Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/167/26. The contractual start date was in October 2014. The draft report began editorial review in October 2018 and was accepted for publication in May 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jane P Daniels declares membership of the Clinical Trials Unit Standing Advisory Committee. Meenakshi Choudhary declares membership of Health Technology Assessment (HTA) Maternal, Newborn and Child Health (MNCH) Panel and the HTA Prioritisation Committee. Jane E Norman declares membership of the HTA MNCH Panel, that she currently receives funding from the National Institute for Health Research Efficacy and Mechanism Evaluation (EME) programme, that she participates in a Data Monitoring and Ethics Committee for GlaxoSmithKline plc (Brentford, UK) and that she is a paid consultant for Dilafor AB (Solna, Sweden). She was a member of the HTA and EME Editorial Board from 2012 to 2014. Caroline Overton declares that she was a Mylan clinical educator for general practitioner education about hormone replacement therapy and incorporated private practice in April 2017 (now called Bristol Women’s Clinic Ltd).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Coomarasamy et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Existing knowledge
Progesterone in pregnancy
Progesterone is an endogenous hormone that is essential to achieve and maintain a healthy pregnancy. Progesterone prepares the lining of the uterus (endometrium) to allow the implantation of the early embryo and stimulates glands in the endometrium to secrete nutrients for the embryo. During the first 8 weeks of pregnancy, progesterone is produced by the corpus luteum; however, between 8 and 12 weeks, the placenta takes over the progesterone-producing role and maintains the pregnancy thereafter.
The physiological importance of progesterone has prompted researchers, physicians and patients to consider progesterone supplementation during early pregnancy to prevent miscarriages. Progesterone supplementation in early pregnancy has been attempted in two contexts: the first is to prevent miscarriages in asymptomatic women with a past history of recurrent miscarriages and the second is to rescue a pregnancy in women who have started to bleed in early pregnancy. 1 Our Progesterone in Recurrent Miscarriage (PROMISE) study, published in the New England Journal of Medicine, addressed the first context. 2 In 2012, the National Institute for Health and Care Excellence (NICE) Clinical Guideline 1543 called for a large randomised placebo-controlled clinical trial to test whether or not progesterone therapy in the first trimester could reduce the risk of miscarriage in women with a history of threatened miscarriage. In response, the current study was designed to address this question and focuses on the rescue context in women with vaginal bleeding in early pregnancy.
Burden of disease
Miscarriage is the most common complication of early pregnancy; one in five clinically recognised pregnancies end in a miscarriage. 4 This has a substantial impact on physical and psychological well-being: research shows that the level of distress associated with miscarriage can be equivalent to that of a stillbirth of a term baby and can induce post-traumatic stress disorder. 5 An estimated 140,000 women per year miscarry in the UK. 3
Costs to the NHS
It is estimated that miscarriage costs the NHS > £350M each year. 3 This value includes the costs of diagnosis (blood tests and ultrasonography), management of miscarriages (expectant, medical or surgical), investigations of causes of miscarriages (e.g. antiphospholipid syndrome, parental karyotype and uterine cavity tests) and hospital inpatient costs. There are also the associated costs of complications following treatment of miscarriages (e.g. uterine perforation, infection, bleeding or visceral damage) and any long-term health consequences of miscarriages or miscarriage management (including complications of intrauterine infections and adhesions). Furthermore, the societal costs (including days lost from work and out-of-pocket expenses for patients and partners) can be expected to be far greater.
Progesterone in clinical use for threatened miscarriage
The Progesterone in Spontaneous Miscarriage (PRISM) study was conceived to address the possibility that progesterone therapy in the first trimester of pregnancy may reduce the risk of miscarriage in women presenting with early pregnancy bleeding. We conducted a UK clinician survey (n = 222) in October 2012. In the UK, the majority of clinicians (212 out of 222; 95.5%) do not use progesterone to prevent miscarriage in women with early pregnancy bleeding. The key reason for non-use is the lack of robust evidence. Therefore, it is not surprising that the majority of clinicians (201 out of 222; 91%) called for a definitive trial. We also conducted a survey of international practitioners at the International Federation of Gynaecology and Obstetrics (FIGO) 2012 conference in Rome. Surprisingly, this survey found that the majority of clinicians (61 out of 68; 90%) already use progesterone in women with early pregnancy bleeding, although the vast majority (56 out of 66; 85%) were willing to recruit into a randomised trial, presumably indicating a lack of confidence in the available evidence.
Effectiveness of progesterone in threatened miscarriage
The first trial of progesterone therapy in women with early pregnancy bleeding was published in 1967, and since then six trials have studied this question, which have previously been summarised in a Cochrane systematic review. 1 In 2014, prior to conducting the PRISM trial, we performed a systematic review of trials on the use of progestogens in women with early pregnancy bleeding, and identified seven studies. 6–12 These studies are listed in Table 1. The seven studies included a total of 744 women. These studies were small and of poor quality, with none reporting the method of allocation concealment. Only three out of seven studies were placebo controlled and five out of seven studies were not blinded. The modified Jadad quality score varied from 1 out of 6 to 3 out of 6. Outcome data were available for miscarriage rates. Individual studies were too small to show an effect, but a meta-analysis of these seven studies (Figure 1) showed a statistically significant reduction in miscarriage rate with progestogen use [relative rate (RR) 0.53, 95% confidence interval (CI) 0.39 to 0.73]. There was no heterogeneity across the studies (I2 = 0%), suggesting that there was consistency across the studies.
| Study | Intervention | Duration of treatment | Comparison | Risk of bias |
|---|---|---|---|---|
| Ehrenskjöld et al., 19676 (n = 153) | 20 mg of oral dydrogesterone | 20 mg then tapering (20 mg after 12 hours/20 mg every 8 hours until symptoms ceased/10 mg twice daily for 5 days/5 mg twice daily for at least 7 days) | No treatment | Method of randomisation unclear, allocation concealment adequate, blinding of patients and study personnel adequate |
| El-Zibdeh and Yousef 20097 (n = 146) | 10 mg of oral dydrogesterone twice daily | From enrolment until 1 week after bleeding stopped | No treatment | Quasi-randomised (allocated according to the day of the week), no allocation concealment, no blinding for participants or study personnel |
| Gerhard et al., 19878 (n = 34) | 25-mg progesterone vaginal suppositories twice daily | Until miscarriage or for 14 days after bleeding stopped | Placebo | Method of randomisation unclear, allocation concealment unclear, no blinding for participants or study personnel |
| Mistò,19679 (n = 16) | 20 to 40 mg of oral dydrogesterone | Once daily for 6–15 days, sometimes for longer periods and for several cycles | Placebo | Method of randomisation unclear, allocation concealment adequate, blinding of patients and study personnel adequate |
| Omar et al., 200510 (n = 154) | Dydrogesterone | 40 mg of dydrogesterone followed by 10 mg twice daily until bleeding stopped | No treatment | Method of randomisation unclear, no allocation concealment, no blinding of patients and study personnel |
| Palagiano et al., 200411 (n = 50) | 90 mg of progesterone (Crinone® 8% Central Pharma Ltd, Bedford, UK) vaginal suppositories | Once daily for 5 days | Placebo | Method of randomisation unclear, allocation concealment adequate, no blinding for participants or study personnel |
| Pandian, 200912 (n = 191) | Oral dydrogesterone | 40 mg of oral dydrogesterone followed by 10 mg of dydrogesterone twice daily, until 16 weeks of gestation | No treatment | Method of randomisation and allocation concealment adequate, no blinding of participants or study personnel |
FIGURE 1.
Meta-analysis of studies of progesterone in women with early pregnancy bleeding (literature review conducted in 2014). M–H, Mantel–Haenszel.
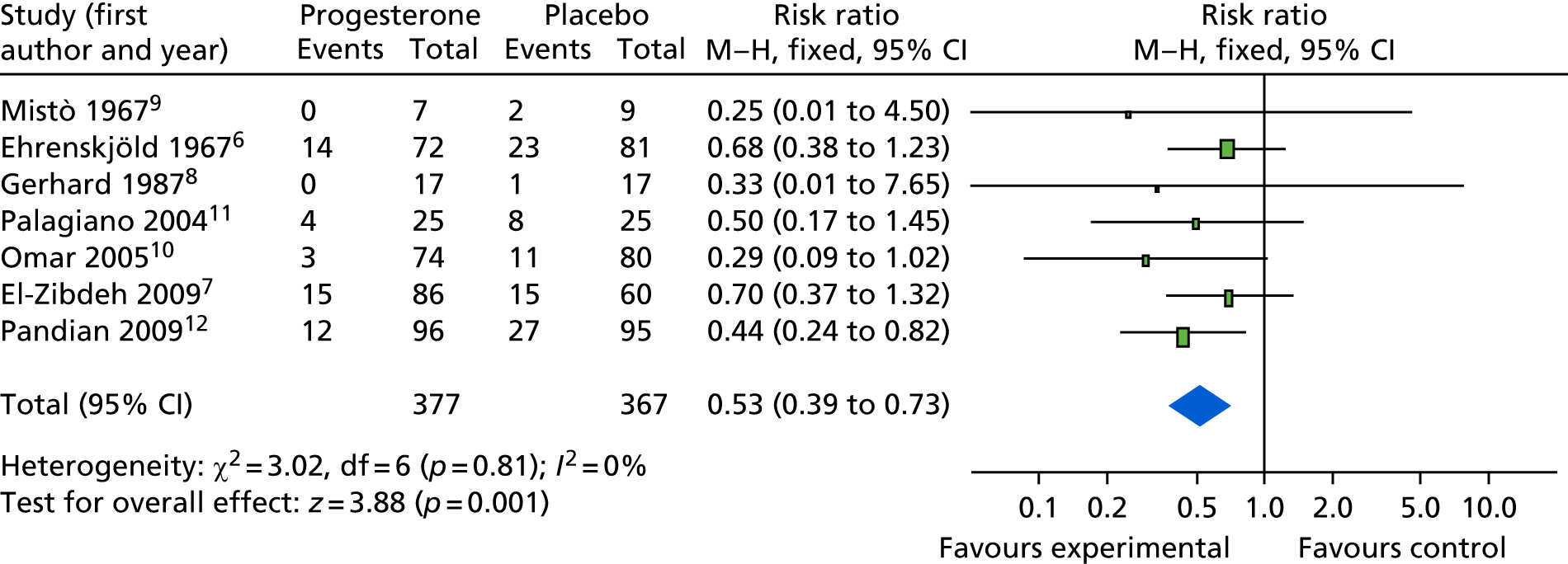
More recently, a Cochrane review on this question summarised evidence from seven studies (see Table 1). The review found that the studies were small with methodological weaknesses (the largest study had a sample size of 191) but the pooled analysis found a significantly lower risk of miscarriages among women who received progesterone than among those who received placebo or no treatment (risk ratio 0.64, 95% CI 0.47 to 0.87). 1
Safety of progesterone supplementation in pregnancy
Our research group previously conducted the PROMISE trial,13 and in the lead-up to this study a full literature review was conducted on the safety of progestogen supplementation in pregnancy. 13 This identified one case–control study that suggested an association between hypospadias and progestogen use. 14 The findings from the case–control study represented weaker evidence than the better-quality evidence from larger cohort studies that did not substantiate this association. Moreover, the PROMISE trial did not show any difference in the incidence of hypospadias between the progesterone and the placebo arms. 13
Rationale
A trial of progesterone therapy in the treatment of threatened miscarriage was required because:
-
A guideline by NICE called for a definitive trial to evaluate the research question:© NICE [2012] Ectopic Pregnancy and Miscarriage: Diagnosis and Initial Management. 3 Available from www.nice.org.uk/guidance/cg154. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication2012NICE
. . . a very large multicentre randomised controlled trial of women treated with either progesterone/progestogen or placebo should be conducted.
-
The Association of Early Pregnancy Units, the Royal College of Obstetrics and Gynaecology Early Pregnancy Clinical Studies Group, the Miscarriage Association and a national team of researchers and clinicians from across the UK prioritised this as an urgent research question.
-
The existing trials, although small and of poor quality, suggest that there is a benefit in a highly prevalent condition with substantial morbidity and costs. If benefit is confirmed in the PRISM trial, both women and the NHS stand to gain substantially. On the other hand, if progesterone is found to be ineffective (or indeed harmful), treatment with progesterone can be avoided. This is relevant given the common use of progesterone for this indication outside the UK.
-
Progesterone treatment is cheap (£0.68 per 400-mg capsule) and safe, and, if benefit is confirmed, we expect the intervention to be taken up rapidly.
-
There is support for the study among UK and international clinicians. In a UK survey of 212 practitioners, 91% believed that a clinical trial is needed to investigate whether or not giving progesterone to women with threatened miscarriage can reduce the risk of miscarriage. In the international survey, 56 out of 66 (85%) respondents were willing to recruit into a randomised trial on this question.
-
A patient survey supports the study. A patient survey (n = 79) showed that 72% of women would consider taking part in this study.
-
The study is supported by the Miscarriage Association (a patient support organisation), The Scottish Early Pregnancy Network, INVOLVE (a national advisory group that supports greater public involvement in health research), PRIME (Public and Researchers Involvement in Maternity and Early Pregnancy), CHARM (Charity for Research into Miscarriage) and Tommy’s charity.
Specific objectives
Primary objective
-
The primary objective of the PRISM trial was to test the hypothesis that in women presenting with vaginal bleeding in the first trimester, receiving progesterone (400 mg vaginal capsules, twice daily) as soon as possible after identification of a visible intrauterine gestation sac with a scan until 16 completed weeks of gestation increases pregnancies with live births at ≥ 34 completed weeks by at least 5% compared with placebo.
Secondary objectives
-
To test the hypothesis that progesterone improves other pregnancy and neonatal outcomes, including gestational age at birth and survival at 28 days of neonatal life.
-
To test the hypothesis that progesterone, compared with placebo, is not associated with serious adverse effects for the mother or the neonate, including chromosomal anomalies in the newborn.
-
To explore differential or subgroup effects of progesterone in prognostic subgroups, including age, fetal heart activity, gestation at presentation, amount of bleeding, body mass index and the number of previous miscarriages.
-
To perform a cost-effectiveness analysis, with cost per additional birth over 34 weeks of gestation from an NHS and NHS/Personal Social Services (PSS) perspective. We will also model longer-term outcomes to the extent that the data permit.
Chapter 2 Methods
Design
The PRISM trial was conducted as a multicentre, double-blind, placebo-controlled randomised trial of progesterone in women with early pregnancy vaginal bleeding. The trial had a favourable ethics opinion from the National Research Ethics Service Committee South Central (Oxford C). The final protocol version was v3.0, 20 July 2016.
Participants
The participants in the PRISM trial were recruited in early pregnancy units in secondary or tertiary care NHS hospitals located across the UK if they fulfilled the following eligibility criteria (see Recruitment for more details on the recruitment process):
-
presented with early pregnancy bleeding that had started in the 4 days prior to screening in the first 12 weeks of pregnancy
-
had intrauterine gestation sac visible on ultrasonography (women were still to be offered the trial in the absence of a visible fetal pole)
-
were aged 16–39 years at randomisation
-
were willing and able to give informed consent.
Participants could not be included if any of the following criteria were applicable:
-
had a crown–rump length measuring ≥ 7 mm with no visible heartbeat; or had a mean gestational sac of ≥ 25 mm with no visible fetal pole on ultrasonography
-
had evidence of ectopic pregnancy
-
presented with life-threatening bleeding
-
currently or had recenly used progesterone supplementation
-
had contraindications to progesterone therapy (progestogens should be avoided in patients with a history of liver tumours; they are also contraindicated in those with genital or breast cancer unless progestogens are being used in the management of these conditions, severe arterial disease, acute porphyria or a history during pregnancy of idiopathic jaundice, severe pruritus or pemphigoid gestations)
-
were participating in any other blinded, placebo-controlled trials of investigational medicinal products (IMPs) in pregnancy.
Recruitment
Potential participants were identified from dedicated early pregnancy units and approached by clinic doctors, research nurses and midwives, after these professionals had received appropriate training relating to the trial. This training included the development of sensitivity in answering questions about the risks of miscarriage, and the intervention that was being used in the trial.
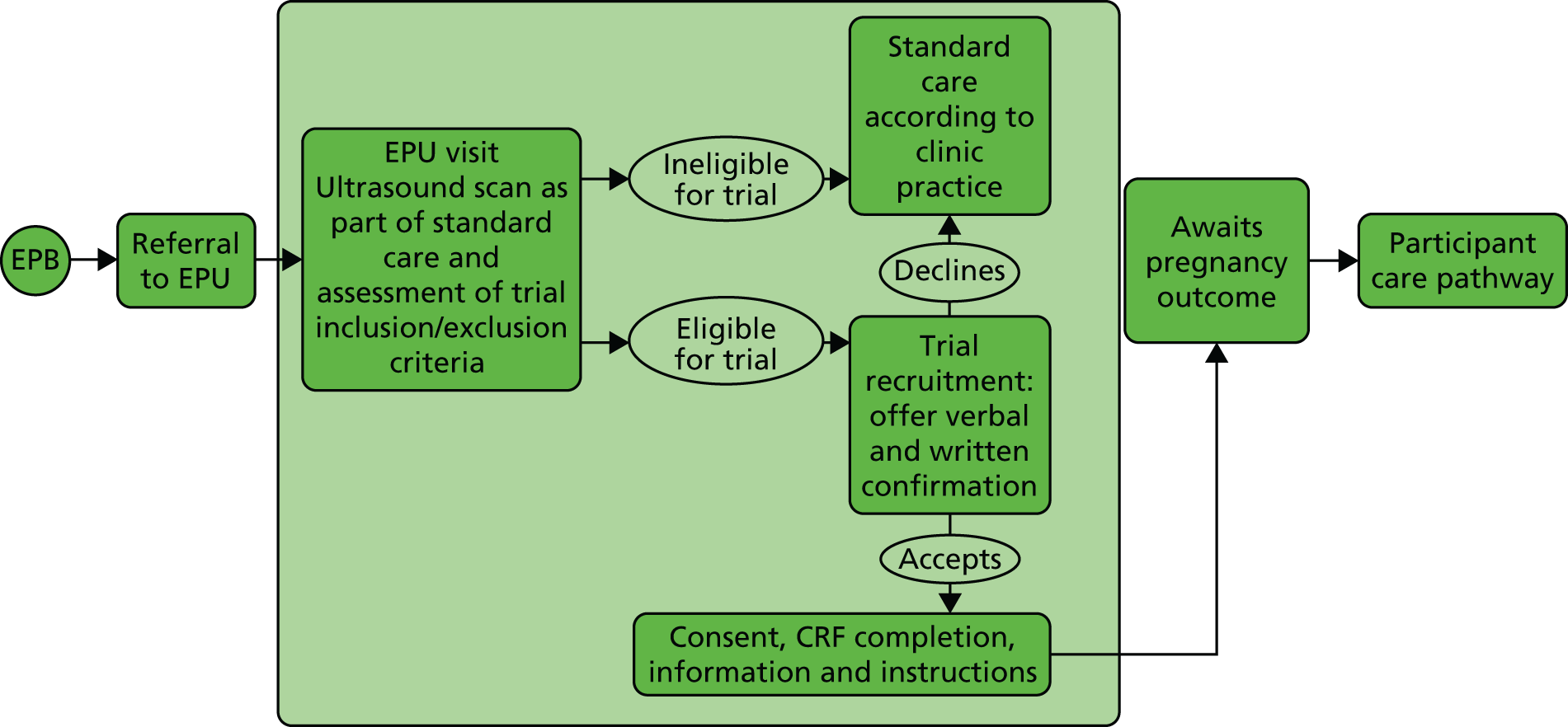
The participant eligibility pathway to recruitment and randomisation is illustrated in Figure 2. Eligible women were given verbal and written explanations about the trial. They were informed clearly that participation in the trial was entirely voluntary, with the option of withdrawing at any stage, and that participation or non-participation would not affect their usual care. They were provided with a participant information sheet. Eligible women were then given the opportunity to decide if they wanted to participate, if they needed more time to consider their decision or if they did not want to participate. In all three scenarios, the decision of the woman was respected. If a woman needed more time to consider her potential involvement, she was asked to call the research nurse or midwife when she had decided. If an undecided woman had not called in 1–2 days, then the research nurse or midwife contacted her. If an initially undecided woman later decided to participate, the research nurse or midwife arranged a mutually convenient opportunity for the woman to be consented, providing she still met the eligibility criteria. A written consent form was provided to each woman who agreed to participate in the trial. The investigator and the participant both signed the consent form. The original copy was kept in the investigator site file, one copy was given to the participant and one copy was retained in the woman’s hospital records. Baseline demographic and medical data were collected, anonymised and stored in an electronic integrated trial management system (ITMS). Any identifying information was collected and stored in a password-protected local database on a secure computer with restricted access.
FIGURE 2.
Eligibility pathway to recruitment and randomisation. CRF, case report form; EPB, early pregnancy bleeding; EPU, early pregnancy unit.
We made provision for translation, if necessary, to communicate with non-English speakers and to accommodate any special communications requirements of potential study participants. Participant information sheets and consent forms were translated from English into Polish, Bengali and Urdu.
Randomisation
Confirmation of eligibility according to inclusion and exclusion criteria was assessed by a medically trained doctor and all of the necessary information was gathered prior to randomisation. Participants were randomised online to receive the trial intervention (either progesterone or placebo) via a purpose-designed ITMS. Each authorised member of the research team was provided with a unique username and password to access the ITMS for this purpose. Online randomisation was available for 24 hours per day, 7 days per week, apart from short periods of scheduled maintenance.
Sequence generation and minimisation
Computer-generated random numbers were used, and participants were randomised online via a secure internet facility. This third-party independent ITMS was designed, developed and delivered by MedSciNet® (MedSciNet UK Ltd, St Thomas’ Hospital, London, UK) in accordance with the standards of the International Organisation for Standardisation 2700015 and the requirements of the US Food and Drug Administration (FDA) CFR21:11. 16,17
Participants were randomised to receive progesterone or placebo in a 1 : 1 ratio. A ‘minimisation’ procedure via computer-based algorithm based on the method described by Pocock and Simon18 was used to avoid chance imbalances in important stratification variables. A random element was incorporated to make the treatment group less predictable. 19 The stratification variables (equally weighted) used for minimisation are listed below:
-
age (< 35 or ≥ 35 years)
-
body mass index (BMI) (< 30 or ≥ 30 kg/m2)
-
fetal heart activity (present or absent)
-
estimated gestational age at presentation (< 42 or ≥ 42 days)
-
amount of bleeding [pictorial bleeding assessment chart (PBAC)]20 score of ≤ 2 or ≥ 3).
Allocation
When all of the eligibility criteria and baseline data items were entered online, the ITMS generated a trial number that took into account the minimisation variables recorded for the individual and that was linked to a specific trial intervention pack. The pack number was revealed via e-mail to the local principal investigator (PI), the relevant trial pharmacist (see Blinding) and the research nurse or midwife performing the randomisation. The trial intervention pack was dispensed to the patient by the clinical trial pharmacist at the randomising hospital. Each trial intervention pack contained either progesterone or an identical-looking placebo pessary.
Interventions
Each participant in the PRISM trial received either progesterone or placebo pessaries, to be administered vaginally. Both products were supplied by Besins Healthcare International (Besins Healthcare, Montrouge, France), a global pharmaceutical company with a manufacturer’s licence for tablets and capsules, in compliance with good manufacturing practice standards,21 good clinical practice requirements22 and Medicines for Human Use (Clinical Trials) Regulations 2004. 23 Besins Healthcare also provided qualified person release of the trial drug under the requirements of the Medicines for Human Use (Clinical Trials) Regulations 2004. 23
Progesterone pessaries
The IMP was a 400 mg dose of progesterone [i.e. two 200 mg pessaries of Utrogestan® (micronised vaginal progesterone, Utrogestan®, Besins Healthcare, Montrouge, France)] taken vaginally twice daily (every morning and every evening) for the duration of treatment. The product had all of the properties of endogenous progesterone with induction of a full secretory endometrium and, in particular, gestagenic, antiestrogenic, slightly antiandrogenic and antialdosterone effects.
Placebo pessaries
Placebo pessaries were vaginal pessaries, composed of sunflower oil, soybean lecithin, gelatin, glycerol, titanium dioxide and purified water, encapsulated in the same form as the IMP, and identical in colour, shape and weight, for use in the placebo arm of the PRISM trial. The dose, route and timing of administration were also identical to those in the active progesterone arm of the study.
Dose
The biologically effective dosage of progesterone pessaries ranged from 200 mg once daily to 400 mg twice daily according to the summary of product characteristics24 and the British National Formulary (BNF). 25 Our choice of 400 mg twice daily was made after a careful review of the existing literature and an extensive survey of clinicians in the UK (see Chapter 1, Progesterone in clinical use for threatened miscarriage). We also reviewed other related evidence. For example, progesterone vaginal capsules are commonly used for luteal support in assisted conception at a treatment dose of 400 mg twice daily, with no specific concerns for safety raised on this dose. 26,27 In addition, the findings from the PROMISE trial, which used the same dose, showed no safety concerns. 13 Therefore, after evaluating the evidence, we considered the dosage of 400 mg vaginal progesterone twice daily to be an acceptable regimen to ensure a clinically effective dose and to minimise the risk of a negative trial result from therapy with a suboptimal dose.
Timing of dose
Treatment commenced as soon as possible after confirmation of an intrauterine pregnancy sac and within 4 days of vaginal bleeding and continued until the gestational age of 16 weeks. Our rationale to discontinue the treatment at 16 weeks was that production of progesterone by the corpus luteum becomes less important when compared with the placental production of progesterone after 16 weeks of gestation. Furthermore, the largest (n = 191) and most recent of the seven previously published trials12 continued treatment until 16 weeks and found a large and statistically significant reduction in miscarriage risk (risk ratio 0.44, 95% CI 0.24 to 0.82). There was also overwhelming agreement among the clinicians and researchers involved in the preparation of this application that we should continue the progesterone until 16 weeks’ gestation.
Route
An immunomodulatory effect of progesterone at the trophoblastic–decidual interface is the key presumed mechanism for preventing miscarriage. 28–31 Our choice to use the vaginal route was, therefore, rational to deliver a greater proportion of the drug to the relevant site (the uterus) using the ‘first uterine pass’ effect. 32,33 Furthermore, studies that have used vaginal progesterone in the prevention of preterm birth have shown its effectiveness when given via this route. 34–36 For example, 14 out of 36 studies of second and/or third trimester progesterone to prevent preterm birth (identified by a recent systematic review) used vaginal progesterone, with significant improvements being observed for various clinical outcomes, confirming the biological effects of vaginal progesterone. 37
The acceptability and availability of interventional drugs were also important considerations supporting the vaginal route of drug delivery. Our discussions with consumer representatives confirmed that a vaginal formulation would be more acceptable to women than an intramuscular injection. These findings were further supported by a study in which 12% of participants were unable to tolerate the intramuscular progesterone preparation and declined participation or withdrew from that trial. 38 Of those who did continue, 34% complained of localised soreness around the injection site. Moreover, the Miscarriage Association conducted a survey to identify women’s opinions regarding acceptability of administering vaginal or rectal medications. The findings showed that the vaginal route of administration of medicines was acceptable to 100 out of 111 (90%) women, and the rectal route was acceptable to 91 out of 111 (82%) women. The pessary formulation of the PRISM trial is widely available in the UK and worldwide.
Instructions to participants
Each participant commenced the trial intervention on the day it was received and continued administration until it was finished, at 16 completed weeks of gestation, unless the pregnancy had ended before this time. Each participant was given instructions on how to administer the pessaries. In addition, each participant was asked for consent to notify her general practitioner (GP) by letter that she was participating in the trial. Moreover, each participant was given a card with contact details of local PRISM investigators and the central trial co-ordinating centre (TCC), the Birmingham Clinical Trials Unit, to inform any directing clinicians in case of potential drug interactions.
Concomitant non-trial treatments
Concomitant therapy was provided at the discretion of the care-providing clinicians, and all concomitant treatment and medications were documented via the ITMS. Other than identified contraindicated drugs (see Participants) and other progestogen preparations, the initiation of treatment for another indication did not necessitate withdrawal from the PRISM trial.
Blinding
Participants, investigators, research nurses, midwives and other attending clinicians remained blind to the trial drug allocation throughout the duration of the trial.
In the case of any serious adverse event (SAE), the general recommendation was to initiate management and care of the participant as though the woman was taking progesterone. Cases that were considered serious, unexpected and possibly, probably or definitely related to the trial intervention were unblinded as appropriate. 39 In any other circumstances, investigators, research nurses and midwives remained blind to drug allocation while the participant remained in the trial. However, if the drug allocation was specifically requested to assist the medical management of a participant, clinicians could contact the trial co-ordinator for this purpose, 24 hours per day, 7 days per week.
Compliance assessment and treatment withdrawal
Compliance monitoring
Our previous experience of research and clinical care for women with miscarriage demonstrated that they would be highly motivated and compliant with therapy advice. However, compliance with the PRISM trial was evaluated by ‘pill counting’ in the first instance. Participants were asked to return completed, partially used and unused treatment packs to the trial centres. The research nurses and midwives at each study centre documented the pessaries returned by each participant, and the trial pharmacists kept their own accountability logs.
In an effort to improve compliance, women who failed to return their empty or unused blister packs were provided with an envelope to return them to the research team. Finally, if neither of these two approaches was successful, where possible, the patients were contacted directly by the research team via telephone and asked to give an honest assessment of their drug compliance in terms of what percentage of treatment they felt that they took.
Good compliance with the intervention was defined as taking > 80% of trial medicines from the date of allocation up to 16 weeks of gestation.
Participant withdrawal from treatment
Following discussion with the trial management group, participants in the PRISM trial could be withdrawn from the trial treatment if it became medically necessary in the opinion of the investigator(s) or clinician(s) providing patient care. In the event of such premature treatment cessation, study nurses and midwives made every effort to obtain and record information about the reasons for discontinuation and to follow-up all safety and efficacy outcomes as appropriate. Providing that the patient gave their continued consent, the follow-up information for these patients was still collected. Participants in the PRISM trial could also voluntarily decide to cease taking the study treatment at any time. If a woman stopped taking the trial treatment but permitted further data collection, she was followed up and outcome assessments were undertaken for the remainder of the study.
Withdrawal from the trial
Participants could voluntarily withdraw their consent to study participation at any time. If a participant did not return for a scheduled visit, attempts were made to contact her and (where possible) to review compliance and adverse events (AEs). We documented the reason(s) for self-withdrawal where possible. Each woman could change her mind about withdrawal, and re-consent to participate in the trial, at any time. If a participant explicitly withdrew consent to any further data recording, then this decision was respected and recorded via the ITMS. All communications surrounding the withdrawal were noted in the study records and no further data were collected for such participants.
Outcomes and assessment
Primary outcome
Live births at or beyond 34 completed weeks of gestation (≥ 34 weeks), as a proportion of all women randomised.
Secondary outcomes
Secondary outcomes were as follows (as a proportion of those randomised unless stated):
-
Time from conception to pregnancy end (any reason). Conception date was estimated using the date of last menstrual period or, failing that, the date from the ultrasound scan at 9–14 weeks.
-
Ongoing pregnancy at 12 weeks of gestation.
-
Miscarriage rate (defined as delivery before 24 weeks of gestation).
-
Other pregnancy end outcomes – live birth at < 34 weeks’ gestation, ectopic pregnancy, termination, stillbirth, molar pregnancy, resolved pregnancy of unknown location (PUL), failed PUL, twin live births, gestational age at miscarriage.
-
When there is live birth at ≥ 24 weeks’ gestation – time from conception to delivery (gestational age), gestational age < 28/< 32/< 37 weeks’ gestation, mode of delivery [unassisted vaginal, instrumental vaginal, elective Caesarean section (C-section), emergency C-section, vaginal breech delivery, other], birthweight adjusted for gestational age and sex, small for gestational age and sex (< 10th centile), arterial and venous cord pH, Apgar scores.
-
Antenatal complications – pregnancy-induced hypertension, pre-eclampsia, obstetric cholestasis, cervical cerclarge, preterm (< 37 weeks’ gestation) pre-labour rupture of membranes, gestational diabetes mellitus (other complications will be tabulated but not formally analysed).
-
Intrapartum complications – chorioamnionitis, intrauterine growth restriction, macrosomia (other complications will be tabulated but not formally analysed).
-
Postpartum complications – haemorrhage (other complications will be tabulated but not formally analysed).
-
Maternal complications – admission to a high-dependency unit (HDU), admission to an intensive therapy unit (ITU) (other complications will be tabulated but not formally analysed).
-
Neonatal complications – discharge to hospital, early infection, retinopathy of prematurity, necrotising enterocolitis, intraventricular haemorrhage, congenital and chromosomal abnormalities, respiratory distress syndrome, ventilation or oxygen support (other complications will be tabulated but not formally analysed).
-
Survival at 28 days of neonatal life.
-
Maternal unexpected AEs (tabulated but not formally analysed).
-
SAEs.
Resource use outcomes
These are detailed in Chapter 4.
Future outcomes
Each participant in the PRISM study was asked to consent for the future evaluation of themselves, the child who was born and the health records of both. Although long-term follow-up will remain outside the scope of this trial, we plan to conduct further studies, as discussed in Chapter 6, Recommendations for research.
Outcome generation
Details of how outcome measures were generated are given in Table 2. The ITMS was utilised to capture baseline and outcome data, and to maintain an audit trail. Relevant trial data were transcribed directly into the ITMS. Source data comprised the research clinic notes, hospital notes, hand-held pregnancy notes, laboratory results and self-reports.
| Outcome assessed | Details | |||
|---|---|---|---|---|
| When? | How? | By whom? | PD or SP? | |
| Ongoing pregnancy | 11–14 weeks | Ultrasound | Ultrasonographer | SP |
|
At or after the end of pregnancy | From:
|
Research nurse or doctor | Both SP and PD |
| Neonatal outcomes | Up to 28 days of neonatal life | From:
|
Research nurse or doctor | Both SP and PD |
| Resource use outcomes | At any time during the conduct of the trial | From:
|
Research nurse or doctor | PD |
First outcome assessment (11–14 weeks of pregnancy)
At the time of randomisation, arrangements for an ultrasound appointment with the woman’s routine care providers were made at between 11 and 14 weeks of gestation. The research nurse or midwife assisted with booking an appointment, if necessary, and was responsible for ensuring that the details of the scan were recorded in the ITMS. If the patient did not have a scan for any reason, this was recorded in the ITMS.
Second outcome assessment (end of pregnancy)
The second outcome assessment was conducted at, or after, birth (Figure 3). The research nurse or midwife at each study site used the patient’s hospital notes to obtain pregnancy outcome data, such as the mode of delivery, gestation, weight and Apgar score at birth. If for any reason the research nurse or midwife was unable to access the hospital records, a telephone call was made to the patient to obtain as much follow-up information as possible.
FIGURE 3.
Participant care pathway and outcome assessment. OA, outcome assessment points; OA1, ongoing pregnancy beyond 12 weeks (range 11–14 weeks); OA2, live birth at > 34 weeks; OA3, survival at 28 days of neonatal life; R, randomisation.
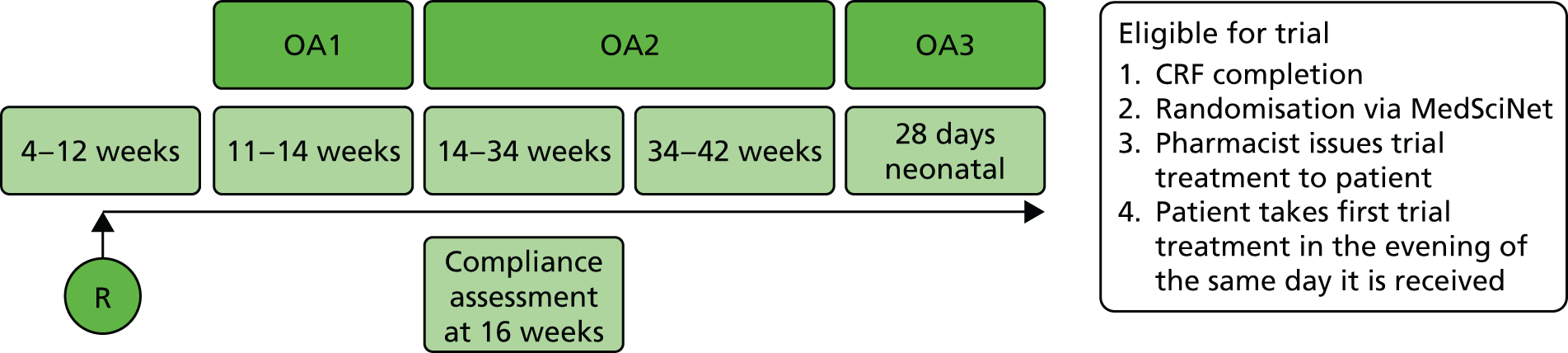
Third outcome assessment (day 28 post birth)
The third and final outcome assessment was conducted to gather neonatal outcomes at 28 days after birth for those patients who had a successful live birth (see Figure 3). The research nurse or midwife at each study site telephoned every participant to ascertain whether or not the baby was still alive at this time point and to enquire about any nights of hospital admission or requirements for ventilation support, and complications (e.g. early infection). Using the full repertoire of evidence-based methods to maximise data collection, the research nurse or midwife also checked birth registers and inpatient records to track hospital admissions and pregnancy outcomes.
Definition of the end of the trial
The observational phase of the trial ceased when the 28-day follow-up had been completed for all surviving babies. The primary analysis was scheduled to occur after all corresponding outcome data had been entered onto the study database and validated as being ready for analysis.
Notes on adverse events and serious adverse events
All of the trial participants were asked to report any hospitalisations, consultations with other medical practitioners, disability, incapacity or any other AEs to their local research team. If the local study nurse or midwife was unavailable for any reason, they were able to report the events to the trial manager or trial co-ordinator via telephone at any time. Moreover, at the time of each outcome assessment, investigators, research nurses and midwives at each study centre proactively asked each participant about any AEs in the preceding weeks. AEs were assessed by clinical investigators, further reported as appropriate and recorded on the ITMS.
Serious adverse events and serious adverse reactions (SARs) were recorded on a purpose-designed SAE form and notified by local investigators to the TCC within 24 hours of the local investigators becoming aware of these events. In addition, local investigators were responsible for reporting SAEs to their host institutions in accordance with local regulations and instituting supplementary investigations as appropriate based on clinical judgement of the causative factors. Any SAE or SAR that was outstanding at the end of the trial treatment period was followed up at least until the final outcome was determined, even if this provision necessitated follow-up beyond 28 days post partum. The TCC reported all SAEs to the Data Monitoring and Ethics Committee (DMEC) approximately every 6 months. The DMEC viewed data blinded to treatment but was able to review unblinded data if requested.
Suspected unexpected serious adverse reactions (SUSARs) were unblinded, as appropriate, reviewed by the trial manager within 24 hours of reporting and further reported to the Medicines and Healthcare products Regulatory Agency and the regional ethics committee by the TCC as soon as possible for any event, within 15 days (or 7 days in the case of fatal or life-threatening SUSARs).
Sample size
The PRISM trial investigators believed that it was important to ensure that the study was large enough to detect reliably moderate but clinically important treatment effects. Our calculations indicated that, to detect a minimally important difference (MID) of 5% in rates of live birth after ≥ 34 weeks (from 60% to 65%), for an alpha error rate of 5% (two sided) with 90% power, it would be necessary to randomise 1970 women to the intervention arm and 1970 women to the placebo arm (3940 women in total). However, assuming and adjusting for a worst-case scenario of a loss to follow-up rate of 5%, the total number of participants required would be 4150 (2075 each in the progesterone and placebo arms). The sample size of the study was planned accordingly. The MID of 5% was defined following consultations among health-care practitioners, patients and representatives of patient bodies as well as through a survey of clinicians. The 60% baseline (placebo) event rate was derived from audits from two of the participating units (Imperial College London and the Royal Infirmary of Edinburgh).
Statistical methods
A comprehensive statistical analysis plan (SAP) was drawn up prior to any analysis and provided to the independent DMEC and Trial Steering Committee (TSC) for review. Full details of the statistical analysis can be found in the SAP. 39
To summarise, categorical baseline data were summarised with frequencies and percentages. Normally distributed continuous variables were summarised as means with standard deviations (SDs), otherwise medians with interquartile ranges (IQRs) were presented. Participants were analysed in the treatment group to which they were randomised in the first instance, irrespective of compliance with the treatment protocol. All estimates of differences between groups are presented with 95%, two-sided CIs. p-values from two-sided tests at the 5% significance level are also included.
For the primary outcome (live birth at ≥ 34 weeks’ gestation), the population was all randomised participants. A Poisson regression model incorporating robust standard errors was used to generate relative risks along with 95% CIs, adjusting for the minimisation parameters. This method has been shown to be appropriate and less prone to convergence issues compared with other comparable methods. 40 Statistical significance of the treatment group parameter was determined through examination of the associated chi-squared statistic.
Analysis was performed as per the primary outcome for the other binary outcomes. For number of twins, mode of delivery, secondary neonatal outcomes, intrapartum complications, postpartum complications and neonatal complications, the analysis population was those with live births at ≥ 24 weeks’ gestation. For secondary neonatal outcomes and neonatal complication rates, twin babies were both counted in the analysis population. For continuous outcomes (e.g. birthweight and birthweight centiles), a linear regression model was used, adjusting for the same minimisation parameters. Here, an F-test was used to test the statistical significance of the estimated treatment group parameter generated from the restricted maximum likelihood estimates. The proportion and percentage of patients experiencing any SAE were presented by group. Statistical significance was determined by chi-squared test.
Sensitivity analysis was performed on the primary outcome and the outcome miscarriage at < 24 weeks’ gestation to test the impact of any missing data. This assumed that all patients lost to follow-up had a negative outcome (i.e. no live birth ≥ 34 weeks’ gestation). An analysis that simulated missing responses using a multiple imputation approach was also performed (Markov chain Monte Carlo method – see SAP for details39). We also repeated the primary analysis, prioritising data scan information over last menstrual period dates (the primary analysis prioritised last menstrual period dates).
Pre-planned subgroup analyses (limited to the primary outcome measure and miscarriage rate) were completed in the following: (1) maternal age (< 35 or ≥ 35 years), (2) BMI ( < 30 or ≥ 30 kg/m2), (3) fetal heart activity (present or absent), (4) estimated gestational age at presentation (< 42 or ≥ 42 days), (5) amount of vaginal bleeding (PBAC score20 of ≤ 2 or ≥ 3), (6) number of previous miscarriages (0, 1/2 or ≥ 3), (7) number of gestational sacs (1 or ≥ 2), (8) ethnicity (white, black, south Asian or other), (9) history of polycystic ovaries (yes or no) and (10) previous cervical excision (yes or no). The effects of these subgroups were examined by adding the subgroup by treatment group interaction parameters to the regression model; a chi-squared test was used to test the statistical significance of this parameter.
Interim analyses of effectiveness and safety end points were performed on behalf of the DMEC on an approximately 6-monthly basis during the period of recruitment. These analyses were performed with the use of the Haybittle–Peto principle41 and hence no adjustment was made in the final p-values to determine significance.
Trial oversight
Study oversight was provided by a TSC (chaired by Professor Siladitya Bhattacharya, University of Aberdeen) and a DMEC (chaired by Professor Andrew Shennan, King’s College London).
The TSC provided independent supervision for the trial, providing advice to the chief investigator and co-investigators and the sponsor on all aspects of the trial throughout the study. The DMEC adopted the DAMOCLES (DAta MOnitoring Committees: Lessons, Ethics, Statistics) charter to define its terms of reference and operation in relation to oversight of the PRISM trial.
Chapter 3 Results
This chapter reports the results of the PRISM trial. It commences with a description of the flow of participants through the trial and is followed by demographic information and results of the primary and secondary outcome measures, including the safety outcomes.
Participant flow
Participant flow is illustrated in Figure 4. A total of 23,775 participants were screened for eligibility to take part in the PRISM trial. Of these, 10,913 participants were not eligible for randomisation and a further 8709 declined to participate in the trial.
FIGURE 4.
The CONSORT flow diagram of participants through the PRISM trial.
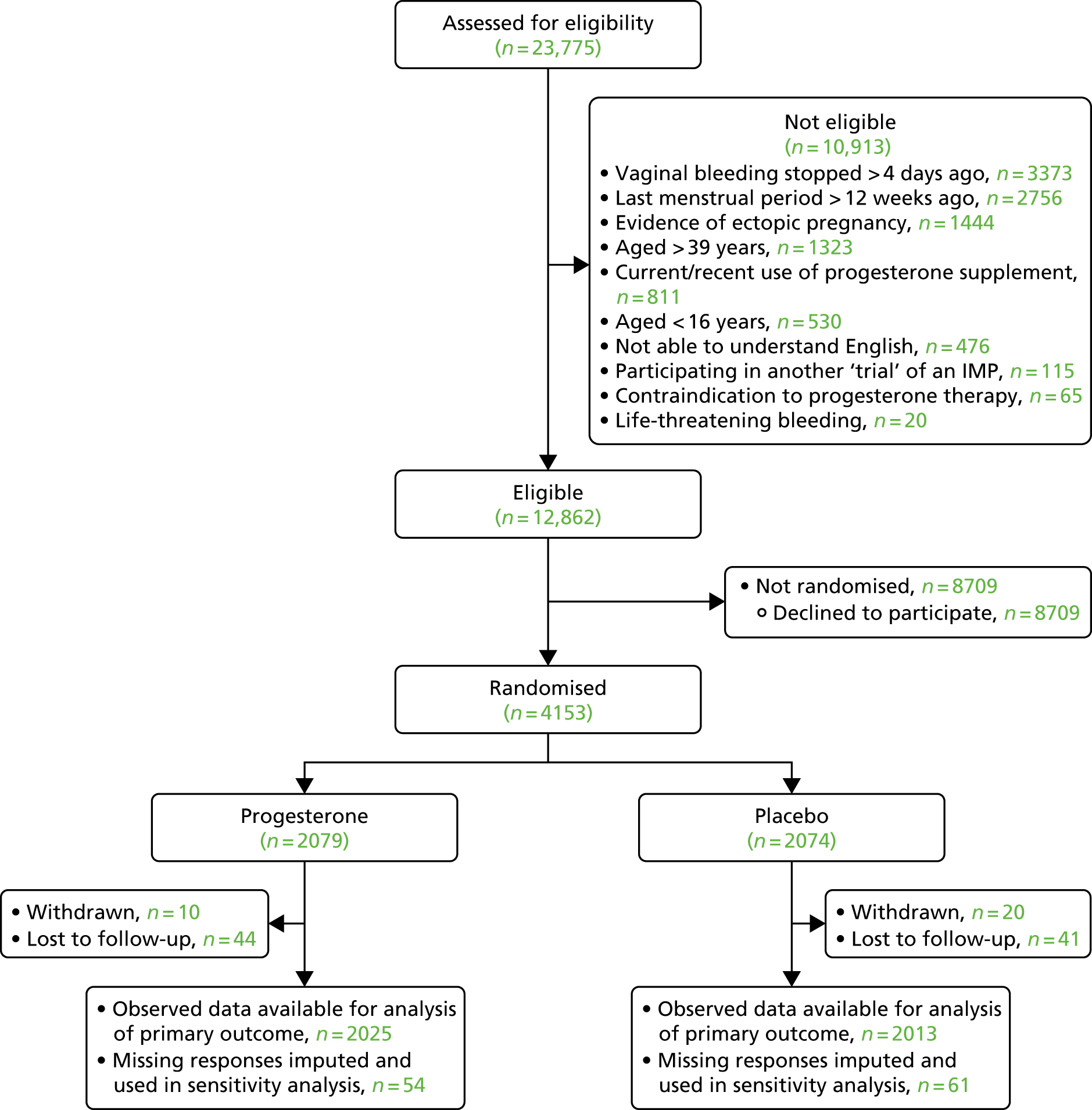
A total of 4153 women proceeded to randomisation, with 2079 allocated to progesterone and 2074 allocated to placebo. Thirty participants were withdrawn from the study and a further 85 were lost to follow-up, meaning that 4038 participants (97.2% of those randomised) were available for analysis of the primary outcome.
Recruitment
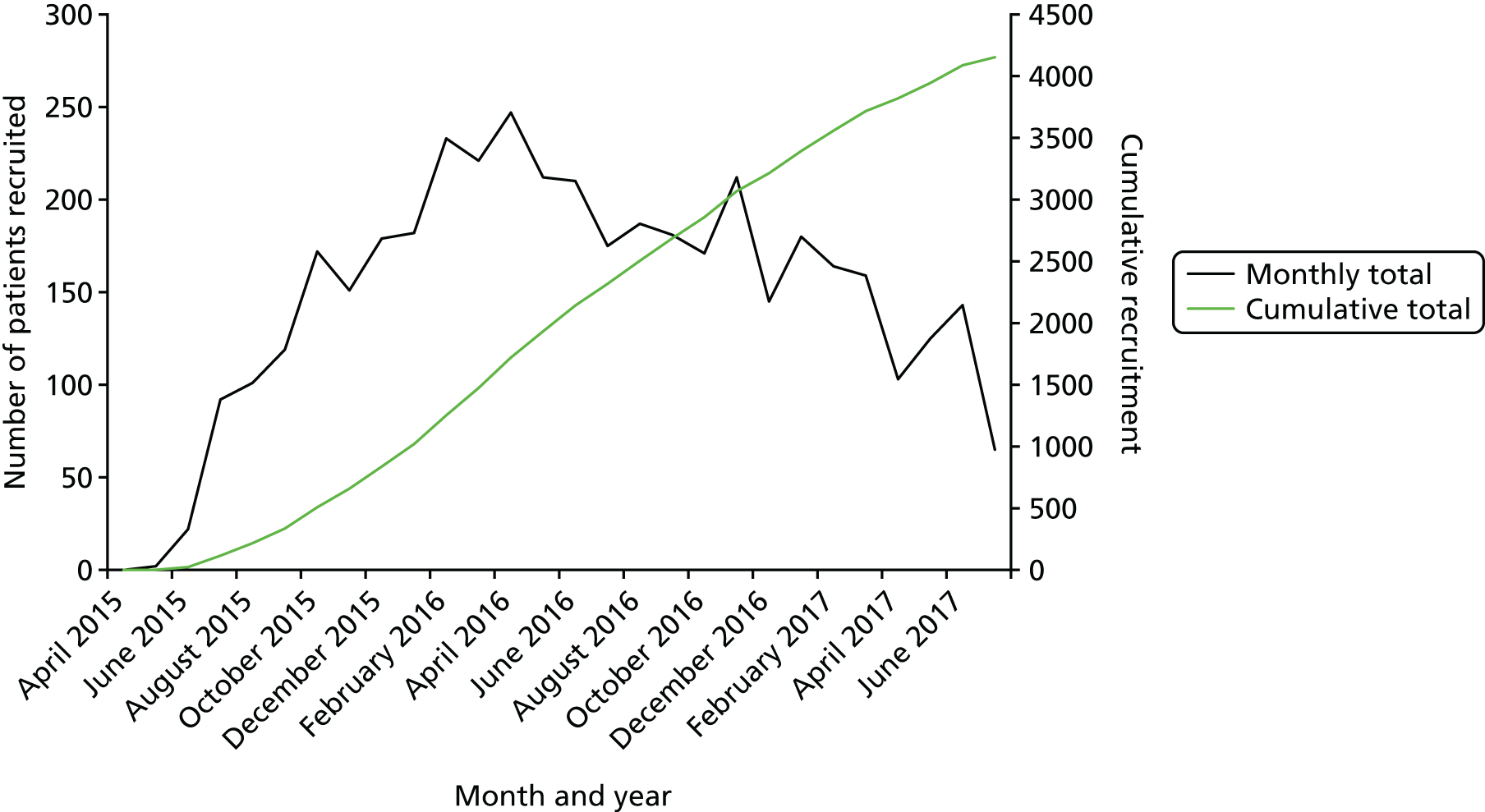
Recruitment and randomisation took place over 27 months in 48 UK NHS hospitals (Figure 5) from May 2015 to July 2017 (Figure 6). Two centres, University College London Hospital and St Michael’s Hospital, Bristol, contributed > 300 enrolled participants each (Table 3).
FIGURE 5.
Map of the PRISM trial recruiting centres.
FIGURE 6.
Rates of recruitment for the PRISM trial.
| Hospital | NHS institution | PI | Number randomised, n (%) |
|---|---|---|---|
| University College Hospital | University College London Hospitals NHS Foundation Trust | Mr Davor Jurkovic | 365 (8.8) |
| St Michael’s Hospital | University Hospitals Bristol NHS Foundation Trust | Mrs Caroline Overton | 313 (7.5) |
| University Hospital Coventry | University Hospitals Coventry and Warwickshire NHS Trust | Mr Feras Izzat | 268 (6.5) |
| Queen’s Medical Centre | Nottingham University Hospitals NHS Trust | Dr Shilpa Deb | 223 (5.4) |
| Sunderland Royal Hospital | City Hospitals Sunderland NHS Foundation Trust | Dr Amna Ahmed | 210 (5.1) |
| Royal Infirmary of Edinburgh | Lothian Health Board | Professor Andrew Horne | 160 (3.9) |
| Glasgow Royal Infirmary | NHS Greater Glasgow and Clyde | Professor Mary-Ann Lumsden | 156 (3.8) |
| King’s College Hospital | King’s College Hospital NHS Foundation Trust | Miss Jemma Johns | 152 (3.7) |
| St Thomas’ Hospital | Guy’s and St Thomas’ NHS Foundation Trust | Dr Thomas Holland | 133 (3.2) |
| Liverpool Women’s Hospital | Liverpool Women’s NHS Foundation Trust | Dr Linda Watkins | 131 (3.2) |
| Queen Alexandra Hospital | Portsmouth Hospitals NHS Trust | Miss Nime Vaithilingam | 130 (3.1) |
| Birmingham Women’s Hospital | Birmingham Women’s and Childrens NHS Foundation Trust | Mr Ismail Hassan | 129 (3.1) |
| West Middlesex University Hospital | Chelsea and Westminster Hospital NHS Foundation Trust | Miss Natalie Nunes | 125 (3.0) |
| Princess Royal Hospital | Shrewsbury and Telford NHS Trust | Mr Martyn Underwood | 118 (2.8) |
| Birmingham Heartlands Hospital | Heart of England NHS Foundation Trust | Dr Pratima Gupta | 117 (2.8) |
| Royal Preston Hospital | Lancashire Teaching Hospitals NHS Foundation Trust | Dr Fiona Crosfill | 110 (2.6) |
| The James Cook University Hospital | South Tees Hospitals NHS Foundation Trust | Dr Padma Manda | 103 (2.5) |
| East Surrey Hospital | Surrey and Sussex Healthcare NHS Trust | Dr Catherine Wykes | 99 (2.4) |
| Chelsea and Westminster Hospital | Chelsea and Westminster Hospital NHS Foundation Trust | Miss Cecilia Bottomley | 91 (2.2) |
| Burnley General Hospital | East Lancashire Hospitals NHS Trust | Miss Kalsang Bhatia | 79 (1.9) |
| Worcestershire Royal Hospital | Worcestershire Acute Hospitals NHS Trust | Mr Samson Agwu | 77 (1.9) |
| Whiston Hospital | St Helen’s and Knowsley NHS Trust | Mrs Sandhy Rao | 73 (1.8) |
| Whipps Cross University Hospital | Barts London NHS Trust | Miss Anupama Shahid | 68 (1.6) |
| Royal Victoria Infirmary | Newcastle Upon Tyne Hospitals NHS Foundation Trust | Dr Meenakshi Choudhary | 62 (1.5) |
| Musgrove Park Hospital, Taunton | Taunton and Somerset NHS Foundation Trust | Dr Hadi Haerizadeh | 61 (1.5) |
| St Peter’s Hospital | Ashford and St Peter’s Hospitals NHS Foundation Trust | Ms Catey Bass | 55 (1.3) |
| Queen’s Hospital, Burton | Burton Hospitals NHS Foundation Trust | Dr Jayasree Srinivasan | 50 (1.2) |
| St Mary’s Hospital, Manchester | Central Manchester University Hospitals NHS Foundation Trust | Dr Ursula Winters | 50 (1.2) |
| Royal London Hospital | Barts London NHS Trust | Mrs Anupama Shahid | 48 (1.2) |
| Scunthorpe General Hospital | Northern Lincolnshire and Goole NHS Foundation Trust | Miss Preeti Gandhi | 39 (0.9) |
| Airedale General Hospital | Airedale NHS Foundation Trust | Miss Sumita Bhuiya | 38 (0.9) |
| John Radcliffe Hospital | Oxford University Hospitals NHS Trust | Dr Ingrid Granne | 35 (0.8) |
| Sheffield Royal Hallamshire Hospital | Sheffield Teaching Hospitals NHS Foundation Trust | Mrs Joanne Fletcher | 35 (0.8) |
| Derriford Hospital, Plymouth | Plymouth Hospitals NHS Trust | Dr Rekha Shrestha | 34 (0.8) |
| Cumberland Infirmary | North Cumbria University Hospitals NHS Trust | Dr Laura Hipple | 33 (0.8) |
| North Devon District Hospital | Northern Devon Healthcare NHS Trust | Mr Samuel Eckford | 33 (0.8) |
| St James University Hospital | Leeds Teaching Hospitals NHS Trust | Ms Jayne Shillito | 25 (0.6) |
| Warrington Hospital | Worcestershire Acute Hospitals NHS Trust | Mrs Rita Arya | 25 (0.6) |
| Royal Stoke University Hospital | University Hospitals of North Midlands NHS Trust | Mr Zeiad El-Gizawy | 24 (0.6) |
| Walsall Manor Hospital | Walsall Healthcare NHS Trust | Mr Jonathan Pepper | 21 (0.5) |
| Hinchingbrooke Hospital | North West Anglia Foundation Trust | Miss Hema Nosib | 14 (0.3) |
| St Mary’s Hospital, London | Imperial College Healthcare NHS Trust | Professor Tom Bourne | 13 (0.3) |
| New Cross Hospital | Royal Wolverhampton Hospitals NHS Trust | Mr Jag Samra | 12 (0.3) |
| Rosie Hospital | Cambridge University Hospitals NHS Foundation Trust | Miss Miriam Baumgarten | 5 (0.1) |
| North Tyneside General Hospital | Northumbria Healthcare NHS Trust | Mr Mamdouh Guirguis | 4 (0.1) |
| Hull Royal Infirmary | Hull and East Yorkshire Hospitals NHS Trust | Mr Piotr Lesny | 3 (0.1) |
| Bradford Royal Infirmary | Bradford Teaching Hospitals NHS Foundation Trust | Professor Derek Tuffnell | 2 (0.05) |
| Royal Devon and Exeter Hospital | Royal Devon and Exeter Hospitals NHS Foundation Trust | Mr James Clark | 2 (0.05) |
Baseline data
The baseline demographic characteristics of participants in the two groups were comparable, with the minimisation algorithm ensuring balance for the factors indicated in Table 4.
| Characteristic | Progesterone (N = 2079) | Placebo (N = 2074) |
|---|---|---|
| General baseline data | ||
| Maternal age (years)a | ||
| < 35, n (%) | 1604 (77) | 1601 (77) |
| ≥ 35, n (%) | 475 (23) | 473 (23) |
| Mean (SD) | 30.6 (5.1) | 30.5 (5.1) |
| BMI (kg/m2)a | ||
| < 30, n (%) | 1589 (76) | 1589 (77) |
| ≥ 30, n (%) | 490 (24) | 485 (23) |
| Mean (SD) | 26.4 (6.2) | 26.5 (6.3) |
| Ethnic group, n (%) | ||
| White | 1714 (82) | 1742 (84) |
| Black | 84 (4) | 79 (4) |
| South Asian | 114 (5) | 102 (5) |
| Other | 165 (8) | 150 (7) |
| Missing | 2 (< 1) | 1 (< 1) |
| Pregnancy history | ||
| Nulliparous, n (%) | 474 (23) | 514 (25) |
| Number of previous miscarriages | ||
| 0, n (%) | 1145 (55) | 1157 (56) |
| 1/2, n (%) | 792 (38) | 758 (37) |
| ≥ 3, n (%) | 142 (7) | 159 (8) |
| Median (IQR) | 0 (0–1) | 0 (0–1) |
| Number of previous miscarriages, median (IQR), n | ||
| First trimester miscarriages (< 14 weeks) in those with ≥ 1 miscarriagesb | 1 (1–2), 891 | 1 (1–2), 878 |
| Second trimester miscarriages (≥ 14 weeks and < 24 weeks) in those with ≥ 1 miscarriagesb | 1 (1–1), 74 | 1 (1–1), 77 |
| Preterm births (≥ 24 weeks and < 34 weeks) | 1 (1–2), 83 | 1 (1–1), 90 |
| Medical history | ||
| Usual length of menstrual cycle (days), median (IQR), n | 28 (28–30), 1947 | 28 (28–30), 1928 |
| Polycystic ovaries, n/N (%) | 226/2077 (11) | 227/2072 (11) |
| Fibroids, n/N (%) | 100/2077 (5) | 78/2072 (4) |
| Endometriosis, n/N (%) | 78/2077 (4) | 68/2072 (3) |
| Pelvic inflammatory disease, n/N (%) | 32/2077 (2) | 33/2072 (2) |
| Uterine abnormalities, n/N (%) | 48/2077 (2) | 53/2072 (3) |
| History associated with previous gynaecological surgeries, n/N (%) | ||
| Previous gynaecological surgeries | 580/2077 (28) | 564/2072 (27) |
| LLETZ | 110/2077 (5) | 103/2072 (5) |
| Surgical management of miscarriages | 118/2077 (6) | 144/2072 (7) |
| Myomectomy | 4/2077 (< 1) | 2/2072 (< 1) |
| Division of intrauterine adhesions | 3/2077 (< 1) | 3/2072 (< 1) |
| Endometrial surgery | 36/2077 (2) | 29/2072 (1) |
| Septum division | 2/2077 (< 1) | 7/2072 (< 1) |
| Tubal surgery | 35/2077 (2) | 29/2072 (1) |
| Ovarian cystectomy | 36/2077 (2) | 40/2072 (2) |
| Other surgeries | 286/2077 (14) | 270/2072 (13) |
| Other disorders | 37/2077 (2) | 44/2072 (2) |
| Family/social history, n/N (%) | ||
| Current smoker | 226/2077 (11) | 249/2072 (12) |
| Partner is a current smoker | 502/2077 (24) | 473/2072 (23) |
| Current alcohol use | 19/2077 (1) | 27/2072 (1) |
| Family history of recurrent miscarriage (≥ 3 miscarriages) | 243/2077 (12) | 257/2072 (12) |
| Current medical data, n/N (%) | ||
| Currently taking metformin | 28/2077 (1) | 20/2073 (1) |
| Current or recent use of aspirin (within 1 week) | 73/2077 (4) | 66/2073 (3) |
| Current or recent use of heparin (within 1 week) | 7/2077 (< 1) | 11/2073 (1) |
| Pregnancy-related information | ||
| Mode of conception, n (%) | ||
| Natural | 2030 (98) | 2036 (98) |
| Fertility treatment | 49 (2) | 38 (2) |
| Number of gestational sacs observed, n (%) | ||
| 1 | 2025 (97) | 2036 (98) |
| 2 | 53 (3) | 38 (2) |
| ≥ 3 | 1 (< 1) | 0 (–) |
| Number of fetuses observed, n (%) | ||
| 0 | 144 (7) | 155 (7) |
| 1 | 1892 (91) | 1887 (91) |
| 2 | 43 (2) | 31 (1) |
| ≥ 3 | 0 (–) | 1 (< 1) |
| Fetal heart activity, n (%) | ||
| Presenta,c | 1710 (82) | 1701 (82) |
| Estimated gestational age at presentation (days)a | ||
| < 42, n (%) | 372 (18) | 374 (18) |
| ≥ 42, n (%) | 1707 (82) | 1700 (82) |
| Median (IQR) | 50 (43–61) | 51 (43–62) |
| Amount of bleeding (PBAC score),a n (%) | ||
| ≤ 2 | 1913 (92) | 1907 (92) |
| ≥ 3 | 166 (8) | 167 (8) |
The randomised participants had an average age of 30.5 years (SD 5.1 years). The mean BMI was 26.5 kg/m2 (SD 6.4 kg/m2) at the time of randomisation. Of those who provided ethnic group data, 3456 (83%) were white, 216 (5%) were South Asian, 163 (4%) were black and 315 (8%) were from other ethnic groups. The majority of the women were non-smokers (3674/4149, 89%).
Of the 4153 randomised women, 2302 (55%) had experienced no previous miscarriage and 301 (7%) had experienced three or more previous miscarriages. A total of 124 (3%) women had previously experienced ectopic pregnancy. Cases of comorbidities included 453 (11%) participants with polycystic ovarian syndrome, 178 (4%) with a fibroid uterus, 146 (4%) with endometriosis and 101 (2%) with an arcuate uterus. Furthermore, 213 (19%) women had previously undergone large loop excision of the cervical transformation zone (LLETZ), 65 (7%) women had previously undergone endometriosis surgery, 64 (6%) women had previously undergone tubal surgery and 76 (7%) women had previously undergone ovarian cystectomy. Study records of concurrent medications showed that 48 (1%) of the randomised women were taking metformin at the time of participation and 139 (3%) were taking low-dose aspirin.
Compliance with treatment
Compliance data were reasonably well determined, with data collected for 72% (2920/4038) of participants. Good compliance (≥ 80% of pills taken) with treatment was higher up to 12 weeks’ gestation (71%) than up to 16 weeks’ gestation (58%), which may reflect an unwillingness of women to take treatment once they felt that their pregnancy was secure following the dating scan at 12–14 weeks (Tables 5 and 6). Compliance levels appeared similar in both groups.
| Compliance | Progesterone (N = 1548) | Placebo (N = 1469) |
|---|---|---|
| ≥ 80%, n (%) | 849 (55) | 854 (58) |
| < 80%, n (%) | 699 (45) | 615 (42) |
| Missing compliance information, n | 477 | 544 |
| Compliance | Progesterone (N = 1548) | Placebo (N = 1469) |
|---|---|---|
| ≥ 80%, n (%) | 1087 (70) | 1066 (73) |
| < 80%, n (%) | 461 (30) | 403 (27) |
| Missing compliance information, n | 477 | 544 |
Results overview
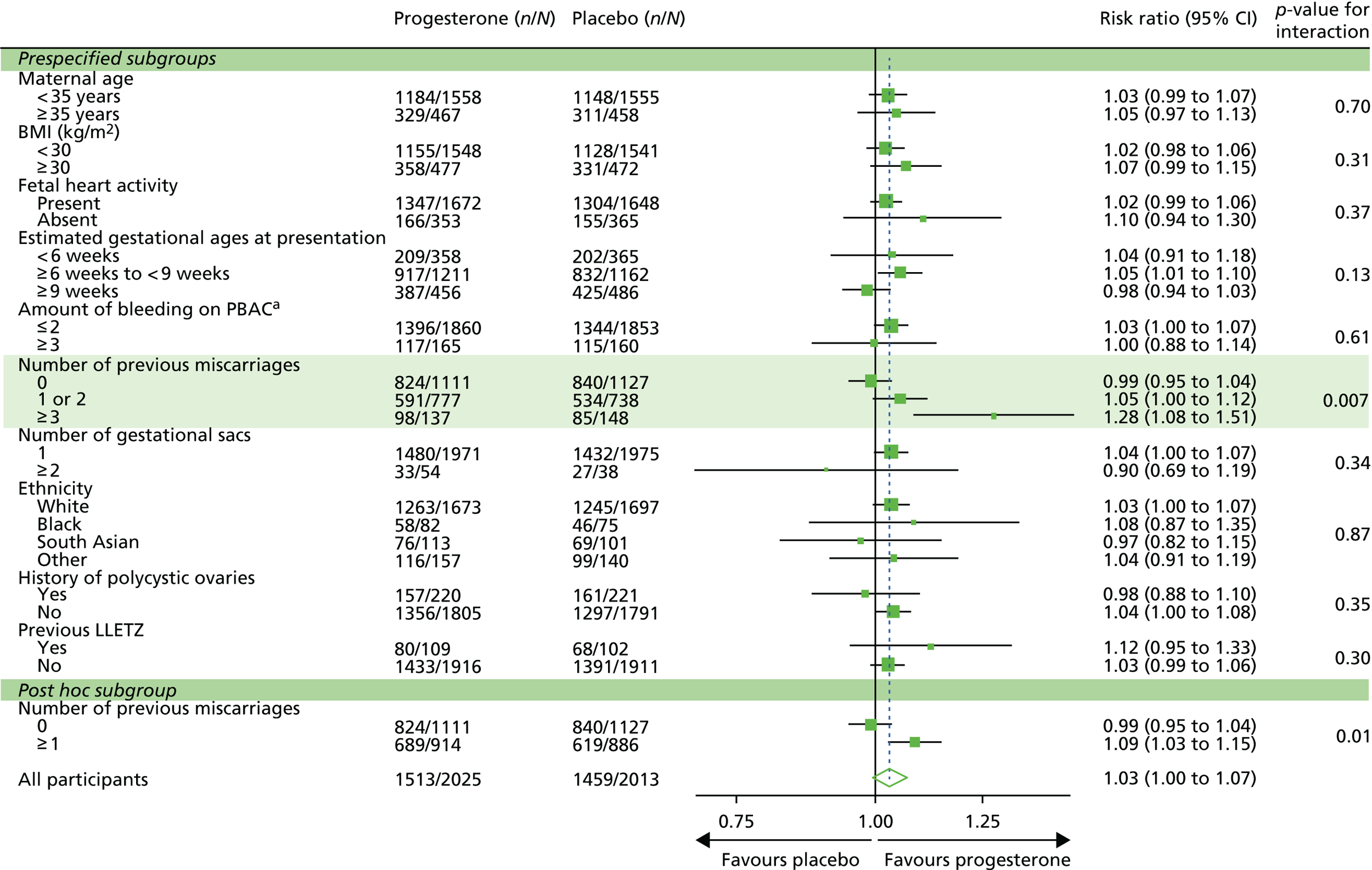
The PRISM trial found no convincing evidence of a difference in the primary outcome (live birth at ≥ 34 weeks) between the two treatment groups. The number of live births was higher in the progesterone group than in the placebo group (75% in the progesterone group vs. 72% in the placebo group; adjusted relative risk 1.03, 95% CI 1.00 to 1.07), but this difference was not statistically significant (p = 0.08). There was evidence that the effect was dependent on the number of previous miscarriages, with a significant (p = 0.007) treatment by subgroup interaction observed. In women with three or more previous miscarriages, the live birth rate was 72% (98/137) with progesterone, compared with 57% (85/148) in the placebo group (relative risk 1.28, 95% CI 1.08 to 1.51; p = 0.004). A post hoc subgroup analysis exploring the effects in the subgroup of women with any number of previous miscarriages found a significant increase in the live birth rate with progesterone (relative risk 1.09, 95% CI 1.03 to 1.15; p = 0.003). For secondary outcomes, there was some evidence that progesterone increased the rate of ongoing pregnancy at 12 weeks (83% in the progesterone group vs. 80% in the placebo group; relative risk 1.04, 95% CI 1.01 to 1.07; p = 0.01) and reduced the rate of emergency C-sections (15% in the progesterone group vs. 19% in the placebo group; adjusted relative risk 0.80, 95% CI 0.69 to 0.94; p = 0.006); there was no evidence of a difference in the other outcomes or in the safety outcomes.
Primary outcome results
Overall, 2972 out of 4038 women (74%) experienced a live birth at ≥ 34 weeks’ gestation. The live birth rate in the progesterone group was 75% (1513/2025) and the rate in the placebo group was 72% (1459/2013) (adjusted relative risk 1.03, 95% CI 1.00 to 1.07), a difference that was not statistically significant (absolute risk difference 2.2%, 95% CI –0.4% to 5.0%; p = 0.08) (Table 7).
| Outcome | Progesterone | Placebo | RRa or mean difference,b 95% CI; p-value |
|---|---|---|---|
| Primary outcome | |||
| Live birth at ≥ 34 weeks, n/N (%) | 1513/2025 (75) | 1459/2013 (72) | 1.03, 1.00 to 1.07; p = 0.08 |
| Secondary maternal outcomes – pregnancy outcomes,c n/N (%) | |||
| Ongoing pregnancy at 12 weeks | 1672/2025 (83) | 1602/2013 (80) | 1.04, 1.01 to 1.07; p = 0.01 |
| Miscarriage at < 24 weeksd | 410/2025 (20) | 451/2013 (22) | 0.91, 0.81 to 1.01; p = 0.09 |
| Live birth at < 34 weeks | 68/2025 (3) | 64/2013 (3) | 1.06, 0.76 to 1.49; p = 0.73 |
| Ectopic pregnancy | 0/2025 (–) | 2/2013 (< 1) | – |
| Stillbirth (interuterine death at ≥ 24 weeks) | 5/2025 (< 1) | 6/2013 (< 1) | 0.82, 0.25 to 2.66; p = 0.74 |
| Terminatione | 34/2025 (2) | 36/2013 (2) | 0.94, 0.59 to 1.50; p = 0.81 |
| Secondary maternal outcomes – other outcomes (in live births at ≥ 24 weeks) | |||
| Twins,f n/N (%) | 29/1581 (2) | 22/1523 (1) | 1.28, 0.74 to 2.22; p = 0.38 |
| Mode of delivery, n/N (%) | |||
| Unassisted vaginal | 845/1577 (53) | 794/1515 (52) | 1.02,0.96 to 1.10; p = 0.39 |
| Instrumental vaginal | 224/1577 (14) | 199/1515 (13) | 1.08, 0.91 to 1.29; p = 0.37 |
| Vaginal breech delivery | 4/1577 (< 1) | 7/1515 (< 1) | 0.55, 0.16 to 1.88; p = 0.34 |
| Elective C-section | 257/1577 (16) | 224/1515 (15) | 1.10, 0.93 to 1.29; p = 0.27 |
| Emergency C-section | 241/1577 (15) | 286/1515 (19) | 0.80, 0.69 to 0.94; p = 0.006 |
| Other | 6/1577 (< 1) | 5/1515 (< 1) | – |
| Missing | 4 (–) | 8 (–) | – |
| Secondary neonatal outcomes (in live births at ≥ 24 weeks) | |||
| Gestation at delivery, weeks [mean (SD), n]g | 38 +4 (2 +4), 1581 | 38 +4 (2 +3), 1521 | 0.11, –0 +1 to 0 +2; p = 0.21 |
| Gestation at delivery | |||
| < 28 weeks, n/N (%) | 19/1581 (1) | 14/1521 (1) | 1.33, 0.67 to 2.65; p = 0.42 |
| < 32 weeks, n/N (%) | 42/1581 (3) | 36/1521 (2) | 1.15, 0.74 to 1.78; p = 0.54 |
| < 37 weeks, n/N (%) | 263/1581 (17) | 235/1521 (15) | 1.07, 0.91 to 1.25; p = 0.42 |
| Birthweight, grams [mean (SD), n]h | 3242 (656), 1604 | 3261 (659), 1539 | –21, –67 to 25; p = 0.37 |
| Birthweight adjusted for gestational age and sex (using intergrowthi standards), centiles [mean (SD), n] | 61.6 (28.2), 1599 | 61.6 (28.2), 1537 | –0.21, –2.16 to 1.74; p = 0.84 |
| Birthweight adjusted for gestational age, sex, parity, maternal BMI and ethnicity (using GROWj standards), centiles [mean (SD), n] | 45.7 (29.4), 1603 | 45.5 (29.4), 1539 | 0.12, –1.91 to 2.15; p = 0.91 |
| Small for gestational age and sex (using intergrowthi standards; proportion < 10th centile), n/N (%) | 78/1599 (5) | 98/1537 (6) | 0.77, 0.57 to 1.03; p = 0.07 |
| Small for gestational age, sex, parity, maternal BMI and ethnicity (using GROWj standards; proportion < 10th centile), n/N (%) | 214/1603 (13) | 199/1539 (13) | 1.02, 0.85 to 1.22; p = 0.81 |
| Large for gestational age and sex (using intergrowthi standards; proportion ≥ 90th centile), n/N (%) | 308/1599 (19) | 295/1537 (19) | 1.01, 0.88 to 1.17; p = 0.86 |
| Large for gestational age, sex, parity, maternal BMI and ethnicity (using GROWi standards; proportion ≥ 90th centile), n/N (%) | 153/1603 (10) | 140/1539 (9) | 1.03, 0.83 to 1.28; p = 0.77 |
| Apgar score at 1 minute [median (IQR), n] | 9 (9–9), 1533 | 9 (9–9), 1477 | 0.05, –0.06 to 0.15; p = 0.37 |
| Apgar score at 5 minutes [median (IQR), n] | 10 (9–10), 1532 | 10 (9–10), 1478 | 0.05, –0.02 to 0.13; p = 0.15 |
| Arterial cord pH [mean (SD), n] | 7.2 (0.1), 474 | 7.2 (0.1), 464 | 0.003, –0.01 to 0.02; p = 0.59 |
| Venous cord pH [mean (SD), n] | 7.3 (0.1), 505 | 7.3 (0.1), 495 | 0.003, –0.01 to 0.01; p = 0.55 |
| Death at 28 days of neonatal life,k n/N (%) | 8/1605 (1) | 2/1533 (< 1) | 3.84, 0.80 to 18.40; p = 0.09 |
Secondary outcome results
Secondary maternal outcome: pregnancy outcomes
There was evidence to suggest that progesterone increased the rate of ongoing pregnancy at 12 weeks: 1672 out of 2025 (83%) women in the progesterone group and 1602 out of 2013 (80%) in the placebo group remained pregnant at 12 weeks (adjusted relative risk 1.04, 95% CI 1.01 to 1.07; p = 0.01). However, there was no convincing evidence of a reduction in the number of miscarriages, with 410 out of 2025 (20%) women in the progesterone group and 451 out of 2013 (22%) in the placebo group experiencing a miscarriage (adjusted relative risk 0.91, 95% CI 0.81 to 1.01; p = 0.09). The median gestational age at the time of miscarriage was 8 weeks (IQR 7–10 weeks) for both groups. Time from conception to the end of pregnancy for any reason is graphically displayed in Figure 7.
Other secondary maternal outcomes
Twenty-nine women in the progesterone group and 22 women in the placebo group gave birth to twins (see Table 7). There was evidence to suggest that women in the progesterone group were less likely to deliver via emergency C-section (15% in the progesterone group vs. 19% in the placebo group; adjusted relative risk 0.80, 95% CI 0.69 to 0.94; p = 0.006). The results of other secondary maternal outcomes appeared similar in both groups, with no significant differences.
Secondary neonatal outcomes
Overall, the distribution of gestational age at delivery in those women with a live birth was very similar in both groups. Live births were delivered at 38 + 4 weeks on average in both groups. There were 498 (16%) preterm births (< 37 weeks) observed, but the numbers were very similar in both groups (17% in the progesterone group vs. 15% in the placebo group; adjusted relative risk 1.07, 95% CI 0.91 to 1.25; p = 0.42). Birthweights appeared similar across both groups (mean difference –21 g, 95% CI –67 to 25 g; p = 0.37), with no evidence of any differences in the numbers of infants being large or small for their gestational age (plus other covariates listed in Table 7). No differences were noted in other outcomes. Eight neonatal deaths were observed by 28 days in the progesterone group, compared with two in the placebo group (adjusted relative risk 3.84, 95% CI 0.80 to 18.40; p = 0.09).
Pregnancy-related complications
Complication rates of antenatal, intrapartum, postpartum and neonatal complications appeared similar for both groups (Table 8). The denominators throughout Table 8 differ across each outcome as they are based on the number of completed responses for that relevant outcome.
| Type of complication | Progesterone | Placebo | RR,a 95% CI; p-value |
|---|---|---|---|
| Maternal antenatal complications (all women randomised), n/N (%) | |||
| Pregnancy-induced hypertension | 46/2019 (2) | 56/2005 (3) | 0.82, 0.56 to 1.21; p = 0.31 |
| Pre-eclampsia | 27/2019 (1) | 43/2005 (2) | 0.63, 0.39 to 1.01; p = 0.06 |
| Obstetric cholestasis | 24/2019 (1) | 27/2005 (1) | 0.89, 0.51 to 1.53; p = 0.67 |
| Cervical cerclargeb | 10/2019 (< 1) | 16/2005 (1) | 0.61, 0.28 to 1.34; p = 0.22 |
| Preterm (< 37 weeks) pre-labour rupture of membranes | 120/2019 (6) | 118/2005 (6) | 1.02, 0.80 to 1.30; p = 0.88 |
| Gestational diabetes mellitus by GTT | 114/2019 (6) | 103/2005 (5) | 1.10, 0.85 to 1.42; p = 0.48 |
| Hyperemesis gravidarum | 36/2019 (2) | 45/2005 (2) | |
| Uterine artery abnormalityc | 7/206 (3) | 11/205 (5) | |
| Umbilical artery raised resistanced | 7/600 (1) | 9/608 (1) | |
| Absent umbilical artery end-diastolic flowd | 2/600 (< 1) | 2/608 (< 1) | |
| Threatened preterm (< 37 weeks) birth requiring tocolysis or steroids | 80/2019 (4) | 84/2005 (4) | |
| Antepartum haemorrhage | 101/2019 (5) | 99/2005 (5) | |
| Psychological conditions | 117/2019 (6) | 120/2005 (6) | |
| Intrapartum complications, n/N (%) | |||
| Chorioamnionitis | 10/1581 (1) | 12/1523 (1) | 0.81, 0.35 to 1.89; p = 0.63 |
| Intrauterine growth restriction | 45/1581 (3) | 42/1523 (3) | 1.03, 0.68 to 1.56; p = 0.88 |
| Macrosomia | 16/1581 (1) | 9/1523 (1) | 1.68, 0.75 to 3.78; p = 0.21 |
| Cord prolapse | 3/1581 (< 1) | 3/1523 (< 1) | |
| Placenta praevia | 7/1581 (< 1) | 12/1523 (1) | |
| Non-reassuring cardiotocography | 194/1581 (12) | 212/1523 (14) | |
| Fetal blood sampling | 12/1581 (1) | 24/1523 (2) | |
| Suspected abruption | 10/1581 (1) | 19/1523 (1) | |
| Failure to progress | 125/1581 (8) | 116/1523 (8) | |
| Abnormal presentation | 45/1581 (3) | 50/1523 (3) | |
| Hypertension/pre-eclampsia | 42/1581 (3) | 37/1523 (2) | |
| Psychosocial problems | 7/1581 (< 1) | 3/1523 (< 1) | |
| Failed induction | 10/1581 (1) | 18/1523 (1) | |
| Meconium | 88/1581 (6) | 86/1523 (6) | |
| Antepartum haemorrhage | 33/1581 (2) | 42/1523 (3) | |
| Uterine rupture | 0/1581 (–) | 0/1523 (–) | |
| Maternal postpartum complications, n/N (%) | |||
| Haemorrhage | 180/1574 (11) | 186/1512 (12) | 0.92, 0.76 to 1.12; p = 0.42 |
| Pre-eclampsia/eclampsia/HELLP | 11/1574 (1) | 10/1512 (1) | |
| Infection | 47/1574 (3) | 39/1512 (3) | |
| Admission to HDU | 69/1569 (4) | 79/1509 (5) | |
| Admission to ITU | 4/1569 (< 1) | 0/1509 (–) | |
| Neonatal complications, n/N (%) | |||
| Discharge to hospital | 16/1562 (1) | 20/1511 (1) | 0.77, 0.39 to 1.52; p = 0.45 |
| Early infection | 72/1595 (5) | 78/1524 (5) | 0.83, 0.60 to 1.14; p = 0.25 |
| Retinopathy of prematurity | 1/1595 (< 1) | 2/1525 (< 1) | 0.48, 0.04 to 5.27; p = 0.55 |
| Necrotising enterocolitis | 4/1594 (< 1) | 8/1525 (1) | 0.48, 0.14 to 1.59; p = 0.23 |
| Intraventricular haemorrhage | 2/1594 (< 1) | 1/1525 (< 1) | 1.91, 0.17 to 21.08; p = 0.60 |
| Congenital abnormalities | 53/1574 (3) | 51/1511 (3) | 1.00, 0.69 to 1.47; p = 0.99 |
| Chromosomal or genetic abnormalities | 10/1576 (1) | 9/1513 (1) | 1.07, 0.43 to 2.62; p = 0.89 |
| Respiratory distress syndrome | 67/1547 (4) | 64/1481 (4) | 0.97, 0.68 to 1.37; p = 0.85 |
| Ventilation or oxygen support | 35/1596 (2) | 47/1525 (3) | 0.71, 0.46 to 1.10; p = 0.13 |
| Severe cranial ultrasound abnormality | 4/1595 (< 1) | 8/1525 (1) | |
| Periventricular leukomalacia | 1/1594 (< 1) | 2/1525 (< 1) | |
| Bell stage 2 or 3 | 0/1594 (–) | 3/1525 (< 1) | |
| Septic screening within 48 hours | 169/1594 (11) | 171/1524 (11) | |
Safety data
The number of SAEs was similar in both groups: 105 out of 2025 (5%) compared with 98 out of 2013 (5%), in the progesterone and placebo groups, respectively (Table 9). The SAEs were categorised in body systems as detailed in Table 10.
| Serious adverse event | Progesterone, n/N (%) | Placebo, n/N (%) | RR, 95% CI; p-value |
|---|---|---|---|
| Total number of participants experiencing a SAE (either maternal or neonatal) | 105/2025 (5) | 98/2013 (5) | 1.07, 0.81 to 0.39; p = 0.65 |
| Total number of SAEs | 133/2025 (7) | 126/2013 (6) | |
| Maternal | 83/2025 (4) | 83/2013 (4) | |
| Neonatal | 50/2025 (2) | 43/2013 (2) |
| Category | Progesterone, n | Placebo, n |
|---|---|---|
| Maternal | ||
| Bone and joint injuries | 2 | 0 |
| Cardiac disorders | 1 | 2 |
| Congenital, familial and genetic disorders | 3 | 0 |
| Endocrine disorders | 1 | 2 |
| Eye disorders | 1 | 0 |
| Gastrointestinal disorders | 0 | 1 |
| General disorders and administration site conditions | 1 | 6 |
| Infections and infestations | 2 | 4 |
| Metabolism and nutrition disorders | 0 | 1 |
| Nervous system disorders | 1 | 2 |
| Pregnancy, puerperium and perinatal conditions | 46 | 46 |
| Renal and urinary disorders | 3 | 2 |
| Reproductive system and breast disorders | 11 | 9 |
| Respiratory, thoracic and mediastinal disorders | 10 | 7 |
| Skin and subcutaneous tissue | 0 | 1 |
| Vascular disorders | 1 | 0 |
| Total | 83 | 83 |
| Neonatal | ||
| Congenital, familial and genetic disorders | 23 | 22 |
| Gastrointestinal disorders | 0 | 1 |
| General disorders and administration site conditions | 1 | 3 |
| Metabolism and nutrition disorders | 2 | 1 |
| Nervous system disorders | 0 | 1 |
| Pregnancy, puerperium and perinatal conditions | 17 | 11 |
| Renal and urinary disorders | 1 | 3 |
| Reproductive system and breast disorders | 1 | 0 |
| Respiratory, thoracic and mediastinal disorders | 3 | 1 |
| Skin and subcutaneous tissue | 1 | 0 |
| Vascular disorders | 1 | 0 |
| Total | 50 | 43 |
Ancillary analyses
Sensitivity analyses
A sensitivity analysis assuming that all missing responses were treatment failures for live birth at ≥ 34 weeks was statistically significant (adjusted relative risk 1.04; 95% CI 1.00 to 1.08; p = 0.04), but not when missing responses were simulated using multiple imputation. An analysis carried out where the dating scan was prioritised over the randomisation scan when calculating gestational age had no effect on the output (Table 11).
| Outcome | Progesterone | Placebo | RR,a 95% CI; p-value |
|---|---|---|---|
| Live birth at ≥ 34 weeks, n/N (%) | |||
| Sensitivity analysis: assume all missing responses are treatment failures | 1513/2079 (73) | 1459/2074 (70) | 1.04, 1.00 to 1.08; p = 0.04 |
| Dating scan prioritised over randomisation scan when calculating gestational age | 1517/2025 (75) | 1463/2013 (73) | 1.03, 1.00 to 1.07; p = 0.08 |
| Simulate missing responses using multiple imputation | – | – | 1.03, 1.00 to 1.07; p = 0.07 |
| Miscarriage at < 24 weeks, n/N (%) | |||
| Sensitivity analysis: assume all missing responses are treatment failures | 464/2079 (22) | 512/2074 (25) | 0.90, 0.81 to 1.01; p = 0.07 |
| Dating scan prioritised over randomisation scan when calculating gestational age | 410/2025 (20) | 451/2013 (22) | 0.91, 0.81 to 1.01; p = 0.09 |
| Simulate missing responses using multiple imputation | – | – | 0.90, 0.79 to 1.01; p = 0.07 |
Subgroup analyses
The output from the subgroup analyses for the primary outcome of live birth at ≥ 34 weeks can be seen in Table 12 and Figures 7 and 8. All tests for subgroup by treatment group interaction were non-significant apart from number of previous miscarriages. Here, there was evidence that the number of live births was higher in the progesterone group than in the placebo group (72% in the progesterone group vs. 57% in the placebo group; relative risk 1.28, 95% CI 1.08 to 1.51; p = 0.007) for those who had three or more previous miscarriages. Further post hoc subgroup analysis on the number of previous miscarriages, where the subgroup was split into none compared with ≥ 1 previous miscarriage, suggested that progesterone was effective in those who had ≥ 1 previous miscarriage (75% in the progesterone group vs. 70% in the placebo group; relative risk 1.09; 95% CI 1.03 to 1.15; p = 0.01).
| Subgroup | Progesterone, n/N (%) | Placebo, n/N (%) | RR,a 95% CI; p-value | Interaction p-value |
|---|---|---|---|---|
| Live birth at ≥ 34 weeks | ||||
| Maternal age (years) | ||||
| < 35 | 1184/1558 (76) | 1148/1555 (74) | 1.03, 0.99 to 1.07; p = 0.16 | p = 0.70 |
| ≥ 35 | 329/467 (70) | 311/458 (68) | 1.05, 0.97 to 1.13; p = 0.27 | |
| BMI (kg/m2) | ||||
| < 30 | 1155/1548 (75) | 1128/1541 (73) | 1.02, 0.98 to 1.06; p = 0.28 | p = 0.31 |
| ≥ 30 | 358/477 (75) | 331/472 (70) | 1.07, 0.99 to 1.15; p = 0.09 | |
| Fetal heart activity | ||||
| Present | 1347/1672 (81) | 1304/1648 (79) | 1.02, 0.99 to 1.06; p = 0.18 | p = 0.37 |
| Absent | 166/353 (47) | 155/365 (42) | 1.10, 0.94 to 1.30; p = 0.23 | |
| Estimated gestational age at presentation | ||||
| < 6 weeks | 209/358 (58) | 202/365 (55) | 1.04, 0.91 to 1.18; p = 0.59 | p = 0.13 |
| ≥ 6 weeks to < 9 weeks | 917/1211 (76) | 832/1162 (72) | 1.06, 1.01 to 1.10; p = 0.02 | |
| ≥ 9 weeks | 387/456 (85) | 425/486 (87) | 0.98, 0.94 to 1.03; p = 0.53 | |
| Amount of bleeding on PBAC20 | ||||
| ≤ 2 | 1396/1860 (75) | 1344/1853 (73) | 1.03, 1.00 to 1.07; p = 0.06 | p = 0.61 |
| ≥ 3 | 117/165 (71) | 115/160 (72) | 1.00, 0.88 to 1.14; p = 0.99 | |
| Number of previous miscarriages | ||||
| 0 | 824/1111 (74) | 840/1127 (75) | 0.99, 0.95 to 1.04; p = 0.72 | p = 0.007 |
| 1/2 | 591/777 (76) | 534/738 (72) | 1.05, 1.00 to 1.12; p = 0.07 | |
| ≥ 3 | 98/137 (72) | 85/148 (57) | 1.28, 1.08 to 1.51; p = 0.004 | |
| Post hoc number of previous miscarriages | ||||
| 0 | 824/1111 (74) | 840/1127 (75) | 0.99, 0.95 to 1.04; p = 0.72 | p = 0.01 |
| ≥ 1 | 689/914 (75) | 619/886 (70) | 1.09, 1.03 to 1.15; p = 0.003 | |
| Number of gestational sacs | ||||
| 1 | 1480/1971 (75) | 1432/1975 (73) | 1.04, 1.00 to 1.07; p = 0.05 | p = 0.34 |
| ≥ 2 | 33/54 (61) | 27/38 (71) | 0.90, 0.69 to 1.19; p = 0.47 | |
| Ethnicity | ||||
| White | 1263/1673 (75) | 1245/1697 (73) | 1.03, 1.00 to 1.07; p = 0.08 | p = 0.87 |
| Black | 58/82 (71) | 46/75 (61) | 1.08, 0.87 to 1.35; p = 0.47 | |
| South Asian | 76/113 (67) | 69/101 (68) | 0.97, 0.82 to 1.15; p = 0.74 | |
| Other | 116/157 (74) | 99/140 (71) | 1.04, 0.91 to 1.19; p = 0.56 | |
| History of polycystic ovaries (yes/no) | ||||
| Yes | 157/220 (71) | 161/221 (73) | 0.98, 0.88 to 1.10; p = 0.74 | p = 0.35 |
| No | 1356/1805 (75) | 1297/1791 (72) | 1.04, 1.00 to 1.08; p = 0.04 | |
| Previous LLETZ | ||||
| Yes | 80/109 (73) | 68/102 (67) | 1.12, 0.95 to 1.33; p = 0.17 | p = 0.30 |
| No | 1433/1916 (75) | 1391/1911 (73) | 1.03, 0.99 to 1.06; p = 0.14 | |
FIGURE 8.
Post hoc subgroup analyses. The results are reported as point estimates and 95% CIs. The widths of the CIs have not been adjusted for multiplicity, so the intervals should not be used to infer definitive treatment effects.
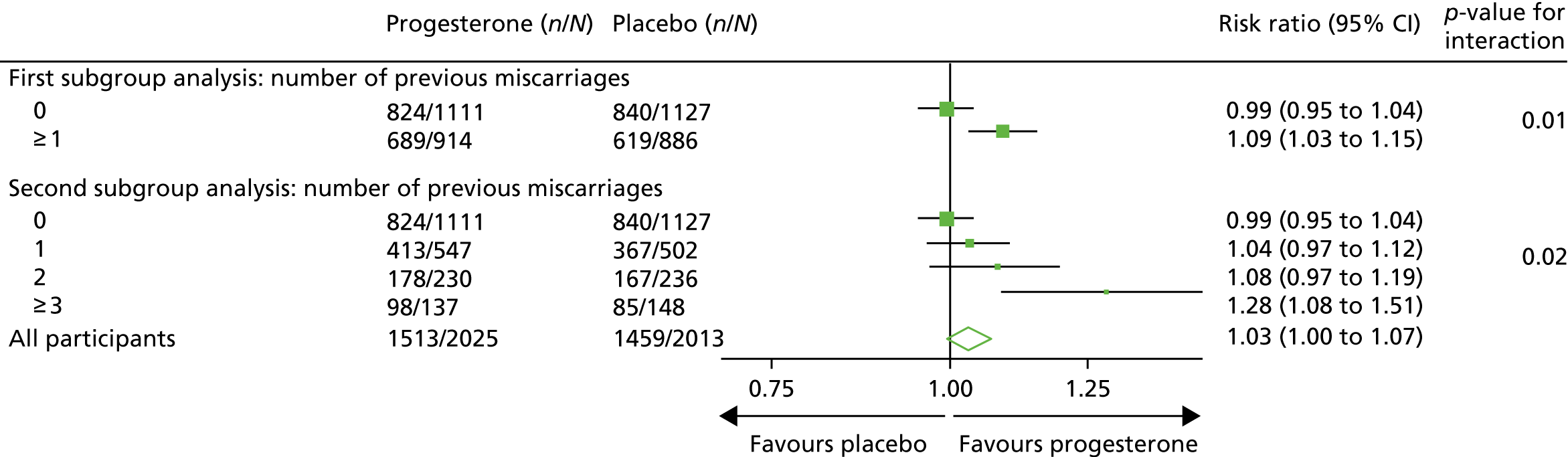
Subgroup analyses for the outcome of miscarriage (at < 24 weeks) can be seen in Table 13. The results were similar to the primary outcome subgroup analyses, with the only evidence of a differential effect observed in the subgroup of number of previous miscarriages. Here, in those with three or more previous miscarriages, there was some evidence that progesterone was effective (23% in the progesterone group vs. 36% in the placebo group; relative risk 0.58, 95% CI 0.40 to 0.83; p = 0.003).
| Subgroup | Progesterone, n/N (%) | Placebo, n/N (%) | RR,a 95% CI; p-value | Interaction p-value |
|---|---|---|---|---|
| Miscarriage at < 24 weeks | ||||
| Maternal age (years) | ||||
| < 35 | 297/1558 (19) | 329/1555 (21) | 0.92, 0.80 to 1.05; p = 0.19 | p = 0.80 |
| ≥ 35 | 113/467 (24) | 122/458 (27) | 0.89, 0.72 to 1.09; p = 0.25 | |
| BMI (kg/m2) | ||||
| < 30 | 315/1548 (20) | 331/1541 (21) | 0.95, 0.83 to 1.08; p = 0.39 | p = 0.20 |
| ≥ 30 | 95/477 (20) | 120/472 (25) | 0.80, 0.64 to 1.00; p = 0.05 | |
| Fetal heart activity | ||||
| Present | 237/1672 (14) | 260/1648 (16) | 0.88, 0.75 to 1.03; p = 0.11 | p = 0.51 |
| Absent | 173/353 (49) | 191/365 (52) | 0.95, 0.81 to 1.10; p = 0.48 | |
| Estimated gestational age at presentation | ||||
| < 6 weeks | 136/358 (38) | 143/365 (39) | 1.01, 0.83 to 1.21; p = 0.96 | p = 0.18 |
| ≥ 6 weeks to < 9 weeks | 237/1211 (20) | 274/1162 (24) | 0.83, 0.72 to 0.96; p = 0.01 | |
| ≥ 9 weeks | 37/456 (8) | 34/486 (7) | 1.10, 0.71 to 1.69; p = 0.68 | |
| Amount of bleeding on PBAC20 | ||||
| ≤ 2 | 374/1860 (20) | 413/1853 (22) | 0.91, 0.81 to 1.02; p = 0.11 | p = 0.90 |
| ≥ 3 | 36/165 (22) | 38/160 (24) | 0.89, 0.61 to 1.28; p = 0.52 | |
| Number of previous miscarriages | ||||
| 0 | 229/1111 (21) | 244/1127 (22) | 0.96, 0.82 to 1.11; p = 0.55 | p = 0.03 |
| 1/2 | 150/777 (19) | 154/738 (21) | 0.95, 0.78 to 1.15; p = 0.57 | |
| ≥ 3 | 31/137 (23) | 53/148 (36) | 0.58, 0.40 to 0.83; p = 0.003 | |
| Number of gestational sacs | ||||
| 1 | 393/1971 (20) | 442/1975 (22) | 0.90, 0.80 to 1.01; p = 0.07 | p = 0.57 |
| ≥ 2 | 17/54 (31) | 9/38 (24) | 1.08, 0.57 to 2.05; p = 0.81 | |
| Ethnicity | ||||
| White | 337/1673 (20) | 370/1697 (22) | 0.92, 0.81 to 1.04; p = 0.16 | p = 0.67 |
| Black | 20/82 (24) | 20/75 (27) | 1.11, 0.65 to 1.88; p = 0.71 | |
| South Asian | 26/113 (23) | 28/101 (28) | 0.89, 0.58 to 1.35; p = 0.57 | |
| Other | 27/157 (17) | 33/140 (24) | 0.73, 0.47 to 1.13; p = 0.16 | |
| History of polycystic ovaries (yes/no) | ||||
| Yes | 43/220 (20) | 50/221 (23) | 0.86, 0.61 to 1.22; p = 0.40 | p = 0.76 |
| No | 367/1805 (20) | 401/1791 (22) | 0.91, 0.81 to 1.03; p = 0.13 | |
| Previous LLETZ | ||||
| Yes | 21/109 (19) | 27/102 (26) | 0.63, 0.40 to 1.00; p = 0.05 | p = 0.12 |
| No | 389/1916 (20) | 424/1911 (22) | 0.93, 0.83 to 1.04; p = 0.19 | |
Chapter 4 Health economic analysis
Introduction
The economic evaluation conducted alongside the PRISM trial is reported in this chapter. The primary objective of the trial was to investigate whether or not progesterone used by women with bleeding in early pregnancy (up to 16 weeks of gestation) can prevent miscarriage and lead to live births at ≥ 34 weeks of pregnancy. The overall aim of the economic evaluation was to assess the relative cost-effectiveness of progesterone compared with placebo in these women.
Methods
A within-trial incremental cost-effectiveness analysis (CEA) was performed from the perspective of the NHS and the NHS/PSS. 44 The CEA results are expressed in terms of cost per additional live birth at ≥ 34 completed weeks of gestation. Given that the duration of the trial was less than 1 year, neither costs nor outcomes were discounted. The health economic analysis was reported following the Consolidated Health Economic Evaluation Reporting Standards (CHEERS). 45
Outcomes
The primary outcome of the CEA was live births at ≥ 34 completed weeks of gestation based on the principal outcome of the clinical trial. Gestational age was estimated based on participants’ ultrasonography result and 11–14 weeks, when available, or otherwise based on the ultrasonography at randomisation. An additional outcome of the PRISM trial was neonatal survival at 28 days post partum, and this was explored as a secondary outcome in the CEA.
Data
Resource use and costs
Data on all resources consumed in the hospital setting by each woman from randomisation to hospital discharge were collected prospectively using researcher-recorded trial collection forms. The use of other services provided by the community during the same period was captured retrospectively via health services self-completed questionnaires. Unit costs for each resource item (Table 14) were identified from established national sources. 47,50 All costs were expressed in 2017–18 Great British pounds. Cost estimates from earlier years were inflated to 2017–18 prices using the Hospital and Community Health Services (HCHS) pay and prices index. 50 Hospital-related unit costs values were obtained from the NHS Reference Costs 2016/1747 where available. Otherwise, these costs were obtained from reference costs for earlier years or from other sources, such as the Personal Social Services Research Unit (PSSRU) costs. In cases where there are different categories associated with a resource use, weighted averages were used (see Table 14).
| Resource use items | Unit cost (£)a | Sourceb |
|---|---|---|
| Intervention | ||
| Progesterone (Utrogestan®) 200 mg | 4 | BNF 201846 |
| Antenatal period | ||
| Antenatal hospital visit | 468 | NHS Reference Costs 2016/1747 (NZ16Z) |
| Antenatal DAU | 125 | NHS Reference Costs 2016/1747 (NZ22Z) |
| Emergency visit | 118 | NHS Reference Costs 2013/1448 (NZ23Z) |
| Inpatient admission (< 24 hours) | 303 | NHS Reference Costs 2016/1747 (NZ20B) |
| Night of patient admission | 395 | PSSRU 200249 |
| Delivery mode | ||
| Unassisted vaginal delivery (without complications) | 1840 | NHS Reference Costs 2016/1747 (NZ30C) |
| Unassisted vaginal delivery (with complications) | 2187 | NHS Reference Costs 2016/1747 (NZ30A NZ30B) |
| Instrumental vaginal delivery (without complications) | 2302 | NHS Reference Costs 2016/1747 (NZ40C) |
| Instrumental vaginal delivery (with complications) | 2446 | NHS Reference Costs 2016/1747 (NZ40A NZ40B) |
| Elective C-section (without complications) | 3257 | NHS Reference Costs 2016/1747 (NZ50C) |
| Elective C-section (with complications) | 4079 | NHS Reference Costs 2016/1747 (NZ50A NZ50B) |
| Emergency C-section (without complications) | 4378 | NHS Reference Costs 2016/1747 (NZ51C) |
| Emergency C-section (with complications) | 5678 | NHS Reference Costs 2016/1747 (NZ51A NZ51B) |
| Vaginal breech delivery (without complications) | 1840 | NHS Reference Costs 2016/1747 (NZ30C) |
| Vaginal breech delivery (with complications) | 2187 | NHS Reference Costs 2016/1747 (NZ30A NZ30B) |
| Management | ||
| Spontaneous resolution | 619 | NHS Reference Costs 2016/1747 (MB08B) |
| Surgical management | 1880 | NHS Reference Costs 2016/1747 (MB08A) |
| Medical management | 1880 | NHS Reference Costs 2016/1747 (MB08A) |
| Postnatal period | ||
| Admission to HDU (level 2 care) | 965 | NHS Reference Costs 2016/1747 (XC06Z) |
| Admission to ITU (level 3 care) | 1586 | NHS Reference Costs 2016/1747 (XC01Z to XC05Z) |
| Hospital visit | 145 | PSSRU 200249 |
| Day assessment unit | 125 | NHS Reference Costs 2016/1747 (NZ22Z) |
| Emergency visit | 98 | NHS Reference Costs 2016/1747 (VB09Z VB11Z) |
| Inpatient admissions (< 24 hours) | 299 | NHS Reference Costs 2016/1747 (NZ26B) |
| Night of inpatient admissions | 395 | PSSRU 200249 |
| Neonatal care | ||
| Neonatal intensive care | 1318 | NHS Reference Costs 2016/1747 (XA01Z) |
| Neonatal high-dependency care | 913 | NHS Reference Costs 2016/1747 (XA02Z) |
| Neonatal special care | 514 | NHS Reference Costs 2016/1747 (XA03Z to XA04Z) |
| Primary care services (contacts) | ||
| GP visits | 39 | PSSRU 201750 |
| Practice/community midwife | 30 | PSSRU 201551 |
| Practice nurse visits | 9.5 | PSSRU 201750 |
| Psychologist (or counsellor) visits | 20 | PSSRU 201750 |
| Health visitor visits | 22 | PSSRU 201551 |
| Social worker visits | 20 | PSSRU 201750 |
| Number of other community services | 21 | PSSRU 201750 |
Resource use data within the hospital setting (inpatient and outpatient) focused on the:
-
quantity of progesterone administered
-
antenatal period
-
intrapartum period
-
postnatal period (maternal and neonatal).
Data were collected for primary care resources such as:
-
contacts with GPs
-
contacts with community midwives.
Progesterone
The quantity of progesterone vaginal pessaries was calculated based on the number of days they were used from randomisation until the end of 16 weeks of gestation (or earlier if miscarriage occurred before 16 weeks). The cost of progesterone was identified from the BNF46 as £21 for a 21-pessary pack. In the trial, each woman used four pessaries daily, which is equivalent to a cost of £4 per day (see Table 14).
Antenatal period
For the antenatal period, resource use data were collected on the number of hospital, day assessment unit (DAU) and emergency visits as well as the number of inpatient day admissions (for a stay of < 24 hours) and nights of inpatient admissions. Based on the descriptions in the trial literature, the costs of antenatal hospital (routine observation) and DAU (antenatal specialised non-routine ultrasound scan) visits were obtained from the NHS Reference Costs 2016/17. 47
For inpatient day admission (< 24 hours), this was described in the trial as a day-case management of an antenatal disorder. 47 Emergency visit (antenatal diagnostic procedures) costs were provided from the NHS Reference Costs 2013 to 2014,48 whereas inpatient night admissions costs were obtained from an earlier PSSRU cost. 49 Because all participants in the trial underwent routine ultrasonography at specified times in the study, the cost of an ultrasound was not included in the analysis.
Intrapartum period
The resource use collected at the end of pregnancy varied depending on whether or not the baby was born alive. Where live births occurred, the onset of labour was recorded as spontaneous, augmented, pre-labour C-section or induced. Information on the mode of delivery included unassisted vaginal deliveries, instrumental vaginal deliveries, elective C-sections, emergency C-sections or vaginal breech deliveries with or without intrapartum complications. Deliveries such as water births and home deliveries were captured as ‘others’.
The Healthcare Resource Group (HRG) unit costs47 for delivery mode are categorised as normal vaginal delivery, assisted vaginal delivery, planned C-section and emergency C-section, corresponding to unassisted vaginal delivery, instrumental vaginal delivery, elective C-section and emergency C-section on the case report form (CRF), respectively. Each category is grouped further as with or without complications. Weighted averages of the unit costs for the different levels of complications were calculated. As there were no HRG unit costs available for breech delivery or ‘other’, in consultation with the clinical team, we assumed that the costs were the same as those for a normal vaginal delivery. For labour onset, we assumed that these costs were already accounted for by the delivery mode and, hence, these were not costed separately.
Where babies were not born alive, the outcome was recorded as miscarriage, ectopic pregnancy, termination or stillbirth. The management of such outcomes was recorded as spontaneous resolution, surgical management or medical management. The costs of management were provided in the NHS Reference Costs as threatened or spontaneous miscarriage with intervention (medical or surgical management) and without intervention (spontaneous resolution). 47
Postnatal period
During the postnatal period, data were collected for both maternal and neonatal resource use, from pregnancy end to 28 days post discharge.
Maternal resource use
The unit costs for hospital visits, DAU visits, emergency visits and others that applied to the antenatal period were also relevant to the postnatal period resource use; relevant unit costs are presented in Table 14. For postnatal DAU visits and inpatient admissions, the corresponding costs for the antenatal indices were used. The cost of a postnatal emergency visit was obtained using a weighted average of emergency medicine for patients requiring category 0–2 treatment and category 0–1 investigation. 47 Postnatal hospital visit costs were obtained from the PSSRU. 49
Data collected for immediate maternal postnatal care resource use included admissions to a HDU (level 2 care) or an ITU (level 3 care). Using the UK definitions for level 2 care (patient receiving a single organ support) and level 3 care (patient receiving at least two organ supports),53 the unit costs for these admissions were obtained from the NHS Reference Costs 2016/17. 47 For level 2 care (HDU admissions), ‘adult critical care – one organ supported’ was used; for level 3 care (ITU admissions), a weighted average was taken across five HRGs [adult critical care (two organs supported) to adult critical care (six or more organs supported)].
Neonatal resource use
The immediate neonatal care resource use included the number of nights receiving intensive care, high-dependency care and special care. Costs for these resources were obtained from the costs schedule. 47
Serious adverse events
Information on SAEs was collected using SAE forms. In this study, a SAE was defined as an untoward event resulting in maternal death, stillbirth, hospitalisation, persistent or significant disability, congenital anomaly or birth defect. Only clinically specified SAEs deemed to have arisen from the trial intervention were considered to be relevant to the economic analysis. Because there were no SAEs that were clearly related to the use of progesterone, we did not include such costs in the analysis.
Primary care services
Service use questionnaires, completed by a subsample of women, captured data on primary care service resource use in the trial period. These included the number of visits to the GP, practice/community midwife, practice nurse, psychologist (or counsellor), health visitor, social worker and other community services. However, these services were recorded as the number of visits without a specified duration.
The costs for primary care services were obtained from the Unit Costs of Health and Social Care 2017. 50 To cost each primary care resource use, we used the recommended average duration of 9.22 minutes for a GP face-to-face visit, 30 minutes for a midwife visit, 15.5 minutes for a GP nurse visit and 20 minutes for the remaining variables. Because no telephone contacts with the GP or practice nurse were recorded, we did not include these costs.
Primary economic analysis
A within-trial incremental CEA was conducted to estimate the relative costs and benefits of progesterone compared with placebo. The cost-effectiveness of progesterone was expressed in terms of the cost per additional live birth after ≥ 34 complete weeks of gestation. The base-case primary analysis focused on the hospital-related (inpatient and outpatient) costs for the participants incurred in the trial period.
Using study-specific resource use and costs, the total cost over the trial period was calculated by multiplying the resource items used by the corresponding unit cost and adding up all items. The mean total costs and mean total resource use for participants across the trial arms were calculated. Given the skewness inherent in most cost data and the concern of economic analyses with mean costs, we calculated 95% CIs around mean differences through the analyses of 1000 resamples using the bias-corrected and accelerated (BCa) bootstrap method. 54
To explore heterogeneity in the trial population, multivariate cost analyses were performed using seemingly unrelated regressions. 55,56 Seemingly unrelated regression has been shown to be robust to skewed data and allows for a correlation in the error terms between costs and outcomes. 57 Model covariates included baseline data on age, BMI, the quantity of bleeding and the number of previous miscarriages. The selection of covariates was informed by the prognostic variables used by the clinical team. All results were presented as mean values with SD and, where applicable, as mean differences in costs and effects with 95% CIs.
Incremental cost-effectiveness ratios (ICERs) were calculated by dividing the difference in mean total cost between the trial arms by the difference in the number of live births at ≥ 34 weeks. The ICER is a measure that depicts the additional cost ascribed to an additional effect. To calculate ICERs, the formula below was used, with C representing cost and E representing effects:
To quantify the uncertainty that typically occurs as a result of variations in sampling, we used non-parametric bootstrapping to resample the joint distribution in the mean cost and outcome difference. 58 This generated 5000 paired estimates of incremental costs and outcomes, which were plotted in a cost-effectiveness plane as a scatterplot. 59 A cost-effectiveness plane is a four-quadrant plane depicting bootstrap estimates. Based on the location of the scatterplot dots on the quadrant, an intervention may be deemed more effective and less costly (south-east), more effective and more costly (north-east), less effective and more costly (north-west) or less effective and less costly (south-west) than the alternative intervention.
A cost-effectiveness acceptability curve (CEAC) was constructed to show the probability that progesterone is a cost-effective intervention compared with placebo across a range of values, representing the decision-maker’s willingness to pay (WTP) for an additional benefit. 44 Currently, there is no standard valuation for an additional live birth. 2,60 In the UK, NICE typically uses a WTP threshold of £20,000–30,000 per quality-adjusted life-year (QALY) in approving a health-care intervention. 44
Secondary economic analyses
Secondary analyses involving ICER calculations on the primary outcome are based on the following costs.
-
Hospital-related costs for resource use for women only: the hospital-related costs for women included antenatal, intrapartum and postnatal care costs. We excluded the neonatal care costs accrued by infants from the total cost for this analysis to examine which aspect of cost had the largest effect on the result – the women’s cost or the neonatal care cost.
-
Hospital and primary care costs: here we added primary care costs such as GP and practice nurse visits to the hospital costs. This analysis was conducted to explore the perspective of the NHS/PSS.
-
Adjusting for missing data: the main base-case analysis was carried out on only individuals for whom there were outcome data. For this analysis, we included participants who were lost to follow-up. We assumed that all women lost to follow-up had a miscarriage and we imputed the costs for this subgroup. Missing costs were imputed using multiple imputations61 by applying chained equations with predictive mean matching across 60 imputations. 62
-
Hospital-related costs for women with three or more previous miscarriages: preliminary results suggested that there was clinical effectiveness for women with three or more previous miscarriages. Therefore, we conducted a CEA for this subgroup.
We also carried out a secondary incremental CEA based on the final end point using hospital-related costs for participants with complete data. We reported the analysis in terms of cost per additional baby who survived beyond 28 days after birth for each woman.
Sensitivity analysis
Deterministic sensitivity analyses were conducted to explore the inherent uncertainty in key assumptions and variations in the analytical methods used, and to consider the broader issue of the generalisability of the results. This involved varying some of the parameters while leaving others at their baseline value. A number of sensitivity analyses were conducted, as detailed below.
-
Fixed cost of treatment until 16 weeks of gestation. In the PRISM trial, women in early pregnancy started treatment from randomisation until 16 weeks’ gestation. If it is assumed that all women started treatment at approximately 7.4 weeks (52 days) with no miscarriage and continued treatment until 16 weeks (112 days) of gestation, this would translate to 60 days of treatment. Based on the progesterone vaginal pessary cost of £4 per day (as described above for four pessaries) and assuming that on day 1 participants used two pessaries, the expected cost of progesterone is £238 [2 + (59 × 4)]. In a real-life scenario, the intervention would be provided for the expected treatment period (from 6 to 8 weeks) until 16 weeks, and hence we explored the impact of a fixed cost of progesterone until 16 weeks of gestation.
-
Unit costs. We explored the impact of alternative cost estimates. For inpatient night of admission for both the antenatal and postnatal periods, we replaced the cost used in the primary analysis with the cost of excess bed-days (£311). 47 The cost of management of miscarriage used in the main analyses was obtained from the NHS Reference Costs. 47 However, the NICE guideline on miscarriage management3,63 provides an estimate of £1522 for medical management and £1827 for surgical management. These values have been used by other studies. 13 For the sensitivity analysis, we replaced the costs of management with these costs.
-
Primary care costs. We imputed the missing costs for primary care costs using multiple imputations61 by applying chained equations with predictive mean matching across 60 imputations. 62 All statistical analyses were performed using Stata version 14 (StataCorp LP, College Station, TX, USA).
Model-based analysis
Preliminary results showed that there was no clinically detectable effect on neonatal outcomes as a result of the PRISM trial that was a result of progesterone. This finding is in keeping with an earlier study in which women were given progesterone. 2,64 In view of this result, modelling costs and outcomes beyond the trial period was not deemed necessary.
Results
A total of 4153 women were recruited to the PRISM trial and randomised to either the progesterone (n = 2079) or the placebo (n = 2074) arm. Among the 4153 women recruited, 30 women withdrew from the trial and 85 women were lost to follow-up. Hence, the base-case primary analysis was conducted for 4038 participants: 2025 in the progesterone arm and 2013 in the placebo arm.
Outcomes
The details of the major outcomes of the trial are presented in Table 15. At the end of pregnancy, 1513 (74.72%) and 1459 (72.48%) women in the progesterone and placebo arms, respectively, had live births after 34 completed weeks of pregnancy. This translates to an effect difference of approximately 2.2% (0.022, 95% CI –0.004 to 0.050). Among women who had live births during the trial period, babies born to 1538 out of 2025 (75.95%) women in the progesterone arm and 1487 out of 2013 (73.87%) women in the placebo arm survived beyond 28 days of birth.
| Outcomes | Progesterone, n/N (%) | Placebo, n/N (%) | Bootstrap difference (adjusted effect, 95% CI) |
|---|---|---|---|
| Primary outcome | |||
| Live birth beyond 34 weeksa | 1513/2025 (74.72) | 1459/2013 (72.48) | 0.022, –0.004 to 0.050 |
| Secondary outcome | |||
| Alive at 28 days post delivery | 1538/2025 (75.95) | 1487/2013 (73.87) | 0.021, –0.005 to 0.048 |
Resource use and costs
A breakdown of the resource use data by trial arm is presented in Table 16. Mean health-care costs per participant by trial arm are presented in Table 17. During the trial, 2023 women in the intervention arm received progesterone and 2009 women in the non-intervention arm received placebo. Based on the mean number of days that participants utilised progesterone pessaries, the average cost of the intervention was calculated to be £204 (95% CI £200 to £207) per woman (see Table 17). The most substantial costs accrued during the trial by participants were from antenatal hospital visits, with a mean cost of £2339 (SD £2672) per woman for the progesterone arm and £2334 (SD £2665) per woman for the placebo arm.
| Resource items | Progesterone (N = 2025), mean (SD), n | Placebo (N = 2013), mean (SD), n | Bootstrap difference (adjusted mean difference, 95% CI) |
|---|---|---|---|
| Days receiving progesterone or placebo | 50.40 (21.11), 2023 | 48.43 (22.01), 2009 | 2.02, 0.72 to 3.31 |
| Antenatal period | |||
| Antenatal hospital visit | 5.00 (5.71), 2020 | 4.99 (5.69), 2005 | 0.01, –0.34 to 0.36 |
| Day assessment unit | 1.32 (2.50), 2020 | 1.26 (2.38), 2005 | 0.06, –0.09 to 0.21 |
| Emergency visit | 0.81 (1.51), 2020 | 0.89 (1.59), 2005 | –0.07, –0.16 to 0.02 |
| Inpatient admission (< 24 hours) | 0.57 (1.02), 2020 | 0.59 (1.08), 2005 | –0.03, –0.09 to 0.04 |
| Nights of admission (duration, days) | 0.86 (2.55), 2020 | 0.96 (2.99), 2005 | –0.09, –0.26 to 0.08 |
| Mode of delivery | |||
| Unassisted vaginal delivery (without cc) | 0.34 (0.48), 695 | 0.33 (0.47), 673 | 0.009, –0.023 to 0.042 |
| Unassisted vaginal delivery (with cc) | 0.07 (0.26), 150 | 0.06 (0.24), 122 | 0.014, 0.000 to 0.03 |
| Instrumental vaginal delivery (without cc) | 0.05 (0.22), 101 | 0.05 (0.21), 93 | 0.004, –0.01 to 0.02 |
| Instrumental vaginal delivery (with cc) | 0.06 (0.24), 123 | 0.05 (0.22), 107 | 0.008, –0.007 to 0.02 |
| Elective C-section (without cc) | 0.10 (0.30), 204 | 0.09 (0.28), 172 | 0.015, –0.004 to 0.03 |
| Elective C-section (with cc) | 0.03 (0.16), 53 | 0.03 (0.16), 52 | 0.000, –0.01 to 0.01 |
| Emergency C-section (without cc) | 0.03 (0.17), 59 | 0.03 (0.16), 56 | 0.001, –0.009 to 0.01 |
| Emergency C-section (with cc) | 0.09 (0.29), 182 | 0.11 (0.32), 230 | –0.024, –0.043 to –0.006 |
| Vaginal breech delivery (without cc) | 0.00 (0.02), 1 | 0.00 (0.03), 3 | 0.000, –0.002 to 0.001 |
| Vaginal breech delivery (with cc) | 0.00 (0.04), 3 | 0.00 (0.05), 5 | –0.001, –0.004 to 0.002 |
| Other (without cc) | 0.00 (0.04), 3 | 0.00 (0.04), 3 | 0.00, –0.002 to 0.002 |
| Other (with cc) | 0.00 (0.04), 3 | 0.00 (0.03), 2 | 0.00, –0.002 to 0.003 |
| Miscarriage management | |||
| Spontaneous resolution | 0.10 (0.30), 197 | 0.12 (0.33), 243 | –0.007, –0.022 to 0.007 |
| Surgical | 0.06 (0.23), 112 | 0.06 (0.24), 125 | –0.023, –0.043 to –0.004 |
| Medical | 0.05 (0.21), 97 | 0.05 (0.21), 91 | 0.003, –0.001 to 0.015 |
| Postnatal period | |||
| Admission to HDU (level 2 care) | 0.05 (0.30), 2006 | 0.06 (0.46), 1991 | –0.01, –0.035 to 0.012 |
| Admission to ITU (level 3 care) | 0.00 (0.04), 2006 | 0.00 (0.03), 1991 | 0.00, –0.001 to 0.003 |
| Hospital visit | 1.03 (2.98), 1984 | 1.02 (2.88), 1963 | 0.01, –0.17 to 0.20 |
| Day assessment unit | 0.30 (1.35), 1984 | 0.27 (1.13), 1963 | 0.03, –0.04 to 0.11 |
| Emergency visit | 0.22 (0.84), 1984 | 0.22 (0.85), 1963 | –0.00, –0.06 to 0.05 |
| Inpatient admission | 0.40 (0.59), 1984 | 0.37 (0.60), 1963 | 0.03, –0.007 to 0.066 |
| Nights of inpatient admission | 1.03 (1.99), 1984 | 0.96 (1.76), 1963 | 0.07, –0.04 to 0.19 |
| Neonatal period | |||
| Neonatal intensive care | 0.48 (4.76), 1565 | 0.48 (4.57), 1502 | –0.00, –0.34 to 0.32 |
| Neonatal high-dependency care | 0.42 (4.02), 1565 | 0.52 (3.90), 1502 | –0.10, –0.38 to 0.18 |
| Neonatal special care | 1.02 (5.07), 1565 | 1.16 (4.95), 1503 | –0.15, –0.50 to 0.21 |
| Primary care services | |||
| GP contact | 0.64 (1.21), 133 | 0.77 (1.25), 133 | –0.12, –0.40 to 0.15 |
| Practice/community midwife contact | 2.69 (3.77), 132 | 1.95 (3.09), 133 | 0.79, –0.01 to 1.60 |
| Practice nurse contact | 0.18 (0.61), 136 | 0.16 (0.47), 136 | 0.02, –0.12 to 0.15 |
| Psychologist (or counsellor) visit | 0.18 (1.05), 136 | 0.05 (0.52), 136 | 0.15, –0.07 to 0.37 |
| Health visitor visit | 0.39 (0.79), 133 | 0.29 (0.69), 136 | 0.10, –0.08 to 0.28 |
| Social worker visit (adult) | 0.04 (0.51), 136 | 0.00 (0.00), 136 | 0.04, –0.02 to 0.11 |
| Other community services | 0.16 (0.78), 134 | 0.13 (0.45), 133 | 0.02, –0.13 to 0.17 |
| Resource items | Progesterone (n = 2025), mean cost (SD) (£) | Placebo (n = 2013), mean cost (SD) (£) | Bootstrap mean cost difference (adjusted mean, 95% CI) (£) |
|---|---|---|---|
| Intervention | 204 (84) | 0 (0) | 204, 200 to 207 |
| Antenatal services | |||
| Hospital visit | 2339 (2672) | 2334 (2665) | 4, –159 to 166 |
| Day assessment unit | 164 (312) | 158 (297) | 8, –11 to 26 |
| Emergency visit | 96 (179) | 105 (188) | –9, –20 to 2 |
| Inpatient admission | 171 (309) | 180 (327) | –8, –28 to 12 |
| Nights of admission | 341 (1006) | 378 (1182) | –36, –102 to 29 |
| Delivery mode | |||
| Unassisted vaginal delivery (without cc) | 632 (874) | 615 (868) | 18, –36 to 71 |
| Unassisted vaginal delivery (with cc) | 162 (573) | 132 (513) | 30, –3 to 63 |
| Instrumental vaginal delivery (without cc) | 115 (501) | 106 (483) | 8, –23 to 39 |
| Instrumental vaginal delivery (with cc) | 149 (584) | 130 (549) | 19, –15 to 53 |
| Elective C-section (without cc) | 328 (981) | 278 (911) | 48, –11 to 108 |
| Elective C-section (with cc) | 107 (651) | 105 (647) | 1, –40 to 41 |
| Emergency C-section (without cc) | 128 (737) | 122 (720) | 5, –38 to 48 |
| Emergency C-section (with cc) | 510 (1624) | 649 (1807) | –137, –246 to –28 |
| Vaginal breech delivery (without cc) | 1 (41) | 2 (58) | –1, –4 to 2 |
| Vaginal breech delivery (with cc) | 3 (84) | 5 (109) | –2, –8 to 4 |
| Other (without cc) | 3 (71) | 3 (71) | 0, –4 to 4 |
| Other (with cc) | 3 (84) | 2 (69) | 1, –4 to 6 |
| Miscarriage management | |||
| Spontaneous resolution | 60 (183) | 75 (202) | –14, –27 to –2 |
| Surgical | 104 (430) | 117 (454) | –13, –40 to 13 |
| Medical | 90 (402) | 85 (391) | 5, –20 to 30 |
| Postnatal services | |||
| Admission to HDU (level 2 care) | 44 (288) | 55 (448) | –11, –34 to 12 |
| Admission to ITU (level 3 care) | 3 (71) | 2 (50) | 2, –2 to 6 |
| Hospital visit | 150 (431) | 148 (417) | 2, –23 to 27 |
| Day assessment unit | 38 (169) | 34 (141) | 4, –5 to 14 |
| Emergency visit | 21 (82) | 22 (83) | 0, –6 to 5 |
| Inpatient admission | 118 (175) | 110 (180) | 9, –3 to 20 |
| Night of inpatient admission | 406 (786) | 378 (694) | 29, –19 to 77 |
| Neonatal services | |||
| Neonatal intensive care | 627 (6275) | 634 (6017) | –10, –442 to 421 |
| Neonatal high-dependency care | 387 (3670) | 477 (3560) | –93, –344 to 159 |
| Neonatal special care | 523 (2605) | 595 (2543) | –76, –260 to 109 |
| Primary care services | n = 136 | n = 136 | |
| GP contacts | 25 (47) | 30 (49) | –5, –16 to 6 |
| Practice/community midwife | 81 (113) | 57 (93) | 24, –1 to 49 |
| Practice nurse contacts | 2 (6) | 2 (5) | 0.18, –1 to 1 |
| Psychologist (or counsellor) visits | 4 (20) | 1 (10) | 3, –1 to 7 |
| Health visitor visits | 9 (17) | 6 (15) | 2, –2 to 6 |
| Social worker visits (adult) | 1 (11) | – (–) | 1, –0 to 2 |
| Number of other community services | 3 (16) | 3 (9) | 0.36, –3 to 3 |
During the antenatal period, women allocated to the progesterone arm had, on average, a higher frequency of antenatal and DAU visits but a smaller number of emergency room visits and hospital admissions than women in the placebo arm. During the postnatal period, women in the progesterone arm utilised similar services more than those in the placebo arm except for emergency hospital visits, which were the same for both arms. However, women in the placebo arm had more admissions to the HDU [mean 0.06 (SD 0.46) for placebo vs. mean 0.05 (SD 0.30) for the progesterone arm]. Similarly, babies born to women in the placebo arm had, on average, a greater number of admissions to the HDU [mean 0.52 (SD 3.90) for placebo vs. mean 0.42 (SD 4.02) for the progesterone arm] and neonatal special care [mean 1.16 (SD 4.95) for placebo vs. mean 1.02 (SD 5.07) for the progesterone arm].
For delivery mode, women in the placebo arm had, on average, more emergency C-sections than women in the intervention arm. On average, women in the placebo arm utilised more neonatal care services than those in the intervention arm.
In keeping with the mean resource use, antenatal care costs for DAU and hospital visits were higher in the intervention arm, whereas emergency visits and hospital admissions costs were higher in the placebo arm.
In terms of cost differences, the greatest mean value for the participants was for emergency C-section with complications, which was greater in the placebo group than in the treatment group (–£137, 95% CI –£246 to –£28). The highest cost difference as a result of the intervention was for elective C-section without complication with a mean difference of £48 (95% CI –£11 to £108) per participant. Neonatal care variables were consistently lower in the progesterone group [ITU, –£10 (95% CI –£442 to £421); HDU, –£93 (95% CI –£344 to £159) and special care unit, –£76 (95% CI –£260 to £109)].
Mean total costs
The mean total costs by trial group for different variables are presented in Table 18. The average hospital-related service costs per woman for the trial period was £7452 in the progesterone group and £7572 in the placebo group, generating a mean cost difference of –£127 (BCa mean –£127, 95% CI –£759 to £505). The inclusion of the intervention cost (£204) generated a mean difference of £83 (adjusted mean £76, 95% CI –£559 to £711) per woman, which increased slightly to £78 (95% CI –£563). The differences in cost were not statistically significant.
| Cost | Progesterone (n = 2025), mean (SD) (£) | Placebo (n = 2013), mean (SD) (£) | Bootstrapped difference, adjusted mean, 95% CI (£) |
|---|---|---|---|
| Intervention | 204 (84) | 0 (0) | 204, 200 to 207 |
| Hospital-related costs | 7452 (9935) | 7572 (10,616) | –127, –759 to 505 |
| Mean cost | 7655 (9952) | 7572 (10,616) | 76, –559 to 711 |
| Intervention, hospital and primary care | 7663 (9953) | 7578 (10,617) | 78, –563 to 718 |
Primary analysis
The primary (base-case) cost-effectiveness outcome of the PRISM trial was the cost for an additional live birth after ≥ 34 completed weeks of pregnancy. The progesterone intervention appeared to be slightly more effective than placebo, resulting in an additional two live births per 100 women (an effect difference of 0.022, 95% CI –0.004 to 0.050) at ≥ 34 weeks’ gestation. The ICER, which combines the differences in costs in both groups, is presented in Table 19. The administration of progesterone resulted in an estimated additional cost of £83 per woman (adjusted mean £76, 95% CI –£559 to £711).
| Treatment | Mean cost (£) | Mean effect | Cost difference (£) (95% CI) | Effect difference (95% CI) | ICER (£) |
|---|---|---|---|---|---|
| Progesterone | 7655 | 0.747 | 76, –559 to 711 | 0.022, –0.004 to 0.0501 | 3305 |
| Placebo | 7572 | 0.725 | – | – | – |
Given the differences in costs and effects, the point ICER estimate for progesterone compared with placebo was calculated at £3305 per additional live birth.
Figure 9 shows the results of 5000 bootstrap replications plotted on the cost-effectiveness plane for the primary analysis. Each point on the plane depicts a pair of incremental cost and incremental effectiveness estimates for the comparison between progesterone and placebo. This suggests that progesterone is likely to be more effective, given that the majority of the scatterplots are in the south-east and north-east quadrants. However, it is uncertain whether the progesterone intervention is likely to be more costly (north-east) or less costly (south-east) than no intervention.
FIGURE 9.
Cost-effectiveness plane for the primary analysis using hospital-related costs for the participants. Reproduced from Okeke Ogwulu et al. 52 © 2020 The Authors. BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd on behalf of Royal College of Obstetricians and Gynaecologists. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
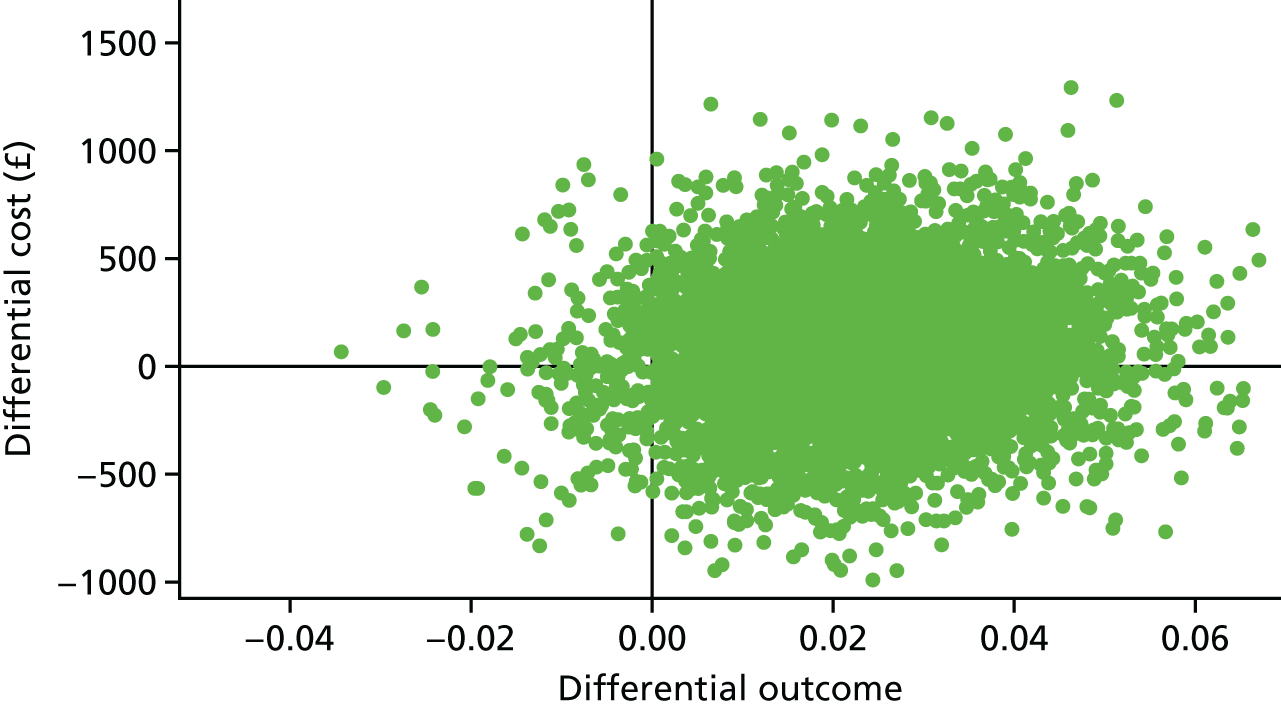
The CEAC for the primary analysis (Figure 10) shows the probability of progesterone being cost-effective at various values of decision-makers’ WTP per additional live birth. Figure 10 indicates that, for thresholds of WTP per additional live birth of > £15,000, there is > 80% probability that progesterone is cost-effective. For WTP thresholds of > £30,000, the probability of cost-effectiveness exceeds 90%.
FIGURE 10.
The CEAC for the primary analysis using hospital-related costs for the participants. Reproduced from Okeke Ogwulu et al. 52 © 2020 The Authors. BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd on behalf of Royal College of Obstetricians and Gynaecologists. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
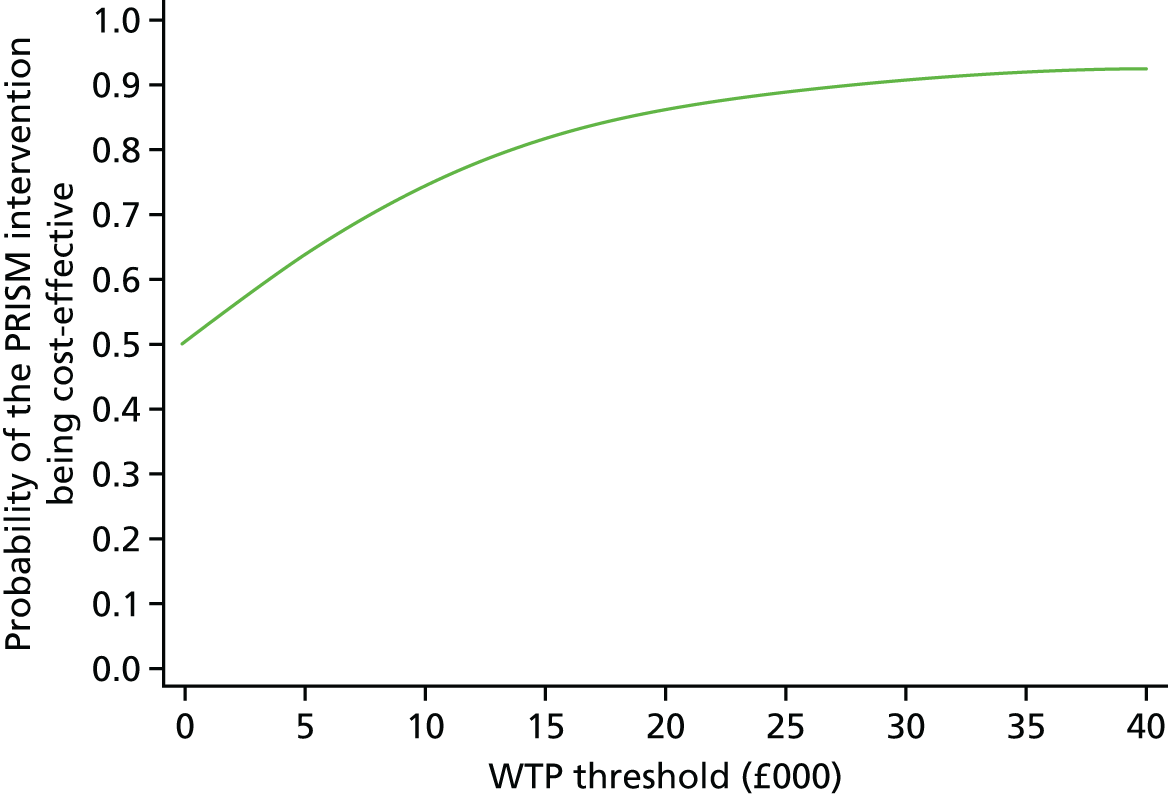
Secondary analyses
A series of secondary analyses were conducted to explore the impact of varying costs on the primary outcome.
Secondary analysis I (hospital-related costs for participants minus neonatal care costs)
For the first secondary analysis, we removed neonatal care costs from the hospital costs. This resulted in an adjusted mean cost difference of £170 (95% CI –£113 to £453) (Table 20).
| Treatment | Mean cost (£) | Mean effect | Cost difference (£) (95% CI) | Effect difference | ICER (£) |
|---|---|---|---|---|---|
| Progesterone | 6467 | 0.745 | 170, –113 to 453 | 0.022 | 7370 |
| Placebo | 6298 | 0.726 | – | – | – |
The ICER was calculated as £7370 per additional live birth beyond 34 weeks of gestation. The increased ICER value for this analysis is probably because, on average, women in the placebo arm utilised more neonatal hospital resources than women in the progesterone arm. Hence, the removal of neonatal care costs resulted in a higher cost difference.
The cost-effectiveness plane (Figure 11) shows that progesterone is the more effective and more costly intervention, with the majority of the bootstrap replications in the south-east quadrant. The CEAC (Figure 12) suggests that, for WTP thresholds of > £12,000 per additional live birth, there is > 95% probability that progesterone is a cost-effective intervention.
FIGURE 11.
Cost-effectiveness plane for secondary analysis I (maternal hospital costs).
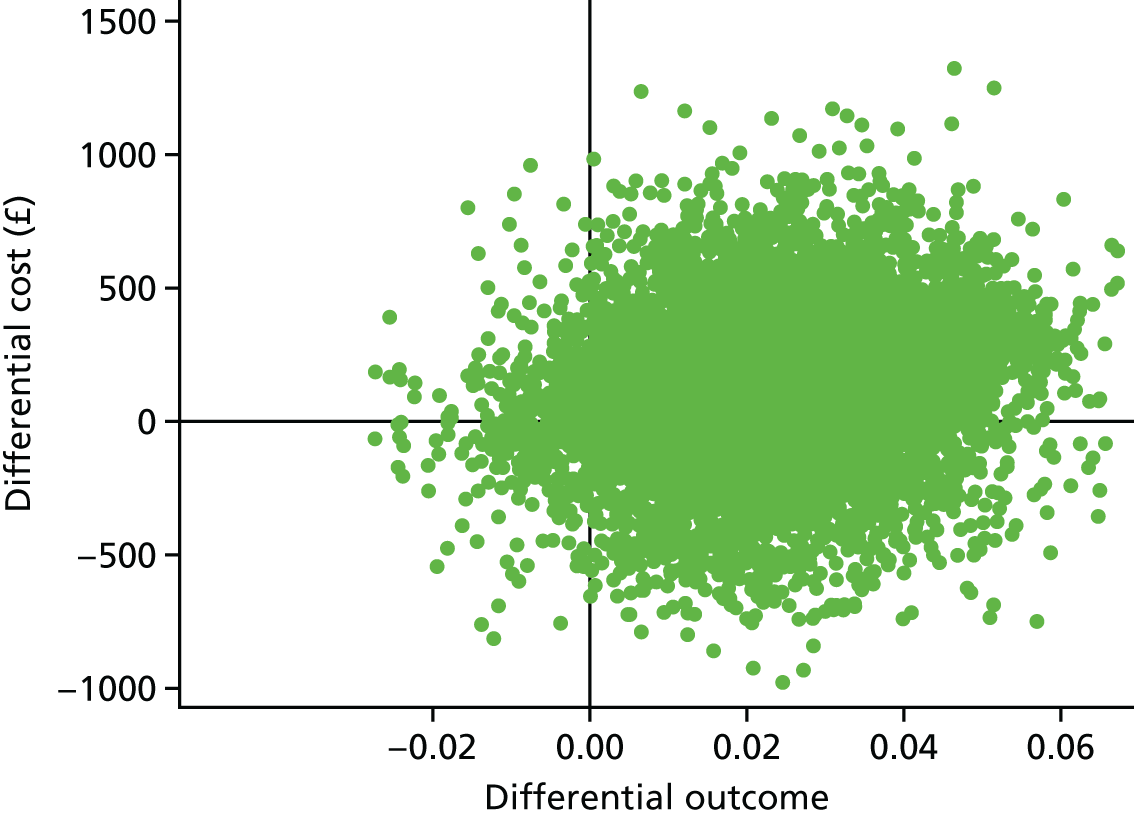
FIGURE 12.
The CEAC for secondary analysis I (maternal hospital costs).
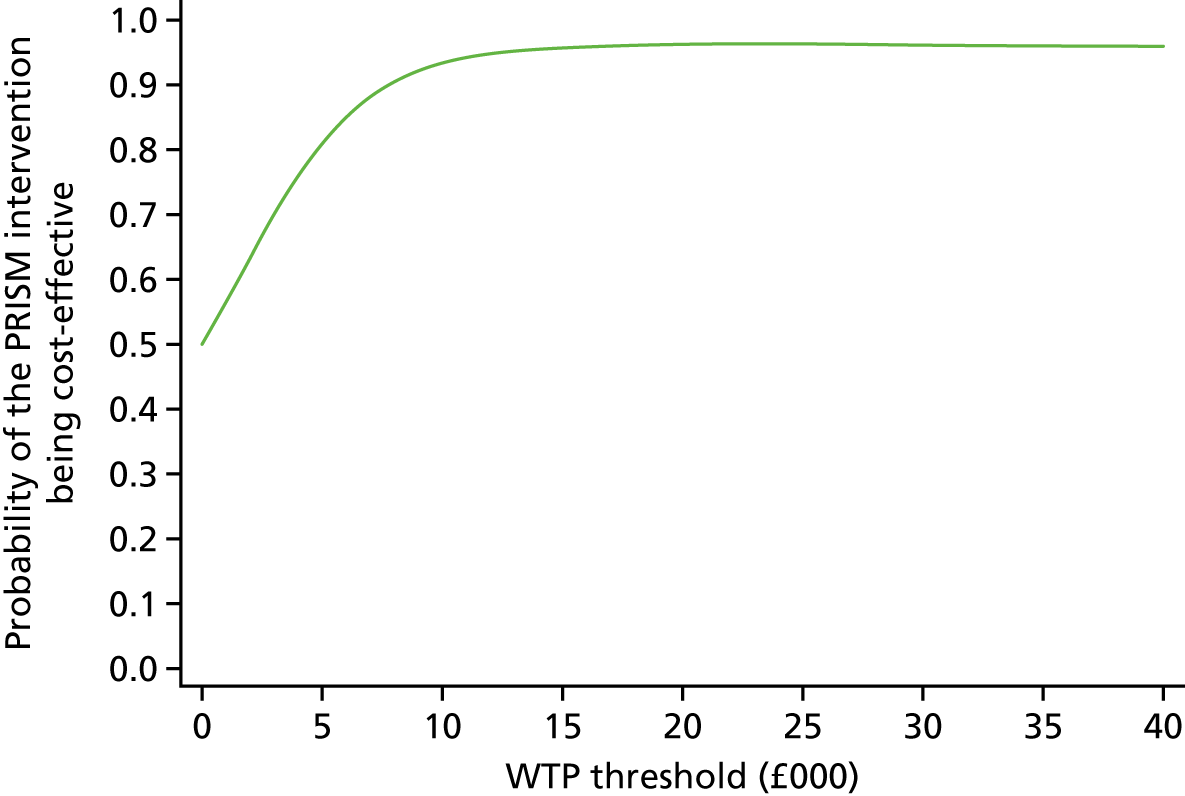
Secondary analysis II (hospital and primary care costs for participants)
In another secondary analysis, we included hospital-related costs and primary care costs for the participants. First, we explored the total primary care cost for women with complete primary care service use data; complete data were available for 272 participants. In this subgroup, the mean total cost was £120 for women in the progesterone arm and £98 for women in the placebo arm. For each woman in the progesterone arm, we added £120 to the total hospital-related cost; for each woman in the placebo arm, we added £98 to the total hospital-related cost (Table 21). We calculated an additional cost of £106 (adjusted mean £98, 95% CI –£537 to £733) and an ICER of £4264 per additional live birth.
| Treatment | Mean cost (£) | Mean effect | Cost difference (£) (95% CI) | Effect difference | ICER (£) |
|---|---|---|---|---|---|
| Progesterone | 7776 | 0.747 | 98, –537 to 733 | 0.022 | 4264 |
| Placebo | 7670 | 0.725 | – | – | – |
Again, the cost-effectiveness plane (Figure 13) clearly suggests that progesterone is more effective. Figure 14 depicts the CEAC for this analysis. For WTP thresholds of > £15,000, per additional live birth beyond 34 weeks’ gestation, the probability of progesterone being more effective than placebo is over 80%. The probability of cost-effectiveness exceeds 90% for WTP thresholds of > £30,000.
FIGURE 13.
Cost-effectiveness plane for secondary analysis II (hospital and primary care costs).
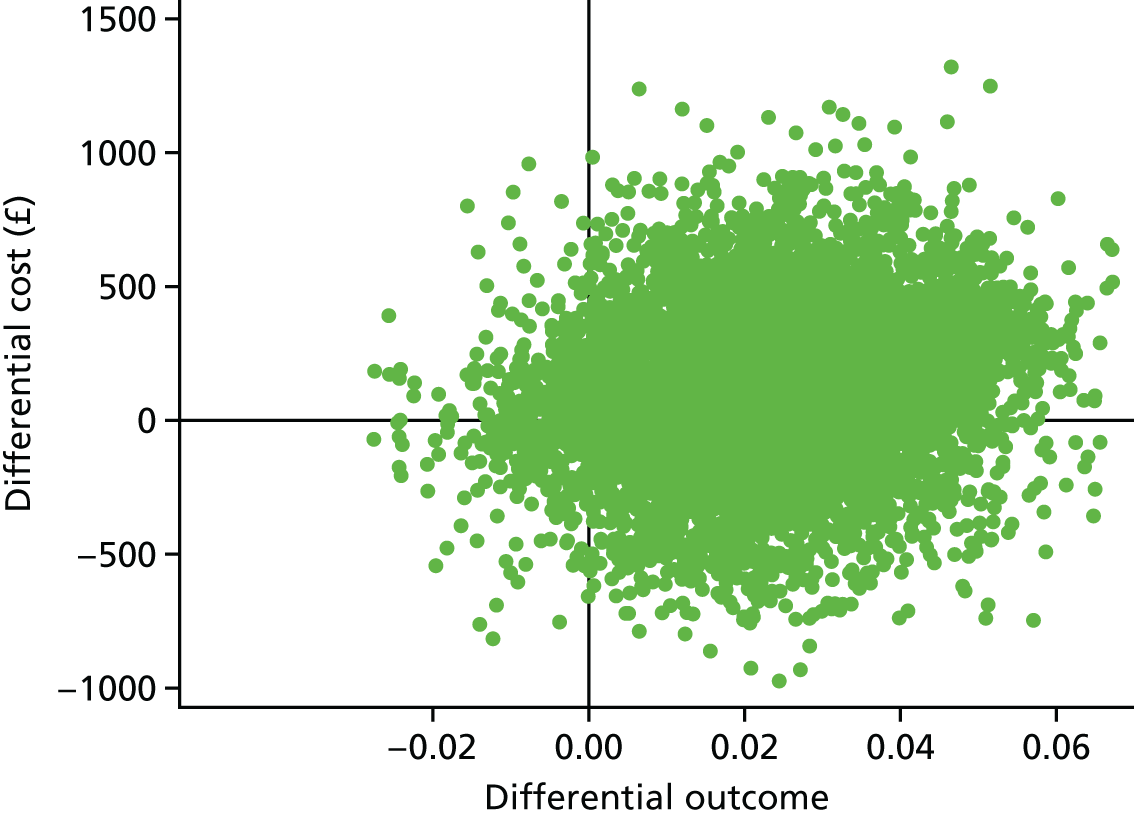
FIGURE 14.
The CEAC for secondary analysis II (hospital and primary care costs).
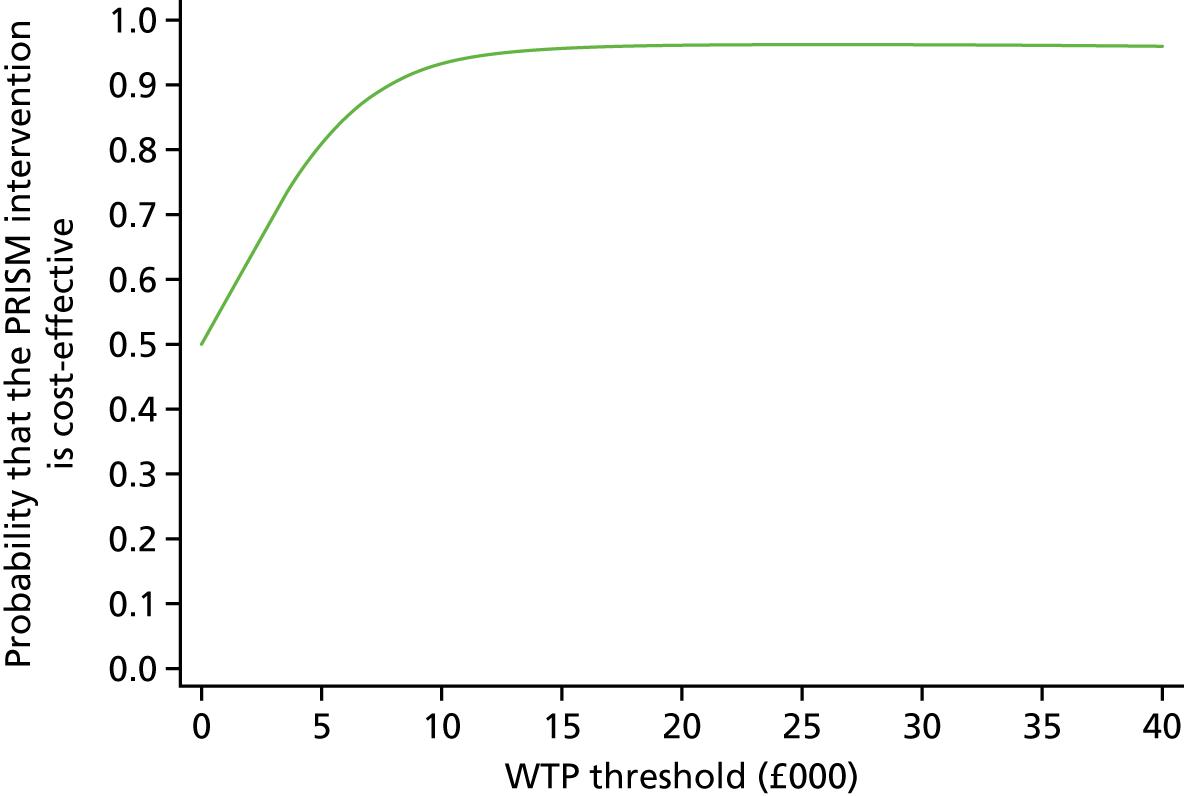
Secondary analysis III (hospital costs for participants including those lost to follow-up)
For this analysis, we included both the women used for the primary base-case analysis and those who were lost to follow-up. We explored a worst-case scenario and assumed that all women lost to follow-up had a miscarriage. We used multiple imputations to impute missing costs and re-ran the primary analysis. This included 2069 participants in the progesterone arm and 2054 participants in the placebo arm.
The results (Table 22) showed that progesterone was slightly more costly (cost difference £29, 95% CI –£593 to £651) and more effective than no progesterone for this subgroup, with an ICER of £1378 per additional live birth beyond 34 weeks of gestation.
| Treatment | Mean cost (£) | Mean effect | Cost difference (£) (95% CI) | Effect difference (95% CI) | ICER (£) |
|---|---|---|---|---|---|
| Progesterone | 7788 | 0.731 | 29, –593 to 651 | 0.021, –0.007 to 0.049 | 1378 |
| Placebo | 7750 | 0.710 | – | – | – |
From the cost-effectiveness plane (Figure 15), it is evident that progesterone is more effective; however, it is uncertain which intervention is more costly. The CEAC (Figure 16) shows that, for WTP thresholds of > £15,000 per additional live birth beyond 34 weeks of gestation, the probability of progesterone being cost-effective is approximately 85%, and the probability exceeds 90% for WTP thresholds of > £30,000.
FIGURE 15.
Cost-effectiveness plane for secondary analysis III (hospital costs for participants including those lost to follow-up).
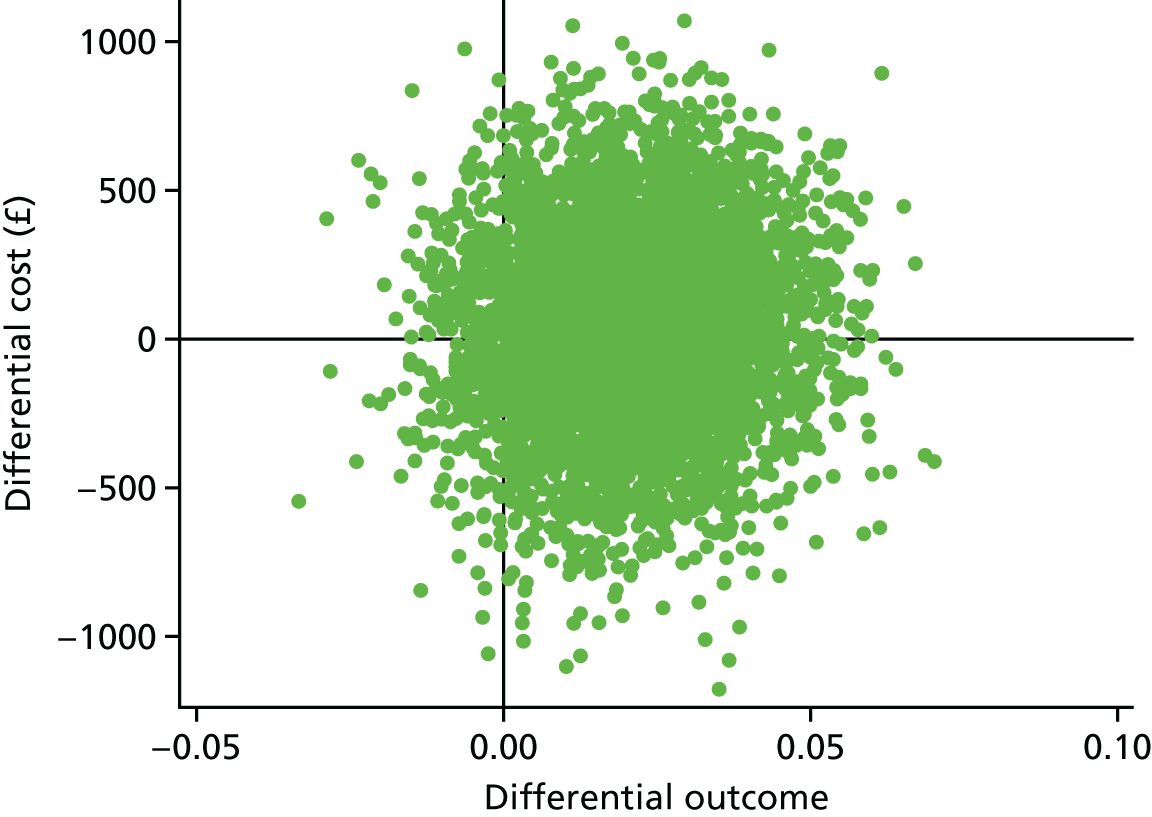
FIGURE 16.
The CEAC for secondary analysis III (hospital costs for participants including those lost to follow-up).
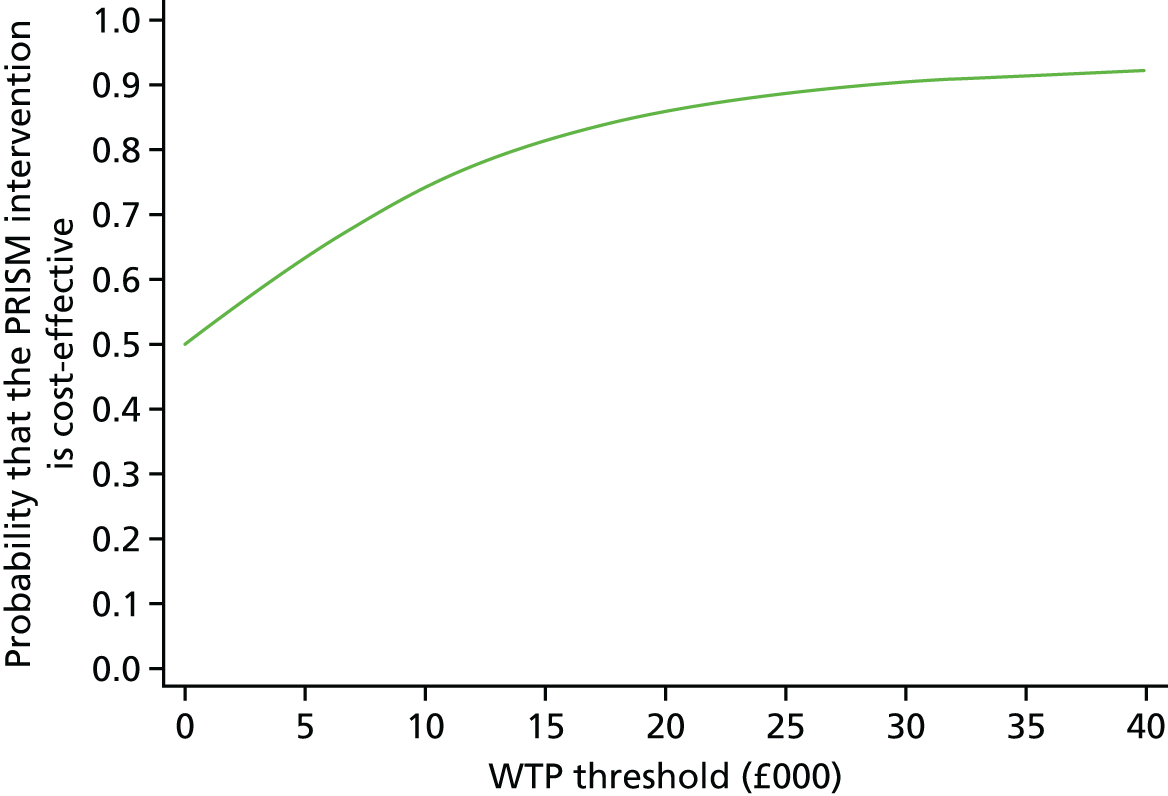
Secondary analysis IV (hospital costs for participants with three or more previous miscarriages)
We conducted a subgroup analysis of women with three or more previous miscarriages. This included 137 women in the intervention arm and 148 women in the placebo arm. The intervention was more effective, with an additional gain of 15 live births per 100 women, beyond ≥ 34 weeks' gestation (Table 23). An ICER of £11,606 per additional live birth beyond 34 weeks’ gestation was calculated for this subgroup.
| Treatment | Mean cost (£) | Mean effect | Cost difference (£) (95% CI) | Effect difference (95% CI) | ICER (£) |
|---|---|---|---|---|---|
| Progesterone | 9304 | 0.715 | 1754, –1041 to 4550 | 0.151, 0.042 to 0.260 | 11,606 |
| Placebo | 7803 | 0.574 | – | – | – |
Similarly, the cost-effective plane (Figure 17) depicts that progesterone is more effective and more costly with the majority of the bootstrap replications in the south-east quadrant. The CEAC (Figure 18) shows over > 90% probability of the intervention being cost-effective for WTP thresholds of > £15,000.
FIGURE 17.
Cost-effectiveness plane for participants with three or more previous miscarriages. Reproduced from Okeke Ogwulu et al. 52 © 2020 The Authors. BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd on behalf of Royal College of Obstetricians and Gynaecologists. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
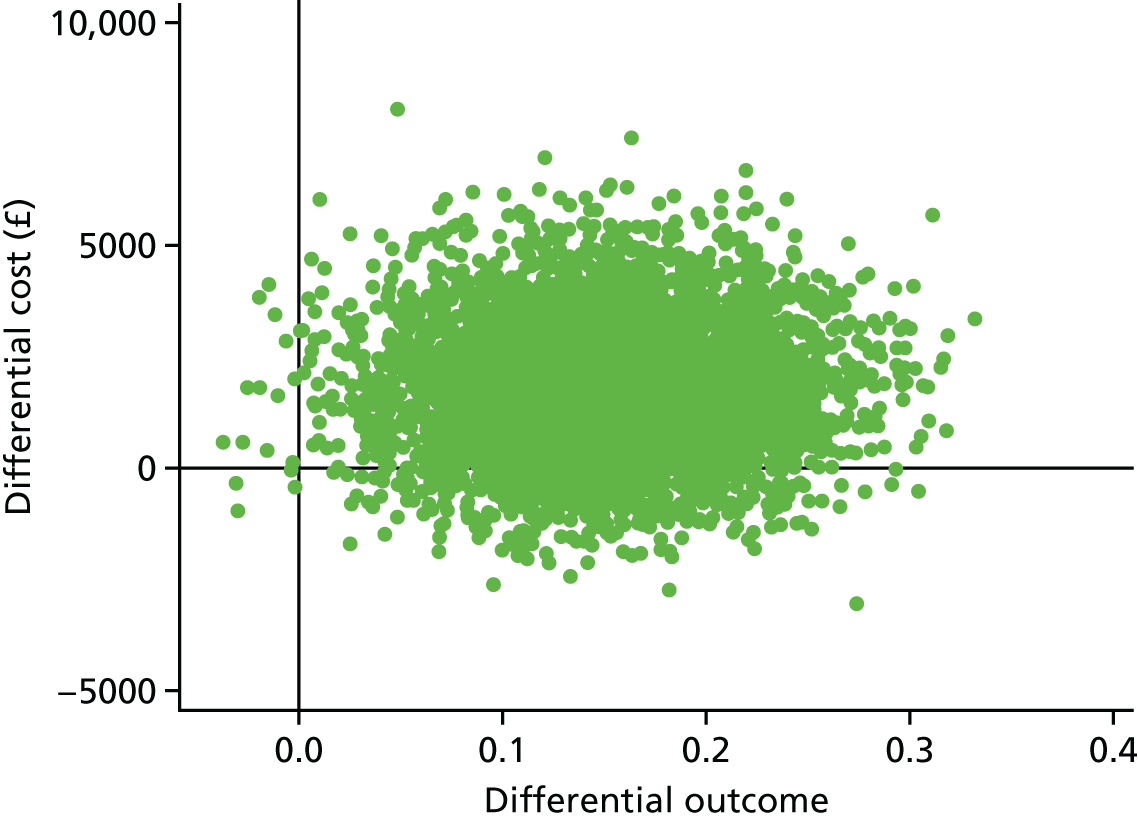
FIGURE 18.
The CEAC for participants with three or more previous miscarriages. Reproduced from Okeke Ogwulu et al. 52 © 2020 The Authors. BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd on behalf of Royal College of Obstetricians and Gynaecologists. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
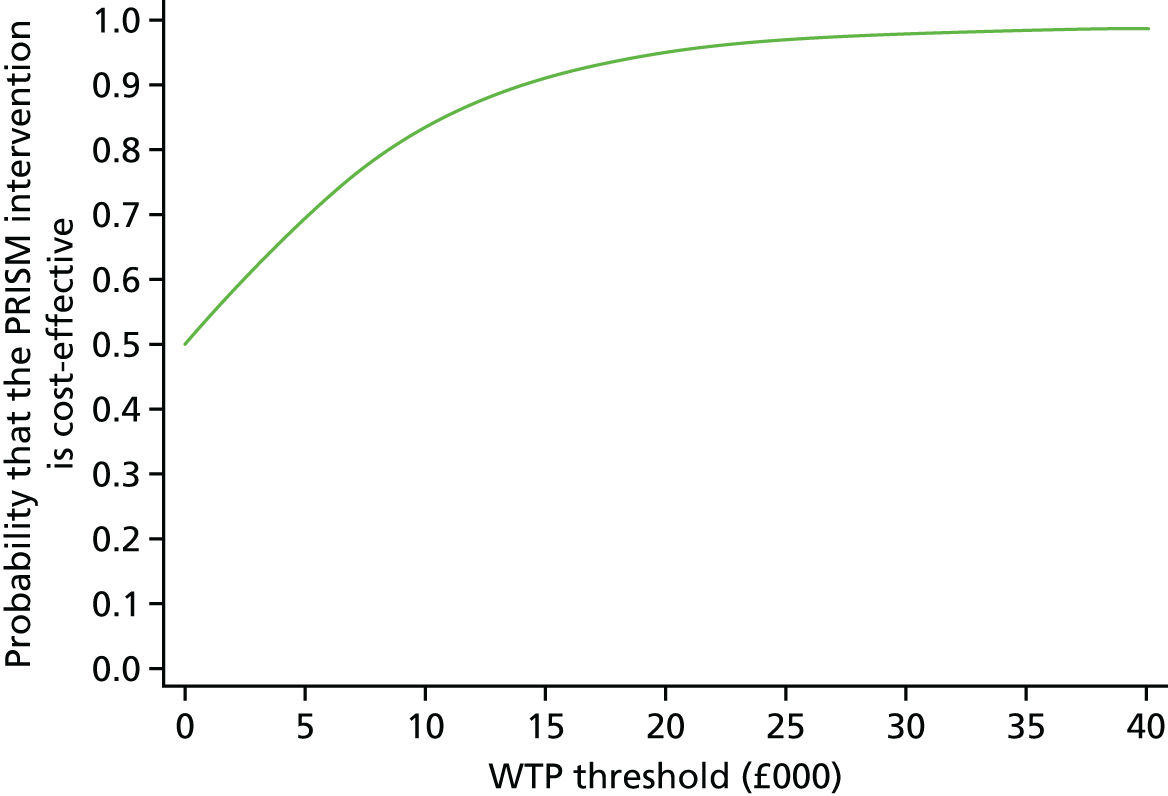
Analyses using secondary outcomes
Incremental CEAs were conducted for the final end point of the PRISM trial (Table 24). The analysis was based on the incremental cost of the intervention for an additional baby survival for each woman at 28 days post partum. The effect difference was 0.021 (95% CI –0.005 to 0.048). The ICER for this analysis was £3037 per additional baby who survived beyond 28 days post birth.
| Treatment | Progesterone, mean effect | Placebo, mean effect | Effect difference (95% CI) | ICER (£) | Cost-effectiveness plane |
|---|---|---|---|---|---|
| Neonatal survival beyond 28 daysa | 0.760 | 0.739 | 0.021, –0.005 to 0.048 | 3037 | North-east dominance |
Sensitivity analyses
We conducted sensitivity analyses in which we explored different scenarios (Table 25). Using alternative cost estimates for nights of hospital admissions and miscarriage management appeared to have a limited effect on the resulting ICER (see Table 25).
| Sensitivity analyses | Mean costs (£) | Cost difference, mean, 95% CI (£) | ICER (£) | |
|---|---|---|---|---|
| Progesterone | Placebo | |||
| Fixed progesterone cost until 16 weeks | 7694 | 7572 | 115, –506 to 735 | 4977 |
| Imputation of primary care costs | 7773 | 7666 | 100, –532 to 731 | 4321 |
| Varying cost of inpatient nights of admission | 7498 | 7413 | 77, –536 to 691 | 3356 |
| Varying cost of miscarriage management | 7635 | 7552 | 76, –546 to 697 | 3282 |
| Removing delivery costs | 5515 | 5421 | 86, –500 to 672 | 3743 |
However, using a fixed cost of progesterone until 16 weeks increased the ICER to £4977 per additional live birth beyond 34 weeks’ gestation (see Table 25). The cost-effectiveness plane (Figure 19) showed dominance in the north-east and south-east quadrants, which indicated that progesterone was more effective and either less costly or more costly. The CEAC (Figure 20) shows over 90% probability of the intervention being cost-effective for WTP thresholds of > £25,000. Likewise, the removal of the cost of delivery increased the ICER to £3743 per additional live birth beyond 34 weeks (see Table 25).
FIGURE 19.
Cost-effectiveness plane for a fixed cost of progesterone until 16 weeks’ gestation.
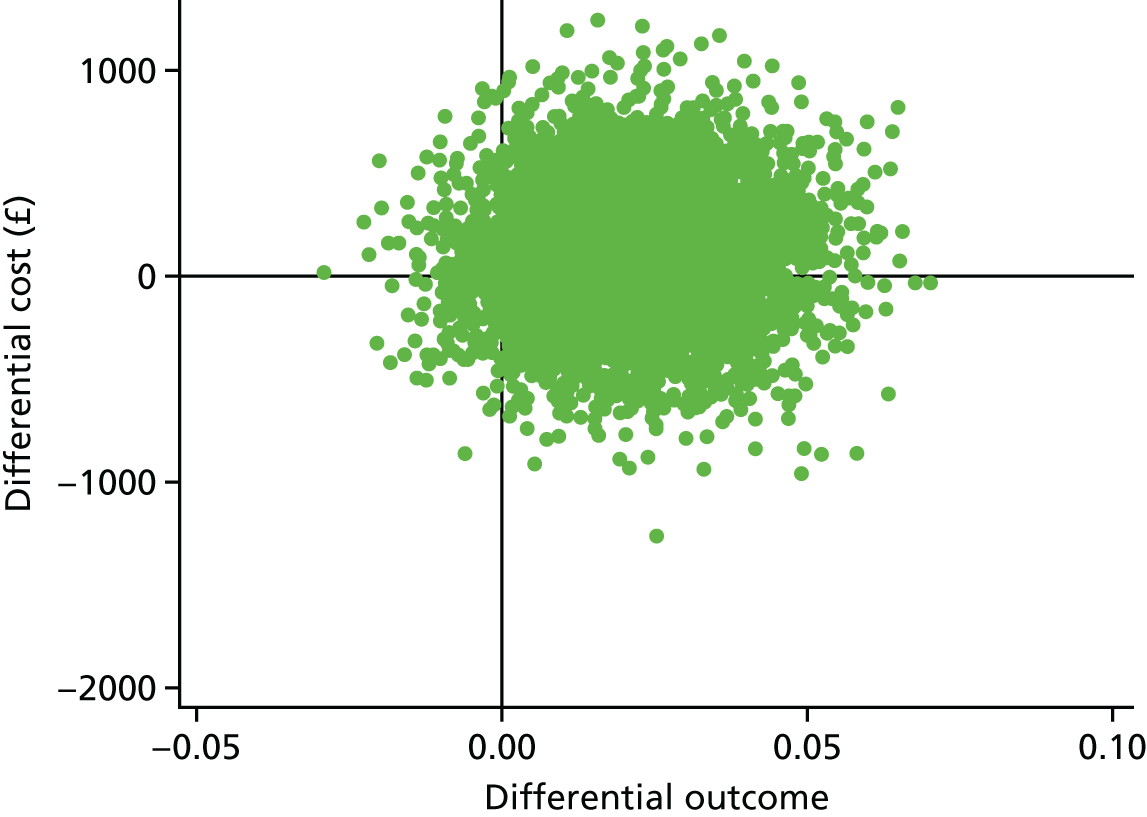
FIGURE 20.
The CEAC for a fixed cost of progesterone until 16 weeks’ gestation.
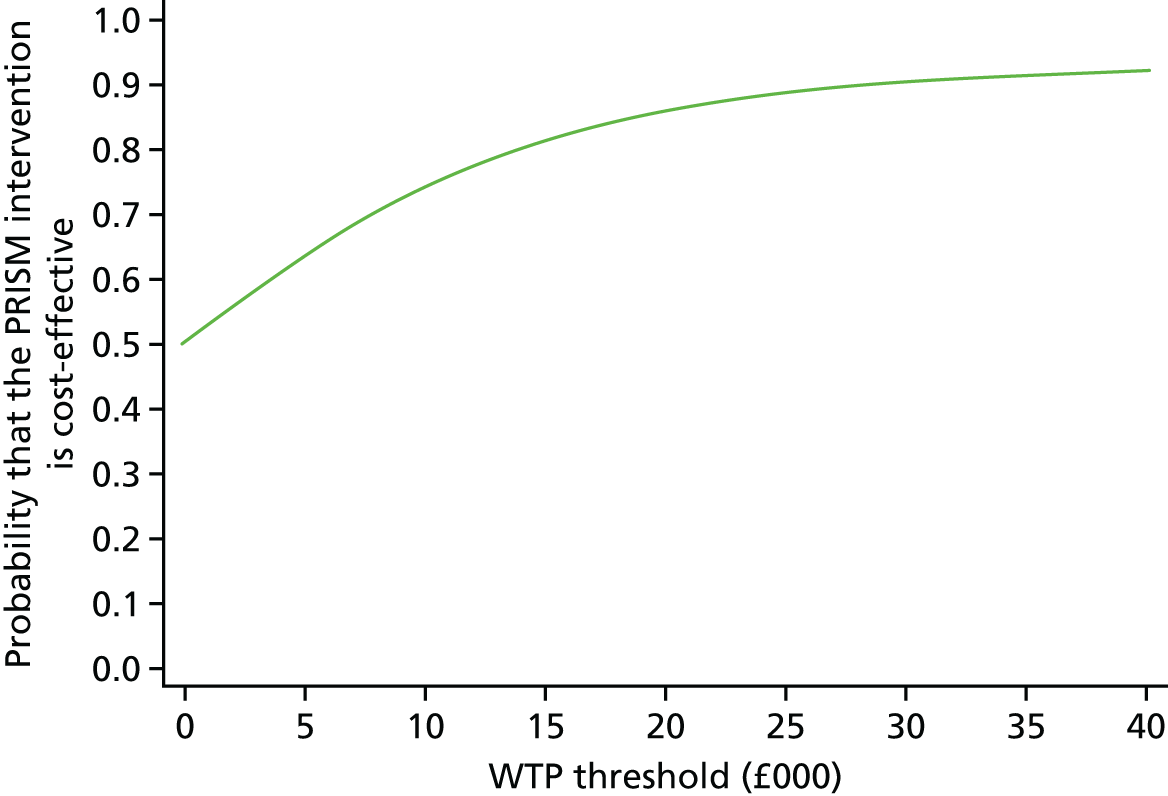
In secondary analysis II, we added mean totals of the primary care costs to the total costs for each woman depending on the trial arm. From this, we calculated an ICER of £4264 per additional live birth beyond 34 weeks of gestation (see Table 21). The sensitivity analysis in which we imputed costs via multiple imputations did not have much effect on the ICER, with a slight increase to £4321 per additional live birth beyond 34 weeks (see Table 25).
Discussion of the health economic findings
Principal findings
We evaluated the cost-effectiveness of progesterone in preventing miscarriage and leading to a live birth at ≥ 34 weeks of pregnancy in women who presented with bleeding in early pregnancy. Our results suggest that progesterone is more effective and slightly more costly than placebo. More specifically, progesterone resulted in an additional two live births per 100 women (0.022, 95% CI –0.004 to 0.050) at ≥ 34 weeks of gestation relative to placebo, with an additional cost of £83 (adjusted mean difference £76, 95% CI –£559 to £711) per woman. The additional cost was mainly attributable to the cost of progesterone administration (mean cost £204). The ICER was estimated at £3305 per live birth at ≥ 34 weeks. A conclusion on the cost-effectiveness of the PRISM trial would depend on the amount that society is willing to pay to increase the chances of an additional live birth at ≥ 34 weeks of pregnancy. For potentially acceptable WTP threshold values for an additional live birth,44 the probability of progesterone being cost-effective for this population group exceeds 90%.
The National Institute for Health and Care Excellence attached a value to an averted stillbirth of 25 QALYs. 65 This assumes that life lost has a typical life expectancy in good health, but when discounting is applied to the expected years in full health, it yields 25 discounted QALYs. To interpret the primary CEA results in relation to QALYs, we used the NICE value (25 QALYs) as a proxy. If we assume that babies born alive at ≥ 34 weeks live in full health and divide the ICER (£3305 per additional live birth) by 25, then the cost per QALY is likely to be £132. If a baby did live in full health for the anticipated life expectancy, then on the basis of this ICER (£132) the intervention is cost-effective. 44 Furthermore, evidence from the NHS Reference Costs schedule47 indicates that the upper cost quartile of the most expensive delivery is about £15,000, which could go much higher if we allow for the cost of excess bed-days. This further suggests that progesterone intervention is cost-effective.
The ICER for the final end point (secondary outcome) of the trial was £3037 per additional baby surviving beyond 28 days after birth. The intervention was more effective, with a gain of three neonates per 100 women surviving beyond 28 days post partum. A subgroup analysis of women with three or more previous miscarriages led to an increase of 15 live births per 100 women in the intervention group.
Strengths and limitations of the economic analyses
The strength of the CEA is that it was based on a large, robust, multicentre randomised controlled trial involving over 4000 participants, making this the largest study to explore whether or not progesterone provides value for the public health-care resources. The outcome and resource use data were prospectively collected at different points in the trial using CRFs. Unit costs were obtained from established national sources. In cases where HRGs did not clearly depict our variables, we liaised with the clinical team to decide on the most appropriate HRG. The CEA also benefited from the robustness of the main analyses and the sensitivity analyses. However, data on primary care services were available for < 10% of the participants: we accounted for this by imputing missing costs in our analyses. A limitation of this analysis was the failure to explore the wider societal costs to the participants. However, this was beyond the scope of the study and beyond the requested resource.
Comparison with the literature
To our knowledge, this is the first UK study to investigate the cost-effectiveness of progesterone in preventing miscarriage and achieving a live birth beyond 34 weeks of gestation. A similar study investigated the cost-effectiveness of progesterone in preventing miscarriages in women with a history of recurrent miscarriages and leading to a live birth beyond 24 weeks of gestation. 2 The authors reported that the total mean cost of the intervention was £332.17 higher in the progesterone arm than in the placebo arm and an ICER of £18,053 per additional live birth beyond 24 weeks for the base-case analysis, with a cost-effectiveness probability of 50% at this value.
Implications for policy
The results of the CEA suggest that progesterone is likely to be considered a cost-effective intervention by decision-makers for women presenting with early pregnancy bleeding (threatened miscarriage) within 12 weeks of gestation.
Summary of health economic findings
-
For the primary analysis, the mean total cost was higher in the progesterone group (£7655) than in the placebo group (£7572), with an additional cost of £83 (1% higher cost than usual care).
-
The additional difference in the mean probability of a live birth beyond ≥ 34 completed weeks of gestation was 0.022 (95% CI –0.004 to 0.050), indicating that the progesterone intervention resulted in an additional two live births per 100 women at ≥ 34 weeks.
-
For the primary analysis, the ICER per additional live birth beyond 34 weeks of gestation was calculated as £3305.
-
There is > 80% confidence that progesterone is cost-effective if decision-makers are prepared to pay £15,000 per additional live birth and > 90% if the WTP threshold is £30,000.
-
Currently, in the UK, progesterone is not routinely given to women who are at high risk of miscarriage. The results of the CEA suggest that progesterone is likely to be considered cost-effective, particularly for women (with one or more miscarriages) who present with bleeding in early pregnancy.
Chapter 5 Discussion
Our large multicentre, double-blind, placebo-controlled, randomised trial showed that vaginal progesterone therapy in the first trimester of pregnancy did not result in a significant increase in the rate of live births at ≥ 34 weeks of gestation in women with early pregnancy bleeding. However, the large sample size of our study allowed investigation of prespecified subgroups, and we found that women with early pregnancy bleeding and a previous history of miscarriages benefited from progesterone therapy. This subgroup effect showed a biological gradient, with those with no previous miscarriages receiving no benefit, those with one or two previous miscarriages receiving some benefit and those with three or more previous miscarriages receiving a substantial benefit. The biological gradient combined with the overall positive direction of effect in the primary analysis gives us confidence in the effects of progesterone in such high-risk women. The findings of the economic evaluation indicate that progesterone is likely to be considered by decision-makers to be cost-effective for any woman with threatened miscarriage, and particularly for women with a history of a previous miscarriage.
Of the 10 prespecified subgroup analyses, one showed differential effects of progesterone; that is, the effects of progesterone in women with early pregnancy bleeding differed according to the number of previous miscarriages, with a suggestion of benefit in women with ≥ 3 miscarriages. Previous data have indicated a steep and proportionate increase in the loss of chromosomally normal pregnancies (euploid miscarriages) with an increasing number of past miscarriages. 66 Given that the potential benefit of progesterone therapy would be expected to be specific to euploid pregnancies, an increasing level of benefit in women with an increasing number of past miscarriages is consistent with our biological understanding of miscarriage risk. Previous miscarriage history is one of only two stratification or prognostic risk factors (the other being maternal age) stated as useful to identify high-risk patients in the 2017 European Society of Human Reproduction and Embryology guideline on recurrent miscarriage. 67 However, we did not identify this subgroup as of special interest a priori in our SAP, and multiple comparisons were performed (without adjustment for multiplicity); thus, this observation requires further validation.
Study strengths
This study is the largest, multicentre, double-blind, placebo-controlled, randomised clinical trial to report on treatment of early pregnancy bleeding with progesterone. The robust study design – including blinding to treatment allocation of both participants and investigators – ensured internal validity, enabling the results to be interpreted with confidence. Randomisation via computer-generated allocation sequence was effective in achieving balanced groups with respect to important prognostic factors.
The size of the study was driven by a MID, determined following consultations with health-care practitioners, patients and representatives of patient bodies, as well as through a clinician’s survey. A consensus of a 5% increase in live birth rates beyond 34 weeks of gestation evolved from this consultation, resulting in a target sample size of 4150 participants with primary outcome data. A total of 4153 women from 48 hospitals in the UK were randomised to receive either progesterone (n = 2079 women) or placebo (n = 2074 women). The follow-up rate for the primary outcome was 97.2% (4038/4153 women).
Our trial design offered a number of other strengths with respect to data collection and analysis. The treatment of participants by a large number of study centres and practitioners allowed intervention impact to be evaluated without confounding by individual variance in clinical practice. The outcome measures selected were routine variables widely used by clinicians who are familiar with early pregnancy care. This ensured that the outcomes were well understood and easy to record. Almost all of the outcome data recorded during the PRISM study were objective outcomes (rather than subjective descriptions) and the study was blinded so there was no risk of incurring assessor bias. The trial intervention was deliverable in the context of customary care without major effects on health service structure. The mode of administration of IMP was designed to reflect the preferences expressed by patients, and most of our data collection could be performed during routine antenatal and postnatal appointments of the study participants.
Limitations and critique
We consider the trial to have been designed and conducted in order to be methodologically robust. Nevertheless, there were some limitations of our study that should be considered. We studied a vaginal preparation of progesterone, at a dose of 400 mg twice daily, and it is possible that the results with this regimen are not generalisable to patients receiving other doses and preparations. However, this route was chosen to deliver a greater proportion of the drug to the biologically relevant site (i.e. the uterus),4,32,33 and the dose used (400 mg twice daily) represents a dose at the top end of the therapeutic window. 2,26 We started progesterone treatment only in women who had an intrauterine sac, and thus our study cannot provide evidence on the effects of earlier use of progesterone before a pregnancy sac is visible on an ultrasound. We discontinued progesterone at 16 weeks of gestation but consider it unlikely that therapy beyond this time would have affected aetiology and outcomes related to miscarriage. We found no increase in the risk of congenital anomalies in the offspring of women treated with progesterone, although the study was not powered for such rare outcomes.
The observed primary outcome rate in the placebo group was slightly higher than that assumed in the sample size calculation (72% vs. 60%). However, an assumed higher rate would have required a smaller sample to detect the same 5% absolute difference and, hence, this is unlikely to have had any impact on the conclusions we have drawn from the study.
Findings in the context of existing literature
The pre-existing evidence, summarised in a recent Cochrane review,1 pooled the results from seven small trials that had substantive methodological limitations; only three of the trials specified the method of concealment of study group assignments, and only four trials used a placebo for comparison. Nevertheless, the pooled analysis did show a benefit in reducing the risk of miscarriages (risk ratio 0.64, 95% CI 0.47 to 0.87). 1 Live birth outcome was not reported in this review. 1
Interpretation of the principal findings
Although there were more live births in the progesterone group than in the placebo group, the trial did not find a statistically significant difference. However, an important subgroup effect by previous history of miscarriage was observed. This subgroup effect is supported by the fact that the risk of a future miscarriage increases proportionately with increasing number of past miscarriages;66 there is a steep and proportionate increase in the loss of chromosomally normal pregnancies (euploid miscarriages) with increasing number of past miscarriages. 66 It is euploid miscarriages that are likely to be helped by progesterone therapy, and thus an increasing level of benefit in women with an increasing number of past miscarriages is consistent with our biological understanding of miscarriage risk. We found no increase in the risk of congenital anomalies among offspring of women treated with progesterone, although the study was not powered for such rare outcomes.
Interpretation of the cost-effectiveness findings
The primary CEA found that the mean total cost per woman was higher in the progesterone group (£7655) than in the placebo group (£7572). Hence, the progesterone intervention led to an additional cost of £76 per woman. The ICER per additional live birth beyond 34 weeks of gestation was calculated as £3305. For potentially acceptable WTP threshold values,44 the probability of progesterone being cost-effective is over 90%. The likelihood of progesterone being cost-effective increased even further for those women with history of repeated miscarriage.
Patient and public involvement
In the PRISM trial, patient and public involvement was utilised at all stages of the study design, development and monitoring. This included questionnaires for patients to assess the acceptability of the intervention, and engagement in the development of patient-facing literature for participants. The patient and public involvement representative was sent study documentation to review at the design stage and attended design meetings to ensure that it was clear and easy to understand. The TSC included a representative of the Miscarriage Association and a representative of Tommy’s charity. We believe that these roles were important to ensure that appropriate communication with study participants and project oversight took place throughout the duration of the research. Dissemination of results will be supported by the international charity Ammalife, the Miscarriage Association and Tommy’s charity.
Generalisability
Centres participating in the study were geographically spread across the UK, improving the generalisability of the results for women with early pregnancy bleeding. Women in the trial did not belong to a ‘selected population’, such as those with a history of previous miscarriage. Therefore, the results of this study are likely to be representative of true unselected ‘low-risk’ women with no gynaecological or obstetric risk factors. The exclusion criteria were kept to a minimum and the heterogeneity of the population was well reflected by trial participants.
Chapter 6 Conclusions
In conclusion, our trial did not find an overall benefit of progesterone supplementation, but identified a subgroup effect in high-risk women, defined as those with early pregnancy bleeding and a previous history of miscarriages. In women with early pregnancy bleeding and a history of previous miscarriages, the number needed to treat to gain an additional live birth at ≥ 34 weeks’ gestation is 18 (95% CI 10 to 71). The results of the economic evaluation, which typically adopts a Bayesian perspective for analysis, suggested that progesterone is likely to be considered cost-effective by decision-makers for women presenting with early pregnancy bleeding, and particularly for women with a history of a previous miscarriage. The final conclusion on the cost-effectiveness of the PRISM trial would depend on the amount that society is willing to pay to increase the chances of an additional live birth at and beyond 34 weeks of pregnancy.
Implications for health care
On the basis of the results of this study, progesterone therapy in the first trimester does not have a significant benefit in women with early pregnancy bleeding overall; however, our study found that women with early pregnancy bleeding and a previous history of miscarriages benefited from progesterone therapy. A biological gradient of effect was observed, with those women with no previous miscarriages receiving no benefit, those with one or two miscarriages receiving some benefit and those with three or more miscarriages receiving a substantial benefit. Furthermore, the findings of the economic evaluation suggest that administering progesterone to women with early pregnancy bleeding (< 12 weeks of gestation) is likely to be considered good value for money.
Recommendations for research
Given the large number of data that now exist on this subject, our research group has previously registered to conduct an individual participant data meta-analysis titled ‘Vaginal progesterone treatment during the first trimester of pregnancy for the prevention of miscarriage: an individual participant data (IPD) meta-analysis’. 68 This analysis will address two key questions:
-
Is treatment with vaginal progesterone during the first trimester of a naturally conceived pregnancy effective for the prevention of miscarriage?
-
Is treatment with vaginal progesterone during the first trimester of a naturally conceived pregnancy for the prevention of miscarriage effective in women with a history of miscarriage(s)?
Furthermore, each participant in the PRISM study was asked to consent for the future evaluation of themselves, the child who is born and the health records of both. Although long-term follow-up will remain outside the scope of this trial, we plan to conduct further studies on outcomes, such as neurodevelopmental outcomes at 7 years of age using aggregated Standard Attainment Tests results from the National Pupil Database, a parent-reported questionnaire to determine levels of cognition, social responsiveness and behaviour, and face-to-face assessments in a subset of children. The NHS number of each baby was recorded to facilitate future follow-up studies.
Acknowledgements
We thank all of the women who participated in this study. We thank the following investigators for supervising recruitment and randomisation at the study centres: Mr Samson Agwu, Mrs Rita Arya, Miss Miriam Baumgarten, Dr Catey Bass, Miss Sumita Bhuiya, Professor Tom Bourne, Mr James Clark, Mr Samual Eckford, Mr Zeiad El-Gizawy, Mrs Joanne Fletcher, Miss Preeti Gandhi, Dr Mary Gbegaje, Dr Ingrid Granne, Mr Mamdough Guirguis, Dr Pratima Gupta, Dr Hadi Haerizadeh, Dr Laura Hipple, Mr Piotr Lesny, Miss Hema Nosib, Mr Jonathan Pepper, Mr Jag Samra, Ms Jayne Shillito, Dr Rekha Shrestha, Dr Jayasree Srinivasan, Dr Ayman Swidan and Professor Derek Tuffnell. We thank all of the PRISM research nurses who assisted in the collection of data; Leanne Beeson, Mary Nulty and Louisa Edwards for their support in managing and co-ordinating the trial; Professor Siladitya Bhattacharya for chairing the TSC; Professor Andrew Shennan for chairing the DMEC; Dr Javier Zamora and Dr Willem Ankum for participating in the DMEC; and all those not otherwise mentioned above who have contributed to the PRISM study.
Contributions of authors
Arri Coomarasamy (https://orcid.org/0000-0002-3261-9807) (Chief Investigator) was involved with the design, implementation and oversight of the trial, and was the primary author of this manuscript.
Hoda M Harb (https://orcid.org/0000-0002-0813-1318) was involved with the design of, implementation of and recruitment to the trial, and was responsible for co-authoring this manuscript.
Adam J Devall (https://orcid.org/0000-0001-5632-079X) was the trial manager and was involved with the implementation and oversight of the trial and was responsible for co-authoring this manuscript.
Versha Cheed (https://orcid.org/0000-0002-6713-0913) was the trial statistician, was responsible for the analysis of the trial data and was responsible for co-authoring this manuscript.
Tracy E Roberts (https://orcid.org/0000-0002-0624-0537) was the senior health economist, was responsible for the health economic analysis and was responsible for co-authoring this manuscript.
Ilias Goranitis (https://orcid.org/0000-0001-7946-8324) was a health economist for the trial and was responsible for the health economic analysis and for co-authoring this manuscript.
Chidubem B Ogwulu (https://orcid.org/0000-0002-8133-7021) was a health economist for the trial and was responsible for the health economic analysis and for co-authoring this manuscript.
Helen M Williams (https://orcid.org/0000-0003-4417-9404) was responsible for implementation and oversight of the trial and was responsible for the critical review of the paper.
Ioannis D Gallos (https://orcid.org/0000-0002-2766-358X) was involved with recruitment to the trial and was responsible for co-authoring this manuscript.
Abey Eapen (https://orcid.org/0000-0003-1951-758X) was involved with recruitment to the trial and was responsible for the critical review of the paper.
Jane P Daniels (https://orcid.org/0000-0003-3324-6771) contributed to the design and critical review of the manuscript.
Amna Ahmed (https://orcid.org/0000-0003-2960-0731) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Ruth Bender-Atik (https://orcid.org/0000-0002-6751-5901) was a patient public involvement representative for the trial and contributed to the critical review of the manuscript.
Kalsang Bhatia (https://orcid.org/0000-0003-0892-4892) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Cecilia Bottomley (https://orcid.org/0000-0003-1914-2482) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Jane Brewin (https://orcid.org/0000-0002-8411-1802) was a patient and public involvement representative for the trial and contributed to the critical review of the manuscript.
Meenakshi Choudhary (https://orcid.org/0000-0003-0526-8965) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Fiona Crosfill (https://orcid.org/0000-0001-6092-3120) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Shilpa Deb (https://orcid.org/0000-0003-4456-8194) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
W Colin Duncan (https://orcid.org/0000-0002-7170-5740) was a co-applicant for the trial and was responsible for the design and contributed to the critical review of the manuscript.
Andrew Ewer (https://orcid.org/0000-0002-3825-4781) was a co-applicant for the trial and was responsible for the design and contributed to the critical review of the manuscript.
Kim Hinshaw (https://orcid.org/0000-0003-0468-4326) was a co-PI for the trial and contributed to the critical review of the manuscript.
Thomas Holland (https://orcid.org/0000-0001-5727-0659) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Feras Izzat (https://orcid.org/0000-0001-9673-4276) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Jemma Johns (https://orcid.org/0000-0003-1079-9170) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Mary-Ann Lumsden (https://orcid.org/0000-0003-2998-9185) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Padma Manda was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Jane E Norman (https://orcid.org/0000-0001-6031-6953) was a co-applicant for the trial, was responsible for the design and contributed to the critical review of the manuscript.
Natalie Nunes (https://orcid.org/0000-0003-1801-6281) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Caroline E Overton (https://orcid.org/0000-0001-5748-9677) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Kathiuska Kriedt (https://orcid.org/0000-0002-9834-7568) was involved with recruitment to the trial and was responsible for the critical review of the paper.
Siobhan Quenby (https://orcid.org/0000-0003-3221-5471) was a co-applicant for the trial, was responsible for the design and contributed to the critical review of the manuscript.
Sandhya Rao was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Jackie Ross (https://orcid.org/0000-0003-4168-6910) was a co-PI for the trial and contributed to the critical review of the manuscript.
Anupama Shahid was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Martyn Underwood (https://orcid.org/0000-0001-6891-7390) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Nirmala Vaithilingham was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Linda Watkins (https://orcid.org/0000-0002-2031-6577) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Catherine Wykes (https://orcid.org/0000-0002-5589-991X) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Andrew W Horne (https://orcid.org/0000-0002-9656-493X) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Davor Jurkovic (https://orcid.org/0000-0001-6487-5736) was a PI for the trial, overseeing recruitment of patients, and contributed to the critical review of the manuscript.
Lee J Middleton (https://orcid.org/0000-0003-4621-1922) was the senior statistician for the trial, was responsible for the analysis of the trial data and was responsible for co-authoring this manuscript.
Publications
Coomarasamy A, Devall AJ, Cheed V, Harb H, Middleton LJ, Gallos ID, et al. A randomised trial of progesterone in women with bleeding in early pregnancy. N Engl J Med 2019;380:1815–24.
Okeke Ogwulu CB, Goranitis I, Devall AJ, Cheed V, Gallos ID, Middleton LJ, et al. The cost-effectiveness of progesterone in preventing miscarriages in women with early pregnancy bleeding: an economic evaluation based on the PRISM trial. BJOG 2020;127:757–67.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Wahabi HA, Fayed AA, Esmaeil SA, Bahkali KH. Progestogen for treating threatened miscarriage. Cochrane Database Syst Rev 2018;8. https://doi.org/10.1002/14651858.CD005943.pub5.
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. A randomized trial of progesterone in women with recurrent miscarriages. N Engl J Med 2015;373:2141-8. https://doi.org/10.1056/NEJMoa1504927.
- National Institute for Health and Care Excellence . Ectopic Pregnancy and Miscarriage: Diagnosis and Initial Management 2012.
- Hemminki E. Treatment of miscarriage: current practice and rationale. Obstet Gynecol 1998;91:247-53. https://doi.org/10.1016/S0029-7844(97)00606-6.
- Farren J, Jalmbrant M, Ameye L, Joash K, Mitchell-Jones N, Tapp S, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011864.
- Ehrenskjöld ML, Bondo B, Weile F. Treatment of threatened abortion with dydrogesterone. Ugeskr Laeg 1967;129:1678-9.
- El-Zibdeh MY, Yousef LT. Dydrogesterone support in threatened miscarriage. Maturitas 2009;65:43-6. https://doi.org/10.1016/j.maturitas.2009.11.013.
- Gerhard I, Gwinner B, Eggert-Kruse W, Runnebaum B. Double-blind controlled trial of progesterone substitution in threatened abortion. Biol Res Pregnancy Perinatol 1987;8:26-34.
- Mistò A. Experiences with 6-dehydro-retroprogesterone int the treatment of placental insufficiency. Ann Ostet Ginecol Med Perinat 1967;89:102-12.
- Omar MH, Mashita MK, Lim PS, Jamil MA. Dydrogesterone in threatened abortion: pregnancy outcome. J Steroid Biochem Mol Biol 2005;97:421-5. https://doi.org/10.1016/j.jsbmb.2005.08.013.
- Palagiano A, Bulletti C, Pace MC, DE Ziegler D, Cicinelli E, Izzo A. Effects of vaginal progesterone on pain and uterine contractility in patients with threatened abortion before twelve weeks of pregnancy. Ann N Y Acad Sci 2004;1034:200-10. https://doi.org/10.1196/annals.1335.022.
- Pandian RU. Dydrogesterone in threatened miscarriage: a Malaysian experience. Maturitas 2009;65:47-50. https://doi.org/10.1016/j.maturitas.2009.11.016.
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. PROMISE: first-trimester progesterone therapy in women with a history of unexplained recurrent miscarriages – a randomised, double-blind, placebo-controlled, international multicentre trial and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20410.
- Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med 2005;159:957-62. https://doi.org/10.1001/archpedi.159.10.957.
- International Organisation for Standardisation (ISO) . ISO 27000 – Information Technology, Security Techniques, Information Security Management Systems, Overview and Vocabulary n.d. www.iso.org/obp/ui/#iso:std:iso-iec:27000:ed-5:v1:en (accessed 22 June 2019).
- e-CFR . Electronic Code of Federal Regulations 2019. https://gov.ecfr.io/cgi-bin/ECFR (accessed 22 August 2019).
- US Food and Drug Administration . CFR – Code of Federal Regulations Title 21 2018. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=11 (accessed 22 June 2019).
- Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103-15. https://doi.org/10.2307/2529712.
- White SJ, Freedman LS. Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer 1978;37:849-57. https://doi.org/10.1038/bjc.1978.124.
- Bottomley C, Van Belle V, Pexsters A, Papageorghiou AT, Mukri F, Kirk E, et al. A model and scoring system to predict outcome of intrauterine pregnancies of uncertain viability. Ultrasound Obstet Gynecol 2011;37:588-95. https://doi.org/10.1002/uog.9007.
- European Commission . EudraLex – Volume 4 – Good Manufacturing Practice (GMP) Guidelines n.d. https://ec.europa.eu/health/documents/eudralex/vol-4_en (accessed 22 June 2019).
- Official Journal of the European Union . Commission Directive 2005 28 EC of 8 April 2005 Laying Down Principles and Detailed Guidelines for Good Clinical Practice As Regards Investigational Medicinal Products For Human Use, As Well As the Requirements for Authorisation of the Manufacturing or Importation of Such Products 2005. https://ec.europa.eu/health//sites/health/files/files/eudralex/vol-1/dir_2005_28/dir_2005_28_en.pdf (accessed 22 June 2019).
- Great Britain . The Medicines for Human Use (Clinical Trials) Regulations 2004 2004. www.legislation.gov.uk/uksi/2004/1031/contents/made (accessed 22 June 2019).
- Besins Healthcare (UK) Ltd . Utrogestan Vaginal 200mg Capsules 2019. www.medicines.org.uk/emc/product/3244 (accessed 22 June 2019).
- National Institute for Health and Care Excellence . Progesterone n.d. https://bnf.nice.org.uk/drug/progesterone.html (accessed 22 June 2019).
- Nosarka S, Kruger T, Siebert I, Grové D. Luteal phase support in in vitro fertilization: meta-analysis of randomized trials. Gynecol Obstet Invest 2005;60:67-74. https://doi.org/10.1159/000084546.
- van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2015;7. https://doi.org/10.1002/14651858.CD009154.pub3.
- Rai R, Regan L. Recurrent miscarriage. Lancet 2006;368:601-11. https://doi.org/10.1016/S0140-6736(06)69204-0.
- Li TC, Spuijbroek MD, Tuckerman E, Anstie B, Loxley M, Laird S. Endocrinological and endometrial factors in recurrent miscarriage. BJOG 2000;107:1471-9. https://doi.org/10.1111/j.1471-0528.2000.tb11670.x.
- Li TC, Tuckerman EM, Laird SM. Endometrial factors in recurrent miscarriage. Hum Reprod Update 2002;8:43-52. https://doi.org/10.1093/humupd/8.1.43.
- Szekeres-Bartho J, Balasch J. Progestagen therapy for recurrent miscarriage. Hum Reprod Update 2008;14:27-35. https://doi.org/10.1093/humupd/dmm035.
- Bulletti C, de Ziegler D, Flamigni C, Giacomucci E, Polli V, Bolelli G, et al. Targeted drug delivery in gynaecology: the first uterine pass effect. Hum Reprod 1997;12:1073-9. https://doi.org/10.1093/humrep/12.5.1073.
- Cicinelli E, Cignarelli M, Sabatelli S, Romano F, Schonauer LM, Padovano R, et al. Plasma concentrations of progesterone are higher in the uterine artery than in the radial artery after vaginal administration of micronized progesterone in an oil-based solution to postmenopausal women. Fertil Steril 1998;69:471-3. https://doi.org/10.1016/S0015-0282(97)00545-1.
- Coomarasamy A, Thangaratinam S, Gee H, Khan KS. Progesterone for the prevention of preterm birth: a critical evaluation of evidence. Eur J Obstet Gynecol Reprod Biol 2006;129:111-18. https://doi.org/10.1016/j.ejogrb.2006.05.013.
- da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol 2003;188:419-24. https://doi.org/10.1067/mob.2003.41.
- Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Fetal Medicine Foundation Second Trimester Screening Group . Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 2007;357:462-9. https://doi.org/10.1056/NEJMoa067815.
- Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst Rev 2013;7. https://doi.org/10.1002/14651858.CD004947.pub3.
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 2003;348:2379-85. https://doi.org/10.1056/NEJMoa035140.
- Coomarasamy A, Devall AJ, Cheed V, Harb H, Middleton LJ, Gallos ID, et al. A randomised trial of progesterone in women with bleeding in early pregnancy. N Engl J Med 2019;380:1815-24. https://doi.org/10.1056/NEJMoa1813730.
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702-6. https://doi.org/10.1093/aje/kwh090.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 1976;34:585-612. https://doi.org/10.1038/bjc.1976.220.
- Chatfield A, Caglia JM, Dhillon S, Hirst J, Cheikh Ismail L, Abawi K, et al. Translating research into practice: the introduction of the INTERGROWTH-21st package of clinical standards, tools and guidelines into policies, programmes and services. BJOG 2013;120:139-42. https://doi.org/10.1111/1471-0528.12416.
- Gardosi J, Mongelli M, Wilcox M, Chang A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol 1995;6:168-74. https://doi.org/10.1046/j.1469-0705.1995.06030168.x.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- Joint Formulary Committee . British National Formulary (Online) 2018. www.medicinescomplete.com (accessed 22 June 2019).
- NHS Improvement . NHS Reference Costs 2016 17 2017.
- Department of Health and Social Care . NHS Reference Costs 2013 to 2014 2014.
- Curtis L, Netten A. Unit Costs of Health and Social Care 2002. Canterbury: PSSRU, University of Kent; 2002.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Okeke Ogwulu CB, Goranitis I, Devall AJ, Cheed V, Gallos ID, Middleton LJ, et al. The cost-effectiveness of progesterone in preventing miscarriages in women with early pregnancy bleeding: an economic evaluation based on the PRISM trial. BJOG 2020;127:757-67. https://doi.org/10.1111/1471-0528.16068.
- Intensive Care Society . Levels of Critical Care for Adult Patients 2009. www.ics.ac.uk/ (accessed 10 May 2018).
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. https://doi.org/10.1002/1097-0258(20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Zellner A. An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. J Am Stat Assoc 1962;57:348-68. https://doi.org/10.1080/01621459.1962.10480664.
- Moon HR, Perron B. Seemingly Unrelated Regressions. The New Palgrave Dictionary of Economics. London: Palgrave Macmillan; 2008.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. https://doi.org/10.1002/hec.843.
- Glick HA, Briggs AH, Polsky D. Quantifying stochastic uncertainty and presenting results of cost-effectiveness analyses. Expert Rev Pharmacoecon Outcomes Res 2001;1:25-36. https://doi.org/10.1586/14737167.1.1.25.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-14. https://doi.org/10.1177/0272989X9001000308.
- Simon J, Petrou S, Gray A. The valuation of prenatal life in economic evaluations of perinatal interventions. Health Econ 2009;18:487-94. https://doi.org/10.1002/hec.1375.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 2004.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Petrou S, Trinder J, Brocklehurst P, Smith L. Economic evaluation of alternative management methods of first-trimester miscarriage based on results from the MIST trial. BJOG 2006;113:879-89. https://doi.org/10.1111/j.1471-0528.2006.00998.x.
- Norman JE, Marlow N, Messow CM, Shennan A, Bennett PR, Thornton S, et al. Does progesterone prophylaxis to prevent preterm labour improve outcome? A randomised double-blind placebo-controlled trial (OPPTIMUM). Health Technol Assess 2018;22. https://doi.org/10.3310/hta22350.
- National Collaborating Centre for Women’s and Children’s Health (UK) . Diabetes in Pregnancy—Management of Diabetes and Its Complications from Preconception to the Postnatal Period 2015.
- Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril 2000;73:300-4. https://doi.org/10.1016/S0015-0282(99)00495-1.
- European Society of Human Reproduction and Embryology . Recurrent Pregnancy Loss: Guideline of the European Society of Human Reproduction and Embryology 2017.
- Devall AJ, Gallos, ID, Middleton, LJ, Coomarasamy A. Vaginal progesterone treatment during the first trimester of pregnancy for the prevention of miscarriage: an individual participant data (IPD) meta-analysis. PROSPERO 2018:CRD42018064560 n.d. www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018064560.
List of abbreviations
- AE
- adverse event
- BCa
- bias-corrected and accelerated
- BMI
- body mass index
- BNF
- British National Formulary
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CHARM
- Charity for Research into Miscarriage
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CRF
- case report form
- C-section
- caesarean section
- DAU
- day assessment unit
- DMEC
- Data Monitoring and Ethics Committee
- FIGO
- International Federation of Gynaecology and Obstetrics
- GP
- general practitioner
- HCHS
- Hospital and Community Health Services
- HDU
- high-dependency unit
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IMP
- investigational medicinal product
- IQR
- interquartile range
- ITMS
- integrated trial management system
- ITU
- intensive therapy unit
- LLETZ
- large loop excision of the cervical transformation zone
- MID
- minimally important difference
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PBAC
- pictorial bleeding assessment chart
- PI
- principal investigator
- PRIME
- Public and Researchers Involvement in Maternity and Early Pregnancy
- PRISM
- Progesterone in Spontaneous Miscarriage
- PROMISE
- Progesterone in Recurrent Miscarriage
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- PUL
- pregnancy of unknown location
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative rate
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SAR
- serious adverse reaction
- SD
- standard deviation
- SUSAR
- suspected unexpected serious adverse reaction
- TCC
- trial co-ordinating centre
- TSC
- Trial Steering Committee
- WTP
- willingness to pay