Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/167/02. The contractual start date was in December 2014. The draft report began editorial review in September 2019 and was accepted for publication in May 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Gilbert et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter have been reproduced from Gilbert et al. 1 © 2019 The Author(s). Published by Elsevier Ltd. This is an open access article distributed under the terms of the CC-BY 4.0 license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium.
Background
In the UK, approximately 8% of all babies are born preterm, defined as delivery before 37 weeks of gestational age. 2 Preterm birth is associated with high rates of neonatal and childhood mortality and with high rates of long-term chronic conditions, manifesting as adverse neurodevelopment and respiratory disease. 3–6 Risks of these outcomes increase steeply with earlier gestational age at birth. Risks are even higher in babies who develop a bloodstream infection (BSI). 7–10 A BSI can be an early-onset infection (i.e. before 2 days of age), which is thought to be predominantly due to organisms transmitted from mother to baby. 11,12 Thereafter, a BSI is commonly caused by organisms that enter the bloodstream through skin or mucosal barriers in babies with immature immune defences, or through contamination of internal devices, such as central venous catheters (CVCs). 13–15 In the UK, 14% of all babies born are admitted to a neonatal unit (NNU), but this proportion is much higher for babies born preterm: all of the 1.4% of babies born before 32 weeks of gestation spend time in a NNU. 16 On average, 5% of all babies admitted to a NNU experience a BSI, but this proportion rises to 22% for babies born before 33 weeks of gestation and to 36% for babies born before 29 weeks of gestation. 17,18 Therefore, prevention of BSI, and its acute and long-term adverse outcomes, is a very high priority for neonatal care.
Central venous catheters are a common source of BSI in babies receiving neonatal care. According to two national surveillance studies,19,20 43–48% of BSIs occurring after the first 2 days of age are considered to be related to CVCs. An estimated 21% of babies admitted born at ≥ 34 weeks of gestation and admitted to a NNU receive a CVC, but this proportion rises to 70% for babies born before 32 weeks of gestational age [based on unpublished analysis of the National Neonatal Research Database (NNRD) (Katie Harron, University College London, 2014, personal communication)]. 21 Peripherally inserted central venous catheters (PICCs) are frequently used in NNUs. The PICC is a very narrow tube placed through the skin and into a central vein, using a needle, which is then removed. The PICC is used to administer medicines, fluids or parenteral nutrition into a large vein near the heart. A PICC can stay in place for several weeks, avoiding the need for repeated procedures, which can be harmful and distressing for small babies.
The mechanism by which CVCs lead to BSI is through microbial colonisation. Bacteria or fungi stick to the catheter tubing inside the vein and secrete a protective biofilm protecting themselves from host defences and any circulating antimicrobial agents, and enabling sustained colonisation. 22,23 The organisms multiply and, in babies with immature immunity, infection spreads via the bloodstream, causing sepsis and infecting other organs, with damaging effects, particularly on the brain and lungs. The organism causing the BSI is important. Gram-positive organisms, such as Group B streptococci, and Gram-negative organisms, including Escherichia coli, Klebsiella and Pseudomonas, are associated with high rates of mortality and morbidity. 7 BSIs due to skin commensals, predominantly coagulase-negative staphylococci (CoNS), can also cause death and adverse neurodevelopmental outcomes, but the risk is lower than for clearly pathogenic organisms. 17 Lower risks of adverse consequences for BSIs due to skin organisms may reflect two factors. First, skin organisms are less virulent than non-skin organisms, evidenced by their status as normal commensal bacteria that colonise the skin and gut of newborn babies. Second, skin organisms frequently contaminate blood culture samples. A positive culture from blood does not, therefore, always reflect the presence of organisms circulating in the bloodstream, and rates of infection with skin organisms may be overestimated.
Bloodstream infections related to PICCs can be treated with intravenous antibiotics, and sometimes require removal of the PICC. 24 Systemic antibiotic treatment alters the microbial ecology of the gut, which increases the risk of necrotising enterocolitis (NEC), an inflammatory condition of gut mucosa, which affects babies born before 32 weeks of gestation. 25,26 Prevention of BSIs in babies who receive a PICC is, therefore, important to avoid these serious and costly consequences.
Antimicrobial impregnation of the tubing of CVCs is widely used to prevent BSIs in adult and paediatric intensive care. Use of antimicrobial-impregnated CVCs is recommended in UK27 and US28 national guidelines for patients at high risk of infection. No such recommendations exist, however, for newborn babies, because of the lack of antimicrobial-impregnated catheters suitable for preterm babies and the lack of evidence from adequately powered randomised trials. 29,30 Various forms of antimicrobial impregnation have been evaluated in 54 randomised controlled trials (RCTs) and numerous systematic reviews, including two systematic reviews combined with network meta-analyses that reported direct and indirect comparisons between types of CVCs. 31,32 The network meta-analyses report the most effective form of antimicrobial impregnation to be a combination of minocycline and rifampicin antimicrobials. However, the only form of impregnated PICC licensed in Europe for newborn babies, and small enough for use in preterm babies, is the Premistar™ catheter, manufactured by Vygon (UK) Ltd (Swindon, UK). The Premistar PICC is impregnated with the antifungal miconazole, in combination with the antibacterial agent rifampicin. In the next section, we summarise findings from an overview of systematic reviews of RCTs that evaluated CVCs impregnated with miconazole and rifampicin, or with minocycline and rifampicin, in any setting or age group.
Evidence review
We searched the Cochrane Central Register of Controlled Trials, EMBASE and MEDLINE on 29 March 2018, and ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform on 3 April 2018, for studies evaluating the effectiveness of antimicrobial-impregnated CVCs compared with any other type of CVC for reducing the rate of BSIs in newborn infants, children and adults. We included systematic reviews or meta-analyses of RCTs of miconazole- and rifampicin-impregnated or minocycline- and rifampicin-impregnated CVCs published since 2008, and RCTs in children or newborn infants published since the searches conducted in the systematic reviews. We used search terms related to CVCs, antimicrobial impregnation and infection. We identified 11 RCTs of CVCs impregnated with rifampicin combined with another antimicrobial agent (two with miconazole and nine with minocycline) (Table 1).
| Comparison | Population | Source | Review/study | Included studies | Number randomised | Outcome | Result, OR/RR/HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Miconazole-and rifampicin-impregnated vs. standard CVCs | Children | Balain 201529 | Review | No RCTs | |||
| Wu 201733 | Review | No RCTs | |||||
| Flemmer 201630 | Study | 1 RCT | 86 | PICC colonisation and signs of sepsis | OR 0.22 (0.02 to 2.27) | ||
| Adults | Chong 201732 | Review | 1 RCT | 223 | Catheter-related BSI | RR 0.89 (0.02 to 45.33) | |
| Minocycline- and rifampicin-impregnated vs. standard CVCs | Children | Wu 201733 | Review | 2 RCTs | 1773 | Catheter-related BSI | OR 0.40 (0.15 to 1.04) |
| Gilbert 201634 | Study | 1 RCT | 1485 | BSI | HR 0.43 (0.20 to 0.96) | ||
| Adults | Lai 201635 | Review | 4 RCTs | 1335 | Catheter-related BSI | RR 0.26 (0.13 to 0.49) | |
| Chong 201732 | Review and network meta-analysis | 7 RCTs | 2724a | Catheter-related BSI | RR 0.29 (0.16 to 0.52) |
Further details of studies and search terms are reported in the appendix to the recent trial report. 1 Of the two trials that compared miconazole- and rifampicin-impregnated CVCs with standard CVCs, one involved newborn infants, but was published as an abstract only. 30 The other was conducted in adult surgical patients. 36 Neither trial reported a significant difference in the rate of catheter-related BSIs. This widely used outcome may give biased catheter-related BSI results because of inhibition of laboratory culture of organisms from the CVC tip due to leaching of the antimicrobial agent from the impregnated CVC tubing onto the culture media. 34 Use of any BSI as an outcome, meaning any positive culture, whether catheter related or otherwise, avoids this problem.
There were nine RCTs34,37–44 of minocycline- and rifampicin-impregnated CVCs. In eight of the RCTs, the comparator was standard CVCs, in the other RCT the comparator was CVCs impregnated with chlorhexidine and silver sulfadiazine. Two RCTs were conducted with children and seven with adults. All nine RCTs reported large reductions in the rates of catheter-related BSIs. The systematic review and network meta-analysis of trials in adults by Chong et al. 32 reported an estimated risk ratio for minocycline and rifampicin impregnation, compared with no impregnation, of 0.29 [95% confidence interval (CI) 0.16, 0.52]. Only one RCT, the CATheter Infections in CHildren (CATCH) trial,34 reported results for BSI from any cause: the rate of BSI was reduced by 57% (see Table 1).
Study rationale
Citing the paucity of evidence for newborn babies, a systematic review published in The Cochrane Library in 2015 recommended that a large, simple and pragmatic RCT of antimicrobial-impregnated CVCs be undertaken to guide policy and practice. 29 The only type of antimicrobial-impregnated CVC developed and licensed to date for use in newborn babies in the UK is the Premistar, which is impregnated with miconazole and rifampicin. Although two RCTs found no evidence of reduced catheter-related BSIs with rifampicin–miconazole impregnation, an in vitro experimental study reported reduced bacterial colonisation of CVCs with rifampicin–miconazole impregnation, compared with no impregnation. 30,36,45
When planning the PREVenting infection using Antimicrobial-Impregnated Long lines (PREVAIL) trial, we sought support for the proposed trial from the Neonatology Clinical Studies Group of the Medicines for Children Research Network. The group emphasised the need for RCT evidence of reduced rates of BSIs in preterm babies, and of safety in relation to antibiotic resistance. Extrapolation of findings from children and adults was not sufficient for clinicians to change practice. Concerns were expressed about the additional costs of impregnated CVCs, and the fact that rates of hospital-acquired infection were declining following implementation of ‘bundles’ of practice designed to improve sterile procedures during catheter insertion and maintenance of the line. 46–48
Combining the overview of published evidence and clinical opinion, it was concluded that:
-
The frequency and serious long-term consequences of BSI in preterm babies could result in significant health gains and reduced health-care costs if impregnated CVCs were found to reduce rates of BSI in babies receiving neonatal intensive care.
-
Evidence would be needed from a large RCT specifically in preterm newborns of the benefits, safety and cost-effectiveness of miconazole- and rifampicin-impregnated PICCs before neonatologists would be willing or able to change purchasing decisions to adopt this type of PICC in NNUs.
-
Reductions over time in BSI rates due to improved infection control practices in neonatal care would need to be taken into account when applying the findings of a large RCT to practice.
Aims and objectives
Aims
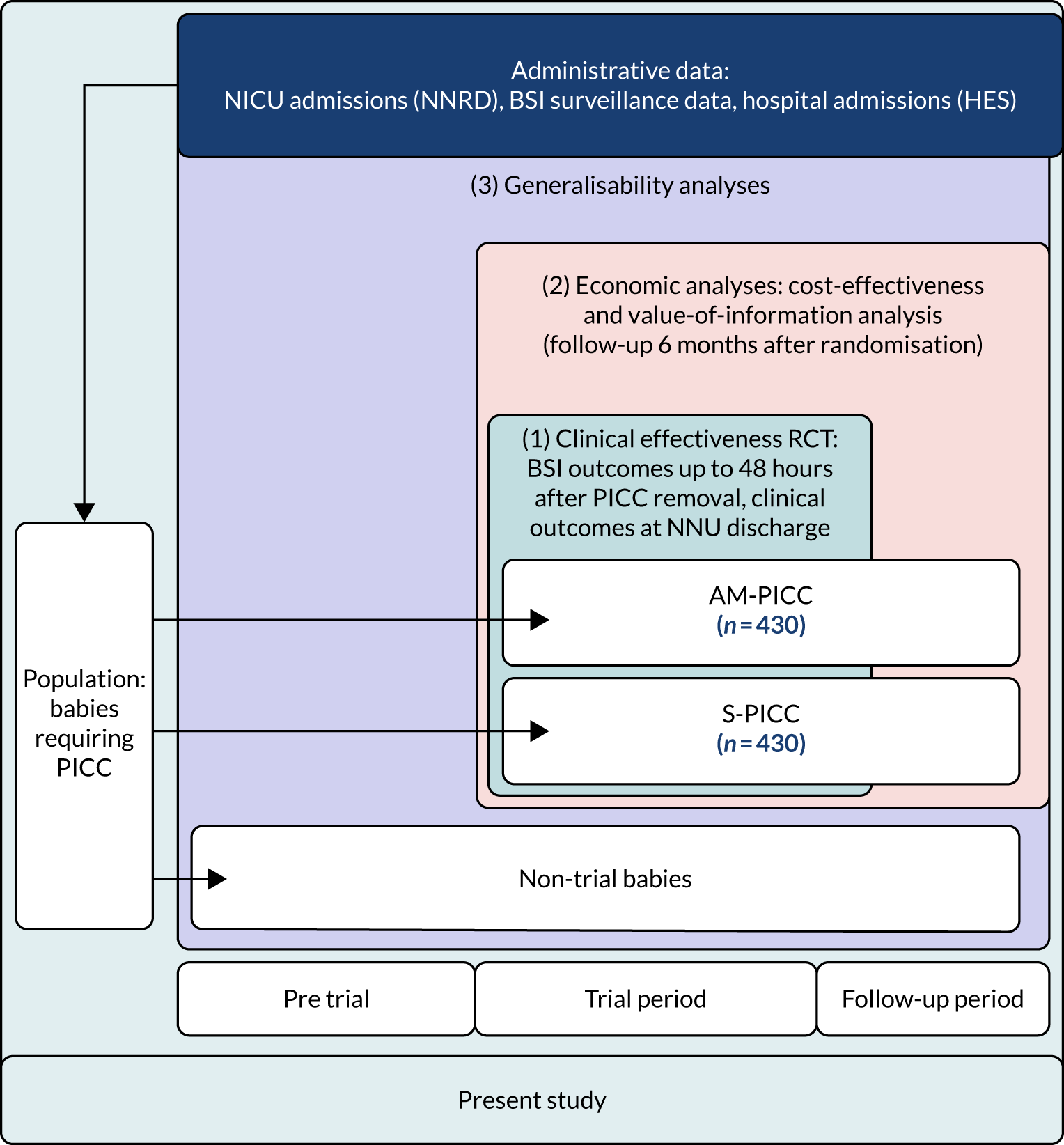
The overall aim of the study was to determine whether or not antimicrobial-impregnated PICCs (AM-PICCs) should be adopted across the NHS for babies receiving neonatal care. We undertook three inter-related analyses to address this aim (see Figure 1):
-
a clinical effectiveness RCT – to determine the clinical effectiveness of AM-PICCs, compared with standard PICCs (S-PICCs), for babies receiving neonatal care
-
an economic analysis – to determine the cost-effectiveness of AM-PICCs, compared with S-PICCs, from an NHS perspective
-
a generalisability analysis – to generalise the trial results to neonatal care in the NHS in England.
Objectives
Clinical effectiveness randomised controlled trial
The objectives of the RCT were to determine the clinical effectiveness of AM-PICCs compared with S-PICCs in terms of:
-
time to BSI (primary outcome), and other measures of BSI (e.g. rate)
-
safety, including measures of rifampicin resistance in blood, cerebrospinal fluid (CSF) and PICC cultures
-
clinical outcomes, for example death before discharge, NEC, time to full enteral feeds and time to PICC removal.
The data sources were case report forms (CRFs) completed by research nurses from routine clinical records, and mortality within 6 months of randomisation based on linked death registration data.
Economic analysis
The economic analysis was conducted to determine:
-
the hospital costs of using AM-PICCs compared with S-PICCs over the 6-month follow-up of the trial
-
the cost-effectiveness of AM-PICCs versus S-PICC in terms of NHS costs and quality-adjusted life-years (QALYs), combined to assess incremental net health benefit over the babies’ expected lifetimes
-
the value of further information from research.
The original study objectives included determining the cost to the NHS of a BSI in preterm babies and the value of implementing the cost-effective PICC type. These objectives were not addressed because we found no evidence of a reduction in BSI rates in babies randomised to AM-PICCs, compared with babies randomised to S-PICCs.
The data sources were as follows: national data from Hospital Episodes Statistics (HES) [all admissions, accident and emergency (A&E), outpatients], paediatric intensive care unit (PICU) admissions [from the Paediatric Intensive Care Audit Network (PICANet)]; and the NNRD for all care in NNUs.
Generalisability analysis
The objectives of the generalisability analysis were as follows:
-
to determine generalisability and applicability by comparing BSI risk factors, causative organisms and rates of BSI among babies in the PREVAIL trial with other babies receiving PICCs
-
to determine the applicability of BSI rates in the PREVAIL trial to rates of BSI in the NHS in England by comparing trends in BSI in babies receiving PICCs in the PREVAIL trial neonatal intensive care units (NICUs), non-PREVAIL trial NICUs and local neonatal units (LNUs)
-
to provide context through understanding of changes in BSI over time by evaluating trends in rates of late-onset BSI per 1000 days of intensive or high-dependency care and per 100 admissions for all babies receiving intensive or high-dependency care in NICUs and LNUs
-
to determine the contribution of PICCs to the overall rate of BSI per admission.
The data source was the NNRD linked to national laboratory infection surveillance data for England.
The three components of the study are illustrated in Figure 1. We also considered further uses of these linked data. First, for ongoing monitoring of BSI rates following implementation of an effective intervention. Second, to link trial and linked administrative health data to school achievement data to assess long-term effects of PICC impregnation on cognitive ability measured in school achievement assessments. These uses have not been pursued, because the infection outcome in the randomised groups did not differ.
FIGURE 1.
Flow diagram to show inter-relationship between the three components of the PREVAIL study.
Chapter 2 Clinical effectiveness randomised controlled trial methods
Parts of this chapter have been reproduced from Gilbert et al. 1 © 2019 The Author(s). Published by Elsevier Ltd. This is an open access article distributed under the terms of the CC-BY 4.0 license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium.
Trial design
We conducted an open-label, two-arm parallel-group RCT in 18 NICUs in England. The trial was a pragmatic trial designed to determine the clinical effectiveness of AM-PICCs in the context of local practice in NICUs. Recruitment was planned over a 24-month period, starting in June 2015. An internal pilot was used to assess recruitment. The threshold for determining that recruitment was feasible was set at 130 participants being randomised after 8 months (to allow the initial sites 6 months of recruiting at full capacity). An interim analysis of the primary outcome took place half-way through the trial (when approximately half of the participants had been randomised), using Haybittle–Peto stopping rule. 49
Ethics approval and research governance
The protocol was approved by the Yorkshire and the Humber Health Research Authority (reference number 14-YH-1202). The trial was funded by the National Institute for Health Research (NIHR) Health Technology Assessment programme and included in the International Standard Randomised Controlled Trial Number registry [https://doi.org/10.1186/ISRCTN81931394 (accessed 22 July 2020)]. Centre-specific approval was obtained at all recruiting centres.
Full details of the amended trial protocol (version 5.0, 26 April 2017), the research ethics approval and the statistical analysis plan are available online [http://prevailtrial.org.uk/ (accessed 22 July 2020)].
A summary of substantial protocol amendments is provided in Appendix 1, Table 26.
Selection of trial sites
Recruitment took place in 18 out of 43 NICUs in England. Overall, there are 154 NNUs in England (Table 2), of which 43 are NICUs that provide intensive care for babies, such as invasive ventilation and organ system support (Table 3). Days of intensive care are supported by nurse staffing ratios of one nurse to one baby, with step-down care for high-dependency (one nurse to two babies) and special care (one nurse to four babies). 51 Of the 154 NNUs, 76 LNUs provide short-term intensive care and high-dependency and special care, and 35 NNUs provide special care only [i.e. special care baby units (SCBUs)]. One in 10 babies admitted to a NNU is transferred to another unit for higher or lower levels of care. Step-up transfers between NNUs within neonatal networks are used to provide centralised intensive care when needed, including neonatal surgery for a small proportion of babies. Step-down transfers are frequently used to allow babies to be cared for near to parents and to reduce pressure for beds in NICUs. Low-intensity neonatal care is also provided in transitional care wards, which are not considered here.
| Type of NNU | Units, n (%) | Babies admitted,a n (%) | Median annual admissions per unit (range) | Percentage of all birthsb (n = 676,794) |
|---|---|---|---|---|
| NICU [provide intensive care, high-dependency care and special care (levels 1–3)] | 43 (27.9) | 22,717 (42.9) | 509 (200–1150) | 3.4% |
| LNU [provide short-term intensive care, high-dependency care and special care (levels 1–2)] | 76 (49.4) | 24,211 (45.8) | 307 (83–749) | 3.6% |
| SCBU [provide special care only (level 1)] | 35 (22.7) | 5972 (11.3) | 154 (46–362) | 0.9% |
| Total | 154 | 52,900 | 319 (46–1150) | 7.8% |
| Level of care | Care received |
|---|---|
| Level 3: intensive care | Any day on which a baby receives/undergoes at least one of the following:
|
| Level 2: high-dependency care | Any day on which a baby who does not meet the criteria for intensive care receives at least one of the following:
|
| Level 1: special care | Any day on which a baby who does not meet the criteria for intensive or high-dependency care receives or has at least one of the following:
|
We invited expressions of interest from NNUs in 2015. We prioritised NICUs with the largest number of babies born before 32 weeks of gestation, using NNRD data, and those sites invited to participate through the Children’s Clinical Research Network.
Participants
Eligible participants were all babies who required the narrowest (1 French gauge) PICC. Only babies who had previously participated in the trial or who were known to have an allergy or hypersensitivity to rifampicin or miconazole were excluded.
The reason for insertion was not requested, but PICCs are primarily used for parenteral nutrition, particularly in preterm babies. As this was a pragmatic trial, designed to reflect routine practice, we did not restrict eligible participants to preterm babies. However, in practice, the 1-French gauge PICC is predominantly used in preterm babies born before 32 weeks of gestation, as more mature and larger babies normally require a wider-gauge PICC to infuse sufficient volumes of fluid. Preliminary, unpublished analyses using the NNRD revealed that 70% of babies in neonatal units born before 32 weeks of gestation had a PICC inserted (unpublished data, NNRD, England).
Recruitment procedure
Screening
The principal investigator (PI) or research nurse (RN) maintained a screening log of babies whose parents were approached about the trial, detailing reasons for declining consent or reasons for no randomisation occurring for those who did consent. They also kept a log of all babies who received a PICC [a 1-French Premicath®; Vygon (UK) Ltd], but whose parents were not approached for the trial, and why they were not approached.
Enrolment
When a baby was likely to require a PICC (Premicath, 1 French), the clinician or RN provided written information and met with the parents to discuss participation in the trial. 52 Written consent was required, along with confirmation of eligibility from the PI or designated other before randomisation could occur. Twins were treated as separate individuals for consent and randomisation, if both babies required a PICC. Babies could be simultaneously enrolled in multiple studies, as agreed between chief investigators.
Randomisation, concealment and blinding
Participants were randomised to either an AM-PICC or a S-PICC using a secure web-based randomisation programme by the PI or delegated other at each of the 18 recruitment sites. PICC insertion was scheduled to occur within 48 hours of randomisation by a designated staff member. Randomisation sequences were computer-generated by an independent statistician in random, variable blocks of two and four, stratified by site. Randomisation was controlled centrally by the Liverpool Clinical Trials Centre (LCTC), at the University of Liverpool (UoL), to ensure allocation concealment. It was impractical to mask clinicians to PICC allocation because rifampicin caused brown staining of the AM-PICC. Participant inclusion in analyses and occurrence of outcome events were determined by following an analysis plan that was specified before individuals saw unblinded data.
Treatments
Trial participants were allocated in a ratio of 1 : 1 to receive either:
-
a miconazole- and rifampicin-impregnated PICC (AM-PICC; Premistar)
-
a standard (non-impregnated) PICC (S-PICC; Premicath).
Miconazole is an antifungal agent that is effective against systemic fungal infection. Rifampicin is an antibacterial agent, previously evaluated as rifampicin–minocycline CVC impregnation in adults and children. The manufacturer, Vygon (UK) Ltd, reported continuing elution from the CVC of rifampicin and miconazole over 21 days. 53 The AM-PICC was marketed after certification under the Conformité Européenne (CE) process in December 2012 (certificate number Z/12/02895).
The method for PICC insertion was according to clinician preference. Either a needle or a 24-gauge intravenous cannula was inserted into a peripheral vein and the PICC tubing was threaded through the stylet until the tip reached a large central vein, such as the inferior or superior vena cava. The position of the PICC was checked by a radiograph. In the case of malposition, for example in a spinal vein or the PICC tip lying within the heart, the catheter was adjusted or withdrawn. For the purposes of the trial, a PICC was deemed to have been successfully inserted if the site of line insertion was dressed in preparation for a radiograph to confirm PICC tip position, even if the result of the radiograph led to the PICC being withdrawn. If PICC insertion was unsuccessful, the clinical team attempted further insertions using the same type of PICC as randomised until 48 hours after randomisation, at which point subsequent PICC insertion attempts were of the S-PICC regardless of the randomised allocation. Babies could not be randomised more than once.
Follow-up
Infection outcomes (including the primary outcome) were captured for all babies until 48 hours after PICC removal, or following the last unsuccessful PICC insertion or randomisation (if insertion was not attempted) (Figure 2). During this time, the RN assessed babies daily to record whether or not any blood or CSF samples were taken for clinical indications of infection (or results received), any concomitant antimicrobials or parenteral nutrition were given, the PICC was removed (and tip cultured) or any adverse events (AEs) were observed.
FIGURE 2.
Schematic representation of the trial procedures.
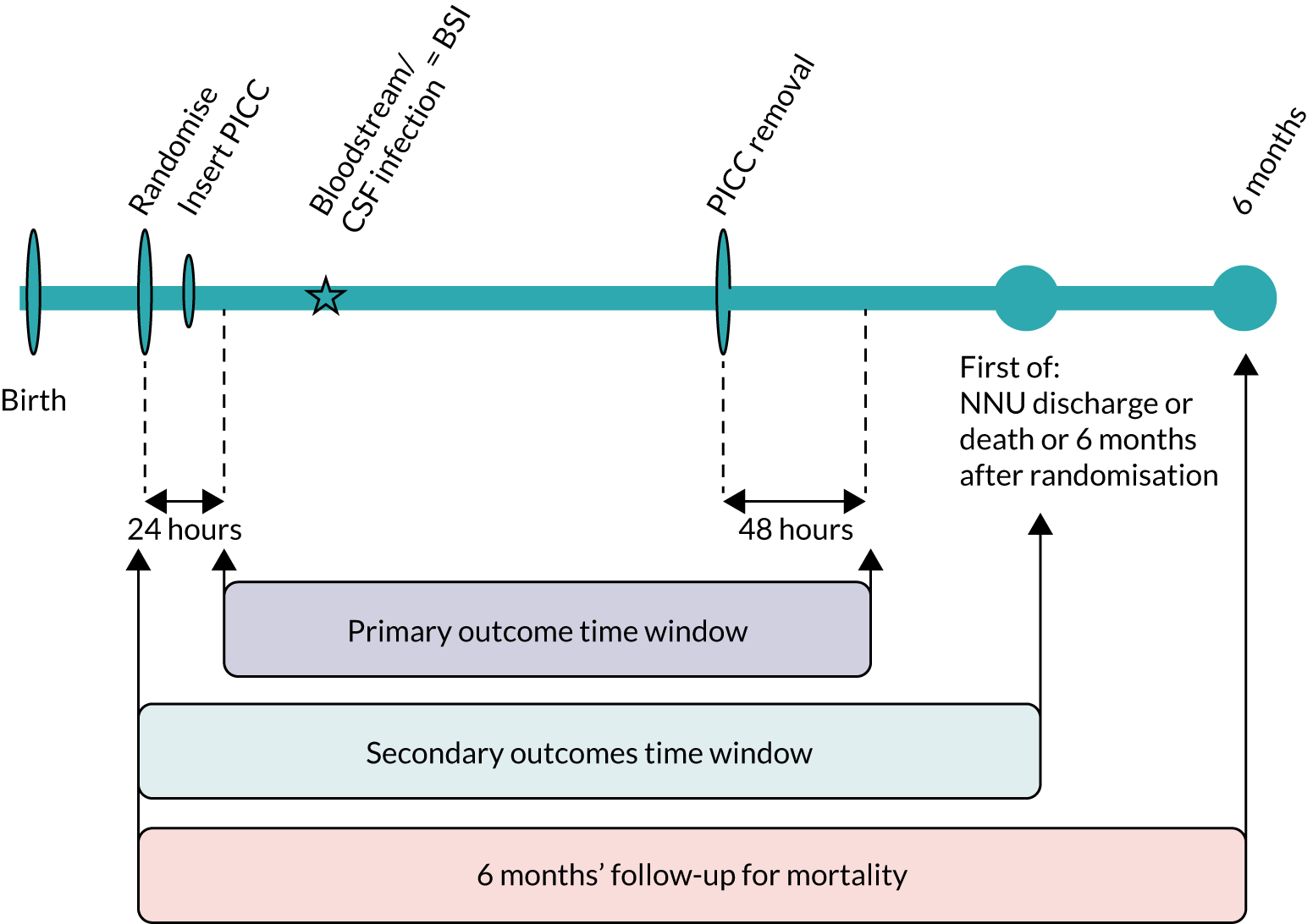
Follow-up for secondary clinical outcomes continued until discharge from the neonatal unit, death or 6 months after randomisation, whichever occurred first (see Figure 2). As babies matured and required less intensive care, some were transferred to a NNU nearer home that was not one of the recruitment centres. We counted date of discharge from the final NNU after all transfers. Information was captured on all deaths within 6 months of randomisation.
Data were collected on paper-based CRFs completed by centre staff authorised to do so and returned to the LCTC. Information on deaths was recorded on the CRF, if death occurred in the NNU, and/or through linked death registration records.
Measures
Baseline characteristics
We recorded the following demographic and relevant clinical characteristics at randomisation.
-
demographics – sex, birthweight, gestational age at birth and age at randomisation
-
delivery characteristics – born in the trial hospital or transferred to the trial hospital after birth, vaginal or caesarean delivery, ruptured membranes > 24 hours before birth, maternal antenatal steroids received or not, and maternal antibiotics received within 12 hours pre delivery
-
characteristics of neonatal care before randomisation – surgery in the previous 14 days, samples taken, antimicrobial medication received and the highest level of respiratory support required, all within the previous 72 hours
-
other clinical characteristics – major congenital anomaly; appearance, pulse, grimace, activity, respiration (Apgar) score at 5 minutes of age; number of invasive devices in situ; and the site where the randomised PICC was successfully inserted.
Adherence to treatment and the protocol
To measure adherence to the intervention, we recorded whether or not a PICC was inserted, and, if so, whether or not it was the allocated PICC, the reasons for removal, the length of time that the PICC was in situ, and whether or not positive samples were tested for resistance.
Protocol deviations were monitored centrally via evaluation of inclusion/exclusion criteria at trial entry and throughout the course of the trial. Some of the secondary outcomes (see the following sections) were also designed to detect potential biases in adherence to the trial protocol that might be influenced by knowledge of the type of PICC.
Primary outcome
The primary outcome was time from randomisation to first bloodstream or CSF infection, defined as a microbiological culture of a bacteria or fungus from blood or CSF sampled for clinical reasons. We use the term BSI to refer to a positive culture from blood or CSF. We defined the primary outcome time window as the period when any positive blood or CSF cultures could be counted in the primary outcome. The time window was from 24 hours post randomisation until 48 hours after PICC removal or death (or 48 hours after randomisation if the PICC was not inserted; see Figure 2). We imposed a priori decision rules to avoid counting pre-existing BSI. We excluded microbial cultures that were within the time window if the same organism was isolated from blood or CSF and samples were taken < 14 days apart, or if a different organism was isolated and samples were < 24 hours apart. When there were multiple positive cultures within the primary outcome time window, each positive culture was assessed and the first one that met the definition was counted.
Secondary outcomes
Secondary outcomes related to infection
These outcomes were measured during the primary outcome time window (see Figure 2):
-
rifampicin resistance in any isolate from blood or CSF culture
-
rifampicin resistance in any isolate from the PICC tip
-
rifampicin resistance in any isolate from blood or CSF culture or from the PICC tip [this was added as an additional analysis in version 2.0 of the statistical analysis plan, which was approved on 7 March 2018, before database lock (study closure)]
-
occurrence of one or more BSI
-
rate of BSIs (including recurrent BSIs) per 1000 PICC days
-
rate of catheter-related BSIs (defined as isolation of the same organism from the PICC tip and blood or CSF) per 1000 PICC days
-
type of organism isolated from BSI meeting primary outcome criteria.
Multiple infection episodes within the time window were considered as distinct infection episodes if positive samples for each episode involved the same organism in samples taken > 14 days apart or involved different organisms in samples taken > 24 hours apart.
Rifampicin resistance was detected using ETEST® (bioMérieux, Marcy-l'Étoile, France) strips. 54
Other clinical secondary outcomes
These outcomes were captured up until neonatal discharge home, death or 6 months after randomisation, whichever occurred first (see Figure 2):
-
chronic lung disease – respiratory support (mechanical ventilation or continuous positive pressure via endotracheal tube or nasal tube) or supplemental oxygen at 36 weeks’ postmenstrual age
-
NEC – Bell’s stage II or III
-
abnormalities on cranial ultrasound (periventricular leukomalacia or intracranial haemorrhage; worst grade of one to four used in analyses)
-
treatment for retinopathy of prematurity (medical or surgical)
-
time from randomisation to full milk feeds (150 ml/kg/day)
-
total duration of parenteral nutrition from randomisation until discharge from neonatal care
-
death –
-
before discharge home from neonatal care
-
within 6 months (26 weeks) of randomisation.
-
Secondary outcomes to detect potential biases in sampling or treatment on the basis of knowledge of peripherally inserted central venous catheter allocation
-
Rate of blood/CSF culture sampling per 1000 PICC days.
-
Duration of antimicrobial exposure from randomisation up to 48 hours after PICC removal.
-
Time from randomisation to PICC removal.
Safety outcomes
All AEs (expected and unexpected) considered to be related to the PICC were reported until 48 hours after PICC removal.
Sample size
The sample size calculation for the primary outcome was based on the log-rank test for equality of survival curves. We hypothesised a similar effect of rifampicin–miconazole impregnation to that of minocycline–rifampicin. We considered a 50% reduction to be conservative, given results of a network meta-analysis by Wang et al. 31 for catheter-related BSI [mean odds ratio (OR) 0.18 and upper 95% CI 0.34], and the results of the CATCH trial. 34 Using a two-sided significance level of 0.05, to detect a reduction in the proportion of babies experiencing a BSI from 14% in the standard arm, which was expected based on audit data from three participating NNUs, to 7% in the antimicrobial-impregnated arm, 79 events were required from 816 babies (408 in each arm) for 90% power. To allow for a 5% loss to follow-up, the target was inflated to 858 babies.
Statistical methods
General statistical considerations
The analysis and reporting of the trial were undertaken in accordance with the Consolidated Standards of Reporting Trials (CONSORT)55,56 and the International Conference on Harmonisation E9 Guidelines. 57 The main features of the statistical analysis plan are included in this section, with a full and detailed statistical analysis plan provided as a supplementary file58 on the PREVAIL trial website.
Outcome data were analysed according to the intention-to-treat principle, which is including all randomised participants in the group to which they were allocated (regardless of whether or not the allocated PICC was inserted) and for whom the outcome was measured/observed. All statistical tests were two-sided and performed using a 5% significance level. We used 95% CIs throughout. There were no adjustments for multiple testing; rather, all secondary analyses were treated as hypothesis-generating.
All analyses were conducted with SAS® version 9.4 (SAS Institute Inc., Cary, NC, USA). Results from the primary outcome and safety analyses were validated by independent programming by another statistician from the point of raw data.
Baseline characteristics
Demographics and other clinical baseline characteristics were summarised for each treatment group and overall, using descriptive statistics. No formal statistical testing was performed on these data. Descriptive statistics, including the number of observations, mean and standard deviation (SD) for continuous variables, and counts and percentages for discrete variables, were presented as appropriate.
Adherence to treatment and the protocol
All protocol deviations were agreed with the co-chief investigators prior to them seeing any unblinded results. These data were summarised for each treatment group and overall, using descriptive statistics, as for the baseline data. No formal statistical testing was performed on these data.
Primary outcome
The primary outcome, time to first BSI, was measured from randomisation to the first sample that met the definition of an independent episode of BSI. Participants not experiencing the primary outcome were censored at death, 48 hours after PICC removal or 48 hours after randomisation (for those with no PICC inserted). The difference between the groups was tested using the log-rank test, and the hazard ratios (HR) and associated 95% CIs obtained from the Cox proportional hazards model were presented. Kaplan–Meier curves were used to present the number of babies at risk. The number of samples taken, the number of babies with samples taken and the number of babies with a BSI within the primary outcome time window were also presented for each treatment group, overall and split by sample type.
Four sensitivity analyses of the primary outcome were prespecified to determine the robustness of the results of the primary analysis:
-
time from randomisation to first clinically serious BSI, defined as treatment with antimicrobials for ≥ 72 hours or death during treatment
-
time from PICC insertion to first BSI
-
time from randomisation to first BSI excluding samples obtained via arterial cannulas or CVCs
-
time from randomisation to first BSI including only clearly pathogenic organisms, as defined in Appendix 1, Table 27.
After seeing the results, we specified an additional analysis of the primary outcome to investigate whether or not the treatment effect varied by gestational age at birth (< 28 weeks or ≥ 28 weeks of gestation at birth) using a Cox proportional hazards model, including an interaction between treatment and gestational age.
Secondary outcomes
Secondary outcomes relating to infection
The analysis of the rifampicin resistance outcomes and occurrence of one or more BSI used Fisher’s exact test to compare the proportions of participants in each group for whom these outcomes (rifampicin resistance and BSI) were observed. Relative risks (RRs) were presented, along with 95% CIs. Frequency tables are also presented for the resistance outcomes, split by treatment and whether samples were Gram positive or negative, along with line listings of the resistant isolates showing the type of PICC and the organism cultured (see Appendix 2, Table 38).
The differences in the rate of BSI (including recurrent BSI) and rate of catheter-related BSI per 1000 PICC days during the primary outcome time window were analysed using Poisson regression. The rate ratios and associated 95% CIs were presented. For comparability with published studies, rates per 1000 PICC days between randomisation and PICC removal were also reported.
The number and proportion of BSIs by type of organism isolated were presented, but no formal statistical analysis of this outcome was performed.
Other clinical secondary outcomes
The proportion of participants in each treatment group experiencing chronic lung disease, NEC, abnormalities on cranial ultrasound and treatment for retinopathy of prematurity were compared using Fisher’s exact test. RRs were presented, along with 95% CIs. We described the type of treatment given for retinopathy of prematurity without formal statistical analysis by treatment allocation.
The time to full milk feeds was compared across the two treatment groups using the log-rank test and Cox proportional hazards models. The HRs and associated 95% CIs were presented, along with a Kaplan–Meier curve stratified by treatment. Survival times were measured from randomisation.
The duration of parenteral nutrition was compared across the two treatment groups using the Mann–Whitney U-test. The medians and interquartile ranges (IQRs) were presented for each group.
Death was analysed in two ways. Fisher’s exact test was used to compare the proportion of deaths in each group before discharge home from neonatal care and within 6 months from randomisation (updated to include data from NHS digital). RRs were presented, along with associated 95% CIs. Time to death was analysed using the log-rank test and Cox proportional hazard models. The HR and associated 95% CIs were presented, along with a Kaplan–Meier curve stratified by treatment. Survival times were measured from randomisation.
Secondary outcomes to detect potential biases in sampling or treatment on the basis of knowledge of peripherally inserted central venous catheter allocation
The difference in rate of blood/CSF culture sampling per 1000 PICC days (during the primary outcome time window) was analysed using Poisson regression. The rate ratio and associated 95% CIs were presented. As with the other rate outcomes, for comparability with published studies, the rate per 1000 PICC days between randomisation and PICC removal was also reported.
Time to PICC removal was compared across the two treatment groups using the log-rank test and Cox proportional hazard models. The HR and associated 95% CIs were presented, along with a Kaplan–Meier curve stratified by treatment. Survival times were measured from randomisation.
The difference between groups in the duration of antimicrobial exposure was analysed using the Mann–Whitney U-test. The medians and IQRs were presented for each group.
Safety analyses
The statistical analysis plan specified that all babies who had a PICC inserted or who had an attempted insertion would be included in the safety analysis population. The plan specified that babies who had a PICC inserted would be analysed according to the treatment they received, and that babies for whom PICC insertion was attempted but unsuccessful would be analysed according to the allocated treatment group, as information was not recorded on the type of PICC that was used for attempted insertion. However, after seeing the trial results, it was deemed more appropriate to exclude babies in whom insertion was unsuccessful from the safety analysis population.
All AEs and serious adverse events (SAEs) reported by the clinical investigator and classified as ‘possibly’, ‘probably’ or ‘almost certainly’ related to the trial treatment were presented. The number (and percentage) of occurrences of each AE/SAE and of babies experiencing each AE/SAE were presented for each treatment arm. The same information was also presented split by severity. For each baby, only the maximum severity that they experienced for each type of AE was displayed. No formal statistical testing was undertaken.
Patient and public involvement
We worked closely with Bliss (registered charity number 1002973) to develop the trial, provide advice to the trial team, identify suitable parent and public representatives and disseminate updates and results. Bliss was involved in the development of the study at an early stage, as one of their members was a co-applicant to the grant. A Bliss representative was a member of the Trial Management Group (TMG) and was involved in the development and review of trial documentation.
A parent of a prematurely born infant who had spent substantial time in a NICU sat on the Trial Steering Committee (TSC) and assisted with decision-making about the trial. The parent representative also aided with the review of trial documentation.
Trial oversight and role of funders
Trial Management Group
The TMG comprised the co-chief investigators, specific co-investigators (clinical and non-clinical), members of the LCTC and members of the chief investigator’s team at University College London (UCL). The TMG was responsible for the month-to-month management of the trial. The TMG proposed the membership of the oversight committees [the TSC and the Independent Data and Safety Monitoring Committee (IDSMC)] to the trial funders, who then made appointments according to their constitutional requirements. Members are listed in the Acknowledgements.
Trial Steering Committee
The TSC consisted of an independent chairperson, an independent expert in the field of neonatology, an independent statistician and a parent representative. One of the co-chief investigators was a non-independent member of the TSC. The TSC was the executive decision-making committee considering the recommendations of the IDSMC. Monitoring reports viewed by the TSC did not present data split by treatment group. Members are listed in the Acknowledgements.
Independent Data and Safety Monitoring Committee
The IDSMC included an independent chairperson, an expert in the field of microbiology and an independent statistician. The IDSMC was responsible for reviewing and assessing recruitment and for interim monitoring of safety and effectiveness, trial conduct and external data. The IDSMC provided recommendations to the TSC concerning the continuation of the trial. Members are listed in the Acknowledgements.
Role of the funding source
The funder appointed independent members to the TSC and the IDSMC, approved all protocol amendments and monitored study progress against agreed milestones. The funder had no involvement in data interpretation or writing of the report. The corresponding author had full access to all outputs from the data in the study.
Dual publication
Chapters 1 and 2 contain information published in the 2019 report of the PREVAIL trial findings, with further details and results. 1 Chapter 2 includes details reported in the PREVAIL trial protocol and statistical analysis plan, which have already been published on the PREVAIL website. 58,59
Chapter 3 Clinical effectiveness randomised controlled trial results
Recruitment
The first participant was randomised on 12 August 2015. The internal pilot at 8 months found that actual recruitment (n = 229) exceeded the target number (n = 179) (see Appendix 2, Figure 15) and the threshold of 130 participants, which was set to demonstrate feasibility. The last participant was randomised on 11 January 2017, 4 months earlier than planned, as the recruitment target had been met (see Appendix 2, Table 28, for additional randomisation information for each site). Clinical follow-up of enrolled participants continued until 30 May 2017.
The CONSORT flow diagram (Figure 3) illustrates the pathway of participants through the trial. Parents or legal guardians of 1404 babies were approached about the trial; consent was provided for 937 (66.7%) babies, and 861 (61.3%) went on to be randomised (see Appendix 2, Table 29, for screening and recruitment details for all sites). The most frequently recorded reason for declining consent was that the parents did not want their baby to participate in research [195/467 (41.8%); see Appendix 2, Table 31]. The most frequently recorded reason for babies not being randomised despite parents providing consent was that the baby no longer required a PICC [49/76 (64.5%); see Appendix 2, Table 32]. There were an additional 487 babies who received a PICC (1 French gauge) at one of the participating sites during the recruitment period, but who were not approached for the trial. The most frequent reasons recorded were parents not available [163/487 (33.5%)] or missed by the clinical team [134/487 (27.5%); see Appendix 2, Table 30].
FIGURE 3.
The CONSORT flow diagram for all trial participants.
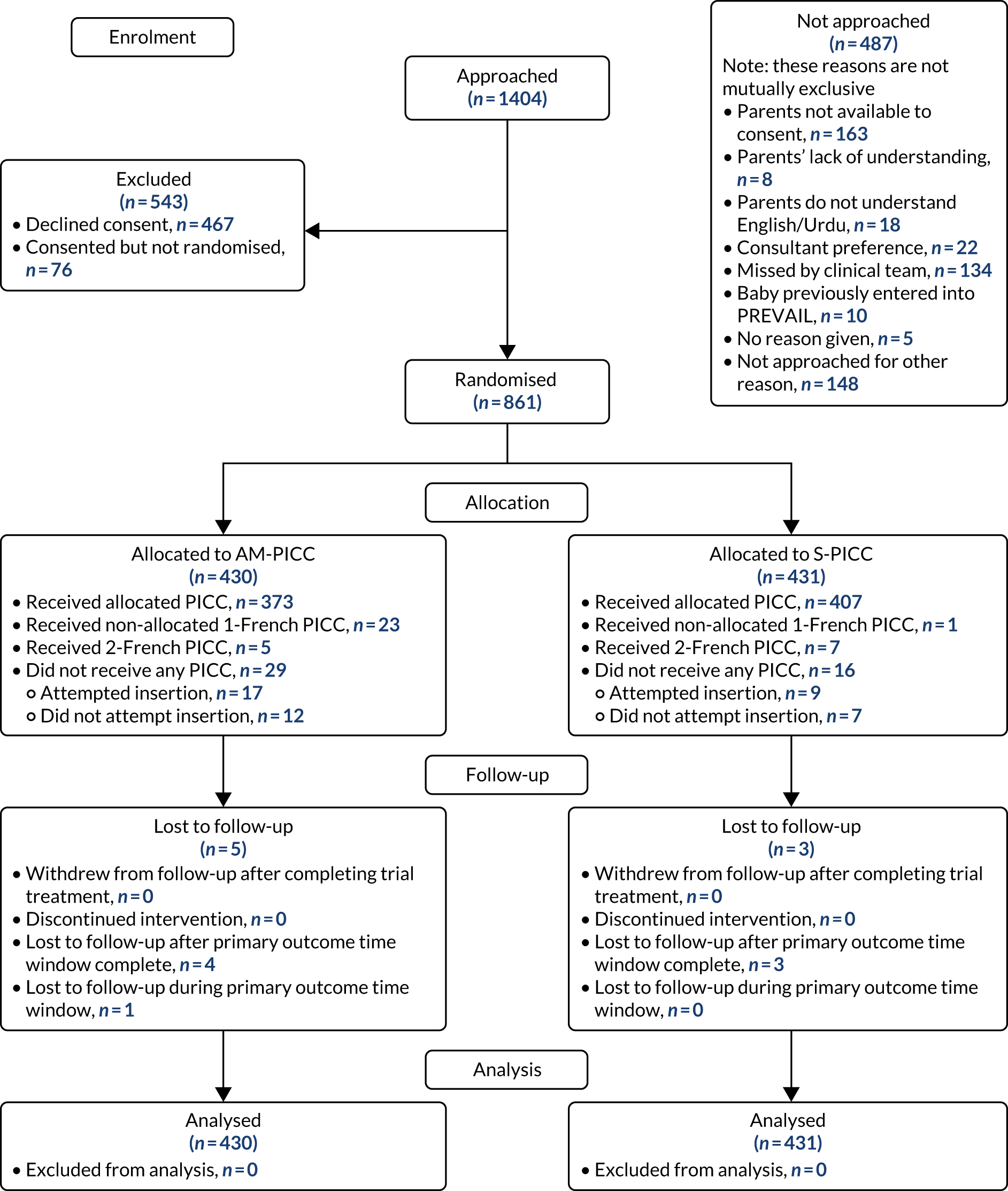
A total of 191 babies (22.2%) were co-enrolled in the PREVAIL trial and at least one other RCT. Appendix 2, Table 33, provides details on the number of babies who were co-enrolled in other trials at each of the recruiting sites.
Interim analysis
An interim analysis was undertaken using a data snapshot taken on 22 June 2016. At this point, 523 participants were randomised. A total of 412 participants were included in the intention-to-treat analysis set (i.e. randomised and consented with sufficient data to analyse), and 411 participants were included in the safety analysis set (i.e. insertion successful or attempted).
In the AM-PICC group, 27 of the 208 participants had experienced at least one BSI (13.0%) and in the S-PICC group, 21 of the 204 participants had experienced at least one BSI (10.3%). The median time to BSI could not be calculated as the event had not been experienced by enough participants. There was no difference between the two groups (HR 1.24, 95% CI 0.70 to 2.19; p-value 0.46).
In the AM-PICC group, 24 related AEs occurred in 22 of the 206 participants (10.7%); in the S-PICC group, 22 related AEs occurred in 20 of the 205 participants (9.8%). One of the related AEs in the AM-PICC group was classified as an unexpected SAE.
The IDSMC did not raise any significant issues of trial conduct, governance or safety and was happy to recommend that the trial continue as planned.
Details of all oversight committee meetings along with recommendations are shown in Appendix 2, Table 34.
Final analysis
Baseline characteristics
The two treatment groups were comparable in terms of baseline characteristics (Tables 4–7). Of the 861 babies who were randomised, 715 (83%) were randomised before 7 days of age and 754 (88%) were born before 32 weeks of gestation.
| Baseline characteristic | Trial group | |
|---|---|---|
| AM-PICC (N = 430) | S-PICC (N = 431) | |
| Sex, n (%) | ||
| Male | 214 (49.8) | 225 (52.2) |
| Female | 216 (50.2) | 206 (47.8) |
| Birthweight (g) | ||
| Median (IQR) | 962.5 (729–1220) | 960 (770–1250) |
| < 750, n (%) | 119 (27.7) | 92 (21.3) |
| 750–999, n (%) | 110 (25.6) | 140 (32.5) |
| 1000–1249, n (%) | 102 (23.7) | 91 (21.2) |
| 1250–1499, n (%) | 52 (12.1) | 62 (14.4) |
| 1500–1749, n (%) | 27 (6.3) | 27 (6.3) |
| 1750–1999, n (%) | 8 (1.9) | 7 (1.6) |
| ≥ 2000, n (%) | 12 (2.8) | 12 (2.8) |
| Gestational age at birth (weeks) | ||
| Median (IQR) | 27.90 (25.78–29.94) | 28.06 (26.23–30.14) |
| < 26, n (%) | 115 (26.7) | 93 (21.6) |
| 26 or 27, n (%) | 101 (23.5) | 110 (25.5) |
| 28 or 29, n (%) | 103 (24.0) | 102 (23.7) |
| 30 or 31, n (%) | 54 (12.6) | 76 (17.6) |
| 32 or 33, n (%) | 28 (6.5) | 15 (3.5) |
| 34 or 35, n (%) | 7 (1.6) | 9 (2.1) |
| 36 or 37, n (%) | 5 (1.2) | 3 (0.7) |
| ≥ 38, n (%) | 7 (1.6) | 11 (2.6) |
| Missing, n (%) | 10 (2.3) | 12 (2.8) |
| < 32, n (%) | 373 (86.7) | 381 (88.4) |
| Age at randomisation (days) | ||
| Median (IQR) | 4.12 (2.04–5.93) | 3.90 (1.90–6.12) |
| < 2, n (%) | 106 (24.7) | 113 (26.2) |
| 2–6, n (%) | 256 (59.5) | 240 (55.7) |
| 7–13, n (%) | 39 (9.1) | 52 (12.1) |
| 14–20, n (%) | 6 (1.4) | 11 (2.6) |
| 21–27, n (%) | 3 (0.7) | 5 (1.2) |
| ≥ 28, n (%) | 20 (4.7) | 10 (2.3) |
| Baseline characteristic | Trial group, n (%) | |
|---|---|---|
| AM-PICC (N = 430) | S-PICC (N = 431) | |
| Location of birth | ||
| Born in study hospital | 340 (79.1) | 367 (85.2) |
| Transferred after birth | 90 (20.9) | 64 (14.8) |
| Mode of delivery | ||
| Vaginal | 196 (45.6) | 198 (45.9) |
| Caesarean | 234 (54.4) | 233 (54.1) |
| Membrane rupture > 24 hours before delivery | ||
| Yes | 111 (25.8) | 104 (24.1) |
| No | 299 (69.5) | 310 (71.9) |
| Missing | 20 (4.7) | 17 (3.9) |
| Maternal antenatal corticosteroids | ||
| Yes | 375 (87.2) | 381 (88.4) |
| No | 53 (12.3) | 50 (11.6) |
| Missing | 2 (0.5) | 0 (0) |
| Maternal antibiotics ≤ 12 hours before delivery | ||
| Yes | 135 (31.4) | 102 (23.7) |
| No | 275 (64.0) | 310 (71.9) |
| Missing | 20 (4.7) | 19 (4.4) |
| Baseline characteristic | Trial group, n (%) | |
|---|---|---|
| AM-PICC (N = 430) | S-PICC (N = 431) | |
| Surgery before randomisation | ||
| > 6 days | 2 (0.5) | 3 (0.7) |
| ≤ 6 days | 15 (3.5) | 10 (2.3) |
| No surgery | 413 (96.0) | 418 (97.0) |
| Positive blood culture < 72 hours prior to randomisation | ||
| Yes | 29 (6.7) | 19 (4.4) |
| No | 401 (93.3) | 412 (95.6) |
| Antibiotics/antifungals < 72 hours prior to randomisationa | ||
| Yes | 367 (85.3) | 363 (84.2) |
| No | 63 (14.7) | 68 (15.8) |
| Respiratory support < 72 hours prior to randomisation | ||
| Invasive ventilation | 262 (60.9) | 257 (59.6) |
| Non-invasive ventilation | 122 (28.4) | 133 (30.9) |
| Oxygen only | 9 (2.1) | 7 (1.6) |
| None | 37 (8.6) | 34 (7.9) |
| Baseline characteristic | Trial group, n (%) | |
|---|---|---|
| AM-PICC (N = 430) | S-PICC (N = 431) | |
| Major congenital anomaly | ||
| Yes | 21 (4.9) | 27 (6.3) |
| No | 408 (94.9) | 404 (93.7) |
| Missing | 1 (0.2) | 0 (0.0) |
| Apgar score at 5 minutes | ||
| 0–3 | 23 (5.3) | 19 (4.4) |
| 4–7 | 138 (32.1) | 140 (32.5) |
| 8–10 | 247 (57.4) | 249 (57.8) |
| Missing | 22 (5.1) | 23 (5.3) |
| Devices in situ at randomisation | ||
| < 4 | 370 (86.0) | 390 (90.5) |
| ≥ 4 | 60 (14.0) | 41 (9.5) |
| PICC insertion site | ||
| No PICC inserted | 29 (6.7) | 16 (3.7) |
| Lower limb | 207 (48.1) | 220 (51.0) |
| Upper limb | 191 (44.4) | 190 (44.1) |
| Scalp | 3 (0.7) | 3 (0.7) |
| Other | 0 (0.0) | 1 (0.2) |
| Missing | 0 (0.0) | 1 (0.2) |
Adherence to treatment and the protocol
Fewer babies randomised to the AM-PICC had the randomly allocated PICC inserted [373 participants (86.7%)] than those randomised to the S-PICC group [407 participants (94.4%)] (Table 8). Approximately half of the babies who did not receive the allocated AM-PICC had no PICC inserted, and half received a different PICC.
| Trial group, n (%) | ||
|---|---|---|
| AM-PICC (N = 430) | S-PICC (N = 431) | |
| PICC status | ||
| Allocated PICC inserted | 373 (86.7)a,b | 407 (94.4)c,d |
| Non-allocated PICC inserted | 28 (6.5)c | 8 (1.9)a |
| 1 French PICC | 23 (5.3)d | 1 (0.2)b |
| 2 French PICC | 5 (1.2) | 7 (1.6) |
| No PICC inserted | 29 (6.7) | 16 (3.7) |
| PICC insertion attempted | 17 (4.0)a | 9 (2.1)c |
| PICC insertion not attempted | 12 (2.8) | 7 (1.6) |
| End of follow-up for infection outcomes | ||
| 48 hours after PICC removal | 387 (90.0) | 398 (92.3) |
| Death with PICC in situ | 13 (3.0) | 18 (4.2) |
| 48 hours after randomisation | 29 (6.7) | 15 (3.5) |
| Lost to follow-up | 1 (0.2) | 0 (0.0) |
| End of follow-up for other outcomes | ||
| Discharge home from neonatal care | 383 (89.1) | 385 (89.3) |
| Transfer to non-participating site | 4 (0.9) | 3 (0.7) |
| Death before discharge | 36 (8.4) | 33 (7.7) |
| 6 months after randomisation | 6 (1.4) | 10 (2.3) |
The intention-to-treat analysis population included all 861 participants (100%) who were randomised, regardless of whether or not they received the allocated PICC. The safety population as defined in the statistical analysis plan (all participants in whom insertion was successful or attempted) included 842 of the 861 randomised participants (97.8%). The modified safety analysis (all participants in whom insertion was successful) included 816 of the 861 randomised participants (94.8%) (see Table 8 for further details on which participants were included in the safety analysis populations).
The time points for end of follow-up were similar in both groups (see Table 8). The majority of participants completed follow-up for infection outcomes (outcomes for which samples were required to be taken) 48 hours after removal of the PICC [785/861 (91.2%)] and follow-up for other outcomes at discharge home from neonatal care [768/861 (89.2%)].
The type and frequency of sampling for microbiological cultures were similar in both groups (see Appendix 2, Table 35); blood/CSF samples were taken from 198 babies in the AM-PICC group (46.0%) and from 190 babies in the S-PICC group (44.1%). The frequency of testing for rifampicin resistance in blood or CSF positive cultures was slightly lower in the AM-PICC group than in the S-PICC group, but similar for PICC tips, although there were half as many positive cultures from PICC tips in the AM-PICC group [47 participants (10.9%)] as in the S-PICC group [90 participants (20.9%)].
Protocol deviations were generally similar across the two treatment groups (see Appendix 2, Table 36). A total of 86 participants (10.0%) had at least one major protocol deviation. The most common major deviation was not receiving the allocated PICC [81 participants (9.4%)], and the most common minor deviations were the PICC tip culture not being taken at removal [167 participants (19.4%)] and resistance testing not being done on positive cultures [135 participants (15.7%)].
Primary outcome
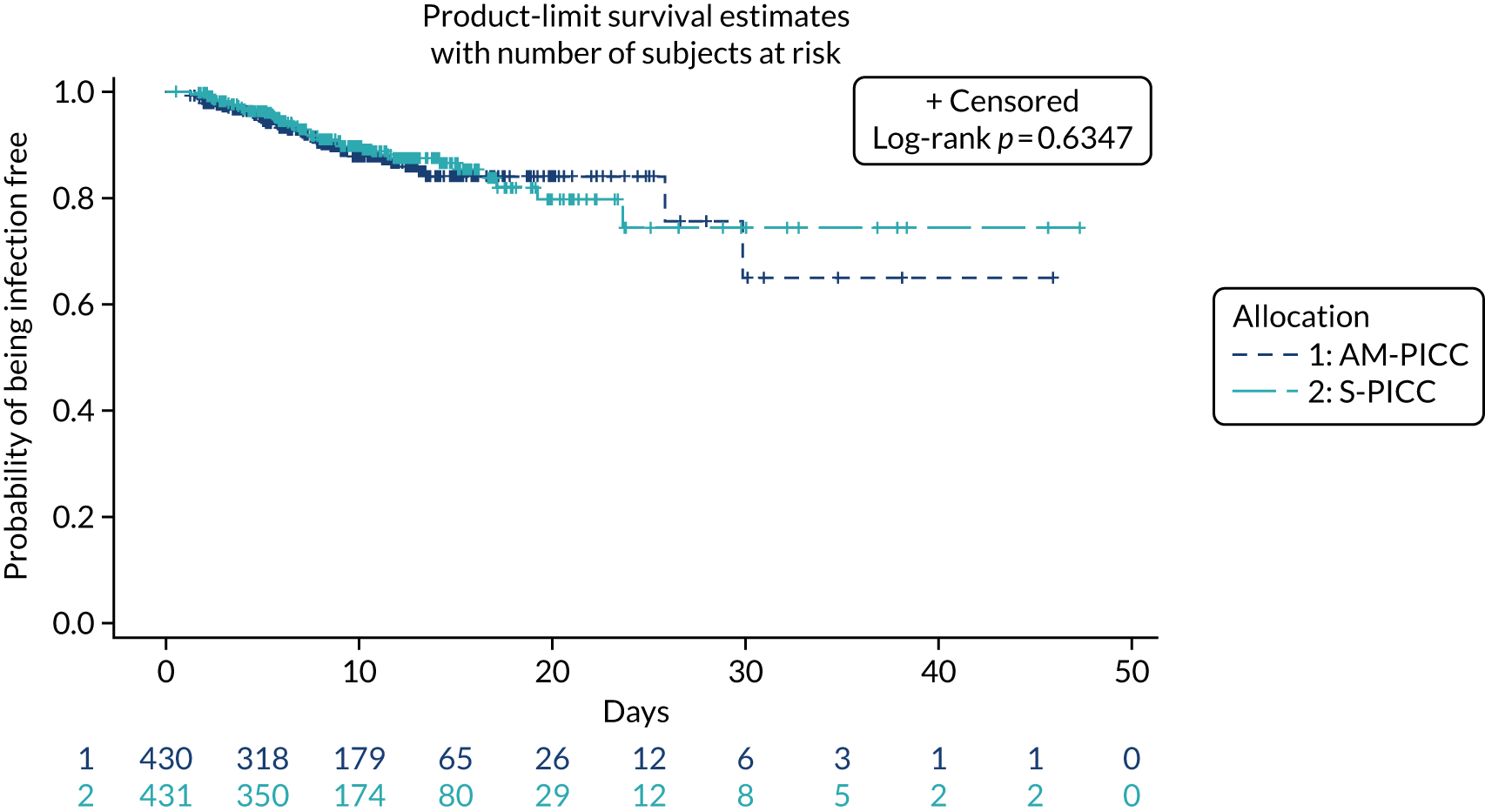
A total of 46 participants in the AM-PICC group (10.7%) and 44 participants in the S-PICC group (10.2%) experienced a BSI in the primary outcome time window. The median time to BSI could not be calculated as not enough participants experienced an event. The time from randomisation until first BSI did not differ between the groups (HR 1.11, 95% CI 0.73 to 1.67) (Table 9 and Figure 4). The Kaplan–Meier curves crossed when the numbers at risk were low. A time-varying coefficient was added to the model to check the assumption of proportional hazards; this was not significant (p = 0.62).
| Primary outcome | Trial group, n (%) | HR (95% CI) | p-value | |
|---|---|---|---|---|
| AM-PICC (N = 430) | S-PICC (N = 431) | |||
| Time to first BSI | 46 (10.7) | 44 (10.2) | 1.11 (0.73 to 1.67) | 0.63 |
| Sensitivity analyses | ||||
| Time to first clinically serious BSI | 42 (9.8) | 40 (9.3) | 1.11 (0.72 to 1.71) | 0.65 |
| Time to first BSI (from insertion)a | 45 (11.2) | 44 (10.6) | 1.08 (0.71 to 1.64) | 0.72 |
| Time to first BSI excluding arterial or PICC samples | 45 (10.5) | 43 (10.0) | 1.11 (0.73 to 1.68) | 0.64 |
| Time to first BSI excluding skin organisms | 16 (3.7) | 9 (2.1) | 1.90 (0.84 to 4.31) | 0.12 |
FIGURE 4.
Kaplan–Meier plot: time to first BSI (primary outcome). The table under the graph presents the numbers of study participants at risk (i.e. not censored) at each time point.
The results from all four sensitivity analyses are consistent with the results from the primary analysis, indicating that the original results are robust regarding the assumptions that were made (see Table 9). The post hoc analysis that was performed to determine if there was evidence of a difference in treatment effect for babies with a gestational age of < 28 weeks compared with babies with a gestational age of ≥ 28 weeks found that this was not the case (p = 0.28). Appendix 2, Figures 16 and 17 show further results from the post hoc analysis.
Secondary outcomes
Secondary outcomes relating to infection
The proportion of babies experiencing rifampicin resistance from PICC tip cultures was significantly higher in the AM-PICC group than in the S-PICC group, but this was not observed in blood or CSF cultures or in the combined outcome of blood or CSF or PICC tip cultures (Table 10). Resistant organisms are categorised and shown by intervention group in Appendix 2, Table 38.
| Secondary outcomes | Trial group | Treatment difference | p-value | |
|---|---|---|---|---|
| AM-PICC (N = 430) | S-PICC (N = 431) | |||
| Rifampicin resistance | n (%) | n (%) | RR (95% CI) | |
| Rifampicin resistance from blood/CSF culture | 4 (0.9) | 7 (1.6) | 0.57 (0.17 to 1.94) | 0.55 |
| Rifampicin resistance from PICC tip culture | 14 (3.3) | 4 (0.9) | 3.51 (1.16 to 10.57) | 0.02 |
| Rifampicin resistance from blood/CSF or PICC tip culturea | 18 (4.2) | 10 (2.3) | 1.80 (0.84 to 3.86) | 0.13 |
| Occurrence of ≥ 1 BSI | 46 (10.7) | 44 (10.2) | 1.05 (0.71 to 1.55) | 0.82 |
| Rate of BSI per 1000 PICC days | Rate ratio (95% CI) | |||
| Rate of BSI | 13.15 | 10.87 | 1.21 (0.78 to 1.88) | 0.40 |
| Rate of BSI (when line is in situ)b | 12.57 | 11.21 | 1.12 (0.73 to 1.12) | 0.60 |
| Rate of catheter-related BSI | 1.84 | 2.35 | 0.78 (0.27 to 2.25) | 0.65 |
| Rate of catheter-related BSI (when line is in situ)b | 1.71 | 2.46 | 0.70 (0.25 to 1.96) | 0.49 |
The number of participants experiencing one or more BSI during the primary outcome time window was not significantly different between the two groups (see Table 10). Details on the number of BSIs that each participant experienced are provided in Appendix 2, Table 39.
There were no significant differences in the rates of BSI or catheter-related BSI (see Table 10). Results from sensitivity analyses of the rates during the time that the PICC was in situ were consistent with the main results.
The types of organisms isolated from BSIs are shown in Table 11.
| Category | Organism | Trial group, n (%) | |
|---|---|---|---|
| AM-PICC (N = 49) | S-PICC (N = 45) | ||
| Gram-positive | CoNS | 31 (63.3)a | 35 (77.8)a |
| Enterococcus spp. | 3 (6.1) | 1 (2.2) | |
| Staphylococcus aureus | 2 (4.1) | 2 (4.4) | |
| Haemolytic streptococcus | 1 (2.0) | 0 (0.0) | |
| Gram-negative | E. coli | 4 (8.2)a | 2 (4.4) |
| Klebsiella spp. | 4 (8.2)a | 1 (2.2) | |
| Enterobacter spp. | 2 (4.1) | 1 (2.2) | |
| Acinetobacter spp. | 0 (0.0) | 1 (2.2) | |
| Moraxella osloensis | 0 (0.0) | 1 (2.2) | |
| Fungi | Candida spp. | 2 (4.1)a | 1 (2.2) |
| Total (n) | 49 | 45 | |
Other clinical secondary outcomes
There were no significant differences in the proportions of participants experiencing chronic lung disease, NEC, abnormality on cranial ultrasound or treatment for retinopathy of prematurity (Table 12). Further information on the type of treatment for retinopathy of prematurity is shown in Appendix 2, Table 40.
| Trial group | Treatment difference | p-value | ||
|---|---|---|---|---|
| AM-PICC (N = 430) | S-PICC (N = 431) | |||
| n (%) | n (%) | RR (95% CI) | ||
| Chronic lung disease | 190 (44.2) | 178 (41.3) | 1.07 (0.92 to 1.25) | 0.41 |
| NEC: Bell’s stage II or III | 41 (9.5) | 46 (10.7) | 0.89 (0.59 to 1.32) | 0.57 |
| Abnormality on cranial ultrasound | 166 (38.6) | 150 (34.8) | 1.11 (0.93 to 1.33) | 0.26 |
| Treatment for retinopathy of prematurity | 40 (9.3) | 30 (7.0) | 1.34 (0.85 to 2.11) | 0.21 |
| Death before discharge | 36 (8.4) | 33 (7.7) | 1.09 (0.70 to 1.72) | 0.71 |
| Death within 6 months of randomisation | 36 (8.4) | 35 (8.1) | 1.03 (0.66 to 1.61) | 0.90 |
| Median (IQR) | Median (IQR) | HR (95% CI) | ||
| Time to death within 6 months of randomisationa | – | – | 1.06 (0.67 to 1.70) | 0.79 |
| Time to full milk feeds (days) | 9.51 (6.37–17.26) | 9.40 (6.32–16.37) | 0.99 (0.86 to 1.14) | 0.85 |
| Days of parenteral nutrition | 11.00 (7.00–19.00) | 10.00 (7.00–18.00) | – | 0.83 |
There was no detectable difference in time to full milk feeds (the median number of days was approximately 9 in each group) or in the duration of parenteral nutrition (the median number of days was 11 in the AM-PICC group and 10 in the S-PICC group) (see Table 12).
There was no difference between the treatment groups in terms of the proportion of deaths before discharge home from neonatal care or within 6 months of randomisation, or in terms of the time to death within 6 months of randomisation (see Table 12).
Five deaths were captured in the CRFs that were not reported in the data provided by NHS Digital, and two deaths were reported in the data provided by NHS Digital that were not captured in the CRFs. There was also a discrepancy in the date of death for one baby; the date reported by NHS Digital was 1 day later than the date captured in the CRF.
Secondary outcomes to detect potential biases in sampling or treatment on the basis of knowledge of peripherally inserted central venous catheter allocation
The rate of blood sampling for signs of infection was significantly higher in the AM-PICC arm than in the S-PICC arm (98/1000 PICC days vs. 80/1000 PICC days, respectively; rate ratio 1.23, 95% CI 1.05 to 1.45; p-value 0.01) (Table 13). There were no differences in the median time to PICC removal (8 days in both groups) or in the median duration of antimicrobial treatment (3 days in both groups) (see Table 13).
| Secondary outcome | Trial group | Treatment difference | p-value | |
|---|---|---|---|---|
| AM-PICC (n = 430) | S-PICC (n = 431) | |||
| Rate per 1000 PICC days | Rate per 1000 PICC days | Rate ratio (95% CI) | ||
| Rate of blood/CSF culture sampling | 97.90 | 79.64 | 1.23 (1.05 to 1.45) | 0.01 |
| Rate of blood/CSF sampling (line in situ)a | 93.72 | 82.01 | 1.14 (0.98 to 1.34) | 0.09 |
| Median (IQR) | Median (IQR) | HR (95% CI) | ||
| Time to PICC removal (days) | 8.20 (4.77–12.13) | 7.86 (5.00–12.53) | 1.03 (0.89 to 1.18) | 0.73 |
| Days of antimicrobial treatment | 3.00 (2.00–6.00) | 3.00 (2.00–6.00) | – | 0.25 |
Safety analysis
All related AEs in participants who had a 1 French PICC successfully inserted are listed in Table 14. A total of 60 events were reported from 49 participants in the AM-PICC group (13.1%), and 50 events were reported from 45 participants in the S-PICC group (10.5%).
| AE | AM-PICC group (N = 374 participants) | S-PICC group (N = 430 participants) | ||
|---|---|---|---|---|
| Events (n) | Participants, n (%) | Events (n) | Participants, n (%) | |
| Any AE | 60 | 49 (13.1) | 50 | 45 (10.5) |
| Evidence of catheter blockage | 15 | 15 (4.0) | 15 | 15 (3.5) |
| Extravasation | 11 | 11 (2.9) | 11 | 11 (2.6) |
| Swelling/haematoma at line site | 10 | 10 (2.7) | 7 | 7 (1.6) |
| Clinically evident thrombophlebitis | 4 | 4 (1.1) | 7 | 7 (1.6) |
| Difficulty removing stylet | 8 | 8 (2.1) | 1 | 1 (0.2) |
| Catheter damage | 3 | 3 (0.8) | 4 | 4 (0.9) |
One SAE involving supraventricular tachycardia following PICC placement was reported in the AM-PICC arm. This event met the seriousness criterion of ‘medically significant’, and the local investigator felt that it was ‘possibly’ related to the recent insertion of the PICC. The event resolved and the PICC was not removed. This event was assessed as ‘unlikely’ to be related to the trial intervention by the chief investigator, but was reported to the Medicines and Healthcare products Regulatory Agency Adverse Incident Centre in line with the local investigator’s assessment.
Chapter 4 Economic evaluation
Introduction
The aim of the economic evaluation was to examine the costs and health benefits of using AM-PICCs rather than S-PICCs in PREVAIL trial babies. In doing so, the economic analysis can inform decisions regarding which type of PICC should be purchased for neonatal care in the NHS, identify the areas where more research is needed and inform future research by providing estimates on the costs of having babies in the NICU.
The economic evaluation planned to include the following elements, as defined in the PREVAIL trial protocol,59 section 2.3.2:
-
the hospital costs of using AM-PICCs compared with using S-PICCs over the time horizon of the trial
-
the cost-effectiveness of AM-PICCs compared with that of S-PICCs over a patient’s expected lifetime, including the minimum clinical effectiveness required for AM-PICCs to be considered cost-effective
-
the value of information (VoI) to assess the benefits of potential additional research
-
the value of implementing the cost-effective PICC type
-
the hospital costs to the NHS of a BSI.
As reported in Chapter 3, the PREVAIL trial found that there was no difference in the risk of BSI or of clinically serious BSI between the AM-PICC and the S-PICC groups. The implication is that AM-PICCs are unlikely to be cost-effective, compared with S-PICCs, given the additional purchasing cost and that the prevention of BSI was expected to be their key benefit. Consequently, the economic analysis plan was revised to include elements 1, 2 and 3 in the preceding list, but not 4 or 5.
The objective of 1, to estimate the hospital costs of using AM-PICCs compared with using S-PICCs, was to understand whether or not the type of PICC had any impact on the costs of hospitalisations. This is henceforth referred to as the ‘cost of hospital care’. Estimates of the cost of hospital care are useful to understand the resources required to improve the health of preterm babies and to inform research on the cost-effectiveness of interventions to reduce hospital length of stay or other hospital use.
Objective 2, to examine the cost-effectiveness of AM-PICCs compared with S-PICCs over a patient’s expected lifetime, required the development of a cost-effectiveness model informed by population-specific inputs to evaluate interventions to prevent BSI. As far as was possible and relevant, the cost-effectiveness model was informed by data collected in the PREVAIL trial. The cost-effectiveness model was used to estimate the cost-effectiveness of AM-PICCs compared with S-PICCs, given the results of the trial, and to determine the minimum effect that would be required so that AM-PICCs were cost-effective given their greater acquisition cost.
The objective of 3, calculating the VoI, was to estimate the impact of parameter uncertainty on the likelihood of not choosing the most cost-effective option and the consequences in terms of health losses and additional costs. These estimates are used to calculate the expected value of further information and the magnitude of the benefits of investing in future research. 60
The economic analysis planned to include objective 4, the value of implementing the cost-effective PICC type. 61 This analysis was not conducted because, as it is unlikely that the AM-PICC is the cost-effective technology and the type of PICC currently implemented is the S-PICC, there is no value in implementation activities.
The estimation of the hospital costs to the NHS of a BSI (objective 5) was not conducted for two reasons. First, this analysis required the use of the random allocation to one of the two PICC types as an instrument to estimate the causal effect of BSI on the costs. As the PREVAIL trial found that there was no difference in the risk of BSI between the two PICC types, the instrumental variable analysis was not feasible. Second, the process for the health economics team to receive the data suffered a considerable delay (see Appendix 3), which did not allow sufficient time to explore alternative statistical models.
Revised objectives
The aim of the economic evaluation was to determine the cost-effectiveness of AM-PICCs compared with S-PICCs over a patient’s lifetime. The specific objectives were to:
-
estimate the cost of hospital care over the follow-up of the trial (reported in Cost of hospital care)
-
predict the long-term costs and quality-adjusted survival of babies with AM-PICCs or S-PICCs (reported in Cost-effectiveness of antimicrobial-impregnated versus standard peripherally inserted central venous catheters over a lifetime)
-
explore the extent to which the consequences of uncertainty indicate that there is value in additional research (reported in Value of information).
Cost of hospital care
Background
This section estimates the cost of hospital care for babies taking part in the PREVAIL trial (hereafter termed ‘PREVAIL babies’) for whom routine health-care data were available and whose parents had consented for their data to be shared with the research team.
Methods
Overview
Figure 5 summarises the methods. The PREVAIL babies may have received a variety of hospital care during the 6-month follow-up period. We sought data on hospitalisations from different data sources, which included stay in the NNU, admission to a PICU, admissions to paediatric hospital care, procedures such as surgery and diagnostic tests, outpatient appointments, and visits to the A&E department. We obtained data on the nature and intensity of hospital care in these different settings and used it to generate standardised units of cost called Healthcare Resource Groups (HRGs). We obtained the unit costs of the HRGs from NHS reference costs 2015–16,62 which represent their average cost to the NHS. The cost of hospital care for each baby is the sum of the costs of the HRGs.
FIGURE 5.
Overview of the methods used to estimate the cost of hospitalisations.
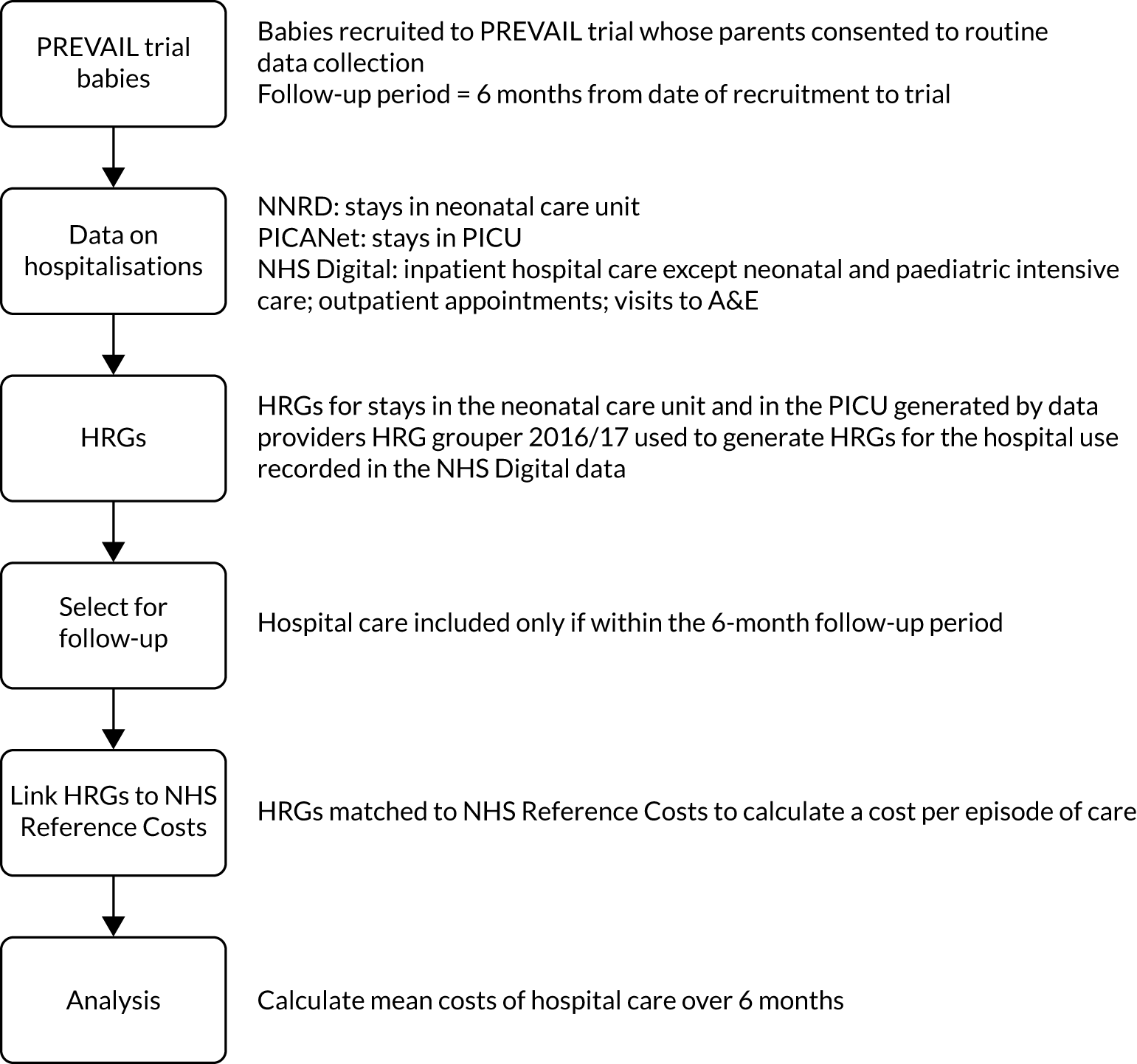
Study population
The study population comprised the PREVAIL babies for whom the parents or guardians consented to share their routine health-care data and for whom routine records were available.
Time horizon
The time horizon was from the randomisation date in the PREVAIL trial to 6 months’ follow-up or death, whichever occurred first.
Data sources
The PREVAIL trial data
The PREVAIL babies’ characteristics, trial group and outcomes were obtained from LCTC. The data were collected by the RNs using CRFs (forms 2, 3, 6, 7a, 7b and 10; details of the variables can be found in Appendix 4. The forms are available on the PREVAIL trial website. 63). The individual-level trial data were linked to data on hospitalisations using the trial participant study number. These data were encrypted, password protected and transferred securely to the University of York (UoY).
Data on hospital care
Hospital care includes care in the NNU and/or PICU, procedures and diagnostic tests, outpatient appointments, and visits to the A&E. To capture the full data on hospital care, data on the PREVAIL babies were extracted from five different databases. The specific data fields obtained from each database are detailed in Appendix 4. The databases and respective data custodians were as follows:
-
Hospital critical care during the stay in the NICU was obtained from the NNRD. The NNRD is a clinical data set (the National Neonatal Data Set) within the NHS Data Model and Dictionary Service. The NNRD compiles information entered by clinicians and nurses onto the electronic patient records of babies in the NNU. The National Neonatal Data Set is extracted quarterly from the electronic records to form the NNRD, which covers England, Wales and Scotland. 64 Details of all data items are searchable online. 65
-
Hospital critical care during any stay in the PICU was obtained from PICANet. PICANet is an audit database comprising details of care, such as need for ventilation, in PICUs in the UK and the Republic of Ireland. 66 PICANet has data on paediatric critical care during each day of hospitalisation in the unit.
-
Information on other hospital care during hospitalisations other than the critical care in the neonatal or paediatric critical care units was obtained from the HES Admitted Patient Care (APC) database. 67
-
Outpatient appointments were obtained from the HES outpatient database.
-
Accident and emergency attendances were obtained from the HES A&E database.
-
Date of randomisation was obtained from the PREVAIL trial data, collected by the UoL.
The HES APC, outpatient and A&E databases contain details of all admissions at NHS hospitals in England. The data are collected during a patient’s time at hospital and submitted to NHS Digital for processing. 67
Data on whether or not death occurred within the 6-month follow-up
Data on deaths occurring within the follow-up period were obtained from both the PREVAIL trial CRFs, which refer to date of death within the primary follow-up period, and from death registrations supplied to the Personal Demographics Service linked to the HES databases. LCTC received death registration data on date of death if death occurred within the 6-month follow-up (see Chapter 2, Secondary outcomes). These data are used to inform the decision model (see Probability of death between peripherally inserted central venous catheter insertion and 6 months of age).
Fair processing
The parents and guardians of the PREVAIL babies were asked for their consent to use data about their babies’ admissions and visits to hospital from the date of PICC insertion for a period of 6 months. For the babies whose parents gave consent, the team at LCTC sent the personal identifiers (NHS number, name, date of birth, sex and postcode) and trial identifier to PICANet (for the stays in the PICU) and NHS Digital (for all hospital stays, outpatient admissions, visits to A&E and date of death). Babies in the PREVAIL trial were linked to the NNRD using patient identifiers (NHS number, sex, date of birth and postcode) within Public Health England (PHE) for the generalisability study (see Chapter 5). The NNRD received de-personalised trial identifiers linked to the NNRD baby identifiers from PHE to identify PREVAIL babies for the NNRD extract for the health economics study.
Individual-level data containing the de-identified hospitalisation records and the trial identifier were transferred from NNRD, PICANet (via LCTC) and NHS Digital to the UoY. These data were password-protected, encrypted and transferred securely. The UoY transferred the data on the date of death, obtained from NHS Digital via the Personal Demographics Service, to LCTC. All data transfers were subject to data-sharing contracts between the various institutions. The privacy notice is available in the parents’ section of the PREVAIL trial website [www.prevailtrial.org.uk/parents.html (accessed 16 August 2020)].
Generating Healthcare Resource Groups from the data on hospital care
The data on hospital care consists of information on the nature and length of the hospital stay. The first step in costing hospital care is to generate HRGs from these hospital records. HRGs are standard groupings of clinically similar treatments that use common levels of health-care resource. 68 They form the payment structure of hospital care, and are relevant to all hospital stays, appointments and A&E visits. The algorithm to generate the relevant HRGs, given the resources involved in the hospitalisations, is released by the National Casemix Office in partnership with NHS Digital. 68
There are two types of HRGs: ‘bundled’ and ‘unbundled’. Bundled HRGs represent the set of procedures, treatments and other interventions for a given diagnosis and case mix. Unbundled HRGs represent high-cost or specialist service elements in addition to the care recorded in the bundled HRG. 68 Examples of unbundled HRGs are diagnostic imaging and high-cost drugs. 69 Activity in critical care units are captured in the critical care HRGs, which are a type of unbundled HRG.
Given that costs can be obtained only for admissions with a HRG, the admissions with insufficient information to derive a HRG were discarded. In principle, the hospitalisation data would be of sufficient quality to derive HRGs for all admissions. In practice, however, errors in coding occur. These errors are likely to be random and unrelated to the babies’ characteristics and the admissions themselves. Therefore, we assumed that the data were missing completely at random and removed missing observations (i.e. the specific hospitalisation) from the analysis. 70
Healthcare Resource Groups for the neonatal care stay (in the National Neonatal Research Database)
The neonatal care HRGs are critical care HRGs that are added to the core HRG relating to the hospitalisation. There are five neonatal critical care HRGs to represent the intensity of care over a 24-hour period, and a neonatal critical-care transport HRG, which is paid per patient journey (see Appendix 1, Table 43). 69 Therefore, each neonatal critical care HRG represents a day in the neonatal care unit or, if a transport HRG, a patient journey. Stays in the neonatal care unit can be aggregated into episodes, which, in turn, can be aggregated into an admission (or spell).
The data on the PREVAIL babies’ stays in the NNU (which were received from NNRD) included two files: the ‘episode file’ and the ‘daily file’. The ‘episode file’ contained the trial identifier, episode number, anonymised date of admission and anonymised date of discharge. Given that the follow-up period was restricted to 6 months after the date of randomisation, some of the episodes for which the date of discharge was outside the follow-up period did not include the record for the date of discharge. The ‘daily file’ contained the trial identifier, the episode number, the anonymised time stamp referring to when the day of care was recorded and the HRGs corresponding to the care on each day. The two files were processed and combined to obtain the length of stay and cost of stays in the neonatal care unit over the 6-month follow-up of the trial.
In principle, all PREVAIL babies have neonatal HRGs referring to their stay in the neonatal care unit. However, some units do not report to NNRD; hence, the data on the PREVAIL babies who had stays in these non-reporting units were not available. Furthermore, errors in entering records or in registering the details of the babies in the PREVAIL trial may result in some babies and some parts of their neonatal care not being recorded in NNRD.
Critical Care Healthcare Resource Groups for the paediatric intensive care stay (in the Paediatric Intensive Care Audit Network)
The paediatric care HRGs are critical care HRGs. Each day in the PICU is assigned to a paediatric critical care HRG. There are eight daily HRGs, in addition to a paediatric transport HRG, which is paid per patient journey. 69
Similarly to the NNRD data, the PICANet data included two files. One file had information on the episodes of care, trial identifier, episode number, date of admission and date of discharge. The other file contained information on the activity in each day of care, trial identifier, episode number, daily date and HRG.
Healthcare Resource Groups for hospital admissions (Hospital Episode Statistics Admitted Patient Care)
The hospital admission comprises the entire continuous stay in a specific hospital. The admission can include one or more finished consultant episodes (FCEs). A FCE is the period of care under a given consultant. Patients can have more than one FCE at the same time. Generally, all FCEs can be assigned to one or more HRGs. Typically, the FCE includes a bundled HRG, as well as one or more unbundled HRGs for high-cost items, and critical care HRGs for stays in critical care units, such as the NNU and PICU. It is not possible to link the FCE admissions in HES APC to the neonatal care episodes in NNRD and paediatric care episodes in PICANet with certainty, because of the lack of a common identifier to link episodes of care in critical care to FCEs in HES APC.
The HES APC data on the PREVAIL babies included the information required to generate HRGs and calculate length of stay for each FCE. The HRG4+ 2016/17 Reference Costs Grouper was used to generate the HRGs at the FCE level. 68 These included the bundled HRG and unbundled HRGs related to high-cost items over and above critical care stays.
Healthcare Resource Groups for outpatient appointments
Outpatient appointments also need to be assigned to a HRG for costing. These were generated with the HRG4+ 2016/17 Reference Costs Grouper. 68
Healthcare Resource Groups for accident and emergency visits
The HRG4+ 2016/17 Reference Costs Grouper was also used to assign each A&E visit to the relevant HRGs. 68
The data-cleaning processes required to prepare the data for the HRG4+ 2016/17 Reference Costs Grouper are detailed in Appendix 5.
Calculating length of stay
In the NNRD and PICANet, the length of stay (in the NNU and PICU, respectively) was calculated by subtracting the date of discharge from the date of admission for each episode of care. In HES APC, length of stay was calculated at the spell level, given that there can be more than one FCE at the same time in the same spell. When the date of admission of the subsequent episode of care or spell was earlier than the date of the discharge of the previous hospitalisation, the date of admission was changed to the previous date of discharge.
The length of stay recorded in HES APC overlaps with the length of stay in the NNUs and PICUs. This is because the days in these units are recorded in the NNRD and PICANet, respectively, in terms of the critical care HRGs, as well as in HES APC for the core hospitalisation. To calculate the cost of hospital care, however, the cost of care recorded in each database needs to be added up together, as the cost of the FCE (from HES APC) reflects only the core HRG and the unbundled HRGs, but not the critical care HRGs (which are recorded in the NNRD and PICANet).
Selecting hospital care that was within the trial follow-up
The period of follow-up was from randomisation for 6 months or until death, whichever happened first. The dates of outpatient and A&E visits were examined to confirm that all referred to the period between date of randomisation and the end of the 6-month follow-up.
The FCEs, which are captured in the HES APC data set, and the episodes of critical care, which are captured in the NNRD and PICANet, span a period of time. This period of time can include days before randomisation and/or days after the end of the follow-up period.
Stays in the NNUs and PICUs have records of daily activity and of the date of admission and discharge. For each database, the records of daily activity and of dates of admission and discharge were compared for consistency. The preferred option was to use the record of daily activity to select the days of care that were within the follow-up period. If these data were inconsistent, the dates of admission and discharge were used to select the episodes of care that were within the follow-up period. The dates of admission and discharge were also used to select the FCEs in the HES APC data set that were within the follow-up period.
The length of stay and cost of FCEs or episodes of care that fell partly outside the trial follow-up were adjusted to account for the time period outside the trial follow-up. This was done to ensure that the costs referred to the same time period for all babies. For the length of stay, this was done by removing the days outside the follow-up period. The costs were rescaled by the proportion of the episode of care or FCE within the follow-up period. The proportion of the episode of care or FCE within the follow-up period was calculated by adding 1 day to the length of stay within and outside the period of follow-up. This was to account for episodes in which the date of discharge corresponds to the date of randomisation or in which the date of admission corresponds to the date of end of follow-up.
Costing hospital care
Data were cleaned prior to costing (see Appendix 5). The HRGs were matched with the relevant costs using the NHS reference costs for 2015–16. 62 NHS reference costs for 2015–1662 were the most recent year for which costs were available when the economic work commenced. HRGs from 2016–17, which were different from 2015–16, were replaced or the costs deflated (see Appendix 5, Tables 41 and 42). Appendix 5, Table 43, reports the unit costs of the HRGs relating to the stay in the neonatal and paediatric critical care unit. The other unit costs are not reported here because of their large number, but are freely available online. 62
Analysis
Hospital care by database
Hospital care was analysed separately by database. For each database, the number of PREVAIL babies with hospital care recorded, the length of days in hospital (or the number of attendances for outpatient and A&E), the HRGs and the cost are reported.
Cost of hospital care
As the FCEs in HES APC cannot be uniquely linked to the episodes of care in the NNRD and PICANet, the cost of hospital care was calculated on a per-baby basis, separately, for each data set. Subsequently, the various data sets were merged to calculate the cost of hospital care in the PREVAIL trial population. The analytical sample comprised the PREVAIL babies with at least one valid record in HES APC and in the NNRD. Babies with no hospital care recorded in the PICANet, HES outpatient and HES A&E were assumed not to have used this care; hence, the cost of hospital care referring to each database was assumed to be zero. This was a complete-case analysis using the valid records in the NNRD and in the HES APC as the reference. It assumed that the likelihood of having none or incomplete information is unrelated to babies’ characteristics and costs. 70
Results
Neonatal care recorded in the National Neonatal Research Database
Data preparation
Appendix 5 details the process of data preparation, including Figure 18. Thirteen PREVAIL babies (1.51%) were not available in the NNRD extract: six were allocated to AM-PICC and seven to S-PICC. After cleaning, the file with the episode dates (the ‘episode file’) contained data on 848 (98.49%) PREVAIL babies, with a total of 1568 episodes. The file with the daily care record (the ‘daily file’) contained data on 848 (98.49%) PREVAIL babies with 57,702 days in NNUs. The date of discharge was imputed using the latest daily care date for 147 episodes without a date of discharge; of these, three episodes of care had their date of admission corrected to the earliest date stamp for the daily care in the episode. Episodes with a date of discharge before the date of randomisation were removed and permanently deleted. The resulting file contained data on 837 PREVAIL babies, with 1388 episodes and 56,610 days in the neonatal care unit.
Length of stay, care activity and cost of care
The breakdown of days of care by HRG code is presented in Appendix 6, Table 44. This breakdown refers to the data without any adjustments for hospitalisations spanning beyond the 6-month follow-up period, because the HRGs are recorded on a daily basis.
Appendix 6, Table 45, shows the length of stay recorded in the NNRD and costs by episode of care, with and without adjusting for the time outside the follow-up period. Without adjusting for the time outside the follow-up period, the average length of stay per episode of care was 40 days and the average cost per episode of care was approximately £36,000. Adjusting for the time outside the follow-up period, the average length of stay per episode of care was 37 days and the average cost per episode of care was approximately £33,000.
Table 15 shows the average length of stay and cost per baby, with adjustment for the follow-up period (see Appendix 6, Table 45, for the results without adjustment). The average length of stay per baby was 61 days and the average cost per baby was £54,086. This was similar for both PICC types.
| Total | AM-PICC | S-PICC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Babies,a n (%) | Mean | SD | Babies,a n (%) | Mean | SD | Babies,a n (%) | Mean | SD | |
| Hospital care recorded in the NNU recorded in NNRDa | |||||||||
| Length of stay (days) | 837 (97) | 61.04 | 35.74 | 420 (98) | 60.70 | 36.31 | 417 (97) | 61.40 | 35.20 |
| Cost (£) | 837 (97) | £54,086.19 | £35,223.79 | 420 (98) | £53,959.65 | £36,046.58 | 417 (97) | £54,213.65 | £34,418.05 |
| Hospital care recorded in the paediatric unit recorded in PICANet | |||||||||
| Length of stay (days) | 94 (11) | 8.29 | 9.56 | 46 (11) | 8.20 | 10.50 | 48 (11) | 8.38 | 8.67 |
| Cost (£) | 94 (11) | £14,547.84 | £17,021.59 | 46 (11) | £14,501.07 | £18,531.86 | 48 (11) | £14,592.66 | £15,636.00 |
| Hospital care recorded in HES APCa | |||||||||
| Length of stay (days) | 828 (96) | 68.65 | 37.91 | 410 (95) | 67.68 | 36.77 | 418 (97) | 69.61 | 39.01 |
| Cost (£) | 828 (96) | £26,332.09 | £18,190.26 | 410 (95) | £26,222.37 | £18,055.68 | 418 (97) | £26,439.72 | £18,342.29 |
| Outpatient appointments recorded in HES outpatient | |||||||||
| Number of attendances | 784 (91) | 7.47 | 7.60 | 394 (92) | 7.65 | 8.03 | 390 (90) | 7.29 | 7.14 |
| Cost (£) | 784 (91) | £1155.97 | £1009.64 | 394 (92) | £1166.57 | £1027.54 | 390 (90) | £1145.27 | £992.44 |
| A&E visits recorded in HES A&E | |||||||||
| Number of attendances | 320 (37) | 1.68 | 1.17 | 163 (38) | 1.72 | 1.19 | 157 (36) | 1.64 | 1.14 |
| Cost (£) | 320 (37) | £223.41 | £162.09 | 163 (38) | £227.34 | £152.69 | 157 (36) | £219.34 | £171.70 |
Paediatric critical care recorded in the Paediatric Intensive Care Audit Network
Data preparation
Appendix 5 details the process of data preparation, including Figure 19. A total of 177 PREVAIL babies were recorded in PICANet, with 184 episodes of care and 1237 days in the PICU. After removing the days of care outside the follow-up period, the PICANet files contained records on 94 (10.92%) PREVAIL babies who had 126 episodes of care and 779 days in the PICU.
Intensity, length of stay and cost of care within the follow-up period
Table 15 shows the results. For the 94 PREVAIL babies for whom PICANet recorded days in paediatric intensive care, the average length of stay, per baby, was 8 days and the average cost was approximately £14,500. This was similar for both PICC types. The breakdown by HRG code is presented in Appendix 6, Table 46.
Hospital care recorded in the Hospital Episode Statistics Admitted Patient Care
Data preparation
Appendix 5 details the process of data preparation, including Figure 20. A total of 834 (96.86%) PREVAIL babies were recorded in HES APC, with a total of 2772 FCEs. During the data preparation process prior to generating HRGs, 58 FCEs were removed, as well as the records referring to two PREVAIL babies. The grouper was fed 2714 FCEs, relating to 832 babies. Of these, 20 FCEs were not grouped into HRGs because of data errors, eight FCEs were removed because of missing admission type, and four FCEs were removed because of lack of unit cost data. As a result, data on the hospital admission for four PREVAIL babies were removed. In total, the HES APC included 2682 FCEs relating to 828 (96.17%) PREVAIL babies.
Intensity, length of stay and cost of care within the follow-up period
All of the 2682 FCEs for the 828 PREVAIL babies were within the follow-up period of 6 months. These corresponded to 2128 spells. The length of stay by spell is presented in Appendix 6, Table 47; it was 29 days, on average, without any adjustment for spells with days outside the follow-up period. There were 744 spells with admissions before the date of randomisation and 21 spells with discharge after the end of follow-up. The average length of stay by spell, adjusting for days outside the follow-up period, was 27 days. These spells with days outside the follow-up period corresponded to 732 FCEs that started before the randomisation date and 21 FCEs that finished after the end of the 6-month follow-up date. Therefore, the cost of these episodes that spanned outside the follow-up period were adjusted, as explained in Selecting hospital care that was within the trial follow-up.
The costs were calculated at the FCE level (results are shown in Appendix 6, Table 48). The average total cost per FCE was £8698, unadjusted, and £8129, adjusted. The number and cost per FCE by admission type is shown in Appendix 6, Table 49. Most of the FCEs were non-elective inpatient long stays [1798 (67.04%)], with an average cost of £12,122, followed by non-elective inpatient short stays [952 (35.50%)], with an average cost of £10,635.
Table 15 shows the length of stay and the costs per baby, after adjusting for the time outside the 6-month follow-up period (unadjusted results are presented in Appendix 6, Table 50). For the 828 PREVAIL babies for whom the hospitalisation was recorded in HES APC, the average length of stay, per baby, was 69 days and the average cost of hospital admission, adjusted for the length of stay outside the 6-month follow-up period, was £26,332 per baby. This is similar across both PICC types.
Outpatient attendances recorded in Hospital Episode Statistics outpatient
Data preparation
Appendix 5 details the process of data preparation, including Figure 21. A total of 799 PREVAIL babies (92.80% of the PREVAIL trial population) were recorded in the HES outpatient data set, with a total 6425 outpatient appointments. Of these, 19 records (i.e. outpatient appointments) did not have valid data to generate a HRG. The grouper derived HRGs for 6406 appointments, which correspond to 798 PREVAIL babies. A total of 547 appointments were removed because they were flagged as either postponed or cancelled; hence, no cost was incurred. One outpatient appointment was associated with an invalid HRG, and so was deleted. As a result, 5858 outpatient appointments, referring to 784 PREVAIL babies (91.06%), were taken forward for costing.
Intensity, length of stay and cost of care within the follow-up period
All 5858 outpatient appointments were within the follow-up period. The average cost per outpatient appointment was £155, and was similar by PICC type (see Appendix 6, Table 51). Table 15 shows the length of stay and the costs per baby. For the 784 PREVAIL babies for whom outpatient appointments were recorded, the average number of outpatient appointments was 7.47 and the average cost per baby was £1156; both values were similar by type of PICC.
Accident and emergency visits recorded in Hospital Episode Statistics accident and emergency
Data preparation
Appendix 5 details the process of data preparation, including Figure 21. A total of 352 PREVAIL babies (40.88% of PREVAIL trial population) were recorded in HES A&E, with a total of 622 A&E attendances. No observations were deleted in the preparation of the data set for the grouper. The grouper derived valid HRGs for 539 A&E attendances among 320 PREVAIL babies (37.17%).
Intensity, length of stay and cost of care within the follow-up period
All 539 A&E attendances, by 320 PREVAIL babies, were within the follow-up period. The average cost per A&E attendance was £132.64, which was similar across both PICC types. Of the 539 A&E attendances, 293 (54%) led to an inpatient admission and 246 (46%) did not; these values were also similar by PICC type. Table 15 shows the number of A&E attendances and A&E costs per PREVAIL baby recorded in HES A&E. For those 320 PREVAIL babies for whom A&E attendances were recorded, the average number of A&E attendances was 1.68 per baby and the average cost was £223 per baby; both values were similar by PICC type.
The number of babies is the number of PREVAIL babies recorded in each database with hospitalisations within the follow-up period of 6 months after randomisation, which had sufficient data for costing (see Appendix 5, Figures 18–21, for details). The percentage refers to the proportion of PREVAIL babies compared with the analysis population of 861 babies (AM-PICC, n = 430; S-PICC, n = 431).
The length of stay and the costs of the hospital care in the NNU recorded in the NNRD and the hospital care recorded in HES APC were adjusted by the proportion of time outside the trial follow-up window.
Cost of hospital care
To calculate the total cost of hospital care, the files with the cost per baby from each data set were merged into one single file. Of the 861 PREVAIL babies, 33 babies had records of hospital use in the NNRD, but not in HES APC, and 24 babies had record of hospital use in HES APC, but not in the NNRD. The analytical sample comprised the PREVAIL babies with data on the cost of hospital care both in HES APC and in the NNRD. The analytical sample comprises 804 PREVAIL babies with data on the cost of hospital care in HES APC and NNRD.
Table 16 summarises the costs of hospital care in the analytical sample. The average costs were similar by PICC type. The average total cost per baby was £83,473. Most of the costs were incurred in the NNU (£54,047; 64.75% of the total cost of hospital care), followed by hospital care other than intensive care (£26,617; 31.89% of the total cost of hospital care). The cost was similar by PICC type. Some of the costs per database are different between Tables 15 and 16. This is because Table 15 shows the average costs per baby with an admission recorded in each database. For example, 94 babies were recorded in the PICANet database, with an average cost each of £14,458 due to their PICU stay. Conversely, Table 16 shows the average costs per baby in the analytical sample. For example, the average cost of a PICU admission is £1641 over the 6 months’ follow-up for all of the 804 PREVAIL babies who comprise the analytical sample, based on a mean of 0.93 days in PICU per baby.
| Type of hospital care | Total (n = 804) | AM-PICC group (n = 400) | S-PICC group (n = 404) | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Length of stay (days) | ||||||
| NICU (recorded in NNRD) | 66.53 | 36.52 | 66.05 | 36.80 | 67.01 | 36.27 |
| PICU (recorded in PICANet) | 0.93 | 4.19 | 0.90 | 4.37 | 0.97 | 4.00 |
| In total for all hospital admissions (recorded in HES APC) | 69.52 | 37.81 | 68.43 | 36.64 | 70.60 | 38.94 |
| Number of outpatient attendances (recorded in HES outpatient) | 7.03 | 7.70 | 7.22 | 8.16 | 6.85 | 7.23 |
| Number of A&E attendances (recorded in HES A&E) | 0.64 | 1.09 | 0.68 | 1.13 | 0.60 | 1.03 |
| Costs | ||||||
| NICU | £54,047.15 | £34,834.76 | £53,526.62 | £35,110.30 | £54,562.53 | £34,595.59 |
| PICU | £1641.37 | £7417.34 | £1590.98 | £7718.18 | £1691.25 | £7116.19 |
| Other inpatient hospital care | £26,616.92 | £18,268.35 | £26,447.75 | £18,152.15 | £26,784.41 | £18,403.65 |
| Outpatient appointments | £1082.63 | £1027.28 | £1098.27 | £1056.66 | £1067.15 | £998.42 |
| A&E appointments | £84.68 | £146.73 | £89.36 | £147.89 | £80.05 | £145.61 |
| Total cost | £83,472.75 | £50,148.48 | £82,752.99 | £49,738.66 | £84,185.39 | £50,602.54 |
Deaths during the 6-month follow-up period
The HES data sets included date of death according to the Personal Demographics Service. Within the follow-up period of 6 months from date of randomisation, 66 deaths were recorded. The PREVAIL trial CRFs recorded whether death had occurred in the neonatal care unit. The CRFs recorded 69 deaths. Five deaths were recorded in the CRFs that were not recorded in the Personal Demographics Service records (which were linked to HES), and two deaths were recorded in the Personal Demographics Service records but not in the CRFs. In total, 71 deaths occurred. These data are used to inform the economic model (see Probability of death between peripherally inserted central venous catheter insertion and 6 months of age).
Cost-effectiveness of antimicrobial-impregnated versus standard peripherally inserted central venous catheters over a lifetime
Background
The primary objective of the cost-effectiveness analysis was to estimate the long-term costs and health benefits of using AM-PICCs, compared with using S-PICCs, in babies in the NICU. The PREVAIL trial provided data on the risk of BSI, the AM-PICC effectiveness and the hospital costs over 6 months of babies who received AM-PICCs, compared with babies who received S-PICCs. To estimate the costs and health benefits of using an AM-PICC or a S-PICC over a baby’s lifetime, information was needed on the long-term consequences of using each PICC. This information was obtained from the published literature and synthesised in a de novo cost-effectiveness model. This section reports the methods used to develop the cost-effectiveness model and the results of the cost-effectiveness analysis.
The model was developed to evaluate the cost-effectiveness of any intervention for the prevention of infection in neonates in the NNU. For the purposes of the cost-effectiveness model, infection is an umbrella term that includes, not only BSI and clinically serious BSI, as defined in the PREVAIL trial, but also sepsis (indicating BSI with clinical signs of tissue damage). The key assumption of the model was that infection increases the risk of death and of developing neurodevelopmental impairment (NDI) in early childhood. NDI is associated with lower life expectancy, worse health-related quality of life (HRQoL) and higher costs to the NHS. 71 Consequently, babies who had a BSI during their stay in the NNU are expected to experience worse health outcomes and higher costs because of the long-term consequences of infection. Therefore, interventions reducing the risk of infection may be cost-effective, depending on the magnitude of risk reduction and costs.
Methods
Overview
The cost-effectiveness model simulated the costs, life expectancy and QALYs of preterm babies who required a PICC during their stay in the neonatal care unit. Costs were expressed in Great British pounds at a 2016 price base, from the perspective of the NHS. Future costs and QALYs were discounted at 3.5% per annum. 72 The time horizon was the babies’ predicted lifetime from birth. The model was built in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA).
Population and subgroups
Gestational age is strongly associated with long-term costs and health outcomes. 71 Therefore, the target population was considered in terms of subgroups defined by gestational age. The gestational age cut-off points were based on Mangham et al. ,71 defined as extremely preterm babies (< 28 weeks), very preterm babies (28–32 weeks) and preterm (33–37 weeks). Given that only 12% of the PREVAIL babies were born at ≥ 33 weeks’ gestation, and the advice by clinical collaborators that the babies who need a PICC of 1 French gauge are unlikely to be representative of the population of babies born at 33–37 weeks, the target population for the cost-effectiveness analysis was restricted to babies born at ≤ 32 weeks’ gestation.
Model conceptualisation
Consequences of infection in preterm neonates
A 2016 study on the clinical effectiveness and cost-effectiveness of AM-PICCs in children reviewed the existing cost-effectiveness studies and found no published reports on the effectiveness in preterm babies. 73 Therefore, a systematic review on cost-effectiveness models in interventions that reduce the risk of BSI in preterm babies was not conducted.
The association between infection in preterm neonates and increased risk of NDI and death is supported by various observational studies. 10,74–77 Published meta-analyses78–80 estimated that infection (specifically sepsis) significantly increases the risk of post-discharge death (RR 2.05, 95% CI 1.28 to 3.28)80 and NDI, which manifests in early childhood (various definitions pooled, OR 2.09, 95% CI 1.65 to 2.65). 78–80 NDI may develop into physical and mental impairment in childhood, adolescence and adulthood, leading to a reduction in HRQoL and life expectancy, and a greater need for health and social care. 9,10,81,82
The long-term outcomes of sepsis have been the subject of various studies, as have the consequences of infection, which is defined as an event requiring the use of antibiotics for at least 3–5 days. 10,78–80 No studies were found that used the same definition of BSI as used in the PREVAIL trial. The PREVAIL trial did collect data on clinically serious BSIs; a clinically serious BSI was defined as a BSI for which a baby is treated for > 72 hours with intravenous antibiotic, or dies during treatment (see section 4.1 of the trial protocol59). Consequently, clinically serious BSI was taken as the key intermediate outcome for the cost-effectiveness model, rather than BSI. In practice, however, there was little difference between the incidence of BSI and clinically serious BSI in the PREVAIL trial (see Chapter 2, Primary outcome). Hence, for consistency with the rest of the report, the term BSI is used throughout, although it refers to clinically serious BSI in this chapter.
Model design
The cost-effectiveness model simulated long-term outcomes in preterm babies by assuming that the occurrence of a BSI during the stay in the NNU increases the risk of developing NDI and increases the risk of death at 2 years of age. The model assumed that having a BSI during the stay in the NNU has no direct consequences after 2 years of age. After 2 years of age, the model was driven by the NDI state that children experience at 2 years of age, which can improve or deteriorate and is associated with an increased risk of death compared with the general population.
In the cost-effectiveness model, NDI was defined as a composite outcome encompassing different types of impairment, namely visual, hearing, mobility and cognitive. 83 Each item is assigned a level ranging from no impairment to severe impairment, following standardised paediatric assessment tests; the most serious level of impairment recorded defines the overall NDI classification for an infant. This classification was previously used and validated as part of the Victorian Infant Collaborative Study Group (VICSG)83–85 and in the UK economic analysis by Mangham et al. 71
A summary of the main model assumptions is available in Appendix 11, Box 1.
Model structure
Predicting outcomes in early childhood
The model included a short- and a long-term component. The short-term component was a decision tree. Babies enter the model at the time of PICC insertion. The model estimated the proportion of children alive at 2 years of age, and their distribution by level of NDI (none, mild, moderate or severe) (Figure 6). PICC type (AM-PICC or S-PICC) determines the probability of BSI and costs, as observed in the PREVAIL trial. BSI is assumed to increase the probabilities of death and of experiencing NDI.
FIGURE 6.
Model structure for predicting outcomes in early childhood. The square denotes the decision: use either an AM-PICC or a S-PICC. The circle denotes a probability (or chance) node. The triangle denotes when the structure is the same in both arms. The diagram details the parameter inputs; their numbers correspond to the numbers in Table 17.
Predicting lifetime costs and health outcomes beyond 2 years of age
The long-term component of the model simulated the lifetime health outcomes and costs given the children’s NDI levels. It assumed that previous BSI does not influence longer-term lifetime costs and health outcomes. The model was a Markov model,86 based on the model depicting the costs of prematurity by Mangham et al. 71 Children enter the model at 2 years of age in one of the NDI states, according to the proportion of babies computed by the decision tree (Figure 7). At each cycle, children can transition between NDI states or die. The transitions represent the improvement or deterioration in the neurodevelopmental state of the child, as well as inaccuracies in the assessment, which might become apparent over time. After reaching 8 years of age, children were assumed to no longer transition between NDI states; therefore, the only transition after age 8 years is to the absorbing death state. The risk of death depends on both age and NDI state.
FIGURE 7.
Model structure for predicting lifetime costs and health outcomes. The arrows depict the possible transitions between model states in each cycle.
Model inputs for predicting outcomes in early childhood
Table 17 presents the short-term model parameters used to predict the outcomes at 2 years of age. Each parameter is shown in Table 17 and discussed in detail in the following text. The base case refers to the primary analysis.
| Parameter | Value (95% CI); distribution | Source |
|---|---|---|
| Effect of AM-PICC on the probability of a BSI occurring | ||
| (1) RR of AM-PICC vs. S-PICC on BSI; same for both gestational age subgroups | 1.06 (0.70 to 1.60); log-normal | PREVAIL trial: calculated from the number of babies who had a clinically serious BSI in the PREVAIL trial |
| Effect of BSI on NDI and death | ||
| (2) RR of the effect of BSI on death at 6 months; same for both gestational age subgroups | Base case: 1 (fixed) | Assumed that BSI has no causal effect on death at 6 months |
| (3) OR of BSI on NDI index at 2 years of age; same for both gestational age subgroups | 1.51 (1.33 to 1.70); log-normal | Meta-analysis of Stoll et al.10 and Schlapbach et al.74 |
| (4) OR of BSI on probability of death at 2 years of age; same for both gestational age subgroups | 2.74 (1.43 to 5.24); log-normal | Meta-analysis of Schlapbach et al.74 and Bassler et al.76 |
| Baseline probabilities | ||
| (5) Probability of having a BSI with S-PICC | ||
| Gestational age ≤ 27 weeks | 0.14 (0.09 to 0.20); beta | PREVAIL trial: proportion of babies who had a BSI who were allocated to S-PICC, by gestational age. For consistency with the costs, the probabilities refer to the subpopulation with gestational age of ≤ 32 weeks for whom costs of hospital care could be calculated |
| Gestational age ≥ 28 weeks | 0.04 (0.02 to 0.08); beta | |
| (6) Probability of death between PICC insertion and 6-months if BSI had not occurred | ||
| Gestational age ≤ 27 weeks | 0.20 (0.16 to 0.23); beta |
|
| Gestational age ≥ 28 weeks | 0.03 (0.03 to 0.04); beta | |
| (7) Probability that children who did not have a BSI during PICC use die between 6 months and 2 years | ||
| Gestational age ≤ 27 weeks | 0.02 (0.01 to 0.03); beta | Mangham et al.71 |
| Gestational age ≥ 28 weeks | 0.01 (0.00 to 0.02); beta | |
| (7) Probability that children aged 2 years who did not have a BSI during PICC use are diagnosed with NDI | ||
| Gestational age ≤ 27 weeks | 0.45 (0.42 to 0.49); Dirichlet | Mangham et al.71 |
| Gestational age ≥ 28 weeks | 0.26 (0.25 to 0.28); Dirichlet | |
| (8) Distribution by NDI levels, given that NDI was diagnosed (independent of previous BSI during PICC use) | ||
| Mild NDI |
|
|
| Gestational age ≤ 27 weeks | 0.54 (0.51 to 0.58); Dirichlet | |
| Gestational age ≥ 28 weeks | 0.73 (0.70 to 0.76); Dirichlet | |
| Moderate NDI | ||
| Gestational age ≤ 27 weeks | 0.29 (0.28 to 0.30); Dirichlet | |
| Gestational age ≥ 28 weeks | 0.16 (0.15 to 0.17); Dirichlet | |
| Costs | ||
| (9) Difference in cost between AM-PICC and S-PICC | £53.70 | Vygon (UK) Ltd, 2015, personal communication |
| (10) Health-care costs between PICC insertion and 6 months | ||
| Base case | PREVAIL trial: generalised linear model with Gaussian distribution and log-link; explanatory variable is gestational age group. Estimated in the subpopulation with gestational age of ≤ 32 weeks for whom costs of hospital care could be calculated | |
| Gestational age 23–27 weeks | £105,873.47 (£101,444.99 to £110,495.27); gamma | |
| Gestational age 28–32 weeks | £62,255.37 (£54,711.87 to £70,838.93); gamma | |
| (11) Health-care costs between 6 months and 2 years | ||
| Gestational age 23–27 weeks | £5989.17 (£5989.14 to £5994.98) |
|
| Gestational age 28–32 weeks | £3026.17 (£3024.21 to £3028.73) | |
Effect of antimicrobial-impregnated peripherally inserted central venous catheter in preventing bloodstream infection
The effect of AM-PICC in preventing BSI was obtained from the PREVAIL trial: RR 1.06 (95% CI 0.70 to 1.60). As discussed earlier, it refers to the RR of clinically serious BSI, as defined in the PREVAIL trial. It refers to the full trial population because gestational age was not expected to be a treatment effect modifier.
Long-term consequences of bloodstream infection
Systematic reviews of the association between BSI during the NNU stay and NDI were used to identify studies using a similar NDI measure as the study by Mangham et al. ,71 the source of the transition probabilities between NDI levels for the Markov model. In Mangham et al. ,71 NDI was defined as an impairment affecting one of four functional dimensions: motor ability (including cerebral palsy), visual ability, hearing ability or development delay and cognitive ability, based on Doyle et al. 83 (see Appendix 7, Table 52, for details). Therefore, only studies that reported the association between infection during the neonatal stay and impairment including all the aforementioned four dimensions were selected and meta-analysed (see Appendix 7, Table 53, for details). 86
Two studies were identified: Stoll et al. 10 and Schlapbach et al. 74 Stoll et al. 10 is a retrospective study describing the outcomes at 18–22 months of age of 6093 babies in a US registry of very low-birthweight infants born between 1993 and 2001 weighing 401–1000 g at birth. Stoll et al. 10 report an adjusted OR for infection on NDI of 1.50 (95% CI 1.20 to 1.70). Schlapbach et al. 74 report a prospective cohort study in Switzerland of 541 babies born between 2000 and 2007 at 24–27 weeks’ gestational age; the OR for sepsis on NDI is 1.69 (95% CI 0.96 to 2.98). The base case uses the meta-analysed OR using Stoll et al. 10 and Schlapbach et al. 74 at 1.51 (95% CI 1.33 to 1.70; fixed effects; see Appendix 7, Table 55). Both estimates used the ORs, adjusted for differences in observed characteristics using multivariate logistic regression.
As discussed in Deaths during the 6-month follow-up period, 71 deaths were recorded in the PREVAIL trial population, according to the PREVAIL trial CRFs and NHS Digital’s Personal Demographics Service. However, some of these deaths occurred in babies who were born at ≥ 33 weeks’ gestation, who are outside the scope of the economic model. In the modelled population of babies born at ≤ 32 weeks’ gestation, 63 deaths occurred among 735 babies (8.57%). There was no clear evidence that BSI (specifically, clinically serious BSI as defined in the PREVAIL trial) increased the risk of death, controlling for gestational age (see Appendix 7). Furthermore, we found no evidence in the literature that BSI increased the risk of death at 6 months of age. Therefore, the model assumes that BSI does not increase the risk of death at 6 months and the assumption is tested in the sensitivity analysis.
Two studies were identified (see Appendix 7, Table 54): Schlapbach et al. ,74 which also provided the link to NDI, and Bassler et al. 76 Stoll et al. 10 did not examine the probability of death. Schlapbach et al. 74 report that the OR for infection on death before 2 years of age (conditional on survival past 36+0/7 weeks) is 5.38 (95% CI 0.55 to 52.07). The study by Bassler et al. 76 is a Canadian prospective cohort study in 944 babies born between 1996 and 1998 weighing 500–999 g at birth who participated in a Trial of Indomethacin Prophylaxis in Preterms. 88 The OR for infection on death before 18 months of age, conditional on survival to 36 weeks, was 2.57 (95% CI 1.31 to 5.07). 76 Schlapbach et al. 74 and Bassler et al. 76 do not report these ORs. Instead, the ORs were calculated for the present study using the count data reported in these studies. 74,76 The base case uses the meta-analysed OR using Schlapbach et al. 74 and Bassler et al. 76 This OR is 2.74 (95% CI 1.43 to 5.24; fixed effects; see Appendix 7, Table 56).
Probability of bloodstream infection with standard peripherally inserted central venous catheter
The probability of BSI with a S-PICC was obtained directly from the PREVAIL trial data: 0.14 (95% CI 0.09 to 0.20) and 0.04 (95% CI 0.02 to 0.08) for babies with gestational age at birth of 23–27 weeks and 28–32 weeks, respectively (see Appendix 8, Table 57).
Probability of death between peripherally inserted central venous catheter insertion and 6 months of age
The probability of death between PICC insertion and 6 months of age for babies with gestational age of 23–27 and 28–32 weeks was obtained from Santhakumaran et al. ,87 which reports the outcomes from the English NNRD for the years 2008–14. The PREVAIL trial data were not used owing to the small sample size for each gestational age subgroup (see Appendix 8, Tables 58 and 59). The probability of death without prior BSI was calculated as 0.20 (95% CI 0.16 to 0.23) and 0.03 (95% CI 0.03 to 0.04) for babies with gestational age at birth of 23–27 and 28–32 weeks, respectively (see Appendix 8, Tables 60 and 61, for details).
Probability of death between 6 months and 2 years of age
The probability of death between 6 months and 2 years of age, without prior BSI, was based on Mangham et al. :71 0.02 (95% CI 0.01 to 0.03) and 0.01 (95% CI 0.00 to 0.02) for babies with gestational age at birth of 23–27 and 28–32 weeks, respectively. Mangham et al. 71 estimated the probability of death for children born preterm between hospital discharge and 2 years of age using individual-level data from three cohort studies: the first EPICure cohort from the UK and Ireland in 1995,89,90 the 1991–2 VICSG cohort in Australia83 and the Oxford Record Linkage Study from 1990 to 1993. 91–93 Hospital discharge in the Mangham et al. 71 study was assumed to occur at 6 months for the purposes of our model, and refers to a mixed population with and without prior BSI.
Probability that children suffered neurodevelopmental impairment who had not suffered prior bloodstream infection
Evidence is available suggesting that NDI can occur even without prior BSI being diagnosed. 10 The occurrence of BSI increases the risk of impairment over and above this baseline level associated with prematurity per se. Three alternative sources for the probability that NDI occurred were identified. 10,71,74 Stoll et al. 10 and Schlapbach et al. 74 report the probability of NDI separately for babies with and babies without prior BSI. 13,94 Mangham et al. 71 report the probability of NDI occurring, the distribution over NDI level and the probability of progression of NDI by gestational age for a mixed population (with and without BSI). 71 Although Mangham et al. 71 refers to the probability of NDI occurring for a mixed population, it was preferred because it provides the probabilities by gestational age subgroup. Hence, the probability of NDI without prior BSI in the model is 0.45 (95% CI 0.42 to 0.49) and 0.26 (95% CI 0.25 to 0.28) for babies with gestational age at birth of 23–27 and 28–32 weeks, respectively.
Distribution of neurodevelopmental impairment by severity level
In the absence of evidence to the contrary, BSI during the neonatal stay was assumed not to change the probability of having a mild, moderate or severe NDI, given that some level of NDI has occurred. NDI encompasses four different outcomes, as defined by Doyle et al. :83 cerebral palsy (classified according to Kitchen et al. 95), blindness (visual acuity of < 20/200 in the better eye), deafness (hearing loss requiring amplification) and developmental delay (Mental Developmental Index, of the Bayley Scales of Infant Development, score of < –1 SD relative to the control group mean). The scale classifies each of these outcomes on a scale: no, mild, moderate or severe NDI. Doyle et al. 83 use the worst recorded severity across the four outcomes as the reference to define the overall level of impairment in an infant. The same scale was subsequently adopted by the Mangham et al. 71 study and, as a result, translates in the current model when babies are assumed to be assigned a level of impairment following a neurological assessment. The proportion of babies at each NDI level, given that NDI was diagnosed, was 0.54 (95% CI 0.51 to 0.58) and 0.29 (95% CI 0.28 to 0.30) for mild and moderate NDI, respectively, for babies with gestational age at birth of 23–27 weeks, and 0.73 (95% CI 0.70 to 0.76) and 0.16 (95% CI 0.15 to 0.17) for mild and moderate NDI for babies with gestational age at birth of 28–32 weeks.
Costs between peripherally inserted central venous catheter insertion and 2 years of age
The cost of S-PICCs and AM-PICCs was provided by their manufacturer [Vygon (UK) Ltd]; each AM-PICC is £53.70 more costly than a S-PICC [Vygon (UK) Ltd, personal communication].
The costs between PICC insertion and 6 months were estimated from the PREVAIL trial data linked to the NNRD; PICANet; and HES APC, outpatient and A&E (see Cost of hospital care). Costs were estimated using a generalised linear model with a Gaussian distribution and log link, controlling for gestational age at birth, survival and BSI status across different specifications (details can be found in Appendix 9, Tables 64 and 65). Base-case estimates amounted to £105,873.47 (95% CI £101,444.99 to £110,495.27) and £62,255.37 (95% CI £54,711.87 to £70,838.93) for gestational age at birth of 23–27 weeks and 28–32 weeks, respectively.
The costs between 6 months and 2 years of age were based on the hospital admissions of babies aged between 6 months and 2 years by gestational age and valued with 2016 NHS reference costs. 69 Estimated costs amounted to £5989.17 (95% CI £5983.44 to £5994.98) for babies born between 23 and 27 weeks of gestation and to £3026.43 (95% CI £3024.21 to £3028.73) for babies born between 28 and 32 weeks of gestation (see Appendix 9, Table 68). Details are in Appendix 9, Tables 66–68.
Model inputs for predicting outcomes beyond 2 years
The parameter inputs for the long-term component of the model include the transition probabilities between NDI states or death, and the respective costs and utilities. Each parameter is shown in Table 18 and discussed in detail in the following text.
| Parameter | Value (95% CI); distribution | Source |
|---|---|---|
| HRQoL values (utilities) | ||
| HRQoL value with no NDI | 0.96 (0.94 to 0.97); beta | Baseline HRQoL from Petrou et al.96 It also corresponds to the utility value for non-impaired babies as their decrement was set at 0 (fixed) |
| Reduction in HRQoL due to mild NDI | 0.18 (0.14 to 0.31); gamma | Petrou et al.96 HRQoL decrement: 0.179 (SE 0.042) |
| Reduction in HRQoL due to moderate NDI | 0.30 (0.24 to 0.46); gamma | Petrou et al.96 HRQoL decrement: 0.298 (SE 0.055) |
| Reduction in HRQoL due to severe NDI | 0.56 (0.44 to 0.77); gamma | Petrou et al.96 HRQoL decrement: 0.558 (SE 0.084) |
| Annual costs between ages 2 and 10 years | ||
| No NDI | £388 (£285 to £509); gamma | Petrou et al.;97 inflated to 2016 prices |
| Mild NDI | £753 (£584 to £946); gamma | Petrou et al.;97 inflated to 2016 prices |
| Moderate NDI | £814 (£560 to £1063); gamma | Petrou et al.;97 inflated to 2016 prices |
| Severe NDI | £1487 (£1096 to £1943); gamma | Petrou et al.;97 inflated to 2016 prices |
| Annual costs from age 11 years | ||
| No NDI | £686 (£440 to £993); gamma | Petrou et al.;96 inflated to 2016 prices |
| Mild NDI | £987 (£782 to £1222); gamma | Petrou et al.;96 inflated to 2016 prices |
| Moderate NDI | £1252 (£933 to £1624); gamma | Petrou et al.;96 inflated to 2016 prices |
| Severe NDI | £1976 (£1411 to £2648); gamma | Petrou et al.;96 inflated to 2016 prices |
| HRs for mortality for all gestational age subgroups | ||
| Type of motor impairment: moderate | 1.51 (0.71 to 3.24); log-normal | Reid et al.98 – baseline: mild impairment |
| Type of motor impairment: severe | 6.21 (3.28 to 11.77); log-normal | Reid et al.98 – baseline: mild impairment |
| Type of cognitive impairment: mild/moderate | 1.11 (0.62 to 1.97); log-normal | Reid et al.98 – baseline: no impairment |
| Type of cognitive impairment: severe/profound | 3.01 (1.74 to 5.22); log-normal | Reid et al.98 – baseline: no impairment |
| Vision impairment: blind | 0.94 (0.58 to 1.53); log-normal | Reid et al.98 – baseline: not blind |
| Hearing impairment: deaf | 2.61 (1.44 to 4.74); log-normal | Reid et al.98 – baseline: not deaf |
Transitions between neurodevelopmental impairment states or death
The 1-year transition probabilities were computed from the counts of babies at each level of NDI from the 1991–2 cohort of the VICSG observed at 2, 5 and 8 years of age in Mangham et al. 71 (see Appendix 8, Tables 62 and 63). 71
The transitions between NDI states stop once a child reach 8 years of age, which corresponds to the last time point for the transition probabilities from Mangham et al. 71 As far as we are aware, no data are available on progression beyond this age. This assumption of no progression beyond 8 years of age was confirmed with the PREVAIL trial clinical team. From this age onwards, the cohort in the model is subject only to the probability of death, which depends on their NDI level.
The probability of death was obtained from the UK lifetables 2013–15,99 to which the excess risk due to NDI is added. The additional risk of death by NDI level was obtained from Reid et al. 98
The study by Reid et al. 98 was a prospective population-based study including 3507 individuals with cerebral palsy related to factors in the antenatal, perinatal or neonatal period born in the state of Victoria (Australia) between 1970 and 2004, and identified from the Victorian Cerebral Palsy Register. It assesses the impact of clinical variables, including motor, visual, hearing and intellectual impairment, on mortality. The classification system is similar between this study and the VICSG one, allowing for the extrapolation of the adjusted HRs from the Reid et al. 98 study to the current model. Each impairment level from the Doyle et al. 83 study can include up to four health outcomes with different level of severity: blindness, deafness, developmental delay and cerebral palsy. For the purpose of this study, we matched each of these outcomes to the covariates used in the Cox regression model performed by Reid et al. ,98 with levels of the categorical covariates corresponding to different level of impairment. For each level of impairment (no, mild, moderate, severe), the largest HR across the health outcomes was then taken as representative of the overall impairment level, and used to adjust the baseline probability of death by NDI level.
Costs and health-related quality of life
Annual costs by NDI level between ages 2 and 10 years were sourced from Mangham et al. ,71 which reports resource use and HRQoL by NDI level collected for the first EPICure study at 6 years of age using a questionnaire completed by the parent(s). 100,101
Annual costs between 11 and 18 years of age and HRQoL by NDI level were obtained from Petrou et al. ,96 who estimated costs and HRQoL at 11 years of age of the children in the first EPICure cohort using a questionnaire filled in by the parent(s) and related it to a NDI classification similar to the one in our model.
Annual costs and HRQoL values after the age of 18 years were assumed to be the same as those used in the model for children aged between 11 and 18 years. These parameters have a minimal impact in the model because of discounting.
Analytical methods
Base case
The model was probabilistic in that the base-case results are computed as the mean costs and QALYs over 10,000 Monte Carlo simulations. 86 Cost-effectiveness was evaluated at two cost-effectiveness thresholds: the cost-effectiveness threshold of the National Institute for Health and Care Excellence (NICE)102 at £20,000 per QALY and the empirical estimate of the opportunity cost to the NHS of bearing additional costs, at £13,000 per QALY. Results are presented in terms of incremental net health benefit, calculated as the difference in expected QALYs between the AM-PICC and S-PICC groups and the difference in costs, expressed in terms of QALYs using the NICE cost-effectiveness threshold and the empirical estimate of the opportunity cost to the NHS. 102,103
Cost-effectiveness acceptability curves are presented, which represent the probability that AM-PICCs are cost-effective for each gestational age subgroup over the range of cost-effectiveness thresholds from null to £30,000 per QALY. The probability that AM-PICCs are cost-effective was calculated as the proportion of simulations in which the AM-PICC has the highest net benefit (i.e. it is the cost-effective option).
Sensitivity analysis
A bivariate sensitivity analysis on the effect of the AM-PICC and its additional cost compared with the S-PICC was conducted to determine the maximum acquisition price of AM-PICCs for a given reduction in the risk of BSI. This analysis can help inform future decisions on whether or not to commission more costly interventions that may reduce the risk of BSI.
A univariate sensitivity analysis was conducted to identify which parameters have the greatest impact on the results in addition to the direct effect and cost of AM-PICCs versus S-PICCs. For this, all parameters predicting the outcomes in early childhood were varied across the extremes of their 95% CIs.
Scenario sensitivity analyses were conducted to understand the impact of the key assumptions (see Appendix 11, Table 69). This includes assuming that BSI has no effect on the risk of NDI or of death, stopping backward transitions from the severe NDI state, employing 3-year transition probabilities, allowing for transitions between NDI states until 18 years of age, applying the age and sex decrement on HRQoL and removing the increased risk of death by NDI level. 104 Additional scenarios regarding the effect of BSI on death and costs before 6 months were also implemented (see Appendix 11, Table 70). One scenario used linked data from the PREVAIL trial to calculate an unadjusted RR of BSI on death before 6 months (2.51, 95% CI 1.44 to 4.38); see Appendix 8, Application to the Model. Two scenarios were tested to consider the possibility that BSI and death before 6 months of age have an impact on the hospital cost over 6 months.
Model validation
Model acceptability and face validity were ascertained during a series of meetings with members of the PREVAIL team. The model underwent quality control to ensure internal consistency and external validity following the recommendations of Vemer et al. 105 This is described in Appendix 10.
Results
Predicting outcomes in early childhood
Table 19 shows the distribution of children at 2 years of age by NDI level and gestational age group, according to S-PICC or AM-PICC allocation. The outcomes are similar between PICC types, although there is a small disadvantage for AM-PICCs given their marginally higher risk of developing BSI, which is not statistically significant.
| Intervention | NDI at 2 years of age | Gestational age (weeks), mean over the probabilistic simulations (%) (95% CI) | |
|---|---|---|---|
| 23–27 | 28–32 | ||
| S-PICC | No | 41.43 (38.36 to 44.79) | 70.41 (68.71 to 72.06) |
| Mild | 19.80 (18.80 to 21.03) | 18.63 (18.08 to 19.16) | |
| Moderate | 10.56 (9.28 to 11.79) | 4.16 (3.59 to 4.75) | |
| Severe | 6.14 (4.75 to 7.50) | 2.80 (2.29 to 3.34) | |
| Dead | 22.07 (18.08 to 25.39) | 3.99 (3.33 to 5.10) | |
| Total | 100% | 100% | |
| AM-PICC | No | 41.32 (38.19 to 44.76) | 70.38 (68.64 to 72.04) |
| Mild | 19.85 (18.80 to 21.14) | 18.65 (18.09 to 19.19) | |
| Moderate | 10.58 (9.29 to 11.81) | 4.16 (3.60 to 4.76) | |
| Severe | 6.15 (4.77 to 7.52) | 2.81 (2.30 to 3.35) | |
| Dead | 22.10 (18.12 to 25.42) | 4.00 (3.34 to 5.11) | |
| Total | 100% | 100% | |
Predicting lifetime costs and health outcomes
Figure 8 shows the outcomes by NDI level over the children’s expected lifetime. The model predicts that children who are assessed as having no NDI at 2 years of age live to 76 years, on average (95% CI 73 to 78 years). As the NDI level increases, life expectancy reduces. Children who are assessed as having a severe NDI at 2 years of age are predicted to live to 61 years (95% CI 47 to 74 years).
FIGURE 8.
Costs and health outcomes by NDI level over expected lifetime. The NHS costs and the lifetime QALYs accrued in the future are discounted at 3.5% in line with current guidelines. The life expectancy from PICC insertion is not discounted. The vertical lines indicate the 95% CIs. Adapted from Grosso et al. 106 © Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. Minor formatting changes have been made.
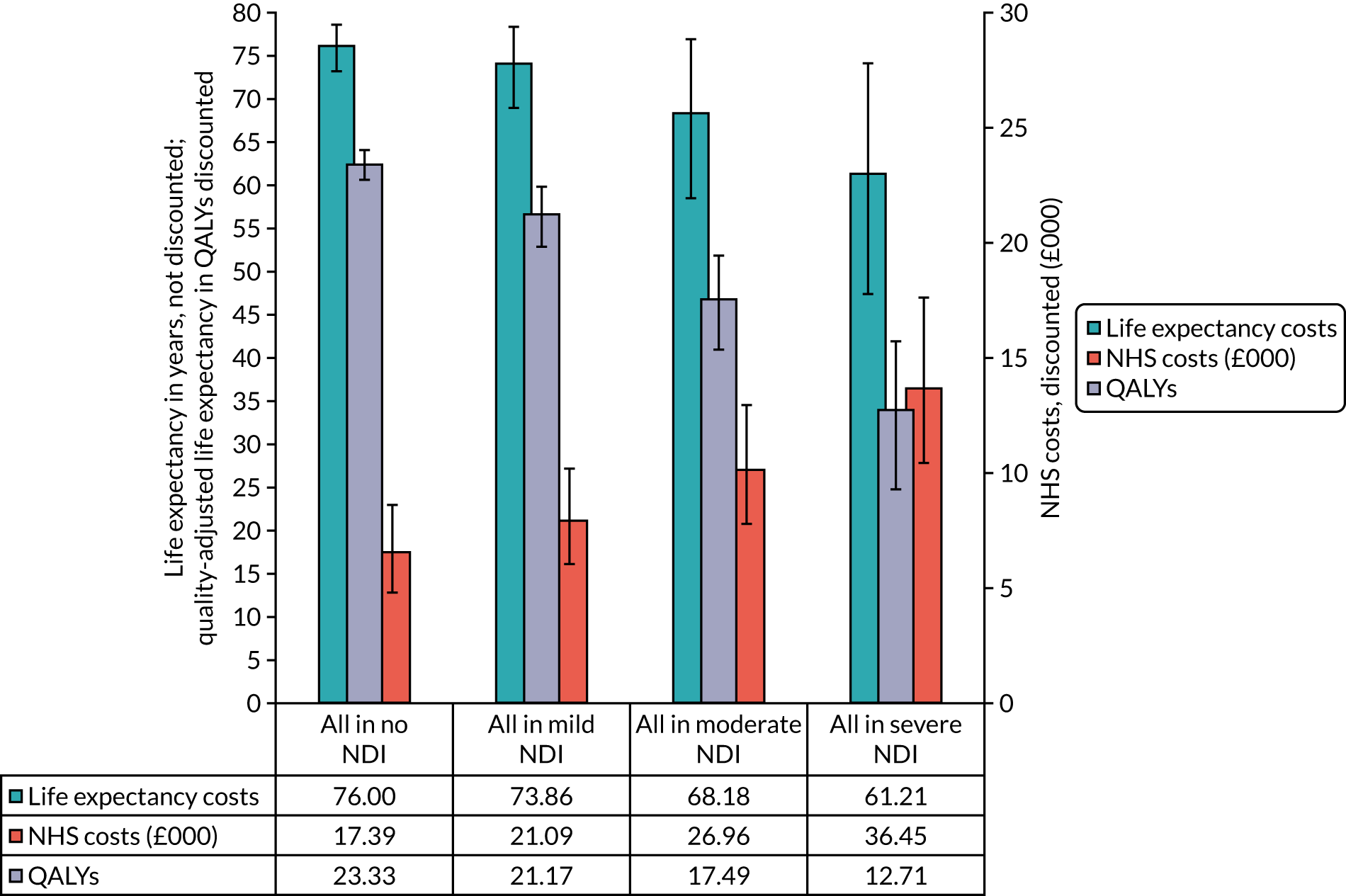
The differences in costs and health outcomes between mild NDI and no impairment are £3690 lower costs and 2.16 additional QALYs. This means that if one child has mild NDI, then, on average, and compared with having no NDI, the child loses 2.16 QALYs over their lifetime and costs an additional £3690 to the NHS. If a QALY is valued at £20,000 by the NHS, avoiding mild NDI in one child warrants £46,890 (2.16 QALYs × £20,000 + £3690) in investment by the NHS.
Cost-effectiveness of antimicrobial-impregnated versus standard peripherally inserted central venous catheters
Table 20 shows the cost-effectiveness results. The AM-PICC is not cost-effective when compared with the S-PICC; rather, the S-PICC dominates at both gestational ages (23–27 and 28–32 weeks). The AM-PICC increases costs by a small extent, to £54.85 (95% CI £25.95 to £89.12) for babies born at 23–27 weeks of gestation and to £54.81 (95% CI £48.21 to £64.28) for those born at 28–32 weeks of gestation. The AM-PICC also reduces health outcomes by a small extent, at –0.01 (95% CI –0.09 to 0.04) and 0.00 (95% CI –0.01 to 0.01), for babies born at 23–27 and 28–32 weeks of gestation, respectively. The cost-effectiveness of an AM-PICC versus a S-PICC can be synthesised in terms of its incremental net benefit, given the cost-effectiveness threshold. 103 At a cost-effectiveness threshold of £13,000 per QALY, the incremental net benefit of AM-PICCs versus S-PICCs is –0.01 QALYS (95% CI –0.09 to 0.04 QALYs) and –0.01 QALYs (95% CI –0.02 to 0.00 QALYs) for babies with gestational age at birth of 23–27 and 28–32 weeks, respectively. This means that using the AM-PICC has a small, non-significant detrimental effect on health outcomes, given its direct impact on babies’ health outcomes and its indirect impact on the health-care system as a result of its additional costs.
| Cost-effectiveness results | Gestational age at birth (weeks), mean (95% CI) | |
|---|---|---|
| 23–27 | 28–32 | |
| AM-PICC | ||
| Total costs (£) | 127,182.65 (120,983.15 to 133,919.12) | 83,588.20 (77,048.17 to 90,839.31) |
| Total health outcomes (QALYs) | 16.48 (15.41 to 17.59) | 21.46 (20.67 to 22.17) |
| S-PICC | ||
| Total costs (£) | 127,127.79 (120,935.61 to 133,866.31) | 83,533.39 (76,993.85 to 90,784.10) |
| Total health outcomes (QALYs) | 16.49 (15.44 to 17.60) | 21.46 (20.67 to 22.17) |
| AM-PICC vs. S-PICC | ||
| Difference in costs (£) | 54.85 (25.95 to 89.12) | 54.81 (48.21 to 64.28) |
| Difference in health outcomes (QALYs) | –0.01 (–0.09 to 0.04) | 0.00 (–0.01 to 0.01) |
| Incremental net health benefit at £13,000 per QALY [probability that AM-PICC is cost-effective] | –0.01 (–0.09 to 0.04) [0.32] | –0.01 (–0.02 to 0.00) [0.09] |
| Incremental net health benefit at £20,000 per QALY [probability that AM-PICC is cost-effective] | –0.01 (–0.09 to 0.04) [0.34] | –0.00 (–0.02 to 0.00) [0.15] |
For babies born at 23–27 weeks’ gestation, the probability that the AM-PICC is cost-effective is < 0.40, irrespective of the cost-effectiveness threshold; for babies born at 28–32 weeks’ gestation, it remains < 0.20 as the cost-effectiveness threshold asymptotes to £30,000 per QALY. The probability of cost-effectiveness reflects the uncertainty in the parameter estimates, particularly the large CI around the RR of AM-PICCs versus S-PICCs in preventing infection.
Sensitivity analysis
The sensitivity analysis results are presented in detail in Appendix 11. The key results are discussed in this section.
Bivariate sensitivity analysis: effectiveness and price
At the current price, the minimum level of effectiveness at which the AM-PICC is cost-effective, at a cost-effectiveness threshold of £20,000 per QALY, is a relative reduction of 3% in the risk of BSI for babies with gestational age at birth of 23–27 weeks, that is a maximum RR of 0.97. For babies with gestational age at birth of 28–32 weeks, the minimum reduction is of 15%, that is a maximum RR of 0.85. At the cost-effectiveness threshold of £13,000 per QALY, the minimum relative reduction is 4% and 20% for babies with gestational age at birth of 23–27 and 28–32 weeks, respectively (Figure 9).
FIGURE 9.
Maximum acquisition price per AM-PICC effectiveness (gestational age at birth of 23–27 weeks). Reproduced from Grosso et al. 106 © Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. Minor formatting changes have been made.
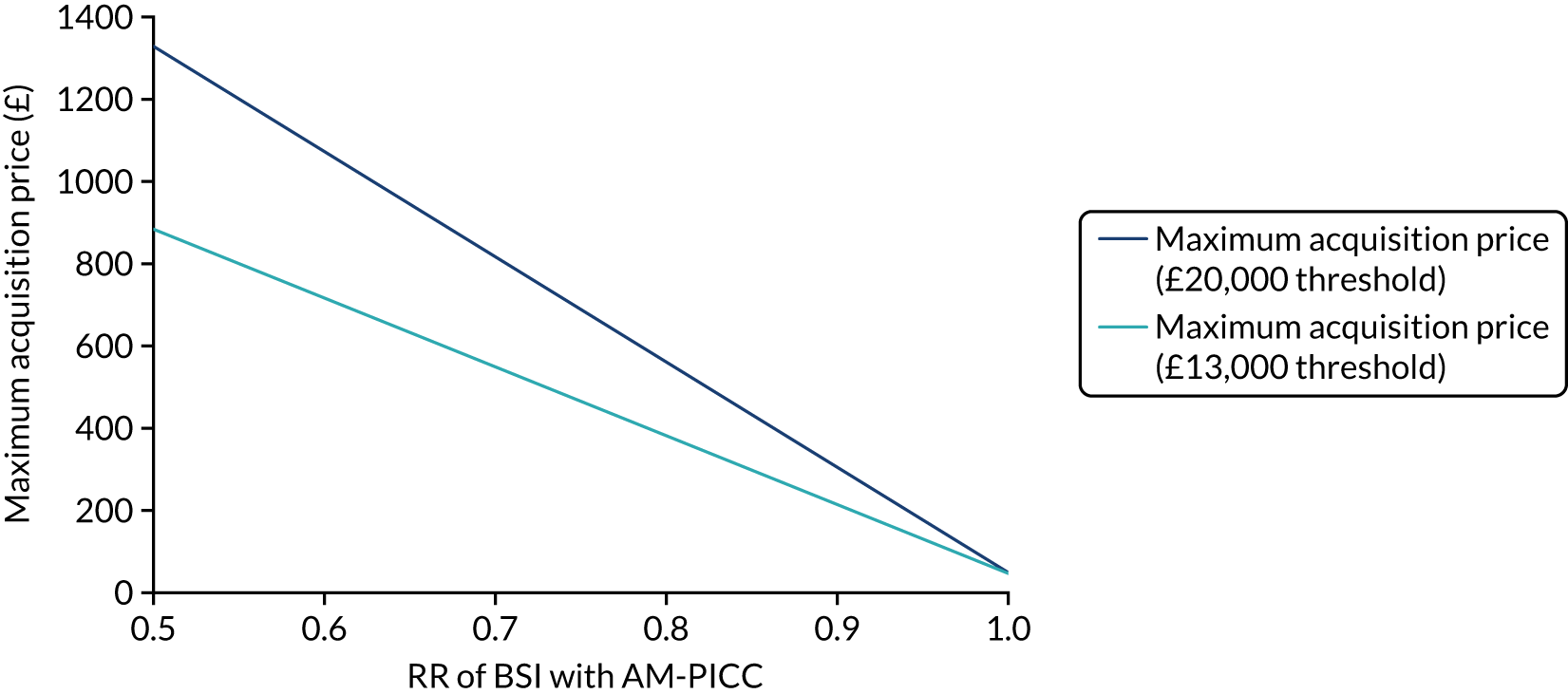
The maximum acquisition price would increase if the effectiveness increased. For example, if the AM-PICC reduced 5% of BSI cases (RR 0.95), the maximum acquisition price at a cost-effectiveness threshold of £20,000 per QALY would be £165, if used solely in babies born at 23–27 weeks’ gestation, or £65 in babies born at 28–32 weeks’ gestation. At a cost-effectiveness threshold of £13,000 per QALY, and for the same effectiveness, the maximum price would be £120 and £55 if used solely in the gestational age groups 23–27 and 28–32 weeks, respectively. At the prices currently charged for the AM-PICC, and at a cost-effectiveness threshold of £20,000 per QALY, the AM-PICC would have to reduce 2% and 15% of cases to be cost-effective for babies born at 23–27 and 28–32 weeks’ gestation, respectively. At a cost-effectiveness threshold of £13,000 per QALY, the AM-PICC would have to reduce at least 3% and 20% of cases to be cost-effective for babies born at 23–27 and 28–32 weeks’ gestation, respectively.
Complete results of this sensitivity analysis are presented in Appendix 11, Tables 71 and 72.
Univariate sensitivity analysis
For both gestational age groups considered, the key cost-effectiveness driver was the RR of the AM-PICC in preventing BSI (see Appendix 11, Figures 23 and 24).
Scenario analysis
The AM-PICC was found to be not cost-effective (i.e. negative incremental net benefit), irrespective of the scenario implemented (see Appendix 11, Table 73). In most scenarios, the incremental net benefit was similar to the base case. Large decreases in incremental net benefit were observed in the scenarios assuming that BSI increased the risk of death, and, to a smaller extent, in the scenarios assuming different hospital costs depending on whether or not death and BSI had occurred. This happens because the PREVAIL trial indicates that the AM-PICC results in a slightly higher number of BSIs (as per Table 17). In scenarios in which BSI increases the risk of death, the costs are lower because children who die do not incur subsequent hospital costs. However, there is a large loss of QALYs, owing to the increased proportion of babies who die in infancy. In these scenarios, the AM-PICC appears to be more detrimental to babies than in the base-case assumption of no direct effect of BSI on death at 6 months.
Value of information
The VoI analysis aims to estimate the health losses, in QALYs, associated with decision uncertainty about which is the most cost-effective PICC type, whether or not future research is worthwhile, and which parameters should be prioritised for research.
The analysis of the value of implementing the cost-effective PICC type (originally planned as objective 4; see Introduction) was not carried out, as the S-PICC was found to be the cost-effective option. Given that the S-PICC is currently in use in the NHS, its value of implementation is, by definition, null. 61
Methods
Expected value of perfect information for the population
The VoI analysis quantifies the consequences of making the wrong decision (using AM-PICCs or S-PICCs) in terms of the health losses if the wrong intervention is deemed cost-effective, and the investment required to resolve this uncertainty. 107 The expected cost of uncertainty is defined as the expected value of perfect information (EVPI), conceived as the difference between the incremental net benefit if every uncertainty was resolved, and the actual incremental net benefit under current uncertainty. 107 A necessary condition for conducting further research is that the cost of research does not exceed the EVPI. The EVPI is computed with the online Shefflied Accelerated Value of Information (SAVI) tool. 108 The analysis is run for a time horizon of 10 years in the population of preterm babies who require a PICC of 1 French.
Expected value of partial perfect information for the population
An approach similar to EVPI can be employed to determine which model parameters are the most important drivers of uncertainty, which are those for which resolving uncertainty would provide the greatest benefit in terms of making the ‘right’ decision. Expected value of partial perfect information (EVPPI) is defined as the difference in net benefits under perfect and current information, but where the uncertainty is resolved for one (or more) parameters at a time only, and averaging across all possible values for the others. 86 As above, EVPPI provides a measure of the maximum amount a funder should be willing to pay for conducting further research related to a specific parameter (or group of parameters).
Patient population
Based on the analysis of the full NNRD by a member of the PREVAIL trial team, 2212 infants with gestational age of 23–27 weeks and 3888 infants with gestational age of 28–32 weeks were estimated to require a PICC each year. Therefore, the population EVPI is presented as a weighted average of the individual EVPI for the two gestational age subgroups considered, using the number of infants requiring a PICC as the weight for each group.
Time horizon
The EVPI and EVPPI are calculated over a time horizon of 10 years, assuming a constant incident population. The value related to future incident populations was discounted to present values at 3.5% per annum. 72
Results
Expected value of perfect information
The EVPI for the annual population of preterm babies who require a PICC is £274,126, or 13.71 QALYs, at the cost-effectiveness threshold of £20,000 per QALY gained. Over 10 years, the EVPI is £2,359,587.27, or 117.98 QALYs. At a cost-effectiveness threshold of £13,000 per QALY, the EVPI is £156,902.00, or 12.07 QALYs, per year, or £1,350,564.85, or 67.53 QALYs, over 10 years.
Expected value of partial perfect information
The main component of the EVPI is the RR parameter; its EVPPI is 99% of the EVPI of the decision. At the cost-effectiveness threshold of £20,000 per QALY, the yearly EVPPI amounts to £268,430.00 (or 13.42 QALYs); over 10 years, it amounts to £2,310,564.28 (115.53 QALYs). At the cost-effectiveness threshold of £13,000 per QALY, the EVPPI on the RR parameter is £151,490.00 (11.65 QALYs) per year, and amounts to £1,303,974.33 (100.31 QALYs) over 10 years.
Summary of findings
Babies born preterm show a high level of resource use over the first 6 months of life, mainly driven by the length of their stays in NICUs and hospital inpatient care (about 70 days on average). This translates to an average total cost per infant of ≈£84,000, of which ≈£54,000 relates to neonatal care and ≈£27,000 relates to costs due to other hospital care during the hospitalisations. The costs of paediatric intensive care, outpatient care and A&E appointments are much lower in comparison. There is no evidence that the type of PICC has any impact on the intensity of hospital care, as measured by the HRGs, or on the length of hospitalisation over the 6-month follow-up. This is consistent with the results of the PREVAIL trial, in that the type of PICC had no effect on either the primary or the secondary outcomes.
Based on information from the trial and on a review of the external literature, a decision-analytic model was built to determine the cost-effectiveness of AM-PICCs compared with S-PICCs, over a baby’s lifetime. The key assumption of the model was that BSI is associated with an increase in the risk of death and of developing NDI. Under this assumption, an intervention preventing BSI can reduce total NHS costs and result in an increase in life expectancy and associated HRQoL, via a reduction in both death and NDI cases.
Findings from the PREVAIL trial suggest that the AM-PICC increases costs and does not improve quality-adjusted survival over the babies’ lifetimes. Therefore, the AM-PICC is not cost-effective. Results are robust with respect to the key model assumptions, as tested through a series of scenarios and sensitivity analyses, which further highlight the pivotal role of PICC effectiveness in driving the model results. At current prices, it would be enough to reduce the probability of BSI by 3% to make the AM-PICC a cost-effective intervention for babies born at 23–27 weeks’ gestational age.
The VoI analysis suggests that, for the population of preterm babies in England and Wales eligible to receiving the 1-French PICC under evaluation, the EVPPI on the RR is £2,310,564.28 over a time horizon of 10 years. This does not represent the value of a RCT concerning the RR for this parameter; instead, it represents the value of knowing this RR with perfect certainty, which may not be possible with further research. This said, the EVPPI suggests that it is worthwhile to invest in additional research on this parameter, which was the primary outcome of the PREVAIL trial.
Chapter 5 Generalisability analysis
Introduction
The PREVAIL trial recruited 861 babies, over 17 months, from 18 NICUs out of a total of 43 NICUs in England. However, if AM-PICCs were effective and adopted, they would be used in all babies who receive the smallest PICC in neonatal care. This is because hospitals are likely to make a policy decision about which PICCs to use and bulk purchase the same type of PICC for all babies who need one. To make this purchasing decision, clinicians need to decide whether or not the results from the trial are generalisable and applicable, and, if so, what is the absolute risk difference given that the baseline risk of BSI may vary between babies and over time. Generalisability refers to whether or not the babies in the PREVAIL trial were similar to the population from which they were drawn, that is, babies in participating NICUs. Applicability refers to whether or not the relative effect can be applied to all babies who receive PICCs in NNUs in England, that is, babies in LNUs in addition to babies in NICUs.
Results from the PREVAIL trial may not be generalisable to other babies in NICUs or applicable to babies in LNUs for several reasons. First, babies in the PREVAIL trial may be treated differently from other babies receiving PICCs in NICUs or LNUs because of trial participation; therefore, they may have a different risk of BSI. Second, there could be differences in characteristics between participating and non-participating babies (e.g. if babies in the PREVAIL trial have lower gestational ages) that were also related to the effectiveness of the AM-PICC (e.g. if the AM-PICC was more effective in babies of a younger gestational age). In this example, we would not be able to extend the results from the trial to other babies in NICUs and LNUs. Third, if BSI risk factors and, hence, the baseline rate of BSI in babies in the PREVAIL trial differed from other babies receiving PICCs in NICUs and LNUs, the absolute benefit (or risk difference) would be different.
We sought the following evidence to indicate whether or not the relative effect of AM-PICCs was likely to differ between subgroups. First, in post hoc analyses of the PREVAIL trial, the effect of AM-PICCs did not differ between babies with a gestational age of < 28 weeks and babies with a gestational age of ≥ 28 weeks (see Chapter 3, Primary outcome). Second, we compared risk factors for BSI and causative organisms in PREVAIL babies randomised to receive S-PICCs with those of other babies with PICCs in NICUs. The proposed mechanism for CVCs leading to BSI is microbial colonisation of the CVC surface spreading to the bloodstream. Certain organisms are more sensitive to the antimicrobials used to impregnate the AM-PICC, for example CoNS is more susceptible to rifampicin–miconazole impregnation than Enterobacteriaceae. 94 Therefore, differences in the distribution of pathogens between PREVAIL and other babies could result in different effects of AM-PICCs than those seen in the PREVAIL trial.
To determine whether or not the baseline rate of BSI differed in babies in the PREVAIL trial compared with other babies receiving PICCs in NICUs and LNUs, we compared crude and risk-adjusted rates during the PREVAIL trial recruitment period. Differences between these groups could reflect selection to the trial based on unmeasured characteristics or different management of PREVAIL babies, resulting in higher or lower rates of BSI in PREVAIL babies than in other babies in participating NICUs. For example, despite the PREVAIL trial protocol requiring blood samples only when clinically necessary, samples may have been taken more often in PREVAIL babies. This could result in detection of asymptomatic BSI or contaminated blood samples that would otherwise not be detected in practice. Conversely, staff may have followed hygiene protocols more closely for babies enrolled in the PREVAIL trial and, therefore, the risk of BSI could be lower.
We also examined trends in BSI over time. Rates of BSI may have declined since the start of the trial because of improved infection control practices. 12,48 Improvements in CVC care may reduce BSI associated with PICCs, but have less effect on BSI due to other causes. To inform targeting of BSI interventions, we determined the contribution of BSI during PICC use to the overall rate of BSI during intensive or high-dependency care in NICUs and LNUs.
Aims and objectives
We aimed to determine whether or not rates of BSI in PREVAIL trial participants were generalisable and applicable to all babies receiving PICCs in NICUs and LNUs in the NHS in England.
Objectives
-
To determine the generalisability and applicability of the trial results by comparing BSI risk factors, causative organisms and BSI rates among babies in the PREVAIL trial with those of other babies receiving PICCs.
-
To determine the applicability of BSI rates in the PREVAIL trial to rates of BSI in the NHS in England by comparing trends in the rates of BSI in babies receiving PICCs in PREVAIL trial NICUs, non-PREVAIL trial NICUs and LNUs.
-
To provide context through understanding of changes in BSI over time by evaluating trends in rates of BSI per 1000 days of intensive or high-dependency care and per 100 admissions for all babies receiving intensive or high-dependency care in NICUs and LNUs.
-
To determine the contribution of PICCs to the overall rate of BSI per admission during intensive and high-dependency care.
The generalisability study originally aimed to predict the number of BSIs prevented and the reduction in cost to the NHS if AM-PICCs were adopted and used in place of S-PICCs. This was not feasible, because the PREVAIL trial detected no difference in the risk of BSI between AM-PICCs and S-PICCs, and, based on these results, AM-PICCs are unlikely to be adopted. A further reason is that the cost of BSI could not be calculated in the health economics study (see Chapter 4).
Methods
Data sources
There is no single data source to study risk-adjusted rates of BSI in NNUs in England. The NNRD provides clinical data on babies in NICUs. Historically, data on BSI in the NNRD was poorly completed, but entry of data on BSI is improving: 74 NNUs reported entering data on all positive cultures in 2017, compared with 25 in 2016. 16,109,110 PHE operate a national infection surveillance system, known as the Second Generation Surveillance System. We linked these two data sources to create a linked data set of babies in all available NICUs and LNUs (112/124) in England. A combination of deterministic and probabilistic linkage methods were used to link babies recorded in the NNRD to records of BSI reported to the national infection surveillance system during their NNU admission. 111 The linkage process is described in Appendix 12. In brief, deterministic links, based on an exact match on NHS number, were used as reference standards to calculate match weights in probabilistic linkage, based on agreement on postcode prefix, postcode suffix, date of birth, laboratory/hospital and sex. 112 Match weights were summed across each comparison pair and the frequency of the summed weights were examined to determine an upper and lower threshold. Pairs with weights above the upper threshold were classified as links, pairs with weights below the threshold were classified as non-links and pairs between the two thresholds were manually reviewed (using rules presented in Appendix 12, Table 74). Links identified in deterministic and probabilistic linkage were combined to produce a NNRD data set enhanced with BSI from the national infection surveillance.
Three NICUs [3/49 (6%)] and nine LNUs [9/78 (12%)] were excluded from our NNRD data. The number of NNUs changed over time: there were 49 NICUs during the generalisability study period (2010–17), but only 43 during the PREVAIL trial. Two NICUs and eight LNUs were excluded because we did not receive responses to our requests for consent to linkage of their data. One NICU and one LNU were excluded because of poor reporting of BSI in the surveillance data. A further three NICUs and six LNUs had reporting gaps in the BSI surveillance data; we excluded months when reporting was poor, but included periods with good reporting. We did not include any SCBUs and we restricted our analyses of the linked data to babies receiving intensive or high-dependency care, as PICCs are not routinely used in special care. Three LNUs became SCBUs during the trial period; they were removed when they became SCBUs. We obtained data from the NNRD in two extractions, (1) March 2010 to December 2015 and (2) January 2016 to June 2017, which resulted in missing data for babies admitted in the first extract but discharged in the second extract. More than 5% of baby days between September 2015 and December 2015 were missing a discharge date, probably due to missing records in 2016. We therefore excluded admissions during the 6 months between September 2015 and February 2016. We excluded all babies who were missing a discharge date as we were unable to correctly calculate time at risk.
Case definition
We defined BSI as a link to a record of positive blood or CSF culture reported to the national infection surveillance system. Multiple cultures of the same organism from the same baby within 14 days were classified as one episode. We use five terms to describe BSI depending on time at risk (Figure 10; see also Appendix 20, Table 81):
-
BSI during PICC days at risk, which included BSI cultured in samples taken between 1 day after PICC insertion and 2 days after PICC removal.
-
Early-onset BSI (no PICC), defined as BSI in babies aged < 2 days. If a baby had a PICC inserted on the day of birth and a BSI on the following day, the BSI would be classified as during PICC days at risk, not as early onset.
-
Late-onset BSI describes BSI from samples taken between 2 days of age and 2 days after discharge from the NICU or LNU, given that the BSI is not during PICC days at risk.
-
Total late-onset BSI included BSIs that were either during PICC days at risk or late onset.
-
Total BSI included all BSI occurring between birth and 2 days after discharge from the NICU or LNU.
FIGURE 10.
Time at risk for PICC days at risk, early-onset BSI (no PICC) and late-onset BSI (no PICC).
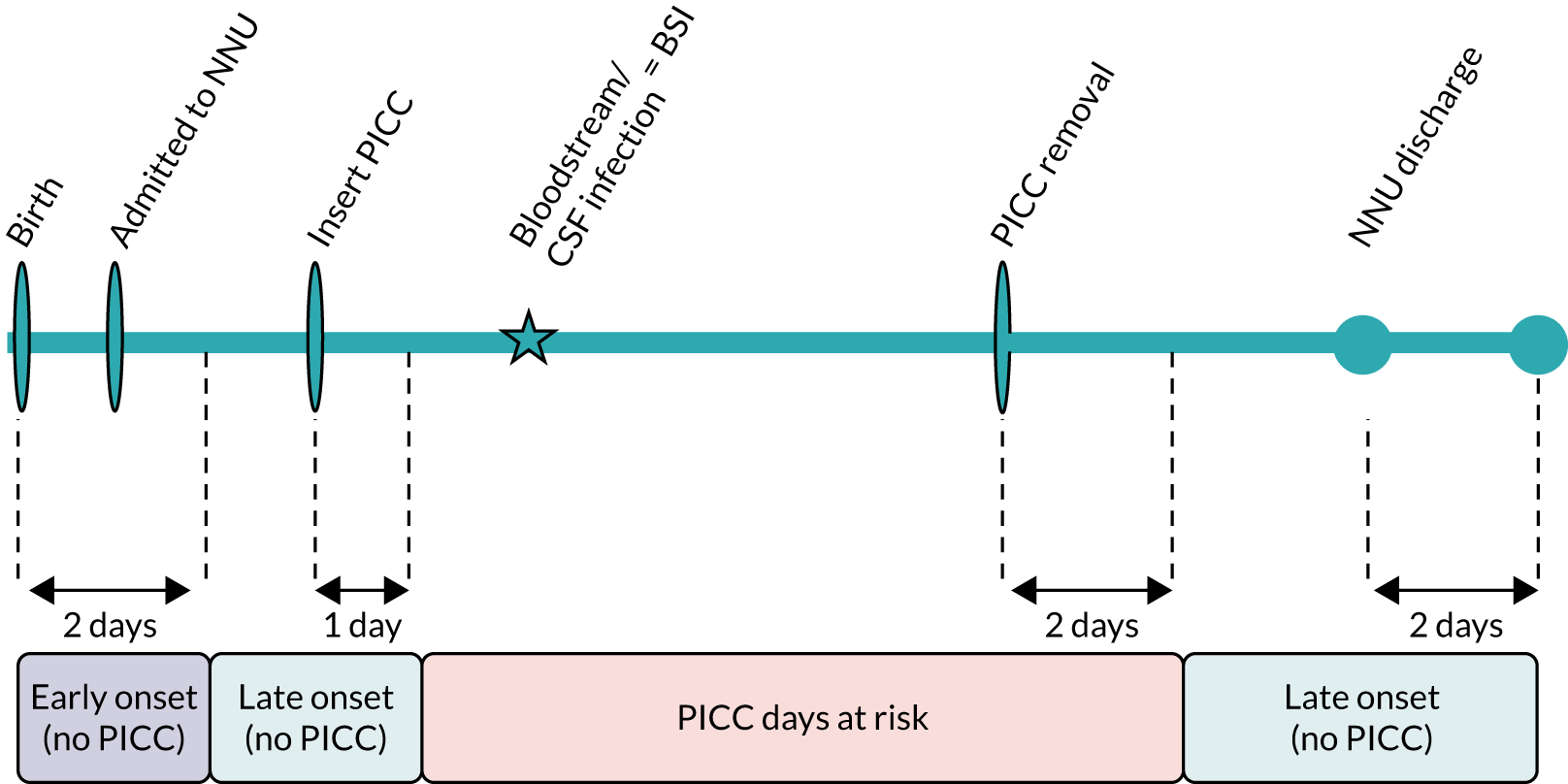
Our definition of PICC days at risk (see Figure 10 and Appendix 20, Table 81) differed slightly from the definition used in the trial (see Figure 2), as we used linked infection surveillance data (not BSI recorded on CRFs in the trial) to ensure comparability between PREVAIL babies and other groups. Surveillance data do not record the exact time of blood or CSF culture sampling, and the NNRD records whether or not a PICC was present each day, but not the time of insertion. Therefore, we used 1 or 2 days, rather than 24 or 48 hours, to define the interval for samples related to the PICC: 1 day after PICC insertion to 2 days after PICC removal (as recorded in the NNRD).
We categorised all organisms recorded in the surveillance data as ‘clearly pathogenic’ or ‘other’ with the guidance of a microbiologist (JG). We used the term ‘clearly pathogenic organisms’ to indicate that a BSI would be diagnosed without the requirement of repeat samples or clinical signs. A full list of clearly pathogenic and other organisms is given in Appendix 13, Boxes 2 and 3. We restricted trend analyses to clearly pathogenic organisms because of a change in the surveillance system in 2014 that resulted in a sharp increase in the reporting of organisms with uncertain clinical significance (e.g. skin commensals) (see Appendix 14, Table 75 and Figure 25).
Identifying babies in the PREVAIL trial
We linked babies who were randomised to receive S-PICCs in the PREVAIL trial to the NNRD. We used deterministic linkage on NHS number or postcode, sex and date of birth. We excluded babies randomised to receive AM-PICCs in the PREVAIL trial and any babies who had no daily records of intensive or high-dependency care, were present only in months excluded because of missing NNRD days between 2015 and 2016, were present only in months excluded because of poor BSI reporting, had missing discharge dates, or had no record of receiving a PICC. We compared the distribution of baseline baby characteristics [gestation, admission to surgical NICU, sex and small for gestational age (birthweight in the < 10th centile for gestation)] using the chi-squared test to determine whether or not there was a significant difference (p < 0.05) between included and excluded PREVAIL babies.
Generalisability and applicability
To determine the generalisability of results of the PREVAIL trial to other babies in NICUs, we compared babies admitted to NICUs who were randomised to receive S-PICCs in the PREVAIL trial with babies who received S-PICCs in the PREVAIL trial NICUs who were not enrolled in the trial, and those in non-PREVAIL trial NICUs, during the recruitment period. Approximately 10% of babies were admitted to more than one NICU or LNU. We evaluated admissions to NNUs, rather than evaluating babies, to ensure that BSI, PICC days and other clinical factors were attributed to the correct NICU or LNU. To determine applicability, we extended the comparison of PREVAIL babies to babies receiving S-PICCs in LNUs during the recruitment period. We compared the prevalence of baby and clinical characteristics, causative organisms and the rate of BSI per 1000 PICC days during the PREVAIL trial recruitment period (August 2015 to January 2017). We used the start and end date specific to each participating NICU, as these dates varied between units. For non-PREVAIL trial NICUs and LNUs, we included the total recruitment period. We also compared trends in rates of BSI per 1000 PICC days from March 2010 to June 2017.
Comparing clinical characteristics in PREVAIL and non-PREVAIL babies
We compared the prevalence of clinical characteristics in the following babies: those receiving S-PICCs who were enrolled in the PREVAIL trial, those who were in a PREVAIL trial NICU but not enrolled in the PREVAIL trial, those who were in a non-PREVAIL trial NICU and those who were in a LNU. The characteristics we included were gestational age at birth (in weeks), sex, small for gestational age, age (in days) at first PICC insertion, PICC days per admission, invasive ventilation days per admission, non-invasive ventilation days per admission and whether or not the NICU provided surgery. We tested for a significant difference (p < 0.05) in the distribution of the characteristics between babies not enrolled in PREVAIL trial NICUs, babies in non-PREVAIL trial NICUs or LNUs and PREVAIL babies, using the chi-squared test.
Comparing causative organisms in PREVAIL and non-PREVAIL trial neonatal intensive care units and local neonatal units
We compared the distribution of causative organisms in PREVAIL trial NICUs, non-PREVAIL trial NICUs and LNUs. In these analyses, we grouped together all babies admitted to NICUs who were participating in the PREVAIL trial, whether or not they were enrolled in the trial, to avoid small cell counts that could be disclosive. We used the chi-squared test to determine whether or not there was a significant difference (p < 0.05) in the distribution of organisms in non-PREVAIL trial NICUs or LNUs, in comparison to PREVAIL trial NICUs.
Comparing rates of bloodstream infection during the PREVAIL trial in PREVAIL and non-PREVAIL babies
We calculated BSI rates per 1000 PICC days for BSI during PICC days at risk during the PREVAIL period separately for all BSI and BSI caused by clearly pathogenic organisms (listed in Appendix 13, Box 2). We calculated risk-adjusted rates of BSI using forward stepwise multilevel Poisson regression to decide which baby characteristics (gestational age at birth in weeks, small for gestational age, age in days at first PICC insertion, invasive ventilation days per admission, non-invasive ventilation days per admission, and whether or not the NICU provided surgery) were significant risk factors for BSI. We considered the risk factor significant if the 95% CI did not include 1. NNU was included as a random effect to account for clustering of admissions in NNUs.
Changes over time in bloodstream infection rates in PREVAIL and non-PREVAIL trial neonatal intensive care units and local neonatal units
To determine trends from March 2010 to June 2017, we calculated the rates of BSI per 1000 PICC days per month of admission for BSI caused by clearly pathogenic organisms during PICC days at risk. We used forward stepwise regression to decide which covariates (as mentioned previously) were significant in the trend analysis, as significant risk factors may differ from the model restricted to the PREVAIL trial period. We included an interaction between NNU group (PREVAIL trial NICU, non-PREVAIL trial NICU and LNU) and month to allow for different trends in BSI rate between the groups. Trends were modelled per admission month, but results are presented per year to aid interpretation.
Trends in rates of bloodstream infection in all babies admitted to neonatal intensive care units and local neonatal units
To contextualise the trial, we extended the analyses to include all babies who received intensive and high-dependency care in NICUs and LNUs, regardless of whether they received a PICC. First, we examined how BSI risk factors had changed from 2010 to 2017 for all babies who received high-dependency or intensive care in LNUs and NICUs. Second, we evaluated trends in total late-onset BSI per 1000 days of intensive and high-dependency care days and per 100 admissions.
Changes over time in bloodstream infection risk factors
We examined how risk factors have changed over time in babies receiving intensive or high-dependency care in NICUs and LNUs from 2010 to 2017 by plotting the proportion of babies admitted to NICUs and LNUs according to gestational age at birth, grouped as: < 26, 26 to < 28, 28 to < 32, 32 to < 37, and ≥ 37 weeks. We plotted the mean number of days of stay, PICC days, central line days, non-invasive ventilation days, invasive ventilation days, and age in days at first PICC insertion by gestational age in weeks in 2010 and 2017. We used two-way analysis of variance (ANOVA) to determine whether or not the mean of each risk factor was significantly different (p < 0.05) by week of gestation in 2010 compared with 2017.
Changes over time in total late-onset bloodstream infection
We evaluated the change over time in rates of total late-onset BSI per 1000 intensive and high-dependency care days and per 100 admissions. In the model per 100 admissions, we included only the first BSI per admission to evaluate whether or not the number of admissions in which a baby develops a BSI has changed over time. We modelled late-onset BSI rates by month of admission restricted to clearly pathogenic organisms. For each model, we used forward stepwise multilevel Poisson regression to identify significant risk factors. We included an interaction between NNU group (PREVAIL trial NICU, non-PREVAIL trial NICU and LNU) and month to allow for different trends in BSI rate between the groups.
The contribution of peripherally inserted central venous catheters to total bloodstream infection
We calculated the proportion of total BSI that was early onset (no PICC), during PICC days at risk or late onset (no PICC). We included BSI caused by all organisms for the period of August 2015 to June 2017. We excluded the period before the start of the PREVAIL trial to give an up-to-date snapshot of recent BSI and to ensure that results were not affected by the change in surveillance methodology in 2014. To determine the contribution of PICCs to the overall rate of BSI per admission, we first calculated the BSI rate per admission for early-onset BSI (no PICC), BSI during PICC days at risk and late-onset BSI (no PICC), for all babies admitted to NICU or LNUs and stratified by gestation at birth (< 32 or ≥ 32 weeks).
Results
Identifying babies in the PREVAIL trial
We identified 423 babies in the NNRD out of the 431 babies recorded as randomised to receive the S-PICC in the PREVAIL trial CRFs. All babies randomised to receive the AM-PICC in the PREVAIL trial were excluded from the generalisability analyses using linked data from the NNRD. We included 269 out of 423 (64%) babies randomised to S-PICCs (see Appendix 21, Figure 26). The reasons for excluding 154 babies were as follows: present only in months excluded because of missing days between 2015 and 2016 (n = 97), present only in months excluded because of poor BSI reporting (n = 24), missing discharge dates (n = 2) and no recording of PICCs (n = 31). Only 16 babies randomised to S-PICCs were recorded as not receiving a PICC in the PREVAIL trial CRFs. Therefore, 15 of the 31 babies with no PICC recorded in the NNRD (4% of the total of 423 babies) had a PICC recorded in the trial CRFs but not in the NNRD. There were no significant differences in the distributions of gestation, small for gestational age or sex between the 269 babies with S-PICCs included in our analysis and the 154 excluded PREVAIL babies for whom we had baseline data available (see Appendix 15, Table 76). Excluded babies were significantly less likely to be in surgical NICUs. Eighteen NICUs participated in the PREVAIL trial; however, only 16 were included in our analysis, as one NICU did not consent to linkage of its data and one was excluded because the corresponding laboratory was a poor reporter during the PREVAIL trial period.
Generalisability and applicability
Peripherally inserted central venous catheters were used for at least 1 day in 8443 admissions involving 8206 babies receiving intensive and high-dependency care during the PREVAIL trial period (August 2015 to January 2017) (see Appendix 25, Figure 35).
Comparing baby and clinical characteristics in PREVAIL and non-PREVAIL babies
Comparing babies in the PREVAIL trial with babies in the same NICUs who were not enrolled in the PREVAIL trial and with babies in other NICUs, more of the PREVAIL babies were born before 32 weeks of gestation, received invasive ventilation and were admitted to a surgical unit (Tables 21 and 22). Admissions to NICUs not in the PREVAIL trial had an earlier median age at first PICC insertion than PREVAIL babies. Use and duration of non-invasive ventilation were significantly higher in NICUs not participating in the PREVAIL trial and in LNUs than among PREVAIL babies (see Table 22). Babies enrolled in the PREVAIL trial were more likely to be born before 32 weeks of gestation, to be older when a PICC was inserted, to retain the PICC for longer, to be small for gestational age and to have more days of ventilation than babies in LNUs (see Tables 21 and 22).
| Characteristic | NICUs (n = 16) participating in the PREVAIL trial | NICUs (n = 28) and LNUs (n = 68) not participating in the PREVAIL trial | ||
|---|---|---|---|---|
| Admissions in the PREVAIL trial | Admissions not in the PREVAIL trial | Admissions to NICU | Admissions to LNU | |
| Total babies (n) | 269 | 1608 | 3745 | 2358 |
| Total admissions (n) | 325 | 1522 | 4051 | 2460 |
| Total PICC days (n) | 3809 | 18,115 | 49,902 | 17,187 |
| Gestation (weeks) | ||||
| < 26, n (%) | 73 (22) | 235 (15) | 776 (19) | 40 (2) |
| 26 to < 28, n (%) | 91 (28) | 195 (12) | 804 (20) | 253 (10) |
| 28 to < 32, n (%) | 136 (42) | 488 (30) | 1320 (33) | 1374 (56) |
| 32 to < 37, n (%) | 17 (5) | 378 (24) | 614 (15) | 610 (25) |
| ≥ 37, n (%) | 8 (2) | 312 (19) | 536 (13) | 183 (7) |
| p-value (chi-squared test)a | < 0.001 | < 0.001 | < 0.001 | |
| Small for gestational age, n (%) | 62 (19) | 251 (16) | 640 (16) | 358 (15) |
| p-value (chi-squared test)a | 0.245 | 0.278 | 0.032 | |
| Male sex, n (%) | 170 (52) | 920 (57) | 2203 (55) | 1361 (55) |
| p-value (chi-squared test)a | 0.094 | 0.423 | 0.283 | |
| Surgical NICU, n (%) | 144 (44) | 1037 (65) | 2056 (51) | N/A |
| p-value (chi-squared test)a | < 0.001 | 0.025 | ||
| Characteristic | NICUs (n = 16) participating in the PREVAIL trial | NICUs (n = 28) and LNUs (n = 68) not participating in the PREVAIL trial | ||
|---|---|---|---|---|
| Babies in PREVAIL trial | Babies not in PREVAIL trial | NICU | LNU | |
| Age at first PICC (days) | ||||
| 0, n (%) | 19 (6) | 115 (7) | 398 (10) | 171 (7) |
| 1–2, n (%) | 56 (17) | 410 (26) | 1144 (28) | 857 (34) |
| 3–5, n (%) | 104 (32) | 482 (30) | 1249 (31) | 773 (31) |
| 6–11, n (%) | 77 (24) | 318 (20) | 707 (17) | 385 (16) |
| ≥ 12, n (%) | 69 (21) | 283 (18) | 553 (14) | 274 (11) |
| Median (IQR) | 5 (3–9) | 4 (2–7) | 3 (1–7) | 3 (2–6) |
| p-value (chi-squared test)a | 0.013 | < 0.001 | < 0.001 | |
| PICC days per admission | ||||
| 1–4, n (%) | 72 (22) | 461 (29) | 905 (22) | 863 (35) |
| 5 or 6, n (%) | 59 (18) | 199 (12) | 571 (14) | 497 (20) |
| 7 or 8, n (%) | 37 (11) | 217 (14) | 575 (14) | 445 (18) |
| 9–12, n (%) | 59 (18) | 281 (17) | 786 (19) | 409 (17) |
| ≥ 13, n (%) | 98 (30) | 450 (28) | 1214 (30) | 246 (10) |
| Median (IQR) | 8 (5–15) | 8 (4–14) | 8 (5–14) | 6 (4–9) |
| p-value (chi-squared test)a | 0.015 | 0.249 | < 0.001 | |
| Invasive ventilation days per admission | ||||
| 0 | 75 (23) | 456 (28) | 1045 (26) | 1368 (56) |
| 1–4, n (%) | 107 (33) | 523 (33) | 1428 (35) | 904 (37) |
| 5–8, n (%) | 37 (11) | 229 (14) | 520 (13) | 129 (5) |
| 9–12, n (%) | 23 (7) | 116 (7) | 282 (7) | 30 (1) |
| ≥ 13, n (%) | 83 (26) | 284 (18) | 776 (19) | 29 (1) |
| Median (IQR) | 3 (1–14) | 3 (0–8) | 3 (0–9) | 0 (0–2) |
| p-value (chi-squared test)a | 0.010 | 0.089 | < 0.001 | |
| Non-invasive ventilation days per admission | ||||
| 0, n (%) | 54 (17) | 528 (33) | 878 (22) | 520 (21) |
| 1 to 5, n (%) | 59 (18) | 397 (25) | 1034 (26) | 783 (32) |
| 6 to 15, n (%) | 63 (19) | 267 (17) | 783 (19) | 622 (25) |
| 16 to 49, n (%) | 107 (33) | 320 (20) | 1035 (26) | 466 (19) |
| ≥ 50, n (%) | 42 (13) | 96 (6) | 321 (8) | 69 (3) |
| Median (IQR) | 14 (3–34) | 3 (0–16) | 6 (1–24) | 5 (1–13) |
| p-value (chi-squared test)a | < 0.001 | < 0.001 | < 0.001 | |
Comparing causative organisms in PREVAIL and non-PREVAIL trial neonatal intensive care units and local neonatal units
We found no differences in the distribution of organisms between PREVAIL trial NICUs, non-PREVAIL trial NICUs and LNUs (see Appendix 22, Table 82). CoNS was the most prevalent organism in all NICUs and LNUs.
Comparing rates of bloodstream infection during the PREVAIL trial in PREVAIL and non-PREVAIL babies
Crude and risk-adjusted rates of BSI per 1000 PICC days were similar in PREVAIL babies compared with non-PREVAIL babies in the PREVAIL trial NICUs and babies in non-PREVAIL trial NICUs (Figure 11) (see Appendix 23, Table 83 and Figure 27). BSI rates were lower in LNUs; however, the difference between babies in LNUs and babies enrolled in the PREVAIL trial was not significant. The risk factors included in the risk-adjusted model were gestational age at birth, age at fist PICC, and days of invasive and non-invasive ventilation. Full details of model-building are presented in Appendix 16, Table 77.
FIGURE 11.
Crude and risk-adjusted rates of BSI (for all organisms) per 1000 PICC days in babies who received S-PICCs in NICUs and LNUs, according to enrolment in the PREVAIL trial during the PREVAIL trial period. Adjusted for gestational age at birth, age at first PICC, and days of invasive and non-invasive ventilation. p-value for effect of group (PREVAIL babies in NICU, babies in non-PREVAIL trial NICUs, babies in LNUs) on BSI rate in comparison to babies in the PREVAIL trial. Period for NICUs that participated in the PREVAIL trial depends on NICU start and end date of recruitment; period for other NICUs and LNUs is August 2015 to January 2017 (whole PREVAIL trial recruitment period).
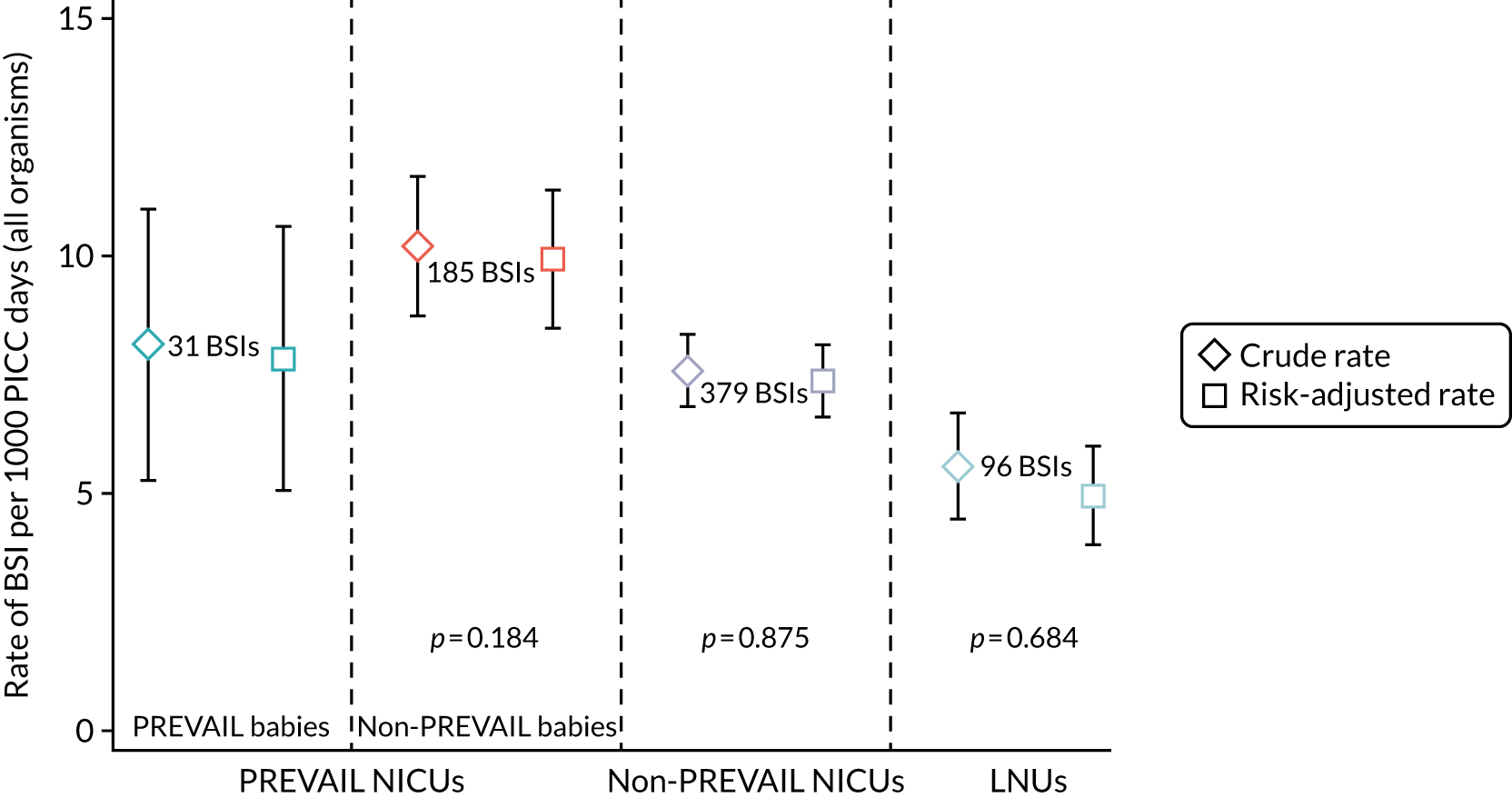
Changes over time in bloodstream infection rates in PREVAIL and non-PREVAIL trial neonatal intensive care units and local neonatal units
From March 2010 to June 2017, there were 55,058 admissions involving 47,669 babies who received PICCs (see Appendix 25, Figure 36). There was no significant change in the risk-adjusted rates of BSI per 1000 PICC days in PREVAIL trial NICUs, non-PREVAIL trial NICUs or LNUs (Table 23 and Figure 12). The trend in non-PREVAIL trial NICUs was not significantly different at the 5% level from the trend in PREVAIL trial NICUs or LNUs. The selected model adjusted for gestational age at birth and days of invasive and non-invasive ventilation (see Appendix 17, Table 78).
| Clearly pathogenic BSI per 1000 PICC days | PREVAIL trial NICUs (n = 16) | Non-PREVAIL trial NICUs (n = 28) | LNUs (n = 68) |
|---|---|---|---|
| Babies (n) | 14,667 | 21,267 | 15,594 |
| Admissions (n) | 15,927 | 23,537 | 14,892 |
| Number of PICC days | 195,360 | 296,824 | 121,750 |
| Number of BSIs | 495 | 736 | 211 |
| Unadjusted | |||
| BSI rate (95% CI) | 2.53 (2.31 to 2.76) | 2.48 (2.30 to 2.66) | 1.73 (1.50 to 1.97) |
| Annual percentage change (95% CI) | 2.33% (–1.98% to 6.82%) | 2.14% (–1.32% to 5.72%) | –5.14% (–11.10% to 1.22%) |
| p-valuea | 0.972 | 0.093 | |
| Risk-adjustedb | |||
| BSI rate (95% CI) | 2.50 (2.28 to 2.72) | 2.44 (2.26 to 2.62) | 1.64 (1.41 to 1.87) |
| Annual percentage change (95% CI) | 2.73% (–1.62% to 7.27%) | 2.63% (–0.86% to 6.25%) | –2.19% (–8.37% to 4.40%) |
| p-valuea | 0.969 | 0.202 | |
FIGURE 12.
Risk-adjusted rate of BSI (clearly pathogenic organisms) during PICC days at risk per 1000 PICC days, by year of admission in PREVAIL trial NICUs, non-PREVAIL trial NICUs and LNUs. Adjusted for gestational age at birth and days of invasive and non-invasive ventilation. Shading denotes the period of enrolment in the PREVAIL trial.
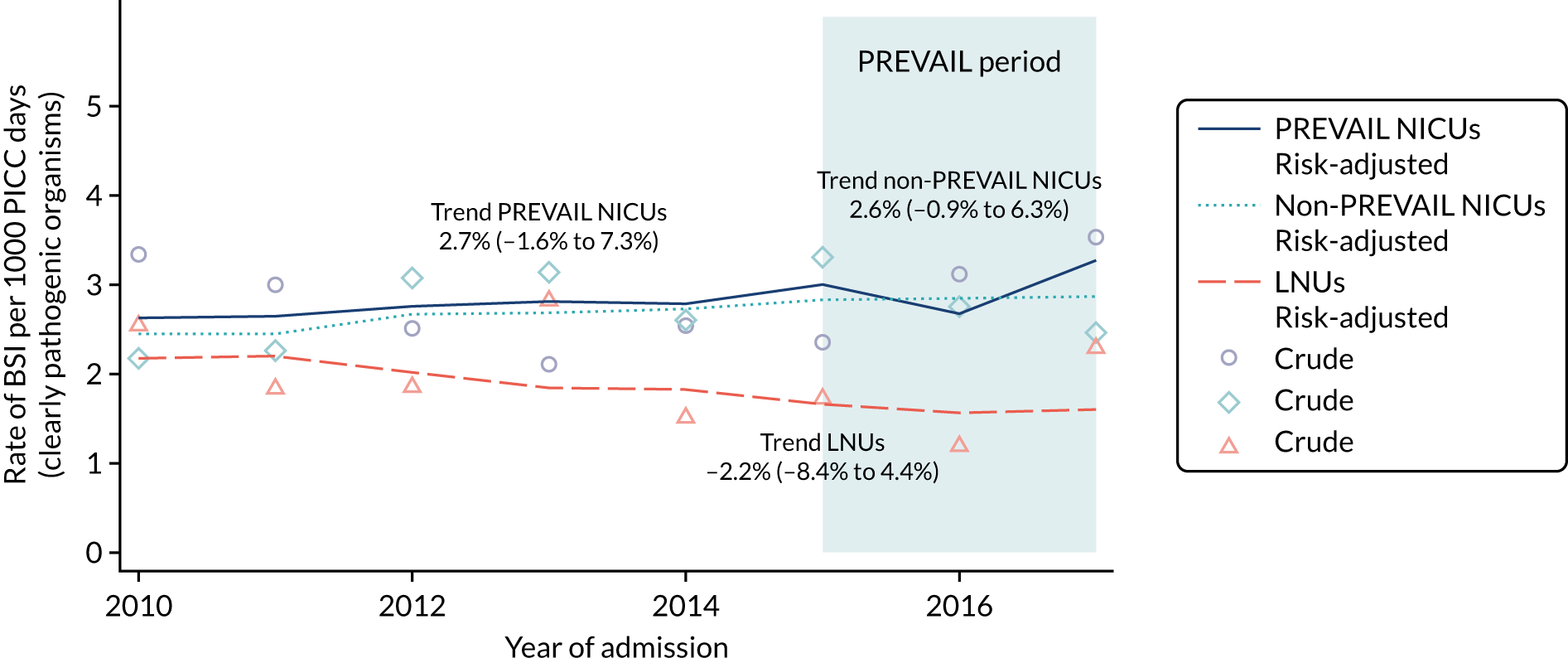
Trends in rates of bloodstream infection in all babies admitted to neonatal intensive care units and local neonatal units
Changes over time in bloodstream infection risk factors
Risk factors for BSI changed from 2010 to 2017 in babies who received intensive or high-dependency care in NICUs and LNUs. First, the proportion of babies born at term or later (≥ 37 weeks’ gestation) increased over time (see Appendix 24, Figure 28). The proportion of babies born at 32 to < 37 weeks and at < 26 weeks of gestation remained fairly stable, but the proportion born at 28 to < 32 weeks and at 26 to < 28 weeks of gestation declined. Second, patterns of care between NICUs and LNUs changed. Extremely preterm babies (< 28 weeks’ gestation) had longer stays in LNUs (see Appendix 24, Figure 29), but fewer PICC and central line days in LNUs in 2017 than in 2010. By contrast, babies in NICUs had more PICC days in 2017 than in 2010 (see Appendix 24, Figures 30 and 31). In both LNUs and NICUs, babies born at ≥ 32 weeks of gestation had fewer PICC and central line days in 2017 than in 2010. Across all gestational ages, PICCs were first inserted at a younger age in 2017 than in 2010 (see Appendix 24, Figure 32). Third, extremely preterm babies in LNUs had more days with non-invasive ventilation and fewer days with invasive ventilation in 2017 than in 2010 (see Appendix 24, Figures 33 and 34).
Changes over time in total late-onset bloodstream infection
From March 2010 to June 2017, there were 161,117 admissions (see Appendix 25, Figure 37) who were at least 2 days old or had a PICC inserted on their day of birth; 2.4% (3798) of these admissions had at least one total late-onset BSI caused by clearly pathogenic organisms. The overall rate was 2.3 (4170/1,849,611) per 1000 days of intensive and high-dependency care.
Risk-adjusted rates of total late-onset BSI (clearly pathogenic organisms) per 1000 days of intensive and high-dependency care remained stable in PREVAIL and non-PREVAIL trial NICUs, but decreased in LNUs (Table 24 and Figure 13). These rates were adjusted for gestational age at birth and days of invasive and non-invasive ventilation (see Appendix 18, Table 79). Risk-adjusted, total late-onset BSI rates per 100 admissions (clearly pathogenic organisms) declined significantly in PREVAIL trial NICUs and LNUs (Table 24 and Figure 14). These rates were adjusted for gestation and for days of invasive and non-invasive ventilation (see Appendix 19, Table 80).
| Total late-onset BSI | PREVAIL trial NICUs (n = 16) | Non-PREVAIL trial NICUs (n = 28) | LNUs (n = 68) |
|---|---|---|---|
| Babies (n) | 37,497 | 53,967 | 58,320 |
| Admissions (n) | 40,787 | 58,845 | 61,485 |
| Number of days | 558,303 | 813,776 | 477,532 |
| Number of BSIs | 1329 | 1791 | 1050 |
| Number of admissions with BSI | 1190 | 1612 | 996 |
| Total late-onset BSI per 1000 intensive and high-dependency care days | |||
| Unadjusted | |||
| BSI rate (95% CI) | 2.38 (2.25 to 2.51) | 2.20 (2.10 to 2.30) | 2.20 (2.07 to 2.33) |
| Annual percentage change (95% CI) | –1.87% (–4.46% to 0.80%) | 1.68% (–0.58% to 3.99%) | –4.44% (–7.21% to –1.59%) |
| p-valuea | 0.036 | 0.233 | |
| Risk-adjustedb | |||
| BSI rate (95% CI) | 2.30 (2.18 to 2.43) | 2.18 (2.08 to 2.28) | 2.11 (1.98 to 2.24) |
| Annual percentage change (95% CI) | –1.40% (–4.01% to 1.29%) | 2.20% (–0.07% to 4.52%) | –2.91% (–5.73% to 0.00%) |
| p-valuea | 0.035 | 0.342 | |
| Total late-onset BSI per 100 admissionsc | |||
| Unadjusted | |||
| Total late-onset BSI per 100 admissions (95% CI) | 2.92 (2.75 to 3.08) | 2.74 (2.61 to 2.87) | 1.62 (1.52 to 1.72) |
| Annual percentage change (95% CI) | –4.84% (–7.48% to –2.12%) | –0.60% (–2.90% to 1.75%) | –7.12% (–9.88% to –4.28%) |
| p-valuea | 0.015 | 0.565 | |
| Risk-adjustedb | |||
| Total late-onset BSI per 100 admissions (95% CI) | 2.83 (2.67 to 2.99) | 2.66 (2.53 to 2.79) | 1.56 (1.46 to 1.65) |
| Annual percentage change (95% CI) | –2.86% (–5.57% to –0.08%) | 1.44% (–0.93% to 3.86%) | –3.28% (–6.16% to –0.32%) |
| p-valuea | 0.015 | 0.774 | |
FIGURE 13.
Risk-adjusted rate of total late-onset BSI (pathogens) per 1000 intensive and high-dependency care days of stay, by year of admission in PREVAIL trial NICUs, non-PREVAIL trial NICUs and LNUs. Rates are adjusted for gestational age at birth and for days of invasive and non-invasive ventilation.
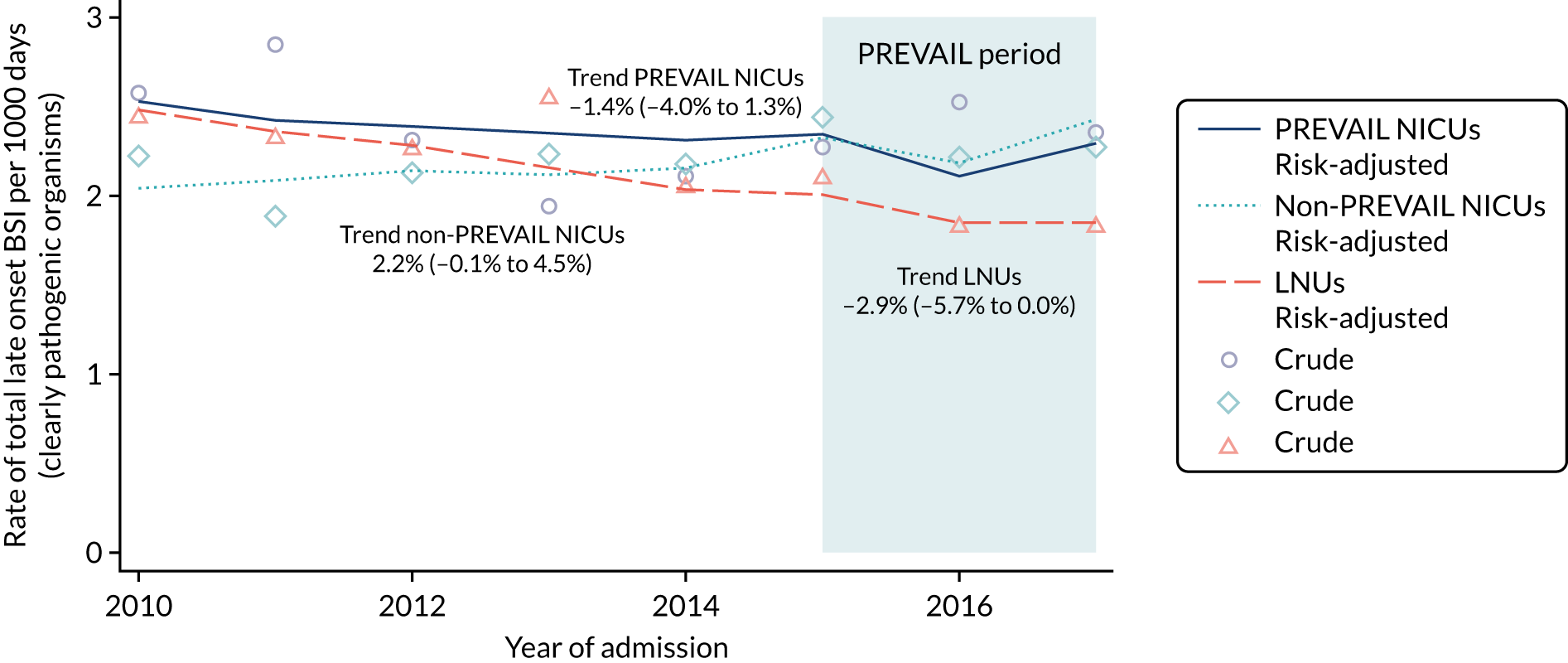
FIGURE 14.
Risk-adjusted rate of late-onset BSI (pathogens) per 100 admissions, by year of admission in PREVAIL trial NICUs, non-PREVAIL trial NICUs and LNUs. Rates are adjusted for gestational age at birth and for days of invasive and non-invasive ventilation; only the first BSI per admission was included.
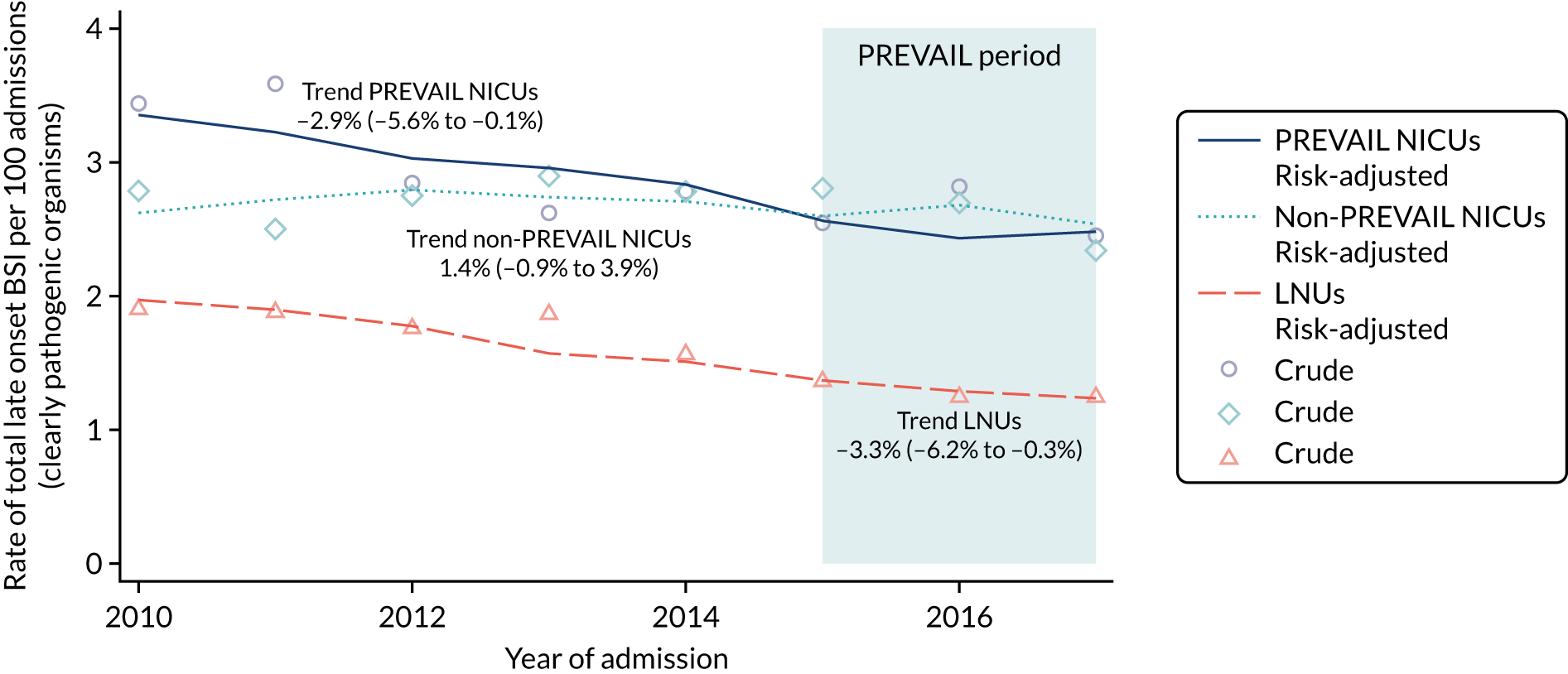
Rates of total late-onset BSI per 100 admissions were lower in LNUs than in NICUs, whereas rates of total late-onset BSI per 1000 days of intensive and high-dependency care were similar. This discrepancy shows that the denominator has an important effect on rates and on trends. In this example, Table 24 shows a similar number of admissions in LNUs and non-PREVAIL trial NICUs, but LNU admissions were shorter, with fewer days of intensive and high-dependency care (477,532 in LNUs, compared with 813,776 in NICUs) (see Table 24).
The contribution of peripherally inserted central venous catheters to total bloodstream infection rates
There was a total of 2476 BSIs in 40,008 admissions receiving intensive and high-dependency care in NICUs and LNUs during the PREVAIL trial recruitment and follow-up period (August 2015 to June 2017): 18% (456) were early onset (no PICC), 46% (1143) were late onset during PICC days at risk, and the remaining 35% (877) were late onset (no PICC) (Table 25). Early-onset BSI was the most frequent type of BSI in babies born at ≥ 32 weeks’ gestation, whereas BSI during PICC days at risk was most frequent in babies born before 32 weeks’ gestation.
| Admissions | Early onset (no PICC, < 2 days of age) | During PICC days at risk | Late onset (no PICC, ≥ 2 days of age) | Total (late plus early onset) |
|---|---|---|---|---|
| All admissions | ||||
| Number of admissions | 31,107 | 12,291 | 26,969 | 40,008 |
| Number of BSIs (% of total BSIs) | 456 (18) | 1143 (46) | 877 (35) | 2476 (100) |
| BSI rate per 100 admissions (95% CI) | 1.5 (1.3 to 1.6) | 9.3 (8.8 to 9.8) | 3.3 (3.0 to 3.5) | 6.2 (6.0 to 6.4) |
| Admissions born before 32 weeks’ gestation | ||||
| Number of admissions | 8891 | 8373 | 11,145 | 13,237 |
| Number of BSIs (% of total BSIs) | 130 (8) | 939 (55) | 631 (37) | 776 (100) |
| BSI rate per 100 admissions (95% CI) | 1.5 (1.2 to 1.7) | 11.2 (10.5 to 11.9) | 5.7 (5.2 to 6.1) | 12.8 (12.3 to 13.4) |
| Admissions born at 32 weeks’ gestation or later | ||||
| Number of admissions | 22,161 | 3918 | 15,824 | 26,771 |
| Number of BSIs (% of total BSI) | 326 (42) | 204 (26) | 246 (32) | 776 (100) |
| BSI rate per 100 admissions (95% CI) | 1.5 (1.3 to 1.6) | 5.2 (4.5 to 5.9) | 1.6 (1.4 to 1.7) | 2.9 (2.7 to 3.1) |
Chapter 6 Discussion
Parts of this chapter have been reproduced from Gilbert et al. 1 © 2019 The Author(s). Published by Elsevier Ltd. This is an open access article distributed under the terms of the CC-BY 4.0 license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium.
Main findings
Clinical effectiveness randomised controlled trial
We found no evidence of benefit or harm from rifampicin- and miconazole-impregnated PICCs in babies receiving intensive or high-dependency neonatal care. The 95% CI for the primary outcome excluded a 27% reduction or 67% increase in the time to BSI associated with using an AM-PICC, compared with using a S-PICC. Sensitivity analyses did not change these results. We found no differences in mortality at 6 months, or in clinical outcomes recorded at discharge home from the NNU.
Economic analysis
Preterm babies in the PREVAIL trial had an average hospital cost of approximately £84,000 over 6 months, mostly related to critical care in the neonatal care unit. The economic model predicted that preventing BSI is both beneficial, as it generates QALY gains, and cost-saving. As AM-PICCs were found in the PREVAIL trial not to prevent BSIs, the model found that AM-PICCs are not cost-effective. At the current price, AM-PICCs would be cost-effective if they achieved a RR reduction of 3% in BSI rate for babies born at < 28 weeks of gestation, or a reduction of 15% for babies born at ≥ 28 weeks of gestation. The largest driver of uncertainty on the costs and health outcomes is whether or not there is any differential effect between AM-PICCs and S-PICCs in preventing BSIs.
Generalisability analysis
The trial findings were generalisable to babies in NICUs in England. This was based on the fact that there were no differences between babies enrolled in the PREVAIL trial and other babies receiving PICCs in NICUs. We found no differences at the 5% level in the distribution of causative organisms isolated from BSI, or in crude and adjusted rates of any BSI per 1000 PICC days. In addition, post hoc trial analyses found no evidence for an interaction between the primary outcome (HR for time to first BSI) and birth before 28 weeks of gestation compared with birth at ≥ 28 weeks of gestation, suggesting that the relative effect observed in the trial could be generalised to babies receiving PICCs, despite varying gestational ages at birth.
We found stable trends in rates over time in BSI per 1000 PICC days (for clearly pathogenic organisms) in PREVAIL trial NICUs, non-PREVAIL trial NICUs and LNUs from 2010 to 2017. The rate of late-onset BSI (for clearly pathogenic organisms) per 100 admissions declined from March 2010 to June 2017 in LNUs and PREVAIL trial NICUs.
Overall, late-onset BSI during PICC days at risk accounted for 46% of BSIs during days of stay for babies admitted to NICUs or LNUs who received intensive or high-dependency care. Corresponding proportions for babies born before and after 32 weeks of gestation were 55% and 26%, respectively. These findings demonstrate that the time when a PICC is present is a high-risk period for BSI; they do not demonstrate that PICCs are necessarily a cause of BSI.
Clinical effectiveness randomised controlled trial
Strengths and limitations
The trial was adequately powered to detect a halving of the BSI risk and included a representative sample of babies receiving PICCs. The trial provides important new evidence for preterm infants born before 32 weeks of gestation, a group at high risk of infection, with frequent use of PICCs, but for whom trial evidence has been lacking. 29,30 The pragmatic design reflected routine practice, as we had no additional sampling, and used a primary outcome that is routinely used to guide antibiotic treatment in neonatal care.
A further strength was the implementation of the trial. Central web-based randomisation ensured allocation concealment and balanced treatment allocation. We achieved near complete follow-up and assessment for the primary outcome, adhered to a prespecified statistical analysis plan for intention-to-treat analyses and halted recruitment once the sample size was achieved. Slightly fewer babies in the antimicrobial arm received the allocated PICC, probably because the randomised PICC had to be inserted within 48 hours, thereafter the standard PICC was used. The sample size and statistical analysis methods were based on the proportional hazards assumption, which was reasonably upheld.
One limitation was that the intervention was open label, so clinicians could distinguish the type of PICC. We found a slightly increased rate of blood culture sampling in the antimicrobial arm, but the proportions of babies with at least one sample or any PICC tip culture were similar and there were no differences in the timing of PICC removal between trial arms. A further limitation was the lack of power to detect significant differences in rifampicin-resistant organisms isolated from blood or CSF cultures. The small number of resistant organisms was due to few positive cultures, and because only 44–54% of these cultures were tested for rifampicin resistance. The risk of rifampicin resistance in isolates from positive blood or CSF cultures did not differ between trial arms, but was significantly increased in positive tip cultures from AM-PICCs. Selection of rifampicin-resistant Gram-positive bacteria during treatment, when rifampicin is used as the sole antibacterial agent, is well recognised. 113 Testing for resistant organisms was considered by the investigators, the TSC and the IDSMC, but the resistance results were not monitored as the risk of AEs arising was viewed as being low because the limited release of rifampicin from the catheter surface would be unlikely to affect bacteria at any site other than the catheter itself. Even if rifampicin-resistant Gram-positive bacteria did cause infection in an individual patient, routine antibiotic use would be unaffected because rifampicin is rarely used for treatment in the neonatal setting.
Findings in context
The finding that rifampicin- and miconazole-impregnation did not reduce BSI is consistent with other studies (see Table 1). 1,30,36,37 In contrast, RCTs of the effectiveness of minocycline- and rifampicin-impregnated CVCs versus standard CVCs have reported substantial reductions in catheter-related BSIs and, in one trial in PICUs, any BSI. 33,34,114 First, these apparent differences in the effectiveness of rifampicin–miconazole and minocycline–rifampicin impregnation may reflect true differences in antibacterial effects. Miconazole is used to prevent invasive fungal infection in preterm babies, but has the disadvantage that rifampicin is then the sole antibacterial agent when combined with miconazole. Rifampicin is more active against Gram-positive than against Gram-negative bacteria and has synergistic action against staphylococci when combined with another antibacterial such as minocycline, especially against meticillin-resistant strains. 113,115
Second, it is possible that, although minocycline–rifampicin is the most effective type of antimicrobial impregnation in systematic reviews,32,35 this type of impregnation might not effectively reduce overall rates of BSI or sepsis. 116 Trials in adults show beneficial effects of antimicrobial impregnation for catheter-related BSI, but few trials measure the effect on any BSI. Catheter-related infection requires the same isolates from blood and CVC tip and could be biased because of inhibition of positive tip cultures by leaching of antimicrobial from the tip during plating-out for culture. Only the large CATCH trial used any clinically indicated BSI as the primary outcome and found a 57% reduction in time to infection (see Table 1)34,38 Third, the reductions in infection rates in NNUs associated with improved catheter asepsis practices and shorter duration of PICC use may have narrowed the potential for further benefits from antimicrobial impregnation. 47 It is also possible that PICCs are not an independent risk factor for infection in sick preterm babies because of the babies’ high susceptibility to infection from multiple sources, including numerous invasive procedures and devices, gut permeability and immune immaturity. 117
Practice context
Since 2012, the Premistar PICC has been the only AM-PICC available for preterm babies in Europe. Its use has been reported in Germany and Italy,30 but use in the UK was limited to the PREVAIL trial. We are not aware of adoption of the AM-PICC by any UK NNUs outside the context of the trial.
Economic evaluation
Strengths and limitations
The aim of the economic evaluation was to inform the decision of whether or not the NHS should purchase AM-PICCs rather than S-PICCs for preterm babies in NNUs. The specific objectives were to estimate the hospital costs of the PREVAIL babies over the time horizon of the trial, to determine the cost-effectiveness of AM-PICCs against S-PICCs and the minimum effectiveness of an AM-PICC required to make it cost-effective, and to assess the value of future research.
The use of routine health-care data to estimate the hospital costs of the PREVAIL babies was a strength of the economic evaluation. The economic evaluation secured access to the routine databases that record admissions to NNUs and to PICUs, hospitalisations involving procedures, outpatient appointments and visits to the A&E department. Accessing data from all these routine databases ensured that the estimation of hospital costs was as thorough as possible. It maximised the accuracy of the data on hospital use by avoiding recall bias and by having access to data on the resources involved with each hospitalisation. Furthermore, using routine health-care data precluded asking the parents and guardians to fill in lengthy questionnaires on the number and duration of hospitalisations at a time of personal distress.
A key strength of the economic evaluation was the development of a new decision-analytic model to predict the quality-adjusted life expectancy and NHS costs of using AM-PICCs or S-PICCs over the babies’ expected lifetimes. The model synthesised the most up-to-date estimates of costs and outcomes of BSI in infants in the neonatal care unit, informed by data from the PREVAIL trial linked to routine health-care databases, and from the published literature. The model also considered the different risks associated with gestational age. 118 Although the model was developed to compare PICC types, it can be used to evaluate any interventions to prevent BSI in babies by estimating the minimal reduction in the risk of BSI that is required so that an intervention could be considered cost-effective, and the relationship between minimal risk reduction and maximum price difference.
A further strength is the use of the economic model to estimate the VoI from future research. Specifically, the VoI analysis quantified the magnitude of the health losses following a wrong adoption decision due to parameter uncertainty, the maximum bound of investment that should be warranted to resolve this uncertainty and the key uncertain parameters for which resolving uncertainty will provide the greatest reduction in the risk of making suboptimal decisions.
There were several limitations to the economic analysis. First, it was not possible to estimate the causal effect of BSI and death on hospital costs over 6 months from PICC insertion, and the causal effect of BSI on the risk of death at 6 months. This was because of the lack of difference between PICC groups (precluding an instrumental variable approach), limited number of events and time constraints (given that the routine health-care data were received 3 months before the project’s deadline). Consequently, the model base case assumes that babies incur the same costs irrespective of BSI or survival status, and assumes that BSI does not cause death at 6 months (although it includes an increased risk of death due to BSI at 2 years). As a consequence, the benefits of preventing BSI may be underestimated.
Second, the model assumes that BSI increases the risk of death and NDI at 2 years of age. The evidence of this causal link was based on the published literature, which, given the enormity of the potential evidence base, was identified from a citation search rather than from a systematic review, which would have been outside the scope and timeline of the project. As a consequence, there is a risk of having omitted some studies in the final evidence review and meta-analysis. Nonetheless, the starting point for the citation search (the so-called ‘pearls’) was informed by the PREVAIL trial clinical team, and great care was taken in matching the outcomes identified in the literature review with those collected in the trial. Moreover, the citation search identified three systematic reviews and one umbrella review, published between 2013 and 2016,78–80,119 thereby providing some reassurance that most, if not all, of the relevant studies were included. The literature on the link of BSI to death and NDI is necessarily observational, and scarce. If this link is not causal, or if it is smaller in magnitude, the value of preventing BSI may have been overestimated. Therefore, the minimum risk reduction for interventions, such as an AM-PICC, to be cost-effective may have been underestimated. This structural uncertainty was explored in the scenario analysis, but was not considered in the VoI framework. This means that the value of future research in the effectiveness of interventions to prevent BSI may have been overestimated, compared with the value of future research on the causes of NDI and death in early years.
Third, the model structure constrains the impact of preventing BSI in increasing the risk of NDI and death at 2 years of age. There are some studies suggesting that antibiotics, given to treat infection, increase the risk of NEC, which would, in turn, increase the risk of NDI. 25,26,120 However, no studies were identified that enable the direct effect of BSI on NDI to be disentangled from the indirect effect via antibiotic treatment; hence, the model structure excludes NEC. If NEC was confirmed to play a role in the pathway leading to NDI over and above the direct effect of BSI, and if BSI causes NEC, preventing BSI may be more cost-effective than this study suggests. Conversely, if NEC is on the causal pathway for NDI and death at 2 years, but it is unrelated to BSI, preventing BSI will be less cost-effective.
A fourth limitation was restriction to the perspective of NHS hospitals for costs during the 6-month follow-up period. Ideally, the model would include all the costs falling on the NHS, including not only hospital costs, but also costs of community health care. This was not possible because there are no routine health-care databases that compile the use of community health-care services. Furthermore, at project conception, it was deemed excessively burdensome to ask parents for details of their use of community health-care services. Nevertheless, the lack of data on community health-care costs is unlikely to have a large impact on the results, given the high costs related to hospitalisations. Costs falling on other sectors, such as social care and education, are likely to be relevant. However, their inclusion would represent a departure from the NHS perspective and would require the consideration of how to account for costs falling on different sectors. 121
Fifth, the model was also restricted to health benefits for the child, by considering the impact of BSI on death and NDI, and its consequences on the babies’ length of life and HRQoL. Children’s health may have spillover effects on their parents. 122,123 Therefore, avoiding BSI may have beneficial consequences to parents’ HRQoL. If this is the case, the benefits in terms of QALYs for parent and child of preventing BSI may have been underestimated and the magnitude of the necessary risk reduction for interventions to be cost-effective may have been overestimated.
A sixth limitation relates to the measure of NDI, which was retained from the previous model of Mangham et al. 71 It is relatively crude and might have been superseded by more recent advances in paediatrics. As far as model results are concerned, most babies are classified as having no impairment at 2 years of age. However, less evident cognitive or motor deficits might become apparent at school age, even in this group, causing additional burden in terms of both health-related and education costs, and QALYs lost. Again, if this is the case, the model will underestimate the benefits from preventing these additional minor deficits related to the occurrence of BSI, which, if accounted for, would further increase the cost-effectiveness of interventions to prevent it.
Using routine health-care data to estimate hospital costs had some limitations. Given that linkage of the PREVAIL babies’ data was conducted by the data providers, there was limited information on the reasons for missing data. Furthermore, as the costing of hospital care is based on HRGs, hospitalisations with insufficient data to derive a HRG were discarded. Nonetheless, complete data were retrieved for most PREVAIL babies. Moreover, any missing data are likely to be related to errors in data entry at the hospital level, which are unlikely to be related to the babies’ characteristics and their hospital care, and hence can be assumed to be missing completely at random. Another limitation was the inconsistencies in the dates in some records, which were needed to calculate the hospital length of stay. However, for the estimation of hospital costs, the dates of hospitalisations are used only to select the hospital care within the trial follow-up period of 6 months. Therefore, the approach taken to analyse the dates is unlikely to have a large impact on the results.
Findings in context
We found no published economic analyses that compared PICC impregnation in newborn babies. Previous cost-effectiveness studies for impregnated PICCs were in older populations and used different antimicrobials for impregnation, precluding meaningful comparisons. 124–127
A previous study71 estimated the health outcomes and costs of care of preterm babies, but it did not assess the cost-effectiveness of interventions to improve outcomes of preterm babies. The results of Mangham et al. 71 are similar to the results of the present study under the standard care (S-PICC) policy, even though some of the model inputs are different. Mangham et al. 71 predicted that at 18 years of age 36% of babies born at gestational age 23–27 weeks (66% of babies born at gestational age 28–32 weeks) would have no NDI, 10% born at gestational age 23–27 weeks (17% born at gestational age 28–32 weeks) would have mild NDI, 5% born at gestational age 23–27 weeks (6% born at gestational age 28–32 weeks) would have moderate NDI and 4% born at gestational age 23–27 weeks (4% born at gestational age 28–32 weeks) would have severe NDI. In the present study, the model predicted that, under the current policy of using S-PICCs, 45% of babies born at gestational age 23–27 weeks (67% of babies born at gestational age 28–32 weeks) would have no NDI, 17% born at gestational age 23–27 weeks (20% born at gestational age 28–32 weeks) would have mild NDI, 9% born at gestational age 23–27 weeks (6% born at gestational age 28–32 weeks) would have moderate NDI and 7% born at gestational age 23–27 weeks (3% born at gestational age 28–32 weeks) would have severe NDI. For babies born at < 28 weeks’ gestation, the Mangham et al. 71 model estimates the total average cost per survivor to 18 years at £136,790 (95% CI £50,222 to £261,873), compared with an average lifetime cost per baby (using S-PICCs) predicted in our model of £126,168 (95% CI £120,133 to £132,655).
Results from the economic analysis show that the RR of BSI is the main contributor to overall parameter and decision uncertainty. If BSI in infancy increases the risk of NDI and death, as suggested by observational studies and included in the model, interventions that prevent such BSI offer a large potential for cost-effectiveness, even if their efficacy is small. 10,74,78 Therefore, future applied research should focus on the evaluation of the effectiveness of interventions to prevent BSIs. However, the increase in risk of NDI and death reported by observational studies may not be a true reflection of the effect of BSI. It is possible that there is some residual confounding, in that babies who have poorer health are more likely to suffer a BSI, as well as being more likely to have NDI or die. If this is the case, the association between BSI and NDI/death may be overestimated, thereby leading the cost-effectiveness model to overestimate the benefits of preventing BSI in the long term. For these reasons, more research is needed on the effect of BSI on long-term health outcomes.
Reflecting the structural uncertainty on the link between BSI, NDI and death is a key challenge. The methodological literature is unclear about how to reflect structural uncertainty from observational studies, although some approaches have been proposed in the past. 128 Further research is warranted to develop adequate methods that are able to incorporate and assess potential biases due to the inclusion of observational evidence, both directly (i.e. in the model structure) and indirectly (i.e. in the evidence synthesis process). In this respect, clinical research could help inform future models, for example via longitudinal studies following preterm babies over time. These would record various types of data, including linkage to hospitalisation, community and primary care data for health outcomes, and measures relevant to health captured in non-health data, for example cognitive function measured through school achievement trajectories, so that causal estimates of the impact of infection could be estimated.
Generalisability analysis
Strengths and limitations
A key strength of our study is the national coverage of 92% of NICUs and LNUs across the NHS in England over 7 years; this minimised referral biases, provided a large enough sample to evaluate subgroups and used routinely submitted laboratory reports, thereby minimising clinician response bias. Our data on BSI are laboratory confirmed and we have data on the organism cultured and dates from both infection surveillance and clinical records, which enables us to tailor specific definitions of BSI.
Limitations include a lack of precise timing of culture sampling (day rather than hour), no consent to use data from 12 units, and lack of full data for 162 PREVAIL trial participants. Furthermore, the presence of a PICC was not recorded in 6% of PREVAIL babies known to have a PICC from trial CRFs. It is unlikely that these errors are limited to PREVAIL babies; hence, some PREVAIL and non-PREVAIL babies were misclassified as not having a PICC.
A further limitation is linkage error, which can have an important impact on trend analyses. In our study, linkage error arises predominantly from missed links, and, to a lesser extent, from false links due to incorrect or missing identifiers. In the linked data, the BSI rate in PREVAIL babies randomised to receive a S-PICC was 8.1 per 1000 PICC days, compared with 10.6 in the PREVAIL trial, highlighting the underestimation of our results. This is probably a result of missed links due to incomplete identifiers or under-reporting to the infection surveillance system. The quality and completeness of identifiers improved in both data sets (NNRD and infection surveillance) over time. This improvement could have led to a spurious increase in BSI rates. Therefore, we need to be cautious in interpreting the stable, increasing and declining BSI rates. Further research is needed to understand the case mix, practice and service factors affecting changing rates of BSI over time, taking into account all periods at risk throughout neonatal care. Stable rates in NICUs may reflect a combination of better infection control, allowing use of PICCs in more high-risk periods. For example, when PICCs are used for shorter durations, it is likely that the PICCs are used during higher-risk periods, such as during stabilisation after birth. We would expect rates of BSI per 1000 PICC days to be higher during shorter, but higher-risk, periods, even though overall BSI risk during NNU stay would decrease. Similarly, replacement of umbilical venous catheters with PICCs may decrease BSI rates if all catheter days were counted. However, the rate of BSI per 1000 PICC days would increase because BSIs that were previously counted during umbilical venous catheter days would be counted while a PICC is in situ.
Findings in context
The total rate of BSI (clearly pathogenic organisms) of 2.4 per 100 admissions was slightly higher than the rate of 1.8 per 100 admissions reported in a retrospective cohort study of 34 NICUs and LNUs in the UK from 2005 to 2014. 12 This may reflect the more stringent definition used in the study of 34 NICUs that required a prescription of at least 5 days of antibiotics, or the reliance on voluntary reporting by clinicians to the study. In addition, we excluded babies who received special care from our study, who would have fewer infections. Excluding special care made the data more comparable between NNUs, and over time, as babies are kept in special care for varying lengths of time; however, this excluded 11.5% of total late-onset BSIs in all NNUs. Our finding of a declining trend in total BSIs per 100 admissions in PREVAIL trial NICUs and LNUs is similar to trends reported in the study of 34 UK NICUs and LNUs. 12
Before-and-after studies have reported reductions in rates of BSI per 1000 PICC days following the introduction of care bundles. 47,48 However, this was not seen in our study. This may be due to the improved ascertainment of BSI over time masking true trends. Alternatively, our study represents all centres across England, where rates may differ from those voluntarily participating in a study. In our study, a mix of decreasing and increasing rates in different NNUs may have resulted in a stable average rate. Furthermore, the BSI rates may have only temporarily decreased, thereby not affecting the long-term trends.
Relevance to future research
Had the trial found one type of PICC to be more effective, the RR could have been applied to rates of BSI per 1000 PICC days at risk in non-participating NICUs and LNUs to estimate the expected risk difference. Our methods for linking NNRD, infection surveillance, and trial data can be used for future trials in contexts of changing infection rates to assess generalisability and applicability. Linkage should be continued to monitor the impact on BSI of infection control, changes in service configuration and practice and to target high-risk groups for infection.
Chapter 7 Conclusions
We found no evidence of benefit or harm of rifampicin- and miconazole-impregnated PICCs during neonatal care. Interventions with a small effect on BSI could be cost-effective over the life course. Our trial findings are generalisable to neonatal care in England.
Implications for practice
-
There is no evidence that PICC impregnation with miconazole and rifampicin is more effective than S-PICCs, and it is more costly; hence, it is not cost-effective. These findings indicate that AM-PICCs should, therefore, not be adopted in neonatal care.
-
Rifampicin resistance in organisms cultured from blood, CSF or PICC tips was not significantly increased in the AM-PICC compared with the S-PICC group, but organisms isolated from only the PICC tip were more likely to be rifampicin resistant. As rifampicin is not routinely used in UK neonatal care, this is probably of limited clinical relevance in the UK setting.
-
The economic model shows that, if there is a causal link between BSI and adverse long-term health outcomes, then interventions that achieve small reductions in the risk of BSI are potentially cost-effective.
-
Findings from the PREVAIL trial are generalisable to neonatal intensive care in the NHS in England.
-
Application of RRs from PICC interventions to predict absolute rate reductions in the future, or locally, need to be based on risk-adjusted rates of BSI per 1000 PICC days.
-
Bloodstream infections that occur during PICC days at risk contribute to nearly half (46%) of total BSIs during neonatal intensive and high-dependency care. Preventative strategies for reducing the risk of BSI in newborn babies should focus on all aspects of care before, during and after the period of PICC use.
Recommendations for research
-
One in 10 babies in the PREVAIL trial had a BSI, and some may suffer serious lifelong NDI or lung disease as a result. Low-cost interventions that reduce the risk of BSI in preterm babies, even by a small amount, would be likely to be cost-effective over a child’s life course, based on the assumption of reduced risk of NDI and death. Investment in further research to develop other types of antimicrobial PICC impregnation or alternative approaches for preventing infection in neonatal care would, therefore, be worthwhile.
-
Our finding of no evidence of benefit associated with rifampicin- and miconazole-impregnated PICCs contrasts with substantial reductions in rates of BSI or catheter-related BSI reported in previous trials in children and adults randomised to rifampicin- and minocycline-impregnated CVCs, compared with standard CVCs. We recommend that further research be undertaken to develop and evaluate rifampicin- and minocycline-impregnated PICCs for use in preterm babies.
-
We recommend that further research be carried out to strengthen evidence on the causal link between BSI and NDI and death, and on methods to reflect the uncertainty in these causal links in cost-effectiveness models.
-
Record-level linked data combining electronic clinical records from neonatal care, HES and infection surveillance data should be made routinely available for research and infection surveillance in England.
-
Further research is required to understand which practices contribute to changes (or lack of changes) in rates of BSI over time in neonatal care.
Acknowledgements
Clinical effectiveness randomised controlled trial
Participants
We would like to thank the 861 babies who participated in the PREVAIL trial, along with their parents and families, for their invaluable contribution to this research.
Trial sites
We are grateful to the PIs and RN teams at each trial site who recruited participants and supported them and their families throughout the trial (in order of number of patients recruited): Bradford Royal Infirmary (Sam J Oddie and Rachel Wane); Leicester Royal Infirmary (Marie Hubbard and Rosalind Astles); Birmingham Women’s Hospital (Andrew Ewer and Rachel Jackson); St Mary’s Hospital, Manchester (Ranganath Ranganna and Nicola Booth); Liverpool Women’s Hospital (Kiran Yajamanyam and Karen Harvey); Homerton University Hospital (Narendra Aladangady and Asha Mathew); The Jessop Wing, Sheffield (Elizabeth Pilling and Pauline Bayliss); Royal Oldham Hospital (Natasha Maddock and Louise Woodhead); The Royal London Hospital (Ajay Sinha and MaySze Chang); Royal Preston Hospital (Sandeep Dharmaraj and Claire Lodge); Queen’s Medical Centre, Nottingham (Jon Dorling and Helen Navarra); John Radcliffe Hospital (Charles Roehr and Sheula Barlow); Royal Bolton Hospital (Mahesh Yadav and Claire Abbott); Leeds General Infirmary (Kathryn Johnson); Nottingham City Hospital (Dushyant Batra and Yvonne Hooton); St Michael’s Hospital, Bristol (Pamela Cairns and Jennifer Chapman); Queen’s Hospital, Romford (Bal Krishnan Sharma and Helen Smith); and Newham General Hospital (Imdad Ali and Ivone Lancoma-Malcolm). We thank the 53 hospital trusts across the UK that agreed to act as continuing care sites for the trial and assisted with collection of important follow-up data when participants were transferred away from their recruiting site.
Liverpool Clinical Trials Centre
We thank the LCTC staff: Zoe Craig, Sue Howlin and Kate Silvera-Cull for their work as data managers on this study; Anna Rosala-Hallas and Elizabeth Conroy who acted as randomising statisticians and produced the randomisation lists; and Ashley Best who undertook quality control checks.
Public engagement
We thank BLISS, a patient advocacy charity for babies born premature or sick, for its support of the trial, assistance with review of public-facing documents and dissemination of results (registered charity number 1002973; www.bliss.org.uk/).
Trial Steering Committee
We thank Mike Sharland (chairperson), Stephanie Chadwick, Edmund Juszczak and Win Tin for their oversight and guidance.
Independent Data and Safety Monitoring Committee
We thank Nicholas Embleton (chairperson), Alison Balfour and Louise Stanton for their oversight and guidance.
Economic and generalisability analyses
We would like to thank María José Aragón for assistance in the analysis of HES data; Lee Norman and Roger Parslow at PICANet for their help in providing data; Neena Modhi, Kayleigh Ougham and Richard Colquhoun at the Neonatal Data Analysis Unit (NDAU) for their help in providing and answering our questions about the data; the NHS Digital data access and data production teams, for similar help; Stavros Petrou for helpful discussions on data sources to inform the model; Nicky Welton and Caroline Clarke for an excellent discussion of our paper at the Health Economics Study Group Summer 2018 meeting and at the 5th EuHEA PhD Student-Supervisor Conference, respectively; and all the colleagues who took part in the discussion. We would like to thank PHE for providing data, the Antimicrobial Resistance team within PHE for their support for the generalisability analyses and Mehdi Minaji for his assistance managing the data. We would also like to thank Ruth Blackburn for her guidance and advice throughout the generalisability analyses.
Other
We thank the manufacturer of the PICCs [Vygon (UK) Ltd] for allowing participating units to purchase AM-PICCs at the same price as S-PICCs during recruitment to the trial. Neither the funder (NIHR) nor the manufacturer had any involvement in the study design, interpretation of the results or writing of the report. Research at UCL Great Ormond Street Institute of Child Health is supported by the NIHR Great Ormond Street Hospital Biomedical Research Centre.
Administrative health records acknowledgements and disclaimers
Clinical effectiveness randomised controlled trial
The use of HES data was approved by the Health and Social Care Information Centre (now known as NHS Digital) for the purpose of this study (DARS-NIC-73974-P0L1Z-v0.9). HES data © 2018, reused with the permission of the Health and Social Care Information Centre. All rights reserved.
Economic analysis
Electronic patient data recorded at participating NNUs that collectively form the UK Neonatal Collaborative (UKNC) are transmitted to the NDAU to form the NNRD. Laura Bojke, Rita Faria and Alessandro Grosso had full access to all the data in the study and take full responsibility for the integrity of the data and accuracy of the data economic analysis. We are grateful to all the families that agreed to the inclusion of their baby’s data in the NNRD, the health professionals who recorded data and the NDAU team.
The PICANet is commissioned by the Healthcare Quality Improvement Partnership (HQIP) as part of the National Clinical Audit and Patient Outcomes Programme; the Welsh Health Specialised Services; NHS Lothian/National Services Division NHS Scotland; the Royal Belfast Hospital for Sick Children; The National Office of Clinical Audit, Republic of Ireland; and HCA Healthcare UK.
The HQIP is led by a consortium of the Academy of Medical Royal Colleges, the Royal College of Nursing, and National Voices. It aims to improve health outcomes and to promote quality improvement. This publication is based on data collected by or on behalf of the HQIP, which has no responsibility or liability for the accuracy, currency, reliability and/or correctness of this publication.
This research was informed by data collected by NHS Digital (formerly the Health and Social Care Information Centre), © 2015–18, the Health and Social Care Information Centre. Re-used with the permission of the Health and Social Care Information Centre. All rights reserved.
Generalisability analysis
Electronic patient data recorded at participating NNUs that collectively form the UKNC are transmitted to the NDAU to form the NNRD. Ruth Gilbert, Katie Harron and Caroline Fraser had full access to all the data in the study and take full responsibility for the integrity of the data and accuracy of the data analysis. We are grateful to all the families that agreed to the inclusion of their baby’s data in the NNRD, the health professionals who recorded data, and Professor Neena Modi and the team at the NDAU. National infection surveillance data from the Second Generation Surveillance System was provided by PHE.
Contributions of authors
Ruth Gilbert (https://orcid.org/0000-0001-9347-2709) (Co-Chief Investigator and Professor of Clinical Epidemiology) developed the study protocol and funding application in collaboration with co-investigators. She oversaw the delivery of the study, assisted with development of the statistical analysis plan and oversaw clinical interpretation of the study data. She led the preparation of the final report (drafting, reviewing and editing) and was co-chairperson of the TMG.
Michaela Brown (https://orcid.org/0000-0002-7772-271X) (Senior Statistician) developed the study protocol and funding application in collaboration with co-investigators, proposed data capture and statistical analysis methods, approved the statistical analysis plan, and oversaw trial monitoring activities and the final statistical analysis. She contributed to the final report (drafting, reviewing and editing) and was a member of the TMG.
Rita Faria (https://orcid.org/0000-0003-3410-1435) (Lead Health Economist) contributed to the study protocol and funding application, led the development of the economic analysis plan, led the applications to access the routine health-care data, led the costing analysis of hospital care, oversaw and collaborated in the model-based economic evaluation, led the interpretation of the economic analysis results, contributed to the preparation of the final report (drafting, reviewing and editing) and was a member of the TMG.
Caroline Fraser (https://orcid.org/0000-0001-7260-7053) (Research Assistant, Epidemiology) contributed to the development of the generalisability study protocol, conducted the generalisability study analysis, contributed to interpretation of the generalisability study results, contributed to the preparation of the final report (drafting, reviewing and editing), and was a member of the TMG.
Chloe Donohue (https://orcid.org/0000-0001-6379-8214) (Trial Co-ordinator) contributed to protocol development and all aspects of governance and study delivery, and led on preparation of progress reports throughout the study. She contributed to the final report (drafting and reviewing) and was a member of the TMG.
Naomi Rainford (https://orcid.org/0000-0002-5876-3946) (Trial Statistician) contributed to protocol development and data capture methods, wrote the statistical analysis plan, prepared data for reports throughout the study and undertook the final statistical analysis under the supervision of Michaela Brown. She was a member of the TMG.
Alessandro Grosso (https://orcid.org/0000-0001-7211-438X) (Health Economist) contributed to the development of the economic analysis plan, contributed to the costing analysis of hospital care, conducted the model-based economic evaluation and contributed to the interpretation of the economic analysis results and to the preparation of the final report (drafting, reviewing and editing).
Ajay K Sinha (https://orcid.org/0000-0001-5201-7718) (Neonatal Consultant) contributed to protocol development and clinical interpretation of the study data. He contributed to the preparation of the final report (reviewing) and was a member of the TMG.
Jon Dorling (https://orcid.org/0000-0002-1691-3221) (Division Head of Neonatal-Perinatal Medicine) contributed to protocol development and clinical interpretation of the study data. He contributed to the preparation of the final report (reviewing) and was a member of the TMG.
Jim Gray (https://orcid.org/0000-0003-4169-3141) (Head of Microbiology) contributed to protocol development, to the classification and interpretation of infections, and to the clinical interpretation of the study data. He contributed to the preparation of the final report (reviewing) and was a member of the TMG.
Berit Muller-Pebody (https://orcid.org/0000-0002-8874-480X) (Antimicrobial Resistance Section Lead) contributed to protocol development of the generalisability study, oversaw data management and linkage of PHE’s national laboratory surveillance data, contributed to the preparation of the final report (reviewing) and was a member of the TMG.
Katie Harron (https://orcid.org/0000-0002-3418-2856) (Senior Lecturer) developed the funding application in collaboration with co-investigators, oversaw the delivery of the generalisability study and contributed data for the economics evaluation. She contributed to the preparation of the final report (reviewing) and was a member of the TMG.
Tracy Moitt (https://orcid.org/0000-0002-5579-996X) (Senior Trials Manager) contributed to protocol development, gave guidance and support on all aspects of governance and study delivery, and supported the preparation of progress reports. She contributed to the final report (reviewing) and was a member of the TMG.
William McGuire (https://orcid.org/0000-0002-3561-514X) (Professor of Child Health) contributed to the development of the study protocol and funding application and interpretation of the study data. He contributed to the preparation of the final report (reviewing) and was a member of the TMG.
Laura Bojke (https://orcid.org/0000-0001-7921-9109) (Senior Health Economist) contributed to the study protocol and funding application; oversaw the development of the economic analysis plan, applications to access the routine health-care data, costing analysis of hospital care and model-based economic evaluation; and contributed to the interpretation of the economic analysis results. She contributed to the preparation of the final report (reviewing) and was a member of the TMG.
Carrol Gamble (https://orcid.org/0000-0002-3021-1955) (Professor of Medical Statistics and Director of LCTC) developed the study protocol and funding application in collaboration with co-investigators. She led a blind review of the data and provided statistical guidance throughout the trial. She also contributed to the preparation of the final report (reviewing and editing).
Sam J Oddie (https://orcid.org/0000-0001-8701-4912) (Co-Chief Investigator and Consultant Neonatologist) developed the study protocol and funding application in collaboration with co-investigators. He oversaw the delivery of the study, assisted with development of the statistical analysis plan and oversaw clinical interpretation of the study data. He contributed to the preparation of the final report (drafting, reviewing and editing) and was co-chairperson of the TMG.
Publications
Fraser C, Harron K, Dalton L, Gilbert R, Oddie SJ. Variation in neonatal unit practices for preventing infection related to central venous catheters. PLOS ONE 2018;13:e0204894.
Brown M, Gilbert R, Oddie SJ, on behalf of the PREVAIL trial team. Reducing catheter-related bloodstream infections in neonates – authors’ reply. Lancet Child Adolesc Health 2019;3:e12.
Gilbert R, Brown M, Rainford N, Donohue C, Fraser C, Sinha A, et al. Antimicrobial-impregnated central venous catheters for prevention of neonatal bloodstream infection (PREVAIL): an open-label, parallel-group, pragmatic, randomised controlled trial. Lancet Child Adolesc Health 2019;3:381–90.
Fraser C, Muller-Pebody B, Blackburn R, Gray J, Oddie SJ, Gilbert RE, Harron K. Linking surveillance and clinical data for evaluating trends in bloodstream infection rates in neonatal units in England. PLOS ONE 2019;14:e0226040.
Grosso A, Faria R, Bojke L, Donohue C, Fraser C, Harron K, et al. The cost-effectiveness of strategies preventing late-onset infection in preterm infants. Arch Dis Child 2020;105:452–7.
Data-sharing statement
All data requests should be submitted to LCTC at UoL for review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Gilbert R, Brown M, Rainford N, Donohue C, Fraser C, Sinha A, et al. Antimicrobial-impregnated central venous catheters for prevention of neonatal bloodstream infection (PREVAIL): an open-label, parallel-group, pragmatic, randomised controlled trial. Lancet Child Adolesc Health 2019;3:381-90. https://doi.org/10.1016/S2352-4642(19)30114-2.
- Office for National Statistics . Birth Characteristics in England and Wales: 2017 2019.
- Blencowe H, Lee AC, Cousens S, Bahalim A, Narwal R, Zhong N, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res 2013;74:17-34. https://doi.org/10.1038/pr.2013.204.
- Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012;345. https://doi.org/10.1136/bmj.e7976.
- Platt MJ. Outcomes in preterm infants. Public Health 2014;128:399-403. https://doi.org/10.1016/j.puhe.2014.03.010.
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261-9. https://doi.org/10.1016/S0140-6736(08)60136-1.
- Piening BC, Geffers C, Gastmeier P, Schwab F. Pathogen-specific mortality in very low birth weight infants with primary bloodstream infection. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0180134.
- Stoll BJ, Gordon T, Korones SB, Shankaran S, Tyson JE, Bauer CR, et al. Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr 1996;129:63-71. https://doi.org/10.1016/S0022-3476(96)70191-9.
- Mitha A, Foix-L’Hélias L, Arnaud C, Marret S, Vieux R, Aujard Y, et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics 2013;132:e372-80. https://doi.org/10.1542/peds.2012-3979.
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004;292:2357-65. https://doi.org/10.1001/jama.292.19.2357.
- Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK, Smith PB, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev 2012;88:69-74. https://doi.org/10.1016/S0378-3782(12)70019-1.
- Cailes B, Kortsalioudaki C, Buttery J, Pattnayak S, Greenough A, Matthes J, et al. Epidemiology of UK neonatal infections: the neonIN infection surveillance network. Arch Dis Child Fetal Neonatal Ed 2018;103:F547-F553. https://doi.org/10.1136/archdischild-2017-313203.
- Ponnusamy V, Perperoglou A, Venkatesh V, Curley A, Brown N, Tremlett C, et al. Skin colonisation at the catheter exit site is strongly associated with catheter colonisation and catheter-related sepsis. Acta Paediatr 2014;103:1233-8. https://doi.org/10.1111/apa.12779.
- Wilson CB, Lewis DB. Basis and implications of selectively diminished cytokine production in neonatal susceptibility to infection. Rev Infect Dis 1990;12:410-20. https://doi.org/10.1093/clinids/12.supplement_4.s410.
- Kalia YN, Nonato LB, Lund CH, Guy RH. Development of skin barrier function in premature infants. J Invest Dermatol 1998;111:320-6. https://doi.org/10.1046/j.1523-1747.1998.00289.x.
- National Neonatal Audit Programme . National Neonatal Audit Programme 2018 Report on 2017 Data 2018.
- Stoll BJ, Hansen N, Fanaroff AA, . Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110:285-91. https://doi.org/10.1542/peds.110.2.285.
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443-56. https://doi.org/10.1542/peds.2009-2959.
- Leistner R, Thürnagel S, Schwab F, Piening B, Gastmeier P, Geffers C. The impact of staffing on central venous catheter-associated bloodstream infections in preterm neonates – results of nation-wide cohort study in Germany. Antimicrob Resist Infect Control 2013;2. https://doi.org/10.1186/2047-2994-2-11.
- Olsen AL, Reinholdt J, Jensen AM, Andersen LP, Jensen ET. Nosocomial infection in a Danish neonatal intensive care unit: a prospective study. Acta Paediatr 2009;98:1294-9. https://doi.org/10.1111/j.1651-2227.2009.01322.x.
- Dubbink-Verheij GH, Bekker V, Pelsma ICM, van Zwet EW, Smits-Wintjens VEHJ, Steggerda SJ, et al. Bloodstream infection incidence of different central venous catheters in neonates: a descriptive cohort study. Front Pediatr 2017;5. https://doi.org/10.3389/fped.2017.00142.
- Machado JD, Suen VM, Figueiredo JF, Marchini JS. Biofilms, infection, and parenteral nutrition therapy. JPEN J Parenter Enteral Nutr 2009;33:397-403. https://doi.org/10.1177/0148607108327526.
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet 2001;358:135-8. https://doi.org/10.1016/S0140-6736(01)05321-1.
- Vasudevan C, Oddie SJ, McGuire W. Early removal versus expectant management of central venous catheters in neonates with bloodstream infection. Cochrane Database Syst Rev 2016;4. https://doi.org/10.1002/14651858.CD008436.pub3.
- Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr 2011;159:392-7. https://doi.org/10.1016/j.jpeds.2011.02.035.
- Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr 2011;159:720-5. https://doi.org/10.1016/j.jpeds.2011.05.033.
- Loveday HP, Wilson JA, Pratt RJ, Golsorkhi M, Tingle A, Bak A, et al. Epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2014;86:S1-70. https://doi.org/10.1016/S0195-6701(13)60012-2.
- O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 2011;39:1-34. https://doi.org/10.1016/j.ajic.2011.01.003.
- Balain M, Oddie SJ, McGuire W. Antimicrobial-impregnated central venous catheters for prevention of catheter-related bloodstream infection in newborn infants. Cochrane Database Syst Rev 2015;9. https://doi.org/10.1002/14651858.CD011078.pub2.
- Flemmer A, De Maio N, Schubert S, Wurster T, Klemme M, Messner H, et al. A randomized controlled trial to evaluate antibiotic impregnated percutaneously introduced central (PIC-) lines in preterm infants. Eur J Pediatr 2016;175.
- Wang H, Huang T, Jing J, Jin J, Wang P, Yang M, et al. Effectiveness of different central venous catheters for catheter-related infections: a network meta-analysis. J Hosp Infect 2010;76:1-11. https://doi.org/10.1016/j.jhin.2010.04.025.
- Chong HY, Lai NM, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy of antimicrobial central venous catheters in reducing catheter-related bloodstream infections in adults: abridged cochrane systematic review and network meta-analysis. Clin Infect Dis 2017;64:S131-S140. https://doi.org/10.1093/cid/cix019.
- Wu G, Chen Z, Sun Y, Xiao S, Xia Z. Impregnated central venous catheters in children: a systematic review of randomized controlled trials. Intensive Care Med 2017;43:1159-61. https://doi.org/10.1007/s00134-017-4777-1.
- Gilbert RE, Mok Q, Dwan K, Harron K, Moitt T, Millar M, et al. Impregnated central venous catheters for prevention of bloodstream infection in children (the CATCH trial): a randomised controlled trial. Lancet 2016;387:1732-42. https://doi.org/10.1016/S0140-6736(16)00340-8.
- Lai NM, Chaiyakunapruk N, Lai NA, O’Riordan E, Pau WS, Saint S. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD007878.pub3.
- Yücel N, Lefering R, Maegele M, Max M, Rossaint R, Koch A, et al. Reduced colonization and infection with miconazole-rifampicin modified central venous catheters: a randomized controlled clinical trial. J Antimicrob Chemother 2004;54:1109-15. https://doi.org/10.1093/jac/dkh483.
- Fraenkel D, Rickard C, Thomas P, Faoagali J, George N, Ware R. A prospective, randomized trial of rifampicin-minocycline-coated and silver-platinum-carbon-impregnated central venous catheters. Crit Care Med 2006;34:668-75. https://doi.org/10.1097/01.CCM.0000201404.05523.34.
- Cox EG, Knoderer CA, Jennings A, Brown JW, Rodefeld MD, Walker SG, et al. A randomized, controlled trial of catheter-related infectious event rates using antibiotic-impregnated catheters versus conventional catheters in pediatric cardiovascular surgery patients. J Pediatric Infect Dis Soc 2013;2:67-70. https://doi.org/10.1093/jpids/pis066.
- Darouiche RO, Berger DH, Khardori N, Robertson CS, Wall MJ, Metzler MH, et al. Comparison of antimicrobial impregnation with tunneling of long-term central venous catheters: a randomized controlled trial. Ann Surg 2005;242:193-200. https://doi.org/10.1097/01.sla.0000171874.29934.61.
- Darouiche RO, Raad II, Heard SO, Thornby JI, Wenker OC, Gabrielli A, et al. A comparison of two antimicrobial-impregnated central venous catheters. Catheter Study Group. N Engl J Med 1999;340:1-8. https://doi.org/10.1056/NEJM199901073400101.
- Hanna H, Benjamin R, Chatzinikolaou I, Alakech B, Richardson D, Mansfield P, et al. Long-term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter-related bloodstream infection in cancer patients: a prospective randomized clinical trial. J Clin Oncol 2004;22:3163-71. https://doi.org/10.1200/JCO.2004.04.124.
- León C, Ruiz-Santana S, Rello J, de la Torre MV, Vallés J, Álvarez-Lerma F, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med 2004;30:1891-9. https://doi.org/10.1007/s00134-004-2378-2.
- Marik PE, Abraham G, Careau P, Varon J, Fromm RE. The ex vivo antimicrobial activity and colonization rate of two antimicrobial-bonded central venous catheters. Critical Care Med 1999;27:1128-31. https://doi.org/10.1097/00003246-199906000-00034.
- Raad I, Darouiche R, Dupuis J, Abi-Said D, Gabrielle A, Hachem R, et al. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. Ann Intern Med 1997;127:267-74. https://doi.org/10.7326/0003-4819-127-4-199708150-00002.
- Schierholz JM, Fleck C, Beuth J, Pulverer G. The antimicrobial efficacy of a new central venous catheter with long-term broad-spectrum activity. J Antimicrob Chemother 2000;46:45-50. https://doi.org/10.1093/jac/46.1.45.
- Lachman P, Yuen S. Using care bundles to prevent infection in neonatal and paediatric ICUs. Curr Opin Infect Dis 2009;22:224-8. https://doi.org/10.1097/QCO.0b013e3283297b68.
- Payne V, Hall M, Prieto J, Johnson M. Care bundles to reduce central line-associated bloodstream infections in the neonatal unit: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2017;103:F422-9. https://doi.org/10.1136/archdischild-2017-313362.
- Sinha AK, Murthy V, Nath P, Morris JK, Millar M. Prevention of late onset sepsis and central-line associated blood stream infection in preterm infants. Pediatr Infect Dis J 2016;35:401-6. https://doi.org/10.1097/INF.0000000000001019.
- Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 1976;34:585-612. https://doi.org/10.1038/bjc.1976.220.
- Office for National Statistics . Births in England and Wales: Summary Tables 2019. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/datasets/birthsummarytables (accessed 22 July 2020).
- NHS England . Neonatal Critical Care 2013. www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/01/e08-serv-spec-neonatal-critical.pdf (accessed 2 August 2020).
- The PREVAIL Trial . Parent Information Sheet and Consent Form Version 6.0: 12 10 2015 2015. http://prevailtrial.org.uk/documents/Binder1.pdf (accessed 2 August 2020).
- Rump AFE, Güttler K, König DP, Yücel N, Korenkov M, Schierholz JM. Pharmacokinetics of the antimicrobial agents rifampicin and miconazole released from a loaded central venous catheter. J Hosp Infect 2003;53:129-35. https://doi.org/10.1053/jhin.2002.1358.
- Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 2009;49:1749-55. https://doi.org/10.1086/647952.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLOS Med 2010;7. https://doi.org/10.1371/journal.pmed.1000251.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-80.
- European Medicines Agency . ICH E9 Statistical Principles for Clinical Trials 1998. www.ema.europa.eu/en/ich-e9-statistical-principles-clinical-trials (accessed 22 July 2020).
- Bacon N, Brown M, Gilbert R, . PREVAIL Statistical Analysis Plan for Final Analysis Version 3.0 28 03 2018 2018. http://prevailtrial.org.uk/documents/ST001TEM01%20Statistical%20Analysis%20Plan%20v3%200%20PREVAIL%20final%20analysis%20v3%200%20280307%20(002).pdf (accessed 2 August 2020).
- Gilbert R, Oddie SJ. PREVenting Infection Using Antimicrobial Impregnated Long Lines (PREVAIL) Protocol v5.0 2017. http://prevailtrial.org.uk/documents/PREVAIL%20Protocol%20V5.0_%20Full%20Signatures.pdf (accessed 2 August 2020).
- Claxton K. Bayesian approaches to the value of information: implications for the regulation of new pharmaceuticals. Health Econ 1999;8:269-74. https://doi.org/10.1002/(SICI)1099-1050(199905)8:3<269::AID-HEC425>3.0.CO;2-D.
- Faria R, Walker S, Whyte S, Dixon S, Palmer S, Sculpher M. How to invest in getting cost-effective technologies into practice? A framework for value of implementation analysis applied to novel oral anticoagulants. Med Decis Making 2017;37:148-61. https://doi.org/10.1177/0272989X16645577.
- Department of Health and Social Care . NHS Reference Costs 2015 to 2016 n.d. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 10 March 2020).
- The PREVAIL trial . Case Report Form Completion Booklet n.d. http://prevailtrial.org.uk/documents/PREVAIL%20CRF%20Completion%20booklet%20%20V6.0.pdf (accessed 16 August 2020).
- Gale C, Morris I. Neonatal Data Analysis Unit (NDAU) Steering Board . The UK National Neonatal Research Database: using neonatal data for research, quality improvement and more. Arch Dis Child Educ Pract Ed 2016;101:216-18. https://doi.org/10.1136/archdischild-2015-309928.
- NHS . National Neonatal Data Set Overview n.d. www.datadictionary.nhs.uk/data_dictionary/messages/clinical_data_sets/data_sets/national_neonatal_data_set/national_neonatal_data_set_-_episodic_and_daily_care_fr.asp?shownav=1 (accessed 16 August 2020).
- Paediatric Intensive Care Audit Network (PICANet) . Paediatric Intensive Care Audit Network: Annual Report 2018 2018.
- NHS Digital . Hospital Episode Statistics (HES) n.d. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics#top (accessed 2 September 2019).
- National Casemix Office, NHS Digital . Casemix Companion – HRG4+ 2016 2017 Reference Costs Grouper 2017. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/grouper-and-tools-archive/costing-hrg4-2016-17-reference-costs-grouper (accessed 2 August 2020).
- Department of Health and Social Care . Reference Costs Guidance 2015 to 2016 2016.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics 2009;123:e312-27. https://doi.org/10.1542/peds.2008-1827.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Harron K, Mok Q, Dwan K, Ridyard CH, Moitt T, Millar M, et al. CATheter Infections in CHildren (CATCH): a randomised controlled trial and economic evaluation comparing impregnated and standard central venous catheters in children. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20180.
- Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 2011;128. https://doi.org/10.1542/peds.2010-3338.
- Hack M, Wilson-Costello D, Friedman H, Taylor GH, Schluchter M, Fanaroff AA. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992–1995. Arch Pediatr Adolesc Med 2000;154:725-31. https://doi.org/10.1001/archpedi.154.7.725.
- Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CM, et al. Trial of Indomethacin Prophylaxis in Preterms Investigators. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics 2009;123:313-18. https://doi.org/10.1542/peds.2008-0377.
- Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr 2008;153:170-5. https://doi.org/10.1016/j.jpeds.2008.02.033.
- van Vliet EO, de Kieviet JF, Oosterlaan J, van Elburg RM. Perinatal infections and neurodevelopmental outcome in very preterm and very low-birth-weight infants: a meta-analysis. JAMA Pediatr 2013;167:662-8. https://doi.org/10.1001/jamapediatrics.2013.1199.
- Alshaikh B, Yusuf K, Sauve R. Neurodevelopmental outcomes of very low birth weight infants with neonatal sepsis: systematic review and meta-analysis. J Perinatol 2013;33:558-64. https://doi.org/10.1038/jp.2012.167.
- Bakhuizen SE, de Haan TR, Teune MJ, van Wassenaer-Leemhuis AG, van der Heyden JL, van der Ham DP, et al. Meta-analysis shows that infants who have suffered neonatal sepsis face an increased risk of mortality and severe complications. Acta Paediatr 2014;103:1211-18. https://doi.org/10.1111/apa.12764.
- Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 2005;115:696-703. https://doi.org/10.1542/peds.2004-0569.
- Martin CR, Dammann O, Allred EN, Patel S, O’Shea TM, Kuban KC, et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J Pediatr 2010;157:751-6.e1. https://doi.org/10.1016/j.jpeds.2010.05.042.
- Doyle L. Victorian Infant Collaborative Study Group . Neonatal intensive care at borderline viability – is it worth it?. Early Hum Dev 2004;80:103-13. https://doi.org/10.1016/j.earlhumdev.2004.05.009.
- Doyle L, Bowman E, Callanan C, Carse E, Charlton MP, Drew J, et al. Outcome at 2 years of children 23–27 weeks’ gestation born in Victoria in 1991–92. J Paediatr Child Health 1997;33:161-5. https://doi.org/10.1111/j.1440-1754.1997.tb01021.x.
- Doyle LW, Anderson PJ. Victorian Infant Collaborative Study Group . Improved neurosensory outcome at 8 years of age of extremely low birthweight children born in Victoria over three distinct eras. Arch Dis Child Fetal Neonatal Ed 2005;90:F484-8. https://doi.org/10.1136/adc.2004.063362.
- Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Santhakumaran S, Statnikov Y, Gray D, Battersby C, Ashby D, Modi N, et al. Survival of very preterm infants admitted to neonatal care in England 2008–2014: time trends and regional variation. Arch Dis Child Fetal Neonatal Ed 2018;103:F208-F215. https://doi.org/10.1136/archdischild-2017-312748.
- Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med 2001;344:1966-72. https://doi.org/10.1056/NEJM200106283442602.
- Marlow N, Wolke D, Bracewell MA, Samara M. EPICure Study Group . Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med 2005;352:9-19. https://doi.org/10.1056/NEJMoa041367.
- Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med 2000;343:378-84. https://doi.org/10.1056/NEJM200008103430601.
- Petrou S, Mehta Z, Hockley C, Cook-Mozaffari P, Henderson J, Goldacre M. The impact of preterm birth on hospital inpatient admissions and costs during the first 5 years of life. Pediatrics 2003;112:1290-7. https://doi.org/10.1542/peds.112.6.1290.
- Goldacre MJ, Simmons H, Henderson J, Gill LE. Trends in episode based and person based rates of admission to hospital in the Oxford record linkage study area. Br Med J 1988;296:583-5. https://doi.org/10.1136/bmj.296.6621.583.
- Gill L, Goldacre M, Simmons H, Bettley G, Griffith M. Computerised linking of medical records: methodological guidelines. J Epidemiol Community Health 1993;47:316-19. https://doi.org/10.1136/jech.47.4.316.
- Schierholz JM, Nagelschmidt K, Nagelschmidt M, Lefering R, Yücel N, Beuth J. Antimicrobial central venous catheters in oncology: efficacy of a rifampicin-miconazole-releasing catheter. Anticancer Res 2010;30:1353-8.
- Kitchen WH, Doyle LW, Ford GW, Murton LJ, Keith CG, Rickards AL, et al. Changing two-year outcome of infants weighing 500 to 999 grams at birth: a hospital study. J Pediatr 1991;118:938-43. https://doi.org/10.1016/S0022-3476(05)82215-2.
- Petrou S, Johnson S, Wolke D, Marlow N. The association between neurodevelopmental disability and economic outcomes during mid-childhood. Child Care Health Dev 2013;39:345-57. https://doi.org/10.1111/j.1365-2214.2012.01368.x.
- Petrou S, Abangma G, Johnson S, Wolke D, Marlow N. Costs and health utilities associated with extremely preterm birth: evidence from the EPICure study. Value Health 2009;12:1124-34. https://doi.org/10.1111/j.1524-4733.2009.00580.x.
- Reid SM, Carlin JB, Reddihough DS. Survival of individuals with cerebral palsy born in Victoria, Australia, between 1970 and 2004. Dev Med Child Neurol 2012;54:353-60. https://doi.org/10.1111/j.1469-8749.2012.04218.x.
- Office for National Statistics . National Life Tables, UK: 2013–2015 2016.
- Mangham L, Petrou S. Modelling the Long-term Costs of Preterm Birth. London: Tommy’s, The Baby Charity; 2008.
- Petrou S, Henderson J, Bracewell M, Hockley C, Wolke D, Marlow N. EPICure Study Group . Pushing the boundaries of viability: the economic impact of extreme preterm birth. Early Hum Dev 2006;82:77-84. https://doi.org/10.1016/j.earlhumdev.2006.01.002.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:68-80. https://doi.org/10.1177/0272989X98018002S09.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Vemer P, Corro Ramos I, van Voorn GA, Al MJ, Feenstra TL. AdViSHE: A validation-assessment tool of health-economic models for decision makers and model users. PharmacoEconomics 2016;34:349-61. https://doi.org/10.1007/s40273-015-0327-2.
- Grosso A, Faria R, Bojke L, Donohue C, Fraser C, Harron K, et al. The cost-effectiveness of strategies preventing late-onset infection in preterm infants. Arch Dis Child 2020;105:452-7. https://doi.org/10.1136/archdischild-2019-317640.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64. https://doi.org/10.1016/S0167-6296(98)00039-3.
- Strong M, Oakley JE, Brennan A, Breeze P. Estimating the expected value of sample information using the probabilistic sensitivity analysis sample: a fast, nonparametric regression-based method. Med Decis Making 2015;35:570-83. https://doi.org/10.1177/0272989X15575286.
- Ismail AQT, Palmer K. Assessing the accuracy of the National Neonatal Audit Programme calculated central line-associated bloodstream infection rate from local data. Arch Dis Child Fetal Neonatal Ed 2017;102. https://doi.org/10.1136/archdischild-2017-313291.
- National Neonatal Audit Programme . National Neonatal Audit Programme 2017 Report on 2016 Data 2017.
- Fraser C, Muller-Pebody B, Blackburn R, Gray J, Oddie SJ, Gilbert RE, et al. Linking surveillance and clinical data for evaluating trends in bloodstream infection rates in neonatal units in England. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0226040.
- Fellegi IP, Sunter AB. A theory for record linkage. J Am Stat Assoc 1969;64:1183-210. https://doi.org/10.1080/01621459.1969.10501049.
- Rothstein DM. Rifamycins, alone and in combination. Cold Spring Harb Perspect Med 2016;6. https://doi.org/10.1101/cshperspect.a027011.
- Kramer RD, Rogers MA, Conte M, Mann J, Saint S, Chopra V. Are antimicrobial peripherally inserted central catheters associated with reduction in central line-associated bloodstream infection? A systematic review and meta-analysis. Am J Infect Control 2017;45:108-14. https://doi.org/10.1016/j.ajic.2016.07.021.
- Segreti J, Gvazdinskas LC, Trenholme GM. In vitro activity of minocycline and rifampin against staphylococci. Diagn Microbiol Infect Dis 1989;12:253-5. https://doi.org/10.1016/0732-8893(89)90022-9.
- Niël-Weise BS, Stijnen T, van den Broek PJ. Anti-infective-treated central venous catheters: a systematic review of randomized controlled trials. Intensive Care Med 2007;33:2058-68. https://doi.org/10.1007/s00134-007-0897-3.
- Wynn JL, Levy O. Role of innate host defenses in susceptibility to early-onset neonatal sepsis. Clin Perinatol 2010;37:307-37. https://doi.org/10.1016/j.clp.2010.04.001.
- Sculpher MJ, Claxton K, Drummond M, McCabe C. Whither trial-based economic evaluation for health care decision making?. Health Econ 2006;15:677-87. https://doi.org/10.1002/hec.1093.
- Haller S, Deindl P, Cassini A, Suetens C, Zingg W, Abu Sin M, et al. Neurological sequelae of healthcare-associated sepsis in very-low-birthweight infants: umbrella review and evidence-based outcome tree. Euro Surveill 2016;21. https://doi.org/10.2807/1560-7917.ES.2016.21.8.30143.
- Berrington JE, Stewart CJ, Cummings SP, Embleton ND. The neonatal bowel microbiome in health and infection. Curr Opin Infect Dis 2014;27:236-43. https://doi.org/10.1097/QCO.0000000000000061.
- Claxton K, Walker S, Palmer S, Sculpher M. Appropriate Perspectives for Health Care Decisions. York: Centre for Health Economics, University of York; 2010.
- Tubeuf S, Saloniki ECC, Cottrell D. Parental health spillover in cost-effectiveness analysis: evidence from self-harming adolescents in England. PharmacoEconomics 2019;37:513-30. https://doi.org/10.1007/s40273-018-0722-6.
- Prosser LA, Lamarand K, Gebremariam A, Wittenberg E. Measuring family HRQoL spillover effects using direct health utility assessment. Med Decis Making 2015;35:81-93. https://doi.org/10.1177/0272989X14541328.
- Hockenhull JC, Dwan KM, Smith GW, Gamble CL, Boland A, Walley TJ, et al. The clinical effectiveness of central venous catheters treated with anti-infective agents in preventing catheter-related bloodstream infections: a systematic review. Crit Care Med 2009;37:702-12. https://doi.org/10.1097/CCM.0b013e3181958915.
- Marin MG, Lee JC, Skurnick JH. Prevention of nosocomial bloodstream infections: effectiveness of antimicrobial-impregnated and heparin-bonded central venous catheters. Crit Care Med 2000;28:3332-8. https://doi.org/10.1097/00003246-200009000-00035.
- Veenstra DL, Saint S, Sullivan SD. Cost-effectiveness of antiseptic-impregnated central venous catheters for the prevention of catheter-related bloodstream infection. JAMA 1999;282:554-60. https://doi.org/10.1001/jama.282.6.554.
- Halton KA, Cook DA, Whitby M, Paterson DL, Graves N. Cost effectiveness of antimicrobial catheters in the intensive care unit: addressing uncertainty in the decision. Crit Care 2009;13. https://doi.org/10.1186/cc7744.
- Jackson CH, Bojke L, Thompson SG, Claxton K, Sharples LD. A framework for addressing structural uncertainty in decision models. Med Decis Making 2011;31:662-74. https://doi.org/10.1177/0272989X11406986.
- The ELFIN trial investigators group . Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet 2019;393:423-33. https://doi.org/10.1016/S0140-6736(18)32221-9.
- National Institute for Health Research . Outcome After Selective Early Closure of Ductus Arteriosus in Extremely Preterm Babies (Baby-OSCAR Trial) n.d. www.journalslibrary.nihr.ac.uk/programmes/hta/119215/#/ (accessed 31 August 2020).
- ClinicalTrials.gov . Neonatal and Paediatric Pharmacokinetics of Antimicrobials Study (NAPPA) n.d. https://clinicaltrials.gov/ct2/show/NCT01975493 (accessed 31 August 2020).
- Great Britain . Data Protection Act 2018 2018.
- Department of Health and Social Care . NHS Reference Costs 2014 to 2015 n.d. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 21 August 2020).
- NHS Improvement . NHS Reference Costs 2016 17 n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed 21 August 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- NHS Digital . Users, Uses and Access to Hospital Episode Statistics n.d. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics/users-uses-and-access-to-hospital-episode-statistics (accessed 21 August 2020).
- Hinde S, Spackman E. Bidirectional citation searching to completion: an exploration of literature searching methods. PharmacoEconomics 2015;33:5-11. https://doi.org/10.1007/s40273-014-0205-3.
- Sayers A, Ben-Shlomo Y, Blom AW, . Probabilistic record linkage. Int J Epidemiol 2016;45:954-64. https://doi.org/10.1093/ije/dyv322.
- Harron K, Dibben C, Boyd J, Hjern A, Azimaee M, Barreto ML, et al. Challenges in administrative data linkage for research. Big Data Soc 2017;4. https://doi.org/10.1177/2053951717745678.
- Public Health England . Laboratory Reporting to Public Health England: A Guide for Diagnostic Laboratories 2016.
- Lopez Bernal J, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2016;46:348-55. https://doi.org/10.1093/ije/dyw098.
Appendix 1 Additional information on clinical effectiveness methods
| Protocol version | Key amendments |
|---|---|
| 2.0 (5 May 2015) |
|
| 3.0 (12 October 2015) |
|
| 4.0 (19 August 2016) |
|
| 5.0 (26 April 2017) |
|
| Organism | Pathogen group |
|---|---|
| Gram positive | |
| CoNS | Potential pathogen or likely contaminant |
| Staphylococcus aureus | Clearly pathogenic organism |
| Group B streptococci | Clearly pathogenic organism |
| Streptococcus pneumoniae | Clearly pathogenic organism |
| Streptococcus (other) | Potential pathogen or likely contaminant |
| Enterococcus spp. | Clearly pathogenic organism |
| Micrococcus sp. | Potential pathogen or likely contaminant |
| Bacillus sp. | Clearly pathogenic organism |
| Diphtheroids | Potential pathogen or likely contaminant |
| Propionibacterium acnes | Potential pathogen or likely contaminant |
| Listeria monocytogenes | Clearly pathogenic organism |
| Other Gram positive | Individual isolates reviewed |
| Gram negative | |
| Pseudomonas sp. | Clearly pathogenic organism |
| Klebsiella spp. | Clearly pathogenic organism |
| Enterobacter spp. | Clearly pathogenic organism |
| Acinetobacter spp. | Clearly pathogenic organism |
| E. coli | Clearly pathogenic organism |
| Enterococcus spp. | Clearly pathogenic organism |
| Serratia sp. | Clearly pathogenic organism |
| Coliform | Clearly pathogenic organism |
| Citrobacter sp. | Clearly pathogenic organism |
| Burkholderia sp. | Clearly pathogenic organism |
| Haemophilus sp. | Clearly pathogenic organism |
| Other Gram negative | Individual isolates reviewed |
| Fungi | |
| Candida albicans | Clearly pathogenic organism |
| Non-candida albicans species | Clearly pathogenic organism |
| Candida (other) | Clearly pathogenic organism |
| Other fungi | Clearly pathogenic organism |
| Other fungal organism | Clearly pathogenic organism |
Appendix 2 Additional information on clinical effectiveness results
| Centre code | Date of site opening | Date of site closure | Planned total recruitment (n) | Date of first randomisation | Date of last randomisation | Number randomised (n) |
|---|---|---|---|---|---|---|
| 00075 | 10 August 2015 | 9 January 2017 | 40 | 24 August 2015 | 6 January 2017 | 99 |
| 00031 | 11 August 2015 | 12 January 2017 | 57 | 12 August 2015 | 11 January 2017 | 92 |
| 00472 | 1 September 2015 | 9 January 2017 | 59 | 2 September 2015 | 25 November 2016 | 52 |
| 00308 | 7 September 2015 | 9 January 2017 | 63 | 9 September 2015 | 12 December 2016 | 65 |
| 00039 | 7 September 2015 | 9 January 2017 | 34 | 18 September 2015 | 28 November 2016 | 31 |
| 00213 | 8 September 2015 | 9 January 2017 | 36 | 13 September 2015 | 10 December 2016 | 40 |
| 00134 | 18 September 2015 | 9 January 2017 | 36 | 26 June 2015 | 6 December 2016 | 47 |
| 18937 | 23 September 2015 | 8 December 2016 | 40 | 3 October 2015 | 4 December 2016 | 50 |
| 00292 | 2 October 2015 | 9 January 2017 | 71 | 2 October 2015 | 6 December 2016 | 57 |
| 00228 | 12 October 2015 | 9 January 2017 | 61 | 23 October 2015 | 9 December 2016 | 58 |
| 18912 | 10 November 2015 | 8 December 2016 | 26 | 27 November 2015 | 12 November 2016 | 23 |
| 18938 | 11 November 2015 | 9 January 2017 | 27 | 15 November 2015 | 7 December 2016 | 44 |
| 00234 | 1 December 2015 | 9 December 2016 | 41 | 4 December 2015 | 23 November 2016 | 24 |
| 00169 | 3 December 2015 | 9 January 2017 | 33 | 11 January 2016 | 29 October 2016 | 37 |
| 20019 | 4 December 2015 | 8 December 2016 | 73 | 4 December 2015 | 5 December 2016 | 35 |
| 18854 | 4 January 2016 | 4 November 2016 | 33 | 4 January 2016 | 13 June 2016 | 8 |
| 00235 | 20 January 2016 | 9 January 2017 | 59 | 22 January 2016 | 11 December 2016 | 60 |
| 00227 | 8 February 2016 | 13 December 2016 | 69 | 18 February 2016 | 30 November 2016 | 39 |
| Total | 858 | 861 | ||||
| Centre code | Number of babies’ parents | Number (%) of babies’ parents consented | Number (%) of babies randomised | |
|---|---|---|---|---|
| Approached for consent | Not approached for consent | |||
| 00075 | 127 | 3 | 104 (81.9) | 99 (95.2) |
| 00031 | 132 | 38 | 105 (79.5) | 92 (87.6) |
| 00472 | 125 | 32 | 65 (52.0) | 52 (80.0) |
| 00308 | 104 | 46 | 69 (66.3) | 65 (94.2) |
| 00039 | 54 | 14 | 31 (57.4) | 31 (100.0) |
| 00213 | 54 | 16 | 40 (74.1) | 40 (100.0) |
| 00134 | 68 | 20 | 47 (69.1) | 47 (100.0) |
| 18937 | 72 | 19 | 50 (69.4) | 50 (100.0) |
| 00292 | 88 | 31 | 61 (69.3) | 57 (93.4) |
| 00228 | 117 | 87 | 81 (69.2) | 58 (71.6) |
| 18912 | 71 | 10 | 24 (33.8) | 23 (95.8) |
| 18938 | 65 | 9 | 48 (73.8) | 44 (91.7) |
| 00234 | 86 | 39 | 31 (36.0) | 24 (77.4) |
| 00169 | 40 | 20 | 37 (92.5) | 37 (100.0) |
| 20019 | 43 | 8 | 36 (83.7) | 35 (97.2) |
| 18854 | 16 | 2 | 8 (50.0) | 8 (100.0) |
| 00235 | 89 | 8 | 60 (67.4) | 60 (100.0) |
| 00227 | 53 | 85 | 40 (75.5) | 39 (97.5) |
| Total | 1404 | 487 | 937 (66.7) | 861 (91.9) |
| Reason | Number (%) of babies (N = 487) |
|---|---|
| Parents not available to consent | 163 (33.5) |
| Parents’ lack of understanding | 8 (1.6) |
| Parents do not understand English/Urdu | 18 (3.7) |
| Consultant preference | 22 (4.5) |
| Missed by clinical team | 134 (27.5) |
| Baby previously entered into the PREVAIL trial | 10 (2.1) |
| No reason given | 5 (1.0) |
| Other reason | 148 (30.4) |
| Reason | Number (%) of babies (N = 467) |
|---|---|
| No reason provided | 67 (14.3) |
| Parent does not want to take part in research | 195 (41.8) |
| Parent does not wish baby to be randomly assigned to treatment | 9 (1.9) |
| Parent does not wish baby to have AM-PICC | 18 (3.9) |
| Other reason | 180 (38.5) |
| Reason | Number (%) of babies (N = 76) |
|---|---|
| Baby requires different size PICC | 8 (10.5) |
| Baby no longer requires a PICC | 49 (64.5) |
| Baby died | 6 (7.9) |
| Trial-trained staff not available | 1 (1.3) |
| Unable to access randomisation system | 0 (0.0) |
| PICC (Premicath 1 French) not available | 0 (0.0) |
| Other reason | 13 (17.1) |
FIGURE 15.
Recruitment graph.
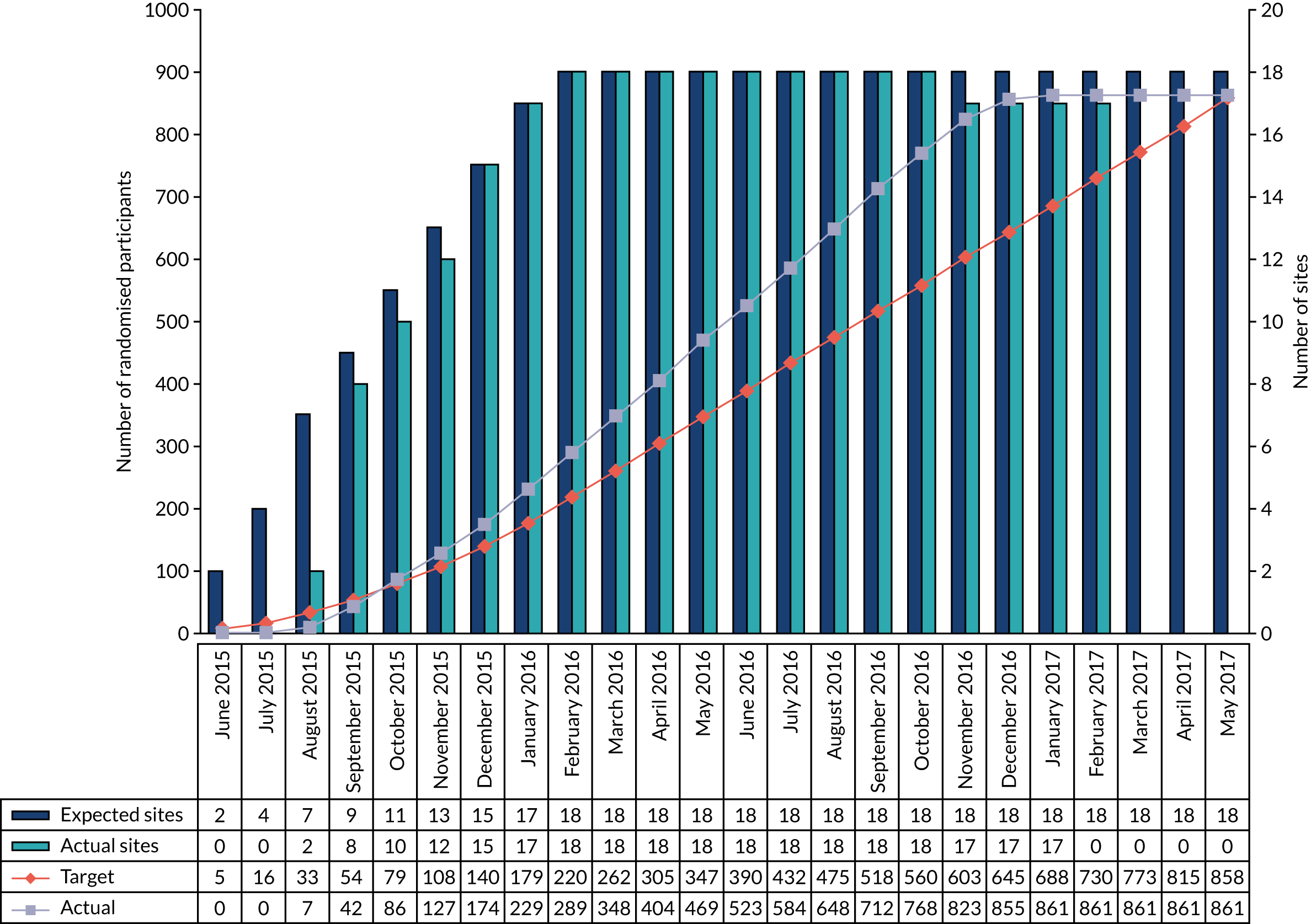
| Site | Total participants randomised (n) | Participants co-enrolled in other trials, n (%) | ||||
|---|---|---|---|---|---|---|
| ELFIN129 | Baby OSCAR130 | NAPPA131 | Other | Total | ||
| Birmingham Women’s Hospital | 65 | 22 (33.8) | 5 (7.7) | 0 (0.0) | 5 (7.7) | 32 (49.2) |
| Bradford Royal Infirmary | 99 | 80 (80.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 80 (80.8) |
| Homerton Hospital | 57 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| John Radcliffe Hospital, Oxford | 39 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Leeds General Infirmary | 35 | 11 (31.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 (31.4) |
| Leicester Royal Infirmary | 91a | 38 (41.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 38 (41.8) |
| Liverpool Women’s Hospital | 58 | 0 (0.0) | 0 (0.0) | 1 (1.7) | 4 (6.9) | 5 (8.6) |
| Newham General Hospital | 8 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Nottingham City Hospital | 31 | 7 (22.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (22.6) |
| Queen’s Hospital Romford | 23 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Queen’s Medical Centre, Nottingham | 40 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Royal Bolton Hospital | 37 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.7) | 1 (2.7) |
| Royal Oldham Hospital | 50 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.0) | 2 (4.0) |
| Royal Preston Hospital | 44 | 9 (20.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (20.5) |
| St Mary’s Hospital, Manchester | 60 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (5.0) | 3 (5.0) |
| St Michael’s Hospital, Bristol | 24 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| The Jessop Wing, Sheffield | 52 | 3 (5.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (5.8) |
| The Royal London Hospital | 47 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total (n) | 860a | 170 | 5 | 1 | 15 | 191 |
| Oversight committee | Meeting dates | Recommendations |
|---|---|---|
| IDSMC | 29 April 2015 (joint with TSC) | |
| 12 May 2016 | Trial to be continued | |
| July 2016 (via e-mail) | Trial to be continued | |
| TSC | 29 April 2015 (joint with IDSMC) | |
| 31 May 2016 | Trial to be continued | |
| 9 August 2016 | Trial to be closed to recruitment when target is met, with no need to continue recruitment until planned closure date |
| AM-PICC (N = 430) | S-PICC (N = 431) | |||
|---|---|---|---|---|
| n | n (%) | n | n (%) | |
| Culture samples takena | ||||
| Blood or CSF | 379 | 198 (46.0) | 329 | 190 (44.1) |
| Peripheral venous blood | 321 | 183 (42.6) | 268 | 178 (41.3) |
| CSF | 40 | 33 (7.7) | 38 | 34 (7.9) |
| Other | 18 | 16 (3.7) | 23 | 20 (4.6) |
| PICC tip | 314 | 313 (72.8) | 310 | 310 (71.9) |
| Rifampicin resistance tested in positive culturesb | ||||
| Blood or CSF | 48 | 21 (43.8) | 46 | 25 (54.3) |
| Peripheral venous blood | 44 | 21 (47.7) | 42 | 23 (54.8) |
| CSF | 0 | 0 (0.0) | 3 | 1 (33.3) |
| Other | 5 | 0 (0.0) | 3 | 2 (66.7) |
| PICC tip | 47 | 32 (68.1) | 90 | 61 (67.8) |
| Protocol deviation | AM-PICC (n = 430 participants), n (%) | S-PICC (n = 431 participants), n (%) |
|---|---|---|
| Any protocol deviation | 171 (39.8) | 175 (40.6) |
| At least one major deviation | 61 (14.2) | 25 (5.8) |
| Baby did not receive allocated 1-French Premicath | 57 (13.3) | 24 (5.6) |
| Blood/CSF samples either not sent for culture, lost, damaged or not analysed | 3 (0.7) | 1 (0.2) |
| Baby was withdrawn from trial prior to completing clinical follow-up | 1 (0.2) | 0 (0.0) |
| Parent/legal representative of the baby did not give informed written consent for the trial | 0 (0.0) | 0 (0.0) |
| Baby was previously entered into this trial | 0 (0.0) | 0 (0.0) |
| Baby had a known allergy or hypersensitivity to rifampicin or miconazole | 0 (0.0) | 0 (0.0) |
| Date and time of PICC removal were not recorded | 0 (0.0) | 0 (0.0) |
| At least one minor deviation | 132 (30.7) | 155 (36.0) |
| PICC tip culture not taken at removal | 72 (16.7) | 95 (22.0) |
| Resistance testing not performed on positive culture | 69 (16.0) | 66 (15.3) |
| Baby has PICC inserted > 48 hours after randomisation | 8 (1.9) | 5 (1.2) |
| Baby was withdrawn from trial follow-up prematurely | 4 (0.9) | 3 (0.7) |
| Subgroup | AM-PICC (N = 46 participants experiencing a BSI) (n) | S-PICC (N = 44 participants experiencing a BSI) (n) |
|---|---|---|
| Gestational age of < 28 weeks | 33 | 35 |
| Gestational age of ≥ 28 weeks | 12 | 8 |
| Gestational age missing | 1 | 1 |
FIGURE 16.
Kaplan–Meier plot for babies born at < 28 weeks of gestation. Values under the graph represent the number of participants remaining at risk at each time point: group 1, AM-PICC; group 2, S-PICC.
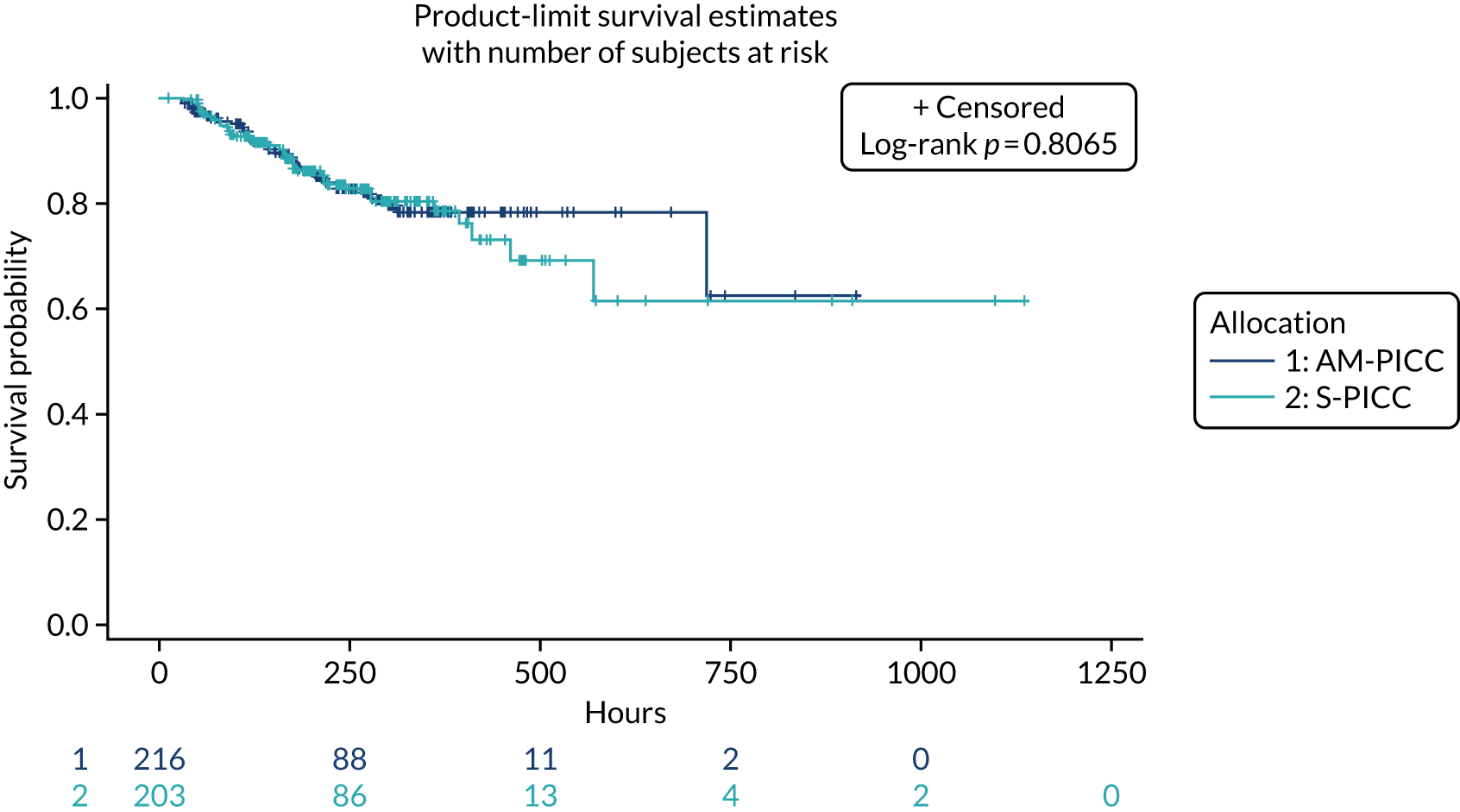
FIGURE 17.
Kaplan–Meier plot for babies born at ≥ 28 weeks of gestation. Values under the graph represent the number of participants remaining at risk at each time point: group 1, AM-PICC; group 2, S-PICC.
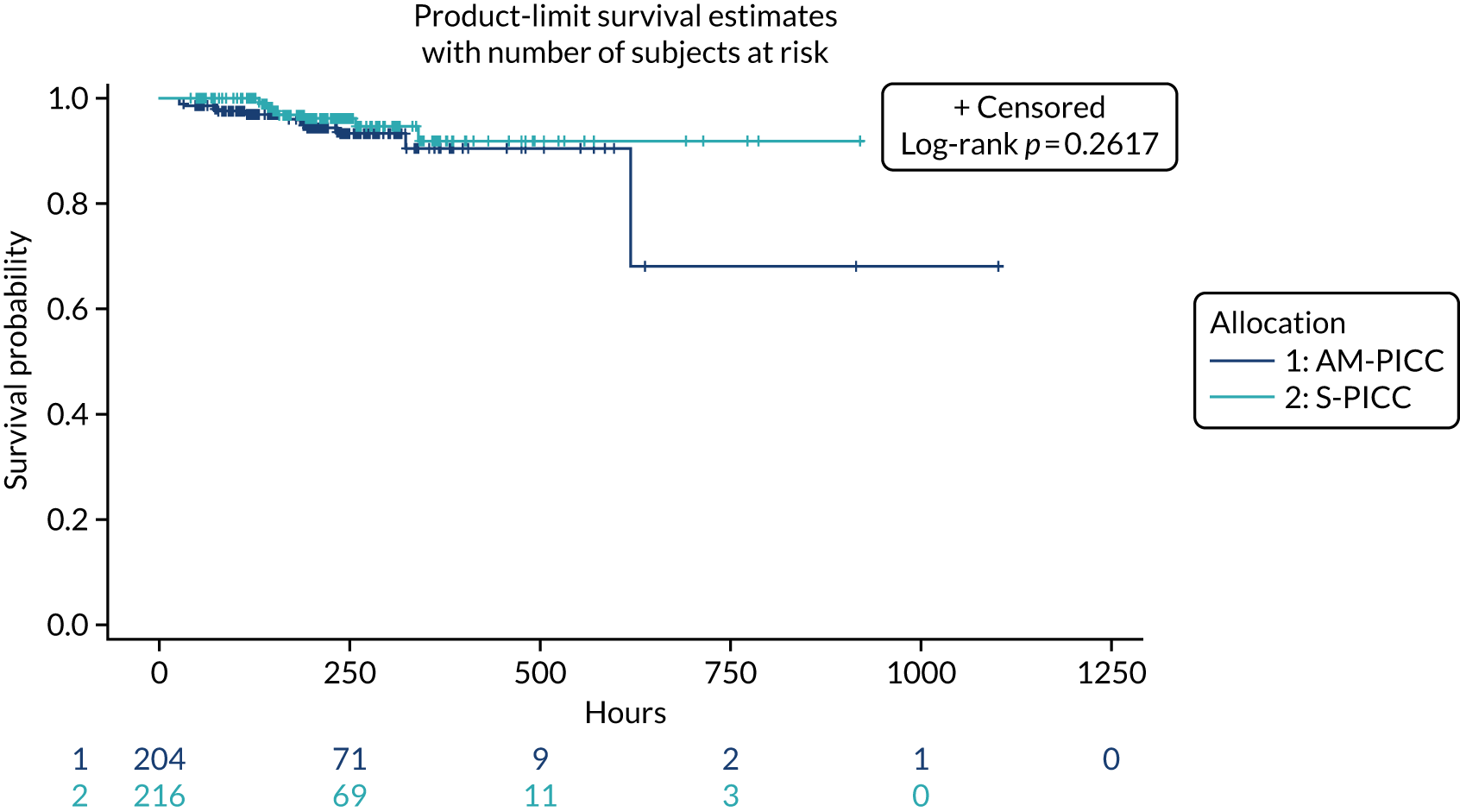
| Sample type | Resistant organism | Participants,a n (%) | |
|---|---|---|---|
| AM-PICC | S-PICC | ||
| Blood/CSF culture | Entercoccus spp. | 3 (75.0) | 2 (22.2)b |
| Klebsiella spp. | 1 (25.0) | 2 (22.2)c | |
| E. coli | 0 (0.0) | 2 (22.2)b | |
| Acinetobacter spp. | 0 (0.0) | 1 (11.1) | |
| CoNS | 0 (0.0) | 1 (11.1) | |
| Enterobacter spp. | 0 (0.0) | 1 (11.1)b | |
| Total (n) | 4 | 9 | |
| PICC tip culture | CoNS | 10 (71.4) | 2 (40.0)b |
| Candida albicans | 0 (0.0) | 1 (20.0)b | |
| Corynebacterium tuberculostearicum | 1 (7.1) | 0 (0.0) | |
| Enterobacteriaceae | 1 (7.1) | 0 (0.0) | |
| Enterobacter spp. | 0 (0.0) | 1 (20.0)b | |
| Enterococcus spp. | 0 (0.0) | 1 (20.0) | |
| Klebsiella spp. | 1 (7.1) | 0 (0.0) | |
| Raoultella ornithinolytica | 1 (7.1) | 0 (0.0) | |
| Total (n) | 14 | 5 | |
| Number of BSIs per baby | Participants, n (%) | |
|---|---|---|
| AM-PICC (N = 430) | S-PICC (N = 431) | |
| 0 | 384 (89.3) | 387 (89.8) |
| 1 | 43 (10.0) | 43 (10.0) |
| 2 | 3 (0.7) | 1 (0.2) |
| Type of treatment | Participants, n (%) | |
|---|---|---|
| AM-PICC (N = 430) | S-PICC (N = 431) | |
| Laser | 38 (8.8) | 28 (6.5) |
| Cryotherapy | 0 (0.0) | 0 (0.0) |
| Injection | 4 (0.9) | 2 (0.5) |
| Missing | 0 (0.0) | 1 (0.2) |
Appendix 3 Additional information on economic analysis: data access
Overview
Data sources
The economic analysis conducted by the team at the UoY for the PREVAIL project required routinely collected health-care data from NHS Digital, PICANet and NDAU, as well as data collected in the clinical trial by the UoL. The team at UCL developed the algorithm to select the babies enrolled in the PREVAIL trial who were recorded in the NNRD held by NDAU.
Data flows
The data flow was as follows: the UoL sent the patient identifiers to NHS Digital, PICANet, NDAU and UCL. NHS Digital selected the cohort using the identifiers and sent the administrative data identified with trial identifier to the UoY; the process was similar with PICANet, but the data were sent to the UoL, then the UoY. The UoY generated a database with the NHS Digital data on date of death and sent it to UoL. UCL linked the PREVAIL trial cohort to NNRD as part of the PREVAIL trial generalisability analysis and sent Badger identifiers (IDs) (a pseudonymised identifier) for PREVAIL babies to NDAU; NDAU used the Badger IDs to select the cohort and sent the data to the UoY. UoL sent the PREVAIL trial data to the UoY.
Data transfer
The UoY submitted the data-sharing applications to NHS Digital, PICANet and NDAU on 21 November 2016. The UoY received the NHS Digital data on hospitalisations on 24 May 2018, and the NHS Digital data on date of death on 27 September 2018, the PICANet data on the 9 May 2018 and the NDAU data on 24 August 2018. The UoY initiated the process to develop the data-sharing agreement between the UoY and the UoL on 3 May 2018. The UoY received the data on the 16 August 2018.
NHS Digital
Preparing the data-sharing application
The UoY submitted the data-sharing application to NHS Digital on 21 November 2016.
Revising the data-sharing application
NHS Digital had a number of queries with the application, which had to be addressed before formal submission to the Independent Group Advising on the Release of Data (IGARD). These issues were related to ensuring that the project had informed consent for sharing of date of death; ensuring that the trial participants had access to information about how their data were being used; processes for withdrawal from the data-sharing process; explaining the rationale for the project in lay terms; and detailing the benefits to the NHS, outputs and target dates of conducting the research. This took place between November 2016 and September 2017.
Meeting the security requirements
The data-sharing application for NHS Digital required answering various information technology queries from the NHS Digital by the UoY and the UoL. This took place between May and July 2017.
Submission to the Independent Group Advising on the Release of Data
The data-sharing application was submitted to the IGARD on 11 September 2017.
Update to the framework contract
The IGARD approved the application on 20 September 2017. At this point, it emerged that the UoY and UoL had not yet signed up to the the Data Sharing Framework Contract version 2. This meant that the data-sharing agreement could not be issued until the two institutions signed up to it, which took place in October.
Signing the data-sharing agreement
In December 2017, the UoY contracts’ office concluded that UCL and UoL had to sign the hard copies of the data-sharing agreement so that UoY could sign it electronically. UCL signed it on 2 January 2018 and UoL on 5 February 2018. The data-sharing agreement was executed on 27 February 2018.
Transfer of hospitalisation data
The data were received at the UoY on 24 May 2018.
Date of death missing
On 6 June 2018, the UoY contacted NHS Digital to enquire about the data on date of death, which were missing from the data sets, but were included in the data-sharing agreement. On 27 September 2018, NHS Digital confirmed that the data were ready for transfer.
Transfer of date of death data
The data were received at Centre for Health Economics on 27 September 2018.
Transfer of the date of death to University of Liverpool
On 24 October 2018, the UoL confirmed that it still required the date of death data; the data were transferred by the UoY on the same day.
Paediatric Intensive Care Audit Network
Data-sharing application to the Paediatric Intensive Care Audit Network
The UoY submitted the data-sharing application to PICANet on 21 November 2016. PICANet replied on 22 November, explaining that data-sharing applications were now processed via HQIP, which required another data-sharing application and agreement forms.
Data-sharing application to the Healthcare Quality Improvement Partnership
The UoY developed the data-sharing application and agreement between 29 November 2016 and 2 March 2018.
Approval from the Healthcare Quality Improvement Partnership
The UoY submitted the data-sharing application and agreement to HQIP on 14 March 2017. HQIP approved the application on 16 June 2017. PICANet signed the agreement on 16 June, and HQIP signed on 19 July 2017.
Data transfer
The UoL transferred PREVAIL trial identifiers to PICANet on 5 March 2018. The UoY received the data on 9 May 2018.
Neonatal Data Analysis Unit
Data-sharing application
The UoY submitted the data-sharing application to NDAU on 21 November 2016. NDAU approved the data-sharing application on 5 May 2017.
Data-sharing contract
The UoY contacted NDAU for the data-sharing contract on 29 June 2017. The data-sharing contract was signed on 10 January 2018.
Data-sharing agreement
On 7 March 2018, NDAU requested a data-sharing agreement in addition to the contract. The UoY signed the data-sharing agreement on 19 March 2018.
New data-sharing agreement
On 23 May 2018, NDAU informed the UoY that, given that the General Data Protection Regulation (GDPR)132 had already come into force, it required a new data-sharing agreement that was GDPR compliant. On 26 June 2018, NDAU concluded that, rather than a new data-sharing agreement, an amendment to the original contract, which had been signed on 10 January 2018, was needed. The addendum to the original contract was executed on 24 August 2018.
Privacy notice
University College London, as the study sponsor, is required to approve the privacy notice. The UoY sent the privacy notice to UCL for approval on 10 July 2018. It was approved on 13 August 2018.
Data transfer
The UoY received the data on 24 August 2018.
The PREVenting infection using Antimicrobial-Impregnated Long lines trial data
Developing the data-sharing agreement
It came to light in early May 2018 that the UoY needed to have an agreement in place with the UoL to receive the PREVAIL trial data, given that the project was contracted separately between UCL and each of the partner organisations. The UoY initiated the data-sharing agreement between the UoY and the UoL on 3 May 2018.
Sign-off by University of Liverpool
The UoL signed off the agreement on 4 July 2018.
Sign-off by sponsor
University College London was contacted on 7 July 2018 to sign off the data-sharing agreement and privacy notice (see Privacy notice). UCL commented on the data-sharing agreement on 1 August 2018.
Execution of contract between all parties
On 16 August 2018, the contract was fully executed.
Data transfer
The UoY received the PREVAIL trial data on 16 August 2018.
Appendix 4 Additional information on economic analysis: data
Hospital Episode Statistics Admitted Patient Care data on the PREVenting infection using Antimicrobial-Impregnated Long lines trial babies from NHS Digital
Admitted Patient Care data included trial identifier; HES identifier (anonymised); provider code; provider type; patient’s sex, month and year of birth; age at admission; dates of admission and discharge; method of admission and discharge; source of admission and discharge destination; dates that the episode started and finished, its duration, and whether or not it finished within the HES data year; episode key; the episode order within the spell and the episode type; indicator of the duration, start and end of the spell; main specialty under which the consultant was contracted and the specialty under which the consultant was working; patient classification; number of bed-days within the HES data year; primary and secondary diagnosis; trust-derived HRG value and its version number; neonatal level of care; procedures codes and dates; post-operative duration; and date of death according to the Personal Demographics Service. The HES APC data do not include information on the stays in intensive care units.
Hospital Episode Statistics outpatient data on the PREVenting infection using Antimicrobial-Impregnated Long lines trial babies from NHS Digital
The outpatient care data included trial identifier, record identifier, patient’s sex, date of the appointment, whether or not the patient attended the appointment, whether it is a first attendance or follow-up, trust-derived HRG value and its version number, main specialty under which the consultant was contracted and the specialty under which the consultant was working, procedure codes, and date of death according to the Personal Demographics Service.
Hospital Episode Statistics accident and emergency data on the PREVenting infection using Antimicrobial-Impregnated Long lines trial babies from NHS Digital
The A&E data included trial identifier, record identifier, age at the time of the A&E visit and at the time of arrival, A&E patient group, date of A&E visit, code of the dominant procedure, trust-derived HRG value and version number, record identifier of the APC episode that the A&E visit was linked to, tests and investigations, and treatment code.
Paediatric intensive care data on the PREVenting infection using Antimicrobial-Impregnated Long lines trial babies from the Paediatric Intensive Care Audit Network
The paediatric intensive care data included trial identifier, record identifier, dates of admission and discharge, admission type, discharge destination, critical care activity, and HRG code and description.
Neonatal care data on the PREVenting infection using Antimicrobial-Impregnated Long lines trial babies from the National Neonatal Research Database
The neonatal intensive care data included trial identifier, record identifier, date and time of birth, date and time of admission, date and time of discharge, HRG code, person accompanying baby (if transported), critical care episode identifier, critical care activity and diagnoses, NHS code of hospital receiving the baby, NHS code of the hospital baby was admitted from, detail of exact location of hospital baby was admitted from, reason for admission, destination on discharge, reason for discharge, type of ward baby is discharged to and NHS code of hospital the baby is transferred to.
PREVenting infection using Antimicrobial-Impregnated Long lines trial data
The PREVAIL trial data included type of allocated PICC; screening number; randomisation number; date of randomisation; time of randomisation; person performing the randomisation; sex; date of birth; time of birth; birthweight; final agreed estimated date of delivery; inborn status; date and time of admission to a NNU; mode of delivery; membrane rupture status; Apgar score; heart rate in beats per minute (bpm) (> 100 bpm at 5 minutes of age); presence of major congenital anomalies and type; use of antenatal corticosteroids; use of antibiotics within the 12 hours prior to delivery; presence of surgical procedure in the 14 days prior to randomisation (and date, if present); presence of samples taken for culture at any period within 72 hours prior to randomisation; antimicrobial use (antibiotics, enteral antifungal) at any period within 72 hours prior to randomisation; respiratory support (and highest level of support) at any period within 72 hours prior to randomisation; presence and type of devices in situ at randomisation; PICC placement details (whether or not a PICC was inserted and the reason for non-insertion, gauge type and justification, insertion site, whether or not allocation respected randomisation, date and time of insertion); date, time, and reason for PICC removal; whether or not the PICC tip was sent for culture and, if not, the reason why it was not sent; baby status (whether discharged, transferred or dead) with date and time; reason for death (PICC use or baby’s underlying conditions); cause of death as per death certificate; date of post-randomisation assessment; in the case of transfer to other hospital, the details of the receiving hospital; PICC line status at transfer; full milk feeds status and date baby first reached full milk feeds; stays in wards other than NNU in admitting hospital; transfers for surgery during NNU stay at admitting hospital; NEC status and date of diagnosis; clinical outcomes in admitting hospital; total duration (in days) of parenteral nutrition in admitting hospital; date of withdrawal (if happened); person who made the withdrawal decision; and reason for withdrawal.
Appendix 5 Additional information on economic analysis: data cleaning
Cleaning of National Neonatal Research Database data
Figure 18 summarises the data preparation. The file with the episode dates (the ‘episode file’) contained data on 848 (98.49%) PREVAIL babies and 1575 episodes of care. Seven episodes were duplicates; for five of these, it was uncertain if these referred to PREVAIL babies. All seven duplicate episodes were permanently deleted. This left 1568 unique episodes about 848 PREVAIL babies.
FIGURE 18.
Preparation of the neonatal care data (received from NNRD).
The file with the daily care record (the ‘daily file’) contained data on 848 (98.49%) PREVAIL babies with 57,702 records; each record represents a day in the neonatal care unit. Of these, 86 records were removed because no HRG was recorded. As a result, records were lost for two PREVAIL babies. According to the anonymised time stamp, 5948 days were outside the follow-up of the trial in the database. If these records were removed, the records on 22 babies would also be removed. Given that the anonymised time stamp records the date and time when the record was made, and not necessarily when the activity took place, the ‘episode file’ was preferred to select the records within the follow-up period.
The subsequent step of data preparation was to merge the two cleaned files: the ‘episode file’ with records of 848 babies with a total of 1568 episodes of care with the ‘daily file’ with records of 846 babies with a total of 57,658 days in the NICU. After merging, 213 days of care, 13 episodes and records on two babies could not be matched. The resulting file contained data on 844 PREVAIL babies, with 1555 episodes and 57,445 days in the neonatal care unit.
Of the 1555 episodes of care, 147 episodes did not have a discharge date. This date was imputed with the date stamp of the last day of care recorded in the episode of care. In 3 (of the 147) episodes with date of discharge imputed, the date of admission was after the date stamp of the last day of care. In these episodes, the date of admission was corrected to the earliest date stamp for the daily care in the episode.
The next step was removing the 167 episodes (and 835 days of care) with a date of discharge before the date of randomisation; this corresponded to the full data on seven PREVAIL babies. These data were permanently deleted. As a result, the data on the NICU stays on the PREVAIL babies consisted of the data on 837 PREVAIL babies, with 1388 episodes of care and 56,610 days in the unit.
Cleaning of Paediatric Intensive Care Audit Network data
Figure 19 summarises the data preparation. The file with the episode dates contained data on 177 (20.56%) PREVAIL babies and 184 episodes of care. The discharge date of three episodes was before the date of randomisation, including of two babies for whom it consisted of all the information in the file. These were removed from the data set. The admission date of 55 episodes was after the end of follow-up, including information on 21 babies for whom it consisted of all the information in the file. As a result, the data set on the episodes of care contained data on 177 babies, with a total of 184 episodes.
FIGURE 19.
Preparation of the paediatric intensive care data (received from PICANet).
The file with the HRGs contained data on 177 PREVAIL babies with 1237 days in the paediatric care unit. No inconsistencies were identified with the date of daily activity; hence, this information was used to select the days of care within the follow-up period. All days of care were assigned to a valid HRG. Thirteen days had an activity date before the randomisation date and 445 days had an activity date after the end of follow-up; these were removed. All daily records were matched to the file with the episode dates. In total, 94 (10.92%) PREVAIL babies had 126 episodes of care and 779 days in the paediatric care unit.
Cleaning of Hospital Episode Statistics data
Cleaning prior to deriving Healthcare Resource Groups
As discussed in Chapter 4, Costing hospital care, hospital care was costed based on the HRGs. The HES data sets, that is HES APC, HES outpatient and HRS A&E, did not include the costing HRGs. Therefore, the hospital care recorded in the HES data sets was passed through the HRG grouper software to derive them.
Before being passed through the grouper software, the HES data sets were cleaned to ensure both internal consistency and to fulfil the grouper software requirements. It was essential to ensure that dates were chronologically sound (e.g. episode start happening before episode end, admission date happening before discharge date, admission date happening before or on episode start date), that poorly coded observations (e.g. record identifier, episode duration, episode end date missing) and duplicates (in particular, episodes sharing the same identifier) were dropped, and that diagnosis and procedure codes included only alphanumeric characters. Episodes sharing the same episode order identifier as at least one other episode in the same spell, but showing an unspecified diagnosis code (‘R69X6’), needed to be dropped. The grouper does not distinguish these particular episodes from the other correct episodes in the same spell and will not generate a HRG for any episode of that spell. Therefore, the data set was first sorted using trial identifier, NHS provider code and relevant dates, and then episodes showing a primary diagnosis code of ‘R69X6’, and sharing the same NHS provider code and episode order identifier of the immediately adjacent episode (above or below) in the data set, were dropped.
Cleaning prior to costing
The data sets required additional cleaning before costing the HRG. Figures 20 and 21 summarise the process.
FIGURE 20.
Preparation of the HES APC data (received from NHS Digital).
FIGURE 21.
Preparation of the HES outpatient and A&E data (received from NHS Digital).
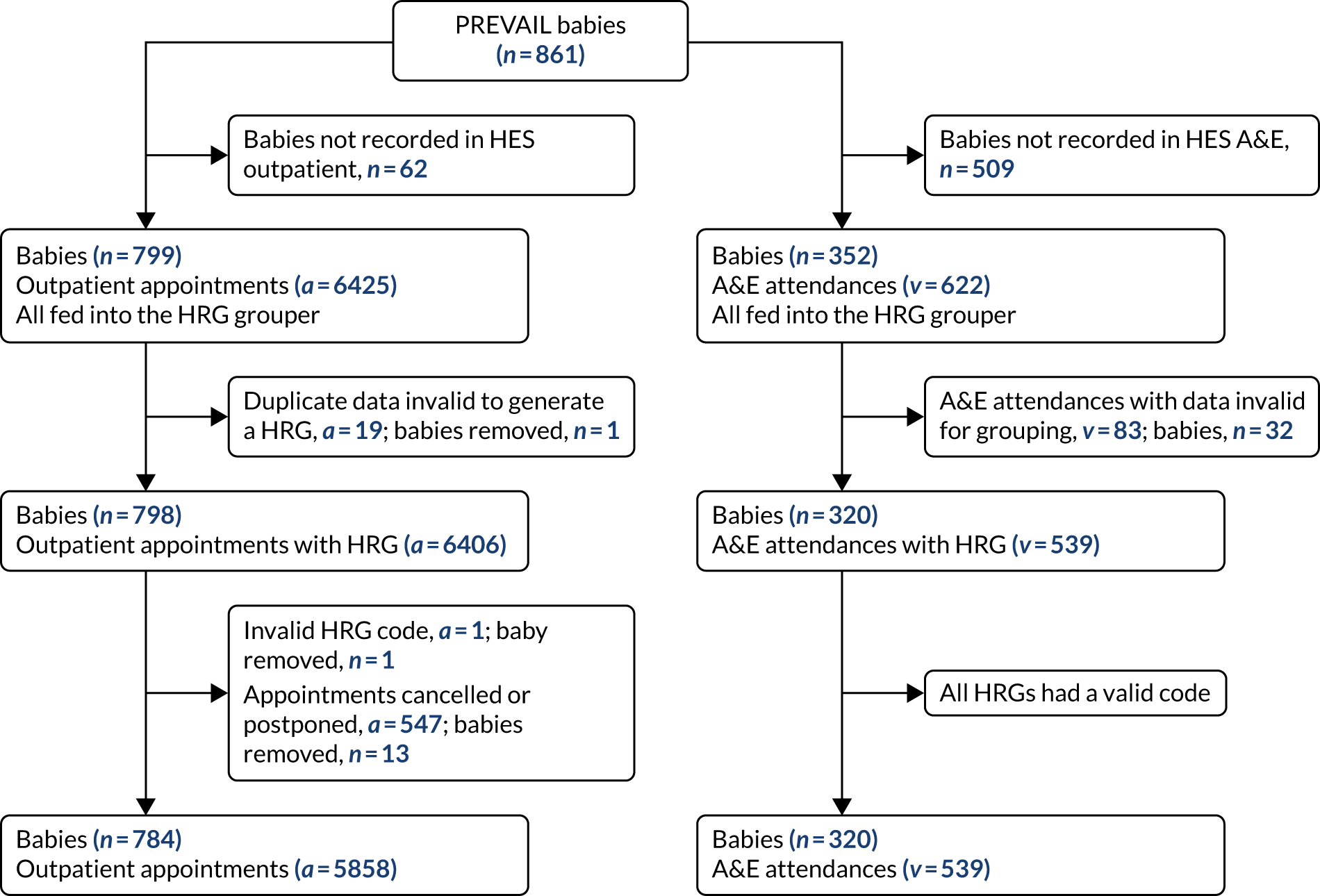
First, it was necessary to create an indicator variable for the type of episode (elective vs. non-elective, long vs. short stay) so that the appropriate unit cost would be attached to its respective HRG. Episodes for which there was not enough information to declare the episode type were dropped from the data set.
Some of the HRG codes were different between the 2016/17134 and the 2015/16 reference costs. 62 Therefore, the ‘new’ codes were replaced with their older counterpart before attaching the reference cost table to the HRG codes (Table 41).
| Data set | 2015/16 HRG code | 2016/17 HRG code |
|---|---|---|
| APC | FE02E | FZ24G |
| APC | FE23B | FZ62B |
| APC | FF03B | FZ82D |
| APC | FF04H | FZ83F |
| APC | FF23E | FZ68L |
| APC | FF35D | FZ78D |
| APC | FF40C | FZ21B |
| APC | FF51J | FZ12U |
| APC | FF52D | FZ27D |
| APC | FF60D | FZ87G |
| APC | FF62F | FZ18F |
| APC | FF63B | FZ19B |
| Outpatient | FZ78D | FF35D |
A small number of HRG codes were introduced in 2016/17 and did not have a corresponding code in the previous year’s schedule. They were therefore deflated to 2015/16 prices using the Hospital and Community Health Service index (Table 42). 135
| Data set | HRG | HCHS index [1987/88 = 100 (i.e. 1987/88 taken as base year)] | Unit cost (£) | ||
|---|---|---|---|---|---|
| 2016/17 | 2015/16 | 2016/17 | 2015/16 | ||
| APC – outpatient | RD97Z | 302.3 | 297.0 | 18.71 | 18.38 |
| Outpatient | JB71B | 302.3 | 297.0 | 26.13 | 25.67 |
Two pieces of information were required to correctly identify the HRG for the A&E data set: the type of department and whether or not patients were admitted for subsequent investigations (Reference Cost Guidance, section 769). It was assumed that all babies were admitted to a ‘Type 01’ structure, representing hospital emergency departments.
In case an A&E admission was linked to a subsequent inpatient admission, the A&E data set presents the unique APC episode identifier. Therefore, a non-missing APC episode identifier was taken as the signal that that A&E admission led to a subsequent inpatient admission, in order to fulfil the second criteria for the correct unit cost identification.
Unit costs
| HRG | Description | National average cost (£) |
|---|---|---|
| Neonatal intensive care | ||
| XA01Z | Neonatal critical care, intensive care | 1218 |
| XA02Z | Neonatal critical care, high-dependency | 872 |
| XA03Z | Neonatal critical care, special care, without external carer | 560 |
| XA04Z | Neonatal critical care, special care, with external carer | 384 |
| XA05Z | Neonatal critical care, normal care | 437 |
| XA06Z | Neonatal critical care, transportation | 990 |
| Paediatric intensive care | ||
| XB01Z | Paediatric critical care, advanced critical care 5 | 5440 |
| XB02Z | Paediatric critical care, advanced critical care 4 | 3748 |
| XB03Z | Paediatric critical care, advanced critical care 3 | 2538 |
| XB04Z | Paediatric critical care, advanced critical care 2 | 2151 |
| XB05Z | Paediatric critical care, advanced critical care 1 | 1899 |
| XB06Z | Paediatric critical care, intermediate critical care | 1448 |
| XB07Z | Paediatric critical care, basic critical care | 1173 |
| XB08Z | Paediatric critical care, transportation | 2966 |
| XB09Z | Paediatric critical care, enhanced care | 870 |
Appendix 6 Additional information on economic analysis: type and length of stay
| HRG code | HRG description | Total days in the neonatal care unit | Days by babies allocated to AM-PICC | Days by babies allocated to S-PICC | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| XA01Z | Neonatal critical care, intensive care | 17,781 | 31.41 | 9125 | 32.13 | 8656 | 30.69 |
| XA02Z | Neonatal critical care, high dependency | 19,107 | 33.75 | 9524 | 33.53 | 9583 | 33.98 |
| XA03Z | Neonatal critical care, special care, without external carer | 18,827 | 33.26 | 9296 | 32.73 | 9531 | 33.79 |
| XA04Z | Neonatal critical care, special care, with external carer | 594 | 1.05 | 283 | 1.00 | 311 | 1.10 |
| XA05Z | Neonatal critical care, normal care | 299 | 0.53 | 174 | 0.61 | 125 | 0.44 |
| Total | 56,608 | 100 | 28,402 | 100 | 28,206 | 100 | |
| XA06Za | Neonatal critical care, transportation | 662 | Not applicable | 361 | Not applicable | 301 | Not applicable |
| Quantity | Total | AM-PICC | S-PICC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Per episode | |||||||||
| Length of stay (days) per episode in NICU without adjustment for follow-up | 1388 | 40.19 | 32.32 | 697 | 40.22 | 33.76 | 691 | 40.16 | 30.82 |
| Length of stay (days) per episode in NICU adjusting for follow-up | 1388 | 36.81 | 30.25 | 697 | 36.57 | 30.92 | 691 | 37.05 | 29.57 |
| Cost per episode in NICU without adjustment for follow-up | 1388 | £35,933.59 | £30,101.02 | 697 | £36,107.66 | £31,930.00 | 691 | £35,758.01 | £28,158.19 |
| Cost per episode in NICU without adjustment for follow-up | 1388 | £32,615.38 | £27,698.45 | 697 | £32,515.14 | £28,712.61 | 691 | £32,716.48 | £26,656.86 |
| Per baby | |||||||||
| Length of stay (days) per baby in NICU without adjustment for follow-up | 837 | 66.65 | 37.38 | 420 | 66.75 | 38.55 | 417 | 66.54 | 36.21 |
| Length of stay (days) per baby in NICU adjusting for follow-up | 837 | 61.04 | 35.74 | 420 | 60.70 | 36.31 | 417 | 61.40 | 35.20 |
| Cost per baby in NICU without adjustment for follow-up | 837 | £59,588.79 | £37,409.92 | 420 | £59,921.52 | £38,893.53 | 417 | £59,253.68 | £35,897.37 |
| Cost per baby in NICU without adjustment for follow-up | 837 | £54,086.19 | £35,223.79 | 420 | £53,959.65 | £36,046.58 | 417 | £54,213.65 | £34,418.05 |
| HRG code | HRG description | Total days in the paediatric intensive care unit | Days by babies allocated to AM-PICC | Days by babies allocated to S-PICC | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| XB04Z | Paediatric critical care, advanced critical care 2 | 124 | 15.92 | 70 | 18.57 | 54 | 13.43 |
| XB05Z | Paediatric critical care, advanced critical care 1 | 361 | 46.34 | 162 | 42.97 | 199 | 49.50 |
| XB06Z | Paediatric critical care, intermediate critical care | 137 | 17.59 | 76 | 20.16 | 61 | 15.17 |
| Total | 779 | 100.00 | 377 | 100.00 | 402 | 100.00 | |
| Quantity | Total | AM-PICC | S-PICC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Per spell | |||||||||
| Length of stay (days) per spell in HES APC without adjustment for follow-up | 2128 | 28.95 | 34.15 | 1080 | 27.96 | 33.84 | 1048 | 29.96 | 34.45 |
| Length of stay (days) per spell in HES APC adjusting for follow-up | 2128 | 26.71 | 31.54 | 1080 | 25.69 | 30.97 | 1048 | 27.77 | 32.09 |
| Per baby | |||||||||
| Length of stay (days) per baby in HES APC without adjustment for follow-up | 828 | 74.39 | 41.19 | 410 | 73.66 | 40.71 | 418 | 75.11 | 41.69 |
| Length of stay (days) per baby in HES APC adjusting for follow-up | 828 | 68.65 | 37.91 | 410 | 67.68 | 36.77 | 418 | 69.61 | 39.01 |
| Results | Total FCEs in PREVAIL babies | FCEs for babies allocated to AM-PICCs | FCEs for babies allocated to S-PICCs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FCEs (n) | Mean cost (£) | SD (£) | FCEs (n) | Mean cost (£) | SD (£) | FCEs (n) | Mean cost (£) | SD (£) | |
| Unadjusted for time outside follow-up | |||||||||
| FCE core cost unadjusted | 2682 | 8592.90 | 10,581.59 | 1332 | 8561.49 | 10,687.94 | 1350 | 8623.88 | 10,479.47 |
| FCE unbundled cost unadjusted | 2682 | 105.40 | 415.90 | 1332 | 95.18 | 378.00 | 1350 | 115.49 | 450.09 |
| FCE total cost unadjusted | 2682 | 8698.30 | 10,582.63 | 1332 | 8656.68 | 10,695.50 | 1350 | 8739.37 | 10,473.87 |
| Adjusted for time outside follow-up | |||||||||
| FCE core cost adjusted | 2682 | 8030.28 | 9962.54 | 1332 | 7981.18 | 9970.79 | 1350 | 8078.71 | 9957.86 |
| FCE unbundled cost adjusted | 2682 | 99.10 | 405.48 | 1332 | 90.27 | 368.81 | 1350 | 107.80 | 438.64 |
| FCE total cost adjusted | 2682 | 8129.37 | 9964.73 | 1332 | 8071.45 | 9981.07 | 1350 | 8186.52 | 9951.95 |
| Admission type | Statistic | Cost of episode (core HRG) | Cost of unbundled HRG | Total episode cost |
|---|---|---|---|---|
| Day case | Number of FCEs | Suppressed as a result of small numbers | ||
| Mean (£) | Suppressed as a result of small numbers | |||
| SD (£) | Suppressed as a result of small numbers | |||
| Elective inpatient | Number of FCEs | 952 | 952 | 952 |
| Mean (£) | 12,753.70 | 47.21 | 12,800.91 | |
| SD (£) | 10,639.15 | 191.24 | 10,635.31 | |
| Non-elective inpatient long stay | Number of FCEs | 643 | 643 | 643 |
| Mean (£) | 880.56 | 121.99 | 1002.55 | |
| SD (£) | 889.51 | 581.24 | 1057.61 | |
| Non-elective inpatient short stay | Number of FCEs | 1798 | 1798 | 1798 |
| Mean (£) | 12,023.43 | 99.16 | 12,122.59 | |
| SD (£) | 10,811.21 | 357.77 | 10,816.73 | |
| Other long admission | Number of FCEs | 175 | 175 | 175 |
| Mean (£) | 4723.34 | 49.20 | 4772.55 | |
| SD (£) | 11,578.98 | 179.26 | 11,568.97 | |
| Other short admission | Number of FCEs | 66 | 66 | 66 |
| Mean (£) | 533.92 | 262.86 | 796.78 | |
| SD (£) | 285.78 | 368.10 | 396.96 | |
| Total | Number of FCEs | 2682 | 2682 | 2682 |
| Mean (£) | 8592.90 | 105.40 | 8698.30 | |
| SD (£) | 10,581.59 | 415.90 | 10,582.63 | |
| Quantity | Total | AM-PICC | S-PICC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Babies (n) | Mean | SD | Babies (n) | Mean | SD | Babies (n) | Mean | SD | |
| Length of stay per baby unadjusted for time outside follow-up (days) | 828 | 74.39 | 41.19 | 410 | 73.66 | 40.71 | 418 | 75.11 | 41.69 |
| Length of stay per baby adjusted for time outside follow-up (days) | 828 | 68.65 | 37.91 | 410 | 67.68 | 36.77 | 418 | 69.61 | 39.01 |
| Total cost per baby unadjusted for time outside follow-up (£) | 828 | £28,174.93 | £19,850.20 | 410 | £28,123.64 | £20,099.41 | 418 | £28,225.24 | £19,626.65 |
| Total cost per baby adjusted for time outside follow-up (£) | 828 | £26,332.09 | £18,190.26 | 410 | £26,222.37 | £18,055.68 | 418 | £26,439.72 | £18,342.29 |
| Results | Total appointments by PREVAIL babies | Appointments by babies allocated to AM-PICCs | Appointments by babies allocated to S-PICCs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of appointments | Mean cost (£) | SD (£) | Number of appointments | Mean cost (£) | SD (£) | Number of appointments | Mean cost (£) | SD (£) | |
| Cost of HRG core | 5858 | 150.29 | 62.59 | 3014 | 148.99 | 63.80 | 2844 | 151.67 | 61.25 |
| Cost of unbundled HRG | 5858 | 4.42 | 46.33 | 3014 | 3.50 | 38.41 | 2844 | 5.38 | 53.45 |
| Total cost per attendance | 5858 | 154.71 | 78.88 | 3014 | 152.50 | 75.00 | 2844 | 157.05 | 82.75 |
Appendix 7 Additional information on economic analysis: review and meta-analysis
Effect of infection on health outcomes
Methods
Pearl-growing review
A ‘pearl-growing’ review was conducted to identify evidence to inform the additional effect of BSI on health outcomes such as death and NDI. 137 Briefly, this technique requires a series of key papers to be identified at the start (the ‘pearls’); from these key references, it is possible to look both forward (through the papers’ citations) and backwards (through their own references). These steps are repeated using the additional papers identified, until a comprehensive evidence base on the topic is gathered and no new publications are found. This particular technique was chosen because a full systematic review was outside the scope and timeline of this report.
Advice from the PREVAIL trial clinical team suggested that the key paper in the area is Stoll et al. 10 This study was used as the starting point for finding studies on the effect of infection on NDI and death. Systematic reviews were preferred.
Studies were included if their definition of infection matched the one used in the trial for clinically serious BSI, and if the definition of NDI could be matched with the one used by Mangham et al. 71 The choice of clinically serious BSI was because literature on the consequences of infection typically uses the term sepsis or defines infection as an event requiring the use antibiotics for at least 3–5 days. 10,78–80 Matching on the definition of NDI used by Mangham et al. 71 was to allow the use of Mangham et al. ’s71 transition probabilities to simulate the progression of impairment over time.
Definition of infection
The definition of infection follows the one of ‘clinically serious BSI’ as defined in the PREVAIL trial protocol,59 section 4.1. A BSI case is defined as clinically serious if a baby is treated for at least 72 hours with intravenous antibiotic or dies during treatment.
Definition of neurodevelopment impairment
The definition of NDI was taken from Mangham et al. ,71 whose source, Doyle et al. ,83 considered the 1991–92 cohort of the Victorian Infant Collaborative Study, covering births of 23–27 weeks’ gestational age in the Australian state of Victoria. Outcomes assessed at 2 years of age included cerebral palsy, blindness, deafness and developmental delay (Table 52).
| Outcome assessed | Method of assessment |
|---|---|
| Cerebral palsy | Criteria for diagnosis and severity assessment present in Kitchen et al.95 |
| Blindness | Visual acuity of < 20/200 in the BSE |
| Deafness | Hearing loss requiring amplification |
| Developmental delay | Assessed using the Bayley Scales MDI. Impairment defined as DQ of < –1 SD computed relative to the mean (SD) for the respective controls on MDI |
Included studies
Three systematic reviews78–80 and an umbrella review119 were found on the effects of infection on health outcomes. Data from the eligible studies were re-extracted and finally synthesised. The final selection of studies for the effect of infection on NDI included Stoll et al. 10 and Schlapbach et al. ,74 whose definition of NDI matched the one used by Doyle et al. 83 and Mangham et al. 71 (Table 53).
| Characteristics of the studies | Composite outcome: NDI | |
|---|---|---|
| Schlapbach et al.74 | Stoll et al.10 | |
| Assessment period | 18–24 months | 18–22 months |
| Definition of infection | Positive culture and ≥ 5 days of antibiotic therapy | Positive culture and ≥ 5 days of antibiotic therapy |
| Cerebral palsy | Presence of cerebral palsy | Presence of cerebral palsy |
| Visual impairment | Bilateral blindness | Bilateral blindness |
| Hearing impairment | Severe hearing loss requiring auditory amplification | Bilateral hearing impairment |
| MDI score | < 70 | < 70 |
| PDI score | < 70 | < 70 |
The review on the effect of infection on the risk of death produced two studies74,76 (Table 54).
Meta-analysis results
The selected studies were meta-analysed following standard inverse variance estimator techniques. 86 Tables 55 and 56 show the results.
Appendix 8 Additional information on economic analysis: probability calculations
Probability of death at 6 months
The objective of this analysis was to obtain the probability of death from PICC insertion to 6 months’ follow-up in babies who did not have a clinically serious BSI, to inform the decision model.
Methods
Population and subgroups
The PREVAIL trial enrolled a mixed population of preterm babies. Of relevance to the model were babies who were born at a gestational age of ≤ 27 weeks and babies who were born at 28–32 weeks of gestation. This analysis uses only the data on the PREVAIL trial babies in these subgroups: 381 PREVAIL babies born at gestational age of ≤ 27 weeks and 354 PREVAIL babies who were born at 28–32 weeks of gestation.
Analysis
The analysis was initially descriptive on:
-
The number of babies who had a clinically serious BSI with S-PICCs by gestational age subgroup, which represents the probability of infection with a S-PICC in the model.
-
The number of babies who died over the time horizon of 6 months, taking death as recorded either in the PREVAIL trial CRFs or in the HES records via the Patient Demographics Service, by gestational age subgroup. This represents the probability of death irrespective of the type of PICC and irrespective of prior clinically serious infection.
In a second stage, a regression analysis was conducted on the probability of death given gestational age subgroup and prior clinically serious infection. The regression analysis explored the impact of using generalised linear models with binomial family or Poisson family and log link.
Results
Tables 57 and 58 show the results of the descriptive analysis. The probability of having a clinically serious BSI with a S-PICC was 14.52% (95% CI 9.79% to 20.41%) in the younger gestational age subgroup and was 3.80% (95% CI 1.54% to 7.68%) in the older gestational age subgroup. The probability of death over the time horizon of 6 months is shown in Table 58.
| Statistics | Gestational age | |
|---|---|---|
| ≤ 27 weeks | 28–32 weeks | |
| Number of babies who had infection and received a S-PICC | 26 | 7 |
| Number of babies who received a S-PICC | 184 | 182 |
| Proportion | 0.14 | 0.04 |
| Standard error | 0.03 | 0.01 |
| 95% CI | 0.09 to 0.20 | 0.02 to 0.08 |
| Statistics | Gestational age | |
|---|---|---|
| ≤ 27 weeks | 28–32 weeks | |
| Number of babies who died | 52 | 11 |
| Number of babies | 381 | 354 |
| Proportion | 0.14 | 0.03 |
| Standard error | 0.02 | 0.01 |
| 95% CI | 0.10 to 0.18 | 0.02 to 0.05 |
Table 59 shows the results of the regression analysis of the probability of death given gestational age and prior clinically serious BSI. The best-fitting regression is the regression including both explanatory variables. It suggests that having a clinically serious BSI is associated with 78.4% greater risk of death (RR 1.784), and that being born at a gestational age of 28–32 weeks is associated with an 85.5% lower risk of death (RR 0.245).
| Explanatory variables | Controlling on only clinically serious BSI | Controlling on only gestational age category | Controlling for both clinically serious BSI and gestational age category | |||
|---|---|---|---|---|---|---|
| Risk ratio | SE | Risk ratio | SE | Risk ratio | SE | |
| Clinically serious BSI | 2.51** | 0.71 | – | – | 1.85* | 0.54 |
| Gestational age category | – | – | 0.23*** | 0.07 | 0.25*** | 0.08 |
| Constant | 0.08*** | 0.01 | 0.14*** | 0.02 | 0.12*** | 0.02 |
| n | 735 | 735 | ||||
| AIC | 0.5793931 | 0.5519772 | 0.5606888 | |||
| BIC | –4415.851 | –4436.002 | –4550.999 | |||
It is not clear whether this is a correlation or a causal association. There may be other factors that increase the risk of clinically serious BSI and increase the risk of death, for example other health problems that the babies may have been born with. Without controlling for these other factors, it is not possible to ascertain whether or not preventing clinically serious BSI reduces the risk of death to the extent that the regression analysis suggests. Given the small number of deaths, the regression analysis is constrained in the number of variables that can be included.
Application to the model
Method
The following equations explain the methodology.
P(death all) is the probability of death in the mixed population of people with and without a clinically serious BSI. P(death|infection) is the probability of death given a prior clinically serious BSI and P(death|no infection) is the probability of death given no prior clinically serious BSI.
P(infection) is the probability of a clinically serious BSI occurring.
RR(infection on death) is the ratio between the probability of death with and death without a clinically serious BSI.
Data inputs
Table 60 shows the distribution of babies based on their survival status at 6 months, conditional on prior infection.
| Occurrence of clinically serious BSI | Death before end of follow-up (n) | ||
|---|---|---|---|
| No | Yes | Total | |
| No | 616 | 50 | 666 |
| Yes | 56 | 13 | 69 |
| Total | 672 | 63 | 735 |
Based on the data from Table 60, it was possible to compute the RR for clinically serious BSI on death before the end of follow-up (RR 2.51, 95% CI 1.44 to 4.38). As the number of events is limited, it was not feasible to compute a gestational age-specific RR by splitting the overall sample. Therefore, the RR calculated using the whole PREVAIL sample was applied to all gestational age groups.
P(death all) was obtained as 1 minus the probability of surviving to discharge extracted from Santhakumaran et al. ,87 and P(Infection) corresponds to the probability of experiencing a clinically serious BSI, as resulted from the PREVAIL trial data. See points (6) and (5), respectively, in Table 17.
Results
The results of Table 61 are used as the baseline probability of death before the end of trial follow-up in the model. The RR of death given clinically serious BSI is then applied to the baseline probability to obtain the probability of death given infection. As detailed in Chapter 4, it was not possible to obtain strong evidence about the existence and the size of this effect, neither from the PREVAIL trial nor from the available literature. Therefore, the base case assumes that a clinically serious BSI does not increase the risk of death at 6 months (i.e. a RR of 1 was assumed), whereas a scenario considers the application of the RR computed from the PREVAIL data, as detailed in Table 60.
| Gestational age (weeks) | P(death|no infection) (95% CI) |
|---|---|
| 23–27 | 0.20 (0.16 to 0.24) |
| 28–32 | 0.034 (0.030 to 0.036) |
Transitions between neurodevelopmental impairment states or death
Mangham et al. 71 report the counts of changes in impairment status for the babies belonging to the VICSG84 1991–92 cohort, which includes all babies born at 23–27 weeks’ gestational age from the state of Victoria in Australia. The impairment levels are classified according to the definition adopted by Doyle et al. 83
The counts reported in Mangham et al. 71 are used to compute 3-year transition probabilities for the period covering ages 2–5 and 5–8 years, respectively. After the age of 8 years, transition across impairment states are assumed to stop; hence, babies either remain in their current impairment level or will transition to the absorbing death model state. The 3-year probabilities are then converted to annual transition probabilities using standard formulas as reported in Briggs et al. ,86 and are reproduced in Tables 62 and 63. The counts reported in Mangham et al. 71 are supplemented by the average probability of death between the ages of 2 and 5 years, 5 and 8 years and 8 and 18 years extracted from the National Life Tables, UK: 2013–2015. 99
| NDI | NDI | |||||
|---|---|---|---|---|---|---|
| No | Mild | Moderate | Severe | Dead | Total | |
| No | 0.91 | 0.08 | 0.01 | 0.00 | 0.00 | 1.00 |
| Mild | 0.17 | 0.75 | 0.07 | 0.01 | 0.00 | 1.00 |
| Moderate | 0.03 | 0.12 | 0.80 | 0.05 | 0.00 | 1.00 |
| Severe | 0.00 | 0.04 | 0.04 | 0.91 | 0.00 | 1.00 |
| NDI | NDI | |||||
|---|---|---|---|---|---|---|
| No | Mild | Moderate | Severe | Dead | Total | |
| No | 0.96 | 0.04 | 0.00 | 0.00 | 0.00 | 1.00 |
| Mild | 0.14 | 0.82 | 0.04 | 0.00 | 0.00 | 1.00 |
| Moderate | 0.01 | 0.08 | 0.83 | 0.07 | 0.00 | 1.00 |
| Severe | 0.00 | 0.02 | 0.06 | 0.92 | 0.00 | 1.00 |
Appendix 9 Additional information on economic analysis: costs
Costs over 6 months from peripherally inserted central venous catheter insertion
The objective of this analysis was to estimate the mean, standard error and appropriate distribution of the costs over 6 months from PICC insertion, to inform the cost-effectiveness model.
Methods
This analysis used the costs of the PREVAIL trial babies, which were calculated as reported in Chapter 4, Costs of hospital care.
Population and subgroups
As these estimates aimed to inform the decision model, the analysis was conducted in the populations with a gestational age of ≤ 27 weeks and with gestational age of 28–32 weeks who had sufficient records in the NNRD and in HES APC data. The analytical sample comprises 381 babies with gestational age of ≤ 27 weeks and 354 babies with gestational age of 28–32 weeks.
Choice of explanatory variables
Five explanatory variables were explored: gestational age subgroup (≤ 27 weeks or 28–32 weeks), PICC type (AM-PICC or S-PICC), occurrence of clinically significant BSI and death at 6 months. Death at 6 months was obtained from the PREVAIL trial CRFs and from the routine health-care records (see Chapter 4, Deaths during the 6-month follow-up period). No other explanatory variables were explored, given that the structure of the cost-effectiveness model for the 6 months from PICC insertion differentiates babies only by these variables.
Description of the costs
The costs in the entire analysis sample and by the various exploratory variables were described in terms of their summary statistics. Their distributions were plotted to understand if the costs followed a normal distribution.
Estimation
Costs were regressed on the various exploratory variables using generalised linear models, namely with identity distribution and Gaussian link (which is equivalent to linear regression) and identity distribution and log link.
Results
Descriptive statistics
Table 64 presents the costs of hospital care in the sample of PREVAIL babies with gestational ages of ≤ 27 weeks and 28–32 weeks, who are those considered in the economic model, by gestational age subgroup, type of PICC, whether or not a clinically serious BSI occurred during the primary follow-up period and by whether or not death occurred during the 6-month follow-up.
| Costs | Number of babies | Mean costs (£) | SE (£) | Number of babies | Mean costs (£) | SE (£) | Number of babies | Mean costs (£) | SE (£) |
|---|---|---|---|---|---|---|---|---|---|
| Total | Gestational age: ≤ 27 weeks | Gestational age: 28–32 weeks | |||||||
| Cost of hospital care | 735 | 85,169.36 | 1848.49 | 381 | 106,240.27 | 2784.30 | 354 | 62,491.35 | 1720.23 |
| Total | AM-PICC | S-PICC | |||||||
| Cost of hospital care | 735 | 85,169.36 | 1848.49 | 366 | 86,045.40 | 2640.83 | 369 | 84,300.44 | 2590.38 |
| Total | No clinically significant BSI | Clinically significant BSI | |||||||
| Cost of hospital care | 735 | 85,169.36 | 1848.49 | 666 | 83,066.31 | 1889.95 | 69 | 105,468.37 | 7002.05 |
| Total | Alive at 6 months | Death during 6-month follow-up | |||||||
| Cost of hospital care | 735 | 85,169.36 | 1848.49 | 672 | 88,146.31 | 1856.02 | 63 | 53,415.21 | 7515.19 |
-
The cost of hospital care is lower for babies in the older gestational age group (£62,491 vs. £106,240).
-
The cost of hospital care is similar, irrespective of the PICC type, at approximately £85,000.
-
The cost of hospital care is higher for babies who had a clinically serious BSI (£105,468 vs. £83,066).
-
The cost of hospital care is lower for the babies who died during the 6-month follow-up period (£53,415 vs. £88,146).
Regression analysis
Model selection
The best-fitting regression to predict costs was the generalised linear model with Gamma distribution and log link according to plot of deviance residuals and normal plot. The best-fitting regression according to the Park test was the Gaussian distribution and log link. The results for both regressions were very similar. Hence, the Gaussian distribution and log link, which produces coefficients that are easier to interpret, was the preferred estimation model.
Results
Table 65 shows the results of four regression equations with various combinations of the candidate explanatory variables: gestational age, clinically significant BSI and death at 6 months. Gestational age is used in all regression equations because it corresponds to the definition of the subgroups. The coefficient on gestational age is similar across the four regression models. It suggests that babies born at 28–32 weeks of gestation have 53–59% lower costs than babies born at younger gestational ages.
| Specification of the regression | Regression | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Gestational age (= 1 if 28–32 weeks) (SE) | –0.580*** (0.0419) | –0.520*** (0.0445) | –0.594*** (0.0417) | –0.531*** (0.0441) |
| Clinically significant BSI ( = 1 if occurred) (SE) | 0.144** (0.0508) | 0.100 (0.0551) | ||
| Death at 6 months (= 1 if occurred) (SE) | –0.726*** (0.104) | –0.714*** (0.104) | ||
| Constant (SE) | 11.63*** (0.0221) | 11.56*** (0.0236) | 11.65*** (0.0205) | 11.57*** (0.0218) |
| N | 735 | 735 | 735 | 735 |
| AIC | 17,753.6 | 17,840.8 | 17,758.8 | 17,841.9 |
| BIC | 17,772.0 | 17,854.6 | 17,772.6 | 17,851.1 |
The association between clinically significant BSI and costs is uncertain. The coefficient is positive, which indicates higher costs in babies who experienced a clinically significant BSI, but not statistically significant. Regression 4, which includes only gestational age as the explanatory variable, has the poorest fit (indicated by the higher Akaike information criterion and Bayesian information criterion). Furthermore, this regression equation does not control for whether or not a clinically significant BSI occurred or for babies’ characteristics that may increase their risk of death, and also increase their costs. Hence, it is unclear if this association is confounded.
The regressions suggest that there is a negative association between whether or not death occurred and costs. This association is statistically significant and stable to the inclusion of clinically significant BSI as an explanatory variable. The association between death and costs may be related with the shorter length of stay of babies who died than those babies who were alive at 6 months. However, the association may be confounded by length of stay and by their characteristics. For example, the babies who died may have been in too poor health to allow for surgical procedures, thereby reducing their costs. Under this scenario, an intervention that prevents their death does not necessarily result in an increase in costs if the babies still do not receive the surgical procedures.
Discussion
The base-case cost-effectiveness model assumes that the costs incurred by babies over 6 months from PICC insertion are the same irrespective of the occurrence of a clinically significant BSI or death. The costs will depend only on their gestational age. The implication is that a reduction in the probability of clinically significant BSI or in the probability of death has no effect on the costs. This is a conservative assumption.
Two scenarios are tested. One scenario assumes that babies who die have lower costs, as suggested by regression equation 3 (see Table 65). The implication is that interventions that reduce the probability of death result in an increase in costs over 6 months. The other scenario assumes that babies who die have lower costs and that babies who have clinically significant BSI have higher costs, as suggested by regression equation 1 (see Table 65). The implication is that interventions that reduce the probability of a clinically significant BSI occurring result in a decrease in costs over 6 months.
Costs between 6 months and 2 years of age
Costs between 6 months and 2 years of age were computed using two sources of information: NHS Reference Costs 2015–16,62 and resource use from an unpublished study about variation in neonatal and paediatric admissions (Katie Harron, personal communication).
Methods
Information from the NHS reference costs was used to compute average costs weighted by level of activity. This results in an estimate of average costs of inpatient care (for elective and non-elective), average outpatient visit cost, and average A&E attendance cost (Table 66).
| Type of hospital use | Average cost (£) |
|---|---|
| Elective inpatient | 2790.72 |
| Non-elective long stay | 2516.04 |
| Paediatric outpatient | 196.50 |
| A&E | 161.60 |
Unpublished results provided information on the number of babies accessing these services, by gestational age groups 23–27 weeks and 28–32 weeks, and their resource use in terms of hospital admission and type (planned vs. unplanned), A&E and outpatient attendances. This was used to compute the number of hospital admissions by type, and visits per baby. Data about the proportion of planned and unplanned admissions were used as weight when averaging the cost of elective and non-elective hospital admission. Average costs were then multiplied by the relevant resource use and summed across type of care to obtain total cost per baby.
Results
| Gestational age at birth (weeks) | Admissions (%) | Number, per baby | |||
|---|---|---|---|---|---|
| Planned | Unplanned | Episodes | A&E visits | Outpatient attendances | |
| 33–36 | 23 | 77 | 0.43 | 1.08 | 1.55 |
| 28–32 | 24 | 76 | 0.74 | 1.34 | 4.61 |
| 23–27 | 28 | 72 | 1.32 | 2.01 | 11.47 |
| Gestational age at birth (weeks) | Cost (95% CI) (£) |
|---|---|
| 23–27 | 5989.17 (5983.44 to 5994.98) |
| 28–32 | 3026.43 (3024.21 to 3028.73) |
Appendix 10 Additional information on economic analysis: model validation
Part A: validation of the conceptual model
A1: Have experts been asked to judge the appropriateness of the conceptual model?
Yes. The experts were Ruth Gilbert, Sam J Oddie and Ajay K Sinha. They were chosen because they were co-applicants in the project and are epidemiologists (RG) or clinicians (SJO, AKS) with extensive experience in this clinical area. The experts found the conceptual model to be generally appropriate, and noted the uncertainty in the link between clinically significant BSI, NEC and NDI. There was some debate about whether or not clinically significant BSI should be assumed to have a causal effect on the risk of death at 6 months, given the limited data in the PREVAIL trial.
A2: Has this model been compared with other conceptual models found in the literature or clinical textbook?
Partly. The model on predicting outcomes in early childhood was informed by the Stoll et al. 10 study on the effect of infection in early infancy on neurodevelopmental outcomes. However, no other cost-effectiveness model on infection in preterm neonates was found for comparison. The model on predicting lifetime costs and health outcomes beyond 2 years of age was informed by the model by Mangham et al. 71
Part B: input data validation
B1: Have experts been asked to judge the appropriateness of the input data?
Yes, the same experts as in A1 reviewed the input data. The experts agreed that the appropriate data were used.
B2: When input parameters are based on regression models, have statistical tests been performed?
Partly; the costs from PICC insertion to 6 months were obtained from the linked data (see Appendix 9). The model type was chosen on the basis of the distribution of the costs, Park test and the plot of deviance residuals versus normal plot. Four model specifications were tested. The most conservative for the cost-effectiveness of interventions to prevent infections was selected for the base case, but the impact of alternative specifications was tested in a sensitivity analysis to the cost-effectiveness model.
Part C: validation of the computerised model
C1: Has the computerised model been examined by modelling experts?
Yes, the cost-effectiveness model was examined by Rita Faria. Rita Faria is not an independent expert, as she supervised the development of the cost-effectiveness model and collaborated in all economic analyses. The cost-effectiveness model is valid.
C2: Has the model been run for specific, extreme sets of parameter values in order to detect any coding errors?
Yes. The tests included:
-
Tree –
-
Baseline risk of infection = 0. If the baseline risk of infection is set to 0, we expect both PICC groups to give identical results, as there will be no difference in the rate of death and NDI at 2 years.
-
RR of infection set to 0: AM-PICC becomes cost-effective.
-
RR of infection, OR of infection on death (6 months), OR of infection on death (2 years) and OR of infection on NDI (2 years) set to 1. AM-PICCs and S-PICCs are identical. There is only a difference of £53.70 in costs, which is the additional cost of an AM-PICC.
-
-
Markov model –
-
Utilities = 1, disutilities = 0. Setting all utilities to 1 means that QALYs accrued in a cycle (without half-cycle correction) should be equal to the number of people alive in a state (i.e. QALYs = life expectancy). Alternatively, that the sum of the QALYs accrued in a cycle and the cumulative number of deaths should always sum to 1. This is indeed the case in our model.
-
Probability of death = 0. Setting UK lifetable value to 0. We expect cycle number of deaths and cumulative number of deaths to remain at 0, with everyone dying in the last cycle. This is indeed the case.
-
No transitions to other states. As transitions are stopped, then babies should not move from the initial distribution.
-
50% no NDI, 50% mild NDI. Results from this test are expected to be identical to the average of having 100% of babies in ‘no NDI’ and 100% of babies in ‘mild NDI’.
-
Setting all HRs to 1 – the mortality rate is identical for all severity levels.
-
Any errors identified were corrected.
C3: Have patients been tracked through the model to determine whether or not its logic is correct?
Yes, the patients were tracked through the model at ages 5, 8 and 18 years. The model logic is correct.
C4: Have individual submodules of the computerised model been tested?
No.
Part D: operational validation
D1: Have experts been asked to judge the appropriateness of the model outcomes?
Yes; Ruth Gilbert, Sam J Oddie and Ajay K Sinha found the results to have face validity.
D2: Have the model outcomes been compared with the outcomes of other models that address similar problems?
Yes. The results were compared with those reported by Mangham et al. ,71 using the same input parameters. The results were very similar.
D3: Have the model outcomes been compared with the outcomes obtained when using alternative input data?
No, as no alternative input data were identified.
D4: Have the model outcomes been compared with empirical data?
No.
Part E: other validation techniques
E1: Have any other validation techniques been performed?
No.
Appendix 11 Additional information on economic analysis: sensitivity analysis
-
The occurrence of infection increases the risk of developing NDI or of dying by 2 years of age.
-
The occurrence of infection has the same effect on the risk of NDI and death for all gestational age subgroups.
-
The occurrence of infection does not increase the risk of death by 6 months of age.
-
The effectiveness of AM-PICCs is the same for all gestational age subgroups.
-
The distribution of infants across levels of NDI at 2 years of age is the same for the two PICCs, irrespective of previous infection.
-
Infants are attributed the same costs between birth and 6 months of age, and between 6 months and 2 years of age, irrespective of the occurrence of infection or death.
-
After age 2 years, long-term costs and health outcomes are influenced only by a child’s NDI level at 2 years of age.
-
Children experience improvements or deteriorations in their NDI state only up to the age of 8 years. After age 8 years, they can transition to the absorbing ‘death’ state only.
| Sensitivity analysis | Justification |
|---|---|
| Effect of infection on NDI and death | |
| Reduce the effect of infection on either death at 2 years or NDI to null effect (OR 1) | To take into account possible bias in the observational nature of the evidence regarding the impact of infection |
| Assume impact of infection on death at 6 months equals the RR from PREVAIL trial data of 2.62 (95% CI 1.52 to 4.51) | To take into account information about the impact of infection coming from the PREVAIL trial follow-up |
| Baseline probabilities | |
| Transitions up to 18 years of age | To test the sensitivity of results to the extrapolation of transition probability data beyond childhood |
| Stop backward transitions for babies with severe NDI | To take into account the clinical implausibility of improvement in severe babies’ classification over time |
| Use 3-year transition probabilities instead of yearly ones | To take into account the actual assessment point in the references’ sources and the likelihood of movement between disability states |
| Costs | |
| Differentiate costs between PICC insertion and 6 months by survival and infection status | To take into account additional evidence coming from the regression analysis of costs (see Appendix 9) |
| Parameters to predict long-term outcomes and costs | |
| Increase/decrease costs after 18 years of age | To take into account the possible changes in costs after 18 years, which were not taken into account in the base case |
| Apply HRQoL age and sex decrements from Ara and Brazier104 | To take into account the impact of age and sex on utility values |
| Assume NDI level does not increase the risk of death after 2 years of age | To take into account the impact of assuming that NDI has an additional impact on mortality according to its level |
| Parameter | Value (95% CI; distribution) | Source |
|---|---|---|
| Effect of infection on NDI and death | ||
| RR of the association of clinically serious BSI on death at 6 months; same for both gestational age subgroups |
|
|
| Costs | ||
| Health-care costs between PICC insertion and 6 months | ||
| Base case | PREVAIL trial | |
| Gestational age: 23–27 weeks | £105,873 (£101,444.99 to £110,485.27; gamma) |
|
| Gestational age: 28–32 weeks | £62,255.37 (£54,711.87 to £70,838.93; gamma) | |
| Scenario 1 | Scenario 1 adjusts for gestational age subgroup and whether or not the baby survived to 6 months | |
| Gestational age: 23–27 weeks |
|
|
| Gestational age: 28–32 weeks |
|
|
| Scenario 2 | Scenario 2 adjusts for gestational age group, whether or not the baby survived to 6 months and whether or not the baby had a clinically significant BSI | |
| Gestational age: 23–27 weeks |
|
|
| Gestational age: 28–32 weeks |
|
|
| RR of infection with AM-PICC vs. S-PICC | Cost-effectiveness (£) at | |
|---|---|---|
| £20,000/QALY | £13,000/QALY | |
| 0.5 | 1245.00 | 830.00 |
| 0.55 | 1125.00 | 750.00 |
| 0.6 | 1005.00 | 670.00 |
| 0.65 | 885.00 | 595.00 |
| 0.7 | 765.00 | 515.00 |
| 0.75 | 645.00 | 435.00 |
| 0.8 | 525.00 | 360.00 |
| 0.85 | 405.00 | 280.00 |
| 0.9 | 285.00 | 200.00 |
| 0.95 | 165.00 | 120.00 |
| 0.96 | 140.00 | 105.00 |
| 0.97 | 115.00 | 90.00 |
| 0.98 | 90.00 | 75.00 |
| 0.99 | 65.00 | 60.00 |
| 1 | 46.00 | 46.00 |
| RR of infection with AM-PICC vs S-PICC | Cost-effectiveness (£) at | |
|---|---|---|
| £20,000/QALY | £13,000/QALY | |
| 0.5 | 250.00 | 180.00 |
| 0.55 | 230.00 | 170.00 |
| 0.6 | 210.00 | 155.00 |
| 0.65 | 190.00 | 140.00 |
| 0.7 | 170.00 | 125.00 |
| 0.75 | 150.00 | 110.00 |
| 0.8 | 125.00 | 100.00 |
| 0.85 | 105.00 | 85.00 |
| 0.9 | 85.00 | 70.00 |
| 0.95 | 65.00 | 55.00 |
| 0.96 | 60.00 | 55.00 |
| 0.97 | 55.00 | 50.00 |
| 0.98 | 50.00 | 50.00 |
| 0.99 | 46.00 | 46.00 |
| 1 | 46.00 | 46.00 |
FIGURE 22.
Maximum acquisition price per AM-PICC effectiveness, gestational age: 28–32 weeks.
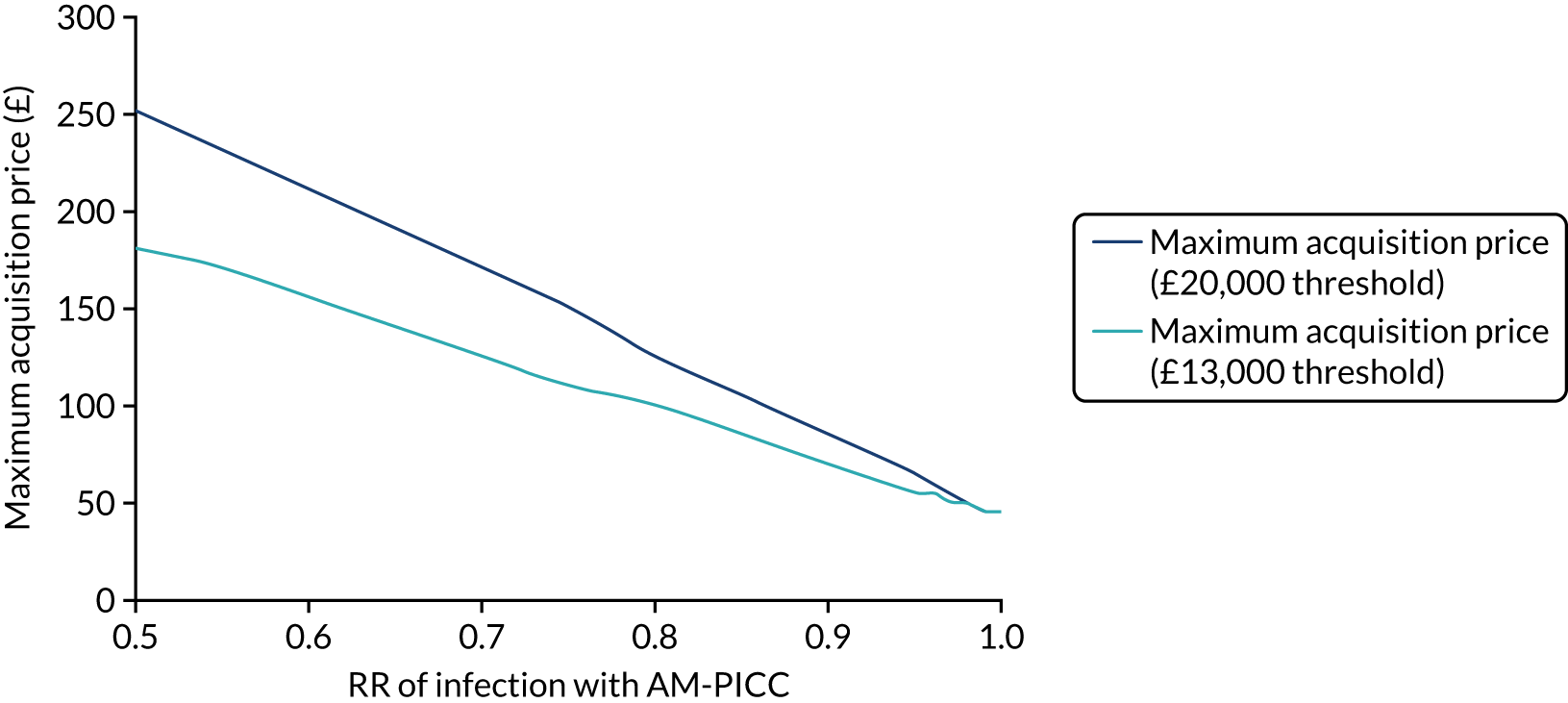
Figures 23 and 24 represent the incremental change of net monetary benefit, with respect to its mean value, over the 95% CI for the model parameters.
FIGURE 23.
Univariate sensitivity analyses for gestational age subgroup 23–27 weeks at the £20,000 per QALY cost-effectiveness threshold. Adapted from Grosso et al. 106 © Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ. This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. Minor formatting changes have been made.
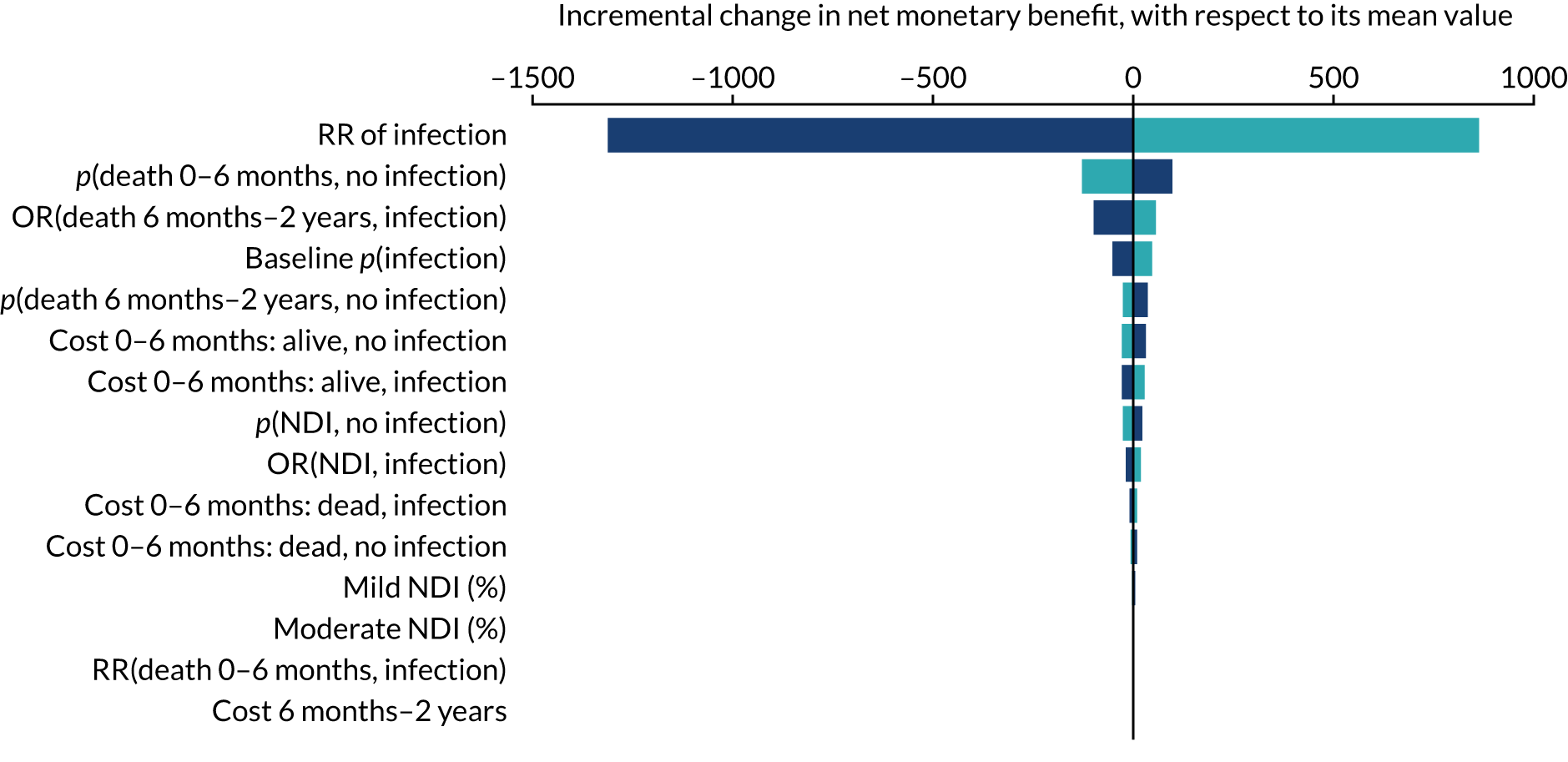
FIGURE 24.
Univariate sensitivity analyses for gestational age subgroup 28–32 weeks at the £20,000 per QALY cost-effectiveness threshold.
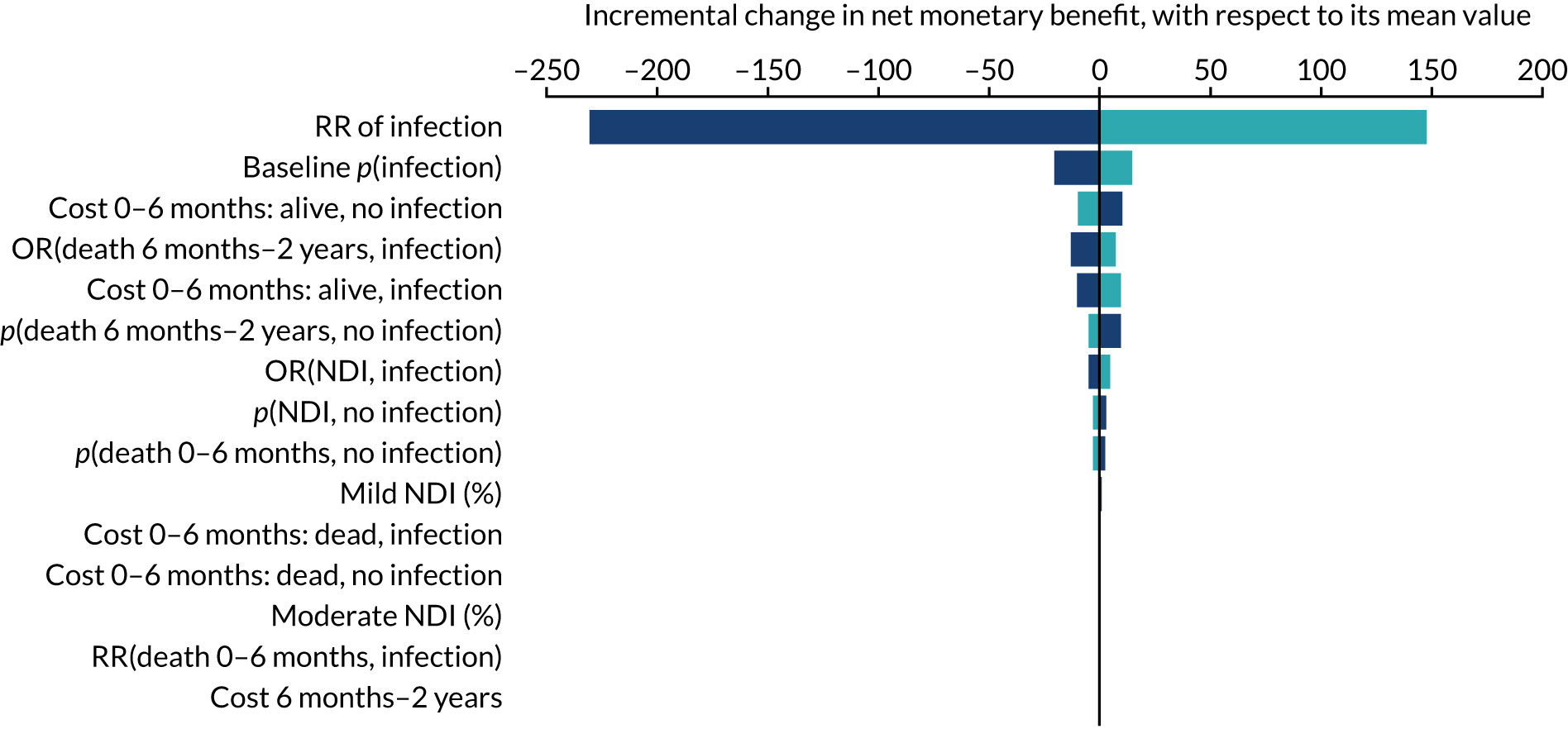
| Sensitivity analysis | Incremental net monetary benefit (£) | |||
|---|---|---|---|---|
| At £13,000 per QALY | At £20,000 per QALY | |||
| Gestational age: 23–27 weeks | Gestational age: 28–32 weeks | Gestational age: 23–27 weeks | Gestational age: 28–32 weeks | |
| Base case | –145.07 | –69.75 | –193.63 | –77.98 |
| No effect of infection on NDI | –101.25 | –59.70 | –128.97 | –63.16 |
| No effect of infection on death between 6 months and 2 years | –98.83 | –63.85 | –120.29 | –68.67 |
| Apply RR on death from PREVAIL trial | –728.45 | –99.40 | –1,127.72 | –124.98 |
| Apply transition probabilities up to 18 years of age | –134.82 | –67.12 | –187.20 | –74.10 |
| Stop backward transitions for babies with severe NDI | –150.80 | –70.98 | –202.04 | –79.80 |
| Differentiate costs between PICC insertion and 6 months of age by survival and gestational age | –134.11 | –69.03 | –182.65 | –77.26 |
| Differentiate costs between PICC insertion and 6 months by survival, gestational age and infection status | –259.43 | –90.26 | –307.97 | –98.49 |
| Use 3-year transition probabilities | –123.08 | –64.44 | –161.17 | –70.14 |
| Double costs after 18 years of age | –145.27 | –70.06 | –193.83 | –78.29 |
| Apply HRQoL age and sex decrement | –147.55 | –70.10 | –197.43 | –78.53 |
| Assume NDI does not increase the risk of death after the age of 2 years | –143.83 | –69.43 | –191.46 | –77.43 |
Appendix 12 Additional information on generalisability study: linkage methods
Parts of this appendix are reproduced from Fraser et al. 111 © 2019 Fraser et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
We used deterministic and probabilistic linkage to link babies in the NNRD to BSI records from national infection surveillance. We first performed deterministic linkage using the NHS number. However, not all babies had a complete NHS number; therefore, we also used probabilistic methods. In both deterministic and probabilistic linkage, links were restricted to those with a sample date from the surveillance data between 7 days before admission and 14 days after discharge from the NNU. Further restrictions to BSI dates were applied depending on the definition of BSI being used (e.g. during PICC time at risk/early onset/late onset). Each BSI could be linked to only one baby; however one baby could be linked to multiple BSI records. Therefore, linkage was one to many.
Deterministic linkage
We first used deterministic linkage to link babies in the NNRD with BSI records in the surveillance data. Records were linked if the same NHS number was recorded in each data set. The NHS number is a unique 10-digit identifier assigned at birth. NHS number matches were manually reviewed to ensure that they represented true matches. NHS number links were then used as the training data set for probabilistic linkage.
Probabilistic linkage
For records that were not linked on NHS number, we used probabilistic linkage based on the remaining common identifiers in each data set. The available identifiers were date of birth, postcode prefix, postcode suffix, sex and hospital/laboratory. In probabilistic linkage, match weights are created that represent the likelihood that two records belong to the same subject, according to the similarity of a set of identifiers. 138 We first used ‘blocking’ to restrict the comparisons to records that agreed on at least one of date of birth, postcode prefix or postcode suffix. 139 This ensured that we compared only records that had a chance of being a match; otherwise, records could, at most, agree on hospital and sex, which would not be sufficient to identify a link.
Next, we used the deterministic links as the reference standard for true matches to calculate probabilistic match weights. 112 Match weights were calculated as log2(m−probabilityu−probability). We estimated the probability that records were a true match, given identifiers were in agreement, disagreement or missing in either data set (m-probabilities). We estimated the probability that records were not a match (i.e. disagreed on NHS number), given identifier agreement, disagreement or missing in either data set (u-probabilities).
Match weights were totalled across identifiers to produce an overall weight (‘summed weight’) for each comparison pair, based on the pattern of agreement of identifiers that represents the likelihood that each pair is a match. A plot of the log frequency of the weights was examined to determine an upper and lower threshold, above which all pairs are classified as links and below which all pairs are classified as non-links. The agreement patterns of the weights close to the thresholds were examined and a second wider set of thresholds was selected to reduce potential missed matches and false matches.
Comparison pairs with a match weight between these two thresholds were reviewed manually by one author (CF) following a set of rules agreed by all authors (Table 74). To aid decision-making, any clinical records indicating BSI in the NNRD were checked as evidence that the baby had an infection. When date of birth was in disagreement, the difference between the dates was examined. When postcode prefix or suffix disagreed, the number of letters that matched was recorded (e.g. RG1 and RK1 would be 2, RG1 and NG2 would be 1).
| Agreement on identifiersa (1 = agree, 0 = disagree, x = missing) | Condition | Allocate | ||||
|---|---|---|---|---|---|---|
| Prefix | Suffix | DOB | Sex | Hospital | ||
| Any | BSI links to any baby with a match weight above the upper threshold | Non-link | ||||
| 1 | 1 | 1 | 0 | 1 | BSI does not link to a different baby above the upper threshold | Link |
| 1 | 0 | 1 | 1 | 1 | BSI does not link to a different baby with a higher summed weight | Link |
| 1 | 0 | x | 1 | 1 | BSI does not link to a different baby with a higher summed weight and baby has a clinical record of BSI with the same sample date | Link |
| 1 | 0 | x | 1 | 1 | BSI does not link to a different baby with a higher summed weight, DOB is recorded in one of the two data sets (missing in other) and age (in data set with complete DOB) is < 28 days | Link |
| 1 | 1 | 0 | x | 1 | BSI does not link to a different baby with a higher summed weight and DOB disagrees, but is within 7 days | Link |
| 1 | x | 0 | 1 | 1 | BSI does not link to a different baby with a higher summed weight and DOB disagrees, but is same month and year, and age at BSI in surveillance data is < 28 days | Link |
| x | x | 1 | 1 | 1 | BSI does not link to a different baby with a higher summed weight and age at BSI in surveillance data is < 28 days | Link |
| x | x | 1 | 1 | 1 | BSI does not link to a different baby with a higher summed weight and age at BSI in surveillance data is ≥ 28 days old | Missing |
| x | x | 1 | x | x | Any given BSI does not link to another baby | Missing |
| x | x | 1 | x | 0 | Any given BSI does not link to another baby | Missing |
| x | x | 1 | 0 | x | Any given BSI does not link to another baby | Missing |
| x | x | 1 | 0 | 0 | Any given BSI does not link to another baby | Missing |
| x | x | 1 | 0 | 1 | Any given BSI does not link to another baby | Missing |
| 0 | x | 1 | x | 0 | Any given BSI does not link to another baby | Missing |
| 0 | x | 1 | 0 | x | Any given BSI does not link to another baby | Missing |
| 0 | x | 1 | 1 | x | Any given BSI does not link to another baby | Missing |
| x | x | 1 | 1 | x | Any given BSI does not link to another baby | Missing |
| x | x | 1 | 1 | 0 | Any given BSI does not link to another baby | Missing |
Following probabilistic linkage, we further inspected unlinked records in the NNRD for babies aged < 28 days with a clinical record of BSI in the NNRD, as we believed that these records should link to a BSI. For these babies, we searched the surveillance data for any BSI reported to the corresponding laboratory in the same month and reviewed the potential links. However, few additional links were identified, suggesting under-reporting of BSI to the surveillance system, or reporting from unexpected laboratories.
Multiple births
We used the same match weights and rules for manual review of records for babies from multiple births as for singletons, but manually reviewed the records separately, as babies born on the same day to the same mother share most of their identifiers. On the few occasions when it was not possible to determine which baby a BSI should match to, we randomly allocated the BSI to one of the babies to avoid duplicates. Multiple births were identified in the NNRD using the foetus number, birth order, date of birth and mother’s NHS number.
Appendix 13 Additional information on generalisability study: clearly pathogenic organisms
-
E. coli.
-
Streptococcus Group B stem.
-
Candida albicans (stellatoidea).
-
Staphylococcus aureus.
-
Enterobacter cloacae.
-
Enterococcus faecalis.
-
Candida parapsilosis.
-
Streptococcus pneumoniae.
-
Klebsiella pneumoniae.
-
Pseudomonas aeruginosa.
-
Serratia marcescens.
-
Haemophilus influenzae.
-
Enterobacter other named.
-
Streptococcus intermedius group.
-
Enterococcus faecium.
-
Coliform.
-
Candida other named.
-
Klebsiella oxytoca.
-
Streptococcus Group D stem.
-
Candida dubliniensis.
-
Enterobacter aerogenes.
-
Acinetobacter baumannii.
-
Streptococcus Group A stem.
-
Clostridium butyricum.
-
Enterococcus sp.
-
Morganella morganii.
-
Bacteroides sp.
-
Listeria monocytogenes.
-
Citrobacter diversus (Citrobacter koseri).
-
Citrobacter freundii.
-
Proteus mirabilis.
-
Gardnerella vaginalis.
-
Candida sp.
-
Streptococcus Group C stem.
-
Candida glabrata.
-
Klebsiella sp.
-
Enterobacter amnigenus.
-
Hansenula sp.
-
Citrobacter farmeri.
-
Streptococcus Group G stem.
-
Cedecea lapagei.
-
Pantoea sp.
-
Clostridium perfringens.
-
Candida guilliermondii.
-
Enterobacter sp.
-
Enterobacter kobei.
-
Enterococcus durans.
-
Streptococcus milleri group.
-
Candida lusitaniae.
-
Enterococcus hirae.
-
Pasteurella sp.
-
Enterococcus gallinarum.
-
Serratia odorifera.
-
Enterobacter cloacae complex.
-
Citrobacter other named.
-
Enterobacter gergoviae.
-
Klebsiella ornithnolytica.
-
Serratia sp.
-
Enterobacter asburiae.
-
Enterobacter agglomerans.
-
Citrobacter sp.
-
Rhodotorula rubra.
-
Candida tropicalis.
-
Klebsiella other named.
-
Coccidioides sp.
-
Neisseria meningitidis.
-
Bacteroides fragilis.
-
Serratia liquefaciens.
-
Citrobacter amalonaticus.
-
Providencia alcalifaciens.
-
Leclercia adecarboxylata.
-
Aspergillus sp.
-
Enterobacter sakazakii.
-
Kluyvera sp.
-
Pantoea septica.
-
Candida fabianii.
-
Klebsiella aerogenes.
-
Enterococcus casseliflavus.
-
Escherichia vulneris.
-
Listeria sp.
-
Bacillus sp.
-
CoNS.
-
Staphylococcus other named.
-
Acinetobacter sp.
-
Staphylococcus sp.
-
Streptococcus alpha and non-haemolytic.
-
Pseudomonas paucimobilis.
-
Actinomyces cardiffensis.
-
Micrococcus sp.
-
Diphtheroids.
-
Phialophora other named.
-
Brevibacterium other named.
-
Acinetobacter junii.
-
Corynebacterium minutissimum.
-
Streptococcus intermedius group.
-
Roseomonas mucosa.
-
Corynebacterium sp.
-
Bacillus cereus.
-
Actinomyces odontolyticus.
-
Acinetobacter other named.
-
Paenibacillus sp.
-
Acinetobacter lwoffii.
-
Corynebacterium striatum.
-
Micrococcus luteus (sarcina).
-
Streptococcus other named.
-
Microbacterium sp.
-
Ochrobactrum anthropi.
-
Pseudomonas fluorescens.
-
Neisseria sp.
-
Rothia sp.
-
Actinomyces other named.
-
Ruminococcus gnavus.
-
Lactobacillus paracasei.
-
Lactobacillus sp.
-
Staphylococcus vitulinus.
-
Stenotrophomonas sp.
-
Comamonas testosteroni.
-
Aerococcus sp.
-
Bacillus other named.
-
Gemella haemolysans.
-
Neisseria flavescens.
-
Streptococcus gordonii.
-
Moraxella sp.
-
Pseudomonas sp.
-
Rhizobium radiobacter.
-
Corynebacterium aurimucosum.
-
Staphylococcus pettenkoferi.
-
Actinomyces viscosus.
-
Brevundimonas sp.
-
Neisseria sicca.
-
Roseomonas sp.
-
Kocuria sp.
-
Stephanoascus ciferrii.
-
Microbacterium aurum.
-
Paracoccus yeeii.
-
Stomatococcus mucilaginosus.
-
Propionibacterium freudenreichii.
-
Acinetobacter calcoaceticus (anitratus).
-
Burkholderia cepacia.
-
Neisseria other named.
-
Bifidobacterium breve.
-
Moraxella osloensis.
-
Lactobacillus other named.
-
Collinsella aerofaciens.
-
Parabacteroides distasonis.
-
Streptococcus sp.
-
Brevibacterium sp.
-
Actinomyces naeslundii.
-
Haemophilus sp.
-
Corynebacterium pseudodiphtheriticum.
-
Hansenula sp.
-
Rothia dentocariosia.
-
Micrococcus lylae.
-
Streptococcus vestibularis.
-
Stenotrophomonas maltophilia.
-
Chryseobacterium meningosepticum.
-
Corynebacterium other named.
-
Brevibacterium casei.
-
Streptococcus alactolyticus.
-
Haemophilus parainfluenzae.
-
Roseomonas gilardii.
-
Moraxella catarrhalis.
-
Massilia timonae.
-
Corynebacterium jeikeium (JK).
-
Corynebacterium amycolatum.
-
Aerococcus viridans.
-
Arcanobacterium haemolyticum.
-
Achromobacter xylosoxidans.
-
Pseudomonas stutzeri.
-
Leuconostoc sp.
-
Brevundimonas vesicularis.
-
Globicatella sanguis.
-
Granulicatella adiacens.
-
Aerococcus other named.
-
Eikenella corrodens.
-
Haematobacter sp.
-
Rhodococcus other named.
-
Abiotrophia defectiva.
-
Paracoccus sp.
-
Acinetobacter johnsonii.
-
Burkholderia gladioli.
-
Acinetobacter parvus.
-
Delftia acidovorans.
-
Lactococcus lactis.
-
Kocuria rhizophila.
-
Streptococcus pseudoporcinus.
-
Pseudomonas luteola.
-
Streptococcus infantarius ssp. nov.
-
Gemella morbillorum.
-
Micrococcus other named.
-
Kocuria kristinae.
-
Pseudomonas other named.
-
Neisseria subflava.
-
Neisseria perflava.
-
Propionibacterium acnes.
-
Chryseobacterium sp.
-
Aggregatibacter (haemophilus) segnis.
-
Pseudomonas oleovorans.
-
Pseudoclavibacter sp.
Appendix 14 Additional information on the generalisability study: changes over time in national infection surveillance
In England, data on BSIs and other infections are collected through a voluntary surveillance system operated by PHE. 140 The current voluntary surveillance system, known as the Second Generation Surveillance System (SGSS), was introduced in 2014 to replace LabBase2, which operated from 2002 to 2014. When LabBase2 ended, data were migrated to SGSS.
In both LabBase2 and SGSS, laboratories are required to provide information on clinically significant BSIs only. PHE do not produce guidance on what is considered clinically significant; therefore, the type of BSI submitted varies by laboratory, depending on decision-making of individual staff or on local procedures. BSIs caused by selected notifiable organisms, for example Streptococcus pneumoniae, were on a fast-track list in LabBase2 and automatically sent to PHE, but BSIs caused by all other organisms required authorisation. In contrast, in SGSS, data on all BSIs are automatically uploaded unless a laboratory requests the ability to manually review a BSI caused by a given organism. It is reported anecdotally that many laboratories upload data on all BSIs to SGSS without any review of clinical significance, to reduce burden on staff. This has resulted in SGSS capturing data on BSIs that would not have been reported to LabBase2, such as positive cultures of skin organisms that have uncertain clinical significance, for example CoNS.
To understand how case ascertainment changed following the system change, we examined the total number of BSIs in the surveillance data over time for all BSIs, for BSIs caused by skin organisms and for BSIs caused by potential pathogens. We used interrupted time series Poisson regression of monthly BSI rates for all BSIs, BSIs caused by skin organisms and BSIs caused by potential pathogens for infants aged < 1 year to quantify the change in the number of BSIs reported to the infection surveillance system. 141 SGSS was first implemented in March 2014 and adoption of SGSS was gradual, owing to the large number of laboratories and their different operating systems; therefore, we examined the time before introduction (January 2010 to March 2014) and after most laboratories were reporting to SGSS (December 2015 to July 2017).
The equation used for interrupted time series regression is given in Equation 5, the interrupted time series Poisson model, where Yt represents the number of BSIs at time t, T represents the months since January 2001, Xt represents a dummy variable for either pre (0) or post (1) the introduction of SGSS. β0 represents the baseline level in January 2010, β1 is the pre-system change trend, β2 is the step change following the introduction of the new system and β3 is the trend change following the introduction of SGSS:
Following the introduction of SGSS, there was an increase in the number of infections reported. The largest increase was seen in BSIs caused by skin organisms, which almost doubled [incidence rate ratio (IRR) 1.96, 95% CI 1.78 to 2.15]. The number of BSIs caused by all organisms increased by 47% (IRR 1.47, 95% CI 1.39 to 1.56) and potential pathogens increased by 20% (IRR 1.20, 95% CI 1.11 to 1.30) (Table 75). The number of BSIs reported to the infection surveillance system was decreasing by 4% for BSIs caused by potential pathogens before the introduction of SGSS (IRR 0.96, 95% CI 0.95 to 0.98), and the decline continued after the introduction of SGSS (IRR 0.89, 95% CI 0.85 to 0.94). However, the number of BSIs reported to the infection surveillance system was stable for BSIs caused by skin organisms before and after the system change.
| Component of time series | Monthly rate ratio (95% CI) | ||
|---|---|---|---|
| Skin organisms | Potential pathogens | All BSIs | |
| Trend pre system change per year | 0.98 (0.96 to 1.00) | 0.96 (0.95 to 0.98) | 0.97 (0.96 to 0.98) |
| Step change in number of BSIs reported | 1.96 (1.78 to 2.15) | 1.20 (1.11 to 1.30) | 1.47 (1.39 to 1.56) |
| Trend post system change per year | 1.04 (0.99 to 1.10) | 0.89 (0.85 to 0.94) | 0.97 (0.94 to 1.01) |
FIGURE 25.
Interrupted time series for the number of BSI episodes reported to the PHE surveillance system over time for infants aged < 1 year, excluding CoNS (aqua) and only CoNS (coral).
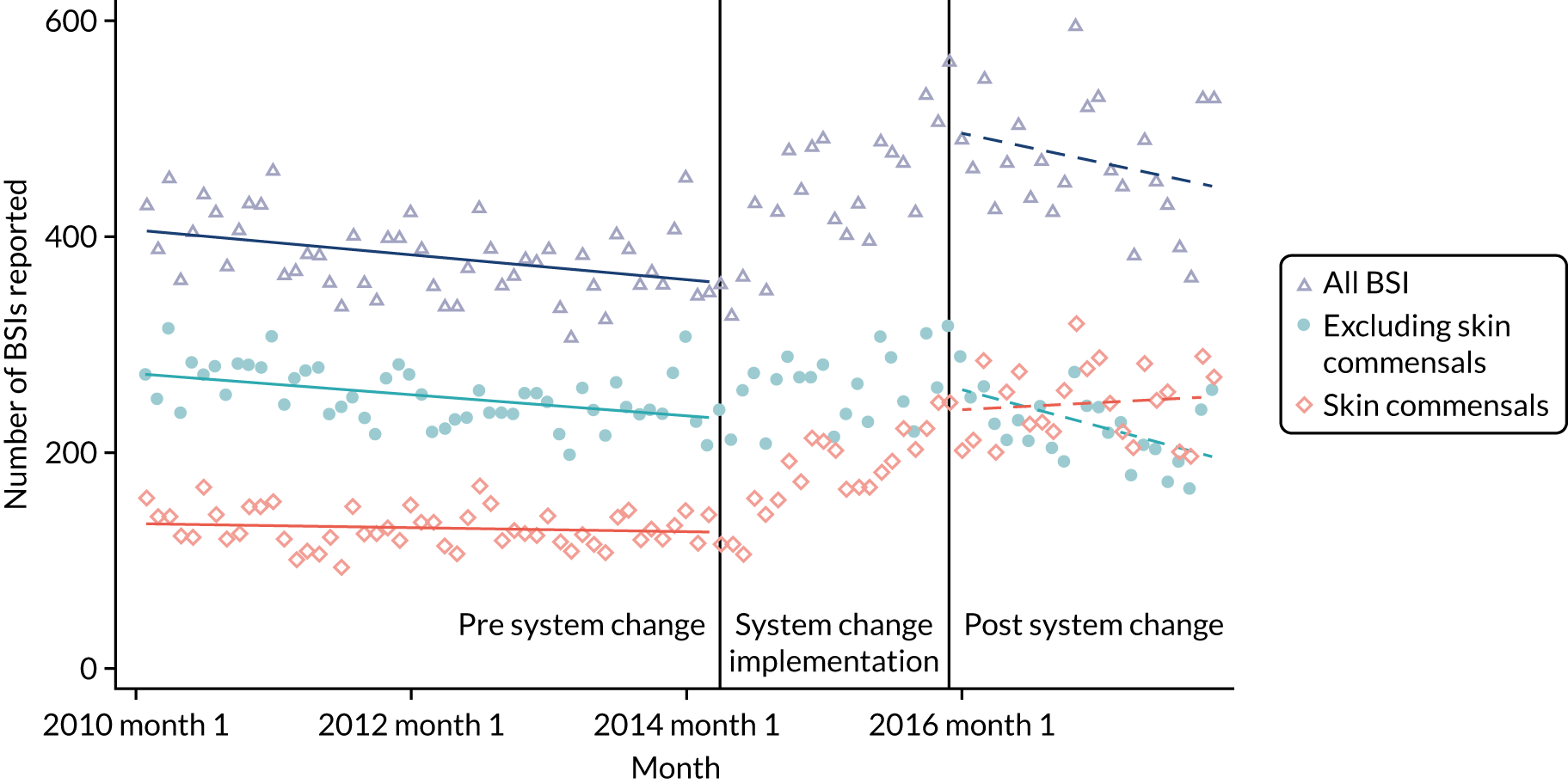
Appendix 15 Additional information on the generalisability study: comparison of included and excluded PREVenting infection using Antimicrobial-Impregnated Long lines trial babies
| Risk factors | Babies in the PREVAIL trial | p-value from chi-squared test | |
|---|---|---|---|
| Included in full analysis | Excluded from full analyses | ||
| Total babies (n) | 269 | 154 | |
| Gestational age (weeks), n (%) | |||
| < 26 | 59 (22) | 38 (25) | 0.882 |
| 26–28 | 75 (28) | 36 (24) | |
| 28–32 | 114 (42) | 67 (44) | |
| 32–37 | 15 (6) | 9 (6) | |
| ≥ 37 | 6 (2) | 4 (3) | |
| Small for gestational age, n (%) | 49 (18) | 25 (16) | 0.606 |
| Male sex, n (%) | 141 (52) | 80 (52) | 0.926 |
| Babies in NICU that provides surgery, n (%) | 144 (54) | 48 (31) | < 0.001 |
Appendix 16 Additional information on the generalisability study: model-building for rate per 1000 peripherally inserted central venous catheter days
| Model, IRR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7a | |
| Group | |||||||
| PREVAIL babies | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| NP babies in PREVAIL NICUs | 1.29 (0.66 to 2.54) | 1.55 (0.78 to 3.06) | 1.60 (0.81 to 3.17) | 1.60 (0.81 to 3.17) | 1.59 (0.80 to 3.16) | 1.58 (0.80 to 3.14) | 1.53 (0.77 to 3.03) |
| NP NICUs | 1.14 (0.57 to 2.26) | 1.16 (0.58 to 2.31) | 1.20 (0.59 to 2.41) | 1.20 (0.60 to 2.42) | 1.20 (0.60 to 2.42) | 1.20 (0.60 to 2.43) | 1.18 (0.59 to 2.37) |
| LNUs | 0.58 (0.27 to 1.24) | 0.87 (0.40 to 1.89) | 0.93 (0.42 to 2.03) | 0.93 (0.43 to 2.04) | 0.96 (0.42 to 2.15) | 1.42 (0.64 to 3.17) | 1.43 (0.64 to 3.20) |
| NNU variance | 0.13 (0.04 to 0.40) | 0.13 (0.04 to 0.40) | 0.15 (0.06 to 0.43) | 0.15 (0.05 to 0.43) | 0.82 (0.50 to 1.34) | 0.15 (0.05 to 0.45) | 0.14 (0.05 to 0.42) |
| Gestational age (weeks) | |||||||
| < 26 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| 26–28 | 0.52 (0.37 to 0.74)b | 0.53 (0.38 to 0.75)b | 0.53 (0.38 to 0.75)b | 0.53 (0.38 to 0.75)b | 0.62 (0.43 to 0.88)b | 0.66 (0.46 to 0.94)b | |
| 28–32 | 0.40 (0.28 to 0.55)b | 0.42 (0.30 to 0.58)b | 0.41 (0.30 to 0.58)b | 0.42 (0.30 to 0.58)b | 0.64 (0.44 to 0.93)b | 0.63 (0.42 to 0.94)b | |
| 32–37 | 0.27 (0.17 to 0.43)b | 0.28 (0.17 to 0.45)b | 0.28 (0.17 to 0.45)b | 0.28 (0.17 to 0.45)b | 0.45 (0.27 to 0.75)b | 0.36 (0.21 to 0.62)b | |
| ≥ 37 | 0.32 (0.18 to 0.56)b | 0.33 (0.18 to 0.58)b | 0.32 (0.18 to 0.58)b | 0.32 (0.18 to 0.57)b | 0.52 (0.29 to 0.95)b | 0.40 (0.21 to 0.74)b | |
| Age (months) at first PICC insertion | |||||||
| 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 1–2 | 0.50 (0.31 to 0.79)b | 0.50 (0.31 to 0.79)b | 0.50 (0.31 to 0.79)b | 0.49 (0.31 to 0.78)b | 0.49 (0.31 to 0.79)b | ||
| 3–5 | 0.62 (0.40 to 0.96)b | 0.62 (0.40 to 0.96)b | 0.62 (0.40 to 0.96)b | 0.59 (0.38 to 0.92)b | 0.60 (0.39 to 0.94)b | ||
| 6–8 | 0.81 (0.50 to 1.33) | 0.82 (0.50 to 1.34) | 0.82 (0.50 to 1.34) | 0.74 (0.45 to 1.21) | 0.72 (0.44 to 1.19) | ||
| 9–11 | 0.85 (0.44 to 1.64) | 0.85 (0.44 to 1.66) | 0.85 (0.44 to 1.65) | 0.74 (0.38 to 1.44) | 0.73 (0.37 to 1.41) | ||
| 12–29 | 0.75 (0.43 to 1.30) | 0.75 (0.43 to 1.30) | 0.75 (0.43 to 1.30) | 0.66 (0.38 to 1.15) | 0.65 (0.38 to 1.14) | ||
| ≥ 30 | 0.91 (0.54 to 1.55) | 0.91 (0.53 to 1.55) | 0.91 (0.53 to 1.55) | 0.92 (0.54 to 1.57) | 0.90 (0.53 to 1.54) | ||
| Small for gestational age | 1.03 (0.73 to 1.45) | ||||||
| Surgery | 1.05 (0.72 to 1.54) | ||||||
| Days of invasive ventilation | |||||||
| 0 | 1.00 | 1.00 | |||||
| 1 | 0.79 (0.34 to 1.88) | 0.89 (0.38 to 2.12) | |||||
| 2–4 | 1.68 (0.95 to 2.98) | 1.88 (1.05 to 3.34)b | |||||
| 5–6 | 2.58 (1.35 to 4.94)b | 3.07 (1.59 to 5.93)b | |||||
| 7–8 | 4.82 (2.57 to 9.02)b | 5.64 (2.99 to 10.65)b | |||||
| 9–12 | 2.86 (1.51 to 5.41)b | 3.54 (1.85 to 6.76)b | |||||
| 13–21 | 4.23 (2.38 to 7.52)b | 5.11 (2.85 to 9.18)b | |||||
| ≥ 22 | 3.06 (1.72 to 5.47)b | 3.73 (2.07 to 6.74)b | |||||
| Days of non-invasive ventilation | |||||||
| 0 | 1.00 | ||||||
| 1 | 0.47 (0.21 to 1.05) | ||||||
| 2–5 | 0.63 (0.39 to 1.03) | ||||||
| 6–9 | 0.96 (0.59 to 1.54) | ||||||
| 10–15 | 0.52 (0.30 to 0.90)b | ||||||
| 16–49 | 0.48 (0.33 to 0.72)b | ||||||
| ≥ 50 | 0.46 (0.29 to 0.71)b | ||||||
Appendix 17 Additional information on the generalisability study: model-building for trend per 1000 peripherally inserted central venous catheter days
| Model, IRR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7a | |
| Trend | |||||||
| PREVAIL NICUs | 1.002 (0.998 to 1.006) | 1.002 (0.999 to 1.006) | 1.002 (0.999 to 1.006) | 1.002 (0.999 to 1.006) | 1.002 (0.999 to 1.006) | 1.002 (0.999 to 1.006) | 1.002 (0.999 to 1.006) |
| Non-PREVAIL NICUs | 1.002 (0.999 to 1.005) | 1.002 (0.999 to 1.005) | 1.002 (0.999 to 1.005) | 1.002 (0.999 to 1.005) | 1.002 (0.999 to 1.005) | 1.002 (0.999 to 1.005) | 1.002 (0.999 to 1.005) |
| LNUs | 0.996 (0.990 to 1.001) | 0.996 (0.991 to 1.002) | 0.996 (0.991 to 1.002) | 0.997 (0.991 to 1.002) | 0.996 (0.991 to 1.002) | 0.998 (0.993 to 1.003) | 0.998 (0.993 to 1.004) |
| Group | |||||||
| PREVAIL NICUs | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Non-PREVAIL NICUs | 1.05 (0.05 to 20.85) | 1.15 (0.06 to 22.87) | 1.14 (0.06 to 22.67) | 1.26 (0.06 to 25.03) | 1.15 (0.06 to 22.89) | 1.02 (0.05 to 20.49) | 0.94 (0.05 to 18.89) |
| LNUs | 35.92 (0.55 to 2 to 348.57) | 34.63 (0.53 to 2 to 273.80) | 36.55 (0.55 to 2 to 409.53) | 34.60 (0.53 to 2 to 264.16) | 34.31 (0.52 to 2 to 256.43) | 16.57 (0.25 to 1 to 100.00) | 15.40 (0.23 to 1 to 023.51) |
| NNU variance | 0.24 (0.14 to 0.39) | 0.21 (0.12 to 0.34) | 0.21 (0.13 to 0.34) | 0.19 (0.11 to 0.31) | 0.21 (0.12 to 0.34) | 0.21 (0.13 to 0.35) | 0.22 (0.13 to 0.36) |
| Gestational age (weeks) | |||||||
| < 26 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| 26–28 | 0.54 (0.47 to 0.63)b | 0.55 (0.48 to 0.64)b | 0.54 (0.47 to 0.62)b | 0.54 (0.47 to 0.63)b | 0.63 (0.54 to 0.73)b | 0.66 (0.56 to 0.76)b | |
| 28–32 | 0.40 (0.35 to 0.46)b | 0.42 (0.36 to 0.48)b | 0.40 (0.35 to 0.46)b | 0.40 (0.35 to 0.46)b | 0.59 (0.51 to 0.70)b | 0.58 (0.49 to 0.68)b | |
| 32–37 | 0.32 (0.27 to 0.39)b | 0.33 (0.28 to 0.40)b | 0.32 (0.26 to 0.38)b | 0.32 (0.27 to 0.39)b | 0.52 (0.42 to 0.64)b | 0.44 (0.36 to 0.55)b | |
| ≥ 37 | 0.26 (0.20 to 0.33)b | 0.26 (0.20 to 0.34)b | 0.25 (0.20 to 0.33)b | 0.26 (0.20 to 0.33)b | 0.39 (0.30 to 0.51)b | 0.32 (0.24 to 0.42)b | |
| Age (months) at first PICC insertion | |||||||
| 0 | 1.00 | ||||||
| 1–2 | 0.88 (0.71 to 1.10) | ||||||
| 3–5 | 0.91 (0.73 to 1.14) | ||||||
| 6–8 | 1.11 (0.88 to 1.39) | ||||||
| 9–11 | 1.16 (0.87 to 1.54) | ||||||
| 12–29 | 0.98 (0.74 to 1.28) | ||||||
| ≥ 30 | 0.93 (0.71 to 1.22) | ||||||
| Small for gestational age | 1.10 (0.96 to 1.26) | ||||||
| Surgery | 0.98 (0.72 to 1.32) | ||||||
| Days of invasive ventilation | |||||||
| 0 | 1.00 | 1.00 | |||||
| 1 | 1.15 (0.86 to 1.53) | 1.22 (0.91 to 1.63) | |||||
| 2–4 | 1.50 (1.20 to 1.87)b | 1.60 (1.28 to 2.00)b | |||||
| 5–6 | 2.27 (1.75 to 2.93)b | 2.46 (1.90 to 3.20)b | |||||
| 7–8 | 2.62 (1.99 to 3.44)b | 2.89 (2.19 to 3.80)b | |||||
| 9–12 | 3.02 (2.37 to 3.85)b | 3.32 (2.60 to 4.25)b | |||||
| 13–21 | 2.74 (2.16 to 3.48)b | 3.03 (2.38 to 3.86)b | |||||
| ≥ 22 | 2.73 (2.17 to 3.43)b | 3.08 (2.44 to 3.89)b | |||||
| Days of non-invasive ventilation | |||||||
| 0 | 1.00 | ||||||
| 1 | 0.85 (0.65 to 1.11) | ||||||
| 2–5 | 0.86 (0.71 to 1.06) | ||||||
| 6–9 | 0.79 (0.63 to 0.99)b | ||||||
| 10–15 | 0.78 (0.63 to 0.97)b | ||||||
| 16–49 | 0.68 (0.57 to 0.80)b | ||||||
| ≥ 50 | 0.61 (0.49 to 0.74)b | ||||||
Appendix 18 Additional information on the generalisability study: model-building for trend per 1000 days
| Model, IRR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7a | |
| Trend | |||||||
| PREVAIL NICUs | 0.998 (0.996 to 1.001) | 0.998 (0.996 to 1.000) | 0.998 (0.996 to 1.000) | 0.998 (0.996 to 1.000) | 0.998 (0.996 to 1.001) | 0.999 (0.997 to 1.001) | 0.999 (0.997 to 1.001) |
| Non-PREVAIL NICUs | 1.001 (1.000 to 1.003) | 1.001 (0.999 to 1.003) | 1.001 (0.999 to 1.003) | 1.001 (0.999 to 1.003) | 1.001 (0.999 to 1.003) | 1.002 (1.000 to 1.003) | 1.002 (1.000 to 1.004) |
| LNUs | 0.996 (0.994 to 0.999)b | 0.996 (0.994 to 0.999)b | 0.996 (0.994 to 0.999)b | 0.996 (0.994 to 0.999)b | 0.997 (0.994 to 0.999)b | 0.998 (0.995 to < 1.000)b | 0.998 (0.995 to < 1.000)b |
| Group | |||||||
| PREVAIL NICUs | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Non-PREVAIL NICUs | 0.13 (0.02 to 0.88) | 0.12 (0.02 to 0.79) | 0.14 (0.02 to 0.94) | 0.14 (0.02 to 0.94) | 0.14 (0.02 to 0.92) | 0.15 (0.02 to 1.03) | 0.13 (0.02 to 0.87) |
| LNUs | 3.70 (0.43 to 31.65) | 3.83 (0.45 to 32.78) | 3.84 (0.45 to 32.79) | 3.78 (0.44 to 32.31) | 2.90 (0.34 to 24.83) | 2.86 (0.33 to 24.48) | 2.84 (0.33 to 24.36) |
| NNU variance | 0.13 (0.09 to 0.19) | 0.13 (0.09 to 0.19) | 0.11 (0.07 to 0.16) | 0.11 (0.07 to 0.16) | 0.10 (0.07 to 0.16) | 0.11 (0.07 to 0.16) | 0.13 (0.09 to 0.19) |
| Gestational age (weeks) | |||||||
| < 26 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| 26–28 | 0.66 (0.60 to 0.72)b | 0.66 (0.60 to 0.73)b | 0.66 (0.60 to 0.73)b | 0.68 (0.62 to 0.75)b | 0.73 (0.66 to 0.81)b | 0.73 (0.66 to 0.80)b | |
| 28–32 | 0.61 (0.56 to 0.67)b | 0.62 (0.56 to 0.68)b | 0.62 (0.56 to 0.68)b | 0.68 (0.62 to 0.76)b | 0.58 (0.52 to 0.64)b | 0.58 (0.52 to 0.64)b | |
| 32–37 | 0.65 (0.59 to 0.72)b | 0.66 (0.59 to 0.73)b | 0.66 (0.60 to 0.73)b | 0.78 (0.70 to 0.88)b | 0.48 (0.43 to 0.55)b | 0.48 (0.42 to 0.54)b | |
| ≥ 37 | 1.00 (0.91 to 1.10) | 1.00 (0.91 to 1.10) | 1.00 (0.91 to 1.10) | 1.16 (1.04 to 1.29) | 0.65 (0.58 to 0.74)b | 0.65 (0.58 to 0.73)b | |
| Small for gestational age | 0.91 (0.83 to 0.99)b | 0.91 (0.83 to 0.99)b | 0.91 (0.83 to 0.99)b | 0.93 (0.85 to 1.02) | |||
| Surgery | 0.97 (0.78 to 1.19) | ||||||
| Days of invasive ventilation | |||||||
| 0 | 1.00 | 1.00 | 1.00 | ||||
| 1 | 1.28 (1.12 to 1.45)b | 1.37 (1.20 to 1.55)b | 1.37 (1.21 to 1.56)b | ||||
| 2–4 | 1.36 (1.22 to 1.50)b | 1.47 (1.33 to 1.63)b | 1.48 (1.33 to 1.64)b | ||||
| 5–6 | 1.51 (1.32 to 1.74)b | 1.71 (1.49 to 1.97)b | 1.72 (1.49 to 1.97)b | ||||
| 7–8 | 1.81 (1.56 to 2.11)b | 2.11 (1.82 to 2.46)b | 2.11 (1.82 to 2.46)b | ||||
| 9–12 | 1.73 (1.51 to 1.99)b | 2.05 (1.78 to 2.36)b | 2.06 (1.79 to 2.36)b | ||||
| 13–21 | 1.68 (1.47 to 1.92)b | 2.04 (1.78 to 2.33)b | 2.04 (1.78 to 2.33)b | ||||
| ≥ 22 | 1.56 (1.38 to 1.75)b | 1.96 (1.74 to 2.21)b | 1.96 (1.73 to 2.21)b | ||||
| Days of non-invasive ventilation | |||||||
| 0 | 1.00 | 1.00 | |||||
| 1 | 1.05 (0.93 to 1.20) | 1.06 (0.93 to 1.20) | |||||
| 2–5 | 0.78 (0.70 to 0.86)b | 0.78 (0.70 to 0.86)b | |||||
| 6–9 | 0.64 (0.56 to 0.73)b | 0.64 (0.56 to 0.73)b | |||||
| 10–15 | 0.58 (0.50 to 0.66)b | 0.58 (0.51 to 0.67)b | |||||
| 16–49 | 0.40 (0.36 to 0.45)b | 0.40 (0.36 to 0.45)b | |||||
| ≥ 50 | 0.28 (0.25 to 0.32)b | 0.28 (0.25 to 0.32)b | |||||
Appendix 19 Additional information on the generalisability study: model-building for rate per 100 admissions
| Model, IRR (95% CI) | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6a | |
| Trend | ||||||
| PREVAIL NICUs | 0.996 (0.994 to 0.998)b | 0.997 (0.995 to 0.999)b | 0.997 (0.995 to 0.999)b | 0.998 (0.995 to < 1.000)b | 0.998 (0.995 to < 1.000)b | 0.998 (0.995 to < 1.000)b |
| Non-PREVAIL NICUs | 0.999 (0.998 to 1.001) | 1.001 (0.999 to 1.003) | 1.001 (0.999 to 1.003) | 1.001 (0.999 to 1.003) | 1.001 (0.999 to 1.003) | 1.001 (0.999 to 1.003) |
| LNUs | 0.994 (0.991 to 0.996)b | 0.995 (0.993 to 0.998)b | 0.995 (0.993 to 0.998) | 0.997 (0.995 to < 1.000)b | 0.997 (0.995 to < 1.000)b | 0.997 (0.995 to < 1.000)b |
| Group | ||||||
| PREVAIL NICUs | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Non-PREVAIL NICUs | 0.09 (0.01 to 0.62) | 0.08 (0.01 to 0.55) | 0.09 (0.01 to 0.67) | 0.10 (0.01 to 0.73) | 0.08 (0.01 to 0.61) | 0.09 (0.01 to 0.62) |
| LNUs | 1.92 (0.21 to 17.75) | 1.90 (0.20 to 17.66) | 2.08 (0.22 to 19.28) | 1.39 (0.15 to 12.80) | 1.38 (0.15 to 12.74) | 1.39 (0.15 to 12.83) |
| NNU variance | 0.16 (0.11 to 0.23) | 0.15 (0.10 to 0.22) | 0.12 (0.08 to 0.17) | 0.10 (0.07 to 0.15) | 0.13 (0.09 to 0.19) | 0.13 (0.08 to 0.19) |
| Gestational age (weeks) | ||||||
| < 26 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| 26–28 | 0.58 (0.53 to 0.64)b | 0.58 (0.53 to 0.64)b | 0.83 (0.75 to 0.92)b | 0.83 (0.75 to 0.92)b | 0.79 (0.71 to 0.87)b | |
| 28–32 | 0.28 (0.25 to 0.31)b | 0.28 (0.25 to 0.31)b | 0.63 (0.57 to 0.70)b | 0.63 (0.57 to 0.70)b | 0.66 (0.60 to 0.74)b | |
| 32–37 | 0.12 (0.11 to 0.13)b | 0.12 (0.11 to 0.13)b | 0.36 (0.31 to 0.40)b | 0.36 (0.32 to 0.40)b | 0.42 (0.37 to 0.48)b | |
| ≥ 37 | 0.15 (0.14 to 0.17)b | 0.15 (0.14 to 0.17)b | 0.42 (0.37 to 0.47)b | 0.42 (0.37 to 0.47)b | 0.49 (0.43 to 0.55)b | |
| Small for gestational age | 1.19 (1.08 to 1.31)b | 1.07 (0.97 to 1.18) | ||||
| Surgery | 1.20 (0.96 to 1.49) | |||||
| Days of invasive ventilation | ||||||
| 0 | 1.00 | 1.00 | 1.00 | |||
| 1 | 1.58 (1.39 to 1.80)b | 1.58 (1.39 to 1.80)b | 1.54 (1.35 to 1.76)b | |||
| 2–4 | 2.26 (2.03 to 2.51)b | 2.26 (2.03 to 2.51)b | 2.17 (1.95 to 2.41)b | |||
| 5–6 | 3.75 (3.25 to 4.33)b | 3.75 (3.25 to 4.32)b | 3.50 (3.03 to 4.05)b | |||
| 7–8 | 5.03 (4.29 to 5.91)b | 5.04 (4.29 to 5.92)b | 4.64 (3.95 to 5.45)b | |||
| 9–12 | 5.82 (5.02 to 6.74)b | 5.83 (5.03 to 6.76)b | 5.28 (4.55 to 6.13)b | |||
| 13–21 | 6.67 (5.79 to 7.69)b | 6.69 (5.80 to 7.71)b | 5.96 (5.16 to 6.89)b | |||
| ≥ 22 | 9.49 (8.34 to 10.80)b | 9.55 (8.40 to 10.86)b | 8.26 (7.22 to 9.44)b | |||
| Days of non-invasive ventilation | ||||||
| 0 | 1.00 | |||||
| 1 | 0.90 (0.79 to 1.03) | |||||
| 2–5 | 0.88 (0.79 to 0.98) | |||||
| 6–9 | 1.06 (0.92 to 1.22) | |||||
| 10–15 | 1.25 (1.09 to 1.45)b | |||||
| 16–49 | 1.39 (1.25 to 1.54)b | |||||
| ≥ 50 | 1.44 (1.25 to 1.65)b | |||||
Appendix 20 Additional information on the generalisability study: bloodstream infection definitions
| BSI term | Definition |
|---|---|
| 1. BSI during PICC days at risk | BSI that occurs from 1 day after PICC insertion to 2 days after PICC removal |
| 2. Early-onset BSI | BSI that occurs in babies aged < 2 days (excluding BSI during PICC days at risk) |
| 3. Late-onset BSI (no PICC) | BSI that occurs from 2 days of age to 2 days after discharge (excluding BSI during PICC days at risk) |
| 4. Total late-onset BSI | BSI that occurs during PICC days at risk plus BSIs that are late onset (no PICC) |
| 5. Total BSI | All BSI that meet any of the criteria for terms 1–3a |
Appendix 21 Additional information on the generalisability study: flow diagram of included PREVAIL babies
FIGURE 26.
Flow diagram to show babies randomised to receive S-PICCs in PREVAIL who were linked to the NNRD and included in the generalisability analysis.
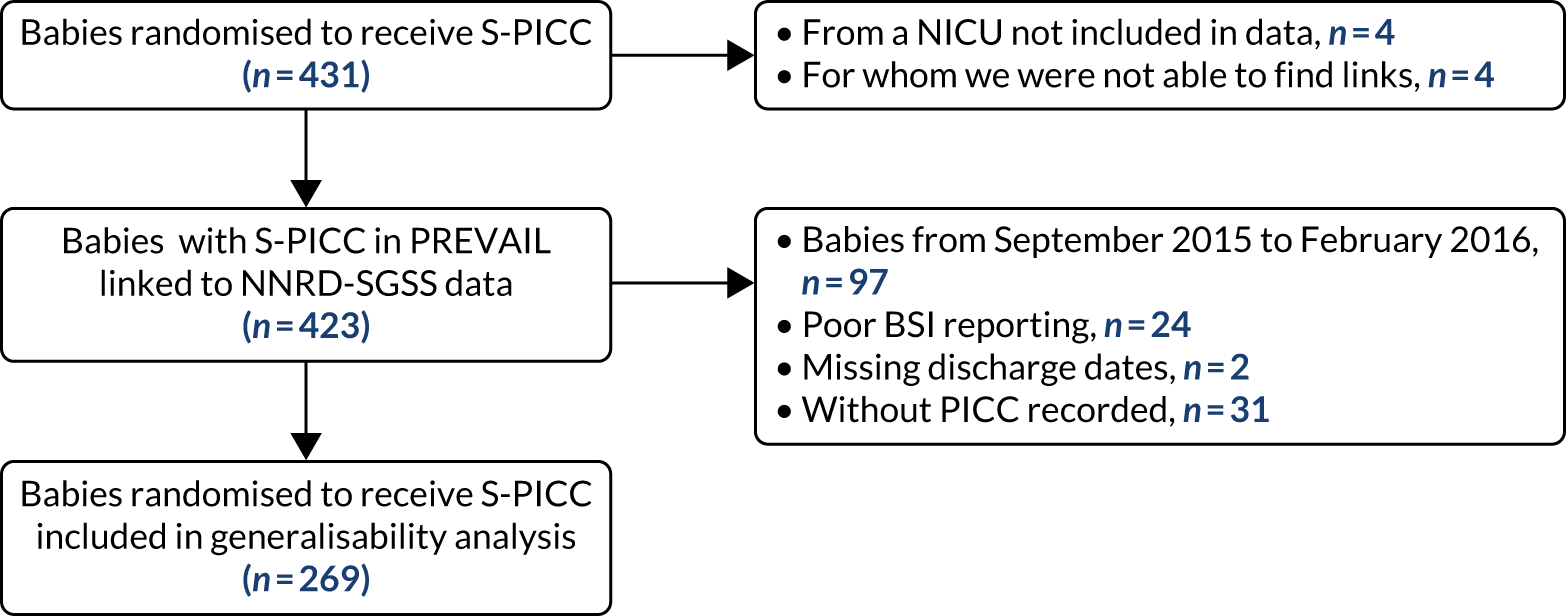
Appendix 22 Additional information on the generalisability study: causative organisms
| Organism | PREVAIL NICUs (n = 44) | Non-PREVAIL NICUs (n = 28) | LNUs (n = 68) |
|---|---|---|---|
| Number of babies | 5433 | 3754 | 2381 |
| Number of admissions | 5958 | 4061 | 2485 |
| Number of admissions with a BSI | 189 | 338 | 92 |
| Clearly pathogenic organisms, n (%) | |||
| E. coli | 22 (8) | 62 (11) | 10 (5) |
| Group B streptococci | 8 (3) | 20 (4) | 11 (5) |
| Other Gram negative | 29 (11) | 55 (10) | 21 (10) |
| Other Gram positive | 17 (6) | 19 (4) | 9 (4) |
| Staphylococcus aureus | 20 (8) | 45 (8) | 14 (7) |
| Other organisms, n (%) | |||
| CoNS | 151 (57) | 322 (58) | 135 (64) |
| Other | 17 (7) | 20 (4) | 11 (5) |
| p-value from chi-squared test comparing with PREVAIL NICUs | 0.593 | 0.238 | |
Appendix 23 Additional information on the generalisability study: rates of bloodstream infection per 1000 peripherally inserted central venous catheter days
FIGURE 27.
Crude and risk-adjusted rates of BSI (pathogens) per 1000 PICC days in babies who received S-PICCs in NICUs and LNUs according to enrolment in the PREVAIL trial during the PREVAIL trial period (August 2015 to January 2017). Adjusted for gestational age at birth, age at first PICC and days of invasive and non-invasive ventilation. p-value for effect of group (PREVAIL babies in NICU, babies in non-PREVAIL trial NICUs, babies in LNUs) on BSI rate in comparison to babies in the PREVAIL trial. Period for NICUs that participated in the PREVAIL trial depends on NICU start and end date of recruitment; period for other NICUs and LNUs is August 2015 to January 2017 (whole PREVAIL trial recruitment period).
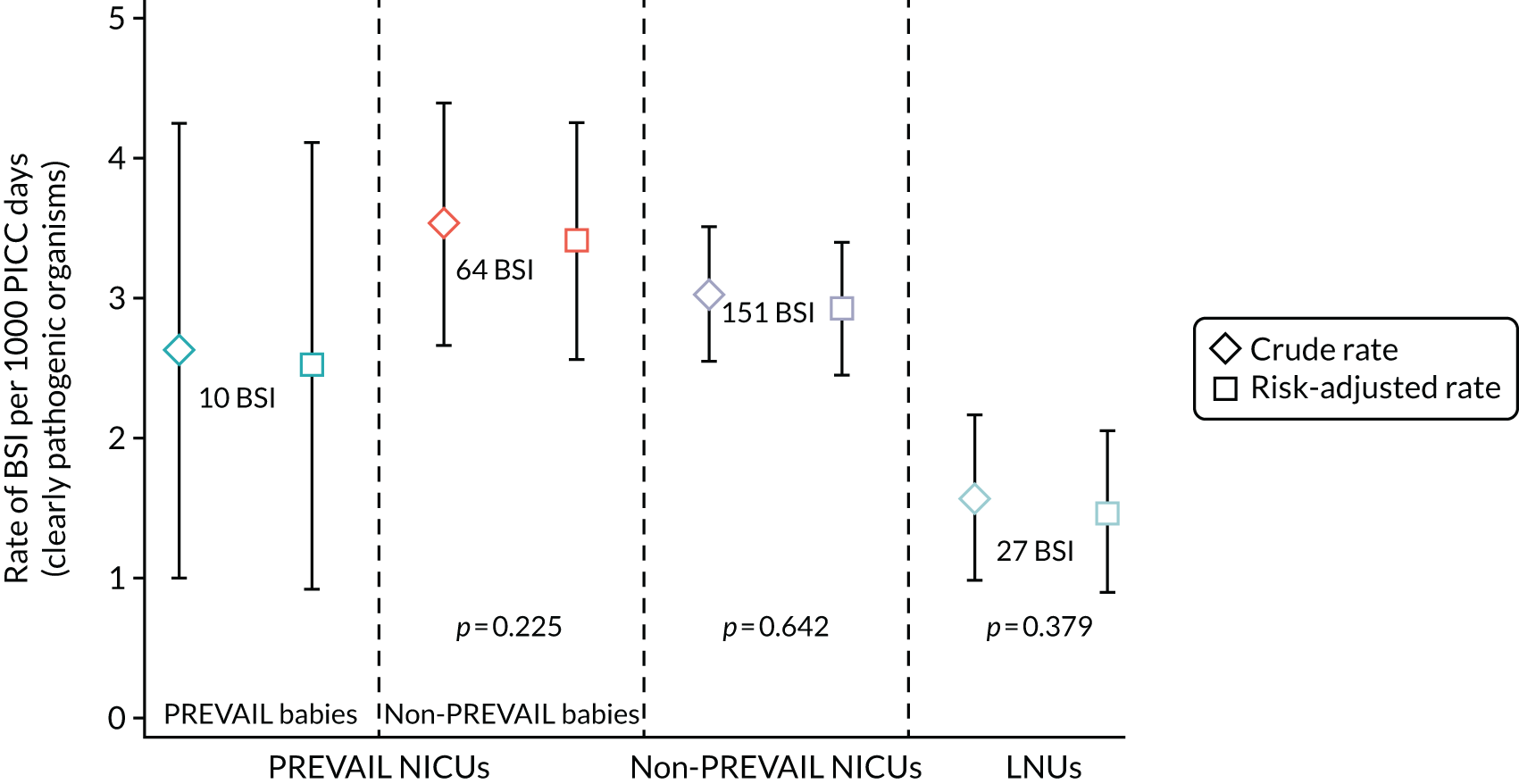
| BSI per 1000 PICC days | NICUs (n = 16) participating in PREVAILa | NICUs (n = 27) and LNUs (n = 67) not participating in PREVAIL | ||
|---|---|---|---|---|
| Admissions in PREVAIL | Admissions not in PREVAIL | Admissions to NICUb | Admissions to LNUb | |
| Babies (n) | 269 | 1608 | 3745 | 2358 |
| Admissions (n) | 325 | 1522 | 4051 | 2460 |
| PICC days (n) | 3809 | 18,115 | 49,902 | 17,187 |
| All BSIs | ||||
| Number of BSIs | 31 | 185 | 379 | 96 |
| Crude BSI rate (95% CI) | 8.1 (5.3 to 11.0) | 10.2 (8.7 to 11.7) | 7.6 (6.8 to 8.4) | 5.6 (4.5 to 6.9) |
| Adjusted BSI rateb (95% CI) | 7.8 (5.0 to 10.6) | 9.9 (8.5 to 11.4) | 7.4 (6.6 to 8.1) | 5.0 (3.9 to 6.0) |
| p-valuec | ||||
| BSI due to clearly pathogenic organisms | ||||
| Number of BSI | 10 | 64 | 151 | 27 |
| Crude BSI rate (95% CI) | 3.2 (1.4 to 4.9) | 3.3 (2.5 to 4.1) | 3.2 (2.7 to 3.7) | 1.5 (0.9 to 2.1) |
| Adjusted BSI rateb (95% CI) | 2.5 (0.9 to 4.1) | 3.4 (2.6 to 4.3) | 2.9 (2.4 to 3.4) | 1.5 (0.9 to 2.0) |
| p-valuec | 0.225 | 0.642 | 0.379 | |
Appendix 24 Additional information on the generalisability study: trends in bloodstream infection risk factors
FIGURE 28.
The percentage of babies admitted to NICUs and LNUs according to gestational age and by year.
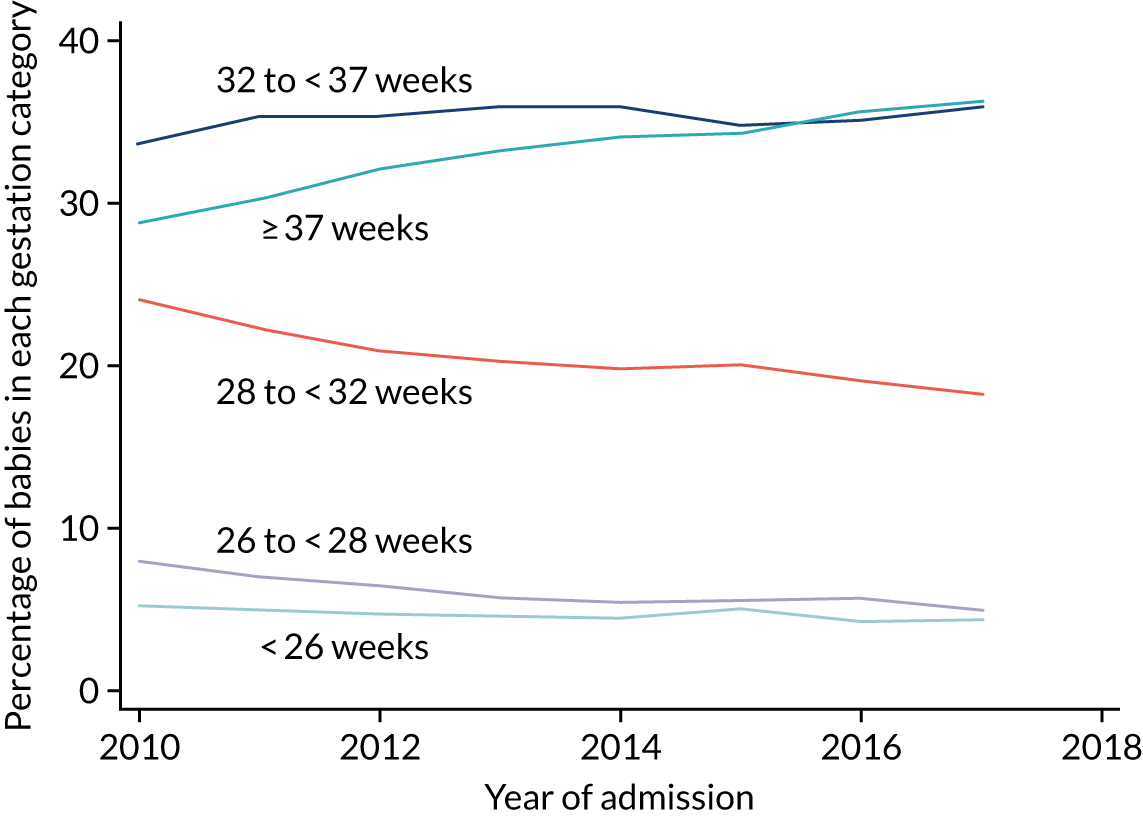
FIGURE 29.
The mean number of days of intensive and high-dependency care per baby in NICUs and LNUs by week of gestational age at birth. (a) Days of stay in NICUs; and (b) days of stay in LNUs. p-value from two-way ANOVA demonstrating whether or not the mean is significantly different by week of gestation in 2010, compared with 2017.
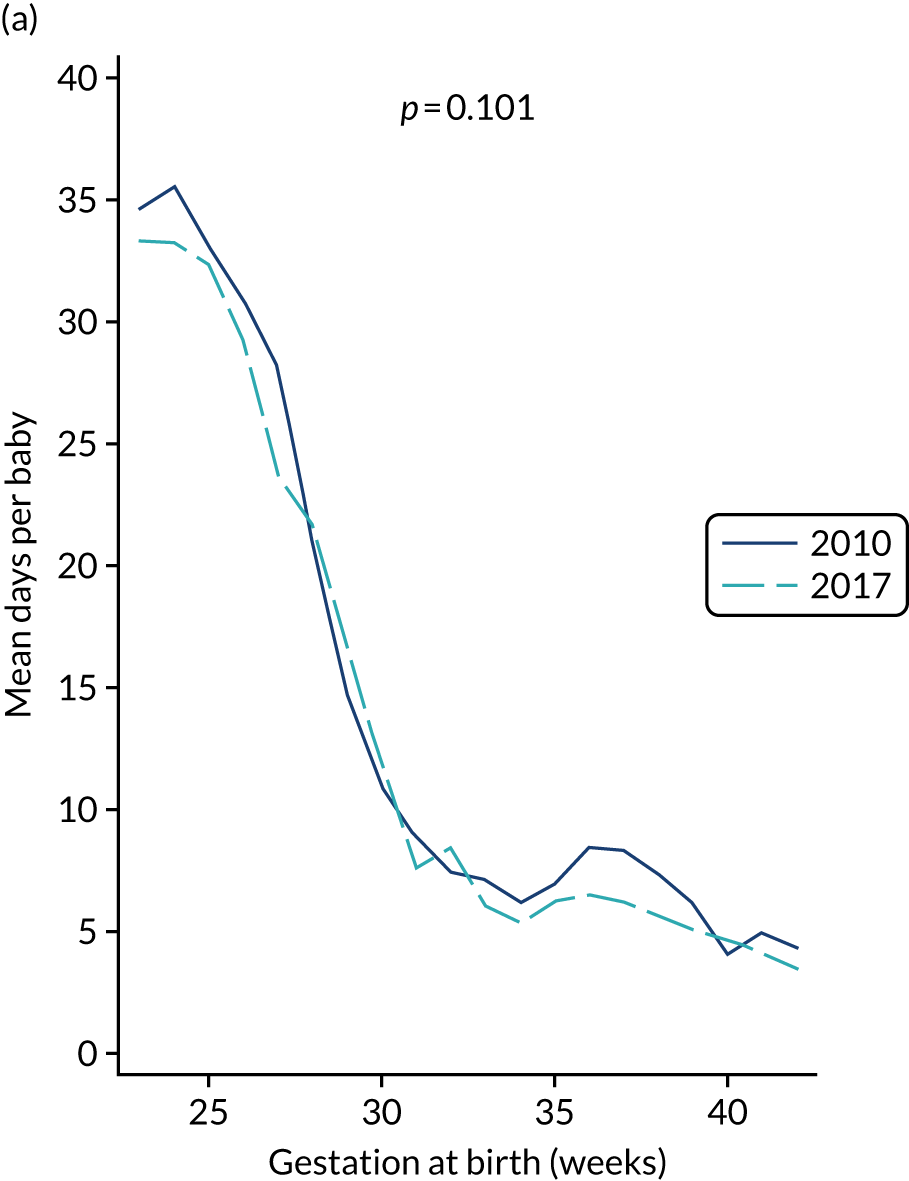
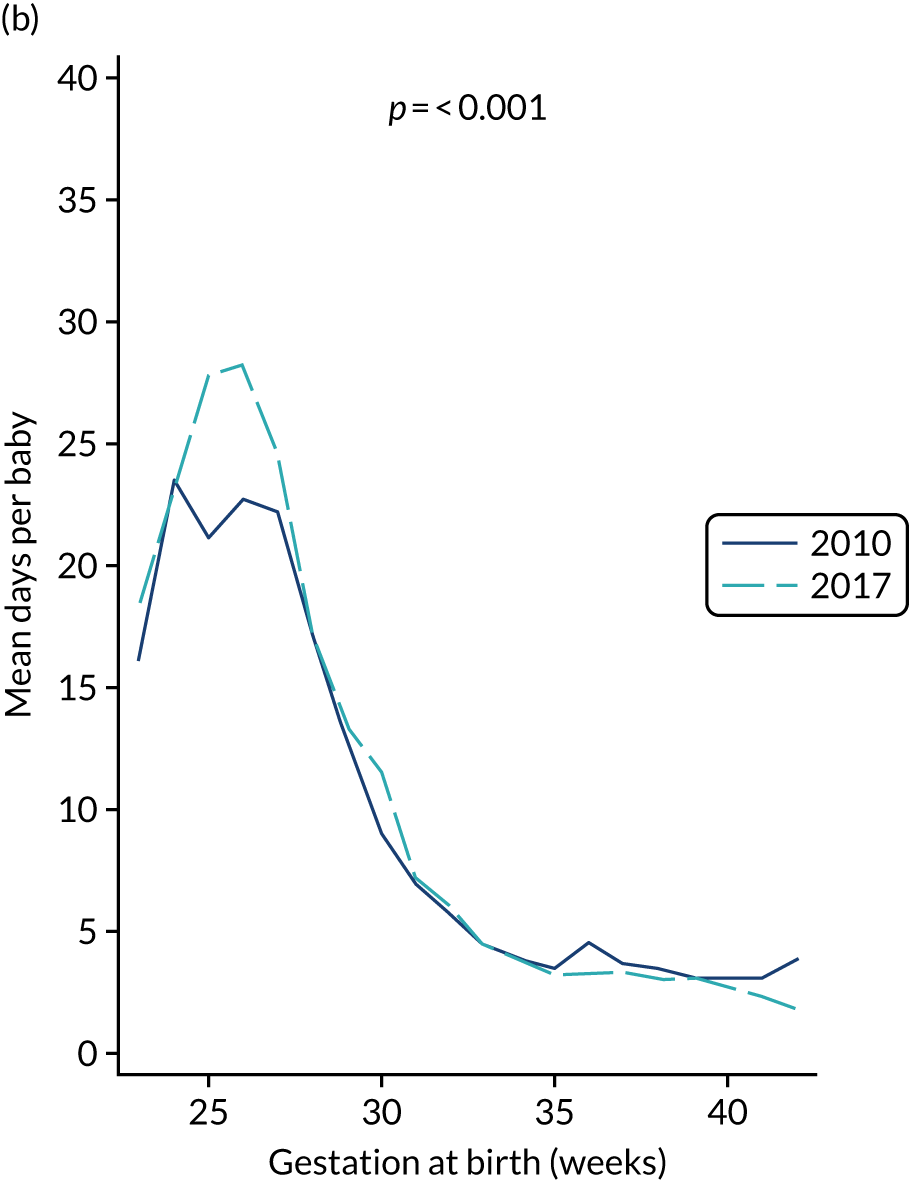
FIGURE 30.
The mean number of PICC days per baby in NICUs and LNUs by week of gestational age at birth. (a) PICCs in NICUs; and (b) PICCs in LNUs. p-value from two-way ANOVA demonstrating whether or not the mean is significantly different by week of gestation in 2010, compared with 2017.
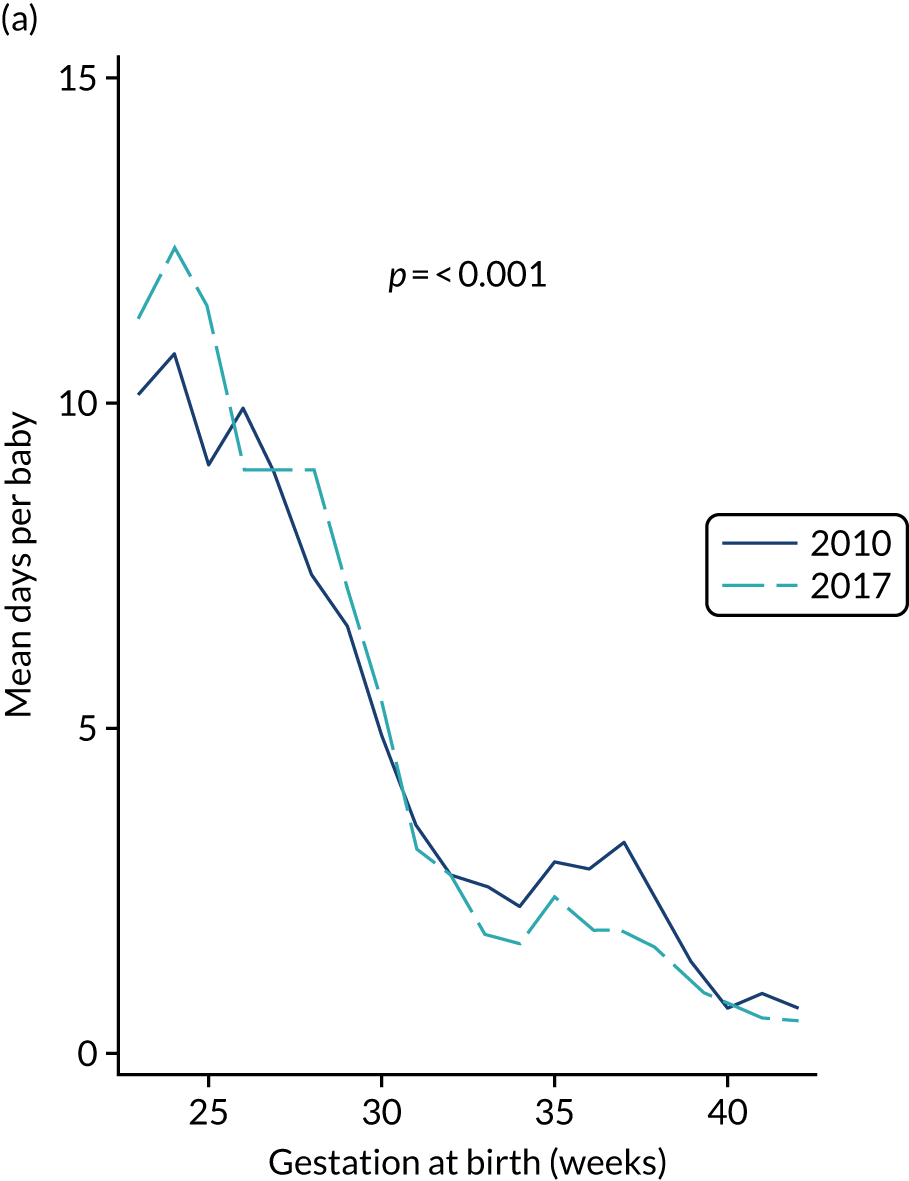
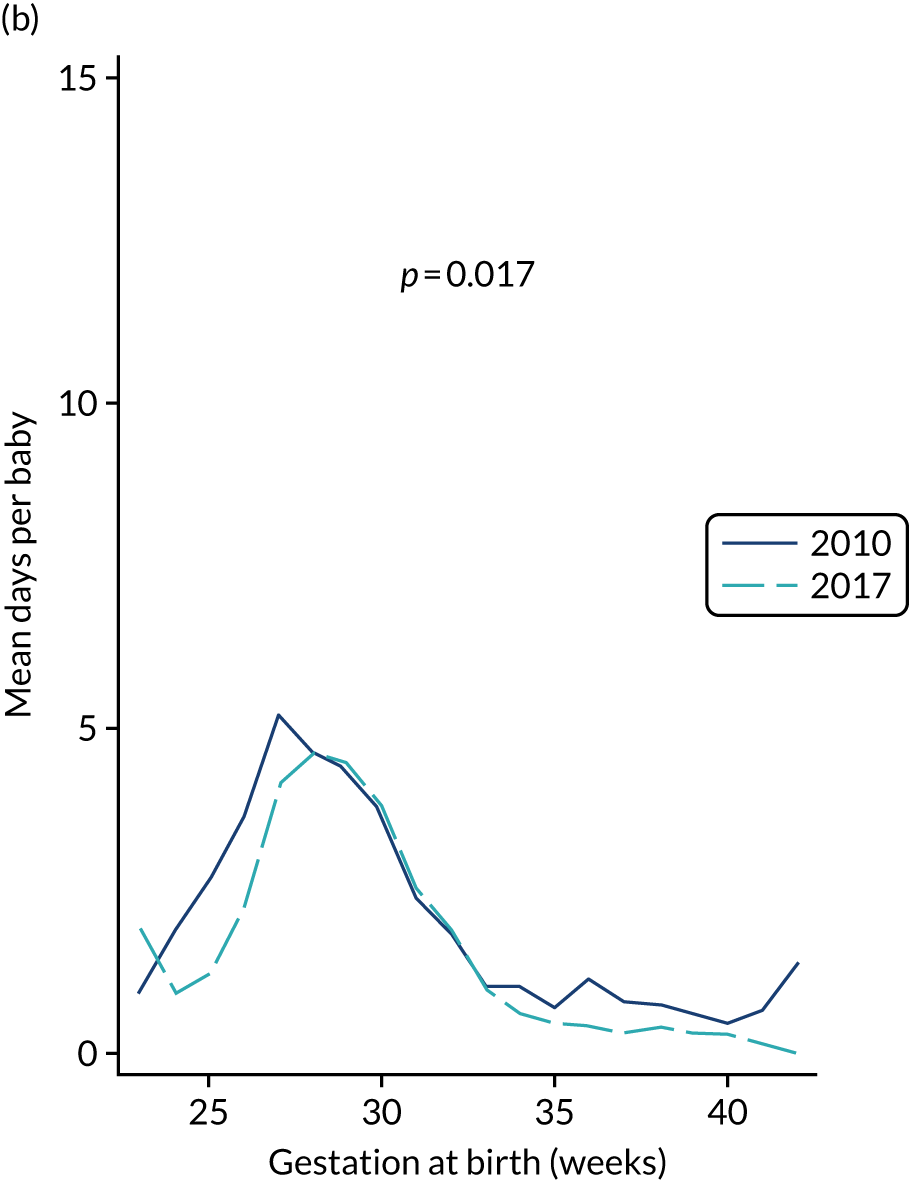
FIGURE 31.
The mean number of central line days per baby in NICUs and LNUs by week of gestational age at birth. (a) Central lines in NICUs; and (b) central lines in LNUs. p-value from two-way ANOVA demonstrating whether or not the mean is significantly different by week of gestation in 2010, compared with 2017.

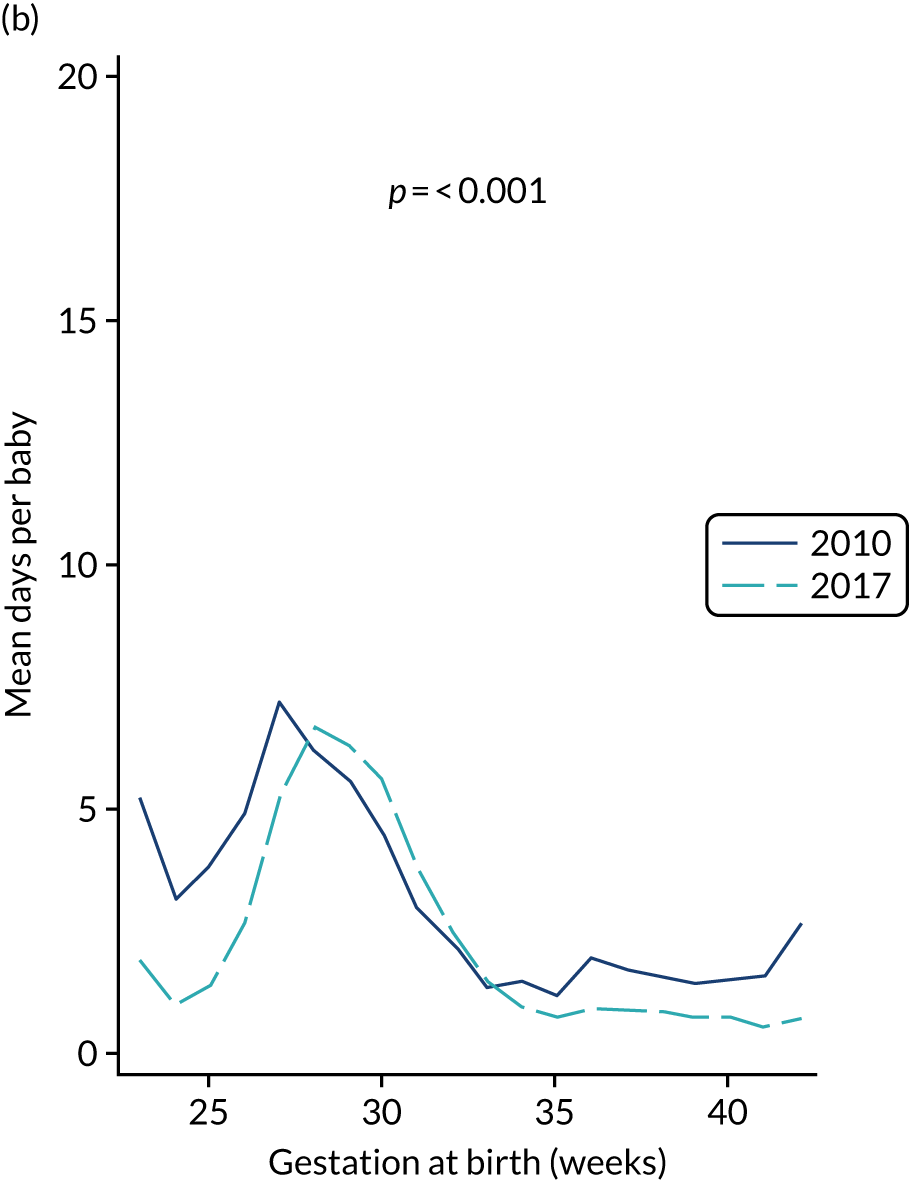
FIGURE 32.
The mean age at first PICC insertion in NICUs and LNUs by week of gestational age at birth. (a) NICUs; and (b) LNUs. p-value from two-way ANOVA demonstrating whether or not the mean is significantly different by week of gestation in 2010, compared with 2017.
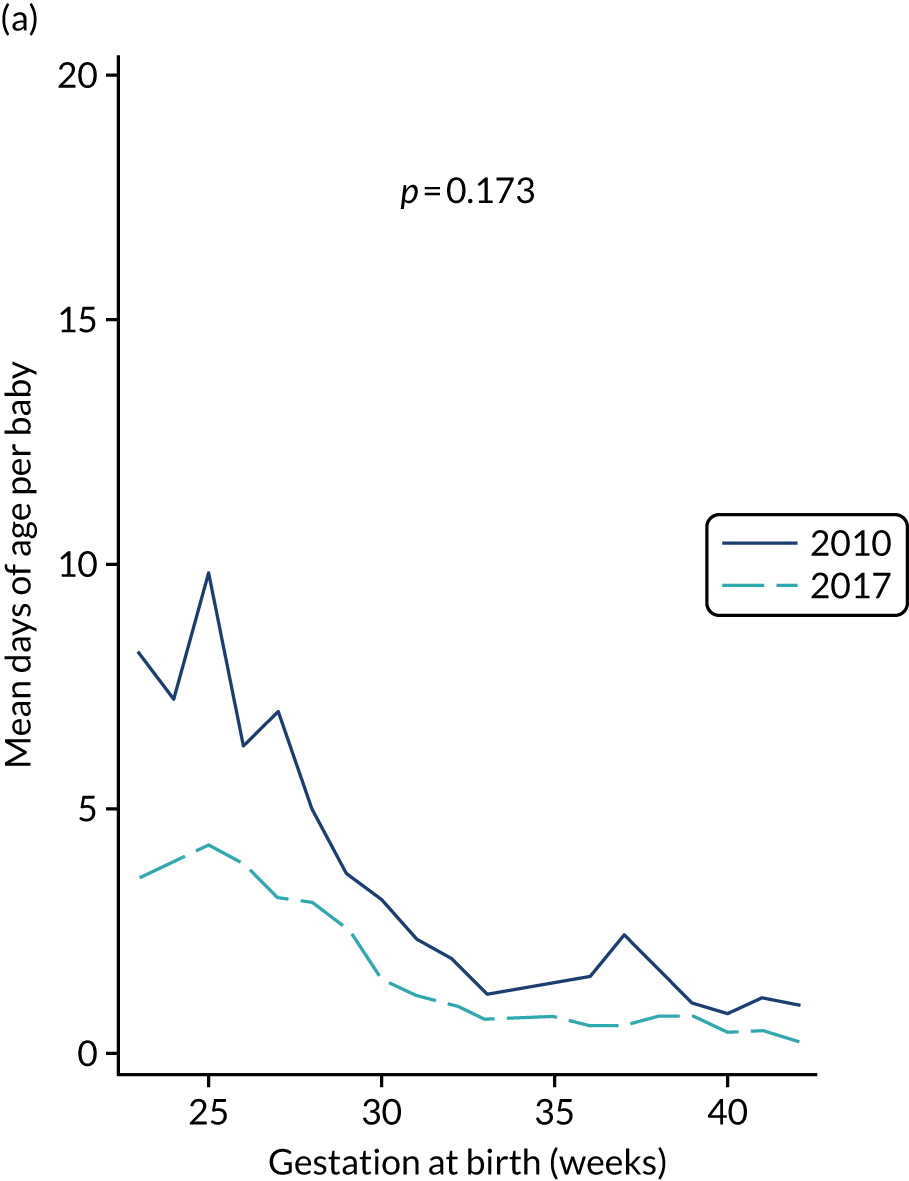
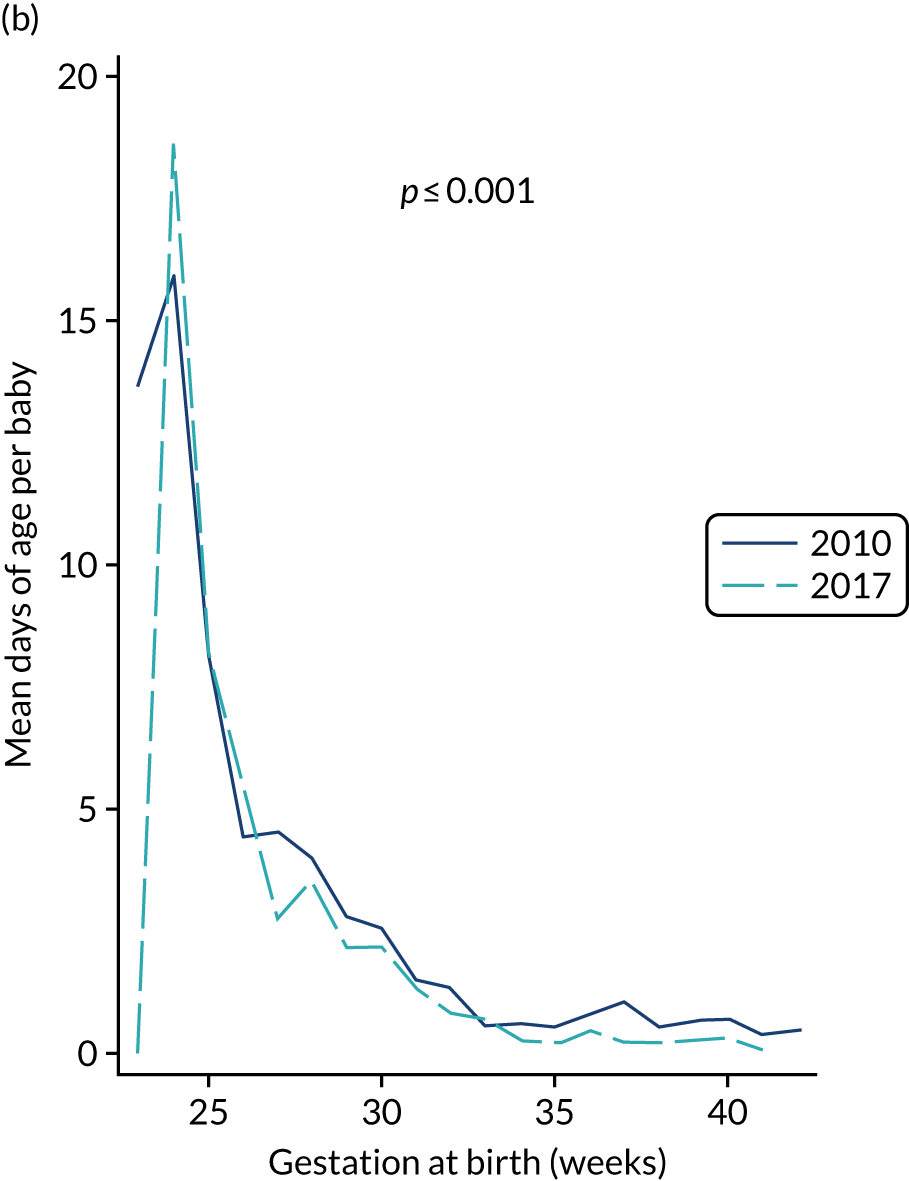
FIGURE 33.
The mean number of non-invasive ventilation days per baby in NICUs and LNUs by week of gestational age at birth. (a) NICUs; and (b) LNUs. p-value from two-way ANOVA demonstrating whether or not the mean is significantly different by week of gestation in 2010, compared with 2017.
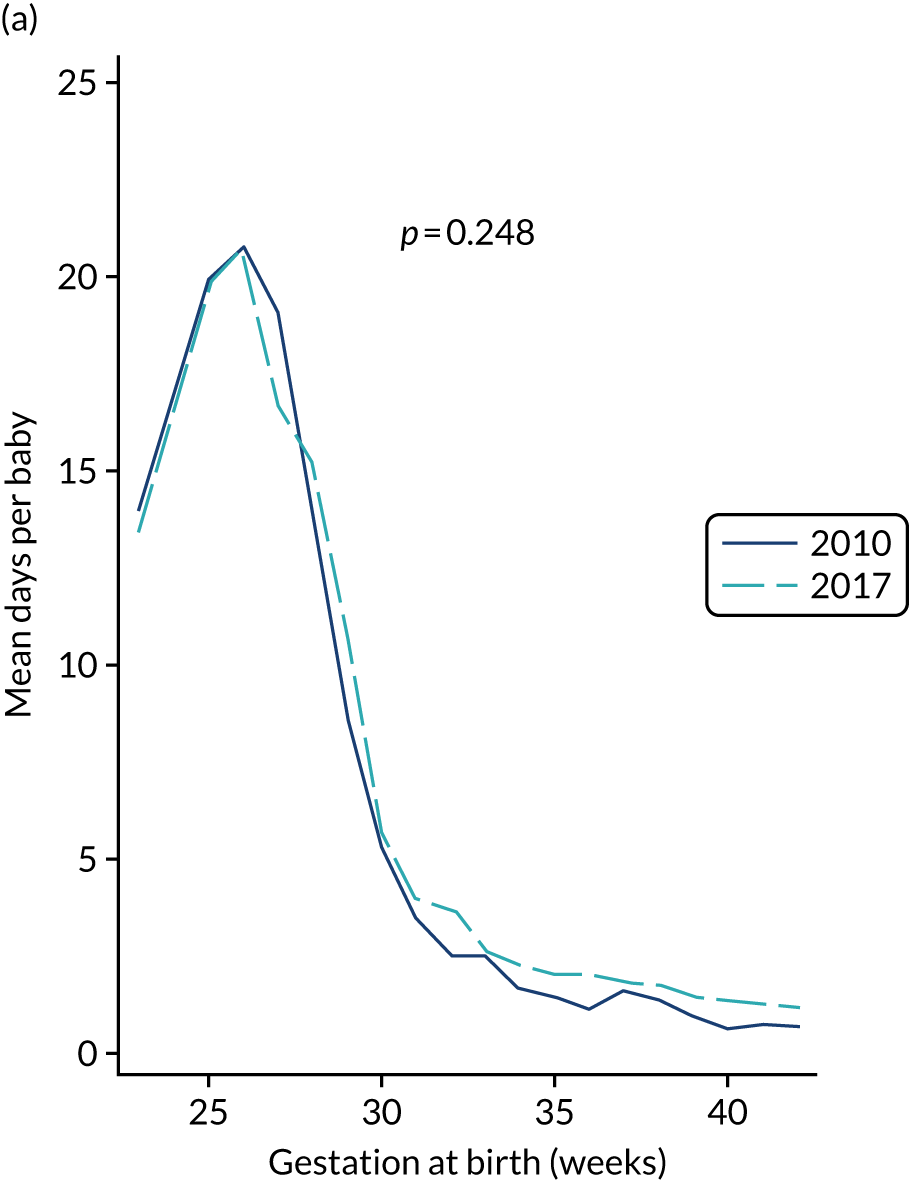
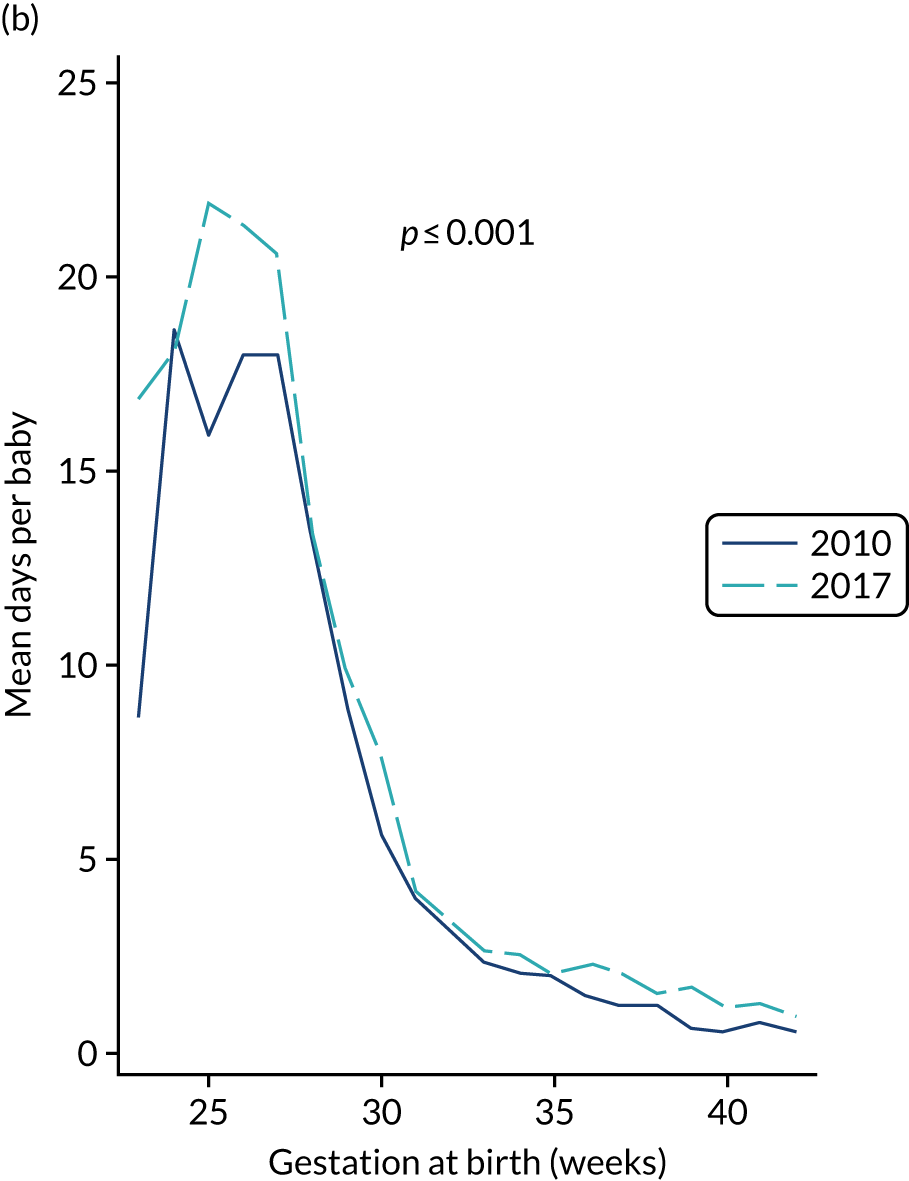
FIGURE 34.
The mean number of invasive ventilation days per baby in NICUs and LNUs by week of gestational age at birth. (a) NICUs; and (b) LNUs. p-value from two-way ANOVA demonstrating whether or not the mean is significantly different by week of gestation in 2010, compared with 2017.
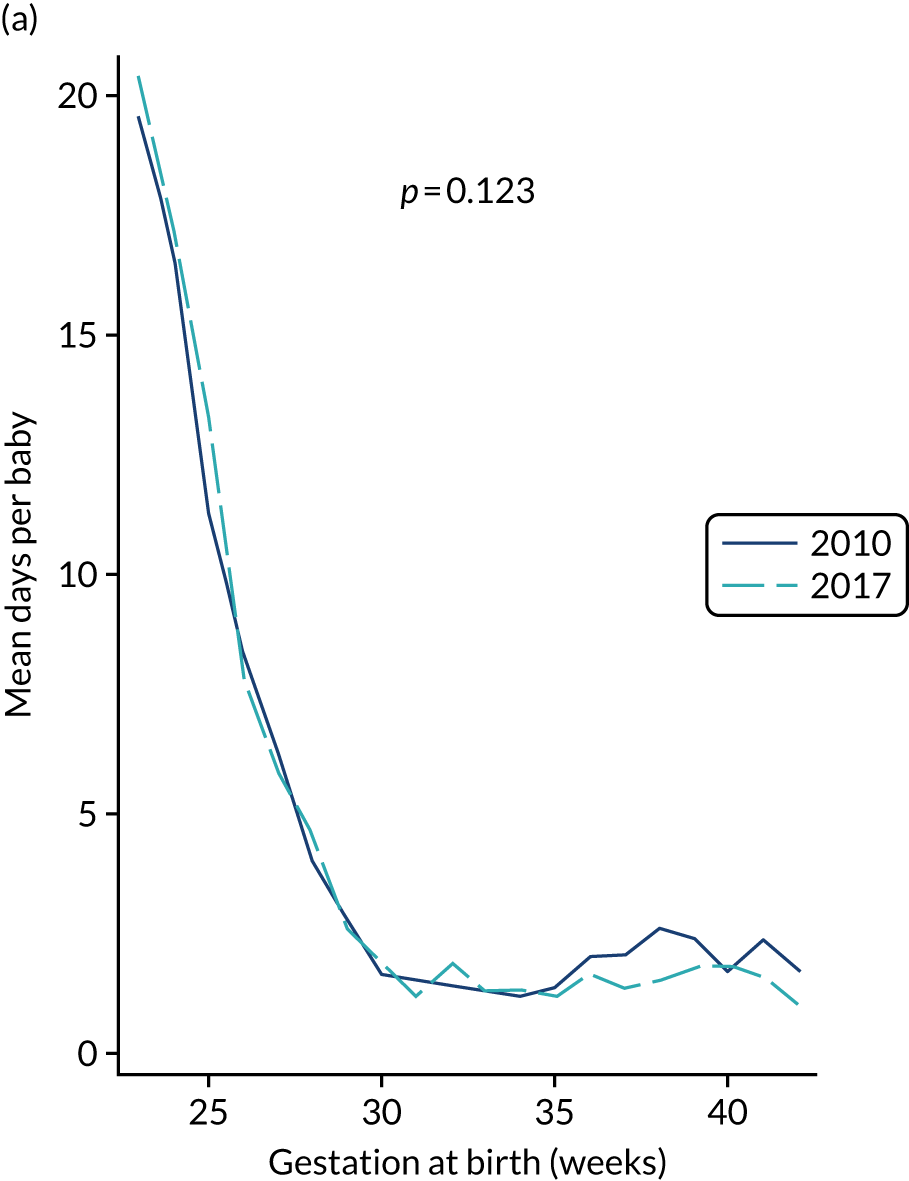
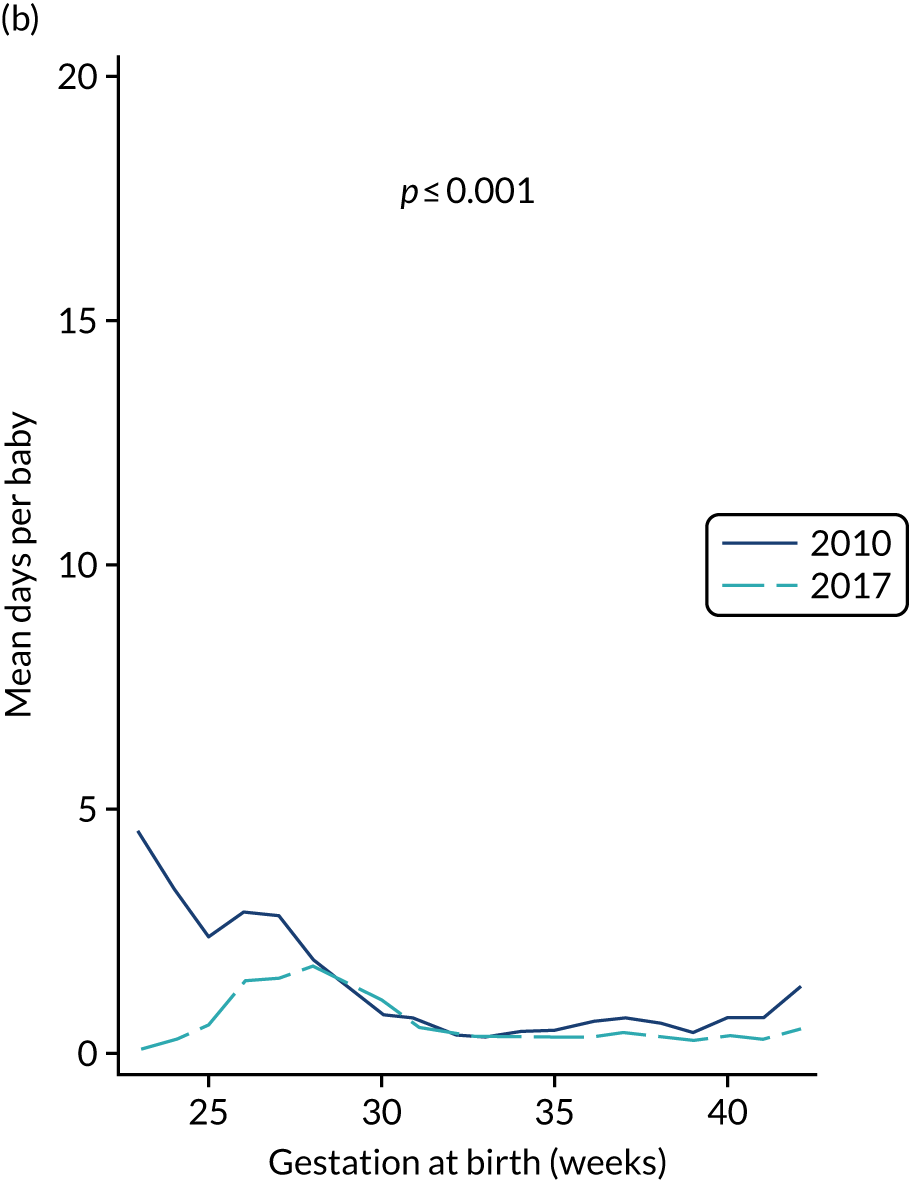
Appendix 25 Additional information on the generalisability study: flow diagrams
FIGURE 35.
The number of admissions and babies receiving S-PICCs according to NNU level and enrolment in the PREVAIL trial for the PREVAIL trial period (August 2015 to January 2017).
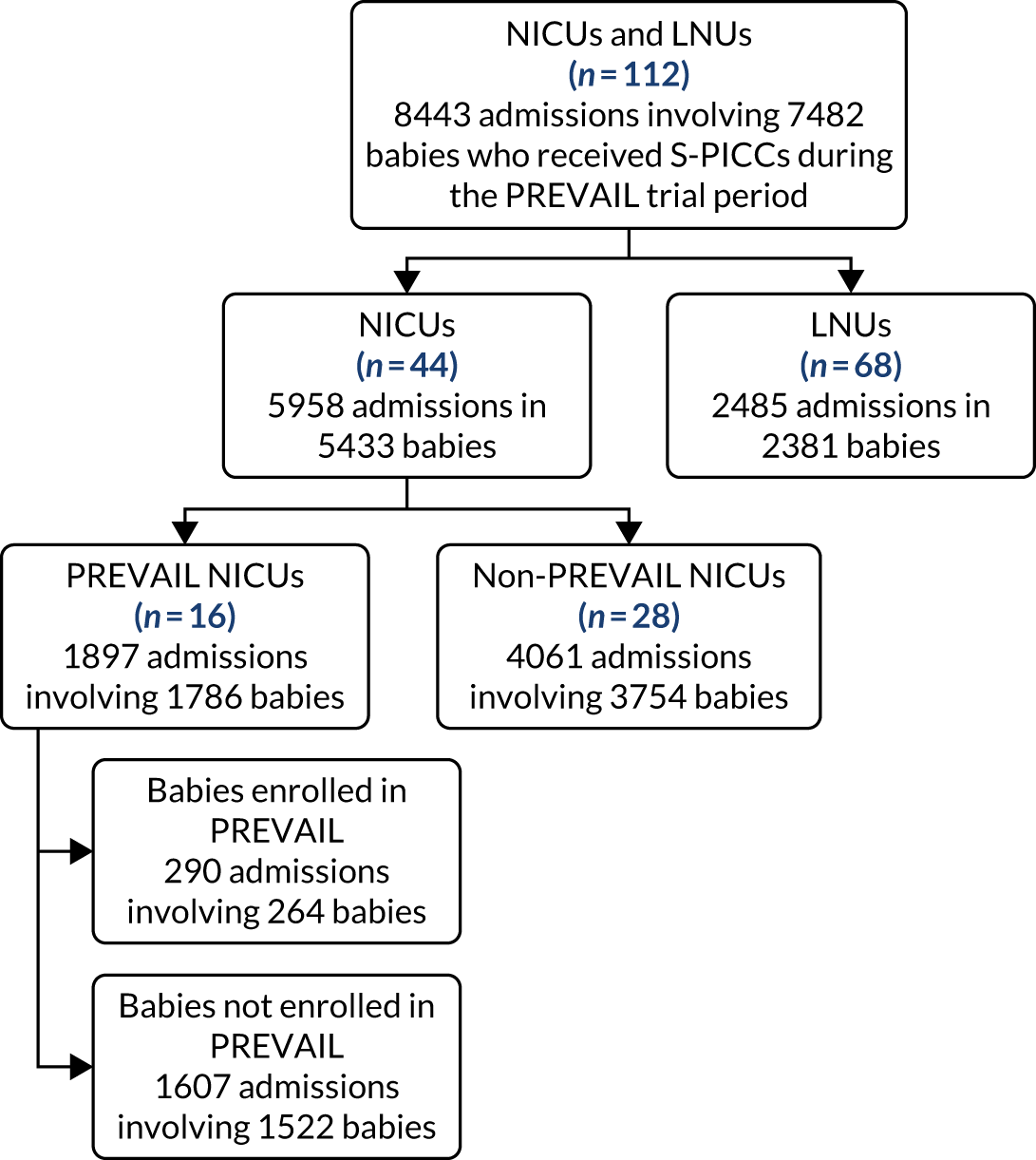
FIGURE 36.
The number of admissions and babies who received PICCs from March 2010 to June 2017 and who were included in the analysis of changes over time in BSI during PICC days at risk.
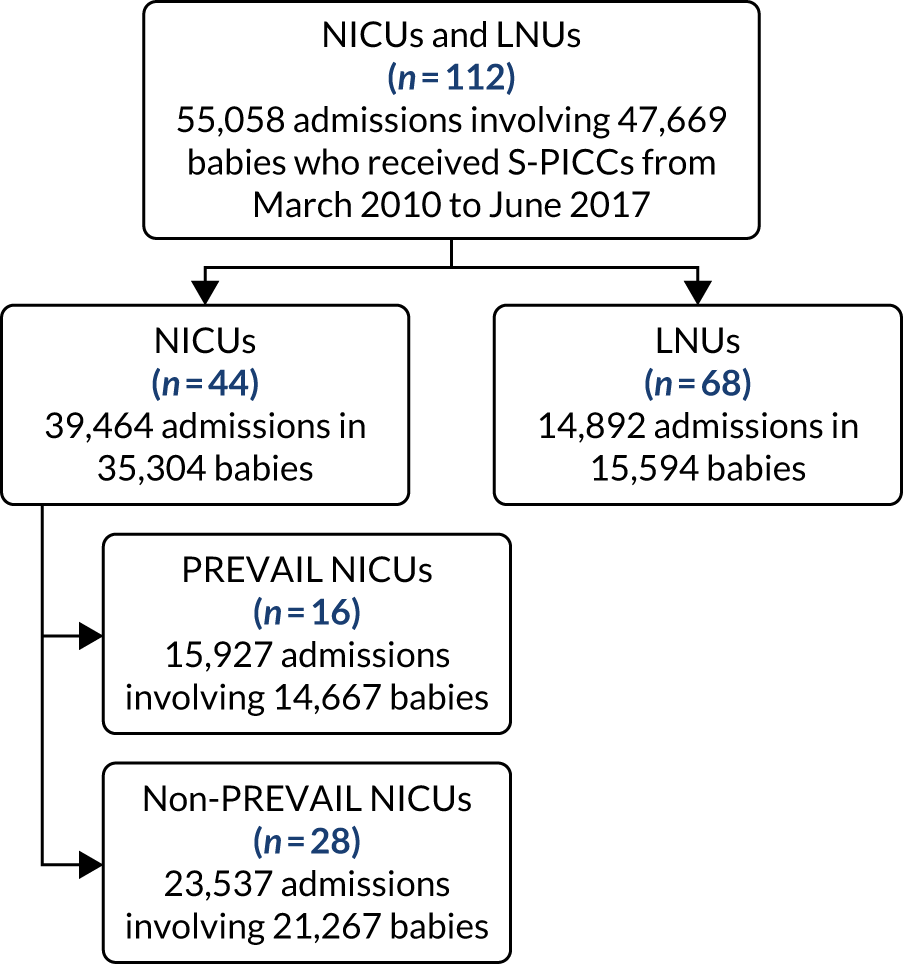
FIGURE 37.
The number of admissions and babies who received intensive or high-dependency care from March 2010 to June 2017 and who were included in the analysis of changes over time in late-onset BSI.
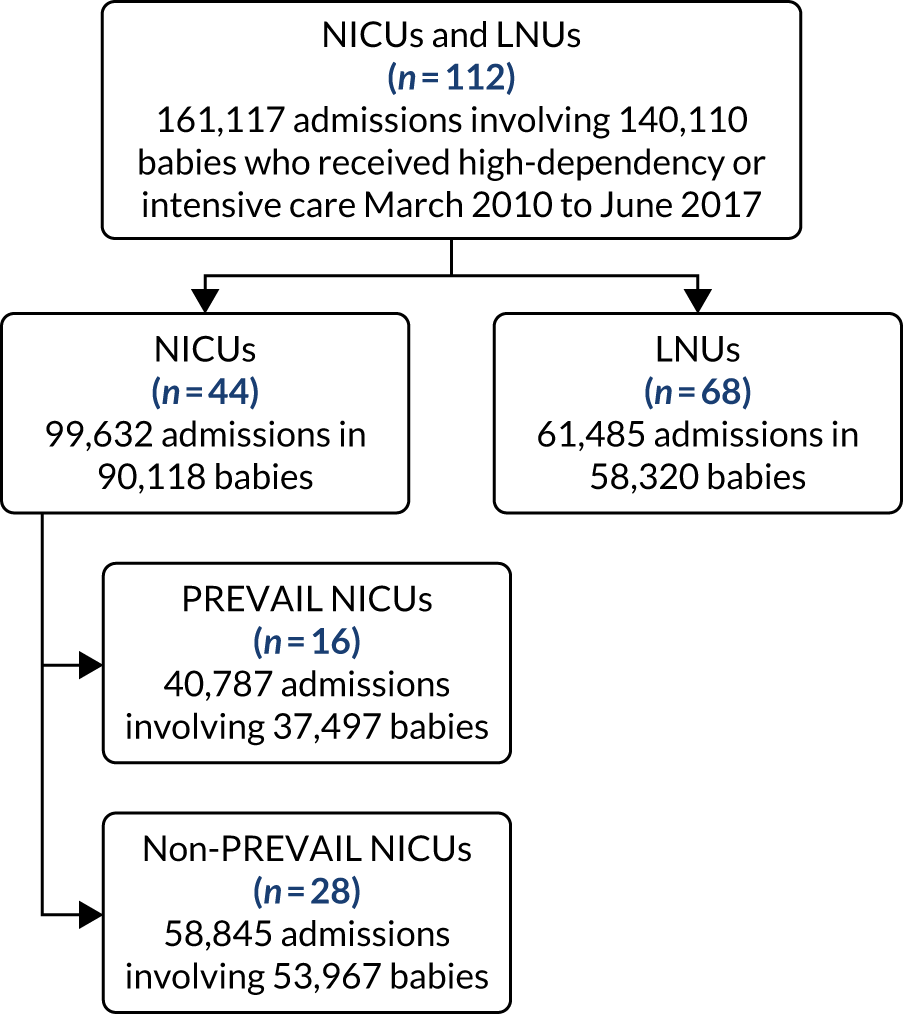
Glossary
- Bloodstream infection
- This refers to any positive bacterial or fungal cultures from blood or cerebrospinal fluid samples. A clinically serious bloodstream infection refers to a bloodstream infection for which the baby was treated for > 72 hours with intravenous antibiotics or died during treatment.
- CATheter Infections in CHildren
- A randomised controlled trial and economic evaluation comparing impregnated and standard central venous catheters in children.
- Catheter-related bloodstream infection
- This refers to a bloodstream infection with isolation of the same organism from the peripherally inserted central venous catheter tip and blood or cerebrospinal fluid.
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AIC
- Akaike information criterion
- AM-PICC
- antimicrobial-impregnated peripherally inserted central venous catheter
- ANOVA
- analysis of variance
- APC
- Admitted Patient Care
- Apgar
- appearance, pulse, grimace, activity, respiration
- BIC
- Bayesian information criterion
- BSI
- bloodstream infection
- CATCH
- CATheter Infections in CHildren
- CI
- confidence interval
- CoNS
- coagulase-negative staphylococcus
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CSF
- cerebrospinal fluid
- CVC
- central venous catheter
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- FCE
- finished consultant episode
- GDPR
- General Data Protection Regulation
- HES
- Hospital Episode Statistics
- HQIP
- Healthcare Quality Improvement Partnership
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- IDSMC
- Independent Data and Safety Monitoring Committee
- IGARD
- Independent Group Advising on the Release of Data
- IQR
- interquartile range
- IRR
- incidence rate ratio
- LCTC
- Liverpool Clinical Trials Centre
- LNU
- local neonatal unit
- MDI
- Mental Development Index
- NDAU
- Neonatal Data Analysis Unit
- NDI
- neurodevelopmental impairment
- NEC
- necrotising enterocolitis
- NICE
- National Institute for Health and Care Excellence
- NICU
- neonatal intensive care unit
- NIHR
- National Institute for Health Research
- NNAP
- National Neonatal Audit Programme
- NNRD
- National Neonatal Research Database
- NNU
- neonatal unit
- OR
- odds ratio
- PDI
- Psychomotor Developmental Index
- PHE
- Public Health England
- PI
- principal investigator
- PICANet
- Paediatric Intensive Care Audit Network
- PICC
- peripherally inserted central venous catheter
- PICU
- paediatric intensive care unit
- PREVAIL
- PREVenting infection using Antimicrobial-Impregnated Long lines
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RN
- research nurse
- RR
- relative risk
- SAE
- serious adverse event
- SCBU
- special care baby unit
- SD
- standard deviation
- S-PICC
- standard peripherally inserted central venous catheter
- SGSS
- Second Generation Surveillance System
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UCL
- University College London
- UKNC
- UK Neonatal Collaborative
- UoL
- University of Liverpool
- UoY
- University of York
- VICSG
- Victorian Infant Collaborative Study Group
- VoI
- value of information
