Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/303/98. The contractual start date was in April 2007. The draft report began editorial review in July 2019 and was accepted for publication in January 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Helena Earl reports grants from Roche (Basel, Switzerland) and Sanofi-Aventis (Paris, France), personal fees and travel expenses from Daiichi Sankyo (Tokyo, Japan), AstraZeneca plc (Cambridge, UK) and Intas Pharmaceuticals (Ahmedabad, India), travel expenses from Pfizer Inc. (New York, NY, USA) and Amgen Inc. (Thousand Oaks, CA, USA) and personal fees from prIME Oncology (Atlanta, GA, USA), all outside the submitted work. Karen McAdam reports grants from Roche and personal fees from Roche, Novartis International AG (Basel, Switzerland), Pfizer and Eisai Co., Ltd (Tokyo, Japan), all outside the submitted work. Daniel Rea reports personal fees and grants from Roche during the conduct of the study, as well as personal fees from Novartis, Pfizer, Genomic Health (Redwood City, CA, USA) and Daiichi Sankyo, and grants from Celgene Corporation (Summit, NJ, USA), all outside the submitted work. Chris Plummer reports personal fees and non-financial support from Roche Products Limited, Novartis UK Limited, Pfizer UK Limited, Celgene and Incyte Corporation (Wilmington, DE, USA) for attending education meetings. He also reports personal fees and non-financial support from Amgen Limited for attending education meetings and advisory boards, all outside the submitted work. Jean Abraham reports fees to her institution, and accommodation and travel expenses from AstraZeneca for session boards and advisory chairs, as well as fees to her institution, and accommodation and travel expenses from Pfizer for a lecture, all outside the submitted work. Carlos Caldas reports grants from Genentech, Inc. (South San Francisco, CA, USA), Roche, Servier Laboratories (Suresnes, France) and AstraZeneca outside the submitted work, and that he is a member of the AstraZeneca iMED External Science Panel. Peter Hall reports grants from Roche, Pfizer, AstraZeneca, Novartis, Eisai and Daiichi Sankyo outside the submitted work. Christopher McCabe’s institution holds research contracts with Roche and reports grants from Roche, all outside the submitted work. David Miles reports personal fees from Roche/Genetech, outside the submitted work. Claire Hulme reports that she is a member of the National Institute for Health Research (NIHR) Health Technology Assessment Commissioning Board. Andrew M Wardley reports personal fees from Roche, Napp Pharmaceuticals Ltd (Cambridge, UK), Amgen, Merck Sharp & Dohme (Hoddesdon, UK), Novartis, Pfizer, AstraZeneca, Laboratoires Pierre Fabre (Paris, France), Accord (Barnstaple, UK), Athenex (Buffalo, NY, USA), Gerson Lehrman Group (New York, NY, USA), Coleman Research Expert Network Group (New York, NY, USA) and Guidepoint Global (New York, NY, USA). He also reports personal fees and other from Eli Lilly and Company (Indianapolis, IN, USA) and Daiichi Sankyo, all outside the submitted work. He is leading the National Cancer Research Institute Breast Group Initiative to develop the next de-escalation trial for HER2-positive breast cancer. David A Cameron reports funds to his institution from Novartis, Astrazeneca, Pfizer, Roche, Eli Lilly and Company, Puma Biotechnology (Los Angeles, CA, USA), Daiichi Sankyo, Synthon (Nijmegen, the Netherlands), SeaGen International GmbH (Zug, Switzerland), Zymeworks (Vancouver, BC, Canada), Elsevier (Amsterdam, the Netherlands), European Cancer Organisation (Brussels, Belgium), Celgene Corporation, Succinct Medical Communications (Wilmington, DE, USA), Immutep (Sydney, NSW, Australia), Oncolytics Biotech (U.S) Inc. (San Diego, CA, USA), Celldex Therapeutics Inc. (Hampton, NJ, USA), San Antonio Breast Cancer Consortium (TX, USA), Highfield Communication (Oxford, UK), Samsung Bioepis Co. Ltd (Incheon, South Korea), prIME Oncology, Merck Sharp & Dohme Ltd, Prima Biomed Ltd, RTI Health Solutions (Research Triangle, NC, USA) and Eisai, all outside the submitted work. Janet A Dunn reports that she is a member of the NIHR Efficacy and Mechanism Evaluation funding board and an NIHR senior investigator.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Earl et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Breast cancer and biology of HER2-positive disease
Breast cancer is a significant health problem worldwide and is the most common cancer occurring in women in the UK, with an incidence of 54,700 diagnoses per year (figures from 2017). 1 This represents 31% of all new cancer diagnoses in women. Although breast cancer also occurs in men it is uncommon, accounting for only 390 cases per year in the UK. A total of around 11,400 deaths are due to breast cancer each year and in women this represents the second most common cancer cause, accounting for 15% of all cancer deaths. 1 Overexpression of human epidermal growth factor receptor 2 (HER2) is recognised as a poor prognostic feature of breast cancer2 and has been found to be present in 12–15% of all breast cancer cases. Subsequently, the molecular intrinsic subtypes3 of luminal A and B, HER2-enriched, and basal-like have helped to define breast cancer prognosis more precisely and have improved clinical management and treatment decisions. These four main intrinsic subtypes are all found in HER2-positive disease, and dominate the biological and clinical phenotype. 4 Patients with HER2+/luminal A disease seem to have a relatively better outcome than patients with the other subtypes; in particular, patients with the HER2-enriched intrinsic subtype were found to have the worst prognosis, a finding confirmed in the integrative cluster of breast cancer analysis. 5
Trastuzumab treatment: metastatic and adjuvant
Significant HER2 overexpression leads to ligand binding and activation of cell division and tumour growth. Treatment of HER2-positive breast cancer with the monoclonal antibody trastuzumab (Herceptin®; Roche, Basel, Switzerland) was one of the first targeted cancer treatments to be developed and tested. It represented a major advance in the management of metastatic disease,6,7 producing significant improvements in response rates and survival. This signal of response soon led to registration studies of trastuzumab given with chemotherapy for HER2-positive breast cancer early in the disease, and cure rates improved. A Cochrane review of all of the adjuvant trastuzumab studies published in 20128 demonstrated that there were 40% fewer cancer recurrences and 34% fewer deaths with adjuvant trastuzumab. The magnitude of this effect has also been demonstrated indirectly in molecular studies of tumours sampled before the introduction of adjuvant trastuzumab. 5,9 These showed that the HER2-enriched group without adjuvant trastuzumab had worse survival than those with nine other molecular subtypes, including basal-type triple-negative breast cancer.
Adjuvant trastuzumab studies
After the success of trastuzumab for treating metastatic disease, registration trials were carried out in the early disease setting. The duration of treatment used in these pivotal registration trials was 12 months, although no evidence existed to support this length of targeted treatment in the adjuvant setting.
HERA
HERA was an international, multicentre, randomised trial that compared 1 year or 2 years of trastuzumab given every 3 weeks with observation in patients with HER2-positive and either node-negative or node-positive breast cancer who had completed locoregional therapy and at least four cycles of neoadjuvant or adjuvant chemotherapy. A total of 1694 patients were randomised to 2 years of trastuzumab, 1694 were randomised to 1 year of trastuzumab, and 1693 were in the observation arm with chemotherapy alone. Results were first reported in the New England Journal of Medicine10 for 1 year of trastuzumab compared with chemotherapy alone. With a median follow-up of 1 year, 347 disease-free survival (DFS) events (recurrence of breast cancer, contralateral breast cancer, second non-breast malignant disease, or death) were observed: 127 events in the trastuzumab group and 220 events in the observation group. The unadjusted hazard ratio (HR) for a DFS event in the trastuzumab group compared with the observation group was 0.54 [95% confidence interval (CI) 0.43 to 0.67; p < 0.0001], representing an absolute benefit in terms of DFS at 2 years of 8.4%. Symptomatic congestive heart failure (CHF), including severe CHF, developed in 1.7% of the women treated with trastuzumab.
Subsequent follow-up confirmed the beneficial effect. The 2-year analysis of the HERA trial demonstrated a benefit for overall survival (OS) as well as for DFS (HR for OS 0.66, 95% CI 0.47 to 0.91; p = 0.0115: updated HR for DFS 0.64, 95% CI 0.54 to 0.76; p < 0.0001). 11 These results confirmed that trastuzumab given sequentially after adjuvant chemotherapy has a significant benefit with a median follow-up of 2 years. The emergence of benefit after a median follow-up of only 1 year suggests that there is a significant effect from the first months of trastuzumab therapy. In addition, it is important to note that during the HERA trial trastuzumab was given sequentially after at least four cycles of standard chemotherapy, demonstrating the same degree of benefit as seen in the trials that used concurrent trastuzumab and chemotherapy.
Longer-term follow-up results of HERA have been published, confirming significant benefit for 12 months’ trastuzumab compared with observation at a median follow-up of 8 years. 12 This is despite nearly 50% of patients in the observation arm ‘crossing over’ to receive adjuvant trastuzumab after the early results were reported in 2005. There is no dispute about the significant benefit of adjuvant trastuzumab in HER2-positive breast cancer.
The HERA trial also tested 24 months’ trastuzumab compared with 12 months’ trastuzumab. The results for the 24-month arm showed no additional benefit in the 2013 publication after 8 years’ follow-up,12 or in the further publication in 2017 after 11 years’ follow-up. 13 Therefore, 12 months remained the standard of care throughout the world. In the accompanying editorial to the 2013 publication,14 Heikki Joensuu discussed the biology of HER2-positive breast cancer that leads to aggressive clinical behaviour and early cancer recurrence. He went on to say ‘The HERA results lend support to the hypothesis that patients with HER2 amplification do not benefit from long treatment durations with HER2-targeted therapy, but might be managed best with effective regimens of short duration’. 14 Interestingly, had the HERA trial been analysed and reported after only 3 years, a benefit for 2 years of trastuzumab would have been found. However, with longer follow-up,13 both 1- and 2-year DFS and OS are identical, and any earlier difference has disappeared.
NSABP B-31 and NCCTG N9831
The second paper published in the New England Journal of Medicine15 included the combined results of two US trials that compared adjuvant chemotherapy with and adjuvant chemotherapy without concurrent trastuzumab in women following surgery for HER2-positive breast cancer. The National Surgical Adjuvant Breast and Bowel Project trial (NSABP B-31) compared group 1 (doxorubicin and cyclophosphamide followed by paclitaxel either every 3 weeks or weekly) with group 2 (the same chemotherapy plus 52 weeks of trastuzumab beginning with the first dose of paclitaxel). The North Central Cancer Treatment Group (NCCTG) trial N9831 compared three regimens: group A (doxorubicin and cyclophosphamide followed by weekly paclitaxel), group B (the same chemotherapy followed by 52 weeks of trastuzumab after paclitaxel) and group C (the same chemotherapy plus 52 weeks of trastuzumab initiated concurrently with paclitaxel). The studies were amended to include a joint analysis comparing groups 1 and A (the control groups) with groups 2 and C (with trastuzumab given concurrently with taxanes). Group B was excluded from this first analysis because trastuzumab was not given concurrently with paclitaxel. The trial was reported when 394 DFS events had occurred, of which 133 were in the trastuzumab group and 261 were in the control group (HR 0.48; p < 0.0001). Three-year DFS was 87.1% in the trastuzumab group, compared with 75.4% in the control group, showing an absolute difference of nearly 12% in favour of trastuzumab. Trastuzumab therapy was associated with a 33% reduction in the risk of death (p = 0.015). The 3-year cumulative incidence of severe congestive heart failure or death from cardiac causes in the trastuzumab group was 4.1% in trial NSABP B-31 and 2.9% in NCCTG N9831.
Subsequently, longer-term follow-up16,17 confirmed the benefits of trastuzumab given for 12 months. At a median follow-up of 8.4 years, there was a 37% relative improvement in OS with the addition of trastuzumab (HR 0.63, 95% CI 0.54 to 0.73; p < 0.001) and an increase in the 10-year OS rate from 75.2% to 84%. DFS rates also improved by 40% (HR 0.60, 95% CI 0.53 to 0.68; p < 0.001), with an increase in the 10-year DFS rate from 62.2% to 73.7%. 17
Analysis of NCCTG N9831 on its own was able to address the comparison of the three arms. 16 The non-trastuzumab control arm was AC-paclitaxel (doxorubicin, cyclophosphamide and paclitaxel) chemotherapy, the second arm was AC-paclitaxel with sequential trastuzumab for 12 months, and the third arm was AC-paclitaxel with concurrent trastuzumab starting with paclitaxel and continuing afterwards to complete 12 months of weekly treatment. With a median follow-up of 6 years, the 5-year DFS rates were 80.1% and 84.4% for the second and third arms, respectively. There was an increase in DFS with concurrent trastuzumab and paclitaxel relative to sequential administration (HR 0.77, 99.9% CI 0.53 to 1.11), but the p-value of 0.02 did not cross the prespecified O’Brien–Fleming boundary (0.00116) required for this arm of the trial to be stopped early in the interim analysis. However, following the publication of these results in 2011, concurrent as well as sequential trastuzumab and chemotherapy were adopted as a ‘standard of care’ and, subsequently, concurrent treatment became the preferred option.
BCIRG-006
The Breast Cancer International Research Group (BCIRG) study of trastuzumab in women with HER2-expressing early breast cancer (BCIRG-006) examined concurrent treatment of trastuzumab with a non-anthracycline-containing chemotherapy regimen (docetaxel and either carboplatin or cisplatin). 18 In BCIRG-006, 3222 patients were recruited, and similar numbers of node-positive and high-risk node-negative women were randomised to each of three arms: adjuvant doxorubicin, cyclophosphamide and then docetaxel plus trastuzumab (AC-TH) or doxorubicin, cyclophosphamide and then docetaxel (AC-T), or docetaxel, carboplatin (or cisplatin) and trastuzumab (TCH). After a median follow-up of 65 months, there was significant benefit in DFS and OS in both groups treated with trastuzumab, compared with the group who received AC-T, who had a 5-year DFS of 75% and an OS of 87%. Among patients receiving AC-TH, the 5-year DFS was 84% (HR for the comparison with AC-T 0.64; p < 0.001) and OS was 92% (HR 0.63; p < 0.001). Among patients receiving TCH, the 5-year DFS was 81% (HR 0.75; p = 0.04) and OS was 91% (HR 0.77; p = 0.04). There was no difference between the two trastuzumab-containing arms, although the study was not sufficiently powered from a statistical point of view to confirm non-inferiority. There was a numerical trend for TCH showing a lower 5-year DFS (81% compared with 84% in the AC-TH arm), although there was significantly less cardiotoxicity among patients receiving TCH than among those receiving AC-TH.
FNCLCC-PACS-04
This was the only study that failed to demonstrate an advantage of adjuvant trastuzumab. 19 Five hundred and twenty-eight patients with operable node-positive breast cancer were randomised (1 : 1) to anthracycline-based chemotherapy with or without trastuzumab and, in a secondary randomisation, with or without docetaxel. The 3-year DFS rates were 78% (95% CI 72.3% to 82.5%) and 81% (95% CI 75.3% to 85.4%) in the observation and trastuzumab arms, respectively. There was a 14% reduction in the risk of relapse (HR 0.86, 95% CI 0.61 to 1.22; p = 0.41, log-rank stratified on pathologic node involvement), which was non-significant. All patients in this trial received sequential trastuzumab, and this result, together with the NCCTG N9831 analysis,16 which was the only trial directly comparing concurrent with sequential trastuzumab, resulted in a gradual shift in UK practice to increase the numbers of patients receiving trastuzumab concurrently with chemotherapy. This coincided with the increasing use of anthracycline-with-taxane chemotherapy combinations, which were approved by the National Institute for Health and Care Excellence (NICE) for the treatment of node-positive breast cancer during the course of the trial.
Shorter-duration studies
FinHer
Soon after the results of the registration trials, the FinHer trial was published. 20 This study compared docetaxel with vinorelbine for the adjuvant treatment of patients with early breast cancer. In this trial, women with HER2-positive tumours were also randomised to receive chemotherapy with or chemotherapy without concurrent trastuzumab. Two hundred and thirty-two women were recruited who had axillary-node-positive or high-risk node-negative HER2-positive cancer. They were randomised to receive three cycles of docetaxel or vinorelbine with or without concurrent weekly trastuzumab for 9 weeks, followed by three cycles of 5-fluorouracil, epirubicin and cyclophosphamide (FEC). In the subgroup of patients who had HER2-positive cancer, those who received trastuzumab had significantly better 3-year recurrence-free survival than those who did not (89% vs. 78%; HR for recurrence or death 0.42, 95% CI 0.21 to 0.83; p = 0.01). Docetaxel was associated with more adverse effects than was vinorelbine. Importantly, despite trastuzumab being given immediately before anthracyclines, this arm was not associated with reduced left ventricular ejection fraction (LVEF) or cardiac failure. The FinHer trial demonstrated that a short course of trastuzumab administered concurrently with docetaxel or vinorelbine was effective in women with HER2-positive breast cancer.
Subsequently, at a median follow-up of 62 months,21 the benefit of 9 weeks’ trastuzumab was maintained in the exploratory comparison of docetaxel and trastuzumab followed by FEC with the same treatment without trastuzumab (HR 0.32, 95% CI 0.12 to 0.89; p = 0.029). However, this was not the case in the comparison of vinorelbine and trastuzumab followed by FEC compared with the same treatment without trastuzumab (HR 0.92, 95% CI 0.47 to 1.83; p = 0.82).
Hence, when the PERSEPHONE trial started in 2007, whether or not similar outcomes could be achieved with durations of trastuzumab treatment of < 12 months was an important question worldwide.
E2198
This was a trial of the Eastern Cooperative Oncology Group (ECOG)22 that was set up to compare the cardiotoxicity of a short course of trastuzumab with that of the conventional 12 months of trastuzumab. This was not published until 2015 and therefore did not inform the design of the PERSEPHONE trial. In addition, it was not powered to demonstrate non-inferiority in the 12-week trastuzumab arm. The trial included 227 women with stage II or IIIa HER2-positive breast cancer. Patients were randomised to 12 weeks of paclitaxel and trastuzumab followed by four cycles of doxorubicin and cyclophosphamide or to the same treatment with a total of 1 year of trastuzumab (standard arm). The primary objective was to assess the safety of the different durations of trastuzumab therapy, in particular with regard to cardiac toxicity, which was defined as CHF or LVEF decrease of ≥ 10%. DFS and OS were secondary end points. The frequency or severity of cardiac toxicity did not increase; three patients in the experimental arm and four in the standard arm experienced CHF. There was no difference in 5-year DFS, which was 76% and 73% for the short and standard arms, respectively, with a HR of 1.3 (95% CI 0.8 to 2.1; p = 0.3). There was also no statistically significant difference in OS (HR 1.4; p = 0.3). The trial was not powered for efficacy; however, the longer duration of trastuzumab therapy did not demonstrate a signal for marked superiority. Retrospectively, this trial provided some additional evidence for the efficacy of shorter-duration trastuzumab.
Duration of trastuzumab studies
PHARE
Based on the FinHer results, the French group Institut National du Cancer developed the PHARE clinical trial to compare 6 months’ trastuzumab with 12 months’ trastuzumab (Table 1). Randomisation into the PHARE trial occurred between 3 and 6 months after a patient had started trastuzumab, which was one of the differences between PHARE and PERSEPHONE. At the start of PERSEPHONE, all patients were randomised before starting trastuzumab, but subsequently randomisation was permitted at any time up to and including the ninth cycle of trastuzumab. In PHARE, patients were stratified by oestrogen receptor (ER) status (positive or negative) and concurrent or sequential trastuzumab and chemotherapy. It mapped on to standard practice in France and was carried out as a multicentre study recruiting 3384 patients: 1690 randomised to 6 months’ trastuzumab and 1690 randomised to 12 months’ trastuzumab. In the original statistical plan outlined in the first publication in 2013,23 a margin of non-inferiority of 2% was calculated on an assumed 2-year DFS of 85%. This was the 2-year DFS figure that had been predicted from the first results of the HERA trial. 10 The number of patients required was 7000 to have a 5% level of significance and 80% power to confirm non-inferiority of the experimental arm with an upper confidence limit below the HR, which was calculated at 1.15. In an international collaboration with the PHARE group, PERSEPHONE will carry out a pre-planned joint analysis of results, which will allow a non-inferiority comparison of 6 and 12 months’ trastuzumab with a margin of non-inferiority of 2%. This requires a total of at least 7000 patients to be entered into the two trials. The PHARE trial defined the HR of non-inferiority in the statistical analysis plan before the start of the trial, and then applied this when the analysis was carried out. This is an important difference between the statistical analysis of PHARE and that of PERSEPHONE. In the latter we defined non-inferiority as an absolute difference of no worse than 3% below the standard arm’s 4-year DFS rate, and the HR limit was to be calculated at the time of analysis using the real, observed DFS of the standard arm.
| Trial name/trial design | PERSEPHONE/duration of trastuzumab with chemotherapy in patients with early breast cancer: 6 vs. 12 months | PHARE/protocol of Herceptin adjuvant with reduced exposure, a randomised comparison of 6 months vs. 12 months in all women receiving adjuvant Herceptin | HORG/6 vs. 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomised study by HORG | SOLD/randomised Phase III study comparing trastuzumab plus docetaxel (HT) followed by 5-FU, epirubicin, and cyclophosphamide (FEC) to the same regimen followed by single-agent trastuzumab as adjuvant treatments for early breast cancer | Short-HER/multicentric randomised phase III trial of two different adjuvant chemotherapy regimens plus 3 vs. 12 months of trastuzumab in HER2-positive breast cancer patients |
| Duration of trastuzumab | 6 months vs. 12 months | 6 months vs. 12 months | 6 months vs. 12 months | 9 weeks vs. 12 months | 9 weeks vs. 12 months |
| Trial recruitment and location | October 2007 to July 2015, UK | May 2006 to July 2010, France | June 2004 to May 2012, Greece | January 2008 to December 2014, international | December 2007 to November 2012, Italy |
| Actual (n)/target (N) | 4088/4000 | 3384/3400 (revised from 7000) | 481/480 | 2174/2168 (revised from 3000) | 1253/2500 |
| ER positive (%) | 69 | 58 | 66 | 56 | 68 |
| Node negative (%) | 58 | 55 | 20 | 60 | 54 |
| Tumour size ≤ 2 cm (%) | 47 | 53 | – | 56 | 41 |
| Adjuvant chemotherapy timing | 85 | 100 | 100 | 100 | 100 |
| Chemotherapy type | |||||
| Anthracyclines (%) | 41 | 16 | |||
| Taxanes without anthracyclines (%) | 10 | 10 | |||
| Anthracyclines and taxanes (%) | 49 | 74 | 100 | 100 | 100 |
| Trastuzumab timing | |||||
| Sequential | 54 | 43 | 0 | 0 | 0 |
| Concurrent | 46 | 57 | 100 | 100 | 100 |
HORG
The Hellenic Oncology Research Group (HORG) study24 randomised HER2-positive patients between 6 and 12 months (see Table 1). Randomisation occurred prior to the commencement of chemotherapy, which was dose-dense FEC given every 2 weeks for four cycles with granulocyte-colony stimulating factor support, followed by docetaxel given every 2 weeks with granulocyte-colony stimulating factor. A total of 481 patients were randomised between trastuzumab for 6 or 12 months, and this was started concurrently with docetaxel chemotherapy. The non-inferiority margin was set with an absolute 8% boundary, and an estimated control arm 3-year DFS of 85%. The authors state that this produced a HR for the non-inferiority boundary of 1.53. With a type I error of 5% and 80% power, 239 patients were required to enrol in each arm within an accrual period of 3 years. The trial recruited 481 patients over nearly 8 years (between June 2004 and May 2012), which was longer than intended.
SOLD
The SOLD (Synergism or Long Duration) trial25 was co-ordinated from Finland and included international centres (see Table 1). This trial arose directly out of the FinHer trial and patients were randomised between standard 12 months’ trastuzumab and a shorter course of 9 weeks’ trastuzumab. All patients received single-agent docetaxel for three cycles concurrently with trastuzumab. This was followed by three cycles of FEC chemotherapy. Patients randomised to the standard arm completed 12 months’ trastuzumab following completion of chemotherapy, whereas patients randomised to 9 weeks’ trastuzumab did not receive more after chemotherapy was completed. Patients were randomised prior to any adjuvant treatment and all patients received the same chemotherapy; all therefore received trastuzumab concurrent with chemotherapy and upfront. Over nearly 7 years (January 2008 to December 2014), 2176 patients were recruited from seven countries. The SOLD trial was originally powered as a superiority trial, as it was anticipated that the standard 12 months’ treatment would lead to a significant increase in cardiac deaths, which was not the case. The original sample size calculation was for a trial of 3000 patients. The primary end point was changed to non-inferiority of DFS in the experimental arm, and a non-inferiority boundary of 4% on the true (observed) 5-year DFS rate of 88.7%. This resulted in a HR limit of 1.385, and the final sample size was 2168 patients (1084 patients in each group). The primary analysis was planned to be an event-driven analysis after 366 DFS events or when the last patient entered had been followed up for 2.0 years after randomisation, whichever occurred first.
Short-HER
Short-HER26 was an Italian government-sponsored trial with a similar design to the SOLD trial (see Table 1). The standard arm consisted of four cycles of anthracycline chemotherapy [doxorubicin and cyclophosphamide (AC) or epirubicin and cyclophosphamide (EC)] followed by four cycles of docetaxel with trastuzumab given 3-weekly, followed by completion of a full 12 months’ trastuzumab. The experimental arm was similar to the SOLD trial, with concurrent docetaxel and weekly trastuzumab for three cycles followed by anthracycline chemotherapy for three cycles (FEC) and then no further trastuzumab. This study was designed to assess if a shorter trastuzumab administration is non-inferior to the standard of 12 months with respect to DFS. Non-inferiority was defined before the trial started as an absolute difference of no worse than 3%, and the non-inferiority HR limit of < 1.29 was set. The sample size was estimated by setting an alpha of 5% and a power of 0.80, which resulted in a requirement for 372 events and 2332 patients. However, accrual of patients was slower than expected and, to comply with timelines set by the government funding body, the trial had to complete recruitment after 1256 patients had been enrolled. The data analysis was carried out after 198 events but, because of lower recruitment than planned, the study was underpowered for its DFS end point (statistical power 56%). As well as the standard frequentist approach, a Bayesian analysis was planned.
Pragmatic design mapping on to standard practice
The PERSEPHONE trial was a pragmatic trial set up in the NHS in 2007 and mapped on to standard practice in the NHS. Therefore, once study sites had been initiated, and had been set up with all the approvals necessary to be part of the trial, all patients who it was planned would receive chemotherapy and trastuzumab could be approached to take part. The advantage of this pragmatic approach is that it is a reasonable assumption that, following completion of the trial, the results would be implementable without concerns that the population of standard patients was in any way different from the population tested in the trial. In licensing trials the entry criteria are necessarily restricted and very tightly controlled to ensure a high level of medical fitness in the patients entered. We were clear that in the PERSEPHONE trial we aimed to investigate how 6 months’ trastuzumab compared with 12 months’ treatment in the patients whom recruiting clinicians on site would be treating as standard in their clinics.
Chapter 2 Methods: recruitment and study conduct
Trial design
PERSEPHONE was a UK prospective, randomised, non-inferiority, multicentre, open-label Phase III clinical trial to examine if the administration of 6 months of trastuzumab is non-inferior to 12 months in patients with histologically confirmed HER2-positive early invasive breast cancer receiving a standard chemotherapy regimen in the adjuvant or neoadjuvant setting.
The key aim of PERSEPHONE was to establish whether or not a shorter duration of trastuzumab (6 months/nine cycles) is non-inferior in terms of efficacy to standard of care (12 months/18 cycles). Eligible patients were randomised equally (1 : 1) to receive 12 or 6 months of trastuzumab as well as chemotherapy. After completing treatment, patients were followed up for 10 years.
The current standard of care for early breast cancer patients with HER2-positive disease is to give chemotherapy and 12 months of trastuzumab, which is given as 17 or 18 cycles depending on local protocols. At the start of the trial, trastuzumab was given mostly sequentially after chemotherapy (in the majority of cases, this was anthracycline based without taxanes), but practice gradually changed during the trial and trastuzumab was increasingly given concurrently with chemotherapy. PERSEPHONE had a safety stage built in to confirm that concurrent administration of chemotherapy and trastuzumab was safe during the trial. Data ‘in real time’ on serious adverse events (SAEs) and treatment delays for the first 100 patients of this cohort were collected and analysed before being discussed by the independent Data and Safety Monitoring Committee (DSMC) (see outcome of the safety phase in Chapter 3, First 100 patients receiving concomitant trastuzumab and chemotherapy).
Research objectives/end points
To compare 6 months (nine cycles) of trastuzumab with 12 months (18 cycles) in terms of non-inferiority and safety in a prospective, randomised, non-inferiority, multicentre, open-label Phase III clinical trial, mapping on to standard practice in the UK.
Primary end point
-
To assess DFS non-inferiority of 6 months’ (nine cycles) compared with 12 months’ (18 cycles) trastuzumab in patients with HER2-positive early breast cancer.
Secondary end points
-
To assess OS non-inferiority of 6 months’ (nine cycles) compared with 12 months’ (18 cycles) trastuzumab in patients with HER2-positive early breast cancer.
-
To assess the expected incremental cost-effectiveness [cost per quality-adjusted life-year (QALY)] of 6 months (nine cycles) of trastuzumab compared with 12 months (18 cycles) of trastuzumab.
-
Cardiac function as assessed by LVEF during trastuzumab therapy, and analysis of predictive factors of development of cardiac damage.
Secondary objectives: substudies
-
Trans-PERSEPHONE: tumour blocks (paraffin-embedded) will be collected prospectively from patients in the study for molecular and candidate gene analysis as prognostic and predictive markers (separate protocol).
-
Trans-PERSEPHONE-SNPs: blood samples will be collected prospectively from patients in the study for single nucleotide polymorphism analysis to research genetic/pharmacogenetic determinants of inherited susceptibility to HER2-positive breast cancer, prognosis and trastuzumab response and toxicity (separate protocol).
Research hypotheses
-
Six months’ trastuzumab in early HER2-positive breast cancer is non-inferior to 12 months’ trastuzumab, which is the standard treatment.
-
The incremental cost-effectiveness of 6 months’ trastuzumab is significant when compared with 12 months’ trastuzumab.
-
Six months’ trastuzumab is significantly less toxic than 12 months’ trastuzumab in terms of clinical cardiac dysfunction, LVEF decrease, and rates of stopping treatment early for reasons of cardiac toxicity.
Study conduct
Sponsorship
Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge are joint sponsors of PERSEPHONE.
Ethics, regulatory and research and development approvals
The trial was approved by the Medicines and Healthcare products Regulatory Agency (MHRA) on 10 May 2007 and received a favourable opinion from the North West – Haydock Research Ethics Committee (REC) (previously named North West REC and then North West 5 REC – Haydock Park) on 9 August 2007. Local research and development department approval was obtained at each participating NHS trust before patients were randomised. The trial was conducted in accordance with the principles and guidelines of the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use,27 Good Clinical Practice (GCP),28 UK legislation,29,30 Cambridge and Warwick Clinical Trials Units Standard Operating Procedures and the REC and MHRA approved protocol. The current trial protocol is available online at the Warwick Clinical Trials Unit website. 31
Management of the trial
The Trial Management Group (TMG) was a multidisciplinary team of clinicians, statisticians, translational scientists and patient advocates who had considerable expertise in all aspects of trial design, conduct, safety, quality assurance and analysis. This group was in charge of running the trial. The TMG met regularly by teleconference to discuss site set-up, recruitment targets, safety and all matters pertaining to efficient conduct of the trial. After recruitment was completed, the focus of the TMG was data collection and case report form (CRF) completion.
The overall supervision of the trial was provided by an independent Trial Steering Committee (TSC) appointed by the National Institute for Health Research (NIHR) Evaluation, Trials and Studies Coordinating Centre, Health Technology Assessment (HTA) programme (the funder of the PERSEPHONE trial). The independent TSC consisted of an independent chairperson (an oncologist) and two other independent members (a breast surgeon and a statistician) and members of the TMG. The NIHR HTA programme and the independent TSC monitored the trial conduct and progress through regular reports, face-to-face meetings and teleconferences about the trial.
The independent monitoring of the trial was undertaken by the independent DSMC, which was established to advise the independent TSC if there was evidence that or a reason why the trial should be amended or terminated based on recruitment rates or safety and efficacy. Reports containing recruitment, protocol compliance, safety data and interim analyses of outcomes (which were confidential and not shared with the investigators) were presented regularly for review along with results from other relevant trials. At each review, the independent DSMC considered whether or not the trial should be stopped prematurely for ethical or safety reasons, including unexpected frequency or severity of toxicity, early indication of an inferior outcome in the experimental arm, unsatisfactory futility analyses or the publication of new data.
At the design stage of the PERSEPHONE trial, it was agreed with the PHARE TMG that one member of the independent DSMC would serve on both trials’ independent DSMCs, with their roles and responsibilities clearly defined for each committee. Details of the membership of the independent TSC, TMG and independent DSMC can be found in the Acknowledgements.
Trial site set-up
A total of 158 hospitals from NHS trusts and health boards in England, Scotland and Wales participated in PERSEPHONE and 152 sites recruited patients (96%); three were screening sites only and three were opened for the purposes of patient follow-up only. A list of all participating sites can be found in the Acknowledgements. Before a site was activated to recruitment, a trial initiation meeting was held, in person or via teleconference, to provide study-specific training to all staff members working on the trial. Continued support was offered to both existing and new staff at participating sites to ensure that they remained fully aware of the trial procedures and requirements.
Monitoring
Several levels of monitoring were applied through the trial.
Remote monitoring of sites was carried out on several occasions and especially after amendments to ensure that the investigator site file (containing the instructional materials and documentation required for the conduct of the trial) was well maintained.
Central monitoring of all data was carried out throughout the trial. Data were routinely cleaned, and queries were sent to sites if needed after (1) automatic validation checks during data entry, (2) manual checks (discrepancies between forms) or (3) annual data freeze to generate interim data-cleaning reports.
To ensure that sites were competent, triggered on-site monitoring was carried out occasionally if serious breaches and/or safety issues were reported.
Since 2010, the sponsor’s regulatory team has been monitoring Addenbrooke’s Hospital on an ongoing 6-monthly basis. Addenbrooke’s has been the highest recruiter throughout the trial. Monitoring involves source data verification for consent, eligibility criteria, LVEF and SAEs. It also includes reviewing the investigator site and pharmacy files. Concurrently, the trial master file held by the Cambridge co-ordinators is reviewed, with a particular focus given to any SAEs and non-compliances reported by all participating sites.
The Warwick trial management file of the PERSEPHONE trial was audited by the MHRA in 2012 and the Cambridge trial management file was reviewed by an independent auditor on behalf of the sponsor in 2015. No critical findings were identified following these reviews.
Patient information and informed consent
Patients potentially eligible to participate in the trial according to the criteria (see Participants) were identified either during a multidisciplinary breast cancer meeting held at each of the sites or, more occasionally, from clinic lists if chemotherapy had already started.
Patients were invited to participate in PERSEPHONE during consultations in oncology clinics, where systemic treatment options were discussed. Here the local principal investigator or co-investigator, or another sufficiently trained individual at the discretion of the principal investigator, discussed the trial with the patient and provided her with a copy of the patient information sheet (see Report Supplementary Material 1). Patients were given sufficient time (at least 24 hours) to discuss participation in the trial with their family, friends and general practitioner (GP) and had the opportunity to ask questions. Patients who were willing to participate were asked to provide written informed consent (see Report Supplementary Material 1) to the principal investigator, co-investigator or other sufficiently trained individual.
Since 2010, consent for sample collections has been included in the main patient information sheet/consent document and made mandatory. This covers a blood sample and the use of the patient’s tissue archival samples removed at the time of surgery (and at diagnosis for neoadjuvant patients). The information and consent related to the collection of quality-of-life data are in a separate optional document.
HER2 testing
HER2 testing was carried out and reported in accordance with the UK Royal College of Pathologists’ HER2 testing guidelines. At the start of the trial, these were the 2004 guidelines,32 which were updated in 200833 and 2014. 34 All laboratories testing for HER2 on samples from NHS patients are required to carry out testing in accordance with this guidance and to be part of the National External Quality Assurance Scheme for both immunohistochemistry (IHC) and in situ hybridisation (ISH). In the lifetime of the trial for recruitment (October 2007 to July 2015), three different guidelines were followed, depending on when a patient entered into the trial.
The 2004 guidelines32 for semiquantitative IHC stated that breast cancer samples should be considered to be HER2 positive if, on formalin-fixed, paraffin-embedded sections, strong, positive, complete membrane staining is apparent in > 10% of cancer cells. IHC was to be considered negative if there was no membrane staining (IHC score of 0), if < 10% tumour cells had membrane staining (IHC score of 0) or if > 10% tumour cells had membrane staining that was faint/barely perceptible and incomplete (IHC score of 1+). IHC was to be considered borderline (IHC score of 2+) when membrane staining was weak to moderate and present in > 10% of tumour cells. According to the guidelines, an IHC score of 2+ should be followed by fluorescence in situ hybridisation (FISH) and conventionally expressed as the ratio of HER2 signal to chromosome 17 signal (HER2 ratio). Tumours showing a ratio of ≥ 2 were considered positive and those with a ratio of < 2 were considered negative regardless of HER2 copy number.
The guidelines were updated in 2008. 33 Minimum workload recommendations were introduced and laboratories were required to perform at least 250 assays per year for IHC and 100 assays per year for ISH. IHC scores of 0 and 1+ were the same as in the 2004 guidelines. The cut-off point for IHC 3+, indicating a positive result, was increased to > 30% of cells with strong, complete membrane staining. The IHC 2+ category was expanded to include cases with 10–30% strong complete staining in addition to the previous definition of > 10% moderate staining. Categories for ISH and gene amplification were refined. A HER2 ratio of < 1.80 was considered HER2 non-amplified (negative). A HER2 ratio of 1.80–2.20 or HER2 gene copy number 4.0–6.0 signals/cell was considered borderline and the test was carried out again. If the HER2 ratio was 1.80–1.99, the tumour was considered non-amplified and therefore HER2 negative, and if the HER2 ratio was 2.00–2.20, the tumour was considered amplified and therefore HER2 positive; this was a subtle difference from the American Society of Clinical Oncology and College of American Pathologists guidelines, which did not further clarify the FISH borderline group. If the HER2 ratio was > 2.20 or the HER2 gene copy number was > 6.0 signals/cell, then the tumour was considered amplified and positive.
Further updates of the guidelines in 201434 redefined an IHC score of 3+ as > 10% strong, complete membrane staining, the same as in the 2004 guidelines. ISH was considered negative with a HER2/CEP17 ratio using a dual probe of < 2.0 and an average HER2 gene copy number of < 4.0 signals/cell. In tumours that were IHC 2+ (borderline), ISH testing was required. In these cases ISH was considered borderline/non-amplified when a dual probe HER2/CEP17 ratio was < 2.0 with either an average HER2 gene copy number of 4.0–6.0 signals/cell or a ratio of 1.80–1.99. In these cases the test was repeated and if the same result was obtained the tumour was regarded as HER2 negative, whereas a dual-probe HER2/CEP17 ratio of ≥ 2.0 or an average gene copy number of ≥ 6.0 signals/cell was considered to indicate HER2-positive cancer.
These changes over the course of the study would have affected only a small percentage of cases. In a review of the pooled data from the BCIRG breast cancer clinical trials, 0.5% of cases had a ratio of < 2.0 and a HER2 gene copy number of ≥ 6.0 (changed from negative to positive in 2008). 35
Screening and randomisation procedures
Sites were asked to screen patients with early breast cancer for their eligibility for the PERSEPHONE trial. We requested that screening logs be completed for all patients who were considered for the PERSEPHONE trial. We asked sites to record on the screening logs the date of screening, the patient’s date of birth, if the patient gave consent, if the patient was randomised and, in the event of patients not being consented or randomised, the reasons. These screening logs were requested from sites every 6 months for 6 years, and then annually for the last 2 years of recruitment.
The trial was an open-label, unblinded trial. Following the assessment of their eligibility during screening and collection of their written informed consent to be entered into the trial, patients were randomised (1 : 1) to either 12 months of trastuzumab (the standard treatment, comprising 18 cycles) or 6 months of trastuzumab (the experimental arm of the trial, comprising nine cycles). Randomisation was carried out by staff at each site telephoning the Warwick Clinical Trials Unit, where a central computerised minimisation procedure used the following stratification variables: ER status (positive or negative); chemotherapy type (anthracyclines without taxanes, anthracyclines with taxanes, taxanes without anthracyclines or neither anthracyclines nor taxanes); chemotherapy timing (adjuvant or neoadjuvant); and trastuzumab timing (concurrent or sequential).
Trial treatment and settings and locations
The trial treatment is trastuzumab and it is considered an Investigational Medicinal Product for the purpose of the PERSEPHONE trial, which is conducted with a Clinical Trial Authorisation in the UK.
Route
Trastuzumab was given intravenously to all patients until 2013, when the subcutaneous formulation became available in NHS hospitals.
Following the approval of amendment 11 by the MHRA at the end of 2013, the PERSEPHONE trial allowed participating sites to use the subcutaneous formulation. Switching between intravenous and subcutaneous administration was at the discretion of the treating clinician.
Setting
Trastuzumab was prescribed neoadjuvantly (pre surgery) or adjuvantly (post surgery).
Trastuzumab was prescribed with chemotherapy (concurrently) or post chemotherapy (sequentially). The PERSEPHONE trial recruited patients in both settings.
Dose
Patients were randomised to:
-
research arm – to receive nine cycles of trastuzumab (6-month arm)
-
standard arm – to receive 18 cycles of trastuzumab (12-month arm).
Each cycle had a 3 week duration with a loading dose given on day 1. Treatment was expected to be administered as per standard practice.
Intravenous route
The starting/loading dose of trastuzumab was 8 mg/kg.
The maintenance dose (6 mg/kg) was given 3 weeks after the starting/loading dose, and subsequent doses were given 3-weekly at 6 mg/kg.
Subcutaneous route
There is no loading dose for subcutaneous trastuzumab administration. Subcutaneous trastuzumab was given at a fixed dose of 600 mg in a volume of 5 ml as per the summary of product characteristics (SmPC).
Trastuzumab doses
The trial mapped on to standard practice and guidelines for dosing followed the SmPC for trastuzumab. In terms of cardiac guidelines, measurement of LVEF using either echocardiography (ECHO) or a multigated acquisition (MUGA) scan was recommended. At the start of the trial this was carried out every 3 months for 1 year in both arms, and then, after a protocol amendment, the interval between ECHO or MUGA scans was increased to 4-monthly. The guidance in the SmPC was to hold trastuzumab if LVEF fell by an absolute value of ≥ 10% below baseline and to < 50%. Cardiac medication could be started by the principal investigator; measurement of LVEF was repeated after 6 weeks, and trastuzumab could be restarted if LVEF was > 50%. The protocol stated that the maximum hold of trastuzumab for cardiac problems should be 12 weeks. If trastuzumab was restarted, then a reloading dose of 8 mg/kg intravenously was to be used with a delay longer than 7 days. In the second half of the study, some sites adopted new national guidelines to use cardiac medication and continue trastuzumab if the patient was asymptomatic and LVEF was > 40%. 36
Treatment location
Most patients were treated in chemotherapy units. However, to relieve these very busy hospital-based facilities, some participating sites started to administer trastuzumab outside chemotherapy units. Some patients were treated at home by an external clinical provider (Healthcare at Home Ltd). A few PERSEPHONE patients were treated in a GP surgery or a mobile unit (chemotherapy bus). As long as this was part of the standard practice at the site, it was allowed once the trials office had given its approval and made sure that the appropriate contracts, training and safety measures were in place.
Other treatments
Chemotherapy: commonly used regimens
Details of the chemotherapy regimens received by all patients (as per local institutional protocols) and whether trastuzumab was given concurrently with or sequentially to their chemotherapy were recorded in full, along with reasons for any early cessation of chemotherapy. Chemotherapy regimens were based on local protocols, which were informed by both licensing and NICE guidance.
Endocrine therapy
For women with ER-positive disease, systemic hormonal therapy is advised following completion of chemotherapy and definitive surgery. Concurrent hormone therapies could be administered with trastuzumab, although not with chemotherapy. The PERSEPHONE protocol stated that all endocrine therapy was at the discretion of the responsible clinician in accordance with standard local therapy protocols. However, the following guidelines were suggested: for women who remained pre-menopausal after completing chemotherapy, hormonal therapy options included ovarian suppression and tamoxifen. Entry into the Breast International Group EORTC trials SOFT (Suppression of Ovarian Function With Either Tamoxifen or Exemestane Compared With Tamoxifen Alone in Treating Premenopausal Women With Hormone-Responsive Breast Cancer Trial) or TEXT (Triptorelin With Either Exemestane or Tamoxifen in Treating Premenopausal Women With Hormone-Responsive Breast Cancer) was suggested. For postmenopausal women, tamoxifen or aromatase inhibitors could be used for a minimum of 5 years (or tamoxifen for 2–3 years, switching to an aromatase inhibitor after 2–3 years). Adjuvant hormonal treatment received by the patient was recorded.
Surgery
The PERSEPHONE protocol did not stipulate surgery as part of the trial. Surgery was carried out in accordance with standard practice at each site. Primary surgery was carried out in all patients receiving adjuvant chemotherapy and trastuzumab, mostly consisting of either wide local excision and axillary surgery (sentinel lymph node biopsy with or without axillary nodal dissection) or mastectomy and axillary surgery. Some patients in the trial received neoadjuvant treatment, and in these patients definitive surgery with wide local excision or mastectomy and axillary surgery took place after chemotherapy was completed.
Radiotherapy
The PERSEPHONE protocol stipulated that radiotherapy should be given after definitive surgery according to local protocols. Radiotherapy could be given concurrently with trastuzumab. All radiotherapy treatment received by the patient was recorded.
Participants
The trial sought to recruit patients with HER2-positive early breast cancer who were or would be receiving trastuzumab. Patients were recruited from oncology departments in NHS hospitals covering England, Scotland and Wales.
Inclusion criteria
Patients with the following characteristics were eligible to enter the trial:
-
A histological diagnosis of invasive breast cancer.
-
No evidence of metastatic disease.
-
Known hormone receptor status.
-
Overexpression of HER2 receptor. Bilateral breast cancers were eligible provided that one of the tumours overexpressed the HER2 receptor.
-
Clear indication for neoadjuvant or adjuvant chemotherapy based on clinical and histopathological features.
-
Fit to receive neoadjuvant or adjuvant chemotherapy and trastuzumab in the opinion of the responsible physician.
-
No previous diagnosis of malignancy unless:
-
managed by surgical treatment only, and disease free for 10 years
-
previous basal cell carcinoma, cervical carcinoma in situ or ductal carcinoma in situ of the breast.
-
-
Not pregnant and not lactating, with no intention of becoming pregnant during chemotherapy, and agreed to adopt adequate contraceptive measures if pre-menopausal and sexually active.
-
No concurrent medical or psychiatric problems that might prevent completion of treatment or follow-up.
-
Aged ≥ 18 years.
-
Gave written informed consent for the study at any time before the 10th cycle of trastuzumab.
Exclusion criteria
Patients with the following characteristics were ineligible to enter the trial:
-
significant concurrent cardiac disease or significant concurrent comorbidity in the opinion of the responsible physician adding to the risks associated with trastuzumab or cytotoxic chemotherapy
-
unable to comply with protocol requirements
-
received more than nine cycles of trastuzumab
-
any other condition that, in the local investigator’s opinion, would make them unsuitable to participate in the trial.
Data collection
Schedule of assessments
Table 2 shows the schedule of assessments for patients considered for entry into the PERSEPHONE trial prior to randomisation, as well as for patients during their treatment and follow-up. Most of the assessments for PERSEPHONE were mapped on to standard practice.
| Event | Prior to randomisation | Trastuzumab treatment visit (every 3 months for a year after starting trastuzumab treatment; patients in the research arm MUST follow the same follow-up schedule as those in the standard arm) | Follow-up visits (every 6 months in year 2; annually thereafter for 8 years) |
|---|---|---|---|
| Informed consent for trial | ✗ | ||
| ER status | ✗ | ||
| HER2 status | ✗ | ||
| Full blood count | ✗ | ||
| Biochemical screen | ✗ | ||
| Chest X-ray (or chest CT if standard practice) | If suspicion of metastases | ||
| Whole-body scintigraphy and liver ultrasound or abdominal CT scan | If suspicion of metastases | ||
| Medical history | ✗ | ||
| Physical examination, weight | ✗a | ✗a | ✗a,b |
| LVEF assessment | Done as per standard practice | ✗c | |
| ECOG performance status | ✗ | ✗ | ✗ |
| Quality of life questionnaire | ✗d | ✗e | ✗f |
| Health-care resource used assessment questionnaire | ✗d | ✗e | ✗f |
| SAEs | ✗ | ||
| Survival/recurrence disease status | ✗ | ✗ | |
Toxicity
Based on the available data on the frequency of toxicities experienced with trastuzumab treatment, the 4000 patients recruited to PERSEPHONE were expected to adequately power the analysis of toxicity to allow detection of any clinically relevant differences between the treatment arms, if they existed.
A change in the eligibility criteria for PERSEPHONE was implemented on 11 September 2009 (after 316 patients had been randomised) to allow patients to be randomised before receiving their 10th cycle of trastuzumab. In the case of patients randomised into PERSEPHONE after the start of their trastuzumab treatment, we did not collect information on the toxicities or SAEs experienced during any treatment administered prior to randomisation. In the case of patients randomised before starting trastuzumab, we planned to collect all toxicities and SAEs experienced during their entire treatment.
Cardiac toxicity
Patients were assessed for symptoms or signs of congestive heart failure and information on new or altered cardiac medication was recorded during follow-up visits while on trastuzumab. Cardiac function was assessed using LVEF, either by ECHO or by MUGA scan depending on standard practice at each hospital. As per the recommendations from the trastuzumab SmPC, we requested scans for each patient at baseline and every 3 months for 12 months from the treatment start date. This was revised to standard practice (minimum of 4-monthly) in PERSEPHONE protocol version 4.0 on 31 October 2013.
Translational research collections
In parallel with the NIHR HTA programme funding, Cancer Research UK supported a translational collection of blood and archival tissue samples through the Trans-PERSEPHONE grant. 37 The patient information sheet detailed collection of blood samples and tumour tissue collection (from the surgical specimen in those receiving adjuvant trastuzumab and from the diagnostic biopsy and surgical specimen in those receiving neoadjuvant chemotherapy). After patient consent was obtained, blood and tumour samples were requested.
Blood collection for pharmacogenetics/genetics study (Trans-PERSEPHONE-SNPs)
Trans-PERSEPHONE-SNPs aimed to collect two tubes of whole blood (2 × 9 ml) from patients, to be collected on one occasion at any time before, during or after treatment.
The purpose of collecting this was to build a bank of germline deoxyribonucleic acid (DNA) from breast cancer patients with HER2-positive disease and to carry out germline genome-wide sequencing for pharmacogenetics studies and prognostic and predictive candidate germline mutations. Analysis is planned of (1) germline single nucleotide polymorphisms (SNPs) predisposing to cardiac and other toxicity from trastuzumab, (2) germline SNPs predisposing to HER2-positive breast cancer, and (3) germline SNPs linked to outcomes from adjuvant trastuzumab and any interaction with duration of therapy.
Archival tissue blocks collection (Trans-PERSEPHONE)
Trans-PERSEPHONE is a collection of archival tissue (formalin-fixed, paraffin-embedded tissue) from PERSEPHONE patients. As per standard of care, following surgery, several formalin-fixed, paraffin-embedded blocks per patient were made at sites for diagnosis purposes. One or two representative block(s) were requested from the site’s pathology department. This bank of HER2-positive tissue is to be used to investigate prognostic and predictive molecular signatures of HER2-positive breast cancer and, in particular, to explore this with respect to treatment duration (comparing 6 and 12 months of treatment). Analysis of tumour and normal tissue will involve (1) tissue microarrays for IHC of protein gene products and in situ hybridisation analysis and (2) whole-genome profiling using expression and DNA microarrays.
Safety
First 100 patients receiving concomitant trastuzumab and chemotherapy
PERSEPHONE had a safety stage built into the trial to confirm that concurrent administration of chemotherapy and trastuzumab was safe within the trial. Data ‘in real time’ on SAEs and treatment delays for the first 100 patients of this cohort were collected and analysed before being discussed by the independent DSMC (see outcome of the safety phase in Chapter 3, First 100 patients receiving concomitant trastuzumab and chemotherapy).
Serious adverse events/reactions
The definition of a SAE for the purpose of the PERSEPHONE trial was any untoward medical occurrence that at any dose resulted in:
-
death
-
a life-threatening experience
-
initial or prolongation of existing hospitalisation (Hospitalisation was defined as an inpatient admission, regardless of length of stay, even if the hospitalisation was a precautionary measure, for continued observation. Hospitalisation for a preexisting condition, including an elective procedure, that had not worsened did not constitute a SAE)
-
persistent or significant disability/incapacity
-
a congenital anomaly/birth defect
-
a new primary malignancy.
-
Any other event that was judged by the responsible investigator to warrant particular attention and for the purposes of the trial included symptomatic LVEF reduction.
Details of all SAEs were documented from the point of randomisation, and therefore not necessarily the start of the trastuzumab treatment, in the trial until 30 days from the last administration of trastuzumab.
SAEs occurring after a patient’s 30-day follow-up assessment were reported only if the investigator believed that the study drug or a protocol procedure may have caused the event.
Each principal investigator was advised to report SAEs using a specific SAE form within 24 hours of becoming aware of the event.
Assessments of all SAEs for expectedness and relatedness were made promptly by the chief investigator. A SAE was deemed to be a serious adverse reaction (SAR) when it was assessed as possibly, probably or likely to be related to trastuzumab.
Listing of SARs was submitted to the MHRA in accordance with national requirements on a yearly basis through development safety update reports/annual safety reports.
Suspected unexpected serious adverse reactions
When the nature and severity of the SAR was not consistent with the trial reference safety information, the event was unexpected and therefore classified as a suspected unexpected serious adverse reaction (SUSAR).
The reference safety information for the PERSEPHONE trial was the trastuzumab SmPC.
The PERSEPHONE Cambridge office reported SUSARs to the MHRA and REC within 7–15 days as per UK regulations.
Serious breaches
To ensure that appropriate action was taken to protect patients, maintain trial integrity and comply with legal requirements and any applicable regulatory guidance, protocol and GCP non-compliances were collected by the trial office before being assessed by the chief investigator and the sponsor.
Any departure from the protocol or regulatory requirements that was likely to affect to a significant degree either the safety of a trial patient or the scientific value of the trial was classified as a serious breach, queried, followed up and reported to the authorities (MHRA and REC) as per UK legislation.
Discontinuation of trial treatment
Patients’ trial treatment was discontinued in the following circumstances, which were reported on the withdrawal CRF:
-
The patient opted to discontinue their randomised treatment arm or chose not to comply with the trial procedures.
-
The patient was found to be ineligible (i.e. they had been randomised inadvertently without meeting the eligibility criteria).
-
The investigator decided that the patient’s trial treatment should be discontinued because of toxicity.
-
The patient did not recover from treatment-related toxicity to an extent that would have allowed further trastuzumab treatment.
-
The patient had disease progression (radiologically confirmed) while on trial treatment.
-
The patient became pregnant while receiving trial treatment and decided to continue her pregnancy.
-
During trial treatment the patient relocated to a site that was not participating in the trial.
Follow-up data were collected for all patients who discontinued treatment. Treatment data were collected for patients who had withdrawn but continued to receive trastuzumab up to 12 months, unless the patient had withdrawn because of relapse.
Withdrawal of consent
Patients could withdraw their consent to participate in the trial at any time by explicitly refusing to receive any further trial treatment, in which case no further trial treatment was given.
Additionally, patients could withdraw their consent for further data or samples to be collected, in which case no further data or samples were collected. However, data and samples collected up to the time consent was withdrawn were included in the data reported for the trial.
Database and data processing
The PERSEPHONE database is held at Warwick Clinical Trial Unit on a Microsoft SQL Server 2012 (Microsoft Corporation, Redmond, WA, USA) system with imposed rules for data entry, which include a valid range for responses, linked dates and patient identification numbers.
Data were single entered into the database by trained study personnel. The trial statistician carried out checks for missing data and plausibility of entered values to enable further queries to be resolved before freezing the data for scheduled analyses.
Protocol amendments
Protocol amendments as well as amendments to key documents such as the patient information sheet and informed consent forms were reviewed by the REC and/or the competent authority before these amendments were implemented. The amendments were also approved locally by sites’ research and development departments.
The key amendments are listed below.
Protocol amendment February 2009
In February 2009, version 2.0 of the protocol was submitted to the authorities. The alterations were to remove limitations of and offer more flexibility in the chemotherapy criteria and options available to patients, such as using the company Healthcare at Home Ltd to administer trastuzumab at patients’ domiciles.
Protocol amendment July 2009
In July 2009, a major amendment was made to the protocol to change randomisation to any point up to and including cycle 9 of the trastuzumab (6 months). Recruitment had been lower than expected and, after extensive consultation with the TMG, TSC, the PHARE group (French trial) and the funder, it was agreed that this was the best option to increase recruitment. As a result of this, changes to the health economic data collection were also made, streamlining it to the patient questionnaires. Detailed health resource use data were collected for the first 300–500 patients only, rather than for all 4000 patients. Mapping the trial on to standard practice also significantly reduced data collection for sites.
Protocol amendment July 2010
A new patient information sheet was designed and submitted to the authorities in July 2010 to improve patient acceptability. Not only was accrual less than expected, but < 60% of the patients consented for the translational substudies, which was lower than in other studies we have conducted. Both the TSC and the patient groups who were contacted for advice strongly believed that (1) the original patient information sheet was too long and complex and (2) when patients agreed to take part in the trial, the substudies should be incorporated into the study as a whole. The TMG, together with patient advisors, therefore developed a more patient-friendly patient information sheet that incorporated the two translational substudies.
Protocol amendment October 2013
The protocol was amended again in October 2013, mainly to reflect the changes to the environment of the trial:
-
A full update on recently published data was added to the introduction of version 4.0 of the protocol.
-
The long-term follow-up data of the HERA trial12 confirmed the significant benefit for 12 months’ trastuzumab compared with no trastuzumab control at median follow-up of 96 months (8 years) but also demonstrated that 24 months’ trastuzumab brought no additional benefit to 12 months’. Interestingly, had the trial been analysed and reported after only 36 months, a benefit of 24 months’ trastuzumab would have been found. However, with longer follow-up, both the 12- and the 24-month DFS and OS curves are identical.
-
Despite a relatively short follow-up, the preliminary results of PHARE were published and showed that the trial had not proven non-inferiority after a median follow-up of 42 months. The PHARE results23 did show that, in a prospectively stratified analysis of subgroups, only patients with ER-negative disease receiving sequential chemotherapy and trastuzumab appeared to be significantly disadvantaged by receiving 6 months of trastuzumab. An examination of the mature DFS and OS curves from the HERA trial shows a clear separation of the 24-month and 12-month curves after 36 months, which resolves with more prolonged follow-up. This time point was the same as that at which PHARE had been analysed.
In addition, following scrutiny of the published PHARE data, the PERSEPHONE Independent Data Monitoring and Safety Committee confidentially examined the data in the PERSEPHONE trial. As a result of this, the committee advised the TMG and Trial Steering Group that there were no adverse signals in the PERSEPHONE trial data and that the trial be continued.
-
The equivalence of the subcutaneous formulation of trastuzumab and the intravenous formulation was reported38 and therefore version 4.0 of the protocol stated that the use of the subcutaneous formulation was allowed.
-
This low-risk trial was made flexible, with the aim of making it as pragmatic as possible (i.e. following standard practice to facilitate recruitment). In particular, cardiac function, which was initially assessed every 3 months for 12 months, was subsequently evaluated at intervals up to 4-monthly in accordance with a change in standard practice guidelines. 36
Protocol amendment December 2018
Protocol version 5.0 included changes to account for the European Union General Data Protection Regulation, revised trial timelines and permission to follow up patients using sites’ standard practice, which could include telephone and e-mail follow-up.
Statistics
Statistical analyses were performed using SAS® version 9.4 software. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration.
Non-inferiority design
The non-inferiority design of clinical trials was introduced to evaluate new treatment approaches, drugs, devices, biologics and other medical treatments to demonstrate whether or not a new treatment is a ‘good substitute’ (i.e. has similar efficacy to that of an established treatment). 39 Increasing in use by a factor of 6 over a 10-year period, this design is especially useful when evaluating whether a new treatment offers greater safety, reduced toxicity and reduced cost, together with confirmation that efficacy is not effectively compromised. It is the favoured design for trials in oncology that seek to de-escalate treatments by reducing either the duration or the intensity and therefore reduce toxicity, improve safety and improve patient experience. Cancer trials that have used this design include the IDEA meta-analysis,40 which established that 3 months rather than 6 months of an adjuvant capecitabine/oxaliplatin regimen for colorectal cancer is non-inferior and significantly less toxic. The non-inferiority of outcomes for head and neck cancer was demonstrated for positron emission tomographic scanning surveillance followed by radical dissection if demonstrated positron emission tomographic scanning positive or equivocal results, rather than routine radical neck dissection. 41 The TAILORx study used a non-inferiority design to compare adjuvant chemotherapy and endocrine therapy with endocrine therapy alone in women with ER-positive, HER2-negative, node-negative breast cancer and a moderate recurrence risk (score of 11–25) on a genomic test (OncotypeDx; Genomic Health, Inc., Redwood City, CA, USA). The trial demonstrated that endocrine therapy alone was non-inferior to adjuvant chemotherapy with endocrine therapy. 42 All of the trials examining reduced durations of trastuzumab in HER2-positive breast cancer have used non-inferiority designs. 23–26
Sample size and non-inferiority limit/margin
The PERSEPHONE trial was designed to allow the non-inferiority of the experimental arm (6 months’ trastuzumab) compared with the control arm (12 months’ trastuzumab) to be demonstrated in terms of the primary end point of DFS. The power calculations assumed that the DFS of the standard treatment of 12 months’ trastuzumab would be 80% at 4 years as this was the result available from the HERA trial. 10 The margin for non-inferiority was set as a 3% level, implying that the 4-year DFS of the experimental arm should not be below 77%, a difference equivalent to a HR of 1.17. On this basis, with 5% one-sided significance and 85% power, a trial randomising 4000 patients in total (2000 to each arm) would be able to prove the non-inferiority of the experimental arm.
The trial was expected to recruit for 4 years, with an additional follow-up period of 5 years, and allowed for loss to follow-up of up to 4%. The sample size was calculated by simulation assuming unadjusted analysis with a Cox’s proportional hazards model. The sample size also allowed for the 4-year DFS rate of the control arm to vary between 77% and 83%. The independent DSMC monitored the assumptions underlying the sample size calculation throughout the study.
Statistical methods
All randomised patients were included in all analyses where possible. Patients were analysed according to the treatment group to which they had been randomised on an intention-to-treat basis. Analyses were guided by the PERSEPHONE statistical analysis plan, which was prepared before data were available, and subsequently agreed by the PERSEPHONE independent DSMC.
Patient and tumour characteristics were presented to evaluate the comparability of the treatment arms and also the generalisability of the results to clinical settings. Categorical variables were presented using counts and percentages, and continuous variables were presented using either mean [standard deviation (SD)] or median [interquartile range (IQR)] depending on normality, and all were tabulated by treatment arm. A Consolidated Standards of Reporting Trials (CONSORT) flow diagram43 was also presented (see Chapter 3).
Primary end point: disease-free survival
The primary end point of DFS was measured for every patient from the date of diagnosis to date of first relapse (local or distant) or death. Patients who were disease free and either on follow-up or lost to follow-up were censored at the latest date at which they were known to be alive and disease free. Survival curves were plotted using the Kaplan–Meier method. Non-inferiority was defined as no worse than 3% below the control arm’s 4-year DFS rate. To test the non-inferiority of the experimental arm (i.e. 6 months’ trastuzumab), the HR was estimated using a Cox’s proportional hazards model containing only the trial treatment effect. If the 95th percentile of the estimated HR was less than the critical value, then the experimental arm (6 months’ trastuzumab) was regarded as non-inferior. Critical values were calculated for different scenarios with regard to the observed 4-year DFS on the control arm, as follows:
-
With a 4-year DFS on the control arm of 80%, the critical value to be used was 1.1712853.
-
With a 4-year DFS on the control arm of 85%, the critical value to be used was 1.2210943.
-
With a 4-year DFS on the control arm of 88%, the critical value to be used was 1.2713341.
-
With a 4-year DFS on the control arm of 90%, the critical value to be used was 1.3217671.
Proportionality of hazards was checked using an assessment of log–log plots.
A secondary analysis adjusting for stratification and baseline prognostic factors was planned. The treatment effect on DFS was also presented for stratification variables (ER status, chemotherapy type, chemotherapy timing and trastuzumab timing) using HR plots with interaction statistics using methods described by the Early Breast Cancer Trialists’ Collaborative Group in 1990. 44
Timing of primary analysis of primary end point
In 2015, the TMG and independent DSMC assessed the latest 4-year DFS rate for trial patients randomised to the control arm. This was found to be approximately 88%. The primary analysis of the primary end point for the 4000 patients was therefore planned to be undertaken when 500 DFS events had been observed, using a conditional power calculation. 45
Pre-planned early stopping guidelines
Initially, the PERSEPHONE protocol stated that, to control the overall alpha level, interim analyses of non-inferiority of the experimental arm with regard to the primary outcome would be reported to the independent DSMC using conservative tests, with significance determined by a p-value of 0.001. In 2015, the TMG and independent DSMC concluded that there was an additional requirement for prospective formal futility stopping rules.
The timings of the interim analyses for futility were configured on the 500 DFS events required for the primary analysis of DFS; these are shown in Table 3, including a recommended harm analysis46 at 25% of the total number of events.
| Percentage of total number of events required (% of 500) | Number of events required | HR limit |
|---|---|---|
| 25 | 125 | 1.52 |
| 50 | 250 | 1.34 |
| 75 | 375 | 1.27 |
Using a p-value of 0.01 (one sided), the HRs in the final column show the limits above which, with the corresponding number of events observed, the trial would have been stopped on the grounds of futility (i.e. concluding that non-inferiority could not reasonably be expected to be achieved).
The three interim analysis times specified above were reached in October 2012, June 2014 and March 2016. The independent DSMC considered the report presented to them and concluded that the data were immature and that there were no signals in the data that caused concern or would warrant a change in the study plan. The results found at these three time points did not cross the boundaries specified in Table 3.
The first of these time points (i.e. October 2012) coincided with the presentation of the PHARE47 and HERA 2-year data. 48
As the PERSEPHONE independent DSMC was confidentially examining the data in the PERSEPHONE trial, it advised the PERSEPHONE Trial Management Group and Trial Steering Committee that there were no adverse signals in these data, and recommended that the trial continue. On the strength of its advice, the NIHR HTA programme agreed a funding grant extension to complete recruitment of 4000 patients and to allow appropriate follow-up.
Landmark analysis of disease-free survival
To remove the effect of timing of randomisation (as patients could be randomised any time during their first 6 months of trastuzumab treatment), an exploratory landmark analysis was planned. Patients were included in the landmark analysis population if they were alive and disease free at least 6 months after the start of their trastuzumab treatment. The cohorts for comparison in the landmark analysis were checked for balance in terms of demographics and baseline disease characteristics. DFS was then recalculated from the landmark time point (6 months after the start of each patient’s trastuzumab treatment) and the number and time of DFS events across the two randomised arms since the landmark time point were assessed. Kaplan–Meier curves were plotted from 6 months after start of trastuzumab and, to test non-inferiority of the experimental arm (6 months’ trastuzumab), the HR was estimated using a Cox’s proportional hazards model containing only the trial treatment effect.
Overall survival
Overall survival (all-cause mortality) and a sensitivity analysis of cause-specific mortality were assessed using Kaplan–Meier curves and a Cox’s proportional hazards model containing only the trial treatment effect. OS across treatment arms was also assessed by stratification variables (ER status, chemotherapy type, chemotherapy timing and trastuzumab timing) using HR plots with interaction statistics. A landmark analysis of OS was also undertaken.
Trastuzumab treatment delivery
The total number of trastuzumab cycles administered per patient is shown graphically, separated by treatment arm. Counts and proportions for the reasons for early discontinuation of trastuzumab treatment as per protocol were documented. The total duration of trastuzumab treatment was calculated from the date of first infusion until 21 days after the date of the last cycle. Counts and proportions of the number of cycle delays/holds are presented, split by treatment arm, along with the stated reasons for them. Following an amendment to the protocol (see Protocol amendment October 2013), trastuzumab was allowed in the trial in its subcutaneous formulation at a fixed dose of 600 mg for each cycle without a loading dose. Method of administration for each trastuzumab cycle was recorded and is presented in tabular form (see Chapter 3).
Adherence to the PERSEPHONE protocol with respect to trastuzumab treatment was also assessed using a subgroup analysis including only cycles of trastuzumab administered to patients after they were randomised into the trial. Therefore, this analysis excluded any variation in trastuzumab treatment given before randomisation that adhered to local practice but was not in accordance with the PERSEPHONE protocol.
Toxicities
Initially, an analysis of all of the toxicity data received from all randomised patients was undertaken. For each toxicity in turn, each patient’s worst reported Common Terminology Criteria for Adverse Events (CTCAE) grade was identified and the frequencies were tabulated.
Following this, an analysis was undertaken on the population who were randomised upfront before any trastuzumab treatment to compare the randomisation arms in terms of the frequency and severity of toxicity through their first 6 months of treatment and, for patients randomised to 12 months’ trastuzumab, through their second 6 months of treatment. The frequency with which patients experienced each CTCAE grade was tabulated.
Serious adverse events
Frequencies of SAEs, SARs and SUSARs are presented by treatment arm. Frequencies of reasons for reporting, severity, causality and outcome are tabulated by treatment arm; a full breakdown of reported primary event categories of the SAEs and SARs is also given.
Patient and public involvement throughout the trial
PERSEPHONE is one of the first of a series of trials in the National Cancer Research Institute cancer portfolio assessing ‘something’ versus ‘something less’ in a non-inferiority context. Essentially, the concept of ‘less than standard’ treatment is worrying for some patients, as they feel that the decision to have less treatment may result in their cancer coming back. Treatment given in a clinical trial setting comes with a ‘comfort blanket’ that it is given per protocol and that the trial team is there to support the patient throughout treatment and beyond. PERSEPHONE benefited from the inclusion of a patient, Maggie Wilcox, who reviewed the trial materials and processes in the early stages as a member of the wider Trial Management Group. Maggie ensured that PERSEPHONE was presented at several early consumer group meetings to elicit opinion about barriers to recruitment and to brainstorm strategies to aid recruitment. In addition, the concept of non-inferiority trials was explained and the acceptable non-inferiority margin of 3% was endorsed, with a view to reducing this to 2% in the pre-planned meta-analysis of this trial with other reduced duration trials. Other patient and public involvement activities were supporting the patient booklets, collecting patient-reported experiences and raising awareness of PERSEPHONE at every opportunity. At the end of the study, Maggie was part of the team that reviewed the patient-reported experience data. The active patient and public involvement has benefited the dissemination and report-writing stages by including the patient voice and resulting in the change to guidelines so that patients do not continue to receive more treatment than they need.
Patient review of trial information and trial conduct
At the start of PERSEPHONE, Maggie, who was the consumer representative in the National Cancer Research Institute Breast Cancer Clinical Studies Group, was co-opted to help with reviewing the patient materials and facilitating the patient focus groups during which the study was explained. These focus groups were instrumental in our revising the patient materials and streamlining the number of consent forms that the ethics committee had mandated (five forms reduced to two) owing to the translational substudies and quality-of-life data collected. Initially, patients were being lost to recruitment as the consent process was too long and the research team did not have time to approach patients. Maggie informally asked various consultants why they were not recruiting patients to this trial, and feedback highlighted the time needed for consent and the burden on the research team and patients when using the five consent forms. Maggie sent a letter to the ethics committee requesting that the consent forms be reduced from five to two and once this was agreed by the committee it greatly increased the numbers of patients approached and recruited.
Throughout the trial, the study team engaged with the charity Independent Cancer Patients’ Voice; Maggie is president of this charity, and it has reviewed patient materials including the patient leaflet and clinic posters. Independent Cancer Patients’ Voice was formed in 2009 by a group of cancer patients with a keen interest in research; it is a patient-led organisation that aims to bring the views and experiences of cancer patients and their families and carers into the cancer research community. Independent Cancer Patients’ Voice believes that clinical research is enhanced when patients are in partnership with health-care professionals, instead of passively receiving health care. 49 As a result of Maggie’s involvement with PERSEPHONE, Independent Cancer Patients’ Voice was invited to work with researchers at the University of Warwick and University of Cambridge; this has been invaluable at all stages of the trial.
Other local groups such as the consumer group at Guilford and the Camazons at Cambridge have contributed to focus groups on the barriers to recruitment. The patients endorsed the trial and the continued importance of the question being addressed.
Patient participation in dissemination
The PERSEPHONE results were first presented at the American Society of Clinical Oncology in 2018, which was followed by a Lancet publication that was e-mailed to all of the recruiting centres. Patients were asked as part of the consent process if they wanted to receive a copy of the results once these were available. A patient-friendly summary of the key findings was sent alongside the main paper. Maggie reviewed the one-page summary and simplified the content for easier understanding. Including patients in the dissemination of patient-approved materials is essential for wider dissemination. Finally, Maggie volunteered to be interviewed by the press and provided a quotation for the PERSEPHONE press release.
The final phase of the patient involvement will be a survey of patients to see what they think about the impact of these results and to endorse a recommended change in the guidelines.
Chapter 3 Results
Screening and recruitment
Screening
Routine screening logs were requested from sites every 6 months for 6 years and then annually for the last 2 years of recruitment. Screening log returns were low; however, analysing the logs that were returned, 1848 out of the 7975 (23%) patients known to have been screened were recruited. The most common reasons that screened patients were not recruited were that they preferred to receive standard treatment (26%), they were ineligible (24.5%) or they declined to take part in the trial without giving a reason (16.5%) (see Appendix 1, Table 26).
Recruitment
Between 4 October 2007 and 31 July 2015, 4089 patients were randomised from 152 sites: 2045 to 12 months’ trastuzumab and 2044 to 6 months’ trastuzumab. Cumulative recruitment over time, compared with predicted accrual revised in October 2009, is shown in Figure 1. There was some slowing of recruitment in the second half of 2012, and it then remained steady at a reduced level. This was when the PHARE trial reported its early findings at the European Society for Medical Oncology,47 a result that failed to demonstrate non-inferiority. The presented data that were subsequently published23 were reviewed in detail by the independent DSMC alongside a confidential report of the PERSEPHONE data. The independent DSMC noted that the results from the PHARE trial had been reported with a median follow-up of 42.5 months and a primary end point of DFS at 2 years, and it interpreted the results as inconclusive. Following a confidential review of our trial data, the independent DSMC reported to the TMG and TSC that, taking into account the new data from PHARE, together with our own safety and efficacy data, there was no reason to stop the trial early, and it encouraged continuing to full recruitment of 4000 patients. All active sites were informed of the independent DSMC review and the advice to continue recruitment.
FIGURE 1.
Recruitment over time.
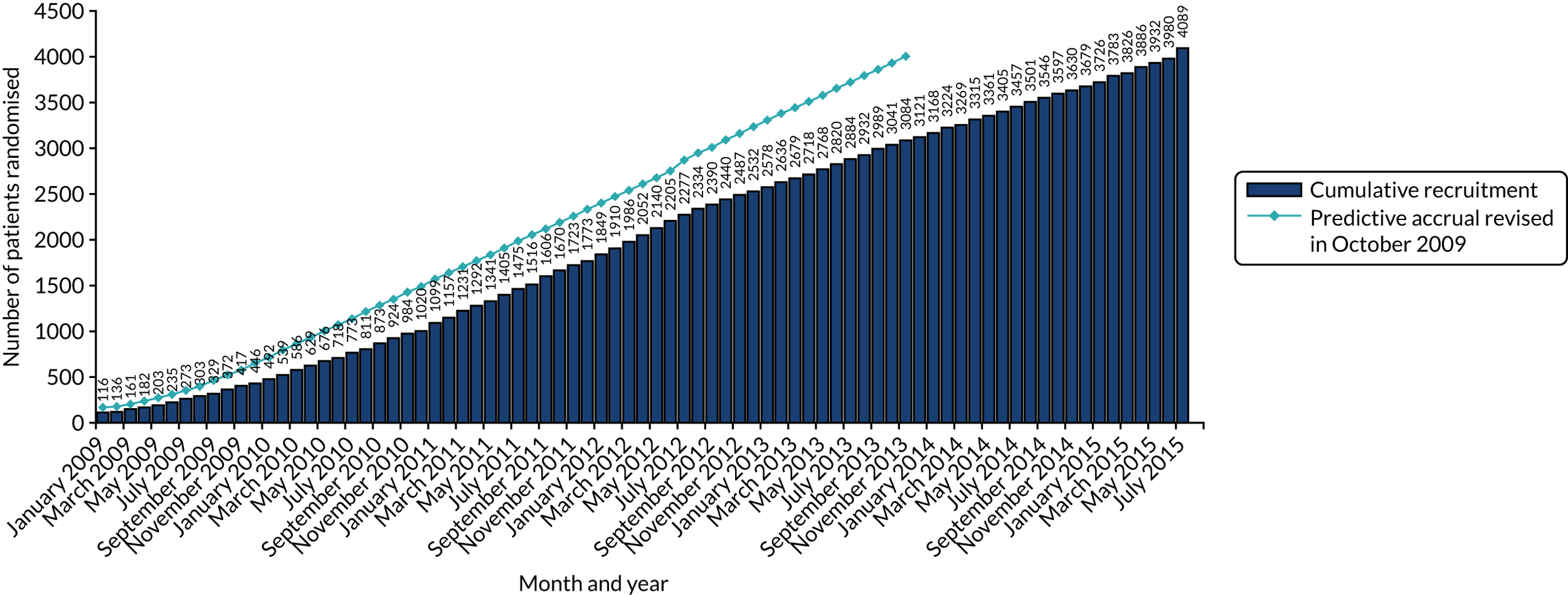
Consolidated Standards of Reporting Trials flow diagram
In total, 2045 patients were randomised to 12 months’ trastuzumab and 2044 patients were randomised to 6 months’ trastuzumab (Figure 2). One patient was double randomised and followed randomisation to 12 months, and this duplicate is the only randomised ‘patient’ excluded from the intention-to-treat analysis. Full treatment details are available for 1942 out of 2045 (95%) 12-month patients and for 1979 out of 2043 (97%) 6-month patients and therefore these are the patients included in the treatment analysis. The numbers of patients in each category of treatment are shown in the CONSORT flow diagram (see Figure 2). Notably, 82% of 12-month patients and 89% of 6-month patients received the number of cycles to which they had been randomised. In the 6-month arm, 5% (102/1979) received more than nine cycles of trastuzumab. Seventeen per cent (336/1942) of 12-month patients received fewer than the randomised 18 cycles, the most common reason being cardiovascular toxicity (146/336; 43%), followed by patient request (94/336; 28%) and relapse or death (26/336; 8%). Among 6-month patients, 6% (113/1979) received fewer than nine cycles, the most common reason being cardiovascular toxicity (60/113; 53%), followed by patient request (26/113; 23%) and relapse or death (12/113; 11%).
FIGURE 2.
The CONSORT flow diagram.
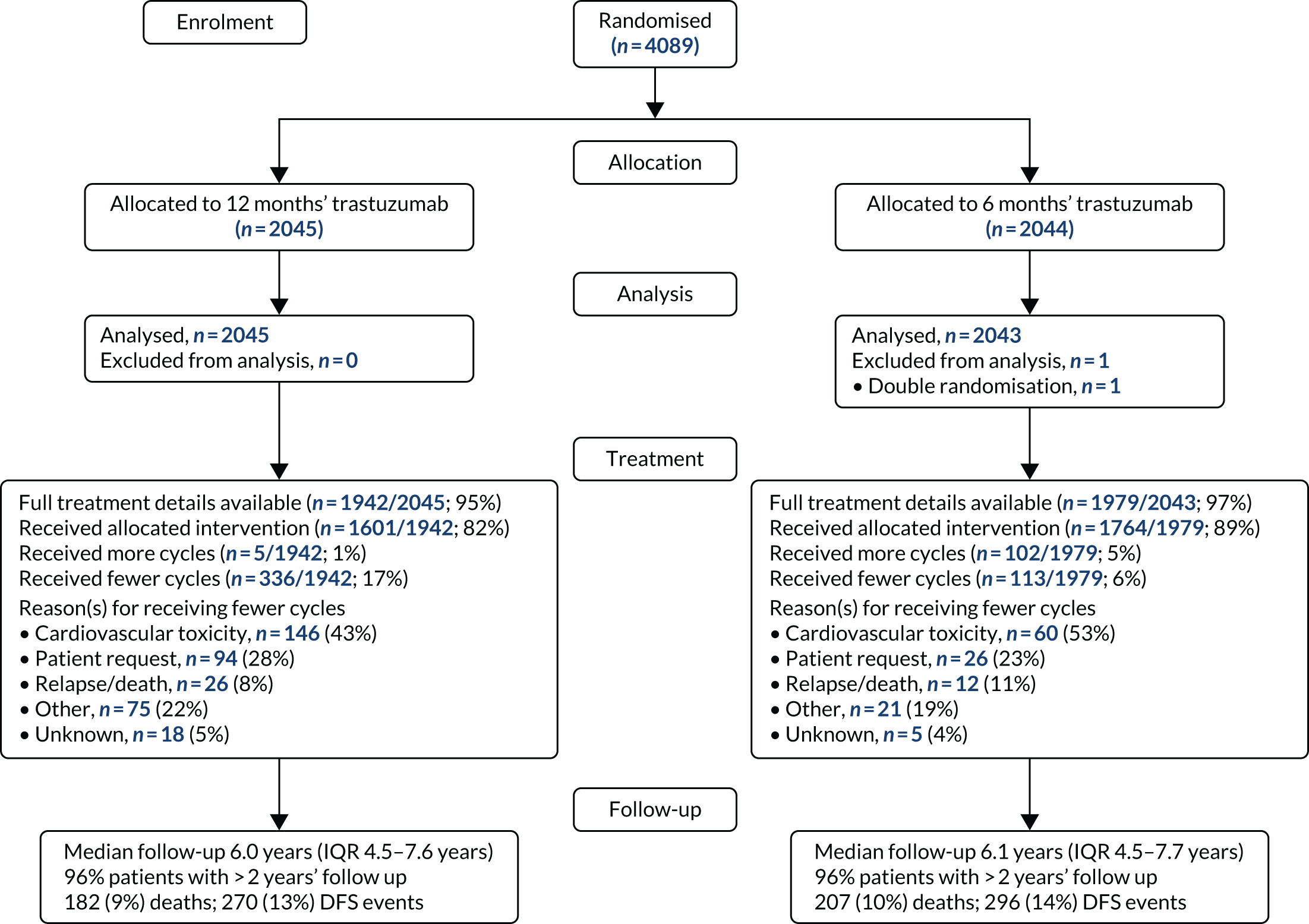
Recruitment by site and across treatment arms
The full recruitment by site is shown in Appendix 1, Table 27, with randomisation to 6 or 12 months, and the percentage of the total recruitment. This demonstrates the importance of recruiting from this large number of cancer units (n = 128) and cancer centres (n = 24) to achieve the high recruitment target. The majority of recruitment was from cancer units, with the research personnel supporting the trial provided initially by the National Cancer Research Network and then by the integrated newly developed Clinical Research Network in England; the NHS Research Networks in Scotland (funded by the Chief Scientist’s Office); and the Health and Care Research Office in Wales.
Withdrawals from trial treatment
Patients were withdrawn from trial treatment for reasons specified in the protocol, and this occurred for 346 (17%) 12-month patients and 223 (11%) 6-month patients. Among 12-month patients, the most common reason for withdrawal was toxicity (8% of patients randomised), followed by patient request (5%) and disease progression (2%) (Table 4). Among 6-month patients, the most common reason was patient request (6%, with 100 patients requesting a change to standard treatment of 12 months), followed by toxicity (3%), disease progression (< 1%) and ineligibility (< 1%). Follow-up data were collected from all withdrawn patients, except 106 patients (12-month patients, n = 65; 6-month patients, n = 41) who also withdrew their consent for further data collection.
| Main reason for withdrawal | 12-month patients | 6-month patients | Total |
|---|---|---|---|
| Toxicity | 171 (8%) | 69 (3%) | 240 (6%) |
| Disease progression | 40 (2%) | 11 (< 1%) | 51 (1%) |
| Ineligibility | 6 (< 1%) | 11 (< 1%) | 17 (< 1%) |
| Non-compliance | 7 (< 1%) | 2 (< 1%) | 9 (< 1%) |
| Relocation | 6 (< 1%) | 2 (< 1%) | 8 (< 1%) |
| Pregnancy | 1 (< 1%) | 1 (< 1%) | 2 (< 1%) |
| Patient request | 94 (5%) | 118 (6%) | 212 (5%) |
| Change to other treatment arm | 9 | 100 | 109 |
| Toxicity | 23 | 4 | 27 |
| Wanted to stop treatment | 18 | 4 | 22 |
| To use Healthcare at Home | 14 | – | 14 |
| Reason not specified | 8 | 4 | 12 |
| Wanted 17 cycles | 9 | – | 9 |
| Other | 13 | 6 | 19 |
| Other | 21 (1%) | 9 (< 1%) | 30 (< 1%) |
Ineligible patients
Nineteen patients were deemed ineligible after randomisation (12-month patients, n = 7; 6-month patients, n = 12), principally for previous cancers or ductal carcinoma in situ treated with radiotherapy as well as surgery:
-
12-month patients –
-
four with previous cancer/ductal carcinoma in situ treated with surgery and radiotherapy
-
two who were HER2 negative
-
one with primary cancer confined to the axilla.
-
-
6-month patients –
-
seven with previous cancer/ductal carcinoma in situ treated with surgery and radiotherapy
-
two with metastatic disease
-
one who was HER2 negative
-
one who had received > 9 cycles of trastuzumab at time of randomisation
-
one who had been double randomised (excluded from analysis).
-
Follow-up data continued to be collected for all patients unless they withdrew from the study and requested not to be followed up. The remainder of this report comprises 4088 randomised patients: 2045 randomised to 12 months’ trastuzumab and 2043 randomised to 6 months’ trastuzumab.
Data return
Return rates of trial CRFs from sites to the Clinical Trials Unit were very high, with > 97% of all expected baseline and treatment CRFs returned and > 90% of all expected annual follow-up forms returned (see Appendix 1, Table 28).
Baseline characteristics
Patient and tumour characteristics, split by randomised treatment arm
Table 5 shows patient and tumour characteristics split by whether patients were randomised to 12 months’ (n = 2045 patients) or 6 months’ (n = 2043 patients) trastuzumab. Patient and tumour characteristics were balanced across the two randomised treatment arms in terms of both minimisation variables and other prognostic factors. Forty-one per cent of patients received anthracycline-based chemotherapy, 10% received taxane-based chemotherapy without anthracyclines and 49% received anthracycline and taxane-based chemotherapy; therefore, 90% received anthracycline-containing chemotherapy. Sixty-nine per cent of patients had ER-positive tumours and 31% had ER-negative tumours. Eighty-five per cent of patients received adjuvant chemotherapy and 15% received neoadjuvant treatment. Of those patients receiving adjuvant chemotherapy and trastuzumab, 58% were node negative and 41% were node positive; 47% had tumours ≤ 2 cm and 50% had tumours > 2 cm. The profile of standard prognostic factors of ER status, nodal status and tumour size for the population included in the trial was considerably better than that for patients in the licensing trials (HERA, NSABP B-31 and NCCTG N9831, and BCIRG-006), and similar to that for patients in other trastuzumab duration trials (see Appendix 1, Table 29).
| Characteristic | 12-month patients, n (%) | 6-month patients, n (%) | Total, N (%) |
|---|---|---|---|
| ER statusa | |||
| Negative | 632 (31) | 631 (31) | 1263 (31) |
| Positive | 1413 (69) | 1412 (69) | 2825 (69) |
| Chemotherapy typea | |||
| Anthracycline based | 851 (41) | 845 (41) | 1696 (41) |
| Taxane based (no anthracycline) | 198 (10) | 202 (10) | 400 (10) |
| Anthracycline and taxane based | 994 (49) | 993 (49) | 1987 (49) |
| No taxane and no anthracycline | 2 (< 1) | 3 (< 1) | 5 (< 1) |
| Chemotherapy timinga | |||
| Adjuvant | 1735 (85) | 1727 (85) | 3462 (85) |
| Neoadjuvant | 310 (15) | 316 (15) | 626 (15) |
| Trastuzumab timinga | |||
| Concurrent | 949 (46) | 951 (47) | 1900 (46) |
| Sequential | 1096 (54) | 1092 (53) | 2188 (54) |
| Sex | |||
| Female | 2041 (99) | 2041 (99) | 4082 (99) |
| Male | 4 (1) | 2 (1) | 6 (1) |
| Age (years) at randomisation | |||
| Median (range) | 56 (23–82) | 56 (23–83) | 56 (23–83) |
| < 35 | 50 (2) | 45 (2) | 95 (2) |
| 35–49 | 552 (27) | 557 (27) | 1109 (27) |
| 50–59 | 608 (30) | 656 (32) | 1264 (31) |
| 60–69 | 617 (30) | 582 (29) | 1199 (30) |
| ≥ 70 | 218 (11) | 203 (10) | 421 (10) |
| Nodal status at surgery (of the 3462 adjuvant patients) | |||
| Negative | 1001 (58) | 1016 (59) | 2017 (58) |
| 1–3 nodes positive | 478 (27) | 486 (28) | 964 (28) |
| ≥ 4 nodes positive | 245 (14) | 210 (12) | 455 (13) |
| Unknown | 11 (1) | 15 (1) | 26 (1) |
| Tumour sizeb (of the 3462 adjuvant patients) | |||
| ≤ 2 cm | 823 (47) | 803 (47) | 1626 (47) |
| > 2 and ≤ 5 cm | 779 (45) | 786 (45) | 1565 (45) |
| > 5 cm | 87 (5) | 82 (5) | 169 (5) |
| Unknown | 46 (3) | 56 (3) | 102 (3) |
| Tumour gradeb | |||
| I (well differentiated) | 28 (1) | 34 (2) | 62 (2) |
| II (moderately differentiated) | 631 (31) | 645 (32) | 1276 (31) |
| III (poorly differentiated) | 1325 (65) | 1297 (63) | 2622 (64) |
| Unknown | 61 (3) | 67 (3) | 128 (3) |
| Ethnicity | |||
| White | 1658 (81) | 1649 (81) | 3307 (81) |
| Asian | 57 (3) | 52 (3) | 109 (3) |
| Black | 52 (3) | 45 (2) | 97 (2) |
| Other | 17 (< 1) | 21 (1) | 38 (1) |
| Unknown | 261 (13) | 276 (13) | 537 (13) |
| Menopausal status before chemotherapy | |||
| Pre | 567 (28) | 579 (28) | 1146 (28) |
| Peri | 110 (5) | 151 (7) | 261 (6) |
| Post | 1145 (56) | 1072 (53) | 2217 (54) |
| Not assessable/not available | 223 (11) | 241 (12) | 464 (12) |
| Reported prior use of cardiac medication | |||
| Yes | 44 (2) | 55 (3) | 99 (2) |
| No | 2001 (98) | 1988 (97) | 3989 (98) |
| IHC-score and FISH positivity (HER2 test result) | |||
| 3+ | 1462 (71) | 1489 (73) | 2951 (72) |
| 2+ and FISH positive | 551 (27) | 510 (25) | 1061 (26) |
| HER2 positive – IHC and FISH score not available | 32 (2) | 44 (2) | 76 (2) |
Patient and tumour characteristics split by adjuvant and neoadjuvant patients
Appendix 1, Table 30, shows the patient and tumour characteristics of all patients, split by whether they received adjuvant (n = 3462 patients) or neoadjuvant (n = 626 patients) chemotherapy. As can be seen, more neoadjuvant chemotherapy patients than adjuvant chemotherapy patients were ER negative (39% vs. 30%: p < 0.0001), received concurrent chemotherapy and trastuzumab (79% vs. 41%: p < 0.0001), and received anthracycline and taxane chemotherapy (89% vs. 41%). In addition, fewer neoadjuvant chemotherapy patients than adjuvant chemotherapy patients received anthracycline-based chemotherapy (9% vs. 48%) or taxane-based chemotherapy (2% vs. 11%: p < 0.0001 for differences in types of chemotherapy). The age profile for neoadjuvant chemotherapy patients was younger than that for adjuvant chemotherapy patients (p < 0.0001), and more neoadjuvant chemotherapy patients were pre-menopausal (39% vs. 26%; p < 0.0001). Follow-up was shorter for neoadjuvant chemotherapy patients (5.4 years, IQR 4.1–7.2 years) than for adjuvant chemotherapy patients (6.2 years, IQR 4.6–7.7 years).
Patient and tumour characteristics split by whether patients received concurrent or sequential trastuzumab and chemotherapy
Appendix 1, Table 31, shows the patient and tumour characteristics of all patients, split by whether they received chemotherapy and trastuzumab concurrently (n = 1900 patients) or sequentially (n = 2188 patients). As had been expected, 79% of concurrent patients received anthracycline and taxane-based chemotherapy compared with 22% of sequential patients, 3% of concurrent patients received anthracycline-based chemotherapy (E-CMF) compared with 75% of sequential patients, and 18% of concurrent patients received taxane-based chemotherapy compared with 3% of sequential patients (p < 0.0001). Patients given trastuzumab and chemotherapy concurrently compared with sequentially were more often node positive (53% and 32%, respectively; p < 0.0001), were more likely to have larger tumours (> 2 cm: 55% vs. 47%, respectively; p < 0.0001), were more likely to receive neoadjuvant treatment (26% vs. 6%, respectively; p < 0.0001) and, in addition, had shorter median follow-up (concurrent 5.3 years, IQR 4.2–6.6 years, vs. sequential 6.7 years, IQR 4.2–6.6 years).
Change in characteristics and standard practice over time throughout the trial
The characteristics of the patients being entered into PERSEPHONE and standard practice by the clinicians over the 8-year recruitment period from 4 October 2007 to 31 July 2015 were seen to change (Figure 3). The rate of ER-positive tumours increased from 62% of all patients randomised in 2008 to 74% of all patients randomised in 2015. The use of concurrent trastuzumab with chemotherapy increased from 24% in 2008 to 70% in 2015. The type of chemotherapy also changed. Anthracycline-based chemotherapy decreased from 63% in 2008 to 25% in 2015, taxane-based chemotherapy without anthracyclines increased from 7% to 18%, and anthracycline and taxane chemotherapy increased from 30% to 57%. In 2008 only 13% of patients received neoadjuvant treatment, but by 2015 this had increased to 20%. The PHARE trial presented early results in October 2012 and published in June 2013, including a subgroup analysis, which supported the use of concurrent chemotherapy particularly in ER-negative patients. The trends continued in the trial for more anthracycline and taxane-based chemotherapy to be used, for more concurrent rather than sequential treatment, and for more ER-positive patients to be included. Among the adjuvant patients the proportion who were node-negative increased from 53% at the start of the trial to 60% in the final year of recruitment, and tumour sizes decreased, with 46% having tumours of ≤ 2 cm at the start of the trial and this increasing to 58% in the final year of the study. The prognosis of patients entered into the study was good at the start and had improved further by the end of the study.
FIGURE 3.
Changing characteristics and treatments of patients randomised into PERSEPHONE: (a) stratification variables for all patients; and (b) prognostic variables for patients receiving adjuvant chemotherapy. CT, chemotherapy.
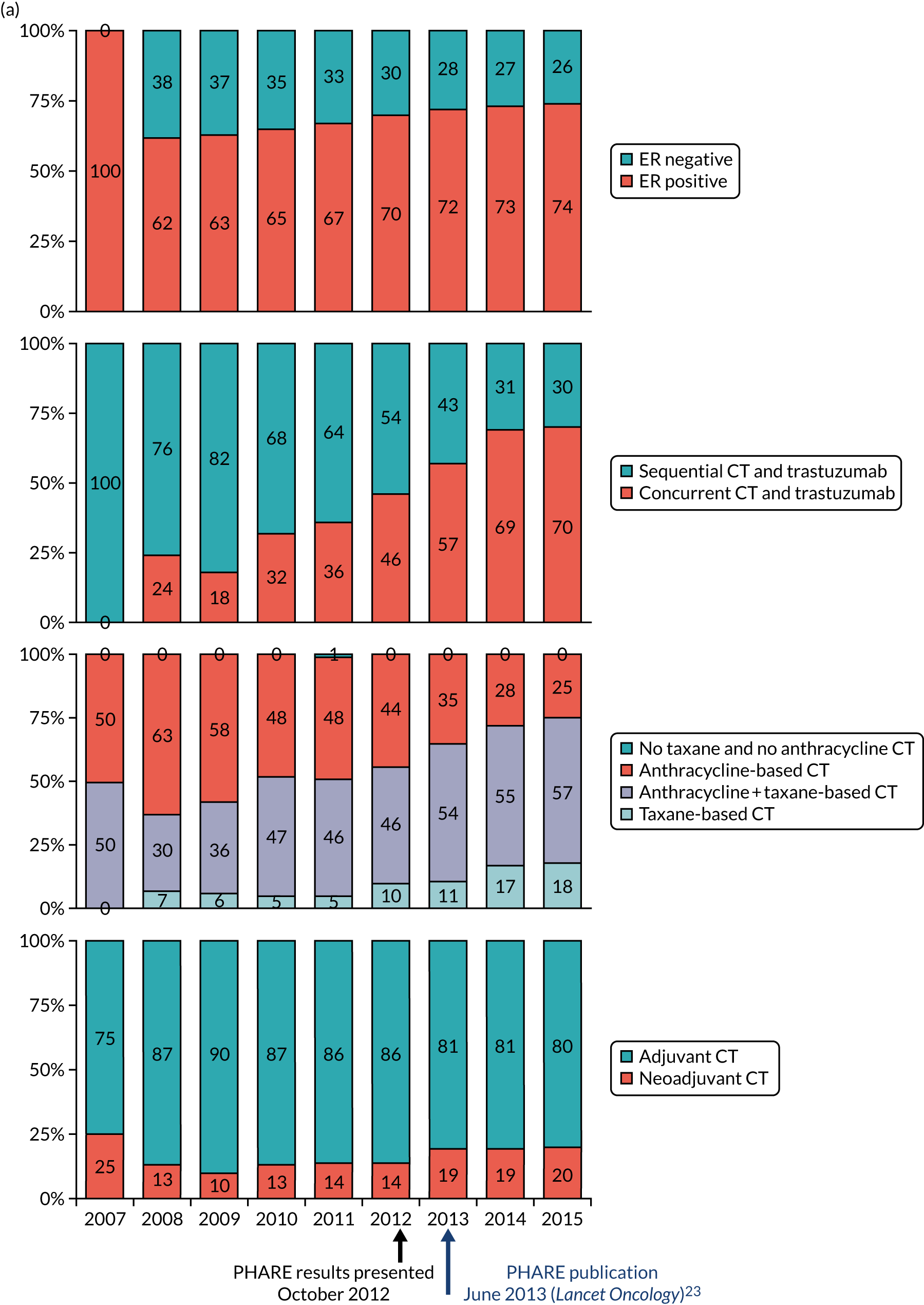
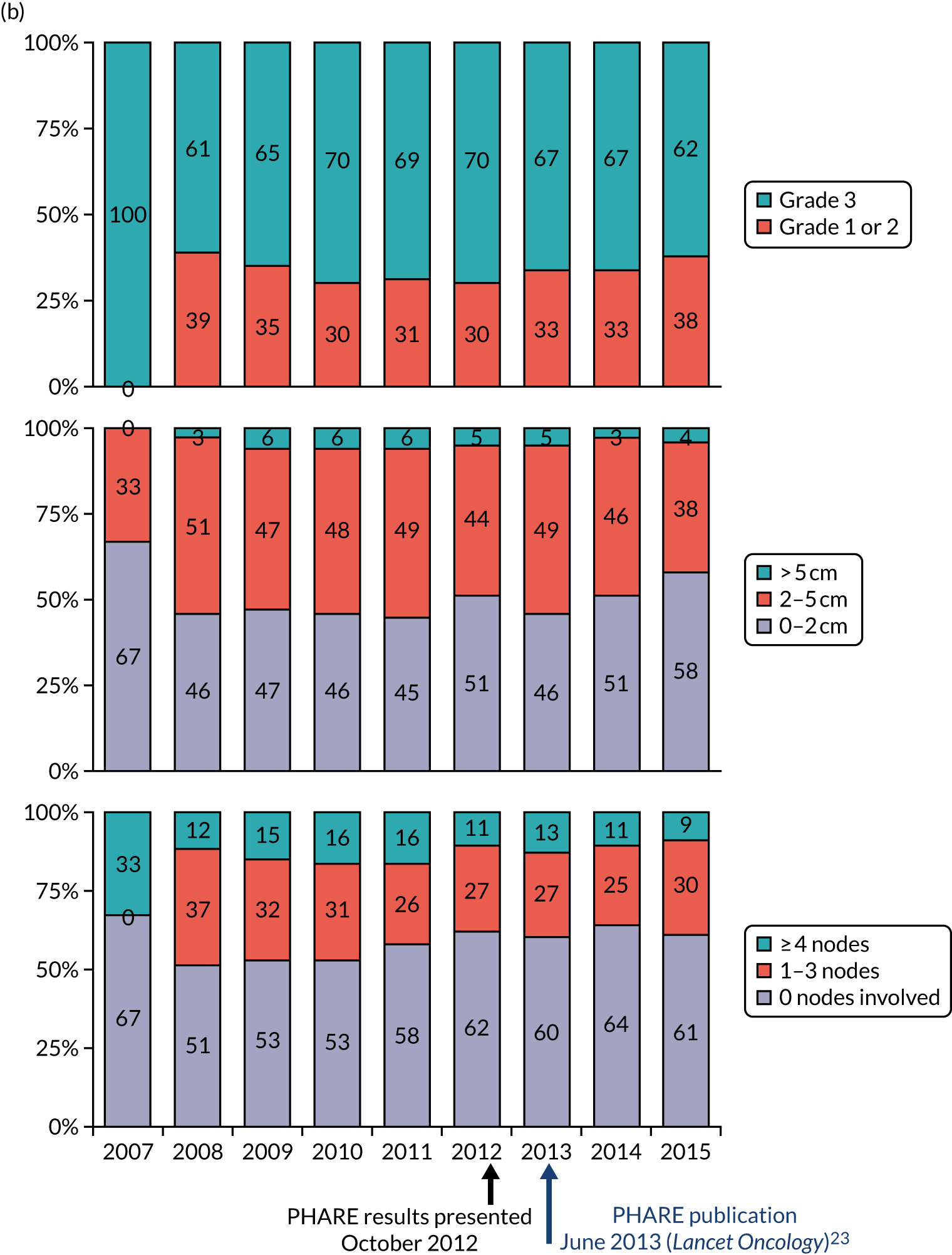
Timing of randomisation
On 11 September 2009, after the first 316 patients had been randomised, eligibility was changed to allow patients to be randomised after receiving up to nine cycles of trastuzumab. Table 6 shows the numbers of trastuzumab cycles that patients had received prior to being randomised into PERSEPHONE, and the numbers are well balanced between the two arms: 44% of 12-month patients and 43% of 6-month patients were randomised prior to any trastuzumab; 81% of 12-month patients and 80% of 6-month patients were randomised up to and including four cycles of trastuzumab; and 98% of patients in both arms were randomised up to and including eight cycles of trastuzumab.
| Trastuzumab cycles received | 12-month patients, n (%) | 6-month patients, n (%) | Total, N (%) |
|---|---|---|---|
| 0 | 898 (44) | 884 (43) | 1782 (44) |
| 1 | 295 (14) | 293 (14) | 588 (14) |
| 2 | 203 (10) | 205 (10) | 408 (10) |
| 3 | 172 (8) | 140 (7) | 312 (8) |
| 4 | 109 (5) | 121 (6) | 230 (6) |
| 5 | 112 (6) | 124 (6) | 236 (6) |
| 6 | 95 (5) | 92 (5) | 187 (4) |
| 7 | 63 (3) | 76 (4) | 139 (3) |
| 8 | 65 (3) | 60 (3) | 125 (3) |
| 9 | 33 (2) | 48 (2) | 81 (2) |
Chemotherapy type
At randomisation:
-
2188 (54%) patients reported receiving sequential chemotherapy and trastuzumab
-
1900 (46%) patients reported receiving concurrent chemotherapy and trastuzumab
-
3462 (85% of the 4088) patients reported receiving adjuvant chemotherapy
-
626 (15% of the 4088) patients reported receiving neoadjuvant chemotherapy.
The chemotherapy regimen received is known for 3968 (97% of the 4088) of PERSEPHONE patients: 1987 (97%) 12-month patients and 1981 (97%) 6-month patients (see Appendix 1, Table 32).
The most commonly given regimens were fluorouracil, epirubicin, cyclophosphamide and docetaxel (FEC-T) (39%), FEC (27%), EC (6%) and TC (5%), and these rates were similar across randomised treatment arms.
-
12-month trastuzumab patients most commonly received FEC-T (40%), FEC (27%), EC (6%) or TC (5%).
-
6-month trastuzumab patients most commonly received FEC-T (39%), FEC (28%), EC (5%) or TC (5%).
FEC-T was the most common regimen received by patients receiving chemotherapy concurrent with trastuzumab (64% of patients) and FEC was the most common regimen received by patients receiving chemotherapy sequential to trastuzumab (51%):
-
Concurrent chemotherapy and trastuzumab patients most commonly received FEC-T (64%), TC (9%) or epirubicin, cyclophosphamide and docetaxel (EC-T) (6%). Trastuzumab was not given concurrently with the anthracycline component of chemotherapy.
-
Sequential chemotherapy and trastuzumab patients most commonly received FEC (51%), FEC-T (18%) or EC (11%).
FEC-T and FEC were the most common regimens received by patients receiving adjuvant chemotherapy (36% and 31%, respectively) and FEC-T the most common regimen received by patients receiving neoadjuvant chemotherapy (61%):
-
Adjuvant chemotherapy patients most commonly received FEC-T (36%), FEC (31%) or EC (7%).
-
Neoadjuvant chemotherapy patients most commonly received FEC-T (61%), EC-T (17%) or AC-T (4%).
In total, 389 (10%) patients reported receiving fewer chemotherapy cycles than initially planned: 197 (10%) 12-month patients and 192 (9%) 6-month patients. The most common reasons provided for this were patient request (n = 92) and sepsis/infection (n = 78).
Of additional interest is the cross-tabulation of chemotherapy type against the timing of the treatments (Table 7).
| Timing | Chemotherapy type, n (%) | |||
|---|---|---|---|---|
| Anthracycline based | Taxane based | Anthracycline and taxane based | No taxane and no anthracycline | |
| Chemotherapy timing | ||||
| Adjuvant | 1642 (48) | 386 (11) | 1429 (41) | 5 (< 1) |
| Neoadjuvant | 54 (9) | 14 (2) | 558 (89) | 0 (0) |
| Trastuzumab timing | ||||
| Concurrent | 55 (3) | 342 (18) | 1503 (79) | 0 (0) |
| Sequential | 1641 (75) | 58 (3) | 484 (22) | 5 (< 1) |
Protocol deviations/non-compliance
To 16 April 2019, 303 protocol non-compliances were reported (see Appendix 1, Table 33). The PERSEPHONE trial treated patients in 152 sites and most protocol non-compliances can be explained by the fact that specific requirements of the trial were missed as a result of the standard practice at the site: the number of cycles, the extra labelling required by the regulatory governance or the 3-monthly LVEF measurement schedule.
Almost half of the non-compliances were linked to trastuzumab treatment. Patients did not always receive the number of cycles prescribed in the randomisation, nine or 18, and reloading doses of 8 mg/kg after breaks of over 28 days were sometimes missed, with patients receiving the standard 6 mg/kg dose instead.
Just over one-quarter of protocol non-compliances were related to Investigational Medicinal Product issues. Most commonly, patients were administered trastuzumab that was not labelled as per GCP, and this was explained by the fact that the patient had not been marked as randomised in the PERSEPHONE trial in clinic and/or in pharmacy. Patients also sometimes received non-Investigational Medicinal Product stock via Healthcare at Home.
Another common non-compliance was in the LVEF measurements, which commonly were incorrectly spaced, were missed or could not be interpreted. The trial required a LVEF measurement every 3 months up to 12 months, whereas standard practice changed in 2010/11, leading to some sites reducing the monitoring to 4-monthly. This change to standard practice was included in the protocol amendment in October 2013. On rare occasions, an abnormal LVEF score was overlooked and trastuzumab treatment continued instead of being interrupted.
Nineteen patients were found retrospectively not to be eligible; one of these was a patient who had been double randomised.
Six protocol non-compliances were considered major and were reported as serious breaches. All of these breaches were separate incidents and are detailed in Serious breaches.
Trastuzumab treatment delivery
Full trastuzumab treatment details are available for 3921 out of 4088 (96%) patients: 1942 out of 2045 (95%) 12-month patients and 1979 out of 2043 (97%) 6-month patients. Between them, 50,856 trastuzumab cycles were given: 32,611 to 12-month patients and 18,245 to 6-month patients. In total, 41,093 (81%) were administered intravenously and 9763 (19%) subcutaneously.
A total of 3365 out of 3921 (86%) patients received the protocol-specified number of trastuzumab cycles as per their randomised arm. Significantly more 6-month patients than 12-month patients received their randomised number of cycles [1764/1979 (89%) and 1601/1942 (82%), respectively; p < 0.0001] (Figure 4).
FIGURE 4.
Number of trastuzumab cycles received, split by randomised treatment arm: (a) 12-month patients; and (b) 6-month patients.
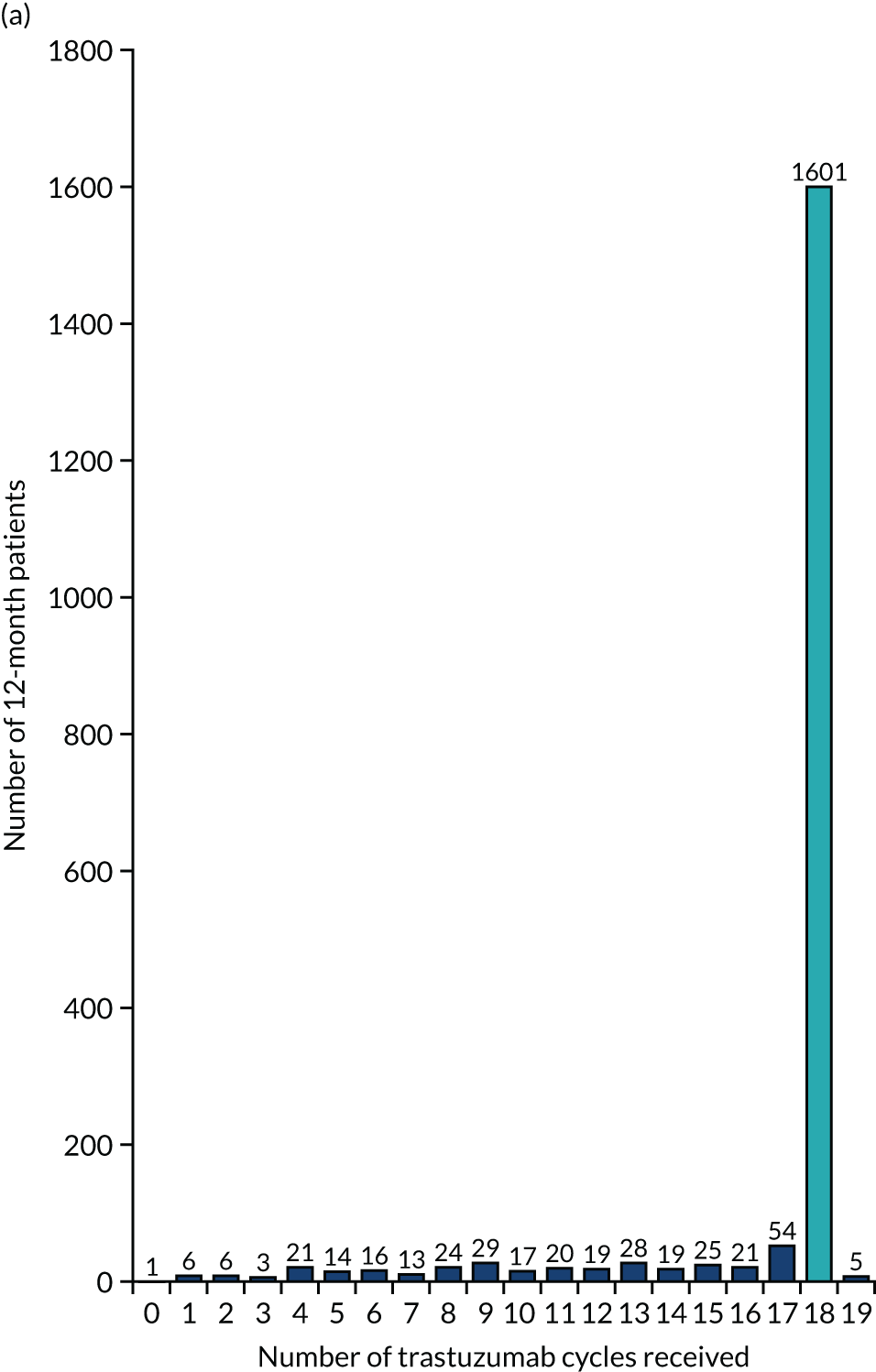
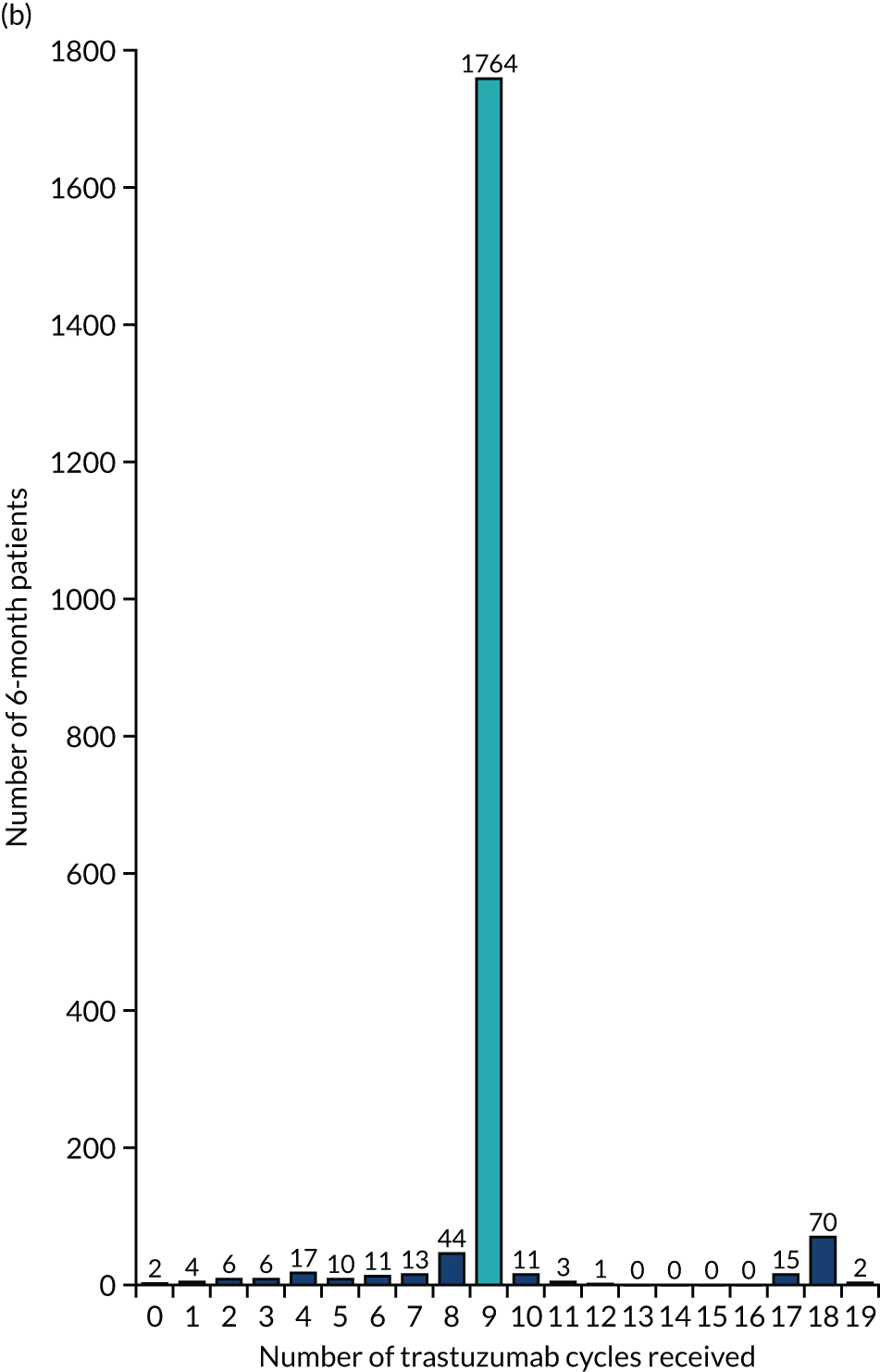
Among those randomised to the 12-month arm:
-
one received no cycles of trastuzumab owing to cardiac dysfunction identified 15 days after randomisation
-
132 (7%) received 1–9 cycles of trastuzumab
-
1809 (93%) received ≥ 10 cycles of trastuzumab
Among those randomised to the 6-month arm:
-
two received no cycles of trastuzumab, one because they were ineligible (HER2 negative) and one because they had a diagnosis of metastatic disease 20 days after randomisation
-
1875 (95%) received 1–9 cycles
-
102 (5%) received ≥ 10 cycles.
The reasons why 449 patients (12-month patients, n = 336; 6-month patients, n = 113) had fewer cycles than the number specified in the protocol for their randomised arm are shown in Figure 2.
The most common reason for receiving fewer than the protocol-specified number of cycles was cardiovascular toxicity [146/336 (43%) 12-month patients, 60/113 (53%) 6-month patients], followed by patient request [94 (28%) 12-month patients, 26 (23%) 6-month patients] and relapse/death [26 (8%) 12-month patients, 12 (11%) 6-month patients].
Length of time on treatment
The protocol-stated total duration of trastuzumab treatment is:
-
378 days for 12 months of trastuzumab (18 cycles each 21 days apart)
-
189 days for 6 months of trastuzumab (nine cycles each 21 days apart).
The median (IQR) time in days on treatment was:
-
383 days (378–392) for 12-month patients
-
190 days (189–197) for 6-month patients.
The good compliance with the protocol reflects the tolerability of each of the treatment arms.
Frequency and reasons for delays/holds of trastuzumab treatment
Of the 50,856 trastuzumab cycles received in total, 34,557 were administered during the first 6 months of treatment and 16,299 were administered during the second 6 months of treatment.
-
In the first 6 months of treatment, delays/holds were reported in 2685 (8%) cycles, by 1769 (45% of the 3918) patients:
-
1420 (8%) delays/holds reported by 920 (47%) 12-month patients
-
1265 (7%) delays/holds reported by 849 (43%) 6-month patients.
-
-
In the second 6 months of treatment, delays/holds were reported in 1203 (7%) cycles, by 802 (42% of the 1911) patients:
-
1132 (7%) delays/holds reported by 753 12-month patients (42% of the 1809 who had ≥ 10 cycles)
-
71 (6%) delays/holds reported by 49 6-month patients (48% of the 102 who had ≥ 10 cycles).
-
The 3968 reasons for the 3888 reported delays/holds throughout treatment are shown in Appendix 1, Table 34. Interestingly, delays/holds were much more likely to be a result of patients’ requests and logistic reasons than because of toxicity. Reasons recorded in the 12-month/6-month groups were, respectively, 23%/22% for patient holidays, 15%/15% for logistical and administrative reasons (rescheduling for bank holidays, change of clinic days, waiting list, poor intravenous access), 7%/5% by patient request for personal reasons and 5%/5% for booked surgery/radiotherapy/procedures (permanent line insertion). Only 5%/6% were due to cardiotoxicity from trastuzumab; 4%/6% were due to sepsis (probably chemotherapy related); 4%/3% were due to unrelated medical problems; and 2%/2% were due to awaiting cardiac function tests.
However, 7798 (15%) of the 50,856 trastuzumab cycles received were given prior to randomisation into PERSEPHONE. Of the 43,058 trastuzumab cycles received under the PERSEPHONE protocol by 3864 patients:
-
delays/holds were reported in 3396 (8%) cycles by 1915 (50%) patients –
-
2289 (8%) by 1170 (60%) 12-month patients
-
1107 (8%) by 745 (39%) 6-month patients.
-
Toxicities
We have full treatment dose data from 3921 randomised patients, totalling 50,856 trastuzumab cycles of information. However, toxicity data were requested only on cycles received after randomisation into the trial. Therefore, 43,058 (85%) of these trastuzumab cycles from 3864 patients have full toxicity information available: 28,856 cycles in 1935 12-month patients and 14,202 cycles in 1929 6-month patients. From these cycles, each patient’s worst reported CTCAE grade for each type of toxicity has been calculated (Table 8).
| Toxicity | CTCAE grade | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12-month patients, n (%) | 6-month patients, n (%) | |||||||||||
| UK | 0 | 1 | 2 | 3 | 4 | UK | 0 | 1 | 2 | 3 | 4 | |
| Chills | – | 1380 (71) | 353 (18) | 133 (7) | 59 (3) | 10 (1) | – | 1549 (80) | 251 (13) | 91 (5) | 31 (2) | 7 (< 1) |
| Cough | – | 1223 (63) | 486 (25) | 146 (8) | 65 (3) | 15 (1) | – | 1436 (74) | 361 (19) | 90 (5) | 37 (2) | 5 (< 1) |
| Diarrhoea | – | 1185 (61) | 497 (26) | 194 (10) | 47 (2) | 12 (1) | – | 1365 (71) | 398 (21) | 117 (6) | 39 (2) | 10 (< 1) |
| Dizziness | – | 1397 (72) | 386 (20) | 121 (6) | 27 (2) | 4 (< 1) | – | 1566 (81) | 281 (15) | 54 (3) | 24 (1) | 4 (< 1) |
| Dyspnoea | – | 1260 (65) | 459 (24) | 157 (8) | 59 (3) | – | 1431 (74) | 365 (19) | 89 (5) | 44 (2) | ||
| Fatigue | 1 (< 1) | 557 (29) | 643 (33) | 511 (26) | 184 (10) | 39 (2) | – | 720 (37) | 642 (33) | 402 (21) | 139 (7) | 26 (2) |
| Fever | – | 1595 (83) | 240 (12) | 72 (4) | 25 (1) | 3 (< 1) | – | 1700 (88) | 147 (8) | 56 (3) | 23 (1) | 3 (< 1) |
| Headache | – | 1207 (62) | 467 (24) | 193 (10) | 61 (3) | 7 (1) | – | 1357 (70) | 403 (21) | 122 (6) | 42 (2) | 5 (1) |
| Hypertension | – | 1750 (90) | 127 (7) | 46 (2) | 10 (1) | 2 (< 1) | – | 1807 (94) | 88 (5) | 28 (1) | 5 (< 1) | 1 (< 1) |
| Hypotension | – | 1881 (97) | 49 (3) | 4 (< 1) | 1 (< 1) | – | – | 1885 (98) | 33 (2) | 8 (< 1) | 3 (< 1) | – |
| Infection | – | 1463 (76) | 190 (10) | 238 (12) | 41 (2) | 3 (< 1) | – | 1606 (83) | 121 (7) | 157 (8) | 41 (2) | 4 (< 1) |
| Muscle/joint pain | 1 (< 1) | 720 (37) | 545 (28) | 450 (24) | 175 (9) | 44 (2) | – | 948 (49) | 480 (25) | 331 (17) | 137 (7) | 33 (2) |
| Nausea | – | 1333 (69) | 448 (23) | 122 (6) | 32 (2) | – | 1503 (78) | 322 (17) | 84 (4) | 20 (1) | ||
| Pain | – | 1123 (58) | 427 (22) | 285 (15) | 86 (4) | 14 (1) | – | 1394 (72) | 296 (15) | 180 (9) | 48 (3) | 11 (1) |
| Palpitations | – | 1540 (79) | 303 (16) | 92 (5) | – | 1661 (86) | 214 (11) | 54 (3) | ||||
| Rash | – | 1512 (78) | 299 (16) | 102 (5) | 21 (1) | 1 (< 1) | – | 1609 (84) | 235 (12) | 63 (3) | 17 (1) | 5 (< 1) |
| Vomiting | – | 1672 (86) | 163 (8) | 86 (5) | 10 (1) | 4 (< 1) | – | 1765 (92) | 117 (6) | 36 (2) | 9 (< 1) | 2 (< 1) |
Categorising patients into whether they had ever reported a toxicity of severe grade (CTCAE ≥ 3, or 2 for palpitations) during the 12-month period from starting trastuzumab, a higher proportion of 12-month patients than 6-month patients reported at least one adverse event of severe grade [460/1935 (24%) vs. 365/1929 (19%) respectively; p = 0.0003]. There was a significant excess of some toxicities in the 12-month patients compared with the 6-month patients. In order of frequency, these were fatigue (11.5% in 12-month patients vs. 8.6% in 6-month patients; p = 0.003), muscle/joint pains (11.3% vs. 8.8%; p = 0.01), pain (5.2% vs. 3.1%; p = 0.001), palpitations (4.8% vs. 2.8%; p = 0.002), cough (4.1% vs. 2.2%; p = 0.0007) and chills (3.6% vs. 2.0%; p = 0.003) (Figure 5).
FIGURE 5.
Patients reporting an adverse event of severe grade (CTCAE ≥ 3, or 2 for palpitations) (all patients).
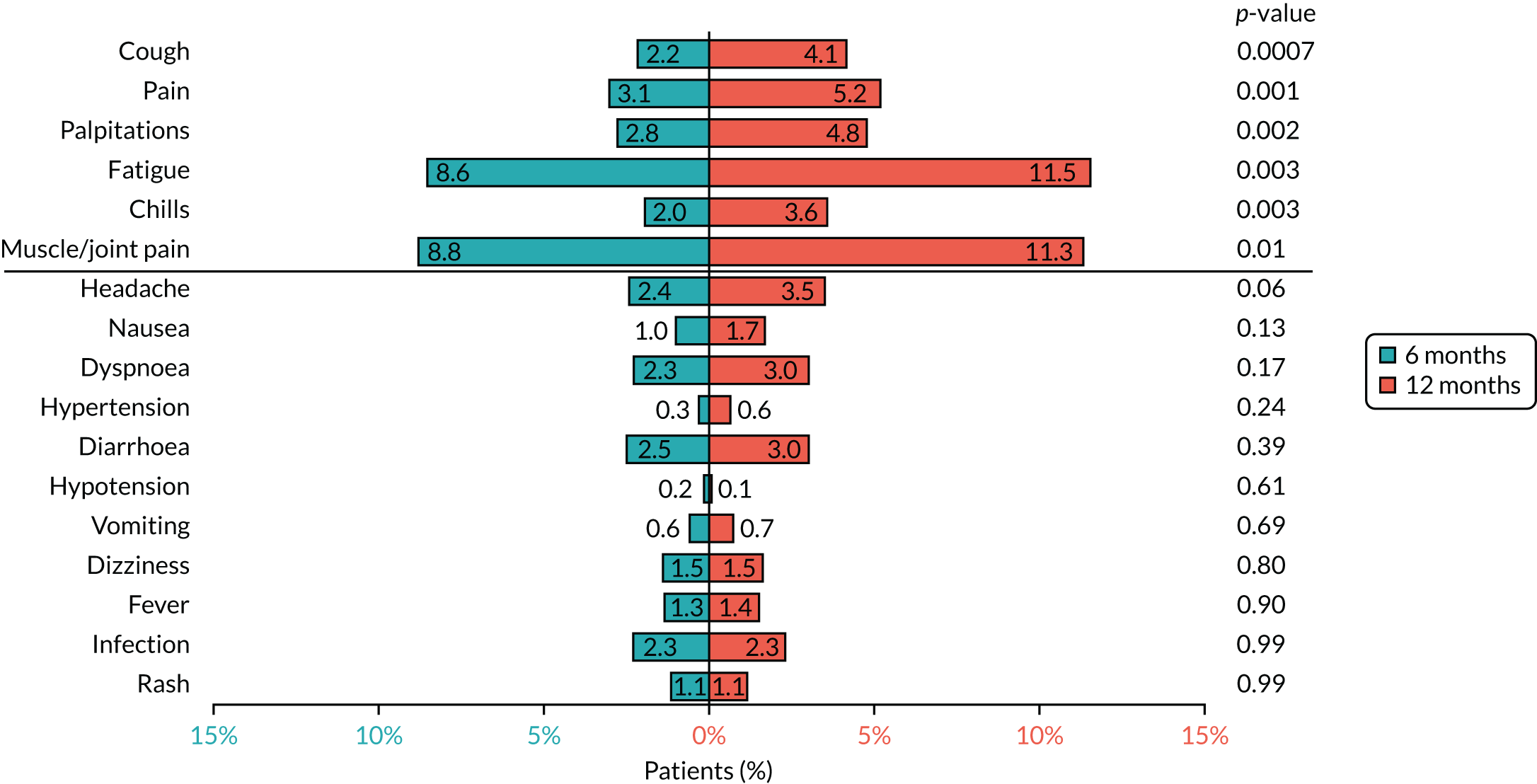
To ensure that the toxicities reported were unlikely to be due to concurrent chemotherapy, analysis was also undertaken on only the 54% of patients who received trastuzumab sequentially after chemotherapy. Similar toxicity profiles to those of all patients were observed (see Appendix 2, Figure 24). In order of frequency, these toxicities were fatigue (12.0% in 12-month patients vs. 8.0% in 6-month patients; p = 0.003), muscle/joint pains (11.2% vs. 9.5%; p = 0.23), pain (6.1% vs. 2.9%; p = 0.0004), palpitations (5.3% vs. 3.3%; p = 0.04), cough (4.3% vs. 2.0%; p = 0.004) and chills (4.5% vs. 2.3%; p = 0.007).
To assess the frequency of toxicity throughout treatment, analysis was also conducted that was restricted to patients randomised upfront before receiving any of their trastuzumab treatment, for whom full toxicity reporting throughout the entire trastuzumab treatment is expected. There are 1727 patients (12-month patients, n = 865; 6-month patients, n = 862) who were randomised prior to receiving any trastuzumab treatment for whom we have full treatment details. These patients received a total of 22,426 trastuzumab cycles (14,458 by 12-month patients and 7968 by 6-month patients).
Frequencies of reported CTCAE toxicity grades were analysed across two 6-month windows. There are toxicity details on:
-
15,179 cycles in the first 6-month treatment period, by 1727 patients –
-
7603 cycles by 865 12-month patients
-
7576 cycles by 862 6-month patients
-
-
7247 cycles in the second 6-month treatment period, by 848 patients –
-
6855 cycles by 800 12-month patients
-
392 cycles by 48 6-month patients.
-
During the first 6 months, when both randomised arms were receiving treatment, as expected, similar rates of each grade of each toxicity were reported across randomised treatment arms (see Appendix 1, Table 35). If we look at 12-month patients’ reporting of toxicities in the first 6 months and the second 6 months, the same three toxicities are shown to be reported at a severe grade (CTCAE 3 and 4, and 2 for palpitations) with a frequency of > 1% of cycles; fatigue in 2.8% followed by 2.8% of cycles in each of the 6-month periods, muscle/joint pain in 2.7% and 2.5% of cycles, and palpitations in 1.2% and 1.1% of cycles. Therefore, among 12-month patients, the frequencies of toxicities during the second 6 months of treatment did not alter substantially from the first 6 months, suggesting that no cumulative toxicity occurred. The frequencies of toxicities during the second 6 months of treatment for 6-month patients were not investigated owing to the small number of patients (n = 48).
Safety
First 100 patients receiving concomitant trastuzumab and chemotherapy
The first 100 patients in PERSEPHONE who received concomitant chemotherapy and trastuzumab provided data ‘in real time’ on SAEs and treatment delays. These data were collected and analysed by the PERSEPHONE trials office and were reported to and discussed by the Independent Data and Safety Monitoring Committee on 15 December 2010. These were confirmed to be within the acceptable safety limits expected for concomitant trastuzumab and chemotherapy.
Serious adverse events
During the trial, 475 SAEs were reported by 384 patients:
-
291 by 227 12-month patients
-
184 by 157 6-month patients.
The most common reason for reporting a SAE was inpatient hospitalisation or prolongation of hospital stay (83%) (see Appendix 1, Table 36). There were nine patients for whom death was either the reason for reporting or the outcome of the SAE, and none of these was judged related to trastuzumab. One sudden death occurred 6 months after completion of trastuzumab, and autopsy revealed a pulmonary embolus with no evidence of metastatic disease. Eight deaths occurred during trastuzumab treatment and causes were as follows: one pulmonary embolus on chemotherapy; one pneumonia with metastatic liver and mediastinal disease; one subarachnoid haemorrhage; two pneumonitis and pulmonary infection related to taxane; one suicide by drug overdose; one death on chemotherapy (autopsy report stated that no cause found); and one patient second primary diagnosis glioblastoma multiforme.
In the 12-month arm the five most common SAEs reported were infection (118, 41% of SAEs reported), cardiac (38, 13%), pain (19, 7%), gastrointestinal (10, 3%) and neurology (14, 5%). In the 6-month arm, the five most common SAEs reported were infection (80, 43%), cardiac (15, 8%), gastrointestinal (12, 7%), pain (11, 6%) and neurology or pulmonary/upper respiratory (both 8, 4%) (see Appendix 1, Table 36). Only 103 (22%) of the SAEs were SARs (see Serious adverse reactions), as the cause of many SAEs was chemotherapy. The severity of the reported SAEs illustrate that only the minority were mild (19% of 12- and 6-month patients), and 5% of SAEs in the 12-month arm and 6% in the 6-month arm were fatal or life-threatening.
Serious adverse reactions
A total of 103 serious adverse reactions (SAEs that were deemed by the PERSEPHONE CI as having a causality either possibly, probably or definitely related to trastuzumab) were reported during the trial. Twice as many 12-month as 6-month patients reported a serious adverse reaction:
-
67 by 63 12-month patients
-
36 by 31 6-month patients.
The primary event categories reported for the 103 SARs are shown in Appendix 1, Table 37. There were no deaths related to trastuzumab. The five most common reasons for reporting a SAR were cardiac [30 (45%) in the 12-month arm, 15 (42%) in the 6-month arm], infection [12 (18%) and 9 (25%), respectively], pulmonary/upper respiratory [8 (12%) and 1 (3%), respectively], cardiac arrhythmia [5 (7%) and 3 (8%), respectively] and allergy/immunology [5 (7%) and 2 (6%), respectively], and these were all more common in 12-month patients.
Suspected unexpected serious adverse reactions
In total, since the trial opened, five SUSARs were reported among the 4088 patients recruited, although two were subsequently downgraded to a SAE:
-
TNO (trial number) 209 – 39-year-old patient had a miscarriage. The patient became pregnant approximately during cycle 14–15 of trastuzumab and subsequently had a miscarriage around her 10th week of pregnancy. The miscarriage was reviewed as possibly related to trastuzumab.
-
TNO 254 – 61-year-old patient presented with idiopathic pulmonary hypertension. The patient was still receiving treatment for this condition in 2018. The event was reviewed as possibly related to trastuzumab.
-
TNO 662 – 61-year-old patient died suddenly at home following cycle 2 of trastuzumab/docetaxel and cyclophosphamide. The postmortem (macroscopic) showed no obvious cause of death. Pneumonia, pulmonary embolism and myocardial infarction were excluded. The toxicology analyses showed no drugs detected in the blood. The death was classified as unlikely to be related to trastuzumab and the event was downgraded to a SAE.
-
TNO 1506 – 48-year-old patient was hospitalised with blistering of mouth and fever 10 days following the fifth cycle of trastuzumab. The event was reviewed as probably related to trastuzumab.
-
TNO 3657 – mild visual symptoms not interfering with function. The event was classified as unlikely to be related to trastuzumab and the event was downgraded to a SAE.
Serious breaches
Six serious breaches were reported to the authorities during the trial. Three were directly linked to clinical incidents and the three others were related to misconduct by teams involved in participation or co-ordination. All breaches were escalated to the sponsor and subsequently reported to the MHRA and the REC. Each breach was investigated and followed up with a corrective and preventative action plan.
-
Patient TNO 858 was administered two cycles of trastuzumab despite having a LVEF measurement of 35%. The patient was admitted to hospital with chest pains. The error was picked up by the trial office 10 months later only after receiving a SAE form for the hospital admission. This error occurred as the principal investigator at the site had not been aware of the LVEF result until two further trastuzumab cycles had been administered, at which point treatment was held. Corrective and preventative actions were discussed with the site and a reminder was sent to all sites regarding LVEF measurements and trastuzumab treatment.
-
Owing to poor weather conditions, patient TNO 1970’s trastuzumab cycle was delayed by 4 days. This dose was manufactured by the company Healthcare at Home on the day of intended administration and had an expiry date of 24 hours later. During administration 4 days later, a research nurse noticed the expiry date and stopped treatment, by which point around 30–40 mg had been given to the patient. The PERSEPHONE trial team was alerted to this mistake only 15 days later. The principal investigator at the site reported no ill effects of this administration. Corrective and preventative actions were discussed with the company, and also with the site, with regard to communication.
-
Patient TNO 2968 had been confused with another patient taking part in another trial. The PERSEPHONE patient had potentially been administered pertuzumab/placebo. The site underwent rigorous monitoring by the PERSEPHONE team and corrective and preventative actions were discussed with staff at the site.
-
An audit review at a participating site uncovered a number of issues related to patient consent. Six patients randomised to the trial were consented by a research nurse who had not been authorised to undertake this responsibility as per protocol. Five additional patient consent forms did not adhere to GCP guidelines (i.e. the dates had been changed and the consent forms had been posted to the patient’s address to be signed). Some issues were also found with co-investigators who had taken consent from patients but were not GCP trained or authorised to take consent as per the site signature and delegation log. Following the event, the site received a monitoring visit to ensure that the corrective and preventative actions were implemented.
-
During the development safety update report 2016 development, the sponsor identified that several updates to the trastuzumab SmPC had been made during the trial. Although important treatment alert letters were sent to all clinical investigators at the time of the changes, these were not amended in the PERSEPHONE protocol and patient information sheet. Corrective and preventative actions were discussed between the sponsor and the trial office. By this time the trial had completed recruitment (completed July 2015), and after discussion it was not felt that an amended patient information sheet would be helpful. All sites who had remaining patients in the trial were contacted with reminders that all patients who were pre-menopausal and could become pregnant were required to take effective contraceptive precautions during trastuzumab treatment and up to and including 7 months after completion. In addition, all sites were asked to identify any other patients who had become pregnant.
-
After her tissue was re-tested during a routine audit of the hospital laboratory, patient TNO 4077 was found to be HER2 negative. The patient was withdrawn from the trial at this point and followed up as per local practice. A protocol non-compliance was not generated after notification of the event and the sponsor was not informed as it was felt that the initial laboratory error occurred outside the trial protocol. The sponsor was made aware of the event when the patient sought legal action against the hospital. By that time, the hospital had already conducted a root cause analysis, and corrective and preventative actions had been taken.
During the trial, several updates were made to the trastuzumab SmPC, but these were not implemented in the PERSEPHONE protocol and patient information sheet. Specifically, the two following major patient safety updates were made:
-
continued use of contraception for 6 months post treatment – SmPC updated August 2010 (up to 7 months in 2016)
-
monitoring of long-term cardiac effects of trastuzumab toxicity – SmPC updated May 2015.
Although the PERSEPHONE trial protocol and patient information sheet were not amended to include these issues, the team was aware of the changes and sent out important treatment alert letters to all clinical investigators at the time of the changes in both 2010 and 2015.
Follow-up and events
Deaths
At database lock on 6 March 2019, 389 deaths (10% of the 4088 patients) had been reported [182 (9%) 12-month patients and 207 (10%) 6-month patients]. Median follow-up of the 3699 alive patients was 6.1 years (IQR 4.5–7.6 years), with 97% of alive patients followed up for at least 2 years.
The reported causes of death (multiple for some patients) illustrate that 77% of deaths were from breast cancer (Table 9). Among the 88 patients who died without breast cancer reported as a cause, two had reported a local relapse and eight had reported a distant relapse.
| Cause(s) of death | 12-month patients, n (%) | 6-month patients, n (%) | Total, N (%) |
|---|---|---|---|
| Breast cancer | 143 (79) | 158 (76) | 301 (77) |
| Other cancer | 13 (7) | 23 (11) | 36 (9) |
| Ovarian | 2 | 5 | 7 |
| Lung | 3 | 3 | 6 |
| Colorectal | 1 | 3 | 4 |
| Breast | 2 | 1 | 3 |
| Stomach | 1 | 1 | 2 |
| Bladder | 1 | – | 1 |
| Renal | 1 | – | 1 |
| Melanoma | 1 | – | 1 |
| Pancreatic | 1 | – | 1 |
| Angiosarcoma | – | 1 | 1 |
| Cholangiocarcinoma | – | 1 | 1 |
| Glioblastoma | – | 1 | 1 |
| Myeloma | – | 1 | 1 |
| Oesophageal | – | 1 | 1 |
| Mesothelioma | – | 1 | 1 |
| Brain | – | 1 | 1 |
| Squamous cell carcinoma/bladder/lung/pleura | – | 1 | 1 |
| Uterus | – | 1 | 1 |
| Lymphoma | – | 1 | 1 |
| Protocol treatment related | 0 (0) | 0 (0) | 0 (0) |
| Other treatment related | 0 (0) | 3 (1) | 3 (1) |
| Neutropenic enterocolitis and neutropenia | – | 1 | 1 |
| Haemolytic transfusion reaction | – | 1 | 1 |
| Docetaxel – drug-related lung injury | – | 1 | 1 |
| Other causes | 27 (15) | 22 (11) | 49 (13) |
| CVDa | 8 | 10 | 18 |
| Infection | 6 | 5 | 11 |
| Liver failure | 2 | 2 | 4 |
| Renal failure | – | 1 | 1 |
| Other | 11 | 4 | 15 |
| Unknown | 3 (2) | 7 (3) | 10 (3) |
Relapses and second primaries
In total, 482 (12%) patients reported a relapse (12-month patients, n = 229; 6-month patients, n = 253) (Table 10). One hundred and sixty-four (4%) patients reported a local relapse [12-month patients, n = 81 (4%); 6-month patients, n = 83 (4%)]. This was most commonly reported in the ipsilateral breast/chest wall (n = 92), ipsilateral axillary nodes (n = 28) and ipsilateral supraclavicular nodes (n = 19) (see Appendix 1, Table 38). Three hundred and ninety-nine (10%) patients reported a distant relapse [12-month patients, n = 192 (9%); 6-month patients, n = 207 (10%)]. This occurred most commonly in the liver [86/192 patients (45%) in the 12-month arm and 91/207 patients (44%) in the 6-month arm], bone [63 (33%) in the 12-month arm and 89 (43%) in the 6-month arm], lung/pleura [74 (39%) in the 12-month arm and 78 (38%) in the 6-month arm] and brain [40 (21%) in the 12-month arm and 43 (21%) in the 6-month arm]. One hundred and thirty-four (3%) patients reported a second primary [12-month patients, n = 67 (3%); 6-month patients, n = 67 (3%)]. This was most commonly reported in the contralateral breast [n = 36 (27%): 12-month patients, n = 23 (34%); 6-month patients, n = 13 (19%)], lung [n = 17 (13%): 12-month patients, n = 9 (13.5%); 6-month patients, n = 8 (12%)] and bowel/colon [n = 15 (11%): 12-month patients, n = 4 (6%); 6-month patients, n = 11 (16.5%)].
| Number of patients with an event reported | 12-month patients (n = 2045) | 6-month patients (n = 2043) | Total (N = 4088) |
|---|---|---|---|
| Death | 182 (9%) | 207 (10%) | 389 (10%) |
| Breast cancer listed as a cause | 143 (79%) | 158 (76%) | 301 (77%) |
| Breast cancer not listed as a cause, but a local or distant relapse reported | 3 (1%) | 7 (4%) | 10 (3%) |
| Breast cancer not listed as a cause and no local/distant relapse reported | 36 (20%) | 42 (20%) | 78 (20%) |
| Relapse | 229 (11%) | 253 (12%) | 482 (12%) |
| Local relapse only | 37 (2%) | 46 (2%) | 83 (2%) |
| Distant relapse only | 148 (7%) | 170 (8%) | 318 (8%) |
| Local and distant relapse | 44 (2%) | 37 (2%) | 81 (2%) |
| Relapse or death | 270 (13%) | 296 (14%) | 566 (14%) |
| Second primary | 67 (3%) | 67 (3%) | 134 (3%) |
Survival outcomes
Disease-free survival
At a median follow-up of 6.1 years, 566 (14%) patients either had experienced a relapse or had died. The 4-year DFS rate was 90.3% (95% CI 88.9% to 91.5%) in the 12-month group and 89.5% (95% CI 88.1% to 90.8%) in the 6-month group (Figure 6). Thus, with the non-inferiority margin of 3%, the non-inferiority limit for the HR was set at 1.33. The HR for relapse or death with 6 months’ compared with 12 months’ trastuzumab was 1.10 (90% CI 0.96 to 1.26). This outcome met the prespecified definition of non-inferiority (non-inferiority p = 0.01). The two-sided p-value for the difference between treatments was 0.26. Adjustment for all stratification factors gave the same results, with a HR of 1.10 (90% CI 0.96 to 1.26; non-inferiority p = 0.01).
FIGURE 6.
Disease-free survival according to randomised group: (a) Kaplan–Meier plot; and (b) HR plot.
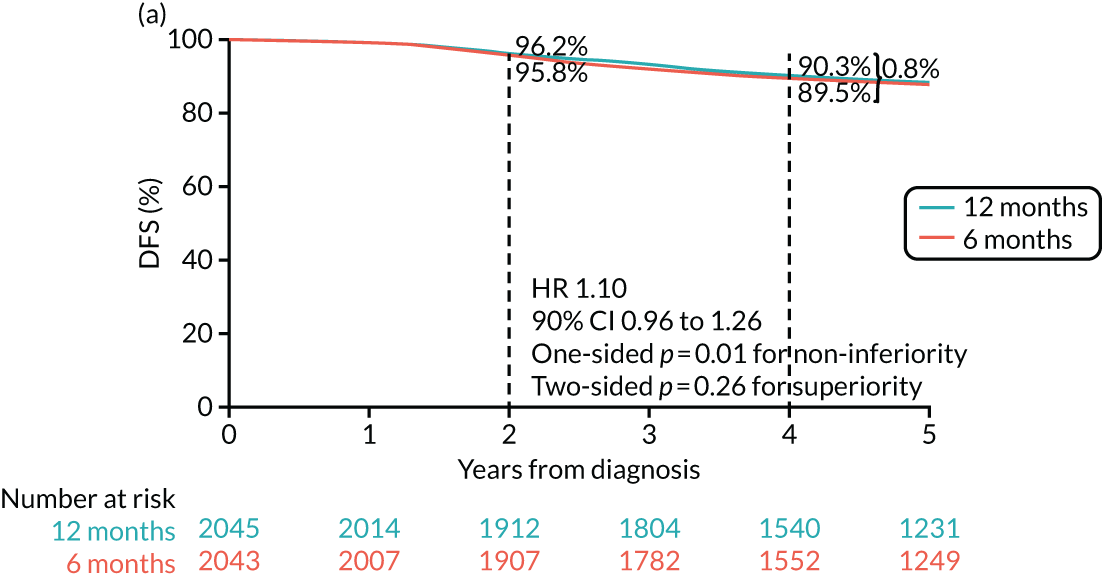
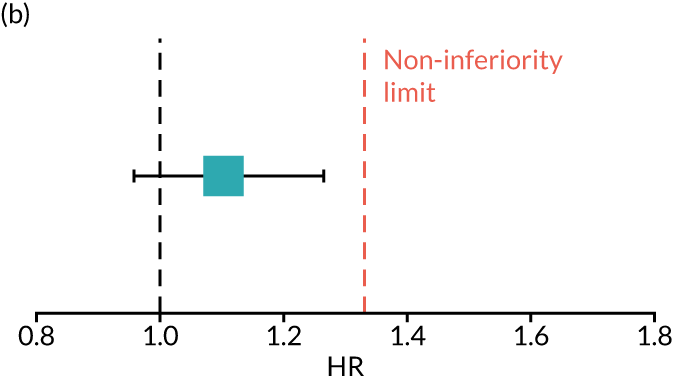
With a difference of 0.008 in absolute risk of a DFS event at 4 years between patients randomised to 6 months and those randomised to 12 months of trastuzumab, the number needed to treat is 125 for the overall population. That is, on average, 125 patients would need to receive 12 months’ trastuzumab for one of them to benefit in terms of a DFS event, compared with the same 125 patients receiving only 6 months of trastuzumab.
Overall survival
Analysis of the secondary end point of OS also met the prespecified definition of non-inferiority. The 4-year OS rate was 94.9% (95% CI 93.9% to 95.8%) in the 12-month group and 94.2% (95% CI 93.0% to 95.1%) in the 6-month group (Figure 7). With the same non-inferiority margin of 3%, the non-inferiority limit for the HR was set at 1.61. The HR for death with 6 months’ trastuzumab compared with 12 months’ trastuzumab was 1.14 (90% CI 0.96 to 1.34), meeting the prespecified definition of non-inferiority (non-inferiority p = 0.0003). The two-sided p-value for the difference between treatments was 0.21, and adjusting for stratification factors gave similar results (HR 1.13, 90% CI 0.95 to 1.33; non-inferiority p = 0.0002).
FIGURE 7.
Overall survival according to randomised group: (a) Kaplan–Meier plot; and (b) HR plot.
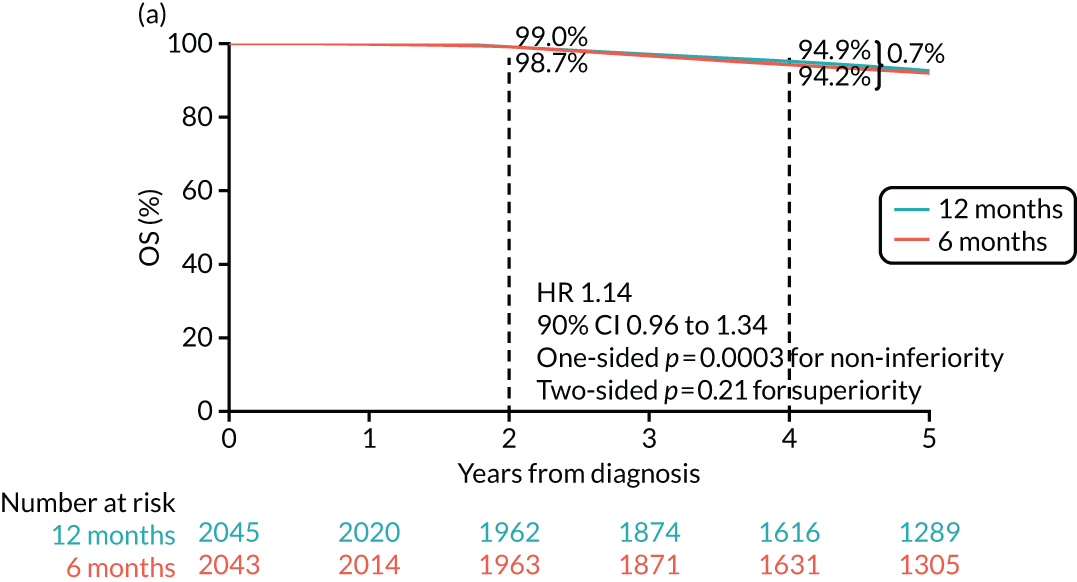
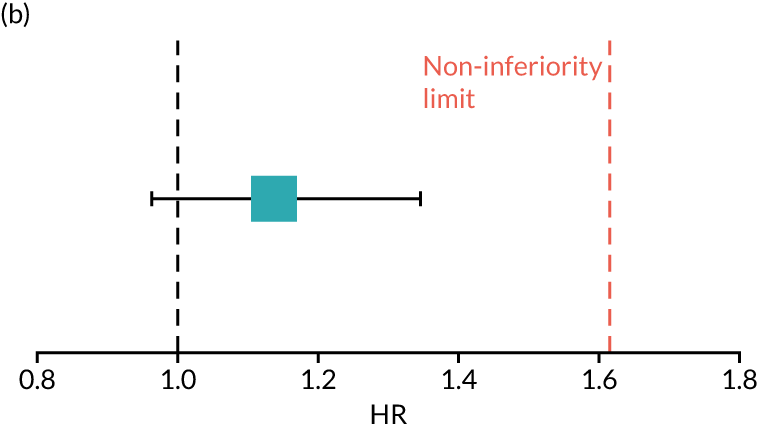
With the difference of 0.007 in absolute risk of an OS event at 4 years between patients randomised to 6 months and those randomised to 12 months of trastuzumab, the number needed to treat is 143 for the overall population. That is, on average, 143 patients would need to receive 12 months’ trastuzumab for one of them to benefit in terms of an OS event, compared with the same 143 patients receiving only 6 months’ trastuzumab.
Landmark analysis of disease-free survival and overall survival
The exploratory landmark analysis, including only patients who were alive and disease free at least 6 months after the start of their trastuzumab treatment, was undertaken on 4008 patients (12-month patients, n = 2007; 6-month patients, n = 2001). The cohort was balanced across randomised arms in terms of demographics and baseline disease characteristics (see Appendix 1, Table 39) and, with a median follow-up of 5.1 years, 365 (9%) deaths and 537 (13%) DFS events have been recorded. DFS and OS were recalculated from the landmark time point (6 months after each patient’s start of trastuzumab treatment).
The landmark 4-year DFS rate was 88.7% (95% CI 87.2% to 90.1%) in the 12-month group and 88.4% (95% CI 86.8% to 89.7%) in the 6-month group, and hence the non-inferiority limit for the HR was set at 1.29 (Figure 8). The calculated HR for relapse or death with 6 months’ trastuzumab compared with 12 months’ trastuzumab was 1.09 (90% CI 0.95 to 1.26), meeting the prespecified definition of non-inferiority (non-inferiority p = 0.03). The two-sided p-value for difference between treatments was 0.30.
FIGURE 8.
Landmark DFS according to randomised group: (a) Kaplan–Meier plot; and (b) HR plot.
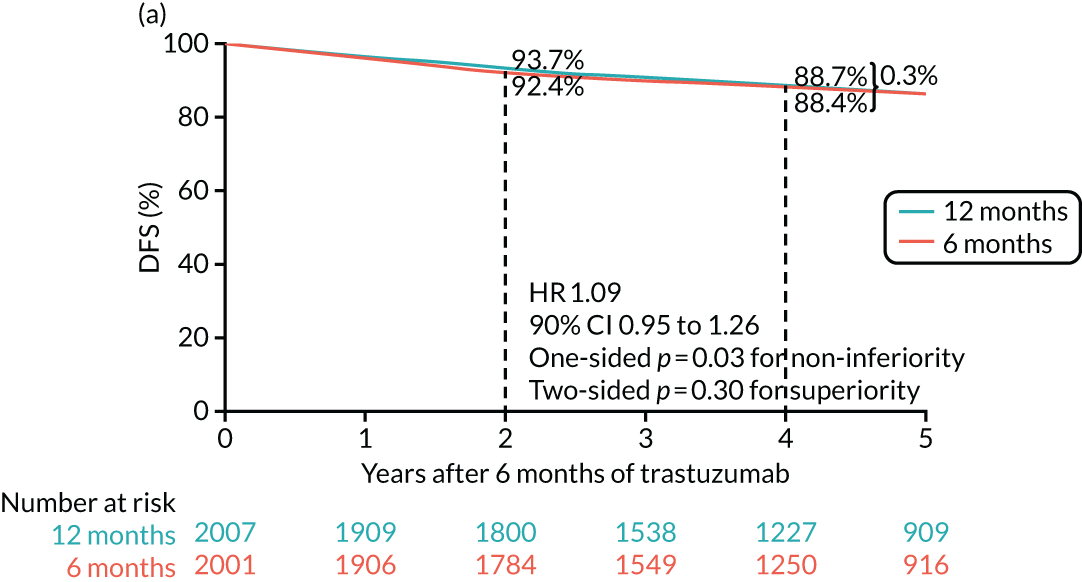
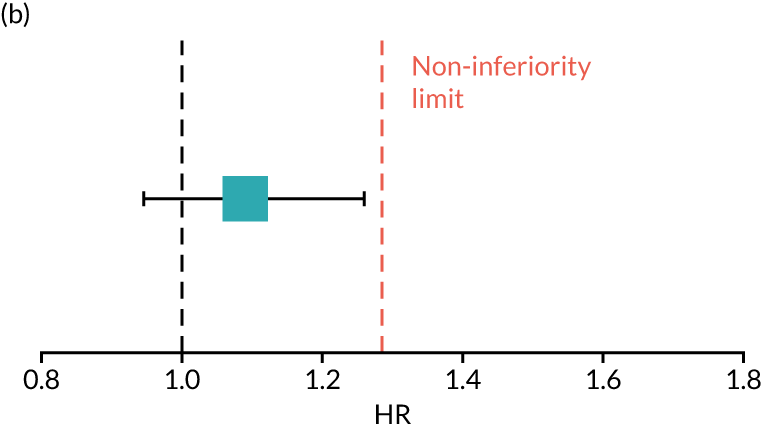
The landmark 4-year OS rate was 93.2% (95% CI 91.9% to 94.3%) in the 12-month group and 92.6% (95% CI 91.3% to 93.7%) in the 6-month group, and hence the non-inferiority limit for the HR was set to 1.46 (Figure 9). The calculated HR for death with 6 months’ trastuzumab compared with 12 months’ trastuzumab was 1.12 (90% CI 0.95 to 1.34), meeting the prespecified definition of non-inferiority (non-inferiority p = 0.006). The two-sided p-value for difference between treatments was 0.27.
FIGURE 9.
Landmark OS according to randomised group: (a) Kaplan–Meier plot; and (b) HR plot.
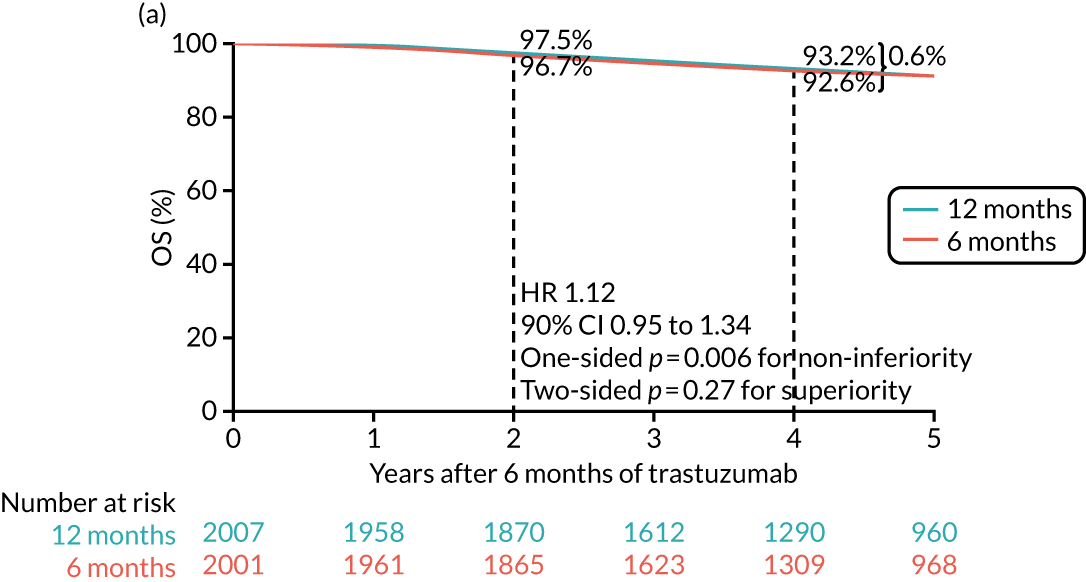
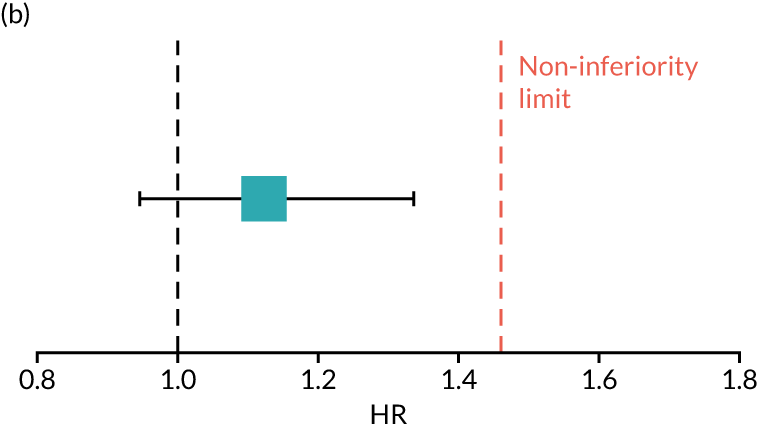
Additional analyses
To ensure the robustness of findings from the PERSEPHONE trial, and to facilitate comparisons across various other studies, additional analyses of invasive DFS [including invasive contralateral breast cancers and second primary invasive cancers (non-breast) according to the STEEP (standardised definitions for efficacy end points in adjuvant breast cancer trials) system], distant DFS, and breast cancer-specific survival were undertaken. Survival curves of these end points were comparable with those of the protocol-specified primary and secondary end points (see Appendix 2, Figures 25–27).
Forest plots
All patients
Forest plots for DFS including all patients (Figure 10) showed heterogeneity in the treatment effect of chemotherapy type (p = 0.01), predominantly driven by the small number of events in the taxane-only group, in which 56% of patients received docetaxel with cyclophosphamide. The timing of trastuzumab relative to chemotherapy (concurrent or sequential) showed heterogeneity in the treatment effect (p < 0.001) favouring 12-month patients receiving concurrent trastuzumab. In terms of DFS, no heterogeneity in the treatment effect was observed for ER status, chemotherapy timing (neoadjuvant or adjuvant), age, grade, menopausal status or IHC status (IHC3+ or IHC2+ and FISH positive). In terms of OS, similar results were found with heterogeneity for concurrent/sequential chemotherapy and trastuzumab, although the heterogeneity in treatment effect was not observed for chemotherapy type (p = 0.11) (see Appendix 2, Figure 28). Forest plots for landmark DFS show heterogeneity for chemotherapy type (p = 0.01), chemotherapy timing (adjuvant/neoadjuvant, p = 0.04) and concurrent/sequential treatment (p = 0.003) (see Appendix 2, Figure 29). Landmark OS showed heterogeneity only for concurrent/sequential trastuzumab and chemotherapy (p = 0.02) (see Appendix 2, Figure 30).
FIGURE 10.
Forest plot of DFS for all patients. CT, chemotherapy; O–E, observed–expected.
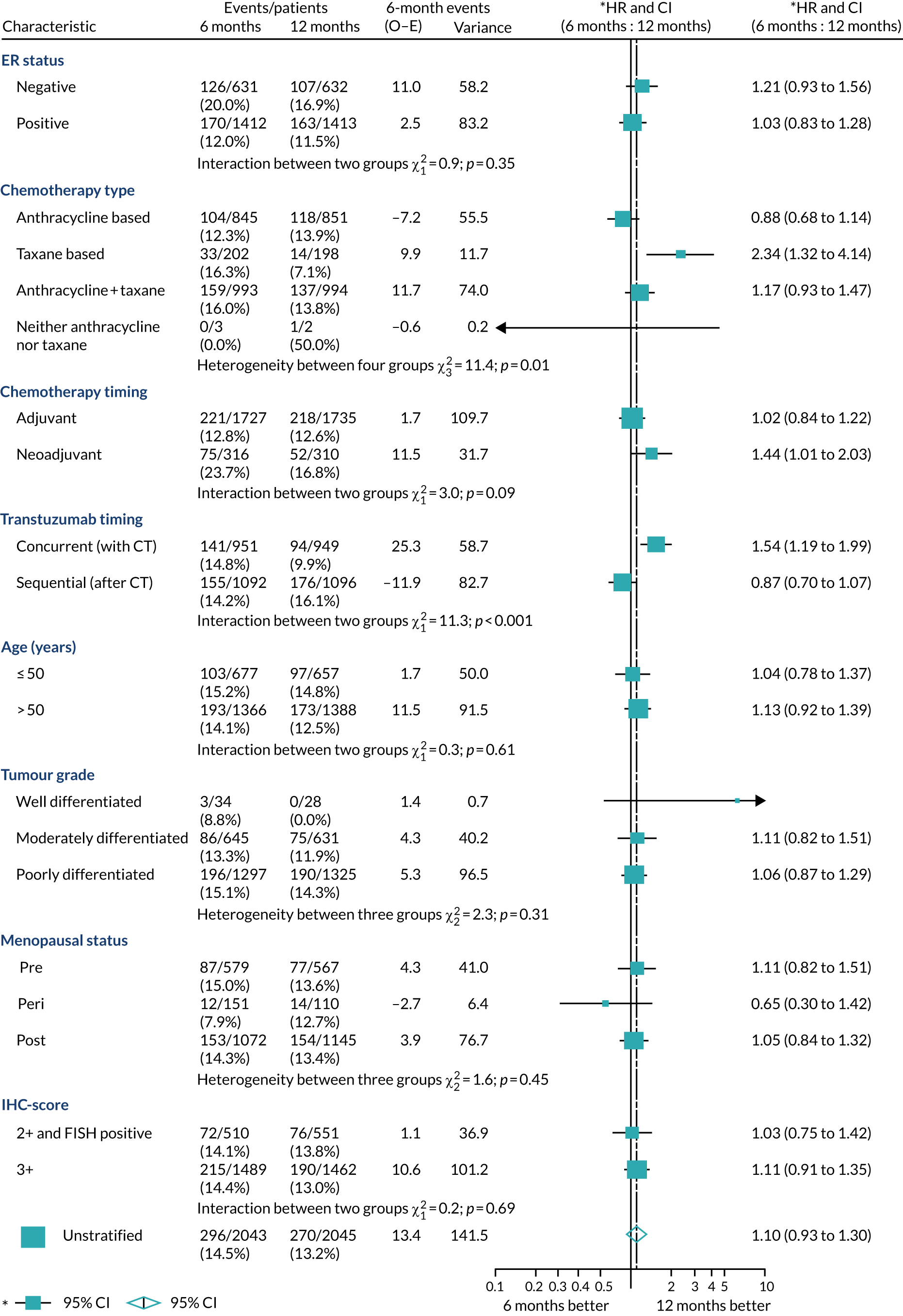
Adjuvant patients
For the subset of patients receiving adjuvant chemotherapy, exploratory forest plots of the randomised treatment effect on DFS (Figure 11) showed heterogeneity for chemotherapy type (p = 0.006) and trastuzumab timing (p = 0.003) and on OS (see Appendix 2, Figure 31) heterogeneity for trastuzumab timing (p = 0.02). There was no additional heterogeneity in the treatment effect for nodal status at surgery, tumour size, or combined ER and nodal status.
FIGURE 11.
Forest plot of DFS for adjuvant patients only. CT, chemotherapy; O–E, observed–expected.
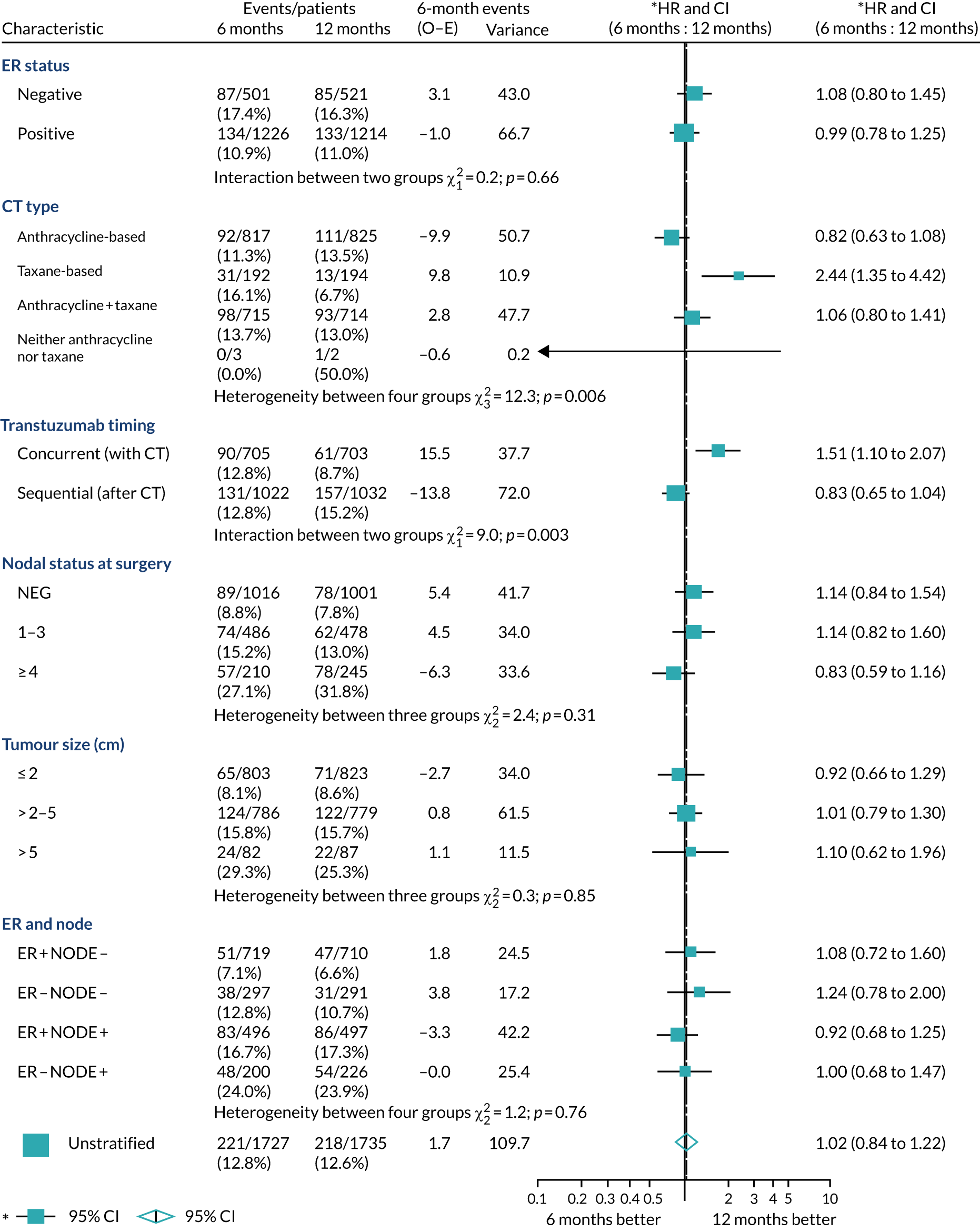
Neoadjuvant patients
For the subset of patients receiving neoadjuvant chemotherapy, exploratory forest plots of the randomised treatment effect on DFS (Figure 12) and OS (see Appendix 2, Figure 32) found no heterogeneity in the treatment effect within the stratification variables. In addition, there was no heterogeneity in the treatment effect for response to neoadjuvant chemotherapy and trastuzumab (pathological complete response vs. non-pathological complete response). Thirty per cent (186/614) of patients achieved pathological complete response (i.e. they had no cancer in breast or axillary lymph nodes) after neoadjuvant chemotherapy and trastuzumab, and to date DFS events have been seen at follow-up in only 6.4% of these patients (12/186), compared with 26% (113/428) of patients who failed to achieve pathological complete response. This is the expected result, as pathological complete response has been shown to predict better outcomes. The numbers in this group are too small to indicate any interaction with treatment duration.
FIGURE 12.
Forest plot of DFS for neoadjuvant patients only. CT, chemotherapy; O–E, observed–expected; pCR, pathological complete response.
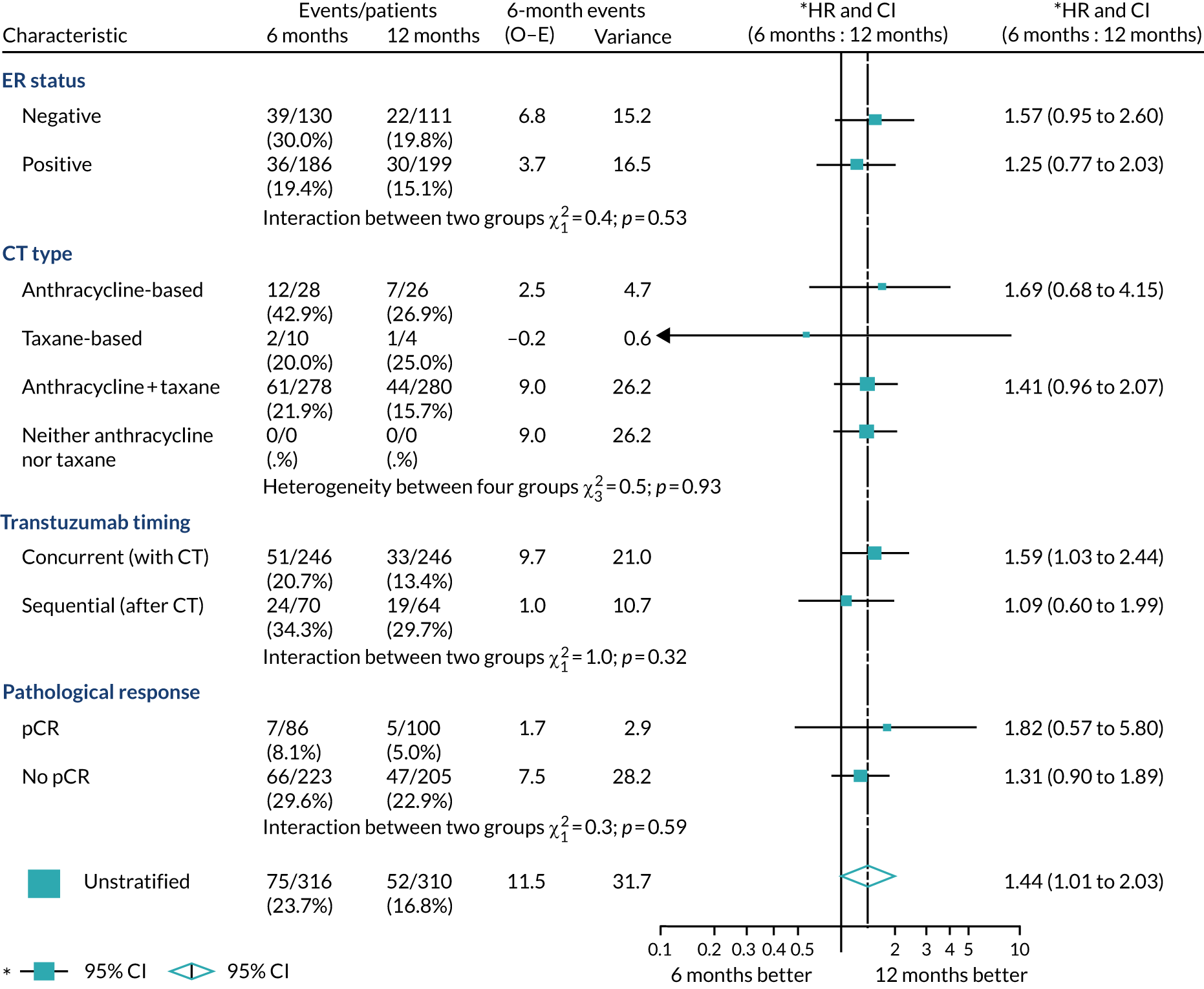
Trial-level meta-analysis of PERSEPHONE and PHARE
A trial-level meta-analysis of non-inferiority trials comparing 6 months with 12 months of trastuzumab treatment was undertaken using DerSimonian and Laird random-effects models50 on the latest published DFS results from the PHARE trial51 (on 3380 patients) and the latest landmark DFS results presented in this report from the PERSEPHONE trial (on 4008 patients). The weighted 4-year DFS rate from 6 months’ trastuzumab for the combined 7388 patient group was 88.9%. Thus, with a non-inferiority margin of 2%, using the methodology used in our study, the non-inferiority limit for the HR is 1.19. There was no detectable difference between the two trials’ results (heterogeneity p = 0.94), and the pooled HR for DFS from 6 months of trastuzumab treatment of 1.08 (90% CI 0.98 to 1.18) met the prespecified definition of 2% non-inferiority (non-inferiority p = 0.04) (Figure 13).
FIGURE 13.
Trial-level meta-analysis of PERSEPHONE and PHARE.
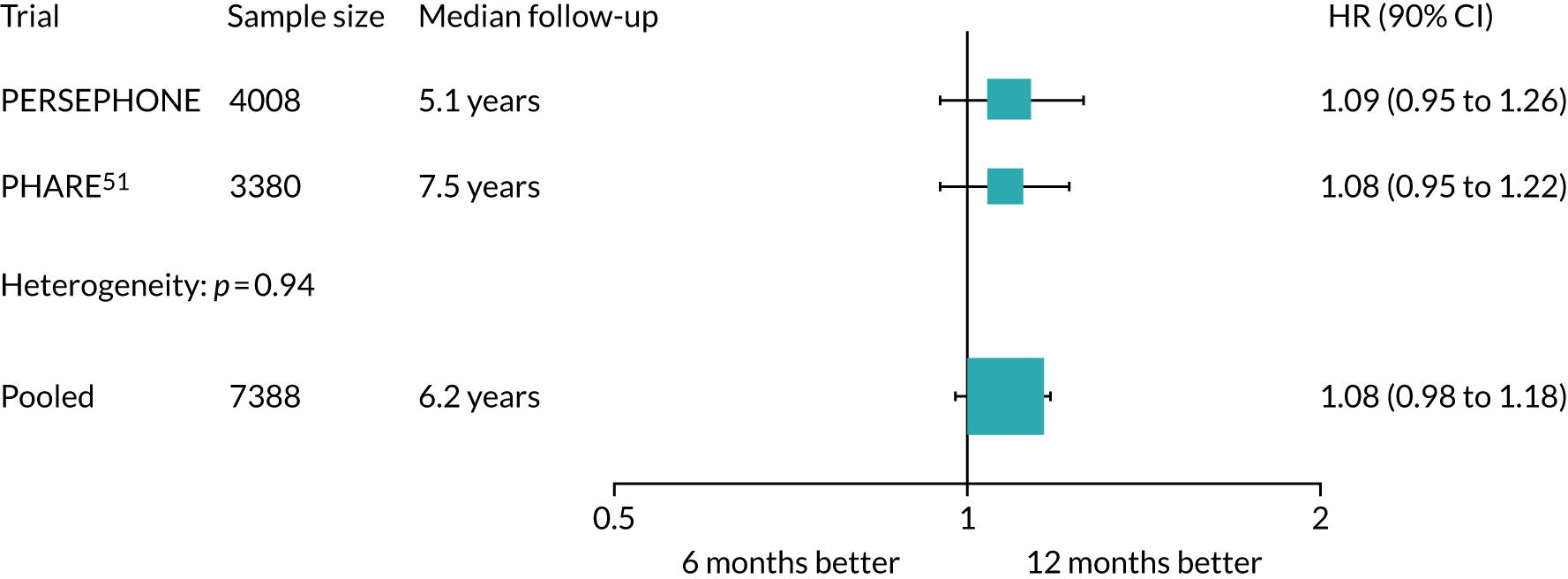
Translational research
The collection of blood and archival tissue samples was conducted in parallel with the PERSEPHONE clinical trial. In total, 3801 (93%) PERSEPHONE patients consented to provide a blood sample to the project but, owing to capacity and logistics at sites, samples from 3392 (83% of all 4088, 89% of those consented) patients were collected. The blood samples were used to extract DNA from peripheral blood mononuclear cells. This DNA has already been profiled with SNP arrays for genome-wide association studies and pharmacogenetic studies. In addition, germline DNA will be sequenced as ‘normal’ to facilitate calling of mutations in DNA extracted from paraffin blocks. Similarly, 3814 (93%) PERSEPHONE patients consented to the accessing of their archival tissue but, owing to capacity and logistics at sites, samples from 2985 (73% of all 4088, 78% of those consented) patients were collected. The tissue cores have been used to construct tissue microarrays, which will be used for digital pathology, IHC and imaging mass cytometry, and for DNA and RNA (ribonucleic acid) extraction (nucleic acids will be used for genomic profiling with DNA and RNA sequencing).
Chapter 4 Cardiac toxicity
Background to cardiac toxicity and trastuzumab
Trastuzumab has transformed the treatment of HER2-positive breast cancer in both the adjuvant and the metastatic setting. However, cardiac toxicity with a reduction in LVEF has been recognised as a side effect of trastuzumab therapy from the early days of its use in metastatic disease52 and has been reported in all adjuvant trials. 53–58 The exact pathophysiology of the observed cardiac dysfunction is not completely understood. HER2 receptors are present on cardiac myocytes, and when normal HER2 function is inhibited by trastuzumab there may be an accumulation of reactive oxygen species, resulting in reduced cardiac function. 59 Trastuzumab may also cause contractile problems by impairing mitochondrial function through intracellular downstream events resulting in depletion of adenosine triphosphate. 60 Anthracyclines, which often precede trastuzumab in the adjuvant setting and have been previously administered when trastuzumab is used in metastatic breast cancer, are also likely to contribute to cardiac problems because of the sequential cardiac stresses. 61,62 The cardiac data from the PHARE trial (6 vs. 12 months of trastuzumab)63 and HERA (12 vs. 24 months)53–56 trials show a clear relationship in the adjuvant setting between the duration of trastuzumab exposure and the incidence of cardiac dysfunction. A recent Cochrane meta-analysis (58 studies in 29,598 patients) of cardiotoxicity of trastuzumab in both randomised controlled trials (RCTs) and observational data64 showed that severe cardiotoxicity occurred in 2.62% (95% CI 1.97% to 3.35%) of early breast cancer patients and in 3.14% (95% CI 2.12% to 4.37%) of metastatic patients. Severe cardiotoxicity was defined as any of the following: symptomatic CHF, myocardial infarction, cardiac dysrhythmia, cardiac toxicity of grade III/IV according to the National Cancer Institute CTCAE or the New York Heart Association (NYHA) classification, or LVEF reduction to ≤ 40%. In early breast cancer, cardiotoxicity occurred in 2.90% of patients treated with taxanes and anthracyclines, compared with 0.92% of patients treated with taxanes without anthracyclines. Notably, the BCIRG-006 study included an experimental arm without anthracyclines and added carboplatin to docetaxel. 18 This combination resulted in a 0.4% incidence of severe congestive heart failure, compared with a 2% incidence in the anthracycline and taxane with trastuzumab group and a 0.7% incidence in the anthracycline and taxane group. 18 Risk factors for cardiotoxicity were older age, smoking, dyslipidaemia, high body mass index, diabetes, hypertension and a positive history of cardiac disease. RCTs have consistently reported lower severe cardiac toxicity rates than observational studies (early breast cancer: 1.7% vs. 3.2%). 64
The PERSEPHONE trial compared 6 months with 12 months of trastuzumab and also carried out detailed cardiac ejection fraction assessments on patients during the 12-month treatment period to assess cardiac toxicity with standard treatment and with reduced treatment. Reducing the risk of cardiac dysfunction would have clear advantages if adjuvant trastuzumab treatment could be shortened to 6 months without a reduction in clinical efficacy. We present here the cardiology substudy with the prespecified secondary end point of cardiac function in all 4088 patients randomised to 6 or 12 months’ trastuzumab. The PERSEPHONE trial was designed to ‘map on to standard practice’ in the UK and therefore our results will be applicable to ‘real-world patients’.
Methods
PERSEPHONE exclusion criteria for cardiac problems
The protocol detailed that patients should have no significant cardiac disease or comorbidity that, in the opinion of the principal investigator, added to the cardiac risks associated with trastuzumab and chemotherapy. PERSEPHONE was a trial designed to ‘map on to standard practice’ and therefore did not exclude any patients who, in the opinion of the principal investigators at recruiting sites, would otherwise be considered fit enough to receive chemotherapy and the standard of 12 months’ adjuvant trastuzumab.
Definition of clinical cardiac dysfunction
‘Clinical cardiac dysfunction’ was defined as any or all of the following: symptoms of cardiac disease or signs of CHF or new/altered cardiac medication prescribed during the 12 months after starting trastuzumab. Following randomisation, side effects specific to trastuzumab were reported in the CRFs with the aid of patient-recorded diary sheets. Every 3 months, symptoms or signs of CHF and new/altered cardiac medication were reported on the CRFs to the trials office.
Left ventricular ejection fraction
Left ventricular ejection fraction measurement
Standard methods for assessing LVEF were used in the study; these were either ECHO or MUGA cardiac scanning. The assessment methods were undertaken as per the standard in each site and, if possible, the same method was used for each individual throughout. Although we recognise that these two methods may produce results that differ in some aspects, ECHO and MUGA scan were treated equivalently in our data analysis.
To start trastuzumab treatment, patients had to have a normal LVEF as defined by the standard at their site. Following randomisation into PERSEPHONE, patients had LVEF measured every 3 months up until 12 months after the start of trastuzumab. In standard practice, cardiac monitoring does not continue after 12 months, and therefore in the study there were no routine measurements of LVEF after 12 months. However, some LVEF measurements after the 12-month time point were available.
In June 2013, the PERSEPHONE independent DSMC reviewed full data on the first 2500 patients randomised. The relative changes over time in LVEF, the frequency of abnormal tests reported over time and the effects of cardiac dysfunction on trastuzumab treatment were scrutinised. For the patients who had ever had reported LVEF of < 50%, all LVEF results over time were reviewed. The independent DSMC did not have any concerns and agreed to a proposed amendment to the protocol from the TMG to reduce the frequency of stipulated cardiology monitoring. The proposal was to change the frequency of LVEF measurement from 3-monthly to a minimum of 4-monthly, in line with standard practice at many sites at that time as recommended in National Cancer Research Institute guidelines,36 which were published after PERSPHONE had been designed. Consequently, more frequent LVEF monitoring was undertaken in the first cohort of PERSEPHONE patients (n = 2500), whereas the second cohort of 1588 patients typically received 4-monthly LVEF monitoring.
Left ventricular ejection fraction end points
‘Low LVEF’ was a cardiac end point defined as a LVEF measurement of < 50%, or systolic function below normal but without LVEF values recorded. Substantial reductions in LVEF were also recorded. ‘Substantial’ was defined as an absolute decrease in LVEF of ≥ 10% from baseline to below 50% or a reduction in LVEF to < 50% after a baseline of ≥ 59%.
Treatment modifications
If LVEF fell below 50%, the protocol advised ‘holding’ trastuzumab and repeating LVEF monitoring at 6-week intervals until LVEF returned to ≥ 50%. After delays for a total of 3 months on account of cardiotoxicity, the protocol advised to discontinue trastuzumab permanently. In the case of NYHA class III/IV heart failure symptoms (breathlessness at rest or on minimal exertion), trastuzumab was permanently discontinued even after resolution of symptoms or normalisation of LVEF with treatment. 65,66 In the later part of the study, standard practice for patients on trastuzumab changed in many sites following the publication of new guidelines. 36 Patients on trastuzumab who had an asymptomatic reduction in LVEF to below the lower limit of normal and ≥ 40% could be treated with cardiac medication while continuing trastuzumab and having their LVEF monitored. Changes to the protocol were not made and therefore these instances were reported as protocol non-compliances.
Statistical analysis
Incidences of clinical cardiac dysfunction, low LVEF, substantial falls in LVEF and treatment modifications as a result of these cardiac events were assessed using chi-squared tests. Changes from patients’ baseline LVEF score (i.e. before they started trastuzumab treatment) were assessed using Wilcoxon signed-rank tests. Random-effects modelling was applied to patients’ LVEF scores over time, and the results are presented graphically as the average patient values over time for each treatment arm as predicted by the model. Further random-effects modelling investigated the influence of chemotherapy type, number of anthracycline cycles and trastuzumab timing (concurrent/sequential). Potential predictive factors of experiencing a low LVEF during the 12-month reporting period were investigated using univariate logistic regressions and are presented with odds ratios (ORs) and 95% CIs.
Results
Chemotherapy, timing of randomisation and prior cardiac medication
Factors relevant to cardiac risk appeared balanced across randomised treatment arms; 90% of patients (12-month patients, 90%; 6-month patients, 90%) received an anthracycline as part of either anthracycline-based (12-month patients, 41%; 6-month patients, 41%) or anthracycline plus taxane-based (12-month patients, 49%; 6-month patients, 49%) chemotherapy (see Table 5). Fifty-four per cent of patients received trastuzumab sequentially after chemotherapy (12-month patients, 54%; 6-month patients, 53%), and 46% received trastuzumab concurrently with the non-anthracycline component of chemotherapy (12-month patients, 46%; 6-month patients, 47%). Only 2% of patients reported being on cardiac medication prior to starting trastuzumab (12-month patients, 2%; 6-month patients, 3%). Randomisation was before the start of trastuzumab or at any time up to and including the ninth cycle. Forty-four per cent (1782/4088) of patients were randomised upfront and 81% (3320/4088) were randomised prior to cycle 5, and the timing of randomisation was balanced between the 6- and 12-month arms (see Table 6).
Cardiac events
Full clinical cardiac data were available for 3995 patients (98% of 4088 randomised): 1987 (97% of 2045 randomised) 12-month patients and 2008 (98% of 2043 randomised) 6-month patients. Clinical cardiac dysfunction was reported in 384 (10% of 3995) patients (Table 11), with 188 (5% of 3995) reporting symptoms of cardiac disease, 65 (2% of 3995) having signs of CHF, and 274 (7%) reporting new or altered cardiac medication (see Appendix 1, Table 40). Clinical cardiac dysfunction was more common in 12-month than in 6-month patients [228/1987 (11%) vs. 156/2008 (8%), respectively; p < 0.0001]. Some differences in rates were apparent in the first 6 months of trastuzumab [168/1987 (8%) 12-month patients vs. 127/2008 (6%) 6-month patients; p = 0.01], which we would assume a chance finding as we would expect these to be similar at this point. A more significant difference between the two arms emerged during the 7- to 12-month period [158/1953 (8%) vs. 97/1914 (5%), respectively; p = 0.0002], as expected.
| Number of patients reporting at least one incidence of | 12-month patients, n/N (%) | 6-month patients, n/N (%) | ||||
|---|---|---|---|---|---|---|
| Overall | In months 1–6 | In months 7–12 | Overall | In months 1–6 | In months 7–12 | |
| Clinical cardiac dysfunctiona | 228/1987 (11) | 168/1987 (8) | 158/1953 (8) | 156/2008 (8) | 127/2008 (6) | 97/1914 (5) |
| Low LVEFb | 228/2042 (11) | 149/2042 (7) | 150/1942 (8) | 175/2038 (9) | 145/2038 (7) | 84/1758 (5) |
| Substantial falls in LVEF | ||||||
| Absolute decrease of ≥ 10% from baseline to < 50% | 164/1964 (8) | 99/1955 (5) | 102/1880 (5) | 131/1961 (7) | 101/1957 (5) | 61/1701 (4) |
| LVEF < 50% after a baseline of ≥ 59% | 109/1964 (6) | 64/1955 (3) | 71/1880 (4) | 86/1961 (4) | 70/1957 (4) | 33/1701 (2) |
| Stopped trastuzumab permanently owing to cardiac toxicity | 146/1941 (8) | 63/1941 (3) | 83/1809 (5) | 61/1977 (3) | 60/1977 (3) | 1/102 (1) |
| Cardiac deathc | 7/2045 | 0/2045 | 0/2018d | 4/2043 | 0/2043 | 0/2017d |
| Cardiac death related to trastuzumabc | 0/2044e | 0/2044e | 0/2018d | 0/2041e | 0/2041e | 0/2017d |
In total, 19,458 LVEF measurements (ECHO, 73%; MUGA, 17%; unknown, 10%) were reported in 4080 patients: 10,193 in 2042 12-month patients and 9265 in 2038 6-month patients. The use of each modality was balanced across the randomised treatment arms (12-month patients: ECHO, 73%, MUGA, 18%, unknown, 9%; 6-month patients: ECHO, 73%, MUGA, 17%, unknown, 10%). MUGA scans appear to have a higher rate of low LVEF readings (< 50%, or percentage unknown but classified on report as abnormal): 10% of MUGA scans compared with 3% of ECHO (see Appendix 1, Table 41). During the first 6 months of treatment, the proportion of patients with low LVEF was 7% in both groups, 149 out of 2042 (7%) in the 12-month arm and 145 out of 2038 (7%) in the 6-month arm (p = 0.87). However, during months 7–12, this proportion increased for 12-month patients (150/1942; 8%) but fell for 6-month patients (84/1758; 5%) (p = 0.0003 for difference between groups). Patients with substantial decreases in LVEF, defined as absolute decrease in LVEF of ≥ 10% from baseline to < 50%, were seen in months 1–6 of treatment in similar numbers: 99 out of 1955 (5%) patients in the 12-month arm and 101 out of 1957 (5%) in the 6-month arm. However, during months 7–12, there were more patients with substantial decreases in the 12-month arm than in the 6-month arm [102/1880 (5%) and 61/1701 (4%), respectively; p = 0.01]. Substantial falls in LVEF, defined as decreasing to < 50% after a baseline of ≥ 59%, occurred in the first 6 months in similar numbers of patients: in 64 out of 1955 (3%) 12-month patients and in 70 out of 1957 (4%) 6-month patients. However, in months 7–12 these figures were 71 out of 1880 (4%) and 33 out of 1701 (2%), respectively (p = 0.0015).
A landmark analysis was carried out on 3415 patients who had not reported any cardiac dysfunction (defined as clinical cardiac dysfunction or low LVEF) within their first 6 months of treatment and for whom data were available in months 7–12. Significantly more 12-month patients (97/1714; 6%) than 6-month patients (39/1701; 2%) reported cardiac dysfunction in the second 6-month period (p < 0.0001).
Eleven deaths were reported to have had a ‘cardiac’ cause, either first cause or contributory cause (see Appendix 1, Table 42). None occurred during the first 12 months after starting trastuzumab treatment. Nine patients died with no metastatic disease and two had metastatic disease. In all cases, the TMG and the cardiologist who reviewed the cases judged trastuzumab as unrelated or unlikely to have been related to cardiac problems. Four deaths were caused by ischaemic heart disease, which has no known association with trastuzumab. One patient developed a decrease in LVEF with CHF; this was very unlikely to have been related to trastuzumab, as the patient received only four cycles, treatment was stopped because of reduced LVEF, and left ventricular function then recovered > 3 months after treatment had stopped. The patient was then diagnosed with chronic obstructive pulmonary disease, which accounted for her breathlessness, and died of chronic obstructive pulmonary disease 78 months after breast cancer diagnosis. There was one case of cardiac amyloid and one of cardiac sarcoid; neither was judged to be related to trastuzumab. One patient was reported to have right ventricular arrhythmic cardiomyopathy, which has a known genetic basis (mostly desmosomal genes) with physiological triggers (e.g. extreme endurance sports), and therefore this was judged unlikely to be related to trastuzumab. One patient had a low LVEF of 48% recorded after three cycles of trastuzumab; the trastuzumab was stopped, and the patient was diagnosed with symptomatic CHF and started cardiac medication. Her LVEF normalised, and she remained on preventative cardiac medication. She had received 300 mg/m2 epirubicin. Her death was due to metastatic breast cancer. One patient had a reduced LVEF during trastuzumab treatment, which was discontinued after four cycles when LVEF was 30%. The patient was started on cardiac medication and further investigations revealed that she had coronary artery disease. After she stopped trastuzumab, her LVEF recovered to normal, at 50%. The cause of her death 6 years later was recorded as metastatic breast cancer, with a secondary cause of death of cardiomyopathy. However, no supporting evidence was provided for cardiomyopathy (no autopsy). Presence of cardiomyopathy was felt unlikely to be related to trastuzumab and most likely to be related to documented coronary artery disease or otherwise anthracyclines (300 mg/m2 of epirubicin).
Trastuzumab modifications
Complete trastuzumab treatment data are available for 3921 (96%) of the 4088 patients (see Figure 2). Delays in trastuzumab cycles were caused by cardiotoxicity in 5% of the 12-month patients (fifth most common reason) and 6% of the 6-month patients (third most common reason) (see Appendix 1, Table 34). However, cardiotoxicity was the most common reason reported for trastuzumab treatment being stopped early [206 (46%) out of 449 patients who received fewer cycles than randomised to receive: (146; 43%) of 336 12-month patients stopping early and 60 (53%) of 113 6-month patients stopping early] (see Figure 4). Most commonly, patients’ trastuzumab treatment was stopped early because of cardiotoxicity (i.e. cardiotoxicity was the reason in over half of cases during that cycle) in cycles 4–8 for both 6- and 12-month patients, and also in cycles 10 and 13 for 12-month patients (Figure 14). Cardiac monitoring with LVEF commenced after four cycles of trastuzumab, and this corresponded to the time point at which we observed an increase in the number of patients stopping trastuzumab early because of cardiac toxicity.
FIGURE 14.
Total number of trastuzumab cycles received, indicating the numbers of patients stopping because of cardiotoxicity: (a) 12-month patients; and (b) 6-month patients. The light blue bar indicates patients who received the exact number of cycles as randomised.
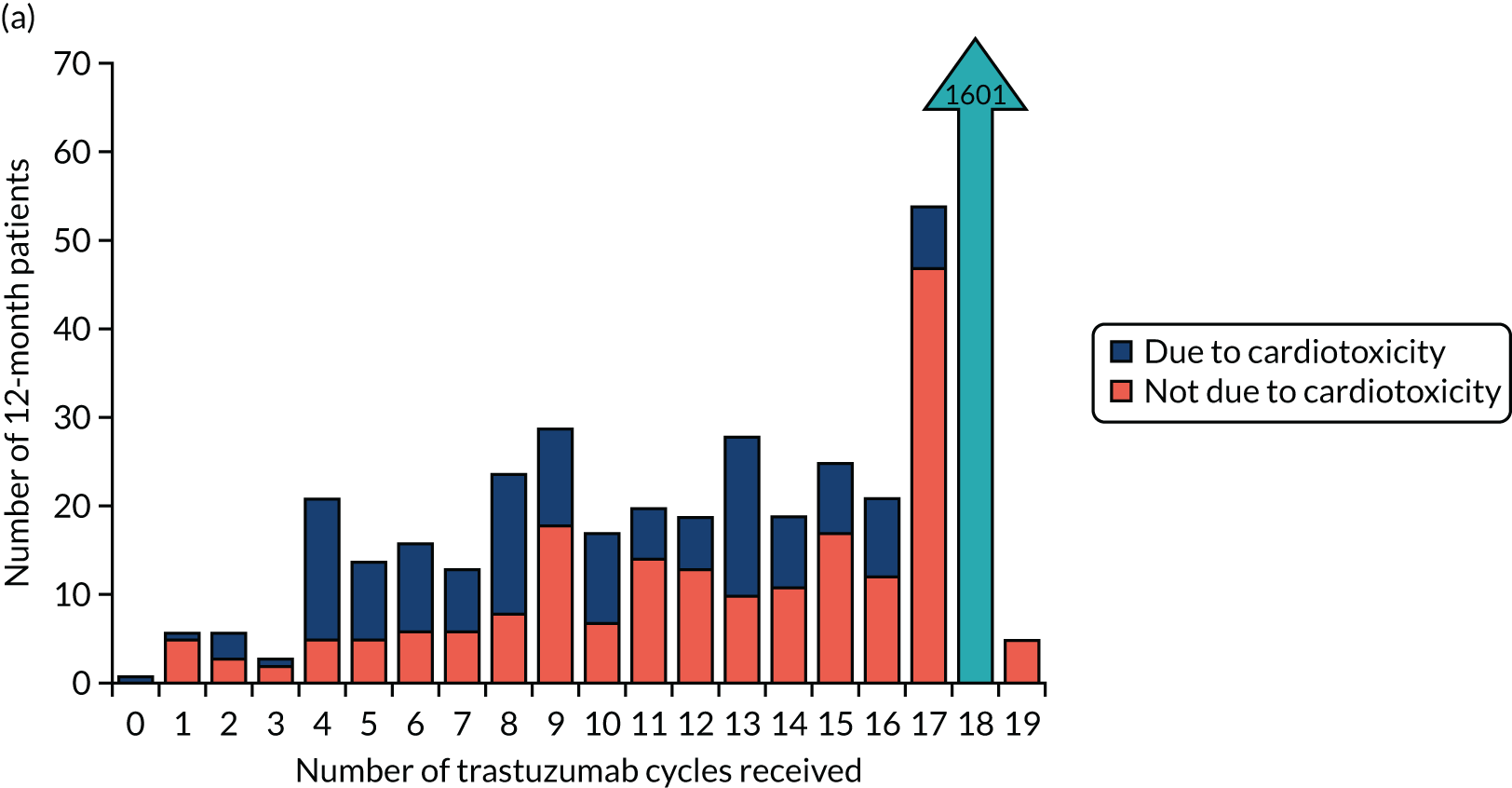
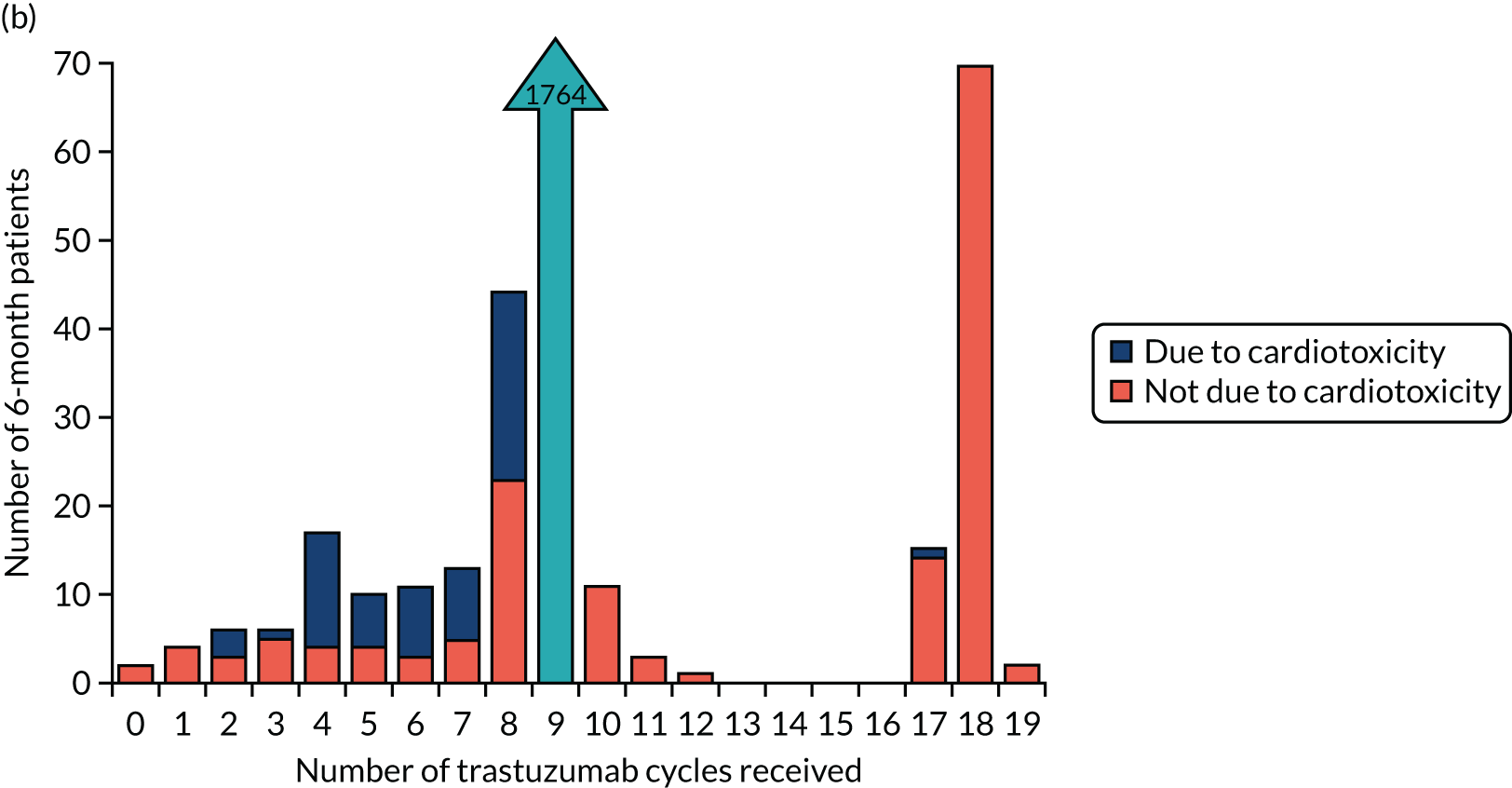
We were interested to see whether cardiac problems occurring within the first 3 months of trastuzumab (early) correlated with early cessation more than those occurring at 4–12 months (late). Clinical cardiac dysfunction occurred early in 138 12-month patients, of whom 82 (59.2%) completed 18 cycles, and late in 217 patients, of whom 105 (48%) completed 18 cycles (see Appendix 1, Table 43). Clinical cardiac dysfunction occurred early in 149 6-month patients, of whom 106 (71%) completed nine cycles, and late in 169 patients, of whom 119 (70%) completed nine cycles. Low LVEF occurred early in 74 12-month patients, of whom 31 (42%) completed 18 cycles, and late in 205 patients, of whom 78 (38%) completed 18 cycles (see Appendix 1, Table 44). Low LVEF occurred early in 81 6-month patients, of whom 53 (65.5%) completed nine cycles, and late in 145 patients, of whom 96 (66%) completed nine cycles. Therefore, patients reporting cardiac dysfunction soon after being randomised to their treatment arm were as likely as those reporting it late to complete their randomised trastuzumab treatment. There was a steady increase in the cumulative number of patients stopping trastuzumab early as treatment continued.
Relative change of left ventricular ejection fraction at 6 and 12 months
A total of 3925 patients had their LVEF percentage reported both at baseline and at least one other time point: 1964 12-month and 1961 6-month patients. The two treatment arms were well matched on baseline LVEF percentages [median (IQR) 63% (59–67%) for 12-month patients and 63% (59–67%) for 6-month patients; p = 0.43]. There were statistically significant reductions in LVEF at the 6- and 12-month time points in both treatment arms (all p < 0.0001). Despite similar relative changes from baseline to 6 months across treatment arms [median (IQR) 0.97 (0.90–1.02) for 12-month patients and 0.97 (0.90–1.02) for 6-month patients; p = 0.50], 12-month patients showed significantly greater reductions in LVEF by 12 months [median (IQR) being 0.97 (0.90–1.02) for 12-month patients and 0.98 (0.92–1.03) for 6-month patients; p = 0.0002].
Random-effects modelling
A quadratic curve was found to fit the LVEF data over all patients, demonstrating that cardiac function recovers post treatment (Figure 15). This was seen for the entire trial population (p < 0.0001) and for each randomised arm separately (both p < 0.0001). There was a significant difference between treatments in terms of change over time (p = 0.016); quadratic modelling predicts an earlier recovery of cardiac function after treatment completion in 6-month patients. No significant differences were found in trastuzumab timing (concurrent or sequential; p = 0.77) in terms of LVEF changes over time (see Appendix 2, Figure 33). Quadratic modelling predicted some differences between chemotherapy types in terms of LVEF changes over time, with anthracycline-based chemotherapy resulting in a lower nadir and a slower recovery of LVEF than anthracycline/taxane chemotherapy (p = 0.04) (see Appendix 2, Figure 34). In addition, in terms of LVEF change over time, a significant difference was found depending on the number of anthracycline cycles patients received, whether three or fewer or more than three (p = 0.04) (see Appendix 2, Figure 35).
FIGURE 15.
Random-effects modelling predicted lines and 95% CIs, by randomised treatment arm.
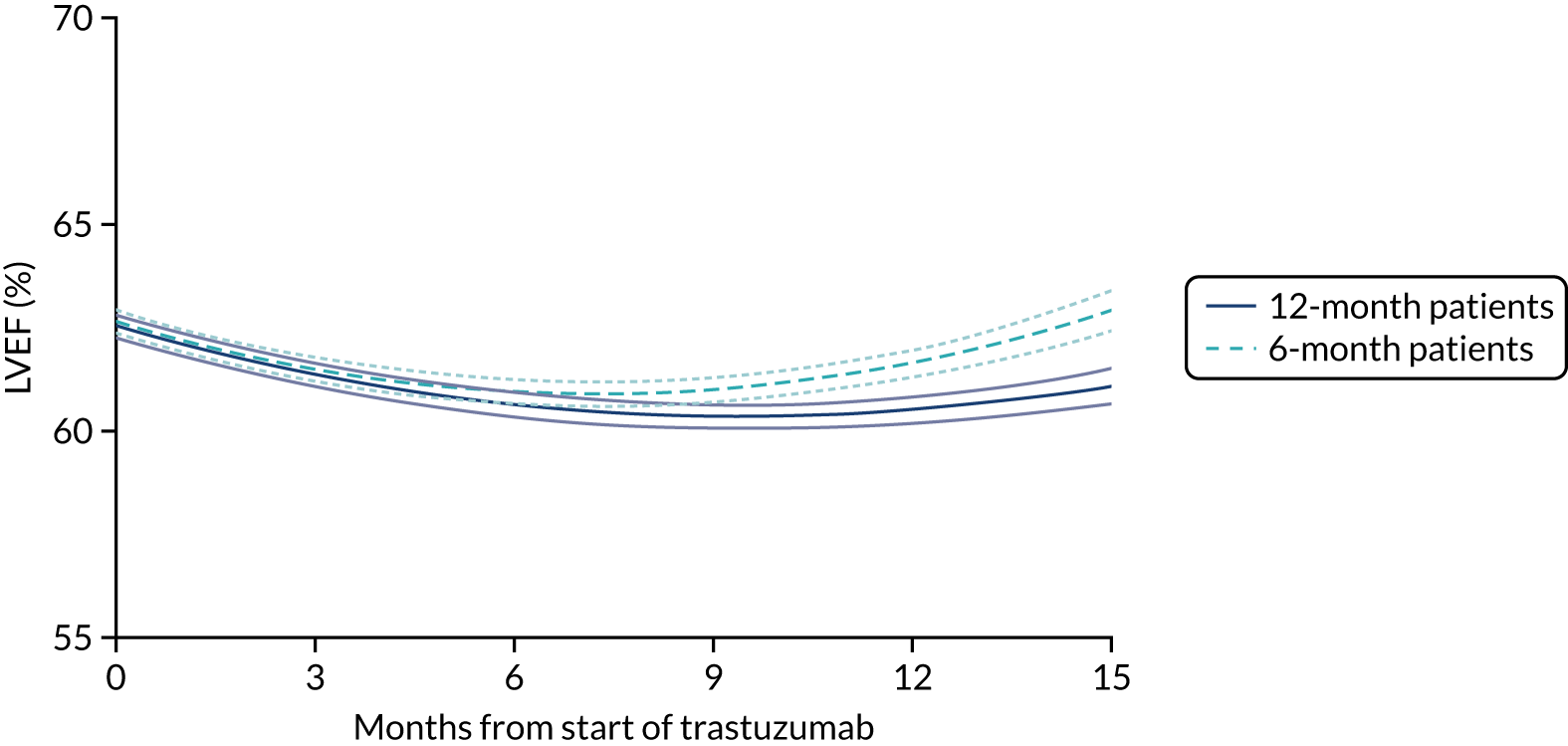
Predictive factors of low left ventricular ejection fraction
Chemotherapy type was found to be predictive of low LVEF (p < 0.0001), with taxane and anthracycline chemotherapy demonstrating an increased risk of low LVEF compared with taxane only (OR 2.30, 95% CI 1.18 to 4.47) in the 12-month group but not in the 6-month group (OR 1.31, 95% CI 0.68 to 2.51) (Table 12). Anthracycline-based chemotherapy without taxanes also demonstrated an increased risk of low LVEF compared with the taxane-only group (OR 2.79, 95% CI 1.43 to 5.44 for 12-month patients, and OR 2.13, 95% CI 1.12 to 4.06 for 6-month patients).
| Factor | 12-month patients, n (%) | 6-month patients, n (%) | Total, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases (N = 228; 11%) | Controls (N = 1814; 89%) | OR (95% CI) | Cases (N = 175; 9%) | Controls (N = 1863; 91%) | OR (95% CI) | Cases (N = 403; 10%) | Controls (N = 3677; 90%) | OR (95% CI) | |
| Chemotherapy | p = 0.008 | p = 0.003 | p < 0.0001 | ||||||
| Taxane based | 10 (5) | 188 (95) | 1.00 | 11 (6) | 189 (94) | 1.00 | 21 (5) | 377 (95) | 1.00 |
| Taxane and anthracycline | 108 (11) | 884 (89) | 2.30 (1.18 to 4.47) | 70 (7) | 921 (93) | 1.31 (0.68 to 2.51) | 178 (9) | 1805 (91) | 1.77 (1.11 to 2.82) |
| Anthracycline based | 110 (13) | 740 (87) | 2.79 (1.43 to 5.44) | 93 (11) | 751 (89) | 2.13 (1.12 to 4.06) | 203 (12) | 1491 (88) | 2.44 (1.54 to 3.88) |
| Anthracyclines | p = 0.0003 | p = 0.0007 | p < 0.0001 | ||||||
| ≤ 3 cycles | 88 (9) | 922 (91) | 1.00 | 63 (6) | 915 (94) | 1.00 | 151 (8) | 1837 (92) | 1.00 |
| > 3 cycles | 136 (14) | 842 (86) | 1.69 (1.27 to 2.25) | 109 (11) | 902 (89) | 1.76 (1.27 to 2.43) | 245 (12) | 1744 (88) | 1.71 (1.38 to 2.12) |
| Baseline LVEF | p < 0.0001 | p < 0.0001 | p < 0.0001 | ||||||
| ≥ 65% | 46 (6) | 760 (94) | 1.00 | 41 (5) | 812 (95) | 1.00 | 87 (5) | 1572 (95) | 1.00 |
| 55 to < 65% | 134 (12) | 961 (88) | 2.30 (1.63 to 3.26) | 93 (9) | 948 (91) | 1.94 (1.33 to 2.84) | 227 (11) | 1909 (89) | 2.15 (1.66 to 2.78) |
| < 55% | 44 (52) | 40 (48) | 18.2 (10.8 to 30.6) | 38 (41) | 55 (59) | 13.7 (8.1 to 23.0) | 82 (46) | 95 (54) | 15.6 (10.8 to 22.5) |
| Left-sided radiotherapy | p = 0.15 | p = 0.69 | p = 0.38 | ||||||
| No | 145 (12) | 1053 (88) | 1.00 | 96 (8) | 1052 (92) | 1.00 | 241 (10) | 2105 (90) | 1.00 |
| Yes | 81 (10) | 728 (90) | 0.81 (0.61 to 1.08) | 75 (9) | 771 (91) | 1.07 (0.78 to 1.46) | 156 (9) | 1499 (91) | 0.91 (0.74 to 1.12) |
| Body mass index | p = 0.90 | p = 0.78 | p = 0.93 | ||||||
| Low/normal | 75 (11) | 596 (89) | 1.00 | 56 (8) | 611 (92) | 1.00 | 131 (10) | 1207 (90) | 1.00 |
| High | 149 (11) | 1206 (89) | 0.98 (0.73 to 1.32) | 119 (9) | 1238 (91) | 1.05 (0.75 to 1.46) | 268 (10) | 2444 (90) | 1.01 (0.81 to 1.26) |
| Age (years) at randomisation | p = 0.33 | p = 0.0009 | p = 0.001 | ||||||
| < 50 | 57 (10) | 543 (90) | 1.00 | 34 (6) | 567 (94) | 1.00 | 91 (8) | 1110 (92) | 1.00 |
| 50 to < 60 | 66 (11) | 541 (89) | 1.16 (0.80 to 1.69) | 52 (8) | 604 (92) | 1.44 (0.92 to 2.25) | 118 (9) | 1145 (91) | 1.26 (0.95 to 1.67) |
| 60 to < 70 | 78 (13) | 539 (87) | 1.38 (0.96 to 1.98) | 61 (11) | 519 (89) | 1.96 (1.27 to 3.03) | 139 (12) | 1058 (88) | 1.60 (1.21 to 2.12) |
| ≥ 70 | 27 (12) | 191 (88) | 1.35 (0.83 to 2.19) | 28 (14) | 173 (86) | 2.70 (1.59 to 4.58) | 55 (13) | 364 (87) | 1.84 (1.29 to 2.63) |
| Ethnicity | p = 0.33 | p = 0.27 | p = 0.15 | ||||||
| White | 192 (12) | 1464 (88) | 1.00 | 147 (9) | 1501 (91) | 1.00 | 339 (10) | 2965 (90) | 1.00 |
| Other | 11 (9) | 115 (91) | 0.73 (0.39 to 1.38) | 7 (6) | 111 (94) | 0.64 (0.29 to 1.41) | 18 (7) | 226 (93) | 0.70 (0.43 to 1.14) |
| Cardiac medication before trastuzumab | p < 0.0001 | p < 0.0001 | p < 0.0001 | ||||||
| No | 213 (11) | 1785 (89) | 1.00 | 160 (8) | 1823 (92) | 1.00 | 373 (9) | 3608 (91) | 1.00 |
| Yes | 15 (34) | 29 (66) | 4.34 (2.29 to 8.22) | 15 (27) | 40 (73) | 4.27 (2.31 to 7.90) | 30 (30) | 69 (70) | 4.21 (2.71 to 6.54) |
The number of anthracycline cycles received was also found predictive of low LVEF across all patients (p < 0.0001). Receiving more than three cycles of anthracycline was associated with a significant increase in the odds of developing low LVEF in both the 12-month arm (OR 1.69, 95% CI 1.27 to 2.25; p = 0.0003) and the 6-month arm (OR 1.76, 95% CI 1.27 to 2.43; p = 0.0007). Baseline LVEF was also found predictive of low LVEF for all patients (reference ≥ 65%: 55% to < 65%, OR 2.15, 95% CI 1.66 to 2.78; < 55%, OR 15.6, 95% CI 10.8 to 22.5; p < 0.0001). This was the case for both 12-month (reference ≥ 65%: 55 to < 65%, OR 2.30, 95% CI 1.63 to 3.26; < 55%, OR 18.2, 95% CI 10.8 to 30.6; p < 0.0001) and 6-month patients (reference ≥ 65%: 55% to < 65%, OR 1.94, 95% CI 1.33 to 2.84; < 55%, OR 13.7, 95% CI 8.1 to 23.0; p < 0.0001). Left-sided radiotherapy (p = 0.38), body mass index (p = 0.93) and ethnicity (p = 0.15) were not found to influence the incidence of low LVEF. Among the small number of patients who were taking cardiac medication before starting trastuzumab (44 in the 12-month arm and 55 in the 6-month arm), those in both arms were at significantly increased risk (over fourfold) of developing cardiac dysfunction (both p < 0.0001). Age at randomisation predicted for low LVEF in the 6-month arm (p = 0.0009) but not in the 12-month arm (p = 0.33), with 6-month patients aged > 60 years having increased risk compared with the reference age of < 50 years (60 to < 70 years; OR 1.96, 95% CI 1.27 to 3.03, and ≥ 70 years, OR 2.70, 95% CI 1.59 to 4.58).
Discussion
Trastuzumab is a highly effective adjuvant treatment for HER2-positive early breast cancer given in addition to chemotherapy. Cardiotoxicity has been demonstrated consistently in patients receiving trastuzumab. In PERSEPHONE, across all the assessments undertaken, less frequent and less severe cardiotoxicity was demonstrated consistently in patients randomised to 6 months’ treatment than in those randomised to 12 months’ treatment. Clinical cardiac dysfunction was seen significantly more often in 12-month patients than in 6-month patients [228/1987 (11%) vs. 156/2008 (8%) respectively; p < 0.0001], and more than twice as many 12-month patients stopped trastuzumab early because of cardiac toxicity (8% vs. 3%; p < 0.0001). Low LVEF measurements were recorded in the PHARE trial,63 and reported reductions in cardiac function (reduced LVEF) occurred in 5.9% (100/1680) of the 12-month arm and in 3.4% (58/1690) of the 6-month arm (p = 0.001). These rates are somewhat lower than those seen in PERSEPHONE and this may be partly because all patients in PHARE were randomised after receiving between 3 and 6 months of trastuzumab and had normal LVEF at that point of randomisation, whereas in PERSEPHONE 44% of patients were randomised before receiving any trastuzumab, and 82% were randomised before they had received five cycles. This means that whereas the PHARE trial excluded all patients who developed low LVEF within the first 3–6 months of treatment, PERSEPHONE did not. The HERA trial55,56 reported cardiac adverse events, which led to trastuzumab being discontinued permanently in 9.4% of patients in the 24-month arm and in 5.2% of patients in the 12-month arm. In PERSEPHONE, 8% of patients in the 12-month arm and 3% of patients in the 6-month arm (p < 0.0001) stopped trastuzumab permanently because of cardiac dysfunction. In the HERA trial, substantial reductions in LVEF (to < 50%, with a decrease of ≥ 10% absolute value) were seen in 7.2% of 24-month patients and 4.1% of 12-month patients. In PERSEPHONE, these substantial reductions were seen in 99 out of 1955 (5%) patients in the 12-month arm and in 101 out of 1957 (5%) in the 6-month arm in months 1–6 of treatment, but, as expected, during months 7–12 there were more in the 12-month arm than in the 6-month arm [102/1880 (5%) and 61/1701 (4%) respectively; p = 0.01]. The lower rates of substantial reductions in LVEF in the HERA trial than in PERSEPHONE are likely to reflect the stringent entry criteria in the former of LVEF ≥ 55%, whereas PERSEPHONE accepted patients who had an institutionally normal LVEF, which could be 50–55%. As in the HERA trial 24 months’ trastuzumab was not shown to be more effective than the standard of 12 months’ trastuzumab,13 but caused more cardiotoxicity, 12 months’ treatment has remained the standard.
Random-effects modelling of LVEF measurements on the whole population provided some interesting comparisons between the 6- and the 12-month arms. Both fit best with a quadratic model during treatment and on recovery up to 15 months after the start of trastuzumab. This suggests recovery from cardiac effects of trastuzumab similar to that reported in other trastuzumab adjuvant studies. 56,58,63 There is a lower LVEF nadir and a slower recovery in 12-month patients than in 6-month patients. This suggests that the longer the trastuzumab treatment, the greater the cardiac dysfunction and the longer the recovery time. In terms of trastuzumab timing with chemotherapy, there were no significant differences between concurrent and sequential treatment delivery. Modelling by type of chemotherapy (anthracycline based, taxane based or anthracycline/taxane) showed that all treatments fit best into the quadratic model, but there was a difference in LVEF changes over time, with a lower nadir and slower recovery with anthracycline-based chemotherapy than with anthracycline/taxane-based treatments. Higher cumulative anthracycline exposure is likely to be the explanation for this finding.
Analysis of LVEF results by assessment method used highlighted some interesting findings. ECHO was more frequently used (in 71% of cases) than MUGA scans (17% of cases) (see Appendix 1, Table 41). However, abnormal values were reported on 10% of MUGA scans compared with only 4% of echocardiograms. It is possible that MUGA was the ‘preferred’ modality when cardiac problems were suspected on clinical grounds, perhaps because it was more easily available in clinical practice. An alternative explanation is that because the abnormal range for LVEF, when measured using MUGA, varies between institutions, and in many cases is lower (e.g. < 43%) than that for ECHO (< 50%), a higher proportion of MUGA-derived LVEFs than ECHO-derived LVEFs will be < 50%, including some patients with normal heart function. In our analysis of monitoring patients on trastuzumab treatment, we took a cut-off point of 50% as the lower limit of normal regardless of the modality of assessment, in accordance with national guidelines, and this is likely to have influenced the incidence of ‘abnormal’ results from the MUGA investigations.
Some researchers have reported genetic alterations within the HER2 receptor that may predispose a patient to cardiac toxicity from trastuzumab. 67–69 Most recently, a meta-analysis of available studies has identified a HER2 variant, HER2 rs1136201, as a risk variant for reduced LVEF with trastuzumab (pooled OR 2.43, 95% CI 1.17 to 5.06; p = 0.018). 70 In a companion translational pharmacogenetic research study (PERSEPHONE PGSNPs), a collection of blood samples for germline analysis has been completed. A total of 3801 (93%) PERSEPHONE patients consented to provide a blood sample to the project but, owing to capacity and logistical issues at sites, samples from 3392 (83% of all 4088, 89% of those who consented) were collected. These samples will allow us not only to validate these potential predictive genetic alterations but also, in a genome-wide association study, to discover other possible genetic factors predisposing to trastuzumab-induced cardiotoxicity.
In terms of predictive factors for cardiac dysfunction, as measured by reduction in LVEF, our study shows that, regardless of whether treatment lasts 6 or 12 months, baseline LVEF measurements are important. As a surrogate for effects of radiotherapy on the heart, we compared patients who had right-sided tumours with those who had left-sided tumours. Despite other reports of increased cardiotoxicity in patients with left-sided HER2-positive breast cancer,71 we did not find an interaction with radiotherapy and sidedness. However, the follow-up in our study is relatively short, and cardiac effects of radiotherapy occur up to 20 years later. 72 In the whole group there is an increasing risk with each decade of age, and those aged > 70 years have an OR of 1.84 for low LVEF, which would suggest that the predicted benefit of trastuzumab in this older age group would have to be larger to balance the risk of cardiac dysfunction. However, in the present analysis this effect is seen in 6-month patients and not in 12-month patients, which is somewhat unexpected. Patients with a lower baseline LVEF, that is between 50% and 55%, have an OR of 15.6 (95% CI 10.8 to 22.5) of developing reductions in LVEF measurements, compared with those with a baseline LVEF of ≥ 65%. These data, however, should be interpreted with caution, as, if baseline values are close to the lower limit of normal LVEF (i.e. 50%), a small absolute percentage LVEF fall, possibly not clinically meaningful, would reduce the LVEF below the normal threshold. Anthracyclines have been demonstrated to cause cardiotoxicity in a dose-dependent way and also to increase trastuzumab cardiotoxicity; this was shown in the BCIRG trial,18 which excluded anthracyclines in one experimental arm (TCH). In many studies this interactive effect is greater when more than three cycles of anthracycline are given. In this analysis our study confirms this in both the 12- and the 6-month arms (OR 1.69, 95% CI 1.27 to 2.25, and OR 1.76, 1.27 to 2.43, respectively). Our previous publication on the first 2500 patients73 suggested that the cardiac effects of more than three cycles of anthracyclines were not seen when 6 months of trastuzumab was given. However, this analysis, which includes all patients and limits cardiac effects to the quantifiable LVEF, does not confirm any differential effect between 6 and 12 months. Although in the BCIRG-006 study Slamon et al. 18 showed that anthracyclines can be replaced by carboplatin, there was nevertheless some reduction in 5-year DFS, 84% in the anthracycline-containing chemotherapy group compared with 81% in the TCH group, although this difference was not statistically significant for superiority of AC-TH compared with TCH, and the study was not powered for non-inferiority. Anthracyclines are highly effective chemotherapeutic agents in the treatment of breast cancer,74,75 and may be particularly so in HER2-positive breast cancer, with induction of immunogenic cell death one of the mechanisms of action (see Chapter 7, Anthracycline effectiveness in HER2-positive breast cancer). Anthracyclines are still received by the majority of breast cancer patients in the adjuvant setting. In addition, CEP17 and topoisomerase II amplification (more common in the HER2-positive population) have been found to be predictive biomarkers of anthracycline benefit. 76 Controversy remains with regard to discontinuing the use of anthracyclines altogether in HER2-positive disease, and therefore we should endeavour to develop guidance to maximise the cardiac safety of the anthracycline–trastuzumab combination.
Clinical trials will often exclude patients who have a number of risk factors for cardiac disease. However, in practice, patients with some risk factors who would not have been included in clinical trials are offered new therapies in the clinic as standard. The PERSEPHONE trial, which ‘maps on to standard practice’, had the advantage that patients in the trial had the same cardiac risk profile as those receiving standard chemotherapy and 12 months’ trastuzumab. This has important implications for interpreting and applying the results of this cardiology substudy to routine patients in the clinic in terms of risk of cardiac toxicity.
Chapter 5 Health economic analysis and cost-effectiveness analysis
Introduction
An economic evaluation was conducted to assess the cost-effectiveness of using trastuzumab for 6 months compared with using it for 12 months in the treatment of patients with HER2-positive early breast cancer. Two types of economic evaluation were performed: (1) a within-trial analysis, in which cost-effectiveness was assessed 2 years after trastuzumab treatment commenced, using individual patient data from the trial; and (2) a decision-analytic modelling analysis, in which cost-effectiveness was estimated over a lifetime horizon using best-practice modelling methods. The primary evaluation adopted an NHS and Personal Social Services perspective. A secondary within-trial analysis was also conducted, adopting a societal perspective.
Methods
Within-trial analysis
Individual patient data from the PERSEPHONE trial (database lock 6 March 2019) were analysed to determine the costs and QALYs associated with the two trastuzumab treatment durations (6 months and 12 months). To boost recruitment, a protocol change was implemented to allow the individuals to be randomised into the trial at any point up to 6 months into their trastuzumab treatment. A landmark analysis from 6 months into trastuzumab treatment was therefore appropriate for the economic analysis as this represented the point at which the treatment pathways diverged between the arms. The analysis followed the intention-to-treat principle to calculate the outcomes and costs incurred by patients in the trial arms during the follow-up period of 18 months. QALYs were estimated using the patient-completed EuroQol-5 Dimensions, three-level version (EQ-5D-3L), questionnaire77 in combination with survival data. Information on resource use and treatments received was recorded in structured CRFs and patient-reported questionnaires, allowing costs to be estimated. In July 2009, a change to the health economics data collection was made (amended in the protocol). This change affected the number of data collected at baseline. These data were poorly completed up to that date and, following the change to the randomisation point, they were no longer needed for the health economic analysis and so were removed. Full details of the changes can be found in Appendix 1, Table 45. Cost-effectiveness is summarised as the incremental cost-effectiveness ratio (ICER) and incremental net health benefit (INB). All analyses were conducted using the statistical package R (The R Foundation for Statistical Computing, Vienna, Austria).
Quality-adjusted life-years
The primary measure of patient health benefit was the QALY. QALYs are a summary measure combining estimates of patient survival (life-years) and the associated quality of life (utility).
Patient health-related quality of life was derived from the EQ-5D-3L questionnaire77 that was filled in by patients at 0, 3 6, 9, 12, 18 and 24 months following the start of trastuzumab. Only the EQ-5D-3L data collected from 6 months onwards are included in this landmark analysis, although the data collected at 0 and 3 months are used in the multiple imputation (see Missing data). The EQ-5D-3L is recommended by NICE as a generic measure of health-related quality of life for cost-effectiveness analyses. 78 It comprises five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each domain consists of three levels: no problems, some problems and severe problems. This is a preference-based instrument whereby the values for each health state are elicited from the UK population using the time trade-off method. 79
Quality-adjusted life-years are then derived from the number of life-years weighted by the utility value during that time. 80 In this analysis, we implemented the area under the curve approach, which assumes a linear transition between each follow-up time point.
If a participant died during the trial, it was assumed that his or her utility score was 0 from the date of death until the end date of the trial and the transition to zero from the last non-zero score was linear. 81,82 Where EQ-5D-3L data were missing and it was known that the individual had either a local recurrence or a distant recurrence during that time period, data were imputed with average utility values identified in the literature for each of these health states. For a distant recurrence, an average utility of 0.69 was imputed. 83 For a local recurrence, an average utility of 0.78 was imputed. 83
The mean QALYs were derived by adjusting the baseline (6 months) utility using a linear regression model. This can account for any potential imbalance between the two arms, as randomisation may have not adequately addressed this issue. 84,85
Resource use and costs data
Resource use data were sourced from the trial CRFs and from patient-completed questionnaires returned at 6, 9, 12, 18 and 24 months. Partially completed patient questionnaires were supplemented by data available in the CRFs. When data were available in both, the reported use was compared between the two sources to account for any potential duplication.
The resource use data and costs can be classified into the following categories: community-based health and social care, including visits or contacts with GPs, district nurses, physiotherapists, occupational therapists, etc.; and hospital services, including outpatients, accident and emergency attendances due to SAEs and hospitalisation costs (inpatients). These data were collected from the patient questionnaires. Data on trastuzumab treatment including acquisition (endocrine therapies and trastuzumab), administration and monitoring (including cardiology assessment) were all collected via CRFs. In addition, details of surgical interventions undertaken during follow-up and the related costs were collected using the CRFs.
The analysis adopted a NHS and Personal Social Services perspective for the cost evaluation, in line with NICE recommendations. 78 All of the unit costs used in the analysis can be found in Table 13 and in Appendix 1, Tables 46–50. Costs were obtained from relevant sources, including the Unit Costs of Health and Social Care,86 NHS Reference Costs 2017–1887 and the British National Formulary (BNF). 88 Given the short time frame of the analysis, a discount rate was not applied to the costs and benefits. The costs used in the analysis were obtained for or inflated to the price year of 2017/18 and reported in Great British pounds.
| Cost (£) | Source | |
|---|---|---|
| 150 mg i.v. vial (Herceptin) | 407.40 | BNF 2018: NHS indicative price88 |
| First dose, i.v. administration | 252.36 | NHS Reference Costs 2017–18:87 deliver complex chemotherapy, including prolonged infusional treatment, at first attendance (OP, SB14Z) |
| Subsequent dose, i.v. administration | 95.24 | NHS Reference Costs 2017–18:87 deliver simple parenteral chemotherapy at first attendance (other, SB12Z) |
| Subcutaneous dose (Herceptin) of 600 mg/5 g | 1222.20 | BNF 2018: NHS indicative price88 |
| Subcutaneous dose administration | 18.45 | Administration cost of €21.07 taken from O’Brien et al.89 and converted from euros to Great British pounds |
Trastuzumab acquisition and administration costs
During the trial, a subcutaneous formulation of trastuzumab became available, allowing trastuzumab to be delivered via a handheld syringe or single-use injection instead of as an intravenous infusion. The difference in cost between the delivery routes was taken into account, in addition to the difference in cost of administration (see Table 13).
For those receiving trastuzumab intravenously, the total dose administered was obtained from the dose used (8 mg for the first and 6 mg for the subsequent doses) multiplied by the patient weight. The difference in time to administer the first dose (which typically takes longer) versus subsequent doses was accounted for. The acquisition cost assigned to trastuzumab is £407.40 for a 150-mg intravenous vial. Assuming vials being shared, the cost of 1 mg was £407.40/150 mg. The cost of administration assigned was £252.36 for the first dose and £95.24 for subsequent doses.
For those receiving trastuzumab subcutaneously, the NHS cost of a fixed dose of 600 mg/5 g was £1222.20.
Cardiology assessment costs
The main tests used for monitoring for cardiac complications as a result of trastuzumab were the MUGA scan and ECHO. However, a number of patients also had other types of cardiology tests reported on the CRF as free text, for example electrocardiography, magnetic resonance imaging (MRI), X-ray and cardiologist opinion. The costs associated with each of these tests were obtained and added to the cost of an outpatient visit (see Appendix 1, Table 46).
Cardiac treatment costs
Cardiac medication prescribed during the trial was also recorded on CRFs as free text, in addition to the start date of treatment and (if relevant) the stop date. The duration of treatment was calculated for each patient. If no stop date was reported, it was assumed that the treatment was taken permanently. The cost of the cardiac medication was obtained by multiplying the cost of the daily dose of the medication (see Appendix 1,Table 47) by the number of days.
Endocrine therapies
Costs associated with hormone therapies typically prescribed to breast cancer patients, including anastrazole, exemestane, letrozole, goserelin and tamoxifen, were included in the analysis. The duration of treatment was obtained from the trial CRFs using the start and stop dates; if the latter was not reported, the treatment was assumed to be taken permanently. The cost of endocrine therapy was obtained by multiplying the cost of the daily dose of the medication (see Appendix 1, Table 48) by the number of days for which the medication was taken.
It was assumed that the bisphosphonate given in the trial as osteoporosis treatment was alendronate; its cost was derived following the same approach.
Surgery costs
Details on surgical interventions related to tumour excision, biopsy and ovarian suppression (oophorectomy) were captured during the follow-up. Surgery data coded in the trial CRF included delayed reconstruction, mastectomy of treated breast, mastectomy of treated breast with reconstruction, mastectomy of contralateral breast, mastectomy of contralateral breast with reconstruction and oophorectomy. The CRF allowed for other surgical interventions relating to the patient’s primary breast cancer to be recorded as free text. These were coded in line with the cost codes reported in NHS Reference Costs 2017–18. 87 Where cost codes were unavailable, surgical interventions were categorised and costed as either minor or intermediate breast surgical procedures, following discussion with clinicians in the team (see Appendix 1, Table 49).
Hospital services and community-based health and social care
Resource use information related to community-based health and social care, including visits or contacts with GPs, district nurses, physiotherapists, occupational therapists and so on, and hospital services, including outpatients, accident and emergency attendances due to SAEs, was collected from patient questionnaires.
Inpatient data were also obtained primarily from the patient questionnaires; if these data were missing, they were supplemented by the hospitalisation data reported in the treatment forms. The data reported in the SAEs report did not include accurate admission and discharge dates and were therefore not used in this analysis.
The unit costs assigned to this resource use can be found in Appendix 1, Table 50.
Missing data
Trastuzumab treatment
The total trastuzumab dose administered intravenously was based on the number of doses and patient’s weight as recorded in the CRFs. Where the weight of the patient was missing but the dose number was available, the weight was imputed with the previous measurement, or, if it was the first dose, the subsequent measurement. Where the dose number and weight were missing, the average cost for that dose across all individuals was imputed.
Cardiac treatment
When the start date of the cardiac treatment was missing, the date of the first abnormal result from cardiology assessment was imputed. When dose information was missing for particular treatments, the most commonly given dose of that medication was imputed. When the stop date of the medication was missing, the medication was assumed to be taken until the end of follow-up and costed accordingly.
Missing data in the patient questionnaires
It was expected that some of the patient questionnaires would not be returned and that some of the questionnaires would not be complete. Multiple imputation was carried out using the ‘mice’ package in R version 3.6.2 (The R Foundation for Statistical Computing, Vienna, Austria), which implements a chained equations approach. Imputation was carried out for each arm of the trial separately, as recommended. 90 Costs were imputed for each patient at a total level for each subcategory (e.g. community health costs or hospital services) at each time point. Utility scores were imputed at each time point.
Cost categories that did not have any missing data (e.g. trastuzumab treatment, surgical costs) were used as predictors in the imputation model, in addition to other patient questionnaire data, DFS and OS, and data collected in the baseline questionnaire (ER status, chemotherapy type, chemotherapy timing, trastuzumab timing). Patient questionnaire data, including utility scores collected prior to the landmark point (i.e. at 0 and 3 months), were also used as predictors. Whether an individual experienced a local recurrence, a distant recurrence or a new primary during the follow-up period was also added as an additional predictor.
A hierarchical multiple imputation approach was attempted to account for correlations between cost categories within individuals at different time points; however, many of the missing data applied to all time points for an individual and, therefore, data were insufficient to support this type of analysis.
Secondary analyses
A secondary analysis was carried out for the within-trial analysis, adopting a societal perspective.
Patients were asked in the patient questionnaires whether or not they had lost earnings because of their diagnosis and, if so, how much. They were also asked whether or not they had had to meet any major one-off expenses of ≥ £50. As a secondary analysis, these data were used to compare out-of-pocket patient expenses across the two arms. Where individuals reported major one-off expenses, these were costed at £50 each (the minimum amount that could have been spent). This was combined with the amount reported in terms of lost earnings. Where lost earnings were reported but the actual amount was not specified, the average for each arm was imputed.
Uncertainty analyses
To calculate the potential impact of sampling uncertainty in the within-trial analysis, bootstrapping with replacement from the full trial data set (including imputed values) using 10,000 simulations was carried out. For each permutation of the data set, the total cost, QALY, ICER and incremental net benefit were calculated. For each of these summary statistics, the median and 95% CIs (based on percentiles) were calculated based on the 10,000 estimates. The incremental QALYs and costs for each simulation were also plotted on a cost-effectiveness plane to provide a visual representation of the impact that uncertainty has on the overall ICER result. These simulations were also used to calculate the probability that 6 months’ trastuzumab treatment is more cost-effective than 12 months’ treatment.
Sensitivity analyses
It was anticipated that the majority of any cost difference between the two arms would result from the difference in trastuzumab treatment costs. At the start of the trial, standard practice was to deliver trastuzumab intravenously. Part-way through the trial, a subcutaneous version of trastuzumab became available and, although the cost of this is higher, the savings in administrative time makes it a cost-effective alternative. Since the end of the trial, the patent for intravenous trastuzumab has expired and a significantly cheaper biosimilar intravenous drug has been released,88 and therefore many hospitals have shifted back to delivering trastuzumab intravenously. In a sensitivity analysis, we will explore the impact on cost and ICERs of the two drug formats: (1) Herceptin, delivered subcutaneously; and (2) biosimilar trastuzumab, delivered intravenously [e.g. Herzuma® (Napp Pharmaceuticals Ltd, Cambridge, UK), Kanjinti (Amgen Ltd, Thousand Oaks, CA, USA), Ontruzant (Merck Sharp & Dohme Ltd, Hoddesdon, UK), Trazimera™ (Pfizer Inc., New York, NY, USA)].
In addition, in the main analysis we assumed that vials were shared for trastuzumab delivered intravenously, as is standard practice at many sites. However, practice varies by site and therefore we explored the impact of this assumption by calculating the cost if vials were not shared.
Lifetime decision model analysis
Some of the long-term health benefits and costs associated with the two trastuzumab treatment durations occur beyond the PERSEPHONE trial follow-up period, such as the costs associated with the treatment of recurrence and secondary primary cancers. A de novo decision-analytic model was therefore developed to extrapolate the costs and health benefits of the two treatment durations over a lifetime horizon. In line with the within-trial analysis, the base case adopts an NHS and social care perspective. Costs and QALYs beyond the first year were discounted at an annual rate of 3.5%. The statistical software R, version 3.6 (The R Foundation for Statistical Computing, Vienna, Austria), was used to build the model.
Modelling approach and structure
A Markov model was built to estimate the total QALYs and costs per patient in each treatment arm. Patients with HER2-positive breast cancer are at long-term risk of relapsing or dying and therefore the model captures the time to these events and the associated quality of life and costs.
The structure of the model was developed in discussion with clinical experts and health economists, and it was adapted from the structure of a previously published model. 91 A 3-month model cycle was selected in line with this model. Patients enter the model 6 months into their trastuzumab treatment. Cost and QALY data were elicited directly from patients and from CRFs for the subsequent 18 months, and these data were used to inform the first six cycles of the model (summarised in the within-trial analysis). During this period, the costs associated with symptomatic or asymptomatic reversible cardiac toxicity were captured. As each state in a Markov model has no memory of ’time in state’, there is also the assumption that each cycle in a recurrence state incurs identical transition probabilities. Where necessary, this was overcome by building in tunnel states that allow transition probabilities, costs and utility parameters to change depending on the number of cycles in that state.
After the treatment period, individuals could move to either a disease-free (follow-up) state or a recurrence state. As data from the PERSEPHONE trial are the primary source of data for the model parameters, the recurrence states and their definitions align with those used in the trial:
-
locoregional (ipsilateral breast/chest wall, axillary and ipsilateral supraclavicular nodes)
-
distant (excluding ipsilateral supraclavicular nodes)
-
second primary (including contralateral malignant breast disease).
There is evidence that the cost of locoregional recurrence is high in the first year after relapse and then falls to minimal levels. 92 For this reason, we incorporated a 1-year tunnel state for non-distant relapses, after which patients move into a ‘disease free after locoregional recurrence state’, provided that no distant relapse has occurred. This also allowed us to build in an ongoing higher risk of developing a distant recurrence for those who had experienced a locoregional recurrence. Those who had a second primary cancer stayed in that state but could move to the locoregional, distant or death state. If individuals developed a distant recurrence, they could stay in the same state or die, but they could not transition to any of the other states. Individuals in the death state could not transition to any of the other states, as death is an absorbing state. In addition, individuals in all states were at risk of dying as a result of breast cancer, chronic heart failure or unrelated causes. The model structure is depicted in Figure 16.
FIGURE 16.
Model structure. BC, breast cancer.
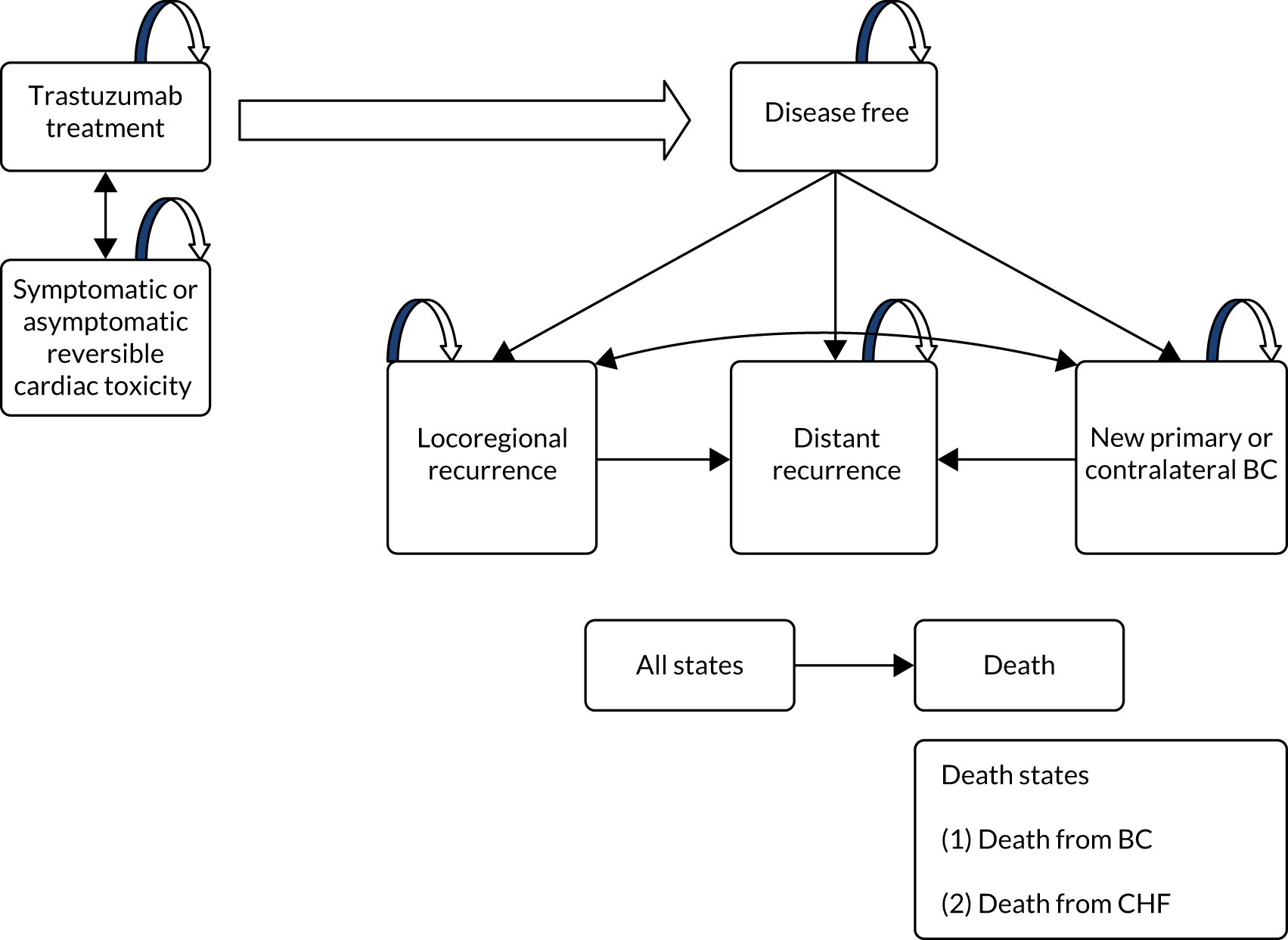
The model was run for the lifetime of the cohort (assuming that no one survived beyond 100 years old), with a start age of 56 years (the median age at the landmark date in the trial). A half-cycle correction was implemented in the model.
Cardiac toxicity
A key side effect of trastuzumab is cardiotoxicity. 93 Throughout trastuzumab treatment, individuals in the PERSEPHONE trial were monitored for signs of cardiotoxicity based on their LVEF. Data on cause of death, including death as a result of chronic heart failure, were collected on a specific CRF that was filled in when required.
Beyond the trial follow-up period, we looked to the literature for evidence of a long-term impact of trastuzumab treatment on cardiovascular outcomes. The HERA trial collected long-term data (up to 11 years) on cardiotoxicity. 13 A primary cardiac end point was defined as NYHA class III or IV toxicity (confirmed by a cardiologist), and a clinically significant LVEF drop of at least 10 percentage points from baseline to an absolute LVEF below 50%, or cardiac death. A secondary cardiac end point was defined as asymptomatic (NYHA class I) or mildly symptomatic (NYHA class II), with a clinically significant LVEF drop of at least 10 percentage points from baseline and to an absolute LVEF below 50%, confirmed by repeat assessment.
After 2 years post randomisation, there was no evidence that trastuzumab had a significant impact on the number of primary cardiac end-point events. After between 4 and 5 years, there was also no evidence that trastuzumab had a significant impact on either primary or secondary cardiac end points. Therefore, in the model, the rates of symptomatic and asymptomatic reversible cardiotoxicity during the trial follow-up period are incorporated, but it is assumed that beyond this point the rate of death as a result of chronic heart failure follows UK population national statistics. 94
Transition probabilities
The probability of being in each health state outlined in Figure 16 was calculated based on three key sources of information: (1) data from the PERSEPHONE trial, (2) data from the HERA trial13 and (3) published UK statistics on mortality rates95 and breast cancer rates96 (see Appendix 1, Table 51). Up to 5.1 years (the median length of follow-up in the PERSEPHONE landmark analysis, i.e. from 6 months into trastuzumab treatment), the probability of being in each health state was based entirely on data from the PERSEPHONE trial. Three-monthly transition probabilities (along with 95% CIs) for each trial arm were estimated using the ‘msm’ package in R, which allows multistate modelling, accounting for the competing risk of being in each health state. 97
Parametric survival analysis was used to extrapolate DFS (which is a composite of locoregional and distant recurrence) observed in the PERSEPHONE trial using the R package ‘flexsurv’. Survival analysis was conducted by arm to relax the proportional hazards assumption. A range of distributions (exponential, Weibull, Gompertz, log-normal and log-logistic) were fitted and compared visually and based on the Akaike information criterion and the Bayesian information criterion. Plots of the extrapolated curves and a table reporting the Bayesian information criterion and Akaike information criterion values can be found in Appendix 2, Figure 36, and Appendix 1, Table 52. The log-normal distribution was the better fit, which makes clinical sense as the peak rate of disease relapse is between 2 and 5 years in the majority of landmark trials in early breast cancer, including the HERA trial. 13 The derived transition probabilities were compared with the numbers of DFS patients at risk for years 5–11 in the HER2-positive subgroup analysis reported for the HERA trial. 13 The proportions of locoregional recurrences and distant recurrences were back-calculated from the DFS transition probabilities by carrying forward the relative proportions from the PERSEPHONE trial period. The probability of a second primary was based on UK national statistics, inflated by a relative risk identified in the literature to account for the increased risk among treated individuals with breast cancer. Once in a recurrence or a secondary primary cancer state, the same transition probabilities observed in the trial were applied to capture movements from one recurrent state to another or transition to death.
After the trial follow-up period, the risk of death from chronic heart failure was based on UK national statistics. The risk of death from other causes was calculated by selecting the remaining individuals (i.e. those who were transitioning to another state or dying from breast cancer or CHF) and multiplying it by UK statistics on background mortality.
Utilities
Health state utility values were identified through a targeted literature review (see Appendix 1, Table 53). The study by Seferina et al. 98 reported utility values for the disease-free state, local recurrence and distant recurrence. In this study, health-related quality of life was based on a cross-sectional survey among breast cancer patients in four Dutch medical sites, using the EQ-5D-3L questionnaire. Utility values were calculated using the UK tariff. 79 The utility value for patients experiencing a new primary or contralateral cancer was based on an alternative study. 83 The utility associated with death was assumed to be zero.
Costs
The assessment of resource use and costs was performed adopting the NHS and social services perspective. Unit costs were reported for 2018 values, where available. Cost estimations based on values from other years were inflated to 2018 values using the Bank of England inflation calculator, which is based on the Office for National Statistics’ composite price index. 99 An annual 3.5% discount rate (calculated monthly) was applied.
Resources used in the disease-free state corresponded to follow-up imaging (i.e. mammography, and clinical follow-up), as indicated by NICE guidelines NG101. 100 The unit costs were retrieved from the literature101 and based on NHS Reference Costs 2017–18. 87 First-year costs as well as annual costs associated with having a local recurrence were taken from a preliminary study of an Optimal Personalised Treatment of early breast cancer (OPTIMA). 102,103 Annual costs associated with having a distant recurrence were taken from the same study. First-year costs and annual costs associated with developing a new primary or contralateral breast cancer were taken from a previous economic evaluation. 91 The cost of a new primary or contralateral breast cancer was estimated by calculating the expected cost for a 5-year period (based on a higher first-year cost and lower subsequent year costs) and converting this to a 3-monthly cost.
Costs of terminal care were applied as a one-off cost to all patients who died from breast cancer. These costs corresponded to costs of care given to patients, in a hospital, in a hospice or at home, in the last 2 weeks before dying. These costs were reported in the single technology appraisal TA563 submitted to NICE. 104 The proportion of patients receiving hospital, hospice or home care was taken from NICE clinical guideline CG81. 105 The cost of a heart failure event was applied to all those who died from chronic heart failure, incurred at the point of death.
Costs falling outside the health-care perspective were not included in the analysis.
Uncertainty analyses
Distributions around all parameters feeding into the lifetime model were modelled (see Appendix 1, Tables 51, 53 and 54). The impact of uncertainty in the model parameters on cost-effectiveness summary statistics was calculated in a probabilistic sensitivity analysis based on 10,000 simulations.
Cost-effectiveness analysis
The relative cost-effectiveness of using trastuzumab for 6 compared with 12 months was assessed using standard decision rules, estimating the pairwise ICER. 106 The ICER examines the additional cost that the use of trastuzumab for 6 months incurs over its use for 12 months and compares this with the additional benefits. The ICER estimate represents the additional cost required to generate one additional unit of health outcome (QALY), which provides the basis for establishing whether or not the new approach appears to provide good value for money to the NHS. Guidance from NICE suggests that an incremental cost per additional QALY of around £20,000–30,000 is considered to represent an appropriate threshold to establish value for money in the NICE Guide to the Methods of Technology Appraisal 2013. 78
To provide monetary value for the effectiveness, net monetary benefits were estimated by assigning a monetary value for the threshold λ, where λ = £20,000 was used:
The analysis followed the intention-to-treat principle, so that patient data were analysed based on the treatment group to which patients were randomised. As the two trial arms were expected to differ after 6 months of trastuzumab treatment onwards, the economic analysis was performed on data reported from that point, consistent with the landmark analysis. The baseline EQ-5D-3L values were obtained from the patient questionnaires returned at 6 months of trastuzumab treatment.
The point estimate of the ICER was obtained from the ratio of the difference between the adjusted mean QALYs in the two trial arms to the difference in the mean costs.
Validation
Internal validation was conducted for both the within-trial analysis and the decision-analytic modelling. Two health economists worked on different parts of the R code necessary to conduct the analyses and verified the equations and parameters used in the analyses relative to their sources. Furthermore, each one explained their part of the code to the other to look for possible computational errors. In the end, all sections of the code were verified by both health economists.
In the discussion, we also compare the results of the present analysis with those of other relevant published studies.
Subgroup analyses
Across both the within-trial analysis and the lifetime decision model, subgroup analyses were conducted for the following groups, in line with the statistical analysis of the main trial:
-
ER status – negative versus positive
-
chemotherapy type – (1) anthracycline-based (no taxanes), (2) taxanes and anthracyclines or (3) taxane-based (no anthracyclines)
-
chemotherapy timing – adjuvant versus neoadjuvant
-
trastuzumab timing – concurrent versus sequential (with respect to chemotherapy).
For the within-trial subgroup analyses, each imputed iteration of the trial data was filtered to those in each subgroup, and a probabilistic sensitivity analysis via bootstrap simulation with replacement (10,000 simulations) was conducted.
For the lifetime model subgroup analyses, the ‘msm’ package in R97 was used to calculate transition probability matrices for each subgroup. Subgroup-specific DFS curves were then estimated and the proportion of local and distant recurrences was calculated using the same method described for the main model analysis (see Transition probabilities).
Results
Within-trial results
Costs
Individual resource use was gathered from CRFs and patient questionnaires. Around one-third of the patient questionnaires were missing at each time point throughout the trial, with the proportion increasing slightly towards the end of the follow-up period (Table 14). For the base-case cost analysis, these missing data were imputed using the methods described previously.
| Follow-up time point (months) | Treatment duration | |||||
|---|---|---|---|---|---|---|
| 12 months | 6 months | |||||
| Received (n) | Expected (n) | Missing (%) | Received (n) | Expected (n) | Missing (%) | |
| 6 | 1439 | 2007 | 568 (28) | 1403 | 2001 | 598 (30) |
| 9 | 1399 | 2007 | 608 (30) | 1347 | 2000 | 653 (33) |
| 12 | 1418 | 2004 | 586 (29) | 1397 | 1997 | 600 (30) |
| 18 | 1303 | 1997 | 694 (35) | 1303 | 1986 | 683 (34) |
| 24 | 1242 | 1977 | 735 (37) | 1189 | 1962 | 773 (39) |
The within-trial costs from the landmark point to the end of follow-up included in the base-case analysis (NHS and Personal Social Services perspective) are reported in Table 15. The total individual costs over the 18-month period were significantly higher in the 12-month trastuzumab arm than in the 6-month trastuzumab arm (mean: £15,298 vs. £5762; p < 0.01 based on Wilcoxon signed-rank test). This cost difference is driven primarily by the reduction in trastuzumab treatment cost (mean: £10,060 vs. £1008).
| Cost category | Trastuzumab duration | Mean difference (£) | p-value (Wilcoxon signed-rank test) | |||||
|---|---|---|---|---|---|---|---|---|
| 12 months (£) | 6 months (£) | |||||||
| Mean | Median | SD | Mean | Median | SD | |||
| Community-based health care | 291.65 | 185.00 | 384.07 | 266.68 | 176.40 | 358.55 | –24.98 | 0.06 |
| Community-based social care | 135.54 | 0.00 | 630.75 | 173.44 | 0.00 | 946.48 | 37.90 | 0.91 |
| Hospital services including inpatient stays | 1418.38 | 804.00 | 1970.13 | 1021.24 | 445.36 | 1582.87 | –397.14 | < 0.001 |
| Trastuzumab treatment | 10,059.73 | 11,087.79 | 4686.66 | 1008.15 | 0 | 2693.31 | –9051.58 | < 0.001 |
| Surgical interventions | 448.12 | 0.00 | 1760.46 | 515.34 | 0.00 | 1812.73 | 67.22 | 0.16 |
| Cardiology assessment and medication | 590.98 | 507.40 | 234.43 | 475.34 | 507.40 | 234.14 | –115.65 | < 0.001 |
| Endocrine therapies | 571.59 | 44.56 | 729.48 | 551.78 | 44.48 | 718.63 | –19.81 | 0.57 |
| Total cost | 15,297.61 | 15,060.00 | 6332.38 | 5761.88 | 4139.25 | 4927.89 | –9535.74 | < 0.001 |
There were some other cost-saving implications of shortening the duration of trastuzumab: lower costs associated with hospital services (mean: £1418 vs. £1021) and lower costs associated with cardiology assessment and medication (mean: £591 vs. £475).
Utilities
Table 16 reports the proportion of missing EQ-5D-3L data at each time point in the trial follow-up. There was no notable difference in the proportion missing between trial arms. The EQ-5D-3L data are presented for the time points included in this analysis (6, 12, 18 and 24 months). We also report the data collected at 0 and 3 months, as these variables were used in the multiple imputation model to predict individual-specific utility scores from 6 months onwards.
| Time point | 12-month patients (N = 2007), n (%) | 6-month patients (N = 2001), n (%) | Total (N = 4008), n (%) |
|---|---|---|---|
| 0 months | 985 (49.1) | 989 (49.4) | 1974 (49.3) |
| 3 months | 764 (38.1) | 710 (35.5) | 1474 (36.8) |
| 6 months (baseline) | 625 (31.1) | 645 (32.2) | 1270 (31.7) |
| 9 months | 655 (32.6) | 702 (35.1) | 1357 (33.9) |
| 12 months | 641 (31.9) | 666 (33.3) | 1307 (32.6) |
| 18 months | 764 (38.1) | 755 (37.7) | 1519 (37.9) |
| 24 months | 811 (40.4) | 864 (43.2) | 1675 (41.8) |
Table 17 provides a summary of the average utilities at each time-point in each arm after multiple imputation for missing data. The SDs are relatively large, representing the uncertainty in these estimates due to the multiple imputation. Individual-reported utilities were similar at baseline. There was evidence of a slight increase in utility for the 6-month arm at 9, 12 and 18 months’ follow-up.
| Time point (months) | Statistic | Treatment duration | p-value (base case; Wilcoxon signed-rank test) | |
|---|---|---|---|---|
| 12 months | 6 months | |||
| 6 (baseline) | Mean | 0.75 | 0.75 | 0.56 |
| Median | 0.76 | 0.76 | ||
| SD | 0.22 | 0.23 | ||
| 9 | Mean | 0.76 | 0.76 | 0.73 |
| Median | 0.80 | 0.80 | ||
| SD | 0.22 | 0.21 | ||
| 12 | Mean | 0.76 | 0.77 | 0.03 |
| Median | 0.80 | 0.80 | ||
| SD | 0.23 | 0.23 | ||
| 18 | Mean | 0.76 | 0.77 | 0.123 |
| Median | 0.80 | 0.80 | ||
| SD | 0.24 | 0.24 | ||
| 24 | Mean | 0.77 | 0.76 | 0.71 |
| Median | 0.80 | 0.80 | ||
| SD | 0.24 | 0.25 | ||
Cost-effectiveness
The within-trial cost-effectiveness results are presented in Table 18 (deterministic results) and Table 19 (probabilistic results). During the 18-month follow-up period from the landmark point (6 months), 6 months of trastuzumab was associated with an estimated incremental cost saving of £9537 (95% CI £9183 to £9890) and an incremental effect of +0.003 QALYs (95% CI −0.015 to 0.021 QALYs) compared with 12 months of trastuzumab. Six months of trastuzumab is, therefore, cost-effective and dominates the longer duration of trastuzumab treatment (INB 0.48, 95% CI 0.45 to 0.51).
| Treatment duration (months) | Total cost (£) | Total QALY | Life-years | Incremental cost (£) | Incremental QALY | ICER (£) | Net benefit (QALYs) | Incremental net benefit (QALYs) |
|---|---|---|---|---|---|---|---|---|
| 12 | 15,298 | 1.14 | 1.472 | – | – | – | 0.38 | – |
| 6 | 5762 | 1.15 | 1.475 | –9536 | 0.003 | Dominant | 0.86 | 0.48 |
| Treatment duration (months) | Total cost, £ (95% CI) | Total QALY (95% CI) | Life-years (95% CI) | Incremental cost, £ (95% CI) | Incremental QALY (95% CI) | ICER (£) | Net benefit (95% CI) | Incremental net benefit (95% CI) |
|---|---|---|---|---|---|---|---|---|
| 12 | 15,298 (15,023 to 15,579) | 1.14 (1.13 to 1.16) | 1.471 (1.464 to 1.478) | – | – | – | 0.38 (0.36 to 0.40) | – |
| 6 | 5762 (5548 to 5981) | 1.15 (1.13 to 1.16) | 1.475 (1.468 to 1.481) | –9537 (–9890 to –9183) | 0.003 (–0.015 to 0.021) | Dominant | 0.86 (0.84 to 0.88) | 0.48 (0.45 to 0.51) |
Figure 17 shows the within-trial probabilistic cost-effectiveness analysis on the cost-effectiveness plane. Each point (light blue) is one of the 10,000 simulated results and the triangle is the average of these points. The spread around this point illustrates the estimated uncertainty around the average cost-effectiveness result. The diagonal line represents a willingness-to-pay threshold of £20,000 per QALY. All of the points fall below this line, indicating 100% probability that 6 months of trastuzumab is more cost-effective than 12 months. There is 100% probability that 6 months of trastuzumab is cost saving compared with 12 months; however, the probability that 6 months is more effective in terms of QALYs is 63%.
FIGURE 17.
Within-trial probabilistic analysis on the cost-effectiveness plane.
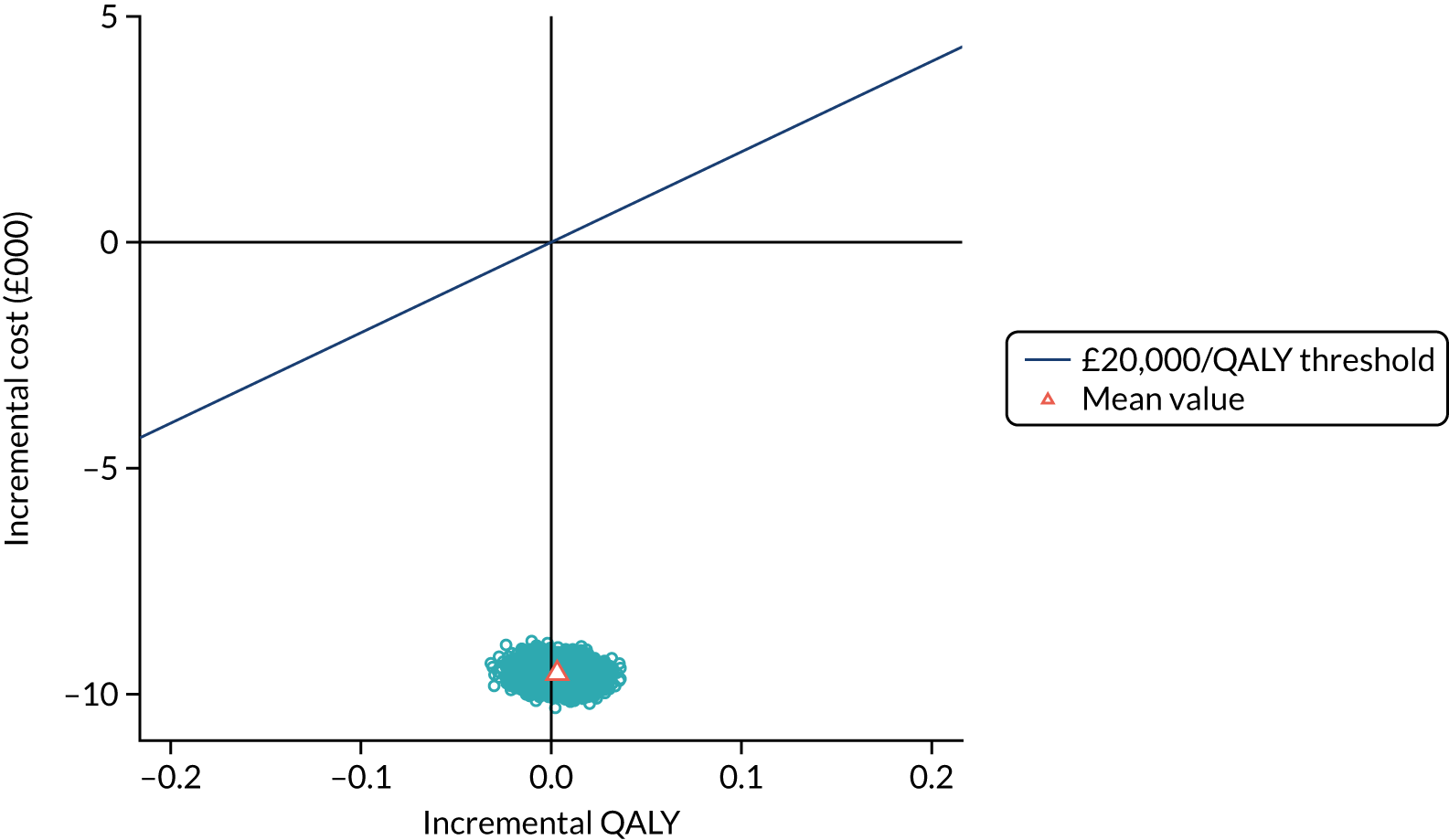
Subgroup analyses
The results of the within-trial subgroup analyses are reported in Appendix 1, Table 55. Across all of the subgroups, there are no notable differences in the main results; the incremental QALY is consistently close to 0 across all groups and 6 months of trastuzumab is consistently cost saving compared with 12 months of trastuzumab.
Sensitivity analyses
Sensitivity analysis 1: societal cost analysis
Costs to the patient were estimated based on the patient-reported loss in earnings and major expenses incurred (Table 20). There was no evidence of a significant difference in costs to the patient between the trial arms, although the mean total cost to the patient was slightly higher in the 12-month arm (£3026 vs. £2048). The total patient costs were combined with the NHS and Personal Social Services costs to provide an overall estimate of the societal cost in each arm.
| Cost category | Trastuzumab duration | Mean difference (£) | p-value (Wilcoxon signed-rank test) | |||||
|---|---|---|---|---|---|---|---|---|
| 12 months (£) | 6 months (£) | |||||||
| Mean | Median | SD | Mean | Median | SD | |||
| Lost earnings | 3008.03 | 0.000 | 8722.83 | 2030.94 | 0.000 | 6900.06 | –977.09 | 0.65 |
| Major expenses | 17.07 | 0.00 | 40.74 | 17.54 | 0.00 | 41.27 | –0.47 | 0.68 |
| Total cost to patient | 3025.57 | 0.00 | 8730.62 | 2048.00 | 0.00 | 6907.32 | –977.56 | 0.44 |
| Total cost to NHS and patient | 18,323.18 | 15,897.79 | 11,416.77 | 7809.88 | 4902.65 | 8984.75 | –10,513.30 | < 0.001 |
Sensitivity analysis 2: comparing trastuzumab drug costs
Given the recent changes to the cost of trastuzumab, we carried out a sensitivity analysis to explore the implications of these changes on the cost of trastuzumab for 6 months compared with 12 months. During the trial, a total of 52,765 doses of trastuzumab were given; 42,044 (80%) doses were administered intravenously and the remaining doses were administered subcutaneously (n = 10,721, 20%). For the within-trial analysis, the cost of intravenous trastuzumab was based on the price of Herceptin, as this was the only intravenous option at the time. The patent for intravenous trastuzumab has since expired, and a number of cheaper biosimilar alternatives are now on the market.
The following analysis compares the cost of trastuzumab if everyone is treated with (1) subcutaneous Herceptin, (2) intravenous Herceptin or (3) biosimilar intravenous trastuzumab.
The results indicate that there remains a significant difference in trastuzumab treatment cost between the arms across all of the different pricing options (Table 21). The average overall cost for the intravenous biosimilar options is lowest, followed by subcutaneous Herceptin.
| Sensitivity analysis | Trastuzumab duration | Mean difference (£) | p-value (Wilcoxon signed-rank test) | |||||
|---|---|---|---|---|---|---|---|---|
| 12 months (£) | 6 months (£) | |||||||
| Mean | Median | SD | Mean | Median | SD | |||
| Within-trial analysis | 10,059.73 | 11,087.79 | 4686.66 | 1008.15 | 0.00 | 2693.31 | –9051.58 | < 0.001 |
| Sensitivity analysis 2: comparing drug costs | ||||||||
| Subcutaneous Herceptin | 9628.48 | 11,165.85 | 4116.19 | 956.06 | 0.00 | 2506.87 | –8672.42 | < 0.01 |
| i.v. Herceptin | 10,188.48 | 11,051.94 | 4756.50 | 1025.23 | 0.00 | 2748.28 | –9163.25 | < 0.01 |
| i.v. biosimilar | 9229.41 | 10,015.94 | 4303.39 | 928.850 | 0.00 | 2488.87 | –8300.56 | < 0.001 |
| Sensitivity analysis 3: impact of vial-sharing assumption | ||||||||
| No vial sharing | 11,263.60 | 11,856.96 | 5334.86 | 1119.80 | 0.00 | 2972.26 | –10,143.80 | < 0.001 |
Sensitivity analysis 3: exploring the impact of the vial-sharing assumption
In the base-case analysis, we assumed that vials were shared when trastuzumab was delivered intravenously, which is standard practice in many sites with a large number of treatments as it minimises wastage and saves costs. However, this may not be standard practice across all sites, and therefore we explored the impact on cost if vials were not shared (see Table 21). The overall cost of trastuzumab treatment increases, in addition to the cost saving between the two arms.
Lifetime model results
Cost-effectiveness results
The results of the lifetime model base-case analysis are presented in Table 22. Compared with 12 months of trastuzumab, reducing treatment duration to 6 months is estimated to produce a lifetime cost saving of £9316 and a slight reduction of 0.01 QALYs per individual. Reducing the length of treatment of trastuzumab therefore has a negligible negative impact on QALYs and is significantly cost saving, with an expected INB of 0.46 QALYs. There is notable uncertainty around this result, however, with a wide 95% CI around this mean value (95% CI –2.21 to 1.98 QALYs).
| Treatment duration (months) | Total cost (95% CI) | Total QALY (95% CI) | Life-years (95% CI) | Incremental cost (95% CI) | Incremental QALY (95% CI) | ICER | Net benefit (95% CI) | Incremental net benefit (95% CI) |
|---|---|---|---|---|---|---|---|---|
| 12 | 25,340 (21,660 to 30,702) | 11.13 (10.35 to 11.85) | 14.018 (13.23 to 14.66) | – | – | – | 9.86 (8.91 to 10.70) | – |
| 6 | 16,024 (10,371 to 28,569) | 11.12 (9.09 to 12.27) | 14.017 (11.57 to 15.14) | –9316 (–16,485 to 2907) | –0.008 (–2.09 to 1.19) | 9016 | 10.32 (7.69 to 11.69) | 0.46 (–2.21 to 1.98) |
The lifetime model base-case analysis results are presented in Figure 18 on the cost-effectiveness plane. The triangle point is the mean incremental cost and QALYs across all of the simulations. Each of the points on the graph represent one of the 10,000 simulated model results. The spread of these points therefore illustrates the uncertainty around the average result. The diagonal line represents the NICE willingness-to-pay threshold of £20,000 per QALY. Points that fall beneath this line are deemed cost-effective; the spread of points above and below the line shows the uncertainty around the lifetime cost-effectiveness of reducing the duration of trastuzumab to 6 months. This uncertainty is driven primarily by uncertainty in the estimated effectiveness of 6 months’ trastuzumab: the probability that reduced treatment duration is cost saving is 95%, whereas the probability that 6 months’ trastuzumab is as effective as or more effective than 12 months’ is 58%.
FIGURE 18.
Lifetime decision model probabilistic sensitivity analysis on the cost-effectiveness plane.
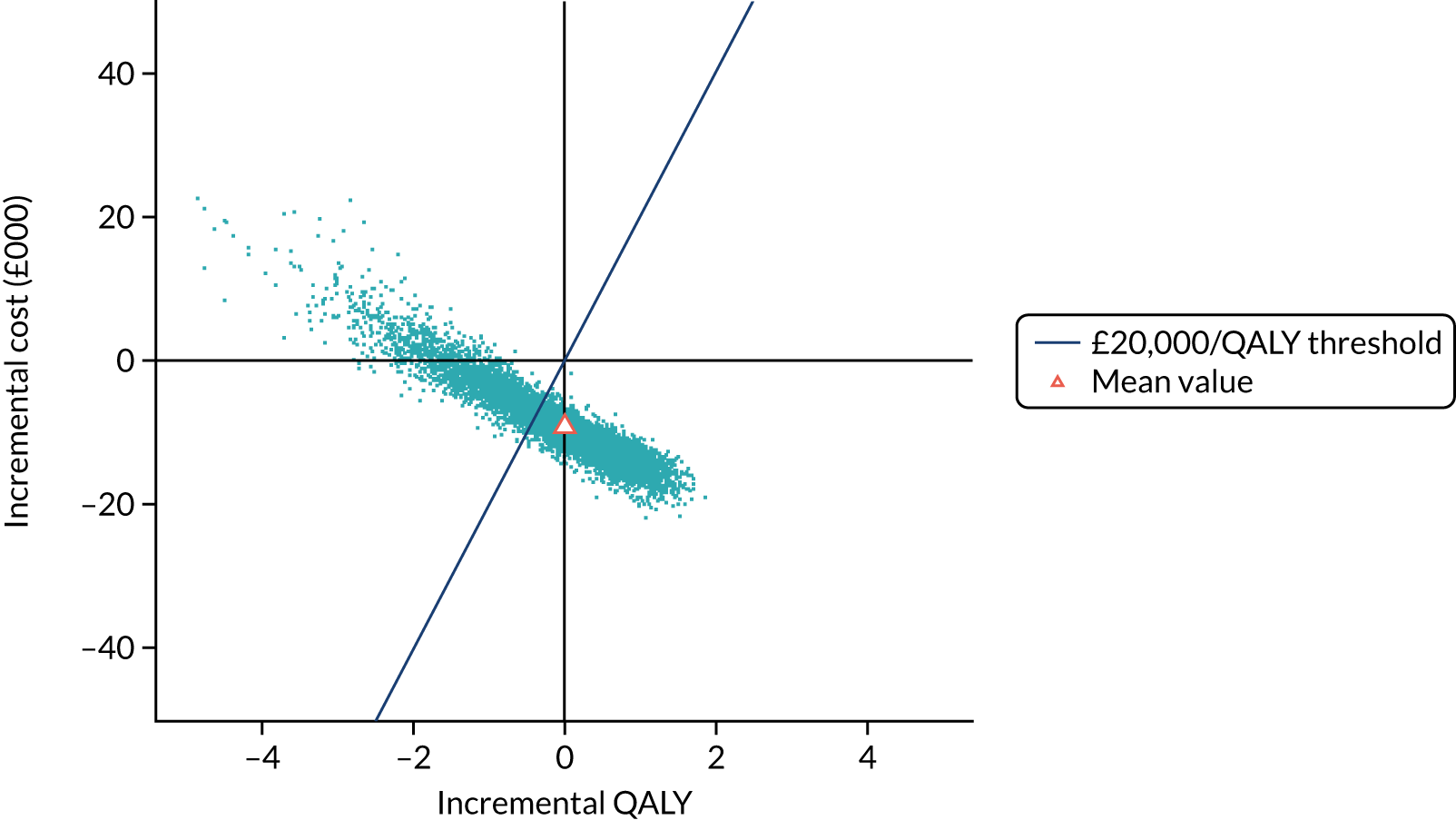
The cost-effectiveness acceptability curve (Figure 19) shows the probability that each treatment duration is the most cost-effective alternative (i.e. has the highest expected net benefit) across varying willingness to pay per QALY thresholds. At a threshold of £20,000, 6 months of trastuzumab has a 73% probability of being cost-effective compared with 12 months of trastuzumab, dropping to 66% at a £50,000 willingness-to-pay threshold and remaining above 61% at a £150,000 threshold.
FIGURE 19.
Lifetime decision model cost-effectiveness acceptability curve.
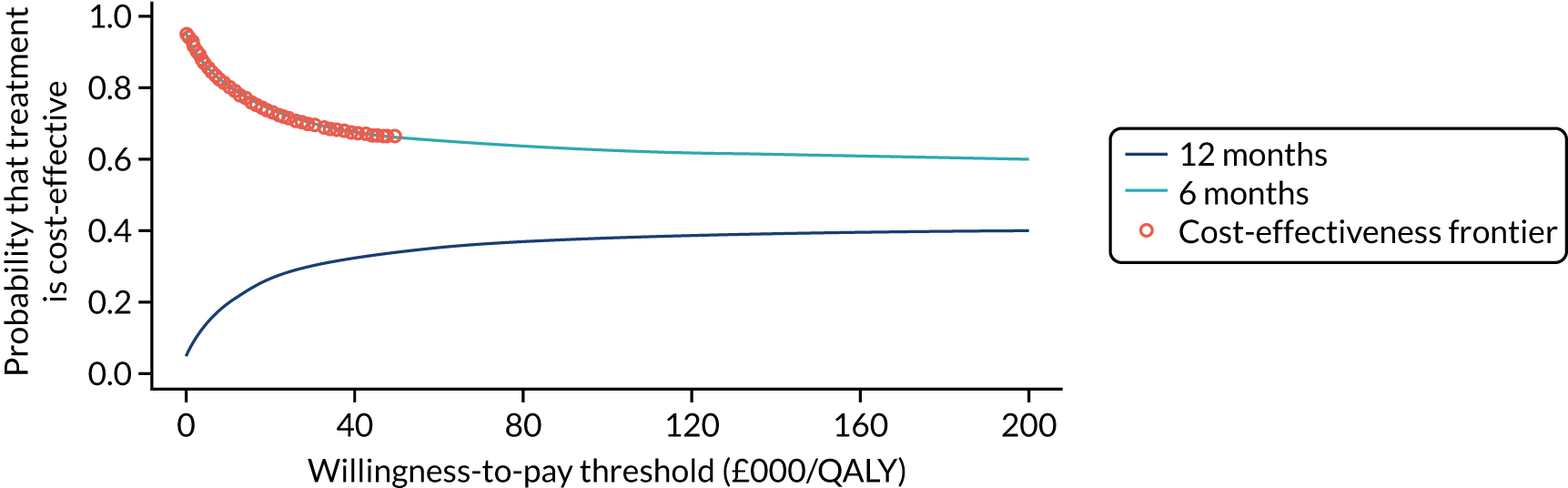
Subgroup analyses
The results of the lifetime decision model subgroup analyses are reported in Table 23 and shown in Figure 20. For some subgroups (concurrent trastuzumab timing, taxane-based chemotherapy and neoadjuvant chemotherapy timing), the incremental net benefits are negative. For these subgroups, the total QALYs for 12 months of trastuzumab treatment compared with 6 months are notably higher. For example, individuals who received taxane-based chemotherapy and 6 months of trastuzumab had an estimated incremental QALY loss of –0.73 (95% CI –1.52 to 0.33) compared with 12 months of trastuzumab. Six months of trastuzumab remained cost-saving, although the cost savings were slightly lower than those observed in the main trial analysis (–£8318 vs. –£9316). The cost-effectiveness results for the remaining subgroups were roughly in line with the main trial analysis results, except for those receiving trastuzumab sequentially to chemotherapy. The incremental QALY gain for the sequential subgroup was 0.32 (95% CI –0.10 to 0.75), with estimated cost savings of £10,871 (95% CI –£13,770 to –£8412). However, the CIs indicate that there is some uncertainty associated around this result.
| Subgroup | Treatment duration | Total cost, £ (95% CI) | Total QALY (95% CI) | Incremental cost, £ (95% CI) | Incremental QALY (95% CI) | ICER (£) | Net benefit (95% CI) | Incremental net benefit (95% CI) |
|---|---|---|---|---|---|---|---|---|
| ER status negative | 12 months | 27,596 (23,612 to 33,069) | 10.67 (10.06 to 11.25) | – | – | – | 9.29 (8.57 to 9.97) | – |
| 6 months | 19,712 (15,284 to 25,747) | 10.38 (9.74 to 10.98) | –7884 (–11,351 to –4108) | –0.29 (–0.88 to 0.28) | 26,851 | 9.39 (8.61 to 10.11) | 0.10 (–0.65 to 0.84) | |
| ER status positive | 12 months | 25,177 (22,385 to 28,980) | 11.24 (10.68 to 11.78) | – | – | – | 9.98 (9.36 to 10.57) | – |
| 6 months | 15,726 (12,837 to 19,699) | 11.24 (10.69 to 11.78) | –9450 (–11,749 to –7109) | –0.002 (–0.39 to 0.38) | 3,924,828 | 10.45 (9.82 to 11.04) | 0.47 (–0.03 to 0.96) | |
| Anthracycline-based chemotherapy | 12 months | 24,299 (21,522 to 27,935) | 11.16 (10.60 to 11.69) | – | – | – | 9.94 (9.33 to 10.52) | – |
| 6 months | 14,767 (12,164 to 18,330) | 11.33 (10.79 to 11.85) | –9532 (–11,701 to –7392) | 0.17 (–0.18 to 0.52) | Dominant | 10.59 (9.99 to 11.16) | 0.65 (0.19 to 1.09) | |
| Concurrent trastuzumab | 12 months | 26,421 (23,685 to 30,084) | 11.30 (10.76 to 11.82) | – | – | – | 9.98 (9.38 to 10.55) | – |
| 6 months | 19,569 (15,766 to 24,875) | 10.75 (10.15 to 11.32) | –6852 (–9521 to –3472) | –0.55 (–1.01 to –0.10) | 12,564 | 9.78 (9.05 to 10.44) | –0.20 (–0.81 to 0.38) | |
| Sequential trastuzumab | 12 months | 26,705 (23,120 to 31,566) | 10.79 (10.23 to 11.34) | – | – | – | 9.45 (8.79 to10.09) | – |
| 6 months | 15,835 (12,789 to 19,975) | 11.11 (10.55 to 11.64) | –10,871 (–13,770 to –8412) | 0.32 (–0.10 to 0.75) | Dominant | 10.32 (9.68 to 10.91) | 0.86 (0.33 to 1.42) | |
| Adjuvant chemotherapy | 12 months | 25,210 (22,189 to 29,350) | 11.12 (10.57 to 11.66) | – | – | – | 9.86 (9.24 to 10.46) | – |
| 6 months | 15,235 (12,344 to 19,235) | 11.18 (10.62 to 11.72) | –9975 (–12,440 to –7560) | 0.05 (–0.34 to 0.45) | Dominant | 10.41 (9.78 to 11.01) | 0.55 (0.05 to 1.06) | |
| Neoadjuvant chemotherapy | 12 months | 29,321 (25,377 to 34,656) | 10.73 (10.12 to 11.33) | – | – | – | 9.26 (8.51 to 9.97) | – |
| 6 months | 25,948 (19,699 to 34,464) | 9.83 (9.09 to 10.52) | –3373 (–7726 to 2340) | –0.91 (–1.64 to –0.22) | 3727 | 8.53 (7.56 to 9.41) | –0.74 (–1.71 to 0.17) | |
| Taxane-based chemotherapy | 12 months | 27,323 (23,167 to 35,160) | 11.01 (9.95 to 11.73) | – | – | – | 9.64 (8.28 to 10.48) | – |
| 6 months | 19,005 (14,524 to 25,428) | 10.27 (9.56 to 10.92) | –8318 (–14,911 to –3011) | –0.73 (–1.52 to –0.33) | 11,344 | 9.32 (8.43 – 10.09) | –0.32 (–1.35 to 1.07) | |
| Anthracycline- and taxane-based chemotherapy | 12 months | 28,146 (24,319 to 33,300) | 10.90 (10.30 to 11.48) | – | – | – | 9.49 (8.79 to 10.16) | – |
| 6 months | 18,995 (15,008 to 24,503) | 10.75 (10.12 to 11.36) | –9151 (–12,479 to –5617) | –0.15 (–0.69 to 0.39) | 63,049 | 9.80 (9.05 to 10.51) | 0.31 (–0.41 to 1.01) |
FIGURE 20.
Lifetime decision model on the cost-effectiveness plane: subgroup analyses. CT, chemotherapy.
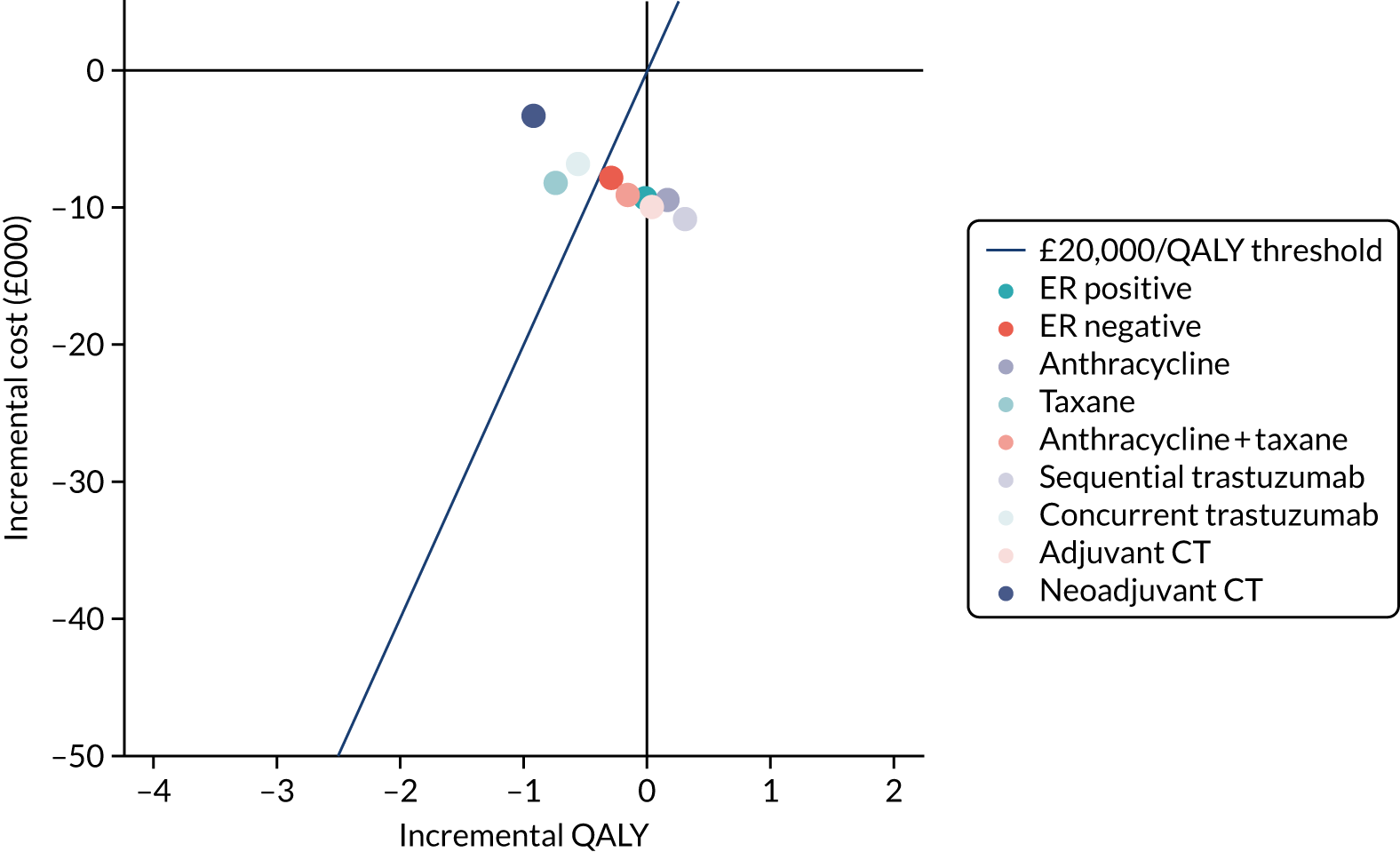
Twelve months of trastuzumab is therefore the preferred option in the three contextual subgroups: neoadjuvant, concurrent and taxane-only chemotherapy.
Sensitivity analyses
One-way sensitivity analyses were conducted by modifying the base-case value for each parameter by 20% higher and lower and assessing the impact on incremental net health benefit. The results can be found in the tornado plot in Figure 37 (see Appendix 2). Varying the cost of trastuzumab had the biggest impact, as expected. Varying the utility associated with the disease-free health state and the new primary cancer health state had the next largest impact on the results, followed by varying the rate of distant recurrence.
Discussion
This chapter has presented an economic evaluation of the cost-effectiveness of 6 months of trastuzumab compared with 12 months for individuals with HER2-positive early breast cancer. The evaluation consists of a within-trial analysis and a lifetime decision model. The within-trial analysis assessed cost-effectiveness using individual patient data collected in the PERSEPHONE trial from the landmark point (6 months post initiation of trastuzumab treatment) for a duration of 18 months. The lifetime decision model evaluated cost-effectiveness over a lifetime horizon by extrapolating data from the main trial. Both analyses were conducted from an NHS and Personal Social Services perspective. Subgroup analyses for both were also conducted in line with the statistical analysis plan for the main trial. For the within-trial analysis, data collected on patient out-of-pocket expenses and lost earnings were used to inform a secondary analysis adopting a societal perspective.
The within-trial cost-effectiveness results estimated that 6 months of trastuzumab was associated with an incremental cost saving of £9537 (95% CI £9183 to £9890) and an incremental effect of +0.003 QALYs (95% CI −0.015 to 0.021 QALYs) compared with 12 months of trastuzumab. Six months of trastuzumab is therefore cost-effective and dominates the longer duration of trastuzumab treatment (INB 0.48, 95% CI 0.45 to 0.51). The probability that 6 months compared with 12 months of trastuzumab was cost-effective was 100%. This result held across all of the subgroup analyses; there were no notable differences in DFS 18 months post the landmark date (2 years after initiation of trastuzumab). However, uncertainty was evident in whether 6 months’ trastuzumab is the more effective strategy (probability of 63%) in terms of incremental QALYs gained. The sensitivity analysis that compared different delivery and drug cost options for trastuzumab indicated that the biosimilar intravenous options recently made available are likely to result in the largest cost savings. A further sensitivity analysis was conducted exploring the impact of the assumption that vials of intravenous trastuzumab would be shared. As expected, the overall costs are higher if vials are not shared, leading to a larger cost saving for the shorter duration of treatment. The results of the within-trial analysis should be interpreted with caution by decision-makers as they do not take account of the long-term consequences of trastuzumab duration for cancer recurrence and survival.
The lifetime decision model, which is the preferred analysis for NHS decision-making, estimated cost savings of £9316 and a slight reduction of 0.01 QALYs per individual, indicating that a reduced length of trastuzumab treatment of 6 months has a negligible impact on QALYs and is significantly cost saving (INB of 0.46 QALYs). The slight negative difference in QALYs compared with the within-trial analysis is likely to be due to the non-significant difference in DFS between the two trial arms. In this analysis, however, there is notable uncertainty around the effectiveness results: the probability that 6 months’ trastuzumab is as effective as or more effective than 12 months’ trastuzumab is 58%. There is, inevitably, large uncertainty around any model extrapolation. If DFS in the 12-month and 6-month trastuzumab arms was to start to diverge as follow-up continues beyond the trial, then the results of the lifetime modelling would be likely to be compromised. The HERA trial compared 1 years’ with 2 years’ trastuzumab treatment and found no additional benefit of the longer treatment duration over a 10-year follow-up,13 suggesting that the main treatment benefits are realised in the first few years of follow-up. Further to this, although the overall trial results demonstrate that 6 months of trastuzumab is non-inferior to 12 months, the subgroup analyses for the lifetime model suggest that some individuals may benefit from a longer duration of trastuzumab treatment. For example, those who received taxane-based chemotherapy had an estimated incremental QALY loss of 0.73 (95% CI –1.52 to –0.33). These results align with the DFS estimates reported for the main trial, but it is important to note the considerable uncertainty around these results, given the much smaller sample sizes, and the significant limitations of subgroup analyses.
A key strength of this analysis is the consideration of both the short- and long-term consequences of different trastuzumab treatment durations. Interestingly, the within-trial subgroup analyses highlighted no notable differences between groups because the differences in DFS emerge only after 2 years of follow-up. This highlights the importance of using caution when interpreting and drawing conclusions from early trial data. We were keen to use the data from the PERSEPHONE trial as far as possible within the model, and the ‘msm’ (which stands for multistate model) package in R97 was incredibly useful for fitting a multistate model to the longitudinal data available and estimating transition probability matrices from the model. The main limitation of the model was that we could not find a study that reported long-term (i.e. beyond 5 years) rates for local and distant recurrence in this patient group with respect to trastuzumab duration. We therefore had to assume the same event rates observed in the trial beyond 5 years, which may not reflect reality.
Clarke et al. 107 recently conducted a three-arm cost-effectiveness analysis comparing different durations (0 weeks, 9 weeks and 12 months) of adjuvant trastuzumab for those with early breast cancer. The evaluation was based on a network meta-analysis of the various clinical trials evaluating the effectiveness of different trastuzumab durations. The conclusions of the authors’ analysis suggested that 9 weeks of trastuzumab was cost saving and led to more QALYs than 12 months of trastuzumab (although the 95% CIs overlapped across all arms). 107 Unfortunately, data from the PHARE trial,47 which evaluated 6 months of trastuzumab, could not be included owing to the late randomisation of individuals recruited. It would be interesting to add the PERSEPHONE trial data to this existing network meta-analysis to include a 6-month arm comparison, although, given the later randomisation of individuals in the trial, the same problems may be incurred as in the PHARE trial.
The next key step in this research is to explore whether or not we can predict who would benefit from 12 months of trastuzumab and who needs a shorter treatment duration. Baseline data collected in the trial could be used to develop a prediction model to start to answer this question, enabling an economic evaluation comparing a stratified-risk approach to determining trastuzumab treatment duration. On a more ambitious scale, an individual patient data meta-analysis of all of the trials evaluating different trastuzumab treatment durations would allow a more powerful exploration of potential predictors and the inclusion of shorter trastuzumab durations (e.g. 9 weeks).
Chapter 6 Quality of life, patient-reported experiences and reporting of results to patients
Introduction
In addition to standard quality-of-life tools and bespoke health economic booklets, patient-reported experiences collected in a clinical trial provide valuable insight into the impact of treatment on patients’ daily lives. In the PERSEPHONE trial, patient-reported experiences were collected at the end of the patient quality-of-life booklet on a free-text page leading with ‘Please use this page to make any comments you would like about the study or your treatment that you think we or future patients should know about’. All patients were encouraged to complete the full patient booklets but this was not a mandatory part of the trial and patients were not excluded if they did not want to complete the patient booklets. Hence there was no bias in the reporting of these data.
Methods
The aim was to assess the quality of life of all patients before they started trastuzumab treatment and then after 3, 6, 9, 12, 18 and 24 months. However, for those entering the trial after their trastuzumab treatment had begun, the same time points throughout their treatment were used but with the initial form(s) missed. The quality-of-life questions comprised a question regarding the patient’s general health and the EQ-5D-3L. Patients were invited to record comments about the study or their treatment on a free-text page at the back of the patient booklet of all questionnaires collected from patients after treatment or at follow-up.
Statistical methods
To assess the quantification of patients’ health, the frequencies of the different responses to the general health question were assessed, and patients’ EuroQol-5 Dimensions (EQ-5D) visual analogue scale scores over time were assessed graphically using box-and-whisker plots.
The free-text data were entered into the NVivo database and coded by treatment arm and time point of the questionnaire. Two researchers and a patient representative looked at the data and began by performing a simple content analysis. Many themes emerged from these data. This broad initial analysis included all free text across all time points, regardless of treatment arm, to explore the whole experience for patients undergoing trastuzumab.
Quality-of-life results
Patients were asked how they would describe their health in general. In total, 3910 patients (12-month patients, n = 1960; 6-month patients, n = 1950) answered this question for at least one time point. In both treatment arms, feelings of general health are seen to decline during the first 3 months of trastuzumab treatment, with the proportion of patients reporting feeling ‘very good’ halving from the baseline time point to the 3-month time point (from 32% to 16% and from 35% to 17% for 12- and 6-month patients, respectively) and the proportion of patients feeling ‘fair’ almost doubling over the same time period (17% to 29% and 14% to 28% for 12- and 6-month patients, respectively) (Figure 21). This period of time is when 46% of patients would have been receiving concurrent chemotherapy. In both arms, feelings of general health are then seen to steadily improve after trastuzumab treatment is completed.
FIGURE 21.
General health: (a) 12-month patients; and (b) 6-month patients.
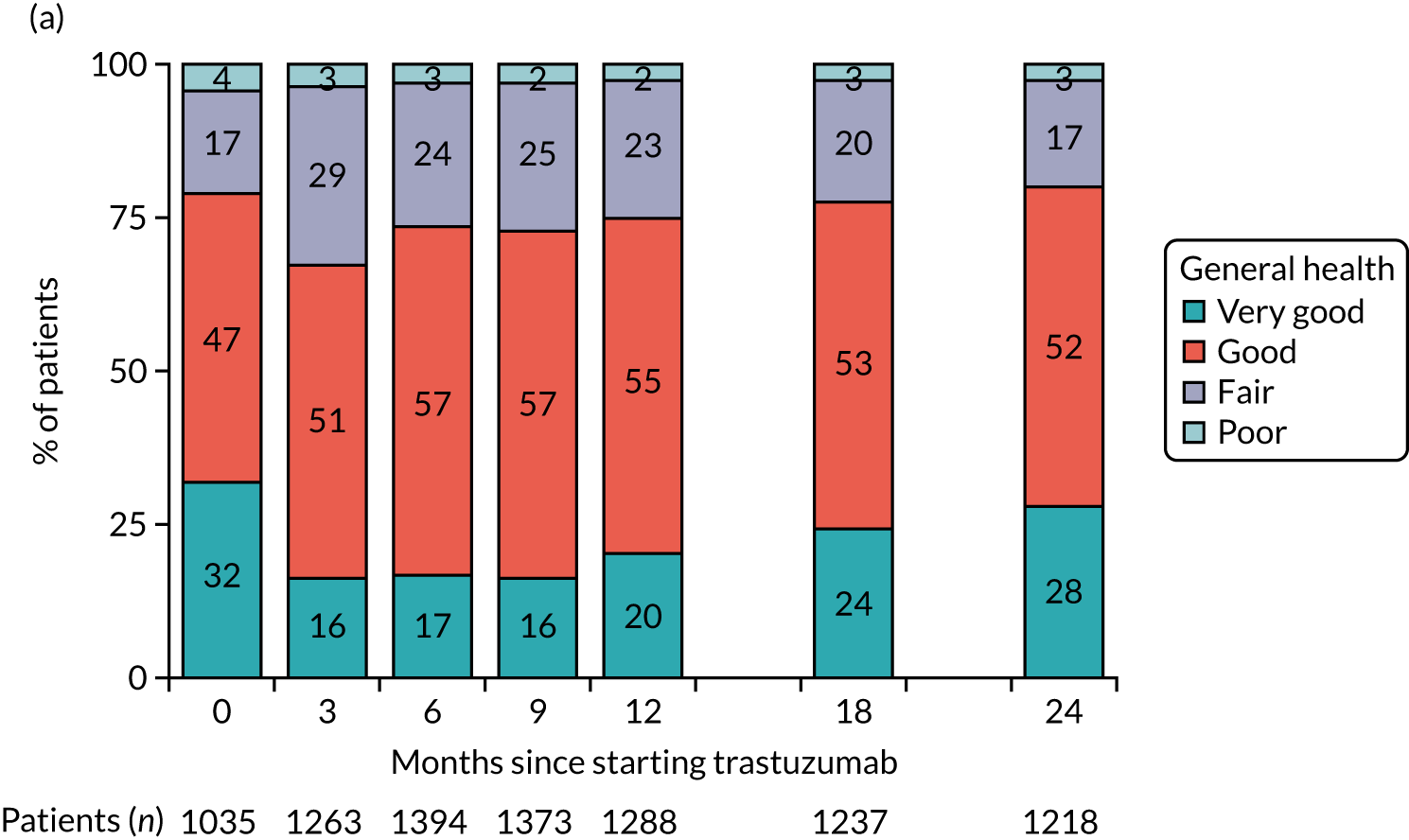
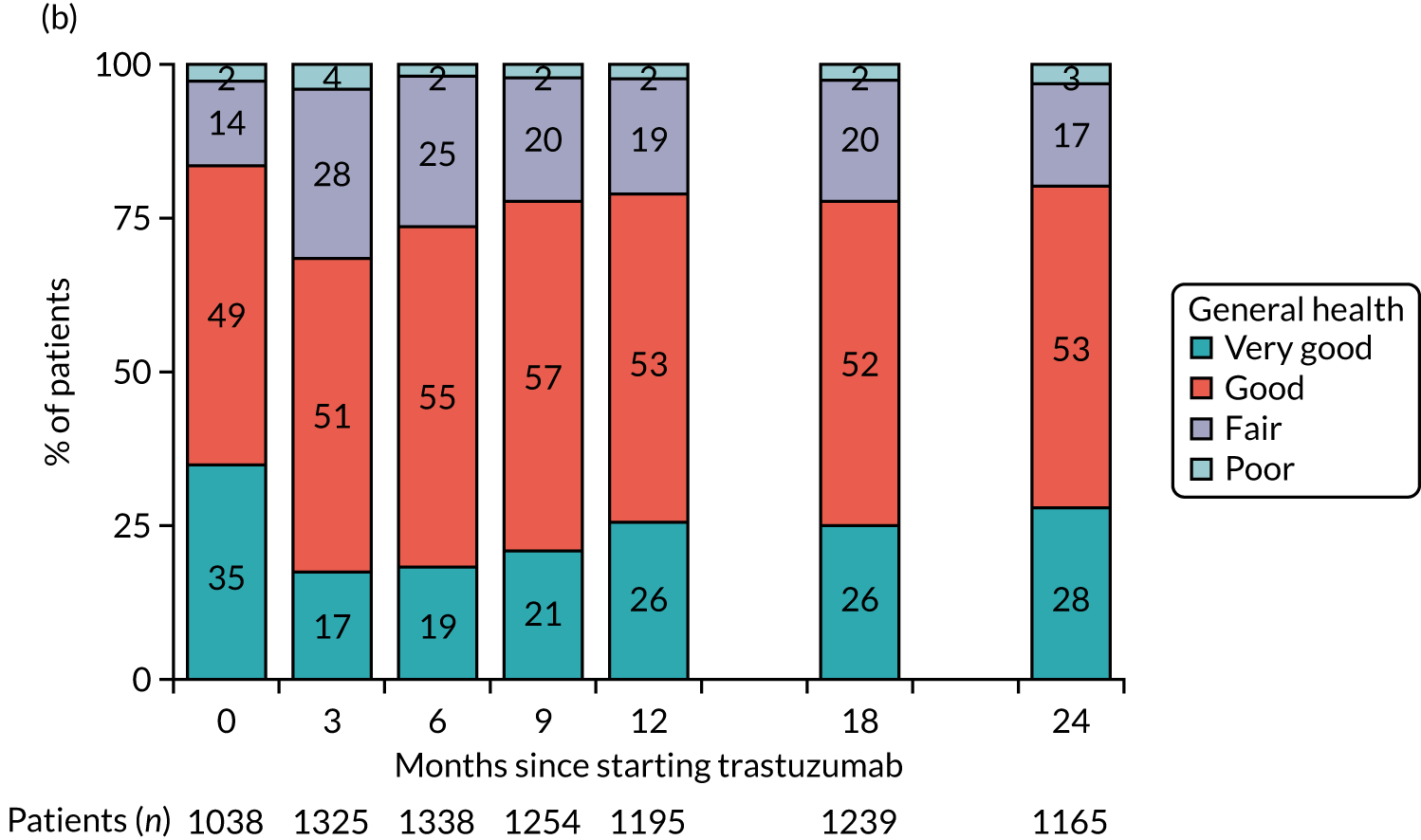
Patients were also asked to rate their own health state using the EQ-5D visual analogue scale. In total, 3902 patients (12-month patients, n = 1958; 6-month patients, n = 1944) completed the visual analogue scale for at least one of the time points. In both randomised treatment arms, health states are seen to remain steady from baseline to 3 months into treatment, with a trend towards a slow increase after this, occurring slightly earlier for 6-month patients (Figure 22).
FIGURE 22.
The EQ-5D visual analogue scale: (a) 12-month patients; and (b) 6-month patients.
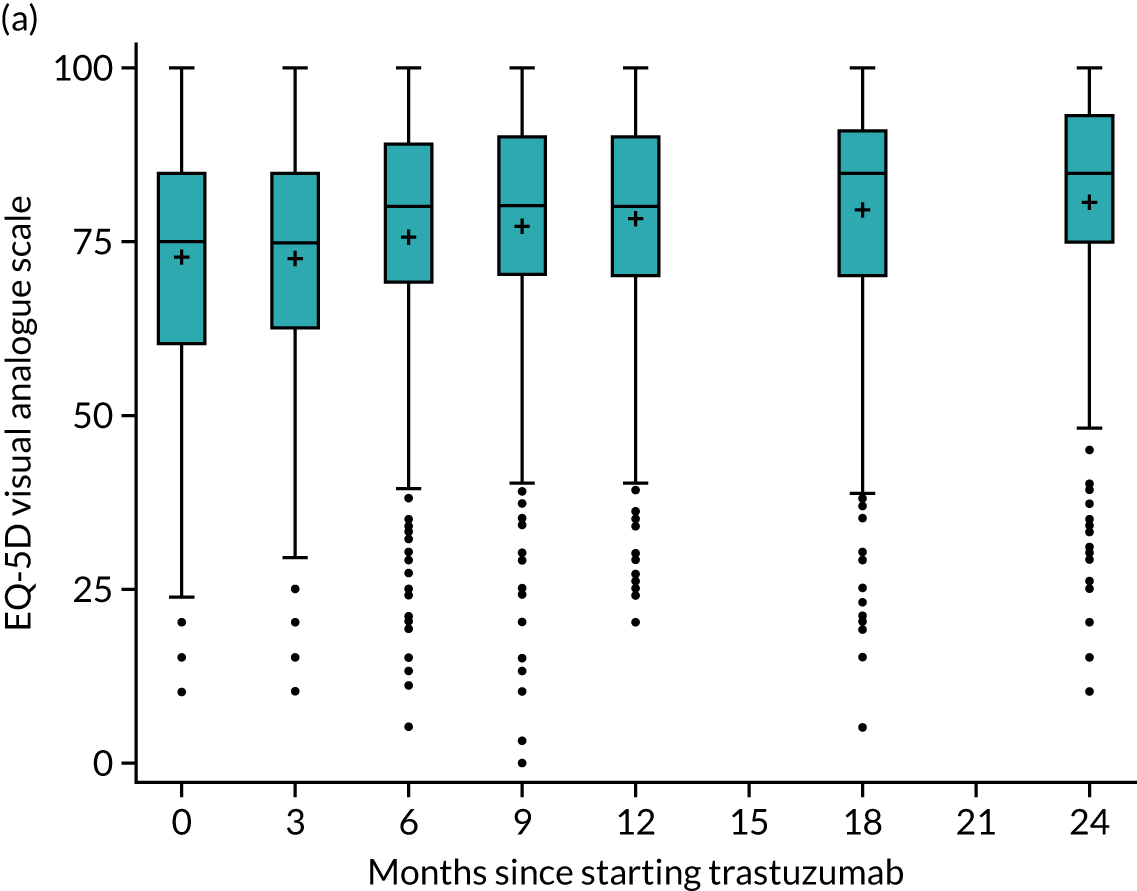
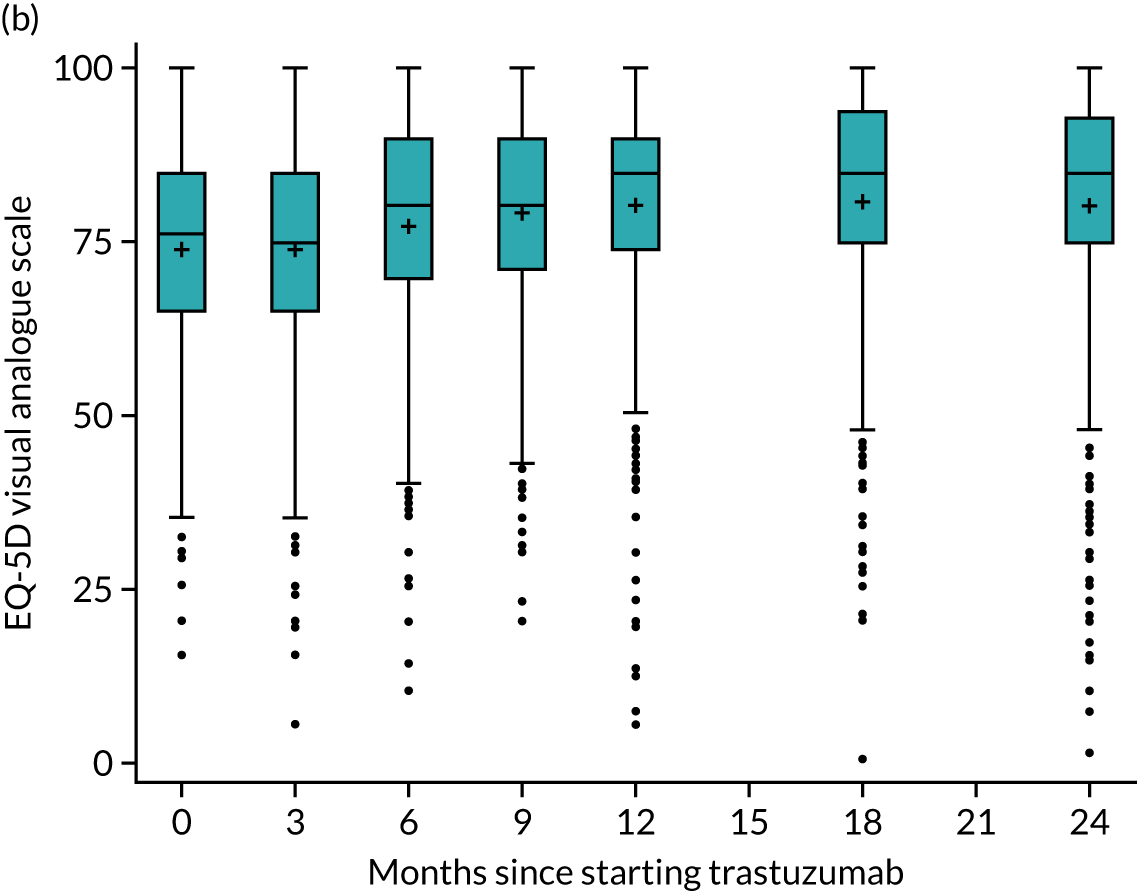
Patient-reported experiences
Across all time points, a total of 5610 free-text fields were completed. Some patients had completed the free text at all or most time points; others had completed it just once, twice or not at all. Patients offered information on all aspects of the study, including their views on the treatment, their care, the questionnaire they were asked to complete and research itself. However, the most often mentioned aspect of the study reported was the impact of the drug on patients personally: physically, psychologically or socially.
Which side effects are due to trastuzumab?
The side effects patients reported were sometimes difficult to attribute to one specific treatment, as many of the treatments were given concurrently or in close succession. This could potentially cause problems with treatment management and should be kept in mind when reviewing the data:
Aches and pains especially below the waist slowing me down considerably; not sure if it’s my age, the Herceptin treatment or the drug Arimidex to be taken for next 5 years. Also not sure if itching on my back and top of arms is caused by Herceptin or Arimidex. I know the Arimidex can cause hot flushes etc. so it could be a combination of each.
878
It is difficult to decide whether tiredness and hot flushes are due to Herceptin or the taking of Anastrozole/Arimidex tablets.
1084
It is really difficult to tell which side effects are due to Herceptin and which ones are due to the chemotherapy – especially when they are taken together.
1052
Results from the simple content analysis
From the first, simple content, analysis, 19 themes emerged (Figure 23).
FIGURE 23.
Number of reports of identified theme of treatment/study.
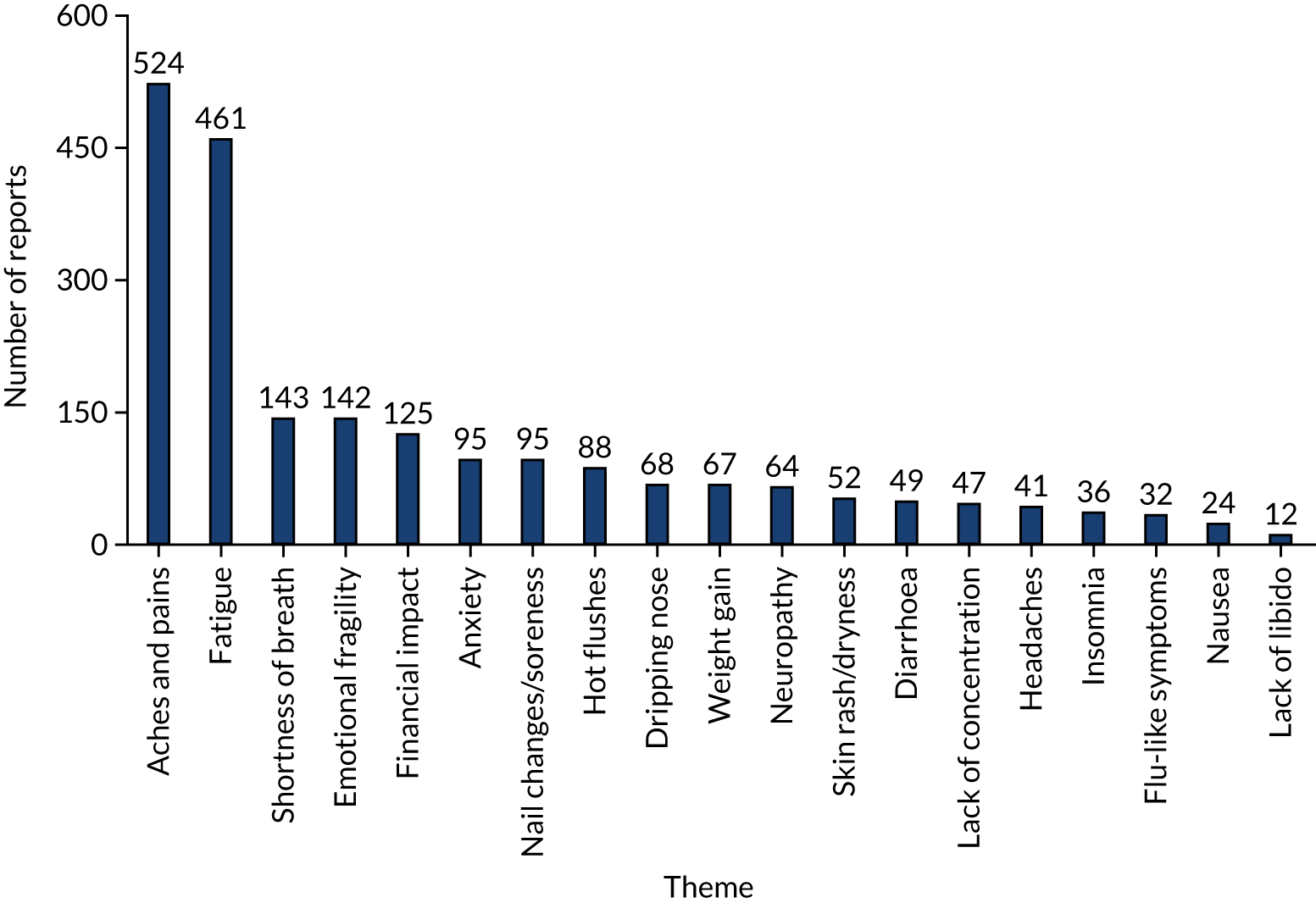
The five most commonly reported aspects were:
-
aches and pains
-
fatigue
-
shortness of breath
-
emotional fragility
-
financial impact.
Aches and pains
Although aches and pains are effects known and reported by health-care organisations (Table 24), they do not appear at the top of lists of side effects and are, arguably, not highlighted, but they appear to have had a huge impact on patients and their daily activities. It is not known whether patients wished to highlight them because of the perceived lack of emphasis elsewhere or because their impact was particularly great but, for many patients, these side effects did appear to be particularly distressing and intractable.
| Genetech 2017 (makers of trastuzumab) | Breast Cancer Care 2016 | NHS Choices 2016 | |
|---|---|---|---|
| 1 | Fever | Flu-like symptoms e.g. chills and mild pain in some parts of the body can occur during or shortly after treatment | Reaction to medication e.g. chills, high temperature, swelling of face and lips, headache, hot flushes, feeling sick, wheezing and breathlessness |
| 2 | Feeling sick to your stomach (nausea) | Nausea (feeling sick) – normally mild and doesn’t last long | Tiredness and difficulty sleeping |
| 3 | Throwing up (vomiting) | Diarrhoea (mild) | Diarrhoea or constipation |
| 4 | Infusion reactions | Soreness at the injection site | Low number of infection-fighting white blood cells which increases risk of infections |
| 5 | Diarrhoea | Less common side effects e.g. headaches, dizziness, joint & muscle pain, rash, vomiting or breathlessness (often mild and don’t usually happen after other treatments) | Loss of appetite and weight loss |
| 6 | Increased cough | Allergic reaction (rare) e.g. flushing, skin rash, itching, back pain, shortness of breath, faintness, fever or chills | Pain in your muscles, joints, chest or tummy |
| 7 | Headache | Heart problems such as abnormal heart rhythm (rare) can cause symptoms such as breathlessness and palpitations | Runny nose |
| 8 | Tiredness | – | Sore red eyes or watery eyes |
| 9 | Shortness of breath | – | Shaking |
| 10 | Rash | – | Dizziness |
| 11 | Low red and white blood cell counts | – | Cough |
| 12 | Muscle pain | – | Increased or decreased blood pressure |
| 13 | – | – | Heart problems |
| Sources (accessed July 2020) | www.gene.com/patients/medicines/herceptin | https://breastcancernow.org/information-support/facing-breast-cancer/going-through-breast-cancer-treatment/targeted-biological-therapy/trastuzumab-herceptin#What%20are%20the%20side%20effects%20of%20trastuzumab | www.nhs.uk/conditions/herceptin/ |
Aches and pains were mentioned in 524 responses. Common descriptions of this included an ‘aching of leg muscles’ (4027), ‘a general feeling of aching in the joints which impedes exercise’ (4001) and ‘joints and muscles hurt most of the time’ (325).
However, this could be extremely severe and debilitating and often seemed to increase over time:
Muscle and bone pain so bad it reduces me to tears.
2489
My body aches more. I feel like a woman of 80 some days.
17
Joint pain has increased as months have passed.
619
Bone pain in the hands and wrists – very painful. Plus occasional bone pain in elbows and ankles. Pain still occurring since finishing Herceptin approximately six weeks ago.
3719
I still suffer with joint pain which I’ve had since I started Herceptin.
481
Achy joints. Shooting pains in arm . . .
860
Fatigue
Fatigue or tiredness was reported in 461 responses. Fatigue is the most common side effect of cancer treatment and is increased with a combination of therapy. 108 Its cause and effects are highly complex and disruptive and have often been, arguably until recently, downplayed or overlooked. 109 Here this was described as ‘always tired’ (379), ‘extreme tiredness’ (489), ‘totally depleted of energy’ (4018) and ‘my energy levels are down’ (14). However, this was again was often experienced as extreme and debilitating:
I do think that people receiving Herceptin should be told that it can cause increasing fatigue because I thought I was going mad until someone told me.
188
Be prepared to be very tired during your treatment, I have found the fatigue hard to deal with. I am usually a very active person and the tiredness has been difficult to deal with. The tiredness is horrid.
3650
Fatigue gets worse as treatment goes on.
3606
The fatigue and anxiety is unreal; I’ve become quite depressed. It’s not easy to explain to people this might happen . . . but more information would be so helpful.
223
Fed up with tiredness & weariness. Sometimes only able to iron or garden for 20 mins then need to sit down.
200
You should put in a question about extreme tiredness which cannot be regarded as an illness but really affects your life.
1977
Shortness of breath
It is not clear whether shortness of breath correlates with the cardiac damage that trastuzumab is known to cause,64 anaemia or infection. However, it is known that shortness of breath is a particularly distressing, uncomfortable and frightening experience that limits both physical and social activity, which may have led to its dominance among the responses:
Following my Herceptin treatment I became breathless very soon after walking (more than 500 yards) or hoovering.
419
I have mitral valve weakening thus I am breathless. I am to visit [a specialist hospital] for this. For these reasons I have to catch a taxi for dentist, doctor, optician etc.
3889
I am still suffering with shortness of breath, extreme tiredness and severe aches in my joints. I had hoped that by now I would have made a far better recovery from my treatment but sadly I feel rather despondent that I am still so low and exhausted.
3626
Shortness of breath may also be significant as it is a reminder of potential cardiac damage, which seemed particularly distressing to some patients:
I was extremely shocked at the damage to my heart as a direct result of Herceptin at such an early stage in treatment. I feel that future patients should be made aware of the risk of heart failure in relation to Herceptin.
1294
Herceptin treatment affected my heart. Although the MRI scan shows heart function is now OK, I get anxiety and fear that the future prognosis is not good due to this . . . and worry constantly that I will have problems in the future. I remain positive on a day to day basis, but have some bleak and despairing moments.
123
I was told about the risks to my heart but I don’t think this was emphasised to the extent it should have been. Because of the affect it has had on my heart I now have to undergo an MRI scan and angiogram. Therefore I have decided to quit the Herceptin programme.
1051
Emotional fragility or depression
‘Emotional fragility’ was a term coined by one participant (1698), who felt that it was a more appropriate descriptor than ‘depression’. Another participant (553) wanted to emphasise that anxiety and depression should never be ‘lumped together’ as she felt that they were significantly different.
Many patients described a ‘black cloud’ (1510) and felt that they had lost all of their self-confidence as a result of the impact of the treatment and side effects. They wanted to warn others that they should be ‘prepared to feel down’ (1) and would ‘cry a lot’ (2627). Although patients seemed to equate this with being in the trial or receiving treatment, arguably it may have been due to the loss and fear that is known to occur after cancer treatment:
As far as the herceptin treatment the side effects are manageable and not as bad as chemo or radiotherapy . . . I find my anxiety and depression increasing now though. I feel a bit lost and my safety nets are being removed one at a time; I find myself dwelling increasingly on the possibility of recurrence of the cancer my moods are often erratic and often unreasonable. I’m snappy and sullen instead of the normal happy and philosophical/positive.
1377
The network & support has been really important e.g. friends/well-being group/family/hospital services/activities etc. Initially for more physical & practical support but towards the end of treatment for mental/emotional support & support in managing long-term recovery which has taken longer than I expected & I don’t feel I’m there yet. Anything you can do to support & help people through this stage I feel is just as important as the medical help & support given at early diagnosis & during treatment.
4061
Not sure whether it is the side effects from the Herceptin but have been feeling really low which is out of character for me. Feeling really tired, depressed and anxious a lot.
381
At times I have been quite depressed for a couple of days after having Herceptin . . . for 2/3 days following treatment I felt quite depressed- on occasions this could be fairly bad: I’d be very tearful, could not face socialising and was pessimistic about the future.
665
I have felt a little stressed and quite tearful over the past weeks.
735
I feel that a little more emphasis should be placed on the psychological effects felt by Cancer patients.
1038
I felt somewhat abandoned as I seemed to fall through the cracks of the support services on offer. I didn’t know or have the emotional strength to call on the breast care nurse service and was unsure whether I was still eligible for such support.
1100
. . . you feel well looked after physically, but the emotional side of the treatment journey is not really talked about. I need and I feel I want to be looked after even now, I need more hugs, cuddles, reassurances.
1344
Financial impact
A total of 125 responses mentioned the importance of subsequent patients being made aware of the financial aspects of treatment and involvement. This impact was from increased costs such as travel and car parking or because of an inability to function and, therefore, to work in the same capacity as before. Although patients seemed to be aware that this was unavoidable, they wanted to raise awareness:
Financially it’s crippling. If you’re low paid there is no help, nearly cost me my home as I wasn’t always well enough to work overtime or do my 2nd job. Still in debt now. But I’m alive!!
2840
I have had increased regular outgoings arising solely from the effects of my illness since diagnosis. I live alone and am not eligible for a social services care plan because I am in my 50s. Consequently I have had to buy in the following services from time to time: home help/meal deliveries, laundry services, taxis to appointments, putting the cat in the cattery during periods of incapacity/hospitalisation. Since diagnosis, I would estimate that these additional regular cost amount to several thousands of pounds. This together with the reduction in my income (end of paid sick pay, return to work on reduced hours, ill health retirement) has had a considerable effect on my standard of living.
1581
Although I have not incurred big one off costs I have had to spend between £4–6 every three weeks whilst having Herceptin. This soon adds up over the year I will be having this treatment. Free parking would be of great benefit.
2262
The financial cost of my diagnosis has been huge to my family. We have had no help with this burden as my husband is working.
3843
The main input on my diagnosis has been on my work/employment. I have had to reduce my work hours from 37 per week to 18 1/2 hours. Although I now spend more time keeping fit i.e. swimming, walking, etc. I no longer feel that I have a career – just a job. I can’t see me going back to full time employment which makes me sad. On the other hand I now have a better work/life balance but this would not be the case if I had children or my husband did not have such a good job. I don’t think I would have had the choice about taking a reduction in hours and would have struggles with the ongoing fatigue.
582
People should be aware of the cost involved in coming to hospital every 3 weeks – car parking, petrol, time off work; it all mounts up.
39
My treatment and care has been excellent, but no-one prepares you for life after. I have been 24 years as a Senior Court Usher and loving my work. Since I returned on a phase return, I can’t do my work because of my pain and disability in my legs after the Herceptin, sleepless nights and this has made me very low although I’m grateful to be here after my treatment. You do need ongoing support to help you through, especially if your work is affected. I’m now doing a job I dislike but as a widow at 47 I have to earn a wage.
2106
The Herceptin injections cause the most trouble. They make you ache to the extreme you can’t walk or stand if at all not for long. Due to my work (HGV driver) I can’t afford to not be able to stand. Now I’ve had 6 months I’m stopping further injections.
3683
Other participant experiences
Between 50 and 100 responses cited another raft of aspects that were arguably more ‘visible’ to others and matched more closely with the side effects described by organisations (see Table 24):
-
Anxiety (95 responses): ‘I have lost a lot of confidence and get very anxious’ (336).
-
Nail changes and soreness (95 responses): ‘Every fingernail was broken/torn (3429)’; ‘I can’t peel an orange or use Sellotape’ (2728); ’very soft nails, sore hands, skin irritation’ (816).
-
Hot flushes (88 responses): ‘I never feel very comfortable! I have to wear cotton/light clothing – not nice in cold weather’ [14].
-
Sore/’dripping’ nose (68 responses): ‘Constantly dripping nose’ [1424]; Very, very, very dry, scabby nose (internal) when having the Herceptin [1412].
-
Weight gain (67 responses): ‘Gradually worsening problem with water retention/swollen feet/legs/body with each of the first 4 Herceptin injections – sometimes gaining over a stone in weight in 3 or 4 days and starting earlier with each cycle. For cycle 5 have swapped to intravenous Herceptin to see if it makes any improvement – watch this space!’ [4081].
-
Neuropathy (64 responses): ‘Continuous pins and needles in hands and feet, with finger tips becoming increasingly sore. Painful balls of feet (like walking on marbles!) making walking uncomfortable – have to wear shoes with very squashy, soft soles’ [4081].
-
Skin rashes/dryness (52 responses): ‘Dry and cracked skin’ [1016]; ‘Skin looks like porridge oats’ [1185].
Other side effects
Although it is impossible to explore issues further with patients, certain aspects stood out during analysis. These were the cumulative impact of the side effects of Herceptin, the perceived ‘downplaying’ of the effects of Herceptin by health-care staff and the impact of Herceptin treatment on other chronic illnesses:
General health has been good. However, the cumulative effect of treatment – chemotherapy, radio-therapy and Herceptin – has been one of fatigue, exhaustion and general debilitation.
1628
Although Herceptin is said to have few side effects (by comparison with chemotherapy), I think it would be helpful to patients if they were made aware of the full range possible. In my own case, I remain very tired after each treatment for several days so that it would not be possible to resume my employment at present. I also continue to have diarrhoea a day or two after each treatment and also disturbed sleeping patterns. Each effect taken singly is not significant but in combination is most significant and does impact on my ’normal life’. My GP – generally sympathetic – reads the current literature provided by Macmillan and does not necessarily appreciate the debilitating effect of this treatment in my case and so is pressing me to return to work. This isn’t helpful – indeed it is very stressful. So, fuller communication to GPs and patients needed I think.
1581
I have found Herceptin equally as challenging as chemo because it has caused significant fluid retention and joint pain in hands, fingers, knees and ankles that makes normal day to day tasks and walking difficult. Unfortunately I have been discriminated against at work and lost my job – I believe as a result of being absent – but couldn’t afford to take my ex-employer to court. I think people should be very wary of taking time off as the law does not protect cancer patients as well as you expect.
2476
I think Herceptin is very ’chemo’ like. My treatment is 3 weekly and the first 2 weeks are miserable. I am bolstered by the fact that after that I begin to feel myself again. I am looking forward to when the Herceptin is out of my system and I’ll begin to feel my old self again – hopefully!
3943
I was given a great deal of information and support to assist with the effects of chemotherapy and radiotherapy but far less on the possible effects of the Herceptin injections. I had assumed that my health would start to improve after the chemotherapy and radiotherapy and was not expecting that the Herceptin alone would have such an impact.
3941
My condition with other problems have become worse. I had problems doing things because of mobility problems before breast cancer but the pain has got worse.
470
As I have chronic fatigue syndrome and fibromyalgia I have found all the treatment for cancer extra hard and very tiring! I can’t live my life because my body doesn’t work properly.
3517
I suffer with osteoarthritis in hands and knees and have noticed a very considerable increase in the pain and stiffness since the start of Herceptin, particularly in my hands, sometimes making it impossible to unlock my hand joints to do simple tasks.
242
The Diabetes that I had under control [has] become uncontrollable. My blood sugar has been between 10 and 20 since last September; now I am type 1 taking insulin.
1606
Additional themes
Having received such a large number of rich, qualitative data from patients, the researchers felt that it would be interesting to share some emerging themes not necessarily related to side effects of the diagnosis or treatment:
-
Taking part in the trial: how does this impact on patients?
-
What do patients on the trial think about receiving less than the standard 12 months’ treatment with Herceptin?
-
Fear of recurrence.
-
Needing more information or support.
-
Patients’ relationships with health-care professionals.
-
Feeling abandoned after treatment.
-
Wanting to move on.
Taking part in the trial: how does this impact on patients?
Reading through the responses from the patients on the trial, it seems that a lot of them felt grateful that they had been given the opportunity to take part. Sometimes this feeling appeared to be attributable to the media attention Herceptin received when it first appeared as a treatment for breast cancer:
I feel very privileged to have this treatment as the press call it the post code lottery.
820
I would like to take the opportunity to say how extremely grateful I am to have been a recipient of Herceptin.
1141
. . . I am alive & very grateful for the treatment & realise I am lucky to have had the opportunity to have it.
1483
I am grateful for the Herceptin & believe it has saved my life.
549
The chance to join the trial was a blessing. Any small inconvenience having the treatment paled at chance to Herceptin . . . and giving hope that my survival could be many more years.
1519
. . . from what I have understood about Herceptin I think it seems a ’wonder drug’. I feel blessed it has been available for my recovery.
1566
For some, being in the trial gave them a sense of being watched more carefully:
I am happy to be a part of your study/clinical trial of Herceptin. It really helps me psychologically because with this treatment I felt safe and secure of not recurring from breast cancer.
1453
I do feel slightly more confident due to the extra care the trial affords.
1377
My family are reassured about my health more because of the extra tests that I have had due to being on the trial.
1016
What do patients on the trial think about having less than the standard 12 months’ treatment with Herceptin?
As the PERSEPHONE trial randomised patients to standard of care (12 months of Herceptin) or a reduced treatment duration, it is interesting to hear how the patients felt about this, especially those who received the shorter duration.
Perhaps surprisingly, not many patients gave negative responses related to risk of receiving fewer Herceptin treatments. This is perhaps a result of careful explanation of the trial given to prospective patients. Just a handful of patients referred to being anxious about being ‘undertreated’:
Not having completed half the treatment has left me somewhat anxious as I feel that somehow I have lowered my chances of the recurrence of cancer. This is having the effect of me feeling vulnerable also.
513
I would sometimes like more re-assurance and information about the 6 months – have I done the right thing??
544
Would have been nice to have a chat at the end of the 9 cycles re the [trial] . . . Reassurance that [trial] was good and results were showing further cycles didn’t not seem to improve survival [rates] would have been nice.
896
Just one participant reported that she withdrew from the trial in order to continue with a full year of Herceptin:
I have decided to stop the trial and continue with 1 years treatment of Herceptin – following discussion with family I feel if the cancer returned within the next five years I would maybe feel that it was partly my fault – by not having the full treatment.
889
Fear of recurrence
Many of the responses indicated a fear of recurrence:
It never leaves you, and I am always thinking it is going to come back . . . I am always thinking on special occasions like Christmas birthdays etc., will I be here for the next one?
470
It’s hard to come to terms with the reality that this ’weight’ will never truly be far from my immediate thoughts.
496
I still think I have cancer or it will come back soon.
1482
Although I do not feel ill. I am finding that my fears of cancer returning are a constant ‘niggle’.
963
This is evidently something that causes a lot of angst for patients with this diagnosis and this manifests in many ways.
Some reported that this fear of recurrence caused them to feel depressed:
Still worry about the cancer coming back which makes me depressed at certain times.
641
Some described it monopolising their life:
I find myself dwelling increasingly on the possibility of recurrence of the cancer. My moods are often erratic and often unreasonable. I’m snappy and sullen instead of the normal happy and philosophical/positive. I am more negative than I have even been.
1377
Many sought more reassurance that their cancer had really gone and was not coming back:
It is frustrating that there is no test to confirm whether the cancer has gone – you are left to see what happens.
1140
I would also like to know when any tests could be performed to see if I’m completely cured or not (always at the back of my mind) . . . You must communicate with sample patients more even if it takes a phone call to put their mind at rest.
608
I finished Herceptin 6 months ago and am coming up to 2 years Post OP. I’m feeling very low and anxious about reoccurrence as I haven’t got an ultrasound or mammogram for another year.
1414
I feel personally that I need to know my cancer has really gone and is not going to return in the other breast/or somewhere else in a few years/months. I have not been given this reassurance yet.
730
Many said that they remained anxious about every symptom and felt the need to be able to ask a health professional about this:
Unfortunately, once having had cancer, one is never free of the worry about it returning, so it is very important to feel able to report any worrying symptoms that may arise.
1166
Some were worried about becoming overly anxious:
The one element that you have to deal with is that of heightened vigilance of any bump, ill feeling. I think it makes you ’ultra-aware’ which inevitably leads to scares/panics about the cancer returning. I am not a hypochondriac – but you begin to feel like one.
1140
Some reported difficulty in remaining objective, with every possible symptom signifying risk of recurrence:
With time, the anxiety over a relapse increases and it becomes difficult to be/remain objective regarding any sensation.
719
Just a few responses suggested feeling safe from the risk of recurrence:
. . . with this treatment I felt safe and secure of not recurring from breast cancer . . . I feel better and worry free now that I finish my dose of Herceptin.
1453
Needing more information or support
A common theme running through the responses is wanting more information. Some wanted more about their own diagnosis and treatment pathway:
It would be good to know how many treatments exactly are involved rather than just a vague 6 or 12 months. I have been asking for 4 months each time I attend for treatment and have been told they don’t know.
740
Please keep patients fully informed about their diagnosis as it is not always easy to know the right questions to ask.
1272
It would have been better if I had known I would not be randomised until after Herceptin (9) – this was not what I was led to expect earlier.
1368
Some felt that the information they had been given was too complicated or difficult to understand:
The information pack I was given was put in medical terms and not lay man’s terms the information should be easy to understand it was far too complicated you needed a medical degree to understand it.
1160]
I think that it would be helpful to the patient if they were told (obviously in layman’s terms) how the Herceptin works in the body. I think that some people are afraid to ask questions.
1243
. . . have treatments explained in layman’s terms.
1524
The time at which information is given is important, as some patients pointed out how difficult it is to absorb information at the point of diagnosis, when they are in shock and struggling to process everything:
After being informed of the diagnosis by one consultant my husband and I were taken to another room and asked if we had any questions. As we were both in a state of shock at the time it was impossible to think clearly.
505
I would appreciate that things be explained more than once as I tend to forget a lot of information.
1470
Some wanted more clarification about the side effects of Herceptin:
The possible side effects of medication should, most definitely, be explained to patients as, given their emotional state of mind after being diagnosed with cancer, could cause some anxiety, the patient believing, as I did, that they could have cancer in their bones!
1038
I wish I had more information about Herceptin and joint pain.
1383
I have peripheral neuropathy in my fingers and toes, and would have liked to be told more about this.
1524
Others wanted more information about Herceptin itself:
I would like to know if they have found out any new information on Herceptin, just interested to find out.
541
Many wanted more information to help them understand the various tests for their heart function as well as the symptoms of heart problems:
I think people should be advised about what symptoms may indicate heart problems. I didn’t know my symptoms of coughing and shortness of breath were due to a lowered E.F. as a result of a reaction to the Herceptin. If I had I would have drawn the Dr’s attention to it sooner.
1355
I think patients should receive more information regarding the effects Herceptin can have on the heart. I feel had I received this I would have declined the treatment.
1051
Several asked to be told the results of the trial, perhaps seeking reassurance that they had made the right decision to take part:
I would like to know the result of trial.
736
If possible, to be kept up to date with findings from trial & outcomes from trial.
928
I would be interested to know what conclusions are reached in the future concerning the Herceptin 6 months vs 1 year trial.
1141
I would be really interested in receiving a copy of the results of the RCT when they are available.
1256
Patients’ relationships with health-care professionals
Many of the responses made reference to the importance of the patient’s relationship with members of the health-care team. A lot of these responses emphasised that a good relationship led to trust and less stress about treatment:
Most of the time I am fine. When I go to the hospital if the doctors and nurses explain things and tell me what is going to happen next I am fine but if I get a letter I am not expecting for the hospital then I get stressed.
582
I have found it useful that I could consult my research nurse as she put my mind at rest over any problems I may be suffering.
807
I also feel as if I don’t want to bother staff with trivial questions, but to me these questions are important.
1041
Where to go what to do if you feel you have a problem between appointments with consultants – leaflet or card . . . Not being treated like an over anxious idiot when asked about ‘new’ lump.
1090
Feeling abandoned after treatment
Some respondents described feeling unsupported or abandoned after completing their treatment. It would seem that the treatment period can be intense and involves a great deal of contact with the health-care team and other patients. Some people have trouble adjusting to the period of follow-up, when they have little contact with others:
I feel any patient who has gone through this experience and treatment need more contact and support after treatment finished. The help + support received during treatment was excellent but it just felt like when treatment was ended I seemed to be abandoned.
1436
After I finished radiotherapy I did tend to feel a bit depressed. Think that was after I had received a great deal of attention attending 5 days over 5 weeks.
1335
The initial flurry of activity for treatment has died down and now I have to deal with the psychological implication of my diagnosis. An issue that I was not expecting.
1100
Wanting to move on
A fairly common theme throughout the responses was patients expressing a wish to ‘move on’ with their lives and to resume some sense of normality or a semblance of ‘pre-diagnosis’ life:
Today is my last treatment and it is with huge relief, as every 3 weeks reminds you, that you are a cancer patient. It will be somehow nice to close this chapter. Emotionally post treatment is harder than the chem + radio time, which was most unexpected.
496
I found it too long a constant reminder of being a patient. I wanted to get on with my life.
533
Had hoped to be selected for 6 month trial . . . want to get on with life and put the cancer into the past.
614
. . . now looking forward to living again.
1502
. . . now it’s time to move on.
644
Some of the respondents felt very positive about this:
I feel the cancer is a thing of the past; I feel positive about my future.
614
Since treatment ceased my feeling of health and wellbeing has improved tremendously and I would say is nearly back to what it was in April 2009 before I knew anything was wrong and that I had breast cancer.
616
My health is doing really well. I am just looking forward to the future.
644
Some suggesting ways of keeping well and staying positive:
I’ve joined a choir which has improved my breathing possibly due to chemo? Being pro-active has definitely been good for me!
1285
Healthy eating and moderate exercise would help.
1324
Reporting back to patients
On the initial trial consent form, patients were asked if they wanted to receive a copy of the trial results at the end of the trial. The patient representative on the PERSEPHONE grant, who was a member of Independent Cancer Patients’ Voice, provided a lay summary of the results. After ethics approval, the lay summary, together with a PDF copy of the published trial manuscript, was sent to all recruiting sites, along with a site-specific list of trial numbers for those patients who had indicated their wish to receive the trial results. As the trial team had not collected the personal identifiable details of patients, the patients received this information through the NHS clinical teams.
Discussion
The quality-of-life analysis demonstrated that, in both randomised treatment arms, the health states are seen to remain steady from baseline to 3 months into treatment, with a trend towards a slow increase after this, occurring slightly earlier for 6-month patients. However, the patient-reported experience data show the impact of the treatment on patients’ lives.
Collecting ‘quasi-qualitative’ data110 using open-ended questions at the end of a questionnaire or survey is becoming increasingly common, although analyses of these data remain novel. 111 This is championed as a way to add depth to assessments112 and complement quantitative survey data. 113
However, there are certain drawbacks using these data. For example, it is important to be aware that free-text comments may not represent the survey population, and just because an issue is raised by one participant does not mean that it is not important to others who did not raise it. 114 In addition, data from general open questions can lack some of the key strengths of qualitative research. It could be argued that the closed questions indicate the legitimate agenda for the responses to the general open question and thus may impose constraints on responses. More importantly, there is a lack of attention to context, and a lack of conceptual richness, because the data in each case often only consist of a few sentences or less and, typically, only a small space is provided for responses. 115
Furthermore, because the data collected are arguably not strictly either qualitative or quantitative, there is a consequent lack of clarity around how to analyse and report them and about the time and expertise needed to do so. 115
Several issues were noted that may have an impact on the usefulness and reliability of the data collected here. These were as follows.
Two hundred and seventy-one patients congratulated or thanked staff in the questionnaire, often mentioning departments and/or staff personally. Another 37 complained about problems that were relevant only to their local treatment, such as delays in departments, inefficiency and lack of communication. Therefore, the reason for the data collection may have been unclear and these patients might not have realised that these data would be going to a central office and not to hospital staff.
Patients replied to the questions at different times throughout their treatment and in a different number of instances. This was further complicated when the side effects of chemotherapy were still having an impact on earlier assessments. Some patients mentioned this themselves:
I have found it difficult to differentiate between the lasting effects of the chemotherapy, steroids and antibiotics (which I don’t usually use) and the effects of herceptin.
3901
Finally, it was difficult to understand the circumstances or state of mind of patients when they were completing the questionnaire and whether or not that had an impact on their responses. Within this framework of patient-reported experience collection, patients could not be asked to expand on, describe or illustrate certain comments that they made, which is the advantage of more in-depth qualitative interviews. However, the data collected are informative and provide an insight into the patient-reported experiences of patients treated within a RCT.
Chapter 7 Discussion
Interpretation
Adjuvant trastuzumab has improved the outcome of HER2-positive early breast cancer, which, before the introduction of trastuzumab, was a molecular subtype associated with a poor prognosis. 5 The pivotal registration trials10,15 marked a revolution in treatment116 and improved outcomes so that they were similar to those of breast cancers with a better prognosis. The registration trials used 12 months as the duration of trastuzumab; this then became the licensed duration, although shorter durations were not tested in these trials. At the special symposium at the American Society of Clinical Oncology in 2005, at which the two licensing trials were presented, views were expressed from the USA that future trials should usefully assess whether or not shorter durations might produce similar benefits. Therefore, from the start of trastuzumab’s use as an adjuvant treatment, its optimal duration was questioned. In addition, in the following year (2006), the publication of the FinHer trial,20 demonstrating efficacy of a significantly shorter duration of trastuzumab (9 weeks) compared with a no-trastuzumab control arm, further stimulated interest in trials of shorter duration. Hence, the PERSEPHONE trial was designed to test 6 months of trastuzumab against the standard duration of 12 months. Using an established non-inferiority design,39,117 PERSEPHONE has demonstrated that 6 months’ trastuzumab is non-inferior to 12 months’, with a 4-year DFS of 90.3% for 12 months compared with 89.5% for 6 months (HR 1.10, 90% CI 0.96 to 1.26). This result is statistically significant for non-inferiority, with a p-value of 0.01, and, in addition, the p-value for superiority (two-sided) of 12 months compared with 6 months is not significant (p = 0.26). Our definition of non-inferiority was an absolute difference of no worse than 3% below the standard group’s 4-year DFS rate, and the non-inferiority limit was calculated as a HR of < 1.33. The upper confidence limit of the HR was 1.26, which does not cross this non-inferiority limit or critical value. This reflects that, although the non-inferiority boundary was set at 3%, the actual point estimate reduction observed was much smaller, at 0.8% for 4-year DFS and 0.3% for the landmark 4-year DFS (from 6 months after the start of trastuzumab). Other outcome analyses, including OS, landmark OS (6 months after the start of trastuzumab), and sensitivity analyses for invasive DFS,118 distant DFS and breast cancer specific survival, are all congruent (see Appendix 2, Figures 25–27). All measures of cardiac and other toxicity showed significantly decreased rates with 6 months’ treatment and, therefore, the balance of risk and benefit favours shorter treatment.
The within-trial cost-effectiveness analysis showed that 6 months of trastuzumab was associated with an estimated incremental cost saving of £9537 (95% CI £9183 to £9890) and an incremental effect of +0.003 QALYs (95% CI −0.015 to 0.021 QALYs) compared with 12 months of trastuzumab. Six months of trastuzumab is therefore cost-effective and dominates the longer duration of trastuzumab (INB 0.48, 95% CI 0.45 to 0.51). The probability that 6 months compared with 12 months of trastuzumab was cost-effective was 100%. The sensitivity analysis that compared different delivery and drug cost options for trastuzumab indicated that the biosimilar intravenous options recently made available are likely to result in the largest cost savings. The lifetime decision model, which is the preferred analysis for NHS decision-making, estimated cost savings of £9316 and a slight reduction of 0.01 QALYs per individual, indicating that a reduced length of trastuzumab treatment of 6 months has a negligible impact on QALYs and is significantly cost saving (INB of 0.46 QALYs).
Overall evidence from trials of reduced duration trastuzumab
PHARE: 6 versus 12 months
The PHARE trial was a French national study, funded by the Institut National du Cancer, that was carried out between May 2006 and July 2010; 3380 patients were randomised to either 6 or 12 months’ trastuzumab (Table 25). Prior to the start of the PERSEPHONE trial and during the trial’s recruitment phase, the trials teams met and agreed on common data collection items so that a joint analysis of individual patient data could occur once both trials had been completed and published. The two trials had significant similarities, including mapping on to standard national practice for the treatment of HER2-positive early breast cancer. Differences included the randomisation time point; in PHARE, this was between 3 and 6 months of trastuzumab, which would have excluded all patients who developed cardiac toxicity in the first 3–6 months, whereas at the start of the PERSEPHONE trial randomisation was carried out prior to any trastuzumab treatment. Subsequently, the PHARE TMG advised that entering patients into a 6 versus 12 months trial was in their opinion more acceptable to patients after they had already received some trastuzumab. As recruitment into PERSEPHONE was slow at this point, the TSC and TMG decided to make the randomisation point more flexible, allowing recruitment up to and including the ninth cycle of trastuzumab. The original PHARE statistical plan was to recruit 7000 patients so that non-inferiority could be detected at the 2% level. With a 2-year DFS estimated at 85%, the HR limit or critical value was predetermined to be 1.15. Shortly after the start of the trial, there was a change in the statistical plan that allowed for longer recruitment time and follow-up, which altered the recruitment target to 3400 patients. The PHARE trial published the results of 2-year DFS in 2013,23 which failed to demonstrate non-inferiority, with 12-month patients having a 2-year DFS of 93.8% (95% CI 92.6% to 94.9%) and 6-month patients having a 2-year DFS of 91.1% (95% CI 89.7% to 92.4%) (HR 1.28, 95% CI 1.05 to 1.56; non-inferiority p = 0.29). More recently, the PHARE trial published long-term follow-up data and demonstrated a HR after 7.5 years’ median follow-up very similar to that in PERSEPHONE (PHARE HR 1.08, 95% CI 0.93 to 1.25; p = 0.39). The statistical analysis plan with a critical value of HR 1.15 does not allow a conclusion of non-inferiority, although the similarity of the results from the two trials is striking.
| Trial characteristic | Trial name/design | ||||
|---|---|---|---|---|---|
| PERSEPHONE | PHARE | HORG | SOLD | Short-HER | |
| Duration of trastuzumab | 6 months vs. 12 months | 6 months vs. 12 months | 6 months vs. 12 months | 9 weeks vs. 12 months | 9 weeks vs. 12 months |
| Recruited (n)/target (N) | 4088/4000 | 3384/3400 (revised from 7000) | 481/480 | 2174/2168 (revised from 3000) | 1253/2500 (funding finished early) |
| Median follow-up (months) | 73 | 42.5a | 49 | 62.4 | 72 |
| 90b | |||||
| Number of DFS events; patients with an event (%) | 566 events; 14% | 175 events; 5.2%a | 45 events; 9.3% | 245 events; 11.2% | 200 events; 15.9% |
| 704 events; 21%b | |||||
| Primary outcome reported | 4-year DFS | 2-year DFSa | 3-year DFS | 5-year DFS | 5-year DFS |
| Design | Non-inferiority | Non-inferiority | Non-inferiority | Non-inferiority | Non-inferiority |
| Absolute non-inferiority margin | < 3% | < 2% | < 8% | < 4% | < 3% |
| HR non-inferiority limit | < 1.33 | < 1.15 | < 1.53 | < 1.385 | < 1.29 |
| Alpha power | 5% one-sided significance, 85% power | 5% one-sided significance, 80% power | 5% one-sided significance, 80% power | 5% one-sided significance | 5% one-sided significance, 56% power for revised sample size of 1250 |
| Time point DFS rates reported at |
2-year DFS: 96.2% vs. 95.8% 4-year DFS: 90.3% vs. 89.5% |
2-year DFS: 93.8% vs. 91.1%a | 3-year DFS: 95.7% vs. 93.3% | 5-year DFS: 90.5% vs. 88% | 5-year DFS: 87.5% vs. 85.4% |
| 5-year DFS: 86.2% vs. 84.2%b | |||||
| HR | 1.10 (90% CI 0.96 to 1.26; non-inferiority p = 0.01) | 1.28 (95% CI 1.05 to 1.56; p = 0.29)a | 1.57 (95% CI 0.86 to 2.10; p = 0.137) | 1.39 (90% CI 1.12 to 1.72) | 1.15 (95% CI 0.91 to 1.46) |
| 1.08 (95% CI 0.93 to 1.25; p = 0.39)b | |||||
| Conclusion | Non-inferior | Not non-inferiorb | Not non-inferior | Not non-inferior | Not non-inferior |
HORG: 6 versus 12 months
This trial from the Hellenic Oncology Research Group reported 3-year DFS in 481 randomised patients (see Table 25); 67% of patients had ER-positive tumours and 79% had malignant involvement of axillary lymph nodes, with 14% having more than four nodes positive, demonstrating a higher risk of relapse profile than patients in the other duration trials. The non-inferiority HR limit of 1.53 was derived from an estimated absolute difference in 3-year DFS of 8%, based on an expected DFS in the 12-month group of 85%. This was calculated before the start of the trial. The 3-year DFS was 95.7% for 12 months’ trastuzumab and 93.3% for 6 months’ trastuzumab, with a HR of 1.57 (95% CI 0.86 to 2.10; non-inferiority p = 0.137), thus failing to demonstrate non-inferiority. Had non-inferiority been demonstrated in this trial, it is debatable whether or not, with a non-inferiority margin of 8%, the result would have been practice-changing.
SOLD: 9 weeks versus 12 months
The SOLD trial has been described in Chapter 1. In a time-driven analysis 2 years after the last patient was randomised, the 5-year DFS of 2174 patients was analysed, with a median follow-up of 5.2 years25 (see Table 25). The non-inferiority limit was derived from the true (observed) standard-arm 5-year DFS of 88.7%, and the HR limit or critical value was 1.385, which was calculated when the statistical design of the trial was changed from superiority to non-inferiority in February 2014. The results showed a 5-year invasive DFS of 90.5% for the 12-month arm and 88.0% for the 9-week arm, with a HR of 1.39 with two-sided 90% CI of 1.12 to 1.72, and therefore the upper CI crossed the non-inferiority boundary, failing to demonstrate non-inferiority. However, distant DFS (94.2% for 12 months and 93.2% for 9 weeks) and OS (95.9% and 94.4%, respectively) did not differ substantially between the groups. The design of the trial was based on the original FinHer experimental arm in which 9 weeks’ trastuzumab was given concurrently with docetaxel and was the first adjuvant treatment, followed by FEC chemotherapy and then completion of 12 months’ trastuzumab in the standard arm. The total dose of trastuzumab in the 9-week arm was 20 mg/kg, in comparison with 56 mg/kg in the 6-month arms of PERSEPHONE, PHARE and HORG, and perhaps this lower dose is insufficient to demonstrate non-inferiority, despite being given upfront and concurrently with docetaxel. An exploratory subgroup analysis with relatively small numbers suggests that docetaxel at a dose of 100 mg/m2 rather than 80 mg/m2 may be associated with non-inferiority of 9 weeks’ trastuzumab, but this would have to be confirmed in a further randomised trial.
Short-HER: 9 weeks versus 12 months
The Short-HER trial has been described in Chapter 1. There were some differences in the chemotherapy given in the experimental arm and the standard arm, which was required by the government funders. The standard arm used chemotherapy that was in the national guidelines and the experimental arm was the same as in the FinHer and SOLD trials. Chemotherapy in the standard arm consisted of AC or EC with a dose of epirubucin of 90 mg/m2 administered every 3 weeks for four cycles followed by 175 mg/m2 paclitaxel or 100 mg/m2 docetaxel every 3 weeks for four cycles. Trastuzumab was administered every 3 weeks for 18 cycles, starting with the first taxane dose (8 mg/kg loading dose at first cycle, and 6 mg/kg thereafter). Chemotherapy in the experimental arm consisted of 100 mg/m2 docetaxel every 3 weeks for three cycles with concurrent trastuzumab weekly for 9 weeks followed by FEC (60 mg/m2 of epirubicin) for three cycles. Therefore, the standard arm had higher doses of epirubucin, and four cycles rather than three of each chemotherapy type. Accrual was planned for 2332 patients, but the trial was required to meet timelines from the funders and therefore had to complete recruitment after 1254 patients, which meant that the trial was underpowered, with a power of 56% for the non-inferiority end point (see Table 25). Five-year DFS was 88% in the standard and 85% in the experimental arm, with a HR of 1.13 (90% CI 0.89 to 1.42) and with the upper limit of the CI crossing the non-inferiority limit, which was set at 1.29. Therefore, the trial could not claim non-inferiority. A Bayesian analysis showed that the probability that the 9-week arm was non-inferior to the 12-month arm was 80%. The 5-year OS did not differ substantially between the groups, at 95.2% in the standard arm and 95.0% in the short arm (HR 1.07, 90% CI 0.74 to 1.56).
Trial-level meta-analysis
In a meta-analysis of the latest published data from the PHARE trial51 and the PERSEPHONE trial (this analysis), we compared 6 months and 12 months of trastuzumab treatment. 119 As the PHARE trial randomised patients to 3 or 6 months of trastuzumab treatment and timed DFS from randomisation, we used the landmark analysis timed from 6 months after the start of trastuzumab as the most appropriate end point comparable with that used in the PHARE trial. The weighted 4-year DFS rate was 88.9%. Thus, with the methodology used in our study, and with a non-inferiority limit of 2%, the non-inferiority limit for the HR was calculated as 1.19. There was no detectable difference between the two trials’ results (heterogeneity p = 0.94), and the pooled HR for DFS of 1.08 (90% CI 0.98 to 1.18) met the prespecified definition of 2% non-inferiority (non-inferiority p = 0.04). The data from both PERSEPHONE and this meta-analysis of trial-level evidence demonstrate that 6 months’ trastuzumab is non-inferior to 12 months’ trastuzumab in the population as a whole. In this population there will, therefore, be a significant number of patients in whom reducing trastuzumab to 6 months should be possible.
Generalisability
Strengths of the study
The PERSEPHONE trial was pragmatic and mapped on to standard practice in the UK. Study sites could approach all patients in whom treatment with adjuvant or neoadjuvant chemotherapy and trastuzumab was planned. It therefore examined the effect of reducing trastuzumab to 6 months in a ‘real world’, post-licensing population treated with chemotherapy and trastuzumab. All types of chemotherapy were included in the trial and therefore as standard chemotherapy practice gradually changed over the course of the trial, continued recruitment was facilitated and the recruitment target was successfully reached. This also means that results will be applicable to this population treated in routine clinics. Detailed cardiology and health economic substudies are further strengths and include within-trial cost-effectiveness analysis and modelling the long-term cost-effectiveness of trastuzumab. Collection of germline blood samples and formalin-fixed paraffin-embedded tissue from around 80% of patients provides an invaluable translational resource to explore pharmacogenetics and trastuzumab cardiotoxicity, inherited germline predisposition to HER2-positive breast cancer and molecular markers predicting outcomes for different durations of trastuzumab.
Limitations of the study
There are some limitations because of the wide range of chemotherapy treatments accepted in the trial, and subgroup analysis based on specific regimens is not possible. However, the recruitment of required numbers for sufficient levels of significance and statistical power outweighs this limitation in our view. The selection of chemotherapy and trastuzumab timing according to perceived risk by the investigators (i.e. concurrent preferred in higher-risk patients) is a further limitation. The changes in standard practice during the trial and, therefore, the different lengths of follow-up also bring some limitations. The variable timing of randomisation potentially introduces ascertainment bias. The analyses were performed in accordance with the intention-to-treat principle. We believe that this is the most appropriate approach to use as it ensures unbiased estimates. There are some concerns that it can potentially underestimate differences between treatment arms and thus drive the results towards non-inferiority. However, this concern is valid only when adherence to treatment is low and/or differential loss to follow-up occurs. In the PERSEPHONE trial, adherence to protocol-mandated treatment is high and loss to follow-up is low and balanced across randomised treatment arms, which we believe mitigates this concern.
Anthracycline effectiveness in HER2-positive breast cancer
Recently, chemotherapy regimens that avoid anthracyclines completely have been introduced in low-risk HER2-positive breast cancer. 120–122 The BCIRG-006 study18 is the randomised Phase III trial that addressed the question of anthracycline-free chemotherapy with trastuzumab. This trial was undertaken in a similar risk population to that in the HERA trial and reported that substituting standard adjuvant chemotherapy of AC-TH for carboplatin and docetaxel with trastuzumab (TCH) resulted in similar 5-year DFS of 84% and 81%, respectively, although the trial was not powered to assess non-inferiority between the two arms. Later follow-up results showed 10-year DFS of 74.6% for AC-TH and 73.0% for TCH. Two other trials are Phase II non-randomised studies in HER2-positive patients,120–122 with a low risk of recurrence (in the trial reported by Tolaney120,121 axillary lymph node negative and tumour size < 3 cm), and, although the reported results are excellent, there is no comparator standard arm. In the UK, at present, for patients at sufficient risk of recurrence to be recommended chemotherapy and trastuzumab, standard practice is to use anthracyclines (usually in combination with taxanes) unless the patient has specific contraindications, for example pre-existing cardiac problems.
There is a very long history of anthracyclines being used in the treatment of breast cancer, including HER2-positive breast cancer. 75 There is evidence of the additive effects of doxorubicin and antibodies to HER2 receptors in cell culture, and significant synergy has been observed in xenografts in mice. 123 Doxorubicin and epirubicin cause cytotoxic activity by DNA intercalation, inhibition of the Type II topoisomerase enzyme, free-radical production, and promotion of histone eviction from transcriptionally active chromatin. 124–127 Additional mechanisms of action for anthracyclines include ‘immunogenic cell death’ and the induction of anticancer immune responses. 128,129 There is evidence that anthracyclines can produce therapy-induced immunosurveillance,130 thereby inducing an immune response to cancer cells. There is also evidence that anthracyclines can stimulate cancer cell-autonomous production of type I interferon in mouse models,131 and recently they have also been found to activate STING (stimulator of interferon genes)-dependent innate immune signalling pathways. 132 There is also some evidence that anthracyclines have an enhanced effect in ER-negative tumours that have CD8+ tumour-infiltrating lymphocytes. 133 The presence of greater numbers of tumour-infiltrating lymphocytes is a good prognostic finding in early HER2-positive breast cancer133,134 and also in metastatic disease, as found in CLEOPATRA (Clinical Evaluation of Pertuzumab and Trastuzumab Trial). 135 Not only are tumour-infiltrating lymphocytes prognostic, but there is evidence that greater numbers of tumour-infiltrating lymphocytes improve the responsiveness to anti-HER2 directed therapy in the metastatic setting. 135 Recently, there has been a report that > 20% tumour-infiltrating lymphocytes in HER2-positive breast cancer provides a prognostic biomarker in the Short-HER trial as well as an indication that 9 weeks of trastuzumab is sufficient, compared with 12 months. 136 The mechanisms of action of trastuzumab include antibody-dependent cell-mediated cytotoxicity and the activation of antigen-specific antitumour immune response. 129 This evidence would point to significant immune mechanisms for activity of both anthracyclines and trastuzumab, as well as that anthracyclines, by producing an induced immune response, could enhance the effect of trastuzumab given after anthracyclines. There is some evidence supporting this in the clinical literature. The BCIRG-006 trial reported initial 5-year DFS results that demonstrated a 3% difference between AC-paclitaxel/trastuzumab (84%) and the non-anthracycline regimen TCH (81%). 18 In the HERA trial, most trastuzumab was given sequentially after chemotherapy, and the subgroup analysis comparing anthracycline only and anthracycline with taxane chemotherapy hinted that the effect of trastuzumab was greater with anthracycline chemotherapy than with the combination. 10 Our own data also point to the activity of anthracyclines given for six cycles with sequential trastuzumab, which was a more common regimen early in the trial, and therefore these patients have the longest median follow-up and yet similar outcomes to the patients receiving anthracycline with taxane chemotherapy and concurrent trastuzumab. Our view remains that, for patients with tumours of enough risk to warrant chemotherapy and trastuzumab, careful consideration needs to be given in discussion with the patients with regard to anthracycline-free chemotherapy regimens.
Concurrent versus sequential trastuzumab and chemotherapy
When the PERSEPHONE trial started, the existing evidence was from two large registration trials that had demonstrated a significant effect of adjuvant trastuzumab compared with a no-treatment control. HERA used trastuzumab sequentially after chemotherapy, and NSABP B-31 and NCCTG N9831 (joint analysis) used only trastuzumab concurrent with the taxane component of chemotherapy. Therefore, when the PERSEPHONE trial started, there was evidence to support both sequential and concurrent trastuzumab with chemotherapy, and in this pragmatic trial, mapping on to standard practice, patients receiving sequential or concurrent trastuzumab and chemotherapy were able to be included. With the publication of PACS-0419 and N983116 evidence was emerging that suggested that delivering trastuzumab concurrently might be the better approach, and so it became more standard in routine clinics. Therefore, as more evidence was published, we saw a gradual decrease in the use of sequential, anthracycline-based chemotherapy and an increase in concurrent treatments with anthracycline and taxane combinations in our trial population.
Subgroup analysis in the trial
Experts including Altman137 and Peto138 have written extensively about the limitations of subgroup analyses, which can lead to subgroup findings being applied incorrectly to change patient treatments, either to introduce ineffective treatments in, or to withhold effective treatments from, certain subgroups.
Ten criteria have been put forward for assessing the credibility of the subgroup effects reported in clinical trials139,140 to judge whether or not it would be appropriate to take these forward into clinical practice. The PERSEPHONE trial has an adequate sample size to answer the question of non-inferiority of 6 months’ trastuzumab compared with 12 months’ in the trial as a whole, but not to answer the question in specific subgroups.
An interaction was seen between the concurrent and sequential administration of trastuzumab and chemotherapy and 6 and 12 months’ trastuzumab. Factors supporting the credibility of this observed effect was that concurrent versus sequential treatment was a stratification factor at randomisation, that the test for interaction was significant (p < 0.001), and that the subgroup effect was consistent across related outcomes. However, factors that did not support credibility of this subgroup finding were that the subgroup variable was not a baseline patient characteristic but was chosen by treating clinicians depending on patient characteristics, centre-based protocols, and the national standards at the time of treatment. In addition, this particular subgroup hypothesis was not specified a priori, and there was no indirect evidence to support the apparent subgroup effect; in fact, the opposite was the case. Concurrent trastuzumab and chemotherapy had been found to be more effective than sequential treatments,16 and a hypothesis emerged that, with the greater effect of concurrent treatment, if there was an interaction between sequential and concurrent trastuzumab and chemotherapy it was likely to be in favour of 6 months’ treatment with concurrent trastuzumab and chemotherapy. Therefore, the subgroup interaction observed in the PERSEPHONE trial was in the opposite direction to that hypothesised and also to that initially seen in the PHARE trial. There is no obvious, plausible biological explanation for our finding. Therefore, although the test of interaction was significant (p < 0.001), this was not seen in other studies (PHARE), and so, on balance, this subgroup analysis is more likely to be misleading and to have been affected by the play of chance.
Evidence for additional anti-HER2 treatments
There have been changes to treatments for HER2-positive early breast cancer since the study started, particularly in the 3 years after the last patient in the trial completed treatment. This has included 11 years’ follow-up of the HERA trial, which included extending adjuvant therapy to 24 months,13 trials testing newly developed HER2-directed therapies both antibodies with pertuzumab,141 (Perjeta®; Roche, Switzerland) and small-molecule treatments with neratinib. 142,143 In addition, there have been successful neoadjuvant trials including studies of pertuzumab (NeoSphere),144,145 and, most recently, studies in the post-neoadjuvant context with the use of trastuzumab emtansine in patients who have not achieved a pathological complete response from neoadjuvant treatment.
Trastuzumab and pertuzumab
The APHINITY (Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer) trial was published in 2017. 141 This compared adjuvant chemotherapy and 12 months’ trastuzumab with or without 12 months’ pertuzumab for the treatment of HER2-positive breast cancer; 63% of patients were node positive and 36% were ER negative. Invasive DFS was timed from randomisation and included local and distant recurrence, contralateral new breast cancer and death from any cause, but did not include second primaries. The estimates of 3-year invasive DFS rate were 94.1% in the pertuzumab group and 93.2% in the placebo group (HR 0.81, 95% CI 0.66 to 1.00; p = 0.045). Among the cohort of patients with node-positive disease, the 3-year rate of invasive DFS was 92.0% in the pertuzumab group, compared with 90.2% in the placebo group (HR for an invasive-disease event 0.77, 95% CI 0.62 to 0.96; p = 0.02). Among patients with node-negative disease there was no improvement in invasive DFS with added pertuzumab at this early time point. In December 2017146 the US Food and Drug Administration (FDA) licensed adjuvant pertuzumab for the treatment of patients who had a high risk of recurrence. In March 2019, NICE approved pertuzumab for use in the NHS with trastuzumab biosimilars147 in patients with node-positive disease. The landscape of the treatment of HER2-positive early breast cancer is therefore very different from that when the trial started.
Neratinib for 12 months after trastuzumab
Neratinib is a potent, irreversible tyrosine kinase inhibitor of HER1, HER2, HER3 and epidermal growth factor receptor. It inhibits the phosphorylation of these receptors by irreversibly binding to the intracellular signalling domain, thereby inhibiting HER-mediated downstream signalling. The ExteNET trial142,143 examined the use of an additional 12 months of neratinib in patients after they had completed 1 year of adjuvant treatment with chemotherapy and 12 months of trastuzumab. A total of 2840 women were randomly assigned up to 2 years after completing primary treatment (amended to 1 year after, during the study) to 12 months’ neratinib or placebo. The 2-year invasive DFS rate was 93.9% (95% CI 92.4% to 95.2%) in the neratinib group and 91.6% (95% CI 90.0% to 93.0%) in the placebo group, with a stratified HR of 0.67 (95% CI 0.50 to 0.91; p = 0.0091). The most common severe adverse event (grade 3 or above) was diarrhoea, with a 40% incidence. In a prespecified subgroup analysis, patients with ER-positive disease seemed to gain more benefit than those with ER-negative disease [ER positive, HR 0.51, 95% CI 0.33 to 0.77, p = 0.0013; ER negative, HR 0.93, 95% CI 0.60 to 1.43, p = 0.74; p (for interaction) = 0.054]. Neratinib’s activity in ER-positive disease has raised questions about the predominant mechanism of action in these tumours and whether or not it also inhibits ER-related signalling pathways. Although 1 year’s neratinib has received marketing authorisation approval from both the FDA and the European Medicines Agency, uptake has been low in the USA and Europe, thought to be because of neratinib’s modest effect and the high incidence of severe diarrhoea. NICE has now appraised neratinib and has approved it for 1-year extended use in patients with ER-positive, HER2-positive breast cancer but only if they had received 1 year of single agent adjuvant trastuzumab and no pertuzumab. 148 However, the NICE appraisal states that it is unclear which patients would receive neratinib in clinical practice.
HERA and 24 months’ trastuzumab
The HERA trial also tested 24 months’ trastuzumab compared with 12 months’ trastuzumab and a no-treatment control arm. 10 The 11-year follow-up, published in 2017,13 showed that 24 months was no better than 12 months in terms of DFS (HR 1.02, 95% CI 0.89 to 1.17), with 10-year DFS of 69% for 1 year and 69% for 2 years of trastuzumab. Extending trastuzumab treatment beyond 12 months did not improve DFS further.
Neoadjuvant trials in HER2-positive disease
Neoadjuvant trials are an excellent route for accelerated drug approval and have been particularly effectively used in HER2-positive breast cancer to speed the process of new drug approval in the adjuvant setting. 149 The NeoSphere trial looked at neoadjuvant trastuzumab and pertuzumab144,145 in a 417-patient randomised Phase II study with four arms. Group A received standard trastuzumab with docetaxel; group B received pertuzumab and trastuzumab with docetaxel; group C received pertuzumab and trastuzumab; and group D received pertuzumab and docetaxel. The primary end point was pathological complete response. This was highest in group B, at 45.8%, followed by group A (29%) and then group D (24%), with the lowest rate in group C (16.8%). The 5-year follow-up analysis confirmed that progression-free survival mirrored the pathological complete response rates and demonstrated differences in 5-year progression-free survival that, because of the small numbers in the trial, did not reach statistical significance. These were 86% (95% CI 77% to 91%) for group B, 81% (95% CI 71% to 87%) for group A, 73% (95% CI 64% to 81%) for group C, and 73% (95% CI 63% to 81%) for group D (group B vs. group A: HR 0.69, 95% CI 0.34 to 1.40; group C vs. group A: HR 1.25, 95% CI 0.68 to 2.30; group D vs. group B: HR 2.05, 95% CI 1.07 to 3.93). NeoSphere prompted the accelerated approval of neoadjuvant pertuzumab with trastuzumab and chemotherapy from the FDA,149 the EMA150 and NICE. 151 The promising early results of neoadjuvant pertuzumab had already led to the APHINITY trial, which tested the addition of pertuzumab to standard chemotherapy and trastuzumab treatment in the adjuvant setting. 141 This trial had already completed recruitment when the FDA stipulated that if the adjuvant APHINITY trial did not demonstrate benefit from the addition of pertuzumab, then approval for its use in the neoadjuvant setting would be withdrawn.
In the recently published Katherine Trial,152 trastuzumab emtansine (Kadcyla®; Roche, Switzerland) was tested against trastuzumab after the failure of neoadjuvant chemotherapy and anti-HER2 therapy to produce a complete pathological response. The estimated proportion of patients who were free of invasive disease at 3 years was 88.3% in the trastuzumab emtansine group and 77.0% in the trastuzumab group. Invasive DFS was significantly higher in the trastuzumab emtansine group than in the trastuzumab group (HR for invasive disease or death 0.50, 95% CI 0.39 to 0.64; p < 0.001). This significant improvement in outcome is likely to lead to an appropriate escalation of standard treatment in the post-neoadjuvant setting, at least for patients who do not achieve a pathological complete response with neoadjuvant treatment.
Trials are now being developed of the de-escalation of adjuvant anti-HER2 therapy after a pathological complete response to neoadjuvant treatment has been achieved. This is an important development in the portfolio of trials for HER2-positive early breast cancer.
Challenges of implementing de-escalation of established treatments
The common pathways for improvements in cancer outcomes are the introduction of additional treatments and the escalation of existing treatments. These often result in more toxicities for patients and a slower return to normal life, and this is accepted when there are proven, clear and significant improvements in outcomes. The introduction of adjuvant trastuzumab led to routine HER2 testing of all breast cancers, and patients with tumours testing positive then received chemotherapy and trastuzumab according to the licence. Many of these patients have small (pT1a–b), node-negative tumours and would not have been considered for chemotherapy prior to 2005 but are now receiving it routinely. Our goals for treatment are to produce best outcomes for patients, while minimising the cost in terms of toxicity, not only treatment side effects but also ‘financial toxicity’ for both patients and health-care systems. However, the challenges of introducing de-escalated treatments remain considerable and are particularly relevant in the case of trastuzumab, as over the past 13 years we have seen this treatment transform the lives of patients with breast cancer. Inevitably, in de-escalation trials there is likely to be some loss of efficacy, however small, and the challenge is to balance this against the gains in terms of reduced toxicity. Even so, there may be an understandable nervousness on the part of specialist teams and some patients to consider a change to practice, despite the potential benefits to the individual patient of reduced toxicity, shorter treatment and a more rapid return to normal life. After reflecting on all of these aspects of the trial, we would argue that registration trials are optimal for evaluating shorter treatment durations, and we would strongly encourage such testing for new, targeted adjuvant cancer therapies. Questions of treatment duration in registration trials could occur only with significant international collaboration between the pharmaceutical industry, international academic groups, and governments/medicines approval bodies such as the European Medicines Agency and FDA, with input from the wider cancer specialist teams and cancer patients, as has been discussed. 153 In registration trials, discussion would be required about whether shorter treatments would have to prove non-inferiority compared with longer treatments, or whether longer treatments would have to prove superiority compared with shorter treatments. In general, the escalating cost of effective novel anticancer treatments is rapidly becoming unsustainable, even for wealthy nations, and we believe that clinical trials designed to test shorter treatments as part of pivotal registration trials should become one of the priorities for cancer research.
Global burden of HER2-positive breast cancer and delivery of trastuzumab as a World Health Organization essential medicine
In 2018, GLOBOCAN (Global Cancer Incidence, Mortality and Prevalence from the International Agency for Research on Cancer) estimated that the incidence of new cases of breast cancer was 2.08 million per year. 154 Assuming 12% of breast cancers are HER2 positive, this means that nearly 250,000 new cases of HER2-positive breast cancer are estimated to occur worldwide every year. In November 2015, trastuzumab was included on the World Health Organization’s essential medicines list for both early and metastatic HER2-positive breast cancer for the first time. 155 Marie-Paule Kieny, World Health Organization Assistant Director General for Health and Innovation, has emphasised that essential medicines should be available everywhere at all times, and are chosen according to evidence of safety, efficacy and public health relevance. 156 It is difficult to access accurate figures for the global delivery of trastuzumab, but in low- and middle-income countries it is likely that many women with HER2-positive breast cancer cannot access trastuzumab in either the early or the metastatic setting. On a recent working visit to India to give talks about the PERSEPHONE trial, the chief investigator, Helena Earl, learnt that 5 years ago only 20% of women with HER2-positive breast cancer had been able to access trastuzumab. There has been some improvement in access recently through the licensing of an increasing number of biosimilars, but even in the largest cancer centre in India (Tata Memorial) the estimated availability is still only 53%. Those women who do receive adjuvant trastuzumab will often receive the FinHer regimen of 9 weeks. Evidence from the PERSEPHONE trial will help to improve delivery of trastuzumab globally to achieve the stated aim of the World Health Organization Essential Medicines listing to deliver the medicine to all women with HER2-positive breast cancer. Global implementation of 6 months’ trastuzumab treatment will save a significant number of lives and there is significant interest globally in the PERSEPHONE results for this reason. Implementing 6 months’ adjuvant trastuzumab in low- and middle-income countries would halve the cost of treatment and produce similar outcomes to 12 months’ treatment.
Trastuzumab biosimilars
As of May 2019, five trastuzumab biosimilars had been approved by the European Medicines Agency (see Appendix 1, Table 56) and four had been approved by the FDA (see Appendix 1, Table 57). There have been Phase III trials of these biosimilars demonstrating non-inferiority of outcomes and safety when compared with the originator trastuzumab in both neoadjuvant and metastatic settings. 157 These biosimilars are marketed at lower cost and are already in widespread use in the UK. NICE’s recent approval of adjuvant pertuzumab and trastuzumab in the treatment of node-positive, HER2-positive breast cancer advises the use of biosimilar trastuzumab with pertuzumab. 147 Therefore, a combination of the introduction of biosimilars and the reduction of trastuzumab duration to 6 months in some patients will significantly reduce the costs of trastuzumab delivery in the NHS.
Conclusions
The PERSEPHONE trial has demonstrated non-inferiority of 6 months’ trastuzumab compared with 12 months’ trastuzumab. DFS at 4 years is 89.5% for 6 months compared with 90.3% for 12 months, a difference of only 0.8%. Cardiotoxicity was reduced in the 6-month arm, with only 3% of patients stopping trastuzumab early on account of cardiotoxicity, compared with 8% of 12-month patients. The incidence of severe adverse events was also lower among the 6-month patients (19%) than among the 12-month patients (24%). Patient-reported experiences showed that the side effects of trastuzumab, particularly fatigue and aches/pains, had a significant impact on daily life. Health economic analysis showed that, with significantly lower lifetime costs and similar lifetime QALYs, there is a high probability that 6 months’ trastuzumab is cost-effective compared with 12 months’ trastuzumab.
Future research
Future research will include a risk-based analysis of non-inferiority using recognised clinical prognostic categories and predictive tools. Multivariable analysis will be carried out to explore whether or not, among the trial population who have superior outcomes with 12 months’ trastuzumab compared with 6 months’ trastuzumab, there are high-risk groups. A joint analysis with the PHARE trial group has been planned from the start of the PERSEPHONE trial. An individual patient data meta-analysis is planned with other trastuzumab duration trials. Translational research exploring tumour biomarkers of response to shorter durations of trastuzumab will be carried out. The collection of blood for germline analysis will allow studies of inherited germline predisposition for HER2-positive breast cancer and pharmacogenetic studies of trastuzumab outcomes and cardiotoxicity.
Acknowledgements
We would especially like to acknowledge the invaluable contribution of the 4088 patients (4082 women and six men) who took part in our study. We would like to thank all of the members of the TSC and independent DSMC for their wise guidance and support throughout the study. We are grateful to the consultants and their teams at all 152 recruiting sites who worked so hard carrying out the study and supporting their patients. We thank the National Cancer Research Network (now part of the Clinical Research Network) for support of research personnel at the recruiting sites in England: the NHS Research Networks in Scotland (funded by the Chief Scientists’ Office ); and the Health and Care Research Office in Wales. We would like to acknowledge our gratitude to the staff of the NIHR HTA programme for their helpful support and guidance throughout.
Contributions of others
Katharina Diernberger (Research Fellow in Health Economics) assisted with the development of the economic model.
Pedro Rodrigues (Research Assistant, University of Leeds) assisted with the cost-effectiveness within-trial and model analysis.
We acknowledge the 210 investigators and their teams from 152 participating UK sites who entered patients into the PERSEPHONE trial.
| Randomising site | Randomising consultant(s) |
|---|---|
| Aberdeen Royal Infirmary | Dr A Radha Todd, Dr Ravi Sharma and Dr Trevor McGoldrick |
| Addenbrooke’s Hospital | Dr Charles Wilson, Dr Elizabeth Cox, Dr Jean E Abraham, Dr Karen McAdam and Dr Luke Hughes-Davies |
| Airedale General Hospital | Dr Chris Bradley and Dr Shazza Rehman |
| Alexandra Hospital | Dr Clive Irwin |
| Ashford Hospital | Dr May Teoh |
| Barnet Hospital | Dr Kin Woo, Dr Peter Ostler and Dr Rob Stein |
| Barnsley District Hospital | Dr Bernadette Birtwhistle, Dr Caroline Lee and Dr Lucy Walkington |
| Basildon and Thurrock University Hospital | Dr Helen Swinburn, Dr Naveed Sarwar and Dr Wendy Ella |
| Basingstoke and North Hampshire Hospital | Dr Felicity Ross and Dr Sandra Tinkler |
| Bedford General Hospital | Dr Robert Thomas and Dr Sarah Smith |
| Birmingham Heartlands Hospital | Dr Indrajit Fernando |
| Bishop Auckland General Hospital | Dr Nicolas J Wadd |
| Blackpool Victoria Hospital | Dr Andrew Hindley, Dr Falalu Danwata, Dr Pavel Bezecny and Dr Shabbir Susnerwala |
| Borders General Hospital | Dr Carolyn Bedi |
| Bradford Royal Infirmary | Dr Chris Bradley and Dr Shazza Rehman |
| Broomfield Hospital | Dr Udaiveer Panwar, Dr Vivienne Loo and Professor Neville Davidson |
| Castle Hill Hospital | Dr Amandeep Dhadda, Dr Georgios Bozas, Dr Penny O’Neill, Dr Saiqa Spensley, Dr Sunil Upadhyay and Professor Michael Lind |
| Charing Cross Hospital | Dr Andrea Zivi, Dr Carlo Palmieri, Dr Charles Lowdell, Dr Conrad Lewanski, Dr Constantine Alifrangis, Dr Hanine Medani, Dr Laura Kenny, Dr Matthew Flook, Dr Sean O’Cathail, Dr Susan Cleator, Professor Charles Coombes, Professor Justin Stebbing and Professor Robert Leonard |
| Cheltenham General Hospital | Dr Jo Bowen, Dr Kim Benstead, Dr Peter Jenkins, Dr Radhika Counsell, Dr Roger Owen and Dr Sean Elyan |
| Chesterfield Royal Hospital | Dr Omar Din |
| Christie Hospital | Dr Abbas Chittalia, Dr Andrew Wardley, Dr Anne Armstrong, Dr Helen Mitchell, Dr Juliette Loncaster and Dr Sasha Howell |
| City Hospital | Dr Daniel Rea and Dr David Spooner |
| Clatterbridge Centre for Oncology | Dr Allison Hall, Dr Douglas Errington, Dr Farida Alam, Dr Khizar Hayat, Dr Susan O’Reilly and Dr Zafar Malik |
| Conquest Hospital | Dr Craig Knighton and Dr Gillian Sadler |
| County Hospital | Dr Sam Guglani |
| Cumberland Infirmary | Dr Paul Dyson and Ms Helen Roe |
| Darent Valley Hospital | Dr Andrew Visioli, Dr Catherine Harper-Wynne and Dr Julia Hall |
| Darlington Memorial Hospital | Dr Alison Humphreys, Dr John Hardman and Dr Sophie Haney |
| Dewsbury and District Hospital | Dr Jay Naik |
| Diana, Princess of Wales Hospital | Dr Mohammad Butt and Dr Sunil Upadhyay |
| Doncaster Royal Infirmary | Dr Kathleen Dunn and Dr Sundareswaran Ramakrishnan |
| Dorset County Hospital | Dr Amitabha Chakrabarti, Dr Perric Crellin and Dr Susan Dean |
| Dumfries and Galloway Royal Infirmary | Dr Carolyn Bedi, Dr Marjory MacLennan and Dr Tamasin Evans |
| Ealing Hospital | Dr Conrad Lewanski |
| East Surrey Hospital | Dr Alexander Lee, Dr Eirini Thanopoulou and Dr Stephen Houston |
| Eastbourne District General Hospital | Dr Alistair Ring, Dr Charlotte Moss and Dr Sarah Westwell |
| Essex County Hospital | Dr Devy Basu, Dr Philip Murray and Dr Vivienne Loo |
| Freeman Hospital | Dr Mark Verrill |
| Friarage Hospital | Dr Hans Van der Voet and Dr Sarah Lawless |
| Furness General Hospital | Dr Geraldine Skailes and Dr Sarah Moon |
| George Eliot Hospital | Dr Lydia Fresco, Dr Medy Tsalic and Dr Susan Lupton |
| Glan Clwyd Hospital | Dr Catherine Bale, Dr Jill Bishop and Dr Win Soe |
| Good Hope Hospital | Dr Andrea Stevens and Dr Medy Tsalic |
| Great Western Hospital | Dr Anne Kendall, Dr David Cole, Dr Kinnari Patel and Dr Shiroma De Silva-Minor |
| Guy’s Hospital | Dr Eleni Karapanagiotou, Dr Janine Mansi, Dr Mark Harries, Dr Paul Ellis and Dr Sarah Harris |
| Halton General Hospital | Professor Peter Clark |
| Hexham General Hospital | Dr Anthony N Branson |
| Hinchingbrooke Hospital | Dr Cheryl Palmer, Dr Clary Evans and Dr Simon Russell |
| Ipswich Hospital | Dr Karen E Sherwin and Dr Ramachandran Venkitaraman |
| James Paget Hospital | Dr Adrian Harnett and Dr May Thu Han |
| Kent and Canterbury Hospital | Dr Carys Thomas, Dr Julia Hall and Dr Natasha Mithal |
| Kidderminster Hospital | Dr Mark Churn |
| King Edward VII Hospital | Dr Narottam Thanvi and Dr Ruth Davis |
| King’s College Hospital | Dr Anne Rigg, Dr Fiona Castell and Dr Vasiliki Michalarea |
| King’s Mill Hospital | Dr Sarah Khan and Dr Victoria Brown |
| Leighton Hospital | Dr Laura Horsley |
| Lincoln County Hospital | Dr Abhro Chaudhuri, Dr Elisabeth Murray and Dr Thiagarajan Sreenivasan |
| Lister Hospital | Dr Nihal Shah |
| Luton and Dunstable Hospital | Dr Mei-Lin Ah-See |
| Macclesfield District General Hospital | Dr Lisa Barraclough and Dr Mark Lawrence |
| Maidstone Hospital | Dr Catherine Harper-Wynne, Dr Charlotte Abson, Dr Rema Jyothirmayi and Dr Russell Burcombe |
| Manor Hospital | Dr Indrajit Fernando and Dr Suhail Anwar |
| Medway Maritime Hospital | Dr Charlotte Abson and Dr Maher Hadaki |
| Milton Keynes Hospital | Dr Hany Eldeeb, Dr Maria Karina and Dr S Azhar Rizvi |
| Mount Vernon Hospital | Dr Amy Guppy, Dr Andreas Makris, Dr Arshi Denton, Dr David Miles, Dr Peter Ostler and Professor Jane Maher |
| Musgrove Park Hospital | Dr Emma Cattell, Dr Hilary Barlow, Dr John Graham, Dr Mohini Varughese and Dr Saiqa Spensley |
| Nevill Hall Hospital | Dr Nayyer Iqbal, Dr Simon Waters and Dr Theresa Howe |
| New Cross Hospital | Dr Caroline Brammer, Dr Georgi Georgiev, Dr Laura Pettit, Dr Mark Churn, Dr Prakash Ramachandra and Dr Rakesh Mehra |
| Newham General Hospital | Dr Chris Gallagher, Dr Karen Tipples and Dr Virginia Wolstenholme |
| Norfolk & Norwich University Hospital | Dr Adrian Harnett, Dr Andrew Bulman, Dr Daniel Epurescu, Dr Dinos Geropantas and Dr Susanna Alexander |
| North Devon District Hospital | Dr Kate Scatchard |
| North Middlesex Hospital | Dr Fharat Raja, Dr Jacqueline Newby and Dr Stephen Karp |
| North Tyneside General Hospital | Dr Anthony N Branson |
| Northampton General Hospital | Dr Craig Knighton, Dr Craig Macmillan and Dr Hany Eldeeb |
| Northwick Park Hospital | Dr Andreas Makris and Dr Arshi Denton |
| Nottingham City Hospital | Dr Sarah Khan and Dr Stephen Chan |
| Peterborough City Hospital | Dr Catherine Jephcott, Dr Karen McAdam and Dr Sarah Ayers |
| Pilgrim Hospital | Dr Chiara Intrivici and Dr Elisabeth Murray |
| Pinderfields Hospital | Dr Jay Naik |
| Poole Hospital | Dr Amitabha Chakrabarti, Dr Darcy Goode, Dr Joanne Brady and Dr Susan Dean |
| Prince Charles Hospital | Dr Helen Passant |
| Princess of Wales Hospital | Dr James Powell and Dr Rosie Stevens |
| Princess Royal University Hospital | Dr Elinor Sawyer and Dr Mark Harries |
| Queen Alexandra Hospital | Dr Caroline Archer, Dr Helen Cooper, Dr Joanna Gale and Dr Timothy Gulliford |
| Queen Elizabeth Hospital (Birmingham) | Dr Andrea Stevens, Dr Daniel Rea and Dr Suhail Anwar |
| Queen Elizabeth Hospital (Gateshead) | Dr Daniela Lee, Dr Goudarz Mazdai, Dr Helen Lucraft, Dr Helen Turnbull, Dr Nicola Cresti, Dr Prithvi Jampana, Dr Rebecca Goranova and Dr Wendy Taylor |
| Queen Elizabeth Hospital (King’s Lynn) | Dr Athar Ahmad, Dr Ellen Gokkel, Dr Margaret Daly, Dr Nicola Ainsworth and Dr Shahzeena Aslam |
| Queen Elizabeth Hospital (London) | Dr Bruce Bryant, Dr Hartmut Kristeleit and Dr Mark Harries |
| Queen Elizabeth The Queen Mother Hospital | Dr Carys Thomas, Dr Jane Brown, Dr Julia Hall, Dr Natasha Mithal and Dr Rohit Malde |
| Queen’s Hospital (Burton) | Dr Ad Chetiyawardana and Dr Mojca Persic |
| Queen’s Hospital (Romford) | Dr Anwar Al-Saffar, Dr Caroline Bridgewater, Dr Eliot Sims, Dr Elizabeth Croydon, Dr Emma Staples, Dr Mark Prentice and Dr Mary Quigley |
| Raigmore Hospital | Dr Alison Nicholls, Dr Carol MacGregor, Dr David Whillis, Dr Kay Kelly, Dr Marion Paterson, Dr Neil McPhail, Dr Pinelopi Gkogkou and Dr Prantik Das |
| Rotherham General Hospital | Dr Alex Bradshaw, Dr Bernadette Birtwhistle and Dr Matthew Hatton |
| Royal Albert Edward Infirmary | Dr Elena Takeuchi and Dr Gregory Wilson |
| Royal Berkshire Hospital | Dr Jocelyn Adams, Dr Madhumita Bhattacharyya and Dr Richard Brown |
| Royal Bournemouth Hospital | Dr Tamas Hickish |
| Royal Derby Hospital | Dr Mojca Persic, Dr Pamela Woodings and Dr Prabir Chakraborti |
| Royal Free Hospital | Dr Alison Jones and Dr Jacqueline Newby |
| Royal Glamorgan Hospital | Dr Jacinta Abraham |
| Royal Gwent Hospital | Dr Carys Morgan, Dr Chris Gaffney, Dr Mohammed Harb, Dr Robert Jones and Dr Simon Waters |
| Royal Hampshire County Hospital | Dr Nicholas Murray, Dr Sanjay Raj and Dr Virginia Hall |
| Royal Lancaster Infirmary | Dr David Eaton and Dr Sarah Moon |
| Royal Liverpool University Hospital | Dr Nicola Thorp and Dr Susan O’Reilly |
| Royal Shrewsbury Hospital | Dr Huzeifa Gadir, Dr Laura Pettit, Dr Rajiv Agrawal and Dr Sheena Khanduri |
| Royal Surrey County Hospital | Dr Anthony Neal, Dr Robert Laing and Dr Stephen Houston |
| Royal Sussex County Hospital | Dr Alistair Ring, Dr David Bloomfield, Dr Gargi Patel, Dr Richard Simcock and Dr Sarah Westwell |
| Royal United Hospital | Dr Abigail Jenner, Dr Hugh Newman, Dr Mark Beresford, Dr Rebecca Bowen, Dr Susan Masson and Dr Susana Mancero |
| Russells Hall Hospital | Dr Georgi Georgiev, Dr Laura Pettit, Dr Muhammad Habibullah Khan, Dr Prakash Ramachandra and Dr Rozenn Allerton |
| Salisbury District Hospital | Dr Clare Crowley, Dr Ellen Copson, Dr Jennifer Bradbury and Dr Melanie Harvey |
| Sandwell General Hospital | Dr David Spooner |
| Scarborough General Hospital | Dr Amandeep Dhadda |
| Scunthorpe General Hospital | Dr Saiqa Spensley |
| Solihull Hospital | Dr Medy Tsalic |
| South Tyneside District Hospital | Dr Goudarz Mazdai, Dr Helen Turnbull, Dr Nicola Cresti and Dr Prithvi Jampana |
| Southampton General Hospital | Dr Clare Crowley, Dr Ellen Copson, Dr Jennifer Marshall, Dr Nicholas Murray, Dr Peter Simmonds and Dr Sanjay Raj |
| Southend Hospital | Dr Anne Robinson, Dr Colin Trask, Dr Hafiz Algurafi and Dr Helena Nam |
| Southport and Formby District General Hospital | Dr Helen Neville-Webbe, Dr Khizar Hayat and Dr Nasim Ali |
| St Bartholomew’s Hospital | Dr Chris Cottrill, Dr Chris Gallagher, Dr Emma Spurrell, Dr John Conibear, Dr Karen Tipples, Dr Nita Patel, Dr Rebecca Roylance and Dr Virginia Wolstenholme |
| St George’s Hospital | Dr Ciara O’Hanlon Brown, Dr Laura Assersohn and Dr Muireann Kelleher |
| St Mary’s Hospital (Isle of Wight) | Dr Jennifer Marshall |
| St Mary’s Hospital (London) | Dr Susan Cleator |
| Stepping Hill Hospital | Dr Abbas Chittalia |
| Stoke Mandeville Hospital | Dr Andrew Theobald, Dr Anthony Kong, Dr Christopher Alcock, Dr Clare Jacobs, Dr Eleanor James and Dr Thinn Pwint |
| Sunderland Royal Hospital | Dr A Radha Todd, Dr Kathryn Wright and Dr Sanjoy Chatterjee |
| The County Hospital (previously Staffordshire General) | Dr Amjad Al-Niaimi, Dr Apurna Jegannathen, Dr Caroline Brammer and Dr Laura Pettit |
| The James Cook University Hospital | Dr Alison Humphreys and Dr Nicola Storey |
| The Tunbridge Wells Hospital (previously Kent & Sussex) | Dr Rema Jyothirmayi |
| The Whittington Hospital | Dr Alison Jones, Dr Emma Spurrell, Dr Mulyati Mohamed and Professor Jayant Vaidya |
| Torbay Hospital | Dr Andrew Goodman and Dr Peter Bliss |
| University Hospital | Dr Clive Irwin, Professor Chris Poole and Professor Robert Grieve |
| University Hospital Aintree | Dr Julie O’Hagan and Dr Peter Robson |
| University Hospital of Hartlepool | Dr Adrian Rathmell, Dr Eleanor Aynsley and Dr Nicola Storey |
| University Hospital of North Durham | Dr Wendy Taylor |
| University Hospital of North Tees | Dr Janine Graham and Dr Nicola Storey |
| Velindre Hospital | Dr Helen Passant, Dr Jacinta Abraham, Dr Nayyer Iqbal and Dr Rosie Stevens |
| Wansbeck General Hospital | Dr Anthony N Branson, Dr Helen Turnbull, Dr Nicola Cresti and Dr Rebecca Goranova |
| Warrington Hospital | Professor Peter Clark |
| Warwick Hospital | Dr Nawaz Walji and Professor Robert Grieve |
| West Cumberland Hospital | Dr Paul Dyson |
| West Middlesex University Hospital | Dr Pippa Riddle and Dr Rizvana Ahmad |
| West Suffolk Hospital | Dr Anne Margaret Moody and Dr Cathryn Woodward |
| Western General Hospital | Dr Angela Bowman, Dr Larry Hayward, Dr Olga Olkonomidou, Dr Peter Hall and Professor David Cameron |
| Weston Park Hospital | Dr Kash Purohit, Dr Matthew Hatton, Dr Matthew Winter and Professor Robert Coleman |
| Wexham Park Hospital | Dr Jocelyn Adams, Dr Narottam Thanvi, Dr Richard Ashford and Dr Ruth Davis |
| Whiston Hospital | Dr Helen Innes and Dr Rajamram Sripadam |
| William Harvey Hospital | Dr Julia Hall and Dr Natasha Mithal |
| Worcester Royal Infirmary | Dr Jo Bowen and Dr Radhika Counsell |
| Worthing Hospital | Dr Ashok Nikapota, Dr Rebecca Herbertson and Dr Sankha Mitra |
| Wrexham Maelor Hospital | Dr Audrey Champion, Dr Niladri Ghosal and Dr Win Soe |
| Wycombe General Hospital | Dr Andrew Weaver, Dr Bernadette Lavery, Dr Ketan Shah, Dr Pattie Beresford and Dr Thinn Pwint |
| Yeovil District Hospital | Dr Geoffrey Sparrow, Dr Julie Walther and Dr Urmila Barthakur |
| Ysbyty Gwynedd | Dr Catherine Bale, Dr Jill Bishop and Dr Rachel Williams |
Independent members of the Trial Steering Committee
We wish to thank our colleagues, the independent members of the TSC, for their expert guidance and support throughout the study: Professor Tim Maughan (chairperson; CRUK/MRC Oxford Institute for Radiation Oncology, Clinical Research Programmes, Department of Oncology, Oxford, UK), Professor Richard Gray (Nuffield Department of Population Health, Medical Sciences Division, University of Oxford, Oxford, UK), and the late Professor Adele Francis (co-chairperson; Department of Surgery, University of Birmingham, Birmingham, UK).
Independent Data Safety and Monitoring Committee
We are very grateful to members of the independent DSMC who provided independent, expert advice throughout the study: Professor Richard Bell (chairperson; Deakin University, Geelong, VIC, Australia), Dr Roger A’Hern (Institute of Cancer Research – Clinical Trials Statistical Unit, Division of Clinical Studies, Royal Marsden NHS Foundation Trust, Sutton, UK) and Professor Marianne Nicolson (University of Aberdeen, and Anchor Unit, Aberdeen Royal infirmary, Aberdeen, UK).
Warwick Trials Unit
We thank the trials unit staff: Phase III co-ordination at Warwick Clinical Trials Unit, Coventry, UK. In addition to the authors, we would like to thank other members of the team as shown below.
| Staff member | Job title |
|---|---|
| Emma Ogburn | Clinical Trials Co-ordinator |
| Jasvinder Sandhu | Clinical Trials Co-ordinator |
| Kate Lamb | Clinical Trials Co-ordinator |
| Tessa Fulton-Lieuw | Clinical Trials Co-ordinator |
| Joanne O’Beirne-Elliman | Clinical Trials Co-ordinator |
| Sarah Duggan | Clinical Trials Unit Manager |
| Asha Chauhan | Data Entry Clerk |
| John Carey | Data Entry Clerk |
| Lisa Poulton | Data Entry Clerk |
| Matthew Dalby | Data Entry Clerk |
| Peter Bell | Data Entry Clerk |
| Sharisse Alleyne | Data Entry Clerk |
| Uzma Manazar | Data Entry Clerk |
| Michelle Davitt | Data Entry Clerk |
| Natalie Strickland | Head of Operations |
| Adrian Willis | Programming Team Manager |
| Claire Daffern | Quality Assurance Manager |
| Amy Ismay | Quality Assurance Support Officer |
| Isabel Wall | Randomisation Officer |
| Nicola Robinson | Randomisation Officer/Data Entry Clerk |
| Rishi Bhandari | Randomisation Officer/Software Tester |
| Scott Booth | Randomisation Officer/Software Tester |
| Caroline Jevons | Recruitment Facilitator |
| Chocks Muthiah | Senior Analyst Programmer |
| Lauren Scott | Trainee Trial Co-ordinator |
Cambridge Trials Unit – Cambridge Cancer Trials Centre
Pharmacovigilance and translational co-ordination was carried out by Cambridge Trials Unit – Cancer Theme, Cambridge University Hospital, and Cancer Research UK Cambridge Institute. In particular, we thank Anne-Laure Vallier (Senior Project Manager), Louise Grybowicz (Trial Co-ordinator), Anita Chhabra (Trials Pharmacy), Mary Kasanicki (Legal, Cambridge University Hospital, Research and Development), Kevin Baker (Data Manager), Edmund Chiu (Data Manager), Stanly Thomas (Trial Co-ordinator), Saba Mahmud (Data Manager), Rebecca Clamp (Data Manager), Heike Templin (Data Manager), Elena Provenzano (Histopathologist), Betania Mahler-Araujo (Histopathologist, slide reviews for tissue microarrays) and Helen Bardwell (tissue microarray preparation, Cancer Research UK Cambridge Institute).
In addition, we especially thank Catherine Durance, personal assistant to Helena Earl, for her expert secretarial assistance in preparing the manuscript and all documentation related to the submission of this report.
Contributions of authors
Professor Helena Earl (https://orcid.org/0000-0003-1549-8094) (Professor of Clinical Cancer Medicine, University of Cambridge Department of Oncology, and Honorary Consultant in Medical Oncology at Cambridge University Hospital) is the chief investigator and lead clinician of the trial. She contributed significantly to the concept and design of the trial, protocol development, grant application, day-to-day clinical management of the study, reviewing the data and writing the report.
Dr Louise Hiller (https://orcid.org/0000-0001-8538-9163) (Associate Professor of Biostatistics, Warwick Clinical Trials Unit, University of Warwick) is the trial statistician and contributed significantly to the concept and design, statistical aspects of protocol development and the statistical analysis plan, confidential analyses for the independent DSMC, data analysis and writing the report.
Mrs Anne-Laure Vallier (https://orcid.org/0000-0001-7879-2552) (Cambridge Clinical Trials Unit – Cancer Theme, Lead and Deputy Operations Manager, previously Senior Project Co-ordinator for the Breast Cancer Team) was the senior trial co-ordinator on the PERSEPHONE trial and was significantly involved in concept and design, grant application, protocol development, day-to-day running of the trial, pharmacovigilance, and writing Chapters 1–3.
Mrs Shrushma Loi (https://orcid.org/0000-0002-3815-2320) (Warwick Clinical Trials Unit, now Senior Trial Manager, University of Birmingham) was trial co-ordinator for the PERSEPHONE trial at the University of Warwick Clinical Trials Unit and made a significant contribution to concept and design, setting up the trial, and day-to-day management of the trial from the start until October 2016. She reviewed the report for important intellectual content.
Dr Karen McAdam (https://orcid.org/0000-0002-5545-2982) (Consultant in Medical Oncology, Cambridge University Hospital and Peterborough City Hospital) was significantly involved in recruitment into the trial, was a member of the TMG and TSC, deputised for the chief investigator and reviewed the report for important intellectual content.
Dr Luke Hughes-Davies (https://orcid.org/0000-0002-2698-818X) (Consultant in Clinical Oncology, Cambridge University Hospital) was significantly involved in recruitment into the trial, was a member of the TMG and TSC, deputised for the chief investigator and reviewed the report for important intellectual content.
Professor Daniel Rea (https://orcid.org/0000-0001-7301-4030) (Professor of Oncology, University of Birmingham, and Honorary Consultant in Medical Oncology, and Chairperson of the National Cancer Research Institute Breast Cancer Clinical Studies Group) was significantly involved in supporting the trial and recruiting patients and reviewed the report for important intellectual content.
Miss Donna Howe (https://orcid.org/0000-0002-8363-8127) (Data Manager, Warwick Clinical Trials Unit) was significantly involved in day-to-day running of the trial and data collection and management, throughout the trial. She contributed to writing Chapters 2 and 3.
Miss Kerry Raynes (https://orcid.org/0000-0002-8493-3144) (Trial Co-ordinator, Warwick Clinical Trials Unit) was significantly involved in co-ordinating the study in the follow-up phase, liaising with sites and supervising data management. She contributed to the writing of Chapters 2 and 3.
Ms Helen B Higgins (https://orcid.org/0000-0002-7095-4542) (Senior Project Manager, Warwick Clinical Trials Unit) was significantly involved in study set-up and running of the study and reviewed the report for important intellectual content.
Mrs Maggie Wilcox (https://orcid.org/0000-0003-3129-2790) (President of Independent Cancer Patients’ Voice) was significantly involved in the study design and advised on patient information and communication, during trial development, study recruitment and after presentation of the primary end-point results. She contributed to the analysis and writing of Chapter 6 and the plain English summary.
Dr Chris Plummer (https://orcid.org/0000-0003-4135-3008) (Consultant Cardiologist, Department of Cardiology, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK, with interests in the cardiovascular effects of cancer treatments including the early detection of toxicity with biomarkers and protective strategies for adults and children) was significantly involved in advising on cardiology aspects of the study as a member of the TMG, and writing Chapter 4.
Dr Betania Mahler-Araujo (https://orcid.org/0000-0001-7952-0292) (Consultant Histopathologist, Cambridge University Hospital) was significantly involved in advising on histopathology aspects of the study, translational aspects and contributed to writing Chapter 2.
Dr Elena Provenzano (https://orcid.org/0000-0003-3345-3965) (Consultant Histopathologist, Cambridge University Hospital, and NIHR Cambridge Biomedical Research Centre) was significantly involved in histopathological and translational aspects of the study and contributed to writing Chapter 2.
Mrs Anita Chhabra (https://orcid.org/0000-0002-6508-7309) (Lead Clinical Trials Pharmacist in Oncology, Cambridge University Hospital) was significantly involved in all pharmacy aspects of the trial with set-up of home delivery of trastuzumab within the trial. She contributed to writing the pharmaceutical aspects of the trial in Chapters 2 and 3.
Mrs Sophie Gasson (https://orcid.org/0000-0003-4648-5229) (Research Fellow at Warwick Clinical Trials Unit) was significantly involved in the patient-facing aspects of the study, communicating the results to patients, analysing data and writing both Chapter 6 and the plain English summary.
Dr Claire Balmer (https://orcid.org/0000-0002-6650-5736) (Warwick Clinical Trials Unit) was significantly involved in the analysis and writing of the section on patient-reported experiences in Chapter 6.
Dr Jean E Abraham (https://orcid.org/0000-0003-0688-4807) (Reader in Precision Breast Cancer Medicine, University of Cambridge, Department of Oncology, Personalised Breast Cancer Programme and NIHR Cambridge Biomedical Research Centre, Honorary Consultant in Medical Oncology, Cambridge University Hospital) contributed significantly from the start of the study, in particular to setting up the translational aspects. She contributed to writing the report and reviewing the report for important intellectual content. She has taken over as chief investigator of the PERSEPHONE trial.
Professor Carlos Caldas (https://orcid.org/0000-0003-3547-1489) (Professor of Cancer Medicine, University of Cambridge, Group Leader Breast Cancer Functional Genomics Laboratory, Cancer Research UK Cambridge Institute, Director Cambridge Breast Research Unit) contributed significantly from the start of the study, in particular to setting up the translational aspects, contributed to writing the report and reviewing the report for important intellectual content.
Dr Peter Hall (https://orcid.org/0000-0001-6015-7841) (Reader in Medical Oncology with a research interest in Health Economics and Health Technology Assessment in Cancer, Honorary Medical Oncologist at Edinburgh Cancer Centre) contributed significantly to the health economic aspects of the trial and the analysis and writing of Chapter 5.
Dr Bethany Shinkins (https://orcid.org/0000-0001-5350-1018) (Associate Professor and Test Evaluation Group Lead, Academic Unit of Health Economics, Leeds Institute of Health Sciences, University of Leeds) contributed significantly to the health economics aspects of the trial and the analysis and writing of Chapter 5.
Professor Christopher McCabe (https://orcid.org/0000-0001-5728-4129) (Executive Director and CEO of the Institute of Health Economics, Alberta, Canada, previously Professor of Health Economics, University of Leeds) was significantly involved in concept and design for the health economics substudy and reviewed Chapter 5 for important intellectual content.
Professor Claire Hulme (https://orcid.org/0000-0003-2077-0419) (Health Economist at the University of Exeter Medical School, and previously of the University of Leeds) significantly contributed to the concept and design of the health economics substudy and was involved in the analysis and writing of Chapter 5.
Professor David Miles (https://orcid.org/0000-0001-5480-2154) (Professor of Medical Oncology and Consultant, Mount Vernon Cancer Centre, Northwood, UK) contributed significantly to the concept and design of the study, was a co-applicant on the grant, was a member of the TMG and TSC and contributed to the running of the study and writing the report in particular Chapters 1, 2 and 7.
Professor Andrew M Wardley (https://orcid.org/0000-0002-9639-0888) (Professor of Medical Oncology, Research & Development, The NIHR Manchester Clinical Research Facility at The Christie NHS Foundation Trust) contributed significantly to the concept and design of the study, was a co-applicant on the grant, was a member of the TMG and TSC and contributed to the running of the study and writing the report, in particular Chapters 1, 4 and 7.
Professor David A Cameron (Professor of Medical Oncology at the Cancer Edinburgh Research Centre, The Institute of Genetics and Molecular Medicine, University of Edinburgh, Western General Hospital, Edinburgh, and Clinical Director of the Cancer Research UK Edinburgh Centre) contributed significantly to the concept and design of the study, was a co-applicant on the grant, was a member of the TMG and TSC and contributed to the running of the study and writing the report in particular Chapters 1, 3 and 7.
Professor Janet A Dunn (https://orcid.org/0000-0001-7313-4446) (Professor of Clinical Trials, University of Warwick) was the Clinical Trials Unit lead and senior statistician for the study. She substantially contributed to the concept and design of the study and monitoring, as well as advising on the statistical analysis plan. She was a member of the TMG and TSC, and contributed to the day-to-day running of the study and writing the report, in particular Chapters 2, 3 and 6.
All authors contributed to writing the report or to reviewing the report for important intellectual content. All authors approved the manuscript for submission.
Publications
Loi S, Vallier AL, Willis A, Earl H, Hiller L, Dunn J. Site Set-up of a Large Multi-centre, Phase III, Randomised Controlled Trial (RCT): A UK Post EU Directive Experience. Proceedings of 29th Annual Meeting Society for Clinical Trials Annual Meeting, May 2008, abstract P07.
Loi S, Hulme C, Vallier AL, Hiller L, Dunn J, McCabe C, Earl H. Health Economics within Large Randomised Phase 3 Trials: The Persephone Health Economic Questionnaire. Proceedings of 29th Annual Meeting Society for Clinical Trials, May 2008, abstract P60.
Earl HM, Loi S, Vallier A-L, Hiller L, Ogburn-Storey E, Higgins H, Dunn J. The PERSEPHONE Trial: Duration of Trastuzumab with Chemotherapy in Women with HER2-positive Early Breast Cancer. Changing the Randomisation Point to Address Potential Barriers to Recruitment. 34th Annual San Antonio Breast Cancer Symposium, 6–10 December 2011, San Antonio, TX, USA. Cancer Res 2011;71(Suppl. 3).
Hiller L, Dunn JA, Higgins HB, Ogburn-Storey E, Loi S, Vallier A-L, Earl HM. Optimising patient recall of adverse events over prolonged time periods. Clinical Trials Methodology Conference, October 2011. Trials 2011;12(Suppl. 1):pA74.
Earl HM, Cameron DA, Miles D, Wardley AM, Ogburn E, Vallier A-L, et al. The PERSEPHONE trial: duration of trastuzumab with chemotherapy in women with HER2-positive early breast cancer. San Antonio Breast Cancer Symposium. Cancer Res 2012;72:OT1–1–03.
Earl HM, Cameron DA, Miles D, Wardley AM, Ogburn E, Vallier A-L, et al. The PERSEPHONE trial: duration of trastuzumab with chemotherapy in women with HER2-positive early breast cancer. J Clin Oncol 2012;30(Suppl.):TPS660.
Earl H, Cameron DA, Miles D, Wardley AM, Ogburn-Storey E, Vallier AL, et al. PERSEPHONE: duration of trastuzumab with chemotherapy in women with HER2-positive early breast cancer: six versus twelve months (Trials in Progress). J Clin Oncol 2013;(Suppl.):abstract TPS667 – poster.
Earl HM, Vallier AL, Ogburn-Storey E, Cameron DA, Wardley AM, Miles D, et al. PERSEPHONE: duration of trastuzumab with chemotherapy in women with HER-2 positive early breast cancer. San Antonio Breast Cancer Symposium, TX, USA, December 2013. Cancer Res 2013;73(Suppl.):OT1-1-08.
Hiller L, Vallier AL, Ogburn E, Wardley A, Cameron D, Miles D, et al. Cardiology monitoring substudy in the PERSEPHONE trial: 6 versus 12 months trastuzumab. J Clin Oncol 2014;32(Suppl.):abstract 552.
Earl HM, Cameron DA, Miles D, Wardley AM, Ogburn E, Vallier A, et al. Persephone: duration of trastuzumab with chemotherapy in patients with HER2-positive early breast cancer: six versus twelve months. J Clin Oncol 2014;32(Suppl.):abstract TPS 656.
Earl HM, Cameron DA, Miles D, Wardley AM, Ogburn E, Vallier AL, Loi S, et al. PERSEPHONE is a Randomised Phase III Controlled Trial Comparing Six Months of Trastuzumab to the Standard 12 Months in Patients with HER2-positive Early Breast Cancer. Association of Breast Surgeons Conference, Liverpool, UK, May 2014.
Hiller L, Vallier A, Ogburn E, Wardley A, Cameron D, Miles D, et al. Cardiology Monitoring Substudy in the PERSEPHONE Trial: 6 versus 12 months Trastuzumab. National Cancer Research Institute Cancer Conference, 2–5 November 2014, Liverpool, UK.
Earl HM, Vallier AL, Dunn JA, Loi S, Ogburn E, McAdam K, et al. Cardiac symptoms, Signs, New Cardiac Medication and Left Ventricular Ejection Fraction (LVEF) Changes with 6 versus 12 months adjuvant Trastuzumab: 2,500 Patients in the Persephone Trial. Global Cardio-Oncology Summit 2016, Vancouver, BC, Canada, 29–30 September 2016.
Earl H, Dunn JA, Vallier A, Loi S, Ogburn E, McAdam K, et al. Trastuzumab-associated cardiac events in the Persephone Trial. Br J Cancer 2016;115:1462–70.
Hiller L, Loi S, Vallier A-L, Howe D, Bell P, Carey J, et al. Measurement methods for eliciting opinions on treatment benefits, toxicities and acceptable trade-offs of the two, within the PERSEPHONE trial. Trials 2017;18(Suppl. 1):P368.
Hiller L, Loi S, Vallier A-L, Howe D, Cameron DA, Miles D, Wardley AM, et al. Adjuvant trastuzumab duration trials in HER2-positive breast cancer – what results would be practice-changing? Persephone investigator questionnaire prior to primary endpoint results. National Cancer Research Institute Cancer Conference, Liverpool, UK, November 2017. No 933.
Hiller L, Dunn JA, Loi S, Vallier AL, Howe DL, Cameron DA, et al. Adjuvant trastuzumab duration trials in HER2-positive breast cancer – what results would be practice-changing? Persephone investigator questionnaire prior to primary endpoint results. BMC Cancer 2018;18:391.
Earl HM, Hiller L, Vallier A, Loi S, Howe D, Higgins HB, et al. , for the PERSEPHONE Trial Investigators. PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2-positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results. J Clin Oncol 2018;36(Suppl.):506abst.
Dunn JA, Hiller L, Balmer C, Wilcox M, Vallier AL, Gasson S, et al. Patients’ perspective of living with and beyond the treatment of Trastuzumab: results from the PERSEPHONE early breast cancer trial. American Society of Clinical Oncology meeting. Chicago, IL, USA. June 2018. J Clin Oncol 2018;36(Suppl.):e22101.
Dunn J, Wilcox M, Gasson S, Balmer C, Hiller L, Vallier A, et al. Patient reported experiences from the PERSEPHONE early breast cancer trial. Melbourne International Breast Cancer Conference, Melbourne, VIC, Australia, October 2018, abstract 213, oral presentation.
Dunn J, Young A, Wilcox M, for PERSEPHONE, MAMMO-50 and OPTIMA Investigators. Working with Patient Advocates to Collect Patient Reported Outcomes within Clinical Trials to Inform Future Follow-up Strategies. International Conference on Cancer Nursing, Auckland, New Zealand, September 2018, abstract 439.
Hall P, Hulme C, Shinkins B, Chehadah F, McCabe C, Dunn J, et al. PERSEPHONE: 6 versus 12 months of adjuvant trastuzumab in patients with HER2-positive early breast cancer: cost-effectiveness analysis results. European Society of Medical Oncology, Munich, Germany, October 2018. Oral Presentation, Early Breast Cancer Session. Ann Oncol 2018;29(Suppl. 8).
Dunn J, Wilcox M, Balmer C, Gasson S, Hiller L, Vallier A, et al. Patient reported Experiences (PREs) from the PERSEPHONE Early Breast Cancer (EBC) Trial. National Cancer Research Institute Cancer Conference, Glasgow, UK, November 2018.
Hulme C, Hall P, Shinkins B, Chehadah F, McCabe C, Dunn J, et al. Cost-effectiveness Analyses of 6 versus 12 Months of Adjuvant Trastuzumab in Patients with HER2-positive Early Breast Cancer: Results from the PERSEPHONE Trial. National Cancer Research Institute Cancer Conference, Glasgow, UK, November 2018.
Earl HM, Hiller L, Vallier AL, Loi S, Howe D, Higgins HB, et al. for the Persephone Trial Investigators. PERSEPHONE: A randomised phase 3 non-inferiority trial of 6 versus 12 months (m) of adjuvant trastuzumab in patients with HER2-positive (+) early breast cancer (EBC). Oral presentation. National Cancer Research Institute Cancer Conference. Glasgow, UK, November 2018.
Earl HM, Hiller L, Vallier AL, Loi S, McAdam K, Hughes-Davies L, et al.; PERSEPHONE Steering Committee and Trial Investigators. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 2019;393:2599–612.
Dunn JA, Hiller L, Howe D, Vallier AL, Higgins H, Raynes K, et al. Patient reported experiences collected in the PERSEPHONE Herceptin duration trial. 5th International Clinical Trials Methodology Conference (ICTMC), Brighton, UK, 7–9 October, 2019. Abstract submission – for publication in Trials.
Dunn JA, Hiller L, Howe D, Vallier AL, Higgins H, Raynes K, et al. Statistical considerations in a non-inferiority trial: results from the PERSEPHONE early breast cancer Herceptin duration trial. 5th International Clinical Trials Methodology Conference, Brighton, UK, 7–9 October, 2019. Abstract submission – for publication in Trials.
Dunn JA, Earl HM, Hiller L, Cameron DA, Wardley A, Miles D. PERSEPHONE: 6 versus 12 months (m) of Adjuvant Trastuzumab in Patients (pts) with HER2-positive (+) Early Breast Cancer (EBC): Randomised Phase 3 Non-inferiority Trial with Definitive 4-year (yr) Disease-free Survival (DFS) Results. Oral presentation. AORTIC 12th International Conference on Cancer in Africa, 6–8 November 2019, Maputo, Mozambique.
Dunn JA, Wilcox M, Gasson S, Hiller L, Young A, Earl HM. Patient Reported Experiences from the PERSEPHONE Trastuzumab Duration Early Breast Cancer Trial. AORTIC 12th International Conference on Cancer in Africa, 6–8 November 2019, Maputo, Mozambique.
Data-sharing statement
The trial is registered on the EU Clinical Trials Register (www.clinicaltrialsregister.eu) (EudraCT number 2006-007018-39) and on ClinicalTrials.Gov (https://clinicaltrials.gov/) (number NCT00712140).
All relevant results-related information regarding the trial will be kept in the EU Clinical Trials Database (EudraCT) and be publicly available through the EU Clinical Trials Register. The information will be posted within 1 year after the end of the trial.
Participant data are stored on a secure server at Warwick Clinical Trials Unit (the Data Processor) on behalf of the sponsor (Cambridge University Hospital and the University of Cambridge). Each participant has been assigned a de-identified trial number. No identifiable data such as name, address, hospital number, NHS number, date of birth or any other identifying data will be shared and should not be requested. A data dictionary will be available and will include descriptions of patient demographics, treatment allocation and primary outcome data. A SAS® Programme will be applied to produce clinical study output together with the statistical analysis plan (plan to analyse data). Any requests for access to the trial data should be sent to the chief investigator, who will inform the data custodians, and agreement will be made through the data access committee, which will comprise the principal investigators from the trial management group. For each data-sharing request it is essential that a pro forma is completed that will describe the purpose, scope, data items requested, analysis plan and acknowledgement of the trial management team. Requestors who are granted access to the data will be required to complete a data-sharing agreement that will be signed by the requester, sponsor and principal investigator(s). The study protocol and statistical analysis plan will be made available on request. Please note that exclusive use will be retained until the publication of major outputs.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatment, monitor safety, and plan NHS services. Patient data should be kept safe and secure to protect everyone’s privacy, and it’s important there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out how patient data are used. #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Cancer Research UK . Breast Cancer Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer (accessed 3 July 2020).
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. https://doi.org/10.1126/science.3798106.
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. https://doi.org/10.1038/35021093.
- Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015;24:26-35. https://doi.org/10.1016/j.breast.2015.07.008.
- Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012;486:346-52. https://doi.org/10.1038/nature10983.
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. https://doi.org/10.1056/NEJM200103153441101.
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 2002;20:719-26. https://doi.org/10.1200/JCO.2002.20.3.719.
- Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012;4. https://doi.org/10.1002/14651858.CD006243.pub2.
- Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 2013;368:1199-209. https://doi.org/10.1056/NEJMoa1213261.
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659-72. https://doi.org/10.1056/NEJMoa052306.
- Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 2007;369:29-36. https://doi.org/10.1016/S0140-6736(07)60028-2.
- Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, de Azambuja E, Procter M, Suter TM, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 2013;382:1021-8. https://doi.org/10.1016/S0140-6736(13)61094-6.
- Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017;389:1195-205. https://doi.org/10.1016/S0140-6736(16)32616-2.
- Joensuu H. Duration of adjuvant trastuzumab: shorter beats longer. Lancet 2013;382:1010-11. https://doi.org/10.1016/S0140-6736(13)61448-8.
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84. https://doi.org/10.1056/NEJMoa052122.
- Perez EA, Suman VJ, Davidson NE, Gralow JR, Kaufman PA, Visscher DW, et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol 2011;29:4491-7. https://doi.org/10.1200/JCO.2011.36.7045.
- Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744-52. https://doi.org/10.1200/JCO.2014.55.5730.
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83. https://doi.org/10.1056/NEJMoa0910383.
- Spielmann M, Roché H, Delozier T, Canon JL, Romieu G, Bourgeois H, et al. Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol 2009;27:6129-34. https://doi.org/10.1200/JCO.2009.23.0946.
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006;354:809-20. https://doi.org/10.1056/NEJMoa053028.
- Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol 2009;27:5685-92. https://doi.org/10.1200/JCO.2008.21.4577.
- Schneider BP, O’Neill A, Shen F, Sledge GW, Thor AD, Kahanic SP, et al. Pilot trial of paclitaxel-trastuzumab adjuvant therapy for early stage breast cancer: a trial of the ECOG-ACRIN cancer research group (E2198). Br J Cancer 2015;113:1651-7. https://doi.org/10.1038/bjc.2015.405.
- Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 2013;14:741-8. https://doi.org/10.1016/S1470-2045(13)70225-0.
- Mavroudis D, Saloustros E, Malamos N, Kakolyris S, Boukovinas I, Papakotoulas P, et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol 2015;26:1333-40. https://doi.org/10.1093/annonc/mdv213.
- Joensuu H, Fraser J, Wildiers H, Huovinen R, Auvinen P, Utriainen M, et al. Effect of adjuvant trastuzumab for a duration of 9 weeks vs. 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2-positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol 2018;4:1199-206. https://doi.org/10.1001/jamaoncol.2018.1380.
- Conte P, Frassoldati A, Bisagni G, Brandes AA, Donadio M, Garrone O, et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: final results of the phase III randomized Short-HER study. Ann Oncol 2018;29:2328-33. https://doi.org/10.1093/annonc/mdy414.
- International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . Efficacy Guidelines n.d. www.ich.org/page/efficacy-guidelines (accessed 3 July 2020).
- NHS Health Research Authority . Good Clinical Practice n.d. www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/good-clinical-practice/ (accessed 3 July 2020).
- Great Britain . The Medicines for Human Use (Clinical Trials) Regulations 2004 2004.
- NHS Health Research Authority . Clinical Trials of Investigational Medicinal Products (CTIMPS) n.d. www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/clinical-trials-investigational-medicinal-products-ctimps/ (accessed 3 July 2020).
- Warwick Clinical Trials Unit . Persephone Protocol v5.0 Sept 2019 n.d. https://warwick.ac.uk/fac/sci/med/research/ctu/trials/cancer/persephone/professionals (accessed 3 July 2020).
- Ellis IO, Bartlett J, Dowsett M, Humphreys S, Jasani B, Miller K, et al. Best Practice No. 176: updated recommendations for HER2 testing in the UK. J Clin Pathol 2004;57:233-7. https://doi.org/10.1136/jcp.2003.007724.
- Walker RA, Bartlett JM, Dowsett M, Ellis IO, Hanby AM, Jasani B, et al. HER2 testing in the UK: further update to recommendations. J Clin Pathol 2008;61:818-24. https://doi.org/10.1136/jcp.2007.054866.
- Rakha EA, Pinder SE, Bartlett JM, Ibrahim M, Starczynski J, Carder PJ, et al. Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol 2015;68:93-9. https://doi.org/10.1136/jclinpath-2014-202571.
- Press MF, Sauter G, Buyse M, Fourmanoir H, Quinaux E, Tsao-Wei DD, et al. HER2 gene amplification testing by fluorescent in situ hybridization (FISH): comparison of the ASCO-College of American Pathologists guidelines with FISH scores used for enrollment in Breast Cancer International Research Group Clinical Trials. J Clin Oncol 2016;34:3518-28. https://doi.org/10.1200/JCO.2016.66.6693.
- Jones AL, Barlow M, Barrett-Lee PJ, Canney PA, Gilmour IM, Robb SD, et al. Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer 2009;100:684-92. https://doi.org/10.1038/sj.bjc.6604909.
- Cancer Research UK . Trans-PERSEPHONE and Trans-PERSEPHONE-SNPs: The Pharmacogenomics and Pharmacogenetics of Adjuvant Trastuzumab. Cancer Research Translational Research in Clinical Trials Committee Project Grant: Funding Reference Number: C507 A9675, 2008–2015 n.d. https://europepmc.org/grantfinder/grantdetails?query=pi%3A%22Caldas%2BC%22%2Bgid%3A%22A18832%22%2Bga%3A%22Cancer%20Research%20UK%22.
- Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim SB, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol 2012;13:869-78. https://doi.org/10.1016/S1470-2045(12)70329-7.
- Mauri L, D’Agostino RB. Challenges in the design and interpretation of noninferiority trials. N Engl J Med 2017;377:1357-67. https://doi.org/10.1056/NEJMra1510063.
- Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 2018;378:1177-88. https://doi.org/10.1056/NEJMoa1713709.
- Mehanna H, Wong WL, McConkey CC, Rahman JK, Robinson M, Hartley AG, et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med 2016;374:1444-54. https://doi.org/10.1056/NEJMoa1514493.
- Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 2018;379:111-21. https://doi.org/10.1056/NEJMoa1804710.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. https://doi.org/10.1016/S0140-6736(00)04337-3.
- Early Breast Cancer Trialists’ Collaborative Group . Treatment of Early Breast Cancer: Worldwide Evidence 1985–1990 1990.
- Jennison C, Turnbull BW. Group Sequential Methods with Applications to Clinical Trials. Boca Raton, FL: Chapman and Hall/CRC; 1999.
- Freidlin B, Korn EL, Gray R. A general inefficacy interim monitoring rule for randomized clinical trials. Clin Trials 2010;7:197-208. https://doi.org/10.1177/1740774510369019.
- Pivot X, Romieu G, Bonnefoi H, Pierga JY, Kerbrat P, Guastalla JP, et al. PHARE trial results comparing 6 to 12 months of trastuzumab in adjuvant early breast cancer. Ann Oncol 2012;23:ixe1-ixe30. https://doi.org/10.1016/S0923-7534(20)34322-2.
- Goldhirsch A, Piccartt MJ, Procter M, de Azambuja E, Weber HA, Untch M, et al. HERA trial: 2 years versus 1 year of trastuzumab after adjuvant chemotherapy in women with HER2-positive early breast cancer at 8 years of median follow up. Ann Oncol 2012;23:ixe1-ixe30. https://doi.org/10.1016/S0923-7534(20)34333-7.
- Independent Cancer Patients’ Voice . About Us n.d. www.independentcancerpatientsvoice.org.uk/about-us/.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. https://doi.org/10.1016/0197-2456(86)90046-2.
- Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet 2019;393:2591-8.
- Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 2002;20:1215-21. https://doi.org/10.1200/JCO.2002.20.5.1215.
- Tan-Chiu E, Yothers G, Romond E, Geyer CE, Ewer M, Keefe D, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 2005;23:7811-19. https://doi.org/10.1200/JCO.2005.02.4091.
- Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 2007;25:3859-65. https://doi.org/10.1200/JCO.2006.09.1611.
- Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol 2010;28:3422-8. https://doi.org/10.1200/JCO.2009.26.0463.
- de Azambuja E, Procter MJ, van Veldhuisen DJ, Agbor-Tarh D, Metzger-Filho O, Steinseifer J, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J Clin Oncol 2014;32:2159-65. https://doi.org/10.1200/JCO.2013.53.9288.
- Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol 2008;26:1231-8. https://doi.org/10.1200/JCO.2007.13.5467.
- Russell SD, Blackwell KL, Lawrence J, Pippen JE, Roe MT, Wood F, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol 2010;28:3416-21. https://doi.org/10.1200/JCO.2009.23.6950.
- Zeglinski M, Ludke A, Jassal DS, Singal PK. Trastuzumab-induced cardiac dysfunction: a ‘dual-hit’. Exp Clin Cardiol 2011;16:70-4.
- Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer 2007;7:332-44. https://doi.org/10.1038/nrc2106.
- Ewer MS, Gibbs HR, Swafford J, Benjamin RS. Cardiotoxicity in patients receiving trastuzumab (Herceptin): primary toxicity, synergistic or sequential stress, or surveillance artifact?. Semin Oncol 1999;26:96-101.
- Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol 2005;23:7820-6. https://doi.org/10.1200/JCO.2005.13.300.
- Pivot X, Suter T, Nabholtz JM, Pierga JY, Espie M, Lortholary A, et al. Cardiac toxicity events in the PHARE trial, an adjuvant trastuzumab randomised phase III study. Eur J Cancer 2015;51:1660-6. https://doi.org/10.1016/j.ejca.2015.05.028.
- Mantarro S, Rossi M, Bonifazi M, D’Amico R, Blandizzi C, La Vecchia C, et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern Emerg Med 2016;11:123-40. https://doi.org/10.1007/s11739-015-1362-x.
- Dolgin M, Fox AC, Levin RI. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. London: Little, Brown; 1994.
- Raphael C, Briscoe C, Davies J, Whinnett ZI, Manisty C, Sutton R, et al. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart 2007;93:476-82. https://doi.org/10.1136/hrt.2006.089656.
- Beauclair S, Formento P, Fischel JL, Lescaut W, Largillier R, Chamorey E, et al. Role of the HER2 [Ile655Val] genetic polymorphism in tumorogenesis and in the risk of trastuzumab-related cardiotoxicity. Ann Oncol 2007;18:1335-41. https://doi.org/10.1093/annonc/mdm181.
- Ng T, Chan M, Khor CC, Ho HK, Chan A. The genetic variants underlying breast cancer treatment-induced chronic and late toxicities: a systematic review. Cancer Treat Rev 2014;40:1199-214. https://doi.org/10.1016/j.ctrv.2014.10.001.
- Halyard MY, Pisansky TM, Dueck AC, Suman V, Pierce L, Solin L, et al. Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG Phase III Trial N9831. J Clin Oncol 2009;27:2638-44. https://doi.org/10.1200/JCO.2008.17.9549.
- Leong SL, Chaiyakunapruk N, Tassaneeyakul W, Arunmanakul P, Nathisuwan S, Lee SWH. Roles of pharmacogenomics in non-anthracycline antineoplastic-induced cardiovascular toxicities: a systematic review and meta-analysis of genotypes effect. Int J Cardiol 2019;280:190-7. https://doi.org/10.1016/j.ijcard.2018.12.049.
- Roca L, Diéras V, Roché H, Lappartient E, Kerbrat P, Cany L, et al. Correlation of HER2, FCGR2A, and FCGR3A gene polymorphisms with trastuzumab related cardiac toxicity and efficacy in a subgroup of patients from UNICANCER-PACS 04 trial. Breast Cancer Res Treat 2013;139:789-800. https://doi.org/10.1007/s10549-013-2587-x.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. https://doi.org/10.1056/NEJMoa1209825.
- Earl H, Hiller L, Dunn J, Blenkinsop C, Grybowicz L, Vallier AL, et al. Disease-free (DFS) and overall survival (OS) at 3.4 years (yrs) for neoadjuvant bevacizumab (Bev) added to docetaxel followed by fluorouracil, epirubicin and cyclophosphamide (D-FEC), for women with HER2 negative early breast cancer: the ARTemis trial. J Clin Oncol 2016;34. https://doi.org/10.1200/JCO.2016.34.15_suppl.1014.
- Poole CJ, Earl HM, Hiller L, Dunn JA, Bathers S, Grieve RJ, et al. Epirubicin and cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy for early breast cancer. N Engl J Med 2006;355:1851-62. https://doi.org/10.1056/NEJMoa052084.
- Early Breast Cancer Trialists’ Collaborative Group . Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. https://doi.org/10.1016/S0140-6736(05)66544-0.
- Bartlett JM, McConkey CC, Munro AF, Desmedt C, Dunn JA, Larsimont DP, et al. Predicting anthracycline benefit: TOP2A and CEP17-not only but also. J Clin Oncol 2015;33:1680-7. https://doi.org/10.1200/JCO.2013.54.7869.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 3 July 2020).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results From a UK General Population Survey. York: Centre for Health Economics, University of York; 1995.
- Shen LZ, Pulkstenis E, Hoseyni M. Estimation of mean quality adjusted survival time. Stat Med 1999;18:1541-54. https://doi.org/10.1002/(SICI)1097-0258(19990630)18:12<1541::AID-SIM139>3.0.CO;2-Z.
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230-5. https://doi.org/10.1136/bmj.300.6719.230.
- Lidgren M, Wilking N, Jonsson B, Rehnberg C. Health related quality of life in different states of breast cancer. Qual Life Res 2007;16:1073-81. https://doi.org/10.1007/s11136-007-9202-8.
- Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. PharmacoEconomics 2015;33:355-66. https://doi.org/10.1007/s40273-014-0247-6.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- NHS Improvement . NHS Reference Costs 2017 18 n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed 3 July 2020).
- Joint Formulary Committee . British National Formulary 2018. www.medicinescomplete.com (accessed 7 July 2020).
- O’Brien GL, O’Mahony C, Cooke K, Kinneally A, Sinnott SJ, Walshe V, et al. Cost minimization analysis of intravenous or subcutaneous trastuzumab treatment in patients with HER2-positive breast cancer in Ireland. Clin Breast Cancer 2019;19:e440-51. https://doi.org/10.1016/j.clbc.2019.01.011.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Hall PS, Hulme C, McCabe C, Oluboyede Y, Round J, Cameron DA. Updated cost-effectiveness analysis of trastuzumab for early breast cancer: a UK perspective considering duration of benefit, long-term toxicity and pattern of recurrence. PharmacoEconomics 2011;29:415-32. https://doi.org/10.2165/11588340-000000000-00000.
- Karnon J, Kerr GR, Jack W, Papo NL, Cameron DA. Health care costs for the treatment of breast cancer recurrent events: estimates from a UK-based patient-level analysis. Br J Cancer 2007;97:479-85. https://doi.org/10.1038/sj.bjc.6603887.
- Mohan N, Jiang J, Dokmanovic M, Wu WJ. Trastuzumab-mediated cardiotoxicity: current understanding, challenges, and frontiers. Antib Ther 2018;1:13-7. https://doi.org/10.1093/abt/tby003.
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, et al. Coronary Heart Disease Statistics 2012 Edition. London: British Heart Foundation; 2012.
- Office for National Statistics . National Life Tables: UK n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (accessed 1 November 2019).
- Cancer Research UK . Breast Cancer Mortality Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/mortality?_ga=2.233072414.1882256152.1588694623-1974509419.1582545276#heading-One (accessed 1 November 2019).
- Jackson CH. Multi-state models for panel data: the msm package for R. Stat Software 2011;38:1-29. https://doi.org/10.18637/jss.v038.i08.
- Seferina SC, Ramaekers BLT, de Boer M, Dercksen MW, van den Berkmortel F, van Kampen RJW, et al. Cost and cost-effectiveness of adjuvant trastuzumab in the real world setting: a study of the Southeast Netherlands Breast Cancer Consortium. Oncotarget 2017;8:79223-33. https://doi.org/10.18632/oncotarget.16985.
- Bank of England . Inflation Calculator n.d. www.bankofengland.co.uk/monetary-policy/inflation/inflation-calculator (accessed 3 July 2020).
- National Institute for Health and Care Excellence (NICE) . Early and Locally Advanced Breast Cancer: Diagnosis and Management 2018. www.nice.org.uk/guidance/ng101 (accessed 3 July 2020).
- Robertson C, Arcot Ragupathy SK, Boachie C, Dixon JM, Fraser C, Hernández R, et al. The clinical effectiveness and cost-effectiveness of different surveillance mammography regimens after the treatment for primary breast cancer: systematic reviews registry database analyses and economic evaluation. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15340.
- Stein RC, Dunn JA, Bartlett JM, Campbell AF, Marshall A, Hall P, et al. OPTIMA prelim: a randomised feasibility study of personalised care in the treatment of women with early breast cancer. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20100.
- Hall P, Walkington L, Newsham A, Hall G, Glaser A. Costs of hospital care over ten years from diagnosis of early breast cancer in England. Eur J Cancer 2014;50:S79-80.
- National Institute for Health and Care Excellence (NICE) . Abemaciclib With an Aromatase Inhibitor for Previously Untreated, Hormone Receptor-Positive, HER2-Negative, Locally Advanced or Metastatic Breast Cancer 2019. www.nice.org.uk/guidance/ta563 (accessed 3 July 2020).
- National Institute for Health and Care Excellence (NICE) . Advanced Breast Cancer: Diagnosis and Treatment 2017. www.nice.org.uk/guidance/cg81 (accessed 3 July 2020).
- Karlsson G, Johannesson M. The decision rules of cost-effectiveness analysis. PharmacoEconomics 1996;9:113-20. https://doi.org/10.2165/00019053-199609020-00003.
- Clarke CS, Hunter RM, Shemilt I, Serra-Sastre V. Multi-arm cost-effectiveness analysis (CEA) comparing different durations of adjuvant trastuzumab in early breast cancer, from the English NHS payer perspective. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0172731.
- Cancer Research UK . Fatigue and Cancer Drugs 2017. www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/side-effects/fatigue (accessed 7 September 2017).
- Narayanan V, Koshy C. Fatigue in cancer: a review of literature. Indian J Palliat Care 2009;15:19-25. https://doi.org/10.4103/0973-1075.53507.
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess 1998;2. https://doi.org/10.3310/hta2160.
- Rich JL, Chojenta C, Loxton D. Quality, rigour and usefulness of free-text comments collected by a large population based longitudinal study – ALSWH. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0068832.
- York GS, Churchman R, Woodard B, Wainright C, Rau-Foster M. Free-text comments: understanding the value in family member descriptions of hospice caregiver relationships. Am J Hosp Palliat Care 2012;29:98-105. https://doi.org/10.1177/1049909111409564.
- Corner J, Wagland R, Glaser A, Richards SM. Qualitative analysis of patients’ feedback from a PROMs survey of cancer patients in England. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002316.
- Parsonage RK, Hiscock J, Law RJ, Neal RD. Patient perspectives on delays in diagnosis and treatment of cancer: a qualitative analysis of free-text data. Br J Gen Pract 2017;67:e49-56. https://doi.org/10.3399/bjgp16X688357.
- O’Cathain A, Thomas KJ. ‘Any other comments?’ Open questions on questionnaires – a bane or a bonus to research?. BMC Med Res Methodol 2004;4. https://doi.org/10.1186/1471-2288-4-25.
- Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med 2005;353:1734-6. https://doi.org/10.1056/NEJMe058196.
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594-604. https://doi.org/10.1001/jama.2012.87802.
- Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 2007;25:2127-32. https://doi.org/10.1200/JCO.2006.10.3523.
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139-45. https://doi.org/10.1016/j.cct.2015.09.002.
- Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015;372:134-41. https://doi.org/10.1056/NEJMoa1406281.
- Tolaney SM, Guo H, Pernas S, Barry WT, Dillon DA, Ritterhouse L, et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2019;37:1868-75. https://doi.org/10.1200/JCO.19.00066.
- Jones SE, Collea R, Paul D, Sedlacek S, Favret AM, Gore I, et al. Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2-amplified early stage breast cancer: a single-group, open-label, phase 2 study. Lancet Oncol 2013;14:1121-8. https://doi.org/10.1016/S1470-2045(13)70384-X.
- Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller WH, et al. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst 1993;85:1327-33. https://doi.org/10.1093/jnci/85.16.1327.
- Momparler RL, Karon M, Siegel SE, Avila F. Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res 1976;36:2891-5.
- Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol 2010;17:421-33. https://doi.org/10.1016/j.chembiol.2010.04.012.
- Pang B, Qiao X, Janssen L, Velds A, Groothuis T, Kerkhoven R, et al. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nat Commun 2013;4. https://doi.org/10.1038/ncomms2921.
- Pang B, de Jong J, Qiao X, Wessels LF, Neefjes J. Chemical profiling of the genome with anti-cancer drugs defines target specificities. Nat Chem Biol 2015;11:472-80. https://doi.org/10.1038/nchembio.1811.
- Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 2011;8:151-60. https://doi.org/10.1038/nrclinonc.2010.223.
- Andre F, Dieci MV, Dubsky P, Sotiriou C, Curigliano G, Denkert C, et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res 2013;19:28-33. https://doi.org/10.1158/1078-0432.CCR-11-2701.
- Kroemer G, Senovilla L, Galluzzi L, André F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med 2015;21:1128-38. https://doi.org/10.1038/nm.3944.
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med 2014;20:1301-9. https://doi.org/10.1038/nm.3708.
- Parkes EE, Walker SM, Taggart LE, McCabe N, Knight LA, Wilkinson R, et al. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst 2017;109. https://doi.org/10.1093/jnci/djw199.
- Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 2014;25:1536-43. https://doi.org/10.1093/annonc/mdu191.
- Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018;19:40-5. https://doi.org/10.1016/S1470-2045(17)30904-X.
- Luen SJ, Salgado R, Fox S, Savas P, Eng-Wong J, Clark E, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol 2017;18:52-6. https://doi.org/10.1016/S1470-2045(16)30631-3.
- Dieci MV, Conte P, Bisagni G, Brandes AA, Frassoldati A, Cavanna L, et al. Association of tumor-infiltrating lymphocytes with distant disease-free survival in the ShortHER randomized adjuvant trial for patients with early HER2+ breast cancer. Ann Oncol 2019;30:418-23. https://doi.org/10.1093/annonc/mdz007.
- Altman DG. Clinical trials: subgroup analyses in randomized trials – more rigour needed. Nat Rev Clin Oncol 2015;12:506-7. https://doi.org/10.1038/nrclinonc.2015.133.
- Peto R. Current misconception 3: that subgroup-specific trial mortality results often provide a good basis for individualising patient care. Br J Cancer 2011;104:1057-8. https://doi.org/10.1038/bjc.2011.79.
- Sun X, Briel M, Busse JW, You JJ, Akl EA, Mejza F, et al. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ 2012;344. https://doi.org/10.1136/bmj.e1553.
- Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users’ guide to the medical literature. JAMA 2014;311:405-11. https://doi.org/10.1001/jama.2013.285063.
- von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017;377:122-31. https://doi.org/10.1056/NEJMoa1703643.
- Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016;17:367-77. https://doi.org/10.1016/S1470-2045(15)00551-3.
- Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1688-700. https://doi.org/10.1016/S1470-2045(17)30717-9.
- Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. https://doi.org/10.1016/S1470-2045(11)70336-9.
- Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016;17:791-800. https://doi.org/10.1016/S1470-2045(16)00163-7.
- Howie LJ, Scher NS, Amiri-Kordestani L, Zhang L, King-Kallimanis BL, Choudhry Y, et al. FDA approval summary: pertuzumab for adjuvant treatment of HER2-positive early breast cancer. Clin Cancer Res 2019;25:2949-55. https://doi.org/10.1158/1078-0432.CCR-18-3003.
- National Institute for Health and Care Excellence (NICE) . Pertuzumab for Adjuvant Treatment of HER2-Positive Early Stage Breast Cancer 2019. www.nice.org.uk/guidance/ta569 (accessed 3 July 2020).
- National Institute for Health and Care Excellence . Neratinib for Extended Adjuvant Treatment of Hormone Receptor-Positive, HER2-Positive Early Stage Breast Cancer After Adjuvant Trastuzumab n.d. www.nice.org.uk/guidance/ta612 (accessed 15 July 2020).
- Esserman LJ, DeMichele A. Accelerated approval for pertuzumab in the neoadjuvant setting: winds of change?. Clin Cancer Res 2014;20:3632-6. https://doi.org/10.1158/1078-0432.CCR-13-3131.
- European Medicines Agency . Perjeta n.d. www.ema.europa.eu/en/medicines/human/EPAR/perjeta#authorisation-details-section.
- National Institute for Health and Care Excellence (NICE) . Pertuzumab for the Neoadjuvant Treatment of HER2-Positive Breast Cancer. 2016. www.nice.org.uk/guidance/ta424 (accessed 3 July 2020).
- von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019;380:617-28. https://doi.org/10.1056/NEJMoa1814017.
- Piccart MJ. Why your preferred targeted drugs may become unaffordable. Cancer Res 2013;73:5849-51. https://doi.org/10.1158/0008-5472.CAN-13-1486.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-42. https://doi.org/10.3322/caac.21492.
- World Health Organization . WHO Model List of Essential Medicines [11 2015] 2015. www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf?ua=1 (accessed 3 July 2020).
- Daily Nation . WHO Updates List of Essential Drugs n.d. www.nation.co.ke/kenya/healthy-nation/who-updates-list-of-essential-drugs-409508.
- Coory M, Thornton K. Randomised clinical endpoint studies for trastuzumab biosimilars: a systematic review. Breast Cancer Res Treat 2019;176:17-25. https://doi.org/10.1007/s10549-019-05227-7.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Marcheselli R, Marcheselli L, Cortesi L, Bari A, Cirilli C, Pozzi S, et al. Risk of second primary malignancy in breast cancer survivors: a nested population-based case-control study. J Breast Cancer 2015;18:378-85. https://doi.org/10.4048/jbc.2015.18.4.378.
- Cancer Research UK . Cancer Incidence by Age n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/age#heading-Zero (accessed 1 November 2019).
- National Institute for Health and Care Excellence (NICE) . Ribociclib With an Aromatase Inhibitor for Previously Untreated, Hormone Receptor-Positive, HER2-Negative, Locally Advanced or Metastatic Breast Cancer 2017. www.nice.org.uk/guidance/ta496 (accessed 3 July 2020).
Appendix 1 Additional tables
| n (%) | |
|---|---|
| Total approached | 7975 |
| Total recruited | 1848 (23) |
| Total not recruited | 6127 (77) |
| Reason given for non-recruitment | |
| Patient would prefer to have standard treatment | 1576 (26) |
| Not eligible | 1502 (24.5) |
| Patient declined, no reason | 1015 (16.5) |
| Awaiting patient’s decision | 783 (13) |
| Patient has no interest in the trial | 204 (3) |
| Clinician’s decision | 92 (1.5) |
| Outside timescale for starting Herceptin | 34 (0.5) |
| Other | 279 (4.5) |
| Unknown | 642 (10.5) |
| Site | 12-month patients (N = 2045) | 6-month patients (N = 2044) | Total (N = 4089) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Aberdeen Royal Infirmary | 10 | 0.5 | 12 | 0.6 | 22 | 0.5 |
| Addenbrooke’s Hospital | 54 | 2.6 | 41 | 2.0 | 95 | 2.3 |
| Airedale General Hospital | 9 | 0.4 | 4 | 0.2 | 13 | 0.3 |
| Alexandra Hospital | 5 | 0.2 | 5 | 0.2 | 10 | 0.2 |
| Ashford Hospital | 2 | 0.1 | 3 | 0.1 | 5 | 0.1 |
| Barnet Hospital | 19 | 0.9 | 25 | 1.2 | 44 | 1.1 |
| Barnsley District Hospital | 8 | 0.4 | 9 | 0.4 | 17 | 0.4 |
| Basildon and Thurrock University Hospitals | 8 | 0.4 | 6 | 0.3 | 14 | 0.3 |
| Basingstoke and North Hampshire Hospital | 5 | 0.2 | 6 | 0.3 | 11 | 0.3 |
| Bedford General Hospital | 14 | 0.7 | 12 | 0.6 | 26 | 0.6 |
| Birmingham Heartlands Hospital | 3 | 0.1 | 5 | 0.2 | 8 | 0.2 |
| Bishop Auckland General Hospital | 1 | 0.05 | 4 | 0.2 | 5 | 0.1 |
| Blackpool Victoria Hospital | 8 | 0.4 | 9 | 0.4 | 17 | 0.4 |
| Borders General Hospital | 12 | 0.6 | 10 | 0.5 | 22 | 0.5 |
| Bradford Royal Infirmary | 7 | 0.3 | 17 | 0.8 | 24 | 0.6 |
| Broomfield Hospital | 8 | 0.4 | 7 | 0.3 | 15 | 0.4 |
| Castle Hill Hospital | 9 | 0.4 | 11 | 0.5 | 20 | 0.5 |
| Charing Cross Hospital | 29 | 1.4 | 28 | 1.4 | 57 | 1.4 |
| Cheltenham General Hospital | 23 | 1.1 | 18 | 0.9 | 41 | 1.0 |
| Chesterfield Royal Hospital | 12 | 0.6 | 17 | 0.8 | 29 | 0.7 |
| Christie Hospital | 22 | 1.1 | 27 | 1.3 | 49 | 1.2 |
| City Hospital | 20 | 1.0 | 21 | 1.0 | 41 | 1.0 |
| Clatterbridge Cancer Centre | 16 | 0.8 | 18 | 0.9 | 34 | 0.8 |
| Conquest Hospital | 21 | 1.0 | 20 | 1.0 | 41 | 1.0 |
| County Hospital | 9 | 0.4 | 13 | 0.6 | 22 | 0.5 |
| Cumberland Infirmary | 24 | 1.2 | 26 | 1.3 | 50 | 1.2 |
| Darent Valley Hospital | 15 | 0.7 | 16 | 0.8 | 31 | 0.8 |
| Darlington Memorial Hospital | 6 | 0.3 | 7 | 0.3 | 13 | 0.3 |
| Dewsbury and District Hospital | 1 | 0.05 | 0 | 0.0 | 1 | 0.02 |
| Diana, Princess of Wales Hospital | 28 | 1.4 | 13 | 0.6 | 41 | 1.0 |
| Doncaster Royal Infirmary | 2 | 0.1 | 8 | 0.4 | 10 | 0.2 |
| Dorset County Hospital | 10 | 0.5 | 11 | 0.5 | 21 | 0.5 |
| Dumfries and Galloway Royal Infirmary | 8 | 0.4 | 9 | 0.4 | 17 | 0.4 |
| Ealing Hospital | 5 | 0.2 | 2 | 0.1 | 7 | 0.2 |
| East Surrey Hospital | 9 | 0.4 | 12 | 0.6 | 21 | 0.5 |
| Eastbourne District General Hospital | 24 | 1.2 | 26 | 1.3 | 50 | 1.2 |
| Essex County Hospital | 17 | 0.8 | 10 | 0.5 | 27 | 0.7 |
| Freeman Hospital | 2 | 0.1 | 1 | 0.05 | 3 | 0.1 |
| Friarage Hospital | 1 | 0.05 | 2 | 0.1 | 3 | 0.1 |
| Furness General Hospital | 6 | 0.3 | 4 | 0.2 | 10 | 0.2 |
| George Eliot Hospital | 11 | 0.5 | 11 | 0.5 | 22 | 0.5 |
| Glan Clwyd Hospital | 9 | 0.4 | 6 | 0.3 | 15 | 0.4 |
| Good Hope Hospital | 5 | 0.2 | 5 | 0.2 | 10 | 0.2 |
| Great Western Hospital | 13 | 0.6 | 33 | 1.6 | 46 | 1.1 |
| Guy’s Hospital | 16 | 0.8 | 21 | 1.0 | 37 | 0.9 |
| Halton General Hospital | 10 | 0.5 | 10 | 0.5 | 20 | 0.5 |
| Hexham General Hospital | 1 | 0.05 | 3 | 0.1 | 4 | 0.1 |
| Hinchingbrooke Hospital | 5 | 0.2 | 6 | 0.3 | 11 | 0.3 |
| Ipswich Hospital | 6 | 0.3 | 8 | 0.4 | 14 | 0.3 |
| James Paget University Hospital | 22 | 1.1 | 21 | 1.0 | 43 | 1.1 |
| Kent and Canterbury Hospital | 15 | 0.7 | 12 | 0.6 | 27 | 0.7 |
| Kidderminster Hospital | 13 | 0.6 | 12 | 0.6 | 25 | 0.6 |
| King Edward VII’s Hospital | 3 | 0.1 | 0 | 0.0 | 3 | 0.1 |
| King’s College Hospital | 16 | 0.8 | 24 | 1.2 | 40 | 1.0 |
| King’s Mill Hospital | 5 | 0.2 | 3 | 0.1 | 8 | 0.2 |
| Leighton Hospital | 1 | 0.05 | 0 | 0.0 | 1 | 0.02 |
| Lincoln County Hospital | 8 | 0.4 | 7 | 0.3 | 15 | 0.4 |
| Lister Hospital | 10 | 0.5 | 11 | 0.5 | 21 | 0.5 |
| Luton and Dunstable University Hospital | 27 | 1.3 | 28 | 1.4 | 55 | 1.3 |
| Macclesfield District General Hospital | 21 | 1.0 | 18 | 0.9 | 39 | 1.0 |
| Maidstone Hospital | 35 | 1.7 | 22 | 1.1 | 57 | 1.4 |
| Medway Maritime Hospital | 16 | 0.8 | 27 | 1.3 | 43 | 1.1 |
| Milton Keynes Hospital | 4 | 0.2 | 7 | 0.3 | 11 | 0.3 |
| Mount Vernon Hospital | 24 | 1.2 | 17 | 0.8 | 41 | 1.0 |
| Musgrove Park Hospital | 16 | 0.8 | 12 | 0.6 | 28 | 0.7 |
| Nevill Hall Hospital | 1 | 0.05 | 5 | 0.2 | 6 | 0.1 |
| New Cross Hospital | 30 | 1.5 | 26 | 1.3 | 56 | 1.4 |
| Newham General Hospital | 9 | 0.4 | 10 | 0.5 | 19 | 0.5 |
| Norfolk and Norwich University Hospital | 36 | 1.8 | 42 | 2.1 | 78 | 1.9 |
| North Devon District Hospital | 10 | 0.5 | 4 | 0.2 | 14 | 0.3 |
| North Middlesex University Hospital | 17 | 0.8 | 14 | 0.7 | 31 | 0.8 |
| North Tyneside General Hospital | 6 | 0.3 | 11 | 0.5 | 17 | 0.4 |
| Northampton General Hospital | 8 | 0.4 | 15 | 0.7 | 23 | 0.6 |
| Northwick Park Hospital | 5 | 0.2 | 2 | 0.1 | 7 | 0.2 |
| Nottingham City Hospital | 4 | 0.2 | 6 | 0.3 | 10 | 0.2 |
| Peterborough City Hospital | 36 | 1.8 | 47 | 2.3 | 83 | 2.0 |
| Pilgrim Hospital | 1 | 0.05 | 7 | 0.3 | 8 | 0.2 |
| Pinderfields Hospital | 6 | 0.3 | 6 | 0.3 | 12 | 0.3 |
| Poole Hospital | 3 | 0.1 | 9 | 0.4 | 12 | 0.3 |
| Prince Charles Hospital | 3 | 0.1 | 2 | 0.1 | 5 | 0.1 |
| Princess of Wales Hospital | 4 | 0.2 | 6 | 0.3 | 10 | 0.2 |
| Princess Royal University Hospital | 20 | 1.0 | 22 | 1.1 | 42 | 1.0 |
| Queen Alexandra Hospital | 14 | 0.7 | 14 | 0.7 | 28 | 0.7 |
| Queen Elizabeth Hospital Birmingham | 21 | 1.0 | 18 | 0.9 | 39 | 1.0 |
| Queen Elizabeth Hospital Gateshead | 13 | 0.6 | 12 | 0.6 | 25 | 0.6 |
| Queen Elizabeth Hospital King’s Lynn | 17 | 0.8 | 17 | 0.8 | 34 | 0.8 |
| Queen Elizabeth Hospital London | 16 | 0.8 | 18 | 0.9 | 34 | 0.8 |
| Queen Elizabeth The Queen Mother Hospital | 7 | 0.3 | 7 | 0.3 | 14 | 0.3 |
| Queen’s Hospital Burton | 30 | 1.5 | 18 | 0.9 | 48 | 1.2 |
| Queen’s Hospital Romford | 29 | 1.4 | 29 | 1.4 | 58 | 1.4 |
| Raigmore Hospital | 18 | 0.9 | 23 | 1.1 | 41 | 1.0 |
| Rotherham General Hospital | 1 | 0.05 | 5 | 0.2 | 6 | 0.1 |
| Royal Albert Edward Infirmary | 5 | 0.2 | 2 | 0.1 | 7 | 0.2 |
| Royal Berkshire Hospital | 9 | 0.4 | 6 | 0.3 | 15 | 0.4 |
| Royal Bournemouth Hospital | 4 | 0.2 | 3 | 0.1 | 7 | 0.2 |
| Royal Derby Hospital | 34 | 1.7 | 26 | 1.3 | 60 | 1.5 |
| Royal Free Hospital | 16 | 0.8 | 17 | 0.8 | 33 | 0.8 |
| Royal Glamorgan Hospital | 5 | 0.2 | 7 | 0.3 | 12 | 0.3 |
| Royal Gwent Hospital | 13 | 0.6 | 13 | 0.6 | 26 | 0.6 |
| Royal Hampshire County Hospital | 24 | 1.2 | 23 | 1.1 | 47 | 1.1 |
| Royal Lancaster Infirmary | 22 | 1.1 | 23 | 1.1 | 45 | 1.1 |
| Royal Liverpool University Hospital | 26 | 1.3 | 28 | 1.4 | 54 | 1.3 |
| Royal Shrewsbury Hospital | 28 | 1.4 | 27 | 1.3 | 55 | 1.3 |
| Royal Surrey County Hospital | 14 | 0.7 | 8 | 0.4 | 22 | 0.5 |
| Royal Sussex County Hospital | 39 | 1.9 | 35 | 1.7 | 74 | 1.8 |
| Royal United Hospital | 10 | 0.5 | 21 | 1.0 | 31 | 0.8 |
| Russells Hall Hospital | 27 | 1.3 | 24 | 1.2 | 51 | 1.2 |
| Salisbury District Hospital | 15 | 0.7 | 13 | 0.6 | 28 | 0.7 |
| Sandwell General Hospital | 2 | 0.1 | 2 | 0.1 | 4 | 0.1 |
| Scarborough General Hospital | 12 | 0.6 | 8 | 0.4 | 20 | 0.5 |
| Scunthorpe General Hospital | 3 | 0.1 | 3 | 0.1 | 6 | 0.1 |
| Solihull Hospital | 4 | 0.2 | 1 | 0.05 | 5 | 0.1 |
| South Tyneside District Hospital | 8 | 0.4 | 12 | 0.6 | 20 | 0.5 |
| Southampton General Hospital | 27 | 1.3 | 34 | 1.7 | 61 | 1.5 |
| Southend Hospital | 34 | 1.7 | 26 | 1.3 | 60 | 1.5 |
| Southport and Formby District General Hospital | 10 | 0.5 | 24 | 1.2 | 34 | 0.8 |
| St Bartholomew’s Hospital | 30 | 1.5 | 35 | 1.7 | 65 | 1.6 |
| St George’s Hospital | 16 | 0.8 | 20 | 1.0 | 36 | 0.9 |
| St Mary’s Hospital Isle of Wight | 10 | 0.5 | 14 | 0.7 | 24 | 0.6 |
| St Mary’s Hospital London | 14 | 0.7 | 8 | 0.4 | 22 | 0.5 |
| Stepping Hill Hospital | 0 | 0.0 | 1 | 0.05 | 1 | 0.02 |
| Stoke Mandeville Hospital | 16 | 0.8 | 18 | 0.9 | 34 | 0.8 |
| Sunderland Royal Hospital | 16 | 0.8 | 15 | 0.7 | 31 | 0.8 |
| The County Hospital | 22 | 1.1 | 15 | 0.7 | 37 | 0.9 |
| The James Cook University Hospital | 17 | 0.8 | 11 | 0.5 | 28 | 0.7 |
| The Tunbridge Wells Hospital | 9 | 0.4 | 4 | 0.2 | 13 | 0.3 |
| The Whittington Hospital | 10 | 0.5 | 11 | 0.5 | 21 | 0.5 |
| Torbay Hospital | 23 | 1.1 | 16 | 0.8 | 39 | 1.0 |
| University Hospital | 12 | 0.6 | 14 | 0.7 | 26 | 0.6 |
| University Hospital Aintree | 7 | 0.3 | 12 | 0.6 | 19 | 0.5 |
| University Hospital of Hartlepool | 13 | 0.6 | 4 | 0.2 | 17 | 0.4 |
| University Hospital of North Durham | 15 | 0.7 | 24 | 1.2 | 39 | 1.0 |
| University Hospital of North Tees | 16 | 0.8 | 11 | 0.5 | 27 | 0.7 |
| Velindre Hospital | 8 | 0.4 | 4 | 0.2 | 12 | 0.3 |
| Walsall Manor Hospital | 28 | 1.4 | 13 | 0.6 | 41 | 1.0 |
| Wansbeck General Hospital | 4 | 0.2 | 7 | 0.3 | 11 | 0.3 |
| Warrington Hospital | 13 | 0.6 | 7 | 0.3 | 20 | 0.5 |
| Warwick Hospital | 17 | 0.8 | 21 | 1.0 | 38 | 0.9 |
| West Cumberland Hospital | 0 | 0.0 | 1 | 0.05 | 1 | 0.02 |
| West Middlesex University Hospital | 7 | 0.3 | 9 | 0.4 | 16 | 0.4 |
| West Suffolk Hospital | 15 | 0.7 | 12 | 0.6 | 27 | 0.7 |
| Western General Hospital | 11 | 0.5 | 9 | 0.4 | 20 | 0.5 |
| Weston Park Hospital | 6 | 0.3 | 5 | 0.2 | 11 | 0.3 |
| Wexham Park Hospital | 31 | 1.5 | 27 | 1.3 | 58 | 1.4 |
| Whiston Hospital | 10 | 0.5 | 14 | 0.7 | 24 | 0.6 |
| William Harvey Hospital | 17 | 0.8 | 15 | 0.7 | 32 | 0.8 |
| Worcester Royal Infirmary | 5 | 0.2 | 6 | 0.3 | 11 | 0.3 |
| Worthing Hospital | 15 | 0.7 | 9 | 0.4 | 24 | 0.6 |
| Wrexham Maelor Hospital | 8 | 0.4 | 9 | 0.4 | 17 | 0.4 |
| Wycombe General Hospital | 13 | 0.6 | 12 | 0.6 | 25 | 0.6 |
| Yeovil District Hospital | 18 | 0.9 | 17 | 0.8 | 35 | 0.9 |
| Ysbyty Gwynedd | 13 | 0.6 | 14 | 0.7 | 27 | 0.7 |
| Total | 2045 | 100 | 2044 | 100 | 4089 | 100 |
| CRF | Received | Expected | % |
|---|---|---|---|
| Eligibility form | 4072 | 4088 | 99.6 |
| Randomisation form | 4080 | 4088 | 99.8 |
| On-study form | 4076 | 4087 | 99.7 |
| Neoadjuvant diagnostic biopsy form | 618 | 625 | 98.9 |
| Surgery form | 4064 | 4083 | 99.5 |
| Trastuzumab treatment form (cycles 1–4) | 4067 | 4083 | 99.6 |
| Trastuzumab treatment form (cycles 5–8) | 4031 | 4048 | 99.6 |
| Trastuzumab treatment form (cycles 9–12) | 4004 | 4027 | 99.4 |
| Trastuzumab treatment form (cycles 13–16/9-month LVEF) | 3849 | 3927 | 98.0 |
| Trastuzumab treatment form (cycles 17–18/12-month LVEF) | 3837 | 3927 | 97.7 |
| Treatment summary form | 4029 | 4080 | 98.8 |
| Radiotherapy form | 4026 | 4065 | 99.0 |
| Annual follow-up forms (years 1–10) | 21,699 | 23,924 | 90.7 |
| Trial | ER positive (%) | Node negative (%) | ≤ 2 cm (%) | Patient numbers (total) |
|---|---|---|---|---|
| SOLD | 66 | 60 | 56 | 2176 |
| Short-HER | 68 | 54 | 41 | 1253 |
| HORG | 67 | 20 | – | 481 |
| PHARE | 58 | 55 | 53 | 3383 |
| PERSEPHONE | 69 | 58 | 47 | 4088 |
| HERA | 46 | 33 | 40 | 5081 |
| NSABP-B31/NCCTG N9381 | 46 | 7 | 38 | 4046 |
| BCIRG-006 | 54 | 29 | 40 | 3222 |
| FINHER | 47 | 16 | 35 | 232 |
| Characteristic | Adjuvant CT, n (%) | Neoadjuvant CT, n (%) | Total, N (%) | p-value |
|---|---|---|---|---|
| Randomised treatment | ||||
| 12 months | 1735 (50) | 310 (50) | 2045 (50) | 0.82 |
| 6 months | 1727 (50) | 316 (50) | 2043 (50) | |
| ER statusa | ||||
| Negative | 1022 (30) | 241 (39) | 1263 (31) | < 0.0001 |
| Positive | 2440 (70) | 385 (61) | 2825 (69) | |
| Chemotherapy typea | ||||
| Anthracycline based | 1642 (48) | 54 (9) | 1696 (41) | < 0.0001 |
| Taxane based (no anthracycline) | 386 (11) | 14 (2) | 400 (10) | |
| Anthracycline and taxane based | 1429 (41) | 558 (89) | 1987 (49) | |
| No taxane and no anthracycline | 5 (< 1) | 0 (0) | 5 (< 1) | |
| Trastuzumab timinga | ||||
| Concurrent | 1408 (41) | 492 (79) | 1900 (46) | < 0.0001 |
| Sequential | 2054 (59) | 134 (21) | 2188 (54) | |
| Sex | ||||
| Female | 3456 (99) | 626 (100) | 4082 (99) | 0.63 |
| Male | 6 (1) | 0 (0) | 6 (1) | |
| Age (years) at randomisation | ||||
| Median (range) | 57 (23–83) | 52 (23–82) | 56 (23–83) | < 0.0001 |
| < 35 | 72 (2) | 23 (4) | 95 (2) | < 0.0001 |
| 35–49 | 867 (25) | 242 (38) | 1109 (27) | |
| 50–59 | 1058 (31) | 206 (33) | 1264 (31) | |
| 60–69 | 1069 (31) | 130 (21) | 1199 (30) | |
| ≥ 70 | 396 (11) | 25 (4) | 421 (10) | |
| Nodal status at surgery (of the 3462 adjuvant patients) | ||||
| Negative | 2017 (58) | – | 2017 (58) | – |
| 1–3 nodes positive | 964 (28) | – | 964 (28) | |
| ≥ 4 nodes positive | 455 (13) | – | 455 (13) | |
| Unknown | 26 (1) | – | 26 (1) | |
| Tumour sizeb (of the 3462 adjuvant patients) | ||||
| ≤ 2 cm | 1626 (47) | – | 1626 (47) | – |
| > 2 and ≤ 5 cm | 1565 (45) | – | 1565 (45) | |
| > 5 cm | 169 (5) | – | 169 (5) | |
| Unknown | 102 (3) | – | 102 (3) | |
| Tumour gradeb | ||||
| I (well differentiated) | 61 (2) | 1 (< 1) | 62 (2) | 0.001 |
| II (moderately differentiated) | 1035 (30) | 241 (39) | 1276 (31) | |
| III (poorly differentiated) | 2282 (66) | 340 (54) | 2622 (64) | |
| Unknown | 84 (2) | 44 (7) | 128 (3) | |
| Ethnicity | ||||
| White | 2851 (83) | 456 (73) | 3307 (81) | < 0.0001 |
| Asian | 83 (2) | 26 (4) | 109 (3) | |
| Black | 74 (2) | 23 (4) | 97 (2) | |
| Other | 25 (1) | 13 (2) | 38 (1) | |
| Unknown | 429 (12) | 108 (17) | 537 (13) | |
| Menopausal status before chemotherapy | ||||
| Pre | 904 (26) | 242 (39) | 1146 (28) | < 0.0001 |
| Peri | 213 (6) | 48 (8) | 261 (6) | |
| Post | 1947 (56) | 270 (43) | 2217 (54) | |
| Not assessable/not available | 398 (12) | 66 (10) | 464 (12) | |
| Reported prior use of cardiac medication | ||||
| Yes | 92 (3) | 7 (1) | 99 (2) | 0.03 |
| No | 3370 (97) | 619 (99) | 3989 (98) | |
| IHC-score and FISH positivity (HER2 test result) | ||||
| 3+ | 2484 (72) | 467 (75) | 2951 (72) | 0.002 |
| 2+ and FISH positive | 935 (27) | 126 (20) | 1061 (26) | |
| HER2 positive – IHC and FISH score not available | 43 (1) | 33 (5) | 76 (2) | |
| Median (IQR) follow-up (years) | 6.2 (4.6–7.7) | 5.4 (4.1–7.2) | 6.1 (4.5–7.6) | |
| Number of deaths reported (%) | 305 (9) | 84 (13) | 389 (10) | |
| Number of DFS events reported | 439 (13) | 127 (20) | 566 (14) | |
| Characteristic | Concurrent patients, n (%) | Sequential patients, n (%) | Total, N (%) | p-value |
|---|---|---|---|---|
| Randomised treatment | ||||
| 12 months | 949 (50) | 1096 (50) | 2045 (50) | 0.95 |
| 6 months | 951 (50) | 1092 (50) | 5043 (50) | |
| ER statusa | ||||
| Negative | 597 (31) | 666 (30) | 1263 (31) | 0.52 |
| Positive | 1303 (69) | 1522 (70) | 2825 (69) | |
| Chemotherapy typea | ||||
| Anthracycline based | 55 (3) | 1641 (75) | 1696 (41) | < 0.0001 |
| Taxane based (no anthracycline) | 342 (18) | 58 (3) | 400 (10) | |
| Anthracycline and taxane based | 1503 (79) | 484 (22) | 1987 (49) | |
| No taxane and no anthracycline | 0 (0) | 5 (< 1) | 5 (< 1) | |
| Chemotherapy timinga | ||||
| Adjuvant | 1408 (74) | 2054 (94) | 3462 (85) | < 0.0001 |
| Neoadjuvant | 492 (26) | 134 (6) | 626 (15) | |
| Sex | ||||
| Female | 1898 (99) | 2184 (99) | 4082 (99) | 0.69 |
| Male | 2 (1) | 4 (1) | 6 (1) | |
| Age (years) at randomisation | ||||
| Median (range) | 54 (23–82) | 57 (23–83) | 56 (23–83) | < 0.0001 |
| < 35 | 65 (3) | 30 (1) | 95 (2) | < 0.0001 |
| 35–49 | 565 (30) | 544 (25) | 1109 (27) | |
| 50–59 | 593 (31) | 671 (31) | 1264 (31) | |
| 60–69 | 525 (28) | 674 (31) | 1199 (30) | |
| ≥ 70 | 152 (8) | 269 (12) | 421 (10) | |
| Nodal status at surgery (of the 3462 adjuvant patients) | ||||
| Negative | 642 (46) | 1375 (67) | 2017 (58) | < 0.0001 |
| 1–3 nodes positive | 506 (36) | 458 (22) | 964 (28) | |
| ≥ 4 nodes positive | 241 (17) | 214 (10) | 455 (13) | |
| Unknown | 19 (1) | 7 (< 1) | 26 (1) | |
| Tumour sizeb (of the 3462 adjuvant patients) | ||||
| ≤ 2 cm | 577 (41) | 1049 (51) | 1626 (47) | < 0.0001 |
| > 2 and ≤ 5 cm | 690 (49) | 875 (43) | 1565 (45) | |
| > 5 cm | 89 (6) | 80 (4) | 169 (5) | |
| Unknown | 52 (4) | 50 (2) | 102 (3) | |
| Tumour gradeb | ||||
| I (well differentiated) | 28 (1) | 34 (2) | 62 (2) | 0.08 |
| II (moderately differentiated) | 560 (30) | 716 (33) | 1276 (31) | |
| III (poorly differentiated) | 1233 (65) | 1389 (63) | 2622 (64) | |
| Unknown | 79 (4) | 49 (2) | 128 (3) | |
| Ethnicity | ||||
| White | 1465 (77) | 1842 (84) | 3307 (81) | < 0.0001 |
| Asian | 69 (4) | 40 (2) | 109 (3) | |
| Black | 55 (3) | 42 (2) | 97 (2) | |
| Other | 24 (1) | 14 (1) | 38 (1) | |
| Unknown | 287 (15) | 250 (11) | 537 (13) | |
| Menopausal status before chemotherapy | ||||
| Pre | 606 (32) | 540 (25) | 1146 (28) | < 0.0001 |
| Peri | 134 (7) | 127 (6) | 261 (6) | |
| Post | 971 (51) | 1246 (57) | 2217 (54) | |
| Not assessable/not available | 189 (10) | 275 (12) | 464 (12) | |
| Reported prior use of cardiac medication | ||||
| Yes | 48 (3) | 51 (2) | 99 (2) | 0.76 |
| No | 1852 (97) | 2137 (98) | 3989 (98) | |
| IHC-score and FISH positivity (HER2 test result) | ||||
| 3+ | 1400 (74) | 1551 (71) | 2951 (72) | 0.01 |
| 2+ and FISH positive | 454 (24) | 607 (28) | 1061 (26) | |
| HER2 positive – IHC and FISH score not available | 46 (2) | 30 (1) | 76 (2) | |
| Median (IQR) follow-up (years) | 5.3 (4.2–6.6) | 6.7 (5.2–8.2) | 6.1 (4.5–7.6) | |
| Number of deaths reported | 148 (8) | 241 (11) | 389 (10) | |
| Number of DFS events reported | 235 (12) | 331 (15) | 566 (14) | |
| Stratification/chemotherapy regimen | 12-month patients, n (%) | 6-month patients, n (%) | Total, N (%) |
|---|---|---|---|
| Anthracycline based (no taxane) | 851 (41) | 845 (41) | 1696 (41) |
| FEC | 544 | 567 | 1111 |
| EC | 124 | 106 | 230 |
| E-CMF | 98 | 84 | 182 |
| AC | 61 | 55 | 116 |
| EX | 7 | 11 | 18 |
| FAC | 4 | 4 | 8 |
| Other | 13 | 19 | 32 |
| Taxane-based (no anthracycline) | 198 (10) | 202 (10) | 400 (10) |
| TC | 112 | 112 | 224 |
| TCH | 38 | 41 | 79 |
| Pac | 29 | 32 | 61 |
| T | 6 | 6 | 12 |
| Other | 13 | 11 | 24 |
| Anthracycline and taxane based | 994 (49) | 993 (49) | 1987 (49) |
| FEC-T | 818 | 795 | 1613 |
| EC-T | 65 | 79 | 144 |
| AC-Paclitaxel | 19 | 16 | 35 |
| AC-T | 14 | 18 | 32 |
| FEC-Pac | 11 | 13 | 24 |
| TAC | 6 | 12 | 18 |
| FEC-TPaca | 9 | 6 | 15 |
| EC-TPaca | 9 | 4 | 13 |
| EC-Pac | 4 | 7 | 11 |
| FEC-TAbraxa | 4 | 6 | 10 |
| ACTPaca | 1 | 1 | 2 |
| EC-GT | 1 | 1 | 2 |
| FEC-Abrax | 1 | 1 | 2 |
| Other | 32 | 34 | 66 |
| Neither anthracycline nor taxanes | 2 (< 1) | 3 (< 1) | 5 (< 1) |
| CMF | 2 | 3 | 5 |
| Type of protocol non-compliance | 12-month patients (n) | 6-month patients (n) | N/A (n) | Total (N) |
|---|---|---|---|---|
| Trastuzumab dose issue (including too few/too many cycles) | 46 | 29 | 0 | 75 |
| Reloading dose of 8 mg/kg not given | 35 | 20 | 0 | 55 |
| Healthcare at Home issue (including dispensing non-Investigational Medicinal Product) | 24 | 16 | 2 | 42 |
| Pharmacy error (including labelling) | 24 | 15 | 1 | 40 |
| Missed or late LVEF | 15 | 22 | 0 | 37 |
| Low LVEF and treatment not held | 13 | 6 | 1 | 20 |
| Ineligible patient | 7 | 12 | 0 | 19 |
| Site administration error (including staff not GCP trained) | 5 | 3 | 1 | 9 |
| Patient identifiers not redacted | 1 | 2 | 0 | 3 |
| Late-reported SAE | 1 | 0 | 0 | 1 |
| > 3 month gap between chemotherapy and trastuzumab | 0 | 1 | 0 | 1 |
| Trial Management Group error | 0 | 0 | 1 | 1 |
| Total | 171 | 126 | 6 | 303 |
| 12-month patients, n (%) | 6-month patients, n (%) | Total, N (%) | |
|---|---|---|---|
| Holiday | 588 (23) | 296 (22) | 884 (23) |
| Administration/clinic dates/i.v. access problems | 376 (15) | 197 (15) | 573 (15) |
| Patient request for family/personal reasons | 178 (7) | 63 (5) | 241 (6) |
| Surgery, radiotherapy or procedure (e.g. line insertion) | 136 (5) | 70 (5) | 206 (5) |
| Cardiotoxicity due to trastuzumab | 120 (5) | 74 (6) | 194 (5) |
| Sepsis/infection/fevera | 113 (4) | 80 (6) | 193 (5) |
| Unrelated medical problems | 112 (4) | 39 (3) | 151 (4) |
| Awaiting cardiac function tests | 52 (2) | 27 (2) | 79 (2) |
| Viral infection | 44 (2) | 18 (1) | 62 (2) |
| Myelosuppression/neutropeniaa | 32 (1) | 26 (2) | 58 (1) |
| Dyspnoea/cough | 27 (1) | 19 (1) | 46 (1) |
| Did not attend | 30 (1) | 12 (1) | 42 (1) |
| Oral/gastrointestinal tract toxicitya | 15 (1) | 12 (1) | 27 (1) |
| Fatigue | 12 (< 1) | 13 (1) | 25 (1) |
| Toxicity due to Herceptin | 6 (< 1) | 12 (1) | 18 (< 1) |
| Adverse weather conditions | 12 (< 1) | 5 (< 1) | 17 (< 1) |
| Toxicity due to chemotherapya | 3 (< 1) | 12 (1) | 15 (< 1) |
| Allergy | 7 (< 1) | 3 (< 1) | 10 (< 1) |
| Other | 38 (1) | 30 (2) | 68 (2) |
| Unknown | 695 (27) | 364 (27) | 1059 (27) |
| Toxicity | CTCAE grade | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycles by 12-month patients, n (%) | Cycles by 6-month patients, n (%) | |||||||||||
| UK | 0 | 1 | 2 | 3 | 4 | UK | 0 | 1 | 2 | 3 | 4 | |
| During the first 6 months of trastuzumab treatment | ||||||||||||
| Chills | 8 (< 1) | 6755 (89) | 590 (8) | 186 (2) | 58 (1) | 6 (< 1) | 1 (< 1) | 6960 (92) | 468 (6) | 105 (1) | 33 (< 1) | 9 (< 1) |
| Cough | 2 (< 1) | 6739 (89) | 708 (9) | 112 (1) | 24 (< 1) | 18 (< 1) | – | 6748 (89) | 695 (9) | 95 (1) | 35 (< 1) | 3 (< 1) |
| Diarrhoea | 5 (< 1) | 6639 (87) | 753 (10) | 169 (2) | 27 (< 1) | 10 (< 1) | – | 6674 (88) | 702 (9) | 160 (2) | 34 (< 1) | 6 (< 1) |
| Dizziness | 5 (< 1) | 7021 (92) | 473 (6) | 85 (1) | 17 (< 1) | 2 (< 1) | – | 7030 (93) | 458 (6) | 54 (1) | 27 (< 1) | 7 (< 1) |
| Dyspnoea | 18 (< 1) | 6675 (88) | 660 (8) | 210 (3) | 40 (1) | 4 (< 1) | 6655 (87) | 753 (10) | 118 (2) | 46 (1) | ||
| Fatigue | 15 (< 1) | 4319 (57) | 2201 (29) | 857 (11) | 177 (2) | 34 (< 1) | 5 (< 1) | 4365 (58) | 2217 (29) | 726 (10) | 234 (3) | 29 (< 1) |
| Fever | 3 (< 1) | 7385 (97) | 163 (2) | 41 (1) | 11 (< 1) | – | 1 (< 1) | 7329 (97) | 164 (2) | 58 (1) | 22 (< 1) | 2 (< 1) |
| Headache | 7 (< 1) | 6482 (85) | 876 (12) | 177 (2) | 58 (1) | 3 (< 1) | 1 (< 1) | 6469 (85) | 894 (12) | 167 (2) | 37 (< 1) | 8 (< 1) |
| Hypertension | 1 (< 1) | 7500 (99) | 72 (1) | 24 (< 1) | 5 (< 1) | 1 (< 1) | 1 (< 1) | 7456 (98) | 87 (1) | 21 (< 1) | 11 (< 1) | – |
| Hypotension | 3 (< 1) | 7569 (99) | 29 (< 1) | 1 (< 1) | 1 (< 1) | – | – | 7543 (99) | 26 (< 1) | 4 (< 1) | 3 (< 1) | – |
| Infection | 1 (< 1) | 7298 (96) | 153 (2) | 132 (2) | 17 (< 1) | 2 (< 1) | 5 (< 1) | 7251 (96) | 160 (2) | 127 (2) | 30 (< 1) | 3 (< 1) |
| Muscle/joint pain | 13 (< 1) | 5096 (67) | 1558 (20) | 727 (10) | 164 (2) | 45 (1) | 2 (< 1) | 5132 (68) | 1487 (20) | 700 (9) | 223 (3) | 32 (< 1) |
| Nausea | 13 (< 1) | 6956 (91) | 534 (7) | 80 (1) | 20 (< 1) | 5 (< 1) | 7022 (93) | 444 (6) | 91 (1) | 14 (< 1) | ||
| Pain | 18 (< 1) | 6696 (88) | 594 (8) | 231 (3) | 57 (1) | 7 (< 1) | 4 (< 1) | 6774 (89) | 492 (6) | 232 (3) | 58 (1) | 16 (< 1) |
| Palpitations | 2 (< 1) | 7111 (94) | 398 (5) | 92 (1) | 3 (< 1) | 7161 (94) | 343 (5) | 69 (1) | ||||
| Rash | 7 (< 1) | 7222 (95) | 301 (4) | 57 (1) | 16 (< 1) | – | 4 (< 1) | 7181 (95) | 291 (4) | 81 (1) | 18 (< 1) | 1 (< 1) |
| Vomiting | 1 (< 1) | 7429 (97) | 127 (2) | 40 (1) | 3 (< 1) | 3 (< 1) | 1 (< 1) | 7433 (98) | 110 (1) | 25 (< 1) | 6 (< 1) | 1 (< 1) |
| During the second 6 months of trastuzumab treatment | ||||||||||||
| Chills | – | 6401 (93) | 348 (5) | 77 (1) | 26 (< 1) | 3 (< 1) | ||||||
| Cough | – | 6174 (90) | 540 (8) | 98 (1) | 35 (1) | 8 (< 1) | ||||||
| Diarrhoea | 1 (< 1) | 6284 (92) | 461 (7) | 88 (1) | 19 (< 1) | 2 (< 1) | ||||||
| Dizziness | 4 (< 1) | 6359 (93) | 396 (6) | 81 (1) | 11 (< 1) | 4 (< 1) | ||||||
| Dyspnoea | 6 (< 1) | 6164 (90) | 522 (8) | 130 (2) | 33 (< 1) | |||||||
| Fatigue | 12 (< 1) | 4165 (61) | 1879 (27) | 610 (9) | 161 (2) | 28 (< 1) | ||||||
| Fever | 1 (< 1) | 6691 (98) | 141 (2) | 16 (< 1) | 5 (< 1) | 1 (< 1) | ||||||
| Headache | – | 5945 (87) | 726 (10) | 140 (2) | 40 (1) | 4 (< 1) | ||||||
| Hypertension | – | 6724 (98) | 100 (1) | 20 (< 1) | 11 (< 1) | – | ||||||
| Hypotension | – | 6826 (99) | 28 (< 1) | 1 (< 1) | – | – | ||||||
| Infection | 2 (< 1) | 6635 (97) | 137 (2) | 77 (1) | 4 (< 1) | – | ||||||
| Muscle/joint pain | 12 (< 1) | 4663 (68) | 1402 (20) | 605 (9) | 139 (2) | 34 (1) | ||||||
| Nausea | – | 6497 (95) | 305 (4) | 48 (1) | 5 (< 1) | |||||||
| Pain | 6 (< 1) | 6063 (88) | 510 (7) | 226 (3) | 42 (1) | 8 (< 1) | ||||||
| Palpitations | 1 (< 1) | 6470 (94) | 311 (5) | 73 (1) | ||||||||
| Rash | 1 (< 1) | 6618 (97) | 198 (3) | 33 (< 1) | 5 (< 1) | – | ||||||
| Vomiting | – | 6737 (98) | 79 (1) | 34 (1) | 4 (< 1) | 1 (< 1) | ||||||
| 12-month patients, n (%) | 6-month patients, n (%) | Total, N (%) | |
|---|---|---|---|
| Reason for reportinga | |||
| Inpatient hospitalisation/prolongation of stay | 242 (83) | 153 (83) | 395 (83) |
| Life-threatening event | 23 (9) | 18 (10) | 41 (9) |
| Persistent or significant disability/incapacity | 11 (4) | 5 (3) | 16 (3) |
| Death | 3 (1) | 3 (2) | 6 (1) |
| New primary | 1 (< 1) | 2 (1) | 3 (< 1) |
| Other | 36 (12) | 19 (10) | 55 (11) |
| Reported severity | |||
| Mild | 55 (19) | 35 (19) | 90 (19) |
| Moderate | 155 (53) | 87 (47) | 242 (51) |
| Severe | 63 (22) | 50 (27) | 113 (24) |
| Fatal/life-threatening | 14 (5) | 11 (6) | 25 (5) |
| Missing | 4 (1) | 1 (1) | 5 (1) |
| Reported causality related to trastuzumab | |||
| Definitely | 27 (9) | 16 (9) | 43 (9) |
| Probably | 19 (7) | 12 (5) | 31 (6) |
| Possibly | 21 (7) | 8 (4) | 29 (6) |
| Unlikely | 48 (17) | 36 (20) | 84 (18) |
| Unrelated | 176 (60) | 112 (61) | 288 (61) |
| Outcome | |||
| Resolved – no sequelae | 208 (71) | 140 (76) | 348 (73) |
| Resolved – with sequelae | 47 (16) | 28 (15) | 75 (16) |
| Unresolved | 32 (11) | 10 (6) | 42 (9) |
| Death | 3 (1) | 6 (3) | 9 (2) |
| Missing | 1 (< 1) | 0 (0) | 1 (< 1) |
| Reported primary CTCAE category | |||
| Infection | 118 (41) | 80 (43) | 198 (42) |
| Cardiac general | 38 (13) | 15 (8) | 53 (11) |
| Pain | 19 (7) | 11 (6) | 30 (6) |
| Gastrointestinal | 10 (3) | 12 (7) | 22 (5) |
| Neurology | 14 (5) | 8 (4) | 22 (5) |
| Pulmonary/upper respiratory | 13 (4) | 8 (4) | 21 (4) |
| Blood/bone marrow | 14 (5) | 6 (3) | 20 (4) |
| Musculoskeletal/soft tissue | 12 (4) | 5 (3) | 17 (4) |
| Constitutional symptoms | 5 (2) | 9 (5) | 14 (3) |
| Vascular | 8 (3) | 5 (3) | 13 (3) |
| Cardiac arrhythmia | 7 (2) | 5 (3) | 12 (3) |
| Allergy/immunology | 6 (2) | 3 (2) | 9 (2) |
| Dermatology/skin | 6 (2) | 3 (2) | 9 (2) |
| Haemorrhage/bleeding | 3 (1) | 2 (1) | 5 (1) |
| Hepatobiliary/pancreas | 4 (1) | 1 (< 1) | 5 (1) |
| Sexual/reproductive | 4 (1) | 1 (< 1) | 5 (1) |
| Secondary malignancy | 3 (1) | 1 (< 1) | 4 (< 1) |
| Death | 1 (< 1) | 2 (1) | 3 (< 1) |
| Endocrine | 1 (< 1) | 2 (1) | 3 (< 1) |
| Metabolic/laboratory | 1 (< 1) | 2 (1) | 3 (< 1) |
| Renal/genitourinary | 2 (1) | 0 (0) | 2 (< 1) |
| Lymphatics | 1 (< 1) | 1 (< 1) | 2 (< 1) |
| Ocular/visual | 1 (< 1) | 0 (0) | 1 (< 1) |
| Auditory/ear | 0 (0) | 1 (< 1) | 1 (< 1) |
| Surgery/intraoperative injury | 0 (0) | 1 (< 1) | 1 (< 1) |
| 12-month patients, n (%) | 6-month patients, n (%) | Total, N (%) | |
|---|---|---|---|
| Cardiac general | 30 (45) | 15 (42) | 45 (43) |
| Infection | 12 (18) | 9 (25) | 21 (20) |
| Pulmonary/upper respiratory | 8 (12) | 1 (3) | 9 (9) |
| Cardiac arrhythmia | 5 (7) | 3 (8) | 8 (8) |
| Allergy/immunology | 5 (7) | 2 (6) | 7 (7) |
| Pain | 3 (5) | 2 (6) | 5 (5) |
| Sexual/reproductive | 1 (1.5) | 1 (2.5) | 2 (2) |
| Dermatology/skin | 1 (1.5) | 1 (2.5) | 2 (2) |
| Constitutional symptoms | 0 (0) | 1 (2.5) | 1 (1) |
| Gastrointestinal | 0 (0) | 1 (2.5) | 1 (1) |
| Lymphatics | 1 (1.5) | 0 (0) | 1 (1) |
| Vascular | 1 (1.5) | 0 (0) | 1 (1) |
| 12-month patients, n (%) | 6-month patients, n (%) | Total, N (%) | |
|---|---|---|---|
| Local relapse | 81 (4%) | 83 (4%) | 164 (4%) |
| Site | |||
| Ipsilateral breast/chest wall | 40 (49) | 52 (63) | 92 (56) |
| Ipsilateral axilla nodes | 18 (22) | 10 (12) | 28 (17) |
| Ipsilateral supraclavicular nodes | 7 (9) | 12 (14) | 19 (12) |
| Contralateral breast disease | 5 (6) | 6 (7) | 11 (7) |
| Other | 11 (14) | 3 (4) | 14 (9) |
| Distant relapse | 192 (9%) | 207 (10%) | 399 (10%) |
| Site | |||
| Liver | 86 (45) | 91 (44) | 177 (44) |
| Bone | 63 (33) | 89 (43) | 152 (38) |
| Lung/pleura | 74 (39) | 78 (38) | 152 (38) |
| Brain | 40 (21) | 43 (21) | 83 (21) |
| Contralateral supraclavicular nodes | 4 (2) | 3 (1) | 7 (2) |
| Other | 27 (14) | 23 (11) | 50 (13) |
| Second primaries | 67 (3%) | 67 (3%) | 134 (3%) |
| Site | |||
| Contralateral breast | 23 (34) | 13 (19) | 36 (27) |
| Lung | 9 (13.5) | 8 (12) | 17 (13) |
| Bowel/colon | 4 (6) | 11 (16.5) | 15 (11) |
| Ovary | 5 (7.5) | 5 (7.5) | 10 (7.5) |
| Bladder/urothelial cells | 2 (3) | 4 (6) | 6 (4) |
| Endometrium | 3 (4.5) | 3 (4.5) | 6 (4) |
| Lymphatic system | 1 (1.5) | 3 (4.5) | 4 (3) |
| Kidney | 4 (6) | – | 4 (3) |
| Skin | 2 (3) | 2 (3) | 4 (3) |
| Skin (melanoma) | 3 (4.5) | – | 3 (2) |
| AML | 2 (3) | 1 (1.5) | 3 (2) |
| Stomach | 1 (1.5) | 2 (3) | 3 (2) |
| Brain | – | 3 (4.5) | 3 (2) |
| Oesophagus | – | 3 (4.5) | 3 (2) |
| Bile duct | 1 (1.5) | 1 (1.5) | 2 (1.5) |
| Head and neck | 2 (3) | – | 2 (1.5) |
| Ipsilateral breast | 2 (3) | – | 2 (1.5) |
| Pancreas | 1 (1.5) | – | 1 (< 1) |
| Soft tissue sarcoma | 1 (1.5) | – | 1 (< 1) |
| Thyroid | 1 (1.5) | – | 1 (< 1) |
| Basal cell carcinoma (skin cancer) | – | 1 (1.5) | 1 (< 1) |
| Bone and liver | – | 1 (1.5) | 1 (< 1) |
| Cervix | – | 1 (1.5) | 1 (< 1) |
| LAMN | – | 1 (1.5) | 1 (< 1) |
| Liver | – | 1 (1.5) | 1 (< 1) |
| Mesothelioma | – | 1 (1.5) | 1 (< 1) |
| Myeloma | – | 1 (1.5) | 1 (< 1) |
| Vulva | – | 1 (1.5) | 1 (< 1) |
| Characteristic | 12-month patients, n (%) | 6-month patients, n (%) | Total, N (%) |
|---|---|---|---|
| ER statusa | |||
| Negative | 615 (31) | 618 (31) | 1233 (31) |
| Positive | 1392 (69) | 1383 (69) | 2775 (69) |
| Chemotherapy typea | |||
| Anthracycline based | 837 (42) | 829 (41) | 1666 (41) |
| Taxane based | 193 (10) | 196 (10) | 389 (10) |
| Anthracycline and taxane based | 975 (48) | 973 (49) | 1948 (49) |
| No taxane and no anthracycline | 2 (< 1) | 3 (< 1) | 5 (< 1) |
| Chemotherapy timinga | |||
| Adjuvant | 1708 (85) | 1693 (85) | 3401 (85) |
| Neoadjuvant | 299 (15) | 308 (15) | 607 (15) |
| Trastuzumab timinga | |||
| Concurrent | 933 (46) | 928 (46) | 1861 (46) |
| Sequential | 1074 (54) | 1073 (54) | 2147 (54) |
| Sex | |||
| Female | 2003 (99) | 1999 (99) | 4002 (99) |
| Male | 4 (1) | 2 (1) | 6 (1) |
| Age (years) at randomisation | |||
| Median (range) | 56 (23–82) | 56 (23–83) | 56 (23–83) |
| < 35 | 48 (2) | 43 (2) | 91 (2) |
| 35–49 | 537 (27) | 545 (27) | 1082 (27) |
| 50–59 | 600 (30) | 641 (32) | 1241 (31) |
| 60–69 | 608 (30) | 574 (29) | 1182 (30) |
| ≥ 70 | 214 (11) | 198 (10) | 412 (10) |
| Nodal status at surgery (of the 3401 adjuvant patients) | |||
| Negative | 987 (58) | 997 (59) | 1984 (58) |
| 1–3 nodes positive | 470 (27) | 479 (28) | 949 (28) |
| ≥ 4 nodes positive | 241 (14) | 207 (12) | 448 (13) |
| Unknown | 10 (1) | 10 (1) | 20 (1) |
| Tumour sizeb (of the 3401 adjuvant patients) | |||
| ≤ 2 cm | 812 (47) | 791 (47) | 1603 (47) |
| > 2 and ≤ 5 cm | 766 (45) | 772 (45) | 1538 (45) |
| > 5 cm | 86 (5) | 80 (5) | 166 (5) |
| Unknown | 44 (3) | 50 (3) | 194 (3) |
| Tumour gradeb | |||
| I (well differentiated) | 28 (1) | 34 (2) | 62 (2) |
| II (moderately differentiated) | 624 (31) | 637 (32) | 1261 (31) |
| III (poorly differentiated) | 1296 (65) | 1271 (63) | 2567 (64) |
| Unknown | 59 (3) | 59 (3) | 118 (3) |
| Ethnicity | |||
| White | 1636 (82) | 1619 (81) | 3255 (81) |
| Asian | 56 (3) | 51 (3) | 107 (3) |
| Black | 51 (3) | 44 (2) | 95 (2) |
| Other | 16 (< 1) | 21 (1) | 37 (1) |
| Unknown | 248 (12) | 266 (13) | 514 (13) |
| Menopausal status before chemotherapy | |||
| Pre | 554 (28) | 567 (28) | 1121 (28) |
| Peri | 108 (5) | 150 (8) | 258 (6) |
| Post | 1127 (56) | 1054 (53) | 2181 (54) |
| Not assessable/not available | 218 (11) | 230 (11) | 448 (11) |
| Reported prior use of cardiac medication | |||
| Yes | 43 (2) | 54 (3) | 97 (2) |
| No | 1964 (98) | 1947 (97) | 3911 (98) |
| IHC-score and FISH positivity (HER2 test result) | |||
| 3+ | 1437 (72) | 1464 (73) | 2901 (72) |
| 2+ and FISH positive | 541 (27) | 499 (25) | 1040 (26) |
| HER2 positive – IHC and FISH score not available | 29 (1) | 38 (2) | 67 (2) |
| Symptoms of cardiac disease | Signs of CHF | New/altered medication for cardiac disease | 12-month patients, n (%) | 6-month patients, n (%) | Total, N (%) |
|---|---|---|---|---|---|
| ✓ | ✓ | ✓ | 20 (1) | 9 (< 1) | 29 (< 1) |
| ✓ | ✓ | 10 (< 1) | 4 (< 1) | 14 (< 1) | |
| ✓ | ✓ | 39 (2) | 23 (1) | 62 (1.5) | |
| ✓ | ✓ | 7 (< 1) | 2 (< 1) | 9 (< 1) | |
| ✓ | 52 (3) | 31 (2) | 83 (2) | ||
| ✓ | 7 (< 1) | 6 (< 1) | 13 (< 1) | ||
| ✓ | 93 (5) | 81 (4) | 174 (4) | ||
| ✗ | ✗ | ✗ | 1759 (89) | 1852 (92) | 3611 (90) |
| LVEF scan type | 12-month patients, n (%) | 6-month patients, n (%) | Total, N (%) |
|---|---|---|---|
| MUGA | |||
| Normal result | 1672 (90) | 1405 (91) | 3077 (90) |
| Low LVEFa | 183 (10) | 141 (9) | 324 (10) |
| ECHO | |||
| Normal result | 7102 (96) | 6610 (97) | 13,712 (97) |
| Low LVEFa | 282 (4) | 213 (3) | 495 (3) |
| TNO | Metastatic (Y/N) | Treatment group | Trastuzumab cycles | Time from diagnosis to death (months) | Cardiac causes | Comment |
|---|---|---|---|---|---|---|
| 144 | N | 12 months | 18 | 44 | Ischaemic heart disease | Unrelated/unlikely. Death due to acute MI 44 months after diagnosis. No association between trastuzumab and coronary artery disease |
| 938 | N | 12 months | 8 | 21 | Ischaemic heart disease | Unrelated/unlikely. Death due to acute MI 21 months after diagnosis. No association between trastuzumab and coronary artery disease |
| 1754 | N | 12 months | 18 | 51 | Cardiac amyloid | Unrelated – no association between trastuzumab and amyloidosis |
| 2003 | N | 12 months | 14 | 29 | Cardiac sarcoid | Unrelated – no association between trastuzumab and sarcoidosis |
| 3021 | N | 12 months | 18 | 46 | Right ventricular arrhythmic cardiomyopathy | Unlikely – arrhythmic cardiomyopathy (ARVC in old terminology) has a known genetic basis (mostly desmosomal genes) with physiological triggers (e.g. extreme endurance sports). It is plausible that cardiac dysfunction caused by trastuzumab could have been a trigger but unlikely in this case as heart function had recovered long before ARVC developed |
| 3463 | N | 6 months | 9 | 53 | Acute heart failure/left ventricular hypertrophy/mitral valve disease | Unrelated/unlikely – there is no known association with left ventricular hypertrophy or mitral valve disease, and it is difficult to think of a mechanism for any effect months after the discontinuation of trastuzumab |
| 35 | N | 6 months | 9 | 111 | Ischaemic heart disease | Unrelated/unlikely. Death due to acute MI 111 months after diagnosis. No association between trastuzumab and coronary artery disease |
| 1051 | N | 6 months | 4 | 78 | CHF and type II respiratory failure | Unrelated/unlikely – very unlikely to be related to trastuzumab – received only four cycles; recovery of LV function > 3 months after cessation; diagnosis of COPD; died 78 months after diagnosis |
| 1086 | N | 6 months | 9 | 43 | Not cardiac | N/A |
| 2654 | N | 6 months | 9 | 41 | Myocardial infarction – pulmonary embolism – atrial myxoma | Unrelated – no effect of trastuzumab during treatment; no association between trastuzumab and atrial myxoma or myocardial infarction |
| 104 | Y | 12 months | 18 | 39 | No cardiac problems – brain metastases – died during a seizure | N/A |
| 625 | Y | 12 months | 3 | 35 | Controlled NYHA class II CHF |
Cause of death was metastatic disease with bone and liver metastases. LVEF pre treatment was 56%; after three cycles of trastuzumab was 51% (abnormal at site). Trastuzumab stopped after three cycles and not restarted. LVEF dropped to 48%, and patient was referred to cardiology and diagnosed with NYHA class II CHF and started on ACE inhibitors and bisoprolol and continued follow-up in heart failure clinic. LVEF was then 51% and then 46%; patient continued on cardiac meds and never restarted trastuzumab. Returned to normal at LVEF 55%, 1 year after stopping trastuzumab; was on cardiac medication at the time and remained on this. Then developed metastatic disease 3 years after diagnosis and deteriorated rapidly with liver impairment and was not fit enough for metastatic treatment that was being considered (capecitabine/lapatinib) Had previously received FEC × 4 cycles and T × 3 cycles (no cycle 8 as allergic reaction) with sequential trastuzumab. Total dose of epirubicin: 75 mg/m2 × 4 = 300 mg/m2 At time of death from metastatic breast cancer had recovery of function and remained on preventative cardiac medication |
| 1279 | Y | 12 months | 4 | 75 | Cardiomyopathy |
Died with metastatic disease in lung/pleura, which was recorded as a cause of death. Cardiomyopathy recorded as contributing cause of death; however, no autopsy was carried out FE(100 mg/m2)C × 3 cycles/T (80 mg/m2) × 3 cycles. Total dose of epirubicin = 300 mg/m2. Trastuzumab given concurrently with docetaxel. Clear effect of trastuzumab causing reduced LVEF during treatment. LVEF baseline normal at 55%; after four cycles fell to 30%, and trastuzumab stopped, and not restarted. Commenced cardiac medication perindopril and bisoprolol. Coronary angiograms at the time showed coronary artery disease. Recovery of LVEF by 1 year later to LVEF 50%; on perindopril and bisoprolol. Death 6 years later judged not related to trastuzumab. No supporting information for cardiomyopathy – no histology, and LVEF had previously recovered. If present, most likely explanation is coronary artery disease or otherwise anthracyclines |
Cardiac deaths recorded without metastatic disease (n = 10):
-
ischaemic heart disease – unrelated/unlikely to be related (n = 4)
-
LVEF decreased and CHF – unrelated/unlikely to be related (n = 1)
-
acute heart failure/left ventricular hypertrophy and mitral valve disease – unrelated/unlikely to be related (n = 1)
-
cardiac amyloid – unrelated/unlikely to be related (n = 1)
-
cardiac sarcoid – unrelated/unlikely to be related (n = 1)
-
right ventricular cardiomyopathy – unrelated/unlikely to be related (n = 1)
-
death after routine computerised tomography scan – clinical anaphylaxis – no sign of this post mortem – no cardiac pathology found. Not cardiac (n = 1).
Deaths with metastatic disease recorded as contributed to by cardiac problems (n = 3):
-
controlled NYHA class II CHF – unrelated/unlikely to be related (n = 1)
-
cardiomyopathy – unrelated/unlikely to be related (n = 1)
-
brain metastases and died during a seizure – unrelated/unlikely to be related. Not cardiac (n = 1).
| Number of trastuzumab cycles received | 12-month patients, n (%) | 6-month patients, n (%) | ||
|---|---|---|---|---|
| Reported clinical cardiac dysfunction in months 0–3 | Reported clinical cardiac dysfunction in months 4–12 | Reported clinical cardiac dysfunction in months 0–3 | Reported clinical cardiac dysfunction in months 4–12 | |
| 1 | 2 (1.5) | 1 (< 1) | – | – |
| 2 | 3 (2) | 1 (< 1) | 2 (1) | 2 (1) |
| 3 | 1 (1) | 1 (< 1) | – | – |
| 4 | 8 (6) | 10 (5) | 10 (7) | 11 (7) |
| 5 | 6 (4) | 8 (4) | 5 (3) | 4 (2) |
| 6 | 6 (4) | 4 (2) | 4 (3) | 5 (3) |
| 7 | 5 (4) | 6 (3) | 7 (5) | 7 (4) |
| 8 | 6 (4) | 13 (6) | 9 (6) | 9 (5) |
| 9 | 2 (1.5) | 7 (3) | 106 (71) | 119 (70) |
| 10 | 2 (1.5) | 7 (3) | – | 1 (< 1) |
| 11 | 3 (2) | 5 (2) | – | – |
| 12 | – | 3 (1) | – | – |
| 13 | 2 (1.5) | 15 (7) | – | – |
| 14 | 2 (1.5) | 10 (5) | – | – |
| 15 | 2 (1.5) | 9 (4) | – | – |
| 16 | 2 (1.5) | 5 (2) | – | – |
| 17 | 3 (2) | 6 (3) | – | 2 (1) |
| 18 | 82 (59.5) | 105 (48) | 4 (3) | 6 (4) |
| 19 | 1 (1) | 1 (< 1) | – | – |
| Unknown | – | – | 2 (1) | 3 (2) |
| Total | 138 (100) | 217 (100) | 149 (100) | 169 (100) |
| Number of trastuzumab cycles received | 12-month patients, n (%) | 6-month patients, n (%) | ||
|---|---|---|---|---|
| Reported low LVEF in months 0–3 | Reported low LVEF in months 4–12 | Reported low LVEF in months 0–3 | Reported low LVEF in months 4–12 | |
| 0 | 1 (1.3) | – | – | – |
| 1 | – | – | – | – |
| 2 | 2 (3) | 1 (1) | 1 (1) | 2 (1) |
| 3 | 1 (1.3) | 1 (1) | 1 (1) | 1 (< 1) |
| 4 | 15 (20) | 11 (5) | 10 (12.5) | 10 (7) |
| 5 | 6 (8) | 8 (4) | 6 (7.5) | 3 (2) |
| 6 | 8 (11) | 8 (4) | 3 (4) | 3 (2) |
| 7 | – | 5 (2) | 4 (5) | 7 (5) |
| 8 | 1 (1.3) | 15 (7) | 2 (2.5) | 17 (12) |
| 9 | 1 (1.3) | 6 (3) | 53 (65.5) | 96 (66) |
| 10 | – | 10 (5) | 1 (1) | 1 (< 1) |
| 11 | 1 (1.3) | 9 (4.5) | – | – |
| 12 | 1 (1.3) | 3 (1) | – | – |
| 13 | – | 17 (8) | – | – |
| 14 | 1 (1.3) | 7 (3) | – | – |
| 15 | 1 (1.3) | 8 (4) | – | – |
| 16 | 1 (1.3) | 9 (4.5) | – | – |
| 17 | 2 (3) | 8 (4) | – | 1 (< 1) |
| 18 | 31 (42) | 78 (38) | – | 4 (3) |
| Unknown | 1 (1.3) | 1 (1) | – | – |
| Total | 74 (100) | 205 (100) | 81 (100) | 145 (100) |
| Old CRFs (baseline at start of trastuzumab) | New CRFs (baseline at 6 months of trastuzumab) |
|---|---|
|
Adjuvant/neoadjuvant eligibility form LVEF measurement |
Eligibility form LVEF measurement prior to trastuzumab dose 1 |
|
Adjuvant/neoadjuvant randomisation form Chemotherapy type, timing |
Adjuvant/neoadjuvant randomisation form Chemotherapy type, timing Trastuzumab start date, number of doses |
|
Neoadjuvant diagnostic biopsy form Planned surgery (could use to check surgery recorded on surgery form) |
Neoadjuvant diagnostic biopsy form Planned surgery (could use to check surgery recorded on surgery form) |
|
Surgery form (for both neoadjuvant and adjuvant) Type of surgery |
Surgery form (for both neoadjuvant and adjuvant) Type of surgery |
|
Chemotherapy form Cycle number Drug dose Dose reduction: plus reason (e.g. cardiotoxicity) Dose delay: plus reason (e.g. cardiotoxicity) Hospital admission: plus reason (e.g. cardiotoxicity) Number of admissions; total days in hospital If patient receiving concurrent trastuzumab with chemotherapy Supportive treatments Toxicity |
|
|
Trastuzumab treatment form Dose Dose reduction: plus reason (e.g. cardiotoxicity) Dose delayed: plus reason (e.g. cardiotoxicity) Dose held: plus reason (e.g. cardiotoxicity) Hospital admission: plus reason (e.g. cardiotoxicity) Number of admissions; total days in hospital Toxicity Cardiology assessment: Symptoms of cardiac disease Signs of congestive heart failure Physical findings of cardiac disease Medication for cardiac disease – all symptomatic signs of cardiotoxicity/cardiac problems LVEF measurement |
Trastuzumab treatment form: over last 3 months whether before or after randomisation Dose Dose reduction: plus reason (e.g. cardiotoxicity) Dose delayed: plus reason (e.g. cardiotoxicity) Dose held: plus reason (e.g. cardiotoxicity) Hospital admission: plus reason (e.g. cardiotoxicity) Number of admissions; total days in hospital Toxicity Cardiology assessment: Symptoms of cardiac disease Signs of congestive heart failure Physical findings of cardiac disease Medication for cardiac disease – all symptomatic signs of cardiotoxicity/cardiac problems LVEF measurement |
|
Treatment summary form Chemotherapy summary Number of cycles Drug If less than intended, reason Trastuzumab summary Number of doses If fewer than randomised to, reason Catheter use |
Treatment summary form Chemotherapy summary Number of cycles Drug If less than intended, reason Trastuzumab summary Number of doses If fewer than randomised to, reason |
|
Radiotherapy form Summary of radiotherapy Modifications to radiotherapy |
Radiotherapy form Summary of radiotherapy Modifications to radiotherapy |
|
Annual follow-up form Medication Toxicities Further chemotherapy Further surgery Ovation suppression/ablation Disease free Dead |
|
|
Relapse/death form Trastuzumab treatment Relapse: locoregional, distant, first/second primary Death, cause of: breast cancer, other cancer, protocol treatment related, other treatment related, other cause |
|
|
Hospital transfer form Reason for transfer Details of transfer |
| Cardiology assessment | Unit cost (£) | Source |
|---|---|---|
| MUGA scan | 252.64 | NHS Reference Costs 2017/18: MUGA scan (RN22Z)87 |
| ECHO | 253.70 | NHS Reference Costs 2017/18: complex echocardiogram for congenital heart disease (EC21Z)87 |
| MRI | 227.60 | NHS Reference Costs 2017/18: cardiac magnetic resonance imaging scan without contrast (RD08Z)87 |
| Electrocardiogram | 120.32 | NHS Reference Costs 2017/18: electrocardiogram monitoring or stress testing, for congenital heart disease (EC22Z)87 |
| X-ray | 31.49 | NHS Reference Costs 2017/18: direct access plain film (DAPF)87 |
| Myocardial perfusion | 290.46 | NHS Reference Costs 2017/18: myocardial perfusion scan, stress only (RN21Z)87 |
| Computerised tomography angiography | 106.22 | NHS Reference Costs 2017/18: computerised tomography scan of one area, with post-contrast only, 19 years and over (RD21A)87 |
| Exercise stress test | 159.53 | NHS Reference Costs 2017/18: cardiopulmonary exercise testing (DZ31Z)87 |
| Blood tests | 1.11 + 2.83 | NHS Reference Costs 2017/18: clinical biochemistry (DAPS04) + phlebotomy (DAPS08)87 |
| Assessment by cardiologist | 128.05 | NHS Reference Costs 2017/18: non-admitted face-to-face attendance, follow-up (WF01A)87 |
| Cardiac catheterisation | 1131.89 | NHS Reference Costs 2017/18: standard cardiac catheterisation with CC score 0–1 (EY43F)87 |
| Cardiac medication | Unit cost (£) | Source |
|---|---|---|
| Warfarin (5 mg) | 0.02 (0.59/28 tablets) | BNF 2018: NHS indicative price88 |
| Carvedilol (3.125 mg) | 0.03 (0.73/28 tablets) | |
| Carvedilol (12.5 mg) | 0.03 (0.75/28 tablets) | |
| Sotalol (40 mg) | 0.04 (1.11/28 tablets) | |
| Clopidogrel (75 mg) | 0.04 (1.16/28 tablets) | |
| Furosemide (40 mg) | 0.01 (3.50/250 tablets) | |
| Amlodipine (5 mg) | 0.02 (0.67/28 tablets) | |
| Amlodipine (10 mg) | 0.02 (0.68/28 tablets) | |
| Aspirin (75 mg) | 0.04 (1.10/28 tablets) | |
| Ramipril (1.25 mg) | 0.05 (1.35/28 tablets) | |
| Ramipril (2.5 mg) | 0.18 (5/28 tablets) | |
| Ramipril (5 mg) | 0.03 (0.81/28 tablets) | |
| Ramipril (10 mg) | 0.03 (0.93/28 tablets) | |
| Celecoxib (200 mg) | 0.05 (1.62/30 capsules) |
| Endocrine medications and bisphosphonates; daily dose | Unit cost (£) | Source |
|---|---|---|
| Anastrozole 1 mg (Arimidex; AstraZeneca UK Limited, Macclesfield, UK) | 2.45 (68.56/28 tablets) | BNF 2018: NHS indicative price88 |
| Exemestane 25 mg (Aromasin; Pfizer Limited, Sandwich, UK) | 2.96 (88.80/30 tablets) | |
| Letrozole 2.5 mg (Femara®; Novartis Pharmaceuticals UK Ltd, London, UK) | 3.03 (90.92/30 tablets) | |
| Goserelin 3.6 mg (Zoladex®; AstraZeneca UK Limited) | 2.50 (70/28 days) | |
| Tamoxifen 20 mg (Nolvadex®; AstraZeneca UK Limited) | 0.08 (2.35/30 tablets) | |
| Bisphosphonates (alendronate 10 mg) | 0.05 (1.29/28 tablets) |
| Surgical intervention | Unit cost (£) | Source |
|---|---|---|
| Delayed reconstruction | 5462.48 | NHS Reference Costs 2017/18: unilateral delayed pedicled myocutaneous breast reconstruction (JA30Z)87 |
| Mastectomy of treated breast | 3490.00 | NHS Reference Costs 2017/18: unilateral major breast procedures with CC score 6+ (JA20D)87 |
| Mastectomy of treated breast with reconstruction | 5462.48 | NHS Reference Costs 2017/18: unilateral delayed pedicled myocutaneous breast reconstruction (JA30Z)87 |
| Mastectomy of contralateral breast | 3490.00 | NHS Reference Costs 2017/18: unilateral major breast procedures with CC score 6+ (JA20D)87 |
| Mastectomy of contralateral breast with reconstruction | 5462.48 | NHS Reference Costs 2017/18: unilateral delayed pedicled myocutaneous breast reconstruction (JA30Z)87 |
| Oophorectomy | 4834.47 | NHS Reference Costs 2017/18: major open upper genital tract procedures with CC score 3–4 (oophorectomy) (MA07F)87 |
| Biopsy | 351 | NHS Reference Costs 2017/18: biopsy of lesion of breast and associated lymph nodes (YJ03Z)87 |
| Mammoplasty | 4331 | NHS Reference Costs 2017/18: unilateral therapeutic mammoplasty (JA40Z)87 |
| Minor breast procedure | 1616 | NHS Reference Costs 2017/18: unilateral minor breast procedures (JA45Z)87 |
| Intermediate breast procedures | 2362 | NHS Reference Costs 2017/18: unilateral intermediate breast procedures with CC score 0–2 (JA43B)87 |
| Lymph node clearance | 3863 | NHS Reference Costs 2017/18: unilateral major breast procedures with lymph node clearance, with CC score 0–1 (JA38C)87 |
| Service | Unit cost (£) | Source |
|---|---|---|
| Hospital services | ||
| Hospital inpatient stay/day | 337.36 | NHS Reference Costs 2017/18: non-elective inpatients – excess bed-days87 |
| Hospital day centre/day | 201.00 | PSSRU Unit Costs of Health and Social Care 2018, p. 89: inpatient, hospital specialist palliative care support (adults only)86 |
| Outpatient clinic visit | 108.00 | PSSRU Unit Costs of Health and Social Care 2018, p. 89: outpatient, medical specialist palliative care attendance (adults and children)86 |
| A&E visit | 153.32 | NHS Reference Costs 2017/18: emergency medicine, category 1 investigation with category 1–2 treatment (T01, VB09Z)87 |
| Community-based health care | ||
| GP surgery face to face | 37.00 | PSSRU Unit Costs of Health and Social Care 2018, p. 127: general practitioner, per surgery consultation lasting 9.22 minutes86 |
| GP surgery telephone-contact | 28.40 | PSSRU Unit Costs of Health and Social Care 2018, p. 127: general practitioner, per patient contact assuming a 7.1-minute call including direct staff costs86 |
| GP home face to face | 120.00 | PSSRU Unit Costs of Health and Social Care 2018, p. 127: general practitioner, per patient contact assuming a 30-minute appointment86 |
| GP home telephone contact | 28.40 | PSSRU Unit Costs of Health and Social Care 2018, p. 127: general practitioner, per patient contact assuming a 7.1-minute call including direct staff costs86 |
| District nurse face to face | 36.48 | PSSRU Unit Costs of Health and Social Care 2015, p. 169: per hour of patient-related work, including qualifications, assuming 30-minute appointment (inflated)86 |
| District nurse telephone contact | 8.51 | PSSRU Unit Costs of Health and Social Care 2015, p. 169: per hour of patient-related work, including qualifications, assuming 7-minute telephone call (inflated)86 |
| Health visitor face to face | 41.39 | PSSRU Unit Costs of Health and Social Care 2015, p. 171: per hour of patient related work, including qualifications, assuming 30-minute appointment (inflated)86 |
| Health visitor telephone contact | 9.66 | PSSRU Unit Costs of Health and Social Care 2015, p. 171: per hour of patient-related work, assuming a 7-minute call (inflated)86 |
| Occupational therapist visit | 23.50 | PSSRU Unit Costs of Health and Social Care 2018, p. 142: NHS community occupational therapist, assuming a 30-minute appointment86 |
| Occupational therapist telephone contact | 5.48 | PSSRU Unit Costs of Health and Social Care 2018, p. 142: NHS community occupational therapist, assuming a 7-minute call86 |
| Physiotherapist visit | 20.67 | PSSRU Unit Costs of Health and Social Care 2017, p. 186: community physiotherapist mean cost of one-to-one contact, assuming a 30-minute appointment (taken out of 2018 edition) (inflated)86 |
| Physiotherapist telephone contact | 5.17 | PSSRU Unit Costs of Health and Social Care 2017, p. 186: assuming a 7-minute call (inflated)86 |
| Community-based social care | ||
| Social worker Macmillan visit | 42.00 | PSSRU Unit Costs of Health and Social Care 2018, p. 139: assuming 30-minute appointment86 |
| Social worker Macmillan telephone contact | 9.80 | PSSRU Unit Costs of Health and Social Care 2018, p. 139: assuming a 7-minute call86 |
| Palliative social worker visit | 42.00 | PSSRU Unit Costs of Health and Social Care 2018: assuming social worker for palliative care same as the Macmillan visit86 |
| Palliative social worker telephone contact | 9.80 | PSSRU Unit Costs of Health and Social Care 2018: assuming social worker for palliative care same as the Macmillan contact86 |
| Counsellor visit | 25.50 | PSSRU Unit Costs of Health and Social Care 2018, p. 50: assuming 30-minute appointment86 |
| Counsellor telephone contact | 5.95 | PSSRU Unit Costs of Health and Social Care 2018, p. 50: assuming 7-minute call86 |
| Citizen adviser visit | 23.50 | PSSRU Unit Costs of Health and Social Care 2018: assuming same as for occupational therapist86 |
| Citizen adviser telephone contact | 5.48 | PSSRU Unit Costs of Health and Social Care 2018: assuming same as for occupational therapist86 |
| Psychiatrist visit | 57.87 | PSSRU Unit Costs of Health and Social Care 2017, p. 62: specialist prescribing, unit costs per patient per week (taken from 2018 edition) (inflated)158 |
| Psychiatrist telephone contact | 28.40 | PSSRU Unit Costs of Health and Social Care 2018, p. 127: per minute of patient contact (7.1 minutes) including direct care staff costs86 |
| Convalescent day | 261.05 | NHS Reference Costs 2017/18: paediatric, convalescent or other relief care (Day Case, PX54Z)87 |
| Convalescent visit | 37.40 | PSSRU Unit Costs of Health and Social Care 2018, p. 127: = GP contact lasting 9.22 minutes86 |
| Nursing home day | 126.86 | PSSRU Unit Costs of Health and Social Care 2018, p. 174: not-for-profit care home fees in England, mid-point of minimum fees for 2017/18 for shared rooms per day (£888/7)86 |
| Nursing home visit | 37.40 | PSSRU Unit Costs of Health and Social Care 2018, p. 127: = GP contact lasting 9.22 minutes86 |
| Parameter | Mean | Standard error | Distribution | Source |
|---|---|---|---|---|
| 5.1 years of follow-up data | ||||
| 6-month arm | ||||
| DF to NP | 0.001693 | 0.002088 | Beta | PERSEPHONE trial |
| DF to LR | 0.001873 | 0.002324 | Beta | PERSEPHONE trial |
| DF to DR | 0.004234 | 0.004889 | Beta | PERSEPHONE trial |
| DF to BC death | 0 | 0 | Fixed | PERSEPHONE trial |
| DF to cardiac death | 0.000048 | 0.000163 | Beta | PERSEPHONE trial |
| DF to background death | 0.000674 | 0.000669 | Beta | PERSEPHONE trial |
| NP to DF | 0 | 0 | Fixed | PERSEPHONE trial |
| NP to LR | 0.003092 | 0.010954 | Beta | PERSEPHONE trial |
| NP to DR | 0.004465 | 0.013006 | Beta | PERSEPHONE trial |
| NP to BC death | 0.000275 | 0.000806 | Beta | PERSEPHONE trial |
| NP to cardiac death | 0.000004 | 0.000034 | Beta | PERSEPHONE trial |
| NP to background death | 0.026313 | 0.026328 | Beta | PERSEPHONE trial |
| LR to DF | 0 | 0 | Fixed | PERSEPHONE trial |
| LR to NP | 0.004199 | 0.015795 | Beta | PERSEPHONE trial |
| LR to DR | 0.054839 | 0.048247 | Beta | PERSEPHONE trial |
| LR to BC death | 0.009398 | 0.029416 | Beta | PERSEPHONE trial |
| LR to cardiac death | 0.002193 | 0.014763 | Beta | PERSEPHONE trial |
| LR to background death | 0.000209 | 0.000428 | Beta | PERSEPHONE trial |
| DR to DF | 0 | 0 | Fixed | PERSEPHONE trial |
| DR to NP | 0 | 0 | Fixed | PERSEPHONE trial |
| DR to LR | 0 | 0 | Fixed | PERSEPHONE trial |
| DR to BC death | 0.109534 | 0.039779 | Beta | PERSEPHONE trial |
| DR to cardiac death | 0 | 0 | Fixed | PERSEPHONE trial |
| DR to background death | 0.005066 | 0.009336 | Beta | PERSEPHONE trial |
| 12-month arm | ||||
| DF to NP | 0.001666 | 0.000845 | Beta | PERSEPHONE trial |
| DF to LR | 0.001439 | 0.000796 | Beta | PERSEPHONE trial |
| DF to DR | 0.003993 | 0.001348 | Beta | PERSEPHONE trial |
| DF to BC death | 0 | 0 | Fixed | PERSEPHONE trial |
| DF to cardiac death | 0.000142 | 0.000292 | Beta | PERSEPHONE trial |
| DF to background death | 0.000692 | 0.000508 | Beta | PERSEPHONE trial |
| NP to DF | 0 | 0 | Fixed | PERSEPHONE trial |
| NP to LR | 0.001584 | 0.011769 | Beta | PERSEPHONE trial |
| NP to DR | 0.003447 | 0.013256 | Beta | PERSEPHONE trial |
| NP to BC death | 0.008326 | 0.015342 | Beta | PERSEPHONE trial |
| NP to cardiac death | 0.000004 | 0.000029 | Beta | PERSEPHONE trial |
| NP to background death | 0.012970 | 0.019627 | Beta | PERSEPHONE trial |
| LR to DF | 0 | 0 | Fixed | PERSEPHONE trial |
| LR to NP | 0 | 0 | Fixed | PERSEPHONE trial |
| LR to DR | 0.045659 | 0.041194 | Beta | PERSEPHONE trial |
| LR to BC death | 0.013969 | 0.023254 | Beta | PERSEPHONE trial |
| LR to cardiac death | 0.000056 | 0.000222 | Beta | PERSEPHONE trial |
| LR to background death | 0.000088 | 0.000269 | Beta | PERSEPHONE trial |
| DR to DF | 0 | 0 | Fixed | PERSEPHONE trial |
| DR to NP | 0 | 0 | Fixed | PERSEPHONE trial |
| DR to LR | 0 | 0 | Fixed | PERSEPHONE trial |
| DR to BC death | 0.099780 | 0.035325 | Beta | PERSEPHONE trial |
| DR to cardiac death | 0 | 0 | Fixed | PERSEPHONE trial |
| DR to background death | 0.003547 | 0.009777 | Beta | PERSEPHONE trial |
| Mortality | ||||
| Cardiac mortality (6-month arm) | 0.0015 | 0.0014 | Beta | PERSEPHONE trial |
| Cardiac mortality (12-month arm) | 0.0040 | 0.0009 | Beta | PERSEPHONE trial |
| Beyond 5.1 years of follow-up data | ||||
| New/second primary cancer | ||||
| Increased risk of NP after BC treatment | 1.57 | 1.07408 | Log-normal | Marcheselli et al.159 |
| Probability of NP | Age-specific | Fixed | UK population rates for females160 | |
| Cardiac toxicity | ||||
| Background levels of CHF | Age-specific | Fixed | Registry data | |
| Mortality | ||||
| Background all-cause mortality in UK females | Age-specific | Fixed | Registry data | |
| Breast cancer-specific mortality in UK females | Age-specific | Fixed | Registry data | |
| DFS | AIC | BIC |
|---|---|---|
| 12 months | ||
| Exponential | 2226.070 | 2231.675 |
| Weibull | 2228.057 | 2239.266 |
| Gompertz | 2226.749 | 2237.958 |
| Log-normal | 2219.691 | 2230.899 |
| Log-logistic | 2226.469 | 2237.678 |
| 6 months | ||
| Exponential | 2260.195 | 2265.796 |
| Weibull | 2261.519 | 2272.722 |
| Gompertz | 2256.466 | 2267.668 |
| Log-normal | 2250.34 | 2261.543 |
| Log-logistic | 2259.327 | 2270.53 |
| Parameter | Mean | Standard error | Distribution | Source |
|---|---|---|---|---|
| Disease free | 0.805 | 0.021 | Beta | Seferina et al.98 |
| Local recurrence | 0.708 | 0.088 | Beta | Seferina et al.98 |
| New/second primary cancer | 0.696 | 0.032 | Beta | Lidgren et al.83 |
| Distant recurrence | 0.604 | 0.046 | Beta | Seferina et al.98 |
| Parameter | Mean cost (£) | Standard error | Distribution | Source |
|---|---|---|---|---|
| Disease-free annual cost | ||||
| Mammogram | 60.29 | 12.06 | Log-normal | Robertson et al.101 (inflated) |
| Breast cancer follow-up clinic | 165.85 | 33.17 | Log-normal | NHS Reference Costs87 |
| Heart failure costs | ||||
| Cost per heart failure event | 1979.71 | 395.94 | Log-normal | NHS Reference Costs87 |
| Cancer recurrence costs | ||||
| Local recurrence first year | 6896.48 | 1379.30 | Log-normal | OPTIMA Prelim HTA model (2013) |
| Local recurrence annual cost | 575.27 | 115.05 | Log-normal | OPTIMA Prelim HTA model (2013) |
| Second primary cancer first year | 26,744.39 | 5348.88 | Log-normal | Hall et al.91 |
| Second primary cancer annual cost | 2659.37 | 531.87 | Log-normal | Hall et al.91 |
| Distant recurrence annual cost | 1893.55 | 378.71 | Log-normal | OPTIMA Prelim HTA model (2013) |
| Terminal care cost | 4687.58 | 937.52 | Log-normal | NICE TA563,104 NICE TA496,161 NICE CG81 Package 3105 (2015/16) (inflated) |
| Subgroup | Treatment duration | Total cost, £ (95% CI) | Total QALY (95% CI) | Incremental cost, £ (95% CI) | Incremental QALY (95% CI) | ICER (£) | NB (95% CI) | Incremental NB (95% CI) |
|---|---|---|---|---|---|---|---|---|
| ER status negative | 12 months (n = 615) | 14,806 (14,291 to 15,312) | 1.14 (1.11 to 1.16) | – | – | – | 0.40 (0.36 to 0.43) | – |
| 6 months (n = 618) | 5632 (5224 to 6052) | 1.13 (1.11 to 1.16) | –9178 (–9826 to –8517) | –0.003 (–0.038 – 0.032) | 2,928,179 | 0.85 (0.81 to 0.89) | 0.46 (0.40 to 0.51) | |
| ER status positive | 12 months (n = 1392) | 15,515 (15,184 to 15,851) | 1.15 (1.13 to 1.16) | – | – | – | 0.37 (0.35 to 0.40) | |
| 6 months (n = 1383) | 5820 (5575 to 6080) | 1.15 (1.14 to 1.17) | –9693 (–10,115 to –9270) | 0.005 (–0.016 – 0.027) | Dominant | 0.86 (0.84 to 0.88) | 0.49 (0.46 to 0.52) | |
| Anthracycline-based chemotherapy | 12 months (n = 837) | 14,285 (13,876 to 14,703) | 1.16 (1.14 to 1.18) | – | – | – | 0.45 (0.42 to 0.47) | – |
| 6 months (n = 829) | 5106 (4801 to 5419) | 1.17 (1.14 to 1.19) | –9182 (–9699 to –8651) | 0.006 (–0.023 – 0.035) | Dominant | 0.91 (0.88 to 0.94) | 0.47 (0.42 to 0.51) | |
| Concurrent trastuzumab | 12 months (n = 933) | 16,119 (15,718 to 16,531) | 1.15 (1.13 to 1.17) | – | – | – | 0.34 (0.31 to 0.37) | – |
| 6 months (n = 928) | 6298 (5969 to 6631) | 1.14 (1.12 to 1.16) | –9823 (–10,339 to –9295) | –0.007 (–0.034 – 0.019) | 1,401,899 | 0.83 (0.80 to 0.85) | 0.48 (0.44 to 0.52) | |
| Sequential trastuzumab | 12 months (n = 1074) | 14,584 (14,209 to 14,952) | 1.14 (1.12 to 1.16) | – | – | – | 0.41 (0.39 to 0.44) | – |
| 6 months (n = 1073) | 5301 (5029 to 5585) | 1.15 (1.14 to 1.17) | –9278 (–9749 to –8805) | 0.011 (–0.014 – 0.037) | Dominant | 0.89 (0.86 to 0.91) | 0.48 (0.44 to 0.51) | |
| Adjuvant chemotherapy | 12 months (n = 1708) | 15,050 (14,759 to 15,342) | 1.15 (1.14 to 1.17) | – | – | – | 0.40 (0.38 to 0.42) | – |
| 6 months (n = 1693) | 5494 (5276 to 5724) | 1.15 (1.14 to 1.17) | –9555 (–9924 – –9184) | 0.000 (–0.020 – 0.020) | Dominant | 0.88 (0.86 to 0.90) | 0.48 (0.45 to 0.51) | |
| Neoadjuvant chemotherapy | 12 months (n = 299) | 16,701 (15,941 to 17,491) | 1.10 (1.07 to 1.14) | – | – | – | 0.27 (0.21 to 0.32) | – |
| 6 months (n = 308) | 7220 (6589 to 7880) | 1.12 (1.09 to 1.16) | –9482 (–10,503 to –8480) | 0.018 (–0.032 – 0.068) | Dominant | 0.76 (0.71 to 0.81) | 0.49 (0.41 to 0.57) | |
| Taxane-based chemotherapy | 12 months (n = 193) | 16,141 (15,287 to 17,055) | 1.13 (1.09 to 1.18) | – | – | – | 0.33 (0.25 to 0.39) | – |
| 6 months (n = 196) | 5446 (4883 to 6070) | 1.14 (1.10 to 1.18) | –10,689 (–11,77 to –9647) | 0.006 (–0.053 – 0.066) | Dominant | 0.87 (0.81 to 0.92) | 0.54 (0.45 to 0.63) | |
| Anthracycline and taxane-based chemotherapy | 12 months (n = 975) | 16,015 (15,625 to 16,406) | 1.13 (1.10 to 1.16)) | – | – | – | 0.33 (0.29 to 0.37) | – |
| 6 months (n = 973) | 6361 (6035 to 6702) | 1.13 (1.10 to 1.17) | –9651 (–10,167 to –9141) | 0.000 (–0.042 – 0.041) | Dominant | 0.82 (0.76 to 0.85) | 0.48 (0.43 to 0.53) |
| Date of authorisation | Product name | Marketing authorisation holder |
|---|---|---|
| 15 November 2017 | Ontruzant | Samsung Bioepis NL B.V. |
| 8 February 2018 | Herzuma | Celltrion Healthcare Hungary Kft. (Budapest, Hungary) |
| 16 May 2018 | Kanjinti | Amgen Europe B.V. (Breda, the Netherlands) |
| 26 July 2018 | Trazimera | Pfizer Europe MA EEIG (Brussels, Belgium) |
| 12 December 2018 | Ogivri | Mylan S.A.S. (Saint-Priest, France) |
| Date of authorisation | Product name | Marketing authorisation holder |
|---|---|---|
| February 2018 | Ogiviri | Mylan S.A.S. |
| 14 December 2018 | Herzuma | Celltrion Inc. |
| January 2019 | Ontruzant | Samsung Bioepis NL B.V. |
| March 2019 | Trazimera | Pfizer Europe MA EEIG |
Appendix 2 Additional figures
FIGURE 24.
Patients (%) reporting at least one adverse event of severe grade (CTCAE ≥ 3, or 2 for palpitations) (patients receiving trastuzumab sequentially after chemotherapy).
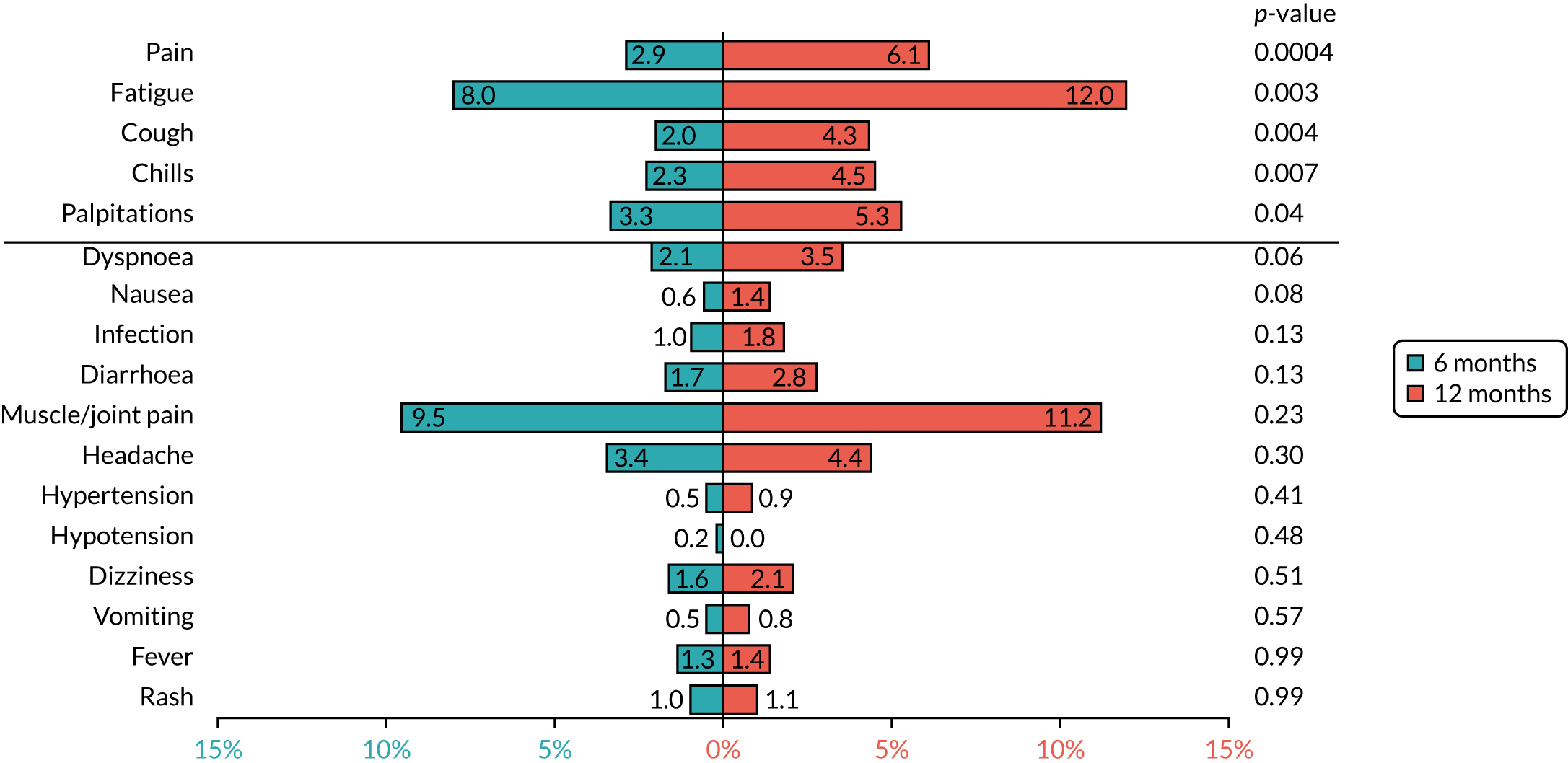
FIGURE 25.
Invasive DFS [including invasive contralateral breast cancers and second primary invasive cancers (non-breast)].
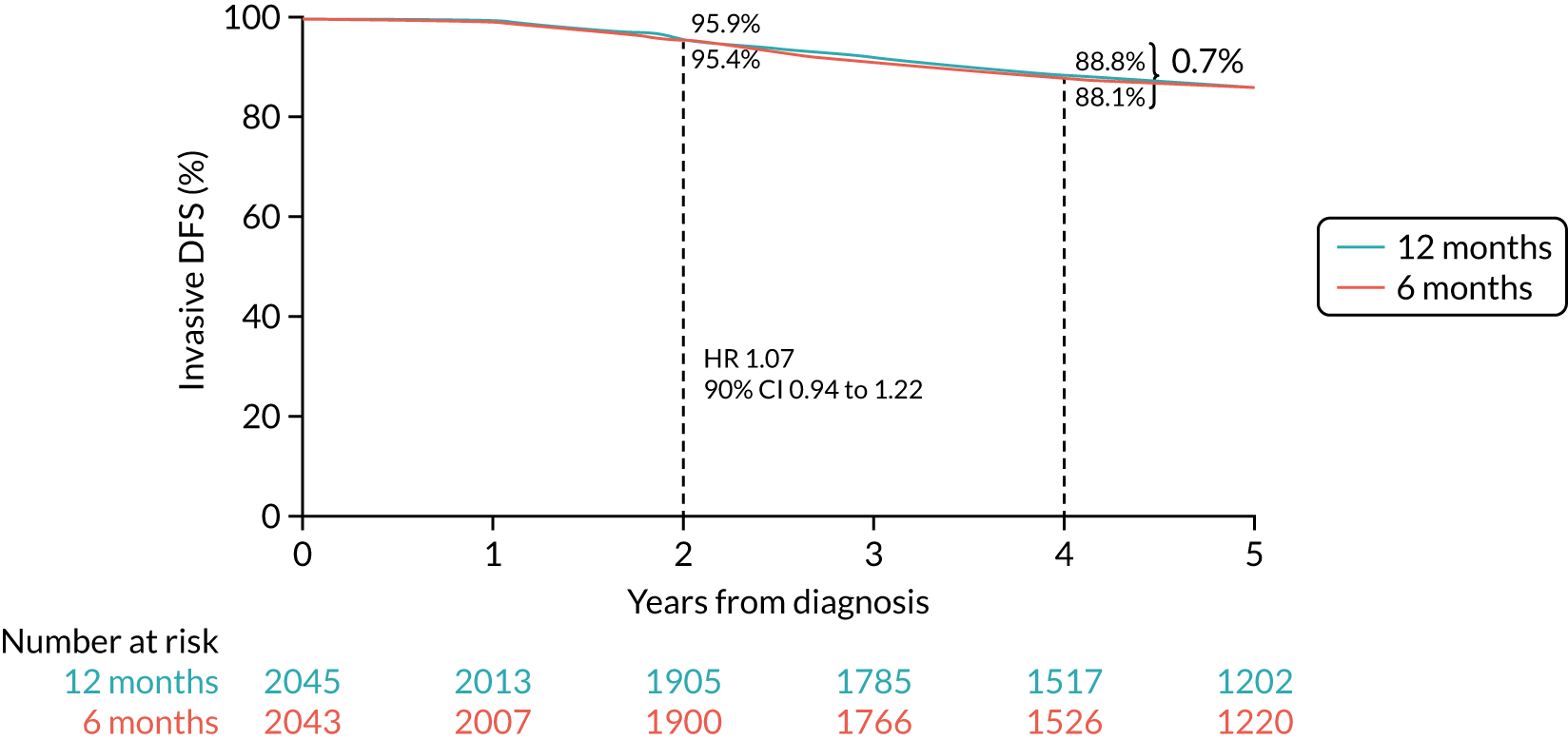
FIGURE 26.
Distant DFS.
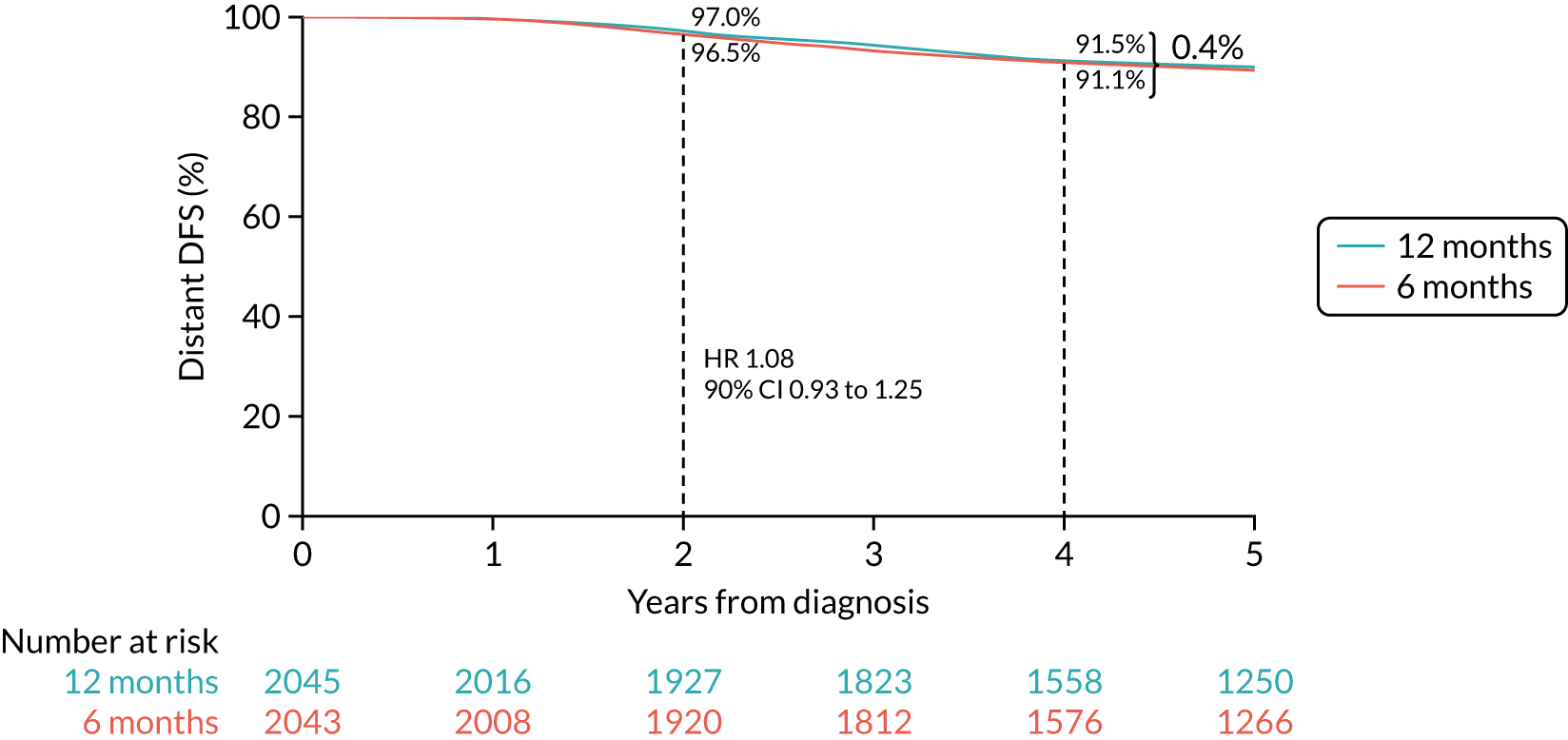
FIGURE 27.
Breast cancer-specific survival.
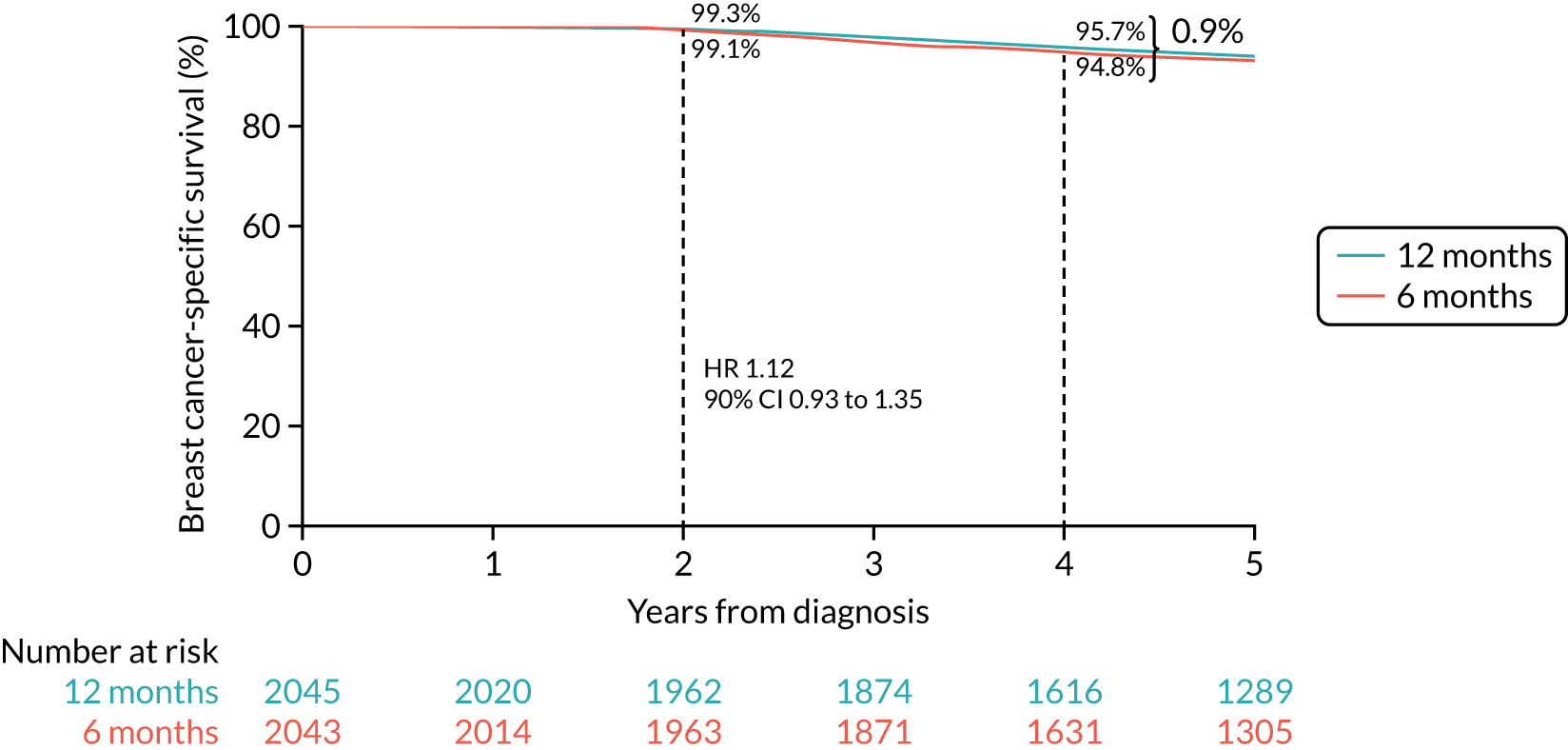
FIGURE 28.
Forest plot of OS for all patients. CT, chemotherapy; O–E, observed–expected.
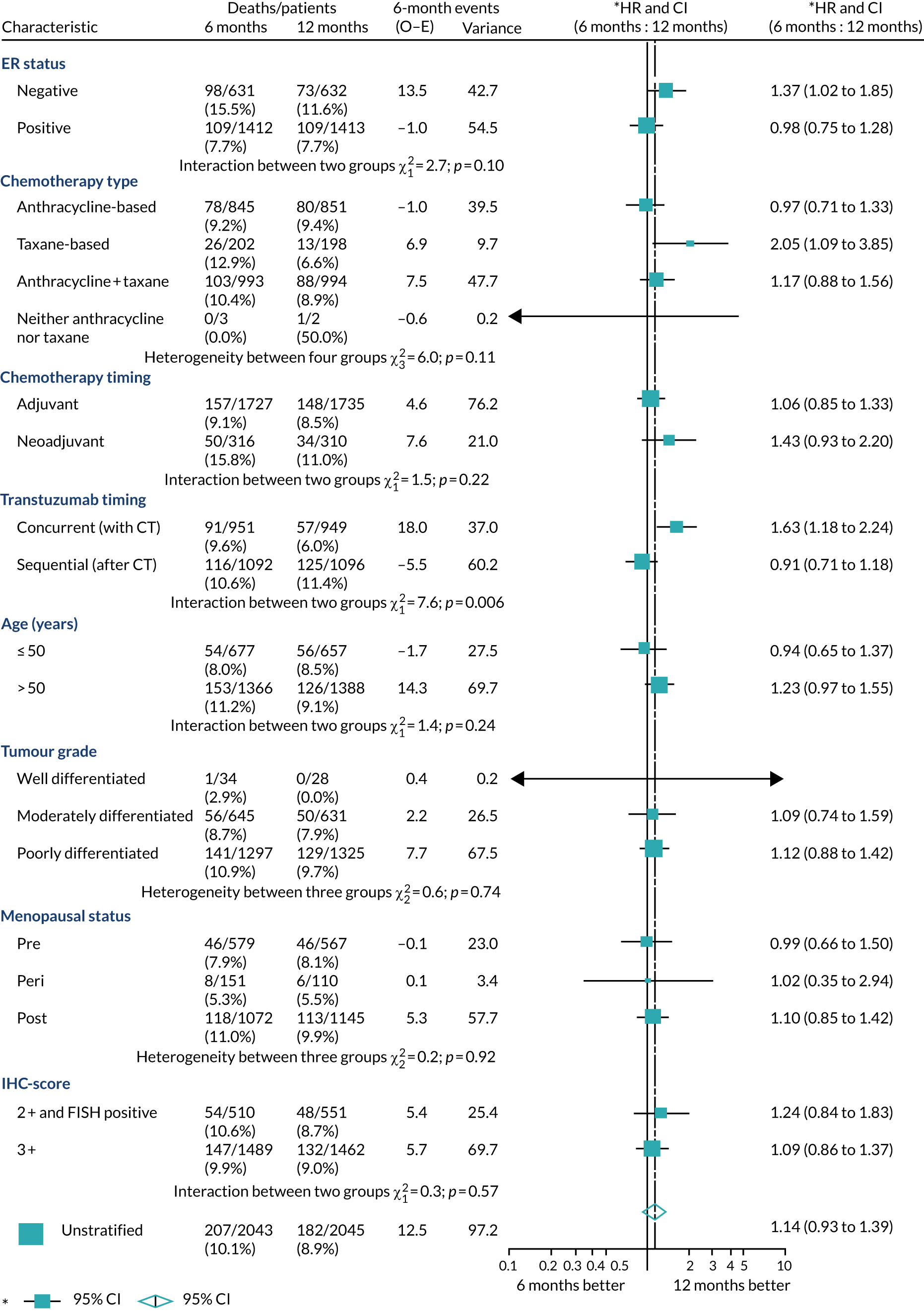
FIGURE 29.
Forest plot of landmark DFS for all patients. CT, chemotherapy; O–E, observed–expected.
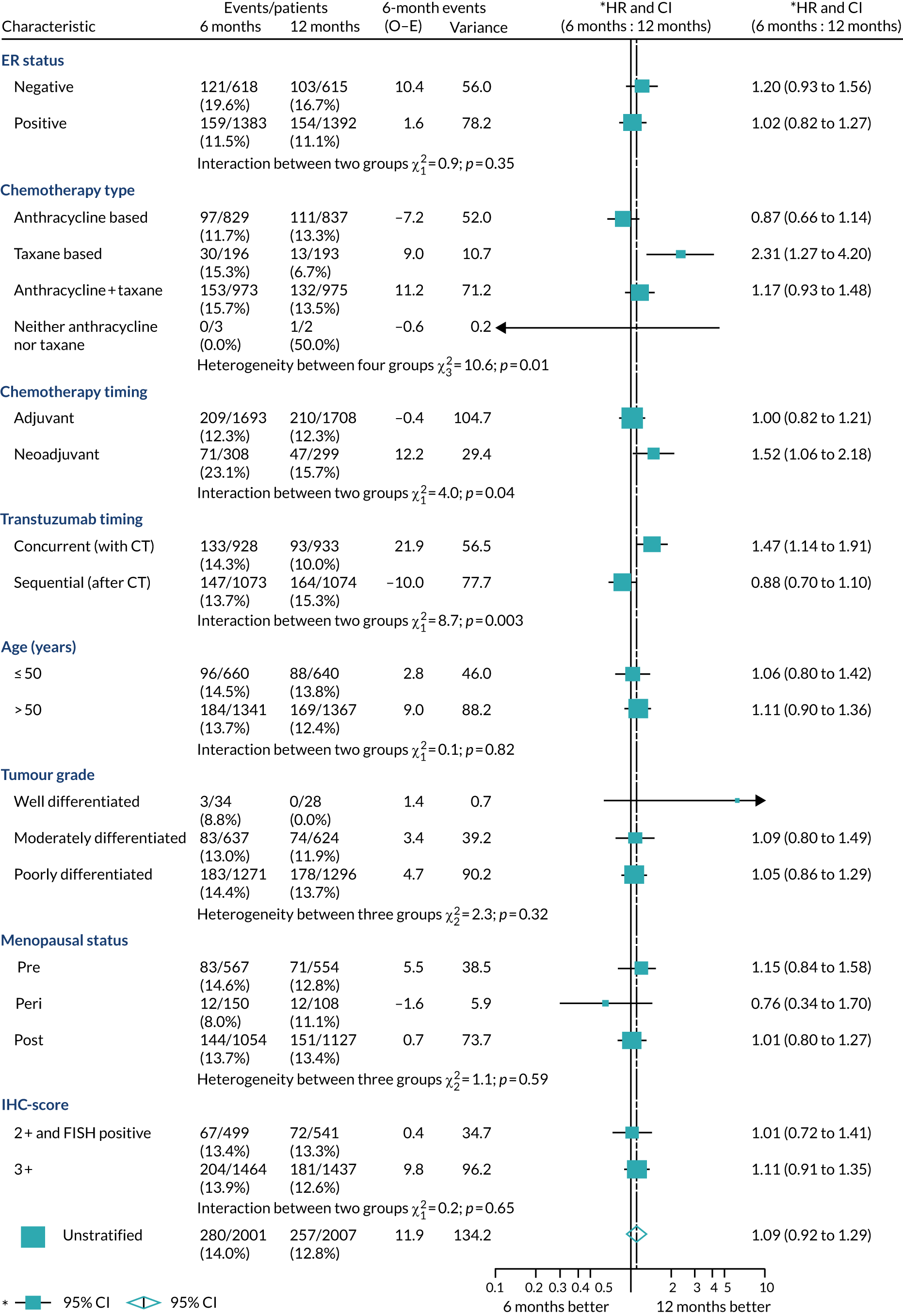
FIGURE 30.
Forest plot of landmark OS for all patients. CT, chemotherapy; O–E, observed–expected.
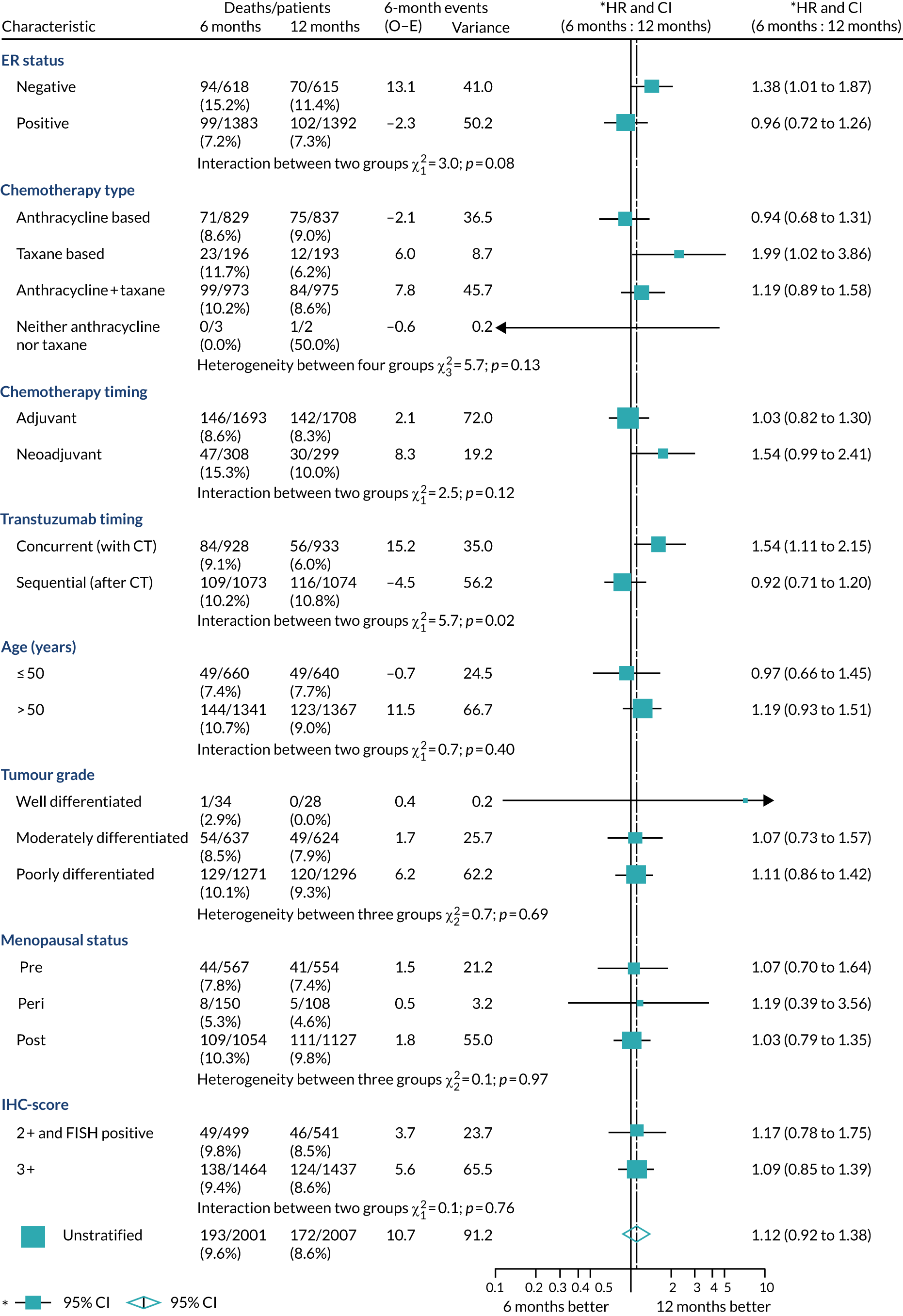
FIGURE 31.
Forest plot of OS for adjuvant patients only. CT, chemotherapy; O–E, observed–expected.
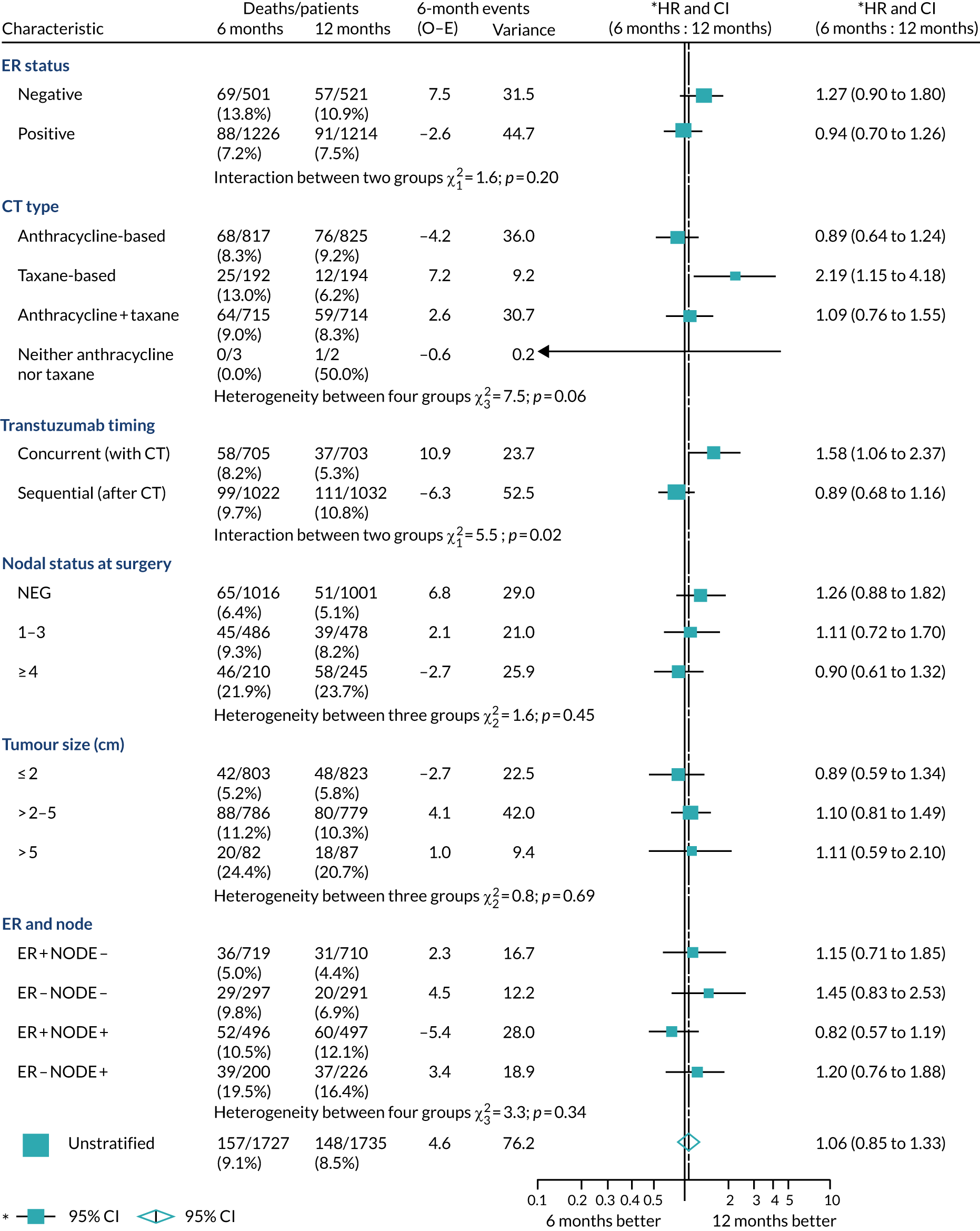
FIGURE 32.
Forest plot of OS for neoadjuvant patients only. CT, chemotherapy; O–E, observed–expected; pCR, pathological complete response.
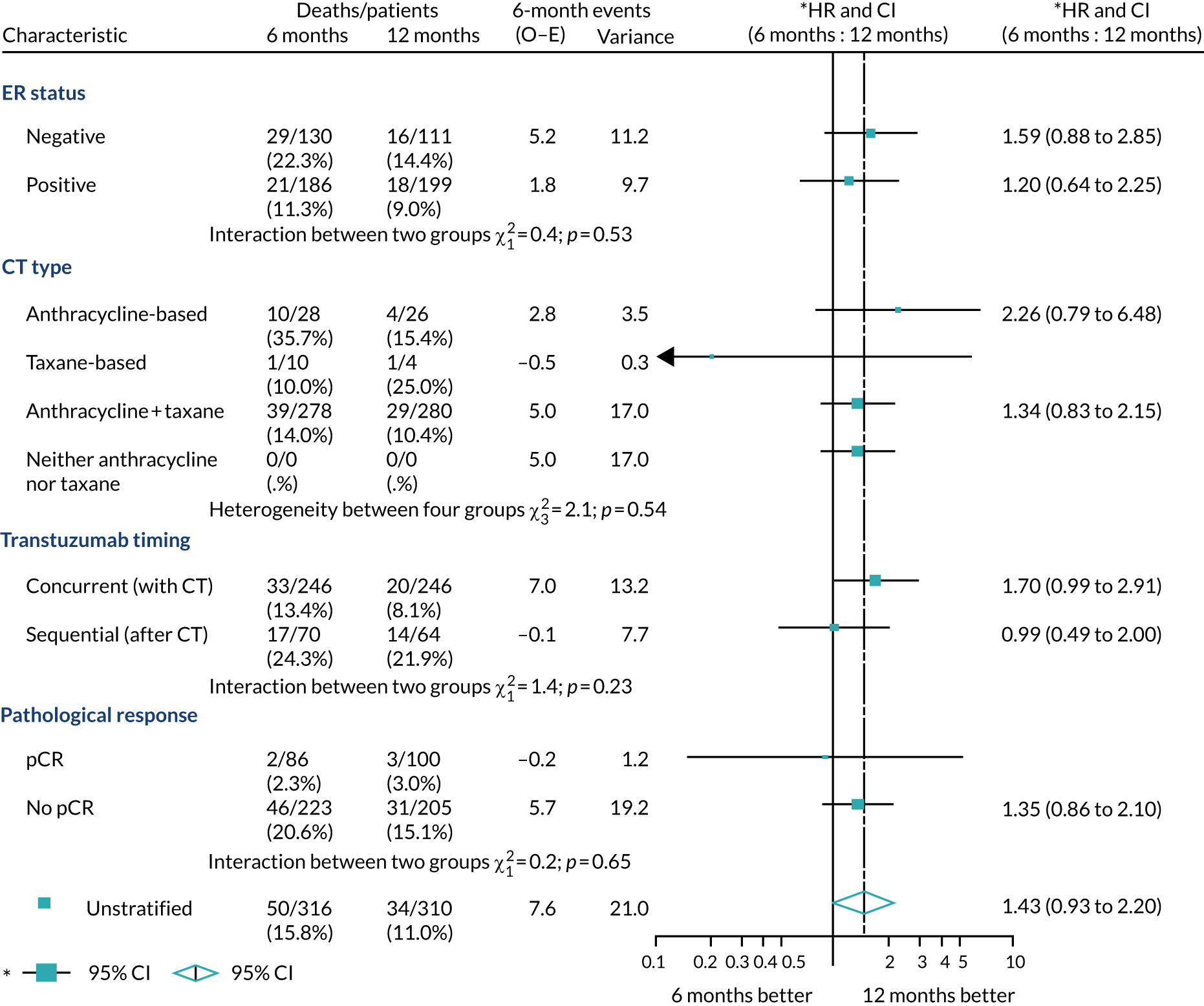
FIGURE 33.
Random-effects modelling predicted lines and 95% CIs, split by trastuzumab timing.
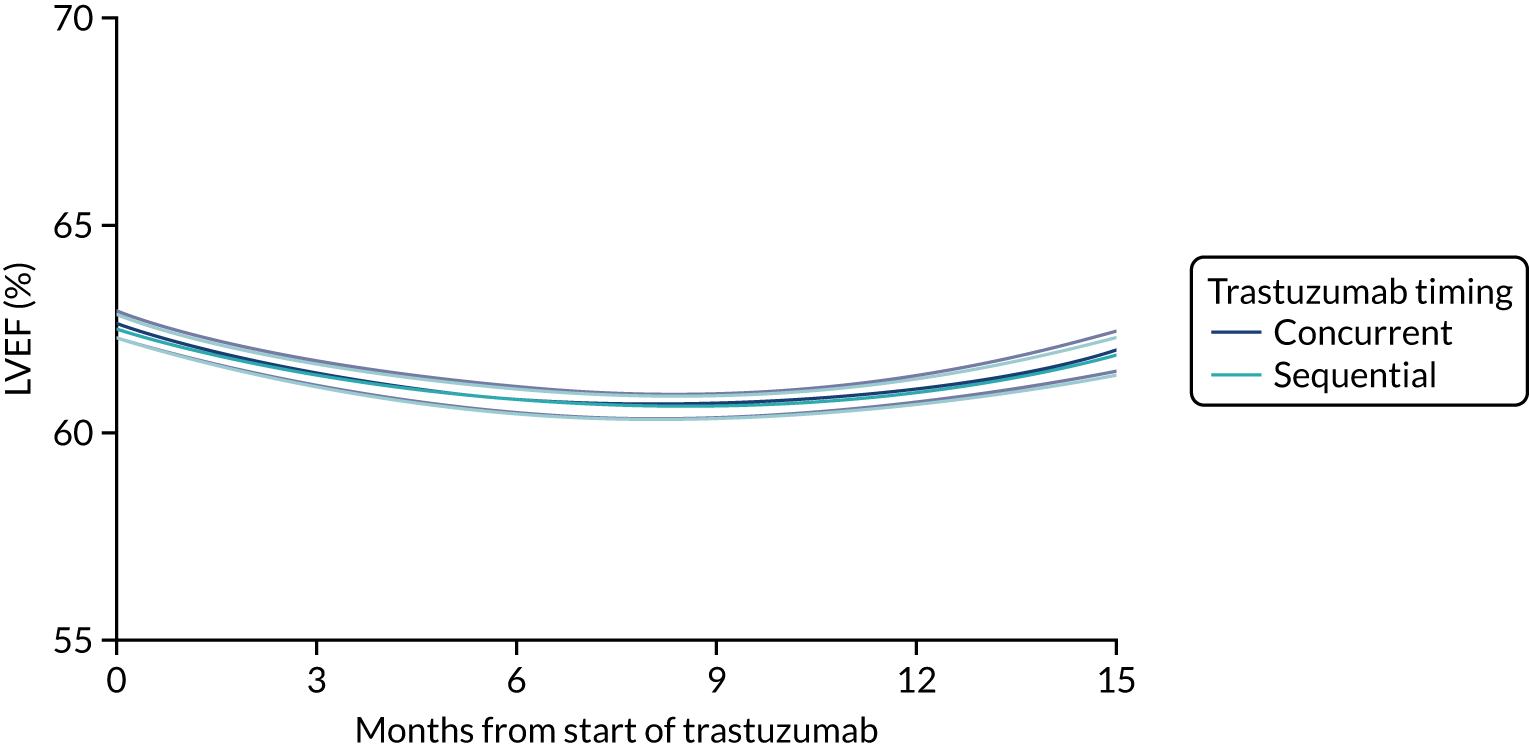
No significant difference was found between trastuzumab timing in terms of LVEF change over time (p = 0.77).
FIGURE 34.
Random-effects modelling predicted lines and 95% CIs, split by chemotherapy type.
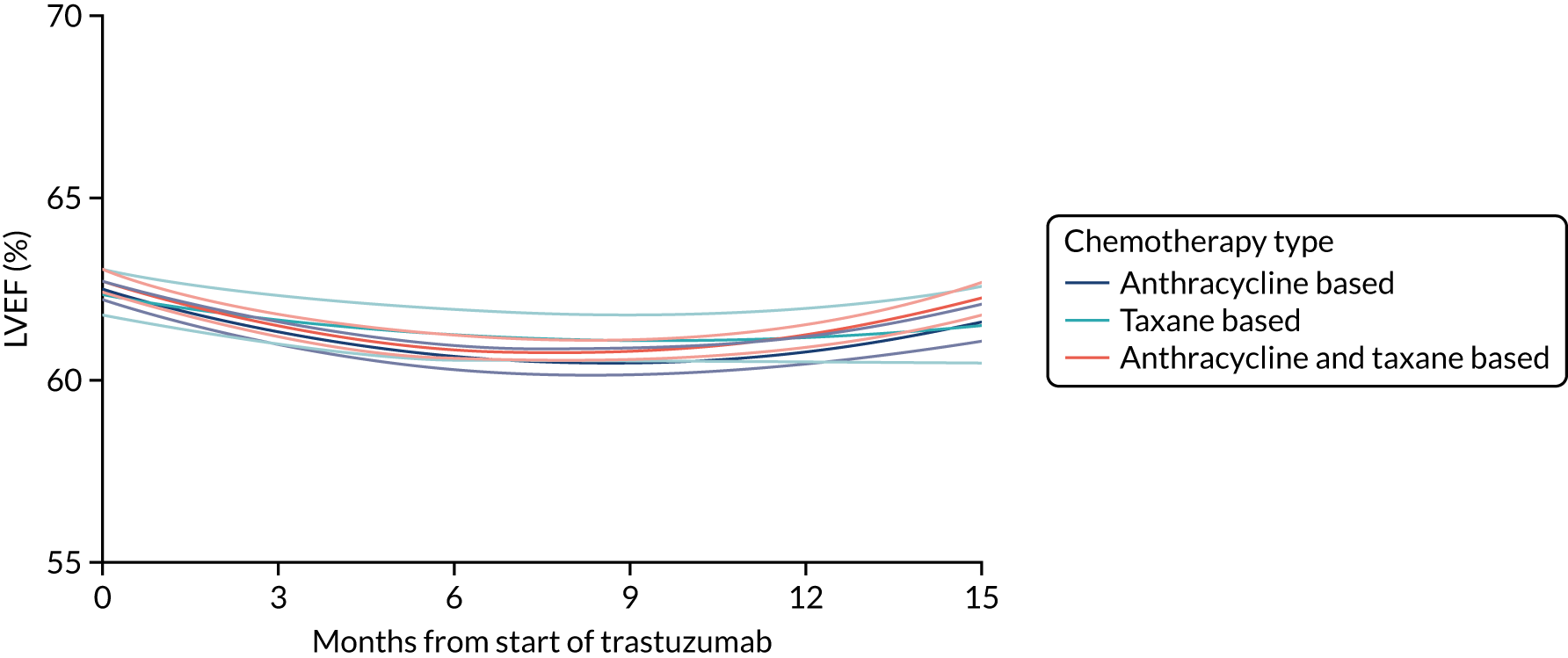
A significant difference was found between chemotherapy types in terms of LVEF change over time (p = 0.04).
FIGURE 35.
Random-effects modelling predicted lines and 95% CIs, split by number of anthracycline cycles.
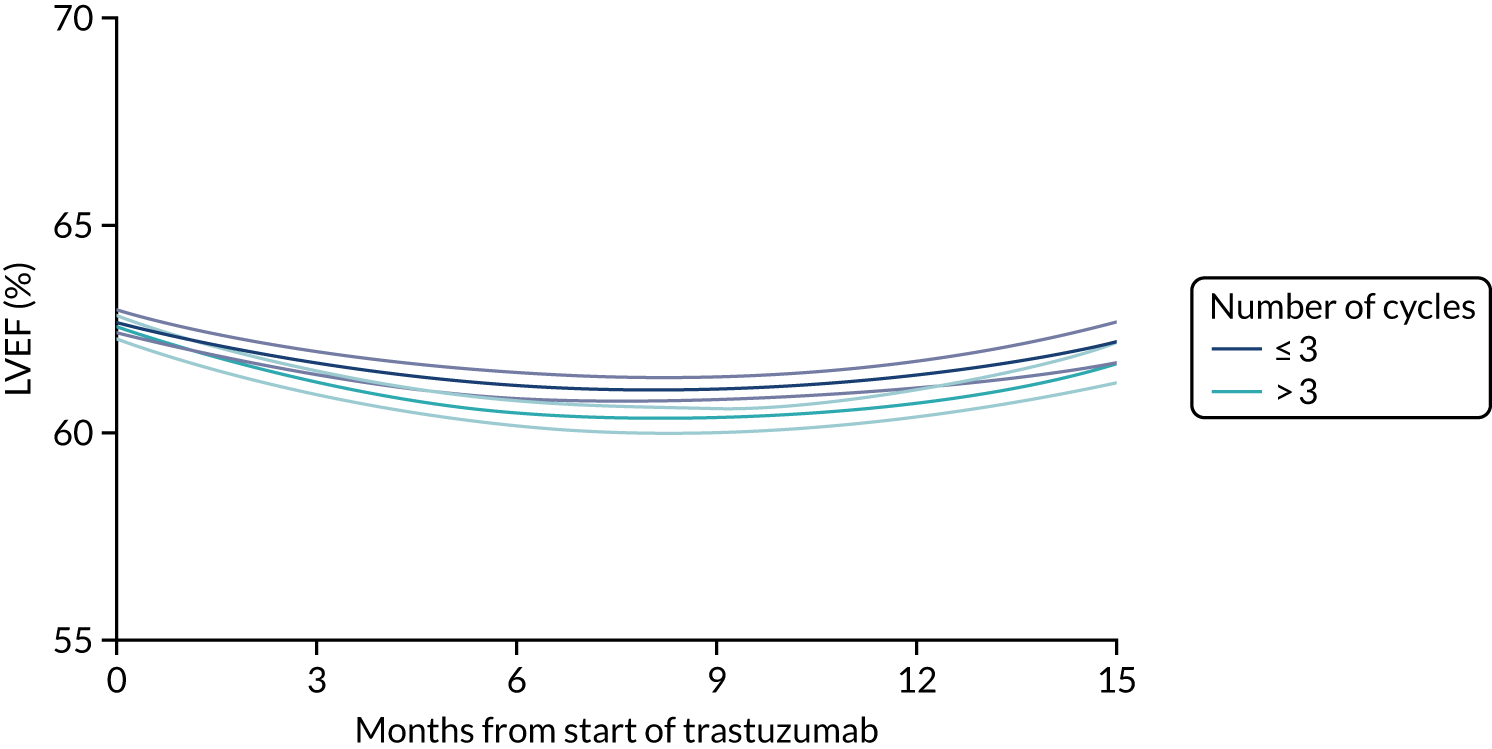
A significant difference was found between number of anthracycline cycles in terms of LVEF change over time (p = 0.04).
FIGURE 36.
Disease-free survival model fits: (a) 6 months exponential; (b) 6 months Gompertz; (c) 6 months log-normal; (d) 6 months log-logistic; (e) 6 months Weibull; (f) 12 months exponential; (g) 12 months Gompertz; (h) 12 months log-normal; (i) 12 months log-logistic; (j) 12 months Weibull.
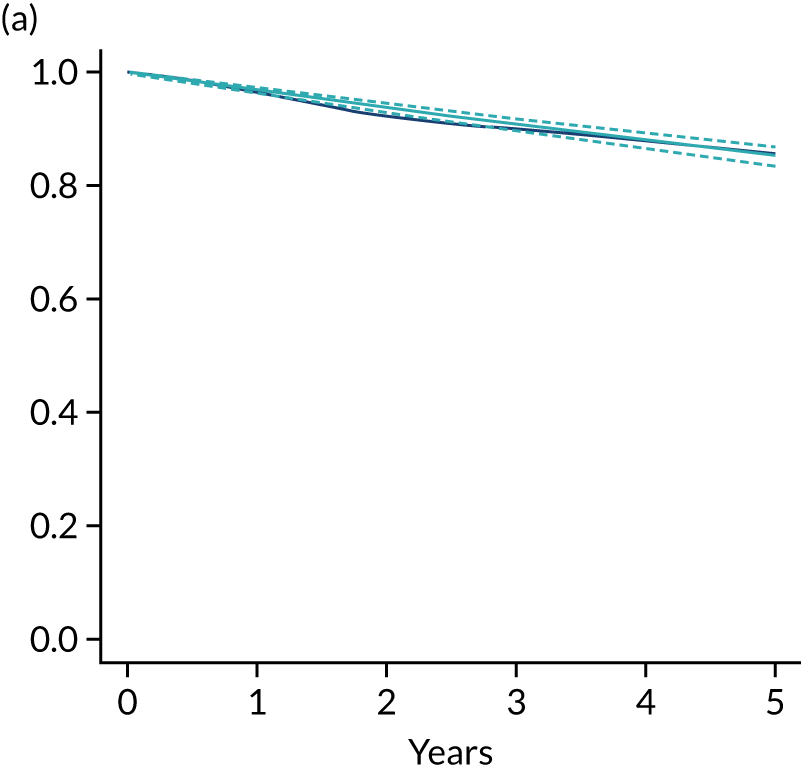
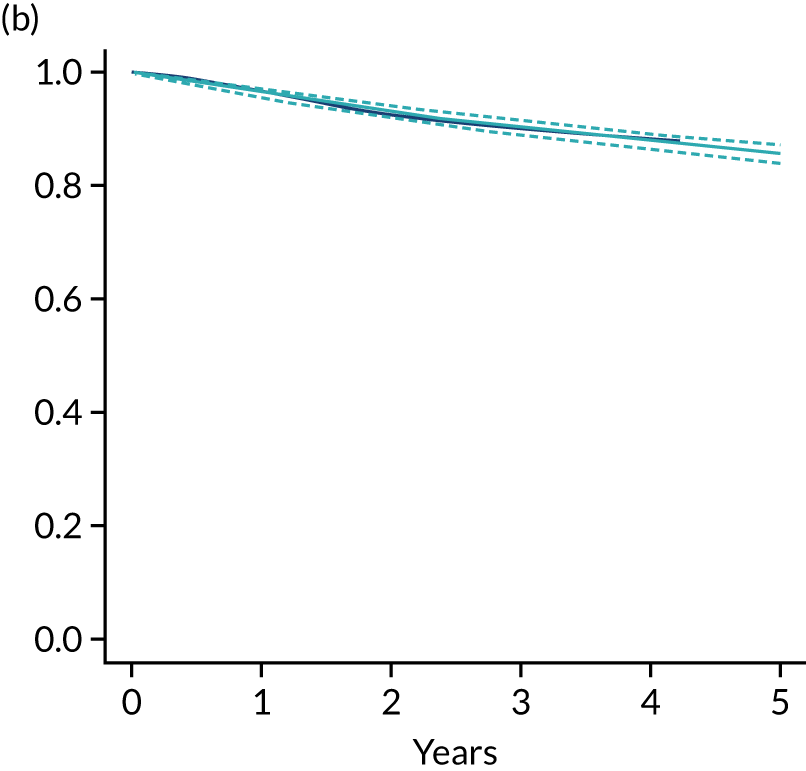
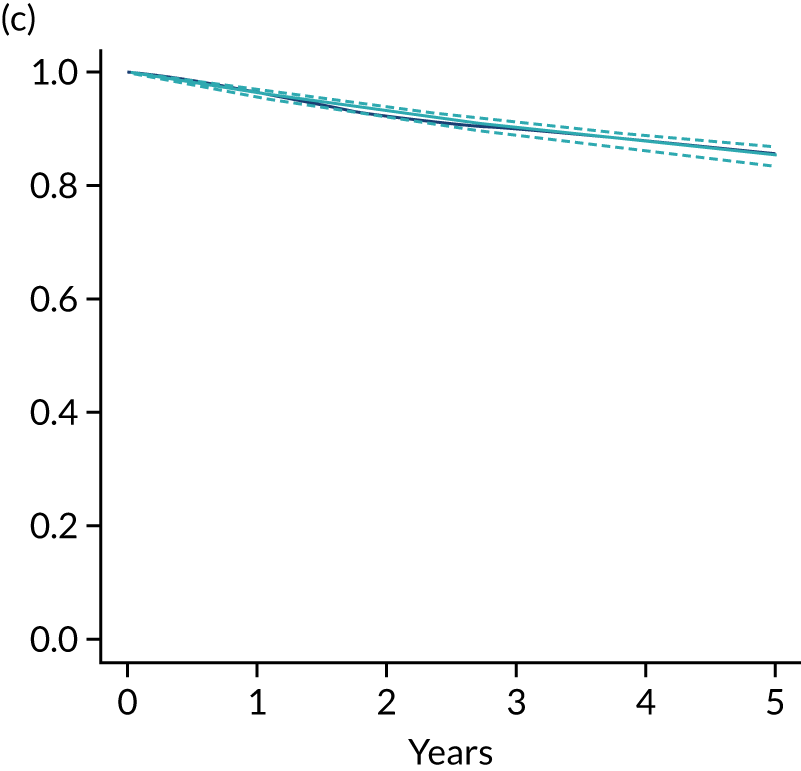
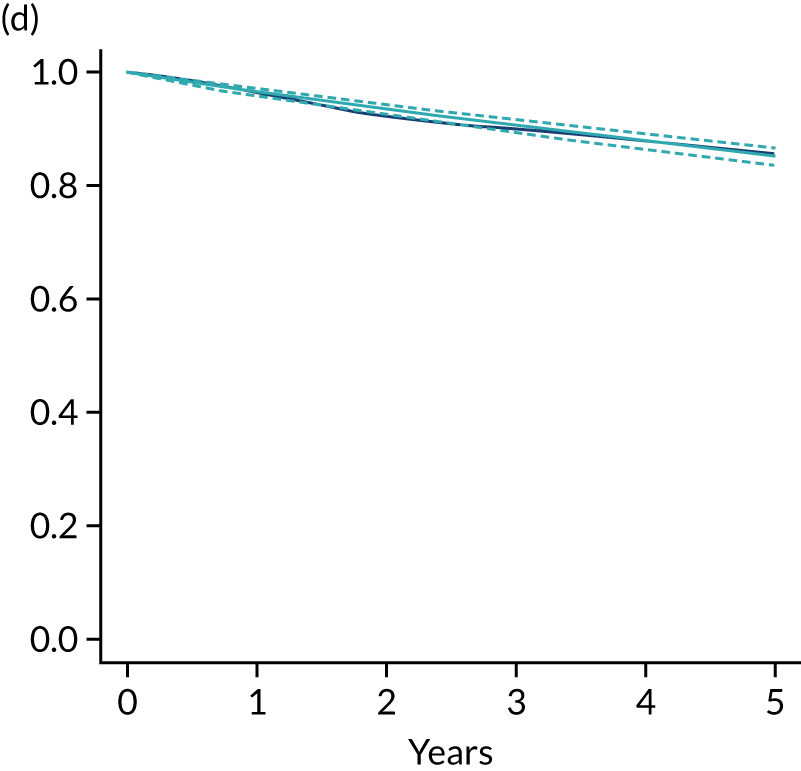
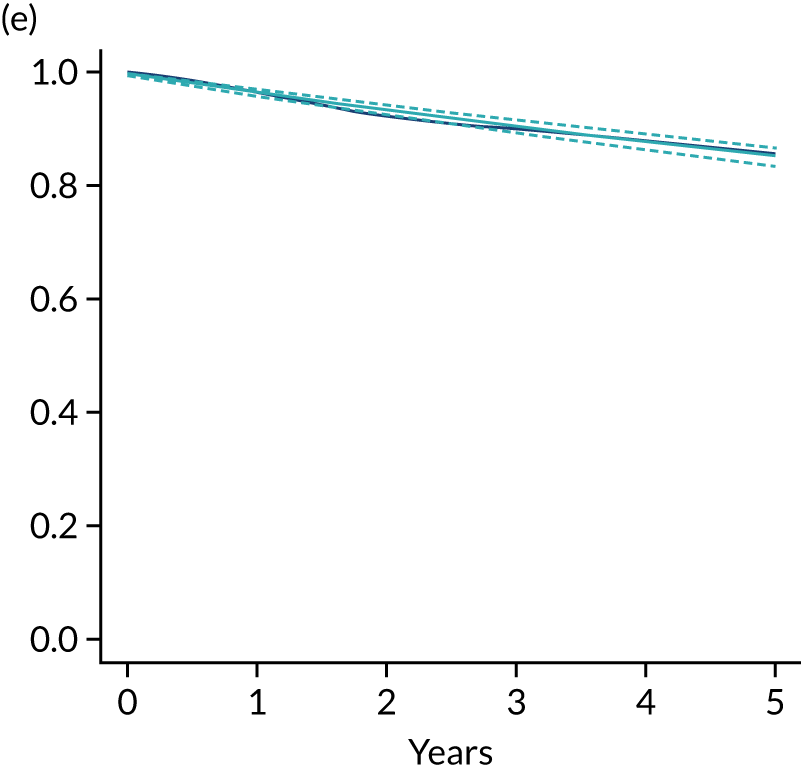
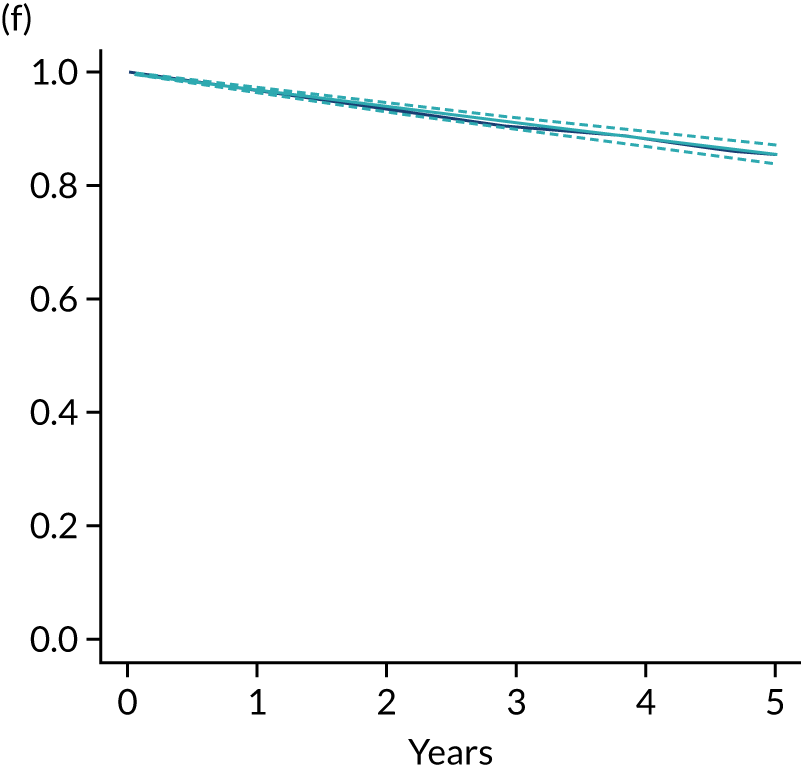
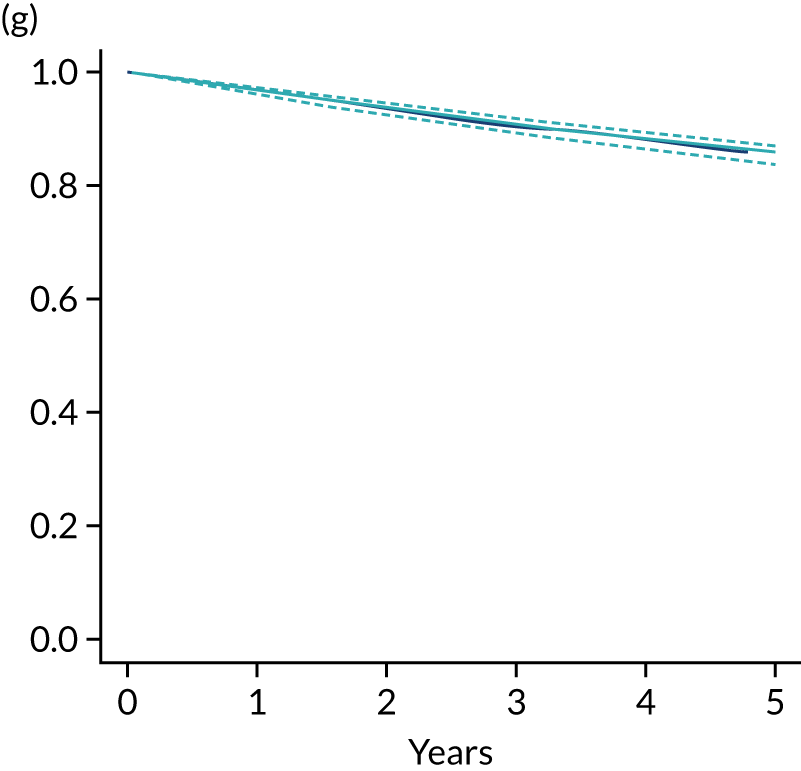
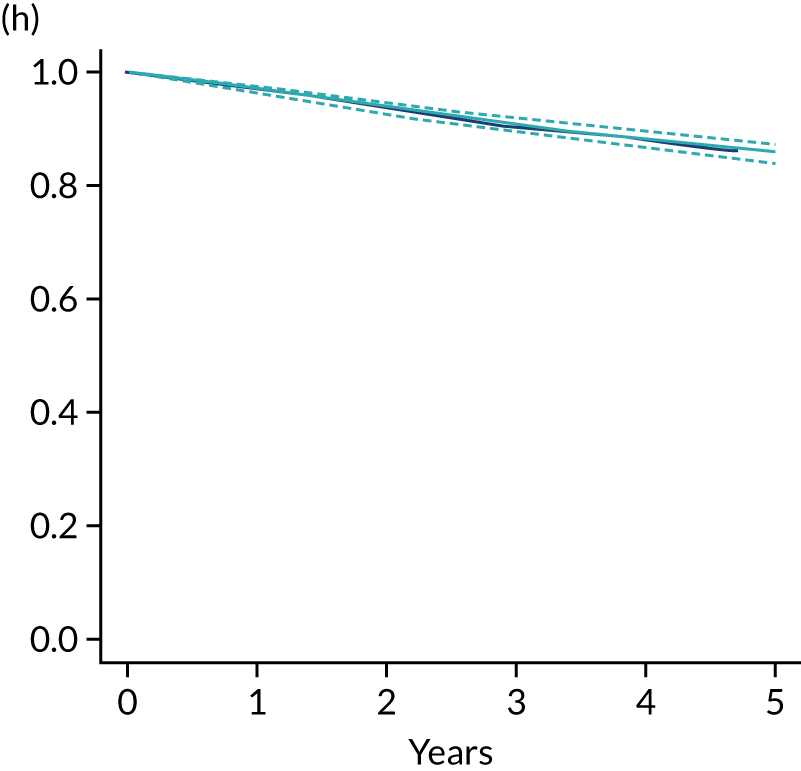
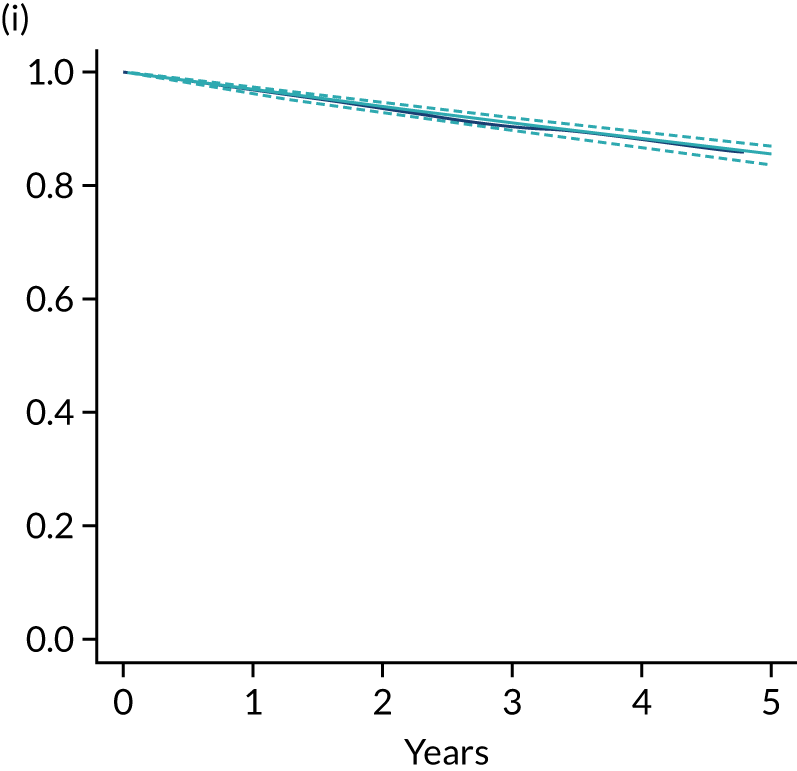
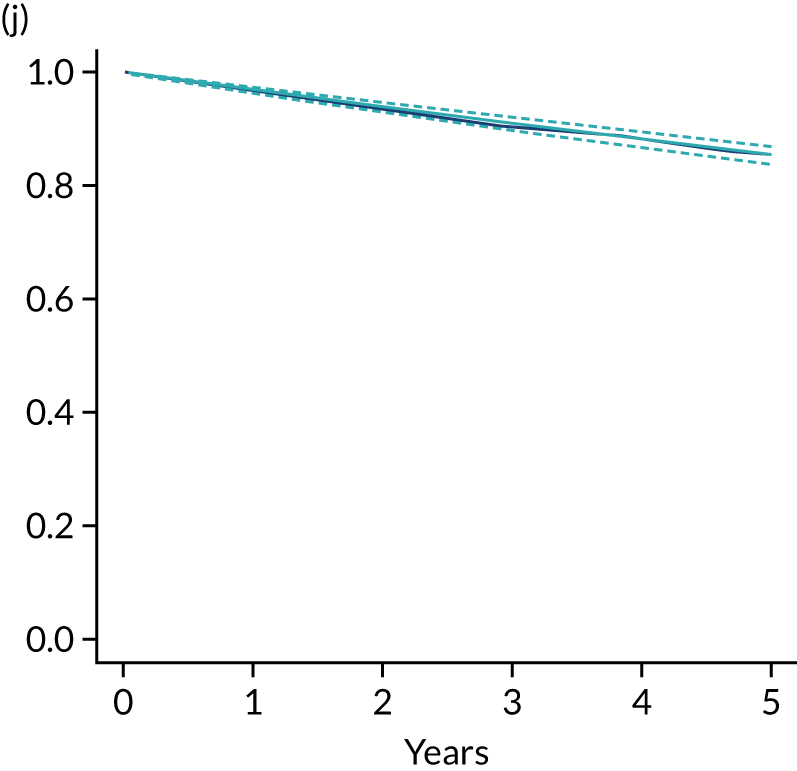
FIGURE 37.
Tornado plot: one-way sensitivity analyses. DF, disease free; DR, distant recurrence; LR, local recurrence; NP, new primary.
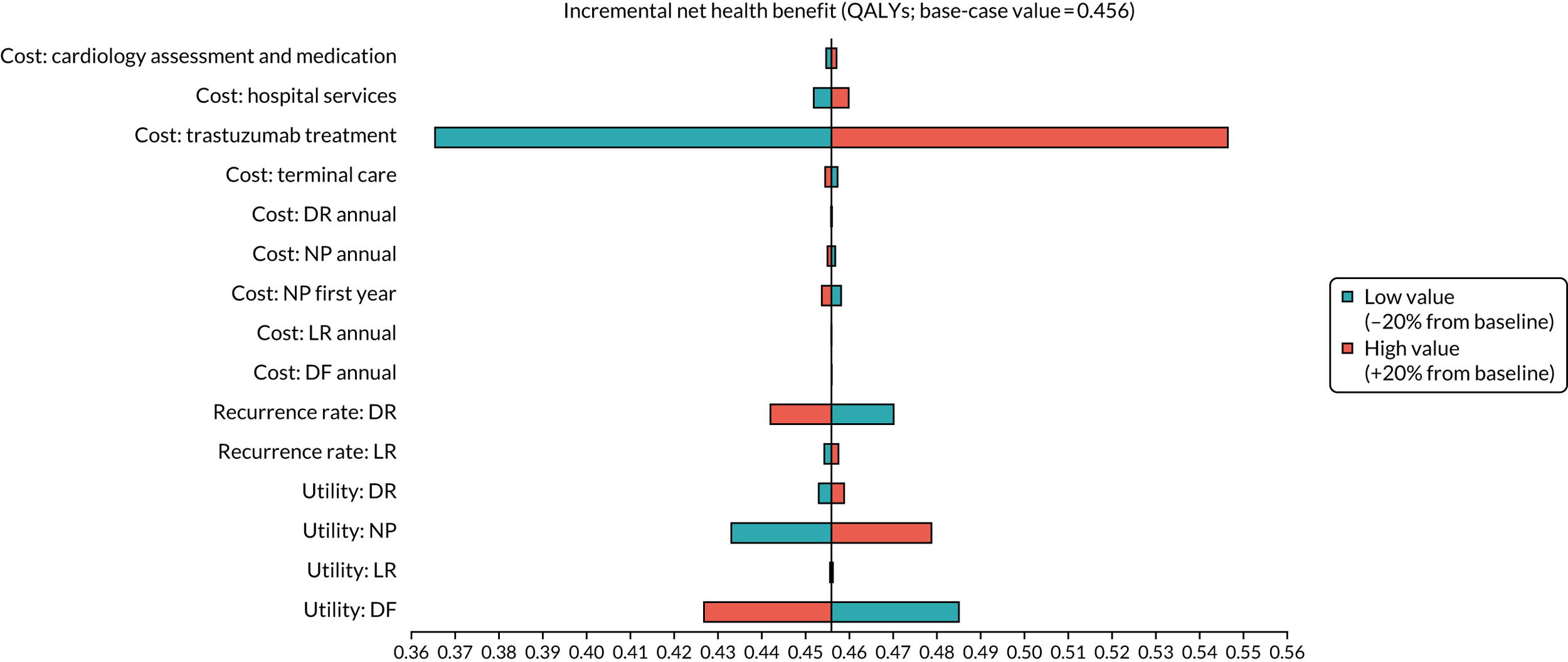
Glossary
- APHINITY
- Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer: phase 3 randomised clinical trial.
- CLEOPATRA
- Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer: phase 3 randomised clinical trial.
- General Data Protection Regulation
- A regulation in EU law (2016/679) on data protection and privacy for all individuals within the European Union and the European Economic Area.
- FNCLCC-PACS 04
- Fédération Nationale des Centres de Lutte Contre le Cancer. Programme Adjuvant Cancer Sein. A randomised controlled trial of trastuzumab in women with axillary lymph node positive, HER2 positive early breast cancer.
- HERA
- A randomised controlled trial of trastuzumab after chemotherapy in HER2-positive early breast cancer. A trial from the Breast International Group.
- HORG
- A Hellenic Oncology Research Group randomised, controlled trial of trastuzumab duration of 6 months versus 12 months in HER2 positive early breast cancer.
- IDEA
- International Duration Evaluation of Adjuvant Therapy collaboration, a meta-analysis of randomised controlled clinical trials of adjuvant chemotherapy duration for colorectal cancer.
- NeoSphere
- Neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial.
- PHARE
- Protocol for Herceptin as Adjuvant therapy with Reduced Exposure. A randomised controlled Phase III trial of 6 versus 12 months trastuzumab in HER2-positive early breast cancer.
- Short-HER
- A randomised controlled trial of 9 weeks’ versus 12 months’ trastuzumab.
- SOFT
- Suppression of Ovarian Function With Either Tamoxifen or Exemestane Compared With Tamoxifen Alone in Treating Premenopausal Women With Hormone-Responsive Breast Cancer Trial. A randomised controlled Phase III International trial.
- SOLD
- Synergism or Long Duration. A randomised Phase III controlled clinical trial of 9 weeks’ versus 12 months’ adjuvant trastuzumab in HER2 positive early breast cancer.
- TEXT
- Triptorelin With Either Exemestane or Tamoxifen in Treating Premenopausal Women With Hormone-Responsive Breast Cancer. A randomised Phase III controlled trial.
List of abbreviations
- AC
- doxorubicin and cyclophosphamide
- AC-paclitaxel
- doxorubicin, cyclophosphamide and paclitaxel
- AC-T
- doxorubicin, cyclophosphamide and then docetaxel
- AC-TH
- doxorubicin, cyclophosphamide and then docetaxel plus trastuzumab
- BCIRG
- Breast Cancer International Research Group
- BNF
- British National Formulary
- CHF
- congestive heart failure
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CTCAE
- Common Terminology Criteria for Adverse Events
- DFS
- disease-free survival
- DNA
- deoxyribonucleic acid
- DSMC
- Data Safety and Monitoring Committee
- EC
- epirubicin and cyclophosphamide
- ECHO
- echocardiography
- EC-T
- epirubicin, cyclophosphamide and docetaxel
- EORTC
- European Organisation for Research and Treatment of Cancer
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- ER
- oestrogen receptor
- FDA
- Food and Drug Administration
- FEC
- 5-fluorouracil, epirubicin and cyclophosphamide
- FEC-T
- 5-fluorouracil, epirubicin, cyclophosphamide and docetaxel
- FISH
- fluorescence in situ hybridisation
- GCP
- Good Clinical Practice
- GP
- general practitioner
- HER
- human epidermal growth factor receptor
- HER2
- human epidermal growth factor receptor 2
- HORG
- Hellenic Oncology Research Group
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IDEA
- International Duration Evaluation of Adjuvant Therapy collaboration
- IHC
- immunohistochemistry
- INB
- incremental net benefit
- IQR
- interquartile range
- ISH
- in situ hybridisation
- LVEF
- left ventricular ejection fraction
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MRI
- magnetic resonance imaging
- MUGA
- multigated acquisition
- NCCTG
- North Central Cancer Treatment Group
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NSABP
- National Surgical Adjuvant Breast and Bowel Project
- NYHA
- New York Heart Association
- OR
- odds ratio
- OS
- overall survival
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SAR
- serious adverse reaction
- SD
- standard deviation
- SmPC
- summary of product characteristics
- SNP
- single nucleotide polymorphism
- SUSAR
- suspected unexpected serious adverse reaction
- TC
- docetaxel and cyclophosphamide
- TCH
- docetaxel, carboplatin (or cisplatin) and trastuzumab
- TMG
- Trial Management Group
- TNO
- trial number
- TSC
- Trial Steering Committee
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta24400).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
