Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/94/02. The contractual start date was in April 2018. The draft report began editorial review in October 2019 and was accepted for publication in January 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Lyvonne N Tume was a National Institute for Health Research (NIHR) Health Technology Assessment (HTA) panel member during the conduct of the study and is the deputy chairperson of the HTA Prioritisation Committee. Chris Gale reports grants from the Medical Research Council during the conduct of the study; and grants from the NIHR (research grant and fellowship for a Doctor of Philosophy student), the Mason Medical Research Foundation (London, UK), Rosetrees Trust (Edgware, UK) and from the Canadian Institute for Health Research (Ottawa, ON, Canada), outside the submitted work. Chris Gale also reports grants and personal fees from Chiesi Pharmaceuticals (Parma, Italy), outside the submitted work (the grant is for a research study and the personal fee was to support attendance at an educational meeting). Chris Gale is vice-chairperson of the NIHR Research for Patient Benefit London Regional Assessment Panel (2016–present). Frederic V Valla reports personal fees from Baxter International (Deerfield, IL, USA) and personal fees from Nutricia (Zoetermeer, the Netherlands), outside the submitted work. Jon Dorling reports grants from the NIHR and from Nutrinia (Nazareth, Israel), outside the submitted work (the grant from Nutrinia in 2018 was for part of his salary to work as an expert advisor on a trial). Jon Dorling was a member of the NIHR HTA General Board (2017–18) and the NIHR HTA Maternity, Neonatal and Child Health Panel (2013–18).
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Tume et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Surveys to establish standard practice
Introduction
Work package (WP) 1 involved an electronic survey for all UK paediatric intensive care units (PICUs) and neonatal units (NNUs), to describe ‘standard care practices’ around enteral feeding and gastric residual volume (GRV) measurement.
Study management
This WP was led by LNT (for PICU) and JD (for NNUs). The Study Management Group (SMG) was responsible for providing input into the survey questions and design, with the SMG statistician (BA) analysing the descriptive results, LNT and JD summarising the guidelines, and members of the qualitative SMG (KW and ED) undertaking qualitative analysis of the free-text responses.
Aims and objectives
To describe current unit (PICU and NNU) practices around the measurement of GRV.
Methods
Two separate cross-sectional surveys were developed by the study team, one for PICUs and one for NNUs, in REDCap,1 an online electronic data-capture tool. The surveys were tested for clarity and face validity on clinicians who did not go on to respond to the final surveys. Minor wording adjustments were made to improve clarity, then the surveys were tested again within the study team. Ethics approval for the surveys was provided by the University of the West of England (reference HAS.18.04.144). The Paediatric Intensive Care Society (PICS) Study Group also reviewed and endorsed the PICU survey, as did the Neonatal Nutrition Network.
All UK PICUs (n = 27) and NNUs (n = 184) were invited to complete a survey. Between May and July 2018, each unit was contacted via professional networks and e-mailed an invitation link. Units were asked to complete the survey as a team consisting of a senior clinical nurse, a consultant and a dietitian (or equivalent leads in these three areas of expertise). They were also asked to upload any written guidance that their unit had around enteral feeding. The target response rate was > 70%. This was maximised by sending out three reminders, 1 week apart. Data were summarised using descriptive statistics for quantitative data and content analysis for qualitative free-text data. 2 The unit guidelines were reviewed and summarised.
The paediatric intensive care unit survey
The PICU survey [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)] consisted of 31 closed questions (tick-box responses, with room for free-text responses whenever a tick-box response ‘other’ was chosen), a ranking question and 19 open-ended questions. It was piloted on 10 PICU clinicians (a mix of doctors, dietitians and nurses). The survey focused on three domains: (1) general enteral feeding and nutrition practices in the respondents’ unit, (2) the GRV measurement technique used in the respondents’ unit and (3) clinical management in response to GRV. Twenty-seven UK PICUs were approached: these were units that admit children for at least 24 hours of intensive care and that are part of the national research network (PICS Study Group).
The neonatal unit survey
The NNU survey [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)] consisted of 10 closed questions (tick-box responses, with room for free-text responses whenever a tick-box response ‘other’ was chosen) and nine open-ended questions. It was piloted on 10 NNU staff (doctors, dietitians and nurses). The survey focused on three domains: (1) general enteral feeding and nutrition practices in the respondent’s unit, (2) the GRV measurement technique used in the respondent’s unit and (3) clinical management in response to GRV. One hundred and eighty-four UK NNUs [special care baby units (SCBUs), local neonatal units (LNUs) and neonatal intensive care units (NICUs) who looked after both medical and surgical babies] were approached. These are NHS units that admit babies for at least 24 hours of neonatal care.
Results
Paediatric intensive care unit
Twenty-four of 27 (89%) UK PICUs completed the survey. These were a mixture of general PICUs (13/24, 54%), mixed cardiac surgical and general PICUs (7/24, 29%) and standalone cardiac intensive care units (ICUs) (4/24, 17%). Collective unit responses were completed by senior doctors (22/24, 92%), nurses (23/24, 96%) and dietitians (23/24, 96%). Almost all (23/24, 96%) responding PICUs reported written guidance regarding enteral feeding and most of these (19/23, 83%) were uploaded for review.
All responding PICUs undertook some nutritional assessment at PICU admission (Table 1).
| Nutritional parameter assesseda | Number (%) assessed (n = 24) |
|---|---|
| Actual weight | 20 (83) |
| Estimated weight | 14 (58) |
| Height or length | 13 (54) |
| z-score/weight | 4 (17) |
| Centile chart | 15 (62) |
| Weight for age | 4 (17) |
| Nutritional assessment score | 9 (37) |
| STAMP | 3/9 (33) |
| PYMS | 5/9 (56) |
| BCH | 1/9 (11) |
The first part of the PICU survey consisted of questions regarding general nutrition practices in units. Most PICUs (15/24, 63%) used the Schofield Equation to predict energy requirements and aimed to achieve full energy targets within 48–72 hours. Over half (14/24, 58%) of PICUs had a target time to initiate enteral feeding, and for half (7/14, 50%) of these this was within 6 hours of admission (total range 2–24 hours). Continuous feeding was delivered in the majority of PICUs (15/24, 63%), and 34% (9/24) used intermittent bolus via the gastric route. Continuous feeding was mostly delivered over 24 hours a day (10/15, 66.6%) or over 20 hours a day (5/15, 33%). When feeding was by intermittent bolus, this was predominantly every 2 hours (6/9, 67%). Most units (15/24, 63%) reported using standard rigid gastric tubes, with eight units (33%) using soft silicone tubes as their standard feeding tube. Most PICUs (18/24, 75%) defined feed tolerance or intolerance in their guidance and, of these, definitions included GRV (18/18, 100%), vomiting (12/18, 67%), diarrhoea (9/18, 50%) and abdominal appearance (8/18, 44%). (See Report Supplementary Material 1 for a table of results and the full qualitative analysis of the text responses.)
The rest of the survey asked questions about GRV technique and management in units. Table 2 presents the key findings and further results are given in Report Supplementary Material 1. Table 3 is a summary of the 19 guidelines that were received.
| Practice | Frequency (N = 24), n (%) |
|---|---|
| GRV is routinely measured | 23 (96) |
| There is an agreed feed intolerance definition | 18 (75) |
| The feed intolerance definition includes GRV | 18/18 (100) |
| Frequency of GRV measurement | |
| Before every bolus feed | 2 (8) |
| 4-hourly | 18 (75) |
| 5-hourly or 6-hourly | 3 (12) |
| Only when child is vomiting | 1 (4) |
| Guidance is in place for GRV measurement technique | 8 (33) |
| The syringe size is specified | 17 (70) |
| Size of syringea | |
| 20 ml | 5/17 (29) |
| 50 ml or 60 ml | 10/17 (59) |
| Size varies according to circumstance | 2/17 (12) |
| GRV is used to define maximum threshold | 21 (88) |
| Type of threshold | |
| Maximum volume in ml/kg body weight | 11/21 (52) |
| Maximum volume percentage of administered feed | 6/21 (29) |
| Other | 4/21 (19) |
| GRV maximal threshold to define ‘intolerance’a | |
| 5 ml/kg | 12 (50) |
| Other: ml/kg threshold (up to 10 ml/kg/other) | 2 (8) |
| Gastric aspirate > 2 hours/4 hours/6 hours | 4 (17) |
| > 50% of previous 4 hours of feed | 3 (13) |
| Reason for discarding GRVa | |
| Abnormal colour | 17 (70) |
| PICU number and type | Default feeding method and route | GRV check frequency | Threshold for stopping feeds | Actions if threshold exceeded | Actions if still not tolerating feeds | Feeding defined by risk level: low- vs. high-risk abdomen |
|---|---|---|---|---|---|---|
| Mixed general and cardiac PICU | Bolus gastric | 3-hourly | 5 ml/kg | Return GRV, stop feeds at 3 hours and recheck GRV | Consider continuous feeding, post-pyloric feeding, PN or prokinetics | No |
| Mixed general and cardiac PICU | Continuous gastric | 4-hourly | 5 ml/kg | Return GRV, stop feeds at 2 hours and recheck GRV | Change to post-pyloric feeding | Yes |
| Mixed general and cardiac PICU | Bolus gastric | 4-hourly | > 4 hours of feed volume given | Replace GRV, continue feeding at same rate, recheck GRV at 4 hours | Stop feeds and review by doctor and dietitian | No |
| Mixed general and cardiac PICU | Continuous gastric, but also uses bolus | 4-hourly | > 4 hours of feed volume given or 200 ml | Return GRV, stop feeds at 2 hours and recheck GRV, restart feed at 0.5–1 ml/hour | Change to post-pyloric feeding | Yes |
| Mixed general and cardiac PICU | Bolus gastric | 2- to 6-hourly to first determine the child’s gastric emptying time and prior to every bolus feed | > 50% of last bolus feed volume | Return GRV, stop feeds at 2 hours and recheck GRV | If GET delayed > 6 hours start post-pyloric feeding | No |
| Mixed general and cardiac PICU | Continuous gastric | 4-hourly | 5 ml/kg or 200 ml | Return GRV, stop feeds at 2 hours and recheck GRV | Withhold and discuss regarding post-pyloric feeding | No |
| Mixed general and cardiac PICU | Bolus gastric | Minimum 8-hourly, but done before every 2-hour feed | 5 ml/kg or 300 ml | Return GRV, stop feeds at 2 hours and recheck GRV | Change to continuous feeds, add oral prokinetic or consider post pyloric feeding | No |
| Mixed general and cardiac PICU | Continuous gastric | 4-hourly | 5 ml/kg | Return GRV, stop feeds at 2 hours and recheck GRV | If in first 48 hours stop feeds, after 48 hours change to post-pyloric feeding | Yes |
| Cardiac ICU | Continuous gastric | 4-hourly | 5 ml/kg | Return GRV, stop feeds at 2 hours and r-check GRV | Discuss with doctor and dietitian | Yes |
| Cardiac ICU | Continuous gastric with somatic NIRS monitoring | 4-hourly | > 4 hours of feed volume given | Replace half GRV, stop feeds at 2 hours and recheck GRV | Consider post-pyloric feeding | Yes |
| General PICU | Continuous gastric | 4-hourly | > 4 hours of feed volume given | Return GRV, stop feeds at 1 hour and recheck GRV | No mention | No |
| General PICU | Continuous gastric | 4-hourly | 5 ml/kg or 200 ml | Return GRV and maintain rate of feed | Consider alternative feed, post-pyloric feeding or PN | No |
| General PICU | Continuous gastric, but do use bolus | 4-hourly | 5 ml/kg or 200 ml | Return GRV, stop feeds 2 hours and recheck GRV | Consider post-pyloric feeding | No |
| General PICU | Continuous gastric | 4-hourly | 5 ml/kg or 200 ml | Change to non-fibre feed then return to half GRV and continue same rate for 4 hours | Consider post-pyloric feeding and prokinetics | No |
| General PICU | Bolus gastric | 4-hourly | > 50% of the feed volume given in last 4 hours | Discard GRV and give the previous amount of feed again, recheck GRV | If still > 50%, change to continuous feeding. If still not tolerating, use IV prokinetic and by 72 hours start post-pyloric feeding | No |
| General PICU | Continuous gastric | 4-hourly | 5 ml/kg | Return GRV, stop feeds at 1 hour and recheck GRV | Start prokinetics and post-pyloric feeding | No |
| General PICU | Continuous or bolus feeds | 4-hourly | 5 ml/kg or 250 ml | Return GRV, stop feeds at 2 hours and recheck GRV | Consider prokinetics, rule out constipation and consider post-pyloric feeding | Yes |
| General PICU | Continuous gastric | 4-hourly | > 50% of the feed given in last 4 hours | Notify medical professional/dietitian, stop feed or reduce rate and recheck GRV | Consider post-pyloric feeding if not tolerating by 24 hours | No |
| General PICU | Continuous gastric | 6-hourly | > 6 hours of feed given | Return GRV, stops feeds at 1 hour and recheck GRV | Does not specify | No |
All but one (23/24, 96%) responding PICU measured GRV routinely as part of their standard practice, and none reported that the policy was different for invasively ventilated compared with non-ventilated children. The frequency of GRV measurement was most commonly reported as 4-hourly (18/24, 75%) in the survey and (15/19, 79%) in the unit guidelines. However, most PICUs (16/24, 67%) reported little guidance around the technique of measuring GRV. Only 71% (17/24) of responding units indicated a specific syringe size to use with GRV measurement (but this was rarely written in their guidelines). When this was specified, this was most commonly (10/17, 59%) a 50- to 60-ml syringe. Most units (15/24, 63%) reported that the feeding method (continuous or intermittent) did not influence the frequency of GRV measurement. Half of responding units (12/24, 50%) reported that the size of the child (i.e. > 40–50 kg) did not affect the frequency of GRV measurement.
Almost all (21/24, 88%) responding units reported that GRV was the main indicator to withhold enteral feeding. The decision to withhold feeds was determined most frequently by a maximum volume in ml/kg body weight (11/21, 52%). Twenty-nine per cent (6/21) of units reported using a maximum percentage of volume of feed given, but this was higher (8/19, 42%) in the unit guidelines. The volume above which feeds were withheld was reported as 5 ml/kg by 52% (11/21) of units in the survey and by 58% (11/19) of guidelines. In the seven units whose guidelines stipulated an upper absolute level (for children > 40–50 kg), this was most frequently 200 ml (5/7, 71%). Of the six guidelines that used a percentage of volume of feed given in previous hours to determine whether or not to withhold feeds, this varied from > 50% of feed given in the previous 4 hours to 100% of the feed given in the previous 2–6 hours. A percentage of the volume of the previous 4 hours of feed given was used in 5 out of 19 (26%) guidelines. More than half (14/24, 58%) of responding units reported that they did not vary the threshold according to the size of the child.
Both volume and/or colour of aspirates affected decisions to stop feeds. Figure 1 shows that most units rated both volume (14/24, 58%) and colour (15/24, 63%) as fairly important.
FIGURE 1.
How much does aspirate volume and aspirate colour affect your decision to stop feeds? (n = 24 paediatric intensive care units.)
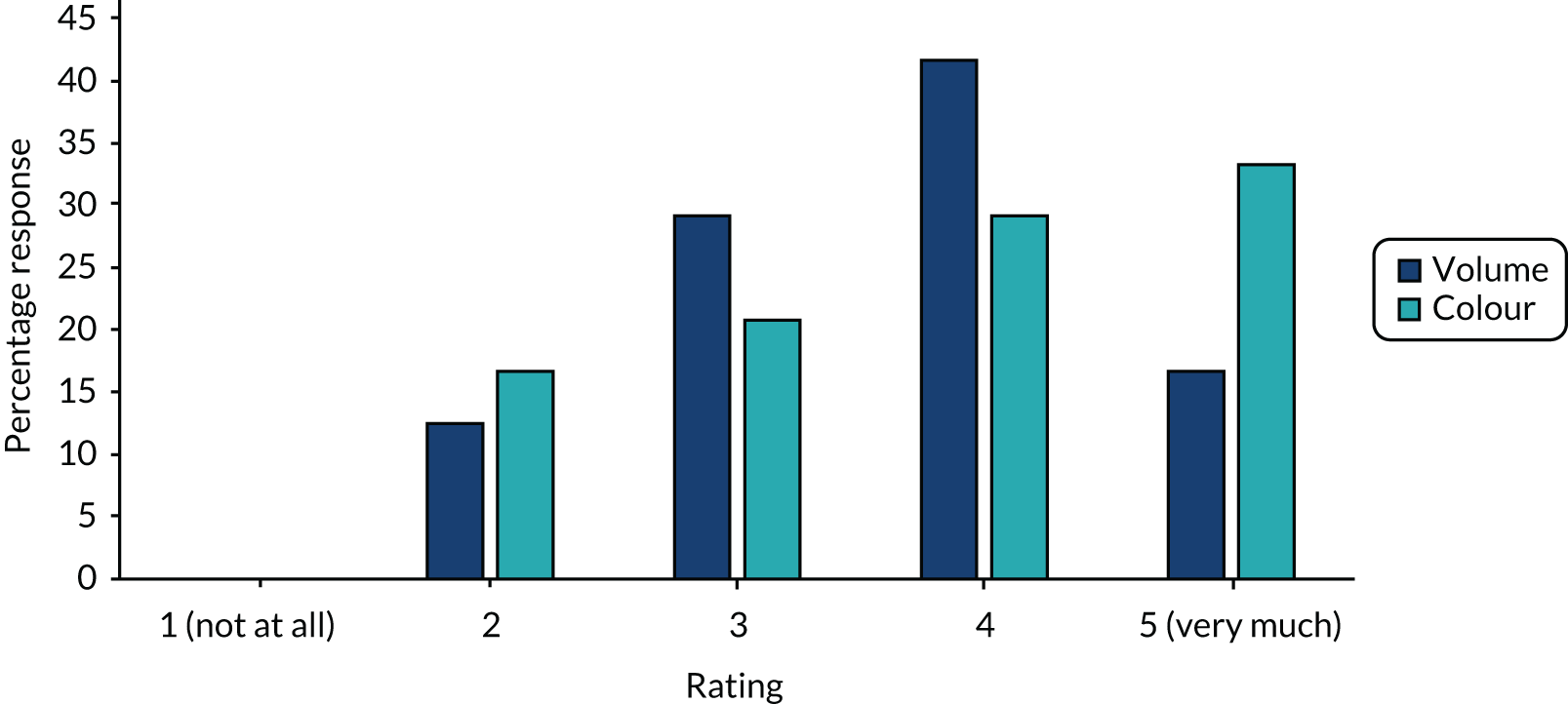
Guideline analysis and free-text responses all cited abnormal colour aspirates being green (bilious), red (bloody) or brown (faecal) in appearance and, even if the volume was not large, aspirates of this appearance would be discarded and indicate the withholding of feeds. Most (15/24, 63%) units reported returning GRV. None reported that GRVs were routinely discarded, but that this was dependent on individual patient factors and aspirate appearance. However, most guidelines (16/19, 84%) required to return the GRV in all patients unless it was abnormal in appearance. In response to obtaining ‘high’ GRVs, PICUs reported their actions by free text and then actions were ranked by frequency in the survey. Qualitative responses indicated that for the majority of PICUs, in the first instance, enteral feeds would be withheld for a period of time (commonly 2 hours) and GRV reassessed. After this, actions ranked by order of priority were most commonly (1) changing the feeding method from bolus to continuous feeds, (2) changing to post-pyloric feeding and/or changing the feed formula, (3) adding prokinetics and persisting with gastric feeding and, lastly, (4) stopping enteral feeds and commencing parenteral nutrition.
For 79% of units (15/19), the initial action in response to a large GRV was to stop feeds for a period of time and recheck the GRV. From the guideline review (see Table 3), six units had defined levels of abdominal risk for enteral feeding of children. Five out of these six units admitted cardiac surgical neonates (83%) and defined low- and high-risk abdomens in their protocols based on the patient profile. Defining features of a high-risk abdomen included infants with hypoplastic left heart syndrome, aortic arch abnormalities, shunts and duct-dependent circulations, gut concerns, including confirmed necrotising enterocolitis (NEC) in the last 4 weeks, high vasopressor support, high lactate concentrations, low somatic near-infrared spectroscopy, and after cardiac arrest and extracorporeal life support. In all situations, even when different feeding regimes were specified in relation to risk, both protocols (for low and high risk) still used routine GRV measurement, but the rate of feed delivery and the speed of advancement was much slower in the high-risk patients.
Neonatal units
Ninety-five of 184 (52%) NNUs in the UK, excluding Northern Ireland, completed the survey. These consisted of 40 NICUs, 42 LNUs and 13 SCBUs,3 giving response rates of 71%, 47% and 33%, respectively. Seventeen of a possible 18 NICUs that routinely care for both immediate postoperative surgical and medical patients responded, as did 23 NICUs that routinely care for only medical cases. LNUs and SCBUs do not provide early postoperative care in the UK. 4,5 Survey responses were received from senior doctors (81/95, 85%), nurses (51/95, 54%) and dietitians (9/95, 10%). Table 4 gives a summary of general feeding practices. Most (81/95, 85%) responding units reported written enteral feeding guidance and 28 unit or local neonatal network guidelines were sent to the authors. Enteral feeding was typically delivered intermittently (90/95, 95%), rather than continuously (5/95, 5%). Forty-two out of 95 units (44%) reported having written guidance for measurement and interpretation of GRVs.
| Practice | Frequency (N = 95), n (%) |
|---|---|
| Units had written feeding guidelines/protocol | 81 (85) |
| Standard gastric feeds were intermittent bolus (not continuous) | 90 (95) |
| There was specific guidance about how GRV should be measured and interpreted (e.g. a protocol or guideline) | 42 (44) |
| NICUs that care for surgical and medical babies (n = 17) | |
| GRV measurement differs between the medical and surgical babies | 5/17 (29) |
Ninety units answered questions about the management of non-surgical babies (Table 5). When asked about how often GRV is measured, 20 out of 90 units (22%) measured aspirates before every feed, 26 (29%) units measured aspirates when it was felt to be clinically indicated and 39 (43%) units measured GRV at regular time intervals [most commonly 4- to 6-hourly, 35/39 (90%), but all more frequently than once per day]. One unit had no guidelines on this and four (4%) reported that they did not measure GRV. Among units that reported having written GRV measurement guidance, 13 out of 39 (33%) indicated that the guidance was ‘always’ followed and 17 (39%) indicated that the guidance was ‘usually’ followed; however, free-text responses suggested that practice was ‘very variable depending on the nurse looking after the baby’ (unit 3, surgical and medical unit). The bedside nurse most commonly made decisions in relation to GRV results (56/90, 62%), followed by middle grade doctors (41/90, 46%) and the senior nurse in charge of the shift (26/90, 29%).
| Practice | Frequency (N = 90), n (%) |
|---|---|
| Frequency that staff in your unit measure GRV | |
| Once a day | 0 (0) |
| Before every feed | 20 (22) |
| Only when clinically indicated | 26 (29) |
| At regular intervals | 39 (43) |
| At least every 3, 4 or 6 hours | 35/39 (90) |
| GRV is not measured | 4 (4) |
| How often is specific guidance for GRV measurement followed and undertaken as per protocol [asked only of units with specific guidance for GRV measurement (n = 39)]? | |
| Always | 13 (43) |
| Usually | 17 (39) |
| Often | 4 (10) |
| Rarely/never | 5 (13) |
| Who usually decides what to do with concerning GRV aspirates in the first instance (more than one response allowed)? | |
| Senior doctor (consultant) | 13 (14) |
| Middle-grade doctor (specialist registrar) | 41 (46) |
| Junior-grade doctor (senior house officer) | 18 (20) |
| Bedside nurse | 56 (62) |
| Nurse in charge of shift (senior nurse) | 26 (29) |
| What is usually done with obtained GRV? | |
| Returned | 44 (49) |
| Discarded | 7 (8) |
| Other | 39 (43) |
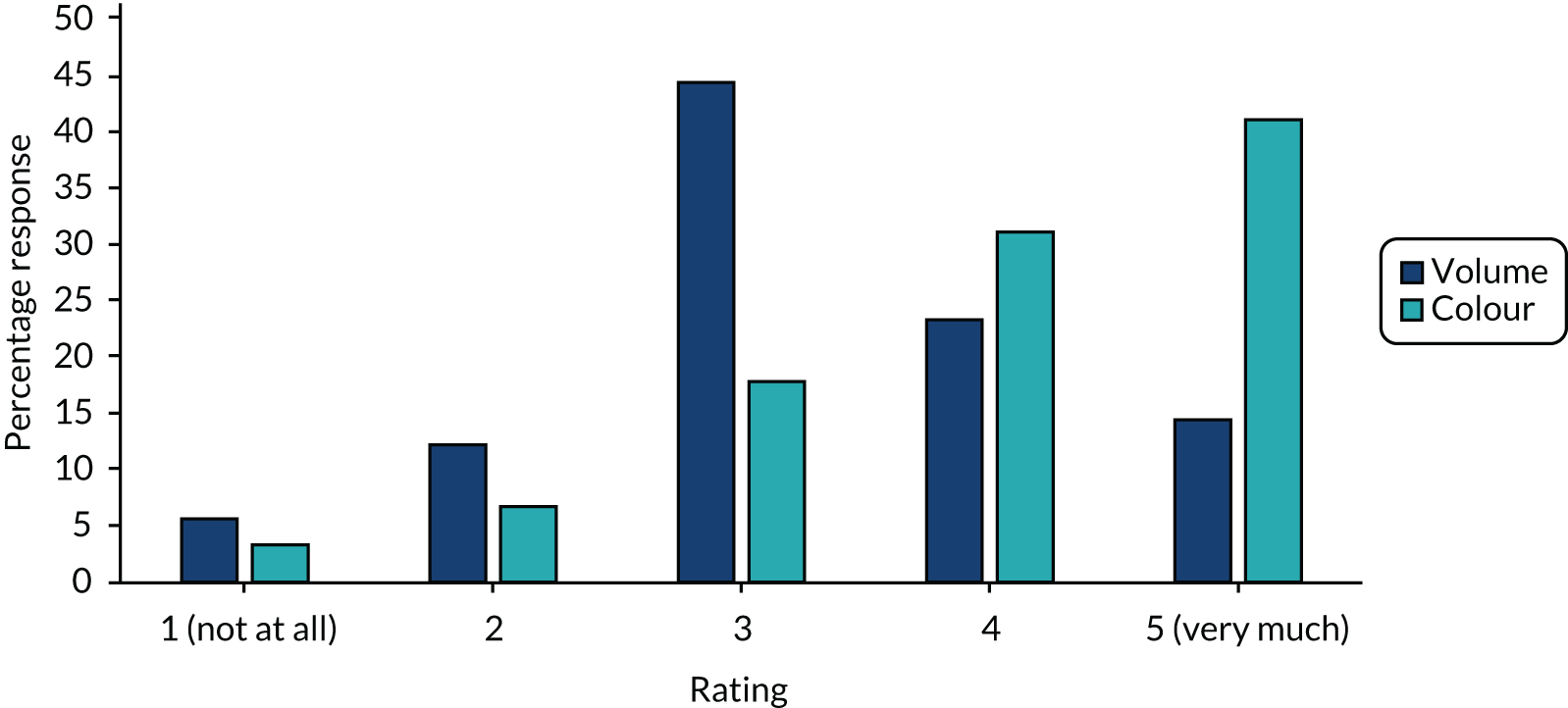
Responding units had mixed views on how useful the volume of the aspirate was for guiding feeding decisions (Figure 2), with 13 out of 90 (14%) units reporting that volume affected clinical decision-making ‘very much’ and the most frequent response was an intermediate score. The colour of the aspirate was felt to be more important, with 37 out of 90 (41%) units reporting that colour influenced clinical decisions ‘very much’ and this was the most frequent response. More detail was obtained from 74 open-text responses to this question. A large volume of aspirate was commonly described as a concern, which would often lead to a clinical review of a baby’s condition and subsequent consideration of how much milk the baby is receiving. The threshold for prompting a feeding review was reported to vary. Some units stated that aspirates > 50% of the feed would ‘prompt a review’ (unit 8, NICU surgical and medical), whereas others stated ‘> 25% of the feed given in previous 6 hours’ (unit 18, NICU medical only), if exceeds ‘25% of the previous 4 hours’ feed volume’ (unit 22, NICU medical only) or ‘if > 25% of the feed volume given since the last assessment was made’ (unit 25, NICU medical only).
FIGURE 2.
How much does aspirate volume and aspirate colour affect your decision to stop feeds? (n = 90 NNUs.)
Almost half (44/90, 49%) routinely returned aspirates to the stomach. Seventy-two nurses gave reasons for seeking medical advice, with 55 (76%) citing increased or large GRVs and 52 (72%) citing bilious colour of the residual or a change in colour. Other reasons were blood-stained aspirates (16/72, 22%), concerns about the condition of the baby, such as arterial oxygen desaturations (16/72, 22%), abdominal distension (11/72, 15%) and vomiting (5/72, 7%). In free-text responses, units stated that that a dark or bilious colour would ‘trigger medical review [by a] middle grade or consultant’ (unit 22, NICU medical only), whereas some described how feeds would be stopped, ‘green aspirate – assess baby and feeds withheld’ (unit 60, LNU). Full summaries of qualitative analyses are presented in Report Supplementary Material 1.
The 28 guidelines received are summarised in Table 6. This shows that 19 out of 28 (68%) guidelines specified a volume of aspirate at which to consider stopping feeds, using a defined proportion of the previous feed. Six guidelines specified this threshold as ≥ 25% of the previous feed, eight guidelines specified ≥ 50% of the previous feed, whereas five guidelines used a level between these. Fourteen guidelines mentioned the bilious green colouring of GRV being an indication to stop enteral feeds, whereas five mentioned blood staining as being important. Vomiting and abdominal distension were also considered important for guiding management, being mentioned by 13 and 12 guidelines, respectively.
| NNU number and levela | Default feeding method | GRV checking | Threshold for stopping feeds |
|---|---|---|---|
| 1. NICU | Bolus feeds | Routinely measured, but no mention of frequency or technique | Aspirate > 50% feed volume in previous 6 hours or bilious aspirates |
| 2. NICU | Bolus feeds | Measured, but no mention of frequency or how | Aspirates > 50% or > 1 ml if aspirate contains blood or bile: discard GRV, stop feeds, wait 2 hours and reassess |
| 3. NICU | Bolus feeds | No mention of frequency or technique | Consider stopping if pre-feed aspirate > 4 ml/kg, heavily bile-stained aspirates or two vomits after consecutive feeds |
| 4. NICU | No mention | No mention | No mention |
| 5. NICU | Bolus feeds and advanced as per SIFT | Check GRV no more than 6-hourly unless concerns | Withhold feeds for 6–12 hours if GRV > 40% of feed given, 2 ml or 3 ml (dependent on infant weight) heavily bile/blood stained or abdominal distension |
| 6. NICU | Bolus feeds | Not stated | GRV > 50% volume of feeds over last 6 hours or vomit of this size |
| 7. NICU | 2-hourly bolus feeds advanced as per SIFT | 4- to 6-hourly | Action with gastric residuals, if:
|
| 8. NICU | Bolus feeds, two risk levels, advanced as per SIFT | Routine measurement of full gastric residuals should be avoided. This should only be done, with discussion, as a part of a full medical review by a doctor or ANNP | Signs of feed intolerance may include clinical observations, such as desaturation and bradycardia events, and increased work of breathing, vomiting, abdominal distension and discolouration |
| 9. NICU | Bolus feeds | Not specified |
Medical babies: 2 ml/kg of milky gastric residual is not important and should simply be replaced. When the gastric residual at higher volumes is equivalent to 100% of the bolus feeds, then the feeds should be stopped and a clinical review should be undertaken Surgical babies: aspirate less than half feed volume since last aspirate, replace the aspirate itself and continue feeding. If aspirate is greater than half feed volume but less than whole feed volume, replace half of the aspirate and discard the rest. If aspirate is greater than whole feed volume since previous aspirate, do not replace the aspirate, stop feeding and obtain senior medical and surgical review |
| 10. NICU | Bolus feeds | 4- to 6-hourly |
Examine and assess the baby if vomiting, GRV is > 25% of the previous 4 hours total feed volume, residuals are persisting or increasing Small milky/yellow aspirates up to 2–3 ml are frequently normal. They can be replaced and feeds continued |
| 11. NICU | Bolus feeds | 4- to 6-hourly |
When babies are on any enteral feeds, only aspirate the stomach contents via a gastric tube every 4–6 hours, to check the residual volume. The assessment of the baby should include any abdominal distension, dark green (bilious) aspirates and bowel opening If < 50% of the previous 4- to 6-hour total feed volume is aspirated, then replace the aspirate and continue enteral feeding, provided the baby is otherwise clinically stable If > 50% of the previous 4- to 6-hour total feed volume is aspirated, then discuss with medical staff; often reasonable to replace the aspirate and omit the feed. If necessary, stop the feeds for 4–6 hours and a senior member of the medical/nursing team should then review |
| 12. NICU | Bolus feeds | 6-hourly until infant is fully fed | Signs of intolerance: vomiting, gastric residuals > 25% of previous 6 hours feed volume, persistent or increasing, abdominal distension/increasing abdominal girth, increase in stool frequency |
| 13. NICU | Bolus feeds | Not specified | Large-volume aspirates or dark-green bile-stained aspirates, particularly in association with abdominal distension and/or tenderness are a cause for concern. Small milky/yellow aspirates up to 2–3 ml are frequently normal. They can be replaced and feeds continued |
| 14. NICU | Bolus feeds | No more than 4- to 6-hourly | If vomit or GRV exceeds 33% of the last feed volume or 3.5 ml in a single aspirate, then examine baby. Small residuals normal |
| 15. NICU | Bolus feeds | Not specified |
Isolated large GRV in the absence of other clinical signs and symptoms should not prevent continued feeding Signs of intolerance: vomiting, GRV > 30% of previous 5 hours feed, abdominal distension, unwell baby |
| 16. LNU | Bolus feeds with advancement strategy as per SIFT | Not specified | Aspirate > 50% feed volume or green aspirates |
| 17. LNU | Bolus feeds with advancement as per SIFT | GRV aspirated 4-hourly | GRV > 25% feeds in previous 4 hours combined with abdominal distension and/or vomiting |
| 18. LNU | Not stated | Not specified | 4-hourly NG aspirates are < 25% of total infused in the preceding 4 hours. No significant abdominal distension. No significant vomiting. No bile-stained aspirates |
| 19. LNU | Bolus feeds | Not specified |
Aspirates up to 2–3 ml or 50% of the previous 4 hours, feed can be normal if the baby is well Aspirates > 50% of the previous 4 hours feed or 2–3 ml (whichever is greater) discard aspirate, hold feed and try again in 2 hours If aspirate contains blood or bile then stop feeds |
| 20. LNU | Bolus feeds | Not specified |
If the aspirates are non-bilious and less than half the volume of previous feed then they can be replaced and feeding continued while observing the infant closely If the aspirates are bilious or > 50% of the previous feed volume, consider withholding the feeds on that occasion and assess for any signs of NEC |
| 21. LNU | Bolus feeds | Not specified | No mention |
| 22. LNU | Bolus feeds | 4- to 6-hourly |
If GRV is 25–50% of total, replace the hourly amount, omit the feed and do not increase If GRV is > 50% of total, stop feeds and medical review |
| 23. SCU | Bolus feeds | Not specified | GRV > 2-hourly amount, vomiting or abdominal distension |
| 24. Mixed network | Bolus feeds | Not specified | GRV > 25% (some > 50%) in previous 4 hours in combination with vomiting and abdominal distension plus bilious aspirates |
| 25. Mixed network | Bolus feeds | 4-hourly | Stop feeds if GRV heavily blood or bile stained. No mention of volume |
| 26. Mixed network | Bolus feeds | Not specified |
GRV should not be used in isolation to determine feed tolerance Intolerance: vomiting plus GRV > 50% in the last 4 hours (especially if increasing) plus abdominal distension |
| 27. Mixed network | Bolus feeds advanced as per SIFT | Not specified |
Infants ‘feed tolerance’ assessed with each set of cares (high risk), assess twice daily (moderate risk) and before making changes in feed volumes (standard risk) Assessing tolerance: undigested gastric residuals using a colour chart, GRV not used in isolation If vomiting, GRV > 25% of feed volume in last 4 hours + bloody or bilious residuals + abdominal distension |
| 28. Mixed network | Bolus feeds | Assess GRV 4- to 6-hourly depending on cares |
If GRV > 50% of total, stop feeds and medical review If GRV 25–50% of total, replace the hourly amount, omit the feed and do not increase An appropriate GRV is < 25% of preceding volume since last replacement of GRV Replace GRV in full A GRV > 25% but < 1.5 ml is unlikely to be problematic A GRV of 25–50% is high, but acceptable if well, replace only normal hourly volume and continue feeds but do not increase A GRV > 50% is excessive, perform a clinical exam and if acceptable hourly volume can be replaced, but with feed withheld |
Summary of findings to inform the GASTRIC trial
Paediatric intensive care unit current practice
The routine and frequent measurement of GRV is embedded into enteral feeding practice and guidelines in UK PICUs, yet little specific guidance is provided about the technique. This is despite a lack of evidence and questionable accuracy of this parameter. For most units, GRV is the main defining assessment of feed tolerance or intolerance and the most commonly used threshold is a GRV ≥ 5 ml/kg. Colour was as important as volume in the decision-making regarding whether to return or discard GRV and to withhold feeds. This survey has established current practice around GRV measurement in UK PICUs, which enabled us to develop a ‘control’ arm of a future trial of not routinely measuring GRV in critically ill children (see Appendix 1 for flow chart). These results have been published. 6
Neonatal unit current practice
Neonatal units show a more mixed practice for both measuring GRV and in how GRV is used to make decisions about enteral feeding. However, around half of UK NNUs still routinely use GRV as a parameter to guide enteral feeding advancement. Health professionals’ views around the importance of the volume compared with colour of the GRV were inconsistent, and importance was defined at different thresholds. Aspirate colour was often cited as more important than volume; however, the importance placed on this was inconsistent. Some unit guidelines specified actions based on bilious or blood staining of the secretions, whereas others did not.
Understanding current practice in both settings has enabled us to construct a ‘control arm’ for a future trial in both settings, which reflects the most prevalent practices across the units (see Appendices 1 and 2).
Chapter 2 Interviews and focus groups involving parents and health-care professionals
Study design
Work package 2 was a mixed-method study involving interviews with parents with relevant PICU or NNU experience, as well as focus groups and interviews with NNU and PICU health-care professionals (HCPs), including nurses, doctors, surgeons and dietitians.
Objectives
To explore the views of parents with NNU and PICU experience on:
-
the acceptability of the proposed trial
-
potential barriers to recruitment
-
participant information
-
whether or not they would be happy to consent to their child’s participation in the trial
-
potential parent-centred outcome measures.
To explore HCPs’ views on:
-
the acceptability of the trial, including the proposed inclusion and exclusion criteria and equipoise
-
the acceptability of not measuring GRV and other measures used to assess feeding tolerance
-
the willingness to randomise to a future trial, including potential barriers to recruitment consent
-
associated training needs.
Study management
The WP 2 team was led by co-investigator (KW). Two experienced research associates (LR and ED) were employed to organise, conduct and analyse interviews and focus groups. The SMG was responsible for overseeing day-to-day management of the entire GASTRIC feasibility study, including the WP 2 qualitative work.
Design and development of the protocol
The design and development of the protocol, including sample estimation, recruitment strategy, information sheets [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)] and interview topic guide [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)], were informed by previous research. 7–11 A review of previous studies of GRV in different populations was conducted prior to this study to develop a list of outcomes to inform outcome-related discussions with parents during interviews. A voting system, using TurningPoint software (Turning Technologies, Youngstown, OH, USA), was used alongside verbally administered questions in practitioner focus groups. This involved some of the key questions [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)] being presented to the group and each participant using a wireless handset to select their answer from those shown on the screen. This method enabled the collection of data from all participants, as well as a means of generating statistical data from all sites alongside qualitative data from group discussions. The same quantitative questions were verbally administered during HCP telephone interviews and recorded by the researcher using a questionnaire [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)], allowing for collation of interview and focus group data.
Recruitment
Participants
Based on our team’s previous relevant studies,7–10 it was anticipated that 20–30 parents (10–15 in each setting) would be recruited to reach data saturation point. This is when the major themes identified in new data are recurring from previous participants or transcripts and no new major themes are being discovered in analysis. 12,13 We aimed to hold between two and four focus groups for HCPs [e.g. two focus groups (one NNU, one PICU) in the north of England and two focus groups (one NNU, one PICU) in the south of England]. It was expected that each focus group would involve 8–12 practitioners. 14 We anticipated conducting up to 10 telephone interviews with HCPs unable to attend the focus groups.
Eligibility criteria
Inclusion criteria
Parents of children with experience of tube feeding in NNU and/or PICU in the last 3 years and HCPs with involvement in decisions about feeding (nurses, doctors or dietitians) currently working in NNUs and PICUs in the UK were included.
Exclusion criteria
Parents who were unable to speak English and HCPs not working in the specialty were excluded.
Recruitment and sampling procedure
Parents
We recruited parents through three routes to maximise the potential sample in the recruitment period and to encourage sample diversity.
Recruitment route 1: social media or website advertising
An advertisement was posted on Twitter (URL: www.twitter.com; Twitter, Inc., San Francisco, CA, USA) and Facebook (URL: www.facebook.com; Facebook, Inc., Menlo Park, CA, USA), which invited parents to register interest in participating in the study. Relevant charities and support groups were asked to place an advertisement on their website and social media [e.g. Bliss (URL: www.bliss.org.uk), Sepsis Trust (URL: https://sepsistrust.org), hospital charities, GASTRIC study (URL: www.grvstudy.com) and Mumsnet (URL: www.mumsnet.com)]. In addition, the study team posted the media advert in relevant university internal newsletters.
Recruitment route 2: national contacts and existing database
E-mails were sent from the study team to their professional national contacts. In addition, e-mail invitations were sent to eligible parents from the FEVER feasibility study7 who had given consent to be contacted about related studies.
Recruitment route 3: national newspaper
To assist recruitment and sample variance, we placed an advertisement in the London Metro newspaper. This helped to balance the predominantly northern sample that was available at that point in recruitment (September 2018).
Health-care professionals
Focus groups were held in different geographical locations (north, north-west and south) to encourage the involvement of HCPs from across the country. We purposively targeted individuals, to include those unable to attend a focus group and include all key professional groups (e.g. doctors, nurses, dietitians and surgeons). We recruited through e-mail invitations and networks known to the co-applicants of the study, including the PICS and British Dietetic Association. HCPs were invited to participate in a telephone interview and if no response was made to the researcher, they were not contacted again.
Screening and conduct of interviews and focus groups
Parent interviews
Screening
Parents’ expressions of interest to participate were responded to in sequential order. Once eligibility was confirmed, an interview date and time was scheduled. A participant information sheet (PIS), a draft GASTRIC randomised controlled trial (RCT) PIS [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)] and list of potential outcomes (see Appendix 3) were e-mailed to parents to read prior to interview. Screening and interview conduct stopped when data saturation and sample variation (e.g. recruitment of parents via multiple recruitment routes) was achieved.
Informed consent
Audio-recorded verbal consent was sought over the telephone before the interview. This involved reading each aspect of the consent form to parents, including consent for audio-recording and to receive a copy of the findings when the study was complete.
Conduct of parent interviews
Interviews began with a discussion about the aims of the study, and included an opportunity for questions and checking that the parent had had enough time to read the information sheet and list of potential outcomes. Interviews commenced using the interview topic guide to explore:
-
experience of having a child with a feeding tube in a NNU or a PICU
-
any previous experience of participation in clinical trials
-
the length and content of the draft GASTRIC study PIS
-
the acceptability of measuring GRV
-
the acceptability of not measuring GRV
-
potential barriers to participation in the trial and how these could be addressed
-
potential facilitators of trial participation
-
trial design, including the selection of outcome measures and randomisation method
-
whether or not parents would (hypothetically) consent to their child taking part in the proposed GASTRIC study.
Respondent validation14 was used to add unanticipated topics to the topic guide as interviewing and analysis progressed. After the interview, participants were sent a copy of the consent form and a thank you letter, including a £30 Amazon voucher (Amazon.com, Inc., Bellevue, WA, USA), to thank them for their time. A copy of the consent form was retained by the University of Liverpool. Researchers (LR and ED) conducted a similar number of parent interviews.
Health-care professional focus groups and interviews
Informed consent
At the start of the focus group or interview the researcher checked that all participants had read the PIS. The focus group or interview aims and topics to be covered were discussed, followed by an opportunity for questions. Participants were asked to provide written consent before the focus group began. Using the same procedure as the parent interviews, audio-recorded verbal consent was sought over the telephone before the HCP interviews.
Conduct of health-care professional focus groups and interviews
Three researchers were involved in the focus group facilitation (LR, n = 3; KW, n = 1; ED, n = 2) and two researchers were involved in conducting interviews (LR, n = 8; ED, n = 2). An ‘ice breaker’ question was used at the beginning of the focus group to help demonstrate how the voting system would work alongside verbally administered questions. Staff were then asked to introduce themselves, their role within the ICU and their experience of recruiting to clinical trials.
Focus group and interviews explored site HCPs’ views and experiences on:
-
the current approach to GRV measurement
-
the acceptability of measuring GRV
-
the acceptability of not measuring GRV
-
potential barriers to the trial and how these could be addressed
-
potential facilitators of trial participation
-
trial design, including randomisation method, inclusion and exclusion criteria
-
perceived training needs
-
the acceptability of the proposed GASTRIC study.
Transcription
Digital audio-recordings were transcribed verbatim by a professional transcription company (Voicescript Ltd., Bristol, UK) in accordance with the Data Protection Act 1998. 15 Transcripts were anonymised and checked for accuracy. All identifiable information (e.g. names of patients, family members or the hospital where their child was admitted) was removed.
Data analysis
Quantitative data from the practitioner-closed interview and focus group questions were entered into Statistical Product and Service Solutions (SPSS) version 20.0 (IBM Corporation, Armonk, NY, USA) and examined using descriptive statistics. Qualitative interview and focus group data analysis was interpretive and iterative. 16,17 Utilising a thematic analysis approach18 (Table 7), the aim was to provide an accurate representation of views on trial design and acceptability. Thematic analysis is a method for identifying, analysing and reporting patterns (or themes) within data. Analysis was informed by the work of Braun and Clarke2 and their guide to thematic analysis. This approach allows for themes to be identified at a semantic level (i.e. surface meanings or summaries) or at a latent level (i.e. interpretive, theorising the significance of the patterns and their broader meanings and implications). 19 NVivo 10 software (QSR International, Warrington, UK) was used to assist in the organisation and coding of data. The researchers (ED and LR) led the analysis and 10% of the analysis was second coded by the qualitative lead (KW).
| Phase | Description |
|---|---|
| 1. Familiarising with data | ED (parents) and LR (practitioners) read and reread transcripts noting down initial ideas |
| 2. Generating initial codes | Initially, two data coding frameworks were developed using a priori codes identified from the project proposal and the interview topic guide. During the familiarisation stage, LR and ED identified additional data-driven codes and concepts not previously captured in the initial coding frame |
| 3. Developing the coding framework | 10% of the transcripts were double coded. LR, ED and KW all reviewed and discussed both initial coding frames (practitioner and parent), making notes on any new themes identified and how the framework could be refined |
| 4. Defining and naming themes | Following review and reconciliation by ED, LR and KW, revised coding frames were subsequently developed and ordered into themes (nodes) within the NVivo database |
| 5. Completing coding of transcripts | LR and ED completed coding of all transcripts in preparation for further analysis |
| 6. Producing the report | ED, LR and KW developed the manuscript using themes that related back to the study aims to ensure key findings and recommendations were relevant to the GASRTIC study. Final discussion and development of selected themes occurred during the write-up phase |
| 7. Participant validation | During PPI webinars and consensus meetings, findings were presented back to parents and practitioners who then had a chance to discuss, validate or disagree with the presented results |
Outcomes analysis
To rank parents’ prioritised outcome measures, we conducted an additional analysis step involving content analysis. 20 Outcomes identified during the thematic analysis were cross-referenced by participant and by question and entered into an Excel® version 1908 (Microsoft Corporation, Redmond, WA, USA) database. The number of times each outcome was prioritised was then counted. A weighted point-based system was used to combine findings from all outcome questions and determine the top prioritised outcomes by group. For example, each time an outcome was ranked first it received 6 points, if it was ranked second it received 5 points and if it was mentioned as an important outcome but subsequently not included in the ranked list it received 1 point.
Results
Participants: parents
A total of 55 parents registered interest (Figure 3), of whom 41 were screened. Five parents were deemed ineligible and five parents did not respond to contact. Two PICU parents were interviewed, but not included in the sample because of recording equipment failure and seven parents were not interviewed as data saturation had been reached. Our sample of 31 parents included 17 parents with experience of NNUs and 14 parents with experience of PICUs. Interviews took place between May and November 2018.
FIGURE 3.
Parent recruitment to WP 2.
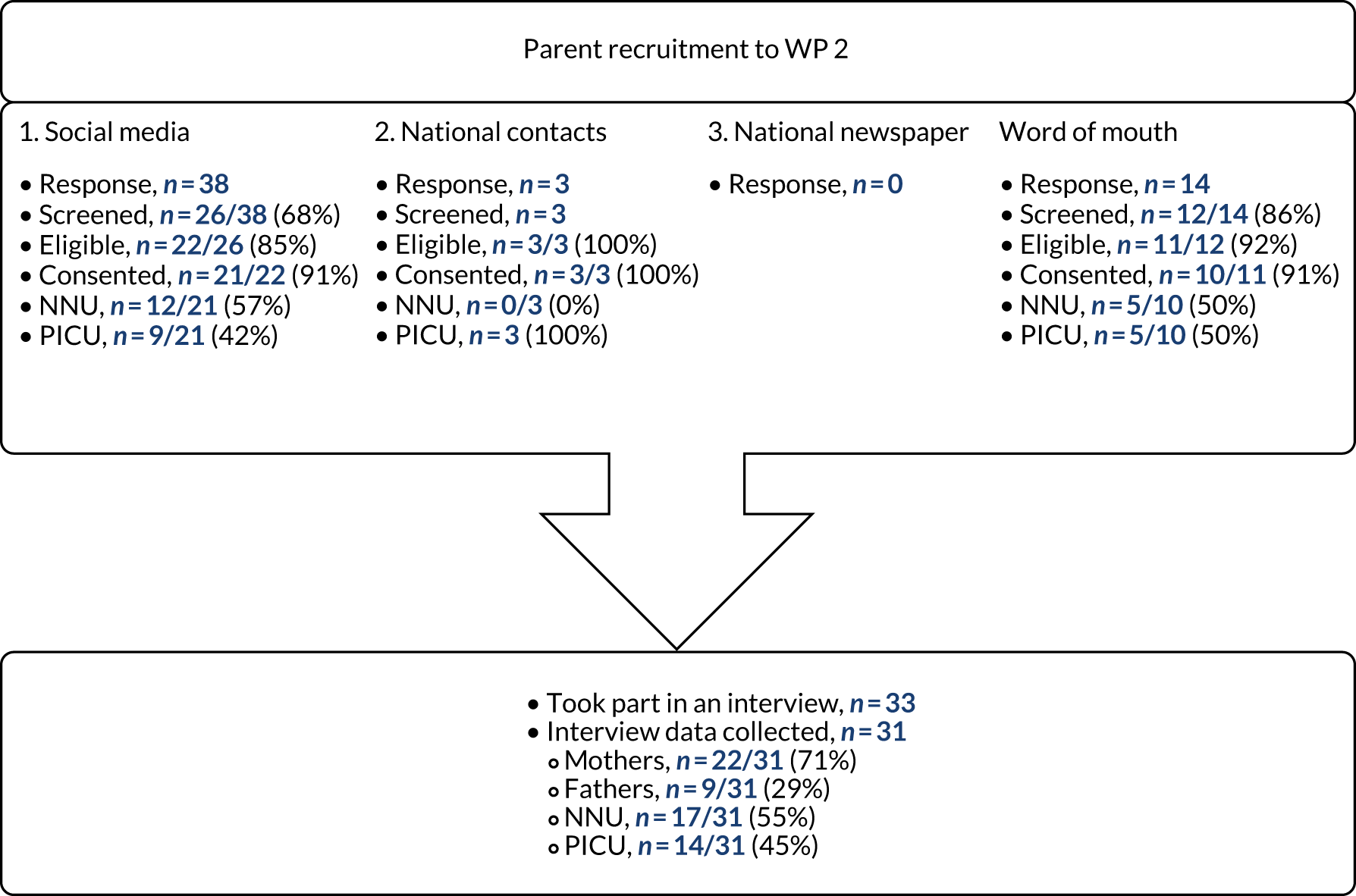
Most participants were recruited through social media (19/31, 61%) and despite advertising nationally, a large proportion of expressions of interest came from the North West. This geographical clustering was reinforced by the WP 2 team’s location in Liverpool and the second most effective recruitment method was word of mouth (9/31, 29%). Although not originally planned as a recruitment route, several parents expressed an interest in taking part in the study after hearing about it from friends, family and other participants. The newspaper advert did not yield any response.
Parent characteristics
The sample included 22 mothers (n = 12, NNU; n = 10, PICU; n = 4, bereaved) and nine fathers (n = 5, NNU; n = 4, PICU; n = 1, bereaved). Most parents lived in the north-west of England (23/31, 74%) and the remainder were from across England (Yorkshire and the Humber, n = 3; South East, n = 2; South West, n = 1; North East, n = 1) and Scotland (n = 1, 3%). The majority (26/31, 84%) of parents did not have medical backgrounds or related occupations. The five parents with health-care-related occupations had roles that included ambulance dispatcher, health-care assistant, physiotherapist, paediatric research nurse and a speech and language specialist.
Neonatal unit parent interviews (n = 17) related to 19 children. Three mothers had twins who were both admitted to NNU and one set of parents were interviewed separately regarding the same child (Table 8). PICU parent interviews (n = 14) related to 10 children, with four sets of parents interviewed separately regarding the same child (see Table 8). Six out of 10 children had also been admitted to NNU at birth and five children had had multiple PICU admissions.
| Characteristic | NNU | PICU |
|---|---|---|
| Child age at hospital admission (or birth) | Median 29 gestational weeks at birth (range 24–41 weeks) | Median 8 months (range 3 weeks to 12 years) |
| Days in unit | Median 21 (range 1–140, missing n = 1) | Median 8 (range 2–72) |
| Days in hospital | Median 57 (range 7–152) | Median 39 (range 3–196) |
| Days on feeding tube | Median 58 (range 2–210) | Median 127.5 (range 5–547) |
| Days on breathing support | Median 56 (range 0–370) | Median 6 (range 0–168) |
| Main reason for admittance |
Prematurity (n = 18) Meconium aspiration syndrome (n = 1) |
Heart conditions (e.g. congenital heart defect, hypoplastic left heart syndrome) (n = 4) Sepsis (n = 2) Reconstruction of airway (n = 1) Complications linked to chronic conditions (e.g. holoprosencephaly, Noonan syndrome, prematurity) (n = 3) |
Collectively, parents had experience of ICU and tube feeding spanning 21 hospitals. This experience varied from hospital admissions due to short-term acute health conditions to chronic conditions, when children had been placed on feeding tubes at birth and were still on them at hospital discharge (see Table 8). Although ventilation was not a separate inclusion criterion, most of the sample (28/31, 90%) had experience of their child being ventilated. Sixteen parents (50%) had experience of their child being approached to take part in a clinical trial (NNU n = 10, PICU n = 6). Telephone interviews took place, on average, 11 months (range 0.8–37 months) from admission to hospital and took a mean of 68 [standard deviation (SD) 12.7] minutes.
Participants: health-care professionals
Five focus groups were conducted at three UK hospitals: two in NNUs and three in PICUs. Forty-two HCPs (NNU, n = 16; PICU, n = 26) participated in one of the five focus groups. As most focus group participants were nursing staff, we purposively contacted doctors (n = 10), surgeons (n = 3) and dietitians (n = 5) and invited them to take part in a telephone interview. Five additional dietitians expressed interest in being interviewed after receiving study information through their professional networks. Of those we directly targeted, 12 out of 21 (57%) did not respond. A total of nine interviews were conducted. Ten hospitals from across England and Wales were represented in the combined interview and focus group sample. Focus groups and interviews were conducted between May 2018 and November 2018.
Health-care professional characteristics
Sixty-two per cent (26/42) of focus group participants were nurses. The remainder were research nurses (5/42, 12%), senior doctors (3/42, 7%) and dietitians (2/42, 5%). Six participants (6/42, 14%) categorised themselves as ‘other’. Three were student nurses and three did not specify. The interview sample included three consultant doctors (NNU, n = 2; PICU, n = 1), four dietitians (NNU, n = 1; PICU, n = 3) and two consultant surgeons (both worked with NNU and PICU patients).
The majority (45/51, 88%) of HCPs were involved in the direct clinical care of children and/or had experience of conducting paediatric clinical trials (39/51, 76%). Focus groups took, on average, 55 minutes (range 49–68 minutes) and telephone interviews took, on average, 32 minutes (range 26–45 minutes).
Parent perspectives
Prior to interviews, parents were sent copies of the draft PIS for a future RCT and a list of potential outcome measures to read. At the start of each interview, parents were asked questions about their experiences of their child’s hospital admission and tube feeding. The interview then moved on to discussions about the proposed GASTRIC RCT.
Views on feeding
Parents’ views around GRV measurement and the perceived acceptability of a future GASTRIC trial was intrinsically linked to their views on the importance of feeding in the ICU. The extent to which parents viewed feeding as a priority appeared to be influenced by their child’s prognosis and associated comorbidities or complications. For example, parents of children who experienced an imminently life-threatening condition, such as sepsis, had not considered feeding to be a priority in the ICU:
Bottom of the pile, yes, you know, the fact is they needed to – well initially we needed to try and get these feet not being black, erm, erm . . . Well initially obviously they were trying to keep her alive, erm, but, but, but the next thing was trying to get these feet, erm, to not be black as I, as, so I’ve said and, and make sure, you know, things like peeing for England, make sure everything was working as it should, and that the, the, the sepsis had gone.
P24, mother, PICU
Once the immediate threat to life had been addressed, parents described how feeding increased in importance, as it was a sign that their child was getting better:
But obviously the main concern at that point was getting her better and then, when we went into HDU [high dependency unit], I was like more worried about like her weight and her getting back to normal.
P21, mother, PICU
By contrast, parents of children with a long-lasting condition, such as prematurity, viewed feeding as very important from the onset of their experience. Weight gain and calorie consumption were seen to have a direct causal relationship with short- and longer-term outcomes. These included reduced length of time on breathing support, time in the PICU or NNU, number of infections, improved kidney function, ability to undertake and survive medical procedures, reduced chance of NEC, and overall health and development:
With our two being premature and being quite small, um, we knew that gaining weight was central to them going home and we also knew that the greater their weight, the less risk there was of an infection or something having a serious impact upon them. We knew that once they got up to full feeds the incidences of NEC reduced greatly as well.
P05, father, NNU
So they just wanted to make sure that she was having enough calories intake in her so that she could thrive basically and, um, she’d get bigger and stronger and, you know, hopefully the, the heart would stabilise.
P27, mother, PICU and NNU
Feeding was particularly pertinent to NNU and PICU parents whose children had experienced difficulties with feeding (e.g. reflux, vomiting, unsafe swallow, aspiration and oral aversion), weight gain or problems related to the child’s bowels (e.g. suspected NEC, bowel loops, bowel perforations, stoma) and breathing. Both NNU and PICU parents also described concerns about their children being force fed or overfed, leading to discomfort, vomiting, aspiration and oral aversion:
He always used to scream after his feeds, and, um, I said, it just feels like I’m force-feeding him; I actually said that to her, I said I feel like, you know, he’s like foie gras or something . . . he’s obviously in pain.
P02, mother, NNU
Yeah, it was a big, big issue, um, for me, because of him being sick. I felt like I was force feeding my child, um, to the point of him being sick and because of the pressure put upon us, um, by kind of the dietitians obviously to get certain amounts . . . of food needed, he needed to be getting his calories, especially as he had more surgery. It was really difficult for me.
P18, mother, PICU and NNU
In addition to being viewed as medically important, feeding of young children was something that parents, particularly mothers, felt was a key part of their parental role and responsibilities:
I think as a mother, it just feels like my primary role in a way, I was supposed to provide milk and ensure he grew.
P19, mother, PICU
During interviews, parents often described how feeding was also one of the few areas of their child’s care that they felt they could be actively involved in within the ICU:
. . . cause everything’s done for your baby other than like you can change a nappy. A lot of the practical care you can’t do, which is . . . it’s quite powerless, isn’t it, so it’s nice to be able to have like some hands-on time so yeah, it was really good.
P16, father, NNU
Children of parents in our NNU sample had a higher proportion of long-lasting illnesses and associated comorbidities than children of parents in the PICU sample. Consequently, it follows that feeding was more of a priority for NNU parents than for PICU parents. However, this prioritisation was formed on experiential knowledge of the importance of feeding gained during their hospital stay. In the first few days of NNU (which would be the point at which children would be eligible for inclusion in the proposed GASTRIC trial), parents would not have such experience. As the following quote illustrates, parents were initially unaware of how difficult, yet important, establishing feeds was at the beginning of their NNU stay and would still be in what feels like an acute situation:
I remember them saying, oh, they’ve coped really well with the last feed, and you think, coped really well? How hard is eating? And they don’t explain to you that it can have a huge a huge impact on their, on their kidneys and everything, because if they’re so premature, then their insides are immature as well, and so they might not even be able to filter through the nutrients, and you’re just left there with your mouth open going, what?
P11, mother, NNU
By comparison, over half of the PICU parents had past NNU experience and would therefore have already developed views on feeding in the early phase of their PICU stay.
Views on gastric residual volume measurement
Twenty-one parents recalled their child’s GRV being measured (NNU, n = 15; PICU, n = 6). Eight reported watching stomach contents being checked for pH and tube placement, but not GRV measurement (NNU, n = 3; PICU, n = 5). Three PICU parents stated that they were certain that GRV measurement had not taken place during the period in which their child was receiving tube feeding in NNU, PICU or at home. Those who did recall GRV being measured had varying understanding of its utility. Some were aware of GRV measurement, but had no understanding of its purpose, risks or benefits, as clinicians had not explained the procedure:
I mean obviously when the procedure was done whilst we were in the NNU, no one really explained why they were doing it, you know, what the benefits were or, um, I suppose what the negatives were for not doing it. I don’t think anyone ever discussed it, it was just a procedure that, er, was carried out.
P07, mother, NNU
Most parents had a basic understanding of its function, which they described as to see if their child’s bowels were working, to check milk tolerance, to guide food volumes or to help detect infections. Some had developed medicalised knowledge of the process and in some cases had performed GRV measurement themselves:
I remember a nurse explaining that they were doing it to see how much milk had been digested and that if all of the milk had been digested it was a good sign that they’re, um, bowels were working normally.
P05, father, NNU
They were, erm, sort of like take it out, erm, and then write it into the computer, erm, and they work it out but if there was so much, if there was too much left then they wouldn’t do the next one as much.
P21, mother, PICU
Some parents noted that GRV process differed between and within hospitals, in terms of frequency and execution. Such differences led parents to comment on how GRV measurement was not a ‘scientific’ (P30, mother, bereaved, PICU and NNU) or consistent process:
I would say it differs quite considerably between hospital to hospital, what the actual practice is. So in [hospital 1] they check the pH of the aspirates. So they take a little aspirate every feed and check the pH, um, to make sure that actually the tube is still located in the stomach, um, and they will check the, um, GRV every 4 hours. So if feeding hourly then they’d check it every fourth feed, then when it goes to two hourly feeds you check it every other feed. So you’re checking the full, um, volume in the stomach at that point. In [hospital 2] it’s completely the opposite. They rarely check the pH of the aspirate and I would say they never check the volume of the fluids in the stomach at all. And in [hospital 3] it is kind of a bit hit and miss. So they would check the pH at every feed, but they only check the volume probably twice a day, yeah, regardless of what kind of, yeah, feeding regime they’re on.
P13, father, NNU
Although GRV measurement was holistically viewed as being a low-risk standard practice, performed for the benefit of the child, some parents also perceived it to be invasive and felt that it had caused vomiting and discomfort:
I don’t see it as being high, high risk. but, um, my husband was quite, um, he was sort of quite worried them actually taking and, taking the fluid out, pulling it out . . . because when you’re, when you, you see your, um, your tiny baby in an incubator, hooked up to tubes and then having it, it, it’s quite invasive when you see, sort of, medical staff going in again, in the incubator when they’re trying to keep them warm and regulate the temperature. It’s sort of quite sort of daunting, um, whereas I’m quite, I’m quite, sort of, matter of fact. If it needs to be done, it needs to be done, and let them crack on.
P03, mother, NNU
Perceived acceptability of the proposed GASTRIC randomised controlled trial
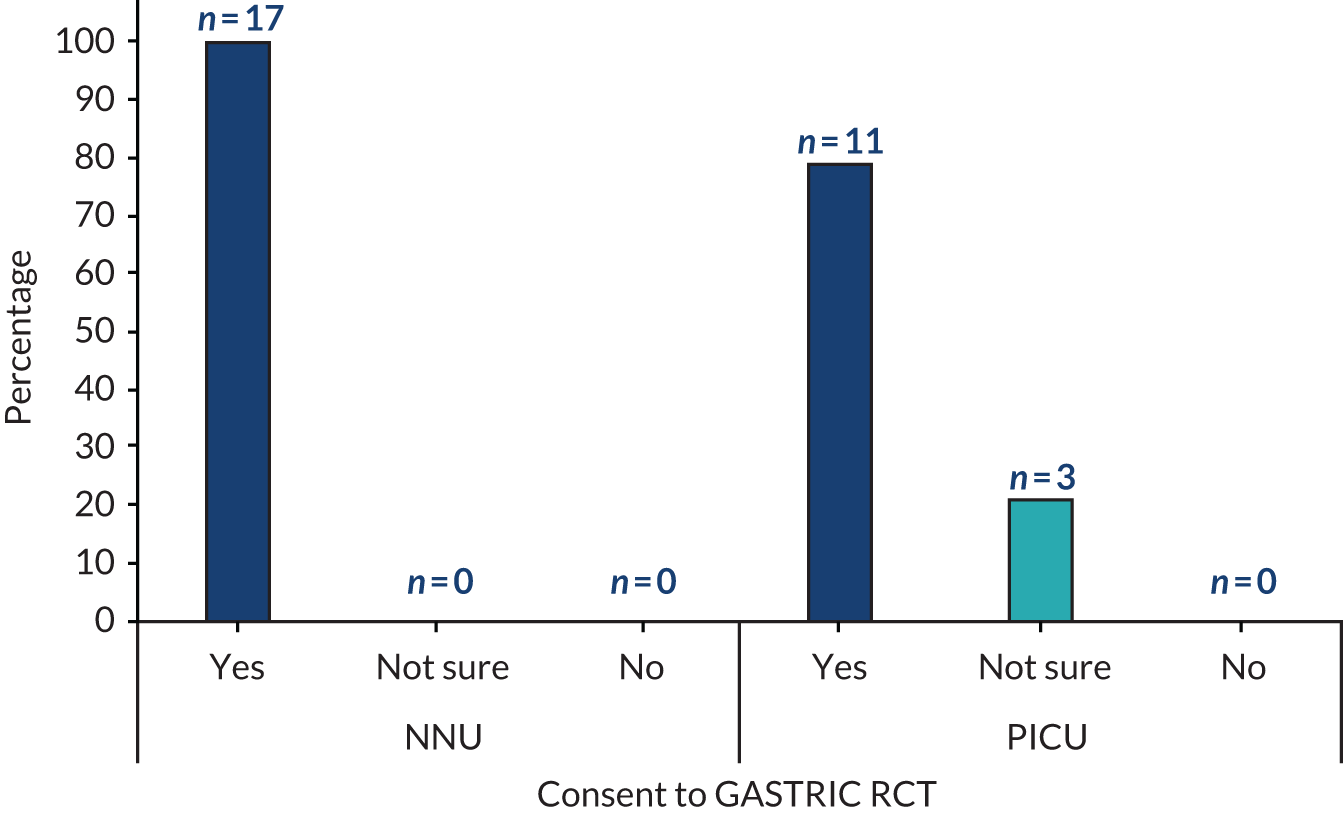
Interviews then explored perceived acceptability of the proposed GASTRIC RCT. Overall, parents supported the trial, with the majority (28/31, 90%) stating that they would hypothetically provide consent for their child to take part if they were approached about the trial (Figure 4).
FIGURE 4.
Parents’ views on whether or not they would have consented to the proposed GASTRIC RCT.
While considering trial acceptability and whether or not they would consent, many parents noted that they would not have pre-existing knowledge or beliefs about tube feeding or GRV measurement at the point they would be approached about the study:
I wouldn’t have known anything about feeding, so I wouldn’t have known any different.
P19, mother, PICU
Consequently, they would not have been aware it was a change in practice or, indeed, its consequences:
A lot of the time you wouldn’t know any different, it was all, all very new to me and if you said, we’re doing a trial where we’re not measuring it and if the nurse said, you know, we can’t see any effect, it wouldn’t, it wouldn’t bother me.
P15, father, NNU
Building on this situational equipoise, acceptability appeared to be influenced by a belief that the proposed study question ‘makes perfect sense’ (P23, father, PICU). NNU and PICU parents described how not measuring GRV might be beneficial because it would reduce potentially unnecessary interventions that may cause infections, discomfort and pain. Some also described how not measuring GRV may improve overall health, due to increased calorie consumption:
I just think that extra intervention, if it’s not actually doing anything positive, then is it really necessary? It’s, you know, it’s, it’s just another issue that can occur, another point of infection, um, and also another thing that the nurses are having to deal with on top of everything else.
P18, mother, PICU and NNU
It is a huge thing for them, to get the feeding established and get the calories on board, because that helps them get better faster as well. So, if you can get them better faster, you’re going to cut down, possibly that they’re blocking a bed as well. You know, like because if they’re getting calories on board quicker, they’ll start to feel better quicker . . . so it’ll have a knock-on effect in that respect. But again, you know, like these wee babies, feeding was one of the issues then that would kind of, I think, have an all-round effect on the overall condition of the, the baby.
P09, mother, NNU
Conversely, measuring GRV was also seen as acceptable, as their child would be receiving normal or ‘standard’ clinical care, because most units in the UK measure GRV:
Well that’s just the way it is. So would they be, would they be told that they’re in a trial and we’re just carrying on doing what we’re doing anyway?
P20, mother, PICU
Two parents described how no GRV measurement was acceptable and ‘normal’ care because, ‘if I was in France, this is what would be happening [not measuring GRV] so I wouldn’t, I wouldn’t worry based on that’ (P17, mother, NNU).
Some parents viewed GRV measurement or no measurement as of little importance, which was potentially influenced by the low significance associated with feeding during a critical care situation. These parents highlighted how, at the beginning of their child’s NNU or PICU stay, their child’s acute condition was the main priority and how a trial involving measuring or not measuring GRV would be seen as ‘low risk’ and therefore acceptable:
Obviously if they weren’t measuring the contents, it wouldn’t bother me ‘cause that’s what we do at home anyway. As I say, I don’t think I’d even be thinking about that at that stage. I don’t think I’d be worried that they weren’t checking it, um, ‘cause I’d be worried about other different, other things. So, um, I think that would be the last thing on me mind. So no, it wouldn’t bother me.
P27, mother, PICU and NNU
Despite parents describing their support for the study, many also voiced concerns and stated that their views on trial acceptability and consent decision-making would be influenced by how such concerns would be addressed. Parents described how they would not have capacity or desire to be approached about a trial in the early stages of their child being admitted to PICU or NNU. They referred to their emotional distress, uncertainty and generally being ‘overwhelmed by everything that’s going on’ (P14, father, NNU) at that point in time. They believed this incapacity would limit their ability to take on board study information and reach an informed decision. Some stated that being approached in the first 24 hours after ICU admission could have led to them to decline consent to a trial, which in different circumstances they would have consented to. Parents stated that any conversations about research in this early phase, when their child was still critically ill, ‘would have to be very carefully approached’ (P15, father, NNU). They also reflected on how the nature of the situation would enhance their desire to protect their child from any perceived risk, and that any trial or change from standard care would be viewed as a risk:
The fear of the impact. I think it’s quite different to ask for that consent for your child than it is like if it was for yourself. I think I’d be more likely to give that consent than I would be for one of my children. Just because, I don’t know. Same reason why you walk on the side of the pavement where the cars are, isn’t it?
P31, father, PICU
I don’t, I don’t feel that those, sort of, children who are in intensive care, I think they should be left alone, you know, because there’s some babies that are being fed by tubes that are absolutely tiny, really, sort of, you know, ill.
P03, mother, NNU
Some parents had described a preference for the ‘standard’ care arm:
Kind of trusting in older practices if that makes sense, especially in that kind of situation, kind of do what you’ve always done to make my child better type, er, feeling towards it.
P30, mother, bereaved, PICU and NNU
However, parents’ consideration of risk appeared to be influenced by the draft trial information provided to parents, which outlined potential risks of GRV measurement (standard care). The PIS raised questions and concerns about a practice that would not otherwise have been brought to their attention. Parents felt that this may result in the need for practitioners to fully explain and be prepared to answer questions on the motivation for both measuring GRV and not measuring GRV:
. . . ’cause it sounds really odd, doesn’t it, sucking up the contents of the stomach. I think it probably would, probably would have drawn more attention to something they do that’s standard practice; I might have been quite, a bit disturbed about that thought, and, and . . . questioned around whether it hurts and so on.
P19, mother, PICU
I suppose in, in general I’m all for, um, no intervention where it’s not necessary and so I think then learning that there was a study, exploring whether, um, we actually needed to measure in the way that it’s being done and she was being measured in the traditional way but there was now some question marks potentially about it because I was made aware of this study. Um, then that, that might make me ask a few extra questions.
P12, mother, NNU
This information was particularly important to PICU parents whose child had been on a feeding tube before admission and who had no previous experience of GRV. From their perspective, GRV measurement was not standard practice, but an additional, invasive and unnecessary procedure:
I would be kind of saying why? He’s never had this done before. This is not something I really want him to have done just for the sake of doing. It’s not necessary . . . But I possibly would say no unless there was some specific benefit to him [from GRV measurement] . . . I’m always kind of aware of not creating an aversion, not doing any more poking and prodding than is strictly necessary because he has plenty already. So I guess I’m probably just weighing it up on, yeah, was it gonna ’cause him any more, sort of any unnecessary discomfort, which I would avoid. Um, but in the main, I would be wanting to take part, I think.
P26, mother, PICU and NNU
Although situational incapacity, risk aversion and concerns about the impact of study participants may be applicable to most trials conducted in an ICU, there were three main trial-specific factors that concerned parents about the proposed GASTRIC trial. First, NNU parents were concerned about the risk of delayed diagnosis of bowel or stomach problems, or missing signs of an infection. Second, NNU and PICU parents worried about the risk of vomiting into lungs, which, as well as being potentially distressing for the child, may cause chest infections and breathing difficulties. Third, both groups of parents focused on the risk of increased pain or discomfort:
I think you’d just be concerned that, like, infections would go unnoticed or that their tube wasn’t in the baby’s stomach and it could be pumping milk in somewhere else.
P01, mother, NNU
Interestingly, parent accounts suggested that both measuring and not measuring GRV may lead to vomiting. Returning stomach contents may cause a child to vomit, ‘9 times out of 10 if they shot it back in, she was sick’ (P15, NNU, father), whereas not measuring GRV may result in overfeeding and vomiting. Similarly, not measuring GRV is believed to cause discomfort or pain if the child cannot be winded, the tube placement is incorrect or increased vomiting, whereas measuring GRV may cause discomfort and pain. In addition, NNU parents were particularly concerned about the risk of NEC not being detected because of not measuring GRV. This information was provided in the information sheet:
From nurses in passing that they’d mention NEC it, it seemed to be, you know, it was quite like, you know, a really, oh god, you don’t want your baby getting NEC and stuff like that, so that spread quite quickly I think, you know, amongst other parents. It was like, oh god NEC, you know, and stuff like that and it was something that god forbid that, that your child would get. So I think, if I was getting this, my question would be, if I hadn’t heard already within those 48 hours, what is NEC and how serious is it?
P14, father, NNU
However, as the previous quote from a father (P14, NNU) helps to illustrate, parents may not be aware of what NEC is at the time of their child’s hospital admission, as this knowledge is often gained from discussions with clinical staff during their child’s hospital stay. Therefore, a concern in retrospect may not influence parents’ views on trial acceptably at the point of consent discussions.
The three PICU parents who were unsure whether or not they would provide consent stated that there was not enough information about the possible benefits of each trial arm for them to predict whether or not they would consent. They described how if additional verbal information, such as the potential benefits of participation, was provided by a trial recruiter then they would potentially consent. However, based on the details provided in the draft PIS alone, they were uncertain about their consent decision:
I don’t think there’s anything you can put in your leaflet in that, in that moment that would have made me sign up to something that effectively felt like a trial or something that is deviating from current practice. Maybe if the doctor had said, had talked through a potential, potential benefit, in terms of the actual ability to get better or fight the infection. If that, if that was, if they were saying, you know, if your child’s taking in those calories he’s going to better fight the infection whatever it is, then maybe I’d have felt differently but the strength of the wording on that doesn’t, doesn’t sort of lead to a conclusion that would have sold it to me at the time, I don’t think.
P31, father, PICU
I think it depends how it, hmm, how it’s explained. There are the other questions I want to know. Sorry, it’s hard to say.
P19, mother, PICU
The proposed GASTRIC randomised controlled trial participant information sheet and consent discussion
Overall, NNU and PICU parents considered the draft PIS [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)] to be ‘very clear’ (P23, father, PICU), ‘very straightforward’ (P01, mother, NNU) and written in a way that would be comprehensible to someone with no pre-existing medical knowledge:
No, I think it’s quite self-explanatory to someone that, doesn’t really know anything. I think it was quite, I think the, the wording and everything, um, was fine. I think, you know, parents would be able to understand what it’s actually about.
P09, mother, NNU
Parents identified parts of the PIS that would benefit from clarification or change (Box 1). Both NNU and PICU parents highlighted the need for additional information regarding potential benefits of not measuring GRV and the adjustment to the wording of potential risks. The majority of NNU parents also reflected that they would require more information around NEC before they could make an informed decision about consenting to the trial.
Additional information, suggested examples:
-
the function of GRV
-
tubes/interventions increasing the likelihood of hospital-related infections
-
reducing procedure-related vomiting and discomfort
-
impact of growth on organ functioning
-
impact of calorie intake on overall health
-
time frame of the study.
Wording of the potential risk section, for example currently it makes the same point twice.
Clarify the difference between GRV, aspirate, tube placement and pH testing.
Discuss other types of monitoring that will be conducted.
Segment larger paragraphs and adjust sentence structures to help clarify content.
Include a glossary of terms (e.g. NG).
NNU PIS onlyNEC:
-
What is the risk of their child getting NEC?
-
How effective is GRV at detecting NEC?
-
If NEC is not detected by GRV, how else would you know?
Possible benefits:
-
Impact of growth on organ functioning and overall health.
NG, nasogastric (tube).
Given the emotive situation in which the PIS would be read, many suggested that the draft PIS was too long. That although ‘I understand obviously you need to get all the information across’ (P30, mother, bereaved, PICU and NNU), parents would be unlikely to process all the information, possibly resulting in them either making an uninformed decision or refusing consent owing to situational incapacity. Therefore, they suggested that all the key information should be summarised on the first page. Parents felt that this format would provide them with essential information needed at the time of the consent discussion, with the option to read the rest of the information later. Essential information was perceived to comprise the purpose of the study, risks and benefits. Therefore, the PISs were amended in response to parental feedback [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)].
When reviewing the PIS, many parents noted that the way they were approached about the trial would probably have more of an impact on their consent decision than written information:
So I just think it’s quite important how you’re approached. Um, and I definitely think, um, information sheets are great but actually being able to ask questions is probably quite important.
P29, mother, bereaved, PICU and NNU
There was disparity in views on when an appropriate time would be to approach parents about the trial; suggestions ranged from 12 to 48 hours from the time of their child’s PICU admission. Views on how long would be needed to make a consent decision ranged from 5 minutes to 24 hours. Parents emphasised the importance of personal and situational factors in facilitating the consent discussions. These included whether or not their child was stable, they were calm, both parents were present, the researcher was caring, supportive and knowledgeable about the trial, and whether the research was already known to them or introduced by a member of the clinical team:
This is it, who’s connected to the trial and the reasons why they’re doing it as well, I mean, that’s hugely beneficial. If someone just comes at you with a form and says, read that, can you sign it and agree to this? That wouldn’t work at all, you need a personal front.
P11, mother, NNU
Some parents questioned if it would be possible to obtain consent prior to being in the NNU, if the admission was planned, for example before a caesarean section or surgery:
For our situation and because there is the time beforehand to be able to look at it, consider it and process it, rather than try and make a decision when you’re in a bit of a crisis really . . . be given it at sort of, with the information pack about having a caesarean.
P06, father, NNU
Views on the use of cluster randomisation in a GASTRIC randomised controlled trial
A description of individual and cluster approaches to randomisation was read to participants (Box 2). They were then asked to reflect on how they think they would feel if they were informed that the unit their child was in was taking part in the GASTRIC trial and all babies or children were or were not having any routine GRV measurement.
The information sheet we sent to you describes how each baby/child will be allocated at random to receive either usual care (regular measurement of GRV every X hours) or no routine GRV measurement. This is called individual randomisation. What happens is, when a child is identified as eligible to take part in the study a doctor will log on to a computer system, or open an envelope, which will state which group that child will take part in. This is completely random, so if the study involved 100 children, the system would randomly allocate 50 children to the GRV group and 50 to the No GRV group. This is how most clinical trials randomise patients as it gives a fair test between the two treatments.
Another method is called cluster randomisation, this is when all patients in one grouping (e.g. a whole PICU) would receive the same treatment. So for the GASTRIC trial, this could mean that children in one ICU (e.g. Liverpool) all have GRV measurement and all children in another unit (e.g. Manchester) do not have GRV measurement. We would then compare the study findings between whole units rather than by individual child. As everyone in a unit will be receiving the same GRV approach, informed consent would not be sought, instead there would be posters about the study on walls and a doctor or nurse may speak to parents about the study at some point. You would have the option to ‘opt out’ or withdraw your child’s information from the study from that point forward.
Overall, parents stated that they would find their child’s involvement in a cluster randomised trial exploring GRV or no GRV measurement acceptable. The main reason provided was that they were happy with both proposed trial arms, as discussed previously. Additionally, parents cited the trust that they have in medical staff expertise to make decisions regarding the care of their child, often concluding that if NNU or PICU HCPs were happy to be part of the trial and not measure GRV, then parents would be satisfied that not measuring GRV as part of a cluster design was acceptable, low risk and therefore perceived to be safe for their child:
The medical staff, they’ve decided that this is something that needs to be done within the unit and they have made that decision. So they obviously think that it is a low risk, you know, to the babies, otherwise they wouldn’t be doing it.
P09, mother, NNU
Parents’ views on consent in a GASTRIC cluster trial design
Current guidance recommends that informed consent is sought for cluster RCTs when practically possible, yet makes provision for research without prior consent in trials that are deemed to be no more than minimal risk to participants. 21–24 We therefore explored parents’ views on approaches to consent if the proposed GASTRIC RCT used a cluster design.
Interview discussions around trial design indicated that parents’ views of the acceptability of a cluster trial design were influenced by the proposed approaches to consent. Parents had mixed views about their child participating in a GASTRIC cluster trial and/or their data being collected without their explicit prior consent. Most parents stated that they do not think it would be necessary to formally give consent for their child’s involvement in the proposed cluster trial, but they do feel that a conversation needs to take place between parents and an informed medical professional about the trial:
Just, yeah, just to be informed, and I think that as, as a parent, whenever you’re on the unit, that’s all that you want, is information. So, so even about a study, you know, when somebody coming up saying, oh, by the way, we’re doing this, um, you know, this is what’s happening on the unit, this is the care that [child’s name] receiving, it’s gonna help us with this study, are you happy with that?
P28, father, bereaved, PICU and NNU
Others described how they ‘would be OK with it [not giving consent], um, as long as I knew that my child’s identity was protected’ (P18, mother, PICU and NNU). Parents described how they trust staff to make the decision about their child’s participation, and as GRV measurement is part of usual clinical care and therefore not something they would normally be asked to provide explicit consent for, ‘it’s sort of happening anyway isn’t it?’ (P21, mother, PICU):
There’s that many procedures involved in every single bit, you know, and, you know, even from, I dunno, taking their temperature to, you know, the administration of drugs and things, that I’m guessing that it’s mainly done on a national basis, isn’t it, every single procedure? Um, I dunno, I mean, I’m not sure how much you, you’d want to . . . actually need to know a-, you know, ‘cause would you have to, to know about every single procedure, about what, what’s happening to your child? See what I mean? You know . . .
P22, father, PICU
One father (P31, PICU) stated that, in general, acceptability of his child’s trial participation without prior consent would depend on whether or not his child had a positive outcome:
I guess if you take the stress out of the situation then, and you look at it logically then, you know, I wouldn’t, about 2 weeks later if he was absolutely fine, I wouldn’t begrudge you having done it that way. But if he’d died, that’d be another thing on your mind, thinking, well did that have an impact on it? And we never got a choice in that.
P31, father, PICU
Others felt that, owing to potential concerns about this trial, such as those described previously, they would want to make an informed decision about their child’s involvement. Some parents described how they would query whether or not the trial was appropriate, as their child was very small and vulnerable:
Just, yeah, like from a, from my sort of controlling me, I can imagine it . . . yeah, whether I would sort of go oh why, why was I not told about this, you know, should I not have been told on day one and then I could have decided whether I wanted to be part of that study or not.
P29, mother, bereaved, PICU and NNU
If somebody said to me oh well we’re treating these children, we’re treating all the children like this in the hospital at the moment, I’d just think oh, but my child’s different, you know. Mine’s only tiny, you know.
P20, mother, PICU
Preferred randomisation method
After discussing the different randomisation approaches, participants were asked ‘which type of randomisation do you think we should use in the GASTRIC study?’ (Figure 5). Twenty-four parents thought that the GASTRIC trial should use a cluster randomisation approach (PICU, n = 9; NNU, n = 15), six preferred individual (PICU, n = 4; NNU, n = 2) and one did not know (PICU).
FIGURE 5.
Parents’ preferred approach to randomisation.
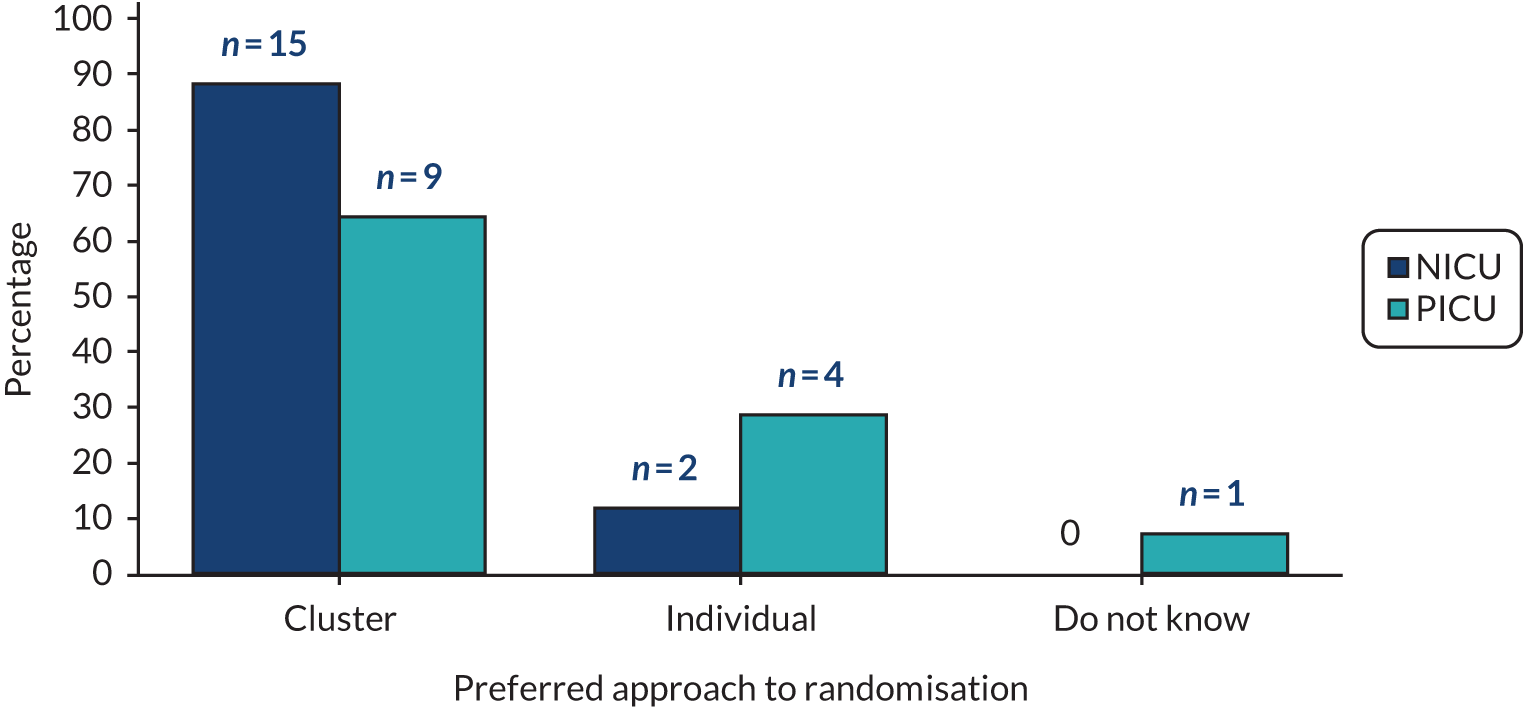
Overall, parents were in favour of a cluster approach. This preference was underscored by trust in medical staff and the perceived acceptability of both study arms. They stated that not seeking full and informed consent would circumvent the previously discussed issues of situational incapacity, timing issues and prevent any concerns about children receiving different care:
I can see the benefit of, um, in that sort of traumatic period of, you know, my child’s been admitted to PICU, not having to make the decision, the decision already being made and everybody else is in the same boat.
P29, mother, bereaved, PICU and NNU
I think I’d be happier knowing that all the babies in the unit were getting the same care, so there’s potentially no disadvantage between babies.
P02, mother, NNU
Um, in some ways I think it would be good because you wouldn’t get parents kind of saying, oh my child doesn’t have that done, and the other one saying mine does. You know, people talk, don’t they? Parents talk particularly in things like that. And I think it would, kind of from an equity point of view, it would just be easier to have one unit doing one thing and one unit doing the other. The only thing I’m not sure about is kind of including people in something without their expressed consent.
P26, mother, PICU and NNU
Nevertheless, parents said that they would still be likely to provide consent if the trial used an individual randomisation approach, although their decision would be dependent on how and when they were approached about the trial. Parents who preferred an individual randomisation approach did so because they wished to provide informed consent owing to concerns about the trial. Parents also stated that they would also be likely to provide consent if the trial used a cluster approach, if the motivation for the methodology was explained by a HCP.
Outcomes of importance to parents
Appendix 3 includes the list of outcomes and accompanying descriptive text sent to parents prior to the interview. In this section of the interview, a definition of an outcome was first read to parents, including an explanation about why it is important to explore parents’ perspectives about important outcomes (Box 3).
As we have discussed, in the GASTRIC study we want to find out whether not measuring stomach contents (GRV) is better than measuring stomach contents (usual care).
To do this, we will collect information on (read through outcome measures list sent prior to interview). By collecting information on these main things, we hope to find out which approach (to measure or not measure GRV) should be used in the future. These are called outcome measures.
However, these outcomes have come from research papers and don’t really give us much information on how children or families feel, or what is important to them. It is important that we include outcome measures that matter to children and their families.
We then asked parents, ‘Thinking about your experience of your child being admitted to the NNU or PICU, what would you hope the GASTRIC study (e.g. the approach to feed measurement) would do to help your child?’.
In response to this question, most parents focused on increased weight gain or growth, time on a feeding tube and, in the case of the NNU patients, ‘reduce the chances of getting NEC’ (P05, father, NNU):
As long as they were putting weight on and progressing . . . with the feeds and having less tube feeds and more breast feeds and bottle feeds. I think that’s definitely one of your biggest concerns, I remember thinking, I just want them to put on weight, because you felt like they were going to get a little bit better every time, if they got a little bit bigger they’d be able to fight infections off.
P01, mother, NNU
From our team’s previous work exploring parents’ prioritised outcomes for trials investigating treatments for sepsis,10,25 we were aware that parents sometimes find this a difficult question to conceptualise and answer. Therefore, all parents were then asked, ‘In general, what would you be looking for as an indicator that your child was getting better?’. The most common responses were breathing support, weight gain, vital signs and time to full feeds:
I don’t know, you sort of know your own child, don’t you? Obviously when she was in intensive care sort of like the ventilation pressures, erm, her having less temperatures, her oxygen requirements coming down. Erm, and then when she sort of was waking up, her wanting a cuddle from me, erm, like she wouldn’t off anybody else. Erm, and just sort of like little sort of indicators that she was feeling a bit better, wanting to watch Peppa Pig and, you know, just little things.
P21, mother, PICU
As illustrated by the quotation from participant 21, PICU parents also prioritised how quickly their child looked and/or behaved like more like their normal self. Examples included improved mood, communication and colour, and being more alert and interested in their surroundings. When directly questioned about the provided outcomes list, both groups of parents thought that they were comprehensive [‘it was quite a, a very thorough list’ (P07, mother, NNU) and contained ‘important’ (P24, mother, PICU) outcomes].
The researcher then repeated back the outcome measures identified by the individual throughout the interview discussion. Parents were then asked to rank their identified outcomes in order of importance for the proposed GASTRIC trial.
As in previous work,25 not all parents included survival as an outcome in their ranked list. When this was the case, the researcher then proceeded with a follow-up question to explore if this is because they did not think it was an appropriate outcome for the GASTRIC trial or if they were other reasons why they had not prioritised survival. One mother reflected that ‘survival has most definitely been probably the most important thing in our journey on PICU. So I don’t know why I ignored it’ (P29, mother, bereaved, PICU and NNU). Some suggested they had not mentioned survival as ‘you don’t think that survival’s even a question’ (P02, mother, NNU). Others explained that survival was not mentioned separately, as it is implicit in the list of outcomes they provided:
I suppose I’ve indirectly, indirectly said that survival is one of my most important things, ‘cause whenever you asked me to identify my top I said, well it’s the ones that’s gonna kill them. So it’s hospital acquired infections and NEC. Yeah, if, if you were saying, first of all it’s survival, um, minimising the things that’s gonna kill them, and then minimising the things that’s gonna cause long-term disabilities. So I think that’s my, that would be my three categories of outcome measures in, um, in, yeah, children in intensive care. So I know infections could kill them, I know NEC could kill them, I know that brain injury could kill them, do you know, so they, they always flagged up quite high for me. But I suppose as a parent you don’t want to say, do you think they’re gonna survive or not, because you don’t want to think, I suppose, that they may not have. So that might be why.
P05, father, NNU
Findings from all outcome questions were then combined to determine the top prioritised outcomes by ICU group (Table 9). Time on ventilator and breathing support, and weight gain both ranked in the top three for both NNU and PICU parents. Prioritised outcome measures did not appear to be condition dependent (e.g. cardiac parents/non-cardiac parents in the PICU).
| NNU parents | PICU parents | All parents |
|---|---|---|
| 1. Time on ventilator and breathing support | 1. Weight gain | 1. Time on ventilator and breathing support |
| 2. Hospital-related infections | 2. Time on ventilator and breathing support | 2. Weight gain/growth |
| 3. Weight gain | 3. Long-term feeding | 3. Survival (first when prompted) |
| 4. NEC | 4. Back to self | 4. Full feeds |
| 5. Time to full feeds | 5. Survival (second when prompted) | 5. Hospital-related infections |
| 6. Survival (first when prompted) | 6. Time to full feeds | 6. Time in hospital |
| 7. Time in hospital | 7. Time in hospital | 7. Long-term feeding |
Health-care professional perspectives
At the start of focus groups and interviews, HCPs were asked questions about their clinical experience of GRV measurement. The discussion then moved on to focus on the proposed GASTRIC RCT. Most HCPs (representing 7 out of 10 hospitals) described how their unit had specific guidelines around the measurement of GRV. Although the guidelines were in place, it was acknowledged that not all staff adhered to the guidelines. For example, two units had recently completed an audit of GRV practice and one found ‘that everyone was doing something different’ (P01, interview, dietitian, PICU). A few of the participants said that they felt ‘very restricted by the guidelines’ (P02, interview, dietitian, PICU).
When describing GRV practice, the frequency of measurements was similar within and across NNUs and PICUs. For example, the majority reported that their unit measured GRV every 4 hours or at every feed. There were differences across units about the amount of volume they would consider ‘too much’ or of concern. Many described how feeds would be discarded if they were large, bilious or green, blood stained or abnormal. Feeds would be returned if ‘it looked like part-digested milk’ (P01, FG3, senior nurse, NNU). These findings were similar to the survey of practice reported in Chapter 1.
Multiple reasons for measuring GRV were provided, including as a guide for tolerance of feed; historical practice, or as a habit; because staff were asked to measure it; clinical judgement reasons; gastric emptying; if child was vomiting, because painkillers reduce gut motility; to aspirate air; to check for irregularities; because a child’s abdomen looks distended; for comfort of the child; or to check tube position. Commonly, practitioners across NNUs and PICUs (including nursing, doctors, dietitians and surgeons) described how GRV measurement provides them with reassurance that a child is tolerating feeds, which then informs decisions about subsequent feeding:
I think it’s a reassurance for the ward that the feed is being tolerated.
P02, interview, dietitian, PICU
In neonatal units [. . .] the kind of culture behaviour is, is quite a complex kind of beast and, um, the nurses clearly feel that it gives them some reassurance about, um, you know, ability to increase feed, or it maybe gives them some reassurance that the baby is, is tolerating milk.
P06, interview, doctor, PICU
To check that the clinical feed is tolerated.
P09, interview, surgeon
In contrast, some medical staff and dietitians stated that measuring GRV was, on its own, ‘completely meaningless’ (P06, interview, doctor, NNU) and that ‘I don’t actually think it really represents anything’ (P01, interview, dietitian, PICU). Some stated that ‘we shouldn’t be measuring it’ (P02, interview, dietitian, PICU) and that if ‘given half a chance, don’t measure it at all’ (P05, interview, surgeon). Only two nurses shared this viewpoint. Most nurses described the value of measuring GRV to inform feeding and their clinical care of children. HCPs reported that parents do not measure GRV in their unit, but might be involved in measuring the pH levels of the aspirate or help with the feeding of the child. This was particularly the case in NNUs.
In two focus groups, neonatal nurses and one PICU dietitian stated that NEC was a consideration when checking GRV and how not measuring GRV may lead to NEC due to increased feeding, or that signs of NEC may be missed:
There is a real potential of NEC as well if you just keep on feeding and feeding and feeding.
P03, FG4, staff nurse, PICU
You’ll have a baby who you’re not measuring residuals anymore, who will end up with NEC and then they’ll say, ‘Oh, this would have been picked up earlier if we’d realised it has bilious aspects’.
P05, FG5, consultant neonatologist, NNU
However, during the telephone interviews, half of participants (including dietitians, doctors and surgeons) described how measuring GRV was not indicative of NEC, ‘babies who are going to develop an important pathology, never present purely with gastric residuals’ (P06, interview, doctor, NNU), and how they ‘don’t think that measuring the GRV is going to be a, um, reliable indicator of whether a baby’s got NEC or is at risk of getting NEC’ (P05, interview, surgeon).
The proposed GASTRIC randomised controlled trial
Perceived benefits and risks of not measuring gastric residual volume
After introducing the proposed trial using the staff PIS [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)], the researcher sought practitioner views on the potential barriers to conducting the trial, including perceived benefits and risks of not measuring GRV. Perspectives were explored using voting in focus groups, as well as through discussion in interviews and focus groups.
As shown in Table 10, the majority (87.5%) of HCPs indicated that there were barriers to not measuring GRV, including concerns about increasing the risk of adverse events (AEs), causing discomfort and pain, or vomiting. There were also concerns about not being able to identify early signs of infections, gut obstructions or feed intolerances, lung injury (acute respiratory distress syndrome), NEC, stenosis of pyloric sphincter or incorrect feeding tube placement. As described previously, not detecting NEC was a concern associated with not measuring GRV and this influenced views on the proposed trial. Although this did not concern PICU staff as much as NNU staff, NEC was a concern discussed by both groups.
| View | Participants, n (% within threshold) | |||||
|---|---|---|---|---|---|---|
| Senior nurse (N = 11) | Junior nurse (N = 15) | Research nurse (N = 4) | Senior doctor (N = 7) | Dietitian (N = 7) | Other (N = 4) | |
| Yes | 9 (82) | 14 (93) | 4 (100) | 5 (72) | 6 (86) | 4 (100) |
| No | 2 (18) | 1 (7) | 0 (0) | 2 (28) | 1 (14) | 0 (0) |
Health-care professionals were then asked to consider any potential barriers to measuring and recording GRV in a set way, in a proposed clinical trial. As shown in Table 11, most staff did not anticipate any barriers with this arm of a proposed trial. However, six out of eight (75%) junior nurses did anticipate barriers. A few were process related, such as ‘measuring and the writing down and stuff’ (P07, FG2, research nurse, PICU) or attitudinal [‘I think staff attitude, yeah, rather than a practical barrier’ (P05, FG2, nurse, PICU)].
| View | Participants, n (% within threshold) | |||||
|---|---|---|---|---|---|---|
| Senior nurse (N = 11) | Junior nurse (N = 14) | Primarily research nurse (N = 4) | Senior doctor (N = 6) | Dietitian (N = 7) | Other (N = 4) | |
| Yes | 2 | 6 | 1 | 0 | 0 | 1 |
| No | 9 | 8 | 3 | 6 | 7 | 3 |
Many HCPs discussed the potential benefits of measuring GRV during the focus groups and interviews, which they perceived included benefits for babies and staff by helping to inform clinical care. Perceived benefits for children included reduced reliance on intravenous lines, having fewer problems with motility and reduced risk of infection. HCPs also described how GRV can help babies ‘be more comfortable, more settled’ (P04, FG1, nurse, NNU), reducing the risk of vomiting and ultimately going home quicker.
The most common benefit of not measuring GRV from HCPs’ perspectives was increased nutritional value of not removing stomach contents. They highlighted the values of increasing ‘nutritional intake by not checking [GRV], in the vast majority of patients’ (P02, interview, dietitian, PICU), with one participant explaining that by not measuring GRV:
. . . well you probably get onto feeds much quicker, because people worry that . . . there are residuals and then start fretting about them and often end up stopping feeds, um, which . . . it takes longer for babies to get onto full feeds.
P04, interview, doctor, NNU
A surgeon described how ‘measuring it [GRV] gets in the way or can get in the way of advancing things, um, because the hesitancy is a baby has a what is felt to be large GRV or a dark green colour to it, to not feed the baby, um, or child’ (P05, interview, surgeon).
Others described the benefits of not measuring something unnecessarily, particularly when there were uncertainties about whether or not the calculations currently used to measure GRV were optimal, owing to lack of evidence to inform practice.
Change in practice
Health-care professionals described how a change in practice, to not measuring GRV, would be a challenge for the proposed trial. As shown in Table 12, 23 staff described how not measuring GRV would require HCP behaviour change. Mainly, nurses indeed valued GRV measurement as a useful practice that informed patient care. Six participants, from a range of clinical backgrounds, described how they would be uncomfortable about changing a ‘normal’ practice for a trial, without evidence to support such a change.
| Behaviour change (n = 23) | Clinical concerns (n = 6) | Current GRV practice is helpful (n = 7) | Wanting evidence first (n = 6) |
|---|---|---|---|
| Um, it’s probably just, um, I can imagine there will be barriers to it because of it’s just the way, the way that things are done and that’s the way, you know, way it’s always beenP02, interview, dietitian, PICU | So it might cause quite a few people to maybe ask some questions, they might not think it’s the best thing to do, for whatever reasonP01, FG2, research nurse, PICU | It’s their safety net for advancing feeds and, and changing things aroundP05, FG5, neonatal consultant, NNU | I tend to think if I was a parent on here . . . I might be more concerned about the fact that you’re changing normal practice without the evidence. And I appreciate you’ve got to get the evidence but . . .P07, FG2, research nurse, PICU |
| You obviously feel dubious about it because it’s something new, and we’re not very good in health care at change anyway are we so when someone comes and says, we’re gonna do this . . . Everyone’s gonna say, well, no, actually I don’t want to do thatP02, FG4, other, NNU | It would be a nerve racking to take that away because we do, as you say, it’s part of the whole pictureP02, FG1, junior nurse, NNU | . . . if it’s a new protocol, then we’d do it, because obviously all the research has been done and proven that that’s better, so even if you feel uncomfortable with it, the research is there; but if the research isn’t there, and you’re just trying something, like, you’d feel quite uncomfortableP06, FG3, senior nurse, PICU |
In contrast, some dietitians reported that they were planning to change their unit practice to not measure GRV, which meant the proposed trial was less of a concern:
I mean we’re going, we’re planning to update ours, um, in the next couple of months cause it’s due for revision. So our next protocol is, is not gonna have measuring GRVs in it.
P01, interview, dietitian, PICU
Others commented on the current debate around GRV measurement and how some units were questioning their practice or were in the process of rewriting their feeding algorithm protocols:
At the moment I’m trying to rewrite our feeding algorithm protocol, there’s some debate on the unit as to whether it’s right.
P03, interview, dietitian, PICU.
There have been discussions over the years and certainly there’s now a lot of questioning about how relevant it is . . . it’s not something that I’ve ever tried to influence or, or change really.
P08, interview, dietitian, NNU
There were mixed views on the importance of the clinical question. During focus groups and interviews, dietitians often commented on the importance of the clinical question and how the proposed trial ‘could change things quite a lot’ (P03, interview, dietitian, PICU). By contrast, medical practitioners (four doctors and one surgeon) described how the question of whether to measure GRV or not was ‘more of an irritant than a, you know, a major uncertainty’ (P04, interview, doctor, NNU). Some stated that it was not a ‘big issue’ (P06, interview, doctor, NNU) or not important ‘in the grand scheme of things’ (P07, interview, doctor, PICU).
Trial acceptability and feasibility
Of the 46 practitioners (95.8%) who answered the question, ‘how acceptable is it to conduct the proposed trial?’, the majority (39/46, 85%) indicated that it was ‘acceptable’ or ‘very acceptable’. Only 15% (7/46) said that it was not acceptable or very unacceptable. Of these seven, six were junior nurses. All practitioners (n = 48) said that the trial was practically possible to conduct and 47 practitioners (98%) said that it was practically possible to not measure GRV.
Health-care professional ‘buy-in’ for the proposed GASTRIC randomised controlled trial
Participants were then asked about whether or not they felt it might be difficult to engage any particular staff groups in conducting the trial. Participants (nurses, dietitians, consultants and surgeons) felt, overall, that all professional groups would support the proposed trial. However, they felt that engaging other staff groups could pose a challenge:
I think some nursing staff would feel deeply uncomfortable about not doing, measuring GRV.
P01, interview, dietitian, PICU
She [doctor] might think it’s, it’s in the baby’s best interest not to follow the protocol but, er, to do what we normally do, so whether you can class that as a barrier or not.
P05, FG1, research nurse, NNU
During two focus groups and two interviews, participants discussed how general surgeons value GRV measurements in some situations (e.g. after babies have had gut surgery):
Surgeons, they’ll really like gastric residual volumes.
P01, interview, dietitian, PICU
Whether it’s just on preterm babies but on your post op babies, the surgeons may feel certain babies you should measure it, and I probably would agree with them. So whether they get enrolled in the trial or whether they’re opted out, that could cause confusion.
P01, FG5, senior nurse, NNU
Health-care professionals highlighted how it was particularly important for senior doctors and consultants to support the trial to help facilitate wider staff engagement and trial conduct:
I think with the right leadership at consultant level, cause that’s, that would be the important thing, you would get a lot of people in the trial.
P06, interview, doctor, NNU
The challenge would be, you know, in a unit with 10 consultants, what’s the dynamic of that unit and, and, and can the PI [principal investigator] really persuade all his consultants to buy-in.
P06, interview, doctor, NNU
Facilitating health-care professional ‘buy-in’ through training
When asked what could help facilitate HCP ‘buy-in’ for the proposed trial, several suggestions were made. These included bespoke site training and providing additional information to support the study rationale, including any evidence to demonstrate why not measuring GRV might be beneficial to patients:
It doesn’t hurt to have an education package.
P02, FG5, junior nurse, NNU
Good data-driven justification to demonstrate why not measuring GRVs would be a sensible thing to do and in fact might be beneficial.
P05, interview, surgeon
Health-care professionals highlighted the need to provide information and education around which clinical parameters would be monitored in the trial when GRV was not being measured, to help inform clinical care. Suggestions included monitoring bowel movements, including if stools were loose or bloody, or bowels were loopy, whether the abdomen was distended or hard, and whether the patient was comfortable was vomiting or had arterial oxygen desaturations.
A few participants were confused about the difference between checking the GRV (by aspirating the entire stomach contents) and simply confirming the position of the feeding tube (by testing the pH, involving testing a small amount of fluid). Guidance and education would be needed about this for a future trial.
Many stated that a good training package would help ensure ‘trial compliance, er, protocol compliance’ (P04, interview, doctor, NNU), whereas face-to-face site training was favoured over ‘e-learning’ (P03, FG2, junior nurse, PICU).
During interviews and focus groups, HCPs described the importance of disseminating training to all staff, ‘including the night staff’ (P08, interview, dietitian, NNU). Suggestions included embedding training into study days, away days and research forum groups:
People struggle . . . sometimes you can have the training on a research study weeks and weeks before we actually start it so by the time you come to start it, then it’s a bit like oh I need a refresher really . . . like maybe the week or 2 weeks before you’re starting it rather than being a big gap before you start it.
P07, FG1, other, NNU
Trial inclusion and exclusion criteria
Neonatal unit and PICU HCPs suggested that babies and children with bronchiolitis, epilepsy, pneumonia, no gastric problems and those with no previous experience of ventilation may be suitable for inclusion. The majority (38/46, 83%) identified some children who should be excluded from a future trial (Table 13).
| Exclusions for both NNU and PICU |
|---|
| Babies or children not being fed |
| Babies or children with feed intolerance |
| Babies or children who have or will have surgery |
| NNU-specific exclusions |
| Congenital gastrointestinal anomaly babies |
| Babies with a risk of NEC |
| Very premature babies |
| Babies with NG on free drainage |
| PICU-specific exclusions |
| Children with brain stem death or awaiting organ donation |
| Children tube fed at home or well-established feeding regime |
| Liver patients |
| Children with oesophageal stenosis |
| Children with reflux |
| Children with tracheoesophageal fistulas |
| Children with tracheal slides |
| Children with traumatic brain injury |
Overall, all HCPs stated that the proposed trial was practically possible to conduct (n = 48). However, many described how flexibility regarding who would be included in the trial was needed and they were keen to consider ‘the clinical picture [of the child] as well though’ (P01, FG1, senior nurse, NNU):
I think there really does need to be a ‘get-out clause’ so that they can choose to start measuring GRV.
P04, FG2, other, PICU
The same participant said:
. . . if you’re going against the clinical judgement of the medical staff and, you know, the child’s vomiting or constantly vomiting or . . . I think there needs to be a get out clause which of course will upset your results completely.
P04, FG2, other, PICU
Potential trial design
Finally, HCPs were provided with a description of individual and cluster randomised trial designs (similar to the description provided to parents) and asked to consider the most appropriate design for the proposed trial. We also explored views on the benefits and challenges of each trial design and approaches to consent. Views on trial design were mixed (Figure 6), with 20 out of 48 (42%) practitioners preferring an individual randomisation design and 25 out of 48 (52%) preferring a cluster randomisation design. One person had no preference and for two people these data were missing. Just over half of nursing staff preferred individual randomisation (14/26, 54%). Three research nurse participants preferred a cluster design (3/26, 12%). ‘Other’ staff groups also preferred a cluster randomisation (11/17, 65%).
FIGURE 6.
Proposed trial randomisation method preference by current job role.
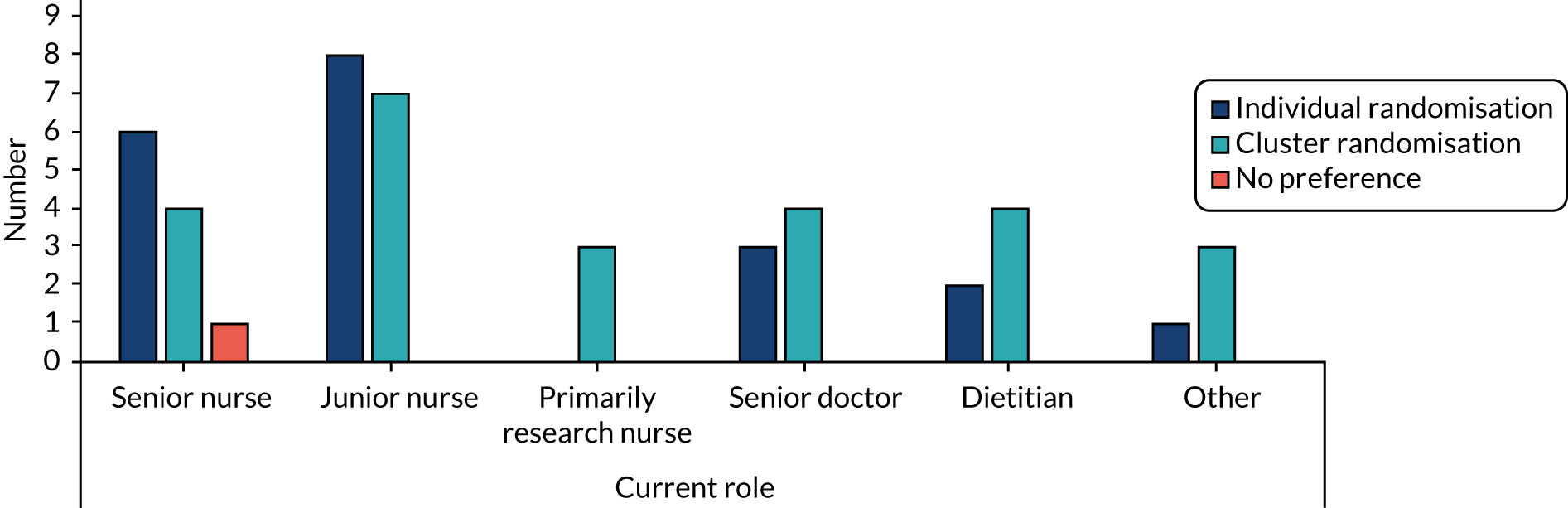
Health-care professionals understood and had experience of an individual randomisation trial design. Most were happy with the trial design because of the familiarity of it, but did report some concerns about protocol adherence due to confusion about which babies were part of the trial:
I mean we all think that we’re excellent at handing over and it would be fine but I think there, there is that potential, more potential for error.
P04, FG2, other, NNU
I think you could get muddled, yeah.
P07, FG3, nurse, PICU
There was also general agreement about the difficulties with an individual randomisation from the parent perspective, which included unease about change in care:
If they know that their child is being treated differently to the [next] child – even if it’s something really small which they probably don’t really understand anyway, the fact that they’re being treated differently . . . [they could be unhappy about it].
P08, FG3, consultant, PICU
Although some stated that ‘there wouldn’t be buy-in at all’ (P06, interview, doctor, NNU) if the whole unit was involved in the cluster design, many were positive about this approach to randomisation. Practitioners said that it would ‘be easier’ (P04, FG3, staff nurse, PICU), particularly ‘to implement for the nurses at the bedside . . . sometimes I think it feels natural; it’s a teaching hospital, and I think people sort of accept that, they know it’s a teaching hospital and they understand that research gets done in teaching hospitals’ (P03, FG3, dietitian, PICU).
Health-care professionals appeared to believe that a cluster design would reduce workload compared with the individual randomisation trial design, particularly in relation to the informed consent process. One participant stated that ‘the nurse looking after the baby on the shop floor, ’cause they’ve got enough to do as it is, the least amount of extra work that it causes the better’ (P05, FG1, research nurse, NNU).
Participants also touched on the perspective of parents and how a cluster design may prevent parental concerns that their baby was being treated in a different way to others. This concern was spoken about more commonly in relation to NNUs than PICUs:
The only different thing is I know from trials is that parents talk a lot and when you’ve got a trial, and you might have two babies in the same room and one of them is not getting the residual volume measured and one of them is and then something goes wrong, that’s when you’ve got, you’ll end up with problems.
P06, FG1, research nurse, NNU
Summary of findings to inform the GASTRIC trial
Overall, most parents supported the proposed GASTRIC RCT and would have provided consent for their child’s participation in the trial if they were approached at an appropriate time, and ideally not in the initial hours of NNU or PICU admission when their child was critically ill. Although GRV measurement was viewed as a ‘low risk’ and standard practice, it was also perceived to be invasive. Therefore, the ‘intervention arm’ (of not measuring GRV) might be seen as non-invasive and potentially beneficial, thus increasing trial acceptability.
Prior knowledge about GRV measurement was one of the main differences between the NNU and PICU parents. Parents with no experience of tube feeding or GRV measurement at the point of their child’s admittance to the NNU or PICU hypothesised that they would have considered the proposed trial as being low risk, without having any preconceptions about measuring, or not measuring, GRV, and thus no preference for either trial arm (equipoise).
Both NNU and PICU parents raised concerns about not measuring GRV and increasing the risk of delayed diagnosis of bowel or stomach problems, vomiting into the lungs and increased pain or discomfort. Parents were also concerned about their situational incapacity, which would make it difficult to make an informed consent decision. In addition, some parents were unable to distinguish between GRV measurement and testing pH and/or tube placement. These concerns could be addressed by adjusting the participant information and through trial protocol changes to help ensure appropriate timing of trial recruitment discussion.
Overall, parents preferred a cluster approach to randomisation, but would probably still consent if the trial used an individual randomisation. Contrastingly, HCPs were split in their preference for individual randomisation or a cluster trial dependent on role and experiential knowledge.
Differences between the outcomes prioritised by NNU and PICU parents were observed. Although both groups ranked time on breathing support and weight gain in the top three outcomes, NNU parents ranked hospital-related infections and PICU parents ranked long-term feeding issues as the most important outcomes.
Multiple reasons were cited by HCPs for measuring GRV, the most common being that it provides reassurance to nurses that a baby or child is tolerating feeds. However, there was also support for not measuring GRV, with some practitioners describing GRV measurement as meaningless.
Concerns about the acceptability and success of conducting the proposed trial mainly related to changing routine practice. ‘Buy-in’ from staff was considered critical in facilitating trial success and some practitioners believed that comprehensive training and defined inclusion and exclusion criteria could address some of the perceived barriers. 10,26 Despite these potential obstacles, most staff felt that the proposed trial was acceptable and all staff felt that it was practically possible to conduct.
From this qualitative work, we have revised, developed and improved the PISs (both for PICUs and NNUs) [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)], and we have developed HCP training packages (see Appendices 4 and 5).
Chapter 3 Trial design survey including e-Delphi
Introduction
A key aspect of assessing the feasibility for a clinical trial is acceptability of the design among HCPs. This WP sought to engage with a broader group of HCPs working in PICUs and NNUs across the UK.
Aims and objectives
To investigate PICU and NNU HCPs’ opinions on issues regarding a potential trial of GRV compared with no GRV measurement, including willingness to randomise and inclusion and exclusion criteria, and to gain consensus on potential primary and secondary outcome measures.
Study management
This WP was led by LNT (for PICUs) and JD and CG (for NNUs). The SMG was responsible for inputting into the survey questions and design, as a member of the Clinical Trials Unit (HE) was responsible for managing the software and collecting responses, and the SMG statistician (BA) analysed the results.
Methods
Survey development
Two separate surveys were developed by the study team: one for PICU clinicians and one for NNU clinicians. Each tool consisted of a cross-sectional survey and a two-round e-Delphi survey [see project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)]. The cross-sectional surveys were developed to look at trial design issues. Each included a set of potential inclusion and exclusion criteria, questions on willingness to randomise and questions on what the primary outcome should be in either PICUs or NNUs.
The list of outcomes for the e-Delphi component of the survey was generated as follows. Phase 1 involved the generation of a list of outcomes used in previous studies of GRV in different populations: 11 for PICUs and 11 different ones for NNUs. 27–32 In WP 2, parents generated seven more important outcomes: (1) length of ventilation, (2) weight gain and growth, (3) time to full feeds, (4) length of hospital stay, (5) long-term feeding issues (PICU), (6) health-care infections (NNU) and (7) survival (of which some were duplicates of our generated list). This resulted in 16 PICU outcomes and 22 NNU outcomes (as the long-term outcomes were broken down into specific outcomes) in round 1. Once developed, both surveys were tested for face validity on 10 individuals (a mix of nurses, doctors and dietitians) and then tested again within the study team. The e-Delphi process was conducted and managed by DelphiManager version 3.0 software (University of Liverpool, Liverpool, UK). The final survey tool can be found at the project web page [www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)]. The outcomes were listed alphabetically to avoid potential weighting of outcomes caused by the order in which they were displayed.
Participants
Key stakeholder groups were identified for the PICU e-Delphi: PICU nurses, PICU doctors, paediatric surgeons and PICU dietitians. We did not involve parents in this process as we had elicited their views on important outcomes in the qualitative work and thought that asking them to comment on trial design issues, such as inclusion and exclusion criteria (without an understanding of the clinical context), was not useful. Members of these groups were invited to take part via e-mail through professional networks (British Dietetic Association, PICS and PICS Study Group). Similarly, stakeholder groups were identified for the NNU e-Delphi: neonatal nurses, neonatal doctors, paediatric surgeons and neonatal dietitians. Members of these groups were invited to take part via e-mail, through professional networks (the UK Neonatal Collaborative and the British Association of Perinatal Medicine). The target number of respondents was 40 for PICU clinicians and 100 for NNU clinicians, reflecting the respective size of the specialty. Automated reminders were sent via the survey software.
Data collection
The survey was electronic, with participants allocated a unique identifier (via name and e-mail address) to allow identification of individuals completing all rounds of the e-Delphi exercise. In round 1, participants completed the cross-sectional survey and then went on to score each of the outcomes listed using a 9-point Likert scale based on the degree of importance, as recommended by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group33 (with 1–3 labelled ‘not important’, 4–6 labelled ‘important but not critical’ and 7–9 labelled ‘critical’). Participants were provided with an option to add additional outcomes in round 1 that they thought were relevant and not listed.
In round 2, participants were presented with a summary of the results of round 1 and asked to rescore these outcomes based on the group score. They were also asked to score any additional outcomes suggested in round 1. Finally, they were asked to specify a single primary outcome.
Data analysis
All question responses were summarised using descriptive statistics for quantitative data and line-listings for qualitative free-text data. For each round of the e-Delphi, the distribution of outcome ratings was assessed using histograms, by stakeholder group. Response rates, attrition bias and the number of questions for which scores were changed between rounds 1 and 2 were assessed. (Note, attrition bias can be incurred if those with specific views are more likely to continue with second or subsequent rounds of an e-Delphi survey. We assessed potential attrition bias between rounds 1 and 2 by comparing the average round 1 score from those responding in both rounds to those responding in round 1 only.) The round 2 scores were used to formulate consensus statistics for each outcome by stakeholder group, and overall by the percentage of respondents who scored 7–9 (outcome rated critical), the percentage who scored 1–3 (outcome rated unimportant) and by consensus status (‘consensus in’, ‘consensus out’ or ‘no consensus’) (Table 14).
| Consensus status | Description | Definition |
|---|---|---|
| Consensus in | Consensus that the outcome should be included in the future study design | ≥ 70% of participants scoring as 7–9 and < 15% of participants scoring as 1–3 in each group |
| Consensus out | Consensus that the outcome should not be included in the future study design | ≥ 70% of participants scoring as 1–3 and < 15% of participants scoring as 7–9 in each group |
| No consensus | Uncertainty about question | Anything else |
Results
Paediatric intensive care unit
A total of 45 PICU HCPs across UK PICUs were contacted via e-mail, via the PICS Study Group, in September 2018. Of these HCPs, 30 (67%) registered for the e-Delphi survey and responded with views regarding inclusion and exclusion criteria. Twenty eight (62%) of those invited went on to score 16 outcomes in round 1 of the e-Delphi survey and 22 (79%) of those who completed round 1 went on to respond to round 2 (Table 15). As there was just one paediatric surgeon taking part, the SMG agreed that a new group should be formed: PICU doctors and one paediatric surgeon. Results are therefore presented throughout for three stakeholder groups instead of four.
| Stakeholder group | Total invited, n | Cross-sectional survey, n (% of those invited) | R1 e-Delphi, n (% of those invited) | R2 e-Delphi, n (% of those that completed R1) |
|---|---|---|---|---|
| PICU doctors | 25 | 14 (56) | 13 (52) | 10 (77) |
| Paediatric surgeons | 2 | 1 (50) | 1 (50) | 1 (100) |
| PICU nurses | 13 | 10 (77) | 9 (69) | 8 (89) |
| PICU dietitians | 5 | 5 (100) | 5 (100) | 3 (60) |
| Total | 45 | 30 (67) | 28 (62) | 22 (79) |
Potential inclusion and exclusion criteria
Wide support across all groups was given for including children admitted to PICU aged from > 37 weeks (term) to 17 years and all children on the PICU who are being fed [intubated, extubated and on non-invasive ventilation (NIV)] (Table 16). Nurses and dietitians were strongly in favour of including cardiac infants and post-surgical patients, but some doctors (5/15, 33%) were not. Mechanical ventilation as a prerequisite had mixed support.
| Criterion | PICU doctors/paediatric surgeon (N = 15), n (%) | PICU nurses (N = 10), n (%) | PICU dietitians (N = 5), n (%) | All (N = 30), n (%) |
|---|---|---|---|---|
| Inclusion criteria | ||||
| Children aged > 37 weeks to 17 years in the PICU | 14 (93) | 8 (80) | 5 (100) | 27 (90) |
| All children, including cardiac infants and post-surgical patients | 10 (67) | 9 (90) | 5 (100) | 24 (80) |
| All children on the PICU who are being fed (intubated, extubated and on NIV) | 11 (73) | 7 (70) | 4 (80) | 22 (73) |
| Only children who are mechanically ventilated in PICU | 6 (40) | 7 (70) | 3 (60) | 16 (53) |
| Children aged > 1 month (> 44 weeks’ GA to 17 years) | 3 (20) | 5 (50) | 2 (40) | 10 (33) |
| Other | 3 (20) | 2 (20) | 1 (20) | 6 (20) |
| Only children with a medical cause of PICU admission | 1 (7) | 0 | 1 (20) | 2 (7) |
| Exclusion criteria | ||||
| Any child with a surgical gut or active GI bleeding | 12 (80) | 8 (80) | 1 (20) | 21 (70) |
| < 24 hours expected PICU admission | 7 (47) | 7 (70) | 3 (60) | 17 (57) |
| Neonates < 37 weeks’ GA (postmenstrual age) | 8 (53) | 6 (60) | 1 (20) | 15 (50) |
| Children on ECMO/ECLS | 5 (33) | 4 (40) | 0 | 9 (30) |
| Children on any vasopressor support | 5 (33) | 1 (10) | 0 | 6 (20) |
| All neonates up to 44 weeks’ GA | 3 (20) | 3 (30) | 0 | 6 (20) |
| Other | 2 (13) | 2 (20) | 2 (40) | 6 (20) |
| Only postoperative cardiac surgical infants after complex surgery | 4 (27) | 1 (10) | 0 | 5 (17) |
| Postoperative cardiac surgical children | 3 (20) | 1 (10) | 0 | 4 (13) |
| All pre- and postoperative cardiac children | 3 (20) | 1 (10) | 0 | 4 (13) |
| Children on NIV | 0 | 3 (30) | 1 (20) | 4 (13) |
| None | 1 (7) | 0 | 3 (60) | 4 (13) |
| Children on HFNC | 0 | 2 (20) | 0 | 2 (7) |
Most doctors (12/15, 80%) and nurses (8/10, 80%) agreed that excluding any child with a ‘surgical gut’ or active gastrointestinal (GI) bleeding was important, although four out of five (80%) dietitians did not (see Table 16). Seven out of 10 (70%) nurses wanted to exclude children who were expected to have a length of stay in PICU of < 24 hours. No other exclusion criteria proposed received strong agreement from any group.
When ‘other’ was selected, participants could offer their own suggestions (see Table 1 in Report Supplementary Material 2).
Willingness to randomise
All respondents except one (29/30, 97%) said that they were willing to randomise children in a trial comparing GRV with no GRV. The one nurse respondent who was not sure elaborated: ‘I struggle to understand how you assess if a child is tolerating feed without using GRV when bolus feeding. Equally I think GRV will be very difficult to interpret if continuous feeding’.
e-Delphi round 1
Sixteen outcomes were scored. These are listed in Appendix 5. Fourteen of these outcomes were scored by 28 respondents and two other outcomes were rated by 27 respondents (one doctor ticked ‘unable to score’ for these). Feedback was offered for most outcomes (see Table 2 in Report Supplementary Material 2).
e-Delphi round 2
Six additional non-duplicate outcomes were suggested in the round 1 survey and added to round 2, to make a total of 22 outcomes to be rated (see Appendix 6). A total of 22 respondents completed round 2. Importantly, the dietitian group was reduced to only three. Although this group is small, we present their round 2 results here, to allow potential differences of opinion from dietitians to be considered (see Table 3 in Report Supplementary Material 2 for summary statistics of the round 2 scores).
Attrition bias
Figure 1 in Report Supplementary Material 2 shows that the three doctors, one nurse and two dietitians who only took part only in round 1 did not have very different views from the 22 HCPs who went on to respond to round 2. We therefore conclude that there was no evidence of attrition bias.
Score changes from round 1 to round 2
Figure 2 in Supplementary Material 2 shows the number of outcomes for which a respondent changed their score between rounds 1 and 2. Thirty-six per cent of respondents (8/22) changed their minds for one, two or no outcomes, which means that the remaining 64% (14/22) changed their minds for at least three outcomes. These results suggest that including a second round in the e-Delphi was useful.
Outcome scores
Table 3 in Supplementary Material 2 shows the median rating given at the end of round 2 for each outcome considered. This shows a little heterogeneity between groups for some outcomes, but also that some outcomes are rated as important by all. No outcomes had a median rating of < 4 in any group, showing that, in general, no outcome was considered as ‘unimportant’ by our respondents.
Consensus statistics
Four outcomes were categorised as ‘consensus in’ in all three stakeholder groups: (1) time to achievement of predicted energy goals, (2) incidence of ventilator-associated pneumonia (VAP), (3) time feed stopped per 24 hours and (4) incidence of GI morbidity (vomiting). No outcomes could be categorised as ‘consensus out’ and the remaining 18 outcomes were categorised ‘no consensus’ (Table 17). If we ignore the stakeholder groups, two outcomes reached ‘consensus in’ [(1) the length of time invasive ventilation (IV) and (2) the incidence of NEC]; however, these did not achieve the a priori threshold. Note, our small sample size of dietitians (n = 3) had undue influence over the ‘consensus in’ threshold: it required only one of the dietitians to score an outcome < 7, regardless of the opinions of other groups, for the outcome to drop below the threshold for ‘consensus in’.
| Outcome | PICU doctors and paediatric general surgeon (n = 11), % | PICU nurses (n = 8), % | PICU dietitians (n = 3), % | All (n = 22), % | Consensus status |
|---|---|---|---|---|---|
| Time to achievement of predicted energy goals (full feeds) | 90.9a | 87.5a | 100.0a | 90.9b | Consensus in |
| Incidence of VAP | 81.8a | 87.5a | 100.0a | 86.4b | Consensus in |
| Time feed stopped per 24 hours | 81.8a | 87.5a | 100.0a | 86.4b | Consensus in |
| Incidence of NEC | 90.9a | 87.5a | 66.7 | 86.4a | No consensus |
| Incidence of GI morbidity: vomiting | 72.7a | 87.5a | 100.0a | 81.8b | Consensus in |
| Length of time: IV | 63.6 | 75.0a | 100.0a | 72.7a | No consensus |
| Mortality | 63.6 | 62.5 | 100.0a | 68.2 | No consensus |
| Length of stay: PICU | 63.6 | 62.5 | 66.7 | 63.6 | No consensus |
| Total length of time respiratory support (IV + NIV) | 63.6 | 50.0 | 100.0a | 63.6 | No consensus |
| Nursing time spent measuring GRV | 54.5 | 50.0 | 100.0a | 59.1 | No consensus |
| Length of stay: hospital | 63.6 | 37.5 | 66.7 | 54.5 | No consensus |
| Administration of parenteral nutrition secondary to feed intolerance | 45.5 | 37.5 | 66.7 | 45.5 | No consensus |
| Post-pyloric feeding (placing a post-pyloric tube) secondary to feed intolerance | 27.3 | 50.0 | 100.0a | 45.5 | No consensus |
| Change in weight (growth) between PICU admission and discharge | 27.3 | 50.0 | 66.7 | 40.9 | No consensus |
| Long-term feeding issues | 27.3 | 37.5 | 33.3 | 31.8 | No consensus |
| GI morbidity: diarrhoea | 9.1 | 37.5 | 66.7 | 27.3 | No consensus |
| Long-term outcomes (after hospital discharge) | 36.4 | 25.0 | 0 | 27.3 | No consensus |
| Administration of prokinetic drugs secondary to feed intolerance | 18.2 | 12.5 | 66.7 | 22.7 | No consensus |
| Looking and/or behaving like their normal self | 18.2 | 25.0 | 0 | 18.2 | No consensus |
| Change in length (growth) between PICU admission and discharge | 18.2 | 12.5 | 33.3 | 18.2 | No consensus |
| Change to feed formula type secondary to feed intolerance | 0 | 25.0 | 66.7 | 18.2 | No consensus |
| Parental satisfaction | 9.1 | 0 | 33.3 | 9.1 | No consensus |
Primary outcome suggestion responses
In round 2, after rating the 22 outcomes, 20 out of 22 (91%) respondents suggested at least one primary outcome, although some suggested more than one (Table 18). Overall, 36% chose ‘time to achievement of predicted energy goals (full feeds)’ and 23% chose ‘incidence of VAP’.
| Primary outcome suggesteda | PICU doctors and one paediatric general surgeon (N = 11), n (%) | PICU nurses (N = 8), n (%) | PICU dietitians (N = 3), n (%) | All (N = 22), n (%) |
|---|---|---|---|---|
| Time to achievement of predicted energy goals (full feeds) | 5 (46) | 3 (38) | 0 | 8 (36) |
| Incidence of VAP | 1 (9) | 3 (37.5) | 1 (33) | 5 (23) |
| Incidence of GI morbidity: vomiting | 1 (9) | 1 (13) | 1 (33) | 3 (14) |
| Time feed stopped per 24 hours | 1 (9) | 1 (13) | 1 (33) | 3 (14) |
| Change in weight (growth) between PICU admission and discharge | 1 (9) | 1 (13) | 0 | 2 (9) |
| GI morbidity: diarrhoea | 0 | 1 (13) | 1 (33) | 2 (9) |
| Length of stay: PICU | 0 | 2 (25) | 0 | 2 (9) |
| Length of time: IV | 1 (9) | 0 | 0 | 1 (5) |
| Long-term feeding issues | 0 | 1 (13) | 0 | 1 (5) |
| Nursing time spent measuring GRV | 0 | 1 (13) | 0 | 1 (5) |
| No outcome suggested | 1 (9) | 1 (13) | 0 | 2 (9) |
Neonatal units
Health-care professionals at all 184 UK NNUs were contacted via e-mail, with a request that the invitation was forwarded on to relevant individuals. A total of 76 HCPs registered for the survey (Table 19) and responded with views regarding inclusion and exclusion criteria. Seventy-four HCPs went on to score 22 outcomes in round 1 of the e-Delphi survey, and 61 (82%) of those who completed round 1 went on to respond to round 2. After round 1, the SMG agreed to collapse the five stakeholder group options into three [only two respondents were paediatric surgeons and the five respondents that selected ‘other’ could all be categorised as nurses (neonatal or paediatric)]. Results are therefore presented throughout for three stakeholder groups instead of five.
| Stakeholder group | Cross-sectional survey, n | R1 e-Delphi, n | R2 e-Delphi, n (% of those that completed R1) |
|---|---|---|---|
| Neonatal/paediatric doctors or paediatric surgeons | 44 | 44 | 40 (91) |
| Neonatal/paediatric nurses | 26 | 25 | 18 (72) |
| Neonatal dietitians | 6 | 5 | 3 (60) |
| Total | 76 | 74 | 61 (82) |
Potential inclusion and exclusion criteria
There was wide support across all groups for including all babies who are mechanically ventilated (78%), or babies who are being tube fed (regardless of whether or not they are ventilated or on respiratory support) (79%) and preterm babies aged < 30 weeks’ gestation (99%) (Table 20). Including only babies with an upper gestational age limit of 32 and 34 weeks’ gestation was also strongly supported. Eighty-five per cent of nurses and 67% of dietitians were in favour of including babies aged 37–40 weeks’ gestation, but half of doctors were not. Most respondents (76%) preferred to exclude neonates who were already suspected of having NEC. No other exclusion criteria proposed received strong agreement from any group (see Table 20). When ‘other’ was selected, participants could offer their own suggestions (see Table 1 in Report Supplementary Material 2).
| Criterion | Neonatal and paediatric doctors/paediatric surgeons (N = 44), n (%) | Neonatal and paediatric nurses (N = 26), n (%) | Neonatal dietitians (N = 6), n (%) | All (N = 76), n (%) |
|---|---|---|---|---|
| Inclusion criteria | ||||
| Preterm babies: < 30 gestational weeks | 42 (95) | 24 (92) | 6 (100) | 72 (95) |
| Preterm babies: < 32 gestational weeks | 39 (89) | 26 (100) | 6 (100) | 71 (93) |
| Preterm babies: < 34 gestational weeks | 34 (77) | 24 (92) | 5 (83) | 63 (83) |
| Preterm babies: < 28 gestational weeks | 37 (84) | 18 (69) | 6 (100) | 61 (80) |
| All babies who are mechanically ventilated | 33 (75) | 21 (81) | 5 (83) | 59 (78) |
| Babies on the NNU who are being tube fed (regardless of whether or not they are ventilated or on respiratory support) | 28 (64) | 25 (96) | 6 (100) | 59 (78) |
| Preterm babies: 34–37 gestational weeks | 22 (50) | 22 (85) | 4 (67) | 48 (63) |
| Only babies with no surgical issues | 22 (50) | 18 (69) | 2 (33) | 42 (55) |
| Babies, including cardiac infants and/or post-surgical patients, so long as being fed | 17 (39) | 14 (54) | 3 (50) | 34 (45) |
| Other | 4 (9) | 6 (23) | 0 | 10 (13) |
| Exclusion criteria | ||||
| Neonates with suspected NEC | 31 (70) | 22 (85) | 5 (83) | 58 (76) |
| Neonates receiving total body hypothermia | 21 (48) | 12 (46) | 2 (33) | 35 (46) |
| Babies with cardiac, neurological, chromosomal or congenital anomalies | 22 (50) | 9 (35) | 0 | 31 (41) |
| Other | 13 (30) | 5 (19) | 1 (17) | 19 (25) |
| Babies with antenatally detected abnormal Doppler studies | 8 (18) | 9 (35) | 0 | 17 (22) |
| Preterm babies: < 34 gestational weeks | 6 (14) | 2 (8) | 1 (17) | 9 (12) |
| Preterm babies: > 32 gestational weeks | 7 (16) | 1 (4) | 1 (17) | 9 (12) |
| Preterm babies: < 28 gestational weeks | 7 (16) | 3 (12) | 0 | 10 (13) |
| Neonates with a birthweight below the 10th centile for gestational age | 4 (9) | 3 (12) | 0 | 7 (9) |
| Preterm babies: > 30 gestational weeks | 4 (9) | 2 (8) | 0 | 6 (8) |
| None | 3 (7) | 1 (4) | 0 | 4 (5) |
| Babies not receiving any respiratory support | 2 (5) | 1 (4) | 0 | 3 (4) |
Willingness to randomise
Most respondents (69/76, 91%) said that they would be willing to randomise babies in a trial comparing GRV with no GRV. One dietitian was not in favour ‘because across our network we do not routinely measure residuals and this is embedded practice’. Another six dietitians were not sure, one stated ‘Because I read the article about time to full feeds with not measuring GRV and prefer to do that. Not sure if would be happy to randomise to the GRV group’, whereas another replied ‘Have never participated in randomisation before’ and another respondent wrote ‘I would be happy as long as all the antenatal information was available, i.e. dopplers, etc., and after discussion with the treating consultant’.
e-Delphi round 1
Following the process described previously (see Chapter 3, Methods), 22 outcomes were included in the round 1 survey [11 from the literature and seven generated from parents (some were duplicates)]; however, other outcomes were broken down into more specific long-term morbidities. These are listed in Appendix 7. Seventeen of these outcomes were scored by 74 respondents and five other outcomes were rated by 73 respondents (one nurse ticked ‘unable to score’ for these). Feedback was offered for most outcomes (see Table 5 in Report Supplementary Material 2).
e-Delphi round 2
Four additional outcomes were suggested in round 1 and added to the round 2 survey, making a total of 26 outcomes to be rated at round 2 (see Appendix 8). A total of 61 respondents completed round 2. Importantly, the dietitian group was reduced to only three. Although this group is small, we present their round 2 results here to allow potential differences of opinion from dietitians to be considered.
Attrition bias
Figure 3 in Report Supplementary Material 2 shows that the four doctors, seven nurses and two dietitians who took part only in round 1 did not have very different views from the 61 HCPs who went on to respond in round 2. We therefore conclude that there was no evidence of attrition bias.
Score changes from round 1 to round 2
Figure 4 in Report Supplementary Material 2 shows the number of outcomes for which a respondent changed their score between rounds 1 and 2. Twenty-five per cent of respondents (15/61) changed their minds for one, two or no outcomes, which means that the remaining 75% changed their minds for at least three outcomes. These results suggest that including a second round in the e-Delphi was a useful exercise.
Outcome scores
Table 6 in Report Supplementary Material 2 shows the median rating given at the end of round 2 for each outcome considered. This shows a little heterogeneity between groups for some outcomes, but also some outcomes that are rated as important by all. No outcomes had a median rating of < 4 in any group, showing that, in general, no outcome was considered as ‘unimportant’ by our respondents.
Consensus statistics
Five outcomes were categorised as ‘consensus in’ in all three stakeholder groups (Table 21): (1) days on parenteral nutrition, (2) incidence of NEC, (3) time feed stopped per 24 hours, (4) time from start of enteral feeding to achieve full (150 ml/kg/day) enteral feeds and (5) mortality. No outcomes could be categorised as ‘consensus out’ and the remaining 17 outcomes were categorised as ‘no consensus’. If we ignore stakeholder groups, four outcomes reached ‘consensus in’: (1) days of central venous line access, (2) change in weight (growth) between birth and NNU discharge, (3) health-care-associated infections and (4) incidence of catheter-associated bloodstream infections, but these did not achieve the a priori threshold. Note, our small sample size for dietitians (n = 3) had undue influence over the ‘consensus in’ threshold: it required only one dietitian to score an outcome < 7, regardless of the opinions of other groups, for the outcome to drop below the threshold for ‘consensus in’.
| Outcome | Neonatal and paediatric doctors/paediatric surgeons (n = 40), % | Neonatal and paediatric nurses (n = 18), % | Neonatal dietitians (n = 3), % | All (n = 61), % | Consensus status |
|---|---|---|---|---|---|
| Mortality | 100a | 100.0a | 100.0a | 100.0b | Consensus in |
| Incidence of NEC | 100a | 94.4a | 100.0a | 98.4b | Consensus in |
| Time from start of enteral feeding to achieve full (150 ml/kg/day) enteral feeds | 92.5a | 88.9a | 100.0a | 91.8b | Consensus in |
| Health-care-associated infections | 87.5a | 88.9a | 66.7 | 86.9a | No consensus |
| Days on parenteral nutrition | 77.5a | 94.4a | 100.0a | 83.6b | Consensus in |
| Incidence of catheter-associated bloodstream infection | 85.0a | 70.6a | 66.7 | 80.0a | No consensus |
| Time feed stopped per 24 hours | 70.0a | 83.3a | 100.0a | 75.4b | Consensus in |
| Change in weight (growth) between birth and NNU discharge | 75.0a | 77.8a | 66.7 | 75.4a | No consensus |
| Days of central venous line access | 75.0a | 72.2a | 66.7 | 73.8a | No consensus |
| Length of stay in hospital | 45.0 | 72.2a | 100.0a | 55.7 | No consensus |
| Incidence of pneumonia due to milk aspiration | 37.5 | 76.5a | 66.7 | 50.0 | No consensus |
| Length of stay: NNU | 35.0 | 72.2a | 66.7 | 47.5 | No consensus |
| Long-term outcomes: problems with mobility like cerebral palsy | 45 | 50.0 | 33.3 | 45.9 | No consensus |
| Long-term outcomes: problems with cognition | 35.0 | 38.9 | 33.3 | 36.1 | No consensus |
| GI morbidity: vomiting | 30.0 | 61.1 | 100.0a | 42.6 | No consensus |
| Change in head circumference between birth and NNU discharge | 42.5 | 23.5 | 100.0a | 40.0 | No consensus |
| Change in length (growth) between birth and NNU discharge | 42.5 | 27.8 | 66.7 | 39.3 | No consensus |
| Long-term outcomes: problems with cognition | 35.0 | 38.9 | 33.3 | 36.1 | No consensus |
| Long-term outcomes: brain injury on imaging | 25.0 | 33.3 | 33.3 | 27.9 | No consensus |
| Time to oral feeding | 27.5 | 23.5 | 33.3 | 26.7 | No consensus |
| Length of time: IV | 12.5 | 50.0 | 66.7 | 26.2 | No consensus |
| Long-term outcomes: hearing loss | 15.0 | 27.8 | 33.3 | 19.7 | No consensus |
| Long-term outcomes: problems with eyesight | 15.0 | 27.8 | 33.3 | 19.7 | No consensus |
| Nursing time spent measuring GRV | 5.0 | 27.8 | 66.7 | 14.8 | No consensus |
| GI morbidity: diarrhoea | 5.0 | 27.8 | 33.3 | 13.1 | No consensus |
| Total length of time on respiratory support (IV + NIV) | 2.5 | 27.8 | 0 | 9.8 | No consensus |
| Time to nasogastric tube removal | 10.0 | 5.6 | 0 | 8.2 | No consensus |
Primary outcome suggestion responses
In round 2, after rating the 26 outcomes, respondents were asked to suggest a primary outcome from the list of 26. Forty-eight out of 61 respondents (79%) suggested at least one primary outcome, although some suggested more than one (Table 22). Overall, 39% of HCPs chose ‘incidence of NEC’ and 30% of HCPs chose ‘time from start of enteral feeding to achieve full (150 ml/kg/day) enteral feeds’.
| Primary outcome suggested | Neonatal and paediatric doctors/paediatric surgeons (N = 40), n (%) | Neonatal and paediatric nurses (N = 18), n (%) | Neonatal dietitians (N = 3), n (%) | All (N = 61), n (%) |
|---|---|---|---|---|
| Incidence of NEC | 19 (48) | 5 (28) | 0 | 24 (39) |
| Time from start of enteral feeding to achieve full (150 ml/kg/day) enteral feeds | 12 (30) | 5 (28) | 1 (33) | 18 (30) |
| Mortality | 8 (20) | 0 | 0 | 8 (13) |
| Days of central venous line access | 4 (10) | 1 (6) | 0 | 5 (8) |
| Days on parenteral nutrition | 2 (5) | 1 (6) | 1 (33) | 4 (7) |
| Length of stay in hospital | 2 (5) | 0 | 0 | 2 (3) |
| Length of stay NNU | 2 (5) | 0 | 0 | 2 (3) |
| Incidence of pneumonia due to milk aspiration | 0 | 2 (11) | 0 | 2 (3) |
| Change in weight (growth) between birth and NNU discharge | 1 (3) | 0 | 0 | 1 (2) |
| Change in length (growth) between birth and NNU discharge | 1 (3) | 0 | 0 | 1 (2) |
| Long-term outcomes: problems with eyesight | 1 (3) | 0 | 0 | 1 (2) |
| Long-term outcomes: problems with cognition | 1 (3) | 0 | 0 | 1 (2) |
| Long-term outcomes: problems with mobility (e.g. cerebral palsy) | 1 (3) | 0 | 0 | 1 (2) |
| Health-care-associated infections | 0 | 1 (6) | 0 | 1 (2) |
| Time to oral feeding | 1 (3) | 0 | 0 | 1 (2) |
| No primary outcome suggested | 6 (15) | 6 (33) | 1 (33) | 13 (21) |
Summary of findings to inform the GASTRIC trial
These trial design surveys and the e-Delphi study have allowed us to generate a list of ‘clinician-acceptable’ inclusion and exclusion criteria for future PICU and NNU trials. This enabled us to request data from national data sets to determine potential eligible numbers of children for a future trial. It has also allowed us to gauge clinician’s acceptability on a wider scale, with regard to willingness to randomise. Most importantly, for both PICUs and NNUs, we have been able to generate a list of outcomes measures for a future trial, including the preferred primary outcome measure. This enabled us to conduct final voting (on the non-consensus outcomes) at the face-to-face consensus meeting.
Chapter 4 Analysis of national data sets for trial feasibility
Introduction
Work package 4 analysed the data gathered and used two existing national databases, the National Neonatal Research Database (NNRD) and the Paediatric Intensive Care Audit Network (PICANet), to explore the size of eligible populations and inform sample sizes for potential future trials of GRV compared with no GRV in UK PICUs and NNUs.
Aims and objectives
To determine potential patient recruitment numbers based on inclusion and exclusion criteria and, when these data are collected, to obtain summary statistics for potential primary and secondary outcomes identified by consensus from WP 3.
Study management
This WP was led by LNT and RP (for PICUs) and CG (for NNUs). The SMG statisticians (BA and APJ) summarised the analysed data.
Methods
Paediatric Intensive Care Audit Network
Data items
The PICANet collects basic demographic and clinical data on all children admitted to designated PICUs in the UK and Ireland. Each admission constitutes an episode, and an individual may have several episodes in the database. Episodes may be aggregated by individual PICUs or by census or administrative geographies using standard postcode lookup tables (the National Statistics Postcode Directory). PICANet has permission to collect patient-identifiable data under section 251 of the National Health Service Act 200634 (originally enacted under section 60 of the Health and Social Care Act 200135). PICANet collects data on demographics, admission characteristics, presenting physiology (to allow calculation of the expected probability of mortality for risk adjustment), diagnostic information, clinical interventions and outcome. A full list of data items and data definitions can be found at www.picanet.org.uk/documentation.
Data quality
Ensuring quality is part of the PICANet process. At input, internal logical, consistency and range checks are carried out within the software, with an on-screen summary of outstanding validation checks on completion of a record for the data entry personnel on the unit. Units can access admission reports (among many others) that allow them to cross-check against admission books and patient administration systems. This system of checks provides an ongoing audit of the quality of the PICANet data. Validation visits are carried out annually to review a sample of records and cross-check that the data submitted to PICANet correspond with the data held in the patient’s clinical records. Detailed feedback is sent to the unit following these visits to ensure that any problems with data collection and abstraction can be dealt with locally.
Data snapshot
A data request was formally made to PICANet in December 2018, based on the results of the e-Delphi survey findings. We received the anonymised data in March 2019.
Inclusion criteria
Admissions to a UK PICU during 2016 and 2017 who were:
-
aged > 37 weeks’ gestation to 17 years
-
intubated and mechanically ventilated.
Exclusion criteria
-
Length of stay in the PICU < 24 hours.
-
Extubated, non-invasive or high-flow nasal cannula ventilation.
-
Admitted after surgical admission with a GI diagnostic group.
Methods
The total number of children fulfilling the inclusion and exclusion criteria was found and split by subgroup: age group and whether a surgical or medical admission. Only two ‘consensus in’ outcomes relevant to the GASTRIC trial are recorded in PICANet: length of stay (categorised as 1 or 2 days, 3–7 days and > 7 days) and length of time intubated [categorised as 1 day, 2 days, 3–7 days and > 7 days, but also as a continuous measure (number of days)]. Numbers and percentages in each outcome category were reported, split by subgroup. Median [interquartile range (IQR)] length of time intubated was reported by subgroup. PICANet also collects mortality data, but as UK PICU mortality is so low (< 4% consistently)36 we did not request these data specifically.
National Neonatal Research Database
The NNRD holds data from all infants admitted to NHS NNUs in England, Scotland and Wales (approximately 90,000 infants annually). The NNRD is formed from data extracted from the neonatal electronic health record system used by HCPs during routine clinical care. Briefly, daily clinical information on NNU admissions is recorded at the point of care in clinician-entered electronic patient records. A defined data extract, the Neonatal Data Set (NHS Information Standard SCCI595), is transmitted quarterly to the Neonatal Data Analysis Unit at Imperial College London and Chelsea and Westminster Hospital NHS Foundation Trust, where patient episodes across different hospitals are linked and data are cleaned and entered into the NNRD. Contributing NNUs are known as the UK Neonatal Collaborative. The NNRD is approved by the National Research Ethics Service (10/H0803/151), Confidentiality Advisory Group of the Health Research Authority [805(f)/2010], and Caldicott Guardians and lead clinicians of contributing hospitals.
Data items
The NNRD holds the Neonatal Data Set, approximately 450 data items that form a NHS data standard. 37
Data items include demographic and admission items (e.g. maternal conditions, birthweight), daily items [entered every day for all infants (e.g. respiratory support, feeding information)], discharge items (e.g. feeding and weight at discharge) and ad hoc items [entered if they occur (e.g. suspected infection, ultrasound scan findings, abdominal radiographic findings)].
Data quality
Data extracted from the neonatal electronic patient record are cleaned and records with implausible data configurations are queried and corrected by the treating clinicians. Cleaning is carried out by the Neonatal Data Analysis Unit before data are incorporated into the NNRD. The robustness of core NNRD data (birth weight, sex, length of stay and death) has been previously demonstrated for research purposes. Accuracy and completeness of NNRD data were confirmed by comparison with case record forms from the National Institute for Health Research Health Technology Assessment Probiotics in Preterms trial, which showed high data completeness and accuracy (> 95%). 38 Data held in the NNRD are used for multiple purposes, including national audit [the Healthcare Quality Improvement Partnership-funded National Neonatal Audit Programme (NNAP)] and analyses for the Department of Health and Social Care, NHS England and the Chief Medical Officer.
Data snapshot
Inclusion criteria
Babies admitted to a NNU in England or Wales during 2017 and 2018, who had at least 1 day of care in which the location was a NNU (NICU, LNU or SCBU). Only days of care when the baby was recorded as being on the NNU were included.
Methods
The total number of children fulfilling each of three separate inclusion criteria was found:
-
gestational age at birth < 32 weeks
-
mechanically ventilated at any point during NNU stay
-
received gastric tube feeds at any point during NNU stay.
The following outcomes measured by NNRD were summarised with descriptive statistics, split by each inclusion criterion:
-
mortality during NNU stay
-
NEC
-
central line-associated bloodstream infection
-
incidence and duration of central line duration
-
incidence and duration of parenteral nutrition
-
incidence and duration of mechanical ventilation
-
time from birth to achieve full enteral feeds (150 ml/kg/day)
-
length of NNU stay.
Outcomes were summarised with descriptive statistics, split by inclusion criteria subgroup. Variable codes used for searches can be found in Appendix 9.
Results
Paediatric Intensive Care Audit Network
In 2016 and 2017 there were 16,122 children treated in PICUs that satisfy all the inclusion and exclusion criteria. Table 23 shows the results of the search, split by age group and whether surgical or medical. Of the children included, 36% were surgical (5813/16,122) and 64% were medical (10,309/16,122). The age distribution is similar for surgical and medical, skewed to younger years (median age category 1–12 months, 21% were aged < 1 month and 87% aged < 11 years).
| Patient population | All | Age group | |||||
|---|---|---|---|---|---|---|---|
| < 1 month | 1–12 months | 1–4 years | 5–10 years | 11–14 years | 15–17 years | ||
| All eligible (surgical and medical), n (%) | 16,122 | 3401 (21) | 5298 (33) | 2804 (17) | 2514 (16) | 1326 (8) | 779 (5) |
| Surgical | |||||||
| Surgical, n (% within age group) | 5813 (36) | 1068 (31) | 2238 (42) | 946 (34) | 820 (33) | 454 (34) | 287 (37) |
| Length of stay (days), n (% of surgical admissions within age group) | |||||||
| 1 or 2 | 1888 (32) | 137 (13) | 680 (30) | 383 (40) | 353 (43) | 209 (46) | 126 (44) |
| 3–7 | 2690 (46) | 615 (58) | 1092 (49) | 366 (39) | 326 (40) | 172 (38) | 119 (41) |
| > 7 | 1235 (21) | 316 (30) | 466 (21) | 197 (21) | 141 (17) | 73 (16) | 42 (15) |
| Length of time (days) intubated, n (% of surgical admissions within age group) | |||||||
| 1 | 1339 (23) | 46 (4) | 432 (19) | 300 (32) | 317 (39) | 147 (32) | 97 (34) |
| 2 | 1800 (31) | 197 (18) | 772 (34) | 304 (32) | 245 (30) | 178 (39) | 104 (36) |
| 3–7 | 1909 (33) | 609 (57) | 741 (33) | 226 (24) | 180 (22) | 92 (20) | 61 (21) |
| > 7 | 765 (13) | 216 (20) | 293 (13) | 116 (12) | 78 (10) | 37 (8) | 25 (9) |
| Length of time intubated (days), median (IQR) | 4 (4) | 2 (3) | 2 (3) | 2 (2) | 2 (2) | 2 (2) | |
| Medical | |||||||
| Medical, n (% within age group) | 10,309 (64) | 2333 (69) | 3060 (58) | 1858 (66) | 1694 (67) | 872 (66) | 492 (63) |
| Length of stay (days), n (% of medical admissions within age group) | |||||||
| 1 or 2 | 1605 (16) | 196 (8) | 390 (13) | 392 (21) | 352 (21) | 175 (20) | 100 (20) |
| 3–7 | 5022 (49) | 1280 (55) | 1553 (51) | 857 (46) | 757 (45) | 379 (43) | 196 (40) |
| > 7 | 3682 (36) | 857 (37) | 1117 (37) | 609 (33) | 585 (35) | 318 (36) | 196 (40) |
| Length of time intubated (days), n (% of medical admissions within age group) | |||||||
| 1 | 876 (8) | 105 (5) | 205 (7) | 208 (11) | 200 (12) | 106 (12) | 52 (11) |
| 2 | 1766 (17) | 302 (13) | 486 (16) | 360 (19) | 348 (21) | 173 (20) | 97 (20) |
| 3–7 | 5117 (50) | 1353 (58) | 1596 (52) | 853 (46) | 738 (44) | 378 (43) | 199 (40) |
| > 7 | 2550 (25) | 573 (25) | 773 (25) | 437 (24) | 408 (24) | 215 (25) | 144 (29) |
| Length of time intubated (days), median (IQR) | 5 (4) | 4 (5) | 4 (5) | 4 (5) | 4 (5) | 4 (7) | |
A total of 12,629 (78%) of the children stayed in a PICU for ≥ 3 days. The most common length of stay was 3–7 days overall (7712/16,122, 48%) and in medical admissions (5022/10,309, 49%), regardless of age. In surgical admissions, children aged < 1 year were also likely to stay 3–7 days (1707/3306, 52%), whereas children aged ≥ 1 year were most likely to stay 1 or 2 days (1071/2507, 43%).
A total of 10,341 (65%) of the children were intubated for ≥ 3 days. The most common length of intubation was 3–7 days overall (7026/16,122, 44%) and in medical admissions (5117/10,309, 50%), regardless of age. In surgical admissions, children aged < 1 month were also likely to be intubated for 3–7 days (609/1068, 57%), whereas children aged ≥ 1 month were most likely to be intubated for 1 or 2 days (2896/4745, 61%). The median intubation duration for medical admissions was 4 days for all age groups except babies aged < 1 month, for whom the median was 5 days. In surgical admissions, median intubation was shorter (2 days for all age groups except babies aged < 1 month, for whom the median was 4 days).
Note that no other GASTRIC-trial relevant outcomes are collected by PICANet.
National Neonatal Research Database
In 2017 and 2018 a total of 129,155 infants were admitted to a NNU in England, Wales or Scotland. Of these infants, 15,375 (12%) had a recorded gestational age at birth < 32 weeks and 23,868 (18%) were recorded to have received mechanical ventilation and 82,555 (64%) to have received nasogastric tube feeds. Further details describing the level of unit of birth (NICU, LNU or SCBU) for these infants is available in Appendix 10. Summary statistics for outcomes, stratified by different potential study populations, are shown in Table 24.
| Outcome | Inclusion criterion | All admissions to NNUs (N = 129,155) | ||
|---|---|---|---|---|
| Born at < 32 gestational weeks (N = 15,375) | Mechanically ventilated (N = 23,868) | Received nasogastric tube feeds (N = 82,555) | ||
| Mortality, n (%) | 1290 (8) | 1978 (8) | 895 (1) | 2072 (2) |
| NEC, n (%) | 806 (5) | 778 (3) | 770 (1) | 806 (1) |
| Catheter-associated bloodstream infection, n (%) | 1004 (7) | 1089 (5) | 1127 (1) | 1180 (1) |
| Had a central venous line in situ, n (%) | 12,517 (81) | 17,505 (73) | 23,322 (28) | 26,332 (20) |
| Median duration in days (IQR) | 9 (4–15) | 6 (0–12) | 0 (0–3) | 0 (0–0) |
| Received parenteral nutrition, n (%) | 12,634 (82) | 13,707 (57) | 19,396 (23) | 20,476 (16) |
| Median duration in days (IQR) | 9 (5–15) | 4 (0–11) | 0 (0–0) | 0 (0–0) |
| Received mechanical ventilation, n (%) | 10,335 (67) | 23,868 (100) | 20,762 (25) | 23,868 (18) |
| Median duration in days (IQR) | 2 (0–6) | 2 (1–5) | 0 (0–1) | 0 (0–0) |
| Time from start of enteral feeding to achieve full (150 ml/kg/day) enteral feeds, median duration in days (IQR) | 12 (9–17) | 11 (8–16) | 6 (4–9) | 5 (3–8) |
| Duration of NNU stay, median duration in days (IQR) | 50 (34–76) | 24 (9–59) | 11 (4–24) | 5 (2–16) |
Table 24 shows that babies born at < 32 weeks’ gestation have different outcomes from the general NNU population. The outcomes of mechanically ventilated babies are also different from those of the general NNU population, but to a lesser extent than babies born at < 32 weeks’ gestation. Infants who receive gastric tube feeds also have a longer length of stay. All duration outcomes are non-normally distributed and skewed. Feeding times vary considerably across each group.
Summary of findings to inform the GASTRIC trial
The analysis of both the national PICU and NNU databases reveal that there are sufficient numbers of potentially eligible patients meeting the inclusion and exclusion criteria to potentially conduct a trial with individual randomisation of patients.
For PICUs, only three of the outcomes are collected routinely in PICANet: (1) length of ventilation, (2) PICU length of stay and (3) mortality. As discussed previously, mortality is so low (4%) so, although collected as part of any trial, would never be a primary outcome. However, few of the data collected by PICANet are relevant to the GASTRIC trial, limiting the usefulness of PICANet as a source of data in any future trial.
For NNUs, five of the six ‘consensus in’ items and all three outcomes deemed to be highly important for two stakeholder groups are routinely extracted and held in the NNRD. This indicates that a data-enabled approach to any future trial would be potentially feasible using existing neonatal electronic patient record systems and data held in the NNRD.
Chapter 5 Consensus and trial design meeting
Introduction
Work package 5 involved separate PICU and NNU face-to-face consensus meetings.
Aims and objectives
To bring together key stakeholders in each specialty, including national societies, parent groups, as well as HCPs and triallists, to:
-
gain stakeholders’ views on the preliminary results from WPs 1–4
-
determine the acceptability of a routine GRV (control arm) and a no GRV (intervention arm) to a wider group of UK HCPs and parents
-
discuss and gain consensus on outcome measures that did not reach consensus in WP 3 of a future trial
-
discuss the feasibility of different study designs and randomisation methods of a future trial.
Study management
This WP was led by LNT (for PICUs) and CG (for NNUs). The SMG was responsible for organising the meeting (HE) and keeping accurate records for the meetings. The whole SMG team attended these meetings. The independent facilitation of the meeting was carried out by Carrol Gamble (director and statistician, Liverpool Clinical Trials Centre) and the summary and analysis of the voting on non-consensus outcomes was done by BA.
Methods
Key stakeholders in both specialties were first identified. These were UK-based NNU and PICU consultants, nurses, dietitians, general surgeons, parents with experience of having a child in NNUs or PICUs, triallists and methodologists from trials units, and key individuals who led these specialist research networks. Individuals were invited to attend a 1-day face-to-face meeting in central London in April 2019. Potential HCPs were contacted through our national networks via e-mail and we tried to get attendance from a range of units geographically and from different types of units. Potential parent participants were invited through our Parent Advisory Group, through our study Twitter account, through the charities and via our Parent Forum Group. The preliminary results of the WPs were presented for discussion, to determine the acceptability of a future trial. Proposed ‘no GRV (intervention) arms’ were presented from two members of the study team who work in a PICU (FVV) and a NNU (AB) in Lyon, France, where GRV is not routinely measured. Following this, outcomes that failed to reach consensus in WP 3 were discussed and voted on using TurningPoint software. With consent from participants, the discussion from the meeting was audio-recorded to ensure no important points were missed. The discussions were summarised and the results of the voting were used to classify the final consensus status of each outcome (in/out/no consensus) using the criteria outlined in Table 14. (Note that all participants were considered as a single group, so results were not split by stakeholder group.)
Paediatric intensive care unit meeting report
Participants
The following members of the SMG attended the NNU meeting:
-
Barbara Arch
-
Frederic Valla (member of the SMG who participated in voting)
-
Helen Eccleson
-
Jennifer Preston
-
Kerry Woolfall
-
Louise Roper
-
Lynne Latten (member of the SMG who participated in voting)
-
Lyvonne Tume (member of the SMG who participated in voting)
-
Nazima Pathan (member of the SMG who participated in voting).
Twenty-two HCPs (three paediatric dietitians, seven paediatric doctors, 11 paediatric nurses and one paediatric general surgeon) attended this meeting, representing 13 of 29 (45%) UK PICUs: Royal Hospital for Children (Glasgow), Leeds General Infirmary (Leeds), Great North Children’s Hospital (Newcastle), Manchester University Foundation Trust (Manchester), Alder Hey Children’s Hospital (Liverpool), Birmingham Children’s Hospital (Birmingham), Nottingham Children’s Hospital (Nottingham), Bristol Royal Hospital for Children (Bristol), Noah’s Ark Children’s Hospital for Wales (Cardiff), University Hospital Southampton NHS Foundation Trust (Southampton), Imperial College Healthcare NHS Trust (London), Evelina London Children’s Hospital (London) and Great Ormond Street Hospital for Children (London).
Discussion areas
Health-care professionals queried whether or not it was important to protocolise the control arm and this may be problematic if the trial was a cluster design. Dietitians confirmed the Schofield equation for estimation of energy requirements would be their standard practice, with the exception of one who expressed concerns that a huge amount of work has been done in their unit with regard to their own nutritional assessment and a change in assessment may cause confusion when patients step down to other wards. There was discussion about units being allowed to set their own energy targets, but that if a predefined energy target was not set, some units may overfeed or underfeed. Further discussion was that the Schofield equation is the internationally accepted and recommended assessment equation for PICU and could be used to standardise the assessment. One unit stated that their PICU would not be willing to increase fluid allowance to achieve targets and thus would not be able to be part of a future trial.
There was also discussion about cluster randomisation and that if a future trial was a cluster trial, then local habits could influence the outcome. However, if a future trial was a RCT, then local habits would be balanced. HCPs queried whether or not the feed volume could be standardised across the control and intervention arms, as the amount of fluid delivered to a child will differ and may have an impact on outcomes and may also have an impact on feed tolerance. It was discussed that there are also differences in feed types, which may have an impact on gastric emptying. It was highlighted that there is variation in feeding method and formula used between units, and it would be impossible to standardise it all. There was discussion about how pragmatic a trial could be when minimising crossover between arms. HCPs discussed that a cluster trial would make the process a lot simpler, as introducing two new practices in a unit would be difficult.
Many HCPs preferred a cluster design trial and there was lots of discussion around this issue. Many believed that this would be better accepted among PICU staff, with views that it was an ‘easier option’ when changing practice. Considerable discussion took place about the feasibility of a cluster design in UK PICUs, especially as unit practices were not the same across the country and the trial results might be criticised because any differences between the two arms could be due to an imbalance in characteristics between the two arms, rather than due to the intervention. It was discussed that cluster trials can be matched on several important prognostic factors; however, there is such a strong level of variation in practice that it would diminish the value of the result, and, with only 15–19 ‘research-active’ PICUs in the UK, powering a cluster design would probably not be possible. There was a question about the possibility of using a stepped-wedge trial design (as in a current UK trial). The team outlined that this trial was different, in that the intervention was a team-based ‘best-practice’ protocol, which would have been impossible to do with individual randomisation, and, although the intervention may not be beneficial, it was ‘best practice’ and thus would be unlikely to cause harm. This cannot be applied to the GASTRIC trial, as we do not know if not measuring GRV will be beneficial or if it may cause harm. In terms of parent-proposed outcomes, one participant raised the issue of potential parent bias in the outcomes, because a proportion (4/10 parents interviewed) were parents of cardiac patients and they have known problems with feeding issues in the longer term and have a big focus on weight gain.
Dr Frederic Valla (Lyon, France), a SMG member, presented a unit protocol that does not measure GRV routinely. Unlike French NNUs, there were no before and after data, as they have not undertaken this practice for nearly 20 years (more detail is presented in Chapter 7).
Paediatric intensive care unit outcomes: consensus results
A total of 22 outcomes were discussed; however, four had previously achieved ‘consensus in’ from the e-Delphi (see Chapter 3). Therefore, 18 outcomes were voted on at the meeting. Of these, eight were voted ‘consensus in’, six were voted ‘consensus out’ and four failed to achieve consensus in or out (Table 25). Full results, including voting statistics, can be found in Table 2 in Report Supplementary Material 3.
| Outcome | Consensus status |
|---|---|
| Administration of parenteral nutrition, secondary to feed intolerance | Consensus in |
| Change to feed formula type, secondary to feed intolerance | Consensus in |
| Change in weight (growth) between PICU admission and discharge | Consensus in |
| Incidence of NEC | Consensus in |
| Length of time: IV | Consensus in |
| Length of stay: PICU | Consensus in |
| Mortality | Consensus in |
| Post-pyloric feeding (placing a post-pyloric tube), secondary to feed intolerance | Consensus in |
| Administration of prokinetic drugs, secondary to feed intolerance | No consensus |
| GI morbidity: diarrhoea | No consensus |
| Length of stay: hospital | No consensus |
| Total length of time on respiratory support (IV + NIV) | No consensus |
| Long-term feeding issues | Consensus out |
| Long-term outcomes (after hospital discharge) | Consensus out |
| Looking and/or behaving like their normal self | Consensus out |
| Nursing time spent measuring GRV | Consensus out |
| Change in length (growth) between PICU admission and discharge | Consensus out |
| Parental satisfaction | Consensus out |
Neonatal unit meeting report
Participants
The following members of the SMG attended the NNU meeting:
-
Anne Beissel (member of the SMG who participated in voting)
-
Barbara Arch
-
Chris Gale (member of the SMG who participated in voting)
-
Helen Eccleson
-
Iza Andrzejewska (member of the SMG who participated in voting)
-
Jennifer Preston
-
Kerry Woolfall
-
Louise Roper
-
Lyvonne Tume.
Nineteen participants (one neonatal charity representative, one clinical triallist and methodologist, two neonatal dietitians, five neonatal doctors, seven neonatal nurses, one paediatric general surgeon and two parents) attended this meeting from 14 out of 184 (8%) UK NNUs, three UK universities and a neonatal charity: Bliss, Bradford Teaching Hospitals NHS Foundation Trust (Bradford), Chelsea and Westminster Hospital (London), Great Ormond Street Hospital (London), NHS Greater Glasgow and Clyde (Glasgow), Lancashire Teaching Hospitals NHS Foundation Trust (Preston), North Bristol NHS Trust (Bristol), Portsmouth Hospitals NHS Trust (Portsmouth), Sheffield Teaching Hospitals NHS Foundation Trust (Sheffield), Shrewsbury and Telford Hospital NHS Trust (Shrewsbury), University Hospitals of Leicester NHS Trust (Leicester), University of Oxford (Oxford), University of Southampton (Southampton), University of West London (London) and William Harvey Hospital (Ashford).
Discussion areas
There was discussion around specifics of a protocolised control arm or whether or not we allow a more pragmatic approach and not ‘define’ a control arm. Specifically, issues were raised about defining a specified time period that feeds would be withheld after large GRVs and what to do with returning aspirates. One consultant said that they would go with a GRV control arm, even though their unit does not currently routinely measure GRV. A nurse stated that in their unit they would not be happy going back to measuring GRV, as this practice stopped many years ago. A good point was raised about not using the term ‘intervention arm’ and instead using ‘aspirate’ or ‘no aspirate’ arms, to acknowledge that around 30% of UK NNUs do not measure it routinely. There was further discussion and debate about a cluster compared with individual randomised trial, with the methodologists, triallists and statisticians strongly recommending a standard trial. This recommendation was on the basis that cluster trials usually require larger sample sizes and are therefore more difficult to fund, there needs to be a large number of units taking part to balance out centre-specific heterogeneity in methods of care and patient populations, and that a cluster design would necessarily exclude Scottish NNUs. Clinicians and some parents preferred a cluster randomisation approach, with factors cited including being a ‘whole unit education and intervention’ being less confusing, easier for practice and fewer problems with agency nursing staff. Additionally, consent could be obtained at an institutional level, rather than needing to be sought from families in a moment of distress. The overall view was that most babies should be included, but any surgical babies and babies with cardiac lesions (awaiting transfer to a PICU) should be excluded (and these would comprise small numbers estimated at < 10% anyway). This would also increase ‘buy-in’ from units for a future trial.
Dr Anne Beissel (Lyon, France), a SMG member, presented a no GRV protocol and the impact of this on their unit after changing in the last 5 years. This NICU had measured the impact of a no GRV protocol on their outcomes of NEC, time to feeding tube removal and hospital length of stay. They found that the incidence of NEC in their unit (in infants < 33 weeks’ gestation) was 2.8–3% (comparable to the 2–7% incidence reported in the recent literature39). The median age of feeding tube removal was 33.8 weeks (or 60 days of life) and the median hospital length of stay was 89.8 days for infants at < 28 weeks’ gestation, 52.2 days for infants between 28 and 31 weeks’ gestation and 25.5 days for infants between 31 and 36 weeks’ gestation. This is comparable to that reported in the literature40 and was not affected by not measuring GRV.
Neonatal unit outcomes: consensus results
A total of 26 outcomes were discussed, but five of these were already voted as ‘consensus in’ from the e-Delphi (see Chapter 3). Voting therefore took place on the remaining 20 no-consensus outcomes. Of these, four were voted ‘consensus in’, four were voted ‘consensus out’ and 12 outcomes remained as ‘no consensus’ (Table 26). Full results including voting statistics can be found in Table 1 in Report Supplementary Material 2.
| Outcome | Consensus status |
|---|---|
| Change in weight (growth) between birth and NNU discharge | Consensus in |
| Health-care-associated infections | Consensus in |
| Incidence of catheter-associated bloodstream infection | Consensus in |
| Incidence of pneumonia due to milk aspiration | Consensus in |
| Change in head circumference between birth and NNU discharge | No consensus |
| GI morbidity: vomiting | No consensus |
| Length of stay: hospital | No consensus |
| Length of stay: NNU | No consensus |
| Length of time: IV | No consensus |
| Long-term outcomes: hearing loss | No consensus |
| Long-term outcomes: problems with eyesight | No consensus |
| Long-term outcomes: problems with cognition | No consensus |
| Long-term outcomes: brain injury on imaging | No consensus |
| Long-term outcomes: problems with mobility (e.g. cerebral palsy) | No consensus |
| Time to oral feeding | No consensus |
| Total length of time on respiratory support (IV + NIV) | No consensus |
| Change in length (growth) between birth and NNU discharge | Consensus out |
| GI morbidity: diarrhoea | Consensus out |
| Nursing time spent measuring GRV | Consensus out |
| Time to nasogastric tube removal | Consensus out |
Summary of findings to inform future GASTRIC trials
The consensus meetings were very useful, enabling us to gain consensus on 12 outcomes that should be measured in a future PICU trial: (1) time to and achievement of predicted energy goals, (2) incidence of VAP, (3) time feed stopped per 24 hours, (4) incidence of GI morbidity (vomiting), (5) length of time on IV, (6) length of stay in a PICU, (7) mortality, (8) post-pyloric feeding (placing a post-pyloric feeding tube), secondary to feed intolerance, (9) administration of parenteral nutrition, secondary to feed intolerance, (10) change to feed formula type, secondary to feed intolerance, (11) change in weight (growth) between PICU admission and discharge and (12) incidence of NEC.
We gained consensus on nine outcomes to be measured in a future NNU trial: (1) mortality, (2) the incidence of NEC, (3) time from start of enteral feeds to achieve full feeds (150 ml/kg/day), (4) days on parenteral nutrition, (5) time feed stopped per 24 hours, (6) change in weight (growth) between birth and unit discharge, (7) health-care-associated infections, (8) incidence of catheter-associated bloodstream infections and (9) incidence of pneumonia due to milk aspiration. Furthermore, they allowed us to determine wider UK acceptability and ‘buy-in’ for a future trial, beyond that ascertained in the focus groups and interviews, and to discuss and debate some of the more contentious issues around a future trial that could be captured in the trial design survey.
Chapter 6 Patient and public involvement
Aim
To gain meaningful PICU and NNU parents’ perspectives and input into a future GASTRIC trial.
Parent involvement objectives
Objective 1
-
Obtain NNU and PICU parents’ views on the design of the study.
-
Include input into the acceptability of the proposed trial.
-
Address outcome measures.
-
Review participant information and to discuss any potential barriers or opportunities to recruitment.
Objective 2
-
To obtain feedback from parents regarding the results of the study. This was delivered via two webinars, one aimed at NNU parents and one aimed at PICU parents. Feedback would be incorporated into the consensus meetings.
Objective 3
-
Parent involvement in NNU and PICU consensus meetings.
Prior to the study commencing, we consulted with parents from both neonatal intensive care (through personal contacts and via the charity Bliss) and paediatric intensive care (through the PICANet parents’ group and personal contacts). We also consulted with an ex-PICU patient (now aged 18 years). All parents agreed that this topic was important to them and that the proposed feasibility study would genuinely elicit parents’ views.
Methods
In the original application, we set out to form a parent advisory group made up of parents who had experience of their child being in a NNU or a PICU, who would meet face to face at least twice during the study. However, it proved difficult to recruit to such a group for face-to-face meetings for various reasons, such as caring responsibilities, juggling work and geographical distance. Therefore, we sought other virtual methods to engage with these parents.
Nine parents (five PICU parents, two NNU parents and two parents having had experience of both NNUs and PICUs) took part in a virtual capacity (five in the study design phase and four in the feedback webinar sessions). One parent became a member of the Study Management Group and two parents attended the face-to-face consensus meetings. Parents were contacted through a variety of channels, including parent support groups, social media and parent charity organisations (e.g. Bliss).
To compensate for the lack of face-to-face meetings, parents were offered support via a parent and carers’ research forum (based at Alder Hey Children’s Hospital, co-ordinated by the patient and public involvement manager, JP). The group offers ongoing support to parent members (with varied experiences of looking after a child with a chronic condition), through training, regular face-to-face meetings (if family commitments allow), joining an online community (closed Facebook group), regular contact with other parents and carers, and various opportunities to attend and present at conferences. One parent is a member of this group and attends regular meetings, and three of the five parents joined the closed Facebook group. The majority, however, preferred to communicate via e-mail or telephone call, if necessary, if they had any questions.
Parent feedback on the design of the study
Five parents contributed to the design of the study. Study documentation, which included a lay summary of the study, draft parent information, proposed outcome measures and the parent interview guide, was shared with each parent. Guidance and prompts were shared with the documentation, to include:
-
Is the wording in the parent information sheet clear?
-
Do you understand what the study team is trying to explain?
-
Have the study team explained the list of possible outcomes well enough? Are they clear?
For the question guide for interviews:
-
Do you think we have missed out any important questions?
-
Are we asking the right questions to gauge parents’ views and opinions on a future trial like this?
-
Do you have any other concerns?
Parent views on study documentation
Lay summary
Overall, parents felt that the lay summary was clear and easy to understand, although two parents (PICU) highlighted their dislike of the phrase ‘sucking it out’ in reference to the withdrawing of the fluid:
I understand it is wanting to not sound too medical but it just sounds a bit brutal when it really isn’t.
NNU parent
This raised a question from one parent, ‘when they suck out the child’s stomach contents do they put it back?’. The parent suggested that maybe the response to this needs to be included in the parent information sheet.
One parent did not like the mention of saving costs to justify the reason for undertaking the study:
I think it’s fine to say it saves time, etc., but no parents/patients ever want to hear costs being discussed as we can’t put a price on our kids’ lives. Much better to phrase it that saving time for nurses leads to better care, etc.
PICU parent
Parent feedback on the proposed GASTRIC RCT participant information sheet
All five parents highlighted the need to explain, in a little more detail, the potential benefits and risks of the study:
It covers benefits well but when a child is on PICU a parent tends to think more about risks and potentially negative outcomes to their own child rather than benefits to children in the future.
PICU parent
Three parents (PICU, n = 2; NNU, n = 1) highlighted their dislike of the word ‘safe’, which appears several times across all the documents (e.g. ‘To find out whether not routinely measuring GRV during is safe and beneficial’). One parent stressed:
I would be inclined to remove the word ‘safe’ and instead talk about the ‘pros and cons’ of not measuring GRV, premature parents make very good armchair medics, and the last thing NNU staff need is parents questioning whether they are putting their baby at undue risk by measuring/not measuring GRV!
NNU parent
Another parent stressed:
We want to see whether it is safe – parents may have the understanding that the trial could potentially be unsafe and could be putting their child at risk. Maybe change the wording in the information to – we want to see whether the child is absorbing enough calories?
PICU parent
The parents felt that if the risks are explained enough then they thought that the study would be OK:
I suppose the main worry for any parent would be the risks associated with taking part, and as long as these are fully discussed and reassurances made, I don’t see a problem.
NNU parent
Obtaining consent and the ability for parents to decide to participate in the study was a concern for some parents. One parent felt that there was quite a lot of information to take in, and that verbally discussing and explaining the study may be more useful to some parents:
There is a lot of text, but I’m not sure how else you could gain full consent without all the information listed? My own experience was that I had pre-eclampsia and was really quite unwell for the first few days my baby was in NNU. The unit was busy, we very rarely saw the same member of staff and some staff were much better at communicating updates on my babies’ care than others. I am not even sure that I would know the answers to a lot of these questions as I was either never given the information or I was too ill to remember.
NNU parent
Another parent felt that the timing of the interview is crucial, especially for mothers of babies being tube fed:
It’s horrible when your baby is ill but actually, from my own memories, the tube feeding is soul destroying. The most natural thing in the world is to feed your baby and so when that is taken away from you feel completely useless as a mother. Parents need some time to adjust to that and really not questioned too heavily on their thoughts on tube feeding and just asked the absolutely essential things for the study and as concisely as possible.
PICU parent
The same parent was also not overly keen on the phrase ‘cannot eat normally’ because for her child, and for many others, tube feeding is normal feeding: ‘Could this be changed to “cannot feed orally” perhaps?’ (PICU parent).
One parent expressed the importance of producing a summary of the results to give to parents who had participated:
There is no mention of follow up feedback or results? If I was consenting to the study I would like to know I will receive some feedback at the end of the study.
PICU parent
Overall, parents considered the draft PIS to be clear and felt that it explained the study well and was written in a way people will find easy to interpret. A summary of what would benefit from further clarification is provided in Box 4.
Additional information, suggested examples:
-
When they suck out the child’s stomach contents do they put it back?
-
Remove the word ‘safe’.
-
Expansion on the proposed ‘risks’ and ‘benefits’.
-
Feedback of the results to parents.
-
Further detail on if parents will know if their child was allocated to the GRV group or the no GRV group.
Parent feedback on the interview questions
The main feedback regarding the interview questions is that there were too many and could be reduced. Parents felt that HCPs carrying out the study will have access to patients’ notes, either electronically or on paper. Some of the questions can already be answered, for example ‘how long was your child on a ventilator (differentiate between mechanical ventilation/ continuous positive airway pressure/high flow)’. Some parents may understand this question, but others may not be able to differentiate, and this information can be obtained beforehand.
One parent felt that some of the questions may be difficult for parents to answer precisely:
From my own experience, I would struggle to tell you exactly the amount of days/months we spent on PICU and definitely struggle to recall the length of ventilation. I’m not sure if this is because we’ve been there so many times or if it’s because I’ve tried to forget about it as it feels like a surreal situation being on the unit. There are also a lot of questions to be asking parents whilst they are sat on PICU during a stressful time. Could some of the information be obtained from the notes with consent?
PICU parent
General concerns about explaining risks and benefits were raised again:
In question 4 of the question guide, I would be inclined to expand the statement ‘we cannot guarantee this will benefit your baby,’ to include an additional comment to the effect of, ‘However, we do guarantee that it won’t cause harm, and won’t negatively affect their treatment’.
NNU parent
It might be worth adding a note to make it clear that the baby’s care will be provided by the same highly qualified NNU/PICU doctors and nurses, irrespective of whether they participate in the study or not, and that their decision about whether or not to participate will not, in any way, affect the care and treatment that their child receives, other than the specific procedure of measuring GRV as outlined in the study notes.
NNU parent
Subtle word changes were also mentioned to avoid raising any further concern with parents:
I would advise changing ‘the babies reached their full levels of feeds and did not get ANY bowel infections . . . ’ to ‘did not get AS MANY bowel infections . . .’ again, this subtle change in wording will avoid parents questioning whether their child contracted NEC/needed bowel surgery as a result of what they now feel (after reading about your study) might have been an unnecessary procedure.
NNU parent
Parent feedback on outcome measures
Following a review of all previous research studies, looking at GRV measurement a list of proposed outcomes for the GASTRIC trial was shared with the parents, to identify if there was anything missing.
The main outcomes parents wanted to add include:
-
Were they able to take their baby home from the hospital?
-
What setbacks did the family encounter during their baby’s stay in the hospital?
-
Does the child have any lasting health issues?
-
Was their child left with any long-term feeding issues as a result of their time on the PICU?
-
Are they still tube fed or struggling to gain weight?
-
Did they have any feeding or GI issues after the PICU stay?
One parent also suggested rephrasing the last point asking about survival, suggesting that this be worded in less clinical, more compassionate terms.
Parent feedback on potential trial barriers to and opportunities for recruitment
Parents perceived that the main potential barrier to recruitment to any proposed study was the timing of the approach.
Several suggestions were made on possible recruitment opportunities, which include:
-
using social media would get attention but maybe not the responses
-
a simple letter or e-mail to parents of the children asking if they would be interested in taking part
-
asking parents whose child is having a planned admission to PICU beforehand
-
involving organisations, such as Bliss, followed by Facebook groups specifically targeting parents of preemies, such as the born-too-soon group on BabyCentre (URL: www.babycentre.co.uk), as well as hospital support groups
-
try to link with parent groups who have communities of > 100,000 mums.
Parent feedback webinars
Two evening webinars were held at a time agreed with the parents, one for PICU parents and one for NNU parents. These webinars were used to thank parents for their contributions to date, feed back the preliminary findings from the study so far and get parents’ feedback on this, which would inform the preparation of the two planned consensus meetings. Despite the teams’ best efforts, only one parent dialled into the NNU webinar (one mother) and four parents (one father and three mothers) dialled into the PICU webinar.
Neonatal unit webinar
Following a short presentation of the results, the parents asked some questions:
-
To clarify, what is meant by ‘surgical’ babies?
-
Why did nurses prefer individual randomisation and parents prefer a cluster trial?
-
Is it correct that feedback, so far, indicates that babies already showing signs of NEC should be excluded from the study?
In addition, the parent recalled the lack of recollection of GRV measurements being taken, and asked whether or not all units in the UK measure GRV. The parent later followed this up in an e-mail regarding the practice of the hospital in which her daughter was cared for:
Going back to the existing results, it would be interesting to see if the results are biased towards units that do currently check GRV, and more specifically, what proportion of the 48% of units that didn’t respond to your survey don’t check GRV (and, therefore, like my daughter’s hospital, felt as though they’d have nothing useful to say on the subject)? Could it be that the checking of GRV isn’t quite as pervasive as your findings suggest? Not sure how relevant this is to the study, but if it is important to know one way or the other, it could be easily checked by running a simple ‘tick-box’ survey of units asking, say, ‘which of the following are routinely practiced in your department . . .’ with a variety of different options, one being the checking of GRV.
NNU parent
The parent also emphasised the point that parents’ experience of early stages of admission are hectic and making decisions about feeds is not always their priority. It is also difficult to understand the terminology in the early stages of admission.
Paediatric intensive care unit webinar
Similarly, a short presentation of the study results was given, then parents shared their experiences and asked questions. Some parents had experience of both PICUs and NNUs and some were still home tube feeding their child. The parents were all positive about the GASTRIC trial, and felt that it was necessary and would benefit both the children and HCPs involved.
Questions and discussions included:
-
Does checking aspirates help to identify issues or conditions such as NEC, bowel issues, etc.?
-
Is the study team proposing measuring volumes at set times or at times of feeds or medication?
One parent discussed their experience of feeds being stopped because of large aspirates and agreed with the suggestion that stopping feeds may not be the best for the child, and highlighted that calories mean growth and growth helps the child get better. In addition, the same parents emphasised that it is important to consider how parents are approached to take part in the trial, which was highlighted in the feedback above and in the qualitative phase of the study.
Parental involvement in the neonatal unit and paediatric intensive care unit consensus meetings
Two NNU parents joined the NNU meeting, but unfortunately the parent who expressed an interest in attending the PICU meeting had to withdraw at the last minute due to her child being unwell. The consensus meetings provided an opportunity for parents to hear the detailed findings from the study and contribute to further discussions to inform the development of a future trial.
Summary of the patient and public input into the GASTRIC feasibility study
Despite using a variety of recruitment methods to involve as many parents with PICU and NNU experience as possible, meeting face to face was extremely challenging for this group of parents. This was more challenging if they are still caring for babies who may have complex care needs, as well as looking after other members of the family, combining this with work and if meetings are geographically distant from parents’ homes. The lack of face-to-face meetings meant that most activities were carried out virtually. The offer of support to the parents via a parent and carers’ research forum was taken up by four of the parents involved, but their preferred method of support was via e-mail or telephone.
Despite these limitations, the information we gathered provided us with valuable insights into the important issues for parents and carers for a future study exploring GRV measurement in UK PICUs and NNUs. The study team acted on this, listened to feedback from parents and revised the lay summary, PISs and interview questions, accordingly. The summary of the research findings will be co-produced by parents in a visual abstract format and disseminated widely among the PICU and NNU parent community.
Chapter 7 Proposed trial designs
This chapter will summarise some of the findings and discuss a proposed trial design, control and intervention arms for a future trial in (1) PICUs and (2) NNUs.
Paediatric intensive care units
As trial design is influenced largely by the choice of primary outcome measure, the two suggested primary outcome measures of VAP and the percentage of energy target achievement will now be discussed.
Ventilator-associated pneumonia
Work package 2 showed that VAP is the AE that most PICU clinicians fear about a full stomach, leading to vomiting and subsequent pulmonary aspiration (resulting in VAP), and adult trials of no GRV have powered their trials on this outcome. However, the incidence of VAP is much lower in paediatric ICUs at 5.6 to 9.2 per 1000 ventilator days (compared with that reported in adult ICUs of 19–27% or 21.3 per 1000 ventilator days). 29,41,42 Currently, few UK PICUs report having an active surveillance programme for VAP (L Tume, University of Salford, 2019, personal communication) and one unit that did, abandoned this altogether. Furthermore, the diagnostic criteria for VAP in paediatrics remains subjective and problematic. 43,44 Therefore, given the low incidence in PICUs, it is unlikely that a non-inferiority trial would have sufficient statistical power on this outcome.
Time to achievement of predicted energy goals
The most commonly preferred primary outcome was time to achievement of predicted energy goals. Published literature reports the achievement of mean energy targets over the first 3 days within the PICU population to be quite variable, ranging from 31% to 82%. 45–48 A study by Tume et al. 31 is the only study that looks at the effect of measuring GRV on the outcome in PICUs, but is a small retrospective observational study comparing measuring GRV to not measuring GRV. This study found that by day 3, the GRV group had achieved 82% (SD 40) of their prescribed feed, compared with 95% (SD 23) in the ‘no GRV’ group. This indicates that there is potential for an improvement in feeding if GRV is not measured, although a larger RCT would be needed to find a statistically significant difference. In adult populations, percentage energy targets achieved seem to be much higher, ranging from 65% to 80%,29,49 and they also provide evidence to suggest that in these populations, measuring GRV has an adverse effect on this outcome. A cohort study of adult ICUs49 showed that over 7 days ‘no GRV’ patients had an average of 95% energy target reached compared with 83% in those ‘with GRV’. Current recommendations50 are to try to achieve no more than the estimated energy requirements using the Schofield equation by 72 hours post PICU admission. All UK PICUs set some energy targets (almost all using the Schofield equation with a variation). In Chapter 4 we present PICANet data for 2016 and 2017. These data show that we can expect > 8000 children in the UK every year that satisfy our potential eligibility criteria and, of these, > 5000 are likely to be intubated for ≥ 3 days (≥ 72 hours).
Sample size calculation
Table 27 below gives a range of possible calculations based on different effect sizes and SDs for the following primary outcome:
| Effect size: mean difference in % energy target achieved by day 3 for ‘no GRV’ vs. GRV | SD | n required per arm | |
|---|---|---|---|
| 80% power | 90% power | ||
| 5% | 20 | 251 | 337 |
| 30 | 564 | 757 | |
| 40 | 1003 | 1345 | |
| 10% | 20 | 63 | 85 |
| 30 | 141 | 190 | |
| 40 | 251 | 337 | |
| 12% | 20 | 44 | 59 |
| 30 | 98 | 132 | |
| 40 | 175 | 234 | |
| 15% | 20 | 28 | 38 |
| 30 | 63 | 85 | |
| 40 | 112 | 150 | |
The calculations assume that the outcome is normally distributed and are based on the independent samples t-test for testing for a difference between means. All the scenarios in Table 27 show that the total numbers needed are feasible within the eligible population. We recommend that a future trial is designed with an in-built pilot phase, to check that SDs are correct, given the variability of estimates of these from the literature.
Trial design
In terms of ‘buy-in’ from PICUs for a future trial, 13 out of 29 PICUs attended the consensus meeting, with all but one demonstrating commitment and interest in this future trial. Additionally, feedback was that this energy target outcome would get significant ‘buy-in’ from the PICU dietitians, many of whom could, and would like to, act as local principal investigators for this study.
There was support at the consensus meeting for utilising a cluster randomised approach with the same outcome measures. Many clinicians favoured this approach, with triallists and statisticians not in favour. With only 29 PICUs in the UK (and 19 of these research active and eliminating the two Scottish PICUs who require individual consent for any trial), it is very unlikely that a cluster trial could be powered in the ≤ 15 PICUs that had showed an interest. However, potential advantages with this method were noted, that is, a whole unit-based education programme and ‘buy-in’ and potentially less risk of crossover between control and intervention trial arms (if the nurse had two or more patients on different arms of the trial). This anticipated problem would need to be investigated in the pilot phase of a future trial.
The most feasible trial design for PICU therefore appears to be an individual consent superiority trial with an inbuilt pilot study, with clear progression targets on the primary outcome of achievement of energy targets by 72 hours after PICU admission, combined with an underlying process evaluation. Secondary outcomes should be those that gained ‘consensus in’ via the e-Delphi study and the final voting at the consensus meeting.
Proposed paediatric intensive care unit GASTRIC trial control and intervention arms
The proposed PICU ‘control arm’ of GRV measurement is as follows:
-
Standardised nutritional assessment: Paediatric Yorkhill Malnutrition Score risk score + weight + length/height + z-score at admission.
-
Energy targets should be calculated based on the Schofield equation, with an aim to achieve but not exceed this target by 72 hours.
-
If no contraindications, start gastric feeding within 6–12 hours of admission (continuous or bolus allowed).
-
Feeding tube position should always be checked and pH tested – this is not GRV measurement and requires only ≈0.3 ml of aspirate (do not aspirate the whole stomach).
-
Routine GRV measurement at least 6-hourly, whether bolus or continuous feeding (always using a 50-ml syringe and aspirate slowly).
-
GRV threshold for stopping enteral feeds: 5 ml/kg or < 250 ml for > 40 kg.
-
If < 5 ml/kg, replace and continue to increase feeds (rate/volume) as per unit protocol.
-
If GRV > 5 ml/kg, replace GRV (unless faecal, bloody or very bilious), withhold feeds for 2 hours and reassess GRV.
-
If two or more GRVs are > 5 ml/kg and fail to tolerate enteral nutrition (EN) consider post-pyloric feeding, adding prokinetics (suggest oral erythromycin), changing feed formula and/or changing to continuous feeding (if bolus fed).
-
In patients requiring frequent fasting (e.g. larger burns) place nasojejunal tube early and commence post-pyloric feeding.
See Appendix 1 for a flow chart.
A proposed no GRV ‘intervention arm’ for the PICU GASTRIC trial is as follows:
-
Define daily nutritional goals according to patient status using the Schofield equation.
-
Prescribe nutritional support according to these goals.
-
Monitor tolerance (vomiting and other GI or systemic signs).
Discuss and agree ‘feed intolerance ‘and any feed withholding with senior colleague or team, and actively search for other causes to treat:
-
iatrogenic sedation withdrawal syndrome
-
malpositioned gastric tube tip (i.e. tip in oesophagus)
-
electrolyte imbalances (e.g. hypokalaemia and/or hypomagnesaemia)
-
intracranial hypertension
-
coughing
-
patient agitation.
See Appendix 2 for a flow chart.
A previous single-centre UK PICU study48 examining PICU nurses’ decision-making around GRV found that most nurses (84%) would be very concerned if they could not measure GRV routinely. However, there was some confusion around what GRV measurement was and what was pH testing to confirm tube position. Many nurses, when presented with some evidence, could understand the need for a trial and would be happy to participate, so long as some education and clear guidance was provided about how to monitor tolerance if not using GRV. The intervention arm would have to be supported by an educational (e-learning) package, which presents the evidence and rationale for the trial, along with clear guidance about pH testing compared with GRV measurement, and a simple flow chart to guide their decision-making and what to do if they were concerned regarding feed intolerance.
The supporting nurse education package (with proposed educational content) is provided in Appendix 4.
Neonatal unit
The two suggested primary outcome measures, incidence of NEC and the time from start of enteral feeding to achieve full (150 ml/kg/day) enteral feeds, will now be discussed.
Incidence of necrotising enterocolitis
Work package 1 showed that NEC is the AE that many NNU clinicians (24/61, 39%) rated as the most important for a primary outcome in a future trial. In Chapter 4 we presented national data on NEC from 2017 and 2018. In those 2 years, the underlying incidence of NEC was 0.6% in NNUs and 5.2% in infants born before 32 weeks’ gestation. Given that neonatal clinicians would not adopt no GRV measurement if there was an appreciable increase in NEC, a non-inferiority analysis would be appropriate for this outcome. If there is truly no difference between the standard and experimental treatments (5.24% in both groups), then 17,018 patients are required to be 90% sure that the upper limit of a one-sided 95% confidence interval (or equivalently a 90% two-sided confidence interval) will exclude a difference in favour of the standard group of > 1%. This is a very large number and would not be a feasible primary outcome measure The UK measures the incidence of NEC annually by NNU for benchmarking, through the NNAP and using data held in the NNRD. Collection of NEC data would therefore be feasible using existing, routinely recorded clinical data held in the NNRD, for a secondary safety outcome in any planned trial.
Time required to achievement of full (150 ml/kg/day) milk feeds
The second most highly ranked outcome from the e-Delphi and consensus process was time to full milk feeds (150 ml/kg/day). This is a very feasible outcome that has already been used for other neonatal trials, including the Speed of Increasing Feeds Trial5 and the Abnormal Doppler Enteral Prescription Trial (ADEPT). 51 The data required for this outcome are routinely recorded using existing electronic patient record systems and held in the NNRD, so the use of this outcome for a trial would be efficient and feasible. In Chapter 4 we present NNRD data for 2017 and 2018. This shows that we can expect > 7500 babies in the UK every year being admitted to a NNU, who are born at < 32 gestational weeks. Many of these babies will be mechanically ventilated and require a nasogastric tube feed. However, even babies who are not mechanically ventilated or who will not require a nasogastric tube feed at these gestational ages, will need to establish milk feeds and could benefit or be harmed by GRV monitoring. The median time it took for these babies to achieve a full feed was 12 (IQR 9–17) days and the mean was 15.3 (SD 11.9) days. As the median is lower than the mean, and these statistics are based on a sample size of 15,375 babies, we can assume that this measure is not normally distributed and is skewed towards a shorter length of time.
Sample size calculation
Table 28 gives a range of possible sample size calculations based on different effect sizes and SDs for the outcome ‘time required for achievement of full (150 ml/kg/day) enteral feeds’. These calculations assume that the outcome has minimal censoring (almost all babies will reach full feed unless transferred or death occurs), so the outcome does not need to be treated as a time-to-event and is positively skewed (has a log-normal distribution). Calculations are based on the methodology given by O’Keefe et al. ,52 which uses medians and SDs to provide sample size calculations. We use the NNRD data to provide estimates for the GRV arm (median 12 days, SD 11.9 days). All the scenarios in Table 28 show that the total numbers needed are feasible within the eligible population. Given that 7500 babies are born each year in the UK at this gestational age, and that the Speed of Increasing Feeds Trial5 recruited 2804 such babies in 25 months, these sample sizes all appear feasible. 5 A total of 3736 infants would give adequate power for most of the probable scenarios. If this was felt to be unfeasible, 1594 infants would give adequate power to detect a 3-day difference.
| Effect size: difference in median days fed between GRVa and no GRV | SD (GRV arm) | SD (no GRV arm) | n required per arm | |
|---|---|---|---|---|
| 80% power | 90% power | |||
| 1 day | 11.9 | 9.0 | 4695 | 6286 |
| 11.9 | 11.9 | 5510 | 7376 | |
| 11.9 | 13.0 | 5799 | 7763 | |
| 2 days | 11.9 | 9.0 | 1129 | 1511 |
| 11.9 | 11.9 | 1326 | 1775 | |
| 11.9 | 13.0 | 1395 | 1868 | |
| 3 days | 11.9 | 9.0 | 482 | 645 |
| 11.9 | 11.9 | 566 | 758 | |
| 11.9 | 13.0 | 595 | 797 | |
| 4 days | 11.9 | 9.0 | 260 | 347 |
| 11.9 | 11.9 | 305 | 408 | |
| 11.9 | 13.0 | 320 | 429 | |
| 5 days | 11.9 | 9.0 | 159 | 212 |
| 11.9 | 11.9 | 186 | 249 | |
| 11.9 | 13.0 | 195 | 261 | |
Proposed neonatal unit ‘no GRV’ intervention arm based on Lyon neonatal unit protocol
A proposed NNU ‘no GRV’ intervention arm was developed and discussed at the consensus meeting. This was based on the protocol in use in the Lyon NNU and is presented here for information. In addition, this would be used as a starting block for discussion and planning of a future trial. For medical babies, this protocol included aspects that would be open to individual site practice variations in a pragmatic trial. These were:
-
enteral feeding from day 1 of life
-
eight feeds per 24 hours
-
oral feeds commenced at 29 weeks’ gestational age
-
feeds increased across the eight daily feeds, according to the weight of the baby (Table 29).
| Weight (g) | Feed increase |
|---|---|
| < 800 | 8 × 0.5 ml extra for each feed |
| 800–1000 | 8 × 1 ml extra for each feed |
| 1000–1200 | 8 × 2 ml extra for each feed |
| 1200–1500 | 8 × 3 ml extra for each feed |
| 1500–1750 | 8 × 4 ml extra for each feed |
| 1750–2000 | 8 × 5 ml extra for each feed |
| 2000–2500 | 7 × 10 ml extra for each feed |
| > 2500 | On demand |
Instead of using GRV, tolerance to feeding is assessed by bowel movements, softness of the abdomen and the absence of erythema or tenderness (Figure 7). If there is emesis, erythema, distension or tenderness, an assessment of severity is made and then feeds are held or reduced if the problems are minor, or a radiograph is performed if NEC is suspected (see Figure 7).
FIGURE 7.
Proposed intervention arm protocol for no GRV measurement.
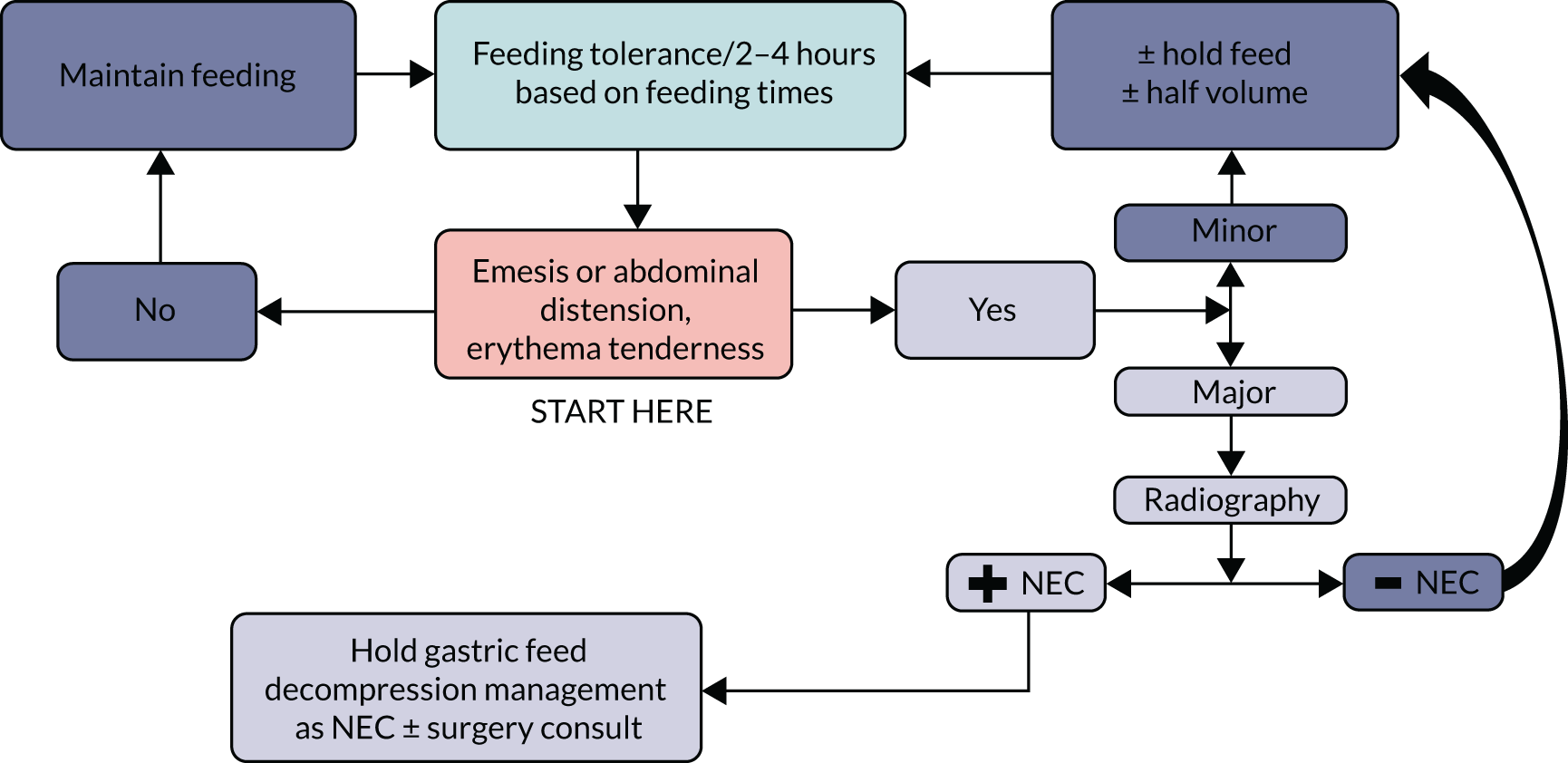
Both proposed trial arms and their associated flow charts are provided in Appendix 2. The supporting staff education package (with proposed educational content) is provided in Appendix 5.
Chapter 8 Discussion and conclusions
Summary of main findings
It was clear at the application stage that although paediatric and neonatal intensive care was ‘combined’ in the commissioning brief, these two specialties are different, with different staff and patients, and thus two separate parallel feasibility studies were conducted: the GASTRIC PICU study and the GASTRIC NNU study. The studies have been reported separately and cannot scientifically or feasibly be combined into one trial.
Surveys of current practice
The survey of PICU practice showed that the regular and frequent measurement of GRV was usual practice in all but one UK PICU. From this survey, we were able to construct a ‘most likely to be accepted’ control arm of a future trial (see Appendix 1).
In NNUs, regular and frequent measurement of GRV was also common, but more mixed than in PICUs, occurring in approximately 66% of responding NNUs in the UK (59/90). We were also able to construct a ‘most likely to be accepted’ control arm of a future trial (see Appendix 2).
Qualitative study
The qualitative work with PICU parents and HCPs provided insight into issues about the information provided and the acceptability of a definitive trial of no routine GRV measurement in UK PICUs. This WP explored the views of 14 PICU parents and 26 PICU HCPs about a future trial. Overall, parents were supportive of a trial but, like other trials in PICU, the timing and manner of the approach for consent was identified as important. Concerns raised by parents related predominantly to delays in identifying AEs or complications (such as vomiting with pulmonary aspiration, leading to pneumonia, and NEC in infants) and unnecessary discomfort. We sought parents’ views about the most important outcome measures to them. The parents prioritised seven key outcomes. These parent-generated outcome measures were added into the e-Delphi survey for HCPs. Twenty-six PICU HCPs’ views from two geographically dispersed PICUs were sought in focus groups. In addition, six telephone interviews were conducted to target key individuals, such as surgeons. Staff were generally positive about a future trial, with the biggest concern being about changing a long-standing and embedded practice (that of routine GRV measurement to guide feeding). The least positive staff were junior nurses. Mixed views existed about the benefit and utility of measuring GRV. Nurses were the most attached to this historical practice, with many feeling that it was reassuring and provided them with a safety net.
Overall, the proposed GASTRIC paediatric intensive care unit trial was viewed as acceptable to most PICU parents and staff, and is feasible to conduct.
For NNUs, the views of 17 parents and 16 HCPs were elicited regarding a future GASTRIC NNU trial. These parents were also supportive of a trial and emphasised that the timing and manner of the approach for consent were important. Similar to PICU parents, concerns were raised by NNU parents around delays in identifying AEs, as well as unnecessary discomfort. Neonatal parents were particularly concerned about NEC, but unlike the parents of PICU patients were not concerned about VAP. Neonatal parents prioritised seven key outcomes and these were added into the e-Delphi survey for HCPs.
The views of 42 HCPs from two NNUs were gained in focus groups and five telephone interviews. Staff, again, were generally positive about a future trial and the biggest concern raised was around changing a historical and embedded practice. As in PICUs, the least positive staff were junior nurses, who were the most attached to this historical practice.
Overall, the proposed GASTRIC neonatal unit trial was also viewed as acceptable to most neonatal parents and staff, and is practically feasible to conduct.
The trial design survey, including the e-Delphi study
The PICU e-Delphi study elicited views from 30 PICU HCPs, regarding who should be included and who should be excluded in a future trial, their willingness to randomise a child into this study, trial design and the ranking of the outcome measures for a future trial. Almost all (97%) staff were willing to theoretically randomise a child in a definitive trial. Nearly three-quarters (73%) thought that all children aged > 37 weeks to 17 years admitted to a PICU who were enterally fed should be included in the definitive trial, not just mechanically ventilated children. Most believed that children expected to stay < 24 hours or who are not expected to survive, would be excluded, along with children with a GI pathology or a ‘surgical gut’ (who would not be enterally fed initially). Sixteen potential outcome measures were rated in the first round of the e-Delphi study, including the seven parent-suggested outcome measures. Six outcome measures were added from participants in rounds 1 and 2, resulting in 22 potential outcomes that were rated in round 2. Consensus was gained on four outcome measures: (1) time to achieve target energy goals, (2) VAP, (3) the incidence of vomiting and (4) time feeds were stopped in each 24-hour period. No items were voted ‘consensus out’, leaving 18 items to be discussed and voted on at the consensus meeting. The two highest scoring primary outcome measures were (1) time to achievement of predicted energy goals (full feeds) (36%) and (2) incidence of VAP (23%).
Seventy-six HCPs participated in the neonatal e-Delphi study, which sought to elicit views on inclusion and exclusion criteria for a future trial, trial design, willingness to randomise a baby into a trial and rating outcome measures for a future trial. Most (91%) staff were willing to theoretically randomise a baby in a definitive trial. Nearly all (93%) HCPs indicated that preterm babies born before 32 weeks’ gestation should be included in the definitive trial, although there was support from many for more mature infants too (e.g. 83% for babies born before 34 weeks). Most believed that babies with NEC or with surgical problems should be excluded, but opinion was mixed on whether to include babies with cardiac problems or those receiving therapeutic-treated hypothermia. Twenty-two potential outcome measures were rated in the first round of the e-Delphi study, including the seven parent-suggested outcome measures. Four outcome measures were added from participants in round 1, resulting in 26 outcomes being rated in round 2. Consensus was achieved on five outcome measures: (1) mortality, (2) incidence of NEC, (3) days on parenteral nutrition, (4) time feeds stopped in each 24-hour period and (5) time from start of enteral feeding to achieve full feeds (150 ml/kg/day). No items were voted ‘consensus out’, leaving 21 items to be discussed and voted on at the consensus meeting. The two highest scoring primary outcome measures were (1) NEC (39%) and (2) time to achieve full feeds (30%).
Analysis of routinely collected national data sets: Paediatric Intensive Care Audit Network and National Neonatal Research Database
Analysis of the national PICU data set (PICANet), based on the outcome of the e-Delphi study, showed that if we included all mechanically ventilated children aged > 37 weeks’ gestational age to 17 years who did not have a primary GI pathology at admission there were 16, 122 children in 2016 and 2017; 36% of these had a surgical admission (n = 5813) and 64% a medical admission (n = 10,309); 84% of these children stayed for > 72 hours (3 days), which would be realistic and enable time to achieve predicted energy targets. This demonstrates that a superiority trial with individual consent is feasible in terms of the number of eligible patients, even with a cautious consent rate of only 60%. This commissioned brief did not allow us to randomise patients; thus, an inbuilt pilot phase, with clear stop/start and progression to full trial, would still be required in a future trial.
Analysis of the NNRD, based on the outcome of the e-Delphi study, showed that in 2017 and 2018 there were 15,375 infants born before 32 weeks’ gestational age and admitted to a NNU in England, Wales and Scotland. Mortality occurred in 1290 (8%) infants and NEC in 806 (5%) infants in this group and their median duration for the time from start of enteral feeding to achieve full (150 ml/kg/day) enteral feeds was 12 (IQR 9–17) days. This demonstrates that a superiority trial with individual consent is also feasible in terms of the number of eligible patients, again, with a cautious consent rate of only 60%. This commissioned brief did not allow us to randomise patients; thus, an inbuilt pilot phase, with clear stop/start and progression to full trial, would still be required in a future trial. The available numbers of eligible patients and neonatal sites would also allow a cluster trial to be undertaken in NNUs, given the necessary increase in required sample size. As described in Chapter 5, there was support from many for this approach.
The consensus meetings
Twenty-two PICU HCPs (a mixture of physicians, nurses and dietitians), triallists and eight clinical members of the study team attended the final stakeholder consensus meeting held on 2 April 2019; no parents were able to attend on the day. After presentation of the findings and discussion, 30 participants voted on 18 outcome measures that did not reach consensus in the e-Delphi study. Following this, consensus (in or out) was reached on all but four items. At this meeting, the proposed intervention arm (of no routine GRV measurement) was presented to the stakeholders by Dr Frederic Valla, a consultant in a large PICU in Lyon, France, where GRV has not routinely been measured for around 20 years. Overall, this was well accepted and participants felt that if the change (to not measuring GRV) was protocolised as part of a trial, it would be easier to implement and nurses would be reassured that they were not to blame if an AE occurred.
Seventeen neonatal HCPs (a mixture of physicians, nurses and dietitians), triallists and nine clinical members of the study team attended the final stakeholder consensus meeting held on 1 April 2019; two parents also attended the day. After presentation of the findings and discussion, 21 participants voted on 21 outcome measures that did not reach consensus in the e-Delphi study. Following this, consensus in or out was reached for four items each, respectively. At this meeting, the proposed intervention arm (of no routine GRV measurement) was presented to the stakeholders by Dr Anne Beissel, a consultant in a large NNU in Lyon, France, where GRV has not routinely been measured for 5 years. Similar to the PICU meeting, this was well accepted and participants felt that if the change (to not measuring GRV) was protocolised as part of a trial, it would be easier to implement and nurses would be reassured that they were not to blame if an AE occurred.
Barriers to delivering the definitive GASTRIC paediatric intensive care unit trial
Synthesising the results from the multiple WPs in this study identifies several barriers to delivering the definitive GASTRIC PICU trial, but has also informed us of how these barriers can be overcome. However, three main barriers to delivering the definitive PICU trial were identified. As we were not allowed to randomise patients and ‘test’ the protocol, these will need to be examined in an inbuilt pilot phase of a future trial.
Parental non-consent is always a risk in trials of critically ill children, because of the parental stress and anxiety associated with a child’s admission to the PICU. A deferred consent approach (which has been used successfully in several trials in critically ill children53,54) allows trial randomisation and allocation, followed by an approach to the parents for consent at a subsequent and, hopefully, less stressful time. However, as enteral feeding is not an ‘urgent’ PICU intervention, and is usually commenced within 12 hours of admission, it would not meet the criteria for deferred consent. 55 Additionally, parental feedback on our information sheet was positive, but recommended some changes (which we have made) [see project web page www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)], to articulate the benefit of both arms of the trial and improve clarity. Finally, parents articulated that although the written information was important, the verbal approach was even more so, and this should be made in a caring and supportive manner by someone knowledgeable about the trial. In UK PICUs this would normally be a PICU research nurse. Some parents suggested that they did not want to read about risks in the PIS, as this was alarming. However, informed consent is an essential ethics requirement for the conduct of research. 56 The parental consent rate should be assessed in the pilot phase of a future definitive trial.
Lack of compliance with the trial protocol is another potential risk in a future trial; this may be more pronounced in this case because it is existing practice to measure GRV routinely. The steps proposed to mitigate this risk of non-compliance would be to focus first on developing an effective trial education package (see Appendix 4) that is adapted and tailored locally to different professional groups. This has been done based on the trial team’s experience and on data from the qualitative work with HCPs. This addresses the rationale for a trial, evidence of the problem and includes simple, visually appealing and easy to follow, one-page trial algorithms (flow charts). Education specifically directed at nurses needs to address other ways to evaluate feed tolerance and to detect signs of AEs. This training should be able to be adapted to each local site to improve site ‘buy-in’ and increase incorporation into existing local processes. Sites should be provided with study materials that act as readily accessible ‘reminders’ of the trial arm algorithms, and inclusion and exclusion criteria. This should be in the form of ‘cheat sheets’ to attach to lanyards and pens with pull out text, along with other important marketing material, to enable easy access to trial protocols. Gaining support and ‘buy-in’ from senior PICU staff is also vital to the success of a trial, as consultants, in particular, are highly influential with all staff within a PICU. Nurses are often reassured of the importance of a trial by PICU consultants and the senior ‘in charge’ nurses. This should be done at the initial site visit and ongoing regular site visits. These site visits will both support and monitor trial compliance. Trial adherence will be evaluated in the pilot phase of a future trial, which we strongly recommend should also include a concurrent process evaluation to robustly evaluate the issues underpinning trial delivery and this change in practice.
Deviation from protocol, specifically by nursing staff, warrants special mentioning as a further risk in a future trial. Data from nurses both in the focus groups and in the e-Delphi process showed the most concern about a future trial and not being able to measure GRV. This was most pronounced with junior nurses. Much of this can be addressed by an effective education package targeted at bedside nurses, but regular compliance monitoring will be essential and a process evaluation will also be invaluable to assess these concerns. The inadvertent crossover between trial arms is a risk when nurses are allocated more than one patient that are on different trial arms. Steps taken to mitigate this risk of inadvertent crossover include visual reminders of the trial arm at each child’s bedside and small ‘cheat sheet’ reminders attached to each nurse’s lanyard or by the bedside, alongside ongoing education and monitoring.
Barriers to delivering the GASTRIC neonatal unit definitive trial
Similarly, synthesising the results from the NNU WPs identified several barriers to delivering the definitive GASTRIC NNU trial, but also informed us of how these barriers can be overcome.
Parental non-consent is also a risk in trials of newborn babies, because of the parental stress and anxiety associated with a baby’s admission to a NNU. Like a PICU setting, a deferred consent approach may improve recruitment rates by allowing more time for careful discussions outside an emergency situation; however, this is unlikely to be acceptable in a trial of no GRV measurement, as feeding decisions are usually not made urgently or as an emergency. Parental feedback on our information sheet was positive. It did, however, recommended changes to articulate the potential benefits of both trial arms and to improve clarity, which we have made [see project web page www.journalslibrary.nihr.ac.uk/programmes/hta/169402/#/documentation (accessed 13 February 2020)]. Parents also articulated that although written information was important, verbal communication was even more so and should be made in a caring and supportive manner by someone knowledgeable about the trial. Several parents suggested they did not want to read about risks in the PIS, as this was alarming. However, informed consent is an essential ethics requirement for the conduct of research. 56 We suggest that parental consent rate should be assessed in an internal pilot phase of any future definitive trial.
In the same way as in PICUs, lack of compliance with the trial protocol is potentially a risk in a future NNU trial, although the more varied practice suggests that this may not be as challenging. The steps proposed to mitigate this risk of non-compliance would also be to focus on developing an effective trial education package (see Appendix 5), adapted and tailored locally to different professional groups. This should first address the rationale for a trial, provide evidence of the problem and include simple, visually appealing and easy to follow, one-page trial algorithms (flow charts). Education, specifically for nurses, needs to address other ways to evaluate feed tolerance and to detect signs of AEs. This training should be adaptable to each local site to improve site ‘buy-in’ and increase incorporation into existing local processes. Sites should be provided with study materials that act as readily accessible ‘reminders’ of the trial arm algorithms, and inclusion and exclusion criteria. This should be in the form of ‘cheat sheets’ to attach to lanyards and pens with pull out text, along with other important marketing material, to enable easy access to trial protocols. Gaining support and ‘buy-in’ from senior NNU staff is also vital to the success of a trial: consultants carry a large amount of influence with all staff. Nurses are often reassured of the importance of a trial from the influence of consultants and the senior ‘in charge’ nurses. Comments collected from focus groups and the e-Delphi process also suggested that some neonatologists in individual units may not all agree with both arms. This brings in the risk of failure to recruit, potential non-compliance and crossover between the allocated groups. Steps taken to mitigate this will include those used for nurses, but also additional leadership from the trial investigators and the local lead investigator. Monitoring of recruitment and retention of participants will be undertaken during the inbuilt pilot phase and throughout the trial.
Obtaining ‘buy-in’ should be the focus of the initial and ongoing regular site visits. These site visits will both support and monitor trial compliance. Trial adherence will be evaluated in the pilot phase of a future trial. For a future trial, we would strongly recommend a concurrent process evaluation to robustly evaluate the issues underpinning trial delivery and this change in practice.
Proposed future trial design: GASTRIC paediatric intensive care unit trial
The most feasible trial design therefore appears to be an individual consent superiority trial, with an inbuilt pilot study and with clear progression targets. The research question for a trial would be as follows.
Population
In mechanically ventilated children aged 37 weeks (term) to 17 years, expected to stay > 24 hours and who can be enterally fed.
Intervention
Does no regular (routine) measurement of GRV to guide enteral feeding.
Comparison
Compared with usual care, the regular 4- to 6-hour measurement of GRV to guide enteral feeding.
Outcomes
Impact on the achievement of predicted energy requirements (using the Schofield equation) by 72 hours after PICU admission?
The trial should also have a concurrent process evaluation and a built-in non-inferiority analysis to determine that not measuring GRV is safe and does not increase the incidence of VAP. This inbuilt pilot phase must assess parental consent rates, clinician study protocol compliance and crossover, and confirm the distribution of the primary outcome measure and feasibility of collection of secondary outcome measures.
Proposed future trial design: GASTRIC neonatal unit trial
The most feasible trial design for NNUs is also an individually randomised superiority trial, with an inbuilt pilot study and clear progression targets and a parallel process evaluation. This research question would be as follows.
Population
In newborn infants born before 32 weeks’ gestation who are ready to start enteral feeds.
Intervention
Does no regular (routine) measurement of GRV to guide enteral feeding.
Comparison
Compared with usual care, the regular 4- to 6-hour measurement of GRV to guide enteral feeding.
Outcomes
Impact of the time from start of enteral feeding to achieve full feeds (150 ml/kg/day)?
This trial should also have a concurrent process evaluation and a non-inferiority analysis built in on the incidence of NEC.
Strengths and limitations of this feasibility study
The PICU survey was comprehensive and obtained a response from 24 (89%) of the 27 units in the UK. Although a larger number of NNUs responded to the neonatal survey, it was less comprehensive (95/184 NNUs, 52% response) owing to the higher number of NNUs in the UK and it may be less likely to be representative of the whole country. The 95 responding NNUs provide a clear signal of mixed practice and identify multiple potential sites for a future trial. In keeping with all surveys of practice, both NNU and PICU surveys are limited by the theoretical, self-reported nature of any responses.
The qualitative work was limited by 74% of the parent sample being based in the north-west of England. However, these participants drew on experience from 21 hospitals from across England and Scotland. Another limitation is that, owing to the purposeful sampling of parents with PICU or NNU experience, we had no ICU-naive parents’ views on the study information, which may be different. A further limitation of WP 2 is its hypothetical, retrospective nature. As reflected in the findings related to the importance placed on feeding, perceptions about measuring GRV and risk assessment of medical factors (hospital-related infections, NEC) were substantially influenced by experiential knowledge. The recruitment of HCPs in both groups was extensive, but targeted interviews drew on the wider GASTRIC teams’ personal contacts, which may have influenced views.
The trial design survey and e-Delphi studies were limited in PICUs by the lower than expected response rate (30/45, 67%) and in both surveys by not including parents and carers: we undertook parent and carer involvement in other ways that we felt was more appropriate. The e-Delphi studies resulted in numerous outcomes not achieving consensus in or out, but we were able to discuss and vote on these at the final consensus meetings. In the PICU e-Delphi study, the wording of the outcome ‘time to achievement of predicted energy goals (full feeds)’ could have been clearer. On further discussion at the consensus meeting, a more appropriately worded outcome is ‘the achievement of a percentage of the predicted goal achieved over time’. It may be that the clinicians completing this part of the study understood what was meant here, and, in the context of the e-Delphi study, the important focus was to gain consensus of whether this or the AE of VAP should be the primary outcome.
The analysis of the national routine data sets was limited by the data collected by these. Specifically, PICANet (vs. the NNRD) collects a very minimal data set and nothing specific to nutrition or feeding. However, the data about what items are collected are robust and reliable.
The consensus meetings were limited by their location (central London), potentially discouraging parents and HCPs from further afield. However, we chose London because transport links meant this was the easiest to reach for the majority. For the PICU meeting, despite our best efforts, no parents were able to attend on the day.
Conclusions and summary of key research recommendations
A definitive trial of no routine GRV measurement in PICUs is feasible to conduct, and this should include an inbuilt pilot phase with clear stop/start progression criteria to full trial and a concurrent process evaluation. Current practice, that is the consistent and frequent routine measurement of GRV, will form the control arm. The intervention arm will be no routine measurement of GRV with an associated education and training package (see Appendix 1 for proposed control and intervention arm study protocol guidance and Appendix 4). This is a clear unmet need of evidence to determine the clinical and cost-effectiveness of this practice.
A separate, definitive trial of no routine GRV measurement in NNU is feasible to conduct. Like the PICU trial, such a trial should include an inbuilt pilot phase, with clear stop/start progression criteria to full trial and a concurrent process evaluation. The frequent routine measurement of GRV, which is current practice in many units, will form the control arm and the intervention arm will be no routine measurement of GRV with an associated education and training package (see Appendix 2 for proposed control and intervention arm study protocol guidance and Appendix 5). This is a clear unmet need for evidence to determine the clinical and cost-effectiveness of this practice.
Further international research is needed to determine and get consensus on core outcome measures for trials of nutritional interventions in critically ill children. Preliminary work is already under way on this (see www.comet-initiative.org/Studies/Details/1106; accessed 22 April 2020), but a large international Delphi study is required to gain consensus, involving families and important outcomes to families and parents. In neonatal care, the Core Outcomes In Neonatology project has completed an international consensus process to identify core outcomes for neonatal care in high-income settings. 57 Any trials of nutritional interventions in neonatal care should ensure that they measure and report these core outcomes. The consensus processes reported in Chapters 3 and 5 of this report provide further evidence to inform choice of additional outcomes more directly relevant to trials examining no measurement of GRV.
Implications for health-care practice
This is a feasibility study that did not recruit patients and therefore has no direct implications for health-care practice at this stage.
Acknowledgements
Contributions of authors
Lyvonne N Tume (https://orcid.org/0000-0002-2547-8209) is an associate professor and a PICU nurse and clinical researcher. She conceived the study, was the chief investigator and led the PICU workstream.
Kerry Woolfall (https://orcid.org/0000-0002-5726-5304) is a senior lecturer and social scientist, was a co-investigator and led the qualitative workstream.
Barbara Arch (https://orcid.org/0000-0001-6060-8091) was the study statistician and was a co-investigator.
Louise Roper (https://orcid.org/0000-0002-2918-7628) is a social scientist and was a co-investigator.
Elizabeth Deja (https://orcid.org/0000-0002-3626-4927) is a social scientist and was a co-investigator.
Ashley P Jones (https://orcid.org/0000-0001-5253-730X) was the senior statistician and was a co-investigator.
Lynne Latten (https://orcid.org/0000-0003-1620-0114) is a paediatric clinical dietitian and was a co-investigator.
Nazima Pathan (https://orcid.org/0000-0002-9447-4252) is a consultant PICU physician and was a co-investigator.
Helen Eccleson (https://orcid.org/0000-0003-0506-8525) was the GASTRIC study co-ordinator at the trials unit and was a co-investigator.
Helen Hickey (https://orcid.org/0000-0003-0467-0362) was the senior study co-ordinator at the trials unit and was a co-investigator.
Roger Parslow (https://orcid.org/0000-0002-3945-5294) was the senior epidemiologist for PICANet and was a co-investigator.
Jennifer Preston (https://orcid.org/0000-0003-4800-234X) is a PPI consultant officer, was the patient and public involvement lead and was a co-investigator.
Anne Beissel (https://orcid.org/0000-0002-1033-451X) is a consultant neonatologist and was a co-investigator.
Izabela Andrzejewska (https://orcid.org/0000-0003-0901-0996) was an expert neonatal nurse and was a co-investigator.
Chris Gale (https://orcid.org/0000-0003-0707-876X) is a consultant neonatologist and is a National Institute for Health Research clinical scientist. He led the NNRD analysis workstream and was a co-investigator.
Frederic V Valla (https://orcid.org/0000-0001-5285-1104) is a consultant PICU physician and expert in clinical nutrition, and was a co-investigator.
Jon Dorling (https://orcid.org/0000-0002-1691-3221) is a professor of neonatal medicine and consultant neonatologist, was a co-chief investigator and led the NNU workstream.
Data-sharing statement
All available data can be obtained by contacting the corresponding author or the Clinical Trials Research Centre at the University of Liverpool for review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81. https://doi.org/10.1016/j.jbi.2008.08.010.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Department of Health and Social Care . Toolkit for High-Quality Neonatal Services 2009. https://webarchive.nationalarchives.gov.uk/20130123200735/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/@sta/@perf/documents/digitalasset/dh_108435.pdf (accessed 1 October 2019).
- Healthcare Quality Improvement Partnership . National Neonatal Audit Programme – 2017 Annual Report on 2016 Data 2017. www.hqip.org.uk/resource/national-neonatal-audit-programme-2017-annual-report-on-2016-data/ (accessed 1 October 2019).
- Dorling J, Abbott J, Berrington J, Bosiak B, Bowler U, Boyle E, et al. Controlled trial of two incremental milk-feeding rates in preterm infants. N Engl J Med 2019;381:1434-43. https://doi.org/10.1056/NEJMoa1816654.
- Tume LN, Arch B, Woolfall K, Latten L, Deja E, Roper L, et al. Gastric residual volume measurement in UK paediatric intensive care units: a survey of practice. Pediatr Crit Care Med 2019;20:707-13. https://doi.org/10.1097/PCC.0000000000001944.
- O’Hara CB, Canter RR, Mouncey PR, Carter A, Jones N, Nadel S, et al. A qualitative feasibility study to inform a randomised controlled trial of fluid bolus therapy in septic shock. Arch Dis Child 2018;103:28-32. https://doi.org/10.1136/archdischild-2016-312515.
- Woolfall K, Frith L, Gamble C, Gilbert R, Mok Q, Young B. CONNECT advisory group . How parents and practitioners experience research without prior consent (deferred consent) for emergency research involving children with life threatening conditions: a mixed method study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008522.
- Woolfall K, Frith L, Gamble C, Young B. How experience makes a difference: practitioners’ views on the use of deferred consent in paediatric and neonatal emergency care trials. BMC Med Ethics 2013;14. https://doi.org/10.1186/1472-6939-14-45.
- Peters MJ, Khan I, Woolfall K, Deja E, Mouncey PR, Wulff J, et al. Different temperature thresholds for antipyretic intervention in critically ill children with fever due to infection: the FEVER feasibility RCT. Health Technol Assess 2019;23. https://doi.org/10.3310/hta23050.
- Molyneux S, Njue M, Boga M, Akello L, Olupot-Olupot P, Engoru C, et al. ‘The words will pass with the blowing wind’: staff and parent views of the deferred consent process, with prior assent, used in an emergency fluids trial in two African hospitals. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0054894.
- Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52:1893-907. https://doi.org/10.1007/s11135-017-0574-8.
- Baker SE, Edwards R. How Many Qualitative Interviews is Enough?. Southampton: National Centre for Research Methods, University of Southampton; 2012.
- Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ 2000;320:50-2. https://doi.org/10.1136/bmj.320.7226.50.
- Great Britain . Data Protection Act 1998 1988.
- Coffey AJ, Atkinson PA. Making Sense of Qualitative Data: Complementary Research Strategies. Thousand Oaks, CA: SAGE Publications Ltd; 1996.
- Silverman D. Interpreting Qualitative Data: Methods for Analysing Talk, Text, and Interaction. London: SAGE Publications Ltd; 2006.
- Boyatzis R. Transforming Qualitative Information: Thematic Analysis and Code Development. London: SAGE Publications Ltd; 1998.
- Braun V, Clarke V. What can ‘thematic analysis’ offer health and wellbeing researchers?. Int J Qual Stud Health Well-Being 2014;9. https://doi.org/10.3402/qhw.v9.26152.
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277-88. https://doi.org/10.1177/1049732305276687.
- Hutton JL. Are distinctive ethical principles required for cluster randomized controlled trials?. Stat Med 2001;20:473-88. https://doi.org/10.1002/1097-0258(20010215)20:3<473::AID-SIM805>3.0.CO;2-D.
- Christie J, O’Halloran P, Stevenson M. Planning a cluster randomized controlled trial: methodological issues. Nurs Res 2009;58:128-34. https://doi.org/10.1097/NNR.0b013e3181900cb5.
- Medical Research Council . Cluster Randomised Trials: Methodological and Ethical Considerations 2002.
- Taljaard M, Weijer C, Grimshaw JM, Eccles MP. Ottawa Ethics of Cluster Randomised Trials Consensus Group . The Ottawa Statement on the ethical design and conduct of cluster randomised trials: precis for researchers and research ethics committees. BMJ 2013;346. https://doi.org/10.1136/bmj.f2838.
- Inwald D, Canter RR, Woolfall K, O’Hara CB, Mouncey PR, Zenasni Z, et al. Restricted fluid bolus versus current practice in children with septic shock: the FiSh feasibility study and pilot RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22510.
- Woolfall K, Roper L, Humphreys A, Lyttle MD, Messahel S, Lee E, et al. Enhancing practitioners’ confidence in recruitment and consent in the EcLiPSE trial: a mixed-method evaluation of site training – a Paediatric Emergency Research in the United Kingdom and Ireland (PERUKI) study. Trials 2019;20. https://doi.org/10.1186/s13063-019-3273-z.
- Riskin A, Cohen K, Kugelman A, Toropine A, Said W, Bader D. The impact of routine evaluation of gastric residual volumes on the time to achieve full enteral feeding in preterm infants. J Pediatr 2017;189:128-34. https://doi.org/10.1016/j.jpeds.2017.05.054.
- Torrazza RM, Parker LA, Li Y, Talaga E, Shuster J, Neu J. The value of routine evaluation of gastric residuals in very low birth weight infants. J Perinatol 2015;35:57-60. https://doi.org/10.1038/jp.2014.147.
- Reigner J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding. JAMA 2013;309:249-56. https://doi.org/10.1001/jama.2012.196377.
- Poulard F, Dimet J, Martin-Lefevre L, Bontemps F, Fiancette M, Clementi E, et al. Impact of not measuring residual gastric volume in mechanically ventilated patients receiving early enteral feeding: a prospective before–after study. JPEN J Parenter Enteral Nutr 2009;34:125-30. https://doi.org/10.1177/0148607109344745.
- Tume LN, Bickerdike A, Latten L, Davies S, Lefèvre MH, Nicolas GW, et al. Routine gastric residual volume measurement and energy target achievement in the PICU: a comparison study. Eur J Pediatr 2017;176:1637-44. https://doi.org/10.1007/s00431-017-3015-8.
- Ozen, N, Nuran T, Yamanel L, Altintas N, Kilciler G, Ozen V. Evaluation of the effect on patient parameters of not monitoring gastric residual volume in intensive care patients on a mechanical ventilator receiving enteral feeding: a randomized clinical trial. J Crit Care 2016;33:137-44. https://doi.org/10.1016/j.jcrc.2016.01.028.
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2 . Framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395-400. https://doi.org/10.1016/j.jclinepi.2010.09.012.
- Great Britain . National Health Service Act 2006 2006.
- Great Britain . Health and Social Care Act 2001 2001.
- Healthcare Quality Improvement Partnership . The Paediatric Intensive Care Audit Network Annual Report 2018. www.picanet.org.uk/wp-content/uploads/sites/25/2018/11/PICANet-2018-annual-report-summary-v1.1.pdf (accessed 1 December 2018).
- NHS Data Model and Dictionary . National Neonatal Data Set – Episodic and Daily Care n.d. www.datadictionary.nhs.uk/data_dictionary/messages/clinical_data_sets/data_sets/national_neonatal_data_set/national_neonatal_data_set_-_episodic_and_daily_care_fr.asp?shownav=0 (accessed 1 October 2019).
- Costeloe K, Bowler U, Brocklehurst P, Hardy P, Heal P, Juszczak E, et al. A randomised controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the Probiotics in Preterm infantS (PiPS) trial. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20660.
- Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed 2018;103:F182-F189. https://doi.org/10.1136/archdischild-2017-313880.
- Manktelow B, Draper ES, Field C, Field D. Estimates of length of neonatal stay for very premature babies in the UK. Arch Dis Child Fetal Neonatal Ed 2010;95:F288-92. https://doi.org/10.1136/adc.2009.168633.
- Richardson M, Hines S, Dixon G, Highe L, Brierley J. Establishing nurse-led ventilator-associated pneumonia surveillance in paediatric intensive care. J Hosp Infect 2010;75:220-4. https://doi.org/10.1016/j.jhin.2009.12.015.
- Ismail N, Darbyshire A, Thorburn K. Ventilator-associated pneumonia (VAP): a UK PICU experience. Arch Dis Child 2012;97. https://doi.org/10.1136/archdischild-2012-301885.361.
- Narayanan A, Dixon G, Chalkley S, Ray S, Brierley J. Ventilator-associated pneumonia in children: comparing the plethora of surveillance definitions. J Hosp Infect 2016;94:163-4. https://doi.org/10.1016/j.jhin.2016.05.006.
- Venkatachalam V, Hendley JO, Willson DF. The diagnostic dilemma of ventilator-associated pneumonia in critically ill children. Pediatr Crit Care Med 2011;12:286-96. https://doi.org/10.1097/PCC.0b013e3181fe2ffb.
- Kerklaan D, Fivez T, Mehta NM, Mesotten D, van Rosmalen J, Hulst JM, et al. Worldwide survey of nutritional practices in PICUs. Pediatr Crit Care Med 2016;17:10-8. https://doi.org/10.1097/PCC.0000000000000542.
- Mehta NM, Bechard LJ, Cahill N, Wang M, Day A, Duggan CP, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children – an international multicenter cohort study*. Crit Care Med 2012;40:2204-11. https://doi.org/10.1097/CCM.0b013e31824e18a8.
- Tume L, Latten L, Darbyshire A. An evaluation of enteral feeding practices in critically ill children. Nurs Crit Care 2010;15:291-9. https://doi.org/10.1111/j.1478-5153.2010.00420.x.
- Tume LN, Latten L, Kenworthy L. Paediatric intensive care nurses’ decision-making around gastric residual volume measurement. Nurs Crit Care 2017;22:293-7. https://doi.org/10.1111/nicc.12304.
- Quenot JP, Plantefeve G, Baudel JL, Camilatto I, Bertholet E, Cailliod R, et al. Bedside adherence to clinical practice guidelines for enteral nutrition in critically ill patients receiving mechanical ventilation: a prospective, multicentre, observational study. Crit Care 2010;14. https://doi.org/10.1186/cc8915.
- Mehta N, Skillman H, Irving S, Coss-Bu JA, Vermilyea S, Farrington EA, et al. Guidelines for the provision and assessment of nutrition support therapy in the pediatric critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr 2017;41:706-42. https://doi.org/10.1177/0148607117711387.
- Leaf A, Dorling J, Kempley S, McCormick K, Mannix P, Linsell L, et al. Early or delayed enteral feeding for preterm growth-restricted infants: a randomized trial. Pediatrics 2012;129:e1260-8. https://doi.org/10.1542/peds.2011-2379.
- O’Keeffe AG, Ambler G, Barber JA. Sample size calculations based on a difference in medians for positively skewed outcomes in health care studies. BMC Med Res Methodol 2017;17. https://doi.org/10.1186/s12874-017-0426-1.
- Harron K, Mok Q, Dwan K, Ridyard CH, Moitt T, Millar M, et al. CATheter Infections in CHildren (CATCH): a randomised controlled trial and economic evaluation comparing impregnated and standard central venous catheters in children. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20180.
- Lyttle MD, Rainford NEA, Gamble C, Messahel S, Humphreys A, Hickey H, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet 2019;393:2125-34. https://doi.org/10.1016/S0140-6736(19)30724-X.
- Furyk J, McBain-Rigg K, Watt K. on behalf of PREDICT . Qualitative evaluation of a deferred consent process in paediatric emergency research: a PREDICT study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-018562.
- Health Research Authority . Consent and Participant Information Guidance n.d. www.hra-decisiontools.org.uk/consent/docs/Consent%20and%20PIS%20Guidance.pdf (accessed 10 January 2019).
- Webbe J, Duffy J, Afonso E, Al-Muzaffar I, Brunton G, Greenough A, et al. Core Outcomes in Neonatology: Development of a Neonatal Core Outcome Set Using an International Delphi Consensus Process. Baltimore, MD: Pediatric Academic Societies; n.d.
Appendix 1 Paediatric intensive care unit: potential trial arms
PICU intervention arm: no routine GRV measurement to guide enteral feeding
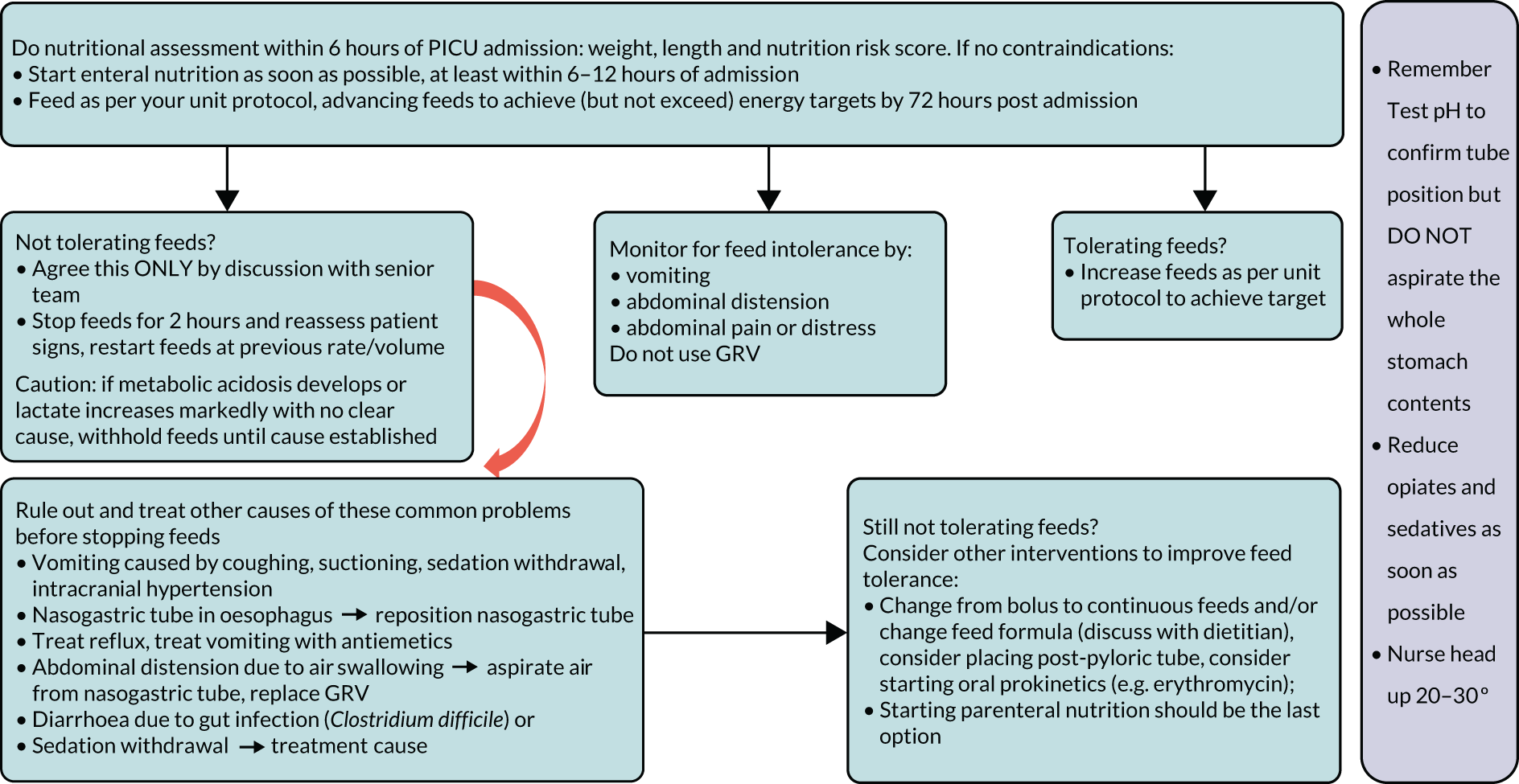
PICU control arm: routine GRV measurement
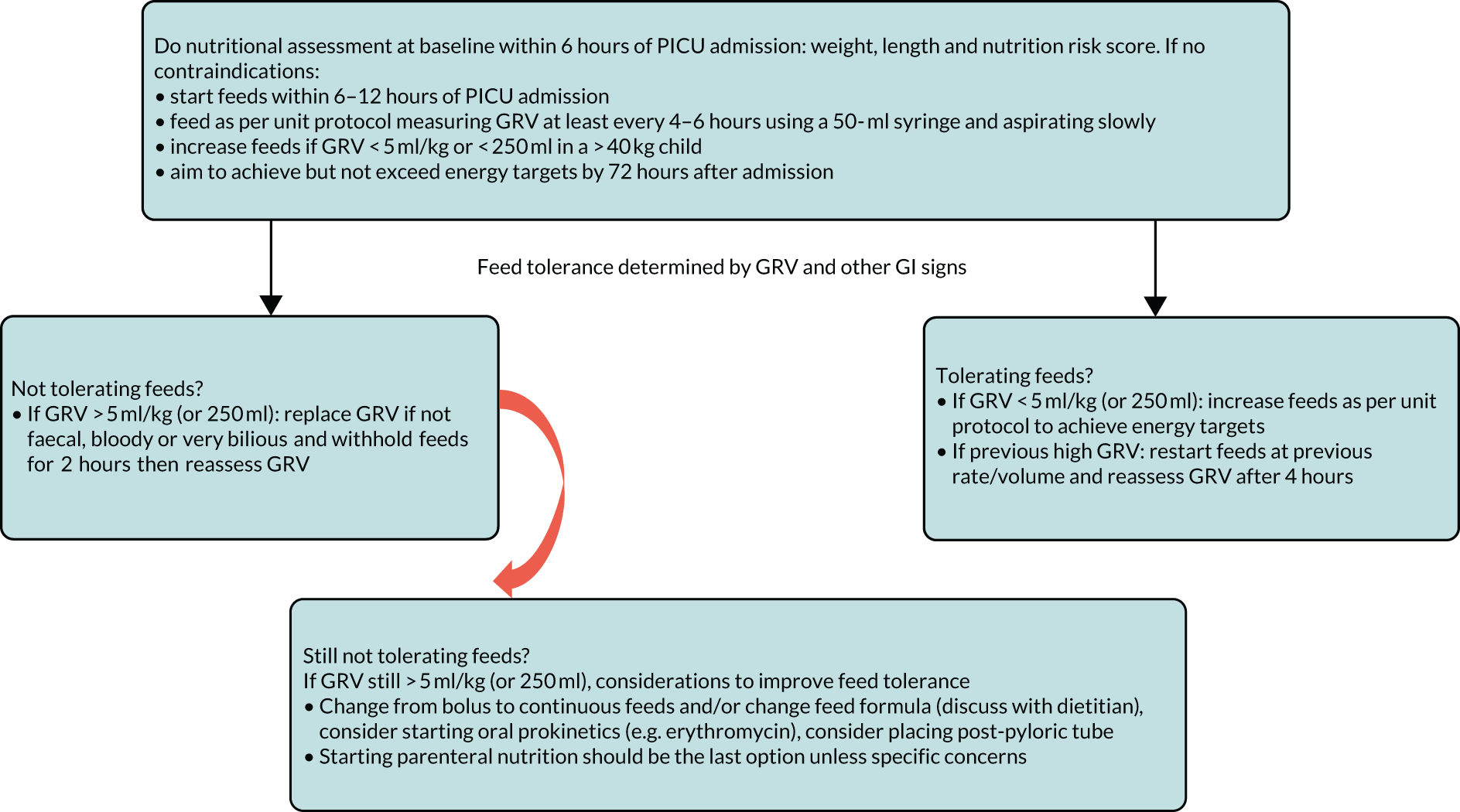
Appendix 2 Flow chart: neonatal unit control and intervention arms
Neonatal unit intervention arm: no GRV to guide enteral feeding
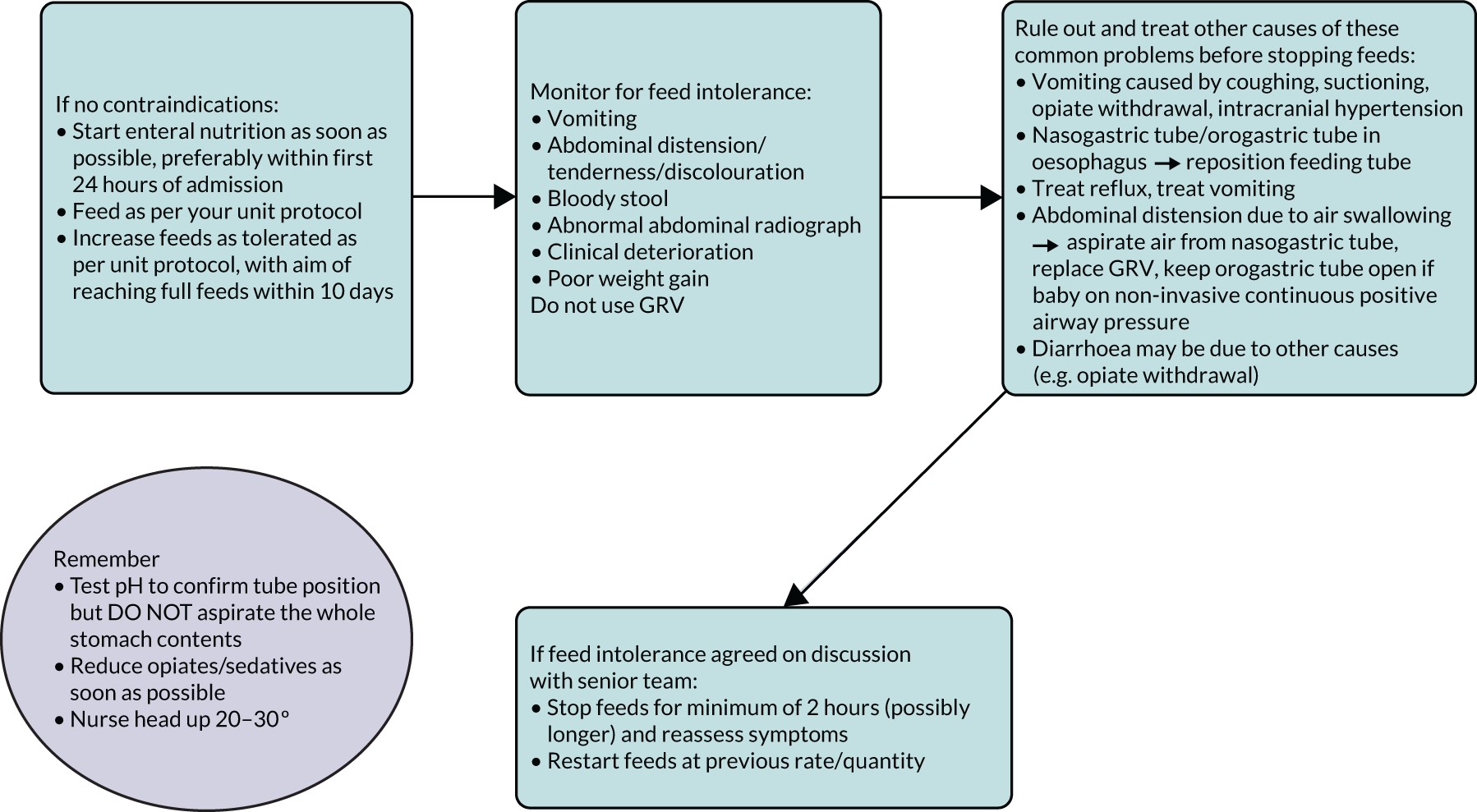
Neonatal unit control arm: routine GRV measurement
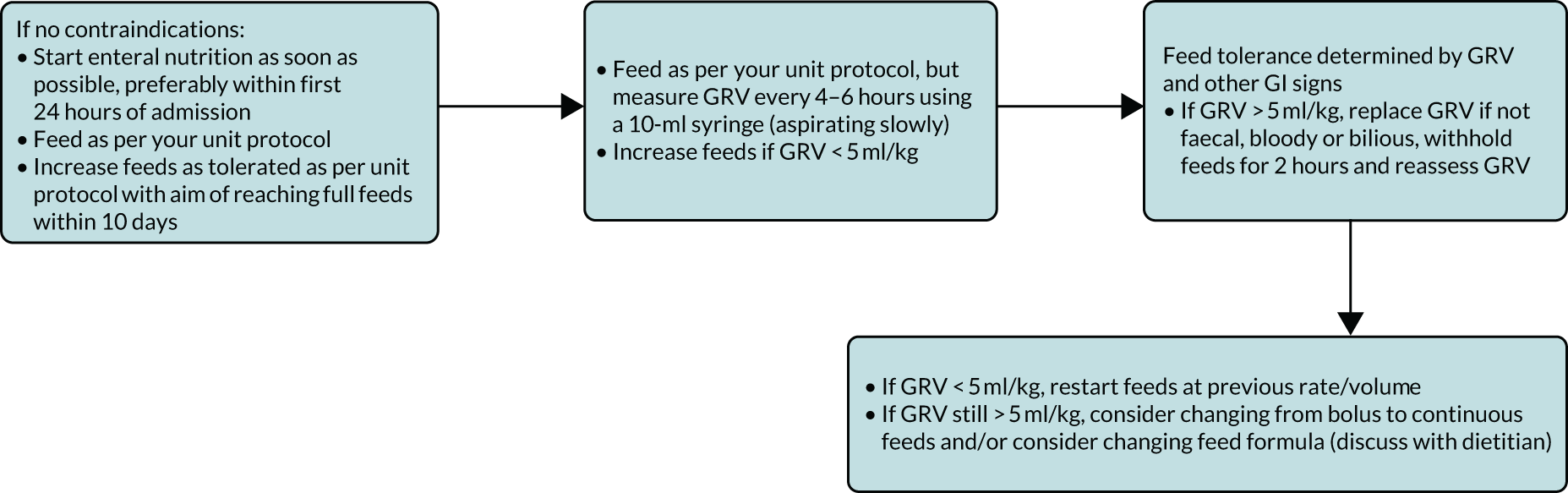
Appendix 3 Potential outcomes
Proposed outcomes of the GASTRIC trial: neonatal intensive care unit
-
An outcome measure refers to ‘what’ should be measured in a research study to find out whether or not an intervention is effective. In this study, we are interested in whether gastric residual volume (GRV) measurement helps to make children better.
-
Studies often have a number of outcome measures to determine whether or not an intervention works. They could be measured during a child’s stay in hospital, at the end of their hospital stay or when they have left hospital.
-
Researchers or doctors often suggest what outcomes should be measured in a research study. However, they do not always fully understand what it is like, either to be a sick child or to be the parent or guardian of a sick child. This is why it is important that we ask parents and guardians about the things that are important to them; the things we should measure from their perspective.
-
For the GASTRIC study, we have reviewed all the previous research studies that have looked at GRV measurement.
-
Below is a list of outcomes that might be useful to measure. During the telephone interview, we will ask you what you think about the outcome measures on this list.
-
It is not a test! We just want to make sure we include outcomes that are important to parents and children.
Outcomes that are already measured during a child’s stay in intensive care
-
Time in the neonatal intensive care unit (NICU).
-
The number of days your child was in NICU within a certain time period (usually period of days/months).
-
-
Time spent on the ventilator or on breathing support.
-
The number of days your child was on breathing support within a certain time period.
-
-
Duration of hospital stay.
-
The time your child has spent in any particular hospital location (e.g. NICU, ward).
-
-
Health-care-associated infections.
-
An infection acquired in hospital, directly as a result of having a breathing tube or central line (large drip) in your child.
-
-
Occurence of necrotising enterocolitis (NEC).
-
We look for any occurrence of a severe bowel infection (called NEC), defined in a specific and consistent way in the infant’s NICU stay (while they are being tube fed).
-
-
We look for the time taken to achieve full feeds.
-
How many days it took to reach 150 ml/kg/day, the agreed definition of full feeds for a preterm infant.
-
-
We measure the frequency of gut problems: vomiting, diarrhoea.
-
How often the baby was recorded as vomiting and had episodes of diarrhoea per day during their time being tube fed on intensive care.
-
-
We count the number of days on total parenteral nutrition (TPN) (intravenous liquid food).
-
How many days the baby received TPN.
-
-
We count the number of days of central line access.
-
The number of days the baby has a central line (a large drip into a big vein) in place.
-
Outcomes that are already measured at the end of care or after a child has left hospital
-
Survival.
-
Whether or not your child survived to a specific event (e.g. hospital discharge).
-
-
Feeding issues.
-
Whether or not your child has any ongoing feeding issues as a result of their time spent on NICU.
-
Whether or not your child is still requiring tube feeding at home.
-
Whether or not your child has achieved their normal weight for age.
-
Proposed outcomes of the GASTRIC trial: paediatric intensive care unit
-
An outcome measure refers to ‘what’ should be measured in a research study to find out whether or not an intervention is effective. In this study, we are interested in whether or not the GRV measurement helps to make children better.
-
Studies often have a number of outcome measures to determine whether or not an intervention works. They could be measured during a child’s stay in hospital, at the end of their hospital stay or when they have left hospital.
-
Researchers or doctors often suggest what outcomes should be measured in a research study. However, they do not always fully understand what it is like, either to be a sick child or to be the parent or guardian of a sick child. This is why it is important we ask parents and guardians about the things that are important to them; the things we should measure from their perspective.
-
For the GASTRIC study, we have reviewed all the previous research studies that have looked at GRV measurement.
-
Below is a list of outcomes that might be useful to measure. During the telephone interview, we will ask you what you think about the outcome measures on this list.
-
It is not a test! We just want to make sure we include outcomes that are important to parents and children.
Outcomes that are already measured during a child’s stay in intensive care
-
Time in the paediatric intensive care unit (PICU).
-
The number of days your child was in PICU within a certain time period (usually period of days/months).
-
-
Time spent on the ventilator or on breathing support.
-
The number of days your child was on breathing support within a certain time period.
-
-
Duration of hospital stay.
-
The time your child spent in any particular hospital location (e.g. PICU, ward).
-
-
Health-care-associated infections.
-
An infection acquired in hospital, directly as a result of having a breathing tube or central line (large drip) in your child.
-
-
The proportion of patients with at least one episode of ventilator-associated pneumonia (VAP).
-
We would measure the length of time your child has a breathing tube in place and if they acquire a chest infection directly related to having this tube in place and being on a ventilator (breathing machine).
-
-
The actual achievement of predicted energy goals.
-
When a child is admitted to the PICU a dietitian calculates how many calories they require each day, so we would calculate how much (what percentage) of the amount that that child should receive that they actually did get.
-
-
The time taken to achieve full feeds.
-
How many days it took to reach the number of calories that the dietitian predicted the child needs.
-
-
The frequency of gut problems: vomiting, diarrhoea.
-
How often the child was recorded as vomiting and had episodes of diarrhoea per day during their time being tube fed on intensive care.
-
-
The weight changes from PICU admission to discharge.
-
Any change in weight (in kilograms) from PICU admission weight to weight at PICU discharge.
-
Outcomes that are already measured at the end of care or after a child has left hospital
-
Survival.
-
Whether or not your child survived to a specific event (e.g. hospital discharge).
-
-
Feeding issues.
-
Whether or not your child has any ongoing feeding issues as a result of their time spent on PICU.
-
Whether or not your child still requires tube feeding at home.
-
Whether or not they have achieved their normal weight for age.
-
Appendix 4 Proposed education package
Proposed health-care professional education package to accompany future GASTRIC trial
To address the concerns raised in staff focus groups and interviews, we have developed two separate proposed educational packages to accompany the GASTRIC trial: one for PICU HCPs and one for NICU HCPs.
There would be four separate short (< 10-minute) educational videos, filmed in the clinical area by a clinician, discussing the topics below. These would be supported by PowerPoint® slide sets (Microsoft Corporation, Redmond, WA, USA) for PICU and NICU champions to deliver in their units.
Paediatric intensive care unit module 1
Why a trial of GRV is needed, why GRV is not a valid marker, how achieving energy targets remains a problem in critically ill children and how suboptimal energy achievement can have an impact on clinical outcomes.
Neonatal intensive care unit module 1
Why a trial of GRV is needed, why GRV is not a valid way to assess feed tolerance, the lack of evidence for GRV as a predictor of NEC, how delaying the time taken to reach full feeds increases the risk of infection can have impacts on clinical outcomes.
Paediatric intensive care unit module 2
The ‘usual care’ arm of the trial in which GRV is routinely measured every 4–6 hours to guide enteral feeding, discussing the technique to do this and actions to take when GRV is high for PICU.
Neonatal intensive care unit module 2
The ‘usual care’ arm of the trial where GRV is routinely measured every 4–6 hours to guide enteral feeding, discussing the technique to do this and actions to take when GRV is high for NICU.
Paediatric intensive care unit module 3
The ‘intervention arm’ of not measuring GRV. How to assess feed tolerance without GRV using clinical signs of vomiting, abdominal distension and abdominal pain or discomfort, or distress. Discuss ruling out and treating of other likely causes of these signs and what to do if feed intolerance is identified in the intervention arm in PICU. If metabolic acidosis develops and levels of lactate rise with no clear cause and no other obvious signs, feeds may need to be withheld until the gut as a cause is ruled out.
Neonatal intensive care unit module 3
The ‘intervention arm’ of not measuring GRV. How to assess feed tolerance without GRV, using clinical signs of vomiting, abdominal distension and abdominal pain or discomfort, or distress. Discuss the ruling out and treating of other likely causes of these signs and what to do if feed intolerance is identified in the intervention arm in NICU. If metabolic acidosis develops and levels of lactate rise with no clear cause and no other obvious signs, feeds may need to be withheld until the gut as a cause is ruled out.
Paediatric intensive care unit module 4
Interventions to be considered if feed intolerance is a problem. Discuss changing from bolus to continuous feeds, consulting with the dietitian to change to a different formula (e.g. semi-elemental), using oral prokinetics or placing a post-pyloric tube, while minimising interventions that reduce gastric motility (high-dose opioids and sedatives, correcting electrolyte abnormalities, etc.) for PICU patients.
Neonatal intensive care unit module 4
Interventions to be considered if feed intolerance is a problem for NICU. Discuss changing feed volume, the time between feeds and rate of increase of feeds. Discuss modifying medications, such as opiates and sedatives, and correcting electrolyte imbalances for NICU patients.
Appendix 5 Paediatric intensive care unit Delphi outcomes
| Name | Help text | Domain name |
|---|---|---|
| GI morbidity: diarrhoea | The intervention may potentially have an impact on the incidence of diarrhoea. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (GI outcomes); AE |
| Incidence of GI morbidity: vomiting | The intervention may potentially have an impact on the incidence of vomiting. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (GI outcomes); AE |
| Incidence of VAP | The intervention may potentially have an impact on the incidence of VAP. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (infection outcomes); AE |
| Length of stay: hospital | The intervention may potentially have an impact on their length of stay in hospital. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Resource use (hospital) |
| Length of stay: PICU | The intervention may potentially have an impact on their length of stay in PICU. Please indicate how important you think it is that this outcome is measured in a future study | Resource use (hospital) |
| Length of time: IV | The intervention may potentially have an impact on the length of time they spend on IV. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (respiratory, thoracic and mediastinal outcomes) |
| Long-term feeding issues | The intervention may potentially have a long-term impact on feeding issues, this includes time spent on tube feedings at home, reflux, colic, vomiting and appetite changes. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was identified by parents as important and prioritised by parents during the qualitative interviews | Life impact (perceived health status) |
| Long-term outcomes (after hospital discharge) | The intervention may potentially have a long-term impact on health, this includes amputation, developmental issues, brain damage and mobility. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was identified by parents as important and prioritised by parents during the qualitative interviews | Life impact (physical and cognitive functioning) |
| Looking and/or behaving like their normal self | The intervention may potentially have an impact on them returning to their ‘normal’ self (e.g. improvement in mood, increased communication, acting more like themselves, increased alertness, starting to sit, starting to eat and drink). Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was identified by parents as important and prioritised by parents during the qualitative interviews | Life impact (perceived health status) |
| Mortality | The intervention may potentially have an impact on mortality/survival rates. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Death (mortality/survival) |
| Nursing time spent measuring GRV | The intervention may potentially have an impact on nursing time taken to perform the procedure, which could otherwise be spent on other things. Please indicate how important you think it is that this outcome is measured in a future study | Resource use (hospital) |
| Time feed stopped per 24 hours | The intervention may potentially have an impact on the amount of time the feed is stopped in the 24-hour period. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Physiological/clinical (metabolism and nutrition outcomes) |
| Time to achievement of predicted energy goals (full feeds) | The intervention may potentially have an impact on the time taken to achieve the child’s predicted energy goals. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (metabolism and nutrition outcomes) |
| Total length of time on respiratory support (IV + NIV) | The intervention may potentially have an impact on the total length of time on any respiratory support (both invasive and non-invasive). Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Physiological/clinical (respiratory, thoracic and mediastinal outcomes) |
| Change in weight (growth) between PICU admission and discharge | The intervention may potentially have an impact on the child’s weight. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Physiological/clinical (metabolism and nutrition outcomes) |
| Change in length (growth) between PICU admission and discharge | The intervention may potentially have an impact on the child’s growth. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was identified by parents as important and prioritised by parents during the qualitative interviews | Physiological/clinical (metabolism and nutrition outcomes) |
Appendix 6 Paediatric intensive care unit additional outcomes
| Name | Help text | Domain name |
|---|---|---|
| Administration of parenteral nutrition secondary to feed intolerance | The intervention may potentially have an impact on whether or not parenteral nutrition is administered. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (GI outcomes) |
| Post-pyloric feeding (placing a post-pyloric tube) secondary to feed intolerance | The intervention may potentially have an impact on whether or not a post-pyloric feeding tube is placed. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (GI outcomes) |
| Parental satisfaction | The intervention may potentially have an impact on how satisfied the parent is with the delivery of care. Please indicate how important you think it is that this outcome is measured in a future study | Delivery of care |
| Change to feed formula type, secondary to feed intolerance | The intervention may potentially have an impact on whether or not there is a change to the type of feed formula. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (GI outcomes) |
| Administration of prokinetic drugs, secondary to feed intolerance | The intervention may potentially have an impact on whether or not prokinetic drugs are administered. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (GI outcomes) |
| Incidence of NEC | The intervention may potentially have an impact on the incidence of NEC. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (infection outcomes); AE |
Appendix 7 Neonatal unit Delphi outcomes
| Name | Help text | Domain name |
|---|---|---|
| Days of central venous line access | The intervention may potentially have an impact on the number of days of central venous line access. Please indicate how important you think it is that this outcome is measured in a future study | Resource use (need for further intervention) |
| Days on parenteral nutrition | The intervention arm may potentially have an impact on the number of days on parenteral nutrition. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (metabolism and nutrition outcomes) |
| GI morbidity: diarrhoea | The intervention may potentially have an impact on the incidence of diarrhoea. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Physiological/clinical (GI outcomes); AE |
| GI morbidity: vomiting | The intervention may potentially have an impact on the incidence of vomiting. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Physiological/clinical (GI outcomes); AE |
| Incidence of NEC | The intervention may potentially have an impact on the incidence of NEC. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Physiological/clinical (infection outcomes); AE |
| Length of stay: hospital | The intervention may potentially have an impact on their length of stay in hospital. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Resource use (hospital) |
| Length of stay: NNU | The intervention may potentially have an impact on their length of stay in the NNU. Please indicate how important you think it is that this outcome is measured in a future study | Resource use (hospital) |
| Length of time: IV | The intervention may potentially have an impact on the length of time they spend on IV. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (respiratory, thoracic and mediastinal outcomes) |
| Mortality | The intervention may potentially have an impact on mortality/survival rates. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Death (mortality/survival) |
| Nursing time spent measuring GRV | The intervention may potentially have an impact on nursing time taken to perform this task. Please indicate how important you think it is that this outcome is measured in a future study | Resource use (hospital) |
| Time feed stopped per 24 hours | The intervention may potentially have an impact on the amount of time the feed is stopped in 24-hour period. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (metabolism and nutrition outcomes) |
| Time from start of enteral feeding to achieve full (150 ml/kg/day) enteral feeds | The intervention may potentially have an impact on the time from start of enteral feeding to achieve full (150 ml/kg/day) enteral feeds. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Physiological/clinical (GI outcomes) |
| Time to nasogastric tube removal | The intervention may potentially have an impact on the time to nasogastric tube removal. Please indicate how important you think it is that this outcome is measured in a future study | Resource use (need for further intervention) |
| Total length of time on respiratory support (IV + NIV) | The intervention may potentially have an impact on the total length of time on respiratory support received (includes both invasive and non-invasive). Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Physiological/clinical (respiratory, thoracic and mediastinal outcomes) |
| Change in weight (growth) between birth and NNU discharge | The intervention may potentially have an impact on the infant’s weight. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was prioritised by parents during the qualitative interviews | Physiological/clinical (metabolism and nutrition outcomes) |
| Change in length (growth) between birth and NNU discharge | The intervention may potentially have an impact on the infant’s growth. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was identified by parents as important and prioritised by parents during the qualitative interviews | Physiological/clinical (metabolism and nutrition outcomes) |
| Long-term outcomes: hearing loss | The intervention may potentially have a long-term impact on the infant’s health. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was identified by parents as important and prioritised by parents during the qualitative interviews | Life impact (physical and cognitive functioning) |
| Long-term outcomes: problems with eye sight | The intervention may potentially have a long-term impact on the infant’s health. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was identified by parents as important and prioritised by parents during the qualitative interviews | Life impact (physical and cognitive functioning) |
| Long-term outcomes: problems with cognition | The intervention may potentially have a long-term impact on the infant’s health. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was identified by parents as important and prioritised by parents during the qualitative interviews | Life impact (physical and cognitive functioning) |
| Long-term outcomes: brain injury on imaging | The intervention may potentially have a long-term impact on the infant’s health. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was identified by parents as important and prioritised by parents during the qualitative interviews | Central nervous system (physical and cognitive functioning) |
| Long-term outcomes: problems with mobility like cerebral palsy | The intervention may potentially have a long-term impact on the infant’s health. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was identified by parents as important and prioritised by parents during the qualitative interviews | Life impact (physical and cognitive functioning) |
| Health-care-associated infections | The intervention may potentially have an impact on the incidence of NEC and other health-care-associated infections. Please indicate how important you think it is that this outcome is measured in a future study. Note, this outcome was identified by parents as important and prioritised by parents during the qualitative interviews | Physiological/clinical (infection outcomes); AE |
Appendix 8 Neonatal unit additional Delphi outcomes
| Name | Help text | Domain name |
|---|---|---|
| Change in head circumference between birth and NNU discharge | The intervention may potentially have an impact on the infant’s head circumference. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (respiratory, thoracic and mediastinal outcomes) |
| Incidence of pneumonia due to milk aspiration | The intervention may potentially have an impact on the incidence of pneumonia due to milk aspiration. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (respiratory, thoracic and mediastinal outcomes) |
| Incidence of catheter-associated bloodstream infection | The intervention may potentially have an impact on the incidence of catheter-associated bloodstream infection. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (infection outcomes); AE |
| Time to oral feeding | The intervention may potentially have an impact on the time taken to start oral feeds. Please indicate how important you think it is that this outcome is measured in a future study | Physiological/clinical (GI outcomes) |
Appendix 9 Neonatal data items extracted from the National Neonatal Research Database for the GASTRIC study
- Aggregate annual data for England and Wales for 2017 and 2018, on the number of infants (data separately for 1-3 below)
1. With a gestation at birth < 32 + 0 (weeks + days)
Defined as: Gestation Weeks < 32 weeks
1. Who received any mechanical ventilation
Defined as any of the following:
Respiratory support = ‘1 – Ventilation via endotracheal tube or tracheostomy’
OR
Ventilation mode equal to either:
‘1 – Conventional’
‘2 – High frequency oscillation’
OR
Added Oxygen = ‘11 – Oxygen given with ventilation’
2. Who are recorded as receiving any enteral feeds via nasogastric tube
Define as: Feeding Method = ‘4 – Nasogastric tube’
4. Aggregate annual data for number of infants that fulfil 1, 2, and 3 above
– All aggregate data (1–4 above) split by the following
-
a. Gestational age < 28 gestational weeks/≥ 28 gestational weeks at birth
-
b. Born in a NICU/born in a LNU/born in a SCBU
– The following number of cases recorded annually for 2017 and 2018 in each population group (1–3 above) – total numbers unless stated otherwise:
-
a. Died prior to neonatal unit discharge
Defined as any of the following:
Discharge Destination = ‘3 – Died’
OR Final NNU Outcome = ‘3 –Died’
-
b. NEC (NNAP definition)
Defined as:
NEC diagnosis = ‘1 = NEC diagnosed this episode’
AND
NEC DiagnosisBasedOn = (11– surgery
12 –post-mortem)
OR
NEC DiagnosisBasedON = ‘10 –clinical signs ‘AND at least one Clinical Features = (10- Bilious gastric aspirate or emesis
11- Abdominal distension
12- Occult or gross blood in stool (no fissure)) AND at least on Radiographic Feature = (10- Pneumatosis intestinalis
11- Hepato-biliary gas
12- Pneumoperitoneum)
-
c. Mean (SD) median (IQR) number of days receiving PN (please also note several babies that did not receive any PN)
Defined as: Parenteral Nutrition = ‘1 – PN given’
-
d. Mean (SD) median (IQR) number of days before a baby is recorded as being fully enterally fed (e.g. no PN or IV fluids for 2 consecutive days)
Defined as:
Parenteral Nutrition = ‘0 – No PN given’
AND
Glucose Electrolyte = ‘0 – None given’
AND
DayEnteralFeed =
1 - Suckling at the breast
2 - Mother’s fresh expressed breast milk
3 - Mother’s frozen expressed breast milk
4 - Donor expressed breast milk
5 - Breast milk fortifier
6 - Formula
8 - Other)
-
e. Bloodstream infection (NNAP definition)
Defined as: SampleType = ‘BLOOD’
AND
Pathogen = pathogenic organisms (considered so from predefined list used for NNAP processing)
OR
Pathogen = skin commensal AND at least 3 clinical signs (including; tachypnoea or clinically relevant increase in oxygen requirement or ventilatory support, clinically relevant increase in apnoea or bradycardia episodes,
Hypotension, glucose intolerance, impaired peripheral perfusion (capillary refill time more than 3 sec, pallor/mottling/core peripheral
temperature gap more than 2 °C), lethargy/irritability/poor handling,
temperature instability, feed intolerance, fall in urine output or metabolic acidosis (base deficit below –10 mmol/l))
OR
Pathogen = mixed growth AND at least 3 clinical signs (as above)
AND
Sample taken time > 72 hours after birth
-
f. Mean (SD) median (IQR) number of days with a central line in (please also note several babies that did not have a central line at all)
Defined as:
Lines in situ equal to any of the following:
B - Umbilical arterial line
C - Umbilical venous line
D - Percutaneous central venous line (long line)
E - Surgically inserted central venous line
These lines are considered central for the NNAP CLABSI item below
-
g. CLABSI (NNAP definition); please also note a number of babies that did not have a central line at any point)
Defined as: Bloodstream infection definition as above
AND
Line in situ equal to the above
-
h. Mean (SD) median (IQR) length of neonatal unit stay
Defined as the total number of days a baby received any level of care where location of care is set to ‘NNU’ (taken from Daily Care General Information - LOCATIONS OF HIGHEST LEVEL OF CARE)
i. Mean (SD) median (IQR) number of days receiving ventilation (also the number of babies not ventilated at all)
Defined as any of the following:
Respiratory support = ‘1 – Ventilation via endotracheal tube or tracheostomy’
OR
Ventilation mode equal to any of the following:
‘1 – Conventional’
‘2 – High frequency oscillation’
OR
Added Oxygen = ‘11 – Oxygen given with ventilation’
-
j. Mean (SD) median (IQR) number of days receiving non-invasive ventilation (also the number of babies not receiving non-invasive ventilation at all)
Defined as any of the following:
Respiratory support = ‘2 – Non-invasive support (inc CPAP)’
OR
Non-Invasive Respiratory Support equal to any of the following:
‘1 - nasal CPAP (prong or mask)’
‘2 - BIPAP/SIPAP’
‘4 - High flow O2/air device’
OR Added Oxygen equal to any of the following:
12 - Oxygen given with CPAP
13 - Oxygen given with nasal ventilation (as of 2012 this option was combined with option ‘12’ to give Oxygen given with CPAP or nasal ventilation)
14 - Headbox oxygen
15 - Nasal cannula oxygen up to 1 lpm
16 - Nasal cannula oxygen above 1 lpm
17 - High flow oxygen/air device used
18 - Oxygen therapy (unspecified)
Unit level aggregate annual numbers for NICU and LNU for 1–3
Appendix 10 Study oversight committees
Study Steering Committee: independent members
Dr Pamela Cairns (chairperson), Consultant Neonatologist, NNU, St Michael’s Hospital, Bristol, UK.
Dr Linda Hunt, Statistician, National Joint Registry Office, Musculoskeletal Research Unit, Translational Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Ms Nailah Brown, public member.
Professor Sharon Irving, Assistant Professor Paediatric Nursing, School of Nursing, University of Pennsylvania, Philadelphia, PA, USA.
Dr Luise Marino, Clinical Academic PICU Dietitian, PICU, Southampton Children’s Hospital, Southampton, UK.
Professor Nilesh Mehta, Associate Professor of Anaesthesia, Harvard Medical School, PICU, Boston Children’s Hospital, Boston, MA, USA.
Professor Namasivayam Ambalavanan, Department of Paediatrics, Women & Infants Centre, Birmingham, AL, USA.
Professor Leslie Parker, Associate Clinical Professor, University of Florida, Gainesville, FL, USA.
Study Management Group
Iza Andrzejewska, NICU nurse, Chelsea and Westminster Hospital, London, UK.
Barbara Arch, Statistician, Liverpool Clinical Trials Centre, University of Liverpool, Liverpool, UK (also a member of the Liverpool Health Partners).
Anne Beissel, Neonatologist, Hospices Civils de Lyon, Lyon-Bron, France.
Ashley Jones, Senior Statistician, Clinical Trials Research Centre, University of Liverpool, Liverpool, UK (also a member of the Liverpool Health Partners).
Beth Deja, Research Associate, Medical Research Centre North West Hub for Trials Methodology, Research Institute of Psychology, Health and Society, Liverpool, Liverpool, UK.
Jon Dorling, Lead NICU Stream Neonatologist, IWK Health Centre, Halifax, NS, Canada.
Helen Eccleson, Study Co-ordinator, Clinical Trials Research Centre, University of Liverpool, Liverpool, UK (also a member of the Liverpool Health Partners).
Chris Gale, Neonatologist and NNRD, Neonatal Medicine, Chelsea and Westminster Hospital, London, UK.
Helen Hickey, Senior Trials Manager, Clinical Trials Research Centre, University of Liverpool, a member of the Liverpool Health Partners, UK.
Clare Jackson, Senior Data Manager, Clinical Trials Research Centre, University of Liverpool, Liverpool, UK (also a member of the Liverpool Health Partners).
Lynne Latten, PICU Dietitian, Nutrition and Dietetics, Alder Hey Children’s NHS Foundation Trust, Liverpool, UK.
Roger Parslow, Senior Lecturer in Epidemiology, Leeds Institute of Cardiovascular and Metabolic Medicine, School of Medicine, University of Leeds, Leeds, UK.
Nazima Pathan, University Lecturer and Honorary Consultant in Paediatric Critical Care University Department of Paediatrics, Addenbrooke’s Hospital, Cambridge, UK.
Jenny Preston, patient and public involvement representative, Department of Women’s and Children’s Health, Institute of Translational Medicine (Child Health), Alder Hey Children’s NHS Foundation Trust, University of Liverpool, Liverpool, UK.
Louise Roper, Research Associate, Medical Research Council North West Hub for Trials Methodology, Research Institute of Psychology, Health and Society, Liverpool, UK.
Lyvonne Tume, chief investigator/Associate Professor in Child Health, School of Health & Society, University of Salford, Manchester, UK.
Frederic Valla, PICU Physician, Hospices Civils de Lyon, Lyon-Bron, France.
Kerry Woolfall, Department of Psychological Sciences, Medical Research Council North West Hub for Trials Methodology, Research Institute of Psychology, Health and Society, Liverpool, UK.
List of abbreviations
- AE
- adverse event
- GI
- gastrointestinal
- GRV
- gastric residual volume
- HCP
- health-care professional
- ICU
- intensive care unit
- IQR
- interquartile range
- IV
- invasive ventilation
- LNU
- local neonatal unit
- NEC
- necrotising enterocolitis
- NICU
- neonatal intensive care unit
- NIV
- non-invasive ventilation
- NNAP
- National Neonatal Audit Programme
- NNRD
- National Neonatal Research Database
- NNU
- neonatal unit
- PICANet
- Paediatric Intensive Care Audit Network
- PICS
- Paediatric Intensive Care Society
- PICU
- paediatric intensive care unit
- PIS
- participant information sheet
- RCT
- randomised controlled trial
- SCBU
- special care baby unit
- SD
- standard deviation
- SMG
- Study Management Group
- VAP
- ventilator-associated pneumonia
- WP
- work package
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta24230).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
