Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/10/37. The contractual start date was in November 2016. The draft report began editorial review in October 2019 and was accepted for publication in March 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Anthony Byrne reports grants from Marie Curie (London, UK), Health and Care Research Wales, the End of Life Board for Wales and the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme outside the submitted work; he is also a member of the End of Life Board for Wales, which is responsible to the Welsh Government for developing and implementing strategy for end-of-life care in Wales. Bee Wee reports that she is National Clinical Director for End of Life Care, Chairperson of the National Institute for Health and Care Excellence Quality Standards Advisory Committee and has a NIHR-funded grant outside the submitted work. She has also received royalties for a book published by Oxford University Press (Oxford, UK). Dyfrig Hughes reports that he was a member of the HTA Programme Pharmaceuticals Panel (2008–12) and member of the HTA Programme Clinical Evaluation and Trials Board (2010–16). Marlise Poolman was a member of the HTA Prioritisation Committee: Integrated Community Health and Social Care (A) from 2013 to 2019. Zoe Hoare reports that she is an associate member of NIHR Health Services and Delivery Research board. Clare Wilkinson reports that she was chairperson of the HTA Commissioning Panel – Primary Care, Community, Preventive Interventions (2013–18) and a member of the HTA Rapid and Add-0n Trials Board (2012–13).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Poolman et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Caring for people who are dying during their last few days of life in a place of their preference is an essential part of health and social care. The majority of people who are dying express a wish to die at home (79%); however, only half of those achieve this. 1 The likelihood of patients remaining at home often depends on the availability of able and willing informal carers. 2–4 These carers take on numerous care tasks, including the responsibility of assisting patients to have their oral as-needed medications. Extending the role of carers to include administering subcutaneous (SC) medication has proven to be key to achieving home death in other countries. 5
Pain, nausea/vomiting, restlessness/agitation and noisy breathing (rattle) are common symptoms in people who are dying. 6,7 In addition to regular (background) medication, which is given via continuous SC infusion using a syringe pump, guidelines suggest using additional (‘as-needed’) medication for symptoms that ‘break through’. 8,9 As dying patients are commonly unable to take oral medication, as-needed medication is most often given as a SC injection by a health-care professional (HCP),8 in the UK usually a district nurse (DN).
Medication for breakthrough symptoms is commonly prescribed in advance (anticipatory prescribing) and kept in the patient’s home. Medication administration can be severely delayed by the HCP’s travel time to the patient’s home and/or the non-availability of anticipatory medication in the home. Delays happen even with dedicated out-of-hours (OOH) ‘rapid response’ nursing services for patients dying at home. Our local audit revealed long waiting times. The median waiting time from the call to OOH services for symptom control to the administration of as-needed medication by a HCP was 86 minutes (range 35–167 minutes), not including the time from administration to the onset of action or symptom control. Breakthrough pain is usually difficult to predict; it is quick in onset and has a median duration of 30 minutes. 10 Long waits mean that pain is often not adequately managed in the home setting, as shown in the National Survey of Bereaved People (VOICES). 1
The CARiAD trial is about exploring and developing:
-
a legal practice in the UK of carer administration of injectable medication (including strong analgesia) to patients who are unable to take oral medication and may not be able to make decisions for themselves (see Appendix 1)
-
a practice that is not yet routine in the UK and needs careful testing to see whether or not the carer role can routinely be extended to include training in the administration of SC medication to a dying patient who is unable to swallow their usual medication.
The CARiAD trial is not about:
-
control of background symptoms that are usually managed by a continuous SC infusion when a patient becomes unable to swallow their usual medication
-
pressurising carers to take on the extended role if that is not right for either the carer or their loved one
-
hastening death or replacing best-quality palliative care from HCPs.
This study focuses on timely administration of as-needed medication for dying patients who are being cared for at home, in particular whether or not lay carer role extension (to be trained to give as-needed SC injections) is feasible and acceptable in the UK.
Rationale
Although carer administration of injectable medication (including strong opioids) is lawful and practical,1 it is not currently part of usual care everywhere in the UK. The national Palliative and End of Life Care Priority Setting Partnership (PeolcPSP) surveyed 1403 people, including patients in the last years of their life, current and bereaved carers and HCPs, on their unanswered questions about palliative and end-of-life care. They accorded highest priority to research into the provision of palliative care, including symptom management outside working hours to avoid crises and help patients to stay in their place of choice. The survey noted the information and training needs of carers and families to provide the best care for their loved one who was dying, including training for administering medicines at home. 11 Data from the PeolcPSP indicated that UK patients may be denied the opportunity to die at home owing to the lack of access to adequate symptom relief. 12,13
Carers across the world embrace this as an option, as evidenced through the published literature. In Australia, the practice is well established (> 30 years) and highly acceptable. 5 A manualised educational package and evidence-based guidelines are available. 14
This practice appears acceptable and has become embedded in Queensland, Australia. However, as per our rapid review (unpublished),5,15–22 to our knowledge there have been no randomised studies testing the clinical effectiveness and cost-effectiveness of carer-administered non-oral medication in the last days of life for patients dying at home anywhere in the world.
Equipoise is emerging on this topic in the UK. Carer administration of as-needed non-oral (including SC) medication for breakthrough symptoms in patients dying at home is practised in a limited way in some areas in the UK, and has been for a number of years. For this to be widely available to more carers who are considering supporting a loved one at home, testing was required in a UK environment with the support of an evidence-based carer education programme and resources. Not all family, carers or patients at home will want to be involved in this practice. This research will help to ascertain the proportion of those who are interested and how to train and support those who are willing to be involved.
How does the existing literature support this study?
Carers prioritise rapid symptom control and are willing and able to administer injectable drugs, including controlled drugs such as morphine: a narrative literature review of family carer perspectives on supporting a dying person at home illustrated the desire of families to be able to provide immediate symptom relief. 23 Our review found that caregivers are willing to learn to overcome their reservations about administering SC medications. 5,15–22 The ability to alleviate their loved one’s symptoms and support them to stay at home was paramount.
There is an existing evidence-based education package and medication resources; a Brisbane group developed and evaluated an educational package and a randomised trial was completed of who prepares the SC injections (carer, nurse or pharmacist). 5,14,24 In Singapore, a colour-coded ‘Comfort Care Kit’ is in use,25 with oral and non-oral as-needed medication provided for caregiver administration. A telephone survey of 49 family carers showed that 67% of them used this kit, all family members found it easy to use and 98% of them found it effective for symptom management. All except one patient died at home. In Canberra, the provision of an emergency medical kit (including for use by lay carers) was largely viewed as an effective strategy to achieve timely symptom control and to prevent inpatient admissions. 19
There is growing UK evidence around the carer role for patients who are in the last months/year of their life, but few studies focus on the last days of life (as reiterated by the Neuberger Review into the Liverpool Care Pathway26). The evidence that is beginning to accumulate mostly focuses on patients who have capacity in the last year of their life. UK and Australian research includes ‘Unpacking the home’,27,28 the Cancer Carers Medication Management work,29,30 the SMART (Self-Management of Analgesia and Related Treatments) study31 and ImPaCCT (Improving Palliative Care through Clinical Trials). 32 Our study, by contrast, focuses on the last days of life at home, when the individual’s capacity is likely to be limited or absent, with very different implications and issues for carer administration.
Community receptivity
The UK was ready for testing this extended lay carer role:
-
Primary care teams and families are used to similar practices in other areas of medicine (e.g. insulin for diabetes mellitus and intravenous antibiotics for children with cystic fibrosis).
-
The PeolcPSP report incorporated the views of 1403 people across the UK and placed great emphasis on empowerment of family carers and symptom management during the last days of life. 11
-
Ambitions for Palliative and End of Life Care: A Framework for Local Action was published in September 2015. 33 It was jointly developed and published by the National Partnership for Palliative and End of Life Care (27 national organisations) and has widespread support in the UK, especially as the partnership included the Patients’ Association and charities with large public contributor groups. They identified eight foundations for their six ambitions; one of these foundations relates to ‘Involving, supporting and caring for those important to the dying person’, acknowledging the importance of lay carers in the caring team. Each ambition has a set of building blocks; the building block of ‘practical support’ in ambition 6 is particularly applicable to the CARiAD trial as it calls for ‘new ways to give the practical support, information and training that enables families . . . to help’. There has been a strong positive response to the framework, and many localities are using it to consider their local strategies. The message about shared ownership and responsibility is particularly pertinent.
-
In the UK, this practice is not widely accepted as usual care; however, over the past few years, we have identified a small number of geographically distinct sites where the practice occurs (< 10 sites). Recently, the Lincolnshire project was showcased on national radio as part of a series of talks on dying. 21,34,35 Since the conception of the CARiAD trial, at least seven other areas have, unsolicited, expressed interest in exploring the practice, including joining as potential recruitment sites in a future definitive trial.
Pressure on health and care services in the UK
Health-care professionals in all three sites were universally positive about testing the intervention; if found to be beneficial, it could make their patients more comfortable and their jobs more manageable. In the longer term, this innovation could relieve some pressure on emergency departments by reducing inappropriate emergency (crisis) admissions due to uncontrolled symptoms. 36,37
Pressure on DN time could also be relieved as extra visits (in addition to the daily check) to administer as-needed medication would decrease, which would contribute to the sustainability of services.
Choice of design
Our team was aware of the challenges that are associated with research in the last days to weeks of life in general, including recruitment and ethics considerations. Although we recognised the benefits of conducting an internal pilot trial with progression rules to a full trial, we felt that an external pilot and feasibility study was more appropriate. Regarding recruitment, an external pilot trial required three sites recruiting 50 patients. A full trial would require at least 30 sites recruiting 520 patients. Although our three sites were confident about recruitment, and a number of other areas had already expressed interest in participating in a future trial, we could not disregard the effect of the other complex factors affecting a broader roll-out.
These specific additional considerations added to the decision to propose a stand-alone (external) pilot trial:
-
The current UK context (post Shipman,38 post Liverpool Care Pathway26 and with the ongoing euthanasia public debate) – this called for careful attention to its affect on consent mechanisms and attitudes of carers, patients and clinicians to this innovation.
-
The lack of clear UK-wide guidance on carer administration of as-needed SC medication to patients dying at home – the practice is legal but current guidance was not detailed or specific enough for wide adoption (see Appendix 1).
-
The lack of a clear and widely accepted training package for lay carers, adapted for the UK context.
-
The uncertainty about the primary outcome measure for a definitive trial.
These were unpredictable barriers until we began to introduce the reworked Australian manualised intervention and tested the trial processes. If the intervention was proven to be feasible and acceptable, we anticipated a phase of ensuring that new guidance is developed and put in place at national level in UK health systems to enable the practice, prior to rolling out a full trial.
We aimed to demonstrate a clear path towards a definitive randomised controlled trial (RCT), as per Medical Research Council Framework, for the evaluation of complex intervention principles, further informed by the MORECare guidance developed for palliative care research. 39,40
Aims and objectives
The research question was ‘is carer administration of as-needed SC medication for common breakthrough symptoms in patients dying at home feasible and acceptable in the UK, and is it feasible to test this intervention in a future definitive randomised controlled trial?’
-
P (Patient) = patients in the last days of their life who are becoming unable to take their usual oral as-needed medication for breakthrough symptoms and are being cared for at home, and their carers.
-
I (Intervention) = carer administration of as-needed SC medication for common breakthrough symptoms, such as pain, restlessness/agitation, nausea/vomiting, and noisy breathing/rattle, supported by tailored education.
-
C (Control) = usual care (HCP administration of as-needed SC medication).
-
O (Outcome) = main outcomes of interest – feasibility and acceptability, recruitment, attrition and contamination.
To inform the design of a Phase III trial we aimed to:
-
Tailor a successful Australian intervention as a standardised, manualised intervention for UK carer administration of as-needed SC medication for breakthrough symptoms in patients dying at home.
-
Establish the feasibility of this standardised manualised package and carer role extension by assessing acceptability, ability to recruit, attrition rates and suitability to UK context. This will be carried out by conducting an external randomised pilot trial with an embedded qualitative component.
-
Identify attributes pertinent to carers’ preferences for HCP versus own administration of as-needed SC medications for patients dying at home (as part of the qualitative component) and to establish the feasibility of completion of the Carer Experience Scale (CES) (assessed in the pilot trial).
The aim of the public contribution was to:
-
conduct the study and be informed and influenced by the lived experience of bereaved carers
-
facilitate meaningful involvement of patient representatives
-
provide an exemplar of a highly integrated and proactive public contributor role in care of the dying trials.
Chapter 2 Developing the intervention
Manualised education package
Introduction
From discussion with our Australian co-investigators, we developed a good understanding of the Caring Safely at Home resources, which consist of carer and HCP resources. When the CARiAD study commenced, carer resources that were part of the Caring@Home study comprised:
-
Illustrated step-by-step guides that provided a simple structured approach to the preparation and administration of SC injections –
-
opening and drawing up from an ampoule
-
10-step plan – blunt needle technique
-
10-step plan – no-needle technique
-
injection via cannula – blunt needle technique
-
injection via cannula – no-needle technique.
-
-
A practice demonstration kit, including a cannula and other equipment that are required for the preparation and delivery of SC injections.
-
Colour-coded medication labels for labelling prepared syringes.
-
A fridge magnet that was colour coded to help the clinician or caregiver match the relevant medication with the patient’s symptoms.
-
A daily diary to document aspects of medication administration.
-
A series of videos, ‘Palliative subcutaneous medication administration: a guide for carers’, that demonstrated aspects of SC medication preparation and administration.
-
A booklet, ‘Subcutaneous medication and palliative care: a guide for caregivers’, that covered topics including frequently asked questions, the importance of symptom control, management of common palliative symptoms, commonly used SC medications and injecting processes.
The HCP resources comprised:
-
the Caring Safely at Home Standardised Education Framework
-
detailed instruction guidelines for the Caring Safely at Home resources and education framework
-
a competency checklist that provides the clinician with a mechanism to ensure that the caregiver is competent to safely inject SC medications
-
a clinician lanyard that provides a useful overview of the educational framework and the colour-coding scheme utilised for the medications.
The Queensland materials continue to be freely available on the project website (www.caringathomeproject.com.au/; accessed 5 January 2020) and are updated frequently (most recent update 12 June 2019). The materials are now available for use in all Australian jurisdictions.
In this chapter, we describe the development of the manualised training package that was used in the CARiAD trial. It does not cover all of the supporting trial documents, for example the participant information sheets (PISs), screening log or daily DN checklist.
Methods
We developed the manualised training package materials in three distinct stages:
-
clarifying focus and scope
-
developing the content
-
deciding the design, colour scheme and layout.
The final materials would be used in the trial and feedback would be obtained on their utility, acceptability and feasibility via qualitative interviews and informal feedback processes.
A more detailed description of each of the three stages is as follows:
-
Clarifying focus and scope.
After detailed review of the suite of Australian materials (including any updates during the time the CARiAD trial materials were developed), and after accounting for practice differences between Australia and the UK, the materials were adapted for a UK trial setting. All references to the Australian practice of drawing as-needed medication up in advance, labelling with colour-coded labels and storing in the fridge (with supporting aide memoires such as fridge magnets and lanyards) were removed. All references to the Queensland-wide framework Guidelines for the Handling of Medication in Community-Based Palliative Care Services in Queensland (March 2015) were removed. Wording was scrutinised and, if needed, aligned with the UK practice of carer administration being tested in the trial. Details in the Australian framework pertinent to the CARiAD trial were included in the trial protocol.
Permission was granted by our Australian co-applicants for us to use the graphics in their materials as appropriate.
The Quality of Life in Life-Threatening Illness--Family Carer Version (QOLLTI-F) questionnaire was added to the diary, to be completed every 48 hours following the first administration of SC as-needed medication (see Appendix 2). 41
-
Developing the content.
This stage required detailed, word-for-word consideration of the Australian materials and adjustments as appropriate. In the first instance this was carried out by the core CARiAD team, who also cross-checked the content against other available documents (specifically the Lincolnshire policy and supporting documentation and the risk assessment tools for self-medicating). 35,42 This was reviewed by the co-applicants who are clinicians. As a final check prior to discussion at the expert consensus workshops, it was considered by our public contributor co-applicants. Overall, minimal adaptation was needed, including of terminology and drug names. Detail included in the Australian Standard Education Framework was incorporated into the training that HCPs received on the trial.
-
Deciding the final design, colour scheme and layout.
The final design stage was an iterative process between the core team and the design company, with invaluable input from the public contributor co-applicants, especially on colour scheme choice (distinctive enough but not intrusive, i.e. not using too strong colours and using different colours for different trial booklets to support ease of recognition for carers and HCPs) and cover graphics.
Results
The final set of documents included in the CARiAD trial manualised training pack for carers comprised:
-
‘Subcutaneous medication for breakthrough symptoms in the last days of life: a guide for carers’ (see Report Supplementary Material 1).
This was adapted from the booklet ‘Subcutaneous medication and palliative care: a guide for caregivers’ and covered similar topic areas. In addition, it included information on how to know which medication to give, how long to wait for medication to work, how often to give medication, which symptoms medication can be given for in the trial, how many doses could be given, what to do if too much medication is given and what to do if the carer is not available to give medications. A section was also included broaching the concept of the ‘last injection’. After careful discussion with our public contributor co-applicants, the following paragraph was added –
There may come a time when you are giving the injections when the person you are caring for is very ill and will soon die. This might mean that the time when they die is near to when you have last given them medication. It is very important for you to know that these two things are not related and the medication has not ended their life. The nurse training you to give injections will discuss any worries or concerns you may have about this.
Rather than leaving it to individual clinicians to find their own way of explaining this, the text was intended to provide a script on which the HCP’s explanation to the carer could be based.
-
A carer diary (intervention or usual care), which was A5 size (see Report Supplementary Material 1). This was divided into the following sections –
-
introduction, explaining provenance and focus
-
contact details of the teams involved in the dying person’s care and space to record medical appointments
-
instructions for use of the diary when recording symptom management
-
information on the prescribed as-needed medication (intervention group diary only)
-
record of symptoms and subsequent management (one page per entry, 15 entries per booklet)
-
QOLLTI-F questionnaire (three copies per diary, additional diaries provided by DN teams as required).
-
Changes to the carer diary included assigning a full A5 page to each administration entry (rather than five administration entries per page, as in the Australian carer diary) and amending the medication information table to align with commonly used medication in the UK. The context of a RCT necessitated the need for carer diaries with different content for the two trial groups; therefore, two versions of the diary were produced.
-
Step-by-step guides with images illustrating the required actions (see Report Supplementary Material 1) –
-
step-by-step guide to opening and drawing-up medications from an ampoule
-
10-step plan for preparing and giving as-needed SC injections using a blunt needle technique
-
10-step plan for preparing and giving as-needed SC injections using a no-needle technique.
-
An injection training pack was also included so that the carer could practise giving needle-less injections (i.e. via a cannula). We listed the suggested content to be assembled by the HCP from their stocks (see Report Supplementary Material 1). Given that ampoule openers are not in widespread use, these were provided to the HCPs for inclusion in the training packs.
In relation to carer resources, we were advised that the video resources were not often used and that carers preferred the printed resources; therefore, video resources were not developed.
More extensive changes were required to the HCP resources, as it was outside the scope of the trial to generate a standardised education framework for a UK context. The framework was therefore removed from the HCP resources along with the detailed instruction guidelines, as HCPs delivering the intervention would be comprehensively trained by the core CARiAD trial team. Additional resources were developed to support HCPs and ensure the safety of patients and carers (see Report Supplementary Material 2) and comprised:
-
risk assessment (RA) tool
-
information for prescribers (intervention group only)
-
daily checklist for DNs – this document was an aide memoire of all the trial-related tasks to be carried out at DN visits
-
adverse event (AE) record – to be kept in the patient’s notes and returned to the trial team at the end of the study. It defined AEs, serious adverse events (SAEs) and related events, included a table to record AEs and provided a prompt of the circumstances in which a separate SAE form should be recorded.
The competency checklist was amended to include the following questions about the nominated carer:
-
Is aware of the symptoms of pain, restlessness/anxiety, nausea/vomiting and noisy breathing and which medications to use for each of these symptoms?
-
Can reconstitute drugs if required?
-
Understands the importance of completing all associated study paperwork?
-
Is aware of how many as-needed doses can be administered of each drug in 24 hours?
-
Understands the importance of contacting the health-care team immediately if an error is made with medications or unusual symptoms develop?
The date, signature and name of the HCP were also included in the competency checklist. In addition, two columns were added so that ongoing checks of competency could be recorded if needed.
The intervention under scrutiny in the CARiAD trial was carer administration of as-needed SC medication and, for this reason, the training to enable carers in this practice was key. The printed documents described supported carer training and, therefore, there was ongoing review of these materials throughout the trial, resulting in minor changes.
Expert consensus work
Introduction
The aim of the expert consensus workshops was to refine trial processes, guide the adaptation of the existing Australian training materials for use in a UK context and inform the development of other supporting documents. The chosen format was based on the successful model used in the Early Lung Cancer Identification and Diagnosis (ELCID) trial. 43
Methods
Two workshops were planned with invited UK experts: one in North Wales and one in the southern part of the UK (either south Wales or Gloucestershire). The invited experts from the three recruitment sites were to include public contributors, primary care clinicians [general practitioners (GPs) and DNs], specialist palliative care (SPC) clinicians, research nurses (RNs), pharmacists and representatives of other support services [including hospice at home and Marie Curie (London, UK)]. Workshops were facilitated by two members of the core CARiAD trial team, one of whom made notes throughout the workshop. Workshop attendees were given background to the trial, including detail on how the question for the research was developed and the existing Australian training package that was to be adapted for the CARiAD trial. The facilitators then led discussions on the following areas:
-
identifying and approaching participants
-
consent processes
-
prescription, supply and storage of drugs
-
delivery of the intervention
-
monitoring and accountability
-
follow-up measures
-
post-bereavement interviews
-
ethics concerns
-
resource pack.
Attendees were given the opportunity to freely discuss, ask for clarification on or raise any queries or concerns about these areas. In addition, the core CARiAD team raised specific questions that required input.
Workshops were audio-recorded and a summary of discussions and resultant actions from each workshop was produced.
Results
Following the first workshop in North Wales, the decision was taken to have two separate workshops in the southern part of the UK (i.e. for both south Wales and Gloucestershire recruitment sites), as it became apparent that the specific details of the local ways of working would be vital in understanding how to deliver the trial intervention. Discussions at each workshop built on and refined those from previous workshops, interpreting them in a local context. There were 10–12 attendees at each of the three workshops, with at least one representative of each of the invited groups present at each meeting. A co-chief investigator for the study (MP) facilitated two of the workshops, supported by the trial manager (JR). The third workshop was facilitated by the principal investigator (PI) for the site (AB) and the qualitative lead for the study (AN).
Identifying and approaching participants
All sites agreed that specific DN teams who were deemed to be stable and had the capacity to take on research should be chosen to take part and be responsible for identifying eligible patient/carer dyads. This decision was to be made with the input of the local DN matrons or equivalent. HCPs who were linked with these teams were to be made aware of the study and that the named DN team was involved. They had to have knowledge of the RA tool (see Report Supplementary Material 2) and would be asked to flag potential patients to the DN team. No concerns were raised about difficulties in identifying dyads suitable for the CARiAD trial by the DN teams, and discussion focused on the practicalities of how, when and where patients and their carers should be approached.
There was considerable discussion around the content of the RA tool. It was noted that there was no need for the RA tool to exclude patients who were known to have human immunodeficiency virus (HIV) or hepatitis as, first, universal precautions should be taken and, second, there was no risk of needle-stick injury as needles would not come into contact with the patient’s blood owing to the no-needle administration technique. It was also suggested that RA using the RA tool should be ongoing to account for changes in circumstances. Other factors discussed included how to include GPs in the RA process, what to do if there was more than one carer, whether or not a question about social support for the carer was appropriate, the inclusion of a free-text box for additional issues and the need for a question about a suitable environment for drug storage.
Consent processes
The benefit of research staff being responsible for the consent process was discussed, as they have experience in explaining trial-specific procedures, such as equipoise and randomisation, to ensure that consent was fully informed and that dyads were clear that agreeing to take part did not guarantee that they would be able to administer as-needed SC medications.
Attendees were asked to consider the balance between the benefits of a RN obtaining informed consent and the potential burden caused by introducing a new colleague (i.e. the RN) to a dyad during such a sensitive time. Public contributors suggested that an acceptable course of action was that, first, the DNs introduced the concept of the study and gave the information sheets and, second, if the dyad was interested in participating, the DN could attend a joint visit with the RN to introduce the RN to the dyad. During subsequent discussion in Gloucestershire, it was suggested that a joint visit might not be necessary and that it would be appropriate for the DNs to ask the dyad if they would be happy for a RN to visit independently.
Prescription, supply and storage of drugs
The legal responsibilities of the prescribers was discussed. If all usual prescribing procedures are followed correctly, training to competency of carers evidenced by the DNs and the delegation of administration duties clearly demonstrated, the legal responsibilities for all HCPs involved remain the same as in usual care. However, as the anticipatory medications prescribed for patients in the intervention group may be administered by a carer rather than a HCP, it was felt that all GPs (the usual prescribers) in the area covered by the DN teams involved in identifying potential participants for CARiAD would need to agree in principle to their patients being approached. Potential other prescribers who should be informed of the study were also identified across the three sites; these included advanced nurse practitioners, DNs, pharmacists and SPC nurses.
The CARiAD team raised the issue of reconstitution of drugs for discussion, as diamorphine is a commonly used drug and needs reconstitution (morphine does not need reconstitution). The workshop attendees confirmed that morphine is used as first-line SC opioid and the use of diamorphine would be limited to a small percentage of cases in which exceptions apply. In North Wales, this was supported by a recent change in the local health board’s official prescribing policy. Therefore, it was felt that these small number of patients should be dealt with on a case-by-case basis, with the DNs liaising with the prescriber to negotiate the use of morphine as an alternative and being able to train carers to reconstitute diamorphine when needed.
It was also noted that because all drugs would be in glass ampoules, blunt filter needles should be used for drawing up medications and it was agreed that a reusable ampoule opener should be provided in the injection training kit, alongside the step-by-step guide to opening an ampoule, to minimise the risk of injury.
Delivery of the intervention
No major concerns were raised about the delivery of the intervention; however, there were certain factors that attendees felt that the research team needed to take into consideration. These mainly centred around support for the carer, including ensuring that they are able to access as-needed support 24/7, and being mindful of carer burden.
Practical issues raised included the need for a second SC butterfly administration site and that carers would need to know how to check when the administration site is compromised. It was also noted that the use of a second SC butterfly site may not be routine in all areas, so this should be emphasised to the DN teams. Similarly, as the research team are relying on DNs to undertake daily visits to these patients, this should be checked with individual teams as this may not always be the case.
Monitoring and accountability
Practicalities around how carer administration of medications would be recorded in patient notes were discussed. It was suggested that information could be transferred to the medication administration chart by the DNs and marked as ‘carer administered’ during the daily checks. Some members felt that a copy of the RA and competency checklist should also be included in the patient notes.
Limits of how many doses of carer-administered as-needed medication that could be given in 24 hours for the same indication were discussed. The agreement was three doses to balance pragmatism and safety considerations. If a further dose of medication for the same indication was needed within 24 hours, this could not be carer administered and a review by a HCP should be sought. The frequency of carer administration of commonly used medication was discussed and agreed that they should, in general, not be given more frequently than every 4 hours. The attendees noted that failure to respond fully to medication may sometimes indicate a different reason for the symptom (e.g. pain could be caused by a new urinary retention, for which a catheter, and not increasing doses of pain relief, is needed) and that clinical review should be sought if symptoms are not settling or are persisting or increasing. This concern was carefully weighed against the need to give timely symptom relief, which is often achieved by increased doses of pain relief medication. Attendees, drawing on experiences of public contributor carers and current usual care in the community setting, agreed that a carer could be given specific permission by the prescriber to give one further dose of morphine or midazolam 1 hour after the previous administration, if required.
For safety reasons, it was felt that prescribers and OOH services should be made aware that dose steps/options should not be prescribed for patients in the intervention group and that dose changes could not be suggested to the carer over the telephone. If the as-needed dose of medication was adjusted, this had to be communicated to the clinical team and the carer. It was suggested that space should be provided in the medication table at the front of intervention carer diaries for updating dose information. A SPC pharmacist at the North Wales workshop suggested including a dose–volume column to aid carers in giving the correct dose of medication.
The patchwork of OOH services available was discussed in some detail, showing the challenges involved in ensuring that there was awareness of the CARiAD study across the board. The group advised that the CARiAD team can flag inclusion on the study to the OOH services themselves, using current mechanisms.
Follow-up measures and post-bereavement interviews
The proposed plans for conducting the post-bereavement follow-up and qualitative interview with the carer were deemed to be an acceptable balance between not overburdening the carer and being soon enough that they would still have accurate recall. Feedback from attendees suggested that earlier time points may also be acceptable. The workshops emphasised the importance of signposting to bereavement services at this visit when appropriate. It was generally felt that the best way to do this would be to signpost via the GP.
It was agreed that, ideally, the follow-up visit should be conducted by the same person who completed the baseline visit, but that if this was not possible it would be acceptable for a different person to do this. As the intention was for the baseline visit to be conducted by the RN, this would require the RN to remain blinded to the randomisation allocation. RNs attending the workshop expressed a strong preference for being unblinded, as it is usually part of the RN role to deliver the randomisation allocation news to a participant and it was felt that their knowledge of allocation would be beneficial to the management of the study processes.
Ethics concerns
No concerns were raised that posed a significant risk to the commencement of the study. For all points raised, the attendees were asked to consider if this was something that was specific to the CARiAD intervention or something that would also be relevant to usual practice in the UK.
The main concern was the potential for carer burden, which was raised in all workshops. This included concerns about randomisation and the potential for distress when families were not randomised to the intervention group. Strategies for minimising this risk were discussed, which focused mainly on establishing an open dialogue between participants and RNs/DNs to allow an explanation of equipoise and the randomisation process. The importance of the initial approach and explanation of the study was discussed, and the reliance on the experience of RNs to do this effectively to ensure that patients and carers understood all of the trial processes as well as difficult events such as the ‘last injection’.
There was a general view that the risk of overdose was no different from the usual risk involved when carers administer oral opioids at home. Similarly, with regard to patients who may attempt to coerce a carer into giving an increased dose, the risk was deemed to be comparable with that in usual practice, as drugs are already present in the home.
There were questions about what would happen if the carer gave the wrong drug, but attendees were reassured by the CARiAD team who reported that there were no recorded incidents of this in the Australian study and that if this were to happen it should be noted by DNs during drug stock checks, which attendees reported were part of usual practice.
There was much discussion at the south Wales workshop about professional accountability and the responsibilities and legal position if things went wrong. The group was informed of research insurance and the sponsor’s role.
There was uncertainty about whether or not there was any issue in taking part in the trial for carers who were also HCPs, and if the relevant medical or nursing councils held a view on their eligibility to take part. It was generally felt that, although HCPs who found themselves in a caring role should not be excluded, it would be beneficial to obtain a supporting statement from the relevant councils if possible.
Discussion
The consensus workshops provided rich, site-specific information from a range of expert stakeholders to inform the trial processes and the development of carer training materials. Attendees were positive and supportive about the trial and its aims, and the majority of concerns that were expressed were addressed by the trial team or other attendees by sharing their experiences.
Suggestions made in the workshops were considered by the core CARiAD team and were incorporated into training materials and other trial documents accordingly. It was apparent that clear guidelines for prescribing for intervention patients would be required and that an information sheet for prescribers should be compiled to enable prescribers to adhere to these. This summarised the issues raised in the workshop and suggested dose regimens for as-needed medications. As reconstitution was not likely to be common, it was felt that no step-by-step guide for this would be needed. Specific changes that were required to the RA tool were removing reference to the risk of transmissible disease, adding a check that there was a suitable place to store medications and amending it to allow it to be used as an ongoing document. Carer burden concerns were addressed as much as possible in the carer handbook and through signposting to OOH services as well as by ensuring that during training carers were aware that there was no obligation to administer as-needed medications, even when trained.
The importance of GP support for the study, particularly regarding the prescribing requests for intervention patients, required all general practices in the areas covered by the chosen DN teams to indicate that they had no objections to patients being approached to take part. Although some DN teams planned to involve the GP in the initial RA, this was unlikely to happen in every case, and for this reason the general practices were targeted by the core CARiAD team prior to opening trial recruitment.
The issue of whether or not the RNs could be unblinded was not resolved, and further discussion of this with the core CARiAD team and trial steering committee was required.
After DN teams were identified at each site, they were trained by the trial manager in how to recruit participants and how to train carers in the intervention group. They were provided with all of the materials and information, and the purpose of the documents was clearly explained, particularly the RA tool and the competency checklists. When usual-care processes already existed and there had been no strong views in the consensus workshops about the need for any changes to these, and as long as there was no impact on safety, DN teams were encouraged to continue with these processes. For this reason, no trial paperwork was provided for drug stock checks because these checks were already being carried out as part of current practice. When DN teams felt that it was appropriate and feasible for the carer, the carer was trained to complete the Medication Administration Record along with the carer diaries.
The General Medical Council (GMC) and Nursing and Midwifery Council (NMC) were contacted to establish their stance on recruiting carers to the CARiAD study who were also HCPs. Although they were unable to offer complete clarity, they did not object to HCPs being included in the study. As all carer participants were to be trained as lay carers, regardless of their background, they were not excluded from taking part.
Chapter 3 Randomised pilot trial design and methods
Design
We conducted a two-arm, parallel-group, individually randomised, multicentre pilot trial of carer-administered as-needed SC medication for common breakthrough symptoms in patients dying at home compared with usual care, with a 1 : 1 allocation ratio and using convergent mixed methods. 44
Qualitative and quantitative data were seen as complementary and were given equal importance. Data analysis of the two data sources was undertaken independently and then combined during the interpretation stage, during which the data were examined for convergence, divergence, contradictions and relationships between the two data sets.
The study was funded by the Health Technology Assessment programme of the National Institute for Health Research (NIHR). It received a favourable ethics opinion from the Wales 1 National Research Ethics Committee (REC) (reference: 17/WA/0208; IRAS project ID: 227970) and the Bangor University REC (project ID: 2016–15826). The UK Medicines and Healthcare products Regulatory Agency has advised that the CARiAD trial was not a Clinical Trial of an Investigational Medicinal Product (CTIMP). The study was registered on the International Standard Randomised Controlled Trials Number (ISRCTN) registry (ISRCTN11211024). Approval was granted from the NHS research and development departments of all three sites. Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 201345 recommendations and Consolidating Standards of Reporting Trials (CONSORT) 2010 statements46 (including those specific to randomised pilot and feasibility trials) guided protocol development. The current version of the trial protocol can be accessed from the NIHR project web page. 47
Trial flow chart
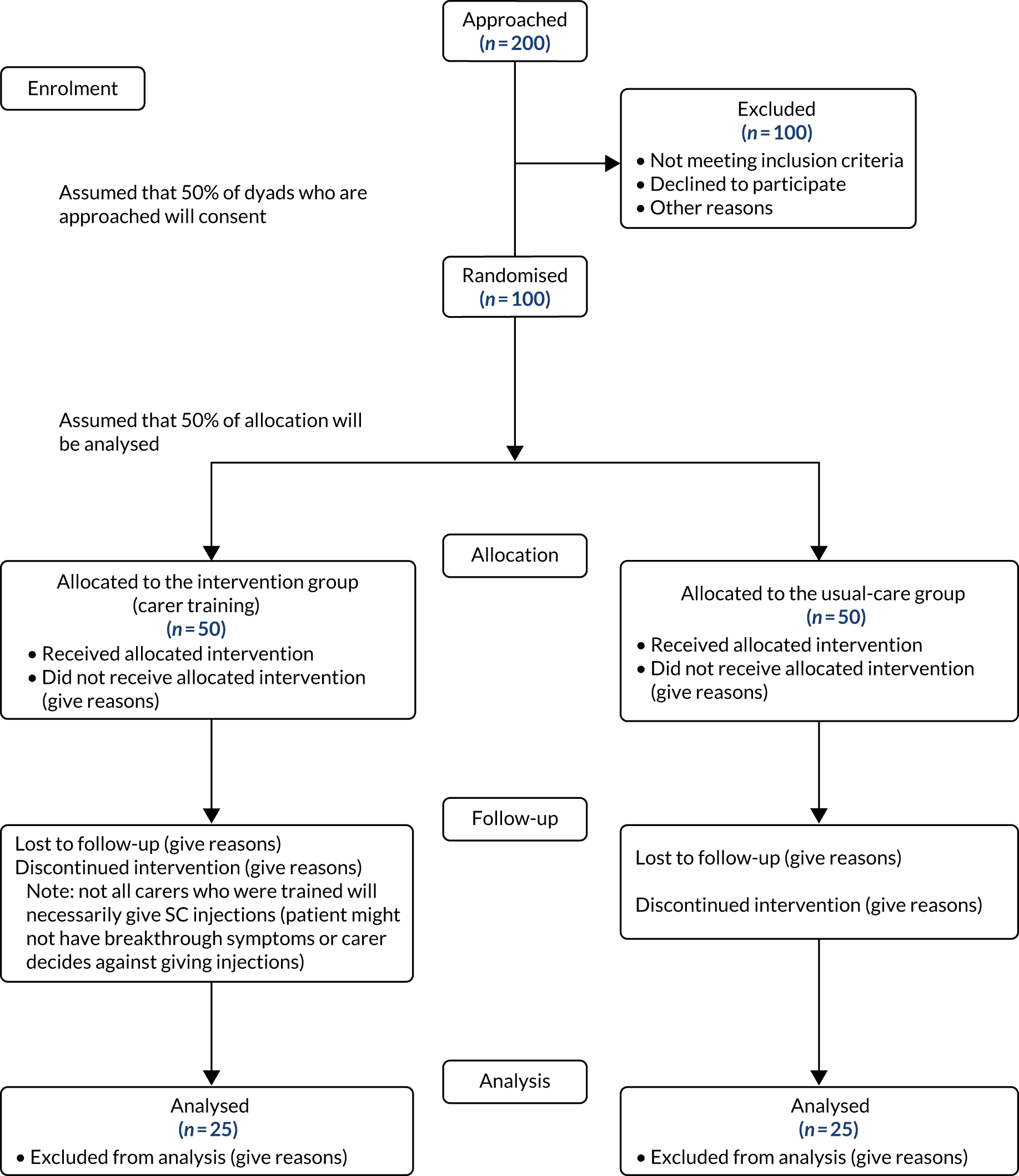
Figure 1 details planned participant flow in the trial.
FIGURE 1.
Anticipated trial flow chart.
Participants
Trial setting/context
The trial was carried out in community settings in North Wales {Betsi Cadwaladr University Health Board [BCUHB], the Vale of Glamorgan [i.e. Cardiff and surrounding areas, Cardiff & Vale University Health Board (CVUHB)] and Gloucestershire [Gloucester Care Services (GCS)]}, where patients were likely to die at home in accordance with their wishes and without the provision of round-the-clock paid care. The three pilot study sites were chosen as they are representative of the range of sites for a future definitive study.
Eligibility criteria
Inclusion criteria
Inclusion criteria for dyads were:
-
an adult (i.e. aged ≥ 18 years) patient in the last weeks of their life who was likely to lose the oral route for administration of medication and who had expressed a preference to die at home
-
their adult (unpaid) lay/family carer, who was willing to have this extended role and receive SC injection training.
Patient inclusion was not reliant on cognitive status.
Prognostication was reliant on the professional judgement of and agreement among the attending HCP team (i.e. clinical estimate of survival). There is an assumption that the carer will spend a significant amount of time with the patient.
In cases where more than one carer was available, we asked the patient to identify which carer they wanted to be included in the study.
Exclusion criteria
A dyad was excluded if any one of the following criteria were present:
-
A patient who –
-
had only paid/formal care
-
had a known allergy/adverse reaction to any one of the usually prescribed anticipatory medication with no suitable alternative
-
had a known history of substance abuse
-
had an objection to the concept of lay carer administration of SC medication
-
was unwilling for available health-care support systems to be accessed (e.g. OOH services).
-
-
A carer who –
-
had cognitive problems (i.e. who was confused, disorientated or forgetful or unable to understand the importance of medications and the information relating to them)
-
had significant visual problems
-
had insufficient literacy skills to understand and complete the study documentation
-
had insufficient dexterity to prepare and give SC injections
-
had a known history of substance abuse
-
had an objection to the concept of lay carer administration of SC medication
-
was unable and unwilling to engage with and access available health-care support systems (e.g. OOH services).
-
-
A context/environment in which –
-
there were known relational issues between the carer and the patient that contraindicated carer administration of medications (e.g. when either the patient or the carer could have assumed this practice supports assisted dying)
-
there were known issues of substance misuse in the immediate circle of family and/or friends
-
there was no suitable place for medications to be stored.
-
The inclusion and exclusion criteria were incorporated in the RA tool (see Report Supplementary Material 2). The aim was to complete the tool:
-
prior to approaching a dyad (if the criteria were not met, dyads were not approached for consent to participate)
-
at intervals (at the discretion of the HCP or local research teams) for dyads included in the trial (if the criteria were not continued to be met, dyads were withdrawn from the trial).
Recruitment
Patient identification
Potential patient/carer dyads were identified consecutively from clinical caseloads in a number of ways: through the hospice, SPC service or DN teams. When a patient was deemed by the HCP team to be in the last weeks of their life and they had expressed a wish to be cared for and die at home, they were screened for approach.
Screening
To be eligible, dyads must have satisfied the RA criteria. A RA screening tool was refined for the CARiAD trial, which was based on existing self-medication tools (see Report Supplementary Material 2). 42 RA took into account several factors as detailed above, and was conducted by the health-care team involved in the patient’s care. If a dyad did not satisfy the RA criteria, they were not approached.
Approach
The patient was approached with written material (PIS; see project web page at www.journalslibrary.nihr.ac.uk/programmes/hta/151037/#/documentation; accessed 5 January 2020) by a member of their health-care team. The initial patient approach was carried out separately from that of the carer, unless otherwise requested by the patient and if the attending HCP deemed this appropriate, that is when there was no perceived risk of patient–carer coercion. As the study involved sites in Wales, the PISs (see project web page at www.journalslibrary.nihr.ac.uk/programmes/hta/151037/#/documentation ; accessed 5 January 2020) and consent forms (see project web page at www.journalslibrary.nihr.ac.uk/programmes/hta/151037/#/documentation; accessed 5 January 2020) were translated into Welsh for the Welsh centres and offered bilingually to comply with the Welsh Language Act 1993. 48 Dyads were given as much time as they needed to consider the information sheets and to discuss with family, friends or the health-care team whether or not to take part. They were told that they could decline to participate without giving reasons.
Informed consent
A RN sought consent at a time that was judged to be suitable by the attending HCP. This gave the patient and carer as much time as they needed to understand the nature of the research, ask questions and make their feelings clear on trial participation. 49,50 If the patient was unable to consent or they had lost the capacity to do so after they had previously given consent, the assent of a personal consultee was sought (as required by the Mental Capacity Act 200551) to the patient’s participation in the trial. 49,50 As the RA excluded dyads for whom there were concerns about relational issues between the patient and the carer, the carer could act as personal consultee.
If the carer did not wish to act as the personal consultee and there was no additional family member or close friend to take on this role, we appointed a nominated consultee (e.g. a HCP not associated with the research) who would act for all patients in this situation in the trial.
The PI retained overall responsibility for the informed consent of participants at their site and was mandated to ensure that any person who was delegated responsibility to participate in the informed consent process was duly authorised, trained and competent to participate according to the ethically approved protocol, principles of Good Clinical Practice and Declaration of Helsinki. 52,53
Randomisation
Method of implementing the allocation sequence
Once the dyad had consented and baseline data collection had been completed, the dyad was randomised to one of the trial arms. Secure online randomisation hosted by the North Wales Organisation for Randomised Trials in Health (NWORTH) Clinical Trials Unit was carried out by the researcher who obtained consent or the trial manager. The system used a dynamic adaptive method of randomisation in which it stratified for recruitment centre and diagnosis (cancer/non-cancer). 54 Confirmation of allocation was sent only to those members of study staff who needed to be aware of the result.
Blinding
The CARiAD trial was an open-label trial in which blinded-outcome assessment was not feasible; therefore, it was important that outcomes were as robust as possible in the light of the lack of blinding. See Appendix 2 and Table 27 for details of the primary outcome contenders, methods of assessment, strategies to reduce bias (by increasing subjectivity) and criteria for assessing feasibility as a primary outcome measure for a future definitive trial. Outcome assessors were experienced RNs.
Data entry was completed unblinded; the trial statistician who carried out the data analysis was the only individual who was blinded to randomisation allocation. The analysis was unblinded at a combined Trial Steering Committee (TSC) and Data Monitoring and Ethics Committee (DMEC) meeting, with independent members present.
Withdrawal criteria
Participants were free to withdraw from the trial at any time without giving reasons and without prejudicing their further treatment. This was made clear to all potential participants at the time that they consented and throughout their time in the trial. Non-completion of the follow-up questionnaires did not constitute formal withdrawal from the trial; unless the participant requested withdrawal of their data completely, all data collected were used for analysis. The RA was reviewed at intervals based on the HCP’s judgement, and if the criteria were not met the dyad was withdrawn from the trial.
Duration of the feasibility study
The study opened to recruitment in BCUHB on 10 January 2018, in CVUHB on 21 March 2018 and in GCS on 1 April 2018. Recruitment closed on 15 March 2019.
Interventions
Schedule of study procedures
Table 1 details the planned study procedures.
| Procedures | Time point | |||
|---|---|---|---|---|
| Screening | Baseline | Study period (last days of life) | Post bereavement | |
| Eligibility assessment | As per dyad inclusion/exclusion criteria | As per dyad inclusion/exclusion criteria | As per dyad inclusion/exclusion criteria | |
| Informed consent | Advance consent from dyad | Check consent | Check consent (personal consultee assent might be required when patient loses capacity) | |
| Demographics | CRF | |||
| Medical history | CRF | |||
| Concomitant medications | CRF | CRF | ||
| Randomisation | ||||
| Assessment | ||||
| Symptom control | ||||
| Symptom scores | Tool: carer diary
|
|||
| Overall symptom burden | Tool: Family MSAS-GDI
|
|||
| Time to symptom relief | Measure: episodes resolved in 30 minutes
|
Tool: qualitative interviewing
|
||
| Safety | ||||
| RA tool | Tool: adapted tool based on Fuller and Watson’s42 self-medication RA screening tool
|
|||
| Competency checklist | Tool: competency checklist
|
Tool: competency checklist
|
||
| SAE reporting | Including appropriateness of administration, proportionality, side effects, drug accountability and carer events | |||
| Evaluation of training package | Tool: qualitative interviewing
|
|||
| Impact on carer | ||||
| Self-efficacy | Tool: QOLLTI-F
|
Tool: QOLLTI-F
|
Tool: qualitative interviewing
|
|
| Confidence | Tool: carer diary
|
|||
| Health economic outcomes | ||||
| Impact on carers | Tool: CES
|
Tool: CES
|
||
| DCE attribute selection | Tool: qualitative interviewing
|
|||
Health technologies being assessed
The technology under scrutiny was the extended role of lay carers to administer as-needed SC medication for common symptoms to a person who was dying at home. Lay carers were trained in this practice, and their training was supported by a manualised training package that was based on the Australian package Caring Safely at Home (see Chapter 2 for details of the development of the intervention).
Of note, it is usual practice in the UK to ensure that there is provision of as-needed medicines for breakthrough symptoms in the patient’s home for administration by the attending HCP. 55–57 The difference in this technology is that lay carers were trained and, therefore, had the option to administer these medicines (instead of and/or in addition to HCP administration).
Carer training
Carers in both groups received training on the trial materials. This was carried out by a DN or RNs at their visit to the dyad’s home to collect baseline information.
In addition, carers in the intervention group received one-to-one face-to-face training that was delivered by a HCP (usually a DN or SPC nurse) and was supported by written materials. The training covered common symptoms that may occur in the last days of life and how to assess if their loved one needed medication for a particular symptom; how to prepare (draw up) medication and dispose of sharps (glass ampoules and drawing-up needles); how to administer SC medication by needle-less technique (using a butterfly SC catheter); how to assess the effect of the medication; and the support that was available, including the primary care team as well as dedicated 24/7 SPC support. If a symptom occurred for which medication was deemed necessary (either as expressed by the patient, if able, or as assessed by the carer), the carer could use the training outlined above to administer the appropriate medication.
Training was to be tailored to the individual lay carer. As competence and confidence in practical tasks develop over time,24 HCPs were asked to be ready to deliver the training over the course of a few visits until the carer attained competency.
When carer competency in the task was attained, HCPs offered ongoing support in building confidence:
-
The first time that the patient needs an injection, the carer should call a HCP and observe administration.
-
The carer should then administer an injection at a subsequent opportunity, while a HCP is present.
-
Finally, the carer should administer an injection and call the HCP.
Medication regimens
Guidelines for anticipatory prescribing for care in the last days of life are in place across the UK. 55,56 They cover common symptoms in the dying phase, such as pain, nausea and/or vomiting, restlessness/agitation and noisy breathing/rattle. The CARiAD trial recruitment sites were advised to follow usual prescribing practice for dosing anticipatory medication. For example, in Wales, as-needed SC medication prescribing advice includes:58
-
for pain – morphine or diamorphine at one-sixth of the 24-hour dose, or if a patient is not on background strong opioids a starting dose of diamorphine of 2.5 mg or morphine of 2.5 mg
-
for nausea and/or vomiting – 50 mg of cyclizine (maximum dose in 24 hours = 150 mg), 6.25 mg of levomepromazine (maximum dose in 24 hours = 25 mg) or 1.25 mg of haloperidol
-
for restlessness/agitation –2.5 mg or 5 mg of midazolam
-
for noisy breathing/rattle – 400 µg of hyoscine hydrobromide (maximum dose in 24 hours = 2.4 mg) or 200 µg of glycopyrronium (maximum dose in 24 hours = 1.2 mg).
For patients in the intervention group only, prescribers were provided with specific additional advice, including instructions not to prescribe dose ranges/steps and that dose changes could be made only after a face-to-face assessment (not remotely, e.g. over the telephone) (see Report Supplementary Material 2).
Care pathways
In both groups, the following aspects of the current care pathway remained in place:
-
Patients were visited regularly (ideally daily) by a member of the health-care team, usually a DN. ‘Usual routes’ for support applied. These usual routes were different across the recruitment sites. For some areas, there was direct access to a 24/7 SPC advice line for carers in addition to support from the patient’s primary care team or OOH. In other areas, support for the carer was via their primary care team, and the GPs and DNs could ask for advice from the SPC clinicians.
-
As per local guidelines for anticipatory care of common symptoms in the last days of life, there was a supply of drugs in the patient’s home and the apparatus needed to administer them. 55,56
-
Measures for managing background symptoms were unchanged (usually through medication delivered via continuous SC infusion). If a patient requires several doses of as-needed medication for a particular symptom in a 24-hour period, it is usual practice for a prescriber to review and either start a continuous SC infusion of a medication or increase the dose if the medication is already given, to reduce the likelihood of further as-needed doses.
In summary, the usual-care group followed an unchanged care pathway for dealing with breakthrough symptoms at home, with the usual palliative care in place and DNs administering as-needed SC medication.
In the intervention group, carers were trained to administer as-needed SC medication, although they were not obliged to actually administer medication. If the carer needed the support of a HCP, either because they would feel more confident having a HCP present when they administered the medication or because they wished the HCP to assess and give the medication, they could obtain it via the usual routes in their area. If the carer had reached the limit of the number of administrations of medication that could be given in 24 hours (maximum of three medication administrations for each indication per 24-hour period, unless the prescribing clinician advised a maximum of fewer than three), they were asked to contact a HCP as review was required. Usual routes for support included the DN team, the GP, the GP/DN OOH, the hospice at home team or a hospice advice line. The use of such support was captured in carer diaries.
For each recruitment site, the following were clearly set out as standard operating procedures:
-
clinical support for dyads and for their primary care HCPs regarding SPC advice (relevant contact details will be recorded in the carer diary)
-
research support, including when, how and in what circumstances the research team should be accessed.
If a patient in the intervention group was admitted to an inpatient unit (including a hospital, hospice or nursing home), carers were made aware that they should not administer SC medications to the patient during the admission.
Health-care professional training requirements
All DNs (or SPC nurses) who were providing carer training had undertaken detailed standardised training on:
-
trial background, including why it is needed, the legal framework (see Appendix 1), ethics considerations and the research support team in their area
-
detailed trial processes, including inclusion/exclusion criteria, screening and approach, consent, randomisation, trial assessments and outcome measurements, safety, recruitment targets, post-trial care and the delegation log
-
training carers in the intervention group, based on the framework developed in Australia detailing the step-by-step processes and materials (see Report Supplementary Material 1 and Report Supplementary Material 2)
-
training the trainers, including detailed guidance on how to disseminate the training within their team and maintain training records.
Training was designed to be delivered by the trial manager to the DN team leads, the DN educator or another nominated individual who would be able to disseminate the training to the rest of the DN team. A record of attendance was kept for each training session. Standardised training documents [Microsoft PowerPoint® (Microsoft Corporation, Redmond, WA, USA) slides and training materials] were made available to the DN team leads for disseminating training.
Randomised pilot trial outcomes
The main outcomes of interest were those appropriate to a pilot trial, including feasibility, acceptability, recruitment rates, attrition and selection of the most appropriate outcomes measures. Outcomes were measured for patients, their lay carers and HCPs. System barriers were also noted. These measurements were made at baseline, on a daily basis for symptom control and lay carer confidence, and at 6–8 weeks post bereavement.
Recruitment measurements
Recruitment measurements were the number of eligible patients who fulfilled the inclusion criteria and were willing to be randomised, which was expressed as a percentage of the number of patients screened, the number who withdrew after baseline assessment and randomisation, the number who completed the various outcome measurements at baseline and at later time points, and reasons for any non-completion.
Patient measurements
Patient measurements were baseline information (including demographic information, medical history, capacity assessment, preferred place of care in the last days of their life and current drug management) and a daily carer diary during the study that was related to the presence and treatment of breakthrough symptoms (for use in both trial groups). Data points in each medication administration diary entry included the initial time when the breakthrough symptom triggered a perceived need for an additional SC dose; whether this was noted by the patient or the lay carer; medication and dose, and time given; reason for medication (e.g. pain, nausea, restlessness or noisy breathing); symptom score before and 30 minutes after medication administration; and when symptom control/reduction of the symptom to an acceptable level was achieved. Hospital or hospice admissions during last illness and the actual place of death were also recorded. Approximately 6–8 weeks post bereavement, carers were asked to complete the Family Memorial Symptom Assessment Score – General Distress Index (MSAS-GDI). 10,59–61
Carer measurements
Carer measurements were demographic information at baseline; QOLLTI-F questionnaire (at baseline, after the first as-needed SC medication, then every 48 hours thereafter until the patient’s death); whether or not HCP support was sought; and Family MSAS-GDI (a measure of the patient’s symptom distress in the last 7 days of life) at the 6–8 week post-bereavement visit. In the intervention group, the confidence in administering SC medication and competence at intervals after training were recorded.
Health-care professional measurements
Health-care professional measurements were the baseline measurements of attending team structure, primary prescriber and carer trainer.
Safety
The CARiAD study contained a number of safety outcome measures at different stages of the clinical journey taken by the patient, carer and HCPs. Safety outcome measures include the RA tool, competency checklist and significant event reporting (see Report Supplementary Material 2). Significant event reporting included the following: the appropriateness of administration (is administration accompanied by evidence of need?); proportionality (has the correct dose been administered?); side effects both anticipated and not anticipated; drug accountability (do stocks tally?); and carer events (e.g. distress, injury, accidental or purposeful self-administration).
An AE was defined as any untoward medical occurrence in a trial participant (either the patient or the carer) and included incidents that were not necessarily caused by or related to the trial. A SAE was any untoward occurrence that resulted in death, was life-threatening, required inpatient hospitalisation or prolonged existing hospitalisation, resulted in persistent or significant disability/incapacity or was otherwise medically significant.
All AEs and SAEs were captured via the significant event form. SAEs were reported to the PI and sponsor within 24 hours. As this was a study involving patients who were close to the end of their lives, death was an expected outcome. It was recorded and reported to the sponsor but was not considered a SAE if, in the opinion of the PI, it was a natural conclusion to a patient’s life-limiting illness. Owing to the nature of the study, events of death did not require immediate reporting to the DMEC.
Exploratory end points/outcomes for a future definitive trial
Core Outcome Measures for Effectiveness Trials (COMET) in patients close to the end of their life were not available at the time of CARiAD trial planning. 2 After scrutiny of available outcome measures, the most likely candidates for primary outcome measures for a future definitive trial of this intervention were Family MSAS-GDI (a measure of overall symptom burden/distress in the last 7 days of life)10,59–61 and QOLLTI-F (a measure of the quality of life of carers looking after someone with a life-threatening illness, incorporating elements of control and self-efficacy). 41
In addition, we measured carer confidence using a seven-point Likert scale, in which the carer is asked after administration of every as-needed SC injection to rate their level of confidence in administering this injection (1 = not at all confident, 7 = extremely confident). We planned to give quantitative and qualitative data equal importance and integrate these at the interpretation stage. For more detail on the rationale for choosing the Family MSAS-GDI and QOLLTI-F, see Appendix 2.
Criteria for assessing feasibility as primary outcome measure
All outcome measures were assessed on the same criteria for consistency.
Applicability:
-
This was assessed by an independent expert panel and was based on feedback from participants (HCPs and carers).
-
Each measure was assessed by the panel with regard to its relevance and applicability to the population, based on the outcomes of the pilot data collection phase.
-
The panel will recommend an ‘accept’ or ‘not accept’ status for each outcome based on the criteria below and their expert opinions, and taking into account the RATIONALE (Risk of Bias Justification Table) statements on outcome measures and assessment of bias risks in ultimate reporting. 62,63
Acceptability:
-
This was assessed by participants and HCPs during the qualitative aspects of the feedback interviews.
Level of completeness:
-
This was assessed by the frequency of missing data during the data collection phase. This would require potential primary outcome measures to have > 70% completeness.
-
An assessment will also be made of the reasons for missing data to establish whether or not anything systematic in the trial design could be adjusted to mitigate for the missing data.
Once the feasibility of the outcomes is established, the design of the definitive trial will consider whether a single or a combined primary outcome of interest is appropriate.
Secondary outcome measures
The potential suitability of the secondary outcomes will be considered, as detailed below.
Time to symptom relief
This outcome measure was collected given the importance of this outcome to carers and patients. It does, however, present significant inherent challenges with potential bias and will not contend as a primary outcome measure for a future definitive trial, unless methodological concerns are resolved. The specific methodological concern is that it will be hard to demonstrate that the measurement of this outcome will be carried out in comparable ways in the two arms of the trials. We acknowledge that these problems arise because the individual who is measuring the outcome (the carer) cannot be blinded to the trial group. The intervention group will have lay carers deciding to dispense treatment, and this could systematically affect their judgement of this outcome.
Carer Experience Scale
See Chapter 7.
Sample size
A fully justified sample size was not required; sample size was justified by estimating what sample size a future definitive RCT will need.
Careful consideration was given to the size of effect that we could potentially see for the Family MSAS-GDI and QOLLTI-F in a potential future definitive trial. There was no definitive statement of what a minimal clinically important difference would be for either of these measures in this context; therefore, we had to make an assumption that was informed by both HCPs and public contributors. It may be that these estimates are considered too conservative/small; however, for the pilot trial we wanted to ensure that we had captured enough precision within any estimates to be confident of capturing any potential indication of a signal. Using a smaller effect size to ensure adequate precision when investigating at this stage would not preclude the use of a larger effect size (and possibly resultant smaller sample), if it were considered more appropriate when designing a definitive study.
Assuming an important difference of 0.4 [standard deviation (SD) = 1)] on the Family MSAS-GDI, a sample of about 216 participants would be required to achieve 90% power to detect a difference of this size with a significance level of 0.05 using a two-sided test. Equivalently, a sample of about 550 participants would be required to detect a difference of 0.5 points (SD = 2) using the QOLLTI-F.
Using the larger of these estimates for the feasibility trial, we assumed approximately 9% of the main trial size, give an 80% confidence interval to exclude a clinically important difference, required approximately 25 participants in each group. 64 Sim and Lewis65 recommend a sample size of approximately 50–55 to ensure robust estimates of the variance. Using estimates of dropouts, we predicted that we needed to approach 200 potential participants to achieve 100 randomised participants, with 50 completers (‘completer’ is defined as a dyad who completed all of the study measures from baseline to follow-up at 6–8 weeks post bereavement). Therefore, we needed to approach 5.5 dyads per month from each of the three sites and randomise 2.7 dyads per month from each of the three sites to meet our recruitment target. Assuming that we would recruit equally between the three sites, we needed to approach 66 dyads, randomise 33–34 and would have had 16–17 dyads available per site for analysis (see Figure 1).
Detailed considerations
The 2013 Office for National Statistics data66 showed that 8.6% of all deaths in those aged > 15 years are home deaths caused by neoplasms. Deaths caused by neoplasms were seen as a useful proxy for expected deaths. Therefore, the three recruitment sites had the following numbers of home deaths caused by neoplasms available per annum: BCUHB, 653; CVUHB, 349; and GCS, 517.
Lay carers of patients who are cared for at home in their last days of life
Drawing on the collective clinical experience of the study team, we knew that it is rare for a patient to successfully fulfil their wish to die at home if they did not have the support of a lay carer. This was because most health and care services (and specifically in the sites that we propose to recruit from) could not provide 24/7 care in a patient’s own home. That said, the best data available on this topic came from a recent quasi-experimental study examining palliative care interventions at home. It noted that of 953 patients who expressed a preference to die at home, 72.2% had an informal carer. 67 It is worth noting that the study did not record where patients who were unable to identify an informal carer actually died, or if previously unidentified lay carers in the patient’s circle stepped in to support their wish to die at home.
The pilot trial
For the pilot trial we proposed to approach 200 dyads (66 or 67 at each site). Therefore, even if only 72.2% of the patients had an identified carer, the numbers of dyads in the three sites that could be eligible were as follows: BCUHB, 471; CVUHB, 251; and GCS, 373. It is also worth noting that all of the participants in the focus group indicated they would take part in a trial if invited (caution: selection bias). Of these, on very conservative calculation, 100 dyads would consent to participate (33 or 34 at each site). The percentage recruited in the Australian study was much higher: 97.6% of those approached consented to participate. In total, 50 of those dyads would be in the intervention group (receiving training) and 50 dyads would be in the usual-care group (i.e. 16 or 17 per group per site), with, again on very conservative calculation, 25 in each group (50 in total) completing the trial (i.e. 8 or 9 per group per site). In total, 50 completers were needed for the analysis (i.e. 25% of those initially approached for participation).
Statistical methods
Statistical analysis
The primary analysis was concentrated on the feasibility metrics and adherence outcomes based on defined thresholds (Table 2). There was limited preliminary analysis of intervention outcomes. Point and 95% confidence interval estimates were calculated and used to estimate the variability and direction of effect to further inform the sample size calculation for a definitive study.
| Proposed primary outcome measure | Outcome measure description | Estimate of a conservative effect | Sample size needed (n) | Rationale |
|---|---|---|---|---|
| Family MSAS-GDI | An 11-item scale rated 0–4 for each item. The score is given as the mean of the 11 items, giving an overall scale of 0–4 | Difference of 0.4 points (SD 1.0 points) | 216 | Hickman et al.59 indicated a SD of 0.87, which reduces the sample size to 164 |
| QOLLTI-F | A 16-item scale rated 0–10 for each item. The score is given as the mean of the 16 items giving an overall scale of 0–10 | Difference of 0.5 points (SD 2 points) | 550 | Cohen et al.41 indicated a 1-point difference between a ‘bad’ and an ‘average’ day or an ‘average’ and a ‘good’ day; using this would reduce the sample size to 140 |
| If feasible, time to symptom relief | Continuous outcome based on the number of minutes from when the need for symptom relief is noted until the time symptoms are resolved | 10 minutes (SD 35 minutes) | 520 | To achieve 90% power to detect a difference of this size with a significance level of 0.05 using a two-sided test |
Summary statistics of all outcomes were used to inform the approximate models of analysis that would be used in a full trial. The results of the feasibility trial were used to inform the most appropriate analysis models (e.g. the number of episodes in which as-needed medication was used and proportion of participants who never required as-needed medication). A preliminary analysis of the outcomes was completed using an intention-to-treat approach. All analysis that was undertaken was prespecified in a statistical analysis plan that was written and agreed before data collection was completed.
As this was a feasibility trial, there was no imputation of missing data. Missing data were considered as a criterion for assessing the suitability of measures. Descriptive statistics were produced for each of the outcome measures to evaluate the appropriateness of the measures for inclusion in a definitive RCT.
Progression to a full trial
Clear progression rules were defined to determine whether or not an application for a future substantive trial that was powered to study clinical effectiveness and cost-effectiveness should proceed. Our progression rules related to the following measure that we considered important to feasibility: reaching our target (16–17) for the number of patients recruited per site.
We also established clear assessment criteria for establishing the acceptability of the potential primary outcome measures.
Table 2 explains sample size calculations and Table 3 summarises the objectives, action plan and criteria for progression to a full trial.
| Objective | Action plan | Threshold for progression to full RCT |
|---|---|---|
| To refine the assessment and outcome measures to be used in any potential RCT | Qualitative feedback will be collected from participants 2–4 months after the intervention, regarding the acceptability of the measures, and will evaluate whether or not all of the intended information was captured | |
| To evaluate the acceptability of the manualised intervention (and potentially refine) | An initial workshop with the Australian team was held (November 2015). Expert consensus workshop discussions led to refined trial processes, education package and resources. A detailed process is described in the study protocol (see NIHR project web page47) clarifying the legal and regulatory framework for the practice | In the feasibility study, the simplest method is for lay carers to draw up medications in immediate form only; a full trial would be more appropriate if it was able to extend this to advance preparation and labelling |
| To evaluate the recruitment process | Referral sites and referral sources, where participants had heard about the study, the number and speed of referrals received and the time elapsed between initial contact made with the study team (for information and consent form) | In the feasibility we have assumed 50% recruitment. We would say that a full trial is not possible if recruitment falls below 30% |
| To estimate participant retention rate for the full RCT | Retention rates will inform the refinement of the sample size calculation for any potential subsequent RCT. Participant engagement will be monitored throughout the pilot trial | In the feasibility we have assumed 50% retention. We would say a full trial is not possible if retention falls below 40% |
| To test the assessment and outcome measures for suitability, relevant change factors, and acceptability to participants | Data from the assessment process will be compared against raw data from the outcomes measures to assess the outcome measures sensitivity to identifying participant change | |
| To identify acceptability and collection of relevant data to inform the data collection and analysis plan for implementation in the subsequent RCT | A review will be completed of each outcome measure of levels of missing data and stability to ensure that the information collected will allow any future main analysis to be feasible and appropriate. Amendments can be suggested where appropriate to amend data collection for any potential future trial. The data available will also inform the details for the analysis plan of any potential full trial | Carer diary data items successfully completed (70%), Family MSAS-GDI successfully completed at bereavement visit (70%) and QOLLTI-F successfully completed at 48-hour intervals (70%) |
Summary of any changes to the study protocol
Version 1 was approved by the funder (October 2016) and detailed the whole CARiAD study (including the work-up phase/expert consensus workshops). The workshops had concluded and the results were incorporated in the trial processes and materials. Version 2 focused on the trial (with embedded qualitative study), which was due to commence in October 2017. Further amendments were made in response to REC comments (July 2017) to clarify details of the qualitative methods (June 2018) to reflect the decision to unblind RNs and the affect on trial processes (August 2018) and to make general corrections to trial processes and reflect the extended trial period (November 2018). The current protocol is version 6 (27 November 2018).
Governance
Trial governance procedures adhere to the NIHR guidelines and include a trial management group, an independent TSC and an independent DMEC. SAEs will be reported to the TSC and DMEC in line with NIHR guidance. 68 The current version of the trial protocol can be accessed via the NIHR project web page. 47
Ethics and regulatory considerations
The current version of the trial protocol details safety outcome measures, REC review, regulatory arrangements, quality assurance and quality control, data handling and publication policy. 47
Public contribution
Public contribution formed an integral part of the CARiAD trial and was part of each stage from development to dissemination. Our reporting of public contribution in the CARiAD trial is based on the GRIPP2 (Guidance for Reporting Involvement of Patients and the Public) (short form) checklist (version 2). 69 A summary, presented in the GRIPP2 (short form) format, is shown in Table 4. This is followed by a more detailed account, organised in accordance with the GRIPP2 (short form) structure.
| Section and topic | Item |
|---|---|
| Aim: report the aim of the study | To collaboratively involve patients as co-applicants and partners at all stages of the study, with the intention of improving the design and conduct of the study and ensuring that it was informed by experiences of giving injections to loved ones dying at home. To involve public contributors who have appropriate experience to ensure full understanding of the needs of research participants |
| Methods: provide a clear description of the methods used for public contribution in the study | A range of methods were used for public contribution at stages throughout the study. These included the following:
|
| Results: outcomes – results of public contribution, including positive and negative outcomes |
Public contribution added to the study in several ways, including a better-designed study, improved quality of public-facing documents, accessible and appropriate lay sections in all study documentation, improved qualitative topic guides and a successful NHS research ethics application owing to the participation of JO’C We identified no negative outcomes from public contribution |
| Discussion: outcomes – comment on the extent to which public contribution influenced the study overall. Describe positive and negative effects |
Patient and public involvement in this study was very effective and influenced the study in important ways. This could have been related to several factors. First, the public contributors both had relevant, direct personal experience of caring for a loved one at the end of life. JO’C had direct experience of administering SC medications to her late husband. Second, effective public contribution processes were in place, for example researchers experienced at involving public contributors and public contributor involvement from the outset, allowing public contributors to fully help shape the study. Third, the study offered the right context for effective public contribution, for example the supportive attitude to their involvement from the research team, two public contributor team members which allowed mutual support, a pre-existing relationship with one of the public contributors (BF) and the opportunity of a ‘fresh pair of eyes’ from the other contributor (JO’C) A more indirect outcome that may have been influenced by participation in this study is that one of the public contributors (JO’C) has decided to embark on a new career in end-of-life care The only possible limitation that we were able to identify was that both of the public contributors were positive about the intervention; however, we did also have group discussions with participants who were not positive |
| Reflections: critical perspective – comment critically on the study |
The public contribution in the study was embedded as far as possible into the research team and into all stages of the research. Two key strengths were the directly relevant experience of the public contributors and the very early public contributor involvement at the pre-design stage, which enabled meaningful and significant involvement In a study that was sensitive and ethically thought-provoking, the public contributors enabled the study team to be better informed and to develop a stronger sense of bereaved carers’ perspectives on the appropriateness of the approaches considered |
Aim
In this study, the aim of public contribution was to collaboratively involve patients as co-applicants and partners at all stages of the study, with the intention of improving the design and conduct of the study and ensuring that it was informed by experiences of giving injections to loved ones dying at home, and to involve public contributors with appropriate experience to ensure that there was full understanding of the needs of research participants.
Methods
A range of methods were used for public contribution at different stages throughout the study. These included the following:
-
The initial decision to develop the CARiAD trial resulted from the Palliative and End of Life Care Priority Setting Partnership report, which incorporated the views of 1403 people across the UK, which placed great emphasis on empowerment of family carers and symptom management during the last days of life. 11
-
The study design was heavily informed by the work-up stages, which comprised two group consultations with carer groups (suggestions on consent mechanisms, drug safety, training and ongoing support were incorporated into the study design), one telephone consultation with a bereaved carer (JO’C) who had given SC medications to her late husband and one telephone consultation with a nurse who had trained a carer to give SC medication to a relative and had supported them in doing so.
-
The CARiAD trial had two public contributors as co-applicants to the study (BF and JO’C) who contributed to the submitted application, including providing the lay summary. Following this, one of the public contributors (JO’C) attended and contributed significantly to the CARiAD REC meeting.
-
Trial materials, including the topic guides for the qualitative interviews, were produced with the input of the public contributors. Public contributors also took part in expert consensus workshops and formed part of the TSC.
-
The public contributors strongly supported the study dissemination. One of the public contributor co-applicants (JO’C) presented the results of the CARiAD study at a national palliative care conference.
Public contribution was of an extremely high standard in this study and public contributors formed an integral part of the research team. When public contributors were able to attend meetings in person, measures were taken for considerate inclusion, such as access, briefing, refreshments and travel provision and expenses. The two public contributor team members/co-applicants were supported in the role directly both by the CARiAD team and by their respective groups (Sue Ryder Hospice and North Wales Cancer Network).
Results
The outcomes of public contribution in the early stages of the study included a better-designed study, particularly based on input during the study work-up phase. This added to, altered or confirmed a number of study design elements. Throughout the study, the study team were better informed through the sustained and active public contribution in meetings and developed a stronger sense of bereaved carers’ perspectives on the appropriateness of the approaches considered.
In more practical ways, it was an enormous advantage to have the presence and participation of Julie O’Connor at the NHS REC meeting. This gave the REC a sense of the meaning of the study and how it may be perceived by bereaved carers, and reassured them that the study intervention could be welcomed by carers. The public contribution improved the quality of a number of the public-facing documents, such as the qualitative topic guide and the study close-out documentation for carers. We identified no negative outcomes from public contribution.
A more indirect outcome that may have been influenced by participating in this study is that one of the public contributors (JO’C) has decided to embark on a new career in end-of-life care.
Reflections
Overall, public contribution influenced this study to a great extent. At all stages, the study was kept on track by ongoing and active public participation. The study team learnt a lot and changed a lot. We were guided through a very sensitive and potentially distressing study and steered towards appropriate and acceptable approaches. Not only was the instructive, beneficial and reassuring for the team but it also resulted in more of the right approaches being used to ensure that the study proceeded with respect and consideration for the study participants at a very difficult, and important, time of their lives.
Chapter 4 Randomised pilot trial results
In this chapter, we report the findings of the quantitative analysis of the pilot trial results that were held in MACRO (version 4.9.0.8594; Elsevier, London, UK), the electronic data capture system that was used to store the trial data. When appropriate, we comment and reflect on CARiAD processes, with information gleaned from the trial manager and the core operational group discussions.
Recruitment
Site initiation
Final REC approval was granted on 3 August 2017 and excess treatment costs were approved on 26 September 2017, both ahead of schedule (Table 5).
| Activity | Proposed deadline | Site | ||
|---|---|---|---|---|
| BCUHB | CVUHB | GCS | ||
| Site agreements signed | 31 September 2017 | 6 October 2017 | 20 December 2017 | 1 December 2017 |
| Local approvals obtained | 31 September 2017 | 25 August 2017 | 3 January 2018 | 8 December 2017 |
| Staff training completed | 31 July 2017 | 19 October 2017 | 8 February 2018 | 6 November 2017 |
| Site opened | 1 October 2017 | 10 January 2018 | 21 March 2018 | 1 April 2018 |
| First dyad recruited | 5 February 2018 | 3 May 2018 | 12 June 2018 | |
| Last dyad recruited | 29 November 2018 | 17 December 2018 | 5 February 2019 | |
| Planned total full months’ recruitment per site (based on recruitment closure date of 15 March 2019) | 12 | 12 | 12 | |
| Total full months’ recruitment until 15 March 2019 (from site opening) | 14 | 11 | 11 | |
| Total full months’ recruitment until 15 March 2019 (from first dyad recruited) | 13 | 10 | 9 | |
Health-care professional training
Initial HCP training was conducted by the trial manager and was given to DN team leads and relevant SPC nurses, who were provided with the training materials to disseminate to DN teams who would be recruiting dyads and training carers. A presentation summarised the background to the study and provided an in-depth discussion of the trial processes. This focused on the trial paperwork that would need to be completed by HCPs, and the training of carers to give SC medications. Training was scheduled to last 2 hours. In BCUHB, further training sessions were requested for the individual DN teams at their bases, alongside the DN team lead.
The training focus was led by the needs of the team and usually focused on the research processes, particularly around identifying and screening dyads and completing the RA. Some teams requested further training sessions in which the trial manager worked through the screening processes with them for the current palliative list of patients, as the team were not confident in carrying out these tasks and required reassurance they were doing this correctly. The training was often led by the questions and concerns of the teams about what would happen in specific situations; these were talked through, outlining the safety measures that were in place and how many of the concerns were also present in the usual-care group. All teams were confident with training carers in the clinical aspects of drawing up and administering medications.
Participant flow
Trial recruitment is presented in Figure 2 and Table 6. There were 189 potential dyads identified at pre-screening in which the patient was in the last weeks of their life and was likely to lose the oral route of medication administration. Of these, 169 dyads were screened.
FIGURE 2.
Trial recruitment overall and by site (BCUHB, CVUHB and GCS).
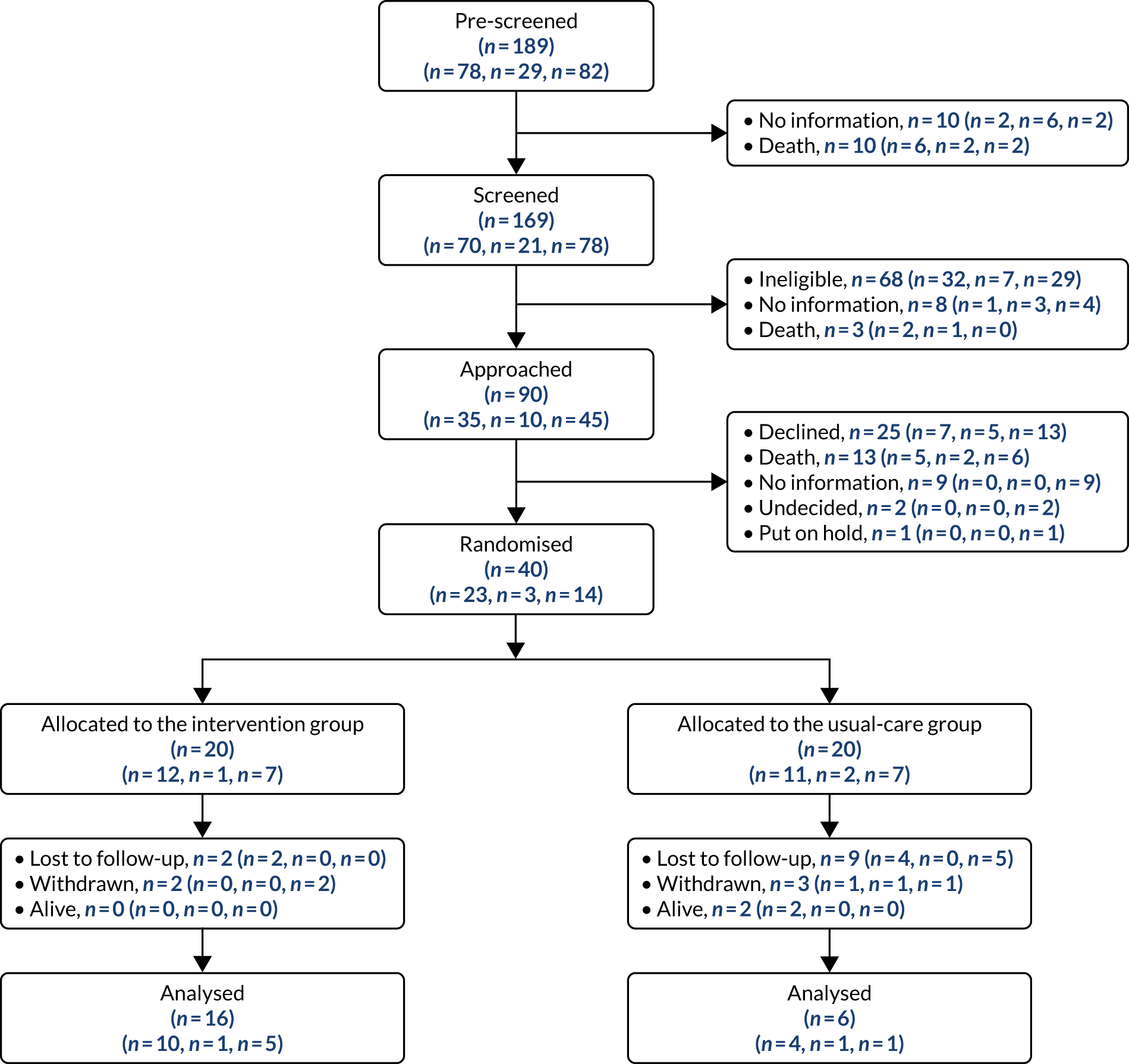
| Patient recruitment | Site (n) | Total (n) | ||
|---|---|---|---|---|
| BCUHB | CVUHB | GCS | ||
| Patients pre-screened | 78 | 29 | 82 | 189 |
| Dyads with no further information | 2 | 6 | 2 | 10 |
| Patients who died before screening | 6 | 2 | 2 | 10 |
| Dyads screened | 70 | 21 | 78 | 169 |
| Dyads eligible | 38 | 14 | 49 | 101 |
| Dyads ineligible | 32 | 7 | 29 | 68 |
| Patients who died after screening but before approach | 2 | 1 | 0 | 3 |
| Eligible dyads with no further information | 1 | 3 | 4 | 8 |
| Dyads approached | 35 | 10 | 45 | 90 |
| Patients who died after approach but before consent | 5 | 2 | 6 | 13 |
| Eligible dyads with no further information | 0 | 0 | 9 | 9 |
| Dyads who declined | 7 | 5 | 13 | 25 |
| Dyads who were undecided | 0 | 0 | 2 | 2 |
| Dyads who were put on hold | 0 | 0 | 1 | 1 |
| Dyads recruited (consented) | 23 | 3 | 14 | 40 |
| Dyads who withdrew | 1 | 1 | 3 | 5 |
| Dyads lost to follow-up | 6 | 0 | 5 | 11 |
| Patients alive | 2 | 0 | 0 | 2 |
| Dyads completed | 15 | 2 | 5 | 22 |
In total, 68 out of 169 dyads were ineligible. The main reasons for ineligibility were that only paid or formal care was in place (n = 18, 26.5%), the patient did not wish to die at home (n = 13, 19.1%), the carer was disorientated, confused or forgetful (n = 5, 7.4%) and there were relationship issues between the patient and the carer (n = 5, 7.4%).
For 11 out of the 169 dyads eligibility was confirmed, but for eight no further information was provided and in three dyads the patient died before approach.
The remaining 90 dyads were approached to participate in the study. Of these, 25 declined to take part. From the patients’ perspective, the reasons given for declining were not wanting to be randomised (n = 1, 4%) and not wanting the carer to administer medications (n = 5, 20%), and two (8%) declined for reasons not specified. From the carers’ perspective, three (12%) carers were concerned about burden, two (8%) did not want to be randomised, six (24%) did not want to administer medications, one (4%) did not want to complete paperwork and five (20%) declined for reasons not specified.
There were 40 dyads (40/101, 39.6% of the eligible population; 40/90, 44.4% of those approached) who completed the baseline visit and were randomised. These 40 dyads were allocated evenly: 20 to the usual-care group and 20 to the intervention group. A total of 22 carers (55% of those randomised) completed the follow-up visit: 16 (80% of those randomised) from the intervention group and six (30% of those randomised) from the usual-care group.
Recruitment site context
Betsi Cadwaladr University Health Board serves a population of 678,000 people in North Wales, providing community and inpatient services. BCUHB employs 16,500 people. Seven DN teams were part of the CARiAD trial from the outset and were chosen after discussion with DN matrons based on their capacity and capability, and were located across a mix of urban and rural areas. One additional team was invited to join the study and opened to recruitment 6 months after the first seven teams.
Cardiff & Vale University Health Board serves a population of 445,000 people in the Vale of Glamorgan and provides community and inpatient services. CVUHB employs 14,500 people. Two DN teams participated and recruitment was also opened via the SPC team, covering the acute hospital and local hospice.
Gloucester Care Services serves a population of 600,000 people in Gloucestershire, providing community services only. GCS employs 2700 people. Five DN teams and one SPC team recruited to the study.
In all sites, the number of DN teams involved in the CARiAD trial was a small proportion of the total number of DN teams across the health authority. This had an impact on the number of potential patients screened compared with our initial assumptions. However, DN matrons and other senior staff members who were involved in the assessment of capacity and capability of the teams were confident that our required numbers could be met from a smaller number of teams, and felt that this was an acceptable compromise in managing the aims of the trial and the capacity of the NHS services.
Measures to support recruitment
Trial manager support
Screening was undertaken by DNs, who were asked to return a screening log via fax each week. The rate of return from all DN teams was low, with the exception of one area (comprising three DN teams) where a weekly log was returned without prompt from the trial team. In addition, the quality of screening logs was sometimes poor, for example using a superseded version, providing incomplete information or documenting items in the incorrect column. Some DNs annotated the logs with their own comments, for example, ‘died before approach’, in which case it was unclear whether or not the patient had died before screening was complete. The trial team made every effort to contact the DN teams to clarify inconsistencies and missing items, but this was not always possible.
To improve screening processes, the trial manager made a weekly call to the DN lead (or designated administrative support) of each team to request an updated screening log. The call provided an informal opportunity to build rapport with the teams, to get updates on the current CARiAD trial dyads, to discuss potential new patients and to identify whether or not there had been any AE/SAEs that should be reported. It was noted that the area in which the screening log was returned weekly had a proactive administrative team; therefore, other teams were encouraged to utilise the skills of non-clinical members of the team. Following feedback, the screening log was also revised to make it more user-friendly. Periods of sickness absence from DN team leads affected the ability of the trial manager to engage with the teams.
Unblinding amendment
The unpredicted increased training needs among overstretched and research-naive DN teams delayed the opening of all three recruitment sites. DNs were happy to teach lay carers to give SC as-needed medication but found research tasks such as screening logs, explaining randomisation and aiding diary completion more difficult. All three RN leads felt that they could help more if they were unblinded and that this would enhance recruitment. RNs were already partially unblinded in this pragmatic trial. We estimated that any bias that ensued from full unblinding would be outweighed by the gains in the feasibility and practicality of the trial. Therefore, we submitted a major amendment to fully unblind RNs to the patient’s allocation in this open-label trial. This was supported by our trial statistician, TSC chairperson and independent TSC statistician. We received a favourable response from the REC on 11 September 2018.
Affect of the measures to support recruitment
A number of measures were taken throughout the recruitment period to try to improve the number of dyads who were recruited and retained (Figure 3). These included the following: refresher training for DN teams in May (three teams), June, July and October; feedback enhancements to all recruiting teams in addition to the regular planned feedback; increased involvement of SPC nursing colleagues; identification of the difficulty for RNs who were partially blinded; the substantial amendment and unblinding; the ability of teams to implement unblinding; opening of new sites in BCUHB and GCS; and the overall no-cost extension to gain more recruitment time.
FIGURE 3.
Measures to support recruitment. SPCN, specialist palliative care nurse.
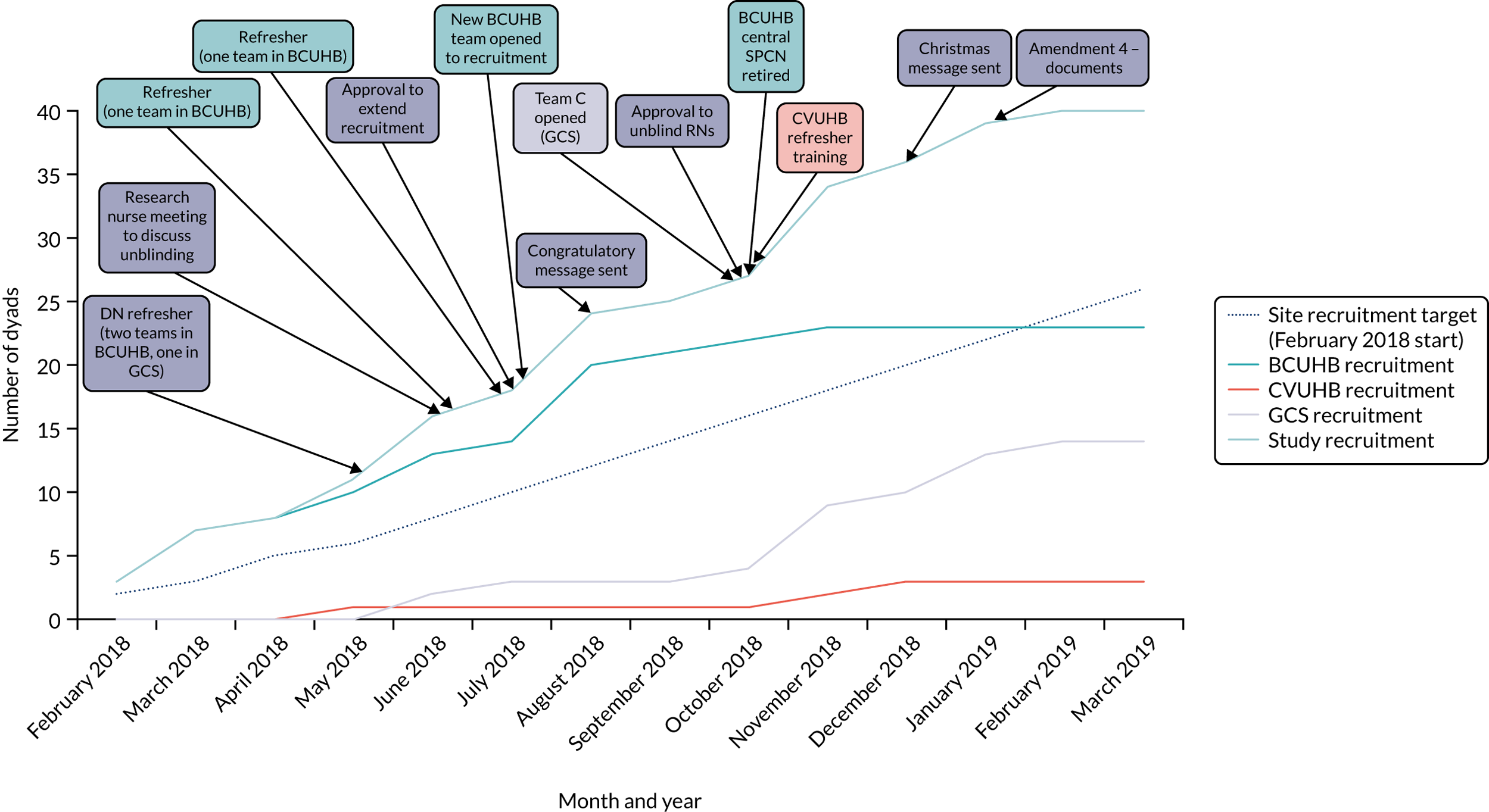
Risk assessment
It was intended that RAs would be carried out by the DN and documented on the CARiAD RA tool before a dyad was approached. RAs for dyads who did not fulfil all of the criteria were disposed of confidentially. We expected to receive completed RA tools for all 40 dyads post bereavement. Unfortunately, a number of copies were misplaced by the DNs or SPC nurses, or not completed at the correct time point; the actions relating to this are reported separately in Protocol deviations.
For BCUHB, nine out of the 23 (39.1%) RAs were returned to the trial office and, of those, one had been completed on an earlier version. In one instance when the RN and SPC nurse visited the dyad for the consent visit, they identified that there was no completed RA tool but completed the document before randomisation. Four RA tools were completed but the DN admitted they had subsequently been misplaced. The remaining 10 RA tools related to dyads from one district nursing team. They had been requested by the trial team repeatedly, but were received only after several reminders. On review, it was noted that they had been completed on versions that were dated later than the date signed off. This was reported as a protocol deviation.
For CVUHB, two out of three (66.7%) RAs were returned; the third was misplaced. For GCS, 12 out of 14 (85.7%) RA tools were returned; a further two could not be located.
Informed consent
Data from the baseline visit identified that 19 patients were able to give initial consent for themselves, but 21 patients required initial consultee assent. Feedback from RNs indicated that some of these 21 patients may have had capacity to give consent but were physically too unwell to sign a consent form.
The intervention (carer administration of as-needed SC medication for common breakthrough symptoms) is applicable to the last few days of a patient’s life, when death is naturally anticipated and accepted. Given the reality of the dying process, it can be assumed that a patient will lose capacity in the last days or hours of their life (i.e. the likelihood of losing capacity to make a decision to continue in the trial is very high). The loss of capacity to make a decision to continue in the trial can happen at any time in the dying process and capacity can fluctuate.
Table 7 details the number of initial patient consents/consultee assents by site and the expected number of consultee forms to be returned. Of the expected 10 consultee declaration forms, none was received after a patient’s death for those patients who had initially consented themselves and had subsequently died. We excluded patients who died in inpatient settings from these figures, as their medication would have been provided exclusively by HCPs and trial processes would not apply.
| Site | Total recruited | Initial consultee assent | Initial patient consent (total) | Initial patient consent categories | |||||
|---|---|---|---|---|---|---|---|---|---|
| Withdrawals | Patient alive | Place of death not known | Place of death not homea | Expected subsequent consultees | Consultee forms received | ||||
| BCUHB | 23 | 15 (15/23, 65.2%) | 8 (8/23, 34.8%) | 0 | 0 | 0 | 2 | 6 | 0 (0/6, 0%) |
| CVUHB | 3 | 1 (1/3, 33.3%) | 2 (2/3, 66.7%) | 1 | 0 | 0 | 0 | 1 | 0 (0/1, 0%) |
| GCS | 14 | 3 (3/14, 21.4%) | 11 (11/14, 78.6%) | 3 | 0 | 0 | 3 | 5 | 0 (0/5, 0%) |
| Total | 40 | 19 (19/40, 47.5%) | 21 (21/40, 52.5%) | 4 | 0 | 0 | 5 | 12 | 0 (0/12, 0%) |
We believed that the consent processes detailed in the CARiAD trial protocol would be fit for purpose in this group of dying patients. However, after retrospective analysis to explore the issues, we now wonder if additional clarification might be needed for any potential future definitive work for the following reasons:
-
Included in the daily checklist was a reminder for DNs to contact the trial manager when capacity changed (to be refreshed on the consultee process), but no DNs did this.
-
Carers are not trained to do mental capacity assessments, yet capacity may change quickly or be fluctuating and there may be no HCPs available.
-
The CARiAD trial took place in patients’ own homes, that is, in a community setting with no 24-hour HCP presence. OOH staff attending the patient may not be familiar with trial processes or may be unaware of the need to obtain consultee assent.
-
The CARiAD trial is a non-CTIMP and the intervention constitutes ‘intrusive research’, defined in the Mental Capacity Act 2005 as when interventions/procedures continue after the patient had lost capacity. Non-CTIMP consent mechanisms apply, that is, consent to participate in a study does not endure after the loss of capacity and, therefore, there is the need for the involvement of either personal or a nominated consultee.
-
The interview topic guide included a prompt to ascertain experiences of/views on consent mechanisms in the trial. The specific issue of consultee assent for those patients who initially consented themselves was not raised by any of the interviewees.
For more detail on the 12 dyads for whom the expected subsequent consultee forms were not received, see Appendix 3.
Randomisation
Dyads were randomised to receive either the intervention (carer training) or the control (usual care). This was completed using a secure online randomisation system at NWORTH. 54 Randomisation was carried out by dynamic allocation to ensure that the trial maintained good balance to the allocation ratio of 1 : 1. The stratification variables were recruitment centre and cancer/non-cancer diagnosis.
The group allocations overall and by stratification variable are presented in Table 8. Equal balance was achieved for the groups. The majority of patients who were recruited had cancer.
| Stratification variable | Intervention | Usual care | Total |
|---|---|---|---|
| Recruitment centre | |||
| BCUHB | 12 | 11 | 23 |
| CVUHB | 1 | 2 | 3 |
| GCS | 7 | 7 | 14 |
| Cancer/non-cancer | |||
| Non-cancer | 3 | 4 | 7 |
| Cancer | 17 | 16 | 33 |
| Total | 20 | 20 | 40 |
In the follow-up data it is important to note that three dyads did not complete the follow-up visit, but the RN was able to obtain the information collected at follow-up from the patients’ medical notes. Of these dyads, one was allocated to the intervention group and two were allocated to the usual-care group.
Patient demographics
There were 40 dyads who were randomised to the study. Among these 40 dyads, 22 carers completed the follow-up and it was possible to obtain some information from medical notes for a further three patients; therefore, partial data are available for 25 patients at follow-up.
The mean age of the patients who were randomised was 68.3 years (SD 12.6 years); 23 out of the 40 (57.5%) patients were male and 17 (42.5%) were female. Information on rurality was available for only three patients (two rural and one semirural) from the quantitative data, although rurality was revealed for a subset who took part in the qualitative study.
There were 18 (out of 25 patients with data, 72%) patients who died at home, with other patients dying in either a hospital (n = 2, 8%) or a hospice (n = 5, 20%). Seven patients (28%) had at least one unscheduled admission to hospital. Of these seven patients, six died during admission and one died at home following admission.
Carer demographics
The mean age of the carers who were randomised to the study was 56.6 years (SD 12.0 years); 35 out of the 40 (87.5%) carers were female and five (12.5%) were male. Half of the 40 patients were cared for by their spouse/partner (n = 20, 50%), 15 (37.5%) were cared for by their child, three (7.5%) were cared for by another relative and two (5%) were cared for by a friend. There were 32 (80%) carers who lived with the patient and eight (20%) who did not live with the patient. Of the 40 carers, 26 (65%) declared that they lived in their usual place of residence and 14 (35%) did not live in their usual place of residence. There were 31 (77.5%) carers who had a role in managing the patient’s medications prior to involvement in the trial and eight (20%) who did not have this role prior to the trial; data were missing for one carer. Half of the carers were the sole carer (n =20, 50%) and 18 (45%) were not the sole carer; data were missing for two carers.
Medications
At baseline, 33 out of the 40 (82.5%) patients had anticipatory prescribing in place and seven (17.5%) did not. Medication was prescribed by a medical prescriber from the primary care team (13/32, 40.6%), a medical (9/32, 28.1%) or non-medical (8/32, 25%) prescriber from the SPC team or a non-medical prescriber from the secondary care team (2/32, 6.3%). At death, all patients (n =25) had anticipatory prescribing in place. Of these patients, nine (36%) had this prescribed by a medical prescriber from the SPC team, six (24%) had this prescribed by a non-medical prescriber in the SPC team, seven (28%) had this prescribed by a medical prescriber in the primary care team, one (4%) had this prescribed by a non-medical prescriber in the primary care team and one (4%) had this prescribed by a medical prescriber in the secondary care team. Information on who prescribed the medication was missing for one patient. Of the 25 patients who had anticipatory prescribing in place at death, four patients did not have this in place at the baseline visit.
At baseline, 27 out of the 40 (67.5%) patients did not have a continuous SC infusion set up and 13 (32.5%) did. Of those who did have a continuous SC infusion, six (46.2%) were prescribed it by a medical prescriber in the primary care team, two (15.4%) were prescribed it by a medical prescriber from the secondary care team, one (7.7%) was prescribed it by a non-medical prescriber in the secondary care team, two (15.4%) were prescribed it by a medical prescriber in the SPC team and one (7.7%) was prescribed it by a non-medical prescriber in the SPC team. Information regarding who prescribed the medication was missing for one patient. In total, 21 out of the 25 (84%) patients had a continuous SC infusion set up at death and four (16%) did not. Among patients who had a continuous SC infusion, eight (38.1%) were prescribed it by a medical prescriber in the primary care team, six (28.6%) were prescribed it by a medical prescriber in the SPC team, three (14.3%) were prescribed it by a non-medical prescriber in the SPC team, two (9.5%) were prescribed it by a non-medical prescriber in the primary care team and one (4.8%) was prescribed it by a medical prescriber in the secondary care team. Information regarding who prescribed the medication was missing for one patient.
At baseline, the numbers of patients who had been prescribed anticipatory SC medication were 36 (out of 40, 90%) for pain, 25 (62.5%) for anxiety/restlessness, 31 (77.5%) for nausea/sickness and 21 (52.5%) for noisy breathing.
At the time of death, of the 25 patients for whom there were data, the numbers of patients who had anticipatory SC medication prescribed were 18 (72%) patients for pain, 19 (76%) for anxiety/restlessness, 14 (56%) for nausea/sickness and 15 (60%) for noisy breathing.
Services
In total, 21 out of the 25 (84%) dyads had accessed GP services and four (16%) had not. DN services were accessed by 21 (84%) dyads and four (16%) had not accessed them. There were 20 (80%) dyads who had accessed nursing SPC and four (16%) who had not; data were missing for one dyad. There were 13 (52%) dyads who did not access medical SPC and 10 (40%) who did; data were missing for two (8%) dyads. Other nursing was not accessed by 14 (56%) dyads and was accessed by 11 (44%); data were missing for one dyad. For health-care support, 16 (64%) dyads did not access this and seven (28%) did access this; data were missing for two (8%) dyads. Other allied health professionals were not accessed by 16 (64%) dyads but were accessed by six (24%), with missing data for three dyads (12%).
Carer training
Intervention
Competency checklist
The competency checklist ensured the safety of dyads in the intervention group. It was signed off by the HCP on completion of training to confirm that the carer was competent in the processes associated with delivering SC medications. It was intended to be reviewed on an ongoing basis and, if necessary, the dyad would be withdrawn.
In BCUHB, eight competency checklists were completed and returned to the trial office after the patient’s death. One additional form was completed at the time but later misplaced (this was reported as a protocol deviation). A further three carers were not trained to competency (two patients were admitted to a hospice and one died soon after randomisation). Some carers were trained to competency but requested that the HCP administered the medication when the patient required treatment for breakthrough symptoms.
In GCS, seven dyads were randomised to the intervention group and there was one competency checklist returned to the trial team. One carer withdrew owing to concerns over the ‘last injection’ and another dyad was withdrawn as part of close-out arrangements before the carer could be trained. Patients in two dyads died soon after randomisation, and for a further two dyads competency training was not completed before the patient died.
The competency checklist was returned for the dyad who was randomised to the intervention in Cardiff.
Although we do not have full information about the time taken to train carers, informal feedback from DNs and competency checklist documentation indicated that, in some cases, multiple visits were needed before a carer achieved competency.
Usual care
Dyads randomised to usual care were provided with a carer diary by the DN or RN and were instructed to complete a medication entry when the patient received medication from the DN, and a QOLLTI-F every 48 hours. The HCPs continued usual-care duties with these patients and a daily checklist was included in the notes to prompt DNs of necessary trial processes for these patients. However, there appeared to be minimal trial-related contact with the usual-care group participants.
Carer diary analyses
Carer diary returns
It was intended that the DN would collect the diaries post bereavement and return them to the trial team, but diaries were also collected by RNs post bereavement or by the qualitative researcher during the carer interview. Carers indicated that they would have appreciated feedback on the diaries for reassurance that these had been completed correctly. Table 9 details reasons why diaries were not returned.
| Site | Usual care | Intervention |
|---|---|---|
| BCUHB |
Received one, expected 11 (9.1%) Reasons for non-return: |
Received nine, expected 12 (75.0%) Reasons for non-return: |
| CVUHB |
Zero received, two expected (0%) Reasons for non-return: |
One received, one expected (100%) |
| GCS |
Two received, seven expected (28.6%). One diary was returned blank (further details unknown) Reasons for non-return: |
One received, seven expected (14.3%) Reasons for non-return: |
General feedback on the carer diaries included that some of the text was too small and that clarification was needed on confidence and symptom scales in the medication administration tables. On the prescribed medication table, additional lines would support dose changes and the option to add more than one medication per symptom when drugs are used in combination (e.g. cyclizine and haloperidol). The table could also be prepopulated with ‘3’ in the maximum number of doses per 24-hour period. One carer was unsure whether this was a rolling 24-hour period or from 00.01 a.m. to midnight. This could be changed in a future version along with the Medication Guide for Carers.
Symptoms resulting in medication administration
The number of occurrences per patient of pain, nausea or vomiting, anxiety/restlessness and noisy breathing that resulted in medication administration is presented in Figure 4. Three dyads in the usual-care group completed 20 medication administration entries and 11 in the intervention group completed 147 medication administration entries. These dyads are labelled UC1 to UC3 for the usual-care group and I1 to I11 for the intervention group. Most patients have zero, one or two occurrences of each symptom resulting in medication administration, with some patients having six or seven occurrences. One patient in the intervention group had 28 occurrences of pain and 43 occurrences of anxiety/restlessness recorded.
FIGURE 4.
Number of medication administrations per dyad.
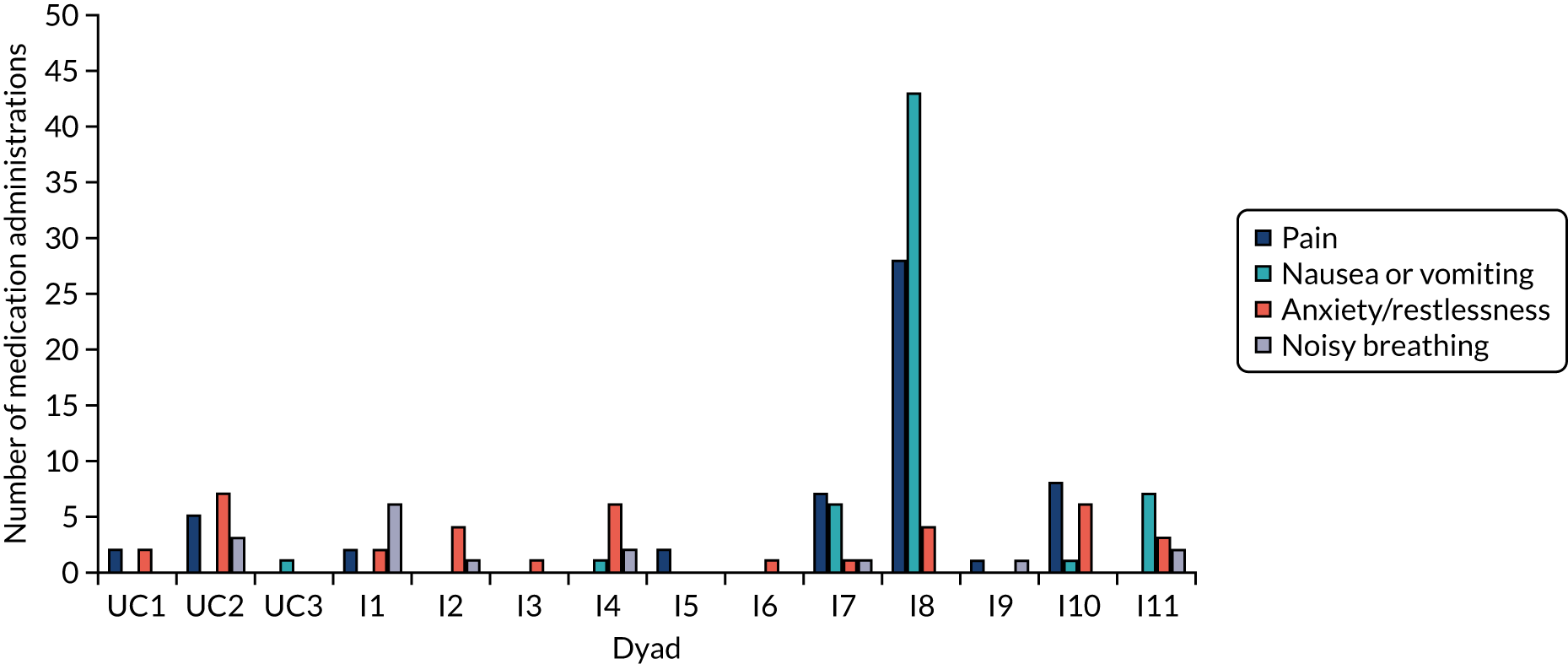
The carer diaries were assessed for completion by considering the medication administration entries only (i.e. documentation regarding administering medications).
In the intervention group, among the 147 medication administration entries, when the carer administered the medication (n = 131, 89.1%) the medication administration documentation was:
-
100% completed in 10 instances (7.6%)
-
70–99% completed in 116 instances (88.5%)
-
50–69% completed in five instances (3.8%).
In the intervention group, when the HCP administered the medication (n = 10, 6.8%) the medication administration documentation was:
-
70–99% completed in nine instances (90%)
-
50–69% completed in one instance (10%).
In the intervention group, when it was not specified who administered the medication (n = 6/147, 4.1%) the medication administration documentation was:
-
70–99% completed in four instances (66.7%)
-
50–69% completed in one instance (16.7%)
-
1–49% completed in one instance (16.7%).
In the usual-care group (n = 20), when the HCP always administered the medication the medication administration documentation was:
-
100% completed in one instance (5%)
-
70–99% completed in 17 instances (85%)
-
50–69% completed in two instances (10%).
Therefore, the medication administration documentation was at least 70% complete for 94.5% of medication administration instances in the intervention group (139/147) and 90% of instances in the usual-care group (18/20). Overall, across both groups, documentation was at least 70% complete for 94% of all medication administration instances (157/167).
Time to symptom relief
Time to symptom relief refers to the time from the onset of symptom to medication administration and the time from medication administration to symptom resolution.
Time from onset of symptom to medication administration
One important item of information was the time period from the onset of symptom to the time of medication administration. For this to be calculated, the time of symptom onset and the time of medication administration were required. The time of symptom onset was available for 131 out of the 147 (89.1%) medication administration entries in the intervention group and 19 out of the 20 (95%) in the usual-care group. The time of medication administration was available for 136 out of 147 (92.5%) medication administration entries in the intervention group and 16 out of 20 (80%) in the usual-care group.
The intervention group had a considerably shorter time from onset of symptom to medication administration than the usual-care group: the median number of minutes [and associated interquartile range (IQR)] from onset of symptom to medication administration in the intervention group was 5 minutes (IQR 0–18 minutes) and in the usual-care group it was 105 minutes (IQR 74–330 minutes). In the intervention group, the median time from onset of symptom to medication administration was 10 minutes (IQR 0–20 minutes) for pain, 0 minutes (IQR 0–7 minutes) for nausea/vomiting, 10 minutes (IQR 0–30 minutes) for anxiety/restlessness and 20 minutes (IQR 15–77.5 minutes) for noisy breathing. In the usual-care group, the median time from onset of symptom to medication administration was 120 minutes (IQR 55–285 minutes) for pain, 120 minutes (IQR not applicable as only one instance) for nausea/vomiting, 92.5 minutes (IQR 70.5–461.25 minutes) for anxiety/restlessness and 80 minutes (IQR not applicable as only two instances) for noisy breathing. The time from onset of symptom to medication administration is presented in Figure 5. This graph presents the median time with whiskers from the lower quartile to the upper quartile.
FIGURE 5.
Median time between symptom onset and medication administration (minutes).
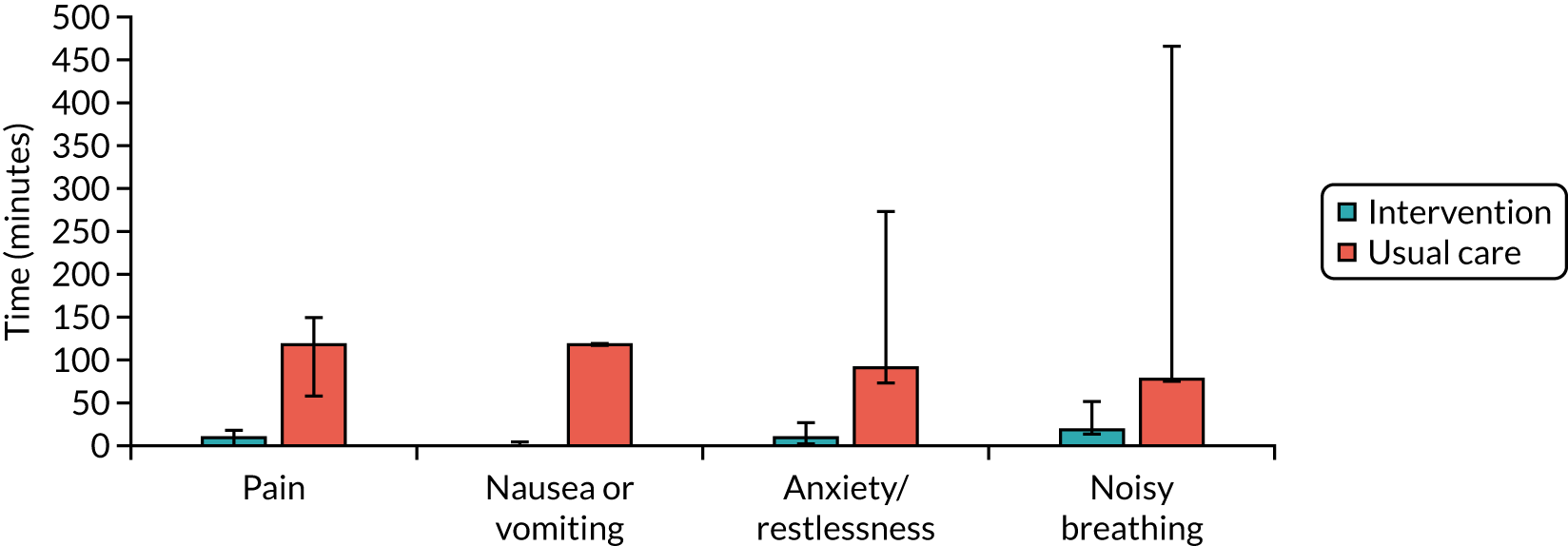
In the usual-care group, it was possible to calculate the time from onset of symptom to medication administration for 15 out of the 20 (75%) medication administration entries. Of these 15 entries:
-
in three instances (20.0%) medication was administered within 1 hour
-
in seven instances (46.7%) medication was administered within 2 hours
-
in one instance (6.7%) medication was administered within 3 hours
-
in one instance (6.7%) medication was administered within 6 hours
-
in one instance (6.7%) medication was administered within 7 hours
-
in two instances (13.3%) medication was administered within 15 hours.
In the intervention group, it was possible to calculate time from onset of symptom to medication administration for 127 out of the 147 (86.4%) medication administration entries. Of these 127 entries, medication was administered by the carer in 114 instances (89.8%) and by a HCP in eight instances (6.3%). In five (3.9%) instances it was not specified who administered the medication.
For the entries in which the carer administered medication (n = 114), it was administered:
-
within 1 hour in 104 instances (91.2%)
-
within 2 hours in five instances (4.4%)
-
within 3 hours in one instance (0.9%)
-
within 4 hours in three instances (2.6%)
-
within 5 hours in one instance (0.9%).
When the HCP administered the medication (8/127, 6.3%) or when it was not specified who administered the medication (5/127, 3.9%), it was administered within 1 hour.
In the intervention group, in 56 out of the 147 (38.1%) medication administration entries the time of medication administration was the same as the time of symptom development. Of these entries, 50 were carer administered, four were HCP administered and for two entries it was not specified who administered the medication.
Symptom scores
The symptom scores before and 30 minutes after treatment are presented in Figure 6. This graph presents the median with whiskers from the lower quartile to the upper quartile. Scores are from 0 to 10, with 0 being no symptom and 10 being the worst the symptom can possibly be.
FIGURE 6.
Median symptom scores before and 30 minutes after treatment.
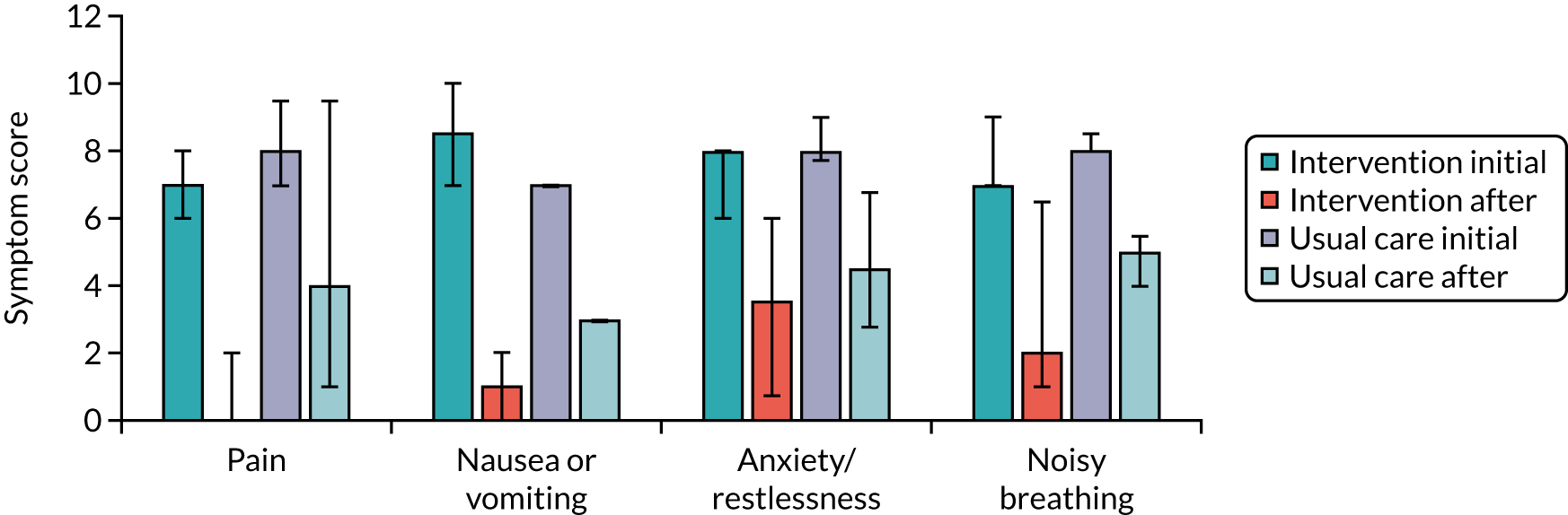
The symptom scores in the intervention group were completed for 81 out of the 147 (55.1%) medication administration entries at the time of symptom development and for 69 (46.9%) 30 minutes after medication administration. The symptom scores in the usual-care group were completed for 19 out of the 20 (95%) medication administration entries at the time of symptom development and for 19 out of the 20 (95%) entries 30 minutes after medication administration.
Both groups had an improvement in symptoms demonstrated by a decrease in the symptom scores.
In the intervention group, the symptom scores were as follows [initial median score (IQR), median score 30 minutes after treatment (IQR)]:
-
pain – 7 (6–8), 0 (0–2)
-
nausea/vomiting – 8.5 (7–10), 1 (0–2)
-
anxiety/restlessness – 8 (6–8), 3.5 (0.25–6)
-
noisy breathing – 7 (7–9), 2 (0–7).
In the usual-care group, the symptom scores were as follows [initial median score (IQR), median score 30 minutes after treatment (IQR)]:
-
pain – 8 (7–10), 4 (1–10)
-
nausea/vomiting – 8.5 (not applicable as only one instance), 1 (not applicable as only one instance)
-
anxiety/restlessness – 8 (7.25–9), 4.5 (2.25–8.25)
-
noisy breathing – 8 (not applicable as only three instances), 5 (not applicable as only three instances).
In the intervention group, the symptoms were noted by the carer in 124 out of the 147 (84.4%) medication administration entries. In the usual-care group, the symptoms were noted by the carer initially in eight out of the 20 (40%) medication administration entries and by the HCP in six out of the 20 (30%) entries.
Time from medication administration to symptom resolution
There was a question in each medication administration entry that asked whether or not symptoms had resolved within 30 minutes of medication administration (yes/no). If the symptoms had not resolved within 30 minutes, a follow-up question asked to record how many minutes had passed until the symptom had been resolved to an acceptable level.
Intervention group
In 127 out of the 147 medication administration entries it was possible to calculate time from onset of symptom to medication administration time, and information was available for 116 (91.3%) entries that made it possible to calculate time to symptom relief.
Of these 116 entries, 103 (88.8%) had resolved to an acceptable level within 30 minutes and 13 (11.2%) had not. Of the 103 entries in which symptoms did resolve to an acceptable level, 92 (89.3%) were administered by the carer, eight (7.8%) by the HCP and in three (2.9%) it was not specified who administered the medication. The 13 that had not resolved to an acceptable level within 30 minutes took a median of 45 minutes (range 40–80 minutes, IQR 41.25–60 minutes) to resolve. For the cases in which the HCP administered the medication, the arrival time was not recorded; therefore, it would not be possible to calculate the time to symptom relief minus the travel time of the HCPs.
We explored the relationship between the initial symptom score and the time to symptom resolution. In the intervention group there were 10 (out of 147, 6.8%) instances in which it was not specified whether or not the symptoms resolved within 30 minutes, which had an initial median symptom score of 7.5 (IQR 6.75–9.25).
In terms of individual symptoms:
-
In 25 out of 48 (52.1%) instances, pain resolved within 30 minutes, with an initial median symptom score of 7 (IQR 6–8). The two instances of pain that took longer than 30 minutes to resolve took a median of 70 minutes to resolve, and had an initial median symptom score of 6 (IQR not applicable).
-
In 17 out of 58 (29.3%) instances, nausea or vomiting resolved within 30 minutes, with an initial median symptom score of 9 (IQR 7–10). The one instance of nausea or vomiting that took longer than 30 minutes to resolve took 45 minutes to resolve, with no symptom score specified.
-
In 10 out of 28 (35.7%) instances, anxiety/restlessness resolved within 30 minutes, with an initial median symptom score of 7.5 (IQR 6–8). The seven instances of anxiety/restlessness that took longer than 30 minutes to resolve took a median of 45 minutes to resolve, based on six results, and had an initial median symptom score of 8 (IQR 7–9).
-
In 7 out of 13 (53.8%) instances, noisy breathing resolved within 30 minutes, with an initial median symptom score of 7 (IQR 5–8). The three instances of noisy breathing that took longer than 30 minutes to resolve took a median of 45 minutes to resolve, and had an initial median symptom score of 9 (IQR not applicable).
From these results there is no relationship between initial symptom score and the time to symptom resolution.
We analysed cases in which medication administration was required for the same symptom/indication within 2 hours. There was only one case in the intervention group in which this occurred. Agitation was noted by the carer at 09:45 (symptom score 8/10) and 2.5 mg of midazolam was administered by the carer at 09:50. Thirty minutes after medication administration the symptom score was 5/10, which the carer assessed to be resolved to an acceptable level. HCP support was not sought at this stage. At 10.45, the carer recorded an agitation symptom score of 7/10 and administered 2.5 mg of oxycodone. It was recorded that the symptom resolved completely within 30 minutes (symptom score 0/10). It is unclear from the entry if HCP support was sought, but the following entry at 12.50 was completed by a HCP. The HCP administered 2.5 mg of midazolam for agitation at 12:50 and the symptom was resolved to an acceptable level within 30 minutes. There were no further medication administration entries for that day.
Usual-care group
In the usual-care group, of the 15 (75%) medication administration entries in which it was possible to calculate the time to symptom resolution, there were four entries (26.7%) in which symptoms resolved within 30 minutes of medication administration and two entries (13.3%) in which symptoms did not. The two entries in which symptoms did not resolve within 30 minutes took 120 minutes and 330 minutes to resolve. There were also two entries in which it was not possible to calculate the time to symptom resolution because the time of medication administration was not recorded, but it was stated that symptoms resolved within 30 minutes of administering medication. It would, therefore, be possible to calculate time to symptom relief for six (40%) entries. The time of contacting the HCP and the arrival time of the HCP were recorded in all six instances; therefore, it would be possible to calculate the time to symptom relief minus the travel time of the HCPs for all cases.
We explored the relationship between the initial symptom score and the time to symptom resolution. In the usual-care group, there were 11 (out of 20 medication administration entries, 55%) instances in which it was not specified whether or not the symptoms resolved within 30 minutes, which had a median initial symptom score of 9 (IQR 8–9).
In terms of individual symptoms:
-
In two out of seven (28.6%) instances, pain resolved within 30 minutes, with an initial median symptom score of 7.5. The two instances of pain that took longer than 30 minutes to resolve took a median of 225 minutes to resolve, and had an initial median symptom score of 8.5.
-
In one (100%) case, nausea or vomiting resolved within 30 minutes, with an initial symptom score of 7.
-
In two out of nine (22.2%) instances, anxiety/restlessness resolved within 30 minutes, with an initial median symptom score of 6.
-
In one out of three (33.3%) cases, noisy breathing resolved within 30 minutes, with an initial symptom score of 8.
From these results there is no relationship between initial symptom score and the time to symptom resolution.
Carer confidence (intervention group)
Carers in the intervention group were asked to note how confident they felt about administering the medication. This was a scale from 1 to 7, with a higher score indicating higher confidence. Of the 11 carers in the intervention group, nine (81.8%) administered medications. There were some entries in which the HCP had administered the medication but a confidence score had been recorded; we report the confidence scores only when the carer administered the medication. Of the 131 instances in which the carer administered the medication, the confidence score was recorded in 128 (97.7%). For pain, nausea or vomiting and noisy breathing the median score was 7, the highest level of confidence, and the median score was 6 for anxiety/restlessness. The confidence scores over time for the nine carers are presented in Figure 7. The final score is 6 or 7 for all carers, but there is fluctuation over time.
FIGURE 7.
Carer confidence scores in the intervention group over time by individual. (a) I1; (b) I2; (c) I3; (d) I4; (e) I5; (f) I6; (g) I7; (h) I8; and (i) I9.
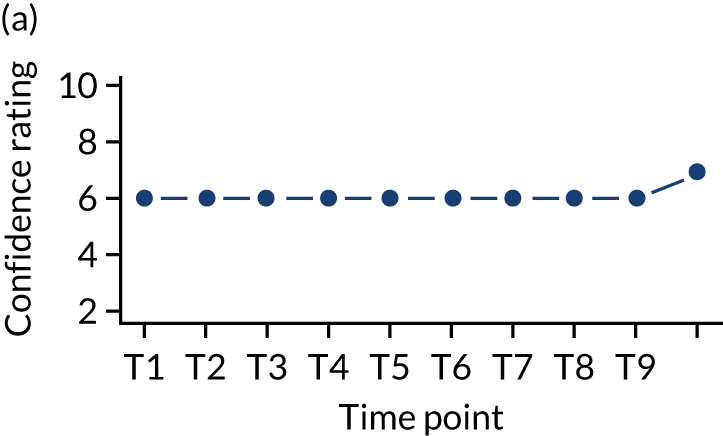
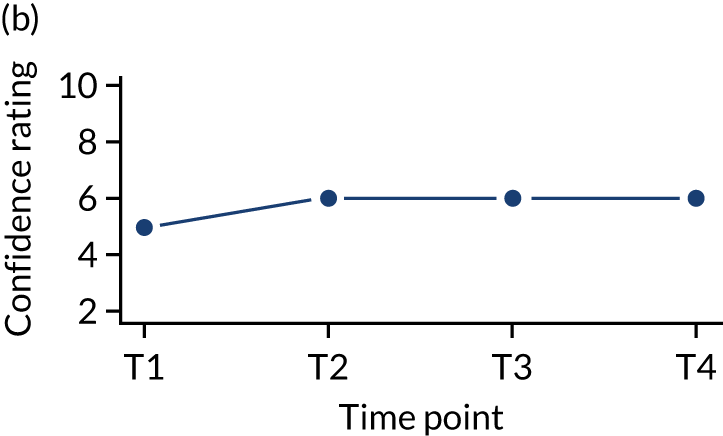
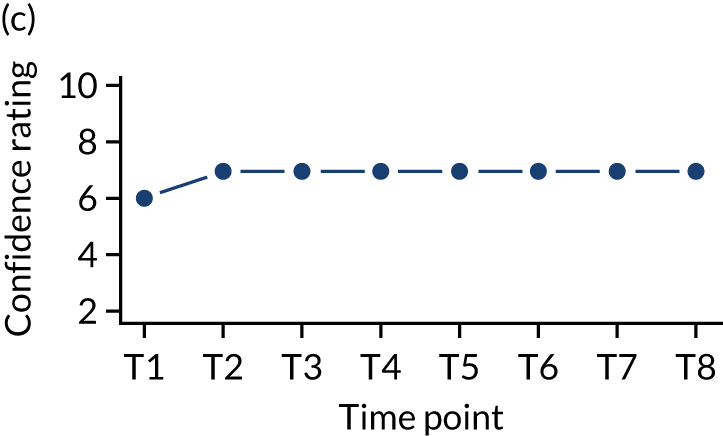
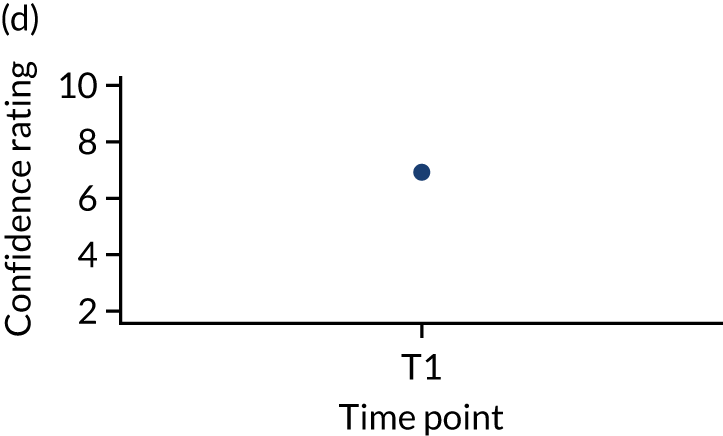
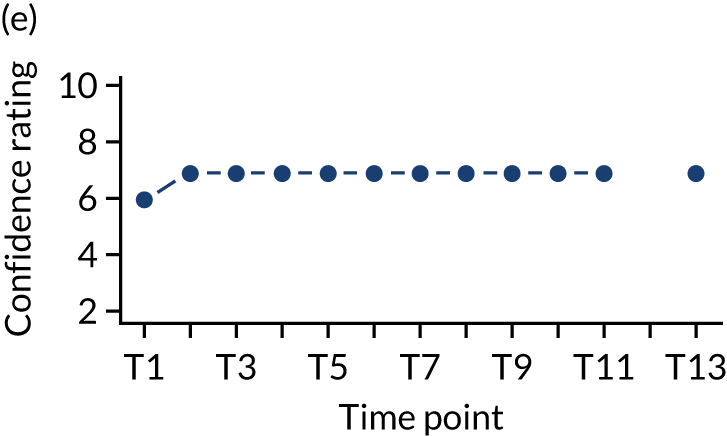
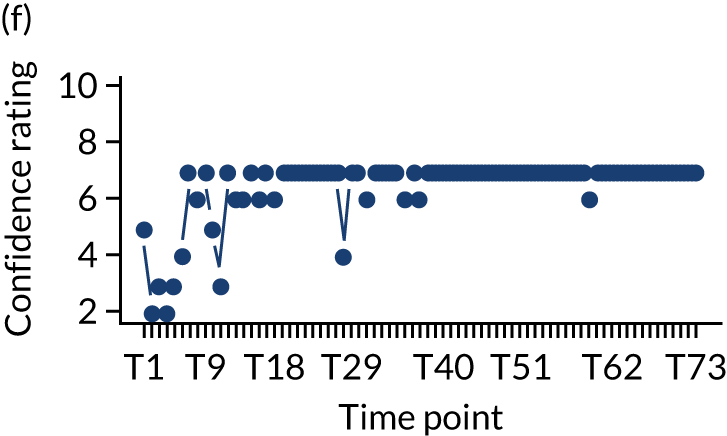
Health-care professional visits
In 24 out of the 147 (16.3%) medication administration entries in the intervention group it was recorded that HCP support was sought. In 119 (81%) instances it was indicated that HCP support was not sought and this question was not answered in four (2.7%) of cases.
In 24 (16.3%) instances in the intervention group it was recorded that HCP support was sought. These were not clustered towards the earlier part of the care episode when the carer administered medication. In 10 instances, medication was administered by a HCP. We did not have access to the patient’s usual medication administration record nor the community/DN notes to comment on the exact amount of times a HCP attended for these 24 instances.
In the usual-care group, a HCP visit was required for all 20 cases.
Withdrawal
In total, four out of the 40 (10%) dyads withdrew from the trial. A further two dyads far exceeded prognostic expectations and were withdrawn from the study by the trial team as part of close-out arrangements. Reasons for withdrawal are documented in Table 10. The GCS dyad who was withdrawn as part of trial close-out had not been trained to administer SC medication, but the local clinical team agreed to assist with carer training off study, without using trial materials, when the patient was at the point of needing SC medication for breakthrough symptoms.
| Randomisation allocation | Site | Reason for withdrawal |
|---|---|---|
| Usual care | CVUHB | Randomisation allocation |
| BCUHB | Randomisation allocation | |
| GCS | Randomisation allocation | |
| BCUHB | Withdrawn by the trial team as part of the close-out arrangements for patient who exceeded prognostic expectations (dyad had been in the study for 18 months at the time of withdrawal) | |
| Intervention | GCS | Concern about the ‘last injection’ |
| GCS | Withdrawn by the trial team as part of the close-out arrangements for patient who exceeded prognostic expectations (dyad had been in the study for 8 months at the time of withdrawal) |
Safety
Adverse event reporting
In total, there were seven AEs and 20 SAEs that we are aware of. Breakdown by site and randomisation allocation can be found in Table 11. Overall, reporting of AEs and SAEs by DNs was poor, particularly in North Wales. In most cases, the trial manager reported the event; therefore, it is possible there were other AEs/SAEs that the trial team were unaware of and that went unreported.
| Site | Intervention | Usual care | Total, n (%) (N = 27) | ||
|---|---|---|---|---|---|
| AE | SAE | AE | SAE | ||
| BCUHB | 4 | 3 | 0 | 9 | 16 (59.3) |
| CVUHB | 0 | 0 | 0 | 0 | 0 (0.0) |
| GCS | 2 | 6 | 1 | 2 | >11 (40.7) |
Of interest in Table 11 is that the total number of AEs/SAEs per site is approximately proportional to the recruitment at each site [23/40 (57.5%) at BCUHB, 3/40 (7.5%) at CVUHB and 14/40 (35%) at GCS].
Table 12 documents the causes of AEs. All were reported according to the BCUHB/Bangor University standard operating procedure (SOP) and, where necessary, further action was taken (e.g. additional team training).
| Treatment group | Summary of event |
|---|---|
| Intervention | The spouse telephoned DNs because the patient was agitated. When the DN attended there were no drugs available in the house. The DN arranged a joint visit with a GP who agreed that the patient needed a continuous SC infusion. The DN returned in the afternoon to commence the continuous SC infusion and the patient died peacefully the next day |
| When the DN attended, the carer advised they had to discard a vial of midazolam that had accidentally been smashed | |
| The RN attended the home of the patient to discuss the trial and obtain informed consent. As the RN prepared to leave, the patient said to the carer ‘now you can finish me off’. The patient left the room but the RN had a thorough and open conversation with the carer about legal and moral responsibilities and DN processes (checking drug stock tallys etc.). This comment and conversation took place before the dyad had been randomised. The RN also discussed with the DN who knew the dyad and she attributed it to their sense of humour but monitored them closely | |
| When the carer diary was returned to the trial team post bereavement, the medication administration entries had been signed by multiple carers | |
| When the diary was returned post bereavement it was noted that the drugs had been prescribed as a dose range rather than as a specific dose | |
| During RN visit the patient deteriorated rapidly, fell on the floor owing to terminal agitation and died. Hospice at home, DN and paramedics all attended | |
| Usual care | Patient fell from bed, ambulance called, crew helped patient back to bed |
All 20 SAEs were inpatient admissions to either a hospital or a hospice. Three patients were admitted once or more and one patient in the usual-care group was admitted eight times. It is understood that six patients died during their admission.
Carers were asked to return their diaries to the trial office as soon as practicable after the patient’s death. However, delays were sometimes unavoidable; for example, one diary was locked in the house of the deceased patient and their CARiAD carer lived elsewhere. Some of the SAEs/deviations were identified only many months post bereavement.
Protocol deviations
In total, 13 protocol deviations were identified. These were related to the recording of the RA process prior to approach (n = 5 deviations across four DN teams), sharing of patient-identifiable information with the central team (n = 2), having more than one carer involved in the trial processes (n = 2), errors on the medication table (n = 2), mismatch between trial documentation and MACRO (n = 1) and declining the use of a continuous SC infusion despite clinical advice (n = 1) (see Appendix 4).
Two were categorised as serious (RA recording = 1, mismatch between trial documentation and MACRO = 1) and all were dealt with according to local BCUHB/Bangor University SOP, and corrective action was taken as appropriate to each case.
The study team noted that the majority of protocol deviations reported were related to recording RAs. Although only one of the deviations related to RA recording was classed as serious, the fact that these issues occurred in two recruitment sites (BCUHB and GCS) and across different DN teams has clear implications for future work.
Survival
The survival times for patients are presented in Table 13 and Figure 8. This is the information that was available at the time of data-set lock; not all patients had died at this point and two were withdrawn from the study owing to longer than expected survival. Eight patients (8/25, 32%) survived for 28 days following the baseline visit and the median survival time was 23 days. Two patients died on the day of their baseline visit and the longest survival time was 153 days.
| Survival time | Total number of patients | Minimum | Maximum | Median | IQR |
|---|---|---|---|---|---|
| Intervention | 17 | 0 | 153 | 19 | 5–62 |
| Usual care | 8 | 1 | 90 | 25 | 4.75–40.5 |
| Overall | 25 | 0 | 153 | 23 | 5–55 |
FIGURE 8.
Proportion of patients surviving over time for both groups.
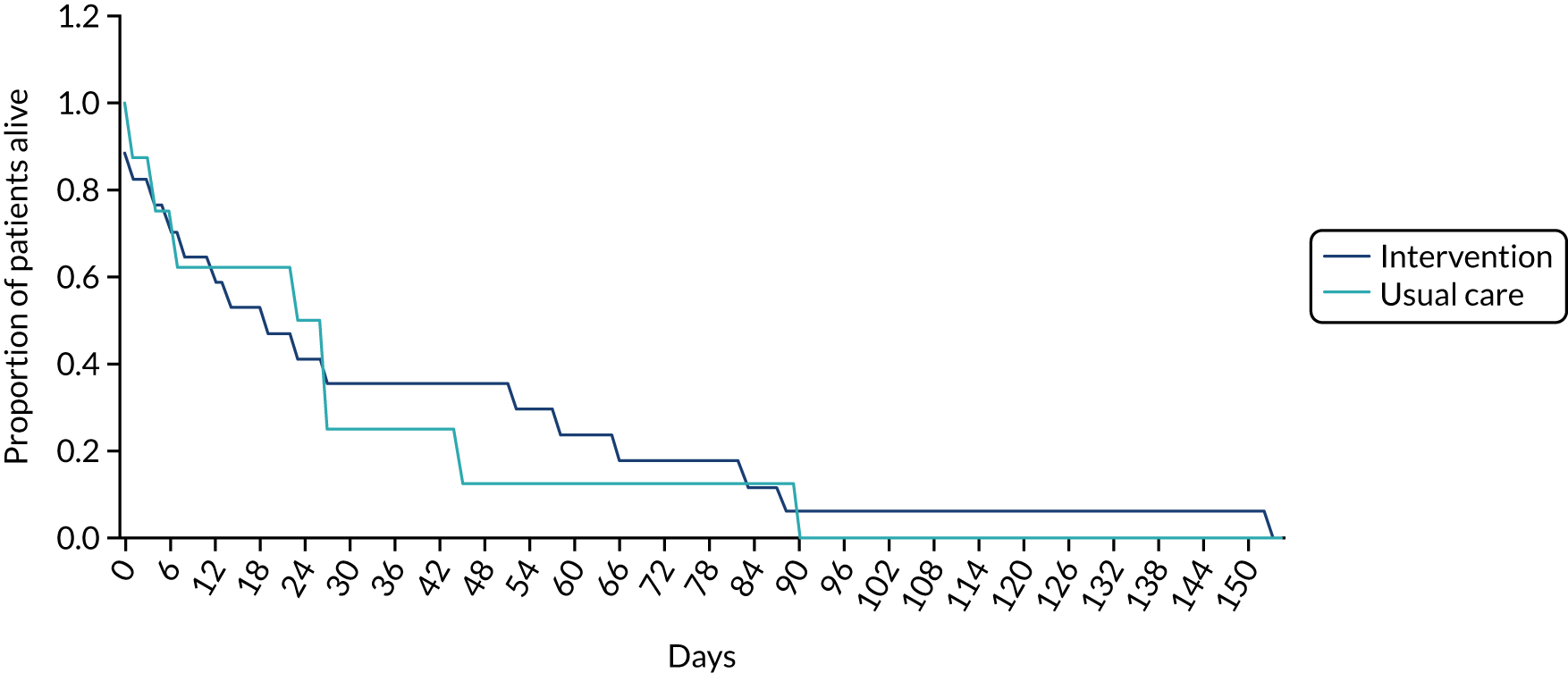
Survival per site
The survival times for the three sites are presented in Figure 9. The points on the plot represent the survival time for each individual patient. The box and whisker plot shows the median, quartiles and ranges of survival times.
FIGURE 9.
Box-and-whisker plot of survival (from consent to death) in days.
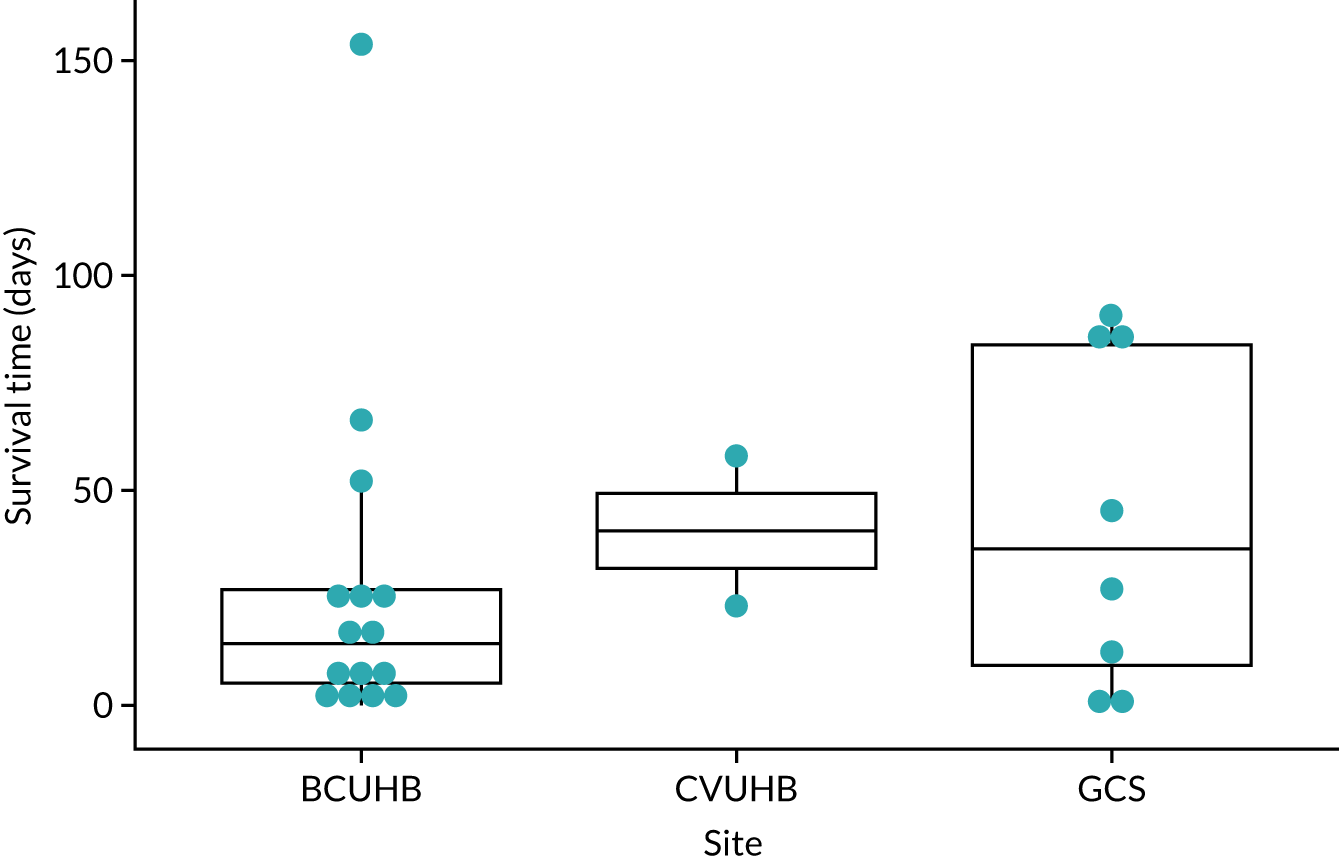
In BCUHB, one patient died on the day of consent, the longest survival time was 153 days and the median survival time for 15 patients (for whom the date of death was available) was 14 days. In CVUHB, two dyads completed the study: one patient survived for 23 days after consent and one patient survived for 58 days after consent. In GCS, one patient died on the day of consent, the longest survival time was 90 days and the median survival time for eight patients (for whom the date of death was available) was 36 days. The cumulative survival times are presented in Table 14. Within 28 days, 17 out of the 25 (68%) patients for whom there are data had died.
| Survival time | Site | ||
|---|---|---|---|
| BCUHB | CVUHB | GCS | |
| Total (n) | 15 | 2 | 8 |
| < 1 day (%) | 6.7 | 0 | 12.5 |
| < 3 days (%) | 13.3 | 0 | 25 |
| < 7 days (%) | 33.3 | 0 | 25 |
| < 14 days (%) | 46.7 | 0 | 37.5 |
| < 21 days (%) | 60 | 0 | 37.5 |
| < 28 days (%) | 80 | 50 | 50 |
| < 35 days (%) | 80 | 50 | 50 |
| < 42 days (%) | 80 | 50 | 50 |
| < 49 days (%) | 80 | 50 | 62.5 |
| < 56 days (%) | 86.7 | 50 | 62.5 |
Trial close-out
The CARiAD trial recruitment ended on 15 March 2019. As of 15 May 2019, three patients of dyads recruited to the trial were still alive. Detailed close-out arrangements were made for these dyads and were approved by the REC (see Appendix 5).
For those dyads for whom survival was longer than anticipated and post-dated the trial end, careful consideration was required on an individual basis to balance the expectations and communication needs of the dyad, burden of trial procedures and safety mechanisms built into the trial. There were also specific considerations based on whether the surviving dyads were in the usual care or intervention group; therefore, in essence, each case was considered separately. To inform these decisions, a detailed document was generated to propose ‘rules’ that differed for the intervention and usual-care group, and informed trial procedures. Specific information sheets for both dyads and HCPs were also generated to ensure that best communication took place.
Exploratory end points/outcomes for a future definitive trial
Family MSAS-GDI
Completion
The Family MSAS-GDI was one of the measures being considered as a primary outcome for a definitive trial. The criterion for considering the Family MSAS-GDI as a primary outcome is that at least 70% of the measure is completed. All carers who completed follow-up completed the Family MSAS-GDI. Of the 22 carers who completed the study, 19 (86.4%) completed all of the Family MSAS-GDI and three (13.6%) completed 75–99% of the measure. From these results, at least 80% of the measure was completed; therefore, from this perspective, the Family MSAS-GDI could be considered as a primary outcome in a definitive trial.
Descriptive statistics
The mean outcome measure scores are presented in Table 15. For the Family MSAS-GDI, a score is calculated as long as no more than three questions are unanswered. It was possible to calculate a score for the 22 carers who completed the study. The mean score for the intervention group was 1.41 (SD 0.65) and for the usual-care group 1.30 (SD 0.87).
| Group | Total number of patients | Minimum | Maximum | Mean | Standard deviation |
|---|---|---|---|---|---|
| Intervention | 16 | 0.55 | 2.82 | 1.41 | 0.65 |
| Usual care | 6 | 0.45 | 2.64 | 1.30 | 0.87 |
| Total | 22 | 0.45 | 2.82 | 1.38 | 0.70 |
Analysis
For the Family MSAS-GDI, the intention was to use an analysis of variance (ANOVA) model to analyse the data. Owing to the small sample size, it would be inappropriate to consider testing using analysis of covariance models as detailed in the analysis plan; therefore, we have provided estimates only of raw group differences in Table 16. For the Family MSAS-GDI, the intervention group had a mean score 0.11 points higher than that of the usual-care group. An increased score indicates increased global distress, although it is difficult to make inferences with the data that are available.
| Group | Total number of patients | Mean | Standard error | 95% confidence interval |
|---|---|---|---|---|
| Total | 22 | 1.38 | 0.15 | 1.07 to 1.69 |
| Intervention | 16 | 1.41 | 0.16 | 1.06 to 1.76 |
| Usual care | 6 | 1.30 | 0.35 | 0.39 to 2.21 |
| Intervention – usual care | 22 | 0.11 | 0.34 | –0.60 to 0.82 |
Quality of Life in Life-Threatening Illness – Family Carer Version
The QOLLTI-F was self-completed by the carer during the baseline visit. It was then anticipated to be completed when the first as-needed medication was administered, and every 48 hours thereafter until the patient’s death.
Completion
The expected and actual number of QOLLTI-F entries completed are presented in Table 17. Each line represents one dyad, with the number of QOLLTI-F entries that were completed and the number of QOLLTI-F entries that were expected to be completed. The expected number of entries was calculated based on the date the first dose of medication administration (by either the HCP or the carer) that was documented in carer diary. From this date to patient death, the QOLLTI-F should have been completed once every 48 hours.
| Dyad (site) | Actual number of QOLLTI-F entries returned | Expected number of QOLLTI-F entries returned (% of expected that were returned) |
|---|---|---|
| Intervention | ||
| BCUHB | 0 | 1 (0) |
| BCUHB | 0 | 1 (0) |
| BCUHB | 0 | 2 (0) |
| BCUHB | 3 | 8 (37.50) |
| BCUHB | 0 | 2 (0) |
| BCUHB | 4 | 8 (50) |
| BCUHB | 1 | 1 (100) |
| BCUHB | 0 | 2 (0) |
| BCUHB | 1 | 9 (11.11) |
| CVUHB | 1 | 3 (33.33) |
| GCS | 2 | 3 (66.67) |
| Total | 12 | 40 (30) |
| Usual care | ||
| BCUHB | 1 | 1 (100) |
| GCS | 0 | 5 (0) |
| GCS | 3 | 10 (30) |
| Total | 4 | 16 (25) |
The small number of diaries received means that it is not possible to make inferences based on differences between sites; however, the following observations were noted:
-
BCUHB – 10 diaries were returned (nine in the intervention group and one in the usual-care group). In the intervention, nine out of the 34 (26.5%) expected QOLLTI-F entries had been completed. One carer stopped completing the QOLLTI-F once they had used all of the space in the diary (three entries). It is possible that they did not alert the DN so that they could be provided with another diary to continue entering data every 48 hours (expected maximum eight entries). There were five carers who did not complete any QOLLTI-F entries and one who completed just one out of the potential nine expected entries.
-
CVUHB – one intervention group diary was returned and one out of the three (33.33%) expected QOLLTI-F entries had been documented.
-
GCS – three diaries contained completed QOLLTI-Fs: one from a dyad in the intervention group (2/3 entries) and two from the usual-care group. One carer had not completed any of the expected five entries and the other had completed three out of 10 (30%).
From the carer diaries alone, it is not clear why carers completed such a low proportion of the QOLLTI-F. After the baseline visit, completion of the QOLLTI-F was intended every 48 hours from the time of first injection for breakthrough symptoms to assess quality of life. However, the QOLLTI-F was completed for only six dyads: four in the intervention group and two in the usual-care group. For three of these dyads, the QOLLTI-F was completed only once after baseline, for one dyad twice and for two dyads three times. One dyad left 5 days and then 3 days between completing the measure. One additional dyad, who was not reported, made partial completions at intervals > 48 hours. The apparent difficulty completing this measure is explored in more detail in the qualitative results.
At baseline, one carer (1/40, 2.5%) did not complete the QOLLTI-F at all, 35 (87.5%) carers completed 100% of the QOLLTI-F and four (10%) completed 70–99% of the QOLLTI-F. From the post-baseline data, there were four instances in which 100% of the QOLLTI-F was completed in the usual-care group. In the intervention group, there were seven instances (7/12, 58.3%) in which 100% of the QOLLTI-F was completed, four (33.3%) instances in which 70–99% of the QOLLTI-F was completed and one instance (8.3%) in which 50–69% of the QOLLTI-F was completed.
The criterion for considering the QOLLTI-F as a primary outcome is that at least 70% is completed, which was true in all but one instance at baseline and one instance post baseline. The QOLLTI-F is, therefore, completed to a satisfactory level to be considered feasible. However, the QOLLTI-F is not completed as many times as it should be. At baseline, 97.5% of carers completed at least 70% of the QOLLTI-F. However, post baseline, 30% of the expected QOLLTI-F entries were completed in the intervention group and 25% were completed in the usual-care group. The carers also left > 48 hours between completing QOLLTI-F entries on some occasions. Therefore, despite being completed to a satisfactory level when it is completed, the QOLLTI-F is not completed as often as it should be. The percentage of the entries that should have been completed is low and so the QOLLTI-F cannot be considered the primary outcome for a definitive trial.
Descriptive statistics
Note that a score for the QOLLTI-F is calculated only if all questions are completed, given that there is no missing value rule. The measure was completed by 39 dyads at baseline and it was possible to compute a score for 35 dyads who fully completed the QOLLTI-F at baseline. The scores are very similar for both groups and overall, and the mean QOLLTI-F score is 7.11 (SD 1.34). The results are presented in Table 18.
| Group | Total number of patients | Minimum | Maximum | Mean | Standard deviation |
|---|---|---|---|---|---|
| Intervention | 16 | 4.68 | 8.97 | 7.22 | 1.09 |
| Usual care | 19 | 4.13 | 9.65 | 7.02 | 1.54 |
| Total | 35 | 4.13 | 9.65 | 7.11 | 1.34 |
Analysis
The intention was to analyse the QOLLTI-F data using a linear mixed model. This was not completed because there were not enough data to test this model. The raw data are presented in Table 19. Another dyad partially completed four instances of the QOLLTI-F after baseline, leaving 3, 4 and 3 days between the entries, respectively (not reported in Table 18, as total score cannot be calculated). These intervals are also longer than required.
| Group | Baseline QOLLTI-F | Diary QOLLTI-F 1 | Days to next QOLLTI-F | Diary QOLLTI-F 2 | Days to next QOLLTI-F | Diary QOLLTI-F 3 |
|---|---|---|---|---|---|---|
| Usual care | 8.35 | 8.58 | 5 | 7.53 | 3 | 7.33 |
| Usual care | 6.90 | 7.06 | N/A | |||
| Intervention | 7.33 | 5.61 | 2 | 7.04 | N/A | |
| Intervention | 6.30 | 7.89 | N/A | |||
| Intervention | 7.86 | 7.68 | 2 | 5.61 | 2 | 7.49 |
| Intervention | 7.98 | 5.77 | N/A | |||
Chapter 5 Embedded qualitative study: carers
Introduction
The first phase of the embedded qualitative study comprised 12 interviews with carers in both groups of the trial: 10 from the intervention group and two from the usual-care group. It was also intended to interview a sample of carers who declined to take part in the trial; however, all of the people who declined to take part in the trial also declined to be interviewed. The qualitative interviews were designed to explore views on the feasibility and acceptability of carer-administered opioids and to gain a deeper understanding of the meaning that delivering these medications has for carers of loved ones in the last days of their life. The interviews asked carers about the practical aspects of the trial, such as the recruitment process, use of trial materials and randomisation. They also explored the emotional impact for carers and the ways in which they were able to negotiate these difficult times.
Methods
Purposive sampling
Purposive sampling was used for the qualitative interviews with carers. The purposive sampling criteria used were geographical location, gender, age and rurality. As patients and carers were recruited to the trial they were added to sample monitoring tables, which were used to monitor participant characteristics and create a purposive sample for interviews. Using this system, carers were purposively identified to provide a range in terms of the purposive sampling criteria. Those carers were then invited to participate. These systems were in place and were followed; however, owing to study attrition, particularly in the usual-care group, carer characteristics (fewer men than women were recruited overall) and difficulty in contacting some carers, there was a need to adopt a more flexible approach to sampling, particularly as the study drew closer to closure. The final sample obtained is outlined in Table 20. Purposive sampling continued until data saturation was reached in the case of the intervention group, but it was not possible to reach this in the usual-care group owing to low numbers resulting from study attrition in the usual-care group.
| Characteristic | Site (n) | Total (n) | ||
|---|---|---|---|---|
| BCUHB | CVUHB | GCS | ||
| Group | ||||
| Intervention | 8 | 1 | 1 | 10 |
| Usual care | 0 | 1 | 1 | 2 |
| Gender | ||||
| Male | 3 | 0 | 0 | 3 |
| Female | 5 | 2 | 2 | 9 |
| Rurality | ||||
| Urban | 4 | 2 | 1 | 7 |
| Rural | 4 | 0 | 1 | 5 |
| Training | ||||
| HCP training | 2 | 2 | 1 | 5 |
| No HCP training | 6 | 0 | 1 | 7 |
| Total | 8 | 2 | 2 | 12 |
Conducting the interviews
A sample of 12 informal carers was contacted by telephone 2–4 months post bereavement. The qualitative researcher telephoned the carers and explained the purpose of the interview and what would happen should they choose to take part. The carers were provided with a PIS and informed consent was taken at the time of interview.
Interviews lasted 30–60 minutes and were carried out in participants’ homes, at the research centre if requested by the carer or by telephone if circumstances necessitated. Face-to-face interviews were used in the study. However, in the case of three carers, interviews were conducted by telephone owing to the carer living overseas and having travelled back home by the time of the interview, or owing to carer request as a result of working unsocial hours. In these three cases, consent was given by post prior to the interview.
All interviews followed a topic guide (see Appendix 6), were audio-recorded and were transcribed verbatim.
The rationale for using interpretive phenomenological analysis
The carer data were analysed using interpretive phenomenological analysis (IPA). 70 The carer aspect of the embedded qualitative component of this study was designed to explore, in depth, the views of carers and the meaning that administering the medication had for them. The intention was not to test a predetermined hypothesis; research questions of this flexible nature are suited to IPA. 71 IPA is an appropriate analysis method for research such as this and seeks to understand how a particular set of circumstances are perceived by an individual and how the person is making sense of those circumstances in the context of their own social world; this was exactly what we were aiming to do in the carer interviews. Semistructured interviews are the best method for IPA because they allow the researcher and participant to engage in dialogue, during which participants are able to give their accounts as they perceive them and the researcher is able to probe areas that provide depth and richness to the data. 71 IPA was chosen as the ideal method for analysis in this study, as it sought to explore personal accounts, views and experiences. 70
The process of interpretive phenomenological analysis
In accordance with the principles of IPA, we followed a process in which transcripts were read thoroughly, case by case, and annotated with notes that were made of observations, insights and points of significance. We also made sure that notice was taken of participants’ use of language and the meanings behind their words and phrases. Each transcript was then re-read and potential emergent themes were noted. IPA follows an idiographic approach, starting with singular examples and slowly building up to wider, more comprehensive categories. 72 In this study, this was achieved by the case-by-case approach to the transcripts and the careful noting of examples. Transcripts were then re-read in more detail and themes organised into groups. The final phase was an in-depth defining and developing of themes and examining relationships between them, which included team involvement. Statements were then made to create a meaningful account. 70
Rigour and reflexivity
During the carer interview phase it was important to consider rigour at all times, which includes reliability and validity that may be translated to trustworthiness in qualitative research. 73 We believe that the methods have been well documented and could be repeated by another researcher, if trustworthiness were to be assessed. The interpretations given within this report are in participants’ own words and illustrate that interpretations reached are grounded in the raw data. 74,75 For this qualitative work, it was also important to consider the role of the researcher in creating the data and care was taken to ensure that no bias was introduced into the data. In this study, rigour and reflexivity were also considered. These were addressed by undertaking several team interpretation workshops to ensure that interpretations were true to the transcripts and analysis was careful and thorough.
Ethics considerations
The interviews with carers were likely to involve emotive topics. It was therefore of utmost importance for the interviewer to carry out the interviews in an appropriately sensitive manner. If a carer became distressed, they were given the opportunity to pause or discontinue the interview. It was also felt important to bear in mind that emotion does not always equal distress,76 so participants were offered the opportunity to continue the interview even if they became emotional, allowing them to decide for themselves whether or not they wanted to continue. Information on signposting to relevant HCPs was also available if necessary.
In the carer interviews, some participants did indeed become emotional; however, no carer decided to discontinue the interview. Rather, they seemed to find the experience somewhat cathartic and described taking comfort in the knowledge that contributing to research may help others in the future.
Sample
The characteristics of the carers interviewed are shown in Table 20.
Results
Summary of key themes
The findings from the carer interviews are presented thematically below. The analysis process revealed key themes and subthemes that held meaning and importance for carers. The initial approach for the study, timing and first impressions were found to be instrumental in influencing decision-making regarding taking part. Carers also expressed their views and experiences of the training provided and of completing the carer diaries. Many of them found that the diaries were a useful tool for reflecting on their experiences and, for those in the usual-care arm, they added a sense of participation. The use of medications and equipment was also discussed during interviews, in terms of both the practical and the emotional aspects of delivery.
Further emotional themes emerged from the analysis, with carers showing concerns regarding the management of symptoms, particularly pain, and their ability to recognise when a patient was in need of medication. This was also bound with concerns about waiting times and delays, as no one wanted prolonged periods of pain or suffering.
The potential of the intervention to empower patients and carers was also of salience in the interviews, as was the deep desire for carers to fulfil the wishes of their loved ones and to keep them at home. However, this also came with the notion of potential carer burden and the possibility of the needs of the patient being prioritised over their own needs as the carer.
First impressions and recruitment
Carers were asked to discuss their first thoughts when approached for recruitment to the trial. Some carers reported initial hesitancy; however, this diminished with further explanation of how the intervention would work. Several carers described feelings of surprise, as they had never before considered the possibility that carers could administer these kinds of medications. There was also relief at the realisation that there was something more that they could do to help keep loved ones at home.
Timing of recruitment
The timing of approaching the carers in this study was complicated. Difficulties of prognostication for the last days of life means that there was no exact time specified for approach. Carers of patients who deteriorated at a slower rate appeared to have had more time to mentally and emotionally prepare for the prospect of administering the medications and were more likely to describe their recruitment as occurring at the right time. Carers of patients who experienced a more rapid deterioration reported that they would have preferred to have been approached earlier than they were. Reasons for this included, as described by one carer, wanting to have had more time to prepare and to be trained:
I think it might’ve been, might’ve been better if it’d come a little bit earlier because [husband] had already gone downhill quite a bit and it was virtually er, you know, the syringe drivers were already – they hadn’t been put up yet but the plan was soon and so he’d already deteriorated and um er I think if it had been a little bit earlier it might’ve been better.
Hillary, urban, intervention
Randomisation
Carers’ accounts indicated a less than complete understanding of the randomisation process. Carers talked of randomisation in terms of being ‘selected’, ‘chosen’ or ‘accepted’ for the trial itself, rather than having been allocated to either group as a result of the randomisation process.
Carers who were allocated to the intervention group reported being pleased with their allocation, although there was misunderstanding of the randomisation process. Carers expressed relief at having been ‘chosen’, as if this had been done on purpose. Some questioned the randomisation and were doubtful of how the trial could be random at all, confusing the different common usages of the word ‘random’. One carer described that, although it had been explained to him that this was a randomised trial, he felt that he knew that he had already been chosen because it would not be possible to do something of this nature at random:
And I’d said from the start, ‘Well, I think it’s random but I bet you’ll choose me’. And then straightaway. So I always felt as though everybody said it was random, but when I were asked, you’d already picked me to do it anyway, sort of thing.
Tom, urban, intervention
Carers who were allocated to the usual-care group expressed disappointment with their allocation and reported feeling that they had been offered an opportunity to do something beneficial that had then been withdrawn. One carer in the usual-care group described her initial disappointment and that the patient had been ‘angry’ about the allocation; however, the carer later felt relieved that she could not administer the drugs because she had the sense that she was being manipulated by the patient. Carers in the intervention group who were asked how they would have felt had they been allocated to the usual-care group explained that they would have been very disappointed at having had their hopes raised by the prospect of the intervention and then being let down, as they would not be in the administering group. The ways in which carers and patients view randomisation is important, as allocation to the usual-care group has the potential to cause distress if participants believe that they have somehow failed to be accepted to the intervention group for reasons other than it being a random process:
The way it was presented to us, it was more that this was something that we could do, rather than something that we might not be able to do. And I think it was quite hard on the district nurse who had to tell to tell [the patient], as I say, she was still an angry person and that made her angry.
Kathy, urban, usual care
Trial materials and training
Trial materials
The trial materials were generally well received. Carers were happy with the guide for carers and appreciated that the DNs or RNs who had explained the study had read through the information with them. Although most carers described the materials as accessible and in lay language, one carer was concerned that, although she herself had understood them, the language in the materials might not be accessible to all.
Carer diaries
The carer diaries appeared to have several functions for the carers in this study. They provided a means of record-keeping for carers themselves as well as for the study. This enabled carers to easily keep track of all of the symptoms and medications. Several carers had already been keeping their own diaries or logs of oral medications and saw this as a useful extension. It also gave them something to show HCPs when they visited to inform them what medications had been given and when.
Keeping the carer diary was also reported by the intervention group carers as a useful means of reflection and a way of providing them with something to focus on. The feeling of actively doing something was often reported by intervention group carers, and the carer diary appeared to have provided an extra way for them to feel that they were actively participating in the care and in the study:
Just to keep a diary, because I’ve kept diaries for donkey’s years. Written down information as I did a job. I didn’t need to do that for a long time, so just to keep that diary was useful. It was good.
David, urban, intervention
Some carers in the intervention group described how, although they had found the carer diary useful, they had found the layout confusing and would have liked to have had more help completing it, particularly at the outset. These carers reported the QOLLTI-F to be a source of confusion, especially as it had to be filled out every 48 hours, which carers found difficult to always achieve, especially in stressful times or during the night. One carer in the intervention group in particular found the QOLLTI-F difficult; given that there was no opportunity to explain why items were scored as they were, she felt that text boxes would have been helpful:
Or to be able to put something underneath might’ve been – made you feel a bit better at answering it. But that’s, I mean, that could be my I’m not very good at answering um I don’t like surveys because I think sometimes you need to explain what you’ve written.
Nicola, urban, intervention
The two carers who were in the usual-care group described a different view of the diaries. One explained that she saw little point in completing the diary, owing to not really feeling like a part of the study after allocation to the usual-care group. The second carer in the usual-care group said that she would have liked to have been able to complete the diary but was not given one until after the patient had passed away.
Training
The training given to carers in the intervention group was largely described as being clear and helpful; however, different carers required different amounts of training to feel confident. Some carers seemed to feel confident after only one training session, particularly those who were comfortable around medications already, such as David, who had been caring for his wife and managing her medications for many years. Other carers, such as Ffion and Josephine, who were both HCPs, also found the training straightforward and easy to understand. However, other carers reported that they required more than one training session. One carer, Myra, for example, had had several sessions but would have liked to feel more confident; however, she also explained that she never needed to administer the drugs and seemed relieved that was the case:
I’d only had it explained to me about a couple of times. Now, they would have done more, but he died. So, there was no point in carrying on that side of it. Um but I think I-I probably had enough confidence to have someone on the phone to actually do it. And if not, you just pray and you do it. It’s amazing what you can do when you have to.
Myra, rural, intervention
Follow-up visit and case report form
The carers who completed the follow-up in person with the nurse found it to be a good way to bring their participation in the trial to a conclusion. However, some of these carers found the follow-up visit to be emotional, as it happened post bereavement and involved reliving the last days of their loved one’s life. However, this was not reported as especially negative; rather, it was a way to reflect positively on the end of their loved one’s life and on the trial. Some carers completed the follow-up over the telephone and this seemed to be less helpful; several of these carers reported having no memory of the follow-up. One carer, Claire, was very distressed that she had been asked to complete a follow-up over the telephone, as she felt that this was insensitive and she had been upset by the unexpected call:
To be on the trial, you’re in a very sad set of circumstances, so the compassion shown by a phone call out of the blue, it’s not a scheduled phone call to say, ‘Would you participate?’ It’s like, ‘Can we do it now?’ And um for me I found that really hard to cope with.
Claire, rural, intervention
Medications and equipment
Storing and managing medications
The SC medications that might be needed to control common symptoms in the last days of life are stored in the home at the end of life as part of routine practice, regardless of trial participation or allocation. Carers reported that they were comfortable managing and storing the medications safely.
Use of equipment
Carers in the intervention group described their views about using the equipment to administer the medications. Although carers expressed initial apprehension about using medical equipment, they reported feeling more confident following training and using the practice kits provided. Carers who had previous medical training reported having no concerns about using the equipment, with one stating that it brought back memories of her previous career in health care. Two carers reported issues with dexterity: one described how he had difficulty opening ampoules early on, but had been reassured by a DN that many people experienced difficulty with this; the other found the equipment to be quite technical and would have liked more training had she gone on to administer any medications:
It was the initial ampoule, breaking one of those ampules, big clumsy hands, but after that when I made the first mistake, I was fine. You realise, because I’d never used an ampoule before. Broke that, ‘Oh god!’. [laughs] Throw that one away. The team said it was quite a common thing when you’re not used to that.
David, urban, intervention
Giving under-the-skin medication
In the intervention group, the exact method of delivery was important to carers and patients. Many were relieved when they realised the difference between the SC cannula (‘Saf-T-Intima’; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and an injection with a needle. The participants’ accounts show that carers have concerns about giving injections, as the word ‘injections’ conjures up images of needles which in turn raises fears over making mistakes and potentially causing harm. Several carers reported feelings of relief when they understood that they would not have to use needles or break the skin. This was often linked to the fact that they wanted to relieve pain, not potentially cause pain. One carer described her loved one as having a fear of needles and explained that part of their desire to take part was so that she could give medications rather than have him have more doses from a HCP. When they both realised that the trial involved no-needle injections, she said that they were greatly comforted. The idea of using needles was even a concern for carers with medical backgrounds. They explained that there was a difference between giving medication to a patient as a professional and giving medication to a loved one as a carer. This difference will be discussed further on in the findings. One ex-HCP carer specifically stated that she was glad she did not have to ‘stick needles’ into her husband:
The difference for me was the emotional side, I think, because with a patient, you’re desperately wanting to make the patient feel better. I also wanted to make my husband feel better. Um but sticking a needle into your husband is a little bit different from-from a patient because of the emotional involvement.
Josephine, urban, intervention
Symptom management
All of the carers interviewed wanted to help their loved one avoid uncontrolled symptoms, particularly pain, in the last weeks and days of their life. A key factor in deciding to participate was the fear of uncontrolled symptoms and the idea that, with the intervention, they would be able to alleviate that. Some patients had already experienced periods of uncontrolled breakthrough symptoms prior to recruitment. This may also have been an influencing factor for study participants, who were hoping to avoid those experiences happening again:
That you weren’t scared of the uncontrollable pain or severe sickness or something that might’ve been there. That-that gave us comfort – comfort – and confidence, I would say, to keep my wife at home, rather than, you know feeling that you had to take her to a hospice to get her symptoms relieved.
George, rural, intervention
One carer in the intervention group reported being upset during times when symptoms were uncontrolled, as he felt that HCPs were blaming him for being unable to manage the symptoms with oral medication. Carers in the usual-care group expressed disappointment, as they had wanted to help patients by managing their symptoms and they then felt that they would not be able to. A usual-care group participant, Paula, had taken part because she wanted to ease uncontrolled pain and was relieved that in the end her loved one did not experience uncontrolled symptoms.
The desire to be able to manage and control symptoms as they occurred was a major contributor to wanting to take part in the trial:
So, yeah, it was just pain relief, you know, in my mind if anything I was doing was gonna relieve it then it was worth doing so I didn’t think of anything else than it was gonna alleviate any pain or suffering for my mum.
Tom, urban, intervention
Concern about delays and rurality
Closely linked to the fear of uncontrolled symptoms is the fear of long waiting times for HCPs to arrive at the home to relieve symptoms. Long periods of waiting for help to arrive, particularly at night, were a source of concern for all carers in the study, particularly those who lived in rural locations. There was a common feeling throughout the accounts that waiting times seem even longer at night and that these long waits during sleepless nights contributed to feelings of powerlessness and isolation. Waiting times were also one of the reasons that carers gave for being disappointed about being allocated to the usual-care group, as they felt that now they were likely to experience delays when others would not:
As I said before, it’s just positive. I was not reliant on waiting for the team to come out to give injections to relieve any symptoms she might have with the vomiting and the anxiety and the pain relief. So that was all positive, not having to wait. It was . . . I could give it as soon as it was required.
David, urban, intervention
Symptom recognition
The ability to recognise breakthrough symptoms, what signs to look out for and when to administer the drugs were of concern to some carers. As the management of symptoms depends on recognising the symptoms, carers worried about leaving loved ones in pain without realising. Recognising the difference between symptoms, such as pain and agitation, was reported as sometimes difficult, particularly if the patient was no longer able to communicate. Some carers in the intervention group described consulting with other relatives about whether or not to give medications. One carer, Josephine, reported having many conversations with her son about when to give medication; although she had a medical background and he did not, she still sought his reassurance. Carers seemed to gain confidence in recognising breakthrough symptoms once they had administered their first dose or once they had been reassured by either family members or HCPs that they were right in their observations:
I was glad that my son was here as well. He-he’s not medically trained at all but (. . .) I-I asked him, ‘Oh, do you think Dad should have something now?’ And-and I valued his input, really, because he said ‘Yes, definitely’. You know, ‘Yes, yes, yes. He’s getting a bit restless now’. Or, ‘His breathing is not so good, you know, and if we can relieve that’.
Josephine, urban, intervention
Administration error, last injections and hastening death
The issue of making mistakes with medication was raised throughout the carer interviews. All of the carers expressed some concern about the potential for errors in administration. Concerns about potentially causing pain were often alleviated by training and the use of no-needle injections. However, the biggest concern for carers was the possibility of causing or hastening death. Although carers knew that their loved ones were in the last stages of their life, there was a desire not to contribute to that in any way and there was a fear among participants of what could happen were they to give an injection and the patient die very soon after: they did not want to take what little life their loved ones had away. There was also a concern among carers that they could be held accountable or be blamed if the patient passed away after an injection that they had administered:
I was concerned if I was to administer morphine and he, say, passed away 10 minutes later, could I be held accountable um that I’d done something slightly wrong?
Claire, rural, intervention
Most carers worried about accidentally hastening the death of the patient; however, two carers in the study raised the concern that patients may ask to be given large doses to end their lives. One carer explained that she remembered a relative in the past who had requested a hospice doctor to assist him with dying; she wondered whether the intervention would increase the chances that patients would make requests like this of their carers. One carer had worried about this happening to her in the trial and was glad to have been allocated to the usual-care group, as she believed that otherwise the patient might have asked her to assist her death:
I think you’re-you’re in a position where you can quite easily be manipulated.
Kathy, urban, usual care
A home death and wish fulfilment
The desire of their loved one to have a home death was something that all carers reported. Carers presented the importance of a home death in a variety of ways by carers: the home was a more comfortable environment, a place where family and pets could be present at all times and a place over which the patient was able to have more control:
Thought of her being in her own bed, surrounded by her own pictures and books and music and, you know, the views. We live in a lovely place and the views and the noises and familiarity of home gave us comfort, really.
George, rural, intervention
Taking part in the trial, if assigned to the intervention group, was seen as an extra way of ensuring that a home death could happen. This was a driving force for many carers, who were seeking above all to fulfil the wishes of their loved one:
And they were all saying that [husband’s] wish was the most important . . . to remain in his home, they could see it was so important. And it’s that reason, you know, obviously I did it [took part in the trial]. Um the hardest thing I’ve ever done. Yeah.
Claire, rural, intervention
It may be that some carers put the wishes of their loved one to stay at home above their own concerns regarding administering medication and being the main carer. One intervention carer whose husband had been admitted to the hospice stated that she felt a great sense of guilt and regret that she had not been able to keep him at home, even though the circumstances of his admission had been out of her control.
Carer and patient empowerment
Carers in the intervention group found a sense of purpose in being able to manage and administer medications rather than have a more traditional, passive role. This led to increased feelings of empowerment and control. Some carers in the intervention group also reported a sense of personal peace in knowing that they were able to do all they could for their loved one during the last days of their life. One carer described how he missed his wife greatly, but was content in the knowledge that he had done his best for her. The intervention was also reported by carers to have been empowering for patients, in that it gave them more control over their own destiny rather than having decisions made for them:
So, those were two really important things for her and CARiAD was part of that, I think. ‘These are things, I can make decisions about. I can’t not have cancer. I can’t not die. But I can make decisions about how that happens’. And that, I think, was really important to her.
Laura, urban, intervention
Potential for carer burden
The potential for increased carer burden arose in several ways, which potentially added to the sense of responsibility for the intervention group carers, particularly in giving medications as part of the intervention. Some carers also expressed that they felt that this increased the time that they needed to spend at home with the patient, especially as only one carer could be trained for the study. It is possible that carers may believe that once they have been trained to give the medications they should try to call on the HCPs less often. Several carers in the intervention group reported feeling reassured by HCPs that they could still call for help whenever they needed to.
The issue of there being only one trained carer was a concern for some, especially those who were used to caring as a family. They worried about who should be the nominated carer and what would happen if they were not always around. Carers in the intervention group who were used to being the sole carer, such as David, were less concerned about being alone, as they were used to being in that situation and preferred it to having control taken away from them by relying on HCPs:
They wanted to put a health-care plan in with people coming in but I’ve always done the cleaning and the cooking and the mending and I just didn’t want people around me when I wanted to cope for myself. I didn’t want things to be taken away from me. To be given this help right at the end, when I did need it, and to be able to do it myself, it was great.
David, urban, intervention
As mentioned above, the possibility of the desire to fulfil a patient’s wish to die at home to be prioritised over the wishes of their carer was alluded to. This may increase burden on carers if they feel that they are obliged to agree to administer medication or to support a home death that they may not feel comfortable with. Carer burden may also increase if the patient wishes to resist, or avoid, contact with HCPs. Several carers in the interviews described their loved ones as being somewhat resistant to medication, particularly strong opioids, or to the more frequent visits from HCPs at the end of life. It may be that some patients are not ready to accept that they are at the end of life, simply prefer to remain in control of their own medications or do not like lots of people visiting. However, although being in the intervention group may reduce the number of visits by HCPs to deal with breakthrough symptoms, it does not mean that visits will stop altogether, although this possibility may be what attracts some patients to the intervention. One carer, Kathy, was glad that she had been allocated to the usual-care group because she realised how resistant the patient was to receiving help from professionals, and how much this would have increased her burden had she and the patient been allocated to the intervention group:
She was very reluctant to accept any external help at all from the district nurses or our more local hospice at home. I think if I had been in the position where I could give her medication, she would have wanted to shut the district nurses out, which would have been very difficult.
Kathy, urban, usual care
Support needed from health-care professionals
The amount and the nature of support from HCPs in both groups of the trial varied according to the carer, the needs of the patients and the home situation. The majority of HCP support, by home visits or telephone, was reported to have been provided by DNs and SPC nurses. Some carers, such as David and George, also said that their GP had been particularly supportive and had made house calls OOH to make sure that things were OK. The support from HCPs was praised by many of the carers, who explained that they felt reassured that they could contact their health-care teams whenever they needed support. Although carers acknowledged that sometimes health-care teams were overstretched and delays occurred, they believed that the teams were always doing everything they could. Carers reported being able to ask any questions that they wanted and that they felt well supported throughout the trial and the palliative care process:
That was why it was so nice for the nurses to say, ‘If you-you get a bit wobbly or you’re a bit worried, ring’. And I thought, ‘Yes, if it comes to it, I shall ring them and they shall talk me through it’. And that was like, ‘Oh, thank you! Sigh of relief’.
Myra, rural, intervention
Family dynamics and health-care professionals as carers
The home circumstances of each dyad in the trial were different. The carer was often the patient’s partner or child. However, that was not always the case, as there was also a neighbour, niece and sister-in-law. Carers reported different circumstances that had led to them being the nominated carer in the trial, and these had caused different dynamics within the home. Ffion, for example, was the niece of the patient and it seemed that her uncle, Phil, the patient’s husband, had been very involved with caring for his wife but the DNs had chosen Ffion to be the carer. During the interview, Ffion and her uncle (Phil) seemed to feel that Phil would have been just as good a choice, but because Ffion had a nursing background she had been selected:
You was in charge of all the medicines. You were helping –
I knew what was what and I did that all the time.
Exactly. I don’t think being a nurse, in that sense, helped. You don’t –
You don’t have to be a nurse.
A carer, Kathy, who was the patient’s neighbour, said that she had been deliberately chosen by the patient because she had a nursing background, even though the patient lived with her husband and had grown-up children. It may be that these carers were chosen because they had a health-care background over somebody else who may have been in a better position. Like Kathy, another carer, Laura, had been approached not by the nurses but by the patient. Laura lived in a large household with her husband and children, as well as her brother and his family. The patient was the wife of Laura’s brother and she and Laura had a close relationship. The patient expressed that she wanted Laura to be the carer who took on the intervention so that her husband (Laura’s brother) would be able to concentrate on providing emotional support and keeping their relationship as ‘normal’ as possible for them and their children.
Paula, another carer and a nurse, was caring for her mother. She described the situation among her brothers and sisters as somewhat tense, as her siblings had not all accepted that their mother was dying. Paula had been expected to move back to her hometown to care for their mother, as she was a palliative care nurse herself. Having a HCP background, however, did not mean that as carers they needed less support from their family and friends. Josephine, as previously mentioned, described consulting her son each time she gave medication, despite having been a palliative care professional in the past. It would seem that the difference between HCPs and lay carers takes on a different meaning when the caring is for a loved one.
A good death
Some of the carers in the study, particularly those with HCP backgrounds, had previous experience of caring for, or being with, people who were in the last days of their life. Previous experiences seemed to have influenced the desire to take part in the trial, especially if during those previous times they had experienced long delays in medication administration or uncontrolled symptoms. Throughout the interviews, carers explained that they did not want their loved one to be in pain or to suffer, as they had seen that happen to others in the past. Taking part in the trial seemed to be a way of trying to help and trying to ensure that a loved one was able to have a ‘good death’:
Well, I had seen people die before and I wanted him to have the best kind of death. I really did.
Josephine, urban, intervention
Summary of findings
Accounts of carers in this study revealed the complexity of issues surrounding caring for a loved one at home during the last days of their life. These interviews also reflect carers’ experiences, both practical and emotional, of trial participation. The recruitment process was well received, although in future work it may be helpful to consider an earlier approach to allow carers more preparation time. Issues with understanding the randomisation process were often mentioned and have the potential for distress and disappointment in carers who are allocated to usual care.
The desire to fulfil the wishes of loved ones who want to have a home death was paramount to carers in these interviews. However, it is possible that patient wishes may overshadow carer needs and it is important to ensure that carers are well supported and are not coerced by patients or overburdened by responsibility.
The acceptability of administering SC medication was driven by the desire to control symptoms as they occurred, particularly in rural dyads, for whom delays may be longer. Carers in the intervention group reported feeling empowered by the ability to assist with symptom control rather than experience the feelings of powerlessness that may come with waiting for HCPs. A key concern for carers in the intervention group was the potential for causing harm or hastening death, showing the importance of reassurance for this.
The experiences of carers in this study have shown the potential for further qualitative work to gain more understanding and potentially improve end-of-life care for those who wish to have a home death.
Implications for feasibility
The qualitative interviews revealed that the intervention was acceptable to carers. Implications for feasibility also arose from the carer interviews, and their accounts show several areas in which changes could be made to improve feasibility for future work.
Many carers in the study had previous health-care training themselves, which may have influenced their decision-making in taking part; however, as evidenced above, this did not mean that they were at an advantage during the study owing to the emotional difference between patients and loved ones. It may improve feasibility if this is taken in to account during recruitment.
One key area that has implications for feasibility is the ways in which the randomisation process is explained to carers. Many carers showed a lack of understanding of how randomisation works, which indicated that a better explanation is needed. This had implications for recruitment and retention to the study; therefore, if randomisation could be explained in a way that would be better understood by participants or if a different strategy could be used, feasibility may be improved. Carers may be less likely to withdraw on allocation to the usual-care group and may engage more with the study if in the usual-care group, which would, therefore, reduce non-completion rates.
Although carer diaries were generally acceptable to carers, the QOLLTI-F was reported to be hard to use, which led to lack of completion. Feasibility may be improved with more training regarding QOLLTI-F or use of an alternative measure.
Chapter 6 Embedded qualitative study: health-care professionals
Introduction
The second part of the embedded qualitative study consisted of 20 interviews with HCPs who were involved with the study in their various capacities. These interviews were designed to assess the feasibility and acceptability to HCPs of carer-administered medications during a patient’s last days of life. They were also designed to explore HCPs’ thoughts and feelings about supporting dyads during the trial; the practical aspects of the process, including recruitment, randomisation, trial materials and training; and the impact on the HCP role when lay carers are trained to administer medications in the home.
Methods
Purposive sampling
A strategy was developed to recruit a purposive sample of HCPs for the qualitative interviews. The purposive sampling criteria were location, rurality, professional role and years in practice. Potential HCPs to interview were identified when their patients (and carers) were recruited to the trial (both groups). They were then added to sample monitoring tables that were used to monitor HCP characteristics and create a purposive sample for the HCP interviews. Using this system, HCPs were purposively identified to provide a range in terms of the above purposive sampling criteria. The identified HCPs were then invited to participate. These systems were in place and were followed; however, owing to lower rates of study recruitment than expected, there were less HCPs taking an active role in the trial than first anticipated. The sample was monitored throughout, but the strategy needed to be applied with more flexibility because of the availability of HCP participants. The final sample (see Table 21) provided a range of perspectives comprising a range of types of nurses and three GPs.
Health-care professionals were first recruited via e-mail and then by telephone. Interviews were conducted by telephone and lasted between 15 and 60 minutes. All of the interviews followed a topic guide (see Appendix 6), were audio-recorded, fully transcribed and analysed using the Framework approach. 77
Analysis
The Framework approach comprises a systematic, five-stage matrix method; this study followed the five stages. 77–81 During the first stages of data familiarisation, the researcher was immersed in the data by re-reading all of the transcripts thoroughly; this was then followed by the construction of a coding structure that was based on key themes emerging from the data. 81 This was used to code all transcripts thematically and charts were created to group and synthesise data according to the coding structure. 81 The final stage was an interpretive stage in which the researcher identified the patterns and links between the themes. 81 The research team also held several group interpretation workshops to discuss the key themes.
Sample
The characteristics of the 20 HCPs who were interviewed as part of the CARiAD trial across the three recruitment sites are shown in Table 21.
| Characteristic | Site | Total | ||
|---|---|---|---|---|
| BCUHB | CVUHB | GCS | ||
| Professional role | ||||
| Registered nurse | 0 | 0 | 2 | 2 |
| DN | 7 | 2 | 3 | 12 |
| SPC nurse | 2 | 0 | 1 | 3 |
| GP | 2 | 0 | 1 | 3 |
| Years in practicea | ||||
| 0–10 | 0 | 0 | 1 | 1 |
| 11–20 | 3 | 0 | 1 | 4 |
| 21–30 | 4 | 0 | 3 | 7 |
| ≥ 31 | 2 | 1 | 1 | 4 |
| Total | 11 | 2 | 7 | |
Results
Summary of key themes
The findings from the 20 HCP interviews are presented thematically below. The HCP interviews revealed a set of key issues that were of importance to those supporting patients and carers throughout the trial. Initial participant identification was discussed by many HCPs who had concerns about screening, RA and choosing the ‘right people’ for approach. A sense of the need to approach the ‘right’ people may have influenced the selection of many carers, for example those who already had some HCP background themselves. HCPs as lay carers became an important topic in the interviews.
As well as considerations about who should be approached, HCPs described concerns about the timing of the approach. Prognostication was a key concern for HCPs who expressed the difficulties in pinpointing the last weeks of life.
Given that randomisation was an issue for carers it was also so for the HCPs, who discussed the disappointment that they saw in those randomised to the usual-care group. HCPs also discussed the training involved with the study, including their own training and the subsequent training of carers to administer medications. Training was often discussed in terms of the amount of time it could take and the needs and feelings of the carers.
Trial materials and carer diaries were also discussed and, although these were generally found to be useful, HCPs were able to give suggestions for delivery and improvements. In practical terms, use and storage of medications and equipment were discussed along with the potential for medication misuse; interestingly, there was less concern in this last area than perhaps expected, as the medications would be in the home anyway and RAs would always be conducted.
Health-care professionals often noted the symbolic difference between a no-needle injection and an injection that breaks the skin, as a no-needle injection may make the delivery of medications less daunting and ease worries about causing pain or injury. Among those HCPs supporting a dyad in the intervention group, there was discussion of family dynamics and household composition, especially when shared caring was happening. A key theme was also the potential for carer burden and distress, particularly in reference to ‘last injections’ and concerns about hastening death.
Finally, it was important to discuss the impact of the intervention on the time and workload of HCPs. Although views differed on this, the most salient point was that HCPs saw things in terms of patient benefit above all.
Participant identification
Screening
The first task for the HCPs who were involved in the early stages of the study was to screen patients to identify potentially eligible patient and carer dyads for RA and recruitment. Screening was often a responsibility of the DNs; however, some HCPs were of the view that DNs were not always in the best position to screen, owing to their heavy workload, and that the SPC nurses would be better placed to undertake the screening process. Some of the BCUHB GPs reported that they would refer to the DNs when they felt that they had identified a potential patient; however, they also expressed a concern that DNs were not always screening patients as required and felt that they needed encouragement to do so:
. . . if we see a patient who we think might be appropriate for it, we then let the district nursing team know who then arrange the actual assessment and recruitment of that patient and their carer.
GP
Risk assessment
Health-care professionals detailed the process that they used for RA. The use of the CARiAD RA tool was often preceded by an informal RA on the suitability of each patient and carer. A major part of this initial assessment focused on the HCP’s perception of whether or not the carer was able to cope with the potential tasks if recruited. An informal assessment of carer coping ability was conducted by looking for characteristics that would indicate an ability to cope. RNs reported that if they felt that a carer would not be able to cope they would not approach them for the study, and DNs similarly explained that if they were not sure about a carer’s coping ability they would approach with caution. There were several reported signs that a carer might be unable to cope. One of these was a perceived difficulty in managing the number of oral medications that a patient was already taking: this indicated to the HCPs a possible inability to cope with further responsibility of administering SC medications. HCPs also described carers who were nervous or anxious about caring at home as being potentially unable to cope, particularly those who were seen to ask the same questions a number of times or frequently seek reassurance from their GP or nursing teams. There was also a view that those who had previous experience of a home death would be able to cope better than those who had not. This could be related to the confidence that they were able to instil in the assessing HCPs. There was concern among HCPs that a distressed or anxious carer would mean a distressed and anxious patient. Therefore, the ideal candidate would be able to remain calm and not panic and be, in the words of one participant, ‘unflappable’:
If somebody is in despair because of a diagnosis then they are not going to . . . They are going to flap. You’ve got to have an unflappable person, if you like. You couldn’t have someone who’s panicking and gets it wrong.
DN
Health-care professionals based their ability to identify carers who could cope on knowing the carers well through home visits or, in the case of GPs, having known the family for a long time prior to care being palliative. This concern for the welfare of patients and carers may also have resulted in some potentially eligible dyads not being approached.
The RA tool was generally found to be straightforward and easy to use. HCPs reported that if they were not the one who carried out the RA they checked it to make sure that they agreed. This may be a form of professional self-protection. Although most HCPs relied on the RA, some expressed dissatisfaction with it. One DN felt that the wording of the RA tool document was confusing. A SPC nurse was unclear about when to use it and a DN reported not having used it at all.
Health-care professionals as lay carers
The HCPs described the type of carer who they were looking for as having good dexterity, good vision and being unafraid of needles. Also seen as important was the ability to understand the responsibilities and retain the information. Possibly related to this, HCPs recruited a number of carers who were current or ex-HCPs themselves. The HCPs interviewed described feeling comfortable with HCPs as carers as they would already possess the necessary skills and the understanding required. It may also be linked to professional self-protection in that they feel that things are less likely to go wrong. However, some of these carers were not as prepared as expected and still required training and reassurance. It is also important to consider that some HCPs may feel obligated to take on the carer role, even if they felt that another family member was more suitable:
So I think from a district nursing perspective, if you’ve got somebody . . . If you’ve got a family member who has some sort of medical background who knows how to give injections then that would be easier in itself.
SPC nurse
Recruitment
Obtaining consent
The RNs responsible for obtaining consent from patients and carers described their processes of doing so. They took copies of the carer diaries with them when gaining consenting to illustrate what the trial would involve. One RN said that this was useful, as it allowed the carer to see that filling in the diary would not be too burdensome. The only reported challenge of obtaining consent was judging whether or not the patients had capacity and whether or not consultee assent would be necessary.
Approaching eligible dyads
Some of the DNs in the study reported an initial apprehension about approaching potential dyads and surprise at how receptive they found dyads to be. SPC nurses tended to approach the topic by discussing end-of-life wishes in general, then introducing the trial if the patient expressed a wish to die at home. This became problematic if patients and carers had not yet discussed end-of-life arrangements:
I think with the patient that I’d spoken to, we’d only seen them once or twice and I was a bit apprehensive about actually approaching them about it, but they do live quite rurally to the area; they’re right on our border. So I think it was quite easy bringing that subject up. They were really open to it straight away.
DN
Prognostication at the end of life
A frequently discussed challenge to recruitment was the difficulty in prognostication at the end of life. There was agreement among the accounts of the HCPs that prognostication is difficult and this perhaps meant that some recruitment opportunities were missed, as the patient deteriorated suddenly before they could be approached or consented. The opposite was also true in some cases; RNs reported that some patients were on care plans for weeks after being thought of as in the last days of their life. One RN was concerned that two patients who had been approached and given trial information were not in the last weeks of their life, which created potential patient distress and a sense that they were closer to death than they had thought:
We missed a few because they deteriorated so rapidly and obviously by the time they deteriorated, we didn’t think it was appropriate to discuss it with them.
DN
The HCPs explained that prognostication difficulties meant that it was hard to get the timing right to discuss the trial. Patients who were not ready to accept that the end of life was near were harder to recruit. However, the HCPs felt that, in general, the earlier end of life was discussed the better as this it could result in improved recruitment and better prepared patients and carers. However, these conversations could be had only if the patient and carer were ready and willing to discuss end-of-life arrangements.
Randomisation
Following recruitment and consent, the dyads were randomised to receive either the intervention or usual care. During the interviews, HCPs were asked for their views on the randomisation process and the effects on patients and carers. The RNs were happier with the randomisation process following their unblinding, as they had found that being blinded to allocation was somewhat problematic. They explained randomisation to dyads carefully, ensuring that it was understood that there was no guarantee of being allocated to the intervention group. One BCUHB GP thought that patients enter trials hoping to gain something beneficial; therefore, if the patients are assigned to usual care they feel that they have missed out on an opportunity. HCPs described the disappointment felt by those allocated to usual care, including being ‘upset’ or ‘angry’ at not being ‘chosen’. The use of words such as ‘chosen’ and ‘selected’ by carers may also indicate that they did not understand randomisation despite the HCPs’ explanations, but may also indicate that HCPs were not always clear about how the process works:
The participants were bitterly disappointed that, having reviewed the information and looking forward to do the care, they weren’t actually randomised to receive it, so that was an issue in itself.
DN
It also appeared that the HCPs themselves were disappointed if they felt that they had identified someone who would benefit from the intervention and that person was allocated to usual care. They expressed sadness that these dyads would not be able to administer the medications. One SPC nurse described the disappointment of a family allocated to usual care as ‘heartbreaking’. Some participants also reported being aware of dyads who had withdrawn from the study following their allocation to usual care, and in one case a dyad had declined to take part at all on hearing that the study would involve randomisation:
The only person that we had that was the male carer, he was keen to do it because he actually was a health-care professional. Different sort of background, but he was in health. And because we couldn’t assure him that he’d be on the intervention arm, he opted out.
DN
Training
Training for health-care professionals
Health-care professionals were asked about their experiences of receiving training and how adequately informed they felt regarding their own roles in the trial. HCPs were generally satisfied with the training they had received from the research team and had found it clear and straightforward. Some felt that, had their training taken place long before recruiting their first patients, refresher training would have been useful. Some DNs felt that they had not received enough training, which they believed may have had an impact on their ability to successfully recruit. Perhaps owing to the structuring of community nursing teams, several DNs reported having had no trial training from the research team because they had not been in their current post at the time of training. This adds emphasis to the point made by some participants that good communication between HCPs is important in case they come across an administering carer. Two SPC clinicians also explained that they had made some mistakes during recruitment that they believed may have been avoided if the training had been delivered in a more accessible way. They described a feeling of being overwhelmed by trial paperwork that gave rise to confusion regarding RAs and carer training:
We’ve had to learn along the way because we’ve made mistakes with the different bits of the paperwork and things like that. It’s just from ourselves, there’s been a lot of learning going along.
DN
The GPs who were interviewed felt that the training they needed was more limited: to be aware of the trial and the eligibility criteria. They would then refer to DNs should they identify an eligible patient.
Training for carers
In this study, HCPs were required to deliver training to the carers who were allocated to the intervention group. HCPs described the training materials as helpful and easy to work with, particularly the practice kit and the picture guides to administering. DNs delivered the majority of carer training, with SPC nurses and RNs also conducting some. HCPs reported that training the carers could be a consuming process, as it was important to ensure that carers understood not only how to administer medication, but also when and in what circumstances. Some carers required training more than once, which was sometimes a result of issues surrounding prognostication and having initially been trained a long time before any injections were needed. In other cases, carers wished for more training to improve confidence. In terms of who is best placed to carry out the training, GPs were of the view that DNs or palliative care nurses would be best, as they were likely to know the family; however, some of the DNs reported difficulty in finding time to train owing to heavy workloads and believed that RNs would be in a better position to deliver training:
I’ve been a bit nervous at first although I’ve been a palliative care nurse myself for 5 years and I still work as a palliative care nurse. It’s something I do do quite often, give people injections. But I think training other people can be a little bit nerve wracking.
RN
Trial materials
Patient information sheet and guide for carers
The trial materials that were provided for patients and carers received good reviews from HCPs. The PIS was described as being informative and written in clear and accessible language. RNs reported that they had found the PIS a useful tool to use during recruitment and consent taking. The guide for carers was also described as clear and useful to patients and carers:
I have to say, I think the patient information sheet, CARiAD information sheet, are fantastic. I think they’re written really well and I work with lots of information sheets that I don’t really like.
RN
Carer diaries
The carer diaries were considered a helpful document that provided carers with a way of keeping track of symptoms and doses, helped them feel that they were actively participating and provided a cathartic means of reflection. In general, the diaries were described by HCPs as clear and easy to use, although they were harder to use in more complex cases. HCPs understood that the diaries were perhaps not always a priority for carers and HCPs during difficult times, such as during OOH or in the event of uncontrolled symptoms, and that this occasionally created more work for nursing staff because they needed to remind carers to fill in the diary. The HCPs reported use of the QOLLTI-F to be the most difficult aspect of the carer diaries. One RN described it as being potentially confusing for carers, as they may not realise how often they need to complete it; this was also reported by SPC nurses:
I find the diaries a bit complicated and I think at the time in the middle of the night when the family are awake with somebody who’s dying and they’re distressed and they give the medication, they’re not always thinking to fill a diary in or a questionnaire at the back.
SPC nurse
Follow-up case report form
The follow-up case report form (CRF) was also reported to be somewhat challenging in some cases, as it was carried out post bereavement with potential for carer distress if not treated sensitively. Some RNs found that the scoring of the CRF was sometimes confusing, which could add to carer distress. Difficulties in completing follow-up visits were described by several HCPs, as carers often became uncontactable post bereavement. This had implications for study retention.
Medications and equipment
Storage and management of medications
The HCPs in this study did not reveal any concerns about carers storing and managing end-of-life medication in the home. Medications are stored in the home anyway, regardless of the trial, so there was no concern as long as there was no perceived risk of drug misuse. Several HCPs argued that the trial may actually make drug storage safer owing to the RA process in place. Some participants also reported that storing and managing medications was a way of empowering the carer and helping them to feel in control of the situation. Medications were often described as being stored in boxes, clearly labelled and out of reach of children. No concerns for safety owing to medication were reported during the trial:
The drugs are usually kept in the district nurse box anyway which isn’t locked, so I don’t think it’s any different to how the drugs are stored in a normal situation, in a situation where the carer isn’t giving those medications.
GP
Use of equipment
The HCPs did not report many concerns regarding the carers using the equipment owing to the RA that required carers to have the vision and the dexterity to be able to administer the medications. Only one RN reported a carer having difficulty with dexterity; although the carer was elderly, she was a former HCP and so the RN was surprised that she experienced difficulty with drawing up medications. Opening ampoules was the one area in which the HCPs expressed concern about carers’ ability; however, this was reported to be an issue not of dexterity but because ampoules are generally hard to open and many HCPs stated that they occasionally struggled themselves. There was also some concern among the participants that measuring doses could be difficult and that if medications could be provided pre-drawn up in some way that might be beneficial in the future:
I think breaking open the ampoules can be quite fiddly if you’re slightly elderly or not used to that sort of thing.
RN
No-needle injections
Several HCPs reported that the use of no-needle injections was a useful element of the trial as carers did not need to break the skin or use needles. HCPs described carers’ relief at realising that they would not be required to actually inject their loved ones, as this meant that there would be no chance of causing injury or pain from needles. HCPs explained that it was usual practice to use a continuous SC infusion using a syringe pump for patients who were nearing the end of their lives and who are unable to take their oral medication; however, some participants reported that some of their colleagues were cautious about the trial because of the lack of clear policy regarding the use of an additional SC cannula (Saf-T-Intima) for as-needed injections. Some HCPs involved in the care of a specific patient in the intervention group reported more difficult circumstances due to the patient’s refusal of the syringe driver:
And the thing is, we call them injections but actually you’re putting it into a bung. And if you talk to patients, like we talk to patients, it’s the breaking of the skin that’s an issue.
RN
Which drug for which indication?
A concern expressed by some HCPs in the interviews was the confidence of the carers in recognising breakthrough symptoms and knowing which drug to administer for which indication. It was explained that some carers may have difficulty in differentiating between some symptoms, such as pain and agitation, and that this may cause anxiety in the carer. HCPs reported that they would try to prevent this by assuring carers that even HCPs make assessments according to their best judgement and that, if one medication does not seem to ease symptoms, then it is acceptable to try another. However, one DN expressed a different view, explaining that district and palliative care nurses are highly trained to recognise breakthrough symptoms and that lay carers would lack expertise:
It would be perhaps difficult for them to establish between terminal agitation and pain but the flipside to that one is that no one knows their loved one more than them.
SPC nurse
Supporting a dyad in the intervention group
Support needed for patients and carers
The accounts of HCPs who were supporting a dyad in the intervention group suggest that, although their roles may change in some ways, their involvement with patients and carers does not lessen. DNs and SPC nurses in particular reported that they still visited the homes every day to help with other caring and clinical needs, and continued to provide support over the telephone to carers who had questions about the trial. GPs reported that their roles in supporting the dyads also did not change and that it was community nursing staff who were providing the majority of the support.
Family dynamics
The HCPs who were interviewed emphasised that understanding the situation with the family in the home was integral to supporting them through the trial. They said that families all have their own unique set of circumstances that are important to consider, especially as family members may have different views regarding end-of-life care. The HCPs reported that families may also differ in their acceptance of HCP presence and that this may influence their decision on whether or not to take part. This may be important, as HCPs will need to explain to families that the intervention does not mean that they will no longer receive visits. An important part of the CARiAD trial was the stipulation that only one carer could be trained to administer medications. This was reported to be a challenge for some HCPs who supported families in which more than one person was involved in care. This may have increased burden on carers who were trained and raise feelings of powerlessness in those who were not. HCPs in this study also discussed the issue of potential drug misuse in the home; however, this was generally described as being filtered by the RA tool:
It’s the whole of the family that you have to take on board their perspectives and what they’re previously agreed as well.
DN
‘Last injections’ and hastening death
The topic of the ‘last injection’ was raised frequently during the HCP interviews owing to concerns over carer distress that was caused by administering the last injection before a patient’s death, which may be interpreted by the carer as having caused or hastened death. It was felt that it was crucial to discuss last injections with carers, ideally early on, and to reassure them that nothing they did would hasten the death of their loved one. It was also the case that HCPs had suffered feelings of burden or guilt themselves after administering the last injection before the patient’s death, and explained that this made them very aware of how a carer may feel if it happened to them:
I think they just don’t want to give the last injection, as it were, which is what this lady wanted, she said ‘I cannot give that last injection, I cannot be the one to do that to my good friend’.
DN
There were also some discussions surrounding the topic of euthanasia during the HCP interviews, a situation in which the carer would hasten death deliberately; however, owing to the RAs in place, this was not reported to be a major concern for most HCPs in this study. A few HCPs said that they would be concerned about euthanasia only if a carer or patient mentioned it. During the study, the only cause for alarm that was reported was when the patient made a joke about the carer ‘finishing me off’ following training. The HCP present was concerned; however, following a reiteration of the legal implications, she was confident that there was no risk of medication misuse.
Carer burden and benefits
A major concern for HCPs in this study was the potential for increased burden on the carers in the intervention group. HCPs described how they were careful to ensure that carers were not feeling under obligation to take part to fulfil the patient’s wishes if they were not genuinely happy to do so. HCPs reported that sometimes carers and patients may have different needs and that, in some instances, carers may feel pressured to keep the patient at home even if they would prefer for them to be in the hospice and that the intervention may increase that pressure:
. . . they can pull back as well because you don’t want them to feel that they have to do it.
DN
There were also concerns regarding patients’ worry about increasing burden on their carer. One DN expressed a particular concern that the intervention, especially if it were to become more widespread, would give a false impression of a home death. She worried that a home death would be presented as the ideal, with none of the associated problems being explained to patients and carers. HCPs confirmed that, during recruitment, it was essential to ensure that patients and carers knew that participating was optional and that usual care would still be in place.
Yet the most important aspect of the trial for all of the HCPs interviewed was the potential benefit to patients and carers, particularly timely symptom control.
Impact on health-care professional time and workload
The impact of the intervention on the workload and time of HCPs was discussed during the interviews. The main concern of the HCPs was timely symptom control and the benefit to the patient and carer, not the possibility of reducing their own workload. In fact, the HCPs did not report that the intervention saved them any time, as they would still visit the home just as often as before, and some of them needed to invest more time in training the carers. Participants reported that the pressures facing the NHS alongside the issues brought about by limited OOH staff and rural patients could mean that patients would face long waiting times during breakthrough symptoms and the key impact of the intervention was that they were reassured that the carer would be able to relieve those symptoms until the HCP arrived:
On one hand I’m saying it is feasible because it is feasible, but it’s a challenge because you do need some time to explain the intervention to the participants and select suitable people and then teaching.
DN
Summary of the findings
Overall, the HCPs involved in this study had a generally positive view of the intervention in terms of its effects on symptom management and benefits to patients and carers. These interviews also identified a need for great sensitivity when recruiting and supporting patients and carers in the trial and showed ways in which carers and patients can be empowered by having a greater control over the last days of the patient’s life. These accounts also revealed concerns regarding carer burden and the ways in which it can, and should, be avoided. There are areas in which improvements could be made, such as more involvement from SPC teams at the outset, some revisions to the trial materials and a focus on recruitment facilitated by earlier discussions regarding end-of-life wishes.
Implications for feasibility
The HCP interviews revealed key areas that have implications for the feasibility of future work. Recruitment in particular seems to have provided HCPs with difficult decisions to make regrading who to approach for the study. It is possible that HCPs are exercising a form of professional self-protection, as mentioned in the findings above; by recruiting carers who have previous HCP experience themselves, they may feel that they are less likely to make mistakes. HCPs may feel that they are in some way accountable for the actions of those they recruit and, therefore, exercise caution and follow their own informal RAs. These informal RAs may be overly cautious and result in potentially eligible dyads being discounted prior to the formal RA. HCPs express concern regarding carers’ ability to ‘cope’ with the intervention, which again may influence their decision-making when deciding who to approach. It may be that in future work HCPs will need further reassurance regarding lay carers and accountability.
Chapter 7 Development and feasibility of preference-based measures for use in economic evaluation
The objectives of the health economic components of the study were to conduct formative and feasibility work to identify preference measures for use in a future definitive trial. Specifically, to:
-
Identify attributes pertinent to carers’ preferences for HCP versus own administration of as-needed SC medications for patients dying at home that could be used in a future discrete choice experiment (DCE) to measure carers’ preferences towards administering SC medications.
-
Establish the feasibility of completion of the CES, a profile measure of the caring experience for use in economic evaluation. The CES was piloted to inform the choice of primary health economic outcome for a cost-effectiveness analysis alongside a future definitive trial.
Identification of attributes for a future discrete choice experiment
Introduction
Discrete choice experiments are a survey method to measure patients’ stated preferences for goods and services (e.g. medicines and health-care provision). 82 Respondents choose between hypothetical but realistic alternatives, which are described in terms of a number of attributes (e.g. waiting time) that are each characterised by specific levels (e.g. 1 hour). This allows for the estimation of the relative importance of each attribute, assessment of any trade-offs between attributes (e.g. waiting time vs. symptom severity) and estimation of respondents’ total satisfaction (utility) with the service. 82
The preferences of carers towards administering SC medications will have a bearing on their willingness to adopt this practice and the effectiveness of carer-administered medication. Evidence on the most important attributes of the intervention may be used to estimate the optimal configuration for service delivery and to explain why, if implemented, the option to administer SC medications is not adopted by all. Although the DCE, aiming to ascertain carers’ preferences for their administration of SC medications, will be conducted as part of a future main study, the formative work required to identify relevant attributes and levels was carried out as part of the embedded qualitative study component of the randomised pilot study.
Objectives of the formative study are to (1) identify and rank factors that are important to carers in guiding their choice between HCP and own administration of SC medications and (2) assess suitability for inclusion in a DCE.
Methods
The process of attribute selection was informed by best practice. 83 A starting list of potential factors was derived using the draft CARiAD study documents/guide for carers and previously published resources for caregivers from an Australian study. 14 This was supplemented with attributes that are commonly used (and found to be significant) in published DCEs of medicinal products. 82,84 The reduction process focused on identifying plausible, salient attributes for a future DCE. Factors from the original starting list that did not qualify as ‘attributes’ for the ranking exercises were discussed in the context of other aspects of the DCE, such as inclusion in the DCE scenario decision question (e.g. appropriate description of breakthrough symptoms) or as candidates for subgroup analysis (e.g. confidence to recognise breakthrough symptoms).
Owing to cognitive burden and the limited time for the ranking exercise (approximately 15 minutes), the study team reduced the starting list of 23 factors to 10 attributes; this was achieved using good practice criteria for what makes a plausible attribute83 and by combining attributes that represented parallel constructs (overlap). A convenience sample of nine university staff and two study team members completed the ranking exercise using the reduced list. Respondents completed the exercise and were asked to give comments/feedback. In response, the list was further reduced to nine attributes, with revised wording, to be used in the embedded qualitative study (Table 22).
| Starting list of factors that were reviewed by the CARiAD study research team | Reduced list of attributes that were used to pilot the ranking exercise | Final list of attributes that were used in the ranking exercise with carers |
|---|---|---|
| Amount of training requireda | Amount of training required | Amount of training required |
| Benefit of the medicationa | Cost to the NHS | Cost to the NHS |
| Breakthrough symptoms | Frequency of additional nurse visits | Frequency of additional home visits by HCP |
| Confidence to recognise symptoms | Frequency of symptoms | Frequency of symptoms |
| Cost of health carea | Number of medications | Number of medications |
| Cost of medicationa | Potential for medication error | Potential for administration error |
| Frequency of administrationa | Risk of serious harm | [Removed] |
| Confidence to give injection | Symptom score | Symptom severity |
| Frequency of additional nurse visits | Waiting time for medication to work | Time for medication to work |
| Distance to nearest inpatient carea | Waiting time for nurse | Waiting time for the HCP |
| Frequency of symptomsa | ||
| Medication concernsa | ||
| Number of medicationsa | ||
| Perceived ability to give injection | ||
| Perceived ability to identify the correct medication | ||
| Potential for medication error | ||
| Preparation time | ||
| Risk of serious harma | ||
| Risk of side effectsa | ||
| Symptom scorea | ||
| Trust in DN | ||
| Waiting time | ||
| Waiting time for medication to work |
A topic guide was developed around the final list of attributes (see Appendix 7). In the interviews, carers were presented with nine attributes that were printed onto show cards that contained the attribute heading and a brief descriptor. Interviewees had the opportunity to nominate additional attributes before selecting those that they considered important in the decision to choose own versus HCP (DN) administration of medications to people dying in their own home. They were then asked to place the selected cards in rank order, from most to least important. An in-depth interview preceded this exercise (see Chapter 5). The ranking was recorded and the corresponding dialogue was transcribed verbatim and analysed independently of the preceding in-depth interview. Interviews were conducted face to face in the respondent’s own home or by telephone (with modified topic guide). Ranking data were entered into Excel® (Microsoft Corporation, Redmond, WA, USA) and analysed descriptively using counts and mean rank scores for each item. Rankings were converted into rank scores using:
where rank 1 = most important. The mean rank score was calculated as the sum of rank scores divided by the number of times that the attribute was selected. The second part of the topic guide was intended to pilot the presentation of the highest ranked attributes and was to be used for respondents 6–10 in the intervention or usual-care group.
Results
In total, eight out of 12 interview participants completed the attribute ranking exercise: seven from the intervention group and four over the telephone. The ranking exercise was the final task of the interview, which on average lasted 51 minutes. All of the interviews used part one of the topic guide, cognitive interviews on attribute presentation were not feasible due to sample size and there was a preference for telephone interview.
The average number of attributes selected for ranking was six (range 3–10). Symptom severity was the most commonly selected attribute. Waiting time for a HCP had the highest rank score (Table 23). Only one respondent selected cost to the NHS and they ranked it the least important. Three additional attributes were suggested, each only once: timing of introducing the intervention, privacy and ownership of decision-making about death and back-up/being able to call someone for support.
| Rank score | Attribute | Count selected |
|---|---|---|
| 11.00 | Waiting time for HCP | 7 |
| 10.25 | Symptom severity | 8 |
| 9.50 | Frequency of additional visits | 6 |
| 9.29 | Frequency of symptoms | 7 |
| 9.17 | Potential for administration error | 6 |
| 7.60 | Number of medications | 5 |
| 6.40 | Time for medication to work | 5 |
| 6.33 | Amount of training required | 3 |
| 3.00 | Cost to the NHS | 1 |
Interpretation of attributes
Waiting time for health-care professional
The waiting time for a HCP was the highest ranked attribute (score of 11/12), with seven participants considering this to be important in their choice for own versus HCP administration of SC injections. Several participants described reduction of waiting time (and associated symptom severity) as their prime motivation for participating in the CARiAD trial.
Symptom severity
Symptom severity was the most commonly selected attribute, with all eight participants considering it important in their choice for own versus HCP (DN) administration of SC injections (score of 10.25/12.00).
Participants aligned symptom severity, frequency and waiting time, but were able to distinguish between them. Severity carried greater importance than frequency, but was often discussed in the context of frequency:
Mm. (. . .) Well, that’s a concern, really, if your symptoms were not being allevied [alleviated] by the um (. . .) by the oral medi-medicines, then (. . .) that would be quite a concern, obviously. That would be eased by having stuff to give at the time, and often how often it was happening.
George, rural, intervention
Future DCE design and analysis needs to allow for potential interaction between the attributes of severity and frequency of symptoms.
Frequency of additional visits
The frequency of additional visits was considered important by six participants (score of 9.50/12.00) and was discussed in relation to the waiting time for HCPs and ‘ownership’, a self-nominated attribute:
And then probably frequency of home visits and ownership um (. . .) and frequency of symptoms are all then tied in to the same thing, that actually needing to reduce how often we were calling – needing to call out the nursing staff um seemed like another-another bonus and that, in turn, would help us maintain that ownership of the process.
Laura, rural, intervention
Ownership overlaps with the underlying construct of the DCE that is the utility associated with self-administration.
Some respondents associated an increased frequency of visits with the need for additional support in the intervention group. This represents a departure from what the attribute intended to capture, which was the reduction in the number of visits associated with medication administration by a HCP. Although it would be acceptable for the direction of preference for additional visits to vary, the context of this attribute would need to be clarified. Overlapping constructs, such as access to support, may also need to be considered to account for the utility that may be derived from alternative interpretations of the original attribute.
Frequency of symptoms
Seven participants considered frequency of symptoms to be important in their choice of own versus HCP (DN) administration of SC injections (score of 9.29/12.00). Frequency of symptoms was linked to ownership of the process and a notion of reducing the number of visits that a HCP would need to make to administer medication.
Potential for administration error
Six participants considered potential for administration error to be important in their choice of own versus HCP (DN) administration of SC injections (score of 9.17/12.00). Two respondents acknowledged that this was important but not personally an issue. This suggests that prior experience has potential to influence preference and should be measured in the definitive survey. Potential for error was also associated with level of training, which implies potential interaction.
Number of medications
Five participants considered the number of medications to be important in their choice of own versus HCP (DN) administration of SC injections (score of 7.60/12.00). The number of medications was discussed in terms of potential for fear, confusion and a factor that could influence self-efficacy:
The number of medications would be important because obviously if there was lots of different ones there then it would make it more, maybe, confusing. You know, the clarity might not be there. I think I’ve given – there was about three or four medications but if somebody said there was a list of eight and all the different quantities, that would have totally, possibly, been out of my depth with that. I think that one is very important.
Tom, urban, intervention
The potential for preferences to be influenced by knowledge and self-efficacy was considered during the attribute selection process and remains important here. This suggests that there is a need to test hypotheses of how psychosocial characteristics influence the outcome of the DCE.
Time for medication to work
Five participants considered time for medication to work to be important in their choice of own versus HCP (DN) administration of SC injections (score of 6.40/12.00). Although the description of ‘time for medication to work’ was intended to distinguish it from waiting time for a HCP, there is evidence that respondents may deconstruct attributes in defining their own preferences. One participant disregarded this attribute, stating that the time for medication to work would be reduced if they were responsible for medication administration, rather than waiting for a HCP to visit. This stresses the importance of the positioning of the description and accompanying instructions: communicating the task using audio or video may reduce this risk.
When time for medication to work was considered important, this was linked to information on indication and potential for death following administration of medication:
Time for medication to work is important, but I think because of the nature of what the medication was for, like agitation or something like that, it wasn’t – I don’t think it had to be instant and I was told, anyway, that it wasn’t instant so I wouldn’t expect it to be instant because that was part of what I was told of why it wouldn’t be anything that I’d done if she did pass soon after giving medication.
Tom, urban, intervention
Throughout the ranking exercise, participants linked attributes to elements of information and training that they had received. Training materials represent a useful resource for developing context and ensuring that hypothetical choices align with plausible scenarios in research and practice.
Amount of training required
Three participants considered training to be important in their choice of own versus HCP (DN) administration of SC injections (score of 6.33/12.00). When ranking the amount of training, one participant described how requirements vary, which shows an important consideration when framing the levels that would accompany this attribute:
So I think it was important, but it wasn’t . . . Because I think people are different so you can’t put a time limit on that. Somebody might have needed twice as much training as somebody else. But in my circumstances, I thought it was adequate. It was important, but it wasn’t, you know, a-a – yeah, I don’t think it was that important because I would’ve sung out if I didn’t think I knew how to do it, and kept going over it.
Tom, urban, intervention
This view has implications for DCE design, in which the attribute would need to be clearly defined, and is potentially associated with knowledge, experience, self-efficacy and capability. Furthermore, a respondent who, when selecting attributes, stated that they were not concerned about the amount of training went on to describe how they asked for further training in specific circumstances.
Cost to the NHS
The cost to the NHS was described as the cost to the NHS of the associated health care. Only one participant considered this to be important in their choice of own versus HCP (DN) administration of these injections (score of 3.00/12.00). Although respondents were clear that they did not see cost as important, discussions around this attribute suggested that they did value the potential for care to be cost saving. The levels associated with this attribute may, therefore, influence acceptance.
Comprehension of task
The majority of respondents were able to consider the attributes and select those that featured in their preference for the intervention. There was an issue with recollection when completing the ranking task over the telephone. There was also a tendency to complete the exercise in relation to participating in the trial, rather than a more generalised preference to adopt the intervention.
The responses to the initial list of attributes suggested that these were appropriate:
. . . [response to showcards] . . . OK. (. . .) Um (. . .) oh, these are good. Very good. Gosh. That’s excellent, isn’t it? We did sort of say that I was worried about the extra workload for the – for the nursing staff. Um (. . .) the number of medications. (. . .) Er oh yes, I hadn’t thought of that one. Um (. . .) [Prolonged pause while reading. Sound of paper shuffling.] Gosh, they’re all very relevant. Um . . .
Josephine, urban, usual care
This concurs with the minimal number of responses to ‘anything missing?’, which generally involved new attributes that encapsulated several existing factors.
Discussion
All nine attributes were selected at least once and appeared valid for inclusion in a DCE. The potential for overlap was observed; however, this could potentially be reduced by further clarification within attribute labels and descriptions that could be obtained from training resources. Respondents were able to select what was important to them and when ‘thinking aloud’ demonstrated a tendency to trade between attributes, which would imply that the DCE would be acceptable. To reduce cognitive burden it is unlikely that all nine attributes would feature in the DCE. Instead it is likely that between four and six would be piloted (Figure 10).
FIGURE 10.
Example of a DCE choice question.
The key strengths of our study are the (1) systematic approach to attribute development and refinement, (2) mixed-methods approach to attribute selection, that enabled greater interpretation of the relationship between attributes and the decision-making process, and (3) conduct of formative work using good practice guidelines. The number of respondents and the proportion of telephone interviews limited the study.
Conclusion
By using a systematic approach to attribute selection, including literature review, expert opinion and ranking exercises, we demonstrated that waiting time for a HCP, symptom severity, frequency of additional HCP visits, frequency of symptoms and potential for administration error are important attributes of the decision of carer administration of medication. These attributes can be constructed into a DCE to quantitatively measure the strength and direction of preferences for each attribute, the willingness to trade between attributes and the total utility and probability of uptake for different service configurations.
Carer Experience Scale
Introduction
The CES is an index measure of the caring experience that focuses on six domains: (1) activities outside caring, (2) support from family and friends, (3) assistance from the government and other organisations, (4) fulfilment from caring, (5) control over caring and (6) getting on with the care recipient. The construct validity of the CES instrument has been demonstrated in a heterogeneous group of 730 carers in the UK,85 and specifically in the context of palliative care. 86 The CES benefits from having preference-based index values, based on 162 unpaid carers of older people from five geographical locations in the UK,87 that allow for calculation of utility for use in economic evaluations that focus on care-related (rather than health-related) quality of life. The CES was piloted to inform the choice of primary health economic outcome for a cost-effectiveness analysis alongside a definitive trial.
Methods
The CES was completed by the carer at baseline and post bereavement. To contextualise the questionnaire post bereavement, the carer was asked to complete the measure reflecting back to ‘during the last few weeks of life’. Completion rates were analysed as counts of expected responses. Missing data were summarised as a percentage of the partially completed measure. A decision threshold of what was considered feasible was not set a priori. A score for the CES may be calculated only if all questions are completed, given that there is no rule for calculating in the presence of missing values. Responses to each of the six CES domains were coded (from 1 for the top level to 3 for the bottom level) to produce a caring state (i.e. 21213) and were then assigned a utility value, which ranged from 0 (for CES state 333333) to 1 (for CES state 111111). The utility values applied represent the mean preferences of a sample of carers aged ≥ 65 years in England, and were derived using best–worse scaling. 87
Results
Table 24 summarises the CES domains by completion at baseline and post bereavement, and the associated utility scores. In total, 21 of the 22 carers who completed follow-up completed the CES. The majority of carers completed all of the CES (21/22) and one carer completed 50% of the measure.
| Domain completeness | Baseline (n = 40) | Follow-up (n = 22) | |
|---|---|---|---|
| Activities | 40 | 21 | |
| Social support | 40 | 21 | |
| Organisational assistance | 39 | 21 | |
| Fulfilment | 40 | 20 | |
| Control | 40 | 20 | |
| Getting-on | 40 | 20 | |
| CES mean score | Baseline utility (n) | Follow-up utility (n) | Incremental utility |
| Intervention | 71.51 (19) | 72.17 (14) | 0.66 |
| Control | 77.87 (20) | 70.94 (6) | –6.93 |
At follow-up, the usual-care group had a mean score of 70.94, which was a decrease of 6.93 from baseline, and the intervention group a mean score of 72.51, which was an increase of 0.66 from baseline. A disutility (negative difference) indicates a decline in carers’ care-related quality of life during the period from baseline to bereavement, and a utility gain (positive difference) indicates an increase in carers’ care-related quality of life; however, it is difficult to make inferences about change from baseline to follow-up because of the small number of participants.
Conclusion
The CES offers a preference-based approach to incorporate the effects on carers in the economic evaluation, which focuses on care-related quality of life (rather than health-related quality of life). In this pilot study, the CES was completed by > 90% of the sample, which would indicate that this was a feasible measure of carer utility that could be used in a future economic evaluation alongside a clinical trial. A future economic analysis would need to consider baseline adjustment and the implications of completing the measure in retrospect at post-bereavement follow-up.
Chapter 8 Interpretation of results in relation to the feasibility of definitive randomised controlled trial
Assessment against progression criteria specified in the protocol
We assessed the feasibility to test the intervention in a future definitive RCT against the progression criteria that we specified in the protocol (Table 25). We concluded that, despite the intervention being deemed acceptable and safe, substantial redesign would be required for a future definitive RCT on the clinical effectiveness and cost-effectiveness of the intervention.
| Objectives | Threshold for progression to a full RCT | Assessment |
|---|---|---|
| To refine the assessment and outcome measures to be used in any potential RCT |
|
|
| To evaluate the acceptability of the manualised intervention (and potentially refine) | In the feasibility study, the simplest method is for lay carers to draw-up medications in immediate form only; a full trial would be more appropriate if able to extend this to advanced preparation and labelling | The intervention was acceptable to carers and patients, in this case carers had to draw up medications in immediate form only |
| To evaluate the recruitment process | In the feasibility study we have assumed 50% recruitment; we would say that a full trial is not possible if recruitment falls below 30% |
|
| To estimate participant retention rate for the full RCT | In the feasibility study, we have assumed 50% retention; we would say that a full trial is not possible if recruitment falls below 40% |
|
| To test the assessment and outcome measures for suitability, relevant change factors, and acceptability to participants | Owing to the small number of data available at the follow-up time point, assessment of sensitivity to change was not possible with any reliability | |
| To identify acceptability and collection of relevant data to inform the data collection and analysis plan for implementation in the subsequent RCT |
|
|
In addition, and to illuminate considerations regarding substantial redesign further, we have undertaken a stepwise approach to interpret the feasibility study results, as suggested by Bugge et al. :88
-
We applied the Shanyinde framework89 (Table 26) and summarised the positive findings and identified problems.
-
We used the ADePT (A Process for Decision-making after Pilot and feasibility Trials) framework to assess the identified problems and suggest solutions – first per problem identified, then in the context of a future definitive study. 88 Examples of the outcomes are given (see Assessment of solutions using ADePT).
| Methodological issues | Summary of findings | Summary of evidence |
|---|---|---|
| Did the feasibility/pilot study allow a sample size calculation for the main trial? | ||
CARiAD protocol:
|
No, calculating a sample size was not possible from the information collected, owing to the small number of participants. Further debate is needed regarding the appropriateness of the outcome measures |
|
| What factors influenced eligibility and what proportion of those approached were eligible? | ||
CARiAD protocol:
|
|
|
| Was recruitment successful? | ||
CARiAD protocol:
|
|
|
| Did eligible participants consent? | ||
| CARiAD protocol outlined consent procedures in the context of adults lacking capacity |
|
|
| Were participants successfully randomised and did randomisation yield equality in groups? | ||
|
|
|
| Were blinding procedures adequate? | ||
| The initial blinding procedures were amended in an attempt to increase recruitment. A future study would need to take this into account or allow for extra research staff to allow blinded assessment |
|
|
| Did participants adhere to the intervention? | ||
|
|
|
| Was the intervention acceptable to the participants? | ||
|
|
|
| Was it possible to calculate intervention costs and duration? | ||
|
|
|
| Were outcome assessments completed? | ||
CARiAD protocol:
|
|
|
| Were outcomes measured those that were the most appropriate outcomes? | ||
|
|
|
| Was retention to the study good? | ||
CARiAD protocol:
|
|
|
| Were the logistics of running a multicentre trial assessed? | ||
|
|
|
| Did all components of the protocol work together? | ||
| Most components of the protocol worked together | Notable exceptions are:
|
|
Findings in the context of the Shanyinde framework
List of positive or acceptable findings
-
The recruitment rate was above what was stated to be necessary for a definitive trial and was close to the 50% rate that was assumed.
-
Retention was above the 50% that was stated to be necessary for a definitive trial.
-
Outcome measures under contention for a main trial did assess main/general areas of interest: overall symptom control and carer self-efficacy/empowerment. When completed, measures were completed well.
-
Acceptability of the intervention was overwhelmingly positive, adherence to the intervention was generally good and the intervention was safe in the study population.
-
There was no evidence of contamination in the usual-care group.
In addition:
-
Overall, eligibility based on clinical estimate of survival worked well.
-
Recruitment appeared to be strongest where the DN team had close links with their local SPC team.
-
Conversion to consent was close to the 50% rate that was assumed.
-
Randomisation yielded equality in groups.
-
The training materials were well received, including by the REC.
-
Secondary outcome measures of carer confidence in administering medication and time to symptom control for patients were appropriate, and fully aligned with importance assigned by carers.
-
Close-out procedures were planned and executed well.
-
The CES provides a feasible preference-based approach to estimating carer utility, for use in an economic evaluation alongside clinical trial.
-
Formative work on DCE attribute identification suggests that it would be feasible and remain very pertinent to quantitatively elicit carers’ preferences as part of the overall research question.
List of ‘problems’ to scrutinise using ADePT
-
Not reaching the target recruitment.
-
Differential retention between groups in the trial.
-
Not able to do a sample size calculation for a main trial.
-
Unclear which outcome measure(s) is/are best to use for clinical effectiveness.
-
Suboptimal HCP adherence to the intervention.
In addition:
-
Sites opened for recruitment later than planned.
-
One site recruited significantly fewer participants.
-
Survival time was short for many potential participants as well as for many participants.
-
Although processes of randomisation were successful, acceptance of the randomisation results proved difficult.
-
A high proportion of carers in the dyads had a HCP background.
-
No consultee declaration forms were received in relation to patients who gave initial consent.
Assessment of solutions using ADePT
Each of the identified ‘problems’ was assessed in detail using ADePT. 80
In this section, we use four examples of the identified ‘problems’ to outline the potential solutions that may be effective and feasible in a trial setting.
Not reaching target recruitment (with one site struggling to significantly to recruit)
These issues are likely to be a trial-specific problem, as recruitment numbers would not be relevant in a real-world context.
The reasons for these observations are mainly the lack of DN research experience and time to commit to the study, which were very marked in the site that struggled to recruit. In addition, we observed a high proportion of carers with a HCP background. This could have been because HCPs appeared to apply the RA only when they felt sure that the carer in a dyad would be able to engage in the intervention. It is, therefore, likely that DN teams did not identify or approach all potentially eligible participants, as suggested by the qualitative findings.
Despite similar levels of input from the central CARiAD research team, CVUHB started recruitment late and recruitment was poor. In addition to pressures on DN time and DN research naivety, the DN teams in CVUHB did not have the same close working relationship with SPC teams as was observed in the well-recruiting DN teams in BCUHB and GCS. Additional factors may have been the different recruitment systems in place in that site and a preference for doing the trial training in a different manner. This site was particularly clear that the partial blinding of RNs created barriers to study progress; however, recruitment did not increase after RNs were unblinded, mainly owing to a long-term sickness absence of the RN that coincided with unblinding. It is not completely clear why there were such marked differences in site recruitment, so it is possible that other issues affected this that are not addressed by the suggested solutions.
Potential solutions include changing aspects of the following:
-
Trial design – rebalance research tasks away from DNs and towards RNs or study-specific SPC nurses (including the identification and recruitment of participants). In addition, teach DNs and SPC clinicians to apply RA to all eligible dyads and not only to those they already feel are competent, and emphasise to carers and HCPs alike that many people are fully capable of engaging in the intervention and not only those with a HCP background.
-
Context – a specific, detailed review of capability and capacity at the site selection stage. Introducing a study-specific SPC nurse to identify and recruit participants could lessen the burden on DN teams and improve engagement.
Potential solution: employ a study-specific specialist palliative care nurse
Could this solution be effective in a trial setting? Yes.
Evidence for this is that we observed in teams in which there were close links with a SPC nurse (e.g. BCUHB Abergele and GCS Team C) that there was a much higher recruitment rate. This may be because the intense SPC involvement enthused a very dedicated DN team with an already-established interest in palliative care or it might have unburdened the DN team of some of the tasks that are associated with the study, or both. The recruitment rate dropped off in BCUHB Abergele when the SPC nurse retired.
Likelihood of effectiveness? High.
If yes, could the solution be feasible in a trial setting? Yes.
Evidence for this is that we have seen that it is feasible for SPC nurses to work with DNs to improve dyad identification and recruitment. Other feasibility trials have successfully moved to full trial and used a study-specific HCP to manage the intervention. However, there can be issues around appointing this person, as it is a fixed-term position that is often part time. There are also additional costs associated with this and logistical considerations of one person covering large recruitment areas.
Likelihood of feasibility? Medium.
Potential solution: detailed review of capability and capacity at recruitment site selection stage
Could this solution be effective in a trial setting? Yes.
Evidence for this is that a detailed review of the capability and capacity at the site selection stage and in the selection of DN teams would draw attention to potential issues, such as lack of sickness cover, or other problems that may affect recruitment, such as suboptimal working relationships between DN teams and SPC teams. Studying the service provision of RNs, SPC nurses, DNs and their relative roles in each prospective recruitment site will be helpful, as will using sites where these service relationships are well understood.
In the two sites where there was more than one RN, recruitment was more successful.
Teams that had strong SPC team links showed the best recruitment.
There will always be some site failures and it is hard to be sure that this can be prevented.
Likelihood of effectiveness? Medium.
It is not completely clear why there were such marked differences in site recruitment, so it is possible that there are other issues affecting this that are not addressed by the suggested solutions.
If yes, could the solution be feasible in a trial setting? Yes.
The evidence for this is that recruitment was higher in other sites where it was possible to conduct a more detailed review of capability and capacity.
Likelihood of feasibility? Medium.
Differential retention between groups
These issues are likely to be a trial-only problem, as trial retention is not relevant in a real-world context.
Only six out of the 20 (30%) dyads in the usual-care group completed the trial. Reasons for non-completion (n = 14) included carers not being contactable post bereavement (n = 6), withdrawals (n = 4: three because they were not randomised to the intervention and one initiated by the trial team as part of close-out arrangements), lost to follow-up on PI advice (n = 3) and one carer declining follow-up.
Differential retention (lower in the usual-care group) is a common observation in randomised trials. In the CARiAD study we observed that carers assigned to the usual-care group were less engaged; this is probably linked to the fact that, to be eligible for the intervention group, the carers had to be willing to be trained to give SC medication. They therefore may not have been in equipoise regarding the intervention and may have felt resentful at not having the opportunity to be trained in the intervention.
Potential solutions include changing aspects of the following:
-
Intervention – if there is more evidence that the intervention is a ‘common good’, a future trial might consider randomising to different intensities of the intervention. Another possibility is to test two different interventions at once, both of which need testing in the end-of-life situation (A vs. B design).
-
Trial design – increased RN attention and follow-up in the usual-care group might improve retention; this role could also be undertaken by a study-specific SPC nurse (see Potential solution: employ a study-specific specialist palliative care nurse). A cluster-randomised design could be considered; however, this might simply move attrition to the level of the cluster.
-
Context – consider recruiting only in areas with explicit SPC nurse support.
Potential solution: cluster-randomised design
Could this solution be effective in a trial setting? Yes.
Evidence for this is that cluster randomisation is generally used in response to concerns regarding contamination bias. In this instance, the aim would be to remove the need for individuals to choose whether or not to be randomised, as this would become standard practice within the cluster. In those clusters in which the intervention was undertaken, the intervention would be offered to all appropriate dyads.
Likelihood of effectiveness? Medium.
If yes, could the solution be feasible in a trial setting? Yes.
Evidence for this is that cluster trials are now relatively standard practice in the realm of clinical trials; the difficulty would be being able to recruit the required number of clusters for power. Careful consideration would need to be given as to what should constitute a cluster (e.g. geographic region or DN team?).
Likelihood of feasibility? Medium, if sufficient clusters of sufficient size can be identified.
Acceptance of randomisation proved difficult
These issues are likely to be a trial-only problem, as randomisation would not be relevant in a real-world context.
Processes for randomisation worked successfully. However, although participants were willing to be randomised, the randomisation results were not universally accepted.
Participants were required to wish to undertake the intervention as part of eligibility for the trial and, although randomisation would have been explained as part of the trial process, participants may not have been in true equipoise about the treatments on offer. Resentful demoralisation may form part of the explanation for disengagement from the usual-care group. In interviews, HCPs said that they believed that dyads allocated to the usual-care group were disappointed, and gave this as a reason for withdrawal and loss to follow-up.
Potential solutions include changing aspects of the following:
-
Trial design – further explanation of the need for randomisation at the point of consent, use of a cluster-randomised design, possible use of Zelen’s randomisation90 and consideration of changing the level of system measurement.
-
Context – assess the policy context to see whether or not this practice is a common good and generate background policy positions and documentation to support more widespread use of the practice prior to further testing.
Potential solution: use of Zelen’s randomisation
Could the solution be effective in a trial setting? Yes.
Evidence for this is that consent would be sought only from those actually randomised to the novel treatment, which, therefore, removes any resentful demoralisation. Consent may still be needed in the usual-care group for data collection at some level.
Likelihood of effectiveness? Medium.
If yes, could the solution be feasible in a trial setting? Yes.
Evidence for this is that Zelen’s randomisation may not work in this exact design, owing to the need for data collection within the usual-care group; however, consideration should be given to what routinely collected data are available.
Likelihood of feasibility? Low; would require further field testing.
Potential solution: consider changing the level of system measurement
Could the solution be effective in a trial setting? Yes.
Evidence for this is that measurement at a different level may remove the need to consent participants.
Likelihood of effectiveness? Medium.
If yes, could the solution be feasible in a trial setting? Yes.
Evidence for this is that measurement at the system level may prove difficult to establish the effect of the intervention.
Likelihood of feasibility? Low; would require further field testing.
Unclear which outcome measure(s) is/are best to use for clinical effectiveness
This issue is likely to be a problem in both a trial and the real world. The latter is because, even if the intervention is implemented, any future service evaluation will face this question.
The outcome measures under contention for a future definitive trial were not deemed appropriate for assessing the effect of the intervention. Although the completion rate of MSAS-GDI was acceptable, assessment of sensitivity to change was not possible with any reliability owing to the small number of data available at the follow-up time point. The completion rate of the QOLLTI-F was lower than anticipated, which may be a result of when this was supposed to be completed. Completion at baseline (when the measure was introduced by a RN) was good, but it may simply be too burdensome to ask carers who are looking after somebody in the last days of their life to complete this, or any other outcome measure, independently and at regular intervals. Feedback from carers was that the QOLLTI-F did not capture the nuances of their situation accurately; although they may not have had the time to do the things that it asked about, they did not necessarily feel burdened by this as they had known to expect this and it was acceptable to them during this time.
At the time of designing the CARiAD trial, there was no suite of COMET measures to apply to studies of the last days of life. In January 2017, development of an international core outcome set of the best care for the dying person commenced. 91 The outcome of this work is still awaited, and may affect any future studies during this care episode.
Potential solutions to the issue of unclear outcome measures include changing aspects of the following:
-
Trial design – identify alternative outcome measures for clinical effectiveness, use ‘time to symptom control’ as a patient-level primary outcome measure. Alternatively, consider the effect of the intervention being measured at an alternative level, such as process- or system-driven outcomes. As this intervention is unlikely to replace any service provision within palliative care teams but become an adjunct to usual care, measuring the effect at this level may be more appropriate and enable those with a desire to support their loved ones in this way to do so.
Potential solution: identify alternative outcome measures for clinical effectiveness
Could the solution be effective in a trial setting? Yes.
Evidence for this is that if an appropriate measure can be identified then it is likely that it could be collected, although some thought needs to go into the perspective and timing of the appropriate measure. Identification of an effective primary outcome is limited by the lack of other candidates observed in the literature.
Likelihood of effectiveness? Low (currently unable to identify an appropriate individual-level outcome measure).
If yes, could the solution be feasible in a trial setting? Yes.
Evidence for this is that if an alternative primary outcome can be identified, the current work has shown that it is likely the participants will complete outcomes of standard questionnaire format.
Likelihood of feasibility? Medium; only if an appropriate outcome can be identified.
Chapter 9 Discussion and conclusions
Discussion
The CARiAD trial was premised on the fact that most people who are dying express a wish to be cared for in their own home in the last days of their life, and that willing and able lay carers play a key role in supporting such a wish. The work of the PeolcPSP11 clearly indicated that people in the UK prioritised the importance of interventions to support home-based care towards the end of someone’s life. Indeed, the first of the top 10 research priorities in end-of-life care asked ‘what are the best ways of providing palliative care outside of “working hours” to avoid crises and help patients to stay in their place of choice?’ and the fourth asked ‘what information and training do carers and families need to provide the best care for their loved one who is dying?’. The intervention of carer administration of as-needed medication for common breakthrough symptoms in people dying at home (henceforth referred to as the intervention) is one of the ways in which the wish for a home death can be supported. This has proven successful in other countries. 5 The study is also set against an emerging cultural backdrop in the UK, where the process of dying is increasingly demystified and demedicalised.
In the UK, however, there are well-described and ongoing challenges in the provision of support to enable home-based care. For example, a recent interview study92 of 30 HCPs and three bereaved carers was carried out to study why patients were transported to hospital to die. The study concluded that home-based end-of-life care in England is structurally precarious owing to poor resources. Hospital admission was considered by the HCPs when there was insufficient nursing provision or when extensive family support was unsupported by home-based services.
By contrast, the intervention studied in the CARiAD trial was already practised in a few areas in the UK prior to this study. At least two other areas implemented the intervention during the time that the CARiAD study was in process, and adoption by new areas is ongoing. However, adoption of the practice is slow, with different policies governing the intervention in each area. The CARiAD study appeared to widen interest and prompted clinicians from many more areas to be in touch and express an interest in the intervention. Most opted to wait for the findings of the CARiAD study to inform their next steps.
Despite this positive approach from many areas, our results shed doubt on whether or not this intervention can be delivered consistently and equitably across the UK, even if it is deemed effective. The reasons for this are likely to be intricate and difficult to solve. This is despite the importance that is assigned to interventions that may support home-based end-of-life care and the specific interest in the intervention studied. Although the CARiAD results suggest that, in the UK, the intervention is practicable, acceptable and safe in the participating dyads, there is uncertainty about whether or not HCPs are in a position to support it. This appears to be due to the current NHS context, with high workloads and pressure on community staff. This would probably apply whether conducting research on the intervention or on potential implementation. Although we note that there were a number of protocol deviations in the trial, the majority of these related to the completion of trial paperwork and did not affect patient safety. This is probably a result of the research naivety of the DNs, who prioritised the record-keeping required as part of usual care, suggesting that this may not be a continuing problem if the intervention were to be implemented in the future.
By contrast, although numbers of CARiAD dyads participating were small, there was almost universal positivity of carers towards the intervention. The high recorded levels of carer confidence over time and the considerable improvements in time to symptom control for patients suggest that the intervention was acceptable, feasible and safe in this small group. Perhaps the research question has changed and the intervention should be argued to be a common good. This means that a full trial of effectiveness may no longer be appropriate. In short, there is a conundrum: people probably want this intervention, but the HCPs in the NHS, as it is at present, may not be able to support it.
The CARiAD team concludes that the results of this feasibility trial are uncertain. We consider here the reasons for this position and articulate the range of additional questions that are raised.
If a future definitive trial of clinical effectiveness and cost-effectiveness of the intervention was still felt to be appropriate (i.e. the UK is not yet ready to consider lay-carer role extension a common good), major redesign would be required. This is detailed in Chapter 8.
Meanwhile, a number of important and timely questions have been raised by our findings that relate to NHS context and HCP perceptions of risk and burden. We consider the main issues under the following subheadings: Current NHS context, Who is burdened?, Cultural influences, Health-care professionals as lay carers, More notes on patient/carer selection and Cost implications.
Current NHS context
The reality of the current pressures on community services was an ongoing thread: DN teams reported being overstretched, resulting in severe time constraints. As DNs were research naive, using any extra time (albeit specifically funded) to support the research study was a considerable additional strain. This aligns with descriptions in the current literature. 92 This leads us to the opinion that there may be insufficient community care to enable consistent, well-supported home-based dying. Furthermore, the research culture among community nurses may not be well-enough developed.
Who is burdened?
During the design stage of the CARiAD study, the burden on carers (real or perceived) was a pivotal issue; this is evident from the methods and materials that we designed. Even at expert consensus workshops, HCPs emphasised this issue and focused their discussions on it. It was also a major theme in the HCP interviews as part of the embedded qualitative study. The subtext, however, of the quantitative and qualitative findings is that, although carer burden (or mitigating it) is very important to HCPs, this might not be the only factor at play. This may be a misperception on the part of HCPs about the willingness of carers to take on the role. It is also possible that HCPs found it easier or more convenient to give this as an explanation than to be more inclusive in their recruitment practices.
The burden that such an intervention placed on HCPs was noted in a number of ways. It was clear that HCPs perceived the recruitment of dyads as ‘risky’ in that they were so carefully chosen. DNs are used to dealing with patients with complex comorbidity issues, and these are things that the DNs deal with on a daily basis and are familiar with the processes for how to deal with them. CARiAD presented a new type of problem for the DNs in terms of decision-making ability. They reported that in the current NHS context they are working under constant strain and they describe being under scrutiny for any mistakes that are being made. Our interpretation is that this culture may have instilled a reluctance in these staff that made it very difficult for them to confidently include people in the study, owing to increasingly risk-averse practices. The high proportion of carers with HCP experience or other clinical experience suggests that they found this to be a reassurance, that is, they would be able to manage the demands of the study and not make any mistakes that may later cause problems for the DN teams. This was a concern raised repeatedly in HCP training sessions, and in the HCP interviews numerous references were made to making sure carers got things ‘right’. Even with continued input and support from the research team and RNs it was not possible to completely overcome these issues, particularly when they were combined with other factors, such as short staffing, limited resources and minimal time to complete all of the required tasks. Our interpretation is that the issue of risk perception was, therefore, undoubtedly augmented by, and intertwined with, the ongoing and severe pressures that community services are facing, which necessitate practitioners to be wary of undue risk.
It was clear that individual HCPs’ level of enthusiasm for the intervention played a big part in how they interacted with the study. In sites where at least one of the HCPs was highly motivated about the intervention, recruitment to the study was good. In the other areas there was always general enthusiasm, but this did not translate into good recruitment to the study. All of the sites reported similar levels of pressure on time and resources. Perhaps further, in-depth research is needed to understand HCPs’ perceptions of risk and attitudes towards such interventions.
From our findings, we concluded that, although HCPs may be ready to support this intervention in theory, they are perhaps not able to do so in practice.
Cultural influences
The CARiAD team comprises UK and Australian colleagues, and the original idea for the intervention was very much informed by the Australian group. When recruitment was much more difficult in the UK setting, we wondered if there may be less resilience or different concepts of burden between different geographies. One clear difference is that the Australian policy landscape makes the practice of the intervention much more straightforward. There may be many other differences at play in our findings that could include different home health-care systems and levels of provision; different grades of community nurses; different caseloads; different approaches to rural medicine in view of great geographical distances in Australia; and possibly cultural differences in UK/Australian public and HCPs regarding resilience and risk aversion. Perhaps more cultural comparisons are needed prior to implementing home-based lay carer roles designed to facilitate home deaths.
Health-care professionals as lay carers
We noted that five out of 12 carers interviewed had a HCP background. This is disproportionate, even allowing for the fact that the NHS is the single biggest employer in the UK. The qualitative findings shed light on this, as there was an assumption that lay carers with a HCP background were best placed to take part in this intervention. This came from several perspectives: from HCPs suggesting dyads for inclusion in the study (see Who is burdened?), from the patients and from other family members with no HCP background, and from those individuals in families who have a HCP background themselves.
Both patients and families seemed to easily assume that a person with a HCP background was the one who should be suggest as the carer in the dyad because the carer would already know how to administer medications. This was despite the fact that trial materials discussed lay carers and it was explained that training would be given. Equally, those with a HCP background in a family often assumed that they were the person to take on the task. Interestingly, and in contrast to what HCPs stated as part of expert consensus work, no HCP who held current registration and acted as a carer in CARiAD mentioned that they were unsure whether or not the GMC/NMC regulations would support their taking on this task. This could have been because they did not know that the regulations might pose an issue or because that they felt protected as the task was to be undertaken as part of a research study.
We reflected on the potential reasons for why we observed the phenomenon of the seemingly disproportionate number of HCPs as lay carers. Although it was not clear that we could easily reach for the constructs of ‘gate-keeping’ or conscious ‘bias’ to explain this, it is perhaps understandable that busy, risk-averse HCPs with the burden of deciding who should or should not take part in the study could be reassured if a carer had a HCP background.
However, it could be considered that HCP behaviour in selecting dyads was highly appropriate and protected against undue carer burden. Alternatively, maybe it was a protective mechanism that led HCPs to choose dyads so carefully. Owing to this careful selection, carers seemed to have the right balance between benefit and burden (as we saw in our study). In short, perhaps HCPs did pick the right dyads; if they did not, we might have observed very different realities of undue carer burden.
More notes on patient/carer selection
For any future work on the topic, whichever view is espoused, careful selection of the patient/carer group will be key in the light of our findings. The actual selection criteria that we observed are possibly much more nuanced than the summary of CARiAD eligibility (i.e. a patient wishing to die at home plus a carer willing to be trained to give these SC medications). Screening to approach a dyad for this intervention, whether as part of research, implementation or routine clinical practice, should probably be a step-wise approach. Such steps could be people who:
-
have a life-limiting diagnosis
-
are aware of their life-limiting diagnosis
-
are aware that their prognosis is limited (i.e. they are coming towards the end of their life)
-
have had the opportunity and been willing to consider their wishes regarding future care (including, specifically, a wish to die at home)
-
have expressed the wish to die at home and hold this view strongly and in a sustained way
-
have a carer/carers who are willing and able to support their wish to die at home (including taking on the additional tasks this requires in the context of not having 24/7 paid care)
-
have a carer who is willing to take on the specific additional task of being trained to give these SC medications.
This careful selection process will still probably yield a sizable number of patients/carers to whom the intervention could be offered, but it aims to strike a balance between more equitable access to the intervention and not overburdening the carer. Therefore, although on face value one might conclude that the intervention should be offered to all who are likely to have a home death (the common good argument), we conclude that a stepwise and thorough approach is preferable.
Cost implications
The intervention is about better care for patients and carers, and may not have any impact on costs to the NHS. The time it took to train carers was mentioned, but this was not perceived as a major barrier. Although some HCPs interviewed believed that the time to train carers and offering additional telephone support when needed would mean that their workload was not reduced, others believed that time taken to train carers would mean that they saved time later owing to reduced visits. However, HCPs were inclined to view the intervention in terms of patient and carer benefit rather than their own workload.
Public contribution
As outlined in the previous sections, public contribution was instrumental in the CARiAD study and was of high value during each stage. Overall, CARiAD was designed and delivered more effectively as a result of the public contributor involvement. The meaningful impact of the presence of bereaved carers as public contributor co-applicants also increased and improved the sensitivity with which CARiAD was conducted. Changes were made to the study design as a direct result of public contributor suggestions and advice, which improved the study as a whole. The two co-applicant public contributors were actively involved as key team members throughout, from before the beginning to after the end of CARiAD; we hope that we facilitated and supported their involvement. We feel that CARiAD offers an example of how public contribution can be invaluable to research and should be included and made full use of during all stages of a study. We believe that public contribution can be particularly advantageous in trials involving end-of-life care.
Strengths and limitations
Strengths
The study illuminated numerous aspects of research related to last-days-of-life research: it illustrated both dying people’s and carers’ willingness to be randomised, strong and successful public contributor involvement at all stages and the value of rich qualitative findings in explaining the quantitative results. It used a structured approach to assess the outcome of such feasibility work.
In addition, it generated a new UK training package and method for lay carer role extension that can be used in any further research or implementation, and clarified limitations of community-based nursing care in three areas in the UK. It also illuminated a number of further research questions, listed above, in recommendations for future research.
The CARiAD study has helped to move research methods for last-days-of-life research forward by providing much-needed evidence of conversion rates from approach to recruitment, which will help future researchers in estimating these rates for their studies. It also gives an example of how such trials can be introduced sensitively to patients and their families, as well as what works in terms of data collection time points and methods. The REC chairperson commended the study’s consideration of these matters and how these were reflected in the trial materials and processes. In particular, she commented on our careful consideration of the consultee process. The results from the trial have further illuminated the realities of this process in the context of last-days-of-life research, as the current legislation does not adequately account for how mental capacity can be assessed in this situation, who it should be assessed by and how consultee assent can be obtained in a way that is sensitive to the difficult emotional situation of patients and their families.
The CARiAD study, like the AMBER study,93 adds weight to the idea that vulnerable patient groups at the end of their life are ready and willing to engage in research of this type. The study showed the tension between the anxieties that carers may have had about taking an extended role and the relief that they felt when they were able to relieve the symptoms of a dying family member.
Limitations
The limitations of this feasibility trial have been fully explored in previous sections (see Chapter 8) and only the main points are summarised here. The key limitations can be summarised as follows:
-
A series of key progression criteria were not met.
-
The differential recruitment to the control group was of particular concern.
-
Overall, the limitations led to an equivocal decision about progression to a full Phase III RCT.
The lack of a policy framework to underpin practice in the UK may have impeded the study in a number of ways; however, if such a policy framework existed, a randomised study would not have been appropriate.
Implications for future research
Future work is needed and will probably comprise several parallel work streams as part of a programme of work. We base our suggestions on the need to unpick the apparent disconnect between what the public wants and what the NHS can deliver.
The research recommendations below are ordered by what the authors currently understand to be the priorities; however, these will undoubtedly evolve as the suggested work progresses.
-
Understand the context of the areas in the UK where the practice has already been adopted (or is soon to be adopted, or implementation has failed):
-
Evaluation of these pilots should be carefully undertaken to assess what conditions allow the practice to be safe for both patients and carers and whether or not carer burden is acceptable. Realist evaluation is a potential future approach to investigating this further.
-
-
Understand the nature of usual practice in terms of community care for people dying at home in the UK:
-
As context was a key problem for the UK, further descriptive work is needed regarding capacity of the existing palliative care provision, team structures and limitations in the community for supporting home deaths. This should include attention to geographical differences in provision.
-
-
Ascertain wide public views on the specific intervention of extending lay carers’ role in this way. We know that the general area of interventions to support a home death is important and that in-depth understanding about public views on the specific intervention is needed:
-
Further in-depth qualitative work with those who have lived experience of supporting a home death in this way. This could explore some of the interesting issues raised in our study, for example what kind of lay carers are happy and able to take on this role (not just those who are also HCPs), and if carers have different views about giving the first injection and giving a top-up when a syringe driver is already in place.
-
Further qualitative work is required to understand public views and the views of bereaved carers about lay role extensions such as CARIAD.
-
Stakeholder workshops or public survey methods could be useful to understand if the UK public is in support of the specific intervention and, if so, how strongly. It is important to ascertain if the public sees this as a common good and if they feel that it should be pursued further.
-
-
Understand HCPs’ motivations and views on burden and risk, and interface with NHS context:
-
An in-depth qualitative study, preferably using IPA rather than framework analysis, is needed to understand HCPs’ views about and attitudes to this lay-carer role extension. This could be a stand-alone study, but if there was a future definitive trial HCP interviews should be equally as deep as carer interviews. HCPs should also be included in attribute selection as part of economic evaluation work-up, as HCPs may rank attributes differently from carers (this would be in contrast to the design of CARiAD).
-
There might also be a literature on HCPs’ perceptions of risk that can support the exploration of HCPs’ views and attitudes; further study of their views of risk of this practice should be explored.
-
-
Explore the most appropriate quantitative outcome measures to use (this is equally important in terms of future research and real-world implementation, as future service evaluation will need to use such appropriate outcomes). In addition, consider at which level these outcomes should be measured.
Conclusion
The CARiAD study explored the feasibility of testing the clinical effectiveness of the intervention of carer administration of as-needed medication for breakthrough symptoms in people dying at home in the UK to inform the design of a future definitive trial. We concluded that the success of a future definitive trial is uncertain. This is owing to the equivocal results in our original progression criteria, particularly the poor recruitment overall and low retention in the usual-care group. The context of the feasibility trial was not ideal, as DNs were seriously overstretched and unfamiliar with research methods; however, the intervention was shown to be acceptable, feasible and safe in the small study population and our criteria for both recruitment and retention overall were satisfied.
Furthermore, noting that the intervention is already spreading across more areas in the UK, consideration should be given to whether or not there is still an unanswered clinical effectiveness question. The CARiAD example lends some weight to this notion by demonstrating considerably shorter time to medication administration and faster symptom control in the intervention group, and almost universal positivity from carers. For this reason, a ‘common good’ argument could increasingly be defended. Moreover, a disparity between carers’ and HCPs’ readiness to consider the intervention was clearly demonstrated.
Future work is clearly needed. This should include ascertaining wider public views on the specific intervention, understanding HCPs’ motivations and views on burden and risk, and interface with NHS context, understanding the context of the areas in the UK where the practice has already been adopted, considering the need for a national policy on the intervention and exploring the most appropriate quantitative outcome measures to use. It may be that there are unanswered questions relating to the intervention that would be best studied in a trial; future work suggested above will help to ascertain if this is the case.
Acknowledgements
Particular thanks go to the CARiAD participants for giving their time when it was at its most precious.
We would also like to thank the following:
-
The DNs at all sites who invested significant time and effort into the study, particularly Karen Evans.
-
The SPC nurses, particularly Jenny Stewart, who showed such enthusiasm for the study and supported the DN teams in identifying patients and training carers.
-
The academic and public contributors who attended the expert consensus workshops, trial steering and data monitoring committees for their knowledge and guidance. Our thanks, in particular, to Christine Hirsch (Senior Lecturer in Clinical Pharmacy) for reviewing the draft report.
-
The SPC clinicians who supported the work-up and championed the study, particularly Susan Phillips, Elaine Sturman, Helen Mitchell, Theresa Richards and Julie Davies.
-
The dedicated RNs (Vicky Saul, Jane Heron, Gwyneth Davies, Mim Evans, Sophie Fletcher and Rebecca Parker) and their administrative support staff (Kelly Andrews, Lewis Waggett and Patricia Thomas) at each site who worked so hard to support this work and recruit the participants. Particular thanks to Jane Stockport (RN), who supported the work-up and conduct of the study.
-
Nic Nikolic, Emma Jones, Richie Evans and Natasha Hulley – the administrative staff at the North Wales Centre for Primary Care Research for every aspect of their organisational and technical support.
-
The staff at NWORTH Clinical Trials Units, particularly Jean Ryan (manager).
Contributions of authors
Marlise Poolman (https://orcid.org/0000-0002-2837-363X) (Clinical Senior Lecturer in Palliative Medicine) was involved in the conception of the study and the design and development of the intervention. She provided SPC clinical expertise (Wales). As co-chief investigator, she had overall responsibility for the co-ordination and delivery of the study. She fulfilled PI duties for BCUHB. She contributed to all outputs from the study, drafted and edited the final report, and is leading the dissemination of results at national and international conferences.
Jessica Roberts (https://orcid.org/0000-0002-3271-1814) (Trial Manager and Health Services Research) assisted with the design practicalities and research ethics applications. She oversaw the co-ordination and delivery of the study including trial set-up and training. She was closely involved in analysis and interpretation. She contributed to all outputs from the study, revising it and providing detailed feedback.
Stella Wright (https://orcid.org/0000-0001-9634-6273) (Trial Manager and Research and Development Officer) oversaw the co-ordination and delivery of the study (maternity leave cover). She was involved in report writing.
Annie Hendry (https://orcid.org/0000-0002-2112-1368) (Research Officer and Qualitative Research) has contributed to matters relating to the embedded qualitative study, and was involved in the conduct, analysis, interpretation and report writing for Chapters 5 and 6. She collected data for the DCE as part of the qualitative interviews.
Nia Goulden (https://orcid.org/0000-0001-6511-3987) (Trial Statistician) was the trial statistician. She provided analysis of the data and contributed to report writing of Chapter 4.
Emily AF Holmes (https://orcid.org/0000-0002-0479-5336) (Research Officer and Health Economics) was involved in the design, conduct, analysis and interpretation of the DCE and health economic outcomes elements. She led on the report writing for Chapter 7.
Anthony Byrne (https://orcid.org/0000-0002-1413-1496) (Consultant in Palliative Medicine) fulfilled PI duties for CVUHB. He provided SPC clinical expertise (Wales) and illuminated Welsh policy context. He assisted with pilot trial design. He gave detailed feedback on the final report.
Paul Perkins (https://orcid.org/0000-0002-6848-967X) (Consultant in Palliative Medicine) fulfilled PI duties for GCS. He provided SPC clinical expertise (England). He assisted with pilot trial design. He gave detailed feedback on the final report.
Zoe Hoare (https://orcid.org/0000-0003-1803-5482) (Principal Statistician), as Clinical Trials Unit lead, advised on matters relating to trial design, including power and sample size calculations and randomisation. She oversaw analysis of the data and contributed to drafting the final report, revising it and providing detailed feedback.
Annmarie Nelson (https://orcid.org/0000-0002-6075-8425) (Marie Curie Professor of Supportive and Palliative Care) was qualitative lead and oversaw the design, conduct, analysis and interpretation of the embedded qualitative study. She provided public contribution expertise. She provided oversight of Chapters 5 and 6 of the final report.
Julia Hiscock (https://orcid.org/0000-0002-8963-2981) (Research Fellow and Qualitative Research) assisted with the design, conduct, analysis and interpretation of the embedded qualitative study. She supported the qualitative lead and had oversight of the qualitative researcher. She advised on sociological aspects and led on public contribution. She contributed to drafting the final report, revising it and providing detailed feedback.
Dyfrig Hughes (https://orcid.org/0000-0001-8247-7459) (Professor of Pharmacoeconomics) was the health economics lead and contributed to matters relating to economic issues. He oversaw the design, conduct, analysis and interpretation of the DCE and health economic outcomes elements. He provided oversight of Chapter 7 of the final report.
Julie O’Connor (Public Contributor) provided valuable insights informing the design and conduct of the study, as well as contributing to interpretation of results. She assisted the drafting of the Plain English summary and supported dissemination at national conferences.
Betty Foster (Public Contributor) provided valuable insights informing the design and conduct of the study, as well as contributing to interpretation of results. She assisted the drafting of the Plain English summary.
Liz Reymond (https://orcid.org/0000-0001-6617-4618) (Professor of Palliative Care) was involved in the design of the pilot RCT, sharing expertise on outcome measures, medicines management and the intervention being tested. She provided SPC clinical and research expertise (Australia). She commented on the final report.
Sue Healy (https://orcid.org/0000-0002-9021-5835) (SPC Nurse Manager) was involved in the design of the pilot RCT as she had direct clinical experience of implementing and supporting the intervention being tested. She provided SPC clinical expertise (Australia).
Penney Lewis (https://orcid.org/0000-0002-7217-5884) (Professor of Law) advised on and drafted the UK legal framework for the intervention being tested. She illuminated ethics issues, including consent procedures. She commented on the final report.
Bee Wee (https://orcid.org/0000-0002-7714-0349) (Professor of Palliative Care) assisted with pilot RCT design. She provided SPC clinical expertise (England) and illuminated UK policy context. She advised on ethics issues including consent mechanism and gave detailed feedback on the final report.
Rosalynde Johnstone (https://orcid.org/0000-0003-1642-5356) (Project Manager and Last-Days-of-Life Care Project) provided guidance on outcome measures and Welsh policy context. She gave detailed feedback on the final report.
Rossela Roberts (https://orcid.org/0000-0003-1775-0986) (Clinical Governance Officer) advised on ethics issues including consent mechanism, as well as research network support.
Anne Parkinson (https://orcid.org/0000-0003-4495-5127) (SPC Nurse) supported the study with SPC clinical and recruitment expertise (England). She commented on the final report.
Sian Roberts (https://orcid.org/0000-0002-4883-4122) (Research and Development Pharmacist) provided medicines management advice.
Clare Wilkinson (https://orcid.org/0000-0003-0378-8078) (Professor of General Practice) was involved in the conception of the study and the design and development of the intervention. She provided UK primary care clinical expertise and advised on matters relating to RCT methodology. As senior co-chief investigator, she had overall responsibility for the co-ordination and delivery of the study. She contributed to all outputs from the study, revising it and providing detailed feedback.
All authors made substantial contributions to conception and design and/or to acquisition of data and/or to analysis and interpretation of data. All authors read and approved the final manuscript.
Publication
Poolman M, Roberts J, Byrne A, Perkins P, Hoare Z, Nelson A, et al. CARer-ADministration of as-needed subcutaneous medication for breakthrough symptoms in homebased dying patients (CARiAD): study protocol for a UK-based open randomised pilot trial. Trials 2019;20:105.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PHR programme or the Department of Health and Social Care.
References
- Office for National Statistics . National Survey of Bereaved People (VOICES): 2013 2014. www.ons.gov.uk/ons/rel/subnational-health1/national-survey-of-bereaved-people--voices-/2013/stb---national-survey-of-bereaved-people--voices-.html?format=print (accessed 3 October 2019).
- Eurocarers . Carers in Europe Factsheet 2009. https://eurocarers.org/publications/carers-in-europe/ (accessed 1 May 2020).
- Thomas C, Morris S, Harman J. Companions through cancer: the care given by informal carers in cancer contexts. Soc Sci Med 2002;54:529-44. https://doi.org/10.1016/S0277-9536(01)00048-X.
- Gomes B, Higginson IJ. Factors influencing death at home in terminally ill patients with cancer: systematic review. BMJ 2006;332:515-21. https://doi.org/10.1136/bmj.38740.614954.55.
- Healy S, Israel F, Charles MA, Reymond L. An educational package that supports laycarers to safely manage breakthrough subcutaneous injections for home-based palliative care patients: development and evaluation of a service quality improvement. Palliat Med 2013;27:562-70. https://doi.org/10.1177/0269216312464262.
- Miaskowski C, Robbins AH. Supportive Care of the Patient with Cancer. New York; 1988.
- Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage 2007;34:94-104. https://doi.org/10.1016/j.jpainsymman.2006.10.015.
- Twycross R, Wilcock A, Howard P. Palliative Care Formulary. Nottingham: Palliativedrugs.com Ltd; 2015.
- NHS England . Leadership Alliance for the Care of Dying People. Engagement With Patients, Families, Carers and Professionals 2013. www.engage.england.nhs.uk/consultation/care-dying-ppl-engage/supporting_documents/lacdpengage.pdf (accessed 3 October 2019).
- Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 1994;30:1326-36. https://doi.org/10.1016/0959-8049(94)90182-1.
- Palliative and End of Life Care Priority Setting Partnership 2015. www.mariecurie.org.uk/globalassets/media/documents/research/PeolcPSP_ExecSummary_English.pdf (accessed 3 October 2019).
- Nelson A. Beyond the Questions: Shared Experiences of Palliative and End of Life Care 2016. www.mariecurie.org.uk/globalassets/media/documents/research/publications/beyond-the-questions-esrc-report.pdf (accessed 3 October 2019).
- Baillie J, Anagnostou D, Sivell S, Van Godwin J, Byrne A, Nelson A. Symptom management, nutrition and hydration at end-of-life: a qualitative exploration of patients’, carers’ and health professionals’ experiences and further research questions. BMC Palliat Care 2018;17. https://doi.org/10.1186/s12904-018-0314-4.
- Caring@home 2019. www.caringathomeproject.com.au/ (accessed 3 October 2019).
- Anderson BA, Kralik D. Palliative care at home: carers and medication management. Palliat Support Care 2008;6:349-56. https://doi.org/10.1017/S1478951508000552.
- Chellappan S, Ezhilarasu P, Gnanadurai A, George R, Christopher S. Can symptom relief be provided in the home to palliative care cancer patients by the primary caregivers?. Cancer Nurs 2013;37:E40-7. https://doi.org/10.1097/NCC.0000000000000098.
- Israel F, Reymond L, Slade G, Menadue S, Charles MA. Lay caregivers’ perspectives on injecting subcutaneous medications at home. Int J Palliat Nurs 2008;14:390-5. https://doi.org/10.12968/ijpn.2008.14.8.30774.
- Letizia M, Shenk J, Jones TD. Intermittent subcutaneous injections of pain medication: effectiveness, manageability, and satisfaction. Am J Hosp Palliat Care 1999;16:585-92. https://doi.org/10.1177/104990919901600407.
- Rosenberg JP, Bullen T, Maher K. Supporting family caregivers with palliative symptom management: a qualitative analysis of the provision of an emergency medication kit in the home setting. Am J Hosp Palliat Care 2015;32:484-9. https://doi.org/10.1177/1049909114531326.
- Sheehy-Skeffington B, McLean S, Bramwell M, O’Leary N, O’Gorman A. Caregivers experiences of managing medications for palliative care patients at the end of life: a qualitative study. Am J Hosp Palliat Care 2014;31:148-54. https://doi.org/10.1177/1049909113482514.
- Lee L, Howard K, Wilkinson L, Kern C, Hall S. Developing a policy to empower informal carers to administer subcutaneous medication in community palliative care; a feasibility project. Int J Palliat Nurs 2016;22:369-78. https://doi.org/10.12968/ijpn.2016.22.8.369.
- Dredge A, Oates L, Gregory H, King S. Effective change management within an Australian community palliative care service. Br J Community Nurs 2017;22:536-41. https://doi.org/10.12968/bjcn.2017.22.11.536.
- Morris SM, King C, Turner M, Payne S. Family carers providing support to a person dying in the home setting: a narrative literature review. Palliat Med 2015;29:487-95. https://doi.org/10.1177/0269216314565706.
- Healy S, Israel F, Charles M, Reymond L. Laycarers can confidently prepare and administer subcutaneous injections for palliative care patients at home: a randomized controlled trial. Palliat Med 2018;32:1208-15. https://doi.org/10.1177/0269216318773878.
- Yap R, Akhileswaran R, Heng CP, Tan A, Hui D. Comfort care kit: use of nonoral and nonparenteral rescue medications at home for terminally ill patients with swallowing difficulty. J Palliat Med 2014;17:575-8. https://doi.org/10.1089/jpm.2013.0364.
- Williams L. More Care, Less Pathway: A Review of the Liverpool Care Pathway 2013. www.gov.uk/government/uploads/system/uploads/attachment_data/file/212450/Liverpool_Care_Pathway.pdf (accessed 3 October 2019).
- Payne S, Brearley S, Milligan C, Seamark D, Thomas C, Wang X, et al. The perspectives of bereaved family carers on dying at home: the study protocol of ‘unpacking the home: family carers’ reflections on dying at home. BMC Palliat Care 2012;11. https://doi.org/10.1186/1472-684X-11-23.
- Payne S, Turner M, Seamark D, Thomas C, Brearley S, Wang X, et al. Managing end of life medications at home – accounts of bereaved family carers: a qualitative interview study. BMJ Support Palliat Care 2015;5:181-8. https://doi.org/10.1136/bmjspcare-2014-000658.
- Hopkinson J, Hughes J, Lowson E, Richardson A, Duke S, Anstey S, et al. Cancer carers medicines management: a feasibility trial of an educational intervention for managing end of life pain medication. BMJ Support Palliat Care 2014;4. https://doi.org/10.1136/bmjspcare-2014-000654.18.
- Latter S, Hopkinson JB, Lowson E, Hughes JA, Hughes J, Duke S, et al. Supporting carers to manage pain medication in cancer patients at the end of life: a feasibility trial. Palliat Med 2018;32:246-56. https://doi.org/10.1177/0269216317715197.
- Bennett MI, Mulvey MR, Campling N, Latter S, Richardson A, Bekker H, et al. Self-management toolkit and delivery strategy for end-of-life pain: the mixed-methods feasibility study. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21760.
- Lovell MR, Luckett T, Boyle FM, Phillips J, Agar M, Davidson PM. Patient education, coaching, and self-management for cancer pain. J Clin Oncol 2014;32:1712-20. https://doi.org/10.1200/JCO.2013.52.4850.
- National Palliative and End of Life Partnership . Ambitions for Palliative and End of Life Care: A Framework for Local Action 2015. http://endoflifecareambitions.org.uk/ (accessed 3 October 2019).
- British Broadcasting Corporation . We Need to Talk About Death 2016. www.bbc.co.uk/programmes/b084ys5v (accessed 3 October 2019).
- Lincolnshire Community Health Services NHS Trust . The Lincolnshire Policy for Informal Carer’s Administration of As Required Subcutaneous Injections in Community Palliative Care 2013. www.eolc.co.uk/uploads/The-Lincolnshire-Policy-for-Informal-Carer%E2%80%99s-Administration-of-As-Required-Subcutaneous-Injections-in-Community-Palliative-Care-August-2015.pdf (accessed 3 October 2019).
- Chitnis X, Georghiou T, Steventon A, Bardsley M. The Impact of the Marie Curie Nursing Service on Place of Death and Hospital Use at the End of Life. London: Nuffield Trust; 2012.
- Georghiou T, Bardsley M. Exploring the Cost of Care at the End of Life 2014. www.nuffieldtrust.org.uk/files/2017-01/end-of-life-care-web-final.pdf (accessed 3 October 2019).
- Queiro A. Shipman effect: how a serial killer changed medical practice forever. BBC News 2014. https://www.bbc.co.uk/news/uk-scotland-30192721 (accessed 5 May 2020).
- Higginson IJ, Evans CJ, Grande G, Preston N, Morgan M, McCrone P, et al. Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-111.
- Medical Research Council . Developing and Evaluating Complex Interventions n.d. www.mrc.ac.uk/documents/pdf/complex-interventions-guidance/ (accessed 3 October 2019).
- Cohen R, Leis AM, Kuhl D, Charbonneau C, Ritvo P, Ashbury FD. QOLLTI-F: measuring family carer quality of life. Palliat Med 2006;20:755-67. https://doi.org/10.1177/0269216306072764.
- Fuller D, Watson R. Validating a self-medication risk assessment instrument. Clin Eff Nurs 2005;9:78-83. https://doi.org/10.1016/j.cein.2004.12.003.
- Hurt CN, Roberts K, Rogers TK, Griffiths GO, Hood K, Prout H, et al. A feasibility study examining the effect on lung cancer diagnosis of offering a chest X-ray to higher-risk patients with chest symptoms: protocol for a randomized controlled trial. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-405.
- Cresswell JW, Plano Clark VL. Designing and Conducting Mixed Methods Research. Los Angeles, CA: SAGE Publications Ltd; 2011.
- Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346. https://doi.org/10.1136/bmj.e7586.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. PAFS consensus group . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355. https://doi.org/10.1136/bmj.i5239.
- NIHR Journals Library . CARer ADministration of As-Needed Subcutaneous Medication for Breakthrough Symptoms in Home-Based Dying Patients: A UK Study (CARiAD). Protocol HTA – 15 10 37 n.d. www.journalslibrary.nihr.ac.uk/programmes/hta/151037/#/ (accessed 3 October 2019).
- Great Britain . Welsh Language Act 1993 1993.
- NHS Health Research Authority . Principles of Consent: Adults Who Are Not Able to Consent for Themselves 2018. www.hra-decisiontools.org.uk/consent/principles-ALC.html (accessed 3 October 2019).
- NHS Health Research Authority . Mental Capacity Act 2018. www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/mental-capacity-act/ (accessed 3 October 2019).
- Great Britain . Mental Capacity Act 2005 2005.
- World Medical Association . World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects 2018.
- Vijayananthan A, Nawawi O. The importance of good clinical practice guidelines and its role in clinical trials. Biomed Imaging Interv J 2008;4. https://doi.org/10.2349/biij.4.1.e5.
- Russell D, Hoare ZS, Whitaker R, Whitaker CJ, Russell IT. Generalized method for adaptive randomization in clinical trials. Stat Med 2011;30:922-34. https://doi.org/10.1002/sim.4175.
- British Medical Association . Anticipatory Prescribing for End-of-Life Care. n.d. http://bma.org.uk/support-at-work/gp-practices/service-provision/prescribing/focus-on-anticipatory-prescribing-for-end-of-life-care (accessed 3 October 2019).
- NHS Scotland . Scottish Palliative Care Guidelines: Anticipatory Prescribing n.d. www.palliativecareguidelines.scot.nhs.uk/guidelines/pain/Anticipatory-Prescribing.aspx (accessed 3 October 2019).
- Bowers B, Ryan R, Kuhn I, Barclay S. Anticipatory prescribing of injectable medications for adults at the end of life in the community: a systematic literature review and narrative synthesis. Palliat Med 2019;33:160-77. https://doi.org/10.1177/0269216318815796.
- Palliative Care Wales . All Wales Guidance: Care Decisions for the Last Days of Life Symptom Control Guidance. n.d. https://wales.pallcare.info/files/docs/Care%20Decisions%20Toolkit/E%20-%20All%20Wales%20Care%20Decisions%20Symptom%20Control%20Guidance%20v10%20June%202019.pdf (accessed 3 October 2019).
- Hickman SE, Tilden VP, Tolle SW. Family reports of dying patients’ distress: the adaptation of a research tool to assess global symptom distress in the last week of life. J Pain Symptom Manage 2001;22:565-74. https://doi.org/10.1016/S0885-3924(01)00299-8.
- Lobchuk MM. The memorial symptom assessment scale: modified for use in understanding family caregivers’ perceptions of cancer patients’ symptom experiences. J Pain Symptom Manage 2003;26:644-54. https://doi.org/10.1016/S0885-3924(03)00205-7.
- Kutner JS, Bryant LL, Beaty BL, Fairclough DL. Symptom distress and quality-of-life assessment at the end of life: the role of proxy response. J Pain Symptom Manage 2006;32:300-10. https://doi.org/10.1016/j.jpainsymman.2006.05.009.
- de Bruin M, McCambridge J, Prins JM. Reducing the risk of bias in health behaviour change trials: improving trial design, reporting or bias assessment criteria? A review and case study. Psychol Health 2015;30:8-34. https://doi.org/10.1080/08870446.2014.953531.
- Johnston BC, Patrick DL, Busse JW, Schünemann HJ, Agarwal A, Guyatt GH. Patient-reported outcomes in meta-analyses – Part 1: assessing risk of bias and combining outcomes. Health Qual Life Outcomes 2013;11. https://doi.org/10.1186/1477-7525-11-109.
- Cocks K, Torgerson DJ. Sample size calculations for pilot randomized trials: a confidence interval approach. J Clin Epidemiol 2013;66:197-201. https://doi.org/10.1016/j.jclinepi.2012.09.002.
- Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol 2012;65:301-8. https://doi.org/10.1016/j.jclinepi.2011.07.011.
- Office for National Statistics . Deaths Registered in England and Wales: 2013 2014. www.ons.gov.uk/ons/rel/vsob1/death-reg-sum-tables/2013/sb-deaths-first-release--2013.html (accessed 3 October 2019).
- Holdsworth LM, Gage H, Coulton S, King A, Butler C. A quasi-experimental controlled evaluation of the impact of a hospice rapid response community service for end-of-life care on achievement of preferred place of death. Palliat Med 2015;29:817-25. https://doi.org/10.1177/0269216315582124.
- National Institute for Health Research . Research Governance Guidelines 2019. www.nihr.ac.uk/documents/research-governance-guidelines/12154 (accessed 3 October 2019).
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- Smith JA, Flowers P, Larkin M. Interpretative Phenomenological Analysis. London: SAGE Publications Ltd; 2009.
- Smith JA, Osborn M, Smith JA. Qualitative Psychology: A Practical Guide To Research Methods. London: SAGE Publications Ltd; 2003.
- Smith JA, Smith JA, Harre R, Van Langenhove L. Rethinking Methods in Psychology. London: SAGE Publications Ltd; 1995.
- Lincoln Y, Guba EG. Naturalistic Enquiry. Newbury Park, CA: SAGE Publications Ltd; 1985.
- Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Qual Res 2006;5:80-92. https://doi.org/10.1177/160940690600500107.
- Patton MQ. Two decades of developments in qualitative enquiry; a personal, experiential perspective. Qual Soc Work 2002;1:261-83. https://doi.org/10.1177/1473325002001003636.
- Dyregrov K. Bereaved parents’ experience of research participation. Soc Sci Med 2004;58:391-400. https://doi.org/10.1016/S0277-9536(03)00205-3.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analysing Qualitative Data. London: Routledge; 1994.
- Dixon-Woods M. Using framework-based synthesis for conducting reviews of qualitative studies. BMC Med 2011;9. https://doi.org/10.1186/1741-7015-9-39.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ 2000;320:114-16. https://doi.org/10.1136/bmj.320.7227.114.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ 2012;21:145-72. https://doi.org/10.1002/hec.1697.
- Coast J, Al-Janabi H, Sutton EJ, Horrocks SA, Vosper AJ, Swancutt DR, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ 2012;21:730-41. https://doi.org/10.1002/hec.1739.
- Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. PharmacoEconomics 2014;32:883-902. https://doi.org/10.1007/s40273-014-0170-x.
- Goranitis I, Coast J, Al-Janabi H. An investigation into the construct validity of the Carer Experience Scale (CES). Qual Life Res 2014;23:1743-52. https://doi.org/10.1007/s11136-013-0616-1.
- Hoefman R, Al-Janabi H, McCaffrey N, Currow D, Ratcliffe J. Measuring caregiver outcomes in palliative care: a construct validation study of two instruments for use in economic evaluations. Qual Life Res 2015;24:1255-73. https://doi.org/10.1007/s11136-014-0848-8.
- Al-Janabi H, Flynn TN, Coast J. Estimation of a preference-based carer experience scale. Med Decis Making 2011;31:458-68. https://doi.org/10.1177/0272989X10381280.
- Bugge C, Williams B, Hagen S, Logan J, Glazener C, Pringle S, et al. A process for Decision-making after Pilot and feasibility Trials (ADePT): development following a feasibility study of a complex intervention for pelvic organ prolapse. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-353.
- Shanyinde M, Pickering RM, Weatherall M. Questions asked and answered in pilot and feasibility randomized controlled trials. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-117.
- Zelen M. A new design for randomised controlled trials. N Engl J Med 1979;300:1242-5. https://doi.org/10.1056/NEJM197905313002203.
- COMET Initiative . Development of an International Core Outcome Set for Best Care for the Dying Person n.d. www.comet-initiative.org/studies/details/967?result=true (accessed 3 October 2019).
- Hoare S, Kelly MP, Barclay S. Home care and end-of-life hospital admissions: a retrospective interview study in English primary and secondary care. Br J Gen Pract 2019;69:e561-e569. https://doi.org/10.3399/bjgp19X704561.
- Koffman J, Yorganci E, Yi D, Gao W, Murtagh F, Pickles A, et al. Managing uncertain recovery for patients nearing the end of life in hospital: a mixed-methods feasibility cluster randomised controlled trial of the AMBER care bundle. Trials 2019;20. https://doi.org/10.1186/s13063-019-3612-0.
- Brisbane South Palliative Care Collaborative . Guidelines for Handling of Medication in Community Based Palliative Care Services in Queensland 2015. www.health.qld.gov.au/cpcre/pdf/medguidepall.pdf (accessed 27 December 2019).
- Great Britain . Misuse of Drugs Regulations 2001 1971.
- Medical Defence Union . Reply to a Request for Information to Dr Lucy Boyland Re: Is It Legal for Carers to Administer Controlled Drugs? 2009. www.palliativedrugs.com/bulletin-board.html (accessed 13 March 2014).
- NHS National Prescribing Centre . A Guide to Good Practice in the Management of Controlled Drugs in Primary Care (England) 2009. www.harrogateandruraldistrictccg.nhs.uk/data/uploads/medicines-management/controlled-drugs/clinical-governance/npc-controlleddrugsthirdedition-dec2009.pdf (accessed 27 December 2019).
- National Institute for Health and Care Excellence . Controlled Drugs and Drug Dependence n.d. https://bnf.nice.org.uk/guidance/controlled-drugs-and-drug-dependence.html (accessed 27 December 2019).
- Nursing and Midwifery Council . Standards for Medicines Management 2010. www.nmc.org.uk/globalassets/siteDocuments/NMC-Publications/NMC-Standards-for-medicines-management.pdf (accessed 15 January 2020).
- Nursing and Midwifery Council . Delegation and Accountability: Supplementary Information to the NMC Code n.d. www.nmc.org.uk/globalassets/sitedocuments/nmc-publications/delegation-and-accountability-supplementary-information-to-the-nmc-code.pdf (accessed 15 January 2020).
- Department of Health, Social Services and Public Safety . Safer Management of Controlled Drugs: A Guide to Good Practice in Primary Care (Northern Ireland) 2013. www.health-ni.gov.uk/sites/default/files/publications/dhssps/safer-management-of-controlled-drugs-a-guide-to-good-practice-in-primary-care-version.pdf (accessed 27 December 2019).
- Durham and Tees Valley Regional Medication Policy Group . Model of Good Practice for the Development of Policy for the Safe Handling, Management and Administration of Medication by Carers Within Domiciliary Care across the North East of England 2008. www.dignityincare.org.uk/_library/Regional_Model_of_Good_Practice_Policy_for_Medication_-_Reviewed_08.pdf (accessed 13 March 2014).
- Great Britain . Mental Capacity Act 2005 2005.
- Department for Constitutional Affairs . Mental Capacity Act 2005 Code of Practice 2007.
- Anderson B, Kralik D. Sterility, stability and potency of medications administered by carers in home-based palliative care setting. Rese Inform Pract 2008;51:349-56.
Appendix 1 Legal framework
Clarity on legal issues is a significant aspect of this research to ensure that lay carers and clinicians alike have legal protection. Our Australian partners have given us full access to their reference resource. 94 Their document covers a broad range of topics on the handling of medication in community-based palliative care services. It covers medication management, drug storage (security of medications, responsibility for medication storage and disposal of medication), prescribing and medication administration (who can administer and record of administration) in the context of lay carer administration.
The premise is that a lay carer can legally administer medication that is individually prescribed for a third party, including controlled drugs such as morphine, as long as the carer has been appropriately trained and assessed as competent, specifically in medication management. This is true even if the medication is given to a patient lacking capacity and/or if the medication is administered via injection. At present, injections are prepared immediately before administration (and not in advance, requiring relabelling). Carers should be trained to assess symptoms and should have access to dedicated support.
In support of these statements, the relevant sections from UK legislation and guidance are detailed below.
A lay carer can administer medication individually prescribed for a third party, including controlled drugs such as morphine:
-
Section 7(3) of the Misuse of Drugs Regulations 2001 states that ‘any person other than a doctor or dentist may administer to a patient, in accordance with the directions of a doctor or dentist, any drug specified in Schedule 2, 3 or 4’ (contains public sector information licensed under the Open Government Licence v3.0). 95
-
This was confirmed by the UK Medical Defence Union. 96
-
NHS National Prescribing Centre guidance (2009) states that ‘a carer/relative can, with consent, administer a controlled drug that has been individually prescribed for a third party. As controlled drugs are included within the legal category of prescription-only medicines, home carers who are competent to administer medicines should also be competent to administer CDs’. 97
-
Morphine is listed in Schedule 2 and midazolam in Schedule 3. 98
As long as the carer has been appropriately trained and assessed as competent:
-
The NMC guidance, Standard 17 (valid until 28 January 2019), delegation states that –NMC. Reproduced with permission99
A registrant is responsible for the delegation of any aspects of the administration of medicinal products and they are accountable to ensure that the patient, carer or care assistant is competent to carry out the task. This will require education, training and assessment of the patient, carer or care assistant and further support if necessary. The competence of the person to whom the task has been delegated should be assessed and reviewed periodically. Records of the training received and outcome of any assessment should be clearly made and be available.
-
From 28 January 2018, NMC delegation and accountability guidance states that –NMC. Reproduced with permission100
These requirements apply, regardless of who the activity is being delegated to. This may be another registered professional, a non-registered colleague, or a patient or carer. These expectations are that people on the NMC register: only delegate tasks and duties that are within the other person’s scope of competence, making sure that they fully understand the instructions; make sure that everyone they delegate tasks to are adequately supervised and supported so they can provide safe and compassionate care; confirm that the outcome of any task delegated to someone else meets the required standard.
-
Department of Health, Social Services and Public Safety (Northern Ireland) guidance states that –Department of Health, Social Services and Public Safety. 101Contains public sector information licensed under the Open Government Licence v3.02013Crown copyrighthttp://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/
. . . home carers who are appropriately trained and assessed as competent are authorised to administer orally prescribed controlled drugs.
-
The Durham and Tees Valley Regional Medication Policy Group102 clarifies that, as long as carers administering medication followed prescriber instructions and local policies, they would not be held responsible for adverse effects that arise from such administration.
Specifically in medication management:
-
The Durham and Tees Valley Regional Medication Policy Group102 emphasises the importance of structured carer training specifically in medicines management in supporting a safe context for carers administering medication.
-
Procedures are already in place in the UK to handle/store medications (including for anticipatory care purposes) in the patient’s home. 55
This is true even if the medication is given to a patient lacking capacity:
-
Medication can be given to a patient who lacks capacity if it is in his or her best interests. The Mental Capacity Act 2005 Section 1(5) states that –Mental Capacity Act. 103Contains public sector information licensed under the Open Government Licence v3.02005Crown copyrighthttp://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/
An act done, or decision made, under this Act for or on behalf of a person who lacks capacity must be done, or made, in his best interests.
-
The Mental Capacity Act 2005 permits the relevant actions to be undertaken by those with appropriate skills or expertise (as long as the carer has been appropriately trained and assessed as competent). The Code of Practice explains:Mental Capacity Act Code of Practice. 104Contains public sector information licensed under the Open Government Licence v3.02005Crown copyrighthttp://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/
To receive protection from liability under section 5, all actions must be related to the care or treatment of the person who lacks capacity to consent. Before taking action, carers must first reasonably believe that: the person lacks the capacity to make that particular decision at the time it needs to be made, and the action is in the person’s best interests.
And/or if the medication is administered via injection:
-
The Durham and Tees Valley Regional Medication Policy Group states that for specialist tasks (including injections) a suitable health professional needs to give additional training and confirm that the carer is competent to provide such care. 102
At present, injections are prepared immediately before administration:
-
NMC guidance, standard 14, states –NMC. Reproduced with permission99
Registrants must not prepare substances for injection in advance of their immediate use or administer medication drawn into a syringe or container by another practitioner when not in their presence.
-
The guidance continues –NMC. Reproduced with permission99
Where a registrant has delegated to a named individual for a named patient’s medication, this may be drawn up in advance to enable the health-care assistant (HCA) or family to administer the medication. The registrant is accountable for the delegation, and a full risk assessment should be documented in the patient’s records ensuring the registrant is aware of the risks before agreeing to delegate.
-
Note that there is evidence that the practice of drawing up and leaving these medications in syringes, for this type of practice, is safe in terms of sterility, potency and stability. 105 The team tested a full range of medications for 28 days.
Carers should be trained to assess symptoms, use the least invasive methods of administration and should have access to dedicated support.
Appendix 2 Outcome measures under contention for a future definitive trial
Qualities of potential future outcome measures under investigation in CARiAD
| Outcome measure | Description | Method(s) of assessment |
|---|---|---|
| Family MSAS-GDI |
|
|
| QOLLTI-F |
|
|
| Time to symptom relief | This measure will be calculated using data items from the carer diary. We will minimise bias by using strict definitions of episode timings:
|
|
Rationale for choice of Family MSAS-GDI and QOLLTI-F
The Memorial Symptom Assessment Scale (32 items) is a valid and reliable patient self-report instrument. 10 The MSAS-GDI has demonstrated reliability and validity in measuring global symptom distress from the patient perspective. Although the scale was designed to produce one single score, the individual items can also be used as single item indicators of burdensome symptoms at the end of life, identifying which symptoms are getting better or worse over time in different patient populations. 59
Hickman et al. 59 showed that the MSAS-GDI is amenable to modification for use in research with recently bereaved family respondents whose family members died from a wide range of causes. Items were modified so that the questions focused on the symptoms experienced by decedents in the last week of their life, as observed by family respondents. The Family MSAS-GDI has good face validity for use in understanding symptoms experienced by patients in the last week of life, regardless of cause of death or role in relation to the patient (carer or HCP). 61
In the Hickman et al. 59 study, in the 103 family members the mean Family MSAS-GDI score was 1.14 (SD 0.87), with a range from 0 to 3.73. The scale demonstrated good internal consistency (α = 0.82). The average item-total correlation was r = 0.49 and the average inter-item correlation was r = 0.30, which suggests that items were moderately correlated with the overall total scale and with each other. They concluded that the Family MSAS-GDI could prove to be a useful tool in assessing and tracking global symptom distress in dying patients. 59
Lobchuk’s work60 corroborates these findings, showing good to excellent intraclass correlations with patients’ ordinal ratings to support the concurrent validity and utility of the MSAS-GDI subscales in family carer populations who care for cancer patients in the home setting.
Rationale for the choice of QOLLTI-F41 is that it has established psychometric properties (reported validity and reliability, demonstrated responsiveness, no floor and ceiling effects), is relatively brief (16 items) and can be administered every 2 days (rather than daily, reducing the risk of overburdening carers). It is broadly aimed at carer quality of life, and incorporates issues of control (reported as paramount by carers) and self-efficacy (conceptualised as ‘a person’s belief about her or his ability to organise and execute courses of action to manage given situations’).
Appendix 3 Additional consultee information
More detail on the 12e dyads in whom the expected subsequent consultee forms were not received:
-
BCUHB 1 – the dyad was randomised to the intervention group 8 weeks before the patient’s death, and the carer was trained. The carer called the DN team to inform them that the patient had deteriorated significantly, anticipatory medication was not in the home at that time (but was promptly arranged), and the patient died within 24 hours of HCPs being made aware of deterioration. The patient had three doses of as-needed medication (two doses for agitation and one dose for pain), all of which were administered by HCPs. All trial paperwork was received.
-
BCUHB 2 – the dyad was randomised to the intervention group 1 week before the patient’s death, and the carer was trained on the same day. The carer administered two injections during the last 2 days of the patient’s life (one for pain and one for noisy breathing). The patient was attended by nurses who might not have had full CARiAD protocol training (in terms of reminding the carer to sign the consultee form). The trial paperwork was received, with the exception of RA tool.
-
BCUHB 3 – the dyad was randomised to the intervention group and both in the dyad had a HCP background. The carer administered eight injections during the last 3 days of the patient’s life (and attending HCPs administered an additional six injections). HCPs were very involved, as the patient was an ex-colleague. All trial paperwork was received.
-
BCUHB 4 – the dyad was randomised to the usual-care group. The patient far exceeded prognostic expectations and lived for 74 weeks following randomisation. The patient died at home. The carer diary was not used and carers are awaiting follow-up.
-
BCUHB 5 – the dyad was randomised to the intervention group and the patient survived for 19 days. The RA was not received but competency checklist and diaries were received. Multiple injections were administered. The family was not contactable post bereavement.
-
BCUHB 6 – the dyad was randomised to the usual-care group and the patient survived for 10 days. The RA was not received and the family were not contactable post bereavement.
-
CVUHB 1 – the dyad was randomised to the intervention group. The carer was a retired doctor. The carer administered four injections during the last 2 days of the patient’s life (three for agitation and one for noisy breathing) and the HCP administered one injection. All trial paperwork was received.
-
GCS 1 – the dyad was randomised to the usual-care group. The carer diary was not used but the RA tool was received.
-
GCS 2 – the dyad was randomised to the intervention group, but the patient deteriorated rapidly (during the same visit as was assigned to carer training) and collapsed and died within minutes. The carer was a retired doctor. The RA tool was received but no carer diary was used and the competency assessment was not completed, as training was abandoned.
-
GCS 3 – the dyad was randomised to the intervention group. The carer administered 10 injections during the last 3 days of the patient’s life (and the attending HCPs administered zero injections). The DN had training on the CARiAD protocol from a colleague in the team, and this was her first patient enrolled in CARiAD. The DN regularly checked in with trial manager. All trial paperwork was received.
-
GCS 4 – the dyad was randomised to the usual-care group. The patient died at home and the carer was lost to follow-up.
-
GCS 5 – the dyad was randomised to the intervention group. The carer was not trained and was lost to follow-up. The patient died at home.
Appendix 4 Details of protocol deviations
-
Patient-identifiable information e-mailed to trial manager.
-
When the carer diary was returned to the trial team post bereavement, it was noted that the medication administration entries had been signed by multiple carers. As a result of this deviation, the DN team received additional support and training. A minor amendment was also submitted and approved to include the name of the carer trained by the DN or RN on the competency checklist.
-
The site trial administrator sent a log on behalf of the DN team that contained sensitive medical and patient-identifiable information.
-
The patient lived with extended family. The family member who completed the follow-up questionnaire via telephone (at their request) was not the designated CARiAD carer.
-
When the carer diary was received by the trial team post bereavement, it was noted that the drugs had been prescribed as a dose range rather than a specific dose.
-
When entering data from the diary of a deceased patient, the trial manager noticed that the medication questions on the MACRO database did not match the questions in the diary. The printed diary was compared with the approved proof and it was noted that the column headed ‘How often can it be given?’ on the medication page had been omitted in error from the printed copy. Any chance of mistakes on the carer part was mitigated by the fact that the diary was a duplicate medication entry area to support the carer. The main medication chart was in the home with the frequency included. In addition, the carer training would have covered information on frequency, and the diary included a column labelled ‘maximum number of doses in 24 hrs’. This deviation was reported as serious to the sponsor’s representative and REC, who both advised that appropriate procedures had been followed to correct the error and no further action was required.
-
The patient was originally approached to take part in CARiAD by a SPC nurse who was not closely involved with CARiAD. A second SPC nurse gave written information but found that the RA tool had not been completed before the dyad were approached.
-
There were multiple RA tools outstanding from one DN team that were returned after several months’ delay. On inspection, the forms had been completed on a version dated later than the date of assessment. When this was discussed with the nurse concerned she admitted that she had forgotten to complete them at the time and forgot that the daily reminder sheet included a prompt about completing the RA. The nurse was also very clear that all of the included dyads continued to satisfy the RA criteria throughout their involvement in the trial (i.e. explaining why there is no review of RA criteria indicated, as this was not needed). This deviation was reported as serious to the sponsors representative and REC, who both advised that appropriate procedures had been followed to correct the error and no further action was required.
-
Following an earlier protocol deviation concerning the RA tool, all sites were asked to return outstanding copies, which identified several minor issues:
-
The date signed by the DN predated the version number. The DN confirmed that a RA did take place at the time but the patient was admitted to hospital and died, and the RA was lost. The DN admitted that she had refreshed a copy on the incorrect version for trial purposes.
-
The trial manager witnessed the RA being completed in DN team offices but the document has since been misplaced.
-
RN confirmed RAs were completed for three dyads but have since been misplaced.
-
RA version 4 was circulated on 11 January 2019 (Friday); however, a RA tool was completed on version 3 on 14 January 2019 (Monday). Version 4 was updated to include the name of the person assessed so there would be no risk to the patient/carer from an earlier version being completed.
-
-
The patient had declined a continuous SC infusion using a syringe pump for personal reasons. When the carer diary was returned post bereavement and the medication entries were reviewed it was noted that more than three injections had been administered within a 24 hour period on one occasion. The CARiAD protocol (and carer documents) state that carers may give a maximum of three doses for each indication. In addition, cyclizine was administered 8 hourly every day except one. CARiAD was not designed to manage background symptoms, rather carers were expected to administer SC medication for breakthrough symptoms only.
-
One column of the medication table in the carer diary is headed ‘maximum number of doses per 24 hour period’. The HCP annotating the chart had documented ‘6’ for each of the four medications. However, the CARiAD protocol states that no more than three carer-administered injections may be given for each indication per 24-hour period. Medication administration entries were checked and all injections were administered safely and as per protocol.
-
RA tool was completed but has since been misplaced.
-
RA tool and competency checklist were completed but have been misplaced. Case notes have been checked and the document cannot be located.
Appendix 5 Trial close-out arrangements
Appendix 6 Interview topic guides
On each topic guide it was noted ‘these are the topics that the researcher will aim to raise in the interview, the topics may be addressed differently or in more or less detail depending on the content from the participant. Topics may be added or deleted in later interviews as we learn from early interviews’.
Topic guide: intervention-group carers
-
Introduction:
-
introduce self, study and purpose of interview
-
explain what will happen – including the length of interview and followed by DCE
-
explain can pause or end interview at any time
-
explain confidentiality and audio-recording
-
take consent.
-
-
Experience of administering the medications:
Possible probes –
-
injecting – attitudes to/experience of
-
pain relief/control – attitudes to
-
if possible probe about attitudes to risk
-
the positive aspects of the experience
-
the most challenging aspects
-
any worries about the process
-
where appropriate probe any concerns about ethical issues
-
-
how they dealt with those worries
-
what might have helped to make it a better experience.
-
-
How it affected themselves/coping mechanisms:
Probe –
-
impact on themselves at the time
-
systems of support for them
-
coping mechanisms
-
what helped/what hindered
-
reaction of other family members.
-
-
Training:
-
views on the training
-
how competent felt after the training?
-
how confident felt
-
most useful aspects of the training (content or method)
-
least useful aspects.
-
-
Support from HCPs:
-
interaction with HCPs
-
relationship
-
type of support received
-
most useful aspects of the support received
-
what could be improved.
-
-
Practicalities:
-
drug storage and management
-
the equipment.
-
-
Trial issues – recruitment and measures:
-
how felt about being approached
-
timing of approach
-
views on recruitment process generally
-
PIS
-
way study was explained
-
consent mechanisms
-
-
randomisation
-
understanding of process of randomisation
-
views on being randomised to intervention
-
how would have felt if had been randomised to usual care
-
what would have done if randomised to usual care
-
-
how recruitment could be improved
-
experience of completing the carer diaries and outcome measures.
-
-
What could be done differently.
-
Thank and explain what will happen to the data.
Topic guide: usual-care carers
-
Introduction:
-
introduce self, study and purpose of interview
-
explain what will happen, including length of interview and followed by DCE
-
explain can pause or end interview at any time
-
explain confidentiality and audio-recording
-
take consent.
-
-
Trial issues – recruitment and measures:
-
how felt about being approached
-
timing of approach
-
views on recruitment process generally
-
PIS
-
way study was explained
-
consent mechanisms
-
-
how recruitment could be improved
-
experience of completing the carer diaries and outcome measures.
-
-
Views on being randomised to usual care:
-
understanding of process of randomisation
-
views on being randomised to usual care
-
which group would have preferred
-
whether tried to get into intervention group
-
or tried to get different care from the usual-care group.
-
-
-
What could be done differently.
-
Thank and explain what will happen to the data.
Topic guide: prescribers
-
Introduction:
-
introduce self, study and purpose of interview
-
explain what will happen, including length of interview
-
explain can pause or end interview at any time
-
explain confidentiality and audio-recording
-
take consent.
-
-
Experience of supporting carers administering the medications:
Possible probes –
-
description of the process
-
the positive aspects of the experience
-
the problems/challenging aspects
-
issues or concerns about the process
-
probe – ethical issues
-
-
how dealt with those concerns
-
what might have helped.
-
-
If provided formal/informal training:
-
what worked well
-
what did not work so well
-
suggested improvements to training.
-
-
Personal and professional impact/views:
Probe –
-
personal experience/impact of providing care this way
-
aspects that were personally challenging/rewarding
-
reflections on professional identity/role
-
support required for prescribers.
-
-
Practical:
-
drug storage and management
-
the equipment
-
other practical.
-
-
Trial processes:
-
observations on trial recruitment and processes
-
observations on the carer diaries and outcome measures data gathering
-
interaction with usual-care group about trial recruitment/randomisation
-
interaction with decliners about trial recruitment.
-
-
Overall views on carers administering the strong opioids:
-
advantages/disadvantages
-
what could be done differently.
-
-
Thank and explain what will happen to the data.
Topic guide: decliners
-
Introduction:
-
introduce self, study and purpose of interview
-
explain what will happen, including length of interview and followed by DCE
-
explain can pause or end interview at any time
-
explain confidentiality and audio-recording
-
take consent.
-
-
Reasons for declining:
Probe reasons –
-
barriers to participating
-
what might have helped.
-
-
Their understanding of the trial.
-
Their understanding of what participation would involve.
-
What could be done differently:
-
to help people make an informed choice about participation.
-
-
Thank and explain what will happen to the data.
Appendix 7 Discrete choice experiment attribute selection topic guide
List of abbreviations
- AE
- adverse event
- ANOVA
- analysis of variance
- BCUHB
- Betsi Cadwaladr University Health Board
- CES
- Carer Experience Scale
- CRF
- case report form
- CTIMP
- Clinical Trial of an Investigational Medicinal Product
- CVUHB
- Cardiff & Vale University Health Board
- DCE
- discrete choice experiment
- DMEC
- Data Monitoring and Ethics Committee
- DN
- district nurse
- GCS
- Gloucester Care Services
- GMC
- General Medical Council
- GP
- general practitioner
- HCP
- health-care professional
- IQR
- interquartile range
- IPA
- interpretive phenomenological analysis
- ISRCTN
- International Standard Randomised Controlled Trials Number
- MSAS-GDI
- Memorial Symptom Assessment Score – General Distress Index
- NIHR
- National Institute for Health Research
- NMC
- Nursing and Midwifery Council
- NWORTH
- North Wales Organisation for Randomised Trials in Health
- OOH
- out of hours
- PeolcPSP
- Palliative and End of Life Care Priority Setting Partnership
- PI
- principal investigator
- PIS
- participant information sheet
- QOLLTI-F
- Quality of Life in Life-Threatening Illness – Family Carer Version
- RA
- risk assessment
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RN
- research nurse
- SAE
- serious adverse event
- SC
- subcutaneous
- SD
- standard deviation
- SOP
- standard operating procedure
- SPC
- specialist palliative care
- TSC
- Trial Steering Committee
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta24250).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
