Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/201/09. The contractual start date was in November 2014. The draft report began editorial review in March 2019 and was accepted for publication in November 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Sue Jowett is (from 2016 to present) a member of the National Institute for Health Research Health Technology Assessment Clinical Trials Committee and reports personal fees as an independent advisor at the Pfizer (Pfizer Inc., New York, NY, USA) chronic pain advisory board meeting in November 2018, outside the submitted work. Kate M Dunn reports grants from the Wellcome Trust (London, UK), during the conduct of the study.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Foster et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
The term sciatica is commonly used to describe symptoms of pain radiating from the lower back to the leg, often extending to the foot. 1,2 Patients may also have other leg symptoms such as pins and needles, numbness or leg muscle weakness. 1,2 Occasionally, there may not be any lower-back pain (LBP), and, commonly, the leg pain is worse than the LBP. 1,2 The most common reason for sciatica is compression or irritation of a lumbar spinal nerve root by a prolapsed or bulging disc; less often, it is due to tightening of the central or lateral spinal canal (spinal stenosis). 1,2 Some clinicians prefer to use only the terms lumbar nerve root pain, lumbar radicular pain or radiculopathy, to distinguish it from non-specific, referred leg pain, which may arise from spinal structures such as muscles, ligaments or facet joints, rather than compression of a nerve root. 3 However, the term sciatica is still used widely to indicate symptoms arising from lumbar nerve root compression, mainly from a disc prolapse,4 and this is the term used throughout this report.
There is significant variation in sciatica prevalence estimates in published studies, from 1.2% to 43%,5 depending on each study’s diagnostic criteria and sampling methods. 5–7 In clinically evaluated general population cohorts, the point prevalence of sciatica was estimated to be 4.8%. 8,9 In a 2015 primary care cohort in the UK, presenting with back and leg pain and attending a clinic for assessment, 74% of participants were suspected to have sciatic pain. 10 Although imaging tests were not used to inform the initial clinical diagnosis in the UK cohort, the prevalence estimate shows that sciatica is commonly encountered or suspected in primary care. 10
Research on characteristics potentially associated with outcome in sciatica has identified a limited number of prognostic factors independently associated with outcome, mainly in studies of secondary care cohorts. 11–14 Only pain and condition-specific disability are consistently associated with having spinal surgery, which, in this secondary care context, is taken as a proxy of poor outcome for natural course and conservative management. 13 In the UK primary care cohort of patients with suspected sciatica previously mentioned, the high impact of sciatic pain on patients and their expectation of non-improvement over time, were independently associated with non-improvement. 14
It is generally believed that, for most patients with sciatica, symptoms improve considerably within 12 weeks of onset, usually without any active treatment. 1,2 Results from both clinical trials and cohort studies involving sciatica populations and utilising a number of different outcomes and definitions of improvement show that the percentage of patients reporting improvement varies between 32% and 65%. 14–18 Therefore, at least one-third of patients continue to suffer with pain for ≥ 1 year. The personal, social and economic burdens are significant,19,20 with UK annual costs estimated at £268M in direct medical costs and £1.9B in indirect costs. 21
Treatments for, and current clinical management of, sciatica
The vast majority of patients with sciatica in the UK are assessed and managed in primary care. Current evidence,22–24 the updated UK National Institute for Health and Care Excellence (NICE) guidance (2016)25 for the treatment of LBP and sciatica in those aged > 16 years and the National Low Back and Radicular Pain Pathway 201726 all support mainly a stepped-care management approach for most patients with sciatica, in the absence of suspected serious pathology. NICE25 recommends considering the use of stratification tools when assessing patients with sciatica to decide on the intensity of conservative management. Current management options may include advice about staying active; non-steroidal anti-inflammatory drugs (NSAIDs); weak opioid or neuropathic pain medications; physiotherapy interventions, such as exercise, with additional options of manual therapy techniques; or psychological therapies. Most sciatica patients will improve over time and will not require specialist care or invasive management options. However, for patients with severe and/or non-resolving sciatica, steroid spinal epidural injections and/or surgery are recommended for pain relief, in the presence of concordant imaging findings, for example from magnetic resonance imaging (MRI). Of all the available treatments for sciatica, surgery has the most robust evidence from research studies; it provides rapid and substantial relief of pain, but outcomes for those having surgery and those managed conservatively have been shown to be similar in the longer term, with approximately one-fifth of patients reporting ongoing complaints, which fluctuate over time, irrespective of which treatment is received. 27 On average, although patients receiving surgical intervention improve rapidly and substantially, they still seem to report mild to moderate pain and disability 5 years after the surgery;28 however, evidence also shows that increased pre-treatment symptom duration is related to worse outcomes following both conservative and surgical management than receiving treatment earlier in a patient’s symptom presentation. 29
In practical terms, stepped care may include an initial ‘wait-and-see’ period in primary care, with advice and pain medication, with subsequent referral to physiotherapy or similar treatments/services (e.g. osteopathy, chiropractic), and, for those not improving or with severe and incapacitating pain, referral to specialist spinal services at the interface and/or the secondary care setting for investigations and further management. 26 In the UK NHS, it is in these specialist settings where treatment options such as spinal injections and/or surgery are considered. 26 However, an immediate referral of all sciatica patients to injections or surgery is not considered a cost-effective model of care,22,23,30 and it is unlikely that it is needed for all patients. Currently, only patients with possible serious spinal pathology are fast-tracked to specialist services. 25,26 For all other sciatica patients, there is variation in clinical practice in terms of referrals from general practice to physiotherapy and specialist services. The UK Spinal Taskforce31 highlighted problems in the management of sciatica caused by variation in clinical practice, specifically delays in the treatment of patients with severe pain, believed to be mainly caused by delays in referral to specialist services. The Spinal Taskforce31 highlighted the need for good-quality trial evidence on the clinical effectiveness and cost-effectiveness of early referral of patients with severe symptoms for consideration of treatments such as surgery or spinal epidural injections. As early referral to surgical services for all sciatica patients cannot be recommended, better, and earlier, identification of patient subgroups in primary care for early matched treatment pathways [stratified care (SC)], including early referral to spinal specialist services for some sciatica patients, may be the key to improving outcomes for sciatica patients. The challenge for primary care clinicians [e.g. general practitioners (GPs) and physiotherapists] is how to identify, early in the presentation, patients who are likely to do well with conservative management in primary care and patients who may need early, fast-track referral to spinal specialist opinion for consideration of more invasive management options, such as spinal injections and surgery.
Stratified care model and rationale for the SCOPiC trial
In the field of non-specific LBP, a SC approach comprising two components – first, subgrouping according to risk of persistent back pain-related disability and, second, matching each subgroup of patients to treatment – demonstrated better clinical and health economic outcomes than non-SC. 32,33 In summary, the approach uses a brief self-report questionnaire, the Subgroups for Targeted Treatment (StarT) Back Screening Tool,34 developed for and validated with primary care patients with non-specific LBP (with and without leg pain), and allocates LBP patients to subgroups of low, medium or high risk of persistent back pain-related disability. The Subgroups for Targeted Treatment (STarT) Back Screening Tool has nine items: four focus on physical constructs and five focus on psychological constructs. A score of < 3 indicates that the patient is, most likely, at a low risk of future persistent back pain-related disability; a score of ≥ 4 of the five psychological items indicates that the patient is, most likely, at a high risk of persistent disability. 34 Any other score identifies patients as being at a medium risk of persistent disability. 34 By estimating the risk of persistent back pain-related disability, the STarT Back Screening Tool supports early clinical decision-making about conservative treatments for patients with non-specific LBP (such as GP care and physiotherapy management). Patients with sciatica have been shown to have more severe symptoms than those with non-specific LBP;20 management options such as injections and spinal surgery may be applicable to sciatica, but are not applicable to non-specific LBP. A SC approach for patients consulting with suspected sciatica in primary care may result in improved outcomes, but evidence is lacking.
Prior to the Sciatica Outcomes in Primary Care (SCOPiC) trial, no tool has yet been developed and tested with which to stratify care specifically for patients with sciatica early in the presentation of symptoms. In particular, it was not possible to predict which patients might benefit from early consideration for invasive treatment such as surgery. 35 In a cohort of sciatica patients with at least 6 weeks’ duration of symptoms, all of whom were surgical candidates, only high levels of pain and disability were associated with having surgery at some point. 12 The lack of clear and consistent factors predicting poor outcomes in patients with sciatica11,13,14 has made it challenging to design prognostic models that can guide early treatment decision-making. 13
In the absence of stratification approaches to help with clinical decision-making in early primary care management of patients with sciatica, we used information about the characteristics of patients referred to specialist spinal services from the only available UK primary care cohort of sciatica patients. 10 Using this, and working closely with clinicians, we developed an algorithm combining information about patients’ risk of persistent pain-related disability (using the STarT Back Screening Tool) and key findings from the clinical assessment that were associated with referral to spinal specialist services to allocate patients to matched care pathways, including an early, fast-track referral to MRI and specialist spinal opinion. 21 The stratification algorithm is described in Chapter 2.
Responding to the National Institute for Health Research (NIHR) Health Technology Assessment programme commissioning call (12/201, February 2013) about research to improve the management and outcomes of patients with sciatica, the aim of this study was to test, in a definitive randomised trial, whether or not the use of a new stratification algorithm and the matched care pathways lead to better outcomes than non-stratified, usual care (UC) for patients consulting with suspected sciatica in primary care.
The SCOPiC trial aims and objectives
The overall aim of this trial was to investigate whether or not a new SC approach for sciatica that combines information on the risk of persistent disability with information about sciatica clinical severity to guide decision-making about matched care pathways results in better clinical and cost outcomes for patients consulting their GP with symptoms of suspected sciatica.
The two main aims were to investigate the:
-
clinical effectiveness of SC versus non-SC for patients consulting with suspected sciatica in primary care, in terms of patient-reported time to symptom resolution
-
cost-effectiveness of SC versus non-SC over a 12-month period.
Further objectives included investigating the clinical effectiveness of SC versus non-SC on sciatica-related disability, LBP, leg pain, quality of life, time lost from work, health-care use and patient satisfaction with care received, and the results of care. In addition, we investigated the impact of SC on service delivery, in terms of referrals to physiotherapy services and spinal specialist services, and the acceptability of this SC approach from the perspective of patients and clinicians.
Chapter 2 The SCOPiC trial methods
Design and setting
The SCOPiC trial was a multicentre, pragmatic, assessor-blinded, randomised controlled trial (RCT), with an internal pilot health economic evaluation and linked qualitative interviews. The trial’s setting included NHS primary care services (general practices), community physiotherapy services and spinal interface services between primary and secondary care.
An economic evaluation was conducted alongside the RCT to evaluate the cost-effectiveness of the intervention over 12 months (see Chapter 4). A nested qualitative study was conducted to explore patients’ and clinicians’ views of the fast-track care pathway associated with the SC model tested in this trial (see Chapter 5).
General practices
We recruited patients from 42 general practices in the areas of North Staffordshire, North Shropshire/Wales and Cheshire. These areas were the three recruiting centres. We initially started with 30 general practices and then added a further 12 practices over the course of the trial, to recruit the number of participants required in the trial.
Physiotherapy sites
Five community NHS physiotherapy services were involved in the trial: two in Staffordshire, one in Shropshire, one in Wales and one in Cheshire. We initially started with three physiotherapy services (those in Staffordshire and Shropshire), then added a further two (in Wales and Cheshire) over the course of the trial.
Spinal specialist sites
Patients recruited to the fast-track pathway were seen by spinal specialists in either Staffordshire (Staffordshire and Stoke-on-Trent Partnership NHS Trust), Shropshire (The Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Trust) or Cheshire (Leighton Hospital, Mid Cheshire Hospitals NHS Trust).
Ethics approval
The trial was registered on the International Standard Randomised Controlled Trial Number (ISRCTN) registry (ISRCTN75449581). Ethics approval was granted by the National Research Ethics Service West Midlands – Solihull (reference number 15/WM/0078). Recruitment commenced in June 2015 and was completed in July 2017. The Trial Steering Committee (TSC) and Data Monitoring Committee (DMC) provided independent oversight of the trial.
Participants
We recruited adults consulting in general practice with symptoms of sciatica of any duration and severity.
Inclusion criteria
Patients were eligible to participate in the trial if the following criteria were satisfied:
-
aged ≥ 18 years
-
had a mobile phone that could receive and send texts or had access to a landline telephone
-
had consulted in general practice with back and/or leg symptoms, and the GP suspected sciatica
-
following clinical assessment in research clinics, the diagnosis of sciatica was confirmed with at least 70% diagnostic confidence by a physiotherapist
-
able to read and communicate in English (to give full informed consent and to complete the baseline and outcome assessments)
-
willing to participate.
Exclusion criteria
Patients were not eligible for participation in the trial if they:
-
had suspected serious spinal pathology or ‘red flags’ (e.g. cauda equina syndrome, progressive/widespread neurological deficit, spinal cord compression, suspicion of malignancy, infection, fractures, inflammatory spondyloarthropathy)
-
had had any previous lumbar spine surgery
-
were currently receiving ongoing care from, or had been in consultation with, a secondary care doctor or physiotherapist for the same problem in the previous 3 months
-
had serious comorbidity preventing them from attending the research clinic and/or being able to undergo assessment and interventions
-
had a severe, enduring mental health condition
-
were pregnant
-
were taking part, at the same time, in another research study relating to symptoms of back and/or leg pain (sciatica).
Interventions
Stratified care (trial arm)
We developed and utilised a stratification algorithm to direct the SC model tested in the SCOPiC trial. The algorithm was used to identify those sciatica patients likely to need a fast-track referral from primary care to specialist spinal services, those who might benefit from a course of physiotherapy treatment and those who may require only minimal intervention to support self-management. The algorithm combined prognostic information, using the STarT Back Screening Tool,34 and information on the following clinical examination findings: level of interference with ability to work (including work around the house), pain below the knee, intensity of leg pain and sensory changes in the painful leg such as loss of, or reduced, pin-prick sensation approximating a dermatomal distribution. The algorithm allocated patients to one of three groups and each group was matched with a care pathway. The matched care for participants in group 1 comprised one or two physiotherapy sessions. Participants in group 2 received a course of up to six physiotherapy sessions; the number and content of treatment sessions was tailored to participants’ individual needs. Participants in group 3 were fast-tracked to have imaging of the lumbar spine (MRI) and to see a spinal specialist, with the MRI results, for further assessment and management. The treatments offered to participants in group 3 (the fast-track pathway) after their appointment at the spinal specialist clinic formed part of current NHS care and were not influenced by the trial’s procedures. All participants in the SC arm received initial advice from the trial’s physiotherapists at the SCOPiC trial research clinic. Full details of the stratification algorithm and care pathways and treatments are given in Description of interventions.
Non-stratified care, usual care (control arm)
The control arm intervention of the SCOPiC trial was based on non-stratified UC, delivered by physiotherapists who were not involved in the delivery of the SC arm of the trial. All participants randomised to the UC arm received their first treatment in the SCOPiC trial research clinic. This included a one-off session of advice and education. If required, the treating physiotherapist could arrange a referral to the local NHS community physiotherapy or interface specialist spinal services, or discharge the patient to the care of their GP. Data were collected on referrals made and subsequent treatments received. The use of any stratification tools was prohibited for participants in the UC arm for the duration of the trial. This was agreed in advance with physiotherapy service managers and physiotherapists in the participating sites, and in sites that expected to see participants in the trial as part of UC.
Trial procedures
Recruitment methods
Two methods were used to identify potentially eligible participants for the SCOPiC trial: (1) electronic ‘pop-up’ prompts in general practice computer software fired by appropriate Read codes (the Read code system is a clinical coded dictionary used for recording consultations in the UK primary care information technology systems)36 for back and/or leg pain and (2) weekly reviews of practice consultation records for those practices not using electronic ‘pop-ups’, with the list of potential participants screened by the GP who identified patients who should be excluded. Patients consulting with a health-care professional (HCP) other than a GP at a general practice (e.g. a practice nurse) could also be recruited using the aforementioned recruitment methods. The list of Read codes used were checked with each general practice. Initially, only Read codes indicative of sciatica were included, but some general practices requested the addition of generic LBP Read codes as well, because a number of GPs used the LBP codes to record sciatica presentations. The list of Read codes used for the trial are presented in Appendix 1.
In the method using electronic ‘pop-up’ prompts, when a patient with back and/or leg pain consulted their GP, and the GP entered an appropriate Read code on the computer system, a ‘pop-up’ screen asked the GP if they thought that the patient might have sciatica, and, if so, to consider whether or not the patient was suitable (yes/no) to be invited to the SCOPiC trial sciatica clinic, taking into account trial inclusion/exclusion criteria. By entering ‘yes’ on the computer system, those patients thought to be suitable for invitation to the clinic were flagged. The GP could briefly inform the patient about the clinic and the study, although this was not a requirement in order to send invitations to potential participants. In addition to using the ‘pop-up’ method for participant identification, the ‘pop-up’ screen asked GPs to record their preferred approach to management of the patient: (1) keep patient under GP care, (2) refer patient to physiotherapy or (3) refer patient to a specialist. This information was intended for use in the SCOPiC trial research clinics for the UC arm treatment decisions, if appropriate, and for descriptive purposes, to compare GP decision-making about management options at first consultation with sciatica patients, versus physiotherapist decision-making in the SCOPiC trial research clinic.
Once a potential participant had been identified, with either method, an invitation letter (on general practice-headed notepaper) and an information sheet about the SCOPiC trial sciatica clinic were posted to them. The letter explained that there was a research study in sciatica and potentially eligible participants were invited, if interested, to telephone an administrator to make an appointment at the SCOPiC trial sciatica clinic to see a physiotherapist. During that telephone call, the administrator carried out a brief check for preliminary eligibility for the trial, to establish the following: presence of leg pain, aged ≥ 18 years, ability to communicate in English, not currently receiving treatment for the same problem by a physiotherapist or secondary care doctor, had not seen a secondary care doctor or physiotherapist for the same problem in the previous 3 months, not currently participating in any other back pain and/or sciatica research study, had not undergone lumbar spine surgery and was not pregnant. Potentially eligible participants receiving or having had received care from alternative or complementary health-care practitioners, such as osteopaths, chiropractors or acupuncturists, were not excluded but were advised, if possible, to keep co-treatments to a minimum during the treatment phase of the trial. The reasons for decline/exclusion were documented. Potential participants were offered a clinic appointment within 10 working days. A letter was then sent to potential participants confirming their appointment details; the pack included the participant information sheet, explaining the SCOPiC trial, and the baseline questionnaire. Approximately 2 days before the clinic appointment, potential participants were telephoned by a clinic administrator to remind them about their appointment and to ask those who were interested in taking part in the trial to bring their completed questionnaire.
Baseline assessment, eligibility screening and informed consent
The SCOPiC trial sciatica clinics were operated as integrated research and NHS service clinics. At these clinics, trial physiotherapists explained the purpose of the clinic and answered questions about the clinic and the trial. Potential participants expressing interest in participating in the trial proceeded to have a standardised clinical assessment by the physiotherapist to confirm the clinical diagnosis of sciatica. The assessment was documented on a standard pro forma. In the context of the trial and according to literature37,38 and clinical guidelines,25 sciatica is, in most cases, indicative of nerve root entrapment due to a disc prolapse, and, less frequently, due to spinal stenosis. 1 Therefore, as well as patients with symptoms thought to be due to disc prolapse, we also included patients with symptoms thought to be due to spinal claudication. Eligibility for the trial was based on the assessing physiotherapist being ≥ 70% confident in their diagnosis of sciatica. We also asked the assessing physiotherapists to record a specific diagnosis of disc prolapse or stenosis for the sciatic symptoms. At least one of the following signs and symptoms contributing to the clinical diagnosis of sciatica7,39,40 had to be present: leg pain approximating a dermatomal distribution; leg pain worse than, or as bad as, back pain; leg pain worse with coughing/sneezing/straining; subjective sensory changes approximating a dermatomal distribution; objective neurological deficits indicative of nerve root compression; positive neural tension test; and (specifically for spinal claudication/spinal stenosis) leg pain worse with weight-bearing activities and better with sitting. Patients were excluded if the assessing physiotherapist thought that the leg pain was a result of causes other than sciatica (e.g. referred leg pain, hip pathology, vascular claudication), if diagnostic certainty was < 70% or if potentially serious pathology was suspected (e.g. malignancy, cauda equina syndrome). All reasons for exclusion were documented.
For those patients who were eligible and interested in taking part in the trial, the trial physiotherapist explained the trial in detail, answered any questions and took written informed consent. The information required from self-report and clinical examination to allocate patients to one of the three groups based on the stratification algorithm was recorded by the assessing physiotherapist on a standard pro forma. Patients had completed and brought with them the baseline questionnaire, or completed the questionnaire during the research clinic visit, along with a second baseline questionnaire. The clinic administrator checked the baseline questionnaires for missing data while the patient was in the clinic.
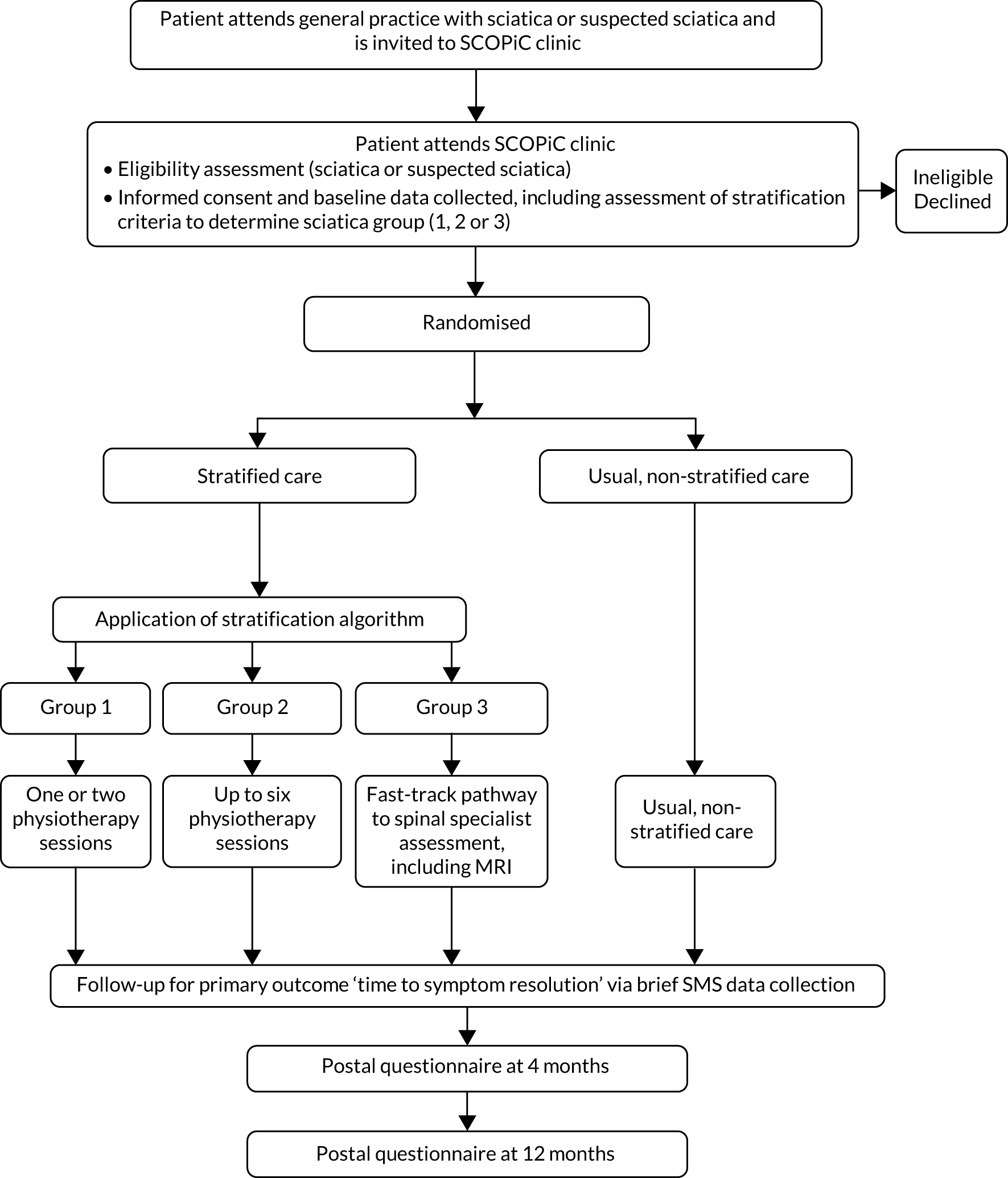
Patients who did not wish to participate or who were ineligible received advice and education from the physiotherapist in the clinic, with the option to be referred for further treatment as appropriate, outside the trial. GPs received information (by a standard letter generated by the trial management database) of the outcome of their patients’ attendance to the SCOPiC trial research clinic, and their care plan. Figure 1 outlines the SCOPiC trial recruitment procedures.
FIGURE 1.
The SCOPiC trial recruitment procedures.
Randomisation
Eligible patients who consented to take part were randomised to one of the two trial arms, in a 1 : 1 ratio, using a web-based randomisation service from Keele Clinical Trials Unit (CTU), which ensured allocation concealment, operated by the clinic administrator. There was a back-up process to telephone the CTU randomisation service in the event of web access failure. The assessing physiotherapist gave the clinic administrator the completed standard pro forma, which included the sciatica group the participant was allocated to according to the stratification algorithm. In each recruiting centre, the clinic administrator used the randomisation service and individual participants were randomised, stratified by centre (North Staffordshire, North Shropshire/Wales and Cheshire) and group allocation according to the stratification algorithm, using random permuted blocks of varying size (two, four and six), to either SC or UC. With three centres and three groups equating to nine stratified cells, participants were randomised in block sequences with random selection from the following blocks: AB, BA, AABB, ABAB, ABBA, BAAB, BABA, BBAA, AAABBB, AABABB, AABBAB, AABBBA, ABAABB, ABABAB, ABABBA, ABBAAB, ABBABA, ABBBAA, BAAABB, BAABAB, BAABBA, BABAAB, BABABA, BABBAA, BBAAAB, BBAABA, BBABAA, BBBAAA.
If a participant was randomised to the SC arm of the trial, the administrator informed the assessing trial physiotherapist, who saw the participant and commenced the care matched to each group. If a participant was randomised to the UC arm of the trial, the administrator informed the physiotherapist delivering care for the UC arm, who saw the patient and initiated treatment. Different physiotherapists delivered treatment to participants in each trial arm, at the research clinic and during subsequent appointments. Information about which arm of the trial a participant was allocated to was not disclosed to either the participant or their GP. A participant’s GP was informed in writing that the individual was participating in the trial, but GPs were not informed about which arm of the trial the participant had been randomised to. Usual clinician-to-clinician correspondence continued as per usual practice; the research did not interfere with this. Each participating general practice had a site file containing contact details of the trial team.
Blinding
The main issue in the SCOPiC trial was the concealment of the stratification algorithm and its use in matching participants to care pathways. Participants were told that the trial was comparing two primary care approaches for the treatment of sciatica: one based on matching patients to treatment using a simple tool that helps to decide on the treatment pathway most likely to help them and one based on the treatment needed as discussed and agreed by the physiotherapist and themselves. To further help with allocation concealment, participants randomised to the UC arm of the trial were seen by physiotherapists who had not carried out their detailed clinical assessment and eligibility screen for the trial. Therefore, physiotherapists in the UC arm remained blinded to both the details of the stratification algorithm and the individual patient’s sciatica group status, hence avoiding contamination between the two trial arms. It was not possible for physiotherapists treating participants in the SC arm to remain blinded. To further protect against contamination, no physiotherapists treating participants in the SC arm were involved in the treatment of participants in the UC arm. Research nurses blinded to treatment allocation conducted the minimum data collection (over the telephone) at 4 and 12 months’ follow-up for participants who did not respond to questionnaires. The risk of contamination from GPs knowing to which arm a participant was randomised was deemed to be very low. In qualitative interviews with the GPs (as part of the nested qualitative interviews), we collected data on whether or not involvement in the trial contributed to changes in GPs’ referral decisions for patients with sciatica (see Chapter 5). Initially, we planned to collect information about GP referral patterns before and during the trial, using anonymised general practice electronic records, to check whether or not trial participation influenced referral patterns for sciatica patients, but this was not technically possible to do.
Statisticians and health economists were blinded to treatment arm during the development of the statistical analysis plan (SAP) (the SAP was agreed in advance with the TSC members) and through monitoring phases of the trial, including the interim data analysis specified in the internal pilot phase of the trial. Furthermore, two statisticians performed the primary analysis of the main trial independently and both were blinded to treatment arm allocation. The trial statistician carrying out further key analyses of primary and secondary outcomes was blinded up to the point of conducting the per-protocol analysis (a sensitivity analysis). Treatment allocation was stored in a secure computer server accessible only by authorised CTU staff, for whom blinding was not relevant. For the quantitative data analysis, throughout the monitoring period, the treatment arm variable was blind dummy-coded until the key primary and secondary data had been analysed.
Follow-up
For the primary outcome, time to resolution of sciatica symptoms, data collection via text messages occurred weekly for the first 4 months for all participants, starting on the first Sunday following the participant’s randomisation at the SCOPiC trial research clinic. Then, between 4 and 12 months’ follow-up, the text message data collection changed to once every 4 weeks, or until ‘stable resolution’ of symptoms (stable resolution was defined as 2 consecutive months’ responses of ‘completely recovered’ or ‘much better’; see Outcome measures and assessments for details on the primary outcome). Once stable resolution occurred, data collection for the primary outcome via text message, beyond the first 4 months, ceased. Non-responders to the first week’s text message received a reminder text 48 hours later, and those who still did not respond were mailed a postcard the next day. Non-responders to the second week’s text message received a reminder message 48 hours later, and those who still did not respond received a telephone call from a research nurse after at least a further 24 hours. For subsequent non-response reminders, the processes described above were repeated. For those participants not providing any response using text messages, there was an option to transfer to data collection by brief telephone call with a research nurse. A small number of participants chose to receive only phone calls for collection of the primary outcome (n = 49).
The secondary clinical outcomes and health economic outcomes were collected at 4 and 12 months using participant self-completed postal questionnaires. Participants who did not respond to the postal questionnaires were sent a reminder postcard after 2 weeks of non-response. After a further 4 weeks, participants received a repeat full postal questionnaire and a short version if they had not responded by 6 weeks. If a total of 8 weeks had passed and no data had been received, the participant was contacted by a research nurse blinded to treatment allocation to collect a minimum data set over the telephone.
Quality assurance
For quality assurance purposes, we implemented monitoring procedures to ensure that all personnel involved in the trial adhered to the trial’s protocol and procedures. All physiotherapists involved in taking participant consent were observed at least once by a senior member of the research team. All physiotherapists involved in eligibility assessment were observed at least once in the research clinic. Administrators involved in the preliminary eligibility criteria checking over the telephone were also observed by a senior member of the research team. Standardised pro formas were used for all trial documentation, to minimise variation in trial procedures. We did not encounter significant problems during quality control monitoring.
Outcome measures and assessments
Baseline
Participants completed a self-report questionnaire at baseline (the questionnaire was posted to participants and they were asked to complete it and bring it with them to the clinic; if they forgot, they were asked to complete a baseline questionnaire in the SCOPiC trial clinic). Following final eligibility screening in the SCOPiC trial clinic, eligible participants completed a second questionnaire, in the SCOPiC trial clinic, prior to randomisation. Demographic data were collected via the baseline questionnaire, and baseline information on analgesic medication was collected during the clinical assessment for eligibility, and recorded on a standard pro forma. Clinical data were also collected prior to randomisation, as part of the eligibility process.
Follow-up
Follow-up for the primary outcome, time to symptom resolution, was carried out via text messages (or brief telephone calls if participants preferred this). Secondary outcomes were collected via postal questionnaires at 4 and 12 months from the baseline clinic appointment.
Clinical outcomes
Primary outcome
The primary outcome measure, collected with text messages, was time to first resolution of symptoms of sciatica, measured on a six-point ordered categorical scale: ‘completely recovered’, ‘much better’, ‘better’, ‘same/no change’, ‘worse’ and ‘much worse’. The scale’s anchor was a participant’s baseline symptoms when he/she attended the SCOPiC trial research clinic. The text message read: ‘Compared to how you were at the SCOPiC clinic X weeks/months ago, how are your back and leg symptoms today?’. This outcome, whereby participants are asked about the change in symptoms compared with baseline assessment, is commonly used in primary care research of musculoskeletal disorders, and was used in a trial comparing early surgery with conservative care for patients with severe sciatica. 4 Patient-reported resolution of symptoms was defined as a response of either ‘completely recovered‘ or ‘much better‘.
Secondary outcomes
We collected secondary clinical outcomes at 4 and 12 months using participant self-completed postal questionnaires, to evaluate pain intensity, function, psychological health, general health status, work status, days lost from work, work productivity loss due to sciatica, satisfaction with care and care results, and quality of life. We also collected information on adverse events (AEs). Resource use information for the duration of the follow-up period of 12 months was also collected for the health economic analysis. This included self-reported information on primary care consultations (GPs and practice nurses, physiotherapists), secondary care consultations (e.g. hospital consultants), prescriptions, hospital-based tests and procedures (investigations such as MRI and blood tests, and procedures such as spinal epidural injections and spinal surgery for sciatica), nature and length of inpatient stays, over-the-counter purchases by participants and out-of-pocket expenses. Participants were asked to distinguish between UK NHS and private provision. Table 1 summarises the demographic characteristics collected, the domains measured, the measures used and the time points of measurement.
| Outcome measure | Instrument | Time points (months) | ||
|---|---|---|---|---|
| 0 | 4 | 12 | ||
| Global perceived change | Six-point Likert scale | ✓ | ✓ | |
| Physical disability | Modified RMDQ for sciatica41 | ✓ | ✓ | ✓ |
| Sciatica symptoms | SBI42 | ✓ | ✓ | ✓ |
| Pain intensity (usual pain) | NRS for back and leg pain43 | ✓ | ✓ | ✓ |
| Sleep interference | Jenkins Sleep Questionnaire44 | ✓ | ✓ | ✓ |
| Risk of poor outcome | STarT Back Screening Tool34 | ✓ | ✓ | ✓ |
| Anxiety and depression | HADS45 | ✓ | ✓ | ✓ |
| Fear of movement | TSK46 | ✓ | ✓ | ✓ |
| Neuropathic symptoms | S-LANSS47 | ✓ | ✓ | ✓ |
| Employment | Questions on employment status and work absence (days) | ✓ | ✓ | ✓ |
| Presenteeism (productivity) | Performance at work: single question with NRS response (0–10 scale) | ✓ | ✓ | ✓ |
| General health | Short Form 148 | ✓ | ✓ | ✓ |
| AEs | Identified by clinicians and through patient self-report via their questionnaires | ✓ | ✓ | |
| Patient satisfaction with care and results of care | 5-point scale | ✓ | ✓ | |
| Economic measures | EQ-5D-5L49 | ✓ | ✓ | ✓ |
| Health-care use questions | ✓ | ✓ | ||
Process outcomes
Process outcomes were collected to investigate the impact of SC on service delivery for both physiotherapy and specialist spinal services. With the use of case report forms (CRFs), data were collected, in each arm of the trial, on the number of participants referred to physiotherapy services, the number of physiotherapy sessions received, treatments provided and the number of participants referred to specialist spinal services and/or secondary care settings. The timing of referral and treatment were also captured, when possible. In addition to CRFs, we captured information on health-care use from participant questionnaires and hospital record reviews from participating NHS specialist services.
Description of the interventions
Stratification algorithm
Figure 2 shows the stratification algorithm used to direct participants’ care in the stratified arm of the trial. The algorithm utilises information on the risk of a poor prognosis from the STarT Back Screening Tool and information on factors associated with referral to spinal specialists to allocate participants with clinically diagnosed sciatica to one of three sciatica groups, each matched to a care pathway, with one of the care pathways being fast-track referral for MRI and to a consultation with a spinal specialist for opinion. We briefly describe here the decisions and methods utilised to derive the stratification algorithm. Full details of the development and internal validation of the stratification algorithm for primary care patients with sciatica are available in a separate peer-reviewed paper. 50
FIGURE 2.
Stratification algorithm for allocating patients to groups and matched care pathways.
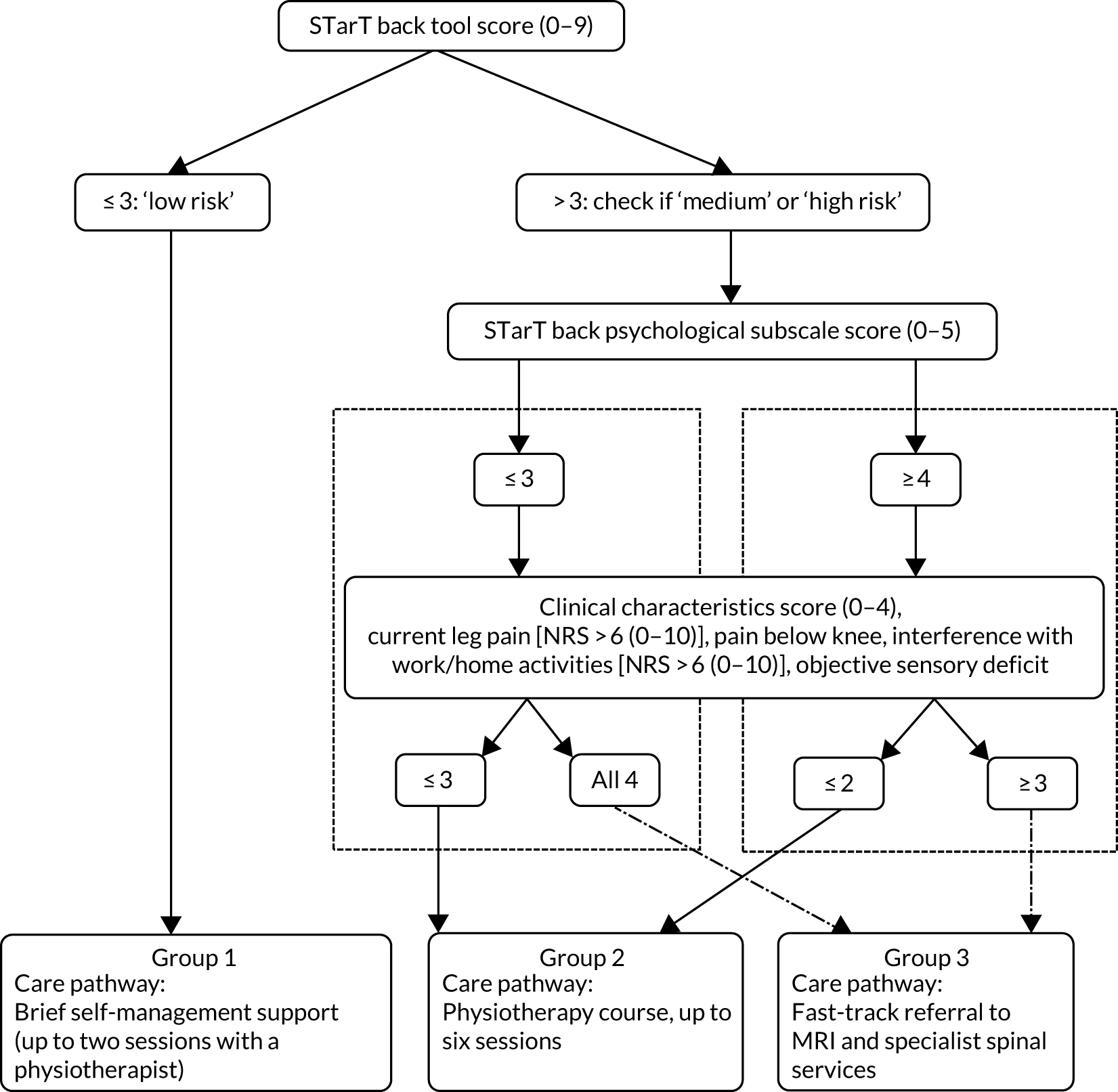
To identify the group of sciatica participants likely to need fast-track referral to imaging tests and a spinal specialist assessment and opinion, we used data on patients with a sciatica clinical diagnosis from the Assessment and Treatment of Leg pain Associated with the Spine (ATLAS) study,10 a prospective, treatment cohort of primary care patients with back and leg pain. The ATLAS cohort included patients with symptoms of any duration and severity, and excluded patients with potential serious pathology. All patients without contraindications to MRI had the scan after their baseline clinical assessment. In the development of the algorithm, ATLAS study participants clinically diagnosed with sciatica with diagnostic confidence of ≥ 70% (as reported by the assessors) were included. The outcome definition was referral to NHS spinal specialist services (yes/no) at some point over the course of 12 months’ follow-up in the ATLAS study. Potential predictors of referral to specialist services that were informed from the literature more broadly and that were available in the ATLAS study data set comprised self-report and clinical examination findings. The selected factors were categorised into five domains: impact of condition, pain levels/symptoms, psychological factors, symptom behaviour and presentation (e.g. increased leg pain with coughing, reporting of numbness in the leg), and clinical examination findings. Logistic regression (univariable and multivariable) was used to determine the association between each factor and the outcome of referral to spinal specialist services. The factors derived from the statistical models as being most strongly associated with referral to specialist services, within each domain and overall, were discussed with clinical and research experts (epidemiologists, triallists, statisticians, spinal surgeons, spinal physiotherapy specialists, rheumatologists, pain specialists, GPs). The final list of factors thought to be most relevant to the decision to refer to spinal specialists was agreed by all clinical stakeholders, and included the following: effect of lower-back and/or leg pain (sciatica) on ability to do one’s job or ability to do jobs around the house [Numerical Rating Scale (NRS) of 0–10, with a cut-off point of > 6], current leg pain intensity (NRS of 0–10, with a cut-off point of > 6), sensory deficits in a dermatomal distribution recorded during the clinical examination (yes/no) and pain below the knee (yes/no). The first three factors were derived from the statistical analyses. The binary cut-off points (NRS scores of > 6) for impact and pain intensity were considered to be reasonable thresholds for pain and functional limitations, and had face validity when considering early referral to specialists. Presence of pain below the knee was subsequently added to the list as it is considered the best proxy indicator of leg pain due to nerve root involvement;37 the clinical experts considered this to be important to combine with the other factors to guide referral decisions for patients with sciatica.
Data from the ATLAS study cohort showed that only one participant (out of 52) with a STarT Back Screening Tool score of up to 3 (out of a total of 9), indicative of low risk of poor prognosis, was referred to spinal specialist services. On the basis of that, patients at a low risk of poor prognosis (STarT Back Screening Tool score of up to 3), irrespective of clinical characteristics, were not considered for referral to specialists or for a full course of physiotherapy management. For the remaining participants, we considered a number of possibilities in terms of combinations of factors from the clinical assessment and information on risk of poor prognosis, using the STarT Back Screening Tool score, for identifying which participants with sciatica should be fast-tracked to spinal specialist services. For each combination of factors, we considered the implications for sensitivity in terms of identifying observed referrals, and feasibility and practicality in relation to use in clinical practice. For all scenarios, sensitivity/specificity, positive/negative predictive values and percentage of the sample fast-tracked were calculated. Based on the results of this analysis, participants at a medium risk of poor prognosis who had all four clinical characteristics described above, and participants at a high risk of poor prognosis with any three of the clinical characteristics, were allocated to group 3 and matched to the care pathway of fast-track referral to MRI and specialist opinion. The remaining participants were allocated to group 2 and matched with a course of physiotherapy management of up to six sessions. Sensitivity, specificity and positive predictive value (PPV) of the algorithm for participant allocation to the fast-track pathway (group 3) were 51%, 73% and 22%, respectively. The algorithm, applied to the ATLAS study cohort, allocated 12% of participants to group 1, 57% to group 2 and 31% to group 3.
Stratified care
Group 1
Participants in this group were expected to have an overall good prognosis and to require only brief support for self-management. However, in contrast with participants with non-specific LBP who have a low risk of poor outcome (according to the STarT Back Screening Tool), patients with sciatica tend to have more severe symptoms; therefore, we decided to offer a brief intervention delivered by physiotherapists for this group of participants. The recommended physiotherapy treatment input was to provide up to two 30-minute sessions, with a target of delivery of 4 weeks, in order to permit review when needed, but no further sessions were advised. The biopsychosocial paradigm guided the care delivered by the physiotherapists, which was tailored to the individual participant’s presentation and specific needs to support self-management and reduce disability. This included advice, information, appropriate reassurance and education about sciatica and focused on the expected good outcome without the need for further tests or investigations. It reinforced the maintenance of activity levels, including return to work, when appropriate; lifestyle advice such as general activity and weight control, as appropriate; and guidance on self-management and the management of future flare-ups of sciatica. Pain relief and discussion about appropriate medication were also part of the treatment, and any suggested changes in analgesia were communicated to the participant and their GP for consideration. A sciatica booklet, developed from existing educational materials, was given to participants along with an information sheet of local contacts for exercise venues, such as swimming pools, exercise classes and physical activity opportunities.
Group 2
Participants allocated to this group received a course of physiotherapy treatment, tailored to their individual needs. This matched treatment package was delivered in up to six sessions of 30 minutes each, over a suggested period of 6–12 weeks. The number and content of treatments were tailored to each participant’s individual needs. The main aims of treatment were to reduce pain and disability and address psychological obstacles to recovery. The STarT Back Screening Tool and clinical assessment findings guided the treating physiotherapist in targeting management towards the physical and psychosocial factors that were found to be particular problems for each patient. Management plans included some or all of the following: advice, explanation, reassurance and education, medication review and advice (suggestions on analgesia were communicated to the participant and their GP for consideration), exercise (e.g. McKenzie ‘directional preference exercises’, muscle-strengthening stability exercises, general fitness and mobility exercises, and guidance on pacing using graded activity principles as applicable), manual therapy techniques (joint and/or soft tissue), advice about and plans for return to normal activities and work as appropriate, and guidance on self-management and the management of future flare-ups. Psychological obstacles to recovery, such as fear-avoidance beliefs, pain-related low mood and distress or anxiety, unhelpful or erroneous beliefs about back pain and sciatica, and catastrophising were also addressed as part of the physiotherapy treatment. The sciatica booklet and information sheet of local contacts for exercise venues, as mentioned in Group 1, were also given to participants in group 2.
Care delivery for groups 1 and 2
The care pathways for groups 1 and 2 were delivered in primary care by NHS physiotherapists. Participants were able to access care via their GP, as per normal. Physiotherapists treating participants in group 1 were able to break protocol and see a participant for more than the recommended two treatment sessions, or refer them to specialist spinal services if they strongly felt that this was clinically necessary. Similarly, physiotherapists treating participants in group 2 could refer a participant to specialist spinal services if it was deemed clinically necessary. Protocol deviations first had to be discussed with the team’s consultant spinal physiotherapist and co-author (KK), and documented.
Group 3
Participants allocated to group 3 were fast-tracked for lumbar spine MRI, providing there were no contraindications to MRI, and for an appointment at the spinal interface service to see a specialist for further assessment and opinion about management. The results and report of the MRI were available to the specialist at the time of the clinic appointment. All MRI was reported by an NHS consultant radiologist. The participant was expected to have the MRI and their appointment at the spinal interface service within 4 weeks from randomisation. Participants with contraindications to MRI were still referred to the spinal interface services to see the spinal specialist, who would decide on alternative imaging tests as necessary.
In the SCOPiC trial, the fast-track pathway to the spinal interface services was for a specialist assessment and opinion about clinical management. After a participant’s assessment at the spinal interface clinic, any referrals required to other services, such as spinal orthopaedics, injections, pain clinic or physiotherapy services, were at the discretion of the treating clinician. The waiting time to treatments offered by those services could not be influenced by the fast-track pathway; however, by ensuring that suitable participants joined these waiting lists earlier than in the UC arm, participants were expected to have these treatments (if they were suitable for them and they wanted to have them) sooner than participants in the UC arm. The spinal interface services in the participating NHS services were specialist clinics at the primary/secondary care interface. In the centres participating in the SCOPiC trial, these services are predominantly delivered by extended scope spinal physiotherapy specialists, who have close links with secondary care spinal services, such as orthopaedics. Specialist physiotherapists working in the spinal interface services collaborating in delivering the SCOPiC trial assessed participants allocated to group 3.
Non-stratified usual care
The control arm of the trial was based on non-stratified primary care and was delivered by different physiotherapists to those who delivered the SC arm of the trial. Patients attending the research clinic and randomised to the UC arm were seen by a physiotherapist in the clinic for an initial physiotherapy session. Then the physiotherapist, in consultation with the patient, decided on further management. Options included discharge back to the care of their GP, referral to community physiotherapy services for further treatment or referral to specialist spinal services. As mentioned previously, no stratification tool was used in the physiotherapist decision-making and delivery of treatment for patients in the UC arm. The physiotherapist was aware of the information on the management options stated by the GP, but did not have to follow the recommendation.
Training and auditing
All personnel (i.e. administrators, trial physiotherapists for intervention and control arms, research nurses) involved in the trial received training in the trial’s procedures and documentation. For audit purposes, treatments delivered to participants in the intervention and control arms of the trial were recorded in a standardised format on CRFs. The information recorded included dates of start and completion of treatment, number of treatments received and types of interventions (i.e. exercise, advice, manual therapy) and the physiotherapist’s clinical grade (NHS Agenda for Change banding). Protocol deviations in both trial arms were recorded and reported. Physiotherapy record reviews were conducted in the cases of missing or incomplete CRFs for both SC and UC participants.
Details of the care of participants in group 3, the fast-track pathway (e.g. referrals for physiotherapy or surgical or injection procedures), were collected from the clinical letters generated in the specialist clinics for each participant and recorded on CRFs. Following the 12-month follow-up, data on treatments actually received for sciatica and the time frame of any interventions delivered were supplemented by reviews of the hospital records at the participating NHS trusts whenever possible. Given that participants could choose to have treatments in other NHS sites, we anticipated that we would not be able to retrieve hospital record data for all participants.
Physiotherapists’ training processes
Physiotherapists who participated in the trial attended training workshops prior to the start of recruitment and treatment. All participating physiotherapists had expertise in assessing and treating musculoskeletal problems, including previous training in the assessment and management of psychological obstacles to improvement, as part of their normal practice. Those delivering the treatment session in the SCOPiC trial clinic for participants in the control arm (eight physiotherapists at NHS band 6, and two at grade 7) took part in a half-day workshop that focused on trial procedures, the importance of avoiding contamination between trial arms and the completion of trial CRFs.
Those physiotherapists involved in the assessment of patients’ eligibility for the SCOPiC trial, who determined the sciatica group status of participants (according to the stratification algorithm) and who delivered matched care pathways in the SC arm attended 3 days of training. Nine physiotherapists at band 6, five at band 7 and six at band 8a participated. Training focused on the standardised assessment to identify sciatica patients for participation in the trial, the stratification algorithm, taking informed consent, the delivery of evidence-based physiotherapy interventions in line with the biopsychosocial model of care and the procedures of the trial, the importance of avoiding contamination between arms, and details of how to complete the trial’s CRFs.
The training was supplemented by comprehensive written material on the trial procedures, practice guidelines and evidence-based management options for the assessment and treatment of patients with sciatica. To maximise protocol fidelity, physiotherapists providing the matched care pathways for participants in groups 1 and 2 had support as required (face to face, over the telephone or by e-mail), provided by the research team’s spinal physiotherapy specialists from the NHS spinal interface services participating in the trial. One refresher training session of 3 hours was held approximately 4 months after the trial commenced.
Adverse events
It was considered unlikely that participants in the SCOPiC trial would be at risk of serious adverse events (SAEs). The trial investigated the approach of matching appropriate care pathways to three groups of sciatica patients. The treatments themselves, in each of the three care pathways, were all part of routine clinical practice. SAEs were defined as those that resulted in death, unscheduled hospitalisation or significant disability as a result of the trial’s interventions or procedures. Any SAEs were documented in CRFs and reported immediately to the trial team. Any SAEs that were considered to be related to the trial procedures or interventions were reported to the Research Ethics Committee by the chief investigator within 15 days of the chief investigator becoming aware of the event, and to the TSC and DMC. We also documented SAEs during hospital records reviews, carried out as part of the trial’s data collection procedures.
Possible potential AEs included worsening of symptoms, for example as a result of an exercise programme prescribed by the physiotherapist. Physiotherapists treating participants in the SCOPiC trial were asked to report potential AEs on the CRFs or to the trial team. Participants were also asked about AEs in the follow-up questionnaires.
Sample size justification
A total sample size of 470 participants was required to test for superiority of SC compared with UC, to detect a hazard ratio (HR) of between 1.4 and 1.5 for time to resolution of symptoms (primary outcome) with 80–90% power (given a two-tailed significance level of 5%), assuming an event (resolution) rate of ≥ 60%, a 20% drop-out rate and intraclass correlation (ICC) for clustering by physiotherapist at the level of 0.01 and allowing for a coefficient of variation in physiotherapist cluster size of 0.65 (Eldridge et al. 51). The sample size allows for a least conservative HR of 1.4 in median survival times with 90% power (if all participants in the trial are recovered by the 12-month follow-up and ICC for physiotherapist effect is < 0.001), and a most conservative HR of 1.5 in median survival times with 80% power (if 60–65% of participants in the trial are recovered by the 12-month follow-up and ICC for physiotherapist effect is 0.01, given an average cluster size of around 12–15).
In this context, a HR of > 1 (denoting a higher ‘successful’ event rate) is a positive outcome.
The sample size of 470 would also provide > 80% power to detect a ‘small’ to ‘moderate’ standardised mean difference (effect size) of 0.3552 between the two trial arms in respect of sciatica-related physical disability [measured using the Roland–Morris Disability Questionnaire (RMDQ), a key secondary outcome], at the 12-month follow-up, allowing for physiotherapist effect and 20% attrition. The trial was not powered to detect differences between the sciatica groups between the trial arms.
Internal pilot phase
The internal pilot phase of the trial was designed to assess participant recruitment and follow-up rates over the first 8 months of recruitment; the success of general practice recruitment and retention; the success of physiotherapy site recruitment, including training and engagement; adherence to the treatment protocols; the proportion of participants allocated to each of the three groups according to the stratification algorithm; the time to MRI and specialist opinion for those in the fast-track pathway (group 3); the event rate of the primary outcome; and rate of missing data (text messages) for the primary outcome up to the 4-month follow-up for all participants recruited during the 8 months of the pilot trial phase.
The following were set as criteria for progression to the main trial from the internal pilot phase, and were agreed with the DMC and TSC:
-
recruitment rate of ≥ 70% of that anticipated; specifically, ≥ 90 patients would need to be recruited over the first 8 months of recruitment (where 130 would have been expected across the centres, taking into account staggered general practice recruitment and set-up)
-
overall loss to follow-up including non-response and dropouts (e.g. withdrawals, deaths, departures) in the primary outcome measure (resolution of symptoms) not exceeding 25% (based on 4 months’ follow-up of participants recruited during the first 7 months).
In the first success criterion, the expected number of 130 participants recruited in the trial for the duration of the internal pilot phase was based on the total number of 470 adults who were to be recruited from approximately 30 general practices over a 22-month recruitment period (or approximately 0.7 participants recruited per practice per month). Recruitment of centres was staggered; active recruitment was expected to start with the first centre (North Staffordshire), from five general practices, during the first month of recruitment, then increase at the first centre with an additional 10 practices by end of the second month. Recruitment would then start at the second centre (North Shropshire) from five practices by end of the third month, and increase at the second centre with an additional 10 practices by end of month 4, with full patient recruitment thereafter. Therefore, expected patient recruitment was (practices × months recruiting in first 8 months × recruitment rate):
The internal pilot did not involve formal interim analysis of between-group effects on the primary or any other outcomes. A review of the internal pilot phase data was undertaken by the Trial Management Group (TMG) and shared with the DMC, which reported their recommendations on progression to the TSC and funder.
Data management
Databases
Electronic data for the trial were stored in a Microsoft SQL Server (Microsoft Corporation, Redmond, WA, USA) database, hosted in a secure infrastructure at Keele CTU. Access to patient-identifiable data was regulated using predefined roles and privileges, and restricted views of the data ensured that authorised trial team members could see only the data required to carry out their role. Data access and entry was fully auditable within this system.
Data verification
Data-checking was carried out on all variables to identify data entry errors and missing data. Any identified errors were cross-checked against returned questionnaires. All manually entered data were verified through a random 10% double-entry validation process (across all returned baseline and follow-up questionnaires). The data entry was considered valid if complete agreement of data entry was verified across ≥ 90% of the (randomly chosen) test questionnaires and if any errors were spread across several variables (as opposed to being present on certain variables). In the case of consistent errors limited to one or a few variables, the team investigated and put in place an appropriate strategy for data entry for the variable(s) in question. If > 10% of questionnaires had one or more data entry errors, then necessary training and re-entry of data were carried out (and further verification checks put in place). All discrepancies were investigated and corrected prior to the final analysis.
For the primary outcome, all data from the text responses were electronically transmitted to the secure patient database. Any telephone responses were manually entered into the patient database by one assessor, blind to intervention allocation.
Missing data
The number of missing data for the primary outcome measure (perceived change in symptoms) was reported by trial arm. Primary data were utilised to the point of ‘resolution of symptoms’ (’completely recovered’ or ‘much better’) or censoring. Participants with no available outcome data were ‘right-censored’ at week 1, and withdrawals/dropouts were ‘right-censored’ on the day they last provided data. In the primary analysis, any missing primary outcome data prior to a recording of ‘resolution of symptoms’ were treated as indicative of no ‘resolution’ of symptoms.
The degree of missingness of primary outcome data was assessed by calculating two statistics, stratified by trial arm. First, the percentage of complete cases (those patients providing full data up to the point of resolution of symptoms or the end of the follow-up period) was reported. Second, we derived the completeness of follow-up (expressed as a percentage): the total observed person-time follow-up relative to the potential person-time follow-up. 53 Both statistics were assessed against baseline characteristics to check the missingness pattern.
Statistical analysis
All analyses were conducted and reported following the Consolidated Standards of Reporting Trials (CONSORT) guidelines. 54–56 The primary analysis was by ‘intention to treat’ (ITT), with analysis being carried out as per randomised allocation.
The Consolidated Standards of Reporting Trials flow diagram
The CONSORT flow diagram shows the numbers and percentages of participants recruited to the trial. It gives the details of preliminary and full eligibility assessment and exclusion reasons at each stage, details the numbers (and percentages) of participants in the two arms of the trial and according to sciatica group (1, 2 or 3) and the follow-up numbers, including the numbers providing data via full questionnaires and via minimum data collection processes.
Baseline data analyses
Participants are described by trial arm with respect to baseline sociodemographic and health-related characteristics (including randomisation stratification variables). Numerical variables are summarised by their mean values [and standard deviation (SD)] or median [and interquartile range (IQR)], depending on skewness of the distribution. Categorical variables are summarised through presentation of their frequency counts and per cent per category (calculated using the number of participants for whom data were available as the denominator). There are no tests of statistical significance of baseline variables between trial arms.
At the suggestion of the TSC, we included comparisons, at baseline, of differences in group allocation (using the stratification algorithm) numbers (percentages) by trial arms, between the three recruitment centres (North Staffordshire, North Shropshire/Wales and Cheshire), by cross-tabulating group by recruitment centre. At the TSC’s suggestion, to explore reasons for any differences, we examined, first, the association between group and socioeconomic status of the participants from each centre (based on the National Statistics Socio-economic Classification57 derived from the job title), and second, area-level deprivation of the participants in each centre.
Primary analysis
The primary analysis was a time-to-event analysis comparing the times to self-reported resolution of symptoms (’completely recovered’ or ‘much better’) between stratified care and usual care arms over 12 months of participant follow-up. The Kaplan–Meier survival analysis estimated the time from randomisation until reporting of first resolution of sciatica symptoms. A life-table review was also carried out to show cumulative event rate over discrete periods. Participants who dropped out of the trial through active withdrawal were censored at the time interval of occurrence, whereas participants who did not respond at any time point continued to be followed up until the point of any notification of withdrawal. From the Kaplan–Meier analysis, we derived and compared the relative mean and median survival times of the two trial arms. A Cox proportional hazards regression analysis was carried out comparing the time to resolution between trial arms by estimating the HR for the rate of resolution along with 95% confidence interval (CI) estimates (and corresponding p-values), adjusted for centre, sciatica group (stratifying variables) and pain duration (fixed effects), and accounting for clustering by physiotherapist (frailty/random effect). A p-value of < 0.05 (two-tailed), based on the Wald test statistic, signified rejection of the null hypothesis of no difference in the rate of recovery across time between the two trial arms. A p-value of < 0.05 along with a HR of > 1 indicated a statistically significant shorter time to recovery (and increased recovery event rate) for the trial arm (SC) than for the control arm (UC). By contrast, a statistically significant p-value (p < 0.05) with a HR of < 1 indicated a (statistically) significantly longer time to recovery for the trial arm (and lower recovery event rate) than for the control arm. The primary analysis was double-analysed by two statisticians working independently (from the source data) following the final agreed SAP [with any differences resolved through consensus agreement and involvement of a third independent statistician (blinded to treatment arm allocation), if needed].
Secondary analysis
Intention-to-treat analyses of between-group differences in secondary outcomes at 4 and 12 months were carried out using longitudinal mixed-effect regression models, as appropriate to the outcome data being analysed (linear regression for numerical measures and logistic regression for categorical measures), adjusting for centre, group (stratifying variables) and pain duration (fixed effects), and accounting for clustering by physiotherapists (random effect). Time-by-trial arm interactions were included, as well as time-by-(baseline) covariates to account for potential attrition bias. Descriptive summaries of mean scores and frequency counts or per cent (as appropriate to the data) are presented for the two trial arms. For the between-trial arm comparisons, mean differences (numerical outcomes) and odds ratios (ORs) (categorical outcomes) are presented, along with 95% CIs and p-values for the test of statistical association.
Per-protocol analysis
Participants in the SC arm who did not receive the matched care pathways were excluded; the remaining sample formed the per-protocol analysis. The physiotherapy session at the initial research clinic was not included in the calculation of total number of physiotherapy contacts. We defined protocol violations or deviations for the participants allocated to the SC arm as follows: (1) those allocated to group 1 who received more than two physiotherapy treatment sessions, (2) participants allocated to group 2 receiving fewer than three physiotherapy treatment sessions, (3) participants allocated to group 3 not referred to (or not attending) spinal specialist services and (4) participants allocated to groups 1 or 2 and referred to spinal specialist services.
Subgroup analyses
Two subgroup analyses were prespecified in relation to re-analysis of the primary outcome: (1) treatment by group evaluation (SC/UC by sciatica groups 1, 2 and 3) and (2) treatment by disc-related sciatica/stenosis ascertained by clinical assessment in the SCOPiC trial research clinic (SC/UC by clinical diagnosis of disc prolapse/stenosis). Descriptive statistical summaries were provided through mean and median time to resolution per trial arm per participant-specified subgroup. The adjusted Cox proportional hazards frailty model was repeated including additional interaction terms for trial arm (SC/UC) by subgroups within the models. Tests of statistical significance were obtained from the p-values for the interaction term for the product of subgroup variable by treatment arm within the Cox model.
Sensitivity analyses
A number of prespecified sensitivity analyses of the primary outcome were carried out to test the rigour and robustness of the main evaluation through the following evaluations:
-
Alternative definitions of good outcome. We used three separate classifications of the self-report data on resolution of symptoms from the text messages based on ‘stable resolution’, ‘improvement’ and ‘stable improvement’. The primary definition of patient-reported resolution of symptoms was defined as a first response of either ‘completely recovered’ or ‘much better’. Sensitivity definitions comprised (1) two consecutive recordings of ‘completely recovered’ or ‘much better’, which was considered indicative of ‘stable resolution’; (2) first response of ‘completely recovered’, ‘much better’ or ‘better’, which was considered indicative of ‘improvement’; and (3) two consecutive recordings of ‘completely recovered’, ‘much better’ or ‘better’, indicative of ‘stable improvement’.
-
Alternative assumptions regarding missing data. For the primary evaluation, missing data were assumed to be synonymous with non-recovery and event definitions were based on ‘recovery’ at the first point of a positive response. Any missing data immediately preceding this response were assumed to be indicative of ‘non-recovery’. Sensitivity analyses set the time interval of recovery as the mean time between the last patient’s response (indicating ‘non-resolution’) and the time at which ‘resolution’ was first classified (for different classifications for the event as noted above). More relaxed sensitivity analyses took the contrary view on missingness, in which it was equated to, and imputed as, an event had occurred, that is ‘resolved’ or ‘improved’ case.
-
Alternative assumption regarding interval-censoring. As we knew only the interval of time during which the resolution occurred and not the exact time (especially after the first 16 weeks, when outcome data were collected monthly rather than weekly), further time-to-event sensitivity analyses were carried out to estimate between-arm comparison of HR that allowed for both left and right censoring through interval-censoring analysis.
-
Alternative (parametric) modelling. Weibull, exponential and log-normal distributions58,59 were considered instead of the Cox proportional hazards approach.
-
Alternative (non-parametric) testing. The log-rank (Mantel–Cox) test, Breslow (Generalised Wilcoxon) and Tarone–Ware tests were carried out.
-
Analysis of groups of participants who completed follow-up (not including censoring) and per-protocol analysis, as previously outlined.
Assumption-checking
For the evaluation of the primary time-to-event analysis, the Cox regression model assumption of proportional hazards was examined in two ways. First, by assessment of the graphs of the survival curves, and, second, through inclusion of a time–trial arm interaction in the regression model, with statistical significance of this term signifying an important deviation from the proportional hazards assumption. In the event that the proportional hazards assumption was not met, greater emphasis on front-line testing would be given to the distribution-free, non-parametric log-rank test result.
For the secondary outcomes, we examined the normality of the residuals in respect of the linear models (in the event of any reasonable violation, we planned to use a suitable data transformation function). In the longitudinal mixed models, we explored different covariance structures to assess for the best goodness-of-fit by comparing likelihood with Bayesian Information Criterion.
Process outcomes analysis
Descriptive statistics were used to examine health-care utilisation by participants in the trial arms and by sciatica groups (1, 2 and 3) in both arms. Numbers (and percentages) of participants referred for physiotherapy treatment or to spinal specialists, and numbers (and percentages) of participants receiving spinal surgery and/or spinal injections, were described. For participants receiving physiotherapy treatment, the numbers (median and IQR) of physiotherapy sessions received and summaries of types of treatments received were also described. Time to physiotherapy treatment and appointments to spinal interface clinics, spinal orthopaedic clinics and pain clinics for participants in both trial arms and by sciatica groups (1, 2 and 3) were also evaluated (median and IQR) when possible, taking the randomisation date as the starting point. Statistical testing was carried out through non-parametric tests of significance, the Mann–Whitney U-test was used for between-arm testing of numerical data, and chi-squared tests were used for between-arm testing of frequency (count) data.
Statistical packages and data considerations
Analyses were conducted using IBM SPSS Statistics version 24 (IBM Corporation, Armonk, NY, USA) and Stata® version 15 (StataCorp LP, College Station, TX, USA). All applicable statistical tests were performed using a two-sided 5% significance level. All CIs of primary and secondary outcomes were presented at the same level of 95%, two-sided. There was no adjustment for multiplicity, as there was only one primary end point evaluation.
Patient and public involvement
Patient and public involvement (PPI) was supported by the PPI infrastructure within the Arthritis Research UK Primary Care Centre at Keele University, which includes a research user group (RUG), made up of 75 members aged from 32 to 82 years, all with experience of living with a long-term condition.
Previous interviews that we conducted with sciatica patients emphasised the impact that it has on them and the requirement for clearer information on treatment and prognosis. 60 Prior to the SCOPiC trial taking place, we held a meeting with three PPI members who were currently experiencing, or previously had experienced, sciatica. Outcomes from this meeting highlighted that prompt pain relief is key, given the severity of the pain. This informed the choice of time to symptoms resolution as the primary outcome of the trial, which emphasised the significance of early assessment and the requirement to match treatments to patients more efficiently, and the need for this trial. Furthermore, it was thought that collecting pain data via regular, brief texts or telephone calls was acceptable.
Further meetings with PPI members were held throughout the trial to seek advice on trial documents, the language of the text messaging and to discuss the findings from the qualitative interviews with patients in the fast-track pathway of the SC arm. A final PPI meeting was held to discuss the overall trial results and agree the key messages for patients and the public.
Two PPI members were part of the TSC and attended each TSC meeting, providing valuable input throughout the trial from a patient perspective.
Trial oversight
The trial was supported by Keele CTU, a UK Clinical Research Collaboration fully registered CTU, and was monitored by the CTU operations group. The conduct of the trial was designed and managed in line with the Keele University Health and Social Care research quality management system and adhered to Keele CTU standard operating procedures.
The trial was overseen by the TMG, which included all key personnel involved in the design and operational management of the trial and its processes. The TMG met on a monthly basis to discuss and monitor progress, including recruitment and follow-up, in line with the agreed trial management plans; to identify solutions to challenges; and to agree actions in the event of any protocol non-compliance issues that might arise. The TMG worked closely with the Clinical Research Network (CRN) West Midlands, CRN Cheshire and CRN Wales in monitoring and facilitating recruitment via general practices and the clinical teams in the participating NHS sites, delivering the care pathways in the SCOPiC trial.
Trial Steering Committee
The TSC included an independent chairperson, five other independent members, including an independent statistician, and two lay representatives. The TSC met initially to review and approve the SCOPiC trial protocol prior to submission to the Research Ethics Committee and then approximately annually to monitor the trial’s overall progress, including agreement on the SAP, and the internal pilot’s success criteria. The TSC had a final meeting to discuss trial findings.
Data Monitoring Committee
A DMC was established to monitor participant safety and data integrity of the trial. The DMC received the trial protocol for review prior to commencement of the trial and then received the required regular DMC reports prior to each TSC meeting. The DMC reports contained a review of recruitment and retention, safety, non-compliance and data from the internal pilot. Recommendations from the DMC were communicated to the chairperson of the TSC and actioned in each TSC meeting.
Chapter 3 Results
Participant flow into the trial
Trial invitation mailing to potentially eligible patients commenced on 28 May 2015; the first participant was randomised on 4 June 2015 and the final participant was randomised on 18 July 2017. The last mailing of follow-up was on 18 July 2018 and the last participant follow-up response was received on 30 August 2018. Three centres participated in the trial: North Staffordshire and North Shropshire were planned centres from the start of the trial, and Cheshire was added as a centre on 1 April 2016 following the internal pilot phase review to ensure continued successful recruitment to the trial. The North Shropshire centre’s general practice numbers were increased to include four general practices from the Shropshire/Welsh border area. By the end of the recruitment phase, 45 general practices had agreed to take part in the trial and SCOPiC trial participants from 42 of those practices consented and were randomised in the trial (range of the number of participants consenting and randomised per practice 1–34). Among those 42 practices, the total number of participants recruited as a percentage of the total number invited to the SCOPiC trial clinics to be screened for eligibility ranged from 4.8% to 35.7%. The observed recruitment rate over the recruitment period was approximately 18 people per month, which was just under the expected number of approximately 20 per month.
Trial population
In total, 2719 potential participants with suspected sciatica were mailed invitations to attend the SCOPiC trial research clinics, 1718 (63%) of whom contacted Keele CTU and were screened for preliminary eligibility over the telephone (median 4.5 days later, IQR 2–11 days later). Of these, 268 were found to be ineligible (the most common reasons being ‘no leg pain’ and ‘ongoing/recent physiotherapist/specialist care’) and 97 declined to attend the research clinics. A further five were screened but had no appointment booked. Hence, 1348 potential participants were booked into the SCOPiC trial research clinics and 1269 (46.7% of invitees) attended (median 14 days after invitation, IQR 9–21 days after invitation). Of these, 552 (43% of attendees) were eligible to take part in the trial and 717 were ineligible. The most common reasons for ineligibility were ‘physiotherapist’s low confidence in sciatica diagnosis’, ‘referred leg pain’ and ‘no symptoms in the leg’. Of the 552 participants attending clinic who were eligible to take part in the trial, 76 declined participation and 476 provided written, informed consent and were randomised to one of the two trial arms: 238 to SC and 238 to non-stratified UC. The flow diagram of participants into and through the trial is shown in Figure 3.
FIGURE 3.
The SCOPiC trial participant flow diagram. a, More than one reason for ineligibility was possible; b, one did not provide any data and nine (five) had experienced the event [resolution (stable resolution)] by the time of withdrawal; c, one did not provide any data and 10 (seven) had experienced the event [resolution (stable resolution)] by the time of withdrawal. FQ, full questionnaire response; MDC, minimal data collection response.
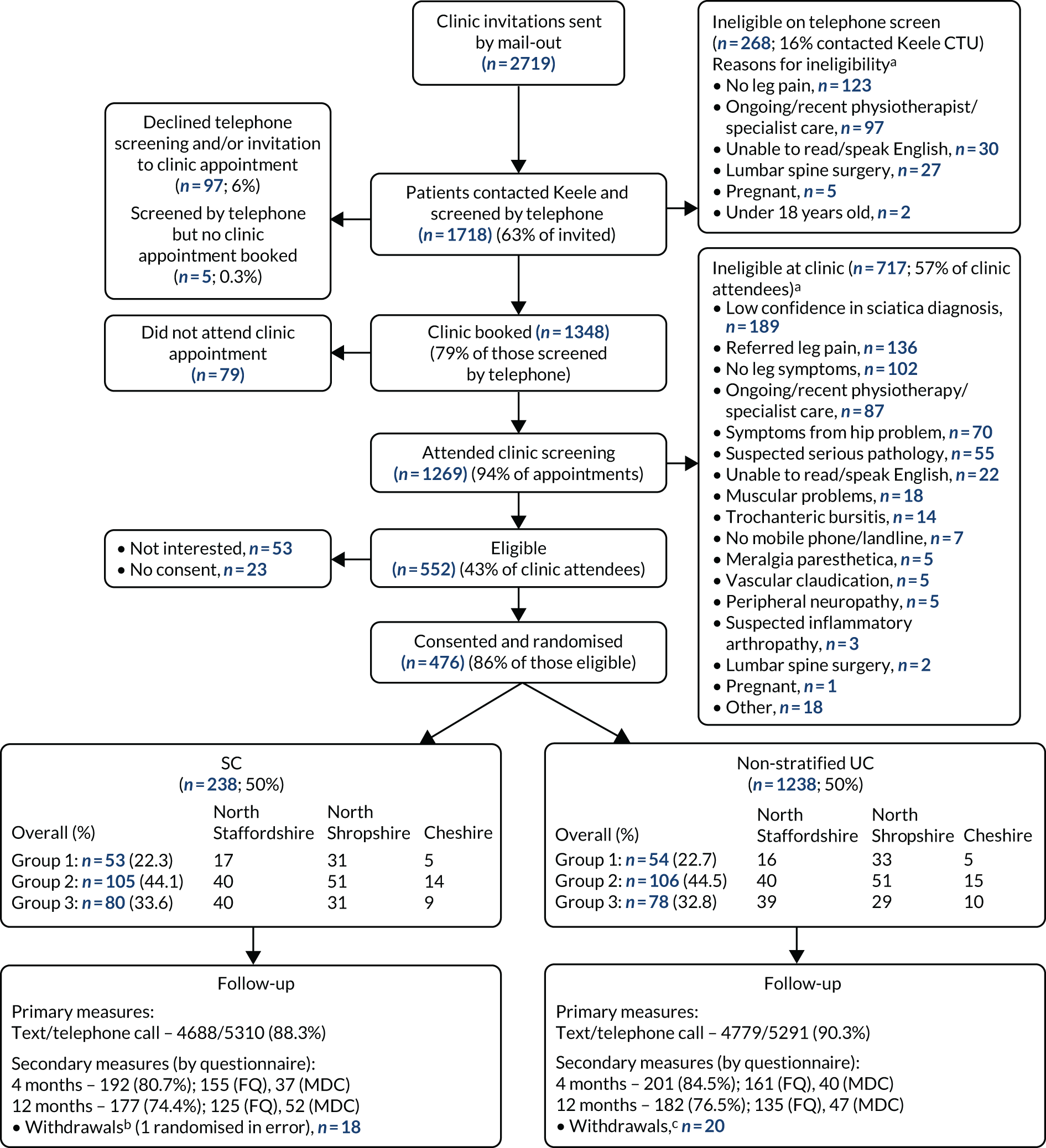
Stratified block randomisation ensured that the number of participants in each arm of the trial was balanced in terms of centre (North Staffordshire/North Shropshire and Wales/Cheshire) and sciatica group (1, 2 and 3). More participants were recruited from North Staffordshire [40.3% of the total sample (192/476)] and North Shropshire [47.5% (226/476)] than from Cheshire [12.2% (58/476)], as the Cheshire centre commenced recruitment after the internal pilot phase. More participants were in sciatica group 2 [44.3% (211/476)] than in group 1 [22.5% (107/476)] or group 3 [33.2% (158/476)] – these proportions were similar in the two trial arms. These observed proportions were somewhat different from our projected estimates before the trial of 12% in group 1, 57% in group 2 and 31% in group 3.
The mean ages of participants who were randomised into the trial (n = 476) and those who were invited but were not randomised (n = 2243) were similar: 52.0 (SD 14.1) years and 52.3 (SD 16.1) years, respectively. There was a similar female-to-male ratio among trial participants (262 : 214, ratio 1.22) and non-participants (1260 : 983, ratio 1.28). Participants had a higher median Index of Multiple Deprivation (IMD) rank value (designating lower social deprivation) than non-participants: 15,304 (IQR 8021–21,790) and 13,353 (IQR 4613–21,294), respectively. There was a difference in participant uptake (i.e. consent rate of those invited) between centres: North Staffordshire [14.9% (192/1291)], North Shropshire/Wales [19.8% (226/1143)] and Cheshire [20.4% (58/285)]. General practice computer templates were completed to capture participating GPs’ preferred treatment choices for patients at that point of consultation. The proportions selecting ‘keep under GP care’, ‘refer to physiotherapy’ or ‘refer to specialist spinal services’ were similar for trial participants and non-participants (30.3% and 32.7% for ‘keep under GP care’, 63.2% and 58.4% for ‘refer to physiotherapy’, and 6.5% and 8.9% for ‘refer to specialist spinal services’, respectively).
Results of the internal pilot phase of the trial
Review of the internal pilot progression criteria took place on two occasions, first on 4 February 2016 (to review progression in relation to participant recruitment) and then on 15 July 2016 (to review progression in relation to follow-up rates for the primary outcome).
Recruitment during the internal pilot phase of the trial (the first 8 months of the recruitment period) was in line with anticipated figures: 129 participants were recruited, compared with an anticipated number of 130 (124 participants within 7.5 months by the time of DMC/TSC reporting). This 99% recruitment rate (against the expected figure) exceeded the threshold criterion for progression to the main trial, which had been set at 70% (i.e. 90/130), thereby fulfilling the first of the two progression criteria. In total, 28 general practices were participating at this stage, only two short of the 30 that had been anticipated by this point (and further practices were ready to join the trial). There was a difference in the observed and expected proportions of participants in sciatica groups 1 and 2, but fidelity checks of research clinic CRFs showed no evidence that suggested that these observed proportions were inaccurate.
It was noted on review of follow-up data that (1) the event rate of the primary outcome (percentage of participants reporting resolution of sciatica symptoms according to the primary definition) exceeded 60%, which satisfied the assumption of the original sample size calculation, and (2) the follow-up rate for the primary outcome data up to 4 months was 88% (1706 responses from a potential total of 1938). Hence, the loss to follow-up was 12%, which was lower than the 25% threshold (thereby fulfilling the second of the two progression criteria). The follow-up rate for the 4-month postal questionnaire (including minimum data collection) was 77%. There was little difference in withdrawal numbers between trial arms (13 participants had withdrawn from one arm at the 4-month follow up, and nine from the other arm).
The DMC and TSC agreed that the internal pilot phase progression criteria had been met and that the trial could proceed to the main trial phase.
Baseline characteristics of randomised participants
A summary of baseline characteristics for the 476 trial participants, overall and by trial arm, is given in Table 2. The mean age was 52.1 years (50.8 years in the SC arm and 53.3 years in the UC arm). The overall percentage of females was 55.0% (262/476): 55.5% and 54.6% in the SC and UC arms, respectively. Data were available from 475 baseline questionnaires, because one participant was withdrawn immediately after randomisation (owing to error, as the participant was ineligible).
| Characteristic | Trial arm | All (SC and UC), n (sample size) | |
|---|---|---|---|
| SC | UC | ||
| Age, mean (SD) | 50.8 (14.6) | 53.3 (13.5) | 476 (238, 238) |
| Sex, n (%) | 476 (238, 238) | ||
| Females | 132 (55.5) | 130 (54.6) | |
| Males | 106 (45.5) | 108 (45.4) | |
| IMD rank value,a median (IQR) | 14,228 (7387–21,790) | 15,614 (8288–21,840) | 425 (213, 212) |
| Opted approach for follow-up, n (%) | 476 (238, 238) | ||
| Text | 213 (89.5) | 213 (89.5) | |
| Telephone | 25 (10.5) | 25 (10.5) | |
| Employed (in paid job), n (%) | 472 (236, 236) | ||
| No | 65 (27.5) | 76 (32.2) | |
| Yes | 171 (72.5) | 160 (67.8) | |
| Full-timeb | 119 (70.0) | 118 (74.2) | |
| Part-timeb | 51 (30.0) | 41 (25.8) | |
| Time off work because of sciatica in the previous 12 months, n (%) | 334 (171, 163) | ||
| No | 87 (50.9) | 67 (41.1) | |
| Yes | 84 (49.1) | 96 (58.9) | |
| WPI (NRS 0–10c), mean (SD) | 5.9 (2.9) | 6.1 (2.8) | 334 (171, 163) |
| Prescribed medication for sciatica, in the previous 3 months,d n (%) | 468 (235, 233) | ||
| No | 41 (17.4) | 50 (21.5) | |
| Yes | 194 (82.6) | 183 (78.5) | |
| Usual back pain intensity (NRS 0–10c), mean (SD) | 5.9 (2.7) | 5.8 (2.9) | 474 (237, 237) |
| Usual leg pain intensity (NRS 0–10c), mean (SD) | 6.8 (2.2) | 6.9 (2.2) | 474 (237, 237) |
| Leg pain interference (yes/no),e n (%) | 474 (236, 238) | ||
| No | 52 (22.0) | 56 (23.5) | |
| Yes | 184 (78.0) | 182 (76.5) | |
| Leg pain interference score (NRS 0–10c), mean (SD) | 6.3 (2.5) | 6.6 (2.4) | 474 (237, 237) |
| Symptom duration, n (%) | 475 (237, 238) | ||
| < 2 weeks | 15 (6.3) | 33 (13.9) | |
| 2–6 weeks | 99 (41.8) | 98 (41.2) | |
| 6–12 weeks | 58 (24.5) | 46 (19.3) | |
| 3–6 months | 31 (13.1) | 29 (12.2) | |
| 6–12 months | 10 (4.2) | 10 (4.2) | |
| > 12 months | 24 (10.1) | 22 (9.2) | |
| Physical function (RMDQ 0–23f), mean (SD) | 11.1 (5.2) | 11.3 (5.4) | 475 (237, 238) |
| SBI score (0–24 composite scorec), mean (SD) | 14.6 (5.0) | 14.5 (5.0) | 475 (237, 238) |
| Neuropathic pain (S-LANSS scoreg), n (%) | 445 (218, 227) | ||
| < 12 | 124 (56.9) | 134 (59.0) | |
| ≥ 12 | 94 (43.1) | 93 (41.0) | |
| Fear of movement (TSK score, 17–64h), mean (SD) | 40.4 (6.1) | 40.8 (6.2) | 475 (237, 238) |
| Anxiety (HADS-A) score, mean (SD) | 7.8 (4.1) | 8.0 (4.1) | 475 (237, 238) |
| HADS-A (0–21i), n (%) | |||
| Normal (0–7) | 118 (49.8) | 120 (50.4) | |
| Possible (8–10) | 64 (27.0) | 51 (21.4) | |
| Probable (≥ 11) | 55 (23.2) | 67 (28.2) | |
| Depression (HADS-D), mean (SD) | 6.5 (3.9) | 6.3 (4.0) | 475 (237, 238) |
| HADS-D score (0–21i), n (%) | |||
| Normal (0–7) | 156 (65.8) | 151 (63.4) | |
| Possible (8–10) | 40 (16.9) | 48 (20.2) | |
| Probable (≥ 11) | 41 (17.3) | 39 (16.4) | |
| Sleep problem, n (%) | 475 (237, 238) | ||
| No | 88 (37.1) | 74 (31.1) | |
| Yes | 149 (62.9) | 164 (68.9) | |
| General health, n (%) | 475 (237, 238) | ||
| Excellent | 11 (4.6) | 13 (5.5) | |
| Very good | 52 (21.9) | 49 (20.6) | |
| Good | 107 (45.1) | 103 (43.3) | |
| Fair | 50 (21.1) | 60 (25.2) | |
| Poor | 17 (7.2) | 13 (5.5) | |
| STarT Back Screening Tool risk subgroup, n (%) | 474 (237, 237) | ||
| Low | 56 (23.6) | 52 (21.9) | |
| Medium | 133 (56.1) | 130 (54.9) | |
| High | 45 (20.3) | 55 (23.2) | |
The majority of the trial participants were employed (68.4%), of whom 72.1% were full-time workers; the majority of these had taken time off work in the previous 12 months because of their sciatica. Around half of the trial participants had acute pain (< 6 weeks’ duration, 51.5%); among the rest, 21.8% and 26.5% had subacute (6–12 weeks’ duration) and chronic (> 12 weeks’ duration) symptoms, respectively. The mean sciatica-related physical disability score (RMDQ score) was 11.2 (SD 5.3): 11.1 for the SC arm and 11.3 for the UC arm. The majority of participants classified their general health as ‘good’, around two-thirds of participants were experiencing significant sleep disturbance due to the sciatic pain, around half were classified as having possible/probable anxiety and one-third were classified as having possible/probable depression at baseline (using the Hospital Anxiety and Depression Scale). For 398 participants, the assessing physiotherapists diagnosed disc prolapse as the reason for the sciatic symptoms; for 63 participants, the recorded clinical diagnosis was spinal stenosis. In approximately 64.3% of the disc-related sciatica cases, the S1 nerve root was suspected of being compressed. In about 85% of the cases clinically diagnosed with stenosis, leg symptoms were unilateral.
The baseline characteristics for each trial arm, stratified by sciatica groups (1, 2 and 3), are summarised in Table 3. Age was similar across all strata (the mean age ranged between 50.1 and 55.3 years). There was a slight trend towards an increasing proportion of females from group 1 (≈50%) through group 2 (≈55%) to group 3 (≈60%). The centre in North Shropshire/Wales had a higher proportion of group 1 participants (28%) than the centres in North Staffordshire and Cheshire (17% each). Furthermore, there was a trend towards a lower proportion of employed participants from group 1 (74%) through group 2 (71%) to group 3 (61%). Trends of greater proportions of time off work and lower work performance levels (indicated by higher Work Performance Index scores) were reported from group 1 through group 2 to group 3. Table 2 shows that the expected trend of worsening health status for all baseline measures of pain levels, disability and mental and general health, from group 1 through group 2 to group 3, was observed in the trial population.
| Characteristic | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|
| SC | UC | SC | UC | SC | UC | |
| Age (years), mean (SD) | 52.2 (14.5) | 55.3 (14.0) | 50.1 (14.5) | 53.4 (13.0) | 50.9 (14.8) | 51.7 (13.9) |
| Sex, n (%) | ||||||
| Females | 27 (51) | 26 (48) | 57 (54) | 59 (56) | 48 (60) | 45 (58) |
| Males | 26 (49) | 28 (52) | 48 (46) | 47 (44) | 32 (40) | 33 (42) |
| IMD rank value,a median (IQR) | 17,910 (10,818–25,484) | 15,436 (8287–21,427) | 13,444 (5953–21,775) | 16,359 (9312–22,887) | 13,107 (5501–19,540) | 14,225 (4813–21,890) |
| Employed, n (%) | ||||||
| No | 11 (21) | 15 (28) | 29 (28) | 30 (29) | 25 (32) | 31 (40) |
| Yes | 42 (79) | 38 (72) | 76 (72) | 75 (71) | 53 (68) | 47 (60) |
| Full-time | 30 (71) | 29 (78) | 53 (70) | 52 (69) | 36 (69) | 37 (79) |
| Part-time | 12 (29) | 8 (22) | 23 (30) | 23 (31) | 16 (31) | 10 (21) |
| Time off work because of sciatica in the previous 12 months, n (%) | ||||||
| No | 29 (69) | 20 (50) | 34 (45) | 33 (43) | 24 (45) | 14 (30) |
| Yes | 13 (31) | 20 (50) | 42 (55) | 43 (57) | 29 (55) | 33 (70) |
| WPI score, mean (SD) | 4.0 (2.4) | 4.4 (2.3) | 6.0 (2.9) | 6.0 (2.6) | 7.2 (2.4) | 7.7 (2.6) |
| Prescription,b n (%) | ||||||
| No | 17 (32) | 17 (33) | 17 (17) | 20 (19) | 7 (9) | 13 (17) |
| Yes | 36 (68) | 35 (67) | 86 (83) | 83 (81) | 72 (91) | 65 (83) |
| Usual back pain NRSc score, mean (SD) | 3.8 (2.2) | 3.5 (2.6) | 5.9 (2.5) | 6.2 (2.6) | 7.1 (2.6) | 6.9 (2.6) |
| Usual leg pain NRSc score, mean (SD) | 4.9 (2.2) | 4.9 (2.3) | 6.5 (1.9) | 6.8 (1.9) | 8.4 (1.2) | 8.5 (1.3) |
| Leg pain interference,d n (%) | ||||||
| No | 33 (62) | 36 (67) | 17 (16) | 19 (18) | 2 (3) | 1 (1) |
| Yes | 20 (38) | 18 (33) | 87 (84) | 87 (82) | 77 (97) | 77 (99) |
| Leg pain interference NRSc score, mean (SD) | 3.5 (2.2) | 4.2 (2.3) | 6.4 (2.1) | 6.6 (2.0) | 8.1 (1.2) | 8.3 (1.2) |
| Duration, n (%) | ||||||
| < 2 weeks | 2 (4) | 11 (20) | 10 (10) | 12 (11) | 3 (4) | 10 (13) |
| 2–6 weeks | 26 (49) | 20 (37) | 36 (34) | 42 (40) | 37 (47) | 36 (46) |
| 6–12 weeks | 13 (25) | 10 (19) | 29 (28) | 25 (24) | 16 (20) | 11 (14) |
| 3–6 months | 4 (7) | 8 (15) | 12 (11) | 9 (8) | 15 (19) | 12 (15) |
| 6–12 months | 2 (4) | 1 (2) | 4 (4) | 7 (7) | 4 (5) | 2 (3) |
| > 12 months | 6 (11) | 4 (7) | 14 (13) | 11 (10) | 4 (5) | 7 (9) |
| RMDQ score,e mean (SD) | 5.1 (3.0) | 5.9 (3.6) | 11.5 (4.3) | 11.7 (4.6) | 14.7 (4.0) | 14.5 (4.6) |
| SBI score,f mean (SD) | 10.8 (4.7) | 10.7 (4.5) | 14.2 (4.6) | 14.6 (4.4) | 17.7 (3.5) | 17.1 (4.4) |
| S-LANSS,g n (%) | ||||||
| < 12 | 35 (74) | 35 (67) | 58 (59) | 62 (61) | 31 (42) | 37 (50) |
| ≥ 12 | 12 (26) | 17 (33) | 40 (41) | 39 (39) | 42 (58) | 37 (50) |
| TSK score,h mean (SD) | 36.2 (4.5) | 36.7 (6.3) | 40.4 (5.8) | 40.7 (5.6) | 43.1 (5.9) | 43.7 (5.2) |
| HADS-A score,i mean (SD) | 5.3 (2.8) | 5.6 (3.4) | 7.6 (3.6) | 7.8 (3.4) | 9.6 (4.5) | 9.9 (4.3) |
| HADS-A, n (%) | ||||||
| Normal | 39 (74) | 42 (78) | 52 (49) | 52 (49) | 27 (34) | 26 (33) |
| Possible | 11 (21) | 7 (13) | 32 (31) | 28 (26) | 21 (27) | 16 (21) |
| Probable | 3 (6) | 5 (9) | 21 (20) | 26 (25) | 31 (39) | 36 (46) |
| HADS-D score,i mean (SD) | 3.3 (2.3) | 3.6 (2.7) | 6.7 (3.5) | 6.0 (3.3) | 8.3 (4.1) | 8.7 (4.3) |
| HADS-D, n (%) | ||||||
| Normal | 51 (96) | 48 (89) | 69 (66) | 73 (69) | 36 (46) | 30 (38) |
| Possible | 2 (4) | 5 (9) | 20 (19) | 22 (21) | 18 (23) | 21 (27) |
| Probable | 0 (0) | 1 (2) | 16 (15) | 11 (10) | 25 (32) | 27 (35) |
| Sleep problem, n (%) | ||||||
| No | 35 (66) | 29 (54) | 35 (33) | 29 (27) | 18 (23) | 16 (21) |
| Yes | 18 (34) | 25 (46) | 70 (67) | 77 (73) | 61 (77) | 62 (79) |
| General health, n (%) | ||||||
| Excellent | 4 (7) | 7 (13) | 4 (4) | 4 (4) | 3 (4) | 2 (3) |
| Very good | 17 (32) | 18 (33) | 15 (14) | 21 (20) | 20 (25) | 10 (13) |
| Good | 28 (53) | 21 (39) | 43 (41) | 47 (44) | 36 (46) | 35 (45) |
| Fair | 4 (7) | 6 (11) | 36 (34) | 33 (31) | 10 (13) | 21 (27) |
| Poor | 0 (0.0) | 2 (4) | 7 (7) | 1 (1) | 10 (13) | 10 (13) |
| STarT Back Screening Tool risk subgroup, n (%) | ||||||
| Low | 52 (98) | 51 (96) | 4 (4)j | 1 (1)j | 0 (0) | 0 (0) |
| Medium | 1 (2)k | 2 (4)k | 93 (89) | 94 (89) | 39 (49) | 34 (44) |
| High | 0 (0) | 0 (0) | 8 (8) | 11 (10) | 40 (51) | 44 (56) |
Trial follow-up
The flow diagram in Figure 3 also summarises the follow-up rate of participants. For the primary outcome (collected through text messages), 9467 responses were received from 10,601 potential texts (or telephone calls) (i.e. completeness, 89.3%). The response rate was similar between the two arms: 88.3% (4688/5310) in the SC arm and 90.3% (4779/5291) in the UC arm. A total of 83.6% (398/476) of participants responded to at least 80% of potential texts/telephone calls, 75.4% (359/476) responded to at least 90% and 60.1% (286/476) responded to all potential texts/calls [138 (58.0%) in the SC arm and 148 (62.2%) in the UC arm]. For the 4-month follow-up questionnaire, the overall response rate was 82.6% (393/476): 80.7% (192/238) in the SC arm and 84.5% (201/238) in the UC arm. For the 12-month follow-up questionnaire, the response rate was 75.4% (359/476) overall: 74.4% (177/238) in the SC arm and 76.5% (182/238) in the UC arm. The summary breakdown of responses as full questionnaire response or minimal data collection by trial arm is shown in the flow diagram (see Figure 3); the proportion of responders who completed the full questionnaires was 80.4% (316/393) at 4 months and 72.4% (260/359) at 12 months. Those who did not complete the 4- and 12-months questionnaires were younger, lived in significantly more deprived neighbourhoods (lower average IMD rank value) and had slightly worse baseline health status than those who completed the questionnaires. Summary baseline characteristics comparing responders to follow-up questionnaires with non-responders are shown in Table 4.
| Key baseline characteristic | Completed follow-up at 4 months (N = 393) | Lost to follow-up at 4 months (N = 83) | Completed follow-up at 12 months (N = 359) | Lost to follow-up at 12 months (N = 117) |
|---|---|---|---|---|
| Age (years), mean (SD) | 53.9 (13.7) | 43.1 (12.3) | 54.1 (13.7) | 45.6 (13.3) |
| Females, n (%) | 214 (54.5) | 48 (57.8) | 192 (53.5) | 70 (59.9) |
| IMD rank value,a median (IQR) | 15,619 (8958–21,903) | 10,569 (3272–18,294) | 15,619 (8920–21,890) | 12,393 (3558–21,427) |
| Employed, n (%) | 263 (67.3) | 61 (75.3) | 240 (67.2) | 84 (73.0) |
| Pain duration of > 12 weeks, n (%) | 191 (48.6) | 39 (47.6) | 168 (46.8) | 62 (53.4) |
| Sciatica group, n (%) | ||||
| 1 | 95 (24.2) | 12 (14.5) | 92 (25.6) | 15 (12.8) |
| 2 | 171 (43.5) | 40 (48.2) | 152 (42.3) | 59 (50.4) |
| 3 | 127 (32.3) | 31 (37.3) | 115 (32.0) | 43 (36.8) |
| Usual back pain (0–10 NRS),b mean (SD) | 5.7 (2.9) | 6.3 (2.5) | 5.6 (2.9) | 6.5 (2.4) |
| Usual leg pain (0–10 NRS),b mean (SD) | 6.8 (2.3) | 7.0 (2.1) | 6.8 (2.3) | 6.9 (2.1) |
| Leg pain interference (0–10 NRS),b mean (SD) | 6.4 (2.5) | 6.9 (2.0) | 6.4 (2.5) | 6.8 (2.2) |
| Physical function (RMDQc), mean (SD) | 10.9 (5.2) | 12.7 (5.7) | 10.8 (5.2) | 12.6 (5.4) |
Loss to follow-up was mainly through non-response; however, there were 38 participant withdrawals over the 12-month follow-up period: 18 in the SC arm and 20 in the UC arm. A summary of the withdrawal reasons (when known), by trial arm, is provided in Table 5 and shows that, as well as the total number, the reasons for withdrawal were similar between the trial arms. As detailed in the legend of the flow diagram in Figure 3, half (i.e. 19) of participants had experienced the primary event (resolution of sciatica symptoms) by the time of withdrawal: nine (half of withdrawals) in the SC arm and 10 (half of withdrawals) in the UC arm. One participant (in the SC arm) was withdrawn immediately post randomisation and was not followed up, as they had been randomised in error (the patient did not fulfil the eligibility criteria).
| Reason | Trial arm (n) | |
|---|---|---|
| SC | UC | |
| Expected more treatment | – | 2 |
| Family commitments | – | 2 |
| Seeing private therapist | 2 | 2 |
| Not interested in further participation | 5 | 4 |
| Poor health/no better | 2 | 3 |
| Randomised in error | 1 | – |
| Reason not known | 7 | 7 |
| Request by clinician | 1 | – |
| Total | 18 | 20 |
Primary outcome
The summary time-to-event data for the primary end point of first resolution of sciatica symptoms are shown in Figure 4. The event rate was rapid over the first few weeks, with 25% of events (sciatica symptom resolution) occurring by week 4 in the SC arm and by week 5 in the UC arm. In addition, inspection of the percentiles presented shows significant data skewness, which explains the difference between the mean and median values. The median time-to-event time (weeks) was slightly shorter in the SC arm than in the UC arm: 10.0 and 12.0 weeks, respectively. Comparing each trial arm, the figure for resolved symptoms (‘completely recovered’ or ‘much better’) by 16 weeks was 137 out of 238 (57.6%) in both arms; by 48 weeks, it was 344 out of 476 (72.3%): 176 out of 238 (73.9%) in the SC arm and 168 out of 238 (70.6%) in the UC arm. Table 6 summarises the Kaplan–Meier event-rate probabilities and event numbers across the time intervals of data collection. For the ratio of the SC arm to the UC arm, the estimate for the primary end point time-to-event HR was 1.14 (95% CI 0.89 to 1.46; p = 0.288) (adjusted for centre, baseline stratification sciatica group and symptom duration, and accounting for clustering by named physiotherapist). There was limited overlap in hazards across time and the statistical interaction term for trial arm by time within the model assessment of proportional hazards was not statistically significant [either when looking at time (p = 0.430) or logetime (p = 0.377)]. Given a total sample size of 476 participants, a follow-up of 89.3%, a primary event (first resolution of sciatica symptoms) rate of 72.3% and a design effect/inflation factor of 1.32 (based on the following: an ICC for clustering by physiotherapist of 2.6% for the cumulated occurrence, or not, of an event by week 48; average cluster size of 4 with coefficient of variation of 1.5), the post hoc power for detecting a HR of between 1.4 and 1.5 ranged from 0.73 to 0.87.
FIGURE 4.
Kaplan–Meier time-to-event analysis of the primary end point (time to first resolution of sciatica symptoms). a, Estimation is limited to the largest event-free time if it is censored. Cumulative proportion of resolved cases by week 48: 0.754 (all): 0.780 (SC) and 0.8729 (UC).
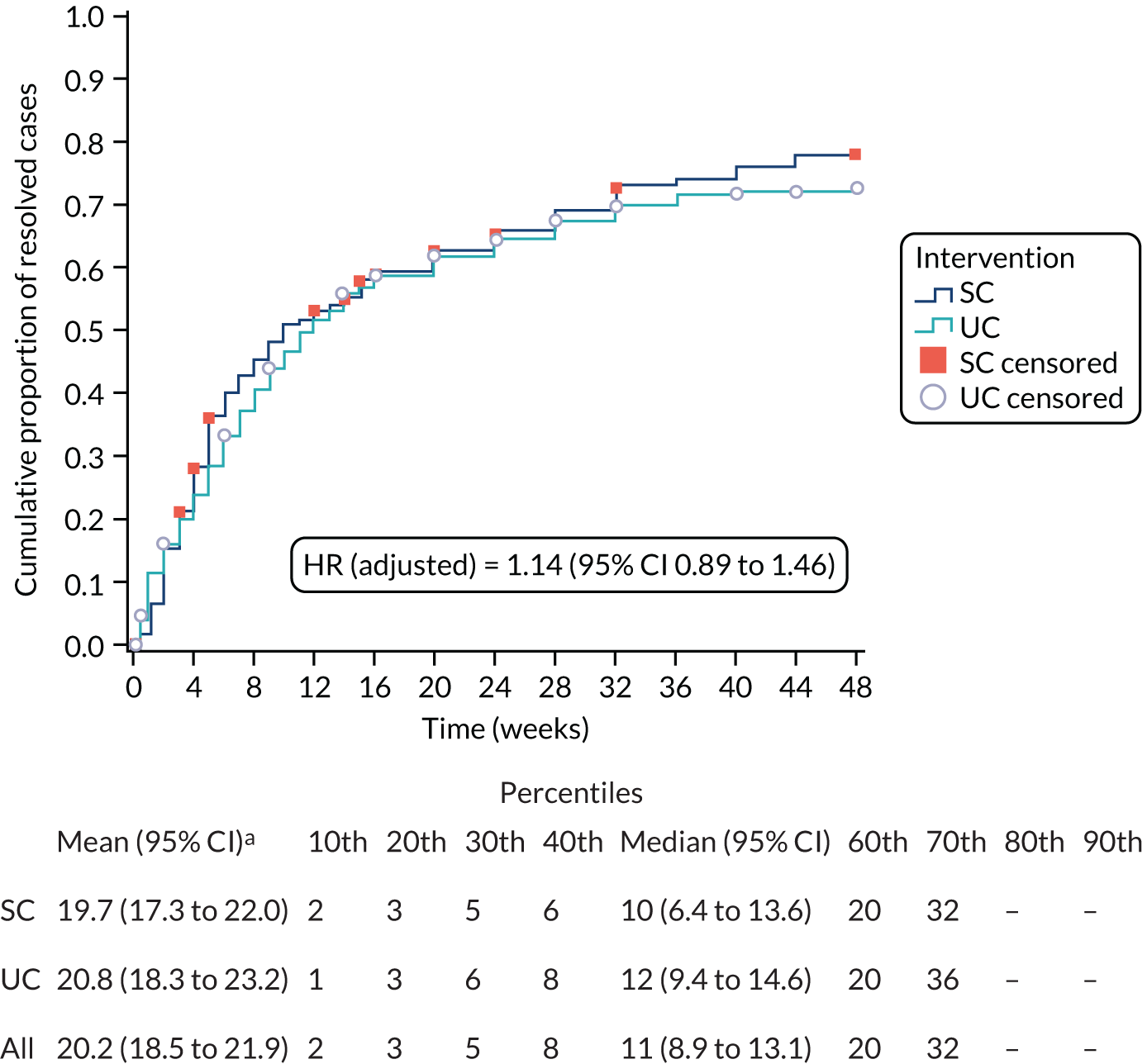
| Arm | Interval start time (weeks) | Number entering interval | Number censored during interval | Number exposed to having event | Number of eventsa | Proportion resolved | Cumulative proportion with resolution |
|---|---|---|---|---|---|---|---|
| SC | 0b | 238 | 1 | 237.5 | 4 | 0.02 | 0.020 |
| 1 | 233 | 0 | 233 | 11 | 0.05 | 0.060 | |
| 2 | 222 | 0 | 222 | 21 | 0.09 | 0.150 | |
| 3 | 201 | 3 | 199.5 | 14 | 0.07 | 0.210 | |
| 4 | 184 | 1 | 183.5 | 16 | 0.09 | 0.280 | |
| 5 | 167 | 1 | 166.5 | 19 | 0.11 | 0.360 | |
| 6 | 147 | 0 | 147 | 9 | 0.06 | 0.400 | |
| 7 | 138 | 0 | 138 | 6 | 0.04 | 0.430 | |
| 8 | 132 | 0 | 132 | 6 | 0.05 | 0.450 | |
| 9 | 126 | 0 | 126 | 7 | 0.06 | 0.480 | |
| 10 | 119 | 0 | 119 | 6 | 0.05 | 0.510 | |
| 11 | 113 | 0 | 113 | 2 | 0.02 | 0.520 | |
| 12 | 111 | 1 | 110.5 | 3 | 0.03 | 0.530 | |
| 13 | 107 | 0 | 107 | 2 | 0.02 | 0.540 | |
| 14 | 105 | 2 | 104 | 3 | 0.03 | 0.550 | |
| 15 | 100 | 1 | 99.5 | 6 | 0.06 | 0.580 | |
| 16 | 93 | 2 | 92 | 2 | 0.02 | 0.590 | |
| 20 | 89 | 2 | 88 | 8 | 0.09 | 0.630 | |
| 24 | 79 | 2 | 78 | 6 | 0.08 | 0.660 | |
| 28 | 71 | 0 | 71 | 8 | 0.11 | 0.690 | |
| 32 | 63 | 4 | 61 | 7 | 0.11 | 0.730 | |
| 36 | 52 | 0 | 52 | 2 | 0.04 | 0.740 | |
| 40 | 50 | 0 | 50 | 4 | 0.08 | 0.760 | |
| 44 | 46 | 0 | 46 | 4 | 0.09 | 0.780 | |
| 48c | 42 | 42 | 21 | 0 | 0 | 0.780 | |
| UC | 0b | 238 | 2 | 237 | 11 | 0.05 | 0.050 |
| 1 | 225 | 0 | 225 | 16 | 0.07 | 0.110 | |
| 2 | 209 | 1 | 208.5 | 11 | 0.05 | 0.160 | |
| 3 | 197 | 0 | 197 | 10 | 0.05 | 0.200 | |
| 4 | 187 | 0 | 187 | 8 | 0.04 | 0.240 | |
| 5 | 179 | 0 | 179 | 11 | 0.06 | 0.280 | |
| 6 | 168 | 1 | 167.5 | 11 | 0.07 | 0.330 | |
| 7 | 156 | 0 | 156 | 9 | 0.06 | 0.370 | |
| 8 | 147 | 0 | 147 | 8 | 0.05 | 0.400 | |
| 9 | 139 | 1 | 138.5 | 8 | 0.06 | 0.440 | |
| 10 | 130 | 0 | 130 | 6 | 0.05 | 0.460 | |
| 11 | 124 | 0 | 124 | 8 | 0.06 | 0.500 | |
| 12 | 116 | 0 | 116 | 4 | 0.03 | 0.520 | |
| 13 | 112 | 0 | 112 | 4 | 0.04 | 0.530 | |
| 14 | 108 | 1 | 107.5 | 6 | 0.06 | 0.560 | |
| 15 | 101 | 0 | 101 | 2 | 0.02 | 0.570 | |
| 16 | 99 | 2 | 98 | 4 | 0.04 | 0.590 | |
| 20 | 93 | 1 | 92.5 | 7 | 0.08 | 0.620 | |
| 24 | 85 | 2 | 84 | 7 | 0.08 | 0.650 | |
| 28 | 76 | 2 | 75 | 6 | 0.08 | 0.680 | |
| 32 | 68 | 1 | 67.5 | 5 | 0.07 | 0.700 | |
| 36 | 62 | 0 | 62 | 3 | 0.05 | 0.720 | |
| 40 | 59 | 2 | 58 | 1 | 0.02 | 0.720 | |
| 44 | 56 | 1 | 55.5 | 0 | 0 | 0.720 | |
| 48c | 55 | 53 | 28.5 | 2 | 0.07 | 0.740 |
Sensitivity analyses for the primary outcome measure
Figures 5–7 show the time-to-event data for the secondary ‘event’ definitions. In all cases, there was no clear graphical indication against proportional hazards and no statistically significant interaction of trial arm by time (hence, proportional hazards were assumed).
-
A total of 286 out of 476 (60.1%) participants had experienced the event ‘stable resolution of symptoms’ (Figure 5) by week 48: 146 out of 238 (61.3%) in the SC arm and 140 out of 238 (58.8%) in the UC arm. The median event times were 24.0 weeks for SC and 24.0 weeks for UC. The HR for SC relative to UC was 1.11 (95% CI 0.87 to 1.43; p = 0.389).
-
A total of 437 out of 476 (91.8%) participants had experienced the event ‘first improvement of symptoms’ (Figure 6) by week 48: 219 out of 238 (92.0%) in the SC arm and 218 out of 238 (91.6%) in the UC arm. The median event times were 2.0 weeks for SC and 2.0 weeks for UC. The HR for SC relative to UC was 0.95 (95% CI 0.79 to 1.15; p = 0.585).
-
A total of 396 out of 476 (83.2%) had experienced the event ‘stable improvement of symptoms’ (Figure 7) by week 48: 198 out of 238 (83.2%) in each trial arm. The median event times were 6.0 weeks for SC and 5.0 weeks for UC. The HR for SC relative to UC was 1.05 (95% CI 0.86 to 1.29; p = 0.606).
FIGURE 5.
Kaplan–Meier time-to-event analysis of the secondary event classification (time to stable resolution of symptoms). a, Estimation is limited to the largest event-free time if it is censored. Cumulative proportion of resolved cases by week 48: 0.636 (all), 0.662 (SC) and 0.613 (UC).
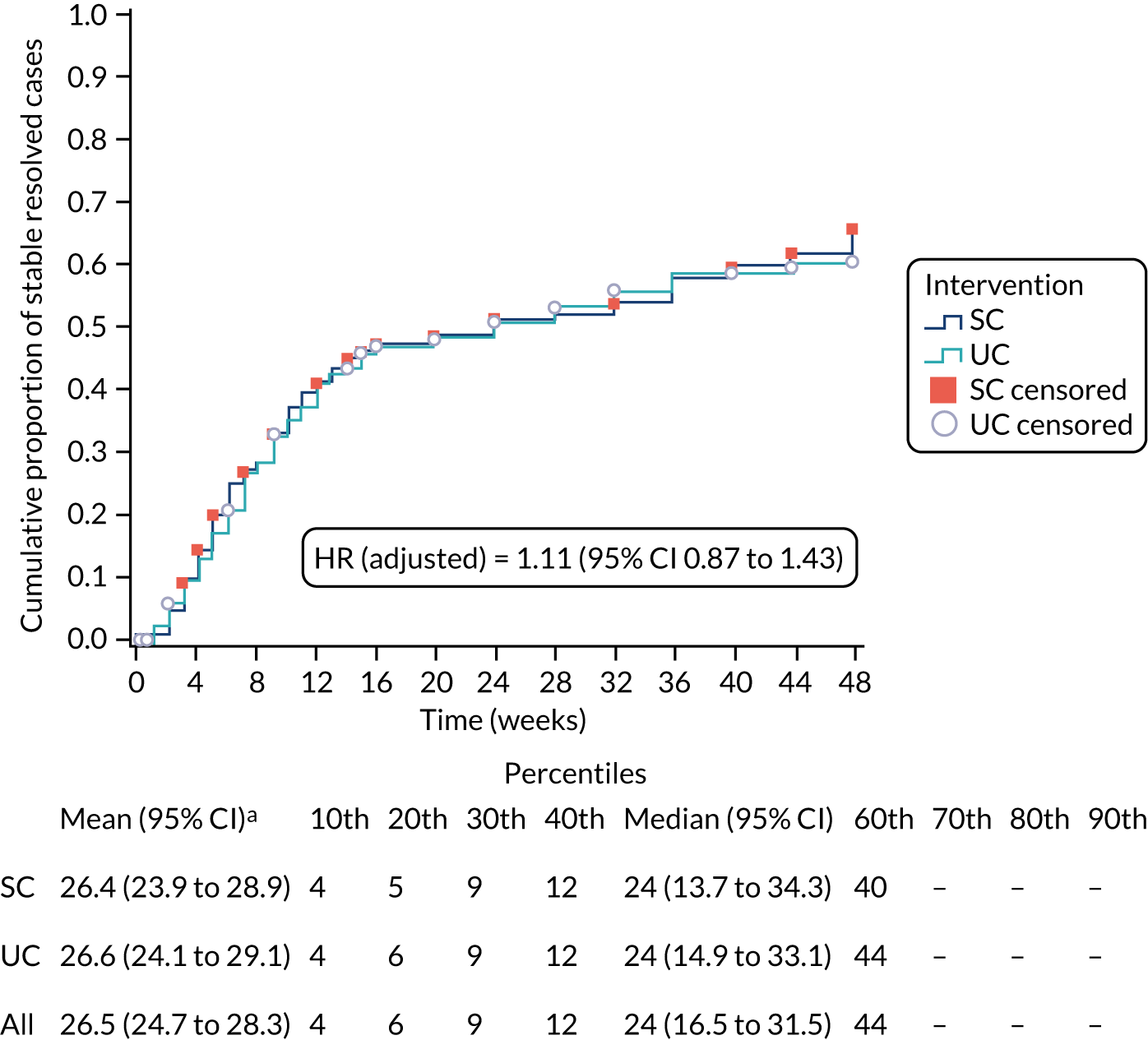
FIGURE 6.
Kaplan–Meier time-to-event analysis of the secondary event classification (time to first improvement of symptoms). a, Estimation is limited to the largest event-free time if it is censored. Cumulative proportion of resolved cases by week 48: 0.935 (all), 0.941 (SC) and 0.929 (UC).
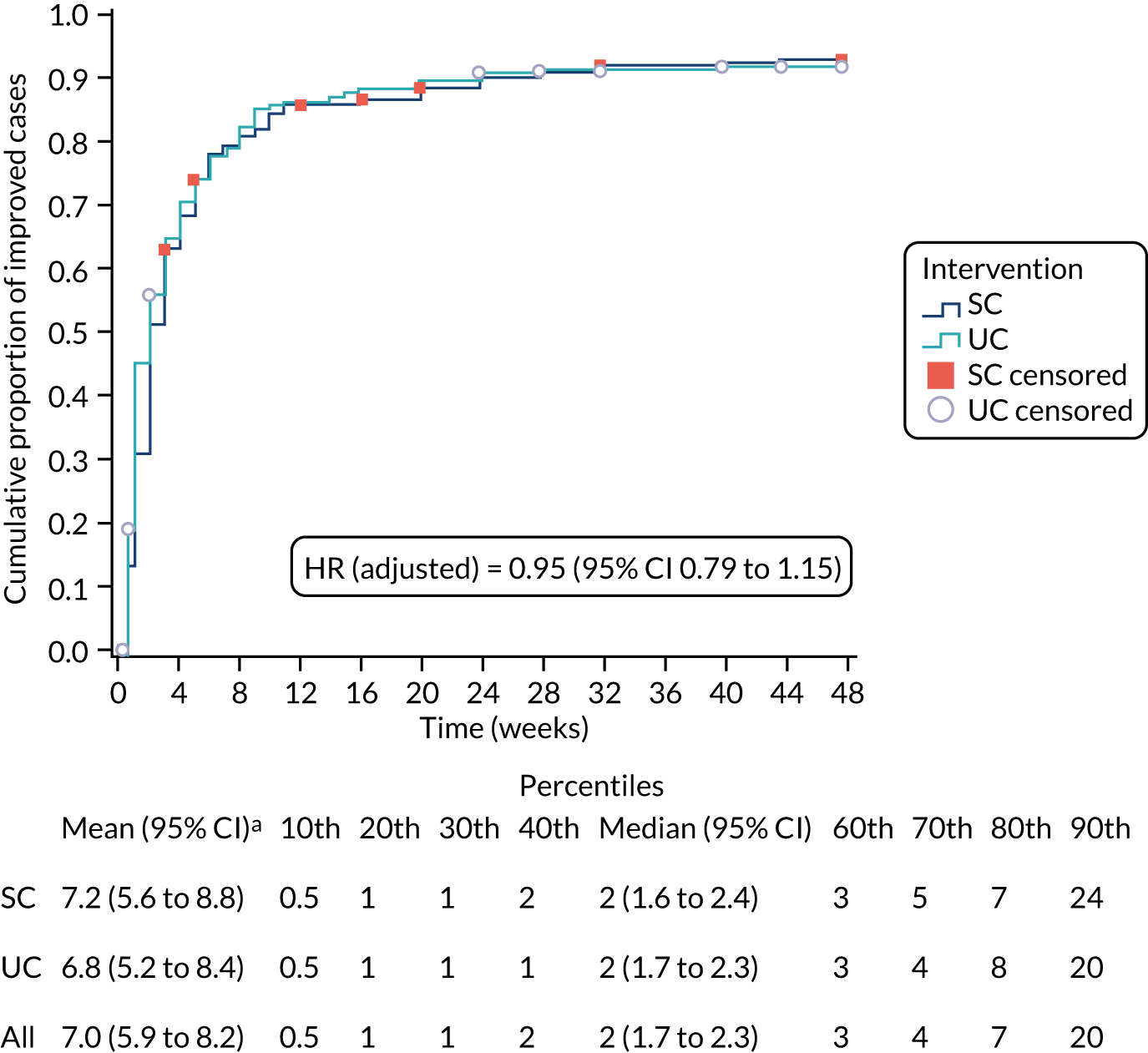
FIGURE 7.
Kaplan–Meier time-to-event analysis of the secondary event classification (time to stable improvement of symptoms). a, Estimation is limited to the largest event-free time if it is censored. Cumulative proportion of resolved cases by week 48: 0.865 (all), 0.875 (SC) and 0.855 (UC).
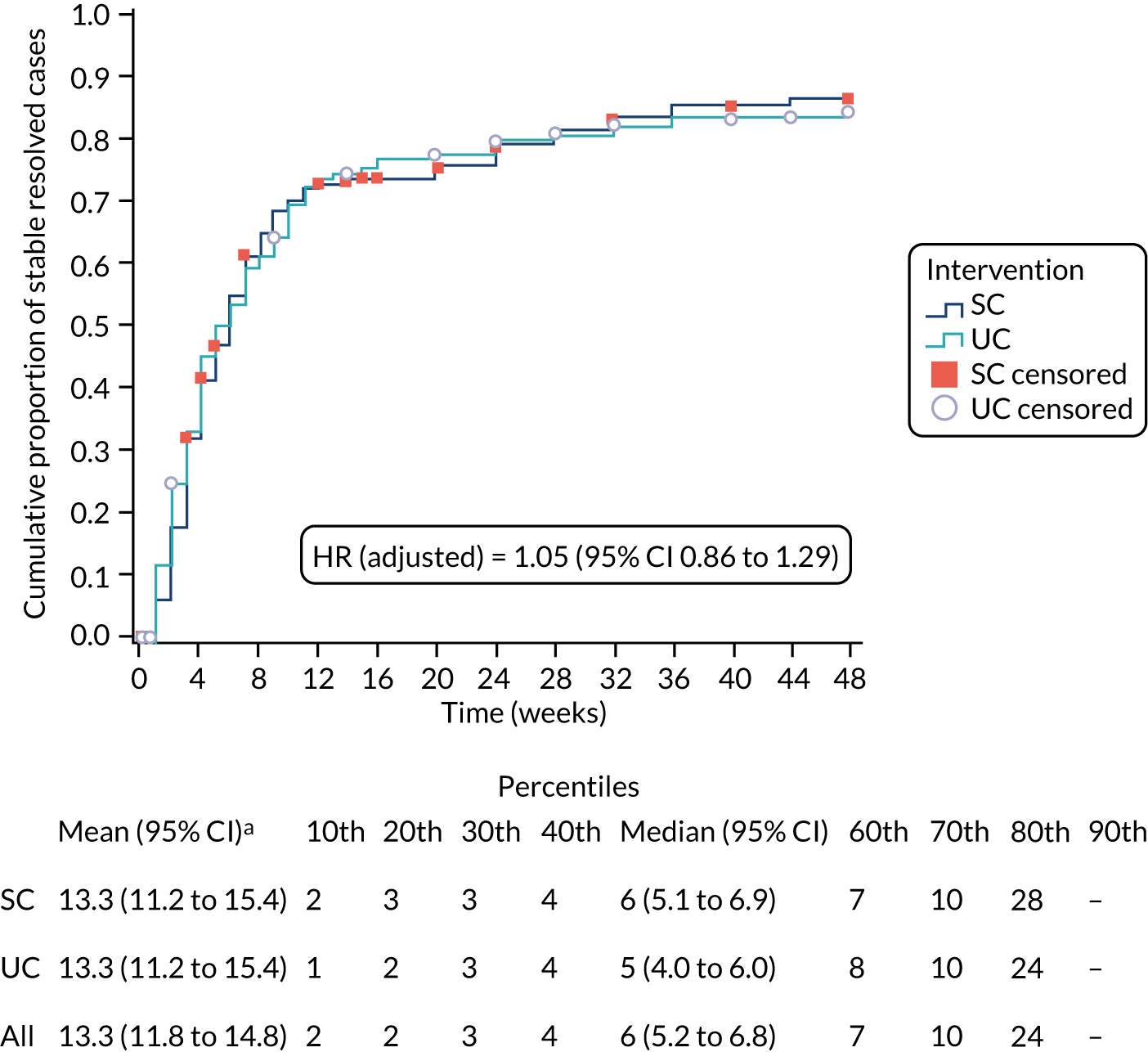
Table 7 summarises the hypothesis test results for the time-to-event sensitivity analyses. Estimates for the HR (SC relative to UC) were close to 1.0 (range 0.86–1.22) in all cases and mostly in the range of 1.0 to 1.2, indicating a slightly faster time to event in the SC arm than in the UC arm. Similar to the primary end point evaluation, all tests showed no statistically significant differences between the trial arms (p > 0.05). Sensitivity analyses addressing different patterns for the missingness of data showed similar HR estimates (and close to 1) across the different scenarios.
| HR (95% CI); p-value | |
|---|---|
| Stable resolutiona | 1.11 (0.87 to 1.43); 0.389 |
| Improvementb | 0.95 (0.79 to 1.15); 0.585 |
| Stable improvementc | 1.05 (0.86 to 1.29); 0.606 |
| Resolution: missing | |
| Mean imputation | 1.15 (0.90 to 1.47); 0.272 |
| Resolved imputation | 1.12 (0.89 to 1.41); 0.324 |
| Stable resolution: missing | |
| Mean imputation | 1.12 (0.87 to 1.43); 0.382 |
| Resolved imputation | 1.11 (0.89 to 1.39); 0.337 |
| Improvement: missing | |
| Mean imputation | 0.95 (0.79 to 1.15); 0.620 |
| Resolved imputation | 0.92 (0.77 to 1.11); 0.386 |
| Stable improvement: missing | |
| Mean imputation | 1.06 (0.86 to 1.29); 0.595 |
| Resolved imputation | 1.07 (0.88 to 1.29); 0.506 |
| Parametric | |
| Weibull model | 1.16 (0.91 to 1.50); 0.237 |
| Exponential model | 1.19 (0.89 to 1.59); 0.249 |
| Log-normal model | 0.88 (0.65 to 1.18); 0.380 |
| Interval-censoring | |
| Weibull | 1.17 (0.91 to 1.49); 0.219 |
| Exponential | 1.20 (0.89 to 1.62); 0.237 |
| Log-normal | 0.86 (0.62 to 1.19); 0.361 |
| Log-rank (non-parametric) test | p = 0.368 |
| Breslow (non-parametric) test | p = 0.489 |
| Tarone–Ware (non-parametric) test | p = 0.432 |
| Complete-case analysisd | 1.22 (0.89 to 1.66); 0.212 |
Per-protocol analysis
A total of 51 participants were judged to not have had care according to the SC intervention protocol and were removed from the per-protocol analysis. The main reason for protocol deviation was participants in sciatica group 2 in the SC arm of the trial receiving fewer than three physiotherapy sessions; this was due to either patients not completing their physiotherapy course of treatment or being discharged by their treating physiotherapist. A further 15 patients, one in sciatica group 1 and 14 in group 2, were eventually referred to spinal specialist services by their treating physiotherapist (for details of protocol deviation reasons, see Appendix 2, Table 25). A total of 138 out of 187 (73.8%) had experienced the event (symptom resolution) by week 48 in the SC arm [compared with 168/238 (70.6%) in the UC arm]. The median event times were 10.0 weeks for the SC and 12.0 weeks in the UC. The HR for SC relative to UC was 1.10 (95% CI 0.84 to 1.45; p = 0.491). Similar to the primary ITT analysis, there was no statistically significant difference between SC and UC.
Subgroup analyses
By sciatica group (1, 2 and 3)
Figure 8 shows the time-to-event data for the primary outcome stratified according to sciatica group (groups 1, 2 and 3) and by trial arm. For group 1, 68 out of 107 (63.6%) participants and 82 out of 107 (76.6%) participants reported that their sciatica symptoms had resolved by weeks 16 and 48, respectively: 31 out of 53 (58.5%) and 38 out of 53 (71.7%), respectively, in the SC arm, and 37 out of 54 (68.5%) and 44 out of 54 (81.5%), respectively, in the UC arm. For group 2, 127 out of 211 (60.2%) and 157 out of 211 (74.4%) participants reported resolved symptoms by weeks 16 and 48, respectively: 66 out of 105 (62.9%) and 81 out of 105 (77.1%), respectively, in the SC arm, and 61 out of 106 (57.5%) and 76 out of 106 (71.7%), respectively, in the UC arm. For group 3, 79 out of 158 (50.0%) and 105 out of 158 (66.5%) participants had resolved symptoms by weeks 16 and 48, respectively: 40 out of 80 (50.0%) and 57 out of 80 (71.3%), respectively, in the SC arm, and 39 out of 78 (50.0%) and 48 out of 78 (61.5%), respectively, in the UC arm. The median event times are displayed in Figure 8 and show that participants in sciatica groups 1 and 3 in the SC arm took slightly longer to reach resolution than participants in sciatica groups 1 and 3 in the UC arm. Conversely, participants in group 2 in the SC arm achieved resolution of sciatica symptoms slightly faster than participants in group 2 in the UC arm. The adjusted HR for group 2 relative to group 1 (ratio SC-to-UC) was 1.46 (95% CI 0.84 to 2.54; p = 0.182), and for group 3 relative to group 1 (ratio SC-to-UC) it was 1.43 (95% CI 0.78 to 2.62; p = 0.243). For the secondary event definitions, the adjusted HRs were, respectively: (i) 1.70 (95% CI 0.94 to 3.09; p = 0.081) for group 2 relative to group 1 and 1.52 (95% CI 0.79 to 2.90; p = 0.209) for group 3 relative to group 1 for ‘stable resolution’; (ii) 1.17 (95% CI 0.72 to 1.90; p = 0.526) for group 2 relative to group 1 and 1.05 (95% CI 0.63 to 1.77; p = 0.849) for group 3 relative to group 1 for first ‘improvement’; (iii) 1.11 (95% CI 0.67 to 1.84; p = 0.689) for group 2 relative to group 1 and 0.96 (95% CI 0.56 to 1.64; p = 0.891) for group 3 relative to group 1 for ‘stable improvement’.
FIGURE 8.
Kaplan–Meier time-to-event (time to first resolution of sciatica symptoms) in each of the sciatica groups (1, 2, and 3).
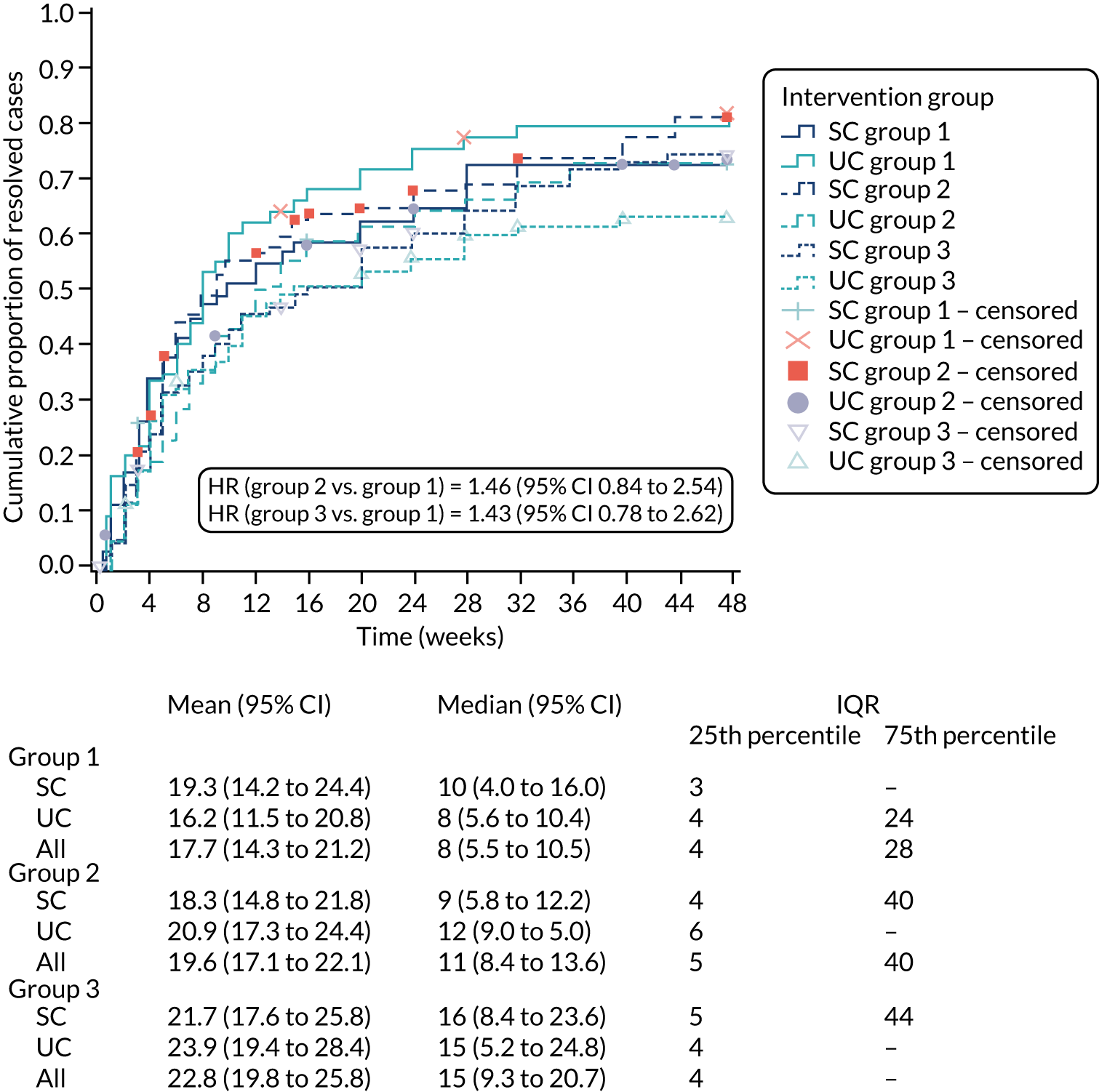
By sciatica clinical diagnostic classification
Figure 9 shows the time-to-event data for the primary outcome stratified according to the clinical diagnosis of either disc-related sciatica or spinal stenosis. Information on clinical diagnosis of sciatica was extracted from the SCOPiC trial clinic physiotherapists’ standardised clinical assessment forms. Physiotherapists recorded a response for this for 461 participants: for 398 out of 461 (86.3%) participants, physiotherapists recorded a clinical diagnosis of disc-related sciatica, and for the remaining 63 (13.7%) participants, a clinical diagnosis of sciatica due to stenosis was recorded. Figure 9 displays the mean and median event times. It shows similar average event times for the SC and UC arms within the subgroup of participants with a clinical diagnosis of disc-related sciatica, but faster resolution for those with clinically suspected stenosis in the SC arm than for those with clinically suspected stenosis in the UC arm. The adjusted HR for the clinically diagnosed spinal stenosis subgroup relative to the group with disc-related sciatica (ratio SC-to-UC) was 1.92 (95% CI 1.01 to 3.65; p = 0.043). For the secondary event definitions, the adjusted HRs were 1.56 (95% CI 0.78 to 3.12; p = 0.213) for ‘stable resolution’, 1.05 95% CI (0.61 to 1.81; p = 0.868) for first ‘improvement’ and 1.18 (95% CI 0.67 to 2.09; p = 0.563) for ‘stable improvement’.
FIGURE 9.
Kaplan–Meier time-to-event (time to first resolution of sciatica symptoms) for those with a clinical diagnosis of disc-related sciatica and those with clinical diagnosis of spinal stenosis.
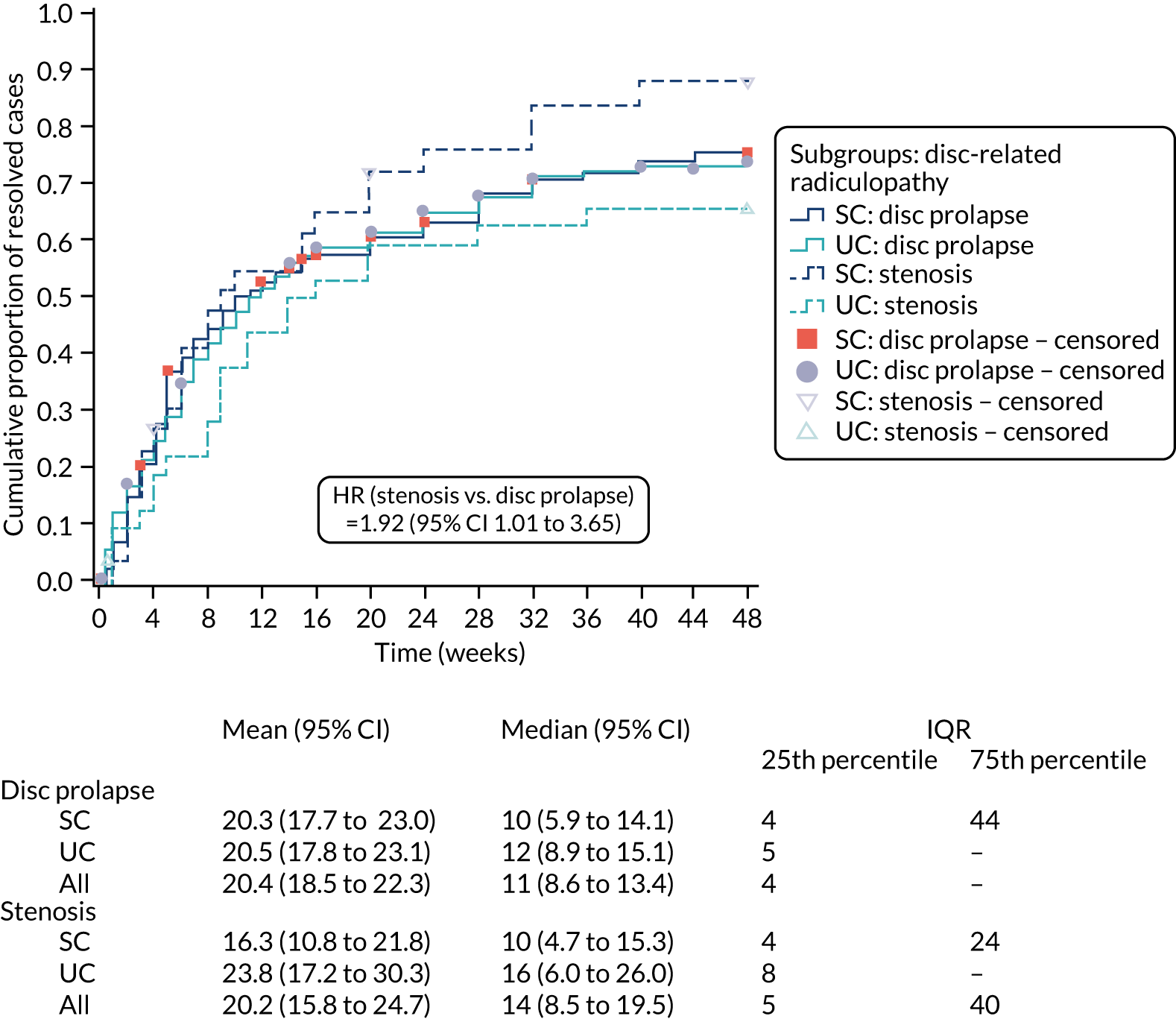
Table 8 displays summary baseline characteristics by clinically diagnosed sciatica due to either disc prolapse or spinal stenosis.
| Characteristic | Clinically diagnosed disc-related sciatica | Clinically diagnosed spinal stenosis | ||||
|---|---|---|---|---|---|---|
| All (N = 398) | SC (N = 203) | UC (N = 195) | All (N = 63) | SC (N = 30) | UC (N = 33) | |
| Age (years), mean (SD) | 49.8 (13.2) | 48.4 (13.6) | 51.3 (12.7) | 66.5 (11.2) | 66.8 (11.2) | 66.2 (11.3) |
| Sex, n (%) | ||||||
| Females | 215 (54) | 108 (53) | 107 (55) | 38 (60) | 21 (70) | 17 (52) |
| Males | 183 (46) | 95 (47) | 88 (45) | 25 (40) | 9 (29) | 16 (48) |
| IMD rank value,a median (IQR) | 14,228 (7193–21,626) | 14,228 (7084–21,790) | 14,604 (7193–21,790) | 20,215 (10,569–22,887) | 18,046 (6767–24,210) | 20,841 (13,447–23,491) |
| Employed, n (%) | 295 (75) | 155 (77) | 140 (72) | 27 (43) | 12 (40) | 15 (45) |
| Time off work because of sciatica, in the previous 12 months, n (%) | 164 (56) | 78 (51) | 86 (61) | 9 (32) | 3 (23) | 6 (40) |
| WPI score, mean (SD) | 6.0 (2.8) | 5.9 (2.8) | 6.1 (2.7) | 5.2 (3.4) | 4.5 (3.3) | 5.8 (3.5) |
| Prescription,b n (%) | 315 (81) | 168 (84) | 147 (77) | 49 (79) | 21 (70) | 28 (88) |
| Sciatica group, n (%) | ||||||
| 1 | 93 (23) | 47 (23) | 46 (24) | 11 (17) | 4 (13) | 7 (21) |
| 2 | 173 (43) | 87 (43) | 86 (44) | 28 (44) | 16 (53) | 12 (36) |
| 3 | 132 (33) | 69 (34) | 63 (32) | 24 (38) | 10 (33) | 14 (42) |
| Usual back pain score, mean (SD) | 5.9 (2.8) | 5.9 (2.7) | 5.8 (2.8) | 5.7 (3.1) | 5.7 (2.9) | 5.6 (3.4) |
| Usual leg pain score, mean (SD) | 6.8 (2.3) | 6.7 (2.3) | 6.8 (2.3) | 7.6 (1.7) | 7.5 (1.6) | 7.7 (1.8) |
| Leg pain interferencec (yes/no), n (%) | 302 (76) | 153 (76) | 149 (76) | 52 (83) | 26 (87) | 26 (79) |
| Leg pain interference score, mean (SD) | 6.5 (2.4) | 6.3 (2.5) | 6.6 (2.4) | 6.4 (2.6) | 6.1 (2.8) | 6.6 (2.5) |
| Symptom duration, n (%) | ||||||
| < 2 weeks | 41 (10) | 13 (6) | 28 (14) | 6 (10) | 2 (7) | 4 (12) |
| 2–6 weeks | 170 (43) | 89 (44) | 81 (42) | 18 (29) | 6 (20) | 12 (36) |
| 6–12 weeks | 85 (21) | 50 (25) | 35 (18) | 15 (24) | 8 (27) | 7 (21) |
| 3–6 months | 50 (13) | 26 (13) | 24 (12) | 10 (16) | 5 (17) | 5 (15) |
| 6–12 months | 15 (4) | 7 (3) | 8 (4) | 5 (8) | 3 (10) | 2 (6) |
| > 12 months | 36 (9) | 17 (8) | 19 (10) | 9 (14) | 6 (20) | 3 (9) |
| RMDQ score,d mean (SD) | 11.1 (5.4) | 11.1 (5.3) | 11.2 (5.5) | 11.5 (4.9) | 11.6 (5.2) | 11.4 (4.7) |
| SBI score,e mean (SD) | 14.5 (5.0) | 14.5 (5.0) | 14.4 (5.1) | 14.8 (4.6) | 15.2 (4.8) | 14.5 (4.5) |
| S-LANSS score,f n (%) | ||||||
| < 12 | 214 (57) | 109 (58) | 105 (56) | 38 (69) | 15 (60) | 23 (77) |
| ≥ 12 | 164 (43) | 80 (42) | 84 (44) | 17 (31) | 10 (40) | 7 (23) |
| TSK score,g mean (SD) | 40.7 (6.0) | 40.2 (6.1) | 41.1 (6.0) | 39.8 (6.8) | 41.1 (6.5) | 38.7 (7.0) |
| HADS-A score,h mean (SD) | 8.0 (4.1) | 7.7 (4.1) | 8.3 (4.0) | 7.2 (3.9) | 8.5 (3.6) | 5.9 (3.7) |
| HADS-A, n (%) | ||||||
| Normal (0–7) | 192 (48) | 102 (51) | 90 (46) | 38 (60) | 12 (40) | 26 (79) |
| Possible (8–10) | 99 (25) | 53 (26) | 46 (24) | 13 (21) | 11 (37) | 2 (6) |
| Probable (≥ 11) | 106 (27) | 47 (23) | 59 (30) | 12 (19) | 7 (23) | 5 (15) |
| HADS-D score,h mean (SD) | 6.3 (4.0) | 6.4 (4.0) | 6.3 (4.0) | 6.6 (3.8) | 6.6 (3.4) | 6.6 (4.2) |
| HADS-D, n (%) | ||||||
| Normal (0–7) | 259 (65) | 133 (66) | 126 (65) | 40 (64) | 20 (67) | 20 (61) |
| Possible (8–10) | 71 (18) | 33 (16) | 38 (19) | 12 (19) | 6 (20) | 6 (18) |
| Probable (≥ 11) | 67 (17) | 36 (18) | 31 (16) | 11 (17) | 4 (13) | 7 (21) |
| Sleep problem, n (%) | 260 (65) | 127 (63) | 133 (68) | 41 (65) | 19 (63) | 22 (67) |
| General health, n (%) | ||||||
| Excellent | 21 (5) | 10 (5) | 11 (6) | 1 (2) | 0 (0) | 1 (3) |
| Very good | 87 (22) | 47 (23) | 40 (21) | 12 (19) | 4 (13) | 8 (24) |
| Good | 173 (44) | 89 (44) | 84 (43) | 32 (51) | 17 (57) | 15 (46) |
| Fair | 88 (22) | 41 (20) | 47 (24) | 16 (25) | 7 (23) | 9 (27) |
| Poor | 28 (7) | 15 (7) | 13 (7) | 2 (3) | 2 (7) | 0 (0) |
| STarT Back Screening Tool risk subgroup, n (%) | ||||||
| Low | 93 (23) | 49 (24) | 44 (23) | 12 (19) | 5 (17) | 7 (21) |
| Medium | 218 (55) | 114 (56) | 104 (54) | 34 (54) | 16 (53) | 18 (55) |
| High | 85 (21) | 39 (19) | 46 (24) | 17 (27) | 9 (30) | 8 (24) |
Secondary outcomes
A summary of overall Global Perceived Change (GPC) ratings from the 4- and 12-month questionnaires is shown in Figure 10. There was no statistically significant difference in GPC ratings between trial arms at either follow-up time point, although, at 12 months, slightly more participants in the SC arm than in the UC arm reported greater GPC ratings. In total, 78 out of 188 (41.5%) in the SC arm reported being ‘completely recovered’ or ‘much better’ at 4 months, compared with 85 out of 194 (43.8%) in the UC arm; at 12 months, these numbers were 97 out of 174 (55.8%) and 88 out of 176 (50.0%), respectively. The summary results for all secondary outcome measures are presented in Table 9. Usual back pain and usual leg pain intensity reduced from 5.8 and 6.9, respectively, at baseline to 3.6 and 3.2, respectively, at 4 months and to 2.9 and 2.8, respectively, at 12 months. The mean reduction in usual back pain was 3.7 at 4 months and 4.1 at 12 months for participants who reported being ‘completely recovered’ or ‘much better’, compared with 0.8 and 0.9 at 4 and 12 months, respectively, for participants who did not report these levels of improvement. There was a similar differential for usual leg pain, which was reduced by 5.6 and 5.8 at 4 and 12 months, respectively, for those who reported being ‘completely recovered’ or ‘much better’, and by 2.2 and 1.8, respectively, for those who were not. As these contrasts in average scale scores between those patients who were completely recovered or much better and those who were not were similar across back and leg pain scales, this would suggest that participants’ rating of GPC of symptoms was similarly driven by both back and leg pain intensity (and not only one of these).
FIGURE 10.
Secondary outcomes: GPC by trial arm at 4 and 12 months. (a) GPC at 4 months; and (b) GPC at 12 months. The ORs are the proportional odds of a favourable outcome relative to not being better, derived through ordinal logistic regression.
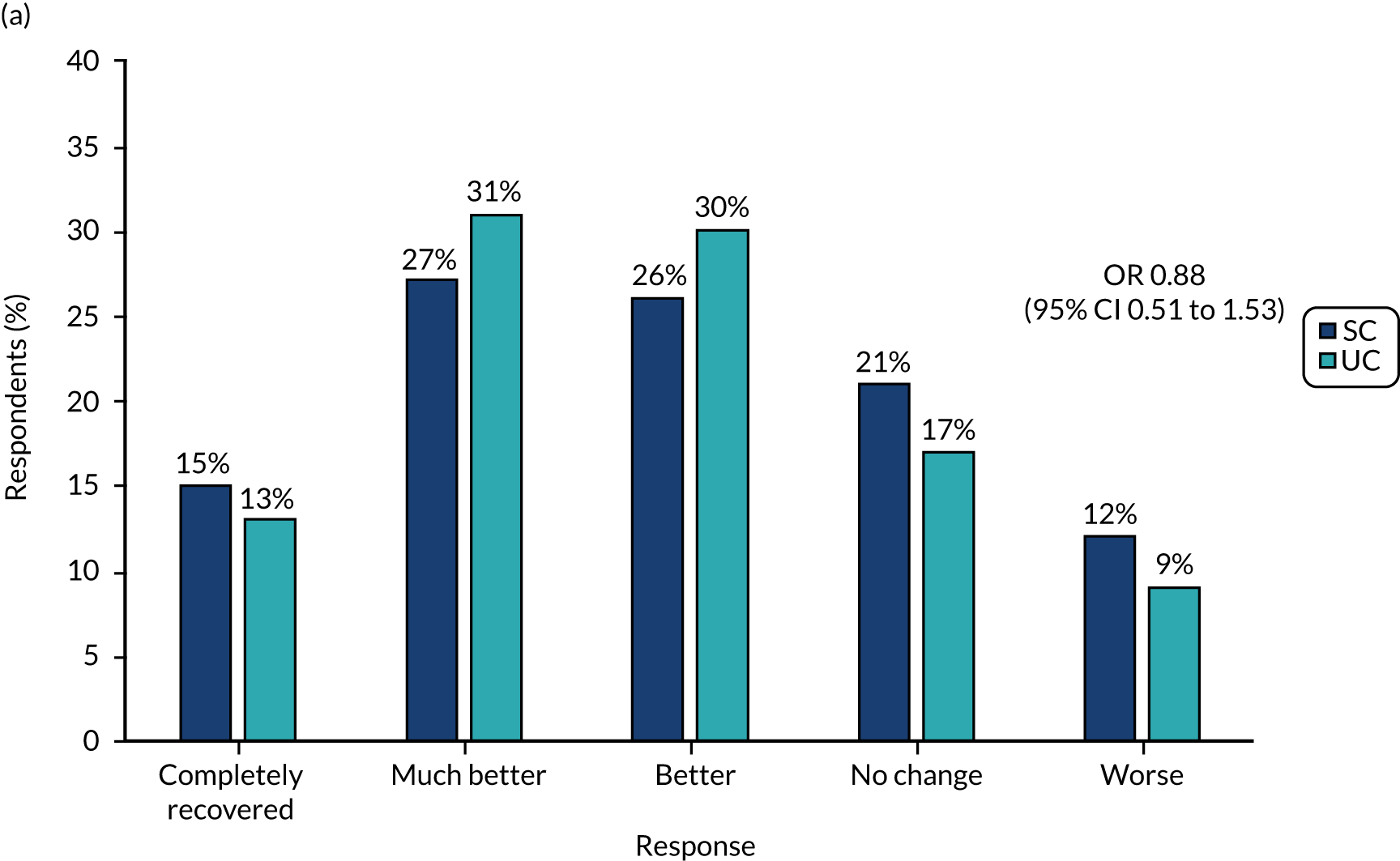
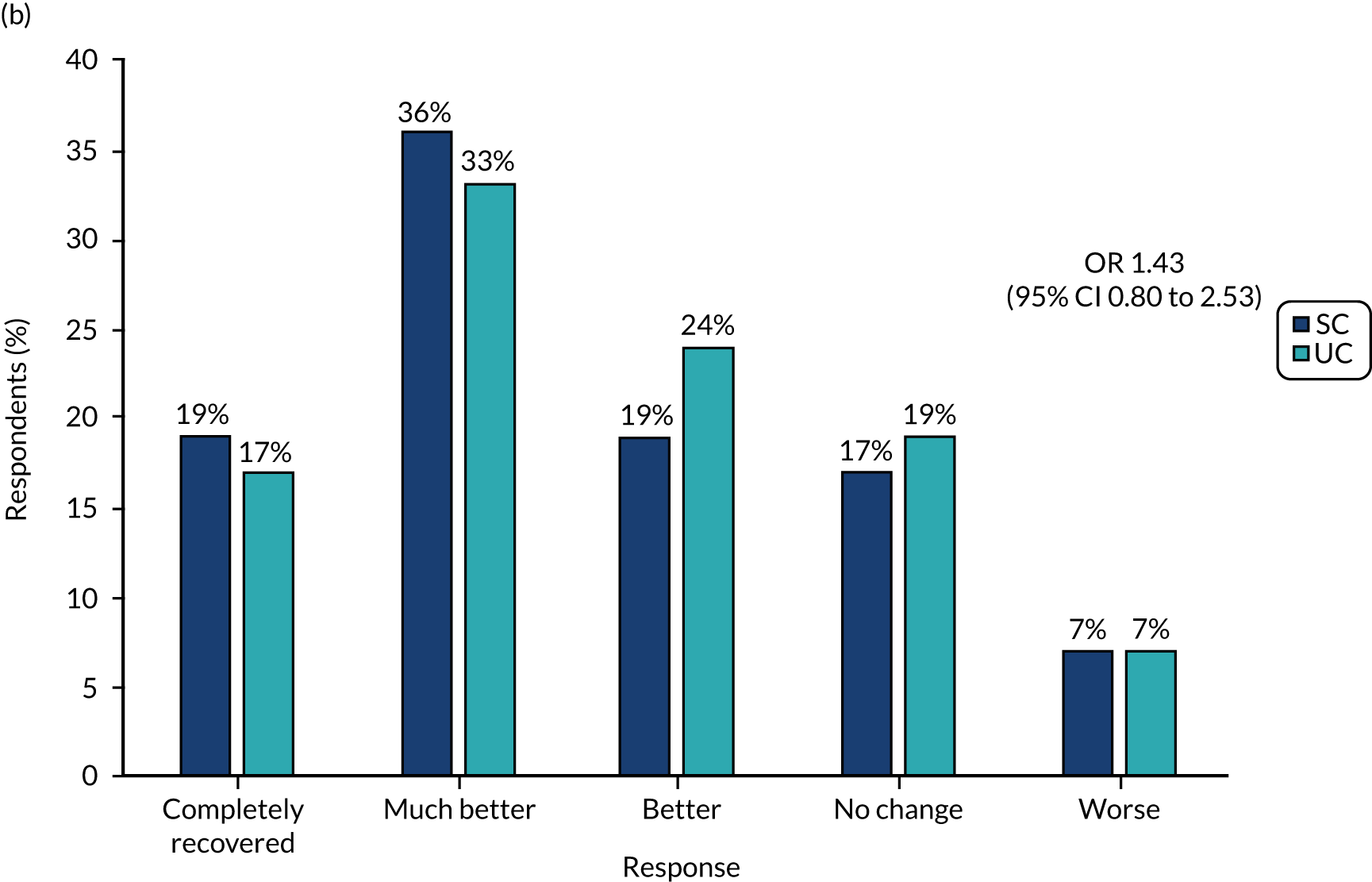
| Health outcome | Trial arm | Between-group effect, MDa/ORb (95% CI); p-value | |
|---|---|---|---|
| SC | UC | ||
| Physical function (RMDQ,c 0–23), mean (SD) | |||
| 4 months | 6.5 (6.3) | 6.2 (6.0) | MD 0.43 (–0.69 to 1.54); 0.451 |
| 12 months | 5.0 (6.2) | 5.5 (6.0) | MD –0.53 (–1.84 to 0.78); 0.429 |
| GPC,d n (%) | |||
| 4 months | |||
| Completely recovered | 28 (15) | 26 (13) | OR 0.88 (0.51 to 1.53); 0.656 |
| Much better | 50 (27) | 59 (31) | |
| Better | 48 (26) | 59 (30) | |
| No change | 39 (21) | 32 (17) | |
| Worse | 23 (12) | 18 (9) | |
| 12 months | |||
| Completely recovered | 34 (19) | 30 (17) | OR 1.43 (0.80 to 2.53); 0.224 |
| Much better | 63 (36) | 58 (33) | |
| Better | 34 (19) | 42 (24) | |
| No change | 30 (17) | 34 (19) | |
| Worse | 13 (7) | 12 (7) | |
| Usual back pain (NRSe 0–10), mean (SD) | |||
| 4 months | 3.8 (2.8) | 3.4 (2.6) | MD 0.32 (–0.30 to 0.94); 0.312 |
| 12 months | 3.2 (2.8) | 2.7 (2.5) | MD 0.26 (–0.48 to 1.01); 0.489 |
| Usual leg pain (NRSe 0–10), mean (SD) | |||
| 4 months | 3.3 (2.9) | 3.1 (2.8) | MD 0.25 (–0.36 to 0.86); 0.421 |
| 12 months | 2.9 (2.9) | 2.8 (2.8) | MD 0.11 (–0.56 to 0.77); 0.752 |
| Leg pain interference (NRSe 0–10), mean (SD) | |||
| 4 months | 2.9 (3.0) | 2.6 (2.8) | MD 0.50 (–0.10 to 1.10); 0.102 |
| 12 months | 2.4 (2.9) | 2.3 (2.7) | MD 0.27 (–0.37 to 0.90); 0.417 |
| SBIf (0–24), mean (SD) | |||
| 4 months | 7.9 (6.0) | 7.5 (5.3) | MD 0.26 (–1.03 to 1.55); 0.692 |
| 12 months | 6.7 (5.7) | 6.5 (6.1) | MD –0.42 (–1.94 to 1.11); 0.591 |
| S-LANSSg (≥ 12), n (%) | |||
| 4 months | 35 (25.7) | 33 (23.9) | OR 1.17 (0.49 to 2.79); 0.724 |
| 12 months | 22 (22.4) | 22 (21.0) | OR 1.08 (0.39 to 2.98); 0.877 |
| TSKh (17–64), mean (SD) | |||
| 4 months | 36.9 (8.4) | 36.2 (7.4) | MD 0.53 (–0.87 to 1.92); 0.461 |
| 12 months | 35.2 (8.5) | 35.3 (7.8) | MD –0.37 (–1.88 to 1.13); 0.625 |
| HADS-Ai score (0–21), mean (SD) | |||
| 4 months | 6.1 (4.3) | 6.0 (4.0) | MD 0.54 (–0.16 to 1.23); 0.132 |
| 12 months | 5.9 (4.6) | 5.3 (4.2) | MD 0.51 (–0.32 to 1.33); 0.227 |
| HADS-A,i n (%) | |||
| 4 months | |||
| Normal (0–7) | 104 (69.3) | 103 (65.6) | OR 1.36 (0.59 to 3.13); 0.476 |
| Possible (8–10) | 26 (17.3) | 37 (23.6) | |
| Probable (≥ 11) | 20 (13.3) | 17 (10.8) | |
| 12 months | |||
| Normal (0–7) | 75 (63.0) | 97 (72.9) | OR 2.30 (0.94 to 5.65); 0.070 |
| Possible (8–10) | 21 (17.6) | 16 (12.0) | |
| Probable (≥ 11) | 23 (19.3) | 20 (15.0) | |
| HADS-D score (0–21),i mean (SD) | |||
| 4 months | 4.7 (3.9) | 4.8 (4.1) | MD –0.37 (–1.19 to 0.44); 0.368 |
| 12 months | 4.5 (4.2) | 4.2 (4.2) | MD –0.29 (–1.14 to 0.57); 0.509 |
| HADS-D,i n (%) | |||
| 4 months | |||
| Normal (0–7) | 117 (78.0) | 121 (76.6) | OR 0.99 (0.41 to 2.42); 0.991 |
| Possible (8–10) | 18 (12.0) | 19 (12.0) | |
| Probable (≥ 11) | 15 (10.0) | 18 (11.4) | |
| 12 months | |||
| Normal (0–7) | 89 (74.8) | 103 (77.4) | OR 1.24 (0.48 to 3.22); 0.655 |
| Possible (8–10) | 18 (15.1) | 15 (11.3) | |
| Probable (≥ 11) | 12 (10.1) | 15 (11.3) | |
| Sleep problem, n (%) | |||
| 4 months | 54 (35.1) | 61 (38.4) | OR 1.59 (0.66 to 3.82); 0.299 |
| 12 months | 42 (33.9) | 41 (31.1) | OR 2.21 (0.85 to 5.72); 0.103 |
| General health, n (%) | |||
| 4 months | |||
| Excellent | 5 (3.3) | 10 (6.3) | OR 1.21 (0.65 to 2.24); 0.556 |
| Very good | 47 (30.7) | 35 (22.2) | |
| Good | 60 (39.2) | 69 (43.7) | |
| Fair | 32 (20.9) | 35 (22.2) | |
| Poor | 9 (5.9) | 9 (5.7) | |
| 12 months | |||
| Excellent | 9 (7.5) | 12 (9.0) | OR 1.49 (0.76 to 2.94); 0.249 |
| Very good | 43 (35.8) | 42 (31.6) | |
| Good | 39 (32.5) | 47 (35.3) | |
| Fair | 27 (22.5) | 24 (18.0) | |
| Poor | 2 (1.7) | 8 (6.0) | |
| Time off work, n (%) | |||
| 4 months | 45 (42) | 47 (49) | OR 1.11 (0.47 to 2.61); 0.817 |
| 12 months | 20 (27) | 15 (19) | OR 2.52 (0.85 to 7.49); 0.095 |
| WPI (NRSe 0–10), mean (SD) | |||
| 4 months | 4.0 (2.9) | 4.2 (3.0) | MD 0.08 (–0.73 to 0.90); 0.841 |
| 12 months | 3.0 (2.8) | 3.0 (2.6) | MD 0.17 (–0.81 to 1.14); 0.737 |
| STarT Back Screening Tool risk subgroup, n (%) | |||
| 4 months | |||
| Low | 85 (61.6) | 100 (68.5) | OR 1.78 (0.78 to 4.06); 0.170 |
| Medium | 34 (24.6) | 33 (22.6) | |
| High | 19 (13.8) | 13 (8.9) | |
| 12 months | |||
| Low | 78 (70.3) | 84 (74.3) | OR 0.92 (0.35 to 2.43); 0.873 |
| Medium | 23 (20.7) | 21 (18.6) | |
| High | 10 (9.0) | 8 (7.1) | |
Sciatica-related physical disability levels (RMDQ mean scores) also reduced, from 11.2 at baseline to 6.3 at 4 months and 5.3 at 12 months. As shown in Table 9, similar improvements, on average, were reported for all outcomes in both trial arms. There were no statistically significant differences in the secondary outcomes between trial arms. Absolute effect sizes for numerical secondary outcomes (standardised mean difference relative to baseline SD) were nearly all lower than the preclassified cut-off point for a ‘small’ effect size (i.e. ≤ 0.2).
Table 10 summarises the data for the secondary outcome measures between trial arms for sciatica groups 1, 2 and 3. There was little difference in health outcomes between interventions arms in each of the three sciatica groups. A trend of worsening health status values was recorded from group 1 through group 2 to group 3. For example, for disability, the 4-month mean value was lowest, at 3.1, in group 1 and highest, at 9.2, in group 3 (similar trends were observed for other measures).
| Secondary outcome | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|
| SC | UC | SC | UC | SC | UC | |
| Physical function (RMDQ),a mean (SD) | n/n = 47/44 | n/n = 48/48 | n/n = 81/74 | n/n = 90/78 | n/n = 64/59 | n/n = 63/56 |
| 4 months | 3.3 (5.0) | 3.0 (3.4) | 6.0 (6.0) | 5.9 (5.6) | 9.4 (6.4) | 9.1 (6.9) |
| 12 months | 1.8 (2.8) | 3.9 (4.5) | 4.9 (6.4) | 5.0 (5.7) | 7.6 (6.8) | 7.6 (7.1) |
| GPC, n (%) | ||||||
| 4 months | N = 45 | N = 46 | N = 79 | N = 87 | N = 64 | N = 61 |
| Recovered | 9 (20) | 7 (15) | 15 (19) | 14 (16) | 4 (6) | 5 (8) |
| Much better | 13 (29) | 18 (39) | 22 (28) | 28 (32) | 15 (23) | 13 (21) |
| Better | 10 (22) | 11 (24) | 20 (25) | 22 (25) | 18 (28) | 26 (43) |
| No change | 8 (18) | 10 (22) | 17 (22) | 13 (15) | 14 (22) | 9 (15) |
| Worse | 5 (11) | 0 (0) | 5 (6) | 10 (11) | 13 (20) | 8 (13) |
| 12 months | N = 44 | N = 46 | N = 71 | N = 78 | N = 59 | N = 52 |
| Recovered | 13 (30) | 9 (20) | 14 (20) | 14 (18) | 7 (12) | 7 (13) |
| Much better | 16 (36) | 13 (28) | 27 (38) | 28 (36) | 20 (34) | 17 (33) |
| Better | 7 (16) | 12 (26) | 17 (24) | 19 (24) | 10 (17) | 11 (21) |
| No change | 7 (16) | 8 (17) | 10 (14) | 16 (21) | 13 (22) | 10 (19) |
| Worse | 1 (2) | 4 (9) | 3 (4) | 1 (1) | 9 (15) | 7 (13) |
| Usual back pain (NRS),b mean (SD) | n/n = 40/30 | n/n = 37/41 | n/n = 67/57 | n/n = 75/49 | n/n = 47/36 | n/n = 46/40 |
| 4 months | 2.8 (2.6) | 2.4 (2.2) | 3.6 (2.6) | 3.3 (2.6) | 5.1 (2.9) | 4.4 (2.8) |
| 12 months | 1.9 (2.3) | 2.7 (2.7) | 3.3 (2.6) | 2.4 (2.1) | 4.2 (3.2) | 3.2 (2.8) |
| Usual leg pain (NRS),b mean (SD) | n/n = 47/43 | n/n = 47/47 | n/n = 80/74 | n/n = 89/76 | n/n = 64/59 | n/n = 61/55 |
| 4 months | 2.3 (2.7) | 2.2 (2.1) | 3.0 (2.7) | 2.9 (2.9) | 4.4 (3.0) | 4.0 (2.9) |
| 12 months | 1.8 (2.3) | 2.8 (2.9) | 2.6 (2.6) | 2.5 (2.5) | 4.0 (3.2) | 3.1 (3.0) |
| Leg pain interference (NRS),a mean (SD) | n/n = 47/43 | n/n = 47/48 | n/n = 80/74 | n/n = 89/76 | n/n = 64/59 | n/n = 61/55 |
| 4 months | 1.7 (2.5) | 1.5 (2.1) | 2.7 (2.8) | 2.4 (2.6) | 4.1 (3.1) | 3.9 (3.1) |
| 12 months | 1.0 (1.8) | 2.1 (2.4) | 2.1 (2.7) | 1.9 (2.6) | 3.6 (3.2) | 3.0 (3.1) |
| SBI,c mean (SD) | n/n = 39/30 | n/n = 37/39 | n/n = 66/55 | n/n = 74/50 | n/n = 45/37 | n/n = 44/37 |
| 4 months | 5.5 (5.6) | 5.1 (3.8) | 8.1 (6.0) | 7.5 (5.4) | 9.8 (5.8) | 9.4 (5.5) |
| 12 months | 4.1 (4.8) | 6.4 (6.3) | 7.0 (5.6) | 5.5 (5.1) | 8.2 (6.1) | 8.0 (6.9) |
| S-LANSS,d n (%) | n/n = 33/25 | n/n = 29/33 | n/n = 59/44 | n/n = 65/39 | n/n = 44/29 | n/n = 44/33 |
| 4 months | 4 (12) | 2 (7) | 16 (27) | 17 (26) | 15 (34) | 14 (32) |
| 12 months | 1 (4) | 5 (15) | 9 (20) | 6 (15) | 12 (41) | 11 (33) |
| TSK,e mean (SD) | n/n = 36/29 | n/n = 36/36 | n/n = 63/52 | n/n = 73/48 | n/n = 46/36 | n/n = 45/38 |
| 4 months | 32.8 (6.3) | 30.3 (6.8) | 34.0 (7.7) | 34.3 (5.7) | 38.9 (8.1) | 38.4 (6.8) |
| 12 months | 29.2 (6.0) | 31.9 (6.8) | 33.7 (8.6) | 32.9 (6.8) | 37.4 (6.7) | 36.9 (7.4) |
| HADS-A,f mean (SD) | n/n = 39/29 | n/n = 38/41 | n/n = 67/55 | n/n = 73/51 | n/n = 44/35 | n/n = 46/41 |
| 4 months | 4.1 (3.0) | 4.5 (3.3) | 6.2 (4.7) | 5.7 (3.3) | 7.6 (4.0) | 7.6 (4.8) |
| 12 months | 4.4 (4.0) | 3.9 (3.8) | 6.0 (4.8) | 4.8 (3.6) | 7.1 (4.2) | 7.4 (4.8) |
| HADS-A,f n (%) | ||||||
| 4 months | N = 39 | N = 38 | N = 67 | N = 73 | N = 44 | N = 46 |
| Normal | 34 (87) | 33 (87) | 46 (69) | 47 (64) | 24 (55) | 23 (50) |
| Possible | 4 (10) | 1 (3) | 9 (13) | 22 (30) | 13 (29) | 14 (30) |
| Probable | 1 (3) | 4 (10) | 12 (18) | 4 (6) | 7 (16) | 9 (20) |
| 12 months | N = 29 | N = 41 | N = 55 | N = 51 | N = 35 | N = 41 |
| Normal | 20 (69) | 35 (85) | 35 (64) | 39 (76) | 20 (57) | 23 (56) |
| Possible | 7 (24) | 3 (7) | 6 (11) | 7 (14) | 8 (23) | 6 (15) |
| Probable | 2 (7) | 3 (7) | 14 (25) | 5 (10) | 7 (20) | 12 (29) |
| HADS-D,f mean (SD) | n/n = 39/29 | n/n = 38/41 | n/n = 65/55 | n/n = 74/51 | n/n = 46/35 | n/n = 46/41 |
| 4 months | 2.5 (2.7) | 2.6 (3.4) | 4.8 (4.2) | 4.7 (3.7) | 6.4 (3.5) | 6.9 (4.4) |
| 12 months | 2.6 (3.2) | 2.8 (3.6) | 4.6 (4.4) | 3.5 (3.2) | 5.8 (4.1) | 6.5 (4.8) |
| HADS-D,f n (%) | ||||||
| 4 months | N = 39 | N = 38 | N = 65 | N = 74 | N = 46 | N = 46 |
| Normal | 38 (97) | 35 (92) | 49 (75) | 60 (81) | 30 (65) | 26 (56) |
| Possible | 0 (0) | 2 (5) | 7 (11) | 8 (11) | 11 (24) | 9 (20) |
| Probable | 1 (3) | 1 (3) | 9 (14) | 6 (8) | 5 (11) | 11 (24) |
| 12 months | N = 29 | N = 41 | N = 55 | N = 51 | N = 35 | N = 41 |
| Normal | 26 (90) | 36 (88) | 39 (71) | 45 (88) | 24 (69) | 22 (54) |
| Possible | 2 (7) | 3 (7) | 9 (16) | 3 (6) | 7 (20) | 9 (22) |
| Probable | 1 (3) | 2 (5) | 7 (13) | 3 (6) | 4 (11) | 10 (24) |
| Sleep problem, n (%) | n/n = 39/30 | n/n = 38/41 | n/n = 68/57 | n/n = 74/50 | n/n = 47/37 | n/n = 47/41 |
| 4 months | 7 (18) | 10 (26) | 23 (34) | 24 (32) | 24 (51) | 27 (57) |
| 12 months | 5 (17) | 9 (22) | 23 (40) | 14 (28) | 14 (38) | 18 (44) |
| General health, n (%) | ||||||
| 4 months | N = 39 | N = 38 | N = 68 | N = 74 | N = 46 | N = 46 |
| Excellent | 3 (8) | 5 (13) | 1 (1) | 5 (7) | 1 (2) | 0 (0) |
| Very good | 16 (41) | 13 (34) | 19 (28) | 14 (19) | 12 (26) | 8 (17) |
| Good | 17 (44) | 15 (39) | 25 (37) | 37 (50) | 18 (39) | 17 (37) |
| Fair | 3 (8) | 4 (11) | 18 (26) | 17 (23) | 11 (24) | 14 (30) |
| Poor | 0 (0) | 1 (3) | 5 (7) | 1 (1) | 4 (9) | 7 (15) |
| 12 months | N = 29 | N = 41 | N = 56 | N = 51 | N = 35 | N = 41 |
| Excellent | 3 (10) | 8 (20) | 5 (9) | 3 (6) | 1 (3) | 1 (2) |
| Very good | 14 (48) | 14 (34) | 17 (30) | 21 (41) | 12 (34) | 7 (17) |
| Good | 10 (34) | 14 (34) | 17 (30) | 15 (29) | 12 (34) | 18 (44) |
| Fair | 2 (7) | 4 (10) | 16 (29) | 10 (20) | 9 (26) | 10 (24) |
| Poor | 0 (0) | 1 (2) | 1 (2) | 2 (4) | 1 (3) | 5 (12) |
| Time off work, n (%) | n/n = 29/19 | n/n = 25/27 | n/n = 45/35 | n/n = 47/32 | n/n = 33/21 | n/n = 24/22 |
| 4 months | 8 (28) | 11 (44) | 20 (44) | 21 (45) | 17 (52) | 15 (63) |
| 12 months | 0 (0) | 5 (19) | 9 (26) | 2 (6) | 11 (52) | 8 (36) |
| WPI, mean (SD) | n/n = 30/20 | n/n = 25/28 | n/n = 46/35 | n/n = 48/33 | n/n = 30/21 | n/n = 24/23 |
| 4 months | 3.2 (2.4) | 3.5 (2.9) | 4.0 (3.1) | 3.9 (2.8) | 4.9 (2.8) | 5.4 (3.2) |
| 12 months | 1.7 (2.0) | 2.6 (2.7) | 2.8 (2.7) | 2.6 (2.0) | 4.5 (2.9) | 4.0 (3.0) |
| STarT Back Screening Tool risk subgroup, n (%) | ||||||
| 4 months | N = 36 | N = 33 | N = 59 | N = 70 | N = 43 | N = 43 |
| Low | 31 (86) | 27 (82) | 37 (63) | 52 (74) | 17 (40) | 21 (49) |
| Medium | 4 (11) | 6 (18) | 14 (24) | 13 (19) | 16 (37) | 14 (33) |
| High | 1 (3) | 0 (0) | 8 (14) | 5 (7) | 10 (23) | 8 (19) |
| 12 months | N = 26 | N = 35 | N = 50 | N = 44 | N = 35 | N = 34 |
| Low | 24 (92) | 28 (80) | 37 (74) | 37 (84) | 17 (49) | 19 (56) |
| Medium | 2 (8) | 7 (20) | 8 (16) | 6 (14) | 13 (37) | 8 (23) |
| High | 0 (0) | 0 (0) | 5 (10) | 1 (2) | 5 (14) | 7 (21) |
Satisfaction with care and with the results of care received
The data for participants’ reported satisfaction with the care received and with the overall results of the care are summarised in Table 11. Most participants reported being ‘very satisfied’ (39%, 122/311) or ‘quite satisfied’ (29%, 90/311) at 4 months with the care they had received; at 12 months, these proportions were 36% (92/255) and 27% (68/255), respectively. Similarly, most participants reported being ‘very satisfied’ (34%, 104/310) or ‘quite satisfied’ (33%, 101/310) at 4 months with the results of the care they had received; at 12 months, these proportions were 34% (86/254) and 29% (73/254), respectively. Although the SC arm had a slightly higher proportion of participants who reported being ‘very satisfied’ at 4 months with the care they had received, there was no statistically significant difference between trial arms; furthermore, data at 12 months about participants’ satisfaction with the care they had received showed little difference between arms. There was an overall significant difference in satisfaction at 4 months with the results of care, mainly because larger numbers in the SC arm than in the UC arm in group 2 reported being ‘very satisfied’. However, at 12 months it was observed that a larger proportion of participants in the UC arm than in the SC arm in group 2 reported being satisfied with the results of their care.
| Satisfaction with | All | Group 1 | Group 2 | Group 3 | ||||
|---|---|---|---|---|---|---|---|---|
| SC | UC | SC | UC | SC | UC | SC | UC | |
| Care | ||||||||
| 4 months | ||||||||
| Very satisfied | 65 (43) | 57 (36) | 18 (45) | 18 (47) | 31 (46) | 25 (34) | 16 (35) | 14 (30) |
| Quite satisfied | 42 (27) | 48 (30) | 12 (30) | 9 (24) | 18 (27) | 27 (37) | 12 (26) | 12 (26) |
| No opinion | 22 (14) | 27 (17) | 6 (15) | 5 (13) | 7 (10) | 14 (19) | 9 (20) | 8 (17) |
| Not satisfied | 24 (16) | 26 (16) | 4 (10) | 6 (16) | 11 (16) | 8 (11) | 9 (19) | 12 (26) |
| p-values | 0.210 | 0.784 | 0.297 | 0.467 | ||||
| 12 months | ||||||||
| Very satisfied | 42 (34) | 50 (38) | 14 (47) | 18 (44) | 17 (30) | 19 (38) | 11 (30) | 13 (32) |
| Quite satisfied | 32 (26) | 36 (27) | 7 (23) | 11 (27) | 15 (27) | 14 (28) | 10 (27) | 11 (27) |
| No opinion | 32 (26) | 28 (21) | 7 (23) | 6 (15) | 17 (30) | 12 (24) | 8 (22) | 10 (24) |
| Not satisfied | 17 (14) | 18 (14) | 2 (7) | 3 (15) | 7 (13) | 5 (10) | 8 (22) | 7 (17) |
| p-values | 0.873 | 0.934 | 0.558 | 0.654 | ||||
| Results of care | ||||||||
| 4 months | ||||||||
| Very satisfied | 62 (41) | 42 (27) | 18 (45) | 15 (40) | 29 (44) | 16 (22) | 15 (33) | 11 (24) |
| Quite satisfied | 43 (28) | 58 (37) | 12 (30) | 10 (26) | 19 (29) | 33 (45) | 12 (26) | 15 (33) |
| No opinion | 20 (13) | 30 (19) | 5 (13) | 6 (16) | 7 (11) | 17 (23) | 8 (17) | 7 (15) |
| Not satisfied | 27 (18) | 28 (18) | 5 (13) | 7 (18) | 11 (17) | 8 (11) | 11 (24) | 13 (28) |
| p-values | 0.030 | 0.379 | 0.065 | 0.367 | ||||
| 12 months | ||||||||
| Very satisfied | 37 (30) | 49 (37) | 15 (52) | 18 (44) | 12 (21) | 19 (38) | 10 (27) | 12 (29) |
| Quite satisfied | 36 (30) | 37 (28) | 7 (24) | 9 (22) | 19 (34) | 15 (30) | 10 (27) | 13 (32) |
| No opinion | 33 (27) | 27 (20) | 6 (21) | 7 (17) | 17 (30) | 11 (22) | 10 (27) | 9 (22) |
| Not satisfied | 16 (13) | 19 (14) | 1 (3) | 7 (17) | 8 (14) | 5 (10) | 7 (19) | 7 (17) |
| p-values | 0.935 | 0.319 | 0.271 | 0.591 | ||||
Process outcomes
Process outcomes comprised data on referrals generated at the SCOPiC trial research clinic (randomisation point) and care delivery data in physiotherapy and specialist spinal settings, including secondary care referrals and treatments. Data were captured with CRFs (at the SCOPiC trial research clinic and at physiotherapy services), patient self-reports and from hospital records review. Waiting times for treatments (other than physiotherapy) and/or tests were collected/calculated from hospital records only.
Referral patterns from SCOPiC trial research clinics
Table 12 presents the referral patterns for participants attending the SCOPiC trial research clinic and randomised to the trial. The stratification algorithm for allocating SC participants to the matched care pathways was followed in all but four cases. In the UC arm, physiotherapists provided initial assessment and advice and directed patients’ care according to clinical judgement, with the majority of participants referred to physiotherapy treatment; only 28 out of 238 (12%) participants were discharged back to GP care from the research clinic. Appendix 3, Table 26, summarises the GPs’ preferred care pathways for UC participants versus care pathways decided by the physiotherapists delivering trial UC at the point of randomisation at the SCOPiC trial research clinic; it shows that, in 62% of cases, GPs said that they would have referred to physiotherapy and, in 32% of cases, they would have continued with GP care.
| Referral | All (n participants) | Group 1 (n participants) | Group 2 (n participants) | Group 3 (n participants) | ||||
|---|---|---|---|---|---|---|---|---|
| SC | UC | SC | UC | SC | UC | SC | UC | |
| Physiotherapy | 153 | 200 | 49 | 41 | 104 | 92 | 0 | 67 |
| Spinal interface clinic | 80 | 10 | 0 | 1 | 0 | 2 | 80 | 7 |
| Discharged to GP carea | 4 | 28 | 4 | 12 | 0 | 12 | 0 | 4 |
Summary of physiotherapy care
Many trial participants were referred for physiotherapy treatment. Data were collected with the use of CRFs, for 214 (89.9%) SC participants and 235 (98.7%) UC participants. In terms of sciatica groups, completed CRFs in the SC arm were obtained for 51 out of 53 (96.2%) participants in group 1, 104 out of 105 (99.2%) participants in group 2 and 59 out of 80 (73.8%) participants in group 3. For the UC arm, these proportions were 54 out of 54 (100%) participants in group 1, 105 out of 106 (99.1%) participants in group 2 and 76 out of 78 (97.4%) participants in group 3.
The overall median number of total treatment sessions was similar for participants in the SC and UC arms [2 (IQR 1–4) and 2 (IQR 0–3), respectively]. Participants in group 2 who were allocated to SC had, on average, more treatment sessions than similar participants in the UC arm. The median time to the first physiotherapy appointment (for those who were referred to physiotherapy) was 9 days (IQR 6–15 days) for SC participants and 21.5 days (IQR 11–46 days) for UC participants. Physiotherapy treatment was delivered over a shorter time frame in the SC arm than in the UC arm [median 38 days (IQR 12.5–70 days) and 66 days (IQR 29–97 days), respectively]. The differences in time to the first physiotherapy appointment and in treatment delivery were driven by SC groups 1 and 2. Appendix 4, Table 27 and Figures 17 and 18, presents information from CRFs that captured physiotherapy treatment data.
Data from spinal interface clinics
Table 13 summarises data on appointment numbers at the spinal interface clinics, and time to appointment, over the trial follow-up period, and data on referral decisions made at the spinal interface clinics. Information was derived from available hospital records review only; therefore data are missing for a number of participants. It should also be noted that, if any participants had their NHS appointment or treatments carried out in a private setting, these are not included in a patients’ NHS records and would not be captured by the hospital records review. Hospital data were available for 427 out of 476 (89.7%) participants in the trial: 215 out of 238 (90.3%) in the SC arm and 212 out of 238 (89.1%) in the UC arm. By arm and sciatica group, data were available for the following numbers of participants: SC group 1, 49 out of 53 (92.5%); SC group 2, 94 out of 105 (89.5%); SC group 3, 72 out of 80 (90.0%); UC group 1, 47 out of 54 (87.0%); UC group 2, 95 out of 106 (89.6%); and UC group 3, 70 out of 78 (89.7%). In line with the trial protocol, a significantly larger proportion of group 3 participants in the SC arm than in the UC arm were referred to and attended the spinal specialist interface clinic (82.0% vs. 58.3%, respectively). The overall time taken to be seen in the interface clinic from the date of randomisation was significantly shorter for the SC arm (median 12 days) than for the UC arm (115 days). The total number of visits to secondary care spinal orthopaedic services (referrals made from spinal interface clinics plus any other referral route) was 32 for the SC arm and 23 for the UC arm. There were five visits to pain clinics (two in the SC arm and three in the UC arm). Overall, there was not much difference in time to appointment in orthopaedics between SC and UC participants, but, as expected in the fast-track pathway, sciatica group 3 participants in the SC arm were seen more quickly than similar participants in the UC arm [87 days from randomisation (IQR 58–105 days), compared with 120 days (IQR 42–151 days), respectively]. In addition, SC participants listed for spinal epidural injections had the procedure more quickly than similar participants in the UC arm [60 days (IQR 41–93 days), compared with 161 days (IQR 113–253 days), respectively]. For spinal surgery, the time to procedure was very similar between SC and UC arms for the small numbers of patients having surgery [236 days (IQR 99–372 days) and 216 days (IQR 102–305 days), respectively]. Appendix 5, Table 28, summarises available data on time to procedures, as captured by hospital records review.
| All (N = 427)a | SC arm (N = 215) | UC arm (N = 212) | |
|---|---|---|---|
| Attended spinal interface clinic (n) | 104 | 80 | 24 |
| Time to appointment from randomisation (days), median (IQR) | 14 (10–69) | 12 (9–15) | 115 (32–191) |
| Group 1 | |||
| n (%) | 2 (1.2) | 1 (1.2) | 1 (4.2) |
| Median (IQR) | 115 (48–181) | 48 (–) | 181 (–) |
| Group 2 | |||
| n (%) | 23 (22.1) | 14 (16.9) | 9 (37.5) |
| Median (IQR) | 88 (34–146) | 83 (50–146) | 88 (25–120) |
| Group 3 | |||
| n (%) | 79 (76.0) | 68 (82.0) | 14 (58.3) |
| Median (IQR) | 12 (9–15) | 11 (8–13) | 119 (51–245) |
| Outcome of spinal interface clinic appointment [numbers referred to other services/interventions] | |||
| Referred to physiotherapy, n (%) | 47 (46.5) | 44 (54.3) | 3 (15.0) |
| Referred to orthopaedics, n (%) | 32 (31.7) | 22 (27.2) | 10 (50.0) |
| Referred to pain clinic, n (%) | 4 (4.0) | 2 (2.5) | 2 (10.0) |
| Referred to receive spinal epidural injection, n (%) | 18 (17.8) | 13 (16.0) | 5 (25.0) |
| Total referralsb (denominator) (n) | 101 | 81 | 20 |
Secondary care interventions
Data from self-report and hospital records review were combined; Table 14 summarises the numbers of participants who underwent MRI, spinal surgery or spinal epidural injection, per trial arm and per sciatica group within each arm. Overall, based on the available data, few participants had spinal surgery and more SC than UC participants had spinal epidural injections. Information about the specifics of injections or surgery was available for a limited number of participants from all those who received these treatments. Seventeen caudal epidural injections and six transforaminal injections were recorded, along with nine discectomies and two decompressions (laminectomies).
| Intervention | Trial arm (n) | |
|---|---|---|
| SC | UC | |
| Self-report | ||
| Lumbar spine surgery | ||
| Group 1 | 0 | 0 |
| Group 2 | 0 | 1 |
| Group 3 | 1 | 2 |
| Spinal epidural injection | ||
| Group 1 | 1 | 0 |
| Group 2 | 4 | 5 |
| Group 3 | 15 | 9 |
| MRI | ||
| Group 1 | 5 | 9 |
| Group 2 | 25 | 22 |
| Group 3 | 14 | 19 |
| Hospital records review | ||
| Lumbar spine surgery | ||
| Group 1 | 1 | 0 |
| Group 2 | 3 | 2 |
| Group 3 | 0 | 3 |
| Spinal epidural injection | ||
| Group 1 | 1 | 0 |
| Group 2 | 0 | 0 |
| Group 3 | 9 | 1 |
| MRI | ||
| Group 1 | 1 | 0 |
| Group 2 | 6 | 6 |
| Group 3 | 5 | 12 |
| Total | ||
| Surgery | 5 | 8 |
| Injectionsa | 30 | 15 |
| MRI | 56 | 68 |
Adverse events
There were no SAEs or AEs in either arm of the trial.
Summary
The SC participants reported improvement a median of 2 weeks earlier than UC participants; however, this difference was small and not statistically significant (HR 1.14, 95% CI 0.89 to 1.46). The trial did not find convincing evidence that this model of SC led to faster improvement or better clinical results than UC, for patients with clinically diagnosed sciatica. The results are discussed in detail in Chapter 6.
Chapter 4 Health economic evaluation
Aim
The overall aim of the health economic evaluation was to compare the resource use, costs and benefits of the SC intervention with UC for participants presenting to primary care with sciatica, and to estimate the cost-effectiveness of SC.
Methods
The primary evaluation was a cost–utility analysis; the base-case analysis was performed from the UK NHS and Personal Social Services (PSS) perspective, with additional analyses for the health-care provider perspective and the societal perspective incorporating productivity costs. The quality-adjusted-life-year (QALY) was used as the measure of health outcome, using data from the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), questionnaire. Participant-level intervention and sciatica-related costs over the 12-month follow-up period were included, with costs reported in 2017 prices. The analysis of cost-effectiveness focused on estimation of the incremental cost per QALY gained.
Costs and outcomes were not discounted, as the trial was limited to a period of 12 months’ follow-up. Trial findings are reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards. 61
Measurement and analysis of outcomes
The EQ-5D-5L questionnaire data were collected at baseline and at 4 and 12 months. Responses were converted to index scores using the interim cross-walk value set for mapping from the EQ-5D-5L to the EuroQol-5 Dimensions, three-level version. 62 QALYs were generated for each participant using the area under the utility curve approach assuming linear interpolation between the three utility measurements. 63 To avoid bias, adjustment for differences between the groups in baseline EQ-5D-5L scores was undertaken using a regression-based adjustment. 64 Health utility values and QALYs accrued over the 12-month follow-up were summarised by trial arm and assessment time point and presented as means and SDs, with between-arm differences compared using bootstrapped mean differences. A univariate and multivariate regression model was performed to estimate QALY differences between the SC and UC arms. In the univariate model, QALY differences over the 12-month follow-up period were regressed against the trial arm variable. In the multivariate model, the QALY differences were regressed against the baseline EQ-5D-5L scores, age and sex variables.
Resource use and costs
Trial interventions resource use
The resource use involved in the delivery of the trial interventions was collected via CRFs. The number and duration of SC and UC physiotherapy sessions attended were recorded for each participant on CRFs. The unit cost of a physiotherapy session was used65 and multiplied by duration of contact (in minutes) to obtain a total treatment cost per patient for SC and UC. The cost of the fast-track care pathway (comprising MRI and appointment at a spinal interface clinic) was calculated using data from NHS Reference Costs. 66
Additional resource use data
Additional health-care resource use data were obtained from responses to the resource use items contained in the 4- and 12-month self-report postal questionnaires. Information was obtained on primary and community care consultations (face to face with GPs, practice nurses, other HCPs, home visits and other primary care contacts), secondary care consultations (hospital consultants, physiotherapists and accident and emergency visits), prescriptions, hospital-based procedures (MRI, computerised tomography, X-rays and injections) and the nature and duration of surgery-related inpatient stays. Participant costs included over-the-counter purchases and out-of-pocket expenditure associated with the purchase of private or alternative treatments, for example private physiotherapy and acupuncture. Participants were asked to distinguish between UK NHS and private provision. Additional information on sciatica-related imaging tests, injections or surgery, obtained from a subset of participants’ hospital records, when available, from participating sites, were used as part of a sensitivity analysis. Information was also collected on prescribed medications, using the reported number of prescriptions and dosages. If these were not reported, the typical dosage and duration of treatment, as reported in the British National Formulary (BNF)67 for each medication, were used to estimate the quantity of medications. Participant-level costs were then generated by multiplying the duration of use with the estimated unit cost.
Resource use valuation
Unit costs were obtained from the Personal Social Services Research Unit’s Unit Costs of Health and Social Care 2017,65 NHS Reference Costs66 and the BNF. 67 Unit costs are reported in Table 15.
| Health-care resource | Unit cost (£) | Source |
|---|---|---|
| Primary care contacts | ||
| GP: surgery consultation | 37.00 | PSSRU 201765 |
| Practice nurse: surgery consultation | 11.00 | PSSRU 201765 |
| Practice nurse: home visit | 94.00 | PSSRU 201765 |
| Hospital-based care | ||
| Consultant:a sciatica pain, first attendance | 167.00 | NHS Reference Costs, 201766 |
| Consultant: sciatica pain, follow-up | 141.00 | NHS Reference Costs, 201766 |
| Consultant:b pain management, first attendance | 177.00 | NHS Reference Costs, 201766 |
| Consultant: pain management, follow-up | 101.00 | NHS Reference Costs, 201766 |
| Physiotherapist: first attendancec | 65.00 | NHS Reference Costs, 201766 |
| Physiotherapist: follow-up attendancec | 49.00 | NHS Reference Costs, 201766 |
| Consultation: A&E | 180.00 | NHS Reference Costs, 201766 |
| Hospital nurse | 89.00 | NHS Reference Costs, 201766 |
| Diagnostic tests: X-ray | 31.00 | NHS Reference Costs, 201766 |
| Diagnostic tests: CT | 103.00 | NHS Reference Costs, 201766 |
| Diagnostic tests: MRI | 169.00 | NHS Reference Costs, 201766 |
| Diagnostic tests: blood test | 6.00 | NHS Reference Costs, 201766 |
| Spinal epidural injection | 575.00 | NHS Reference Costs, 201766 |
| Surgery (microdiscectomy) | 6663.00 | NHS Reference Costs, 201766 |
| Surgery (discectomy) | 5298.00 | NHS Reference Costs, 201766 |
| Out-of-pocket treatments | Participant-reported costs | Not applicable |
| Prescribed medication | Participant-specific | BNF 201767 |
| Work absence/reduced productivity | Participant-specific | ONS 201768 |
NHS costs
The unit costs of primary care consultations, including GP consultations and visits to practice nurses or physiotherapists, were sourced from Curtis and Burns. 65 The costs of secondary care outpatient consultations, investigations and procedures (including blood tests, injections and scans, e.g. spinal imaging tests such as MRI) were obtained from NHS Reference Costs 2016–17. 66 The costs of surgery and inpatient stays were obtained from the same source. 66 The unit costs of surgery were estimated at £6663 and £5298 per participant for discectomy and microdiscectomy, respectively. The unit cost was based on the weighted average for procedures for complications of the following Healthcare Resource Group (HRG) codes:
-
very major extradural spinal procedures with a complications or comorbidities (CC) score of ≥ 4 (HC62 A)
-
very major extradural spinal procedures with a CC score of 2–3 (HC62B)
-
very major extradural spinal procedures with a CC score of 0–1 (HC62C)
-
major extradural spinal procedures with a CC score of ≥ 4 (HC63 A)
-
major extradural spinal procedures with a CC score of 2–3 (HC63B)
-
major extradural spinal procedures with a CC score of 0–1 (HC63C).
Other surgical procedures are included in the same HRG codes; therefore, the surgeries did not differ in unit cost.
Broader health-care costs
Participant-reported costs of over-the-counter medicines were used, when available; if costs were not detailed, unit costs were obtained from online sources. Participant-level costs were then generated by multiplying the duration of use with the estimated unit cost.
Private health-care costs
Owing to the lack of nationally representative unit cost estimates for private health-care use, private health-care use was costed as the NHS equivalent.
Productivity costs
Information about whether or not an individual had experienced any loss of earnings due to work absence (absenteeism) associated with their sciatica was obtained directly from participants through their postal questionnaires at the 12-month follow-up. Information associated with reduced productivity while at work (presenteeism) was obtained at 4 and 12 months. Participants were initially asked if they had taken any time off work as a result of their sciatica symptoms. Those who responded positively were then asked to report the amount of time lost from work during the follow-up period. Individuals were also asked to report the rate at which their sciatica had affected their work ability while at work (presenteeism) on a previously validated 0–10 single-item presenteeism scale. 69 Presenteeism time loss was generated by translating the self-reported scores to work loss based on the actual number of days worked during this period. 70 Costs were assigned to the total amount of lost productivity by use of the human capital approach and average national wage estimates identified from annual earnings data. 68,70,71
Analysis
Analysis of costs
The total NHS, health-care and societal costs were estimated over a period of 12 months. NHS costs included the intervention, NHS primary and secondary care, and medication costs. Health-care costs included participant-incurred costs such as out-of-pocket expenses and private health care. Care was taken to avoid double-counting for all patients, especially in relation to visits to physiotherapists, by cross-checking self-reported records with information obtained from CRFs that captured sessions delivered as part of the trial protocol. Similar to the estimate of between-arm QALY differences, the mean cost difference between the SC and UC arms was estimated using regression methods.
Missing data analysis
To minimise bias, multiple imputation techniques were used to handle missing costs and missing EQ-5D-5L data at each follow-up time point. Resource use and, therefore, cost data were considered missing if participants did not complete and return their follow-up questionnaire. In returned questionnaires, missing individual resource use items were treated as not to have occurred and were consequently treated as a zero cost. For the EQ-5D-5L, however, non-response on one or more items meant that the overall score was deemed missing, as all items are necessary for the overall index score. Multiple imputation was performed by the predictive mean matching method to account for the non-normality of the distribution of costs and the EQ-5D-5L values for missing total costs and missing EQ-5D-5L items. 72 An imputation was fitted and included 25 imputed data sets. Rubin’s rule was used to combine the imputed data sets into one final imputed variable. 73 This regression method is based on the values of the available data to estimate missing values. The imputational model used to impute missing data included age, sex and trial arm. The model for imputing missing EQ-5D-5L scores also included the baseline EQ-5D-5L scores. The imputed data were used to inform the base-case, sensitivity and all subgroup analyses, with the exception of the complete data set.
Cost-effectiveness analysis
The within-trial health economic analysis was used to determine the cost-effectiveness of SC compared with UC. A cost–consequence analysis was initially reported, describing all the important disaggregated results relating to resource use, costs and outcomes. The analysis was performed according to the ITT principle. An incremental cost–utility analysis was undertaken to calculate the incremental cost-effectiveness ratio (ICER) as the cost per additional QALY gained. This is the ratio of the mean difference in the cost and mean difference in QALYs between the two trial arms. The base-case analysis adopted a UK NHS perspective; the health-care and societal perspectives were included in sensitivity analyses. Because cost and QALY data were skewed, all estimates are presented as means with bootstrapped 95% CIs, each with 5000 replications.
To represent the overall uncertainty in the trial cost and outcome data, a probabilistic sensitivity analysis was then undertaken by jointly bootstrapping mean cost and QALY differences to generate 5000 paired ICER estimates. The 5000 paired bootstrap estimate pairs of the mean costs against mean QALYs (paired differences) were used to provide a graphical display of a cost–utility plane. Uncertainty was also estimated by constructing cost-effectiveness acceptability curves (CEACs). The CEAC shows the probability of SC being cost-effective at different cost-per-QALY thresholds. In the UK, interventions are deemed cost-effective if the cost per QALY gained is < £20,000 or, in some circumstances, < £30,000. 74 All statistical analyses were performed using Stata version 14.
Sensitivity analyses
Four sensitivity analyses were performed to assess the robustness of the base-case results:
-
Health-care and societal perspectives – this analysis was based on alternative costing perspectives to assess the impact of including patient-incurred health-care costs and wider societal costs, such as work-related costs.
-
Incorporating additional hospital record data – a review of trial participants’ hospital records was undertaken to collect data on sciatica-related tests and treatments over the 12 months of follow-up, to supplement the information collected through self-report in the 4- and 12-month questionnaires. The records accessed included information on spinal surgeries, spinal injections and MRI. The costs related to the additional tests or treatments identified from the hospital records review were then added to the total costs.
-
Complete-case analysis – the analysis was re-run using a complete data set to investigate the impact of imputing missing data.
-
Analysis of the three sciatica groups – prespecified exploratory analyses considered the cost-effectiveness of SC compared with UC for participants in each sciatica group (1, 2 and 3). Details of the three sciatica groups and matched care pathways are reported in Chapter 2.
Results
Response rates and data completion
Resource use and cost per participant are reported by category for all those for whom complete data were available at 4 and 12 months. For total cost per participant estimation, the base-case analysis included the information imputed at the total cost level for all those with data available at 4 and 12 months. Base-case NHS costs used the imputed participants’ data for NHS cost and QALY data (n = 476).
The proportion of the trial population returning questionnaires at each time point is reported in Table 16. Data for participants for whom complete cost data (n = 236, 50%) were available at 4 and 12 months were used in the cost imputation model. Complete items on all EQ-5D-5L domains were required for the calculation of the QALY. After excluding missing items, 471 (99%) participants had a valid EQ-5D-5L score at baseline and 334 (70%) participants had a valid EQ-5D-5L score at month 12. In total, 301 participants (63%) had a valid EQ-5D-5L score at all three time points. Missing data for the EQ-5D-5L values were imputed at each time point.
| Questionnaires returned | Questionnaires returned (including MDC) | Valid EQ-5D-5L responses | |
|---|---|---|---|
| Baseline, n (%) | 471 (99) | ||
| 4 months, n (%) | 316 (66) | 393 (83) | 369 (78) |
| 12 months, n (%) | 260 (55) | 359 (75) | 334 (70) |
| Available case (full EQ-5D-5L scores), n (%) | 301 (63) | ||
| Complete case (costs and QALYs), n (%) | 236 (50) | ||
| SC arm | UC arm | Mean differencea (95% CI) | |
| Primary (imputed) analysis | n = 238 | n = 238 | |
| EQ-5D-5L score, mean (SD) | |||
| Baseline | 0.5168 (0.2109) | 0.5201 (0.2300) | –0.003 (–0.043 to 0.037) |
| 4 months | 0.6710 (0.2006) | 0.6806 (0.1955) | –0.009 (–0.046 to 0.026) |
| 12 months | 0.7138 (0.2137) | 0.7320 (0.1879) | –0.0182 (–0.054 to 0.0176) |
| QALYs (imputed) | |||
| Unadjusted QALYs,b mean (SD) | 0.6599 (0.1731) | 0.6713 (0.1685) | –0.0114 (–0.042 to 0.019) |
| Adjusted QALYsb | – | – | –0.0115 (–0.036 to 0.013) |
| Available case | n = 147 | n = 154 | |
| QALYs over 12 months, mean (SD) | 0.6609 (0.1857) | 0.6950 (0.1641) | –0.0239 (–0.056 to 0.007) |
| Complete-case analysisc | n = 114 | n = 122 | |
| Unadjusted QALYs, mean (SD) | 0.6751 (0.1682) | 0.7005 (0.1601) | –0.0253 (–0.068 to 0.016) |
| Adjusted QALYsb | – | – | –0.0255 (–0.061 to 0.008) |
Health-related quality-of-life outcomes
Table 16 also describes the EQ-5D-5L scores at baseline and at the two follow-up time points, along with unadjusted QALYs over the 12-month period for the imputed data set (n = 476) and adjusted QALY differences. At baseline, participants in the SC arm had a slightly lower unadjusted EQ-5D-5L score than those in UC arm (0.5168 and 0.5201, respectively), and at 12 months they had a slightly lower score than those in the UC arm (0.6599 and 0.6713, respectively). At 12 months, the mean adjusted QALY difference between the two arms was –0.0115 (95% CI –0.036 to 0.013) in favour of UC; this difference was not significant. The differences in age, sex and baseline EQ-5D-5L scores were adjusted for in the main cost–utility analyses. 64 Overall, on average, participants in both arms improved over the 12-month follow-up period (i.e. EQ-5D-5L scores increased from baseline to 4 and 12 months in both trial arms).
Resource use and costs
Intervention costs
Unit costs and total costs associated with the different aspects of SC and UC are shown in Table 17. The cost of care for participants in SC group 3 (those on the fast-track pathway) included the costs of MRI and the consultation in the spinal interface services. Costs associated with the provision of the SC intervention were higher than those associated with UC.
| Health-care resource | Unit cost (£) | Source |
|---|---|---|
| UC intervention | ||
| Physiotherapy session (30 minutes) | 24.50 | PSSRU 201765 |
| SC intervention | ||
| Group 1 | ||
| Physiotherapy (up to two sessions of 30 minutes each) | 24.50 | PSSRU 201765 |
| Group 2 | ||
| Physiotherapy (up to six sessions of 30 minutes each) | 24.50 | PSSRU 201765 |
| Group 3 | ||
| MRI cost | 169.00 | NHS Reference Costs, 201766 |
| Spinal interface clinic cost | 141.00 | NHS Reference Costs, 201766 |
| Mean cost per participant | ||
| ITT | ||
| Total cost of UC (£), mean (SD) | 54.48 (56.98) | |
| Total cost of SC (£), mean (SD) | 155.79 (142.64) | |
| Mean difference (SC – UC) (£) (95% CI) | 101.31 (81.52 to 120.55) | |
Sciatica-related health-care resource use and costs
In the following sections, the mean cost per participant by category is given for complete 4- and 12-month data for specific primary and secondary care resource use.
Primary care consultations
Information about the mean (and SD) number of primary care consultations, including GP, nurse practitioner and other HCP consultations, and the corresponding disaggregated mean per-participant cost by trial arm, is reported in Table 18. There were slight differences in resource use and cost, but, overall, the primary care costs for UC were slightly higher than for SC.
| Health-care resource | SC arm (N = 114) | UC arm (N = 122) | ||
|---|---|---|---|---|
| Average number of visits per participant (SD) | Mean cost per participant (SD) | Average number of visits per participant (SD) | Mean cost per participant (SD) | |
| Primary care GP | 1.44 (1.88) | 52.58 (69.62) | 1.53 (2.01) | 56.71 (74.49) |
| Primary care nurse | 0.09 (0.39) | 0.97 (4.28) | 0.13 (0.49) | 1.44 (5.47) |
| Primary care othera | 0.05 (0.39) | 2.58 (19.38) | 0.23 (0.98) | 11.25 (48.30) |
| Prescriptions | 4.70 (8.50) | 15.51 (29.04) | 3.79 (5.79) | 12.81 (20.68) |
| NHS consultant | 0.57 (1.28) | 77.57 (173.07) | 0.43 (1.33) | 65.34 (222.93) |
| Private consultant | 0.03 (0.20) | 3.26 (25.87) | 0.04 (0.37) | 5.08 (46.19) |
| NHS physiotherapist | 1.57 (2.88) | 76.93 (141.17) | 2.79 (3.08)b | 136.55 (151.09) |
| Private physiotherapist | 0.32 (1.69) | 15.47 (82.79) | 0.61 (2.15) | 29.72 (105.33) |
| NHS hospital nurse | 0.15 (1.05) | 16.40 (116.36) | 0.01 (0.09) | 0.90 (9.95) |
| Private hospital nurse | 0.02 (0.18) | 1.93 (20.60) | 0.00 (0.00) | 0.00 (0.00) |
| NHS chiropractor | 0.03 (0.28) | 1.29 (13.76) | 0.05 (0.54) | 2.41 (26.61) |
| Private chiropractor | 0.67 (3.08)b | 32.67 (151.29) | 0.12 (0.71) | 5.62 (34.61) |
| NHS acupuncturist | 0.03 (0.16) | 1.29 (7.88) | 0.07 (0.53) | 3.21 (25.77) |
| Private acupuncturist | 0.54 (3.25)b | 26.64 (159.43) | 0.03 (0.20) | 1.21 (9.88) |
| NHS osteopath | 0.02 (0.13) | 0.86 (6.46) | 0.03 (0.27) | 1.21 (13.30) |
| Private osteopath | 0.08 (0.66) | 3.86 (32.68) | 0.07 (0.37) | 3.62 (18.00) |
| NHS X-rays | 0.09 (0.28) | 2.72 (8.80) | 0.11 (0.42) | 3.30 (13.10) |
| NHS scans | 0.07 (0.29) | 7.23 (29.76) | 0.01 (0.09) | 0.84 (9.33) |
| NHS blood tests | 0.16 (0.18) | 0.95 (2.59) | 0.10 (0.37) | 0.59 (2.24) |
| NHS MRI investigations | 0.32 (0.64) | 53.37 (108.56) | 0.33 (0.65) | 55.41 (109.59) |
| Private MRI investigation | 0.02 (0.13) | 2.97 (22.28) | 0.02 (0.18) | 2.77 (30.60) |
| NHS epidural injections | 0.14 (0.44) | 80.70 (252.29) | 0.10 (0.35) | 56.56 (201.23) |
| Resource use: other, n (%) | ||||
| NHS sciatica-related surgery | 1 (0.9) | 46.47 (496.20) | 3 (2.5) | 130.27 (823.89) |
| Prescriptions | 40 (35.09) | 36 (29.51) | ||
| Over-the-counter treatments | 28 (24.56) | 4.78 (10.35) | 31 (25.41) | 10.00 (21.81) |
Prescribed medication
Table 18 summarises information (from complete cases at 4 and 12 months) about resource use and costs related to prescribed medication in primary care. The proportions of individuals reporting that they had prescribed medications were very similar in both trial arms (SC arm: 35.09%; UC arm: 29.51%). The mean number of self-reported medication prescriptions was very similar across the two arms, with mean prescription costs for the SC arm being slightly lower than those for the UC arm (see Appendix 6, Table 29).
Secondary care-related NHS costs
Resource use associated with hospital visits, including visits to NHS consultants, private consultants, other outpatient consultations, investigations and associated procedures, sciatica-related surgeries and epidural injections, is reported in Table 18. Resource use and costs between the two trial arms were very similar, but costs associated with NHS physiotherapy treatment in the UC arm were higher than those in the SC arm (£136.55 and £76.93, respectively), whereas the costs of average visits to an NHS consultant were slightly higher in the SC arm than in the UC arm (£77.57 and £65.34, respectively). Although the numbers are small, the variable demonstrating the most difference in secondary resource use between the trial arms was the number of spinal surgeries, with more surgeries in the UC arm than in the SC arm. This was also the case when hospital records review data were used to supplement self-reported data in the sensitivity analysis. More SC participants than UC participants received spinal epidural injections.
Private health-care use and out-of-pocket health-care costs
The proportions of individuals reporting expenditure on over-the-counter medication and other out-of-pocket hospital-related expenditure are given in Table 18. The costs reported are for those who provided detail of costs incurred. The biggest area of difference in resource use and cost was in the use of private chiropractic care and acupuncture among those allocated to SC (£32.67 for the SC arm and £5.62 for the UC arm). However, further exploration of the data indicated that this was due to a few outlier participants (two people) with large numbers of these health-care visits.
Imaging and other tests
Information about resource use associated with hospital tests is shown in Table 18. The costs of tests (excluding costs directly related to the intervention) in the two trial arms were similar, but, overall, costs associated with SC were slightly higher than those associated with UC (NHS epidural injections: £80.70 and £56.56, respectively; NHS MRI: £53.37 and £55.41, respectively).
Hospital records resource use
Information about additional resource use from hospital records review (over and above self-report data) in relation to key cost drivers contributing to the total costs is reported in Table 14 (see Chapter 3). Similar results to those of the self-report data were observed for hospital records data, with more spinal surgeries among UC participants and more spinal injections among SC participants.
Work-related outcomes
Results regarding paid employment, work status, sciatica-related work absence and reduced productivity are reported in Table 19. Overall, sciatica-related work absence costs were slightly lower, and presenteeism costs were slightly higher, in the SC arm than in the UC arm.
| Work-related outcome | Trial arm | |
|---|---|---|
| SC | UC | |
| Baseline: working in paid employment, n (%) | 166 (70.3) | 158 (66.4) |
| Baseline: reported time off work in the previous 12 months, n (%) | 84 (49.4) | 96 (58.9) |
| Working in paid employment at 12 months, n (%) | 75 (60) | 81 (60) |
| Reported time off work owing to sciatica at 12 months, n (%) | 20 (26.7) | 15 (18.5) |
| Sciatica-related absence days,a mean (SD) | 5.48 (18.14) | 5.67 (17.08) |
| Reduced productivity days owing to sciatica,a mean (SD) | 68.06 (46.04) | 65.51 (44.43) |
| Cost (£) of sciatica-related absence,a mean (SD) | 590.42 (1955.13) | 610.53 (1840.98) |
| Cost (£) of sciatica-related presenteeism,a mean (SD) | 7332.21 (4960.96) | 7057.90 (4787.33) |
Incremental costs
Table 20 summarises the total costs per participant for the imputed and complete-case analysis for the NHS, health-care and societal perspectives. The costs per participant are also shown, including additional hospital records data on health-care use, from the sensitivity analysis. The results show that, although the SC and UC costs to the NHS were very similar, the cost of SC was slightly higher, overall, than the UC cost (difference £46.21, 95% CI –£110.60 to £187.06).
| Imputed analysis | SC (£) | UC (£) |
|---|---|---|
| Total NHS cost (base case), mean (SD) | 663.58 (737.14) | 617.37 (935.50) |
| Mean difference (95% CI) | 46.21 (–110.60 to 187.06) | |
| Total health-care cost, mean (SD) | 743.23 (764.05) | 665.55 (978.15) |
| Mean difference (95% CI) | 77.67 (–70.49 to 232.94) | |
| Total societal cost, mean (SD) | 1229.90 (1984.34) | 1246.63 (2211.85) |
| Mean difference (95% CI) | –16.73 (–422.66 to 337.76) | |
| Total societal cost (absenteeism and presenteeism), mean (SD) | 6336.50 (5327.85) | 6292.25 (5580.80) |
| Mean difference (95% CI) | 44.25 (–1028.76 to 1005.16) | |
| Sensitivity analysis: incorporating hospital records for participants | ||
| Total NHS cost, mean (SD) | 786.72 (1016.23) | 746.00 (1238.81) |
| Mean difference (95% CI) | 40.72 (–168.20 to 250.08) | |
| Total health-care cost, mean (SD) | 866.37 (1031.85) | 794.18 (1272.62) |
| Mean difference (95% CI) | 72.18 (–150.36 to 276.88) | |
| Complete-case analysis | ||
| Total NHS cost, mean (SD) | 587.10 (901.10) | 601.13 (1105.14) |
| Mean difference (95% CI) | –14.03 (–310.17 to 254.26) | |
| Description | Mean incremental costs (£) (95% CI) | Mean incremental QALYs (95% CI) |
| Cost-effectiveness analysis results | ||
| Base-case analysisa | 46.21 (–110.60 to 187.06) | –0.011 (–0.035 to 0.013) |
| ICER | Dominatedb | |
| Sensitivity analyses: alternative perspectives | ||
| Health care | 77.67 (–70.49 to 232.94) | –0.011 (–0.035 to 0.013) |
| ICER | Dominatedb | |
| Societal perspective | –16.73 (–422.66 to 337.76) | –0.011 (–0.035 to 0.013) |
| ICER (£) | 1520.00c | |
| Health-care costs including hospital records datad | ||
| NHS | 40.72 (–168.20 to 250.08) | –0.011 (–0.035 to 0.013) |
| ICER | Dominatedb | |
| Health care | 72.18 (–150.36 to 276.88) | –0.011 (–0.035 to 0.013) |
| ICER | Dominatedb | |
| Complete-case analysis | –14.03 (–310 to 254) | –0.0255 (–0.061 to 0.008) |
| ICER (£) | 550.20b | |
Cost-effectiveness analysis
Table 20 provides a summary of the cost-effectiveness analysis using data from the base-case NHS perspective. Results from the health-care and societal perspective are also reported. In the base-case analysis, SC was associated with an adjusted mean increase in costs of £46.21 (95% CI –£110.60 to £187.06) and an adjusted mean reduction in QALYs of –0.011 (95% CI –0.035 to 0.013) per participant, compared with UC, over the 12 month follow-up. The SC intervention was therefore dominated by UC; the net monetary benefit (NMB) was –£275 if society’s willingness to pay for a QALY (λ) is valued at £20,000. Uncertainty around the value is illustrated in Figure 11 (NHS perspective) and Figure 12 (health-care perspective). The majority of the points are in the north-west quadrant, showing that SC was more costly and less effective than UC and is unlikely to be cost-effective at the £20,000–30,000 per QALY threshold range, which is currently used by NICE to determine the cost-effectiveness of health-care interventions for the NHS. The CEACs show the probability that the SC intervention is cost-effective at different levels of willingness to pay for a QALY. For the NHS perspective, at £20,000 and £30,000 per QALY, the probability that the SC intervention is cost-effective was 0.18 and 0.19, respectively.
FIGURE 11.
Cost-effectiveness from the NHS perspective. (a) Cost-effectiveness plane comparing the SC intervention with UC, showing 5000 bootstrapped replicates of the ICER; and (b) CEAC for SC (intervention) compared with UC.
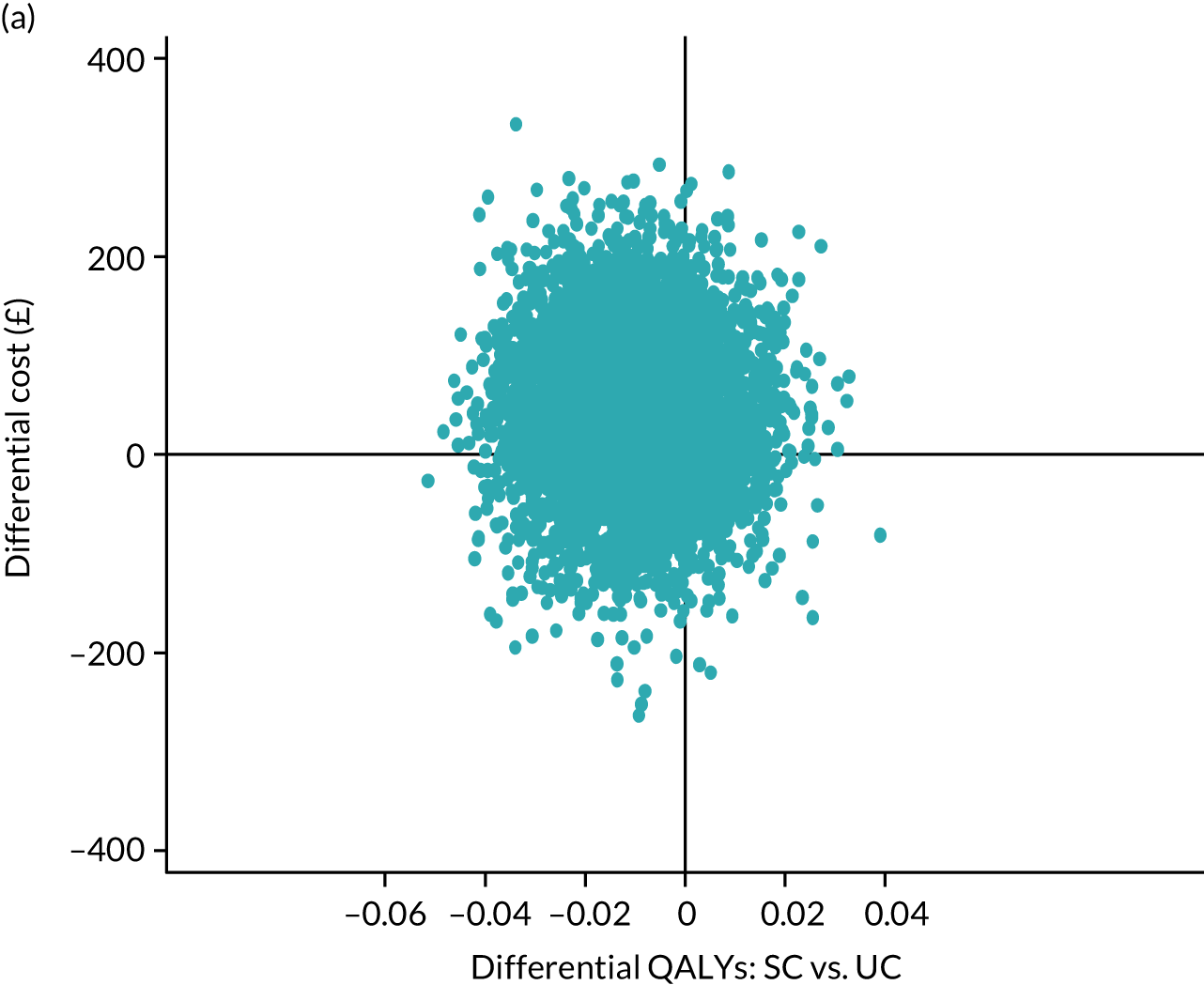
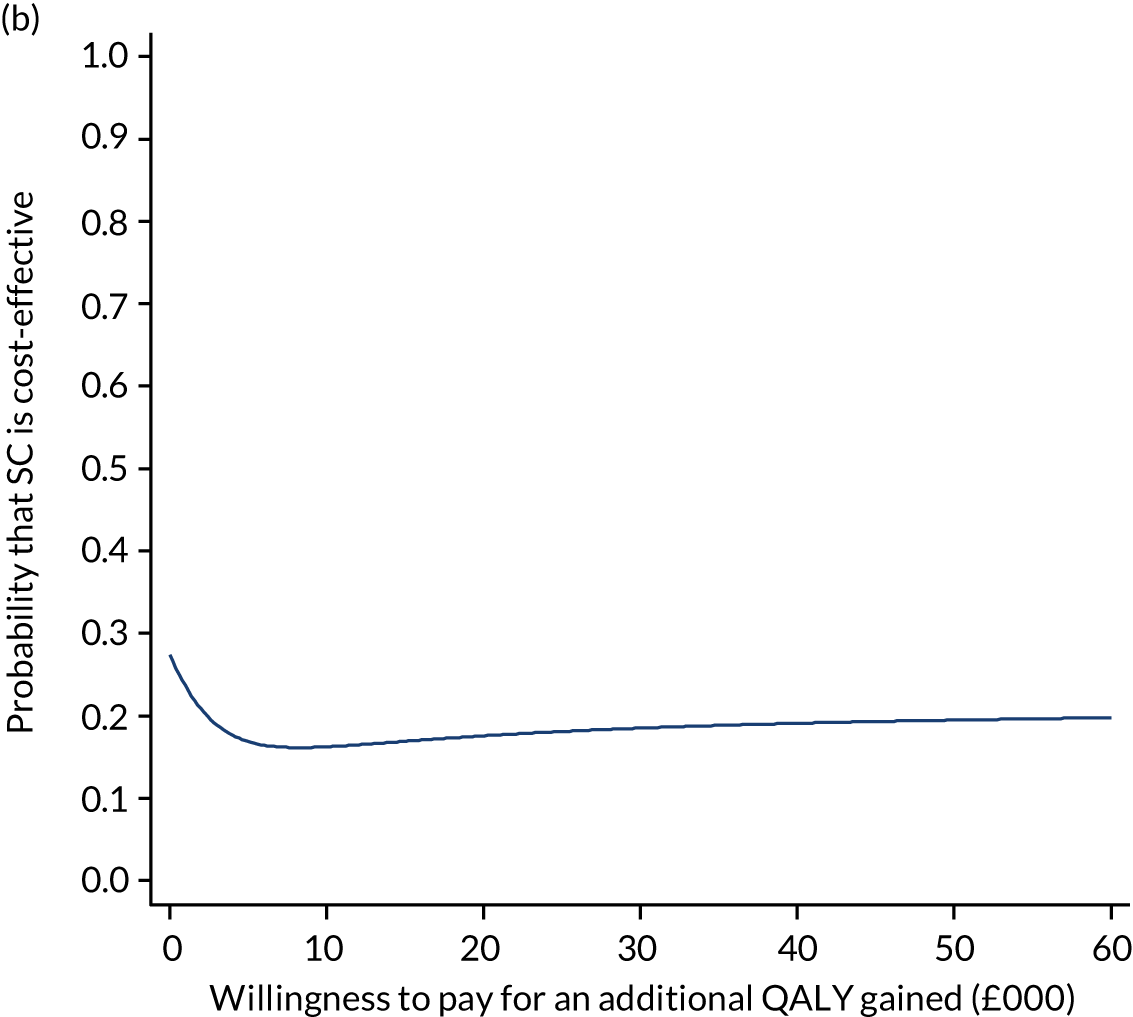
FIGURE 12.
Cost-effectiveness from the health-care perspective. (a) Cost-effectiveness plane comparing the SC intervention with UC; and (b) CEAC for SC (intervention) compared with UC.
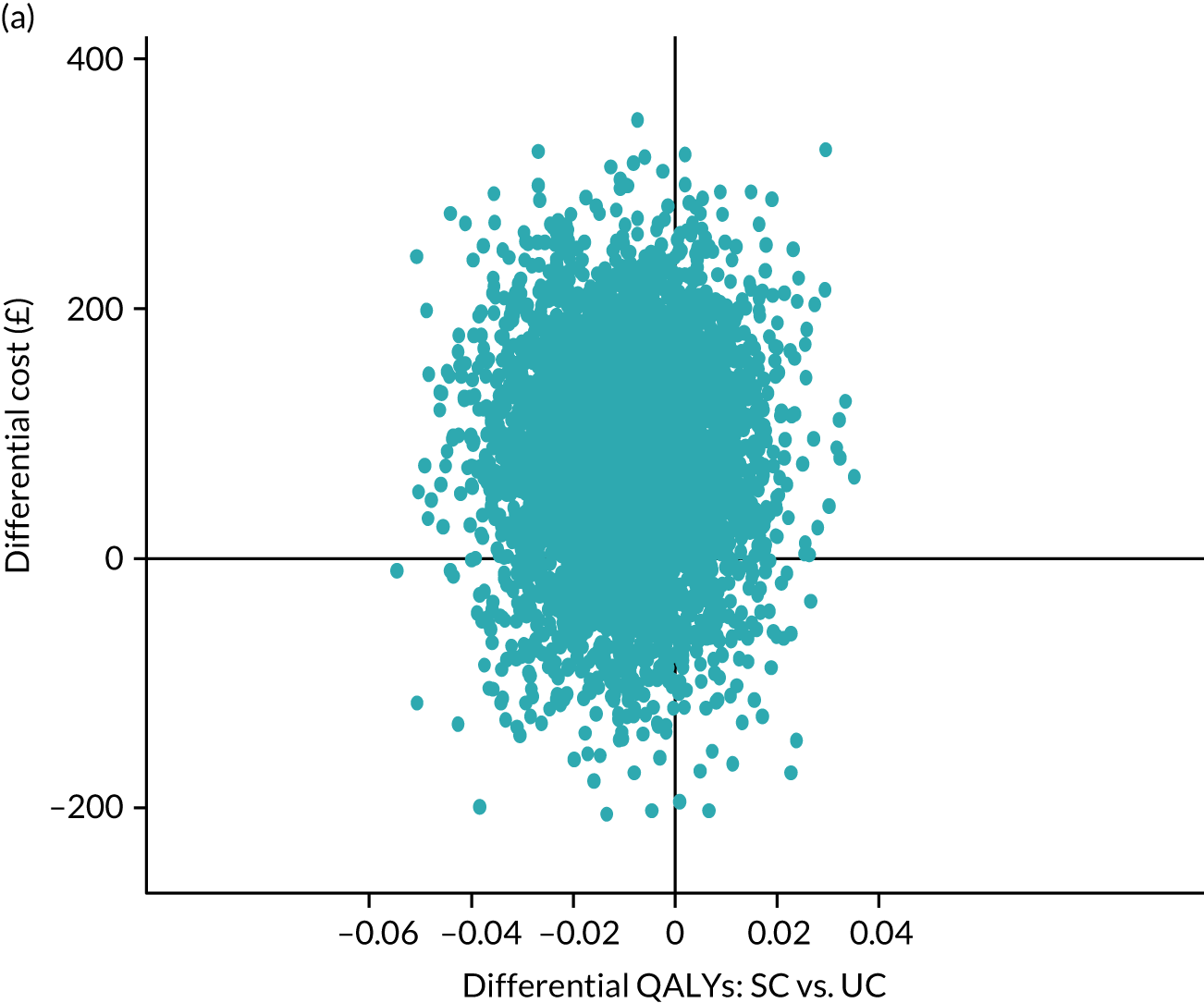
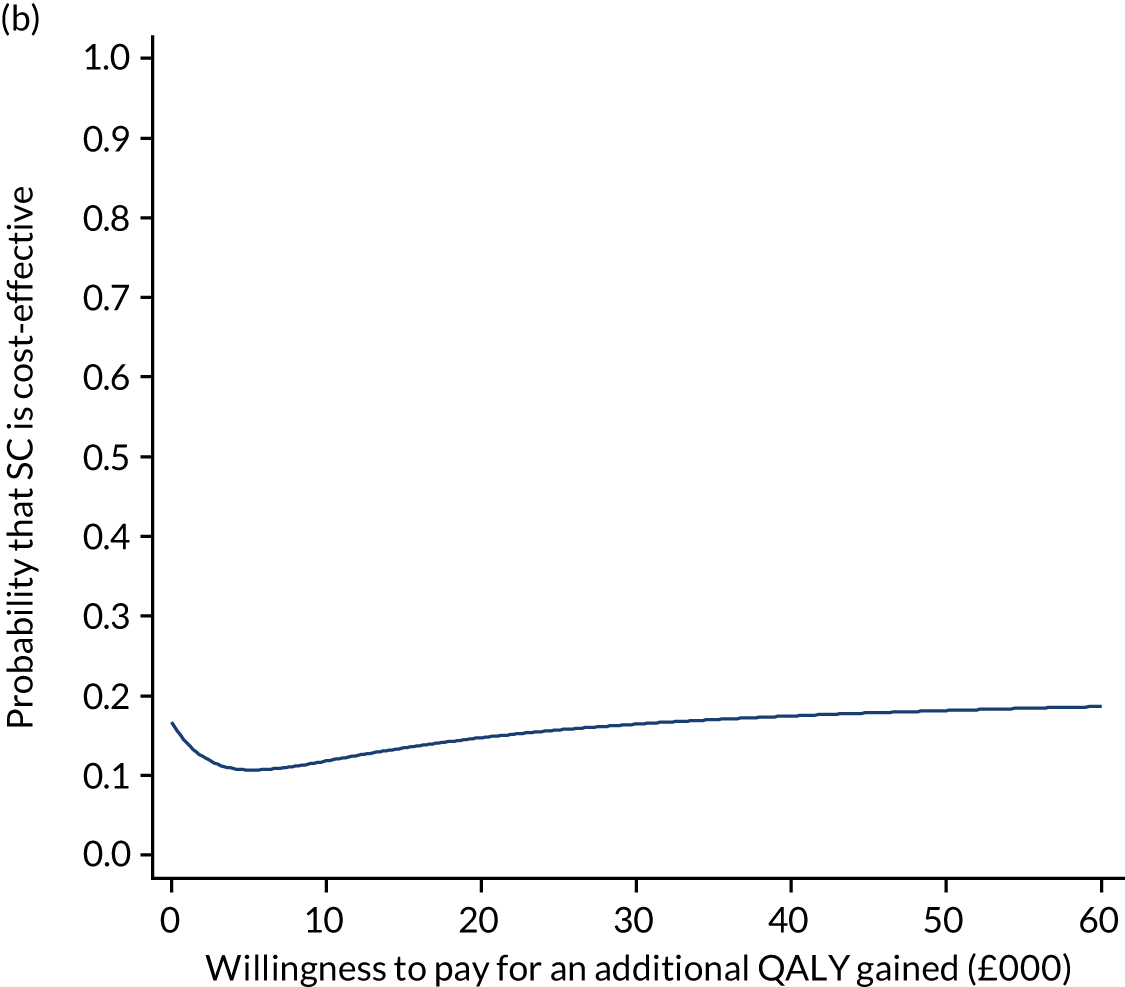
Sensitivity analyses
Health-care and societal perspectives
Details of the sensitivity analysis from alternative perspectives are presented in Table 20. The results between the two trial arms showed that SC was slightly more expensive than UC from a health-care perspective owing to slightly more visits to private physiotherapists; SC was slightly cheaper than UC from a societal perspective owing to lower work absence-related costs. From the health-care perspective, the probability that SC was cost-effective at £20,000 per QALY was 0.15; at £30,000 per QALY, that probability was 0.17 (see Figure 12).
Total costs incorporating additional hospital record data
Similar results to the base-case were observed when health-care use data (MRI, or spinal injections or spinal surgery) were included from the hospital records review for participants in the total cost analysis (see Table 20). The results between the two arms were similar, with the cost of SC being slightly higher, overall, than the UC cost.
Complete-case analysis
Under this scenario, from an NHS perspective, SC was slightly less costly (–£14.03) and less effective (–0.025 QALYs) when the analysis was restricted to participants with complete cost and outcome data (see Table 20). The ICER was therefore lower, at £550.10, and the NMB was –£461 (λ = £20,000). The probability of SC being cost-effective at λ = £20,000 was 0.15; at £30,000, it was 0.14. This is illustrated in Figure 13.
FIGURE 13.
Cost-effectiveness for the complete-case analysis, from the NHS perspective. (a) Cost-effectiveness plane comparing the SC intervention with UC; and (b) CEAC for SC (intervention) compared with UC.
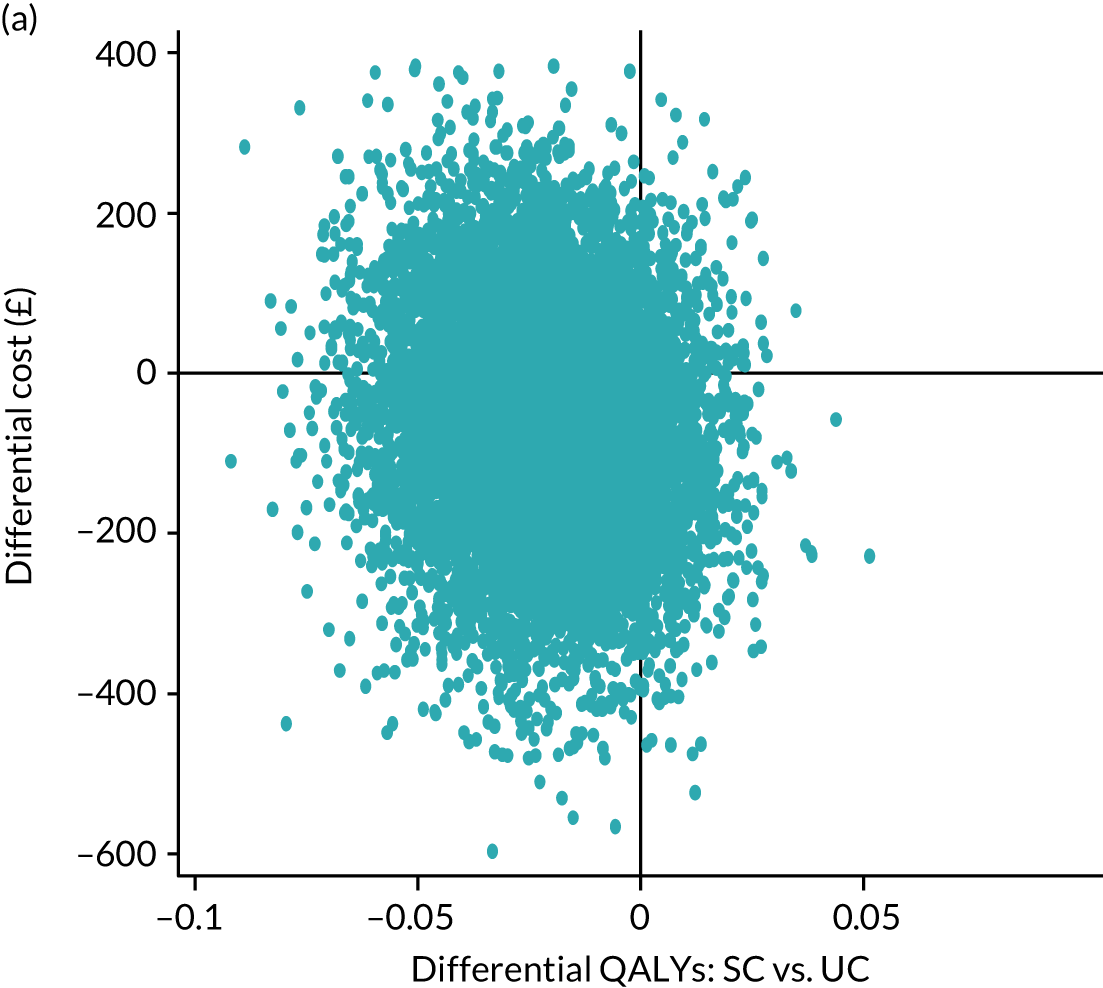
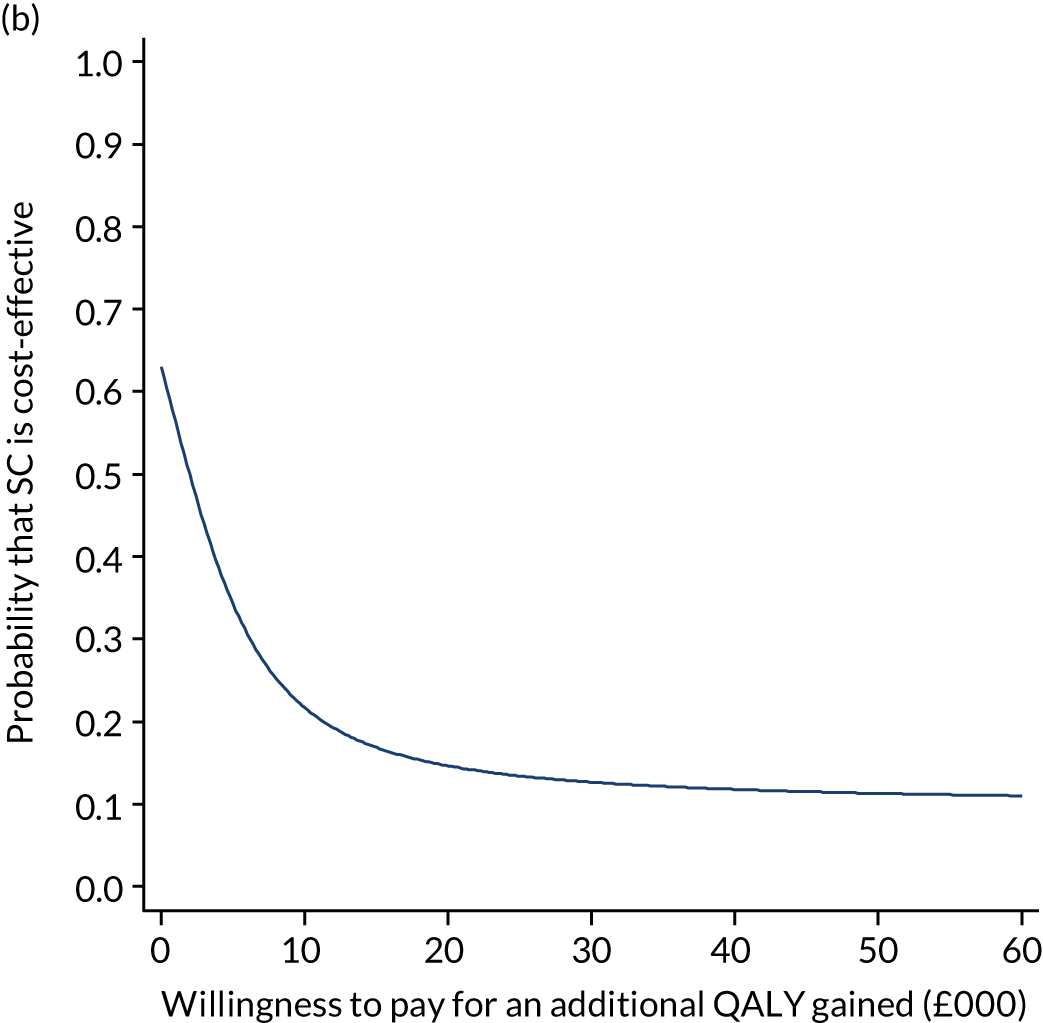
Sciatica group analysis
The primary base-case results were also examined in exploratory subgroup analyses considering the cost-effectiveness of SC compared with UC for each of the three sciatica groups separately (groups 1, 2 and 3, according to the stratification algorithm).
Impact of stratified care on health-related quality of life by sciatica group
Quality-of-life data (EQ-5D-5L and QALY scores) at each time point by trial arm and sciatica group are provided in Table 21. At baseline and at the end of 12 months’ follow-up, participants in the SC arm had slightly lower EQ-5D-5L scores than participants in the UC arm, in all sciatica groups, with the exception of group 1. At 12 months, QALY outcomes in all sciatica groups were similar (very slightly in favour of UC: –0.0004, –0.0198 and –0.008 for groups 1, 2 and 3, respectively), with all CIs crossing zero.
| Health outcomes | Trial arm, mean (SD) | Mean differencea (95% CI) | |
|---|---|---|---|
| SC | UC | ||
| Primary (imputed) analysis | |||
| Sciatica group 1 (n = 107) | |||
| EQ-5D-5L score | |||
| Baseline | 0.7039 (0.097) | 0.7017 (0.112) | 0.002 (–0.0386 to 0.0420) |
| 4 months | 0.7669 (0.172) | 0.7799 (0.131) | –0.013 (–0.0751 to 0.0427) |
| 12 months | 0.8235 (0.136) | 0.7992 (0.148) | 0.024 (–0.0284 to 0.0765) |
| QALYs over 12 months | 0.7755 (0.118) | 0.7735 (0.108) | –0.0004b (–0.0447 to 0.0392) |
| Sciatica group 2 (n = 211) | |||
| EQ-5D-5L score | |||
| Baseline | 0.5193 (0.194) | 0.5278 (0.208) | –0.009 (–0.061 to 0.0482) |
| 4 months | 0.6858 (0.160) | 0.7016 (0.162) | –0.016 (–0.0597 to 0.0286) |
| 12 months | 0.7284 (0.173) | 0.7589 (0.150) | –0.031 (–0.0766 to 0.0129) |
| QALYs over 12 months | 0.6726 (0.135) | 0.6921 (0.125) | –0.0198b (–0.051 to 0.010) |
| Sciatica group 3 (n = 158) | |||
| EQ-5D-5L score | |||
| Baseline | 0.3896 (0.193) | 0.3840 (0.230) | 0.006 (–0.0608 to 0.0707) |
| 4 months | 0.5881 (0.232) | 0.5834 (0.229) | 0.005 (–0.0645 to 0.0784) |
| 12 months | 0.6219 (0.261) | 0.6489 (0.227) | –0.027 (–0.100 to 0.0537) |
| QALYs over 12 months | 0.5667 (0.196) | 0.5724 (0.200) | –0.008b (–0.0566 to 0.0433) |
Incremental costs
Point estimates of incremental costs for each sciatica group are reported in Table 22. Results for NHS costs showed that SC was slightly cheaper for participants in sciatica groups 1 and 2, but more expensive for participants in sciatica group 3, because of the higher number of spinal injections and costs associated with the fast-track pathway (MRI and specialist consultation). Similar results were observed from the societal perspective, with SC being less expensive than UC for participants in sciatica groups 1 and 2, because of lower work absence-related costs, but more expensive for participants in sciatica group 3 because of the costs associated with the fast-track pathway.
| Base case: NHS cost | SC arm | UC arm |
|---|---|---|
| Sciatica group 1 (n = 107) | 403.73 (530.94) | 413.19 (515.36) |
| Mean difference (95% CI) | –9.45 (–205.67 to 203.42) | |
| Sciatica group 2 (n = 211) | 572.50 (546.83) | 626.95 (886.55) |
| Mean difference (95% CI) | –54.45 (–274.91 to 141.32) | |
| Sciatica group 3 (n = 158) | 955.27 (953.13) | 745.70 (1180.25) |
| Mean difference (95% CI) | 209.56 (–142.47 to 521.72) | |
| Secondary analysis: health-care perspective | ||
| Sciatica group 1 (n = 107) | 516.68 (612.05) | 448.25 (526.69) |
| Mean difference (95% CI) | 68.43 (–152.92 to 292.94) | |
| Sciatica group 2 (n = 211) | 631.76 (565.85) | 676.29 (931.82) |
| Mean difference (95% CI) | –44.52 (–282.40 to 130.95) | |
| Sciatica group 3 (n = 158) | 1039.61 (971.23) | 801.41 (1232.04) |
| Mean difference (95% CI) | 238.19 (–133.71 to 558.49) | |
| Secondary analysis: societal perspective | ||
| Sciatica group 1 (n = 107) | 759.53 (932.05) | 783.52 (1233.20) |
| Mean difference (95% CI) | –23.99 (–454.31 to 373.61) | |
| Sciatica group 2 (n = 211) | 1114.19 (1923.22) | 1232.37 (2156.06) |
| Mean difference (95% CI) | –118.17 (–674.79 to 443.20) | |
| Sciatica group 3 (n = 158) | 1693.38 (2447.02) | 1586.63 (2721.13) |
| Mean difference (95% CI) | 106.75 (–726.51 to 931.14) | |
| Sensitivity analysis: NHS perspective including hospital records data | ||
| Sciatica group 1 (n = 107) | 517.73 (981.84) | 413.19 (515.37) |
| Mean difference (95% CI) | 104.54 (–141.18 to 475.53) | |
| Sciatica group 2 (n = 211) | 735.14 (1027.96) | 739.67 (1170.73) |
| Mean difference (95% CI) | –4.53 (–308.90 to 259.97) | |
| Sciatica group 3 (n = 158) | 1032.63 (979.40) | 985.01 (1592.50) |
| Mean difference (95% CI) | 47.61 (–361.53 to 459.75) | |
| Sensitivity analysis: health-care perspective including hospital records data | ||
| Sciatica group 1 (n = 107) | 630.68 (1017.62) | 448.25 (526.69) |
| Mean difference (95% CI) | 182.43(–60.68 to 574.07) | |
| Sciatica group 2 (n = 211) | 794.40 (1037.22) | 789.01 (1214.32) |
| Mean difference (95% CI) | 5.39 (–313.39 to 316.64) | |
| Sciatica group 3 (n = 158) | 1116.96 (994.23) | 1040.71 (1624.07) |
| Mean difference (95% CI) | 76.25 (–424.32 to 445.56) | |
| Cost-effectiveness analysis | Mean incrementala costs (£) (95% CI) | Mean incrementala QALYs (95% CI) |
| NHS perspectivea | ||
| Sciatica group 1 | –9.45 (–205.67 to 203.42) | –0.00041 (–0.0447 to 0.0392) |
| ICER | 23,048b | |
| Sciatica group 2 | –54.45 (–274.91 to 141.32) | –0.01981 (–0.051 to 0.010) |
| ICER | 2748b | |
| Sciatica group 3 | 209.56 (–142.47 to 521.72) | –0.00841 (–0.0567 to 0.04) |
| ICER | Dominatedc | |
Cost-effectiveness analysis by sciatica group
Table 22 reports the point estimates of incremental costs, incremental QALYs and ICERs by each sciatica group. These analyses revealed considerable uncertainty around the central estimates of the incremental costs and QALYs because of the reduced sample size in each group, but SC remained unlikely to be cost-effective. Overall, the results demonstrated that SC generated slightly fewer QALYs, on average, and was slightly cheaper than UC for sciatica groups 1 and 2, but not for group 3. Based on conventional willingness-to-pay thresholds for additional QALYs (approximately £20,000 per QALY),75 SC was dominated by UC over 12 months for those in group 3, that is a slightly lower mean health benefit (0.00841 additional QALYs) and a higher mean NHS cost (£209.56). For participants in groups 1 and 2, SC was associated with a slightly lower mean NHS cost (–£9.45 and –£54.45 for groups 1 and 2, respectively) and a lower mean health benefit (–0.00041 and –0.01981 QALYs for groups 1 and 2, respectively). Figures 14–16 present the uncertainty around the cost-effectiveness scenarios, along with the cost-effectiveness planes for the analysis per sciatica group. The location of the ‘swarm’ of bootstrapped cost–utility pairs explains the nature of the uncertainty in the generated incremental cost and QALY estimates. The CEACs in Figures 14–16 show the probability that SC is cost-effective compared with UC for a range of cost-effectiveness thresholds. In sciatica group 1, minimal cost savings were realised, but with considerable uncertainty regarding both costs and health benefits, although the ICER (in the south-west quadrant) was > £20,000 per QALY. Similarly, for group 2, cost savings were realised, with the majority of bootstrapped cost-effectiveness mean differences falling in the south-west quadrant of the £30,000 and £50,000 per QALY cost-effectiveness threshold lines. For sciatica group 3, the majority of the replicates of the ICERs were located in the north-west quadrant, with SC dominated by UC. The results suggest that SC is unlikely to be cost-effective at the £20,000–30,000 per QALY threshold currently used by NICE to determine the cost-effectiveness of health technologies. 75 The CEACs in Figures 14–16 show that the probability that SC is cost-effective compared with UC is 0.51, 0.19 and 0.26 for sciatica groups 1, 2 and 3, respectively.
FIGURE 14.
Sciatica group 1: comparing SC with UC. (a) Cost-effectiveness plane; and (b) CEAC.
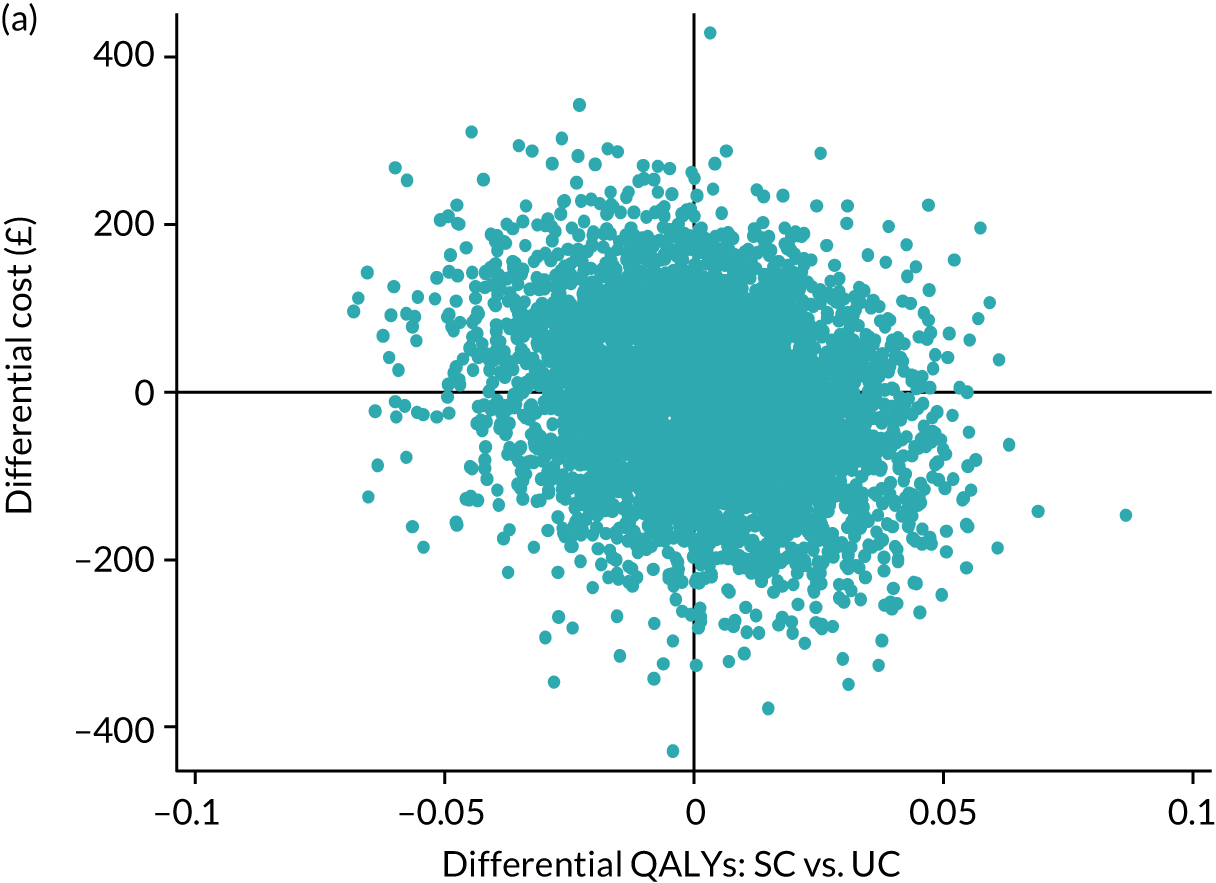
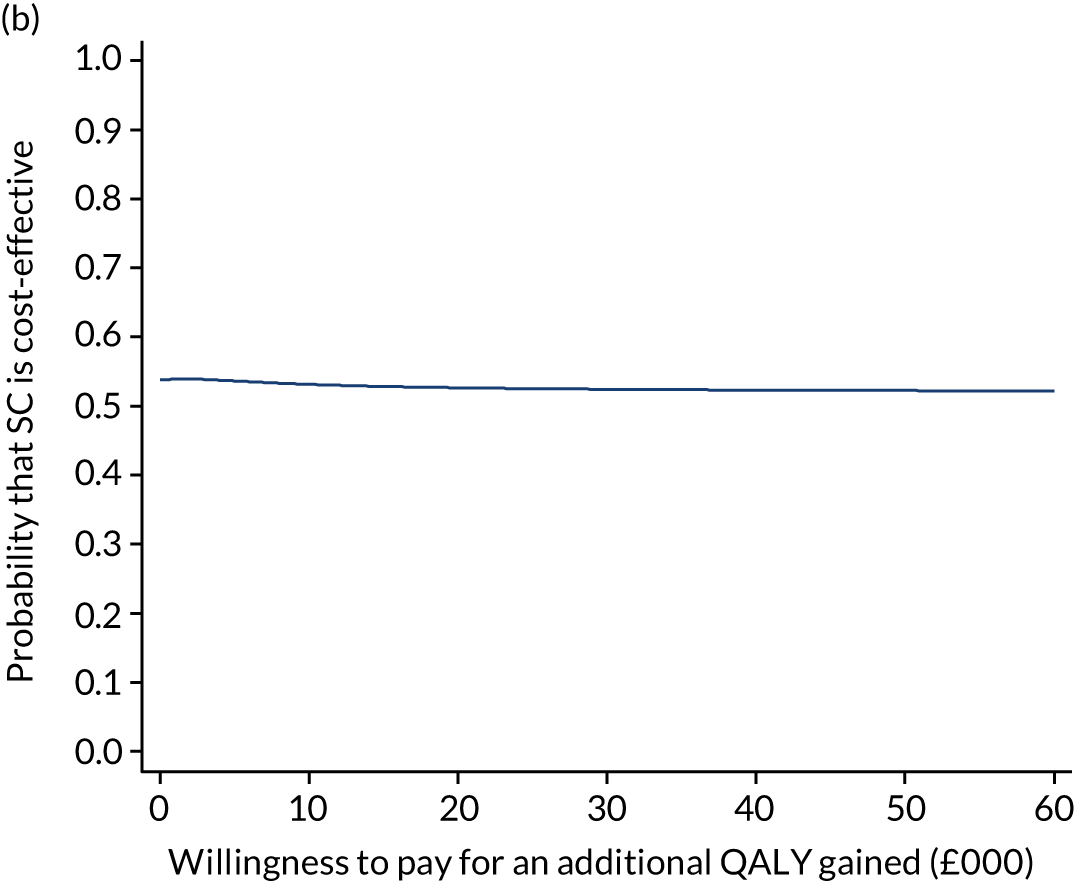
FIGURE 15.
Sciatica group 2: comparing SC with UC. (a) Cost-effectiveness plane; and (b) CEAC.
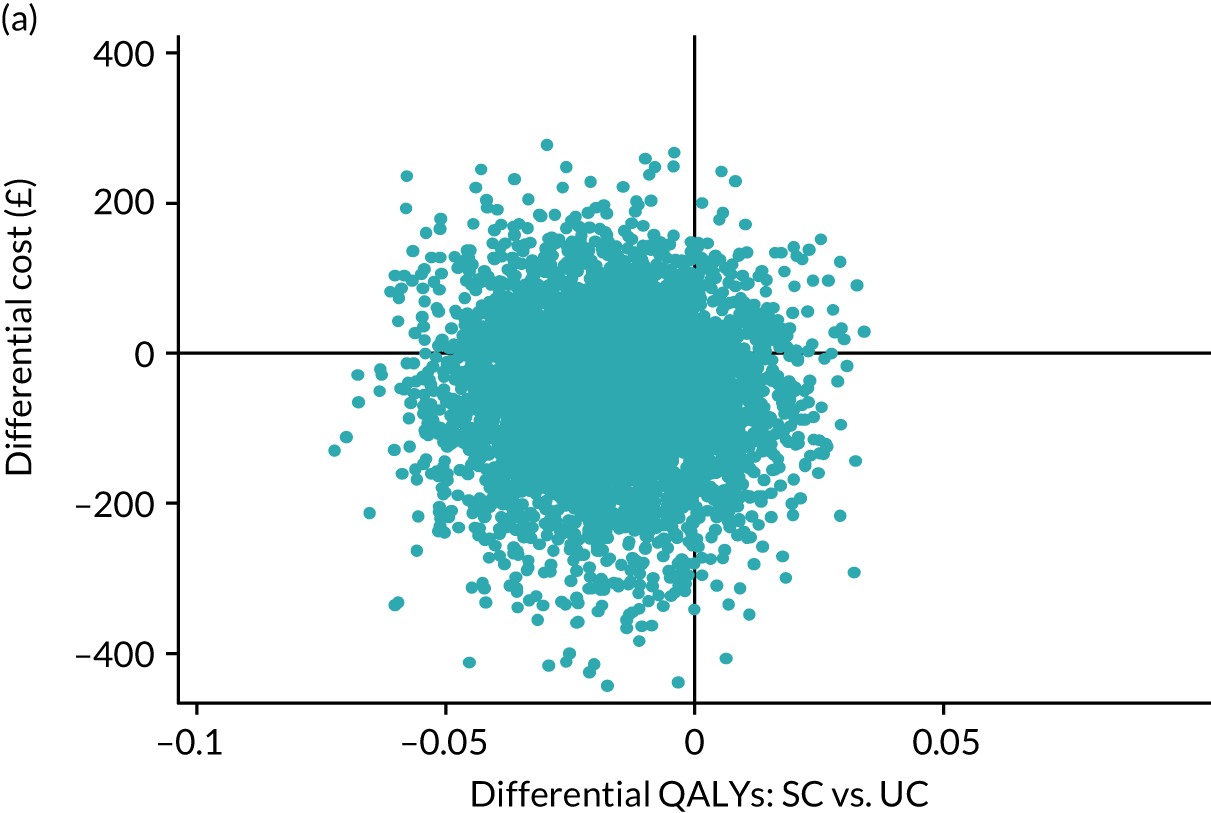
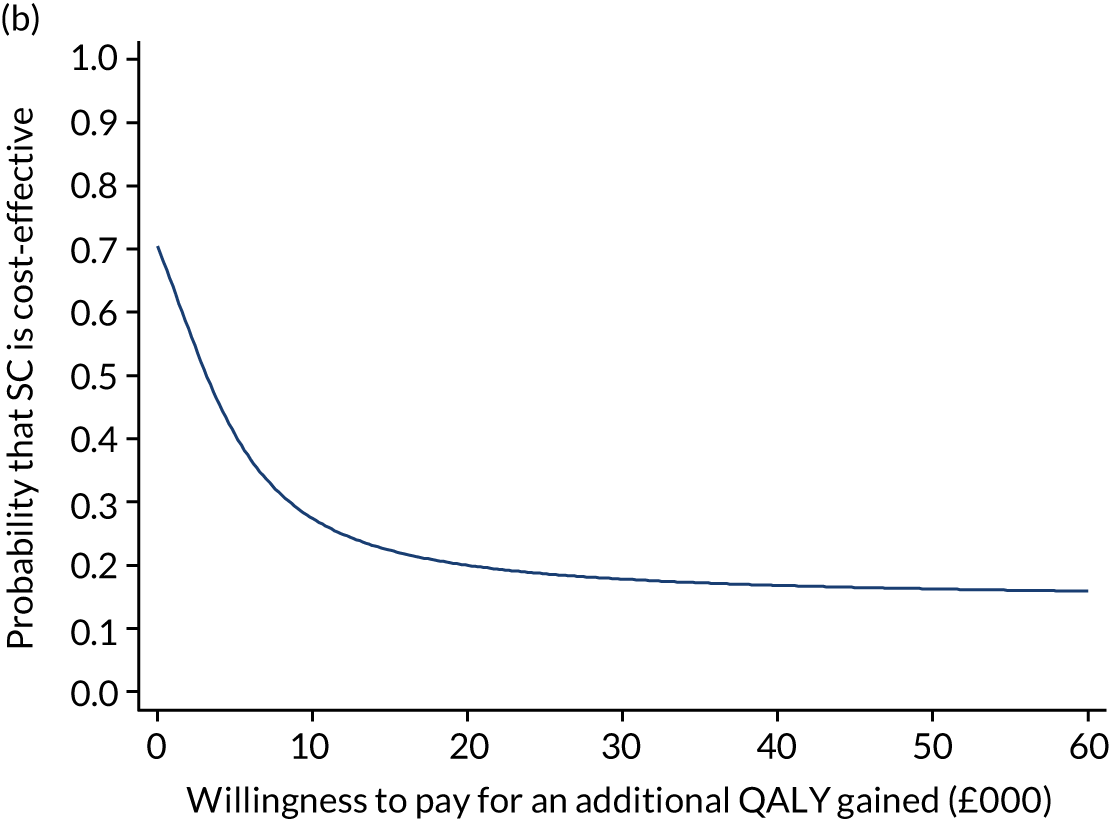
FIGURE 16.
Sciatica group 3: comparing SC with UC. (a) Cost-effectiveness plane; and (b) CEAC.
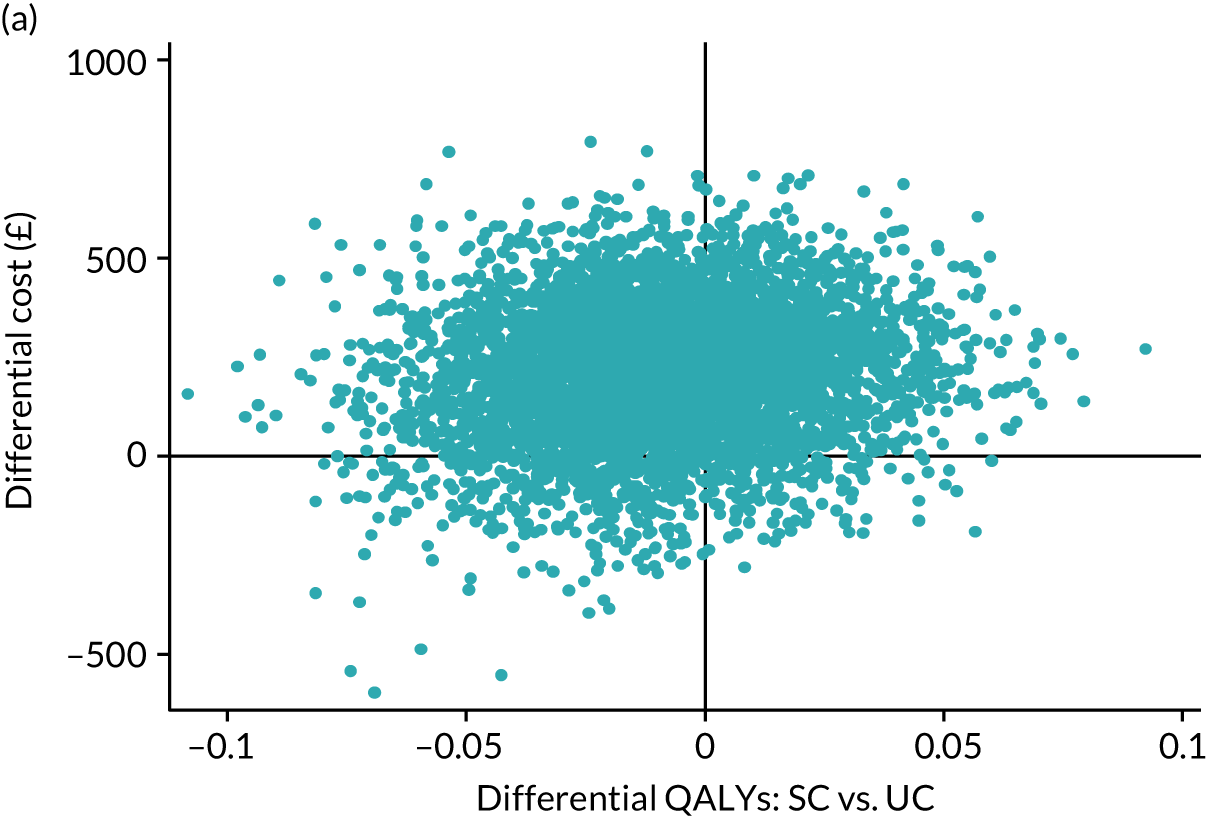
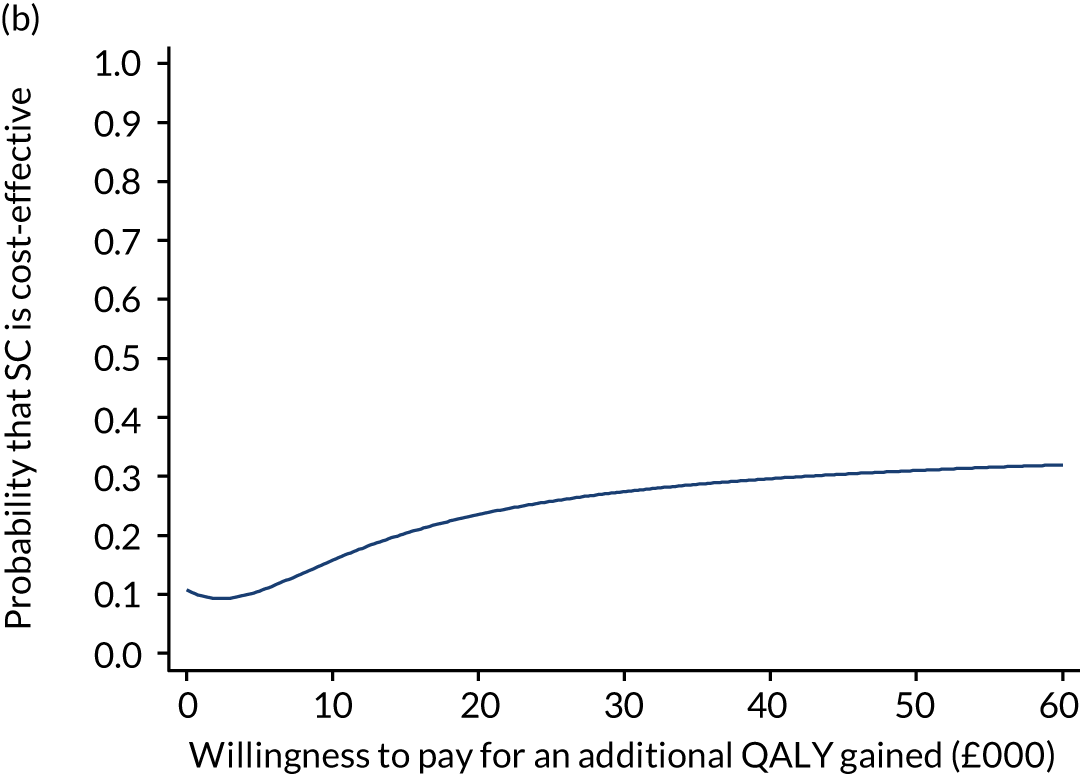
Summary
In the context of the economic analysis results, the SC model tested in the trial is unlikely to be a cost-effective option for the NHS, using commonly applied willingness-to-pay threshold values of £20,000–30,000 per QALY gained.
Chapter 5 Nested qualitative study
Parts of this chapter are from Saunders et al. 76 © The Author(s). 2020 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Aim and objectives
The aim of the nested qualitative research was to determine, and understand, the acceptability of the fast-track care pathway to patients and the clinicians who either were directly involved in patient care in this pathway or would be if this were to become incorporated into current clinical management in future, that is GPs, spinal specialist physiotherapists and spinal surgeons. As outlined in the Chapter 2, GPs identified patients with suspected sciatica for invitation to the SCOPiC trial research clinics for assessment of their eligibility to participate in the RCT. Spinal specialist physiotherapists were directly involved in the management of patients in the fast-track pathway: they provided specialist assessment and opinion at spinal interface clinics about further management, including consideration for more invasive treatments. Spinal surgeons were not involved in assessing patients as part of the fast-track pathway following the MRI scan, but they would assess patients who were referred for surgical opinion following spinal specialist opinion in interface clinics. Spinal surgeons were, however, not aware of which patients had been referred as a result of the trial’s fast-track pathway.
Although previous research has explored the views of clinicians and patients on the clinical management of sciatica,60,77,78 it is the fast-track pathway to MRI and spinal specialist assessment and opinion that is the novel addition in the SCOPiC trial. Gaining an understanding of how the fast-track pathway is perceived by patients and clinicians can provide important insights into this element of the SC model tested in the trial. It is for this reason that the fast-track pathway is the focus of the qualitative research.
This aim was addressed through the following specific objectives:
-
to explore and understand patients’ –
-
views of their clinical care and its appropriateness and timeliness for addressing their problem
-
views of the time taken for assessment, treatment and symptom resolution
-
experiences of sciatica symptoms and their impact, to provide context in terms of the treatments received.
-
-
to explore and understand clinicians’ views of –
-
the suitability of patients on the fast-track pathway
-
how the fast-track pathway compared with usual care
-
the added value of the fast-track pathway for this group of patients with sciatica
-
the limitations of the fast-track pathway for this group of patients with sciatica.
-
In addition, interviews with GPs explored perceptions of whether or not their involvement in the trial contributed to changes in their referral decisions for patients with sciatica.
Theoretical frameworks
The introduction of the fast-track care pathway clearly represents a change to usual care for a group of patients with sciatica. Changing health-care practice brings diverse challenges,79,80 including whether or not an intervention is considered meaningful and relevant to clinicians and patients, and the degree to which the intervention is seen to ‘fit’ within established ways of working. 81 Examining such issues using a theoretically underpinned approach can extend the scope of purely descriptive approaches, enabling a more cogent and coherent explanation of the issues identified in the data. Therefore, we drew on two theoretical frameworks to guide the qualitative research. The first of these is normalisation process theory (NPT),82 a theoretical framework that has been widely used to explore the introduction of new health-care interventions across a diverse range of settings, for example cancer care,83 hypertension84 and mental health. 85 NPT provides a framework for understanding why some health-care interventions are accepted and successfully embedded in routine practice, yet others are less successful in their uptake. 82 NPT aims to identify and explain ‘factors that promote and inhibit the routine incorporation of complex interventions into everyday practice’,86 through highlighting the ‘ways in which work must be reconfigured both individually and collectively by multiple stakeholders involved in the work of implementation’,87 thus taking account of both the individual-level and broader organisation-level factors. This aim is reflected in the framework’s four main components, as outlined in Murray et al:86 coherence (or sense-making), cognitive participation (or engagement), collective action (work done to enable the intervention to happen) and reflexive monitoring (formal and informal appraisal of the benefits and costs of the intervention).
Normalisation process theory aims to address both the initial introduction of a new intervention to the clinical setting, namely implementation, as well as its subsequent routinisation, namely its embedding, and the sustaining and reproduction of behaviours that allow for the longer-term integration of the intervention in everyday clinical work. Given that the aims of the nested qualitative research were to explore and understand patients’ and clinicians’ views about the acceptability of the fast-track pathway in the context of the trial, our focus was on the implementation stage of the normalisation process; we could not assess the degree to which the fast-track pathway was embedded or integrated into routine working at this early stage. Of the four key components of NPT outlined in the previous paragraph, it is the concept of ‘coherence’ that we drew on as a lens through which to interpret the interview findings. Coherence relates to the degree to which a new intervention is seen to be ‘meaningful’ to key stakeholders, and whether it ‘makes sense’ in the context of existing ways of working. The meaning attached to a clinical intervention and whether or not clinicians and patients ‘buy in’ to a new way of working is key to its early adoption and implementation. The concept of coherence thus aligns closely with our aims, as the degree to which clinicians and patients perceive the fast-track pathway as making sense in the context of the existing care pathway (i.e. ‘stepped’ care) for sciatica is key to understanding its overall acceptability.
The second theoretical framework that we drew on was Allen’s88 conceptualisation of care pathways as ‘boundary objects’. Allen88 defines a ‘boundary object’ as a loose concept, but with strong cohesive power, which enables the bringing together of the interests of different groups, while still allowing these groups to maintain their respective social identities. Allen88 argues that care pathways can be considered as ‘boundary objects’ in that they have the ability to ‘align clinical, management and service user interests around a healthcare quality agenda’. 88 She also points out, however, that they can, at the same time, give rise to conflicting agendas among different stakeholders (e.g. clinicians, patients, managers), which results in challenges when trying to meet the needs of multiple groups in the design and operationalisation of new care pathways. 88 This ‘boundary object’ conceptualisation was also drawn on as a lens through which to interpret the findings, enabling the investigation of how the fast-track pathway addressed the shared goals of patients and the three clinician groups (GPs, spinal specialist physiotherapists and spinal surgeons), aspects in which the fast-track pathway did not align with the priorities of these respective groups and where competing agendas were present in terms of the care pathway’s goals, components and operationalisation.
Methods
Individual semistructured interviews were conducted with patients in the SC care arm of the trial who were allocated to group 3’s matched fast-track pathway (n = 20) and with participating clinicians (n = 20: seven spinal physiotherapists, nine GPs and four spinal surgeons) between January 2016 and February 2018.
Sampling
Patients were purposively sampled to capture diverse characteristics including the treatment centre attended and participant demographics such as age, sex, leg pain intensity, treatments received (including patients who had received invasive treatments such as spinal injections or surgery) and response to treatment. Clinicians were sampled for variation in geographical location across the different practice areas from which patients were recruited to the trial (North Staffordshire, North Shropshire/Wales and Cheshire). GPs were further purposively sampled based on the number of patients during the trial for whom a sciatica-related diagnostic Read code had been entered, prompting the electronic ‘pop-up’ on the GP’s online system indicating that the patient may be eligible for invitation to the SCOPiC trial clinics. It was felt that interviewing those GPs who had had the most opportunity to engage with the trial, in terms of identifying patients to be invited to the SCOPiC research clinics, might yield rich interview data. Sampling based on opportunity to engage with the trial was not relevant for the other clinician groups: the spinal physiotherapists had, by definition, been involved in the management of this group of patients in terms of providing specialist opinion and assessment as part of the fast-track pathway, and spinal surgeons would not have been aware of which patients they had seen that were part of the fast-track pathway.
Recruitment
Patients were initially sent, via post, an interview invitation letter along with a participant information leaflet about the qualitative study, having consented to contact as part of their participation in the RCT. A member of the research team then telephoned these patients to ask if they would be interested in taking part in an interview. For those who were willing to take part, a convenient time and location for the interview was arranged and a confirmation letter was sent via post.
Clinicians were initially contacted by e-mail or, in the case of some GPs, via their practice manager and were subsequently posted an invitation letter and information leaflet about the qualitative study. Clinicians who agreed to take part were contacted again to arrange the interview.
Semistructured interviews
Interviews with patients, spinal physiotherapists and spinal surgeons were conducted after the 4-month follow-up time point of the RCT. This was to allow patients to reflect on their experiences of the fast-track pathway up to that point, and for clinicians to have had the opportunity to see patients as part of the fast-track pathway. Interviews with GPs were conducted once recruitment to the trial had finished (November 2017 onwards), to avoid the possibility of influencing their normal referral patterns of patients with sciatica.
Of the 20 patient interviews, 13 took place at participants’ homes, two took place at the university and five were conducted via telephone, in line with participants’ preferences. The duration of the patient interviews ranged between 21 minutes and 1 hour 15 minutes (average 48 minutes). Of the 20 clinician interviews, eight were carried out at the clinician’s practice or hospital, four took place at the university and eight were conducted via telephone. The duration of clinician interviews ranged between 19 minutes and 32 minutes in length (average 26 minutes).
Interviews were audio-recorded, with the exception of one GP interview as the GP consented to take part in an interview but not to the interview being audio-recorded. This GP reported that, although they were willing to have their views included in the research, they felt uncomfortable being audio-recorded. Instead, the interviewer took detailed notes during and immediately after the interview to capture the GP’s views. All participants provided written, informed consent prior to the start of interviews, or audio-recorded consent in the case of telephone interviews. Consent was reaffirmed verbally at the end of each interview.
Topic guides
Topic guides were used in interviews, which covered a range of areas relevant to the qualitative study aims. Separate topic guides were used for patient and clinician interviews. The clinician topic guide was adapted for the three clinician groups to reflect the differing roles of these clinicians in patient assessment and management and in the trial’s fast-track care pathway (as outlined in Aim and objectives in this Chapter). For instance, interviews with spinal surgeons placed more emphasis on exploring the suitability of patients in the fast-track pathway for surgical opinion, whereas interviews with GPs explored the impact of their participation in the trial on their management of patients with sciatica in general practice. Spinal physiotherapist topic guides placed more emphasis on asking the participants to reflect on concrete experiences of managing specific patients in the trial, as a result of their role providing specialist spinal opinion as part of the fast-track care pathway.
Topic guides were used primarily as an aide-memoire; the interviewer retained flexibility to follow up on any unexpected findings emerging during the interviews, allowing aspects of the interviews to be participant led. Early findings informed subsequent interviews, with the topic guides iteratively revised throughout the data collection process. An example of this was in early GP interviews when the GP participants highlighted that, as part of usual care, they could often identify the more ‘severe’ patients with sciatica who they felt were particularly in need of early investigation and consideration for more invasive treatments. Based on those early findings, the topic guide was revised to prompt the interviewer to explore in more detail, in subsequent interviews, the characteristics that GPs felt make patients’ presentation more ‘severe’ and, thus, their condition more suitable for early investigation, as well as the potential implications of the fast-track pathway for managing this group of patients.
Data analyses
Audio-recordings of interviews were transcribed in full and anonymised by replacing names with pseudonyms and removing other potentially identifiable information. A two-stage analysis framework was adopted that incorporated an inductive thematic analysis followed by mapping the identified themes onto the two theoretical frameworks: the ‘coherence’ construct in NPT and the conceptualisation of care pathways as ‘boundary objects’. Adopting this two-stage framework reflected a desire to avoid imposing meaning on the data, or potentially missing important findings, which can be risked when pre-established theories are applied in a deductive manner. Analysis was an iterative process and data collection continued until saturation was judged to have been reached, defined as ‘informational redundancy’: the point at which additional data no longer offers new insights. 89
Anonymised transcripts were first systematically coded on a line-by-line basis by one of the authors (BS) with the aid of the software program NVivo 10 (QSR International, Warrington, UK), to identify recurrent concepts inductively. Coding was, at first, largely descriptive, and later became more conceptual as interpretations of the data moved towards a higher level of theoretical abstraction. Coding was reflexive and recursive, with codes being revisited in the light of the findings of subsequent data collection. A random sample of six patient transcripts and six clinician transcripts was independently coded by three other members of the team to check for inter-coder reliability. Coders brought different disciplinary perspectives to the data (BS: medical sociology; BB: social gerontology; MA: clinical academic and general practice expertise; and KK: clinical academic physiotherapy and spinal specialist expertise). The aim of independent coding was also, therefore, to understand cross-disciplinary perspectives on the data and, through discussion, to come to an agreement on shared meanings and interpretations. Early findings from the patient interview data, along with a sample of three interview transcripts, were also shared with four members from the Research Institute’s PPI group. A meeting was held in which the researchers looked through these transcripts with the PPI members and explored whether or not their interpretations of the data aligned with the emergent findings. All four PPI members were broadly in agreement with the early interpretations of this interview data.
Data were analysed thematically using the constant comparison method. 90 This involved looking for connections within and across interviews, and across codes, highlighting data consistencies and variations. Initially, patient and clinician data were coded separately; these data sets were then mapped onto one another, looking at how each of the main themes that was identified did or did not manifest across both the clinician and patient interviews. Three of the four main themes that were identified could be neatly mapped across both data sets; one theme identified in the patient interviews did not emerge in the clinician data: the impact of sciatica symptoms on everyday life. Although clinicians demonstrated understanding and awareness of the impact of sciatica on patients’ lives, they discussed this mainly in relation to how the impact of symptoms on patients informed their clinical management. Whereas the other three themes relate directly to the experiences and perceptions of the fast-track pathway, this theme, as it emerged in relation to the patient data, is broader in scope and focused primarily on patients’ experiences outside clinical/health-care settings. For this reason, this theme is reported on only briefly here to provide context for understanding patients’ views and experiences of treatments received during the trial; the theme is explored in greater detail in Saunders et al. 91
The second stage of the analysis involved mapping the three themes identified in both the patient and clinician interviews onto the ‘coherence’ construct in NPT and the conceptualisation of care pathways as ‘boundary objects’. We explored the degree to which the identified themes could be seen to ‘fit’ within these frameworks, and how the theoretical constructs manifested in relation to these themes. Exploring the findings in the context of these theoretical frameworks allowed us to move beyond a purely descriptive analysis, developing insights at a higher level of abstraction and, thus, allowing for the development of a more robust understanding of the identified issues. In what follows, we outline the characteristics of the sample, before reporting on the findings in relation to each of the key themes: in brief, the one theme identified only in the patient data, followed by the three key themes identified in both the patient and clinician interviews, in turn.
Results
Patient participant characteristics
Ten patient participants were male and 10 were female, aged from 28 to 86 years (average age 52 years), with a range of occupational backgrounds. Leg pain intensity reported at the interview after the 4-month follow-up time point in the trial varied widely between 1 out of 10 and 10 out of 10, with an average of 5.3 out of 10 (higher scores indicate worse symptoms). Symptom duration reported at the interview after the 4-month time point in the trial ranged from symptoms having resolved by 3 months to symptoms still experienced at 11–16 months (average symptom duration: 5–6 months). Table 23 summarises the characteristics of the 20 patients interviewed.
| Patient ID | Age (years) | Sex | Self-reported occupation type | Duration (months) of current symptoms at the time of interview (4-month follow-up)a | Leg pain intensity score (out of 10) over the previous 2 weeks (at the 4-month follow-up) | Self-reported symptoms at the 4-month follow-up compared with baseline |
|---|---|---|---|---|---|---|
| 1 | 62 | F | Unemployed as a result of sciatica | 5–6 | 9 | Worse |
| 2 | 36 | F | Radiographer | Symptoms resolved prior to interview; duration: 3 months | 0 | Completely recovered |
| 3 | 53 | M | Compliance director | 7–10 | 4 | Better |
| 4 | 49 | F | Pottery worker | 7–10 | 7 | Same |
| 5 | 44 | M | Assistant manager | 9–12 | 7 | Same |
| 6 | 60 | F | Dining hall assistant | Symptoms resolved prior to interview; duration: 3 months | 3 | Better |
| 7 | 64 | M | Retired | 7–10 | 9 | Much worse |
| 8 | 42 | M | Builder | 9–12 | 10 | Much worse |
| 9 | 44 | F | Early years practitioner | 7–10 | 7 | Same |
| 10 | 57 | M | Pottery worker | 5–6 | 4 | Better |
| 11 | 66 | M | Ambulance driver | 6–8 | 3 | Better |
| 12 | 86 | F | Retired | Symptoms resolved prior to interview; duration: 4 months | 2 | Much better |
| 13 | 45 | F | Data claims manager | 5–6 | 7 | Better |
| 14 | 69 | F | Retired | 11–16 | 6 | Better |
| 15 | 55 | M | Machine driver | 5–6 | 2 | Better |
| 16 | 67 | F | Unpaid carer | 7–10 | 7 | Same |
| 17 | 70 | F | Retired | 5–6 | 6 | Better |
| 18 | 67 | M | Office manager | Symptoms resolved prior to interview; duration: 4 months | 2 | Much better |
| 19 | 46 | M | Police service staff | 5–6 | 5 | Better |
| 20 | 28 | M | Left skilled labour job because of sciatica | Symptoms resolved prior to interview; duration: 5 months | 1 | Completely recovered |
In relation to the investigations and treatments patients received throughout the course of the trial, information on referrals immediately following patients’ appointments at the spinal interface clinics was available for all 20 participants; hospital records review data on management over the 12-month follow-up period in the trial was available for 17 of the 20 patients. Because patient interviews were conducted after the 4-month follow-up, some hospital records data post date the interviews (Table 24 shows the full breakdown of referrals, investigations and treatments received by each patient participant, along with the dates these were received, when available). These data show that 16 out of the 20 patients interviewed were referred for physiotherapy as a first management option, following fast-track to MRI and the spinal interface clinic. Although 12 of these 16 were referred only for physiotherapy at that stage, the other four patients were also referred for a caudal epidural injection at the same time that their physiotherapy referral was made. Of these four, one patient went on to have a further injection (transforaminal epidural injection) at a later date.
| Patient ID | Date randomised to the RCT | Date of MRI | Date of spinal assessment at spinal interface clinic | Onward referral from interface clinic and date referral was made | Date of referral appointment and any treatment received | Any further referral and date referral was made | Date of referral appointment | Treatment received and date of this treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | 7 July 2015 | 8 July 2015 | 20 July 2015 | Physiotherapy, 20 July 2015 | First appointment: 28 July 2015 (four physiotherapy treatment sessions in total) | N/A | N/A | N/A |
| 2 | 10 September 2015 | 16 September 2015 | 22 September 2015 | Physiotherapy, 22 September 2015 | Information not available | N/A | N/A | N/A |
| 3 | 9 June 2015 | 17 June 2015 | 25 June 2015 | Physiotherapy, 25 June 2015 | First appointment: 7 July 2015 (seven physiotherapy treatment sessions in total) | Spinal surgeon, 12 August 2015 | No data available | No data available |
| 4 | 27 August 2015 | 28 August 2015 | 11 September 2015 | Spinal surgeon, 16 September 2015 | Appointment: 24 October 2015 | Second MRI scan: 1 April 2016 | No data available | Second transforaminal epidural injection: 18 May 2016 |
| Received transforaminal epidural injection: 24 November 2015 | ||||||||
| 5 | 3 August 2015 | 5 August 2015 | 13 August 2016 | Physiotherapy, 13 August 2016 | Information not available | N/A | N/A | N/A |
| 6 | 18 September 2015 | 18 September 2015 | 2 October 2015 | Spinal surgeon, 2 October 2015 | Appointment: 14 December 2015 | Second MRI scan; referral date not available | Underwent MRI: 30 October 2015 (following orthopaedic referral but prior to appointment) | N/A |
| 7 | 23 July 2015 | 28 July 2015 | 4 August 2015 | Physiotherapy, 4 August 2015 | First appointment: 13 August 2015 (three physiotherapy treatment sessions in total) | Spinal surgeon: 22 September 2015 | No data available | No data available |
| 8 | 22 September 2015 | 27 September 2015 | 5 October 2015 | Physiotherapy and spinal injection, 5 October 2015 | First physiotherapy appointment: 7 October 2015 (four physiotherapy treatment sessions in total) | Spinal surgeon: 16 December 2015 | No data available | No data available |
| Caudal epidural injection: 4 November 2015 | ||||||||
| 9 | 15 October 2015 | 23 October 2015 | 26 October 2015 | Physiotherapy and spinal injection, 26 October 2015 | First physiotherapy appointment: 5 November 2015 (three physiotherapy treatment sessions in total) | Second spinal injection: 3 February 2016 | No data available | Transforaminal epidural injection: 20 April 2016 |
| Caudal epidural injection: 16 December 2015 | ||||||||
| 10 | 14 January 2016 | 21 January 2016 | 19 January 2016 (not known why patient underwent MRI after spinal specialist appointment) | Physiotherapy and spinal injection, 25 January 2016 | No information available regarding physiotherapy appointments | N/A | N/A | N/A |
| Caudal epidural injection: 17 February 2016 | ||||||||
| 11 | 17 March 2016 | Could not undergo MRI owing to metal plate in arm | 4 April 2016 | Physiotherapy, 15 April 2016 | First appointment: 3 May 2015 (four physiotherapy treatment sessions in total) | N/A | N/A | N/A |
| 12 | 25 February 2016 | 3 March 2016 | 4 March 2016 | Physiotherapy, 4 March 2016 | First appointment: 17 March 2016 (four physiotherapy treatment sessions in total) | N/A | N/A | N/A |
| 13 | 6 May 2016 | 12 May 2016 | 20 May 2016 | Spinal surgeon; referral date not available | Information not available – patient saw chiropractor privately (five sessions in total) | No data available | No data available | No data available |
| 14 | 3 June 2016 | 7 June 2016 | 17 June 2016 | Spinal surgeon; referral date not available | Date of first appointment not known (four physiotherapy treatment sessions in total) | N/A | N/A | N/A |
| 15 | 20 May 2016 | 27 May 2016 | 3 June 2016 | Physiotherapy, 3 June 2016 | First appointment: 7 June 2016 (four physiotherapy treatment sessions in total) | N/A | N/A | N/A |
| 16 | 24 May 2016 | 6 June 2016 | 7 June 2016 | Physiotherapy, 7 June 2016 | Information not available | N/A | N/A | N/A |
| 17 | 22 August 2016 | 23 August 2016 | 30 August 2016 | Physiotherapy, 30 August 2016 | First appointment: 3 February 2017 (four physiotherapy treatment sessions in total) | N/A | N/A | N/A |
| 18 | 9 August 2016 | Did not want to be scanned | 22 August 2016 | Physiotherapy, 22 August 2016 | First appointment: 6 September 2016 (three physiotherapy treatment sessions in total) | N/A | N/A | N/A |
| 19 | 30 August 2016 | 4 September 2016 | 9 September 2016 | Physiotherapy and spinal injection, 9 September 2016 | No information available regarding physiotherapy appointments | N/A | N/A | N/A |
| Date of caudal epidural injection not available | ||||||||
| 20 | 14 October 2016 | 18 October 2016 | 25 October 2016 | Physiotherapy; referral date not available | One physiotherapy treatment session: 22 February 2017 (patient did not attend follow-up appointment) | No data available | No data available | No data available |
Four out of the 20 patients interviewed were referred to a spinal orthopaedic surgeon as the first management option following their appointment at the spinal interface clinics. One of these four was later referred for a transforaminal epidural injection and one went on to receive physiotherapy. Information about onward management as part of the trial beyond the initial spinal surgeon consultation was not available for the other two patients. Three additional patients, out of the 17 for whom hospital records review data were available, were also referred to a spinal surgeon, but at a later stage: two of these patients were referred having first received physiotherapy, and one was referred after receiving physiotherapy and a caudal epidural injection. Although four patients reported in interviews that they had either undergone or were waiting for spinal surgery, this information was not available from the hospital records data.
Clinician participant characteristics
Eleven clinicians were female and nine were male. Of these, five GPs were female and four were male, six spinal specialist physiotherapists were female and one was male, and all four spinal surgeons were male. The length of time that clinicians had been practising ranged from 9 to 31 years. In relation to the different practice areas in the trial, nine clinicians were located in Staffordshire, six were in North Shropshire and five were in Cheshire. Despite inviting clinicians (GPs) from practices in Wales, we did not recruit any clinicians for interview from this area; however, Welsh general practices represented only a small number of those participating in the trial (four out of 42).
Main themes
The four main themes identified through the thematic analysis were:
-
the impact of sciatica symptoms on everyday life (identified in patient data only)
-
the acceptability of the fast-track care pathway
-
the perceived benefits of the fast-track care pathway
-
the waiting times for onward treatment following fast-track.
Impact of sciatica symptoms on everyday life
As mentioned, this theme focused primarily on patients’ experiences outside clinical/health-care settings and did not directly relate to experiences of the fast-track care pathway. For this reason, this theme is reported on only briefly here, to provide context for understanding patients’ views and experiences of treatments received during the trial; the theme is explored in greater detail in Saunders et al. 91
All patients who were interviewed emphasised the extreme severity of their symptoms, including intense leg pain and restricted mobility, often leading to distress and low mood. For example, one patient articulated the intensity and impact of his pain, and the extreme lengths he felt that it could have pushed him to:
I can’t describe the pain, that sort of pain where you want to throw up . . . constant, there was no relief from it and I’ve still got it now . . . some nights I was suicidal. If they’d put a gun at the side of the bed, I’d have sooner shot myself.
Male, aged 64 years, patient identifier (ID) 7
Such symptoms were reported as permeating all aspects of life, preventing individuals from going about their usual routines, or, in some cases, preventing them even leaving the house, resulting in considerable frustration:
Financially it’s hit us massively. Health-wise, I’m not sleeping; I haven’t been able to go to functions, go to the pub, I haven’t done nothing like that . . . and you can’t go out, not that you don’t want to go out, you can’t . . . And frustration, that’s the biggest one.
Male, aged 42 years, patient ID 8
Patients appeared to often respond to the impact of sciatica symptoms through placing all of their hope on available treatment options to resolve or improve their symptoms. One patient highlighted how pain and restricted function meant that she was currently unable to work, which was an aspect of her life that was particularly salient to her. She expressed a sense of desperation that her upcoming spinal injection (that she was waiting for at the time of interview) would improve her symptoms to the point that she would be able to get back to work:
I love my job working with children [as an early years practitioner]. The thought of not being able to ever go back is soul-destroying. I can’t think of that . . . I’ve got to get back to work . . . I’m just hoping to God this injection works. It’s all fingers crossed . . . and just hope.
Female, aged 44 years, patient ID 9
Acceptability of the fast-track care pathway
All patients who were interviewed reported being pleased and surprised by how quickly they underwent MRI after randomisation (which was, on average, 5 days following randomisation to the trial) and how quickly they had a subsequent spinal specialist appointment at the interface clinics (on average, 11.5 days following randomisation). Patients drew contrast between this and their previous experiences of long NHS waiting times for treatments and investigations:
I went for my scan more or less straight after [the SCOPiC clinic appointment]. I even commented how quick it was, because normally you wait and wait and wait, don’t you? I was shocked and pleased because it seemed like I’d jumped the queue!
Female, aged 49 years, patient ID 4
The MRI was within the 2 weeks. I felt absolutely like a VIP [very important person] because I have a friend who is a GP and quite often he says that a lot of his patients would take 6 to 12 months to get to where I did in just a few weeks.
Male, aged 46 years, patient ID 19
Clinicians reported finding it acceptable for patients identified as needing spinal specialist assessment and consideration for more invasive treatments to be seen by those specialists sooner. In particular, clinicians expressed positive views that the fast-track pathway can enable earlier access to more invasive treatments, potentially leading to pain being resolved more quickly and, therefore, allowing patients to get back to work and usual activities sooner:
There was a patient I saw 6 or 7 weeks down the line, and they were struggling. They were quite severe and their scan findings said they had a big disc problem and they went on to have a caudal epidural injection. They got that injection a lot quicker than they would probably for the normal route, and that helped them massively in the sense that they’ve got back to work.
Spinal physiotherapist 2
From the GPs’ perspectives, all nine reported positive views towards the short time frame for the fast-track pathway, in that they felt able to take a more active management approach than currently available to them as part of usual, stepped care. They reported that, previously, they would have reviewed patients for a period of time before referring them for investigation and onward treatments, but, as part of the trial, they could now take a more ‘proactive’ approach by identifying patients for invitation to the SCOPiC trial research clinics. GPs highlighted particularly ‘severe’ patients as being those who were absent from work or struggling at work as a result of their pain; it was these patients who the GPs felt were most in need of early intervention:
The time scale is vastly different [between onward referral as part of usual care and onward referral as part of the fast-track pathway]. I think prior to SCOPiC being around, we would maybe sort of review people for a bit longer. But if someone is unable to do their work, then that makes me feel that I need to do something more proactive; so we are now actually practically doing something to try and help them.
GP 2
However, there was some variation among clinicians in views about the timing of the fast-track pathway, based on a patient’s symptom duration. Thirteen clinicians (seven GPs and six spinal physiotherapists) felt that the fast-track pathway was less acceptable for managing patients with short symptom durations (i.e. < 6 weeks), and expressed concern that some of these patients may be ‘fast-tracked’ too soon and that their symptoms could still resolve naturally without the need for imaging:
My cut-off is if things are no better after 6 to 8 weeks, then it’s looking more like a chronic issue that’s maybe not going to resolve easily or quickly. So I would say patients are being fast-tracked for a scan earlier than I would [fast-track them normally]. Potentially, you may be seeing people that would have gone on to just resolve in another 2 or 3 weeks.
GP 7
I’d say really acute, early on where you’ve still got natural resolution within that first 4 weeks; I wouldn’t normally scan someone at 3 weeks with sciatica that have not probably had sciatica before. It’s more likely they’re going to be getting better.
Spinal physiotherapist 6
The view that some patients with short symptom duration may be less suitable for fast-track was reflected in examples given by spinal physiotherapists of patients seen in the spinal interface clinics as part of the fast-track pathway. Although the spinal physiotherapists felt that most patients were ‘fast-tracked’ appropriately, they reported seeing some patients who had had symptoms for only a few weeks, and, based on their assessment, they felt that these patients did not need to see a spinal specialist at that early stage, particularly if they were beginning to show signs of improvement:
If you had a DVD [digital versatile disc] that went through everything, then that would have been just as good as me being there. As long as they were getting better at that point, I don’t really feel they needed to be seen by me really, in terms of a spinal specialist opinion.
Spinal physiotherapist 5
In line with the views in the preceding three quotations, several clinicians felt that ‘fast-tracking’ may be more beneficial only after patients have had symptoms beyond a certain duration, most commonly reported by clinicians to be between 6 and 10 weeks:
The only place where it [the fast-track pathway] maybe falls down is them being referred into that service too soon. I saw a lady who had only had a 2-week history of symptoms and, for me, that’s too early to refer into that kind of service. But the ones that are a little bit later down the line, then it’s great to be able to assess them and have all the investigations done relatively rapidly because then you can make a decision quickly for that patient. It’s just where the starting point begins, and making sure the patients don’t come into it too early . . . I’d say anyone that’s coming through [for spinal specialist opinion] with less than 2 months’ or even up to 10 weeks’ worth of symptoms, I’d like them to have longer to settle conservatively beforehand.
Spinal physiotherapist 7
There was some variation in views, however, as seven clinicians (all four surgeons, one spinal physiotherapist and two GPs) did see it as acceptable for patients with severe symptoms, even if of short duration, to be ‘fast-tracked’ as early as possible (i.e. even within a few weeks of symptom onset). However, this was primarily for the purpose of providing patient reassurance rather than to inform treatment decisions (the concept of reassurance is discussed in greater detail Perceived benefits of the fast-track care pathway):
I don’t think getting MRIs done earlier would be a bad idea. Patients like to have that reassurance. They’re not reassured by a GP, physio[therapist] or you and me, they like to know a scan was done and it said it was fine. So I think the MRI scan is more of a tool to address their mental state rather than to decide treatment from that point of view. So I don’t think that getting MRIs done earlier would be a bad idea, but I don’t think it will change my surgical plan or decision-making or time to treat.
Spinal surgeon 4
Although there was variation among clinicians as to their views on the acceptability of the fast-track pathway for patients with short symptom durations, there was greater agreement across the three clinician groups about the management of these ‘acute’ patients following fast-track for MRI and specialist opinion at the spinal interface clinics. Clinicians expressed reluctance to consider invasive treatment options too early for these ‘acute’ patients, particularly until conservative treatment had first been tried and failed. This appears to reflect the expectation among the clinicians that, for many patients, their symptoms will resolve naturally, and also shows the extent to which the current ‘stepped’-care approach is strongly embedded as part of their routine usual care. There was clearly a reluctance among clinicians to move away from their usual stepped-care model for sciatica patients with short symptom durations:
If they’ve not had appropriate conservative treatment, I wouldn’t want to rush down those management options too early [i.e. more invasive options] . . . In my own mind, I want to make sure I’ve ticked all those boxes, make sure that I know my patient has been conservatively managed as best as we can.
Spinal physiotherapist 3
Even if I see patients privately, I tell them to wait for 2 to 3 months anyway; even if they are paying me, I will say wait 2 or 3 months, so that is our standard approach, that’s what we follow.
So if a patient did come to you earlier, would you still take that kind of conservative viewpoint of ‘let’s see if it settles down by itself’?
Yes, we always do that.
I wouldn’t normally recommend any form of invasive treatment, or even an injection, unless they’ve had symptoms for 6 weeks or the symptoms were getting worse and were at a level where they were needing hospital admission.
Spinal surgeon 2
There was a concern expressed by the GPs that, if spinal specialists continue to adopt this conservative ‘wait-and-see’ approach for some patients, this may result in spinal surgeons repeating a patient’s original MRI scan:
The difficulty is, sometimes, if the spinal surgeons see people at too early a stage, they will just want to try conservative management anyway, and leave them waiting to see if the symptoms improve and then repeat the scan.
GP 5
The embeddedness of the stepped-care model for those patients with short symptom duration was also reflected in how some patients understood the treatment options available to them following fast-track:
They [the spinal physiotherapist] said ‘well, this is the course of how we treat sciatica’. You wouldn’t go for the big drug [an analogy for the most intensive treatment] as a first option, you would go for the small one to see if it had any effect. The big drug would be the last option, because if that doesn’t work, where do you go from there?
Male, aged 42 years, patient ID 8
Perceived benefits of the fast-track care pathway
Patients and all three clinician groups perceived benefits from the fast-track pathway, in terms of providing earlier patient reassurance based on MRI scan findings, particularly in enabling patients to understand the cause of their pain and assuring them that there was no serious underlying pathology:
I was happy when I had the MRI because I knew what it was [causing the pain]; I think that’s half the problem, because you worry about it otherwise. You think ‘oh my god, what’s going on there?’. But he [the spinal specialist physiotherapist] showed me the MRI scan and showed me exactly where the disc bulge was, so at least then you know exactly what was going on. They assured me and explained everything, what had happened. So at least I know why the pain’s happening . . . at least I know there could be a solution to it. I know what I’m coping with and I just feel easier now that I know what it is. That’s 90% of the battle really, that I know that it’s nothing too sinister.
Female, aged 60 years, patient ID 6
With a lot of these patients, it’s the first episodes of these types of problems that they’ve ever had and they can be quite dramatic. So there is a huge amount of anxiety . . . and one of the benefits, certainly, of ‘fast-tracking’ them is at least we can show them, ‘yes, there is something’, because if they’ve had a scan that confirms the changes . . . there’s nothing sinister or nasty going on. So you can offer a level of reassurance.
Spinal physiotherapist 1
Patients also highlighted that being able to see on the scan a finding that could explain an underlying cause of their pain, along with the accompanying explanation from the clinician, allowed them to gain a better understanding of their diagnosis of sciatica:
Was it helpful in any way actually being able to see the picture of the scan?
Yes, because being able to actually see a very clear picture and seeing the nerves in the lower back being tweaked or pressured, it then becomes very clear what sciatica is, because sciatica is often a very generic term, if someone’s got a sore leg. But you can then see the physical nerves themselves are trapping which explains why it [the pain] goes down the leg, it’s very good for explanation and understanding what you’ve got, it’s not just something theoretical . . . And he [the spinal specialist physiotherapist] said, despite the fact that you could see a slight pinching, the joints of the spine are very healthy, there’s no deterioration through most of them . . . he was quite surprised by how healthy they actually looked.
Several patients also highlighted that the fast-track pathway led to a greater sense of satisfaction with the care they had received than with previous experiences of NHS care. Notably, four patients reported satisfaction with the fast-track pathway despite not having experienced any improvement in their sciatica symptoms at the point of their interview. All four had a history of episodic sciatica, and reported satisfaction in feeling that something active was being done to address their pain, which to them represented progress compared with previous care received:
I feel like I’ve got further with you this time than I’ve got with anyone else. In previous years, I’ve been to so many people for help and everyone seems to close the door on me, basically; that’s how I feel. This time it just seems like I’m getting somewhere.
Female, aged 49 years, patient ID 4
Although all clinicians thought that the fast-track pathway could benefit patient outcomes in the short term, there was some variation in the clinician data about the potential of the fast-track pathway to benefit patient outcomes over time. One spinal surgeon felt that the main benefits would be limited to short-term patient outcomes, such as getting patients back to work sooner, and that there may be less impact in the longer term:
If the question is: a year down the line will they be better off if they’d been treated sooner rather than treated later?, the answer is probably no. The long-term outcome varies very little according to the speed at which they’re treated, but, in terms of getting them back to work, the sooner we treat them, the sooner we will get them back to that happy situation.
Spinal surgeon 2
However, four GPs highlighted the potential for longer-term benefits of ‘fast-tracking’ in terms of intervening earlier to prevent patients from developing a chronic pain problem and becoming dependent on medication:
I think it’s preventing people from going down into that chronicity and all the other issues that go alongside that, with dependency, like diazepam, health-seeking . . . the behaviour around being an invalid.
GP 8
Waiting times for onward treatment following fast-track
The final theme relates to patients’ and clinicians’ views on waiting times for treatment following assessment at the spinal interface clinics and onward referral. Although patient management following fast-track was not altered as part of the trial (i.e. patients referred for onward treatments would join usual NHS waiting lists), understanding views on the fast-track pathway in the context of the broader care pathway for patients with sciatica is an important part of understanding its overall acceptability.
Patients generally reported feeling satisfied with decisions about onward referrals that arose following their assessment at the spinal interface clinics. However, they highlighted the significant difference between the short time frame for the initial fast-track to MRI and spinal specialist opinion at the interface clinics, and the usual NHS waiting times after onward referral for more invasive treatments. For instance, the average waiting time for the four interview participants who received an epidural injection during the trial was 49.6 days (i.e. 7 weeks) from the time of referral, whereas the average waiting time, for the two patients for whom hospital records data were available, for those who were referred straight for an orthopaedic consultation was 57 days (i.e. 8 weeks). Interview participants who joined these usual NHS waiting lists reported uncertainty about how long they would be waiting to receive further treatments, leading to a sense of dissatisfaction. One patient reported initially being told that there would be an approximate waiting time of 5 weeks for an epidural injection, but eventually she waited > 7 weeks from the time of referral, which she explained would have been longer were it not for a last-minute cancellation:
And how quickly did you go from the clinic to the MRI?
Very quickly, that was a matter of weeks, it was very quick. But then waiting for the injection they said would be about a 5-week waiting list . . . So 5 weeks came, and 6 weeks came, which kept going on and on. And I was phoning them and I was still seeing the doctor, my own doctor, and he was like ‘We need to get something sorted with this.’. And I was phoning and phoning them and an appointment then came through as a cancellation. If there hadn’t been a cancellation, I’d still be waiting, I’d wait until I don’t know when. There was quite a wait. So, after initially saying it’s sort of about a 5-week wait, it was longer and it would have been even longer still.
For four patients, the speed of their initial fast-track, coupled with what they perceived as the thorough nature of the assessment by the spinal specialist in the interface clinic, led them to believe that their condition must be severe to warrant such prompt attention. Thus, having been ‘fast-tracked’ for MRI and spinal specialist assessment, they expected they would similarly receive any required further treatment quickly. This suggests that although MRI findings can provide reassurance, as highlighted earlier, for some patients, being ‘fast-tracked’ had the opposite effect by leading them believe that they had a particularly bad problem. As a result, joining usual NHS waiting lists for further treatment led to a degree of frustration and distress:
Because of the speed I went at from the doctor to there [the SCOPiC trial clinic], to the MRI to the specialist; I thought ‘yeah there’s got to be something wrong here’, for them to have spent the 9 hours and so on, on treatment to get me to this point. And then for it to stop, you think, ‘well, where do I go from here? Who can I talk to just to get things moving?’.
Female, aged 45 years, patient ID 13
Having to wait that long for that injection was a long time and, mentally, that had an impact as well, because you’re like, ‘OK, I’m waiting, I’m waiting, I’m waiting’ and it’s like, ‘When’s it coming, when’s it coming, when’s it coming?’. And all the time, like, the physio[therapist] said ‘I can’t do any more’ and I’m like, ‘What’s happening next, what can I do?’.
Male, aged 46 years, patient ID 19
As outlined above, following fast-track, four patients received a physiotherapy referral at the same time as being referred for a caudal epidural injection. The explanation patients gave about this decision was that, owing to the anticipated waiting time for epidural injections, they had been referred for physiotherapy to see if this might improve their symptoms while they waited for the injection:
Going for the physio[therapist] they sort of said, ‘Right, the next step is physio[therapy] and we’ll wait for the injection’. We’ll do that while we wait for that one [i.e. the epidural injection].
So they sort of said have the physio[therapy] while you’re waiting?
While we’re waiting, yeah.
To see if that can help in the meantime?
Yes, that’s right.
However, not all patients expressed dissatisfaction with waiting times for further treatments following their fast-track MRI and assessment. In fact, some patients had been offered more invasive treatments but decided against these following discussions with the clinician. These decisions were often informed by patients weighing up the perceived risks and benefits associated with different treatment options:
I was offered the steroid treatment [i.e. epidural steroid injection] by the physio[therapist]. I didn’t really want to take it on because she said there was risks involved, because I said at the moment I can tolerate it, it’s not that bad. If it got to the stage where it was crippling and I couldn’t get out of bed and stuff and couldn’t dress myself, I’d have to think about having something done. That would forfeit the risk involved then.
Male, aged 44 years, patient ID 5
Clinicians across all three groups also expressed concerns about patients having to go on usual NHS waiting lists to receive further treatments following fast-track. Echoing the views of patients in previous quotations, four clinicians (two surgeons and two GPs) felt that ‘fast-tracking’ patients for MRI and spinal specialist assessment could result in patients anticipating that their sciatic pain will be resolved quickly, leading to unmet expectations if they then have to wait several months for further treatments:
There’s no point in investigating these patients quickly if you then have to put them on a long waiting list before they’re allowed to have them [i.e. spinal injections]. It’s unfair to get patients’ expectations up.
Spinal surgeon 3
If you’re referring people quickly, potentially, people might get a false sense of hope that they are going to be cured immediately. That’s the only thing in the back of my mind, thinking, well, they are not necessarily going to be able to fix you straightaway.
GP 1
Not being able to influence the timing of further treatment was seen as a potential limitation of the fast-track pathway:
We can fast-track it to a point, but then we can’t then necessarily influence the speed with which the next step, the next intervention occurs. So if we do deem that person as being appropriate for having an injection, but then there’s a 10-week waiting time for injections, that’s out of our hands. So whilst we can get them to this point [i.e. MRI and spinal specialist opinion] quicker, we can’t then necessarily get them onto the next stage any quicker.
Spinal physiotherapist 4
However, seven clinicians (two GPs, three spinal physiotherapists and two spinal surgeons) acknowledged that, despite not being able to influence waiting times for further treatment, ‘fast-tracking’ patients through the initial phase of the care pathway and onto NHS waiting lists sooner reduces the overall time period for patients to receive treatments, and, therefore, could improve patient outcomes:
Having an MRI scan at an earlier stage and then being seen by a spinal specialist without having a 3-, 4-, 5-, 6-month wait to get to that point is better for the individual if they are going to require the intervention, even if they have to then wait [to actually receive the onward treatment]. Because, for a lot of patients, there is a lot of waiting for investigations and that is part of the usual care, isn’t it?, that we wait and it may well be that that is all we can do. So hopefully [having been ‘fast-tracked’] their symptoms will be resolved more quickly and they can get back to work or to their usual activities.
GP 3
In contrast with the views of spinal surgeon 3, GP 1 and spinal physiotherapist 4 (in the previous quotations), two spinal physiotherapists did not perceive waiting times for onward referral following fast-track as overly problematic in the case of some patients, as they reported that a patient’s symptoms can begin to settle during this waiting period. They emphasised that, by highlighting this possibility to patients, clinicians can provide reassurance:
We could give them that reassurance that, even if they are referred on for a surgical opinion, that things may well improve and settle down in that time whilst they’re then waiting to see the surgeons. And that does happen quite a lot, you know, we refer people on with quite severe symptoms even past that sort of initial 10- to 12-week period of onset and, by the time they’ve actually seen the surgeons, their symptoms have settled down.
Spinal physiotherapist 1
Discussion
The nested qualitative research showed that patients and all three clinician groups experienced benefit from the fast-track pathway, particularly in providing reassurance based on MRI scans. This reassurance related primarily to patients feeling assured that there was no serious underlying pathology related to their pain, such as cancer. They also reported feeling reassured that they had been given an explanation for the cause of their pain, which they could see on the scan, and, as a result, they felt more hopeful that their condition was treatable, a view supported by the availability of viable treatment options discussed with clinicians. This aligns with Pincus et al. ’s 92 classification of ‘cognitive reassurance’, relating to reassurance provided through the giving of information about aetiology and diagnosis, and discussion of suitable treatment options. The finding contrasts with recent arguments by Wheeler et al. 93 that imaging patients with LBP can lead to patient distress if degenerative changes or aberrant findings are identified. However, the difference in our findings may be explained by the clear biomedical cause that is more often present with sciatica than with non-specific LBP, which can lead to patients feeling a greater degree of certainty about their condition and, thus, stronger cognitive reassurance. These findings align with those of Ryan and Roberts,94 who found that patients with sciatica experienced ‘relief’ when investigations led to the identification of a cause of their pain. However, although the MRI findings led to reassurance, for four patients in our study, the speed with which they underwent MRI and specialist assessment led them to believe that their condition must be particularly severe and, therefore, had the opposite effect of leading to concerns.
There were differences in views identified regarding when to offer the fast-track pathway in terms of sciatica symptom duration. For patients, early MRI investigation and assessment by spinal specialists was viewed very positively compared with stepped care, which shows similarity with previous research findings on the importance patients with sciatica place on undergoing early MRI. 60,94 Among some clinicians, however, there was a concern that those with short symptom duration (i.e. < 6 weeks) might be inappropriately ‘fast-tracked’, as their symptoms may well resolve without the need for imaging and specialist assessment. Spinal surgeons and some GPs felt that it was acceptable for patients with short symptom duration to be ‘fast-tracked’, but only to provide early reassurance rather than to direct treatment. In general, there was a reluctance among participating clinicians to consider more invasive treatments for patients with symptom durations of < 6 weeks, or even 2–3 months in the view of spinal surgeons. This suggests that the overall expectation of a favourable natural course for most patients with sciatica, and the embeddedness of the current ‘stepped’-care approach in routine clinical care, makes clinicians reluctant to consider most patients with short symptom duration for more invasive treatment options early on. This shows similarity with findings in other health-care contexts: for instance, in the Dutch health-care context, Hofstede et al. 78 also observed a reluctance on the part of clinicians to refer patients with short symptom duration for investigations and specialist opinion before a period of conservative management has first been tried, and failed.
Both patients and clinicians also highlighted limitations in the fast-track pathway in not being able to influence waiting times for further treatment.
Exploring the identified themes in relation to the normalisation process theory and the ‘boundary object’ concept
When explored through the lens of NPT, the findings presented on the three key themes that were identified in both the patient and clinician data suggest that the fast-track pathway was able to establish a degree of ‘coherence’ (i.e. made sense) among patients and the three clinician groups. The short time frame for fast-track to MRI and appointment at the spinal interface clinics was seen as representing the most significant change from usual care, and this was seen as having the potential to make a meaningful contribution to the existing care pathway, both through providing early patient reassurance and having potential benefits for clinical outcomes. In this sense, then, patients and clinicians appeared to ‘buy in’ to the aims of the fast-track pathway. Considered in relation to Allen’s88 conceptualisation of care pathways as ‘boundary objects’, the fast-track pathway can be seen to have, at least in part, successfully enabled cohesion of the interests of the different stakeholder groups, through bringing together their shared goal of ensuring that patients in need of specialist assessment and consideration for more invasive treatments are seen by those specialists sooner.
Where the fast-track pathway appeared less successful in achieving coherence was in relation to its timeliness for patients with short symptom duration. For some clinicians, the fast-track pathway made less ‘sense’ as a way of managing these more acute patients, particularly when considered in the context of their existing ways of working, that is to initially adopt a conservative, ‘wait-and-see’ approach until sufficient time has passed to allow for a patient’s symptoms to settle naturally, in line with the clinical expectation of natural improvement in most cases. There were no such reservations from patients regarding the speed of fast-track referral; all of the patients were pleased with the short time frame for undergoing MRI and receiving specialist opinion at the interface clinics. This may represent a mismatch between the respective goals/agendas of the patients and clinicians that is highlighted in the ‘boundary object’ concept. Although patients may perceive waiting times for investigations from a mind-set of ‘the sooner, the better’, some clinicians showed concern about the suitability of patients for early investigations, and a reticence towards sending patients for MRI unnecessarily, which was not a concern that emerged in the patient data. Clinicians also raised the point of repeating MRI if patients were investigated too early in the course of symptoms and having to wait too long to see a spinal surgeon. This, however, was not confirmed by the trial data, as only a handful of patients in the fast-track group underwent MRI a second time.
Although the spinal specialist physiotherapists judged most patients to have been appropriately ‘fast-tracked’, seeing patients with short symptom duration in their capacity as a spinal specialist did not appear to ‘make sense’ to them in terms of how they perceive their own role in the sciatica care pathway. This is because they felt that some of these patients did not require this level of expertise at this early stage; this could, therefore, indicate a lack of coherence at an ‘individual level’82 in terms of the spinal physiotherapists’ perceptions of their own professional identities in their role as spinal specialists (i.e. as distinct from the more general role of physiotherapist). The fact that all clinicians indicated a reluctance to consider invasive treatment options for patients with short symptom duration suggests that intervening too early with these patients would represent a misalignment with their current practice, thus, again indicating a lack of coherence with usual ways of working.
A lack of coherence of the fast-track pathway was also evident in views towards broader organisational-/system-level factors,82 that is in relation to waiting times for further, more invasive, treatments following fast-track. Some clinicians did show a recognition that ‘fast-tracking’ patients through the initial phase of the care pathway should result in them waiting for less time, overall, for treatments than those receiving usual care. However, clinicians and patients highlighted the incongruence between the fast-track short time frame for patients undergoing MRI and receiving a specialist appointment (on average 11.5 days), when compared with the usual NHS waiting times for receiving further treatments such as epidural injections (on average, 7 weeks for the four interview participants who received an epidural injection during the trial). Particularly for some patients, the fast-track pathway was seen as less meaningful given that fast-track did not extend to the receipt of further treatments.
Contribution of the qualitative findings in providing insights on the fast-track pathway
As outlined in Chapter 3, for patients in the SC arm, there was a small but not statistically significant difference in time to resolution of symptoms (a median of 2 weeks faster than the UC arm). The qualitative research focused only on views and experiences of the fast-track pathway (group 3); the findings presented can therefore provide useful insights for understanding this element of the SC model that was tested. Although the majority of patients in the qualitative study (18 out of 20) underwent MRI as part of the fast-track pathway, and all 20 patients went for spinal specialist assessment, 16 patients (i.e. more than three-quarters of those interviewed) initially received conservative treatment, being referred for physiotherapy as a first option following their spinal specialist assessment, whereas only four were referred for a consultation with a spinal surgeon. This could be, in part, down to the lack of ‘coherence’ that clinicians indicated in considering invasive treatment options for patients with short symptom duration, and is indicative of the overall preference of clinicians to allow nature some time to take its course before more invasive treatments are used. Furthermore, it is acknowledged that not all patients in this sample would have findings on the MRI scan that could be potentially targeted by available invasive treatments. However, even when invasive treatments were recommended by spinal specialists, some patients reported instead choosing to opt for conservative treatment, for example physiotherapy. Therefore, although the qualitative research findings showed that both patients and clinicians did report benefits of the fast-track pathway, particularly in providing early ‘cognitive reassurance’92 to patients based on MRI findings, there was collective clinical reluctance, and reluctance from some patients, to consider invasive treatments early on. In this respect, therefore, this facet of the SC model tested in the trial was not consistently helpful to clinicians in their discussions with their patients about management options.
In addition, the lack of coherence identified at an organisational/system level, highlighted above, could represent a limitation of the fast-track pathway. Although patients in the fast-track pathway joined the NHS lists and received the intervention much quicker than patients in the UC arm, it is acknowledged that patients were ‘fast-tracked’ through only the initial part of the care pathway before joining the usual NHS waiting lists for further treatment; this could suggest that the scope of the fast-track pathway was not broad enough to fully enable the cohesion of patients’ and clinicians’ shared goals/agendas,88 that is even quicker access for suitable patients to further invasive treatments. Therefore, in cases for which referral for invasive treatment was agreed by the clinician and patient, there was often some delay in receiving these treatments, albeit less delay than for those in the UC arm. In some cases, this led to patients receiving conservative management while they waited for invasive treatment, such as in the case of the four patients interviewed who were referred for a spinal injection at the same time as their physiotherapy referral, to see if physiotherapy might help to improve their symptoms while they waited for the injection.
Strengths and limitations
A strength of this qualitative research is the parallel investigation of both patients’ and clinicians’ views, allowing access to a range of different perspectives about the fast-track pathway that was tested as part of the SC model in the SCOPiC trial. The sample of 40 interview participants (20 patients and 20 clinicians) was suitably large to allow for the identification of trends across the data set, and the use of the two theoretical frameworks (NPT and the ‘boundary objects’ concept) enabled us to develop a more robust understanding of the identified issues. The multidisciplinary team involved in data analysis was a further strength, allowing us to establish a good level of intercoded reliability. PPI input into the interpretation of the patient data further increases the trustworthiness of the findings presented.
A potential limitation is that interviews with spinal surgeons and GPs commonly involved hypothetical discussions about patients in the fast-track pathway, rather than reflecting on concrete experiences of specific patients in the SCOPiC trial. Whereas spinal physiotherapists were able to draw on examples of patients they had seen in spinal interface clinics through the fast-track pathway, spinal surgeons were not aware of which patients, if any, referred to them had originally been ‘fast-tracked’ as part of the trial. Although GPs initially identified potential participants to be invited to the SCOPiC trial research clinics for potential inclusion in the trial, they were not directly involved in delivering care as part of the trial. However, the adoption of the fast-track pathway requires communication between clinicians across the care pathway; therefore, gaining the views of these different clinician groups as to the acceptability of this approach holds important insights.
When interpreting these findings it is also important to acknowledge the influence of the researchers’ contributions on participants’ responses. Interviews were conducted by experienced qualitative researchers from a social science background; however, the researchers were part of the wider SCOPiC trial TMG, which means that their close involvement with the trial could have had the potential to influence the way in which participants’ views were elicited. However, the role of the researchers’ own subjectivities in the research process does not represent a limitation, but can be seen as an integral part of that process. 95
Summary
-
Most patients and all three clinician groups saw potential added value in the fast-track care pathway, particularly for providing early reassurance to patients who had no serious underlying pathology and who had a clear explanation for their symptoms. However, conversely, for some patients, the speed of undergoing MRI and spinal specialist assessment led to concerns that their condition was particularly severe.
-
Patients reported positive views on the short time frame for fast-track; however, there was variation in views among clinicians about the suitability for fast-track of patients with short symptom duration (i.e. those with pain lasting < 6 weeks), and some clinicians expressed concern that such patients may be inappropriately ‘fast-tracked’.
-
Clinicians reported a reluctance to move from the usual ‘stepped’-care model for patients with short symptom duration, expressing reservations about considering ‘acute’ patients for invasive treatments. Some patients chose to opt for conservative management even when offered invasive treatment options. This reluctance on the part of clinicians and patients, which is based on the hopeful expectation of natural resolution, is evidenced in the fact that most of the patients interviewed (16/20) were initially referred for physiotherapy as a first treatment option following fast-track.
-
Patients and clinicians expressed concerns that the ‘fast-track’ pathway did not extend to shortening the time that patients waited for further treatments after their MRI and initial specialist assessment at the spinal interface clinics.
Chapter 6 Discussion and conclusion
Overview of the SCOPiC trial aim, justification and key findings
Research justification and aims
Our aim was to compare a SC model for the management of patients consulting their GP with symptoms of suspected sciatica with non-stratified UC, and to investigate the SC model’s clinical effectiveness and cost-effectiveness in a large, pragmatic RCT. SC, based on prognosis, for patients presenting to primary care with non-specific LBP has previously been shown to be clinically effective and cost-effective compared with best current practice32 and usual, non-stratified, primary care. 33 However, to the best of our knowledge, a model of SC had not yet been specifically developed or tested for patients in primary care with sciatica. We developed a SC model for sciatica, which we tested in the SCOPiC RCT, taking into account that patients with sciatica have more severe symptoms overall than those with non-specific LBP,20 and that there are other treatment options for patients with sciatica, such as spinal injections and spinal surgery, in addition to conservative treatment.
Sciatica is associated with the highest societal expenses of all LBP presentations. 19 At a population level, research evidence about the natural history of sciatica suggests that symptoms improve within 2–3 months of onset,96–100 and that up to about one-third of people with severe sciatica will still have significant pain 1 year after the onset of symptoms. 100 When comparing outcomes between spinal surgery and conservative management options for the treatment of disc-related sciatica, it is clear that patients treated surgically recover more quickly. 4,101 However, studies have shown that longer-term outcomes are the same, on average, whether patients are treated surgically or conservatively, with about one-quarter of patients having a poor outcome over time, irrespective of treatment received. 4,27 A key limitation of available RCTs comparing spinal surgery with conservative management is patient crossover during the trial timeline, which does not help in confidently deciding which treatment option is most effective. Current clinical management of sciatica tends to follow a stepped-care model for most patients, in which those who do not improve with earlier conservative interventions, such as medication and physiotherapy treatment, are eventually ‘stepped up’ or referred for imaging tests and spinal specialist opinion for consideration of other, more invasive, treatments. 22,25 It has not yet been possible to identify, early, those patients with sciatica who are likely to do well either by natural resolution of symptoms or with conservative management, and those who might benefit from early referral to spinal specialists for tests and consideration of more invasive management options. 35 Patients with severe pain and high disability levels may be offered surgery sooner, but not all patients with severe symptoms will require surgery. 12,13,35,102
In the absence of (1) stratification tools to help with clinical decision-making regarding patients with sciatica at the point of primary care consultation and (2) predictive models to identify which patient might subsequently need to consider invasive management options, we developed a stratified care algorithm, prior to the SCOPiC trial, specifically for primary care patients with sciatica. We used data from a primary care sciatica cohort in UK primary care to identify factors associated with referral to spinal specialist services. Combining information on prognosis (risk of persistent back pain-related disability), using the STarT Back Screening Tool34 and clinical characteristics associated with referral to a spinal specialist (leg pain severity, impact of sciatica on normal life, whether or not pain is below the knee and dermatomal sensory deficits in the lower limb) using the ATLAS cohort,10 the algorithm allocated patients to one of three groups, each with a matched care pathway (see Chapter 2, Stratification algorithm). For group 1, this included up to two sessions (delivered by a physiotherapist) of advice and support to self-manage; group 2 were allocated a course of physiotherapy treatment of up to six sessions; and group 3 were allocated a fast-track referral to MRI and an appointment with a spinal specialist for further assessment and opinion about clinical management, within 4 weeks of randomisation to the trial.
We compared this SC model with non-stratified UC with 476 randomised participants (n = 238 in the SC arm, n = 238 in the UC arm) and investigated whether or not SC led to faster resolution of symptoms and better outcomes, overall, over a 12-month period. We also investigated the cost-effectiveness of the SC model compared with UC and explored the acceptability of the fast-track care pathway to participants in group 3 of the SC arm.
Summary of the key findings
We did not find convincing evidence, overall, that the SC model tested in this RCT led to better outcomes for patients than UC. On average, participants in both arms of the trial improved substantially from baseline, and to a similar degree, over time. In relation to care pathways, at the SCOPiC trial research clinics and at the point of randomisation, all trial participants received a one-off physiotherapy session, including assessment and advice tailored to each individual patient. Following this, the management of participants (n = 237) in the SC arm was directed by the use of the stratification algorithm (see Chapter 2): 153 participants were referred for physiotherapy treatment (49 participants were scheduled to receive up to two physiotherapy sessions to help with self-management and 104 were scheduled to receive up to six physiotherapy sessions), four patients were discharged back to their GP (as it was deemed that they did not require any further treatment) and 80 were fast-tracked to MRI and an appointment with a spinal specialist physiotherapist at the spinal interface clinics, for further assessment and management. In the UC arm (n = 238), 200 patients were referred for further physiotherapy treatment, 28 were discharged back to their GP and 10 were referred to the spinal interface clinics. Data on the care received by participants in both trial arms showed that, during the 12-month follow-up period, 22 participants in the SC arm went on to receive spinal epidural injections and five underwent spinal surgery (two from group 3), whereas 13 participants in the UC arm received spinal injections and eight underwent surgery (five from group 3). On average, participants in the SC arm received their treatments sooner than participants in the UC arm, including both primary and secondary care interventions.
In the primary trial data analysis of time to first self-reported resolution of symptoms, participants in both the SC and UC arms reported improvements in a similar time frame. In the SC arm, the median time to first resolution of symptoms was slightly shorter (2 weeks), with a non-statistically significant ‘time to event’ HR of 1.14 (95% CI 0.89 to 1.46). Approximately one-quarter of participants in the SC and UC arms reported ‘resolution of symptoms’ by week 4 and week 5 from randomisation, respectively. By 12 weeks, approximately 50% of participants in both arms had reported first ‘resolution of symptoms’. By the end of the follow-up period at 12 months, 74% of participants in the SC arm and 71% in the UC arm had reported resolution of symptoms. Adopting a more inclusive definition of improvement, to include ‘better’ in addition to ‘completely recovered’ and ‘much better’, just over 90% of participants in both trial arms had reported improvement by the end of the follow-up period.
Analysis of the secondary outcomes also showed no statistically significant differences between participants in the two trial arms; this included the key secondary outcome of sciatica-related physical disability (mean difference in RMDQ score at 12 months –0.53 points, 95% CI –1.84 to 0.78). The number of days lost from work due to sciatica over the 12-month follow-up was also similar in both arms (SC arm, mean 5.48 days and UC arm, means 5.67 days).
There was a trend for larger numbers of participants in the SC arm to report being satisfied with care, and with the results of care, at the 4-month follow-up, yet this was reversed at the 12-month follow-up. However, any between-arm differences were small and statistically non-significant, and likely to be driven by a few individuals. At 12 months, fewer than 20 participants in each arm reported not being satisfied with the care they had received or the results of the care received.
Subgroup analyses, by sciatica group (1, 2 and 3), showed similar improvements in both trial arms, in terms of time to symptom resolution, with HRs for all definitions of improvement showing no statistically significant differences between SC and UC. There were statistically non-significant trends in the data: the median time to ‘symptom resolution’ for groups 1 and 3 in the SC arm was slightly longer than for those in the UC arm, whereas the median time to ‘symptom resolution’ for group 2 was slightly faster than for those in the UC arm. Although prespecified, these subgroup analyses were exploratory as the trial was not powered to detect differences at the level of the sciatica groups (1, 2 and 3).
Similarly, exploratory subgroup analysis by the clinical diagnosis of disc-related sciatica/spinal stenosis showed that the between-arm effect for SC over UC was significantly higher for the subgroup of patients with a clinical diagnosis of stenosis than for those with a clinical diagnosis of disc prolapse. SC participants with a clinical diagnosis of spinal stenosis reported faster resolution of symptoms than UC participants with a clinical diagnosis of spinal stenosis (HR 1.92, 95% CI 1.01 to 3.65). However, this was an exploratory analysis, the reasons for the difference are unclear, and given the small sample size, it may be a chance finding.
The primary health economic analysis found that the adjusted mean difference between SC and UC in health-related quality of life (measured by QALYs) at 12 months was small, at –0.011 (95% CI –0.036 to 0.013), in favour of UC. The mean difference in NHS and PSS costs at 12 months was also small: £46.21 (95% CI –£110.60 to £187.06) in favour of UC. Compared with UC, the SC model tested in the SCOPiC trial was marginally more costly and associated with slightly lower health-related quality-of-life outcomes over 12 months. For both QALYs and costs, CIs were wide and crossed zero, highlighting the uncertainty in the cost-effectiveness estimate. Given these results, SC is unlikely to be a cost-effective option using commonly applied willingness-to-pay threshold values of £20,000–30,000 per QALY gained. Extrapolation beyond 12 months’ follow-up was not required as the health outcomes and costs for SC and UC were very similar at 12 months, and unlikely to change over a longer time period.
The qualitative investigation of the acceptability of the fast-track care pathway in the SC arm showed that both patients and clinicians reported some benefits, particularly in providing reassurance early in the presentation of symptoms, based on MRI results, and also in considering more invasive treatment options, if appropriate and desired, for patients with persistent symptoms. However, for patients with a short duration of symptoms, there was collective clinical reluctance to consider tests or invasive treatments early on, with spinal specialists reporting that they would choose to wait at least 6 weeks, or even as long as 2–3 months before considering surgical options. Some patients, even when offered treatments such as injection or surgery, also chose to continue with conservative treatment options or to ‘wait and see’ whether or not their pain would improve over time. Data from the ‘time-to-event’ analysis showed that approximately 25% of participants in sciatica group 3 had reported improvement by 4–5 weeks (in both trials arms). So, in that respect, although ‘fast-tracking’ some sciatica patients (mainly those with more severe symptoms) to MRI and specialist opinion was considered by most as a positive action, it was not consistently helpful to clinicians in their discussions with their patients about management options when the duration of symptoms was short.
Discussion of trial findings
A number of reasons may be considered in explaining the trial results and the key finding of no difference, overall, in clinical outcomes between the SC and UC arms of the trial. The first is the stratification algorithm we used to subgroup patients and allocate them to one of three matched care pathways. The sensitivity, specificity and PPV of the algorithm for patient allocation to group 3, namely the fast-track pathway, were 51%, 73% and 22%, respectively. 50
In the absence of factors consistently associated with outcomes specifically in sciatica patients, the stratification algorithm combined prognostic data from a previously developed tool for non-specific LBP patients (the STarT Back Screening Tool34) with data associated with referral to specialist spinal services, in order to identify sciatica patients for the fast-track pathway (group 3). Those with a good prognosis using the STarT Back Tool (group 1) and, irrespective of clinical characteristics, had a low likelihood of being referred to spinal specialists and were matched with a brief intervention comprising two sessions with a physiotherapist. All other patients (group 2) were matched with a course of up to six physiotherapy sessions. Participants in both trial arms still had access to GP care. Participants in group 3 (the fast-track) scored consistently higher on all measures than participants in groups 1 and 2; it is clear that group 3 is at the worse end of the symptoms spectrum, based on the specific algorithm used in this trial. In terms of further health-care resource utilisation, only small numbers of participants from groups 1 and 2 had spinal injections and/or spinal surgery. Nevertheless, the algorithm’s ability to correctly identify those patients most likely to need or benefit from referral to a spinal specialist may be limited (PPV was 22%),50 and a number of participants in group 3 either improved by the time they attended the spinal interface clinic or had no evidence of nerve root compression on their MRI scans. Data from other studies of sciatica in primary and secondary care settings show that, in about 20–40% of clinically diagnosed sciatica cases, MRI scans do not show evidence of nerve root compression;4,10,96,97 therefore, invasive management options are deemed inappropriate. Similarly, the algorithm’s sensitivity of 51%50 indicates that it is possible that a number of patients who were likely to have been appropriate for the fast-track pathway were not identified using the algorithm. Future research may result in a different algorithm for directing care for patients with sciatica; however, we believe that the approach taken here, which combined prognostic information and sciatica severity characteristics identified from the clinical assessment, is a reasonable approach to have developed and tested for this group of patients.
Another consideration is the effectiveness of the UC intervention in this trial. All UC participants received treatment in the SCOPiC trial. Participants consulted their GP and then attended the SCOPiC trial research clinic where they were assessed by a physiotherapist, and all had advice tailored to them at this visit. From the research clinic, 84% (n = 238) in the UC arm were referred for further physiotherapy; only 4% were referred to specialist spinal services and only 12% were discharged back to their GP (indicating that the assessing physiotherapist felt that these participants did not require any further treatment). This compares to data on GP-suggested referral decisions (for UC participants) at the point of identifying potential participants for the trial, where 32% said they would recommend that the patient continue with GP care, 62% to be referred to physiotherapy and 6% to be referred to spinal specialists. Thus, in the operationalisation of the trial from SCOPiC trial research clinics, where physiotherapists assessed patients and made decisions about clinical management, more participants received physiotherapy intervention than would have been the case had GPs made decisions about usual care. However, it is important to point out that these decisions by GPs and physiotherapists about onward treatment were made at different time points, with the difference in time ranging from a few days to a couple of weeks from when each patient consulted their GP to when decisions were made about onward care by physiotherapists in the SCOPiC trial clinics. It is possible that symptoms changed between the two assessment points and this may be reflected in the different decisions about management in 36% of UC participants. Overall, as regards primary care management, more participants in the UC arm received treatment in addition to GP care than would have been observed in normal, usual GP-led primary care practice. The consequence is that the UC intervention in the SCOPiC trial may have been more effective than the care usually received in true usual primary care practice. Participants in the UC arm, on average, waited longer before being referred to specialist services than those in the SC arm; this was expected, taking into account the inclusion of the fast-track pathway for participants in group 3 of the SC arm. The time to be seen in physiotherapy services was also somewhat longer for participants in the UC arm than for those in the SC arm, but, overall, the SCOPiC trial UC arm may have provided an enhanced level of care compared with true usual GP-led primary care, and that may help to explain the findings of the trial. However, this finding in itself is important, as it indicates that good clinical care for this population leads to good results overall, and that the SC model we tested does not lead to better outcomes for patients.
From the qualitative research findings, it was evident that, for patients who might be potential candidates for injection and/or surgery but have symptoms for a short time only, both clinicians and patients preferred to try conservative management while giving time for improvement of symptoms. Whether or not this had any bearing on the trial results is unclear and, on balance, unlikely, as the numbers that proceeded to have invasive procedures were small.
Comparison with existing studies
We are not aware of any studies that have investigated a SC model for sciatica management, and, generally, very few sciatica studies have been successfully conducted in primary care. Comparisons are not straightforward between studies owing to differences in characteristics, for example levels of disability and/or pain, duration of symptoms, variation in condition case definitions and availability of similar outcome measures. We could not identify any primary care studies that used ‘time to improvement’ as their outcome. However, we can make some comparisons in terms of improvements in pain intensity and disability levels between the SCOPiC trial participants and the participants of a recent primary care study, as the latter study has a broadly similar population, including in terms of the duration of symptoms. Mathieson et al. 99 investigated the effectiveness of pregabalin versus placebo medication for primary care sciatica patients; similar improvements were seen in both trial arms, with approximately two-thirds of participants using additional health services, including physiotherapy treatment by approximately one-third. The mean leg pain intensity score reduced from 6.3 and 6.1 at baseline to 3.4 (percentage change: 46%) and 3.0 (50.9% change) at 1 year for the active drug and placebo groups, respectively. 99 In the SCOPiC trial, the mean change was slightly greater: in the SC arm, the leg pain intensity score reduced from 6.8 to 2.9 (57.3% change) and, in the UC arm, it reduced from 6.9 to 2.8 (59.4% change). Similar changes were observed in disability scores: 54.9% and 51.3% change in the SCOPiC trial SC and UC arms, respectively, and 44.6% and 52.3% change in the active drug and placebo arms of the Mathieson et al. 99 trial, respectively. Data from the ATLAS study,14 a clinical cohort that included sciatica patients presenting to primary care, all seeing a physiotherapist and mostly treated according to a stepped-care model, showed a 38.8% change for disability score and a 55.3% change for leg pain intensity score at 1 year, which is slightly less change than the SCOPiC trial or the Mathieson et al. 99 trial. However, a larger number of patients in the ATLAS study had longer pain duration; this may explain the difference in disability results between this population and the trial populations described earlier in this section. These comparisons with other similar sciatica studies set in primary care provide some evidence that the UC arm in the SCOPiC trial can be considered a best-care comparison, which, without the use of any stratification tools, seems to have worked well for approximately 75% of participants. The percentage change in disability score in both arms of the SCOPiC trial (SC arm, 54.9%; UC arm, 51.3%) was considerably higher than that achieved in a study of SC for non-specific LBP (SC, 43.9%; best-care control, 34%). 32
Strengths and limitations
The CONSORT guidelines were followed both in the conduct and in the reporting of the trial. To protect against bias, randomisation was carried out by means of a computer-generated code to ensure concealment and balance of allocation, participants (and their GPs) were not told of their allocated trial arm and validated outcome measures were used to reduce measurement error. However, the design of the trial makes it impossible to conceal allocation of trial arm for the physiotherapists delivering the interventions. We protected against contamination by having different physiotherapists deliver treatment for the SC and UC arms for the duration of the trial. The main analyses were conducted according to the ITT principle and the overall statistical and health economics analyses plan was agreed in advance with the TSC.
The SC model tested, with its two components, the stratification algorithm and the matched care pathways for each of the three sciatica groups, which were developed prior to the trial and were agreed with input from all stakeholders in the management of patients with sciatica (i.e. GPs, spinal surgeons, spinal physiotherapists, rheumatologists, patients and researchers). The primary outcome (time to symptom resolution) was chosen in consultation with patients with experience of either current or previous sciatica. For patients, it was important to gain pain relief as swiftly as possible, and clinicians were comfortable with the characteristics of patients who would then be fast-tracked to MRI and specialist assessment. As part of this, clinicians involved in developing the matched interventions for sciatica groups in our SC model agreed that symptom duration should not be a key factor in a model of SC. However, it is known that short symptom duration reduces the predictive ability of stratification tools more generally. 103
A further strength is the high follow-up rate (88.3% for the SC arm and 90.3% for the UC arm) for the primary outcome in the SCOPiC trial, obtained via text messages. A sensitivity analysis assessing the impact of missing data on the primary outcome did not affect the trial results.
One of the limitations is that the stratification algorithm that we developed and internally validated prior to the trial, to match patients to the trial care pathways, was not externally validated in an independent primary care sciatica cohort before being tested in the trial. 50 We were not aware of any similar primary care data set that we could use to do this that was available at the time before starting this trial. We acknowledge that the algorithm’s sensitivity, specificity and PPV were not optimal; however, in the absence of any other methods to guide matching sciatica patients to care pathways early on, including a referral for MRI and specialist assessment, this stratification algorithm was the first step in investigating the effectiveness of SC for this population. It is acknowledged that the design of the trial does not allow differentiation between the effect of the stratification algorithm (the subgrouping) and the matched care pathways (matched treatments). Any effects observed can be attributed only to the combination of using the algorithm and the matched treatment pathways.
A further limitation is that health-care resource use information was based on self-reported data, which can potentially be affected by under-reporting. In addition, the number of missing data for the health economic analysis (up to 50% for cost data) was a weakness. A sensitivity analysis, however, showed comparable results. We also acknowledge that the trial was not powered to detect differences at the level of each sciatica group (1, 2 and 3) or in the suspected specific clinical diagnosis of disc-related sciatica or spinal stenosis between the trial arms. Therefore, caution is needed in the interpretation of the results of the subgroup analyses, and we recommend that these are seen as very exploratory findings.
Implications for current practice
Contrary to the evidence for superior clinical effectiveness and cost-effectiveness of a prognostic model of SC for patients with non-specific LBP,32,33 the new SC model specifically developed for patients with sciatica and tested in the SCOPiC trial (comprising both prognosis and factors associated with referral to spinal specialists) was not superior to non-stratified UC. Overall, SC participants reported symptom improvement a median of 2 weeks earlier than UC participants, and, although this is important for individual patients, on a population level, this difference is small and most likely to be of little consequence in terms of overall improvement over time. The UC arm involved all patients having a session with a physiotherapist for assessment and advice, and most were referred for further physiotherapy input; therefore, the improvements observed in the UC arm could be attributed to the physiotherapy intervention received. However, it is difficult to disentangle the treatment effect from the improvement due to the natural history of sciatica. A few more participants in the SC arm than in the UC arm received epidural injections, but this did not lead to better outcomes compared with UC.
In contrast to patients with non-specific LBP for whom invasive treatments are not appropriate, for sciatica patients, these are potential management options, depending on MRI findings and a patient’s overall health and preferences. However, lessons learned from the crossover levels observed in sciatica trials comparing surgery with conservative management4,104 clearly indicate that a number of patients improve over time without treatment and others decide against the risk of invasive procedures if there is chance of improvement by other means. It may be, therefore, that, for patients with suspected sciatica, a SC model in which all care pathways are considered early on will not be appropriate for most patients, because some of the available treatments carry risks (and are expensive) and both patients and clinicians prefer not to consider these options early in the presentation of symptoms, as was evident from the qualitative research results. Taking into account the significant burden sciatica has on patients and society, trying to identify which patients need earlier or more intensive or invasive treatment makes intuitive sense, but this trial shows that, in reality, this is difficult to achieve. It could be that our SC algorithm has potential but needs to have different cut-off points for allocation to sciatica groups and matched care pathways, or it could be that different approaches to subgrouping and targeting treatment for patients with sciatica need to be developed and tested, other than the approach we used in this trial. Further research should attempt to identify factors consistently and differentially associated with outcome or treatment effect in the sciatica population, to facilitate construction of better predictive models for use in clinical decision-making. In the meantime, efforts to systematise clinical care delivery for patients with sciatica may help reduce unhelpful practice variation.
Suggestions for further research
As the common prognostic factors shown to be relevant in non-specific LBP are not consistent prognostic factors of pain persistence in sciatica,11,13 avenues for further research in prognostic factors in sciatica may include investigation of sensory profiles based on the hypothesis that differences in these profiles are associated with different pathophysiological mechanisms of the involved nerve fibres, and, therefore, differential prognoses or response to treatments may follow. 105–107 There is some evidence from the treatment of other neuropathic conditions that pharmacological treatment stratification according to sensory profiling has a differential treatment effect. 108
Further research that develops and tests new SC models, for example symptom-based and pathophysiological mechanisms stratification using sensory profiling, might have potential to better inform treatment decisions early on.
Acknowledgements
We are grateful to all the patients, clinicians and managers who took part in and supported this research.
We would like to thank the following organisations and colleagues who contributed to the SCOPiC trial, through recruitment, treatment and administrative support.
Staffordshire and Stoke-on-Trent Partnership NHS Trust (currently known as Midlands Partnership NHS Foundation Trust): Kevin Gorski, Frances Davies, Alison Wallbanks and Ellie Delicata for facilitating approvals and room space in the organisation; Emma Hall, manager of the Spinal Interface Service at Haywood Hospital; the trial physiotherapists – Lucy Huckfield, Siobhan Stynes, Paul Delicata, Steph Cooper, Alan Nagington, Liz Hallam, Joe Wright, Jo Banham and Carol Doyle; and the clinic administrators – Lauren Bradbury, Amy Thompson, Susan Woodroffe and Chantel-Lea Grocott.
Mid Cheshire Hospitals NHS Foundation Trust: Paula Salmon, Ruth Heaton and Chris Smith for facilitating approvals and room space in the organisation; the trial physiotherapists – Vicky Hope, Alex Latham, Gemma Sellors, Sophie Barltrop, Sian French, Stuart Cameron and Elizabeth Mason; and the clinic administrators – Becky Carter and Jane Mason.
Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust and Shropshire Community Health NHS Trust (Shropcom): Teresa Jones, Paula Jefferson, Philip Hulse and Lorraine Lewis for facilitating approvals and room space in the organisation; the trial physiotherapists – Gill Lawrence, Lucy Rice, Yvonne Rimmer, Beth Keddie, Martin Latham, Jean Denton, Lisa Stevenson, Wendy Jones, Andy Laing, Michael Delahay, Eirian Jones and Lis Wharton; and the clinic administrators – Lucie Bishop, Lorraine Thomas and Rachel Evans.
Betsi Cadwaladr University Health Board: Jane Fereday-Smith and Nefyn Williams for facilitating approvals in the organisation.
Physiotherapists in all of these locations that assessed and treated participants in the UC arm of the SCOPiC trial.
Participating general practices and staff members at the following practices: Earnswood Medical Centre, Grosvenor Medical Practice, The Delamere Practice, Hungerford Medical Centre, Beeches Medical Practice, Cambrian House Surgery, Claremont Bank Surgery, Clive Surgery, Ellesmere Medical Practice, Severn Fields Medical Practice, Hope Family Medical Centre, Marysville Medical Centre, Plas Ffynnon Medical Centre, Dr RM Pickles’ Practice, Prescott Medical Practice, Radbrook Green Surgery, Riverside Medical Practice, Dr CC Jones’ Practice, The Caxton Surgery, Gardden Road Surgery, Worthen Medical Practice, Baddeley Green Surgery, Birches Head Medical Centre, Brook Medical Centre, Dr PD Miles, Dr Pathak and Partners, Dunrobin Street, Five Towns Surgery, Golden Hill Medical Centre, Haymarket Health Centre, Higherland Surgery, Dr McCarthy and Partners, Middleport Medical Practice, Orchard Surgery, Dr AK Sinha, Lucie Wedgwood Health Centre, Dr Sonnathi’s Surgery, Trinity Medical Centre, Adderley Green Surgery and Cambridge Surgery.
Clinical Research Network physiotherapy research facilitators: Lucy Huckfield, Yvonne Rimmer, Gemma Sellors and Katrina Humphries for their contribution in setting up and liaising with our NHS partners, facilitating the running of the SCOPiC trial community research clinics and auditing CRFs.
Clinical Research Network staff: Jenny Stevens, Natalie Burgess, Stella Wright, Arwell Lloyd, Jane Davies and Kate Maitland for being the link with the SCOPiC trial general practices and all of the CRN West Midlands research nurses for conducting telephone calls and data collection with participants.
The SCOPiC trial team would like to acknowledge our clinical advisors and collaborators in secondary care: Mr Vinay Jasani, Mr Birender Balain and Dr Julie Ashworth. We would like to thank Lucy Huckfield, Carol Doyle, Paula Salmon and Yvonne Rimmer for their contributions to the training aspects delivered as part of the trial protocols and procedures.
We would like to thank and acknowledge the members of the TSC [Professor Suzanne McDonough (chairperson), Professor Phil Hannaford, Professor Jeremy Fairbank, Professor Catrin Tudur-Smith, Mr Stephen Tatton and Mr Robert Taylor] and the DMC [Dr Ricky Mullis (chairperson), Professor Nicola Walsh, Professor Karla Hemming and Professor Terence O’Neill] for their valuable advice and support during the trial.
The trial team would like to thank and acknowledge the support of the sponsor and staff from Keele CTU: Ruth Beardmore, Alison Lloyd, Sarah Lawton and Susie Hennings; the West Midlands CRN: Helen Duffy, Head of NHS Partnerships & Engagement, Keele University; and lay members from the Keele RUG. Furthermore, we thank Dr Tom Sanders for his contribution to the early plans for the qualitative research in the trial.
Contributions of authors
Nadine E Foster (https://orcid.org/0000-0003-4429-9756) (NIHR Research Professor of Musculoskeletal Health in Primary Care, NIHR Senior Investigator and Director of Keele CTU) was responsible for the original proposal, securing funding for the trial and drafting the original proposal. As chief investigator, she had overall responsibility for the trial and was a member of the TMG. She also critically revised all chapters of the report and edited several versions of the report.
Kika Konstantinou (https://orcid.org/0000-0003-4228-9386) (Senior Clinical Lecturer and Consultant Spinal Physiotherapist) contributed to the original funding proposal. She was a member of the TMG and led the trial on a day-to-day basis as principal investigator, provided training for the trial physiotherapists and oversaw quality control for the trial. She drafted the final report and led the critical revision and editing of the final report.
Martyn Lewis (https://orcid.org/0000-0002-3667-132X) (Reader in Biostatistics) contributed to the original funding proposal, was a member of the TMG, wrote the SAP, carried out data cleaning, was responsible for the statistical analysis and reporting, drafted the trial’s clinical outcomes results chapter and contributed to the methods chapter.
Reuben Ogollah (https://orcid.org/0000-0002-5777-4117) (Research Fellow in Biostatistics) wrote the SAP, was a member of the TMG (until September 2017), carried out data cleaning, was responsible for the statistical analysis and reporting, and contributed to the trial’s methods and clinical outcomes results chapters.
Benjamin Saunders (https://orcid.org/0000-0002-0856-1596) (Research Associate: Health Services Research) was a member of the TMG, conducted the qualitative interviews, co-led the qualitative data analysis and drafted the qualitative chapter.
Jesse Kigozi (https://orcid.org/0000-0001-7608-4923) (Research Fellow in Health Economics) was a member of the TMG, conducted the cleaning of the health economic data, worked on the health economic analyses and data interpretation and drafted the health economics chapter.
Sue Jowett (https://orcid.org/0000-0001-8936-3745) (Reader in Health Economics) was a member of the TMG, contributed to the original funding proposal, developed the economic data collection and analysis plan, and oversaw the health economic analysis and the drafting of the health economics chapter.
Bernadette Bartlam (https://orcid.org/0000-0002-8557-6222) (Senior Lecturer, Applied Health Research) was the senior qualitative research team member, was responsible for the data collection and analyses and was a member of the TMG (until February 2018). She conducted a number of the qualitative interviews and contributed to the drafting of the qualitative chapter.
Majid Artus (https://orcid.org/0000-0001-5319-9015) (NIHR Clinical Lecturer in General Practice) contributed to the original funding proposal, provided general practice expertise, was a member of the TMG and contributed to the conduct and analysis of the qualitative interview research and the drafting of the qualitative chapter.
Jonathan C Hill (https://orcid.org/0000-0001-6246-1409) (Senior Lecturer in Physiotherapy) contributed to the original funding proposal and was a member of the TMG.
Gemma Hughes (https://orcid.org/0000-0003-0056-9291) (Trial Manager) was responsible for overseeing the day-day-running of the trial, was a member of the TMG, contributed to drafting the report and formatted the final report.
Christian D Mallen (https://orcid.org/0000-0002-2677-1028) (Director, Institute for Primary Care and Health Sciences and NIHR Research Professor in General Practice) contributed to the original funding proposal and provided general practice expertise.
Elaine M Hay (https://orcid.org/0000-0002-9545-4296) (Professor of Community Rheumatology) contributed to the original funding proposal.
Danielle A van der Windt (https://orcid.org/0000-0002-7248-6703) (Professor of Primary Care Epidemiology) contributed to the original funding proposal.
Michelle Robinson (https://orcid.org/0000-0002-2266-8250) (Study Co-ordinator) was responsible for the day-to-day co-ordination of the trial and was a member of the TMG. She also carried out data cleaning and provided reports.
Kate M Dunn (https://orcid.org/0000-0002-6202-2606) (Professor of Epidemiology) contributed to the original funding proposal, was a member of the TMG and contributed to the drafting of the methods chapter.
All authors contributed to the final report and approved the final version.
Publications
Konstantinou K, Lewis M, Dunn KM, Ogollah R, Artus M, Hill JC, et al. Stratified care versus usual care for management of patients presenting with sciatica in primary care (SCOPiC): a randomised controlled trial. Lancet Rheumatol 2020;2:e401–11.
Saunders B, Konstantinou K, Artus M, Foster NE, Bartlam B. Patients’ and clinicians’ perspectives on a ‘fast-track’ pathway for patients with sciatica in primary care: qualitative findings from the SCOPiC stratified care trial. BMC Musculoskelet Disord 2020;21:469.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Koes B, van Tulder M, Peul W. Diagnosis and treatment of sciatica. BMJ 2007;334:1313-7. https://doi.org/10.1136/bmj.39223.428495.BE.
- Valat JP, Genevay S, Marty M, Rozenberg S, Koes B. Sciatica. Best Pract Res Clin Rheumatol 2010;24:241-52. https://doi.org/10.1016/j.berh.2009.11.005.
- Fairbank JC. Sciatic: an archaic term. BMJ 2007;335. https://doi.org/10.1136/bmj.39275.951343.BE.
- Peul WC, van Houwelingen HC, van den Hout WB, Brand R, Eekhof JA, Tans JT, et al. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med 2007;356:2245-56. https://doi.org/10.1056/NEJMoa064039.
- Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine 2008;33:2464-72. https://doi.org/10.1097/BRS.0b013e318183a4a2.
- Genevay S, Atlas SJ, Katz JN. Variation in eligibility criteria from studies of radiculopathy due to a herniated disc and of neurogenic claudication due to lumbar spinal stenosis: a structured literature review. Spine 2010;35:803-11. https://doi.org/10.1097/BRS.0b013e3181bc9454.
- Lin CW, Verwoerd AJ, Maher CG, Verhagen AP, Pinto RZ, Luijsterburg PA, et al. How is radiating leg pain defined in randomized controlled trials of conservative treatments in primary care? A systematic review. Eur J Pain 2014;18:455-64. https://doi.org/10.1002/j.1532-2149.2013.00384.x.
- Heliövaara M, Impivaara O, Sievers K, Melkas T, Knekt P, Korpi J, et al. Lumbar disc syndrome in Finland. J Epidemiol Community Health 1987;41:251-8. https://doi.org/10.1136/jech.41.3.251.
- Heliövaara M, Mäkelä M, Knekt P, Impivaara O, Aromaa A. Determinants of sciatica and low-back pain. Spine 1991;16:608-14. https://doi.org/10.1097/00007632-199106000-00002.
- Konstantinou K, Dunn KM, Ogollah R, Vogel S, Hay EM. ATLAS study research team . Characteristics of patients with low back and leg pain seeking treatment in primary care: baseline results from the ATLAS cohort study. BMC Musculoskelet Disord 2015;16. https://doi.org/10.1186/s12891-015-0787-8.
- Ashworth J, Konstantinou K, Dunn KM. Prognostic factors in non-surgically treated sciatica: a systematic review. BMC Musculoskelet Disord 2011;12. https://doi.org/10.1186/1471-2474-12-208.
- Peul WC, Brand R, Thomeer RT, Koes BW. Improving prediction of ‘inevitable’ surgery during non-surgical treatment of sciatica. Pain 2008;138:571-6. https://doi.org/10.1016/j.pain.2008.02.011.
- Verwoerd AJ, Luijsterburg PA, Lin CW, Jacobs WC, Koes BW, Verhagen AP. Systematic review of prognostic factors predicting outcome in non-surgically treated patients with sciatica. Eur J Pain 2013;17:1126-37. https://doi.org/10.1002/j.1532-2149.2013.00301.x.
- Konstantinou K, Dunn KM, Ogollah R, Lewis M, van der Windt D, Hay EM. ATLAS Study Team . Prognosis of sciatica and back-related leg pain in primary care: the ATLAS cohort. Spine J 2018;18:1030-40. https://doi.org/10.1016/j.spinee.2017.10.071.
- Vroomen PC, de Krom MC, Slofstra PD, Knottnerus JA. Conservative treatment of sciatica: a systematic review. J Spinal Disord 2000;13:463-9. https://doi.org/10.1097/00002517-200012000-00001.
- Haugen AJ, Brox JI, Grøvle L, Keller A, Natvig B, Soldal D, et al. Prognostic factors for non-success in patients with sciatica and disc herniation. BMC Musculoskelet Disord 2012;13. https://doi.org/10.1186/1471-2474-13-183.
- Iversen T, Solberg TK, Wilsgaard T, Waterloo K, Brox JI, Ingebrigtsen T. Outcome prediction in chronic unilateral lumbar radiculopathy: prospective cohort study. BMC Musculoskelet Disord 2015;16. https://doi.org/10.1186/s12891-015-0474-9.
- Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double blind placebo-controlled trial of evaluating the effect of piroxicam (NSAID). Spine 1993;18:1433-8. https://doi.org/10.1097/00007632-199309010-00006.
- van Tulder MW, Koes BW, Bouter LM. A cost-of-illness study of back pain in the Netherlands. Pain 1995;62:233-40. https://doi.org/10.1016/0304-3959(94)00272-G.
- Konstantinou K, Hider SL, Jordan JL, Lewis M, Dunn KM, Hay EM. The impact of low back-related leg pain on outcomes as compared with low back pain alone: a systematic review of the literature. Clin J Pain 2013;29:644-54. https://doi.org/10.1097/AJP.0b013e31826f9a52.
- Foster NE, Konstantinou K, Lewis M, Ogollah R, Dunn KM, van der Windt D, et al. The clinical and cost-effectiveness of stratified care for patients with sciatica: the SCOPiC randomised controlled trial protocol (ISRCTN75449581). BMC Musculoskelet Disord 2017;18. https://doi.org/10.1186/s12891-017-1513-5.
- Lewis R, Williams N, Matar HE, Din N, Fitzsimmons D, Phillips C, et al. The clinical effectiveness and cost-effectiveness of management strategies for sciatica: systematic review and economic model. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15390.
- Fitzsimmons D, Phillips CJ, Bennett H, Jones M, Williams N, Lewis R, et al. Cost-effectiveness of different strategies to manage patients with sciatica. Pain 2014;155:1318-27. https://doi.org/10.1016/j.pain.2014.04.008.
- Fernandez M, Hartvigsen J, Ferreira ML, Refshauge KM, Machado AF, Lemes ÍR, et al. Advice to stay active or structured exercise in the management of sciatica: a systematic review and meta-analysis. Spine 2015;40:1457-66. https://doi.org/10.1097/BRS.0000000000001036.
- National Institute for Health and Care Excellence (NICE) . Low Back Pain and Sciatica in Over 16s: Assessment and Management 2016.
- NHS England . National Low Back and Radicular Pain Pathway 2017.
- Lequin MB, Verbaan D, Jacobs WC, Brand R, Bouma GJ, Vandertop WP, et al. Surgery versus prolonged conservative treatment for sciatica: 5-year results of a randomised controlled trial. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002534.
- Machado GC, Witzleb AJ, Fritsch C, Maher CG, Ferreira PH, Ferreira ML. Patients with sciatica still experience pain and disability 5 years after surgery: a systematic review with meta-analysis of cohort studies. Eur J Pain 2016;20:1700-9. https://doi.org/10.1002/ejp.893.
- Rihn JA, Hilibrand AS, Radcliff K, Kurd M, Lurie J, Blood E, et al. Duration of symptoms resulting from lumbar disc herniation: effect on treatment outcomes: analysis of the Spine Patient Outcomes Research Trial (SPORT). J Bone Joint Surg Am 2011;93:1906-14. https://doi.org/10.2106/JBJS.J.00878.
- Lewis RA, Williams NH, Sutton AJ, Burton K, Din NU, Matar HE, et al. Comparative clinical effectiveness of management strategies for sciatica: systematic review and network meta-analyses. Spine J 2015;15:1461-77. https://doi.org/10.1016/j.spinee.2013.08.049.
- Department of Health and Social Care (DHSC) . National Spinal Taskforce. Commissioning Spinal Services – Getting the Service Back on Track: A Guide for Commissioners of Spinal Services 2013.
- Hill JC, Whitehurst DG, Lewis M, Bryan S, Dunn KM, Foster NE, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet 2011;378:1560-71. https://doi.org/10.1016/S0140-6736(11)60937-9.
- Foster NE, Mullis R, Hill JC, Lewis M, Whitehurst DG, Doyle C, et al. Effect of stratified care for low back pain in family practice (IMPaCT Back): a prospective population-based sequential comparison. Ann Fam Med 2014;12:102-11. https://doi.org/10.1370/afm.1625.
- Hill JC, Dunn KM, Lewis M, Mullis R, Main CJ, Foster NE, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum 2008;59:632-41. https://doi.org/10.1002/art.23563.
- Arts MP, Peul WC. Leiden-Hague Spine Intervention Prognostic Study Group . Timing and minimal access surgery for sciatica: a summary of two randomized trials. Acta Neurochir 2011;153:967-74. https://doi.org/10.1007/s00701-011-0983-8.
- Hassey A, Gerrett D, Wilson A. A survey of validity and utility of electronic patient records in a general practice. BMJ 2001;322:1401-5. https://doi.org/10.1136/bmj.322.7299.1401.
- Konstantinou K, Hider SL, Vogel S, Beardmore R, Somerville S. Development of an assessment schedule for patients with low back-associated leg pain in primary care: a Delphi consensus study. Eur Spine J 2012;21:1241-9. https://doi.org/10.1007/s00586-011-2057-2.
- Stynes S, Konstantinou K, Dunn KM. Classification of patients with low back-related leg pain: a systematic review. BMC Musculoskelet Disord 2016;17. https://doi.org/10.1186/s12891-016-1074-z.
- Verwoerd AJH, Mens J, El Barzouhi A, Peul WC, Koes BW, Verhagen AP. A diagnostic study in patients with sciatica establishing the importance of localization of worsening of pain during coughing, sneezing and straining to assess nerve root compression on MRI. Eur Spine J 2016;25:1389-92. https://doi.org/10.1007/s00586-016-4393-8.
- Germon T, Singleton W, Hobart J. Is NICE guidance for identifying lumbar nerve root compression misguided?. Eur Spine J 2014;23:20-4. https://doi.org/10.1007/s00586-014-3233-y.
- Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 1983;8:141-4.
- Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB. Assessing health-related quality of life in patients with sciatica. Spine 1995;20:1899-908. https://doi.org/10.1097/00007632-199509000-00011.
- Dunn KM, Jordan KP, Croft PR. Recall of medication use, self-care activities and pain intensity: a comparison of daily diaries and self-report questionnaires among low back pain patients. Prim Health Care Res Dev 2010;11:93-102. https://doi.org/10.1017/S1463423609990296.
- Jenkins DC, Stanton B, Niemcryk SJ, Rose RM. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol 1988;41:313-21. https://doi.org/10.1016/0895-4356(88)90138-2.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Kori SH, Miller RP, Todd DD. Kinesphobia: a new view of chronic pain behaviour. Pain Manag 1990;3:35-43.
- Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain 2005;6:149-58. https://doi.org/10.1016/j.jpain.2004.11.007.
- Ware JNJ. SF-36 health survey update. Spine 2000;25:3130-39. https://doi.org/10.1097/00007632-200012150-00008.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new a five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Konstantinou K, Dunn KM, van der Windt D, Ogollah R, Jasani V, Foster NE. SCOPiC study team . Subgrouping patients with sciatica in primary care for matched care pathways: development of a subgrouping algorithm. BMC Musculoskelet Disord 2019;20. https://doi.org/10.1186/s12891-019-2686-x.
- Eldridge SM, Ashby D, Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol 2006;35:1292-300. https://doi.org/10.1093/ije/dyl129.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1998.
- Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet 2002;359:1309-10. https://doi.org/10.1016/S0140-6736(02)08272-7.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. CONSORT Group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. https://doi.org/10.7326/0003-4819-148-4-200802190-00008.
- Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337. https://doi.org/10.1136/bmj.a2390.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012;10:28-55. https://doi.org/10.1016/j.ijsu.2011.10.001.
- Office for National Statistics . Earnings and Hours Worked, Region By Occupation By Two-Digit SOC: ASHE Table 3 2019. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/regionbyoccupation2digitsocashetable3 (accessed 1 March 2019).
- Royston P, Lambert P. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model. College Station, TX: Stata Press; 2011.
- Pourhoseingholi MA, Hajizadeh E, Moghimi Dehkordi B, Safaee A, Abadi A, Zali MR. Comparing Cox regression and parametric models for survival of patients with gastric carcinoma. Asian Pac J Cancer Prev 2007;8:412-16.
- Ong BN, Konstantinou K, Corbett M, Hay E. Patients' own accounts of sciatica: a qualitative study. Spine 2011;36:1251-6. https://doi.org/10.1097/BRS.0b013e318204f7a2.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50. https://doi.org/10.1016/j.jval.2013.02.002.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Morris S, Devlin N, Parkin D. Economic Analysis in Health Care. Chichester: John Wiley & Sons Ltd; 2007.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Department of Health and Social Care . NHS Reference Costs 2016–17 2017.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2017.
- Office for National Statistics . Annual Survey of Hours and Earnings 2017. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2017provisionaland2016revisedresults (accessed 1 March 2019).
- Kigozi J, Lewis M, Jowett S, Barton P, Coast J. Construct validity and responsiveness of the single-item presenteeism question in patients with lower back pain for the measurement of presenteeism. Spine 2014;39:409-16. https://doi.org/10.1097/BRS.0000000000000162.
- Krol M, Brouwer W. How to estimate productivity costs in economic evaluations. PharmacoEconomics 2014;32:335-44. https://doi.org/10.1007/s40273-014-0132-3.
- Sculpher M, Drummond M, McGuire A. Economic Evaluation in Health Care: Merging Theory with Practice. Oxford: Oxford University Press; 2001.
- Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3-15. https://doi.org/10.1177/096228029900800102.
- Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991;10:585-98. https://doi.org/10.1002/sim.4780100410.
- McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. PharmacoEconomics 2008;26:733-44. https://doi.org/10.2165/00019053-200826090-00004.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Saunders B, Konstantinou K, Artus M, Foster NE, Bartlam B. Patients’ and clinicians’ perspectives on a ‘fast-track’ pathway for patients with sciatica in primary care: qualitative findings from the SCOPiC stratified care trial. BMC Musculoskelet Disord 2020;21. https://doi.org/10.1186/s12891-020-03483-z.
- Boote J, Newsome R, Reddington M, Cole A, Dimairo M. Physiotherapy for patients with sciatica awaiting lumbar micro-discectomy surgery: a nested, qualitative study of patients’ views and experiences. Physiother Res Int 2017;22. https://doi.org/10.1002/pri.1665.
- Hofstede SN, van Bodegom-Vos L, Wentink MM, Vleggeert-Lankamp CL, Vliet Vlieland TP, Marang-van de Mheen PJ. DISC study group . Most important factors for the implementation of shared decision making in sciatica care: ranking among professionals and patients. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0094176.
- Porcheret M, Main C, Croft P, McKinley R, Hassell A, Dziedzic K. Development of a behaviour change intervention: a case study on the practical application of theory. Implement Sci 2014;9. https://doi.org/10.1186/1748-5908-9-42.
- Sanders T, Foster NE, Ong BN. Perceptions of general practitioners towards the use of a new system for treating back pain: a qualitative interview study. BMC Med 2011;9. https://doi.org/10.1186/1741-7015-9-49.
- Finch TL, Rapley T, Girling M, Mair FS, Murray E, Treweek S, et al. Improving the normalization of complex interventions: measure development based on normalization process theory (NoMAD): study protocol. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-43.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-29.
- Myall M, May CR, Grimmett C, May CM, Calman L, Richardson A, et al. RESTORE: an exploratory trial of a web-based intervention to enhance self-management of cancer-related fatigue: findings from a qualitative process evaluation. BMC Med Inform Decis Mak 2015;15. https://doi.org/10.1186/s12911-015-0214-y.
- Band R, Bradbury K, Morton K, May C, Michie S, Mair FS, et al. Intervention planning for a digital intervention for self-management of hypertension: a theory-, evidence- and person-based approach. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0553-4.
- Coupe N, Anderson E, Gask L, Sykes P, Richards DA, Chew-Graham C. Facilitating professional liaison in collaborative care for depression in UK primary care; a qualitative study utilising normalisation process theory. BMC Fam Pract 2014;15. https://doi.org/10.1186/1471-2296-15-78.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- Finch TL, Girling M, May CR, Mair FS, Murray E, Treweek S, et al. Improving the normalization of complex interventions: part 2 – validation of the NoMAD instrument for assessing implementation work based on normalization process theory (NPT). BMC Med Res Methodol 2018;18. https://doi.org/10.1186/s12874-018-0591-x.
- Allen D. From boundary concept to boundary object: the practice and politics of care pathway development. Soc Sci Med 2009;69:354-61. https://doi.org/10.1016/j.socscimed.2009.05.002.
- Sandelowski M, Given LM. The SAGE Encyclopedia of Qualitative Research Methods: Volume 2. Thousand Oaks, CA: SAGE Publications, Inc.; 2008.
- Glaser B, Strauss A. Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine Publishing Company; 1967.
- Saunders B, Bartlam B, Artus M, Konstantinou K. Biographical suspension and liminality of Self in accounts of severe sciatica. Soc Sci Med 2018;218:28-36. https://doi.org/10.1016/j.socscimed.2018.10.001.
- Pincus T, Holt N, Vogel S, Underwood M, Savage R, Walsh DA, et al. Cognitive and affective reassurance and patient outcomes in primary care: a systematic review. Pain 2013;154:2407-16. https://doi.org/10.1016/j.pain.2013.07.019.
- Wheeler L, Karran E, Harvie D. Low back pain: can we mitigate the inadvertent psycho-behavioural harms of spinal imaging?. Aust J Gen Pract 2018;47:610-3. https://doi.org/10.31128/AJGP-03-18-4525.
- Ryan C, Roberts L. Investigations for sciatica: the patient’s perspective. A qualitative, interpretative inquiry. Musculoskelet Sci Pract 2018;33:71-6. https://doi.org/10.1016/j.msksp.2017.11.005.
- Holstein J, Gubrium J, Holstein J, Gubrium J, Silverman D. Qualitative Research: Theory, Method and Practice. London: SAGE Publications Ltd; 1997.
- Vroomen PC, de Krom MC, Wilmink JT, Kester AD, Knottnerus JA. Lack of effectiveness of bed rest for sciatica. N Engl J Med 1999;340:418-23. https://doi.org/10.1056/NEJM199902113400602.
- Hofstee DJ, Gijtenbeek JM, Hoogland PH, van Houwelingen HC, Kloet A, Lötters F, et al. Westeinde sciatica trial: randomized controlled study of bed rest and physiotherapy for acute sciatica. J Neurosurg 2002;96:45-9. https://doi.org/10.3171/spi.2002.96.1.0045.
- Awad JN, Moskovich R. Lumbar disc herniations: surgical versus nonsurgical treatment. Clin Orthop Relat Res 2006;443:183-97. https://doi.org/10.1097/01.blo.0000198724.54891.3a.
- Mathieson S, Maher CG, McLachlan AJ, Latimer J, Koes BW, Hancock MJ, et al. Trial of pregabalin for acute and chronic sciatica. N Engl J Med 2017;376:1111-20. https://doi.org/10.1056/NEJMoa1614292.
- Balagué F, Nordin M, Sheikhzadeh A, Echegoyen AC, Brisby H, Hoogewoud HM, et al. Recovery of severe sciatica. Spine 1999;24:2516-24. https://doi.org/10.1097/00007632-199912010-00014.
- Jacobs WC, van Tulder M, Arts M, Rubinstein SM, van Middelkoop M, Ostelo R, et al. Surgery versus conservative management of sciatica due to a lumbar herniated disc: a systematic review. Eur Spine J 2011;20:513-22. https://doi.org/10.1007/s00586-010-1603-7.
- Peul WC, Arts MP, Brand R, Koes BW. Timing of surgery for sciatica: subgroup analysis alongside a randomized trial. Eur Spine J 2009;18:538-45. https://doi.org/10.1007/s00586-008-0867-7.
- Khan Y. The STarT back tool in chiropractic practice: a narrative review. Chiropr Man Therap 2017;25. https://doi.org/10.1186/s12998-017-0142-2.
- Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson AN, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. JAMA 2006;296:2451-9. https://doi.org/10.1001/jama.296.20.2451.
- Mahn F, Hüllemann P, Gockel U, Brosz M, Freynhagen R, Tölle TR, et al. Sensory symptom profiles and co-morbidities in painful radiculopathy. PLOS ONE 2011;6. https://doi.org/10.1371/journal.pone.0018018.
- Baron R, Förster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol 2012;11:999-1005. https://doi.org/10.1016/S1474-4422(12)70189-8.
- Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain 2017;158:261-72. https://doi.org/10.1097/j.pain.0000000000000753.
- Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 2014;155:2263-73. https://doi.org/10.1016/j.pain.2014.08.014.
Appendix 1 List of Read codes
| Read code | Read term |
|---|---|
| N143 | Sciatica |
| N143-1 | Acute back pain with sciatica |
| N1420 | Lumbago with sciatica |
| N12C4 | Prolapsed lumbar intervertebral disc with sciatica |
| 16C3 | Backache with radiation |
| N12C2 | Lumbar disc prolapse with radiculopathy |
| N11C | Lumbosacral spondylosis with radiculopathy |
Including child codes of:
|
|
| N1402 | Lumbar spinal stenosis |
| N1407 | Idiopathic lumbar spinal stenosis |
| N1408 | Degenerative lumbar spinal stenosis |
| N140A | Lumbar spinal stenosis secondary to other disease |
| N140z | Spinal stenosis NOS |
| 16CA | Mechanical back pain |
| N142-1 | LBP |
Appendix 2 Protocol deviations
| Deviation | Sciatica group (n) | Total (n) | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Fewer than three treatment (physiotherapy) sessions | 23 | 23 | ||
| Three or more treatment (physiotherapy) sessions | 3 | 3 | ||
| Incorrect sciatica group allocation (correct = group 1) | 4 | 4 | ||
| Incorrect sciatica group allocation (correct = group 2) | 1 | 1 | ||
| Participant did not attend the spinal interface clinic (group 3) | 7 | 7 | ||
| Participant attended spinal interface clinic > 4 weeks after randomisation | 1 | 1 | ||
| Participant referred to spinal interface clinic | 1 | 14 | 15 | |
| Participant referred for MRI | 1 | 1 | ||
| Total | 5 | 42 | 8 | 55a |
Appendix 3 General practitioners’ preferred management option
| Physiotherapist management option | GP management optiona (n participants) | Total (n participants) | ||
|---|---|---|---|---|
| Keep under GP care | Refer to physiotherapy | Refer to spinal specialist services | ||
| Keep under GP care | 14 | 9 | 1 | 24 |
| Refer to physiotherapy | 51 | 121 | 9 | 181 |
| Refer to spinal specialist services | 3 | 3 | 2 | 8 |
| Total | 68 | 133 | 12 | 213 |
Appendix 4 Physiotherapy session details
| Physiotherapy sessions detail | All (N = 449) | SC arm (N = 214) | UC arm (N = 235) |
|---|---|---|---|
| Count of treatment sessions, median (IQR) | 2 (0–4) | 2 (1–4) | 2 (0–3) |
|
1 (0–2) | 1 (1–1) | 1 (0–3) |
|
3 (1–4) | 3 (2–5) | 2 (0–3) |
|
2 (0–4) | 2 (0–4) | 2 (0–4) |
| Participants who had three or more treatment sessions, n (%) | 194 (43) | 95 (44) | 99 (42) |
|
20 (19) | 3 (6) | 17 (31) |
|
114 (55) | 64 (62) | 50 (48) |
|
60 (44) | 28 (47) | 32 (42) |
| Days to first appointment,a median (IQR) | 14 (7–25) | 9 (6–14) | 21.5 (11–46) |
|
14 (7–28) | 10 (6–14) | 32 (21–69) |
|
10 (6–20) | 7 (3–10) | 21 (11–49) |
|
20 (12–28) | 21 (15–28) | 16 (10–32) |
| Days to last session,a,b median (IQR) | 49 (14–87) | 38 (12.5–70) | 66 (29–97) |
|
15.5 (7–56) | 11.5 (7–18) | 67.5 (28–97) |
|
53.5 (24–84) | 44 (21–73) | 65 (35–97) |
|
66 (35–97) | 66 (42–85) | 67.5 (22–99) |
FIGURE 17.
Summary of modalities provided in physiotherapy intervention treatment sessions.
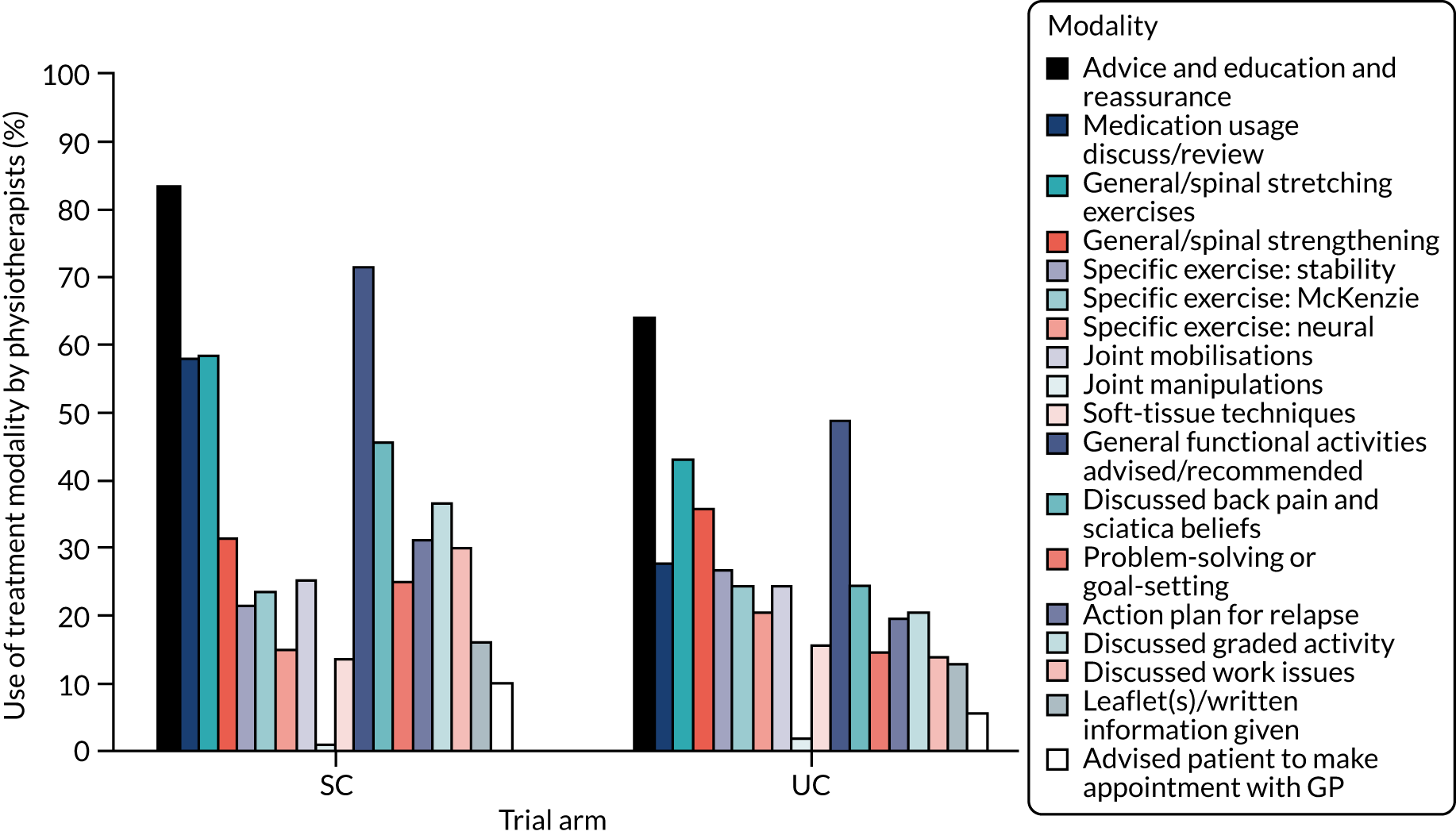
FIGURE 18.
Summary of modalities provided in physiotherapy intervention treatment sessions (by sciatica group).
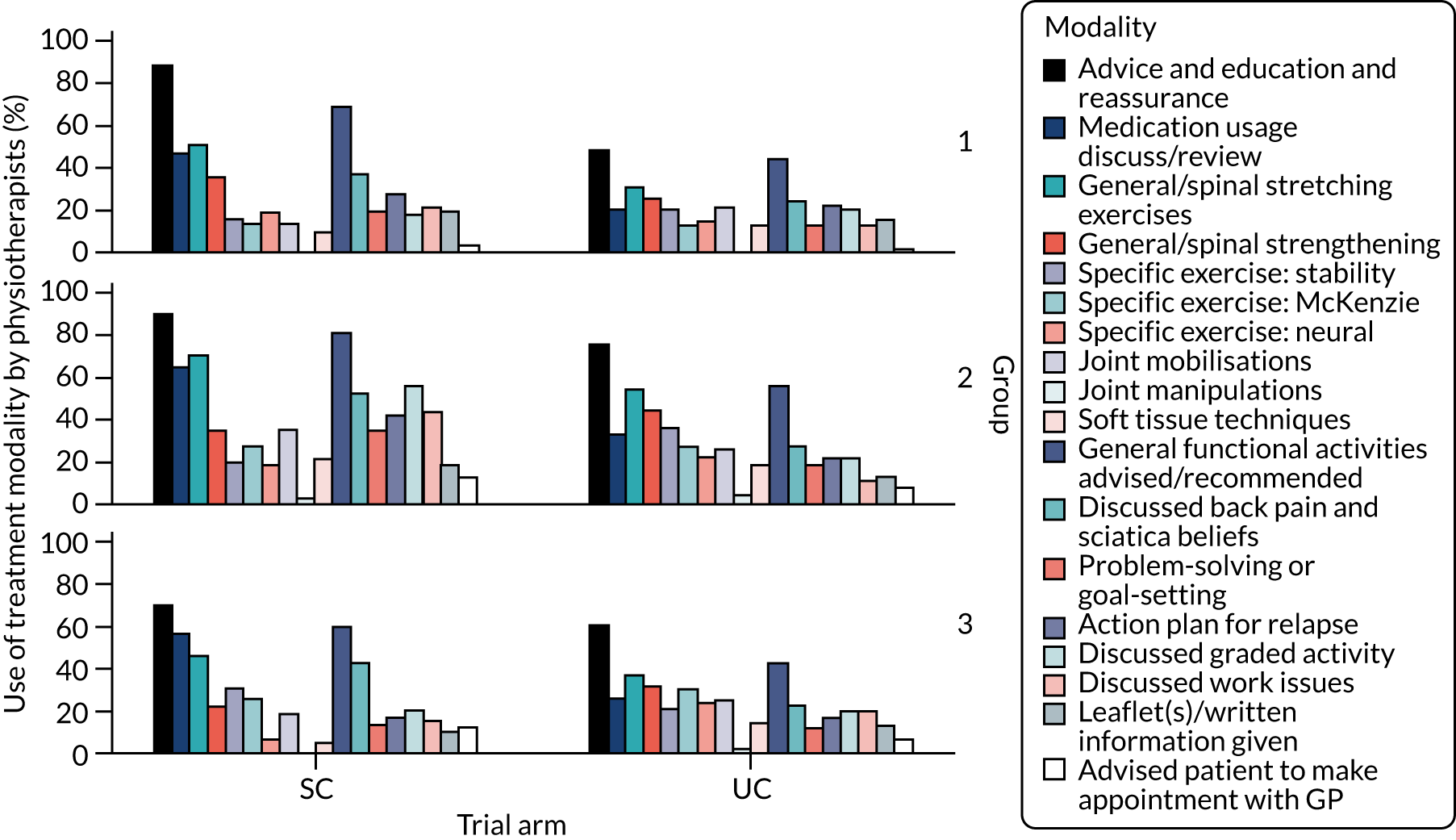
Appendix 5 Hospital records review data
| Hospital records review data | All (N = 427)a | SC (N = 215) | UC (N = 212) |
|---|---|---|---|
| Referred to orthopaedics services | |||
| Participants, n (%) | 55 (13) | 32 (15) | 23 (11) |
| Days to be seen, median (IQR) | 120 (75–179) | 123 (87–179) | 119 (66–151) |
| Group 1 | |||
| Participants, n (%) | 8 (8) | 4 (8) | 4 (9) |
| Days to be seen, median (IQR) | 135 (115–150) | 135 (120–160) | 133 (115–150) |
| Group 2 | |||
| Participants, n (%) | 19 (10) | 9 (10) | 10 (11) |
| Days to be seen, median (IQR) | 155 (66–308) | 209 (123–304) | 101 (66–228) |
| Group 3 | |||
| Participants, n (%) | 28 (20) | 19 (27) | 9 (13) |
| Days to be seen, median (IQR) | 95 (58–120) | 87 (58–105) | 120 (42–151) |
| Number of spinal injections | |||
| Participants, n (%) | 22 (5) | 15 (7) | 7 (3) |
| Days to first injection, median (IQR) | 90 (43–151) | 60 (41–93) | 161 (113–253) |
| Group 1 | |||
| Participants, n (%) | 1 (1) | 1 (2) | 0 (0) |
| Days to first injection, median (IQR) | 226 (–) | 226 (–) | – |
| Group 2 | |||
| Participants, n (%) | 4 (2) | 1 (1) | 3 (3) |
| Days to first injection, median (IQR) | 251 (151–253) | 251 (–) | 202 (151–253) |
| Group 3 | |||
| Participants, n (%) | 17 (12) | 13 (18) | 4 (6) |
| Days to first injection, median (IQR) | 62 (41–99) | 48 (41–89) | 142 (106–251) |
| Spinal surgery | |||
| Participants, n (%) | 11 (3) | 5 (2) | 6 (3) |
| Days to surgery, median (IQR) | 216 (99–321) | 236 (99–372) | 216 (102–305) |
| Group 1 | |||
| Participants, n (%) | 1 (1) | 1 (2) | 0 (0) |
| Days to surgery, median (IQR) | b | b | – |
| Group 2 | |||
| Participants, n (%) | 5 (3) | 3 (3) | 2 (2) |
| Days to surgery, median (IQR) | 144 (99–372) | 236 (99–372) | 144 (–) |
| Group 3 | |||
| Participants, n (%) | 5 (4) | 1 (1) | 4 (6) |
| Days to surgery, median (IQR) | 288 (59–321) | b | 288 (59–321) |
Appendix 6 Prescription data
| Medication type | SC arm (N = 114) | UC arm (N = 122) | ||
|---|---|---|---|---|
| n (%)a | Mean (SD)b | n (%)a | Mean (SD)b | |
| Paracetamol | 14 (6) | 0.32 (1.454) | 19 (8) | 0.27 (0.89) |
| Ibuprofen | 19 (8) | 0.48 (1.86) | 17 (7) | 0.16 (0.48) |
| Co-codamol | 62 (26) | 1.58 (3.41) | 47 (20) | 1.04 (2.41) |
| Co-dydramol | 4 (2) | 0.01 (0.09) | 7 (3) | 0.15 (0.92) |
| Diclofenac sodium | 4 (2) | 0.05 (0.48) | 5 (2) | 0.11 (0.64) |
| Tramadol | 13 (5) | 0.11 (0.39) | 18 (8) | 0.40 (1.59) |
| Naproxen | 30 (13) | 0.48 (1.54) | 32 (13) | 0.66 (1.49) |
| Amitriptyline | 38 (16) | 0.98 (3.15) | 26 (11) | 0.67 (2.02) |
| Gabapentin | 24 (10) | 0.68 (2.39) | 18 (8) | 0.32 (1.15) |
List of abbreviations
- AE
- adverse event
- ATLAS
- Assessment and Treatment of Leg pain Associated with the Spine
- BNF
- British National Formulary
- CC
- complications or comorbidities
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CRN
- Clinical Research Network
- CTU
- Clinical Trials Unit
- DMC
- Data Monitoring Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- GPC
- Global Perceived Change
- HCP
- health-care professional
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- ICC
- intraclass correlation
- ICER
- incremental cost-effectiveness ratio
- ID
- identifier
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- ITT
- intention to treat
- LBP
- lower-back pain
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NPT
- normalisation process theory
- NRS
- Numerical Rating Scale
- OR
- odds ratio
- PPI
- patient and public involvement
- PPV
- positive predictive value
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised control trial
- RMDQ
- Roland–Morris Disability Questionnaire
- RUG
- research user group
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SC
- stratified care
- SCOPiC
- Sciatica Outcomes in Primary Care
- SD
- standard deviation
- STarT BacK Screening Tool
- Subgroups for Targeted Treatment (STarT) Back questionnaire
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UC
- usual care
