) | 0.250 (–0.022 to 0.521) | –3390 |
| Female | 111 | 111 | 5124 (53) | 6153 (58) | –1042 (–1791 to –293) | 3.411 (0.965) | 3.175 (1.028) | 0.271 (0.043 to 0.499) | –3840 |
| OKS group | |||||||||
| 0–14 | 75 | 74 | 5136 (66) | 6104 (64) | –950 (–1883 to –16) | 3.048 (0.983) | 2.672 (1.096) | 0.334 (–0.077 to 0.745) | –2841 |
| 15–21 | 102 | 100 | 5275 (50) | 6150 (66) | –882 (–1582 to –181) | 3.399 (0.954) | 3.130 (1.052) | 0.252 (–0.077 to 0.581) | –3503 |
| 22–48 | 87 | 90 | 5012 (63) | 5890 (65) | –864 (–1937 to 209) | 3.849 (0.888) | 3.691 (0.916) | 0.164 (–0.107 to 0.436) | –5252 |
Table 25 presents the complete-case analysis results at 5 years. Only 297 participants (57%) contributed data to the complete-case analysis (PKR, 57%; TKR, 55%). Compared with the multiple imputation analysis, PKR is still the most effective intervention albeit associated with fewer additional QALYs, but with greater cost savings. The qualitative conclusions remain the same, with PKR dominating TKR.
| Outcomes | PKR, mean (SD) | TKR, mean (SD) | Difference (PKR vs. TKR), mean (95% CI) |
|---|---|---|---|
| n | 151 | 146 | – |
| Life-years | 5.000 (0.000) | 4.921 (0.526) | 0.065 (–0.002 to 0.132) |
| QALYs | 3.575 (0.930) | 3.413 (1.016) | 0.150 (–0.103 to 0.402) |
| Total costs (£) | 5040 (2947) | 6327 (3681) | –1232 (–1995 to –468) |
| Initial surgery | 3947 (820) | 4711 (1683) | –779 (–1110 to –447) |
| Follow-up | 1093 (2755) | 1616 (3307) | –453 (–1140 to 234) |
| ICER (£) | – | – | –8224 |
Summary
Over 5 years of follow-up in TOPKAT, we found that PKR led to improved health-related quality of life and reduced health-care costs compared with TKR in individuals with OA of the medial compartment of the knee, even when assuming equal device costs. The cost-effectiveness results are consistent with a recent modelling study based on data from the National Joint Registry (NJR),54 which found that PKR is expected to generate better health outcomes at lower costs over a patient’s lifetime compared with TKR. 54
Following the index surgery, participants in both treatment groups saw considerable improvements in their EQ-5D utility, reflecting the known clinical benefits of knee replacement surgery in patients with OA and poor functional outcomes. 89 Differences in EQ-5D utility at all follow-up time points favoured PKR but were not typically significantly different by treatment group. However, QALYs, which reflect cumulative health-related quality-of-life adjusted for survival, were found to be significantly higher among those allocated to the PKR group than those allocated to the TKR group beyond 1 year.
Costs of the index surgery were significantly lower among those allocated to the PKR group than those allocated to the TKR group. Follow-up health-care costs were significantly lower among those allocated to PKR than among those allocated to TKR over the first 2 years of follow-up, driven predominantly by fewer outpatient visits. Cumulative health-care costs were significantly different through the trial follow-up and favoured PKR, even when assuming equal device costs.
Our cost-effectiveness analysis is based on the largest, to our knowledge, RCT comparison of PKR and TKR in the UK. The analysis has several limitations, including the sizeable number of missing data on use of health-care resources and EQ-5D-3L, particularly in the later years of follow-up. We accounted for this using multiple imputation. 86 This assumes that data are missing at random, conditional on modelled covariates. We found no strong evidence to contradict this assumption and indeed found that the qualitative conclusions were the same in the multiple imputation and complete cases analyses. In addition, results were calculated over 5 years only. Longer follow-up could confirm whether or not the observed differences in costs and quality of life are maintained.
Chapter 6 Discussion and conclusion
TOPKAT was designed as a large, multicentre, parallel group, superiority RCT to address the uncertainty around which type of knee replacement treatment provides the most benefit for patients with medial compartmental OA of the knee. The trial evaluated the clinical effectiveness and cost-effectiveness of PKR compared with TKR for this condition. To our knowledge, the trial, which randomised 528 patients, is the largest of its kind to date and provides the longest follow-up (5 years) for this size of cohort.
Summary of main findings
The trial showed that at the 5-year follow-up both types of operation had good outcomes. Patients in both groups had a superior outcome to their preoperative baseline status. The changes in OKS following PKR and TKR intervention were substantial (mean OKS change > 18 points for both procedures). There was no evidence of difference in the primary outcome of mean OKS between groups at the 5-year time point, despite there being evidence of a difference at 1 year. However, a post hoc AUC analysis of the primary outcome over the duration of the study did show significant benefit in favour of PKR over TKR, although this difference was smaller than the minimal clinically important difference in the score. All analyses are consistent with a small difference (around 1-point OKS) in favour of PKR.
The secondary outcome measures all showed a consistent pattern of benefit in the direction of PKR, although most differences were small and many were not statistically significant. This included measures of self-reported activity (OKS-APQ, AKSS, HAAS and UCLA), global health (EQ-5D-3L) and patient satisfaction. The EQ-5D VAS global health instrument revealed significant differences in favour of PKR. Anchor questions of transition (how are your problems now compared with before the operation?) and self-reflection on benefit (would you have the operation again?) suggested that PKR was superior to TKR, but both variables are open to some bias as the study was not blinded.
The reoperation rate (including revision) was similar for both groups: 22 out of 245 PKR participants and 28 out of 269 TKR participants. The 5-year revision rates were 10 PKR revisions from 245 patients (4.1%) and 10 TKR revisions from 269 patients (4%). The 5-year revision rate for PKR in the NJR (and other worldwide registries) is 6.1%, which is substantially higher than that found in TOPKAT (discussed further below). 6,61,62 In view of the limitation of using revision data alone to indicate ‘failure’ of an implant or operation, a conservative measure of failure was also employed in this study. This composite outcome (with failure defined as reoperation/revision and/or poor improvement in OKS) showed that there was 28 failures of PKR and 38 failures of TKR over 5 years. This difference of 10 fewer failures of PKR was not found to be significant.
Independent opinion was also sought to aid wide-ranging and unbiased interpretation of the trial. Although TKR was not seen to be advantageous to PKR using any variable (significantly or otherwise), the trial also did not reveal any convincing clinical advantage of PKR over TKR, including for the primary outcome measure. However, informed by the consistent patterns of direction of effect across all variables in favour of PKR and the lack of perceived advantage of TKR in any domain, both independent opinion and investigators not clinically aligned with any implant or type were inclined to suggest that there was a modest benefit of PKR over TKR from these mid-term clinical results.
The health economic evaluation provided very strong evidence in favour of PKR, with PKR being found to be more cost-effective than TKR. This finding was primarily driven by slightly better quality-of-life outcome (as measured by the EQ-5D), lower surgery costs and lower follow-up health-care costs. PKR, therefore, dominated TKR with lower costs and higher QALYs. Even assuming equal costs of the implant device, the MD in total costs remained substantial [£619 (95% CI –£6 to £1244)] and PKR remained cheaper and more effective than TKR.
Discussion of findings in the light of other literature
The previous evidence and literature, and their limitations, were presented in Chapter 1. Most of the previously published cohort data provided results in favour of PKR for early outcome, including the work of Liddle et al. ,60,90 findings that have been echoed by previous and recent systematic reviews. 47,63,91 The current study provides further evidence that PKR provides modest clinical advantages and larger health-care economic benefits over TKR for this condition. The robust study design and execution provided strong protection against selection bias potentially influencing other results. This large and definitive randomised study was deemed necessary as any previous cohort data, no matter how well matched, will have the inherent risk of selection and likely residual bias even after adjustment for this. Selection bias is important, as the decision-making for which patients should undergo TKR and PKR outside a randomised study is influenced by multiple factors and characteristics. For example, not all surgeons would offer a PKR to all patients with medial compartment OA. To date, it has been considered as an operation for younger patients with less widespread disease and fewer comorbidities, all of which have the potential to influence outcome.
There are several issues regarding the trial findings that warrant specific discussion.
The observed consistent patterns in the direction of PKR found in the primary and secondary outcome variables was striking (although many effects were small and not statistically significant). Possible explanations could include either a lack of sensitivity in the measurement instruments used or a true underlying, but very modest, difference between TKR and PKR. Although the outcome measures may not be perfectly sensitive for this particular comparative research question, as TKR and PKR are both widely used interventions of known benefit, they were chosen carefully and represent the best indicators of outcome available when the study was designed.
The magnitude of the difference observed in the primary outcome at 5 years (a MD of 1.04 points in OKS) is worthy of comment. This level of difference lies well within a revised estimate of the true minimally important difference in OKS,66 and is unlikely to have any discernible impact on practice. However, clinicians and patients themselves must decide whether or not this level of difference, and the evidence from other variables, would constitute enough of a difference to change or support their current practice.
Some discussion is warranted over the contrasting significance of the primary analysis using cross-sectional means (at each year and finally at 5 years) and the AUC analysis. There is an argument that the AUC analysis is more appropriate and takes into account the cumulative changes over the duration of the study. As can be seen in Figure 1 there were more patients with OKS outcome scores of ≥ 40 in the PKR group than in the TKR group. If the objective is to deliver more patients with higher scores, then PKR appears to be able to deliver this. The earlier cross-sectional data are also less informative from a clinical perspective. Arthroplasty is an intervention aimed at long-term benefit, and 5 years is often considered the minimum follow-up required to make reasonable conclusions. That said, the early (1 year) revision data do provide insight into early failure. It was previously thought that most failures occur within the first year of implantation and that PKR was more susceptible to this. This study has shown that failure of intervention in the first year can be more common than in subsequent years, but there was no obvious difference between implant types.
When exploring confounders, it was important to assess the effect of any delay to undergoing surgery. As seen in Figure 4, it was reassuring to see that the delays in surgery had similar effects with neither PKR nor TKR patients being disadvantaged by a delay in surgery.
In terms of mortality after knee replacement, this was low overall and similar in both groups. The study was not designed to substantiate the findings of higher mortality seen for TKR shown by other large cohort studies. 61,92
The reoperation and revision data are a point of notable interest. The trial findings differ significantly from previously reported worldwide joint registry data and from the perceived clinical understanding of the performance of the procedure. PKR is generally thought to have a much higher revision rate (at least 6%) than TKR. 6 The high revision rate is one reason put forward in opposition to more frequent use of PKR. This trial has shown that evaluation in a controlled trial (where selection bias is minimised) yields revision and reoperation rates of PKR and TKR that are very similar. This demands consideration, and there are several possible reasons for the contrast.
First, the threshold for revision, outside a closely monitored trial, is much lower for PKR than for TKR. Surgeons are more likely to offer revision of partial to total replacement (PKR to TKR) than total to total replacement (TKR to TKR) because of the likely outcome of these revision scenarios. 93 This normal threshold may have been disrupted in TOPKAT, with surgeons less likely to revise without high levels of justification. An important side issue, therefore, is whether or not the routine clinical pathway and threshold for revision surgery outside a trial should always be as well justified as it appears to have been for the patients in this trial.
Second, the issue of surgeon experience or expertise may influence personal surgeon thresholds for revision. The trial involved surgeons who had to have met a minimum experience and qualification threshold and were all self-selected and volunteered to be part of the study. Although they were not considered (or desired) to be top-level experts, by definition, all involved surgeons considered themselves to be highly competent at the knee replacements they were performing (either both types if in equipoise or one of PKR/TKR if not). PKR is considered to be more technically demanding than TKR, and patients undergoing PKR outside the study (and who will thus contribute to the registry data) are likely to have had surgeons less experienced in the technique. The analysis that adjusted for the learning suggested that this has a small impact on OKS at 1 year but not at 5 years. Similarly, there was no clear evidence of a difference between equipoise and expertise surgeons. Many of the surgeons outside the trial will have been on a learning curve or perform the operation infrequently. This is known to affect success94 and could account for the disparity in revision data between the trial and registries. The UK NJR shows that, over a 3-year period (1 January 2015 to 31 December 2017), 272,133 primary TKR procedures were performed by 1976 surgeons (median = 103, IQR 29–202.5 procedures) in 403 separate units (median = 599 cases per unit, IQR 278–944 cases per unit). In the same time period, 28,573 primary PKR procedures were performed by 809 consultant surgeons (median = 15, IQR 4–39 procedures) in 358 units (median = 42 cases per unit, IQR 16–92 cases per unit). This difference in average arthroplasties performed per surgeon per year for each implant type (TKR, n = 34; PKR, n = 5) is marked and could account for the differences between registry and TOPKAT data. 6
The side issue to this point is that a minimal level of surgeon competence or experience is required to undertake PKR to achieve success. The revision rate for the lowest volume surgeons has been shown to be four times higher than that for the highest volume surgeons, with a minimal annual volume number of 13 procedures stated for competence. 95–97
The numbers of reoperations and revisions were too low to draw many conclusions above those involving simple frequency. However, it was noted that more PKR patients underwent subsequent revision surgery for bearing dislocation (as the majority of PKRs were mobile bearing implants) and more TKR patients underwent revision for unexplained pain. Notably, 12 patients who had undergone TKR had a manipulation under anaesthetic for stiffness compared with no patients who had undergone PKR. Although manipulation under anaesthetic may be considered a relatively benign non-invasive reoperation procedure (compared with component revision), it could be argued that the long-term consequences of a stiff knee after arthroplasty are likely to have more of an impact on function than a single operation to perhaps replace a bearing to establish a normal functioning arthroplasty in the future.
There was some debate as to whether or not bearing exchange should be classified as true revision surgery. However, it is defined in the protocol as a reoperation and, therefore, reported as such for this study.
The issue of surgeon experience in relation to external validity and generalisation is dealt with in Strengths below.
Strengths
There are many general strengths of this study. Globally, to the best of our knowledge, it is the largest, most complete and longest randomised follow-up of any comparison of TKR with PKR to date. The study was sufficiently large to have detected clinically meaningful differences in OKS and probably all of the other key outcomes also. Well-established and well-validated outcome measures were used. Follow-up of the primary outcome variable at 5 years was 88% (94% of those who were sent a questionnaire returned it). The use of simple patient-reported success questions (e.g. satisfaction, transition and reflection) was found to be particularly useful. There was little issue over fidelity, with highly visible signals as to whether or not the intervention (operation) had been carried out. The conduct of the trial was considered a strength with strong support from the knee surgery community (British Association for Surgery of the Knee) and the same trial co-ordination and management team throughout. The trial was completed in two trials units with strong expertise in rigorous surgical evaluation (the Royal College of Surgeons of England Surgical Intervention Trials Unit surgical trial unit in Oxford and the CHaRT in Aberdeen). It was well supported by the funder [National Institute for Health Research (NIHR)]; long-term studies such as this one are expensive and require commitment. It is entirely independent of commercial interests, a problem for many similar comparative studies of implantable devices.
Some strengths of the study demand exploration in more detail. The external validity of the study is high and the findings are considered generalisable across the UK. It involved a large number of sites, from varied geographic regions, and had surgeons with different levels of experience. Uniquely, for arthroplasty, the study includes surgeons both in equipoise and not in equipoise regarding their beliefs about the benefit for each operation type.
Another strength of the study was that it was designed as a pragmatic study. This permitted and took into account a level of acceptable local variation with clinical arthroplasty pathways and expected variation of expertise within the surgeons. However, a minimum threshold for training and experience level was stipulated for the study, and each surgeon was screened before being admitted to recruit to TOPKAT. This aspect was essential to ensure that the procedures were performed competently. Such minimum experience thresholds were especially important for the PKR procedure, as the technically challenging nature of this type of knee replacement would have allowed criticisms about recruiting surgeons who could be considered ill-equipped to perform the operation. The competence for performing the procedure across the trial would have been imbalanced. TKR operations are performed by all knee surgeons in training and are routine and regular operations that they perform. The same cannot be said of PKR. The potential for imbalance in surgical expertise for two or more different procedures is a problem for all surgical trials and methodologies are continually being refined to address this.
The inclusion of an expertise randomisation component to the study did, in part, assuage the above threats and strengthened external validity further. The study was able to reflect current practice by allowing any surgeon who believed strongly in one technique over another, or who considered themselves imbalanced in their own competency for performing the operation, to participate and randomise patients. The expertise aspect of the study was also thought to offer insights into the possible negative effects of dilution of expertise (for surgeons in equipoise) or any positive effects of superspecialisation (for surgeons out of equipoise). It can be suggested, a priori, that having self-declared experts in one operation over another would presuppose greater effects; surgeons implanting their device of choice and performing their operation of highest competency would be expected to produce superior results. However, in reality, surgeons performing TKR only (and not PKR) may have considered themselves ill at ease with the more challenging PKR operation for a variety of reasons. It follows that overall capability (and surgical expertise) could be be elevated in the equipoise group of surgeons as they are the ones who are more able, and comfortable, to perform both procedures. The counter argument, alluded to above, is that the expertise surgeons performing TKR have independently decided on the benefits of TKR outside their ability to perform PKR. Regardless, the analysis between expert and non-expert randomisation groups did not show any strong effects.
External validity of the trial results is further supported by wide range of implants used in the study; all common implants could be used in the study, again reflecting current practice. The demographic data for implant type for the study closely mirrored that of the UK NJR (see Appendix 6). This meant that both fixed and mobile bearing devices were evaluated and some implants that are less frequently used are also included in the study. The limitation of this approach (revisited in Limitations) is that some types of implant have a naturally greater prevalence (i.e. the Low Contact Stress implant for TKR and the Oxford mobile bearing for PKR). Although deliberately pragmatic, with the most common implants dominating, if more of the other types of implants had been included it is possible that different results could have been generated.
The composite score for failure is a strength and a particularly judicious outcome (and conservative for success), as it does not rely solely on reoperation or revision to define lack of success. The score allows patients who may not have undergone reoperation or have been revised (obvious failure), but who have not improved sufficiently by a specified number of points on self-reported outcome (for success), to be classified as ‘failures’. Few other studies have used this metric.
A final notable strength of the study was the employment of external independent assessors to help interpret the results and their review of the data blind to allocation. This was considered especially important to neutralise any real, or perceived, personal bias (of persons involved in the study with conflicts of interest) or institutional bias (the lead institution has long-standing connections with various arthroplasty manufacturers and specific devices). It will be impossible to remove every aspect of perceived bias, but the work has benefited from considerable effort throughout the course of the study to minimise such potential bias.
Limitations
Several limitations to TOPKAT should also be outlined.
First, the study was not blinded. Patients were aware of which implant had been inserted. Any bias of expectation, on behalf of the patient, could have influenced the self-reported measurements and affected clinical results. It was not considered feasible to blind patients to their implant for ≥ 5 years (now 10 years). Although this remains a design limitation, it was considered in great detail at the outset and was thought to be a relatively minor threat to conclusions. Outside self-report, objective measures of function (AKSS), the incidence of complications, the need for reoperation and revision and the health economics message are relatively immune to this sort of expectancy bias.
There was some non-compliance with allocated intervention. Some patients allocated to the TKR group underwent PKR and some patients allocated to the PKR group underwent TKR. The main reason for this was a change in clinician equipoise during the operation. Having previously considered individual patients suitable for randomisation (and allocation), surgeons observed interoperative features that influenced the indications for a specific procedure and reversed their original decision. For patients who were allocated to the PKR group but underwent TKR, these were features related to the indications for PKR, such as an intact anterior cruciate ligament or greater degeneration of other compartments in the knee than expected. For patients who were allocated to TKR but underwent PKR, these were features such as finding a relatively healthy knee and being uncomfortable removing large amounts of healthy tissue. Usefully, these events were in the minority with only 31 patients (12%) moving from PKR to TKR and 13 patients (5%) moving from TKR to PKR. Understandably, the higher percentage of the total number of patients who were non-compliant with allocated treatment was from PKR to TKR (71%).
Although only a small number of patients had compliance with allocation issues, this problem did pose some minor difficulties with the analysis. Correctly, the primary analysis of the trial was ITT throughout. For the primary outcome, a CACE analysis allowed for this non-compliance and showed that the possible impact on magnitude of effect was very low. However, this non-compliance had a disproportionate effect on low-frequency events such as reoperation and revision. The possibility existed (and occurred) that out of only a very small number of revision procedures of one type of implant, several of these had actually had the contrasting device implanted at primary operation (for the reasons explained above). The use of allocated classifications for revision could have been misleading. This was especially important as the revision data in TOPKAT was different from that found in other registries and cohorts. A compromise of reporting both ‘as allocated’ and ‘as treated’ analyses appears to satisfy this issue.
The experience of surgeons implanting PKR could be listed as a limitation, especially when considering extrapolation to routine NHS care. All surgeons providing PKR were experienced at this procedure and had self-identified as PKR surgeons. In routine NHS care, this may not be the case at the present time. Many of the revision cases in the NJR may be the result of ‘learning curve cases’ that would not be seen in TOPKAT. Therefore, extrapolation of management recommendation into the NHS must be cautious. Although having generally strong external validity (from multisite data), the caveat that the results here apply to experienced surgeons only is important. Rolling out more PKR without adequate training or expertise would likely have negative consequences.
Follow-up data were largely of good quality and were complete. The 88% follow-up of the primary variable at 5 years (94% of questionnaires sent out at this time point were returned) is considered exceptional. There was, however, some loss to follow-up of other variables, particularly the AKSS score, which provided objective clinical outcome data. The reason for this was that completion of the AKSS score required a clinic appointment, and many patients, although willing to complete self-reports over the telephone, were less willing to attend hospital 5 years into the study. In addition, the AKSS required some unfamiliar physical measurements to be taken, and these were often carried out by research support teams at sites, who, despite efforts to provide training, still often found this instrument demanding. The lower levels of follow-up for the AKSS has little impact on the study, as this was a secondary variable and the manifestations of some of the clinical deficiencies expressed in the AKSS are reflected in other, better completed, outcome measures, such as the activity scores. The clinical effectiveness and cost-effectiveness analyses dealt with the potential impact of missing outcome data in different ways (use of multiple imputation or not for the principal analyses), but both sets of analyses suggest robustness of the findings to this.
Other limitations include, as reported previously, high prevalence of specific implants. Most notable are the Oxford mobile bearing partial knee implant and the Low Contact Stress total knee implant. The justification of this is that the study was pragmatic and sampled those implants in current use. The distribution mirrored that from national joint registries. Having said that, it could be argued that different results may have been obtained if the study had been limited or had set criteria for implant type. It is suggested that TOPKAT, in its highly pragmatic form, is more informative for the HTA programme, NIHR and the NHS than a tightly controlled more mechanistic study.
The length of hospital stay data showed that a benefit for PKR led to lower costs for the PKR group. Length of hospital stay was not controlled or strongly advised for either implant type. However, there is an anecdotal sentiment and observation in normal practice that PKR patients are expected to be discharged earlier than TKR patients. 98 The reasons for this are complex and may include habit, shibboleths, the TKR population being more aged in general and clinical reasons related to a decreased insult to the tissues of PKR compared with TKR. Regardless, length of hospital stay should be viewed cautiously as an outcome variable for clinical success by itself. Until the same discharge criteria are applied to TKR (if this is possible), the shorter length of stay may have been dictated by uncontrolled factors within the study. Note that this does not make the health economics data less valid; moreover, the length of hospital stay can be considered more of a fixed variable, similar to cost data, rather than an independent outcome-related variable.
Some secondary outcome variables proved difficult to collect. Radiographic data were insufficiently complete across sites and, therefore, unreliable and added little to the study findings. This was often the result of differences in clinical practice across sites.
Clinical implications
Although one overall message could be that PKR has advantages for patients with medial compartment OA over TKR, such a conclusion must be made cautiously. There are some nuances associated with both the interpretation and further issues about provision of PKR that would affect any clinical recommendation.
On a clinical basis only, given the small and non-significant difference in OKS, it could also be argued that TKR is an effective and reliable operation and there is no evidence from this study to discontinue it. Surgeons using TKR are providing a good clinical service and good outcome with this operation in this population. Similarly, the study has shown that PKR is an effective operation and it may achieve slightly improved clinical outcome over TKR. Clinicians themselves must decide whether or not this level of difference would constitute enough to change or support their current practice. Interestingly, the risk of revision, previously thought to be much higher for PKR, is not apparent in this study (providing that surgeons are equipped and have sufficient expertise to conduct the operation, as here in TOPKAT). Although the power for this finding is low, it has a substantial impact on the choice of implant once combined with the health economics data. The cost-effectiveness analysis shows clear superiority of PKR over TKR and, in the absence of any negative factors such a risk of revision, would imply that PKR should be considered a valuable treatment for patients requiring arthroplasty.
Integrating such findings and recommendations into the NHS is not straightforward. It has already been discussed that PKR can be more technically challenging operation and, if not performed correctly, could produce inferior results, especially outside a trial. TKR is more forgiving in terms of precision of implantation and technical skill requirements. More knee surgeons are likely to be more comfortable with TKR. There is a danger that, if widespread encouragement and promotion of PKR were established, without checks on experience and capability the outcomes and benefits seen in TOPKAT, and especially the revision data, would not been seen universally. Indeed, this is what is believed to have happened to some extent already at a national level96,99 and is reflected in the high revision rates seen in the national registry data.
The solution may be to still recommend PKR but to ensure that it is performed only by suitably qualified personnel. This may mean an improved training emphasis and higher levels of quality control on PKR implantation. Surgeons without capability or who perform the operation infrequently should perhaps consider other options. The obvious alternative is a TKR, which this study has shown to be nearly as clinically effective as PKR.
Recommendations for future work
Further follow-up is required to assess the longer-term stability of these findings over time (10-year follow-up in progress). Further research is also required into the effect of experience and the disparity between trial and cohort data. Which set of data should be used to formulate policy? There may also be a case to discuss which subgroups of patients might be more appropriately treated with one approach or the other and this might be the subject of further research.
The trial clearly shows results for a comparison in which experienced surgeons conducted the operations. The input needed to bring all knee surgeons to a level of capability and competency, particularly for PKR, is yet to be completed and could be an avenue for further work. In pools of surgeons without the necessary expertise, results may be different from TOPKAT, and this could also be explored. It is even possible that a new trial may be required in which centres with experience in using PKR allow PKR to be carried out with various types of supervision or training given to young surgeons to understand how to make the learning curve as short as possible.
The outcome measures used for TOPKAT may not have been ideal. Further work is also required to generate more sensitive outcome measures and also to decide which measures are most appropriate to answer the primary research question.
The expertise/equipoise aspect of the trial was of great interest and could be explored further. It may be a useful model for other research questions in surgery. The issues around why expertise randomisation models do not recruit as well as device-based requires further work.
Further work will also be required to investigate any changes in practice as a result of the trial. The overall message is that PKR may be more suitable than TKR but there are many caveats to this position and such discussions are required with any potential patient about to undergo arthroplasty. Research work around decision-making for knee replacement type should, therefore, continue but hopefully utilise some of the output from TOPKAT.
Conclusions
To our knowledge, TOPKAT is the largest RCT of longest follow-up to date and provides the most robust evidence on which to choose between TKR and PKR. Both TKR and PKR are effective and they offer similar clinical outcomes and have similar reoperation/revision and complication rates. Some patient-reported outcome measures were significantly higher for PKR. PKR had substantial cost-effectiveness benefits over TKR at 5 years.
Acknowledgements
TOPKAT study group
Chief investigator
David Beard.
Trial co-investigators
Nigel Arden (Oxford), Helen Campbell (Oxford), Marion Campbell (Aberdeen), Andrew Carr (Oxford), Jonathan Cook (Aberdeen then Oxford), Helen Doll (Oxford), Ray Fitzpatrick (Oxford), David Murray (Oxford) and Andrew Price (Oxford).
Trial management
Mayret Castillo (until 2011), Cushla Cooper, Loretta Davies, Anne Duncan (until 2017), Gordon Fernie, Sophie Halpin (until 2015) and Alison McDonald.
Trial administration
Katie Chegwin, Jiyang Li (until 2018), Elena Rabaiotti (until 2013), Sandra Regan (until 2012) and Victoria Stalker (until 2014).
Data management
Diana Collins (until 2013), Janice Cruden, Akiko Greshon, Kay Holland and Beverley Smith (until 2017).
Database/programming management
Gladys McPherson.
Trial statisticians
Charles Boachie (until 2013), Jemma Hudson and Graeme MacLennan.
Health economists
Helen Campbell (until 2015), Francesco Fusco (until 2018), Seamus Kent and Jose Leal.
We would also like to thank Hannah Wilson (DPhil student, University of Oxford) for her help with the update to the literature search.
Research teams
We are grateful to the participants and research teams at collaborating hospital sites:
Aneurin Bevan University Health Board, Royal Gwent Hospital
Ruth Jenkins, Mark Lewis [principal investigator (PI)] and Witek Mintowt-Czyz.
Belfast Health and Social Care Trust, Musgrove Park Hospital, Belfast
David Beverland (PI), Leeann Bryce, Julie Catney, Ian Dobie, Emer Doran and Seamus O’Brien.
Chesterfield Royal Hospital NHS Foundation Trust
Fazal Ali, Heather Cripps, Amanda Whileman, Phil Williams (PI) and Julie Toms.
County Durham and Darlington NHS Foundation Trust
Ellen Brown, Gillian Horner, Andrew Jennings (PI) and Glynis Rose.
East Lancashire Hospitals NHS Trust, Royal Blackburn Hospital
Frances Bamford, Wendy Goddard, Hans Marynissen (PI), Haleh Peel and Lyndsey Richards.
Great Western Hospitals NHS Foundation Trust, Swindon
Amanda Bell, Sunny Deo, Sarah Grayland, David Hollinghurst, Suzannah Pegler, Venkat Satish (PI) and Claire Woodruffe.
Harrogate and District NHS Foundation Trust, Harrogate
Nick London (PI), David Duffy, Caroline Bennett and James Featherstone.
Hull and East Yorkshire Hospitals NHS Trust
Joss Cook, Kim Dearnley, Nagarajan Muthukumar (PI), Laura Onuoha and Sarah Wilson.
Maidstone and Tunbridge Wells NHS Trust, Medway
Sandhu Banher, Eunice Emeakaroha, Jamie Horohan, Sunil Jain (PI) and Susan Thompson.
Mid Yorkshire Hospitals NHS Trust
Sarah Buckley, Aaron Ng (PI), Ajit Shetty and Karen Simeson.
Milton Keynes University Hospital NHS Foundation Trust
Julian Flynn, Meryl Newsom, Cheryl Padilla-Harris and Oliver Pearce (PI).
NHS Grampian, Woodend Hospital, Aberdeen
James Bidwell (PI), Alison Innes, Winifred Culley and Bill Ledingham and Janis Stephen.
North Bristol NHS Trust
Rachel Bray, Hywel Davies, Debbie Delgado, Jonathan Eldridge, Leigh Morrison, James Murray (PI), Andrew Porteous and James Robinson.
North Cumbria University Hospitals NHS Trust, Carlisle
Matt Dawson (PI), Raj Dharmarajan, David Elson, Will Hage, Nicci Kelsall and Mike Orr.
North Tees and Hartlepool NHS Foundation Trust, Stockton-On-Tees
Jackie Grosvenor, SS Maheswaran (PI), Claire McCue, Hemanth Venkatesh, Michelle Wild and Deborah Wilson.
Oxford University Hospitals NHS Trust, Nuffield Orthopaedic Centre
Chris Dodd, William Jackson (PI), Pam Lovegrove, David Murray, Jennifer Piper and Andrew Price.
Royal United Hospitals Bath NHS Foundation Trust, Bath
Neil Bradbury, Lucy Clark, Stefanie Duncan, Genevieve Simpson and Allister Trezies (PI).
Sherwood Forest Hospitals NHS Foundation Trust, Kings Mill Hospital, Sutton in Ashfield
Vikram Desai (PI), Cheryl Heeley, Kramer Guy and Rosalyn Jackson.
South Devon Healthcare NHS Foundation Trust, Torbay
Alan Hall, Gordon Higgins (PI), Michael Hockings, David Isaac and Pauline Mercer.
Stockport NHS Foundation Trust, Stockport
Lindsey Barber, Helen Cochrane, Janette Curtis, Julie Grindey, David Johnson (PI), and Phil Turner.
The Hillingdon Hospitals NHS Trust
David Houlihan-Burne (PI), Briony Hill, Ron Langstaff and Mariam Nasseri.
The Ipswich Hospital NHS Trust, Ipswich
Mark Bowditch, Chris Martin, Steven Pryke, Bally Purewal, Chris Servant (PI), Sheeba Suresh and Claire Tricker.
University Hospitals of Leicester NHS Trust, Leicester
Robert Ashford, Manjit Attwal, Jeanette Bunga, Urjit Chatterji, Susan Cockburn, Colin Esler (PI), Steven Godsiff, Tim Green, Christina Haines and Subash Tandon.
University Hospitals of North Midlands NHS Trust, Stoke on Trent
Racquel Carpio, Sarah Griffiths, Natalie Grocott and Ian dos Remedios (PI).
University Hospital Southampton NHS Foundation Trust
David Barrett, Phil Chapman-Sheath, Caroline Grabau, Jane Moghul, William Tice (PI) and Catherine Trevithick.
United Lincolnshire Hospitals NHS Trust, Boston
Rajiv Deshmukh, Mandy Howes, Kimberley Netherton, Dipak Raj (PI) and Nikki Travis.
United Lincolnshire Hospitals NHS Trust, Lincoln
Mohammad Maqsood, Rebecca Norton, Farzana Rashid, Alison Raynor, Mark Rowsell and Karen Warner.
We would like to thank the external members of the TSC and DMC for their advice and support for the project.
Trial Steering Committee
Donna Dodwell as our patient representative, Simon Donell (chairperson) (University of East Anglia), Shawn Tavares (Royal Berkshire Hospital) and Jonathan Waite (South Warwickshire NHS Foundation Trust).
Data Monitoring Committee
Karen Barker (Oxford University Hospitals NHS Foundation Trust), Gordon Murray (chairperson) (University of Edinburgh) and Hamish Simpson (University of Edinburgh).
Independent review and interpretation of results
-
Professor David Torgerson (University of York).
-
Professor Chris Maher (University of Sydney).
-
Mr Peter Brownson (The Royal Liverpool and Broadgreen University Hospitals NHS Trust).
-
Professor Simon Donell (University of East Anglia, Norwich).
-
Mr Mark Mullins (Abertawe Bro Morgannwg University Health Board).
-
Professor Jane Blazeby (Bristol University).
Ethics
Approval obtained from the UK National Research Ethics Service, Oxfordshire REC C, September 2009 (09/H0606/88).
Contributions of authors
David J Beard (https://orcid.org/0000-0001-7884-6389) (Professor of Musculoskeletal and Surgical Science, University of Oxford) was the chief investigator, led the funding application, study conception and design, provided overall study supervision, and prepared and reviewed the report.
Loretta J Davies (https://orcid.org/0000-0002-4721-356X) (Trial Manager, University of Oxford) contributed to overall study conduct, and preparation and review of the report.
Jonathan A Cook (https://orcid.org/0000-0002-4156-6989) (Associate Professor, University of Oxford) contributed to study design, advised on expertise-based design, overall study conduct, review of statistical aspects, and preparation and review of the report.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (Director of the CHaRT, University of Aberdeen) was responsible for overseeing the clinical analysis, contributed to overall study design, and preparation and review of the report.
Andrew Price (https://orcid.org/0000-0002-4258-5866) (Professor of Orthopaedic Surgery, University of Oxford) contributed to study design, advised on clinical aspects of trial, overall study conduct and review of the report. (Note that he did not contribute to analysis or interpretation of data.)
Seamus Kent (https://orcid.org/0000-0001-7298-3163) (Senior Researcher, University of Oxford) conducted the health economic evaluation and contributed to preparation and review of the report.
Jemma Hudson (https://orcid.org/0000-0002-6440-6419) (Research Fellow, University of Oxford) conducted the clinical analysis and contributed to preparation and review of the report.
Andrew Carr (https://orcid.org/0000-0001-5940-1464) (Nuffield Professor of Orthopaedics, University of Oxford) contributed to study design, was the orthopaedic clinical advisor and reviewed the report.
Jose Leal (https://orcid.org/0000-0001-7870-6730) (Senior Research Officer, University of Oxford) was responsible for overseeing the health economic evaluation and contributed to preparation and review of the report.
Helen Campbell (https://orcid.org/0000-0003-2070-7794) (Senior Researcher, University of Oxford) contributed to early study design regarding health economic evaluation.
Ray Fitzpatrick (https://orcid.org/0000-0003-0607-0563) (Professor of Public Health and Primary Care, University of Oxford) contributed to study design, outcome choice and review of the report.
Nigel Arden (https://orcid.org/0000-0002-3452-3382) (Professor in Rheumatic Diseases, University of Oxford) contributed to study design and review of the report.
David Murray (https://orcid.org/0000-0002-0839-3166) (Professor of Orthopaedic Surgery, University of Oxford) contributed to study design (particularly early in grant history), clinical guidance and review of the report. (Note that he did not contribute to analysis or interpretation of data.)
Marion K Campbell (https://orcid.org/0000-0001-5386-4097) (Professor of Health Services Research, University of Aberdeen) contributed to study design, advised on methodological aspects and overall study conduct and contributed to preparation and review of the report.
Publications
Beard DJ, Holt MD, Mullins MM, Malek S, Massa E, Price AJ. Decision making for knee replacement: variation in treatment choice for late stage medical compartment osteoarthritis. Knee 2012;19:886–9.
Beard D, Price A, Cook J, Fitzpatrick R, Carr A, Campbell M, et al. Total or Partial Knee Arthroplasty Trial – TOPKAT: study protocol for a randomised controlled trial. Trials 2013;14:292.
Beard DJ, Davies LJ, Cook JA, MacLennan G, Price A, Kent S, et al. The clinical and cost-effectiveness of total versus partial knee replacement in patients with medial compartment osteoarthritis (TOPKAT): 5-year outcomes of a randomised controlled trial. Lancet 2019;394:746–56.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Beard D, Price A, Cook J, Fitzpatrick R, Carr A, Campbell M, et al. Total or Partial Knee Arthroplasty Trial – TOPKAT: study protocol for a randomised controlled trial. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-292.
- Bennell KL, Hunter DJ, Hinman RS. Management of osteoarthritis of the knee. BMJ 2012;345. https://doi.org/10.1136/bmj.e4934.
- Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull 2013;105:185-99. https://doi.org/10.1093/bmb/lds038.
- Willis-Owen CA, Brust K, Alsop H, Miraldo M, Cobb JP. Unicondylar knee arthroplasty in the UK National Health Service: an analysis of candidacy, outcome and cost efficacy. Knee 2009;16:473-8. https://doi.org/10.1016/j.knee.2009.04.006.
- National Institute for Health and Care Excellence (NICE) . Guideline Scope Hip, Knee and Shoulder Joint Replacement (GID-NG10084) 2018. www.nice.org.uk/guidance/gid-ng10084/documents/draft-scope (accessed December 2018).
- National Joint Registry . National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 15th Annual Report 2018 n.d. https://reports.njrcentre.org.uk/Portals/7/PDFdownloads/NJR%2015th%20Annual%20Report%202018.pdf (accessed January 2019).
- Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am 2004;86:963-74. https://doi.org/10.2106/00004623-200405000-00012.
- Kane RL, Saleh KJ, Wilt TJ, Bershadsky B. The functional outcomes of total knee arthroplasty. J Bone Joint Surg Am 2005;87:1719-24. https://doi.org/10.2106/00004623-200508000-00008.
- Beard DJ, Holt MD, Mullins MM, Malek S, Massa E, Price AJ. Decision making for knee replacement: variation in treatment choice for late stage medial compartment osteoarthritis. Knee 2012;19:886-9. https://doi.org/10.1016/j.knee.2012.05.005.
- Weale AE, Murray DW, Baines J, Newman JH. Radiological changes five years after unicompartmental knee replacement. J Bone Joint Surg Br 2000;82:996-1000. https://doi.org/10.1302/0301-620x.82b7.10466.
- Khan OH, Davies H, Newman JH, Weale AE. Radiological changes ten years after St. Georg Sled unicompartmental knee replacement. Knee 2004;11:403-7. https://doi.org/10.1016/j.knee.2004.07.003.
- Pearse AJ, Hooper GJ, Rothwell A, Frampton C. Survival and functional outcome after revision of a unicompartmental to a total knee replacement: the New Zealand National Joint Registry. J Bone Joint Surg Br 2010;92:508-12. https://doi.org/10.1302/0301-620X.92B4.22659.
- Cameron HU, Jung YB. A comparison of unicompartmental knee replacement with total knee replacement. Orthop Rev 1988;17:983-8.
- Price AJ, Webb J, Topf H, Dodd CA, Goodfellow JW, Murray DW. Oxford Hip and Knee Group . Rapid recovery after oxford unicompartmental arthroplasty through a short incision. J Arthroplasty 2001;16:970-6. https://doi.org/10.1054/arth.2001.25552.
- Brown NM, Sheth NP, Davis K, Berend ME, Lombardi AV, Berend KR, et al. Total knee arthroplasty has higher postoperative morbidity than unicompartmental knee arthroplasty: a multicenter analysis. J Arthroplasty 2012;27:86-90. https://doi.org/10.1016/j.arth.2012.03.022.
- Hassaballa MA, Porteous AJ, Newman JH. Observed kneeling ability after total, unicompartmental and patellofemoral knee arthroplasty: perception versus reality. Knee Surg Sports Traumatol Arthrosc 2004;12:136-9. https://doi.org/10.1007/s00167-003-0376-5.
- Newman JH, Ackroyd CE, Shah NA. Unicompartmental or total knee replacement? Five-year results of a prospective, randomised trial of 102 osteoarthritic knees with unicompartmental arthritis. J Bone Joint Surg Br 1998;80:862-5. https://doi.org/10.1302/0301-620X.80B5.0800862.
- Newman J, Pydisetty RV, Ackroyd C. Unicompartmental or total knee replacement: the 15-year results of a prospective randomised controlled trial. J Bone Joint Surg Br 2009;91:52-7. https://doi.org/10.1302/0301-620X.91B1.20899.
- Dennis D, Komistek R, Scuderi G, Argenson JN, Insall J, Mahfouz M, et al. In vivo three-dimensional determination of kinematics for subjects with a normal knee or a unicompartmental or total knee replacement. J Bone Joint Surg Am 2001;83–A:104-15. https://doi.org/10.2106/00004623-200100022-00008.
- Isaac SM, Barker KL, Danial IN, Beard DJ, Dodd CA, Murray DW. Does arthroplasty type influence knee joint proprioception? A longitudinal prospective study comparing total and unicompartmental arthroplasty. Knee 2007;14:212-17. https://doi.org/10.1016/j.knee.2007.01.001.
- Gill T, Schemitsch EH, Brick GW, Thornhill TS. Revision total knee arthroplasty after failed unicompartmental knee arthroplasty or high tibial osteotomy. Clin Orthop Relat Res 1995;321:10-8. https://doi.org/10.1097/00003086-199512000-00003.
- Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am 1991;73:186-90. https://doi.org/10.2106/00004623-199173020-00005.
- Saldanha KA, Keys GW, Svard UC, White SH, Rao C. Revision of Oxford medial unicompartmental knee arthroplasty to total knee arthroplasty - results of a multicentre study. Knee 2007;14:275-9. https://doi.org/10.1016/j.knee.2007.03.005.
- Cameron HU, Park YS. Total knee replacement following high tibial osteotomy and unicompartmental knee. Orthopedics 1996;19:807-8. https://doi.org/10.3928/0147-7447-19960901-30.
- Jackson M, Sarangi PP, Newman JH. Revision total knee arthroplasty. Comparison of outcome following primary proximal tibial osteotomy or unicompartmental arthroplasty. J Arthroplasty 1994;9:539-42. https://doi.org/10.1016/0883-5403(94)90102-3.
- Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee 2007;14:154-7. https://doi.org/10.1016/j.knee.2006.11.012.
- Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty 1996;11:797-801. https://doi.org/10.1016/S0883-5403(96)80179-3.
- Myers TG, Cui Q, Kuskowski M, Mihalko WM, Saleh KJ. Outcomes of total and unicompartmental knee arthroplasty for secondary and spontaneous osteonecrosis of the knee. J Bone Joint Surg Am 2006;88:76-82. https://doi.org/10.2106/00004623-200611001-00012.
- Fisher DA, Watts M, Davis KE. Implant position in knee surgery: a comparison of minimally invasive, open unicompartmental, and total knee arthroplasty. J Arthroplasty 2003;18:2-8. https://doi.org/10.1016/S0883-5403(03)00291-2.
- Jenny JY, Boeri C. Accuracy of implantation of a unicompartmental total knee arthroplasty with 2 different instrumentations: a case-controlled comparative study. J Arthroplasty 2002;17:1016-20. https://doi.org/10.1054/arth.2002.34524.
- Manzotti A, Confalonieri N, Pullen C. Unicompartmental versus computer-assisted total knee replacement for medial compartment knee arthritis: a matched paired study. Int Orthop 2007;31:315-19. https://doi.org/10.1007/s00264-006-0184-x.
- Engh GA, Dwyer KA, Hanes CK. Polyethylene wear of metal-backed tibial components in total and unicompartmental knee prostheses. J Bone Joint Surg Br 1992;74:9-17. https://doi.org/10.1302/0301-620X.74B1.1732274.
- Weale AE, Murray DW, Newman JH, Ackroyd CE. The length of the patellar tendon after unicompartmental and total knee replacement. J Bone Joint Surg Br 1999;81:790-5. https://doi.org/10.1302/0301-620x.81b5.9590.
- Murray DW, MacLennan GS, Breeman S, Dakin HA, Johnston L, Campbell MK, et al. A randomised controlled trial of the clinical effectiveness and cost-effectiveness of different knee prostheses: the Knee Arthroplasty Trial (KAT). Health Technol Assess 2014;18. https://doi.org/10.3310/hta18190.
- Amin AK, Patton JT, Cook RE, Gaston M, Brenkel IJ. Unicompartmental or total knee arthroplasty?: Results from a matched study. Clin Orthop Relat Res 2006;451:101-6. https://doi.org/10.1097/01.blo.0000224052.01873.20.
- Laurencin CT, Zelicof SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. A comparative study. Clin Orthop Relat Res 1991;273:151-6. https://doi.org/10.1097/00003086-199112000-00023.
- Soohoo NF, Sharifi H, Kominski G, Lieberman JR. Cost-effectiveness analysis of unicompartmental knee arthroplasty as an alternative to total knee arthroplasty for unicompartmental osteoarthritis. J Bone Joint Surg Am 2006;88:1975-82. https://doi.org/10.2106/00004623-200609000-00011.
- Slover J, Espehaug B, Havelin LI, Engesaeter LB, Furnes O, Tomek I, et al. Cost-effectiveness of unicompartmental and total knee arthroplasty in elderly low-demand patients. A Markov decision analysis. J Bone Joint Surg Am 2006;88:2348-55. https://doi.org/10.2106/00004623-200611000-00005.
- Costa CR, Johnson AJ, Mont MA, Bonutti PM. Unicompartmental and total knee arthroplasty in the same patient. J Knee Surg 2011;24:273-8. https://doi.org/10.1055/s-0031-1280970.
- Sun PF, Jia YH. Mobile bearing UKA compared to fixed bearing TKA: a randomized prospective study. Knee 2012;19:103-6. https://doi.org/10.1016/j.knee.2011.01.006.
- Kulshrestha V, Datta B, Kumar S, Mittal G. Outcome of Unicondylar Knee Arthroplasty vs Total Knee Arthroplasty for Early Medial Compartment Arthritis: A Randomized Study. J Arthroplasty 2017;32:1460-9. https://doi.org/10.1016/j.arth.2016.12.014.
- ClinicalTrials.gov . Unicompartmental Knee Arthroplasty (UKA) Versus Total Knee Arthroplasty (TKA) of Medial Osteoarthritis n.d. www.ClinicalTrials.gov/show/NCT03457051 (accessed December 2018).
- ClinicalTrials.gov . Finnish Unicompartmental and Total Knee Arthroplasty Investigation (FUNCTION) n.d. www.clinicaltrials.gov/ct2/show/NCT02481427 (accessed 18 December 2018).
- ClinicalTrials.gov . Medial Unicondylar Knee Arthroplasty Vs Total Knee Arthroplasty n.d. www.clinicaltrials.gov/ct2/show/NCT03396640 (accessed December 2018).
- ClinicalTrials.gov . Unicondylar Knee Arthroplasty Versus Total Knee Arthroplasty in Patients With Anteromedial Osteoarthritis of the Knee n.d. https://clinicaltrials.gov/ct2/show/NCT02430129 (accessed March 2020).
- ClinicalTrials.gov . Unicondylar- or Total Knee Replacement? Patient Satisfaction, Function and Muscle Mass n.d. www.clinicaltrials.gov/ct2/show/NCT02563756 (accessed December 2018).
- Arirachakaran A, Choowit P, Putananon C, Muangsiri S, Kongtharvonskul J. Is unicompartmental knee arthroplasty (UKA) superior to total knee arthroplasty (TKA)? A systematic review and meta-analysis of randomized controlled trial. Eur J Orthop Surg Traumatol 2015;25:799-806. https://doi.org/10.1007/s00590-015-1610-9.
- Schwab PE, Lavand’homme P, Yombi JC, Thienpont E. Lower blood loss after unicompartmental than total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2015;23:3494-500. https://doi.org/10.1007/s00167-014-3188-x.
- Siman H, Kamath AF, Carrillo N, Harmsen WS, Pagnano MW, Sierra RJ. Unicompartmental knee arthroplasty vs total knee arthroplasty for medial compartment arthritis in patients older than 75 years: comparable reoperation, revision, and complication rates. J Arthroplasty 2017;32:1792-7. https://doi.org/10.1016/j.arth.2017.01.020.
- Bolognesi MP, Greiner MA, Attarian DE, Watters TS, Wellman SS, Curtis LH, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among Medicare beneficiaries, 2000 to 2009. J Bone Joint Surg Am 2013;95. https://doi.org/10.2106/JBJS.L.00652.
- Duchman KR, Gao Y, Pugely AJ, Martin CT, Callaghan JJ. Differences in short-term complications between unicompartmental and total knee arthroplasty: a propensity score matched analysis. J Bone Joint Surg Am 2014;96:1387-94. https://doi.org/10.2106/JBJS.M.01048.
- Drager J, Hart A, Khalil JA, Zukor DJ, Bergeron SG, Antoniou J. Shorter hospital stay and lower 30-day readmission after unicondylar knee arthroplasty compared to total knee arthroplasty. J Arthroplasty 2016;31:356-61. https://doi.org/10.1016/j.arth.2015.09.014.
- Burn E, Liddle AD, Hamilton TW, Pai S, Pandit HG, Murray DW, et al. Choosing between unicompartmental and total knee replacement: what can economic evaluations tell us? A systematic review. Pharmacoecon Open 2017;1:241-53. https://doi.org/10.1007/s41669-017-0017-4.
- Burn E, Liddle AD, Hamilton TW, Judge A, Pandit HG, Murray DW, et al. Cost-effectiveness of unicompartmental compared with total knee replacement: a population-based study using data from the National Joint Registry for England and Wales. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-020977.
- Wiik AV, Aqil A, Tankard S, Amis AA, Cobb JP. Downhill walking gait pattern discriminates between types of knee arthroplasty: improved physiological knee functionality in UKA versus TKA. Knee Surg Sports Traumatol Arthrosc 2015;23:1748-55. https://doi.org/10.1007/s00167-014-3240-x.
- Fabre-Aubrespy M, Ollivier M, Pesenti S, Parratte S, Argenson JN. Unicompartmental knee arthroplasty in patients older than 75 results in better clinical outcomes and similar survivorship compared to total knee arthroplasty. A matched controlled study. J Arthroplasty 2016;31:2668-71. https://doi.org/10.1016/j.arth.2016.06.034.
- Lum ZC, Lombardi AV, Hurst JM, Morris MJ, Adams JB, Berend KR. Early outcomes of twin-peg mobile-bearing unicompartmental knee arthroplasty compared with primary total knee arthroplasty. Bone Joint J 2016;98-B:28-33. https://doi.org/10.1302/0301-620X.98B10.BJJ-2016-0414.R1.
- Zuiderbaan HA, van der List JP, Khamaisy S, Nawabi DH, Thein R, Ishmael C, et al. Unicompartmental knee arthroplasty versus total knee arthroplasty: which type of artificial joint do patients forget?. Knee Surg Sports Traumatol Arthrosc 2017;25:681-6. https://doi.org/10.1007/s00167-015-3868-1.
- Burn E, Sanchez-Santos MT, Pandit HG, Hamilton TW, Liddle AD, Murray DW, et al. Ten-year patient-reported outcomes following total and minimally invasive unicompartmental knee arthroplasty: a propensity score-matched cohort analysis. Knee Surg Sports Traumatol Arthrosc 2018;26:1455-64. https://doi.org/10.1007/s00167-016-4404-7.
- Kim MS, Koh IJ, Choi YJ, Lee JY, In Y. Differences in patient-reported outcomes between unicompartmental and total knee arthroplasties: a propensity score-matched analysis. J Arthroplasty 2017;32:1453-9. https://doi.org/10.1016/j.arth.2016.11.034.
- Liddle AD, Judge A, Pandit H, Murray DW. Adverse outcomes after total and unicompartmental knee replacement in 101,330 matched patients: a study of data from the National Joint Registry for England and Wales. Lancet 2014;384:1437-45. https://doi.org/10.1016/S0140-6736(14)60419-0.
- Chawla H, van der List JP, Christ AB, Sobrero MR, Zuiderbaan HA, Pearle AD. Annual revision rates of partial versus total knee arthroplasty: a comparative meta-analysis. Knee 2017;24:179-90. https://doi.org/10.1016/j.knee.2016.11.006.
- Migliorini F, Tingart M, Niewiera M, Rath B, Eschweiler J. Unicompartmental versus total knee arthroplasty for knee osteoarthritis. Eur J Orthop Surg Traumatol 2019;29:947-55. https://doi.org/10.1007/s00590-018-2358-9.
- Cook JA, Elders A, Boachie C, Bassinga T, Fraser C, Altman DG, et al. A systematic review of the use of an expertise-based randomised controlled trial design. Trials 2015;16. https://doi.org/10.1186/s13063-015-0739-5.
- Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br 1998;80:63-9. https://doi.org/10.1302/0301-620x.80b1.7859.
- Beard DJ, Harris K, Dawson J, Doll H, Murray DW, Carr AJ, et al. Meaningful changes for the Oxford hip and knee scores after joint replacement surgery. J Clin Epidemiol 2015;68:73-9. https://doi.org/10.1016/j.jclinepi.2014.08.009.
- Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res 1989;248:13-4. https://doi.org/10.1097/00003086-198911000-00004.
- Martimbianco AL, Calabrese FR, Iha LA, Petrilli M, Lira Neto O, Carneiro Filho M. Reliability of the ‘American Knee Society Score’ (AKSS). Acta Ortop Bras 2012;20:34-8. https://doi.org/10.1590/S1413-78522012000100007.
- Zahiri CA, Schmalzried TP, Szuszczewicz ES, Amstutz HC. Assessing activity in joint replacement patients. J Arthroplasty 1998;13:890-5. https://doi.org/10.1016/S0883-5403(98)90195-4.
- Talbot S, Hooper G, Stokes A, Zordan R. Use of a new high-activity arthroplasty score to assess function of young patients with total hip or knee arthroplasty. J Arthroplasty 2010;25:268-73. https://doi.org/10.1016/j.arth.2008.09.019.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Robertsson O, Dunbar M, Pehrsson T, Knutson K, Lidgren L. Patient satisfaction after knee arthroplasty: a report on 27,372 knees operated on between 1981 and 1995 in Sweden. Acta Orthop Scand 2000;71:262-7. https://doi.org/10.1080/000164700317411852.
- Dawson J, Beard DJ, McKibbin H, Harris K, Jenkinson C, Price AJ. Development of a patient-reported outcome measure of activity and participation (the OKS-APQ) to supplement the Oxford knee score. Bone Joint J 2014;96–B:332-8. https://doi.org/10.1302/0301-620X.96B3.32845.
- Murray DW, Fitzpatrick R, Rogers K, Pandit H, Beard DJ, Carr AJ, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br 2007;89:1010-14. https://doi.org/10.1302/0301-620X.89B8.19424.
- Cook JA, Ramsay CR, Fayers P. Statistical evaluation of learning curve effects in surgical trials. Clin Trials 2004;1:421-7. https://doi.org/10.1191/1740774504cn042oa.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2016 to 2017 2017. https://improvement.nhs.uk/resources/reference-costs/#archive (accessed September 2018).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Information Services Division (ISD) . Scottish Health Service Costs 2018. www.isdscotland.org/Health-Topics/Finance/Publications/2018-11-20/2018-11-20-Costs-Report.pdf (accessed September 2018).
- Stokes EA, Wordsworth S, Staves J, Mundy N, Skelly J, Radford K, et al. Accurate costs of blood transfusion: a microcosting of administering blood products in the United Kingdom National Health Service. Transfusion 2018;58:846-53. https://doi.org/10.1111/trf.14493.
- World Health Organization (WHO) . International Classification of Diseases 2016.
- NHS Digital . Costing – HRG4+ 2016 17. Reference Costs Grouper 2018. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/grouper-and-tools-archive/costing-hrg4-2016-17-reference-costs-grouper (accessed September 2018).
- NHS Improvement . Reference Costs 2017 18: Highlights, Analysis and Introduction to the Data 2018. https://improvement.nhs.uk/documents/1972/1_-_Reference_costs_201718.pdf (accessed September 2018).
- Leal J, Manetti S, Buchanan J. The impact of hospital costing methods on cost-effectiveness analysis: a case study. PharmacoEconomics 2018;36:1263-72. https://doi.org/10.1007/s40273-018-0673-y.
- Office of Population Censuses and Surveys . OPCS Classification of Surgical Operations and Procedures, Fourth Revision 1992.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed September 2018).
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- Skou ST, Roos EM, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O, et al. A randomized, controlled trial of total knee replacement. N Engl J Med 2015;373:1597-606. https://doi.org/10.1056/NEJMoa1505467.
- Liddle AD, Pandit H, Judge A, Murray DW. Patient-reported outcomes after total and unicompartmental knee arthroplasty: a study of 14,076 matched patients from the National Joint Registry for England and Wales. Bone Joint J 2015;97–B:793-801. https://doi.org/10.1302/0301-620X.97B6.35155.
- Wilson HA, Middleton R, Abram SGF, Smith S, Alvand A, Jackson WF, et al. Patient relevant outcomes of unicompartmental versus total knee replacement: systematic review and meta-analysis. BMJ 2019;364. https://doi.org/10.1136/bmj.l352.
- Hunt LP, Ben-Shlomo Y, Clark EM, Dieppe P, Judge A, MacGregor AJ, et al. 45-day mortality after 467,779 knee replacements for osteoarthritis from the National Joint Registry for England and Wales: an observational study. Lancet 2014;384:1429-36. https://doi.org/10.1016/S0140-6736(14)60540-7.
- Rothwell AG, Hooper GJ, Hobbs A, Frampton CM. An analysis of the Oxford hip and knee scores and their relationship to early joint revision in the New Zealand Joint Registry. J Bone Joint Surg Br 2010;92:413-18. https://doi.org/10.1302/0301-620X.92B3.22913.
- Zhang Q, Zhang Q, Guo W, Liu Z, Cheng L, Yue D, et al. The learning curve for minimally invasive Oxford phase 3 unicompartmental knee arthroplasty: cumulative summation test for learning curve (LC-CUSUM). J Orthop Surg Res 2014;9. https://doi.org/10.1186/s13018-014-0081-8.
- Liddle AD, Pandit H, Judge A, Murray DW. Optimal usage of unicompartmental knee arthroplasty: a study of 41,986 cases from the National Joint Registry for England and Wales. Bone Joint J 2015;97–B:1506-11. https://doi.org/10.1302/0301-620X.97B11.35551.
- Liddle AD, Pandit H, Judge A, Murray DW. Effect of surgical caseload on revision rate following total and unicompartmental knee replacement. J Bone Joint Surg Am 2016;98:1-8. https://doi.org/10.2106/JBJS.N.00487.
- Baker P, Jameson S, Critchley R, Reed M, Gregg P, Deehan D. Center and surgeon volume influence the revision rate following unicondylar knee replacement: an analysis of 23,400 medial cemented unicondylar knee replacements. J Bone Joint Surg Am 2013;95:702-9. https://doi.org/10.2106/JBJS.L.00520.
- Reilly KA, Beard DJ, Barker KL, Dodd CA, Price AJ, Murray DW. Efficacy of an accelerated recovery protocol for Oxford unicompartmental knee arthroplasty – a randomised controlled trial. Knee 2005;12:351-7. https://doi.org/10.1016/j.knee.2005.01.002.
- Liddle AD, Judge A, Pandit H, Murray DW. Determinants of revision and functional outcome following unicompartmental knee replacement. Osteoarthritis Cartilage 2014;22:1241-50. https://doi.org/10.1016/j.joca.2014.07.006.
- Hobbs FDR, Bankhead C, Mukhtar T, Stevens S, Perera-Salazar R, Holt T, et al. National Institute for Health Research School for Primary Care Research . Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007–14. Lancet 2016;387:2323-30. https://doi.org/10.1016/S0140-6736(16)00620-6.
- The Information Centre, Primary Care Statistics . 2006/07/UK/General/Practice/Workload/Survey 2007.
- National Joint Registry . National Joint Registry for England and Wales: 9th Annual Report. 2012. Prostheses Used in Hip, Knee, Ankle, Elbow and Shoulder Replacement Procedures 2011 n.d. www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/Prostheses%20used%20in%20hip%20knee%20and%20ankle%20replacements%202011.pdf (accessed November 2018).
- National Joint Registry . National Joint Registry for England and Wales: 11th Annual Report. 2014. Prostheses Used in Hip, Knee, Ankle, Elbow and Shoulder Replacement Procedures 2013 n.d. www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/11th_annual_report/NJR%2011th%20AR%20Prostheses%20used%20in%20hip,%20knee,%20ankle,%20elbow%20and%20shoulder%20replacements%202013.pdf (accessed November 2018).
- National Joint Registry . National Joint Registry For England And Wales: 10th Annual Report. 2013. Prostheses Used in Hip, Knee, Ankle, Elbow and Shoulder Replacement Procedures 2012 n.d. www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/10th_annual_report/NJR%20Prostheses%20used%20in%20hip,%20knee,%20ankle,%20shoulder%20and%20elbow%20replacements%202012.pdf (accessed November 2018).
- National Joint Registry . National Joint Registry For England And Wales: 8th Annual Report. 2011. Prostheses Used in Hip, Knee, Ankle, Elbow and Shoulder Replacement Procedures 2010 n.d. www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/Prostheses%20used%20in%20hip%20knee%20and%20ankle%20replacements%202010.pdf (accessed November 2018).
Appendix 1 Changes to TOPKAT protocol
| Protocol version number | Date issued | Details of changes made |
|---|---|---|
| 1 | 9 June 2009 | Original protocol |
| 2 | 10 June 2010 | Inclusion of the HAAS to the secondary outcome measures |
| Revision of criteria on surgeon/site inclusion form | ||
| 3 | 3 June 2012 | Inclusion of additional OKS questionnaire at 1 year post surgery for participants with a time between randomisation and surgery > 12 weeks |
| Revision of primary procedure hospital form and inclusion of re-admission form | ||
| Clarity given to the inclusion and exclusion criteria | ||
| 4 | 4 April 2014 | Inclusion of the OKS-APQ to the secondary outcome measures |
| Clarity given to the reporting procedures for serious adverse events and post-surgical complications. Inclusion of post-operative event non-re-admission form |
Appendix 2 The CONSORT time points 2 months to 5 years
| Time point (post randomisation unless stated otherwise) | PKR (n) | TKR (n) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Questionnaire | Primary analysis | Clinical assessments | Withdrawn | Deceased | Questionnaire | Primary analysis | Clinical assessments | Withdrawn | Deceased | |||
| Responded | Non-response | Responded | Non-response | |||||||||
| 2 months (post surgery) | 249 | 14 | 247 | 254 | 0 | 0 | 239 | 12 | 239 | 244 | 0 | 0 |
| 1 year | 248 | 15 | 247 | 239 | 1 | 0 | 246 | 14 | 244 | 238 | 4 | 2 |
| 1 year (post surgery) | 54 | – | 54 | – | 2 | 0 | 40 | – | 40 | – | 4 | 1 |
| 2 years | 240 | 21 | 240 | – | 2 | 1 | 238 | 19 | 238 | – | 4 | 3 |
| 3 years | 219 | 41 | 218 | – | 3 | 2 | 228 | 27 | 226 | – | 6 | 3 |
| 4 years | 209 | 49 | 205 | – | 4 | 3 | 219 | 34 | 219 | – | 7 | 4 |
| 5 years | 235 | 18 | 233 | 201 | 5 | 6 | 232 | 11 | 231 | 196 | 10 | 11 |
Appendix 3 Suspected complications
| Measure | PKR (N = 264), n (%) | TKR (N = 264), n (%) |
|---|---|---|
| Received surgery | 263 | 251 |
| Postoperative | ||
| Number of participants with complications | 0 | 2 (0.8) |
| Thrombolytic | – | 2 |
| Required re-admission | ||
| Required medical treatment only | ||
| Number of participants | 2 (0.8) | 4 (1.6) |
| Number of complications | 2 | 5 |
| Details of complications | ||
| DVT | 1 | 4 |
| Infection | 1 | – |
| DVT and infection | – | 1 |
| Did not require re-admission | ||
| 2-month follow-up | ||
| Number of participants and complications | 2 (0.8) | 2 (0.8) |
| Infection | – | 1 |
| DVT | 2 | 1 |
| 1-year follow-up | ||
| Number of participants and complications | – | 1 (0.4) |
| DVT | – | 1 |
Appendix 4 Health-care costs
| Type of implant | PKR (n) | TKR (n) | Cost per device (£) |
|---|---|---|---|
| PKR | |||
| Oxford Partial Knee | 150 | 7 | 812.73 |
| Zimmer | 36 | 4 | 993.23 |
| M/G Unicompartmental Knee System | 22 | 1 | 988.07 |
| Uniglide | 9 | 0 | 633.27 |
| AMC | 5 | 0 | 1082.96 |
| Du Puy | 4 | 0 | 896.28 |
| Mathys | 4 | 0 | 931.34 |
| Mecdata | 1 | 0 | 930.31 |
| Sigma | 1 | 0 | 896.28 |
| Vanguard | 0 | 1 | 1310.89 |
| TKR | |||
| Low Contact Stress | 10 | 61 | 1441.88 |
| PFC/Sigma | 3 | 54 | 1047.89 |
| Vanguard | 3 | 41 | 1310.89 |
| NexGen | 8 | 29 | 1480.04 |
| Triathlon | 4 | 27 | 1291.30 |
| Genesis | 2 | 7 | – |
| Scorpio/Kinemax | 1 | 7 | 1528.52 |
| ACS | 0 | 6 | 650.81 |
| EUROS | 0 | 2 | – |
| AGC | 0 | 1 | 1099.46 |
| AllPoly | 0 | 1 | – |
| Oxinium xlpe poly | 0 | 1 | – |
| Unknown | 0 | 1 | – |
| None | 1 | 13 | 0 |
| Resource type | Unit cost (£) | Details |
|---|---|---|
| Theatre time (per minute) | 17.03 | Table R142X from Scottish cost tables79 |
| Blood transfusion | 49.00 | Red blood cell costs from a bottom-up UK costing study80 |
| Hospital bed-day | 327.61 | Weighted average cost per excess bed data for elective HRG HN22 (major knee procedures) from the NHS Reference Costs 2016–17,77 where the weights reflect national activity levels |
| Day in critical care | 699.11 | From NHS Reference Costs 2016–17,77 currency code CCU02 (i.e. surgical adult patient, unspecified specialty, with zero organs supported) |
| GP consultation | 38.00 | Annual cost from table 10.3b of Unit Costs of Health and Social Care,78 and an average duration of consultation of 9.22 minutes100 |
| Practice nurse consultation | 15.50 | Annual cost from table 10.2 of Unit Costs of Health and Social Care78 and average duration of contact of 15.50 minutes101 |
| Physiotherapist or occupational therapist appointment | 50.74 | Weighted average of costs of physiotherapy and occupational therapy appointments from NHS Reference Cost Schedule 2016–1777 (service codes 650–651) for non-admitted patient follow-up face-to-face attendances, non-consultant led, and community health-care services (allied health-care professionals, physiotherapists and occupational therapists, adults only) |
| Outpatient appointments | 133.05 | Non-admitted patient follow-up face-to-face attendance from NHS Reference Cost Schedule 2016–1777 for rheumatology (service code 410). Weighted average of consultant and non-consultant appointments and procedures |
| Complication | n | ICD-10 | HRG code | HRG description | Cost (£) |
|---|---|---|---|---|---|
| Acute respiratory failure | 1 | J960 | DZ27 | Respiratory failure | 1533.20 |
| Atrial fibrillation | 1 | I489 | EB07 | Arrhythmia or conduction disorders | 1239.46 |
| Acute kidney injury | 2 | N179 | LA07 | Acute kidney injury | 2261.14 |
| Chest infection | 2 | J22X | DZ22 | Unspecified acute lower respiratory infection | 2344.26 |
| Dural tear | 1 | G961 | AA25 | Cerebral degenerations or miscellaneous disorders of the nervous system | 2412.83 |
| DVT | 1 | I829 | YQ51 | DVT | 928.44 |
| Hyponatraemia | 1 | E871 | KC05 | Fluid or electrolyte disorders | 1546.12 |
| Hypoxaemia | 3 | J960 | DZ27 | Respiratory failure | – |
| PE | 3 | I269 | DZ09 | Pulmonary embolus | 1493.75 |
| Pneumonia | 1 | J198 | DZ11 | Lobar, atypical or viral pneumonia | 2952.03 |
| Pyrexia | 1 | R509 | WJ07 | Fever of unknown origin | 1476.70 |
| Urinary retention | 1 | R398 | LB37 | Miscellaneous urinary tract findings | 1404.79 |
| Unallocateda | 5 | – | – | – | – |
| Complication | n | ICD-10 | ICD-10 description |
|---|---|---|---|
| Unexplained pain | 28 | M255 | Pain in joint |
| Knee stiffness | 16 | M256 | Stiffness of joint |
| Bearing dislocation | 7 | T840 | Mechanical complication of internal joint prosthesis |
| Suspected DVT | 5 | I829 | Embolism and thrombosis of unspecified vein |
| Device loosening (tibia) | 3 | T840 | Mechanical complication of internal joint prosthesis |
| Infection | 2 | T845 | Infection and inflammatory reaction due to internal joint prosthesis |
| Suspected infection | 2 | A499 | Bacterial infection, unspecified |
| Ligamentous instability | 2 | M235 | Chronic instability of knee |
| Wound breakdown | 2 | T813 | Disruption of operation wound, not elsewhere classified |
| Mechanical failure | 2 | T840 | Mechanical complication of internal joint prosthesis |
| Renal and urological problems | 2 | N298 | Other disorders of kidney and ureter in other diseases classified elsewhere |
| Treated DVT or PE | 1 | I269 | Pulmonary embolism without mention of acute cor pulmonale |
| Cellulitis | 1 | L039 | Cellulitis, unspecified |
| Periprosthetic fracture | 1 | M966 | Fracture of bone following insertion of orthopaedic implant, joint prosthesis, or bone plate |
| Bronchopneumonia | 1 | J180 | Bronchopneumonia, unspecified |
| Pain from trauma | 1 | S899 | Unspecified injury of lower leg |
| Superficial infection | 1 | – | – |
| Swelling | 1 | M254 | Effusion of joint |
| Skin complications | 1 | – | – |
| Treatment | n | OPCS-4 | OPCS-4 description |
|---|---|---|---|
| Revisiona | 20 | O183 | Revision of hybrid prosthetic replacement of knee joint using cement |
| Manipulation under anaesthetic | 13 | W789 | Unspecified release of contracture of joint |
| Arthroscopy | 7 | W852 | Endoscopic irrigation of knee joint |
| Aspiration | 4 | W901; Z846 | Aspiration of joint; knee joint |
| Debridement/exploration/washout | 4 | W851 | Endoscopic removal of loose body from knee joint |
| Patella resurfacing | 3 | W851; Z787 | Endoscopic removal of loose body from knee joint; patella |
| Polyethylene exchange | 3 | W424; Y036; Z949 | Attention to total prosthetic replacement of knee joint NEC; adjustment to prosthesis in organ NOC; other branch of thoracic aorta NEC |
| Open reduction and internal fixation of avulsion fracture (tibial tuberosity) | 1 | W201 | Primary open reduction of fracture of long bone and extramedullary fixation using plate NEC |
| Marcain injection | 2 | W903 | Injection of therapeutic substance into joint |
| Wound debridement/management | 1 | S571 | Debridement of skin NEC |
| Examination under anaesthetic | 1 | W789 | Unspecified release of contracture of joint |
| Biopsy | 1 | W871 | Diagnostic endoscopic examination of knee joint and biopsy of lesion of knee joint |
| Partial medial meniscectomy | 1 | W822 | Endoscopic resection of semilunar cartilage NEC |
| HRG code | HRG description | n | Cost (£) |
|---|---|---|---|
| HD24 | Non-inflammatory bone or joint disorders | 27 | 1235.47 |
| HN81 | Complex hip or knee procedures for non-trauma | 18 | 9336.47 |
| HN24 | Intermediate knee procedures for non-trauma | 4 | 1960.64 |
| HN25 | Minor knee procedures for non-trauma | 4 | 1579.58 |
| YH30 | Image guided aspiration of joint | 4 | 825.34 |
| HN80 | Very complex hip or knee procedures for non-trauma | 2 | 14,174.96 |
| HN93 | Other muscle, tendon, fascia or ligament procedures | 2 | 1614.49 |
| JC42 | Intermediate skin procedures | 1 | 1120.93 |
Appendix 5 Economic analysis
| Dimension and level | Baseline | 2 months | 1 year | 2 years | 3 years | 4 years | 5 years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PKR | TKR | PKR | TKR | PKR | TKR | PKR | TKR | PKR | TKR | PKR | TKR | PKR | TKR | |
| Mobility (% responses) | ||||||||||||||
| No problems | 7 | 9 | 44 | 36 | 54 | 48 | 59 | 50 | 48 | 48 | 46 | 48 | 51 | 47 |
| Some problems | 91 | 87 | 47 | 53 | 37 | 41 | 30 | 42 | 34 | 37 | 33 | 36 | 37 | 37 |
| Extreme problems | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Missing | 3 | 5 | 10 | 11 | 9 | 11 | 11 | 8 | 18 | 14 | 21 | 16 | 13 | 16 |
| Self-care (% responses) | ||||||||||||||
| No problems | 65 | 66 | 76 | 74 | 80 | 72 | 77 | 75 | 71 | 68 | 67 | 66 | 73 | 66 |
| Some problems | 33 | 29 | 14 | 16 | 11 | 17 | 12 | 17 | 11 | 18 | 12 | 18 | 14 | 18 |
| Extreme problems | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Missing | 3 | 5 | 10 | 11 | 9 | 11 | 11 | 8 | 18 | 14 | 21 | 16 | 13 | 16 |
| Usual activities (% responses) | ||||||||||||||
| No problems | 14 | 11 | 35 | 30 | 51 | 45 | 56 | 48 | 48 | 47 | 49 | 45 | 51 | 47 |
| Some problems | 76 | 77 | 49 | 54 | 38 | 41 | 31 | 39 | 32 | 37 | 27 | 35 | 33 | 32 |
| Extreme problems | 8 | 7 | 6 | 5 | 2 | 3 | 2 | 5 | 3 | 2 | 3 | 4 | 4 | 4 |
| Missing | 3 | 5 | 10 | 11 | 9 | 11 | 11 | 8 | 18 | 14 | 21 | 16 | 13 | 16 |
| Pain (% responses) | ||||||||||||||
| No problems | 1 | 1 | 19 | 13 | 36 | 30 | 41 | 36 | 35 | 35 | 36 | 35 | 42 | 38 |
| Some problems | 62 | 51 | 67 | 72 | 51 | 53 | 44 | 48 | 43 | 44 | 40 | 42 | 39 | 37 |
| Extreme problems | 35 | 43 | 5 | 5 | 3 | 5 | 5 | 7 | 4 | 7 | 3 | 7 | 7 | 9 |
| Missing | 3 | 5 | 10 | 11 | 9 | 11 | 11 | 8 | 18 | 14 | 21 | 16 | 13 | 16 |
| Anxiety/depression (% responses) | ||||||||||||||
| No problems | 60 | 56 | 69 | 63 | 72 | 69 | 71 | 67 | 68 | 66 | 63 | 65 | 67 | 64 |
| Some problems | 33 | 34 | 19 | 24 | 17 | 18 | 15 | 23 | 13 | 16 | 15 | 16 | 17 | 17 |
| Extreme problems | 4 | 6 | 3 | 2 | 2 | 2 | 3 | 2 | 1 | 4 | 2 | 4 | 3 | 3 |
| Missing | 3 | 5 | 10 | 11 | 9 | 11 | 11 | 8 | 18 | 14 | 21 | 16 | 13 | 16 |
| Follow-up time | EQ-5D utility, mean (SD) | Health-care costs,a mean (SD) | Total costs, mean (SD) | |||
|---|---|---|---|---|---|---|
| PKR | TKR | PKR | TKR | PKR | TKR | |
| 2 months | 0.428 (0.545) | 0.381 (0.563) | 423 (17) | 450 (19) | 651 (46) | 508 (41) |
| 1 year | 0.680 (0.499) | 0.656 (0.488) | 292 (20) | 339 (18) | 4018 (50) | 4622 (63) |
| 2 years | 0.759 (0.499) | 0.711 (0.521) | 221 (17) | 379 (24) | 422 (48) | 501 (54) |
| 3 years | 0.763 (0.518) | 0.715 (0.532) | 93 (13) | 144 (18) | 240 (39) | 397 (64) |
| 4 years | 0.766 (0.501) | 0.723 (0.539) | 59 (14) | 86 (16) | 225 (57) | 342 (70) |
| 5 years | 0.772 (0.498) | 0.724 (0.542) | 54 (14) | 83 (16) | 396 (67) | 294 (53) |
Appendix 6 Distribution of knee replacement implants
The study was pragmatic in terms of implant selection for the knee replacement operation. The distribution of implants used in the study is detailed in Tables 37 and 38. Data on implant use in clinical practice over the recruitment period of the trial, obtained from the NJR,102–105 is also presented in Tables 39 and 40.
| PKR implants used in TOPKAT | N = 245, n | % |
|---|---|---|
| Oxford Partial Knee | 157 | 64.1 |
| Zimmer unicompartmental | 40 | 16.3 |
| M/G Unicompartmental Knee System | 23 | 9.4 |
| Uniglide | 9 | 3.7 |
| AMC | 5 | 2 |
| DePuy | 4 | 1.6 |
| Mathys unicondylar knee | 4 | 1.6 |
| Sigma HP | 1 | 0.4 |
| Medacta | 1 | 0.4 |
| Vanguard | 1 | 0.4 |
FIGURE 7.
The PKR implants used in TOPKAT from 2010 to 2014.
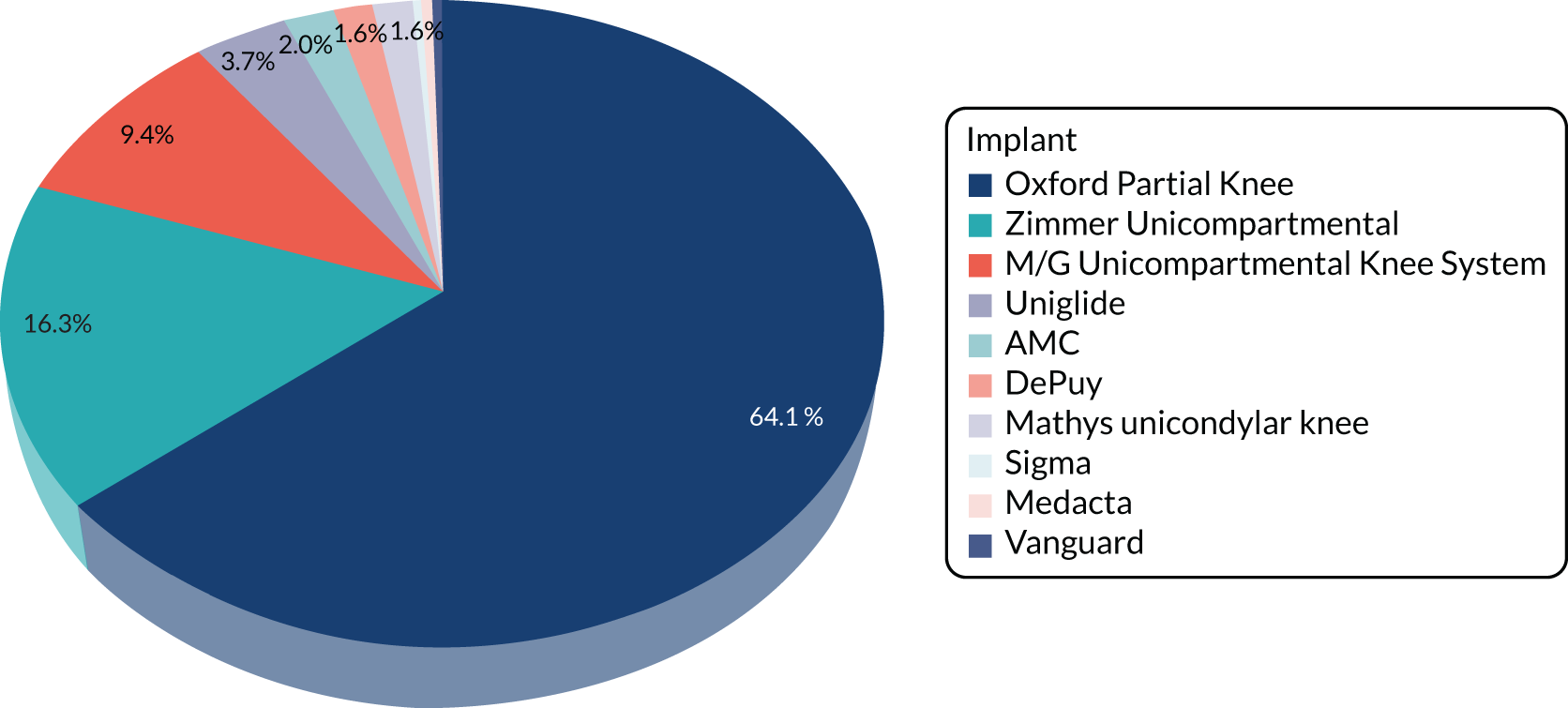
| TKR implants TOPKAT | N = 269, n | % |
|---|---|---|
| Low Contact Stress complete | 71 | 26.4 |
| PFC/sigma bicondylar knee | 51 | 21.2 |
| Vanguard | 44 | 16.4 |
| NexGen | 37 | 13.8 |
| Triathlon | 31 | 11.5 |
| Scorpio/Kinemax | 8 | 3.0 |
| Genesis | 9 | 3.3 |
| ACS | 6 | 2.2 |
| AGC | 1 | 0.4 |
| Euros bicondylar | 1 | 0.4 |
| AllPoly | 1 | 0.4 |
| Oxinium | 1 | 0.4 |
| Unknown | 1 | 0.4 |
FIGURE 8.
The TKR implants used in TOPKAT from 2010 to 2014.
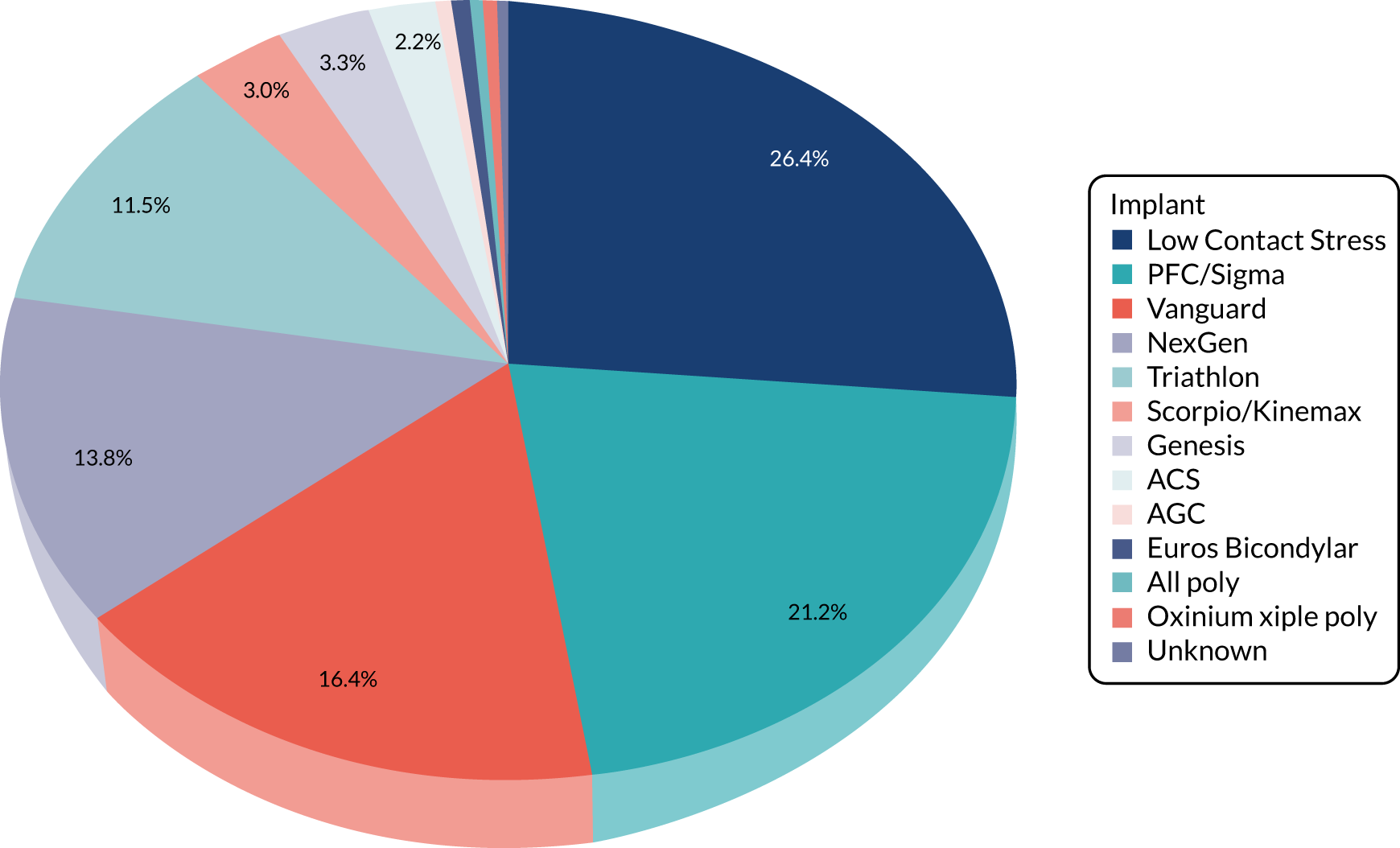
| PKR implants NJR 2010–14 | N = 6984, n | % |
|---|---|---|
| Oxford partial knee | 4520 | 65 |
| Zimmer unicompartmental | 864 | 12 |
| AMC/Uniglide | 291 | 4 |
| Mathys unicondylar knee | 54 | 1 |
| Sigma HP | 881 | 13 |
| Sled | 40 | 1 |
| Triathlon uni | 96 | 1 |
| Othera | 238 | 3 |
FIGURE 9.
The PKR implants used in clinical practice (NJR) from 2010 to 2014.
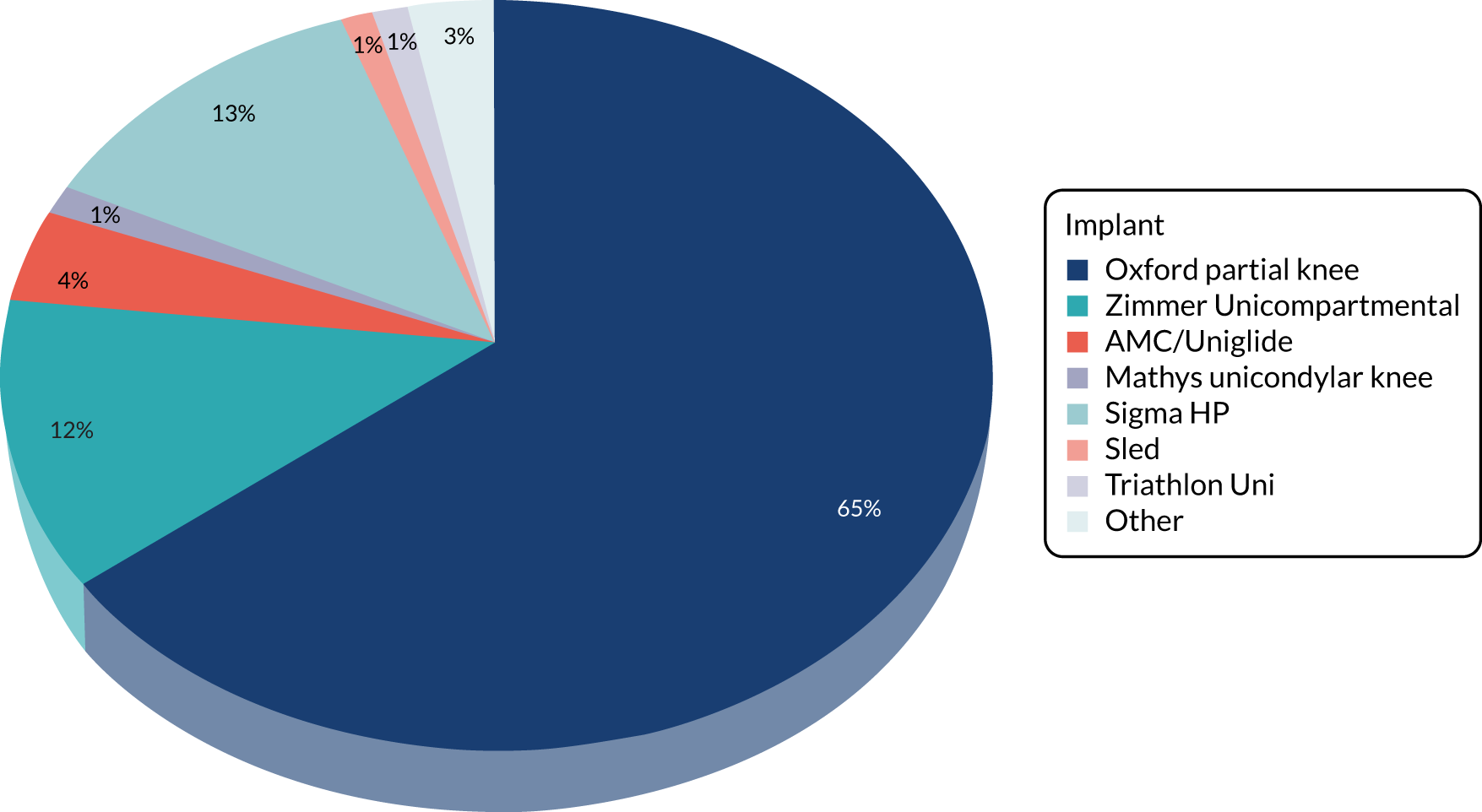
| TKR implants NJR 2010–14 | N = 72,762, n | % |
|---|---|---|
| LCS complete | 2279 | 3 |
| PFC/Sigma bicondylar knee | 26,007 | 36 |
| Vanguard | 5247 | 7 |
| Nexgen | 11,048 | 15 |
| Triathlon | 8056 | 11 |
| Genesis 2 | 6265 | 9 |
| AGC | 5414 | 7 |
| Othera | 8446 | 12 |
FIGURE 10.
The TKR implants used in clinical practice (NJR) from 2010 to 2014.
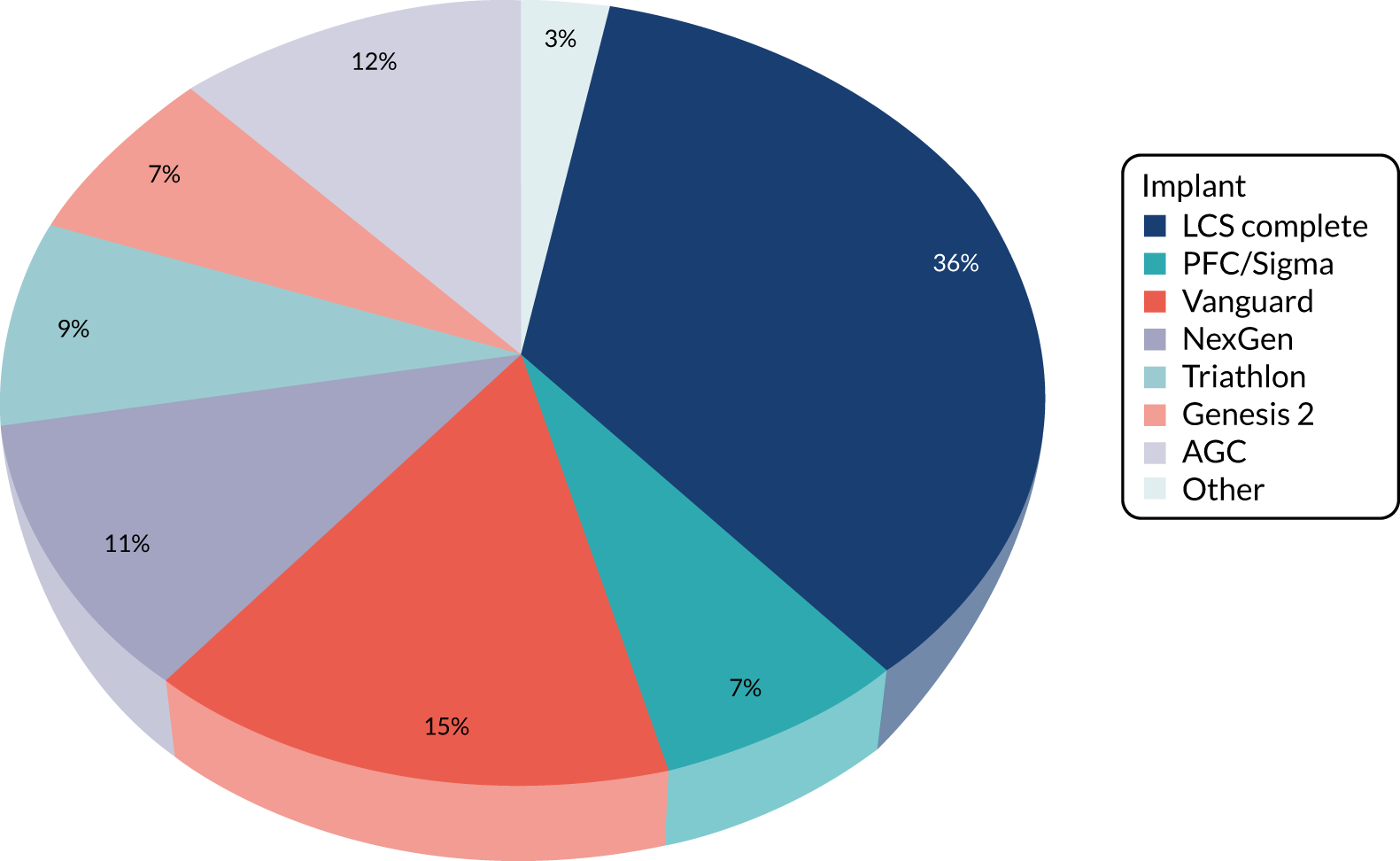
List of abbreviations
- AKSS
- American Knee Society Score
- ASA
- American Society of Anesthesiologists
- AUC
- area under the curve
- BMI
- body mass index
- CACE
- complier-average causal effect
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DMC
- Data Monitoring Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GP
- general practitioner
- HAAS
- High Activity Arthroplasty score
- HRG
- Healthcare Resource Group
- ICD-10
- International Classification of Diseases, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- IRR
- incidence rate ratio
- ITT
- intention to treat
- KAT
- Knee Arthroplasty Trial
- MD
- mean difference
- NIHR
- National Institute for Health Research
- NJR
- National Joint Registry
- NMB
- net monetary benefit
- OA
- osteoarthritis
- OKS
- Oxford Knee Score
- OKS-APQ
- Oxford Knee Score – Activity and Participation Questionnaire
- OPCS-4
- Office of Population, Censuses and Surveys Classification of Surgical Operations and Procedures, Fourth Revision
- PI
- principal investigator
- PKR
- partial knee replacement
- PMG
- Project Management Group
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- rate ratio
- SD
- standard deviation
- SITU
- Surgical Intervention Trials Unit
- TKR
- total knee replacement
- TOPKAT
- Total or Partial Knee Arthroplasty Trial
- TSC
- Trial Steering Committee
- UCLA
- University of California, Los Angeles
- VAS
- visual analogue scale
