Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/37/01. The contractual start date was in December 2012. The draft report began editorial review in November 2018 and was accepted for publication in May 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Gary A Ford declares personal fees from AstraZeneca (Cambridge, UK), Bayer AG (Leverkusen, Germany), Medtronic (Dublin, Ireland), Pfizer (New York, NY, USA), Pulse Therapeutics Euphrates Vascular (St Louis, MO, USA), Stryker Corporation (Kalamazoo, MI, USA) and Amgen (Thousand Oaks, CA, USA), and grants from Daiichi Sankyo (Tokyo, Japan), Medtronic and Pfizer outside the submitted work. Anne Forster declares grants from the National Institute for Health Research (NIHR) and The Stroke Association (London, UK) outside the submitted work. She reports membership of the Health Services and Delivery Research (HSDR) Researcher-led Prioritisation Committee. Denise Howel was a member of the NIHR Programme Grants for Applied Research panel (2016 to present) and NIHR HSDR Commissioning Board (2012–15) during this research project. Luke Vale was a member the NIHR Health Technology Assessment (HTA) Clinical Evaluation and Trials panel (2014–18) during this research project. Helen Rodgers declares fees from Bayer and that during this research project she was a member of the British Association of Stroke Physicians (president) (2014–17), NIHR HTA Clinical Evaluation and Trials Board (2010–14), Intercollegiate Stroke Working Party (2002 to present), National Stroke Programme (chairperson of rehabilitation and ongoing care working group) (2018 to present) Joint Stroke Medicine Committee Royal College of Physicians London (chairperson) (2018 to present) and Steering Group member VISTA (Virtual International Stroke Trials Archive) (2015 to present).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Shaw et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Some parts of this report are based on Rodgers et al. 1 © 2019 The Authors. Stroke is published on behalf of the American Heart Association, Inc., by Wolters Kluwer Health, Inc. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution, and reproduction in any medium, provided that the original work is properly cited. In 2010, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme identified the need to evaluate the clinical effectiveness and cost-effectiveness of rehabilitation interventions that aimed to improve ability to perform extended activities of daily living (EADL) in the longer term after stroke. EADL include mobility, housework, hobbies and leisure interests. 2 This report describes findings of the research commissioned for this evaluation.
Problems after stroke
In the UK, approximately 113,000 strokes currently occur per year. 3 Although the incidence of stroke has fallen, survival rates have improved and the prevalence of stroke has increased. 4 At present, there are over 1.2 million stroke survivors living in the UK. 5
The effects of stroke are diverse and, despite recent improvements in acute treatments (e.g. stroke unit care, thrombolysis and thrombectomy), currently around two-thirds of survivors leave hospital with a disability. 5 Some people will make a full recovery but for others disabilities persist long term. 6,7 At the time of planning this research project in 2010, a national survey of stroke survivors more than 1 year after stroke had shown that nearly half of respondents reported unmet needs in relation to issues such as personal care, mobility, emotional problems and leisure activities. 8,9 Later work continues to report similar findings. 10–12 Stroke survivors, carers and health-care professionals are repeatedly requesting that this situation is improved.
Rehabilitation after stroke
Rehabilitation is a broad term that encompasses specific interventions to target specific issues (e.g. robot-assisted training for upper limb function) through to complex care systems that involve teams of health-care professionals delivering a package of interventions. 13
It is well established that hospital stroke unit care and early supported discharge (ESD) services are both effective and cost-effective ways to improve patient outcomes following stroke. 14,15 The ESD services provide ongoing rehabilitation in a patient’s own home following the period of stroke unit care. They enable patients to leave hospital earlier than would be possible without such a service.
Stroke units and the ESD services are referred to as ‘organised stroke care’ and their key features are multidisciplinary stroke specialist expertise and co-ordination of care. 16,17 The National Clinical Guideline for Stroke recommends specialist stroke unit care for the duration of the inpatient stay unless stroke is not the main illness. 16 In 2016/17, 83.8% of patients spent at least 90% of their stay In a stroke unit. 18 ESD is recommended for patients with mild to moderate disability16 and in 2016 81% of stroke units had access to an ESD service. 19
Evidence and guidelines also continue to grow about specific rehabilitation interventions for different problems after stroke. 13,16,20 However, most research to date has focused on the acute and subacute phases of stroke, and there is less evidence to guide rehabilitation in the longer term following stroke. 16 When planning this research project, several publications were noting this lack of evidence for longer-term rehabilitation and the need for further research. 21,22
A 2008 Cochrane review of therapy-based rehabilitation services for patients living at home > 1 year after stroke found only five trials suitable for inclusion and reported that there was inconclusive evidence about whether or not these services improved patient outcomes. 21 Although a 2010 Cochrane review of community stroke liaison workers reported that there was no evidence that this intervention could improve outcomes for all stroke patients, it showed that people with milder disability had a reduction in death and dependence. 23 Another Cochrane review, which included 14 trials of non-ESD therapy-based rehabilitation services, which were provided at home for patients within 1 year of stroke, reported that these services decreased the odds of a poor outcome (defined as death or deterioration in ability to perform activities of daily living) and had a beneficial effect on performance of activities of daily living. 24 However, most of the services included in this review commenced soon after discharge rather than later after stroke.
Probably in part a result of the lack of evidence of the effectiveness of rehabilitation in the longer term after stroke, at the time of planning this research project, it was recognised that provision of longer-term services for stroke patients was limited and varied across the UK. 25 Following discharge from ESD services, the concept of ‘organised stroke care’ disappears and patients who have ongoing rehabilitation needs may be referred to a range of services, if available (e.g. neurorehabilitation teams, day hospital and community rehabilitation services). Reports from both the National Audit Office and Care Quality Commission highlighted that the longer-term care after stroke needed to be improved. 22,25
The literature about interventions to improve longer-term outcomes after stroke has of course expanded since 2010; however, the evidence is still mixed. For example, a 2014 review of several specific physical therapy interventions concluded that those involving repetitive task practice improved outcomes, including outcomes for patients later after stroke. 26 A 2015 Cochrane review that examined interventions that aimed to improve ambulation in the community reported that there was insufficient evidence to determine if these interventions were beneficial. 27 A review examining the effect of physiotherapy interventions that aimed to improve mobility or independence in activities of daily living in patients at least 6 months after stroke showed a significant improvement in mobility but no improvement in independence in activities of daily living. 28 A 2016 Cochrane review of self-management programmes for stroke survivors living in the community found that these interventions led to improved quality of life, but not to improvements in activities of daily living or mood. 29 A large cluster randomised controlled trial published in 2015 evaluated a system of Longer-Term Stroke (LoTS) care in which stroke care co-ordinators, who were community-based health-care professionals with experience in stroke care, were trained to follow a structured assessment and treatment plan working with patients and carers after discharge from hospital (29 centres, 800 patients, 208 carers). 30 Follow-up was at 6 months and 12 months following patient recruitment, and included assessment of quality of life and performance in EADL. Neither patients nor carers in the intervention group had improved outcomes compared with usual care.
Although improvements in stroke services have taken place since this research was commissioned,18 the evidence base supporting longer-term rehabilitation is still limited and post acute stroke care remains variable. 31
The EXTRAS trial
This research project has evaluated an extended stroke rehabilitation service (EXTRAS). The service consisted of five rehabilitation reviews conducted at 1, 3, 6, 12 and 18 months post discharge from ESD services. Reviews were undertaken by senior members of ESD teams and consisted of a structured assessment of rehabilitation needs, goal-setting and action-planning. Reviews covered mobility; personal care; mealtimes; domestic activities; work and volunteering; hobbies and interests; driving and transport, communication; memory and concentration; mood, anxiety and depression; medical issues; pain and other issues. Both stroke patients and their carers were included in the study and outcomes were assessed at 12 and 24 months post randomisation. Although EXTRAS did not include any assessment of carer needs or specific interventions for carers, we hypothesised that the new service may also influence carer outcomes. An evaluation of a longer-term stroke specialist service was undertaken because of the strong evidence showing that stroke units and ESD services improve patient outcomes and are cost-effective. If EXTRAS is effective and affordable, it could extend ‘organised stroke care’ and improve longer-term outcomes after stroke.
Chapter 2 Methods
Parts of this chapter have been reproduced from Rodgers et al. 32 © 2015 Crown copyright; licensee BioMed Central. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Study aim and objectives
1. Aim
To determine the clinical effectiveness and cost-effectiveness of an extended stroke rehabilitation service (EXTRAS).
2. Objectives
-
To determine whether and extended stroke rehabilitation service (intervention) improved patient outcomes compared to usual care (control). The primary outcome was extended activities of daily living at 24 months following randomisation. Secondary outcomes were health status, quality of life, mood and experience of services (12 and 24 months following randomisation).
-
To determine whether an extended stroke rehabilitation service improved carer outcomes compared to usual care. Outcomes were quality of life, carer stress, experience of services (12 and 24 months following randomisation).
-
To determine the cost-effectiveness of an extended stroke rehabilitation service.
-
To document how the extended stroke rehabilitation service was implemented and delivered in different settings.
-
To seek the views and experiences of patients, carers and rehabilitation staff about the community rehabilitation that they received or provided.
-
To explore the impact of the severity of activity limitation, pre-stroke health status and comorbidity on the effectiveness of the intervention.
Study design
The study was a pragmatic, observer-blind, parallel-group, multicentre randomised controlled trial with health economic and process evaluations. The study protocol has been published. 32
Study setting
The study was conducted in 19 NHS study centres in the UK. All study centres provided an ESD service. To be eligible to take part, the ESD service had to meet the following criteria:
-
had a multidisciplinary stroke team that provided community rehabilitation following discharge from hospital
-
was able to provide stroke rehabilitation at home within 48 hours of patient discharge from hospital
-
was able to provide stroke rehabilitation for a specified period of time and/or had clear criteria for discharge of patients from the service.
Study participants
Adults with a stroke who fulfilled the following criteria were eligible.
1. Inclusion criteria
-
Aged ≥ 18 years.
-
Confirmed diagnosis of new stroke (first ever or recurrent).
-
Planned discharge from hospital under the care of ESD or currently receiving ESD.
2. Exclusion criteria
-
Unable to participate in a rehabilitation programme that focused on EADL.
A carer was the main family member or friend who provided support after stroke. He or she was not required to be a co-resident of the patient. If an eligible patient had no carer or a carer who did not wish to participate in the study, the patient was still able to take part.
Case ascertainment, recruitment and consent
1. Patients
Potential patients were identified and recruited by NHS staff (clinicians, staff from the Local Clinical Research Network and senior members of an ESD team). Potential patients could be recruited prior to discharge from hospital or while receiving care from an ESD service. Although EXTRAS did not commence until routine ESD services ended, identification and recruitment of patients in hospital or during ESD was used to maximise recruitment opportunities.
It was intended that screening data would be collected to report eligibility and subsequent recruitment or reason for lack of recruitment. Unfortunately, collecting these data was not possible owing to workload at the NHS study centres.
As stroke survivors can have impairments that result in difficulties with communication and/or cognition, a number of consent methods were used to facilitate participation.
For patients with mental capacity to consent to research, a standard research information sheet and consent form were used by NHS staff. If a person had capacity to consent to research but was unable to sign the consent form (e.g. because of weakness of the dominant hand due to stroke), consent was confirmed orally in the presence of a witness (an individual not involved in the trial) who signed the consent form on behalf of the participant. For patients with communication difficulties owing to aphasia, an ‘easy access’ study information sheet and consent form were used. For patients without mental capacity to consent to research, a personal consultee was identified, was provided with a consultee information sheet and signed a consultee declaration form if they believed that the patient would have no objection to taking part in the study. Due to the nature of this study, potential patients lacking in capacity also needed to have a relative/friend (carer) who was prepared to assist with EXTRAS reviews and outcome assessments, as these were unlikely to be possible without their support.
2. Carers
Potential carers were identified by ESD senior team members while the patient was receiving routine ESD care. At the time of patient discharge from routine ESD services, if the patient had an identified carer, she/he was provided with an invitation letter, study information sheet, study carer baseline questionnaire and prepaid envelope (addressed to the study co-ordinating centre). Provision of the invitation letter and study documents could be in person by an ESD senior team member, by post by the local study team, or by a consented patient. These three options were used to maximise potential opportunities for carers to take part in the study, as carers were not always present at staff visits. The invitation letter asked the carer to complete and return the baseline questionnaire if she/he was willing to participate in the study.
Recruitment and baseline assessments
Patient baseline data were collected during face-to-face recruitment and baseline assessments. Both assessments were performed by NHS staff after informed consent had been obtained. The recruitment assessment could be performed within 4 days prior to planned discharge from hospital, or during routine ESD care. The baseline assessment was performed at discharge from routine ESD services and immediately prior to randomisation. Both assessments could be conducted together at discharge from ESD if preferred. Data collection was divided in this format for logistical ease. As patient recruitment could be during hospital stay, data that were more easily obtainable by hospital staff were collected in the recruitment assessment. Data pertaining to patient characteristics at discharge from ESD were collected at the baseline assessment.
The following data were collected during the recruitment assessment: demographic data, pre-stroke performance in EADL [Nottingham Extended Activities of Daily Living (NEADL) Scale33], pre-stroke health status [Oxford Handicap Scale (OHS)34], date of hospital admission, date of stroke, stroke type and subtype,35 National Institutes of Health Stroke Scale (NIHSS),36 comorbidity and pre-stroke resource usage [adaptation of the Client Service Receipt Inventory (CSRI)37–39].
The following data were collected during the baseline assessment: date of hospital discharge, date of ESD discharge, Abbreviated Mental Test Score,40 Sheffield Aphasia Screening Test,41 EADL (NEADL Scale),33 health status (OHS),34 mood [Hospital Anxiety and Depression Scale (HADS)42] and quality of life [EuroQol-5 Dimensions, five-level version (EQ-5D-5L)43].
Carers received a baseline questionnaire with the study invitation letter. The questionnaire collected the following data: demographic, carer stress [Caregiver Strain Index (CSI)44] and quality of life (EQ-5D-5L). 43
Randomisation
Randomisation was conducted by NHS staff at participating study centres using a central independent web-based service hosted by Newcastle University Clinical Trials Unit. Participants were stratified according to study centre and randomised to intervention and control in a 1 : 1 ratio using permuted block sequences. Stroke patients and their carers were randomised as a single unit.
Study control treatment
Stroke patients in the control group received usual ESD care with subsequent referral to other rehabilitation services post discharge from ESD if required, and in accordance with the local usual care. Patients who had ongoing rehabilitation needs following completion of ESD could be referred to a range of services, for example neurorehabilitation teams, day hospital and community rehabilitation services. The availability of rehabilitation services for patients taking part in this study was recorded during a mapping exercise (see Chapter 7).
Control participants also received the booklet Care After Stroke or Transient Ischaemic Attack. Information for Patients and their Carers written by the Intercollegiate Stroke Working Party. 45 This booklet described what a stroke is and its assessment, acute management and rehabilitation. It was based on the National Clinical Guideline for Stroke in 200846 updated in 2012. 47
Study intervention treatment
Stroke patients in the intervention group received EXTRAS for 18 months following completion of rehabilitation with their ESD team. This was in addition to usual care. They also received the Intercollegiate Stroke Working Party booklet about stroke care and rehabilitation. 45
EXTRAS was developed by the investigator team and consisted of reviews conducted by a senior member of the ESD team at 1, 3, 6, 12 and 18 months post discharge from routine ESD (randomisation).
Each EXTRAS review consisted of:
-
Identification of rehabilitation needs. A semistructured interview was conducted to determine the patient’s progress, current rehabilitation needs and service provision. The interview addressed daily activities (mobility, personal care, mealtimes, domestic activities), social participation (work and volunteering, hobbies and interests, driving and transport) and wider issues (communication, memory and concentration, mood, medical issues, pain), which may be problematic for stroke survivors. The views of both the patient and the carer (where appropriate) could be sought. The interview topics were based on the literature about stroke survivors’ long-term needs including the UK Stroke Survivor Needs Survey8 and input from stroke survivors, carers and health-care professionals.
-
Joint rehabilitation goal-setting. From the identified progress and rehabilitation needs, up to five individual rehabilitation goals could be set by the patient (and carer) in collaboration with the senior ESD team member who conducted the review. The focus of joint goal-setting was intended to be on increasing participation in everyday activities. From the second review, progress towards the goals set previously was assessed prior to further goal-setting. Achievement of goals was recorded using a Goal Attainment Scale. 48
-
Action-planning. The patient (and carer) agreed an action plan for each rehabilitation goal. Action plans could include:
-
Verbal advice and encouragement.
-
Discussion with the stroke team, rehabilitation team, primary care team or social services involved in care.
-
Signposting to local activities, community organisations or voluntary services.
-
Referral to stroke services, rehabilitation services or primary care services for further assessment and treatment if required according to local guidelines and/or service provision.
-
Subsequent to each review, the ESD therapist/nurse could contact the services currently involved in the patient’s care to discuss progress, goals and care plan.
The EXTRAS reviews were intended to be predominantly undertaken by telephone. The senior ESD team member would know the patient and carer, as he/she had treated the patient as part of the ESD service. However, if the patient and/or carer was unable to participate in a telephone review, a home visit could be undertaken. On randomisation to the intervention group, patients were given a study appointment card that also contained a short checklist of rehabilitation issues to be covered in each review. This was intended to allow patients (and carers) time to consider the topics to be discussed prior to each review. Patients with aphasia received an ‘easy access’ version of the appointment card. Following each review, a summary of the interview and recommendations for rehabilitation were sent to the patient by post and could be copied to other health-care professionals involved in care as appropriate. Patients with aphasia received an ‘easy access’ version of the summary.
On opening a study centre, all senior ESD staff taking part in delivery of the new service received face-to-face training from the trial co-ordinating centre team. As delivery of the intervention for this study was conducted for almost 5 years (recruitment commenced in late 2012 and delivery of the intervention ceased in mid-2017), inevitable staffing changes took place in ESD teams. New members of staff received cascade training from existing trained staff and/or further training visits from the trial co-ordinating centre.
A manual describing EXTRAS was also provided to each member of staff. This manual described how to conduct the reviews and included guidance on exploring rehabilitation needs, goal-setting and appropriate interventions to meet a patient’s needs. Study-specific paperwork was completed for each review, including documentation of identified needs, goals set and action plans made.
The choice of ESD team staff member (e.g. physiotherapist, occupational therapist, nurse) to conduct each individual review was at the discretion of each participating centre. The trial co-ordinating centre sent all participating study centres weekly reminders about forthcoming reviews and a regular data report that illustrated which review data were entered onto the study database, therefore highlighting overdue or missing reviews.
A description of EXTRAS using the Template for Intervention Description and Replication (TIDieR) checklist49 is shown in Appendix 1, Table 31.
EXTRAS did not include any assessment of carer needs or specific interventions for carers.
Outcome assessments
Outcomes were intended to be assessed at 12 months (± 7 days) and 24 months (± 7 days) post randomisation.
1. Patients
Patient outcome assessments could be undertaken over the telephone, by postal questionnaire or during a face-to-face visit. The preferred method was telephone questionnaire but postal or face-to-face options were possible where use of a telephone was unfeasible. Postal questionnaires were also used when it had not been possible to make contact by telephone.
Telephone interviews were conducted by a researcher based in the study co-ordinating centre at Newcastle University, Newcastle upon Tyne, UK. This researcher also co-ordinated any postal questionnaires and conducted any required face-to-face visits for patients in north-east England. When patients required a face-to-face visit outside north-east England, this was undertaken by staff from the local participating centre following training by the co-ordinating centre researcher.
The following data were collected: EADL (NEADL Scale),33 health status (OHS),34 mood (HADS),42 experience of services (adaption of an experience survey designed by Northumbria Healthcare NHS Foundation Trust based on Picker Institute questions,50 quality of life (EQ-5D-5L)43 and resource utilisation (adaptation of the CSRI). 37–39 The primary outcome was the NEADL Scale. 33 The OHS,34 HADS42 and experiences of services survey were secondary outcome data. The EQ-5D-5L43 and resource utilisation were collected for the economic evaluation.
The NEADL Scale is a 22-item scale to assess performance in carrying out EADL. The items are ordered as four subscales covering ‘mobility’, ‘in the kitchen’, ‘domestic tasks’ and ‘leisure activities’. Respondents are asked to indicate what they have actually done, not what they perceive they could do, ought to do or would like to do. Each item is scored as follows: 0 is ‘no’, 1 is ‘with help’, 2 is ‘on my own with difficulty’ and 3 is ‘on my own’. Responses are summed, giving a score of 0–66, where higher scores indicate greater activity.
The OHS34 is a derivative of the Modified Rankin Scale,51 which, in turn, is a derivative of the original Rankin Scale. 52 The OHS used in this study was a self-report version. The scale has ordinal scale scoring: 0 is ‘I have no symptoms at all and cope well with life’, 1 is ‘I have a few symptoms but these do not interfere with my everyday life’, 2 is ‘I have symptoms which have caused some changes in my life but I am still able to look after myself’, 3 is ‘I have symptoms which have significantly changed my life, prevent me coping fully on my own, and I need some help in looking after myself’, 4 is ‘I have quite severe symptoms which mean I need to have help from other people but I am not so bad as to need attention day and night’, 5 is ‘I have major symptoms which severely handicap me and I need constant attention day and night’. A score of 6 is used to indicate death.
The HADS42 is made up of anxiety and depression subscales of seven items each. Each item is scored 0–3, so patients can score from 0 to 21 on each subscale. Higher scores are indicative of anxiety and depression. In addition, ‘cut-off’ scores have been defined as follows: a score of 0–7 is ‘non-case’, 8–10 is ‘doubtful case’ and 11–21 is ‘definite case’.
The experience of services scale was an adaptation of an experience survey designed by Northumbria Healthcare NHS Foundation Trust based on Picker Institute questions. 50 It had three parts. Part 1 involved 15 questions about services received with responses on a five-point Likert scale: ‘strongly disagree’, ‘disagree’, ‘agree’, ‘strongly agree’ and ‘does not apply’. Part 2 was about overall satisfaction. Participants were asked ‘Overall, how satisfied are you with the services you received?’. There were five responses from ‘extremely satisfied’ to ‘extremely unsatisfied’. Part 3 contained three questions about meeting needs in relation to speaking, mobility and emotional problems. Responses were ‘yes, definitely’, ‘yes, to some extent’, ‘no I did not get enough help from the NHS’ and ‘I did not have any difficulties’. There was no numerical scoring for this questionnaire, so questions were dichotomised to create proportions of patients answering ‘strongly agree’ and ‘agree’, for comparison with ‘disagree’ and ‘strongly disagree’; or, ‘extremely satisfied’, ‘very satisfied’ and ‘quite satisfied’, for comparison with ‘not very satisfied’ and ‘extremely unsatisfied’.
The EQ-5D-5L43 instrument has two parts: a descriptive system comprising five dimensions (i.e. mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and a visual analogue scale (VAS). In the descriptive system, each dimension has five responses: no problems, slight problems, moderate problems, extreme problems and unable to do. The VAS is vertical with ‘the best health you can imagine’ at the top (score of 100) and ‘the worst health you can image’ at the bottom (score of 0). Responses to the EQ-5D-5L were converted into utility values, which were combined with observed mortality to create quality-adjusted life-years (QALYs). Data are presented in Chapter 8.
An adaption of the CSRI37–39 was used to measure resource utilisation. This instrument captures data about accommodation and living, hospital care, primary care, other NHS services (e.g. physiotherapy, occupational therapy) and social services.
2. Carers
Carers’ outcome assessments were undertaken by a postal questionnaire sent by and returned to the study co-ordinating centre. The following data were collected from carers: carer stress (CSI),44 experience of services (adaption of an experience survey designed by Northumbria Healthcare NHS Foundation Trust based on Picker Institute questions)50 and quality of life (EQ-5D-5L). 43 Responses to the EQ-5D-5L were used to create QALYs, and these data are presented in the health economic evaluation chapter (see Chapter 8).
The CSI44 is a tool used to identify strain in informal carers for physically ill and functionally impaired elderly adults. It comprises 13 items, each answered as ‘no’ (score of 0) or ‘yes’ (score of 1). Scores are summed and a higher score indicates more strain. Because the CSI asks some sensitive questions about the impact of stroke on the carer, it was not felt appropriate to conduct telephone interviews as carers may have modified their answers if they could be overheard on the telephone by the patient.
Blinding
Owing to the nature of the study intervention, it was not possible to blind stroke patients or carers to treatment allocation. Where patient outcome assessments were undertaken by telephone or face-to-face visit, it was intended that they were conducted blinded to treatment allocation. After each assessment, the assessor was asked to record whether or not they had unintentionally become aware of treatment allocation as a result of conversation with the participant.
Study withdrawal
No specific study withdrawal criteria were pre-set. Stroke patients and/or carers could withdraw from the study at any time for any reason. Reasons for withdrawal were sought but participants could withdraw without providing an explanation. Investigators, senior ESD team members and/or a patient’s consultee (in the case of mental incapacity) could also withdraw participants from the study at any time if they felt that it was no longer in their interest to continue, for example because of intercurrent illness. Participants were informed that data collected prior to withdrawal would be used in the study analysis, unless consent for this was specifically withdrawn. Participants who wished to receive no further intervention were not withdrawn from study follow-up unless they specifically requested this.
Safety evaluation
Adverse events were collected by including the following questions in the study outcome questionnaires at 12 and 24 months:
-
Have you suffered any new medical illnesses in the last 12 months?
-
Have you suffered any falls resulting in injury in the last 12 months?
Any event potentially fulfilling the criteria to be a serious adverse event (SAE) was further investigated by local study centre staff who had access to medical records, and fully detailed on a separate study SAE form as appropriate. As local study centre staff could become aware of events fulfilling the criteria to be SAEs at any time during a participant’s involvement in the study, the study SAE form was also used to directly capture any such events. A causality and expectedness assessment was undertaken for all SAEs.
The standard definition for SAE was used. A SAE is an untoward occurrence that:
-
results in death
-
is life-threatening
-
requires hospitalisation, or prolongation of existing hospitalisation
-
results in persistent or significant disability or incapacity
-
consists of a congenital anomaly or birth defect
-
is otherwise considered medically significant by the investigator.
Data management
Study data were collected onto study-specific paper case-record forms and subsequently entered onto an online database by NHS staff at participating centres and/or staff at the study co-ordinating centre.
Sample size
A difference of 6 points on the NEADL Scale (scored 0–66, SD 18) is considered to be clinically important, and power calculations for previous multicentre rehabilitation trials had been based on this difference. 38,53 Responses from 382 patients who were split equally between intervention and control groups would provide 90% power to detect a difference in mean NEADL Scale of 6 points. Based on attrition in other stroke rehabilitation trials, it was estimated that there could be up to 25% attrition between study randomisation and the 24-month (primary) outcome assessment. To allow for this, 510 patients were required to be randomised into the study.
Because patients could be recruited at any time from within 4 days prior to hospital discharge to discharge from ESD, there could be several weeks between recruitment and randomisation. As dropout was observed during this time interval, it was planned that recruitment would cease when it was estimated that at least 510 patients would be randomised. Reasons for loss from the trial were recorded.
Statistical analysis of primary and secondary outcome data
1. Analysis populations
Analyses were carried out on an intention-to-treat (ITT) basis, retaining patients and carers in their randomisation groups, and including protocol violators and ineligible patients.
2. Analysis data sets
The pattern and extent of missing observations because of loss to follow-up was examined. Unless specified by the scale developers, where no more than 20% of questions were missing or uninterpretable on specific scales, the score was calculated by using the mean or median value (as appropriate) of the respondent-specific completed responses on the rest of the scale to replace the missing items (i.e. simple imputation). 54 It was planned to investigate the use of multiple imputation techniques if the primary outcome was still missing for > 20% of patients after the use of simple imputation; however, this was not necessary. Complete-case, simple imputation and a sensitivity analysis data set were analysed. The sensitivity analysis data set excluded data from patients who had follow-up conducted 1 month before and 3 months after the due date for their 12- and 24-month assessments, and patients whose NEADL Scale scores recorded post stroke were higher than those recorded prior to their stroke.
3. Descriptive analyses
Characteristics of study patients and carers were summarised separately for each randomisation group. This included primary and secondary outcome variables and covariates. Continuous variables were summarised by the numbers of observations and the mean and SD, or the median and interquartile range (IQR), depending on whether or not the distribution was symmetric. Numbers of observations and percentages were reported for categorical variables. No significance testing for any baseline imbalance was carried out, but any noted differences were reported descriptively.
4. Inferential analyses
Mean scores on the NEADL Scale (the primary outcome) were compared at 24 months between the intervention and the control groups using multiple linear regression, including terms for centre, baseline OHS, age and sex. Mean scores with bootstrapped confidence intervals (CIs) are reported for:
-
data with complete-case NEADL Scale scores
-
a complete-case set adjusted for centre, baseline OHS, age and sex
-
data with simple imputation for partially completed NEADL Scale scores
-
a simple imputation set, adjusted for centre, baseline OHS, age and sex (primary analysis)
-
a sensitivity analysis of above, excluding data from patients outside –1 and + 3 months of their expected visit date and those whose NEADL Scale scores appeared to be unlikely as they had increased (improved) post stroke.
This analysis was repeated for the 12-month NEADL Scale scores for:
-
data with simple imputation for partially completed NEADL Scale scores
-
a simple imputation set adjusted for centre, baseline OHS, age and sex
-
a sensitivity analysis of above, excluding data from patients outside –1 and +3 months of their expected visit date and those whose NEADL scores appeared to be unlikely as they had increased (improved) post stroke.
In terms of secondary outcomes, ordinal regression was used to analyse the 12- and 24-month OHS scores by randomisation group, using the same covariates as for the primary analysis. This was carried out for categories 0–5, with category 0 set as the reference group. To come closer to a full ITT analysis, patients who had died were included in a second analysis in a separate category, as a score of 6. Odds ratios with 95% CIs were reported for an unadjusted model and one adjusted for centre, baseline OHS, age and sex.
The HADS scores were analysed as the two separate domains of anxiety and depression. Multiple linear regression was used to compare the intervention and the control groups at 12 and 24 months. Mean scores with bootstrapped CIs were reported for data with simple imputation for partially completed HADS scores, and the simple imputation set adjusted for centre, baseline OHS, age and sex. In addition, a post hoc analysis considered those patients scoring ≥ 8 to have cases of anxiety or depression, and logistic regression was used to compare the randomisation groups on this binary variable at 12 and 24 months. Odds ratios with 95% CIs were reported for an unadjusted model and one adjusted for centre, baseline OHS, age and sex. The HADS can be presented as either scores or ‘cases’, with the latter being more meaningful to a clinical audience. During the trial, the Trial Steering Committee (TSC) and the Data Monitoring Committee (DMC) reports had included both methods as they are considered to be of equal importance. This post hoc analysis of ‘cases’ was undertaken owing to an omission from the statistical analysis plan. This was the only post hoc analysis that was undertaken for this report.
Experience of service questions for both patients and carers at 12 and 24 months were reported as a difference in the proportion of patients and carers satisfied or in agreement between the control and the intervention groups with a 95% CI. No adjustment for covariates was undertaken for this scale analysis.
For the CSI, mean scores at 12 and 24 months were compared between carers whose patients were in the control group and those whose patients were in the intervention group, using multiple linear regression and adjusting for the age and sex of the carer, and using the patients’ baseline OHS score as a measure of the patient’s health status.
5. Exploratory analyses
Pre-planned exploratory descriptive analyses examined the association of the severity of activity limitation measured by the baseline NEADL Scale score and the pre-stroke health status measured by the pre-stroke OHS, with the effectiveness of the intervention (24-month NEADL Scale score). These are reported as separate scatterplots of the 24-month NEADL Scale score against the severity variables. A boxplot showing 24-month NEADL Scale scores by pre-stroke OHS category and randomisation group is also reported.
The study protocol included a third pre-planned exploratory analysis, which was to examine the impact of comorbidity on the effectiveness of the intervention. On preparing the detailed statistical analysis plan, it was decided that this would not be conducted because although free-text comorbidity had been recorded for patients, a quantifiable measure of comorbidity had not been included.
In preparing the detailed statistical analysis plan, it was decided to include an additional analysis to examine the effect of time in organised stroke care (time as an inpatient plus the time in ESD) on the effectiveness of the intervention. This is illustrated as a scatterplot of the 24-month NEADL Scale score against the duration of organised stroke care.
All analyses were carried out using Stata® version 15 (StataCorp LP, College Station, TX, USA).
Economic analysis
The economic evaluation consisted of a cost-effectiveness analysis (CEA) and a cost–utility analysis. 55 Methods are described in the health economic evaluation chapter (see Chapter 8).
Parallel process evaluation
Parallel process evaluations of complex interventions being tested by randomised controlled trials are increasingly recommended. 56 They can provide information about unanticipated consequences, reasons for success and how an intervention can be improved, and can identify contextual factors associated with variations in outcome. 57 The study process evaluation consisted of the following components, and the methods are described at the start of each respective chapter:
-
mapping the routine rehabilitation and follow-up services provided for stroke patients in each study centre (see Chapter 7)
-
analysis of the study intervention paperwork completed by ESD staff to understand implementation and delivery of the new service in the different study settings (see Chapter 5)
-
conducting interviews to seek the views and experiences of patients and carers about the rehabilitation services they received (see Chapter 6)
-
conducting interviews to seek the views and experiences of senior members of the ESD teams and community rehabilitation staff about the services provided to the intervention and control groups (see Chapter 6).
Ethics and regulatory issues
The study sponsor was Northumbria Healthcare NHS Foundation Trust. Ethics approval was granted by the National Research Ethics Committee North-East – Newcastle and North Tyneside 1 (reference 12/NE/0217). Local NHS approvals were obtained from all participating NHS organisations. The study was conducted in accordance with the Research Governance Framework for Health and Social Care58 and Good Clinical Practice. 59 Monitoring of study conduct and data collection was performed by regular visits to all participating study centres. The trial was managed by a co-ordinating centre based at Newcastle University, Newcastle upon Tyne, UK. An independent DMC and TSC were in place for the duration of the project.
Amendments made to the study after it commenced
1. Modification of the enrolment process for carers
When the study commenced, carer enrolment was in keeping with the patient enrolment procedures described above. There was a face-to-face approach and written consent to participate in the study was sought. This was followed by a face-to-face recruitment assessment and then a separate self-completion baseline questionnaire. It was soon noted that carer recruitment rates were not as high as had been anticipated and it appeared that this may have been due to the enrolment procedures. The procedures appeared to be proving logistically challenging due to carers and NHS staff not being available at mutually convenient times. The carer enrolment procedure was, therefore, amended to invitation by letter and return of one self-completion baseline questionnaire (as described above).
2. Inclusion of stroke patients with communication difficulties (aphasia)
The study commenced without inclusion of patients with significant communication difficulties. The documentation required to support their involvement (‘easy access’) was developed during the first year of the trial.
3. Widening of options for patient outcome assessments
The original study protocol did not include postal questionnaires when telephone contact had failed or the option to mail reminder questionnaires for non-response. These options were later included.
4. Revision of recruitment target
The sample size of 510 patients included inflation for 25% attrition. This was based on the estimated loss between study randomisation and the 24-month (primary) outcome assessment. Randomisation for this study was at discharge from ESD services, but to maximise recruitment opportunities, patients could be recruited from 4 days prior to hospital discharge until discharge from ESD services. This could lead to a delay of up to several weeks between recruitment and randomisation, and dropout was observed during this time, which had not been anticipated. The protocol was therefore revised to clarify that 510 patients would need to be randomised to a study group and that recruitment would be kept under review, ceasing when it was estimated that this would be achieved.
In total, 573 patients were randomised. This is a larger number of participants than described in the sample size calculation, as during the study we felt that there was a risk that attrition at 24 months could be greater than anticipated and, as a result, the primary analysis would be underpowered. The ethics committee approved a minor amendment to increase the number of participants recruited and randomised.
5. Increasing number of study centres
The original study protocol proposed 12 study centres. The number of centres was increased to maintain the proposed recruitment rate.
Chapter 3 Randomised controlled trial results: patients
Patient recruitment and randomisation
Between 15 November 2012 and 6 July 2015, 674 patients were recruited to the trial from 19 participating study centres. Patients could be invited and consent to take part in the trial from 4 days prior to hospital discharge to discharge from an ESD service. Randomisation to a study group was conducted at discharge from an ESD service. The duration of ESD services ranged from a few days to several months. A total of 101 patients dropped out of the trial during the time between recruitment and randomisation. Reasons for this included the choice of patients [49/101 (49%)] and study centre staff [20/101 (20%)] to discontinue involvement. Full details about reasons for dropout are shown in Appendix 2, Table 32.
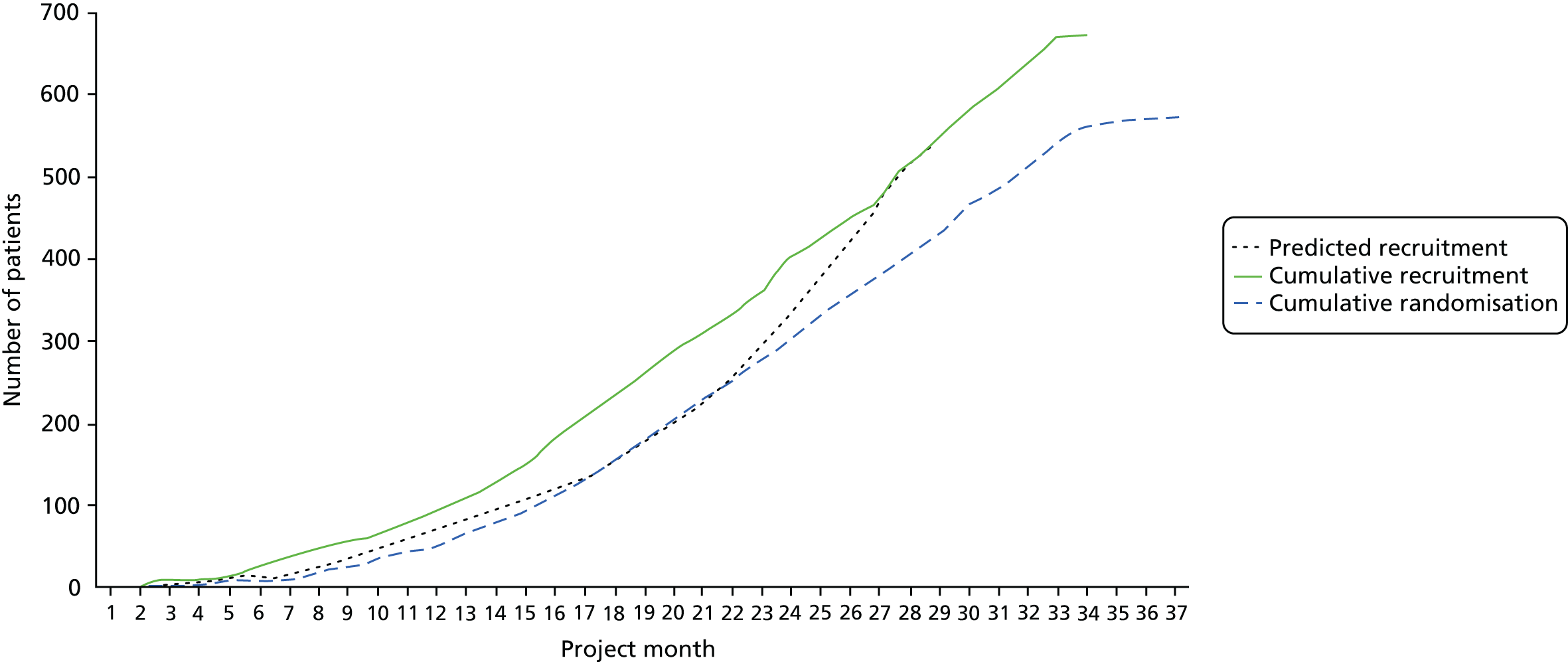
A total of 573 patients were randomised to a study group. Randomisation occurred at a median of 73 days (IQR 48–111.5 days) post stroke in the intervention group and 70 days (IQR 48–106.5 days) post stroke in the control group. Predicted and actual cumulative recruitment and actual cumulative randomisation are shown in Figure 1. Recruitment and randomisation per study centre are illustrated in Appendix 2, Table 33.
FIGURE 1.
Predicted and actual cumulative recruitment, and actual cumulative randomisation.
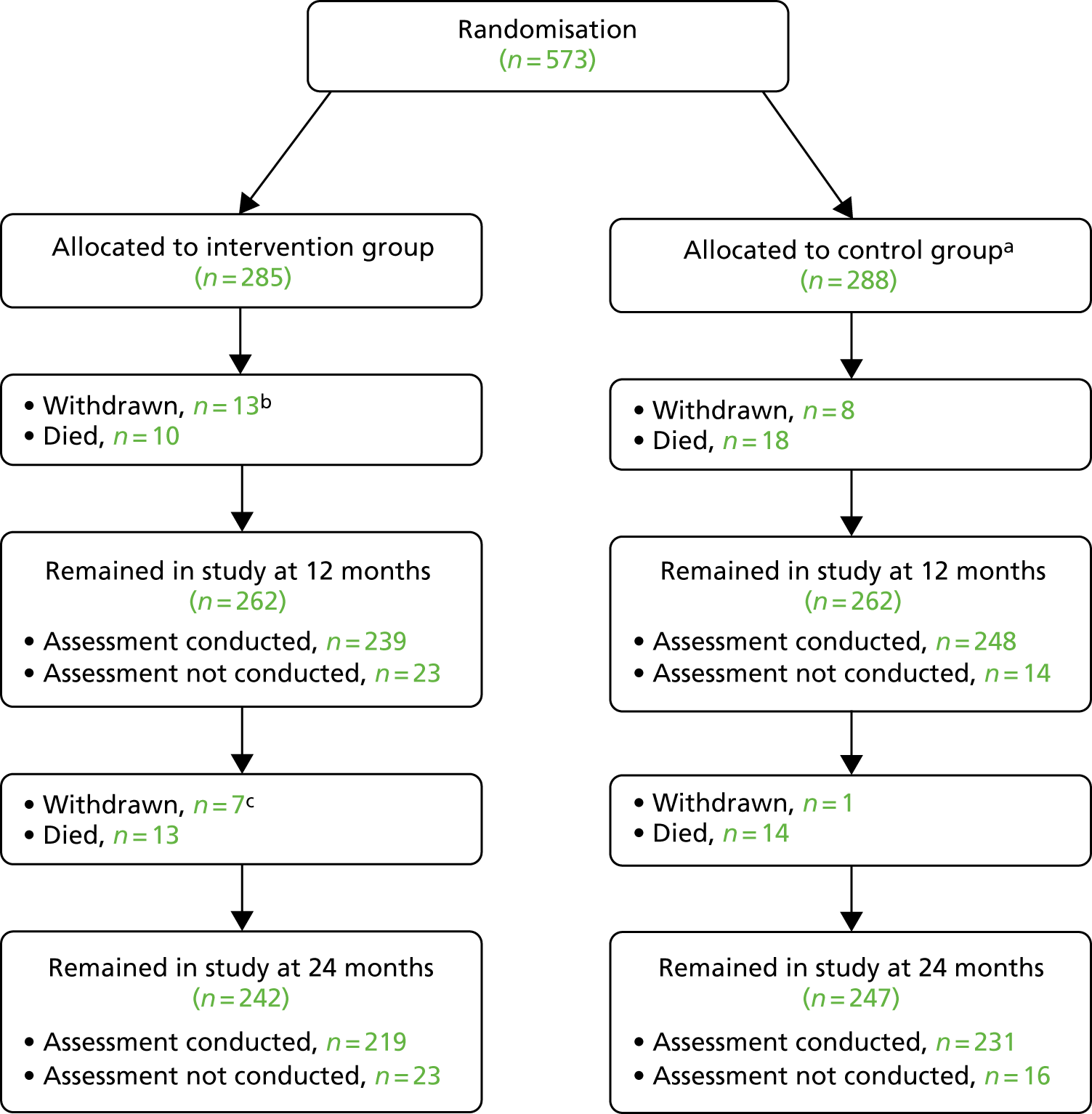
Patient retention and follow-up
Of the 573 randomised patients, 285 were allocated to the intervention group and 288 were allocated to the control group. Patient retention is illustrated in Figure 2. Outcome data were collected for 487 out of 573 (85%) patients at 12 months and 450 out of 573 (78%) patients at 24 months. Further detail about the patients who were alive and did not have follow-up data collected is shown in Appendix 2, Table 34.
FIGURE 2.
Patient retention. a, One patient allocated to control received intervention in error; b, study SAE information indicates that 2 out of 13 patients died after withdrawal and within the 24-month follow-up period; and c, study SAE information indicates that one out of seven patients died after withdrawal and within the 24-month follow-up period.
Timing of patient outcome assessments
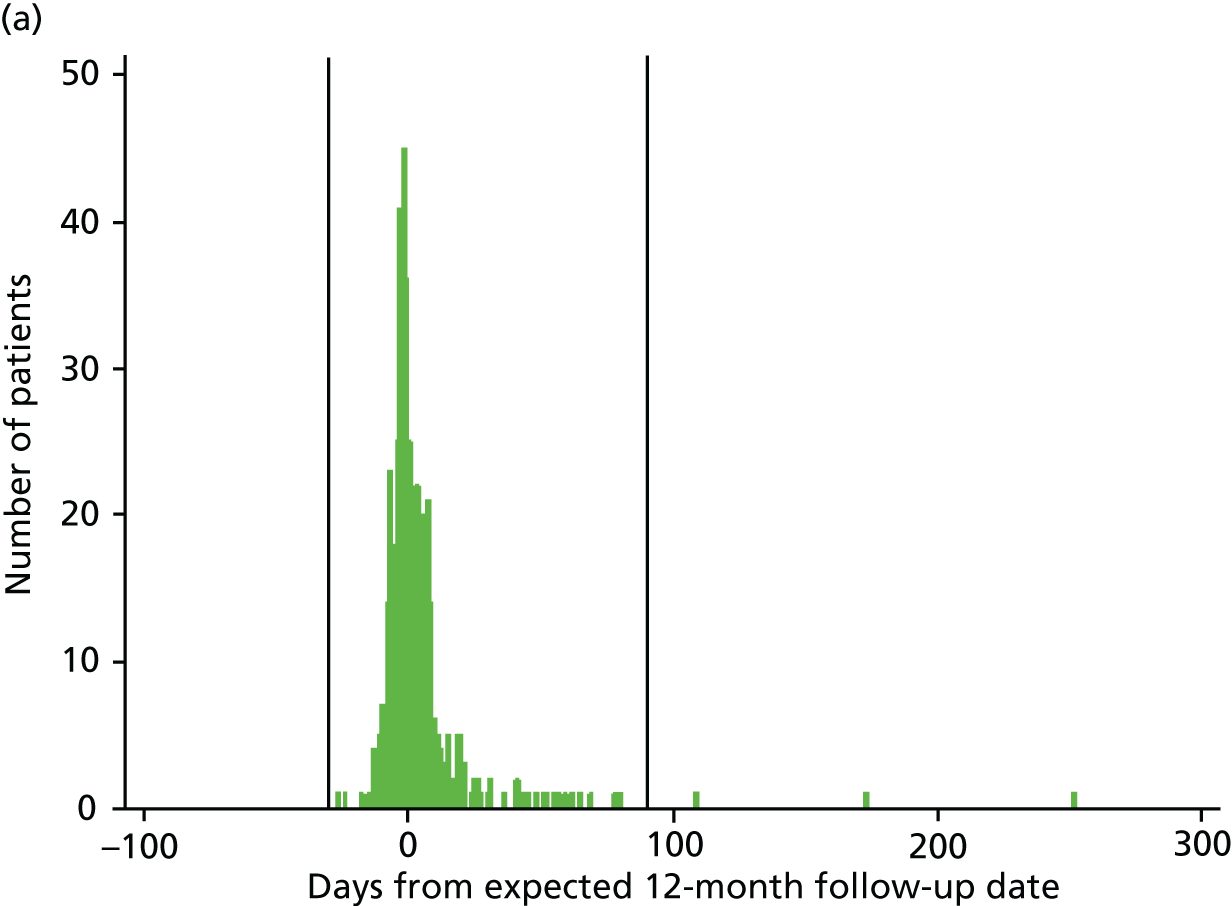
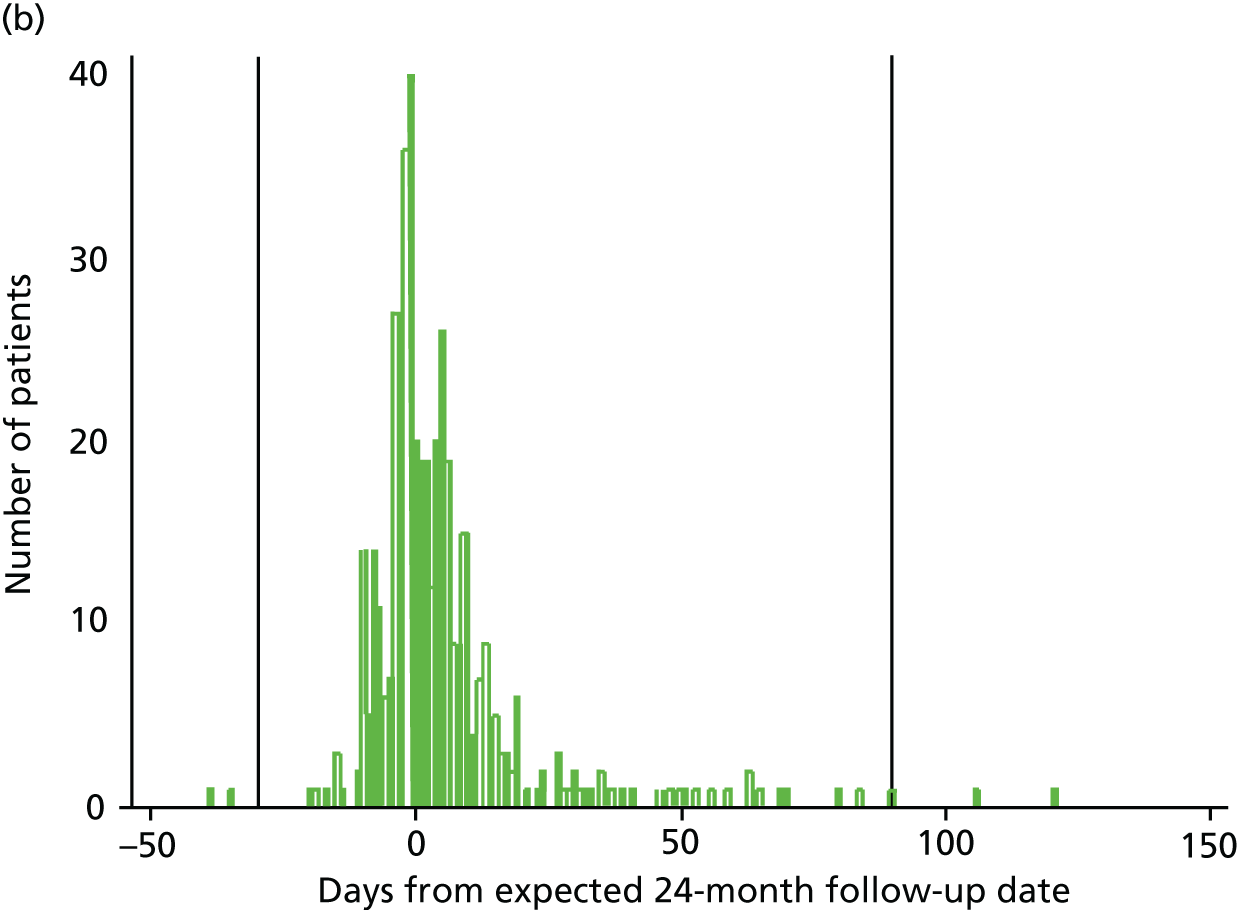
According to the study protocol, assessments were due at 12 and 24 months (± 7 days) post randomisation. At 12 months, 351 out of 487 (72%) assessments took place within this time frame; at 24 months this was 295 out of 450 (66%). The investigators felt that data collected more than 1 month before the assessment was due and 3 months after it was due may not reflect the outcomes that would have been collected at the planned time points. Figures 3a and 3b show how closely data were collected in relation to the 12-month and 24-month due dates, with reference lines indicating the –1/+3 months outside the time window. A sensitivity analysis was conducted excluding people whose data collection fell outside the –1/+3 months.
FIGURE 3.
Time from randomisation to patient outcome assessments. (a) 12-month follow-up date; and (b) 24-month follow-up date.
Patient baseline characteristics
Demographic, stroke and baseline characteristics for the 573 randomised patients are shown in Table 1. The groups were well matched. Most people had suffered a cerebral infarction that was a first stroke. Just over half of the patients were male, with a median age of 71 years at the stroke. The median duration of hospital stay was 14 days and the median duration of ESD services was 43 days, and the time between stroke and randomisation was 72 days. Most people were living in their own home at randomisation.
| Characteristic | Intervention | Control |
|---|---|---|
| Sex, n (%) | n = 285 | n = 288 |
| Male | 174 (61.1) | 168 (58.3) |
| Female | 111 (39.0) | 120 (41.7) |
| Age (years) | n = 285 | n = 288 |
| Median (IQR) | 71 (60–77) | 71 (62–79) |
| Pre-stroke NEADL Scale, mean (SD) | n = 285 | n = 288 |
| Mobility (scored 0–18) | 16.7 (3.1) | 16.1 (3.8) |
| Kitchen (scored 0–15) | 14.7 (1.5) | 14.5 (2.1) |
| Domestic tasks (scored 0–15) | 13.7 (2.8) | 13.5 (3.3) |
| Leisure activities (scored 0–18) | 16.0 (3.1) | 15.6 (3.4) |
| Total (scored 0–66) | 61.0 (8.6) | 59.7 (10.6) |
| Pre-stroke OHS (scored 0–5) score, n (%) | n = 285 | n = 287 |
| 0 | 179 (62.8) | 170 (59.2) |
| 1 | 52 (18.3) | 56 (19.5) |
| 2 | 39 (13.7) | 43 (15.0) |
| 3 | 14 (4.9) | 15 (5.2) |
| 4 | 1 (0.4) | 3 (1.1) |
| 5 | 0 (0.0) | 0 (0.0) |
| Stroke type, n (%) | n = 285 | n = 288 |
| Cerebral infarction | 250 (87.7) | 253 (87.9) |
| Intracerebral haemorrhage | 30 (10.5) | 29 (10.1) |
| Subarachnoid haemorrhage | 5 (1.8) | 5 (1.8) |
| Unknown | 0 (0.0) | 1 (0.4) |
| Stroke subtype, n (%) | n = 284 | n = 286 |
| TACS | 55 (19.4) | 60 (21.0) |
| PACS | 125 (44.0) | 133 (46.5) |
| LACS | 56 (19.7) | 44 (15.4) |
| POCS | 47 (16.6) | 49 (17.1) |
| Uncertain | 1 (0.4) | 0 (0.0) |
| First ever stroke, n (%) | n = 285 | n = 288 |
| Yes | 235 (82.5) | 227 (78.8) |
| NIHSS scorea | n = 285 | n = 288 |
| Median (IQR) | 2 (1–4) | 2 (1–4) |
| Comorbidity, n (%) | n = 285 | n = 288 |
| Ischaemic heart disease | 45 (15.8) | 47 (16.3) |
| Previous myocardial infarction | 25 (8.8) | 29 (10.1) |
| Peripheral arterial occlusive disease | 11 (3.9) | 9 (3.1) |
| Previous transient ischaemic attack | 46 (16.1) | 53 (18.4) |
| Diabetes mellitus | 51 (17.9) | 53 (18.4) |
| Hypertension | 166 (58.3) | 170 (59.0) |
| Hyperlipidaemia | 92 (32.4) | 98 (34.2) |
| Atrial fibrillation | 59 (20.7) | 65 (22.6) |
| Congestive heart failure | 15 (5.3) | 7 (2.4) |
| Thromboembolism | 15 (5.3) | 13 (4.5) |
| Valvular heart disease | 15 (5.3) | 7 (2.4) |
| Other | 205 (71.9) | 199 (69.1) |
| Duration of hospital stay (days) | n = 282 | n = 286 |
| Median (IQR) | 13.5 (6–33) | 14 (6–35) |
| Duration of ESD (days) | n = 283 | n = 285 |
| Median (IQR) | 43 (36–68) | 43 (31–68) |
| Time (days) from stroke to randomisation | n = 284 | n = 288 |
| Median (IQR) | 73 (48–111.5) | 70 (48–106.5) |
| Residence at randomisation, n (%) | n = 283 | n = 283 |
| Own house | 261 (92.2) | 261 (92.2) |
| Living with family/friends | 13 (4.6) | 14 (5.0) |
| Sheltered accommodation | 4 (1.4) | 5 (1.8) |
| Residential care/nursing home | 2 (0.7) | 2 (0.7) |
| Other | 3 (1.1) | 1 (0.4) |
| Lived alone at randomisation, n (%) | n = 283 | n = 284 |
| Yes | 94 (33.2) | 78 (27.5) |
| Total patients | 283 | 284 |
| Abbreviated Mental Test Score (scored 0–10) | n = 281 | n = 285 |
| Median (IQR) | 9.9 (9–10) | 9 (9–10) |
| Sheffield Screening test for acquired language disorders | ||
| Receptive (scored 0–9) | n = 282 | n = 285 |
| Median (IQR) | 8 (7–9) | 8 (7–9) |
| Expressive (scored 0–11) | n = 282 | n = 285 |
| Median (IQR) | 11 (10–11) | 11 (10–11) |
| Total (scored 0–20) | n = 282 | n = 285 |
| Median (IQR) | 19 (18–20) | 19 (18–20) |
| Baseline NEADL Scale | ||
| Mobility (scored 0–18) | n = 283 | n = 285 |
| Mean (SD) | 10.5 (5.5) | 10.3 (5.5) |
| Kitchen (scored 0–15) | n = 283 | n = 286 |
| Mean (SD) | 12.1 (4.0) | 11.8 (4.3) |
| Domestic tasks (scored 0–15) | n = 283 | n = 285 |
| Mean (SD) | 8.0 (5.2) | 7.8 (5.2) |
| Leisure activities (scored 0–18) | n = 281 | n = 284 |
| Mean (SD) | 9.3 (4.2) | 9.3 (4.1) |
| Total (scored 0–66) | n = 281 | n = 282 |
| Mean (SD) | 39.8 (16.1) | 39.1 (16.1) |
| Baseline OHS (scored 0–5) score, n (%) | n = 283 | n = 285 |
| 0 | 9 (3.2) | 8 (2.8) |
| 1 | 42 (14.8) | 43 (15.1) |
| 2 | 104 (36.8) | 95 (33.3) |
| 3 | 100 (35.3) | 99 (34.7) |
| 4 | 18 (6.4) | 34 (11.9) |
| 5 | 10 (3.5) | 6 (2.1) |
| HADS | ||
| Anxiety (scored 0–21) | n = 282 | n = 285 |
| Mean (SD) | 5.7 (4.2) | 5.6 (3.9) |
| Score, n (%) | ||
| 0–7 (non-case) | 203 (72.0) | 201 (70.5) |
| 8–10 (doubtful case) | 39 (13.8) | 50 (17.5) |
| 11–21 (definite case) | 40 (14.2) | 34 (11.9) |
| Depression (scored 0–21) | n = 282 | n = 285 |
| Mean (SD) | 5.4 (3.8) | 5.4 (3.7) |
| Score, n (%) | ||
| 0–7 (non-case) | 215 (76.2) | 211 (74.0) |
| 8–10 (doubtful case) | 40 (14.2) | 42 (14.7) |
| 11–21 (definite case) | 27 (9.6) | 32 (11.2) |
The distributions of performance in EADL (NEADL Scale), health status (OHS) and mood (HADS) at baseline (and pre stroke for NEADL Scale and OHS) were well matched between the intervention and the control groups. The majority of people had a high level of performance in activities of daily living before their stroke and this decreased post stroke.
A comparison of the characteristics of patients who dropped out of the trial between recruitment and randomisation with those of patients who were randomised is shown in Appendix 2, Table 35. Patients who were not randomised tended to be slightly older and have poorer function prior to stroke (pre-stroke NEADL Scale and OHS).
Primary outcome
The distribution of the NEADL Scale scores (primary outcome) at pre stroke, baseline, 12 months and 24 months is shown in Table 2. Patients reported that they undertook the most activities before their stroke, but the mean scores at baseline decreased to 39.9 in the intervention group and 39.1 in the control group. Thereafter, the mean scores remained very similar over time for those patients still providing data in the intervention group and decreased very slightly in the control group.
| Time point | Intervention | Control | Difference in mean score (intervention – control) (95% CI) | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | Unadjusted | Adjusted for centre, baseline OHS, age and sex | |
| Pre stroke | 285 | 61.0 (8.6) | 288 | 59.7 (10.6) | NA | NA |
| Baseline: simple imputation | 281 | 39.8 (16.1) | 282 | 39.1 (16.1) | NA | NA |
| 12 months: simple imputation | 239 | 40.6 (17.7) | 247 | 38.3 (17.0) | 2.3 (–0.5 to 5.2) | 1.5 (–0.8 to 3.7) |
| 12 months: simple imputation and sensitivity | 229 | 40.9 (17.7) | 228 | 38.5 (17.2) | 2.5 (–0.8 to 5.7) | 1.5 (–1.0 to 4.0) |
| 24 months: complete cases | 217 | 39.9 (18.1) | 230 | 37.3 (18.4) | 2.5 (–0.5 to 5.6) | 1.6 (–0.7 to 3.8) |
| 24 months: simple imputation | 219 | 40.0 (18.1) | 231 | 37.2 (18.5) | 2.8 (–0.6 to 6.2) | 1.8 (–0.7 to 4.2) |
| 24 months: complete case and sensitivity | 208 | 40.4 (17.9) | 214 | 37.6 (18.3) | 2.9 (0.0 to 5.7) | 2.0 (–0.9 to 4.9) |
| 24 months: simple imputation and sensitivity | 210 | 40.5 (17.9) | 215 | 37.4 (18.4) | 3.1 (–0.4 to 6.6) | 2.2 (–0.7 to 5.2) |
The primary comparison was at 24 months, adjusting for covariates and using simple imputation for partially completed scores. The mean NEADL Scale scores at 24 months were 40.0 in the intervention group and 37.2 in the control group: an adjusted difference in means of 1.8 (95% CI –0.7 to 4.2). The minimum clinically important difference on the NEADL Scale is 6 units, so the results were not consistent with a meaningful change on this scale.
Other analyses of the NEADL Scale were complete-case data and a sensitivity analysis that excluded both patients providing data outside the –1 and +3 months of the expected assessment date and those with improbable NEADL Scale scores. The differences in means between trial groups remained similar at both time points and for each analysis group.
The distribution of the subscales of the NEADL Scale at each time point is shown in Appendix 2, Table 36.
Patient secondary outcomes
1. Health status (Oxford Handicap Scale)
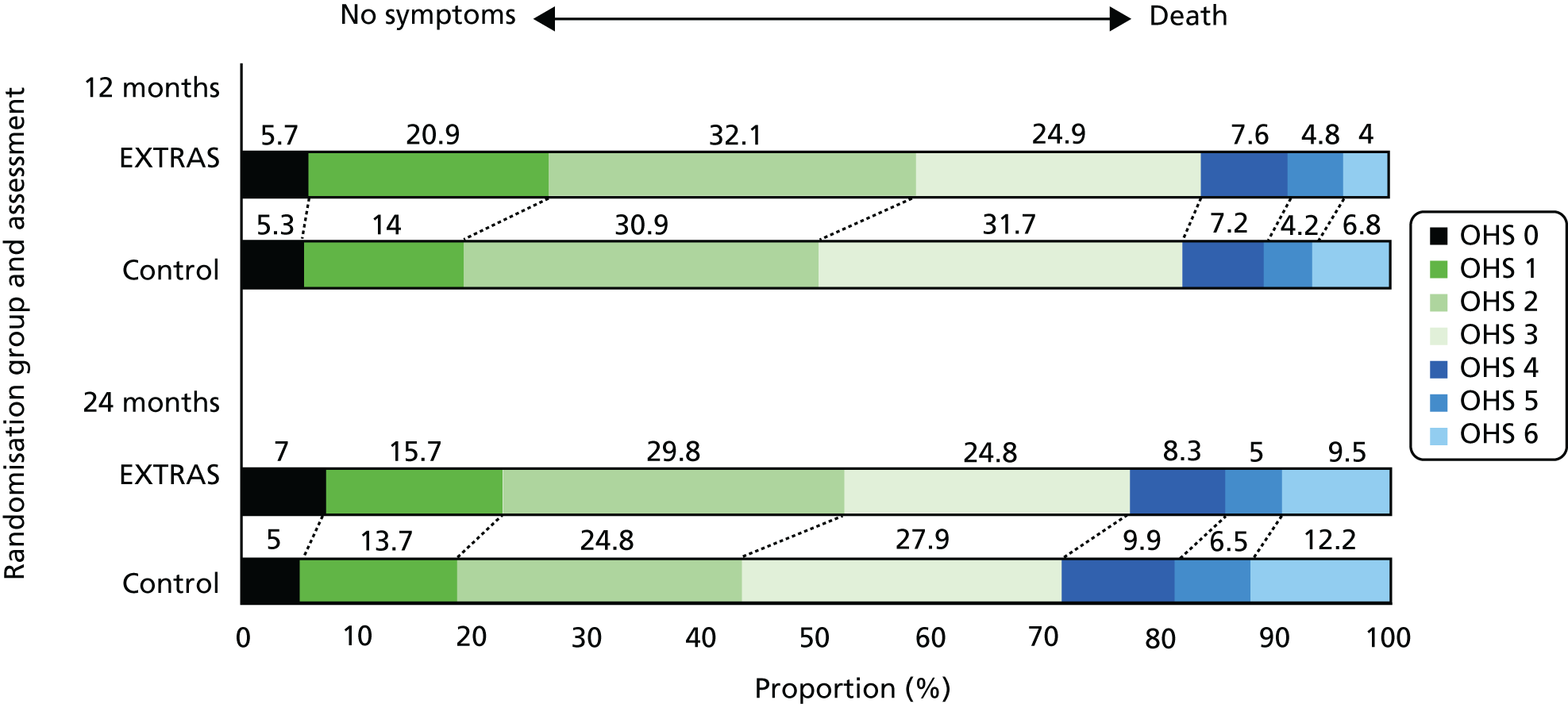
The distribution of the health status score (OHS) at pre stroke, baseline, 12 months and 24 months is shown in Table 3. Relatively few patients reported significant symptoms or disability pre stroke, but by baseline the majority of patients reported symptoms that were affecting their well-being. This distribution changed little over time in those providing data, except for a small number of deaths.
| Oxford Handicap Scale (score) | Time point, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre stroke | Baseline | 12 months | 24 months | |||||
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Total patients, n | 285 | 287 | 283 | 285 | 249 | 265 | 242 | 262 |
| No symptoms (0) | 179 (62.8) | 170 (59.2) | 9 (3.2) | 8 (2.8) | 14 (5.7) | 14 (5.3) | 17 (7.0) | 13 (5.0) |
| Few symptoms (1) | 52 (18.3) | 56 (19.5) | 42 (14.8) | 43 (15.1) | 52 (20.9) | 37 (14.0) | 38 (15.7) | 36 (13.7) |
| Symptoms caused some change (2) | 39 (13.7) | 43 (15.0) | 104 (36.8) | 95 (33.3) | 80 (32.1) | 82 (30.9) | 72 (29.8) | 65 (24.8) |
| Symptoms caused significant change (3) | 14 (4.9) | 15 (5.2) | 100 (35.3) | 99 (34.7) | 62 (24.9) | 84 (31.7) | 60 (24.8) | 73 (27.9) |
| Severe symptoms (4) | 1 (0.4) | 3 (1.1) | 18 (6.4) | 34 (11.9) | 19 (7.6) | 19 (7.2) | 20 (8.3) | 26 (9.9) |
| Major symptoms (5) | 0 | 0 | 10 (3.5) | 6 (2.1) | 12 (4.8) | 11 (4.2) | 12 (5.0) | 17 (6.5) |
| Death (6) | – | – | – | – | 10 (4.0) | 18 (6.8) | 23 (9.5) | 32 (12.2) |
| Intervention: control, OR (95% CI) | Intervention: control, OR (95% CI) | |||||||
| Unadjusted | NA | NA | 0.8 (0.6 to 1.1) | 0.7 (0.5 to 1.0) | ||||
| Unadjusted including death | NA | NA | 0.7 (0.5 to 1.0) | 0.7 (0.5 to 1.0) | ||||
| Adjusteda | NA | NA | 0.8 (0.5 to 1.1) | 0.7 (0.5 to 1.0) | ||||
| Adjusteda including death | NA | NA | 0.7 (0.5 to 1.0) | 0.7 (0.5 to 1.0) | ||||
The OHS scale was compared in two ways: first using the 0–5 scale (i.e. not including death) and second including deaths (i.e. scoring 6 for death). At 24 months, ordinal regression adjusting for covariates and using the 0–6 scale estimated that the odds of the intervention group patients having a worse health status (compared with better) was 0.7 times as high as those for control patients (95% CI 0.5 to 1.0). This was not significantly different from equal odds in both groups. There were very similar results at both time points and for each analysis group. Figure 4 shows the unadjusted distribution of OHS scores at 12 and 24 months.
FIGURE 4.
Distribution of OHS scores at 12 and 24 months.
2. Mood (Hospital Anxiety and Depression Scale)
Tables 4 and 5 show the distribution of the anxiety and depression scores (HADS) at baseline, 12 months and 24 months. Scores at baseline showed relatively low levels of both anxiety and depression, and the mean scores changed very little over time in those who remained in the trial.
| Time point | Intervention | Control | Difference in means (intervention – control) (95% CI) | |||
|---|---|---|---|---|---|---|
| n | Mean score (SD) | n | Mean score (SD) | Unadjusted | Adjusted for centre, baseline, OHS, age and sex | |
| Anxiety at baselinea | 282 | 5.7 (4.2) | 285 | 5.6 (3.9) | NA | NA |
| Anxiety at 12 monthsa | 238 | 5.8 (4.3) | 246 | 6.5 (4.7) | –0.7 (–1.5 to 0.0) | –0.7 (–1.3 to 0.0) |
| Anxiety at 24 monthsa | 217 | 5.5 (4.3) | 230 | 6.4 (4.6) | –0.9 (–1.8 to 0.0) | –0.6 (–1.4 to 0.1) |
| Depression at baselinea | 282 | 5.4 (3.8) | 285 | 5.4 (3.7) | NA | NA |
| Depression at 12 monthsa | 239 | 5.7 (4.3) | 247 | 6.5 (4.2) | –0.8 (–1.6 to 0.0) | –0.7 (–1.5 to 0.0) |
| Depression at 24 monthsa | 217 | 5.9 (4.3) | 230 | 6.7 (4.6) | –0.8 (–1.5 to –0.1) | –0.7 (–1.4 to 0.0) |
| Time point | Intervention | Control | OR (intervention/control) (95% CI)a | |||
|---|---|---|---|---|---|---|
| n | Cases (%)b | n | Cases (%)b | Unadjusted | Adjusted for centre, baseline, OHS, age and sex | |
| Anxiety at baselinea | 282 | 28 | 285 | 29 | NA | NA |
| Anxiety at 12 monthsa | 238 | 34 | 246 | 39 | 0.79 (0.55 to 1.15) | 0.83 (0.55 to 1.25) |
| Anxiety at 24 monthsa | 217 | 28 | 230 | 38 | 0.62 (0.41 to 0.92) | 0.64 (0.41 to 0.99) |
| Depression at baselinea | 282 | 24 | 285 | 26 | NA | NA |
| Depression at 12 monthsa | 239 | 29 | 247 | 40 | 0.61 (0.42 to 0.89) | 0.59 (0.39 to 0.90) |
| Depression at 24 monthsa | 217 | 34 | 230 | 39 | 0.80 (0.55 to 1.18) | 0.81 (0.52 to 1.24) |
The mean scores at 24 months for anxiety were 5.5 in the intervention group and 6.4 in the control group: an adjusted difference in means of –0.6 (95% CI –1.4 to 0.1). The mean scores for depression at 24 months were 5.9 in the intervention group and 6.7 in the control group: an adjusted difference in means of –0.7 (95% CI –1.4 to 0.0). The differences at 12 months were very similar. The differences in means between trial groups were consistently small with narrow CIs.
3. Experience of services (experience survey)
Table 6 shows the dichotomised responses to the experiences of services questionnaire at 12 and 24 months. There were high levels of satisfaction for all aspects of experiences of care. The difference in the percentage of patients who were satisfied between study groups was usually quite small, but the 95% CIs were quite wide. The wider CIs occurred when there was a high proportion of patients either not completing that question or saying that it did not apply. The detailed breakdown of scores is shown in Appendix 2, Tables 37 and 38. These tables show that by 24 months, approximately half of the patients were reporting that each question ‘did not apply’. This means that the comparisons between study groups were based on much reduced sample sizes.
| About the services received in the last 12 months, to what extent do you agree that | 12 months | 24 months | ||||
|---|---|---|---|---|---|---|
| Patients satisfied/in agreement | Difference in proportion of patients satisfied (intervention – control) (95% CI) | Patients satisfied/in agreement | Difference in proportion of patients satisfied (intervention – control) (95% CI) | |||
| Intervention, n (%) | Control, n (%) | Intervention, n (%) | Control, n (%) | |||
| Staff were welcoming and friendly | 230 (99.1) | 230 (97.5) | 1.7 (–0.7 to 4.0) | 114 (98.3) | 123 (96.9) | 1.4 (–2.4 to 5.3) |
| Staff treated you with dignity and respect | 227 (98.3) | 230 (97.1) | 1.2 (–1.5 to 4.0) | 117 (100.0) | 122 (96.8) | 3.2 (0.1 to 6.2) |
| Staff assessed your needs | 219 (95.6) | 229 (96.6) | –1.0 (–4.5 to 2.5) | 113 (96.6) | 114 (92.7) | 3.9 (–1.8 to 9.6) |
| Staff met your needs | 217 (93.9) | 222 (93.7) | 0.3 (–4.1 to 4.6) | 114 (97.4) | 111 (88.8) | 8.6 (2.4 to 14.9) |
| You have been involved as much as you wanted to be in decisions about your care | 211 (91.3) | 213 (91.4) | –0.1 (–5.2 to 5.0) | 110 (94.0) | 108 (89.3) | 4.8 (–2.2 to 11.8) |
| You were able to discuss your preferences, beliefs and concerns as part of your care | 211 (93.0) | 208 (92.4) | 0.5 (–4.3 to 5.3) | 106 (93.0) | 112 (91.8) | 1.2 (–5.6 to 7.9) |
| You were told who to contact if you had any worries or concerns | 220 (94.4) | 218 (93.6) | 0.9 (–3.5 to 5.2) | 110 (94.0) | 116 (92.8) | 1.2 (–5.0 to 7.5) |
| You were confident that the staff you saw had the right skills and knowledge to help you | 223 (97.0) | 223 (94.1) | 2.9 (–0.9 to 6.6) | 116 (97.5) | 119 (94.4) | 3.0 (–1.9 to 7.9) |
| You were treated fairly, regardless of your age, race, sex, belief, sexual orientation or disability | 223 (96.5) | 228 (96.6) | –0.1 (–3.4 to 3.2) | 116 (99.2) | 124 (96.9) | 2.3 (–1.2 to 5.7) |
| You were given the information you wanted | 220 (94.8) | 219 (93.6) | 1.2 (–3.0 to 5.5) | 114 (96.6) | 115 (92.0) | 4.6 (–1.2 to 10.4) |
| You were able to see the same health-care professional/team whenever possible | 211 (92.5) | 203 (87.1) | 5.4 (–0.1 to 10.9) | 103 (92.0) | 107 (87.7) | 4.3 (–3.4 to 12.0) |
| If you had important questions to ask, you got answers that you could understand | 212 (93.0) | 212 (95.5) | –2.5 (–6.8 to 1.8) | 107 (93.0) | 113 (92.6) | 0.4 (–6.1 to 7.0) |
| If you needed more than one service, staff made sure they were well co-ordinated | 196 (91.2) | 189 (88.7) | 2.4 (–3.3 to 8.1) | 91 (91.9) | 95 (91.4) | 0.6 (–7.0 to 8.2) |
| If you needed more than one service, staff made sure that your care information was clearly and accurately shared | 197 (92.1) | 192 (89.7) | 2.3 (–3.1 to 7.8) | 91 (91.0) | 94 (90.4) | 0.6 (–7.4 to 8.6) |
| You were told who to contact if you had any ongoing health-care needs | 217 (93.1) | 215 (91.5) | 1.6 (–3.2 to 6.5) | 110 (94.8) | 115 (92.7) | 2.1 (–4.0 to 8.2) |
| Overall, how satisfied are you with the services you received? | 228 (95.4) | 227 (92.3) | 3.1 (–1.1 to 7.4) | 208 (97.7) | 189 (87.5) | 10.2 (5.3 to 15.0) |
| In the last 12 months, have you had enough help with speaking difficulties from the NHS? | 79 (87.8) | 89 (84.8) | 3.0 (–6.6 to 12.7) | 29 (69.1) | 35 (63.6) | 5.4 (–13.5 to 24.3) |
| In the last 12 months, have you had enough treatment to help improve your mobility from the NHS? | 167 (82.7) | 166 (76.2) | 6.5 (–1.2 to 14.2) | 95 (67.4) | 81 (48.5) | 18.9 (8.0 to 29.7) |
| In the last 12 months, have you had enough help with emotional problems from the NHS? | 80 (70.8) | 112 (76.7) | –5.9 (–16.7 to 4.9) | 52 (71.2) | 59 (62.1) | 9.1 (–5.1 to 23.4) |
Nevertheless, there were 4 out of 19 aspects of care at 24 months where the 95% CI for the differences in percentage satisfied between groups did not cover the value zero. These were ‘staff treated you with dignity and respect’, ‘staff met your needs’, ‘overall satisfaction’ and ‘help with mobility’. However, these must be interpreted with some caution because of multiple significance testing. In addition, the largest difference at 24 months came from the question about overall satisfaction (‘Overall, how satisfied are you with the services you received?’): 97.7% in the intervention group compared with 87.5% in the control group, which is a difference of 10.2% (95% CI 5.3% to 15.0%).
Patient post hoc analysis
Post hoc analyses of the HADS used established ‘cut-off’ scores where patients scoring ≥ 8 on a subscale are considered to have a case of anxiety or depression (see Tables 4 and 6). For anxiety, the proportion of cases was slightly lower in the intervention group (34%) than in the control group (39%) at 12 months, with an adjusted OR of 0.83 (95% CI 0.55 to 1.25). This proportion was lower again by 24 months with 28% of the intervention group versus 38% of the control group considered to be cases, with an adjusted OR of 0.64 (95% CI 0.41 to 0.99). For depression, the proportion of cases was lower in the intervention group (29%) than the control group (40%) at 12 months, with an adjusted OR of 0.59 (95% CI 0.39 to 0.90), but only slightly lower by 24 months, with 34% in the intervention group versus 39% in the control group adjusted OR 0.64, (95% CI 0.52 to 1.24).
Patient exploratory analyses
A set of prespecified exploratory analyses investigated whether or not there was an association between the effectiveness of EXTRAS and pre-stroke health status (OHS), and baseline performance in activities of daily living (NEADL Scale) and time in organised stroke care (inpatient and ESD).
Figure 5 shows the NEADL Scale scores at 24 months plotted against baseline NEADL Scale scores with different symbols for study groups. Although this shows, not surprisingly, that those who had higher NEADL Scale scores at baseline tended to have higher scores at 24 months, there does not seem to be an indication that the relationship differs between study groups.
FIGURE 5.
Association between severity of activity limitation and effectiveness of EXTRAS.
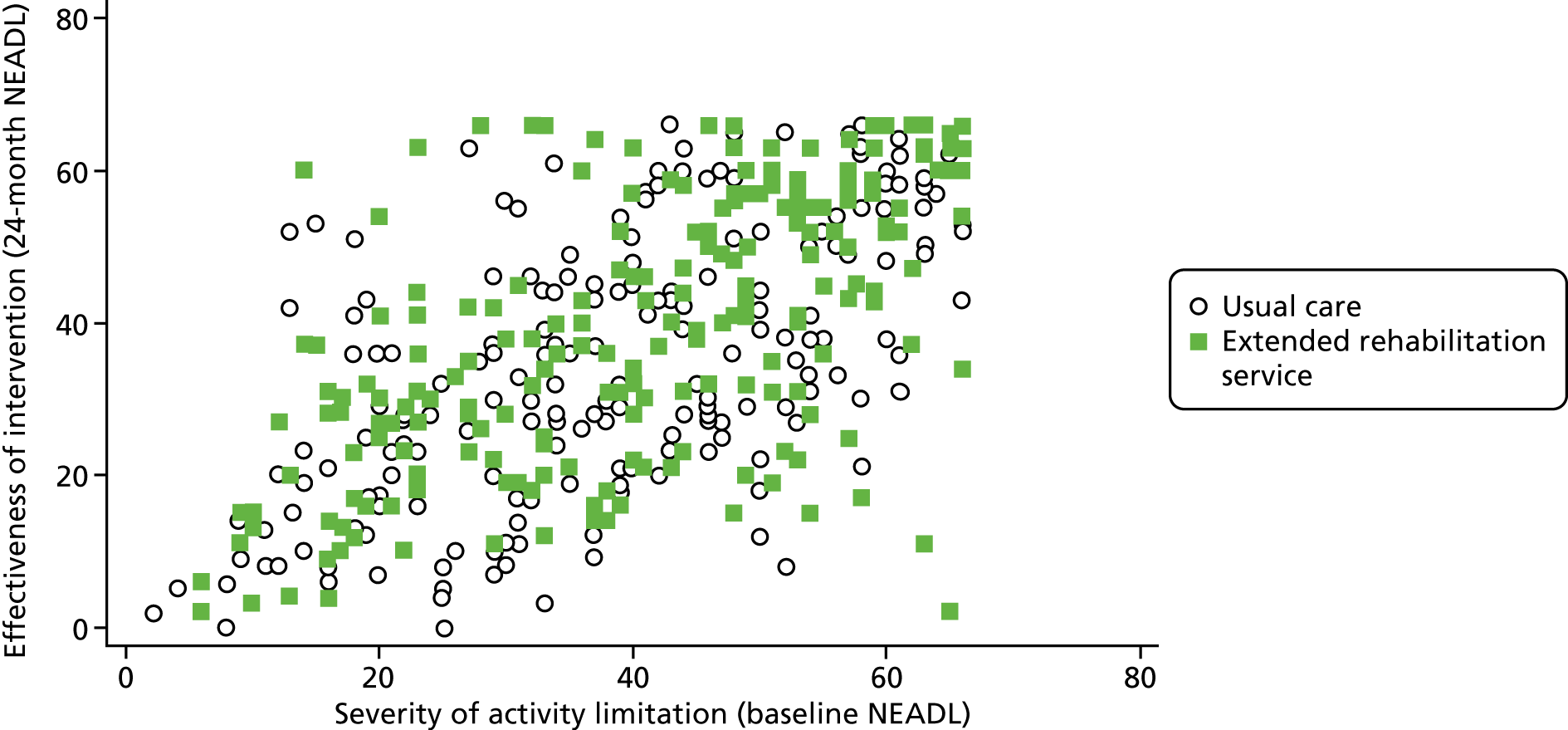
Figure 6 shows the NEADL Scale scores at 24 months plotted against pre-stroke health status (OHS) as pairs of boxplots for intervention and control groups. Those patients with better health status pre stroke tended to have higher NEADL Scale scores at 24 months, but there was little indication that the relationship differed between study groups.
FIGURE 6.
Association between pre-stroke health status and effectiveness of EXTRAS.
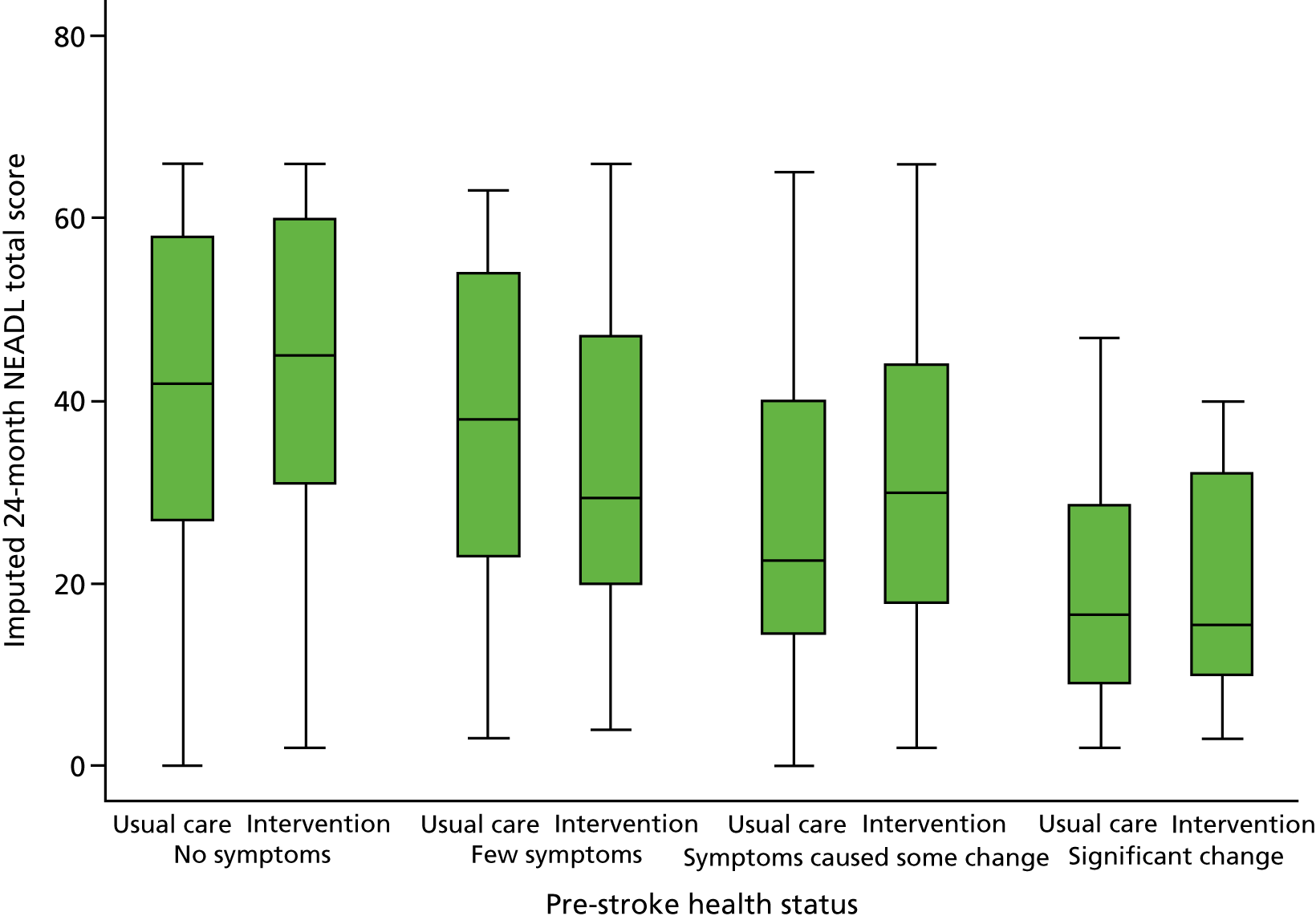
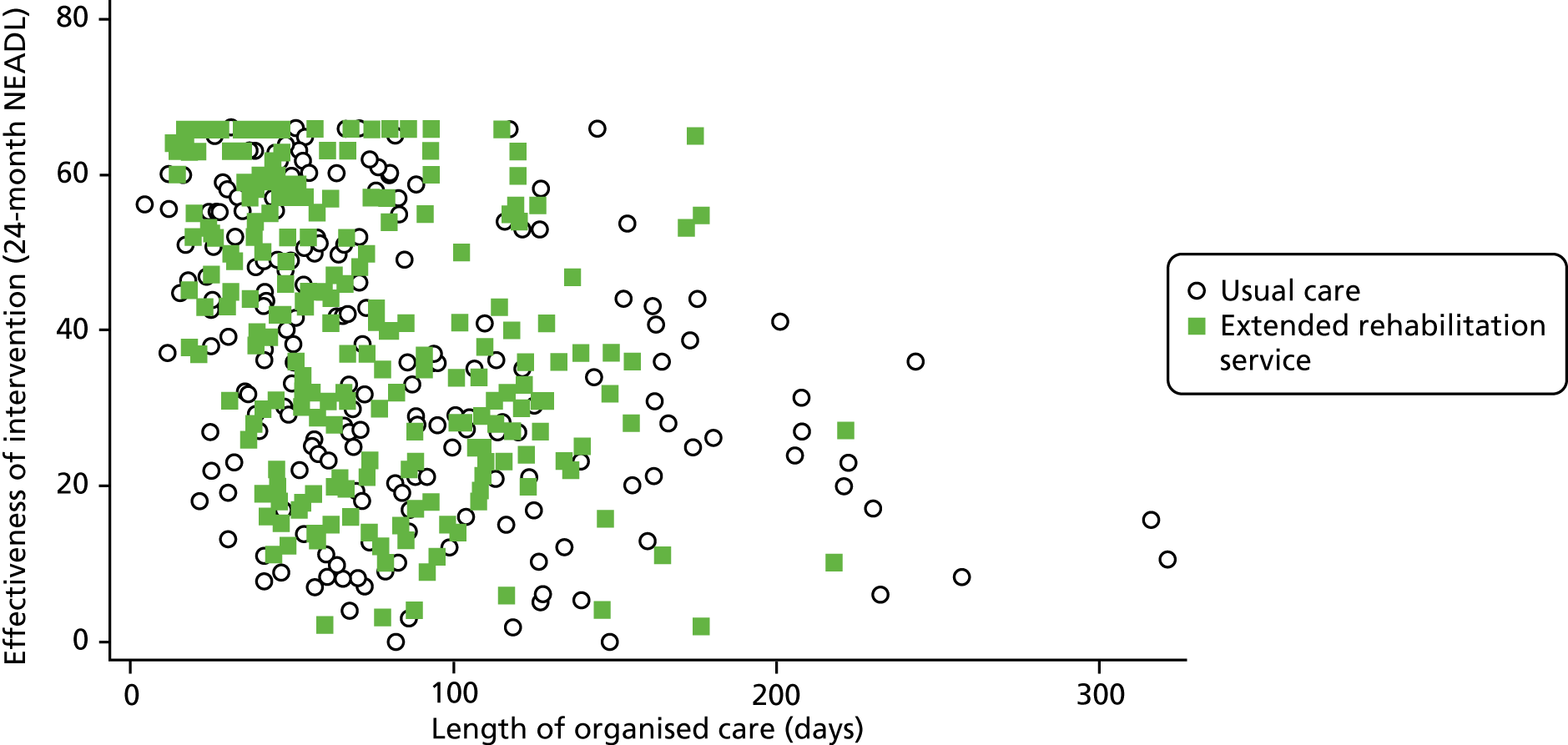
Figure 7 shows the NEADL Scale scores at 24 months plotted against length of organised stroke care with different symbols for study groups. This shows, not surprisingly, that those who had experienced longer organised stroke care tended to have lower (worse) NEADL Scale scores at 24 months, but there does not seem to be an indication that the relationship differs between study groups.
FIGURE 7.
Association between length of time of organised care and effectiveness of EXTRAS.
Patient outcome assessment blinding
Outcome assessments were conducted by telephone interview [12 months: n = 408/487 (84%); 24 months: n = 371/450 (82%)], face-to-face assessment [12 months: n = 8/487 (2%); 24 months: n = 10/450 (2%)] or postal questionnaire [12 months: n = 71/487 (15%); 24 months: n = 69/450 (15%)]. For assessments conducted by telephone and face to face, it was intended that the researcher was blinded to study group. At 12 months, 371 out of 416 (89%) assessments undertaken by phone or face to face were conducted blinded to study group and at 24 months this was 363 out of 450 (81%). Data are shown in Appendix 2, Table 39.
Patient safety data
1. Serious adverse events
Serious adverse events were reported for 125 out of 285 (43.9%) patients in the intervention group (total 250 events) and 130 out of 288 (45.1%) patients in the control group (total 254 events). The number of events per patient is shown in Table 7. No significant differences were seen between the study groups.
| Number of SAEs per patient | Number of patients, n (%) | Difference in proportion (95% CI) | |
|---|---|---|---|
| Intervention (N = 285) | Control (N = 288) | ||
| 0 | 160 (56.1) | 158 (54.9) | 1.3 (–6.9 to 9.4) |
| 1 | 70 (24.6) | 69 (24.0) | 0.6 (–6.4 to 7.6) |
| 2 | 30 (10.5) | 31 (10.8) | –0.2 (–5.3 to 4.8) |
| 3 | 11 (3.9) | 17 (5.9) | –2.0 (–5.6 to 4.8) |
| 4 | 4 (1.4) | 3 (1.0) | 0.3 (–1.4 to 2.2) |
| 5 | 3 (1.1) | 3 (1.0) | For > 4 events: 0.0 (–2.9 to 3.0) |
| 6 | 2 (0.7) | 4 (1.4) | |
| 7 | 1 (0.4) | 3 (1.0) | |
| 8 | 2 (0.7) | 0 (0.0) | |
| 9 | 1 (0.4) | 0 (0.0) | |
| 12 | 1 (0.4) | 0 (0.0) | |
| Total events | 250 | 254 | |
The reasons for the reported events being considered to be SAEs are shown in Appendix 2, Table 40. The most common reason for an event to be reported as a SAE was because it resulted in hospitalisation (intervention group, n = 163; control group, n = 168). A total of 58 reported events resulted in death (intervention group, n = 26; control group, n = 32). Note that in the retention flow diagram (see Figure 2), 23 participants in the intervention group and 32 participants in the control group are reported to be deceased. The SAEs in the control group (n = 32) match the control group deaths (n = 32). In the intervention group, the cause of death for two participants could not be established and no SAE form was received. However, for a further five participants, a SAE was initially reported during their involvement in the study but the date of death from this event was after their involvement ceased. For two out of five participants, the date of death was after the 24-month outcome assessment; for three out of five participants, the date of death was after withdrawal but within the study period.
A summary of the events that resulted in death is shown in Appendix 2, Table 41. A summary of all other events is shown in Appendix 2, Table 42.
During SAE reporting, a standard causality assessment was undertaken for all SAEs and none were reported to be related to the study intervention.
As safety monitoring started from participant consent and this could be several weeks before randomisation to a study group, some patients had SAEs reported before randomisation (16 patients, 19 events). These data are shown in Appendix 2, Tables 43–45. In addition, some patients who were recruited but dropped out of the study before randomisation had SAEs reported before their involvement in the study ended (14 patients, 22 events). These data are shown in Appendix 2, Tables 46–48.
2. Non-serious adverse events
At each outcome assessment, patients were asked to self-report adverse events by means of the following question: ‘Have you suffered any new medical illnesses in the last 12 months?’. Any events potentially fulfilling the criteria to be a SAE were investigated and reported as SAEs, as appropriate. Such events are included in Serious adverse events. All other events are reported here.
At 12 months, 66 out of 239 (27.6%) participants in the intervention group who had the assessment conducted reported at least one non-serious AE. For the control group, this was 57 out of 248 (22.9%) participants. The difference in proportions was 4.6% (95% CI –3.1% to 12.3%).
At 24 months, 53 out of 219 (24.2%) participants in the intervention group who had the assessment conducted reported at least one non-serious AE. For the control group, this was 50 out of 231 (21.6%) participants. The difference in proportions was 2.6% (95% CI –5.2% to 10.3%).
The number of events per participant is shown in Appendix 2, Table 49, and events are summarised in Appendix 2, Table 50.
3. Injurious falls
Participants were asked to self-report injurious falls at outcome assessments by means of the following question: ‘Have you suffered any falls resulting in injury in the last 12 months?’. To avoid undercounting falls where an outcome assessment had not been conducted, SAE data were also examined. Participants were recorded as suffering from ‘at least one’ injurious fall if they answered ‘yes’ to the self-report question at either 12 or 24 months or if any SAE reports indicated that an injurious fall had occurred. Where a SAE report was unclear about an injurious fall (e.g. the report documented a fractured hip but did not state whether or not a fall had occurred), adjudication took place to determine whether or not the event should be considered an injurious fall.
There were slightly more patients in the intervention group [82/285 (29%)] than in the control group [74/288 (26%)] who suffered at least one injurious fall, but this difference was not significant: difference in proportions 3.1 (95% CI –4.2 to 10.4).
Chapter 4 Randomised controlled trial results: carers
Carer enrolment
In the early months of the study, carer enrolment included a face-to-face consent process and a carer recruitment assessment that was conducted at a similar time to the corresponding patient recruitment assessment. Recruited carers were subsequently asked to self-complete a baseline questionnaire at the time of the patient baseline assessment and randomisation. Owing to difficulties with identifying and contacting carers to conduct the face-to-face consent and recruitment assessment, carer enrolment was later modified to invitation by letter, with return of a self-completion baseline questionnaire indicating consent to participate. The time point for this revised invitation was the patient baseline assessment and randomisation (further details are given in Chapter 2).
Recruitment and/or baseline data were returned for 200 carers. Return per study centre is shown in Appendix 2, Table 51. When the enrolment process for carers was modified (January 2014), staff collecting patient baseline data were asked to record whether or not a carer had been identified and study invitation paperwork had been issued. These data were not collected before this time and are therefore not available for all patients. However, when these data were collected, staff reported inviting carers for 301 out of 440 patients (68%). For 80 out of 139 patients (58%) where a carer was not reported to be invited, the reason provided was that no carer was identified. A full summary of reasons provided for not inviting a carer to participate is shown in Appendix 2, Table 52.
Carer retention
Carer retention is illustrated in Figure 8. Where a recruited patient did not subsequently undergo randomisation into the trial, the corresponding carer data collection also ceased. Of 200 enrolled carers, six were associated with patients who were not randomised, resulting in 194 carers taking part in the trial. Thirty-four percent of patients, therefore, had a carer who participated. Of the 194 participating carers, 103 were associated with patients allocated to the intervention group and 91 were associated, with patients allocated to the control group. Follow-up data were available for 166 out of 194 (86%) carers at 12 months and 153 out of 194 (79%) carers at 24 months.
FIGURE 8.
Carer retention.

Carer baseline characteristics
The baseline characteristics of carers are shown in Table 8. Study groups were well matched. There were more female carers, with the majority being retired, the spouse/partner of the patient and co-resident.
| Characteristic | Patients allocated to intervention | Patients allocated to control |
|---|---|---|
| Sex, n (%) | n = 102 | n = 89 |
| Male | 30 (29.4) | 24 (26.9) |
| Female | 72 (70.6) | 65 (73.0) |
| Age (years) | n = 100 | n = 82 |
| Median (IQR) | 66.5 (53.5–72.5) | 67 (58–74) |
| Employment, n (%) | n = 98 | n = 84 |
| Retired | 63 (64.3) | 59 (70.2) |
| Working full time | 16 (16.3) | 12 (14.3) |
| Working part time | 19 (19.4) | 13 (15.5) |
| Relationship to patient, n (%) | n = 102 | n = 87 |
| Spouse/partner | 86 (84.3) | 76 (87.4) |
| Son/daughter (in-law) | 11 (10.8) | 7 (8.0) |
| Brother/sister | 1 (1.0) | 2 (2.3) |
| Other | 4 (3.9) | 2 (2.3) |
| Currently living with patient, n (%) | n = 102 | n = 87 |
| Yes | 91 (89.2) | 82 (94.3) |
| No | 11 (10.8) | 5 (5.8) |
| If ‘yes’ | ||
| Lived with patient before stroke | 87 (95.6) | 79 (98.8) |
| Patient moved in with carer after stroke | 2 (2.2) | 1 (1.3) |
| Carer moved in with patient after stroke | 2 (2.2) | 0 (0.0) |
| If ‘no’ | ||
| Time to travel to patient’s home, median (IQR) | 7.5 (4–15.5) | 15 (6–30) |
| CSI (scored 0–13)a | n = 99 | n = 83 |
| Mean (SD) | 4.9 (3.6) | 5.3 (4.0) |
| Time (days) after patient randomisation that carer baseline questionnaire was completed | n = 96 | n = 80 |
| Median (IQR) | 2 (0–6) | 1 (0–3) |
Carer outcomes
1. Caregiver strain
Table 9 shows the distribution of the CSI at baseline, 12 months and 24 months. At baseline, carers typically reported low levels of strain, and there was little change over time for those who continued to provide data. There was little difference in mean scores between study groups at any time point. The mean scores at 24 months were 4.2 (SD 3.9) in the intervention group and 5.0 (SD 3.9) in the control group: an adjusted difference in means of –1.0 (95% CI –2.3 to 0.2).
| Time point | Intervention (n) | Mean (SD) | Control (n) | Mean (SD) | Adjusteda mean difference (intervention – control) (95% CI) |
|---|---|---|---|---|---|
| Baselineb | 99 | 4.9 (3.6) | 83 | 5.3 (4.0) | NA |
| 12 monthsb | 92 | 4.8 (3.8) | 74 | 5.3 (4.0) | –0.7 (–1.9 to 0.5) |
| 24 monthsb | 80 | 4.2 (3.9) | 67 | 5.0 (3.9) | –1.0 (–2.3 to 0.2) |
2. Experience of services
Table 10 shows the dichotomised responses to the experiences of services questionnaire for carers at 12 and 24 months. There were high levels of satisfaction for all aspects of experiences of care. The differences in the percentage of carers who were satisfied between study groups were usually quite small, but the 95% CIs were quite wide. The wider CIs occurred when there was a high proportion of carers either not completing that question or saying that it did not apply. The detailed breakdown of scores is shown in Appendix 2, Table 53. This table shows that by 24 months, approximately half of the carers were reporting that each question ‘did not apply’.
| About the services, to what extent do you agree that | 12 months | 24 months | ||||
|---|---|---|---|---|---|---|
| Carers satisfied/in agreement | Difference in proportion of carers satisfied (intervention – control) (95% CI) | Carers satisfied/in agreement | Difference in proportion of carers satisfied (intervention – control) (95% CI) | |||
| Intervention,a n (%) | Control,a n (%) | Intervention,a n (%) | Control,a n (%) | |||
| Staff were welcoming and friendly | 83 (100) | 69 (100) | 0.0 | 52 (98.1) | 34 (97.1) | 1.0 (–5.7 to 7.6) |
| Staff treated your relative/friend with dignity and respect | 83 (98.8) | 68 (98.7) | 0.3 (–3.4 to 3.9) | 52 (98.1) | 35 (100.0) | –1.9 (–5.6 to 1.8) |
| Staff assessed your relative’s/friend’s needs | 81 (96.4) | 68 (98.6) | –2.1 (–7.0 to 2.7) | 48 (94.1) | 32 (91.4) | 2.7 (–8.6 to 14.0) |
| Staff met your relative’s/friend’s needs | 77 (92.8) | 66 (97.1) | –4.3 (–11.2 to 2.6) | 47 (90.4) | 28 (80.0) | 10.4 (–5.1 to 25.9) |
| You have been involved as much as you wanted to be in decisions about their care | 76 (92.7) | 64 (95.5) | –2.8 (–10.3 to 4.7) | 47 (92.2) | 32 (88.9) | 3.3 (–9.4 to 15.9) |
| You were able to discuss your preferences, beliefs and concerns as part of their care | 71 (88.8) | 58 (89.2) | –0.5 (–10.7 to 9.8) | 46 (95.8) | 31 (91.2) | 4.7 (–6.4 to 15.7) |
| You were told who to contact if you had any worries or concerns | 75 (88.2) | 65 (92.9) | –4.6 (–13.7 to 4.5) | 52 (94.6) | 29 (80.6) | 14.0 (–0.3 to 28.2) |
| You were confident that the staff your relative/friend saw had the right skills and knowledge to help them | 81 (96.4) | 67 (97.1) | –0.7 (–6.3 to 4.9) | 52 (94.6) | 34 (100.0) | –5.5 (–11.5 to 0.5) |
| Your relative/friend was treated fairly, regardless of their age, race, sex, belief, sexual orientation or disability | 84 (97.7) | 71 (100.0) | –2.3 (–5.5 to 0.9) | 56 (96.6) | 35 (94.6) | 2.0 (–6.7 to 10.6) |
| You were given the information you wanted | 77 (92.8) | 64 (92.8) | 0.02 (–8.3 to 8.3) | 50 (92.6) | 31 (91.2) | 1.4 (–10.4 to 13.2) |
| Your relative/friend was able to see the same health-care professional/team whenever possible | 73 (89.0) | 58 (86.6) | 2.5 (–8.2 to 13.1) | 50 (94.3) | 29 (85.3) | 9.0 (–4.4 to 22.5) |
| If you had important questions to ask, you got answers that you could understand | 77 (95.1) | 61 (91.0) | 4.0 (–4.3 to 12.3) | 47 (95.9) | 32 (97.0) | –1.1 (–9.1 to 7.0) |
| If your relative/friend needed more than one service, staff made sure they were well co-ordinated | 70 (88.6) | 51 (85.0) | 3.6 (–7.8 to 15.0) | 41 (93.2) | 25 (89.3) | 3.9 (–9.8 to 17.6) |
| If your friend/relative needed more than one service, staff made sure that their care information was clearly and accurately shared | 72 (92.3) | 54 (88.5) | 3.8 (–6.2 to 13.7) | 39 (90.7) | 25 (89.3) | 1.4 (–13.0 to 15.8) |
| You were told who to contact if your relative/friend had any ongoing health-care needs | 74 (88.1) | 62 (91.2) | –3.1 (–12.7 to 6.6) | 45 (88.2) | 31 (88.6) | –0.3 (–14.1 to 13.4) |
| Overall, how satisfied are you with the services your friend/relative received? | 84 (92.3) | 65 (91.6) | 0.8 (–7.7 to 9.2) | 68 (94.4) | 40 (78.4) | 16.0 (3.6 to 28.5) |
| In the last 12 months, has your relative/friend had enough help with speaking difficulties from the NHS? | 23 (74.2) | 21 (80.8) | –6.6 (–28.2 to 15.0) | 16 (72.7) | 12 (70.6) | 2.1 (–26.4 to 30.7) |
| In the last 12 months, has your relative/friend had enough treatment to help improve their mobility from the NHS? | 65 (83.3) | 43 (69.4) | 14.0 (–0.2 to 28.1) | 39 (75.0) | 18 (40.0) | 35.0 (16.5 to 53.5) |
| In the last 12 months, has your relative/friend had enough help with emotional problems from the NHS? | 46 (79.3) | 30 (68.2) | 11.1 (–6.1 to 28.4) | 24 (66.7) | 22 (61.1) | 5.6 (–16.6 to 27.7) |
Nevertheless, there were 2 out of 19 aspects of care at 24 months where the 95% CI for the differences in percentage satisfied between groups did not cover the value zero. The ‘overall satisfaction’ at 24 months (question ‘Overall, how satisfied are you with the services your friend/relative received?’) was 94.4% in the intervention group compared with 78.4% in the control group, a difference of 16.0% (95% CI 3.6% to 28.5%). The satisfaction with ‘help with mobility’ was 75.0% in the intervention group compared with 40.0% in the control group, a difference of 35% (95% CI 16.5% to 53.5%).
Chapter 5 Delivery and implementation of EXTRAS
Staff delivering EXTRAS reviews completed study-specific paperwork and data were uploaded onto the study database. These data are reported in this chapter.
The EXTRAS paperwork recorded the content of each review, including the method of conduct (telephone or face to face), rehabilitation issue identification, goals or action points set and action plans made. A record of why a review was not undertaken was documented if relevant. Data that concerned the rehabilitation issues identified, goals/action points set and action plans made were free text. To analyse these data, the free text for each rehabilitation issue and goal/action point was examined and coded to map onto the rehabilitation areas, which were prespecified for discussion in the semistructured interview (e.g. mobility issue, personal care goal). Free text for action plans was also examined and coded. Full details of this analysis are given in Action plans.
EXTRAS reviews also included the assessment of goal and action point achievement, and the details of these data and analysis are provided in Goal and action point achievement.
EXTRAS reviews conducted
It was intended that each patient in the intervention group would participate in an EXTRAS review at five time points during their involvement in the trial. These reviews were scheduled for 1, 3, 6, 12 and 18 months following randomisation (which occurred at discharge from ESD). However, because of death or withdrawal from the study, the expected number of reviews decreased for some participants. Table 11 illustrates the number of reviews expected to be conducted at each time point, the number actually conducted and the mode of the reviews. Overall, 86% (1155/1338) of expected EXTRAS reviews were undertaken. Per participant, the median number of EXTRAS reviews received was five (full details of expected and undertaken reviews per participant are found in Appendix 2, Table 54).
| Review stage (month) | Reviews expected to be conducted,a n | Reviews conducted, n (% of expected) | Mode of review, n (%) |
|---|---|---|---|
| 1 | 282 | 258 (91) | Telephone: 201 (78) |
| Face to face: 47 (18) | |||
| Missing: 10 (4) | |||
| 3 | 276 | 245 (89) | Telephone: 191 (78) |
| Face to face: 45 (18) | |||
| Missing: 9 (4) | |||
| 6 | 269 | 231 (86) | Telephone: 185 (80) |
| Face to face: 40 (17) | |||
| Missing: 6 (3) | |||
| 12 | 262 | 211 (81) | Telephone: 167 (79) |
| Face to face: 43 (20) | |||
| Missing: 1 (< 1) | |||
| 18 | 249 | 210 (84) | Telephone: 170 (81) |
| Face to face: 40 (19) |
In addition to study withdrawal and death, reasons were recorded for other lack of review conduct. The most common reason for a review not being conducted was inability to contact the patient, in that they did not answer the telephone when the reviewer called. A summary of the reasons for all non-conducted reviews is shown in Appendix 2, Table 55.
EXTRAS reviews could be conducted by any senior member of the ESD team. In total, 649 (56%) reviews were conducted by a physiotherapist, 320 (28%) were conducted by an occupational therapist, 98 (8%) were conducted by a nurse, and 58 (5%) were conducted by a speech and language therapist (SLT). A detailed breakdown of staff involved in each review, including banding, is shown in Appendix 2, Table 56.
Services were encouraged to provide the same reviewer for each participant’s EXTRAS review. Of 258 patients who completed at least two reviews, 167 (65%) had the same reviewer for all their reviews, 77 (30%) had two reviewers and 13 (5%) had three reviewers. One patient received reviews from four different individuals.
Rehabilitation issues and goals/action points
Rehabilitation issues were assessed over 12 prespecified domains plus an ‘other’ category to capture any issues identified that fell outside those 12 domains. The domains were mobility; personal care; mealtimes; domestic activities; work and volunteering; hobbies and interests; driving and transport; communication; memory and concentration; mood, anxiety and depression; medical issues; and pain. Each domain was further categorised into prespecified ‘activity issues’ to guide assessment. Following identification of rehabilitation issues, it was intended that up to five would be prioritised and used to formulate goals or action points.
Table 12 shows the number of rehabilitation issues identified and prioritised at each EXTRAS review. Most patients identified rehabilitation issues at each review, but some did not prioritise any issues. At the 1-month review, the median number of priority rehabilitation issues was three; at the 18-month review, it was zero.
| 1 month (N = 258)a | 3 months (N = 245)a | 6 months (N = 231)a | 12 months (N = 211)a | 18 months (N = 210)a | |
|---|---|---|---|---|---|
| One or more issues identified, n (%) | 247 (96) | 230 (94) | 211 (91) | 184 (87) | 148 (70) |
| One or more priority rehabilitation issues, n (%) | 187 (72) | 164 (67) | 145 (63) | 108 (51) | 74 (35) |
| No issues, n (%) | 11 (4) | 15 (6) | 20 (9) | 27 (13) | 62 (30) |
| Number of priority rehabilitation issues | |||||
| Minimum | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 3 (0–4) | 2 (0–3) | 1 (0–3) | 1 (0–2) | 0 (0–1) |
| Maximum | 6 | 7 | 8 | 8 | 7 |
Patients could have multiple priority rehabilitation issues in multiple domains. Proportions of patients with issue(s) in each domain are illustrated in Figure 9. A breakdown of the identified activity issues within a domain is shown in Appendix 2, Table 57. Mobility issues were the most common priority rehabilitation issue at each review stage. Hobbies and interests were also a common priority. Medical issues increased over the duration of the study.
FIGURE 9.
Priority rehabilitation issues.
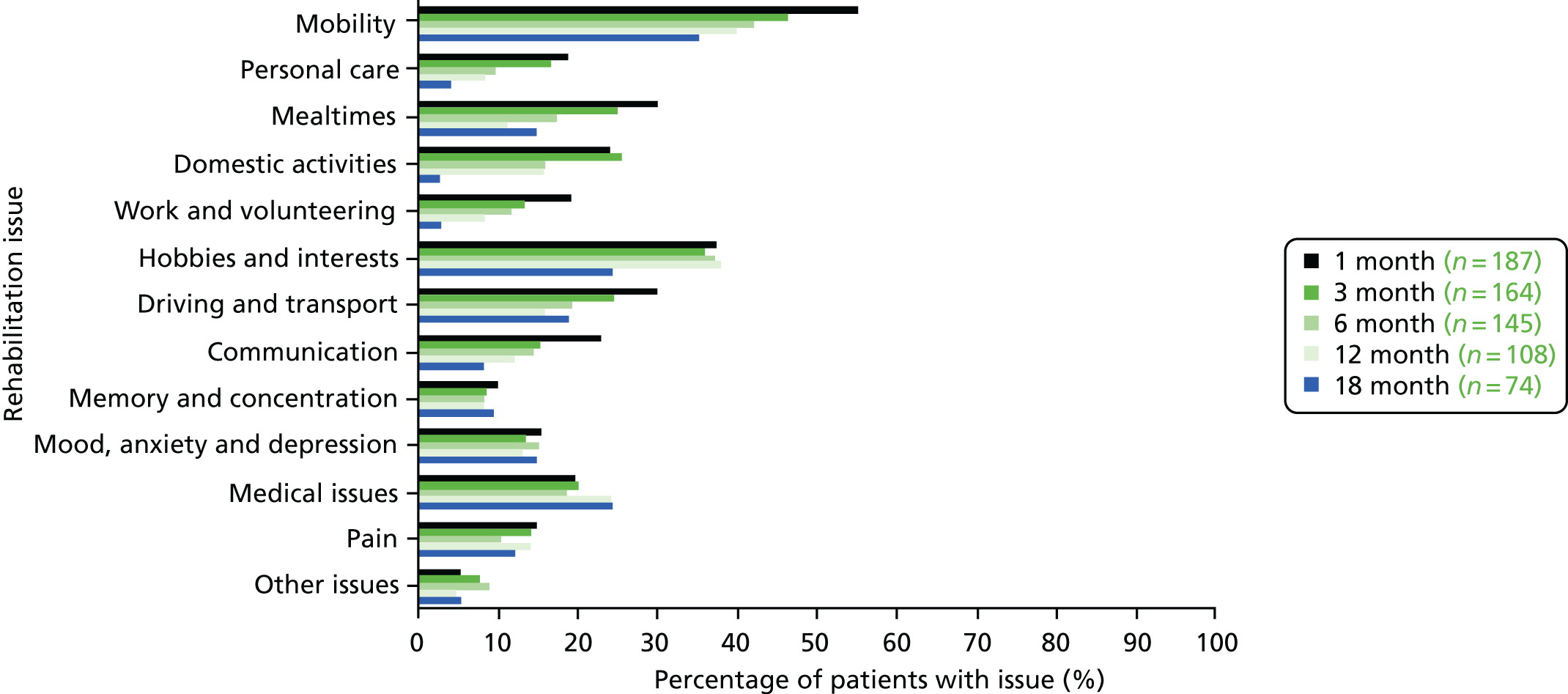
Table 13 shows the numbers of goals/action points set at each review. More patients had goals/action points recorded at each review stage than had priority issues recorded (see Table 12). This is probably because of an administrative error, in that issues were identified as priorities during the review (and used to formulate goals/action points) but were not marked as priority issues in the study paperwork.
| 1 month (n = 258)a | 3 months (n = 245)a | 6 months (n = 231)a | 12 months (n = 211)a | 18 months (n = 210)a | |
|---|---|---|---|---|---|
| Patients with one or more goals/action points recorded, n (%) | 233 (90) | 200 (82) | 180 (78) | 142 (67) | 79 (38) |
| Number of goals/action points | |||||
| Minimum | 0 | 0 | 0 | 0 | 0 |
| Median (IQR) | 3 (2–4) | 2 (1–3) | 2 (1–3) | 1 (0–3) | 0 (0–1) |
| Maximum | 9 | 8 | 8 | 5 | 6 |
The goals/action points per domain is illustrated in Figure 10. Unsurprisingly, the nature of goals/action points set at each review stage mirrored the priority rehabilitation issues.
FIGURE 10.
Goals/action points by domain at each EXTRAS review. (a) 1 month, 742 goals/action points were set in total; (b) 3 months, 547 goals/action points were set in total; (c) 6 months, 427 goals/action points were set in total; (d) 12 months, 313 goals/action points were set in total; and (e) 18 months, 146 goals/action points were set in total.
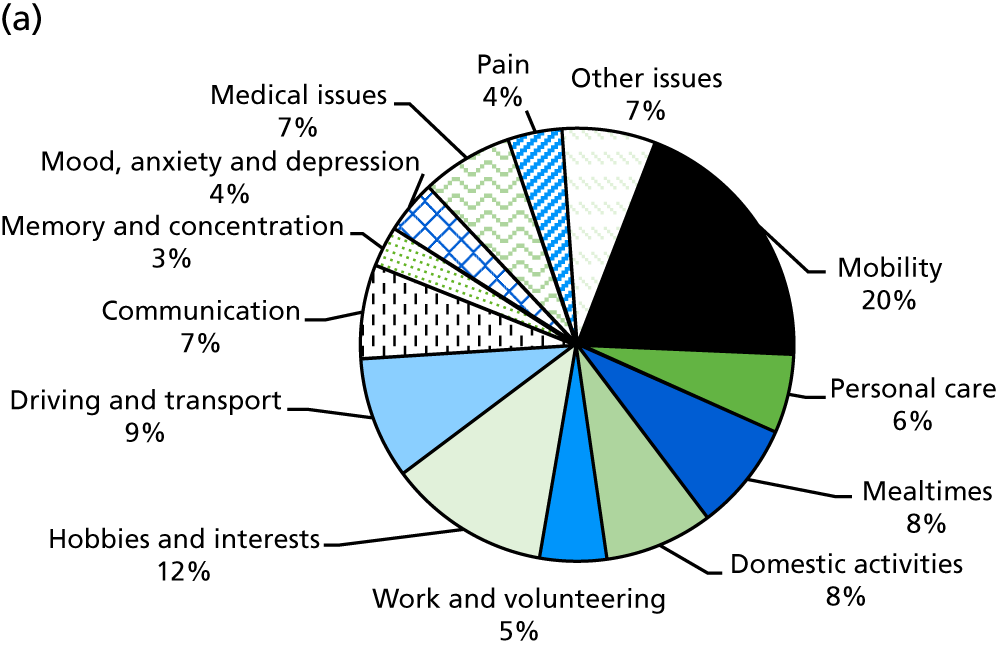
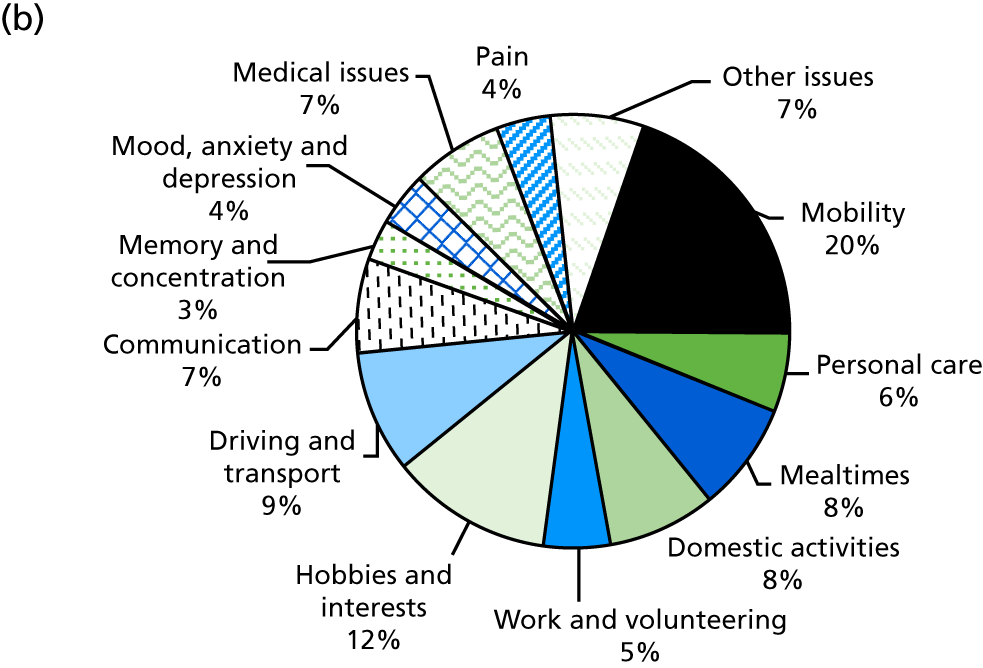
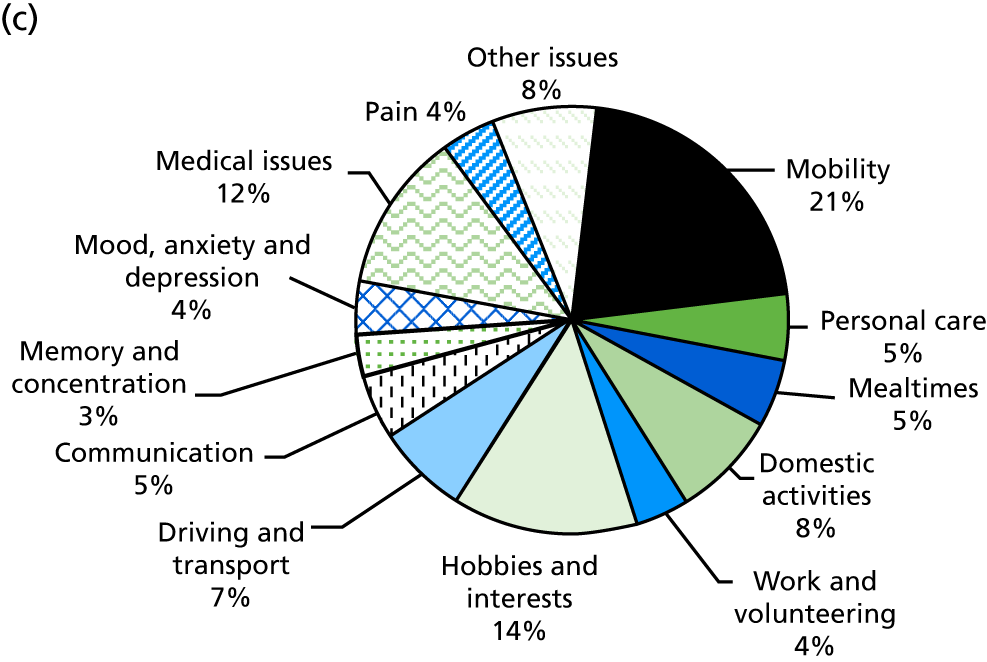
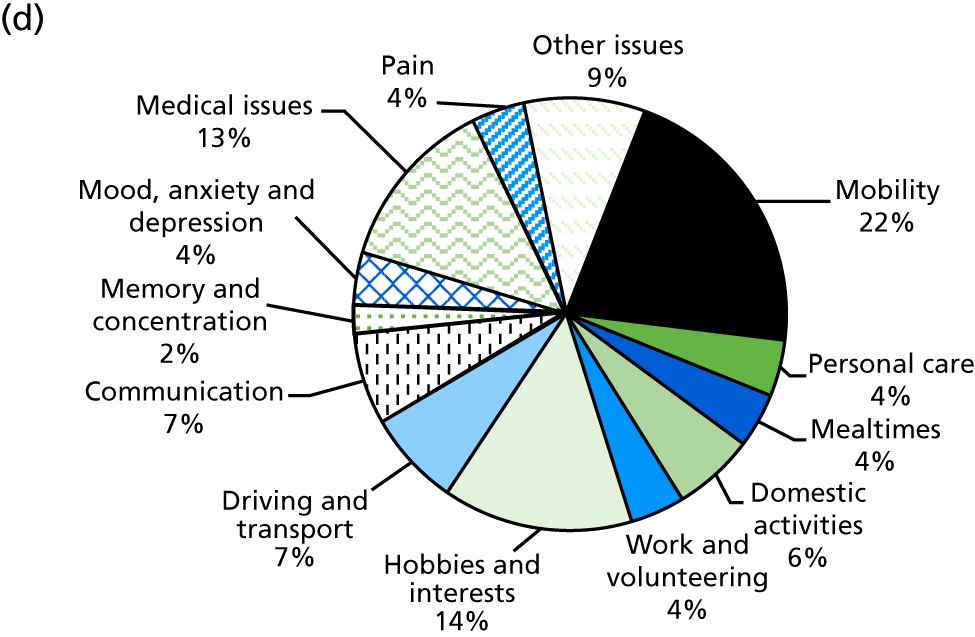
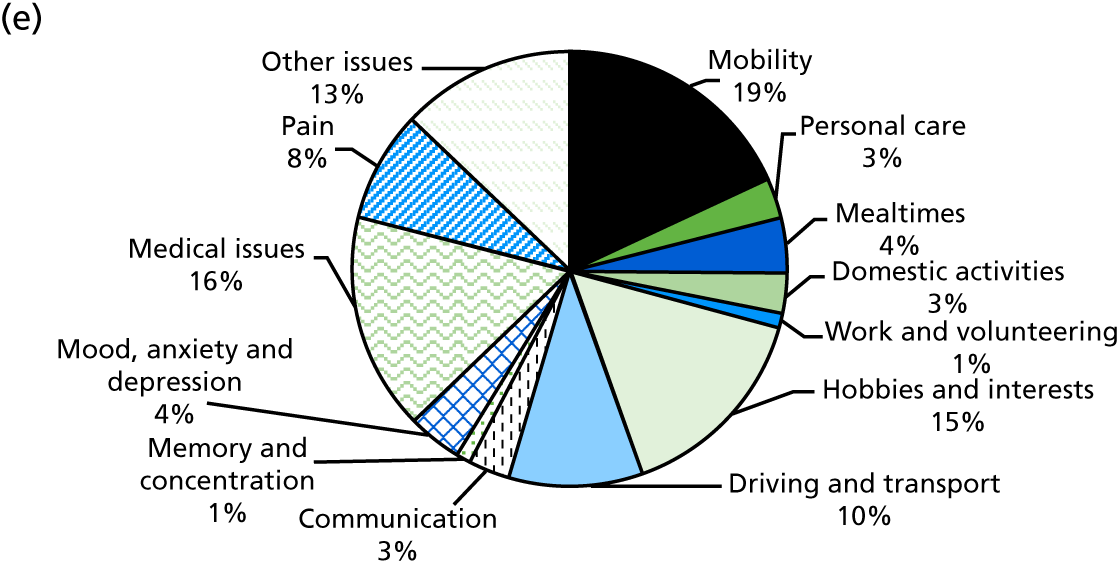
Action plans
Action plans were intended to be set for each goal/action point. Depending on the review stage, between 97% and 99% of goals/action points had an action plan (see Appendix 2, Table 58). The free text of the action plans was coded into different ‘actions’ and these actions were categorised as the responsibility of the reviewer, patient or carer. Each action plan could have multiple actions. The analysis includes actions that were completed during the review (e.g. advice to patient, information to patient) as well as actions to be carried out at some point after the review. Table 14 shows descriptions and/or examples of the free text that was coded as each action where this was not evident from the action code.
| Action code | Description/example of free text (if required) |
|---|---|
| Reviewer actions | |
| Advice to patient | Advice/encouragement given |
| Liaise with HSCP | Liaise with the HSCPs treating the patient |
| Referral to make | |
| Referral to follow-up | |
| Provide aid | Source aid/equipment for the patient |
| Information to patient | |
| Signposting | Signposted the patient to local/community/voluntary service |
| Patient actions | |
| Attend therapy | (Continue to) attend therapy session/group or review with the therapist (PT, OT, SLT, sensory team, counsellor/psychologist) |
| Liaise with employment contact | Discuss with employer/mentor/human resources/advisor |
| Review information | Seek/review information (e.g. internet search), self-monitor symptoms (e.g. fatigue) |
| Obtain aid/equipment | Obtain aid/adaptation/equipment (e.g. adapted cutlery, memory aid) |
| Practise activity | The activity that the goal relates to |
| Practise exercises | For example, home exercise programme, memory strategies |
| Lifestyle change | For example, healthy eating, fitness, exercise referral scheme |
| Arrange activity | Arrange/attend activity, for example social activity (not therapy) |
| Liaise with HCP | Seek advice/treatment from non-therapy health-care professional (e.g. GP, dietitian, orthoptist, audiologist) |
| Liaise with other | Seek advice/help from non-health-care professional (e.g. DVLA, mobility centre, social worker) |
| Carer action | |
| Carer action | For example, make appointment on patient’s behalf |
Figure 11 shows that the most common action taken by reviewers was giving advice to the patient. Advice was recorded in 71–75% of action plans (depending on the review stage). Referrals to other services were recorded in 5–9% of action plans. The most common action to be taken by patients was to practise the activity that the goal related to (e.g. outdoor mobility). This featured in 22–34% of action plans.
FIGURE 11.
Action plan composition. HCP, health-care professional; HSCP, health and social care professional.
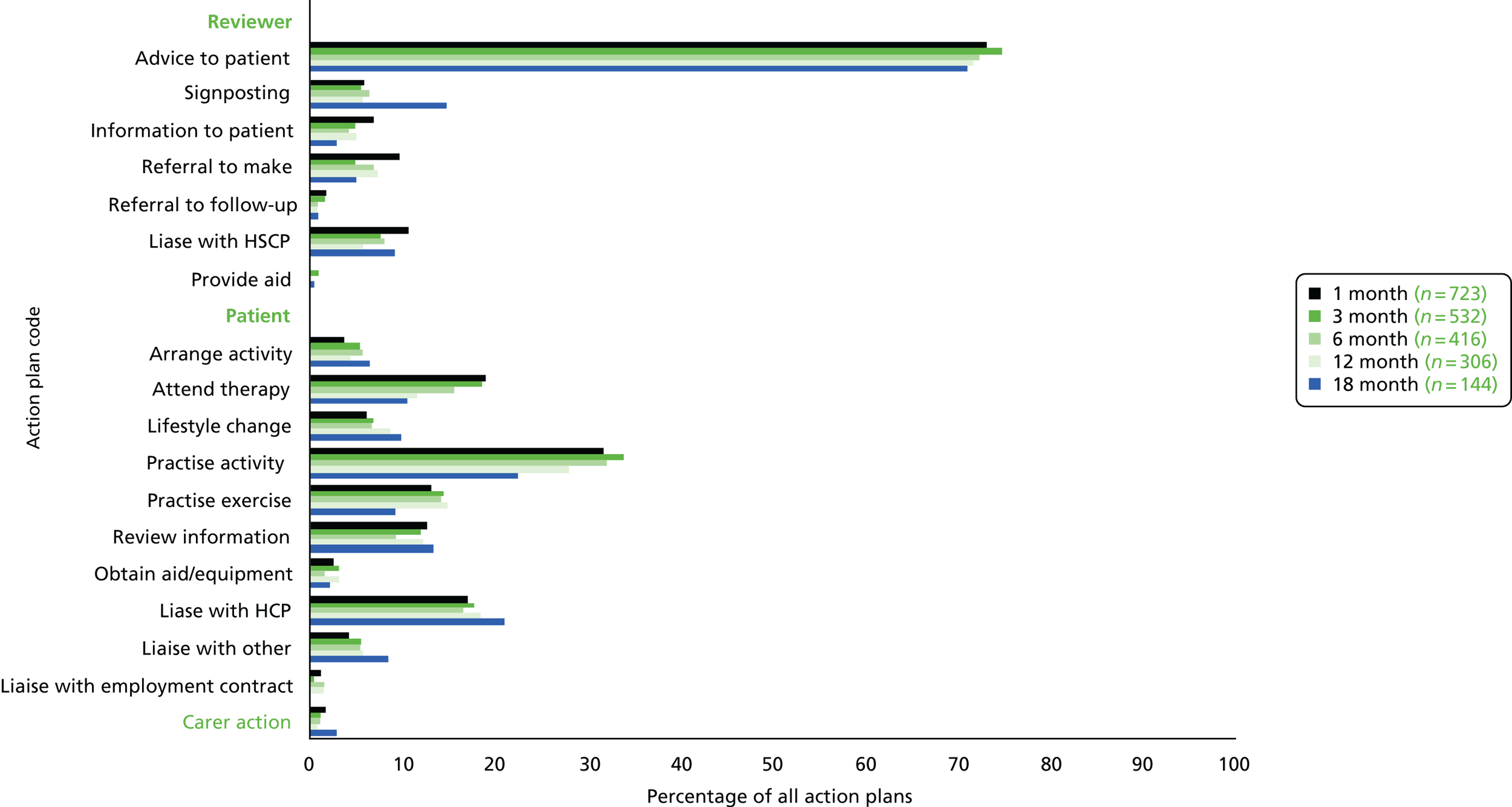
Table 15 shows the breakdown of action plans by the domains of the associated goals/action points.
| Action | Mobility (n = 446) | Personal care (n = 118) | Mealtimes (n = 141) | Domestic activities (n = 159) | Work and volunteering (n = 106) | Hobbies and interests (n = 276) | Driving and transport (n = 178) | Communication (n = 127) | Memory and concentration (n = 58) | Mood, anxiety and depression (n = 75) | Medical issues (n = 220) | Pain (n = 98) | Other issues (n = 172) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reviewer actions, n (%) | |||||||||||||
| Advice to patient | 322 (72) | 86 (73) | 113 (80) | 131 (82) | 72 (68) | 193 (70) | 103 (58) | 104 (82) | 41 (71) | 54 (72) | 140 (64) | 75 (77) | 113 (66) |
| Signposting | 10 (2) | 11 (9) | 6 (4) | 4 (3) | 8 (8) | 9 (3) | 28 (16) | 2 (2) | 2 (3) | 14 (19) | 15 (7) | 7 (7) | 18 (10) |
| Information | 13 (3) | 5 (4) | 16 (11) | 6 (4) | 2 (2) | 5 (2) | 9 (5) | 9 (7) | 2 (3) | 4 (5) | 19 (9) | 1 (1) | 19 (11) |
| Referral to make | 52 (12) | 6 (5) | 7 (5) | 4 (3) | 6 (6) | 7 (3) | 5 (3) | 10 (8) | 5 (9) | 9 (12) | 17 (8) | 7 (7) | 17 (10) |
| Referral to follow-up | 5 (1) | 1 (1) | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 2 (1) | 1 (1) | 4 (7) | 1 (1) | 5 (2) | 1 (1) | 3 (2) |
| Liaise with HSCP | 38 (9) | 20 (17) | 15 (11) | 9 (6) | 5 (5) | 9 (3) | 10 (6) | 9 (7) | 7 (12) | 9 (12) | 21 (10) | 11 (11) | 15 (9) |
| Provide aid | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 1 (1) |
| Patient actions, n (%) | |||||||||||||
| Arrange activity | 2 (< 0.5) | 0 (0) | 2 (1) | 2 (1) | 11 (10) | 57 (21) | 7 (4) | 0 (0) | 1 (2) | 4 (5) | 7 (3) | 0 (0) | 6 (3) |
| Attend therapy | 140 (31) | 19 (16) | 24 (17) | 25 (16) | 10 (9) | 24 (9) | 12 (7) | 26 (20) | 6 (10) | 5 (7) | 10 (5) | 28 (29) | 20 (12) |
| Lifestyle change | 27 (6) | 2 (2) | 16 (11) | 6 (4) | 1 (1) | 10 (4) | 3 (2) | 0 (0) | 0 (0) | 1 (1) | 68 (31) | 2 (2) | 9 (5) |
| Practise activity | 205 (46) | 47 (40) | 44 (31) | 95 (60) | 13 (12) | 113 (41) | 37 (21) | 59 (46) | 17 (29) | 2 (3) | 9 (4) | 3 (3) | 15 (9) |
| Practise exercises | 83 (19) | 22 (19) | 27 (19) | 24 (15) | 5 (5) | 20 (7) | 5 (3) | 33 (26) | 9 (16) | 3 (4) | 5 (2) | 19 (19) | 31 (18) |
| Review information | 24 (5) | 9 (8) | 19 (13) | 17 (11) | 21 (20) | 27 (10) | 26 (15) | 9 (7) | 4 (7) | 10 (13) | 31 (14) | 2 (2) | 48 (28) |
| Obtain aid/equipment | 7 (2) | 5 (4) | 18 (13) | 4 (3) | 0 (0) | 4 (1) | 1 (1) | 2 (2) | 2 (3) | 0 (0) | 6 (3) | 0 (0) | 2 (1) |
| Liaise with HCP | 38 (9) | 14 (12) | 11 (8) | 3 (2) | 17 (16) | 23 (8) | 51 (29) | 11 (9) | 6 (10) | 40 (53) | 75 (34) | 49 (50) | 31 (18) |
| Liaise with other | 7 (2) | 7 (6) | 5 (4) | 3 (2) | 13 (12) | 6 (2) | 44 (25) | 1 (1) | 0 (0) | 4 (5) | 3 (1) | 0 (0) | 16 (9) |
| Liaise with employment contact | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 18 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Carer action, n (%) | 6 (1) | 1 (1) | 3 (2) | 2 (1) | 1 (1) | 4 (1) | 1 (1) | 1 (1) | 1 (2) | 0 (0) | 2 (1) | 0 (0) | 4 (2) |
| No action plan, n (%) | 14 (3) | 1 (1) | 2 (1) | 6 (4) | 4 (4) | 15 (5) | 4 (2) | 1 (1) | 1 (2) | 2 (3) | 1 (< 0.5) | 0 (0) | 3 (2) |
During the coding of individual action plans, it was noted that advice, information and/or signposting offered by the reviewers was usually complemented by an action for the patient to do something based on that advice. Given this, it was felt that presenting these separately (see Figure 11) was masking the extent to which the action plans concerned what the patient was going to do. A second analysis was therefore undertaken where each action plan was placed into a single category that was felt to best describe the main action(s) in the plan. For example, if the plan involved only the patient/carer undertaking actions following advice/information/signposting from the reviewer, this was categorised as a patient/carer action only. This analysis is shown in Table 16 and illustrates that 80% of the action plans focused on what the patient was going to do.
| Action | Number of goals (N = 2175), n (%) |
|---|---|
| Patient/carer action(s) only | 1735 (80) |
| Reviewera action(s) only | 202 (9) |
| Both reviewera and patient/carer actions | 155 (7) |
| Reviewer advice, signposting or information only | 29 (1) |
| No action plan | 54 (2) |
One of the actions that a reviewer could take was to refer the patient to another service. During the trial, 117 unique referrals (intentions to refer) were recorded. A total of 162 action plans included a referral, but some referrals were recorded more than once for the same patient at different review stages. The most common referrals were to physiotherapy (n = 20 patients; 7%), occupational therapy (n = 16 patients; 6%) or an exercise group/referral scheme (n = 14 patients; 5%). Full details about referrals are shown in Appendix 2, Table 59.
Goal and action point achievement
Over the five EXTRAS reviews, a total of 2175 goals/action points were recorded. The process for recording and evaluating goals was as follows:
-
Goals were given a baseline score indicating the patient’s status regarding that goal.
-
Goals that were set at the first four review stages were evaluated in the subsequent review and rated on the extent to which they had been achieved. The exception was goals with a longer time frame than the next review, for example a goal set at the 1-month review that was to be achieved by the 6-month review would not need to be evaluated at the 3-month review.
-
The goal evaluation rating is on a six-point scale representing descriptors of progress towards the goal, from ‘yes (achieved), much better than expected’ to ‘no (not achieved), worse than baseline’.
-
Goals/action points set at the 18-month review (n = 146) were not evaluated.
The process for evaluating action points was to assign as ‘yes (achieved)’ or ‘no (not achieved)’. Neither a baseline score nor a rating was required.
Unfortunately, the study paperwork did not prompt the reviewer to record whether an entry was a goal or an action point. Because of this, these data were analysed in two ways.
First, Table 17 shows whether any entry was reported as achieved or not, broken down across the domains.
| Domain | Combined goals and action points, n | Achieved,a n (%) | Not achieved,b n (%) | Not recorded,c n (%) |
|---|---|---|---|---|
| Mobility | 419 | 224 (53) | 152 (36) | 43 (10) |
| Personal care | 113 | 52 (46) | 44 (39) | 17 (15) |
| Mealtimes | 135 | 75 (56) | 45 (33) | 15 (11) |
| Domestic activities | 155 | 93 (60) | 47 (30) | 15 (10) |
| Work and volunteering | 105 | 53 (50) | 41 (39) | 11 (10) |
| Hobbies and interests | 254 | 105 (41) | 120 (47) | 29 (11) |
| Driving and transport | 164 | 81 (49) | 59 (36) | 24 (15) |
| Communication | 122 | 65 (53) | 35 (29) | 22 (18) |
| Memory and concentration | 57 | 26 (46) | 16 (28) | 15 (26) |
| Mood, anxiety and depression | 69 | 44 (64) | 16 (23) | 9 (13) |
| Medical issues | 196 | 99 (51) | 52 (27) | 45 (23) |
| Pain | 86 | 50 (58) | 22 (26) | 14 (16) |
| Other issues | 153 | 89 (58) | 39 (25) | 25 (16) |
| Total | 2029 | 1057 (52) | 688 (34) | 284 (14) |
Second, some assumptions were made to determine which entries were goals and which entries were action points for separate analyses. An entry was defined as a goal where the record included a baseline score and an evaluation rating on the six-point scale described above. An entry was defined as an action point if there was no baseline score and there was an evaluation of ‘yes (achieved)’ or ‘no (not achieved)’. Using these definitions, 524 out of 2029 (26%) of the entries were unclassifiable as goals or action points. Table 18 shows the separated goal and action point evaluation.
| Mobility | Personal | Mealtimes | Domestic activities | Work and volunteering | Hobbies and interests | Driving and transport | Communication | Memory and concentration | Mood, anxiety and depression | Medical issues | Pain | Other issues | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Goal achievement rating | ||||||||||||||
| Total goals | 325 | 80 | 103 | 129 | 78 | 199 | 116 | 87 | 30 | 41 | 92 | 52 | 88 | 1420 |
| Achieved, n (%) | 189 (58) | 45 (56) | 65 (63) | 85 (66) | 40 (51) | 94 (47) | 66 (57) | 55 (63) | 17 (57) | 28 (68) | 48 (52) | 33 (63) | 55 (63) | 820 (58) |
| Much better than expected, n (%) | 41 (13) | 5 (6) | 7 (7) | 9 (7) | 4 (5) | 26 (13) | 13 (11) | 7 (8) | 3 (10) | 2 (5) | 7 (8) | 6 (12) | 8 (9) | 138 (10) |
| Little better than expected, n (%) | 38 (12) | 10 (13) | 14 (14) | 28 (22) | 8 (10) | 21 (11) | 10 (9) | 17 (20) | 7 (23) | 7 (17) | 3 (3) | 12 (23) | 10 (11) | 185 (13) |
| As expected, n (%) | 110 (34) | 30 (38) | 44 (43) | 48 (37) | 28 (36) | 47 (24) | 43 (37) | 31 (36) | 7 (23) | 19 (46) | 38 (41) | 15 (29) | 37 (42) | 497 (35) |
| Not achieved, n (%) | 136 (42) | 35 (44) | 38 (37) | 44 (34) | 38 (49) | 105 (53) | 50 (43) | 32 (37) | 13 (43) | 13 (32) | 44 (48) | 19 (37) | 33 (38) | 600 (42) |
| Partially achieved, n (%) | 59 (18) | 14 (18) | 19 (18) | 19 (15) | 14 (18) | 40 (20) | 9 (8) | 17 (20) | 6 (20) | 4 (10) | 14 (15) | 9 (17) | 13 (15) | 237 (17) |
| Same as baseline, n (%) | 58 (18) | 19 (24) | 18 (17) | 23 (18) | 21 (27) | 59 (30) | 37 (32) | 15 (17) | 7 (23) | 8 (20) | 29 (32) | 9 (17) | 19 (22) | 322 (23) |
| Worse than baseline, n (%) | 19 (6) | 2 (3) | 1 (1) | 2 (2) | 3 (4) | 6 (3) | 4 (3) | 0 (0) | 0 (0) | 1 (2) | 1 (1) | 1 (2) | 1 (1) | 41 (3) |
| Action point rating | ||||||||||||||
| Total action points, n | 9 | 1 | 3 | 3 | 8 | 3 | 6 | 6 | 2 | 9 | 20 | 6 | 8 | 85 |
| Achieved, n (%) | 9 (100) | 1 (100) | 3 (100) | 3 (100) | 6 (75) | 2 (67) | 4 (67) | 5 (58) | 1 (50) | 9 (100) | 14 (70) | 6 (100) | 8 (100) | 71 (84) |
| Not achieved, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (25) | 1 (33) | 2 (33) | 1 (17) | 1 (50) | 0 (0) | 6 (30) | 0 (0) | 0 (0) | 14 (16) |
When goals/action points were not achieved by the next review (and the goal/action point was not intended to be ongoing beyond that review), the reviewer was prompted to record a reason. This was completed for 594 out of 688 (86%) of the non-achieved goals/action points. For 73% (500/688) of the non-achieved goals/action points, the reason seemed to be related to the patients’ circumstances. Reasons included patients not being motivated or changing their mind about the goal; co-existing conditions or illness; and having limited opportunities to carry out the action plan (e.g. lack of time or poor weather). In 7% (49/688) of cases, the reason was service related; most often the patient was awaiting input or a decision from a service such as community therapy or the Driver and Vehicle Licensing Agency. Another 7% (45/688) of comments reported that the patient had not made sufficient progress to meet a goal. For the remaining 14% (94/688), no reason was recorded.
Differences between study centres
The number of patients randomised to receive EXTRAS at each study centre ranged from 3 to 41. The proportion of reviews that were conducted (of those expected to be conducted, i.e. removing deaths and withdrawals) ranged from 53% to 100%, as shown in Figure 12. Detailed data, including reasons for non-conduct of reviews by study centres, are available in Appendix 2, Table 60.
FIGURE 12.
Review completion as a proportion of review completion expected, per study centre.
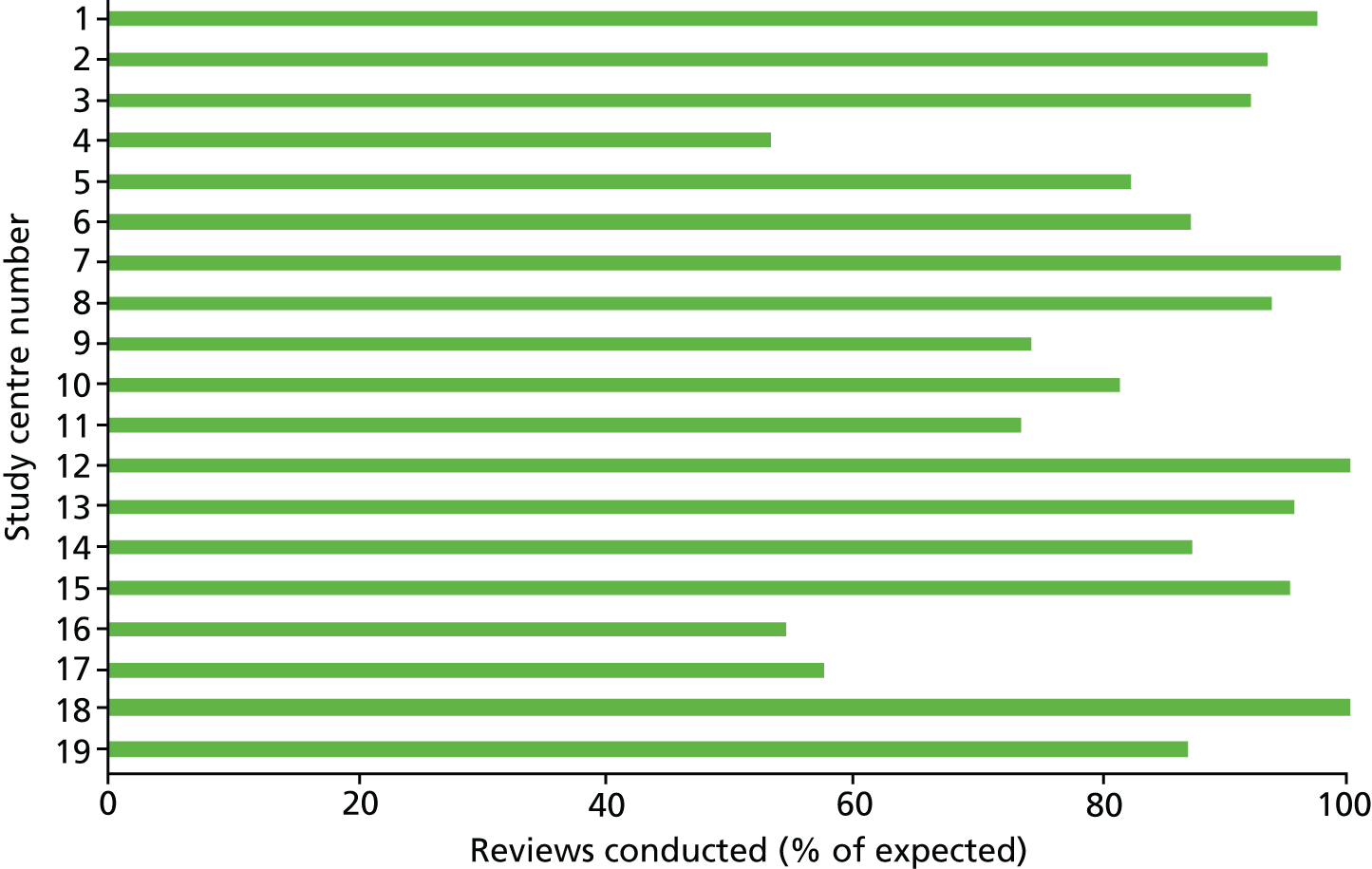
Study centres could choose which staff to involve in the delivery of EXTRAS. In some centres, reviews were predominantly undertaken by one member of staff and in others it could be multiple members of staff; one study centre involved 16 members of staff at the 1-month review stage. In 7 out of the 19 study centres, at least half of the patients had the same reviewer over the course of the intervention (see Appendix 2, Table 61).
Individual staff members conducted a minimum of 1 and a maximum of 28 reviews at one specific review stage (see Appendix 2, Table 62).
The number of goals/action points set per patient over the reviews varied across the study centres, for example the median number of goals/action points ranged from 2 to 13 (Figure 13).
FIGURE 13.
Goals/action points set per patient, by study centre.
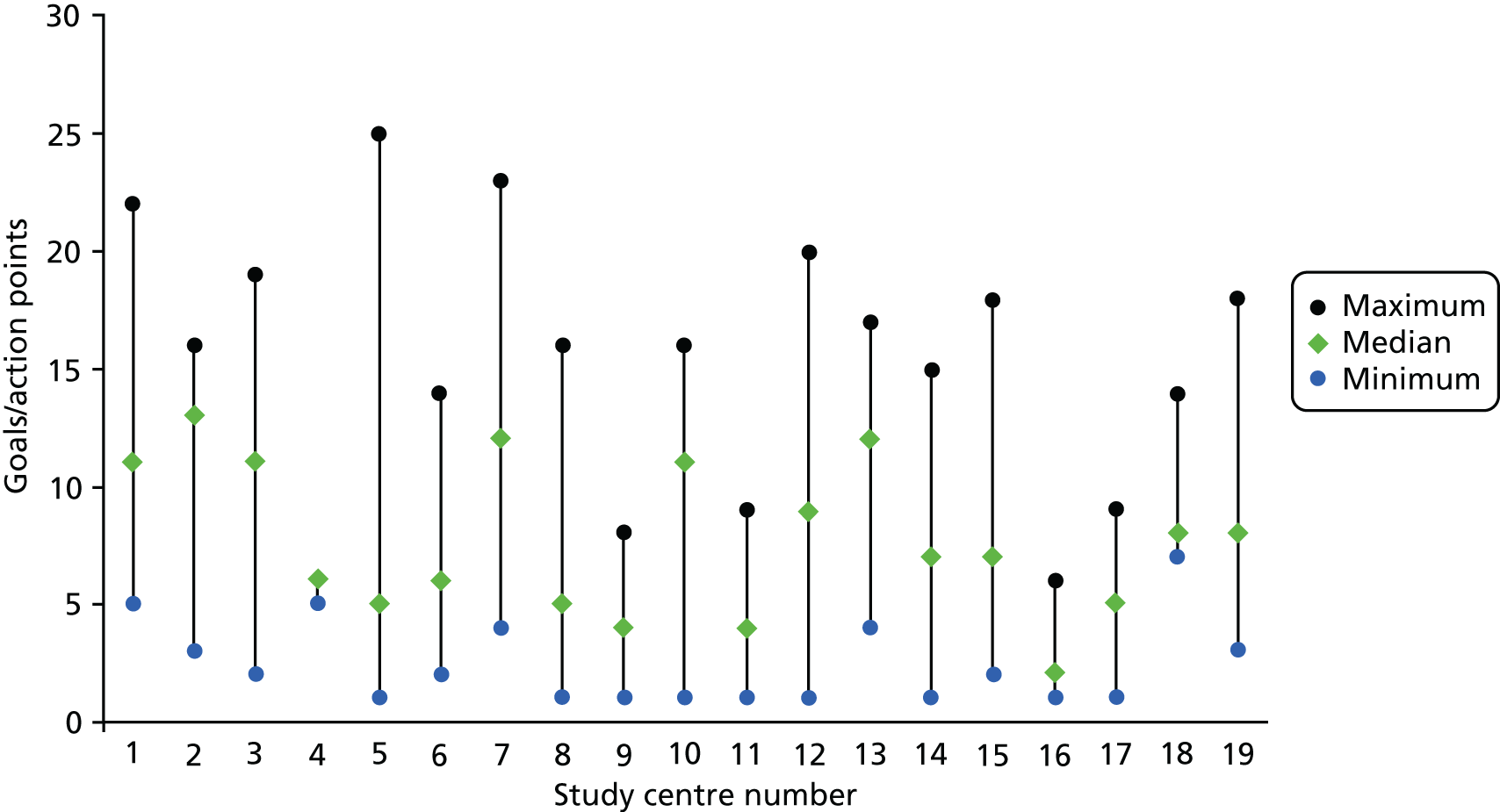
In most of the action plans, it was recorded that reviewers offered advice, signposting and/or information, and this was generally complemented by an action for the patient to do something based on that advice. In Figure 14, advice, signposting and information from the reviewer are excluded from the ‘reviewer action’ category. Figure 14 shows that, at most study centres, most of the action plans focus on what the patient is going to do.
FIGURE 14.
Ownership of action plans by study centre. n = number of goals/action points set at the study centre. Advice, signposting and information from the review are excluded from the ‘reviewer action’ category.
Chapter 6 Patient, carer and staff interviews
As part of the process evaluation, we undertook interviews with samples of patients and carers participating in the trial, staff involved in delivering EXTRAS and therapists working for community rehabilitation services.
Aim and objectives
The overall aim was to seek the views and experiences of patients, carers and rehabilitation staff about the community rehabilitation that they received or provided. Underpinning this aim were three objectives with associated question areas, shown below. The objectives/questions map on to sections of this chapter.
-
Objective 1: to understand rehabilitation staff, patient and carer experiences of usual care.
Question areas –
-
Staff experiences of delivering usual care to stroke patients.
-
Patient and carer experiences of usual care after stroke.
-
-
Objective 2: to understand staff experiences of EXTRAS.
Question areas –
-
Staff experiences of preparing for and delivering EXTRAS, including the service context.
-
Staff views on factors that promoted or challenged the delivery of EXTRAS.
-
Staff views on the benefits and/or disadvantages experienced by patients and carers receiving EXTRAS.
-
Staff views on the impact of provision of EXTRAS on other community stroke services.
-
-
Objective 3: to understand patient and carer experiences of EXTRAS.
Question areas –
-
Patient and carer experiences of EXTRAS.
-
If EXTRAS was found to be effective, the interview study would help us to understand for which patients and under which circumstances the new service appeared to ‘work’, and to identify improvements that could be made to promote provision of EXTRAS in clinical practice. If the service was not found to be effective, the interview study would help to identify possible causes, such as poor delivery, poor patient response, an inadequate design or another reason.
Patient and carer interviews: methods
Interviews were semistructured and conducted in the patients’ own home or care home by the research assistant (RA) (patients and carers had the option to request an alternative venue, e.g. their local hospital). Interviews lasted between 25 and 40 minutes (median 30 minutes).
The topic guide was based on examples used in previous studies that were led by the process evaluation lead (CM) and was informed by literature on stroke rehabilitation experiences. 60–62
Patients and carers were asked about their rehabilitation needs after their current stroke (physical, emotional, social), their experience of health care and rehabilitation, and about any unmet needs. Intervention group patients and carers were asked for their views about the reviews that they received as part of EXTRAS.
The RA piloted the topic guide in face-to-face interviews with three stroke survivors not involved in the EXTRAS trial (all male, aged 30–75 years) who were recruited from the King’s College London Stroke Research Patients and Family Group. A carer participated in one of the interviews. As volunteers reported that the questions were clear and they did not find the interview burdensome, no changes were made to the topic guide.
We included a limited number of study centres in the patient and carer study to ensure that fieldwork was feasible. Nine study centres were selected. Study centre selection was based on:
-
number of recruited participants (centres that had recruited higher numbers of participants were preferred as they created a larger potential sampling frame)
-
achieving a range of regions and urban/rural settings.
We decided not to devise an overall sampling frame at the outset of data collection; for pragmatic reasons, we wanted to identify groups of patients in the same geographical region who could potentially be interviewed within an agreed time window (3 months after their 24-month assessment). Multiple sampling frames were, therefore, identified over the course of the trial. At the start of the study, all patients and carers in the frame were sampled because of small numbers. As recruitment progressed, we prioritised sampling patients with characteristics under-represented among those interviewed in each study group [i.e. age, sex and health status at baseline (OHS34)]. As the interview data analysis proceeded concurrently, the RA continued to sample patients until no new themes relevant to the research aim and objectives were created.
Inclusion criteria for inviting patients to interview:
-
current participant in the EXTRAS trial at one of the nine selected study centres
-
able to give consent to participate in the patient and carer interview study.
Exclusion criteria:
-
withdrawn from the study
-
refused contact about the interview study when asked during the 24-month outcome assessment
-
cognitive impairment or aphasia of a degree that they would be unable to participate in an interview (identified by comments on outcome assessment records).
In total, 34 patients and their carers (when the carer was participating in the study, n = 6) were informed about the patient and carer interview study and invited to take part by letter (with a study information sheet) and a follow-up telephone call. Patients and carers received separate letters. All except one of the carers were cohabiting with the patient. One carer lived separately from the patient so was telephoned separately. A total of 20 patients and 6 carers participated.
In total, 10 intervention patients, 10 control patients and 6 carers participated in the interview study. Table 19 shows respondent characteristics, including baseline and 24-month data, although only patient sex, age and baseline health status (OHS34) were considered when sampling.
| Characteristic | Intervention patients (n = 10) | Control patients (n = 10) |
|---|---|---|
| Sex (n) | ||
| Male | 8 | 5 |
| Female | 2 | 5 |
| Age at interview (years) | ||
| Median (IQR) | 73 (60–78) | 70 (69–74) |
| Range | 48–86 | 56–92 |
| OHS baseline assessment scorea | ||
| Median (IQR) | 2 (1–3) | 2 (2–3) |
| Range | 1–4 | 1–5 |
| OHS 24-month assessment score | ||
| Median (IQR) | 2 (1–3) | 3 (2–3) |
| Range | 1–4 | 1–5 |
| NEADL Scale baseline assessment score | ||
| Median (IQR) | 43 (30–63) | 42 (30–50) |
| Range | 17–66 | 8–66 |
| NEADL Scale 24-month outcome assessment score | ||
| Median (IQR) | 57 (28–65) | 34 (21–54) |
| Range | 16–66 | 6–66 |
| Carer participation | ||
| Carer took part in study and participated in interview (n) | 4 (1 daughter, 3 female spouses/partners) | 2 (1 male spouse/partner, 1 female spouse/partner) |
Interviews were conducted after patients’ final (24-month) outcome assessment was completed to avoid influencing their outcome assessment responses. At the end of the outcome assessment, the assessor informed patients that they might be contacted by another RA (Eleanor Stevens) about the patient and carer study; any comments or refusals were recorded.
The RA confirmed and sought signed consent, including permission to record the interview, from the patient and separately from their carer (where applicable) using standard forms at the interview appointment. Carers were asked whether or not they would prefer to be interviewed separately, but all preferred to be interviewed jointly with the patient. In the interviews with two intervention and two control patients whose carers were not participating in the study, it was their preference that their carer (spouse/partner) was present during the interview.
The RA was given only the names and contact details of patients and carers who were part of the interview sample and who the trial co-ordinating centre confirmed would be able to take part in an interview. These details were passed to the RA via telephone calls from the trial co-ordinating centre and held in a password-protected computer file, with access limited to the RA only.
Staff interviews: methods
The staff interviews were semistructured and conducted by telephone. They were intended to take place within 6 weeks of the last 18-month (final) review of EXTRAS at a study centre. Owing to a combination of a delay in finalising the interview topic guides and staff unavailability, four interviews with EXTRAS staff were conducted much later than this time window. Identifying and recruiting community rehabilitation staff tended to take several weeks, so only two of the community rehabilitation staff interviews were conducted within 6 weeks of the last 18-month review. Interviews lasted between 20 and 50 minutes (median 25 minutes).
Two versions of the topic guide were developed for staff who provided EXTRAS and for community rehabilitation staff, respectively. These were informed by literature on community stroke rehabilitation and process evaluation. 61,63–65 Both versions included questions about the provision of longer-term support for stroke patients and carers in their locality (i.e. usual care), and any changes to their service during the intervention delivery period.
EXTRAS staff were asked to describe and give their opinions on the preparation for and implementation and impact of the new service, including how patients and carers responded, and their experiences of trial procedures such as data management (if applicable).
Community rehabilitation staff were additionally asked about their experiences of delivering rehabilitation to stroke patients, focusing on EADL and perceived barriers or facilitators.
The first two EXTRAS staff interviews and the first two community rehabilitation staff interviews were used to pilot the topic guides. In response to their responses, a change was made to the EXTRAS staff topic guide: one unclear/redundant question was omitted.
Sampling was purposive and aimed to include:
-
One key member of EXTRAS staff from each of the 20 ESD services that participated in the trial (at 1 of the 19 study centres, ESD was provided by two separate teams).
-
Two or three community rehabilitation staff in each ESD service locality. These were staff in services to which participating ESD teams regularly referred patients in usual care. These staff were not involved in delivering EXTRAS.
EXTRAS staff were invited to participate by e-mail. If unwilling or unable to participate, they were asked to nominate a colleague who had delivered the intervention. In the case of two ESD services, two staff members were interviewed as the first staff member suggested that the second would be able to ‘fill gaps’ in responses.
EXTRAS staff were asked to nominate one or more community rehabilitation staff members as potential respondents. The RA gained the nominees’ e-mail address where possible. Three community rehabilitation staff did not respond to direct e-mail contact. At seven study centres, EXTRAS staff agreed to forward by e-mail a summary of the interview to potential community rehabilitation staff respondents, which meant that the RA did not have direct contact. This produced a nomination in three of those seven study centres.
Respondent characteristics are shown in Table 20. In total, 22 EXTRAS staff were interviewed; in two of the ESD teams, two staff members were interviewed individually. At one study centre, the EXTRAS staff respondent had conducted some of the EXTRAS reviews but was based in a neurology outpatient service, not in an ESD team. A second person, who was a member of the ESD team, conducted the remaining reviews at that study centre but was unavailable for interview. A total of 19 community rehabilitation staff were interviewed. Five community rehabilitation staff worked in a participating ESD team for at least part of the trial period but did not conduct EXTRAS reviews.
| Characteristic | EXTRAS staff (N = 22 interviewed, from 19 ESD services and 1 neurology outpatient service) | Community rehabilitation staff (N = 19 interviewed, nominations from 14/20 study centres) |
|---|---|---|
| Discipline, n (%) | ||
| Physiotherapy | 10 (45) | 14 (74) |
| Occupational therapy | 6 (27) | 3 (16) |
| Nursing | 5 (23) | 1 (5) |
| Speech and language therapy | 1 (5) | 1 (5) |
| Years in post | ||
| Median (IQR) | 8 (3–8) | 8 (3–13) |
| Range | 3–23 | 3–23 |
| Service type, n (%) | ||
| ESD | 21 (95) | 6 (32) |
| Community: generic | 0 (0) | 4 (21) |
| Community: neurology | 0 (0) | 3 (16) |
| Outpatients: neurology | 1 (5) | 5 (26) |
| Outpatients: generic | 0 (0) | 1 (5) |
Invited staff were asked to consent via an e-mail to the RA once any queries were resolved. Consent, including to record the discussion, was confirmed at the start of the interview. Respondent identities and contact details were held in a password-protected computer file, with access limited to the RA.
Patient, carer and staff interviews: data analysis
Interviews were transcribed verbatim. A thematic approach to analysis was used. 66 The analysis approach was a combination of inductive (i.e. derived from the data themselves) and deductive (i.e. looking for evidence pertaining to the study objectives and question areas, and to the key components of the intervention). The key components were identified through reviewing the trial protocol and the EXTRAS manual, and through discussion with the study team:
-
ongoing contact with advice, encouragement and signposting as appropriate
-
regular structured review of rehabilitation needs
-
goal-setting and action-planning
-
route to referral.
An analysis framework was retrospectively applied to the staff interview data to structure the evidence on promoters of and challenges to implementation of the EXTRAS intervention. Normalisation process theory (NPT) was chosen because it is a general theory of how complex interventions become routinely embedded in health services,67 and it has previously been applied retrospectively in a study of a community-based stroke intervention. 68 NPT categorises the operationalisation of implementing interventions into four mechanisms: (1) coherence, (2) cognitive participation, (3) collective action and (4) reflexive monitoring. Identifying staff responses relating to these mechanisms gives some insight into factors that could lead to implementation success or failure.
Interview transcripts were managed and coded using NVivo qualitative data analysis software version 11 (QSR International, Warrington, UK). The RA (ES) checked and corrected transcripts and read them closely, annotating as necessary to clarify responses. The meanings of responses were summarised into initial codes. The lead investigator for the process evaluation (CM) independently coded a sample of transcripts. The resulting codes were similar to those generated by the RA; a few additional codes were incorporated into the coding process for other transcripts.
The initial codebooks developed as further transcripts were analysed and comparisons were made between them: codes were added, amended and grouped into themes. ES and Christopher McKevitt discussed themes generated from the data to agree which were highly relevant to the study aim and objectives and would be included in this report, and which were emergent findings that could be explored in further work. The analysis of the interview study and drafting of this chapter except the discussion was done prior to the intervention outcomes being known.
Staff experiences of delivering usual care
This section draws on the interviews with staff who provided EXTRAS and community rehabilitation staff.
All but one EXTRAS staff who were interviewed were working in stroke ESD teams while the intervention was being delivered. The community rehabilitation staff worked in a variety of services – stroke, neurological and generic – and were therefore seeing different proportions of stroke patients in their caseloads. Some staff dealt with mild–moderately affected stroke patients only, whereas others also treated more severely affected patients. Some worked only with patients who had recently had a stroke; others (also) worked with patients who had been referred back into their service several months post stroke.
Staff described supporting stroke patients and carers to address physical, emotional and psychosocial rehabilitation issues and the impact of post-stroke fatigue. Some described seeing patients who had been discharged home very quickly from hospital without any follow-up. After a period of ‘natural recovery’, some of these patients would gain ‘rehabilitation potential’ or they would exhibit signs of cognitive impairment that had not been identified or addressed during their inpatient stay. Patients would then be referred [usually by their general practitioner (GP)] to their service.
In some areas, staff reported that stroke patients and carers who received usual care could expect good-quality longer-term care with a range of services available and a clear pathway. For example, one area offered an outpatient ‘life after stroke’ course that included monitored rehabilitation gym sessions, relevant talks and peer support.
In other areas, staff described usual follow-up care after ESD as un-co-ordinated, unavailable or ‘stop-start’, with patients experiencing delays while being referred between different services. Lack of services for ‘hidden’ (less apparent) issues such as fatigue and health anxieties was highlighted.
Staff mentioned that it could be challenging and demotivating for patients when they were stepped down from intensive ESD therapies to community services, which are likely to be at a much lower intensity. Staff said that they wanted to encourage self-management but there were not always services available to support patients in this. Some staff also commented that it was hard to keep track of available patient and family support services because their availability tended to change often.
The most commonly cited challenge to delivering rehabilitation for stroke patients was the time that staff spent in meetings and undertaking administrative and management tasks. Individual staff reported other challenges, such as:
-
referrals not being appropriate or timely
-
being the sole member of staff in their profession in a service
-
travel time (domiciliary services in large catchment areas)
-
in integrated teams – allocating resources between ESD patients and longer-term patients.
Patient and carer experiences of usual care
Patients and carers were asked about their experiences of health care and other forms of support provided by health and social care professionals after their stroke. It is possible that some intervention patients/carers could not distinguish rehabilitation provided as part of usual care from rehabilitation provided through EXTRAS. Their views are included in this section if it was clear from the community services mapping work (see Chapter 7) and from individuals’ intervention records that they were referring to services provided as part of usual care in their area.
Most patients and carers reported that therapy was provided very quickly after discharge from hospital. Four patients (two from each of the control and the intervention samples) felt that delays in starting rehabilitation in hospital, or recommencing rehabilitation once at home, had hindered their recovery.
Some patients and carers in both the intervention and the control samples described the care that they received from the community stroke service and/or social services after the stroke as ‘faultless’. They believed that enough therapy had been provided and that their level of recovery was as good as they could expect. They tended to describe rehabilitation as ‘tailing off’ as the patient recovered. This group included patients with ongoing disability from the stroke that significantly restricted their activities, as well as patients who had made a near-complete recovery. It also included control patients who reported having no professional rehabilitation and/or point of contact with stroke services beyond ESD: they did not feel that these things were needed, and if they had any residual impairments they perceived that they were managing well. Carers in this group felt well supported by the health and social care professionals involved in the patients’ care.
However, in both samples, some patients and carers were dissatisfied with the intensity/duration of therapy and some felt that they had been ‘left to manage’ without adequate follow-up from health-care professionals. This included control patients who had accessed NHS community rehabilitation for several weeks beyond ESD. Some patients and carers felt this way despite reporting, or intervention records indicating, that they had follow-up contact from social services or EXTRAS. For instance, one control group patient felt ‘abandoned’ by the community rehabilitation team but described ongoing reablement support from social services. Some carers reported that patients’ motivation and confidence to practise an activity, for example walking outdoors, decreased once ESD ended and patients had no further therapy or less intense therapy. These carers thought that ‘more therapy’ would have helped to sustain patients’ motivation to practise activities of daily living, ultimately reducing dependence on the carer.
Although some patients and carers felt discouraged once therapy was withdrawn and there was no follow-up contact (that they recalled), several patients in both the intervention and the control samples were not discouraged and sustained activities that they understood would promote recovery or maintenance after ESD or further therapy had ended (e.g. gym sessions, ‘brain training’ puzzles). Some of these activities/changes were prompted by health-care professionals (it is unclear whether or not these were EXTRAS staff), whereas others were reported to be self-directed.
Apart from some patients’ and carers’ wish for further physical therapy, other needs not met by usual care were counselling for health anxiety that had been exacerbated by the stroke (an intervention patient did not want to wait several weeks for an NHS psychology appointment so used a private provider) and the opportunity for further discussion with a stroke health-care professional about their risk factors for a further stroke or potential for further recovery (these were control patients). One control patient who did not report specific needs said he would have liked reassurance via follow-up contact:
I think they [stroke team staff] should spend a little bit more time with you. Even to the effect of phoning you up and just saying, ‘How are you going? Any problems or anything we can do?’. A courtesy call, that’s the main thing. I don’t think they should be spending a lot of their time coming in and out, no. I’ve got everything.
C10, control patient
Staff experiences of EXTRAS
This section describes staff experiences of and views about delivering EXTRAS. Across the study centres, staff reported a mixture of factors that promoted (‘promoters’) and factors that challenged (‘challenges’) the delivery of EXTRAS and/or influenced their appraisal of it. These have been identified and framed by drawing on NPT67 to analyse the 22 staff interviews. In the NPT framework, the work that people do around implementing an intervention can be described using four areas of work (‘constructs’) and activities within these areas of work (‘components’). These are shown in Appendix 2, Table 63.
We report promoters and challenges identified by staff under relevant components of NPT. The interview data did not include a promoter and challenger for every component. Inclusion of a promoter or challenge does not imply that it was experienced by most staff or study centres, only that staff perceived it to have potentially influenced intervention fidelity or the effectiveness of EXTRAS.
Coherence or sense-making
Differentiation
Promoters
-
Perception that EXTRAS addressed an unmet need for ongoing contact and support, especially in areas with limited access to follow-up.
-
Observation that EXTRAS reviews were comprehensive, whereas reviews in usual care tended to focus on medical matters or a single issue, for example mobility.
-
Perception that goal-setting and action-planning was patient led and aimed to encourage self-management. This was not always practised in usual care (e.g. action plans for goals set in usual care might focus on what the therapists would do in therapy sessions, rather than what the patient would do independently of the therapy sessions).
Challenge
-
Observation by respondents in six services that similar reviews (at 6 months) and/or other ongoing contact, such as a stroke co-ordinator, that offered a route to referral were already offered to patients in their area.
Communal specification
Promoter
-
Following training, staff understood that the objectives of EXTRAS corresponded to its key components.
Individual specification
Promoters
-
Attending training provided by the trial co-ordinating centre. Occasionally, staff acted outside their remit for the trial (conducted an additional review or follow-up call for a patient; conducted physiotherapy reviews when a need was identified), but staff were aware that this was not expected.
-
Prior experience in reviewing patients and in goal-setting.
Challenges
Some staff were uncertain about:
-
Whether or not they always needed to ask about all domains of need in the review when they were confident that the patient had no needs in one or more domains. Staff tended to tailor the review based on their familiarity with the specific patient.
-
How to document the goal-setting and action-planning.
-
When to arrange a home visit instead of a telephone call (some felt that a home visit might be more appropriate for reasons other than patients’ difficulties with cognition, speech or hearing).
Internalisation
Promoters
-
Confidence that enhanced follow-up (beyond usual care) would benefit some/all patients based on their understanding of existing research evidence.
-
Perception that they could use the review data as evidence of unmet needs to inform future service provision.
Cognitive participation or relational work
Initiation
Promoter
-
Each study centre had a named EXTRAS lead.
Challenge
-
At a few study centres, when the original lead staff member left their post the handover of the research/intervention was not smooth.
Enrolment
Promoter
-
Perception of the intervention as an extension of ESD services.
Challenge
-
View of staff or their managers that the intervention should be delivered by a community-based team rather than a hospital-based ESD team.
Legitimation
Challenge
-
Disapproval of any patient receiving the intervention only by telephone. A few staff thought that at least one visit was necessary to understand how a patient coped in their home environment, and to identify safety risks or carer strain. This was felt to be necessary so that advice given and goals set were appropriate. Face-to-face interactions were felt to be important for encouraging discussion if patients were reticent:Study location 15, EXTRAS staff
Yes, I think if you are face to face with people, you can tease things out. You can see things, you can introduce an idea, a suggestion to try to get them to open up. You can use all your counselling skills for something like that, which over a phone is difficult, because you can only go on what you’re hearing over the phone and only feedback on what they are giving you.
Activation
Promoter
-
Belief in the importance of the research for stroke care in general, even if staff did not anticipate that EXTRAS would make a difference to patients locally.
Challenges
-
Sense of frustration if unable to contact therapists involved in a patient’s care to co-ordinate action plans.
-
View that being an ‘advice’ or ‘support’ service for those patients without rehabilitation goals was a poor use of their time.
-
Emotional discomfort or (in one case) the sense that the intervention was unethical if staff were unable to offer appropriate support for an identified rehabilitation need:Study location 8A, EXTRAS staff
The problem is, we felt that we were offering them something that we didn’t have. So we were identifying they had rehab needs which we had to do as part of the trial, and then we were coming to do a goal, action plan or whatever. It could have been like we would recommend this person might be referred to neuropsychology, well we haven’t got one. So it was a little bit like dangling the carrot and then not being able to give them it. So sometimes from an ethical point of view, we felt a little bit uncomfortable [. . .] sometimes it was really awkward because we just sort of said, ‘Well obviously we have identified there is a need there’, but we could not refer them on to anybody.
Collective action or operational work
Relational integration
Challenge
-
The perception that staff responsible for recruitment had ‘selected’ too many high-functioning patients who did not have rehabilitation goals.
Interactional workability
Promoters
-
Positive working relationships within the service and with other services – this was important for referring appropriately and communicating goals/action plans to others treating the patient.
-
Having sufficient time during the review contact to ‘tease out’ problems and explore solutions with patients.
Challenges
-
Time: each review plus the associated paperwork and actions took an average of 2 hours.
-
Needing to prioritise clinical work over EXTRAS, so some reviews were missed.
-
Frequent difficulties in contacting patients and needing to rearrange appointments, adding to the burden on staff.
Contextual integration
Challenge
-
Staff not being allocated protected time for providing EXTRAS.
Skill-set workability
Promoters
-
There was good communication between EXTRAS and other services involved in rehabilitating patients:Study location 5, community staff
[. . .] just three or four patients from EXTRAS have come to us, but anyway they’ve been on the books so it didn’t make much difference, actually. It was just like, after [intervention staff] used to see [a patient] she [informed my team], ‘The patient has highlighted this need’. And the patient was already on my books, I used to tell [intervention staff], ‘Yes, we have addressed those things’.
-
Having knowledge about relevant local services and referral routes into these.
-
Staff being allocated patients that they knew from ESD, or at least being matched according to their discipline and the patient’s primary need.
Challenges
-
Poor communication between EXTRAS and other services involved in rehabilitating patients. There was the potential for unrealistic or inappropriate goals to be set in reviews:Study location 12, EXTRAS staff
[. . .] to be able to set goals when somebody’s still having ongoing therapy is extremely hard and also, doing it blind, so over the phone you’re setting goals. You have no idea which are appropriate or not [. . .] because 18 months down the line, you’ve got no idea what’s been tried and failed, because we really struggle to have contact with physios that were seeing them in outpatients. [. . .] I know we’re led by the patients, but if they don’t answer questions [about their active therapy, abilities] as we need them to, then we’re possibly giving inappropriate advice.
-
Discomfort discussing with patients matters outside the staff member’s own discipline.
-
Staff changes within EXTRAS – it was not always possible to hand over the conduct of reviews to someone who knew the patient.
Reflexive monitoring or appraisal work
Individual appraisal and collective appraisal
Promoters
-
Individual staff perceived benefits for patients: being a ‘sounding board’ for concerns; reducing isolation by connecting patients and carers to services; offering an opportunity for the patient to reflect on their status and achievements and ‘realise they were doing OK’; and supporting patients to ‘push themselves’ and gain confidence in their abilities:Study location 8A, EXTRAS staff
I think from a kind of psychological point of view they were benefiting because I think they felt that, ‘Oh this is contact and it is still contact from the stroke team’, and that we were still caring and listening to them. [. . .] they felt that they were getting some benefit even if it was just somebody sitting down having a chat. Because sometimes it might not have even been around the goals or the action plan, it might have been just the fact you are sitting having a chat, or you are giving them some advice.
-
Individual staff perceived that early intervention in psychological or emotional issues probably prevented referrals.
-
Individual staff valued the opportunity to identify and address risks (safety/falls) or higher cognitive problems that only became apparent several months after a patient’s stroke.
Challenges
Individual staff observed that some patients:
-
Did not share staff’s understanding of the reviews’ purpose and viewed them as a ‘check-up’ rather than a ‘goal-setting exercise’.
-
Did not want to/were not able to fully participate in goal-setting because of cognitive impairment, limited insight into their own abilities or lack of motivation. Patients sometimes agreed to goals and then, when these were evaluated at the next review, admitted that they were not personally meaningful.
-
Became dependent on the review for help with problem-solving or for motivation (e.g. asking for reviews between or beyond the scheduled reviews).
-
Did not benefit in terms of outcomes:Study location 15, EXTRAS staff
[. . .] when I did their very last reviews, [patients] were very grateful; they wanted to thank the team for all the support that they had had and one of them was quite sorry that the calls would be finishing. [. . .] But from an outcome point of view, I didn’t feel anything had necessarily changed for them.
-
Found the review appointments inconvenient (one respondent reported consensus on this among EXTRAS staff at her study centre), tiring or even distressing if they did not remember that they were a trial participant or did not want to be reminded about their stroke.
At one study centre, the respondent said that staff agreed that later reviews (12-month and especially 18-month reviews) were less useful. Few patients had new goals and needs became predominantly non-stroke related, for example comorbidity, age-related decline in mobility or senses, or family problems.
Reconfiguration
Promoter
-
Perceptions that the review content was useful, especially around emotional and social issues. In at least two study centres, this resulted in changes to usual care being implemented after the study had finished, that is incorporating some of the questions into 6-month reviews or planning a 6-week telephone review based on the EXTRAS format.
Impact of EXTRAS on community rehabilitation staff and their services
According to the interviewed community rehabilitation staff, EXTRAS had little impact on community staff and their services. The intervention data reported in Chapter 5 confirm that a very small number of new referrals were made at any study centre.
At some study centres, more contact than usual was reported between EXTRAS staff and community rehabilitation staff to discuss patient goals or referrals. This was not perceived as a substantial burden as patient numbers were small.
Patient and carer experiences of EXTRAS
Patients tended to have poor recall of EXTRAS. The interviews took place at least 6 months after their last EXTRAS review. Some patients whose records showed that they completed reviews and set goals did not remember participating in any reviews and/or did not remember goal-setting . Patients’ responses sometimes indicated that they confused EXTRAS reviews with the study outcome assessments. None of the carers reported having participated in an EXTRAS review (this is confirmed by the study records). Carers’ knowledge of the reviews was based on what the patient had told them, so they tended to share the patient’s view of the service.
Patients who remembered their reviews and were able to reflect on their experience of them, were typically relatively young and had recovered well. They reported that:
-
arrangements were convenient
-
reviewers were personable and ‘helpful’ in terms of advice given
-
reviews were reassuring and thorough in covering potential post-stroke issues
-
repeated reviews over an extended period were targets/milestones for ‘weaning off’ support (e.g. improving mobility to not need a walking stick).
Patients who had made a near-full recovery within the first 6 months or so of their stroke tended to feel that the later reviews were not necessary for them.
A few patients described their reviews as a series of reviewer’s questions rather than as opportunities to discuss current rehabilitation issues and personal goals. Most of these patients expressed satisfaction with this approach. No patient or carer reported any disadvantage of participation.
Three patients in the intervention group complained about a lack of follow-up, yet records showed that they had received EXTRAS reviews and had set goals. Two of these patients perceived an unmet need for further therapy. One had completed all five reviews but said that he had not been contacted by any health-care professional since ESD and was unhappy that his mobility had deteriorated since discharge from hospital. He recalled ‘filling in a questionnaire’ that seemed to describe the study outcome assessment rather than a review:
Well you get the feeling from the hospital’s point of view, that once you’re discharged you’ve, goodbye cheerio. [. . .] I signed up for this [the study] in hospital. The girl that presented it, I thought I was going to have more and more OT [occupational therapy] attention throughout a long period. But all it seems to be is just a talking shop, and filling in a questionnaire; ‘How do you feel about this, that and the other?’. As far as I’m concerned nothing has ever come about it, you know?
I7, intervention patient
The other patient was uncertain whether or not she had participated in reviews:
I think they [unidentified health-care professional] just rang us up and asked us different things. That’s about it, I can’t remember anyone actually visiting us. I’m not saying they haven’t, but I can’t remember. [. . .] Because I think if they had [provided ongoing rehabilitation], I’d have got this back [arm function].
I3, intervention patient
The patients who recalled goal-setting in the reviews said that it motivated them. The evaluation of their goals was an opportunity to reflect on their progress and have a sense of achievement:
[. . .] it felt like a continuing care, even if it is just a phone call. I hadn’t realised, I hadn’t thought about it like that actually. It felt very important [. . .] it helped me see the improvement that I was making.
I1, intervention patient
These patients said that they had achieved what they wanted to in terms of their recovery.
Discussion
The interview study has several findings that add to our understanding of how and in what context the service was delivered, and that help to interpret the intervention data.
1. EXTRAS was not clearly distinct from usual care in some study centres.
Overall, staff bought into the general rationale for EXTRAS when they commenced the trial, but a minority were not convinced that it was needed in their location as they felt that patients’ needs were already being addressed and that goals were being set in community rehabilitation. In some areas, local services already offered patients and carers an ongoing point of contact with a stroke-specialist health-care professional or volunteer support officer; furthermore, in most areas, patients could be referred back (usually via their GP) into a community rehabilitation service several months or even years after their stroke. Nevertheless, several staff acknowledged that in their localities there were gaps in meeting some longer-term needs, particularly psychological/emotional needs and higher-level cognitive impairment. Some thought that an extended community stroke service could help to close those gaps, but this would not necessarily use the EXTRAS design (e.g. it could be provided by a service other than ESD).
One of the aims of the intervention was to provide a route to referral, but study centres made very few new referrals as a result of EXTRAS reviews. Interviewed staff indicated that this was mostly because patients’ needs were already being addressed in community rehabilitation or occasionally because there was a lack of suitable services to refer to. Consequently, the impact of EXTRAS on individual community rehabilitation services appeared to be negligible.
The control group participants did not all experience an abrupt end to ongoing rehabilitation and care after ESD, which was initially identified as a motivation for this trial in the protocol. A few control group patients and carers described having been followed up by health and/or social care professionals for as long as they needed. In addition, although some patients and carers in both groups expressed an unmet need for follow-up or further health care, others reported that they had had no need for further support after ESD and did not feel concerned when therapy finished: this reflects a finding of another interview study with community-dwelling stroke survivors in the UK. 69
The intervention patients and carers interviewed were not consistently more satisfied with their care than the control sample, although some found that the intervention was helpful in terms of reassurance or motivation to continue their self-practice. In contrast, the trial outcomes data show that the intervention group was more likely to be satisfied with services. This was a relatively small interview sample and some of the intervention sample did not recall having received the intervention. Interviews were conducted > 6 months after the last EXTRAS review so as not to affect the 24-month outcome assessment.
2. EXTRAS was delivered with good fidelity to the protocol.
Evidence from the interview study corresponds to the intervention data findings that the fidelity of intervention delivery was good, that is that EXTRAS was delivered as per protocol. However, some staff at some study centres tailored the delivery of the EXTRAS review based on their prior knowledge of patients’ current rehabilitation status and issues, which meant that they did not ask questions they perceived to be irrelevant. Staff were trained to deliver EXTRAS reviews and most had prior experience in conducting reviews and/or goal-setting with stroke patients. The intervention was designed to be delivered by senior members of ESD teams. In one study centre, several reviews were conducted by someone other than a member of the ESD team owing to a lack of capacity in the ESD team: that reviewer was based in a service that worked closely with ESD and saw patients as they transitioned from ESD. This arrangement made it possible for the trial to run at that study centre, and as the centre randomised only a small number of patients it seems unlikely that this exception would have affected overall trial outcomes.
Patients were offered a regular structured review with ongoing support, advice and encouragement. The intervention was designed to include carers, but carers were rarely involved in the reviews. According to staff, the main reason for carers’ lack of involvement was that most reviews were conducted by telephone with the patient only: carers could more readily be involved if reviews were conducted as home visits.
3. The context of the early supported discharge services affected delivery of EXTRAS.
Staff turnover meant that handover to other staff in the trial (if the lead staff member left) or handover of patients was not always smooth, and there were sometimes time pressures where staff needed to prioritise clinical duties over EXTRAS reviews. Nevertheless, the intervention data show that staff unavailability or miscommunication regarding which patients were due to have a review did not significantly reduce the rate of completion of reviews.
Positive working relationships within the service and with other services, and knowledge of local non-statutory services, were perceived as important in being able to deliver the intervention. Conversely, poor communication with other rehabilitation services meant that at some study centres the reviewers did not know patients’ current rehabilitation plans and this made it difficult to co-ordinate care and to set goals.
4. Staff perceived some problems with the design of and patient recruitment to EXTRAS.
The primary outcome for the trial was the NEADL Scale at 24 months post randomisation. EXTRAS did focus on EADL as well as emotional/psychosocial aspects of recovery. However, intervention staff were not directly providing therapy, supervising practice of EADL activities or routinely conducting face-to-face patient assessments. Some staff, therefore, believed that any benefit was likely to be psychological or emotional and did not perceive that EXTRAS would have an effect on physical outcomes. The support, advice, encouragement and reassurance offered to patients was perceived by staff to be the component of EXTRAS from which patients were most likely to have benefited. Their view seems to be supported by post hoc analysis of trial outcome data, which found fewer cases in the intervention group of anxiety at 24 months and depression at 12 months. Staff did consider that it was important that stroke survivors received emotional/psychosocial support; however, some staff thought that patients’ need for this support could be met without using so much of therapists’ time, for example by voluntary sector support services or a stroke co-ordinator/specialist nurse.
Staff believed that the ongoing telephone contact from a stroke service was generally appreciated by patients and carers but was inappropriate for some patients because of their cognitive problems. EXTRAS did not have sufficient flexibility (resources) to offer domiciliary assessments routinely but the protocol did enable face-to-face assessments to be undertaken at the discretion of a reviewer. Staff described clinical rehabilitation activity in the community as being under various competing pressures, particularly because of administrative and ‘information exchange’ activities. The time spent in information exchange has also been found to limit therapy in stroke units. 69,70
Staff gave other reasons for anticipating that the intervention would not be more effective on the NEADL Scale than usual care. Some thought that the ‘wrong patients’ had been recruited at their study centre: patients without significant rehabilitation needs who recovered quickly and patients with cognitive difficulties who staff perceived could not engage in goal-setting. The goal-setting component of the intervention raised issues for some staff who conducted the reviews. Staff described wanting to take a patient-led approach because they believed that if patients set goals that were personally meaningful then they would have more intrinsic motivation to achieve them. However, some staff also expressed concern about goals being appropriate and achievable if they had not recently (or ever) seen the patient in their home environment and were not sure what was being addressed in any community rehabilitation the patient was receiving. In some circumstances, it may not be desirable for goals to be patient led if, for example, patients lack insight or safety is a concern. 71,72 As reviews were not directly observed, it is unknown what proportion of goal-setting was patient led rather than therapist led. Other research suggests that therapists are not always good judges of whether rehabilitation is patient led or therapist led,73 and tend to overestimate the collaborative nature of goal-setting in stroke rehabilitation. 74
Apart from the issue of setting appropriate goals by telephone, staff reflected that goal-setting was not appropriate for all patients (whether in the intervention or usual care study group). Some staff described this as patients ‘not engaging with’ goal-setting or not being ‘motivated’ to complete action plans. The concept of ‘patient motivation’ has been critically evaluated in the literature;75 ‘unmotivated’ patients may in fact be unclear on their role or feel that they do not have the knowledge necessary to take the lead. 74 The problem of role is apparent in this interview study: patients did not always share staff’s understanding of the purpose of the review. Some patients saw the reviews as check-ups and ‘not about goal-setting’. In the interview sample, patients who remembered that they had been encouraged to set goals found it helpful. If they instead described the reviews as check-ups, some valued this, but some did not because it did not result in more therapy or a better understanding of their prognosis.
Strengths and limitations of the interview study
We conducted interviews with at least one EXTRAS staff member at all of the study centres (in one case the respondent was not a member of an ESD team) and with community rehabilitation staff at most locations. This enabled us to gather views about EXTRAS from staff working in a range of settings and with varying levels of professional experience.
A smaller sample of patients and carers was recruited than envisaged in the protocol. There was some evidence of patients having ‘research fatigue’76 by the point of recruitment to this interview study; some who declined the interview said that they felt that they had contributed enough or had nothing further to say. As with all voluntary research participation, there is the potential for non-response bias. However, each sample did produce a range of opinions about the intervention and usual care.
The intervention patient sample was disproportionately male (80%) compared with the trial participants overall (60% male). The control patient sample (50% male) was more similar to trial participants overall. The interviews with patients, carers and staff, however, did not indicate that there was a sex difference in response to the intervention.
Most patients in the intervention sample were not able to discuss EXTRAS in much detail (if at all). Those who did recall the intervention in more detail tended to be younger and had made a good recovery; in that sense they were not representative of the wider intervention group. The interviews took place after the patients’ and carers’ 24-month outcome assessment, that is at least 6 months after the final rehabilitation review, to avoid influencing participation in the outcome assessment. It was difficult for respondents to recall the reviews, which were telephone conversations, after so much time had elapsed. To have a more comprehensive understanding of patients’ and carers’ perceptions of what was helpful or not about the rehabilitation reviews, it may have been better to schedule the interviews shortly after the final review and risk a small proportion of patients/carers not wishing to complete the 24-month outcome assessment. Given that these patients and carers agreed to an interview of up to 1 hour, it is possible that they would still feel engaged enough with the study to complete the (shorter) outcome assessment some months after the interview.
None of the carers (of intervention group patients) who participated in an interview had taken part in any of the rehabilitation reviews. Our sampling approach excluded patients with cognitive or communication impairments of a degree that they could not consent to or participate in an interview. This also excluded the carers of those patients, who were perhaps more likely (than carers of patients without these impairments) to have participated in a review alongside or on behalf of the patient. Carers participated in only 10% of reviews overall and, given the other (temporal and geographical) restrictions on sampling, this targeting may not have proved helpful in practice.
Conclusion
The interpretation of the interview study findings concurs with the intervention data findings in pointing to the intervention concept and design, rather than fidelity of delivery, as being an important reason for the neutral result of the trial on the primary outcome and primary analyses. The support and advice offered by the intervention was perceived to be beneficial for patients’ emotional health, but review, goal-setting and evaluation without direct therapeutic intervention (whether or not the patient was still receiving therapy in usual care) was not perceived to be necessarily effective in improving EADL. The recruitment of some study centres that already offered extended contact with a community stroke service may also have limited the potential intervention effect. Some staff thought that the patient case mix was likely to reduce the potential of seeing an intervention effect as some patients had mild symptoms and recovered quickly, whereas others had a cognitive impairment that made them unsuitable to receive a telephone review.
Chapter 7 Routine rehabilitation service mapping
Aim and objectives
During the trial, a mapping exercise was undertaken to determine the routine rehabilitation and follow-up services provided for stroke patients by each participating study centre, or by other organisations within the vicinity of the study centre to which patients had access.
The objectives were:
-
to describe the characteristics of ESD services at each participating study centre
-
to identify the services to which patients may be signposted or referred for ongoing rehabilitation and support in the longer term, after ESD.
Methods
Between May and September 2016, a RA (ES) conducted structured telephone interviews with a senior member of each participating ESD team. Following an e-mail invitation, the interview questions were sent to staff in advance and they were asked to read through the document in case any clarification was needed and to obtain any readily available data relevant to the questions.
Interviews were digitally recorded and data were entered into a pro forma Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) sheet for analysis. Prior to the start of the interview, all participants provided consent to take part. Although there were 19 study centres taking part in the EXTRAS trial, 20 interviews were undertaken. This was because ESD was provided by two teams at one of the study centres.
Information about the ESD service was sought, including the criteria that patients needed to meet to be eligible for the ESD service, the professional disciplines within the team, the service provision (e.g. duration of rehabilitation) and the options available at discharge from ESD.
Information was also sought about routine stroke reviews undertaken post hospital discharge. The English National Stroke Strategy 2007–201777 recommends that stroke patients should be reviewed at 6 weeks and 6 months post discharge from hospital to assess their health and social needs and to optimise secondary prevention. 77 This is supported by a National Institute for Health and Care Excellence (NICE) Quality Standard78 that requires provision of a structured health and social care review at 6 months and 1 year after stroke, and annually thereafter. 78 Interviewees were asked about the local arrangements for providing these reviews.
The interview also contained several prompt questions to identify and describe the community services that were available to address one or more of the needs of stroke survivors after the ESD service was withdrawn. Fourteen domains of need were included: (1) mobility and movement, (2) communication, (3) everyday activities, (4) emotional and psychosocial issues, (5) impaired cognition, (6) nutrition, (7) visual disturbance, (8) incontinence management, (9) support with relationships and sex, (10) pain management, (11) return to work/volunteering, (12) return to driving, (13) healthier lifestyles and (14) support services (e.g. stroke-specific information and advice). Interviewees were also asked if any services had been introduced or had been withdrawn over the course of the trial.
Results: characteristics of early supported discharge services at participating study centres
Early supported discharge services were multidisciplinary teams (MDTs) that were provided as either a stand-alone ESD service for stroke patients (n = 11, 55%) or an integrated ESD and community stroke service (n = 9, 45%); that is, the same service provides both ESD and less intensive community stroke rehabilitation.
The criteria that patients had to meet to be eligible to receive the ESD services are shown in Tables 21 and 22. A total of 19 (95%) ESD services cared exclusively for stroke patients. One service provided ESD to both stroke patients and those with brain injury. Services varied in terms of the patients’ level of independence in transfers and in how recent the stroke must have been for the patient to be accepted into the service. Some services required patients to have agreed rehabilitation goals by the time they were seen by ESD staff. A total of 11 (55%) ESD teams used other criteria to identify patients who could receive their service, for example continence managed.
| Location | Eligibility criteria | Flexible with criteria | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Confirmed diagnosis of stroke | Medically stable | Transfers independently/assistance of one person | Identified rehabilitation goals | Aged ≥ 18 years | Able to provide informed consent | Limit to time since stroke | Other criteria (see Table 22) | ||
| 1 | Yes but see functional stroke mimic patients | Yes | No: accept if transfers with assistance of two people and safe between visits | Yes | Yes | No: best interest basis if cognitive impairment | No | Yes | No |
| 2 | Yes | Yes | Yes | Yes | Yes | Yes | Yes: up to 12 weeks from date of stroke | No | No |
| 3 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
| 4 | Yes | Yes | Yes | Yes | Yes | No: next of kin accepted | No | Yes | Yes: around assistance of two people to transfer |
| 5 | Yes | Yes | No: transfers must be manageable | Yes | Yes | No: best interest basis if cognitive impairment | No | Yes | Yes: if caseload allows |
| 6 | Yes | Yes | Yes | Yes | Yes | No: next of kin accepted | Yes: within 14 days of stroke onset | Yes | No |
| 7 | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| 8A | Yes | Yes | Yes | No | Yes | Yes | No | Yes | No |
| 8B | Yes | Yes | No: will accept if unable to transfer with assistance of one person | No | Yes | Yes | No | Yes | Yes: if caseload allows |
| 9A | Yes | Yes | Yes | Yes | Yes | Yes | Yes: within 14 days of stroke onset | No | Yes: if caseload allows |
| 9B | Yes | Yes | No: up to with assistance with two people | No | Yes | No: best interest basis if cognitive impairment | No | Yes | No |
| 10 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes: if caseload allows |
| 11 | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| 12 | Yes | Yes | Yes | No | Yes | Yes | Yes: 30 days post onset | No | No |
| 13 | Yes or acquired brain injury | Yes | Yes | Yes | Yes | No: next of kin accepted | No | Yes | Yes |
| 14 | Yes | Yes | Yes | Yes | Yes | No: next of kin accepted | Yes: within 14 days of stroke onset | No | No |
| 15 | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| 16 | Yes | Yes | No | No | Yes | No: next of kin accepted | No | No | Yes |
| 17 | Yes but see patients with stroke mimics | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| 18 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
| Criterion | Number of ESD teams (N = 20), n (%) |
|---|---|
| Continence managed | 8 (40) |
| Home environment suitable/risk assessed | 3 (15) |
| Nutrition managed | 3 (15) |
| Cognitively able to cope at home | 2 (10) |
| Medication managed | 2 (10) |
| Risks minimised between visits | 2 (10) |
| Able to communicate basic needs/raise alert | 1 (5) |
| Able to cope with intensity of rehabilitation (physically/cognitively) | 2 (10) |
| Care package arranged if needed | 1 (5) |
| Lived in own home prior to stroke (not care home) | 1 (5) |
| Low carer strain | 1 (5) |
| Tissue viability | 1 (5) |
Table 23 illustrates the professional disciplines in the ESD teams, the service provision and the options for rehabilitation after ESD. All ESD teams that participated in the trial had physiotherapy and occupational therapy professionals. Two services did not have SLTs but accepted patients with speech disorders who were treated by a separate service. Within the ESD teams, most of the direct patient care was provided by rehabilitation assistants/associate practitioners/technical instructors, who followed plans set by therapists. Provision of nursing and psychology expertise within the ESD team was not universal and was available in 16 (80%) and 8 (40%) of the services, respectively. When nursing was not part of the ESD service, most teams had access to community nursing services, but the availability of psychological services was very limited.
| Location | ESD disciplines | Provision | Rehabilitation after ESD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PT | OT | SLT | Rehabilitation assistants | Psychology | Nursing | Other | Maximum interval from hospital discharge to ESD assessment/visit (hours) | Service availability (days per week) | Duration of treatment in this service (initial, most intensive period) | ||
| 1 | Yes | Yes | Yes | Yes | No | Yes | 24 | 7 | 2 weeks (some flexibility to extend) | Separate community services | |
| 2 | Yes | Yes | Yes | Yes | Yes | Yes | Stroke care co-ordinator | 48 | 5 | 6 weeks (some flexibility to extend) | Separate community services – ESD may continue while patient on waiting list |
| 3 | Yes | Yes | Yes | Yes (technicians) | No | Yes | Administrator | 24 | 5 | 6 weeks | Separate community services |
| 4 | Yes | Yes | Yes | Yes (associate practitioners) | Yes | Yes | 24 | 7 | 6 weeks | Step down to community strand within same service as ESD – no wait | |
| 5 | Yes | Yes | Yes | Yes | No | Yes | Dietitian | 48 | 7 | 10 weeks | Separate community services |
| 6 | Yes | Yes | Yes | Yes | Yes | Yes | Technical instructor, social worker, stroke information co-ordinator | 24 | 7 | 6 weeks | Step down to community strand within same service as ESD – no wait |
| 7 | Yes | Yes | Yes | Yes | Yes | Yes | 24 | 7 | 6 weeks (some flexibility to extend) | Separate community services | |
| 8A | Yes | Yes | Yes | Yes | No | Yes | Stroke care co-ordinator (nurse) | 24 | 7 | No time limit | Separate community services – ESD continues to see patients who need OT |
| 8B | Yes | Yes | Yes | Yes | No (access to old-age psychiatry) | No (access to district nurse) | 24 | 7 | 6 weeks (some flexibility to extend) | Separate community services – no wait | |
| 9A | Yes | Yes | Yes | Yes | No | Yes | Stroke service team lead | 24 | 7 | 6 weeks | Step down to community strand within same service as ESD – no wait |
| 9B | Yes | Yes | No | Yes (associate practitioners) | No | Yes | Health-care support workers | 24 | 7 | No time limit | Separate community services |
| 10 | Yes | Yes | Yes | Yes | Yes | Yes | 24 | 6 | 6 weeks (some flexibility to extend) | Separate community services | |
| 11 | Yes | Yes | Yes | Yes | No (access to psychology)a | Yes | 24 | 7 | 6 weeks (some flexibility to extend) | Separate community services | |
| 12 | Yes | Yes | Yes | Yes | Yes | Yes | 24 | 6 | 6 weeks | Separate community services | |
| 13 | Yes | Yes | Yes | Yes | Yes | Yes | Stroke co-ordinator | 24 | 7 | 6 weeks (some flexibility to extend) | Step down to community strand within same service as ESD |
| 14 | Yes | Yes | Yes | Yes (technical instructors) | No | Yes | Stroke co-ordinator (nurse) | 48 | 5 | 6 weeks | Step down to community strand within same service as ESD |
| 15 | Yes | Yes | Yes | Yes | Yes | Yes | 24 | 7 | 6 weeks | Separate community services | |
| 16 | Yes | Yes | No | Yes | No | No | 48 | 5 | 6 weeks (some flexibility to extend) | Separate community services | |
| 17 | Yes | Yes | Yes | Yes | No | No (access to stroke nurse and nutrition nurse) | 24 | 7 | 6 weeks (some flexibility to extend, except strict limit for OT) | Separate community services | |
| 18 | Yes | Yes | Yes | Yes | No | No (access to nurse) | 24 | 7 | 12 weeks | Step down to community strand within same service as ESD | |
A total of 14 (70%) of the ESD services operated 7 days per week, four (20%) services operated 5 days per week and two (10%) operated 6 days per week. All services provided stroke rehabilitation at home within 48 hours of patient discharge from the hospital as this was a criterion for being an EXTRAS site. A total of 13 out of 14 services that were provided 7 days per week were able to visit patients at home within 24 hours of hospital discharge.
Most (n = 18/20, 90%) of the services provided rehabilitation that they considered to be an ESD service (i.e. intense rehabilitation following early discharge from hospital) for a time-limited period. The maximum treatment period ranged from 2 to 12 weeks. Most commonly, the treatment period was 6 weeks (n = 15/20, 75% of services), with some flexibility on this limit in some services. However, two services did not have a limit for how long they treated patients, instead discharging patients once agreed goals had been met or once patients were judged to have no further rehabilitation potential.
After ESD rehabilitation was completed, if patients were judged to have further rehabilitation potential, the next stage of the pathway varied according to the local service model and disciplines required. Patients could be:
-
referred to other stroke-specialist, neurospecialist or generic community services and discharged from the service that delivered ESD (n = 12, 60%)
-
referred to other community services but could continue with ESD therapists in specific circumstances, such as if they needed an occupational therapist and no community occupational therapist was available, or if there was thought to be a risk of deterioration during a long wait for community services (n = 2, 10%)
-
stepped down to the community strand of the service (n = 6, 30%).
Results: post hospital discharge routine stroke reviews
Figure 15 shows whether or not routine reviews were offered to stroke patients in study locations at around 6 weeks, 6 months, 12 months and annually thereafter, after discharge from hospital. Seventeen (85%) locations offered 6-week reviews. These were provided by a consultant (n = 13), ESD team (n = 1), specialist nurse (n = 1), stroke co-ordinator (n = 1) and the Stroke Association (n = 1). Seventeen locations (85%) offered 6-month reviews; however, in one location, 6-month reviews were available for only city-based patients. Six-month reviews were provided by a nurse (n = 8), the Stroke Association (n = 3), ESD team (n = 2), stroke co-ordinator (n = 2), community stroke team (n = 1) and consultant (n = 1). The type of nurse who provided the 6-month review was a stroke specialist nurse (n = 3), a district nurse (n = 1) and for the other locations the type of nurse was not specified.
FIGURE 15.
Post hospital discharge routine stroke reviews in study locations.
Staff interviewed in this mapping exercise had less knowledge about the provision of the 12-month and the subsequent annual reviews. Respondents were aware that a 12-month review service was offered at seven locations, not offered in nine locations and for the remaining four locations it was unknown if a 12-month review was offered. Reviews were provided by GPs (n = 4), combined GP and Stroke Association (n = 1) Stroke Association (n = 1) and stroke co-ordinator (n = 1). Ongoing annual reviews were reported as not being available in 13 (65%) locations, unknown in four locations, and, in the two locations where these were offered, they were not universally available, being offered by only some general practices.
In the first year after discharge from hospital, two study locations offered one review, 12 locations offered two reviews and five offered three reviews (however, note that at four locations, it is not known if a 12-month review was offered). One of the study locations did not routinely offer any reviews at the time of data collection but was due to start offering 6-week reviews later that year.
Although not a specific question on the structured interview pro forma, review of the data collected indicated that at 13 study locations a ‘stroke co-ordinator’ was available. These staff were commissioned under various arrangements and had different remits. As above, in some areas they conducted post-discharge routine reviews. In other areas, they provided information and support to patients, and in some areas they appeared to be a point of contact should the patient wish to get in touch.
Results: community services available to stroke patients post early supported discharge
Data collected about community service availability in terms of the 14 domains of need investigated are summarised in Figure 16. A green entry indicates that a service(s) was available, a black entry indicates that a service(s) was available but may not have been accessible by all patients treated by the ESD team because of differing geographical boundaries for care, an amber entry indicates that available services provided some limited care for this need and a red entry indicates no service provision.
FIGURE 16.
Summary of availability of community services to meet needs post stroke in study locations.
Although many community services were available to support post-stroke needs in the different study locations, several area respondents reported limited or no provision for emotional issues, relationship issues, pain management and return to work. In addition, access to the available services could be complex. Data about eligibility criteria were available for 116 out of 280 community services described in this mapping exercise: 23 out of the 116 (20%) had criteria that excluded some stroke patients. Examples included patients aged < 65 years who were excluded from psychology or psychiatry because the service was for elderly patients; individuals with aphasia and/or who were housebound who were excluded from counselling services as the service was by telephone or in an outpatient clinic. In 13 (65%) locations there was at least one community service that would not be available to patients who lived in care homes.
Services varied in terms of stroke specificity, waiting times and duration of access. A summary of these data for services addressing five key domains is illustrated in Table 24. In each of these five domains, where waiting list information was available, approximate waiting times ranged from 1 to 26 weeks between services waiting times could also vary within services as patients were prioritised according to urgency of need. Details of the stroke specificity of services addressing all domains can be found in Appendix 2, Tables 64–67.
| Mobility and movement (n = 54) | Communication (n = 27) | Everyday activities (n = 32) | Emotional and psychosocial issues (n = 31) | Impaired cognition (n = 30) | |
|---|---|---|---|---|---|
| Specificity, n (%) | |||||
| Generic service | 16 (30) | 12 (44) | 3 (41) | 18 (58) | 4 (13) |
| Neurological specific | 28 (52) | 7 (26) | 11 (34) | 8 (26) | 16 (53) |
| Stroke specific | 10 (19) | 8 (30) | 8 (25) | 5 (16) | 10 (33) |
| Waiting lists, n (%) | |||||
| No waiting list | 4 (7) | 3 (11) | 5 (16) | 2 (6) | 5 (17) |
| Waiting list used | 29 (54) | 19 (70) | 17 (53) | 15 (48) | 15 (50) |
| Do not know | 21 (39) | 5 (19) | 10 (31) | 14 (45) | 10 (33) |
| Duration of access, n (%) | |||||
| Open-ended duration | 29 (54) | 16 (59) | 22 (69) | 10 (32) | 15 (50) |
| Limited duration | 6 (11) | 4 (15) | 5 (16) | 7 (23) | 5 (17) |
| Do not know duration | 19 (35) | 7 (26) | 5 (16) | 14 (45) | 10 (33) |
In terms of referral to community services, this could be undertaken directly by the ESD for most services. For some services, referrals needed to be made via the patient’s GP (e.g. specialist clinics for spasticity and/or pain) or via a consultant (e.g. neuro-outpatient service). In some areas, referrals could be made by a stroke co-ordinator. Most community services would, however, accept referrals at any time after stroke.
Using the data obtained about the community services available in each study location, a series of diagrams have been drawn to illustrate the referral options available at the end of ESD. Figure 17 shows study location 1, which had an integrated ESD and community stroke team, and Figure 18 shows study location 5, which had a stand-alone ESD team. Diagrams for other locations can be found in Appendix 2, Figures 22–39.
FIGURE 17.
Referral options available at the end of ESD at study location 1. The arrows indicate that patients can self-refer back into the service. HSCP, health and social care professional; IAPT, Improving Access to Psychological Therapies; OT, occupational therapist; PT, physiotherapist; SPoA, single point of access.
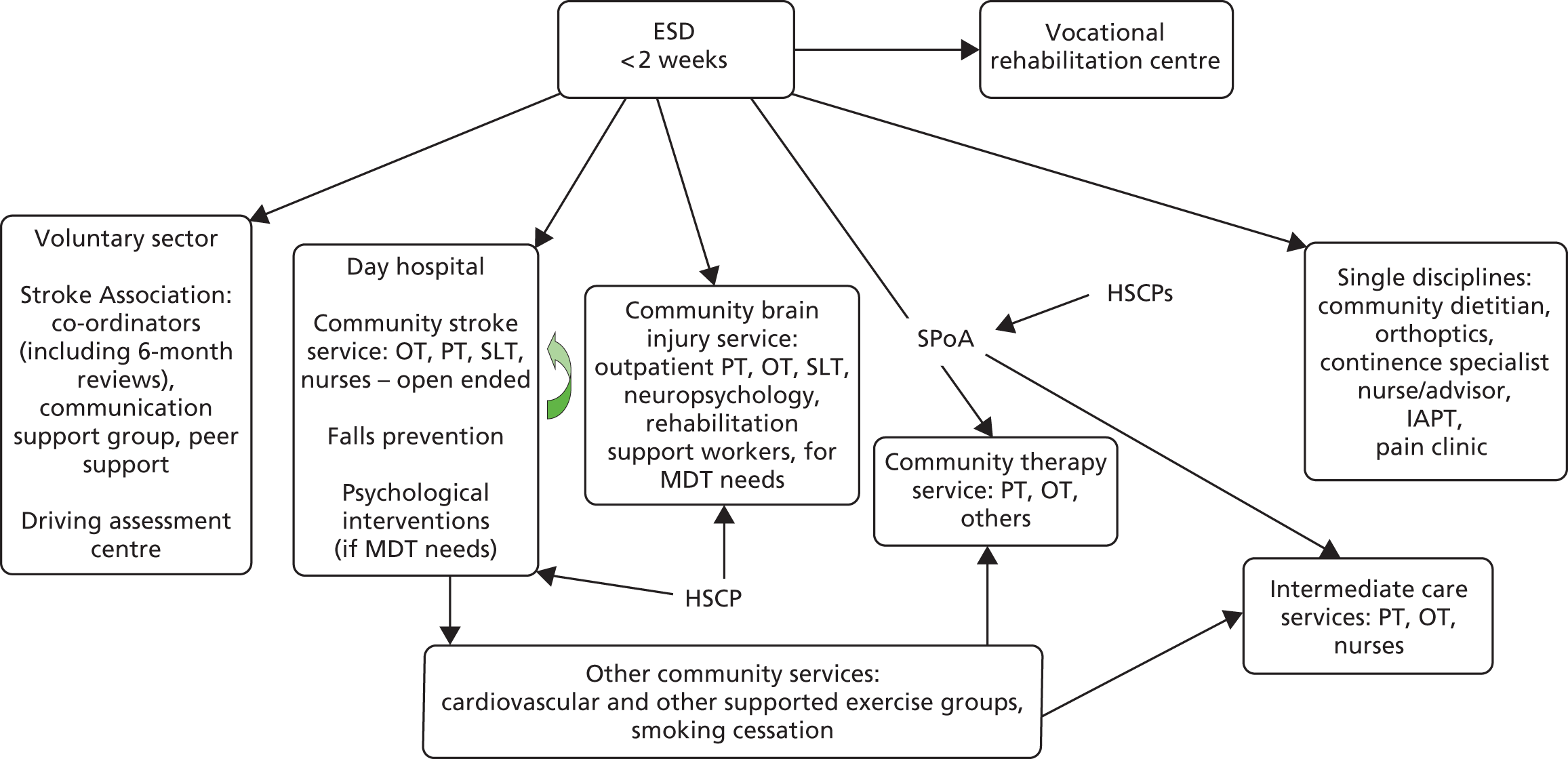
FIGURE 18.
Referral options available at the end of ESD at study location 5. The arrows indicate that patients can self-refer back into the service. a, Part of community neurorehabilitation service. CST, community stroke team; HSCPs, health and social care professionals; IAPT, Improving Access to Psychological Therapies; OT, occupational therapist; PT, physiotherapist.
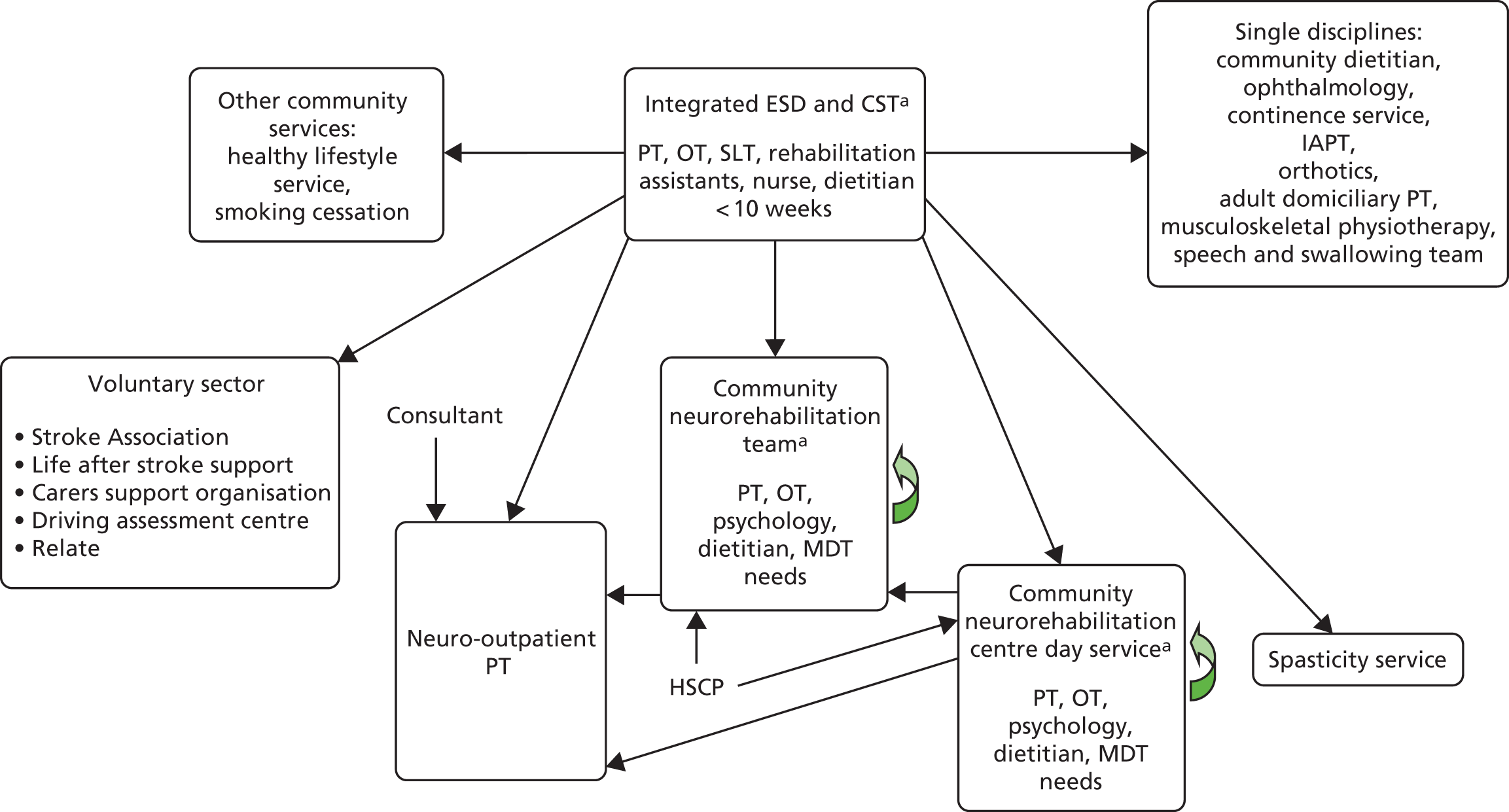
Discussion
This mapping exercise has provided information about ESD services, availability of post hospital discharge routine stroke reviews and community services in participating study centre locations.
To take part in the study, ESD services had to be MDTs that provided rehabilitation at home within 48 hours of patient discharge from hospital for a time-limited period or had clear criteria for discharge of patients from the service. All participating ESD teams continued to meet these criteria at the time of the mapping exercise in 2016.
Although all participating ESD services met the criteria necessary to take part in the study, there were some differences in their organisation and operation. The types of patients accepted differed, for example in terms of required level of independence and time after stroke. Some teams were stand-alone ESD teams and other teams were a combined ESD and community stroke service. The duration of rehabilitation provided also varied. Some teams provided only 2 weeks of ESD rehabilitation whereas other teams had no fixed time limit but discharged patients once agreed goals were met or there was judged to be no further rehabilitation potential.
When compared with available national data on ESD services, the study ESD services were very similar, except more study teams operated 7 days per week (see Appendix 2, Table 68).
Most, but not all, study locations provided 6-week and 6-month reviews post hospital discharge. Because some data are missing about the provision of 12-month or later reviews, it is more difficult to be certain about similarities or differences. However, it appears that fewer than half of the study locations were providing 12-month reviews and possibly only two locations provided a later review. Overall, the majority of study locations provided two post hospital discharge reviews in the first year after the patient left hospital and there was very limited provision for such reviews thereafter. In most areas, the first of these two reviews was undertaken at 6 weeks post discharge. As most ESD services were still providing support at 6 weeks, reviews undertaken at this time would probably be conducted while patients were still in ESD care. This indicates that routine structured follow-up after discharge from ESD services was limited. This is of importance because EXTRAS commenced once ESD services ended and, therefore, was unlikely to significantly overlap with regular routine follow-up services. Although 13 study locations had stroke co-ordinators, their roles outside contributing to these routine reviews appeared to be variable; data provided by respondents did not suggest that they were involved in further regular contact.
National data indicate that 49% of hospitals offered all patients a 6-month review, 43% offered a 6-month review to only some patients and the remaining 8% of hospitals did not offer a review to any patients. 79 This suggests that study locations were performing better in this regard.
The data collected about community services indicate that, in general, most study locations had services to meet the majority of the needs explored. Exceptions to this were emotional needs, support with relationships, pain management and return to work, where several locations had limited provision for these issues. However, access to and operation of the services were very variable and some had lengthy waiting lists. For example, patients could be waiting to access services such as physiotherapy, speech and language therapy or psychology for up to 26 weeks in some places. This variability in community services was not surprising and is in keeping with various publications from both before and during the trial.
Variability in community service availability and operation in the study locations suggests that care for individuals post ESD was likely to differ. This has implications for both the control and the intervention groups in the trial. Control group patients may have received different usual care and intervention group patients may have received both different usual care and different care in relation to EXTRAS, which interacted with existing services. However, location differences were expected and a priori it was planned that all trial analyses would be adjusted for study centre.
This mapping exercise has some limitations. The data were collected from interviews with a senior member of each ESD team participating in the trial. Although these staff were likely to be very knowledgeable about their individual ESD service, their awareness of post hospital discharge reviews and community service availability/operation was likely to have been more limited. Knowledge may also have been variable depending on how an individual ESD team interacted with surrounding services. Indeed, some respondents expressed uncertainty about describing the community services available and how they operated. This was more noticeable with respondents who worked in ESD services where patients were discharged to community services and ESD staff generally had no further contact with patients beyond the duration of their ESD treatment. It would probably have been preferable to obtain mapping information from individuals working within community services, but this would have been a considerably larger study and would have required additional resources.
The diagrams illustrating the summary of community service availability and referral options after ESD were assimilated by one researcher who pulled together the provided interview information. In retrospect, it may have been useful to share these diagrams with the individual teams to check and improve accuracy, but time did not allow for this to occur.
Chapter 8 Health economic evaluation
Summary
The economic evaluation of the trial was undertaken to assess the value of an extended stroke rehabilitation service provided for 18 months after ESD.
Cost per unit change in the NEADL Scale and QALYs for EXTRAS were assessed. Costs (in 2017 prices) are presented from a health and social care perspective and uncertainty is presented as a cost-effectiveness acceptability curve (CEAC) and as deterministic sensitivity analyses.
Over 2 years, EXTRAS was associated with lower costs than usual care (–£311, 95% CI –£3392 to £2787 less per person). Patients randomised to receive EXTRAS used, on average, more health-care services but less social care services, mainly in the form of reduced home help and allowances. There was a 68% chance that EXTRAS was cost saving. The CIs around the difference between the mean NEADL Scale scores for the intervention and the control groups ruled out a clinically important difference. However, in terms of the NEADL Scale, there was an 84% chance that EXTRAS would be cost saving.
EXTRAS provided more QALYs than usual care (0.07, 95% CI 0.01 to 0.12). Given the gain in QALYs and the findings on cost differences, EXTRAS has a 90% chance of being considered cost-effective at 2 years should society be willing to pay £20,000 per QALY. Sensitivity analyses, removal of outliers, alternative EQ-5D-5L tariffs and removing imputed values did not alter this finding.
Despite no evidence of a meaningful difference in the primary outcome (NEADL Scale score) between the study groups, provision of EXTRAS had a 68% chance of being cost saving in terms of QALYs. The changing resource use pattern suggests that it is likely that provision of EXTRAS resulted in cost saving overall, but there were likely to be extra costs to health services, which were offset by savings in social care. QALYs were greater for patients who received EXTRAS. The QALY estimates arguably may capture wider impacts of rehabilitation services beyond those captured by the NEADL Scale on patient outcomes. Given the impact on costs and QALYs, there is a very high chance that EXTRAS would be considered cost-effective at conventional thresholds for society’s willingness to pay (WTP) for a QALY.
Outline
EXTRAS provided regular contact between the patient and a senior ESD team member, usually by telephone, at 1, 3, 6, 12 and 18 months post discharge from ESD. Each assessment consisted of a semistructured interview to identify the patient’s progress, current rehabilitation needs and service provision. There was then joint rehabilitation goal-setting and action-planning, which could consist of verbal advice and encouragement; discussion with others involved in the patient’s care; signposting to local activities or voluntary services; or referral to other services. The evaluation took an NHS and social care perspective to judge whether or not EXTRAS was cost-effective, as judged by the incremental cost per QALY compared with usual care after ESD services. A within-trial economic evaluation of costs per QALY over the 24 months of the trial with a secondary analysis of cost per incremental increase in the primary outcome (the NEADL Scale) was undertaken. The population was that of the main trial and their characteristics are shown in Table 1.
We also assessed the effect of EXTRAS on carers’ health-related quality of life (HRQoL) and QALYs.
Methods
Overview
The evaluation was a within-trial analysis conducted over a 24-month time horizon, without extrapolation or modelling. The EQ-5D-5L was used to estimate QALYs. 43,80 The cost–utility analysis was reported as the incremental cost per QALY gained over the 24-month trial period. QALYs were generated from individual participant responses to the EQ-5D-5L. 43 These responses were converted into utility values using the appropriate tariffs for England (UK tariffs were not available). 80 A CEA was also carried out, with results reported as the incremental cost per unit gain in the NEADL Scale score during the trial period. Changes in NEADL Scale scores were generated for each participant in both study groups.
Costs were based on the NHS and social care resources used by participants in each group of the trial. These data were collected during the trial at individual participant level. Prices were obtained from NHS Reference Costs 2015–1681 and the Personal Social Services Research Unit (PSSRU). 82
Where relevant, stochastic sensitivity analysis, as recommended by NICE in Guide to the Method of Technology Appraisal,83 was conducted. The analyses were conducted on an intention-to-treat basis.
In line with the good practice guidelines,83 both cost and health outcomes were discounted at the rate of 3.5% per annum in year 2, which is the recommended discount rate. The following equation was used for generating a discounted outcome:
where R is the discount rate used and n is the number of years.
All health economic outcomes are reported using descriptive statistics appropriate to the type of data: continuous variables or counts are reported as means and standard deviations (SDs) or medians and IQRs, as appropriate to the distribution of the data. Dichotomous variables are reported as absolute numbers and percentages. Differences in mean resource use, NEADL Scale scores and QALYs are reported, together with bootstrapped 95% CIs.
The EQ-5D-5L was used to estimate QALYs. 43,80 The EQ-5D-5L was completed by each participant at three time points: baseline, 12 months and 24 months. As noted above, responses to the EQ-5D-5L were converted into utility values using appropriate tariffs. The utility values were then converted into QALYs using the area under the curve approach. 84
All analyses were undertaken using Stata version 15.
Health-care resource use
Resource utilisation was recorded at baseline, 12 months and 24 months using an adapted version of the CSRI. 37–39 The following types of resource were included in each group of the trial.
EXTRAS:
-
implementation costs along with the costs of senior ESD member involvement
-
health and social care resources utilised by each patient over the 24-month trial period
-
hospital resources utilised from post randomisation to death for patients who died within the trial period.
Usual care:
-
health-care resources utilised by each patient over the 24-month trial period
-
hospital and social care resources utilised from post randomisation to death for patients who died within the trial period.
For patients who died during the 24-month follow-up period, post-randomisation costs were based on use of hospital services until their death.
Costs: valuing resources
Unit costs for the resources used were obtained from routine sources, for example the PSSRU82 and the NHS Reference Costs,81 when appropriate. Costs were those required to support one patient, which were estimated as the average amount of resources used weighted by their unit costs. Costs were taken from the most recent available price year (2016/17). Where the most recent cost data were not available, available costs were adjusted to 2016/17 prices using the appropriate inflation index. 83
EXTRAS reviews were provided at five time points: 1, 3, 6, 12 and 18 months post randomisation. Each assessment required 2 hours of therapist time, which was costed at £44 per hour. 82 The hourly cost was assumed to be the cost per working hour of NHS band 6 staff. The cost of training professionals was not considered in the analysis. Costs in the second year were discounted,83 which equates to a total intervention cost of £437 per patient, using a 3.5% discount factor.
Outcome measures
Changes in HRQoL were derived from responses to the EQ-5D-5L at baseline, 12 months and 24 months. 43,80 The EQ-5D-5L measures the health of the patient (utility) at that point in time over five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), with each dimension having five levels; therefore, a total of 3125 health states can be defined. Responses were valued using the England population tariffs. 80 The analysis utilised the EQ-5D-5L responses collected at baseline, 12 months and 24 months to generate within-trial differences in HRQoL expressed as QALYs for each patient using the area under the curve approach. 85 One QALY is equivalent to 1 year lived with full health.
Using the QALY estimates derived for each participant, the mean QALY score for each group was calculated. Differences in the patient characteristics between the two groups at baseline in terms of baseline utility, centre, age and sex were accounted for when estimating QALY differences between groups. Information about self-assessed health status was also collected using the VAS component as part of EQ-5D-5L. The VAS asks respondents to rate their health on the day of completion on a scale of between 0 and 100, where 0 represents the worst imaginable state of health and 100 represents the best imaginable state of health.
Missing data
Complete-case analyses, using only cases with complete records of health outcomes and resource utilisation, are likely to suffer from biases as there may be reasons why respondents were unable to provide data. Therefore, our primary CEA used imputation methods to impute missing data. Missing variables were tested for any pattern of missingness across both trial groups. When a cost item, or component of HRQoL, was missing at random it is acceptable to exclude the missing cases if the regression controls for all of the variables associated with the probability of missingness. In the event of partially completed measures, the mean number of missing items was reported. Simple imputation based on means was used because of the small numbers of missing data and this was tested with sensitivity analyses. In the case of EQ-5D-5L, imputation was carried out at the utility score level. The missing resource use data were assumed to be missing completely at random and the reported total costs are based on the non-imputed missing resource use data. The available case analysis approach,86 which is considered more efficient than the complete-case analysis, was followed for resource utilisation.
Regression analysis
Differences in costs were estimated using a general linear model with a gamma distribution, with an identity link function controlling for characteristics such as baseline costs, centre, age and sex to allow for any heterogeneity between the intervention and control group populations.
Health outcomes were estimated by a linear regression based on an area under the curve technique, controlling for baseline utility, age and sex to allow for any heterogeneity between treatment and control populations.
Cost-effectiveness analysis
Costs for each participant were used to generate the average cost per participant in each group of the trial, and thereby the incremental costs. QALYs estimated for each participant were used to generate the average QALYs gained in each of the trial groups, along with the incremental QALYs. The same approach was applied to the NEADL Scale scores. Possible imprecision around costs and QALYs was estimated as 95% CIs using parametric bootstrapping. 87 An incremental cost-effectiveness ratio (ICER) was calculated as the ratio of the difference in mean costs divided by the difference in mean effects between the intervention and the control groups of the trial.
The primary analyses were based on data where imputation was used for missing NEADL Scale and QALY data. Sensitivity analyses were conducted using non-imputed data (i.e. complete cases: patients with complete outcome data), with the removal of any outliers in the cost and using an alternative variation tariff for EQ-5D-5L. The net benefit when society was willing to pay £20,000 per QALY was estimated. Net benefit involves putting a monetary value on any QALY differences by multiplying the mean QALY per person by the value for a QALY. Net costs are then subtracted from this to give a net benefit value that is reported in monetary terms.
Uncertainty and sensitivity analyses
The EXTRAS trial was not powered on health economic outcomes and may, therefore, be underpowered for these outcomes. Therefore, estimation rather than hypothesis testing is appropriate when reporting results. The focus of the analyses is on estimating cost-effectiveness, even when either the potential cost or the effect differences lack conventional statistical significance. This is a standard way of conducting economic evaluations and consistent with the approach adopted by NICE.
As well as a sensitivity analysis around the imputation of missing values, sensitivity analyses were performed around key parameters affecting incremental cost-effectiveness. These are presented as a series of one-way sensitivity analyses to test the impact of changes in one specific parameter on the results, for example exploration of the impact of using alternative unit costs of resource utilisation.
A stochastic sensitivity analysis was also undertaken to explore the impact of uncertainties relating to within-trial estimates of costs, effect and cost-effectiveness. As noted earlier, a parametric bootstrapping technique was used to address the uncertainties around costs and effects and the results were used to plot the cost-effectiveness plane and estimate the CIs in the costs and effects in both groups of the trial. CEACs88 were used to visualise the probability that EXTRAS was cost-effective over a range of maximum monetary values the decision-maker/NHS is willing to pay for a particular unit change in outcome.
Carers’ health-related quality of life
EQ-5D-5L responses were also collected from the carers of trial participants at baseline, 12 months and 24 months. The purpose of estimating any effect there might be on carers’ quality of life is that this could be a factor for decision-makers to consider in relation to future provision of EXTRAS. As for patients, an area under the curve method, controlling for baseline utility, was used to estimate QALYs and QALY differences. Information about self-assessed health status was also collected from the VAS included as part of the EQ-5D-5L. These data are not formally integrated into the cost-effectiveness or cost–utility analysis; rather, they are used to aid interpretation of findings.
Deviations from the study protocol
In the light of the results of the primary outcome, the plan for the economic evaluation was revisited and two changes were made to the plan described in the study protocol. First, it was decided that the extrapolation of long-term outcomes was not relevant because no significant difference was found between randomisation groups at 24 months and, as such, no long-term differences would arise. Second, it was decided to restrict the scope of the analyses to an NHS and Personal Social Services perspective. Extending the scope of the evaluation towards a more societal one was intended to provide decision-makers with additional information about the effects of EXTRAS. Given no significant change was found in the primary outcome, we did not feel that this information would be of value to decision-makers.
Results
Overview
A total of 573 patients were randomised; 285 patients were randomised to receive EXTRAS and 288 were randomised to receive usual care. Details of assessments conducted, deaths and withdrawals are shown in the Consolidated Standards of Reporting Trials flow chart (see Figure 2). A 24-month assessment was conducted for 219 out of 285 (76.8%) patients in the intervention group and 23 out of 285 (8.1%) patients in this group died during the trial period. For patients randomised to receive usual care, 231 out of 288 (80.2%) patients completed a 24-month assessment and 32 out of 288 (11.1%) patients died.
Imputed data
During the trial period, a small number of the patients who completed the assessments (the population informing the primary economics analysis) did not complete the EQ-5D-5L. Only 10 utility scores were imputed (see Appendix 2, Table 69) and there were no apparent differences in response rates between the study groups.
Costs and outcomes
Table 25 shows the results of the primary economic evaluation using the imputed data set. The imputed data set for the CEA is the subset of participants with completed resource utilisation forms until death or trial ending. Utility values were imputed for this group. A total of 235 participants who were in receipt of EXTRAS and 259 participants who received usual care contributed data to this evaluation. Table 25 shows the adjusted means for costs, by health care and social care, and total costs and their adjusted differences. Likewise, adjusted utilities at each time point are shown along with their differences and resulting QALYs.
| Outcome | Intervention (n = 235), mean (95% CI) | Control (n = 259), mean (95% CI) | Adjusted difference:a EXTRAS minus usual care, mean (95% CI) |
|---|---|---|---|
| Costs (£) | |||
| Health care | 5667 (3175 to 8711) | 5943 (4839 to 6495) | 963 (–160 to 2087) |
| Social care | 7708 (5998 to 11,077) | 9656 (7797 to 11,514) | –1788 (–4381 to 806) |
| Intervention | 437 | – | 437 |
| Total | 13,375 (11,381 to 15,369) | 15,599 (12,018 to 19,180) | –311 (–3392 to 2787)b |
| EQ-5D-5L43,80 | |||
| Baseline | 0.60 (0.56 to 0.63) | 0.60 (0.55 to 0.62) | 0.01 (NA) |
| Utility year 1 | 0.58 (0.54 to 0.62) | 0.52 (0.48 to 0.56) | 0.05 (0.00 to 0.09) |
| Utility year 2 | 0.53 (0.49 to 0.58) | 0.48 (0.44 to 0.53) | 0.04 (0.01 to 0.07) |
| Total as QALY | 1.12 (1.06 to 1.19) | 1.04 (0.98 to 1.10) | 0.07 (0.01 to 0.12) |
| Mean net benefit at £20,000 WTP for QALY | £1711 | ||
| Mean cost-effectiveness ratio | On average EXTRAS dominates usual care | ||
| Probability of being cost-effective at £20,000 WTP per QALY | 90% | ||
| Probability that EXTRAS is cost saving | 68% | ||
The mean cost of health and social care resources used by patients in the intervention group was less than those who received usual care (–£311, 95% CI –£3292 to £2787 less per person). The reduced mean costs were due to reduced social care use but were partially offset by increased health-care use. Increased health-care use was mainly a result of increased outpatient costs, day patient costs and primary health-care costs, whereas reduced social care use was a result of the reduction in home help (personal care, home services, shopping) and allowances. In all cases, the 95% CI around the difference includes zero. The CIs are sufficiently wide to include economically important differences that could favour either study group.
A breakdown of the health-care and social care costs between the randomisation groups is shown in Appendix 2, Table 70. Standard resource item costs are shown in Appendix 2, Table 71. It is not possible to show all of the resource costs because some were disease specific. For example, for the inpatient cost of a particular patient-reported condition we used the weighted average of the costs in the NHS Reference Costs,81 and for another condition for another patient, the respective average cost for that condition was used.
The utility and QALY differences, however, did show a statistically significant difference between the groups, with patients in the intervention group experiencing between 0.01 and 0.12 additional QALYs over 2 years. At the mean value, 0.07, this equates to 25 additional days in ‘full health’ over the 24-month trial follow-up period. A breakdown of EQ-5D-5L by dimension and trial group at baseline, 12 months and 24 months for patients who contributed to the CEA is given in Appendix 2, Tables 72–74.
Tables 78–80 in Appendix 2 show a breakdown of the EQ-5D-5L by dimension and trial group at baseline, 12 months and 24 months for all patients who provided EQ-5D-5L data. Tables 81 and 82 show a breakdown of the utilisation of major resource items for all patients who completed the CSRI at each time point. Note that these tables do not match the numbers included in the cost-effectiveness analyses that required CSRI data at all time points until death or trial end.
Figures 19 and 20 show the cost-effectiveness plane and CEAC, respectively. Both figures illustrate the uncertainty around the estimates of cost-effectiveness. On the bootstrapped cost-effectiveness plane, points on the negative vertical axis favour EXTRAS as cost saving (68%) and positive values favour usual care (32%). Positive values (98.5%) on the horizontal (QALY) axis favour EXTRAS and negative values favour usual care (1.5%). The average ICER falls in the south-east quadrant of the cost-effectiveness plane, suggesting that, on average, EXTRAS dominates usual care. The ellipse depicts the 95% CI around the ratio of costs to QALYs. The proportions are reflected in the CEAC where at a zero value for society’s WTP for a 1-QALY gain, the CEAC is shown to cross the vertical axis at 68%, rising to 90% at £20,000 per QALY. The CEAC shows the probability that EXTRAS would be deemed cost-effective across a range of values for WTP for 1 QALY. As WTP for 1 QALY increases, so does the probability that EXTRAS would be judged cost-effective.
FIGURE 19.
Cost-effectiveness plane.
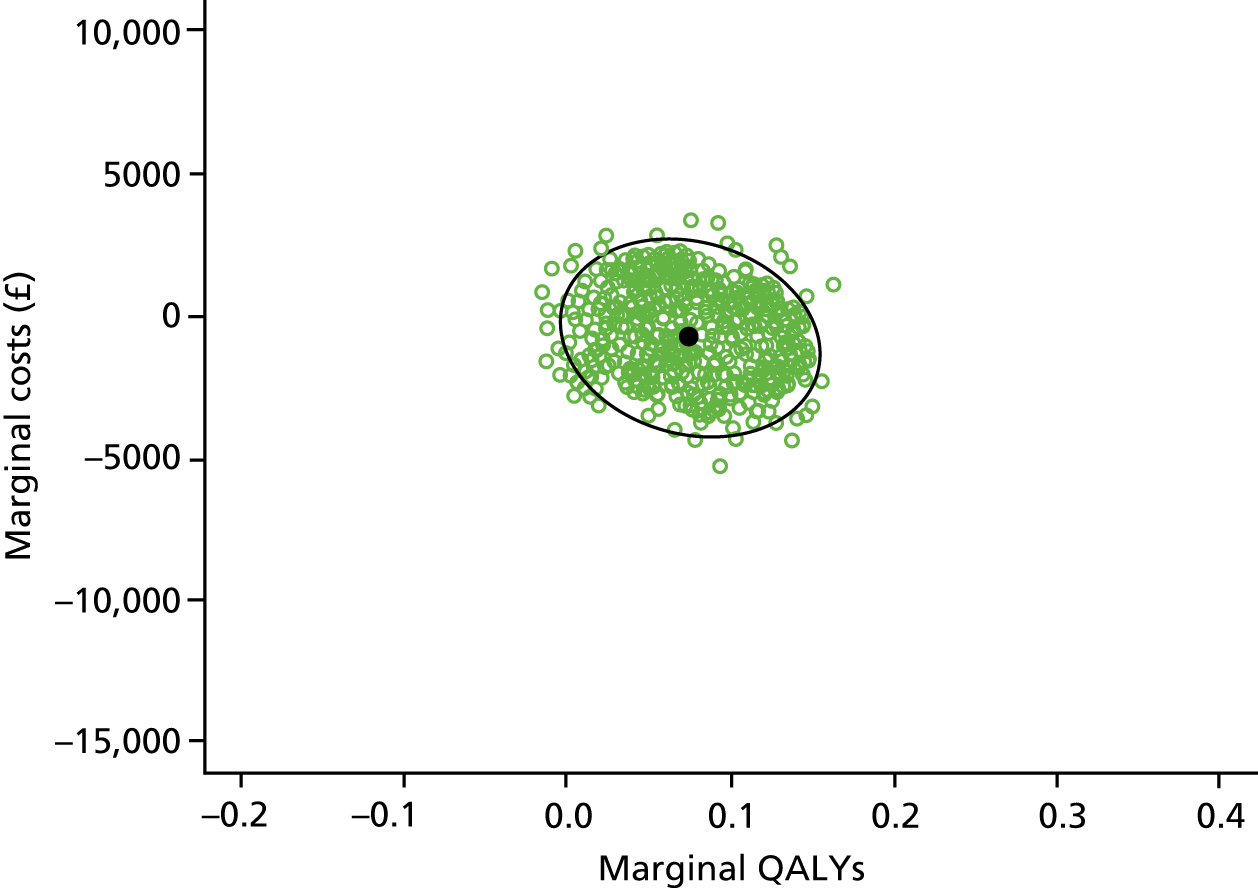
FIGURE 20.
Cost-effectiveness acceptability curve.
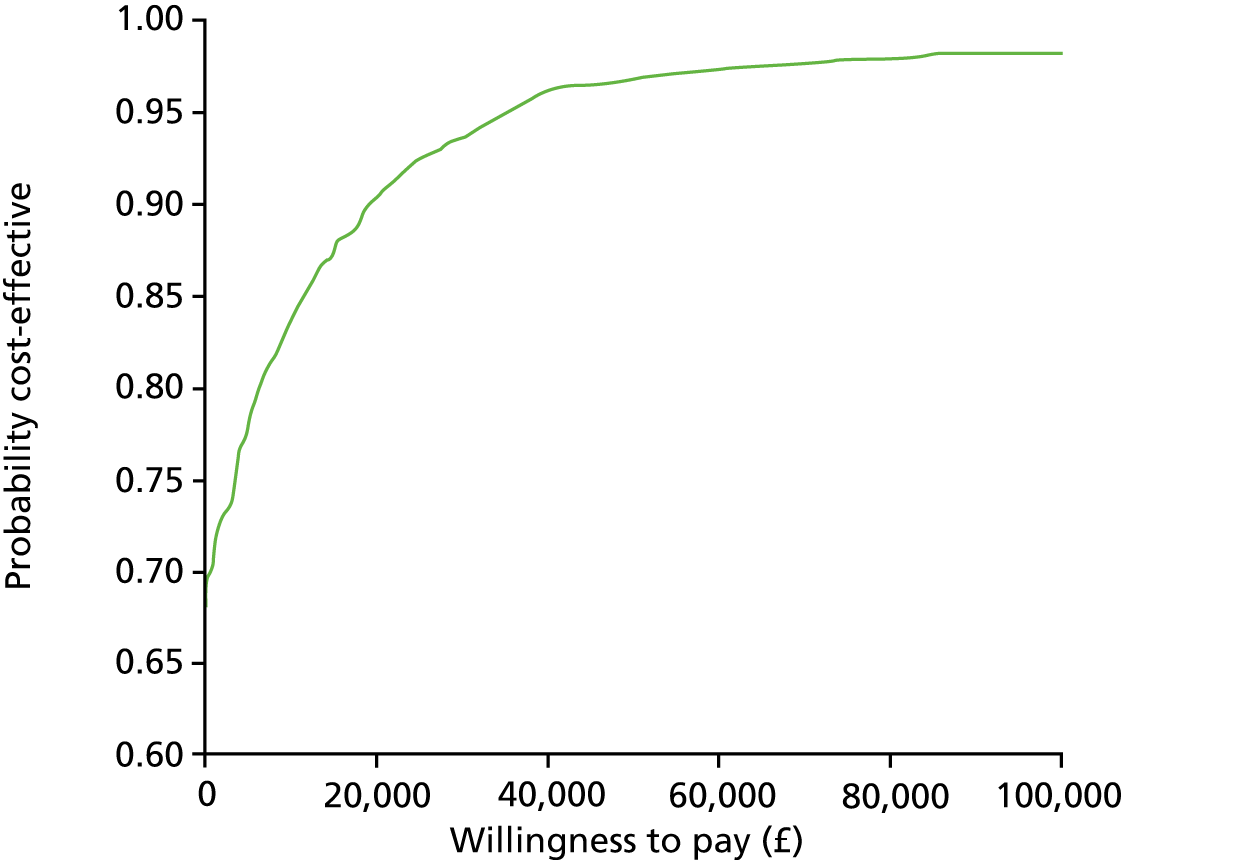
A summary of the self-reported quality of life on the VAS score in EQ-5D-5L (for patients who contributed to the CEA) is shown in Table 26. There was no significant difference between the self-reported health scores at baseline, 12 months and 24 months between trial groups.
| Time point | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Baseline | ||||
| Usual care | 64.40 | 20.80 | 0 | 100 |
| EXTRAS | 67.34 | 19.20 | 5 | 100 |
| 12 months | ||||
| Usual care | 64.52 | 21.45 | 0 | 100 |
| EXTRAS | 68.39 | 20.81 | 5 | 100 |
| 24 months | ||||
| Usual care | 63.71 | 22.20 | 0 | 100 |
| EXTRAS | 67.62 | 21.67 | 5 | 100 |
| Changes from baseline, mean (95% CI) | ||||
| Usual care | –0.69 (–4.57 to 3.18) | |||
| EXTRAS | 0.28 (–3.55 to 4.12) | |||
Nottingham Extended Activities of Daily Living Scale scores
The economic analysis considered only those patients who reported completing the CSRI forms at 24 months. Among these, there were only two patients missing data in the intervention group and one in the usual care group. CIs around the mean difference in NEADL Scale score (1.98, 95% CI –0.17 to 4.14) rule out a clinically important difference on this measure (Table 27). The results did not change when the analysis was carried out using the complete data (1.94, 95% CI –0.3 to 4.18). Figure 21 shows the cost-effectiveness plane for the NEADL Scale scores. In terms of the NEADL Scale, there was an 84% chance that EXTRAS would be cost saving.
| Intervention, mean (95% CI) | Usual care, mean (95% CI) | Adjusted difference, mean (95% CI) | |
|---|---|---|---|
| Imputed data set | n = 219 | n = 231 | – |
| NEADL Scale score | 39.96 (37.56 to 42.37) | 37.19 (34.78 to 39.59) | 1.98 (–0.17 to 4.14) |
| Total costs (£) | 13,749 (11,701 to 15,797) | 16,881 (12,908 to 20,854) | –1265 (–4223 to 1693) |
| Complete cases | n = 217 | n = 230 | – |
| NEADL Scale score | 39.87 (37.45 to 42.29) | 37.33 (34.93 to 39.72) | 1.94 (–0.3 to 4.18) |
| Total costs (£) | 13,739 (11,677 to 15,802) | 16,615 (12,660 to 20,571) | –1001 (–4043 to 2041) |
FIGURE 21.
Cost-effectiveness plane: NEADL Scale.
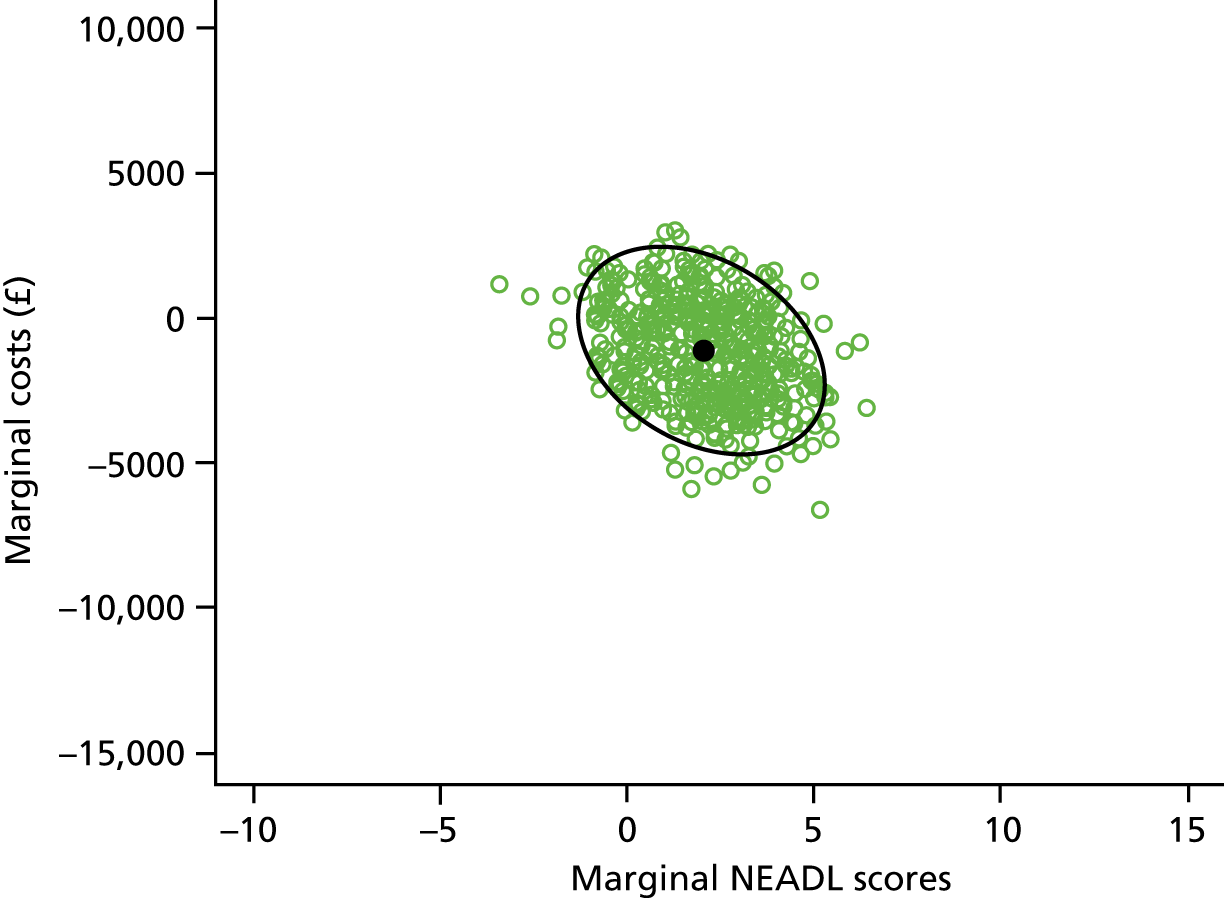
Sensitivity analyses
In the light of the findings of the primary health economic analysis that EXTRAS was very likely to be deemed cost-effective according to current thresholds, three sensitivity analyses were carried out. These were:
-
on the non-imputed data set
-
with the removal of any cost outliers
-
using an alternative valuation tariff for the EQ-5D-5L.
Despite the relatively few imputed values used to generate QALYs, it was important to establish the effect that imputation had on estimates. Likewise, it was also important to establish the effect that any cost outliers (in this analysis those with costs of ≥ £100,000 were excluded) might have on our estimates. In the non-parametric analysis of costs, it was noted that a group of patients fell into this category and it was necessary to determine whether or not they occurred disproportionately in one group of the trial. Finally, the EQ-5D-5L crosswalk tariff89 was used as an alternative to the tariff used in the primary analysis to establish what effect alternative valuations of the EQ-5D-5L health states might have on estimates of effect.
The results of the sensitivity analysis are shown in Table 28. In addition the CEAC and cost-effectiveness planes are presented in Appendix 2, Figures 40–45. In all sensitivity analyses, the average ICER remained in the south-east quadrant of the cost-effectiveness plane, suggesting that, on average, EXTRAS dominated usual care.
| Analysis/metric | Estimate |
|---|---|
| Primary analysis | |
| Mean net benefit at £20,000 WTP for QALY | £1711 |
| Mean cost-effectiveness ratio | On average EXTRAS dominates usual care |
| Probability of being cost-effective at £20,000 WTP per QALY | 90% |
| No imputed data | |
| Mean net benefit at £20,000 WTP for QALY | £2238 |
| Mean cost-effectiveness ratio | On average EXTRAS dominates usual care |
| Probability of being cost-effective at £20,000 WTP per QALY | 88% |
| Outliers excluded | |
| Mean net benefit at £20,000 WTP for QALY | £2692 |
| Mean cost-effectiveness ratio | On average EXTRAS dominates usual care |
| Probability of being cost-effective at £20,000 WTP per QALY | 96% |
| Crosswalk EQ-5D-5L tariff | |
| Mean net benefit at £20,000 WTP for QALY | £1911 |
| Mean cost-effectiveness ratio | On average EXTRAS dominates usual care |
| Probability of being cost-effective at £20,000 WTP per QALY | 96% |
In each case, the mean cost-effectiveness ratio did not change. It remained that, on average, EXTRAS was associated with an increase in estimated HRQoL and reduced net health and social care costs.
Carers’ health-related quality of life
Information about carers’ HRQoL was also collected at baseline and at 12 and 24 months. The carers’ own valuations of their quality of life as captured by the EQ-5D-5L VAS are shown in Table 29. There was no significant difference in the self-reported health scores of carers of patients at baseline and at 12 and 24 months in both groups of the trial.
| Time point | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Baseline | ||||
| Usual care | 79.02 | 15.16 | 28 | 100 |
| EXTRAS | 76.11 | 18.58 | 10 | 100 |
| 12 months | ||||
| Usual care | 74.78 | 18.07 | 30 | 100 |
| EXTRAS | 72.45 | 19.55 | 10 | 100 |
| 24 months | ||||
| Usual care | 75.39 | 18.76 | 15 | 100 |
| EXTRAS | 78.02 | 16.12 | 30 | 100 |
| Changes from baseline, mean (95% CI) | ||||
| Usual care | –3.64 (–9.16 to 1.88) | |||
| EXTRAS | –0.73 (–6.48 to 5.03) | |||
The associated QALYs are shown in Table 30. Two estimates of QALY differences were made. The first used the imputed data set, where predictive mean matching was used when a utility value existed for at least one data point (baseline, 12 months or 24 months). Each estimate controlled for baseline utility. Only 135 carers (76 from the usual care group and 59 from the intervention group) completed the EQ-5D-5L at each time point out of 185 carers considered for imputation. Missing data from nine from the usual care group and 41 from the intervention group were imputed. EQ-5D-5L scores by domain at each time point are given in Appendix 2, Tables 75–77.
| Intervention | Usual care | Difference | |
|---|---|---|---|
| Imputed data set | n = 100 | n = 85 | – |
| Mean (95% CI) | 1.52 (1.52 to 1.58) | 1.57 (1.54 to 1.60) | –0.05 (–0.14 to 0.04) |
| Complete cases | n = 59 | n = 76 | – |
| Mean (95% CI) | 1.52 (1.43 to 1.68) | 1.60 (1.52 to 1.68) | –0.08 (–0.20 to 0.04) |
In each analysis, the 95% CI around the estimated differences in QALYs included zero but the CI may be sufficiently wide to include clinically important differences favouring either group. Despite the small but significant QALY gain for patients estimated in the primary economic analysis, there is no evidence that this translated into HRQoL gains for their carers.
Discussion
Despite no evidence of a meaningful difference in the primary outcome (NEADL Scale score) between study groups, provision of EXTRAS had a 68% chance of being cost saving in terms of QALYs. The change in resource use suggests that it is likely that EXTRAS would result in cost saving overall, but most likely with extra costs to health services, which would be offset by savings in social care. Although from a societal perspective how savings are made is relevant, in practice it raises the issue of cost shifting, whereby savings may accrue to one service or organisation that may not be paying for the intervention, causing disincentives and barriers to the adoption of a more cost-effective intervention.
Quality-adjusted life-years were greater for patients who were randomised to receive EXTRAS and this outcome measure may arguably have captured wider impacts of the new service. Given the impact of EXTRAS on costs and QALYs, there is a very high chance that this service would be considered cost-effective at conventional thresholds for society’s WTP for 1 QALY (currently £20,000 per QALY).
It is possible in this case, as with the majority of economic evaluations undertaken alongside clinical trials, that findings are subject to a type 1 error: a false-positive finding. However, the economic evaluation estimates that EXTRAS was associated with HRQoL gains and net health-care and social care cost savings. Sensitivity analyses confirmed this analysis as robust. Furthermore, focusing only on cost savings and the NEADL Scale score implied that there was an 84% chance that EXTRAS would be considered cost-effective.
Other considerations, strengths and weaknesses
The economic evaluation of EXTRAS was based on data completed by a vast majority of participants in the trial and the conclusions from the economic evaluation were supported by those from the sensitivity analyses. We also conclude that the difference in estimated marginal QALY gains did not appear to be driven by any differential rates of death between the two groups. However, the EXTRAS group appeared to have more people reporting ‘no problems’ for all EQ-5D-5L domains at baseline and during follow-up. This would potentially bias results against EXTRAS, as such cases represent a ceiling on possible gains in HRQoL that could be derived from EXTRAS.
Within the analyses, we took account of clustering at the univariate level when estimating the association between EXTRAS and the differences in costs and differences in QALYs. However, no bivariate adjustment for clustering was made in the generation of the CEACs. It is methodologically unclear how such an analysis could be performed, although it would have been possible to generate a CEAC per site and develop a weighted cost-effectiveness ratio and CEAC, but as there was a relatively large number of sites with differing numbers of patients at each site we felt that this was not a practical approach. The CEACs themselves were produced from parametric bootstrapped samples. For this reason, they were affected by the distributions used on the generalised linear models to estimate costs and QALYs. However, given the relatively large sample sizes we felt that this parametric approach was appropriate despite the potential for non-normally distributed data.
Chapter 9 Discussion
Key findings
Primary outcome: extended activities of daily living
EXTRAS did not improve stroke patients’ performance in EADL following discharge from ESD services when compared with usual care. The mean 24-month NEADL Scale score was 40.0 (SD 18.1) for intervention group participants and 37.2 (SD 18.5) for those who received usual care, giving an unadjusted mean difference of 2.8 (95% CI –0.6 to 6.2). The primary outcome, that is the mean difference in NEADL Scale score at 24 months adjusted for baseline OHS, age and sex was 1.8 (95% CI –0.7 to 4.2).
Secondary outcomes: mood, health status, carer stress and satisfaction with services
Depression and anxiety are common after stroke. The mean anxiety and depression HADS scores were lower for patients in the intervention group at 12 and 24 months, but the 95% CIs for the difference in scores included zero.
Provision of the new service did not lead to significantly improved patient health status (OHS) or a reduction in carer stress. Patients who received EXTRAS and their carers reported that they were more satisfied with some aspects of their care than those who received usual care. For patients, these aspects were overall satisfaction with care; staff met their needs; staff treated them with dignity and respect; and they received sufficient help with mobility. Carers in the intervention group were more satisfied with overall care and more felt that sufficient help had been provided for mobility issues.
Subgroup analyses
No association was found between the effectiveness of the intervention and the pre-stroke OHS score, baseline NEADL Scale score and time in organised stroke care (defined as time as an inpatient and time in ESD services), which were prespecified exploratory analyses.
Post hoc analysis
Post hoc analysis of the HADS score case versus non-case found that there were significantly fewer cases of depression at 12 months in the intervention group (29% intervention group vs. 40% control group; adjusted OR 0.59, 95% CI 0.39 to 0.90) and significantly fewer cases of anxiety at 24 months (28% intervention group vs. 38% control group; adjusted OR 0.64, 95% CI 0.41 to 0.99).
Adverse events
A total of 504 SAEs were identified for 573 participants over 24 months. There were no significant differences in the number of SAEs between those who received EXTRAS and those who received usual care. None of the SAEs was attributed to the intervention.
Health economic evaluation
Stroke survivors who were randomised to receive EXTRAS had a better HRQoL and experienced 0.07 additional QALYs (95% CI 0.01 to 0.12). The mean cost of resource utilisation was lower in the intervention group (–£311, 95% CI –£3292 to £2787), with a 68% chance of being cost saving. The changing resource use pattern suggests that it is likely that additional costs to health services were offset by savings in social care. At the standard WTP of £20,000 per QALY,90 there is a 90% probability that EXTRAS is cost-effective for the NHS. No differences were seen for the HRQoL of carers.
Potential explanations of divergent results
Researchers and decision-makers will probably be most interested in the finding that, although there is no evidence of any treatment effect on the primary outcome, the cost-effectiveness analyses are consistent with EXTRAS being associated with cost saving and HRQoL gain. This combination of results has been reported in other settings, for example psychological treatments for obsessive–compulsive disorder,91 but we are not aware of any stroke rehabilitation trials that have reported this combination of findings.
One explanation of this finding is that the EQ-5D-5L captured wider impacts of EXTRAS than the NEADL Scale. Another possibility is that although both the EQ-5D-5L and the NEADL Scale are ordinal scales, the NEADL Scale lacks the cardinal property derived from patient and public preferences: a unit change in utility measured by the EQ-5D-5L is of the same value to a patient, wherever it occurs on the scale. This is not the case for the NEADL Scale.
In clinical trials, the primary outcome is chosen as it is believed to be the outcome of greatest importance. 92 Our intervention sought to improve performance in EADL, but it did not. Secondary outcomes, including health economic outcomes, are used to evaluate additional effects of an intervention that may be related to the primary question or other hypotheses. These effects are also important, but secondary outcome results in the context of a negative or neutral primary outcome are generally regarded as hypothesis generating rather than hypothesis testing. Nevertheless, there is consistency between the results of the health economic analyses, some secondary outcomes and a post hoc analysis; our findings suggest that EXTRAS may improve stroke survivors’ quality of life (QALYs) and mood (anxiety and depression cases on the HADS) and some aspects of satisfaction with services. In addition, qualitative interviews analysed prior to the randomised trial results becoming available reported that staff who delivered the reviews felt that, as EXTRAS provided support and advice without direct therapeutic intervention, it was more likely to have an impact on mood and general well-being rather than to improve performance in EADL.
The EXTRAS trial results in the context of other studies
Systematic reviews of stroke units and ESD services have shown that ‘organised stroke care’ is clinically effective and cost-effective. 14,15 However, it is unclear how best to provide rehabilitation and support to meet the needs of stroke patients and carers beyond ESD services. A number of studies have identified that stroke survivors have a wide range of longer-term physical, psychological and social needs. 6,8–10,12,25,60,93,94 A recent systematic review and meta-ethnography study12 reported that ‘stroke survivors and care givers feel abandoned because they have been marginalised by services and they do not have the knowledge or skills to re-engage’.
Trials that evaluated community-based interventions that sought to address either single or multiple longer-term needs have used a range of approaches and outcomes and are of variable quality. Individual community-based rehabilitation studies and subsequent meta-analyses have been largely neutral across a range of interventions and outcomes. Few studies included a health economic evaluation. 30,95 Given our findings, it is possible that potential benefits of some of these interventions could have been overlooked as the trials did not measure QALYs.
Therapy-based services
Like many previous studies, EXTRAS did not lead to improved performance in EADL. When designing the EXTRAS trial in 2010, we were encouraged by the results of a 2003 Cochrane review24 (14 trials, 1617 participants) that reported that therapy-based services (excluding ESD services) provided to stroke patients soon after discharge from the hospital reduced the odds of a poor outcome (OR 0.72, 95% CI 0.57 to 0.92) and increased performance in personal activities of daily living compared with usual care. However, a 2008 Cochrane review21 (five trials, 487 participants) found inconclusive evidence that similar interventions provided 1 year or more after stroke were effective and the authors concluded that ‘this review highlights the dearth of evidence investigating long-term therapy-based rehabilitation interventions for patients with stroke’. The findings of the 2008 Cochrane review led to the NIHR HTA commissioned call that funded the EXTRAS trial (see the project web page: www.journalslibrary.nihr.ac.uk/programmes/hta/103701/#/; accessed 9 April 2020).
Stroke liaison workers and stroke care co-ordinators
EXTRAS aimed to help individuals to maximise their recovery in the context of their day-to-day activities and/or adjust to their residual disability. Our intervention was broader than trying to improve performance in EADL, as we sought to address the full range of long-term needs experienced by stroke survivors. Therefore, EXTRAS has some similarities to the interventions provided by stroke liaison workers who are health-care workers or volunteers who provide education, social support and liaison with services. A 2010 Cochrane review23 of trials that evaluated stroke liaison worker services (16 studies, 4759 participants) found no benefit in terms of perceived health, mood, activities or participation. As with EXTRAS, intervention participants were more satisfied with some aspects of service provision. Patients with mild to moderate disability (Barthel activities of daily living score96 of 15–19) who were supported by a stroke liaison worker were reported to have reduced death and disability (OR 0.62, 95% CI 0.44 to 0.87). We did not measure the Barthel activities of daily living score in the EXTRAS trial, but our study population is not directly comparable with this subgroup as some of the EXTRAS participants would have had a Barthel activities of daily living score of < 15, that is they had more severe disability.
The Longer Term Stroke (LoTS) Care trial30 was published after the start of the EXTRAS trial. This large cluster randomised controlled trial (32 sites, 800 patients, 208 carers) evaluated a system of care where stroke care co-ordinators, who were community-based health-care professionals with experience in stroke care, were trained to follow a structured assessment and treatment plan working with patients and carers after discharge from hospital. Neither patients nor carers in the intervention group had improved outcomes (including quality of life and EADL) when compared with usual care. The total health-care and social care costs were similar between both randomisation groups.
Self-management interventions
Self-management interventions seek to encourage people with chronic conditions to take an active part in their own care;69,97–99 this was one of the components of our intervention. Approaches to self-management include problem-solving, goal-setting, shared decision-making, self-monitoring and coping with the condition. A 2016 Cochrane review29 of self-management programmes for people with stroke (14 studies, 1863 participants) reported that these programmes improved quality of life [standardised mean difference (SMD) random effects 0.34, 95% CI 0.05 to 0.62] and improved self-efficacy (SMD random effects 0.33, 95% CI 0.04 to 0.61) but that they had no effect on activities of daily living or mood. Studies have shown that self-management programmes are likely to be cost-effective for some long-term conditions, for example diabetes mellitus,100,101 but studies evaluating the cost-effectiveness of self-management for stroke survivors are lacking. 29
The EXTRAS intervention
Development and content of EXTRAS
The intervention was developed following an evidence review and the domains were comprehensive in covering the range of priorities for stroke patients and carers. 6,8,9,22,25,60,77,102 Stroke therapists, patients and carers were involved in designing and piloting study materials. A domain potentially missing from the reviews was fatigue. Fatigue was not included as a specific domain, as at the time of designing the intervention the importance of this symptom had not been fully appreciated. 103 Reviews were multifaceted, reflecting current clinical practice. Manuals and detailed study documentation were produced along with a training programme for participating ESD staff, enabling the intervention to be replicated in clinical practice. The duration of the intervention was purposely much longer than most previous studies, as patients and carers have recommended that care and support should be ongoing in the longer term. 12 In our qualitative study, ESD team members felt that provision of ongoing contact and support was an important component of stroke care, which was not provided in many settings. Some felt that the EXTRAS trial would provide valuable data to inform the development of community-based stroke services.
Delivery of EXTRAS
EXTRAS was embedded within NHS care and was delivered as it would be in clinical practice. We have described EXTRAS according to the TIDieR checklist and guide, enabling the intervention to be replicated and compared with other interventions. 49 We have detailed quantitative and qualitative data about how EXTRAS was delivered, which have helped us to interpret our divergent results.
One of the potential criticisms of our approach to providing a new community stroke service is that reviews were primarily undertaken by telephone. Telephone reviews were selected not only because they were more affordable, but also because they were likely to be less disruptive to a participant’s daily routine than a clinic visit. In addition, they optimised the use of the reviewer’s time as no travel was needed. We did not use video or more sophisticated technology, as many participants would have not had access to or experience of using them.
As EXTRAS reviews were undertaken by telephone, we felt that it was important that they were carried out by a stroke specialist with experience of community rehabilitation who knew the patient, who had expertise in goal-setting and who had detailed knowledge of local services. It was important that the reviewer had the skills and expertise to work in partnership with the patient to identify ongoing needs and determine ways to address them. Reviewers came from a range of health-professional backgrounds and their experience within an ESD team would have equipped them to take a holistic approach, as well as using the knowledge of their discipline. Reviewers were also able to seek support, information and advice from other members of the ESD team and those currently involved in the patient’s care. However, our process evaluation found that not all of the EXTRAS reviewers were comfortable in addressing issues beyond their own discipline, and some reported difficulties contacting others involved in the patient’s care. Some ESD team members felt that EXTRAS reviews could be carried out by voluntary sector support services or a stroke co-ordinator or specialist nurse.
There was an option within the EXTRAS protocol to undertake face-to-face reviews but this was used infrequently. Some ESD team members felt that some patients would have benefited from one or more home visits over the 18-month review period, as this would have enabled them to see how the patient coped within their environment, assess any safety risks and discuss issues with carers. Face-to-face reviews were thought to be more appropriate than telephone reviews if the patient had residual physical or cognitive problems, or if he/she found it difficult to participate in telephone reviews.
Completion of EXTRAS reviews was good, with between 81% and 91% of expected reviews being undertaken at each of the five time points. Although reviews could be undertaken by any member of the ESD team, they were predominantly undertaken by physiotherapists (56%) or occupational therapists (28%). Reduced mobility was the most common problem raised, followed by hobbies and leisure. It was envisaged that goals and action plans would be jointly set by reviewers and patients but we did not assess whether or not this partnership occurred. It may be that some of the priorities selected reflected the skills and interests of the reviewer.
Action plans could include providing advice or encouragement; self-practice; liaising with services currently involved in the patient’s care; or making a new referral. The most common action taken by a reviewer was to provide the patient with advice (71–75% of action plans, depending on review stage) and this could be linked to an action for the patient, for example practise an activity relating to an agreed goal (22–34% of action plans). The EXTRAS reviewers felt that patient-led goal-setting and action-planning were important components of the intervention and that these activities were not always undertaken or patient led in usual care. They felt that EXTRAS promoted self-management. Some patients had little recollection of goal-setting and some did not recall that reviews had taken place. Qualitative interviews took place 6 months after the 18-month EXTRAS review (to enable prior completion of the 24-month outcome assessment), so it is not surprising that some people had poor recall. In addition, 30% of EXTRAS participants did not identify any issues at their 18-month review, compared with 4% at their 1-month review. It may be inferred from the limited recall of reviews that the intervention was not meaningful or important to patients, or, more positively, that the intervention was not burdensome.
A potential criticism of EXTRAS is the lack of provision of direct rehabilitation interventions following a review (e.g. referrals for therapy). Action plans and referrals were at the discretion of the ESD staff who delivered EXTRAS reviews and data were not captured about the clinical reasoning processes behind these decisions. It is possible that a number of factors were influencing the choices made, including judgement about a perceived lack of effect from further direct intervention for the stroke survivor or knowledge about the lack or limited availability of local rehabilitation services.
As the availability of some longer-term rehabilitation services was limited in some areas, this could have led to needs being identified at EXTRAS reviews that could not be addressed and this could have negatively affected study participants. Alternatively, being informed about the help and support that was and was not available may have helped some participants to revise their expectations and adjust to the situation. In our process evaluation, ESD staff who were interviewed did not widely report that they felt that referral for rehabilitation was limited or not possible in their area, although one interviewee did highlight a lack of psychological services.
One explanation for the lack of a treatment effect on the primary outcome is that direct therapeutic intervention may be required to improve performance in EADL. However, it appears that more general support and advice has an effect on overall well-being and satisfaction. This could be an effect of stroke-specific support and advice or a more non-specific effect from talking regularly to an individual about ongoing issues. It is likely that stroke-specific expertise is an important component, as previous studies of support services have been neutral. 23
There was some overlap between EXTRAS and existing services, for example 6-month reviews; however, few of the EXTRAS study centres offered routine reviews at ≥ 12 months after stroke. Nationally, only 28% of stroke survivors receive a 6-month review, despite NICE guidance. 78,104 Nevertheless, if EXTRAS is adopted into clinical practice, any overlap will need to be addressed.
One of the concerns raised by ESD teams who expressed an interest in participating in the trial was that EXTRAS reviews could lead to an increased workload for health and social care services, but this was not the case. Similar concerns were also raised when ESD services were first evaluated; however, ESD services were also found to be cost saving. 105 During the EXTRAS trial, only 117 unique referrals were intended to be made by reviewers to other services and these intended referrals formed < 10% of action plans at each review. The effect on individual services was minute; overall, 20 new referrals were intended to be made to physiotherapy, 16 to occupational therapy, six to a community rehabilitation team, nine to speech and language therapy and nine to social services.
Usual care
Participants in the control group received usual care post ESD. Usual care is perhaps a misnomer, as NHS and social care are not standardised and, therefore, the care received by participants in the control group is likely to have varied according to local service provision. Similarly, the availability of services to which EXTRAS patients could be referred following each review will also have varied between study centres.
Our service mapping found that structured reviews of patients’ health and social care needs beyond 6 months were rarely provided, despite recommendations from the NHS National Stroke Strategy and NICE. 77,78 Provision of services to meet the longer-term needs of stroke patients was variable, and access could be complex and confusing. In our process evaluation, some ESD team members reported that their area already had comprehensive co-ordinated services to meet the longer-term needs of stroke survivors, whereas others felt that community-based rehabilitation was un-co-ordinated, often with long waiting times, or unavailable. Interviewees highlighted the lack of service provision and support for some of the ‘hidden consequences’ of stroke, such as anxiety and fatigue. Some participants who received usual care reported that their care had been faultless, whereas others commented on a lack of therapy and they felt that they had been ‘left to manage’ without follow-up from health-care professionals.
Research studies and national audit results consistently report that stroke patients receive very little longer-term rehabilitation. 12,60,104 However, a number of EXTRAS participants were in receipt of long-term therapy: 47% of patients in the intervention group and 48% of patients in the control group saw a physiotherapist in the 12 months following discharge from ESD services, and 26% and 23%, respectively, saw a physiotherapist in the subsequent 12 months. In total, 31% of patients in the intervention group reported that they had seen an occupational therapist in the 12 months post ESD, compared with 33% of the usual-care group. Over the following 12 months, contact with an occupational therapist was 14% for the intervention group and 11% for the control group. Contact with a SLT was 16% for the intervention group and 19% for the usual care group over the first 12 months post randomisation and 5% for both intervention and control groups over the following 12 months.
Methodological considerations
The EXTRAS trial is one of the largest randomised controlled trials of a new community stroke service undertaken to date. Multicentre studies of community rehabilitation are uncommon and to participate in the EXTRAS trial each study site was required to identify funding/resource to establish a new service specifically for the trial.
Study setting
All participants received stroke unit and ESD care prior to participating in the trial, in accordance with best practice. 16,20 Sites were from a range of urban and rural settings around the UK and were keen to further develop stroke care in their area, so these were likely to be progressive services. Most study sites reported that EXTRAS was an important addition to their stroke service that would lead to improvements in patient care, but a few felt that EXTRAS would not offer benefit over and above their current service provision.
Participants
Our inclusion criteria were broad and inclusive. Participants were typical of stroke survivors treated by ESD services. 14 We did not collect screening data because of the additional workload for recruiting staff and we do not have data about the proportion of ESD service patients who participated in the trial at each site. Participant numbers differed between study centres, which could represent a selection bias. However, this is more likely to be a reflection of the duration of recruitment (longest 32 months, shortest 6 months), the size of the stroke service, the availability of staff to identify and consent patients and the availability of ESD team members to provide the intervention. The availability of ESD members was a particular consideration for recruitment at each individual site. Some ESD staff felt that provision of EXTRAS was unlikely to benefit patients with mild symptoms or cognitive deficits.
When designing the study, we anticipated that most participants would have a relative or carer who would be invited to participate in the study. Only 194 out of 573 (34%) stroke survivors had a relative or carer taking part in the study. Various unsuccessful attempts were made to increase the number of relatives/carers participating in the study.
Outcomes
The primary outcome for this study was performance in activities of daily living measured by the NEADL Scale. This scale assesses mobility, kitchen tasks, domestic tasks and leisure activities. Although labelled slightly differently, the EXTRAS reviews covered all of these domains. Mobility was identified as a priority issue for 55% of participants at 1 month and 35% at 18 months. Mealtimes were a priority for 30% of participants at 1 month and 15% at 18 months, and these values were 24% and 3% for domestic activities, and 37% and 24% for hobbies and interests, respectively.
Being able to undertake everyday activities is only one of a range of outcomes that are valued by stroke patients and carers, and the EXTRAS intervention purposely aimed to cover a wide range of potential rehabilitation and other ongoing needs. Therefore, a broad range of secondary outcome measures were selected, covering health status, mood, quality of life and experience of services. The outcome measures used in the EXTRAS trial are widely used in stroke and rehabilitation research and are well validated, and the satisfaction scale has been used in national service improvement initiatives.
In retrospect, it could be argued that a more global outcome measure rather than EADL would be a more appropriate primary outcome. Some staff interviewed in our process evaluation felt that EXTRAS would not lead to improvements in EADL, as it did not involve face-to-face assessments, provide therapy or involve supervised practice of EADL. The ESD team members felt that the reviews were comprehensive but were more likely to affect emotional and social issues, rather than physical recovery.
Economic evaluation
As with the majority of economic evaluations conducted as part of a randomised controlled trial, comparisons of the cost and cost-effectiveness outcomes in the EXTRAS trial are secondary outcomes and power calculations were not undertaken. 106 It is likely that the economic analyses are underpowered. For this reason, health economic analyses focus on estimation rather than hypothesis testing. The width of the CIs around these results suggest that care must be taken when interpreting these findings. The CIs around the QALY difference suggest that EXTRAS was associated with an improvement in HRQoL for patients. The magnitude of the gains, although small, would be judged as economically significant. 107
Sources of bias
We carried out the EXTRAS trial in accordance with current best practice. 65,108 The trial was conducted in line with a published protocol32 and reported according to international standards. 49,109,110 A statistical analysis plan and health economics analysis plan were finalised prior to data lock. Thanks to the efforts of study participants, local research network staff, ESD teams and co-ordinating centre team members, one of the strengths of the EXTRAS trial is the completeness and high quality of the data. Our findings are robust and there is a low risk of bias. 111
When designing the trial, we did consider including an attention control study group or another intervention group that provided face-to-face EXTRAS reviews, but either of these options would have significantly increased the sample size and complexity of the study.
Randomisation was by an independent web-based service with allocation concealment.
All but one of the outcomes (number of cases of anxiety and depression) have been reported according to statistical and health economic analysis plans. The number of cases of anxiety and depression analysis (HADS caseness) was undertaken post hoc and therefore these results should be treated with caution.
As with many rehabilitation studies, it was not possible to blind study participants or those delivering the intervention to the participants’ study group. However, outcome assessments were usually undertaken by a member of the co-ordinating centre who was blinded to treatment allocation. Outcome assessor blinding rates were reported and are acceptable. As it was not possible to blind study participants to their study group, this could have led to disappointment for those who were randomised to usual care and, in turn, poorer outcomes because of resentful demoralisation. 112 We did not record the views of control group participants to determine if this was an issue.
Overall, the attrition rates in the EXTRAS trial are acceptable. However, there was differential attrition between patient study groups at 24 months. In the intervention group, 66 out of 285 (23%) patients did not provide data for the 24-month assessment and in the control group this was 57 out of 288 (20%). Removing those patients who did not provide data because they were known to be deceased, the dropout is 43 out of 262 (16%) in the intervention group and 25 out of 256 (10%) in the control group. The differential attrition rate between study groups could potentially have biased our results. The increased attrition rate in the intervention group may be due to trial fatigue because of the number of clinical assessments that had been completed during the intervention period, and this was mentioned by some participants who were invited to participate in the interview study. It could be also be argued that some of the dropout could be a result of those in the intervention group being dissatisfied with the care that they had received (or not received). Alternatively, the intervention may have helped individuals to adjust to life after stroke and move on.
One patient who was randomised to usual care received the intervention in error. However, contamination or competitive therapy bias was otherwise unlikely, as ESD teams had limited capacity for additional work. Most sites would agree to recruit only two participants per month because of the capacity to provide the EXTRAS intervention. Although this low recruitment rate probably contributed to the high fidelity to the intervention seen in the study, it may have meant that reviewers were inexperienced and we evaluated the new service on a learning curve. Evaluation of EXTRAS after a learning period may have been a preferable approach. However, as EXTRAS was a research intervention, this design would probably have been challenging to implement.
Patient and public involvement
In 2011, the Care Quality Commission25 reported that stroke patients and carers identified the need for ongoing rehabilitation, provision of information and contact with a named person as priorities for improving stroke services. EXTRAS was developed based on the expressed needs of stroke survivors identified in the Stroke Association UK Survivor Needs Survey,8 the Care Quality Commission report25 and other research evidence relating to the longer-term needs of stroke patients.
Stroke survivors and carers were involved in developing our outline and full NIHR HTA proposals. Prior to each submission, our application was discussed with the Lay Member Panel of the NIHR Stroke Research Network and the North-east Stroke Patient and Carer Panel. They were very positive about the proposed new service and evaluation. They agreed that it was reasonable to undertake telephone rather than face-to-face reviews.
Patients and carers were actively involved in the development of the intervention materials. This was discussed with the patient and carer groups who were involved in developing the research proposal and we also sought the opinions of individual patients who were being cared for by Northumbria Healthcare NHS Foundation Trust stroke service and their patient experience team.
The NIHR Stroke Research Network Lay Member Panel and the North-east Patient and Carer Panel commented on the study information sheets and data collection forms. The topic guide for qualitative interviews with patients and carers was piloted with the King’s College London Stroke Research Patients and Family Group. Stroke survivors and carers provided advice and practice interviews with the outcome assessor about how to conduct telephone and face-to-face assessments. Dysphasia-friendly study materials were produced by Speakeasy (Bury, UK), and stroke survivors and carers were involved in the development of these materials. We feel that patient and carer involvement facilitated recruitment to the study. Their involvement ensured that study documents and assessments were appropriate and acceptable.
The study was adopted by the NIHR Stroke Research Network and we provided updates to their Lay Member Panel until 2015, when research networks were reorganised and the panel ceased to exist. Many local NIHR Clinical Research Network: Stroke networks have established patient and carer panels that comment on issues relating to adopted studies, but we did not set up a system to enable regular contact with these panels. During the course of the trial, we intermittently presented progress to the North-east Patient and Carer Panel. In particular, we discussed ways to increase the recruitment of carers.
A service user (RC) was part of the investigator team and was involved in the study from its design at grant application stage to reviewing this final report. He was involved in regular investigator group meetings, commented on the study design and documents, and was involved in the final data interpretation. Another service user (David Burgess) was a member of the TSC and advised on a number of issues, particularly the recruitment of carers.
We did not have a documented patient and carer involvement and evaluation plan and, in retrospect, this would have been helpful. Once recruitment and other parts of the study were progressing well, patient and carer involvement was sparse. We should have systematically recorded the changes made to the study based on patient and carer involvement. In future studies, we will follow GRIPP2 (Guidance for Reporting Involvement of Patients and the Public) guidance113 and will also consider establishing a patient and carer panel specifically for the study.
We have not yet shared our findings with patient and carer groups, but we will do so once this report is published. We will seek their advice about the wider dissemination of results and we will work with the Stroke Association and with the North-east Patient and Carer Panel to look at ways to ensure that the results reach a broad audience.
It is important that study participants are informed about the results of the EXTRAS trial. Once the trial is published, study results will be presented at local meetings to study participants who will also be sent a summary of the results of the study and a copy of the final NIHR report (for those who wish to receive it).
Chapter 10 Conclusions
EXTRAS did not improve stroke survivors’ performance in EADL. The lack of effect on the primary outcome is likely to be because of the concept of EXTRAS rather than the fidelity of the intervention. Patients and carers in the intervention group were more satisfied with their overall care, but carer strain and carer quality of life were not influenced by EXTRAS. The post hoc analysis found fewer cases of anxiety and depression in the intervention group than in the usual care group. The impact on patient costs and QALYs resulted in a high chance that EXTRAS could be considered cost-effective at the current conventional thresholds of the NHS/UK society’s WTP for 1 QALY, despite there being no difference in the primary outcome.
Self-management was an important part of EXTRAS, as we sought to improve the stroke survivors’ knowledge, skills and confidence to manage their condition in the context of their day-to-day activities. 114 The EXTRAS reviews offered stroke survivors an opportunity to have regular contact with a stroke specialist who supported them to reflect on their situation and to be realistic about their progress and expectations, as well as to become more knowledgeable about stroke and the help that was and was not available.
Implications for health and social care
The main challenge of this study is the interpretation of the results, given the apparent divergence between the primary outcome and the health economic outcomes. Although there is no evidence of any treatment effect on the primary outcome, the cost-effectiveness analyses are consistent with EXTRAS being associated with cost saving and quality-of-life gain. Our findings suggest that EXTRAS may be an affordable addition to improve stroke care.
Recommendations for further research
The EXTRAS trial has demonstrated that a large multicentre trial to evaluate a new innovative community stroke service is achievable. Robust development and evaluation of stroke units and ESD services, supported by national audit, have led to major improvements in stroke care over the last 20 years. There remains an urgent need to develop, provide and evaluate community services to meet the long-term and ongoing needs of stroke survivors and carers. Unless this is achieved, community-based rehabilitation will continue to lag behind other aspects of stroke care.
Further research is required to identify whether or not community-based interventions tailored to the individual needs of stroke survivors can improve performance in EADL, and to understand the mechanisms by which quality of life is improved and costs are reduced by provision of intermittent longer-term specialist review.
The James Lind Alliance (Southampton, UK) has identified 10 top priorities relating to life after stroke115 and many of these priorities influence performance in EADL. For example, recovery of upper limb function and improving mobility, cognition and speech. These priorities are currently being reviewed by the James Lind Alliance Priority Setting Partnership and the Stroke Association, but addressing the longer-term consequences of stroke is likely to remain a priority.
Acknowledgements
We would like to thank all of the patients, carers and stroke services who participated in this research project. The project was a large collaborative team effort involving 21 NHS trusts. The following NHS trusts were involved in recruiting participants, collecting data and delivery of EXTRAS: Northumbria Healthcare NHS Foundation Trust; Leeds Teaching Hospitals NHS Trust; Leeds Community Healthcare NHS Trust; The Newcastle upon Tyne Hospitals NHS Foundation Trust; Pennine Acute Hospitals NHS Trust; Pennine Care NHS Foundation Trust; South Tyneside NHS Foundation Trust; Royal Cornwall Hospitals NHS Trust; Solent NHS Trust; Portsmouth Hospitals NHS Trust; Plymouth Community Healthcare; Norfolk Community Health and Care NHS Trust; Staffordshire and Stoke-on-Trent Partnership NHS Trust; The Royal Bournemouth and Christchurch Hospitals NHS Foundation Trust; Hull and East Yorkshire Hospitals NHS Trust; Humber NHS Foundation Trust; York Teaching Hospital NHS Foundation Trust; Sherwood Forest Hospitals NHS Foundation Trust; Somerset Partnership NHS Foundation Trust; Wrightington, Wigan and Leigh NHS Foundation Trust; and Cardiff and Vale University Health Board. Many thanks to members of the ESD teams, stroke unit teams, NIHR Stroke Research Network and NIHR Clinical Research Network: Stroke at each site for their help and contributions.
Particular thanks is owed to colleagues in the EXTRAS co-ordinating centre at Newcastle University and Newcastle Clinical Trials Unit, who assisted with the delivery of the project: Dawn Greene, Anne Harrison, Deborah Jones, Jacqueline Price, Chris Speed and Ruth Wood. Also thanks to Caroline Potts and members of the research and development team at Northumbria Healthcare NHS Foundation Trust (study sponsor).
Thanks to members of the NIHR Stroke Research Network Lay Member Panel, North-east Stroke Patient and Carer Panel and King’s College London Stroke Research Patients and Family Group for their help in designing and developing this research project and EXTRAS.
We would also like to thank Gill Pearl and her team at Speakeasy who designed the ‘easy access’ study documentation for patients with communication difficulties and the trial oversight committees. The Data Monitoring and Ethics Committee members were Professor Ian Ford, Professor Gillian Mead, Professor Catherine Sackley and Dr Ailie Turton. The TSC members were Mr David Burgess, Professor Peter Langhorne, Professor Jonathan Mant and Professor Andy Vail.
Contributions of authors
Dr Lisa Shaw (https://orcid.org/0000-0002-3435-9519) (Principal Research Associate) was a co-investigator and managed the project. She was involved in the study design and delivery, and interpretation of data and led the drafting of the report.
Mr Nawaraj Bhattarai (https://orcid.org/0000-0002-1894-2499) (Research Assistant) was a study health economist who conducted the health economic analyses and drafted the health economic chapter of the report.
Mr Robin Cant (Service User) was a co-investigator and is a service user. He was involved in the study design, delivery and interpretation of data, and drafting the report.
Professor Avril Drummond (https://orcid.org/0000-0003-1220-8354) (Professor of Healthcare Research) was a co-investigator, involved in the study design and delivery, the interpretation of data and drafting the report.
Professor Gary A Ford (https://orcid.org/0000-0001-8719-4968) (Professor of Clinical Pharmacology) was a co-investigator, involved in the study design and delivery, the interpretation of data and drafting the report.
Professor Anne Forster (https://orcid.org/0000-0001-7466-4414) (Professor of Stroke Rehabilitation) was a co-investigator, involved in the study design and delivery, the interpretation of data and drafting the report.
Dr Richard Francis (https://orcid.org/0000-0002-6568-1295) (Research Associate) was a researcher working on the project who contributed to data management and analyses during the study delivery period.
Mrs Katie Hills (https://orcid.org/0000-0002-0319-2701) (Research Physiotherapist) was a researcher working on the project who led the design of EXTRAS evaluated in this study.
Ms Denise Howel (https://orcid.org/0000-0002-0033-548X) (Senior Lecturer) was a co-investigator and the senior statistician. She was involved in the study design and delivery, the interpretation of data and drafting the report, and supervised the main patient and carer analyses.
Ms Anne Marie Laverty (https://orcid.org/0000-0002-0941-5849) (Director of Patient Experience) was a co-investigator and involved in the study design and delivery, the interpretation of data and drafting the report.
Professor Christopher McKevitt (https://orcid.org/0000-0002-5290-4613) (Professor of Social Sciences and Health) was a co-investigator and lead for the parallel process evaluation. He was involved in the study design and delivery, the interpretation of data and drafting the report, and supervised the process evaluation.
Dr Peter McMeekin (https://orcid.org/0000-0003-0946-7224) (Reader) was a co-investigator and senior study health economist. He was involved in the study design and delivery, the interpretation of data and drafting the report, and supervised the health economic analyses.
Dr Christopher Price (https://orcid.org/0000-0003-3566-3157) (Reader) was a co-investigator and involved in the study design and delivery, the interpretation of data and drafting the report.
Mrs Elaine Stamp (https://orcid.org/0000-0001-5915-8636) (Research Associate) was the study statistician who conducted the main patient and carer data analyses, and contributed to drafting of the corresponding chapters of this report.
Ms Eleanor Stevens (https://orcid.org/0000-0002-1578-8449) (Research Assistant) was a researcher working on the project who undertook the parallel process evaluation data collection and analyses, and drafted the corresponding chapters of the report.
Professor Luke Vale (https://orcid.org/0000-0001-8574-8429) (Health Foundation Chairperson in Health Economics) was a senior study health economist, and was involved in interpretation of data and drafting the report.
Professor Helen Rodgers (https://orcid.org/0000-0003-3433-4175) (Professor of Stroke Care) was the chief investigator and lead grant holder. She was involved in the study design and delivery, the interpretation of data and drafting the report.
Publications
Rodgers H, Shaw L, Cant R, Drummond A, Ford GA, Forster A, et al. Evaluating an extended rehabilitation service for stroke patients (EXTRAS): study protocol for a randomised controlled trial. Trials 2015;16:205.
Rodgers H, Howel D, Bhattarai N, Cant R, Drummond A, Ford GA, et al. Evaluation of an Extended Stroke Rehabilitation Service (EXTRAS): a randomized controlled trial and economic analysis. Stroke 2019;50:3561–8.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review. Exclusive use will be retained until the publication of major outputs.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Rodgers H, Howel D, Bhattarai N, Cant R, Drummond A, Ford GA, et al. Evaluation of an Extended Stroke Rehabilitation Service (EXTRAS): a randomized controlled trial and economic analysis. Stroke 2019;50:3561-8. https://doi.org/10.1161/STROKEAHA.119.024876.
- Wade DT, Wade DT. Measurement in Neurological Rehabilitation. Oxford: Oxford University Press; 1992.
- Stroke Association . Current, Future and Avoidable Costs of Stroke in the UK 2017.
- Lee S, Shafe AC, Cowie MR. UK stroke incidence, mortality and cardiovascular risk management 1999–2008: time-trend analysis from the General Practice Research Database. BMJ Open 2011;1. https://doi.org/10.1136/bmjopen-2011-000269.
- Stroke Association . State of the Nation. Stroke Statistics January 2017 2017.
- Murray J, Young J, Forster A. Review of longer-term problems after a disabling stroke. Rev Clin Gerontol 2007;17:277-92. https://doi.org/10.1017/S0959259808002608.
- Hartman-Maeir A, Soroker N, Ring H, Avni N, Katz N. Activities, participation and satisfaction one-year post stroke. Disabil Rehabil 2007;29:559-66. https://doi.org/10.1080/09638280600924996.
- McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Wolfe C. UK Stroke Survivor Needs Survey. London: The Stroke Association; 2010.
- McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Rudd AR, et al. Self-reported long-term needs after stroke. Stroke 2011;42:1398-403. https://doi.org/10.1161/STROKEAHA.110.598839.
- Sumathipala K, Radcliffe E, Sadler E, Wolfe CD, McKevitt C. Identifying the long-term needs of stroke survivors using the International Classification of Functioning, Disability and Health. Chronic Illn 2012;8:31-44. https://doi.org/10.1177/1742395311423848.
- Stroke Association . Struggling to Recover. Life After Stroke Campaign Briefing 2012.
- Pindus DM, Mullis R, Lim L, Wellwood I, Rundell AV, Abd Aziz NA, et al. Stroke survivors’ and informal caregivers’ experiences of primary care and community healthcare services – a systematic review and meta-ethnography. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0192533.
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377:1693-702. https://doi.org/10.1016/S0140-6736(11)60325-5.
- Langhorne P, Baylan S. Early Supported Discharge Trialists. Early supported discharge services for people with acute stroke. Cochrane Database Syst Rev 2017;7. https://doi.org/10.1002/14651858.CD000443.pub4.
- Stroke Unit Trialists Collaboration . Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev 2013;9.
- Intercollegiate Stroke Working Party . National Clinical Guideline for Stroke 5th Edition 2016.
- Fisher RJ, Gaynor C, Kerr M, Langhorne P, Anderson C, Bautz-Holter E, et al. A consensus on stroke: early supported discharge. Stroke 2011;42:1392-7. https://doi.org/10.1161/STROKEAHA.110.606285.
- Royal College of Physicians . Sentinal Stroke National Audit Programme (SSNAP) Changes Over Time: 4 Years of Data April 2013 – March 2017 2017.
- Royal College of Physicians . Sentinal Stroke National Audit Programme (SSNAP) Acute Organisational Audit Report November 2016 2016.
- National Institute for Health and Care Excellence . Stroke Rehabilitation in Adults. 2013.
- Aziz NA, Leonardi-Bee J, Phillips M, Gladman JR, Legg L, Walker MF. Therapy-based rehabilitation services for patients living at home more than one year after stroke. Cochrane Database Syst Rev 2008;2. https://doi.org/10.1002/14651858.CD005952.pub2.
- National Audit Office . Department of Health: Progress in Improving Stroke Care. 2010.
- Ellis G, Mant J, Langhorne P, Dennis M, Winner S. Stroke liaison workers for stroke patients and carers: an individual patient data meta-analysis. Cochrane Database Syst Rev 2010;5. https://doi.org/10.1002/14651858.CD005066.pub2.
- Outpatient Service Trialists . Therapy-based rehabilitation services for stroke patients at home. Cochrane Database Syst Rev 2003;1.
- Care Quality Commission . Supporting Life After Stroke. A Review of Services for People Who Have Had a Stroke and Their Carers 2011.
- Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0087987.
- Barclay RE, Stevenson TJ, Poluha W, Ripat J, Nett C, Srikesavan CS. Interventions for improving community ambulation in individuals with stroke. Cochrane Database Syst Rev 2015;3. https://doi.org/10.1002/14651858.CD010200.pub2.
- Ferrarello F, Baccini M, Rinaldi LA, Cavallini MC, Mossello E, Masotti G, et al. Efficacy of physiotherapy interventions late after stroke: a meta-analysis. J Neurol Neurosurg Psychiatry 2011;82:136-43. https://doi.org/10.1136/jnnp.2009.196428.
- Fryer CE, Luker JA, McDonnell MN, Hillier SL. Self management programmes for quality of life in people with stroke. Cochrane Database Syst Rev 2016;8. https://doi.org/10.1002/14651858.CD010442.pub2.
- Forster A, Young J, Chapman K, Nixon J, Patel A, Holloway I, et al. Cluster randomized controlled trial: clinical and cost-effectiveness of a system of longer-term stroke care. Stroke 2015;46:2212-19. https://doi.org/10.1161/STROKEAHA.115.008585.
- Royal College of Physicians . Sentinal Stroke National Audit Programme (SSNAP) Post-Acute Organisational Audit Phase 1: Post-Acute Stroke Service Commissioning Audit 2015. www.strokeaudit.org/Documents/National/PostAcuteOrg/2015/2015-PAOrgGenericReport.aspx.
- Rodgers H, Shaw L, Cant R, Drummond A, Ford GA, Forster A, et al. Evaluating an extended rehabilitation service for stroke patients (EXTRAS): study protocol for a randomised controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0704-3.
- Nouri FM, Lincoln NB. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987;1:301-5. https://doi.org/10.1177/026921558700100409.
- Bamford JM, Sandercock PA, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1989;20. https://doi.org/10.1161/01.STR.20.6.828.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337:1521-6. https://doi.org/10.1016/0140-6736(91)93206-O.
- Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864-70. https://doi.org/10.1161/01.STR.20.7.864.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. Cambridge: Cambridge University Press; 2001.
- Forster A, Dickerson J, Young J, Patel A, Kalra L, Nixon J, et al. A cluster randomised controlled trial and economic evaluation of a structured training programme for caregivers of inpatients after stroke: the TRACS trial. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17460.
- Patel A, Knapp M, Evans A, Perez I, Kalra L. Training care givers of stroke patients: economic evaluation. BMJ 2004;328. https://doi.org/10.1136/bmj.328.7448.1102.
- Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972;1:233-8. https://doi.org/10.1093/ageing/1.4.233.
- Al-Khawaja I, Wade DT, Collin CF. Bedside screening for aphasia: a comparison of two methods. J Neurol 1996;243:201-4. https://doi.org/10.1007/BF02444015.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Robinson BC. Validation of a Caregiver Strain Index. J Gerontol 1983;38:344-8. https://doi.org/10.1093/geronj/38.3.344.
- Intercollegiate Stroke Working Party . Care After Stroke or Transient Ischaemic Attack. Information for Patients and Their Carers 2012.
- Intercollegiate Stroke Working Party . National Clinical Guideline for Stroke 2008.
- Intercollegiate Stroke Working Party . National Clinical Guideline for Stroke 2012.
- Hurn J, Kneebone I, Cropley M. Goal-setting as an outcome measure: a systematic review. Clin Rehabil 2006;20:756-72. https://doi.org/10.1177/0269215506070793.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- The Picker Institute . Working With Us: Surveys. Acute Care n.d. www.picker.org/working-with-us/surveys/ (accessed 30 October 2018).
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7. https://doi.org/10.1161/01.STR.19.5.604.
- Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J 1957;2:200-15. https://doi.org/10.1177/003693305700200504.
- Parker CJ, Gladman JR, Drummond AE, Dewey ME, Lincoln NB, Barer D, et al. A multicentre randomized controlled trial of leisure therapy and conventional occupational therapy after stroke. TOTAL Study Group. Trial of Occupational Therapy and Leisure. Clin Rehabil 2001;15:42-5. https://doi.org/10.1191/026921501666968247.
- Peyre H, Leplège A, Coste J. Missing data methods for dealing with missing items in quality of life questionnaires. A comparison by simulation of personal mean score, full information maximum likelihood, multiple imputation, and hot deck techniques applied to the SF-36 in the French 2003 decennial health survey. Qual Life Res 2011;20:287-300. https://doi.org/10.1007/s11136-010-9740-3.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Hawe P, Shiell A, Riley T, Gold L. Methods for exploring implementation variation and local context within a cluster randomised community intervention trial. J Epidemiol Community Health 2004;58:788-93. https://doi.org/10.1136/jech.2003.014415.
- Medical Research Council (MRC) . Developing and Evaluating Complex Interventions: New Guidance 2008.
- Department of Health and Social Care (DHSC) . Research Governance Framework for Health and Social Care. Second Edition 2005.
- International Conference for Harmonisation . Good Clinical Practice n.d. www.ich.org/home.html (accessed 30 October 2018).
- McKevitt C, Wolfe C. Community support after stroke: patient and carer views. British Journal of Therapy and Rehabilitation 2000;7:6-10. https://doi.org/10.12968/bjtr.2000.7.1.13907.
- Hart E. The use of pluralistic evaluation to explore people’s experiences of stroke services in the community. Health Soc Care Community 1999;7:248-56. https://doi.org/10.1046/j.1365-2524.1999.00183.x.
- Dowswell G, Lawler J, Young J, Forster A, Hearn J. A qualitative study of specialist nurse support for stroke patients and care-givers at home. Clin Rehabil 1997;11:293-301. https://doi.org/10.1177/026921559701100405.
- Fisher RJ, Walker MF, Golton I, Jenkinson D. The implementation of evidence-based rehabilitation services for stroke survivors living in the community: the results of a Delphi consensus process. Clin Rehabil 2013;27:741-9. https://doi.org/10.1177/0269215512473312.
- Clarke DJ, Hawkins R, Sadler E, Harding G, McKevitt C, Godfrey M, et al. Introducing structured caregiver training in stroke care: findings from the TRACS process evaluation study. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004473.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- May C, Finch T. Implementing, embedding and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Clarke DJ, Godfrey M, Hawkins R, Sadler E, Harding G, Forster A, et al. Implementing a training intervention to support caregivers after stroke: a process evaluation examining the initiation and embedding of programme change. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-96.
- Jones F, McKevitt C, Riazi A, Liston M. How is rehabilitation with and without an integrated self-management approach perceived by UK community-dwelling stroke survivors? A qualitative process evaluation to explore implementation and contextual variations. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014109.
- Clarke DJ, Burton LJ, Tyson SF, Rodgers H, Drummond A, Palmer R, et al. Why do stroke survivors not receive recommended amounts of active therapy? Findings from the ReAcT study, a mixed-methods case-study evaluation in eight stroke units. Clin Rehabil 2018;32:1119-32. https://doi.org/10.1177/0269215518765329.
- Taylor E, McKevitt C, Jones F. Factors shaping the delivery of acute inpatient stroke therapy: a narrative synthesis. J Rehabil Med 2015;47:107-19. https://doi.org/10.2340/16501977-1918.
- Scobbie L, Dixon D, Wyke S. Goal setting and action planning in the rehabilitation setting: development of a theoretically informed practice framework. Clin Rehabil 2011;25:468-82. https://doi.org/10.1177/0269215510389198.
- Mudge S, Kayes N, McPherson K. Who is in control? Clinicians’ view on their role in self-management approaches: a qualitative metasynthesis. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007413.
- Sugavanam T, Mead G, Bulley C, Donaghy M, van Wijck F. The effects and experiences of goal-setting in stroke rehabilitation – a systematic review. Disabil Rehabil 2013;35:177-90. https://doi.org/10.3109/09638288.2012.690501.
- Maclean N, Pound P. A critical review of the concept of patient motivation in the literature on physical rehabilitation. Soc Sci Med 2000;50:495-506.
- Clark T. ‘We’re over-researched here!’ Exploring accounts of research fatigue within qualitative research engagements. Sociology 2008;42:953-70. https://doi.org/10.1177/0038038508094573.
- Department of Health and Social Care (DHSC) . National Stroke Strategy 2007.
- National Institute for Health and Care Excellence . Stroke in Adults. Quality Standard 7: Regular Review of Health and Social Care Needs 2010.
- Royal College of Physicians . Sentinel Stroke National Audit Programme (SSNAP). Acute Organisational Audit: National Results – Full Results Portfolio 2016.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015–16 2016.
- Curtis L, Burns B. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull 2010;96:5-21. https://doi.org/10.1093/bmb/ldq033.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res 2006;6. https://doi.org/10.1186/1472-6963-6-52.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal. Reference N0515 2004.
- Lovell K, Bower P, Gellatly J, Byford S, Bee P, McMillan D, et al. Clinical effectiveness, cost-effectiveness and acceptability of low-intensity interventions in the management of obsessive-compulsive disorder: the Obsessive-Compulsive Treatment Efficacy randomised controlled Trial (OCTET). Health Technol Assess 2017;21. https://doi.org/10.3310/hta21370.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726-32. https://doi.org/10.7326/0003-4819-152-11-201006010-00232.
- Hare R, Rogers H, Lester H, McManus R, Mant J. What do stroke patients and their carers want from community services?. Fam Pract 2006;23:131-6. https://doi.org/10.1093/fampra/cmi098.
- Shannon RL, Forster A, Hawkins RJ. A qualitative exploration of self-reported unmet need 1 year after stroke. Disabil Rehabil 2016;38:2000-7. https://doi.org/10.3109/09638288.2015.1107784.
- Tummers JF, Schrijvers AJ, Visser-Meily JM. Economic evidence on integrated care for stroke patients; a systematic review. Int J Integr Care 2012;12. https://doi.org/10.5334/ijic.847.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965;14:61-5. https://doi.org/10.1037/t02366-000.
- Jones F, Gage H, Drummond A, Bhalla A, Grant R, Lennon S, et al. Feasibility study of an integrated stroke self-management programme: a cluster-randomised controlled trial. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-008900.
- Parke HL, Epiphaniou E, Pearce G, Taylor SJ, Sheikh A, Griffiths CJ, et al. Self-management support interventions for stroke survivors: a systematic meta-review. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0131448.
- Sadler E, Wolfe CD, McKevitt C. Lay and health care professional understandings of self-management: a systematic review and narrative synthesis. SAGE Open Med 2014;2. https://doi.org/10.1177/2050312114544493.
- Gillett M, Dallosso HM, Dixon S, Brennan A, Carey ME, Campbell MJ, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ 2010;341. https://doi.org/10.1136/bmj.c4093.
- Li J, Parrott S, Sweeting M, Farmer A, Ross J, Dack C, et al. Cost-effectiveness of facilitated access to a self-management website, compared to usual care, for patients with type 2 diabetes (HeLP-Diabetes): randomized controlled trial. J Med Internet Res 2018;20. https://doi.org/10.2196/jmir.9256.
- National Audit Office . Reducing Brain Damage: Faster Access to Better Stroke Care 2005.
- Wu S, Mead G, Macleod M, Chalder T. Model of understanding fatigue after stroke. Stroke 2015;46:893-8. https://doi.org/10.1161/STROKEAHA.114.006647.
- King’s College London . Sentinel Stroke National Audit Programme (SSNAP). Results Portal 2018. www.strokeaudit.org/results (accessed 30 October 2018).
- Saka O, Serra V, Samyshkin Y, McGuire A, Wolfe CC. Cost-effectiveness of stroke unit care followed by early supported discharge. Stroke 2009;40:24-9. https://doi.org/10.1161/STROKEAHA.108.518043.
- Briggs A. Economic evaluation and clinical trials: size matters. BMJ 2000;321:1362-3. https://doi.org/10.1136/bmj.321.7273.1362.
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523-32. https://doi.org/10.1007/s11136-004-7713-0.
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200-7. https://doi.org/10.7326/0003-4819-158-3-201302050-00583.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. https://doi.org/10.1136/bmj.f1049.
- CONSORT . Consolidated Standards of Reporting Trials. n.d. www.consort-statement.org (accessed 30 October 2018).
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Onghena P, Everitt BS, Howell D. Encyclopedia of Statistics in Behavioural Science. Hoboken, NJ: Wiley-Blackwell; 2005.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- de Iongh A, Fagan P, Fenner J, Kidd L. A Practical Guide To Self-Management Support. London: The Health Foundation; 2015.
- Pollock A, St George B, Fenton M, Firkins L. Top ten research priorities relating to life after stroke. Lancet Neurol 2012;11. https://doi.org/10.1016/S1474-4422(12)70029-7.
- Royal College of Physicians . Sentinel Stroke National Audit Programme (SSNAP). Post-Acute Organisational Audit. Public Report. Phase 2 2015.
- Royal College of Physicians . National Results – Post Acute Organisation. Phase 2 Post Acute Stroke Service Provider Audit: Summary Spreadsheet 2015.
- Royal College of Physicians . Mind the Gap! The Third SSNAP Annual Report. Care Received Between April 2015 to March 2016 2016.
- Department for Work and Pensions . Benefit and Pension Rates 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/524117/benefit-and-pension-rates-from-6-april-2016.pdf (accessed 30 October 2018).
Appendix 1 Description of EXTRAS using the TIDieR checklist
| Item | Description |
|---|---|
| 1. Brief name: ‘provide the name or phrase that describes the intervention’ | Extended stroke rehabilitation service (EXTRAS) |
| 2. Why: ‘describe any rationale, theory, or goal of the elements essential to the intervention’ |
The EXTRAS consisted of rehabilitation reviews conducted by a senior member of the ESD team at 1, 3, 6, 12 and 18 months post discharge from routine ESD (trial randomisation). Each review consisted of a semistructured interview to identify rehabilitation issues, goal-setting and action-planning The elements of this service are established rehabilitation practice |
| 3. What materials: ‘describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed’ |
A study-specific manual described how to conduct the reviews and included guidance on exploring rehabilitation needs, goal-setting and appropriate interventions to meet a patient’s needs Study-specific paperwork was completed for each review and required documentation of identified needs, goals set and action plans made Staff delivering the reviews received specific training Participants received an appointment card that documented review dates and also contained a short checklist of rehabilitation issues to be covered in each review Following a review, participants received a summary of the interview and recommendations for rehabilitation by post |
| 4. What (procedures): ‘describe each of the procedures, activities and/or processes used in the intervention, including any enabling support activities’ |
The EXTRAS reviews were intended to be conducted by telephone. However, if participation in a telephone interview was not possible, a home visit could be undertaken. Each review consisted of: Identification of rehabilitation needs. A semistructured interview was conducted to determine the patient’s progress, current rehabilitation needs and service provision. The interview addressed daily activities (mobility, personal care, mealtimes, domestic activities), social participation (work and volunteering, hobbies and interests, driving and transport) and wider issues (communication, memory and concentration, mood, medical issues, pain) that may be problematic for stroke survivors. The views of both the patient and carer (where appropriate) could be sought. The interview topics were based on the literature about long-term needs after stroke, including the UK Stroke Survivor Needs Survey 7 and input from stroke survivors, carers and health-care professionals Joint rehabilitation goal-setting. From the identified progress and rehabilitation needs, up to five individual rehabilitation goals could be set by the patient (and carer) in collaboration with the senior ESD team member who conducted the review. The focus of joint goal-setting was intended to be on increasing participation in everyday activities. From the second review, progress towards goals set previously was assessed prior to further goal-setting. Achievement of goals was recorded using a Goal Attainment Scale48 Action-planning. The patient (and carer) agreed an action plan for each rehabilitation goal. Action plans could include: Verbal advice and encouragement Discussion with the stroke team, rehabilitation team, primary care team or social services involved in care Signposting to local activities, community organisations or voluntary services Referral to stroke services, rehabilitation services or primary care services for further assessment and treatment if required according to local guidelines and/or service provision |
| 5. Who provided: ‘for each category of intervention provider (for example, psychologist, nursing assistant) describe their expertise, background and any specific training given’ |
Senior members of ESD teams delivered the reviews. The choice of ESD team staff member (e.g. physiotherapist, occupational therapist, nurse) to conduct each individual review was at the discretion of each participating centre On opening a study centre, all senior ESD staff taking part in delivery of the new service received face-to-face training from the trial co-ordinating centre team. As delivery of the intervention for this study was conducted for almost 5 years (recruitment commenced in late 2012 and delivery of the intervention ceased in mid-2017), inevitable staffing changes took place in ESD teams. New members of staff received cascade training from existing trained staff and/or further training visits from the trial co-ordinating centre were undertaken |
| 6. How: ‘describe the modes of delivery (such as face to face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group’ | One-to-one telephone interviews were intended. However, if participation in a telephone interview was not possible, a home visit could be undertaken |
| 7. Where: ‘describe the type of location(s) where the intervention occurred’ | Telephone calls were made from ESD team facilities |
| 8. When and how much: ‘describe the number of times the intervention was delivered and over what period of time including the number of sessions, their schedule, duration, intensity or dose’ | Five reviews were conducted over 18 months. These took place at 1, 3, 6, 12 and 18 months post discharge from routine ESD services. Review duration was individual and according to the length of time it took to complete |
| 9. Tailoring: ‘if intervention was planned to be personalised or adapted, then describe what, why, when and how’ | Every review was personalised as individual needs were identified, goals set and actions planned |
| 10. Modifications: ‘if intervention was modified during the course of the study, describe the changes what, why, when and how’ | No modifications were made |
| 11. How well (planned): ‘if intervention adherence or fidelity was assessed, describe how and by whom, and if any strategies were used to maintain or improve fidelity, describe them’ |
Each review was documented onto study-specific paperwork that asked for rehabilitation issues, goals set and action plans made to be recorded The paperwork for all five reviews was provided as a booklet and at the start of each time point space was provided to document a reason if the review could not be undertaken Data from the study paperwork was uploaded onto an online database and the study co-ordination centre used this to send monthly reports to all participating teams highlighting overdue or missing reviews |
| 12. How well (actual): ‘If intervention adherence or fidelity was assessed, describe the extent to which the intervention was delivered as planned’ | Details of delivery of the intervention are provided in Chapter 5 |
Appendix 2 Supplementary data
| Reasons recruited patients were not randomised | Number of patients (N = 101), n |
|---|---|
| Patient decision to withdraw | 49 |
| Deterioration in cognition or health | 5 |
| Did not want further rehabilitation | 13 |
| No reason provided | 18 |
| Too many other commitments/time commitment | 10 |
| Too many questions/questions too challenging | 3 |
| Study centre staff decision to withdraw | 20 |
| Staff considered patient no longer appropriate because of a deterioration in health | 8 |
| Staff considered patient no longer appropriate because of the cognitive function of patient and/or carer | 8 |
| Patient recruited in error as they did not fulfil eligibility criteria (not a diagnosis of stroke) | 4 |
| Other decision | 28 |
| Patient moving away from study centre area | 2 |
| Patient discharged from routine ESD and baseline assessment/randomisation not conducted in error | 9 |
| Patient unavailable/uncontactable for baseline assessment and randomisation | 6 |
| Staff unavailable to conduct baseline assessment and randomisation | 6 |
| Patient recruited in error did not fulfil eligibility criteria (not discharged with ESD team) | 5 |
| Patient died | 4 |
| Study centre | Opening date | Number of patients recruited (n) | Number of patients randomised | ||
|---|---|---|---|---|---|
| Total, n (%) | Intervention (n) | Control (n) | |||
| North Tyneside | 5 November 2012 | 42 | 36 (86) | 17 | 19 |
| Wansbeck | 5 November 2012 | 35 | 24 (69) | 12 | 12 |
| Leeds | 3 December 2012 | 132 | 81 (61) | 41 | 40 |
| Newcastle | 2 January 2013 | 36 | 27 (75) | 14 | 13 |
| Pennine | 12 February 2013 | 23 | 15 (65) | 8 | 7 |
| South Tyneside | 1 August 2013 | 28 | 27 (96) | 14 | 13 |
| Cornwall | 28 August 2013 | 68 | 61 (90) | 29 | 32 |
| Southampton | 7 October 2013 | 21 | 21 (100) | 11 | 10 |
| Plymouth | 24 October 2013 | 60 | 60 (100) | 30 | 30 |
| Portsmouth | 7 November 2013 | 29 | 27 (93) | 14 | 13 |
| Norfolk | 29 November 2013 | 57 | 56 (98) | 28 | 28 |
| Staffordshire | 5 December 2013 | 21 | 18 (86) | 9 | 9 |
| Bournemouth | 25 February 2014 | 32 | 32 (100) | 16 | 16 |
| Hull | 10 March 2014 | 8 | 8 (100) | 3 | 5 |
| York | 21 May 2014 | 11 | 11 (100) | 5 | 6 |
| Sherwood Forest | 4 July 2014 | 24 | 22 (92) | 11 | 11 |
| Somerset | 25 July 2014 | 19 | 19 (100) | 9 | 10 |
| Wigan | 7 August 2014 | 10 | 10 (100) | 5 | 5 |
| Cardiff | 23 December 2014 | 18 | 18 (100) | 9 | 9 |
| Group | Withdrawn at or before the 12-month assessment | Assessment not conducted at 12 months | Withdrawn between 12 and 24 months | Assessment not conducted at 24 months |
|---|---|---|---|---|
| Intervention | Total: N = 13
|
Total: N = 23
|
Total: N = 7
|
Total: N = 23
|
| Control | Total: N = 8
|
Total: N = 14
|
Total: N = 1
|
Total: N = 16
|
| Characteristic | Randomised | Not randomised |
|---|---|---|
| Sex, n (%) | n = 573 | n = 99 |
| Male | 342 (59.7) | 50 (50.5) |
| Female | 231 (40.3) | 49 (49.4) |
| Age (years) | n = 573 | n = 99 |
| Median (IQR) | 71 (61–78) | 75 (66–82) |
| Pre-stroke NEADL Scale score | n = 573 | n = 99 |
| Mean (SD) | 60.4 (9.6) | 54.8 (13.7) |
| Pre-stroke OHS score, n (%) | n = 572 | n = 99 |
| 0 | 349 (61.0) | 50 (50.5) |
| 1 | 108 (18.9) | 20 (20.2) |
| 2 | 82 (14.3) | 17 (17.2) |
| 3 | 29 (5.1) | 11 (11.1) |
| 4 | 4 (0.7) | 1 (1.01) |
| 5 | 0 (0.0) | 0 (0.0) |
| Stroke type, n (%) | n = 573 | n = 99 |
| Cerebral infarct | 503 (87.8) | 92 (92.9) |
| Intracerebral haemorrhage | 59 (10.3) | 5 (5.1) |
| Subarachnoid haemorrhage | 10 (1.8) | 1 (1.0) |
| Unknown | 1 (0.2) | 1 (1.0) |
| Stroke subtype, n (%) | n = 570 | n = 99 |
| TACS | 115 (20.2) | 20 (20.2) |
| PACS | 258 (45.3) | 41 (41.4) |
| LACS | 100 (17.5) | 24 (24.2) |
| POCS | 96 (16.8) | 14 (14.1) |
| Uncertain | 1 (0.2) | 0 (0.00) |
| First ever stroke, n (%) | n = 573 | n = 99 |
| Yes | 462 (80.6) | 78 (78.8) |
| NIHSS score at recruitment (scored 0–42) | n = 563 | n = 99 |
| Median (IQR) | 2 (1–4) | 2 (2–4) |
| Subscale | Pre stroke | Baseline | 12 months | 24 months | ||||
|---|---|---|---|---|---|---|---|---|
| Intervention, mean (SD) | Control, mean (SD) | Intervention, mean (SD) | Control, mean (SD) | Intervention, mean (SD) | Control, mean (SD) | Intervention, mean (SD) | Control, mean (SD) | |
| n = 285 | n = 288 | n = 283 | n = 285 | n = 239 | n = 248 | n = 219 | n = 231 | |
| Mobility (0–18) | 16.7 (3.1) | 16.1 (3.8) | 10.5 (5.5) | 10.3 (5.5) | 11.0 (5.6) | 10.3 (5.7) | 10.7 (5.8) | 9.8 (6.0) |
| n = 285 | n = 288 | n = 283 | n = 286 | n = 239 | n = 247 | n = 219 | n = 231 | |
| Kitchen (0–15) | 14.7 (1.5) | 14.5 (2.1) | 12.1 (4.0) | 11.8 (4.3) | 11.6 (4.3) | 10.9 (4.4) | 11.3 (4.4) | 10.8 (4.8) |
| n = 285 | n = 288 | n = 283 | n = 285 | n = 239 | n = 248 | n = 219 | n = 231 | |
| Domestic tasks (0–15) | 13.7 (2.8) | 13.5 (3.3) | 8.0 (5.2) | 7.8 (5.2) | 8.1 (5.2) | 7.4 (5.0) | 7.8 (5.1) | 7.1 (5.0) |
| n = 285 | n = 288 | n = 281 | n = 284 | n = 239 | n = 248 | n = 219 | n = 231 | |
| Leisure activities (0–18) | 16.0 (3.1) | 15.6 (3.4) | 9.3 (4.2) | 9.3 (4.1) | 10.0 (4.8) | 9.5 (4.5) | 10.2 (5.0) | 9.6 (4.9) |
| n = 285 | n = 288 | n = 281 | n = 282 | n = 239 | n = 247 | n = 219 | n = 231 | |
| Total (0–66) | 61.0 (8.6) | 59.7 (10.6) | 39.8 (16.1) | 39.1 (16.1) | 40.6 (17.7) | 38.3 (17.0) | 40.0 (18.1) | 37.2 (18.5) |
| About the services, to what extent do you agree that: | Intervention, n (%) | Control, n (%) |
|---|---|---|
| Staff were welcoming and friendly | n = 238 | n = 246 |
| Strongly disagree | 1 (0.4) | 3 (1.2) |
| Disagree | 1 (0.4) | 3 (1.2) |
| Agree | 83 (34.9) | 112 (45.5) |
| Strongly agree | 147 (61.8) | 118 (48.0) |
| Does not apply | 6 (2.5) | 10 (4.1) |
| Missing | 1 | 2 |
| Staff treated you with dignity and respect | n = 238 | n = 246 |
| Strongly disagree | 1 (0.4) | 3 (1.2) |
| Disagree | 3 (1.3) | 4 (1.6) |
| Agree | 81 (34.0) | 107 (43.5) |
| Strongly agree | 146 (61.3) | 123 (50.0) |
| Does not apply | 7 (2.9) | 9 (3.7) |
| Missing | 1 | 2 |
| Staff assessed your needs | n = 237 | n = 246 |
| Strongly disagree | 1 (0.4) | 2 (0.8) |
| Disagree | 9 (3.8) | 6 (2.4) |
| Agree | 93 (39.2) | 130 (52.9) |
| Strongly agree | 126 (53.2) | 99 (40.2) |
| Does not apply | 8 (3.4) | 9 (3.7) |
| Missing | 2 | 2 |
| Staff met your needs | n = 238 | n = 246 |
| Strongly disagree | 0 (0.0) | 2 (0.8) |
| Disagree | 14 (5.9) | 13 (5.3) |
| Agree | 97 (40.8) | 134 (54.5) |
| Strongly agree | 120 (50.4) | 88 (35.8) |
| Does not apply | 7 (2.9) | 9 (3.7) |
| Missing | 1 | 2 |
| You have been involved as much as you wanted to be in decisions about your care | n = 238 | n = 246 |
| Strongly disagree | 1 (0.4) | 3 (1.2) |
| Disagree | 19 (8.0) | 17 (6.9) |
| Agree | 102 (42.9) | 121 (49.2) |
| Strongly agree | 109 (45.8) | 92 (37.4) |
| Does not apply | 7 (2.9) | 13 (5.3) |
| Missing | 1 | 2 |
| You were able to discuss your preferences, beliefs and concerns as part of your care | n = 238 | n = 244 |
| Strongly disagree | 1 (0.4) | 3 (1.2) |
| Disagree | 15 (6.3) | 14 (5.7) |
| Agree | 107 (45.0) | 129 (52.9) |
| Strongly agree | 104 (43.7) | 79 (32.4) |
| Does not apply | 11 (4.6) | 19 (7.8) |
| Missing | 1 | 4 |
| You were told who to contact if you had any worries or concerns | n = 238 | n = 244 |
| Strongly disagree | 2 (0.8) | 2 (0.8) |
| Disagree | 11 (4.6) | 13 (5.3) |
| Agree | 106 (44.5) | 144 (59.0) |
| Strongly agree | 114 (47.9) | 74 (30.3) |
| Does not apply | 5 (2.1) | 11 (4.5) |
| Missing | 1 | 4 |
| You were confident that the staff you saw had the right skills and knowledge to help you | n = 236 | n = 246 |
| Strongly disagree | 1 (0.4) | 4 (1.6) |
| Disagree | 6 (2.5) | 10 (4.1) |
| Agree | 100 (42.4) | 136 (55.3) |
| Strongly agree | 123 (52.1) | 87 (35.4) |
| Does not apply | 6 (2.5) | 9 (3.7) |
| Missing | 3 | 2 |
| You were treated fairly regardless of your age, race, sex, belief, sexual orientation or disability | n = 237 | n = 246 |
| Strongly disagree | 1 (0.4) | 2 (0.8) |
| Disagree | 7 (3.0) | 6 (2.4) |
| Agree | 96 (40.5) | 127 (51.6) |
| Strongly agree | 127 (53.6) | 101 (41.1) |
| Does not apply | 6 (2.5) | 10 (4.1) |
| Missing | 2 | 2 |
| You were given the information you wanted | n = 237 | n = 246 |
| Strongly disagree | 1 (0.4) | 2 (0.8) |
| Disagree | 11 (4.6) | 13 (5.3) |
| Agree | 113 (47.7) | 140 (56.9) |
| Strongly agree | 107 (45.2) | 79 (32.1) |
| Does not apply | 5 (2.1) | 12 (4.9) |
| Missing | 2 | 2 |
| You were able to see the same health-care professional/team whenever possible | n = 238 | n = 246 |
| Strongly disagree | 2 (0.8) | 3 (1.2) |
| Disagree | 15 (6.3) | 27 (11.0) |
| Agree | 108 (45.6) | 135 (54.9) |
| Strongly agree | 103 (43.5) | 68 (27.6) |
| Does not apply | 9 (3.8) | 13 (5.3) |
| Missing | 1 | 2 |
| If you had important questions to ask, you got answers that you could understand | n = 236 | n = 245 |
| Strongly disagree | 2 (0.9) | 0 (0.0) |
| Disagree | 14 (5.9) | 10 (4.1) |
| Agree | 109 (46.2) | 136 (55.5) |
| Strongly agree | 103 (43.6) | 76 (31.0) |
| Does not apply | 8 (3.4) | 23 (9.4) |
| Missing | 3 | 3 |
| If you needed more than one service, staff made sure they were well co-ordinated | n = 238 | n = 246 |
| Strongly disagree | 3 (1.3) | 3 (1.2) |
| Disagree | 16 (6.7) | 21 (8.6) |
| Agree | 104 (43.7) | 126 (51.4) |
| Strongly agree | 92 (38.7) | 63 (25.7) |
| Does not apply | 23 (9.7) | 32 (13.1) |
| Missing | 1 | 2 |
| If you needed more than one service, staff made sure that your care information was clearly and accurately shared | n = 238 | n = 245 |
| Strongly disagree | 4 (1.7) | 3 (1.2) |
| Disagree | 13 (5.5) | 19 (7.7) |
| Agree | 104 (43.7) | 129 (52.4) |
| Strongly agree | 93 (39.1) | 63 (25.6) |
| Does not apply | 24 (10.1) | 32 (13.0) |
| Missing | 1 | 3 |
| You were told who to contact if you had any ongoing health-care needs | n = 238 | n = 246 |
| Strongly disagree | 1 (0.4) | 2 (0.8) |
| Disagree | 15 (6.3) | 18 (7.3) |
| Agree | 107 (45.0) | 147 (59.8) |
| Strongly agree | 110 (46.2) | 68 (27.6) |
| Does not apply | 5 (2.1) | 11 (4.5) |
| Missing | 1 | 2 |
| Overall, how satisfied are you with the services you received? | n = 239 | n = 246 |
| Extremely satisfied | 77 (32.2) | 64 (26.0) |
| Very satisfied | 112 (46.9) | 102 (41.5) |
| Quite satisfied | 39 (16.3) | 61 (24.8) |
| Not very satisfied | 9 (3.8) | 16 (6.5) |
| Extremely unsatisfied | 2 (0.8) | 3 (1.2) |
| Missing | 0 | 2 |
| In the last 12 months, have you had enough help with speaking difficulties from the NHS? | n = 239 | n = 246 |
| Yes, definitely | 48 (20.1) | 54 (22.0) |
| Yes, to some extent | 31 (13.0) | 35 (14.2) |
| No I did not get enough help from the NHS | 11 (4.6) | 16 (6.5) |
| I did not have any speaking difficulties | 149 (62.3) | 141 (57.3) |
| Missing | 0 | 2 |
| In the last 12 months, have you had enough treatment to help improve your mobility from the NHS? | n = 239 | n = 247 |
| Yes, definitely | 103 (43.1) | 80 (32.4) |
| Yes, to some extent | 64 (26.8) | 86 (34.8) |
| No I did not get enough help from the NHS | 35 (14.6) | 52 (21.1) |
| I did not have any mobility difficulties | 37 (15.5) | 29 (11.7) |
| Missing | 0 | 1 |
| In the last 12 months, have you had enough help with emotional problems from the NHS? | n = 239 | n = 246 |
| Yes, definitely | 38 (15.9) | 52 (21.1) |
| Yes, to some extent | 42 (17.6) | 60 (24.4) |
| No I did not get enough help from the NHS | 33 (13.8) | 34 (13.8) |
| I did not have any emotional problems | 126 (52.7) | 100 (40.7) |
| Missing | 0 | 2 |
| About the services, to what extent do you agree that: | Intervention, n (%) | Control, n (%) |
|---|---|---|
| Staff were welcoming and friendly | n = 216 | n = 227 |
| Strongly disagree | 0 (0.0) | 2 (0.9) |
| Disagree | 2 (0.9) | 2 (0.9) |
| Agree | 51 (23.6) | 65 (28.6) |
| Strongly agree | 63 (29.2) | 58 (25.6) |
| Does not apply | 100 (46.3) | 100 (44.1) |
| Missing | 3 | 4 |
| Staff treated you with dignity and respect | n = 216 | n = 225 |
| Strongly disagree | 0 (0.0) | 0 (0.0) |
| Disagree | 0 (0.0) | 4 (1.8) |
| Agree | 53 (24.5) | 64 (28.4) |
| Strongly agree | 64 (29.6) | 58 (25.9) |
| Does not apply | 99 (45.8) | 99 (44.0) |
| Missing | 3 | 6 |
| Staff assessed your needs | n = 216 | n = 225 |
| Strongly disagree | 0 (0.0) | 2 (0.9) |
| Disagree | 4 (1.9) | 7 (3.1) |
| Agree | 56 (25.9) | 61 (27.1) |
| Strongly agree | 57 (26.4) | 53 (23.6) |
| Does not apply | 99 (45.8) | 102 (45.3) |
| Missing | 3 | 6 |
| Staff met your needs | n = 217 | n = 226 |
| Strongly disagree | 0 (0.0) | 2 (0.9) |
| Disagree | 3 (1.4) | 12 (5.3) |
| Agree | 63 (29.0) | 60 (26.6) |
| Strongly agree | 51 (23.5) | 51 (22.6) |
| Does not apply | 100 (46.1) | 101 (44.7) |
| Missing | 2 | 5 |
| You have been involved as much as you wanted to be in decisions about your care | n = 216 | n = 226 |
| Strongly disagree | 0 (0.0) | 1 (0.4) |
| Disagree | 7 (3.2) | 12 (5.3) |
| Agree | 61 (28.2) | 59 (26.1) |
| Strongly agree | 49 (22.7) | 49 (21.7) |
| Does not apply | 99 (45.8) | 105 (46.5) |
| Missing | 3 | 5 |
| You were able to discuss your preferences, beliefs and concerns as part of your care | n = 216 | n = 226 |
| Strongly disagree | 0 (0.0) | 1 (0.4) |
| Disagree | 8 (3.7) | 9 (4.0) |
| Agree | 64 (29.6) | 68 (30.1) |
| Strongly agree | 42 (19.4) | 44 (19.5) |
| Does not apply | 102 (47.2) | 104 (46.0) |
| Missing | 3 | 5 |
| You were told who to contact if you had any worries or concerns | n = 216 | n = 226 |
| Strongly disagree | 1 (0.5) | 4 (1.8) |
| Disagree | 6 (2.8) | 5 (2.2) |
| Agree | 58 (26.9) | 66 (29.2) |
| Strongly agree | 52 (24.1) | 50 (22.1) |
| Does not apply | 99 (45.8) | 101 (44.7) |
| Missing | 3 | 5 |
| You were confident that the staff you saw had the right skills and knowledge to help you | n = 216 | n = 226 |
| Strongly disagree | 0 (0.0) | 1 (0.4) |
| Disagree | 3 (1.4) | 6 (2.7) |
| Agree | 62 (28.7) | 68 (30.1) |
| Strongly agree | 54 (25.0) | 51 (22.6) |
| Does not apply | 97 (44.9) | 100 (44.3) |
| Missing | 3 | 5 |
| You were treated fairly regardless of your age, race, sex, belief, sexual orientation or disability | n = 216 | n = 227 |
| Strongly disagree | 0 (0.0) | 1 (0.4) |
| Disagree | 1 (0.5) | 3 (1.3) |
| Agree | 58 (26.9) | 68 (30.0) |
| Strongly agree | 58 (26.9) | 56 (24.7) |
| Does not apply | 99 (45.8) | 99 (43.6) |
| Missing | 3 | 4 |
| You were given the information you wanted | n = 216 | n = 227 |
| Strongly disagree | 0 (0.0) | 0 (0.0) |
| Disagree | 4 (1.9) | 10 (4.4) |
| Agree | 65 (30.1) | 70 (30.8) |
| Strongly agree | 49 (22.7) | 45 (19.8) |
| Does not apply | 98 (45.4) | 102 (44.9) |
| Missing | 3 | 4 |
| You were able to see the same health-care professional/team whenever possible | n = 215 | n = 226 |
| Strongly disagree | 0 (0.0) | 4 (1.8) |
| Disagree | 9 (4.2) | 11 (4.9) |
| Agree | 63 (29.3) | 66 (29.2) |
| Strongly agree | 40 (18.6) | 41 (18.1) |
| Does not apply | 103 (47.9) | 104 (46.0) |
| Missing | 4 | 5 |
| If you had important questions to ask, you got answers that you could understand | n = 216 | n = 226 |
| Strongly disagree | 0 (0.0) | 1 (0.4) |
| Disagree | 8 (3.7) | 8 (3.5) |
| Agree | 64 (29.6) | 66 (29.2) |
| Strongly agree | 43 (19.9) | 47 (20.8) |
| Does not apply | 101 (46.8) | 104 (46.0) |
| Missing | 3 | 5 |
| If you needed more than one service, staff made sure they were well co-ordinated | n = 216 | n = 227 |
| Strongly disagree | 1 (0.5) | 2 (0.9) |
| Disagree | 7 (3.2) | 7 (3.1) |
| Agree | 50 (23.2) | 59 (26.0) |
| Strongly agree | 41 (19.0) | 36 (15.9) |
| Does not apply | 117 (54.2) | 123 (54.2) |
| Missing | 3 | 4 |
| If you needed more than one service, staff made sure that your care information was clearly and accurately shared | n = 216 | n = 226 |
| Strongly disagree | 1 (0.5) | 1 (0.4) |
| Disagree | 8 (3.7) | 9 (4.0) |
| Agree | 52 (24.1) | 58 (25.7) |
| Strongly agree | 39 (18.1) | 36 (15.9) |
| Does not apply | 116 (53.7) | 122 (54.0) |
| Missing | 3 | 5 |
| You were told who to contact if you had any ongoing health-care needs | n = 216 | n = 226 |
| Strongly disagree | 1 (0.5) | 3 (1.3) |
| Disagree | 5 (2.3) | 6 (2.6) |
| Agree | 62 (28.6) | 75 (32.9) |
| Strongly agree | 48 (22.1) | 40 (17.5) |
| Does not apply | 101 (46.5) | 104 (45.6) |
| Missing | 3 | 5 |
| Overall, how satisfied are you with the services you received? | n = 213 | n = 216 |
| Extremely satisfied | 56 (26.3) | 45 (20.8) |
| Very satisfied | 98 (46.0) | 91 (42.1) |
| Quite satisfied | 54 (25.4) | 53 (24.5) |
| Not very satisfied | 5 (2.4) | 20 (9.3) |
| Extremely unsatisfied | 0 (0.0) | 7 (3.2) |
| Missing | 6 | 15 |
| In the last 12 months, have you had enough help with speaking difficulties from the NHS? | n = 215 | n = 225 |
| Yes, definitely | 15 (7.0) | 18 (8.0) |
| Yes, to some extent | 14 (6.5) | 17 (7.6) |
| No, I did not get enough help from the NHS | 13 (6.1) | 20 (8.9) |
| I did not have any speaking difficulties | 173 (80.5) | 170 (75.6) |
| Missing | 4 | 6 |
| In the last 12 months, have you had enough treatment to help improve your mobility from the NHS? | n = 215 | n = 224 |
| Yes, definitely | 31 (14.4) | 35 (15.6) |
| Yes, to some extent | 64 (29.8) | 46 (20.5) |
| No, I did not get enough help from the NHS | 46 (21.4) | 86 (38.4) |
| I did not have any mobility difficulties | 74 (34.4) | 57 (25.5) |
| Missing | 4 | 7 |
| In the last 12 months, have you had enough help with emotional problems from the NHS? | n = 216 | n = 223 |
| Yes, definitely | 22 (10.2) | 31 (13.9) |
| Yes, to some extent | 30 (13.9) | 28 (12.6) |
| No, I did not get enough help from the NHS | 21 (9.7) | 36 (16.1) |
| I did not have any emotional problems | 143 (66.2) | 128 (57.4) |
| Missing | 3 | 8 |
| 12 months | 24 months | |||
|---|---|---|---|---|
| Intervention (n = 239) | Control (n = 248) | Intervention (n = 219) | Control (n = 231) | |
| Assessment by telephone or face to face, n (%) | 212 (89) | 204 (82) | 195 (89) | 186 (81) |
| Assessor reported certainty for randomisation group, n (%) | ||||
| Yes | 37 (17) | 7 (3) | 17 (9) | 2 (1) |
| No | 173 (82) | 197 (97) | 178 (91) | 184 (99) |
| Missing | 2 (1) | |||
| Where assessor reported certainty, correct randomisation group was recorded, n (%) | n = 37 | n = 7 | n = 17 | n = 2 |
| Yes | 37 (17) | 6 (3) | 17 (9) | 1 (1) |
| No | 0 (0) | 1 (0) | 0 (0) | 1 (1) |
| Assessments conducted blinded, n (%) | 173 (82) | 198 (97) | 178 (91) | 185 (99) |
| Reason | Intervention (N = 250) | Control (N = 254) |
|---|---|---|
| Event resulted in death, n (%) | 26 (10.4) | 32 (12.6) |
| Event was life-threatening, n (%) | 6 (2.4) | 5 (2.0) |
| Event resulted in admission to hospital or prolongation of hospitalisation, n (%) | 163 (65.2) | 168 (66.1) |
| Event resulted in persistent or significant disability or incapacity, n (%) | 14 (5.6) | 9 (3.5) |
| Event was ‘otherwise considered significant’ by the investigator, n (%) | 41 (16.4) | 40 (15.7) |
| Intervention (N = 26), n | Control (N = 32), n | |
|---|---|---|
| Cardiovascular | ||
| Total | 7 | 6 |
| Cardiorespiratory failure | 1 | |
| Coronary heart disease | 1 | |
| Heart failurea | 4a | 2 |
| Hypertensive heart disease | 1 | |
| Ischaemic heart disease | 1 | 1 |
| Myocardial infarction | 1 | |
| Peripheral vascular disease | 1 | |
| Dermatological | ||
| Total | 1 | |
| Cellulitis and septic shock | 1 | |
| Gastrointestinal | ||
| Total | 1 | 4 |
| Bowel cancer | 1 | 1 |
| Gastric outlet obstruction and metastatic gastric cancer | 1 | |
| Ischaemic bowel | 1 | |
| Pancreatic cancer | 1 | |
| Musculoskeletal | ||
| Total | 2 | |
| Fracture: hip | 1 | |
| Sarcomab | 1b | |
| Musculoskeletal/respiratory | ||
| Total | 2 | |
| Fractured hip and chest infection/pneumoniab | 2b | |
| Neurological | ||
| Total | 1 | 6 |
| Stroke (new) | 1 | 3 |
| Subarachnoid haemorrhage | 1 | |
| Subdural haematoma | 2 | |
| Respiratory | ||
| Total | 8 | 11 |
| Acute respiratory distress syndrome postoperative | 1 | |
| Chest infection/pneumoniaa | 6a | 6 |
| Chronic obstructive pulmonary disease | 1 | |
| Lung cancer | 1 | |
| Metastatic lung cancer | 1 | |
| Pulmonary embolus | 1 | |
| Respiratory failure from bilateral pneumothoraces from ribs following fall | 1 | |
| Sarcoidosis | 1 | |
| Urinary tract | ||
| Total | 1 | 1 |
| Urinary tract infection | 1 | 1 |
| Miscellaneous | ||
| Total | 4 | 3 |
| Complications of stroke | 1 | |
| Death: cause could not be established | 3 | 1 |
| Frailty of old age | 1 | |
| Old age | 1 | |
| Intervention | Control | |||
|---|---|---|---|---|
| Number of participants who experienced event, n | Number of events (N = 224), n | Number of participants who experienced event, n | Number of events (N = 222), n | |
| Cardiovascular | ||||
| Total | 18 | 22 | 20 | 26 |
| AF or other arrhythmia | 5 | 4 | ||
| Angina/ischaemic chest pain | 3 | 3 | ||
| Aortic valve replacement | 1 | |||
| Atrial septal defect repair | 1 | |||
| Cardiac resynchronisation therapy and defibrillator insertion | 1 | |||
| Carotid sinus syncope | 1 | |||
| Foramen ovale closure | 2 | |||
| Heart failure | 2 | 8 | ||
| ICD insertion | 1 | |||
| Implantation of loop recorder | 1 | |||
| Insertion of left ventricular assistive device | 1 | |||
| Insertion of stent graft for thoracic aneurysm | 1 | |||
| Ischaemic toe | 1 | |||
| Leg angioplasty | 1 | |||
| Malignant hypertension | 1 | |||
| Myocardial infarction | 3 | 2 | ||
| Pacemaker insertion | 1 | 2 | ||
| Postural/orthostatic hypotension | 2 | |||
| Dermatological | ||||
| Total | 7 | 7 | 3 | 3 |
| Abscess | 2 | |||
| Burns | 1 | |||
| Cellulitis | 4 | |||
| Infected diabetic foot ulcer | 1 | |||
| Infected sebaceous cyst | 1 | |||
| Leg ulcers | 1 | |||
| Endocrine | ||||
| Total | 3 | 3 | 2 | 2 |
| Hyperglycaemia | 2 | |||
| SIADH | 1 | 1 | ||
| Thyroid lobectomy | 1 | |||
| ENT | ||||
| Total | 3 | 3 | ||
| Ear infection | 1 | |||
| Labyrinthitis | 1 | |||
| Vertigo | 1 | |||
| Eye | ||||
| Total | 1 | 1 | ||
| Cataract surgery | 1 | |||
| Gastrointestinal | ||||
| Total | 16 | 18 | 17 | 26 |
| Alcoholic liver disease | 1 | |||
| Appendicitis | 1 | |||
| Biliary sepsis related pancreatic cancer | 1 | |||
| Bowel cancer surgery | 2 | 2 | ||
| Campylobacter diarrhoea | 1 | |||
| Cholangitis | 1 | |||
| Cholecystitis | 1 | 1 | ||
| Cholecystitis related to pancreatic cancer | 1 | |||
| Constipation | 3 | |||
| Diarrhoea secondary to chemotherapy | 1 | |||
| Diverticulitis | 1 | |||
| ERCP | 1 | |||
| Faecal impaction | 1 | |||
| Gastric cancer | 1 | |||
| Gastritis | 1 | |||
| Gastroenteritis | 2 | 2 | ||
| GI bleed | 2 | |||
| Haemorrhoids | 1 | |||
| Hernia repair | 3 | 1 | ||
| Laparoscopic cholecystectomy | 1 | |||
| Liver biopsy | 1 | |||
| Liver injury and bleed | 1 | |||
| New diagnosis pancreatic cancer | 1 | |||
| Oesophageal spasm | 1 | |||
| Oesophageal stricture | 1 | |||
| OGD | 1 | |||
| OGD and endoscopic mural resection | 1 | |||
| PR bleed | 1 | |||
| Recurrent ascites | 1 | |||
| Symptoms because of progressive pancreatic cancer | 1 | |||
| Symptoms related to pancreatic cancer | 1 | |||
| Gynaecological | ||||
| Total | 1 | 1 | ||
| Vaginal wall laxity | 1 | |||
| Haematological | ||||
| Total | 1 | 1 | 4 | 4 |
| DVT | 4 | 4 | ||
| Follicular lymphoma | 1 | |||
| Musculoskeletal | ||||
| Total | 32 | 34 | 24 | 31 |
| Accidental laceration | 1 | |||
| Disc generation and neural compromise | 1 | |||
| Dislocated hip | 1 | |||
| Dog bite to hand | 1 | |||
| Femoral reconstruction | 1 | |||
| Fracture: ankle | 1 | 1 | ||
| Fracture: clavicle | 1 | |||
| Fracture: femur | 1 | 1 | ||
| Fracture: finger | 1 | |||
| Fracture: hip | 9 | 6 | ||
| Fracture: hip and metacarpal | 1 | |||
| Fracture: humerus | 3 | 1 | ||
| Fracture: metatarsal | 1 | |||
| Fracture: pelvis | 1 | |||
| Fracture: pubic rami and wrist | 1 | |||
| Fracture: ribs and finger | 1 | |||
| Fracture: wedge L1 | 1 | |||
| Fracture: wrist | 3 | |||
| Head/facial injury | 1 | |||
| Infected hip replacement and washout | 1 | |||
| Knee effusion | 1 | |||
| Knee haemarthrosis | 1 | |||
| Knee replacement | 1 | 5 | ||
| L4/5 decompression surgery | 1 | |||
| Limb pain following fall | 1 | |||
| Lumbar facet joint disease | 1 | |||
| Muscular leg pain | 1 | |||
| Musculoskeletal back pain | 2 | |||
| Musculoskeletal hip pain | 1 | |||
| Musculoskeletal leg pain | 1 | |||
| Nerve root entrapment | 1 | |||
| Pain post knee replacement | 1 | |||
| Pseudogout | 1 | |||
| Psoas abscess | 1 | |||
| Revision of infected hip replacement | 1 | |||
| Rheumatoid arthritis flare | 1 | |||
| Seroma related to recent hip replacement | 1 | |||
| Spinal decompression surgery | 1 | |||
| Neurological | ||||
| Total | 31 | 52 | 40 | 57 |
| Balance and mobility issues because of old stroke | 1 | |||
| Brain tumour removal | 1 | |||
| Cerebral amyloid and subarachnoid haemorrhage | 1 | |||
| Cerebral arteriography | 1 | |||
| Decompensation/exacerbation of stroke | 2 | 2 | ||
| Focal neurology related to previous stroke | 1 | |||
| Head injury | 2 | |||
| Increased tone/spasticity | 2 | 1 | ||
| Insertion of cranioplasty plate | 1 | |||
| Memory loss/dementia | 1 | 3 | ||
| Migraine | 1 | |||
| Neurological symptoms: cause unknown | 1 | |||
| Neuropathic pain post shingles | 1 | |||
| New stroke | 15 | 14 | ||
| Occipital fracture and subarachnoid bleed | 1 | |||
| Occipital fracture/subdural haematoma | 1 | |||
| Parkinson’s disease | 1 | |||
| Possible seizure | 2 | |||
| Seizure(s) | 13 | 16 | ||
| Stereotactic radiosurgery | 1 | |||
| Stereotactic surgery | 1 | |||
| Subdural haematoma | 2 | |||
| TIA | 8 | 8 | ||
| TIA or seizure | 2 | 1 | ||
| Unexplained neurological symptoms | 1 | |||
| Psychiatry | ||||
| Total | 3 | 3 | ||
| Anxiety | 1 | |||
| Self-harm | 1 | |||
| Severe depression | 1 | |||
| Respiratory | ||||
| Total | 17 | 38 | 19 | 22 |
| Breathlessness: under investigation | 1 | |||
| Bronchoscopy | 1 | |||
| Chest infection/pneumonia | 22 | 16 | ||
| Elective mediastinoscopy and biopsy | 1 | |||
| Exacerbation of asthma | 1 | |||
| Exacerbation of COAD | 5 | 4 | ||
| Pleural effusion | 1 | |||
| Pleural effusion and thorascopy | 1 | |||
| Pleural effusion drained (lymphoma) | 3 | |||
| Pulmonary embolus | 2 | 1 | ||
| Respiratory dyspnoea: cause not given | 1 | |||
| Urinary tract | ||||
| Total | 10 | 14 | 17 | 20 |
| Bladder tumour resection | 1 | |||
| Collection post kidney transplant | 1 | |||
| Hydrocele repair | 1 | |||
| Kidney infection | 1 | |||
| Kidney transplant | 1 | |||
| Renal acidosis | 1 | |||
| Renal colic | 1 | |||
| Renal haematoma and ureter calculi post lithotripsy | 1 | |||
| TURP | 1 | 1 | ||
| Urinary retention | 2 | 2 | ||
| UTI | 8 | 12 | ||
| Miscellaneous | ||||
| Total | 28 | 29 | 23 | 29 |
| Abdominal pain: cause unknown | 1 | |||
| Anaphylaxis | 1 | |||
| Back pain: cause unknown | 1 | |||
| Basilic vein transposition | 1 | |||
| Blackout/collapse/unresponsive episode: cause unclear | 2 | 1 | ||
| Bleed from PICC line | 1 | |||
| Bleeding: high INR | 1 | |||
| Chest pain: cause unknown | 1 | |||
| Collapse: low BP from medication | 1 | |||
| Dehydration | 1 | |||
| Drug-induced electrolyte imbalance | 1 | |||
| Fall: cause unclear – injury documented | 1 | |||
| Fall: cause unknown – injury documented | 1 | |||
| Fall: no cause documented – injury documented | 1 | |||
| Fall: no cause documented – no injury documented | 1 | 2 | ||
| Fall because of stroke weakness and shoulder pain: no injury documented | 1 | |||
| Falls secondary to cardiovascular disease: no injury documented | 1 | |||
| Functional weakness | 1 | |||
| Granulomatosis | 1 | |||
| Headache: muscular | 1 | |||
| Headache: no cause established | 1 | 1 | ||
| Headache and dizziness: no cause found | 1 | |||
| Hypotensive episode | 1 | |||
| Injury to finger and face following fall | 1 | |||
| Mastectomy | 1 | |||
| Mechanical fall: no injury documented | 2 | |||
| Micturition syncope | 1 | |||
| Musculoskeletal chest pain | 1 | |||
| Nausea and bradycardia following ERCP anaesthesia | 1 | |||
| Neurological symptoms because of fibromyalgia | 1 | |||
| Non-cardiac chest pain | 3 | |||
| Off legs: no cause found | 1 | 1 | ||
| Overdose | 1 | |||
| Pain after OGD | 1 | |||
| Pain from bone metastasis | 1 | |||
| Parotitis | 1 | |||
| Peripheral oedema because of hemiparesis | 1 | |||
| Postural hypotension | 1 | |||
| Sepsis: source not documented | 1 | |||
| Sepsis: source unknown | 1 | |||
| Syncopal episode | 1 | |||
| Tesio line insertion | 1 | |||
| Unsteadiness: cause unclear | 1 | |||
| Vasovagal | 2 | 1 | ||
| Vomiting: no cause documented | 1 | |||
| Vomiting: no cause established | 1 | 1 | ||
| Number of SAEs reported per patient | Number of patients (N = 573), n |
|---|---|
| 0 | 557 |
| 1 | 14 |
| 2 | 1 |
| 3 | 1 |
| Total SAEs | 19 |
| Reason | Number of events (N = 19), n |
|---|---|
| Event resulted in death | 0 |
| Event was life-threatening | 2 |
| Event resulted in admission to hospital or prolongation of hospitalisation | 14 |
| Event resulted in persistent or significant disability or incapacity | 1 |
| Event was ‘otherwise considered significant’ by the investigator | 2 |
| Number of patients who experienced event, n | Number of events (N = 19), n | |
|---|---|---|
| Cardiovascular | ||
| Total | 2 | 2 |
| Heart failure | 1 | |
| Hypotensive collapse | 1 | |
| Eye | ||
| Total | 1 | 1 |
| Corneal ulcer | 1 | |
| Gastrointestinal | ||
| Total | 2 | 2 |
| Choledocholithiasis | 1 | |
| Diverticulitis | 1 | |
| Haematological | ||
| Total | 1 | 1 |
| DVT | 1 | |
| Musculoskeletal | ||
| Total | 1 | 1 |
| Fracture: hip | 1 | |
| Neurological | ||
| Total | 5 | 6 |
| Cerebellitis | 1 | |
| New stroke | 2 | |
| Seizure(s) | 3 | |
| Respiratory | ||
| Total | 2 | 3 |
| Chest infection/pneumonia | 2 | |
| Pulmonary embolus | 1 | |
| Urinary tract | ||
| Total | 3 | 3 |
| Bladder tumour resection | 1 | |
| Urinary retention | 1 | |
| UTI | 1 | |
| Number of SAEs per patient | Number of patients (N = 101), n |
|---|---|
| 0 | 87 |
| 1 | 8 |
| 2 | 5 |
| 4 | 1 |
| Total SAEs | 22 |
| Reason | Number of events (N = 22), n |
|---|---|
| Event resulted in death | 4 |
| Event was life-threatening | 1 |
| Event resulted in admission to hospital or prolongation of hospitalisation | 14 |
| Event resulted in persistent or significant disability or incapacity | 0 |
| Event was ‘otherwise considered significant’ by the investigator | 3 |
| Number of patients who experienced event, n | Number of events (N = 22), n | |
|---|---|---|
| Cardiovascular | ||
| Total | 5 | 5 |
| Carotid endarterectomy | 1 | |
| Heart failure | 1 | |
| MI | 1 | |
| Mitral value repair (Libman–Sacks endocarditis) | 1 | |
| Pacemaker insertion | 1 | |
| Gastrointestinal | ||
| Total | 2 | 2 |
| Gastritis or reflux | 1 | |
| Ischaemic bowel | 1 | |
| Heamatological | ||
| Total | 1 | 1 |
| DVT | 1 | |
| Miscellaneous | ||
| Total | 1 | 1 |
| Overdose | 1 | |
| Neurological | ||
| Total | 4 | 4 |
| Complications of stroke | 1 | |
| New stroke | 1 | |
| Seizure(s) | 1 | |
| Stroke | 1 | |
| Respiratory | ||
| Total | 4 | 6 |
| Chest infection/pneumonia | 5 | |
| Pulmonary embolus | 1 | |
| Urinary tract | ||
| Total | 3 | 3 |
| UTI | 3 | |
| Number of events (n) | Number of patients, n (%) | |||
|---|---|---|---|---|
| 12 months | 24 months | |||
| Intervention (N = 239) | Control (N = 248) | Intervention (N = 219) | Control (N = 231) | |
| 0 | 173 (72.4) | 191 (77.0) | 166 (75.8) | 181 (78.4) |
| 1 | 56 (23.4) | 43 (17.3) | 39 (17.8) | 37 (16.0) |
| 2 | 8 (3.3) | 12 (4.8) | 11 (5.0) | 11 (4.8) |
| 3 | 1 (0.4) | 1 (0.4) | 2 (0.9) | 1 (0.4) |
| 4 | 1 (0.4) | 0 (0.0) | 1 (0.5) | 1 (0.4) |
| 5 | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) |
| Total events | 79 | 75 | 71 | 66 |
| 12 months | 24 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |||||
| Number of participants who experienced event | Number of events (N = 79) | Number of participants who experienced event | Number of events (N = 75) | Number of participants who experienced event | Number of events (N = 71) | Number of participants who experienced event | Number of events (N = 66) | |
| Cardiovascular | ||||||||
| Total | 8 | 8 | 5 | 5 | 4 | 4 | 4 | 5 |
| Angina | 1 | 0 | 1 | 0 | ||||
| Atrial fibrillation/other arrhythmia | 0 | 3 | 1 | 0 | ||||
| Blood pressure issues | 1 | 0 | 1 | 2 | ||||
| Heart attack | 0 | 2 | 0 | 0 | ||||
| Heart failure | 2 | 0 | 0 | 0 | ||||
| Heart monitoring | 0 | 0 | 0 | 1 | ||||
| Hole in heart | 1 | 0 | 0 | 0 | ||||
| Leaking valve | 1 | 0 | 0 | 0 | ||||
| Pacemaker insertion/replacement | 1 | 0 | 0 | 1 | ||||
| Peripheral vascular disease | 1 | 0 | 1 | 0 | ||||
| Suspected heart attack | 0 | 0 | 0 | 1 | ||||
| Dermatological | ||||||||
| Total | 3 | 4 | 4 | 4 | 2 | 2 | 4 | 4 |
| Callous on foot | 0 | 0 | 0 | 1 | ||||
| Cellulitis | 0 | 2 | 1 | 0 | ||||
| Facial rashes | 0 | 0 | 0 | 1 | ||||
| Infection in feet | 1 | 0 | 0 | 0 | ||||
| Injury to skin with poor healing | 0 | 1 | 0 | 0 | ||||
| Issues with skin graft for BCC | 1 | 0 | 0 | 0 | ||||
| Itchy skin because of medication | 0 | 0 | 1 | 0 | ||||
| Psoriasis | 0 | 1 | 0 | 0 | ||||
| Rodent ulcer face | 1 | 0 | 0 | 0 | ||||
| Rodent ulcer leg | 1 | 0 | 0 | 0 | ||||
| Toe ulcers | 0 | 0 | 0 | 1 | ||||
| Toenail removal | 0 | 0 | 0 | 1 | ||||
| Endocrine | ||||||||
| Total | 8 | 8 | 1 | 1 | 0 | 0 | 3 | 3 |
| Diabetes mellitus | 7 | 1 | 0 | 3 | ||||
| Swelling on thyroid | 1 | 0 | 0 | 0 | ||||
| ENT | ||||||||
| Total | 3 | 4 | 0 | 0 | 1 | 2 | 2 | 2 |
| Cancerous growth in ear | 1 | 0 | 0 | 0 | ||||
| Ear infection | 2 | 0 | 0 | 0 | ||||
| Nose bleeds | 0 | 0 | 0 | 1 | ||||
| Tinnitus | 0 | 0 | 1 | 0 | ||||
| Vertigo | 1 | 0 | 1 | 1 | ||||
| Eye | ||||||||
| Total | 1 | 1 | 5 | 5 | 2 | 3 | 5 | 6 |
| Blurred vision | 0 | 0 | 0 | 1 | ||||
| Burst blood vessels in eye | 0 | 1 | 0 | 1 | ||||
| Cataract/cataract surgery | 0 | 2 | 1 | 2 | ||||
| Diabetic macular oedema | 0 | 0 | 0 | 1 | ||||
| Diabetic maculopathy | 0 | 1 | 0 | 0 | ||||
| Eyesight deterioration | 0 | 0 | 1 | 0 | ||||
| Glaucoma | 0 | 1 | 0 | 0 | ||||
| Macular degeneration | 0 | 0 | 1 | 1 | ||||
| Reconstruction of eyelids | 1 | 0 | 0 | 0 | ||||
| Gastrointestinal | ||||||||
| Total | 2 | 2 | 9 | 9 | 5 | 5 | 8 | 8 |
| Bowel cancer | 0 | 0 | 2 | 0 | ||||
| Bowel problems | 0 | 0 | 0 | 1 | ||||
| Constipation | 0 | 1 | 0 | 0 | ||||
| Diverticulitis | 0 | 1 | 1 | 1 | ||||
| Gall stones | 0 | 0 | 0 | 1 | ||||
| Hernia/hernia repair | 2 | 4 | 2 | 2 | ||||
| Irritable bowel syndrome | 0 | 0 | 0 | 1 | ||||
| Polyps/polyp removal | 0 | 2 | 0 | 2 | ||||
| Rectal bleeding | 0 | 1 | 0 | 0 | ||||
| Gynaecological | ||||||||
| Total | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| Benign ovarian cyst | 0 | 0 | 0 | 1 | ||||
| Cyst | 0 | 0 | 1 | 0 | ||||
| Ovarian cyst | 1 | 0 | 0 | 0 | ||||
| Possible endometrial cancer | 0 | 0 | 0 | 1 | ||||
| Womb prolapse | 0 | 1 | 0 | 0 | ||||
| Haematological | ||||||||
| Total | 1 | 1 | 2 | 2 | 3 | 3 | 1 | 1 |
| Anaemia | 0 | 0 | 2 | 0 | ||||
| Blood clot | 0 | 1 | 0 | 0 | ||||
| Blood clots in leg | 0 | 1 | 0 | 0 | ||||
| Blood disorder | 0 | 0 | 1 | 0 | ||||
| Low platelets | 1 | 0 | 0 | 0 | ||||
| Monoclonal gammopathy of unknown significance | 0 | 0 | 0 | 1 | ||||
| Musculoskeletal | ||||||||
| Total | 8 | 8 | 4 | 4 | 9 | 9 | 4 | 4 |
| Arthritis | 3 | 2 | 3 | 1 | ||||
| Back injury | 1 | 0 | 0 | 0 | ||||
| Baker’s cyst | 1 | 0 | 1 | 0 | ||||
| Fibromyalgia flare | 1 | 0 | 0 | 0 | ||||
| Fractured wrist | 0 | 0 | 1 | 0 | ||||
| Frozen shoulder | 1 | 1 | 0 | 0 | ||||
| Golfer’s elbow | 0 | 0 | 0 | 1 | ||||
| Gout | 0 | 1 | 1 | 0 | ||||
| Hip joint problems | 0 | 0 | 0 | 1 | ||||
| Paget’s disease | 1 | 0 | 0 | 0 | ||||
| Prolapsed disc | 0 | 0 | 1 | 0 | ||||
| Sore muscles and joint | 0 | 0 | 1 | 0 | ||||
| Spinal and knee injuries | 0 | 0 | 0 | 1 | ||||
| Tennis elbow | 0 | 0 | 1 | 0 | ||||
| Neurological | ||||||||
| Total | 19 | 21 | 12 | 12 | 16 | 18 | 8 | 8 |
| Carpal tunnel operation | 0 | 0 | 1 | 0 | ||||
| Central post-stroke pain | 0 | 0 | 1 | 0 | ||||
| Memory loss/dementia | 0 | 2 | 3 | 3 | ||||
| Nerve release surgery | 0 | 0 | 1 | 0 | ||||
| Neurological symptoms? Cause | 1 | 0 | 0 | 0 | ||||
| Numbness over mouth and face | 0 | 0 | 0 | 1 | ||||
| Parkinson’s disease | 2 | 0 | 1 | 0 | ||||
| Possible seizure/epilepsy | 1 | 1 | 0 | 0 | ||||
| Possible TIA | 1 | 0 | 0 | 0 | ||||
| Sciatica | 0 | 1 | 3 | 0 | ||||
| Seizure(s) | 7 | 4 | 5 | 1 | ||||
| Senile amyloidosis | 0 | 0 | 1 | 0 | ||||
| Shingles | 2 | 0 | 0 | 2 | ||||
| Stroke | 1 | 1 | 0 | 0 | ||||
| Surgery of nerve damage to arm | 0 | 1 | 0 | 0 | ||||
| TIA | 6 | 2 | 1 | 0 | ||||
| Trigeminal neuralgia | 0 | 0 | 0 | 1 | ||||
| Psychiatric | ||||||||
| Total | 5 | 5 | 1 | 1 | 1 | 1 | 3 | 3 |
| Anxiety | 2 | 0 | 0 | 2 | ||||
| Depression | 2 | 1 | 0 | 1 | ||||
| Panic attacks | 1 | 0 | 0 | 0 | ||||
| Suicidal thoughts | 0 | 0 | 1 | 0 | ||||
| Respiratory | ||||||||
| Total | 3 | 3 | 5 | 5 | 3 | 3 | 3 | 3 |
| Asthma | 0 | 0 | 1 | 0 | ||||
| Breathing issues under investigation | 0 | 1 | 0 | 0 | ||||
| Breathlessness | 2 | 0 | 0 | 0 | ||||
| COPD | 1 | 0 | 0 | 0 | ||||
| Pneumonia/chest infection | 0 | 4 | 1 | 3 | ||||
| Sleep apnoea | 0 | 0 | 1 | 0 | ||||
| Urinary tract | ||||||||
| Total | 10 | 10 | 11 | 12 | 9 | 10 | 6 | 6 |
| Angiomyolipoma of kidney | 0 | 0 | 1 | 0 | ||||
| Bladder problem | 0 | 2 | 0 | 0 | ||||
| Bladder problems under investigation | 0 | 1 | 0 | 0 | ||||
| Bladder prolapse | 0 | 1 | 0 | 0 | ||||
| Erectile dysfunction | 0 | 0 | 0 | 1 | ||||
| Haematuria under investigation | 1 | 0 | 0 | 0 | ||||
| Incontinence | 1 | 0 | 1 | 0 | ||||
| Kidney cancer and surgery | 0 | 0 | 1 | 0 | ||||
| Kidney problems | 1 | 0 | 0 | 0 | ||||
| Kidney stone(s) | 1 | 2 | 0 | 1 | ||||
| Metastatic kidney cancer | 0 | 0 | 1 | 0 | ||||
| Prostate cancer | 0 | 1 | 1 | 0 | ||||
| Prostate problem | 1 | 2 | 1 | 2 | ||||
| Renal cancer | 2 | 0 | 0 | 0 | ||||
| Urinary retention | 1 | 0 | 0 | 0 | ||||
| UTI(s) | 2 | 3 | 4 | 2 | ||||
| Miscellaneous | ||||||||
| Total | 3 | 3 | 11 | 14 | 10 | 10 | 11 | 11 |
| Back and joint pain: side effects of statins | 1 | 0 | 0 | 0 | ||||
| Blackout/collapse | 1 | 3 | 2 | 2 | ||||
| Breast cancer surgery | 0 | 0 | 1 | 0 | ||||
| Chemotherapy side effects | 0 | 1 | 0 | 0 | ||||
| Cyst in abdomen | 0 | 0 | 1 | 0 | ||||
| Cysts on liver and kidneys | 0 | 0 | 0 | 1 | ||||
| Dehydration | 0 | 0 | 0 | 1 | ||||
| Dizziness | 0 | 1 | 0 | 0 | ||||
| Drug intolerances | 0 | 1 | 0 | 0 | ||||
| Fall | 0 | 0 | 0 | 1 | ||||
| Flu | 0 | 1 | 0 | 0 | ||||
| Fluid retention and kidney weakness | 0 | 0 | 1 | 0 | ||||
| Growth removed | 0 | 0 | 1 | 0 | ||||
| Head pains because of drug side effect | 0 | 1 | 0 | 0 | ||||
| Hypercholesterolaemia | 0 | 0 | 0 | 1 | ||||
| Infection source not documented | 0 | 0 | 1 | 1 | ||||
| Joint/muscle/back pain | 1 | 1 | 0 | 0 | ||||
| Left side of mouth dribbles | 0 | 1 | 0 | 0 | ||||
| Leg oedema | 0 | 0 | 0 | 2 | ||||
| Pain from neck problems | 0 | 0 | 0 | 1 | ||||
| Removal of metal plate in foot | 0 | 0 | 0 | 1 | ||||
| Swollen abdomen | 0 | 1 | 0 | 0 | ||||
| Swollen leg(s) | 0 | 2 | 0 | 0 | ||||
| Unsteady and falls | 0 | 1 | 0 | 0 | ||||
| Unwell cause unclear | 0 | 0 | 1 | 0 | ||||
| Vacant episodes | 0 | 0 | 1 | 0 | ||||
| Virus | 0 | 0 | 1 | 0 | ||||
| Study centre | Opening date | Number of carers enrolled (N = 200), n |
|---|---|---|
| North Tyneside | 5 November 2012 | 16 |
| Wansbeck | 5 November 2012 | 11 |
| Leeds | 3 December 2012 | 6 |
| Newcastle | 2 January 2013 | 11 |
| Pennine | 12 February 2013 | 1 |
| South Tyneside | 1 August 2013 | 11 |
| Cornwall | 28 August 2013 | 24 |
| Southampton | 7 October 2013 | 5 |
| Plymouth | 24 October 2013 | 30 |
| Portsmouth | 7 November 2013 | 8 |
| Norfolk | 29 November 2013 | 26 |
| Staffordshire | 5 December 2013 | 9 |
| Bournemouth | 25 February 2014 | 8 |
| Hull | 10 March 2014 | 1 |
| York | 21 May 2014 | 5 |
| Sherwood Forest | 4 July 2014 | 10 |
| Somerset | 25 July 2014 | 7 |
| Wigan | 7 August 2014 | 2 |
| Cardiff | 23 December 2014 | 9 |
| Reason why a carer was not invited | n (%) |
|---|---|
| No carer identified | 80 (58) |
| Carer or patient declined carer involvement | 17 (12) |
| Carer unwell | 5 (4) |
| Patient or researcher felt inappropriate to burden carer | 18 (13) |
| No reason provided/reason unclear | 19 (14) |
| About the services, to what extent do you agree that: | 12 months, n (%) | 24 months, n (%) | ||
|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |
| Staff were welcoming and friendly | ||||
| Strongly disagree | ||||
| Disagree | 1 (1.3) | 1 (1.6) | ||
| Agree | 26 (28.6) | 33 (45.8) | 23 (29.1) | 16 (25.0) |
| Strongly agree | 57 (62.6) | 36 (50.0) | 29 (36.7) | 18 (28.1) |
| Does not apply | 8 (8.8) | 3 (4.2) | 26 (32.9) | 29 (45.3) |
| Missing | 1 | 2 | 4 | 6 |
| Staff treated your relative/friend with dignity and respect | ||||
| Strongly disagree | ||||
| Disagree | 1 (1.1) | 1 (1.4) | 1 (1.3) | 0 (0) |
| Agree | 24 (26.7) | 33 (45.8) | 22 (28.2) | 18 (28.1) |
| Strongly agree | 59 (65.6) | 35 (48.6) | 30 (38.5) | 17 (26.6) |
| Does not apply | 6 (6.7) | 3 (4.2) | 25 (32.1) | 29 (45.3) |
| Missing | 2 | 2 | 5 | 6 |
| Staff assessed your relative/friend’s needs | ||||
| Strongly disagree | 1 (1.3) | 2 (3.1) | ||
| Disagree | 3 (3.3) | 1 (1.4) | 2 (2.6) | 1 (1.6) |
| Agree | 29 (32.2) | 40 (55.6) | 21 (27.3) | 17 (26.6) |
| Strongly agree | 52 (57.8) | 28 (38.9) | 27 (35.1) | 15 (23.4) |
| Does not apply | 6 (6.7) | 3 (4.2) | 26 (33.8) | 29 (45.3) |
| Missing | 2 | 2 | 6 | 5 |
| Staff met your relative/friend’s needs | ||||
| Strongly disagree | 1 (1.3) | 1 (1.6) | ||
| Disagree | 6 (6.7) | 2 (2.8) | 4 (5.1) | 6 (9.4) |
| Agree | 29 (32.6) | 40 (56.3) | 23 (29.5) | 13 (20.3) |
| Strongly agree | 48 (53.9) | 26 (36.6) | 24 (30.8) | 15 (23.4) |
| Does not apply | 6 (6.7) | 3 (4.2) | 26 (33.3) | 29 (45.3) |
| Missing | 3 | 3 | 5 | 6 |
| You have been involved as much as you wanted to be in decisions about their care | ||||
| Strongly disagree | 1 (1.1) | 1 (1.4) | ||
| Disagree | 5 (5.6) | 2 (2.8) | 4 (5.1) | 4 (6.3) |
| Agree | 31 (34.4) | 35 (49.3) | 21 (26.9) | 14 (21.9) |
| Strongly agree | 45 (50.0) | 29 (40.9) | 26 (33.3) | 18 (28.1) |
| Does not apply | 8 (8.9) | 4 (5.6) | 27 (34.6) | 28 (43.8) |
| Missing | 2 | 3 | 5 | 6 |
| You were able to discuss your preferences, beliefs and concerns as part of their care | ||||
| Strongly disagree | 2 (2.3) | 3 (4.2) | ||
| Disagree | 7 (7.9) | 4 (5.6) | 2 (2.6) | 3 (4.8) |
| Agree | 32 (36.0) | 36 (50.7) | 23 (29.5) | 16 (25.4) |
| Strongly agree | 39 (43.8) | 22 (31.0) | 23 (29.5) | 15 (23.8) |
| Does not apply | 9 (10.1) | 6 (8.5) | 30 (38.5) | 29 (46.0) |
| Missing | 3 | 3 | 5 | 7 |
| You were told who to contact if you had any worries or concerns | ||||
| Strongly disagree | 3 (3.3) | 1 (1.4) | 0 (0) | 1 (1.6) |
| Disagree | 7 (7.8) | 4 (5.6) | 3 (3.8) | 6 (9.4) |
| Agree | 36 (40.0) | 41 (56.9) | 30 (39.0) | 15 (23.4) |
| Strongly agree | 39 (43.3) | 24 (33.3) | 22 (27.9) | 14 (21.9) |
| Does not apply | 5 (5.6) | 2 (2.8) | 24 (30.4) | 28 (43.8) |
| Missing | 2 | 2 | 4 | 6 |
| You were confident that the staff who saw your relative/friend had the right skills and knowledge to help them | ||||
| Strongly disagree | 1 (1.1) | 0 (0) | ||
| Disagree | 2 (2.2) | 2 (2.8) | 3 (3.8) | 0 (0) |
| Agree | 35 (38.9) | 38 (53.5) | 28 (35.4) | 20 (31.8) |
| Strongly agree | 46 (51.1) | 29 (40.9) | 24 (30.4) | 14 (22.2) |
| Does not apply | 6 (6.7) | 2 (2.8) | 24 (30.4) | 29 (46.0) |
| Missing | 2 | 3 | 4 | 7 |
| Your relative/friend was treated fairly regardless of their age, race, sex, belief, sexual orientation or disability | ||||
| Strongly disagree | ||||
| Disagree | 2 (2.2) | 0 (0) | 2 (2.5) | 2 (3.1) |
| Agree | 25 (27.8) | 32 (44.4) | 24 (30.4) | 19 (29.7) |
| Strongly agree | 59 (65.6) | 39 (54.2) | 32 (40.5) | 16 (25.0) |
| Does not apply | 4 (4.4) | 1 (1.4) | 21 (26.6) | 27 (42.2) |
| Missing | 2 | 2 | 4 | 6 |
| You were given the information you wanted | ||||
| Strongly disagree | 1 (1.1) | 2 (2.9) | ||
| Disagree | 5 (5.7) | 3 (4.3) | 4 (5.1) | 3 (4.7) |
| Agree | 33 (37.1) | 38 (54.3) | 24 (30.8) | 18 (28.1) |
| Strongly agree | 44 (49.4) | 26 (37.1) | 26 (33.3) | 13 (20.3) |
| Does not apply | 6 (6.7) | 1 (1.4) | 24 (30.8) | 30 (46.9) |
| Missing | 3 | 4 | 5 | 6 |
| Your relative/friend was able to see the same health-care professional/team whenever possible | ||||
| Strongly disagree | 0 (0) | 1 (1.6) | ||
| Disagree | 9 (10.1) | 9 (12.7) | 3 (3.8) | 4 (6.3) |
| Agree | 35 (39.3) | 37 (52.1) | 25 (32.1) | 20 (31.3) |
| Strongly agree | 38 (42.7) | 21 (29.6) | 25 (32.1) | 9 (14.1) |
| Does not apply | 7 (7.9) | 4 (5.6) | 25 (32.1) | 30 (46.9) |
| Missing | 3 | 3 | 5 | 6 |
| If you had important questions to ask, you got answers that you could understand | ||||
| Strongly disagree | 2 (2.2) | 0 (0) | ||
| Disagree | 2 (2.2) | 6 (8.5) | 2 (2.6) | 1 (1.6) |
| Agree | 37 (41.1) | 34 (47. 9) | 22 (28.2) | 21 (33.3) |
| Strongly agree | 40 (44.4) | 27 (38.0) | 25 (32.1) | 11 (17.5) |
| Does not apply | 9 (10.0) | 4 (5.6) | 29 (37.2) | 30 (47.6) |
| Missing | 2 | 3 | 5 | 7 |
| If your relative/friend needed more than one service, staff made sure they were well co-ordinated | ||||
| Strongly disagree | 2 (2.2) | 1 (1.4) | ||
| Disagree | 7 (7.9) | 8 (11.4) | 3 (3.9) | 3 (4.8) |
| Agree | 36 (40.5) | 29 (41.4) | 21 (27.3) | 13 (20.6) |
| Strongly agree | 34 (38.2) | 22 (31.4) | 20 (26.0) | 12 (19.1) |
| Does not apply | 10 (11.2) | 10 (14.3) | 33 (42.9) | 35 (55.6) |
| Missing | 3 | 4 | 6 | 7 |
| If your relative/friend needed more than one service, staff made sure that your care information was clearly and accurately shared | ||||
| Strongly disagree | 1 (1.1) | 1 (1.4) | ||
| Disagree | 5 (5.6) | 6 (8.5) | 4 (5.3) | 3 (4.7) |
| Agree | 40 (44.9) | 33 (46.5) | 21 (27.6) | 14 (21.9) |
| Strongly agree | 32 (36.0) | 21 (29.6) | 18 (23.7) | 11 (17.2) |
| Does not apply | 11 (12.4) | 10 (14.1) | 33 (43.4) | 36 (56.3) |
| Missing | 3 | 3 | 7 | 6 |
| You were told who to contact if your relative/friend had any ongoing health-care needs | ||||
| Strongly disagree | 2 (2.2) | 1 (1.4) | ||
| Disagree | 8 (9.0) | 5 (6.9) | 6 (7.7) | 4 (6.3) |
| Agree | 43 (48.3) | 38 (52.8) | 24 (30.8) | 19 (29.7) |
| Strongly agree | 31 (34.8) | 24 (33.3) | 21 (26.9) | 12 (18.8) |
| Does not apply | 5 (5.6) | 4 (5.6) | 27 (34.6) | 29 (45.3) |
| Missing | 3 | 2 | 5 | 6 |
| Overall, how satisfied are you with the services you received? | ||||
| Extremely satisfied | 35 (38.5) | 18 (25.4) | 22 (30.6) | 7 (13.7) |
| Very satisfied | 34 (37.4) | 27 (38.0) | 29 (40.3) | 18 (35.3) |
| Quite satisfied | 15 (16.5) | 20 (28.2) | 17 (23.6) | 15 (29.4) |
| Not very satisfied | 7 (7.7) | 6 (8.5) | 2 (2.8) | 10 (19.6) |
| Extremely unsatisfied | 2 (2.8) | 1 (2.0) | ||
| Missing | 1 | 3 | 11 | 19 |
| In the last 12 months, has your relative/friend had enough help with speaking difficulties from the NHS? | ||||
| Yes, definitely | 14 (15.2) | 11 (15.5) | 7 (9.2) | 5 (7.8) |
| Yes, to some extent | 9 (9.8) | 10 (14.1) | 9 (11.8) | 7 (10.9) |
| No, I did not get enough help from the NHS | 8 (8.7) | 5 (7.0) | 6 (7.9) | 5 (7.8) |
| I did not have any speaking difficulties | 61 (66.3) | 45 (63.4) | 54 (71.1) | 47 (73.4) |
| Missing | 0 | 3 | 7 | 7 |
| In the last 12 months, has your relative/friend had enough treatment to help improve your mobility from the NHS? | ||||
| Yes, definitely | 44 (48.9) | 21 (29.2) | 18 (24.7) | 4 (6.6) |
| Yes, to some extent | 21 (23.3) | 22 (30.6) | 21 (28.8) | 14 (23.0) |
| No, I did not get enough help from the NHS | 13 (14.4) | 19 (26.4) | 13 (17.8) | 27 (44.3) |
| I did not have any mobility difficulties | 12 (13.3) | 10 (13.9) | 21 (28.8) | 16 (26.2) |
| Missing | 2 | 2 | 10 | 9 |
| In the last 12 months, has your relative/friend had enough help with emotional problems from the NHS? | ||||
| Yes, definitely | 15 (17.1) | 11 (15.9) | 7 (9.5) | 7 (11.5) |
| Yes, to some extent | 31 (35.2) | 19 (27.5) | 17 (23.0) | 15 (24.6) |
| No, I did not get enough help from the NHS | 12 (13.6) | 14 (20.3) | 12 (16.2) | 14 (23.0) |
| I did not have any emotional problems | 30 (34.1) | 25 (36.2) | 38 (51.4) | 25 (41.0) |
| Missing | 4 | 5 | 9 | 9 |
| Number of reviews | Patients expected to have a review, n (%) | Patients who received a review, n (%) |
|---|---|---|
| 0 | 3 (1) | 12 (4) |
| 1 | 6 (2) | 15 (5) |
| 2 | 7 (2) | 21 (7) |
| 3 | 7 (2) | 24 (8) |
| 4 | 13 (5) | 39 (14) |
| 5 | 249 (87) | 174 (61) |
| Reason | Stage (N = 285 randomised) (n) | ||||
|---|---|---|---|---|---|
| 1 month | 3 months | 6 months | 12 months | 18 months | |
| Total reviews not conducted, n (%) | 27 (9) | 40 (14) | 54 (19) | 74 (26) | 75 (26) |
| Died (new case) | 0 | 3 | 3 | 4 | 8 |
| Died (prior case) | 0 | 0 | 3 | 6 | 10 |
| Withdrawn (new case) | 3 | 3 | 4 | 3 | 5 |
| Withdrawn (prior case) | 0 | 3 | 6 | 10 | 13 |
| Admitted to hospital/unwell | 2 | 2 | 7 | 10 | 4 |
| Refused review when contacted | 3 | 6 | 3 | 4 | 4 |
| Uncontactable | 10 | 15 | 22 | 24 | 20 |
| Staff decided the review was inappropriate | 0 | 2 | 1 | 2 | 2 |
| Staff unavailable to conduct the review | 4 | 3 | 3 | 9 | 7 |
| Miscommunication and staff unaware of patient involvement in trial | 2 | 0 | 0 | 0 | 0 |
| No reason provided | 3 | 3 | 2 | 2 | 2 |
| Profession | Time point, n (%) | Total, n (%) | ||||
|---|---|---|---|---|---|---|
| 1 month (N = 258) | 3 months (N = 245) | 6 months (N = 231) | 12 months (N = 211) | 18 months (N = 210) | ||
| All physiotherapist bands | 138 (53) | 140 (57) | 132 (57) | 120 (57) | 119 (57) | 649 (56) |
| Physiotherapist band 5 | 1 (0) | 0 (0) | 1 (0) | 3 (1) | 1 (0) | 6 (1) |
| Physiotherapist band 6 | 59 (23) | 65 (27) | 46 (20) | 27 (13) | 26 (12) | 223 (19) |
| Physiotherapist band 7 | 78 (30) | 75 (31) | 85 (37) | 90 (43) | 92 (44) | 420 (36) |
| All occupational therapist bands | 76 (29) | 71 (29) | 64 (28) | 51 (24) | 58 (28) | 320 (28) |
| Occupational therapist band 5 | 6 (2) | 6 (2) | 5 (2) | 2 (1) | 2 (1) | 21 (2) |
| Occupational therapist band 6 | 40 (16) | 35 (14) | 33 (14) | 26 (12) | 25 (12) | 159 (14) |
| Occupational therapist band 7 | 28 (11) | 29 (12) | 25 (11) | 20 (9) | 25 (12) | 127 (11) |
| Occupational therapist band 8 | 2 (1) | 1 (0) | 1 (0) | 3 (1) | 6 (3) | 13 (1) |
| All SLT bands | 13 (5) | 11 (4) | 12 (5) | 13 (6) | 9 (4) | 58 (5) |
| SLT band 6 | 7 (3) | 6 (2) | 8 (3) | 6 (3) | 7 (3) | 34 (3) |
| SLT band 7 | 2 (1) | 3 (1) | 2 (1) | 4 (2) | 1 (0) | 12 (1) |
| SLT band 8A | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 1 (0) |
| SLT band 8C | 4 (2) | 2 (1) | 2 (1) | 2 (1) | 1 (0) | 11 (1) |
| All nurse bands | 24 (9) | 20 (8) | 18 (8) | 20 (9) | 16 (8) | 98 (8) |
| Nurse band 5 | 1 (0) | 2 (1) | 2 (1) | 1 (0) | 0 (0) | 6 (1) |
| Nurse band 6 | 13 (5) | 12 (5) | 9 (4) | 12 (6) | 11 (5) | 57 (5) |
| Nurse band 7 | 10 (4) | 6 (2) | 6 (3) | 7 (3) | 5 (2) | 34 (3) |
| Nurse band 8 | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 1 (0) |
| All dietitian bands | 2 (1) | 1 (0) | 1 (0) | 2 (1) | 2 (1) | 8 (1) |
| Dietitian band 5 | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) |
| Dietitian band 6 | 1 (0) | 1 (0) | 1 (0) | 2 (1) | 2 (1) | 7 (1) |
| All stroke co-ordinator bands | 2 (1) | 1 (0) | 3 (1) | 2 (1) | 2 (1) | 10 (1) |
| Not recorded | 12 (1) | |||||
| Total (n) | 1155 | |||||
| Time point, n (%) | |||||
|---|---|---|---|---|---|
| 1 month (N = 187) | 3 months (N = 164) | 6 months (N = 145) | 12 months (N = 108) | 18 months (N = 74) | |
| Mobility | 103 (55) | 76 (46) | 61 (42) | 43 (40) | 26 (35) |
| Transfers | 6 (3) | 6 (4) | 5 (3) | 3 (3) | 1 (1) |
| Walking indoors | 28 (15) | 19 (12) | 23 (16) | 13 (12) | 5 (7) |
| Stairs | 11 (6) | 6 (4) | 5 (3) | 2 (2) | 2 (3) |
| Walking outdoors | 66 (35) | 54 (33) | 43 (30) | 29 (27) | 16 (22) |
| Falls | 11 (6) | 9 (5) | 7 (5) | 7 (6) | 8 (11) |
| Personal care | 35 (0) | 27 (0) | 14 (0) | 9 (0) | 3 (4) |
| Washing | 17 (9) | 10 (6) | 7 (5) | 2 (2) | 2 (3) |
| Dressing | 16 (9) | 14 (9) | 7 (5) | 2 (2) | 0 (0) |
| Toileting | 6 (3) | 6 (4) | 5 (3) | 4 (4) | 2 (3) |
| Personal grooming | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 0 (0) |
| Mealtimes | 56 (30) | 41 (25) | 25 (17) | 12 (11) | 11 (15) |
| Eating | 20 (11) | 11 (7) | 8 (6) | 2 (2) | 5 (7) |
| Swallowing | 5 (3) | 3 (2) | 2 (1) | 2 (2) | 1 (1) |
| Appetite/healthy eating | 8 (4) | 9 (5) | 7 (5) | 4 (4) | 5 (7) |
| Meal/drink preparation | 29 (16) | 19 (12) | 9 (6) | 5 (5) | 1 (1) |
| Domestic activities | 45 (24) | 42 (26) | 23 (16) | 17 (16) | 2 (3) |
| Inside the home | 19 (10) | 17 (10) | 6 (4) | 5 (5) | 0 (0) |
| Outside the home | 15 (8) | 20 (12) | 15 (10) | 10 (9) | 1 (1) |
| Shopping | 17 (9) | 12 (7) | 6 (4) | 2 (2) | 1 (1) |
| Work and volunteering | 36 (19) | 22 (13) | 17 (12) | 9 (8) | 2 (3) |
| Return to work | 27 (14) | 17 (10) | 13 (9) | 7 (6) | 2 (3) |
| Volunteering | 10 (5) | 8 (5) | 4 (3) | 3 (3) | 0 (0) |
| Hobbies and interests | 70 (37) | 59 (36) | 54 (37) | 41 (38) | 18 (24) |
| Quiet leisure | 10 (5) | 5 (3) | 6 (4) | 5 (5) | 3 (4) |
| Active leisure | 46 (25) | 38 (23) | 36 (25) | 28 (26) | 11 (15) |
| Social participation | 22 (12) | 21 (13) | 17 (12) | 11 (10) | 5 (7) |
| Driving and transport | 56 (30) | 40 (24) | 28 (19) | 17 (16) | 14 (19) |
| Driving | 46 (25) | 33 (20) | 24 (17) | 13 (12) | 13 (18) |
| Travel on public transport | 8 (4) | 3 (2) | 3 (2) | 3 (3) | 3 (4) |
| Getting in/out of car or on/off public transport | 6 (3) | 6 (4) | 3 (2) | 3 (3) | 0 (0) |
| Communication | 43 (23) | 25 (15) | 21 (14) | 13 (12) | 6 (8) |
| Reading | 14 (7) | 6 (4) | 3 (2) | 4 (4) | 2 (3) |
| Writing | 25 (13) | 19 (12) | 16 (11) | 8 (7) | 3 (4) |
| Comprehension and understanding | 4 (2) | 2 (1) | 1 (1) | 3 (3) | 0 (0) |
| Accounting and money management | 4 (2) | 2 (1) | 1 (1) | 0 (0) | 0 (0) |
| Speech | 2 (1) | 0 (0) | 1 (1) | 1 (1) | 0 (0) |
| Hearing | 0 (0) | 0 (0) | 2 (1) | 8 (7) | 1 (1) |
| Memory and concentration | 19 (10) | 14 (9) | 12 (8) | 9 (8) | 7 (9) |
| Memory | 10 (5) | 10 (6) | 8 (6) | 7 (6) | 5 (7) |
| Concentration and attention | 12 (6) | 4 (2) | 7 (5) | 4 (4) | 3 (4) |
| Mood, anxiety and depression | 29 (16) | 22 (13) | 22 (15) | 14 (13) | 11 (15) |
| Low mood | 19 (10) | 19 (12) | 18 (12) | 13 (12) | 9 (12) |
| Anxiety | 11 (6) | 5 (3) | 3 (2) | 3 (3) | 5 (7) |
| Changes in personality | 5 (3) | 0 (0) | 3 (2) | 1 (1) | 2 (3) |
| Medical issues | 37 (20) | 33 (20) | 27 (19) | 26 (24) | 18 (24) |
| Recent hospital admission | 0 (0) | 0 (0) | 3 (2) | 2 (2) | 1 (1) |
| Lifestyle management | 10 (5) | 13 (8) | 13 (9) | 15 (14) | 6 (8) |
| Medication management | 8 (4) | 6 (4) | 2 (1) | 3 (3) | 1 (1) |
| Contact with health-care professional) | 9 (5) | 6 (4) | 6 (4) | 9 (8) | 5 (7) |
| Other relevant past medical history | 13 (7) | 11 (7) | 8 (6) | 4 (4) | 7 (9) |
| Pain | 28 (15) | 23 (14) | 15 (10) | 15 (14) | 9 (12) |
| Pre-existing pain | 7 (4) | 4 (2) | 5 (3) | 3 (3) | 4 (5) |
| Musculoskeletal pain | 20 (11) | 13 (8) | 9 (6) | 11 (10) | 4 (5) |
| Neuropathic pain | 1 (1) | 6 (4) | 1 (1) | 3 (3) | 2 (3) |
| Other issues | 10 (5) | 12 (7) | 13 (9) | 5 (5) | 4 (5) |
| Fatigue | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Finance | 5 (3) | 2 (1) | 1 (1) | 0 (0) | 0 (0) |
| Independent living | 1 (1) | 4 (2) | 1 (1) | 0 (0) | 1 (1) |
| Relationships | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Upper limb | 3 (2) | 3 (2) | 5 (3) | 2 (2) | 2 (3) |
| Vision | 0 (0) | 3 (2) | 6 (4) | 2 (2) | 0 (0) |
| Review stage | Goals/action points set (n) | Goals/action points with action plans, n (%) |
|---|---|---|
| 1 month | 742 | 723 (97) |
| 3 months | 547 | 532 (97) |
| 6 months | 427 | 416 (97) |
| 12 months | 313 | 306 (98) |
| 18 months | 146 | 144 (99) |
| Total | 2175 | 2121 (98) |
| Service | Number of referrals madea | |||||
|---|---|---|---|---|---|---|
| 1 month (n = 233) | 3 months (n = 200) | 6 months (n = 180) | 12 months (n = 142) | 18 months (n = 79) | Across review stagesb | |
| Age UK, n (%) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 1 |
| Community rehabilitation team, n (%) | 3 (1) | 0 (0) | 2 (1) | 0 (0) | 1 (1) | 6 |
| Dietitian, n (%) | 1 (< 0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 |
| District nursing, n (%) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 |
| Driving assessment centre, n (%) | 1 (< 0.5) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 4 |
| Exercise group or referral scheme, n (%) | 7 (3) | 3 (2) | 4 (2) | 3 (2) | 0 (0) | 14 |
| Falls team/group, n (%) | 1 (< 0.5) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 2 |
| Gait analysis clinic, n (%) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 |
| General practitioner, n (%) | 1 (< 0.5) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 2 |
| Health trainers, n (%) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 |
| Independent living team, n (%) | 0 (0) | 1 (1) | 1 (1) | 1 (1) | 0 (0) | 2 |
| Library service, n (%) | 1 (< 0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Long-term conditions team, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 |
| Ophthalmology, n (%) | 1 (< 0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Orthotics, n (%) | 1 (< 0.5) | 1 (1) | 0 (0) | 0 (0) | 2 (3) | 4 |
| Occupational therapist, n (%) | 8 (3) | 4 (2) | 6 (3) | 2 (1) | 0 (0) | 16 |
| Psychologist, n (%) | 2 (1) | 0 (0) | 2 (1) | 3 (2) | 0 (0) | 6 |
| Physiotherapist, n (%) | 10 (4) | 3 (2) | 4 (2) | 5 (4) | 1 (1) | 20 |
| Sensory team/sight service, n (%) | 2 (1) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 3 |
| Speech and language therapist, n (%) | 6 (3) | 1 (1) | 1 (1) | 1 (1) | 0 (0) | 9 |
| Social services, n (%) | 6 (3) | 1 (1) | 2 (1) | 1 (1) | 1 (1) | 9 |
| Stroke Association, n (%) | 2 (1) | 3 (2) | 1 (1) | 3 (2) | 0 (0) | 7 |
| Stroke consultant, n (%) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 2 |
| Stroke nurse, n (%) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 |
| Vocational rehabilitation, n (%) | 1 (< 0.5) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 2 |
| Total (n) | 117 | |||||
| Study centre | Reviews expected to be conducted (n)a | Reviews conducted, n (% of expected) | Reviews not conducted (excluding deaths/withdrawals) (n) | Reasons reviews not conducted (excluding deaths/withdrawals) (number of reviews) |
|---|---|---|---|---|
| 1 | 74 | 72 (97) | 2 |
|
| 2 | 45 | 42 (93) | 3 |
|
| 3 | 138 | 127 (92) | 11 |
|
| 4 | 15 | 8 (53) | 7 |
|
| 5 | 181 | 149 (82) | 32 |
|
| 6 | 62 | 54 (87) | 8 |
|
| 7 | 136 | 135 (99) | 1 |
|
| 8 | 78 | 73 (94) | 5 |
|
| 9 | 35 | 26 (74) | 9 |
|
| 10 | 150 | 122 (81) | 28 |
|
| 11 | 64 | 47 (73) | 17 |
|
| 12 | 49 | 49 (100) | 0 | NA |
| 13 | 44 | 42 (95) | 2 |
|
| 14 | 55 | 48 (87) | 7 |
|
| 15 | 61 | 58 (95) | 3 |
|
| 16 | 44 | 24 (55) | 20 |
|
| 17 | 59 | 34 (58) | 25 |
|
| 18 | 25 | 25 (100) | 0 | NA |
| 19 | 23 | 20 (87) | 3 |
|
| Study centre | Number of different reviewers who conducted reviews for an individual patient, n (% of patients randomised) | |||
|---|---|---|---|---|
| 1 member of staff | 2 members of staff | 3 members of staff | 4 members of staff | |
| 1 | 11 (69) | 5 (31) | 0 | 0 |
| 2 | 7 (78) | 2 (22) | 0 | 0 |
| 3 | 1 (3) | 18 (62) | 10 (34) | 0 |
| 4 | 0 | 2 (67) | 1 (33) | 0 |
| 5 | 9 (22) | 21 (51) | 10 (24) | 1 (2) |
| 6 | 6 (43) | 7 (50) | 1 (7) | 0 |
| 7 | 25 (89) | 3 (11) | 0 | 0 |
| 8 | 10 (59) | 5 (29) | 2 (12) | 0 |
| 9 | 2 (25) | 2 (25) | 4 (50) | 0 |
| 10 | 12 (40) | 15 (50) | 3 (10) | 0 |
| 11 | 3 (21) | 7 (50) | 3 (21) | 1 (7) |
| 12 | 3 (27) | 7 (64) | 1 (9) | 0 |
| 13 | 4 (36) | 5 (45) | 2 (18) | 0 |
| 14 | 6 (67) | 3 (33) | 0 | 0 |
| 15 | 1 (11) | 8 (89) | 0 | 0 |
| 16 | 7 (50) | 7 (50) | 0 | 0 |
| 17 | 5 (42) | 7 (58) | 0 | 0 |
| 18 | 3 (60) | 1 (20) | 1 (20) | 0 |
| 19 | 2 (40) | 3 (60) | 0 | 0 |
| Study centre | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
| 1 month | |||||||||||||||||||
| Number of staff | 2 | 2 | 3 | 1 | 14 | 8 | 1 | 4 | 3 | 16 | 8 | 7 | 7 | 2 | 2 | 6 | 2 | 2 | 2 |
| Number reviews per staff member | |||||||||||||||||||
| Minimum | 8 | 4 | 8 | 3 | 1 | 1 | 28 | 3 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 4 | 2 | 1 |
| Median | 8 | 5 | 8 | 3 | 1 | 1 | 28 | 4 | 3 | 2 | 1 | 1 | 1 | 4 | 3 | 2 | 5 | 3 | 3 |
| Maximum | 8 | 5 | 13 | 3 | 7 | 4 | 28 | 5 | 4 | 3 | 2 | 2 | 3 | 6 | 4 | 3 | 5 | 3 | 4 |
| 3 months | |||||||||||||||||||
| Number of staff | 2 | 2 | 3 | 1 | 10 | 7 | 1 | 4 | 4 | 14 | 9 | 6 | 6 | 2 | 1 | 5 | 2 | 2 | 2 |
| Number reviews per staff member | |||||||||||||||||||
| Minimum | 4 | 3 | 6 | 3 | 1 | 1 | 28 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 1 | 3 | 2 | 1 |
| Median | 7 | 4 | 11 | 3 | 3 | 1 | 28 | 4 | 2 | 2 | 1 | 2 | 2 | 5 | 4 | 3 | 4 | 3 | 2 |
| Maximum | 10 | 5 | 15 | 3 | 7 | 4 | 28 | 4 | 3 | 4 | 2 | 2 | 3 | 8 | 4 | 3 | 4 | 3 | 3 |
| 6 months | |||||||||||||||||||
| Number of staff | 2 | 2 | 3 | 1 | 11 | 7 | 1 | 4 | 4 | 12 | 7 | 6 | 5 | 2 | 1 | 5 | 2 | 3 | 2 |
| Number reviews per staff member | |||||||||||||||||||
| Minimum | 5 | 4 | 6 | 1 | 1 | 1 | 27 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 1 | 3 | 1 | 1 |
| Median | 8 | 5 | 7 | 1 | 2 | 1 | 27 | 4 | 1 | 2 | 1 | 1 | 2 | 5 | 4 | 3 | 4 | 2 | 2 |
| Maximum | 10 | 5 | 13 | 1 | 10 | 4 | 27 | 4 | 2 | 6 | 2 | 2 | 2 | 8 | 4 | 3 | 4 | 2 | 3 |
| 12 months | |||||||||||||||||||
| Number of staff | 2 | 2 | 3 | 1 | 10 | 7 | 1 | 3 | 1 | 9 | 6 | 6 | 5 | 1 | 1 | 4 | 2 | 2 | 2 |
| Number reviews per staff member | |||||||||||||||||||
| Minimum | 5 | 4 | 2 | 1 | 1 | 1 | 27 | 4 | 2 | 1 | 1 | 1 | 1 | 8 | 4 | 1 | 2 | 2 | 1 |
| Median | 7 | 4 | 3 | 1 | 3 | 1 | 27 | 5 | 2 | 2 | 1 | 2 | 2 | 8 | 4 | 3 | 3 | 3 | 2 |
| Maximum | 9 | 4 | 17 | 1 | 6 | 3 | 27 | 5 | 2 | 8 | 2 | 3 | 3 | 8 | 4 | 4 | 4 | 3 | 2 |
| 18 months | |||||||||||||||||||
| Number of staff | 2 | 2 | 2 | 0 | 9 | 4 | 1 | 4 | 3 | 8 | 4 | 6 | 4 | 1 | 1 | 4 | 2 | 2 | 2 |
| Number reviews per staff member | |||||||||||||||||||
| Minimum | 4 | 4 | 6 | 0 | 1 | 1 | 25 | 2 | 1 | 1 | 1 | 1 | 1 | 8 | 7 | 1 | 2 | 1 | 1 |
| Median | 7 | 4 | 11 | 0 | 2 | 2 | 25 | 4 | 1 | 2 | 2 | 2 | 3 | 8 | 7 | 3 | 3 | 3 | 2 |
| Maximum | 9 | 4 | 16 | 0 | 10 | 3 | 25 | 4 | 2 | 11 | 3 | 3 | 3 | 8 | 7 | 4 | 3 | 4 | 3 |
| NPT construct | Components (work done by staff implementing the intervention) |
|---|---|
| Coherence or sense-making |
|
| Cognitive participation or relational work |
|
| Collective action or operational work |
|
| Reflexive monitoring or appraisal work |
|
| Location | Mobility and movement | Communication | Everyday activities | Emotional and psychosocial issues | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Psychology/psychiatry | IAPT/well-being/counselling | |||||||||||||||||
| Any | Generic | Neurological | Stroke | Any | Generic | Neurological | Stroke | Any | Generic | Neurological | Stroke | Generic | Neurological | Stroke | Generic | Neurological | Stroke | ||
| 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| 8A | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| 8B | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| 9A | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| 9B | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| 12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| 13 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| 15 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||
| 16 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| 17 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| 18 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Totals (n) | 20 | 8 | 14 | 6 | 20 | 11 | 6 | 9 | 20 | 9 | 9 | 7 | 20 | 2 | 5 | 4 | 12 | 1 | 9 |
| Location | Impaired cognition | Nutrition | Visual disturbance | Incontinence management | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Memory clinic | Other | Any | Generic | Neurological | Stroke | Any | Generic | Neurological | Any (all generic) | |||
| Generic | Neurological | Stroke | |||||||||||
| 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 8A | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 8B | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 9A | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 9B | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 13 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 15 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 16 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 17 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 18 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Totals (n) | 20 | 5 | 3 | 8 | 8 | 20 | 16 | 2 | 3 | 20 | 30 | 19 | 20 |
| Location | Support with relationships and sex | Pain management | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Psychology/psychiatry | IAPT/well-being/counselling | Medical | Any | Generic | Neurological | Stroke | ||||||
| Generic | Neurological | Stroke | Generic | Neurological | Stroke | Neurological | Stroke | ||||||
| 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| 2 | ✓ | ✓ | ✓ | ✓ | |||||||||
| 3 | ✓ | ✓ | ✓ | ✓ | |||||||||
| 4 | ✓ | ✓ | No | ||||||||||
| 5 | ✓ | ✓ | ✓ | ✓ | |||||||||
| 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| 8A | ✓ | ✓ | ✓ | ✓ | |||||||||
| 8B | Not known | No | |||||||||||
| 9A | ✓ | ✓ | ✓ | No | |||||||||
| 9B | ✓ | ✓ | ✓ | No | |||||||||
| 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| 11 | ✓ | ✓ | ✓ | ✓ | |||||||||
| 12 | ✓ | ✓ | ✓ | ✓ | |||||||||
| 13 | ✓ | ✓ | ✓ | ✓ | |||||||||
| 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| 15 | ✓ | ✓ | ✓ | ✓ | |||||||||
| 16 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| 17 | No | ✓ | ✓ | ||||||||||
| 18 | ✓ | ✓ | ✓ | ✓ | |||||||||
| Totals (n) | 18 | 1 | 1 | 4 | 5 | 0 | 11 | 3 | 3 | 16 | 1 | 9 | 7 |
| Location | Return to work/volunteering | Return to driving | Healthier lifestyles | Support services | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Vocational rehabilitation | General occupational therapy support | Generic | Stroke | Any | Generic | Stroke | Any | Generic | Stroke | |||
| Generic | Neurological | Stroke | Stroke | ||||||||||
| 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 4 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 8A | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 8B | ✓ | ✓ | ✓ | Not known | ✓ | ✓ | ✓ | ||||||
| 9A | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 9B | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| 11 | ✓ | ✓ | ✓ | No | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 13 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| 15 | ✓ | ✓ | ✓ | No | ✓ | ✓ | |||||||
| 16 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 17 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| 18 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Totals (n) | 20 | 3 | 7 | 8 | 7 | 19 | 18 | 16 | 8 | 20 | 13 | 19 | |
FIGURE 22.
Referral options available at the end of ESD at study location 2. HSCP, health and social care professional; OT, occupational therapist; PT, physiotherapist.
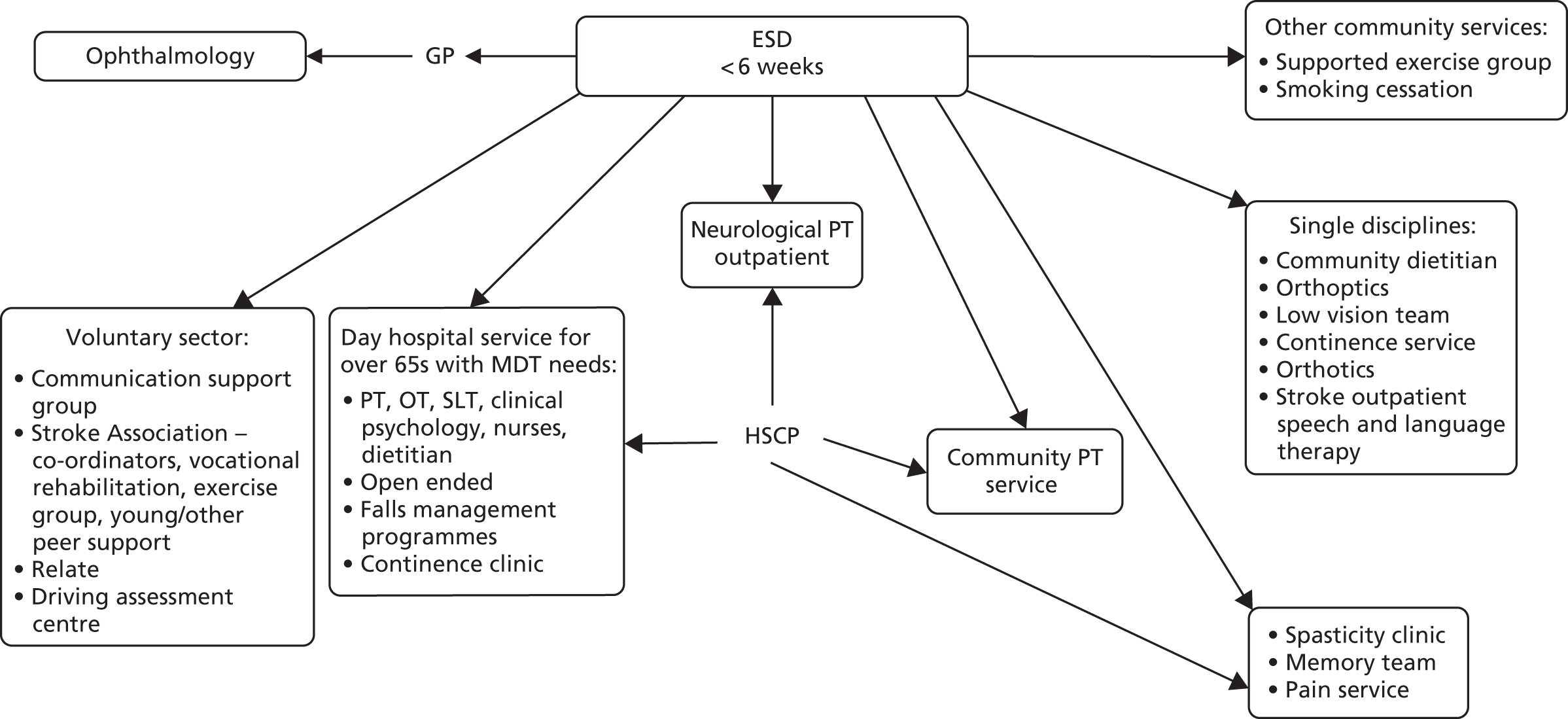
FIGURE 23.
Referral options available at the end of ESD at study location 3. HSCP, health and social care professional; OT, occupational therapist; PT, physiotherapist.
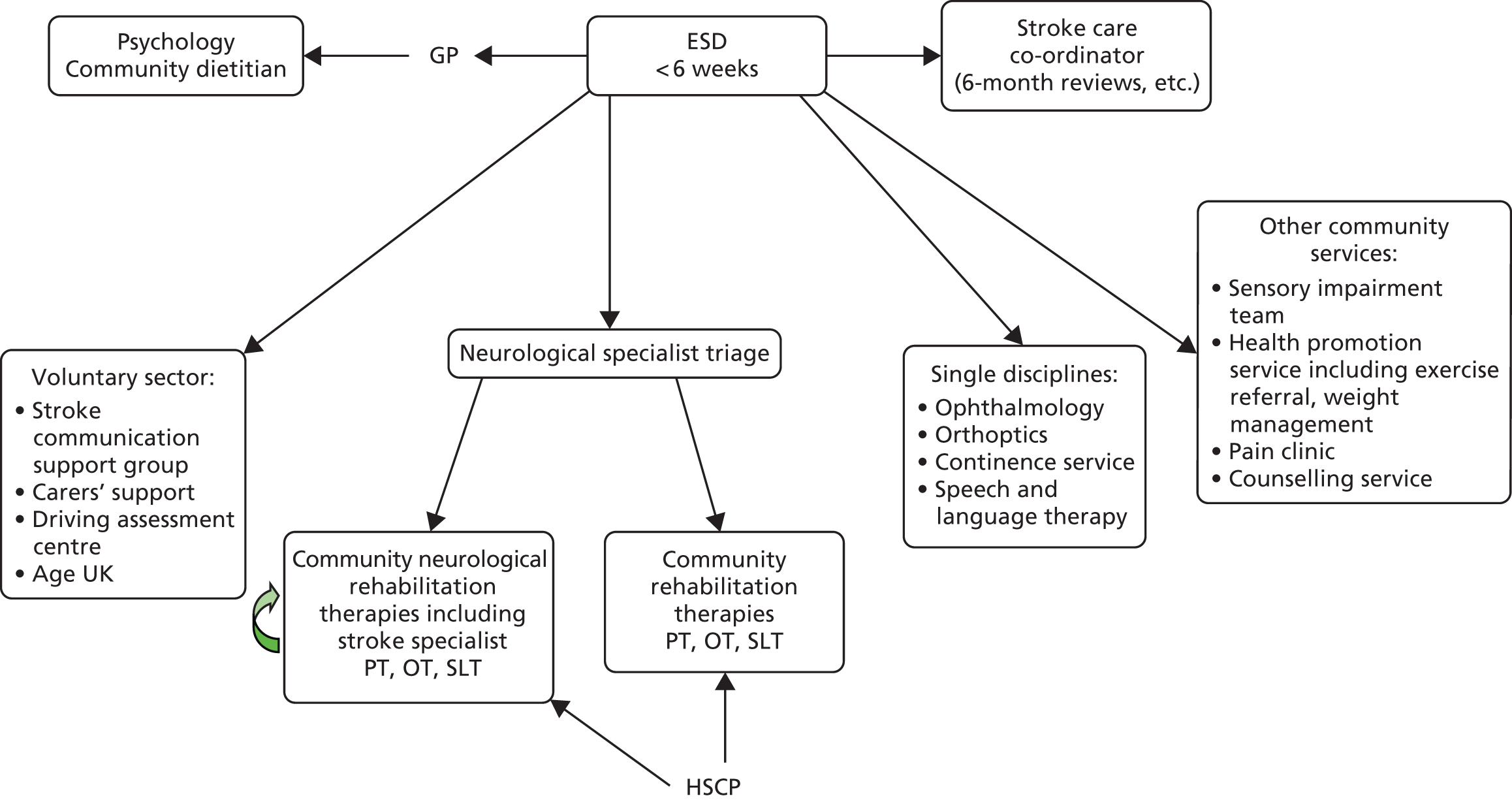
FIGURE 24.
Referral options available at the end of ESD at study location 4. CST, community stroke team; HSCP, health and social care professional; OT, occupational therapist; PT, physiotherapist.
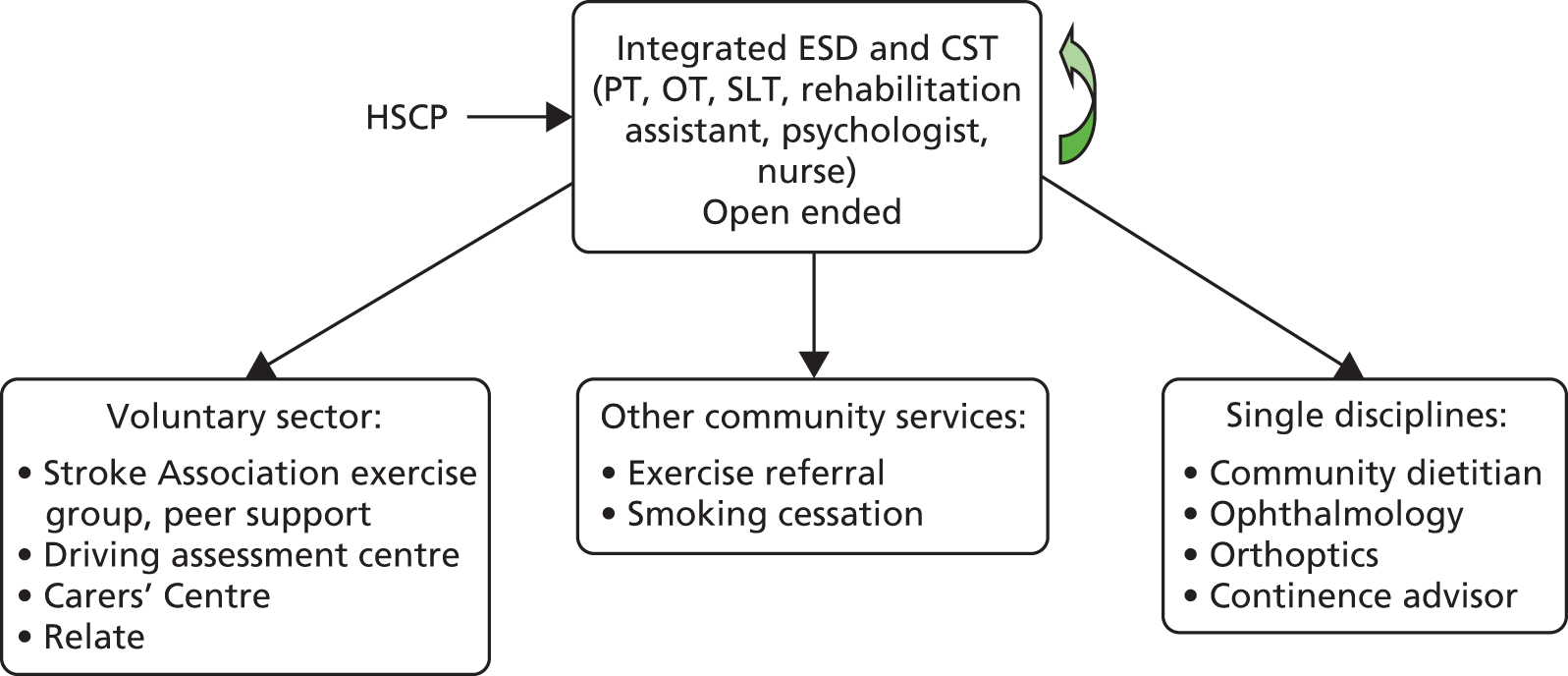
FIGURE 25.
Referral options available at the end of ESD at study location 6. CST, community stroke team; HSCP, health and social care professional; OT, occupational therapist; PT, physiotherapist.
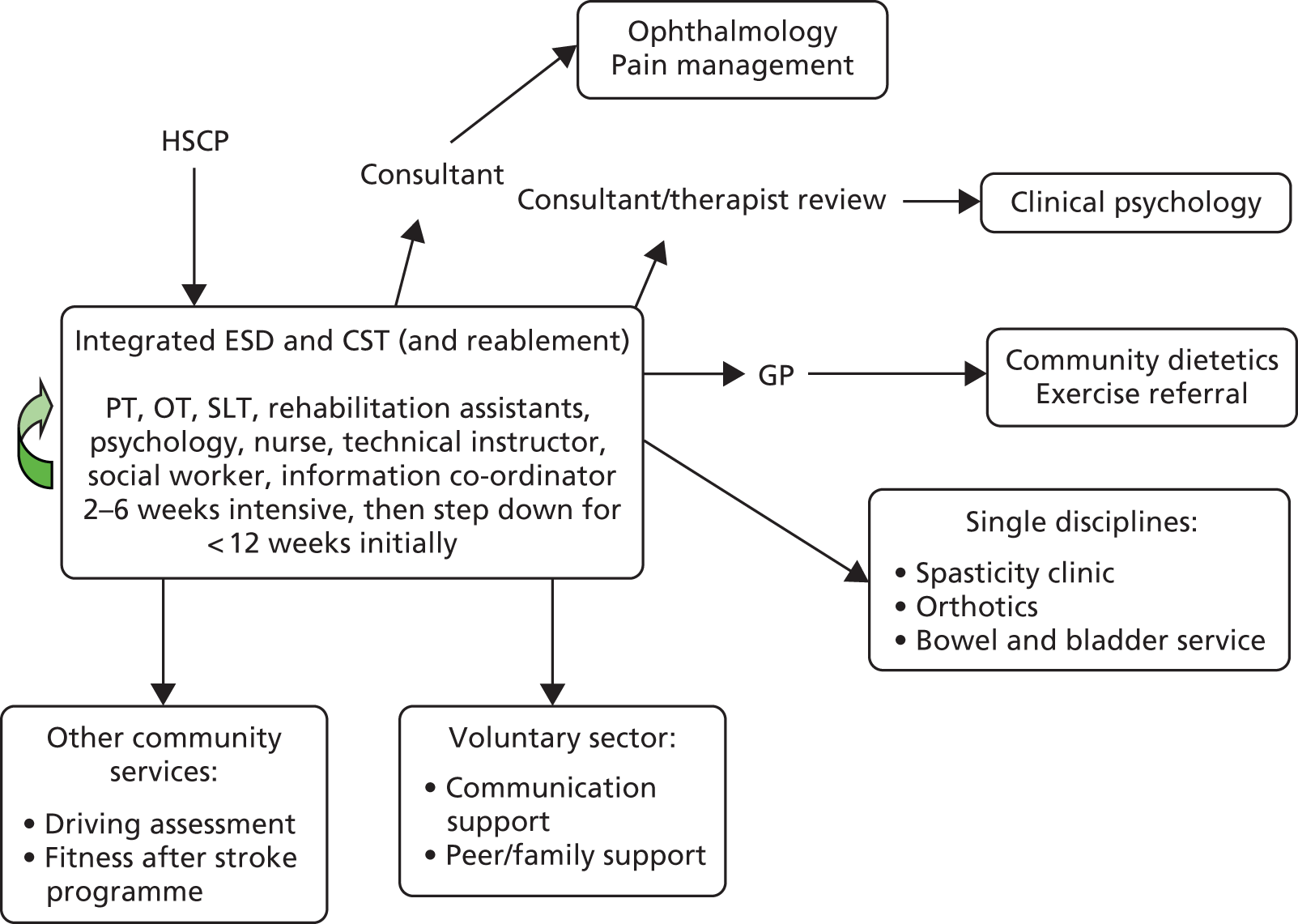
FIGURE 26.
Referral options available at the end of ESD at study location 7. HSCP, health and social care professional; OT, occupational therapist; PT, physiotherapist.
FIGURE 27.
Referral options available at the end of ESD at study location 8A. HSCP, health and social care professional; OT, occupational therapist; PT, physiotherapist.
FIGURE 28.
Referral options available at the end of ESD at study location 8B. HSCP, health and social care professional; OT, occupational therapist; PT, physiotherapist.
FIGURE 29.
Referral options available at the end of ESD at study location 9A. CST, community stroke team; HSCP, health and social care professional; OT, occupational therapist; PT, physiotherapist.
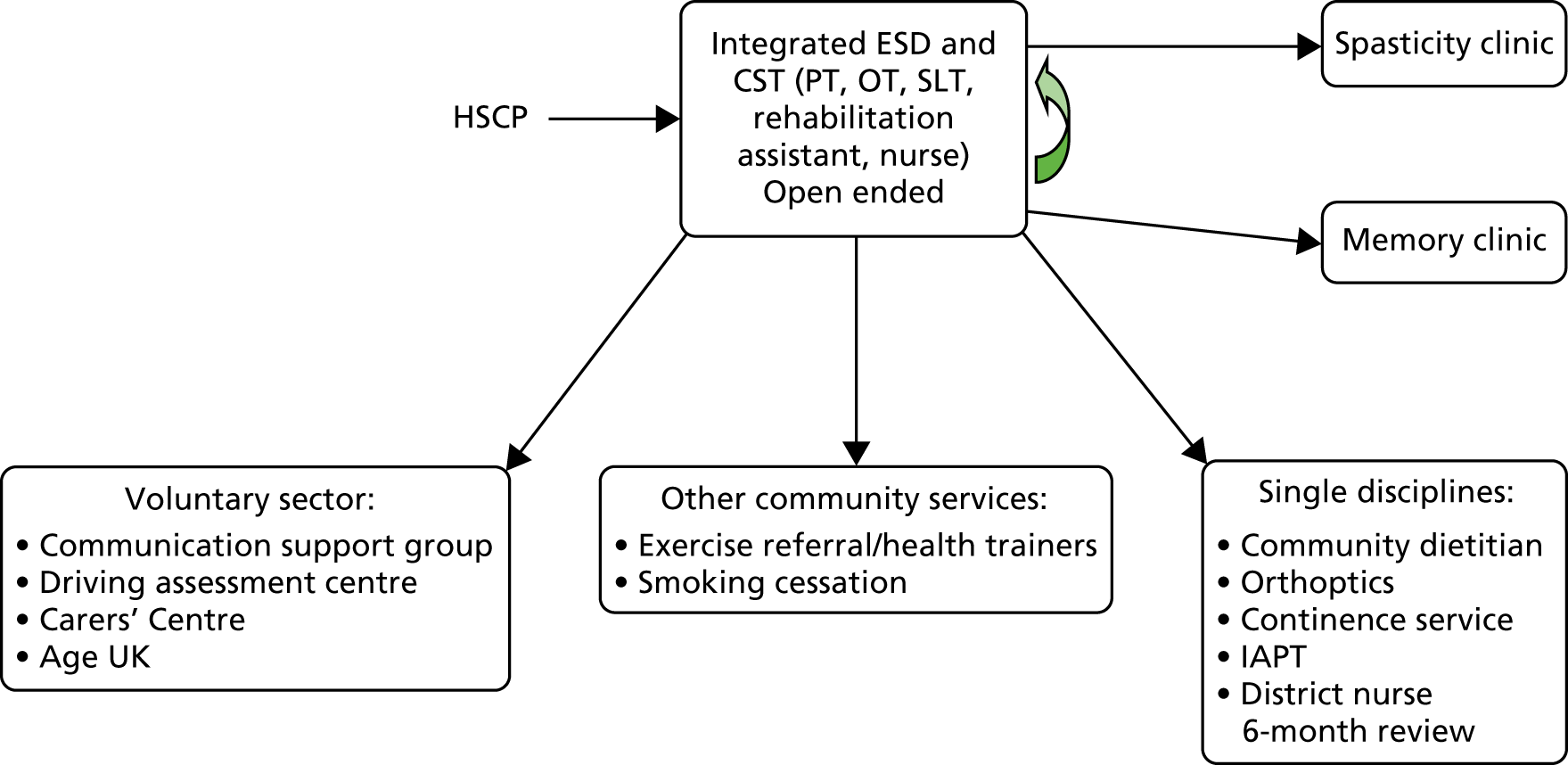
FIGURE 30.
Referral options available at the end of ESD at study location 9B. HSCP, health and social care professional; OT, occupational therapist; PT, physiotherapist.
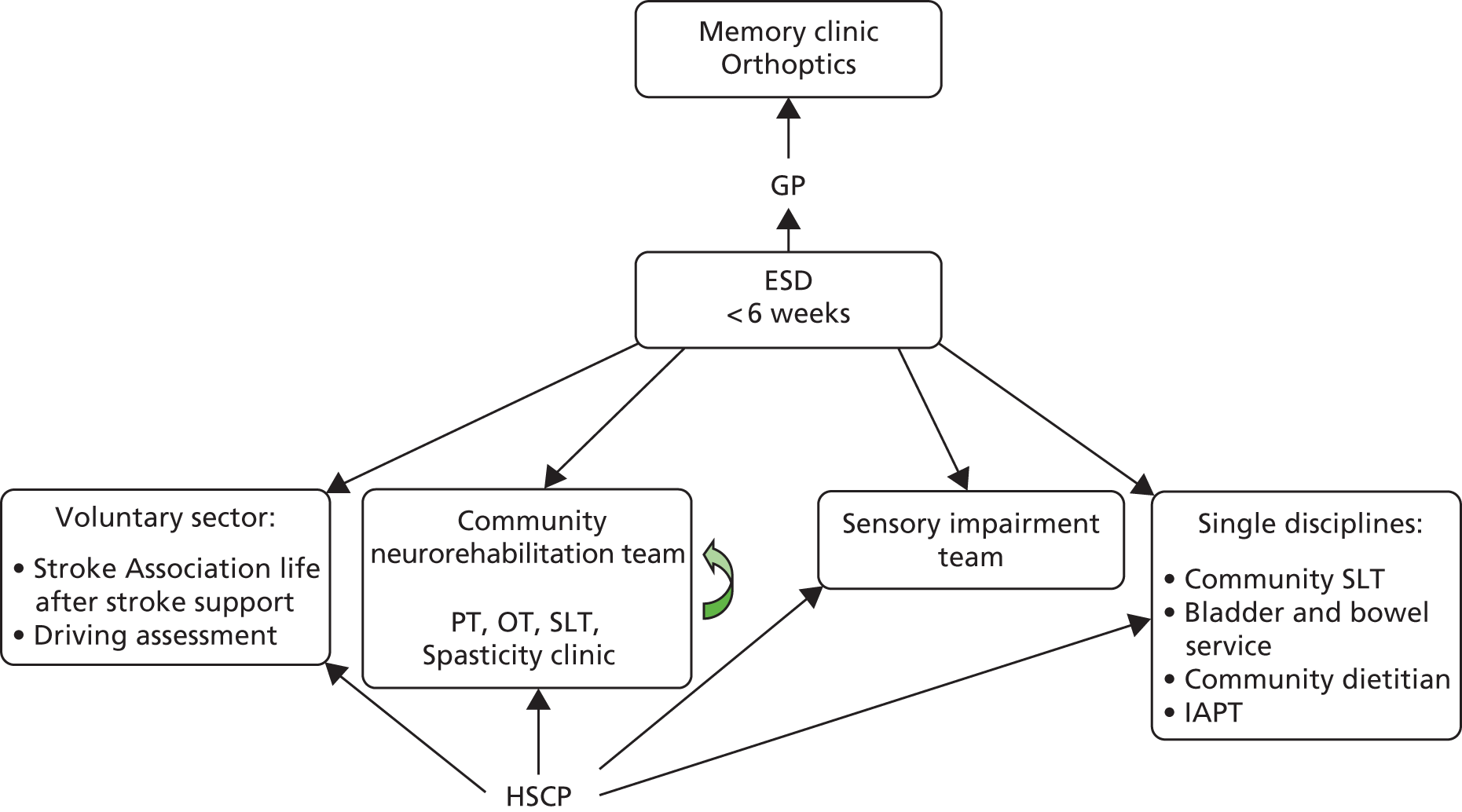
FIGURE 31.
Referral options available at the end of ESD at study location 10. HSCP, health and social care professional; OT, occupational therapist; PT, physiotherapist.
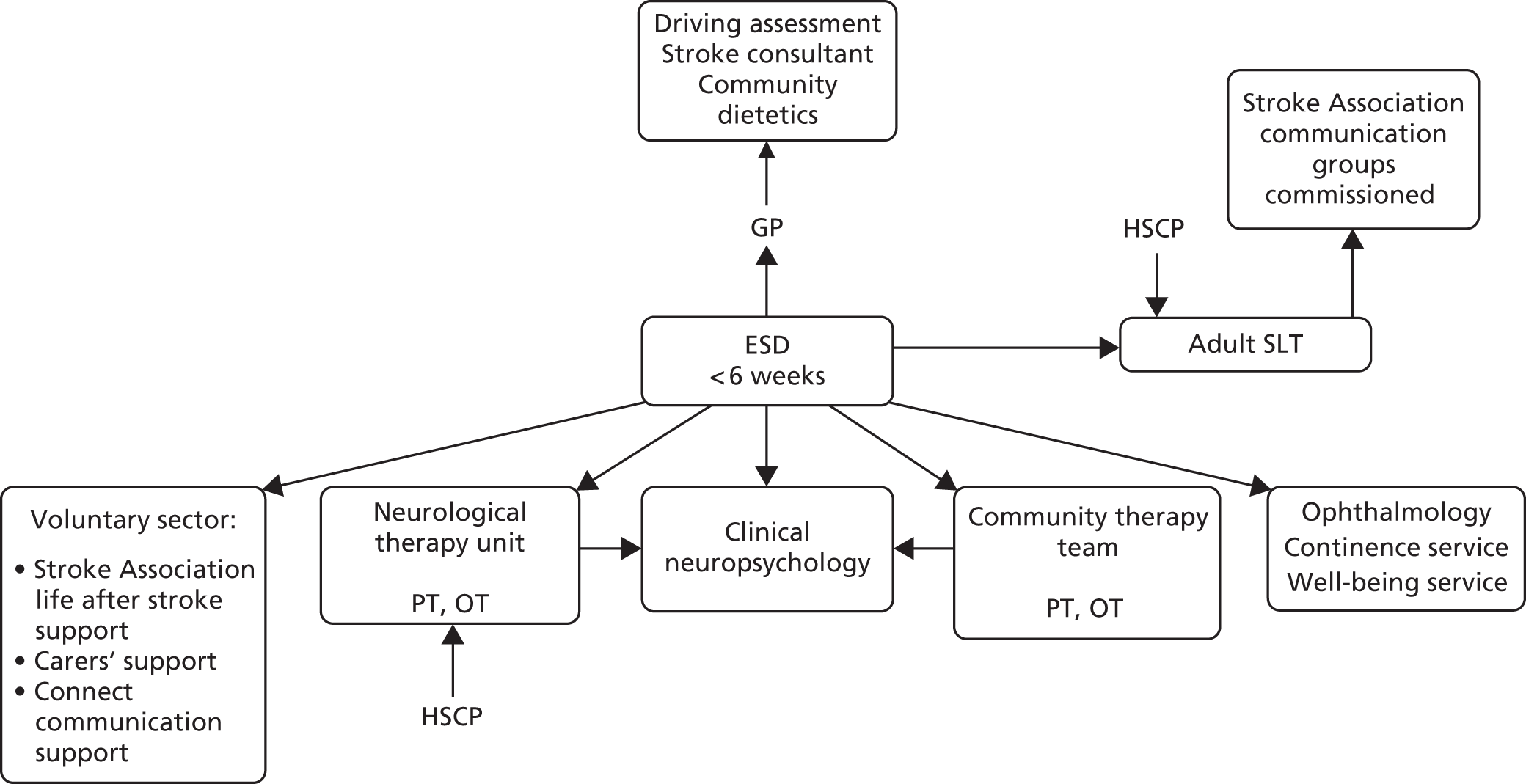
FIGURE 32.
Referral options available at the end of ESD at study location 11. HSCP, health and social care professional; IAPT, Improving Access to Psychological Therapies; OT, occupational therapist; PT, physiotherapist; SPoA, single point of access.
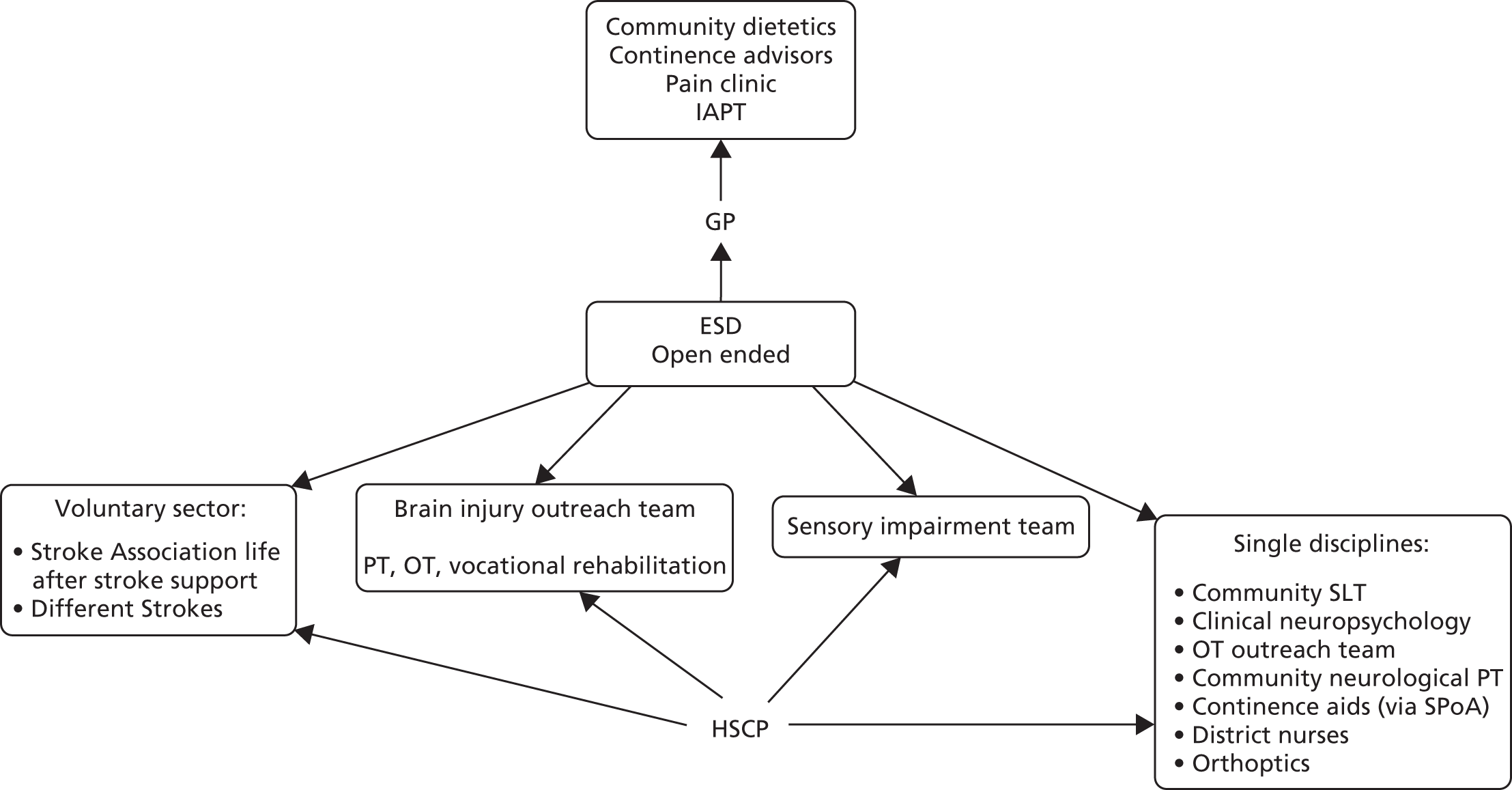
FIGURE 33.
Referral options available at the end of ESD at study location 12. HSCP, health and social care professional; IAPT, Improving Access to Psychological Therapies; OT, occupational therapist; PT, physiotherapist; SPoA, single point of access.
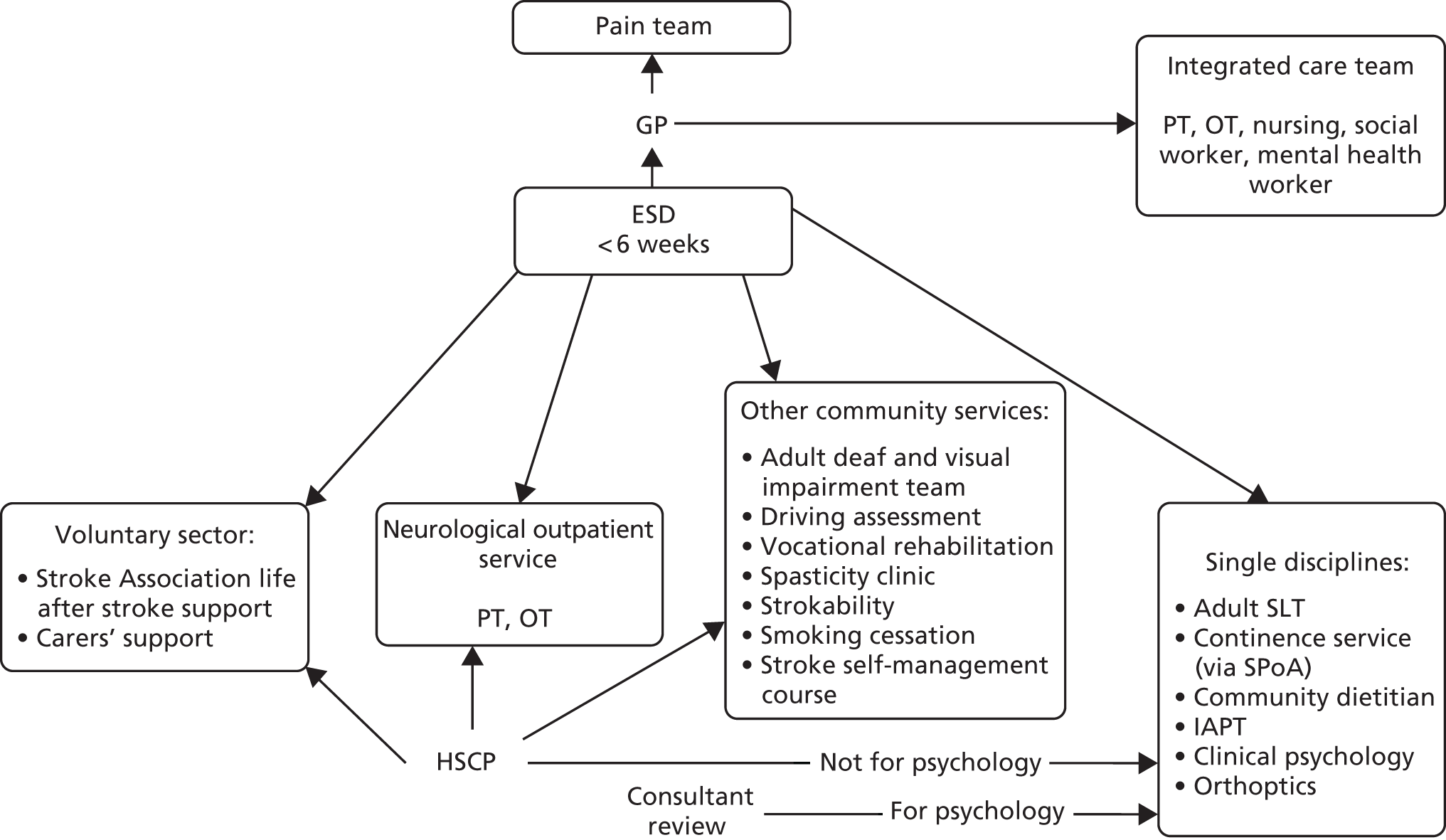
FIGURE 34.
Referral options available at the end of ESD at study location 13. CST, community stroke team; HSCP, health and social care professional; OT, occupational therapist; PT, physiotherapist.
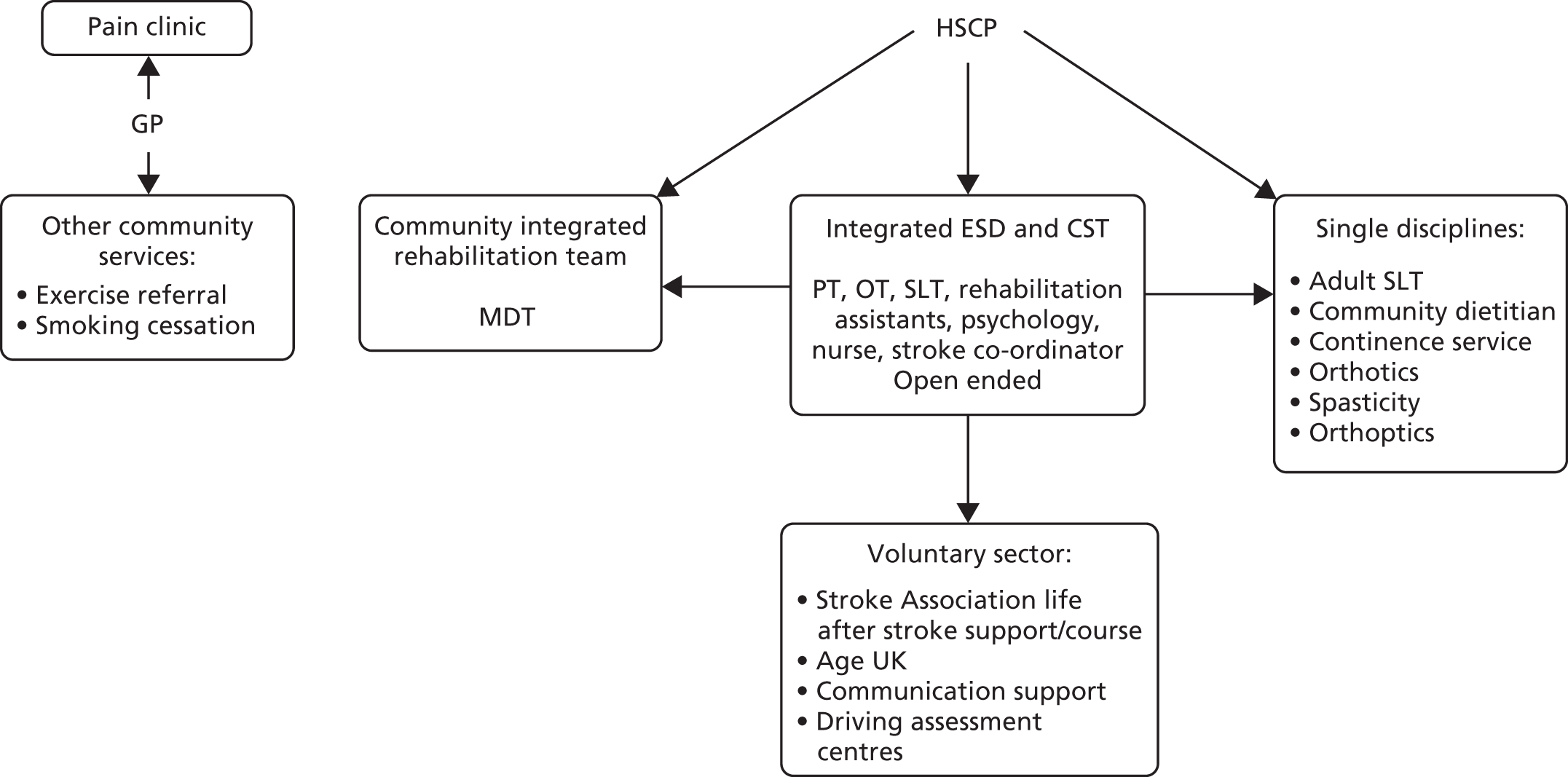
FIGURE 35.
Referral options available at the end of ESD at study location 14. CST, community stroke team; OT, occupational therapist; PT, physiotherapist.
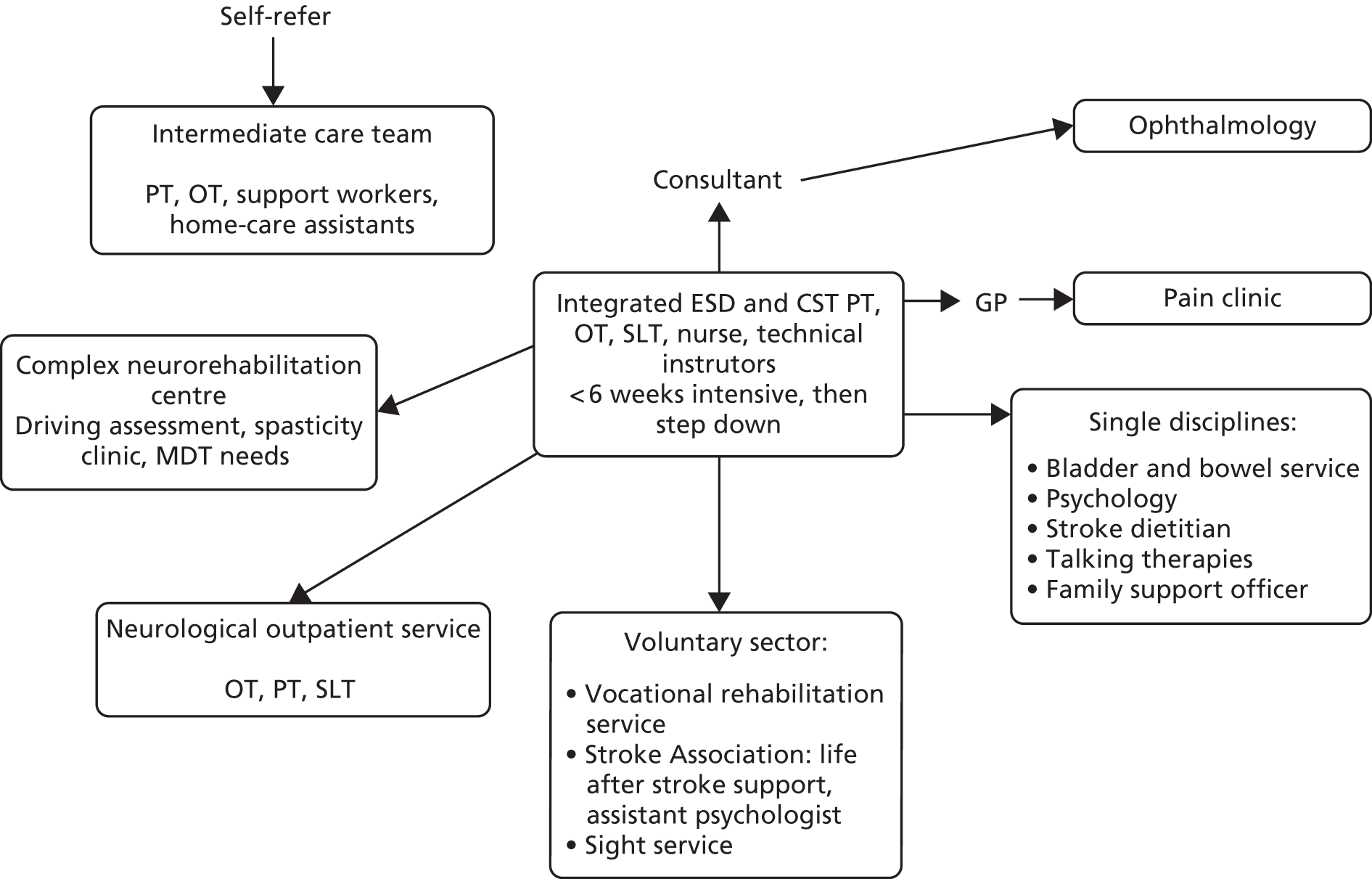
FIGURE 36.
Referral options available at the end of ESD at study location 15. CST, community stroke team; HSCP, health and social care professional; IAPT, Improving Access to Psychological Therapies; OT, occupational therapist; PT, physiotherapist.
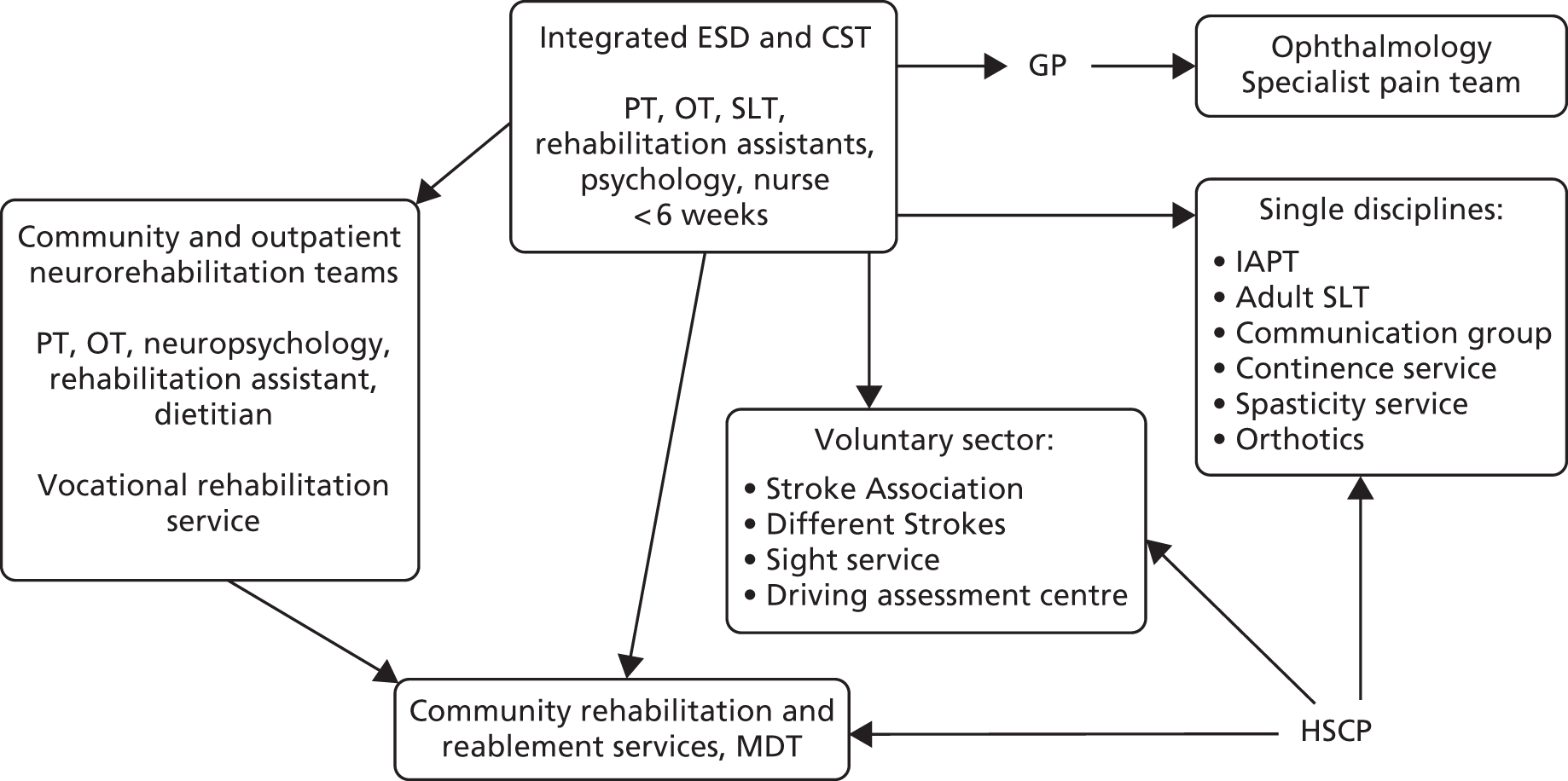
FIGURE 37.
Referral options available at the end of ESD at study location 16. HSCP, health and social care professional; IAPT, Improving Access to Psychological Therapies; OT, occupational therapist; PT, physiotherapist.
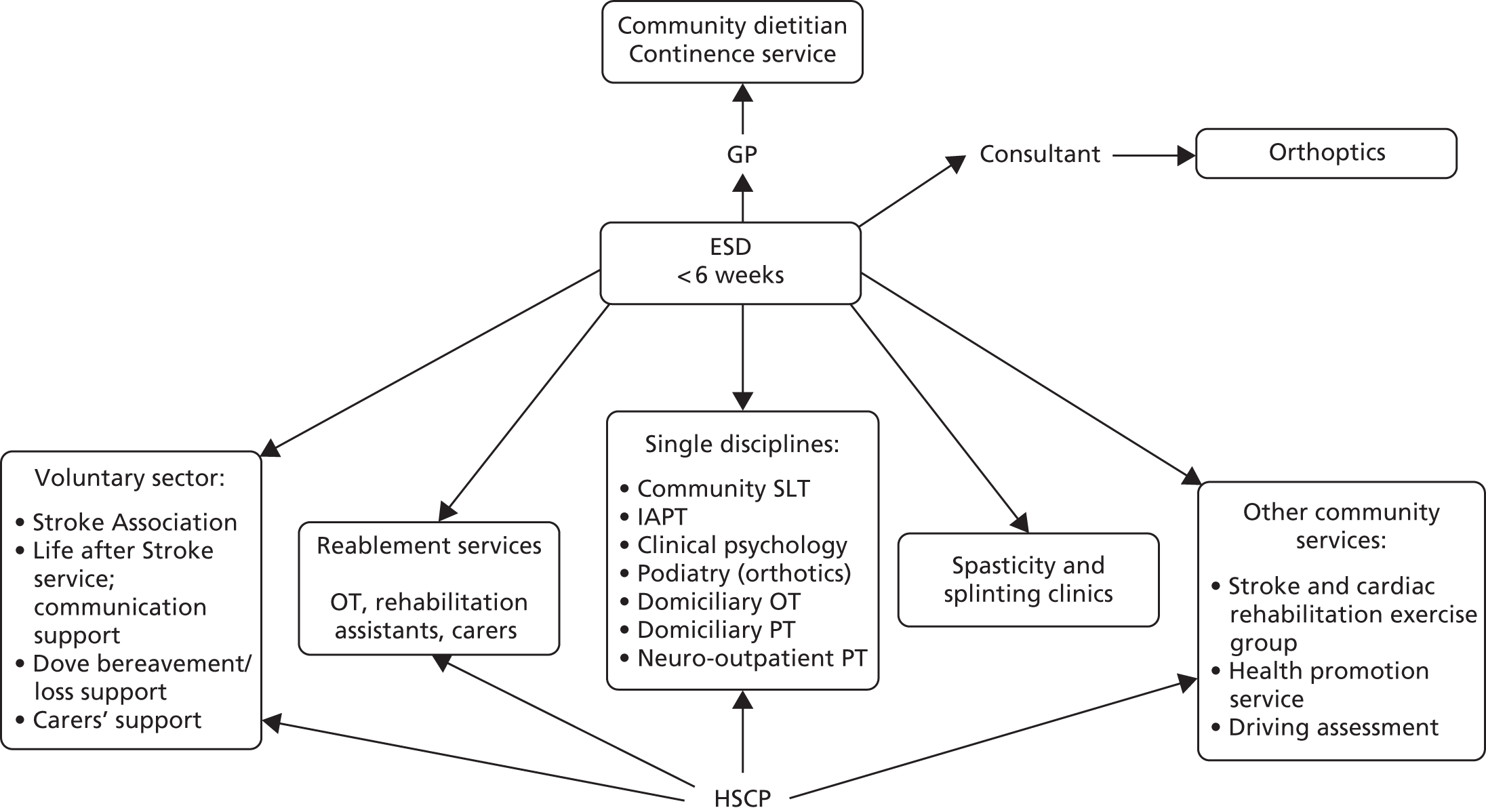
FIGURE 38.
Referral options available at the end of ESD at study location 17. CST, community stroke team; HSCP, health and social care professional; IAPT, Improving Access to Psychological Therapies; OT, occupational therapist; PT, physiotherapist.
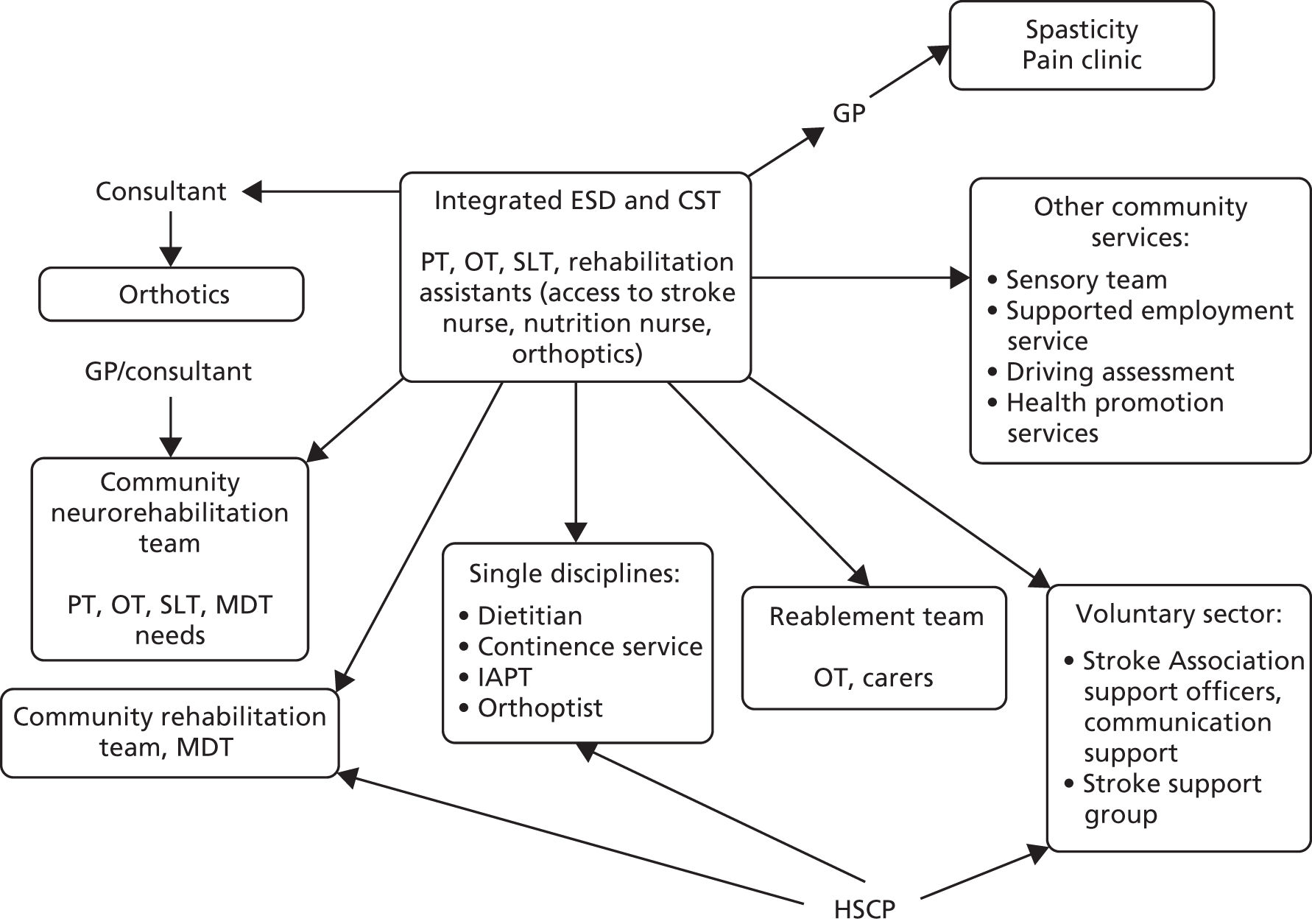
FIGURE 39.
Referral options available at the end of ESD at study location 18. CST, community stroke team; HSCP, health and social care professional; IAPT, Improving Access to Psychological Therapies; OT, occupational therapist; PT, physiotherapist.
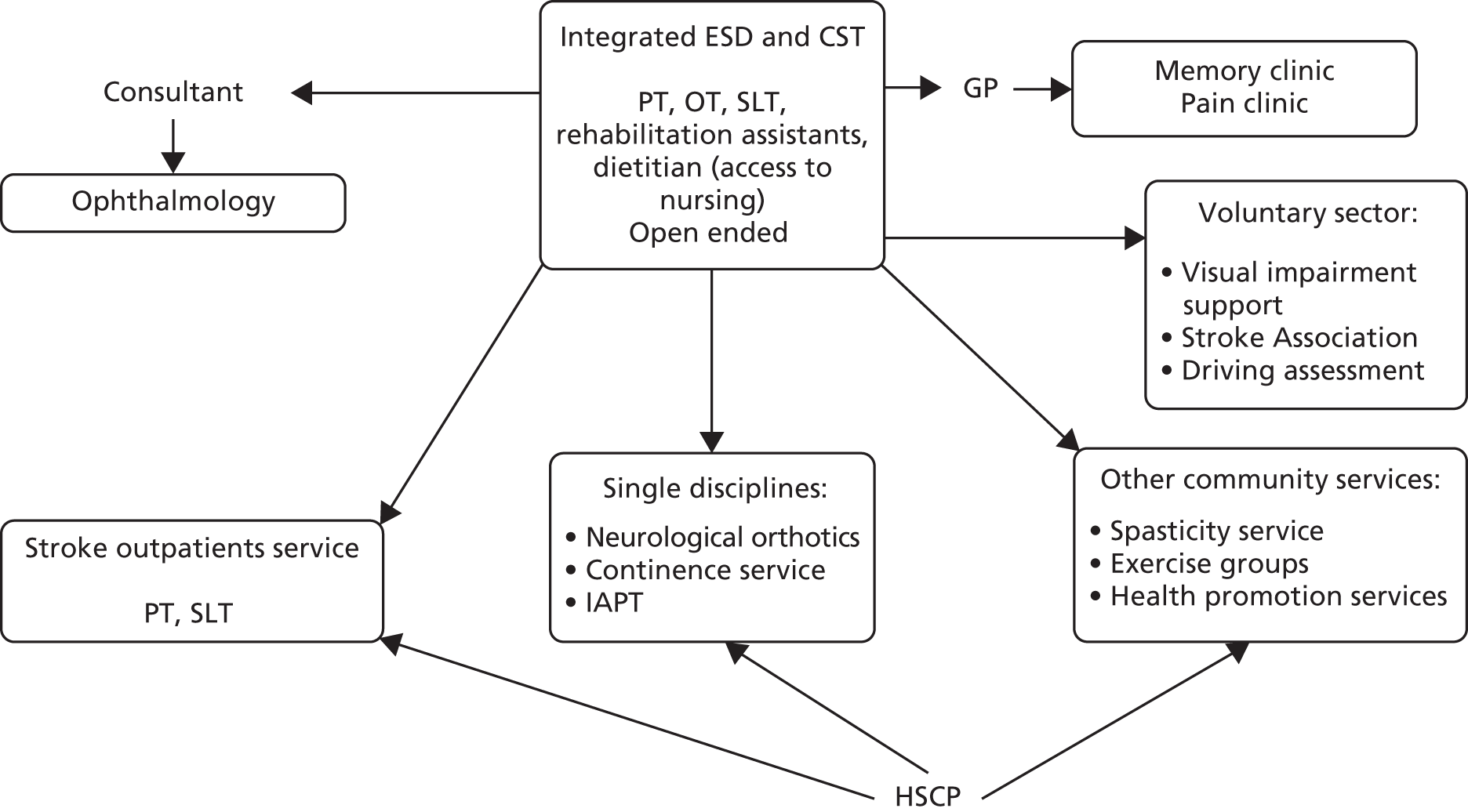
| Characteristic | Study ESD services (n = 20, 2016) | National data ESD services (n = 142, 2015/16) |
|---|---|---|
| Nursing | 2 ESD services (10%) did not have a nurse | 39% of ESD services did not have a nurse116 |
| Physiotherapy | 20 ESD services (100%) had a physiotherapist(s) | 100% of ESD services had a physiotherapist117 |
| Occupational therapy | 20 ESD services (100%) had an occupational therapist(s) | 100% of ESD services had an occupational therapist117 |
| Speech and language therapy | 2 ESD services (10%) did not have a SLT | 3% of ESD services did not have a SLT117 |
| Psychology | 11 ESD services (55%) did not have a psychologist (or access to one)a | 58% of ESD services did not have a psychologist116 |
| Referral to treatment time | 1–2 days | 1–2 days116 |
| 7-day working | 15 (75%) ESD teams operated 7 days a week | 29% of ESD teams operated 7 days a week118 |
| Group | Utility (n) | ||
|---|---|---|---|
| Baseline | 12 months | 24 months | |
| Usual care | 2 | 3 | 1 |
| Intervention | 1 | 3 | 0 |
| Total | 3 | 6 | 1 |
| Cost (£) | Intervention (n = 235), mean (95% CI) | Control (n = 259), mean (95% CI) | Adjusted difference,a EXTRAS minus usual care, mean (95% CI) |
|---|---|---|---|
| NHS costs | |||
| Outpatient costs | 795 (675 to 915) | 848 (677 to 1020) | 80 (–99 to 259) |
| Inpatient costs | 1898 (1469 to 2327) | 1922 (1507 to 2338) | –122 (–932 to 687) |
| Day patient costs | 500 (225 to 776) | 1529 (–938 to 3995) | 117 (–40 to 274) |
| Primary health costs | 1469 (1210 to 1727) | 1493 (1247 to 1739) | 50 (–252 to 352) |
| Social care costs | |||
| Home help (home service, personal care, shopping) | 54 (12 to 95) | 397 (41 to 754) | –216 (–419 to –13)b |
| Benefit/allowance | 4900 (4103 to 5607) | 5782 (4972 to 6592) | –727 (–1478 to 24) |
| Resource utilisation unit | Cost (£) | Reference | Notes |
|---|---|---|---|
| GP surgery visit | 42.68 | PSSRU 2016/1782 | |
| GP home visit | 142.28 | PSSRU 2016/1782 | |
| GP telephone call | 26.08 | PSSRU 2016/1782 | |
| GP nurse surgery visit | 14.23 | PSSRU 2016/1782 | |
| Physiotherapist hospital | 47.43 | PSSRU 2016/1782 | |
| Physiotherapist surgery | 20.16 | PSSRU 2016/1782 | |
| Physiotherapist home | 55.73 | PSSRU 2016/1782 | |
| Physiotherapist elsewhere | 20.16 | PSSRU 2016/1782 | Assuming the cost of surgery visit |
| Occupational therapist hospital | 50.98 | PSSRU 2016/1782 | |
| Occupational therapist surgery | 20.16 | PSSRU 2016/1782 | |
| Occupational therapist home | 54.54 | PSSRU 2016/1782 | |
| Occupational therapist elsewhere | 20.16 | PSSRU 2016/1782 | |
| Speech therapist hospital | 49.80 | PSSRU 2016/1782 | |
| Speech therapist surgery | 20.16 | PSSRU 2016/1782 | |
| Speech therapist home | 55.73 | PSSRU 2016/1782 | |
| Speech therapist elsewhere | 20.16 | PSSRU 2016/1782 | |
| Community nurse | 28.85 | PSSRU 2016/1782 | |
| Health visitor | 49.80 | PSSRU 2016/1782 | |
| Geriatrician | 42.68 | PSSRU 2016/1782 | |
| Psychiatrist | 388.90 | PSSRU 2016/1782 | |
| Psychologist | 54.54 | PSSRU 2016/1782 | |
| Chiropodist | 13.04 | PSSRU 2016/1782 | |
| Optician | 21.31 | PSSRU 2016/1782 | |
| Meals on wheels | 4.23 | PSSRU 2016/1782 | Assuming £25.00 per week |
| Help (personal care) | 26.00 | PSSRU 2016/1782 | Assuming cost of 1 hour per day during the weekdays |
| Help (household) | 26.00 | PSSRU 2016/1782 | Assuming cost of 1 hour per day during the weekdays |
| Help (shopping) | 31.00 | PSSRU 2016/1782 | Assuming cost of 1 hour per day during the weekdays |
| Attendance allowance (lower) | 55.65 | Department for Work and Pensions119 | Per week |
| Attendance allowance (higher) | 83.10 | Department for Work and Pensions119 | Per week |
| Disability allowance (mobility) | 58.00 | Department for Work and Pensions119 | Per week: personal independence payment |
| Disability allowance (care) | 83.10 | Department for Work and Pensions119 | |
| Employment and support allowance | 73.10 | Department for Work and Pensions119 | Per week |
| Statutory sick pay | 89.35 | Department for Work and Pensions119 | Per week; paid maximum 28 weeks |
| Personal independence payment | 58.00 + 73.10 | Department for Work and Pensions119 | Per week, sum of disability allowance (mobility) and disability allowance (care) |
| Nursing allowance | 155.05 | PSSRU 2016/1782 | Per week |
| Nursing nights | 122.80 | PSSRU 2016/1782 | Per night, derived from weekly cost of £725 |
| Resident nights | 87.74 | PSSRU 2016/1782 | Per night, derived from weekly cost of £518 |
| A&E attendance | 115.01 | PSSRU 2016/1782 |
| Levela | Mobility, n (%) | Self-care, n (%) | Usual activities, n (%) | Pain/discomfort, n (%) | Anxiety/depression, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | |
| 1 | 50 (21.37) | 48 (18.68) | 119 (50.85) | 113 (43.97) | 34 (14.53) | 32 (12.45) | 95 (40.60) | 101 (39.30) | 100 (42.74) | 116 (45.14) |
| 2 | 78 (33.33) | 76 (29.57) | 64 (27.35) | 75 (29.18) | 76 (32.48) | 75 (29.18) | 73 (31.20) | 67 (26.07) | 87 (37.18) | 90 (35.02) |
| 3 | 86 (36.75) | 99 (38.52) | 37 (15.81) | 53 (20.62) | 64 (27.35) | 80 (31.13) | 46 (19.66) | 70 (27.24) | 37 (15.81) | 39 (15.18) |
| 4 | 12 (5.13) | 29 (11.28) | 9 (3.85) | 8 (3.11) | 25 (10.68) | 30 (11.67) | 17 (7.26) | 17 (6.61) | 5 (2.14) | 11 (4.28) |
| 5 | 8 (3.42) | 5 (1.95) | 5 (2.14) | 8 (3.11) | 35 (14.96) | 40 (15.56) | 3 (1.28) | 2 (0.78) | 5 (2.14) | 1 (0.39) |
| Total (n) | 234 | 257 | 234 | 257 | 234 | 257 | 234 | 257 | 234 | 257 |
| Patients reporting some problems (%) | 78.63 | 81.32 | 49.15 | 56.03 | 85.47 | 87.55 | 59.40 | 60.70 | 57.26 | 54.86 |
| Levela | Mobility, n (%) | Self-care, n (%) | Usual activities, n (%) | Pain/discomfort, n (%) | Anxiety/depression, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | |
| 1 | 53 (23.77) | 53 (22.18) | 111 (49.78) | 102 (42.86) | 52 (23.32) | 47 (19.67) | 76 (34.23) | 59 (24.69) | 101 (45.29) | 89 (37.24) |
| 2 | 79 (35.43) | 65 (27.20) | 58 (26.01) | 57 (23.95) | 78 (34.98) | 58 (24.27) | 65 (29.28) | 79 (33.05) | 75 (33.63) | 87 (36.40) |
| 3 | 57 (25.56) | 74 (30.96) | 36 (16.14) | 50 (21.01) | 49 (21.97) | 65 (27.20) | 56 (25.23) | 72 (30.13) | 36 (16.14) | 45 (18.83) |
| 4 | 27 (12.11) | 44 (18.41) | 10 (4.48) | 18 (7.56) | 23 (10.31) | 33 (13.81) | 24 (10.81) | 23 (9.62) | 9 (4.04) | 14 (5.86) |
| 5 | 7 (3.14) | 3 (1.26) | 8 (3.59) | 11 (4.62) | 21 (9.42) | 36 (15.06) | 1 (0.45) | 6 (2.51) | 2 (0.90) | 4 (1.67) |
| Total (n) | 223 | 239 | 223 | 238 | 223 | 239 | 222 | 239 | 223 | 239 |
| Patients reporting some problems (%) | 76.23 | 77.82 | 50.22 | 57.14 | 76.68 | 80.33 | 65.77 | 75.31 | 54.71 | 62.76 |
| Levela | Mobility, n (%) | Self-care, n (%) | Usual activities, n (%) | Pain/discomfort, n (%) | Anxiety/depression, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | |
| 1 | 56 (26.42) | 43 (18.45) | 102 (48.11) | 100 (44.05) | 52 (24.53) | 44 (19.38) | 73 (34.43) | 54 (23.89) | 98 (46.23) | 90 (39.82) |
| 2 | 48 (22.64) | 53 (22.75) | 43 (20.28) | 43 (18.94) | 62 (29.25) | 54 (23.79) | 56 (26.42) | 75 (33.19) | 63 (29.72) | 77 (34.07) |
| 3 | 64 (30.19) | 70 (30.04) | 44 (20.75) | 44 (19.38) | 54 (25.47) | 66 (29.07) | 57 (26.89) | 64 (28.32) | 39 (18.40) | 51 (22.57) |
| 4 | 35 (16.51) | 57 (24.46) | 11 (5.19) | 27 (11.89) | 21 (9.91) | 26 (11.45) | 21 (9.91) | 26 (11.50) | 8 (3.77) | 4 (1.77) |
| 5 | 9 (4.25) | 10 (4.29) | 12 (5.66) | 13 (5.73) | 23 (10.85) | 37 (16.30) | 5 (2.36) | 7 (3.10) | 4 (1.89) | 4 (1.77) |
| Total (n) | 212 | 233 | 212 | 227 | 212 | 227 | 212 | 226 | 212 | 226 |
| Patients reporting some problems (%) | 73.58 | 81.55 | 51.89 | 55.95 | 75.47 | 80.62 | 65.57 | 76.11 | 53.77 | 60.18 |
FIGURE 40.
Cost-effectiveness plane: no imputed data.
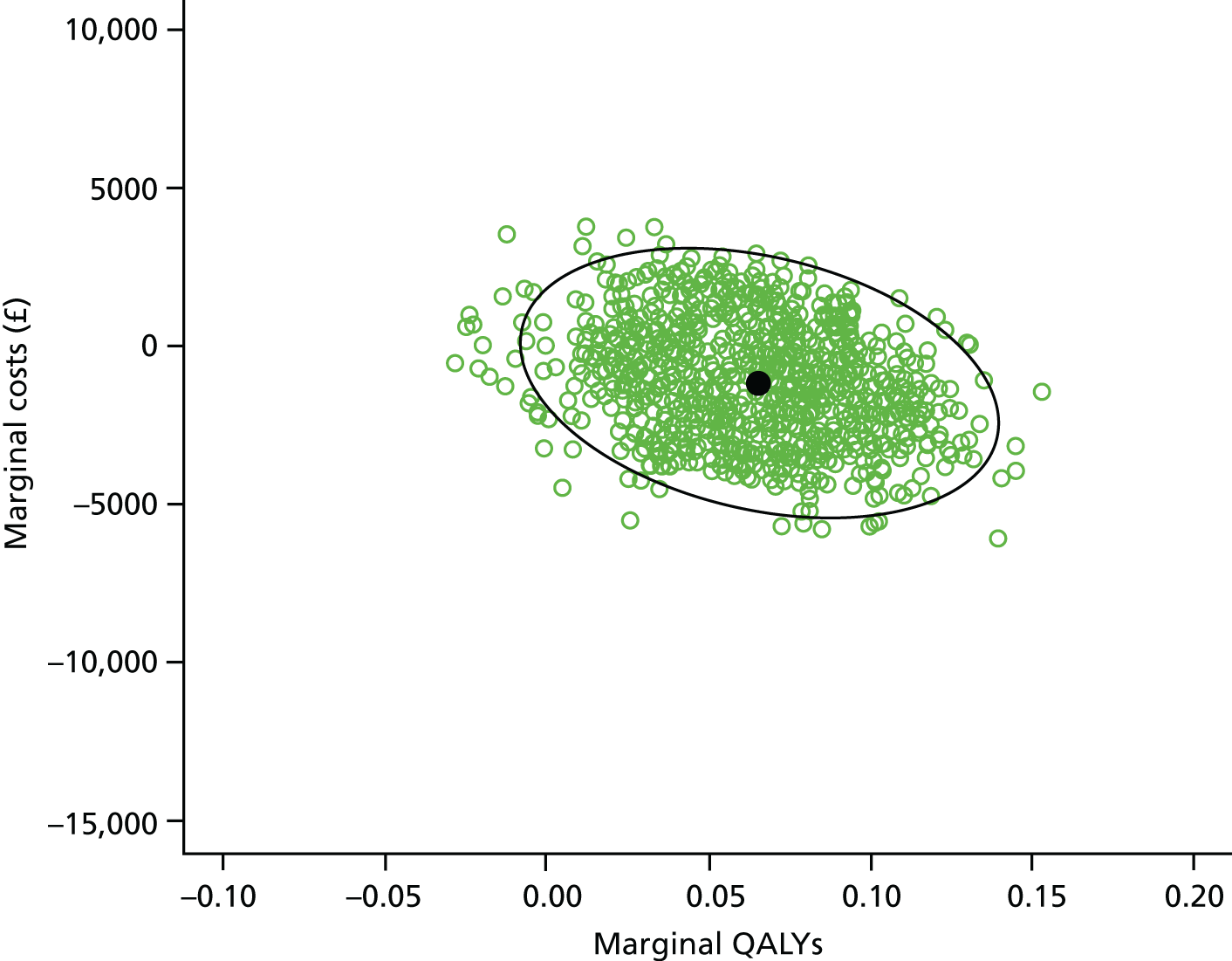
FIGURE 41.
Cost-effectiveness acceptability curve: no imputed data.
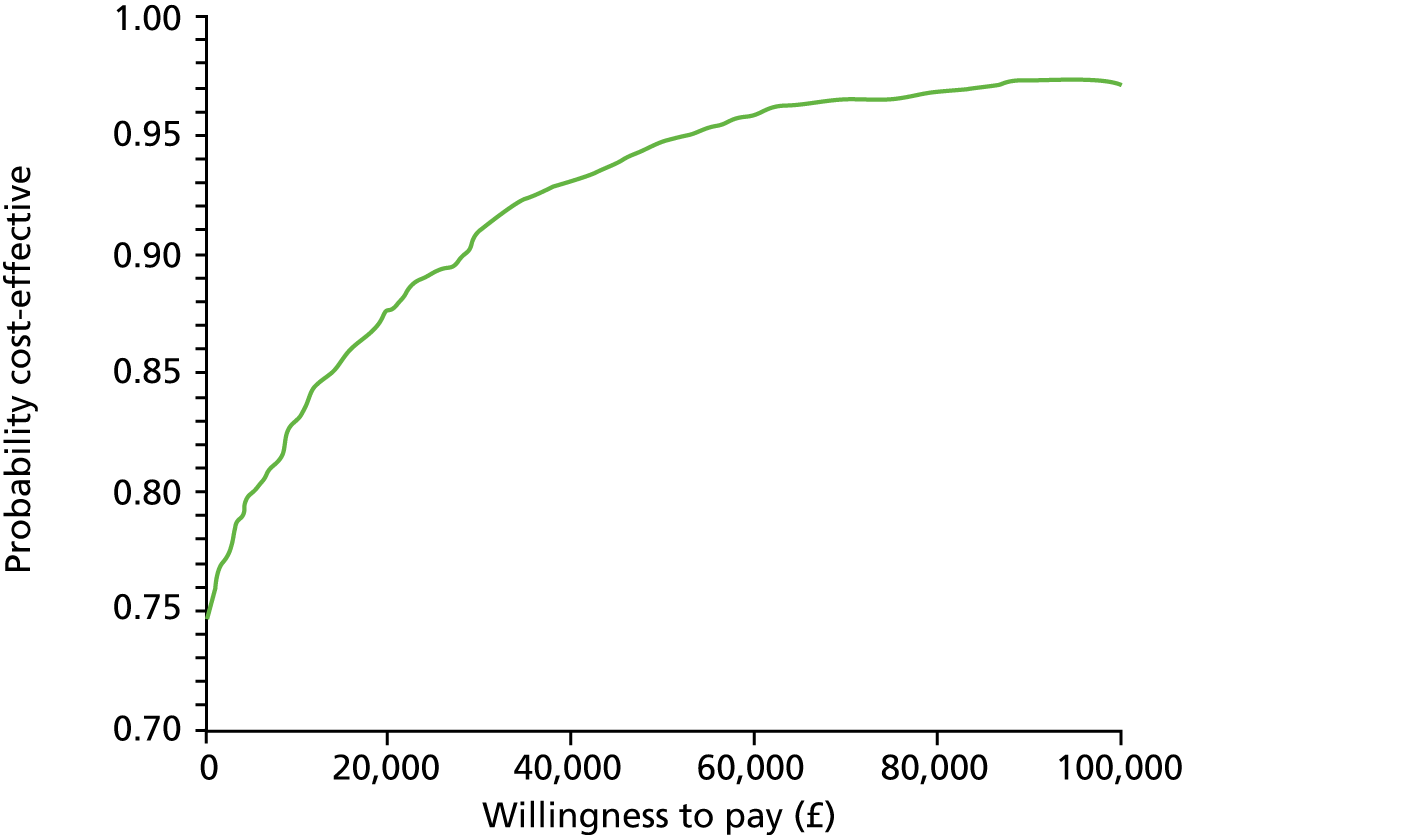
FIGURE 42.
Cost-effectiveness plane: outliers excluded.
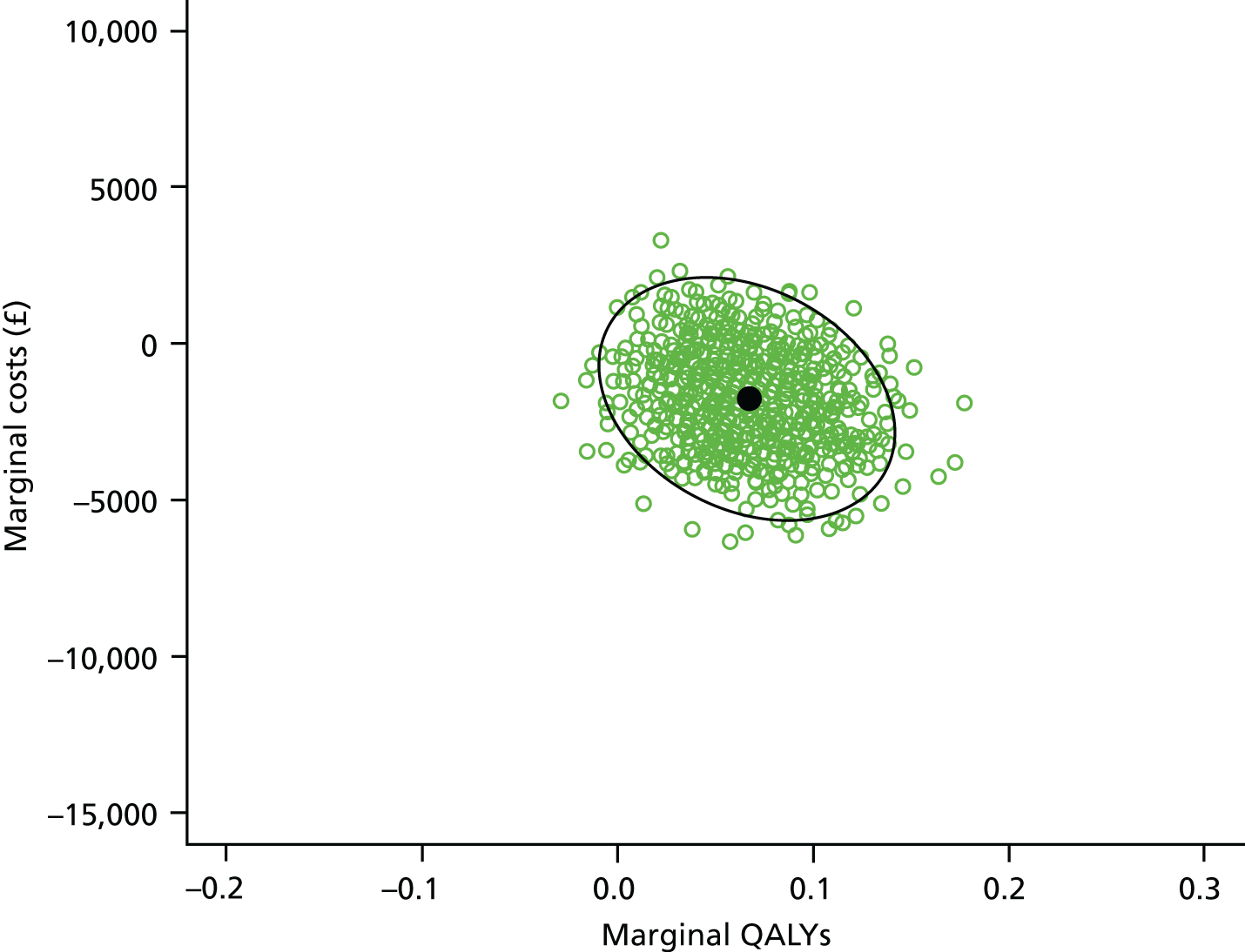
FIGURE 43.
Cost-effectiveness acceptability curve: outliers excluded.
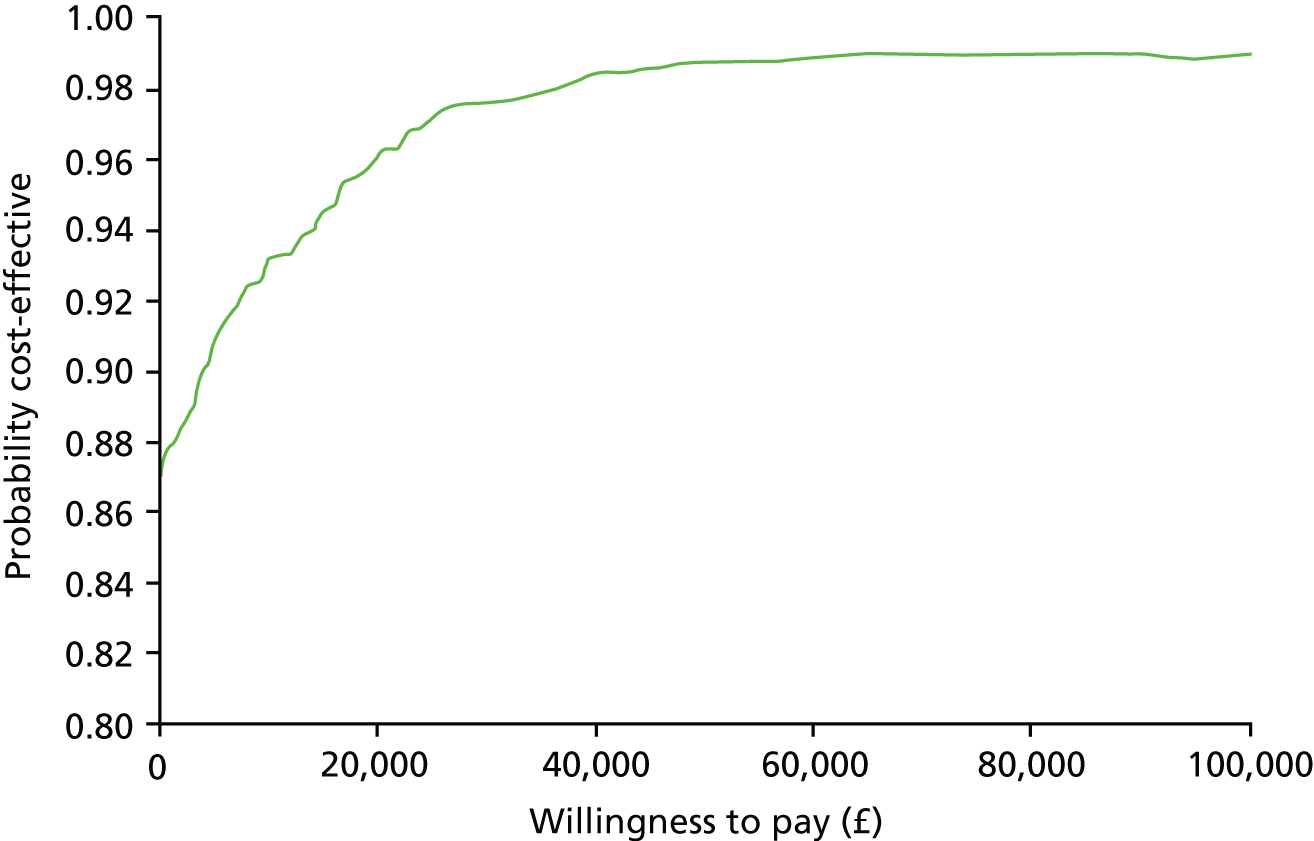
FIGURE 44.
Cost-effectiveness plane: cross-walk EQ-5D-5L tariff.
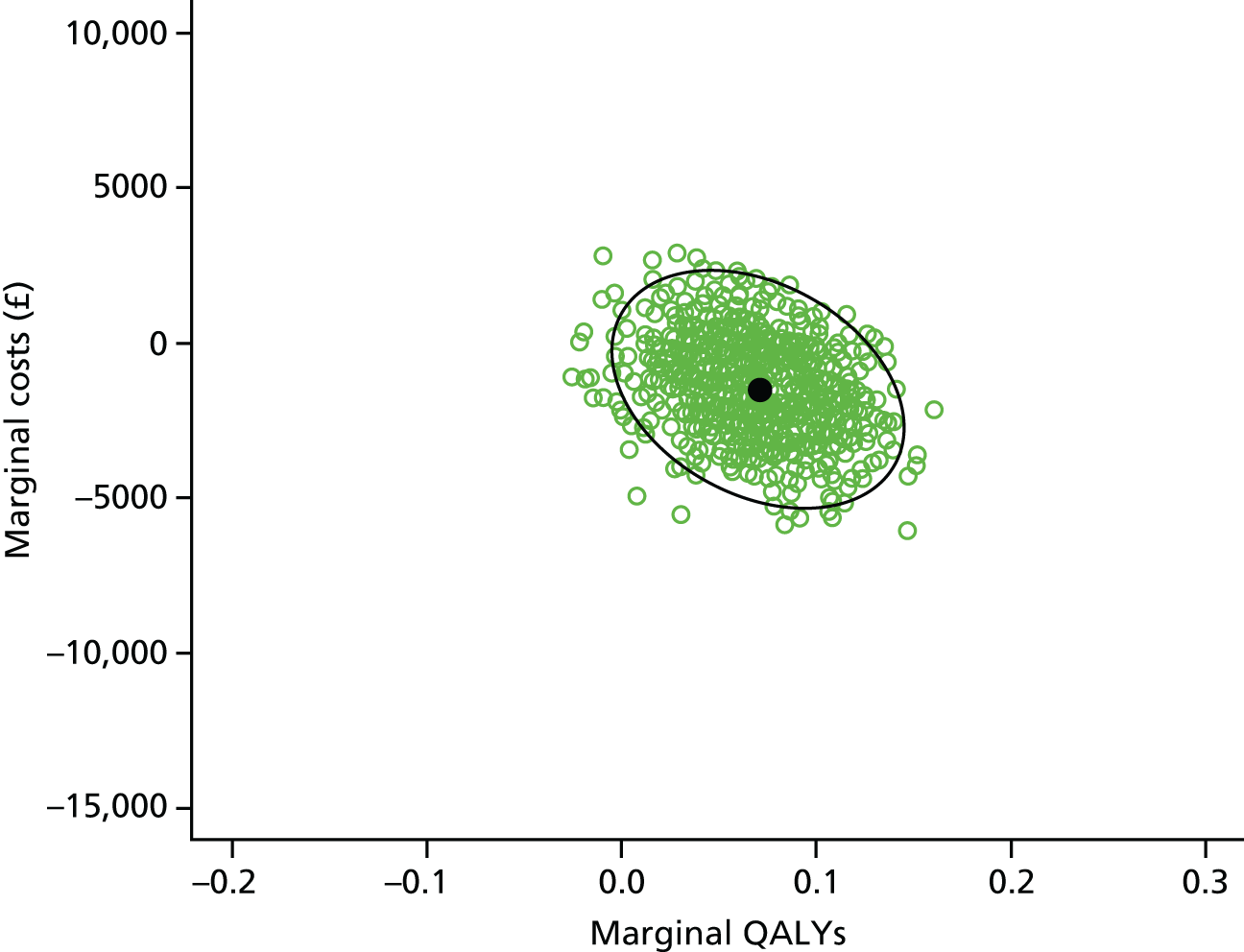
FIGURE 45.
Cost-effectiveness acceptability curve: cross-walk EQ-5D-5L tariff.
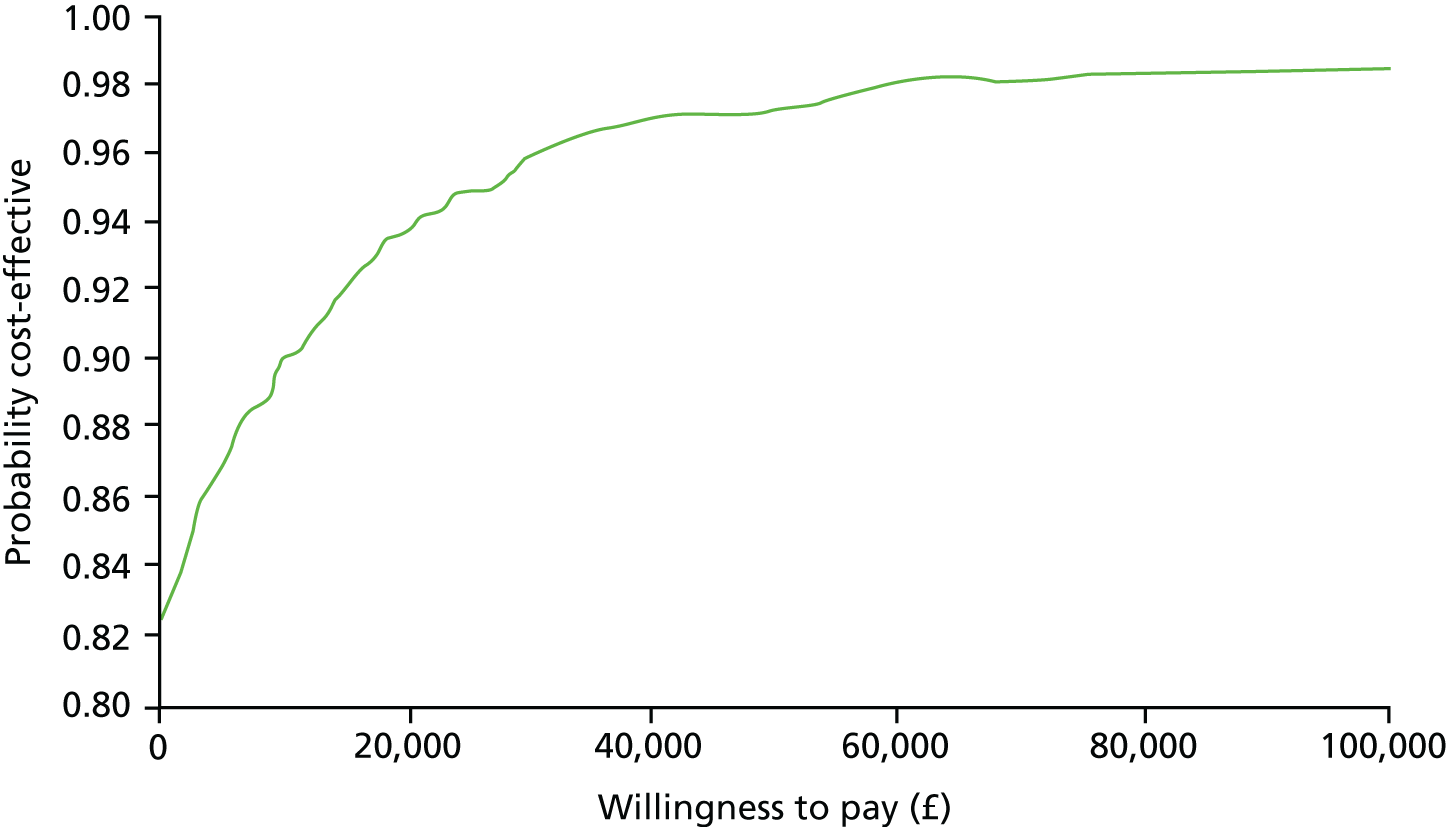
| Levela | Mobility, n (%) | Self-care, n (%) | Usual activities, n (%) | Pain/discomfort, n (%) | Anxiety/depression, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | |
| 1 | 70 (70.71) | 62 (74.70) | 89 (89.90) | 82 (98.80) | 50 (49.50) | 51 (63.75) | 42 (41.58) | 37 (45.68) | 38 (37.62) | 35 (42.68) |
| 2 | 15 (15.15) | 11 (13.25) | 7 (7.07) | 1 (1.20) | 29 (28.71) | 13 (16.25) | 34 (33.66) | 23 (28.40) | 47 (46.53) | 36 (43.90) |
| 3 | 8 (8.08) | 8 (9.64) | 3 (3.03) | 0 (0) | 16 (15.84) | 13 (16.25) | 20 (19.80) | 15 (18.52) | 12 (11.88) | 10 (12.20) |
| 4 | 6 (6.06) | 2 (2.41) | 0 (0) | 0 (0) | 3 (2.97) | 2 (2.5) | 5 (4.95) | 4 (4.94) | 3 (2.97) | 1 (1.22) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1.98) | 1 (1.25) | 0 (0) | 2 (2.47) | 1 (0.99) | 0 (0) |
| Total (n) | 99 | 83 | 99 | 83 | 100 | 80 | 101 | 81 | 101 | 82 |
| Carers reporting some problems (%) | 29.29 | 25.30 | 10.10 | 1.20 | 50.00 | 36.25 | 58.42 | 54.32 | 62.38 | 57.32 |
| Levela | Mobility, n (%) | Self-care, n (%) | Usual activities, n (%) | Pain/discomfort, n (%) | Anxiety/depression, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | |
| 1 | 55 (59.78) | 54 (73.97) | 81 (88.04) | 70 (95.89) | 42 (45.65) | 38 (52.05) | 35 (38.04) | 29 (39.73) | 38 (41.30) | 35 (47.95) |
| 2 | 19 (20.65) | 11 (15.07) | 8 (8.70) | 2 (2.74) | 25 (27.17) | 16 (21.92) | 31 (33.70) | 25 (34.25) | 32 (34.78) | 25 (34.25) |
| 3 | 14 (15.22) | 7 (9.59) | 3 (3.26) | 1 (1.37) | 19 (20.65) | 11 (15.07) | 18 (19.57) | 15 (20.55) | 19 (20.65) | 11 (15.07) |
| 4 | 4 (4.35) | 1 (1.37) | 0 (0) | 0 (0) | 5 (5.43) | 7 (9.59) | 7 (97.61) | 4 (5.48) | 2 (2.17) | 2 (2.74) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.09) | 1 (1.37) | 1 (1.09) | 0 (0) | 1 (1.09) | 0 (0) |
| Total (n) | 92 | 73 | 92 | 73 | 92 | 73 | 92 | 73 | 92 | 73 |
| Carers reporting some problems (%) | 40.22 | 26.03 | 11.96 | 4.11 | 54.35 | 47.95 | 61.96 | 60.27 | 58.70 | 52.05 |
| Levela | Mobility, n (%) | Self-care, n (%) | Usual activities, n (%) | Pain/discomfort, n (%) | Anxiety/depression, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | |
| 1 | 54 (65.85) | 48 (68.57) | 73 (89.02) | 67 (95.71) | 47 (57.32) | 38 (54.29) | 35 (42.68) | 27 (39.13) | 42 (51.22) | 33 (48.53) |
| 2 | 14 (17.07) | 10 (14.29) | 5 (6.10) | 1 (1.43) | 15 (18.29) | 13 (18.57) | 27 (32.93) | 21 (30.43) | 25 (30.49) | 23 (33.82) |
| 3 | 11 (13.41) | 9 (12.86) | 4 (4.88) | 2 (2.86) | 15 (18.29) | 13 (18.57) | 15 (18.29) | 16 (23.19) | 12 (14.63) | 10 (14.71) |
| 4 | 3 (3.66) | 3 (4.29) | 0 (0) | 0 (0) | 4 (4.88) | 3 (4.29) | 4 (4.88) | 5 (7.25) | 2 (2.44) | 2 (2.94) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.22) | 3 (4.29) | 1 (1.22) | 0 (0) | 1 (1.22) | 0 (0) |
| Total (n) | 82 | 70 | 82 | 70 | 82 | 70 | 82 | 69 | 82 | 68 |
| Carers reporting some problems (%) | 34.15 | 31.43 | 10.98 | 4.29 | 42.68 | 45.71 | 57.32 | 60.87 | 48.78 | 51.47 |
| Levela | Mobility, n (%) | Self-care, n (%) | Usual activities, n (%) | Pain/discomfort, n (%) | Anxiety/depression, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | |
| 1 | 60 (21.2) | 54 (18.9) | 135 (47.7) | 126 (44.1) | 39 (13.8) | 40 (14) | 113 (39.9) | 114 (39.9) | 123 (43.5) | 127 (44.4) |
| 2 | 91 (32.2) | 82 (28.7) | 76 (26.9) | 82 (28.7) | 89 (31.4) | 80 (28) | 88 (31.1) | 74 (25.9) | 96 (33.9) | 99 (34.6) |
| 3 | 102 (36) | 107 (37.4) | 54 (19.1) | 60 (21) | 82 (29) | 88 (30.8) | 59 (20.8) | 74 (25.9) | 49 (17.3) | 45 (15.7) |
| 4 | 20 (7.1) | 37 (12.9) | 12 (4.2) | 10 (3.5) | 32 (11.3) | 37 (12.9) | 20 (7.1) | 22 (7.7) | 6 (2.1) | 14 (4.9) |
| 5 | 10 (3.5) | 6 (2.1) | 6 (2.1) | 8 (2.8) | 41 (14.5) | 41 (14.3) | 3 (1.1) | 2 (0.7) | 9 (3.2) | 1 (0.3) |
| Total (n) | 283 | 286 | 283 | 286 | 283 | 286 | 283 | 286 | 283 | 286 |
| Patients reporting some problems (%) | 78.8 | 81.1 | 52.3 | 55.9 | 86.2 | 86 | 60.1 | 60.1 | 56.5 | 55.6 |
| Levela | Mobility, n (%) | Self-care, n (%) | Usual activities, n (%) | Pain/discomfort, n (%) | Anxiety/depression, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | |
| 1 | 55 (23) | 55 (22.3) | 116 (48.5) | 106 (43.1) | 53 (22.2) | 50 (20.2) | 78 (32.8) | 62 (25.1) | 107 (44.8) | 93 (37.7) |
| 2 | 82 (34.3) | 66 (26.7) | 63 (26.4) | 61 (24.8) | 82 (34.3) | 59 (23.9) | 72 (30.3) | 81 (32.8) | 78 (32.6) | 90 (36.4) |
| 3 | 63 (26.4) | 79 (32) | 38 (15.9) | 50 (20.3) | 54 (22.6) | 69 (27.9) | 60 (25.2) | 75 (30.4) | 40 (16.7) | 46 (18.6) |
| 4 | 32 (13.4) | 44 (17.8) | 13 (5.4) | 18 (7.3) | 28 (11.7) | 33 (13.4) | 27 (11.3) | 23 (9.3) | 10 (4.2) | 14 (5.7) |
| 5 | 7 (2.9) | 3 (1.2) | 9 (3.8) | 11 (4.5) | 22 (9.2) | 36 (14.6) | 1 (0.4) | 6 (2.4) | 4 (1.7) | 4 (1.6) |
| Total (n) | 239 | 247 | 239 | 246 | 239 | 247 | 238 | 247 | 239 | 247 |
| Patients reporting some problems (%) | 77 | 77.7 | 51.5 | 56.9 | 77.8 | 79.8 | 67.2 | 74.9 | 55.2 | 62.3 |
| Levela | Mobility, n (%) | Self-care, n (%) | Usual activities, n (%) | Pain/discomfort, n (%) | Anxiety/depression, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | |
| 1 | 56 (25.6) | 43 (18.6) | 103 (47) | 102 (44.2) | 52 (23.7) | 45 (19.5) | 74 (33.8) | 54 (23.5) | 101 (46.1) | 93 (40.3) |
| 2 | 48 (21.9) | 54 (23.4) | 44 (20.1) | 44 (19.0) | 62 (28.3) | 54 (23.4) | 57 (26) | 76 (33) | 65 (29.7) | 77 (33.3) |
| 3 | 67 (30.6) | 72 (31.2) | 46 (21) | 45 (19.5) | 54 (24.7) | 68 (29.4) | 62 (28.3) | 66 (28.7) | 40 (18.3) | 51 (22.1) |
| 4 | 38 (17.4) | 52 (22.5) | 13 (5.9) | 27 (11.7) | 25 (11.4) | 27 (11.7) | 21 (9.6) | 27 (11.7) | 9 (4.1) | 5 (2.2) |
| 5 | 10 (4.6) | 10 (4.3) | 13 (5.9) | 13 (5.6) | 26 (11.9) | 37 (16.0) | 5 (2.3) | 7 (3) | 4 (1.8) | 5 (2.2) |
| Total (n) | 219 | 231 | 219 | 231 | 219 | 231 | 219 | 230 | 219 | 231 |
| Patients reporting some problems (%) | 74.4 | 81.4 | 53 | 55.8 | 76.3 | 80.5 | 66.2 | 76.5 | 53.9 | 59.7 |
| Resource | Sample at 12 months, n | Participants with at least one contact between randomisation and 12 months, n (%) | Mean number of contacts between randomisation and 12 months, median (IQR) | Sample at 24 months, n | Participants with at least one contact between 12 months and 24 months, n (%) | Mean number of contacts between 12 months and 24 months, median (IQR) |
|---|---|---|---|---|---|---|
| GP | 245 | 232 (94.69) | 7.66, 5 (7) | 229 | 214 (93.45) | 6.33, 4 (6) |
| Physiotherapist | 242 | 115 (47.52) | 8.10, 0 (6) | 227 | 52 (22.91) | 2.62, 0 (0) |
| Occupational therapist | 242 | 79 (32.64) | 2.36, 0 (2) | 228 | 24 (10.53) | 0.62, 0 (0) |
| Speech and language therapist | 244 | 46 (18.85) | 1.92, 0 (0) | 226 | 11 (4.87) | 0.23, 0 (0) |
| Community or district nurse | 242 | 68 (28.10) | 2.42, 0 (1) | 229 | 42 (18.34) | 1.79, 0 (0) |
| Health visitor | 234 | 6 (2.56) | 0.047, 0 (0) | 221 | 3 (1.36) | 0.05, 0 (0) |
| Geriatrician | 235 | 2 (0.85) | 0.021, 0 (0) | 220 | 3 (1.36) | 0.041, 0 (0) |
| Psychiatrist | 235 | 6 (2.55) | 0.089, 0 (0) | 219 | 4 (1.83) | 0.023, 0 (0) |
| Psychologist | 235 | 17 (7.23) | 0.40, 0 (0) | 222 | 8 (3.60) | 0.1, 0 (0) |
| Chiropodist | 235 | 85 (36.17) | 1.51, 0 (2) | 224 | 82 (36.61) | 1.71, 0 (3) |
| Optician | 236 | 131 (55.51) | 0.74, 1 (1) | 223 | 127 (56.95) | 0.67, 1 (1) |
| Other NHS and social care services | 244 | 45 (18.44) | NAa | 228 | 39 (17.11) | NAa |
| Residential care | 246 | 7 (2.85) | 1.3, 0 (0) | 229 | 9 (3.93) | 3.08, 0 (0) |
| Nursing home | 245 | 2 (0.82) | 0.18, 0 (0) | 229 | 1 (0.44) | 0.04, 0 (0) |
| A&E | 246 | 81(32.93) | 0.54, 0 (1) | 230 | 66 (28.70) | 0.49, 0 (0) |
| Outpatients | 248 | 175 (70.56) | 3.19, 2 (4) | 231 | 126 (54.55) | 2.27, 1 (3) |
| Meals on wheels | 245 | 2 (0.82) | 0.20, 0 (0) | 225 | 1 (0.44) | 0.44, 0 (0) |
| Home help (personal care) | 243 | 6 (2.47) | 1.19, 0 (0) | 225 | 4 (1.78) | 9.65, 0 (0) |
| Home help (household) | 244 | 4 (1.64) | 0.75, 0 (0) | 224 | 5 (2.23) | 2.33, 0 (0) |
| Home help (shopping) | 244 | 2 (0.82) | 0.29, 0 (0) | 222 | 2 (0.90) | 0.49, 0 (0) |
| Receiving health benefits | 245 | 132 (53.88) | NAa | 228 | 132 (57.89) | NAa |
| Inpatient | 247 | 62 (25.10) | NAa | 230 | 49 (21.30) | NAa |
| Day patient | 246 | 40 (16.26) | NAa | 229 | 32 (13.97) | NAa |
| Resource | Sample at 12 months, n | Participants with at least one contact between randomisation and 12 months, n (%) | Mean number of contacts between randomisation and 12 months, median (IQR) | Sample at 24 months, n | Participants with at least one contact between 12 months and 24 months, n (%) | Mean number of contacts between 12 months and 24 months, median (IQR) |
|---|---|---|---|---|---|---|
| GP | 237 | 227 (95.78) | 7.46, 5 (7) | 217 | 205 (94.47) | 6.6, 5 (7) |
| Physiotherapist | 236 | 111 (47.03) | 7, 0 (7) | 215 | 56 (26.05) | 2.32, 0 (10) |
| Occupational therapist | 236 | 72 (30.51) | 3.4, 0 (2) | 214 | 30 (14.02) | 0.58, 0 (0) |
| Speech and language therapist | 235 | 38 (16.17) | 0.87, 0 (0) | 214 | 11 (5.14) | 0.43, 0 (0) |
| Community or district nurse | 231 | 54 (23.38) | 5.4, 0 (0) | 215 | 38 (17.67) | 3.38, 0 (0) |
| Health visitor | 226 | 4 (1.77) | 0.03, 0 (0) | 209 | 3 (1.44) | 0.02, 0 (0) |
| Geriatrician | 225 | 1 (0.44) | 0.02, 0 (0) | 210 | 2 (0.95) | 0.03, 0 (0) |
| Psychiatrist | 225 | 6 (2.67) | 0.08, 0 (0) | 210 | 4 (1.90) | 0.03, 0 (0) |
| Psychologist | 226 | 16 (7.08) | 0.32, 0 (0) | 210 | 7 (3.33) | 0.05, 0 (0) |
| Chiropodist | 179 | 62 (34.64) | 1.03, 0 (1) | 212 | 70 (33.02) | 1.55, 0 (2.55) |
| Optician | 230 | 127 (55.22) | 0.99, 1 (1) | 213 | 113 (53.05) | 0.76, 1 (1) |
| Other NHS and social care services | 235 | 42 (17.87) | NAa | 215 | 37 (17.21) | NAa |
| Residential care | 235 | 8 (3.40) | 3.56, 0 (0) | 217 | 6 (2.76) | 5.53, 0 (0) |
| Nursing home | 235 | 4 (1.70) | 2.5, 0 (0) | 217 | 4 (1.84) | 1.32, 0 (0) |
| A&E | 237 | 78 (32.91) | 0.5, 0 (1) | 217 | 59 (27.19) | 0.42, 0 (1) |
| Outpatients | 239 | 172 (71.97) | 3.1, 2 (4) | 219 | 138 (63.01) | 2.5, 1 (3) |
| Meals on wheels | 236 | 2 (0.85) | 1.56, 0 (0) | 211 | 1 (0.47) | 0.005, 0 (0) |
| Home help (personal care) | 236 | 5 (2.12) | 0.63, 0 (0) | 210 | 3 (1.43) | 1.15, 0 (0) |
| Home help (household) | 236 | 0 (0) | 0, 0 (0) | 209 | 0 (0) | 0, 0 (0) |
| Home help (shopping) | 236 | 0 (0) | 0, 0 (0) | 208 | 0 (0) | 0, 0 (0) |
| Receiving health benefits | 238 | 118 (49.58) | NAa | 228 | 132 (57.89) | NAa |
| Inpatient | 239 | 61 (25.52) | NAa | 218 | 46 (21.10) | NAa |
| Day patient | 239 | 48 (20.08) | NAa | 217 | 46 (21.20) | NAa |
List of abbreviations
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CSI
- Caregiver Strain Index
- CSRI
- Client Service Receipt Inventory
- DMC
- Data Monitoring Committee
- EADL
- extended activities of daily living
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ESD
- early supported discharge
- EXTRAS
- extended stroke rehabilitation service
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- MDT
- multidisciplinary team
- NEADL
- Nottingham Extended Activities of Daily Living
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NIHSS
- National Institutes of Health Stroke Scale
- NPT
- normalisation process theory
- OHS
- Oxford Handicap Scale
- OR
- odds ratio
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RA
- research assistant
- SAE
- serious adverse event
- SD
- standard deviation
- SLT
- speech and language therapist
- SMD
- standardised mean difference
- TIDieR
- Template for Intervention Description and Replication
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- WTP
- willingness to pay
