Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/95/01. The contractual start date was in May 2018. The draft report began editorial review in January 2020 and was accepted for publication in May 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Michael J Crawford is Director of the College Centre for Quality Improvement at the Royal College of Psychiatrists (London, UK) (from 2011 to present) and has been a member of the National Institute for Health Research (NIHR) Health Technology Assessment General Committee (2017–18). Elizabeth Hughes received personal fees for speaking at a Lundbeck (Copenhagen, Denmark)-sponsored event. Zoe Hoare has been a member of the NIHR Health Services and Delivery Research panel (2016–20). Carol Paton received personal fees as an advisory board member for Allergan Ltd (Marlow, UK). Sofia Pappa received personal fees as a speaker for Janssen Pharmaceutica (Beerse, Belgium), Sunovion (Marlborough, MA, USA) and Recordati (Milan, Italy). Thomas RE Barnes received personal fees as a speaker for Janssen and as an advisory board member for Lundbeck, Newron Pharmaceuticals (Milan, Italy) and Gedeon Richter (Budapest, Hungary).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Crawford et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Schizophrenia is a severe mental health condition that affects almost 250,000 people in Britain. The illness usually starts in early adult life and can have a major impact on a person’s quality of life and social functioning. 1,2 Antipsychotic medication can improve the mental health of most people with the condition, but it can have a range of side effects that can impair a person’s quality of life. Over recent years, interventions have been developed that counteract some of the adverse effects (e.g. weight gain and changes in blood glucose) of antipsychotic medications. 3,4 By contrast, sexual dysfunction is a common side effect of antipsychotic medication, and there is no clear evidence to guide clinicians and patients seeking to manage this side effect. 5
Impact of sexual dysfunction associated with antipsychotic medication
At least half of people who take antipsychotic medication for schizophrenia experience sexual dysfunction. 6–8 The mechanisms through which antipsychotic drugs cause sexual dysfunction are varied. 9,10 Many antipsychotic drugs block dopamine receptors. This can lead to increased levels of prolactin, which reduces sex drive. 11 Some antipsychotic drugs have sedative effects, which may also reduce libido. Others have direct effects on blood flow to reproductive organs and can impair sexual functioning. Changes in blood lipids and glucose metabolism associated with antipsychotic medications may also lead to peripheral vascular disease and associated impairments to sexual functioning over the longer term. 12
Sexual dysfunction can have a negative impact on a person’s well-being and quality of life. 13–15 In a study of 139 men with schizophrenia in North America, those with sexual dysfunction reported lower overall quality of life than those with no sexual dysfunction. 16 Among those with sexual partners, people experiencing sexual dysfunction reported having poorer-quality relationships and being less likely to discuss their illness with their partner. 16
Sexual dysfunction can also affect adherence to antipsychotic medication. Surveys of people with psychosis indicate that sexual dysfunction is a common reason for stopping antipsychotic medication, which increases the likelihood of relapse and readmission to hospital. 17,18 Many patients find it difficult to talk to their mental health team about sexual dysfunction. For example, in one survey 80% of women who had experienced sexual dysfunction associated with use of antipsychotic medication had not discussed this with their mental health nurse or psychiatrist. 17 This means that people may stop their medication without seeking advice about how to manage these important problems.
Assessment of sexual dysfunction among people taking antipsychotic medication
Although sexual side effects of antipsychotic drugs are those that people with psychosis are most troubled by,18 there is evidence that clinicians find it difficult to discuss them. 19 Data from audits of prescribing practice show that sexual side effects of antipsychotic drugs are those least likely to be recorded in a patient’s clinical records. 20 It is unclear why mental health professionals have paid less attention to sexual side effects of antipsychotic medications than to other adverse effects. It is possible that some find it difficult to talk to patients about sexual side effects or are not aware how common they are. By contrast, most people using mental health services consider their sexual health and functioning to be important for their quality of life16 and expect mental health professionals to enquire about sexual matters. 21
Management of sexual dysfunction associated with antipsychotic medication
When sexual dysfunction is associated with the use of antipsychotic medication, clinicians may try reducing the dose of medication they prescribe. 9 This can be an effective strategy when it is possible to do this without having a negative impact on the person’s mental health. 22
When reducing the dose of medication is not possible, an alternative approach is to switch to an antipsychotic that is associated with a lower incidence of sexual dysfunction. Open-label studies of switching a person’s antipsychotic medication to aripiprazole23 or quetiapine24 have demonstrated beneficial effects. However, to the best of our knowledge, only two small randomised trials have compared the effects of switching medication with no switch for the management of sexual dysfunction associated with antipsychotic medication. Kinon et al. 25 randomised 54 men and women who were taking antipsychotic medication for schizophrenia and who had sexual dysfunction to either a switch to olanzapine or continuation of their current drug regimen. The study25 noted improved self-reported sexual functioning among those switched to olanzapine at 4 months. Byerly et al. 26 compared switching from risperidone to quetiapine with maintenance risperidone on sexual functioning of 42 men and women with schizophrenia. Participants were followed up for only 6 weeks. No statistically significant differences were found, but a non-statistically significant trend towards improved sexual functioning was seen in those who switched to quetiapine. Neither study was sufficiently powered to assess harms associated with switching antipsychotic drugs.
Switching a person’s antipsychotic medication may increase the risk of relapse,27,28 and different side effects associated with the medication may lead to poorer health outcomes or reduced quality of life. 3 A Cochrane review29 of the management of sexual dysfunction associated with antipsychotic medication among people with schizophrenia concluded that switching to another drug may provide an effective way to manage sexual side effects. An update5 of this review in 2018 found no new trials that tested the effects of switching medication.
The REMEDY trial
Switching medication may provide an effective strategy for improving sexual functioning and quality of life for people with schizophrenia who experience sexual dysfunction associated with use of antipsychotic medication. However, uncertainty about how effective this strategy is, together with concerns about increased risk of relapse and emergence of new side effects, means that clinicians and patients do not know whether or not this is an effective approach to help people who experience these problems.
The Randomised Evaluation of Management of sExual DYsfunction (REMEDY) trial was designed to address this uncertainty. The main aim of the study was to test whether or not switching a person’s antipsychotic medication to one considered to have a relatively low propensity for sexual side effects plus brief psychoeducation and support improved patient-reported sexual dysfunction compared with brief psychoeducation and support alone.
Trial objectives
-
To examine whether or not switching medication is a clinically effective and cost-effective strategy for improving sexual functioning among people with psychosis who experience sexual dysfunction associated with the antipsychotic medication they are prescribed.
-
To examine whether or not switching antipsychotic medication leads to changes in mental health, side effects of medication, health-related quality of life or service utilisation for people with psychosis who report sexual side effects.
-
To examine barriers to recruitment when conducting a trial of an intervention to reduce sexual dysfunction among people with psychosis.
Chapter 2 Methods
Design
The study was a multicentre, two-arm, parallel-group, researcher-blind randomised controlled trial with an internal pilot and a parallel qualitative study.
Study setting
The study setting was secondary care NHS community mental health services in England. We planned to recruit participants from all those in contact with community mental health services in five Trusts in the north and north-east of England and west and north-west of London.
Participants
To be eligible to take part in the study, potential participants had to be aged ≥ 18 years, have a diagnosis of schizophrenia, schizophreniform disorder, schizoaffective disorder or psychosis not otherwise specified, as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,30 and have sexual dysfunction associated with their use of antipsychotic medication, for which reducing the dose they were taking was either not effective or not appropriate.
Potential participants were excluded if:
-
they were acutely psychotic or had been acutely psychotic
-
their clinical team judged that they had an underlying physical health condition that was responsible for their sexual dysfunction
-
their current sexual problems began prior to their starting antipsychotic medication
-
they were already taking part in another clinical trial
-
they were unable to speak sufficient English to complete the baseline assessment
-
they were currently prescribed clozapine (a medication used when people have not responded to other antipsychotic medications).
Interventions
All study participants were offered enhanced standard care comprising treatment as usual plus brief psychoeducation and support to discuss their sexual health and functioning. The content of these sessions was based on that published by NHS Choices. 31 Brief psychoeducation and support included written information on specific psychosexual issues and psychoeducation, and signposting to the NHS Choices website and their primary care team should participants want additional help. Those delivering the brief psychoeducation and support were asked to keep a record of the time taken to deliver it and the content of the session. All those who took part in the study continued to have access to mental health services. No restrictions were placed on the use of other treatments.
Treatment in the switch arm of the trial
In addition to enhanced standard care, those in the switch arm of the trial were offered a switch from their current antipsychotic medication to an equivalent dose of another antipsychotic medication that is considered to have a lower propensity to cause sexual dysfunction. Evidence from observational and experimental studies suggest that improvements in sexual dysfunction associated with antipsychotic medication may result from swapping to aripiprazole,23,32 quetiapine24,26 or olanzapine. 25 A meta-analysis33 of the occurrence of sexual dysfunction among people taking antipsychotic medication found that two of these drugs (i.e. aripiprazole and quetiapine) were among those least likely to be associated with sexual side effects and that these three medications (i.e. aripiprazole, quetiapine and olanzapine) were the least likely to be associated with arousal dysfunction.
We asked clinicians to use their clinical judgement to select which medication to switch to, based on the known side effect profiles of these medications, patient preference, previous response to antipsychotic medication and potential interactions with other drugs the patient may be taking. 34 For patients who were being prescribed long-acting injectable antipsychotic preparations, clinicians could decide to switch the participant’s medication to oral aripiprazole, quetiapine or olanzapine, or aripiprazole long-acting injection. This was because quetiapine is not available as a long-acting injection and participating Trusts had not approved the use of olanzapine long-acting injection in community settings during the recruitment phase of the trial.
Prescribers overseeing any switch in medication were asked to gradually discontinue the participant’s current antipsychotic while at the same time gradually introducing the new antipsychotic medication (i.e. cross-tapering) to reach an equivalent dose. The equivalent was based on the Prescribing Observatory for Mental Health-UK’s Antipsychotic Dosage Ready Reckoner – Version 6.1. 35 We asked prescribers to aim to complete this cross-tapered switch of antipsychotic medication over a 4-week period (Table 1).
| Week | Recommended dose of pre-study antipsychotic medication | Recommended dose of switch medication |
|---|---|---|
| 1 | 80% of pre-study dose | British National Formulary-recommended starting dose for the switch drug36 |
| 2 | 60% of pre-study dose | Adjust based on clinical judgement |
| 3 | 40% of pre-study dose | Adjust based on clinical judgement |
| 4 | 20% of pre-study dose | Adjust based on clinical judgement |
| 5 | Nil | Equivalent dose of switch medication |
Assessments
Assessment of eligibility and covariates
A summary of all data collected from study participants is presented in Table 2. In addition to collecting basic demographic data on age, sex, ethnicity, marital/relationship status, socioeconomic status and education level, we assessed eligibility using the Arizona Sexual Experience Scale (ASEX) and a question on the onset of sexual dysfunction. We collected additional information on the participant’s sex life using questions from the National Survey of Sexual Attitudes and Lifestyles (Natsal-3). 37
| Assessment | Time point | |||
|---|---|---|---|---|
| Post-consent screening | Baseline | 3-month follow-up | 6-month follow-up | |
| Demographic information | ✗ | |||
| Participant contact details | ✗ | ✗ | ||
| Sexual functioning (ASEX) | ✗ | ✗ | ✗ | |
| Sexual behaviour (Natsal-3) | ✗ | ✗ | ||
| Question on onset of sexual dysfunction | ✗ | |||
| Alcohol and drug use (ASSIST) | ✗ | |||
| BARS | ✗ | ✗ | ||
| Service use (AD-SUS) | ✗ | ✗ | ||
| Depression (CDSS) | ✗ | ✗ | ||
| CGI for Sexual Functioning scale | ✗ | |||
| Symptoms of schizophrenia (PANSS) | ✗ | ✗ | ||
| Side effects of antipsychotic drugs (ANNSERS) | ✗ | ✗ | ||
| Health-related quality of life (EQ-5D-5L) | ✗ | ✗ | ||
| Recovery and well-being (ReQoL) | ✗ | ✗ | ||
The ASEX is a five-item self-report measure of sexual dysfunction that is widely used, sensitive to change and takes < 5 minutes to complete. 26,38 To take part in the study, potential participants had to have a total ASEX score of ≥ 19 points or a score of ≥ 5 points on one of item of the ASEX. Potential participants were then asked when their sexual problems started. Only those who stated that their problem began after they started medication for their mental health condition were judged eligible to take part in the study. Those who were ineligible were thanked for their time, informed of the reason(s) why they were ineligible and offered a leaflet containing information about sexual dysfunction and sources of help.
In keeping with recommended practice we used computerised-assisted direct interview for obtaining information about sexual functioning during study visits. 39 Participants used the Bristol Online Survey tool to complete the ASEX and to answer additional questions on sexual behaviour [URL: www.onlinesurveys.ac.uk/ (accessed 11 June 2020)].
Primary outcome
The primary outcome was patient-reported sexual dysfunction, measured at 6 months using the ASEX. The ASEX is the most widely used measure of sexual dysfunction in this field. 40 It also provides a reliable assessment of sexual dysfunction among people with psychosis. 41,42
Secondary outcomes
-
Total score on the ASEX at 3 months.
-
Researcher-rated sexual functioning at 6 months, using the Clinical Global Impression (CGI) for Sexual Functioning. 43
-
Mental health, using the total score on the Positive And Negative Syndrome Scale (PANSS). 44 This is a 30-item rating scale that is accompanied by a structured interview. It has been widely used to examine changes in symptoms in people with schizophrenia and related psychoses.
-
Side effects of medication, using the Antipsychotic Non-Neurological Side Effects Rating Scale (ANNSERS). The ANNSERS covers a range of negative subjective experiences as well the cardiovascular, gastrointestinal and metabolic side effects of antipsychotic medication. 45
-
Medication adherence at baseline and 6 months, using the Brief Adherence Rating Scale (BARS). 46
-
Resource use data collected at baseline and 6 months, assessed using the Adult Service Use Schedule. The Adult Service Use Schedule is an instrument designed on the basis of previous studies in adult mental health populations. 47
-
Health-related quality of life, assessed using the EuroQol-5 Dimensions (EQ-5D)48 and the Recovering Quality of Life (ReQoL) questionnaire49 at 3 and 6 months.
Blinding
All researchers who assessed study participants were masked to allocation status. Participants and clinical staff were aware of which arm of the trial people had been allocated to. To help maintain blinding researchers were not based with unblinded clinical team members. Prior to follow-up interviews participants were asked not to reveal the arm they have been allocated to. If any researcher became inadvertently unmasked, we arranged for another researcher to collect all further data.
Study logistics
The flow of participants through the study is presented in Figure 1.
FIGURE 1.
Study flow chart.
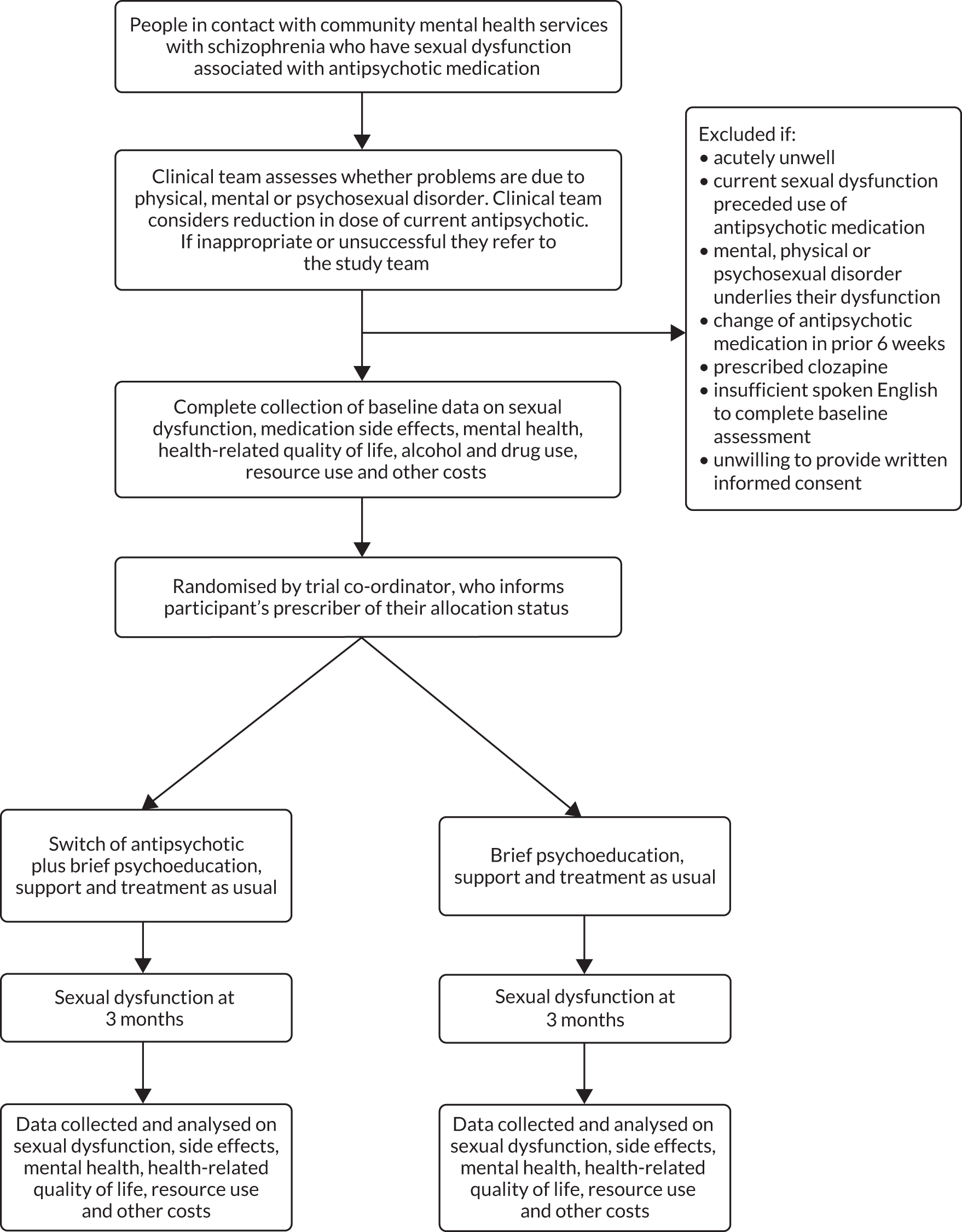
Recruitment
We contacted clinical teams and asked them to identify potential participants. Researchers and other members of the study team, including people with lived experience of poor mental health, gave presentations at team meetings to explain the study rationale and inclusion criteria. Following feedback that staff wanted advice about how to raise the topic of sexual dysfunction with their patients, we distributed a short script by e-mail and circulated a link to a video illustrating how people could be asked about sexual side effects of their medication. We also used a broad range of methods to communicate directly with people with psychosis to let them know about the study. We asked clinicians to seek verbal consent from people they believed met our inclusion criteria, so that they could be approached by a member of the research team.
We also used a range of direct marketing strategies, including displaying posters in clinics, day care and residential services; giving presentations to patients attending NHS, social care and voluntary sector groups; and writing to people who had reported sexual dysfunction in the past. Approved study posters asked potential participants either to speak to their psychiatrist/mental health team about the possibility of their taking part in the study or to contact a member of the REMEDY research team. We also worked with clinical teams to identify people who had already undergone screening for side effects of medication and helped them send letters to patients who had indicated that they had experienced sexual side effects. Prior to meeting potential participants, researchers liaised with clinicians to check whether or not the person’s sexual dysfunction could be the result of a physical health condition and whether or not they felt that they needed to have a trial of a reduction in the dose of their medication prior to taking part in the study.
If potential participants appeared to meet study inclusion criteria, arrangements were made for them to receive a copy of a patient information sheet and meet with a researcher to obtain written informed consent and complete a screening assessment. Researchers encouraged potential participants to spend as much time as they wanted asking questions about the study and considering if they wished to take part. In all instances potential participants were given at least 24 hours before deciding whether or not to take part in the study. Those who declined to take part were asked if they would be willing to take part in a short interview to establish their reasons for deciding not to take part. This interview took up to 10 minutes. Researchers took contemporaneous hand-written notes, which were subsequently transcribed. If potential participants declined to take part in an interview we asked them to provide a brief comment on their reason for deciding not to take part in the study.
Screening and baseline assessment
If written informed consent was given and documented, the referring clinician completed a document to confirm their medical opinion of the participant’s eligibility and a researcher then completed the post-consent screening assessment (see Table 1). Potential participants were screened using the ASEX and asked about the onset of their sexual dysfunction in relation to the antipsychotic medication they were prescribed. If the participant fulfilled all the eligibility criteria then the baseline assessment was also completed and he/she was randomised into the trial. Following randomisation, the participant’s general practitioner (GP) and consultant were informed of their enrolment into the trial.
Assignment of interventions
Study participants were randomly allocated to study interventions using a remote web-based system developed and maintained by the North Wales Organisation for Randomised Trials in Health. 50 The system uses a sequentially randomised, dynamic and adaptive algorithm which randomises participants in a 1 : 1 allocation ratio to either enhanced standard care plus a medication switch or enhanced standard care alone, while balancing for stratification variables. Stratification was by age (i.e. < 40 or ≥ 40 years), gender (i.e. male or female), Trust and relationship status (i.e. steady relationship or no steady relationship). For each participant randomised, the likelihood of their allocation to each treatment arm was recalculated based on the participants already recruited and allocated. This recalculation was done at the overall allocation level, within stratification variables and within strata. By undertaking this recalculation the algorithm ensured that balance was maintained within acceptable limits of the assigned allocation ratio while also maintaining unpredictability.
Following randomisation a member of the trial co-ordination team contacted the doctor or nurse who had agreed to oversee the delivery of study interventions. This clinician then made arrangements to offer the participant the brief psychoeducation and support and, for those in the switch arm of the trial, a switch in their medication.
Follow-up
Participants received subsequent assessments 3 and 6 months after randomisation. The timing and content of all assessments are summarised in Table 1. Participants were asked to complete the ASEX 3 months after randomisation, either in person using the touch-screen computer or over the telephone (in which case the researcher completed a paper version of the questionnaire).
Data management
All quantitative data were entered onto a secure web-based database. Access was restricted by encrypting the files. Audio-recordings of interviews with patient and staff were stored at local Trusts on encrypted files. Recordings were professionally transcribed verbatim and quality checked for accuracy. Once the transcriptions were verified, the recordings were deleted. Hand-written notes were taken during interviews with patients who declined to participate in the trial. These notes were then typed up, the files encrypted and the hand-written notes destroyed.
Sample size
We based the sample size calculation on our primary hypothesis: for people who experience sexual dysfunction associated with use of antipsychotic medication, switching to alternative medication that is considered to have a lower propensity to cause these side effects will result in improved self-reported sexual functioning (as rated using the ASEX).
In their randomised trial of switching antipsychotics among 42 people with sexual dysfunction associated with use of antipsychotic medication, Byerly et al. 26 reported a mean ASEX score of 19.5 points at 6-weeks follow-up and a standard deviation (SD) of 5.7 points.
We based the sample size calculation on having 90% power to detect a 3-point difference in the ASEX score between study arms. Three points on this scale equates to a clear improvement in one of the five domains in the ASEX or marginal improvement in three areas. In addition, it is at the lower end of the range of differences (2.0–5.6 points) seen in previous small-scale studies of switching antipsychotic medication that have used ASEX. 26,51 Feedback from our service user advisory group was that a change of this magnitude would represent an important change in sexual functioning.
Using these data we calculated that a total sample of 172 participants (86 participants in the switch arm and 86 participants in the control arm) would be needed to have 90% power to detect a 3-point difference in total ASEX score (SD 6.0 points) at 26 weeks, using a 0.05 significance level. To allow for a 20% loss to follow-up we aimed to recruit 216 participants.
Statistical analyses
We were not able to analyse differences in outcomes between study arms because of the very small sample size and limited number of follow-up data collected. Instead, we report details of participant flow, recruitment figures, demographic data, outcome measure descriptive statistics and details of safety events in Chapter 3. No inferences should be made from these data regarding differences between treatment arms, and summary statistics should be treated with caution because of low samples, especially when split by randomised allocation arm.
Health economics analysis
Data on the use of health and social services were collected using a modified version of the Adult Service Use Schedule, which has been used extensively in research in similar populations. 52,53 Prior to recruitment starting during the set-up phase of the trial, the questionnaire was modified based on a literature review and in collaboration with clinical and service user members of the research team to ensure that it included all relevant services. Use of services was collected in interview using patient recall, which we considered to be sufficient for the 6-month follow-up.
For each service use item a relevant and suitable unit cost was identified, and these costs are listed in Table 3. All unit costs were for the financial year 2017/18. Costs for NHS hospital contacts were extracted from NHS reference costs,56 community health and social care costs were taken from the Unit Costs of Health and Social Care 201854 and the cost of antipsychotic medication was estimated using costs per dose per item. 36
| Service | Unit cost (£) | Source |
|---|---|---|
| GP (per contact) | 31.30 | Unit Costs of Health and Social Care 2018 54 |
| Practice nurse (per contact) | 9.30 | Unit Costs of Health and Social Care 2018 54 |
| Key worker/community psychiatric nurse (per contact) | 37.00 | Unit Costs of Health and Social Care 2018 54 |
| Drug/alcohol support worker/advice worker (per contact) | 31.00 | Unit Costs of Health and Social Care 2018 54 |
| Social worker (per contact) | 61.00 | Unit Costs of Health and Social Care 2018 54 |
| NHS Direct (per telephone call) | 19.00 | Evaluation of NHS 11155 |
Health-related quality-of-life data were collected using the EuroQol-5 Dimensions, five-level version,57 and the ReQoL. 58 The EQ-5D is the measure preferred by the National Institute for Health and Care Excellence,59 although there are concerns about its lack of sensitivity in people with psychotic symptoms. 60 To mitigate these concerns, we included the ReQoL,49 which is a self-report outcome measure for use in people experiencing poor mental health.
The final sample size was too small to conduct the planned economic analysis. Instead, we conducted descriptive statistics on service use data, cost data at 6-month follow-up by randomised arm and quality-of-life data at baseline and 6-month follow-up. No inferences should be made from these data regarding the size and direction of differences in service use and costs, and summary statistics should be considered in the context of very small sample sizes.
Parallel qualitative study
We originally set out to conduct a parallel process evaluation in keeping with Medical Research Council guidelines. 61 However, as the study progressed and we realised how challenging it was to recruit study participants, we changed the focus of the qualitative component of the study to explore these barriers.
We aimed to interview as many as possible of the potential participants who were eligible to take part in the study but who declined to do so. We also interviewed a purposive sample of NHS staff to ensure that the views of people with a range of different backgrounds and seniority were captured. We developed semistructured interview schedules in consultation with the service user panel. Interview schedules were designed to explore patient views about reasons for not taking part in the study, and staff views about factors that promoted and hindered their referring people to the study team. Interviews lasted for up to 1 hour. Face-to-face interviews with staff were recorded, professionally transcribed and quality checked prior to data analysis. Researcher notes taken during interviews with patients were immediately typed up and stored securely following interviews.
Data were analysed using thematic analysis. 62 The researcher (LT) used an interpretative approach for analysing the data and developed themes concerning contextual factors, mechanisms and associations that may be inherent in the interviewee’s descriptions but not overtly stated.
The results of an interim analysis of data from the first 14 interviews (11 interviews with staff and three interviews with patients) were presented to members of the Trial Management Committee in June 2019. Following this, changes were made to the content of the interview guides, to take account of feedback from the group, as well as the emerging themes. These revised guides were then used for the remaining interviews with patients and staff. In the final data collection and analysis phase, themes were subjected to critical scrutiny, revision and refinement as well as restructuring of the thematic hierarchy where more prominent themes were generated.
Service user involvement
Patients identified this topic as a research priority through their membership of the James Lind Alliance Schizophrenia Priority Setting Partnership. This group, which included patient and carer representatives, rated the question ‘How can sexual dysfunction due to antipsychotic drug therapy be managed?’ as one of their top 10 research priorities. 63
Patients helped us to develop plans for this study through taking part in a survey and a focus group. Nine people attended the focus group. Members expressed enthusiasm for the topic and said that it was often neglected by mental health services. Members felt that the primary outcome should be patient-rated sexual functioning, which should be assessed over months rather than weeks. They asked us to also include assessments of people’s mental health and side effects of medication. They told us that everyone who agreed to take part in the study should be offered some support for their difficulties.
A co-applicant with lived experience of using mental health services (CG) contributed to the development of the study through attending regular Trial Management Group meetings. After the study had started, we set up a service user panel, which met on three occasions during the first 18 months of the trial. Members of the panel helped us design a leaflet for patients and a study poster and made suggestions for community-based groups among which the study could be publicised. Panel members also helped generate a list of topics to be covered in qualitative interviews with patients and staff.
Later in the study another member of the group (SJ) joined researchers in publicising the study and meeting clinical teams to help raise the profile of the trial and encourage clinicians to refer people to the study. At the final meeting of the group, members commented on the quantitative and qualitative results of the study, helped revise a draft version of the lay summary and made suggestions for future research in this field.
Ethics approval and governance
The trial was approved by the West Midlands – Solihull Research Ethics Committee (reference 18/WM/0076). In accordance with the current revision of the Declaration of Helsinki (amended October 2000,64 with additional footnotes added 2002 and 2004), a participant had the right to stop trial treatment and to withdraw from the trial at any time for any reason, without prejudice to his or her future treatment. The investigator could also withdraw a participant from trial treatment at any time in the interests of the participant’s health and well-being or for administrative reasons. Trial follow-ups continued after treatment was withdrawn unless the participant withdrew consent.
All potential participants were provided with written and verbal information about the study before being asked to provide written informed consent to take part. All participants were offered a £10 voucher at 3 months and a £20 voucher following completion of the follow-up interview at 6 months. Participants were also reimbursed for any travel expenses.
Study progress was overseen by a Trial Steering Committee and a Data Monitoring and Ethics Committee.
Changes to trial design
-
During the preparatory phase of the trial we encountered problems when trying to use software for generating an Operational Criteria Checklist for Psychotic Illness and Affective Illness diagnosis from data in case records. We therefore dropped plans to confirm the clinical diagnosis assigned to the patient by the referring team.
-
Following a request from the Trial Steering Committee we sent a reminder to all doctors overseeing medication of participants in the switch arm of the trial. The doctors were advised about the importance of monitoring body weight, blood glucose, blood pressure and blood lipids in the period following any change in medication.
-
In the initial phase of recruitment it became clear that some patients who would otherwise be eligible to take part in the study had been psychotic within the 3 months prior to being asked about taking part in the study. We therefore changed this criterion from ‘acutely psychotic within the last 3 months’ to ‘judged by the clinical team to be sufficiently stable to take part in the study’.
-
We obtained ethics and other approvals to contact all patients who were sent a letter about the study by telephone. We attempted to contact potential participants 7–14 days after sending them a letter to check that they had received it and to ask if they were interested in finding out more about the study.
-
Because it became apparent that we were struggling to recruit study participants, we changed the focus of the qualitative component of the study from a formal process evaluation to a qualitative exploration of barriers to recruitment.
-
We originally planned to recruit patients from three Trusts in England during the pilot phase of the trial; however, we extended this to four Trusts in the final 3 months of the recruitment phase of the study.
-
Two study participants were due to have both a 3- and a 6-month follow-up assessment when the decision was made to stop the study. We obtained approvals to complete all 6-month assessments with these participants at the 3-month visit to reduce the time and cost of a longer study.
Chapter 3 Results
Six participants were recruited during the first 6 months of the pilot phase of the trial. A decision was made to increase the pilot phase for a further 6 months, during which time an additional four patients were recruited. A decision was made to stop recruitment at this time. Between 1 July 2018 and 30 June 2019, 98 patients were referred to the study, of whom 61 (62%) met a researcher to assess eligibility. Of these 98 referrals, 85 (87%) were from staff and 13 (13%) were self-referrals. Five self-referred participants contacted the team after seeing a study poster or leaflet. Eight (2%) patients were among the 341 patients who were sent a letter about the study. None of the five self-referred participants who responded to a poster or leaflet had a diagnosis of psychosis, and none of the eight patients who responded to a letter agreed to take part in the trial after finding out more about it.
Participant flow
Table 4 and Figure 2 detail patient flow through the study. Among the 61 patients who met with a researcher, 15 (25%) consented to be screened and 46 (75%) did not. Among the 46 patients who declined to be screened, 28 (61%) provided a brief comment about their reasons for not wanting to take part in the trial. Of these, 10 (36%) patients stated that their sexual dysfunction was not important enough to need to do something about it, 11 (39%) patients said that they were worried about the possibility of having to switch their medication, three (11%) patients wanted their medication to be changed and were worried about being randomised to the control arm of the trial. The three remaining patients declined to take part, one because of work commitments, one because they did not want to be asked ‘intrusive questions’ and one patient stated that they had started taking Viagra® (Pfizer Inc., New York, NY, USA) and told us that this had improved their sexual functioning.
| Patient recruitment | Total | Trust | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Number of patients identified and referred | 98 | 56 | 28 | 10 | 4 |
| Number of patients not approached for consent | 37 | 18 | 13 | 5 | 1 |
| Number of patients approached for consent | 61 | 38 | 15 | 5 | 3 |
| Number of patients who did not consent | 46 | 28 | 12 | 4 | 2 |
| Number of patients who did consent | 15 | 10 | 3 | 1 | 1 |
| Number of patients screened (of those who consented) | 15 | 10 | 3 | 1 | 1 |
| Number eligible (of those screened) | 11 | 7 | 3 | 0 | 1 |
| Number ineligible (of those screened) | 4 | 3 | 0 | 1 | 0 |
| Number of participants recruited (of those eligible) | 10 | 6 | 3 | 0 | 1 |
| Number of patients not recruited (of those eligible) | 1 | 1 | 0 | 0 | 0 |
| Number of participants withdrawn before the 3-month follow-up | 2 | 2 | 0 | 0 | 0 |
| Number of participants withdrawn before the 6-month follow-up | 0 | 0 | 0 | 0 | 0 |
FIGURE 2.
Diagram of participant flow: guided by Consolidated Standards of Reporting Trials (CONSORT) guidelines. ITT, intention to treat; NA, not applicable.
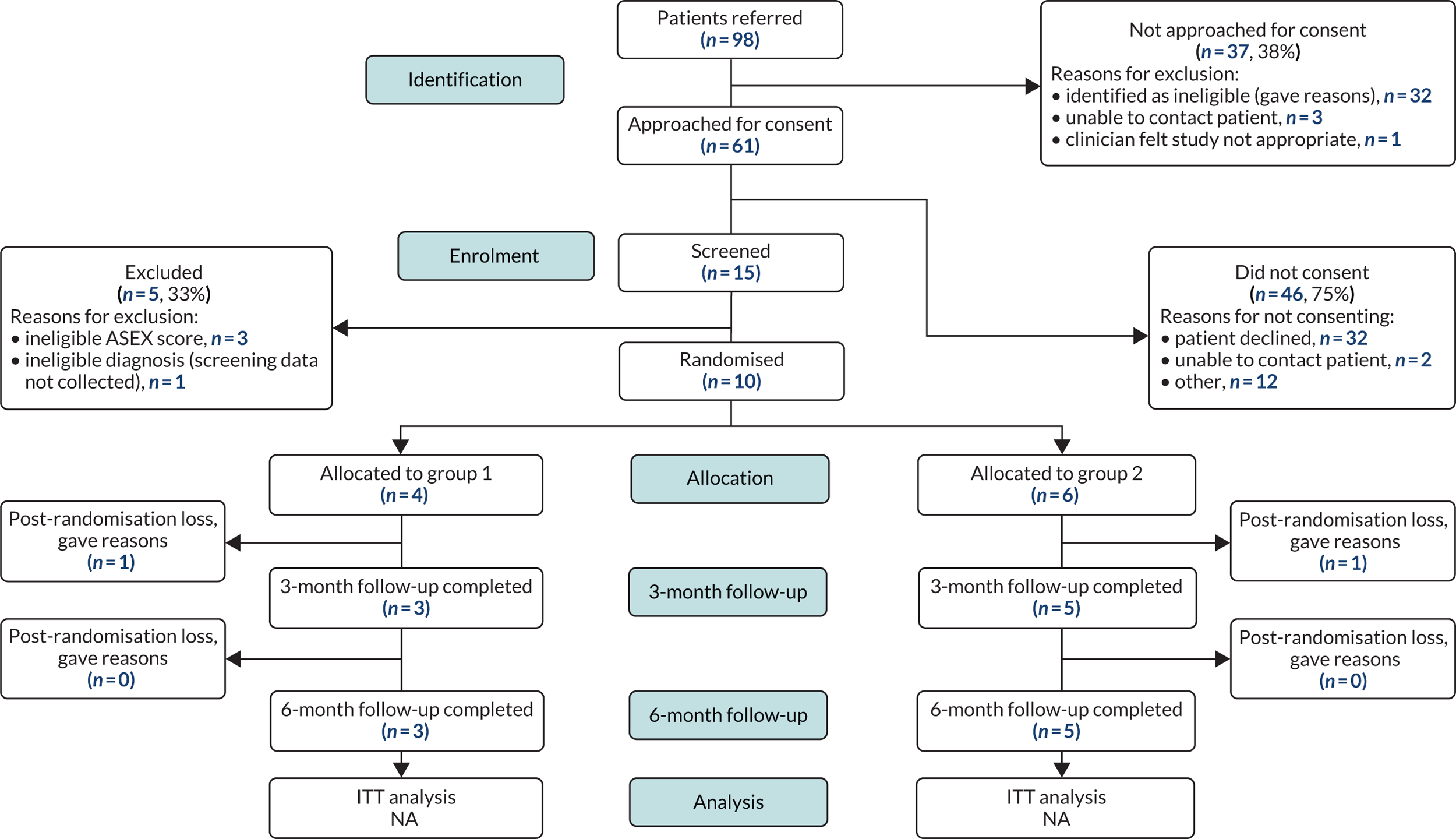
Of the 15 patients who consented to be screened, 11 (73%) were eligible and four (27%) were ineligible. Of the four patients who were ineligible, one did not have psychosis and should not have been screened. No screening data for this patient have been included in the remainder of this report. Of the 11 eligible patients, 10 (91%) were recruited and randomised into the study and one (9%) withdrew consent prior to baseline assessment and randomisation. Of the 10 patients randomised, four were allocated to enhanced standard care and six were randomised to enhanced standard care plus switch of medication (see Figure 2). Following randomisation, a further two patients withdrew from the study (one from the control arm and one from the switch arm).
A breakdown detailing the reasons for non-approach, non-consent, ineligibility, non-recruitment and post-randomisation loss is given in Figure 2. The most common reason for a researcher not approaching a patient was that they were found to be ineligible (n = 32, 86%). The main reasons for ineligibility prior to consent were not having a primary diagnosis of schizophrenia or related psychoses (n = 11, 34%), being prescribed clozapine (n = 7, 22%) or not having a suitable switch medication (n = 4, 13%). Three (9%) patients were excluded because they were being prescribed aripiprazole long-acting injection, three (9%) patients were judged by the clinical team to be insufficiently stable to take part in the study, two (6%) patients had an underlying physical health condition responsible for their dysfunction and one (3%) patient had not had a trial of dose reduction to see if this improved their sexual functioning.
The main reason for non-consent was the participant declining (n = 32, 70%). Following consent one participant was found not to have a primary diagnosis of psychosis and three patients did not meet the threshold score on the ASEX for sexual dysfunction.
Descriptive statistics
Patient and demographic details of the 14 study participants are detailed in Table 5. For patients who were randomised (n = 10) the data are split into randomised allocation arm. As indicated in Participant flow, 15 patients were screened; however, data for one patient, who should not have been screened, were not entered into the database, and therefore data on 14 screened patients are presented. Of these patients, four were not randomised (three were ineligible and one withdrew from the study) and 10 were randomised.
| Variable | Overall screened (N = 14), n (%) | Randomisation status, n (%) | Randomised arm, n (%) | ||
|---|---|---|---|---|---|
| Not randomised (N = 4) | Randomised (N = 10) | Control (N = 4) | Switch (N = 6) | ||
| Gender | |||||
| Male | 11 (79) | 3 (75) | 8 (80) | 2 (50) | 6 (100) |
| Female | 3 (21) | 1 (25) | 2 (20) | 2 (50) | 0 (0) |
| Trust | |||||
| 1 | 9 (64) | 3 (75) | 6 (60) | 2 (50) | 4 (67) |
| 2 | 3 (21) | 0 (0) | 3 (30) | 2 (50) | 1 (17) |
| 3 | 1 (7) | 1 (25) | 0 (0) | 0 (0) | 0 (0) |
| 4 | 1 (7) | 0 (0) | 1 (10) | 0 (0) | 1 (17) |
| Marital status | |||||
| Single | 11 (79) | 4 (100) | 7 (70) | 2 (50) | 5 (83) |
| Married and living with partner | 1 (7) | 0 (0) | 1 (10) | 0 (0) | 1 (17) |
| Divorced | 2 (14) | 0 (0) | 2 (20) | 2 (50) | 0 (0) |
| Relationship status | |||||
| Living with a partner | 2 (14) | 0 (0) | 2 (20) | 1 (25) | 1 (17) |
| Steady relationship, not cohabiting | 1 (7) | 1 (25) | 0 (0) | 0 (0) | 0 (0) |
| No steady relationship, previously cohabiting | 3 (21) | 1 (25) | 2 (20) | 2 (50) | 0 (0) |
| No steady relationship, never cohabited | 7 (50) | 2 (50) | 5 (50) | 1 (25) | 4 (67) |
| Missing | 1 (7) | 0 (0) | 1 (10) | 0 (0) | 1 (17) |
| Sexuality | |||||
| Heterosexual/straight | 12 (86) | 3 (75) | 9 (90) | 3 (75) | 6 (100) |
| Gay/lesbian | 1 (7) | 0 (0) | 1 (10) | 1 (25) | 0 (0) |
| Bisexual | 1 (7) | 1 (25) | 0 (0) | 0 (0) | 0 (0) |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Ethnicity | |||||
| White | 6 (43) | 1 (25) | 5 (50) | 2 (50) | 3 (50) |
| Mixed | 1 (7) | 1 (25) | 0 (0) | 0 (0) | 0 (0) |
| Asian (British Asian or other) | 3 (21) | 1 (25) | 2 (20) | 1 (25) | 1 (17) |
| Black (black British or other) | 4 (29) | 1 (25) | 3 (30) | 1 (25) | 2 (33) |
| Age (years)a | |||||
| < 40 | 2 (20) | 1 (25) | 1 (17) | ||
| ≥ 40 | 8 (80) | 3 (75) | 5 (83) | ||
| Medical conditionsb | |||||
| Hypertension (high blood pressure) | 3 | 1 | 2 | 0 | 2 |
| Diabetes (high blood sugar) | 3 | 0 | 3 | 0 | 3 |
| Chronic lung disease | 2 | 1 | 1 | 0 | 1 |
| Epilepsy | 2 | 1 | 1 | 0 | 1 |
| None of the above | 1 | 1 | 0 | 0 | 0 |
Data from the Alcohol, Smoking and Substance Involvement Screening Test revealed that six participants smoked tobacco (three in the control arm and three in the switch arm) and eight participants consumed alcohol (three in the control arm and five in the switch arm). No participant was currently using any other substance but five had a lifetime history of cannabis use, three had a lifetime history of amphetamines use, three a lifetime history of hallucinogenic drugs use, two a lifetime history of cocaine use and two had a lifetime history of opiates use.
The mean ASEX score for the 14 participants who were screened was 20.6 points (SD 4.97 points). Among the four participants who were not randomised, the mean score was 17.0 points (SD 6.88 points) and among the 10 participants who were randomised the mean score was 22.0 points (SD 3.46 points).
Table 6 describes the status of the patients screened according to whether or not they were eligible and, if eligible, whether or not they were randomised and, if randomised, whether or not they completed baseline data collection, and 3- (the primary outcome only) and 6-month follow-up data collection. Complete baseline data were collected for all 10 randomised participants, complete 3-month follow-up data were collected for eight participants and complete 6-month follow-up data were collected for seven participants.
| Screening status | Reason for ineligibility | Randomised | Study arm | Time point | Status | ||
|---|---|---|---|---|---|---|---|
| Baseline | 3-month follow-up (primary outcome) | 6-month follow-up | |||||
| Eligible | Yes | Switch | Yes | Yes | Yes (primary outcome only) | Completed | |
| Ineligible | ASEX | No | |||||
| Eligible | Yes | Control | Yes | No | No | Withdrew | |
| Eligible | Yes | Control | Yes | Yes | Yes | Completed | |
| Eligible | No (withdrawn before randomisation) | ||||||
| Ineligible | Diagnosis | No (screened when should not have been) | |||||
| Ineligible | ASEX | No | |||||
| Eligible | Yes | Switch | Yes | Yes | Yes | Completed | |
| Eligible | Yes | Switch | Yes | Yes | Yes | Completed | |
| Eligible | Yes | Switch | Yes | No | No | Withdrew | |
| Eligible | Yes | Switch | Yes | Yes | Yes | Completed | |
| Eligible | Yes | Control | Yes | Yes | Yes | Completed | |
| Eligible | Yes | Control | Yes | Yes | Yesa (secondary outcomes only) | Completed | |
| Ineligible | ASEX | No | |||||
| Eligible | Yes | Switch | Yes | Yes | Yesa (secondary outcomes only) | Completed | |
Six-month follow-up data could not be collected for the two participants randomised in the final months of the study (see footnote a in Table 6) but instead 6-month secondary outcome data were collected at the 3-month follow-up time point. Therefore, ASEX score was available for all 10 participants at baseline, for eight participants at 3 months and for six participants at 6 months (two participants did not reach the point when their 6-month interview was due because the study closed earlier than originally planned).
Uptake of allocated treatments
Antipsychotic medication was switched for five out of the six participants in the switch arm of the trial (three to oral aripiprazole and two to aripiprazole long-acting injection) and one six participant withdrew from the study before a switch could be initiated. None of those in the control arm of the trial had a change in their antipsychotic medication during the follow-up period.
Nine study participants were offered brief psychoeducation and support and one six participant withdrew from the study before they could be offered it. Seven (78%) of these nine participants attended a session. Sessions lasted between 30 and 60 minutes (mean duration of 45 minutes). The main problems discussed at sessions were lack or loss of desire for sex (n = 4), erectile dysfunction (n = 3) and orgasmic dysfunction (n = 2). All participants who attended a session were told about the prevalence and possible causes of sexual dysfunction and given information about the NHS Choices website and other sources of help. All seven participants were offered the possibility of a follow-up session. One participant accepted this offer.
Primary outcome
Table 7 contains the summary statistics of the ASEX scores (primary outcome) at baseline, and at 3- and 6-month follow-ups, presented overall and split by randomisation allocation arm. The ASEX data presented are the total scores calculated by summing the five items. The mean and median scores for the ASEX measure have little variation between time points and arms; however, the summary statistics should be treated with caution because small numbers represent the samples (especially when split by arm).
| Outcome | Allocation | Time point | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline, N = 10 (control, n = 4; switch, n = 6) | 3-month follow-up, N = 8 (control, n = 3; switch, n = 5) | 6-month follow-up, N = 8 (control, n = 3; switch, n = 5) | |||||||||||
| n | Mean score (points) (SD) | Observed score (points) range | Median score (points) (IQR 25%, 75%) | n | Mean score (points) (SD) | Observed score (points) range | Median score (points) (IQR 25%, 75%) | n | Mean score (points) (SD) | Observed score (points) range | Median score (points) (IQR 25%, 75%) | ||
| ASEX | Overall | 10 | 22.0 (3.46) | 18.0–30.0 | 21.0 (19.75, 24.0) | 8 | 21.3 (5.12) | 13.0–28.0 | 20.5 (17.5, 26.0) | 6 | 22.2 (4.49) | 17.0–29.0 | 22.0 (17.75, 26.0) |
| Control | 4 | 21.8 (2.06) | 20.0–24.0 | 21.5 (20.0, 23.75) | 3 | 23.0 (4.36) | 20.0–28.0 | 21.0 (20.0, –) | 2 | 22.0 (1.41) | 21.0–23.0 | 22.0 (21.0, –) | |
| Switch | 6 | 22.2 (4.36) | 18.0–30.0 | 21.0 (18.75, 25.5) | 5 | 20.2 (5.72) | 13.0–26.0 | 19.0 (15.0, 26.0) | 4 | 22.3 (5.34) | 17.0–29.0 | 21.5 (17.25, 28.0) | |
Table 8 contains the summary statistics of the continuous secondary outcomes [i.e. BARS, PANSS, Calgary Depression Scale for Schizophrenia (CDSS) and ANNSERS]. The total scores for each and, if applicable, the total subscale scores are presented. As with the ASEX data, the summary statistics represent very small samples and should be interpreted as such. The BARS data presented in Table 8 relate to the adherence percentage (the third item of the measure), which is calculated by dividing the proportion of doses taken by the patient in the past month by the total number of prescribed antipsychotic medication doses over the same period. The PANSS, CDSS and ANNSERS data are summary scores calculated from the individual measure items. The completion rates of the measures are high (relative to expected n) for all measures, with 100% completion at baseline for PANSS, CDSS and ANNSERS, and just one missing case at the 6-month follow-up for all. The BARS completion rates are a little lower, with 7 out of 10 participants completing at baseline and five out of eight participants completing at follow-up.
| Outcome | Randomised arm | Time point | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline, N = 10 (control, n = 4; switch, n = 6) | 6-month follow-up, N = 8 (control, n = 3; switch, n = 5) | ||||||||
| n | Mean (SD) | Observed range | Median (IQR 25%, 75%) | n | Mean (SD) | Observed range | Median (IQR 25%, 75%) | ||
| BARS | Overall | 7 | 96.6 (6.27) | 83 to 100 | 100 (95.0, 100) | 5 | 98.0 (4.47) | 90 to 100 | 100 (95.0, 100) |
| Control | 3 | 99.3 (1.16) | 98 to 100 | 100 (98.0, –) | 3 | 100.0 (0.00) | 100 to 100 | 100 (100, 100) | |
| Switch | 4 | 94.5 (8.02) | 83 to 100 | 97.5 (86.0, 100) | 2 | 95.0 (7.07) | 90 to 100 | 95.0 (90.0, –) | |
| PANSS: total | Overall | 10 | 59.6 (13.83) | 41.0 to 83.0 | 59.5 (46.0, 70.0) | 7 | 55.9 (9.32) | 44.0 to 69.0 | 55.0 (49.0, 67.0) |
| Control | 4 | 61.25 (18.23) | 41.0 to 83.0 | 60.5 (44.0, 79.25) | 3 | 55.3 (11.50) | 56.3 (9.22) | 55.0 (44.0, –) | |
| Switch | 6 | 58.5 (11.90) | 43.0 to 73.0 | 59.5 (46.0, 70.0) | 4 | 56.3 (9.22) | 49.0 to 69.0 | 53.5 (49.25, 66.0) | |
| PANSS: index | Overall | 10 | 4.1 (8.16) | –7.0 to 15.0 | 5.5 (–5.0, 11.0) | 7 | 1.14 (6.47) | –8.0 to 11.0 | 0.00 (–3.0, 8.0) |
| Control | 4 | 8.5 (9.26) | –5.0 to 15.0 | 12.0 (–1.25, 14.75) | 3 | 3.67 (10.21) | –8.0 to 11.0 | 8.0 (–8.0, –) | |
| Switch | 6 | 1.17 (6.52) | –7.0 to 9.0 | 1.5 (–5.5, 7.5) | 4 | –0.75 (1.71) | –3.0 to 1.0 | –0.5 (–2.5, 0.75) | |
| PAN: positive subscale | Overall | 10 | 16.2 (6.78) | 8.0 to 28.0 | 15.0 (10.75, 22.0) | 7 | 13.9 (4.14) | 8.0 to 18.0 | 14.0 (9.0, 18.0) |
| Control | 4 | 19.5 (8.96) | 8.0 to 28.0 | 21.0 (10.25, 27.25) | 3 | 14.7 (5.77) | 8.0 to 18.0 | 18.0 (8.0, –) | |
| Switch | 6 | 14.0 (4.47) | 10.0 to 21.0 | 12.0 (10.75, 18.75) | 4 | 13.3 (3.30) | 9.0 to 17.0 | 13.5 (10.0, 16.25) | |
| PANS: negative subscale | Overall | 10 | 12.1 (3.76) | 7.0 to 18.0 | 11.5 (10.0, 14.25) | 7 | 12.7 (3.68) | 7.0 to 18.0 | 12.0 (10.0, 16.0) |
| Control | 4 | 11.0 (2.83) | 7.0 to 13.0 | 12.0 (8.0, 13.0) | 3 | 11.0 (4.58) | 7.0 to 16.0 | 10.0 (7.0, –) | |
| Switch | 6 | 12.8 (4.36) | 7.0 to 18.0 | 11.5 (10.0, 18.0) | 4 | 14.0 (2.83) | 12.0 to 18.0 | 13.0 (12.0, 17.0) | |
| PANSS: general psychopathy subscale | Overall | 10 | 31.3 (7.07) | 20.0 to 42.0 | 30.5 (25.75, 38.5) | 7 | 29.3 (6.21) | 20.0 to 39.0 | 29.0 (24.0, 34.0) |
| Control | 4 | 30.8 (9.07) | 20.0 to 42.0 | 30.5 (22.25, 39.5) | 3 | 29.7 (9.50) | 20.0 to 39.0 | 30.0 (20.0, –) | |
| Switch | 6 | 31.7 (6.35) | 25.0 to 40.0 | 30.5 (25.75, 38.5) | 4 | 29.0 (4.08) | 24.0 to 34.0 | 29.0 (25.25, 32.75) | |
| CDSS: total | Overall | 10 | 6.8 (4.42) | 1.0 to 15.0 | 6.0 (3.5, 10.25) | 7 | 5.7 (4.50) | 0.0 to 12.0 | 5.0 (1.0, 10.0) |
| Control | 4 | 5.0 (3.46) | 2.0 to 10.0 | 4.0 (2.5, 8.5) | 3 | 5.7 (6.03) | 0.0 to 12.0 | 5.0 (0.0, –) | |
| Switch | 6 | 8.0 (4.86) | 1.0 to 15.0 | 8.0 (4.0, 12.0) | 4 | 5.8 (4.03) | 1.0 to 10.0 | 6.0 (1.75, 9.5) | |
| CDSS: mild | Overall | 10 | 2.1 (1.20) | 1.0 to 5.0 | 2.0 (1.0, 2.25) | 7 | 1.9 (1.07) | 0.0 to 3.0 | 2.0 (1.0, 3.0) |
| Control | 4 | 2.0 (0.00) | 2.0 to 2.0 | 2.0 (2.0, 2.0) | 3 | 1.7 (1.53) | 0.0 to 3.0 | 2.0 (0.0, –) | |
| Switch | 6 | 2.2 (1.60) | 1.0 to 5.0 | 1.5 (1.0, 3.5) | 4 | 2.0 (0.82) | 1.0 to 3.0 | 2.0 (1.25, 2.75) | |
| CDSS: moderate | Overall | 10 | 2.6 (2.32) | 0.0 to 8.0 | 2.0 (1.5, 4.0) | 7 | 1.7 (2.43) | 0.0 to 6.0 | 0.0 (0.0, 4.0) |
| Control | 4 | 1.5 (1.00) | 0.0 to 2.0 | 2.0 (0.5, 2.0) | 3 | 0.0 (0.00) | 0.0 to 0.0 | 0.0 (0.0, 0.0) | |
| Switch | 6 | 3.3 (2.73) | 0.0 to 8.0 | 3.0 (1.5, 5.0) | 4 | 3.0 (2.58) | 0.0 to 6.0 | 3.0 (0.5, 5.5) | |
| CDSS: severe | Overall | 10 | 2.1 (2.85) | 0.0 to 6.0 | 0.0 (0.0, 6.0) | 7 | 2.1 (3.34) | 0.0 to 9.0 | 0.0 (0.0, 3.0) |
| Control | 4 | 1.5 (3.00) | 0.0 to 6.0 | 0.0 (0.0, 4.5) | 3 | 4.0 (4.58) | 0.0 to 9.0 | 3.0 (0.0, –) | |
| Switch | 6 | 2.5 (2.95) | 0.0 to 6.0 | 1.5 (0.0, 6.0) | 4 | 0.8 (1.50) | 0.0 to 3.0 | 0.0 (0.0, 2.25) | |
| ANNSERS: total | Overall | 10 | 22.9 (11.28) | 6.0 to 40.0 | 21.5 (15.0, 32.25) | 7 | 14.4 (10.60) | 5.0 to 36.0 | 10.0 (7.0, 18.0) |
| Control | 4 | 18.0 (15.38) | 6.0 to 40.0 | 13.0 (6.75, 34.25) | 3 | 10.0 (5.57) | 5.0 to 16.0 | 9.0 (5.0, –) | |
| Switch | 6 | 26.2 (7.41) | 17.0 to 36.0 | 27.0 (18.5, 32.25) | 4 | 17.8 (13.02) | 7.0 to 36.0 | 14.0 (7.75, 31.5) | |
| ANNSERS: present | Overall | 10 | 14.9 (7.52) | 3.0 to 31.0 | 14.5 (10.5, 19.0) | 7 | 11.14 (5.96) | 5.0 to 19.0 | 7.0 (7.0, 18.0) |
| Control | 4 | 12.0 (7.39) | 3.0 to 19.0 | 13.0 (4.5, 18.5) | 3 | 9.0 (5.29) | 5.0 to 15.0 | 7.0 (5.0, –) | |
| Switch | 6 | 16.8 (7.60) | 11.0 to 31.0 | 14.5 (11.0, 22.0) | 4 | 12.8 (6.65) | 7.0 to 19.0 | 12.5 (7.0, 18.75) | |
| ANNSERS: mild | Overall | 10 | 3.7 (4.00) | 0.0 to 12.0 | 2.5 (0.75, 6.0) | 7 | 4.9 (0.90) | 3.0 to 6.0 | 5.0 (5.0, 5.0) |
| Control | 4 | 3.3 (1.71) | 1.0 to 5.0 | 3.5 (1.5, 4.75) | 3 | 4.3 (1.16) | 3.0 to 5.0 | 5.0 (3.0, –) | |
| Switch | 6 | 4.0 (5.18) | 0.0 to 12.0 | 1.5 (0.0, 9.75) | 4 | 5.3 (0.50) | 5.0 to 6.0 | 5.0 (5.0, 5.75) | |
| ANNSERS: moderate | Overall | 10 | 10.7 (7.63) | 1.0 to 24.0 | 9.0 (5.5, 17.5) | 7 | 7.1 (7.73) | 0.0 to 22.0 | 4.0 (2.0, 12.0) |
| Control | 4 | 9.3 (9.29) | 1.0 to 22.0 | 7.0 (1.75, 19.0) | 3 | 4.67 (3.06) | 2.0 to 8.0 | 4.0 (2.0, –) | |
| Switch | 6 | 11.7 (7.09) | 6.0 to 24.0 | 9.0 (6.0, 18.0) | 4 | 9.0 (10.13) | 0.0 to 22.0 | 7.0 (0.50, 19.5) | |
| ANNSERS: severe | Overall | 10 | 8.2 (8.03) | 0.0 to 24.0 | 7.5 (0.75, 12.75) | 7 | 2.43 (3.51) | 0.0 to 9.0 | 0.0 (0.0, 5.0) |
| Control | 4 | 4.8 (6.95) | 0.0 to 15.0 | 2.0 (0.25, 12.0) | 3 | 1.0 (1.73) | 0.0 to 3.0 | 0.0 (0.0, –) | |
| Switch | 6 | 10.5 (8.43) | 0.0 to 24.0 | 12.0 (2.25, 15.0) | 4 | 3.5 (4.36) | 0.0 to 9.0 | 2.5 (0.0, 8.0) | |
Table 9 contains the frequencies of the categorical outcomes for CGI at 6-month follow-up and the Natsal-3 questionnaire at baseline and 6-month follow-up and Table 10 reports the Natsal-3 count data summary statistics. No participant reported being satisfied with their sex lives either at the start of the study or at follow-up. Only one participant, in the switch arm of the trial, was rated by the researcher as having improved sexual functioning on the CGI at follow-up, but the improvement was judged to be minimal.
| Stratification variable | Time point, n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline | 6-month follow-up | |||||
| Overall (N = 10) | Randomised arm | Overall (N = 8) | Randomised arm | |||
| Control (N = 4) | Switch (N = 6) | Control (N = 3) | Switch (N = 5) | |||
| CGI: clinical judgement rating | ||||||
| Not assessed | 0 (0) | 0 (0) | 0 (0) | |||
| Very much improved | 0 (0) | 0 (0) | 0 (0) | |||
| Much improved | 0 (0) | 0 (0) | 0 (0) | |||
| Minimally improved | 1 (12.5) | 0 (0) | 1 (20) | |||
| No change | 5 (62.5) | 3 (100) | 2 (40) | |||
| Minimally worse | 1 (12.5) | 0 (0) | 1 (20) | |||
| Much worse | 0 (0) | 0 (0) | 0 (0) | |||
| Very much worse | 0 (0) | 0 (0) | 0 (0) | |||
| Missing (data not completed) | 1 (12.5) | 0 (0) | 1 (20) | |||
| Natsal-3 question 1: I feel satisfied with my sex life | ||||||
| Agree strongly | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Agree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Neither agree nor disagree | 1 (10) | 1 (25) | 0 (0) | 1 (12.5) | 0 (0) | 1 (20) |
| Disagree | 3 (30) | 1 (25) | 2 (33) | 3 (37.5) | 1 (33) | 2 (40) |
| Disagree strongly | 6 (60) | 2 (50) | 4 (67) | 4 (50) | 2 (67) | 2 (40) |
| Natsal-3 question 2: I feel distressed or worried about my sex life | ||||||
| Agree strongly | 5 (50) | 2 (50) | 3 (50) | 3 (37.5) | 1 (33) | 2 (40) |
| Agree | 2 (20) | 0 (0) | 2 (33) | 1 (12.5) | 2 (67) | 1 (20) |
| Neither agree nor disagree | 2 (20) | 1 (25) | 1 (17) | 3 (37.5) | 0 (0) | 1 (20) |
| Disagree | 1 (10) | 1 (25) | 0 (0) | 1 (12.5) | 0 (0) | 1 (20) |
| Disagree strongly | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Natsal-3 question 3: I have avoided sex because of sexual difficulties, either my own or those of my partner | ||||||
| Agree strongly | 2 (20) | 1 (25) | 1 (17) | 3 (37.5) | 1 (33) | 2 (40) |
| Agree | 4 (40) | 1 (25) | 3 (50) | 0 (0) | 0 (0) | 0 (0) |
| Neither agree nor disagree | 3 (30) | 2 (50) | 1 (17) | 3 (37.5) | 2 (67) | 1 (20) |
| Disagree | 1 (10) | 0 (0) | 1 (17) | 1 (12.5) | 0 (0) | 1 (20) |
| Disagree strongly | 0 (0) | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) | 1 (20) |
| Natsal-3 question 4: sought help regarding your sex life from a friend, family members, GP, doctor or nurse in the last 6 months? | ||||||
| No | 5 (50) | 3 (75) | 2 (33) | 5 (62.5) | 2 (67) | 3 (60) |
| Yes | 5 (50) | 1 (25) | 4 (67) | 3 (37.5) | 1 (33) | 2 (40) |
| Natsal-3 question 5: thinking of the way things are for you these days, which one would you really prefer? | ||||||
| To have sex much more often than I do now | 6 (60) | 2 (50) | 4 (67) | 3 (37.5) | 1 (33) | 2 (40) |
| To have sex a bit more often | 3 (30) | 2 (50) | 1 (17) | 4 (50) | 2 (67) | 2 (40) |
| It is about right as it is | 0 (0) | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) | 1 (10) |
| To have sex a little less often | 1 (10) | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) |
| Natsal-3 question 6: thinking about your sex life in the last 6 months, how many people have you had sexual intercourse with? | ||||||
| None | 5 (50) | 2 (50) | 3 (50) | 5 (62.5) | 2 (67) | 3 (60) |
| One | 4 (40) | 1 (25) | 3 (50) | 3 (37.5) | 1 (33) | 2 (40) |
| Two | 1 (10) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Three | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Four | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Five | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Six | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Six or more | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Natsal-3 continuous measure | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | 6-month follow-up | |||||
| Overall (n = 10) | Randomised arm | Overall (n = 8) | Randomised arm | |||
| Control (n = 4) | Switch (n = 6) | Control (n = 3) | Switch (n = 4) | |||
| Question 7: in the last month, how many times have you had sexual intercourse? | ||||||
| Mean (SD) | 0.9 (1.52) | 0.5 (1.00) | 1.2 (1.84) | 0.9 (1.45) | 0.3 (0.58) | 1.2 (1.79) |
| Observed range | 0.0–4.0 | 0.0–2.0 | 0.0–4.0 | 0.0–4.0 | 0.0–1.0 | 0.0–4.0 |
| Median (IQR 25%, 75%) | 0.0 (0.0, 2.25) | 0.0 (0.0, 1.5) | 0.0 (0.0, 3.25) | 0.0 (0.0, 1.75) | 0.0 (0.0, –) | 0.0 (0.0, 3.0) |
| Question 8: thinking about your sex life in the last month, how many times have you masturbated?a | ||||||
| Mean (SD) | 19.6 (36.7) | 32.0 (58.7) | 11.3 (12.5) | 57.3 (138.79) | 136.7 (228.1) | 9.6 (11.44) |
| Observed range | 0.0–120.0 | 1.0–120.0 | 0.0–31.0 | 0.0–400.0 | 2.0–400.0 | 0.0–28.0 |
| Median (IQR 25%, 75%) | 5.0 (0.75, 22.75) | 3.5 (1.0, 91.5) | 8.5 (0.0, 22,75) | 9.0 (0.5, 23.5) | 8.0 (2.0, –) | 10.0 (0.0, 19.0) |
Side effects of medication
Table 11 provides the frequencies that patients scored ‘absent’, ‘mild’, ‘moderate’ or ‘severe’ to side effects at baseline and at 6-month follow-up using ANNSERS. The most common side effects reported at baseline were lethargy/lassitude, daytime sleepiness or difficulty waking, loss of energy or drive, problems with memory, problems with concentration and dry mouth.
| Side effect | Time point, n (%) | |
|---|---|---|
| Baseline (N = 10) | 6-month follow-up (N = 8) | |
| Headache | ||
| Absent | 6 (60) | 6 (75.0) |
| Mild | 2 (20) | 0 (0.0) |
| Moderate | 2 (20) | 1 (12.5) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Lethargy/lassitude | ||
| Absent | 3 (30) | 3 (37.5) |
| Mild | 1 (10) | 3 (37.5) |
| Moderate | 3 (30) | 1 (12.5) |
| Severe | 3 (30) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Disturbed sleep | ||
| Absent | 3 (30) | 2 (25.0) |
| Mild | 2 (20) | 2 (25.0) |
| Moderate | 2 (20) | 3 (37.5) |
| Severe | 3 (30) | 0 (0.0) |
| Missing | 0 (0) | 1 (13.0) |
| Daytime sleepiness | ||
| Absent | 3 (30) | 4 (50.0) |
| Mild | 2 (20) | 1 (12.5) |
| Moderate | 2 (20) | 0 (0.0) |
| Severe | 3 (30) | 2 (25.0) |
| Missing | 0 (0) | 1 (12.5) |
| Loss of energy/drive | ||
| Absent | 3 (30) | 5 (62.5) |
| Mild | 1 (10) | 1 (12.5) |
| Moderate | 4 (40) | 1 (12.5) |
| Severe | 2 (20) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Problems with memory | ||
| Absent | 3 (30) | 2 (25.0) |
| Mild | 2 (20) | 1 (12.5) |
| Moderate | 3 (30) | 3 (37.5) |
| Severe | 2 (20) | 1 (12.5) |
| Missing | 0 (0) | 1 (12.5) |
| Impaired concentration | ||
| Absent | 4 (40) | 6 (75.0) |
| Mild | 2 (20) | 0 (0.0) |
| Moderate | 3 (30) | 0 (0.0) |
| Severe | 1 (10) | 1 (12.5) |
| Missing | 0 (0) | 1 (12.5) |
| Dysphoria | ||
| Absent | 5 (50) | 5 (62.5) |
| Mild | 0 (0) | 2 (25.0) |
| Moderate | 4 (40) | 0 (0.0) |
| Severe | 1 (10) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Emotional numbing | ||
| Absent | 6 (60) | 4 (50.0) |
| Mild | 3 (30) | 3 (37.5) |
| Moderate | 1 (10) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Tachycardia | ||
| Absent | 8 (80) | 4 (50.0) |
| Mild | 0 (0) | 2 (25.0) |
| Moderate | 2 (20) | 1 (12.5) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Postural hypotension | ||
| Absent | 8 (80) | 5 (62.5) |
| Mild | 1 (10) | 1 (12.5) |
| Moderate | 1 (10) | 1 (12.5) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Hypertension | ||
| Absent | 9 (90) | 6 (75.0) |
| Mild | 0 (0) | 1 (12.5) |
| Moderate | 1 (10) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| ECG abnormality | ||
| Absent | 9 (90) | 7 (87.5) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 1 (10) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Peripheral oedema | ||
| Absent | 6 (60) | 4 (50.0) |
| Mild | 1 (10) | 2 (25.0) |
| Moderate | 0 (0) | 1 (12.5) |
| Severe | 3 (30) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Breathlessness | ||
| Absent | 8 (80) | 6 (75.0) |
| Mild | 1 (10) | 0 (0.0) |
| Moderate | 0 (0) | 1 (12.5) |
| Severe | 1 (10) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Nausea/vomiting | ||
| Absent | 6 (60) | 6 (75.0) |
| Mild | 1 (10) | 0 (0.0) |
| Moderate | 3 (30) | 1 (12.5) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Constipation | ||
| Absent | 8 (80) | 7 (87.5) |
| Mild | 1 (10) | 0 (0.0) |
| Moderate | 1 (10) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Diarrhoea | ||
| Absent | 4 (40) | 5 (62.5) |
| Mild | 1 (10) | 1 (12.5) |
| Moderate | 3 (30) | 0 (0.0) |
| Severe | 2 (20) | 1 (12.5) |
| Missing | 0 (0) | 1 (12.5) |
| Weight gain | ||
| Absent | 9 (90) | 5 (62.5) |
| Mild | 1 (10) | 2 (25.0) |
| Moderate | 0 (0) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Weight loss | ||
| Absent | 6 (60) | 4 (50.0) |
| Mild | 2 (20) | 1 (12.5) |
| Moderate | 1 (10) | 1 (12.5) |
| Severe | 1 (10) | 1 (12.5) |
| Missing | 0 (0) | 1 (12.5) |
| Gynaecomastia | ||
| Absent | 10 (100) | 6 (75.0) |
| Mild | 0 (0) | 1 (12.5) |
| Moderate | 0 (0) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Galactorrhoea | ||
| Absent | 10 (100) | 7 (87.5) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 0 (0) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Onset/worsening of diabetes | ||
| Absent | 9 (90) | 7 (87.5) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 0 (0) | 0 (0.0) |
| Severe | 1 (10) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Dyslipidaemia | ||
| Absent | 8 (80) | 7 (87.5) |
| Mild | 1 (10) | 0 (0.0) |
| Moderate | 1 (10) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Blurred vision | ||
| Absent | 8 (80) | 6 (75.0) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 2 (20) | 1 (12.5) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Dry mouth | ||
| Absent | 3 (30) | 4 (50.0) |
| Mild | 3 (30) | 1 (12.5) |
| Moderate | 2 (20) | 2 (25.0) |
| Severe | 2 (20) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Hypersalivation | ||
| Absent | 8 (80) | 7 (87.5) |
| Mild | 2 (20) | 0 (0.0) |
| Moderate | 0 (0) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Sweating | ||
| Absent | 5 (50) | 5 (62.5) |
| Mild | 2 (20) | 1 (12.5) |
| Moderate | 3 (30) | 1 (12.5) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Fever | ||
| Absent | 10 (100) | 7 (87.5) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 0 (0) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Nocturnal enuresis | ||
| Absent | 8 (80) | 6 (75.0) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 1 (10) | 1 (12.5) |
| Severe | 1 (10) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Difficulty passing urine | ||
| Absent | 6 (60) | 7 (87.5) |
| Mild | 2 (20) | 0 (0.0) |
| Moderate | 2 (20) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Change in menstruation (females only) | ||
| Absent | 1 (50) | 2 (100.0) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 0 (0) | 0 (0.0) |
| Severe | 1 (50) | 0 (0.0) |
| Missing | 0 (0) | 0 (0.0) |
| Confusion | ||
| Absent | 8 (80) | 5 (62.5) |
| Mild | 1 (10) | 2 (25.0) |
| Moderate | 1 (10) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Fits | ||
| Absent | 10 (100) | 7 (87.5) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 0 (0) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Neuroleptic malignant syndrome | ||
| Absent | 10 (100) | 7 (87.5) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 0 (0) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Hepatic dysfunction | ||
| Absent | 10 (100) | 7 (87.5) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 0 (0) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Skin rash | ||
| Absent | 9 (90) | 4 (50.0) |
| Mild | 1 (10) | 2 (25.0) |
| Moderate | 0 (0) | 1 (12.5) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Blood dyscrasias | ||
| Absent | 10 (100) | 7 (87.5) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 0 (0) | 0 (0.0) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Facial oedema | ||
| Absent | 10 (100) | 6 (75.0) |
| Mild | 0 (0) | 0 (0.0) |
| Moderate | 0 (0) | 1 (12.5) |
| Severe | 0 (0) | 0 (0.0) |
| Missing | 0 (0) | 1 (12.5) |
| Other side effects | ||
| Absent | 6 (60) | 4 (50.0) |
| Mild | 2 (20) | 1 (12.5) |
| Moderate | 1 (10) | 1 (12.5) |
| Severe | 1 (10) | 1 (12.5) |
| Missing | 0 (0) | 1 (12.5) |
Safety data, protocol compliance and withdrawals
Tables 12 and 13 contain data on the number of adverse events, protocol deviations and withdrawals noted in the study. There was one adverse event and one serious adverse event, both in the switch arm of the trial. Neither were thought to be related to study procedures. The serious adverse event was for hospitalisation for an unrelated physical health condition. Three withdrawals occurred, one before randomisation and two after, one of which was because of the serious adverse event. The other two withdrawals were due to patients withdrawing consent. One protocol deviation was noted, which occurred when a patient was screened who did not meet eligibility criteria because they did not have schizophrenia or a related psychosis.
| Event | Allocation arm (n) | Total (n) | |
|---|---|---|---|
| Control | Switch | ||
| Adverse event | 0 | 1 | 1 |
| Serious adverse event | 0 | 1 | 1 |
| Protocol deviation | NA | NA | 1 |
| Withdrawal (prior to randomisation) | NA | NA | 1 |
| Withdrawal (after to randomisation) | 1 | 1 | 2 |
| Adverse event | Arm | Trust | Serious | Severity | Relationship with trial | Adverse reaction | Expectedness | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | Yes | Severe | Unrelated | No | Unexpected | Worsened |
| 2 | 2 | 2 | No | Moderate | Unlikely | No | Expected | Persisting |
Economic evaluation
Service use data were collected at follow-up for eight participants (four participants in the control arm and four participants in the switch arm of the trial). All resources used by study participants over the follow-up are summarised in Table 14 and the costs of these services are provided in Table 15.
| Service | Allocation arm | |||
|---|---|---|---|---|
| Control (n = 4) | Switch (n = 4) | |||
| Mean (SD) | % | Mean (SD) | % | |
| Brief psychoeducation and support sessions (number) | 1.25 (0.50) | 100 | 0.60 (0.55) | 60 |
| Inpatient (nights) | 45.00 (90.00) | 25 | 0.00 (0.00) | 0 |
| Outpatient (appointments) | 1.50 (1.29) | 75 | 1.00 (1.41) | 50 |
| A&E (attendances) | 0.00 (0.00) | 0 | 0.25 (0.50) | 25 |
| GP (contacts) | 1.50 (1.73) | 50 | 1.00 (1.41) | 50 |
| Practice nurse (contacts) | 0.25 (0.50) | 25 | 1.50 (3.00) | 25 |
| Key worker (contacts) | 3.50 (3.11) | 75 | 4.25 (2.63) | 100 |
| Psychiatrist (contacts) | 0.50 (1.00) | 25 | 0.75 (0.50) | 75 |
| Social worker (contacts) | 0.00 (0.00) | 0 | 1.25 (2.50) | 25 |
| NHS Direct (contacts) | 0.25 (0.50) | 25 | 0.25 (0.50) | 25 |
| Cost | Allocation arm, mean (SD) (£) | |
|---|---|---|
| Control (n = 4) | Switch (n = 4) | |
| Advice sessions | 102.19 (78.27) | 54.50 (44.50) |
| Hospital services | 18,660.00 (36,987.00) | 165.00 (194.04) |
| Community services | 233.22 (249.39) | 418.62 (359.01) |
| Medication | 162.19 (320.76) | 270.23 (299.14) |
| Total | 19,157.60 (317,171.33) | 908.35 (691.91) |
The substantial difference in total costs over the 6-month follow-up period between randomised arms is the result of one participant, in the control arm, being an inpatient throughout this period. Mean scores on measures of health-related quality of life at baseline and follow-up are presented in Table 16.
| Outcome | Allocation arm | Time point | |||
|---|---|---|---|---|---|
| Baseline | 6-month follow-up | ||||
| n | Mean (SD) | n | Mean (SD) | ||
| EQ-5D utility score | Control | 6 | 0.788 (0.180) | 5 | 0.848 (0.290) |
| Switch | 4 | 0.653 (0.162) | 3 | 0.853 (0.114) | |
| ReQoL | Control | 6 | 48.82 (15.93) | 5 | 46.67 (17.95) |
| Switch | 4 | 40.67 (14.61) | 3 | 47.50 (11.00) | |
Chapter 4 Qualitative findings
Fifty-one members of staff and four patients took part in a qualitative interview. Characteristics of staff and patient participants are presented in Tables 17 and 18. All four patients who were interviewed were eligible to take part in the study but declined to do so.
| Identification number | Gender | Ethnicity | Professional background | Job title | Time (years) spent working in mental health |
|---|---|---|---|---|---|
| 1ST001 | Female | White (other) | Doctor | Consultant psychiatrist | 10–15 |
| 1ST002 | Doctor | Psychiatrist | 10–15 | ||
| 1ST003 | Doctor | Psychiatrist | > 15 | ||
| 1ST004 | Male | Doctor | Psychiatrist | 10–15 | |
| 1ST005 | Male | Doctor | Psychiatrist | 5–10 | |
| 1ST006 | Doctor | Psychiatrist | 10–15 | ||
| 1ST007 | Female | Mental health nurse | Mental health nurse | < 5 | |
| 1ST008 | Female | Mental health nurse | Community psychiatric nurse | 10–15 | |
| 1ST009 | Female | Mental health nurse | Mental health nurse | < 5 | |
| 1ST010 | Female | British Asian | Mental health nurse | Mental health nurse | < 5 |
| 1ST011 | Male | White (other) | Doctor | Consultant psychiatrist | < 5 |
| 1ST012 | Male | Asian (other) | Mental health nurse | Mental health nurse | > 15 |
| 1ST013 | Male | Other Chinese | Doctor | Specialist registrar | < 5 |
| 1ST014 | Female | White (other) | Doctor | Psychiatrist | > 15 |
| 1ST015 | Male | White (other) | Doctor | Consultant psychiatry | > 15 |
| 1ST016 | Male | White British | Social worker | Care co-ordinator/social worker | > 15 |
| 1ST017 | Male | Black African | Mental health nurse | Mental health nurse | > 15 |
| 1ST018 | Female | Mixed | Mental health nurse | Mental health nurse | > 15 |
| 1ST019 | Female | White (other) | Doctor | Psychiatrist | > 15 |
| 1ST020 | Male | Asian (other) | Doctor | Consultant psychiatrist | > 15 |
| 1ST021 | Male | British Asian | Mental health nurse | Mental health nurse | > 15 |
| 1ST022 | Female | White British | Mental health nurse | Mental health nurse | < 5 |
| 1ST023 | Male | Black African | Mental health nurse | Nurse manager | > 15 |
| 1ST024 | Female | White (other) | Social worker | Social worker | > 15 |
| 1ST025 | Male | White British | Mental health nurse | Staff nurse | 5–10 |
| 1ST026 | Female | White British | Pharmacist | Pharmacist | > 15 |
| 1ST027 | Female | Black British | Mental health nurse | Senior staff nurse | 10–15 |
| 1ST028 | Female | White (other) | Doctor | Consultant psychiatrist | > 15 |
| 1ST029 | Female | British Asian | Pharmacy technician | Pharmacy technician | 10–15 |
| 1ST030 | Female | White (other) | Doctor | Psychiatrist | < 5 |
| 1ST031 | Female | White British | Peer support worker | Peer support co-ordinator | < 5 |
| 1ST032 | Female | Black African | Pharmacist | Pharmacist | 10–15 |
| 1ST033 | Female | White British | Peer support worker | Peer worker | 5–10 |
| 1ST034 | Female | White British | Peer support worker | Peer support worker | < 5 |
| 2ST001 | Female | White British | Doctor | Specialty registrar | < 5 |
| 2ST002 | Female | White (other) | Doctor | Consultant psychiatrist | > 15 |
| 2ST003 | Male | Doctor | Specialty doctor | < 5 | |
| 2ST004 | Male | British Asian | Doctor | Consultant psychiatrist | > 15 |
| 2ST005 | Male | Nurse | Senior nurse practitioner | ||
| 2ST006 | Male | Nurse | Community psychiatric nurse | ||
| 2ST007 | Female | Sudanese | Doctor | Specialty doctor | |
| 3ST001 | Male | White British | Doctor | Consultant psychiatrist | > 15 |
| 3ST002 | Female | Black African | Doctor | Consultant psychiatrist | 10–15 |
| 3ST003 | Female | White British | Doctor | Consultant psychiatrist | > 15 |
| 3ST004 | Female | African | Doctor | Consultant psychiatrist | > 15 |
| 3ST005 | Male | White, non-British | Doctor | Consultant psychiatrist | 10–15 |
| 3ST006 | Male | British Asian | Doctor | Psychiatry | 5–10 |
| 3ST007 | Male | White British | Nurse | Senior care co-ordinator | 10–15 |
| 3ST008 | Male | Indian | Doctor | Consultant psychiatrist | > 15 |
| 3ST009 | Female | White British | Doctor | Consultant psychiatrist | > 15 |
| 3ST010 | Female | White British | Mental health nurse | Team manager | > 15 |
| Identification number | Age (years) | Gender | Ethnicity |
|---|---|---|---|
| 1PT001 | Male | ||
| 1PT002 | Female | White British | |
| 1PT003 | 40–50 | Male | Black (other) |
| 2PT001 | 30–40 | Male | White British |
Emerging themes from interviews with patients
The small number of qualitative interviews we conducted with patients means that we are unable to report fully developed themes. Instead, we present emerging themes. Potential participants who decided not to take part in the study did so having weighed up concerns about their sexual functioning with concerns about their current mental health and loss of control about their treatment if they took part in the trial. Because all the qualitative patient interviews were with those who declined to take part, these emerging themes are from a biased sample and reflect barriers to taking part and not the thoughts of all patients prescribed antipsychotic medication who experience sexual side effects. Three main themes emerged and these are discussed below.
Previous adverse experiences when medication was switched
Patients reported that previous negative experiences with changes to their medication put them off taking part in the study. Patients were worried about the side effects of any new medication they were prescribed (e.g. weight gain). Patients also reported concerns about jeopardising their mental health. One patient was concerned that a switch in their medication could lead to them having a mental health crisis and one patient stated that they would be ‘unhappy’ on any new medication.
Not a priority
Patients reported that sexual dysfunction was important to them; however, they had to weigh this up against what might happen if their medication was switched. Two of the four patients said that sexual dysfunction was not a ‘pressing issue’: one patient was not in a sexual relationship and the other patient did not feel that that their sexual problems affected their relationship.
Lack of control/choice
Patients reported concerns about being randomised to one of the two treatment arms of the study. They were concerned that randomisation limited their choice about what treatment they would be given and would reduce the control they had over this. Making a switch in medication was significant event. Patients stated that they wanted as much control over their treatment as they could have, so it made sense for them not to restrict this by taking part in the study.
Staff perspectives: barriers to talking about sexual functioning and the study
Staff highlighted the complex nature of sexual dysfunction among people with psychosis and discussed a range of reasons why it was difficult to refer people to the trial. Many reported that they had not referred anyone to the study despite being aware that recruitment was taking place. Barriers included reluctance to discuss sex in general as well as specific factors concerning sexual dysfunction among people with psychosis and involving people with psychosis in clinical trials. Eight main themes emerged and these are discussed below.
Not a common problem
Staff views about the prevalence of sexual dysfunction among people with psychosis differed greatly. Some believed that it was a frequent occurrence and others believed that it was rare. Clinicians who said that they often or routinely asked patients about sexual dysfunction were more likely to consider it a frequent occurrence. Clinicians who thought it was common for people with psychosis to experience sexual side effects of antipsychotic medication felt that it was under-reported:
I think it’s very difficult to ascertain, actually. I think it’s a much bigger problem than it appears.
1ST033
Timing
Clinicians said that it was difficult to find the right time to talk to patients about sexual dysfunction. Much of the contact they had with patients was when they were acutely unwell. Clinicians reported that the priority at that stage was trying to help stabilise a person’s mental state. They reported a ‘trade-off’ between concerns about side effects and concerns about mental health, in which side effects were unlikely to be discussed until mental health had improved:
It’s just not the time or place, you really want to get the person well, regardless of whatever side effects.
1ST004
However, when asked about what happened when patients were more stable, some staff stated that they still had to weigh up having this conversation with the possibility of relapse if the medication needed to be changed or the dose reduced. Staff said that it may be necessary for a patient to be stable over a longer period of time to be able to tolerate a switch in medication. Some reported that patients tended to ‘ride on the side of caution’ in relation to switching medication:
[Patients will say] ‘No, I don’t want to change my medication, I’m stable on it’ and you can understand that . . . Really they’ve been quite unwell in the past and, they’ve become stable . . .
3ST010
A ‘taboo’ issue
Although staff reported being able to discuss a range of other side effects with their patients, most reported discomfort and some reported ‘ignoring’ issues around sexual functioning when meeting their patients. Staff said that it was not part of their professional culture or ‘routine practice’ to ask such questions.
Moreover, many staff reported that these feelings of discomfort or embarrassment were mutual, and they thought that patients also experienced difficulties in discussing these ‘sensitive’, ‘personal’ and ‘taboo’ subjects:
. . . I think it’s a two-way thing. The patients tend to be finicky about reporting it because it can be quite personal and we sometimes feel finicky in asking it because it can be quite personal. So both things don’t help. It becomes a hidden thing, that’s something you don’t talk about.
1ST004
. . . so you see it’s a big taboo for our patients to come [to] us and talk about it. That’s my experience on it.
1ST017
When conversations with patients about sexual functioning did take place, staff reported that they may need to cover a range of sensitive subjects, including masturbation, sexual intercourse with partners and risky behaviours resulting from sexual disinhibition. Staff reported that they avoided such conversations to avoid upsetting or unsettling patients:
. . . many patients suffer in silence due to embarrassment or not hav[ing] any opportunity to discuss this.
1ST034
. . . personal embarrassment, or subconsciously or deliberately ignoring [sexual dysfunction], I think I’m guilty of [that] . . .
3ST007
Staff said that it was ‘safer’ to discuss other side effects of medication (e.g. weight gain) or psychological factors (e.g. low self-esteem) that could be linked to sexual dysfunction.
Social and demographic factors, such as the patients’ gender, age, ethnicity and relationship status, affected the likelihood of discussions about sexual functioning. Staff reported that it was easier to discuss the topic with younger males, particularly those who were sexually active, and others said that they avoided taking about these issues with female patients altogether. Several staff said that it was easier to talk to men about erectile dysfunction than it was to talk to women about loss of libido. Male members of staff were particularly concerned about speaking to female patients about sexual matters:
It’s something I discuss more with men than women, maybe for women with a male care co-ordinator it’s more difficult to bring up.
1ST016
A few staff questioned the relevance of sexual functioning for older adults; however, others acknowledged that an active sex life was part of general well-being at any age:
I’ve noticed that a lot of our ladies who are older are very reluctant to talk about it. They don’t want to talk about their sexuality let alone their sexual functioning.
1ST031
It doesn’t seem to be something that older service users, by that I mean 50 plus, find easy to talk about, maybe that’s because younger people are more sexually active.
1ST016
In addition, most staff said that they avoided raising the topic of sex with those from cultural/religious backgrounds that are perceived to be more ‘traditional’ or conservative, such as Asian, Middle Eastern and/or Muslim and Jewish, and particularly females from those cultures. Staff were of the view that discussion of the topic might be considered ‘taboo’ or shameful in these cultures.
‘Reactive’ stance
Staff appeared to adopt a passive rather than an active role in relation to their patients’ sexual functioning. They often said that they relied on patients raising the subject themselves and took a ‘reactive’ stance. Some clinicians felt that it was up to the patient to flag pertinent side effects:
Sexual side effects I don’t usually discuss – I am more reactive.
3ST005
To me, [what is of] importance is what the patient tells me.
2ST003
I don’t really feel like it’s appropriate as it’s such a personal and sensitive issue. I’d discuss healthy eating and sleep and topics like that but I wouldn’t just bring up someone’s sexual functioning without them discussing it first.
1ST016
However, some staff acknowledged disconnect between what they ‘should’ ideally be doing and what actually took place in practice. Although staff often said that they waited for patients to raise any problems they had with sexual side effects of medication, they acknowledged that patients rarely raised these issues themselves. Other staff reported that if a patient did not raise the issue of sexual dysfunction then they concluded that the patient was not affected by these problems.
Distinguishing different causes of sexual dysfunction
Another primary concern for clinicians in identifying potential participants was their ability to investigate and eliminate other underlying causes of sexual dysfunction. Clinicians raised concerns that other factors, such as co-prescribed medication, diabetes, obesity (which can have an impact on self-image and self-esteem) and depression, made it difficult to identify people who experienced sexual side effects of antipsychotic drugs. Staff stated that untangling the varied and multifactorial potential causes of sexual dysfunction was a difficult undertaking. It sometimes involved periods of trial and error, which added another layer of complexity to the process of identifying patients for the study:
Some sexual issues, it can be related to . . . [issues] of a psychological nature, because the illness might impact their self-confidence . . . self-esteem or can be of organic cause. So if it’s an organic cause you’re trying to establish the possible factors or co-factors that might be the reason for their sexual dysfunction.
1ST015
It seems for some people that the antipsychotic medications that they are on are clearly linked to their sexual dysfunction but for others who may have so many side effects including potentially developing diabetes/obesity that may contribute to sexual dysfunction too as well as personal issues and juggling mental health symptoms sometimes it’s difficult to pin down what exactly is causing the dysfunction in some. Some service users are on antidepressants and antipsychotics so both could be contributing . . .
1ST016
Keeping the study ‘in mind’
Many staff reported finding it difficult to keep the study in mind. They described being in a position of ‘spinning multiple plates’, suggesting that it was often necessary to ‘fight fires’ and focus their efforts on priorities. For example, some staff reported that working in crisis care settings meant that there was not enough time to explore side effects and the focus had to be on stabilising mental state:
I don’t know if I’m a bit skewed by being in the home treatment team, seeing people who are acutely psychotic. And almost giving them minimal reason not to take treatment because we might be the ones just starting it or getting them to restart treatment when they’ve had a serious relapse . . .
1ST003
Staff often described how pressures on mental health services meant that they struggled to remember the study or make time to discuss it with their patients. A few participants discussed working in teams that were understaffed and had high levels of staff turnover, which had an impact on their ability to keep the study in mind:
That’s purely maybe because there’s a lack of time, not enough manpower to review side effects for patients.
1ST029
Some staff felt that they did not know enough about the study and wanted more contact with the research team to remind them about the study protocol and eligibility criteria. These staff said that it would have been helpful to have more frequent contact with researchers, more guidance on recruitment procedures and more reminders about the study. Other staff felt that the study was well advertised and said that they had received a prompt response to any queries that they had raised:
The researchers have done their best to be present; however . . . more presence would have been much more important. So that we have in mind that we need to tackle these questions because as I said not many professionals go in depth with regard to sexual life.
1ST030
Fear of unwanted consequences
Some staff were concerned about potential consequences of having conversations with their patients about sexual functioning. A few suggested that raising the topic could have a negative impact on their efforts to maintain trust and rapport with patients, especially for patients with psychotic symptoms:
[Patients could become] agitated, angry and argumentative and might create unpleasant situations and then you have to avoid it.
2ST007
Some staff expressed concern about complaints from patients who had psychotic symptoms and questioned whether or not it was appropriate to discuss sex with these patients.
Clinicians also raised concerns about speaking about sex to patients who had a history of sexual abuse. Staff were worried that such discussions could trigger painful memories and emotions related to these experiences. Staff feared that raising the study with such patients could negatively affect their relationship with them, with some staff stating that they had avoided talking to patients about the study as a result.
Other staff reported reluctance to speak to patients about the study because it could lead patients to stop taking their medication, leading to relapse. These concerns were most pronounced in the case of patients who had been treatment resistant, because staff had often spent a lot of time and energy identifying a drug regime that was effective in reducing psychotic symptoms in these patients.
‘Risky’ patients
Staff were particularly cautious about discussing the study with patients who were currently sexually disinhibited or had been so in the past:
The only moment when I’m cautious is if the person is unwell or sexually disinhibited.
1ST028
There were concerns that improving the sexual functioning of such patients could even be ‘detrimental’ because increased risk-taking behaviours could place sexually disinhibited patients in dangerous situations. Staff reported that discussion of sexual functioning with risky patients would require extra precautions, such as the presence of a chaperone:
We need to be cautious depending on every case . . . Everybody is, as I said, different. We’ve got different patients, and we need to be cautious . . . on what kind of, part of the life we are going to improve.
1ST030
Other staff expressed concern about raising the topic of sexual functioning or the study with patients who had deviant sexually fantasies (e.g. paedophilia) or with women who had thoughts around harming children.
Staff perspectives: facilitators of discussions about sexual functioning and the study
Raising the topic indirectly
Staff reported that it was easier to discuss sexual functioning in the context of other subjects that were less taboo. For example, exploring self-esteem linked to weight gain and difficulties establishing romantic relationships could open up channels for conversations about sex and sexual dysfunction:
It’s a big issue; however it’s difficult to record. So in my career hardly ever do I get patients say[ing] they suffer from sexual dysfunction directly. You can get the information indirectly that it’s making them not have energy, making them unmotivated, making them not have a relationship.
1ST017
Clinicians reported that including questions about sexual functioning as part of a battery of questions that patients were asked to complete during medication reviews could be helpful:
. . . sometimes it’s difficult to bring it up in general conversation so if you have a form that they can then fill in then you can see whether they’ve ticked it or not and bring it up that way.
1ST018
Quality of the therapeutic relationship
Many staff reported that it was necessary to establish trust and rapport with patients prior to discussing these types of side effects. This was often difficult in circumstances when patients were acutely unwell and required crisis intervention because of the short-term nature of the work. In contrast, a few inpatient staff suggested that it was easier to discuss with patients who were well known to them on the wards:
Someone’s likelihood to speak to you and have an open conversation about their sexual functioning will also very much depend on the relationship that the staff member has with the individual. Although we work as a team, as an MDT [multidisciplinary team], often cases particularly with females clients and patients build up rapport in particular with one individual . . . all patients will have one nurse that’s their favourite to go to talk to.
1ST031
Other staff perspectives
During the course of the interviews some staff made suggestions for future studies of sexual dysfunction among people with psychosis. Suggestions included staff training and studies examining add-on medications. Staff suggested that pharmacists, because they focus on side effects of medication, may find it easier to ask patients about these side effects. Others highlighted the importance of staff training and guidance to help facilitate discussions about sexual dysfunction.
Clinicians reported that it would be easier to start discussions about sexual dysfunction if they knew that switching medication provided an effective approach to helping people who experience these problems.
Some staff said that they already offered additional medication, such as sildenafil citrate (Viagra), and asked if this could be a topic for a future study. They suggested that offering additional medication could avoid some of the concerns they and their patients had about switching their antipsychotic medication:
When we have tried medications . . . with less side effects and these medications haven’t worked because [we] haven’t been able to control the psychic symptoms then we know we probably need something else, and the Viagra and other drugs maybe would be an alternative.
1ST028
Chapter 5 Discussion
Recruitment to this trial was very challenging. Over the course of an extended 12-month pilot trial we recruited only 10 participants. This led to the early termination of the study, and we were unable to collect sufficient data to test study hypotheses. We explored reasons why recruitment was so difficult in qualitative interviews with 51 members of staff and four patients who declined to take part in the study. Themes derived from these interviews highlighted the discomfort and embarrassment that many staff and patients felt when talking about sex. Staff also had concerns about talking to patients about side effects of antipsychotic medication and patients were uncomfortable about the idea of switching medication, especially if they felt that the one they were already taking had helped them achieve better mental health.
Staff reported finding it difficult to talk to people with psychosis about side effects of medication when patients were acutely unwell. They told us that the priority at this point was to help the patient achieve better mental health. However, staff said that, even when patients had achieved better mental health, they found it difficult to talk about sexual functioning because they were concerned how patients would experience this. Some believed that attempts to discuss sexual functioning could damage their relationship with a patient. Staff reported finding it especially difficult to talk to people of the opposite sex, to older adults and to people with religious beliefs that they felt made them less open to discussing sex. Staff were also worried about raising the topic with people who had a history of sexual abuse, people with paranoid thoughts and people with a history of sexual disinhibition or paraphilia. Most staff therefore waited for patients to raise the topic of sexual functioning themselves, although many recognised that patients were unlikely to do this. This meant that problems with sexual functioning often went undetected. Some staff preferred to avoid the topic of sexual dysfunction altogether. One consultant psychiatrist advised ‘none of my patients have these problems’.
Staff beliefs that patients can find it difficult to talk about sexual dysfunction were supported by (1) the poor response we had to direct marketing of the study and (2) the high proportion of people who were eligible to take part in the study but who declined to do so. Of 341 patients who were identified as potential candidates for the study, only eight responded to a letter from their clinical team with information about the study. None of these eight patients agreed to take part in the trial after they were provided with additional information about it. Despite the fact that as many as half of people with psychosis reported experiencing sexual dysfunction associated with use of antipsychotic medication,7 only five people contacted the study team having seen one of the many posters and leaflets that were distributed at clinics, day services and community support services for people with psychosis. None of these patients had schizophrenia or a related psychosis and were therefore ineligible to take part in the study.
Data from four qualitative interviews with patients and brief comments from 28 others who declined to take part suggest that, although sexual dysfunction is important, it is a problem that people often feel they can live with. Many of those interviewed were concerned about the impact that switching medication could have on their mental health or were worried about new side effects they might experience with the drug they were switched to. Others did not like the lack of control they would have about whether or not their medication was switched. A minority of patients wanted to have a change in their medication in an effort to improve their sexual functioning and were not prepared to take part in a study in which there might not be a change. These data highlight the barriers to recruiting to a study of the management of sexual dysfunction among people with psychosis, especially one that involves randomising people to a switch in their current medication.
Strengths and weaknesses
To the best of our knowledge, the REMEDY trial is the first trial in the UK to test the impact of an intervention aimed at reducing sexual dysfunction associated with use of antipsychotic medication. We used a broad range of strategies to publicise the study. We encouraged staff to refer potential participants to the trial by presenting it at local and regional meetings. Researchers attended clinical meetings to remind staff about the study and encouraged the use of screening tools for assessing sexual and other side effects of antipsychotic medication. A person with lived experience joined some of these meetings and talked to staff about the importance of sexual functioning for people with psychosis. We also used a broad range of methods to communicate directly with people with psychosis to let them know about the study.
The main limitation of the study was the small sample size, which meant that we were unable to test differences in study outcomes between those who were and were not offered a switch in medication. We do not know how successful we would have been had we tried to recruit participants at other services. After 9 months of failing to recruit to target at three Trusts we decided to open recruitment up in a fourth Trust that had expressed particular interest in the study. Part of the rationale for this was to check whether or not the difficulties we had recruiting study participants were specific to the places where we initially tried to recruit from. This fourth Trust had a track record of successfully recruiting to other clinical trials involving people with psychosis, and feedback from local clinicians was that they could recruit patients to the REMEDY trial. Although one participant was recruited over a 3-month period, the slow rate of recruitment at this fourth Trust and the findings from our qualitative interviews with staff suggest that the barriers to recruitment we experienced were ones that are likely to exist across secondary care mental health services in England.
Another limitation was the small number of qualitative interviews we completed with patients. It is inherently challenging to recruit people to take part in an in-depth interview when they had already indicated that they do not want to take part in a study. We attempted to overcome this limitation by gathering brief comments from all potential participants, including those who did not want to take part in an in-depth interview. We were able to collect brief comments from 28 patients who may have been eligible but declined to be interviewed. These comments supported the views of staff, that is, patients may not consider problems with sexual functioning to be a sufficiently important to risk the potential for negative effects of a switch in their medication. Others patients were anxious about not having a change in their treatment when they did want something to be done to try to improve their sexual functioning.
Comparison with previous literature
A recent systematic review5 of interventions for managing sexual dysfunction associated with antipsychotic medication identified six RCTs. The authors concluded that there was insufficient evidence to make recommendations for clinical practice based on the results of these trials. This was, in part, because previous studies have been small and underpowered. The six trials had between 10 and 50 participants, and the authors speculated that it ‘may be difficult to recruit participants with antipsychotic-related sexual dysfunction’ to clinical trials. 5 The results of the REMEDY trial provides further support for this suggestion and, to the best of our knowledge, for the first time, provide a rich source of data about possible reasons for this.
One of the main reasons for poor recruitment was that clinical staff found it difficult to ask patients about sexual functioning. A number of surveys have reported that clinical staff believe that sexual functioning is an important component of health and well-being, but they are reluctant to raise the subject with their patients. For instance, a survey of 100 mental health nurses in Sweden found that two-thirds believed that they had a responsibility to talk to patients about sexual concerns, but only 20% did so in practice. 65 Similar surveys in the UK also report that mental health nurses believe that it is important to discuss sexual functioning with patients. 66 By contrast, retrospective audits of clinical records find that patients are unlikely to be asked about sexual functioning unless they raise it themselves. 19,20 In-depth interviews with 14 mental health nursing staff members in the UK found that staff avoided talking to patients about sex because they did not think it is an important priority or believed that others were better placed to have these discussions. 67
When staff were able to talk to patients about sexual side effects of antipsychotic medication and identify potential participants, many patients were reluctant or unwilling to take part because of concerns about a possible switch in their medication. Very little research has examined the personal experiences of people prescribed antipsychotic medication. Research that has been conducted suggests that patient experiences are complex and that problems with side effects are a major concern. 68–70 Patients who feel settled on their medication may be fearful about the consequences of a change. 71 Such concerns are well founded given evidence that switching antipsychotic medication may increase the risk of relapse27,28 or cause new side effects. 3
Implications for future research
Although the costs and benefits of switching antipsychotic medication to help people with psychosis who experience sexual dysfunction associated with the use of these medications remain unclear, we are not able to recommend future studies of using this approach in the NHS because of the substantial problems we had with recruitment. Indeed, any trials of interventions to manage sexual dysfunction among people with psychosis are likely to be challenging unless there are changes within mental health services and in society at large to make it easier for staff and patients to talk about sex and sexual functioning.
Patient and staff concerns about switching antipsychotic medication were an important barrier to recruitment in this trial. Patients may be more willing to consider taking part in studies examining the use of adjunctive medication. Although adjunctive antipsychotic medications (e.g. aripiprazole) may lead to reductions in prolactin, which have the potential to improve sexual functioning,72 concerns about co-prescribing different antipsychotic drugs73 may limit the willingness of patients and staff to support such a study. There is evidence from a small randomised trial74 that phosphodiesterase inhibitors can improve sexual function among married men with schizophrenia who experience sexual dysfunction associated with antipsychotic medication. There is also evidence that phosphodiesterase inhibitors can improve sexual dysfunction among women in the general population. 75 If it were possible to overcome barriers to assessing sexual dysfunction among people with psychosis then consideration could be given to testing the acceptability, clinical effectiveness and cost-effectiveness of phosphodiesterase inhibitors.
Acknowledgements
We thank all those who took part in the study and clinical staff at Central and North West London NHS Foundation Trust; Cumbria, Northumberland, Tyne and Wear NHS Foundation Trust; Tees Esk and Wear Valleys NHS Foundation Trust; and West London NHS Trust for their help with completing the study, especially Kirsty Allen, Shakina Bellam, Daisy Mbwanda, Laxmi Patel, Abhishek Shastri and Chizoba Unigwe.
We thank the Clinical Research Network for publicising the study and supporting the recruitment and follow-up of study participants. We would especially like to thank Lauren Stevens, Susan Wilson, Harriet Martin and Mary-Josephine Doyle for their help with recruitment.
We are grateful to John Green and Channa Jayasena for contributing to the development of the brief psychoeducation and support that we used in the study and to Alan Quirk for advising on the qualitative component of the study.
We are grateful to all members of the Trial Steering Committee [Helen Killaspy (chairperson), Angela Etherington, Khalida Ismail, Richard Keers and David Shiers], the Data Monitoring and Ethics Committees [David Osborn (chairperson), Paul Bassett and David Kessler] and our Service User Panel (Manisha Ahya, John Clarke, A Fletcher, Robert Koch, Ivan Moore, Vickas Sharma, Jenny Trite and Niruben Patel) for their support and guidance.
The Imperial Biomedical Research Centre Facility, which is funded by the National Institute of Health Research, also provided support that has contributed to the research results reported in this paper.
Contributions of authors
Michael J Crawford (https://orcid.org/0000-0003-3137-5772) (Professor of Mental Health, Centre for Psychiatry, Imperial College London) is the chief investigator of the study and contributed to the design of the study, the study protocol and the preparation of this report.
Lavanya Thana (https://orcid.org/0000-0002-8343-5549) (Research Associate, Imperial College London) developed plans for the qualitative component of the study, contributed to the design of the study, led the analysis of qualitative data and contributed to the preparation of this report.
Rachel Evans (https://orcid.org/0000-0003-0960-9843) (Statistician, University of Bangor) led the development of the analysis plan for the study and the analysis of quantitative data and contributed to the preparation of this report.
Alexandra Carne (https://orcid.org/0000-0001-7526-8077) (Research Associate, Tees Esk and Wear Valleys NHS Foundation Trust) recruited and followed up study participants and contributed to the preparation of this report.
Lesley O’Connell (https://orcid.org/0000-0002-4580-0840) (Research Associate, Imperial College London) recruited and followed up study participants and contributed to the preparation of this report.
Amy Claringbold (https://orcid.org/0000-0001-5041-4621) (Senior Clinical Trials Co-ordinator, Imperial College London) co-ordinated the trial, contributed to the development of the study protocol and contributed to the preparation of this report.
Arunan Saravanamuthu (https://orcid.org/0000-0002-8924-724X) (Research Associate, Central and North West London NHS Foundation Trust) recruited and followed up study participants and contributed to the preparation of this report.
Rebecca Case (https://orcid.org/0000-0003-4080-4807) (Research Associate, Central and North West London NHS Foundation Trust) recruited and followed up study participants and contributed to the preparation of this report.
Jasna Munjiza (https://orcid.org/0000-0003-2368-9464) (Consultant Psychiatrist, Central and North West London NHS Foundation Trust; Honorary Senior Clinical Lecturer, Imperial College London) was the principal investigator and contributed to the conduct of the study and to the preparation of this report.
Sandra Jayacodi (https://orcid.org/0000-0001-5028-3465) (expert by experience) helped to publicise the study and interpret study findings and contributed to the preparation of this report.
Joseph G Reilly (https://orcid.org/0000-0002-2469-9878) (Consultant Psychiatrist, Tees Esk and Wear Valleys NHS Foundation Trust) was the principal investigator and contributed to the design and conduct of the study and to the preparation of this report.
Elizabeth Hughes (https://orcid.org/0000-0002-4480-0806) (Professor of Mental Health, University of Leeds) was the principal investigator and contributed to the design and conduct of the study and to the preparation of this report.
Zoe Hoare (https://orcid.org/0000-0003-1803-5482) (Principal Statistician, Senior Lecturer, University of Bangor) contributed to the design of the study and study protocol, to development of the analysis plan, to the analysis of quantitative data and to the preparation of this report.
Barbara Barrett (https://orcid.org/0000-0002-0270-6256) (Reader, King’s College London) developed plans for the economic evaluation, contributed to the design of the study and the study protocol and contributed to the preparation of this report.
Verity C Leeson (https://orcid.org/0000-0001-7548-8096) (Clinical Trials Manager, Imperial College London) contributed to the development of the study protocol and the preparation of this report.
Carol Paton (https://orcid.org/0000-0001-7756-1031) (Honorary Research Fellow, Imperial College London) was the lead pharmacist on the project and contributed to the design of the study, the study protocol and the preparation of this report.
Patrick Keown (https://orcid.org/0000-0003-4727-5880) (Honorary Clinical Senior Lecturer Newcastle University) was the principal investigator and contributed to the design and conduct of the study and the preparation of this report.
Sofia Pappa (https://orcid.org/0000-0002-6303-1547) (Consultant Psychiatrist, West London NHS Trust; Honorary Senior Clinical Lecturer, Imperial College London) was the principal investigator and contributed to the conduct of the study and the preparation of this report.
Charlotte Green (https://orcid.org/0000-0002-1781-3070) (expert by experience) led patient involvement in the study and helped interpret study findings and prepare this report.
Thomas RE Barnes (https://orcid.org/0000-0002-2324-656X) (Professor of Psychiatry, Imperial College London) contributed to the design of the study, the study protocol and the preparation of this report.
Data-sharing statement
Anonymised data can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Eack SM, Newhill CE. Psychiatric symptoms and quality of life in schizophrenia: a meta-analysis. Schizophr Bull 2007;33:1225-37. https://doi.org/10.1093/schbul/sbl071.
- Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol 2014;29:63-76. https://doi.org/10.1097/YIC.0b013e32836508e6.
- Barnes TR. Schizophrenia Consensus Group of British Association for Psychopharmacology . Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2011;25:567-620. https://doi.org/10.1177/0269881110391123.
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry 2013;14:2-44. https://doi.org/10.3109/15622975.2012.739708.
- Allen K, Baban A, Munjiza J, Pappa S. Management of antipsychotic-related sexual dysfunction: systematic review. J Sex Med 2019;16:1978-87. https://doi.org/10.1016/j.jsxm.2019.08.022.
- Macdonald S, Halliday J, MacEwan T, Sharkey V, Farrington S, Wall S, et al. Nithsdale Schizophrenia Surveys 24: sexual dysfunction. Case–control study. Br J Psychiatry 2003;182:50-6. https://doi.org/10.1192/bjp.182.1.50.
- Smith SM, O’Keane V, Murray R. Sexual dysfunction in patients taking conventional antipsychotic medication. Br J Psychiatry 2002;181:49-55. https://doi.org/10.1192/bjp.181.1.49.
- Young SL, Taylor M, Lawrie SM. ‘First do no harm.’ A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol 2015;29:353-62. https://doi.org/10.1177/0269881114562090.
- Baldwin D, Mayers A. Sexual side-effects of antidepressant and antipsychotic drugs. Adv Psychiatr Treat 2003;9:202-10. https://doi.org/10.1192/apt.9.3.202.
- de Boer MK, Castelein S, Wiersma D, Schoevers RA, Knegtering H. The facts about sexual (Dys)function in schizophrenia: an overview of clinically relevant findings. Schizophr Bull 2015;41:674-86. https://doi.org/10.1093/schbul/sbv001.
- Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs 2004;64:2291-314. https://doi.org/10.2165/00003495-200464200-00003.
- Stadler T, Bader M, Uckert S, Staehler M, Becker A, Stief CG. Adverse effects of drug therapies on male and female sexual function. World J Urol 2006;24:623-9. https://doi.org/10.1007/s00345-006-0136-5.
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44. https://doi.org/10.1001/jama.281.6.537.
- Mallis D, Moisidis K, Kirana PS, Papaharitou S, Simos G, Hatzichristou D. Moderate and severe erectile dysfunction equally affects life satisfaction. J Sex Med 2006;3:442-9. https://doi.org/10.1111/j.1743-6109.2005.00173.x.
- Ostman M. Low satisfaction with sex life among people with severe mental illness living in a community. Psychiatry Res 2014;216:340-5. https://doi.org/10.6/j.psychres.2014.02.009.
- Olfson M, Uttaro T, Carson WH, Tafesse E. Male sexual dysfunction and quality of life in schizophrenia. J Clin Psychiatry 2005;66:331-8. https://doi.org/10.4088/jcp.v66n0309.
- Rosenberg KP, Bleiberg KL, Koscis J, Gross C. A survey of sexual side effects among severely mentally ill patients taking psychotropic medications: impact on compliance. J Sex Marital Ther 2003;29:289-96. https://doi.org/10.1080/00926230390195524.
- Finn SE, Bailey JM, Schultz RT, Faber R. Subjective utility ratings of neuroleptics in treating schizophrenia. Psychol Med 1990;20:843-8. https://doi.org/10.1017/s0033291700036539.
- Singh SP, Beck AJ. ‘No sex please we’re British’ – taking a sexual history from in-patients. Psychiatr Bull 1997;21:99-101. https://doi.org/10.1192/pb.21.2.99.
- Barnes T, Paton C. The Prescribing Observatory for Mental Health 10-Year Report: Supporting Rational, Effective and Safe Prescribing in Mental Health Services 2016.
- Crawford MJ, Shaw T. How do psychiatric out patients feel about the sexual history?. Psychiatr Bull 1998;22:365-7. https://doi.org/10.1192/pb.22.6.365.
- Bo Q, Dong F, Li X, Wang Z, Ma X, Wang C. Prolactin related symptoms during risperidone maintenance treatment: results from a prospective, multicenter study of schizophrenia. BMC Psychiatry 2016;16. https://doi.org/10.1186/s12888-016-1103-3.
- Mir A, Shivakumar K, Williamson RJ, McAllister V, O’Keane V, Aitchison KJ. Change in sexual dysfunction with aripiprazole: a switching or add-on study. J Psychopharmacol 2008;22:244-53. https://doi.org/10.1177/0269881107082901.
- Byerly MJ, Lescouflair E, Weber MT, Bugno RM, Fisher R, Carmody T, et al. An open-label trial of quetiapine for antipsychotic-induced sexual dysfunction. J Sex Marital Ther 2004;30:325-32. https://doi.org/10.1080/00926230490465082.
- Kinon BJ, Ahl J, Liu-Seifert H, Maguire GA. Improvement in hyperprolactinemia and reproductive comorbidities in patients with schizophrenia switched from conventional antipsychotics or risperidone to olanzapine. Psychoneuroendocrinology 2006;31:577-88. https://doi.org/10.1016/j.psyneuen.2005.12.006.
- Byerly MJ, Nakonezny PA, Rush AJ. Sexual functioning associated with quetiapine switch vs. risperidone continuation in outpatients with schizophrenia or schizoaffective disorder: a randomized double-blind pilot trial. Psychiatry Res 2008;159:115-20. https://doi.org/10.1016/j.psychres.2007.02.014.
- Essock SM, Covell NH, Davis SM, Stroup TS, Rosenheck RA, Lieberman JA. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163:2090-5. https://doi.org/10.1176/ajp.2006.163.12.2090.
- Faries DE, Ascher-Svanum H, Nyhuis AW, Kinon BJ. Clinical and economic ramifications of switching antipsychotics in the treatment of schizophrenia. BMC Psychiatry 2009;9. https://doi.org/10.1186/1471-244X-9-54.
- Berner MM, Hagen M, Kriston L. Management of sexual dysfunction due to antipsychotic drug therapy. Cochrane Database Syst Rev 2007;1. https://doi.org/10.1002/14651858.CD003546.pub2.
- McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry 1991;48:764-70. https://doi.org/10.1001/archpsyc.1991.01810320088015.
- NHS Choices . Sexual Health n.d. www.nhs.uk/live-well/sexual-health/?tabname=sex-facts (accessed 4 August 2020).
- Casey DE, Carson WH, Saha AR, Liebeskind A, Ali MW, Jody D, et al. Aripiprazole Study Group . Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology 2003;166:391-9. https://doi.org/10.1007/s00213-002-1344-3.
- Serretti A, Chiesa A. Sexual side effects of pharmacological treatment of psychiatric diseases. Clin Pharmacol Ther 2011;89:142-7. https://doi.org/10.1038/clpt.2010.70.
- National Institute for Health and Care Excellence (NICE) . Schizophrenia: Core Interventions in the Treatment and Management of Schizophrenia in Adults in Primary and Secondary Care 2009.
- Prescribing Observatory for Mental Health-UK . Antipsychotic Dosage Ready Reckoner–Version 6.1 n.d. www.cntw.nhs.uk/content/uploads/2015/09/PPT-PGN-10-App1-Ready-Reckoner-V06.1-BNF.pdf (accessed 12 June 2020).
- Joint Formulary Committee . British National Formulary 2018.
- Mitchell KR, Mercer CH, Ploubidis GB, Jones KG, Datta J, Field N, et al. Sexual function in Britain: findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Lancet 2013;382:1817-29. https://doi.org/10.1016/S0140-6736(13)62366-1.
- McGahuey CA, Delgado PL, Gelenberg AJ. Assessment of sexual dysfunction using the Arizona Sexual Experiences Scale (ASEX) and the implications for the treatment of depression. Psychiatr Ann 1999;29:39-42. https://doi.org/10.3928/0048-5713-19990101-10.
- Fenton KA, Johnson AM, McManus S, Erens B. Measuring sexual behaviour: methodological challenges in survey research. Sex Transm Infect 2001;77:84-92. https://doi.org/10.1136/sti.77.2.84.
- van Strien AM, Keijsers CJ, Derijks HJ, van Marum RJ. Rating scales to measure side effects of antipsychotic medication: a systematic review. J Psychopharmacol 2015;29:857-66. https://doi.org/10.1177/0269881115593893.
- Nakhli J, El Kissi Y, Bouhlel S, Amamou B, Nabli TA, Nasr SB, et al. Reliability and validity of the Arizona sexual experiences scale – Arabic version in Tunisian patients with schizophrenia. Compr Psychiatry 2014;55:1473-7. https://doi.org/10.1016/j.comppsych.2014.04.006.
- Nunes LVA, Mann L, Lacaz FS, Bressan R, Matsuo T, de Jesus Mari J. The accuracy of the Arizona Sexual Experience Scale (ASEX) to identify sexual dysfunction in patients of the schizophrenia spectrum. Rev Psiquiatr Clin 2009;6:182-9. https://doi.org/10.1590/S0101-60832009000500002.
- Guy W. Assessment Manual for Psychopharmacology – Revised. Rockville, MD: United States Department of Health, Education and Welfare; 1976.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76. https://doi.org/10.1093/schbul/13.2.261.
- Ohlsen RI, Williamson RJ, Yusufi B, Mullan J, Irving D, . Interrater reliability of the Antipsychotic Non-Neurological Side-Effects Rating Scale (ANNSERS). Psychopharmacology 2008;22:323-9. https://doi.org/10.1177/0269881108091069.
- Byerly MJ, Nakonezny PA, Rush AJ. The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophr Res 2008;100:60-9. https://doi.org/10.1016/j.schres.2007.12.470.
- Barrett B, Byford S, Crawford MJ, Patton R, Drummond C, Henry JA, et al. Cost-effectiveness of screening and referral to an alcohol health worker in alcohol misusing patients attending an accident and emergency department: a decision-making approach. Drug Alcohol Depend 2006;81:47-54. https://doi.org/10.1016/j.drugalcdep.2005.05.015.
- Kind P, Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott-Raven; 1996.
- Keetharuth A, Brazier JE, Connell J, Carlton J, Taylor Buck L, Ricketts T, et al. Development and Validation of the Recovering Quality of Life (ReQoL) Outcome Measures. York: University of York; 2017.
- Russell D, Hoare ZSJ, Whitaker R, Whitaker CJ, Russell IT. Generalised method for adaptive randomisation in clinical trials. Stat Med 2011;30:922-34. https://doi.org/10.1002/sim.4175.
- Chen CY, Lin TY, Wang CC, Shuai HA. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci 2011;65:95-7. https://doi.org/10.1111/j.1440-1819.2010.02156.x.
- Crawford MJ, Killaspy H, Barnes TR, Barrett B, Byford S, Clayton K, et al. Group art therapy as an adjunctive treatment for people with schizophrenia: multicentre pragmatic randomised trial. BMJ 2012;344. https://doi.org/10.1136/bmj.e846.
- Barrett B, Waheed W, Farrelly S, Birchwood M, Dunn G, Flach C, et al. Randomised controlled trial of joint crisis plans to reduce compulsory treatment for people with psychosis: economic outcomes. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0074210.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2019.
- Turner J, O’Cathain A, Knowles E, Nicholl J, Tosh J, Sampson F, et al. Evaluation of NHS 111 Pilot Sites. Sheffield: University of Sheffield; 2012.
- Department of Health and Social Care (DHSC) . National Schedule of Reference Costs 2017–18 2018.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Keetharuth AD, Brazier J, Connell J, Bjorner JB, Carlton J, Taylor Buck E, et al. Recovering Quality of Life (ReQoL): a new generic self-reported outcome measure for use with people experiencing mental health difficulties. Br J Psychiatry 2018;212:42-9. https://doi.org/10.1192/bjp.2017.10.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Mulhern B, Mukuria C, Barkham M, Knapp M, Byford S, Soeteman D, et al. Using generic preference-based measures in mental health: psychometric validity of the EQ-5D and SF-6D. Br J Psychiatry 2014;205:236-43. https://doi.org/10.1192/bjp.bp.112.122283.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- James Lind Alliance . Schizophrenia Top 10 n.d. www.jla.nihr.ac.uk/priority-setting-partnerships/schizophrenia/top-10-priorities/ (accessed 22 June 2020).
- World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4.
- Saunamäki N, Andersson M, Engström M. Discussing sexuality with patients: nurses’ attitudes and beliefs. J Adv Nurs 2010;66:1308-16. https://doi.org/10.1111/j.1365-2648.2010.05260.x.
- Cort EM, Attenborough J, Watson JP. An initial exploration of community mental health nurses’ attitudes to and experience of sexuality-related issues in their work with people experiencing mental health problems. J Psychiatr Ment Health Nurs 2001;8:489-99. https://doi.org/10.1046/j.1351-0126.2001.00425.x.
- Quinn C, Happell B, Browne G. Talking or avoiding? Mental health nurses’ views about discussing sexual health with consumers. Int J Ment Health Nurs 2011;20:21-8. https://doi.org/10.1111/j.1447-0349.2010.00705.x.
- Carrick R, Mitchell A, Powell RA, Lloyd K. The quest for well-being: a qualitative study of the experience of taking antipsychotic medication. Psychol Psychother 2004;77:19-33. https://doi.org/10.1348/147608304322874236.
- Moncrieff J, Cohen D, Mason JP. The subjective experience of taking antipsychotic medication: a content analysis of Internet data. Acta Psychiatr Scand 2009;120:102-11. https://doi.org/10.1111/j.1600-0447.2009.01356.x.
- Gray R, Deane K. What is it like to take antipsychotic medication? A qualitative study of patients with first-episode psychosis. J Psychiatr Ment Health Nurs 2016;23:108-15. https://doi.org/10.1111/jpm.12288.
- Murawiec S, Rajewska-Rager A, Samochowiec J, Kalinowska S, Kurpisz J, Krzyzanowska J, et al. Pharmacy switch of antipsychotic medications: patient’s perspective. Ann Gen Psychiatry 2015;14. https://doi.org/10.1186/s12991-015-0066-y.
- Shim JC, Shin JG, Kelly DL, Jung DU, Seo YS, Liu KH, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry 2007;164:1404-10. https://doi.org/10.1176/appi.ajp.2007.06071075.
- Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs 2011;25:383-99. https://doi.org/10.2165/11587810-000000000-00000.
- Gopalakrishnan R, Jacob KS, Kuruvilla A, Vasantharaj B, John JK. Sildenafil in the treatment of antipsychotic-induced erectile dysfunction: a randomized, double-blind, placebo-controlled, flexible-dose, two-way crossover trial. Am J Psychiatry 2006;163:494-9. https://doi.org/10.1176/appi.ajp.163.3.494.
- Gao L, Yang L, Qian S, Li T, Han P, Yuan J. Systematic review and meta-analysis of phosphodiesterase type 5 inhibitors for the treatment of female sexual dysfunction. Int J Gynaecol Obstet 2016;133:139-45. https://doi.org/10.1016/j.ijgo.2015.08.015.
List of abbreviations
- ANNSERS
- Antipsychotic Non-Neurological Side Effects Rating Scale
- ASEX
- Arizona Sexual Experience Scale
- BARS
- Brief Adherence Rating Scale
- CDSS
- Calgary Depression Scale for Schizophrenia
- CGI
- Clinical Global Impression
- EQ-5D
- EuroQol-5 Dimensions
- GP
- general practitioner
- Natsal-3
- National Survey of Sexual Attitudes and Lifestyles
- PANSS
- Positive And Negative Syndrome Scale
- REMEDY
- Randomised Evaluation of Management of sExual DYsfunction
- ReQoL
- Recovering Quality of Life
- SD
- standard deviation
