Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 96/20/99. The contractual start date was in June 2001. The draft report began editorial review in March 2018 and was accepted for publication in November 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Malcolm Mason reports personal fees from Sanofi (Paris, France), Bayer (Leverkusen, Germany) and Janssen Pharmaceutica (Beerse, Belgium) outside the submitted work. Derek Rosario reports grants from Bayer and personal fees from Ferring Pharmaceuticals (Saint-Prex, Switzerland) outside the submitted work. Jane Blazeby was a member of the following during the project: National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Clinical Trials and Evaluation Committee (2009–2013), HTA NIHR Obesity (2010–2012), Commissioning Board for HTA Surgery Themed Call Board and the NIHR Clinical Trials Unit (CTU) Standing Advisory Committee (2015–2019). Jenny Donovan was a member of the following during the project: HTA Commissioning Board (2006–12), Rapid Trials and Add-on Studies Board (2012), NIHR Senior Investigator panel (2009–12) and NIHR Health Services and Delivery Research board (Deputy Chairperson) (2010–11). Freddie C Hamdy was a member of the following during the project: HTA Commissioning Board (2007–12) and HTA Surgery Themed Call Board (2012–13). J Athene Lane was a member of the following during the project: CTUs funded by NIHR (2017 to present). Chris Metcalfe was a member of the following during the project: CTUs funded by NIHR (2010 to present). Tim Peters was a member of the following during the project: HTA Medicines for Children Themed Call (2005–6) and NIHR CTU Standing Advisory Committee (2008–14). John Staffurth reports support for travel to conferences and attendance on an advisory board from Bayer. Emma L Turner reports grants from Cancer Research UK (London, UK) during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Hamdy et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
Prostate cancer is the most common cancer among men in the UK. In 2014, there were 46,610 new cases of diagnosed prostate cancer, and 11,287 men died from the disease that year. Incidence rates for prostate cancer are projected to rise by 12% in the UK between 2014 and 2035, to 233 cases per 100,000 males by 2035. 1 The lifetime risk of being diagnosed with prostate cancer is one in eight for men in the UK, and, although it is often overtreated, many men are undertreated. In the USA alone, 26,730 deaths from the disease were expected in 2017. 2 It is estimated that there were approximately 330,000 men living with prostate cancer in the UK in 2015, and this number is expected to rise to around 830,000 by 2040. 3 The incidence of prostate cancer is increasing, with wider use of prostate-specific antigen (PSA) testing in asymptomatic men and with the population ageing, and has tripled over the past 35 years in many countries in Europe. 4 Although prostate cancer can be lethal, the majority of men who are diagnosed through PSA testing will not suffer clinically significant consequences from the disease during their lifetime, and evidence that treating such men improves survival or quality of life (QoL) is weak. Consequently, there are concerns that increasing PSA testing in the community is resulting in overdiagnosis, overtreatment, and an increasing burden on the NHS in the UK and other health providers elsewhere. Despite its high incidence and social and economic impact, prostate cancer continues to be under-researched, and only a limited number of studies are addressing the issues of screening and long-term comparison of treatment modalities.
In particular, there have been very few studies of the longer-term impact on QoL of the major initial treatments for localised prostate cancer. Only one trial has reported on this: the Scandinavian Prostate Cancer Group-4 (SPCG-4) trial5 from the pre-PSA era comparing passive ‘watchful waiting’ with open prostatectomy for clinically detected prostate cancer. This trial’s follow-up, 12 years after randomisation, showed that urinary incontinence was persistently worse among those who had undergone prostatectomy rather than watchful waiting and sexual dysfunction was similarly poor in both groups. In addition, rates of sexual dysfunction, urinary leakage and anxiety were found to be much higher in SPCG-4 trial participants than in an age-matched cohort, with decreasing QoL reported from the increasing use of hormone therapy for progressing prostate cancer. 5,6 However, the SPCG-4 trial did not collect these data with a validated patient-reported outcome measure (PROM), the trial compared only surgery and passive ‘watchful waiting’ [not radiotherapy or active monitoring (AM)], participants had clinically detected not PSA-detected disease at diagnosis and treatments for advancing prostate cancer during their follow-up were very different from current options.
Most comparative cohort studies have analysed PROMs in only the short term,7 with only a small number extending this to the medium term of 5–6 years. 8–10 These cohort studies have produced somewhat contradictory findings, most likely because of baseline differences in PROMs between men receiving different treatments. Miller et al. 8 found that sexual, urinary and bowel dysfunction remained more prevalent and bothersome in men receiving surgery or radiotherapy than in an age-matched control group, and that sexual function remained stable after radical prostatectomy (RP) but continued to decline over time after radical radiotherapy (RT), whereas general QoL remained stable. They commented that they had found ‘evolving and potentially unexpected changes in long-term, patient-reported QoL as patients proceed from earlier to later phases of survivorship’ that required further research. 8 Potosky et al. 9 also reported large declines in sexual function following RT but Fransson and Widmark10 found no such worsening. There have been even fewer studies in the longer term, and these have also produced conflicting results. Resnick et al. 11 reported that differences in levels of urinary incontinence and erectile dysfunction for RT and RP at 5 years reduced to become similar by 15 years, but noted that the specific contribution of prostate cancer treatments could not be distinguished from age-dependent changes in the longer term. In contrast, a population-based study12 comparing prostate cancer survivors with an age-matched control group found similar levels of global QoL in the longer term, but with prostate cancer survivors reporting severe and persistent urinary, bowel and sexual adverse effects, particularly if they had received combined treatments.
There is a paucity of research on the QoL impacts of progressing prostate cancer. Recent large trials have focused on mortality and clinical outcomes without parallel reporting of QoL impacts [e.g. CHAARTED (ChemoHormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer)13]. This hinders men when making informed choices between treatments because there is a lack of information about the potential magnitude of QoL benefits or the likelihood of a man realising those benefits. A small number of older trials have included PROMs, and their results have been helpful. For example, the Medical Research Council RT01 trial14 found that sexual functioning deteriorated, urinary function did not change and there was a slight decline in physical well-being, although not in overall QoL, after androgen deprivation therapy (ADT) was received in advance of high- or standard-dose RT. A pooled analysis showed that denosumab prevented progression of pain severity and pain interference more effectively than zoledronic acid in patients with advanced solid tumours (including some with prostate cancer) and bone metastases. 15 A systematic review concluded that effects on QoL of prostate cancer and its treatments varied across treatments and disease stage, and that there was a ‘pressing need for more research in men with advanced disease’. 16 There is some evidence that better information can improve patient satisfaction and even QoL. 17 A more recent review by the American Cancer Society18 noted that there remains very limited evidence to underpin guidelines for the long-term care of men with prostate cancer. The majority of articles found up to 2014 were case–control studies with fewer than 500 participants or reviews without PROMs, and there were only a small number of higher-quality studies using population-based data. In particular, the review stated that ‘the lack of clinical trials is a limitation of the current state of the science for survivorship’. 18
Diagnosis of prostate cancer
When the Prostate testing for cancer and Treatment (ProtecT) trial was designed in the late nineties, prostate cancer was diagnosed following serum PSA testing, with transrectal ultrasound (TRUS)-guided biopsies in most cases. Although this allowed many early cancers to be detected, the majority represented low-risk disease, which does not tend to progress. Intermediate-risk, high-risk and locally advanced cancers were also detected, but in smaller numbers, and lethal cancers could be missed. More recently, multiparametric magnetic resonance imaging (mpMRI) technology and dissemination has been reaching a level at which accurate assessment of the location and grade of prostate cancer using imaging and biopsies is possible, as demonstrated in the recently published National Institute for Health Research (NIHR) Health Technology Assessment (HTA) PROstate Magnetic resonance Imaging Study (PROMIS);19 this was a definitive validating cohort study evaluating mpMRI as a triage test. PROMIS demonstrated higher levels of accuracy in the detection of clinically important disease, and found that ‘TRUS-biopsy performs poorly as a diagnostic test for clinically significant prostate cancer. mpMRI, used as a triage test before first prostate biopsy, could identify a quarter of men who might safely avoid an unnecessary biopsy and might improve the detection of clinically significant cancer’. 20 This is transforming the diagnostic pathway for prostate cancer, and it is likely that if it were available during the conduct of ProtecT, it would have had an impact on the disease-risk composition of our cohorts.
Screening and the linked CAP (Cluster randomised trial of PSA testing for Prostate cancer) trial
Evidence from randomised controlled trials (RCTs) in Europe [European Randomized Study of Screening for Prostate Cancer (ERSPC), n = 162,24321] and the USA [Prostate, Lung, Colorectal, and Ovarian (PLCO), n = 76,69322] has not resolved the controversies surrounding PSA-based prostate cancer screening, resulting in different recommendations worldwide. 23,24 The prognosis for low- and intermediate-risk localised prostate cancer is excellent,25 and although there is fair-quality evidence that screening by PSA testing reduces prostate cancer deaths,26 debate continues about the trade-off between the mortality benefit and risks of harm from overdetection and overtreatment. 22–24
Current UK policy does not advocate screening. 27 The proposed 2017 update from the US Preventative Services Task Force recommends individualised decision-making for men between the ages of 55 and 69 years after a discussion of risks and harms with their physician. 26 This latest guidance comes amid concerns about the quality of previous evidence,24 favourable modelling projections,28 new secondary analyses,28 greater absolute risk (but not rate) benefits with long-term follow-up,29 the use of active surveillance (AS) to avoid radical treatment unless cancer is progressing30 and long-term data on the effects of different treatment options for localised prostate cancer. 25,30 The PLCO22 and ERSPC21 trials undertook repeated PSA testing at intervals of 1, 2 or 4 years. Less intensive strategies, such as longer screening intervals or one-off screens, have been predicted to reduce overdetection, overtreatment and costs relative to more frequent screening. 31,32 However, opportunistic screening may increase overdetection without reducing prostate cancer mortality. 33
We undertook the primary-care-based Cluster randomised trial of PSA testing for Prostate cancer (CAP),34 within which the ProtecT trial of treatments for localised prostate cancer was embedded. CAP was designed to determine the effects of a low-intensity, single-invitation PSA test and standardised diagnostic pathway on prostate cancer-specific and all-cause mortality while minimising overdetection and overtreatment.
Between 2001 and 2009, general practices (the clusters) around eight hospital centres in England and Wales were randomised before recruitment (‘Zelen’ design) to intervention or control groups and approached for consent to participate. In the intervention group, men aged 50–69 years received a single invitation to a nurse-led clinic appointment (the intervention) at which they were provided with information about PSA testing and the treatment trial. Screened men with a PSA level of ≥ 3.0 ng/ml were offered a standardised 10-core TRUS-guided biopsy. Those diagnosed with clinically localised prostate cancer were offered recruitment to the ProtecT treatment trial comparing RP, radical conformal radiotherapy with neoadjuvant ADT and AM. 25 Control practices provided standard NHS management, with information about PSA testing provided to only men who requested it. 35
The primary outcome was prostate cancer mortality at a median of 10 years of follow-up (reached by March 2016), analysed by intention to screen. Prespecified secondary outcomes were diagnostic stage and grade of prostate cancers identified, all-cause mortality at 10 and 15 years, prostate cancer mortality at 15 years, health-related QoL, cost-effectiveness and instrumental variable analysis estimating the causal effect of attending PSA screening.
Study population
Parts of this section have been reproduced with permission from Martin et al. 36 JAMA 2018;319(9):883–95. Copyright © 2018 American Medical Association. All rights reserved.
In total, 911 general practices were randomised in 99 geographical areas. Of these, 126 were subsequently excluded as ineligible. 34 Consent rates among the remaining eligible intervention (n = 398) and control (n = 387) group general practices were 68% (n = 271) and 78% (n = 302), respectively; 195,912 and 219,445 men registered with these 573 practices were eligible for the intervention and control groups, respectively. After exclusions, the main analysis was based on 189,386 men in the intervention group and 219,439 men in the control group. There were some differences between numbers of participants in the intervention group of this trial and the published ProtecT study population. 25 There were no important differences between the measured characteristics of practices that did agree to participate and those that did not agree to participate. 34 There were also no important differences in measured baseline characteristics between intervention group and control group practices or men, indicating that post-randomisation exclusions did not introduce detectable selection biases.
Among 189,386 intervention group men in the CAP study, 75,707 (40%) attended the PSA testing clinic, 67,313 (36%) had a blood sample taken and 64,436 had a valid test result. Of these 64,436 men, 6857 (11%) had a PSA level of between 3 ng/ml and 20 ng/ml (eligible for ProtecT), of whom 5850 (85%) had a prostate biopsy. Intervention group men who attended PSA testing clinics were sociodemographically similar to non-attenders. 37 Cumulative contamination (PSA testing in the control group) was indirectly estimated at ≈10–15% over 10 years, based on previously reported diagnostic referral rates and ≈20% of follow-up being subsequent to a PSA test undertaken for screening. 38–40
The results of the primary analysis have been published. 36 After an average of 10 years of follow-up, there were 8054 (4.3%) cases of cancer in the screened group and 7853 (3.6%) cases in the control group. Crucially, both groups had the same percentage of deaths (0.29%). This demonstrated that the single PSA test followed by TRUS-guided biopsy diagnostic pathway does not appear to improve disease-specific survival at a median follow-up of 10 years, but longer follow-up is needed. Critically, the testing missed the diagnosis of an important number of lethal cancers, confirming the necessity to improve our diagnostic pathway, perhaps by incorporating imaging in the form of mpMRI upstream of biopsies, in order to triage men at risk of significant disease, and targeting significant cancers.
Treatment options for localised prostate cancer
Parts of this section are reproduced from Hamdy et al. 41 Reproduced with permission from JAMA 2017;317:1121–3. Copyright © 2017 American Medical Association. All rights reserved.
Conventional treatment options for men with clinically localised prostate cancer include AM/AS, RP, now most commonly carried out as robot-assisted laparoscopic procedures, intensity-modulated radiotherapy (IMRT) and brachytherapy, which appear to have similar short- to medium-term oncological outcomes in non-randomised studies.
Active monitoring/surveillance protocols involve regular clinical examination, PSA measurements, mpMRI and repeat biopsies. If these parameters suggest the risk of progression, men are offered radical treatment. A number of Phase II studies have shown that delayed intervention due to signs of progression takes place in approximately one-third of AS groups within 5 years of diagnosis. 42,43 For those with intermediate disease, AM has been reported as conferring an 84% 5-year metastasis-free survival rate. 44 However, observational strategies can lead to significant anxiety. 45
Active monitoring has been tested in clinically-localised prostate cancer in the context of the ProtecT study,46 which reported that although RP and radiotherapy were associated with lower rates of disease progression, 44% of men assigned to AM did not receive radical treatment and, thus, avoided side effects. Men with newly diagnosed, localised prostate cancer therefore need to consider the critical trade-off between the short-term and long-term effects of radical treatments on urinary, bowel and sexual function and the higher risks of disease progression with AM, as well as the effects of each of these options on QoL.
Radical prostatectomy involves total open, laparoscopic or robot-assisted surgery to remove the entire prostate gland and seminal vesicles. The proportion of prostate cancer patients receiving surgery varies with age: 8% of prostate cancer patients receive a major surgical resection as part of their cancer treatment, with fewer resections in the oldest age group (0% in those aged ≥ 85 years) than in the youngest age group (29% in those aged 15–54 years). 1 Until recent years, most RPs were carried out using conventional open surgery. Laparoscopic RP was developed in the nineties, and evolved to robot-assisted techniques, which are now used almost exclusively. A recent RCT compared open with robotic techniques and showed no differences in short-term oncological and functional outcomes. 47
Radical radiotherapy in the form of external beam radiotherapy (EBRT) is a common treatment in the UK for men diagnosed with localised prostate cancer. It is usually preceded by 3–6 months of neoadjuvant androgen suppression, and is given in daily fractions over 4–8 weeks as an outpatient. In large reported series, EBRT conferred a 5-year disease-free survival of between 78% and 80%, or 88% and 94% in combination with hormone therapy. 48–51 IMRT, an optimised form of EBRT, is delivered in some centres. 52
Brachytherapy can be given either as permanent radioactive seed implantation or as high-dose brachytherapy using a temporary source. For localised prostate cancer, the 5-year biochemical failure rates are similar for permanent seed implantation, high-dose (> 72 Gy) external radiation, combination seed/external irradiation and RP. 53
Radical, extensive treatments carry the potential for significant short-, medium- and long-term morbidity, such as urinary leakage, erectile dysfunction and radiotherapy toxicity. At present, there is little difference between RP and RT in terms of cancer control in the short to medium term; much of the decision-making process that governs treatment allocation is based on the differences in the side effect profiles associated with the various interventions. 52 The recently published clinical and patient-reported outcomes from the ProtecT study30,41 demonstrated that each treatment option has a particular pattern of adverse effects on QoL in the short term. Urinary incontinence and sexual dysfunction were worst after surgery, followed by recovery but persistent difficulties for some men; bowel problems were worst after radiation, with sexual dysfunction mostly related to neoadjuvant ADT. Although adverse effects of interventions can be avoided initially with AS, there is a natural decline in urinary and sexual function symptoms over time, and the adverse impacts of radical treatments will be experienced when those treatments are received. 30,41 Findings from the ProtecT trial described in this report have therefore established the true side effect profiles of the various treatment options. 54 These side effect profiles have been consistently described even with more modern and contemporary radical treatment options, such as robot-assisted surgery, and different forms of radiation, including brachytherapy. It is therefore true to state that contemporaneous men who are treated with current forms of radical therapy will continue to suffer from the now well-described and well-documented side effect profile patterns related to these treatments, substantiated by more recent PROMs reported by two large prospective observational cohorts from the USA. 55,56
Alternatives to conventional therapies
Alternative, targeted focal ablative therapies are being developed in an attempt to reduce treatment burden, improve QoL and reduce adverse events associated with radical treatment, while retaining at least equivalent cancer control. Focal therapies should minimise morbidity by lowering the chance of damage to the neurovascular bundles responsible for erectile function, and the urinary continence mechanism, and may help to avoid the psychological morbidity associated with surveillance, but their long-term oncological effectiveness remains untested.
These alternative technologies are being used as primary ablative therapy in a number of centres worldwide, but have been introduced without robust Phase III RCT validation. Examples include high-intensity focused ultrasound (HIFU), cryotherapy, vascular-targeted photodynamic therapy (VTP), radiofrequency interstitial tumour ablation (RITA), laser photocoagulation and irreversible electroporation. Each one is at a different stage in its evaluation and application to clinical practice. The current evidence for focal therapy for prostate cancer is mostly provided from non-randomised Phase I and II trials in single centres and from case series with small numbers of patients. 57 A previous Phase I/II study has demonstrated that as few as 5% of men suffered from genitourinary side effects after focal therapy, with absence of clinically significant cancer in all treated patients. A manufacturer-sponsored Phase III RCT of VTP versus AS in 413 men with very low-risk disease has been recently published with 2-year follow-up data; this is discussed later in this section. 58
High-intensity focused ultrasound uses ultrasound energy focused by an acoustic lens to cause tissue damage as a result of thermal coagulative necrosis and acoustic cavitation. The procedure is undertaken using a transrectal approach and may be carried out under general or spinal anaesthesia as a day-case procedure.
Cryotherapy is the localised destruction of tissue by extremely low temperatures followed by thawing, and may be undertaken under general or regional anaesthesia. Cryoneedles or probes are inserted into the prostate via the perineum, using image guidance.
Vascular-targeted photodynamic therapy uses light to activate an intravenously administered photosensitising drug to produce instantaneous vessel occlusion and subsequent tissue necrosis. The light is delivered by optical fibres placed transperineally under transrectal ultrasound guidance. VTP is given under general anaesthesia and can be undertaken as a day-case procedure.
A manufacturer-sponsored Phase III RCT of VTP versus AS in 413 men with very low-risk disease has been recently published, with 2-year follow-up data. 59 It found VTP to be safe and effective, with a 66% reduced risk of treatment failure [adjusted hazard ratio (HR) 0.34, 95% confidence interval (CI) 0.24 to 0.46] compared with AS; however, the VTP group experienced more frequent and severe side effects, although these were mostly mild and of short duration.
Other ablative technologies are currently under evaluation but without sufficient evidence to be used within the context of a RCT. Examples include radiofrequency ablation, which acts by converting radiofrequency waves to heat, resulting in thermal damage. RITA has recently been proposed for the treatment of prostate cancer. 60–63 Interstitial laser photocoagulation was reported by Amin et al. ,64 who described a percutaneous technique for local ablation. Irreversible electroporation is a new non-thermal ablation modality that uses short pulses of DC (direct current) electric current to create irreversible pores in the cell membrane, thus causing cell death. 65,66
These newer techniques have been evaluated in a systematic review by Ramsay et al. ,67 who conclude that they have not been evaluated with sufficient reliability to inform their utilisation in the NHS.
Ongoing and recently completed studies clearly indicate that the evidence base for partial ablation therapies is increasing, particularly the evidence for focal ablative therapies. However, the quality of the evidence base will not improve substantially given that the majority of these studies are case series. Research efforts in the use of ablative therapies in the management of prostate cancer should focus on conducting more rigorous, high-quality studies. A NIHR HTA feasibility trial was completed successfully by the authors of this report to assess rates of recruitment and randomisation to a trial comparing radical treatments with partial ablation of the prostate,68 and a full RCT application is currently being reviewed by the NIHR HTA programme.
Implications for research
The lack of RCT-based evidence for the treatment effectiveness of radical therapeutic options with AM for PSA-detected clinically localised prostate was the main driver for the inception and conduct of the ProtecT trial. The overarching aim was to provide robust evidence to inform patients, clinicians and policy-makers to develop optimal guidelines and recommendations for the management of this common and ubiquitous disease.
At the same time, NIHR supported a feasibility study of creating an overarching screening trial to provide the recruitment framework for ProtecT. A previous trial, ERSPC,21 had shown a 20% reduction in mortality after 13 years with repeated PSA testing, but with unacceptable levels of overdetection and overtreatment. In 2003, Cancer Research UK agreed to support the CAP trial. The goal of CAP, with its low-intensity, one-off PSA testing, was to try to avoid unnecessary detection of low-risk cancers while still identifying men with dangerous disease for whom screening and early treatment could be beneficial. The trial’s median 10-year outcomes have been summarised earlier in this section. 36
A further important strategy, which remains untested, is to use novel technologies to target and treat all clinically significant cancers in the gland focally, with careful follow-up and repeat treatments as necessary, particularly of emerging new lesions detected by biopsy. The strategy may obviate the need for any radical therapies.
Identifying men at high risk of harbouring significant prostate cancer is essential to achieve a reduction in overdetection, subsequent overtreatment as well as improving undertreatment of aggressive cancers. This requires comprehensive high-throughput platform investigation of large, well-phenotyped cohorts of men and their biobanked material with accurate and long-term follow-up, which the ProtecT cohort offers, and the investigators are poised to undertake this next critical step in patient stratification pending appropriate funding for the research.
Rationale for the ProtecT trial and summary of the feasibility study
Rationale for the ProtecT trial
The discovery and wide clinical use of serum PSA for over three decades to detect asymptomatic cancers in fit men, combined with the continuous refinement of curative radical treatments including anatomical RP, has led to a substantial increase in the detection and treatment of early prostate cancer. In countries where PSA testing has been encouraged, overdetection and overtreatment of prostate cancer has prevailed, with unnecessary adverse events caused by treatments and cost pressures for health-care providers. Unlike most developed countries, the UK’s policy on screening for prostate cancer has been not to recommend its use.
The ERSPC21 investigated the effects of screening versus no screening in a large multicentre European cohort. The study reported an advantage in favour of screening, which improved survival and reduced disease progression, but at a substantial cost of overdetection and overtreatment. 31,69 In contrast, the US screening study (PLCO22) showed no beneficial effect of screening for prostate cancer, but suffered serious limitations because of contamination in both groups. 70
In large reported series, RP conferred a 5-year disease-free survival of between 69% and 84%. 71–75 The Scandinavian SPCG-4 RCT comparing surgery and watchful waiting showed an absolute risk reduction in preventing cancer mortality within 8 years of 5% (from 14% to 9%). 76 An update has shown that this absolute difference remains unchanged with longer follow-up of 14 years. 77,78 However, the Prostate Intervention versus Observational Treatment (PIVOT) study in the USA compared RP with watchful waiting in over 700 men with localised PSA-detected prostate cancers,79 concluding that RP did not significantly reduce all-cause or prostate cancer mortality, compared with observation, through at least 12 years of follow-up. There were fewer prostate cancer deaths with RP (21/364 for RP vs. 31/367 for watchful waiting, HR 0.63, 95% CI 0.36 to 1.09; p = 0.09). In other large reported series, radiotherapy conferred a 5-year disease-free survival of 78–94% in combination with hormone therapy. 48–51
Although prostate cancer can be lethal, the majority of men who are diagnosed through PSA testing will not suffer clinically significant consequences from the disease during their lifetime, and evidence that treating such men improves survival or QoL is weak. Consequently, there have been serious concerns that increasing PSA testing in the community is resulting in overdiagnosis, overtreatment and an increasing burden to the NHS. Despite its high incidence and social and economic impact, prostate cancer continues to be under-researched and only a limited number of studies are addressing the issues of screening and long-term comparison of treatment modalities. Following a successful feasibility study, described in the following section, the NIHR HTA ProtecT study conducted by the authors of this report aimed to test the value of a single round of PSA testing in the community, and compared the effectiveness of the three conventional treatment options of AM, RP and EBRT in clinically localised disease at all risk levels. A large prostate cancer detection programme was essential to conduct the main RCT of treatment, in order to diagnose men and counsel them in advance of the diagnostic pathway, which allowed them to become well-informed participants to the treatment trial.
The ProtecT feasibility study
The ProtecT feasibility study, conducted between 1999 and 2001, was undertaken in three clinical centres to provide evidence to underpin the design and conduct of the main ProtecT trial. The methods of the study and its findings have been published in detail. 80 In brief, the feasibility study showed that men aged 50–69 years could be identified in primary care practices, invited to attend a prostate check clinic appointment to discuss having a PSA test and potentially participating in a RCT of treatment, and that sufficient numbers would attend and follow the diagnostic pathway to feed into the proposed treatment trial. By the end of the feasibility study, 8505 men from 18 primary care centres attended clinics (56% of those invited) and 7383 had a PSA test. Of these men, 861 (12%) had a raised PSA level and, after biopsy, 224 were found to have prostate cancer, with 165 clinically localised and eligible for the treatment RCT. These response rates were largely reflected in the main trial. The feasibility study also showed that men were willing to complete detailed questionnaires before the PSA test and biopsy. These questionnaires comprised patient-reported outcomes that were then used in the main trial.
As it was expected that recruitment to the trial would be extremely difficult, the feasibility study was innovatively embedded in a qualitative research study to explore a wide range of issues with the urologists and nurses undertaking recruitment and among men participating in the study. 81 In addition, the feasibility study included a RCT comparing the effectiveness and efficiency of recruiting with nurses or urologists. In total, 167 men with localised prostate cancer were identified and 150 (90%) took part. There was a 4.0% difference between nurses and surgeons in recruitment rates (67% nurses, 71% urologists, 95% CI −10.8% to +18.8%; p = 0.60). Cost minimisation analysis showed that nurses spent longer with patients but surgeon costs were higher and nurses often supported surgeon-led clinics. We concluded that nurses were as effective and more cost-effective recruiters than surgeons, and so nurses became the major recruiters in the main RCT.
The qualitative research involved in-depth interviews with 39 men before and after the PSA result, case studies of four men interviewed several times after the PSA result as they progressed through the study, 20 audio-recorded recruitment appointments and subsequent interviews, and 15 other audio-recordings of recruitment appointments with nurses or urologists attempting to recruit men to the treatment trial. Findings from these data included:
-
Men perceived the offer of PSA testing as an opportunity to discover cancer early, most did not want to consider the implications of a positive result and most expected to receive a negative test result. 82
-
Although most men could recall and understand the reason for the treatment RCT, they did not always find it acceptable, and most found the concept of randomisation difficult to accept. However, those who understood and were able to believe in clinical equipoise were most likely to consent to randomisation. 83 These findings formed a detailed basis for developing suitable and effective trial information in relation to clinical equipoise in the main RCT.
-
In recruitment appointments, surgeon and nurse recruiters initially found it difficult to present the treatment options equivalently and many were not in equipoise, particularly in relation to the non-radical treatment group. These findings were documented and fed back to recruiters. A plan to improve recruitment was designed by Jenny L Donovan and implemented by Freddie C Hamdy: to change the order of presenting treatments to encourage greater emphasis on equivalence, avoiding terms commonly misinterpreted by patients (such as ‘trial’ and ‘watchful waiting’) and describing a redefined and reconceptualised ‘active monitoring’ group. Presentations about the plan and workshops on recruitment practice were followed by the randomisation rate increasing from 40% to 70%. In further analyses of appointments, treatments were described more clearly and with greater balance, patients became better informed and the three-group trial (including AM) became the preferred design. Embedding the trial recruitment process within this innovative and detailed qualitative research study enabled efficient recruitment that was acceptable to patients and clinicians in the three feasibility study centres. 81
The feasibility study thus showed that, against all expectations, it was possible to introduce PSA testing in primary care practices, implement a standardised diagnostic pathway, identify sufficient numbers of men with clinically localised prostate cancer and mount a full-scale three-group RCT to evaluate the major treatment options: radical surgery, radiotherapy and a reconfigured ‘active monitoring’.
Aim and objectives of the main ProtecT trial
Aim
The overarching aim of the main ProtecT trial was to provide robust evidence of the comparative treatment effectiveness of the three conventional treatment options in the management of clinically localised prostate cancer.
Objectives
-
To evaluate the comparative treatment effectiveness of the three conventional options (AM, RP and RT) for men with clinically localised prostate cancer, with a first analysis at the 10-year median follow-up.
-
To assess QoL measures and patient-reported outcomes related to the three treatment options.
-
To inform patients, clinicians and policy-makers about the optimal management of patients with clinically localised prostate cancer.
-
To develop a comprehensive biorepository of biobanked material donated by patients, associated with an electronic clinicopathological database for conducting effective translational prostate cancer research.
Chapter 2 Methods
Study design, ethics approvals and management
Trial design
ProtecT is a multicentre, pragmatic, parallel-group RCT that compared RP, external beam three-dimensional (3D)-conformal radiotherapy and AM for clinically localised prostate cancer detected through population-based PSA testing in primary care. The trial was conducted in nine UK centres based at hospitals. A feasibility trial conducted at three centres between June 1999 and September 2001 preceded the main trial, which recruited until January 2009, with follow-up for the primary analysis completed in November 2015. The primary outcome of prostate cancer-specific mortality was evaluated at a median of 10 years’ follow-up. Secondary outcomes analysed included disease progression and patient-reported outcomes, as well as cost-effectiveness.
Ethics approvals and research governance
The trial was approved by the NHS Multicentre Research Ethics Committee (Trent Multicentre Research Ethics Committee reference number 01/04/025). An independent Data Monitoring Committee (DMC) oversaw the trial to the primary outcome analysis and met annually. The Trial Steering Committee (TSC), with an independent chairman and seven independent members, monitored the conduct and progress of the trial annually. The DMC would have recommended changes to the TSC if clear evidence (of the order of p < 0.001) of a positive or negative balance of risks and benefits emerged for one intervention in comparison with the others. Study training programmes (described in Research nurses’ role in recruitment, randomisation and follow-up and The ProtecT recruitment story) and on-site monitoring visits were used to standardise trial conduct. 84 The main trial is registered with Controlled Clinical Trials ISRCTN20141297 (feasibility trial: ISRCTN08435261). The protocol is available at www.journalslibrary.nihr.ac.uk/programmes/hta/962099/#/.
Study setting and participants
The feasibility study recruitment was conducted in three English cities (in 24 primary care centres linked to three hospitals) from June 1999 to September 2001. The main phase of recruitment was conducted from October 2001 to January 2009 in nine cities (seven in England, one in Scotland and one in Wales) where around 100,000 men were recruited in primary care.
The CAP trial (ISRCTN92187251)34 commenced in 2001; it randomly assigned 911 primary care centres in eight UK centres to undertake either the ProtecT trial or standard UK NHS management to assess population-based screening for prostate cancer (Figure 1).
FIGURE 1.
Flow chart of the ProtecT and CAP trials.
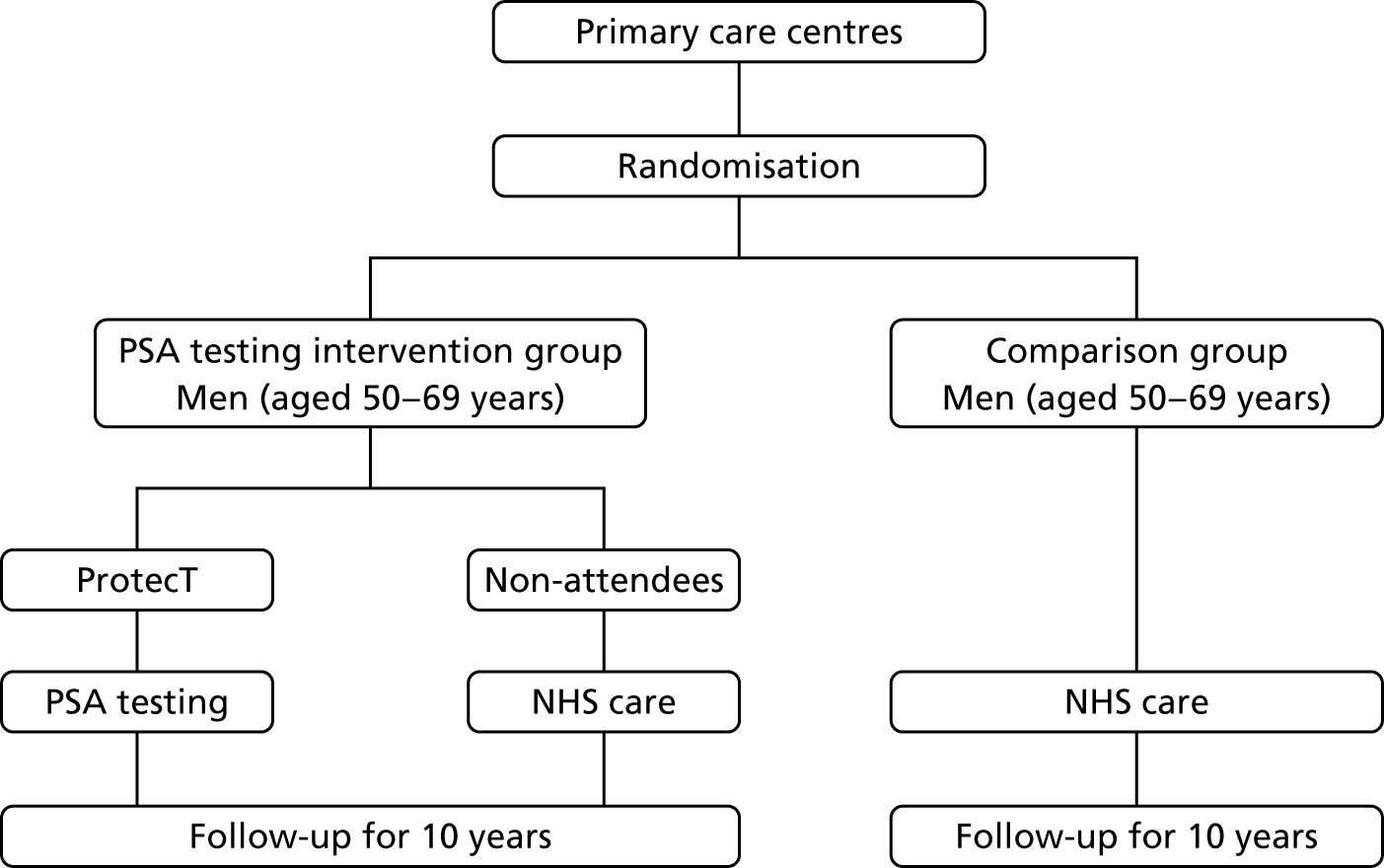
A written invitation was sent by 337 primary care centres participating in the ProtecT trial to registered men aged 50–69 years (inclusion criterion). Trial exclusion criteria were a previous malignancy (apart from skin cancer), renal transplant or current renal dialysis, major cardiovascular or respiratory comorbidities, bilateral hip replacement or an estimated life expectancy of < 10 years. Men who responded received a ProtecT patient information sheet and an appointment with a specialist research nurse, who explained the complexities of PSA testing, assessed trial eligibility and sought written informed consent. Previous PSA test results were checked in the medical records (not an exclusion criterion). On postal receipt of a second consent form, total PSA level was analysed at hospital laboratories audited by the NHS External Quality Assessment Service.
Diagnosis of prostate cancer
Participants with a PSA concentration of ≥ 3.0 µg/l were invited to attend secondary care centres within the nine participating cities for a physical and digital rectal examination and standardised 10-core TRUS-guided prostate biopsies. Participants with an initial PSA concentration of ≥ 20.0 µg/l at diagnosis were excluded because of the high likelihood that they had more advanced cancer. Patients were staged using a combination of digital rectal examination, PSA concentration, TRUS-guided biopsies and isotope bone scanning (if their PSA level was ≥ 10 µg/l). Magnetic resonance imaging (MRI) was used for staging at the discretion of individual investigators because this imaging technique was not available in all centres during recruitment. Men diagnosed with clinically localised prostate cancer and deemed fit for radical treatment received a ProtecT treatment patient information sheet and were subsequently invited to discuss randomisation with the specialist nurses. Men with a PSA concentration of ≥ 10µg/l or a Gleason score of ≥ 7 points underwent an isotope bone scan to exclude metastatic disease. Men who initially had benign biopsy samples or who were diagnosed with locally advanced or advanced prostate cancer were managed in the NHS and excluded from the trial. Men with a benign first biopsy sample and a free-to-total PSA ratio of < 11%, or atypical small acinar proliferation or high-grade prostatic intraepithelial neoplasia, were offered further biopsies; if these repeat biopsy samples were benign, these men were managed in primary care and excluded from the trial. No further trial follow-up took place after the one round of PSA testing or identification of cancers after referral to the NHS. Histopathologists at each site reported pathology findings on standardised forms and participated in trial quality-control processes and those of the NHS Uropathology External Quality Assessment Scheme. At baseline, height and weight were measured and blood samples were taken (whole blood, plasma and serum). Biopsy and prostatectomy tissue, if relevant, were biobanked for participants who also entered the ongoing linked translational Prostate Mechanisms of Progression and Treatment study (see Chapter 7).
Interventions
Participants were randomised to one of three treatments: AM, radiotherapy or RP.
In the AM group, the aim was to avoid immediate radical treatment while assessing the disease over time, with radical treatment offered if disease progression was evident. PSA concentrations were reviewed every 3 months in the first year and twice yearly thereafter (frequency was changed as indicated). The specialist nurses also met with participants yearly to assess their overall health and discuss graphical displays of PSA results and any concerns raised, overseen by each centre’s local clinical investigator. Changes in PSA concentrations were assessed at each visit, and a rise of ≥ 50% during the previous 12 months triggered repeat testing within 6–9 weeks. If the PSA concentrations were persistently raised, or the patient had any other concerns, a review appointment was made with the centre urologist for discussion of further tests including re-biopsy and all relevant management options. A site monitoring and review team comprising trial research nurses and the trial manager visited sites annually; these visits included observation of the nurse-led AM appointments as per the protocol. 85
In radiotherapy, neoadjuvant androgen suppression was given for 3–6 months before and concomitantly with 3D-conformal radiotherapy delivered at 74 Gy in 37 fractions at each of the nine centres. Quality assurance followed RT01 trial procedures. PSA concentrations were measured every 6 months for the first year and then yearly. The study oncologist held a review appointment with participants if the PSA concentrations rose by ≥ 2.0 µg/l post nadir or if concerns were raised about disease progression. Management options were discussed, including monitoring, tests and salvage, radical or palliative treatments as indicated. 34
The predominant approach for RP was open retropubic with individual-level quality assurance to published standards at each of the nine centres. 86 Participants with a baseline PSA concentration of ≥ 10 µg/l or a biopsy Gleason score of at least 7 points received bilateral lymphadenectomy. Postoperatively, PSA concentrations were measured every 3 months for the first year, every 6 months for the subsequent 2 years and then yearly. Adjuvant radiotherapy was discussed and offered to patients with positive surgical margins or extracapsular disease. The centre urologist held a review appointment with participants if their postoperative PSA concentrations reached ≥ 0.2 µg/l to discuss adjuvant radiotherapy.
In all treatment groups, ADT was offered when serum PSA reached a concentration of 20.0 µg/l, or less if indicated. Imaging of the skeleton was recommended if the serum PSA level reached 10.0 µg/l, using isotope bone scintigraphy, plain radiographs and MRI as necessary.
Primary and secondary outcomes
Primary outcome
The primary outcome was definite or probable prostate cancer mortality, including intervention-related deaths, evaluated at a median of 10 years’ follow-up. Participants were linked to the NHS national registry for vital status information, which was updated quarterly. The process used to assess cause of death was adapted from the PLCO algorithm,70,87 used in the CAP and ProtecT trials. In brief, hospital medical records were summarised by trained CAP researchers onto vignettes, anonymised and reviewed by an independent endpoint committee masked to the ProtecT and CAP trials. 34
Secondary outcomes
These outcomes were analysed at a median of 10 years and included overall mortality (from death certificates), metastases, disease progression, treatment complications (including adverse events) and resource use for the cost-effectiveness analysis (described in Chapter 5). Outcomes were collected on case report forms (CRFs) by research nurses annually, based on medical record reviews and participant information gained at an appointment.
Patient-reported QoL outcomes were focused on symptoms, condition-specific and overall QoL and psychological status. The measures and their foci are summarised in Table 1 and included the Expanded Prostate Index Composite (added in 2005 for rectal complications), the International Consultation on Incontinence Questionnaire (ICIQ), the International Continence Society (ICS) urinary ICSmaleSF (International Continence Society male Short-Form) and sexual function ICSsex measures, the EORTC-QLQ Q30 (European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 module) (added in 2007 for cancer-specific effects), the Hospital Anxiety and Depression Scale (HADS) for psychosocial effects and the Short Form questionnaire-12 items (SF-12) and EuroQol-5 Dimensions generic health status measures. 88–94 These validated questionnaires were completed at recruitment and at first biopsy appointments then at 6 months after randomisation and yearly thereafter for at least 10 years (see Table 1).
| Outcome | PROM and its focus (time frame)a | PROM scales/items presented in main analysis (range/categories) [domain MID utilised or calculated] | Completion rate (n completed/n given the PROM)b |
|---|---|---|---|
| Urinary | ICIQ-UI: incontinence (4 weeks) |
|
82% (1244/1508) |
| EPIC: prostate cancer treatment (4 weeks) |
|
88% (745/849) | |
| ICSmaleSF: LUTS (4 weeks) |
|
86% (1413/1643) | |
| Bowel | EPIC: prostate cancer treatment (4 weeks) |
|
88% (748/849) |
| Sexual | EPIC: prostate cancer treatment (4 weeks) |
|
85% (719/849) |
| Psychological | HADS: anxiety and depression (1 week) |
|
85% (1399/1643) |
| SF-12: overall mental health (4 weeks) | Mental component subscore (0–100) [3.8] | 77% (1260/1643) | |
| Physical | SF-12: overall physical health (4 weeks) | Physical component subscore (0–100) [4.0] | 77% (1260/1643) |
| Generic health | EQ-5D-3L: health utility (today) | Health utility score (–0.594 to 1.0 scored as perfect health) [0.09] | 86% (1413/1643) |
| Cancer-related QoL | EORTC-QLQ Q304,126 (1 week) | Global health, five functional and three symptom scales, five single items, completed after randomisation |
Questionnaires in the follow-up period were posted with a Freepost envelope, with a reminder questionnaire sent after 3 weeks, a telephone call from research nurses after another 3 weeks and a short version of the questionnaire sent at around 8 weeks if there was no reply. Qualitative interviews investigated participants’ experiences of treatments and outcomes over time and are described fully in Chapter 3.
Data-collection schedule
The data-collection schedule related to trial outcomes is summarised in Appendix 1, Table 23.
Sample size
Before the start of the trial, a sample size target of 1434 randomly assigned men (478 in each group) was identified as sufficient to estimate the absolute difference in mortality probability between two treatment groups with a 95% CI of ±0.045, on the basis of an assumed prostate cancer mortality risk of 15% at 10 years, consistent with prostate cancer-specific mortality in men aged 55–69 years, with clinically detected disease managed conservatively at that time and a difference that would be deemed clinically significant by the NHS. The pilot study recruitment data were used to calculate the number of sites and duration of recruitment needed to meet the sample size target. However, more-recent data suggested that disease-specific mortality with non-radical treatment was likely to be closer to 10% at 10 years because of improvements in disease management. 34 As a result, the DMC advised in 2008 that recruitment should continue to the planned end date, with 1590 men (530 per group) expected to be randomly allocated by that point. This sample size would enable a 46% relative reduction in prostate cancer mortality to be detected with 80% power at a 5% significance level for a pairwise comparison of a radical treatment with AM. This calculation assumes a 10% prostate cancer-specific mortality at 10 years with AM, and hence a 5.4% risk with radical treatment (an absolute difference very similar to the margin of error specified in the first calculation). These sample size targets are based on differences in and ratios of risk rather than the HRs planned for the primary analysis, because the resulting calculations are simpler and more flexible. When a high survival rate is expected, calculations based on risk ratios will be a close approximation to those based on HRs.
Randomisation
Patients were randomised on an equal basis (1 : 1 : 1) to AM, surgery or radiotherapy by a remote randomisation service. Allocation concealment was assured by the treatment being assigned only after the nurse telephoned the research centre and participant identifiers and key baseline data were logged on the computerised randomisation system held at the University of Bristol. Allocations were computer generated as required for each participant, originally using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) functions and subsequently in C++ by an independent programmer, stratified by site with stochastic minimisation (maintaining a random element to the allocation) to improve the balance across the groups in relation to age at primary care patient-identification date, Gleason sum score (< 7, 7 or 8–10 points) and mean of baseline and first biopsy PSA results (< 6.0, 6.0–9.9 or > 9.9 µg/l). The allocation was revealed after the entry of participant details and given to the participant by the nurse. Men who declined randomisation were offered trial follow-up and formed a comprehensive cohort. 95
Blinding
Clinicians, participants and researchers were not masked to group assignment. All investigators remained blinded to outcomes by group throughout recruitment and analysis. The statistical analysis plan was written by the senior statistician and was agreed by the trial team post recruitment end but prior to any statistical analysis. 96 The primary outcome was assessed by a committee independent of the trial team. 97
Statistical methods and analysis plan
The primary analyses utilised an intention-to-treat (ITT) approach comparing three treatment groups as allocated. At a median of 10 years of follow-up (November 2015), the primary outcome measure of prostate cancer mortality was compared between treatment groups using a survival analysis (Cox proportional hazards regression model) adjusted for stratification and minimisation variables. The estimated relative treatment effect for each pairwise comparison of treatments was captured as a HR, and presented with a 95% CI. Pairwise significance tests were planned if a test of an equal 10-year disease-specific mortality risk across all three groups yielded a p-value of < 0.05. 98 This was used for event-based secondary outcomes (i.e. grouped analyses of definite, probable or possible prostate cancer, all-cause mortality and metastatic disease).
Pairwise comparisons of symptom burden utilised multilevel models for repeated measures to estimate the average treatment effect over the median 10-year follow-up. Further analyses will investigate the relative burden between treatment groups over time. Prespecified subgroup analyses will investigate whether treatment effectiveness in the reduction of prostate cancer-specific mortality is modified by clinical stage, Gleason score, age or PSA concentration using stratified analyses for descriptive statistics and by formally including interaction terms in the relevant regression models. Secondary analyses will estimate the efficacy of radical treatment versus AM in the reduction of prostate cancer mortality in individuals who complied with their allocated treatment, by using a method to derive an unbiased estimate in parallel with the per-protocol analysis originally specified in the trial protocol. 99,100
Data from the recruitment, diagnostic and randomisation phases are summarised, and categorisation of continuous variables was based on either clinical thresholds (e.g. for PSA) or the aim of equal group sizes (other measures). Resident area-based material and social deprivation scores (the proportion of people living in an area of material deprivation) were derived using lower super output areas, each equating to around 1500 residents, for England, Scotland and Wales separately. Analyses were carried out in Stata® version 10 (StataCorp LP, College Station, TX, USA).
Summary of changes to the protocol
The main changes in chronological order were:
-
An alternative two-group randomisation between RP and radiotherapy was stopped in 2003 by the TSC because of limited uptake (24 participants in total).
-
An additional exclusion criterion of bilateral hip replacement (precluded radiotherapy) was added in 2003.
-
A pilot study of men aged 45–49 years in one centre that recruited around 1300 participants between 2003 and 2005 was discontinued because of lower recruitment and identification of mostly low-risk disease. 101
-
There was a recruitment extension from June 2006 to June 2008 resulting from contractual delays in opening centres in the main trial.
-
Additional PROMs were added for bowel effects [Expanded Prostate Cancer Index Composite (EPIC) added in 2005] and cancer-specific QoL (EORTC-QLQ Q30 in 2007).
-
Sample size calculation changed on the advice of the DMC and TSC, as described earlier in 2008.
-
The 5-year survival analysis was removed in 2009 on DMC advice.
-
The chief investigator (and trial sponsorship) moved from the University of Sheffield to the University of Oxford in 2010.
The role of research nurses in recruitment, randomisation and follow-up
Establishment, development and preservation of the local ProtecT teams
The results of the feasibility study102 provided an opportunity for nurses, with the support of secretaries, to be involved in all aspects of the trial: initial recruitment of general practitioner (GP) surgeries (later taken on by the CAP study34), recruitment of participants, assisting with the diagnostic/eligibility phase, randomisation and follow-up. Although commonplace now, at the time this level of involvement by nurses was unusual. Each centre had a senior ProtecT ‘lead nurse’, research nurses (during recruitment) and a trial secretary. The feasibility study showed that nurses were as effective as urologists in randomising participants and they became central to the success of the main trial. The nurses helped recruit general practices, enrolled participants in primary care, assisted with the cancer diagnosis phase, offered randomisation to participants and conducted AM follow-up and research follow-up. At the start of the trial in the early 2000s, nurses rarely took consent, which was questioned by at least one local ethics review board when opening the centres and also by the sponsors. Recruitment took place mainly at GP surgeries, but on occasions at other venues such as sports centres or church halls, requiring the nurses to be adaptable and autonomous. In addition, the nurses sometimes travelled widely during recruitment, particularly where there were few surgeries near the hospital or if they had taken part early on in recruitment.
In order to increase trial activity, particularly recruitment, six additional clinical centres were added at the start of the main trial in two separate three-centre ‘waves’. Practical on-site support was provided to these clinical centres by two of the lead nurses who had gained experience of the trial during the feasibility phase. These ‘co-ordinating’ lead nurses assisted with the initial office set-up, appointment of new staff and on-site staff training: at times this included recruitment of the first participants at a fledgling centre while the team settled into their new role. Each centre first employed a dedicated lead nurse and lead secretary and then new team members were added as trial workload increased. Although each centre was different and had its own challenges, once established these new teams became integrated within the urology department. In order to keep recruitment on schedule and to deal with increasing numbers of men in follow-up, each centre had a team of four or five nurses and two or three secretaries; numbers in centres varied depending on the ratio of full- and part-time staff and the number of participants in follow-up. At the height of recruitment, there were around 45 nurses working across the UK. In total, more than 80 nurses were involved in the trial at some point. Some staff stayed for only a few weeks, leaving once they realised that the repetition of recruitment was not for them; others stayed for many years, often citing the annual nurse-led follow-up clinics and the strong bonds that they subsequently established with the participants as a major factor in their decision to continue working on the trial.
Many of the nurses had previous urology experience when they started on the trial; few had previous research experience. National training meetings were provided by the research hub at the University of Bristol for new and existing staff, with an initial focus on recruitment/randomisation, standard operating procedure (SOP) development, data collection and research methods. As the trial matured, the training increasingly focused on the treatment pathways and research/clinical follow-up issues. The training meetings were an opportunity for nurses and secretaries from across the UK to meet and share experiences and ideas.
Two trial-specific ‘jamborees’ brought together staff from all disciplines and further engendered a national and team approach. Centralised training for nurses was also delivered on an individualised basis as the appointments with participants who had been newly diagnosed with prostate cancer and considering entry into the main treatment trial (‘information appointment’) were, with the participant’s consent, audio-recorded. This material provided a platform for individual and group feedback to improve randomisation. 102
A small peer-review team was established to visit clinical centres and provide on-site support and training, audit, increase protocol/good clinical practice adherence, maintain ‘target’ recruitment/follow-up and to help with any centre-specific issues. 85 The training and on-site review meetings were on a rolling programme to accommodate changes in staff and different phases of the trial. Lead nurse meetings were chaired by the trial co-ordinator and hosted by the University of Bristol (three per year) to encourage cross-centre support, provide feedback on performance, assist with trial development/protocol changes and to work through current issues of concern. In addition, each centre had a nurse attend the national trial meetings, for instance the investigator and radiotherapy meetings. During the follow-up phase, a third co-ordinating lead nurse was added to help develop the trial in the light of new challenges, for instance national changes to trial governance, the move from paper to electronic records across NHS trusts and the complexities of maintaining high follow-up rates in an ageing population. A summary of current ‘nursing issues’ was presented annually at the TSC meetings, at which the emphasis of discussion moved from recruitment to follow-up as the trial matured.
Many teams remained quite constant over time, often citing the trial follow-up clinics and strong bonds that they established with participants as major factors in continuing to work on the trial. ProtecT staff were respected within urology departments for their research and clinical experience, and they advised on other trials and helped to train staff. Several nurses undertook additional qualifications, such as nursing diplomas, Bachelor of Science degrees and one Master of Science degree.
Recruitment/randomisation
Drawing on the experience of the feasibility study, recruitment was carried out by qualified nurses and took place mainly in primary care/community settings, such as GP surgeries, sports centres and church halls. Recruitment centred on the main ProtecT research office/urology department, graduating outwards to ensure that recruitment targets were met. Towards the end of the recruitment phase, many of the nurses regularly had a daily commute of up to 80 miles, particularly to reach centres with a smaller core population, such as Cambridge. The timing and content of appointments were as described in the feasibility report. Methods to ensure standardisation across centres are described previously. Additional support was provided to centres that were underperforming in relation to recruitment and/or randomisation. One centre was withdrawn from recruitment 2 years ahead of the other centres owing to futility despite intensive support, but then became, by default, a pilot for the follow-up phase.
Follow-up
Annual nurse-led follow-up clinics were driven by the trial protocol and were developed using the ‘treatment pathways’ and SOPs as the focus of activity. Each centre ensured that clinics were compliant with local hospital and urology/oncology policies. Follow-up included face-to-face (including ‘outreach’) and telephone appointments and were tailored to the circumstances and wishes of participants. The individualised approach to follow-up facilitated continued participant contact even in the event of participants moving ‘out of area’, helping to reduce levels of trial attrition. In addition to the annual research follow-up, the nurses were responsible for the ‘clinical’ follow-up of the AM cohort, with accountability/oversight provided by the local investigator. In some centres, the nurses provided ongoing clinical support to participants following primary surgery and radiotherapy treatments. The clinical component was perhaps the most important factor in ensuring job satisfaction of the nurses working on the trial. ProtecT nurse-led clinics were demonstrated as being acceptable to patients, nurses and investigators owing to the perceived quality and continuity of service provided. 103 If trial participants moved away, the nurses tried to conduct research follow-up remotely; with participants who lived overseas, this was when they returned to visit the area. Several urology nurse-led clinics have been established at these hospitals using the ‘ProtecT model’. In some centres, nurses who left the trial have drawn on experiences from the ProtecT study and there are examples of departmental-led, nurse-led clinics run on the ‘ProtecT model’. At the time of the primary analysis, plans were being made for a ‘transition’ phase whereby participants would be referred back to usual NHS follow-up – this will be described in the next HTA report.
Chapter 3 Trial results at baseline and randomisation
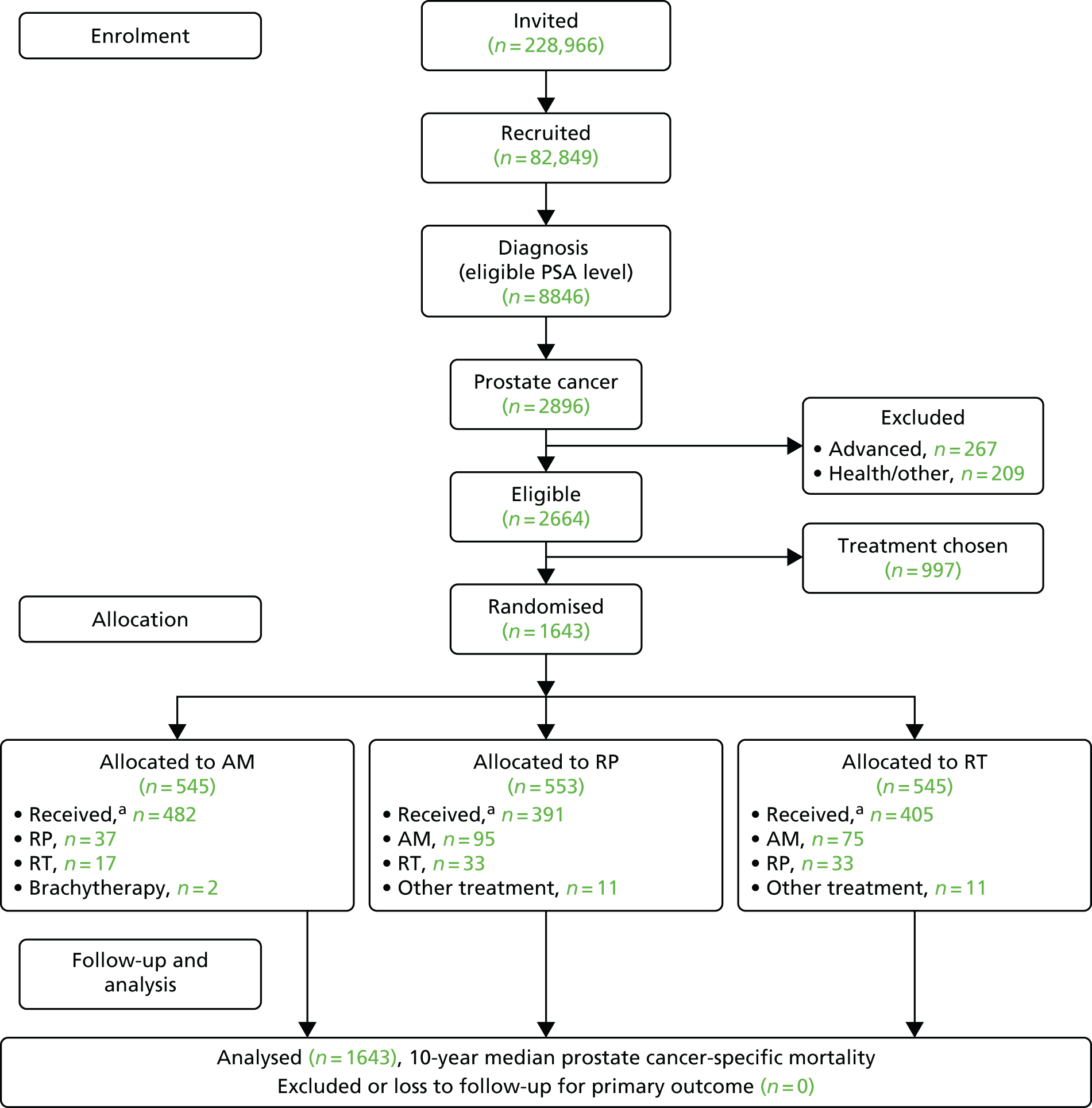
Participant flow
The CONSORT flow chart is presented in Figure 2. All participants were included in the primary outcome analysis, which was based on follow-up from June 1999 to November 2015.
FIGURE 2.
The ProtecT CONSORT flow chart. a, Within 9 months of randomisation.
The ProtecT recruitment story
Recruitment in the feasibility study
Figure 2 shows the flow of participants through the ProtecT study. The recruitment process through the prospective cohort of PSA testing and prostate cancer diagnosis has been described elsewhere. 95,104 This section details the process and outcome of recruitment to the ProtecT trial from the feasibility study until completion in January 2009.
The ProtecT feasibility study was undertaken in three clinical centres from 1999 to 2001 to explore the issues that were expected to make recruitment challenging, if not impossible. The feasibility study was innovatively embedded in a qualitative (ethnographic) study of the perspectives of recruiters and potential participants, identifying obstacles and challenges to successful recruitment. Recruitment in the feasibility study was not easy, but it was eventually successful. The findings produced several recommendations for the main trial: that research nurses should undertake most recruitment activities, supported by urologists who would conduct the ‘eligibility’ assessment appointments; that the comparison should be between three treatments – surgery, radiotherapy and ‘active monitoring’ (as reconceptualised by the qualitative research); that AM should be presented first in the list of treatments; and that nurses would be trained to present the study in a carefully balanced way, avoiding terms such as ‘trial’ and ‘random’ and attempting to explain equipoise and randomisation clearly. 80,83,105 At the beginning of the feasibility study, recruitment struggled, with only 30–40% of eligible patients consenting to randomisation, and 60–70% accepting the allocation, but this rose to a randomisation rate of 70% (95% CI 62% to 77%) by the end of the feasibility study in May 2001, with around 70% accepting the allocation (Table 2).
| Dates | Number of eligible patients | Eligible patients consenting to randomisation, n (%) | Randomised patients immediately accepting the allocation, n (%) |
|---|---|---|---|
| October 1999–May 2000 | 30 | 9–12 (30–40) | 18–21 (60–70) |
| June 2000–August 2000 | 45 | 23 (51) | 18 (78) |
| September 2000–November 2000 | 67 | 39 (58) | 30 (77) |
| December 2000–January 2001 | 83 | 51 (61) | 38 (75) |
| February 2001–May 2001 | 155 | 108 (70)a | 76 (70) |
Recruitment to the main ProtecT trial: 2001–9
The feasibility study had been undertaken in three clinical centres; the main trial required the addition of six further clinical centres. The target for accrual was to randomise 1590 men with localised prostate cancer (including the 146 recruited in the feasibility study). The clinical centres were set two simultaneous targets:
-
Achieve randomisation of at least 60% of eligible patients.
-
Ensure that participants were well informed so that at least 70% would accept the random allocation.
The recruitment intervention that had been successful in the feasibility study was further developed and extended into a complex intervention in the main trial. 102 Initially, the following actions were proposed as part of the intervention, building on the findings from the feasibility study:
-
regular training for nurses
-
centre reviews if study targets were not met
-
provision of documents to support recruitment
-
individual feedback to recruiters as required.
In addition, the lead investigators in the clinical centres were interviewed to capture their views about participating in the trial and confidence with eligibility assessments and recruitment, and their ‘eligibility’ appointments were audio-recorded if recruitment rates fell below the target rate. Nurses were asked to audio-record all of their recruitment appointments. Patient consent for audio-recording was almost universal. Recordings were selected for scrutiny when staff commenced recruitment or when rates of randomisation or acceptance of allocation fell below study targets. Some recordings were selected at random for trial monitoring, or consecutively for analysis in particular studies. Audio-recordings and interviews were analysed in accordance with methods of constant comparison based on grounded theory, with thematic methods used for interviews and a mixture of content, thematic and targeted conversation analysis methods used for audio-recordings. Centres failing to reach both study targets were selected for review, in which J Athene Lane undertook an audit of consent procedures and Jenny L Donovan completed a focused qualitative analysis of recruitment practice.
Figure 3 shows numbers of patients agreeing to randomisation over time and Table 3 shows recruitment over time according to the rates of randomisation and immediate acceptance of allocation. By the end of 2001, although the randomisation rate was ahead of the target of 60%, the rate of acceptance of allocation had slipped to 64%. The TSC advised that all efforts should be devoted to improving the acceptance rate, and suggested the need to aim to reach a rate of 75–80%. This was investigated in detail through reviews of two recruiting centres with recruitment considerably lower than the targets: centre A with 45% randomisation and 55% acceptance rates, and centre B at 50% and 65%, respectively. Details of the reviews have been reported elsewhere. 102 In brief, after these reviews, rates of randomisation and acceptance of allocation increased markedly to 86% and 78% for randomisation and 67% and 87% for acceptance, with evidence of improvement beyond chance after 12 months. These changes were sustained at 24 months, although numbers were small. In one of the centres, most of those declining randomisation chose surgery, and so training and feedback were targeted towards clearer explanation of all treatments. In the other centre, the nurse recruiters referred to prostate cancer as ‘early’, leading to men expressing a preference for AM (radical treatment was seen as using ‘a sledgehammer to crack a nut’), with nurses then accepting their preference without further discussion. These issues were dealt with by avoiding the term ‘early’ and referring instead to mostly ‘small’ or ‘slow-growing’ cancers, asking patients to keep an ‘open mind’ while they listened to information about treatments, and by acknowledging the need for nurses to explore the reasons for patients’ preferences.
FIGURE 3.
Numbers of patients agreeing to randomisation over time. Q, quarter.
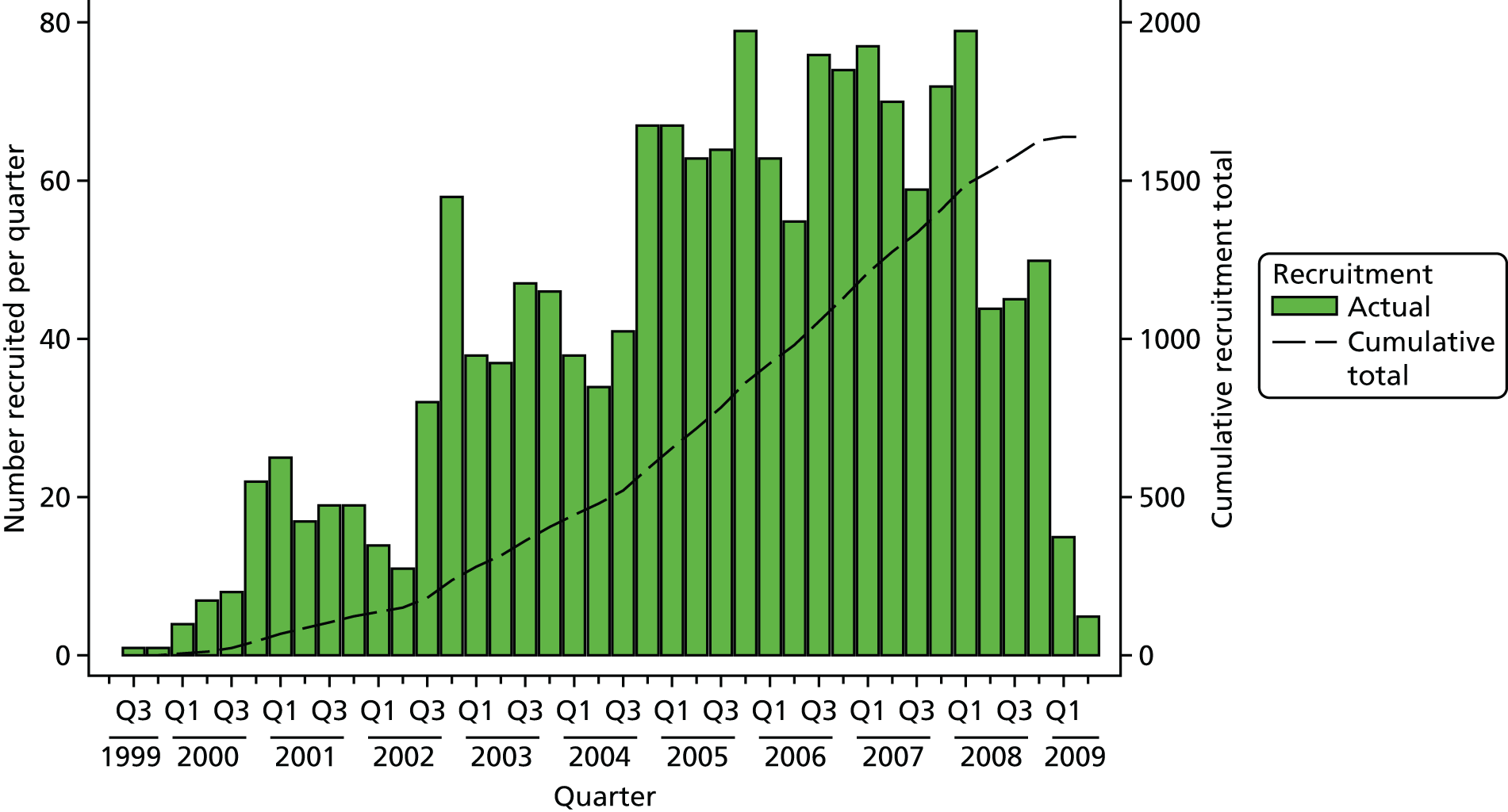
| Year(s) of recruitment | Cumulative number of eligible men with clinically localised prostate cancer | Cumulative number (%) of eligible men agreeing to randomisation | Cumulative number (%) of randomised men accepting allocation |
|---|---|---|---|
| 1999–2001 (includes feasibility) | 200 | 137 (69) | 88 (64) |
| 2002 | 381 | 263 (69) | 173 (66) |
| 2003 | 622 | 430 (69) | 303 (71) |
| 2004 | 914 | 621 (68) | 455 (76) |
| 2005 | 1319 | 889 (67) | 702 (79) |
| 2006 | 1762 | 1153 (65) | 918 (80) |
| 2007–9 | 2664 | 1643 (62) | 1336 (81) |
Information about these issues was then included in nurse training sessions that were held in March 2002, February and June 2003, January and October 2004 and annually thereafter. In October 2002, the training was supplemented with a document of ‘tips’ for recruitment, which was sent to all nurse recruiters and urologists carrying out eligibility appointments. This document advised:
-
mentioning the purpose of the study and randomisation early in the appointment
-
avoiding the use of terms such as ‘early’ to avoid misunderstanding
-
describing the treatments succinctly and with balance, including potential advantages as well as adverse effects
-
eliciting and exploring participants’ treatment preferences, particularly if based on incorrect information (such as radiotherapy resulting in hair loss)
-
providing details about what would happen next after advising the participant of the treatment allocation.
A further document (‘Tips 2’) was produced in December 2004, focusing more on the structure and content of appointments, with the following advice:
-
Explain the purpose and rationale of the study, inform patients about advantages and disadvantages of treatments so that they could perceive them as equivalent in terms of outcomes in the long term, obtain consent from randomisation only after checking that the participant was likely to accept all three treatments and ensure that they were comfortable with the conduct and outcome of the appointment.
-
Conduct appointments in three basic stages: opening (points 1–3 above, and empathising with the patient’s situation), process (point 4 above) and ending (ensure that all preferences are addressed, explain purpose and advantages of randomisation, only gain consent if patient indicates willingness to accept all options – or arrange preferred treatment).
-
Examples of difficult situations were given.
-
Essential things to do and avoid were given.
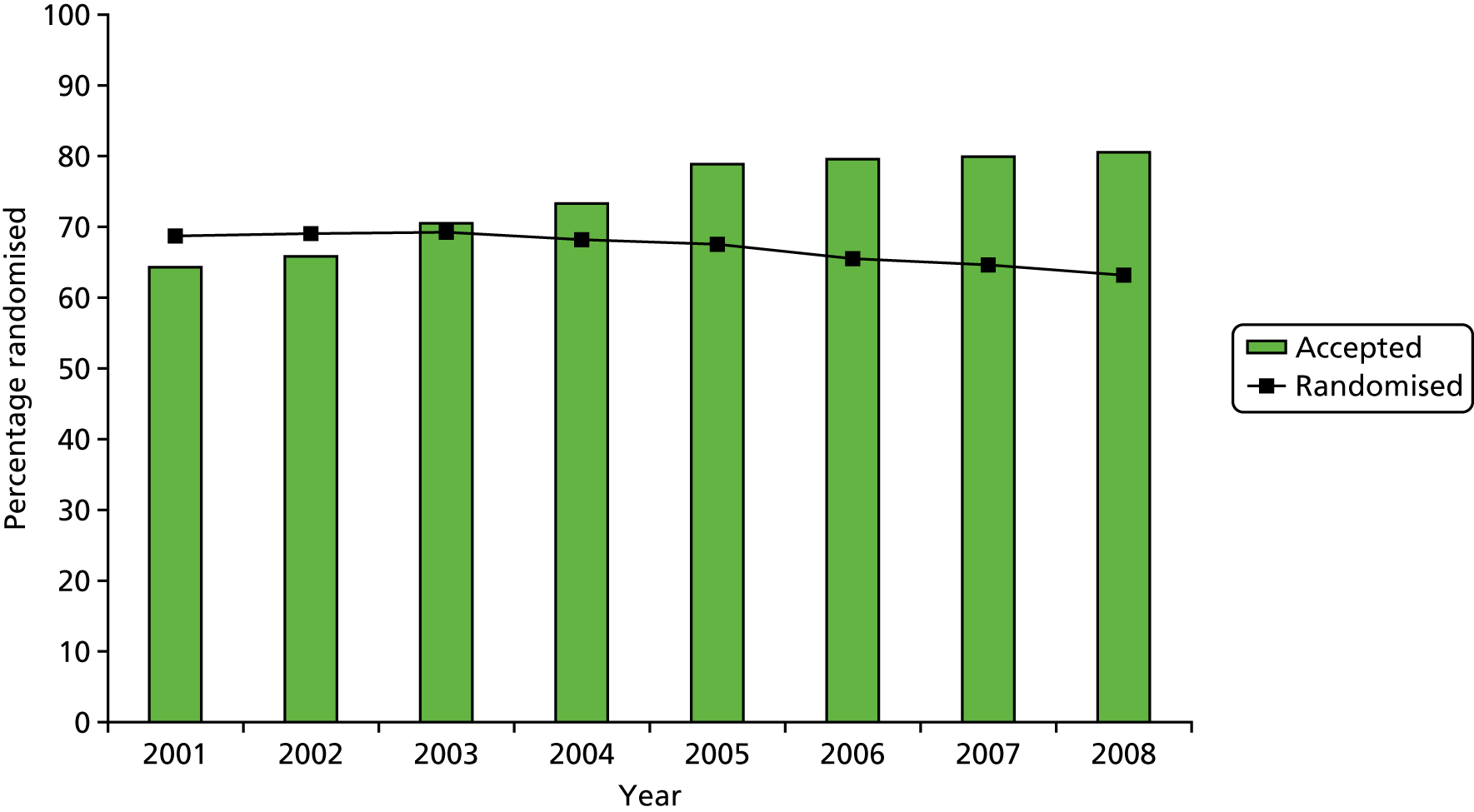
As can be seen in Table 3 and Figure 4, the rate of acceptance of allocation increased sharply in 2002 and continued to increase, reaching 70% in 2003 and rising to 80% in 2005, before stabilising at that level to the end of recruitment. The rate of randomisation began to decline gradually from its high point of 69% in 2003 as the acceptance of allocation increased. Both original targets were thus met from 2003 onwards, as was the revised TSC target for acceptance of allocation from 2005 onwards.
FIGURE 4.
Randomisation and acceptance of allocation over time.
Years
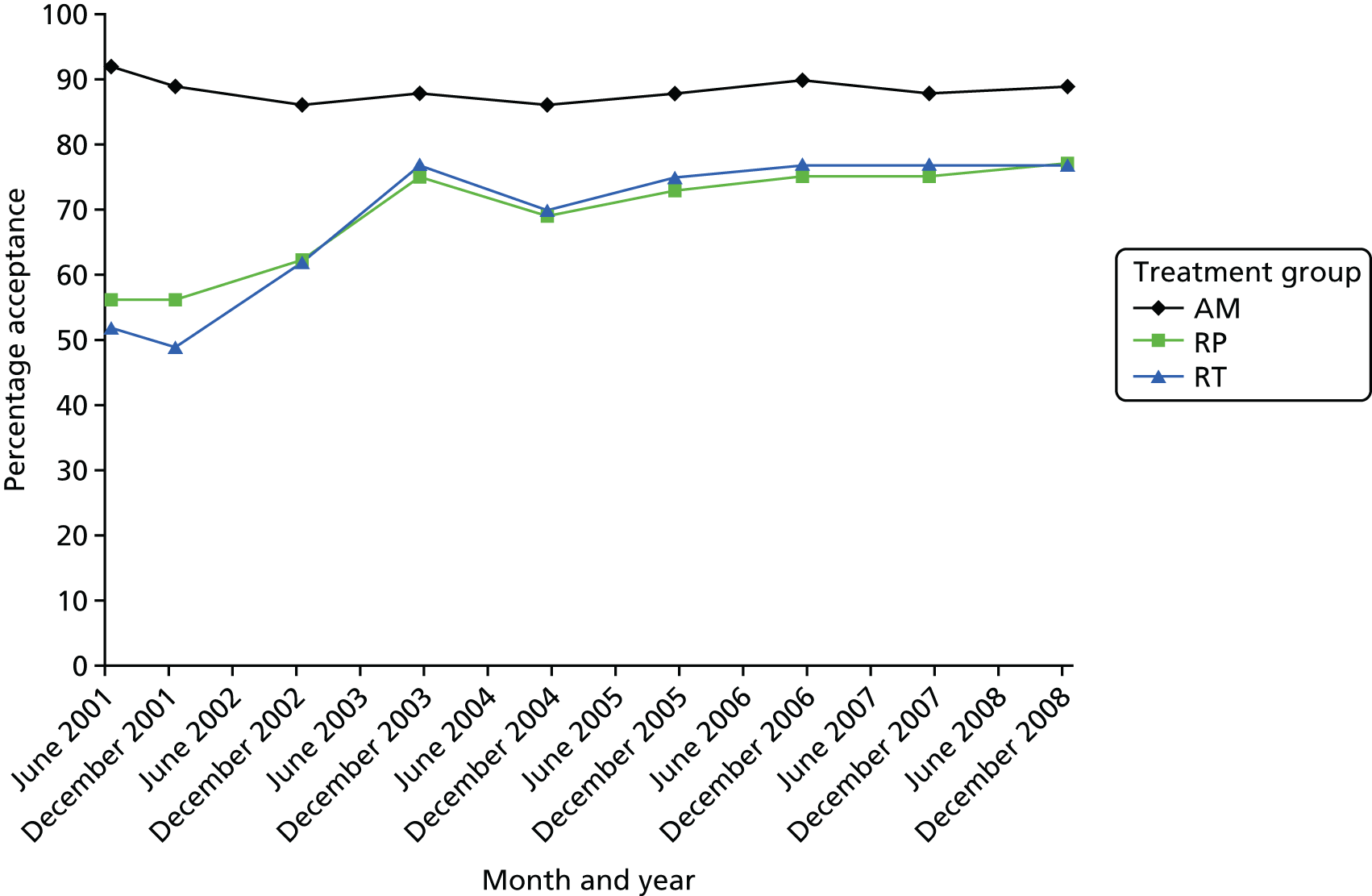
We also investigated acceptance of allocation by group. Figure 5 shows how the low level of acceptance in 2001 was related to the radical treatments, particularly radiotherapy. Nurses were encouraged to visit oncology units and improve information provision about radiotherapy in addition to providing more balanced information about all three treatments. As can be seen in Figure 5, the acceptability of allocation to both radical treatments became similar to but remained somewhat lower than the rate for AM, reflecting the differences in the immediate consequences of these options.
FIGURE 5.
Acceptance of allocation by group over time.
Development and refinement of the ProtecT recruitment intervention
The centre reviews, nurse training, provision of ‘tips’ documents and individual confidential feedback constituted the ProtecT trial recruitment intervention. The intervention was further developed through two qualitative research studies undertaken alongside the recruitment:
-
A qualitative analysis of audio-recordings of recruitment appointments revealed that their structure was mostly either recruiter led or participant led. Recruiter-led appointments followed a more typical script of issues to be covered, whereas the participant-led appointments were driven by the questions that the participant wanted to raise. The structure was associated with how well it was possible to judge whether or not participants understood the information provided by the recruiters and whether or not they reached equipoise. In the recruiter-led appointments, patients tended to spend most of the time listening and making only occasional utterances, whereas they were able to contribute much more in participant-led appointments. The qualitative analysis suggested that informed consent was enhanced by tailored information provision including strategic use of open questions, pauses and ceding the floor to participants in the interaction. These techniques facilitated detailed and systematic exploration of each participant’s concerns and facilitated truly informed consent. In contrast, interruptions and failing to address participants’ questions were unhelpful. 106 These techniques were presented to recruiters during training and were taken up by many recruiters over time.
-
A qualitative analysis of audio-recordings of 93 consecutive recruitment appointments explored treatment preferences expressed by men, reactions to these expressed preferences by recruiters and what the outcome of the appointment was (i.e. consent to participate in the trial or selection of a treatment). This study showed that treatment preferences were more complex and dynamic than previously assumed. Most participants expressed views about treatments early in appointments, ranging on a continuum from hesitant to well-formed opinions. As recruiters explored men’s views and provided detailed evidence-based treatment and study information, some men opted for their preference, but many became uncertain and open to RCT recruitment, often accepting a different treatment from their original ‘preference’. It was clear that expressed preferences were not as strong as they originally seemed, and could be explored. Unexpectedly, we concluded that the discussion of treatment preferences did not act as a barrier to recruitment but actively enabled many men to express their concerns and reach an informed decision that often included RCT participation and acceptance of allocation, even if this was not to their originally expressed preference. 107 This nuanced understanding of expressed preferences was used in training in role plays, and there were detailed discussions about the importance of gently exploring preferences to ensure that participants were fully informed about the treatment options and could then make an informed decision about trial participation or choosing a treatment. Discussions about appropriately exploring and addressing treatment preferences took up a large proportion of training sessions, and advice on this issue formed a substantial element in the ‘tips’ documents.
ProtecT trial recruitment outcome and completion
The original projection of recruitment for ProtecT assumed that the six centres added after the feasibility study would come on stream promptly and recruit at the same level as the three feasibility centres. Unfortunately, 2001/2 was when new rules came into operation in relation to research governance in NHS organisations, and this severely delayed the opening of the new centres. At this point, recruitment fell behind target. A revised projection was made in 2005, when an extension was also applied for. Once all centres were operating, recruitment increased significantly and met and then exceeded the target (see Appendix 1, Figure 28).
By the end of the recruitment period in early 2009, there were 2664 men with clinically localised prostate cancer who were eligible for the trial and who were informed about it by ProtecT study research nurses. Of these, 1643 (62%) agreed to be randomised and 997 selected a treatment of their choice (24 had opted for the discontinued two-group trial). Among the 1643 patients who agreed to be randomised, 1336 (81%) accepted their allocation immediately. The vast majority of these men then went on to have the treatment as allocated: 1278 (78% of those randomised) commenced the allocated treatment within 9 months. The original targets of randomising at least 60% of eligible patients and for at least 70% to accept the allocation were thus achieved.
Subsequent development of the recruitment intervention for use in other randomised controlled trials
As the ProtecT recruitment intervention showed promise, its methods were codified and the transferability of the intervention was assessed in four further RCTs in the Medical Research Council Quartet (Qualitative Research to Improve Recruitment to RCTs) study54 and many more independent RCTs. Collaborations were often initiated by trial investigators who anticipated that they would encounter recruitment obstacles in the RCTs because comparisons were controversial and/or included very different treatments (e.g. current standard treatment vs. ‘less’ or ‘no’ treatment, or radiotherapy vs. surgery). These collaborations usually included some aspects of the intervention from the outset of the RCT’s recruitment, often during feasibility or pilot phases. Other collaborations began part way through the RCT recruitment period by the trial investigators or following encouragement by the funder in response to recruitment falling short of targets. Some of these RCTs were at risk of early closure, and most had already applied for funded extensions and/or revised their target sample sizes. These different contexts – the intervention integrated from the outset or inserted into the recruitment at a later stage – provided interesting and sometimes different challenges that further encouraged the development of aspects of the intervention.
The four RCTs in the Quartet study showed that the intervention could be transferred in some circumstances, particularly where aspects were integrated with the main RCT, as had been possible in ProtecT. 108–110 At this stage in the development of the intervention, involvement seemed to lead to improvements in recruitment in most RCTs. 111–114 However, there were also some RCTs in which insurmountable issues were found, leading to the early closure of the RCT. 109,115,116 These were difficult for the researchers and chief investigators, although the intervention did clearly identify the source of the insurmountable difficulties: a lack of equipoise was evident among clinicians involved in all of these trials, and there were also insufficient numbers of eligible patients or problems with the timing of intervention delivery. 109,115,116 These collaborations were certainly instrumental in refining the content of the intervention. Some collaborations at this stage also enabled further methodological development, alongside that being undertaken in ProtecT (as described previously), for example measurements of timing of recruitment appointments and content related to particular interventions or the study itself, and the identification of methods of communication. 117,118
There were considerable challenges in integrating the intervention part way through recruitment, especially in relation to research governance approvals, and opportunities to optimise recruitment were often constrained by delays and difficulties in data collection, most notably in obtaining audio-recorded recruitment appointments. 108 These audio-recorded appointments underpinned the understanding of and then feedback and training delivered in ProtecT, but a routine audio-recording process proved difficult to operationalise in other RCTs. Interviews with recruiters, scrutiny of trial documentation, focus groups and informal discussions with the TMGs were the key sources of data collection in most of these trials, and it became clear that this was limited compared with the ProtecT responsive and tailored support.
The emergence of the Quintet Recruitment Intervention
A large number of data were collected and analysed during the application of the ProtecT intervention and its translation in five further RCTs. These data were synthesised in two papers published in 2014. 119,120 The first paper showed how RCT recruiters readily identified organisational difficulties, fewer than expected eligible patients and patients’ treatment preferences as the key barriers to recruitment. 119 These were ‘clear obstacles’. As recruiters described their experiences of recruitment and data from appointments were synthesised, several otherwise ‘hidden challenges’ related to their roles as researchers and clinicians emerged, imbued with discomfort and emotion. The synthesis went on to show that doctors were most uncomfortable about aspects of patient eligibility and the effectiveness of interventions, whereas nurses were more anxious about approaching potential RCT participants and conflicts between their research and clinical responsibilities. It was notable that recruiters were unaware that their views could have an impact on recruitment. These hidden challenges were not shared with RCT chief investigators. The synthesis also showed how the feedback and training developed in the intervention could enable most recruiters to become more comfortable with key RCT concepts including equipoise, uncertainty, patient eligibility and randomisation. 119 In the second paper, the discomfort and emotion in relation to community equipoise and ‘hunches’ about particular treatments and patients was explored in greater detail. 119 Both papers concluded that recruitment to RCTs is a fragile process that is difficult for doctors and nurses intellectually and emotionally, and that they required support.
The synthesis, including and initiated by ProtecT, led directly to the formalisation of the intervention as the Qualitative Research Integrated in Trials (Quintet) Recruitment Intervention (QRI). 54 By this time, the intervention had been developed further in 13 RCTs in addition to ProtecT. The final version of the QRI uses mixed research methods in two major phases: phase I is to understand recruitment as it happens and then phase II is for developing and implementing a plan of actions to address identified difficulties and optimise informed consent and recruitment in collaboration with the RCT chief investigator and clinical trials unit. The QRI can be integrated into the feasibility/pilot or main phase of a RCT to prevent difficulties developing and to optimise recruitment from the start, or applied in a RCT experiencing shortfalls with a view to rapidly improve recruitment and informed consent or to gather evidence to justify RCT closure.
The QRIs (or their developmental precursor) have now been used in 30 RCTs and have produced a number of insights into recruitment. These include how recruiters can be trained to explore patients’ treatment preferences;121 how equipoise is conveyed during recruitment and how it can facilitate or hinder recruitment;122 the development of a measure of informed consent;123 how training can increase recruiter confidence;124 and the value of collecting data logging patients at the various stages of screening, eligibility, approach and recruitment. 125
Baseline data
Baseline characteristics of ineligible and eligible participants
There were over 10,000 ProtecT participants with a raised PSA level (around 11% of recruited participants), of whom 8566 in the main trial had at least one biopsy and about 3000 were diagnosed with prostate cancer; of these 3000, around 400 had advanced disease (12%) (see Figure 2). The characteristics of men with prostate cancer were similar to the characteristics of those without diagnosed cancer, except for the expected greater frequency of a family history of prostate cancer and higher initial PSA levels (Table 4).
| Characteristics | Ineligible participants: no prostate cancer (N = 79,208)a | All participants with prostate cancer (localised, advanced and ineligible) (N = 2896) | p-value |
|---|---|---|---|
| Age (years), mean (SD) | 58.3 (5.4) | 61.4 (5.0) | < 0.001 |
| Ethnicity,b n (%) | |||
| White | 77,486 (97.8) | 2839 (98.0) | 0.10 |
| African Caribbean | 215 (0.3) | 11 (0.4) | |
| Married/living with partner, n (%) | 66,178 (83.5) | 2420 (83.6) | 0.88 |
| Living in an area of social deprivation,c n (%) | 10,706 (13.5) | 407 (14.1) | 0.34 |
| Family history of prostate cancer, n (%) | 4082 (5.2) | 220 (7.6) | < 0.001 |
| PSA level (ng/ml), mean (SD) | 1.3 (1.3) | 6.0 (3.3) | < 0.001 |
Eligible participants with localised prostate cancer who selected their treatment had similar measured clinical and sociodemographic characteristics to the characteristics of those who accepted randomisation, except that they were less likely to reside in an area of material deprivation [odds ratio (OR) of increased deprivation in randomised vs. non-randomised participants 0.74, 95% CI 0.58 to 0.94] and may have differed in unmeasured characteristics (Table 5). These characteristics have been reported previously, including the PROMs for men with advanced prostate cancer or health-related exclusions. 95
| Characteristics | Participants | p-value | |
|---|---|---|---|
| Randomised (N = 1643) | Non-randomiseda (N = 997) | ||
| Age (years), mean (SD) | 61.2 (5.0) | 61.1 (5.0) | 0.60 |
| Material and social deprivation,b n (%) | 239 (14.6) | 111 (11.1) | 0.02 |
| Ethnicity,c n (%) | |||
| White | 1606 (98.7) | 984 (99.1) | 0.31 |
| African Caribbean | 10 (0.6) | 2 (0.2) | |
| Married/living with partner, n (%) | 1375 (84.3) | 841 (84.8) | 0.72 |
| Family history of prostate cancer, n (%) | 119 (7.2) | 83 (8.3) | 0.28 |
| PSA level (ng/ml), mean (SD) | 5.8 (3.0) | 5.8 (3.1) | 0.67 |
| Gleason score (points),d n (%) | |||
| 6 | 1266 (77.1) | 755 (75.7) | 0.42 |
| 7 | 339 (20.6) | 218 (21.9) | |
| 8–10 | 37 (2.3) | 24 (2.4) | |
| Clinical stage, n (%) | |||
| T1c | 1249 (76.0) | 758 (76.0) | 0.96 |
| T2 | 394 (24.0) | 239 (24.0) | |
Demographic and clinical features of randomised participants
The median age of randomly assigned participants was 62 years (range 50–69 years), and the median PSA level was 4.6 µg/l. Most randomised participants had clinical T1c disease and a Gleason score of 6 points. The distributions of age, PSA levels, Gleason scores and disease stage were well balanced across the randomised groups (Table 6).
| Characteristics | Treatment group | RP (N = 553) | |
|---|---|---|---|
| AM (N = 545) | RT (N = 545) | ||
| Age band at invitation (years), n (%) | |||
| 49–54 | 58 (10.6) | 62 (11.4) | 69 (12.5) |
| 55–59 | 140 (25.7) | 141 (25.9) | 137 (24.8) |
| 60–64 | 184 (33.8) | 176 (32.3) | 172 (31.1) |
| 65–69 | 163 (29.9) | 166 (30.5) | 175 (31.6) |
| Age (years), mean (SD) | 61.3 (5.0) | 61.3 (5.0) | 61.2 (5.1) |
| PSA level (ng/ml), n (%) | |||
| 3.0–5.9 | 373 (68.4) | 373 (68.4) | 371 (67.1) |
| 6.0–9.9 | 116 (21.3) | 121 (22.0) | 123 (22.2) |
| ≥ 10.0 | 56 (10.3) | 51 (9.4) | 59 (10.7) |
| PSA level (ng/ml), mean (SD) | 5.7 (3.0) | 5.7 (2.9) | 5.7 (3.0) |
| Gleason score (points), n (%) | |||
| 6 | 421 (77.3) | 423 (77.6) | 422 (76.3)a |
| 7 | 111 (20.4) | 108 (19.8) | 120 (23.5) |
| 8–10 | 13 (2.4) | 14 (2.6) | 10 (1.8) |
| Clinical stage, n (%) | |||
| T1c | 410 (75.2) | 429 (78.7) | 410 (74.1) |
| T2 | 135 (24.8) | 116 (21.3) | 143 (25.9) |
Baseline levels of patient-reported outcomes for randomised participants
Urinary, bowel and sexual dysfunction and their impact on quality of life
Levels of incontinence were low: fewer than 1% of men reported incontinence as a large problem (4/1259; Table 7) and fewer than 1% of men reported using at least one pad per day. Seventy per cent of participants reported being incontinence-free (873/1244). Urinary function was good (95.1% of participants), and bother related to urinary symptoms was low (91.0% of participants), with few irritative/obstructive symptoms (93.0% of participants did not report these) as measured by the EPIC summary scores. Nocturia was more frequent, affecting around one-fifth of men (312/1423, measured using the ICSmaleSF), and around one-third also reported a regular daytime frequency (460/1410), although only 3% of men (44/1427) reported these lower urinary tract symptoms (LUTS) as being a moderate or severe problem (measured using the ICSmaleSF). Bowel EPIC symptom scores indicated few problems, and only 3% of men (20/751) reported a moderate or large problem due to bowel symptoms (see Appendix 1, Table 25). The frequency of faecal incontinence or bloody stools was also very low, although 16% of participants reported having loose stools at least half of the time (118/754). Around one-third of men (241/735) reported erectile dysfunction and for some of the participants this was a moderate or large problem (118/731). Sexual function and bother EPIC summary scores were much lower than those for urinary and bowel symptoms (see Appendix 1, Tables 24 and 25). 126
| Summary scores | Treatment group | RP (N = 553) | Total (N = 1643) | |
|---|---|---|---|---|
| AM (N = 545) | RT (N = 545) | |||
| SF-12 minimum analysed, n (%) | 418 (77) | 410 (75) | 432 (78) | 1260 (77) |
| Mental health score, mean (SD) | 53. 4 (8.2) | 54.5 (6.3) | 53.9 (7.9) | 53.9 (7.5) |
| Physical health score, mean (SD) | 50.4 (8.7) | 51.7 (7.0) | 51.4 (7.9) | 51.2 (7.9) |
| HADS minimum analysed, n (%) | 469 (86) | 454 (83) | 472 (85) | 1399 (85) |
| Anxiety score, mean (SD) | 5.1 (3.6) | 4.5 (3.2) | 5.0 (3.6) | 4.9 (3.5) |
| Depression score, mean (SD) | 2.7 (2.7) | 2.3 (2.4) | 2.4 (2.5) | 2.5 (2.5) |
| Anxiety possible case, n/N (%) | 107/472 (23) | 74/454 (16) | 97/477 (20) | 278/1403 (20) |
| Depression possible case, n/N (%) | 37/469 (8) | 17/458 (4) | 26/472 (6) | 80/1399 (6) |
| EQ-5D-3L minimum analysed, n (%) | 474 (87) | 458 (84) | 481 (87) | 1413 (86) |
| Health utility, mean (SD) | 0.87 (0.19) | 0.90 (0.16) | 0.88 (0.17) | 0.89 (0.17) |
Overall health status, anxiety and depression
Overall physical and mental health scores were comparable with UK normative data (SF-12 subscores of 50)93 (see Table 7). The EuroQol-5 Dimensions, three-level version (EQ-5D-3L) health utility scale also indicated good overall health (mean 0.89). Mean anxiety and depression scores were low, although one-fifth of men (278/1403) could be classed as having possible clinical levels of anxiety and around 6% of men could be classed as having depression (80/1399) (see Table 7). 126
Numbers analysed and return of data-collection forms
Primary outcome
As there were no missing data for the primary outcome analysis, we did not compare the analysable sample with those who withdrew or were lost to follow-up.
Secondary outcomes
Response rates for annual follow-up questionnaires and completion of the CRFs by research nurses remained at around 90% for men still in follow-up over 10 years (see Appendix 1, Figure 29). CRFs (see Appendix 1, Figure 29a) and questionnaires (see Appendix 1, Figure 29b) were completed annually (also at 6 months for questionnaires). Results are presented for participants still agreeing to data collection for secondary outcomes. The rates of completion of exemplar outcome measures at 6 years (time point of analysis for PROMs) were 87% for the ICIQ (1369/1572), 86% for EPIC sexual function (1352/1572) and 88% (1388/1572) for men alive at completion.
Chapter 4 Trial results at the 10-year median follow-up
Primary and secondary clinical outcomes
The primary outcome measure was defined as definite or probable prostate cancer mortality, including intervention-related deaths, at a median follow-up point of 10 years. To ascertain cause of death, summaries of anonymised medical records were reviewed by members of the independent Cause of Death Evaluation (CoDE) Committee, who were blinded to treatment assignment. The process was adapted from the PLCO Cancer Screening Trial22 and the ERSPC. 21 Deaths were categorised as definitely, probably, possibly, probably not or definitely not attributable to prostate cancer. 97,127
Secondary outcomes included all-cause mortality, metastases, clinical disease progression, primary treatment failure and treatment complications. Metastatic disease was defined as imaging showing bony, visceral or lymph node metastases, or PSA levels of > 100 µg/l. Clinical disease progression was defined by any of the following:
-
clinical and/or imaging evidence of soft tissue or skeletal metastases
-
diagnosis of clinical T3 or T4 disease (locally advanced prostate cancer outside the confines of the prostate)
-
initiation of long-term ADT
-
ureteric obstruction due to tumour growth along the ureteric orifices
-
rectal fistula due to overgrowth of the tumour
-
the need for urinary catheterisation due to local tumour growth causing bladder outflow obstruction.
Primary treatment failure following RP was defined as a PSA level of ≥ 0.2 µg/l at 3 months post surgery. Following RT, the RTOG-ASTRO (Radiation Therapy Oncology Group – American Society for Radiation Oncology) Phoenix Consensus Conference recommendations were used. 128 Change of management to ADT, RT or RP in the AM group was recorded. Serious intervention-related complications occurring within 90 days were recorded following completion of RP or RT.
Following RP, the complications recorded were:
-
death
-
more than 3 units of blood transfused
-
thromboembolic-cardiovascular event
-
rectal injury
-
anastomotic problems requiring intervention.
Following RT, the complications recorded were:
-
death
-
any treatment toxicity resulting in major surgical intervention.
Quality assurance
Radical prostatectomy was carried out using the open retropubic technique, and pelvic lymphadenectomy was undertaken at the discretion of individual urologists. A quality-assurance exercise was undertaken at the beginning of the trial to ascertain the competency levels of urologists involved in delivering surgical treatments. Twenty consecutive procedures undertaken by each trial urological surgeon and the outcomes were reviewed independently using standardised published criteria. 86 The Data Monitoring and Safety Committee reviewed all outcomes regularly to identify ‘outliers’ if outcomes throughout the trial appeared negative and outside the norm. No surgeons were stopped from offering the procedure throughout the duration of the trial.
Radical radiotherapy included neoadjuvant ADT for 3–6 months before and concomitantly with 3D-conformal RT delivered at 74 Gy in 37 fractions. Quality assurance followed the RT01 trial procedures. 129–131 There has been an increasingly sophisticated quality assurance programme in prostate radiotherapy trials over the last 15 years, reflecting dose escalation and treatment complexity. In ProtecT, machine dosimetry results were comparable between trial centres and with the UK RT01 trial. 129–131 The outlining review showed that most deviations were clinically acceptable, although three (1.4%) may have been of clinical significance and were related to outlining of the prostate. Seminal vesicle outlining varied, possibly due to several prostate trials running concurrently with different protocols. Adherence to dose constraints in ProtecT was considered acceptable, with 80% of randomised participants having two or fewer deviations, and planning target volume coverage was excellent. The ProtecT trial quality assurance results were satisfactory and comparable with trials of its era. 131
Results
Of the 1643 men randomised, 545 were allocated to AM, 553 were allocated to RP and 545 were allocated to RT. Contact was lost with 14 men (0.9%), but mortality data were captured for all participants. Following randomisation, 482 men (88%) who were assigned to AM, 391 (71%) who were assigned to RP and 405 (74%) who were assigned to RT received the allocated treatment within 9 months of randomisation, as illustrated in the CONSORT flow diagram (see Figure 2).
By the end of our reported follow-up, over 85% of men assigned to RT or RP received a radical intervention (Figure 6). Of the 545 men assigned to AM, 290 received a radical treatment by the end of November 2015 (Kaplan–Meier estimate 54.6%, 95% CI 50.2% to 59.2%). Of those 290 men, 142 (49%) received RP (37 within 9 months of allocation), 97 (33%) received radiotherapy per protocol (17 within 9 months of allocation), 22 (8%) received brachytherapy (two within 9 months of allocation), 26 (9%) received non-protocol radiotherapy and three (1%) received HIFU beyond 9 months from allocation.
FIGURE 6.
Cumulative probability of receiving radical treatment in the three allocation groups: AM (n = 545), RP (n = 553) and RT (n = 545).
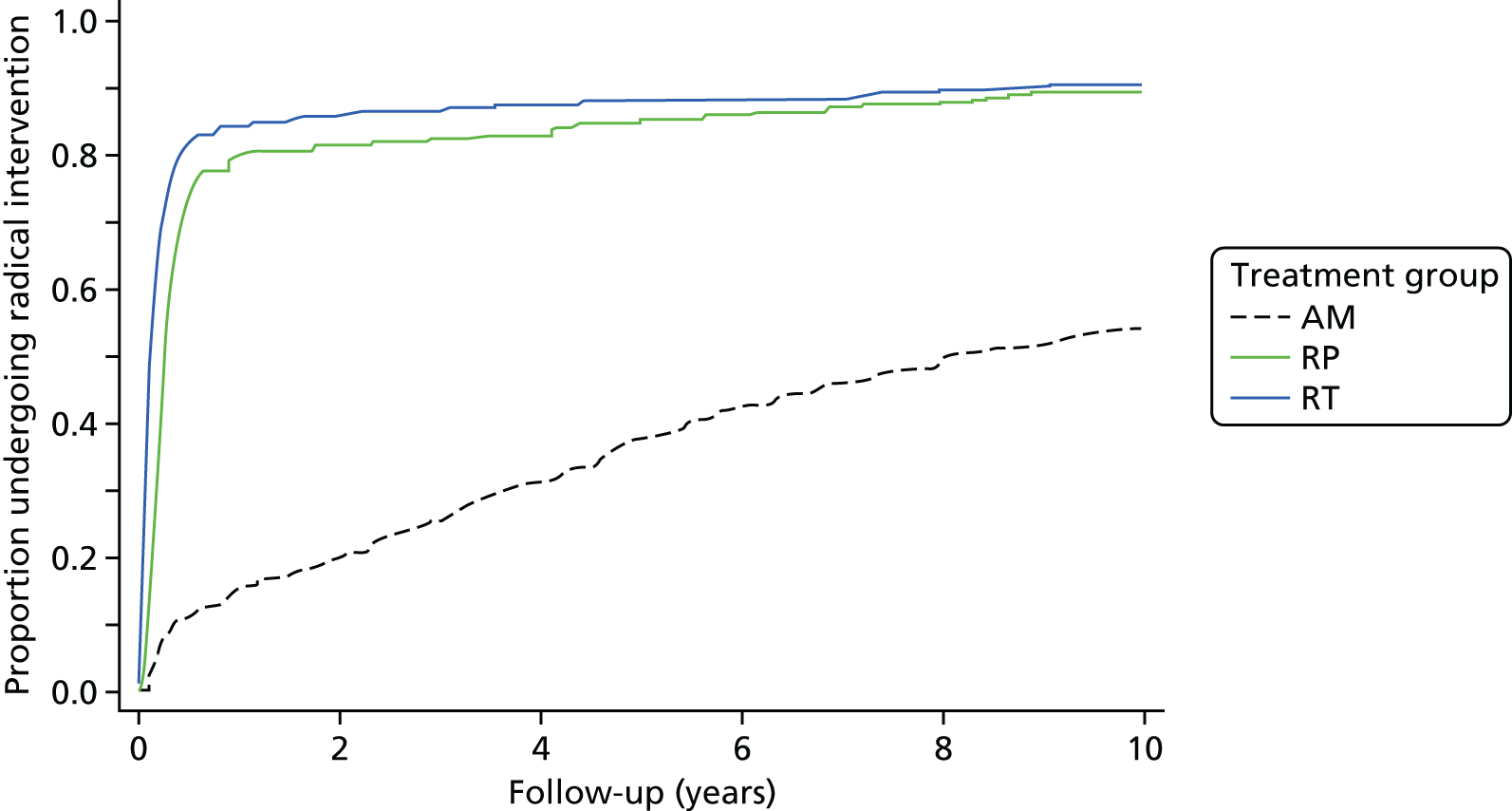
Of the 391 men who underwent RP within 9 months of allocation, nine (2%) had a PSA level of ≥ 0.2 µg/l between 31 and 183 days following surgery; five of those men received salvage RT and one received long-term ADT within 1 year of surgery. A further nine men received adjuvant RT within 1 year of surgery because of pT3 disease (n = 8) or positive surgical margins (n = 7). pT3 disease was present in 114 of the 391 men (29%), and 93 (24%) had a positive surgical margin. Four out of 280 patients (1%) who received lymphadenectomy had lymph node involvement. Of the 405 men who started RT within 9 months of allocation, 55 (14%) had a PSA increase of ≥ 2 ng/ml above the nadir following RT. Of those 55 men, three received salvage RP, 14 started long-term ADT and one underwent HIFU.
Prostate cancer-specific and all-cause mortality
The independent CoDE Committee ascertained seven definite prostate cancer-specific deaths and one probable prostate cancer-specific death in the AM group, three definite and two probable prostate cancer-specific deaths in the RP group and four definite prostate cancer-specific deaths in the RT group (Table 8 and Figure 7). Prostate cancer-specific survival was > 98.8% in all groups, and there was no evidence of difference between the three randomised groups (log-rank test p = 0.48). The HR of prostate cancer-specific mortality for the RT group was 0.45 (95% CI 0.14 to 1.47) compared with AM and 0.80 (95% CI 0.22 to 2.99) compared with RP; for RP compared with AM, it was 0.56 (95% CI 0.19 to 1.67) (Figure 8). Subgroup analyses showed no evidence of any subgroup modifying the relative effectiveness of the three treatments in terms of prostate cancer mortality (see Table 2). All-cause deaths were evenly distributed across the treatment groups (likelihood ratio test p = 0.87). Of the patients with ascertained prostate cancer-specific deaths, 7 out of 17 had baseline features whereby most international guidelines would recommend AS, and 12 out of 17 had received AM (three out of five had been randomised to RP and two out of 45 had been randomised to RT), as shown in Table 9.
| Characteristics | Treatment group, number of deaths attributable to prostate cancer | p-value | ||
|---|---|---|---|---|
| AM (n = 545) | RP (n = 553) | RT (n = 545) | ||
| Age (years) at randomisation | ||||
| < 65 | 1 | 3 | 1 | 0.095 |
| ≥ 65 | 7 | 2 | 3 | |
| PSA level (µg/l) at diagnosis | ||||
| < 6 | 5 | 3 | 4 | 0.72 |
| ≥ 6 | 3 | 2 | 0 | |
| Gleason score (points) at diagnosis | ||||
| 6 | 3 | 3 | 2 | 0.69 |
| ≥ 7 | 5 | 2 | 2 | |
| Clinical stage at diagnosis | ||||
| T1c | 5 | 3 | 3 | 0.95 |
| T2 | 3 | 2 | 1 | |
FIGURE 7.
Kaplan–Meier estimates of deaths from prostate cancer in the AM, RP and RT treatment groups.
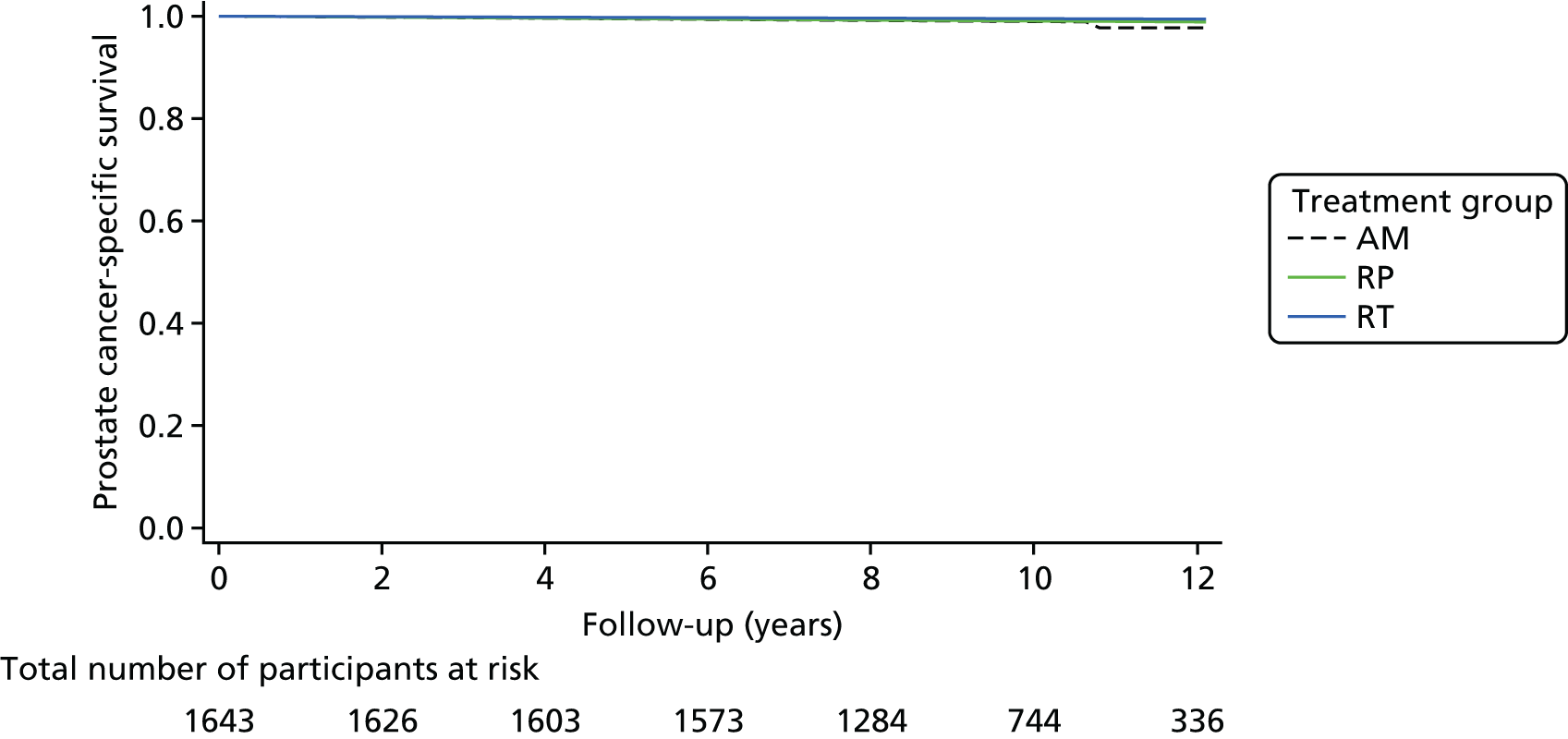
FIGURE 8.
Kaplan–Meier estimates of death from any cause in the AM, RP and RT treatment groups.
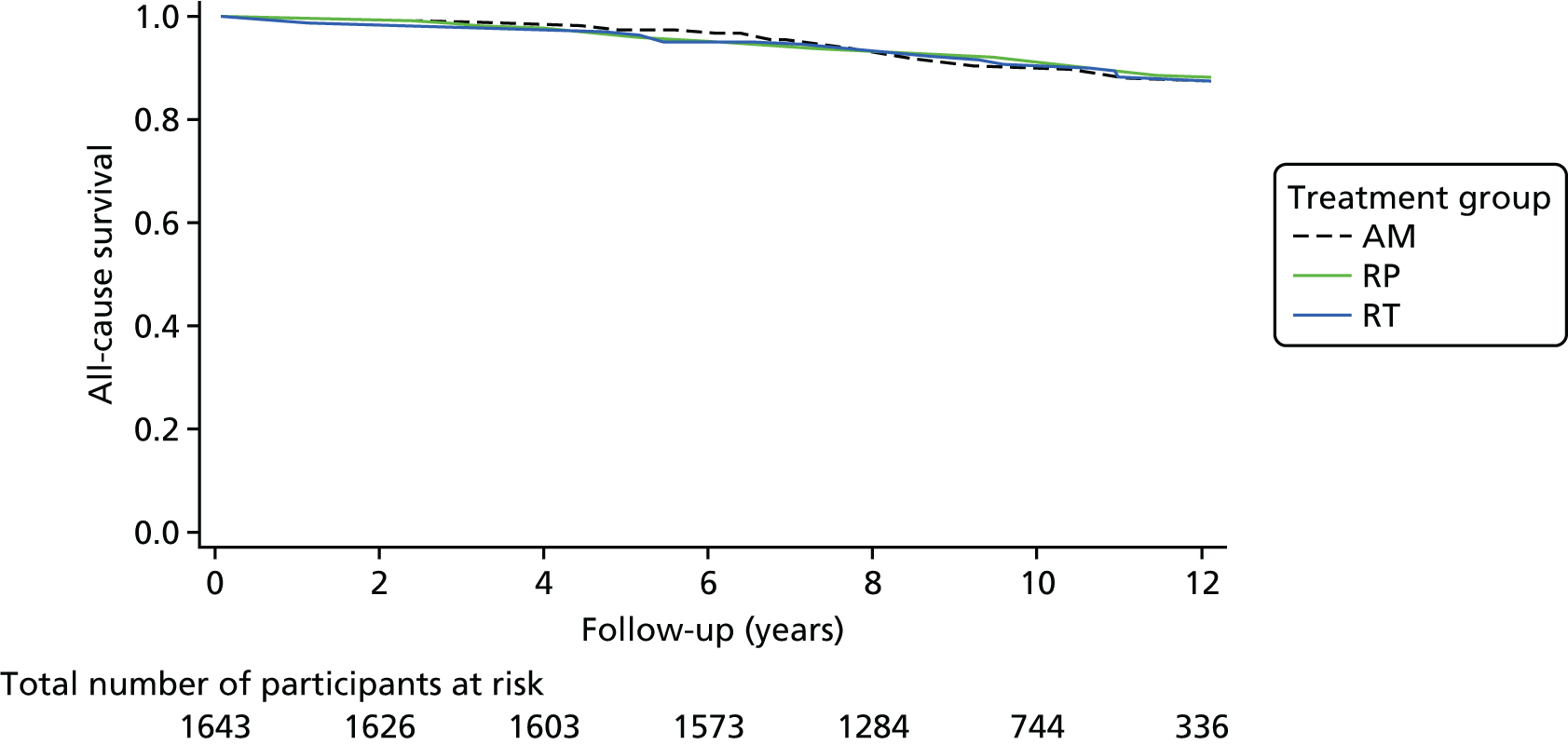
| Allocation | Age (years) at diagnosis | Gleason score (points) at diagnosis | PSA level (µg/l) at diagnosis | Number of biopsy cores with tumour | Stage at diagnosis | Date of allocation | First treatment received | Date of first treatment | Second treatment received | Date of second treatment | Date PSA level was ≥ 10 ng/ml | Date of death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | 60–64 | 6 | 6–9.99 | 3 | T2 | March 2004 | AM | April 2004 | RT | October 2004 | June 2004 | October 2009 |
| AM | 65–69 | 6 | < 6 | 1 | T1c | September 2003 | AM | September 2003 | ADT | September 2005 | August 2005 | December 2006 |
| AM | 65–69 | 6 | < 6 | 1 | T1c | December 2003 | AM | December 2003 | – | – | November 2009 | August 2014 |
| AM | 65–69 | 7 | < 6 | 6 | T2 | October 2004 | AM | October 2004 | ADT | June 2007 | March 2007 | December 2012 |
| AM | 65–69 | 7 | < 6 | 2 | T1c | March 2004 | AM | March 2004 | RT | February 2007 | March 2005 | December 2014 |
| AM | 65–69 | 7 | < 6 | 5 | T1c | June 2005 | AM | June 2005 | RT | November 2010 | April 2010 | February 2013 |
| AM | 65–69 | 7 | 6–9.99 | 6 | T2 | October 2006 | AM | October 2006 | ADT | April 2009 | March 2009 | March 2010 |
| AM | 65–69 | 7 | 6–9.99 | 7 | T1c | July 2008 | AM | July 2008 | ADT | May 2009 | October 2008 | December 2009 |
| RP | 55–59 | 6 | < 6 | 3 | T1c | January 2001 | AM | January 2001 | ADT | March 2008 | January 2008 | July 2010 |
| RP | 60–64 | 6 | 6–9.99 | 1 | T1c | August 2000 | AM | August 2000 | RT | May 2001 | January 2001 | June 2014 |
| RP | 60–64 | 7 | < 6 | 3 | T2 | August 2003 | RP | October 2003 | SRT | June 2005 | October 2007 | October 2009 |
| RP | 65–69 | 6 | 6–9.99 | 1 | T1c | September 2001 | AM | September 2001 | – | – | August 2007 | October 2007 |
| RP | 65–69 | 7 | < 6 | 4 | T2 | August 2004 | RP | August 2004 | SRT | January 2006 | March 2010 | October 2013 |
| RT | 55–59 | 6 | < 6 | 3 | T2 | July 2005 | RT | August 2005 | ADT | July 2009 | January 2009 | February 2013 |
| RT | 65–69 | 6 | < 6 | 2 | T1c | June 2001 | AM | June 2001 | ADT | August 2005 | May 2012 | October 2013 |
| RT | 65–69 | 7 | < 6 | 2 | T1c | June 2006 | AM | June 2006 | RP | July 2008 | February 2008 | July 2013 |
| RT | 65–69 | 7 | < 6 | 2 | T1c | November 2001 | RT | January 2002 | ADT | January 2012 | February 2009 | April 2014 |
Causes of death unrelated to prostate cancer over a 10-year median follow-up, ascertained by the CoDE Committee, are listed by allocation group in Appendix 1, Table 26.
Disease progression
A total of 204 men showed progression including distant metastases (Table 10 and Figure 9), which was higher in the AM group than in the RP and RT groups (AM, n = 112; RP, n = 46; and RT, n = 46; p < 0.001). Evidence of disease progression included the presence of metastases (AM, n = 33; RP, n = 13; and RT, n = 16; p = 0.004), clinical T3 or T4 disease (AM, n = 79; RP, n = 24; and RT, n = 21) or initiation of long-term ADT (AM, n = 47; RP, n = 26; and RT, n = 30), with evidence of more than one criterion for some men.
| Parameter | Treatment group | RT (N = 545) | p-value | |
|---|---|---|---|---|
| AM (N = 545) | RP (N = 553) | |||
| Total number of person-years in follow-up | 5393 | 5422 | 5339 | |
| Number of deaths due to prostate cancera | 8 | 5 | 4 | |
| Prostate cancer survivala at 5 years (%) (95% CI) | 99.4 (98.3 to 99.8) | 100 | 100 | |
| Prostate cancer survivala at 10 years (%) (95% CI) | 98.8 (97.4 to 99.5) | 99.0 (97.2 to 99.6) | 99.6 (98.4 to 99.9) | |
| Number of prostate cancer deathsa per 1000 person-years (95% CI) | 1.5 (0.7 to 3.0) | 0.9 (0.4 to 2.2) | 0.7 (0.3 to 2.0) | 0.48 |
| Number of person-years of follow-up free of clinical progressionb | 4893 | 5174 | 5138 | |
| Number of men with clinical progressionb | 112 | 46 | 46 | |
| Number of men with clinical progressionb per 1000 person-years (95% CI) | 22.9 (19.0 to 27.5) | 8.9 (6.7 to 11.9) | 9.0 (6.7 to 12.0) | < 0.001 |
| Number of person-years of follow-up free of metastatic disease | 5268 | 5377 | 5286 | |
| Number of men with metastatic disease | 33 | 13 | 16 | |
| Number of men with metastatic disease per 1000 person-years (95% CI) | 6.3 (4.5 to 8.8) | 2.4 (1.4 to 4.2) | 3.0 (1.9 to 4.9) | 0.004 |
| Total number of person-years in follow-up | 5393 | 5422 | 5339 | |
| Number of deaths attributable to any cause | 59 | 55 | 55 | |
| Number of all-cause deaths per 1000 person-years (95% CI) | 10.9 (8.5 to 14.1) | 10.1 (7.8 to 13.2) | 10.3 (7.9 to 13.4) | 0.87 |
FIGURE 9.
Cumulative proportion of participants free of disease progression in the AM, RP and RT treatment groups.
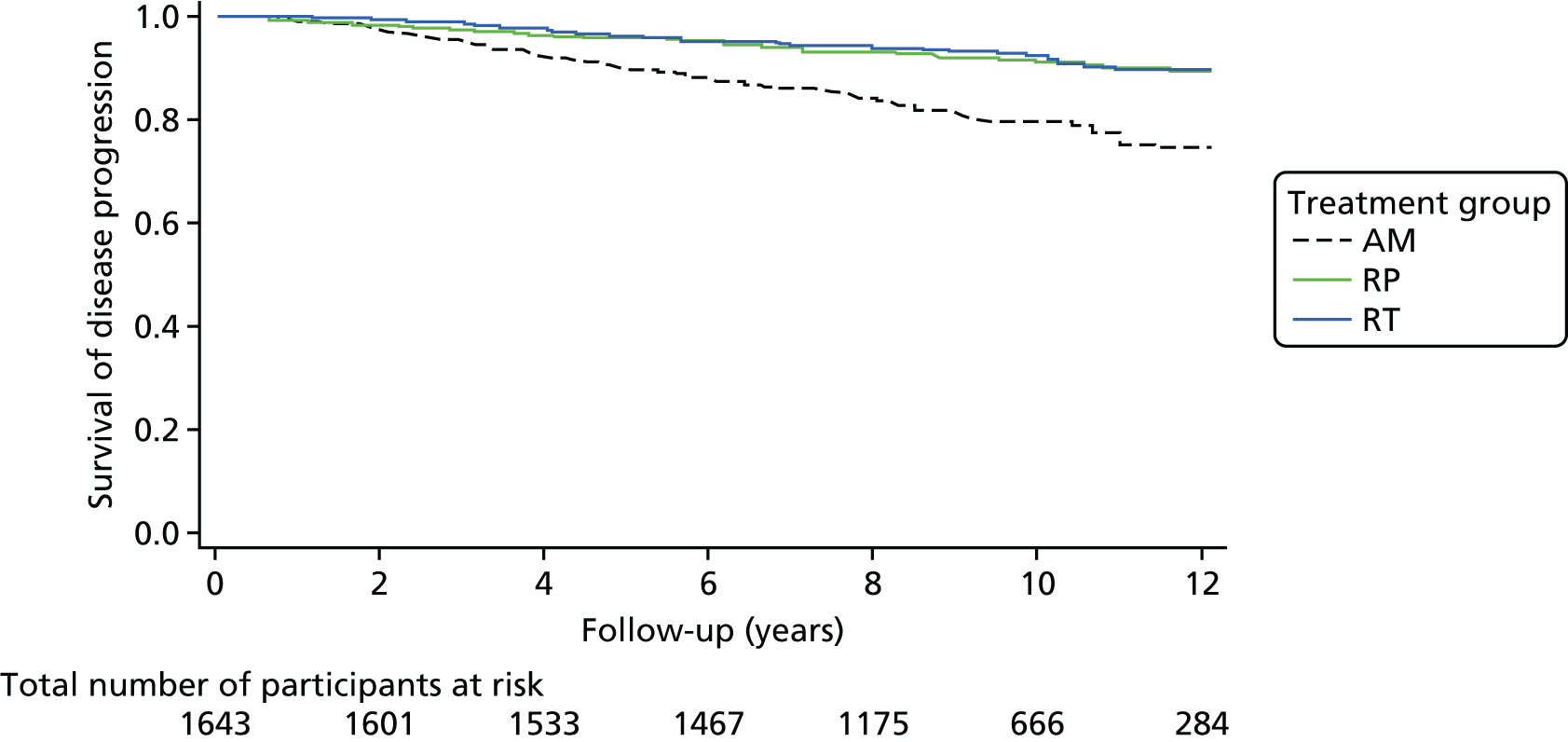
Treatment complications
There were no deaths related to RP; nine men suffered thrombo-embolic or cardiovascular events, 14 required more than 3 units of blood transfused, one suffered a rectal injury and nine required intervention for anastomotic problems. There were three deaths unrelated to prostate cancer within 90 days of completing RT, and no cases of radiation toxicity requiring major intervention.
Numbers needed to treat
From these data, compared with AM, 178 and 137 men would need to be treated with RP and RT, respectively, to avoid one prostate cancer death; 27 and 33 men would need to be treated with RP and RT, respectively, to avoid one patient progressing to metastases; and nine men would need to be treated with either RP or RT to avoid one patient developing clinical disease progression.
Analysis of disease characteristics in progressing and non-progressing patients
We have reviewed the baseline risk classification of all ProtecT participants using the D’Amico criteria132–134 and other clinicopathological features, and compared men who developed disease progression with those whose disease remained stable to determine if there were any features associated with clinical outcomes. Furthermore, we report detailed pathological information for men who received surgery and the impact of these features on outcome. The results of these recent analyses are presented in Baseline clinicopathological features of participants with disease progression.
Men who had started a protocol treatment within 12 months were included in this analysis (n = 1585 of 1643 men). Patients were categorised as receiving AM if they had started formal treatment under this name and had at least two PSA tests within 1 year of eligibility (diagnosis). Patients were categorised as receiving RP or RT if they started treatment within 12 months and, for RT, completed it within 15 months.
Risk status
D’Amico scoring was defined as low risk if the Gleason score was ≤ 3 + 3 points, the PSA level was < 10 ng/ml and there was stage T1c/T2a disease, intermediate risk if the Gleason score was 7 points or the PSA level was ≥ 10 ng/ml and < 20 ng/ml or there was stage T2b disease, and high risk if the Gleason score was ≥ 8 points or the PSA level was ≥ 20 ng/ml or there was stage T2c disease. Twenty-five men were classed as having cT2 disease according to a previous TNM staging system and so could not be given a D’Amico score.
Pathology
Expert histopathologists at each centre reported prostate biopsy and RP pathology findings on standardised pro formas. 135 RP specimens were whole embedded and the volume of the tumour was calculated. 135,136 Surgical margins were recorded as positive if the tumour was seen at an inked margin and classified as apical, basal, intraprostatic or extraprostatic. The ProtecT pathology group conducted internal audits of both biopsy cores and RP specimens to minimise variation in assessment.
Progression and metastasis
Metastatic prostate cancer was defined as the presence of bony, visceral, or lymph node metastases on imaging, or a PSA level of > 100 ng/ml. Clinical progression was defined as the presence of any of (1) evidence of metastases, (2) progression to clinical T3 or T4 disease, (3) long-term ADT, (4) ureteric obstruction, (5) rectal fistula or (6) need for a urinary catheter owing to local tumour growth. Primary treatment failure after RP was defined as a PSA level of ≥ 0.2 ng/ml 3 months after surgery, and primary treatment failure after RT was defined in accordance with the Phoenix Consensus Conference recommendations. 128
Statistical analysis
All analyses were carried out using Stata version 14.2. Logistic regression was used to estimate the odds of progression for baseline characteristics, adjusting for age and treatment received when appropriate. When model assumptions could not be met, the log of the exposure was used. The odds of progression were calculated for participants with baseline Gleason scores of ≤ 3 + 3 points. Gleason score values for the prostate biopsy cores were derived using the sum of the primary Gleason grade and the greater of the secondary or tertiary Gleason grades in order to reflect current practice. Aggregate and maximum lengths of tumours were measured in millimetres. The aggregate length of tumours was calculated as the summation of the lengths on the right, left and unknown sides and targeted biopsy, adding 0.5 mm for each small additional tumour (up to four). The Cox proportional hazards model and likelihood ratio test were used to test the interaction between treatment and each of baseline Gleason score (≤ 3 + 3, 3 + 4, 4 + 3 or ≥ 8 points), length of tumour (≤ 4 mm or > 4 mm) and age (< 65 or ≥ 65 years), and their impact on time to progression.
The odds of progression were compared across RP pathological features adjusted for participant age at recruitment. Logistic, ordinal logistic and linear regression were used to test the association between treatment pathway and binary, ordered categorical and continuous surgical characteristics, respectively. Upgrading was defined as an increase from a Gleason score of ≤ 3 + 3 points at baseline to > 3 + 3 points at treatment with RP, or moving from a Gleason score of 3 + 4 at baseline to ≥ 4 + 3 points at treatment with RP. Upstaging was defined as moving from T1/T2 disease at baseline to T3/T4 at RP. A positive surgical margin was categorised if any of the basal, apical, intraprostatic or extraprostatic margins were positive, and as negative if all four margins were negative or not applicable. A full statistical analysis plan is included in the protocol (see www.journalslibrary.nihr.ac.uk/programmes/hta/962099/#/).
Risk categorisation
On average, randomised participants (n = 1643) were 62 years old, of white ethnicity, with a median PSA level of 4.6 ng/ml, and their clinicopathological characteristics were reported previously. 104 These were reviewed and this showed that 1192 men (75%) had Gleason score ≤ 3 + 3 points disease and 1203 (76%) had clinical stage T1c disease. Using the D’Amico classification, 1031 participants (66%) were defined as being at low risk, 490 (31%) were defined as being at intermediate risk and 40 (3%) had features of high-risk disease (see Table 10).
Baseline clinicopathological features of participants with disease progression
A total of 199 out of 1585 (12.5%) randomised participants who commenced prostate cancer management within 12 months developed prostate cancer progression during a median follow-up of 10 years (see Table 1). There were 17 prostate cancer-specific deaths, a further 44 men developed metastatic disease and 138 men developed other clinical evidence of disease progression (see Tables 1 and 10). Older age (≥ 65 vs. < 65 years), baseline PSA level, Gleason score, cT stage, number of prostate cancer-involved biopsy cores, length of prostate cancer in any core (median 4.5 mm vs. 2.0 mm), aggregate length of tumour (median 8.0 mm vs. 4.0 mm) and presence of perineural invasion were each associated with an increased risk of subsequent disease progression (p < 0.001 for each) (see Figure 1). Thirty-nine per cent of men (n = 74) with disease progression were initially categorised with D’Amico low-risk disease, whereas 54% (n = 104) had intermediate-risk and 7% (n = 13) had high-risk disease, compared with respective values of 70% (n = 957), 28% (n = 386) and 2% (n = 27) for men without progression (p < 0.001).
Disease progression of participants with a baseline Gleason score of ≤ 3 + 3 points
A total of 101 out of 1192 men (8.5%) with a Gleason score of ≤ 3 + 3 points at baseline developed disease progression during follow-up; eight of these men died of prostate cancer, a further 20 developed metastases and 73 developed clinical progression (see Table 10). The odds of disease progression were higher among men who initially received AM than in men who initially received radical treatment (RP or RT) (p < 0.001) (Figure 10). For men with a Gleason score of ≤ 3 + 3 points at baseline, higher median PSA level (5.7 vs. 4.3 ng/ml), higher cT stage, higher D’Amico risk group, increased number of prostate cancer-involved biopsy cores, increased maximum length of prostate cancer in any core (median 3.0 vs. 2.0 mm) and increased aggregate length of tumour (median 5.5 vs. 3.0 mm) were each associated with disease progression (at least p < 0.05 for each comparison) (Figure 11).
FIGURE 10.
Cumulative risk of progression, by treatment group and age group. (a) Age < 65 years; and (b) age ≥ 65 years. a, n (n censored by time point).
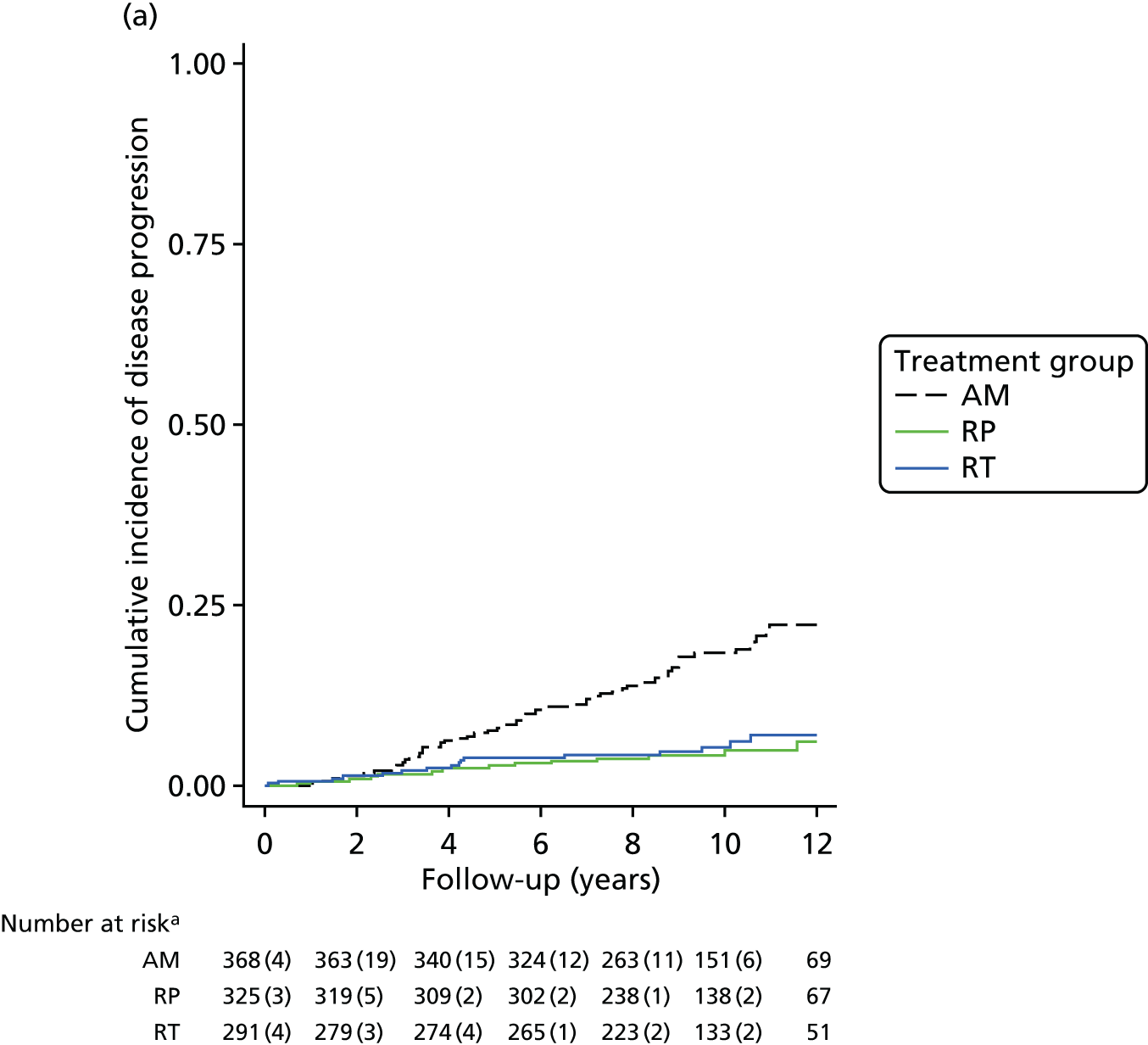
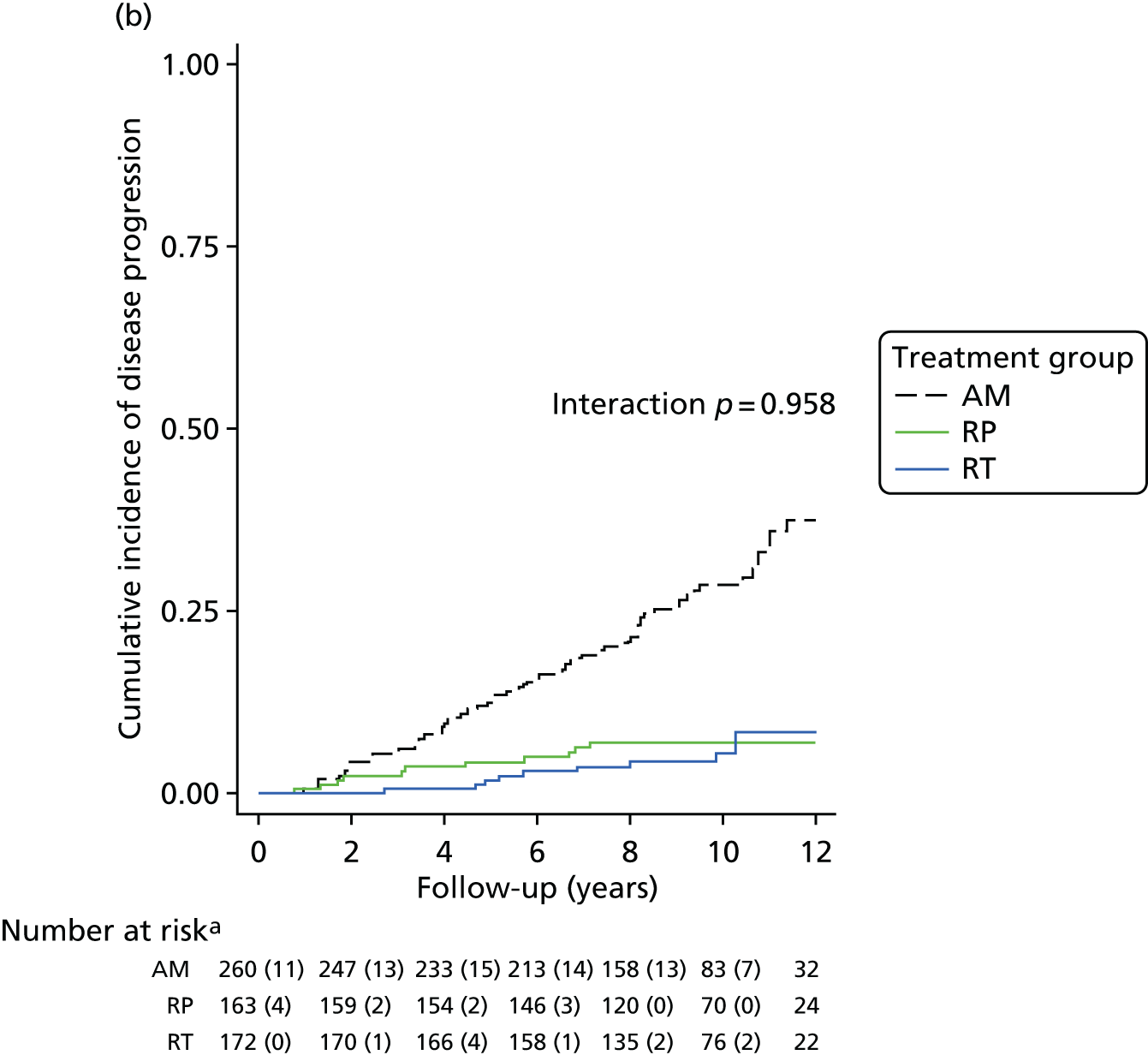
FIGURE 11.
Cumulative risk of progression, by treatment group, Gleason scores and PSA levels. (a) Gleason score of < 7 points; (b) Gleason score of ≥ 7 points; (c) PSA level of < 10 ng/ml; and (d) PSA level of ≥ 10 ng/ml. a, n (n censored by time point).
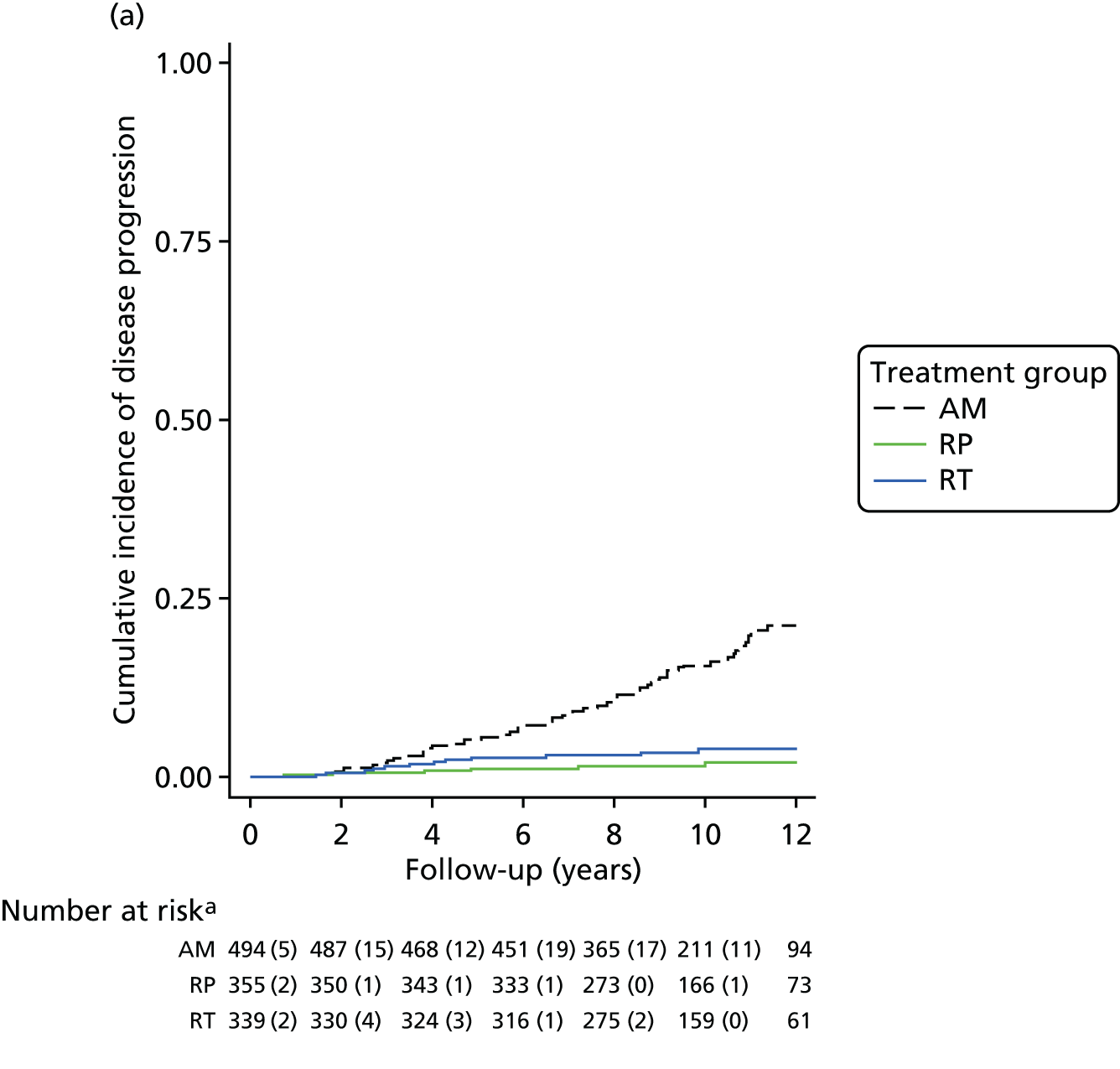
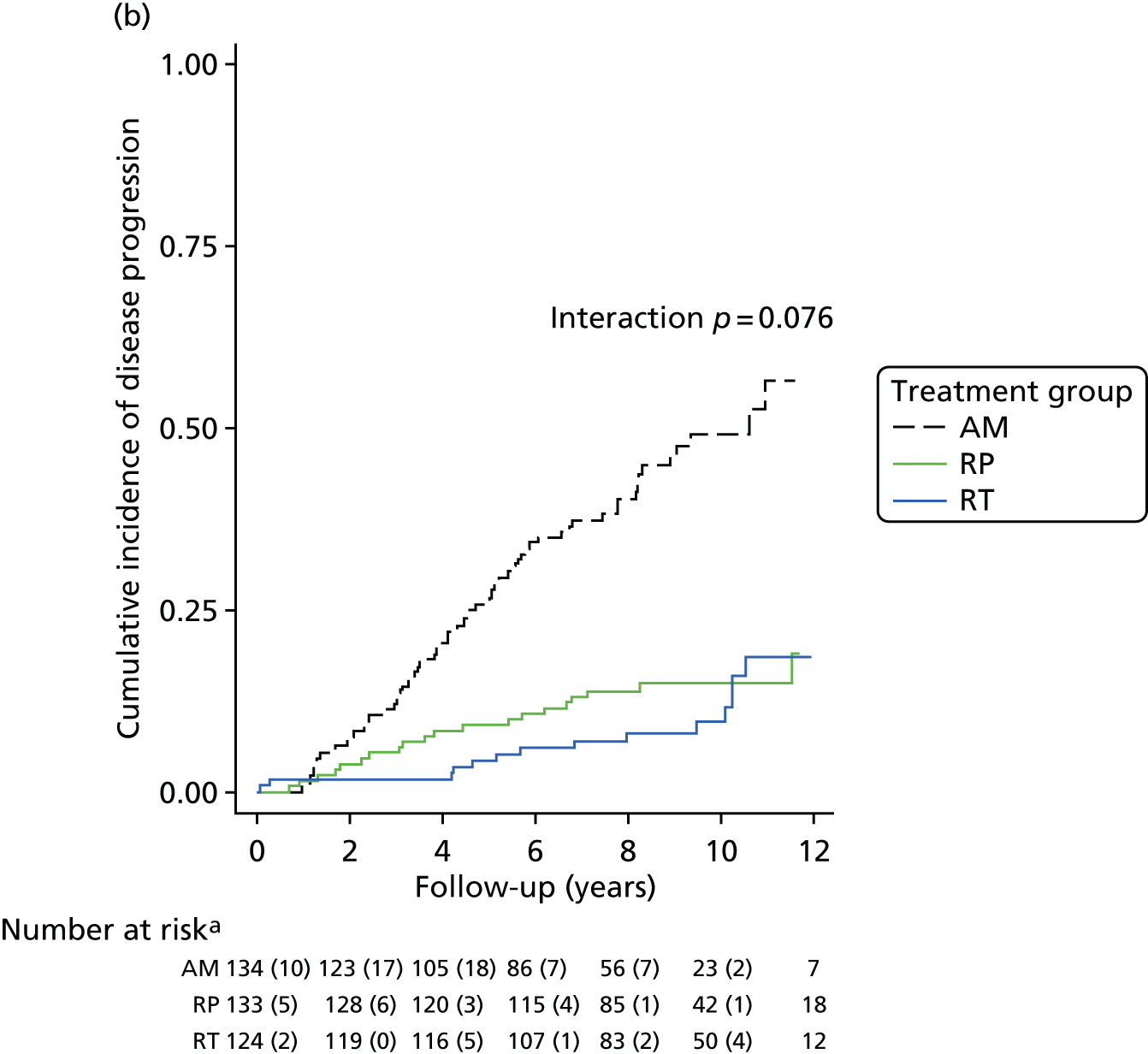
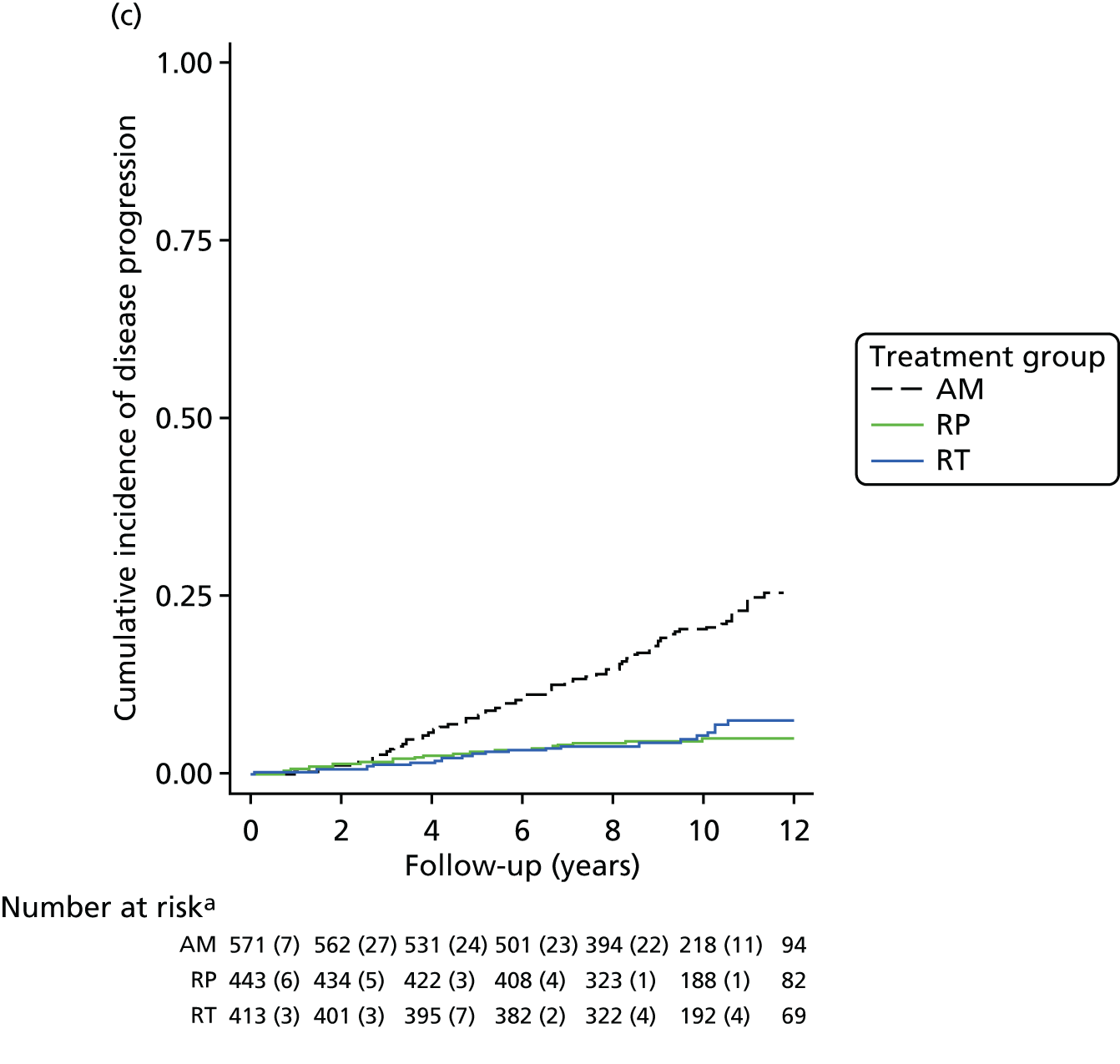
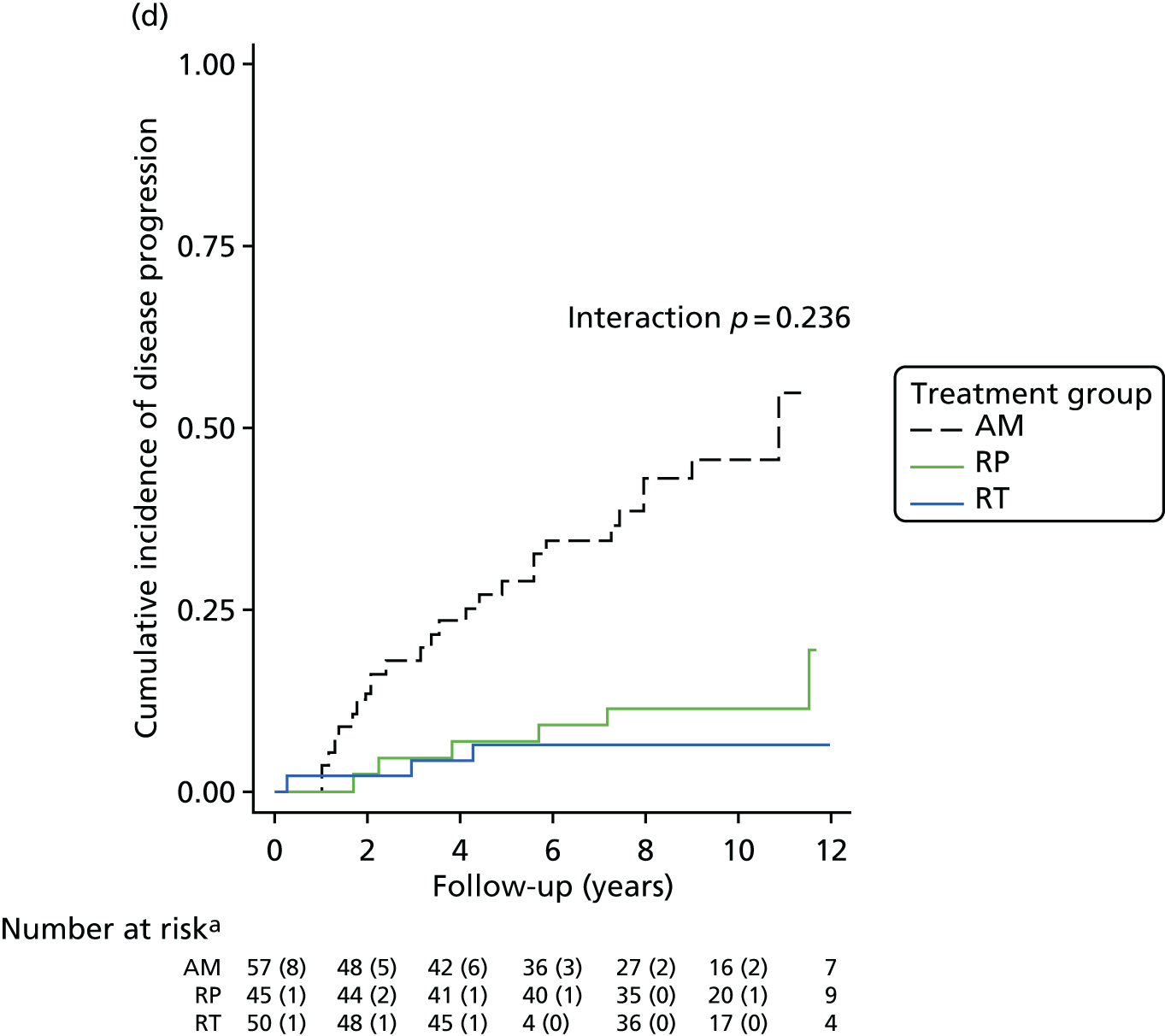
Radical prostatectomy pathology characteristics
A total of 396 out of 553 men randomised to receive RP underwent this allocated treatment within 12 months. Of the 19 men who subsequently developed prostate cancer progression following surgery, none had a Gleason score of ≤ 3 + 3 points on examination of the resected prostate, whereas eight had a Gleason score of 3 + 4 points, seven had a Gleason score of 4 + 3 prostate cancer and four had a Gleason score of ≥ 8 points, which differed from men without disease progression (p < 0.001) (see Table 3). RP pathology features associated with subsequent disease progression included pathological Gleason score, pathological tumour stage, largest tumour volume, lymph node involvement, perineural and/or vascular invasion and apical or any margin positive status (p < 0.05 for each comparison). There was no evidence to suggest that the number of tumours was related to disease progression. Prostate cancer upstaging, but not upgrading, at surgery was associated with subsequent disease progression (p < 0.001).
Discussion
According to the D’Amico risk stratification criteria, 66% of ProtecT participants had low-risk prostate cancer and 34% had intermediate- or high-risk disease. The reported analysis of baseline clinical and demographic characteristics of randomised ProtecT participants demonstrates several baseline features associated with disease progression, including increased age, PSA level, Gleason score, cT stage, number of prostate cancer-involved biopsy cores, length of prostate cancer in any core, aggregate length of tumour (Figure 12) and perineural invasion.
FIGURE 12.
Cumulative risk of progression, by treatment group and length of tumour. (a) Length of tumour < 4 mm; and (b) length of tumour ≥ 4 mm. a, n (n censored by time point).
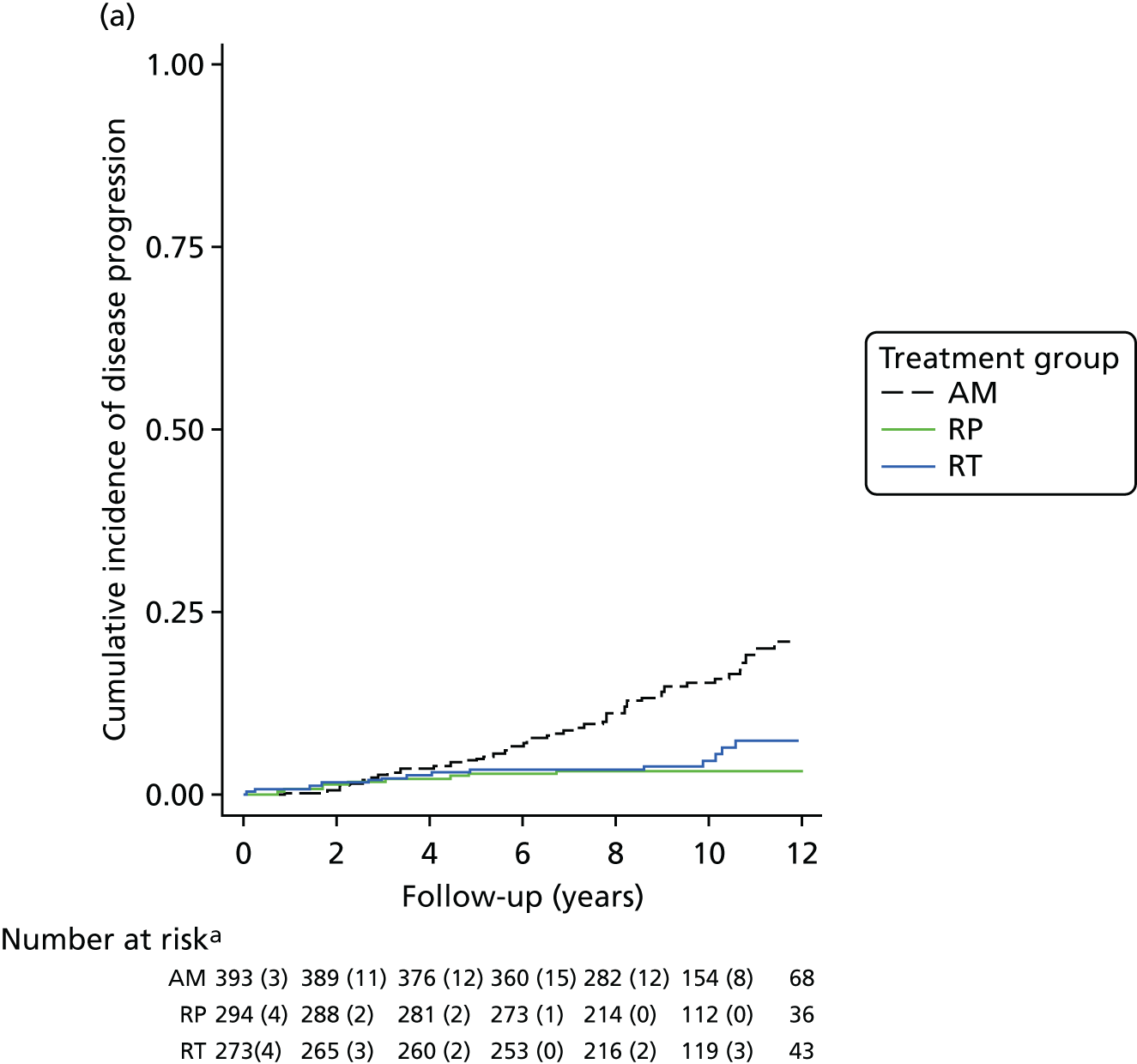
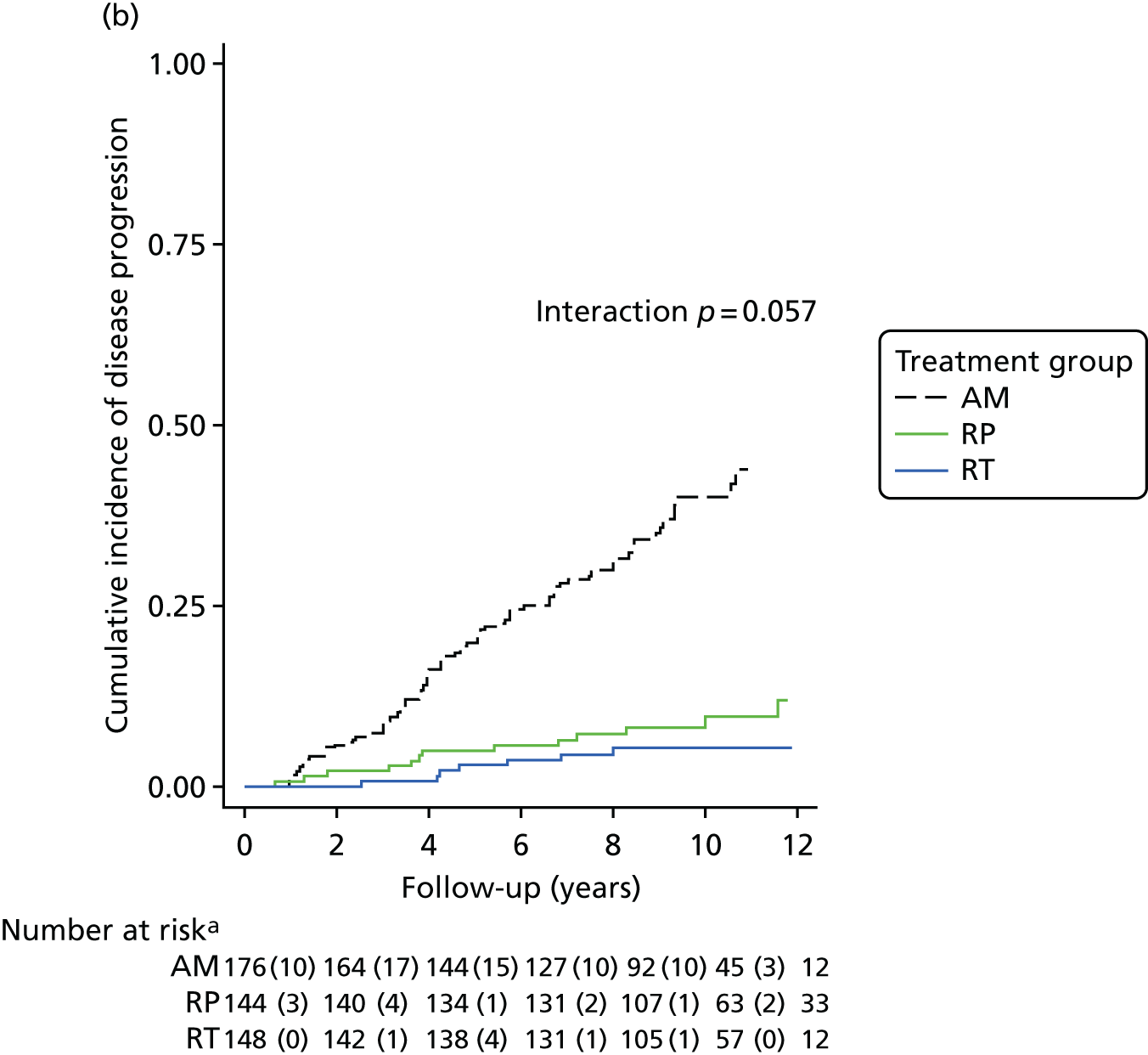
The observation that baseline Gleason score is associated with post-treatment recurrence/progression concurs with previously reported evidence,137–140 but 51% of participants with prostate cancer progression in ProtecT had a Gleason score of ≤ 3 + 3 points at baseline. It is unclear if this represents inadequate sampling or that this disease may progress over time. It is well recognised that low-risk, Gleason score ≤ 3 + 3 prostate cancer does not behave aggressively. However, the data supporting this are largely based on men who receive RP as their primary treatment, and it may mean that men with a Gleason score of ≤ 3 + 3 points are therefore cured by radical intervention even though their disease is very unlikely to progress, and thus they may not have needed radical treatment. It has been suggested that Gleason score ≤ 3 + 3 prostate tumours may no longer need to be called cancerous, but there is evidence suggesting that relatively low-grade prostate cancer foci can progress to lethal disease. 141,142 It is possible that Gleason score ≤ 3 + 3 lesions may comprise a spectrum of phenotypes and genotypes, indicating the need to delineate molecular features associated with prostate cancer progression rather than relying on conventional histological evaluation of biopsy alone.
The protocol for prostate cancer detection in ProtecT was designed in the late 1990s, before the routine use of imaging and refinement of mpMRI. Results of the recent PROMIS study suggest that mpMRI can aid prostate cancer diagnosis,20 albeit with an associated false negative rate. It is unclear whether or not new diagnostic pathways including pre-biopsy imaging and targeted biopsies, as well as existing genomic assays, will improve the prediction of outcomes in newly diagnosed patients. 20,143,144 Molecular-based risk stratification based on diagnostic samples aims to improve on the performance of current risk stratification tools. 145 Incorporation of molecular tumour profiling at baseline may possibly lead to more accurate and personalised risk stratification than is currently achievable using relatively limited clinicopathological features, but these remain to be tested prospectively in well-conducted evaluation studies.
In ProtecT, men with cT2 disease were more likely to progress than those with cT1 tumours, and a proportion of patients (29%) with cT2b tumours were found to have extraprostatic extension when operated on. The observation that an increased number of positive cores in the diagnostic biopsies and an increased maximum tumour length were associated with an increased risk of progression is also consistent with evidence from AS cohorts. 146,147 Baseline PSA levels were higher in men with disease progression in ProtecT than in men who had stable disease, consistent with studies suggesting PSA as a prognostic factor for lethal or recurrent prostate cancer following radical treatment. 73,148–150
Of the men who progressed, 101 had a Gleason score of ≤ 3 + 3 points at baseline, and eight of these participants died of prostate cancer, suggesting that transrectal 10-core prostate biopsy probably undersampled and/or underdetected high-grade prostate cancer in some patients. Among ProtecT participants with a Gleason score of ≤ 3 + 3 points at diagnosis, 87% were in the low-risk category at baseline using the D’Amico risk classification, and 13% had intermediate- or higher-risk disease.
There are limitations to our analysis and interpretation of these results. The ProtecT trial was designed in the late nineties, when the standard diagnostic pathway was a combination of digital rectal examination, serum PSA testing and TRUS-guided biopsies. It is now known that these methods lead to overdetection of indolent prostate cancer and underdetection of significant disease compared with more modern imaging techniques, such as mpMRI and targeted biopsies. The AM protocol in ProtecT has been less intensive than most contemporary AS regimes, although none of the current methods have been validated to demonstrate an improvement in outcomes. Finally, there were very few patients with high-risk disease at baseline (3%), and men from diverse ethnic origins, in particular African Caribbean men, were under-represented.
The main strengths of ProtecT are threefold: (1) its size, with over 82,000 men tested in the community, (2) the standardised diagnostic approach to detect clinically localised disease using a minimum of 10-core biopsies and (3) the high randomisation rate of men enrolled to evaluate the treatment effectiveness of RP, RT and AM. Although a reduction in the rate of metastasis and disease progression in men receiving radical treatment was demonstrated compared with AM, there was no evidence of a difference in mortality, and longer-term follow-up is needed to investigate whether or not this will change at the median 15-year follow-up.
Although the majority of ProtecT participants had a Gleason score of ≤ 3 + 3 points at diagnosis, when PSA levels and tumour volume were also taken into consideration it has become apparent that the actual prevalence of low-risk prostate cancer in the ProtecT randomised cohort was 66%, with 34% of participants having at least intermediate-risk disease.
None of the 19 ProtecT participants with a pathological Gleason score of ≤ 3 + 3 points following RP showed disease progression, suggesting that surgery cures definite low-risk Gleason score ≤ 3 + 3 disease using current criteria. These patients, if accurately identified, may benefit from AM by avoiding treatment side effects without deleterious oncological outcomes, as currently advocated in AS protocols. 151,152 The clinical dilemma is that we do not know whether or not men receiving AM or RT who subsequently developed disease progression actually had a true Gleason score of ≤ 3 + 3 points at baseline. It is recognised that around half of men with PSA-detected prostate cancer may be undergraded or overgraded by TRUS-guided needle biopsy. Although recent data evaluating pre-biopsy mpMRI suggests that this appears to improve the detection of high-grade disease compared with the diagnostic pathway used in ProtecT,20 it remains to be shown whether or not mpMRI is sufficiently reliable to avoid the overdetection of pathological Gleason score ≤ 3 + 3 prostate cancer.
The absence of any association between the number of tumour foci in the RP specimen and disease progression contrasts with published data suggesting that multifocal prostate cancer is associated with an increased risk of recurrence, although previous evidence from ProtecT suggests that solitary tumours were found in only one-fifth of RP specimens with PSA-detected localised prostate cancer.
In conclusion, baseline clinicopathological features of men with localised prostate cancer in ProtecT differed in men who developed disease progression compared with those with stable disease, but associations were not strong enough to reliably predict progression in individuals. As our understanding of the biology of prostate cancer behaviour improves, and its genomic diversity is elucidated, it is becoming clear that current methods of stratification need refinement with pre-biopsy imaging and targeted sampling, as well as through utilisation of validated genomic and other emerging biomarkers. This will need to be assessed in new, large-scale, prospective early-detection programmes. Only then will clinicians and patients be able to improve and refine the complex decision-making processes needed for the management of this ubiquitous malignancy.
General conclusions from clinical outcomes
The ProtecT trial has demonstrated no differences in survival at an average of 10 years from randomisation in clinically localised prostate cancer, irrespective of treatment allocation, in an ITT analysis. Baseline characteristics were insufficient to stratify patients and to predict outcomes of their disease. Radical treatments provided an oncological benefit compared with AM by reducing the incidence of metastases and disease progression by approximately 50%. At a median of 3 years’ follow-up, approximately 25% of men had moved out of AM to receive radical treatments, and, by 10 years, 55% of AM participants had received a radical intervention. Further analysis is under way to investigate the reasons for that change in management, and will be published and reported in due course. Over 80% of men receiving AM have no evidence of prostate cancer progression and thus have avoided side effects of radical treatments for prostate cancer.
Main ProtecT trial patient-reported outcome results
This section of the report focuses on the comprehensive evaluation of the effectiveness and acceptability of treatments through the prospective assessment by men of the impacts of treatments on urinary, sexual and bowel function, and specific and general aspects of QoL in regularly completed PROMs. The main outcome findings after 6 full years of follow-up were published in 2016, with considerable details in the online supplement. 30 Additional analyses have also been subsequently undertaken and are reported in Methods: patient-reported outcome measures and statistical approaches and Quality of life associated with the experience of urinary incontinence after treatment for prostate cancer.
Main patient-reported outcome measure analysis after 6 years of follow-up
Systematic reviews153–156 and large prospective cohorts7 have identified particular impacts on urinary, bowel and sexual function and little impact on generic QoL following radical treatments, but clear comparisons between contemporary treatments have been hindered by differences in outcome definitions, limited use of validated PROMs, short-term follow-up and sparse data on RT or AM/AS programmes. 157 RCT data have also been limited. The SPCG-4 trial showed greater impact on sexual and urinary function and QoL following RP than the impact following watchful waiting using the trial’s study questionnaire, and limited impact of RP on anxiety and depression. 158 PIVOT,79 comparing RP with watchful waiting for men with PSA-detected prostate cancer but with only three symptom items, reported similar results. These RCTs did not collect the full range of validated PROMs.
Methods: patient-reported outcome measures and statistical approaches
The Consolidated Standards of Reporting of Trials (CONSORT) guidelines for PROMs was used. 159 PROMs were prespecified secondary outcomes in the statistical analysis plan (see www.journalslibrary.nihr.ac.uk/programmes/hta/962099/#/), collected by validated questionnaires in four key domains:
-
urinary function and QoL impact – including urinary incontinence and LUTS (ICIQ,88 ICSmaleSF89 and EPIC160)
-
sexual function and QoL impact – including erectile function (EPIC160)
-
bowel function and QoL impact – including loose/bloody stools and incontinence (EPIC160)
-
health-related QoL – comprising generic health status (SF-1293), anxiety/depression (HADS92) and cancer-related QoL (EORTC-QLQ Q3091).
As indicated previously, study questionnaires were completed at baseline (at biopsy, before knowledge of diagnosis), at 6 and 12 months after randomisation and annually thereafter. The ICSmaleSF,89 SF-1293 and HADS92 were always included in study questionnaires, the ICIQ88 was added from 2001 and EPIC160 was added from 2005. As it concerned cancer-related QoL, EORTC-QLQ C3091 was included at year 5 only. PROMs from baseline to the complete 6 years’ follow-up were scored and analysed as recommended, with key items identified to aid interpretation of clinical relevance. Analyses were by ITT, with summary statistics and 95% CIs presented by randomised group. Multilevel models were employed to accommodate correlations between repeated measurements and test for treatment differences in follow-up assessments, and included covariates for the variables stratified by or minimised in the random allocation: age and PSA level at baseline (continuous variables), and Gleason score and study centre (dummy variables). Baseline PROMs were not included as a covariate as EPIC and ICIQ scores were not available for all men at baseline. PROMs data indicated that the allocated groups were comparable at baseline (see Chapter 3, Baseline level of patient-reported outcomes of randomised participants).
Results
Follow-up response rates were > 80% for all PROMs, without decline over time. Outcomes in the four domains are presented in the following sections and in Table 1, with selected scores and items presented graphically in Figures 13–16 (with time since randomisation on the x-axis, full measurement scale on the y-axis and scores representing ‘better’ function/health towards the top). All outcomes are reported in full in the online supplement of Donovan et al. 30
Patient-reported outcome measure domains, scores and items
Domain A: urinary function and impact on quality of life (see Figure 13)
-
Scores: ICIQ88 score (see Figure 13a), EPIC160 incontinence subscore (supplement) and ICSmaleSF questionnaire incontinence score. 89
-
Items: EPIC pad use (see Figure 13b).
-
QoL impact item: ICIQ impact of incontinence on QoL (see Figure 13c).
-
Scores: EPIC urinary summary (see Figure 13d), EPIC bother (supplement), EPIC obstruction/irritation (supplement) and ICSmaleSF voiding score (see Figure 13e).
-
Items: ICSmaleSF nocturia (see Figure 13g) and daytime frequency (supplement).
-
QoL impact item: ICSmaleSF item on the impact of urinary symptoms on QoL (see Figure 13f).
Domain B: sexual function and impact on quality of life (see Figure 14)
-
Items: EPIC item on erections firm enough for intercourse (see Figure 14a).
-
QoL impact: EPIC item on problem with erectile dysfunction (see Figure 14b).
-
Scores: EPIC sexual summary (supplement), sexual function (see Figure 14c) and sexual bother (see Figure 14d).
-
QoL impact: EPIC item on impact of sexual function on QoL (see Figure 14e).
Domain C: bowel function and impact on quality of life (see Figure 15)
-
Scores: EPIC bowel summary (supplement), bowel function (see Figure 15a) and bowel bother (see Figure 15b).
-
Items: EPIC items on loose stools (see Figure 15c), faecal incontinence (see Figure 15d) and bloody stools (see Figure 15e).
-
QoL impact: EPIC item on impact of bowel habits on QoL (see Figure 15f).
Domain D: health-related quality of life (see Figure 16)
-
Generic health status: SF-1293 physical health (see Figure 16a) and mental health (see Figure 16b).
-
HADS92 mean anxiety score, mean depression score, percentage of potentially significant clinical cases of anxiety (see Figure 16c) and depression (see Figure 16d).
-
Cancer-related QoL: EORTC-QLQ C30. 91
Domain A: urinary function and quality-of-life impact
All measures of urinary incontinence showed that there was greater impact in the RP group at 6 months, with some recovery, but urinary incontinence remained worse in the RP group than in the RT and AM groups at every time point (Figure 13a–c; all p < 0.001). Urinary incontinence rates were similar and little affected in the RT and AM groups, with a little worsening discernible in the AM group over time as surgery was taken up. Pad use increased from 1% at baseline to 47% in the RP group, compared with 4% in the AM group and 5% in the RT group at 6 months. By year 6, 18% of men in the RP group used pads, compared with 10% in the AM group and 3% in the RT group (see Figure 13b). There was a greater impact on urinary incontinence-related QoL in the RP group for 2 years, but this improved to become similar to that in the AM and RT groups (see Figure 13c). A similar pattern was shown for LUTS including urinary incontinence (see Figure 13d and f). Voiding LUTS were a little worse in the RT group at 6 months but then returned to close to baseline levels and similar to those in the RP and AM groups (see Figure 13e). Urinary frequency remained similar across the groups, with nocturia increasing in all groups at 6 months, particularly in the RT group, but recovering and returning closest to baseline in the RP group (see Figure 13g).
FIGURE 13.
Impact on urinary function (including urinary incontinence) and QoL. (a) ICIQ incontinence score; (b) EPIC item (one or more pads per day); (c) ICIQ incontinence problem; (d) EPIC urinary score; (e) ICSmaleSF Voiding Scale; (f) ICSmaleSF QoL item; and (g) ICSmaleSF nocturia item.
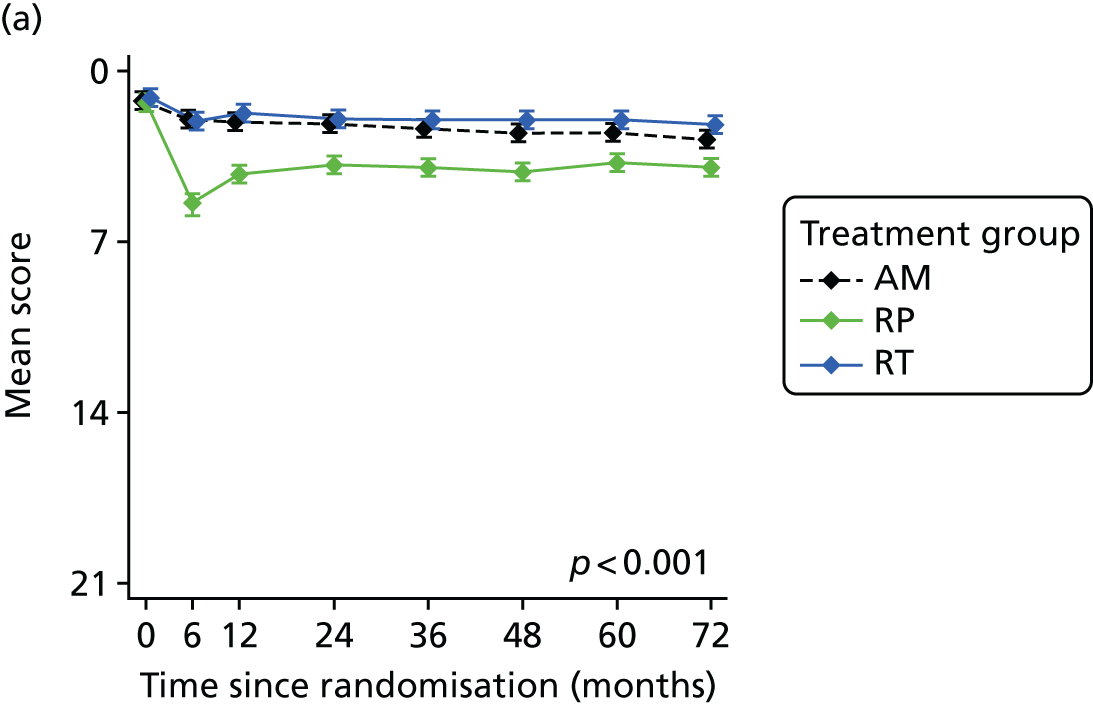
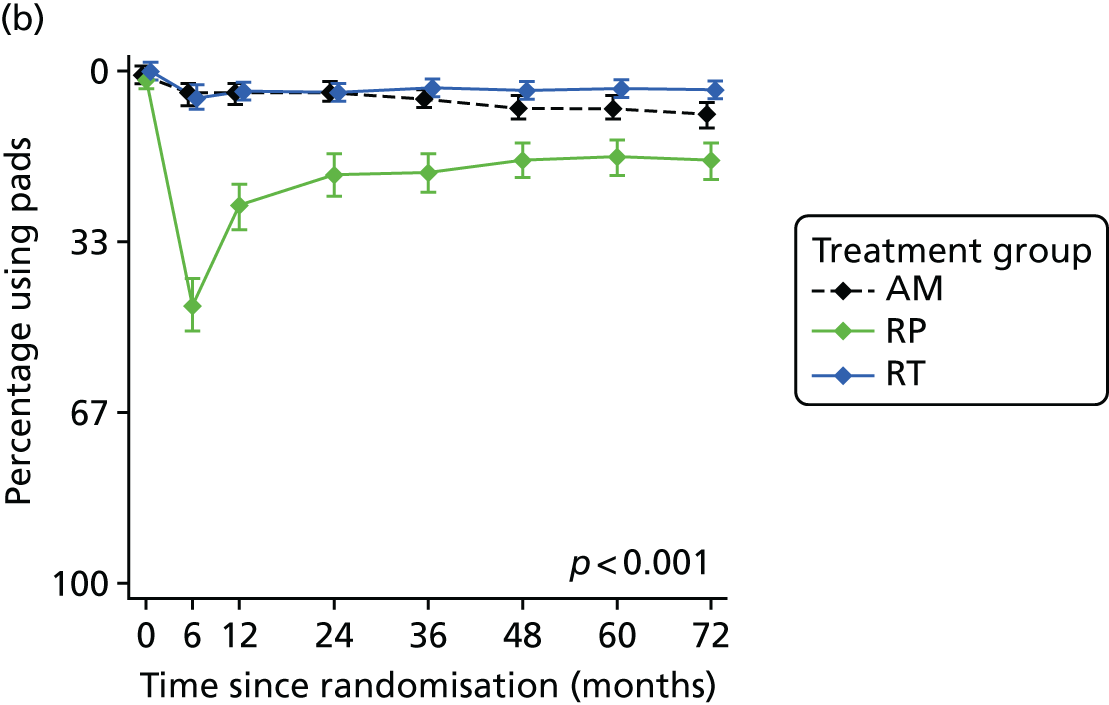
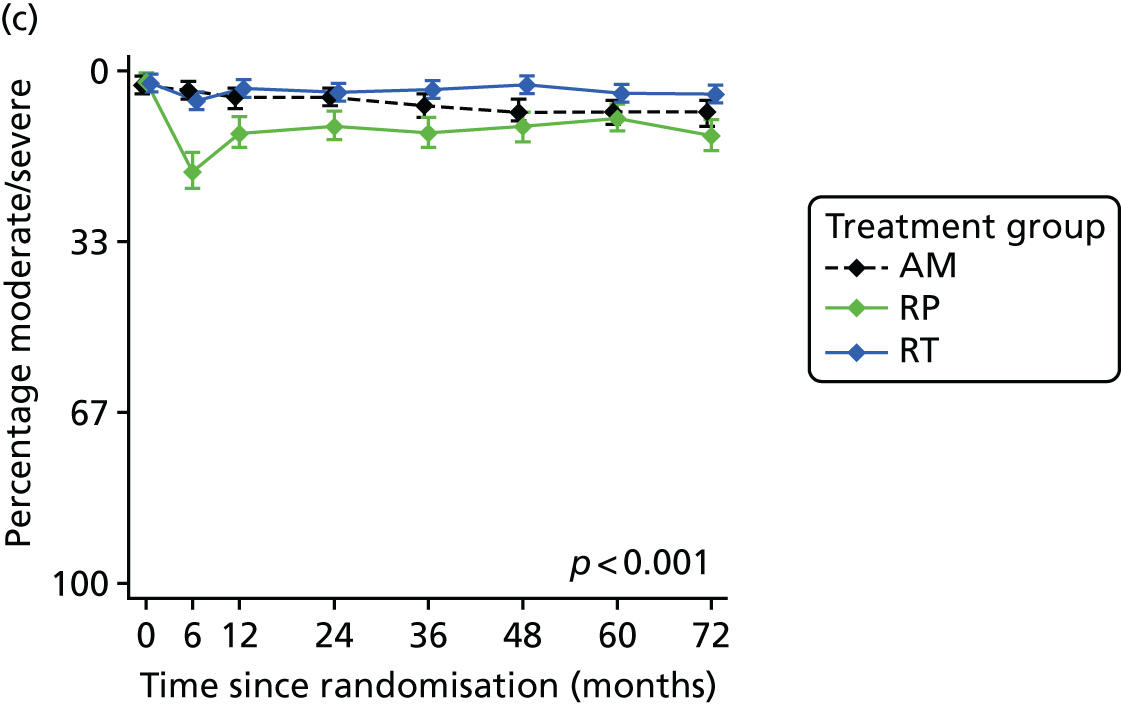
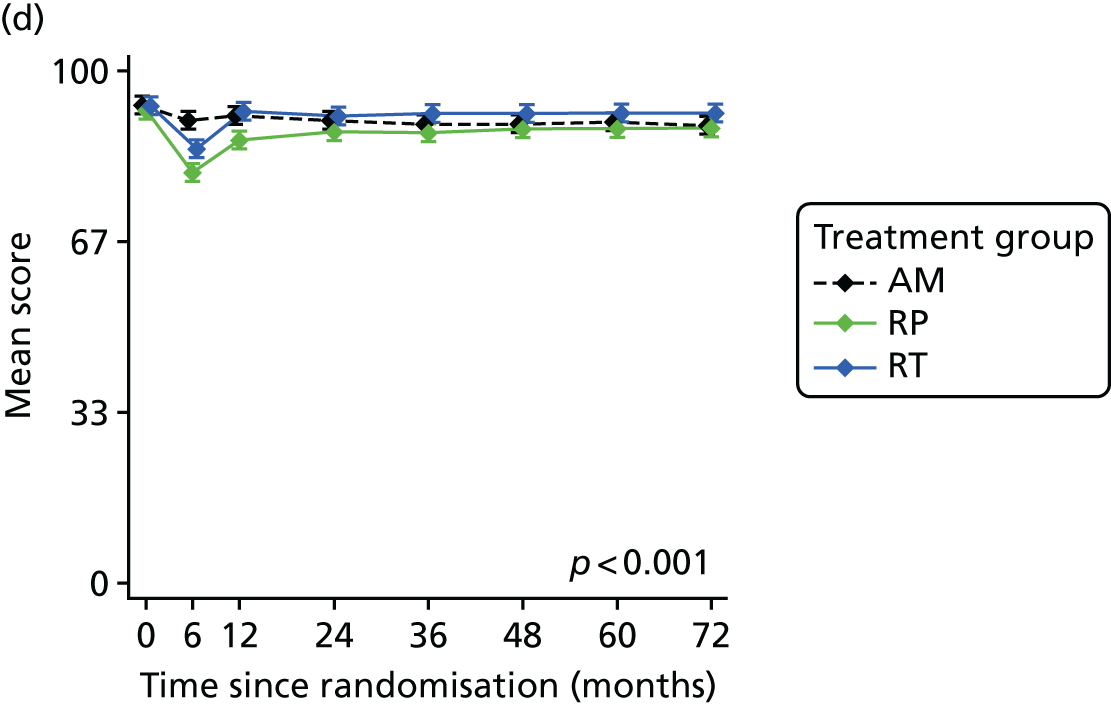
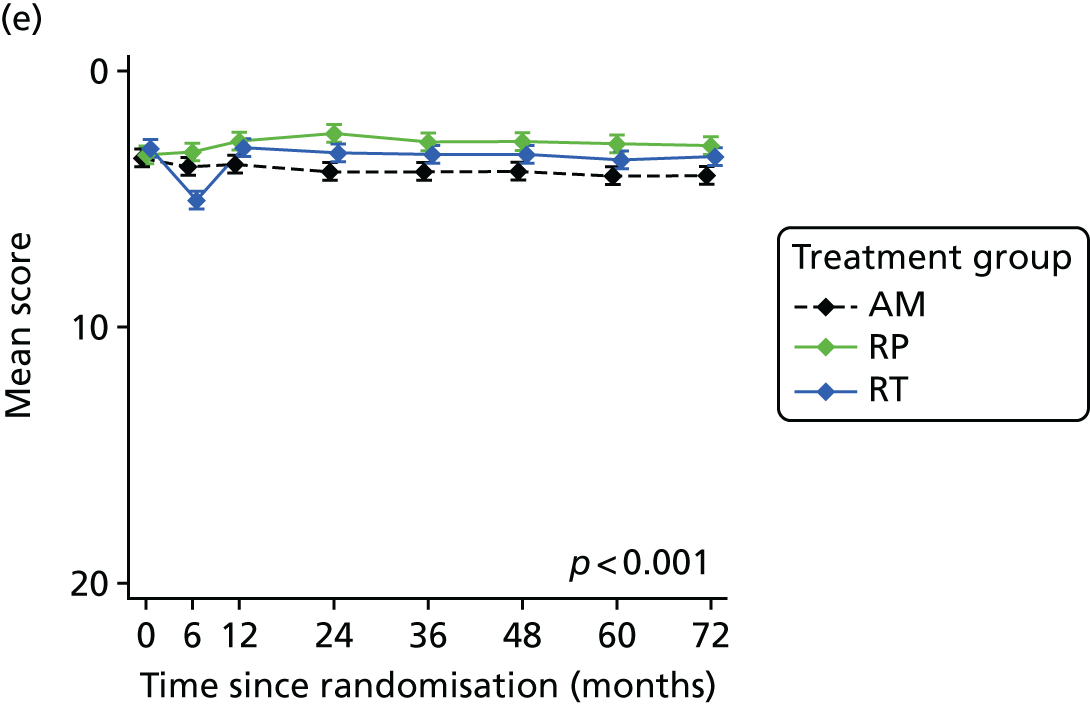
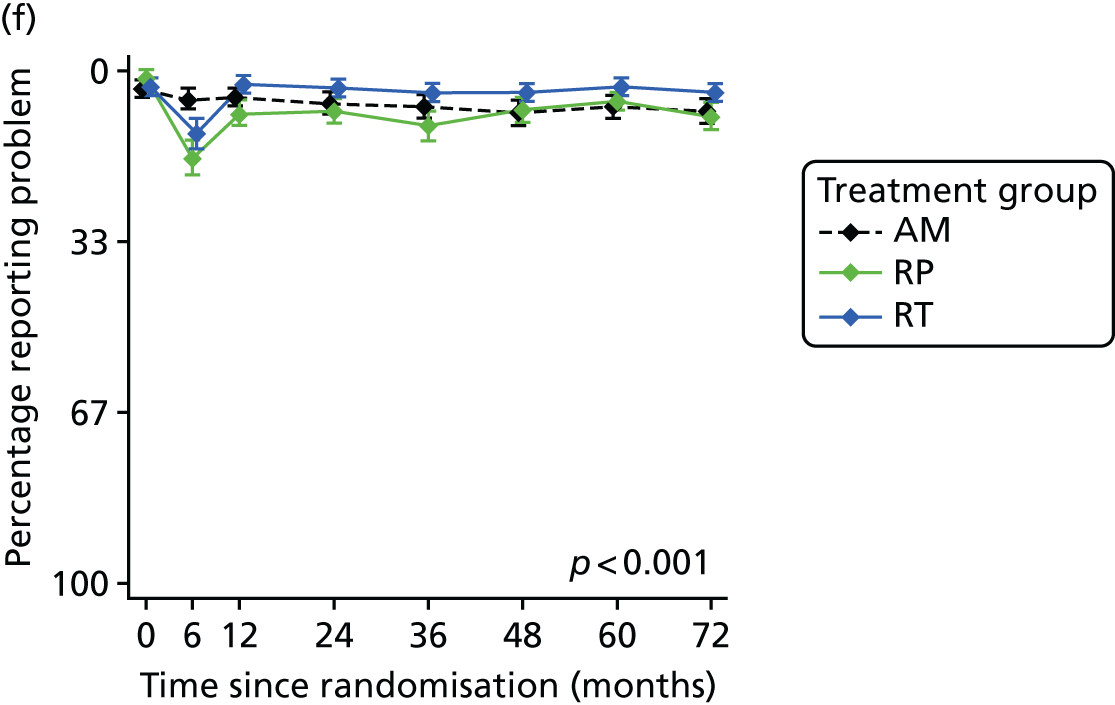
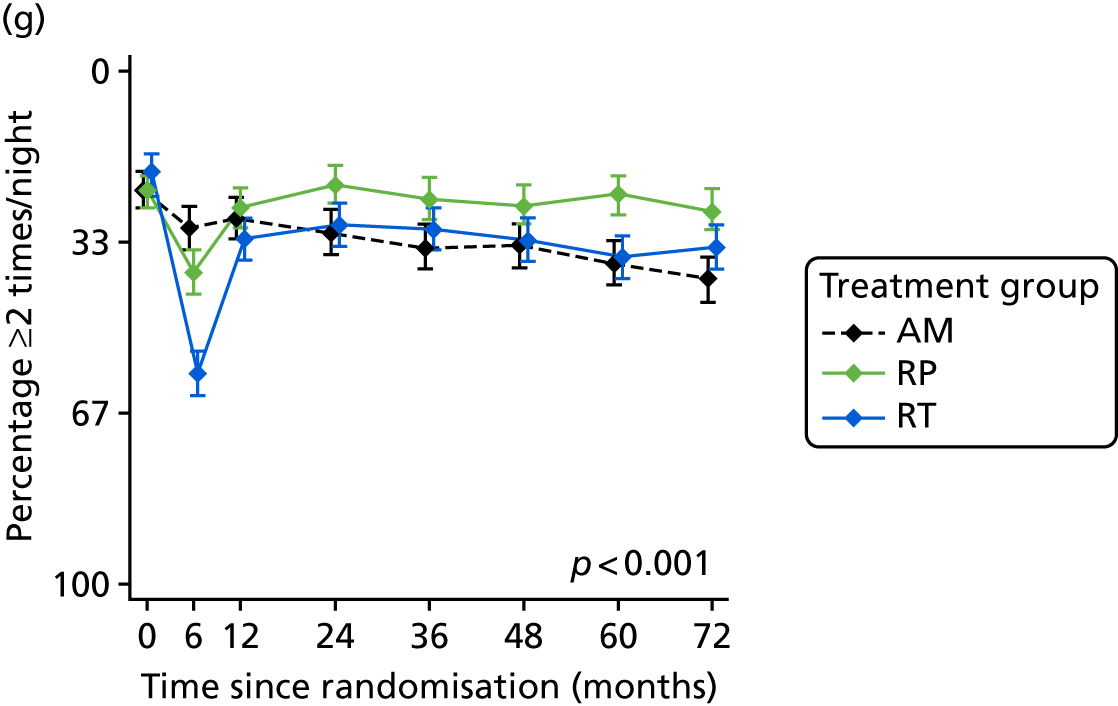
Domain B: sexual function and quality-of-life impact (including erectile function)
Erectile function reduced for all men at 6 months, with clear differences between the groups (Figure 14a; p < 0.001). At baseline, 67% of all men reported erections firm enough for intercourse, but by 6 months this reduced to 50% in the AM group, 24% in the RT group and 11% in the RP group. Erectile function remained worse in the RP group at all time points, with some recovery over 2 years but then further decline to 15% at 6 years, compared with recovery followed by decline to 29% in the RT group and a gradual year-on-year decline in the AM group (40% at year 3 to 28% by year 6). Very similar patterns of impact across the groups and over time were seen in the other measures of sexual function, bother and QoL impact of erectile dysfunction and sexual function (see Figure 14b–e).
FIGURE 14.
Impact on sexual function (including erectile function) and QoL. (a) EPIC item (erection firmness); (b) EPIC problem with erectile dysfunction; (c) EPIC sexual function score; (d) EPIC sexual bother score; and (e) EPIC sexual QoL.
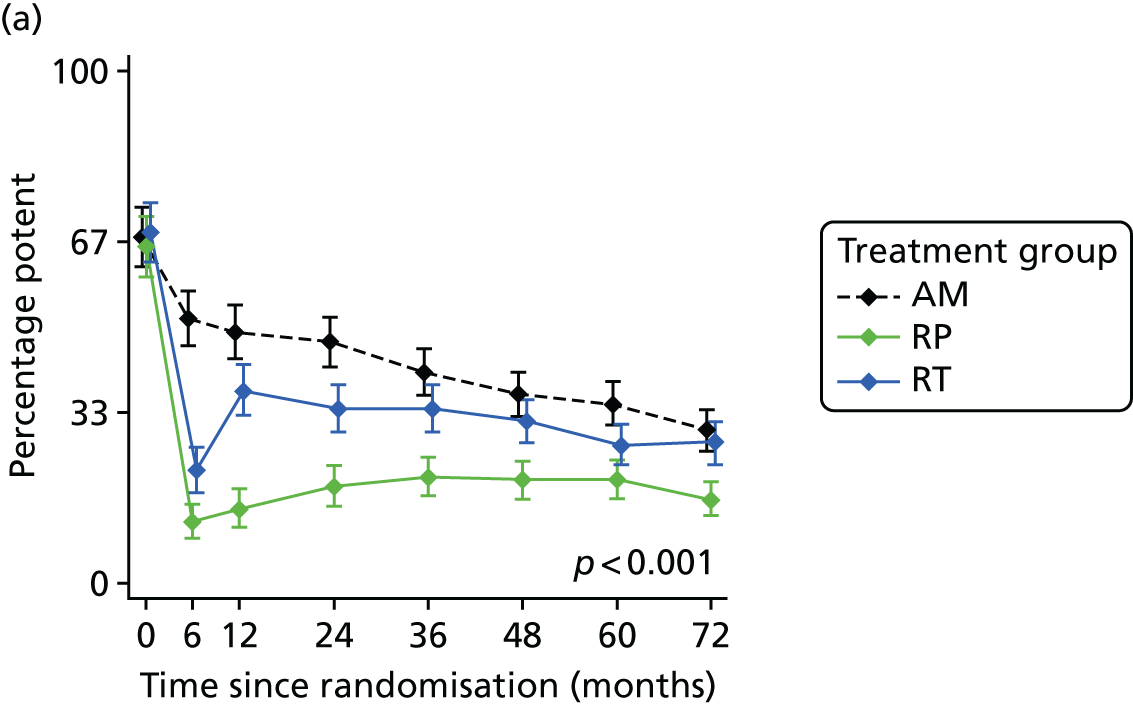
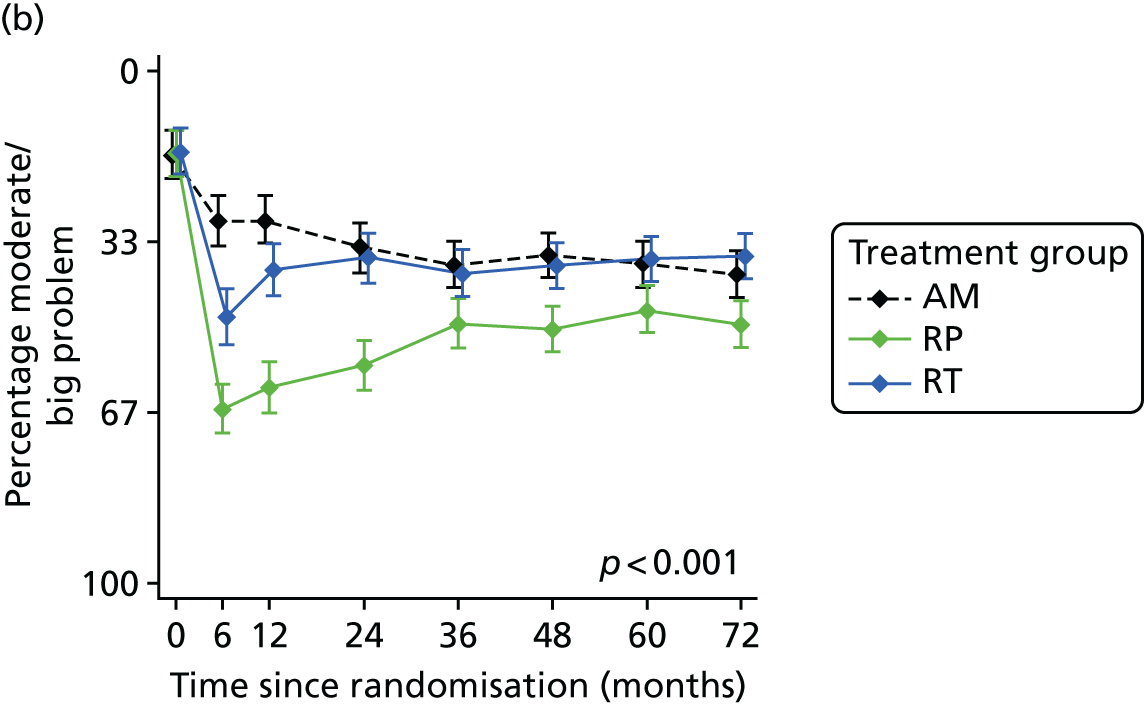
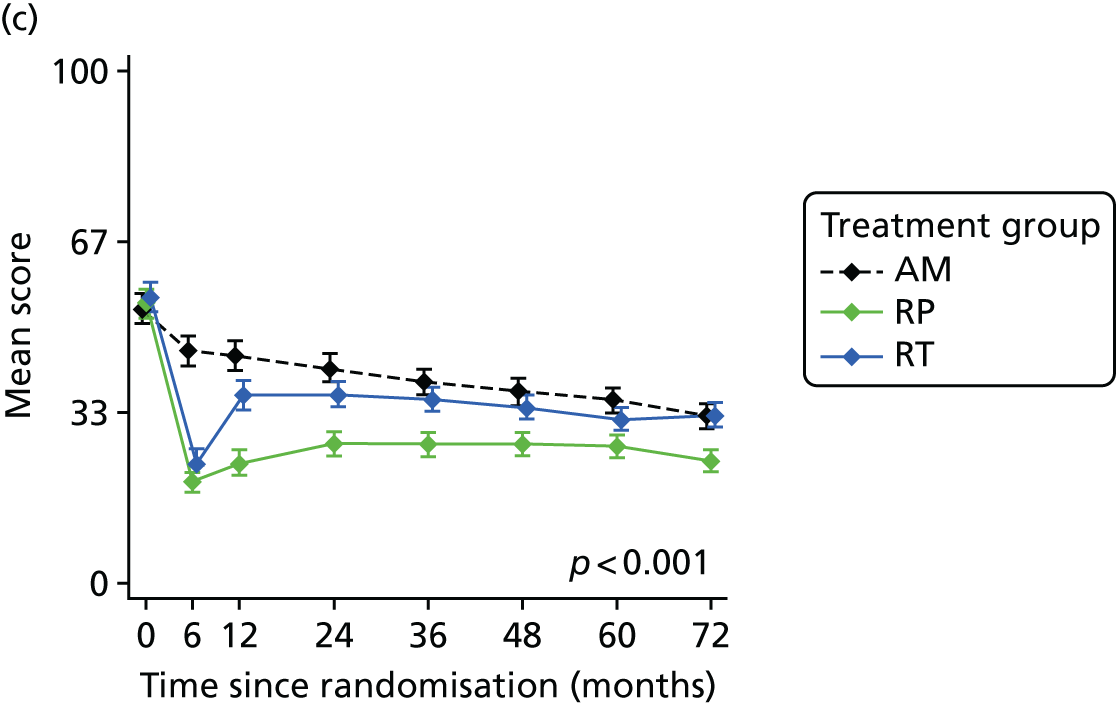
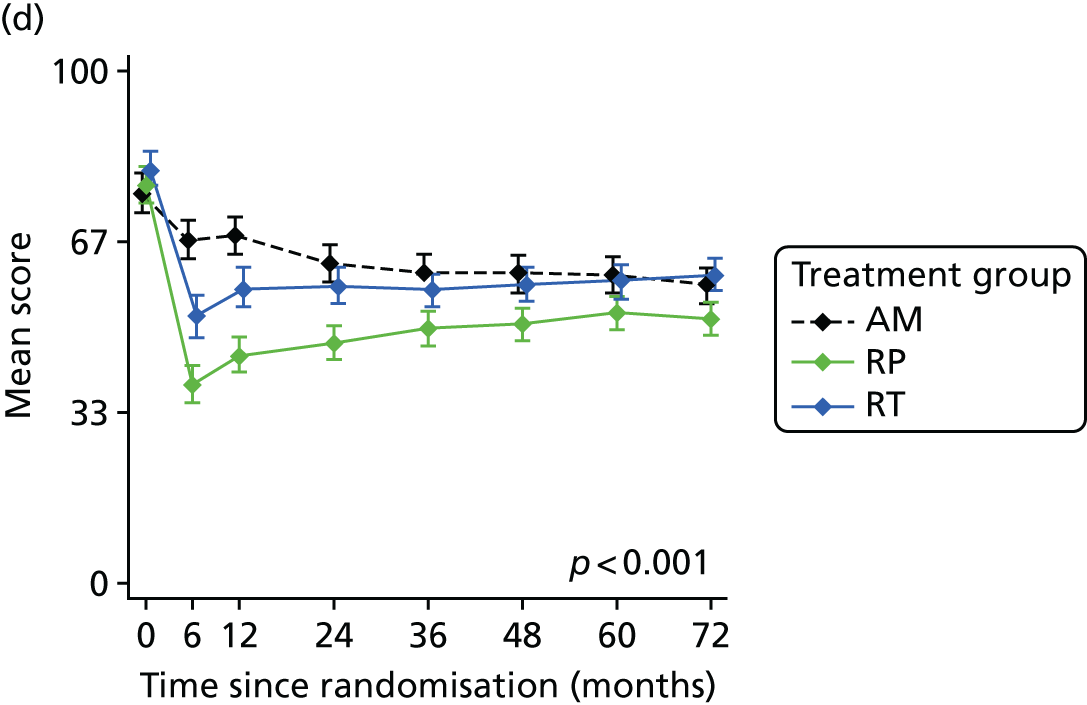
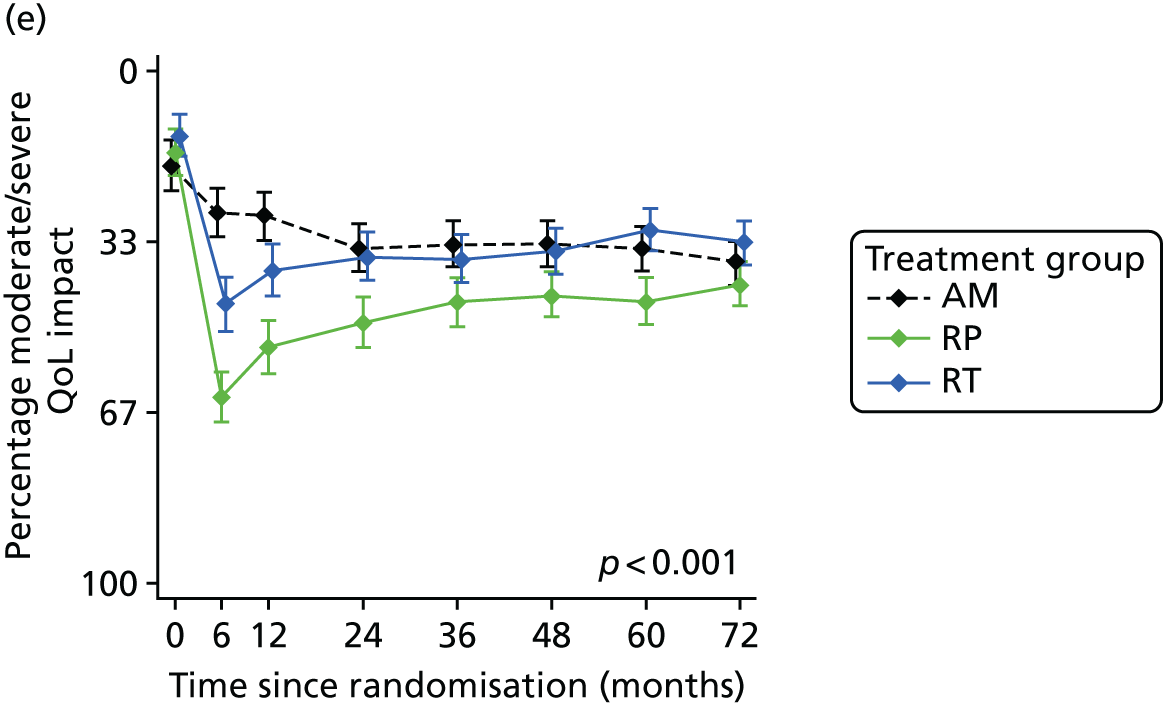
Domain C: bowel function and quality-of-life impact
Bowel function and bother, and the impact of bowel habits on QoL were unchanged in the RP and AM groups, but worse in the RT group, particularly at 6 months (Figure 15a, b and f). Faecal incontinence and loose stools were similar across the groups (see Figure 15c and d), but bloody stools were experienced more in the RT group from year 2 onwards (see Figure 15e; p < 0.001). Bowel bother and QoL impact scores were a little worse in the RT group than in the other groups.
FIGURE 15.
Impact on bowel function and QoL. (a) EPIC bowel function score; (b) EPIC bowel bother score; (c) EPIC item (loose stools); (d) EPIC item (faecal incontinence); (e) EPIC item (bloody stools); and (f) EPIC item (bowel habits).
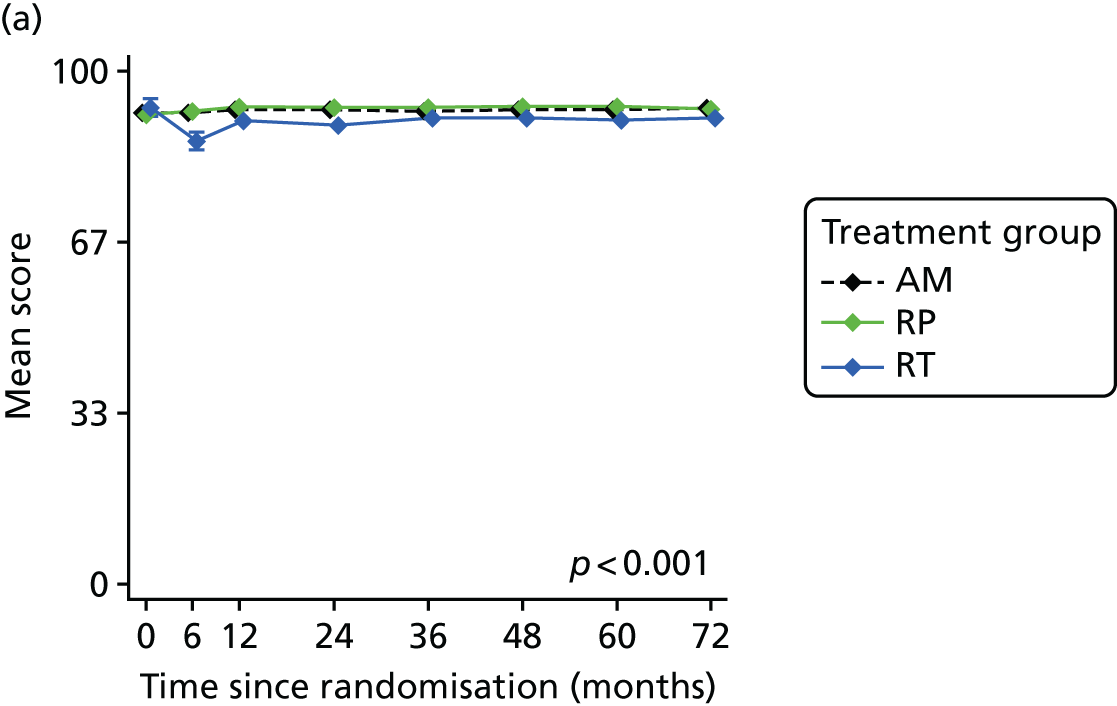
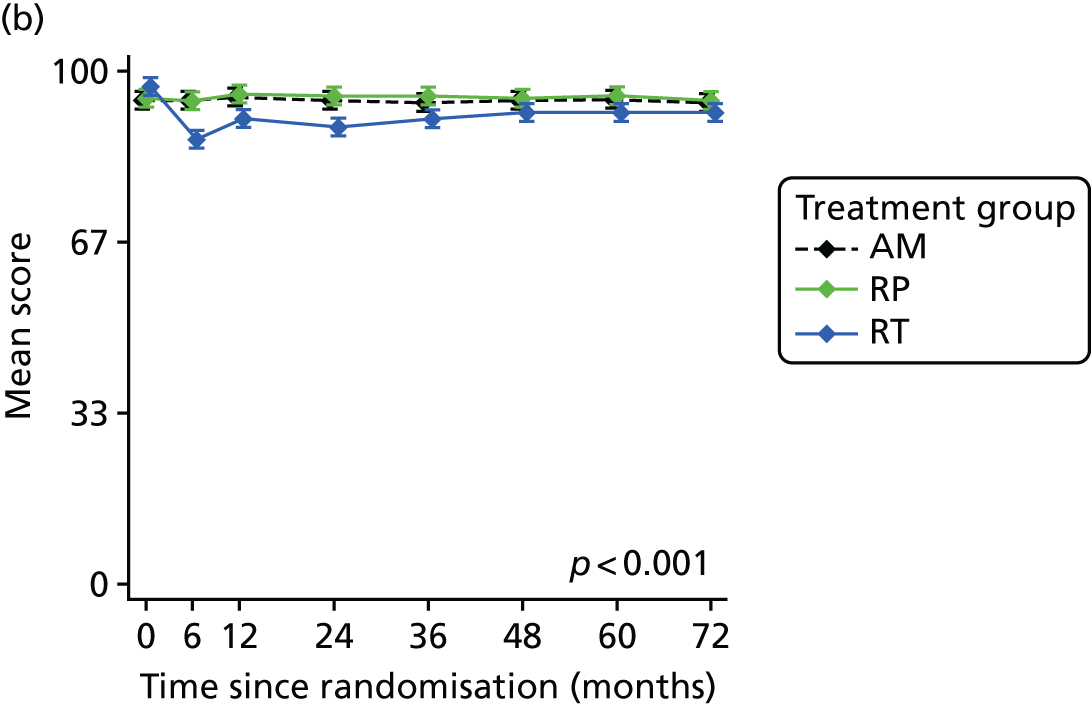
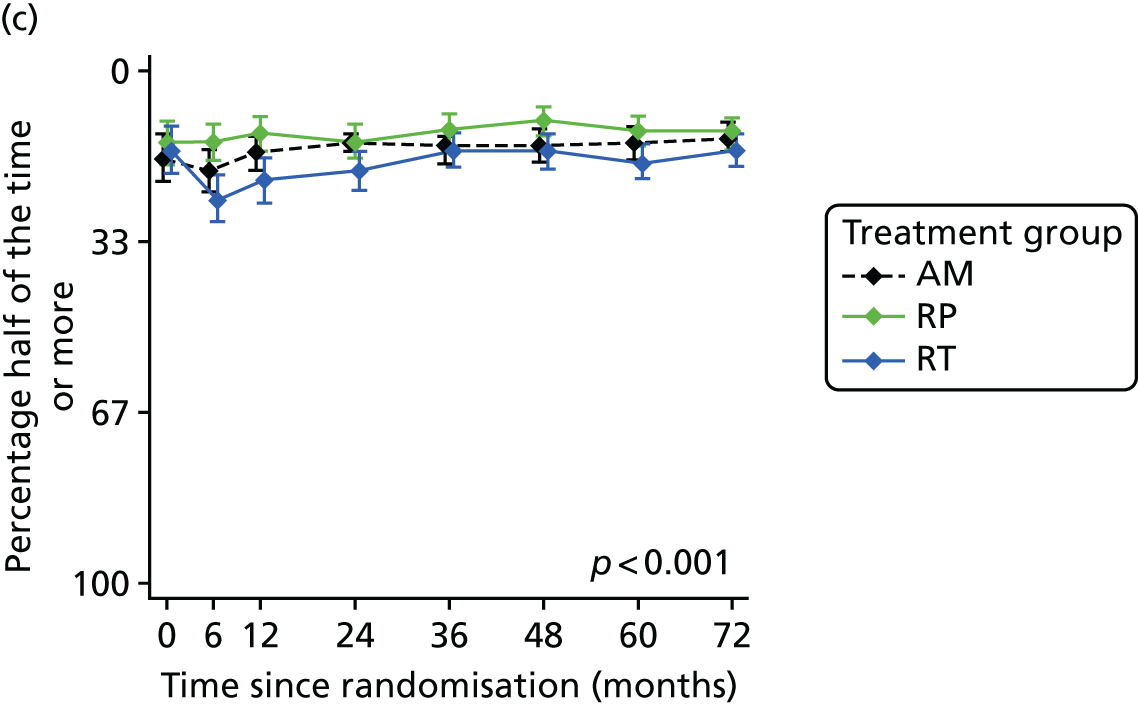
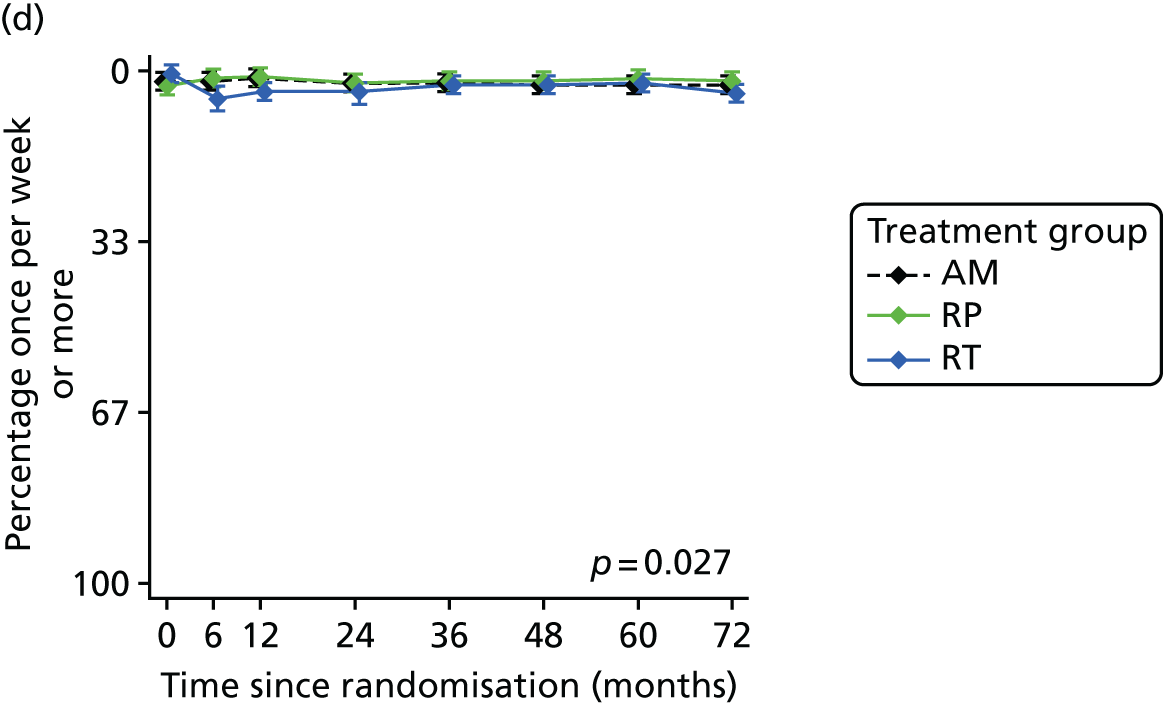
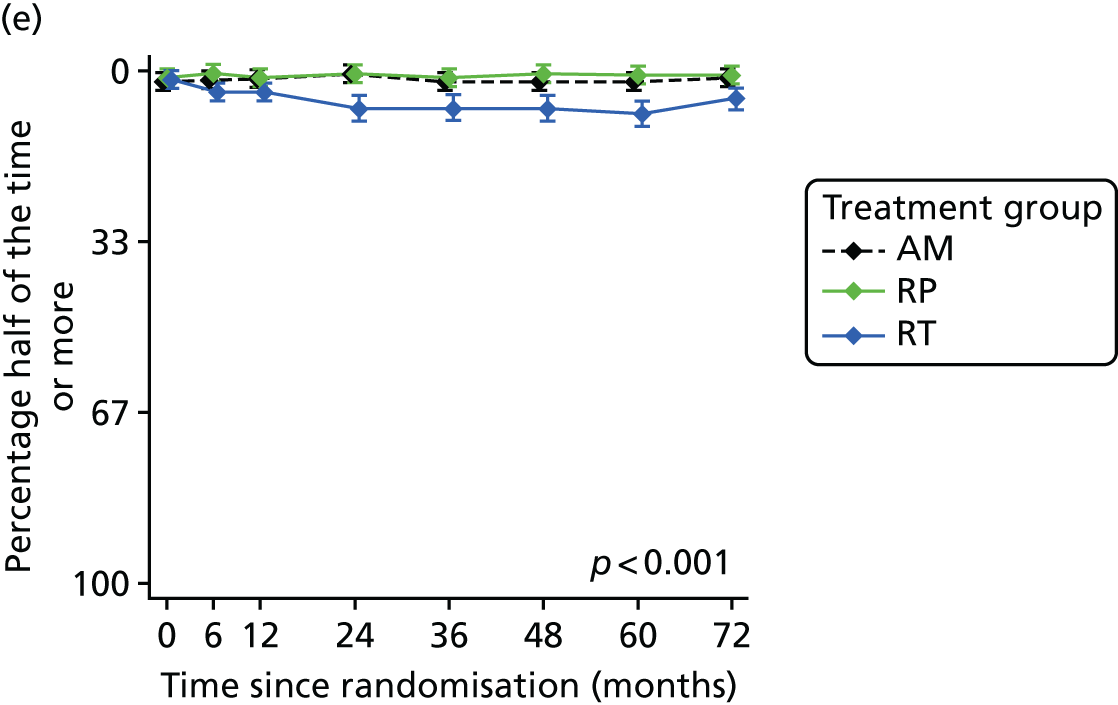
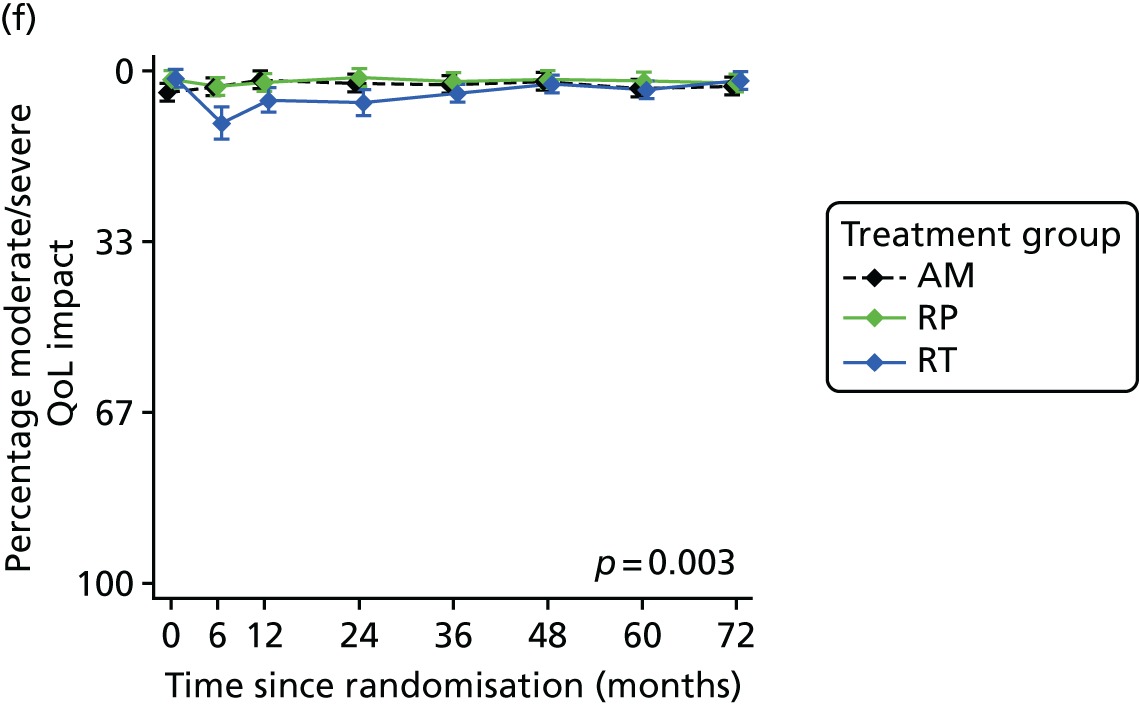
Domain D: health-related quality of life
There was no evidence of differences between the groups according to physical and mental health subscores in the generic health measure SF-12, anxiety or depression measured by the HADS or any of the symptom or function scales of the EORTC-QLQ C30 at year 5 (Figure 16a–d).
FIGURE 16.
Impact on health-related QoL. (a) SF-12 physical health score; (b) SF-12 mental health score; (c) HADS anxiety; and (d) HADS depression.
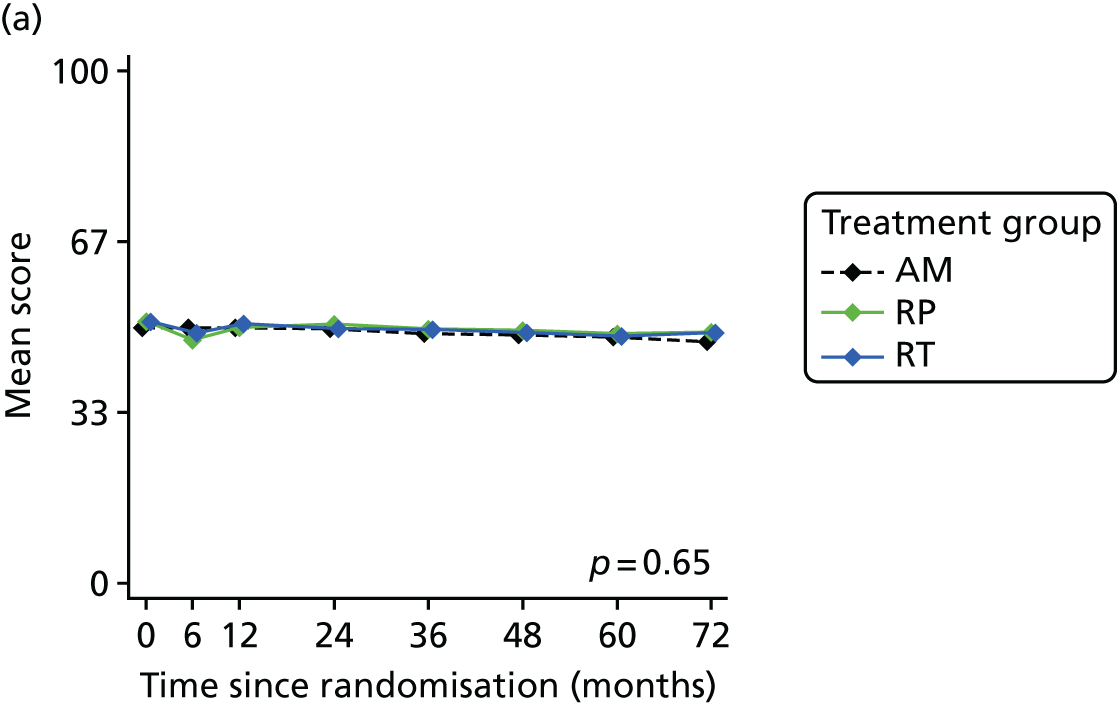
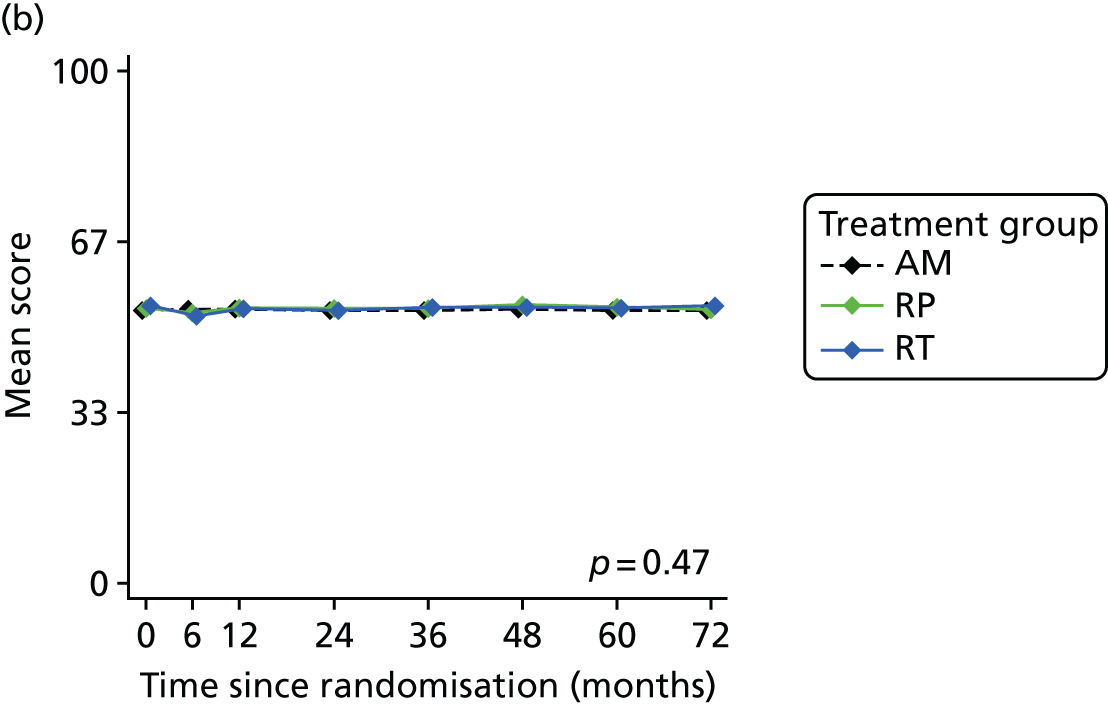
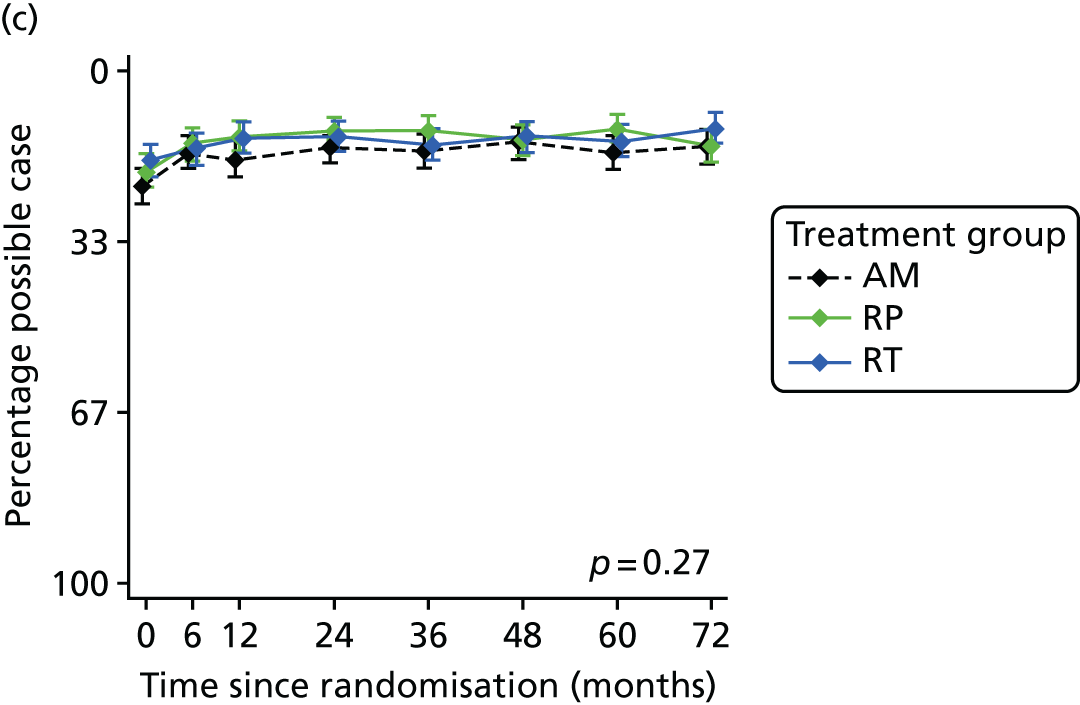
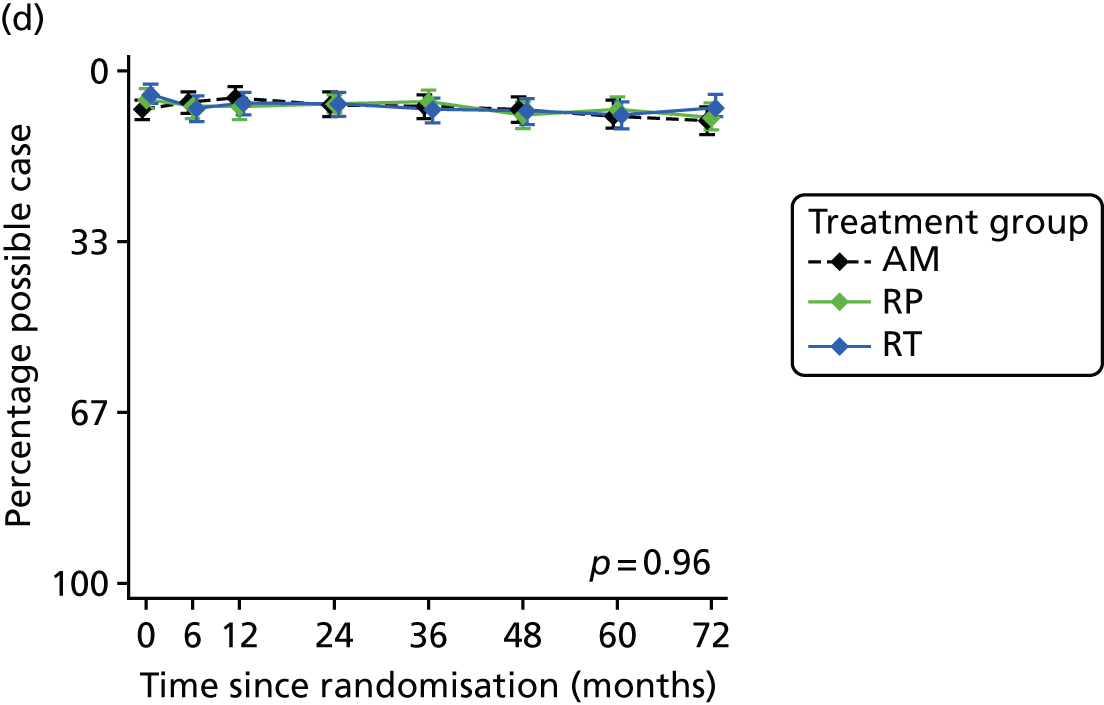
Conclusions
As described elsewhere in this report, the ProtecT RCT showed similar very high survival rates following all three allocated treatments but higher rates of metastases and disease progression in the AM group than in the radical treatment groups (metastases per 100 person-years: AM 0.63, RP 0.24, RT 0.30; p = 0.004; and disease progression per 100 person-years: AM 2.29, RP 0.89, RT 0.90; p < 0.001). In this context, understanding the profiles and levels of impact of the treatments on men’s lives becomes even more crucial for decision-making. ProtecT PROMs matched key domains recommended by international groups,155,161 and followed reporting guidelines,159 to provide definitive and unbiased comparisons of the impacts following standardised protocols for RP, RT and AM management strategies in the PSA era.
These ProtecT study findings definitively clarified the distinct impacts of prostate cancer treatments on urinary, sexual and bowel function and condition-specific QoL. RP’s impact on urinary incontinence and sexual function, particularly erectile function, was most severe at 6 months and although there was some recovery, it was worse than in the other groups over 6 years; there was no change in bowel function and some improvement in voiding LUTS. At 6 months, the impact of RT with neoadjuvant hormones on sexual function, particularly erectile function, was nearly as severe as RP and there was worse bowel function and LUTS than with either RP or AM, but there was gradual recovery, and, after 2 years, RT’s sexual and urinary impacts, including erectile function and urinary incontinence, were much less than for RP, and bowel function (apart from worsening bloody stools) was similar to AM and RP. In the AM group, impacts accumulated over time with ageing and the addition of radical treatments, with sexual and urinary function (including erectile function and urinary incontinence) gradually worsening year on year; bowel function was unchanged.
In terms of numbers needed to treat, treating four men with RP or eight men with RT rather than AM would cause one additional case of erectile dysfunction at 2 years; treating five men with RP or 143 men with RT rather than AM would cause one additional case of urinary incontinence at 2 years. By the end of follow-up at 6 years, urinary and sexual function had stabilised in the RT group after recovering for 2 or 3 years, and with the steady decline evident in the AM group, outcomes became similar for AM and RT, but remained worse in the RP group. These profiles of functional impact were mirrored in the sexual, urinary and bowel QoL items, with some evidence of accommodation to changes over time. No effects were seen on generic health status (mental or physical) or anxiety or depression in any group at any time, or in cancer-related QoL at 5 years.
The paucity of published data and the lack of consistency and variability in timing of measurements severely constrained the capacity to compare ProtecT findings directly with other RCTs or major studies of treatments at the time of publication. Direct comparisons were only possible for two specific (EPIC) items: erectile function and pad use. These showed that ProtecT findings were similar to the SPCG-4 and PIVOT studies in relation to erectile function in the RP and AM/watchful waiting groups. 79,158,162 Slightly worse results in observational cohorts7,11,163,164 would probably be related to age or selection biases. ProtecT results for pad use were considerably better than those from SPCG-4 for RP and watchful waiting, and broadly comparable to PIVOT’s watchful waiting; RT outcomes were similar at all time points in the observational cohorts. 30 Broadly comparable results were also found for bowel function and LUTS following RT155,163 and voiding after RP. EPIC scores were similar in other studies. 164,165 Further studies also found no differences in generic health or psychological/QoL assessments. 154,158,166
In terms of limitations, aspects of the ProtecT treatment policies may have affected some PROM scores. For example, the assessment at 6 months post randomisation will have captured the impact of neoadjuvant androgen suppression on sexual function at 6 months among those in the RT group. It was anticipated that the different treatments in ProtecT would lead to variation in the acceptance of allocation, and so the immediate receipt of RP by 68% and RT by 73% in those allocated groups may have attenuated some PROM comparisons. The take-up rate of AM was higher (89%), but because an integral part of the policy was the opportunity for reassessment and change of management, triggers including PSA level rise or evidence of progression led to increasing numbers of men receiving RP or RT over time. The rate of change was similar to many other AS programmes, but impacts from radical treatments combined with increased disease progression may have contributed to the gradual worsening of PROMs over time in the AM group. The AM findings also indicate a gradual decline in sexual and urinary function over time that would have occurred to some degree in the other groups.
The interventions in ProtecT remain the three most common contemporary types of treatments, but there have been considerable developments in treatment techniques since the study began, including the introduction of robot-assisted and laparoscopic surgery and brachytherapy or IMRT, and protocols for AS that exclude many of those included in ProtecT and using different strategies for monitoring and triggering change of management. Two large observational cohort studies were established in 2011–13 in the USA to evaluate short-term oncological outcomes and PROMs, explicitly expecting to show better results for PROMs with these newer treatment technologies. 55,56 The findings from these cohorts were remarkably consistent with those from ProtecT in terms of overall rates of symptoms and adverse effects. These findings, alongside randomised evidence from ProtecT, confirmed that all options carry risks of adverse effects that affect QoL, with each treatment option having a particular pattern in the short term: urinary incontinence and sexual dysfunction worst after surgery, followed by some recovery but persistent difficulties for some men; bowel problems worst after radiotherapy; and sexual dysfunction mostly related to neoadjuvant ADT. Even with AS, although adverse effects of interventions can be initially avoided, there is a natural decline in urinary and sexual function symptoms over time, and the adverse effects of radical treatments will be experienced when those treatments are received. 41
In the ProtecT RP group, 324 men received open retropubic procedures, with 23 receiving laparoscopic and 25 receiving robotic-assisted laparoscopic prostatectomy (19 were not specified), and most were nerve sparing (205 bilateral, 53 unilateral and 12 unspecified). In the two cohort studies above, most patients received minimally invasive surgery, and so these findings, combined with those of ProtecT, add to the evidence from the very short-term follow-up of a randomised trial of open surgery compared with robot-assisted surgery, also showing no evidence of differences in PROMs between open and minimally-invasive procedures. 47
There were strengths and limitations in the design and conduct of the ProtecT PROMs study. Strengths included the use of validated PROMs, well-balanced baseline data, high response rates and concordance between measures across the range of domains expected to be affected by treatments for localised prostate cancer. In addition, there was a high rate of randomisation of eligible participants – 62% (compared with 14.6% in PIVOT, for example) – here because of integrated recruitment research and enhanced team-working. The wider generalisability of ProtecT is assured by its PSA testing phase being a population-based programme embedded in a screening RCT – the CAP, which was recently published. 36,95
An important limitation was that only a small number of men of non-white ethnicity were included, although this reflected the population in the recruitment areas. 15 Other limitations relate to changes in diagnostic and treatment strategies since the study’s inception, and that populations with regular PSA testing might be different from this predominantly untested population, although on biopsy, compared with other PSA era treatment or screening RCTs, ProtecT had similar or higher numbers of participants with stage T1 (76%) and a Gleason score of 6 points (77%).
The ITT analysis undertaken as part of the ProtecT trial reflected important aspects for policy implementation; in future publications, we will present PROMs according to treatment received, and investigate reasons for management change in the AM group to further inform decision-making.
The ProtecT RCT has provided data that clarify the comparative impact of the major contemporary treatment options for localised PSA-detected prostate cancer on urinary, sexual and bowel function and QoL, including rates of recovery and outcomes up to 6 years after treatment allocation. Longer-term follow-up is needed to document the changes in PROMs and oncological outcomes that will emerge from the originally randomised groups. Although the current findings do not yet provide a complete picture, men making decisions about treatments for newly diagnosed localised prostate cancer or contemplating PSA testing can use these profiles of impact for decision-making with clinicians. As indicated above, the longer-term impacts of treatments are unknown. Follow-up for a further 5 to 10 years is required to fully inform the balance between the shorter-term impacts of treatments shown here and the longer course of prostate cancer progression, death from prostate cancer or competing causes and QoL impacts that may emerge in the longer term from these treatments or from the sequelae of treatments for metastatic or progressing prostate cancer. However, the NIHR HTA programme declined to support longer-term follow-up in ProtecT using PROMs. Although we have attempted to seek funding from other sources, as of March 2018 no such support has been found.
Optimal measurement of urinary incontinence and lower urinary tract symptoms after treatment for prostate cancer
Urinary incontinence and other LUTS can be assessed following treatment for prostate cancer with several different questionnaires. In this substudy, we compared their effectiveness and aimed to identify the most suitable questionnaire or questions to be recommended for use in research and clinical practice in future.
Methods
Urinary incontinence and its impact on QoL were assessed by the ICIQ score and QoL item, the EPIC 50-item urinary incontinence subscore and item on pad use, and the ICSmaleSF questionnaire urinary incontinence score. LUTS and their impact on QoL were assessed by the ICSmaleSF questionnaire voiding score and individual symptoms of frequency, nocturia and a QoL item, and with the EPIC urinary summary, bother and obstruction/irritation subscores. The PROMs were compared with each other.
Results
Response rates were higher than 85% for all questionnaires and did not decline over time.
All measures of urinary incontinence (ICIQ, ICSmaleSF urinary incontinence and EPIC urinary incontinence scores and EPIC item on pad use) showed that surgery had the greatest negative effect at 6 months, and that urinary incontinence remained worse in the surgery group than in the radiotherapy and AM groups at all time points over 6 years (p < 0.001 for each measure) (Figure 17). There was little difference in urinary incontinence between the radiotherapy and AM groups over 6 years. Only the ICIQ measure had a specific item assessing the QoL impact of urinary incontinence. This showed that men in the surgery group experienced most impact at 6 and 12 months after randomisation; after 24 months, the QoL impact of urinary incontinence recovered and became similar to the other groups, although the profiles were different (p < 0.001).
FIGURE 17.
Measuring urinary incontinence. (a) ICIQ incontinence score; (b) EPIC urinary incontinence; (c) ICSmaleSF incontinence; and (d) EPIC item (one or more pads per day).
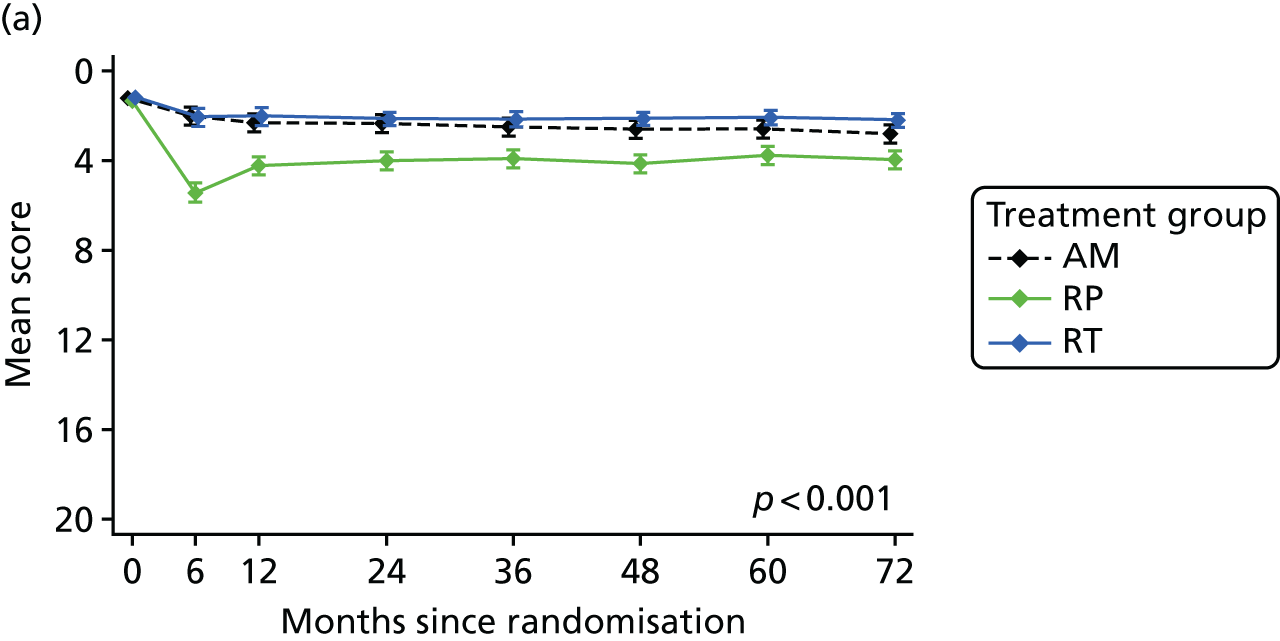
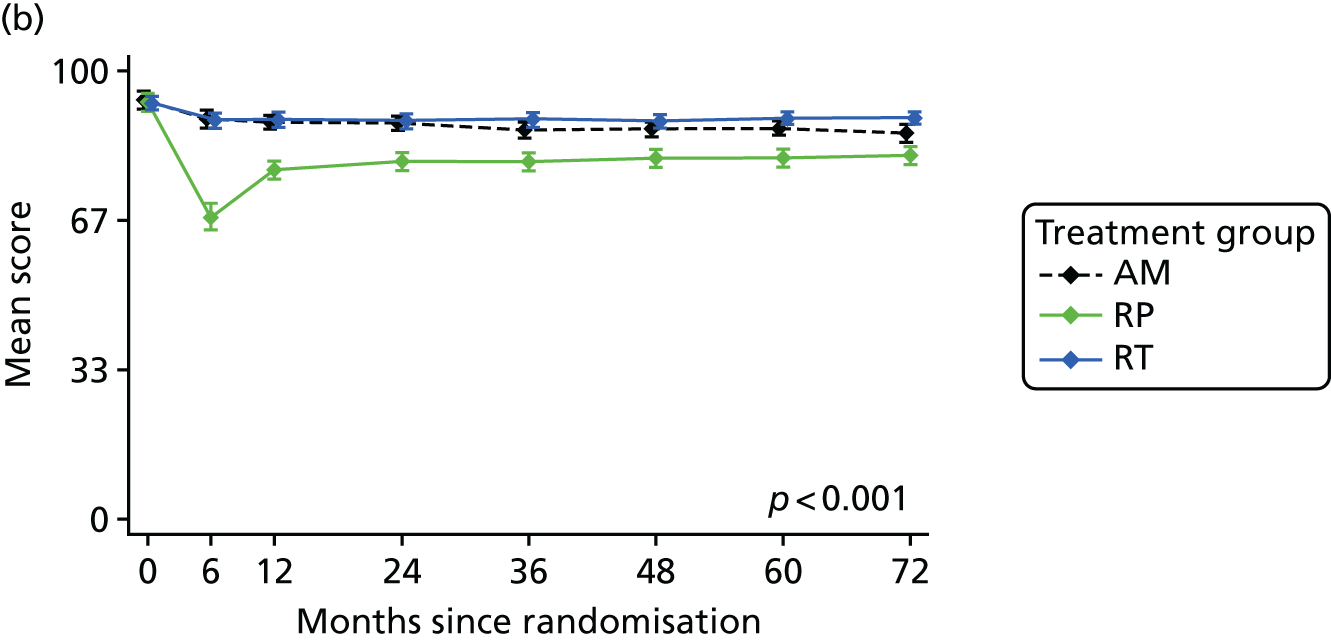
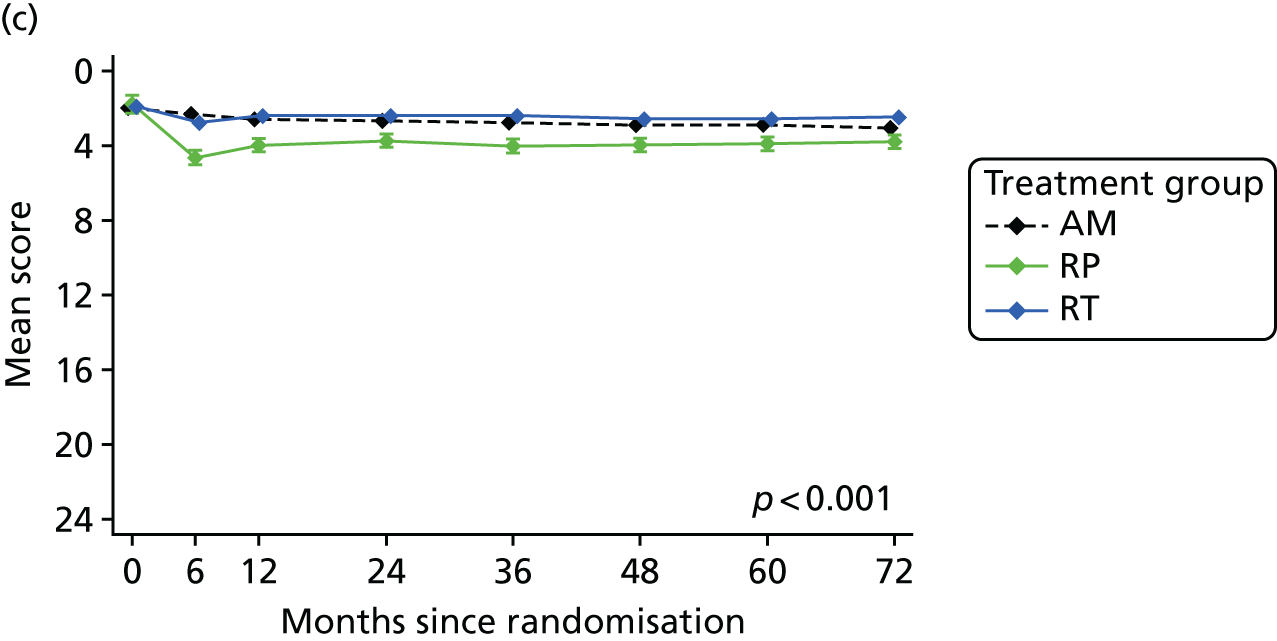
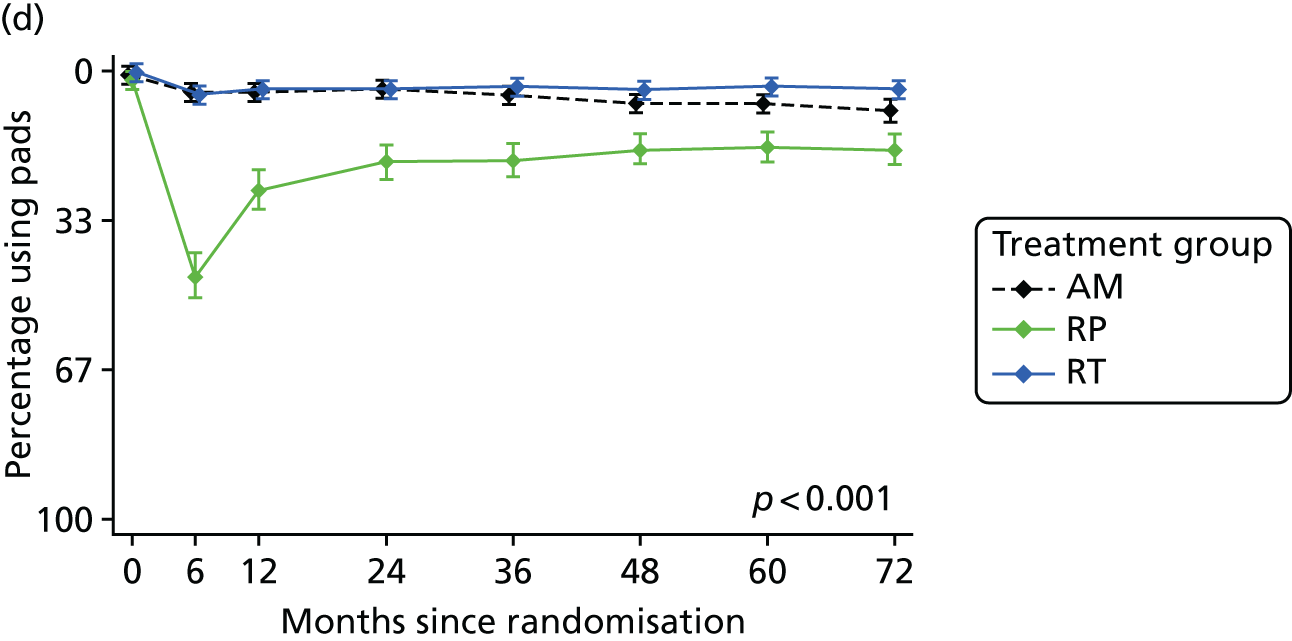
The ICSmaleSF voiding score showed differences between the groups (p < 0.001), with worse voiding symptoms in the RT group at 6 months, returning to be similar to the other groups from 12 months onwards (Figure 18). The EPIC obstruction/irritative subscore did not detect these differences (p = 0.77). The ICSmaleSF illustrated differences between the groups for nocturia (p < 0.001), with radiotherapy worse at 6 months, but no differences in daytime frequency (p = 0.47). The distinct effect of voiding difficulties on QoL was unclear, however, as the question in both measures – EPIC urinary bother and ICSmaleSF QoL score – referred to the impact of all urinary symptoms including urinary incontinence. The profiles from these scores, as well as the EPIC urinary summary score, were very similar to the profiles related to urinary incontinence. The EPIC urinary bother subscore was not clearly different between the groups (p = 0.095).
FIGURE 18.
Measuring LUTS and impact on QoL. (a) ICSmaleSF voiding; (b) EPIC urinary summary score; (c) EPIC urinary irritative score; (d) ICSmaleSF nocturia item; (e) ICSmaleSF effect of urinary symptoms on QoL; and (f) EPIC urinary bother score.
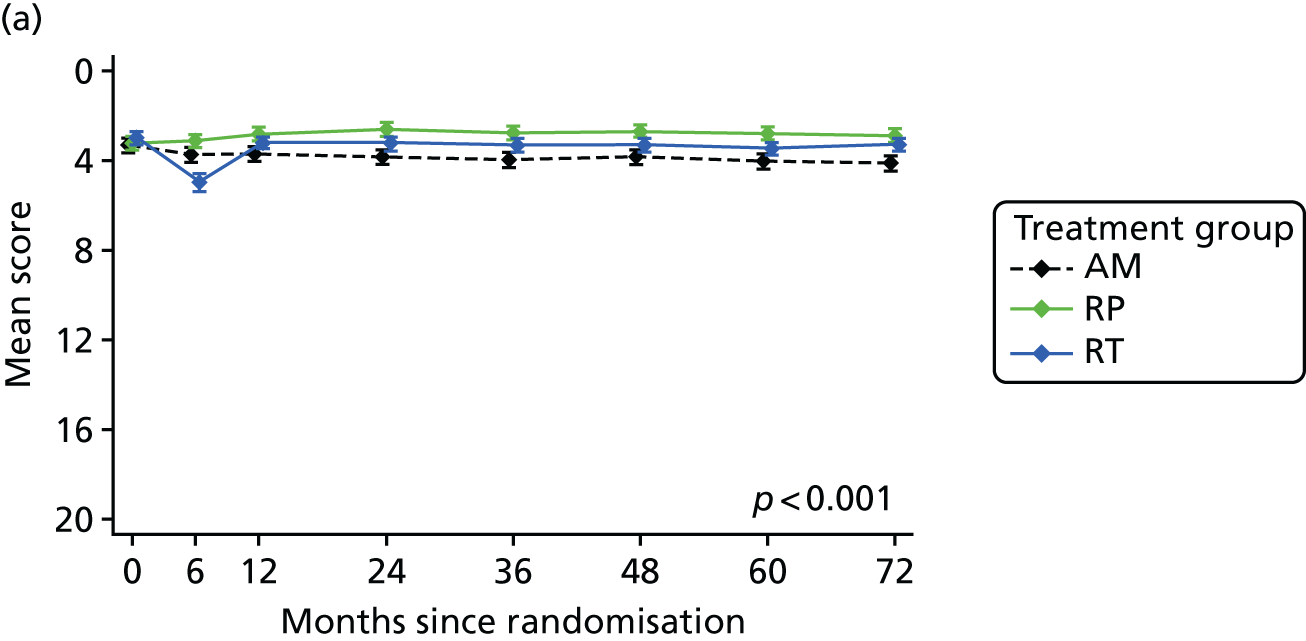
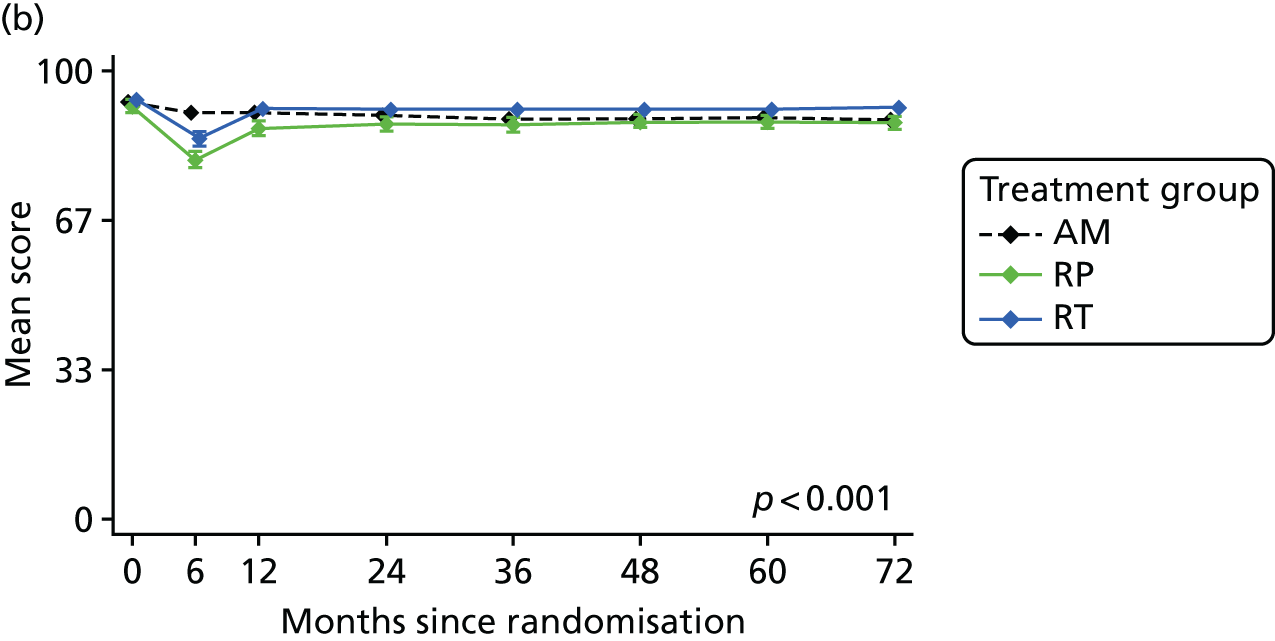
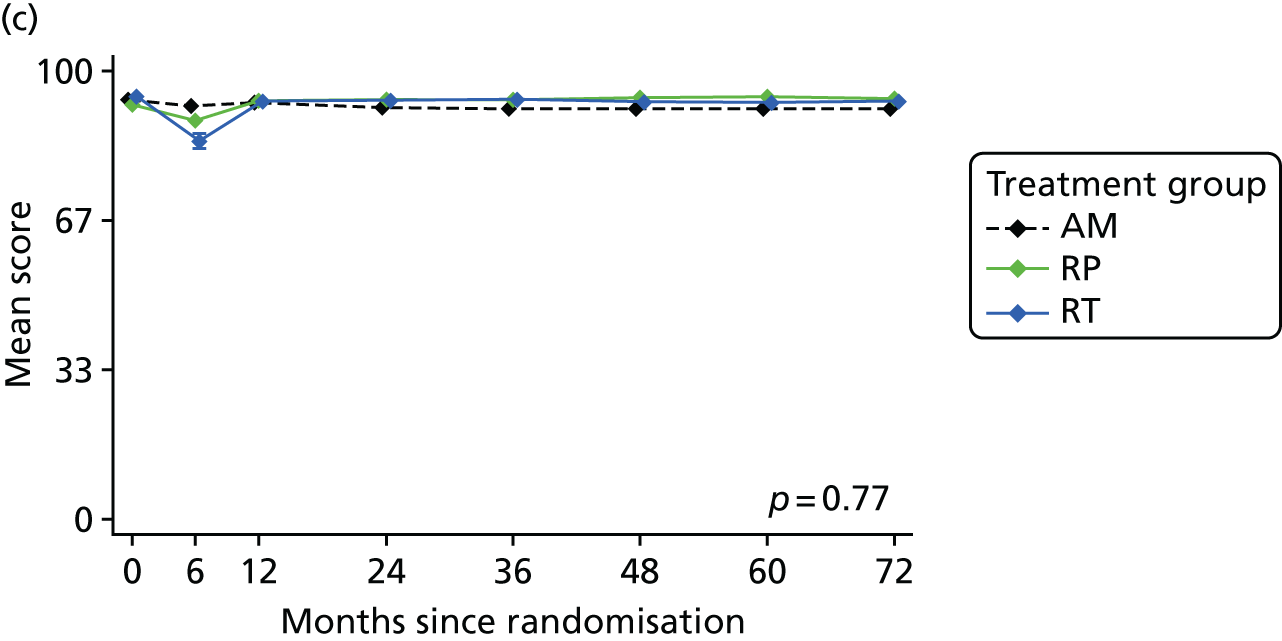
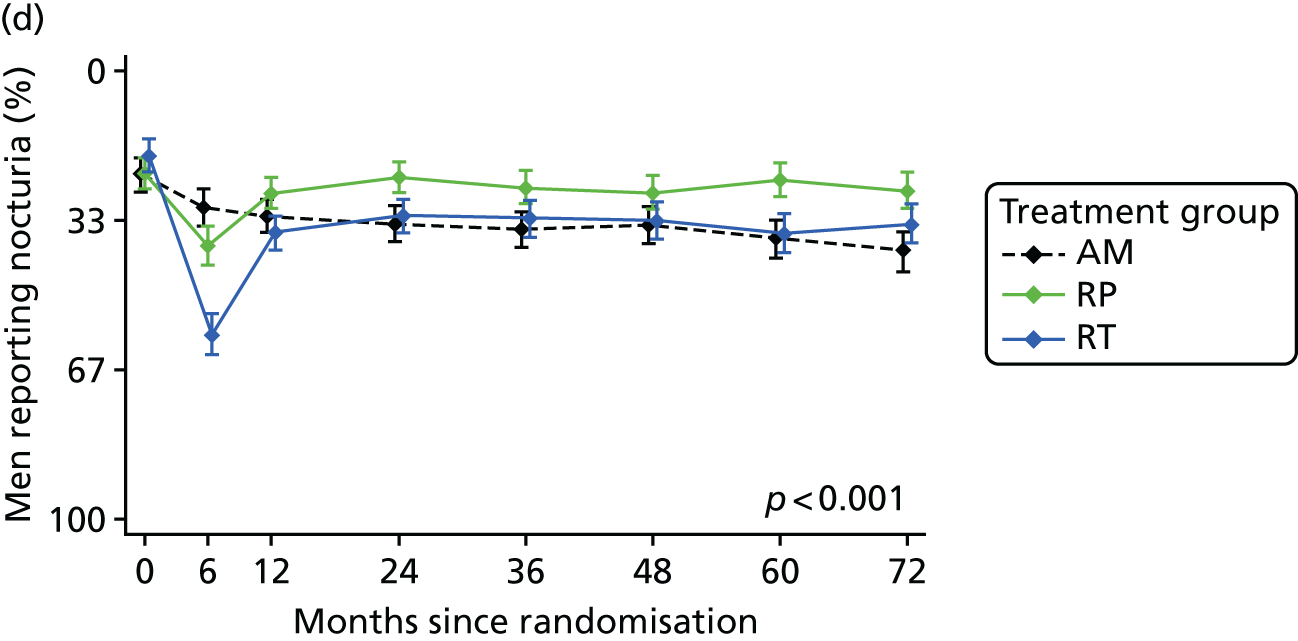
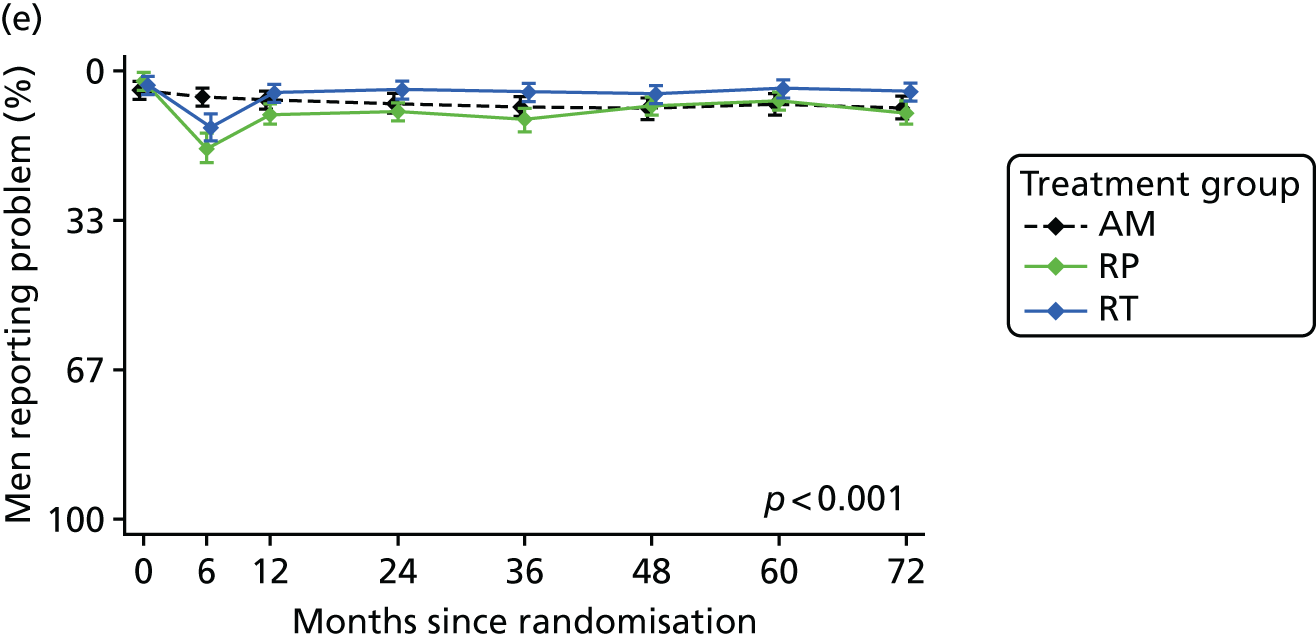
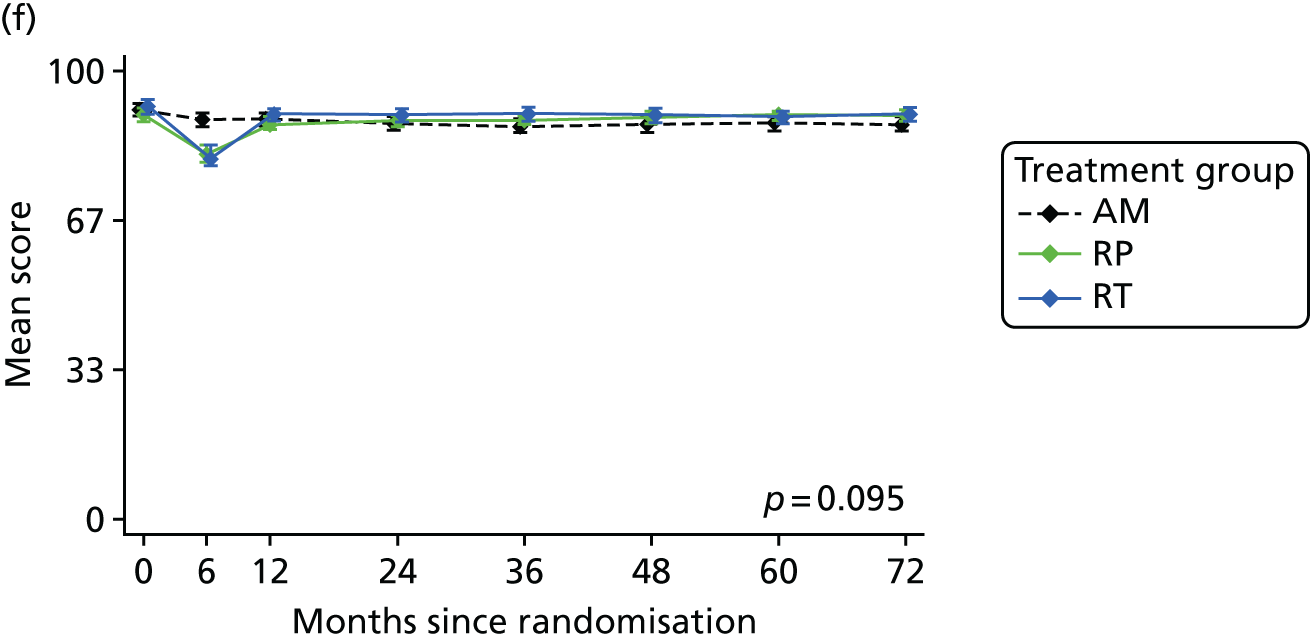
Conclusion
The EPIC, ICIQ and ICSmaleSF measures produced very similar profiles for the effects of treatments on urinary incontinence. Although the magnitude of differences was a little different between the measures, they were equally effective at measuring the effects of treatments on levels of urinary incontinence. However, the only measure that assessed the impact of urinary incontinence on QoL was the ICIQ questionnaire.
The ICSmaleSF and EPIC measures did not concur over the assessment of LUTS. The main EPIC scores (urinary summary and bother) included items on urinary incontinence, and so their profiles conflated urinary incontinence with other LUTS. The EPIC measure also did not have a specific voiding score, as its subscore assessed obstructive and irritative symptoms combined (dysuria, haematuria, weak stream and frequency). ICSmaleSF had separate voiding and incontinence scores, as well as specific items for nocturia and frequency, which it recommends reporting separately. ICSmaleSF did not include items on dysuria or haematuria in its assessment. EPIC and ICSmaleSF both conflated urinary incontinence and LUTS in their QoL impact item/scores.
Thus, there are clear similarities between measures in their assessment of urinary incontinence, and so any of these validated PROMs could be used: ICIQ, EPIC or ICSmaleSF. However, the assessment of QoL related to urinary incontinence is only assessed by the ICIQ, so this should be included in studies wishing to assess this. For the assessment of LUTS, the measures have differences that need to be taken into account when deciding which measure to use and in interpreting the results. The EPIC and ICSmaleSF questionnaires include different LUTS in their assessments of voiding, urinary incontinence and obstruction/irritation. Care needs to be taken when using scores as different measures include different items within similarly named scores. Particular issues arise for the assessment of voiding or storage symptoms, which are likely to become important for men undergoing AM/AS programmes or radiotherapy. In this situation, ICSmaleSF offers a specific voiding score and items.
Quality of life associated with the experience of urinary incontinence after treatment for prostate cancer
As indicated previously, ProtecT and many cohort studies have concluded that overall QoL, in terms of physical, social or emotional well-being, is not affected by surgery, radiotherapy or AM for localised prostate cancer. However, each treatment is associated with a particular set of side effects that can affect specific aspects of QoL. For example, erectile dysfunction is a relatively common side effect of radical treatment (surgery or radiotherapy), and raised levels of bother or problem are found when comparing groups on specific QoL measures [see Domain B: sexual function and quality-of-life impact (including erectile function)]. Urinary incontinence is a less common side effect of surgery, but this is also associated with raised levels of specific bother on specific QoL measures (see Figure 14).
Methods
The aim of this substudy was to investigate this seeming contradiction by examining levels of general QoL (physical and mental health, anxiety and depression) among men reporting urinary incontinence or erectile dysfunction following treatment in ProtecT, compared with those not reporting these symptoms. Urinary incontinence was assessed by the EPIC items on absorbent pad use and erectile dysfunction by the firmness of erections for intercourse item. Physical and mental health were assessed by two domains of the SF-12 generic health measure. Anxiety and depression were assessed by the HADS. Study questionnaires were completed at baseline before the diagnosis was known, at 6 and 12 months after randomisation and annually thereafter. PROMs were scored and analysed as recommended by their authors. Means and standard deviations (SDs) were calculated for the HADS and SF-12, with p-values testing the null hypothesis of equal population means across groups without the symptom or with the symptom at 6 months, or with the symptom at 6, 12 and 24 months, assessed over the duration of the study (6 years).
Results
There were 133 men (13%) with urinary incontinence who needed to use pads at 6 months and 62 men (6%) who needed to use pads at 6, 12 and 24 months, compared with 845 (81%) who did not use pads for urinary incontinence. Men who needed to use pads for urinary incontinence had higher depression scores than those who did not need to use pads at all time points, with scores highest of all for those who needed to use pads for longer. There was strong evidence that depression scores were worse in those using pads at 6 and 12 months (p < 0.001). The pattern for anxiety scores was somewhat similar. The highest anxiety scores were evident among men needing to use pads for longer, with those using pads at only 6 months having scores more similar to those not needing pads. There was some evidence that anxiety scores were particularly worse for those using pads at 6 and 12 months (p = 0.02 at 6 months and p = 0.03 at 12 months).
The impact of urinary incontinence (pad use) on depression and physical health is shown in Appendix 1, Figures 30 and 31, and the impact of erectile dysfunction on depression and physical health is shown in Appendix 1, Figures 32 and 33.
The SF-12 scores for physical health were different at only 6 months, when there was slightly worse physical health in those who needed pads compared with those who did not (p < 0.001). After this, physical health was similar between the groups. Reflecting the HADS scores above, SF-12 scores for mental health were worse among those needing pads at 6 months (p = 0.005), with some weaker evidence of worse mental health at 12 months among those needing pads longer term (p = 0.07).
There were 278 men (27%) who had erectile dysfunction at 6 months and 454 (44%) who had erectile dysfunction at 6, 12 and 24 months, compared with 289 (28%) who did not report erectile dysfunction. HADS depression and anxiety scores were much higher in men reporting longer-term erectile dysfunction than in those with erectile dysfunction at only 6 months or with no erectile dysfunction. For depression, this evidence was very strong (p < 0.001 at each time point); it was slightly less strong for anxiety (at p = 0.001 to p = 0.07). Depression and anxiety levels did not decline over time among those with long-term erectile dysfunction, but there was some evidence that those who recovered function after 6 months experienced less anxiety and depression. These patterns were mirrored in the SF-12 mental health scores. Perhaps more surprisingly, the experience of erectile dysfunction at 6 months or longer term was associated with a small reduction in physical functioning, and this was maintained for 6 years (p < 0.001).
Conclusions
High levels of erectile dysfunction were experienced by men who underwent treatment for localised prostate cancer and, for those with these symptoms, this was associated with worse physical and mental health, including anxiety and depression. Erectile dysfunction continued to have a small but measurable impact on these aspects of general QoL for the duration of the study (6 years). Urinary incontinence affected a smaller number of men, but had a clear impact on anxiety, depression and physical and mental health at 6 months. This impact on physical and overall mental health reduced after 6 months, but urinary incontinence continued to be associated with slightly higher levels of depression and anxiety over time.
Therefore, although it has been found that treatments for localised prostate cancer do not affect overall QoL on average across the treatment groups in ITT analyses, men experiencing erectile dysfunction or urinary incontinence had worse physical and mental health, particularly anxiety and depression, than men without these side effects in a secondary analysis. Men undergoing treatment should be made aware of the likelihood of these symptoms and their potential impact, and may need additional support when they experience them.
Chapter 5 Economic evaluation: the cost-effectiveness of treatments in the ProtecT trial
Parts of this chapter are reproduced or adapted from Noble et al. 167 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Introduction
The aim of this within-trial economic evaluation is to establish the cost-effectiveness of the three treatment strategies (3D-conformal radiation therapy with neoadjuvant ADT, RP and AM) in men with PSA-detected localised prostate cancer in the UK at a median of 10 years’ follow-up from a NHS perspective.
Methods
Perspective
The primary economic evaluation is from a NHS perspective. A preliminary analysis of a cross-sectional survey of the ProtecT men (years 1–5) in which Personal Social Services costs in the form of home care were obtained showed that home care was not a main cost driver; therefore, a NHS perspective was chosen.
Time horizon
The economic evaluation compared the costs and outcomes of each group from randomisation until the closure of the ProtecT study database (November 2015): a median of 10 years’ follow-up.
Identification of relevant resource use
The scope of the economic evaluation was defined as the main NHS resources used relating to prostate cancer and the three treatments and their follow-up. Data were collected on the use of hospital inpatient, outpatient, clinic and GP services for the initial trial interventions and associated long-term follow-up.
Measurement and valuation of relevant resource use
Resource use information in relation to the initial treatments given and any subsequent RP or radiotherapy was recorded by trial research nurses onto study-specific pro formas. For surgery, information was recorded in relation to the pre-operative assessments, the actual operation itself and complications. For radiotherapy, information was recorded in relation to the pre-treatment planning of radiotherapy and relating to the actual delivery of the radiotherapy including number of fractions given. For AM, all visits for the duration of the time the patient was on the AM treatment were recorded.
Information on all follow-up outpatient visits, inpatient stays and accident and emergency attendance, and, from April 2005, primary care attendances, was recorded onto research follow-up schedules, which were completed by research nurses using information from appointments with the patient and their medical records at 12 months from randomisation and then annually thereafter. Telephone appointments were held with men unable to attend the hospital and participants were given an annual resource use log to aid recollection of events at appointments.
To ensure completeness of resource use, initial treatment pro formas and annual schedules were compared, duplicated data were dropped and then the combined sources of data were used. In addition, the mainly administrative trial clinical centres database, which contained some clinical information, was used for validation purposes to ensure that all prostate cancer treatments were recorded and took place post randomisation.
In order to value the initial treatments, in relation to surgery, information relating to the type of procedure conducted and any complications incurred was used to assign a Healthcare Resource Group (HRG) 4 code (these are groups of events that consume similar levels of resources). This was then valued using the UK NHS Reference Costs 2014 to 2015. 168
In relation to radiotherapy treatment, the neoadjuvant deprivation and hormone injections were valued for primary care delivery using the BNF. 169 Hormone injections delivered in secondary care were valued using the UK NHS Reference Costs 2014 to 2015. 168 Pre-treatment planning, including scans, was assigned a HRG4 code, as were the fractions used for each dose of radiotherapy. These were valued using the NHS Reference Costs 2014 to 2015. 168
The AM visits were valued using the average time for a face-to-face or telephone consultation given in the AM schedules and the cost of a nurse team leader (including qualifications) based on national estimates. 170
All other inpatient stays and day cases were assigned HRG codes; initially, the text cited in the questionnaires as the reason for admission was used to map the event to Office of Population Censuses and Surveys Classification of Interventions and Procedures (OPCS-4) codes. The HRG4+ 2014/15 Reference Costs Grouper Code to Group v1.0 workbook171 was then used to allocate the HRG codes. NHS Reference Costs 2014 to 2015168 were applied to the HRGs on a fixed consultant episode basis. Day-case costs were assigned when the patient did not stay in hospital overnight. In the absence of information to identify whether the admission was elective or non-elective, stays were assigned elective inpatient costs.
All outpatient visits were valued using the Department of Health and Social Care reference costs relevant to the specialty. 168
Outpatient procedures were allocated HRG codes. The ProtecT annual research follow-up was valued at half the cost of an outpatient visit to account for both the clinical and research element of this visit.
Appendix 1, Table 27, gives further details of how the resources were measured, coded and valued. All costs were valued in 2014/15 Great British pounds. Data cleaning and coding was conducted prior to the unblinding of the health economist. A number of assumptions were made in relation to missing resource use-data, which are given in Table 11.
| Resource | Assumptions made in relation to missing resource use data |
|---|---|
| Delivery of hormones | For participants, where hormones were recorded but no method of delivery was given, the first injection was assumed to be delivered in outpatients. From the second injection onwards, the hormones were assumed to be delivered in primary care and the number of appointments was determined by the number of hormone injections |
| Hormones delivered in primary care | If the medication name was missing from the radiotherapy schedule, goserelin (Zoladex®, AstraZeneca) was assumed (most common), and if the frequency was missing, monthly was assumed (from ProtecT protocol). Hormones from follow-up schedule data were costed as Zoladex injections. If there were more than four injections per annum, a monthly injection regime was assumed, otherwise a 3-monthly injection regime was assumed |
| Radiotherapy delivery | Where number of fractions was missing, it was assumed that participants who had protocol radiotherapy had protocol number of fractions (37 fractions). Participants who had salvage radiotherapy but a missing number of fractions following clinician opinion were assumed to have 20 fractions |
| Inpatients stays, day cases (no overnight stay), outpatient procedures and consultations | If nothing was recorded for these sections, it was assumed following consultation with research nurses that no events occurred. If the number of outpatient visits was missing, one visit was assumed as most research nurses questioned believed that this would have been the case. For all events in which there was not enough information in the text to map onto a HRG code, these were allocated on the basis of the diagnosis. If type of inpatient admission was absent, it was assumed to be an elective admission. If type of outpatient specialty was missing, a urology specialty code was used |
Measurement and valuation of outcomes
The outcome for the economic analysis as recommended by the National Institute for Health and Care Excellence (NICE) is the quality-adjusted life-year (QALY) at a median of 10 years following randomisation into the trial. The utility values for each year that the participant could have been a trial participant were estimated from the EQ-5D-3L health status questionnaire and the associated published societal utility tariffs. 172 The EQ-5D-3L was completed at baseline (at the confirmatory biopsy), 6 months and 12 months following randomisation and annually thereafter. Participants who had died were assigned a zero utility value for the remaining years that they could have been trial participants (i.e. from time of death until the closure date of the database). The total number of QALYs for each participant was calculated using the area under the curve approach. For men who had missing EQ-5D-3L scores in the year prior to the year of death but available for the preceding year, the area under the curve methodology used the preceding year’s EQ-5D-3L score. Where a EQ-5D-3L time point was missing and the adjacent year’s values, other than death, were available, the mean of the adjacent year’s values was used for the analysis.
Analysis
The analysis was conducted on an ITT basis comparing the three treatment groups as randomised, considering prostate cancer or its treatment-related NHS costs in relation to the QALY. Total mean adjusted costs and outcomes were discounted at 3.5% as recommended by NICE. 173 All analyses were conducted in Stata version 14.1.
The total number of times each item of resource use was used was calculated for each participant. Participants who had died were assigned zero resource use for the remaining years that they could have been trial participants (i.e. from time of death until the closure date of the database). The total mean resource use by resource use category and by trial group was then calculated.
The cost of each item of resource use was calculated as the resource use (e.g. number of GP visits) multiplied by its relevant unit cost (e.g. cost of GP visit). The costs for each aspect of resource use were summed annually, across time and by resource use category for each participant.
Seemingly unrelated regressions, which control for the correlation between costs and QALYs, were used to estimate total adjusted mean costs and QALYs. Adjustments were made for study centre and the minimisation variables of the randomisation process: age at baseline, Gleason score (< 7, 7 or 8–10 points) and the mean of the baseline and first biopsy PSA test results together (< 6.0, 6.0–9.9 or > 9.9 ng/ml). QALYs were also adjusted for baseline EQ-5D-3L result. 174 The adjusted mean costs of the three treatment groups were compared with the adjusted mean QALYs to assess if any of the treatments were dominated (i.e. less effective and more expensive than one or both of the other two treatments). Incremental adjusted mean costs and QALYs, bias-corrected and accelerated CIs (to account for non-normal distributions) and incremental cost-effectiveness ratios (ICERs) were estimated for non-dominated treatments using seemingly unrelated regression and non-parametric bootstrapping (5000 model iterations). Regression outputs were used to estimate parametrically the net monetary benefit (NMB) statistic and associated CIs at a willingness to pay of £20,000 per QALY.
Cost-effectiveness acceptability curves (CEACs) were generated to explore uncertainty. 175 The CEAC graphs the probability that each trial group is the cost-effective option at a range of willingness to pay thresholds. A willingness to pay threshold is defined as the maximum that society would be willing to pay for an improvement in health, measured by the QALY. The CEAC was calculated as follows: NMB values were calculated for each participant at willingness to pay per QALY thresholds from £0 to £100,000 at £1000 intervals; 5000 bootstrap model iterations of the adjusted linear regression models of NMBs were performed. At each threshold, bootstrapped NMBs were compared between groups to calculate the proportion of times each group had the highest NMB. CEACs were plotted to present the probability that each trial group is the most cost-effective compared with the other two groups at a range of monetary values.
Sensitivity analyses
One-way sensitivity analyses in which the value of one of the variables was changed, and the analysis rerun, and scenario sensitivity analyses in which the values of more than one of the variables were changed simultaneously and the analysis rerun, were used to account for any methodological uncertainty or assumptions made during the course of the study and analysis.
One-way sensitivity analyses
Given the long period of follow-up, multiple imputation on total costs and total QALYs would have led to a great loss of information. The first four sensitivity analyses were therefore conducted to explore the effect of missing data:
-
The imputed mean QALYs for those who had one time point missing was replaced with a QALY score 10% lower than this mean score to take into account the possibility that non-completion of questionnaires resulted from illness and treatment.
-
An analysis excluding the cases in which the QALY had been imputed.
-
To examine the impact of not capturing information on primary care visits until 2005, the analysis was rerun for those participants who received their first annual follow-up following this date.
-
To examine whether or not administrative censoring affected the results, the analysis was rerun on the first 6 years of follow-up, the time point at which no administrative censoring had taken place. The analysis was run again on the first 6 years adjusting for the information appointment date. The results of these two analyses were compared.
-
During the feasibility period of the study, initial follow-up was not as robust as for the main trial; the men recruited during this period were therefore excluded from this sensitivity analysis.
-
The appropriate discount rate for economic evaluations in health care is the subject of debate internationally, and varies between jurisdictions. For this reason, the impact of a 1.5% discount rate on both costs and benefits was explored.
-
It was sometimes difficult to distinguish between a day-case procedure and an outpatient procedure. All day cases were therefore costed as outpatient procedures in the sensitivity analysis.
-
Prostate cancer-related resource use was used for this analysis; however, unrelated resource use was also recorded. There was some discrepancy over whether a transurethral resection of the prostate (TURP) was or was not related to the AM treatment. The increased surveillance of these men could have meant that more LUTS may have been identified, which could have meant that more TURPs were conducted. In the base-case analysis, all TURPS were included, but they were excluded from this sensitivity analysis.
-
In economic evaluations, research-based appointments are not usually costed. Following discussion with the trial research nurses, it was discovered that clinical follow-up also took place in annual research follow-up appointments. In the base-case analysis, these appointments were costed as half an outpatient visit. In the sensitivity analysis, these appointments were excluded.
-
The trial research nurses were asked to record all non-admission secondary care attendances in one section of the pro forma. It was discovered through discussion with the nurses that some nurses at certain times only recorded clinician follow-up in that section, and any outpatient procedures were recorded elsewhere in the pro forma. Although the main procedures (e.g. bone scans, biopsies, MRI and computerised tomography) were accounted for, other procedures (e.g. cystoscopy) may not have been included by some nurses in the outpatient section and were not included in the base-case analysis. This sensitivity analysis therefore includes the costs of all other procedures recorded elsewhere in the pro forma.
Scenario analyses
-
A high-cost option, whereby all trial participants without catheters were costed as day cases, an outpatient visit cost was assigned for extracting blood for PSA tests, all procedures recorded in only the follow-up schedules were costed as the highest complications and comorbidities split, annual research follow-up appointments were costed as an outpatient visit and initial and salvage radiotherapy planning were costed using technical support.
-
A current initial treatment option, whereby radiotherapy was costed as IMRT and RP was costed as a robotic RP.
Results and discussion
Resource use data [given the assumptions in relation to missing data (see Table 11)] were obtained for 1556 men (95%), and QALY data, given the assumptions made, were obtained for 1132 men (69%) randomised into the ProtecT study. The total adjusted cost and QALY data are based on 1101 out of 1643 men (67%).
In terms of resources used (Table 12), the treatment groups, as expected, varied by resources related to the individual treatment. Men in the RT group had more outpatient visits and fewer inpatient stays than men in the other two groups, and more primary care resources were used in the AM group (this group also had more biopsies and MRI scans). Resources relating to treatment complications related to initial treatments received and progression varied by group. More men in the RP group had inpatient stays related to infection and urinary sphincter procedures, more men in the RT group had colonoscopies, sigmoidoscopies and chemotherapy and men in the AM group had more bone scans and TURPs.
| Resource | Treatment group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AM (n = 513) | RT (n = 516) | RP (n = 527) | |||||||
| Mean number of unitsa | Mean cost (£) | SD of mean cost (£) | Mean number of unitsa | Mean cost (£) | SD of mean cost (£) | Mean number of unitsa | Mean cost (£) | SD of mean cost (£) | |
| Hospital outpatients visits | |||||||||
| Procedure-driven appointments | |||||||||
| Protocol radiotherapy | 7.772 | 846 | 1608 | 28.374 | 3090 | 1665 | 3.691 | 402 | 1190 |
| Non-protocol radiotherapy | 1.008 | 133 | 631 | 0.374 | 49 | 377 | 1.863 | 245 | 835 |
| CT scan | 0.287 | 26 | 53 | 0.882 | 81 | 56 | 0.216 | 20 | 52 |
| Radiotherapy preparation | 0.269 | 169 | 279 | 0.800 | 503 | 252 | 0.194 | 122 | 249 |
| TRUS | 0.353 | 19 | 33 | 0.291 | 16 | 30 | 0.292 | 16 | 30 |
| Trial without a catheter | 0.172 | 24 | 57 | 0.091 | 13 | 43 | 0.526 | 73 | 73 |
| Bone scan | 0.279 | 56 | 176 | 0.112 | 23 | 97 | 0.127 | 26 | 102 |
| MRI scan | 0.199 | 27 | 59 | 0.126 | 17 | 53 | 0.112 | 15 | 45 |
| TRUS-guided biopsy | 0.156 | 35 | 83 | 0.068 | 15 | 113 | 0.042 | 9 | 47 |
| Chemotherapy | 0.035 | 58 | 663 | 0.087 | 145 | 1570 | 0.030 | 50 | 844 |
| Other procedures | 0.499 | 60 | 250 | 0.318 | 57 | 346 | 0.336 | 55 | 569 |
| Specialty-driven appointments | |||||||||
| Urology | 8.992 | 868 | 787 | 9.564 | 913 | 795 | 11.636 | 1122 | 810 |
| Oncology | 1.076 | 156 | 463 | 2.764 | 400 | 646 | 1.313 | 190 | 536 |
| Uro-oncology | 0.624 | 80 | 205 | 1.072 | 137 | 235 | 0.803 | 102 | 199 |
| Other specialitiesb | 16.563 | 912 | 519 | 4.791 | 374 | 577 | 3.981 | 260 | 493 |
| Total outpatient cost | 3469 | 2754 | 5832 | 3106 | 2707 | 2748 | |||
| Hospital day-case stays: top 10 reasons | |||||||||
| Flexible cystoscopy | 0.111 | 51 | 165 | 0.064 | 29 | 119 | 0.076 | 35 | 142 |
| Colonoscopy | 0.041 | 21 | 126 | 0.066 | 34 | 185 | 0.015 | 8 | 63 |
| TWOC | 0.043 | 16 | 83 | 0.006 | 2 | 29 | 0.051 | 19 | 92 |
| Sigmoidoscopy | 0.012 | 5 | 46 | 0.045 | 19 | 100 | 0.017 | 7 | 81 |
| Rigid cystoscopy | 0.031 | 27 | 160 | 0.012 | 10 | 107 | 0.038 | 33 | 189 |
| TRUS-guided biopsy | 0.047 | 25 | 117 | 0.010 | 5 | 52 | 0.008 | 4 | 46 |
| Education | 0.021 | 10 | 70 | 0.010 | 4 | 52 | 0.021 | 9 | 75 |
| Chemotherapy | 0.006 | 13 | 285 | 0.010 | 21 | 390 | 0.027 | 57 | 936 |
| Urodynamics | 0.006 | 2 | 25 | 0.006 | 2 | 25 | 0.017 | 6 | 43 |
| Brachytherapy | 0.010 | 10 | 97 | 0.006 | 6 | 75 | 0.006 | 6 | 75 |
| Other day-case reasons | 0.142 | 99 | 427 | 0.118 | 81 | 417 | 0.156 | 119 | 505 |
| Total day-case cost | 277 | 689 | 214 | 715 | 303 | 1299 | |||
| Hospital inpatient stays: top 10 reasons | |||||||||
| Prostatectomy | 0.246 | 1332 | 2380 | 0.107 | 578 | 1709 | 0.769 | 4013 | 2347 |
| Infection | 0.019 | 31 | 278 | 0.016 | 42 | 721 | 0.044 | 76 | 509 |
| TURP | 0.051 | 142 | 667 | 0.017 | 44 | 333 | 0.004 | 21 | 379 |
| Brachytherapy | 0.033 | 61 | 347 | 0.017 | 32 | 240 | 0.017 | 31 | 237 |
| Bladder neck procedure | 0.010 | 18 | 213 | 0.014 | 30 | 278 | 0.021 | 44 | 443 |
| Insertion of urinary sphincter | 0.014 | 129 | 1238 | 0.008 | 70 | 796 | 0.023 | 206 | 1464 |
| Pain | 0.008 | 17 | 243 | 0.008 | 10 | 115 | 0.015 | 28 | 323 |
| Urinary retention | 0.014 | 17 | 143 | 0.006 | 7 | 94 | 0.011 | 14 | 131 |
| Rigid cystoscopy | 0.002 | 3 | 60 | 0.014 | 19 | 158 | 0.011 | 16 | 168 |
| Blood transfusion | 0.018 | 18 | 255 | 0.000 | 0 | 0 | 0.004 | 4 | 90 |
| Other inpatient reasons | 0.152 | 434 | 2298 | 0.083 | 235 | 1458 | 0.176 | 403 | 2316 |
| Total inpatient cost | 2202 | 3956 | 1068 | 2854 | 4855 | 4055 | |||
| General practice visitsc by health-care professional | |||||||||
| GP | 0.815 | 36 | 79 | 0.928 | 41 | 86 | 1.087 | 48 | 92 |
| General practice nurse | 0.908 | 13 | 38 | 0.661 | 10 | 34 | 0.685 | 10 | 36 |
| Other | 0.049 | 1 | 5 | 0.074 | 1 | 9 | 0.121 | 2 | 16 |
| General practice visitsa by reason | |||||||||
| PSA test | 27.407 | 435 | 142 | 20.911 | 332 | 140 | 20.799 | 330 | 138 |
| Hormone delivery | 1.973 | 44 | 146 | 4.236 | 94 | 87 | 0.850 | 19 | 87 |
| Total general practice cost | 528 | 243 | 477 | 205 | 409 | 231 | |||
| Medications | |||||||||
| Cyproterone acetate daysd | 0.228 | 1 | 5 | 0.374 | 1 | 7 | 0.159 | 0 | 4 |
| Hormone injections | 2.476 | 277 | 770 | 3.963 | 331 | 515 | 1.197 | 154 | 606 |
| Total medication cost | 277 | 770 | 332 | 515 | 154 | 606 | |||
In terms of annual mean unadjusted costs (Table 13 and Figure 19), over half of the radical groups’ costs from the first 12 years were within the first year (£4910 and £4707 for RP and radiotherapy, respectively).
| Year | Treatment group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AM | RT | RP | |||||||
| n | Mean cost (£) | SD of mean cost (£) | n | Mean cost (£) | SD of mean cost (£) | n | Mean cost (£) | SD of mean cost (£) | |
| 1 | 513 | 1155 | 2075 | 516 | 4707 | 2053 | 527 | 4910 | 2593 |
| 2 | 513 | 660 | 1309 | 516 | 465 | 817 | 527 | 530 | 1045 |
| 3 | 513 | 670 | 1470 | 516 | 350 | 606 | 527 | 493 | 1322 |
| 4 | 513 | 714 | 1662 | 516 | 346 | 712 | 527 | 474 | 1799 |
| 5 | 513 | 765 | 1680 | 516 | 356 | 1031 | 527 | 436 | 1393 |
| 6 | 513 | 710 | 2405 | 516 | 435 | 1608 | 527 | 354 | 1093 |
| 7 | 510 | 574 | 1376 | 514 | 233 | 593 | 521 | 347 | 1076 |
| 8 | 445 | 570 | 1436 | 451 | 420 | 1740 | 450 | 261 | 866 |
| 9 | 356 | 466 | 1205 | 363 | 239 | 584 | 362 | 321 | 1211 |
| 10 | 273 | 481 | 1317 | 270 | 296 | 878 | 279 | 272 | 845 |
| 11 | 180 | 467 | 1220 | 184 | 406 | 1226 | 189 | 298 | 1150 |
| 12 | 123 | 478 | 1519 | 122 | 301 | 1063 | 128 | 258 | 836 |
| 13 | 75 | 611 | 1357 | 69 | 636 | 3349 | 74 | 496 | 1492 |
| 14 | 36 | 519 | 1648 | 38 | 262 | 404 | 37 | 489 | 1517 |
| 15 | 13 | 582 | 1436 | 12 | 636 | 839 | 14 | 127 | 183 |
FIGURE 19.
Mean unadjusted costs per year of follow-up, by treatment group.
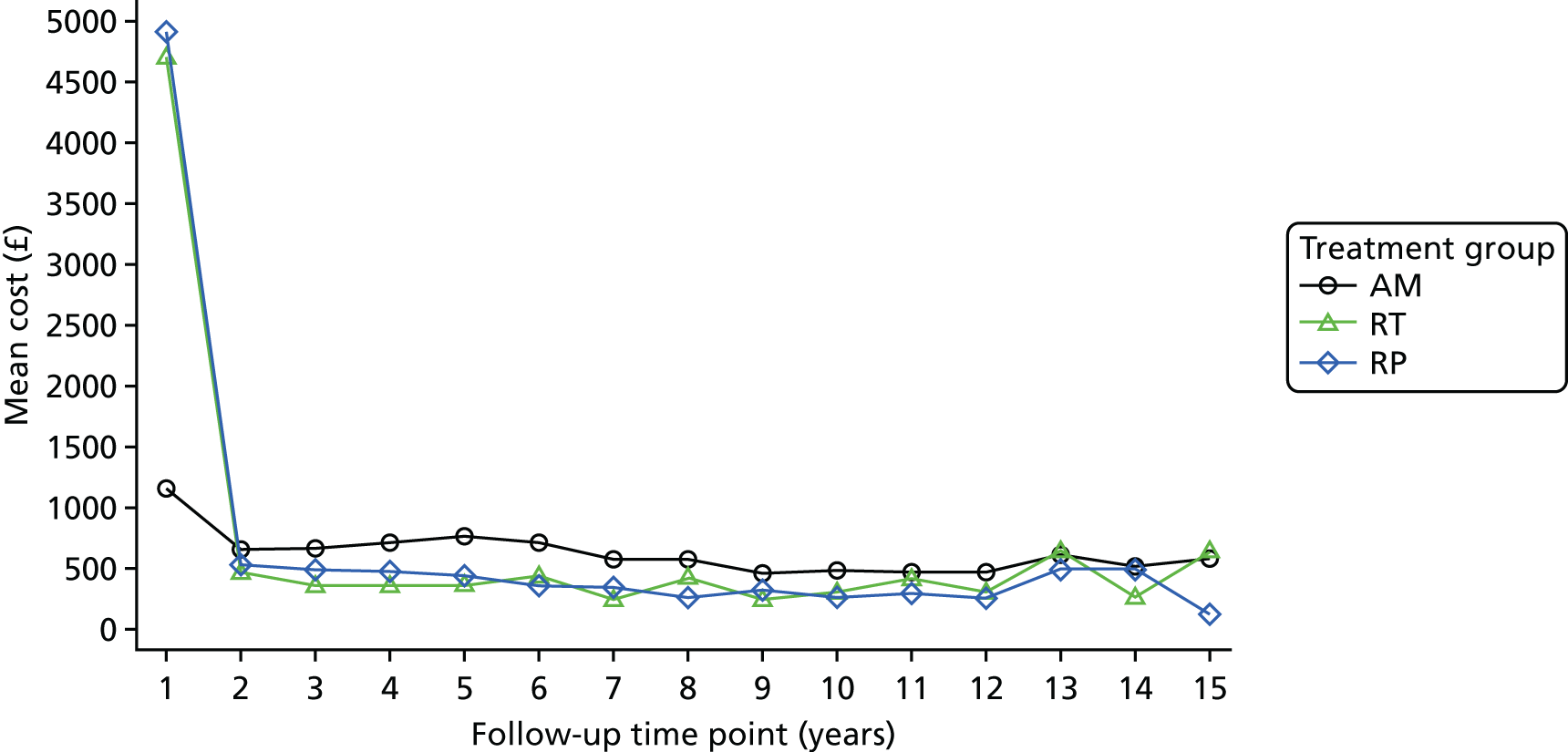
The total mean adjusted cost for the RP group (£7519, 95% CI £7099 to £7940) was slightly higher than for the RT group (£7361, 95% CI £6938 to £7783), and both radical groups were more expensive than the AM group (£5913, 95% CI £5494 to £6332) (Table 14).
| Allocation arm | n a | Adjustedb costs (£), mean (95% CI) | Adjustedb QALYs, mean (95% CI) | Comparison | Incremental costs (£) (95% CIc) | Incremental QALYs (95% CIc) | ICER (£/QALY)d | NMB (£) at £20,000/QALY (95% CI) |
|---|---|---|---|---|---|---|---|---|
| AM | 370 | 5913 (5494 to 6332) | 6.976 (6.798 to 7.154) | |||||
| RT | 364 | 7361 (6938 to 7783) | 7.093 (6.914 to 7.273) | RT vs. AM | 1448 (803 to 2061) | 0.118 (–0.141 to 0.368) | 12,310 | 904 (–4181 to 5990) |
| RPe | 367 | 7519 (7099 to 7940) | 6.909 (6.731 to 7.087) | RP vs. RT | 159 (–410 to 747) | –0.184 (–0.431 to 0.073) | RT dominates RP | –3847 (–8940 to 1245) |
The total number of adjusted mean QALYs for radiotherapy (7.093 QALYs, 95% CI 6.914 to 7.273 QALYs) was higher than that of AM (6.976 QALYs, 95% CI 6.976 to 7.154 QALYs) and RP (6.909 QALYs, 95% CI 6.731 to 7.087 QALYs). The difference between the RT group and those allocated to AM equates to an extra 43 days, and between the RT and RP groups, this equates to an extra 67 days of perfect health for men over a median 10-year period. (see Table 14).
The RT group dominated the RP group in that it was slightly more expensive and less effective. The RP group was also dominated by AM; therefore, it is unlikely that RP would be the cost-effective option. This leaves the choice between radiotherapy (slightly more beneficial but more expensive) and AM (slightly less beneficial but less expensive). The ICER comparing radiotherapy with AM was therefore calculated as £12,310 per QALY. The CEACs (Figure 20) show that, at the NICE threshold of £20,000 per QALY, at a median 10-year follow-up, the probabilities that each group is the cost-effective option are 58% (radiotherapy); 32% (AM) and 10% (RP). At the £30,000-per-QALY threshold, the figures are 64% (radiotherapy), 23% (AM) and 13% (RP).
FIGURE 20.
Cost-effectiveness acceptability curves displaying the probability that each treatment is cost-effective at different willingness-to-pay thresholds, based on the primary analysis. Reproduced from Noble et al. 167 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original.
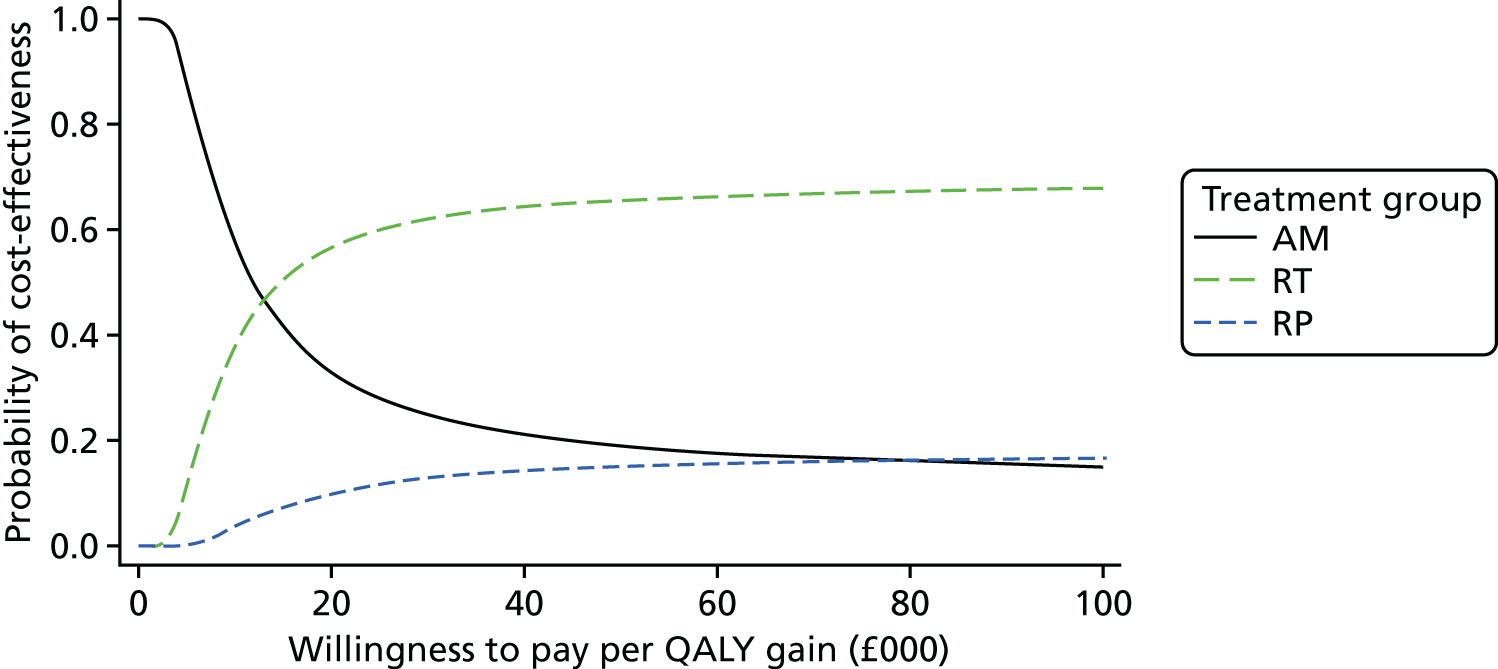
The sensitivity analyses (Table 15) showed some uncertainty in the results. The results differed when the QALY data were not imputed (ICER for RT vs. AM = £27,673), when only including participants following the introduction of primary care data collection (ICER for RT vs. AM = £27,009), when excluding men recruited during the feasibility period (ICER for RT vs. AM = £20,195) and for the current treatment cost scenario (ICER for RT vs. AM = £22,519), although all analyses indicated that radiotherapy would still be cost-effective at the upper NICE willingness-to-pay threshold of £30,000 per QALY.
| Allocation arm | n a | Adjustedb costs (£), mean (95% CI) | Adjustedb QALYs, mean (95% CI) | Comparison | Incremental costs (£) (95% CIc) | Incremental QALYs (95% CIc) | ICER (£/QALY)d | Incremental NMB (£) at £20,000/QALY (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Sensitivity analysis 1: imputed QALYs assumed to be 10% lower | ||||||||
| AM | 370 | 5913 (5494 to 6332) | 6.957 (6.780 to 7.134) | |||||
| RT | 364 | 7361 (6938 to 7783) | 7.073 (6.895 to 7.252) | RT vs. AM | 1448 (771 to 2065) | 0.117 (–0.130 to 0.375) | 12,397 | 888 (–4177 to 5953) |
| RP | 367 | 7519 (7099 to 7940) | 6.889 (6.712 to 7.067) | RP vs. RT | 159 (–417 to 727) | –0.184 (–0.433 to 0.068) | RT dominates RP | –3838 (–8910 to 1234) |
| Sensitivity analysis 2: no imputed QALY data | ||||||||
| AM | 284 | 5861 (5366 to 6355) | 6.808 (6.613 to 7.002) | |||||
| RT | 273 | 7384 (6879 to 7889) | 6.863 (6.664 to 7.061) | RT vs. AM | 1523.34 (709 to 2225) | 0.055 (–0.242 to 0.341) | 27,673 | –422 (–6028 to 5183) |
| RP | 276 | 7478 (6975 to 7981) | 6.751 (6.553 to 6.949) | RP vs. RT | 94.32 (–566 to 765) | –0.112 (–0.391 to 0.164) | RT dominated RP | –2327 (–7982 to 3327) |
| Sensitivity analysis 3: including only participants recruited following the introduction of primary care data | ||||||||
| AM | 274 | 5672 (5169 to 6175) | 6.417 (6.248 to 6.586) | |||||
| RT | 244 | 7122 (6588 to 7656) | 6.471 (6.292 to 6.650) | RT vs. AM | 1450 (694 to 2143) | 0.054 (–0.189 to 0.303) | 27,009 | –376 (–5351 to 4599) |
| RP | 269 | 7691 (7182 to 8199) | 6.363 (6.193 to 6.534) | RP vs. RT | 569 (–122 to 1277) | –0.107 (–0.341 to 0.119) | RT dominates RP | –2714 (–7707 to 2279) |
| Sensitivity analysis 4a: only including data from the first 6 years | ||||||||
| AM | 393 | 4464 (4128 to 4801) | 4.798 (4.724 to 4.872) | |||||
| RT | 383 | 6481 (6140 to 6822) | 4.835 (4.760 to 4.910) | RT vs. AM | 2017 (1479 to 2461) | 0.037 (–0.073 to 0.144) | 54,283 | –1274 (–3472 to 924) |
| RP | 388 | 6769 (6431 to 7108) | 4.823 (4.749 to 4.897) | RP vs. RT | 288 (–141 to 720) | –0.012 (–0.108 to 0.088) | RT dominates RP | –524 (–2728 to 1679) |
| Sensitivity analysis 4b: only including data from the first 6 years, controlling for the information appointment date | ||||||||
| AM | 393 | 4466 (4131 to 4801) | 4.797 (4.724 to 4.871) | |||||
| RT | 383 | 6488 (6148 to 6828) | 4.834 (4.759 to 4.908) | RT vs. AM | 2022 (1469 to 2465) | 0.036 (–0.065 to 0.144) | 55,860 | –1298 (–3487 to 891) |
| RP | 388 | 6761 (6424 to 7099) | 4.824 (4.750 to 4.899) | RP vs. RT | 273 (–154 to 712) | –0.009 (–0.108 to 0.093) | RT dominates RP | –454 (–2649 to 1741) |
| Sensitivity analysis 5: excluding men recruited during the feasibility period | ||||||||
| AM | 350 | 5854 (5423 to 6285) | 6.816 (6.644 to 6.987) | |||||
| RT | 334 | 7285 (6844 to 7727) | 6.887 (6.711 to 7.062) | RT vs. AM | 1431 (793 to 2056) | 0.071 (–0.169 to 0.317) | 20,195 | –14 (–4943 to 4915) |
| RP | 347 | 7568 (7134 to 8001) | 6.766 (6.594 to 6.938) | RP vs. RT | 282 (324 to 839) | –0.120 (–0.367 to 0.116) | RT dominates RP | –2692 (–7629 to 2246) |
| Sensitivity analysis 6: discount rates of 1.5% for costs and QALYs | ||||||||
| AM | 370 | 6345 (5889 to 6801) | 7.560 (7.356 to 7.765) | |||||
| RT | 364 | 7618 (7158 to 8077) | 7.696 (7.490 to 7.903) | RT vs. AM | 1273 (563 to 1933) | 0.136 (–0.154 to 0.432) | 9358 | 1447 (–4388 to 7283) |
| RP | 367 | 7745 (7287 to 8202) | 7.476 (7.270 to 7.681) | RP vs. RT | 127 (–471 to 738) | –0.221 (–0.515 to 0.069) | RT dominates RP | –4541 (–10,383 to 1302) |
| Sensitivity analysis 7: day cases costed as outpatients | ||||||||
| AM | 370 | 5774 (5375 to 6173) | 6.976 (6.798 to 7.154) | |||||
| RT | 364 | 7247 (6845 to 7650) | 7.094 (6.915 to 7.273) | RT vs. AM | 1473 (810 to 2059) | 0.118 (–0.153 to 0.368) | 12,531 | 878 (–4204 to 5961) |
| RP | 367 | 7375 (6974 to 7776) | 6.909 (6.730 to 7.087) | RP vs. RT | 128 (–422 to 647) | –0.185 (–0.430 to 0.068) | RT dominates RP | –3830 (–8919 to 1260) |
| Sensitivity analysis 8: excluding TURPs | ||||||||
| AM | 370 | 5814 (5396 to 6232) | 6.976 (6.798 to 7.154) | |||||
| RT | 364 | 7323 (6901 to 7745) | 7.094 (6.915 to 7.273) | RT vs. AM | 1509 (834 to 2106) | 0.118 (–0.143 to 0.374) | 12,829 | 843 (–4243 to 5930) |
| RP | 367 | 7520 (7100 to 7940) | 6.909 (6.730 to 7.087) | RP vs. RT | 197 (–373 to 767) | –0.185 (–0.445 to 0.055) | RT dominates RP | –3899 (–8992 to 1195) |
| Sensitivity analysis 9: excluding annual/research follow-ups | ||||||||
| AM | 370 | 5893 (5475 to 6312) | 6.976 (6.798 to 7.154) | |||||
| RT | 364 | 7330 (6908 to 7752) | 7.094 (6.915 to 7.273) | RT vs. AM | 1436 (790 to 2046) | 0.118 (–0.137 to 0.370) | 12,215 | 915 (–4170 to 6001) |
| RP | 367 | 7495 (7075 to 7915) | 6.909 (6.730 to 7.087) | RP vs. RT | 165 (–402 to 745) | –0.185 (–0.439 to 0.059) | RT dominates RP | –3867 (–8960 to 1225) |
| Sensitivity analysis 10: including data from the procedures section of the annual follow-up schedule | ||||||||
| AM | 370 | 5946 (5526 to 6367) | 6.976 (6.798 to 7.154) | |||||
| RT | 364 | 7391 (6967 to 7815) | 7.094 (6.915 to 7.273) | RT vs. AM | 1445 (798 to 2035) | 0.118 (–0.142 to 0.369) | 12,284 | 907 (–4178 to 5993) |
| RP | 367 | 7552 (7130 to 7975) | 6.909 (6.730 to 7.087) | RP vs. RT | 161 (–413 to 744) | –0.185 (–0.429 to 0.069) | RT dominates RP | –3863 (–8955 to 1229) |
| Scenario analysis 1: outpatient TWOCs costed as day cases, outpatient, non-consultant-led visits for PSAs, inpatient procedures costed at highest complications and comorbidities split, annual/research follow-ups costed at full cost, protocol radiotherapy preparation includes technical support | ||||||||
| AM | 370 | 8525 (7905 to 9145) | 6.976 (6.798 to 7.154) | |||||
| RT | 364 | 9343 (8718 to 9969) | 7.094 (6.914 to 7.273) | RT vs. AM | 818 (–118 to 1670) | 0.117 (–0.122 to 0.368) | 6967 | 1531 (–3569 to 6630) |
| RP | 367 | 9905 (9282 to 10,528) | 6.909 (6.730 to 7.087) | RP vs. RT | 561 (–296 to 1405) | –0.185 (–0.437 to 0.063) | RT dominates RP | –4263 (–9369 to 843) |
| Scenario analysis 2: current treatment costs – protocol radiotherapy costed as IMRT and radical prostatectomies costed as robotic RP | ||||||||
| AM | 370 | 6828 (6363 to 7292) | 6.976 (6.798 to 7.154) | |||||
| RP | 367 | 9417 (8951 to 9884) | 6.909 (6.730 to 7.087) | RP vs. AM | 2590 (1941 to 3247) | –0.068 (–0.327 to 0.195) | AM dominates RP | –3940 (9023 to 1144) |
| RT | 364 | 9476 (9007 to 9944) | 7.094 (6.915 to 7.273) | RT vs. AM | 2648 (1943 to 3312) | 0.118 (–0.137 to 0.374) | 22,519 | –296 (–5391 to 4799) |
Including only data for the first 6 years showed that AM was the most cost-effective treatment at this time point (6 years). Controlling for the date of the information appointment did not affect the results.
The sensitivity analyses also showed that, apart from the current treatment cost scenario, the RP group was always dominated by the RT group. It was also dominated by the AM group, apart from the analyses using the first 6 years of data, in which, although the RP group was more costly, it was also more effective.
The cost-effectiveness analysis of the ProtecT treatment trial showed that at a median of 10 years’ follow-up the AM group was the least costly group and the RT group was the most cost-effective group at the NICE threshold of £20,000 per QALY. There does remain some uncertainty in relation to this result given that there was some uncertainty within the sensitivity analyses and that the probability that the RT group was the cost-effective option at this threshold was only 58%.
The RP group, with the exception of the ‘current treatment costs’ sensitivity analysis scenario, was dominated throughout in that it was slightly more expensive (£7519 vs. £7361) and less effective (7.09 vs. 6.91 QALYs) than the RT group. The cost difference between these two groups was only £159, but the QALY difference was equivalent to a reduction of 67 days in perfect health.
Strengths
This use of individual patient data and the study’s longevity are its core strengths. The use of medical record review in conjunction with a participant visit meant that secondary care missing data were kept to a minimum and are likely to be missing completely at random; missingness did not differ by group.
Limitations
Primary care resource use was not recorded at the beginning of the trial; thus, an appropriate primary care delivery cost was applied to hormone injections prior to radiotherapy and to each PSA test. All other missing primary care data were assumed to be missing completely at random as there was no reason to believe that primary care costs collected from the annual schedules would differentially differ by group over the trial’s duration. Sensitivity analysis 3, which only included participants who were asked about primary care visits, showed that the RT group was cost-effective only at the higher, £30,000-per-QALY, threshold; however, this could be indicative of a shorter period of follow-up in keeping with other sensitivity analyses.
There were missing data in the follow-up pro formas, and reasonable assumptions, often following discussions with nurses and clinicians, were used to impute these data.
There was the potential for incomplete EQ-5D-3L data not to be missing at random, resulting from non-completion of questionnaires due to illness and treatment. Reducing the mean QALY score by 10% for those participants who had mean imputed QALY scores for 1 year of the trial did not affect the overall result (sensitivity analysis 1).
Future work
During this long-term study, different and potentially more expensive treatments (e.g. robotic prostatectomies, IMRT and brachytherapy) have become more prevalent. The most recent costs that allowed for the coding of HRGs onto NHS Reference Costs were used,168 and a sensitivity analysis in which prostatectomies were costed as robotic and radiotherapy was coded as IMRT showed the initial results to be robust; however, future modelling using these data will allow for other treatments to be compared.
Although a NHS perspective is of most interest to decision-makers, patient costs are still important. A cross-sectional analysis that aimed to capture participant costs was carried out at two different time points of the ProtecT study. Future work will include analyses using these data as well as information in relation to indirect patient costs in terms of time off work and time lost for other activities, which was collected in the annual self-completed questionnaires administered to the participants.
Conclusion
At a median 10-year follow-up, AM is the least costly of the three treatment groups. Surgery was dominated by both RT and AM in that it was more expensive and less effective than RT and AM. The ICER comparing the RT group with the AM group was £12,310 per QALY, but there is uncertainty in relation to this result as there is only a 58% chance that the RT group is the cost-effective option at the NICE threshold of £20,000 per QALY.
Chapter 6 Integrated qualitative research
Introduction and design
Following the successful embedding of the ProtecT feasibility study within a qualitative (ethnographic) study of recruitment, qualitative research was integrated throughout the main trial. The qualitative research used a combination of single and serial (repeated) in-depth qualitative interviews to conduct in-depth cross-sectional and longitudinal qualitative research into men’s experiences of screening, diagnosis, treatment and trial participation, with an emphasis on longitudinal data collection to capture how men’s experiences evolved over time during the 5- to 15-year follow-up period. This design allowed targeted insight into key points on the patient pathway through PSA testing, biopsy and diagnosis and into participants’ experiences of treatment side effects over the long term. 176
The aims were to investigate, in dedicated qualitative studies, participants’ experiences in the following key areas:
-
PSA testing, including reasons for accepting or refusing a PSA test
-
diagnostic testing, particularly the standardised protocol of 10-core TRUS-guided biopsies
-
recruitment to the treatment trial
-
outcomes from each of the three study treatments (AM, surgery and radiotherapy) from the point of randomisation or choice of option and longitudinally throughout the follow-up period of up to 14 years
-
taking part in the ProtecT study [also incorporating ProtecT’s version of patient and public involvement (PPI)].
The following sections report on the sampling of the participants interviewed in the qualitative research and the major methods used.
Sampling of core participants
In the original study design, it was the intention to recruit and follow a core set of around 50 men from the time of initial attendance at the PSA testing clinic, through diagnosis and treatment, participation in the treatment RCT or having chosen a treatment, and then regularly over time through to the end of follow-up. However, out of 39 men initially recruited at the time of PSA testing, only seven were later diagnosed with prostate cancer, so the strategy was inefficient. A new recruitment strategy was therefore initiated in 2007 to establish a pool of patients for recruitment among those who had accepted the random treatment allocation or who had chosen a treatment and agreed to be followed up. Purposive sampling was used to include men across the range of ages, cancer risk statuses and socioeconomic backgrounds, whether they accepted randomisation or chose a treatment and from several of the clinical centres. Fifty-nine men who fitted this sampling frame were sent written information about the qualitative interview study, followed by a telephone call to establish willingness to participate. Two men refused, four did not respond and six were ineligible owing to treatment received outside the study or too much time elapsing since treatment started. In total, 47 participants (alongside the seven recruited prior to diagnosis) were thus recruited to the core qualitative interview study and were interviewed several times over a period of up to 14 years (Table 16). As we were unsure whether or not we would capture enough experiences of adverse treatment outcomes with this sample size, the core group was then enriched with a sample of 11 participants identified by study nurses as having reported side effects related to one of the radical treatments or having changed management from AM, and these participants were asked to consent to participate in a single interview (see Table 16).
| Characteristics | Number of participants | |
|---|---|---|
| Accepted random allocation | Chose own treatment | |
| A: participants recruited pre diagnosis (n = 7) | n = 2 | n = 5 |
| Age at referral (years) | ||
| 50–59 | 1 | 2 |
| 60–69 | 1 | 3 |
| Social class | ||
| Managerial/professional | 0 | 3 |
| Other | 2 | 2 |
| Study centre | ||
| 1 | 1 | 2 |
| 2 | 0 | 0 |
| 3 | 1 | 2 |
| 4 | 0 | 0 |
| 8 | 0 | 1 |
| Cancer status: grade and stage | ||
| Low risk | 1 | 4 |
| Intermediate risk | 1 | 1 |
| Primary treatment group | ||
| RT | 0 | 1 |
| AM | 2 | 3 |
| B: serial interview study participants recruited post diagnosis (n = 47) | n = 31 | n = 16 |
| Age at referral (years) | ||
| 50–59 | 10 | 3 |
| 60–69 | 21 | 13 |
| Social class | ||
| Managerial/professional | 14 | 6 |
| Other | 16 | 9 |
| Missing | 1 | 1 |
| Study centre | ||
| 2 | 7 | 2 |
| 3 | 18 | 12 |
| 4 | 6 | 2 |
| Cancer status: grade and stage | ||
| Low risk | 19 | 12 |
| Intermediate risk | 12 | 4 |
| Primary treatment group | ||
| RP | 10 | 7 |
| RT | 11 | 4 |
| AM | 10 | 5 |
| C: participants with adverse experiences taking part in a single interview (n = 11) | n = 4 | n = 7 |
| Age at referral (years) | ||
| 50–59 | 2 | 2 |
| 60–69 | 2 | 5 |
| Social class | ||
| Managerial/professional | 1 | 3 |
| Other | 3 | 4 |
| Study centre | ||
| 2 | 0 | 2 |
| 4 | 2 | 4 |
| 6 | 2 | 0 |
| 9 | 0 | 1 |
| Cancer status: grade and stage | ||
| Low risk | 2 | 6 |
| Intermediate risk | 2 | 1 |
| Primary treatment group | ||
| RP | 1 | 0 |
| RT | 2 | 0 |
| AM | 1 | 7 |
Overall, 88 men were invited to take part in the core qualitative interview study and a total of 65 participants were recruited and had one or more interviews. The characteristics of those interviewed are shown in Table 16. Approval for all qualitative data collection and analysis was obtained from the UK East Midlands (formerly Trent) Multicentre Research Ethics Committee (reference number 01/4/025).
Methods of data collection and analysis
Interviews were conducted either face to face in the location chosen by participants (e.g. home, hospital clinic or university premises) or by telephone if participants preferred or if distance made telephone interviews more practical and participants agreed. Interviews were semistructured, following a topic guide that was individually structured for men according to primary treatment received and derived from a review of existing literature on experiences and beliefs about prostate cancer diagnosis and treatments (see Appendix 2 for an overview of topics covered). Interviewers also encouraged men to introduce topics of relevance to their own personal experience and topic guides were revised during data collection to include issues raised by the participants.
The main method of analysis was the thematic approach, applying the principles of constant comparison to all data. 177,178 Findings were developed iteratively and adjusted as new data were added. All interviews were audio-recorded and transcribed verbatim. Transcripts were read and re-read to code data and identify emerging themes. A subset of interviews in each analysis was coded in detail by at least two researchers. Coding and emerging themes were then discussed and differences in interpretation compared and resolved to enhance the credibility of the findings. Data collection and analysis proceeded iteratively and purposive sampling was used to identify further cases to test emerging hypotheses (e.g. men experiencing negative effects following treatments and men changing from AM to radical treatments). Qualitative data-analysis software (NVivo 10; QSR International, Warrington, UK) was used to organise the large numbers of data collected.
Qualitative studies
In the sections that follow, each of the studies itemised previously (apart from recruitment) is described in terms of the specific background, particular methods employed and participants included, major findings and conclusions.
Experiences of prostate-specific antigen testing
Background
Prostate-specific antigen testing remains controversial, with the most recent study showing no benefits of PSA screening on mortality over 10 years’ follow-up. 36 The European Association of Urology Guidelines179 and the UK National Screening Committee through the Prostate Cancer Risk Management Programme180 recommend that men who request a PSA test are given full information about the relative risks and benefits of screening to enable them to make an informed choice. An understanding of men’s beliefs and experiences about prostate cancer screening and their reasons for attending or not attending screening are needed to inform optimum support services to support decision-making by patients. 181
Methods in the ProtecT study
Men consenting to or refusing PSA testing in the ProtecT study were invited to take part in a single interview study investigating men’s decision-making about whether or not to undergo PSA testing. 116 Interviews were undertaken with men accepting a PSA test or not responding to the invitation for PSA testing. Interviews were conducted face to face or by telephone by Dr Kerry Avery (University of Bristol, ProtecT study group) and lasted for a mean of 34 minutes (range 7–70 minutes) (Table 17).
| Parameter | Accepting PSA test | Not responding to PSA test invite |
|---|---|---|
| Number interviewed (number invited) | 14 (16) | 7 (8) |
| Mean age (years) | 61.5 | 59.5 |
| Mean PSA level (ng/ml) | 1.1 | – |
| Number of centres | 3 | 1 |
Men consenting to take part as core participants in the serial interview study were also invited to recount their reasons for responding to the invitation to take part in the study, whether at the time of the prostate check clinic (n = 7), following random allocation to a treatment (n = 31) or after choosing treatment (n = 16). The data relevant to this topic were combined.
Results
Men who declined the invitation to attend for the PSA test believed that a test was not needed and justified this belief with the view that they mostly perceived themselves to be at low risk of prostate cancer or in good health. Some also believed that prostate cancer was not life-threatening, had been advised against a PSA test (in some cases by a medical practitioner) or considered the PSA test not to be sufficiently accurate. In contrast, those who accepted the invitation to attend for PSA testing reported that they had done so because they felt that they had nothing to lose because they expected a clear result as they were in good health or lacked symptoms. Attenders indicated that a friend or family member had recommended that they attend, in some cases because of a family history of prostate cancer or previous PSA. 116
In conclusion, most men in both groups perceived themselves to be at low risk of having prostate cancer, which was accurate in that 3% were eventually diagnosed.
Experiences of prostate biopsy
Background
Men’s views and experiences of undergoing the standardised 10-core TRUS-guided prostate biopsies were investigated in ProtecT. Previous research, including in ERSPC, suggested that sextant (six-core) prostate biopsy carried a risk of sepsis, pain, bleeding and, rarely, death. 182,183 There was evidence of variation in practice in the use of local anaesthesia during prostate biopsy. 184,185
The experience of pain or other side effects during or after the biopsy procedure was poorly investigated. Two studies suggested that around 18–19% of men refused to undergo repeat biopsy. 185,186 Limited qualitative research found that although most men reported biopsy to be uncomfortable rather than painful, some found it acutely stressful, exhausting or painful. 187 It was suggested in one study that pain was influenced by psychosocial factors. 188
Methods in ProtecT
Experiences of diagnostic testing were explored in two substudies to supplement the quantitative data generated in the Prostate Biopsy Effects (ProBE) study. 189 In the first study, men were purposively sampled to include those agreeing (n = 24) or refusing (n = 13) to undergo prostate biopsy in ProtecT from a range of study centres (Table 18). 116
| Characteristics | Accepted biopsy | Refused biopsy | |
|---|---|---|---|
| Negative biopsy | Prostate cancer diagnosis | ||
| Number interviewed (number invited) | 13 (17) | 11 (13) | 13 (18) |
| Mean age (years) | 64.6 | 61.6 | 63.0 |
| Mean PSA level (ng/ml) | 6.5 | 5.1 | 4.8 |
| Number of centres | 7 | 6 | 8 |
In the second study, men taking part in the quantitative ProBE study189 were invited to participate in a single interview (n = 33), and a subset of men was sampled specifically to contribute their experience of post-biopsy infection (n = 5). These interviews were also supplemented with information about biopsy from the 47 men taking part in serial in-depth interviews (Table 19).
| Characteristics | Participants | |||
|---|---|---|---|---|
| ProBE | ProtecT (n = 47) | All (n = 85) | ||
| No infection (n = 33) | Infection (n = 5) | |||
| Mean age at time of first biopsy (years) | 64.3 | 60.8 | 63.5 | 63.6 |
| Ethnicity (n) | ||||
| White | 33 | 5 | 46 | 84 |
| Other | 0 | 0 | 1 | 1 |
| Centres from which men were sampled (n) | 8 | 2 | 3 | 8 |
| Biopsy result (n) | ||||
| Benign | 12 | 1 | 0 | 13 |
| Localised prostate cancer | 12 | 4 | 47 | 63 |
| Advanced prostate cancer | 9 | 0 | 0 | 9 |
| Interview type (n) | ||||
| Telephone | 18 | 5 | 0 | 23 |
| Face to face | 15 | 0 | 47 | 62 |
Results
The findings showed that most patients tolerated prostate biopsy reasonably well. However, as many as one-quarter reported problematic side effects (such as pain and bleeding) and anxiety. Side effects were more likely to be perceived as being problematic if experiences were not in line with expectations derived from the information provided in preparation for biopsy. Where men were not sufficiently prepared for the experience or severity of side effects following biopsy, they responded by contacting health professionals and asking for reassurance. They also expressed frustration that they were not adequately prepared for these side effects.
The number of patients who refused biopsy when offered it was relatively small. However, those who did refuse tended to do so because they perceived themselves to be at low risk of having cancer, or because they were anxious about the test. The perception of low risk was often associated with not having urinary symptoms.
Conclusions
Both substudies of the experience of biopsy concluded that men were insufficiently informed about the biopsy procedure, its relationship with urinary symptoms and the potential incidence and severity of side effects. 116,190 These qualitative findings were combined with quantitative data from the ProBE189 to propose an updated and evidence-based set of information for men undergoing prostate biopsy to support more informed decision-making about whether or not to attend for a biopsy, what to expect and how to respond to side effects. 190
Experiences of outcome
Background
Secondary outcomes in the ProtecT trial included disease progression, treatment complications, urinary, bowel and sexual function symptoms and impact on QoL, psychological status and general health status. The study design also included qualitative assessment of these outcomes through in-depth interviews, with sampling of participants across the randomised groups and of those choosing a treatment. When the ProtecT main study started data collection in 2001, the literature reporting men’s views and experiences of undergoing testing, diagnosis and treatment for localised prostate cancer was extremely limited. The little research that had been conducted was usually in small cross-sectional studies within a short time of treatment; for example, there were small qualitative studies of men’s short-term experiences of surgery191,192 or radiotherapy. 193
As the strategy of active monitoring/surveillance was only initiated in the late 1990s, at the time of the start of the ProtecT study, studies of this option have only been published in recent years. Some have focused on levels of anxiety and depression, with some suggesting increased levels of distress,45,194 but others, including a systematic review, reported not finding adverse effects on QoL measures. 157,195,196 Qualitative research, again cross-sectional and small scale, revealed that men adopted coping strategies to deal with the uncertainty inherent in a monitoring/surveillance protocol by blocking off the diagnosis or rationalising the level of threat posed or engaging in lifestyle change as a method to counter the cancer. 188,197,198 Therefore, in relation to all outcomes from treatment options, previous qualitative studies were mostly restricted to short-term effects, usually without comparisons or long-term follow-up. In ProtecT, we were able to sample across all treatment options and explore experiences related to each of the treatments.
In a further study to evaluate the management of AM, the views of 22 of the core participants in the qualitative serial interview study were combined with data from 11 urologists and 23 nurses delivering ProtecT trial AM and 20 men, with prostate cancer managed in urology clinics elsewhere in the UK, with in-depth interviews with urologists and three specialist nurses working in these clinics. 199
Methods in ProtecT
Experiences of following AM were explored with 20 men included in the core group of patients who were seen over a period of up to 14 years, supplemented with five more who were identified by study nurses as having changed management to a radical treatment. The characteristics of the men sampled across centres, ages, cancer risk groups and who were randomised to or chose AM are given in Table 20.
| Characteristics | AM group | ||
|---|---|---|---|
| Randomly allocated | Men choosing | Total | |
| Participants in the core serial interview study | N = 12 | N = 8 | N = 20 |
| Age (years) (n) | |||
| 50–59 | 2 | 1 | 3 |
| 60–69 | 10 | 7 | 17 |
| Social class (n) | |||
| Managerial/professional | 5 | 4 | 9 |
| Other | 7 | 3 | 10 |
| Missing | – | 1 | 1 |
| Study centre (n) | |||
| 1 | 1 | 1 | 2 |
| 2 | 3 | 0 | 3 |
| 3 | 6 | 7 | 13 |
| 4 | 2 | 0 | 2 |
| Grade and stage (n) | |||
| Gleason 6, T1 | 9 | 6 | 15 |
| Other | 3 | 2 | 5 |
| Characteristics of men who changed to a radical treatment | N = 5 | N = 4 | N = 9 |
| Number of interviews while on AM, median (range) | 3 (2–6) | 2/3 (1–5) | 2 (1–6) |
| Time (years, months) in follow-up before radical treatment, median (range) | 6, 5 (4, 1 to 13, 9) | 2, 11 (0, 9 to 8, 11) | 4, 1 (0, 9 to 13, 5) |
| Treatment (n) | |||
| Prostatectomy | 1 | 1 | 2 |
| Radiotherapy | 4 | 3 | 5 |
| Number of interviews post treatment (range) | 0–3 | 1–5 | 0–5 |
| Total time (years, months) in follow-up at 10-year median follow-up, median (range) | 7, 9 (7, 4 to 15, 1) | 13, 0 (7, 02 to 15, 6) | 1, 7 (7, 2 to 15, 4) |
| Characteristics of men who remained on AM | N = 7 | N = 4 | N = 11 |
| Number of interviews while on AM (median) | 3 | 3 | 3 |
| Total time (years, months) in follow-up at 10-year median follow-up, median (range) | 7, 6 (6, 8 to 7, 9) | 7, 3 (7, 2 to 7, 5) | 7, 5 (6, 8 to 7, 9) |
| Men who changed from AM to a radical treatment | N = 1 | N = 4 | N = 5 |
| Time in (years, months) follow-up before active treatment, median (range) | 7, 12 | 3, 6 (2, 10 to 5, 4) | 4, 11 (2, 10 to 7, 11) |
| Treatment (n) | |||
| Prostatectomy | 1 | 1 | 2 |
| Radiotherapy | – | 3 | 3 |
Sixteen men recruited to the core serial interview study received radiotherapy treatment, and their experiences of neoadjuvant ADT and RT were explored over a period of up to 8 years post treatment. The characteristics of these men are given in Table 21.
| Characteristics | Study participants (n) | |
|---|---|---|
| Accepted random allocation (N = 11) | Chose treatment (N = 5) | |
| Age at referral (years) | ||
| 50–69 | 4 | 1 |
| 60–69 | 7 | 4 |
| Social class | ||
| Managerial/professional | 6 | 1 |
| Other | 5 | 4 |
| Study centre | ||
| 1 | 0 | 1 |
| 2 | 3 | 1 |
| 3 | 5 | 2 |
| 4 | 3 | 1 |
| Grade and stage | ||
| Gleason 6, T1 | 7 | 3 |
| Other | 4 | 2 |
| Number of interviews | ||
| 2 | 0 | 1 |
| 3 | 11 | 4 |
There were 20 men who were recruited to the core serial interview study who underwent RP (see Table 21). Their experiences were explored over a period of up to 8 years. Two men who were recruited following adverse experiences of surgery were interviewed on one occasion and their data were included in this analysis (one was randomly allocated to surgery and the other chose surgery).
Findings: active monitoring
Men in the core serial interview group took part in up to six interviews over 14 years about their experiences of being assigned to or choosing AM, following the monitoring protocol and decision-making around any change to radical treatments. Their characteristics are summarised in Table 22. A manuscript is under preparation considering the multiplicity of issues for this group; in the manuscript we present the key findings.
| Characteristics | Men receiving nurse-led AM within ProtecT (N = 22)a | Men receiving usual AS protocol outside ProtecT (N = 20) |
|---|---|---|
| Mean age at time of first interview (years) | 64.7 | 65.0 |
| Ethnicity (n) | ||
| White | 21 | 20 |
| Other | 1 | 0 |
| Treatment decision-making (n) | ||
| Random allocation | 12 | NA |
| Chose treatment | 10 | 20 |
| Interview (n) | ||
| Face to face | 33a | 0 |
| Telephone | 49 | 20 |
All participants were aware of the uncertainties inherent in following an AM protocol, namely uncertainty surrounding cancer progression and whether or not monitoring would prove effective in prompting timely curative treatment if needed and desired. They experienced a key contradiction following this pathway: feeling well (many were symptom free) yet with a potentially life-threatening cancer. They employed a range of strategies to minimise the impact of living with these uncertainties and contradictions: seeking to assert control over the disease and the monitoring process, seeking reassurance from health professionals and their wider social networks and contextualising/normalising their prostate cancer. Each PSA test or review in the early stages of participation brought potential anticipation that radical treatment might be needed, and although over time this threat reduced, it did not disappear. Trust was invested in health professionals to maintain the delicate balance between anxiety and reassurance, and to recommend remaining on AM or changing to a radical treatment. The information exchanged in review appointments and the attitudes of clinical staff were pivotal in enabling men to maintain this delicate balance.
Patient-reported outcomes reported in questionnaires showed no evidence of greater anxiety among men randomised to AM than among those randomised to RP or RT at any time up to 6 years. 30 The qualitative research revealed that there was anxiety associated with the uncertainties of living with prostate cancer while on AM but it varied between men, fluctuated over time and was mostly temporary and successfully managed. In this study, men acknowledged uncertainties and most described some anxiety, but their strategies helped to maintain anxiety levels within tolerable limits. Over time, anxiety lessened to some degree as confidence in AM increased or reassurance was gained from trusted health professionals who advised whether to continue or change treatment.
ProtecT trial participants valued nurse-led AM within the study for its flexibility, its accessibility and the continuity of the service. 103 They felt confident about the quality of care. ProtecT consultant urologists and nurses also valued the nurse-led service highly, identifying continuity of care and potential resource savings as key attributes. Clinicians and patients outside the ProtecT trial believed that nurse-led AM could relieve pressure on urology clinics without compromising patient care.
Findings: radiotherapy
Sixteen participants in the core serial interview study recounted their experiences of following the ProtecT radiotherapy protocol, which involved 3 to 6 months of neoadjuvant ADT (hormone therapy) and external beam conformal radiotherapy delivered every weekday over a period of several weeks. Most men took part in three interviews. A manuscript detailing experiences is in preparation, and so only the key findings are presented here.
Men reported impacts on urinary, bowel and sexual function similar to those shown in the patient-reported questionnaires (see Chapter 4). Men considered the possibility that some loss of function was the consequence of normal ageing, and so it was difficult for them to separate out what was the effect of normal ageing and what was the effect of hormone therapy/radiotherapy, especially with regard to fatigue and sexual function. Although some experienced socially embarrassing side effects (urinary and bowel urgency, breast enlargement and hot flushes), most were able to accommodate these over the long term without significant changes to their lifestyle. On the whole, men did not seek help to regain sexual function and many reported prioritising recovery from the prostate cancer and being disease free over sexual function.
Findings: surgery
Eighteen participants in the core serial interview study and one man recruited to take part in a single interview following adverse experiences post surgery were asked about their experiences following surgery. A manuscript including experiences is in preparation, and so only the key findings are presented here. The main concern of men post surgery was urinary control. Several men reported surprise at the degree of incontinence experienced in the immediate period following removal of the catheter, although for most men the level of control improved to a level that allowed them to be able to function normally. A small number experienced significant problems that had a major impact on daily life over the longer term, with two men considering whether or not to opt to have a further procedure to implant an artificial sphincter. An important issue for men experiencing major difficulties with urinary continence was coping with the unknown trajectory of future function. They had not known in advance of surgery what level of function they would experience post surgery, and then post surgery it was also unclear what level of function would be regained.
Those who had difficulties with urinary control explained that this overshadowed other issues, such as sexual difficulties. Those who regained urinary control became more concerned about sexual function difficulties. Contextual issues (e.g. whether or not a man was in a relationship) affected men’s levels of concern about sexual function. Men who were not in relationships expressed more concern about loss of sexual function than those who were in stable relationships.
Conclusions
Appointments with health professionals (primarily nurses, supported by urologists and oncologists as required) to review PSA levels and disease progression were critical for the management of men in the AM group. These appointments enabled and supported men to achieve their particular balance between uncertainty and reassurance, and staying on AM or changing to a radical treatment. The information exchanged in review appointments and the attitudes of clinical staff were crucial in maintaining this delicate balance. The ProtecT AM service protocol is available for implementation. 190
Men undergoing radical treatments felt that they could have been better informed, particularly about the timing of when hormone or radiotherapy started and finished, and also, particularly for surgery patients, about the levels of impairment they might face and for how long it would last. Work is continuing to ensure that the ProtecT outcome data will now be presented to support decision-making.
Experiences of participation in the ProtecT study
Background
It is now standard practice to integrate PPI in trial design, conduct, reporting and dissemination. At the time that the ProtecT study was designed, PPI was not generally integrated in trial designs. In this study, PPI has not been added in the now standard way but was integrated into the design and conduct from the outset using qualitative research methods. It has been highlighted many times as an exemplar of PPI. 200–202 Men who declined randomisation in the feasibility study explained in interviews how the study should be organised, and we incorporated these into the design: the conservative study group was called ‘active monitoring’ rather than ‘watchful waiting’, there were three rather than two groups and nurses were the primary recruiters and reviewers of men’s progress. The participants in the core serial interview study have formed a PPI-like ‘sounding board’ to give views about possible changes to the study, such as e-mail contact would not be acceptable, questionnaires needed to be shorter and that they highly valued the study and contact with research nurses during the follow-up process.
Experiences of trial participation
Men’s experiences of trial participation were collected throughout the study and contributed to improvement of recruitment processes, questionnaire revision and the evaluation of nurse-led AM.
Patient and public involvement consultation in 2016
A PPI consultation was conducted when the median 10-year follow-up outcomes were published. 54 The aim of the PPI consultation was to ask men in ProtecT what information they thought should be provided to patients newly diagnosed with localised prostate cancer regarding possible treatments in order to support better-informed treatment decisions. The aim was to establish what information men are likely to want and how best to present this information.
This consultation involved ProtecT study participants and men with prostate cancer outside the study. Two patient advisory groups (PAGs) were convened in June 2017, comprising:
-
nine ProtecT participants and three female partners
-
two men (who were not ProtecT study participants) from the NIHR Bristol Nutrition Biomedical Research Unit (BRU) prostate cancer PPI group – a well-established local group.
The ProtecT study results newsletter (originally sent to all ProtecT study participants in September 2016) was given to PAG participants to read in advance. Group meetings began with a short presentation summarising the ProtecT study and its key findings. PAG participants then commented on study findings, presented as profiles relating to each treatment. For example, for men aged 60–69 years, profiles showed the risks of experiencing treatment harms (erectile dysfunction, urinary incontinence or bloody stools) and risk of cancer spread and risk of death from prostate cancer or other causes according to allocation group. We asked group participants to comment on how helpful these tables might be to men, in particular for men making decisions regarding treatment for newly diagnosed localised prostate cancer. We asked group participants for their views on what information should be included and how best to present it.
All participants found the study findings reassuring, particularly the number of ProtecT study participants alive 10 years after their prostate cancer diagnosis. One man was concerned that these positive findings might lead to complacency about the threat posed by prostate cancer. Both groups believed that awareness of prostate cancer had grown over the previous 10 years, partly because of media and charity campaigns. Some participants suggested that the impact of treatment side effects needed to be clearer, for example that findings need to be backed up by ‘facts and figures’ and that more information was needed on the impact across ethnic groups. One of the BRU PAG participants felt that it was important to recognise that prostate cancer was not exclusively an ‘old man’s disease’. Participants argued that it was important to be aware that individuals will interpret findings from their own perspective, for example thinking of 1 in 100 men in their own age group. They suggested that messages from the ProtecT study should make clear that findings only included men with localised prostate cancer and referred only to men between the ages of 50 and 69 years at diagnosis. They thought that showing findings relevant to a particular age group (e.g. men aged 50–59 years) would be more helpful than showing findings for all ProtecT participants overall. They agreed that headline findings should make clear that the same favourable survival rates were found for all three treatment groups. Participants discussed their understanding of the terminology used in the reports of study findings, in particular the use of the terms ‘cancer growth’ and ‘cancer spread’ to refer to cancer progression and cancer metastases. Most participants generally understood these terms and found them acceptable. One man who worked with a prostate cancer support group felt more comfortable with the terms ‘progression’ and ‘metastases’, whereas several felt that using all of the terms was useful. Some were uncertain regarding whether or not when prostate cancer spread it always spread to the bones. The numbers in the study experiencing cancer growth and spread were thought to be clear, but use of a diagram to illustrate growth within and spread beyond the prostate gland was found to be very helpful.
Some participants found that use of the word ‘bothersome’ in relation to the impact of treatment side effects diminished their experiences: ‘I think the problems you have after surgery are slightly more than bothersome’. They felt that it would be sufficient to state that side effects experienced were different between groups and to give numbers. One participant described ‘risk’ as an emotive word. Men observed that referring to ‘increased risk’ with regard to cancer growth and spread was unhelpful if not accompanied by numbers giving the difference in risk, for example stating that the risk was ‘doubled with AM and the original risk is 1%, you’ve [only] got a 2% chance’. Others argued that the figures ‘speak for themselves’. Participants highlighted that there had been changes in treatments and technology, such as the development of robotic surgery. Group members argued that this had an impact on the way that men interpreted the findings of ProtecT and the way that they considered future treatment choices for men diagnosed with localised prostate cancer, and so they thought that information in relation to these changes would be needed.
The PAG participants were also asked to indicate what they felt was the best way of providing information to men diagnosed with localised prostate cancer to support treatment decision-making. They varied considerably in their views on the format and content of the outcome profiles. Some argued that profiles were helpful, that it was important to have ‘facts and figures’ about possible side effects to help men with treatment decision-making and that presenting data on side effects ‘brings home quite dramatically what the key problems are’. They were reassured about the credibility of the data presented as data came from the ProtecT study, which was perceived as a trusted source. Others felt that there was ‘too much’ information, with one of the patients’ partners commenting that they would ‘immediately switch off, because numbers mean nothing to me’ and that a ‘picture’ would be better.
Participants commented on the presentation of side effects. One man argued that it was important to include the need for pads as this represented a significant ‘lifestyle change’ and also to include data on erectile dysfunction, but noted the lack of information on psychological impact.
In terms of the delivery of information, some participants felt that although written profiles were helpful, it was important that any such information was presented by a qualified health professional, such as a clinical nurse consultant, who could talk through the data and answer questions. Men could then make an ‘informed choice’. One participant explained that he would rather ‘leave it to the expert’ to give him advice on the best treatment for him, ‘not look at a sheet of figures’.
They considered that the timing of information-giving was important, with men likely to be very anxious following diagnosis and finding it difficult to retain information: ‘at the beginning, too much – information overload’.
The PAG participants were shown existing examples of information that used visual representations to convey messages and they were asked to comment on the formats. One BRU group member commented that using symbols such as smiley faces would be more appropriate for conveying information to children or people with learning difficulties. Some group members found it difficult to comment on examples that were not relevant to their own experience; others felt that cancer progression could be usefully explained using visual aids. There was a recognition that people have ‘different ways of absorbing data’ so that ‘a graph like this might be good for some people, straight pictures for others and others might like a good description’ and that any presentation of information should cover all of these options.
Chapter 7 Epidemiological and translational research
Biorepository
As described previously, ProtecT is the active intervention group of the CAP screening study and is itself a randomised trial of treatment. Within ProtecT, we have tissue [formalin-fixed, paraffin-embedded (FFPE) material] and blood samples [plasma, serum and germline deoxyribonucleic acid (DNA)] from 2500 patients with prostate cancer, blood from 60,000 controls, plus full demographic, lifestyle, dietary and epidemiologic data. One of the reasons why ProtecT is important is that the PSA history is so well documented, that men were recruited in the community and that treatment decisions were based on randomisation. Consequently, and uniquely, the future use of the ProtecT biorepository will allow investigation of whether or not a given biomarker (or biomarkers) could predict response to a particular treatment and better outcomes (fewer deaths and/or fewer men treated). This differs from a usual cohort study, which can only predict outcome within a given treatment plan. 42,203
Risk stratification of prostate cancer: the unmet need
Accurate stratification of risk at the time of diagnosis is problematic for several reasons. There may be staging errors on MRI, biopsies may not always provide a reliable estimate of the risk of the cancer132–134,204–207 and PSA is not a reliable marker of metastasis, particularly at levels below 20 ng/ml. We need better methods of stratifying risk of prostate cancer, particularly for men at low and intermediate risk, so that we can advise men about appropriate treatment. As noted in earlier chapters, such methods will rely on well-annotated biorepositories derived from RCTs to provide samples for development and validation of biomarkers. Even for men at high risk, we cannot accurately identify those who require multimodal treatment, although some men benefit from early docetaxel (Taxotere®, Sanofi-Aventis) treatment. 208
Recent translational integrative genomic studies demonstrate that prostate cancer can be categorised into subgroups209,210 that may respond to therapy in different ways. Our contention is that the ProtecT biorepository addresses the unmet need of providing samples with the right context and annotation to allow better biomarkers to be developed.
ProtecT is the largest RCT on the management of localised prostate cancer that compares surgery, radiotherapy and active monitoring/surveillance, which was designed using robust epidemiological and statistical approaches. ProtecT is unique because it has randomised men recruited from the community between three treatments and because it has generated a bioresource from asymptomatic, community-based men whose prostate cancer was diagnosed following PSA testing and whose PSA history is fully understood and documented. In the UK generally, opportunistic PSA testing is uncommon (≈8% of all men of that age). 38 As a consequence of randomisation, ProtecT differs from cohort studies used to develop and test biomarkers. A cohort study might allow analyses to show that a marker predicts mortality and outcome within treatment groups. However, ProtecT would allow investigation of the more important question of whether or not using that marker (or markers) to determine if a particular treatment would have led to better outcomes (fewer deaths and/or fewer men treated). It should provide precision genomic or protein biomarkers to assist men and their clinical advisors to choose the best individualised approach to management.
In addition, within ProtecT we have large numbers of community-based cancer-free controls who also add significant value to the repository. These controls have been flagged with the Office for National Statistics (ONS) to determine their cause of death and with the NHS Digital to determine details of any cancer diagnosis and treatment via the appropriate cancer registries. We estimate that ≈5% of these 60,000 men (≈3000) will eventually be diagnosed with prostate cancer, particularly those with a PSA level of between 1 µg/l and 3 µg/l. At the time of writing, 1391 of these men had been diagnosed with prostate cancer (126 had an initial PSA level of < 1 µg/l, 780 had an initial PSA level of 1–3 µg/l and 485 had an initial PSA level of > 3 µg/l but a negative biopsy) and 97 of them had died and had prostate cancer reported as the immediate or underlying cause of death (16 had an initial PSA level of < 1 µg/l, 62 had an initial PSA level of 1–3 µg/l and 19 had an initial PSA level of > 3 µg/l but a negative biopsy). Over 80% of this cohort of control men who later developed prostate cancer have baseline blood samples that are included in our biorepository.
Advanced cases of prostate cancer excluded from the ProtecT trial
A further 479 men in ProtecT were diagnosed with locally advanced prostate cancer and so were excluded from the randomised treatment trial. This is reported in more detail later in this chapter. These advanced cases are very important because these men were diagnosed months or years earlier than they would have been without PSA testing, being asymptomatic at the time of diagnosis. They were thus able to receive treatment earlier than those presenting with clinical symptoms. To our knowledge, no other trials have published data on such men. Figure 21 shows the outcome of this important group over 10 years, compared with a comparison group of patients with locally advanced prostate cancer from the National Cancer Registration Office (Eastern Office). Over 300 of these men have samples in the ProtecT biorepository.
FIGURE 21.
Survival in ‘advanced’ cases compared with a matched cohort from the Anglia Cancer Registry. 211 3A prostate cancer specific survival. ACN, Anglia Cancer Network.
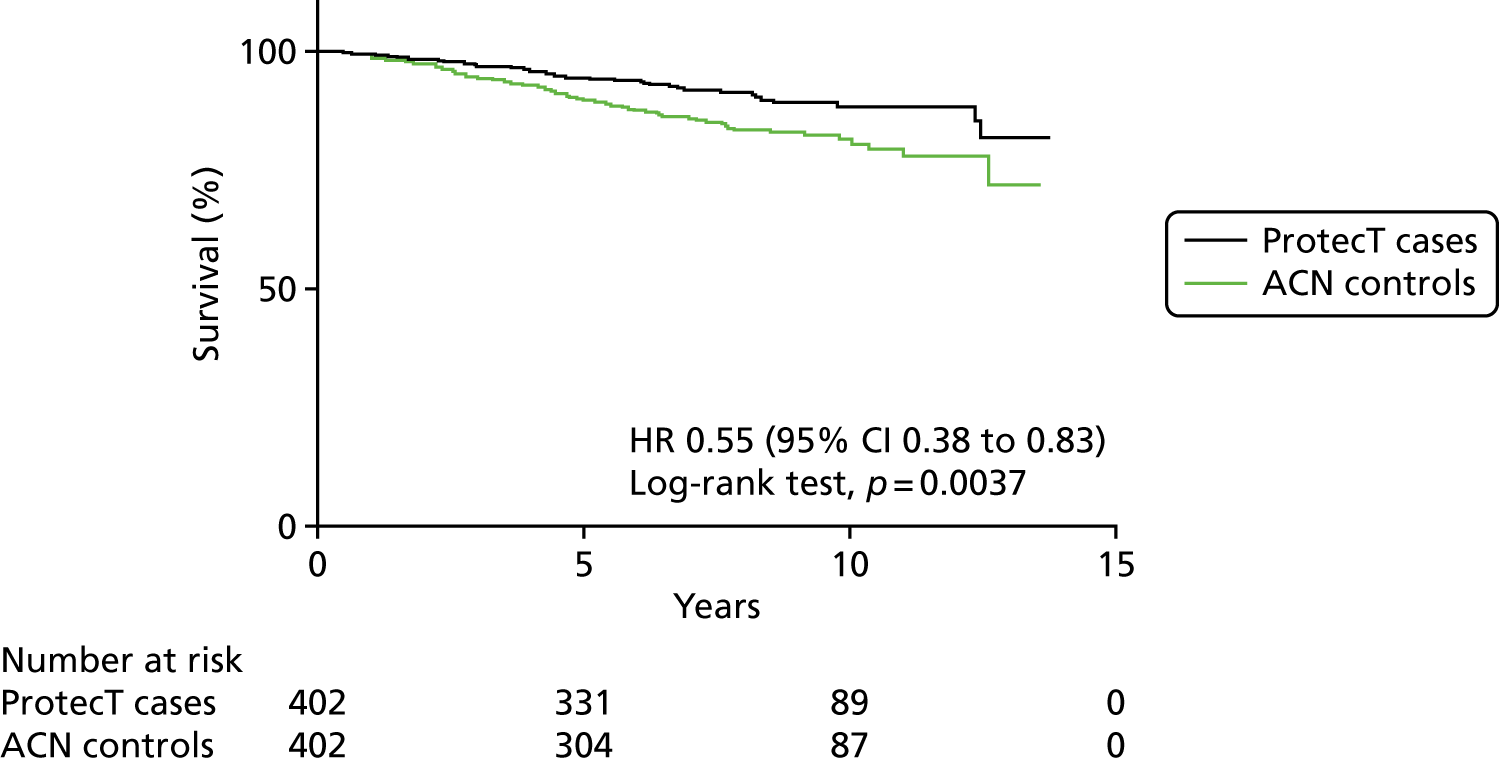
What fluid samples do we have?
The following minimum samples were collected at baseline venepuncture, with up to 50 ml of blood taken and samples being stored at the University of Oxford:
-
× 1 whole blood for germline DNA (≈2.5 ml)
-
× 1 serum sample (≈4 ml)
-
× 2 plasma (× 2 EDTA and × 2 heparin plasma) (≈4 ml each)
-
urine for proteins and cells (50 ml) – the majority taken only during follow-up.
As noted above, baseline samples (whole blood, two plasma samples and one serum sample) were obtained for over 3000 cases in ProtecT (including the excluded men and those with advanced prostate cancer). For baseline samples, three of the four tubes still required aliquoting (serum × 1, plasma × 2) as most of the whole blood in the samples that were stored in Cambridge [by Thermo Fisher Scientific (Waltham, MA, USA)] had been converted already to germline DNA. For the 3000 cases, there are about 9000 tubes of samples.
For the 60,000 control men, ≈14,000 men have had whole blood converted to germline DNA, and there are 180,000 tubes of serum and plasma samples. There are four baseline samples on 1000 additional control men who subsequently developed prostate cancer during the 10-year follow-up period. In ProtecT, we have additional follow-up serial sample sets on the majority of men (with an average of five serial sample sets in ProtecT men; ≈37,000 plasma and serum samples over a 10-year period), which have been collected. There are also 13,500 follow-up urine samples for ProtecT cases.
In ProtecT, 2694 individual men with prostate cancer donated baseline samples that have been aliquoted. A further 417 men who donated baseline samples remain to be aliquoted. Over 8000 tubes from participants with prostate cancer and 13,000 tubes from control participants have been aliquoted. Germline DNA has been extracted from ProtecT cases (2505 men) and controls (13,844 men), and aliquoted into 96 well plates.
Method of processing of samples
The sample processing laboratory incorporates automated liquid-handling robots, a –80 °C freezer store and liquid nitrogen storage. Barcoded, standardised input serum and plasma samples are thawed as per relevant SOPs and aliquoted into final working volumes by sample-formatting robots. Each sample is barcoded with a unique Cryo-Safe number and automatically logged by the sample-formatting robot into the Prostate Cancer: Mechanisms of Progression and Treatment (ProMPT) sample tracking database so that there is a full audit trail from venepuncture at clinic to use in the laboratory. Whole-blood samples are frozen and stored in vacutainers until they are required for extraction (Figure 22).
FIGURE 22.
Method of sample processing.
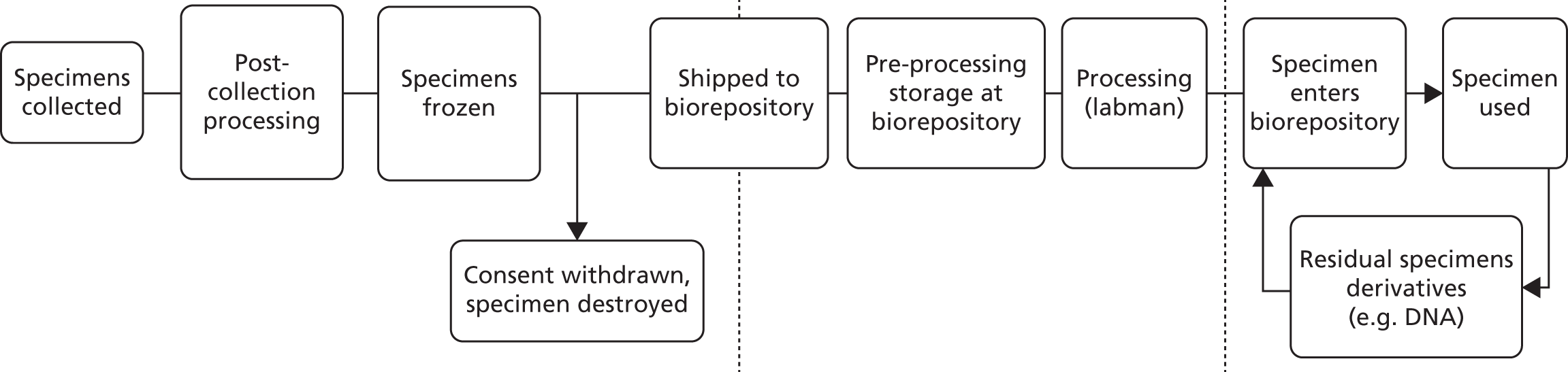
The availability of the samples is coded by a ‘traffic light system’ according to value and amount:
-
red (≤ 10 tubes of 110 µl)
-
amber (11–20 tubes of 110 µl)
-
green (≥ 21 tubes of 110 µl).
Quality control and standard operating procedures for stored materials
Each stage of sample collection, processing and storage is standardised and quality assured by an internal quality management system encompassing all relevant quality documentation, such as policies, SOPs, risk assessment and COSHH (Control of Substances Hazardous to Health Regulations). Audits of the sample collection are carried out to a predetermined schedule. In Oxford, samples derived from blood or urine are kept in cryogenic (–80 °C) freezers. Each freezer is supported by an around-the-clock temperature monitoring and reporting system, and the facility has an automatic switch system generator that is capable of running for 72 hours in the event of power failure. Biorepository staff are on hand 365 days a year to attend emergency freezer call-outs and there are dedicated emergency freezers available for the transfer of samples from failed units. In Cambridge, the samples are stored by Thermo Fisher Scientific at the Bishop’s Stortford site, but these are then transferred to Oxford in the near future. Thermo Fisher Scientific is the leading provider of biospecimen storage and biobanking services, storing over hundreds of millions of samples in more than 20 global repositories. Thermo Fisher Scientific complies with current Good Manufacturing Practices (cGMPs), Good Tissue Practices (GTPs) and Good Laboratory Practices (cGLPs). Cold storage units in the biorepository are monitored at all times. The quality assurance department certifies the temperature probes to National Institute of Standards and Technology (NIST) traceable standards every 6 months to ensure correct temperature readings. Thermo Fisher Scientific also meet US Food and Drug Administration (FDA) requirements for temperature compliance and has data to show that materials have been maintained at appropriate temperatures. Should the temperature of any unit deviate from its acceptable range, the monitoring system will automatically notify repository personnel or on-call staff, day or night. The exterior perimeters are typically monitored by video cameras. The facilities are protected from fire by sprinkler systems that automatically call the local fire station on activation.
Tissue resource
Our collaboration includes strong and long-standing input from the pathology subgroup of ProtecT (chaired initially by Dr Mary Robinson, now by Dr Jon Oxley). There was also strong input from two histopathologists in Oxford and Cambridge (Dr Clare Verrill and Dr Anne Warren). The FFPE histopathological material is being centralised. The material from the FFPE material from the RP samples can be used for both tissue microarrays and extracting DNA. We have developed methods for processing fresh tissue from radical samples, which has already been shown to be of great use in terms of next-generation sequencing approaches that have led to high-impact papers. This has been fully described previously,212,213 and is shown in brief in Figure 23. This careful approach has allowed multiple samples to be taken from each prostate and allowed preservations of margins for routine pathological assessment as well as appropriate tissue for the extraction of nucleic acids. These have been used in recent high-impact publications. 209,210
FIGURE 23.
Tissue sampling from fresh RP specimens. The prostate is shown intact (A) and inked (B). It was then sliced using a parallel-blade device (C). Multiple punch biopsies were removed and the sites of the punched cores marked on a ‘map’ diagram (D and E), leaving margins intact. The holes in the transverse slice were inked, using two coloured inks in a random pattern that was recorded on the map to enable easy identification. The cylindrical cores of tissue were placed onto individual, pre-numbered foil squares and snap frozen in liquid nitrogen before transferring immediately to pre-cooled vials and stored at −80 °C (F). The fresh slice and other portions were pinned onto a cork board to avoid warping during subsequent fixation (G). Republished with permission of Wiley Periodicals, Inc., from Method for sampling tissue for research which preserves pathological data in radical prostatectomy, Warren et al. ,212 Volume 73, edition 2, © 2012; permission conveyed through Copyright Clearance Center, Inc.
Data-sharing policies and Material Transfer Agreements
The biorepository contains demographic, clinical, specimen and scientific data. Even though the final data set will be stripped of personal identifiers prior to release for sharing, we believe that there remains the possibility of deductive disclosure of patients with unusual characteristics. Thus, we will make the data and associated documentation available to users only under a data-sharing agreement that provides for (1) a commitment to using the data only for research purposes and not to identify any individual participant, (2) a commitment to securing the data using appropriate computer technology and (3) a commitment to destroying or returning the data after analyses are completed.
Applications for accessing samples from the biorepository will be assessed by an independent scientific advisory group. Once the samples have been analysed, investigators will be required to upload the data into the ProMPT/ProtecT database. This will include genomic data or other high-throughput data. Such data will be made available to other academic investigators.
Studies of men with advanced disease and excluded men
We have published two recent papers on men with advanced disease who were excluded from the main trial. 95,211 The abstract and summary of the findings of the men with advanced disease are given in this section; Figures 24–26 show the outcomes at 10 years.
FIGURE 24.
Diagram of patient flow through the study of the men with advanced disease. KM, Kaplan–Meier. Reproduced with permission from Johnston et al. 211 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license. See: http://creativecommons.org/licenses/by-nc-nd/4.0/.
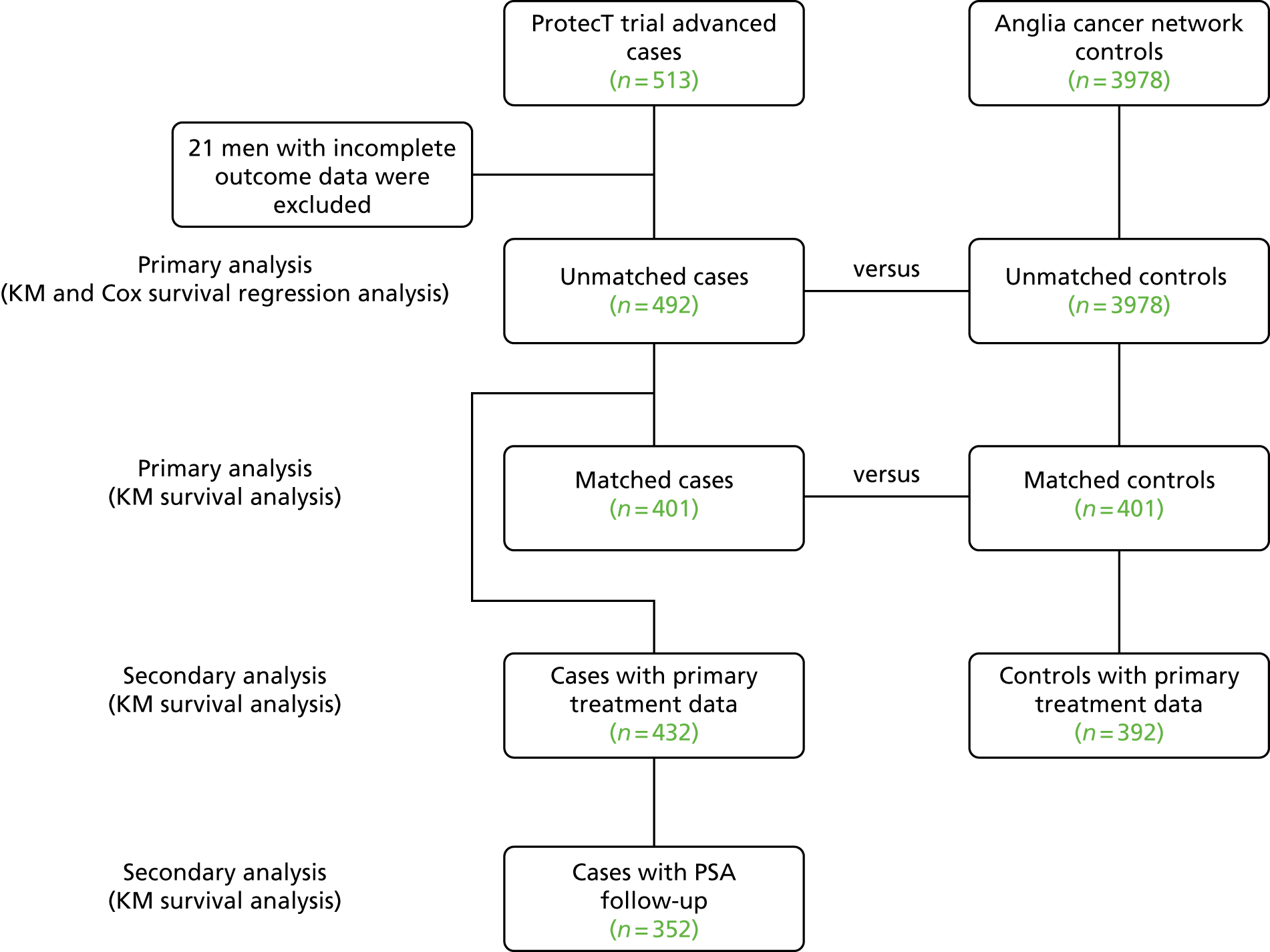
FIGURE 25.
(a) Prostate cancer-specific survival and (b) overall survival according to primary treatment groups among ProtecT cases. Reproduced with permission from Johnston et al. 211 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license. See: http://creativecommons.org/licenses/by-nc-nd/4.0/.
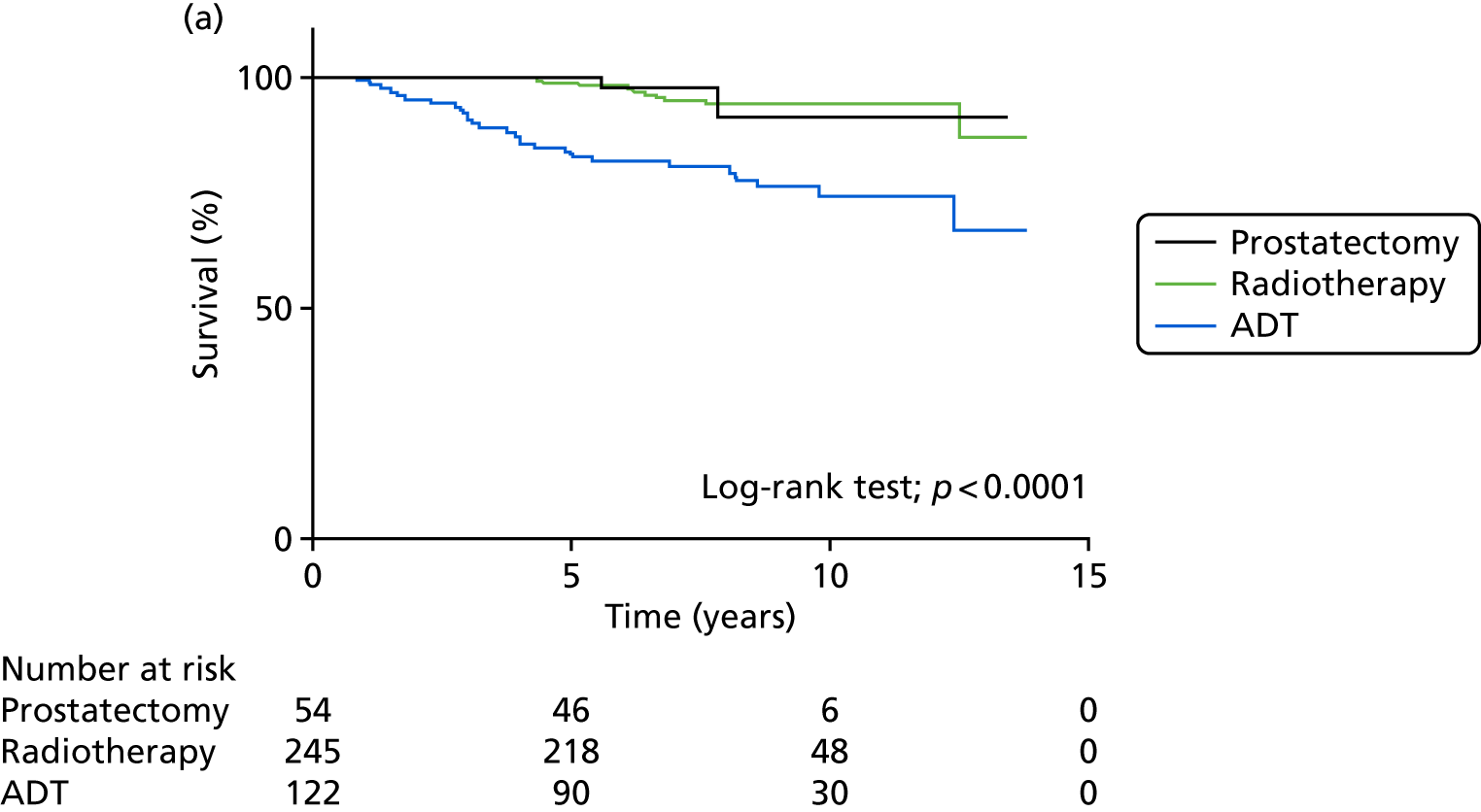
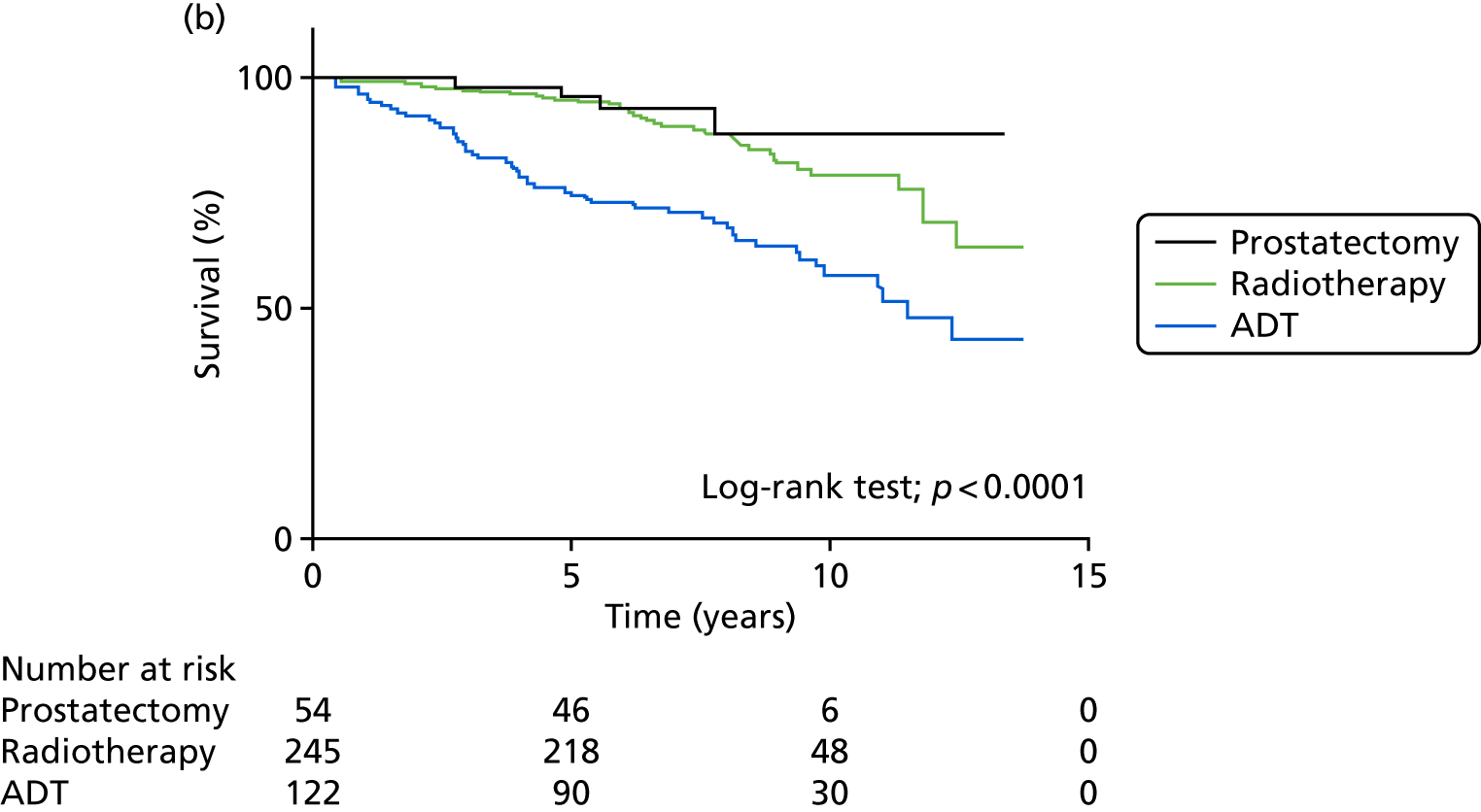
FIGURE 26.
Kaplan–Meier plots of (a) prostate cancer-specific survival and (b) overall survival among matched ProtecT cases and ACN controls. ACN, Anglia Cancer Network. Reproduced with permission from Johnston et al. 211 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license. See: http://creativecommons.org/licenses/by-nc-nd/4.0/.
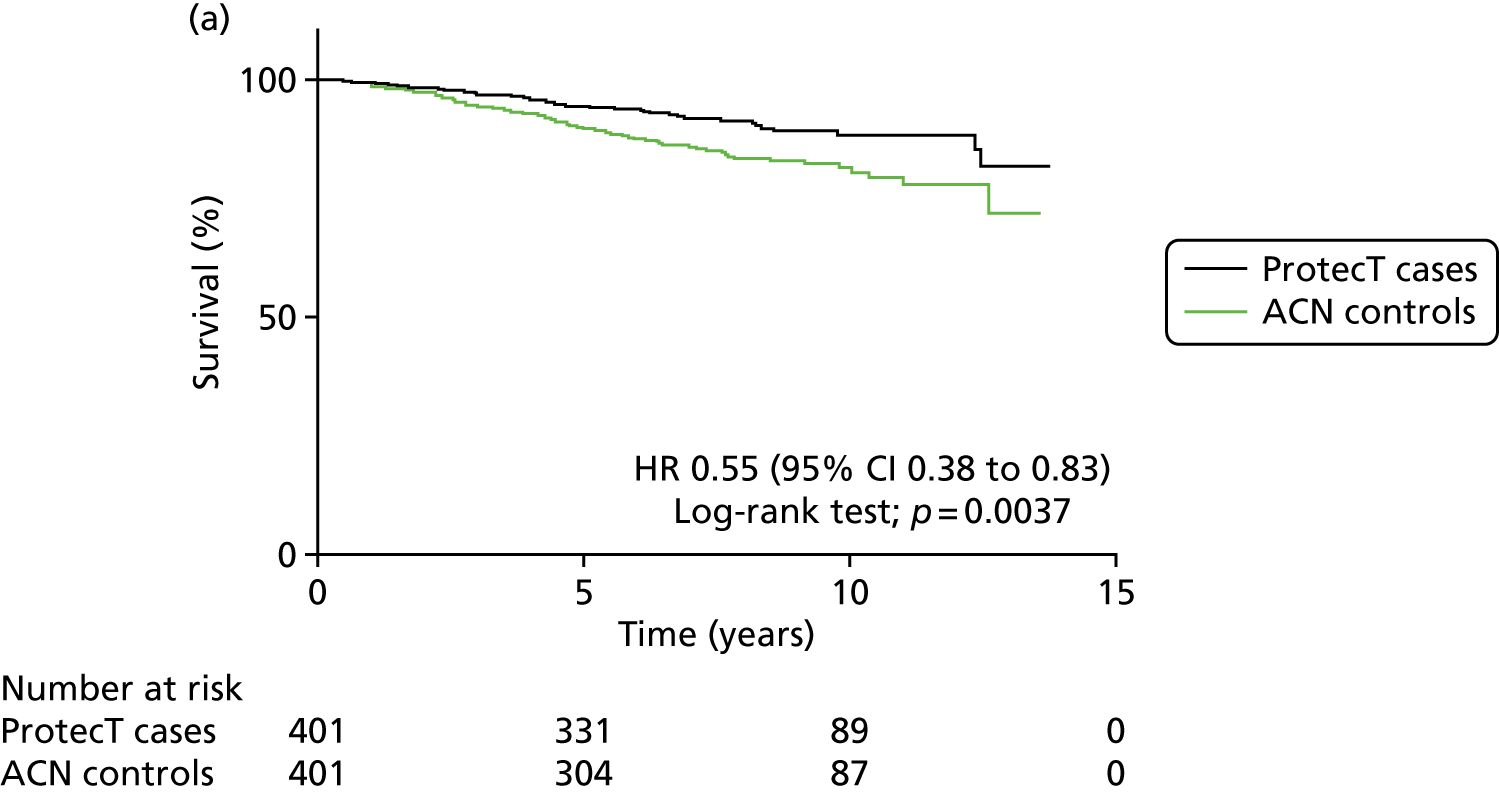
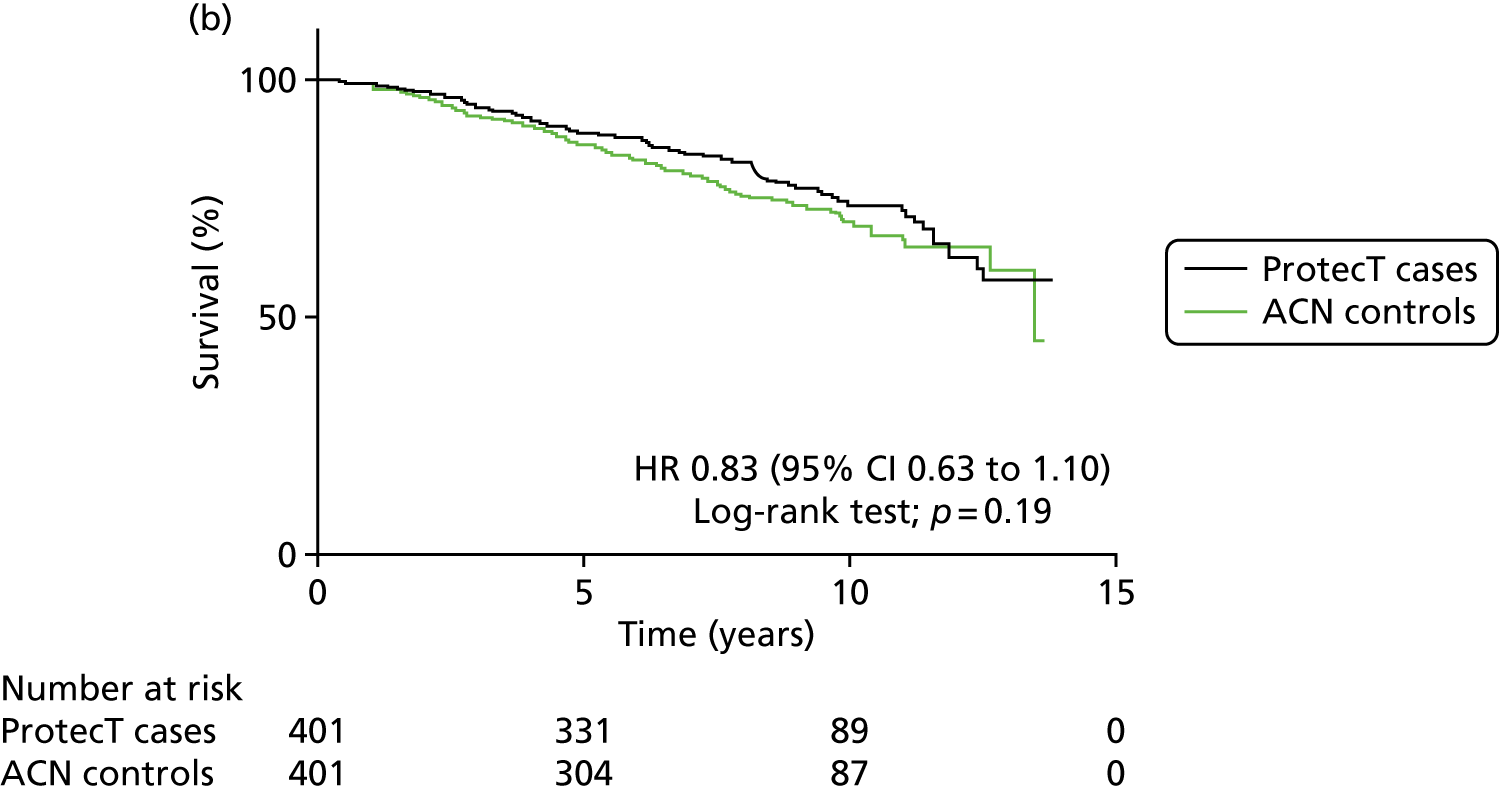
Abstract
Reproduced from Johnston et al. 211 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license. See: http://creativecommons.org/licenses/by-nc-nd/4.0/.
Background: Early detection and treatment of asymptomatic men with advanced and high-risk prostate cancer (PCa) may improve survival rates.
Objective: To determine outcomes for men diagnosed with advanced PCa following prostate-specific antigen (PSA) testing who were excluded from the ProtecT randomised trial.
Design, setting, and participants: Mortality was compared for 492 men followed up for a median of 7.4 yr to a contemporaneous cohort of men from the UK Anglia Cancer Network (ACN) and with a matched subset from the ACN. Outcome measurements and statistical analysis: PCa-specific and all-cause mortality were compared using Kaplan–Meier analysis and Cox’s proportional hazards regression.
Results and limitations: Of the 492 men excluded from the ProtecT cohort, 37 (8%) had metastases (N1, M0 = 5, M1 = 32) and 305 had locally advanced disease (62%). The median PSA was 17 mg/l. Treatments included radical prostatectomy (RP; n = 54; 11%), radiotherapy (RT; n = 245; 50%), androgen deprivation therapy (ADT; n = 122; 25%), other treatments (n = 11; 2%), and unknown (n = 60; 12%). There were 49 PCa-specific deaths (10%), of whom 14 men had received radical treatment (5%); and 129 all-cause deaths (26%). In matched ProtecT and ACN cohorts, 37 (9%) and 64 (16%), respectively, died of PCa, while 89 (22%) and 103 (26%) died of all causes. ProtecT men had a 45% lower risk of death from PCa compared to matched cases (hazard ratio 0.55, 95% confidence interval 0.38–0.83; p = 0.0037), but mortality was similar in those treated radically. The non-randomised design is a limitation.
Conclusions: Men with PSA-detected advanced PCa excluded from ProtecT and treated radically had low rates of PCa death at 7.4-yr follow-up. Among men who underwent nonradical treatment, the ProtecT group had a lower rate of PCa death. Early detection through PSA testing, leadtime bias, and group heterogeneity are possible factors in this finding. Patient summary: Prostate cancer that has spread outside the prostate gland without causing symptoms can be detected via prostate-specific antigen testing and treated, leading to low rates of death from this disease.
Death from prostate cancer occurred in two (4%) of the RP and 12 (5%) of the RT group (HR 0.95, 95% CI 0.22–4.12; p = 0.94). Death from all causes occurred in four (7%) of the RP and 37 (15%) of the RT group (HR 0.69, 95% CI 0.29–1.67; p = 0.41). A significantly greater proportion of the ADT group died from prostate cancer (n = 27, 22%) and all causes (n = 49, 40%) compared to men treated with radical therapy (p < 0.0001). RP = radical prostatectomy; RT = radical radiotherapy; ADT = androgen deprivation therapy; HR = hazard ratio; CI = confidence interval.
By the end of the study, 37 matched cases (9%) and 64 controls (16%) died from prostate cancer. Death from all causes occurred in 89 cases (22%) and 103 controls (26%). HR = hazard ratio; CI = confidence interval.
In summary then, this paper has added significantly to our understanding about what happens following a single round of PSA testing in a community which essentially had not previously been exposed to such testing. The advanced men have done extremely well from this early detection and achieved good survival rates.
Comprehensive cohort
Another recent paper reviewed the whole cohort of men. 95 The abstract is presented here and has been reproduced from Donovan et al. 95 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license. See: http://creativecommons.org/licenses/by-nc-nd/4.0/.
Objectives: Randomised controlled trials (RCTs) deliver robust internally valid evidence but generalizability is often neglected. Design features built into the ProtecT RCT of treatments for localised prostate cancer (PCa) provided insights into its generalizability.
Study Design and Setting: Population-based cluster-randomisation created a prospective study of PSA-testing and a comprehensive-cohort study including groups choosing treatment or excluded from the RCT, as well as those randomised. Baseline information assessed selection and response during RCT conduct.
Results: The prospective study (82,430 men PSA-tested) represented healthy men likely to respond to a screening invitation. The extended comprehensive-cohort comprised 1,643 randomised, 997 choosing treatment, and 557 excluded with advanced cancer/comorbidities. Men choosing treatment were very similar to randomised men except for having more professional/managerial occupations. Excluded men were similar to the randomised socio-demographically but different clinically, representing less healthy men with more advanced PCa.
Conclusion: The ProtecT RCT’s design features provided data to assess the representativeness of the prospective cohort and generalizability of the RCT’s findings. Greater attention to collecting data at the design stage of pragmatic trials would better support later judgements by clinicians/policy-makers about the generalizability of RCT findings in clinical practice.
Figure 27 shows how the cohort was developed and how the excluded men and men with advanced disease fit into the whole group.
FIGURE 27.
Description of the extended comprehensive cohort in ProtecT.
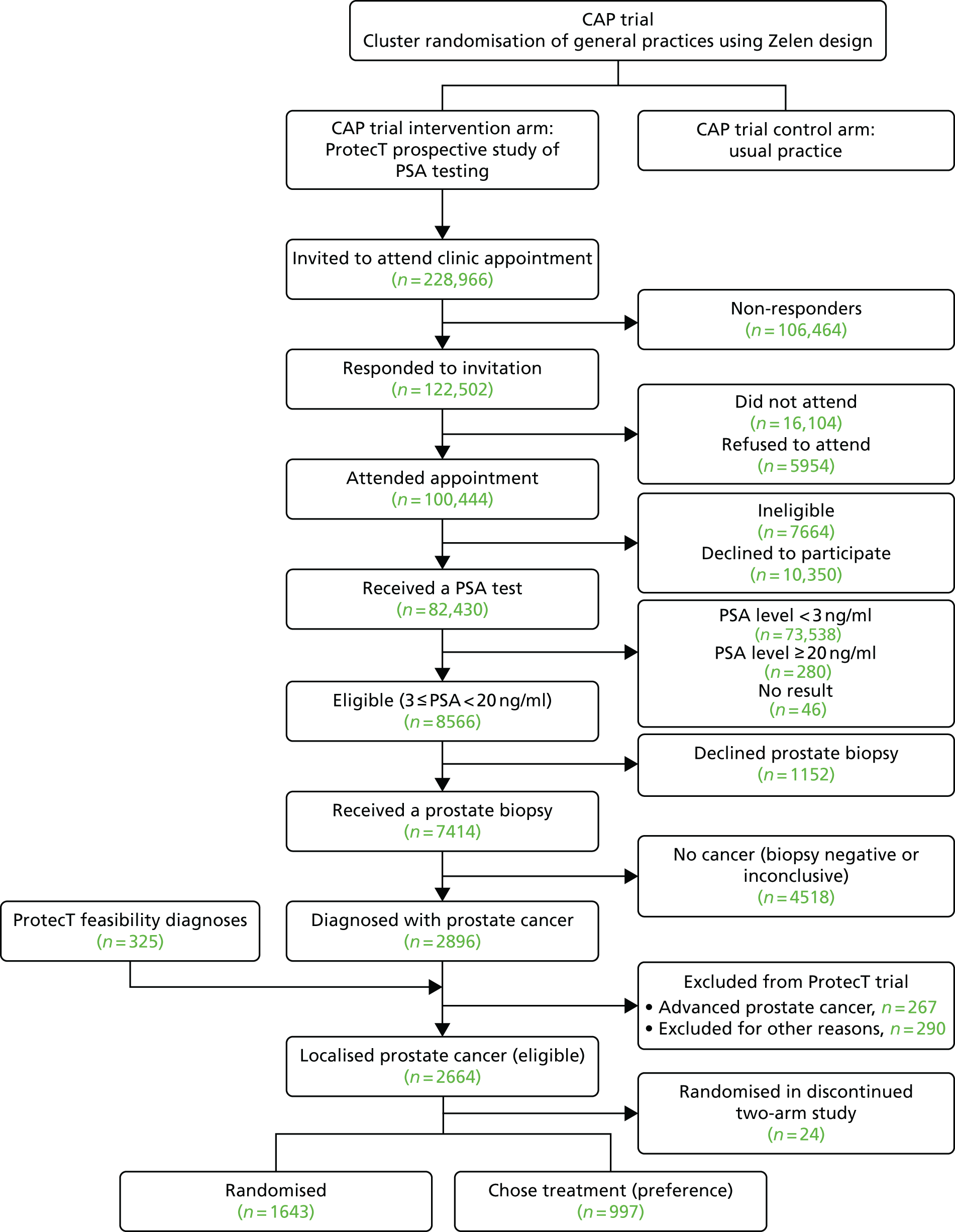
Importantly, this paper highlighted the following points:
-
Decisions taken when designing the ProtecT prostate cancer treatment and linked CAP screening RCTs enabled the collection of data to assess the representativeness of the prospective study of PSA testing and generalisability of the ProtecT RCT’s findings.
-
Adding the extended comprehensive-cohort study comprising all participants diagnosed with prostate cancer, including those who chose a treatment or were ineligible for the RCT, with advanced cancer or comorbidities as well as those randomised in ProtecT, allowed the assessment of the generalisability of the trial’s findings to patients diagnosed with prostate cancer in routine care.
-
Aspects of the generalisability of pragmatic RCTs can be evaluated through initiatives in the design phase, such as assessing factors associated with participation at various stages through a preceding prospective study and/or collecting data from those choosing treatments or who were excluded from the trial according to eligibility criteria, although these decisions will have time and resource implications.
-
Including an innovatively extended comprehensive-cohort study of all men diagnosed with a condition such as prostate cancer can enable assessment of important similarities and differences between the randomised group and those who choose a treatment in standard practice or with aspects of advanced cancer or comorbidities that preclude trial participation, thus providing insights about the RCT’s applicability to patients in routine practice.
-
Greater attention should be devoted at the design stage of pragmatic RCTs to ensure that appropriate data are collected to support later judgements by clinicians and policy-makers about the generalisability of the RCT’s findings to patients in routine clinical practice.
Genetic studies
These have been a very productive aspect of the ProtecT trial, in which whole blood was stored and germline DNA was extracted. The collaboration with Professor Ros Eeles, Institute of Cancer Research, London, has been a critical aspect of this. 214–251
There have been over 40 publications (one cited 570 times and marked as a ‘highly cited paper’ by Web of Science), with seven papers cited over 100 times each. Samples from the ProtecT trial participants have significantly added to our knowledge about genetic predisposition for prostate cancer.
So far as germline genetics is concerned, a strong family history of prostate cancer roughly doubles the risk in first-degree relatives. Previous studies have shown that there are a large number of moderate- to high-risk loci, which predispose to prostate cancer development. Recent studies have identified common single-nucleotide polymorphisms (SNPs), which confer an increasing risk of prostate cancer with an increasing number of risk alleles. There is some recent evidence that germline mutations in DNA repair genes [e.g. BRCA1 (breast cancer gene 1) and BRCA2 (breast cancer gene 2)] predisposes to high risk and metastatic prostate cancer. Over 100 SNPs have been found, which account for ≈33% of all genetic prostate cancer risk. 252 Rare, but high-risk, SNPs occur in HOXB13 (homeobox B13), BRCA1 and BRCA2. However, many of the SNPs occur in intronic areas of the genome and may be more concerned with transcriptional regulation than with structural changes in messenger ribonucleic acid (mRNA) and proteins. Other SNPs are found in the promoter regions of genes, such as those in MSMB (beta-microseminoprotein) or PSP94 (prostate Secretory protein of 94 amino acids), which results in altered tissue-specific regulation of a protein. In addition, SNPs account for a significant impact on the expression and secretion of PSA, where it is thought that ≈44% of the variation in serum PSA may be the result of genetic variation.
One important aspect of this has been the availability of DNA from men with low PSA levels as ‘extreme controls’. As the outcomes of the ProtecT trial become available (along with the incidence and death rate from prostate cancer in the control participants with low PSA levels), these studies will become more meaningful in determining the impact of genetic predisposition on prostate cancer outcomes. It is true that ‘attribution bias’ resulting from prostate cancers only being diagnosed because of a raised PSA level is an issue in the ProtecT trial.
Biomarker studies
Biomarker measurement can include a number of different approaches, including the following:
-
germline genetics, as discussed previously
-
liquid protein and nucleic acid (circulating cell-free DNA) markers in blood and urine
-
tissue-based biomarkers using nucleic acid extraction or protein immunohistochemistry
-
imaging-based biomarkers.
Good reviews of the role of biomarkers in the diagnosis and management of prostate cancer have been published in recent years. 252–256 The studies using the ProtecT biorepository have thus far been productive, with many of the papers resulting from a longstanding collaboration with Richard Martin and colleagues in the University of Bristol. 84,199,238–240,249,257–295
A theme of this work has been to look through the lens of Mendelian randomisation with a focus on the insulin-like growth factor (IGF) axis, the vitamin D axis and also PSA variants. In brief, the top-cited outcomes include:
-
Vitamin B12 and (in cohort studies) folate were associated with increased prostate cancer risk. 282
-
Lower 25(OH)D (calcifediol) concentrations were associated with more-aggressive cancers (advanced vs. localised cancers and high vs. low Gleason grades), but there was no evidence of an association with overall prostate cancer risk. 273
-
Diabetes mellitus was associated with a reduced risk of prostate cancer (OR 0.78, 95% CI 0.61 to 0.99). There was weak evidence that the inverse association was greater for well-differentiated cancers versus poorly differentiated cancers (p = 0.07). These data add to the evidence of the association of diabetes mellitus with prostate cancer in the PSA era. 279
-
The measurement of four kallikreins enhanced prostate cancer detection compared with PSA level and age alone. Area under the curve (AUC) for the four kallikreins was 0.719 (95% CI 0.704 to 0.734) vs. 0.634 (95% CI 0.617 to 0.651) (p < 0.001) for PSA level and age alone for any-grade cancer, and 0.820 (95% CI 0.802 to 0.838) vs. 0.738 (95% CI 0.716 to 0.761) for high-grade cancer. Using a 6% risk of high-grade cancer as an illustrative cut-off point, for 1000 biopsied men with PSA levels of ≥ 3.0 ng/ml, the model would reduce the need for biopsy in 428 men, detect 119 high-grade cancers and delay diagnosis of 14 out of 133 high-grade cancers. 258
-
MSMB expression in benign and malignant prostate tissue was examined using immunohistochemistry and compared with the rs10993994 genotype. MSMB levels in prostate tissue and urine were greatly reduced in prostate cancer patients. Urinary MSMB was better than urinary PSA level at differentiating men with prostate cancer at all Gleason grades. The high-risk allele was associated with heterogeneity of MSMB staining and loss of MSMB in both tissue and urine in benign prostates. 296
-
Around 40% of the variability of PSA levels in the general population is accounted for by inherited factors. We undertook a genome-wide association study and follow-up analysis using PSA information from 15,757 Icelandic and 454 British men not diagnosed with prostate cancer. Overall, we detected a genome-wide significant association between PSA levels and SNPs at six loci {5p15.33 [rs2736098], 10q11 [rs10993994], 10q26 [rs10788160], 12q24 [rs11067228], 17q12 [rs4430796] and 19q13.33 [rs17632542 (KLK3: I179T)]}, each with P(combined) < 3 × 10(–10). Among 3834 men who underwent a biopsy of the prostate, the 10q26, 12q24 and 19q13.33 alleles that are associated with high PSA levels are associated with higher probability of a negative biopsy (OR between 1.15 and 1.27). We propose that a personalised PSA cut-off value, based on genotype, might be a promising addition to decisions about whether or not to perform a prostate biopsy. 297
-
Raised levels of IGF-I and/or its molar ratio with insulin-like growth factor-binding protein-3 (IGFBP-3) were associated with higher intakes of milk, dairy products, calcium, carbohydrate and polyunsaturated fat; lower levels were associated with high vegetable consumption, particularly tomatoes. These patterns support the possibility that IGFs may mediate some diet–cancer association. 298
-
We used a population-based case-finding exercise using the PSA test to examine whether or not associations between the IGF axis and cancer risk were apparent. A matched case–control study was conducted with 7383 men (aged 50–70 years) receiving a PSA test as part of a case-finding exercise. The risk of prostate cancer increased across quartiles of IGF-1 [highest vs. lowest quartile, OR 2.34, 95% CI 1.26 to 4.34; p(trend) = 0.02] and IGF-II [OR 1.78, 95% CI 0.94 to 3.15; p(trend) = 0.09]. Controlling for smoking history and IGFBP-3 strengthened associations with cancer for both IGF-1 [OR 3.00, 95% CI 1.50 to 6.01; p(trend) 0.005] and IGF-11 [OR 2.02, 95% CI 1.07 to 3.84; p(trend) = 0.04]. Associations between the IGFs and cancer risk were stronger for advanced cases. Our findings suggest that both IGF-1 and IGF-11 are associated with an increased risk of screen-detected prostate cancer. 299
Conclusion
The ProtecT trial participants form a unique cohort of men, recruited within a community setting where PSA testing was rare. Because of the randomised trial element, the biorepository will offer unique insights about the role of particular biomarkers in the response to particular methods of management. The fact that we have a comprehensive cohort of men including those with more-advanced disease adds significantly to the value of the sample collections.
Already, high-impact papers have resulted from the use of this repository and these will continue to be a very important legacy for future studies of prostate cancer (see Appendix 3).
Chapter 8 General conclusion
Design and objectives
The ProtecT RCT was supported by the NIHR HTA programme to evaluate the clinical effectiveness and cost-effectiveness of the three major treatment modalities for prostate cancer localised within the prostate gland. No previous trials had compared surgery, radiotherapy and AM, and the two previous published trials comparing surgery with outdated watchful waiting had produced conflicting results.
ProtecT was designed as a prospective cohort study of PSA testing, leading to the diagnosis of prostate cancer, with an embedded randomised trial of treatments for men with clinically localised prostate cancer and follow-up of all men diagnosed with prostate cancer (including those choosing a treatment or those who were excluded because of advanced cancer or comorbidities) in a novel extended comprehensive cohort design. A successful feasibility study embedded in a qualitative study of recruitment conducted between 1999 and 2001 showed that men aged 50–69 years could be identified in general practices and invited to attend a prostate check clinic appointment to discuss having a PSA test and potentially participating in a RCT of treatment, and that sufficient numbers would attend and follow the diagnostic pathway to support recruitment into the proposed main treatment trial.
Recruitment of participants to the main ProtecT prospective cohort study of PSA testing was itself embedded as the intervention group of a larger trial of prostate cancer screening (the CAP, supported by Cancer Research UK and the Department of Health and Social Care). In total, 911 general practices were randomised, 189,386 men were included in the intervention group (ProtecT) and 219,439 were included in the control group (receiving usual NHS care).
In total, combining the ProtecT feasibility and main recruitment phases from 1999 to 2009, 228,966 men aged between 50 and 69 years in nine UK clinical centres were invited to participate in ProtecT. Of these, 100,444 attended a prostate check appointment and were provided with information about the PSA test and ProtecT treatment trial by a study nurse. A total of 82,430 men had a PSA test, 7414 received prostate biopsies and 3221 received a diagnosis of prostate cancer and were followed up in the extended comprehensive cohort. There were 2664 men with a diagnosis of clinically localised prostate cancer who were eligible to take part in the ProtecT treatment trial, and 1643 agreed to be randomised (62% of eligible patients). In the ProtecT trial, 545 participants were randomly assigned to AM, 553 to RP and 545 to radiotherapy. All measured sociodemographic, clinical and patient-reported measures were balanced between the groups at baseline.
The aims of the ProtecT trial were to:
-
evaluate the clinical effectiveness of the three options for men with clinically localised prostate cancer at a median of 10 and 15 years of follow-up, including the primary outcome of prostate cancer-specific mortality and secondary outcomes of all-cause mortality and the incidence of metastases and disease progression
-
evaluate the impact of the three options on patient-reported symptoms and QoL outcomes using standardised and validated PROMs
-
develop a comprehensive biorepository of biobanked material donated by patients for translational research.
Analysis was by ITT. To ascertain cause of death accurately, summaries of anonymised medical records were reviewed and categorised by members of the independent CoDE Committee, blinded to treatment assignment.
Conclusions from the ProtecT trial of treatments at a median of 10 years’ follow-up
Mortality from prostate cancer was very low, at 1%, in all three randomised groups. There were also no differences in all-cause mortality between the groups. Disease progression and metastases were reduced by approximately half in the surgery and radiotherapy groups compared with AM. PROMs analysis through to 6 years showed that each group had particular profiles of side effects. Urinary incontinence and erectile symptoms were worse in the surgery group, with some symptoms persisting through the 6 years. Erectile symptoms and some bowel symptoms were experienced in the RT group. In the AM group, men who stayed on AM avoided the side effects of radical treatments, but as around half had received radical treatment over the median 10-year period, overall there was a year-on-year decline in the AM group in terms of urinary and sexual function. Generic health status, anxiety and depression and cancer-related QoL were not different between the groups.
The interventions in ProtecT remain the three most common contemporary modalities of prostate cancer treatment, but there have been considerable developments in treatment techniques since the study began, including the introduction of robot-assisted and laparoscopic surgery and brachytherapy or IMRT, and protocols for AS that exclude many of those included in ProtecT and using different strategies for monitoring and triggering change of management. However, the most recent large observational cohort studies evaluating robot-assisted and laparoscopic surgery, brachytherapy, IMRT and more-stringent polices of AS, as well as a small trial comparing open surgery with robot-assisted surgery, all found side effects with remarkably similar patterns to those found in ProtecT, suggesting that the ProtecT results are generalisable to contemporary treatments. Health economics analysis demonstrates that the total mean adjusted cost for the surgery group was slightly higher than that for radiotherapy, and both radical groups were more expensive than AM. The total adjusted mean QALYs were higher for the RT group than for the AM and surgery groups. The RT group was thus slightly more expensive and less effective than the surgery group and so it is unlikely that surgery would be the cost-effective option. Radiotherapy was slightly more beneficial but more expensive, and AM was slightly less beneficial but less expensive. The CEAC showed that, at the NICE threshold of £20,000 per QALY, at a median 10-year follow-up, the probabilities that each group was the most cost-effective option were 58% (radiotherapy), 32% (AM) and 10% (RP).
Conclusions from substudies in ProtecT
Disease progression
Baseline clinicopathological features of men with localised prostate cancer within ProtecT differed in men who developed disease progression compared with those with stable disease, but associations were not strong enough to reliably predict progression in individuals. This indicates that methods of stratification that are currently employed need to be refined with pre-biopsy imaging and targeted sampling, as well as utilisation of validated genomic and other emerging biomarkers. These will, however, need to be evaluated in new large-scale prospective early-detection programmes.
Qualitative research
The qualitative research integrated into the feasibility study and main recruitment enabled a nuanced understanding of the recruitment process, and the information developed from that understanding and the training and support given to research nurses and urologists enabled the ProtecT trial recruitment to be completed successfully. The recruitment intervention was later developed into the QRI, now being applied in 30 other RCTs with difficult recruitment challenges. Other qualitative research uses a combination of single and serial (repeated) in-depth interviews to conduct cross-sectional and longitudinal research to explore ProtecT study participants’ experiences of PSA testing, biopsy and outcomes following each of the treatments. Most men taking part in PSA testing perceived themselves to be at low risk of having prostate cancer. The studies of the experience of biopsy concluded that, in future, men needed to be better informed about the biopsy procedure, the relationship with urinary symptoms and the potential incidence and severity of side effects, and a proposal for such information was developed and published. 186
The longitudinal research captured how men’s experiences evolved over up to 14 years of follow-up. Appointments with health professionals (primarily nurses, supported by urologists and oncologists as required) to review PSA levels and disease progression were found to be critical for the management of men in the AM group. These appointments enabled and supported men to achieve their particular balance between uncertainty and reassurance and staying on AM or changing to a radical treatment. The ProtecT AM service protocol is available for implementation. Men undergoing radical treatments felt that they could have been better informed about the timing of hormone therapy or radiotherapy, the levels of impairment they might face and for how long impairment might last after radical treatment. Work is continuing to include ProtecT outcome data in materials provided to men to support decision-making.
Patient and public involvement
Patient and public involvement was not undertaken in the standard way in ProtecT, but the views and opinions of men involved in the qualitative research have been integrated into the design and conduct of the study from the outset. In addition, in 2016, a PPI consultation was undertaken with ProtecT patient representatives and members of the public about the interpretation of the main ProtecT outcome findings. This consultation provided a wealth of information about the type of information about prostate cancer and its treatment that men would like to have access to for decision-making, including the timing of provision and modes of presentation, which will feed into the materials being developed.
Translational research
The ProtecT trial participants were a unique cohort of men, recruited in a community setting where PSA testing was rare. Tissue from biopsies and surgery specimens (FFPE material) were collected from 2500 men with prostate cancer, and blood samples (plasma, serum and germline DNA) were also collected from these men and from ≈60,000 men who underwent PSA testing but were below the threshold (‘controls’). These samples have been carefully stored and 14,000 have had whole blood converted to germline DNA. With full sociodemographic, lifestyle, dietary, clinical, genetic, epidemiological and outcomes data, the ProtecT biorepository represents a key resource for future testing of biomarkers and screening and treatment strategies.
Strengths and limitations
There are limitations to the analysis and interpretation of the results. The ProtecT trial was designed in the late 1990s, when the standard diagnostic pathway was a combination of digital rectal examination, serum PSA testing and TRUS-guided biopsies. It is now known that these methods lead to overdetection of indolent prostate cancer and underdetection of significant disease compared with more modern imaging techniques, such as mpMRI and targeted biopsies. There have also been changes in Gleason grading over time, which may have resulted in an underestimate of the effectiveness of AM. The AM protocol in ProtecT was less intensive than many contemporary AS regimes, although none of the current methods has been validated and there is no consensus about optimal protocols. There were very few patients with high-risk disease at baseline – although this would be expected from PSA testing – and those with advanced prostate cancer who were excluded from the trial have been followed up observationally. There were only a small number of men of non-white ethnicity, although the study group was representative of the source population.
The main strengths of ProtecT were its size (with over 82,000 men tested and 1643 randomised); population base (recruited from GP surgeries across nine cities in the UK); standardised diagnostic approach using a consistent PSA threshold of 3.0 ng/ml and a minimum of 10-core biopsies; the high rate of randomisation of eligible patients; the use of standardised treatment pathways; and the use of validated PROMs for a wide range of generic and symptomatic impacts on QoL. All measured socioeconomic measures, clinical measures and PROMs were balanced at baseline between the allocated groups, and response rates were very high for PROMs, at over 85% in all allocation groups and without discernible decline over 6 years of follow-up. The trial was rated ‘good’ quality by the US Prostate Cancer Taskforce (on a scale where ‘good’ is the highest grade).
Final overall conclusions
Screening for prostate cancer and prostate cancer management are important public health topics. Prostate cancer is the most common cancer in men in the UK. 300 In 2014, 46,610 new cases of prostate cancer were diagnosed, and 11,287 men died from the disease that year, with approximately 330,000 men living with the disease in the UK. 300 Deaths from prostate cancer were higher than deaths from breast cancer in the most recent figures. 300
The ProtecT trial has shown that death from localised prostate cancer is low, at approximately 1% at a median of 10 years of follow-up, irrespective of the treatment assigned. All-cause mortality was also low. However, the rate of disease progression and metastases among men assigned to surgery or radiotherapy was half of the rate among men in the AM group. The ProtecT trial has also clarified the comparative impact of the major contemporary treatment options for localised prostate cancer on urinary, sexual and bowel function and QoL, including rates of recovery and outcomes up to 6 years after treatment allocation.
Longer-term follow-up is needed to document the changes in PROMs and oncological outcomes that will emerge from the originally randomised groups. Although the current findings do not yet provide a complete picture, men making decisions about treatments for newly diagnosed localised prostate cancer or contemplating PSA testing can use the information from ProtecT for decision-making with clinicians. More than 200 scientific publications have been produced from the study, some with very high impact, to support clinicians, researchers and policy decision-makers. However, follow-up for a further 5 to 10 years is required to fully inform the balance between the shorter-term impacts of treatments shown here and the longer course of prostate cancer progression, death from prostate cancer or competing causes, and QoL impacts that may emerge longer term from these treatments or from the sequelae of treatments for metastatic or progressing prostate cancer. The NIHR HTA programme has supported the collection of data to evaluate mortality and metastases up to the median 15-year follow-up, but not follow-up of PROMs.
Acknowledgements
We would like to express our deep gratitude to the more than 82,000 men who volunteered to take part in the PSA check clinics, to the 3321 men who were found to have prostate cancer, to the 1643 men who agreed to be randomised to the ProtecT study and who continue to provide precious information and follow-up data, and to those who volunteered to donate samples and material for translational research.
We would like to thank the wonderful ProtecT study team of research nurses and administrators at all levels, who have been a real inspiration and formed an unprecedented community and working team over the years.
We thank the funders, NHS Research and Development, the HTA programme, then the NIHR HTA programme and all the staff and directors over the years who have enabled and supported the inception, execution and continuation of the study, namely Professor Sir John Pattison, Professor Sir Miles Irving, Professor Sir Kent Woods, Professor Tom Walley, Professor Dame Sally Davies, Dr Russell Hamilton, Professor Jon Nicholl, Professor Hywel Williams, Professor Chris Whitty, Dr Louise Wood, Mrs Lesley Dodd and Mrs Kim Wherry.
We are also very grateful for the tremendous help and assistance in preparing this report provided by Ms Claire Thomson and Mrs Nicky Iyer. Thank you to Marta Tazewell for contributing to data management.
We could not have carried out this work, spanning almost 20 years, without the gigantic patience, dedication and support of our respective families.
We would like to give our sincere thanks to the following groups of experts who have provided tireless support and advice throughout the conduct of the ProtecT trial.
The University of Oxford was the sponsor.
Project study group
Principal investigators
Freddie C Hamdy (Chief Investigator), Jenny L Donovan and David E Neal.
Trial co-ordinator
J Athene Lane.
Trial statisticians
Tim J Peters and Chris Metcalfe.
Urologists
Leads: Prasad Bollina, Andrew Doble, Alan Doherty, David Gillatt, Roger Kockelbergh, Howard Kynaston, Alan Paul, Philip Powell, Stephen Prescott, Derek Rosario and Edward Rowe.
Others: John B Anderson (deceased), Jonathan Aning, James Catto, Garett Durkan, Owen Hughes, Anthony Kouparis, Hing Leung, Param Mariappan, Alan McNeill, Edgar Paez, Raj Persad, Hartwig Schwaibold, David Tulloch and Michael Wallace.
Nurses
Leads: Susan Bonnington, Lynne Bradshaw, Deborah Cooper, Emma Elliott, Phillipa Herbert, Peter Holding, Joanne Howson, Amanda Jones, Teresa Lennon, Norma Lyons, Hilary Moody, Claire Plumb, Tricia O’Sullivan, Elizabeth Salter, Pauline Thompson, Sarah Tidball, Jan Blaikie, Catherine Gray.
Others: Tonia Adam, Sarah Askew, Sharon Atkinson, Tim Baynes, Carole Brain, Viv Breen, Sarah Brunt, Sean Bryne, Jo Bythem, Jenny Clarke, Jenny Cloete, Susan Dark, Gill Davis, Rachael De La Rue, Jane Denizot, Elspeth Dewhurst, Anna Dimes, Nicola Dixon, Penny Ebbs, Ingrid Emmerson, Jill Ferguson, Ali Gadd, Lisa Geoghegan, Alison Grant, Collette Grant, Rosemary Godfrey, Louise Goodwin, Susie Hall, Liz Hart, Andrew Harvey, Chloe Hoult, Sarah Hawkins, Sharon Holling, Alastair Innes, Sue Kilner, Fiona Marshall, Louise Mellen, Andrea Moore, Sally Napier, Julie Needham, Kevin Pearse, Anna Pisa, Mark Rees, Elliw Richards, Lindsay Robson, Janet Roxburgh, Nikki Samuel, Irene Sharkey, Michael Slater, Donna Smith, Pippa Taggart, Helen Taylor, Vicky Taylor, Ayesha Thomas, Briony Tomkies, Nicola Trewick, Claire Ward, Christy Walker, Ayesha Williams, Colin Woodhouse and Elizabeth Wyber.
Oncologists
Lead: Malcolm Mason.
Others: Amit Bahl, Richard Benson, Mark Beresford, Catherine Ferguson, John Graham, Chris Herbert, Grahame Howard, Nick James, Peter Kirkbride, Alastair Law, Cgroupel Loughrey, Duncan McClaren, Helen Patterson (deceased), Ian Pedley, Trevor Roberts (deceased), Angus Robinson, Simon Russell, John Staffurth, Paul Symonds, Narottam Thanvi, Subramaniam Vasanthan and Paula Wilson.
Histopathologists
Leads: Jon Oxley and Mary Robinson.
Others: Selina Bhattarai, Neeta Deshmukh, John Dormer, Malee Fernando, John Goepel, David Griffiths, Ken Grigor, Patricia Harnden, Nick Mayer, Murali Vgroupa and Anne Warren.
Radiologists and medical physics
Helen Appleby, Dominic Ash, Dean Aston, Steven Bolton, Graham Chalmers, John Conway, Nick Early, Tony Geater, Lynda Goddall, Claire Heymann, Deborah Hicks, Liza Jones, Susan Lamb, Geoff Lambert, Gill Lawrence, Geraint Lewis, John Lilley, Aileen MacLeod, Pauline Massey, Alison McQueen, Rollo Moore, Lynda Penketh, Janet Potterton, Neil Roberts, Helen Showler, Pam Shuttleworth, Stephen Slade, Alasdair Steele, James Swinscoe, Marie Tiffany, John Townley, Jo Treeby, Michael Weston, Joyce Wilkinson, Lorraine Williams, Lucy Wills, Owain Woodley and Sue Yarrow.
Other researchers and data managers
Lucy Brindle, Linda Davies, Michael Davis, Dan Dedman, Elizabeth Down, Kirsty Garfield, Hanan Khazragui, Richard M Martin, Sian Noble, Hilary Taylor, Marta Tazewell, Emma L Turner, Julia Wade, Eleanor Walsh and Grace Young.
Administrative support
Susan Baker, Elizabeth Bellis-Sheldon, Chantal Bougard, Joanne Bowtell, Catherine Brewer, Chris Burton, Jennie Charlton, Nicholas Christoforou, Rebecca Clark, Susan Coull, Christine Croker, Rosemary Currer, Claire Daisey, Gill Delaney, Rose Donohue, Jane Drew, Rebecca Fgrouper, Susan Fry, Jean Haddow, Alex Hale, Susan Halpin, Belle Harris, Barbara Hattrick, Sharon Holmes, Helen Hunt, Vicky Jackson, Donna Johnson, Mandy Le Butt, Jo Leworthy, Tanya Liddiatt, Alex Martin, Jainee Mauree, Susan Moore, Gill Moulam, Jackie Mutch, Kathleen Parker, Christopher Pawsey, Michelle Purdie, Teresa Robson, Lynne Smith, Carole Stenton, Tom Steuart-Feilding, Beth Stott, Chris Sully, Caroline Sutton, Carol Torrington, Zoe Wilkins, Sharon Williams, Andrea Wilson and Ashleigh Weaver.
Trial Steering Committee
Chairperson: Michael Baum.
Members: Jan Adolfsson, Peter Albertsen, David Dearnaley, Fritz Schröder, Tracy Roberts and Anthony Zietman.
Joint ProtecT and CAP Cause of Death Evaluation Committee
Research lead: Richard M Martin.
Chairperson: Peter Albertsen.
Members: Jan Adolfsson, Amit Bahl, Michael Baum, Anthony Koupparis, Jon McFarlane, Jon Oxley, Colette Reid, Mary Robinson, Emma Turner and Anthony Zietman.
Data Monitoring Committee
Chairpersons: Adrian Grant (deceased) and Ian Roberts.
Members: Deborah Ashby, Richard Cowan, Peter Fayers, Killian Mellon, James N’Dow, Tim O’Brien and Michael Sokhal.
Contributions of authors
Conception and design of work, acquisition of funding, interpretation of data, writing, reviewing and final approval of report: Freddie C Hamdy, Jenny L Donovan and David Neal.
Trial co-ordination: J Athene Lane.
Statistical expertise and analysis: Tim J Peters, Chris Metcalfe and Grace Young.
Qualitative research, QRI: Julia Wade and Jenny L Donovan.
Quality-of-life measurements and patient-reported outcomes: Jenny L Donovan, J Athene Lane and Jane Blazeby.
Health economics analysis: Sian Noble and Kirsty Garfield.
Cause-of-death ascertainment and link with CAP: Richard M Martin and Emma L Turner.
Nursing lead and co-ordination: Peter Holding.
Clinical centre investigators (recruitment, diagnosis, treatment and follow-up of participants): Prasad Bollina, James Catto, Andrew Doble, Alan Doherty, David Gillatt, Vincent Gnanapragasam, Owen Hughes, Edgar Paez, Freddie C Hamdy, Roger Kockelbergh, Howard Kynaston, David Neal, Alan Paul, Philip Powell, Stephen Prescott, Derek Rosario and Edward Rowe.
Radiotherapy leads and expert advisors, quality-assurance analysis: Malcolm Mason and John Staffurth.
Pathology leads and expert advisors, co-chairs of ProtecT pathology group: Jon Oxley and Mary Robinson.
Data management: Michael Davis and Eleanor Walsh.
Disease progression analysis: Richard Bryant.
Publications
Neal DE, Leung HY, Powell PH, Hamdy FC, Donovan JL. Unanswered questions in screening for prostate cancer. Eur J Cancer 2000;36:1316–21.
Donovan JL, Frankel SJ, Neal DE, Hamdy FC. Screening for prostate cancer in the UK. Seems to be creeping in by the back door. BMJ 2001;323:763–4.
Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766–70.
Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al. Prostate testing for cancer and treatment (ProtecT) feasibility study. Health Technol Assess 2003;7(14).
Donovan JL, Peters TJ, Noble S, Powell P, Gillatt D, Oliver SE, et al. Who can best recruit to randomized trials? Randomized trial comparing surgeons and nurses recruiting patients to a trial of treatments for localized prostate cancer (the ProtecT study). J Clin Epidemiol 2003;56:605–9.
Gunnell D, Oliver SE, Peters TJ, Donovan JL, Persad R, Maynard M, et al. Are diet-prostate cancer associations mediated by the IGF axis? A cross-sectional analysis of diet, IGF-I and IGFBP-3 in healthy middle-aged men. Br J Cancer 2003;88:1682–6. https://doi.org/10.1038/sj.bjc.6600946
Hamdy FC, Donovan JL, Lane JA, Neal DE. Ethics of clinical trials from bayesian perspective: randomisation to clinical trials may solve dilemma of treatment choice in prostate cancer. BMJ 2003;326:1456. https://doi.org/10.1136/bmj.326.7404.1456
Mills N, Donovan JL, Smith M, Jacoby A, Neal DE, Hamdy FC. Perceptions of equipoise are crucial to trial participation: a qualitative study of men in the ProtecT study. Control Clin Trials 2003;24:272–82.
Oliver SE, Donovan JL, Peters TJ, Frankel S, Hamdy FC, Neal DE. Recent trends in the use of radical prostatectomy in England: the epidemiology of diffusion. BJU Int 2003;91:331–6.
Gunnell D, Oliver SE, Donovan JL, Peters TJ, Gillatt D, Persad R, et al. Do height-related variations in insulin-like growth factors underlie the associations of stature with adult chronic disease? J Clin Endocrinol Metab 2004;89:213–18.
Oliver SE, Barrass B, Gunnell DJ, Donovan JL, Peters TJ, Persad RA, et al. Serum insulin-like growth factor-I is positively associated with serum prostate-specific antigen in middle-aged men without evidence of prostate cancer. Cancer Epidemiol Biomarkers Prev 2004;13:163–5.
Oliver SE, Gunnell D, Donovan J, Peters TJ, Persad R, Gillatt D, et al. Screen-detected prostate cancer and the insulin-like growth factor axis: results of a population-based case-control study. Int J Cancer 2004;108:887–92. https://doi.org/10.1002/ijc.11631
Oliver SE, Holly J, Peters TJ, Donovan J, Persad R, Gillatt D, et al. Measurement of insulin-like growth factor axis does not enhance specificity of PSA-based prostate cancer screening. Urology 2004;64:317–22.
Donovan JL, Hamdy FC, Neal DE, Prostate Testing for Cancer and Treatment study group. Screening for prostate cancer. The case against. Ann R Coll Surg Engl 2005;87:90–1.
Donovan JL, Martin RM, Neal DE, Hamdy FC. Prostate cancer: screening approaches. Br J Hosp Med 2005;66:623–6. https://doi.org/10.12968/hmed.2005.66.11.20023
Brindle LA, Oliver SE, Dedman D, Donovan JL, Neal DE, Hamdy FC, et al. Measuring the psychosocial impact of population-based prostate-specific antigen testing for prostate cancer in the UK. BJU Int 2006;98:777–82.
Martin RM, Gunnell D, Hamdy F, Neal D, Lane A, Donovan J. Continuing controversy over monitoring men with localized prostate cancer: a systematic review of programs in the prostate specific antigen era. J Urol 2006;176:439–49.
Metcalfe C, Lane JA, Hamdy F, Neal D, Donovan JL. A model of the natural history of screen-detected prostate cancer. Br J Cancer 2006;95:1122–3.
Mills N, Metcalfe C, Ronsmans C, Davis M, Lane JA, Sterne JA, et al. A comparison of socio-demographic and psychological factors between patients consenting to randomisation and those selecting treatment (the ProtecT study). Contemp Clin Trials 2006;27:413–19.
Lane JA, Howson J, Donovan JL, Goepel JR, Dedman DJ, Down L, et al. Detection of prostate cancer in unselected young men: prospective cohort nested within a randomised controlled trial. BMJ 2007;335:1139.
Avery KN, Blazeby JM, Lane JA, Neal DE, Hamdy FC, Donovan JL. Decision-making about PSA testing and prostate biopsies: a qualitative study embedded in a primary care randomised trial. Eur Urol 2008;53:1186–93.
Avery KN, Metcalfe C, Blazeby JM, Lane JA, Neal DE, Hamdy FC, Donovan JL. Prostate-specific antigen testing and prostate biopsy: are self-reported lower urinary tract symptoms and health-related quality of life associated with the decision to undergo these investigations? BJU Int 2008;102:1629–33.
Collin SM, Martin RM, Metcalfe C, Gunnell D, Albertsen PC, Neal D, et al. Prostate-cancer mortality in the USA and UK in 1975–2004: an ecological study. Lancet Oncol 2008;9:445–52. https://doi.org/10.1016/S1470-2045(08)70104-9
Collin SM, Metcalfe C, Donovan J, Lane JA, Davis M, Neal D, et al. Associations of lower urinary tract symptoms with prostate-specific antigen levels, and screen-detected localized and advanced prostate cancer: a case-control study nested within the UK population-based ProtecT (prostate testing for cancer and treatment) study. BJU Int 2008;102:1400–6. https://doi.org/10.1111/j.1464-410X.2008.07817.x
Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet 2008;40:316–21. https://doi.org/10.1038/ng.90
Ghoussaini M, Song H, Koessler T, Al Olama AA, Kote-Jarai Z, Driver KE, et al. Multiple loci with different cancer specificities within the 8q24 gene desert. J Natl Cancer Inst 2008;100:962–6. https://doi.org/10.1093/jnci/djn190
Hussain S, Gunnell D, Donovan J, McPhail S, Hamdy F, Neal D, et al. Secular trends in prostate cancer mortality, incidence and treatment: England and Wales, 1975–2004. BJU Int 2008;101:547–55. https://doi.org/10.1111/j.1464-410X.2007.07338.x
Kote-Jarai Z, Easton DF, Stanford JL, Ostrander EA, Schleutker J, Ingles SA, et al. Multiple novel prostate cancer predisposition loci confirmed by an international study: the PRACTICAL Consortium. Cancer Epidemiol Biomarkers Prev 2008;17:2052–61.
Metcalfe C, Martin RM, Noble S, Lane JA, Hamdy FC, Neal DE, Donovan JL. Low risk research using routinely collected identifiable health information without informed consent: encounters with the Patient Information Advisory Group. J Med Ethics 2008;34:37–40.
Rosario DJ, Lane JA, Metcalfe C, Catto JW, Dedman D, Donovan JL, et al. Contribution of a single repeat PSA test to prostate cancer risk assessment: experience from the ProtecT study. Eur Urol 2008;53:777–84.
Zuccolo L, Harris R, Gunnell D, Oliver S, Lane JA, Davis M, et al. Height and prostate cancer risk: a large nested case-control study (ProtecT) and meta-analysis. Cancer Epidemiol Biomarkers Prev 2008;17:2325–36.
Al Olama AA, Kote-Jarai Z, Giles GG, Guy M, Morrison J, Severi G, et al. Multiple loci on 8q24 associated with prostate cancer susceptibility. Nat Genet 2009;41:1058–60. https://doi.org/10.1038/ng.452
Chen L, Davey Smith G, Evans DM, Cox A, Lawlor DA, Donovan J, et al. Genetic variants in the vitamin D receptor are associated with advanced prostate cancer at diagnosis: findings from the prostate testing for cancer and treatment study and a systematic review. Cancer Epidemiol Biomarkers Prev 2009;18:2874–81.
Collin SM, Metcalfe C, Donovan JL, Athene Lane J, Davis M, Neal DE, et al. Associations of sexual dysfunction symptoms with PSA-detected localised and advanced prostate cancer: a case-control study nested within the UK population-based ProtecT (prostate testing for cancer and treatment) study. Eur J Cancer 2009;45:3254–61. https://doi.org/10.1016/j.ejca.2009.05.021
Collin SM, Metcalfe C, Zuccolo L, Lewis SJ, Chen L, Cox A, et al. Association of folate-pathway gene polymorphisms with the risk of prostate cancer: a population-based nested case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev 2009;18:2528–39.
Donovan JL, Lane JA, Peters TJ, Brindle L, Salter E, Gillatt D, et al. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. J Clin Epidemiol 2009;62:29–36. https://doi.org/10.1016/j.jclinepi.2008.02.010
Down L, Metcalfe C, Avery K, Noble S, Lane JA, Neal DE, et al. Factors distinguishing general practitioners who more readily participated in a large randomized trial were identified. J Clin Epidemiol 2009;62:67–73. https://doi.org/10.1016/j.jclinepi.2008.02.014
Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet 2009;41:1116–21. https://doi.org/10.1038/ng.450
Gilbert R, Metcalfe C, Oliver SE, Whiteman DC, Bain C, Ness A, et al. Life course sun exposure and risk of prostate cancer: population-based nested case-control study and meta-analysis. Int J Cancer 2009;125:1414–23. https://doi.org/10.1002/ijc.24411
Guy M, Kote-Jarai Z, Giles GG, Al Olama AA, Jugurnauth SK, Mulholland S, et al. Identification of new genetic risk factors for prostate cancer. Asian J Androl 2009;11:49–55. https://doi.org/10.1038/aja.2008.18
Kote-Jarai Z, Jugurnauth S, Mulholland S, Leongamornlert DA, Guy M, Edwards S, et al. A recurrent truncating germline mutation in the BRIP1/FANCJ gene and susceptibility to prostate cancer. Br J Cancer 2009;100:426–30. https://doi.org/10.1038/sj.bjc.6604847
Macefield RC, Lane JA, Metcalfe C, Down L, Neal DE, Hamdy FC, Donovan JL. Do the risk factors of age, family history of prostate cancer or a higher prostate specific antigen level raise anxiety at prostate biopsy? Eur J Cancer 2009;45:2569–73.
Metcalfe C, Tilling K, Davis M, Lane JA, Martin RM, Kynaston H, et al. Current strategies for monitoring men with localised prostate cancer lack a strong evidence base: observational longitudinal study. Br J Cancer 2009;101:390–4. https://doi.org/10.1038/sj.bjc.6605181
Moore AL, Dimitropoulou P, Lane A, Powell PH, Greenberg DC, Brown CH, et al. Population-based prostate-specific antigen testing in the UK leads to a stage migration of prostate cancer. BJU Int 2009;104:1592–8. https://doi.org/10.1111/j.1464-410X.2009.08652.x
Murad A, Lewis SJ, Smith GD, Collin SM, Chen L, Hamdy FC, et al. PTGS2-899G>C and prostate cancer risk: a population-based nested case-control study (ProtecT) and a systematic review with meta-analysis. Prostate Cancer Prostatic Dis 2009;12:296–300. https://doi.org/10.1038/pcan.2009.18
Neal DE, Donovan JL, Martin RM, Hamdy FC. Screening for prostate cancer remains controversial. Lancet 2009;374:1482–3. https://doi.org/10.1016/S0140-6736(09)61085-0
Noble S, Donovan J, Turner E, Metcalfe C, Lane A, Rowlands MA, et al. Feasibility and cost of obtaining informed consent for essential review of medical records in large-scale health services research. J Health Serv Res Policy 2009;14:77–81. https://doi.org/10.1258/jhsrp.2008.008085
Pashayan N, Duffy SW, Pharoah P, Greenberg D, Donovan J, Martin RM, et al. Mean sojourn time, overdiagnosis, and reduction in advanced stage prostate cancer due to screening with PSA: implications of sojourn time on screening. Br J Cancer 2009;100:1198–204. https://doi.org/10.1038/sj.bjc.6604973
Pashayan N, Pharoah P, Neal DE, Hamdy F, Donovan J, Martin RM, et al. Stage shift in PSA-detected prostate cancers – effect modification by Gleason score. J Med Screen 2009;16:98–101. https://doi.org/10.1258/jms.2009.009037
Turner EL, Lane JA, Metcalfe C, Down L, Donovan JL, Hamdy F, et al. Psychological distress and prostate specific antigen levels in men with and without prostate cancer. Brain Behav Immun 2009;23:1073–8. https://doi.org/10.1016/j.bbi.2009.01.009
Wade J, Donovan JL, Lane JA, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018–28.
Collin SM, Metcalfe C, Refsum H, Lewis SJ, Smith GD, Cox A, et al. Associations of folate, vitamin B12, homocysteine, and folate-pathway polymorphisms with prostate-specific antigen velocity in men with localized prostate cancer. Cancer Epidemiol Biomarkers Prev 2010;19:2833–8.
Collin SM, Metcalfe C, Refsum H, Lewis SJ, Zuccolo L, Smith GD, et al. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev 2010;19:1632–42.
Elliott KS, Zeggini E, McCarthy MI, Gudmundsson J, Sulem P, Stacey SN, et al. Evaluation of association of HNF1B variants with diverse cancers: collaborative analysis of data from 19 genome-wide association studies. PLOS ONE 2010;5:e10858. https://doi.org/10.1371/journal.pone.0010858
Gudmundsson J, Besenbacher S, Sulem P, Gudbjartsson DF, Olafsson I, Arinbjarnarson S, et al. Genetic correction of PSA values using sequence variants associated with PSA levels. Sci Transl Med 2010;2:62ra92. https://doi.org/10.1126/scitranslmed.3001513
Kote-Jarai Z, Leongamornlert D, Tymrakiewicz M, Field H, Guy M, Al Olama AA, et al. Mutation analysis of the MSMB gene in familial prostate cancer. Br J Cancer 2010;102:414–18. https://doi.org/10.1038/sj.bjc.6605485
Lane JA, Hamdy FC, Martin RM, Turner EL, Neal DE, Donovan JL. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. Eur J Cancer 2010;46:3095–101. https://doi.org/10.1016/j.ejca.2010.09.016
Lewis SJ, Murad A, Chen L, Davey Smith G, Donovan J, Palmer T, et al. Associations between an obesity related genetic variant (FTO rs9939609) and prostate cancer risk. PLOS ONE 2010;5:e13485. https://doi.org/10.1371/journal.pone.0013485
Macefield RC, Metcalfe C, Lane JA, Donovan JL, Avery KN, Blazeby JM, et al. Impact of prostate cancer testing: an evaluation of the emotional consequences of a negative biopsy result. Br J Cancer 2010;102:1335–40. https://doi.org/10.1038/sj.bjc.6605648
Murad AS, Smith GD, Lewis SJ, Cox A, Donovan JL, Neal DE, et al. A polymorphism in the glucokinase gene that raises plasma fasting glucose, rs1799884, is associated with diabetes mellitus and prostate cancer: findings from a population-based, case-control study (the ProtecT study). Int J Mol Epidemiol Genet 2010;1:175–83.
Rowlands MA, Holly JM, Gunnell D, Gilbert R, Donovan J, Lane JA, et al. The relation between adiposity throughout the life course and variation in IGFs and IGFBPs: evidence from the ProtecT (prostate testing for cancer and treatment) study. Cancer Causes Control 2010;21:1829–42. https://doi.org/10.1007/s10552-010-9610-x
Tilling K, Garmo H, Metcalfe C, Holmberg L, Hamdy FC, Neal DE, et al. Development of a new method for monitoring prostate-specific antigen changes in men with localised prostate cancer: a comparison of observational cohorts. Eur Urol 2010;57:446–52. https://doi.org/10.1016/j.eururo.2009.03.023
Vickers AJ, Cronin AM, Roobol MJ, Hugosson J, Jones JS, Kattan MW, et al. The relationship between prostate-specific antigen and prostate cancer risk: The Prostate Biopsy Collaborative Group. Clin Cancer Res 2010;16:4374–81.
Batra J, Lose F, O’Mara T, Marquart L, Stephens C, Alexander K, et al. Association between Prostinogen (KLK15) genetic variants and prostate cancer risk and aggressiveness in Australia and a meta-analysis of GWAS data. PLOS ONE 2011;6:e26527. https://doi.org/10.1371/journal.pone.0026527
Catto JW, Robinson MC, Albertsen PC, Goepel JR, Abbod MF, Linkens DA, et al. Suitability of PSA-detected localised prostate cancers for focal therapy: experience from the ProtecT study. Br J Cancer 2011;105:931–7. https://doi.org/10.1038/bjc.2011.314
Collin SM, Metcalfe C, Palmer TM, Refsum H, Lewis SJ, Smith GD, et al. The causal roles of vitamin B12 and transcobalamin in prostate cancer: can Mendelian randomization analysis provide definitive answers? Int J Mol Epidemiol Genet 2011;2:316–27.
Dimitropoulou P, Martin RM, Turner EL, Lane JA, Gilbert R, Davis M, et al. Association of obesity with prostate cancer: a case-control study within the population-based PSA testing phase of the ProtecT study. Br J Cancer 2011;104:875–81. https://doi.org/10.1038/sj.bjc.6606066
Down L, Metcalfe C, Martin RM, Neal DE, Hamdy FC, Donovan JL, Lane JA. Seasonal variation in prostate-specific antigen levels: a large cross-sectional study of men in the UK. BJU Int 2011;108:1409–14. https://doi.org/10.1111/j.1464-410X.2011.10174.x
Kote-Jarai Z, Amin Al Olama A, Leongamornlert D, Tymrakiewicz M, Saunders E, Guy M, et al. Identification of a novel prostate cancer susceptibility variant in the KLK3 gene transcript. Hum Genet 2011;129:687–94. https://doi.org/10.1007/s00439-011-0981-1
Kote-Jarai Z, Olama AA, Giles GG, Severi G, Schleutker J, Weischer M, et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet 2011;43:785–91. https://doi.org/10.1038/ng.882
Lane JA, Wade J, Down L, Bonnington S, Holding PN, Lennon T, et al. A Peer Review Intervention for Monitoring and Evaluating sites (PRIME) that improved randomized controlled trial conduct and performance. J Clin Epidemiol 2011;64:628–36. https://doi.org/10.1016/j.jclinepi.2010.10.003
Mills N, Donovan JL, Wade J, Hamdy FC, Neal DE, Lane JA. Exploring treatment preferences facilitated recruitment to randomized controlled trials. J Clin Epidemiol 2011;64:1127–36. https://doi.org/10.1016/j.jclinepi.2010.12.017
Murad AS, Down L, Davey Smith G, Donovan JL, Athene Lane J, Hamdy FC, et al. Associations of aspirin, nonsteroidal anti-inflammatory drug and paracetamol use with PSA-detected prostate cancer: findings from a large, population-based, case-control study (the ProtecT study). Int J Cancer 2011;128:1442–8. https://doi.org/10.1002/ijc.25465
Pashayan N, Pharoah P, Neal DE, Hamdy F, Donovan J, Martin RM, et al. PSA-detected prostate cancer and the potential for dedifferentiation – estimating the proportion capable of progression. Int J Cancer 2011;128:1462–70. https://doi.org/10.1002/ijc.25471
Pashayan N, Pharoah P, Tabár L, Neal DE, Martin RM, Donovan J, et al. Validation of a modelling approach for estimating the likely effectiveness of cancer screening using cancer data on prevalence screening and incidence. Cancer Epidemiol 2011;35:139–44. https://doi.org/10.1016/j.canep.2010.07.012
Schumacher FR, Berndt SI, Siddiq A, Jacobs KB, Wang Z, Lindstrom S, et al. Genome-wide association study identifies new prostate cancer susceptibility loci. Hum Mol Genet 2011;20:3867–75. https://doi.org/10.1093/hmg/ddr295
Stacey SN, Sulem P, Jonasdottir A, Masson G, Gudmundsson J, Gudbjartsson DF, et al. A germline variant in the TP53 polyadenylation signal confers cancer susceptibility. Nat Genet 2011;43:1098–103. https://doi.org/10.1038/ng.926
Turner EL, Lane JA, Donovan JL, Davis MJ, Metcalfe C, Neal DE, et al. Association of diabetes mellitus with prostate cancer: nested case-control study (Prostate testing for cancer and treatment study). Int J Cancer 2011;128:440–6. https://doi.org/10.1002/ijc.25360
Williams N, Hughes LJ, Turner EL, Donovan JL, Hamdy FC, Neal DE, et al. Prostate-specific antigen testing rates remain low in UK general practice: a cross-sectional study in six English cities. BJU Int 2011;108:1402–8. https://doi.org/10.1111/j.1464-410X.2011.10163.x
Ankerst DP, Boeck A, Freedland SJ, Jones JS, Cronin AM, Roobol MJ, et al. Evaluating the prostate cancer prevention trial high grade prostate cancer risk calculator in 10 international biopsy cohorts: results from the prostate biopsy collaborative group. World J Urol 2014;32:185–91.
Ankerst DP, Boeck A, Freedland SJ, Thompson IM, Cronin AM, Roobol MJ, et al. Evaluating the PCPT risk calculator in ten international biopsy cohorts: results from the Prostate Biopsy Collaborative Group. World J Urol 2012;30:181–7. https://doi.org/10.1007/s00345-011-0818-5
Avery KN, Metcalfe C, Vedhara K, Lane JA, Davis M, Neal DE, et al. Predictors of attendance for prostate-specific antigen screening tests and prostate biopsy. Eur Urol 2012;62:649–55. https://doi.org/10.1016/j.eururo.2011.12.059
Burton AJ, Martin RM, Donovan JL, Lane JA, Davis M, Hamdy FC, et al. Associations of lifestyle factors and anthropometric measures with repeat PSA levels during active surveillance/monitoring. Cancer Epidemiol Biomarkers Prev 2012;21:1877–85.
Cheng I, Chen GK, Nakagawa H, He J, Wan P, Laurie CC, et al. Evaluating genetic risk for prostate cancer among Japanese and Latinos. Cancer Epidemiol Biomarkers Prev 2012;21:2048–58.
Gilbert R, Martin RM, Fraser WD, Lewis S, Donovan J, Hamdy F, et al. Predictors of 25-hydroxyvitamin D and its association with risk factors for prostate cancer: evidence from the prostate testing for cancer and treatment study. Cancer Causes Control 2012;23:575–88. https://doi.org/10.1007/s10552-012-9919-8
Gilbert R, Metcalfe C, Fraser WD, Donovan J, Hamdy F, Neal DE, et al. Associations of circulating 25-hydroxyvitamin D with prostate cancer diagnosis, stage and grade. Int J Cancer 2012;131:1187–96. https://doi.org/10.1002/ijc.27327
Gilbert R, Metcalfe C, Fraser WD, Donovan J, Hamdy F, Neal DE, et al. Associations of circulating retinol, vitamin E, and 1,25-dihydroxyvitamin D with prostate cancer diagnosis, stage, and grade. Cancer Causes Control 2012;23:1865–73. https://doi.org/10.1007/s10552-012-0052-5
Gudmundsson J, Sulem P, Gudbjartsson DF, Masson G, Agnarsson BA, Benediktsdottir KR, et al. A study based on whole-genome sequencing yields a rare variant at 8q24 associated with prostate cancer. Nat Genet 2012;44:1326–9. https://doi.org/10.1038/ng.2437
Roobol MJ, Schröder FH, Hugosson J, Jones JS, Kattan MW, Klein EA, et al. Importance of prostate volume in the European Randomised Study of Screening for Prostate Cancer (ERSPC) risk calculators: results from the prostate biopsy collaborative group. World J Urol 2012;30:149–55. https://doi.org/10.1007/s00345-011-0804-y
Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, Goodwin L, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 2012;344:d7894. https://doi.org/10.1136/bmj.d7894
Rowlands MA, Holly JM, Gunnell D, Donovan J, Lane JA, Hamdy F, et al. Circulating insulin-like growth factors and IGF-binding proteins in PSA-detected prostate cancer: the large case-control study ProtecT. Cancer Res 2012;72:503–15. https://doi.org/10.1158/0008-5472.CAN-11-1601
Rowlands MA, Holly JM, Hamdy F, Phillips J, Goodwin L, Marsden G, et al. Serum insulin-like growth factors and mortality in localised and advanced clinically detected prostate cancer. Cancer Causes Control 2012;23:347–54. https://doi.org/10.1007/s10552-011-9883-8
Young NJ, Metcalfe C, Gunnell D, Rowlands MA, Lane JA, Gilbert R, et al. A cross-sectional analysis of the association between diet and insulin-like growth factor (IGF)-I, IGF-II, IGF-binding protein (IGFBP)-2, and IGFBP-3 in men in the United Kingdom. Cancer Causes Control 2012;23:907–17. https://doi.org/10.1007/s10552-012-9961-6
Amin Al Olama A, Kote-Jarai Z, Schumacher FR, Wiklund F, Berndt SI, Benlloch S, et al. A meta-analysis of genome-wide association studies to identify prostate cancer susceptibility loci associated with aggressive and non-aggressive disease. Hum Mol Genet 2013;22:408–15. https://doi.org/10.1093/hmg/dds425
Avery KN, Donovan JL, Gilbert R, Davis M, Emmett P, Down L, et al. Men with prostate cancer make positive dietary changes following diagnosis and treatment. Cancer Causes Control 2013;24:1119–28. https://doi.org/10.1007/s10552-013-0189-x
Bonilla C, Gilbert R, Kemp JP, Timpson NJ, Evans DM, Donovan JL, et al. Using genetic proxies for lifecourse sun exposure to assess the causal relationship of sun exposure with circulating vitamin D and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2013;22:597–606.
Burton A, Martin RM, Holly J, Lane JA, Donovan JL, Hamdy FC, et al. Associations of adiponectin and leptin with stage and grade of PSA-detected prostate cancer: the ProtecT study. Cancer Causes Control 2013;24:323–34.
Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet 2013;45:385–91. https://doi.org/10.1038/ng.2560
Gilbert R, Metcalfe C, Fraser WD, Lewis S, Donovan J, Hamdy F, et al. Associations of circulating 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and vitamin D pathway genes with prostate-specific antigen progression in men with localized prostate cancer undergoing active monitoring. Eur J Cancer Prev 2013;22:121–5. https://doi.org/10.1097/CEJ.0b013e3283584954
Kote-Jarai Z, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Dadaev T, Jugurnauth-Little S, et al. Fine-mapping identifies multiple prostate cancer risk loci at 5p15, one of which associates with TERT expression. Hum Mol Genet 2013;22:2520–8. https://doi.org/10.1093/hmg/ddt086
Lose F, Batra J, O’Mara T, Fahey P, Marquart L, Eeles RA, et al. Common variation in Kallikrein genes KLK5, KLK6, KLK12, and KLK13 and risk of prostate cancer and tumor aggressiveness. Urol Oncol 2013;31:635–43. https://doi.org/10.1016/j.urolonc.2011.05.011
Orozco G, Goh CL, Al Olama AA, Benlloch-Garcia S, Govindasami K, Guy M, et al. Common genetic variants associated with disease from genome-wide association studies are mutually exclusive in prostate cancer and rheumatoid arthritis. BJU Int 2013;111:1148–55. https://doi.org/10.1111/j.1464-410X.2012.11492.x
Pooley KA, Bojesen SE, Weischer M, Nielsen SF, Thompson D, Amin Al Olama A, et al. A genome-wide association scan (GWAS) for mean telomere length within the COGS project: identified loci show little association with hormone-related cancer risk. Hum Mol Genet 2013;22:5056–64. https://doi.org/10.1093/hmg/ddt355
Rodriguez S, Al-Ghamdi OA, Burrows K, Guthrie PA, Lane JA, Davis M, et al. Very low PSA concentrations and deletions of the KLK3 gene. Clin Chem 2013;59:234–44.
Rowlands MA, Tilling K, Holly JM, Metcalfe C, Gunnell D, Lane A, et al. Insulin-like growth factors (IGFs) and IGF-binding proteins in active monitoring of localized prostate cancer: a population-based observational study. Cancer Causes Control 2013;24:39–45. https://doi.org/10.1007/s10552-012-0087-7
Simpkin AJ, Metcalfe C, Martin RM, Lane JA, Donovan JL, Hamdy FC, et al. Longitudinal prostate-specific antigen reference ranges: choosing the underlying model of age-related changes. Stat Methods Med Res 2016;25:1875–91.
Wade J, Rosario DJ, Macefield RC, Avery KN, Salter CE, Goodwin ML, et al. Psychological impact of prostate biopsy: physical symptoms, anxiety, and depression. J Clin Oncol 2013;31:4235–41. https://doi.org/10.1200/JCO.2012.45.4801
Zuccolo L, Lewis SJ, Donovan JL, Hamdy FC, Neal DE, Smith GD. Alcohol consumption and PSA-detected prostate cancer risk – a case-control nested in the ProtecT study. Int J Cancer 2013;132:2176–85. https://doi.org/10.1002/ijc.27877
Al Olama AA, Kote-Jarai Z, Berndt SI, Conti DV, Schumacher F, Han Y, et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet 2014;46:1103–9. https://doi.org/10.1038/ng.3094
Ankerst DP, Boeck A, Freedland SJ, Jones JS, Cronin AM, Roobol MJ, et al. Evaluating the prostate cancer prevention trial high grade prostate cancer risk calculator in 10 international biopsy cohorts: results from the Prostate Biopsy Collaborative Group. World J Urol 2014;32:185–91. https://doi.org/10.1007/s00345-012-0869-2
Avery KN, Donovan JL, Horwood J, Neal DE, Hamdy FC, Parker C, et al. The importance of dietary change for men diagnosed with and at risk of prostate cancer: a multi-centre interview study with men, their partners and health professionals. BMC Fam Pract 2014;15:81. https://doi.org/10.1186/1471-2296-15-81
Er V, Lane JA, Martin RM, Emmett P, Gilbert R, Avery KN, et al. Adherence to dietary and lifestyle recommendations and prostate cancer risk in the prostate testing for cancer and treatment (ProtecT) trial. Cancer Epidemiol Biomarkers Prev 2014;23:2066–77.
Gilbert R, Bonilla C, Metcalfe C, Lewis S, Evans DM, Fraser WD, et al. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: a nested case-control study. Cancer Causes Control 2015;26:205–18.
Gilbert R, Martin RM, Donovan J, Lane JA, Hamdy F, Neal DE, Metcalfe C. Misclassification of outcome in case-control studies: methods for sensitivity analysis. Stat Methods Med Res 2016;25:2377–93.
Horwood JP, Avery KN, Metcalfe C, Donovan JL, Hamdy FC, Neal DE, Lane JA. Men’s knowledge and attitudes towards dietary prevention of a prostate cancer diagnosis: a qualitative study. BMC Cancer 2014;14:812.
Knipe DW, Evans DM, Kemp JP, Eeles R, Easton DF, Kote-Jarai Z, et al. Genetic variation in prostate-specific antigen-detected prostate cancer and the effect of control selection on genetic association studies. Cancer Epidemiol Biomarkers Prev 2014;23:1356–65.
Lane JA, Donovan JL, Davis M, Walsh E, Dedman D, Down L, et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014;15:1109–18. https://doi.org/10.1016/S1470-2045(14)70361-4
Mills N, Blazeby JM, Hamdy FC, Neal DE, Campbell B, Wilson C, et al. Training recruiters to randomized trials to facilitate recruitment and informed consent by exploring patients’ treatment preferences. Trials 2014;15:323. https://doi.org/10.1186/1745-6215-15-323
Oxley J, Simpkin A, Goepel J, Varma M, Griffiths D, Grigor K, et al. Gleason drift in the NIHR ProtecT Study. Histopathology 2015;66:438–46.
Saunders EJ, Dadaev T, Leongamornlert DA, Jugurnauth-Little S, Tymrakiewicz M, Wiklund F, et al. Fine-mapping the HOXB region detects common variants tagging a rare coding allele: evidence for synthetic association in prostate cancer. PLOS Genet 2014;10:e1004129. https://doi.org/10.1371/journal.pgen.1004129
Turner EL, Metcalfe C, Donovan JL, Noble S, Sterne JA, Lane JA, et al. Design and preliminary recruitment results of the Cluster randomised triAl of PSA testing for Prostate cancer (CAP). Br J Cancer 2014;110:2829–36. https://doi.org/10.1038/bjc.2014.242
Weng PH, Huang YL, Page JH, Chen JH, Xu J, Koutros S, et al. Polymorphisms of an innate immune gene, toll-like receptor 4, and aggressive prostate cancer risk: a systematic review and meta-analysis. PLOS ONE 2014;9:e110569. https://doi.org/10.1371/journal.pone.0110569
Mills N, Blazeby JM, Hamdy FC, Neal DE, Campbell B, Wilson C, et al. Training recruiters to randomized trials to facilitate recruitment and informed consent by exploring patients’ treatment preferences. Trials 2014;15:323. https://doi.org/10.1186/1745-6215-15-323
Amin Al Olama A, Benlloch S, Antoniou AC, Giles GG, Severi G, et al. Risk analysis of prostate cancer in PRACTICAL, a multinational consortium, using 25 known prostate cancer susceptibility loci. Cancer Epidemiol Biomarkers Prev 2015;24:1121–9.
Amin Al Olama A, Dadaev T, Hazelett DJ, Li Q, Leongamornlert D, Saunders EJ, et al. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum Mol Genet 2015;24:5589–602.
Bryant RJ, Sjoberg DD, Vickers AJ, Robinson MC, Kumar R, Marsden L, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. J Natl Cancer Inst 2015;107:djv095. https://doi.org/10.1093/jnci/djv095
Gilbert R, Bonilla C, Metcalfe C, Lewis S, Evans DM, Fraser WD, et al. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: a nested case-control study. Cancer Causes Control 2015;26:205–18. https://doi.org/10.1007/s10552-014-0500-5
Hackshaw-McGeagh LE, Penfold CM, Walsh E, Donovan JL, Hamdy FC, Neal DE, et al. Physical activity, alcohol consumption, BMI and smoking status before and after prostate cancer diagnosis in the ProtecT trial: opportunities for lifestyle modification. Int J Cancer 2015;137:1509–15. https://doi.org/10.1002/ijc.29514
Kote-Jarai Z, Mikropoulos C, Leongamornlert DA, Dadaev T, Tymrakiewicz M, Saunders EJ, et al. Prevalence of the HOXB13 G84E germline mutation in British men and correlation with prostate cancer risk, tumour characteristics and clinical outcomes. Ann Oncol 2015;26:756–61. https://doi.org/10.1093/annonc/mdv004
Oxley J, Simpkin A, Goepel J, Varma M, Griffiths D, Grigor K, et al. Gleason drift in the NIHR ProtecT study. Histopathology 2015;66:438–46. https://doi.org/10.1111/his.12549
Pashayan N, Duffy SW, Neal DE, Hamdy FC, Donovan JL, Martin RM, et al. Implications of polygenic risk-stratified screening for prostate cancer on overdiagnosis. Genet Med 2015;17:789–95. https://doi.org/10.1038/gim.2014.192
Simpkin AJ, Rooshenas L, Wade J, Donovan JL, Lane JA, Martin RM, et al. Development, validation and evaluation of an instrument for active monitoring of men with clinically localised prostate cancer: systematic review, cohort studies and qualitative study. Health Serv Deliv Res 2015;3(30).
Simpkin AJ, Tilling K, Martin RM, Lane JA, Hamdy FC, Holmberg L, et al. Systematic Review and Meta-analysis of Factors Determining Change to Radical Treatment in Active Surveillance for Localized Prostate Cancer. Eur Urol 2015;67:993–1005.
Szulkin R, Karlsson R, Whitington T, Aly M, Gronberg H, Eeles RA, et al. Genome-wide association study of prostate cancer-specific survival. Cancer Epidemiol Biomarkers Prev 2015;24:1796–800.
Szulkin R, Whitington T, Eklund M, Aly M, Eeles RA, Easton D, et al. Prediction of individual genetic risk to prostate cancer using a polygenic score. Prostate 2015;75:1467–74. https://doi.org/10.1002/pros.23037
Wade J, Rosario DJ, Howson J, Avery KN, Salter CE, Goodwin ML, et al. Role of information in preparing men for transrectal ultrasound guided prostate biopsy: a qualitative study embedded in the ProtecT trial. BMC Health Serv Res 2015;15:80. https://doi.org/10.1186/s12913-015-0729-z
Williams NJ, Hill EM, Ng SY, Martin RM, Metcalfe C, Donovan JL, et al. Standardisation of information submitted to an endpoint committee for cause of death assignment in a cancer screening trial – lessons learnt from CAP (Cluster randomised triAl of PSA testing for Prostate cancer). BMC Med Res Methodol 2015;15:6. https://doi.org/10.1186/1471-2288-15-6
Simpkin AJ, Tilling K, Martin RM, Lane JA, Hamdy FC, Holmberg L, et al. Systematic review and meta-analysis of factors determining change to radical treatment in active surveillance for localized prostate cancer. Eur Urol 2015;67:993–1005.
Amin Al Olama A, Dadaev T, Hazelett DJ, Li Q, Leongamornlert D, Saunders EJ, et al. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum Mol Genet 2015;24:5589–602. https://doi.org/10.1093/hmg/ddv203
Amin Al Olama A, Benlloch S, Antoniou AC, Giles GG, Severi G, Neal DE, et al. Risk analysis of prostate cancer in PRACTICAL, a multinational consortium, using 25 known prostate cancer susceptibility loci. Cancer Epidemiol Biomarkers Prev 2015;24:1121–9. https://doi.org/10.1158/1055-9965.EPI-14-0317
Key TJ, Appleby PN, Travis RC, Albanes D, Alberg AJ, Barricarte A, et al. Carotenoids, retinol, tocopherols, and prostate cancer risk: pooled analysis of 15 studies. Am J Clin Nutr 2015;102:1142–57. https://doi.org/10.3945/ajcn.115.114306
Gilbert R, Martin RM, Evans DM, Tilling K, Davey Smith G, Kemp JP, et al. Incorporating known genetic variants does not improve the accuracy of PSA testing to identify high risk prostate cancer on biopsy. PLOS ONE 2015;10:e0136735.
Davies NM, Gaunt TR, Lewis SJ, Holly J, Donovan JL, Hamdy FC, et al. The effects of height and BMI on prostate cancer incidence and mortality: a Mendelian randomization study in 20,848 cases and 20,214 controls from the PRACTICAL consortium. Cancer Causes Control 2015;26:1603–16. https://doi.org/10.1007/s10552-015-0654-9
Thorn JC, Turner E, Hounsome L, Walsh E, Donovan JL, Verne J, et al. Validation of the hospital episode statistics outpatient dataset in England. PharmacoEconomics 2016;34:161–8. https://doi.org/10.1007/s40273-015-0326-3
Wade J, Holding PN, Bonnington S, Rooshenas L, Lane JA, Salter CE, et al. Establishing nurse-led active surveillance for men with localised prostate cancer: development and formative evaluation of a model of care in the ProtecT trial. BMJ Open 2015;5:e008953. https://doi.org/10.1136/bmjopen-2015-008953
Szulkin R, Karlsson R, Whitington T, Aly M, Gronberg H, Eeles RA, et al. Genome-wide association study of prostate cancer-specific survival. Cancer Epidemiol Biomarkers Prev 2015;24:1796–800. https://doi.org/10.1158/1055-9965.EPI-15-0543
Simpkin AJ, Rooshenas L, Wade J, Donovan JL, Lane JA, Martin RM, et al. Development, validation and evaluation of an instrument for active monitoring of men with clinically localised prostate cancer: systematic review, cohort studies and qualitative study. Health Serv Deliv Res 2015;3(30).
Thorn JC, Turner EL, Hounsome L, Walsh E, Down L, Verne J, et al. Validating the use of hospital episode statistics data and comparison of costing methodologies for economic evaluation: an end-of-life case study from the Cluster randomised triAl of PSA testing for Prostate cancer (CAP). BMJ Open 2016;6:e011063. https://doi.org/10.1136/bmjopen-2016-011063
Price AJ, Travis RC, Appleby PN, Albanes D, Barricarte Gurrea A, Bjørge T, et al. Circulating folate and vitamin B12 and risk of prostate cancer: a collaborative analysis of individual participant data from six cohorts including 6875 cases and 8104 controls. Eur Urol 2016;70:941–51.
Gusev A, Shi H, Kichaev G, Pomerantz M, Li F, Long HW, et al. Atlas of prostate cancer heritability in European and African-American men pinpoints tissue-specific regulation. Nat Commun 2016;7:10979. https://doi.org/10.1038/ncomms10979
Bonilla C, Lewis SJ, Martin RM, Donovan JL, Hamdy FC, Neal DE, et al. Pubertal development and prostate cancer risk: Mendelian randomization study in a population-based cohort. BMC Med 2016;14:66. https://doi.org/10.1186/s12916-016-0602-x
Walsh EI, Turner EL, Lane JA, Donovan JL, Neal DE, Hamdy FC, Martin RM, the CAP and ProtecT Trial Groups. Characteristics of men responding to an invitation to undergo testing for prostate cancer as part of a randomised trial. Trials 2016;17:497. https://doi.org/10.1186/s13063-016-1624-6
Travis RC, Appleby PN, Martin RM, Holly JMP, Albanes D, Black A, et al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res 2016;76:2288–300. https://doi.org/10.1158/0008-5472.CAN-15-1551
Simpkin AJ, Donovan JL, Tilling K, Athene Lane J, Martin RM, Albertsen PC, et al. Prostate-specific antigen patterns in US and European populations: comparison of six diverse cohorts. BJU Int 2016;118:911–18. https://doi.org/10.1111/bju.13422
Stacey SN, Kehr B, Gudmundsson J, Zink F, Jonasdottir A, Gudjonsson SA, et al. Insertion of an SVA-E retrotransposon into the CASP8 gene is associated with protection against prostate cancer. Hum Mol Genet 2016;25:1008–18. https://doi.org/10.1093/hmg/ddv622
Bonilla C, Lewis SJ, Rowlands MA, Gaunt TR, Davey Smith G, Gunnell D, et al. Assessing the role of insulin-like growth factors and binding proteins in prostate cancer using Mendelian randomization: genetic variants as instruments for circulating levels. Int J Cancer 2016;139:1520–33. https://doi.org/10.1002/ijc.30206
Castro E, Mikropoulos C, Bancroft EK, Dadaev T, Goh C, Taylor N, et al. The PROFILE feasibility study: targeted screening of men with a family history of prostate cancer. Oncologist 2016;21:716–22. https://doi.org/10.1634/theoncologist.2015-0336
Harrison S, Tilling K, Turner EL, Lane JA, Simpkin A, Davis M, et al. Investigating the prostate specific antigen, body mass index and age relationship: is an age-BMI-adjusted PSA model clinically useful? Cancer Causes Control 2016;27:1465–74. https://doi.org/10.1007/s10552-016-0827-1
Kar SP, Beesley J, Amin Al Olama A, Michailidou K, Tyrer J, Kote-Jarai Z, et al. Genome-wide meta-analyses of breast, ovarian, and prostate cancer association studies identify multiple new susceptibility loci shared by at least two cancer types. Cancer Discov 2016;6:1052–67. https://doi.org/10.1158/2159-8290.CD-15-1227
Mason MD, Moore R, Jones G, Lewis G, Donovan JL, Neal DE, et al. Radiotherapy for prostate cancer: is it ‘what you do’ or ‘the way that you do it’? A UK perspective on technique and quality assurance. Clin Oncol 2016;28:e92–e100. https://doi.org/10.1016/j.clon.2016.05.011
Lane JA, Metcalfe C, Young GJ, Peters TJ, Blazeby J, Avery K, et al. Patient-reported outcomes in the ProtecT randomised trial of clinically localised prostate cancer treatments: design and baseline urinary, bowel and sexual function and quality of life. BJU Int 2016;118:869–79.
Turner EL, Metcalfe C, Donovan JL, Noble S, Sterne JA, Lane JA, et al. Contemporary accuracy of death certificates for coding prostate cancer as a cause of death: Is reliance on death certification good enough? A comparison with blinded review by an independent cause of death evaluation committee. Br J Cancer 2016;115:90–4. https://doi.org/10.1038/bjc.2016.162
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415–24.
Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–37.
Hamdy FC. The prostate testing for cancer and treatment (ProtecT) study: what have we learnt? BJU Int 2016;118:843.
Johnston TJ, Shaw GL, Lamb AD, Parashar D, Greenberg D, Xiong T, et al. Mortality among men with advanced prostate cancer excluded from the ProtecT trial. Eur Urol 2017;71:381–8.
Lane JA, Oliver SE, Appleby PN, Lentjes MA, Emmett P, Kuh D, et al. Prostate cancer risk related to foods, food groups, macronutrients and micronutrients derived from the UK Dietary Cohort Consortium food diaries. Eur J Clin Nutr 2017;71:567. https://doi.org/10.1038/ejcn.2017.17
Vickers A, Vertosick EA, Sjoberg DD, Roobol MJ, Hamdy F, Neal D, et al. Properties of the 4-Kallikrein panel outside the diagnostic gray zone: meta-analysis of patients with positive digital rectal examination or prostate specific antigen 10 ng/m and above. J Urol 2017;197:607–13.
Brunner C, Davies NM, Martin RM, Eeles R, Easton D, Kote-Jarai Z, et al. Alcohol consumption and prostate cancer incidence and progression: a Mendelian randomisation study. Int J Cancer 2017;140:75–85. https://doi.org/10.1002/ijc.30436
Leal J, Welton NJ, Martin RM, Donovan J, Hamdy F, Neal D, et al. Estimating the sensitivity of a prostate cancer screening programme for different PSA cut-off levels: a UK case study. Cancer Epidemiol 2018;52:99–105.
Merriel SWD, Turner EL, Walsh E, Young GJ, Metcalfe C, Hounsome L, et al. Cross-sectional study evaluating data quality of the National Cancer Registration and Analysis Service (NCRAS) prostate cancer registry data using the Cluster randomised trial of PSA testing for Prostate cancer (CAP). BMJ Open 2017;7:e015994. https://doi.org/10.1136/bmjopen-2017-015994
Young GJ, Harrison S, Turner EL, Walsh EI, Oliver SE, Ben-Shlomo Y, et al. Prostate-specific antigen (PSA) testing of men in UK general practice: a 10-year longitudinal cohort study. BMJ Open 2017;7:e017729. https://doi.org/10.1136/bmjopen-2017-017729
Camacho N, Van Loo P, Edwards S, Kay JD, Matthews L, Haase K, et al. Appraising the relevance of DNA copy number loss and gain in prostate cancer using whole genome DNA sequence data. PLOS Genet 2017;13:e1007001. https://doi.org/10.1371/journal.pgen.1007001
Er V, Biernacka K, Simpkin AJ, Martin RM, Jeffreys M, Emmett P, et al. Post-diagnosis serum insulin-like growth factors in relation to dietary and lifestyle changes in the Prostate testing for cancer and Treatment (ProtecT) trial. Cancer Causes Control 2017;28:877–88. https://doi.org/10.1007/s10552-017-0910-2
Lin HY, Chen DT, Huang PY, Liu YH, Ochoa A, Zabaleta J, et al. SNP interaction pattern identifier (SIPI): an intensive search for SNP-SNP interaction patterns. Bioinformatics 2017;33:822–33. https://doi.org/10.1093/bioinformatics/btw762
Donovan JL, Young GJ, Walsh EI, Metcalfe C, Lane JA, Martin RM, et al. A prospective cohort and extended comprehensive-cohort design provided insights about the generalizability of a pragmatic trial: the ProtecT prostate cancer trial. J Clin Epidemiol 2018;96:35–46.
Elliott D, Husbands S, Hamdy FC, Holmberg L, Donovan JL. Understanding and improving recruitment to randomised controlled trials: qualitative research approaches. Eur Urol 2017;72:789–98.
Hamdy FC, Donovan JL, Neal DE. 10-year outcomes in localized prostate cancer. N Engl J Med 2017;376:180. https://doi.org/10.1056/NEJMc1614342
Hamdy FC, Donovan JL. Patient-reported outcomes following treatment for localized prostate cancer: helping decision making for patients and their physicians. JAMA 2017;317:1121–3. https://doi.org/10.1001/jama.2017.1703
Wilson C, Rooshenas L, Paramasivan S, Elliott D, Jepson M, Strong S, et al. Development of a framework to improve the process of recruitment to randomised controlled trials (RCTs): the SEAR (Screened, Eligible, Approached, Randomised) framework. Trials 2018;19:50. https://doi.org/10.1186/s13063-017-2413-6
Seibert TM, Fan CC, Wang Y, Zuber V, Karunamuni R, Parsons JK, et al. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ 2018;360:j5757. https://doi.org/10.1136/bmj.j5757
Lophatananon A, Stewart-Brown S, Kote-Jarai Z, Al Olama AA, Garcia SB, Neal DE, et al. Height, selected genetic markers and prostate cancer risk: results from the PRACTICAL consortium. Br J Cancer 2018;118:e16. https://doi.org/10.1038/bjc.2018.6
Gilbert R, Tilling K, Martin RM, Lane JA, Davis M, Hamdy FC, et al. Developing new age-specific prostate-specific antigen thresholds for testing for prostate cancer. Cancer Causes Control 2018;29:383–8. https://doi.org/10.1007/s10552-018-1014-3
Mikropoulos C, Hutten Selkirk CG, Saya S, Bancroft E, Vertosick E, Dadaev T, et al. Prostate-specific antigen velocity in a prospective prostate cancer screening study of men with genetic predisposition. Br J Cancer 2018;118:e17. https://doi.org/10.1038/bjc.2018.11
Vickers A, Vertosick EA, Sjoberg DD, Hamdy F, Neal D, Bjartell A, et al. Value of intact prostate specific antigen and human kallikrein 2 in the four kallikrein predictive model: an individual patient data meta-analysis. J Urol 2018;199:1470–4.
Martin RM, Donovan JL, Turner EL, Metcalfe C, Young GJ, Walsh EI, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018;319:883–95. https://doi.org/10.1001/jama.2018.0154
Noble SM, Garfield K, Lane JA, Metcalfe C, David M, Walsh EI, et al. The ProtecT randomised trial cost-effectiveness analysis comparing active monitoring, surgery, or radiotherapy for prostate cancer. Br J Cancer 2020. https://doi.org/10.1038/s41416-020-0978-4
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Please note that exclusive use will be retained until the publication of major outputs. Access to anonymised data may be granted following review. Data will be managed and shared in a way that safeguards the confidentiality and anonymity of patients and is consistent with the terms of consent signed by patients.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Public Health England and Cancer Research UK . Major Resections by Cancer Site, in England; 2006 to 2010 n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/diagnosis-and-treatment#heading-Two (accessed 15 March 2018).
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. https://doi.org/10.3322/caac.21387.
- Macmillan Cancer Support . People With Prostate Cancer 2014. http://be.macmillan.org.uk/Downloads/CancerInformation/RichPicture/RP-People-with-prostate-cancer.pdf (accessed 15 March 2018).
- Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer 2010;46:3040-52. https://doi.org/10.1016/j.ejca.2010.09.013.
- Johansson E, Steineck G, Holmberg L, Johansson JE, Nyberg T, Ruutu M, et al. SPCG-4 Investigators . Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol 2011;12:891-9. https://doi.org/10.1016/S1470-2045(11)70162-0.
- Hamdy FC. Long-term quality of life in prostate cancer. Lancet Oncol 2011;12:832-3. https://doi.org/10.1016/S1470-2045(11)70187-5.
- Smith DP, King MT, Egger S, Berry MP, Stricker PD, Cozzi P, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ 2009;339. https://doi.org/10.1136/bmj.b4817.
- Miller DC, Sanda MG, Dunn RL, Montie JE, Pimentel H, Sandler HM, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol 2005;23:2772-80. https://doi.org/10.1200/JCO.2005.07.116.
- Potosky AL, Davis WW, Hoffman RM, Stanford JL, Stephenson RA, Penson DF, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst 2004;96:1358-67. https://doi.org/10.1093/jnci/djh259.
- Fransson P, Widmark A. Late side effects unchanged 4-8 years after radiotherapy for prostate carcinoma: a comparison with age-matched controls. Cancer 1999;85:678-88. https://doi.org/10.1002/(SICI)1097-0142(19990201)85:3<678::AID-CNCR18>3.0.CO;2-E.
- Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med 2013;368:436-45. https://doi.org/10.1056/NEJMoa1209978.
- Kerleau C, Guizard AV, Daubisse-Marliac L, Heutte N, Mercier M, Grosclaude P, et al. French Network of Cancer Registries (FRANCIM) . Long-term quality of life among localised prostate cancer survivors: QALIPRO population-based study. Eur J Cancer 2016;63:143-53. https://doi.org/10.1016/j.ejca.2016.05.020.
- Greasley RU, Turner R, Collins K, Brown J, Bourke L, Rosario DJ. Treatment in the STAMPEDE era for castrate resistant prostate cancer in the UK: ongoing challenges and underappreciated clinical problems. BMC Cancer 2018;18. https://doi.org/10.1186/s12885-018-4527-y.
- Stephens RJ, Dearnaley DP, Cowan R, Sydes M, Naylor S, Fallowfield L. RT01 collaborators . The quality of life of men with locally advanced prostate cancer during neoadjuvant hormone therapy: data from the Medical Research Council RT01 trial (ISRCTN 47772397). BJU Int 2007;99:301-10. https://doi.org/10.1111/j.1464-410X.2006.06560.x.
- von Moos R, Body JJ, Egerdie B, Stopeck A, Brown JE, Damyanov D, et al. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support Care Cancer 2013;21:3497-507. https://doi.org/10.1007/s00520-013-1932-2.
- Eton DT, Lepore SJ. Prostate cancer and health-related quality of life: a review of the literature. Psycho-Oncology 2002;11:307-26. https://doi.org/10.1002/pon.572.
- Husson O, Mols F, van de Poll-Franse LV. The relation between information provision and health-related quality of life, anxiety and depression among cancer survivors: a systematic review. Ann Oncol 2011;22:761-72. https://doi.org/10.1093/annonc/mdq413.
- Skolarus TA, Wolf AM, Erb NL, Brooks DD, Rivers BM, Underwood W, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin 2014;64:225-49. https://doi.org/10.3322/caac.21234.
- Brown LC, Ahmed HU, Faria R, El-Shater Bosaily A, Gabe R, Kaplan RS, et al. Multiparametric MRI to improve detection of prostate cancer compared with transrectal ultrasound-guided prostate biopsy alone: the PROMIS study. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22390.
- Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. https://doi.org/10.1016/S0140-6736(16)32401-1.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-8. https://doi.org/10.1056/NEJMoa0810084.
- Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 2009;360:1310-19. https://doi.org/10.1056/NEJMoa0810696.
- Moyer VA. Screening for prostate cancer: U.S. preventive services task force recommendation statement. Ann Intern Med 2012;157:120-34. https://doi.org/10.7326/0003-4819-157-2-201207170-00459.
- Pinsky PF, Prorok PC, Kramer BS. Prostate cancer screening - a perspective on the current state of the evidence. N Engl J Med 2017;376:1285-9. https://doi.org/10.1056/NEJMsb1616281.
- Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415-24. https://doi.org/10.1056/NEJMoa1606220.
- Bibbins-Domingo K, Grossman DC, Curry SJ. The US preventive services task force 2017 draft recommendation statement on screening for prostate cancer: an invitation to review and comment. JAMA 2017;317:1949-50. https://doi.org/10.1001/jama.2017.4413.
- UK National Screening Committee (NSC) . The UK NSC Recommendation on Prostate Cancer Screening PSA Testing in Men Over the Age of 50 2016. http://legacy.screening.nhs.uk/prostatecancer (accessed 15 March 2018).
- Tsodikov A, Gulati R, Heijnsdijk EAM, Pinsky PF, Moss SM, Qiu S, et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO Trials. Ann Intern Med 2017;167:449-55. https://doi.org/10.7326/M16-2586.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384:2027-35. https://doi.org/10.1016/S0140-6736(14)60525-0.
- Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425-37. https://doi.org/10.1056/NEJMoa1606221.
- Heijnsdijk EA, Wever EM, Auvinen A, Hugosson J, Ciatto S, Nelen V, et al. Quality-of-life effects of prostate-specific antigen screening. N Engl J Med 2012;367:595-60. https://doi.org/10.1056/NEJMoa1201637.
- Pataky R, Gulati R, Etzioni R, Black P, Chi KN, Coldman AJ, et al. Is prostate cancer screening cost-effective? A microsimulation model of prostate-specific antigen-based screening for British Columbia, Canada. Int J Cancer 2014;135:939-47. https://doi.org/10.1002/ijc.28732.
- Arnsrud Godtman R, Holmberg E, Lilja H, Stranne J, Hugosson J. Opportunistic testing versus organized prostate-specific antigen screening: outcome after 18 years in the Göteborg randomized population-based prostate cancer screening trial. Eur Urol 2015;68:354-60. https://doi.org/10.1016/j.eururo.2014.12.006.
- Turner EL, Metcalfe C, Donovan JL, Noble S, Sterne JA, Lane JA, et al. Design and preliminary recruitment results of the Cluster randomised triAl of PSA testing for Prostate cancer (CAP). Br J Cancer 2014;110:2829-36. https://doi.org/10.1038/bjc.2014.242.
- Burford D, Kirby M, Austoker J. Prostate Cancer Risk Management Programme. Information for Primary Care; PSA Testing in Asymptomatic Men. Evidence Document 2010. https://webarchive.nationalarchives.gov.uk/20150505144744/www.cancerscreening.nhs.uk/prostate/pcrmp02.pdf (accessed 15 March 2018).
- Martin RM, Donovan JL, Turner EL, Metcalfe C, Young GJ, Walsh EI, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018;319:883-95. https://doi.org/10.1001/jama.2018.0154.
- Walsh EI, Turner EL, Lane JA, Donovan JL, Neal DE, Hamdy FC, et al. the CAP. ProtecT Trial Groups . Characteristics of men responding to an invitation to undergo testing for prostate cancer as part of a randomised trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1624-6.
- Williams N, Hughes LJ, Turner EL, Donovan JL, Hamdy FC, Neal DE, et al. Prostate-specific antigen testing rates remain low in UK general practice: a cross-sectional study in six English cities. BJU Int 2011;108:1402-8. https://doi.org/10.1111/j.1464-410X.2011.10163.x.
- Young GJ, Harrison S, Turner EL, Walsh EI, Oliver SE, Ben-Shlomo Y, et al. Prostate-specific antigen (PSA) testing of men in UK general practice: a 10-year longitudinal cohort study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-017729.
- Moss S, Melia J, Sutton J, Mathews C, Kirby M. Prostate-specific antigen testing rates and referral patterns from general practice data in England. Int J Clin Pract 2016;70:312-18. https://doi.org/10.1111/ijcp.12784.
- Hamdy FC, Donovan JL. Patient-reported outcomes following treatment for localized prostate cancer: helping decision making for patients and their physicians. JAMA 2017;317:1121-3. https://doi.org/10.1001/jama.2017.1703.
- Klotz L. Active surveillance for prostate cancer: trials and tribulations. World J Urol 2008;26:437-42. https://doi.org/10.1007/s00345-008-0330-8.
- van As NJ, Parker CC. Active surveillance with selective radical treatment for localized prostate cancer. Cancer J 2007;13:289-94. https://doi.org/10.1097/PPO.0b013e318156ff65.
- Chodak GW, Thisted RA, Gerber GS, Johansson JE, Adolfsson J, Jones GW, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med 1994;330:242-8. https://doi.org/10.1056/NEJM199401273300403.
- van den Bergh RC, Essink-Bot ML, Roobol MJ, Wolters T, Schröder FH, Bangma CH, et al. Anxiety and distress during active surveillance for early prostate cancer. Cancer 2009;115:3868-78. https://doi.org/10.1002/cncr.24446.
- Klotz L. Active surveillance with selective delayed intervention: a biologically nuanced approach to favorable-risk prostate cancer. Clin Prostate Cancer 2003;2:106-10. https://doi.org/10.3816/CGC.2003.n.017.
- Yaxley JW, Coughlin GD, Chambers SK, Occhipinti S, Samaratunga H, Zajdlewicz L, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet 2016;388:1057-66. https://doi.org/10.1016/S0140-6736(16)30592-X.
- Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Miller DW, Adams JA, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 2005;294:1233-9. https://doi.org/10.1001/jama.294.10.1233.
- D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA 2004;292:821-7. https://doi.org/10.1001/jama.292.7.821.
- Speight JL, Roach M. Radiotherapy in the management of clinically localized prostate cancer: evolving standards, consensus, controversies and new directions. J Clin Oncol 2005;23:8176-85. https://doi.org/10.1200/JCO.2005.03.4629.
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002;360:103-6. https://doi.org/10.1016/S0140-6736(02)09408-4.
- Heidenreich A, Bolla M, Joniau S, Mason MD, Matveev V, Mottet N, et al. Guidelines on Prostate Cancer. European Association of Urology; 2010.
- Kupelian PA, Potters L, Khuntia D, Ciezki JP, Reddy CA, Reuther AM, et al. Radical prostatectomy, external beam radiotherapy < 72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int J Radiat Oncol Biol Phys 2004;58:25-33. https://doi.org/10.1016/S0360-3016(03)00784-3.
- Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet recruitment intervention (QRI). Trials 2016;17. https://doi.org/10.1186/s13063-016-1391-4.
- Barocas DA, Alvarez J, Resnick MJ, Koyama T, Hoffman KE, Tyson MD, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA 2017;317:1126-40. https://doi.org/10.1001/jama.2017.1704.
- Chen RC, Basak R, Meyer A, Kuo T, Carpenter WR, Agans RP, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA 2017;317:1141-50.
- Ahmed HU, Hindley RG, Dickinson L, Freeman A, Kirkham AP, Sahu M, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol 2012;13:622-32. https://doi.org/10.1016/S1470-2045(12)70121-3.
- Azzouzi AR, Vincendeau S, Barret E, Cicco A, Kleinclauss F, van der Poel HG, et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol 2017;18:181-91. https://doi.org/10.1016/S1470-2045(16)30661-1.
- Kim K, Zhang H, La Rosa S, Jebiwott S, Desai P, Kimm S, et al. Bombesin antagonist-based radiotherapy of prostate cancer combined with WST-11 vascular targeted photodynamic therapy. Clin Cancer Res 2017;23:3343-51. https://doi.org/10.1158/1078-0432.CCR-16-2745.
- Zlotta AR, Djavan B, Matos C, Noel JC, Peny MO, Silverman DE, et al. Percutaneous transperineal radiofrequency ablation of prostate tumour: safety, feasibility and pathological effects on human prostate cancer. Br J Urol 1998;81:265-75. https://doi.org/10.1046/j.1464-410X.1998.00504.x.
- Djavan B, Zlotta AR, Susani M, Heinz G, Shariat S, Silverman DE, et al. Transperineal radiofrequency interstitial tumor ablation of the prostate: correlation of magnetic resonance imaging with histopathologic examination. Urology 1997;50:986-92. https://doi.org/10.1016/S0090-4295(97)00540-2.
- Tucker RD, Huidobro C, Larson T. Ablation of stage T1/T2 prostate cancer with permanent interstitial temperature self-regulating rods. J Endourol 2005;19:865-7. https://doi.org/10.1089/end.2005.19.865.
- Shariat SF, Raptidis G, Masatoschi M, Bergamaschi F, Slawin KM. Pilot study of radiofrequency interstitial tumor ablation (RITA) for the treatment of radio-recurrent prostate cancer. Prostate 2005;65:260-7. https://doi.org/10.1002/pros.20242.
- Amin Z, Lees WR, Bown SG. Technical note: interstitial laser photocoagulation for the treatment of prostatic cancer. Br J Radiol 1993;66:1044-7. https://doi.org/10.1259/0007-1285-66-791-1044.
- Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat 2007;6:295-300. https://doi.org/10.1177/153303460700600405.
- Rubinsky J, Onik G, Mikus P, Rubinsky B. Optimal parameters for the destruction of prostate cancer using irreversible electroporation. J Urol 2008;180:2668-74. https://doi.org/10.1016/j.juro.2008.08.003.
- Ramsay CR, Adewuyi TE, Gray J, Hislop J, Shirley MD, Jayakody S, et al. Ablative therapy for people with localised prostate cancer: a systematic review and economic evaluation. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19490.
- Hamdy FC, Elliott D, le Conte S, Davies LC, Burns RM, Thomson C, et al. Partial ablation versus radical prostatectomy in intermediate-risk prostate cancer: the PART feasibility RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22520.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 2012;366:981-90. https://doi.org/10.1056/NEJMoa1113135.
- Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, Church TR, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up. J Natl Cancer Inst 2012;104:125-32. https://doi.org/10.1093/jnci/djr500.
- Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am 2001;28:555-65. https://doi.org/10.1016/S0094-0143(05)70163-4.
- Catalona WJ, Smith DS. 5-year tumor recurrence rates after anatomical radical retropubic prostatectomy for prostate cancer. J Urol 1994;152:1837-42. https://doi.org/10.1016/S0022-5347(17)32397-2.
- Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol 2002;167:528-34. https://doi.org/10.1016/S0022-5347(01)69079-7.
- Trapasso JG, deKernion JB, Smith RB, Dorey F. The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J Urol 1994;152:1821-5. https://doi.org/10.1016/S0022-5347(17)32394-7.
- Zincke H, Oesterling JE, Blute ML, Bergstralh EJ, Myers RP, Barrett DM. Long-term (15 years) results after radical prostatectomy for clinically localized (stage T2c or lower) prostate cancer. J Urol 1994;152:1850-7. https://doi.org/10.1016/S0022-5347(17)32399-6.
- Bill-Axelson A, Holmberg L, Ruutu M, Häggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-84. https://doi.org/10.1056/NEJMoa043739.
- Bill-Axelson A, Holmberg L, Filén F, Ruutu M, Garmo H, Busch C, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst 2008;100:1144-54. https://doi.org/10.1093/jnci/djn255.
- Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2011;364:1708-17. https://doi.org/10.1056/NEJMoa1011967.
- Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012;367:203-13. https://doi.org/10.1056/NEJMoa1113162.
- Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al. Prostate testing for cancer and treatment (ProtecT) feasibility study. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7140.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70. https://doi.org/10.1136/bmj.325.7367.766.
- Brindle LA, Oliver SE, Dedman D, Donovan JL, Neal DE, Hamdy FC, et al. Measuring the psychosocial impact of population-based prostate-specific antigen testing for prostate cancer in the UK. BJU Int 2006;98:777-82. https://doi.org/10.1111/j.1464-410X.2006.06401.x.
- Mills N, Donovan JL, Smith M, Jacoby A, Neal DE, Hamdy FC. Perceptions of equipoise are crucial to trial participation: a qualitative study of men in the ProtecT study. Control Clin Trials 2003;24:272-82. https://doi.org/10.1016/S0197-2456(03)00020-5.
- Burton AJ, Martin RM, Donovan JL, Lane JA, Davis M, Hamdy FC, et al. Associations of lifestyle factors and anthropometric measures with repeat PSA levels during active surveillance/monitoring. Cancer Epidemiol Biomarkers Prev 2012;21:1877-85. https://doi.org/10.1158/1055-9965.EPI-12-0411.
- Lane JA, Wade J, Down L, Bonnington S, Holding PN, Lennon T, et al. A Peer Review Intervention for Monitoring and Evaluating sites (PRIME) that improved randomized controlled trial conduct and performance. J Clin Epidemiol 2011;64:628-36. https://doi.org/10.1016/j.jclinepi.2010.10.003.
- Van Poppel H, Collette L, Kirkali Z, Brausi M, Hoekstra W, Newling DW, et al. EORTC GU Group . Quality control of radical prostatectomy: a feasibility study. Eur J Cancer 2001;37:884-91. https://doi.org/10.1016/S0959-8049(01)00056-9.
- De Koning HJ, Blom J, Merkelbach JW, Raaijmakers R, Verhaegen H, Van Vliet P, et al. Determining the cause of death in randomized screening trial(s) for prostate cancer. BJU Int 2003;92:71-8. https://doi.org/10.1111/j.1465-5101.2003.04402.x.
- Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004;23:322-30. https://doi.org/10.1002/nau.20041.
- Donovan JL, Peters TJ, Abrams P, Brookes ST, de aa Rosette JJ, Schäfer W. Scoring the short form ICSmaleSF questionnaire. International Continence Society. J Urol 2000;164:1948-55. https://doi.org/10.1016/S0022-5347(05)66926-1.
- Frankel SJ, Donovan JL, Peters TI, Abrams P, Dabhoiwala NF, Osawa D, et al. Sexual dysfunction in men with lower urinary tract symptoms. J Clin Epidemiol 1998;51:677-85. https://doi.org/10.1016/S0895-4356(98)00044-4.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European-organization-for-research-and-treatment-of-cancer Qlq-C30: a quality-of-life instrument for use in international clinical-trials in oncology. J Natl Cancer I 1993;85:365-76. https://doi.org/10.1093/jnci/85.5.365.
- Zigmond AS, Snaith RP. The Hospital Anxiety And Depression Scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA Project. International quality of life assessment. J Clin Epidemiol 1998;51:1171-8. https://doi.org/10.1016/S0895-4356(98)00109-7.
- EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Donovan JL, Young GJ, Walsh EI, Metcalfe C, Lane JA, Martin RM, et al. A prospective cohort and extended comprehensive-cohort design provided insights about the generalizability of a pragmatic trial: the ProtecT prostate cancer trial. J Clin Epidemiol 2018;96:35-46. https://doi.org/10.1016/j.jclinepi.2017.12.019.
- Metcalfe C, Peters T, Hamdy FC. Prostate Testing for Cancer and Treatment (ProtecT) Study: Statistical Analysis Plan. Bristol: University of Bristol; 2015.
- Turner EL, Metcalfe C, Donovan JL, Noble S, Sterne JA, Lane JA, et al. Contemporary accuracy of death certificates for coding prostate cancer as a cause of death: is reliance on death certification good enough? A comparison with blinded review by an independent cause of death evaluation committee. Br J Cancer 2016;115:90-4. https://doi.org/10.1038/bjc.2016.162.
- Bauer P. Multiple testing in clinical trials. Stat Med 1991;10:871-89. https://doi.org/10.1002/sim.4780100609.
- Hampson LV, Metcalfe C. Incorporating prognostic factors into causal estimators: a comparison of methods for randomised controlled trials with a time-to-event outcome. Stat Med 2012;31:3073-88. https://doi.org/10.1002/sim.5411.
- Loeys T, Goetghebeur E. A causal proportional hazards estimator for the effect of treatment actually received in a randomized trial with all-or-nothing compliance. Biometrics 2003;59:100-5. https://doi.org/10.1111/1541-0420.00012.
- Lane JA, Howson J, Donovan JL, Goepel JR, Dedman DJ, Down L, et al. Detection of prostate cancer in unselected young men: prospective cohort nested within a randomised controlled trial. BMJ 2007;335. https://doi.org/10.1136/bmj.39381.436829.BE.
- Donovan JL, Lane JA, Peters TJ, Brindle L, Salter E, Gillatt D, et al. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. J Clin Epidemiol 2009;62:29-36. https://doi.org/10.1016/j.jclinepi.2008.02.010.
- Wade J, Holding PN, Bonnington S, Rooshenas L, Lane JA, Salter CE, et al. Establishing nurse-led active surveillance for men with localised prostate cancer: development and formative evaluation of a model of care in the ProtecT trial. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008953.
- Lane JA, Donovan JL, Davis M, Walsh E, Dedman D, Down L, et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014;15:1109-18. https://doi.org/10.1016/S1470-2045(14)70361-4.
- Donovan J. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70. https://doi.org/10.1136/bmj.325.7367.766.
- Wade J, Donovan JL, Lane JA, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018-28. https://doi.org/10.1016/j.socscimed.2009.02.023.
- Mills N, Donovan JL, Wade J, Hamdy FC, Neal DE, Lane JA. Exploring treatment preferences facilitated recruitment to randomized controlled trials. J Clin Epidemiol 2011;64:1127-36. https://doi.org/10.1016/j.jclinepi.2010.12.017.
- de Salis I, Tomlin Z, Toerien M, Donovan J. Qualitative research to improve RCT recruitment: issues arising in establishing research collaborations. Contemp Clin Trials 2008;29:663-70. https://doi.org/10.1016/j.cct.2008.03.003.
- Hamilton DW, de Salis I, Donovan JL, Birchall M. The recruitment of patients to trials in head and neck cancer: a qualitative study of the EaStER trial of treatments for early laryngeal cancer. Eur Arch Otorhinolaryngol 2013;270:2333-7. https://doi.org/10.1007/s00405-013-2349-8.
- Howard L, de Salis I, Tomlin Z, Thornicroft G, Donovan J. Why is recruitment to trials difficult? An investigation into recruitment difficulties in an RCT of supported employment in patients with severe mental illness. Contemp Clin Trials 2009;30:40-6. https://doi.org/10.1016/j.cct.2008.07.007.
- Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016;388:2004-14. https://doi.org/10.1016/S0140-6736(16)30825-X.
- Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 2014;311:263-70. https://doi.org/10.1001/jama.2013.285718.
- Hay AD, Redmond NM, Costelloe C, Montgomery AA, Fletcher M, Hollinghurst S, et al. Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial. Health Technol Assess 2009;13. https://doi.org/10.3310/hta13270.
- Griffin D, Wall P, Realpe A, Adams A, Parsons N, Hobson R, et al. UK FASHIoN: feasibility study of a randomised controlled trial of arthroscopic surgery for hip impingement compared with best conservative care. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20320.
- Paramasivan S, Huddart R, Hall E, Lewis R, Birtle A, Donovan JL. Key issues in recruitment to randomised controlled trials with very different interventions: a qualitative investigation of recruitment to the SPARE trial (CRUK/07/011). Trials 2011;12. https://doi.org/10.1186/1745-6215-12-78.
- Avery KN, Blazeby JM, Lane JA, Neal DE, Hamdy FC, Donovan JL. Decision-making about PSA testing and prostate biopsies: a qualitative study embedded in a primary care randomised trial. Eur Urol 2008;53:1186-93. https://doi.org/10.1016/j.eururo.2007.07.040.
- Paramasivan S, Strong S, Wilson C, Campbell B, Blazeby JM, Donovan JL. A simple technique to identify key recruitment issues in randomised controlled trials: Q-QAT - Quanti-Qualitative Appointment Timing. Trials 2015;16. https://doi.org/10.1186/s13063-015-0617-1.
- Realpe A, Adams A, Wall P, Griffin D, Donovan JL. A new simple six-step model to promote recruitment to RCTs was developed and successfully implemented. J Clin Epidemiol 2016;76:166-74. https://doi.org/10.1016/j.jclinepi.2016.02.002.
- Avery KN, Donovan JL, Horwood J, Neal DE, Hamdy FC, Parker C, et al. The importance of dietary change for men diagnosed with and at risk of prostate cancer: a multi-centre interview study with men, their partners and health professionals. BMC Fam Pract 2014;15. https://doi.org/10.1186/1471-2296-15-81.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15:1-12. https://doi.org/10.1186/1745-6215-15-5.
- Mills N, Blazeby JM, Hamdy FC, Neal DE, Campbell B, Wilson C, et al. Training recruiters to randomized trials to facilitate recruitment and informed consent by exploring patients’ treatment preferences. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-323.
- Rooshenas L, Elliott D, Wade J, Jepson M, Paramasivan S, Strong S, et al. Conveying equipoise during recruitment for clinical trials: qualitative synthesis of clinicians’ practices across six randomised controlled trials. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002147.
- Wade J, Elliott D, Avery KNL, Gaunt D, Young GJ, Barnes R, et al. Informed consent in randomised controlled trials: development and preliminary evaluation of a measure of Participatory and Informed Consent (PIC). Trials 2017;18. https://doi.org/10.1186/s13063-017-2048-7.
- Mills N, Gaunt D, Blazeby JM, Elliott D, Husbands S, Holding P, et al. Training health professionals to recruit into challenging randomized controlled trials improved confidence: the development of the QuinteT randomized controlled trial recruitment training intervention. J Clin Epidemiol 2018;95:34-4. https://doi.org/10.1016/j.jclinepi.2017.11.015.
- Wilson C, Rooshenas L, Paramasivan S, Elliott D, Jepson M, Strong S, et al. Development of a framework to improve the process of recruitment to randomised controlled trials (RCTs): the SEAR (Screened, Eligible, Approached, Randomised) framework. Trials 2018;19. https://doi.org/10.1186/s13063-017-2413-6.
- Lane A, Metcalfe C, Young GJ, Peters TJ, Blazeby J, Avery KN, et al. Patient-reported outcomes in the ProtecT randomized trial of clinically localized prostate cancer treatments: study design, and baseline urinary, bowel and sexual function and quality of life. BJU Int 2016;118:869-79. https://doi.org/10.1111/bju.13582.
- Williams NJ, Hill EM, Ng SY, Martin RM, Metcalfe C, Donovan JL, et al. Standardisation of information submitted to an endpoint committee for cause of death assignment in a cancer screening trial – lessons learnt from CAP (Cluster randomised triAl of PSA testing for Prostate cancer). BMC Med Res Methodol 2015;15. https://doi.org/10.1186/1471-2288-15-6.
- Roach M, Hanks G, Thames H, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965-74. https://doi.org/10.1016/j.ijrobp.2006.04.029.
- Sydes MR, Stephens RJ, Moore AR, Aird EG, Bidmead AM, Fallowfield LJ, et al. Implementing the UK Medical Research Council (MRC) RT01 trial (ISRCTN 47772397): methods and practicalities of a randomised controlled trial of conformal radiotherapy in men with localised prostate cancer. Radiother Oncol 2004;72:199-211. https://doi.org/10.1016/j.radonc.2004.04.007.
- Mayles WP, Moore AR, Aird EG, Bidmead AM, Dearnaley DP, Griffiths SE, et al. RT01 collaborators . Questionnaire based quality assurance for the RT01 trial of dose escalation in conformal radiotherapy for prostate cancer (ISRCTN 47772397). Radiother Oncol 2004;73:199-207. https://doi.org/10.1016/j.radonc.2004.08.017.
- Mason MD, Moore R, Jones G, Lewis G, Donovan JL, Neal DE, et al. Radiotherapy for prostate cancer: is it ‘what you do’ or ‘the way that you do it’? A UK perspective on technique and quality assurance. Clin Oncol 2016;28:e92-e100. https://doi.org/10.1016/j.clon.2016.05.011.
- D’Amico AV, Desjardin A, Chung A, Chen MH, Schultz D, Whittington R, et al. Assessment of outcome prediction models for patients with localized prostate carcinoma managed with radical prostatectomy or external beam radiation therapy. Cancer 1998;82:1887-96. https://doi.org/10.1002/(SICI)1097-0142(19980515)82:10<1887::AID-CNCR11>3.0.CO;2-P.
- D’Amico AV, Hui-Chen M, Renshaw AA, Sussman B, Roehl KA, Catalona WJ. Identifying men diagnosed with clinically localized prostate cancer who are at high risk for death from prostate cancer. J Urol 2006;176:S11-5. https://doi.org/10.1016/j.juro.2006.06.075.
- D’Amico AV, Whittington R, Malkowicz SB, Cote K, Loffredo M, Schultz D, et al. Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localized prostate carcinoma in the prostate specific antigen era. Cancer 2002;95:281-6. https://doi.org/10.1002/cncr.10657.
- Oxley J, Simpkin A, Goepel J, Varma M, Griffiths D, Grigor K, et al. Gleason drift in the NIHR ProtecT study. Histopathology 2015;66:438-46. https://doi.org/10.1111/his.12549.
- Chen ME, Johnston D, Reyes AO, Soto CP, Babaian RJ, Troncoso P. A streamlined three-dimensional volume estimation method accurately classifies prostate tumors by volume. Am J Surg Pathol 2003;27:1291-301. https://doi.org/10.1097/00000478-200310000-00001.
- Epstein JI, Carmichael M, Partin AW, Walsh PC. Is tumor volume an independent predictor of progression following radical prostatectomy? A multivariate analysis of 185 clinical stage B adenocarcinomas of the prostate with 5 years of followup. J Urol 1993;149:1478-81. https://doi.org/10.1016/S0022-5347(17)36421-2.
- Bostwick DG. Grading prostate cancer. Am J Clin Pathol 1994;102:38-56.
- Sogani PC, Israel A, Lieberman PH, Lesser ML, Whitmore WF. Gleason grading of prostate cancer: a predictor of survival. Urology 1985;25:223-7. https://doi.org/10.1016/0090-4295(85)90316-4.
- Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol 2004;17:292-306. https://doi.org/10.1038/modpathol.3800054.
- Kulac I, Haffner MC, Yegnasubramanian S, Epstein JI, De Marzo AM. Should Gleason 6 be labeled as cancer?. Curr Opin Urol 2015;25:238-45. https://doi.org/10.1097/MOU.0000000000000165.
- Haffner MC, Mosbruger T, Esopi DM, Fedor H, Heaphy CM, Walker DA, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest 2013;123:4918-22. https://doi.org/10.1172/JCI70354.
- Moschini M, Spahn M, Mattei A, Cheville J, Karnes RJ. Incorporation of tissue-based genomic biomarkers into localized prostate cancer clinics. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0613-7.
- Cucchiara V, Cooperberg MR, Dall’Era M, Lin DW, Montorsi F, Schalken JA, et al. Genomic markers in prostate cancer decision making. Eur Urol 2018;73:572-82. https://doi.org/10.1016/j.eururo.2017.10.036.
- Cancer Genome Atlas Research Network . The molecular taxonomy of primary prostate cancer. Cell 2015;163:1011-25. https://doi.org/10.1016/j.cell.2015.10.025.
- Iremashvili V, Manoharan M, Rosenberg DL, Soloway MS. Biopsy features associated with prostate cancer progression in active surveillance patients: comparison of three statistical models. BJU Int 2013;111:574-9. https://doi.org/10.1111/j.1464-410X.2012.11127.x.
- Barayan GA, Brimo F, Bégin LR, Hanley JA, Liu Z, Kassouf W, et al. Factors influencing disease progression of prostate cancer under active surveillance: a McGill University Health Center cohort. BJU Int 2014;114:E99-E104. https://doi.org/10.1111/bju.12754.
- Fall K, Garmo H, Andrén O, Bill-Axelson A, Adolfsson J, Adami HO, et al. Prostate-specific antigen levels as a predictor of lethal prostate cancer. J Natl Cancer Inst 2007;99:526-32. https://doi.org/10.1093/jnci/djk110.
- Mohan R, Schellhammer PF. Treatment options for localized prostate cancer. Am Fam Physician 2011;84:413-20.
- Zagars GK. Prostate-specific antigen as a prognostic factor for prostate cancer treated by external beam radiotherapy. Int J Radiat Oncol Biol Phys 1992;23:47-53. https://doi.org/10.1016/0360-3016(92)90542-P.
- Bokhorst LP, Valdagni R, Rannikko A, Kakehi Y, Pickles T, Bangma CH, et al. PRIAS study group . A decade of active surveillance in the PRIAS Study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol 2016;70:954-60. https://doi.org/10.1016/j.eururo.2016.06.007.
- Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33:272-7. https://doi.org/10.1200/JCO.2014.55.1192.
- Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med 2008;148:435-48. https://doi.org/10.7326/0003-4819-148-6-200803180-00209.
- Chou R, Croswell JM, Dana T, Bougatsos C, Blazina I, Fu R, et al. Screening for prostate cancer: a review of the evidence for the U.S. preventive services task force. Ann Intern Med 2011;155:762-71. https://doi.org/10.7326/0003-4819-155-11-201112060-00375.
- Chen RC, Chang P, Vetter RJ, Lukka H, Stokes WA, Sanda MG, et al. Recommended patient-reported core set of symptoms to measure in prostate cancer treatment trials. J Natl Cancer Inst 2014;106. https://doi.org/10.1093/jnci/dju132.
- Whiting PF, Moore TH, Jameson CM, Davies P, Rowlands MA, Burke M, et al. Symptomatic and quality-of-life outcomes after treatment for clinically localised prostate cancer: a systematic review. BJU Int 2016;118:193-204. https://doi.org/10.1111/bju.13499.
- Bellardita L, Valdagni R, van den Bergh R, Randsdorp H, Repetto C, Venderbos LD, et al. How does active surveillance for prostate cancer affect quality of life? A systematic review. Eur Urol 2015;67:637-45. https://doi.org/10.1016/j.eururo.2014.10.028.
- Steineck G, Helgesen F, Adolfsson J, Dickman PW, Johansson JE, Norlén BJ, et al. Scandinavian prostatic cancer group study number 4 . Quality of life after radical prostatectomy or watchful waiting. N Engl J Med 2002;347:790-6. https://doi.org/10.1056/NEJMoa021483.
- Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. CONSORT PRO Group . Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013;309:814-22. https://doi.org/10.1001/jama.2013.879.
- Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000;56:899-905. https://doi.org/10.1016/S0090-4295(00)00858-X.
- Martin NE, Massey L, Stowell C, Bangma C, Briganti A, Bill-Axelson A, et al. Defining a standard set of patient-centered outcomes for men with localized prostate cancer. Eur Urol 2015;67:460-7. https://doi.org/10.1016/j.eururo.2014.08.075.
- Bill-Axelson A, Garmo H, Holmberg L, Johansson JE, Adami HO, Steineck G, et al. Long-term distress after radical prostatectomy versus watchful waiting in prostate cancer: a longitudinal study from the Scandinavian Prostate Cancer Group-4 randomized clinical trial. Eur Urol 2013;64:920-8. https://doi.org/10.1016/j.eururo.2013.02.025.
- Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250-61. https://doi.org/10.1056/NEJMoa074311.
- Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, Litwin MS, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol 2002;20:557-66. https://doi.org/10.1200/JCO.2002.20.2.557.
- Parker PA, Davis JW, Latini DM, Baum G, Wang X, Ward JF, et al. Relationship between illness uncertainty, anxiety, fear of progression and quality of life in men with favourable-risk prostate cancer undergoing active surveillance. BJU Int 2016;117:469-77. https://doi.org/10.1111/bju.13099.
- Buckley BS, Lapitan MC, Glazener CM. MAPS Trial Group . The effect of urinary incontinence on health utility and health-related quality of life in men following prostate surgery. Neurourol Urodyn 2012;31:465-9. https://doi.org/10.1002/nau.21231.
- Noble SM, Garfield K, Lane JA, Metcalfe C, David M, Walsh EI, et al. The ProtecT randomised trial cost-effectiveness analysis comparing active monitoring, surgery, or radiotherapy for prostate cancer. Br J Cancer 2020. https://doi.org/10.1038/s41416-020-0978-4.
- Department of Health and Social Care . NHS Reference Costs 2014 to 2015 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 15 March 2018).
- NHS Business Services Authority . Dictionary of Medicines and Devices n.d. https://www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/dictionary-medicines-and-devices-dmd (accessed 18 April 2018).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- NHS Digital. HRG4+ 2014/15 . Reference Cost Grouper 2014 2015 n.d. http://webarchive.nationalarchives.gov.uk/20171012004847/http://content.digital.nhs.uk/article/6226/HRG4-201415-Reference-Cost-Grouper (accessed 18 April 2018).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- NICE . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/process/pmg9/chapter/the-reference-case#framework-for-estimating-clinical-and-cost-effectiveness (accessed 18 April 2018).
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Briggs AH. A Bayesian approach to stochastic cost-effectiveness analysis. Health Econ 1999;8:257-61. https://doi.org/10.1002/(SICI)1099-1050(199905)8:3<257::AID-HEC427>3.0.CO;2-E.
- Murray SA, Kendall M, Carduff E, Worth A, Harris FM, Lloyd A, et al. Use of serial qualitative interviews to understand patients’ evolving experiences and needs. BMJ 2009;339. https://doi.org/10.1136/bmj.b3702.
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. London: SAGE Publications Ltd; 2014.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory. New York, NY: Aldine; 1967.
- Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017;71:618-29. https://doi.org/10.1016/j.eururo.2016.08.003.
- GOV.UK . Prostate Cancer Risk Management Programme: Overview n.d. www.gov.uk/guidance/prostate-cancer-risk-management-programme-overview (accessed 2 September 2019).
- Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al. The impact of screening on future health-promoting behaviours and health beliefs: a systematic review. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7420.
- Raaijmakers R, Kirkels WJ, Roobol MJ, Wildhagen MF, Schrder FH. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology 2002;60:826-30. https://doi.org/10.1016/S0090-4295(02)01958-1.
- Djavan B, Waldert M, Zlotta A, Dobronski P, Seitz C, Remzi M, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol 2001;166:856-60. https://doi.org/10.1016/S0022-5347(05)65851-X.
- Leippold T, Preusser S, Engeler D, Inhelder F, Schmid HP. Prostate biopsy in Switzerland: a representative survey on how Swiss urologists do it. Scand J Urol Nephrol 2008;42:18-23. https://doi.org/10.1080/00365590701520503.
- Lee G, Attar K, Laniado M, Karim O. Trans-rectal ultrasound guided biopsy of the prostate: nationwide diversity in practice and training in the United Kingdom. Int Urol Nephrol 2007;39:185-8. https://doi.org/10.1007/s11255-006-6654-7.
- Irani J, Fournier F, Bon D, Gremmo E, Doré B, Aubert J. Patient tolerance of transrectal ultrasound-guided biopsy of the prostate. Br J Urol 1997;79:608-10. https://doi.org/10.1046/j.1464-410X.1997.00120.x.
- Chapple AB, Ziebland S, Brewster S, McPherson A. Patients’ perceptions of transrectal prostate biopsy: a qualitative study. Eur J Cancer Care 2007;16:215-21. https://doi.org/10.1111/j.1365-2354.2006.00766.x.
- Oliffe JL, Davison BJ, Pickles T, Mróz L. The self-management of uncertainty among men undertaking active surveillance for low-risk prostate cancer. Qual Health Res 2009;19:432-43. https://doi.org/10.1177/1049732309332692.
- Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, Goodwin L, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 2012;344. https://doi.org/10.1136/bmj.d7894.
- Wade J, Rosario DJ, Howson J, Avery KN, Salter CE, Goodwin ML, et al. Role of information in preparing men for transrectal ultrasound guided prostate biopsy: a qualitative study embedded in the ProtecT trial. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-0729-z.
- Walsh E, Hegarty J. Men’s experiences of radical prostatectomy as treatment for prostate cancer. Eur J Oncol Nurs 2010;14:125-33. https://doi.org/10.1016/j.ejon.2009.10.003.
- Petry H, Berry DL, Spichiger E, Kesselring A, Gasser TC, Sulser T, et al. Responses and experiences after radical prostatectomy: perceptions of married couples in Switzerland. Int J Nurs Stud 2004;41:507-13. https://doi.org/10.1016/j.ijnurstu.2003.11.005.
- Kelsey SG, Owens J, White A. The experience of radiotherapy for localized prostate cancer: the men’s perspective. Eur J Cancer Care 2004;13:272-8. https://doi.org/10.1111/j.1365-2354.2004.00487.x.
- Watson E, Hewitson P, Brett J, Bukach C, Evans R, Edwards A, et al. Informed decision making and prostate specific antigen (PSA) testing for prostate cancer: a randomised controlled trial exploring the impact of a brief patient decision aid on men’s knowledge, attitudes and intention to be tested. Patient Educ Couns 2006;63:367-79. https://doi.org/10.1016/j.pec.2006.05.005.
- Ruane-McAteer E, Porter S, O’Sullivan JM, Santin O, Prue G. Active surveillance for favorable-risk prostate cancer: Is there a greater psychological impact than previously thought? A systematic, mixed studies literature review. Psycho-Oncology 2017;26:1411-21. https://doi.org/10.1002/pon.4311.
- Carter G, Clover K, Britton B, Mitchell AJ, White M, McLeod N, et al. Wellbeing during active surveillance for localised prostate cancer: a systematic review of psychological morbidity and quality of life. Cancer Treat Rev 2015;41:46-60. https://doi.org/10.1016/j.ctrv.2014.11.001.
- O’Callaghan C, Dryden T, Hyatt A, Brooker J, Burney S, Wootten AC, et al. ‘What is this active surveillance thing?’ Men’s and partners’ reactions to treatment decision making for prostate cancer when active surveillance is the recommended treatment option. Psychooncology 2014;23:1391-8. https://doi.org/10.1002/pon.3576.
- Volk RJ, McFall SL, Cantor SB, Byrd TL, Le YC, Kuban DA, et al. ‘It’s not like you just had a heart attack’: decision-making about active surveillance by men with localized prostate cancer. Psychooncology 2014;23:467-72. https://doi.org/10.1002/pon.3444.
- Simpkin AJ, Rooshenas L, Wade J, Donovan JL, Lane JA, Martin RM, et al. Development, validation and evaluation of an instrument for active monitoring of men with clinically localised prostate cancer: systematic review, cohort studies and qualitative study. Health Serv Deliv Res 2015;3. https://doi.org/10.3310/hsdr03300.
- NIHR Cancer Research Network . Impact of Patient, Carer and Public Involvement in Cancer Research 2012. www.ncri.org.uk/wp-content/uploads/2013/07/2012-NCRI-PPI-report.pdf (accessed 15 March 2018).
- n.d. www.crn.nihr.ac.uk/wp-content/uploads/cancer/sites/7/Introductory-day-28.05.14-slides.pdf (accessed 15 March 2018).
- Pavitt S. The Impact of Patient Public Involvement on Clinical Research 2011. https://ec.europa.eu/research/health/pdf/event05/sue-pavitt_en.pdf (accessed 15 March 2018).
- Bostwick DG, Waters DJ, Farley ER, Meiers I, Rukstalis D, Cavanaugh WA, et al. Group consensus reports from the consensus conference on focal treatment of prostatic carcinoma, celebration, Florida, February 24, 2006. Urology 2007;70:42-4. https://doi.org/10.1016/j.urology.2007.07.037.
- Wong LM, Neal DE, Finelli A, Davis S, Bonner C, Kapoor J, et al. Evaluation of models predicting insignificant prostate cancer to select men for active surveillance of prostate cancer. Prostate Cancer Prostatic Dis 2015;18:137-43. https://doi.org/10.1038/pcan.2015.1.
- Shaw GL, Thomas BC, Dawson SN, Srivastava G, Vowler SL, Gnanapragasam VJ, et al. Identification of pathologically insignificant prostate cancer is not accurate in unscreened men. Br J Cancer 2014;110:2405-11. https://doi.org/10.1038/bjc.2014.192.
- Wong LM, Johnston R, Sharma N, Shah NC, Warren AY, Neal DE. General application of the National Institute for Health and Clinical Excellence (NICE) guidance for active surveillance for men with prostate cancer is not appropriate in unscreened populations. BJU Int 2012;110:24-7. https://doi.org/10.1111/j.1464-410X.2011.10732.x.
- Johnston R, Wong LM, Warren A, Shah N, Neal D. The role of 1.5 Tesla magnetic resonance imaging in staging prostate cancer. ANZ J Surg 2013;83:234-8. https://doi.org/10.1111/ans.12094.
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387:1163-77. https://doi.org/10.1016/S0140-6736(15)01037-5.
- Ross-Adams H, Lamb AD, Dunning MJ, Halim S, Lindberg J, Massie CM, et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: a discovery and validation cohort study. EBioMedicine 2015;2:1133-44. https://doi.org/10.1016/j.ebiom.2015.07.017.
- Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015;520:353-7. https://doi.org/10.1038/nature14347.
- Johnston TJ, Shaw GL, Lamb AD, Parashar D, Greenberg D, Xiong T, et al. Mortality among men with advanced prostate cancer excluded from the ProtecT trial. Eur Urol 2017;71:381-8. https://doi.org/10.1016/j.eururo.2016.09.040.
- Warren AY, Whitaker HC, Haynes B, Sangan T, McDuffus LA, Kay JD, et al. Method for sampling tissue for research which preserves pathological data in radical prostatectomy. Prostate 2013;73:194-202. https://doi.org/10.1002/pros.22556.
- Gill PS, Roberts IS, Browning L, Perera R, Warren AY, Hamdy FC, et al. The handling and sampling of radical prostatectomy specimens for reporting and research: the Oxford approach. J Clin Pathol 2012;65:1057-61. https://doi.org/10.1136/jclinpath-2012-200923.
- Elliott KS, Zeggini E, McCarthy MI, Gudmundsson J, Sulem P, Stacey SN, et al. Evaluation of association of HNF1B variants with diverse cancers: collaborative analysis of data from 19 genome-wide association studies. PLOS ONE 2010;5. https://doi.org/10.1371/journal.pone.0010858.
- Kote-Jarai Z, Leongamornlert D, Tymrakiewicz M, Field H, Guy M, Al Olama AA, et al. Mutation analysis of the MSMB gene in familial prostate cancer. Br J Cancer 2010;102:414-18. https://doi.org/10.1038/sj.bjc.6605485.
- Kote-Jarai Z, Amin Al Olama A, Leongamornlert D, Tymrakiewicz M, Saunders E, Guy M, et al. Identification of a novel prostate cancer susceptibility variant in the KLK3 gene transcript. Hum Genet 2011;129:687-94. https://doi.org/10.1007/s00439-011-0981-1.
- Schumacher FR, Berndt SI, Siddiq A, Jacobs KB, Wang Z, Lindstrom S, et al. Genome-wide association study identifies new prostate cancer susceptibility loci. Hum Mol Genet 2011;20:3867-75. https://doi.org/10.1093/hmg/ddr295.
- Cheng I, Chen GK, Nakagawa H, He J, Wan P, Laurie CC, et al. Evaluating genetic risk for prostate cancer among Japanese and Latinos. Cancer Epidemiol Biomarkers Prev 2012;21:2048-58. https://doi.org/10.1158/1055-9965.EPI-12-0598.
- Al Olama AA, Kote-Jarai Z, Schumacher FR, Wiklund F, Berndt SI, Benlloch S, et al. A meta-analysis of genome-wide association studies to identify prostate cancer susceptibility loci associated with aggressive and non-aggressive disease. Hum Mol Genet 2013;22:408-15. https://doi.org/10.1093/hmg/dds425.
- Pooley KA, Bojesen SE, Weischer M, Nielsen SF, Thompson D, Amin Al Olama A, et al. A genome-wide association scan (GWAS) for mean telomere length within the COGS project: identified loci show little association with hormone-related cancer risk. Hum Mol Genet 2013;22:5056-64. https://doi.org/10.1093/hmg/ddt355.
- Orozco G, Goh CL, Al Olama AA, Benlloch-Garcia S, Govindasami K, Guy M, et al. Common genetic variants associated with disease from genome-wide association studies are mutually exclusive in prostate cancer and rheumatoid arthritis. BJU Int 2013;111:1148-55. https://doi.org/10.1111/j.1464-410X.2012.11492.x.
- Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet 2013;45:385-91. https://doi.org/10.1038/ng.2560.
- Al Olama AA, Kote-Jarai Z, Berndt SI, Conti DV, Schumacher F, Han Y, et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet 2014;46:1103-9. https://doi.org/10.1038/ng.3094.
- Knipe DW, Evans DM, Kemp JP, Eeles R, Easton DF, Kote-Jarai Z, et al. Genetic variation in prostate-specific antigen-detected prostate cancer and the effect of control selection on genetic association studies. Cancer Epidemiol Biomarkers Prev 2014;23:1356-65. https://doi.org/10.1158/1055-9965.EPI-13-0889.
- Weng PH, Huang YL, Page JH, Chen JH, Xu J, Koutros S, et al. Polymorphisms of an innate immune gene, toll-like receptor 4, and aggressive prostate cancer risk: a systematic review and meta-analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0110569.
- Al Olama AA, Dadaev T, Hazelett DJ, Li Q, Leongamornlert D, Saunders EJ, et al. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum Mol Genet 2015;24:5589-602. https://doi.org/10.1093/hmg/ddv203.
- Al Olama AA, Benlloch S, Antoniou AC, Giles GG, Severi G, Neal DE, et al. Risk analysis of prostate cancer in PRACTICAL, a multinational consortium, using 25 known prostate cancer susceptibility loci. Cancer Epidemiol Biomarkers Prev 2015;24:1121-9. https://doi.org/10.1158/1055-9965.EPI-14-0317.
- Pashayan N, Duffy SW, Chowdhury S, Dent T, Burton H, Neal DE, et al. Polygenic susceptibility to prostate and breast cancer: implications for personalised screening. Br J Cancer 2011;104:1656-63. https://doi.org/10.1038/bjc.2011.118.
- Yu OH, Foulkes WD, Dastani Z, Martin RM, Eeles R, Consortium P, et al. An assessment of the shared allelic architecture between type II diabetes and prostate cancer. Cancer Epidemiol Biomarkers Prev 2013;22:1473-5. https://doi.org/10.1158/1055-9965.EPI-13-0476.
- Ju YS, Alexandrov LB, Gerstung M, Martincorena I, Nik-Zainal S, Ramakrishna M, et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. Elife 2014;3. https://doi.org/10.7554/eLife.02935.
- Tubio JMC, Li Y, Ju YS, Martincorena I, Cooke SL, Tojo M, et al. Mobile DNA in cancer. Extensive transduction of nonrepetitive DNA mediated by L1 retrotransposition in cancer genomes. Science 2014;345. https://doi.org/10.1126/science.1251343.
- Sud A, Thomsen H, Law PJ, Försti A, Filho MIDS, Holroyd A, et al. Genome-wide association study of classical Hodgkin lymphoma identifies key regulators of disease susceptibility. Nat Commun 2017;8. https://doi.org/10.1038/s41467-017-00320-1.
- Taylor AE, Martin RM, Geybels MS, Stanford JL, Shui I, Eeles R, et al. Investigating the possible causal role of coffee consumption with prostate cancer risk and progression using Mendelian randomization analysis. Int J Cancer 2017;140:322-8. https://doi.org/10.1002/ijc.30462.
- Seibert TM, Fan CC, Wang Y, Zuber V, Karunamuni R, Parsons JK, et al. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ 2018;360. https://doi.org/10.1136/bmj.j5757.
- Pashayan N, Duffy SW, Neal DE, Hamdy FC, Donovan JL, Martin RM, et al. Implications of polygenic risk-stratified screening for prostate cancer on overdiagnosis. Genet Med 2015;17:789-95. https://doi.org/10.1038/gim.2014.192.
- Szulkin R, Karlsson R, Whitington T, Aly M, Gronberg H, Eeles RA, et al. Genome-wide association study of prostate cancer-specific survival. Cancer Epidemiol Biomarkers Prev 2015;24:1796-800. https://doi.org/10.1158/1055-9965.EPI-15-0543.
- Szulkin R, Whitington T, Eklund M, Aly M, Eeles RA, Easton D, et al. Prediction of individual genetic risk to prostate cancer using a polygenic score. Prostate 2015;75:1467-74. https://doi.org/10.1002/pros.23037.
- Bonilla C, Lewis SJ, Martin RM, Donovan JL, Hamdy FC, Neal DE, et al. Pubertal development and prostate cancer risk: Mendelian randomization study in a population-based cohort. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0602-x.
- Bonilla C, Lewis SJ, Rowlands MA, Gaunt TR, Davey Smith G, Gunnell D, et al. Assessing the role of insulin-like growth factors and binding proteins in prostate cancer using Mendelian randomization: Genetic variants as instruments for circulating levels. Int J Cancer 2016;139:1520-33. https://doi.org/10.1002/ijc.30206.
- Bull CJ, Bonilla C, Holly JM, Perks CM, Davies N, Haycock P, et al. Blood lipids and prostate cancer: a Mendelian randomization analysis. Cancer Med 2016;5:1125-36. https://doi.org/10.1002/cam4.695.
- Castro E, Mikropoulos C, Bancroft EK, Dadaev T, Goh C, Taylor N, et al. The PROFILE feasibility study: targeted screening of men with a family history of prostate cancer. Oncologist 2016;21:716-22. https://doi.org/10.1634/theoncologist.2015-0336.
- Khankari NK, Shu XO, Wen W, Kraft P, Lindström S, Peters U, et al. Association between adult height and risk of colorectal, lung, and prostate cancer: results from meta-analyses of prospective studies and Mendelian randomization analyses. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1002118.
- Lophatananon A, Stewart-Brown S, Kote-Jarai Z, Olama AAA, Garcia SB, Neal DE, et al. Height, selected genetic markers and prostate cancer risk: results from the PRACTICAL consortium. Br J Cancer 2017;117:734-43. https://doi.org/10.1038/bjc.2017.231.
- Stegeman S, Amankwah E, Klein K, O’Mara TA, Kim D, Lin HY, et al. A large-scale analysis of genetic variants within putative miRNA binding sites in prostate cancer. Cancer Discov 2015;5:368-79. https://doi.org/10.1158/2159-8290.CD-14-1057.
- Gusev A, Shi H, Kichaev G, Pomerantz M, Li F, Long HW, et al. Atlas of prostate cancer heritability in European and African-American men pinpoints tissue-specific regulation. Nat Commun 2016;7. https://doi.org/10.1038/ncomms10979.
- Ju YS, Tubio JM, Mifsud W, Fu B, Davies HR, Ramakrishna M, et al. Corrigendum: frequent somatic transfer of mitochondrial DNA into the nuclear genome of human cancer cells. Genome Res 2016;26. https://doi.org/10.1101/gr.206557.116.
- Marzec J, Mao X, Li M, Wang M, Feng N, Gou X, et al. A genetic study and meta-analysis of the genetic predisposition of prostate cancer in a Chinese population. Oncotarget 2016;7:21393-403. https://doi.org/10.18632/oncotarget.7250.
- Southey MC, Goldgar DE, Winqvist R, Pylkäs K, Couch F, Tischkowitz M, et al. PALB2, CHEK2 and ATM rare variants and cancer risk: data from COGS. J Med Genet 2016;53:800-11. https://doi.org/10.1136/jmedgenet-2016-103839.
- Brunner C, Davies NM, Martin RM, Eeles R, Easton D, Kote-Jarai Z, et al. Alcohol consumption and prostate cancer incidence and progression: a Mendelian randomisation study. Int J Cancer 2017;140:75-8. https://doi.org/10.1002/ijc.30436.
- Kote-Jarai Z, Mikropoulos C, Leongamornlert DA, Dadaev T, Tymrakiewicz M, Saunders EJ, et al. Prevalence of the HOXB13 G84E germline mutation in British men and correlation with prostate cancer risk, tumour characteristics and clinical outcomes. Ann Oncol 2015;26:756-61. https://doi.org/10.1093/annonc/mdv004.
- Saunders EJ, Dadaev T, Leongamornlert DA, Al Olama AA, Benlloch S, Giles GG, et al. Gene and pathway level analyses of germline DNA-repair gene variants and prostate cancer susceptibility using the iCOGS-genotyping array. Br J Cancer 2016;114:945-52. https://doi.org/10.1038/bjc.2016.50.
- Benafif S, Eeles R. Genetic predisposition to prostate cancer. Br Med Bull 2016;120:75-89. https://doi.org/10.1093/bmb/ldw039.
- Cheng HH, Pritchard CC, Montgomery B, Lin DW, Nelson PS. Prostate cancer screening in a new era of genetics. Clin Genitourin Cancer 2017;15:625-8. https://doi.org/10.1016/j.clgc.2017.05.024.
- Wu D, Ni J, Beretov J, Cozzi P, Willcox M, Wasinger V, et al. Urinary biomarkers in prostate cancer detection and monitoring progression. Crit Rev Oncol Hematol 2017;118:15-26. https://doi.org/10.1016/j.critrevonc.2017.08.002.
- Curtius K, Wright NA, Graham TA. An evolutionary perspective on field cancerization. Nat Rev Cancer 2018;18:19-32. https://doi.org/10.1038/nrc.2017.102.
- Chistiakov DA, Myasoedova VA, Grechko AV, Melnichenko AA, Orekhov AN. New biomarkers for diagnosis and prognosis of localized prostate cancer. Semin Cancer Biol 2018;52:9-16. https://doi.org/10.1016/j.semcancer.2018.01.012.
- Vickers AJ, Cronin AM, Roobol MJ, Hugosson J, Jones JS, Kattan MW, et al. The relationship between prostate-specific antigen and prostate cancer risk: The prostate biopsy collaborative group. Clin Cancer Res 2010;16:4374-81. https://doi.org/10.1158/1078-0432.CCR-10-1328.
- Bryant RJ, Sjoberg DD, Vickers AJ, Robinson MC, Kumar R, Marsden L, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. J Natl Cancer Inst 2015;107. https://doi.org/10.1093/jnci/djv095.
- Vickers A, Vertosick EA, Sjoberg DD, Roobol MJ, Hamdy F, Neal D, et al. Properties of the 4-kallikrein panel outside the diagnostic gray zone: meta-analysis of patients with positive digital rectal examination or prostate specific antigen 10 ng/ml and above. J Urol 2017;197:607-13. https://doi.org/10.1016/j.juro.2016.09.086.
- Vickers A, Vertosick EA, Sjoberg DD, Hamdy F, Neal D, Bjartell A, et al. Value of intact prostate specific antigen and human kallikrein 2 in the 4 kallikrein predictive model: an individual patient data meta-analysis. J Urol 2018;199:1470-4. https://doi.org/10.1016/j.juro.2018.01.070.
- Dimitrakopoulou VI, Tsilidis KK, Haycock PC, Dimou NL, Al-Dabhani K, Martin RM, et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ 2017;359. https://doi.org/10.1136/bmj.j4761.
- Price AJ, Travis RC, Appleby PN, Albanes D, Barricarte Gurrea A, Bjørge T, et al. Circulating folate and vitamin B12 and risk of prostate cancer: a collaborative analysis of individual participant data from six cohorts including 6875 cases and 8104 controls. Eur Urol 2016;70:941-51. https://doi.org/10.1016/j.eururo.2016.03.029.
- Simpkin AJ, Donovan JL, Tilling K, Athene Lane J, Martin RM, Albertsen PC, et al. Prostate-specific antigen patterns in US and European populations: comparison of six diverse cohorts. BJU Int 2016;118:911-18. https://doi.org/10.1111/bju.13422.
- Harrison S, Tilling K, Turner EL, Lane JA, Simpkin A, Davis M, et al. Investigating the prostate specific antigen, body mass index and age relationship: is an age-BMI-adjusted PSA model clinically useful?. Cancer Causes Control 2016;27:1465-74. https://doi.org/10.1007/s10552-016-0827-1.
- Simpkin AJ, Tilling K, Martin RM, Lane JA, Hamdy FC, Holmberg L, et al. Systematic review and meta-analysis of factors determining change to radical treatment in active surveillance for localized prostate cancer. Eur Urol 2015;67:993-1005. https://doi.org/10.1016/j.eururo.2015.01.004.
- Key TJ, Appleby PN, Travis RC, Albanes D, Alberg AJ, Barricarte A, et al. Carotenoids, retinol, tocopherols, and prostate cancer risk: pooled analysis of 15 studies. Am J Clin Nutr 2015;102:1142-57. https://doi.org/10.3945/ajcn.115.114306.
- Gilbert R, Bonilla C, Metcalfe C, Lewis S, Evans DM, Fraser WD, et al. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: a nested case-control study. Cancer Causes Control 2015;26:205-18. https://doi.org/10.1007/s10552-014-0500-5.
- Hackshaw-McGeagh LE, Penfold CM, Walsh E, Donovan JL, Hamdy FC, Neal DE, et al. Physical activity, alcohol consumption, BMI and smoking status before and after prostate cancer diagnosis in the ProtecT trial: opportunities for lifestyle modification. Int J Cancer 2015;137:1509-15. https://doi.org/10.1002/ijc.29514.
- Er V, Lane JA, Martin RM, Emmett P, Gilbert R, Avery KN, et al. Adherence to dietary and lifestyle recommendations and prostate cancer risk in the prostate testing for cancer and treatment (ProtecT) trial. Cancer Epidemiol Biomarkers Prev 2014;23:2066-77. https://doi.org/10.1158/1055-9965.EPI-14-0322.
- Young NJ, Metcalfe C, Gunnell D, Rowlands MA, Lane JA, Gilbert R, et al. A cross-sectional analysis of the association between diet and insulin-like growth factor (IGF)-I, IGF-II, IGF-binding protein (IGFBP)-2, and IGFBP-3 in men in the United Kingdom. Cancer Causes Control 2012;23:907-17. https://doi.org/10.1007/s10552-012-9961-6.
- Bonilla C, Gilbert R, Kemp JP, Timpson NJ, Evans DM, Donovan JL, et al. Using genetic proxies for lifecourse sun exposure to assess the causal relationship of sun exposure with circulating vitamin D and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2013;22:597-606. https://doi.org/10.1158/1055-9965.EPI-12-1248.
- Burton A, Martin RM, Holly J, Lane JA, Donovan JL, Hamdy FC, et al. Associations of adiponectin and leptin with stage and grade of PSA-detected prostate cancer: the ProtecT study. Cancer Causes Control 2013;24:323-34. https://doi.org/10.1007/s10552-012-0118-4.
- Gilbert R, Metcalfe C, Fraser WD, Donovan J, Hamdy F, Neal DE, et al. Associations of circulating 25-hydroxyvitamin D with prostate cancer diagnosis, stage and grade. Int J Cancer 2012;131:1187-96. https://doi.org/10.1002/ijc.27327.
- Rowlands MA, Tilling K, Holly JM, Metcalfe C, Gunnell D, Lane A, et al. Insulin-like growth factors (IGFs) and IGF-binding proteins in active monitoring of localized prostate cancer: a population-based observational study. Cancer Causes Control 2013;24:39-45. https://doi.org/10.1007/s10552-012-0087-7.
- Simpkin AJ, Metcalfe C, Martin RM, Lane JA, Donovan JL, Hamdy FC, et al. Longitudinal prostate-specific antigen reference ranges: choosing the underlying model of age-related changes. Stat Methods Med Res 2016;25:1875-91. https://doi.org/10.1177/0962280213503928.
- Gilbert R, Martin RM, Fraser WD, Lewis S, Donovan J, Hamdy F, et al. Predictors of 25-hydroxyvitamin D and its association with risk factors for prostate cancer: evidence from the prostate testing for cancer and treatment study. Cancer Causes Control 2012;23:575-88. https://doi.org/10.1007/s10552-012-9919-8.
- Rowlands MA, Holly JM, Hamdy F, Phillips J, Goodwin L, Marsden G, et al. Serum insulin-like growth factors and mortality in localised and advanced clinically detected prostate cancer. Cancer Causes Control 2012;23:347-54. https://doi.org/10.1007/s10552-011-9883-8.
- Rowlands MA, Holly JM, Gunnell D, Donovan J, Lane JA, Hamdy F, et al. Circulating insulin-like growth factors and IGF-binding proteins in PSA-detected prostate cancer: the large case-control study ProtecT. Cancer Res 2012;72:503-15. https://doi.org/10.1158/0008-5472.CAN-11-1601.
- Turner EL, Lane JA, Donovan JL, Davis MJ, Metcalfe C, Neal DE, et al. Association of diabetes mellitus with prostate cancer: nested case-control study (prostate testing for cancer and treatment study). Int J Cancer 2011;128:440-6. https://doi.org/10.1002/ijc.25360.
- Down L, Metcalfe C, Martin RM, Neal DE, Hamdy FC, Donovan JL, et al. Seasonal variation in prostate-specific antigen levels: a large cross-sectional study of men in the UK. BJU Int 2011;108:1409-14. https://doi.org/10.1111/j.1464-410X.2011.10174.x.
- Murad AS, Down L, Davey Smith G, Donovan JL, Athene Lane J, Hamdy FC, et al. Associations of aspirin, nonsteroidal anti-inflammatory drug and paracetamol use with PSA-detected prostate cancer: findings from a large, population-based, case-control study (the ProtecT study). Int J Cancer 2011;128:1442-8. https://doi.org/10.1002/ijc.25465.
- Collin SM, Metcalfe C, Refsum H, Lewis SJ, Smith GD, Cox A, et al. Associations of folate, vitamin B12, homocysteine, and folate-pathway polymorphisms with prostate-specific antigen velocity in men with localized prostate cancer. Cancer Epidemiol Biomarkers Prev 2010;19:2833-8. https://doi.org/10.1158/1055-9965.EPI-10-0582.
- Collin SM, Metcalfe C, Refsum H, Lewis SJ, Zuccolo L, Smith GD, et al. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev 2010;19:1632-42. https://doi.org/10.1158/1055-9965.EPI-10-0180.
- Murad AS, Smith GD, Lewis SJ, Cox A, Donovan JL, Neal DE, et al. A polymorphism in the glucokinase gene that raises plasma fasting glucose, rs1799884, is associated with diabetes mellitus and prostate cancer: findings from a population-based, case-control study (the ProtecT study). Int J Mol Epidemiol Genet 2010;1:175-83.
- Rowlands MA, Holly JM, Gunnell D, Gilbert R, Donovan J, Lane JA, et al. The relation between adiposity throughout the life course and variation in IGFs and IGFBPs: evidence from the ProtecT (Prostate testing for cancer and Treatment) study. Cancer Causes Control 2010;21:1829-42. https://doi.org/10.1007/s10552-010-9610-x.
- Tilling K, Garmo H, Metcalfe C, Holmberg L, Hamdy FC, Neal DE, et al. Development of a new method for monitoring prostate-specific antigen changes in men with localised prostate cancer: a comparison of observational cohorts. Eur Urol 2010;57:446-52. https://doi.org/10.1016/j.eururo.2009.03.023.
- Collin SM, Metcalfe C, Palmer TM, Refsum H, Lewis SJ, Smith GD, et al. The causal roles of vitamin B12 and transcobalamin in prostate cancer: can Mendelian randomization analysis provide definitive answers?. Int J Mol Epidemiol Genet 2011;2:316-27.
- Dimitropoulou P, Martin RM, Turner EL, Lane JA, Gilbert R, Davis M, et al. Association of obesity with prostate cancer: a case-control study within the population-based PSA testing phase of the ProtecT study. Br J Cancer 2011;104:875-81. https://doi.org/10.1038/sj.bjc.6606066.
- Noble S, Donovan J, Turner E, Metcalfe C, Lane A, Rowlands MA, et al. Feasibility and cost of obtaining informed consent for essential review of medical records in large-scale health services research. J Health Serv Res Policy 2009;14:77-81. https://doi.org/10.1258/jhsrp.2008.008085.
- Murad A, Lewis SJ, Smith GD, Collin SM, Chen L, Hamdy FC, et al. PTGS2-899G>C and prostate cancer risk: a population-based nested case-control study (ProtecT) and a systematic review with meta-analysis. Prostate Cancer Prostatic Dis 2009;12:296-300. https://doi.org/10.1038/pcan.2009.18.
- Metcalfe C, Martin RM, Noble S, Lane JA, Hamdy FC, Neal DE, et al. Low risk research using routinely collected identifiable health information without informed consent: encounters with the patient information advisory group. J Med Ethics 2008;34:37-40. https://doi.org/10.1136/jme.2006.019661.
- Zuccolo L, Harris R, Gunnell D, Oliver S, Lane JA, Davis M, et al. Height and prostate cancer risk: a large nested case-control study (ProtecT) and meta-analysis. Cancer Epidemiol Biomarkers Prev 2008;17:2325-36. https://doi.org/10.1158/1055-9965.EPI-08-0342.
- Chen L, Davey Smith G, Evans DM, Cox A, Lawlor DA, Donovan J, et al. Genetic variants in the vitamin D receptor are associated with advanced prostate cancer at diagnosis: findings from the prostate testing for cancer and treatment study and a systematic review. Cancer Epidemiol Biomarkers Prev 2009;18:2874-81. https://doi.org/10.1158/1055-9965.EPI-09-0544.
- Collin SM, Metcalfe C, Donovan JL, Athene Lane J, Davis M, Neal DE, et al. Associations of sexual dysfunction symptoms with PSA-detected localised and advanced prostate cancer: a case-control study nested within the UK population-based ProtecT (Prostate testing for cancer and Treatment) study. Eur J Cancer 2009;45:3254-61. https://doi.org/10.1016/j.ejca.2009.05.021.
- Gilbert R, Metcalfe C, Oliver SE, Whiteman DC, Bain C, Ness A, et al. Life course sun exposure and risk of prostate cancer: population-based nested case-control study and meta-analysis. Int J Cancer 2009;125:1414-23. https://doi.org/10.1002/ijc.24411.
- Whitaker HC, Kote-Jarai Z, Ross-Adams H, Warren AY, Burge J, George A, et al. The rs10993994 risk allele for prostate cancer results in clinically relevant changes in microseminoprotein-beta expression in tissue and urine. PLOS ONE 2010;5. https://doi.org/10.1371/journal.pone.0013363.
- Gudmundsson J, Besenbacher S, Sulem P, Gudbjartsson DF, Olafsson I, Arinbjarnarson S, et al. Genetic correction of PSA values using sequence variants associated with PSA levels. Sci Transl Med 2010;2. https://doi.org/10.1126/scitranslmed.3001513.
- Gunnell D, Oliver SE, Peters TJ, Donovan JL, Persad R, Maynard M, et al. Are diet-prostate cancer associations mediated by the IGF axis? A cross-sectional analysis of diet, IGF-I and IGFBP-3 in healthy middle-aged men. Br J Cancer 2003;88:1682-6. https://doi.org/10.1038/sj.bjc.6600946.
- Oliver SE, Gunnell D, Donovan J, Peters TJ, Persad R, Gillatt D, et al. Screen-detected prostate cancer and the insulin-like growth factor axis: results of a population-based case-control study. Int J Cancer 2004;108:887-92. https://doi.org/10.1002/ijc.11631.
- Cancer Research UK . Prostate Cancer Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer (accessed 16 September 2019).
- Joint Formulary Committee . British National Formulary (online) n.d. https://about.medicinescomplete.com/publication/british-national-formulary/ (accessed 26 November 2015).
Appendix 1 Supplementary tables and figures
| Outcome | Time point | ||
|---|---|---|---|
| Recruitment | Baseline (first biopsy) | Post allocation (6 months then annually) | |
| Demographics | CRF | – | – |
| Prostate cancer mortality | – | – | CRFa |
| Overall survival | – | – | Death certificate |
| Metastasis | – | – | CRF |
| Disease progression | – | – | CRF |
| Treatment complications | – | – | CRF |
| Resource use | – | – | CRF |
| Symptoms | Questionnaireb | Questionnaire | Questionnairec |
| QoL | Questionnaire | Questionnaire | Questionnairec |
| Psychological status | Questionnaire | Questionnaire | Questionnaire |
| Treatment experience | Interviewd | Interviewd | Interviewd |
FIGURE 28.
Projected recruitment and actual accrual in ProtecT.
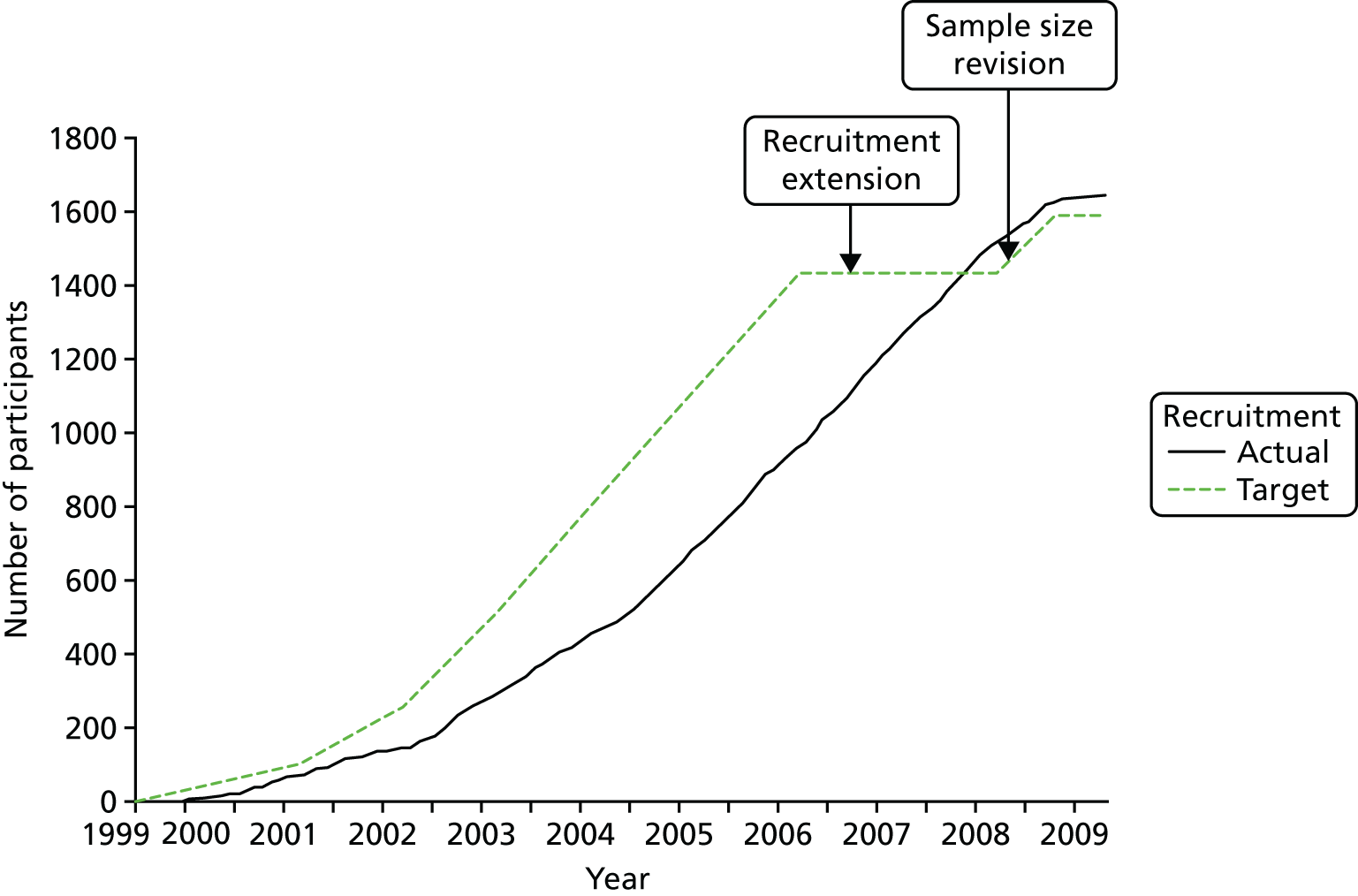
FIGURE 29.
Response rates for PROMs questionnaires and nurse-completed CRFs by years of follow-up for randomised and treatment selection participants. (a) Percentage CRFs returned (bars) and numbers of alive men (line) in follow-up; and (b) percentage questionnaires returned (bars) and numbers of alive men (line) in follow-up.
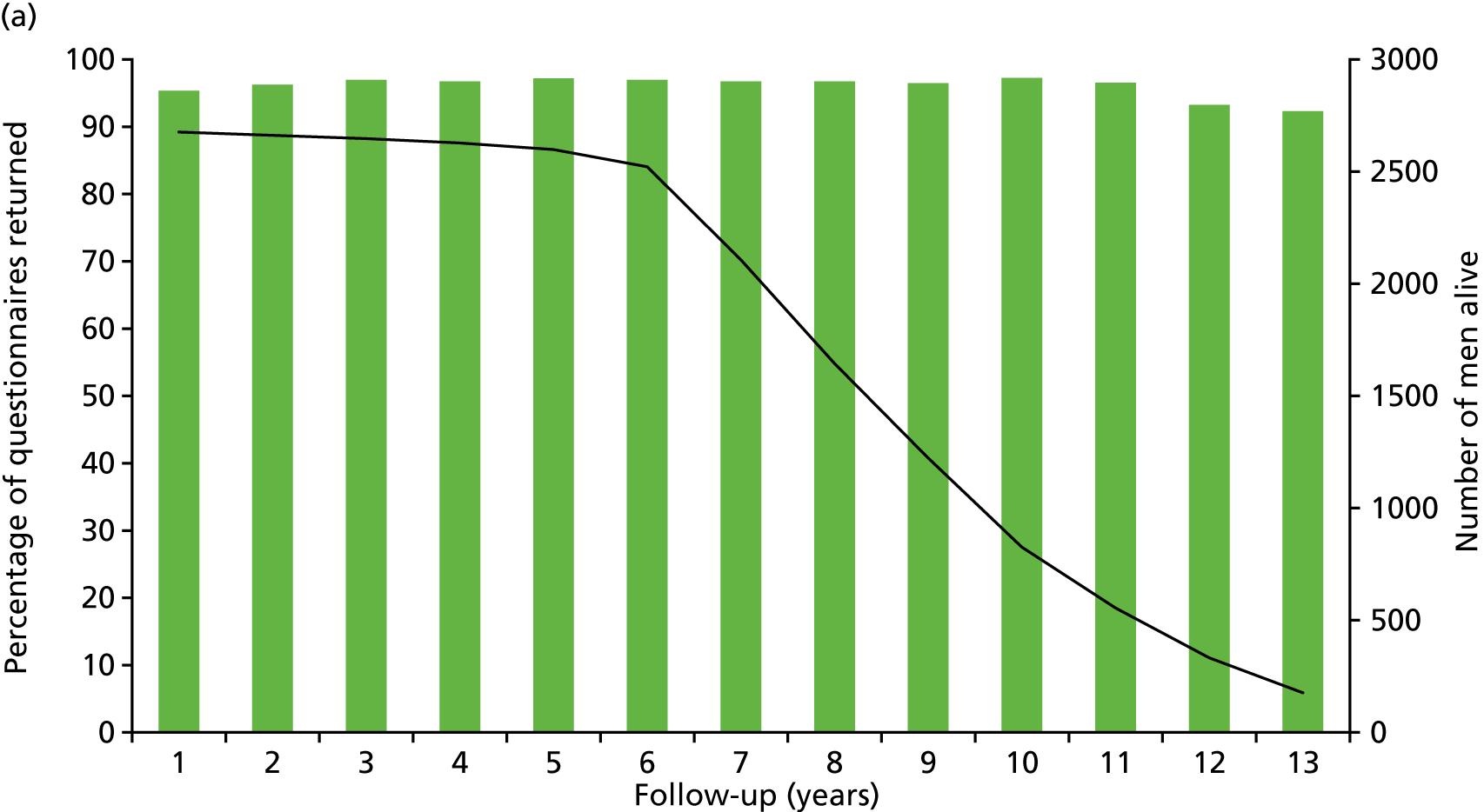
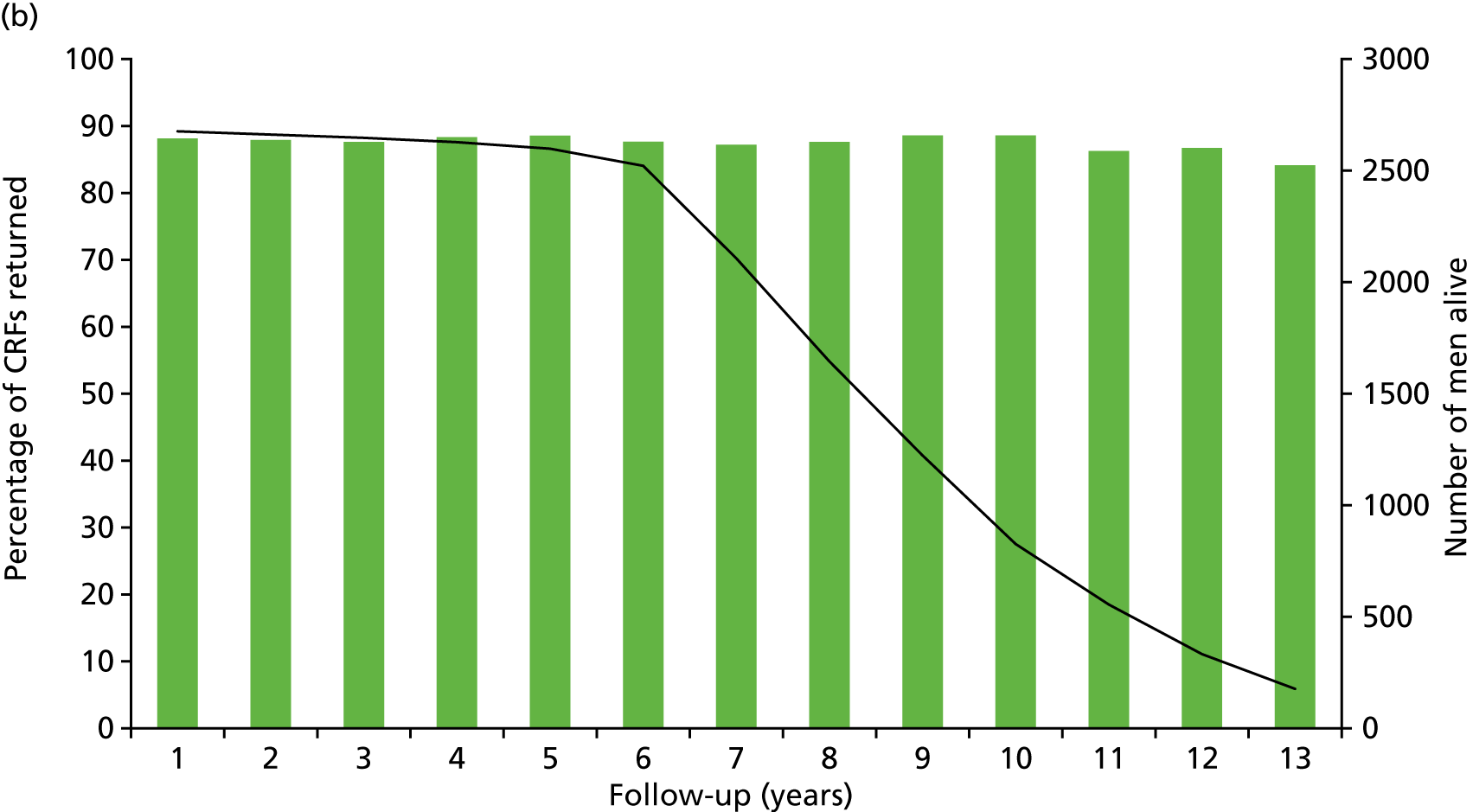
| Cause of death | Number of deaths | ||
|---|---|---|---|
| AM | RP | RT | |
| Cardiovascular system | 16 | 14 | 13 |
| Digestive system | 2 | 1 | 3 |
| Musculoskeletal system | 0 | 0 | 1 |
| Nervous system | 3 | 2 | 5 |
| External causes | 8 | 8 | 6 |
| Neoplasms other than prostate cancer | 22 | 25 | 23 |
| Total deaths unrelated to prostate cancer | 51 | 50 | 51 |
| PROMs,a summary scores, symptoms or QoL impact | Treatment group | |||
|---|---|---|---|---|
| AM (N = 545) | RT (N = 545) | RP (N = 553) | Total (N = 1643) | |
| ICIQ-UI minimum analysed/asked, n/N (%) | 422/500 (84) | 408/498 (82) | 414/510 (81) | 1244/1508 (82) |
| Incontinence score, mean (SD) | 1.3 (2.5) | 1.2 (2.0) | 1.3 (2.4) | 1.3 (2.3) |
| No incontinence, n (%) | 305 (72) | 280 (69) | 288 (70) | 873 (70) |
| Moderate QoL impact, n/N (%) | 28/428 (7) | 24/413 (6) | 29/418 (7) | 81/1259 (6) |
| Large QoL impact, n/N (%) | 3/428 (1) | 0/413 (0) | 1/418 (< 1) | 4/1259 (< 1) |
| EPIC minimum analysed/asked, n/N (%) | 244/280 (87) | 246/283 (87) | 255/286 (89) | 745/849 (88) |
| Urinary summary (SD) | 93.0 (9.6) | 93.2 (8.3) | 91.9 (9.4) | 92.7 (9.1) |
| Function score (SD) | 95.6 (8.0) | 94.7 (8.3) | 94.8 (8.9) | 95.1 (8.4) |
| Bother score (SD) | 91.2 (12.3) | 92.1 (10.3) | 89.9 (12.2) | 91.0 (11.7) |
| Incontinence score (SD) | 93.5 (11.3) | 92.8 (11.0) | 92.8 (11.6) | 93.0 (11.3) |
| Irritative/obstructive score (SD) | 93.2 (9.5) | 93.8 (7.9) | 92.0 (9.9) | 93.0 (9.2) |
| Pad use, n/N (%) | 1/250 (< 1) | 0/248 (0) | 4/256 (2) | 5/754 (1) |
| ICSmaleSF minimum analysed, n/N (%) | 471/545 (86) | 462/545 (85) | 480/553 (87) | 1413/1643 (86) |
| Incontinence score (SD) | 1.9 (2.1) | 1.8 (1.8) | 1.8 (1.8) | 1.8 (1.9) |
| Voiding score (SD) | 3.4 (2.9) | 3.1 (2.9) | 3.3 (3.1) | 3.3 (3.0) |
| Daytime frequency, n/N (%) | 147/470 (31) | 150/457 (33) | 163/483 (34) | 460/1410 (33) |
| Nocturia, n/N (%) | 111/475 (23) | 89/463 (19) | 110/485 (23) | 312/1423 (22) |
| Little urinary QoL impact, n/N (%) | 109/475 (23) | 99/466 (21) | 115/486 (24) | 323/1427 (23) |
| Somewhat/a lot QoL impact, n/N (%) | 20/475 (4) | 14/466 (3) | 10/486 (2) | 44/1427 (3) |
| EPICa scores and symptoms | Treatment group | Total (N = 1643) | ||
|---|---|---|---|---|
| AM (N = 545) | RT (N = 545) | RP (N = 553) | ||
| Bowel function | ||||
| Minimum analysed/asked, n/N (%) | 247/280 (88) | 247/283 (87) | 254/286 (89) | 748/849 (88) |
| Summary score (SD) | 92.8 (9.1) | 94.8 (6.9) | 93.1 (8.9) | 93.6 (8.4) |
| Function score (SD) | 91.6 (9.0) | 92.9 (8.0) | 91.4 (9.3) | 92.0 (8.8) |
| Bother score (SD) | 94.0 (11.8) | 96.8 (7.1) | 94.7 (10.3) | 95.1 (10.0) |
| Bloody stools, n/N (%) | 18/247 (7) | 18/250 (7) | 16/255 (6) | 52/752 (7) |
| Loose stools, n/N (%) | 43/249 (17) | 39/250 (16) | 36/255 (14) | 118/754 (16) |
| Stool leakage, n/N (%) | 10/249 (4) | 3/250 (1) | 10/255 (4) | 23/754 (3) |
| Overall bowel problems: small, n/N (%) | 32/249 (13) | 18/247 (7) | 35/255 (14) | 85/751 (11) |
| Moderate/big bowel problems, n/N (%) | 11/249 (4) | 4/247 (2) | 5/255 (2) | 20/751 (3) |
| Sexual function | ||||
| Minimum analysed/asked, n/N (%) | 236/280 (84) | 241/283 (85) | 240/286 (84) | 719/849 (85) |
| Summary score (SD) | 60.3 (23.5) | 63.6 (23.1) | 61.4 (22.7) | 61.8 (23.1) |
| Function score (SD) | 53.5 (22.8) | 55.7 (23.0) | 54.4 (22.9) | 54.5 (22.9) |
| Bother score (SD) | 76.0 (30.5) | 80.5 (29.2) | 77.6 (28.9) | 78.0 (29.5) |
| Erectile function, n/N (%) | 79/243 (33) | 78/247 (32) | 84/245 (34) | 241/735 (33) |
| Erectile problems: small, n/N (%) | 70/239 (29) | 55/245 (22) | 63/247 (26) | 188/731 (26) |
| Erectile problems: moderate/big, n/N (%) | 39/239 (16) | 39/245 (16) | 40/247 (16) | 118/731 (16) |
| Overall sexual function problem: small, n/N (%) | 55/239 (23) | 58/244 (24) | 69/245 (28) | 182/728 (25) |
| Moderate/big sexual function problems, n/N (%) | 44/239 (18) | 31/244 (13) | 39/245 (16) | 114/728 (16) |
FIGURE 30.
Impact of urinary incontinence (pad use) on depression.
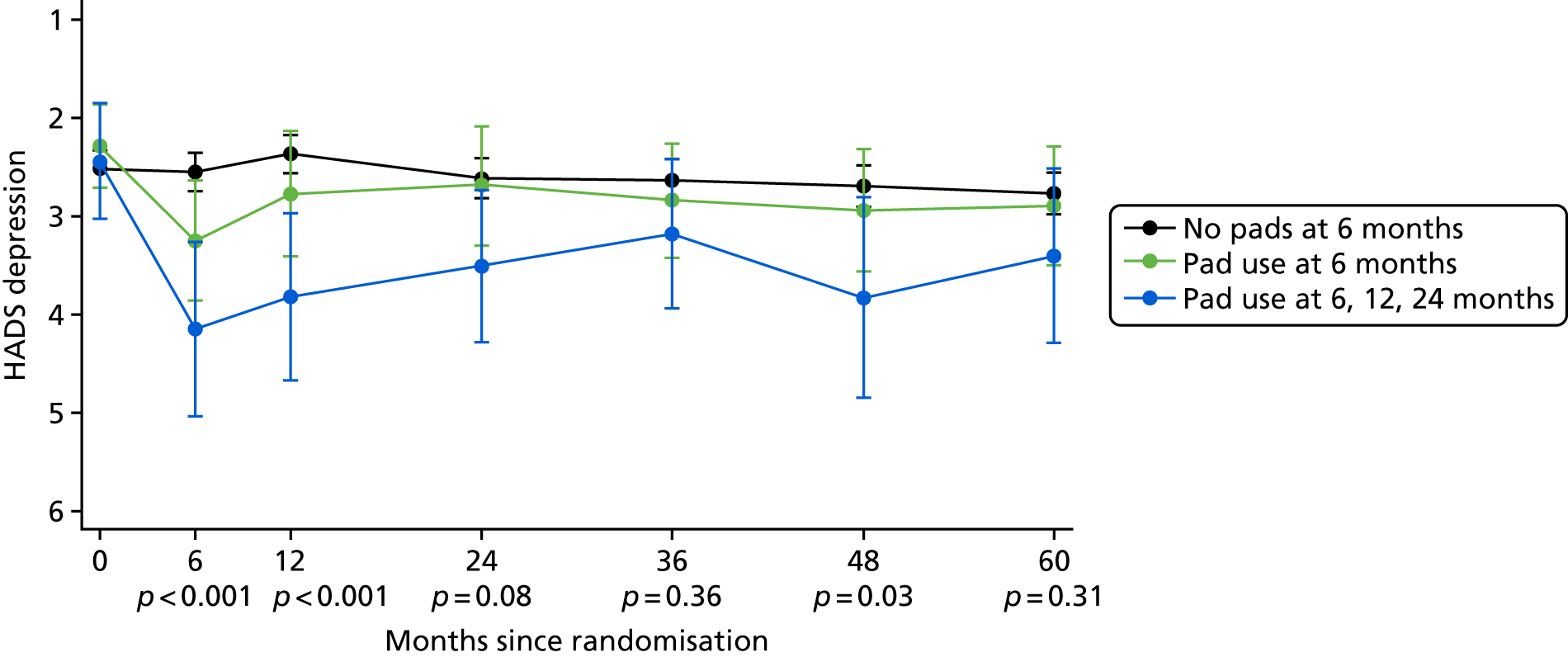
FIGURE 31.
Impact of urinary incontinence (pad use) on physical health.
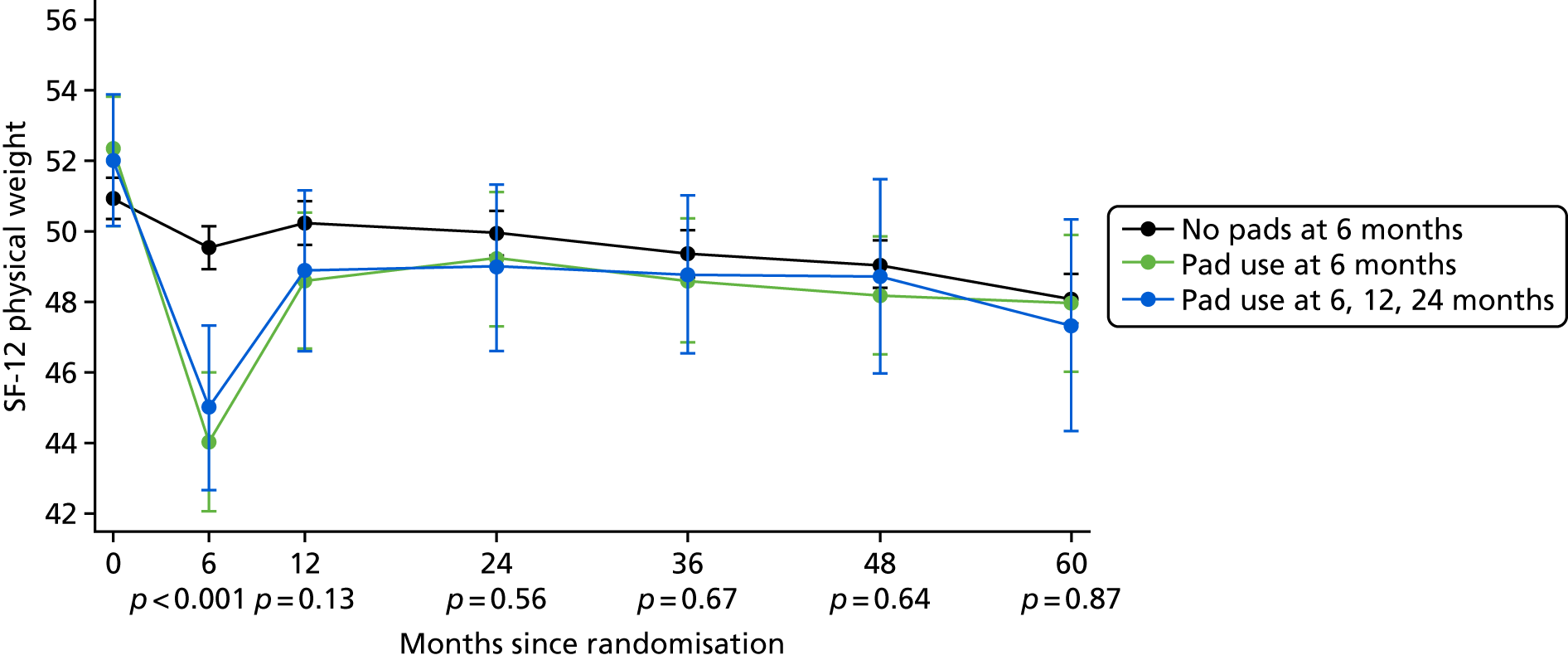
FIGURE 32.
Impact of erectile dysfunction (erection firmness) on depression. ED, erectile dysfunction.
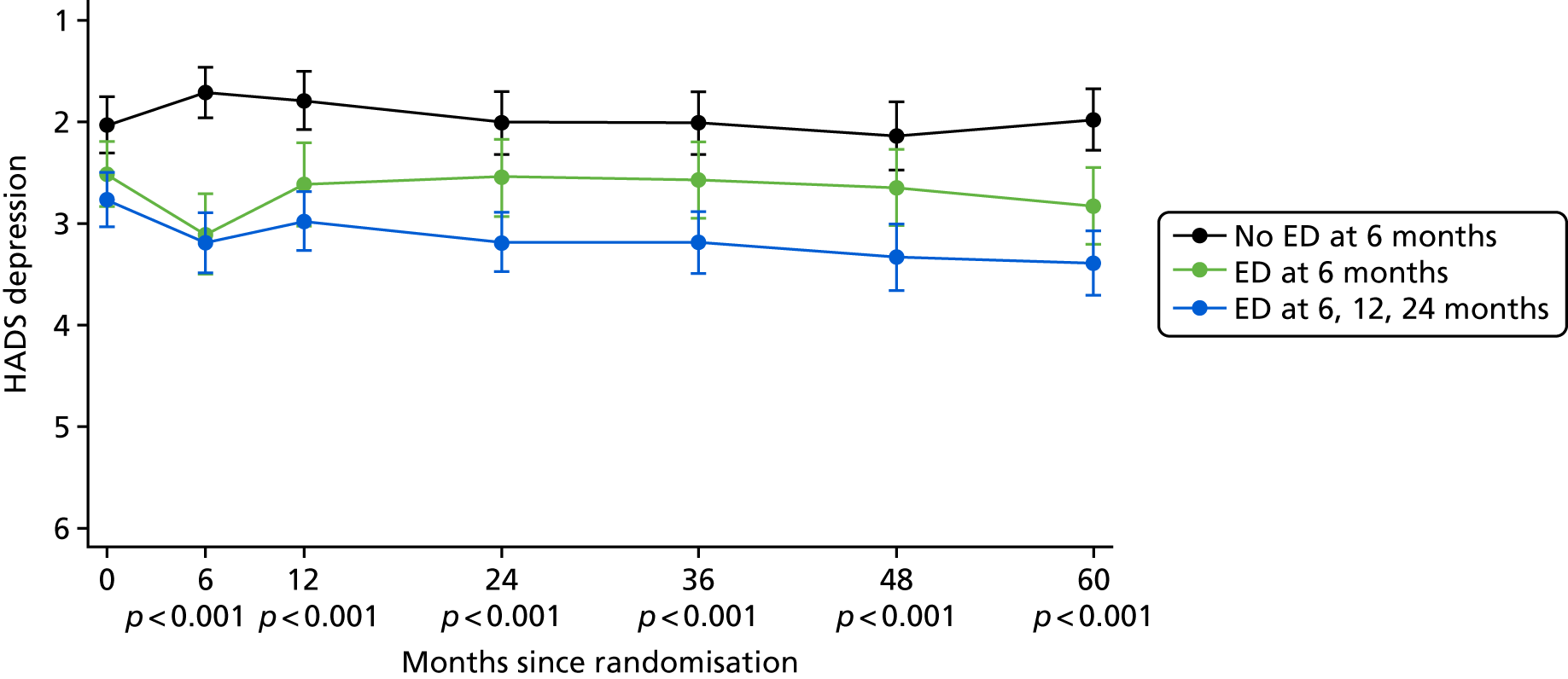
FIGURE 33.
Impact of erectile dysfunction (erection firmness) on physical health. ED, erectile dysfunction.
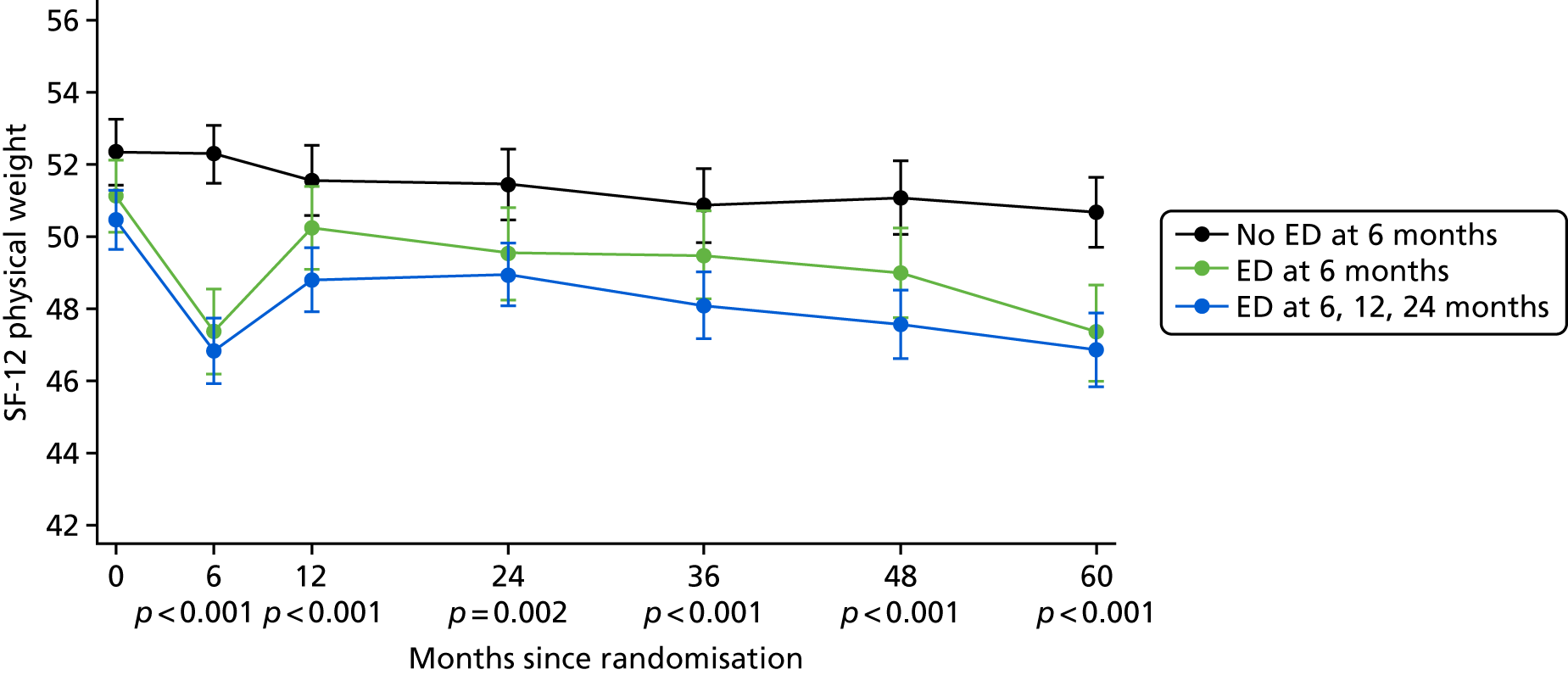
| Resource | How it was measured | Pre-valuation coding and costing | How it was valued |
|---|---|---|---|
| RP | Number of each type of procedure | Standard open prostatectomies were assigned a HRG4 code LB21A or LB21B dependent on complications and comorbidities, human keyhole prostatectomies were assigned LB22Z and robotic keyhole prostatectomies were assigned LB69Z | NHS Reference Costs 2014 to 2015 168 |
| Outpatient delivery of hormones | Number of appointments | For participants, where hormones were recorded but no method of delivery was given, the first depot was assigned a non-consultant led (WF01A) and clinical oncology outpatient specialty (800) code2 | NHS Reference Costs 2014 to 2015 168 |
| Primary care delivery of hormones | Number of appointments | The proportions of injections recorded that were delivered by GPs (26%) and practice nurses (74%) were used to create a weighted average cost |
|
| Hormones delivered in primary care | Number of injections | Zoladex injections were costed using one 3.6-mg pre-filled disposable injection for monthly injections and using one 10.8 mg pre-filled disposable injection for 3-monthly injections. Leuprolide injections were costed using one 3.75-mg pre-filled disposable injection for monthly injections and using one 11.25-mg pre-filled disposable injection for 3-monthly injections | BNF online (current price was valid for 2015 Dictionary of Medicines and Devices)301 |
| Pre-treatment planning | Allocated to each radiotherapy treatment | The HRG4 code SC51Z was used for protocol radiotherapy and salvage radiotherapy | NHS Reference Costs 2014 to 2015 168 |
| Radiotherapy delivery | Number of fractions | The HRG4 code SC22Z was used for protocol radiotherapy and SC23Z for salvage radiotherapy | NHS Reference Costs 2014 to 2015 168 |
| AM visits | Number of visits | The average time of a face-to-face and telephone consultation were calculated from the AM schedules | Cost of a nurse team leader per minute of face-to-face contact4 |
| Taking of blood for PSA test | Number of PSA tests | All PSA tests recorded in the clinical centres’ databases were allocated a weighted GP/nurse visit average cost using the recorded proportion of blood taken by GPs (4.7%) and practice nurses, phlebotomists and other staff (95.3%) |
|
| Other inpatient stays and day cases (no overnight stay) | Number of each type of procedure | Reasons for admission were mapped to OPCS-4 codes. The HRG4+ 2014–15 Reference Costs Grouper Code to Group v1.0 workbook171 was used to allocate the HRG codes.5 Assumption of no comorbidities or complications made where a HRG split was made on comorbidities or complications | Non-elective and elective inpatient costs assigned for overnight stays; day-case costs assigned,1 otherwise NHS Reference Costs 2014 to 2015168 |
| Study annual research/clinical follow-up visit | Number of visits | Half of the cost of an outpatient visit | NHS Reference Costs 2014 to 2015 168 |
| Chemotherapy | Number of visits |
Outpatient chemotherapy was valued using an assumption of a 21-day cycle of docetaxel and a weighted average cost of chemotherapy delivery HRG4s (SB12Z, SB13Z, SB14Z) plus a weighted average cost of chemotherapy procurement HRG4s (SB05Z, SB06Z, SB07Z, SB08Z, SB09Z, SB010Z) Inpatient/day-case chemotherapy was valued assuming docetaxel using a chemotherapy delivery HRG4 of SB12Z and chemotherapy procurement HRG4 of SB08Z |
NHS Reference Costs 2014 to 2015 168 |
| Other outpatient consultations | Number of outpatient consultations for each specialty | NHS Reference Costs 2014 to 2015 168 | |
| Outpatient procedures | Number of each type of procedure | Procedures were assigned a HRG4 code. An average outpatient procedure HRG cost was created from all relevant specialty outpatient procedure HRG costs | NHS Reference Costs 2014 to 2015 168 |
| Other primary care consultations | Number of each type of consultation |
|
Appendix 2 Topics: qualitative interviews
Introductions
Interviewer’s professional background, purpose and length of interview and explanation of how confidentiality will be maintained.
First interviews explored
-
Decision-making whether or not to participate in the ProtecT study.
-
Experiences of the diagnostic process including reasons for responding to the study invitation and experiences of biopsy.
-
Impact of prostate cancer diagnosis at time of diagnosis and ongoing.
-
Decision-making whether to accept random allocation to a treatment or to choose a treatment and views on study treatments.
-
Experiences of primary treatment received and changes over time.
-
Experiences and views on trial processes including recruitment and follow-up.
Following interviews explored
-
Experiences since last contact, positive and negative, prostate cancer related or not and including secondary treatments received.
-
Reflections on treatment decision-making and any changes in views with time/events.
-
Decision-making whether to initiate radical treatment (for men following active monitoring).
-
Experiences and views on trial processes of follow-up including study questionnaires, annual review appointments and follow-up.
Appendix 3 The ProtecT bibliography
Neal DE, Leung HY, Powell PH, Hamdy FC, Donovan JL. Unanswered questions in screening for prostate cancer. Eur J Cancer 2000;36:1316–21.
Donovan JL, Frankel SJ, Neal DE, Hamdy FC. Screening for prostate cancer in the UK. Seems to be creeping in by the back door. BMJ 2001;323:763–4.
Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766–70.
Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al. Prostate testing for cancer and treatment (ProtecT) feasibility study. Health Technol Assess 2003;7(14).
Donovan JL, Peters TJ, Noble S, Powell P, Gillatt D, Oliver SE, et al. Who can best recruit to randomized trials? Randomized trial comparing surgeons and nurses recruiting patients to a trial of treatments for localized prostate cancer (the ProtecT study). J Clin Epidemiol 2003;56:605–9.
Gunnell D, Oliver SE, Peters TJ, Donovan JL, Persad R, Maynard M, et al. Are diet-prostate cancer associations mediated by the IGF axis? A cross-sectional analysis of diet, IGF-I and IGFBP-3 in healthy middle-aged men. Br J Cancer 2003;88:1682–6. https://doi.org/10.1038/sj.bjc.6600946
Hamdy FC, Donovan JL, Lane JA, Neal DE. Ethics of clinical trials from bayesian perspective: randomisation to clinical trials may solve dilemma of treatment choice in prostate cancer. BMJ 2003;326:1456. https://doi.org/10.1136/bmj.326.7404.1456
Mills N, Donovan JL, Smith M, Jacoby A, Neal DE, Hamdy FC. Perceptions of equipoise are crucial to trial participation: a qualitative study of men in the ProtecT study. Control Clin Trials 2003;24:272–82.
Oliver SE, Donovan JL, Peters TJ, Frankel S, Hamdy FC, Neal DE. Recent trends in the use of radical prostatectomy in England: the epidemiology of diffusion. BJU Int 2003;91:331–6.
Gunnell D, Oliver SE, Donovan JL, Peters TJ, Gillatt D, Persad R, et al. Do height-related variations in insulin-like growth factors underlie the associations of stature with adult chronic disease? J Clin Endocrinol Metab 2004;89:213–18.
Oliver SE, Barrass B, Gunnell DJ, Donovan JL, Peters TJ, Persad RA, et al. Serum insulin-like growth factor-I is positively associated with serum prostate-specific antigen in middle-aged men without evidence of prostate cancer. Cancer Epidemiol Biomarkers Prev 2004;13:163–5.
Oliver SE, Gunnell D, Donovan J, Peters TJ, Persad R, Gillatt D, et al. Screen-detected prostate cancer and the insulin-like growth factor axis: results of a population-based case-control study. Int J Cancer 2004;108:887–92. https://doi.org/10.1002/ijc.11631
Oliver SE, Holly J, Peters TJ, Donovan J, Persad R, Gillatt D, et al. Measurement of insulin-like growth factor axis does not enhance specificity of PSA-based prostate cancer screening. Urology 2004;64:317–22.
Donovan JL, Hamdy FC, Neal DE, Prostate Testing for Cancer and Treatment study group. Screening for prostate cancer. The case against. Ann R Coll Surg Engl 2005;87:90–1.
Donovan JL, Martin RM, Neal DE, Hamdy FC. Prostate cancer: screening approaches. Br J Hosp Med 2005;66:623–6. https://doi.org/10.12968/hmed.2005.66.11.20023
Brindle LA, Oliver SE, Dedman D, Donovan JL, Neal DE, Hamdy FC, et al. Measuring the psychosocial impact of population-based prostate-specific antigen testing for prostate cancer in the UK. BJU Int 2006;98:777–82.
Martin RM, Gunnell D, Hamdy F, Neal D, Lane A, Donovan J. Continuing controversy over monitoring men with localized prostate cancer: a systematic review of programs in the prostate specific antigen era. J Urol 2006;176:439–49.
Metcalfe C, Lane JA, Hamdy F, Neal D, Donovan JL. A model of the natural history of screen-detected prostate cancer. Br J Cancer 2006;95:1122–3.
Mills N, Metcalfe C, Ronsmans C, Davis M, Lane JA, Sterne JA, et al. A comparison of socio-demographic and psychological factors between patients consenting to randomisation and those selecting treatment (the ProtecT study). Contemp Clin Trials 2006;27:413–19.
Lane JA, Howson J, Donovan JL, Goepel JR, Dedman DJ, Down L, et al. Detection of prostate cancer in unselected young men: prospective cohort nested within a randomised controlled trial. BMJ 2007;335:1139.
Avery KN, Blazeby JM, Lane JA, Neal DE, Hamdy FC, Donovan JL. Decision-making about PSA testing and prostate biopsies: a qualitative study embedded in a primary care randomised trial. Eur Urol 2008;53:1186–93.
Avery KN, Metcalfe C, Blazeby JM, Lane JA, Neal DE, Hamdy FC, Donovan JL. Prostate-specific antigen testing and prostate biopsy: are self-reported lower urinary tract symptoms and health-related quality of life associated with the decision to undergo these investigations? BJU Int 2008;102:1629–33.
Collin SM, Martin RM, Metcalfe C, Gunnell D, Albertsen PC, Neal D, et al. Prostate-cancer mortality in the USA and UK in 1975–2004: an ecological study. Lancet Oncol 2008;9:445–52. https://doi.org/10.1016/S1470-2045(08)70104-9
Collin SM, Metcalfe C, Donovan J, Lane JA, Davis M, Neal D, et al. Associations of lower urinary tract symptoms with prostate-specific antigen levels, and screen-detected localized and advanced prostate cancer: a case-control study nested within the UK population-based ProtecT (prostate testing for cancer and treatment) study. BJU Int 2008;102:1400–6. https://doi.org/10.1111/j.1464-410X.2008.07817.x
Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet 2008;40:316–21. https://doi.org/10.1038/ng.90
Ghoussaini M, Song H, Koessler T, Al Olama AA, Kote-Jarai Z, Driver KE, et al. Multiple loci with different cancer specificities within the 8q24 gene desert. J Natl Cancer Inst 2008;100:962–6. https://doi.org/10.1093/jnci/djn190
Hussain S, Gunnell D, Donovan J, McPhail S, Hamdy F, Neal D, et al. Secular trends in prostate cancer mortality, incidence and treatment: England and Wales, 1975–2004. BJU Int 2008;101:547–55. https://doi.org/10.1111/j.1464-410X.2007.07338.x
Kote-Jarai Z, Easton DF, Stanford JL, Ostrander EA, Schleutker J, Ingles SA, et al. Multiple novel prostate cancer predisposition loci confirmed by an international study: the PRACTICAL Consortium. Cancer Epidemiol Biomarkers Prev 2008;17:2052–61.
Metcalfe C, Martin RM, Noble S, Lane JA, Hamdy FC, Neal DE, Donovan JL. Low risk research using routinely collected identifiable health information without informed consent: encounters with the Patient Information Advisory Group. J Med Ethics 2008;34:37–40.
Rosario DJ, Lane JA, Metcalfe C, Catto JW, Dedman D, Donovan JL, et al. Contribution of a single repeat PSA test to prostate cancer risk assessment: experience from the ProtecT study. Eur Urol 2008;53:777–84.
Zuccolo L, Harris R, Gunnell D, Oliver S, Lane JA, Davis M, et al. Height and prostate cancer risk: a large nested case-control study (ProtecT) and meta-analysis. Cancer Epidemiol Biomarkers Prev 2008;17:2325–36.
Al Olama AA, Kote-Jarai Z, Giles GG, Guy M, Morrison J, Severi G, et al. Multiple loci on 8q24 associated with prostate cancer susceptibility. Nat Genet 2009;41:1058–60. https://doi.org/10.1038/ng.452
Chen L, Davey Smith G, Evans DM, Cox A, Lawlor DA, Donovan J, et al. Genetic variants in the vitamin D receptor are associated with advanced prostate cancer at diagnosis: findings from the prostate testing for cancer and treatment study and a systematic review. Cancer Epidemiol Biomarkers Prev 2009;18:2874–81.
Collin SM, Metcalfe C, Donovan JL, Athene Lane J, Davis M, Neal DE, et al. Associations of sexual dysfunction symptoms with PSA-detected localised and advanced prostate cancer: a case-control study nested within the UK population-based ProtecT (prostate testing for cancer and treatment) study. Eur J Cancer 2009;45:3254–61. https://doi.org/10.1016/j.ejca.2009.05.021
Collin SM, Metcalfe C, Zuccolo L, Lewis SJ, Chen L, Cox A, et al. Association of folate-pathway gene polymorphisms with the risk of prostate cancer: a population-based nested case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev 2009;18:2528–39.
Donovan JL, Lane JA, Peters TJ, Brindle L, Salter E, Gillatt D, et al. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. J Clin Epidemiol 2009;62:29–36. https://doi.org/10.1016/j.jclinepi.2008.02.010
Down L, Metcalfe C, Avery K, Noble S, Lane JA, Neal DE, et al. Factors distinguishing general practitioners who more readily participated in a large randomized trial were identified. J Clin Epidemiol 2009;62:67–73. https://doi.org/10.1016/j.jclinepi.2008.02.014
Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet 2009;41:1116–21. https://doi.org/10.1038/ng.450
Gilbert R, Metcalfe C, Oliver SE, Whiteman DC, Bain C, Ness A, et al. Life course sun exposure and risk of prostate cancer: population-based nested case-control study and meta-analysis. Int J Cancer 2009;125:1414–23. https://doi.org/10.1002/ijc.24411
Guy M, Kote-Jarai Z, Giles GG, Al Olama AA, Jugurnauth SK, Mulholland S, et al. Identification of new genetic risk factors for prostate cancer. Asian J Androl 2009;11:49–55. https://doi.org/10.1038/aja.2008.18
Kote-Jarai Z, Jugurnauth S, Mulholland S, Leongamornlert DA, Guy M, Edwards S, et al. A recurrent truncating germline mutation in the BRIP1/FANCJ gene and susceptibility to prostate cancer. Br J Cancer 2009;100:426–30. https://doi.org/10.1038/sj.bjc.6604847
Macefield RC, Lane JA, Metcalfe C, Down L, Neal DE, Hamdy FC, Donovan JL. Do the risk factors of age, family history of prostate cancer or a higher prostate specific antigen level raise anxiety at prostate biopsy? Eur J Cancer 2009;45:2569–73.
Metcalfe C, Tilling K, Davis M, Lane JA, Martin RM, Kynaston H, et al. Current strategies for monitoring men with localised prostate cancer lack a strong evidence base: observational longitudinal study. Br J Cancer 2009;101:390–4. https://doi.org/10.1038/sj.bjc.6605181
Moore AL, Dimitropoulou P, Lane A, Powell PH, Greenberg DC, Brown CH, et al. Population-based prostate-specific antigen testing in the UK leads to a stage migration of prostate cancer. BJU Int 2009;104:1592–8. https://doi.org/10.1111/j.1464-410X.2009.08652.x
Murad A, Lewis SJ, Smith GD, Collin SM, Chen L, Hamdy FC, et al. PTGS2-899G>C and prostate cancer risk: a population-based nested case-control study (ProtecT) and a systematic review with meta-analysis. Prostate Cancer Prostatic Dis 2009;12:296–300. https://doi.org/10.1038/pcan.2009.18
Neal DE, Donovan JL, Martin RM, Hamdy FC. Screening for prostate cancer remains controversial. Lancet 2009;374:1482–3. https://doi.org/10.1016/S0140-6736(09)61085-0
Noble S, Donovan J, Turner E, Metcalfe C, Lane A, Rowlands MA, et al. Feasibility and cost of obtaining informed consent for essential review of medical records in large-scale health services research. J Health Serv Res Policy 2009;14:77–81. https://doi.org/10.1258/jhsrp.2008.008085
Pashayan N, Duffy SW, Pharoah P, Greenberg D, Donovan J, Martin RM, et al. Mean sojourn time, overdiagnosis, and reduction in advanced stage prostate cancer due to screening with PSA: implications of sojourn time on screening. Br J Cancer 2009;100:1198–204. https://doi.org/10.1038/sj.bjc.6604973
Pashayan N, Pharoah P, Neal DE, Hamdy F, Donovan J, Martin RM, et al. Stage shift in PSA-detected prostate cancers – effect modification by Gleason score. J Med Screen 2009;16:98–101. https://doi.org/10.1258/jms.2009.009037
Turner EL, Lane JA, Metcalfe C, Down L, Donovan JL, Hamdy F, et al. Psychological distress and prostate specific antigen levels in men with and without prostate cancer. Brain Behav Immun 2009;23:1073–8. https://doi.org/10.1016/j.bbi.2009.01.009
Wade J, Donovan JL, Lane JA, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018–28.
Collin SM, Metcalfe C, Refsum H, Lewis SJ, Smith GD, Cox A, et al. Associations of folate, vitamin B12, homocysteine, and folate-pathway polymorphisms with prostate-specific antigen velocity in men with localized prostate cancer. Cancer Epidemiol Biomarkers Prev 2010;19:2833–8.
Collin SM, Metcalfe C, Refsum H, Lewis SJ, Zuccolo L, Smith GD, et al. Circulating folate, vitamin B12, homocysteine, vitamin B12 transport proteins, and risk of prostate cancer: a case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev 2010;19:1632–42.
Elliott KS, Zeggini E, McCarthy MI, Gudmundsson J, Sulem P, Stacey SN, et al. Evaluation of association of HNF1B variants with diverse cancers: collaborative analysis of data from 19 genome-wide association studies. PLOS ONE 2010;5:e10858. https://doi.org/10.1371/journal.pone.0010858
Gudmundsson J, Besenbacher S, Sulem P, Gudbjartsson DF, Olafsson I, Arinbjarnarson S, et al. Genetic correction of PSA values using sequence variants associated with PSA levels. Sci Transl Med 2010;2:62ra92. https://doi.org/10.1126/scitranslmed.3001513
Kote-Jarai Z, Leongamornlert D, Tymrakiewicz M, Field H, Guy M, Al Olama AA, et al. Mutation analysis of the MSMB gene in familial prostate cancer. Br J Cancer 2010;102:414–18. https://doi.org/10.1038/sj.bjc.6605485
Lane JA, Hamdy FC, Martin RM, Turner EL, Neal DE, Donovan JL. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. Eur J Cancer 2010;46:3095–101. https://doi.org/10.1016/j.ejca.2010.09.016
Lewis SJ, Murad A, Chen L, Davey Smith G, Donovan J, Palmer T, et al. Associations between an obesity related genetic variant (FTO rs9939609) and prostate cancer risk. PLOS ONE 2010;5:e13485. https://doi.org/10.1371/journal.pone.0013485
Macefield RC, Metcalfe C, Lane JA, Donovan JL, Avery KN, Blazeby JM, et al. Impact of prostate cancer testing: an evaluation of the emotional consequences of a negative biopsy result. Br J Cancer 2010;102:1335–40. https://doi.org/10.1038/sj.bjc.6605648
Murad AS, Smith GD, Lewis SJ, Cox A, Donovan JL, Neal DE, et al. A polymorphism in the glucokinase gene that raises plasma fasting glucose, rs1799884, is associated with diabetes mellitus and prostate cancer: findings from a population-based, case-control study (the ProtecT study). Int J Mol Epidemiol Genet 2010;1:175–83.
Rowlands MA, Holly JM, Gunnell D, Gilbert R, Donovan J, Lane JA, et al. The relation between adiposity throughout the life course and variation in IGFs and IGFBPs: evidence from the ProtecT (prostate testing for cancer and treatment) study. Cancer Causes Control 2010;21:1829–42. https://doi.org/10.1007/s10552-010-9610-x
Tilling K, Garmo H, Metcalfe C, Holmberg L, Hamdy FC, Neal DE, et al. Development of a new method for monitoring prostate-specific antigen changes in men with localised prostate cancer: a comparison of observational cohorts. Eur Urol 2010;57:446–52. https://doi.org/10.1016/j.eururo.2009.03.023
Vickers AJ, Cronin AM, Roobol MJ, Hugosson J, Jones JS, Kattan MW, et al. The relationship between prostate-specific antigen and prostate cancer risk: The Prostate Biopsy Collaborative Group. Clin Cancer Res 2010;16:4374–81.
Batra J, Lose F, O’Mara T, Marquart L, Stephens C, Alexander K, et al. Association between Prostinogen (KLK15) genetic variants and prostate cancer risk and aggressiveness in Australia and a meta-analysis of GWAS data. PLOS ONE 2011;6:e26527. https://doi.org/10.1371/journal.pone.0026527
Catto JW, Robinson MC, Albertsen PC, Goepel JR, Abbod MF, Linkens DA, et al. Suitability of PSA-detected localised prostate cancers for focal therapy: experience from the ProtecT study. Br J Cancer 2011;105:931–7. https://doi.org/10.1038/bjc.2011.314
Collin SM, Metcalfe C, Palmer TM, Refsum H, Lewis SJ, Smith GD, et al. The causal roles of vitamin B12 and transcobalamin in prostate cancer: can Mendelian randomization analysis provide definitive answers? Int J Mol Epidemiol Genet 2011;2:316–27.
Dimitropoulou P, Martin RM, Turner EL, Lane JA, Gilbert R, Davis M, et al. Association of obesity with prostate cancer: a case-control study within the population-based PSA testing phase of the ProtecT study. Br J Cancer 2011;104:875–81. https://doi.org/10.1038/sj.bjc.6606066
Down L, Metcalfe C, Martin RM, Neal DE, Hamdy FC, Donovan JL, Lane JA. Seasonal variation in prostate-specific antigen levels: a large cross-sectional study of men in the UK. BJU Int 2011;108:1409–14. https://doi.org/10.1111/j.1464-410X.2011.10174.x
Kote-Jarai Z, Amin Al Olama A, Leongamornlert D, Tymrakiewicz M, Saunders E, Guy M, et al. Identification of a novel prostate cancer susceptibility variant in the KLK3 gene transcript. Hum Genet 2011;129:687–94. https://doi.org/10.1007/s00439-011-0981-1
Kote-Jarai Z, Olama AA, Giles GG, Severi G, Schleutker J, Weischer M, et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat Genet 2011;43:785–91. https://doi.org/10.1038/ng.882
Lane JA, Wade J, Down L, Bonnington S, Holding PN, Lennon T, et al. A Peer Review Intervention for Monitoring and Evaluating sites (PRIME) that improved randomized controlled trial conduct and performance. J Clin Epidemiol 2011;64:628–36. https://doi.org/10.1016/j.jclinepi.2010.10.003
Mills N, Donovan JL, Wade J, Hamdy FC, Neal DE, Lane JA. Exploring treatment preferences facilitated recruitment to randomized controlled trials. J Clin Epidemiol 2011;64:1127–36. https://doi.org/10.1016/j.jclinepi.2010.12.017
Murad AS, Down L, Davey Smith G, Donovan JL, Athene Lane J, Hamdy FC, et al. Associations of aspirin, nonsteroidal anti-inflammatory drug and paracetamol use with PSA-detected prostate cancer: findings from a large, population-based, case-control study (the ProtecT study). Int J Cancer 2011;128:1442–8. https://doi.org/10.1002/ijc.25465
Pashayan N, Pharoah P, Neal DE, Hamdy F, Donovan J, Martin RM, et al. PSA-detected prostate cancer and the potential for dedifferentiation – estimating the proportion capable of progression. Int J Cancer 2011;128:1462–70. https://doi.org/10.1002/ijc.25471
Pashayan N, Pharoah P, Tabár L, Neal DE, Martin RM, Donovan J, et al. Validation of a modelling approach for estimating the likely effectiveness of cancer screening using cancer data on prevalence screening and incidence. Cancer Epidemiol 2011;35:139–44. https://doi.org/10.1016/j.canep.2010.07.012
Schumacher FR, Berndt SI, Siddiq A, Jacobs KB, Wang Z, Lindstrom S, et al. Genome-wide association study identifies new prostate cancer susceptibility loci. Hum Mol Genet 2011;20:3867–75. https://doi.org/10.1093/hmg/ddr295
Stacey SN, Sulem P, Jonasdottir A, Masson G, Gudmundsson J, Gudbjartsson DF, et al. A germline variant in the TP53 polyadenylation signal confers cancer susceptibility. Nat Genet 2011;43:1098–103. https://doi.org/10.1038/ng.926
Turner EL, Lane JA, Donovan JL, Davis MJ, Metcalfe C, Neal DE, et al. Association of diabetes mellitus with prostate cancer: nested case-control study (Prostate testing for cancer and treatment study). Int J Cancer 2011;128:440–6. https://doi.org/10.1002/ijc.25360
Williams N, Hughes LJ, Turner EL, Donovan JL, Hamdy FC, Neal DE, et al. Prostate-specific antigen testing rates remain low in UK general practice: a cross-sectional study in six English cities. BJU Int 2011;108:1402–8. https://doi.org/10.1111/j.1464-410X.2011.10163.x
Ankerst DP, Boeck A, Freedland SJ, Jones JS, Cronin AM, Roobol MJ, et al. Evaluating the prostate cancer prevention trial high grade prostate cancer risk calculator in 10 international biopsy cohorts: results from the prostate biopsy collaborative group. World J Urol 2014;32:185–91.
Ankerst DP, Boeck A, Freedland SJ, Thompson IM, Cronin AM, Roobol MJ, et al. Evaluating the PCPT risk calculator in ten international biopsy cohorts: results from the Prostate Biopsy Collaborative Group. World J Urol 2012;30:181–7. https://doi.org/10.1007/s00345-011-0818-5
Avery KN, Metcalfe C, Vedhara K, Lane JA, Davis M, Neal DE, et al. Predictors of attendance for prostate-specific antigen screening tests and prostate biopsy. Eur Urol 2012;62:649–55. https://doi.org/10.1016/j.eururo.2011.12.059
Burton AJ, Martin RM, Donovan JL, Lane JA, Davis M, Hamdy FC, et al. Associations of lifestyle factors and anthropometric measures with repeat PSA levels during active surveillance/monitoring. Cancer Epidemiol Biomarkers Prev 2012;21:1877–85.
Cheng I, Chen GK, Nakagawa H, He J, Wan P, Laurie CC, et al. Evaluating genetic risk for prostate cancer among Japanese and Latinos. Cancer Epidemiol Biomarkers Prev 2012;21:2048–58.
Gilbert R, Martin RM, Fraser WD, Lewis S, Donovan J, Hamdy F, et al. Predictors of 25-hydroxyvitamin D and its association with risk factors for prostate cancer: evidence from the prostate testing for cancer and treatment study. Cancer Causes Control 2012;23:575–88. https://doi.org/10.1007/s10552-012-9919-8
Gilbert R, Metcalfe C, Fraser WD, Donovan J, Hamdy F, Neal DE, et al. Associations of circulating 25-hydroxyvitamin D with prostate cancer diagnosis, stage and grade. Int J Cancer 2012;131:1187–96. https://doi.org/10.1002/ijc.27327
Gilbert R, Metcalfe C, Fraser WD, Donovan J, Hamdy F, Neal DE, et al. Associations of circulating retinol, vitamin E, and 1,25-dihydroxyvitamin D with prostate cancer diagnosis, stage, and grade. Cancer Causes Control 2012;23:1865–73. https://doi.org/10.1007/s10552-012-0052-5
Gudmundsson J, Sulem P, Gudbjartsson DF, Masson G, Agnarsson BA, Benediktsdottir KR, et al. A study based on whole-genome sequencing yields a rare variant at 8q24 associated with prostate cancer. Nat Genet 2012;44:1326–9. https://doi.org/10.1038/ng.2437
Roobol MJ, Schröder FH, Hugosson J, Jones JS, Kattan MW, Klein EA, et al. Importance of prostate volume in the European Randomised Study of Screening for Prostate Cancer (ERSPC) risk calculators: results from the prostate biopsy collaborative group. World J Urol 2012;30:149–55. https://doi.org/10.1007/s00345-011-0804-y
Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, Goodwin L, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 2012;344:d7894. https://doi.org/10.1136/bmj.d7894
Rowlands MA, Holly JM, Gunnell D, Donovan J, Lane JA, Hamdy F, et al. Circulating insulin-like growth factors and IGF-binding proteins in PSA-detected prostate cancer: the large case-control study ProtecT. Cancer Res 2012;72:503–15. https://doi.org/10.1158/0008-5472.CAN-11-1601
Rowlands MA, Holly JM, Hamdy F, Phillips J, Goodwin L, Marsden G, et al. Serum insulin-like growth factors and mortality in localised and advanced clinically detected prostate cancer. Cancer Causes Control 2012;23:347–54. https://doi.org/10.1007/s10552-011-9883-8
Young NJ, Metcalfe C, Gunnell D, Rowlands MA, Lane JA, Gilbert R, et al. A cross-sectional analysis of the association between diet and insulin-like growth factor (IGF)-I, IGF-II, IGF-binding protein (IGFBP)-2, and IGFBP-3 in men in the United Kingdom. Cancer Causes Control 2012;23:907–17. https://doi.org/10.1007/s10552-012-9961-6
Amin Al Olama A, Kote-Jarai Z, Schumacher FR, Wiklund F, Berndt SI, Benlloch S, et al. A meta-analysis of genome-wide association studies to identify prostate cancer susceptibility loci associated with aggressive and non-aggressive disease. Hum Mol Genet 2013;22:408–15. https://doi.org/10.1093/hmg/dds425
Avery KN, Donovan JL, Gilbert R, Davis M, Emmett P, Down L, et al. Men with prostate cancer make positive dietary changes following diagnosis and treatment. Cancer Causes Control 2013;24:1119–28. https://doi.org/10.1007/s10552-013-0189-x
Bonilla C, Gilbert R, Kemp JP, Timpson NJ, Evans DM, Donovan JL, et al. Using genetic proxies for lifecourse sun exposure to assess the causal relationship of sun exposure with circulating vitamin D and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2013;22:597–606.
Burton A, Martin RM, Holly J, Lane JA, Donovan JL, Hamdy FC, et al. Associations of adiponectin and leptin with stage and grade of PSA-detected prostate cancer: the ProtecT study. Cancer Causes Control 2013;24:323–34.
Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet 2013;45:385–91. https://doi.org/10.1038/ng.2560
Gilbert R, Metcalfe C, Fraser WD, Lewis S, Donovan J, Hamdy F, et al. Associations of circulating 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and vitamin D pathway genes with prostate-specific antigen progression in men with localized prostate cancer undergoing active monitoring. Eur J Cancer Prev 2013;22:121–5. https://doi.org/10.1097/CEJ.0b013e3283584954
Kote-Jarai Z, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Dadaev T, Jugurnauth-Little S, et al. Fine-mapping identifies multiple prostate cancer risk loci at 5p15, one of which associates with TERT expression. Hum Mol Genet 2013;22:2520–8. https://doi.org/10.1093/hmg/ddt086
Lose F, Batra J, O’Mara T, Fahey P, Marquart L, Eeles RA, et al. Common variation in Kallikrein genes KLK5, KLK6, KLK12, and KLK13 and risk of prostate cancer and tumor aggressiveness. Urol Oncol 2013;31:635–43. https://doi.org/10.1016/j.urolonc.2011.05.011
Orozco G, Goh CL, Al Olama AA, Benlloch-Garcia S, Govindasami K, Guy M, et al. Common genetic variants associated with disease from genome-wide association studies are mutually exclusive in prostate cancer and rheumatoid arthritis. BJU Int 2013;111:1148–55. https://doi.org/10.1111/j.1464-410X.2012.11492.x
Pooley KA, Bojesen SE, Weischer M, Nielsen SF, Thompson D, Amin Al Olama A, et al. A genome-wide association scan (GWAS) for mean telomere length within the COGS project: identified loci show little association with hormone-related cancer risk. Hum Mol Genet 2013;22:5056–64. https://doi.org/10.1093/hmg/ddt355
Rodriguez S, Al-Ghamdi OA, Burrows K, Guthrie PA, Lane JA, Davis M, et al. Very low PSA concentrations and deletions of the KLK3 gene. Clin Chem 2013;59:234–44.
Rowlands MA, Tilling K, Holly JM, Metcalfe C, Gunnell D, Lane A, et al. Insulin-like growth factors (IGFs) and IGF-binding proteins in active monitoring of localized prostate cancer: a population-based observational study. Cancer Causes Control 2013;24:39–45. https://doi.org/10.1007/s10552-012-0087-7
Simpkin AJ, Metcalfe C, Martin RM, Lane JA, Donovan JL, Hamdy FC, et al. Longitudinal prostate-specific antigen reference ranges: choosing the underlying model of age-related changes. Stat Methods Med Res 2016;25:1875–91.
Wade J, Rosario DJ, Macefield RC, Avery KN, Salter CE, Goodwin ML, et al. Psychological impact of prostate biopsy: physical symptoms, anxiety, and depression. J Clin Oncol 2013;31:4235–41. https://doi.org/10.1200/JCO.2012.45.4801
Zuccolo L, Lewis SJ, Donovan JL, Hamdy FC, Neal DE, Smith GD. Alcohol consumption and PSA-detected prostate cancer risk – a case-control nested in the ProtecT study. Int J Cancer 2013;132:2176–85. https://doi.org/10.1002/ijc.27877
Al Olama AA, Kote-Jarai Z, Berndt SI, Conti DV, Schumacher F, Han Y, et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet 2014;46:1103–9. https://doi.org/10.1038/ng.3094
Ankerst DP, Boeck A, Freedland SJ, Jones JS, Cronin AM, Roobol MJ, et al. Evaluating the prostate cancer prevention trial high grade prostate cancer risk calculator in 10 international biopsy cohorts: results from the Prostate Biopsy Collaborative Group. World J Urol 2014;32:185–91. https://doi.org/10.1007/s00345-012-0869-2
Avery KN, Donovan JL, Horwood J, Neal DE, Hamdy FC, Parker C, et al. The importance of dietary change for men diagnosed with and at risk of prostate cancer: a multi-centre interview study with men, their partners and health professionals. BMC Fam Pract 2014;15:81. https://doi.org/10.1186/1471-2296-15-81
Er V, Lane JA, Martin RM, Emmett P, Gilbert R, Avery KN, et al. Adherence to dietary and lifestyle recommendations and prostate cancer risk in the prostate testing for cancer and treatment (ProtecT) trial. Cancer Epidemiol Biomarkers Prev 2014;23:2066–77.
Gilbert R, Bonilla C, Metcalfe C, Lewis S, Evans DM, Fraser WD, et al. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: a nested case-control study. Cancer Causes Control 2015;26:205–18.
Gilbert R, Martin RM, Donovan J, Lane JA, Hamdy F, Neal DE, Metcalfe C. Misclassification of outcome in case-control studies: methods for sensitivity analysis. Stat Methods Med Res 2016;25:2377–93.
Horwood JP, Avery KN, Metcalfe C, Donovan JL, Hamdy FC, Neal DE, Lane JA. Men’s knowledge and attitudes towards dietary prevention of a prostate cancer diagnosis: a qualitative study. BMC Cancer 2014;14:812.
Knipe DW, Evans DM, Kemp JP, Eeles R, Easton DF, Kote-Jarai Z, et al. Genetic variation in prostate-specific antigen-detected prostate cancer and the effect of control selection on genetic association studies. Cancer Epidemiol Biomarkers Prev 2014;23:1356–65.
Lane JA, Donovan JL, Davis M, Walsh E, Dedman D, Down L, et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014;15:1109–18. https://doi.org/10.1016/S1470-2045(14)70361-4
Mills N, Blazeby JM, Hamdy FC, Neal DE, Campbell B, Wilson C, et al. Training recruiters to randomized trials to facilitate recruitment and informed consent by exploring patients’ treatment preferences. Trials 2014;15:323. https://doi.org/10.1186/1745-6215-15-323
Oxley J, Simpkin A, Goepel J, Varma M, Griffiths D, Grigor K, et al. Gleason drift in the NIHR ProtecT Study. Histopathology 2015;66:438–46.
Saunders EJ, Dadaev T, Leongamornlert DA, Jugurnauth-Little S, Tymrakiewicz M, Wiklund F, et al. Fine-mapping the HOXB region detects common variants tagging a rare coding allele: evidence for synthetic association in prostate cancer. PLOS Genet 2014;10:e1004129. https://doi.org/10.1371/journal.pgen.1004129
Turner EL, Metcalfe C, Donovan JL, Noble S, Sterne JA, Lane JA, et al. Design and preliminary recruitment results of the Cluster randomised triAl of PSA testing for Prostate cancer (CAP). Br J Cancer 2014;110:2829–36. https://doi.org/10.1038/bjc.2014.242
Weng PH, Huang YL, Page JH, Chen JH, Xu J, Koutros S, et al. Polymorphisms of an innate immune gene, toll-like receptor 4, and aggressive prostate cancer risk: a systematic review and meta-analysis. PLOS ONE 2014;9:e110569. https://doi.org/10.1371/journal.pone.0110569
Mills N, Blazeby JM, Hamdy FC, Neal DE, Campbell B, Wilson C, et al. Training recruiters to randomized trials to facilitate recruitment and informed consent by exploring patients’ treatment preferences. Trials 2014;15:323. https://doi.org/10.1186/1745-6215-15-323
Amin Al Olama A, Benlloch S, Antoniou AC, Giles GG, Severi G, et al. Risk analysis of prostate cancer in PRACTICAL, a multinational consortium, using 25 known prostate cancer susceptibility loci. Cancer Epidemiol Biomarkers Prev 2015;24:1121–9.
Amin Al Olama A, Dadaev T, Hazelett DJ, Li Q, Leongamornlert D, Saunders EJ, et al. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum Mol Genet 2015;24:5589–602.
Bryant RJ, Sjoberg DD, Vickers AJ, Robinson MC, Kumar R, Marsden L, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. J Natl Cancer Inst 2015;107:djv095. https://doi.org/10.1093/jnci/djv095
Gilbert R, Bonilla C, Metcalfe C, Lewis S, Evans DM, Fraser WD, et al. Associations of vitamin D pathway genes with circulating 25-hydroxyvitamin-D, 1,25-dihydroxyvitamin-D, and prostate cancer: a nested case-control study. Cancer Causes Control 2015;26:205–18. https://doi.org/10.1007/s10552-014-0500-5
Hackshaw-McGeagh LE, Penfold CM, Walsh E, Donovan JL, Hamdy FC, Neal DE, et al. Physical activity, alcohol consumption, BMI and smoking status before and after prostate cancer diagnosis in the ProtecT trial: opportunities for lifestyle modification. Int J Cancer 2015;137:1509–15. https://doi.org/10.1002/ijc.29514
Kote-Jarai Z, Mikropoulos C, Leongamornlert DA, Dadaev T, Tymrakiewicz M, Saunders EJ, et al. Prevalence of the HOXB13 G84E germline mutation in British men and correlation with prostate cancer risk, tumour characteristics and clinical outcomes. Ann Oncol 2015;26:756–61. https://doi.org/10.1093/annonc/mdv004
Oxley J, Simpkin A, Goepel J, Varma M, Griffiths D, Grigor K, et al. Gleason drift in the NIHR ProtecT study. Histopathology 2015;66:438–46. https://doi.org/10.1111/his.12549
Pashayan N, Duffy SW, Neal DE, Hamdy FC, Donovan JL, Martin RM, et al. Implications of polygenic risk-stratified screening for prostate cancer on overdiagnosis. Genet Med 2015;17:789–95. https://doi.org/10.1038/gim.2014.192
Simpkin AJ, Rooshenas L, Wade J, Donovan JL, Lane JA, Martin RM, et al. Development, validation and evaluation of an instrument for active monitoring of men with clinically localised prostate cancer: systematic review, cohort studies and qualitative study. Health Serv Deliv Res 2015;3(30).
Simpkin AJ, Tilling K, Martin RM, Lane JA, Hamdy FC, Holmberg L, et al. Systematic Review and Meta-analysis of Factors Determining Change to Radical Treatment in Active Surveillance for Localized Prostate Cancer. Eur Urol 2015;67:993–1005.
Szulkin R, Karlsson R, Whitington T, Aly M, Gronberg H, Eeles RA, et al. Genome-wide association study of prostate cancer-specific survival. Cancer Epidemiol Biomarkers Prev 2015;24:1796–800.
Szulkin R, Whitington T, Eklund M, Aly M, Eeles RA, Easton D, et al. Prediction of individual genetic risk to prostate cancer using a polygenic score. Prostate 2015;75:1467–74. https://doi.org/10.1002/pros.23037
Wade J, Rosario DJ, Howson J, Avery KN, Salter CE, Goodwin ML, et al. Role of information in preparing men for transrectal ultrasound guided prostate biopsy: a qualitative study embedded in the ProtecT trial. BMC Health Serv Res 2015;15:80. https://doi.org/10.1186/s12913-015-0729-z
Williams NJ, Hill EM, Ng SY, Martin RM, Metcalfe C, Donovan JL, et al. Standardisation of information submitted to an endpoint committee for cause of death assignment in a cancer screening trial – lessons learnt from CAP (Cluster randomised triAl of PSA testing for Prostate cancer). BMC Med Res Methodol 2015;15:6. https://doi.org/10.1186/1471-2288-15-6
Simpkin AJ, Tilling K, Martin RM, Lane JA, Hamdy FC, Holmberg L, et al. Systematic review and meta-analysis of factors determining change to radical treatment in active surveillance for localized prostate cancer. Eur Urol 2015;67:993–1005.
Amin Al Olama A, Dadaev T, Hazelett DJ, Li Q, Leongamornlert D, Saunders EJ, et al. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum Mol Genet 2015;24:5589–602. https://doi.org/10.1093/hmg/ddv203
Amin Al Olama A, Benlloch S, Antoniou AC, Giles GG, Severi G, Neal DE, et al. Risk analysis of prostate cancer in PRACTICAL, a multinational consortium, using 25 known prostate cancer susceptibility loci. Cancer Epidemiol Biomarkers Prev 2015;24:1121–9. https://doi.org/10.1158/1055-9965.EPI-14-0317
Key TJ, Appleby PN, Travis RC, Albanes D, Alberg AJ, Barricarte A, et al. Carotenoids, retinol, tocopherols, and prostate cancer risk: pooled analysis of 15 studies. Am J Clin Nutr 2015;102:1142–57. https://doi.org/10.3945/ajcn.115.114306
Gilbert R, Martin RM, Evans DM, Tilling K, Davey Smith G, Kemp JP, et al. Incorporating known genetic variants does not improve the accuracy of PSA testing to identify high risk prostate cancer on biopsy. PLOS ONE 2015;10:e0136735.
Davies NM, Gaunt TR, Lewis SJ, Holly J, Donovan JL, Hamdy FC, et al. The effects of height and BMI on prostate cancer incidence and mortality: a Mendelian randomization study in 20,848 cases and 20,214 controls from the PRACTICAL consortium. Cancer Causes Control 2015;26:1603–16. https://doi.org/10.1007/s10552-015-0654-9
Thorn JC, Turner E, Hounsome L, Walsh E, Donovan JL, Verne J, et al. Validation of the hospital episode statistics outpatient dataset in England. PharmacoEconomics 2016;34:161–8. https://doi.org/10.1007/s40273-015-0326-3
Wade J, Holding PN, Bonnington S, Rooshenas L, Lane JA, Salter CE, et al. Establishing nurse-led active surveillance for men with localised prostate cancer: development and formative evaluation of a model of care in the ProtecT trial. BMJ Open 2015;5:e008953. https://doi.org/10.1136/bmjopen-2015-008953
Szulkin R, Karlsson R, Whitington T, Aly M, Gronberg H, Eeles RA, et al. Genome-wide association study of prostate cancer-specific survival. Cancer Epidemiol Biomarkers Prev 2015;24:1796–800. https://doi.org/10.1158/1055-9965.EPI-15-0543
Simpkin AJ, Rooshenas L, Wade J, Donovan JL, Lane JA, Martin RM, et al. Development, validation and evaluation of an instrument for active monitoring of men with clinically localised prostate cancer: systematic review, cohort studies and qualitative study. Health Serv Deliv Res 2015;3(30).
Thorn JC, Turner EL, Hounsome L, Walsh E, Down L, Verne J, et al. Validating the use of hospital episode statistics data and comparison of costing methodologies for economic evaluation: an end-of-life case study from the Cluster randomised triAl of PSA testing for Prostate cancer (CAP). BMJ Open 2016;6:e011063. https://doi.org/10.1136/bmjopen-2016-011063
Price AJ, Travis RC, Appleby PN, Albanes D, Barricarte Gurrea A, Bjørge T, et al. Circulating folate and vitamin B12 and risk of prostate cancer: a collaborative analysis of individual participant data from six cohorts including 6875 cases and 8104 controls. Eur Urol 2016;70:941–51.
Gusev A, Shi H, Kichaev G, Pomerantz M, Li F, Long HW, et al. Atlas of prostate cancer heritability in European and African-American men pinpoints tissue-specific regulation. Nat Commun 2016;7:10979. https://doi.org/10.1038/ncomms10979
Bonilla C, Lewis SJ, Martin RM, Donovan JL, Hamdy FC, Neal DE, et al. Pubertal development and prostate cancer risk: Mendelian randomization study in a population-based cohort. BMC Med 2016;14:66. https://doi.org/10.1186/s12916-016-0602-x
Walsh EI, Turner EL, Lane JA, Donovan JL, Neal DE, Hamdy FC, Martin RM, the CAP and ProtecT Trial Groups. Characteristics of men responding to an invitation to undergo testing for prostate cancer as part of a randomised trial. Trials 2016;17:497. https://doi.org/10.1186/s13063-016-1624-6
Travis RC, Appleby PN, Martin RM, Holly JMP, Albanes D, Black A, et al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res 2016;76:2288–300. https://doi.org/10.1158/0008-5472.CAN-15-1551
Simpkin AJ, Donovan JL, Tilling K, Athene Lane J, Martin RM, Albertsen PC, et al. Prostate-specific antigen patterns in US and European populations: comparison of six diverse cohorts. BJU Int 2016;118:911–18. https://doi.org/10.1111/bju.13422
Stacey SN, Kehr B, Gudmundsson J, Zink F, Jonasdottir A, Gudjonsson SA, et al. Insertion of an SVA-E retrotransposon into the CASP8 gene is associated with protection against prostate cancer. Hum Mol Genet 2016;25:1008–18. https://doi.org/10.1093/hmg/ddv622
Bonilla C, Lewis SJ, Rowlands MA, Gaunt TR, Davey Smith G, Gunnell D, et al. Assessing the role of insulin-like growth factors and binding proteins in prostate cancer using Mendelian randomization: genetic variants as instruments for circulating levels. Int J Cancer 2016;139:1520–33. https://doi.org/10.1002/ijc.30206
Castro E, Mikropoulos C, Bancroft EK, Dadaev T, Goh C, Taylor N, et al. The PROFILE feasibility study: targeted screening of men with a family history of prostate cancer. Oncologist 2016;21:716–22. https://doi.org/10.1634/theoncologist.2015-0336
Harrison S, Tilling K, Turner EL, Lane JA, Simpkin A, Davis M, et al. Investigating the prostate specific antigen, body mass index and age relationship: is an age-BMI-adjusted PSA model clinically useful? Cancer Causes Control 2016;27:1465–74. https://doi.org/10.1007/s10552-016-0827-1
Kar SP, Beesley J, Amin Al Olama A, Michailidou K, Tyrer J, Kote-Jarai Z, et al. Genome-wide meta-analyses of breast, ovarian, and prostate cancer association studies identify multiple new susceptibility loci shared by at least two cancer types. Cancer Discov 2016;6:1052–67. https://doi.org/10.1158/2159-8290.CD-15-1227
Mason MD, Moore R, Jones G, Lewis G, Donovan JL, Neal DE, et al. Radiotherapy for prostate cancer: is it ‘what you do’ or ‘the way that you do it’? A UK perspective on technique and quality assurance. Clin Oncol 2016;28:e92–e100. https://doi.org/10.1016/j.clon.2016.05.011
Lane JA, Metcalfe C, Young GJ, Peters TJ, Blazeby J, Avery K, et al. Patient-reported outcomes in the ProtecT randomised trial of clinically localised prostate cancer treatments: design and baseline urinary, bowel and sexual function and quality of life. BJU Int 2016;118:869–79.
Turner EL, Metcalfe C, Donovan JL, Noble S, Sterne JA, Lane JA, et al. Contemporary accuracy of death certificates for coding prostate cancer as a cause of death: Is reliance on death certification good enough? A comparison with blinded review by an independent cause of death evaluation committee. Br J Cancer 2016;115:90–4. https://doi.org/10.1038/bjc.2016.162
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415–24.
Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–37.
Hamdy FC. The prostate testing for cancer and treatment (ProtecT) study: what have we learnt? BJU Int 2016;118:843.
Johnston TJ, Shaw GL, Lamb AD, Parashar D, Greenberg D, Xiong T, et al. Mortality among men with advanced prostate cancer excluded from the ProtecT trial. Eur Urol 2017;71:381–8.
Lane JA, Oliver SE, Appleby PN, Lentjes MA, Emmett P, Kuh D, et al. Prostate cancer risk related to foods, food groups, macronutrients and micronutrients derived from the UK Dietary Cohort Consortium food diaries. Eur J Clin Nutr 2017;71:567. https://doi.org/10.1038/ejcn.2017.17
Vickers A, Vertosick EA, Sjoberg DD, Roobol MJ, Hamdy F, Neal D, et al. Properties of the 4-Kallikrein panel outside the diagnostic gray zone: meta-analysis of patients with positive digital rectal examination or prostate specific antigen 10 ng/m and above. J Urol 2017;197:607–13.
Brunner C, Davies NM, Martin RM, Eeles R, Easton D, Kote-Jarai Z, et al. Alcohol consumption and prostate cancer incidence and progression: a Mendelian randomisation study. Int J Cancer 2017;140:75–85. https://doi.org/10.1002/ijc.30436
Leal J, Welton NJ, Martin RM, Donovan J, Hamdy F, Neal D, et al. Estimating the sensitivity of a prostate cancer screening programme for different PSA cut-off levels: a UK case study. Cancer Epidemiol 2018;52:99–105.
Merriel SWD, Turner EL, Walsh E, Young GJ, Metcalfe C, Hounsome L, et al. Cross-sectional study evaluating data quality of the National Cancer Registration and Analysis Service (NCRAS) prostate cancer registry data using the Cluster randomised trial of PSA testing for Prostate cancer (CAP). BMJ Open 2017;7:e015994. https://doi.org/10.1136/bmjopen-2017-015994
Young GJ, Harrison S, Turner EL, Walsh EI, Oliver SE, Ben-Shlomo Y, et al. Prostate-specific antigen (PSA) testing of men in UK general practice: a 10-year longitudinal cohort study. BMJ Open 2017;7:e017729. https://doi.org/10.1136/bmjopen-2017-017729
Camacho N, Van Loo P, Edwards S, Kay JD, Matthews L, Haase K, et al. Appraising the relevance of DNA copy number loss and gain in prostate cancer using whole genome DNA sequence data. PLOS Genet 2017;13:e1007001. https://doi.org/10.1371/journal.pgen.1007001
Er V, Biernacka K, Simpkin AJ, Martin RM, Jeffreys M, Emmett P, et al. Post-diagnosis serum insulin-like growth factors in relation to dietary and lifestyle changes in the Prostate testing for cancer and Treatment (ProtecT) trial. Cancer Causes Control 2017;28:877–88. https://doi.org/10.1007/s10552-017-0910-2
Lin HY, Chen DT, Huang PY, Liu YH, Ochoa A, Zabaleta J, et al. SNP interaction pattern identifier (SIPI): an intensive search for SNP-SNP interaction patterns. Bioinformatics 2017;33:822–33. https://doi.org/10.1093/bioinformatics/btw762
Donovan JL, Young GJ, Walsh EI, Metcalfe C, Lane JA, Martin RM, et al. A prospective cohort and extended comprehensive-cohort design provided insights about the generalizability of a pragmatic trial: the ProtecT prostate cancer trial. J Clin Epidemiol 2018;96:35–46.
Elliott D, Husbands S, Hamdy FC, Holmberg L, Donovan JL. Understanding and improving recruitment to randomised controlled trials: qualitative research approaches. Eur Urol 2017;72:789–98.
Hamdy FC, Donovan JL, Neal DE. 10-year outcomes in localized prostate cancer. N Engl J Med 2017;376:180. https://doi.org/10.1056/NEJMc1614342
Hamdy FC, Donovan JL. Patient-reported outcomes following treatment for localized prostate cancer: helping decision making for patients and their physicians. JAMA 2017;317:1121–3. https://doi.org/10.1001/jama.2017.1703
Wilson C, Rooshenas L, Paramasivan S, Elliott D, Jepson M, Strong S, et al. Development of a framework to improve the process of recruitment to randomised controlled trials (RCTs): the SEAR (Screened, Eligible, Approached, Randomised) framework. Trials 2018;19:50. https://doi.org/10.1186/s13063-017-2413-6
Seibert TM, Fan CC, Wang Y, Zuber V, Karunamuni R, Parsons JK, et al. Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ 2018;360:j5757. https://doi.org/10.1136/bmj.j5757
Lophatananon A, Stewart-Brown S, Kote-Jarai Z, Al Olama AA, Garcia SB, Neal DE, et al. Height, selected genetic markers and prostate cancer risk: results from the PRACTICAL consortium. Br J Cancer 2018;118:e16. https://doi.org/10.1038/bjc.2018.6
Gilbert R, Tilling K, Martin RM, Lane JA, Davis M, Hamdy FC, et al. Developing new age-specific prostate-specific antigen thresholds for testing for prostate cancer. Cancer Causes Control 2018;29:383–8. https://doi.org/10.1007/s10552-018-1014-3
Mikropoulos C, Hutten Selkirk CG, Saya S, Bancroft E, Vertosick E, Dadaev T, et al. Prostate-specific antigen velocity in a prospective prostate cancer screening study of men with genetic predisposition. Br J Cancer 2018;118:e17. https://doi.org/10.1038/bjc.2018.11
Vickers A, Vertosick EA, Sjoberg DD, Hamdy F, Neal D, Bjartell A, et al. Value of intact prostate specific antigen and human kallikrein 2 in the four kallikrein predictive model: an individual patient data meta-analysis. J Urol 2018;199:1470–4.
Martin RM, Donovan JL, Turner EL, Metcalfe C, Young GJ, Walsh EI, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA 2018;319:883–95. https://doi.org/10.1001/jama.2018.0154
FIGURE 34.
Number of ProtecT publications vs. the rate of citation. The H index is shown where the lines cross (44 papers had a citation count of 44 or more).
Altmetric 2016 scoring of main ProtecT trial 10-year clinical outcomes publication:
-
www.altmetric.com/details/12003554 (accessed 15 March 2018).
New England Journal of Medicine Metrics for both 10-year clinical outcomes and patient reported outcomes:
-
www.nejm.org/doi/metrics/10.1056/NEJMoa1606220 (accessed 15 March 2018)
-
www.nejm.org/doi/metrics/10.1056/NEJMoa1606221 (accessed 15 March 2018)
-
www.h2mw.eu/redactionmedicale/2017/01/Notable-Articles-2016.pdf (accessed 15 March 2018).
Glossary
- Active monitoring/active surveillance
- Protocols involving regular clinical examination, imaging in the form of multiparametric magnetic resonance imaging, prostate-specific antigen measurements and repeat biopsies. If these parameters suggest the risk of progression, men are offered radical treatment.
- Focal therapy
- A general term for a variety of non-invasive techniques for destroying small tumours inside the prostate while leaving the remaining gland intact and sparing most of its normal tissue.
- Gleason grade
- When cells are seen under the microscope, they have different glandular patterns, depending on how quickly they are likely to grow. The pattern is given a grade (called the Gleason grade) from 1 to 5. The higher the grade, the more likely the cancer is to spread outside the prostate.
- Gleason score
- There may be more than one grade of cancer in biopsy samples. An overall Gleason score is worked out by adding together two Gleason grades. One is the most common grade in all of the samples. The other is the second most common grade seen in the tissue examined.
- Minimally invasive technique
- The use of a variety of energy sources to ablate prostate tissue using advanced imaging technology and protective devices to reduce treatment-related morbidity.
- Watchful waiting
- This term is sometimes used to describe a less intensive type of follow-up than active surveillance/active monitoring, which may involve fewer tests and rely more on changes in symptoms to decide whether or not treatment is needed.
List of abbreviations
- 3D
- three-dimensional
- ADT
- androgen deprivation therapy
- AM
- active monitoring
- AS
- active surveillance
- BRCA1
- breast cancer gene 1
- BRCA2
- breast cancer gene 2
- BRU
- Bristol Nutrition Biomedical Research Unit
- CAP
- Cluster randomised triAl of PSA testing for Prostate cancer
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CoDE
- Cause of Death Evaluation
- CRF
- case report form
- DMC
- Data Monitoring Committee
- DNA
- deoxyribonucleic acid
- EBRT
- external beam radiotherapy
- EORTC-QLQ Q30
- European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 module
- EPIC
- Expanded Prostate Cancer Index Composite
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- ERSPC
- European Randomized Study of Screening for Prostate Cancer
- FFPE
- formalin fixed, paraffin embedded
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HIFU
- high-intensity focused ultrasound
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICIQ
- International Consultation on Incontinence Questionnaire
- ICS
- International Continence Society
- ICSmaleSF
- International Continence Society male Short-Form
- IGF
- insulin-like growth factor
- IGFBP
- insulin-like growth factor-binding protein
- IMRT
- intensity-modulated radiotherapy
- ITT
- intention to treat
- LUTS
- lower urinary tract symptoms
- mpMRI
- multiparametric magnetic resonance imaging
- MRI
- magnetic resonance imaging
- MSMB
- beta-microseminoprotein gene
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OPCS-4
- Office of Population Censuses and Surveys Classification of Interventions and Procedures
- OR
- odds ratio
- PAG
- patient advisory group
- PIVOT
- Prostate Intervention versus Observational Treatment
- PLCO
- Prostate, Lung, Colorectal, and Ovarian
- PPI
- patient and public involvement
- ProBE
- Prostate Biopsy Effects
- PROM
- patient-reported outcome measure
- PROMIS
- PROstate Magnetic resonance Imaging Study
- ProMPT
- Prostate Cancer: Mechanisms of Progression and Treatment
- ProtecT
- Prostate testing for cancer and Treatment
- PSA
- prostate-specific antigen
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QRI
- Quintet Recruitment Intervention
- Quartet
- Qualitative Research to Improve Recruitment to RCTs
- Quintet
- Qualitative Research Integrated in Trials
- RCT
- randomised controlled trial
- RITA
- radiofrequency interstitial tumour ablation
- RP
- radical prostatectomy
- RT
- radical radiotherapy
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SNP
- single-nucleotide polymorphism
- SOP
- standard operating procedure
- SPCG-4
- Scandinavian Prostate Cancer Group-4
- TRUS
- transrectal ultrasound
- TSC
- Trial Steering Committee
- TURP
- transurethral resection of the prostate
- VTP
- vascular-targeted photodynamic therapy
