Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/35/99. The contractual start date was in September 2011. The draft report began editorial review in August 2019 and was accepted for publication in June 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Clarkson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Introduction
The subsequent chapters of this monograph describe the INTERVAL (Investigation of NICE Technologies for Enabling Risk-Variable-Adjusted-Length) Dental Recalls Trial, a National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme-funded trial testing of the effectiveness and cost–benefit of dental check-ups at different recall intervals. The trial protocol has been published. 1
The reason for the trial
Background
Traditionally, patients have been encouraged to attend dental recall appointments at regular intervals of 6 months between appointments – irrespective of the individual’s risk of developing dental disease, the principal function of the dental recall being prevention and early detection of oral disease, in particular dental caries (tooth decay) and periodontal disease. 2 The traditional clinical rationale, developed at times of higher caries levels and progression rates, was the early detection of caries lesions while they were small in order to restore them before lesion progression resulted in extensive destruction of tooth tissue. This has evolved to a modern philosophy that seeks to detect small lesions at an early stage in order to provide preventative interventions prior to lesion cavitation. The preventative advice provided at recall examinations varies between practitioners and, indeed, between a practitioner’s individual patients and may incorporate instruction on appropriate oral hygiene practices and dietary advice for the prevention of dental disease, as well as advice aimed at modifying risk factors for oral disease such as smoking cessation advice and alcohol-related health advice. The dental recall examination may, therefore, be understood as having a dual function as a primary preventative (the prevention of oral disease before it occurs) and a secondary preventative (limiting the progression and effect of oral diseases at an early stage through early diagnosis) measure. The evolution of implementing the change from surgical to preventative treatment philosophies has been, and continues to be, complex and slow.
The recommendation of a 6-month recall interval has become established practice in primary dental care in many countries3–7 and has probably been a cornerstone of dental practice since it was mentioned by Pierre Fauchard in 1746. 8 There has been a longstanding international debate regarding the clinical effectiveness and cost-effectiveness of different recall intervals for routine dental check-up examinations,2,3,5,9–16 particularly in the light of changes in the epidemiology of dental diseases and in the interests of careful resource management. 9,17–19 This debate has been fuelled by conflicting evidence from observational studies on the effects of regular attendance and by the subsequent diverging interpretations of that conflicting evidence. 20
Epidemiology and pathogenesis
Periodontal disease is an inflammatory disease of the soft and hard tooth-supporting tissues. Periodontal diseases comprise gingivitis and periodontitis. Gingivitis is a reversible condition characterised by gingival redness and oedema, and absence of periodontal attachment loss. 21 The 2017 world workshop and classification system gives clear definition of a gingivitis site and a gingivitis case (patient). 21 A patient can be defined as a ‘gingivitis case’ when bleeding on probing at > 30% of sites is evident at a minimum of 10% of sites. 21 Bleeding at between 10% and 30% of sites is defined as localised gingivitis and bleeding on probing at > 30% of sites is generalised gingivitis. Gingival health is defined as bleeding on probing at < 10% of sites. Gingivitis is a pre requisite for periodontitis and is also a risk indicator for dental caries progression.
Periodontitis is the irreversible destruction of the tooth-supporting periodontal structures (periodontal ligament, cementum and alveolar bone) due to inflammation. 22 Periodontitis is characterised by periodontal pocket formation and gingival recession. In addition, tooth mobility and migration may occur as a result, as well as dentine hypersensitivity of the exposed root surface, root caries and, ultimately, tooth loss.
Gingivitis and periodontitis are a continuum of the same inflammatory disease process,23 with evidence that gingivitis is a risk factor for periodontitis,24 and that absence of gingival bleeding is a reliable predictor for the maintenance of periodontal health. 25 However, it is not currently possible to predict progression from gingivitis to periodontitis at either the individual or the site-specific level. Accumulation of microbial dental plaque is the primary aetiological factor for gingivitis and periodontitis, as well as dental caries. 26–28 Disease progression is also known to be affected by genetic factors (host defence mechanism), calculus, smoking and systemic comorbidities, including type 2 diabetes. 29–32
Despite the largely preventable nature of periodontal disease, it is considered the most common disease of mankind,33 remains the major cause of poor oral health globally and is the primary cause of tooth loss in older adults. 22,34 Global estimates of gingivitis range from 50% to 90% of populations. 35–37 Severe periodontitis is the sixth most prevalent human disease globally, with a prevalence of 11.2%,38 which appears to be rapidly increasing. 33
Dental caries is a multifactorial chronic oral disease that affects most populations globally and is considered the most important global oral health burden. 39 Dental caries results from production of organic acids by acidogenic bacteria within dental plaque (a biofilm formed on the tooth surface soon after tooth cleaning). Dental caries is considered a consequence of an ecological shift in the balance of the normally beneficial oral microbiota driven by a change in lifestyle and in the oral environment. 40 Organic acids can cause mineral loss from the tooth surface by removing calcium and phosphate ions from surface apatite crystals (demineralisation). In favourable conditions, a reversal of this process is possible (remineralisation). The development of a carious lesion is a dynamic process that may progress, halt or reverse. Progression of the carious lesion occurs where the demineralisation process prevails over remineralisation. Carious lesions can range from early non-detectable mineral loss restricted to enamel, through lesions that extend into dentine without any surface cavitations, to cavitated lesions visible as holes in the teeth. Progression rates of carious lesions appear to be more rapid in dentine than in enamel, with variable rates between individuals as well as between lesions within an individual. 41,42 Dental caries and its consequences are considered the most important burden of oral health, affecting up to 100% of adults in most countries. 43 It is not just a disease of children, but appears to occur at a relatively constant rate throughout the life course. 44
Where gingival recession has migrated apical to the amelocemental junction, the exposed root surface of the tooth may be susceptible to root caries. Like coronal caries, the main aetiological factor for the initiation and progression of root caries is the presence of a cariogenic biofilm and fermentable carbohydrates. Owing to the lower level of mineralisation of dentine, a smaller decrease in pH will induce demineralisation of the root surface. 45 As with coronal caries, the formation of root caries is a dynamic process of demineralisation and remineralisation, with progression of caries occurring where the balance of factors favours demineralisation. 46 Importantly, and unlike caries in enamel, coronal dentine and root caries both involve not only demineralisation but also collagen degradation,47 resulting in a demineralisation process that is approximately twice as rapid on enamel. 48
Root caries, like other forms of the disease, can be associated with pain, discomfort and tooth loss,49,50 which has the most significant impact on the oral health-related quality of life (OHRQoL) of the elderly. 51,52 Although a well-recognised disease, its prevalence is increasing as populations age and retain more of their natural teeth into older life. 47,53,54 There is a wide range in the reported global prevalence of root caries for diverse populations ranging from 29% to 89%. 55
Individuals and dental care professionals have different roles to play in the prevention and control of periodontal diseases and dental caries. Consistent removal of the intraoral plaque biofilm by means of personal tooth brushing and interdental cleaning is considered the foundation of successful primary prevention of periodontal disease and dental caries. 56,57 The dental care professionals’ role in primary prevention involves assessment of an individual’s risk of developing oral disease and tailoring preventative advice, including oral hygiene and dietary advice based on this risk assessment, although the evidence relating to the beneficial effects of chairside provision of dental health education advice is conflicting. 58,59
In 2004, the National Institute for Health and Care Excellence (NICE) published a guideline entitled Dental Recall: Recall Interval Between Routine Dental Examinations,60 following a remit received from the Department of Health and Social Care and the Welsh Assembly Government. Within this guideline, the role of the oral health review, or dental check-up, in providing primary prevention and secondary prevention is highlighted. Subsequently, in 2011, the Scottish Dental Clinical Effectiveness Programme (SDCEP) published their guidance on oral health assessment and review. The SDCEP guidance was based on and was a tool to assist implementation of the NICE recommendations. 61
Given that the global burden of oral disease is not shared evenly, the risk of developing oral disease between patients is clearly variable. It has, therefore, been suggested that the preventative needs of patients are also variable and that intervals between oral health reviews should be appropriate for the needs of individual patients.
Dental check-ups at 6-month intervals have been customary in the general dental service (GDS) in the UK since the inception of the NHS. Although a recall interval of 6 months is not explicitly recommended by the NHS, this practice is implicitly recognised by NHS regulations that have remunerated dental practitioners for providing a dental check-up at 6-month intervals for decades, and, since 2006, the dental check-up has been free in Scotland. It has been argued that a 6-month dental recall policy is too rigid and that recall intervals should match the individual needs of patients more closely – needs that may change over time. Analysis of dental attendance patterns in NHS primary dental care using the Dental Practice Board’s longitudinal data demonstrates an attendance pattern that is variable, with many patients attending less frequently than every 6 months. 59 Data from the Information Services Division (ISD) in Scotland show that the vast majority of Scottish adults did not attend NHS primary care dental services on an annual basis, with only 23% attending at least once per year in each of the previous 6 years, and 21% not attending at all in the past 6 years. 62
In addition, it has been consistently observed that caries experience is generally more extensive in lower socioeconomic status groups,63 reinforcing the case for patient-specific recall intervals, based on an assessment of the patient’s risk of oral disease. 3,60,64 Evidence from the Dutch health system suggests that there is an increase in general dentists moving away from recalling all patients at the same interval in favour of applying an individualised recall interval, resulting in more frequent screening for periodontal disease than with those dentists using a fixed-period recall protocol. 65
One of the persistent arguments in favour of maintaining 6-month dental check-ups is that dentists may miss the opportunity to diagnose oral cancer lesions at an early stage in patients who attend at longer recall intervals. The incidence of oral cavity cancer in the UK is highest in Scotland, at 10.0 per 100,000 males,66 and has been relatively stable in Scotland since 2000 but rising in England and Wales. However, it has been reported that 53.7% of patients diagnosed with oral cancer had not attended an NHS primary care dentist in the 2 years preceding diagnosis,67 thus radically decreasing the opportunity for early detection. From these data it is estimated that a dentist potentially encounters one case of oral cancer every 10 years. 67 Brocklehurst and Speight68 reviewed the pros and cons of a national screening programme for mouth cancer, concluding that studies into mouth cancer screening have provided evidence to satisfy only 5 of the 20 criteria required by the UK Screening Committee, with no evidence of a test effective in the detection of oral lesions in the context of a screening programme,69 and that more research is needed to develop diagnostic tests more specific than conventional oral examination. 68 The authors also report that screening programmes have not resulted in a demonstrable reduction in mortality, apart from in high-risk groups, in which there is some evidence that screening may be effective and cost-effective. 68 Instead of asserting the need for shorter intervals between dental recall appointments, these papers67–69 highlight the need for oral health services to develop strategies to reach out to populations that do not attend primary care dental services regularly, instruct patients about high-risk habits including alcohol and tobacco use, and better network with other primary care services.
Evidence base
The recommendations in the NICE guideline on dental recall60 are designed to aid dentists in assigning individualised recall intervals to patients based on their risk of developing oral disease. The Guideline Development Group produced a checklist for use by dentists when assessing risk, including specific risk factors for consideration. 60 The guideline recommends an adjustable recall interval for adults, ranging from a minimum of 3 months to a maximum interval of 24 months between recall appointments for patients who have repeatedly demonstrated an ability to maintain oral health. The guideline panel recommended that the recall interval be regularly assessed, discussed and agreed based on each individual’s oral health risk profile and amended accordingly. The Guideline Development Group considered a balance of benefits and harms related to caries, periodontal disease and also oral mucosal lesions in making its recommendations. The recommendations are, however, based on low-quality evidence and the clinical experience of the Guideline Development Group. This lack of evidence has complicated the implementation of this guideline for dentists and health service commissioners.
Although the concept of assigning risk-based recall intervals has gained increasing standing internationally, the clinical effectiveness of this recall protocol is not supported by scientific evidence from clinical trials. Furthermore, there remains significant variation in professional recommendations within and between countries regarding the maximum time interval between dental check-ups that can reasonably be assigned for patients at low risk of oral disease. This can be considered inevitable given that many guideline recommendations regarding this issue have been informed primarily by professional consensus and are subject to variation in interpretation. 70
There is also a paucity of reliable scientific evidence to support the effectiveness of routine dental checks of differing recall frequencies in adults. The scientific basis for the 6-month dental examination was questioned more than 30 years ago. 2 Since then, systematic reviews investigating this key question have reported limited evidence of poor overall quality, which is insufficient to reach any conclusions regarding the potential beneficial and harmful effects of varying recall intervals between dental check-ups, and concluding that there is no evidence to support or refute the practice of encouraging patients to attend for dental check-ups at 6-month intervals. 71,72 The NICE guideline on dental recall reiterated the need for research in this area to examine the effects of varying dental recall intervals on oral health. 59 The NICE guideline also concluded that the research base was severely lacking in terms of determination of the optimal dental recall intervals on the basis of cost-effectiveness. 60
There is, therefore, an urgent need to assess the relative effectiveness and cost-effectiveness of different dental recall intervals in a robust, sufficiently powered randomised control trial (RCT) in primary dental care.
The questions addressed by the INTERVAL trial
Aim
The aim of this trial was to compare the effectiveness and cost–benefit of dental check-ups at different recall intervals (fixed-period 6-month recall, risk-based recall or fixed-period 24-month recall) for maintaining optimum oral health in dentate adults attending general dental practice.
Objectives
The primary objectives were to compare the three recall strategies on:
-
gingival bleeding on probing
-
oral health-related quality of life
-
value for money in terms of (1) cost per quality-adjusted life-year (QALY) gained, (2) incremental net (societal) benefit and (3) incremental net (dental health) benefits.
The secondary objectives were to compare the three recall strategies on:
-
periodontal probing depths
-
dental caries
-
calculus
-
preventative and interventive dental treatment
-
patient anxiety
-
patient satisfaction with care
-
oral health knowledge, attitudes and behaviours and to explore dentists’ attitudes towards dental recall intervals. 10
Chapter 2 Trial design and methods
Study design
The trial was a UK-wide [England (London, Manchester, Birmingham, North East), Wales (Cardiff), Northern Ireland (Belfast, County Down) and Scotland] multicentre, parallel-group, RCT with blinded outcome assessment at 4-year follow-up.
The trial interventions were three recall intervals – a fixed-period 24-month recall interval, a risk-variable adjusted-length recall interval (risk-based recall) based on the NICE guideline60 and a fixed-period 6-month recall interval.
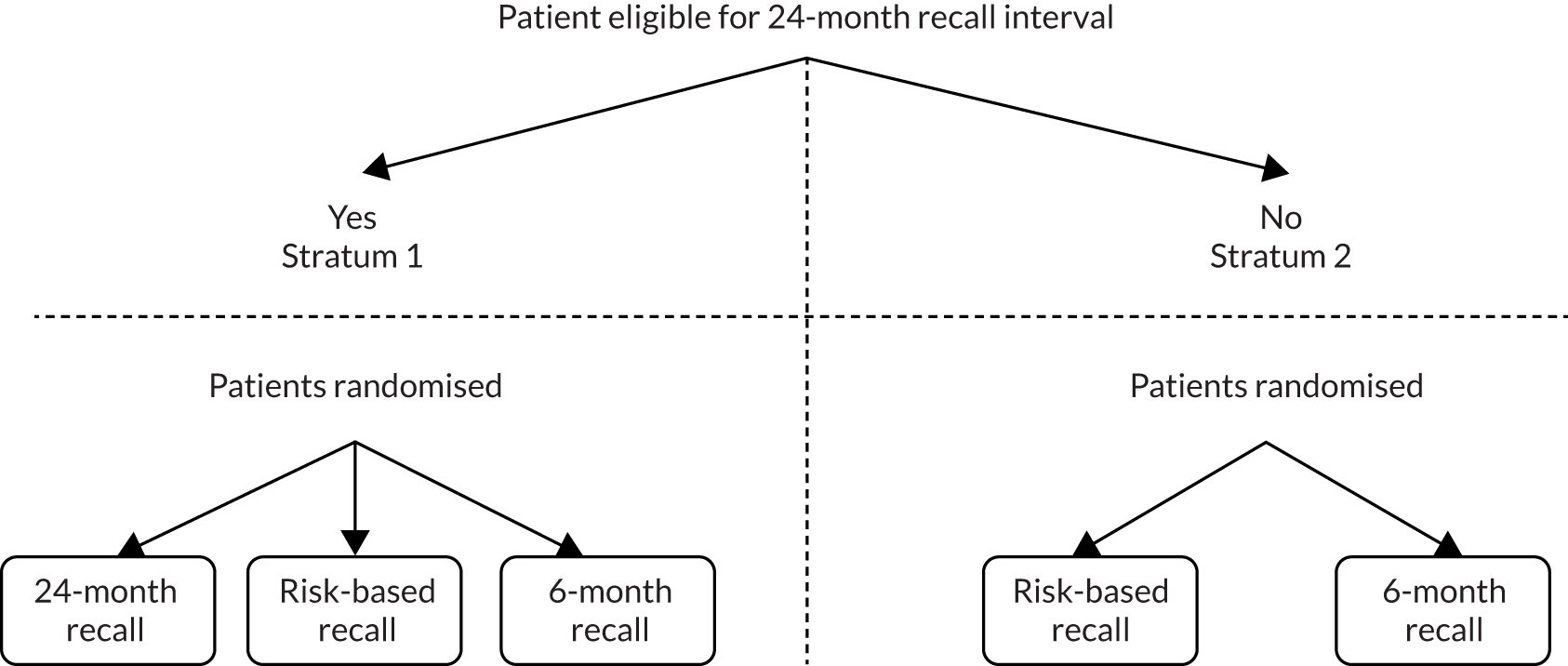
A two-stratum trial design was proposed to overcome potential ethical considerations and dental clinician and/or participant concerns. Participants were randomised to the fixed-period 24-month recall interval only if the recruiting dentist considered them clinically suitable.
Randomisation was organised within the two strata (Figure 1):
-
For those participants considered suitable for a fixed-period 24-month recall (stratum 1), randomisation was to one of three groups:
-
Fixed-period 24-month recall versus risk-variable-adjusted-length recall (risk-based recall) versus fixed-period 6-month recall.
-
-
For those participants not considered suitable for a fixed-period 24-month recall (stratum 2), randomisation was to one of two groups:
-
Risk-variable-adjusted-length recall (risk-based recall) versus fixed-period 6-month recall.
-
FIGURE 1.
Study design.
An economic evaluation to determine the cost-effectiveness of different recall intervals and to compare NHS and patient-incurred costs and benefits is included in Chapter 5.
Ethics approval and protocol amendments
Favourable ethical opinions were granted for the INTERVAL Dental Recalls Trial by the Fife and Forth Valley Research Ethics Committee (feasibility study Research Ethics Committee reference number 09/S0501/1; main study Research Ethics Committee reference number 09/S0501/1).
The trial was registered with the International Standard Randomisation Controlled Trial Register (ISRCTN), reference number 95933794.
Amendments to the protocol were made after recruitment of practices and participants, and on conclusion of the feasibility study. These included an increase of more than one dentist per practice able to participate in consenting, recruiting, randomising and establishing risk-based recall intervals for participants in the risk-based arm and the assistance of dental postgraduate research networks to identify and recruit potential dentists and identify and approach potential participants.
Additional amendments, notified to the funder, included an increase in the number of practices recruited and increased numbers of participants per practice, extension of the recruitment period, changes of Trial Steering Committee (TSC) and Data Monitoring Committee (DMC) members, lengthening from 3 to 4 months plus or minus the 4-year anniversary of participant randomisation for final year assessments, and adaptions to study administrative processes. All changes were in accordance with approved contract variations.
Recruitment and consent of dental practices
The trial sought to recruit general dental practitioners/practices from across the UK (i.e. England, Wales, Northern Ireland and Scotland), representing a cross-section of practitioners in terms of urban/rural areas, community-level sociodemographics and fluoridated or non-fluoridated communities.
Dentists were recruited through local postgraduate dental research networks, by advertising in professional dental publications and through presentations at dental conferences and dental events. Trial information and recruitment evenings were organised in Birmingham and Cardiff and across Scotland.
The Trial Office in Dundee (TOD) sent potential dentist participants a personalised invitation letter for the dentist and their staff to attend a local information and recruitment session, at which the reasons for and design of the trial and practice involvement were described. Dental professionals were given the opportunity to discuss participation with the trial team. For dentists and teams that could not attend, information packs about the trial were posted/e-mailed from the TOD.
Trial team members telephoned dental practices to follow up the notes of interest of involvement. A site briefing/training session was arranged with the dentist, practice staff and TOD staff (see Training of dentists). Following the site briefing, dentists who were interested in the trial were asked to provide written consent to participate, a signed declaration agreeing to adhere to the trial protocol and a completed clinician beliefs questionnaire (see the NIHR project web page for details: www.journalslibrary.nihr.ac.uk/programmes/hta/063599/#/documentation; accessed October 2020). Original signed and dated dentist consent forms and declarations were held securely as a part of the trial site file at the TOD. Copies were made and returned to dentists.
Inclusion/exclusion criteria: dental practices
The inclusion criteria were:
-
NHS provider for adult patients
-
primary care provider: salaried service, corporate and independent operators
-
willingness to follow trial protocol.
The exclusion criteria were:
-
providing only private dental services to adults
-
unwilling to follow trial protocol.
Recruitment and consent of participants
Recruitment of patient participants was achieved through standard procedures and agreements for primary care research in the four nations. In some areas of England, Wales and Northern Ireland, regional Clinical Local Research Networks assisted dental practice staff to identify eligible patients and facilitate an approach by including information about the trial in the appointment letter for their routine dental examination. In Scotland, co-ordinators from the Scottish Primary Care Research Network, when invited, provided a similar service. The appointment letter included an invitation to participate, the patient information leaflet and the baseline patient and cost questionnaires (see the project web page for copies of questionnaires: www.journalslibrary.nihr.ac.uk/programmes/hta/063599/#/documentation).
In instances where the Clinical Local Research Networks and Scottish Primary Care Research Network were not available at an agreeable time, or practices did not request or require assistance, practice staff undertook the duties of identifying and contacting eligible patients and inviting them to participate in the trial using the same paperwork.
At the routine appointment, discussion about the trial was held between the dentist and patient. If agreeable, potential participants were screened for suitability prior to their routine dental examination. Patients who contacted the practice to advise that they were not interested in taking part in the trial were reassured that they would still receive a dental examination appointment with their dentist as per practice policy.
There are a variety of patient recall appointment management strategies utilised within dental practices across the UK. Some dental practices arrange routine examination appointments for their patients up to 6 months or a year in advance. Some practices send letters, e-mail, telephone or text reminders to their patients when their routine dental examinations are due, asking them to contact the dental practice to make an appointment, whereas other practices pre-allocate the date and time of appointments and ask patients to contact the practice if the appointment is not suitable. The INTERVAL Dental Recalls Trial utilised a flexible and pragmatic participant recruitment strategy that aimed to be suitable for each practice’s usual recall procedure.
Eligibility of those who expressed an interest in taking part was confirmed against the trial inclusion and exclusion criteria. The dentist confirmed consent with those eligible and willing to participate in the trial. A signed participant consent form was obtained in triplicate. The participant retained a copy, the practice retained a copy in the patient’s notes in the site file, and the original copy was sent to the TOD.
Following consent, the dentist clinically examined the participant to establish suitability for randomisation to the 24-month arm (see Randomisation). If participants had not completed the questionnaires provided with the appointment letter, they were asked to complete the baseline questionnaire and cost questionnaire in the waiting room of the dental practice before placing them in a sealed opaque envelope and returning them to practice staff. Questionnaires were returned to the TOD by the dental practice in a sealed envelope.
The TOD staff did not have access to any participant data prior to the participants consenting to take part in the trial.
Inclusion/exclusion criteria: participants
The inclusion criteria were adult patients (≥ 18 years of age) who:
-
were dentate
-
had visited their dentist in the previous 2 years
-
received their dental care in part or fully as an NHS patient, including dental examination.
The exclusion criteria were:
-
patients who had a medical condition indicating increased risk of bleeding
-
immunocompromised patients.
Participants whose medical condition changed during the follow-up period were not prohibited from continuing in the trial. Provision was made for dentists to record changes and rationale to the length of the recall interval, on the patient attendance data (PAD) form, but such participants remained within the allocated stratum.
Training of dentists
In England, Wales, Northern Ireland and Scotland, the process of training recruited dentists took the same format.
Training in trial procedures
Trial staff visited the practice by arrangement for a 1- to 2-hour site briefing/training session at an agreed and convenient time, attended by the participating dentist and practice staff. After a brief review of the trial aim and objectives, trial procedures were described and discussed.
Recruited dentists were defined as local investigators within the dental practice, and were responsible for recruiting, consenting and protecting the personal data of trial participants within the dental practice. Local Investigators signed an agreement to conduct the trial in compliance with applicable legislation including (1) the International Conference on Harmonisation Good Clinical Practice guideline73 and (2) the Department of Health and Social Care’s Research Governance Framework for Health and Social Care (April 2005)74 or the Scottish Executive Health Department’s Research Governance for Health and Community Care (2nd edition 2006),75 whichever was relevant.
Dentists and practice staff were advised at the trial briefing/training and in monthly practice newsletters that the International Conference on Harmonisation Good Clinical Practice guideline training was available in their local area and that attendance at these sessions could be arranged through the TOD. The TOD also signposted to the online NIHR Introduction to Good Clinical Practice e-learning (primary care) module. 76
Training in determining risk-based recall intervals
Following the site briefing/training session, each dentist was sent a link to an online training package. The online training package presented risk-based recall interval determination according to the NICE guideline,60 with written instruction, audio and video components, examples and test assessments. It described a systematic approach dentists could follow to consider setting and review of individualised patient recall intervals and to enable discussion and explanation between patient and dentist of the risk-based recall interval. Dentists were instructed to complete this training before screening any potential patient participants for the trial, and reminders via letter, e-mail, telephone and trial newsletter requested that dentists complete it on an annual basis during follow-up.
On completion of the online training package, and before being awarded with a certificate of training and 2 hours continuing professional development (CPD), dentists were required to complete an evaluation form that asked users to measure to what extent they felt that the training programme met the learning objectives, and how easy it was to access and understand. They were also asked to suggest improvements regarding content, design, navigability and length.
Feedback from users was mixed; some found it difficult to access and to navigate, whereas others found it user-friendly and were reassured that they could use the tool with patients. Some users found the content appropriate, easy to understand and a good support for participating in the trial, whereas others found some aspects to be basic to a practising dentist or felt that it could be shortened for the experienced practitioner.
For additional reference on risk-based interval determination, practices in England, Wales and Northern Ireland were provided with a link to the NICE guideline Dental Recall: Recall Interval Between Routine Dental Examinations. 60 Practices in Scotland were supplied with an electronic link and hard copy of the SDCEP Oral Health Assessment and Review (OHAR) guidance. 60,61 Both documents contained templates for checklists to record variables identified as potential modifying factors (risk variables) that influence the setting of recall intervals (see Trial interventions for more detail on risk-based templates).
Clinical outcome assessor training
The clinical outcome training was delivered by trial collaborators with expertise and experience of training assessors in periodontal and caries measures. The clinical outcome assessors and scribes for this trial were qualified, General Dental Council-registered dentists, dental hygienists/therapists and dental nurses employed by the trial.
The emphasis of the training was consistency of the examination process and agreement of scoring criteria. Following the didactic face-to-face and online training, the outcome assessors, with their research nurse, examined 15 patient volunteers in a clinical setting similar to that of a dental practice. The cohort of patient volunteers were similar in age and dental attendance behaviour to those recruited to the trial. The clinical outcome assessments were conducted at Dundee Dental Hospital and School, and each participant was examined by all outcome assessors.
The processes of clinical outcome assessment were agreed in advance, including the order of outcome measure assessment, time allocation, sequence around the mouth and moisture control. The primary clinical outcome of gingival bleeding on probing is a measure of gingival inflammation. It is described as ‘gingival inflammation/bleeding on probing’ in the study protocol;1 for clarity within this report it will be described as gingival bleeding on probing, the definition and outcome measurement remaining the same as outlined in the protocol. This clinical outcome does not allow for repeat assessment; therefore, neither intra-assessor nor interassessor reliability measurements were possible. Training77 for the primary outcome involved face-to-face discussion with the assessors and scribes about the assessment technique and scoring criteria. Prior to the assessment of the cohort of volunteer patients by the outcome assessment teams, a slide presentation developed for training in commercial clinical trials was presented by a periodontal clinical trial expert, and this was supplemented with group discussion about clinical photographs and clinical cases. Periodontal training included positioning, angulation of instrument and pressure of the University of North Carolina-15 (UNC15) periodontal probe, to ensure a standardised approach by the outcome assessors. Periodontal probing depths were recorded at six sites on erupted teeth using a probing force of approximately 25 g.
Training in caries assessment consisted of several components that have been developed and used in other clinical trials and epidemiological studies. This included an online training programme for the International Caries Detection and Assessment System (ICDAS), a half-day of slide presentations and discussions of the ICDAS codes and protocol for the clinical examination. The assessor training included both theoretical aspects and discussions regarding patient participants within the clinical trial setting. Practical training included simulation of the assessment protocol on extracted carious teeth representing carious lesions at all stages of lesion progression included in the ICDAS scale, as well as clinical assessment of a cohort of volunteers similar to the trial population, who had been specifically recruited for trial assessor training. All training in the use of ICDAS was completed under the supervision of a trial collaborator experienced in the use of ICDAS in clinical research.
The caries detection elements of the ICDAS criteria are now well tested and are advocated for general use as well as for use in the clinical trials and in dental epidemiology. 78,79 The ICDAS criteria measure both early stages of caries and more advanced stages of caries. For early caries, ICDAS measures the surface changes and potential histological depth of carious lesions by relying on surface characteristics related to the optical properties of sound and demineralised enamel prior to cavitation. Advanced stages are recorded when cavitation is evident. The trial utilised a modified ICDAS as clinical data were collected only on the caries experience. Restorations and non-carious tooth loss were not recorded.
The intensive face-to-face and online training was provided a month before the first trial outcome assessment to provide sufficient time for additional training if required. The training was repeated mid-way through the INTERVAL trial clinical outcome assessment period, to reinforce standardisation in the process and clinical measures. Throughout the clinical outcome collection period, the assessment team met regularly to confirm the outcome assessment processes and data collection methods to achieve the highest level of standardisation possible.
Randomisation
Eligible and consenting patient participants were clinically examined by their dentist to determine suitability for randomisation to the 24-month recall arm (yes/no). The decision that a patient was eligible for a 24-month recall was based on routine clinical examination and risk assessment. Dentists were instructed not to apply the detailed risk-based variable assessment unless randomised to the risk-based arm in either stratum.
There were separate, identical algorithms in the trial design for the two strata. Eligible participants were randomised in equal numbers within each of the two strata according to a minimisation algorithm including:
-
dentist
-
participant age (18–40 years/≥ 40 years)
-
filled teeth (n ≤ 8/n > 8)
-
absence of gingival bleeding on probing (yes/no)
-
exempt from dental charges (yes/no).
Random allocation occurred via telephone, after the decision by the dentists about the patient’s suitability for a 24-month recall. The trial utilised the automated central randomisation service at the Centre for Healthcare Randomised Trials (CHaRT), University of Aberdeen, which had 24-hour telephone access. The service prompted dental practice staff to enter and confirm details by entering numbers (i.e. 1 = yes, 0 = no) on the telephone touch pad.
The dentist communicated the allocation outcome and confirmed trial details with the participant. Participants randomised to a fixed-period recall interval, either 24 or 6 months, were managed according to routine practice regarding the practice recall management system. For participants randomised to receive a risk-based recall, further history taking, examination and assessments were undertaken, if required, to determine the appropriate variable risk-based recall interval. This was discussed and agreed with the patient participant prior to the recall interval being entered into the routine practice management system (see NICE guideline60).
Owing to the nature of the interventions, it was not possible to blind participants and dentists to allocated recall intervals. TOD staff received an e-mail notification when a successful randomisation had taken place, providing practice and participant ID numbers, and trial arm allocation. Following randomisation, dental practice staff were asked to send the original signed patient consent form, baseline patient questionnaire and cost questionnaire, and screening/patient case report form (PCRF) to the TOD.
Trial interventions
The trial interventions recall intervals were a fixed-period 24-month recall interval, a risk-variable-adjusted-length recall interval based on the NICE guideline60 and a fixed-period 6-month recall interval.
Fixed-period recall intervals (24 months, 6 months)
Patient participants allocated to the fixed-period 24-month recall interval and the fixed 6-month recall interval groups attended their dentist at the scheduled time intervals for a routine dental check-up. The content of this check-up remained as per current practice. A recognised definition of a routine NHS dental check-up is clinical examination, advice, charting including monitoring of periodontal status and report. 71
Risk-variable-adjusted-length recall interval (National Institute for Health and Care Excellence guideline)
Patient participants allocated to the risk-based recall interval group attended their dentist at time intervals determined by the evidence-based process outlined in the 2004 NICE guideline on dental recall. 60 The essential steps of the procedure and the risk factors collected at recall examinations are outlined (from the guideline) in Figure 2.
FIGURE 2.
The NICE risk-based dental recall procedure and risk factors. Reproduced with permission. © NICE 2004 Dental Recall – Recall Interval Between Routine Dental Examinations. Available from www.nice.org.uk/guidance/cg19/evidence/full-guideline-pdf-193348909. 80 All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
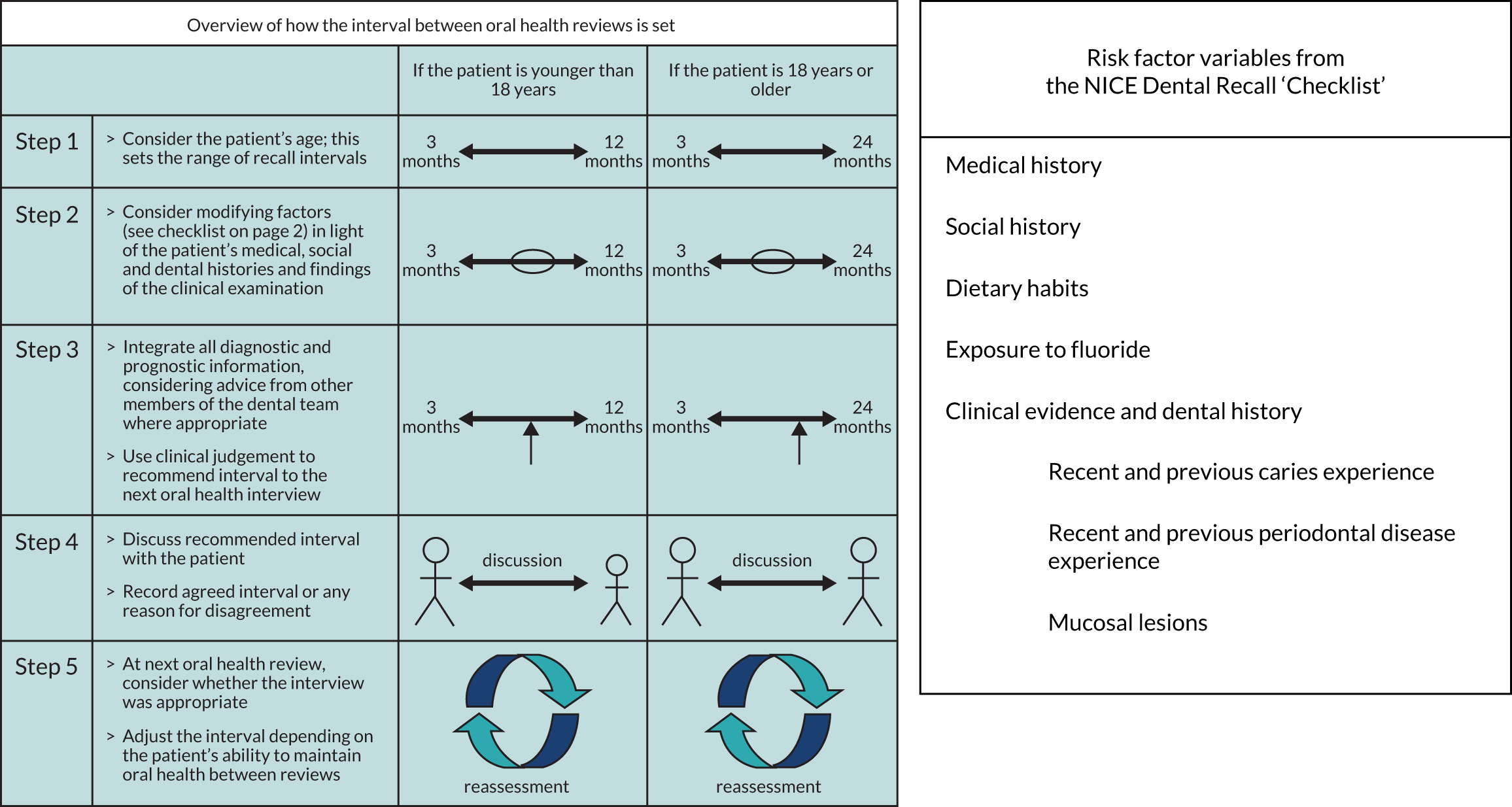
The recommended steps in establishing the appropriate recall interval were:
-
Consider the age range – in the case of this trial, all patients were adults of ≥ 18 years.
-
Consider risk variables – identification of the pertinent risk and protective factors present for each patient from both the checklist and a comprehensive oral health assessment, leading to the evaluation of the impact of these factors in the context of the patient’s past levels of oral health and current disease experience, and then consideration of a likely range of recall intervals.
-
Integrate prediction of recall need – use of all the information obtained by the dental team in order to predict the potential level of threat to maintaining oral health and controlling disease for this patient and, from this, judge the most appropriate next recall interval.
-
Discuss with patient – to explicitly discuss the recommended recall interval with the patient, explain the influencing factors in setting the recall and record the agreed interval (or any reason given by a patient in disagreement).
-
Review – at each check-up review (oral health review), the appropriateness of the preceding interval is reviewed by the dentist and patient and the recall interval is reset according to the experience from the last period along with any change in the risk and protective variables identified at re-examination.
The frequency of recall interval appropriate for an individual patient depends on the likelihood that specific diseases or conditions may develop or progress beyond the control of secondary prevention. The selection of an appropriate recall interval for a patient is a multifaceted clinical decision that involves judgement and cannot be decided mechanistically.
The NICE guideline60 was developed using extensive consensus methods and the limited evidence available. The recommendation was that the recall interval range for adults should vary from 3 to 24 months according to risk.
The NICE guideline checklist60 was intended to be used as a guide to assist the dentist in setting an appropriate recall interval. It is not an exhaustive list of all factors that may influence the choice of a recall interval for a patient. There is insufficient evidence to assign a ‘weight’ to individual factors in the checklist and dentists must use their clinical judgement to weigh the risk and protective factors for each patient. The same checklist is in the SDCEP Oral Health Assessment and Review guidance61 and, therefore, for dentists across the UK guidance is consistent with the content of the trial training material including the online resource.
It was anticipated that by taking a comprehensive history and carrying out a comprehensive oral health assessment the dentist would be better informed to provide an accurate risk assessment and more appropriate preventative and interventive treatment recommendations including advice.
It was envisaged that, once trained, the time taken to complete this process would be 20 minutes for the first risk-setting visit and 15 minutes for subsequent recall examinations (oral health reviews).
Outcome measures
All primary and secondary outcome measures were measured at 4 years’ follow-up and are outlined below.
Primary outcomes
Clinical:
-
gingival bleeding on probing.
Patient centred:
-
OHRQoL [Oral Health Impact Profile-14 (OHIP-14)]. 81
Secondary outcomes
Clinical:
-
dental caries
-
periodontal probing depth
-
calculus
-
preventative and interventive care.
Patient centred:
-
dental anxiety82
-
oral health-related knowledge, attitudes and behaviours
-
generic quality of life, measured using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L)
-
use of, and reason for use of, dental services
-
satisfaction with care.
Economic outcomes
-
NHS costs.
-
Patient-incurred costs.
-
General population preferences, willingness to pay (WTP) calculated from a discrete choice experiment (DCE) to value service delivery and outcomes.
-
Incremental net benefits (INBs) (WTP minus costs), measured as societal net benefit (WTP for health and non-health aspects) and dental health net benefit (WTP for health outcomes, bleeding on brushing and caries experience only).
-
Generic quality of life, measured using the EQ-5D-3L.
-
QALYs.
-
Incremental cost per QALY.
(See Chapter 3 for further details.)
Service provider measures
-
Dentist attitude towards dental recall strategies.
Post hoc outcomes
-
Self-reported bleeding.
Measurement of clinical outcomes
Clinical outcomes were assessed at 4 years post randomisation ± 4 months by trained outcome assessors who were blinded to allocation. The blinding of the outcome assessors was achieved by non-disclosure of the practice or patient to the allocated recall interval. The flexibility around the 4-year anniversary was to accommodate participant and practice factors influencing the convenience of attending the outcome assessment visit. Each tooth was examined, except third molars, unless a second molar was absent and the third molar tooth had drifted mesially to occupy the second molar position. The periodontal examination was performed first and the sequence of assessment was gingival bleeding on probing, periodontal probing depths and calculus. Teeth then were cleaned with a manual toothbrush by the outcome assessor and an ICDAS caries examination was carried out. More details on clinical data collection are outlined below.
Periodontal
Gingival bleeding on probing was measured according to the Gingival Index of Löe83 by running a colour-coded UNC15 periodontal probe circumferentially around each tooth just within the gingival sulcus or pocket. After 30 seconds, bleeding was recorded as being present or absent on the buccal and lingual surfaces. The primary outcome was calculated by adding all the sites where bleeding was observed and dividing it by the number of sites (twice the number of teeth) and was presented as a percentage.
Periodontal probing depth was measured using a colour-coded UNC15 periodontal probe. Clinical probing depths were measured for all teeth at six sites per tooth (mesiobuccal, midbuccal, distobuccal, mesiolingual/palatal, midlingual/palatal and distolingual/palatal). Clinical probing depth was calculated as the mean of the six sites measured per tooth and is presented in millimetres.
Calculus was detected using a colour-coded UNC15 periodontal probe as being present or not. Calculus was calculated by adding all the sites where calculus was observed and dividing it by the number of teeth and presented as a percentage.
Caries
We measured caries at the enamel and dentine threshold using ICDAS. After manual tooth brushing by the outcome assessor, an examination of clean and wet/dry teeth (according to ICDAS procedure) was performed. Examination was aided by a ball-ended explorer used to remove any remaining plaque and debris and to check for surface contour, minor cavitation or sealants. All surfaces of all teeth were examined and the status of each recorded in terms of caries detection. A score between 0 and 6 was recorded for each surface.
Preventative and interventive care
Practice-reported data were collected throughout the 4-year trial including recall appointments on the PAD forms completed by practice staff. The PAD form was used to collect data on intended recall appointment dates, actual date of appointment, any rescheduling, length of time of appointment, any further treatment required as an outcome of the recall and, if known, the reason why a participant had not adhered to the recall interval. It also captured the expected date of the next recall appointment, after taking into account any course of treatment that might be required.
NHS routine data reported details of the treatment provided under the NHS during the trial period and was accessed through the routinely collected data in all participating regions in the UK, NHS Business Services Authority (NHSBSA) England and Wales, Business Services Organisation (BSO) Northern Ireland and ISD Scotland. Further details are provided in Chapter 3.
Measurement of patient-centred outcomes
Patient-centred outcomes were measured at 4 years and also collected annually, including at baseline, through a self-administered questionnaire.
Annual questionnaires were mailed to participants from the TOD on the anniversary of their randomisation. A Freepost envelope was included for ease of return. If the questionnaire had not been returned within 4 weeks, a reminder letter and another copy of the questionnaire were sent to the participants.
The full details of the calculations used to generate each patient-centred outcome are shown in Appendix 1, Table 26. We used the relevant publication to inform the calculation of validated scores, such as OHIP-14 and the dental anxiety scale.
Oral health-related quality of life
Quality of life was measured using OHIP-14. 81 The OHIP-14 is a 14-question oral health-specific patient-centred measure referring to symptoms in the past 12 months. The questions are scored from 0 (never) to 4 (very often) and summed to produce a score ranging from 0 to 56, with 56 being the worst outcome.
Dental anxiety
Patient dental anxiety status was measured using recognised and validated psychological inventories [Modified Dental Anxiety Scale (MDAS)]. 82,84 Five questions relating to different dental treatments and situations were posed. The answers are scored on a scale from not anxious (rated as 1) to extremely anxious (rated as 5). The range of the summed total of five items was 5–25.
Oral health knowledge, attitudes and behaviours
The questions for measuring patient-centred belief outcomes and beliefs [attitude and perceived behavioural control (PBC)] outcomes were derived from social cognitive theory and the theory of planned behaviour. 85,86
Oral health knowledge outcome
Knowledge was measured using four questions related to oral health (frequency of brushing, duration of brushing, frequency of flossing and frequency of interdental brush usage). The best value between flossing and interdental brush usage was used as a measure of interdental cleaning knowledge. Each response varied from 0 to 3, with a score of 3 being the highest level of knowledge. The responses were summed to produce a total score ranging from 0 to 9, with 9 being the best outcome.
Oral health beliefs (attitude and perceived behavioural control) outcomes
Oral health-related attitude was measured using a 7-point scale varying from 1 to 7 (strongly disagree to strongly agree) and the higher the score, the better (i.e. the more positive) the attitude. The scale comprised seven questions and the final score was the average of the individual item scores.
Perceived behaviour control was measured using a 7-point scale varying from 1 to 7 (strongly disagree to strongly agree) with higher scores indicating more perceived behaviour control. The scale comprised four questions and the final score was the average of the individual item scores.
Oral health behaviours
Patient-reported oral health behaviour outcomes were measured using four questions (frequency of brushing, duration of brushing, frequency of flossing and frequency of interdental brush usage). Each response ranged from 0 to 3, with a score of 3 being the best possible behaviour. The best value between flossing and interdental brushes was used as a measure of interdental cleaning behaviour. The responses for each question were summed to produce a total score ranging from 0 to 9, with 9 being the best outcome.
Use of and reason for use of dental services
A question within the annual patient questionnaire asked participants to record the number of times they had attended the dental practice and the type of treatment received (NHS, private or combination), and their payment cost of this treatment. Questions were also asked about frequency and treatment data on non-scheduled attendance at services for dental problems (e.g. hospital accident and emergency, hospital outpatients or general medical practitioners).
Satisfaction with care was a score averaging 12 items, each varying on a scale of 1 to 7 (strongly disagree to strongly agree) and the higher the score, the more satisfied participants were with care. The satisfaction measure was developed with dental patients in Scotland.
Service provider measures
Dentists were asked to complete a clinician belief questionnaire at baseline prior to the online risk-based training and randomisation of participants.
The questionnaire collected data on the dentist’s professional history and profile, practice profile, professional engagement and factors such as decision-making, confidence and workplace stress. The majority of questions (n = 21) related to their attitude towards dental recall interval, ease of its determination and the consequence for patients. A 7-item scale was developed from 1 (strongly disagree) to 7 (strongly agree). Full details of the calculations used to generate dentist belief outcomes are shown in Appendix 1, Table 27.
Post hoc outcome
Self-reported bleeding was included as a post hoc outcome and measured via patient questionnaire at 4 years post randomisation. It was measured by asking patients ‘Have you had bleeding from your gums when brushing your teeth?’ The answer could vary from 0 (never) to 4 (very often).
Demographic characteristics
Participant demographic characteristics were collected at baseline and annually using a self-administered postal questionnaire. The demographic characteristics included the most recent visit to the dental practice, type of attender (regular/non-regular), type of toothbrush (manual/electric) and smoking status. Participants also provided details on difficulty of travelling to the dental practice, which was scored from 1 to 7 on a Likert scale, where the higher the score, the easier participants found travelling to their dentist. Demographic characteristics are presented by year and randomised group, using either mean, standard deviation (SD) or n (%) as appropriate.
Fidelity measures
Dental practice compliance with the protocol was monitored through face-to-face practice visits by a member of the trial office team, regular telephone contact from the TOD to practices and an audit of six participants per practice at the mid-point in the trial. The PAD forms were reviewed as part of the audit and a judgement was made on whether or not they were compliant with the allocated recall interval. Dentists were also reminded to complete the online risk-based variable training package annually to reinforce the review needed for the participants randomised to a variable risk-based recall.
The audit of six participants (two participants from each of the three recall intervals or three each from risk-based and 6 months if no participants had been allocated to 24 months) was conducted with each practice to check if participants had been contacted to attend an appointment according to their allocated treatment group. If ≥ 50% of these random six participants had not been contacted or invited to attend, this triggered a telephone call to the practice to check the trial processes and, if required, a visit to review protocol.
All practices received at least one face-to-face visit. This was to ensure practice compliance with the protocol and confirm staff understanding of their role. It provided a valuable opportunity to answer any queries the practice staff had and to build and maintain a rapport to ensure a smooth transition into the follow-up phase of the trial. Practice staff were encouraged to flag INTERVAL participants in their electronic system as an aide-mémoire to following up participants.
Regular telephone calls were made by TOD administration staff to designated main contacts in practices during the course of follow-up to keep practice staff on board and on track, to provide an opportunity to discuss queries and for TOD staff to seek updates on PAD forms where necessary. Practices were encouraged to contact the TOD for guidance on any aspect of the trial.
An INTERVAL-branded site folder was prepared for each practice containing copies of their completed screening log/PCRFs and participant consent forms, and a section in which to file copies of completed PAD forms.
The TOD e-mailed and posted monthly newsletters to practices to remind dentists and staff of procedures and processes for recruitment and training, and trial updates.
Number of check-ups received (routine data and patient attendance data forms)
The PAD forms were used to collect information on intended and actual appointment dates. The information about the first intended appointment date per recall group was used to assess dentists’ intended compliance with the protocol.
Routine treatment data were obtained from the NHSBSA in England and ISD in Scotland for the time period 2010 to 2018. 62 The routine data provided information about the number of dental recalls received throughout the trial by counting the number of claims for treatment made by dentists for each participant.
Data collection
Baseline
Dentists were asked to complete the baseline clinician belief questionnaire after consenting to participate in the trial.
Patient-centred outcomes were collected at baseline using a self-administered questionnaire. Questionnaires were returned to the TOD by the dental practice in a sealed envelope.
Ongoing
Recall appointment dates and times and further treatment information were collected by practices on PAD forms. Routine NHS treatment data were obtained from national-level dental claims data held by the ISD (Scotland), NHSBSA (England) and BSO (Northern Ireland).
Annual follow-up
Patient-centred outcomes were collected annually using self-administered postal questionnaires.
The annual follow-up questionnaire combined questions on patient-centred outcomes, OHRQoL (OHIP-14), generic health (collected using the EQ-5D-3L), dental anxiety, oral health-related knowledge, attitudes and behaviours, use of dental services and satisfaction with care.
Questions collecting descriptive measures were also included in this questionnaire. The annual patient questionnaire also included questions for the health economic analysis, relating to patient-incurred dental costs, and patient attendance at general NHS services (e.g. hospital accident and emergency) for dental-related problems. Questionnaires were issued annually to participants at their home address from the TOD with a covering letter and a Freepost envelope for return of the completed questionnaire.
Those participants who failed to return their questionnaire within 4 weeks were sent a reminder, a further copy of the questionnaire and a Freepost envelope.
On the second anniversary of their recruitment to the trial, participants were sent a £15 gift voucher for their participation in the trial.
Final/fourth-year follow-up
Dentists completed the end-of-trial clinician belief questionnaire after all clinical assessments had been completed in their practice.
Patient-centred outcomes were collected at 4-year follow-up using the annual patient questionnaire. To maximise return, the final questionnaire was sent to participants at least 6 weeks before the date of their final year clinical assessment appointment, with a reminder sent 2 weeks before the appointment. Participants who had not returned a questionnaire by the time of their follow-up assessment appointment were asked to complete the questionnaire at that appointment prior to being assessed. A summary of the data collection items and time point is presented in Figure 3.
FIGURE 3.
Data collection schedule summary.
All participants were invited to attend a trial final-year clinical assessment appointment by their dental practice either at the time of their routine check-up or as a separate appointment. A brief medical history was undertaken about bleeding disorders or immunocompromised disorders. The assessor also confirmed continuing consent with the participants. Gingival bleeding on probing scores, periodontal probing depths, calculus and caries detection were measured by the assessors and recorded on a clinical chart by the dental research nurse, who was a member of the trial team.
Participants who could not attend were contacted and given the option of attending at least one other day or time. Patients who were no longer registered at the practice were offered the opportunity to return to the practice (with practice permission) for an assessment examination, or at another INTERVAL practice (if appropriate) or at a suitable satellite location.
All participants who attended the follow-up assessment received a letter of appreciation for their participation in the trial, details of where the trial results would be published and a final £15 gift voucher in recognition of their contribution. Letters and vouchers were subsequently posted, where possible, to participants who did not attend a follow-up assessment.
Sample size
An exploratory trial in a similar population reported that 35% of gingival sites were bleeding on probing (SD 25%). 87 The Cochrane review of periodontal instrumentation (PI) suggested that 6-month PI versus no PI reduces bleeding sites by 15%. 88 The recall interval was expected to produce an effect lower than this given that the majority of participants in all arms would still receive PI at some time during follow-up. Assuming that either risk-based versus 24-month recall or 6-month versus 24-month recall could reduce/increase the percentage of sites bleeding by 7.5%, the study with 235 participants in each arm could detect such a difference with 90% power at 5% significance, and, likewise, detect a difference of 0.3 of the SD of the OHIP-14 score or any other global measure of OHRQoL. For the caries clinical outcome, assuming a SD of 3.5, the study with 235 participants per arm would detect a shift in white spot lesions (from 3.3 to 4.2) at 80% power and 5% significance. 79 We combined the two strata, without introducing bias, to estimate this comparison. We anticipated smaller effect sizes for the 6-month versus risk-based recall comparison than 6-month versus 24-month recall given that many of the participants in the risk-based group would be seen more frequently than 24 months. A study with 750 participants in each arm could detect a difference in bleeding scores of 4.5% with at least 90% power and a 5% significance level,89 and likewise detect a difference of 0.17 of the SD of the OHIP-14 score. For the caries clinical outcome, assuming a SD of 3.5, a study with 750 participants per arm could detect a 20% relative shift in white spot lesions from 3.3 to 3.9 at 90% power and 5% significance. 79 Although there was no reason to be concerned about contamination effects in this trial or clustering by dentist, the sample size was conservatively estimated such that if contamination occurred with 15% of the control participants or the intracluster correlation was 0.03, the study would still have 80% power to detect the hypothesised changes in the bleeding score. Our sample size calculations indicated that we needed to randomise 705 participants to stratum 1 (235 in each arm) and 1030 to stratum 2 (515 in each arm).
Contamination effects
There were no obvious mechanisms for contamination to occur in this trial. Our experience of simultaneously conducting an educational cluster and patient randomised trial in dentistry suggested that contamination occurred in at most 15% of participants (if any);89 therefore, fewer participants are required to perform a patient randomised trial than a cluster randomised trial. 90 There is no perceived direct influence of skill on the patient outcomes and, even if that were hypothesised, the intracluster correlation would be very low (< 0.03).
The sample size has been conservatively estimated such that, if contamination occurred with 15% of the control participants or the intracluster correlation was 0.03, the study would still have 80% power to detect the hypothesised changes in the bleeding score.
Statistical analyses of outcomes
Baseline data were explored to better understand dentists’ decision-making process when allocating participants to different strata (eligible to be randomised to 24-month recall vs. not eligible). The primary analysis used an intention-to-treat framework and all participants with available data remained in their allocated groups. The participant outcomes listed above were compared between 24-month, risk-based and 6-month recall groups (for the stratum eligible for a 24-month recall) and risk-based versus 6-month recall (for the stratum ineligible for a 24-month recall). Outcomes collected at year 4 (clinical outcomes, behaviour, knowledge and PBC scores) were analysed using a generalised linear model with a random effect for dental practice. Outcomes collected at years 1, 2, 3 and 4 were analysed using a mixed-effects model with two random effects: participant and practice. A time by treatment interaction term was included in the models. The appropriate effect sizes and 95% confidence intervals were derived. All analyses were adjusted for the protocol minimisation variables [age, dentist, filled teeth (≤ 8 or > 8) and absence of gingival bleeding on probing]. Stata 15 (StataCorp LP, College Station, TX, USA) was used to undertake the analysis.
Missing data
Missing items in scales were dealt with as recommended in the literature by their authors, when recommendations were available. Otherwise, a complete-case approach was used where, in the presence of any missing items in a patient’s score, the score was considered missing. Continuous missing data at baseline were imputed for modelling purposes as recommended in the literature,91 and categorical missing data at baseline used the missing indicator method. 92 The primary intention-to-treat analysis was on observed data.
Sensitivity analysis
We explored differences between responders and non-responders to inform our missing data model. As a sensitivity analysis, missing primary outcome data were imputed using a predictive mean matching multiple imputation approach. 92
Subgroup analysis
A pre-planned subgroup analysis explored the effect modification of age (< 45 years, 45–64 years, ≥ 65 years) and social class (exempt from payment and not exempt from payment) by including a subgroup-by-treatment interaction term in the primary clinical outcome model described in Statistical analyses of outcomes. Further pre-planned subgroup analyses included residence in a fluoridated area, and dentist characteristics were included in the protocol; however, there were too few participants (2%) in fluoridated areas to make a subgroup analysis meaningful. The dentist characteristics subgroup analysis was an error in the protocol. A post hoc subgroup analysis by country was also included.
Trial oversight
The University of Dundee acted as the sponsor for the study. The trial was co-ordinated from the TOD in the Dental Health Services Research Unit, Dundee, which provided day-to-day support for the dental practices and outcome assessors/research nurses. The TOD was responsible for issuing and collecting trial documentation from practices and participants (including annual questionnaires and reminders) and co-ordination of participant follow-up. The TOD was also responsible for the entry of collected data into the database, including screening logs/PCRFs, PAD forms, baseline, costs and annual patient questionnaires, baseline and follow-up dentist questionnaires and clinical data from outcome assessments. Clinical data were entered by the dentally qualified assessment team.
CHaRT, in the Health Services Research Unit, University of Aberdeen, provided the database applications and information technology programming for the trial, and hosted the randomisation system, provided experienced trial management guidance, and took responsibility for all statistical aspects of the trial (including interim reports to the TSC and the DMC).
Trial Operational Committee meetings were held weekly and attended by Co-Chief Investigators, the Trial Manager and key TOD staff.
The Operational Management Committee met monthly, chaired by one of the Co-Chief Investigators, and comprised co-investigators in TOD and CHaRT.
The Trial Management Committee (TMC) met approximately annually, chaired by the Co-Chief Investigators, and comprised co-investigators, key members of the TOD and CHaRT and a lay representative.
The TSC comprised an independent chairperson and two further independent members, plus a member of the public acting as a lay patient representative. The TSC met approximately annually.
The DMC comprised an independent chairperson and two further independent members. The DMC met approximately annually.
Patient and public involvement
Prior to the start of the trial, advice on the design and conduct of the study was sought from members of the public partnership groups and from similar patient groups in other parts of the UK sourced under guidance from INVOLVE (UK National Advisory group).
These independent public partnership groups comprise volunteers who work in partnership with NHS Tayside and aim to provide a conduit for the views of people about their local services.
Patient advisors were a valuable resource at the outset of the trial and helped to ensure good conduct and patient-friendly practice throughout the duration of the trial. Patient advisors were involved with the trial design and provided invaluable feedback on trial recruitment and communication strategies. Patient advisors also contributed to the content and layout of trial invitation, trial newsletters and the design of patient participant questionnaires. This ensured that trial participants could understand and easily complete these materials.
As quality of life was a primary outcome of the trial, patient advisors’ input to the proposed questionnaire design was essential. Qualitative work with patients was carried out to ensure that the outcome measures were patient centred.
Lay representatives on the TSC and TMC actively contributed to trial oversight, processes and procedures, including helping to interpret the trial findings, preparation of the monograph and assisting with the review of the Plain English summary.
Chapter 3 Health economics methods
Introduction
In this chapter we report the methods used to conduct the within-trial health economic analysis comparing different dental recall strategies as follows: eligible for 24-month recall – 24-month versus risk-based versus 6-month recall; and ineligible for 24-month recall – risk-based versus 6-month recall. All analyses are completed according to the intention to treat principle at 4 years’ follow-up. The health economic analysis compares the costs and benefits of different dental care recall strategies. The results are presented using different perspectives. Economic evaluations to inform UK health-care decision-making, for example through NICE, typically take the form of cost–utility (i.e. cost per QALY) analyses, reporting incremental cost-effectiveness ratios (ICERs). However, in the context of dentistry, there are concerns that generic EQ-5D-3L-based QALYs lack the sensitivity to capture the processes and outcomes of care that are of value to patients and decision-makers. 93 Furthermore, it is argued that the time dimension embedded in QALY calculations may underestimate the impact of acute health effects such as painful caries. 94,95
Similarly, from a cost point of view, dentistry is unique within health care as the majority of patients pay a contribution towards their NHS care. It is, therefore, crucial to consider both the costs falling directly on the NHS dental budget and the impact on patients in terms of co-charges for NHS care and the opportunity cost of time and travel to dental care appointments when making recommendations about the most efficient dental recall strategy.
For these reasons, it is necessary to consider different frameworks of evaluation that (a) are sensitive to changes in important dental health outcomes such as bleeding or caries, (b) value outcomes that are relevant to service users and (c) capture the full cost burden to both patients and the NHS of different recall strategies. Providing results from a range of perspectives of benefits and cost is essential to equip decision-makers with all the relevant information necessary to reach informed decisions about the efficient allocation of scarce dental care resources.
In terms of the benefits, the scopes are WTP for dental recall interval and associated outcomes [cost–benefit analysis, including all benefits (health and non-health) that are important to individuals], QALYs and WTP for dental health outcomes (caries and self-reported bleeding gums). The preferred analysis depends on what outcome a decision-maker wishes to maximise: social welfare, QALYs or (WTP for) defined dental health (caries and bleeding). In terms of costs, the perspectives are NHS dental budget, NHS total budget and societal perspective (NHS and participant).
Resource use and costs
Resource use and cost data are collected from an NHS and dental patient participant perspective. NHS costs include provision of dental care services (obtained from data linkage to routinely collected claims data) and costs of attending non-dental health professionals for dental problems (obtained from participant questionnaires). Patient participant costs include co-payments for dental care, purchase of dental care products, and the travel costs and the opportunity cost of time spent attending dental appointments obtained from a combination of routinely collected data and participant self-reported questionnaires.
NHS dental costs
Resource use and costs associated with the use of NHS dental services are obtained through data linkage for each randomised trial participant to national-level dental claims data held by the ISD (Scotland), NHSBSA (England) and BSO (Northern Ireland). For the 13 participants recruited from a single Welsh practice, resource use is obtained from practice note data extraction. The actual cost to the NHS of providing treatments, the split of cost burden between the NHS and the patient, and the level of granularity of data available for analysis is dependent on different remuneration systems and contracting arrangements in place for payment of NHS dental care across different UK countries. For example, dentists in Scotland and Northern Ireland are reimbursed on the basis of fee for service, whereas in England contract payments are based on banded categories of treatment complexity. This means that there is a greater granularity of data available for Scotland and Northern Ireland to inform resource use and costing. There are also differences in patient co-charges for dental check-ups ranging from £0.00 (Scotland) to £19.70 (England), which have implications for the interpretation of NHS versus patient perspective costs across the regions. Tables 1 and 2 describe the NHS and patient breakdown of treatment charges across the UK regions for fee-paying adults. All cost data are reported in 2016/17 values based on the regional dental payment contracts. Regional specific variation in the dental payment systems is discussed in the following paragraphs.
| England and Wales activity-based banding96,97 | |||||||
|---|---|---|---|---|---|---|---|
| Band | Number of UDAs | UDA unit value | Band treatment value | England (2016/17 charges)a,b | Wales (2016/17 charges)a,b | ||
| Patient charge | NHS charge | Patient charge | NHS chargec | ||||
| 1 (e.g. check-up/X-ray/advice/PI) | 1 | £25 | £25 | £19.70 | £5.30 | £13.50 | £6.20 |
| 2 (band 1 treatments + fillings, extractions, root canal treatments) | 3 | £25 | £75 | £53.90 | £21.10 | £43.00 | £10.90 |
| 3 (band 1 and 2 work + crowns, dentures and bridges) | 12 | £25 | £300 | £233.70 | £66.30 | £185.00 | £48.70 |
| Urgent | 1.2 | £25 | £30 | £19.70 | £10.30 | £13.50 | £6.20 |
| Scotland/Northern Irelanda | ||
|---|---|---|
| Treatment | Patient charge (2016/17 charges) | NHS charge (2016/17 charges) |
| Check-ups and case assessments | Scotland: 0% of full cost | Scotland: 100% of full cost |
| Northern Ireland: 80% of full cost | Northern Ireland: 20% of full cost | |
| Other treatments | 80% of full cost | 20% of full cost |
| Maximum charge per course of treatment |
Scotland: £384.00 Northern Ireland: unlimited |
Unlimited |
Use of NHS dental services: England and Wales (activity-based treatment bands)
In England, dental payment contracts are negotiated at either a national (GDS contracts) level or a local (personal dental service contracts) level. GDS contracts are the most common across England, accounting for approximately 85% of negotiated contracts, and are used to inform the costing analysis. 98 Under GDS arrangements, each dental practice is contracted to provide a prespecified quantity of NHS care, measured in a currency called units of dental activity (UDAs). All dental treatments are classified into four treatment bands based on treatment complexity, with more complex treatment bands attracting a greater number of UDAs.
The value of a UDA may vary across dental practices, depending on contract negotiations, population-level general health and dental health in a catchment area and area deprivation. A value of £25 per UDA is used for the analysis, based on the Steele report in 200999 and maintaining consistency with NICE public health guidance on oral health promotion100 and the published Improving the Quality of Dentistry (IQuaD) study. 101 If patients are exempt from paying treatment charges, the NHS covers the full value of treatment. It should be noted that, in England and Wales, dental check-ups without further treatment requirement incur a band 1 charge.
Routine data downloads for England were taken on 4 May 2018. NHSBSA data are often entered, cleaned and processed towards the end of the financial year, meaning that the quality of the data is probably high for all claims starting prior to the end of March 2018. Some participants [n = 43/1031 (4.2%)] were recruited to the trial after April 2014. Routine data for resource consumed by these participants may be missing for the 3-month period from April to June 2018; however, this applies to a very small proportion of participants over a very short time frame, so such missing data are unlikely to bias the costing analysis. For the remainder of the linked participants in England, the routine data capture a complete, and accurate, record of all primary NHS dental care received for the full 4-year duration of follow-up in the trial.
Use of NHS dental services: Scotland and Northern Ireland
The remuneration systems are similar in Scotland and Northern Ireland. Dentists are paid on a fee-for-service basis, with payment attached to over 300 different treatment codes. Payment contracts are updated and refined on a regular basis. Costing data for this report are sourced from the statements of dental remuneration in Scotland and Northern Ireland, with all treatment costs inflated to 2016/17 values using an online tool. 102 Dental check-ups are free of charge to the patient in Scotland. In Northern Ireland, the patient pays a co-charge of £6.74 (2016/17 statements of dental remuneration), which is approximately 80% of the total check-up value. For other items of service (e.g. scale and polish, fillings and extractions), patients in Scotland and Northern Ireland pay approximately 80% of the item of service value, unless they are exempt from payment charges. The payment mechanism in Scotland and in Northern Ireland means that there is a high level of detail available to inform the costing analysis.
Routine data downloads were taken on 15 May 2018 (Scotland) and 29 August 2018 (Northern Ireland). Communication with ISD and BSO indicates that the routine data are an accurate and complete representation of all treatment and resource use up until 3 months prior to the data download. On this basis, we are confident that the routine data are a complete representation of all primary dental care contacts for 1157/1188 (97.4%) participants with known robustness of data quality for 990/1188 (83%) participants for the full duration of follow-up; however, any biases are likely to be small in magnitude because of the short period of time in which the data exist, but the quality cannot be guaranteed. For Northern Ireland, all data are a complete and accurate reflection of the full record for all patients over the 4-year follow-up.
Costing of NHS dental services
Costs to the NHS are calculated for each individual trial participant as the region-specific total treatment value minus any patient charge contributions. For patients who are exempt from paying dental charges (e.g. those on a low income), the full value is assigned to the NHS. Exemption status is, therefore, an important determinant of NHS perspective costs, and the analysis tests for any differences in status across the randomised groups.
The costing analysis excludes any treatment claims relating to or originating from examinations at the baseline trial visit. To do this, all treatment claims with a start date prior to 3 months (specifically 90 days) post randomisation are excluded. This ensures that any treatment costs are driven by the decision to randomise patients to different recall intervals and not driven by unrelated treatment need identified on entry to the trial. In addition, all treatment claims with a start date occurring more than 4 years post randomisation are excluded. Some final outcome assessments may have occurred prior to 4 years post randomisation. The outcome assessment data were not routinely passed to the participant’s dental practice, and it is not anticipated that having the assessment early had a significant impact on costs. However, to ensure that the analysis does not create any bias by identifying treatment need at the final outcome assessment, the proportion of respondents for whom this occurred is compared across trial arms. If differences are identified, these are controlled for in the cost regression analyses.
Costs are reported from both an NHS and a societal perspective (NHS + patient participant). All costing is reported in 2016/17 Great British pounds and costs occurring beyond the first year of follow-up are discounted at a rate of 3.5% per annum. 103,104
Costs of other NHS services during follow-up
Information about the use of other NHS services related to dental health was obtained from participant-completed annual postal questionnaires. NHS resource use falling outside the dental budget included secondary care visits (hospital inpatient admission, outpatient consultations, day case procedures, and accident and emergency attendances) together with general practitioner (GP) appointments and contact with out-of-hours services such as NHS 24 (Scotland), NHS Direct (England/Wales) or NIdirect (Northern Ireland). The following approach was used to deal with instrument and item non-response as well as inconsistent reporting on the participant-completed questionnaires:
-
Where an instrument was not returned, data were treated as missing, and non-dental NHS costs were imputed using multiple imputation.
-
Where respondents ticked ‘No’ to an item of resource use, but entered a number of contacts, it was assumed that no resource use was incurred.
-
Where respondents stated that they attended hospital inpatient services for problems related to their teeth but provided no further information, it was conservatively assumed that such responses should be treated as day case admissions. National data show that the majority of hospital admissions in the UK for dental problems are day-case procedures. 104
-
Where respondents stated that they attended secondary care, and provided information regarding the reasons for attendance, these reasons were assessed for their link to dental care, and if clearly not related to dental care, were excluded from analysis.
The reported rates of contact with secondary care (e.g. inpatient/outpatient) were compared with average attendance rates obtained from ISD/Hospital Episode Statistics to determine their face validity. 104 It would be anticipated that, in a general dental population, as included in the trial, the rates of admission should be similar to national-level data.
Participant costs
Three sources of participant cost are included in the analysis: (a) participant co-charges for NHS dental care, (b) direct out-of-pocket expenses for private dental care and (c) the opportunity cost of participant’s (and any companion’s/carer’s) time spent attending dental appointments.
Co-payments for NHS dental care
Routine dental claims data were used to determine participant perspective charges for each treatment provided to each trial participant. These were estimated based on treatment banding and exemption status. In England, explicit recording of the patient charge is not necessarily required for NHS payment and this may explain why, in some cases, the English data set indicates £0 co-payment despite the patient not having any exemptions indicated, or the patient charges are missing. This is likely to be an administration error and it is, therefore, assumed that the practice collected the appropriate patient charge.
Self-purchased dental care products
Participants reported whether or not and how frequently they used/replaced electric toothbrushes, electric toothbrush heads and manual toothbrushes. The cost of a new electric toothbrush was obtained from participant-reported data in each annual questionnaire. Costs of manual toothbrushes and replacement heads for electric brushes were assumed to be the cheapest available prices for commonly used toothbrush products using an online search of multiple retail stores conducted on 20 September 2018. The unit costs sourced are £8.48 [pack of four Oral-B electric heads (Procter & Gamble, Cincinnati, OH, USA), sourced from Superdrug (Superdrug Stores plc, Croydon, UK) online] and £2 [manual Colgate 360 (Colgate–Palmolive Company, New York, NY, USA), sourced from Boots (Boots UK Limited, Beeston, UK)], adjusted back to 2016 values to account for inflation and to ensure a common year of currency for all costs.
Private dental care costs
Participants reported the total cost of out-of-pocket spending on private dental care in each annual questionnaire. Reported data are summed across each questionnaire to obtain the full cost of private dental care over the trial time horizon and data are described descriptively as mean (SD) and median (interquartile range) to demonstrate the impact of a small number of high-cost items (e.g. dental implants) on the cost analysis.
Costs of time and travel
There is a direct cost to the participant for travelling to every dental appointment and an opportunity cost of their time (and their companion’s/carer’s time, if accompanied) spent travelling when they are away from their usual activities (e.g. work, leisure time and child care). Routine dental claims are used to identify the number of attendances for NHS dental treatment. It is conservatively assumed that each unique treatment’s start/acceptance date (rather than each item of service claimed in Scotland/Northern Ireland) relates to a single dental visit. This approach probably underestimates the true participant perspective costs if more than one visit was required for a course of treatment. The unit time and travel cost to participants and companions of a return journey to the dental practice is calculated using data provided by respondents in the baseline questionnaire, using a subset of questions obtained from a standardised patient costs questionnaire. 105 The cost of a visit is assumed to remain constant at the individual level across the time horizon of the trial to avoid overburdening respondents with multiple similar questions in each annual questionnaire. Unit costs of travel and the opportunity cost of time commitment of travelling to and attending dental appointments are detailed in Table 3. The total opportunity cost of time and travel is obtained by multiplying the number of treatment courses by the unit opportunity cost of time and travel for each participant.
| Activity | Assumptions made | Unit cost | Reference |
|---|---|---|---|
| Paid work | Median UK gross weekly wage: £550; 39.1 hours per week | £14.07 | Office for National Statistics; Annual Survey of Hours and Earnings, 2017106 |
| Transport | Cost per milea | £0.45 | HM Revenue & Customs (approved mileage rate, 2016/17)107 |
| Caring for a relative or friend | Median gross weekly pay: £341; 39.2 hours per weekb | £8.70 | NHS Pay Review Body report 2016, page 17108 |
| Leisure activities | Value of non-working timec | £7.79 | Transport Analysis Guidance (TAG) Data Book, July 2017109 |
| Child care | Assumed as paid work | £14.07 | Office for National Statistics; Annual Survey of Hours and Earnings, 2017106 |
| Voluntary work | Assumed as paid work | £14.07 | Office for National Statistics; Annual Survey of Hours and Earnings, 2017106 |
| Unemployed | Value of non-working time | £7.79 | Transport Analysis Guidance (TAG) Data Book, July 2017109 |
| Retired | Value of non-working time | £7.79 | Transport Analysis Guidance (TAG) Data Book, July 2017109 |
| Parking | Participant’s individual costs | Various | Participant questionnaires |
| Housework | Cost of housework in the NHS, assumed annual salary £21,000 gross, 2012 values inflated to 2016/17 | £10.56 | NHS pay review body, 2016108 |
Statistical analysis and reporting of cost data
Cost models are estimated using generalised linear models, specifying a log link function and gamma family to account for a skewed distribution with a minimum of zero cost, and a long tail reflecting a small proportion of participants with high costs. The chosen family (gamma) is based on the results of a modified Park test, and the chosen link function is based on a combination of Akaike information criterion (AIC) score and p-values from the Pearson correlation test, the Pregibon link test and the modified Hosmer–Lemeshow test110,111 obtained using the glmdiag program for Stata. Costing models are adjusted for the trial minimisation variables, region and baseline EQ-5D-3L score, and a random effect for centre to control for practice-specific variation in treatment decisions. For the comparison of treatment options across all trial participants, a fixed effect is included to account for randomised stratum in the trial. The cost models are estimated using Stata’s meglm command. Recycled predictions of the coefficient on randomised treatment determine the incremental costs compared with standard care (currently 6-month recall). Two sets of results are reported. First, results are reported for all data comparing risk-based recall with 6-month recall. Second, a three-way comparison is presented for those participants randomised to the stratum of participants who are eligible for 24-month recall. Costs associated with risk-based and 24-month recall are compared against each other, and with the assumed standard care (i.e. 6-month recall).
Descriptive statistics for cost data (mean, SD, N) are reported for all resource use items from both an NHS and a patient participant perspective based on complete-case data for information. NHS perspective costs to the dental budget are relatively complete because of a high match rate across the regions between the routine dental claims data and the recruited trial population. However, participant perspective costs (based on questionnaire response data) are subject to significant missing data. For the statistical analysis of costs, the mechanism of data missingness was investigated using logistical regression analysis, where the dependent binary variable (missing or not) was regressed on sociodemographic characteristics (age, gender), minimisation variables, region, baseline EQ-5D-3L utility score, and baseline measures of clinical outcome. Statistically significant explanatory variables were assumed to indicate that data were missing at random as opposed to missing completely at random. Best practice was applied, imputing data using multiple imputation of missing data following Stata’s MI procedure. 112 Missing cost data are imputed by component of cost (e.g. dental product costs) for each questionnaire, using predictive mean matching (average of five closest values), accounting for the repeated measures nature of the costs from the annual participant questionnaires. M = 5 imputed data sets are used to ensure the stability of results, and the data sets were combined using Rubin’s rules to obtain a single estimate of costs. 113 All data imputation models account for randomised group and adjust for explanatory variables as described above.
Benefits
Three different measures of benefit are included in the economic evaluation as alternative analyses. The analyses differ in terms of the scope and definition of the benefits included. Benefits are considered as (a) WTP for both dental recall frequency and associated dental health outcomes to inform a cost–benefit analysis (CBA) that includes all perceived benefits, health and non-health, to the general population, (b) QALYs to inform a cost–utility analysis (CUA) and (c) WTP for dental health outcomes, specifically ICDAS and bleeding gums on brushing, where the utility of dental health outcomes is measured in terms of WTP. The preferred analysis depends on what outcome a decision-maker wishes to maximise: social welfare, QALYs or (WTP for) dental health outcomes. All benefits are valued using general population preferences.
Willingness to pay: discrete choice experiment
Willingness to pay is calculated based on responses to an online DCE conducted with a nationally representative sample of the UK population, with oversampling in Scotland to determine any subgroup effects on preferences driven by different, regional-specific payment systems. A DCE is a survey designed to elicit respondents’ preferences through the choices they make between different hypothetical goods or services. In the context of this study, respondents make choices between different packages of dental care that vary in terms of their recall frequency, health outcomes (bleeding gums when brushing and caries experience over a 4-year period, as informed by ICDAS classification groups) and cost. Each package comes at an annual cost over 4 years, reflecting the trial’s time horizon.
Discrete choice experiments assume that a treatment or service’s value depends on its component attributes and the levels of those attributes. By including the price proxy within the DCE (i.e. the annual cost of each dental package), we can obtain a monetary valuation for any given dental recall package. These WTP estimates are used as a measure of benefit within the CBA, and can be used to value all outcomes or a subset of those outcomes (e.g. health related only). The DCE received ethics approval from the College Ethics Review Board at the University of Aberdeen (ref: 2015/12/1278). The main considerations for the DCE design are as follows.
Discrete choice experiment selection of attributes and levels
The trial protocol sets the primary research question, and the DCE therefore seeks to value general population preferences for different frequencies of dental recall (24 month, 12 month, 6 month, or risk based). The selection of outcome attributes and levels was partially determined by the trial (e.g. valuing avoidance of caries), but the selection of outcomes for valuation was also based on engagement with key stakeholders (in formal and informal discussion), literature review and primary qualitative (focus group) work with members of the general population to derive a set of attributes and levels meaningful and important to respondents. It became clear from the focus group work that respondents would have difficulty valuing and understanding the primary clinical trial outcome (gingival bleeding on probing). The DCE attribute for bleeding is, therefore, bleeding on brushing (this is mapped to a post hoc trial outcome that asks respondents to report on the frequency of bleeding when they brush their teeth). The final list of attributes and levels included in the DCE is provided in Table 4 and an example choice set can be found in Figure 4.
| Attributes | Levels |
|---|---|
| You have a dental check-up |
Every 2 years Every year Every 6 months It varies depending on your risk |
| In 4 years’ time, your gums will bleed |
Never Hardly ever Occasionally Fairly often Very often |
| Over the next 4 years, you will have |
No dental decay Early dental decay Moderate dental decay Advanced dental decay |
| The annual cost to you |
£20 per year (total cost: £80 over 4 years) £50 per year (total cost: £200 over 4 years) £100 per year (total cost: £400 over 4 years) £200 per year (total cost: £800 over 4 years) |
FIGURE 4.
Example choice task. Note: the example choice set shown in Figure 4 is for a respondent categorised as having moderate dental health. If this hypothetical respondent chose dental check-up package B, they would be willing to pay £200 per year for that package.

Discrete choice experiment experimental design
A pivoted, segmented design was generated using Ngene™ experimental design software (ChoiceMetrics Pty Ltd, Sydney, NSW, Australia) to select the combination of treatment descriptions, and to create the choice sets, which minimised the design’s D-error. First, respondents were assigned to one of three segments (good, moderate or poor dental health) based on their self-reported dental health as measured using stated caries experience and bleeding frequency.
The opt-out scenario described the implications of choosing the ‘no dental check-up’ package (i.e. receiving none of the services within the package and at no cost). Outcome attributes in the reference case were tailored to each segment, reflecting a modest reduction in the outcome attributes if no dental package was selected. The levels of the outcome attributes were then pivoted around these baseline (opt-out) levels, to ensure that only realistic choice tasks were presented to respondents. The pivoting ensured that respondents were not presented with situations in which their health could get worse by opting into one of the dental packages.
The DCE has a single five-level attribute with three four-level attributes, resulting in 320 (51 × 43) possible combinations and (320 × 319)/2 = 5040 unique choice sets. The resultant chosen main effects D-optimal design consisted of 24 choices, split equally across three blocks of eight choices. Two further choice tasks were added to test for consistency and dominance to each block of choices, meaning that each respondent completed a total of 10 choice tasks. The responses to the internal validity choice tasks were not included in the analysis models. The experimental design was based on vague priors, specifying only the expected coefficient sign for outcome attributes and null priors for the recall frequency attribute.
Discrete choice experiment questionnaire development
There are three sections to the survey. Section 1 asks about respondents’ experiences of dental care, current dental health, how often they attend their dental practice and by whom they are treated. Responses to dental health questions are used to inform the pivoting of the design as described in Discrete choice experiment experimental design. Section 2 asks the respondents to complete the DCE, and section 3 concludes the survey with a series of demographic and attitudinal questions that may help explain choices. In addition, there are debriefing questions about ease of understanding, realism and appropriateness of the choice task questions, as well as questions designed to understand respondents’ experiences of taking online surveys more generally. The questionnaire was designed using Qualtrics (Qualtrics, Provo, UT, USA) online survey design platform.
Discrete choice experiment data collection
Data were collected using Qualtrics online panels. Data collection for the DCE was conducted in parallel with the trial follow-up period and responses to the survey were provided between 7 July and 5 August 2016. The survey was nationally representative, with population census quotas sought for age (among adult population), sex and region. We oversampled in Scotland (n = 183) to enable regional-specific subgroup analysis split by contract type (UDA, England/Wales; fee for service, Scotland/Northern Ireland). At the pilot stage, 30% of responses were sought from non-regular attenders (defined as not having seen the dentist in the previous 2 years),114 but it was not possible to achieve this target and, instead, a relaxed quote of 10% was sought for the main data collection phase. All responses were anonymised and respondents could leave the survey at any time and did not need to provide a reason for doing so. Those who completed the survey were reimbursed in a manner determined by the survey panel.
Discrete choice experiment data analysis
The DCE data are analysed using best practice methods and followed random utility maximisation theory. 115 In brief, all data are analysed using a mixed logit model, specifically an error components logit model, allowing for multiple choices per respondent. The observable, individual specific systematic component of the utility function (Vnjt) for individual n, choosing dental check-up package j, in each choice task t is specified as a linear additive function of the attributes and levels presented to the respondents:
where α is the alternative specific constant (ASC), included as a random (normally distributed) parameter in the model. The ASC accounts for latent or unobserved utility associated with choosing any package, regardless of the attribute levels, and β represents the marginal utilities associated with the attributes and levels.
All categorical variables are effects coded where the β coefficients represent the effect of a unit change in an attribute level (away from the grand mean) on utility. The advantage of effects coding is that the reference level of an attribute is uncorrelated with the ASC. The reference categories for the effects coded attributes are 24-month recall, never bleeding and having teeth that have no decay. The utility of the reference category, relative to the grand mean of the attributes, is obtained as the negative sum of the estimated coefficients for that attribute. The coefficient on cost indicates the marginal change in utility for a £1 increase in annual cost. The error term is split into two, where µn is the individual specific random effect and ϵj, the unobserved component of the error term, is independent Type I extreme value distributed. The final model is estimated using the maximum simulated likelihood method, with 200 Halton draws.
The marginal WTP of an attribute level is equal to the negative of the marginal rate of substitution between that level and the cost attribute. For the purposes of result reporting, confidence intervals (CIs) are based on the delta method, calculated using Stata’s nlcom command which computes point estimates and CIs for potentially non-linear combinations of parameter estimates, implemented after running the corresponding error components logit models.
The models are re-estimated separately using interaction terms for several predetermined subgroups, including (1) sex; (2) region, to determine if system of reimbursement, and patient co-charge (£0 in Scotland) has an impact on preferences; (3) smoking status, to determine if current or previous smokers’ preferences differ from the overall group; (4) household income, to determine if lower ability to pay (annual income < £20,800) has an impact on preferences; (5) experience of dental check-ups (attending at least every 6 months); and (6) experience of dental decay, to determine if having experience of dental decay has an impact on preferences for its avoidance. The impact of subgroups on main attribute-level effects is assessed by the significance of interaction terms between subgroup identifier and attribute level coefficient. Likelihood ratio tests are used to test the joint significance of the interaction terms.
Estimating benefits (willingness to pay) for each trial participant
Benefits in terms of WTP, are calculated for each trial participant by mapping the WTP tariffs obtained from the DCE to the trial outcomes. Following an intention-to-treat principle, WTP values are attached to the services provided (i.e. the number of check-ups received at a trial participant level, regardless of randomised group). This means that the tariff for risk-based recall in the DCE was not directly assigned to the trial interventions. This is in line with an intention-to-treat approach, but it is possible that patients attach value directly to being allocated to a risk-based interval; therefore, sensitivity analysis explores a per protocol approach to attaching DCE WTP values to trial interventions. For check-up frequencies observed in the trial that extend beyond the valuation scope of the DCE (e.g. four check-ups per year), a stepwise linear utility function is used to extrapolate the WTP tariffs predicted using the DCE. WTP tariffs were assigned directly to the health outcome attributes observed in the trial (i.e. self-reported bleeding on brushing and caries experience). Caries experience is defined as a composite of treated caries assumed equal to the number of fillings provided over the follow-up phase in addition to the untreated caries detected at the final outcome assessment. The marginal WTP tariffs attached to each service and outcome are summed to obtain a total WTP measure of benefit. Two different measures are presented: (A) total WTP calculated as the sum of marginal WTPs for mapped health outcomes only (i.e. caries and bleeding), generating a dental health perspective of benefits, and (B) total WTP calculated as A + the marginal WTP attached to service frequency, generating a broader and more holistic measure of benefit for use in the CBA.
Quality-adjusted life-years
The EQ-5D-3L is used to capture generic health-related quality of life in the trial. Respondents complete the EQ-5D-3L at baseline and in each annual follow-up questionnaire. Responses to the EQ-5D-3L are then valued using UK general population tariffs, estimated using the time trade-off method. 116 The valuation generates a utility score for each EQ-5D-3L response on a scale of –0.564 (worst possible health state) to 1 (best possible health state). QALYs are then calculated using an area under the curve approach with linear extrapolation between the annual data collection time points. Any respondents who died over the course of the trial are assigned a zero utility score from the follow-up time point after death to the end of the trial follow-up.
Statistical analysis of benefits data
The approach for the analysis of benefits data is similar for calculations of both WTP and QALYs. All benefits, regardless of measurement approach, are discounted at a rate of 3.5% per annum in line with NICE guidance. 103 Total WTP and QALY data are both analysed using a generalised linear regression model, adjusting for minimisation covariates, region and baseline EQ-5D-3L utility score, and include a random effect for centre. For the comparison of all data in a single model, a fixed effect for stratum is added. For each benefit measure, models are estimated using Stata’s meglm command with a Gaussian family and identify link. The chosen family (Gaussian) is based on the results of a modified Park test, and the chosen link function is based on a combination of AIC score, and p-values from the Pearson correlation test, the Pregibon link test and the modified Hosmer–Lemeshow test.
Missing data exist for benefits because participants may not have attended their final year clinical assessment appointment or may not have returned questionnaires detailing experience of bleeding on brushing. As with costs, the mechanism of missingness was explored using logistical regression models to determine any predictors of missingness. Presence of significant explanatory variables was taken as an indication that data were missing at random and could not be assumed missing completely at random, and so multiple imputation would be required. Missing data are imputed using Stata’s MI procedure112,117 (by randomised group) using predictive mean matching (five closest values), and accounting for minimisation variables and baseline EQ-5D-3L utility score. M = 5 imputed data sets are used and results obtained from the combined data using Rubin’s rules. 113
Economic evaluation
Cost–benefit analysis
The CBA compares the costs and benefits of alternative policies of dental recall against standard care (currently 6-month scheduled recall). Both costs and benefits are measured in monetary terms and net benefits can, therefore, be directly estimated (benefits minus cost). The INBs indicate whether moving from standard care to an alternative policy increases or decreases overall societal welfare. The imputed data sets are used to estimate net benefits and INBs. Net benefit is calculated for each participant in the trial according to Equation 2:
Cost–utility analysis: generic
This analysis is based on the cost of achieving changes in generic health profile, as measured using the EQ-5D-3L, and uses QALYs as the measure of benefits. An incremental comparison of costs and QALYs between each recall strategy is reported, and ICERs are calculated as the mean difference in costs divided by the mean difference in QALYs. The ICER can then be compared with a threshold value of WTP for a QALY gained, as is commonly considered by NICE. If the ICER is < £20,000 to £30,000 per QALY gained, an intervention is typically considered to be an efficient use of resources. In cases where there are additional costs but QALY losses, an intervention is said to be ‘dominated’ and is less likely to provide good value for money.
Dental health-specific evaluation
Quality-adjusted life-years are unlikely to be sufficiently sensitive to capture changes in dental health; therefore, an ‘alternative’ analysis is conducted using WTP for dental health outcomes (based on a composite measure of caries experience as measured using ICDAS/fillings received and self-reported bleeding gums on brushing) as the measure of benefit. INBs are estimated as described in the CBA.
Statistical analysis of costs and benefits together
For each analysis framework, data are analysed using multiple imputation of missing data, and regression analyses as described in the preceding sections. The point estimate of INB, or incremental cost per QALY gained, is obtained by dividing the treatment coefficient on costs by the treatment coefficient on benefits regressions. This is done for each cost perspective (NHS dental perspective, NHS perspective and societal perspective). The results should be considered in the light of the uncertainty surrounding the point estimate. Uncertainty is illustrated using scatterplots of costs and benefits (WTP or QALYs) on the cost-effectiveness (benefit) plane and cost-effectiveness (benefit) acceptability curves. The cost-effectiveness acceptability curves (CEACs)/cost–benefit acceptability curves (CBACs) and scatterplots are obtained using 1000 bootstraps of the corresponding analysis models, with imputation included within each bootstrapped loop where required, following the guidance outlined in Brand 2019. 118
For the CBA, the CBACs illustrate the probability that INB is positive at various threshold values of the benefit–cost ratio. A positive INB would be associated with a benefit–cost ratio > 1. The higher the benefit–cost ratio, the more likely the intervention is to be cost-beneficial (i.e. an efficient use of resources). For the CUA, CEACs illustrate the probability that each intervention is the optimal (most efficient) strategy at increasing threshold values of society’s WTP for a QALY gained. These analyses illustrate the impact of sampling uncertainty on the results, but it is also necessary to consider uncertainty driven by key analysis assumptions. The range of deterministic and scenario analyses carried out are described, with a justification for each, in Table 5.
| Topic | Scenarios considered | Justification for approach |
|---|---|---|
| Impact of costing perspective on results | ||
| Costing perspective | Costs are considered from an NHS dental care perspective, based on NHS payment for dental care obtained from routine claims data | To determine the direct impact of different recall frequencies on the NHS dental budget |
| Costing perspective | Costs are considered from both an NHS dental care perspective and a broader NHS health-care perspective including costs falling outwith the dental budget (e.g. hospital attendance, GP care and out-of-hours service contacts) | To determine the impact of a narrow vs. wider perspective of NHS-incurred costs on results |
| Costing perspective | Costs are considered from an NHS dental care, a broader NHS health-care perspective and include all patient participant-incurred costs (including costs directly associated with attendance at NHS dental appointments and all other private dental care) |
To determine the impact of different recall strategies on costs from both the NHS perspective and the patient perspective To more fully understand the impact on patients of attendance for dental visits |
| Costing perspective | Costs are considered from both an NHS dental care perspective and including patient participant perspective costs directly associated with attendance at dental appointments | An even wider perspective of costs, including all NHS and participant perspective costs related to dentistry |
| Methodological issues | ||
| Reference analysis: multiple imputation, 3.5% discount rate for costs and benefits, WTP mapped to caries experience (routine data) + untreated caries (trial outcome), ITT analysis, based on all UK regions combined | ||
| Discounting of costs and benefits | Assume undiscounted costs and benefits | Standard exploration of methodological uncertainty |
| Imputation | Complete cases | |
| Mapping of WTP to trial outcomes | Exploring the impact of mapping WTP for caries avoidance to clinical trial outcome (untreated caries) only | The clinical outcome assessment captures untreated caries, but not caries experience for participants when they obtained fillings over the trial follow-up. Sensitivity analysis (untreated caries only) may underestimate value of caries experience, but may be more robust given that it is unclear from routine data if fillings are provided for caries (or replacements/trauma) |
| ITT vs. per protocol analysis | ITT analysis values the number of services received in the CBA. The ‘per protocol’ analysis attaches WTP tariffs from the DCE to randomised arms, regardless of services delivered | Analysis conducted to explore the impact of explicit valuation of risk-based recall on the CBA results |
| UK vs. regional analyses | Region-specific analyses for Scotland and England | Regional-specific analyses conducted to determine if the payment system for dentistry (e.g. fee for service and free check-ups for patients in Scotland) has an impact on cost-effectiveness results. This analysis additionally includes regional-specific subgroup WTP tariffs from the DCE applied to trial participant services and outcomes (by region) |
Chapter 4 Trial results and clinical effectiveness
Introduction
This chapter describes the study groups at trial entry, followed by a description of the interventions received, the results at the annual follow-up points and a statistical analysis of the primary and secondary outcomes. Randomised groups are presented separately dependent on eligibility stratum: whether the patient participant was eligible or not to be randomised to a 24-month recall. If the participant was eligible, three treatment options were available and are presented in this chapter (i.e. risk-based recall, 24-month recall and 6-month recall). If the participant was not eligible to be recruited to the 24-month recall stratum, two randomised groups are presented (risk-based recall and 6-month recall).
Recruitment to the study
Participants were recruited between July 2010 and July 2014. Data were closed to follow-up on 13 August 2018. The flow of participants for each INTERVAL eligibility stratum is shown separately in Figure 5 (eligible to be randomised to 24-month recall) and Figure 6 (ineligible to be randomised to 24-month recall), with information on primary outcome collection at the different follow-up time points in the form of a Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
FIGURE 5.
The CONSORT flow diagram for participants eligible for the 24-month recall stratum.
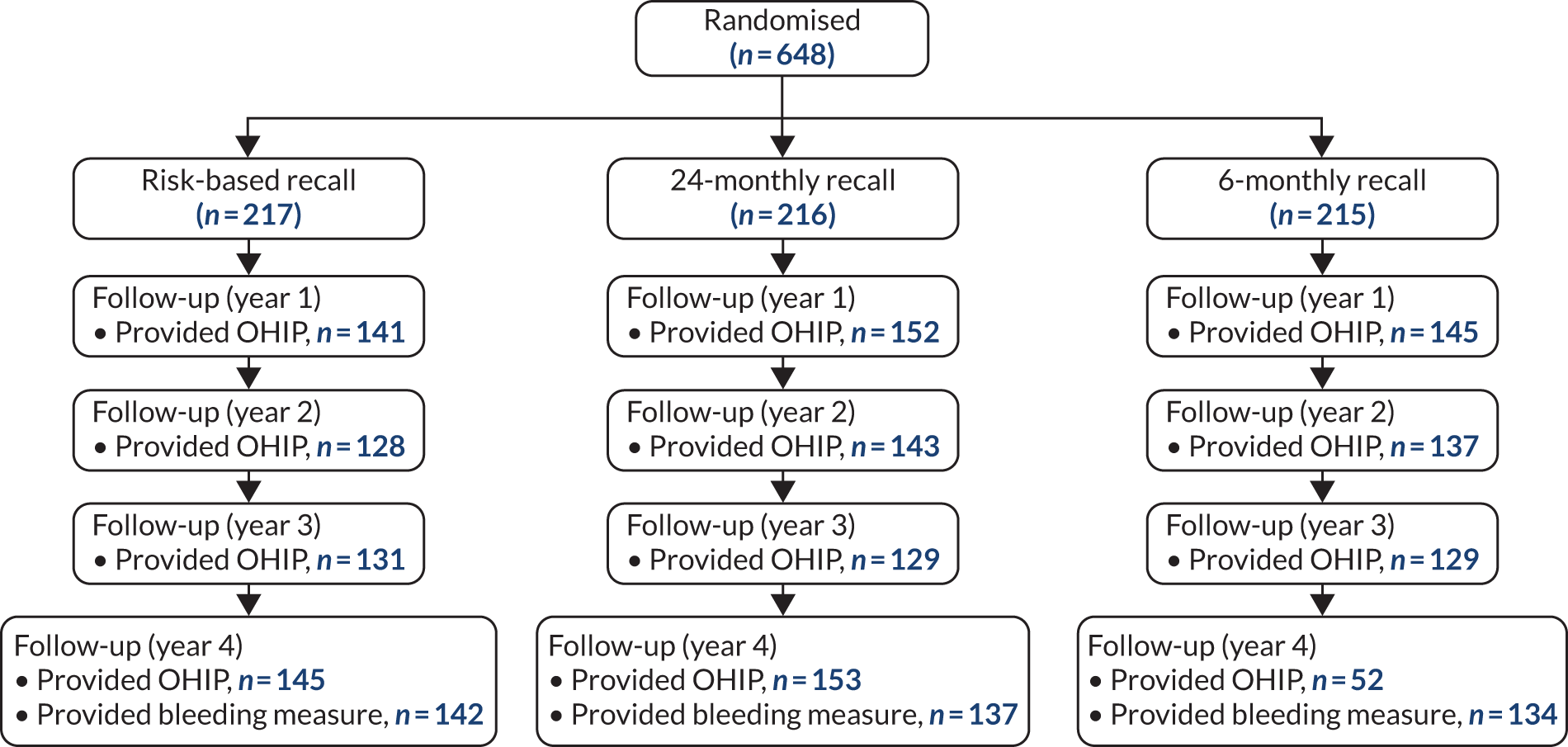
FIGURE 6.
The CONSORT flow diagram for participants ineligible for the 24-month recall stratum.
Participants
Participants were recruited from 51 practices (see Appendix 1, Table 28). A total of 2372 participants were recruited to the trial. Of the 648 participants recruited to the 24-month recall stratum, 217 were allocated to risk-based recall, 216 to 24-month recall and 215 to 6-month recall. Of the 1724 participants ineligible for 24-month recall stratum, 861 were allocated to risk-based recall and 863 were allocated to 6-month recall. In total, 1078 participants received a risk-based recall and 1078 received a 6-month recall. Half of all participants (1188) were recruited from Scotland, 1031 were recruited from England and Wales (43%) and 153 were recruited from Northern Ireland (7%).
Description of the groups at trial entry
Participant characteristics
Sociodemographic factors and dental characteristics
Participants and sociodemographic characteristics are shown in Table 6. The average age of participants was 45 years (participants in the eligible for 24-month recall stratum were younger, on average by around 4 years, than those ineligible for 24-month recall); the majority were women (around 55%) and regular attenders, although there was a difference between the eligible for 24-month recall stratum (around 74% self-reported having been to the dentist in the last year) and the ineligible stratum (around 86% had been to the dentist in the last year). Approximately 15% of participants self-identified as smokers in the last 12 months. Fifty-five per cent of the participants used manual dental brushes. Participants found it easy to travel to the dentist (on average scoring 6 out of 7, where 7 is the easiest to travel). Randomised groups were balanced on the sociodemographic factors and dental characteristics collected.
| Participant characteristic | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Randomised, n (%) | 217 (100.0) | 216 (100.0) | 215 (100.0) | 861 (100.0) | 863 (100.0) |
| Baseline questionnaire returned, n (%) | 181 (83.4) | 186 (86.1) | 187 (87.0) | 810 (94.1) | 803 (93.0) |
| Age (years), mean (SD), n | 43.3 (15.1), 217 | 44.2 (15.2), 216 | 43.5 (14.5), 215 | 49.3 (14.1), 861 | 50.1 (15.3), 863 |
| Gender, n (%) | |||||
| Male | 87 (40.1) | 100 (46.3) | 94 (43.7) | 356 (41.3) | 366 (42.4) |
| Female | 128 (59.0) | 115 (53.2) | 121 (56.3) | 498 (57.8) | 491 (56.9) |
| Missing | 2 (0.9) | 1 (0.5) | 0 (0.0) | 7 (0.8) | 6 (0.7) |
| Smoking status, n (%) | |||||
| Smoked in the last 12 months | 32 (14.7) | 27 (12.5) | 32 (14.9) | 145 (16.8) | 130 (15.1) |
| Missing | 38 (17.5) | 32 (14.8) | 30 (14.0) | 53 (6.2) | 69 (8.0) |
| Time since previous visit to dentist, n (%) | |||||
| < 1 year | 165 (76.0) | 157 (72.7) | 165 (76.7) | 737 (85.6) | 741 (85.9) |
| 1–2 years | 13 (6.0) | 24 (11.1) | 16 (7.4) | 62 (7.2) | 51 (5.9) |
| > 2 years | 1 (0.5) | 4 (1.9) | 4 (1.9) | 4 (0.5) | 0 (0.0) |
| Missing | 38 (17.5) | 31 (14.4) | 30 (14.0) | 58 (6.7) | 71 (8.2) |
| Patient status, n (%) | |||||
| NHS | 152 (70.0) | 154 (71.3) | 155 (72.1) | 695 (80.7) | 677 (78.4) |
| Private | 8 (3.7) | 12 (5.6) | 7 (3.3) | 33 (3.8) | 32 (3.7) |
| Combination | 12 (5.5) | 8 (3.7) | 14 (6.5) | 54 (6.3) | 56 (6.5) |
| Missing | 45 (20.7) | 42 (19.4) | 39 (18.1) | 79 (9.2) | 98 (11.4) |
| Type of toothbrush, n (%) | |||||
| Manual | 114 (52.5) | 131 (60.6) | 113 (52.6) | 489 (56.8) | 496 (57.5) |
| Electric | 64 (29.5) | 53 (24.5) | 74 (34.4) | 318 (36.9) | 298 (34.5) |
| Missing | 39 (18.0) | 32 (14.8) | 28 (13.0) | 54 (6.3) | 69 (8.0) |
| Regular attender: self-report | 158 (72.8) | 163 (75.5) | 168 (78.1) | 740 (85.9) | 735 (85.2) |
| Missing | 39 (18.0) | 31 (14.4) | 28 (13.0) | 60 (7.0) | 71 (8.2) |
| Difficulty travelling to dentist, mean (SD), n | 6.4 (1.2), 179 | 6.5 (1.2), 186 | 6.4 (1.2), 186 | 6.4 (1.2), 805 | 6.3 (1.2), 791 |
Participants’ dental behaviour at baseline
Most participants (around 70%) brushed twice a day or more often and believed that this is what they should do (around 86%). Around 56% of the participants reported taking 2 minutes or longer to brush. Around one-quarter of the participants reported that they spit but do not rinse, and one-third reported that they believed that is what they should do (Table 7).
| Oral health-related behaviour | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Frequency of brushing, n (%) | |||||
| Less than once per day | 0 (0.0) | 1 (0.5) | 5 (2.3) | 9 (1.0) | 11 (1.3) |
| Once per day | 33 (15.2) | 42 (19.4) | 42 (19.5) | 151 (17.5) | 116 (13.4) |
| Twice per day | 123 (56.7) | 128 (59.3) | 132 (61.4) | 560 (65.0) | 587 (68.0) |
| More than twice per day | 23 (10.6) | 15 (6.9) | 8 (3.7) | 82 (9.5) | 80 (9.3) |
| Missing | 38 (17.5) | 30 (13.9) | 28 (13.0) | 59 (6.9) | 69 (8.0) |
| Duration of brushing, n (%) | |||||
| < 1 minute | 9 (4.1) | 3 (1.4) | 10 (4.7) | 24 (2.8) | 25 (2.9) |
| 1 to 2 minutes | 56 (25.8) | 55 (25.5) | 62 (28.8) | 252 (29.3) | 254 (29.4) |
| 2 minutes | 87 (40.1) | 99 (45.8) | 79 (36.7) | 362 (42.0) | 383 (44.4) |
| > 2 minutes | 26 (12.0) | 29 (13.4) | 36 (16.7) | 163 (18.9) | 132 (15.3) |
| Missing | 39 (18.0) | 30 (13.9) | 28 (13.0) | 60 (7.0) | 69 (8.0) |
| After brushing, n (%) | |||||
| Rinse with water | 78 (35.9) | 100 (46.3) | 97 (45.1) | 432 (50.2) | 435 (50.4) |
| Rinse with mouthwash | 37 (17.1) | 33 (15.3) | 26 (12.1) | 154 (17.9) | 165 (19.1) |
| Spit not rinse | 64 (29.5) | 53 (24.5) | 64 (29.8) | 213 (24.7) | 190 (22.0) |
| Missing | 38 (17.5) | 30 (13.9) | 28 (13.0) | 62 (7.2) | 73 (8.5) |
Patients’ reported outcomes
The baseline patient-reported outcomes summary is shown in Table 8. More details about individual questions that produced the scales are given in Appendix 1, Table 26. OHIP-14 score, the patient-reported primary outcome, was, on average, low, indicating good OHRQoL in both strata. OHIP-14 was around 4.5 in the eligible for 24-month recall stratum and 5.9 in the ineligible stratum, on a scale that goes up to 56 for worst quality of life. Participants were, in general, satisfied with their dental services (giving, on average, a score of 5 out of 7 on the satisfaction scale, where 7 is maximum satisfaction with the service). Their anxiety was on average around 10 points, on a scale ranging from 5 to 25, where 25 is the maximum anxiety.
| Patient-reported outcome | Eligible for 24-month recall, mean (SD), n | Ineligible for 24-month recalll, mean (SD), n | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Attitude (score from 1 to 7) | 4.2 (0.8), 178 | 4.3 (0.9), 186 | 4.1 (0.7), 187 | 4.1 (0.9), 806 | 4.0 (0.9), 795 |
| Satisfaction (score from 1 to 7) | 5.2 (0.8), 147 | 5.2 (0.8), 153 | 5.2 (0.7), 158 | 5.3 (0.6), 703 | 5.3 (0.6), 701 |
| OHIP-14 (score from 0 to 56) | 4.5 (7.0), 175 | 4.7 (6.4), 183 | 4.4 (6.1), 182 | 5.8 (6.9), 778 | 6.1 (7.7), 778 |
| Overall anxiety (score from 5 to 25) | 10.4 (4.6), 176 | 10.7 (4.4), 186 | 10.5 (4.5), 185 | 10.2 (4.5), 801 | 10.1 (4.5), 798 |
Baseline overview
Overall, participants in the ineligible for 24-month recall stratum were older, self-reported to attend the dentist more regularly and had higher OHIP-14 scores than those in the eligible stratum. Participants were, in general, satisfied with the dental services received and had low dental anxiety and a good knowledge about the frequency and duration of brushing; however, they were less informed about what to do after brushing (i.e. spit, but not rinse). There were no important differences or imbalances across randomised groups in each of the eligibility strata. Appendix 1, Tables 29–37, display all baseline tables by country: Scotland, England and Northern Ireland. Average age is similar across countries, as is the proportion of regular smokers. The percentage of regular attenders was higher in Scotland than in England in the stratum eligible for the 24-month recall (85% vs. 70%). Around 80% of the participants in Scotland reported that they were fully NHS; in England, around 72% of patients eligible for a 24-month recall were fully NHS, as were 80% of patients in the ineligible for a 24-month recall stratum. In Northern Ireland, around one-third of patients in the eligible for a 24-month recall stratum were fully NHS, as were 65% of the ineligible patients. Patient-reported outcomes and behaviour and knowledge questions produced similar results across countries, except for ‘What do you do after brushing?’ or ‘What should you do after brushing?’ (i.e. spit, not rinse). In Scotland, around 35% of participants spit but do not rinse and around 45% of participants know that this should be the case. In England, around 14% of participants reported that they spit but do not rinse and around 16% of participants said that they know that this should be the case. In Northern Ireland, around 16% of participants in the eligible for 24-month recall stratum spit but do not rinse or think that they should do that whereas in the ineligible stratum this value is around 50% for both questions.
Trial follow-up
Description of the intervention
Using routine data from Scotland, England and Northern Ireland, the number of routine check-ups reported during the trial is presented in Table 9. Participants eligible for 24-month recall, and who had a clinical outcome assessment, had on average 3.7 check-ups in the risk-based group, 2.5 in the 24-month recall group and 5.1 in the 6-month recall group. Participants who were ineligible for the 24-month recall, and who had a clinical outcome assessment, had 5.0 check-ups on average during the trial in the risk-based group and 5.4 check-ups in the 6-month group.
| Intervention | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based (n = 137) | 24 month (n = 128) | 6 month (n = 128) | Risk based (n = 582) | 6 month (n = 578) | |
| Check-ups received | |||||
| 0 | 1 (0.7) | 8 (5.8) | 0 (0.0) | 1 (0.2) | 0 (0.0) |
| 1 | 15 (10.5) | 50 (36.2) | 7 (5.2) | 14 (2.3) | 7 (1.2) |
| 2 | 27 (18.9) | 22 (15.9) | 9 (6.7) | 44 (7.3) | 30 (5.0) |
| 3 or more | 94 (65.7) | 48 (34.8) | 112 (83.0) | 523 (86.3) | 541 (89.9) |
| Missing | 6 (4.2) | 10 (7.2) | 7 (5.2) | 24 (4.0) | 24 (4.0) |
| Number of check-ups, mean (SD), n | 3.7 (1.9), 137 | 2.5 (2.2), 128 | 5.1 (3.7), 128 | 5.0 (2.3), 582 | 5.4 (2.0), 578 |
Once a participant had been randomised, dentists were asked to record the date of their first intended appointment, which took account of any expected course of treatment. Participants in the eligible for 24-month recall stratum were assigned recall appointments that were intended to be at, on average, 13 months (risk-based recall), 24 months (24-month recall) and 7 months (6-month recall). Participants in the ineligible for 24-month recall stratum were advised by clinicians to wait 9 months until their first appointment if in the risk-based group and 8.5 months if in the 6-month recall group. Appendix 1, Tables 38–40, presents the description of the intervention by country. Participants from England and Northern Ireland had, on average, more check-ups than Scottish participants, but the discrepancy between randomised groups was similar across countries.
Overall, there was a clear difference in terms of number of check-ups received between the different randomised groups in the eligible for 24-month recall stratum. The number of check-ups received in the risk-based and 6-month recall was similar in the ineligible for 24-month recall stratum: there was a higher number of check-ups in the risk-based group of the ineligible stratum than of the eligible stratum. In terms of clinicians’ intentions at the beginning of the trial, there is a similar pattern on the PAD forms: a clear separation in time between randomisation and first intended appointment between randomised groups. However, participants ineligible for a 24-month recall had a similar interval between randomisation and first intended appointment across the different groups.
Attendance at 4-year clinical examination and questionnaire returns
The primary clinical outcome was collected at the 4-year clinical follow-up. Overall, around 64% and 71% of the participants attended their appointment and replied to the year 4 questionnaire, respectively, in the eligible for 24-month recall stratum. In the ineligible stratum, 70% of participants attended the clinical appointment and 76% replied to the year 4 questionnaire (Table 10). For most participants (around 89% of the participants randomised), reasons for non-attendance were unknown. When a reason was obtained from the practices, the most common reason for non-attendance was inability to contact the patient (around 9%). This finding was similar across groups and in both strata. The mean follow-up time was 122 months with a SD of 6.2 months. Approximately 71% of participants in the eligible for 24-month recall stratum and 76% of participants in the ineligible for 24-month recall stratum returned their year 4 questionnaire.
| Time point | Eligible for 24-month recall, n (%) | Ineligible for 24-month recall, n (%) | |||
|---|---|---|---|---|---|
| Risk based (N = 217) | 24 month (N = 215) | 6 month (N = 216) | Risk based (N = 861) | 6 month (N = 863) | |
| Attended clinical follow-up | 143 (65.9) | 138 (63.9) | 135 (62.8) | 606 (70.4) | 602 (69.8) |
| Baseline | 181 (83.4) | 186 (86.1) | 187 (87.0) | 810 (94.1) | 803 (93.0) |
| Year 1 | 145 (66.8) | 155 (71.8) | 152 (70.7) | 638 (74.1) | 640 (74.2) |
| Year 2 | 135 (62.2) | 144 (66.7) | 142 (66.0) | 610 (70.8) | 619 (71.7) |
| Year 3 | 134 (61.8) | 131 (60.6) | 134 (62.3) | 571 (66.3) | 584 (67.7) |
| Year 4 | 151 (69.6) | 153 (70.8) | 156 (72.6) | 650 (75.5) | 655 (75.9) |
Participants’ characteristics at 4 years
Participants’ characteristics at 4 years are shown in Table 11. A lower percentage of participants reported smoking in the last 12 months at year 4 than at baseline (12% vs. 15% approximately). The majority of participants stated that they had been to dentist in the last year (around 90%); however, there were important differences between groups. The group randomised to 24-month recall had a lower percentage of participants that reported that they had attended in the last year (76%) and a significantly higher percentage of participants reporting that the time since their previous visit was between 1 and 2 years. Around the same percentage of participants across groups reported that they used the NHS (70%) and used a manual toothbrush (50%). Most of the participants reported to be regular attenders across the groups with similar percentages in the 6-month recall groups for both strata and in the risk-based group in the ineligible stratum (around 90%). Around 84% of the participants in the risk-based group eligible for a 24-month recall and in the 24-month recall stratum reported the same. Participants, overall, found travelling to the dentist easy and with similar scores to baseline (6 out of 7 on a Likert scale, where 7 is the easiest to travel). Participant characteristics at years 1, 2 and 3 are presented in Appendix 1, Tables 41–43. Participant behaviour and knowledge related to oral health at years 1, 2, 3 and 4 are presented in Appendix 1, Tables 44–47.
| Participant characteristic | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Number randomised | 217 | 215 | 216 | 861 | 863 |
| Replied to year 4 questionnaire | 151 (100.0) | 153 (100.0) | 156 (100.0) | 650 (100.0) | 655 (100.0) |
| Smoked in the last 12 months | 18 (11.9) | 18 (11.8) | 19 (12.2) | 86 (13.2) | 74 (11.3) |
| Missing | 1 (0.7) | 0 (0.0) | 1 (0.6) | 6 (0.9) | 5 (0.8) |
| Time since previous visit to dentist | |||||
| < 1 year | 130 (86.1) | 116 (75.8) | 143 (91.7) | 604 (92.9) | 621 (94.8) |
| 1–2 years | 19 (12.6) | 32 (20.9) | 8 (5.1) | 33 (5.1) | 28 (4.3) |
| > 2 years | 2 (1.3) | 4 (2.6) | 3 (1.9) | 5 (0.8) | 2 (0.3) |
| Missing | 0 (0) | 1 (0.7) | 2 (1.3) | 8 (1.2) | 4 (0.6) |
| Patient status | |||||
| NHS | 104 (68.9) | 94 (61.4) | 105 (67.3) | 460 (70.8) | 469 (71.6) |
| Private | 8 (5.3) | 5 (3.3) | 3 (1.9) | 17 (2.6) | 22 (3.4) |
| Combination | 16 (10.6) | 16 (10.5) | 23 (14.7) | 92 (14.2) | 92 (14.0) |
| Missing | 23 (15.2) | 38 (24.8) | 25 (16.0) | 81 (12.5) | 72 (11.0) |
| Type of toothbrush | |||||
| Manual | 72 (47.7) | 86 (56.2) | 82 (52.6) | 328 (50.5) | 339 (51.8) |
| Electric | 73 (48.3) | 54 (35.3) | 69 (44.2) | 275 (42.3) | 274 (41.8) |
| Both | 6 (4.0) | 13 (8.5) | 4 (2.6) | 42 (6.5) | 36 (5.5) |
| Missing | 0 (0.0) | 0 (0.0) | 1 (0.6) | 5 (0.8) | 6 (0.9) |
| Regular attender: self-report | 128 (84.8) | 127 (83.0) | 142 (91.0) | 612 (94.2) | 614 (93.7) |
| Missing | 0 (0.0) | 1 (0.7) | 1 (0.6) | 7 (1.1) | 5 (0.8) |
| Difficulty travelling to dentist, mean (SD), n | 6.2 (1.4), 151 | 6.4 (1.1), 151 | 6.3 (1.2), 154 | 6.3 (1.3), 644 | 6.3 (1.3), 646 |
Clinical outcomes
Table 12 summarises the clinical outcomes mean per stratum and per randomised group. On average, participants had 34% of sites bleeding and 37% of surfaces with calculus across the randomised groups in both the eligible and the ineligible stratum. The average number of teeth was around 24. Moderate lesions were the most serious carious lesion found in about 65% of the participants. The ineligible stratum had a slightly higher percentage of participants with extensive caries (14%) than the eligible stratum (18%). Around 15–23% of participants had root caries. Around half of the participants presented generalised gingivitis according to the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases. 119 In Appendix 1, Table 48 presents the same clinical outcomes combining risk-based and 6-monthly groups from the eligible and ineligible strata.
| Clinical outcomes | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Attended clinical follow-up, n | 143 | 138 | 135 | 606 | 602 |
| Primary clinical outcome, mean (SD), n | |||||
| Gingival bleeding: mean percentage of sites bleeding | 35.6 (19.1), 142 | 34.4 (20.1), 137 | 35.6 (21.7), 134 | 33.4 (22.2), 599 | 32.8 (22.1), 597 |
| Secondary clinical outcomes, mean (SD), n | |||||
| Calculus: mean proportion of surfaces with calculus | 34.1 (26.0), 142 | 38.2 (28.3), 138 | 37.4 (24.9), 133 | 37.3 (27.8), 604 | 38.0 (27.8), 600 |
| Mean pocket depth (mm) | 2.2 (0.5), 142 | 2.1 (0.3), 137 | 2.1 (0.4), 133 | 2.2 (0.4), 594 | 2.2 (0.4), 594 |
| Number of teeth | 24.2 (4.8), 143 | 24.0 (4.5), 138 | 24.8 (4.0), 135 | 23.9 (4.6), 606 | 23.3 (5.2), 602 |
| Most advanced carious lesion per person, n (%) | |||||
| Sound surfaces (ICDAS 0) | 1 (0.7) | 1 (0.7) | 0 (0.0) | 3 (0.5) | 8 (1.3) |
| Initial lesions (ICDAS 1–2) | 22 (15.4) | 28 (20.3) | 33 (24.4) | 100 (16.5) | 107 (17.8) |
| Moderate lesions (ICDAS 3–4) | 98 (68.5) | 87 (63.0) | 87 (64.4) | 393 (64.9) | 376 (2.5) |
| Extensive caries or treatment needed (ICDAS 5–6) | 20 (14.0) | 22 (15.9) | 13 (9.6) | 110 (18.2) | 107 (17.8) |
| Missing | 2 (1.4) | 0 (0.0) | 2 (1.5) | 0 (0.0) | 4 (0.7) |
| Mean number of surfaces with caries, mean (SD), n | |||||
| Any caries | 15.5 (9.8), 143 | 14.1 (7.9), 138 | 14.7 (8.4), 135 | 14.7 (8.9), 606 | 14.7 (9.2), 602 |
| Initial lesions | 12.4 (8.8), 143 | 11.7 (7.4), 138 | 12.4 (7.5), 135 | 11.2 (7.5), 606 | 11.3 (7.8), 602 |
| Moderate lesions | 2.8 (2.5), 143 | 2.1 (1.9), 138 | 2.2 (2.2), 135 | 3.1 (3.1), 606 | 3.0 (3.0), 602 |
| Extensive caries or treatment needed | 0.30 (0.9), 143 | 0.28 (0.85), 138 | 0.16 (0.60), 135 | 0.43 (1.5), 606 | 0.36 (1.3), 602 |
| Root caries, n (%) | |||||
| Yes | 26 (18.2) | 21 (15.2) | 17 (12.6) | 121 (20.0) | 137 (22.8) |
| No | 99 (69.2) | 101 (73.2) | 99 (73.3) | 417 (68.8) | 390 (64.8) |
| Missing | 18 (12.6) | 16 (11.6) | 19 (14.1) | 68 (11.2) | 75 (12.5) |
| Gingivitis severity,a n (%) | |||||
| No gingivitis (< 10% sites bleeding) | 10 (7.0) | 14 (10.1) | 18 (13.3) | 100 (16.5) | 96 (15.9) |
| Localised gingivitis (10–30% sites bleeding) | 58 (40.9) | 50 (36.5) | 42 (31.3) | 183 (30.2) | 201 (33.4) |
| Generalised gingivitis (more than 30% sites bleeding) | 74 (52.1) | 73 (53.3) | 74 (55.2) | 316 (52.1) | 300 (49.8) |
| Missing | 1 (0.7) | 1 (0.7) | 1 (0.7) | 7 (1.2) | 5 (0.8) |
Statistical analyses
Primary clinical outcome (gingival bleeding)
The treatment effects for the primary clinical outcome and secondary outcomes can be found in Table 13. Three treatment comparisons are presented for the eligible for 24-month recall stratum: 24-month recall versus 6-month recall; risk-based recall versus 6-month recall and 24-month recall versus risk-based recall. For the primary outcome, the adjusted difference between interventions was < 1% and the CIs excluded the possibility of a 7.5% difference between groups. There was no evidence of a significant difference between the groups in any comparison: the 24-month group versus 6-month group had an adjusted mean difference of –0.91 (95% CI –5.02 to 3.20; p-value = 0.66); the risk-based versus 6-month recall had an adjusted difference of –0.98 (95% CI –5.05 to 3.09; p-value = 0.64); and the 24-month versus risk-based recall had an adjusted mean difference of 0.07 (95% CI –3.99 to 4.12; p-value = 0.97). For the overall sample (combining eligible and ineligible strata), one comparison is presented for each outcome: risk-based recall versus 6-month recall. The adjusted mean difference between the groups was 0.78 (95% CI –1.17 to 2.72; p-value = 0.43).
| Outcome | Comparison | Effect size (95% CI); p-value |
|---|---|---|
| Eligible for 24-month recall stratum | ||
| Primary clinical outcome | ||
| Gingival bleeding: mean percentage of sites bleeding | 24 month vs. 6 month | –0.91 (–5.02 to 3.20); 0.66 |
| Risk based vs. 6 month | –0.98 (–5.05 to 3.09); 0.64 | |
| 24 month vs. risk based | 0.07 (–3.99 to 4.12); 0.97 | |
| Secondary clinical outcomes | ||
| Calculus: mean proportion of surfaces with calculus | 24 month vs. 6 month | 0.19 (–5.46 to 5.83); 0.95 |
| Risk based vs. 6 month | –2.92 (–8.52 to 2.67); 0.31 | |
| 24 month vs. risk based | 3.11 (–2.45 to 8.67); 0.27 | |
| Mean pocket depth (mm) | 24 month vs. 6 month | –0.03 (–0.12 to 0.06); 0.51 |
| Risk based vs. 6 month | 0.07 (–0.02 to 0.15); 0.14 | |
| 24 month vs. risk based | –0.10 (–0.18 to –0.01); 0.03 | |
| Most serious level of caries found per person | Risk based vs. 6 month | 1.58 (0.96 to 2.62); 0.07 |
| 24 month vs. 6 month | 1.38 (0.83 to 2.29); 0.22 | |
| 24 month vs. risk based | 0.87 (0.53 to 1.44); 0.59 | |
| Root caries | 24 month vs. risk based | 0.86 (0.40 to 1.83); 0.70 |
| Risk based vs. 6 month | 1.69 (0.75 to 3.78); 0.20 | |
| 24 month vs. 6 month | 1.45 (0.64 to 3.32); 0.37 | |
| Overall sample (eligible and ineligible for 24-month recall stratum) | ||
| Primary clinical outcome | ||
| Gingival bleeding: mean percentage of sites bleeding | Risk based vs. 6 month | 0.78 (–1.17 to 2.72); 0.43 |
| Secondary clinical outcomes | ||
| Calculus: mean proportion of surfaces with calculus | Risk based vs. 6 month | –1.30 (–3.68 to 1.08); 0.29 |
| Mean pocket depth (mm) | Risk based vs. 6 month | 0.03 (–0.01 to 0.07); 0.14 |
| Most serious level of caries found per person | Risk based vs. 6 month | 1.18 (0.96 to 1.46); 0.12 |
| Root caries | Risk based vs. 6 month | 0.86 (0.64 to 1.14); 0.29 |
Sensitivity analysis
We used multiple imputation for the primary clinical outcome (gingival bleeding). The treatment effects for the eligible for 24-month recall stratum were as follows: 6-month recall versus risk-based recall –0.05 (95% CI –4.9 to 4.8, p = 0.98); 24-month recall versus risk-based recall –1.34 (95% CI –5.8 to 3.1, p = 0.55); 24-month recall versus 6-month recall –1.3 (95% CI –6.5 to 3.9, p = 0.62). For the ineligible for 24-month recall stratum, the treatment effects were as follows: 6-month recall versus risk-based recall 0.57 (95% CI –1.5 to 2.6, p = 0.58). The sensitivity analyses did not change the interpretation of the results.
Secondary clinical outcomes
The remaining treatment effects for secondary clinical outcomes are also presented in Table 13. There was no evidence of a significant difference between the groups in any comparison in either eligibility stratum. The clinical outcomes are presented by country in Appendix 1, Tables 49–51.
Patient-reported outcomes at 4 years
Table 14 shows the patient-reported outcomes at 4 years. Participants in the eligible for 24-month recall group had lower OHIP-14 scores than ineligible participants, as observed at baseline. Participants remained positive about dental services, with a high level of satisfaction on average (around 5 out of 7, where 7 represents the maximum satisfaction with the service). Anxiety levels remained similar, and on average around 10 out of 25 on the MDAS, ranging from 5 to 25 (maximum anxiety). Outcomes measured only at 4 years included PBC, attitude, oral health behaviour and knowledge. The first two ranged from 1 to 7 (where 7 means maximum PBC and most positive attitude); participants had, on average, a score of 4.8 for PBC and 4.2 for attitude.
| Patient-reported outcome | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Primary patient-reported outcome, mean (SD), n | |||||
| OHIP-14 (score 0–56) | 4.1 (5.7), 145 | 4.8 (6.4), 153 | 4.8 (6.2), 152 | 5.5 (6.8), 624 | 5.8 (8.3), 630 |
| Secondary patient-reported outcomes, mean (SD), n | |||||
| Satisfaction (score 1–7) | 5.2 (0.7), 151 | 5.0 (0.7), 153 | 5.1 (0.7), 155 | 5.3 (0.6), 647 | 5.2 (0.6), 654 |
| Dental anxiety (score 5–25; MDAS) | 10.5 (4.5), 150 | 10.9 (4.7), 153 | 11.0 (4.8), 155 | 10.3 (4.5), 645 | 10.5 (4.7), 649 |
| Perceived behaviour control (score 1–7) | 4.8 (1.4), 149 | 4.6 (1.4), 153 | 4.5 (1.5), 155 | 4.7 (1.5), 647 | 4.7 (1.4), 654 |
| Attitude (score 1–7) | 4.3 (0.9), 151 | 4.3 (0.9), 153 | 4.1 (0.8), 155 | 4.1 (0.9), 647 | 4.1 (0.8), 654 |
| Behaviour (score 1–9) | 5.2 (1.8), 151 | 4.9 (1.7), 153 | 5.3 (1.6), 155 | 5.5 (1.7), 648 | 5.4 (1.7), 654 |
| Knowledge (score 1–9) | 6.5 (1.5), 150 | 6.6 (1.3), 153 | 6.8 (1.2), 155 | 6.6 (1.4), 648 | 6.7 (1.4), 651 |
| Self-reported bleeding (score 1–5) | 2.0 (1.0), 148 | 2.0 (0.9), 151 | 2.2 (1.0), 154 | 2.1 (1.0), 590 | 2.1 (1.0), 603 |
| EQ-5D-3L utility (score –0.594 to 1) | 0.893 (0.208), 145 | 0.884 (0.209), 151 | 0.856 (0.268), 157 | 0.866 (0.230), 637 | 0.867 (0.237), 645 |
Patient-reported outcomes means and standard errors per year and per stratum are presented in Figures 7–10. Overall, the scores remained unchanged during the trial and are similar across groups. Patient-reported outcomes by year and stratum are presented in Appendix 1, Tables 54–56.
FIGURE 7.
Primary participant reported outcome: OHRQoL (OHIP-14; mean and standard error) by randomised allocation and risk stratum. (a) Eligible for 24-month recall; and (b) ineligible for 24-month recall.
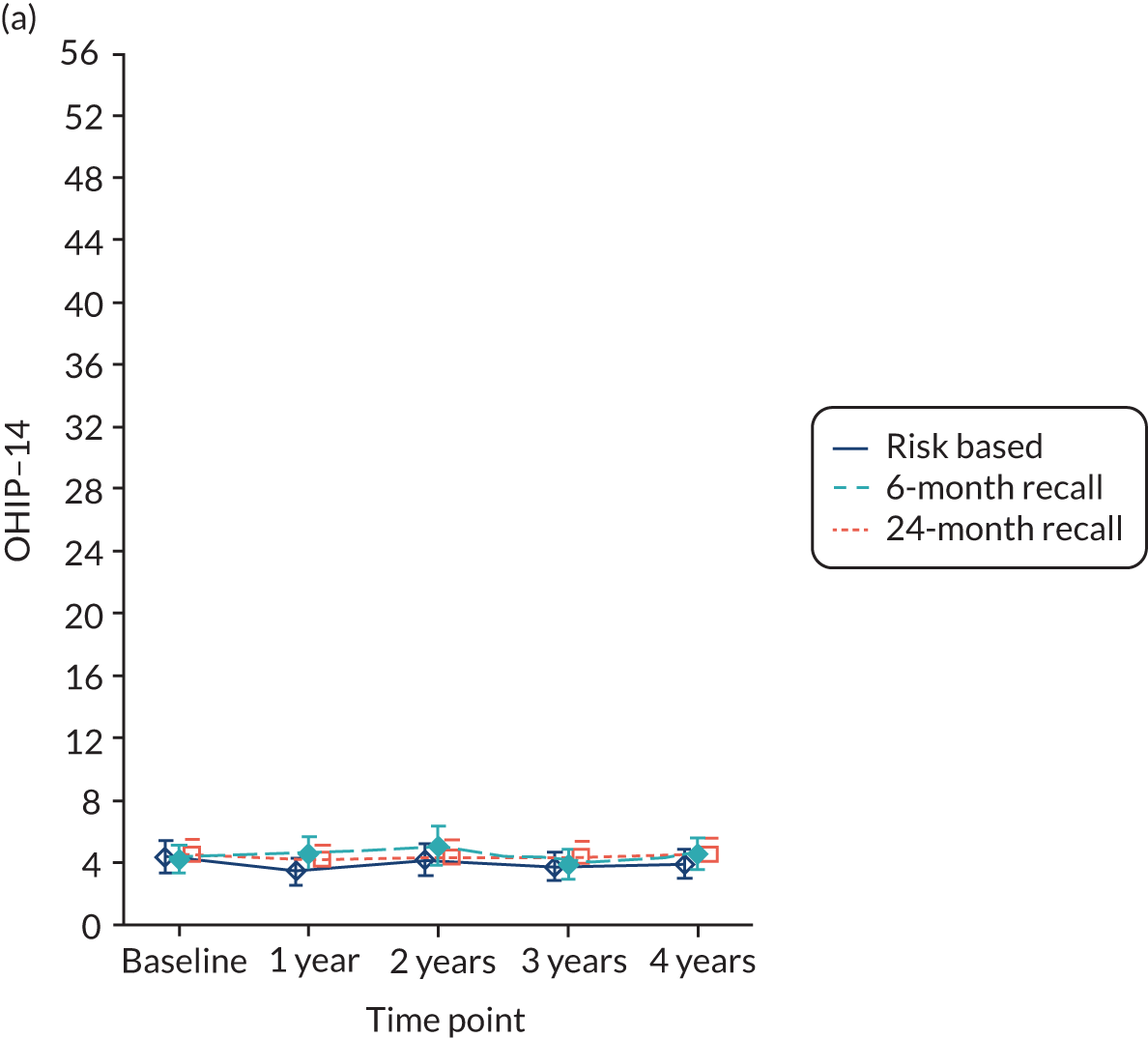
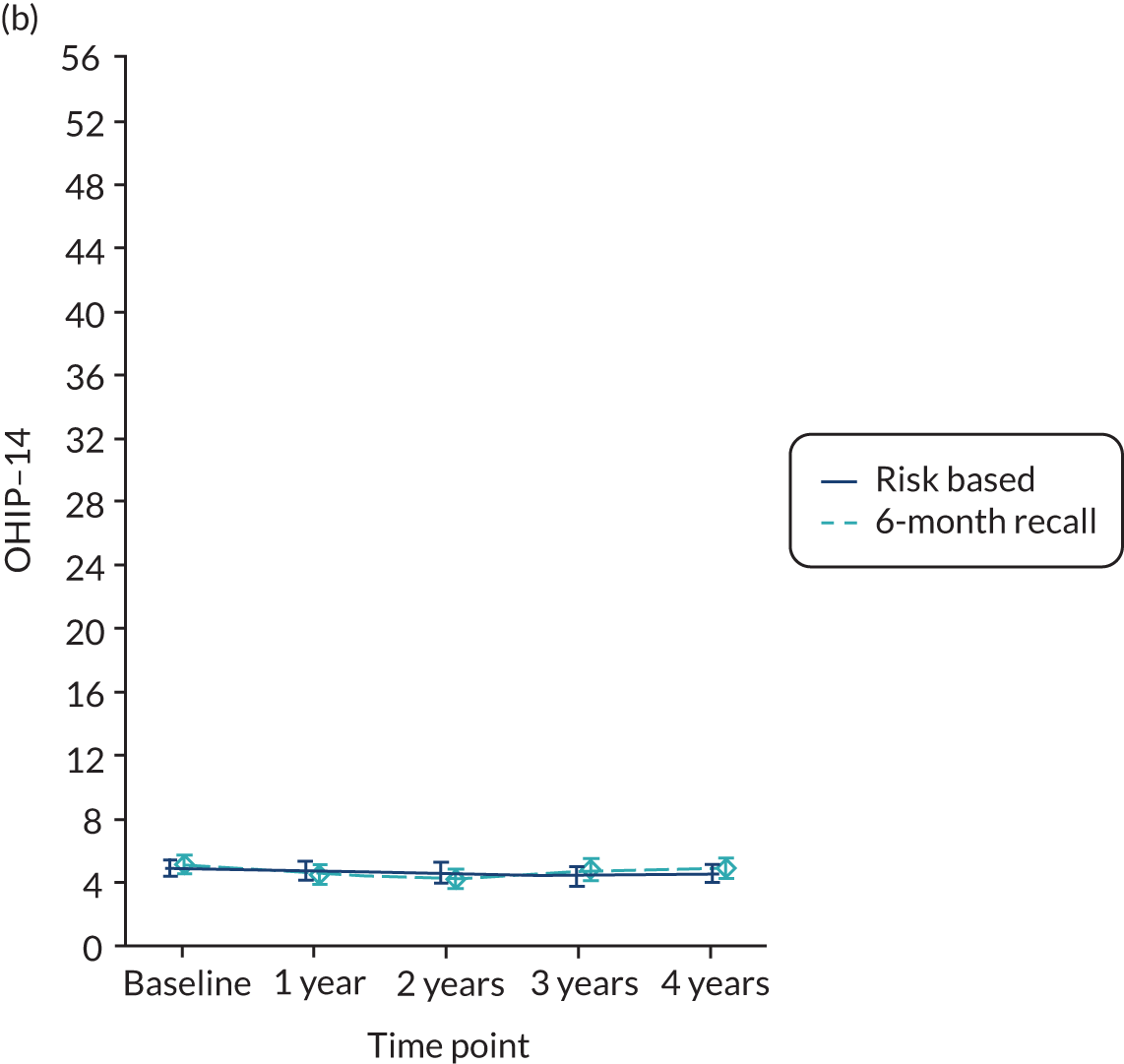
FIGURE 8.
Anxiety (mean and standard error) by randomised allocation and risk stratum. (a) Eligible for 24-month recall; and (b) ineligible for 24-month recall.
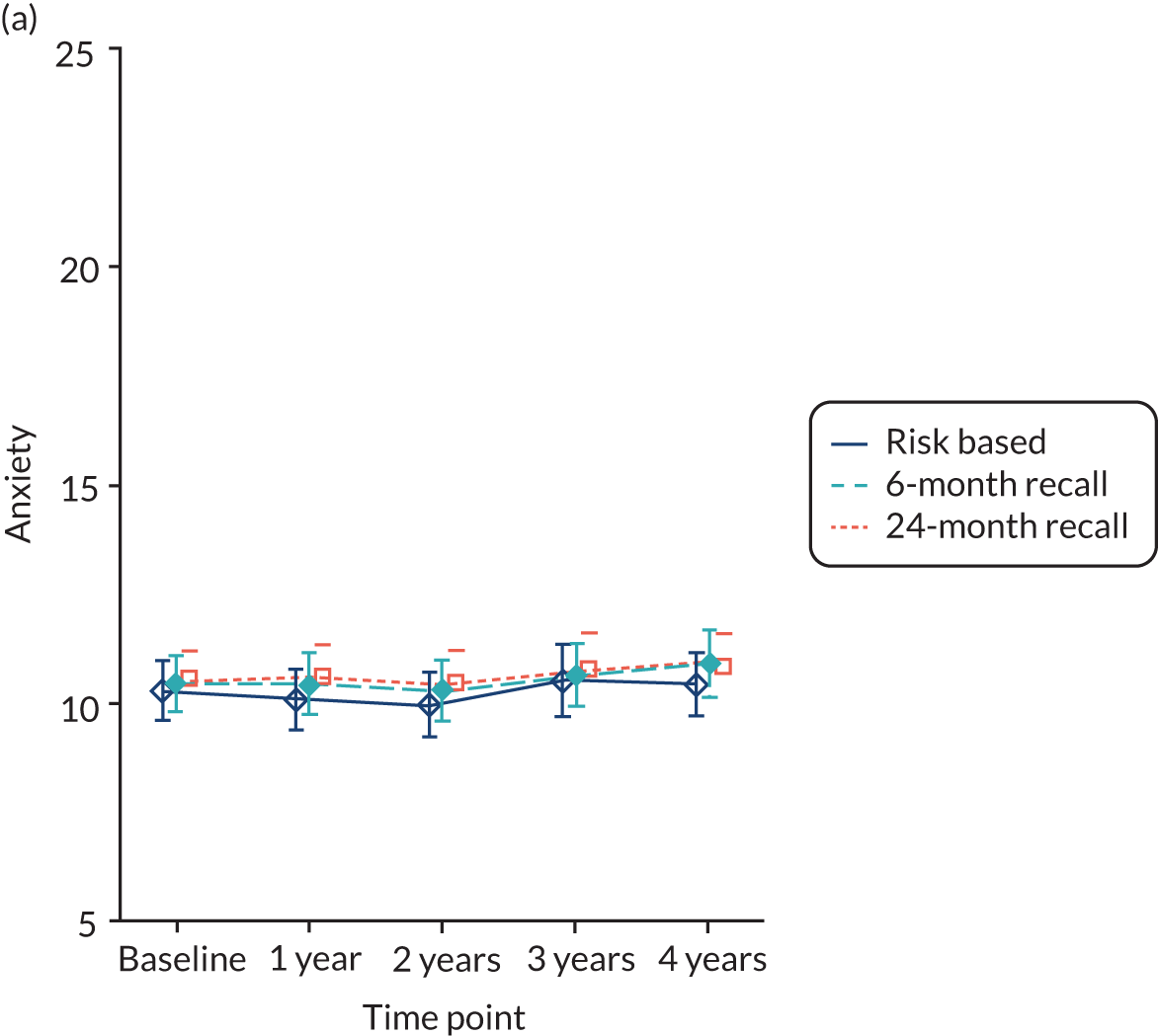
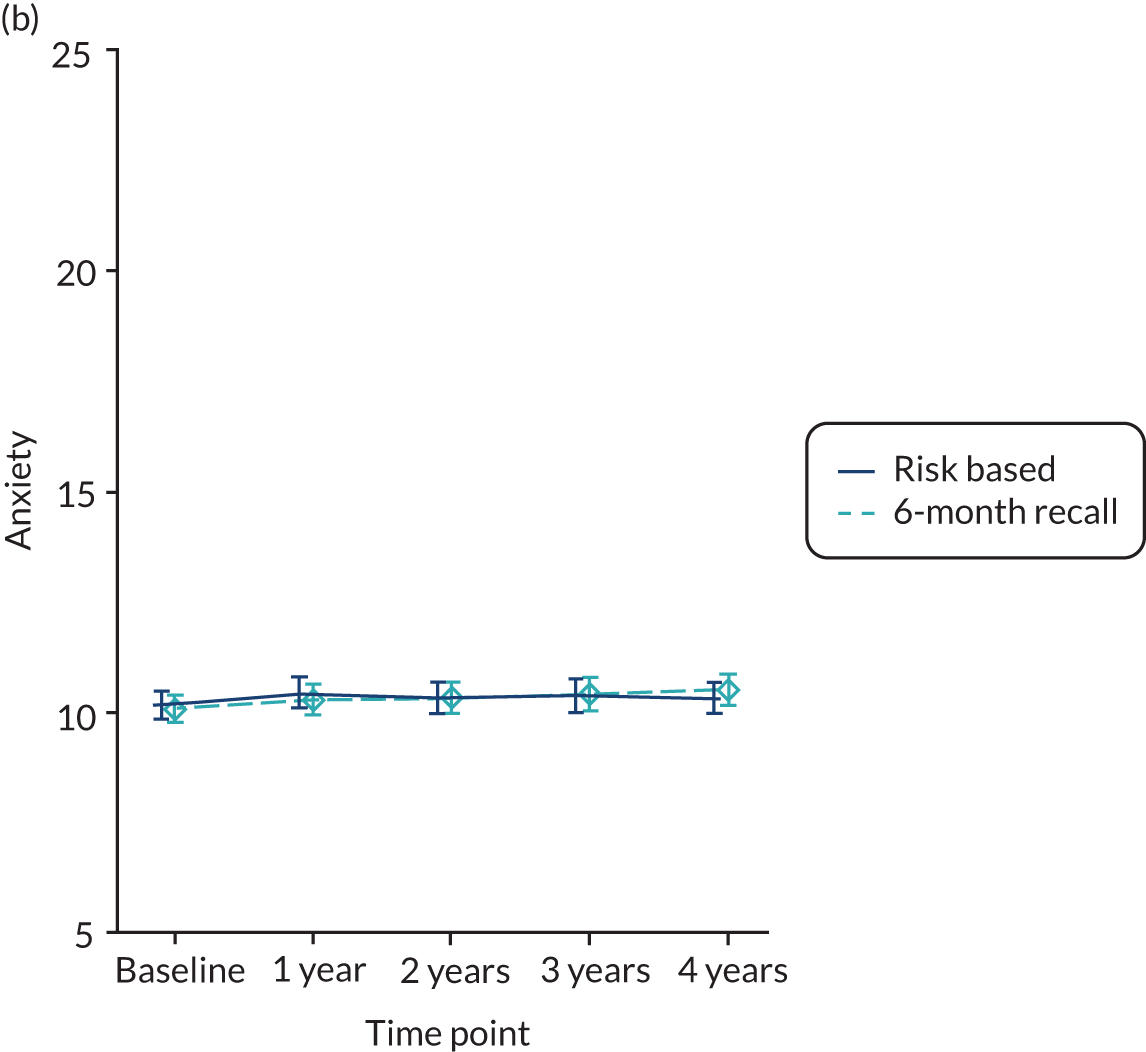
FIGURE 9.
Attitude scale (mean and standard error) by randomised allocation and risk stratum. (a) Eligible for 24-month recall; and (b) ineligible for 24-month recall.
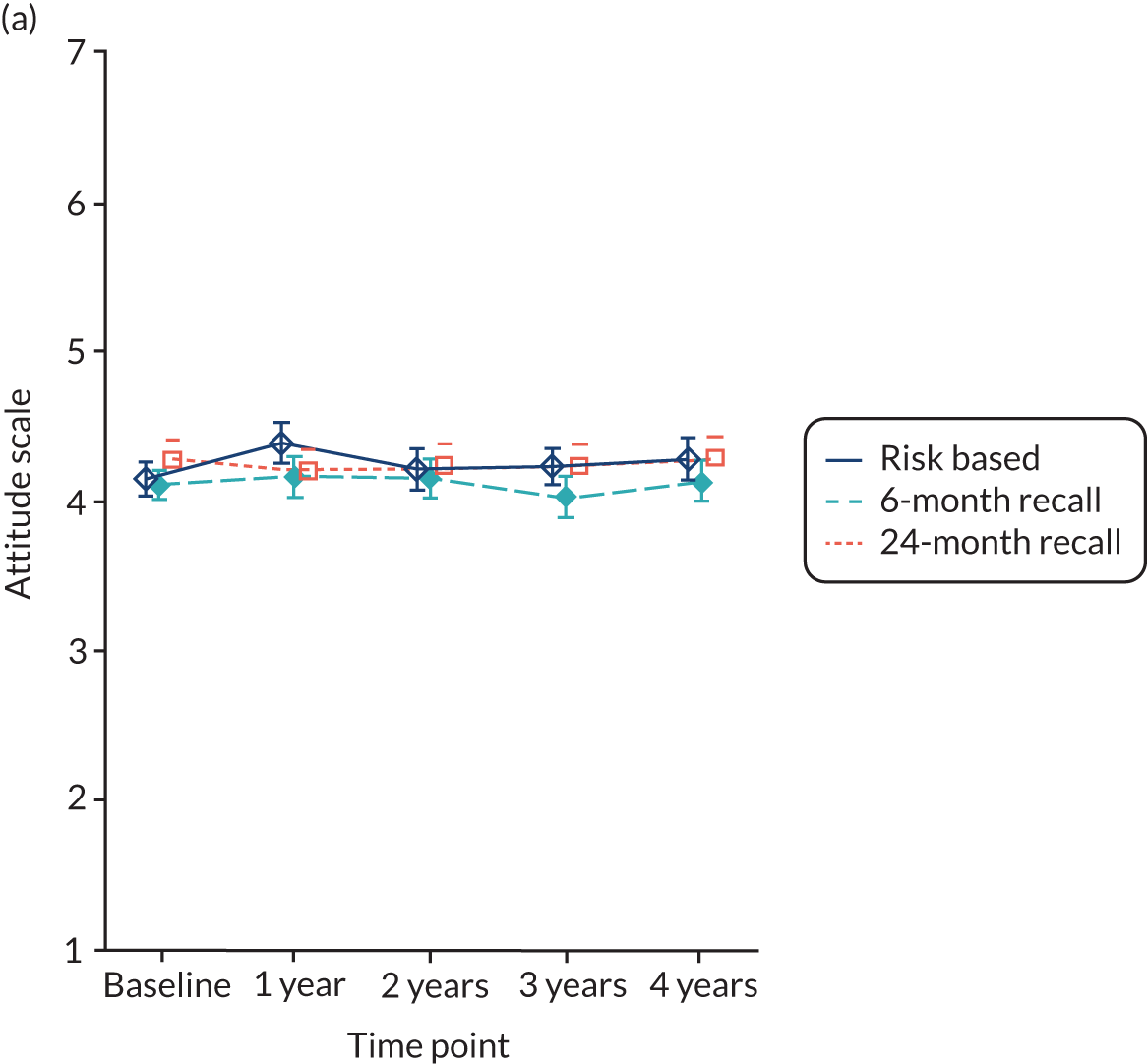
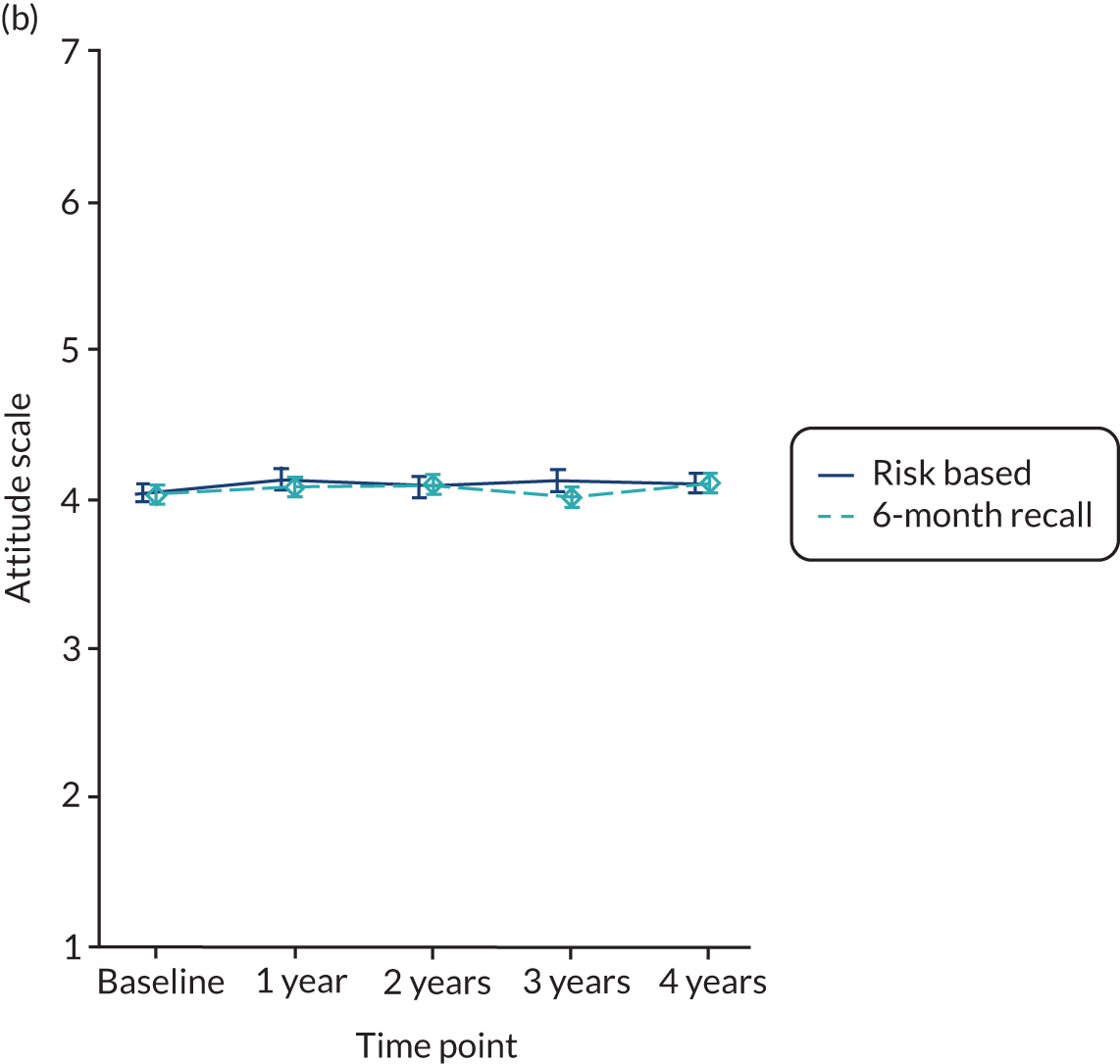
FIGURE 10.
Satisfaction scale (mean and standard error) by randomised allocation and risk stratum. (a) Eligible for 24-month recall; and (b) ineligible for 24-month recall.
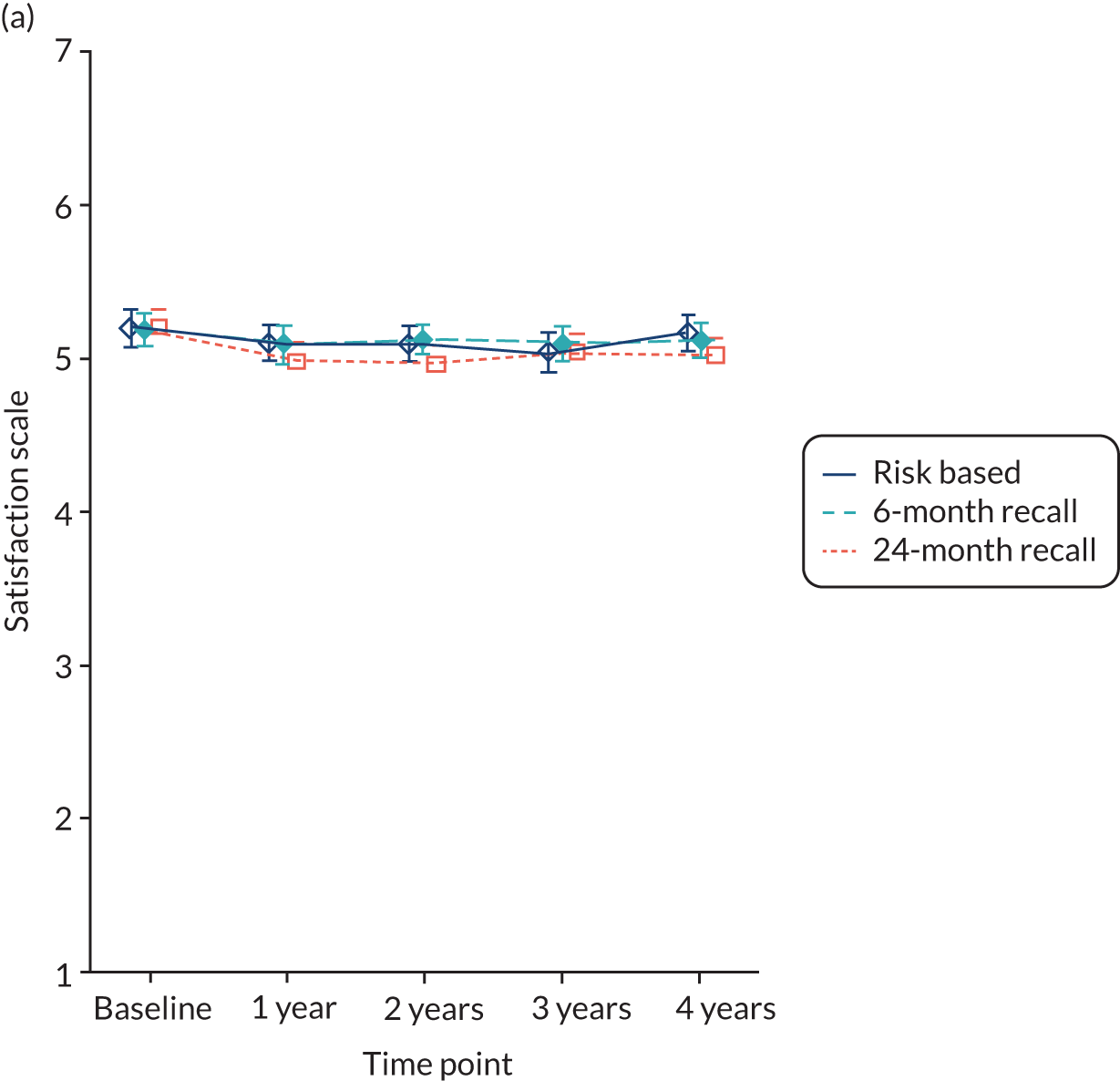
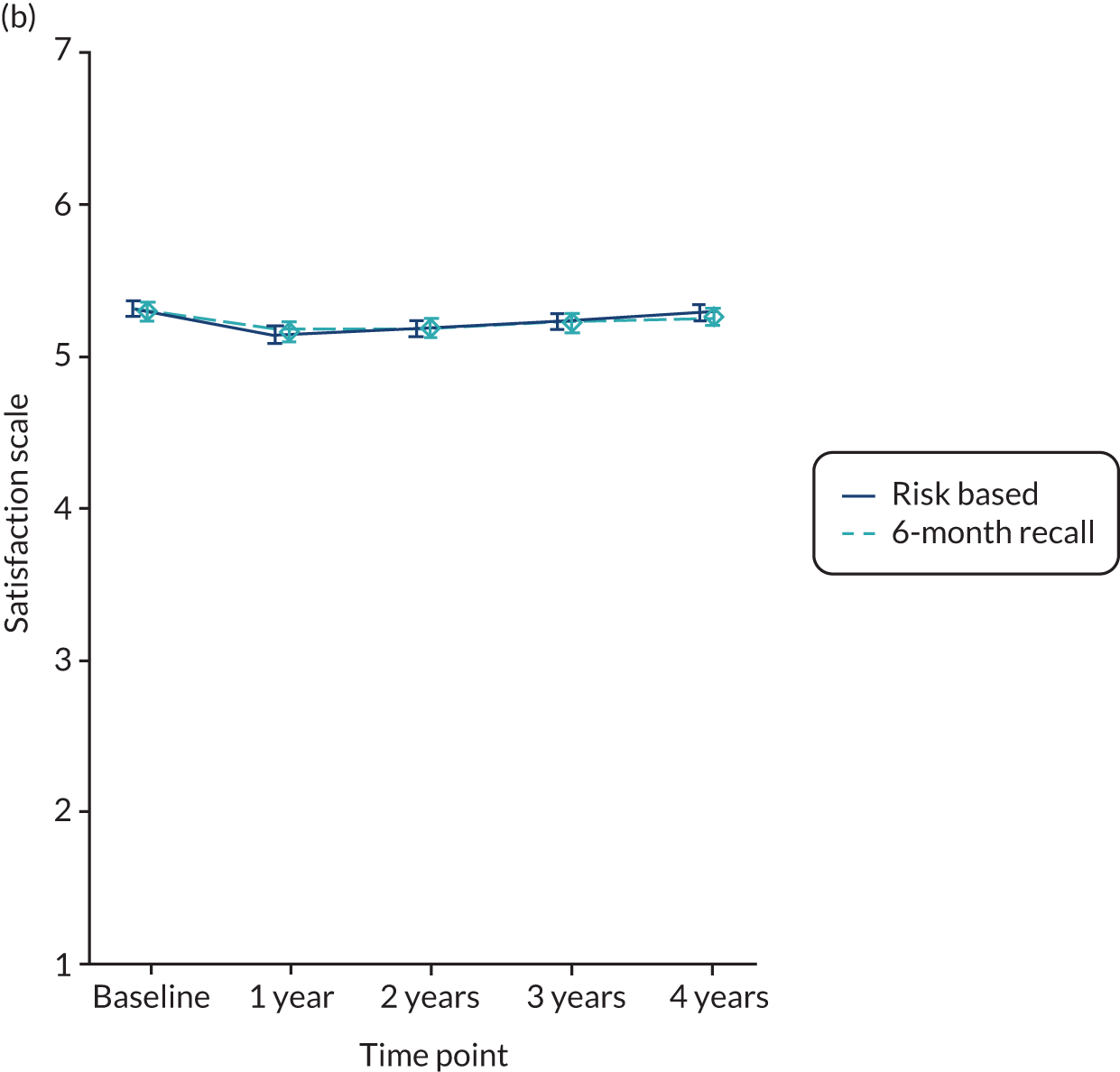
Statistical analyses of patient-reported outcomes
Table 15 presents the treatment effects for the patient-reported outcomes. There was no evidence of a difference across comparisons for oral health impacts (OHIP-14), the patient-reported primary outcome, with adjusted treatment differences smaller than one-quarter of a SD. Overall, there were no important differences between the groups across all the patient-reported outcomes and in the eligible for 24-month recall stratum and overall samples (eligible and ineligible strata).
| Outcome/status | Comparator | Effect size (95% CI); p-value |
|---|---|---|
| Eligible for 24-month recall stratum | ||
| Primary patient-reported outcome | ||
| OHIP-14 | Risk based vs. 6 month | –0.61 (–1.93 to 0.71); 0.37 |
| 24 month vs. 6 month | –0.24 (–1.55 to 1.07); 0.72 | |
| 24 month vs. risk based | 0.37 (–0.95 to 1.69); 0.58 | |
| Secondary patient-reported outcomes | ||
| Anxiety | Risk based vs. 6 month | –0.31 (–1.22 to 0.61); 0.51 |
| 24 month vs. 6 month | 0.02 (–0.90 to 0.93); 0.97 | |
| 24 month vs. risk based | 0.32 (–0.60 to 1.24); 0.49 | |
| Attitude | Risk based vs. 6 month | 0.15 (–0.03 to 0.32); 0.11 |
| 24 month vs. 6 month | 0.17 (–0.01 to 0.35); 0.06 | |
| 24 month vs. risk based | 0.02 (–0.15 to 0.20); 0.79 | |
| Behaviour | Risk based vs. 6 month | 0.01 (–0.02 to 0.04); 0.53 |
| 24 month vs. 6 month | 0.00 (–0.03 to 0.03); 0.89 | |
| 24 month vs. risk based | –0.01 (–0.04 to 0.02); 0.62 | |
| Knowledge | Risk based vs. 6 month | –0.03 (–0.06 to 0.01); 0.10 |
| 24 month vs. 6 month | –0.01 (–0.05 to 0.02); 0.42 | |
| 24 month vs. risk based | 0.01 (–0.02 to 0.05); 0.41 | |
| PBC | Risk based vs. 6 month | 0.30 (–0.02 to 0.61); 0.06 |
| 24 month vs. 6 month | 0.09 (–0.22 to 0.40); 0.59 | |
| 24 month vs. risk based | –0.21 (–0.52 to 0.10); 0.19 | |
| Satisfaction | Risk based vs. 6 month | 0.05 (–0.10 to 0.20); 0.51 |
| 24 month vs. 6 month | –0.11 (–0.26 to 0.04); 0.16 | |
| 24 month vs. risk based | –0.16 (–0.31 to –0.01); 0.04 | |
| Self-reported bleeding | Risk based vs. 6 month | –0.16 (–0.37 to 0.06); 0.15 |
| 24 month vs. 6 month | –0.22 (–0.43 to –0.01); 0.04 | |
| 24 month vs. risk based | –0.06 (–0.28 to 0.15); 0.57 | |
| EQ-5D-3L | Risk based vs. 6 month | 0.032 (–0.013 to 0.076); 0.165 |
| 24 month vs. 6 month | 0.024 (–0.021 to 0.069); 0.290 | |
| 24 month vs. risk based | –0.008 (–0.053 to 0.037); 0.741 | |
| Overall sample (eligible and ineligible for 24-month recall) | ||
| Primary patient-reported outcome | ||
| OHIP-14 | Risk based vs. 6 month | –0.35 (–1.02 to 0.32); 0.30 |
| Secondary patient-reported outcomes | ||
| Anxiety | Risk based vs. 6 month | –0.11 (–0.52 to 0.29); 0.59 |
| Attitude | Risk based vs. 6 month | 0.04 (–0.04 to 0.11); 0.38 |
| Behaviour | Risk based vs. 6 month | 0.00 (–0.01 to 0.02); 0.52 |
| Knowledge | Risk based vs. 6 month | –0.01 (–0.03 to 0.00); 0.15 |
| PBC | Risk based vs. 6 month | 0.06 (–0.08 to 0.20); 0.38 |
| Satisfaction | Risk based vs. 6 month | 0.03 (–0.03 to 0.09); 0.31 |
| Self-reported bleeding | Risk based vs. 6 month | –0.02 (–0.12 to 0.08); 0.72 |
| EQ-5D-3L | Risk based vs. 6 month | 0.008 (–0.012 to 0.029); 0.432 |
Subgroup analyses
Bleeding on probing outcome
Figures 11 and 12 show the means and 99% CIs for the differences in bleeding at 4 years in the subgroups for recall frequency and strata, respectively. There was no evidence of treatment heterogeneity by age category or by payment for treatment at the 1% level (see Appendix 1, Table 52). There was also no evidence of treatment heterogeneity for country in the overall sample (eligible for 24-month recall stratum and ineligible for 24-month recall stratum); however, in the eligible for a 24-month recall stratum, participants in England who were randomised to a 6-month recall showed a significant improvement compared with those randomised to a risk-based recall (mean difference 4.98, 95% CI 1.14 to 8.83; p < 0.001).
FIGURE 11.
Subgroup results for recall allocation in the eligible for 24-month recall stratum for bleeding on probing: difference between arms by subgroup.
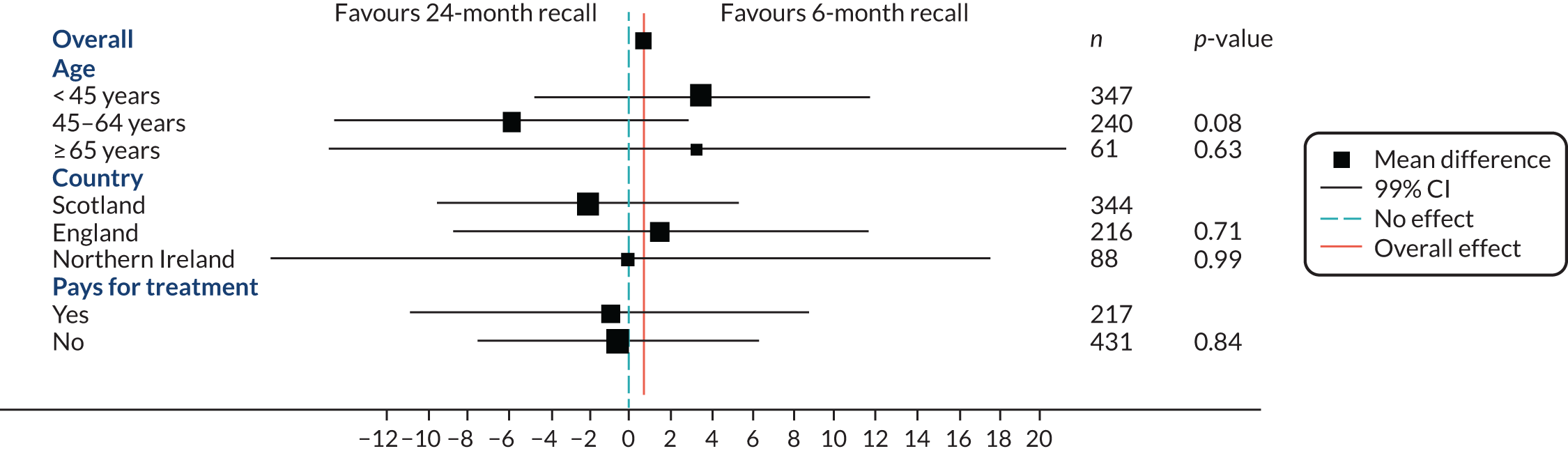
FIGURE 12.
Subgroup results for recall allocation in the overall sample (eligible and ineligible strata) for bleeding on probing: difference between arms by subgroup. RB, risk-based.
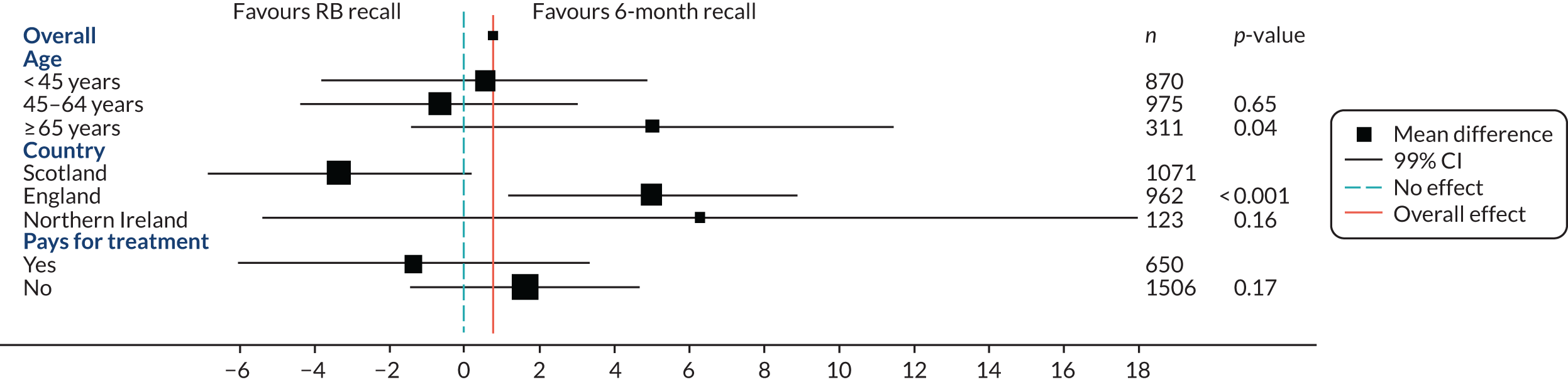
Caries outcome (post hoc subgroup analyses)
Figures 13 and 14 show the odds ratios and 99% CIs for caries at 4 years, comparing by recall frequency and by stratum. For this purpose, caries were grouped into two categories: sound surfaces/initial lesions (n = 303) versus moderate or serious lesions (n = 1313). There was no evidence of treatment heterogeneity by age category, country or payment for treatment at the 1% level; in the overall sample in England, patients randomised to 6-month recall were less likely to have serious carious lesions than those randomised to risk-based recall (odds ratio 1.66, 95% CI 0.98 to 2.81; p = 0.01). Interaction coefficients are shown in Appendix 1, Table 53.
FIGURE 13.
Subgroup results for recall allocation in the eligible for 24-month recall stratum for caries: odds ratio by subgroup (CIs with cap have been truncated to fit the plot). RB, risk based.
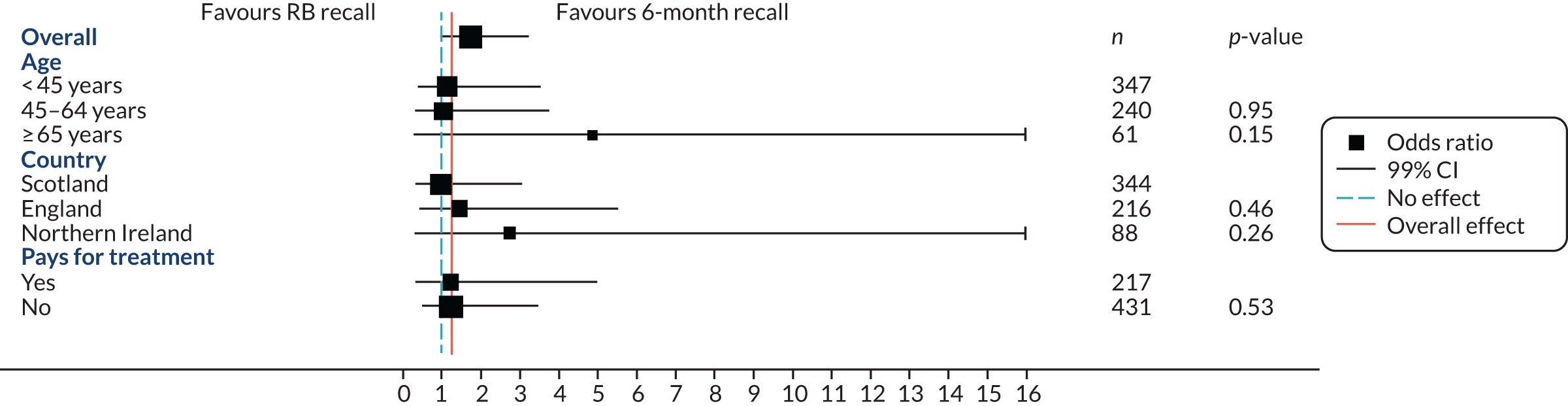
FIGURE 14.
Subgroup results for recall allocation in the overall sample (eligible and ineligible strata) for caries: odds ratio by subgroup. RB, risk based.
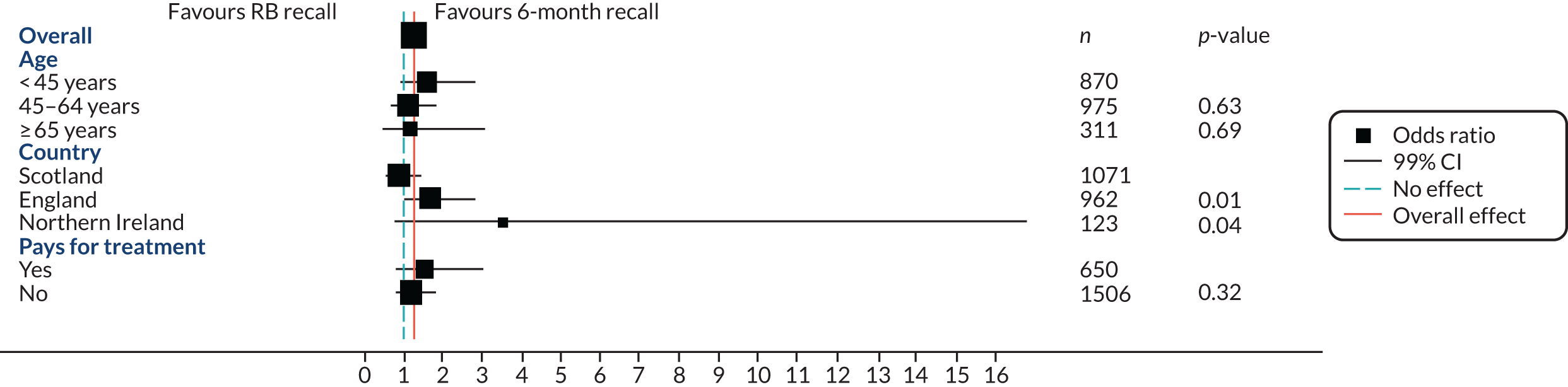
Service providers’ measures
Baseline
Table 16 provides dentists’ and practices’ characteristics at baseline, for dentists who replied to both the baseline questionnaire and the follow-up questionnaire (out of the 68 responders, 49 replied to both questionnaires, 16 to the baseline questionnaire only and three to the follow-up questionnaire only). Most practices had one dentist responding. About 55% of the respondents were male, had a median of 16 years being qualified as a dentist and 69% were principal dentists or practice owners. The median practice list size was 5000 with considerable variability. Most of the dentists considered their practices to be non-rural (86%). There was a median of three other dentists working in the practice and five nurses. Most of the practices had a mixture of NHS and private patients (65%).
| Characteristic | Median (percentile 25 – percentile 75), count or n (%) |
|---|---|
| Number of dentist respondents | 49 |
| Gender | |
| Male | 27 (55.1) |
| Female | 21 (42.9) |
| Missing | 1 (2.0) |
| How long have you been qualified as a dentist? | 16.0 (10.0–23.0), 49 |
| Within this practice are you | |
| Principal/practice owner | 34 (69.4) |
| Associate | 10 (20.4) |
| Other | 5 (10.2) |
| What is your approximate total practice list size? | 5000.0 (2400.0–8000.0), 41 |
| Do you consider your practice to be rural/remote? | |
| Yes | 5 (10.2) |
| No | 42 (85.7) |
| Missing | 2 (4.1) |
| How many other members of the dental team in the practice are dentists? | 3.0 (2.0–4.0), 46 |
| How many other members of the dental team in the practice are nurses? | 5.0 (3.0–6.0), 46 |
| How many other members of the dental team in the practice are hygienists? | 1.0 (0.0–1.0), 25 |
| Is your practice | |
| NHS patients only | 16 (32.7) |
| Mixture of NHS and private | 32 (65.3) |
| Missing | 1 (2.0) |
| Before this study, how did you usually set your patient recall appointments? | |
| Routinely, every 6 months | 29 (59.2) |
| Other | 15 (30.6) |
| Missing | 5 (10.2) |
Follow-up
Table 17 presents dentists’ beliefs at baseline and at follow-up, and their change from baseline. Dentists’ attitudes regarding a 24-month recall improved, as did their attitude regarding a 6-month recall. Overall, their general attitude about the patients’ ability to assess risk and perceived ability to assess risk decreased from baseline. However, dentists who deemed at least one patient eligible for the 24-month recall (n = 40) increased slightly in their perceived ability to judge risk, whereas those who did not consider any patient eligible for a 24-month recall (n = 6) decreased their perceived ability. Appendix 1, Table 57, presents more details about dentists’ beliefs by whether or not the dentist deemed at least one of their patients eligible for a 24-month recall.
| Dentists’ beliefs | Baseline, mean (SD), n | Follow-up, mean (SD), n | Change from baseline (follow-up–baseline), mean (SD), n |
|---|---|---|---|
| Attitude regarding a 24-month recall | 3.8 (1.2), 46 | 4.4 (1.2), 48 | 0.6 (1.1), 45 |
| Attitude regarding a 6-month recall | 3.7 (1.0), 46 | 4.0 (1.2), 48 | 0.2 (0.9), 45 |
| General attitude to the patient‘s ability to maintain self-care | 4.6 (1.1), 46 | 4.0 (1.1), 48 | –0.6 (1.4), 45 |
| Perceived ability to assess risk | 4.1 (0.9), 46 | 4.1 (1.1), 48 | –0.1 (1.2), 45 |
Chapter 5 Health economics results
Introduction
This chapter reports the results of the within-trial health economic analysis (4-year follow-up). Descriptive statistics for resource use, costs and outcomes (QALYs, WTP) are presented separately by randomised stratum as follows: [eligible for 24-month recall (24 month; risk based; 6 month) and ineligible for 24-month recall (risk based; 6 month)] using complete-case data. The chapter then reports the findings of the online general population DCE. The economic evaluation results, combining incremental costs and benefits, are then reported under the three different evaluation frameworks (see Chapter 3) based on multiple imputation of missing data. A range of alternative scenario analyses are provided to investigate the impact of different costing perspectives and methodological issues on the results.
Resource use and costs
Resource use and costs are reported for NHS dental care, NHS other care and participants. This section details descriptive statistics from complete-case data. The reader is referred to Appendix 2, Table 59, for descriptive statistics based on multiple imputation of missing data.
Costs of NHS dental services
NHS dental care costs are based on routinely collected dental claims in the four different UK regions. Data were successfully linked for n = 1121/1188 (94%), n = 932/1031 (90%), n = 143/153 (93%) and collected from practice notes for 13/13 (100%) participants in Scotland, England, Northern Ireland and Wales, respectively. Given the high linkage rate, missing dental claims data were not considered to have an important impact on cost. Overall, n = 552/2209 (25%) participants with dental claims data were exempt from payment of dental charges for the majority of treatment claims. The proportion of participants exempt from charges was evenly balanced across the randomised groups. For the ineligible for 24-month recall stratum, 186/861 (22%) and 161/863 (19%) were exempt in the risk-based and 6-month recall groups, respectively. For the stratum where participants were eligible for 24-month recall, 74/217 (34%), 57/215 (27%) and 74/216 (34%) were exempt from charges in the risk-based, 6-month and 24-month recall groups, respectively. There are no statistically significant differences in the proportions exempt across groups, providing reassurance that any treatment effects on NHS costs are not biased by eligibility for fully funded NHS care. Table 18 reports details of treatment claims and costs to the dental budgets of the respective regions.
| Category | Ineligible | Eligible | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk based | 6 month | Risk based | 6 month | 24 month | ||||||||||||||||||||||||||
| Resource use | Cost | Resource use | Cost | Resource use | Cost | Resource use | Cost | Resource use | Cost | |||||||||||||||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | |
| UK-wide data: total NHS dental costs | ||||||||||||||||||||||||||||||
| Check-ups | 4.53 | 2.48 | 800 | – | – | – | 4.84 | 2.23 | 804 | – | – | – | 3.44 | 2.14 | 201 | – | – | – | 4.32 | 3.42 | 201 | – | – | – | 2.38 | 2.19 | 203 | – | – | – |
| Fillings | 1.63 | 1.95 | 800 | – | – | – | 1.48 | 1.90 | 804 | – | – | – | 1.21 | 1.93 | 201 | – | – | – | 1.29 | 1.71 | 201 | – | – | – | 1.14 | 1.69 | 203 | – | – | – |
| Extractions | 0.22 | 0.54 | 800 | – | – | – | 0.20 | 0.54 | 804 | – | – | – | 0.07 | 0.26 | 201 | – | – | – | 0.21 | 0.70 | 201 | – | – | – | 0.17 | 0.47 | 203 | – | – | – |
| Dental budget costs | – | – | – | £130 | £188 | 800 | – | – | – | £113 | £149 | 804 | – | – | – | £97 | £174 | 201 | – | – | – | £96 | £214 | 201 | – | – | – | £80 | £146 | 203 |
| UK-wide data: other NHS resource use | ||||||||||||||||||||||||||||||
| GP consultations | 0.76 | 1.76 | 371 | £29 | £66 | 371 | 0.80 | 1.58 | 393 | £30 | £60 | 393 | 0.94 | 1.51 | 69 | £36 | £57 | 69 | 0.73 | 1.36 | 80 | £27 | £51 | 80 | 0.81 | 1.99 | 77 | £30 | £75 | 77 |
| Inpatient admissions | 1.61 | 12.51 | 432 | £7 | £67 | 425 | 1.10 | 10.33 | 455 | £9 | £78 | 450 | 0.01 | 0.11 | 85 | £8 | £74 | 85 | 1.04 | 10.05 | 97 | £14 | £97 | 96 | 2.98 | 16.97 | 100 | £7 | £71 | 97 |
| Outpatient consultations | 0.03 | 0.29 | 374 | £5 | £42 | 374 | 0.08 | 0.52 | 400 | £12 | £77 | 400 | 0.01 | 0.12 | 69 | £2 | £18 | 69 | 0.05 | 0.22 | 82 | £7 | £30 | 82 | 0.03 | 0.16 | 78 | £3 | £22 | 78 |
| Accident and emergency attendances | 0.01 | 0.10 | 376 | £1 | £13 | 376 | 0.01 | 0.07 | 399 | £1 | £10 | 399 | 0.00 | 0.00 | 68 | £0 | £0 | 68 | 0.01 | 0.11 | 82 | £1 | £13 | 82 | 0.00 | 0.00 | 78 | £0 | £0 | 78 |
| Total (other NHS resource use) | £34 | £96 | 331 | £46 | £116 | 355 | £49 | £106 | 55 | £50 | £112 | 69 | £24 | £49 | 66 | |||||||||||||||
| Total NHS costs (dental + other) | £136 | £164 | 323 | £147 | £209 | 347 | £114 | £130 | 54 | £131 | £169 | 69 | £90 | £143 | 66 | |||||||||||||||
| NHS dental costs: Scotland | ||||||||||||||||||||||||||||||
| Diagnosis | 4.75 | 2.31 | 393 | £34 | £16 | 393 | 5.22 | 2.16 | 400 | £38 | £14 | 400 | 3.79 | 2.38 | 107 | £27 | £17 | 107 | 4.87 | 2.19 | 111 | £36 | £15 | 111 | 3.10 | 2.84 | 110 | £21 | £20 | 110 |
| Check-ups (1A 1B 1C) | 3.59 | 1.69 | 393 | – | – | – | 4.09 | 1.59 | 400 | – | – | – | 2.82 | 1.78 | 107 | – | – | – | 3.88 | 1.62 | 111 | – | – | – | 2.10 | 2.05 | 110 | – | – | – |
| Periodontal | 3.06 | 2.56 | 393 | £15 | £26 | 393 | 3.10 | 2.52 | 400 | £14 | £20 | 400 | 2.47 | 2.85 | 107 | £15 | £26 | 107 | 2.80 | 2.38 | 111 | £14 | £24 | 111 | 1.64 | 2.11 | 110 | £10 | £22 | 110 |
| Conservative | 2.08 | 2.57 | 393 | £20 | £44 | 393 | 2.20 | 2.98 | 400 | £20 | £59 | 400 | 1.71 | 2.66 | 107 | £28 | £69 | 107 | 1.86 | 2.25 | 111 | £22 | £67 | 111 | 1.64 | 2.72 | 110 | £21 | £71 | 110 |
| Fillings (14A, 14C1, 14C2) | 1.47 | 1.92 | 393 | – | – | – | 1.58 | 2.18 | 400 | – | – | – | 1.25 | 2.15 | 107 | – | – | – | 1.35 | 1.64 | 111 | – | – | – | 1.20 | 1.77 | 110 | – | – | – |
| Surgical | 0.45 | 1.09 | 393 | £2 | £7 | 393 | 0.39 | 1.03 | 400 | £1 | £4 | 400 | 0.16 | 0.53 | 107 | £0 | £2 | 107 | 0.32 | 0.87 | 111 | £1 | £7 | 111 | 0.45 | 1.07 | 110 | £2 | £6 | 110 |
| Extractions (21/22) | 0.22 | 0.52 | 393 | – | – | – | 0.19 | 0.49 | 400 | – | – | – | 0.07 | 0.26 | 107 | – | – | – | 0.14 | 0.40 | 111 | – | – | – | 0.22 | 0.51 | 110 | – | – | – |
| Prosthesis | 0.25 | 0.79 | 393 | £7 | £29 | 393 | 0.26 | 0.85 | 400 | £5 | £24 | 400 | 0.34 | 1.25 | 107 | £8 | £40 | 107 | 0.11 | 0.51 | 111 | £3 | £22 | 111 | 0.13 | 0.47 | 110 | £4 | £20 | 110 |
| Orthodontic | 0.00 | 0.00 | 393 | £0 | £0 | 393 | 0.00 | 0.05 | 400 | £0 | £1 | 400 | 0.00 | 0.00 | 107 | £0 | £0 | 107 | 0.00 | 0.00 | 111 | £0 | £0 | 111 | 0.00 | 0.00 | 110 | £0 | £0 | 110 |
| Other | 1.09 | 2.52 | 393 | £1 | £5 | 393 | 0.81 | 1.69 | 400 | £1 | £4 | 400 | 1.65 | 4.15 | 107 | £2 | £7 | 107 | 1.07 | 2.37 | 111 | £1 | £4 | 111 | 1.06 | 2.42 | 110 | £2 | £6 | 110 |
| Total Scotland | £79 | £85 | 393 | £79 | £79 | 400 | £80 | £130 | 107 | £77 | £98 | 111 | £59 | £114 | 110 | |||||||||||||||
| NHS dental costs: Northern Ireland | ||||||||||||||||||||||||||||||
| Diagnosis | 6.33 | 2.60 | 30 | £17 | £19 | 30 | 7.33 | 2.63 | 30 | £19 | £14 | 30 | 4.54 | 2.94 | 28 | £13 | £15 | 28 | 4.96 | 3.00 | 26 | £16 | £19 | 26 | 3.79 | 2.78 | 29 | £16 | £17 | 29 |
| Check-ups (101/111) | 5.07 | 1.80 | 30 | – | – | – | 5.83 | 2.00 | 30 | – | – | – | 3.46 | 2.15 | 28 | – | – | – | 3.92 | 2.62 | 26 | – | – | – | 2.93 | 2.03 | 29 | – | – | – |
| Periodontal | 3.33 | 2.54 | 30 | £15 | £20 | 30 | 4.80 | 2.30 | 30 | £23 | £19 | 30 | 2.21 | 2.23 | 28 | £12 | £20 | 28 | 2.23 | 2.07 | 26 | £14 | £20 | 26 | 2.34 | 2.04 | 29 | £20 | £25 | 29 |
| Conservative | 3.10 | 3.57 | 30 | £53 | £129 | 30 | 2.93 | 4.31 | 30 | £43 | £85 | 30 | 2.29 | 3.02 | 28 | £32 | £61 | 28 | 2.42 | 2.93 | 26 | £28 | £46 | 26 | 2.48 | 3.18 | 29 | £35 | £70 | 29 |
| Fillings (1401–04; 1421; 1426) | 2.20 | 2.31 | 30 | – | – | – | 1.57 | 2.11 | 30 | – | – | – | 1.57 | 1.83 | 28 | – | – | – | 1.69 | 1.95 | 26 | – | – | – | 1.76 | 2.01 | 29 | – | – | – |
| Surgical | 0.53 | 1.17 | 30 | £5 | £23 | 30 | 0.40 | 0.77 | 30 | £2 | £5 | 30 | 0.14 | 0.45 | 28 | £1 | £3 | 28 | 0.35 | 0.75 | 26 | £3 | £11 | 26 | 0.10 | 0.41 | 29 | £2 | £9 | 29 |
| Extractions (2101) | 0.20 | 0.48 | 30 | – | – | – | 0.13 | 0.35 | 30 | – | – | – | 0.04 | 0.19 | 28 | – | – | – | 0.15 | 0.37 | 26 | – | – | – | 0.03 | 0.19 | 29 | – | – | – |
| Prosthesis | 0.20 | 0.76 | 30 | £5 | £16 | 30 | 0.17 | 0.53 | 30 | £4 | £14 | 30 | 0.00 | 0.00 | 28 | £0 | £0 | 28 | 0.00 | 0.00 | 26 | £0 | £0 | 26 | 0.41 | 1.38 | 29 | £10 | £30 | 29 |
| Orthodontic | 0.00 | 0.00 | 30 | £0 | £0 | 30 | 0.00 | 0.00 | 30 | £0 | £0 | 30 | 0.00 | 0.00 | 28 | £0 | £0 | 28 | 0.00 | 0.00 | 26 | £0 | £0 | 26 | 0.00 | 0.00 | 29 | £0 | £0 | 29 |
| Other | 0.20 | 0.48 | 30 | £1 | £3 | 30 | 0.83 | 1.09 | 30 | £2 | £4 | 30 | 0.32 | 0.98 | 28 | £1 | £2 | 28 | 0.27 | 0.60 | 26 | £1 | £2 | 26 | 0.21 | 0.49 | 29 | £1 | £2 | 29 |
| Total Northern Ireland | – | – | – | £96 | £186 | 30 | – | – | – | £94 | £117 | 30 | – | – | – | £58 | £83 | 28 | – | – | – | £62 | £88 | 26 | – | – | – | £85 | £120 | 29 |
| NHS dental costs: England/Wales | ||||||||||||||||||||||||||||||
| Band 1 | 3.37 | 2.39 | 377 | £29 | £28 | 377 | 3.87 | 2.57 | 374 | £30 | £33 | 374 | 3.17 | 1.98 | 66 | £25 | £24 | 66 | 3.86 | 3.01 | 64 | £30 | £38 | 64 | 1.56 | 1.45 | 64 | £14 | £20 | 64 |
| Check-up | 5.46 | 2.83 | 377 | – | – | – | 5.56 | 2.54 | 374 | – | – | – | 4.44 | 2.32 | 66 | – | – | – | 5.23 | 5.34 | 64 | – | – | – | 2.61 | 2.45 | 64 | – | – | – |
| Band 2 | 1.95 | 2.04 | 377 | £76 | £106 | 377 | 1.52 | 1.58 | 374 | £52 | £75 | 374 | 1.20 | 1.60 | 66 | £55 | £98 | 66 | 1.30 | 2.29 | 64 | £54 | £146 | 64 | 1.05 | 1.88 | 64 | £56 | £132 | 64 |
| Fillings | 1.75 | 1.94 | 377 | – | – | – | 1.37 | 1.53 | 374 | – | – | – | 1.00 | 1.56 | 66 | – | – | – | 1.02 | 1.72 | 64 | – | – | – | 0.75 | 1.27 | 64 | – | – | – |
| Extractions | 0.23 | 0.58 | 377 | – | – | – | 0.23 | 0.59 | 374 | – | – | – | 0.09 | 0.29 | 66 | – | – | – | 0.34 | 1.10 | 64 | – | – | – | 0.14 | 0.47 | 64 | – | – | – |
| Band 3 | 0.40 | 0.78 | 377 | £68 | £172 | 377 | 0.37 | 0.76 | 374 | £56 | £143 | 374 | 0.26 | 0.69 | 66 | £49 | £173 | 66 | 0.22 | 0.55 | 64 | £45 | £143 | 64 | 0.17 | 0.42 | 64 | £32 | £88 | 64 |
| Band: urgent | 0.46 | 0.92 | 377 | £13 | £26 | 377 | 0.40 | 0.82 | 374 | £11 | £23 | 374 | 0.36 | 0.74 | 66 | £10 | £21 | 66 | 0.42 | 1.97 | 64 | £12 | £56 | 64 | 0.41 | 1.05 | 64 | £12 | £30 | 64 |
| Band: free | 0.02 | 0.32 | 377 | £1 | £9 | 377 | 0.03 | 0.22 | 374 | £1 | £6 | 374 | 0.00 | 0.00 | 66 | £0 | £0 | 66 | 0.02 | 0.13 | 64 | £0 | £4 | 64 | 0.00 | 0.00 | 64 | £0 | £0 | 64 |
| Band: other | 0.01 | 0.12 | 377 | £0 | £1 | 377 | 0.01 | 0.07 | 374 | £0 | £0 | 374 | 0.00 | 0.00 | 66 | £0 | £0 | 66 | 0.02 | 0.13 | 64 | £0 | £0 | 64 | 0.02 | 0.13 | 64 | £0 | £0 | 64 |
| Total England and Wales | – | – | – | £187 | £243 | 377 | – | – | – | £151 | £194 | 374 | – | – | – | £140 | £244 | 66 | – | – | – | £141 | £350 | 64 | – | – | – | £114 | £195 | 64 |
Resource use data are reported separately for check-ups, fillings and extractions (for the UK as a whole and at a regional level). Participants ineligible for 24-month recall incurred greater resource use than the eligible stratum. The average number of check-ups for respondents in the risk-based group was closer to the 6-month group in the ineligible stratum, but approximately mid-way between the 6-month and 24-month group in the eligible stratum. This indicates the likelihood of greater need for more frequent checks in the risk-based group in the ineligible stratum. A similar pattern of greater treatment consumption in the ineligible than the eligible stratum is also observed for other treatments such as fillings and extractions, and appears consistent across the regions.
Other NHS resource use and costs (descriptive data)
Table 18 also indicates the resource use and costs associated with care for dental health problems provided by services other than dental practices (e.g. GP consultations, inpatient admissions, outpatient consultations and accident and emergency admissions). Emergency dental consultations (with community dental practices) are included in the dental budget and, therefore, will have been picked up through the routine data linkage. There are no obvious differences in the use of non-dental NHS services for dental problems across the randomised groups; however, it should be noted that complete data regarding use of non-dental NHS services for dental problems are available for only 37% of participants (n = 876/2372). A total of 385 (16%) randomised participants (between 15% and 20% across the groups) returned no questionnaires, and so have provided no resource use data regarding the use of non-dental services for dental problems. Missing data for the costs associated with the use of other (non-dental) NHS services appear to be evenly distributed across the randomised groups, and additional logistical regression analyses do not indicate that the probability of missingness is significantly predicted by any patient characteristics or dental health outcomes. An assumption of missing completely at random is therefore plausible; however, given the high proportion of missing data, multiple imputation is undertaken to complete the data set and improve the precision of the results.
Participant costs (descriptive statistics)
Table 19 reports participant cost data, specifically payment of dental co-charges, consumer out-of-pocket expenditure on dental care (electric toothbrushes, heads and other private treatment) and the opportunity cost of time and travel incurred while attending dental appointments.
| Category | Ineligible | Eligible | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk based | 6 month | Risk based | 6 month | 24 month | |||||||||||
| Cost | Cost | Cost | Cost | Cost | |||||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | |
| Costs directly associated with attendance for NHS dental care | |||||||||||||||
| Participant co-charges | £108 | £129 | 800 | £115 | £128 | 804 | £71 | £101 | 201 | £77 | £85 | 201 | £51 | £82 | 203 |
| Scotland | £84 | £101 | 393 | £89 | £97 | 400 | £57 | £90 | 107 | £65 | £73 | 111 | £51 | £85 | 110 |
| England/Wales | £131 | £151 | 377 | £140 | £148 | 374 | £87 | £103 | 66 | £99 | £98 | 64 | £49 | £73 | 64 |
| Northern Ireland | £117 | £104 | 30 | £138 | £142 | 30 | £86 | £129 | 28 | £77 | £89 | 26 | £60 | £91 | 29 |
| Opportunity cost of time and travel | £98 | £50 | 775 | £99 | £44 | 787 | £76 | £51 | 191 | £88 | £65 | 191 | £59 | £43 | 184 |
| Scotland | £92 | £47 | 383 | £95 | £45 | 396 | £71 | £57 | 103 | £84 | £41 | 109 | £60 | £44 | 99 |
| England/Wales | £104 | £52 | 362 | £102 | £42 | 362 | £83 | £41 | 63 | £96 | £100 | 59 | £56 | £42 | 57 |
| Northern Ireland | £107 | £53 | 30 | £122 | £48 | 29 | £79 | £51 | 25 | £81 | £42 | 23 | £61 | £41 | 28 |
| Total costs (co-charges plus opportunity costs) of attending NHS dental care | £209 | £159 | 775 | £216 | £154 | 787 | £150 | £128 | 191 | £169 | £125 | 191 | £116 | £111 | 184 |
| Other participant-incurred costs | |||||||||||||||
| Private dental care | £109 | £842 | 388 | £99 | £828 | 403 | £23 | £77 | 71 | £13 | £54 | 79 | £95 | £745 | 81 |
| Electric toothbrushes | £39 | £56 | 204 | £41 | £54 | 204 | £38 | £50 | 35 | £40 | £74 | 45 | £24 | £31 | 38 |
| Brush heads | £16 | £11 | 217 | £15 | £11 | 228 | £18 | £10 | 39 | £15 | £11 | 44 | £13 | £11 | 44 |
| Manual brushes | £19 | £10 | 283 | £20 | £10 | 297 | £20 | £10 | 47 | £22 | £10 | 56 | £22 | £10 | 64 |
| Total (all participant costs) | £430 | £581 | 76 | £420 | £724 | 70 | £278 | £104 | 12 | £232 | £167 | 16 | £188 | £213 | 8 |
| Total cost of attending NHS appointments | £343 | £261 | 775 | £332 | £224 | 787 | £252 | £237 | 191 | £269 | £291 | 191 | £204 | £212 | 184 |
It was possible to ascertain opportunity costs of time and travel, and co-charges for NHS dental care for 90% (2128/2372) of participants following the assumptions detailed in Chapter 3. However, data completion was poor for annual questionnaires asking about private care costs, and purchase of dental products such as electric and manual toothbrushes. As a result, total complete-case participant costs across all items and questionnaires were calculable for only 8% (182/2372) of the sample. Missing participant cost data do not appear to be driven by randomised group or patient characteristics; however, multiple imputation is still required to complete the data set, given the significant proportions of missing data.
Benefits
Benefits are assessed in this study using a DCE with the general population to obtain WTP tariffs to map to clinical trial outcomes (see Chapter 3) and services received and in terms of EQ-5D-3L-based QALYs.
Quality-adjusted life-years
Table 20 details descriptive statistics for the EQ-5D-3L and calculated QALYs from the trial. Across all time points, full EQ-5D-3L profile response data were available for only 35% (836/2372) of the randomised trial population. However, there were only 151 (6%) members of the trial population missing all EQ-5D-3L time points (including baseline). Missing data were evenly distributed across the randomised groups. Additional regression analysis found no evidence that missingness was determined by any participant characteristics or dental health outcomes. QALY data were, therefore, assumed to be missing at random.
| Timepoint | Ineligible for 24-month recall | Eligible for 24-month recall | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk based | 6 month | Risk based | 6 month | 24 month | |||||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | |
| Undiscounted QALYs | |||||||||||||||
| Baseline | 0.898 | 0.208 | 602 | 0.903 | 0.196 | 590 | 0.931 | 0.135 | 134 | 0.891 | 0.235 | 140 | 0.927 | 0.172 | 140 |
| 1 year | 0.882 | 0.207 | 613 | 0.889 | 0.204 | 620 | 0.902 | 0.187 | 140 | 0.872 | 0.224 | 148 | 0.920 | 0.174 | 149 |
| 2 years | 0.874 | 0.217 | 597 | 0.881 | 0.226 | 603 | 0.887 | 0.225 | 133 | 0.887 | 0.201 | 143 | 0.891 | 0.195 | 142 |
| 3 years | 0.878 | 0.217 | 561 | 0.872 | 0.226 | 574 | 0.889 | 0.235 | 129 | 0.863 | 0.264 | 137 | 0.907 | 0.174 | 124 |
| 4 years | 0.866 | 0.230 | 637 | 0.867 | 0.237 | 645 | 0.893 | 0.208 | 145 | 0.856 | 0.268 | 157 | 0.884 | 0.209 | 151 |
| QALY | 3.523 | 0.715 | 310 | 3.491 | 0.801 | 319 | 3.507 | 0.761 | 65 | 3.483 | 0.906 | 71 | 3.522 | 0.781 | 71 |
| Discounted QALYs | |||||||||||||||
| Baseline | 0.898 | 0.208 | 602 | 0.903 | 0.196 | 590 | 0.931 | 0.135 | 134 | 0.891 | 0.235 | 140 | 0.927 | 0.172 | 140 |
| 1 year | 0.882 | 0.207 | 613 | 0.889 | 0.204 | 620 | 0.902 | 0.187 | 140 | 0.872 | 0.224 | 148 | 0.920 | 0.174 | 149 |
| 2 years | 0.845 | 0.210 | 597 | 0.851 | 0.219 | 603 | 0.857 | 0.217 | 133 | 0.857 | 0.195 | 143 | 0.861 | 0.189 | 142 |
| 3 years | 0.820 | 0.202 | 561 | 0.814 | 0.211 | 574 | 0.830 | 0.220 | 129 | 0.806 | 0.246 | 137 | 0.847 | 0.162 | 124 |
| 4 years | 0.781 | 0.207 | 637 | 0.782 | 0.213 | 645 | 0.805 | 0.188 | 145 | 0.772 | 0.242 | 157 | 0.797 | 0.188 | 151 |
| QALY | 3.392 | 0.688 | 310 | 3.363 | 0.769 | 319 | 3.378 | 0.728 | 65 | 3.355 | 0.871 | 71 | 3.392 | 0.751 | 71 |
Respondents were generally in good health, with greater than 3.5 undiscounted QALYs gained over 4-year time horizon across the randomised arms and strata.
Discrete choice experiment results
Sample characteristics
The online DCE was completed by 597 respondents sampled from the general population. The median survey completion time was 16.03 minutes (interquartile range 11.85–21.98 minutes; minimum 3.95 minutes, maximum 1640.10 minutes). Table 21 compares the sociodemographic characteristics and dental health experiences of the sample with the same characteristics in the general population. The sample was generally a good representation of the UK general population and satisfactorily matched the sought quotas for the survey. There is, however, over-representation of respondents from Scotland relative to the general population. These respondents were purposely oversampled to enable subgroup analysis of preferences across the UK regions and, in particular, to detect any impact of free check-ups in Scotland on preferences; therefore, it is less appropriate to compare the survey sample against the general population on regional distribution. Respondents were generally more regular attenders than the general population, and it is possible that the decision to participate in the survey was driven by this in part. Despite multiple invitations to the survey panel seeking at least 10% of our sample with their last attendance > 2 years ago, this was not achievable, and only 4% of the sample had their last dental check-up more than 2 years prior to completing the DCE.
| Characteristic | General populationa | DCE sample, n (%)b (N = 597) | |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age (years), mean (SD)a,c | ≈47 | 49.54 (16.60) | |
| Sexa,c | Male | 49% | 292 (49) |
| Female | 51% | 305 (51) | |
| Country of residencec | England | 84% | 392 (66) |
| Scotland | 8% | 183 (31) | |
| Wales | 5% | 13 (2) | |
| Northern Ireland | 3% | 9 (2) | |
| Smoker statusd | Yes | 18% | 128 (21) |
| No | 59% | 355 (59) | |
| Previous | 23% | 114 (19) | |
| Annual gross incomee | Low | 54% | 248 (42) |
| Medium | 37% | 189 (32) | |
| High | 8% | 92 (15) | |
| Refused to answer | N/A | 62 (10) | |
| Left blank | N/A | 6 (1) | |
| Educational attainmentc | SVQ (level 1/2) | 29% | 210 (35) |
| SVQ (level 3) | 12% | 83 (14) | |
| Degree | 27% | 151 (15) | |
| Professional | 6% | 64 (11) | |
| Apprentice | 4% | 12 (2) | |
| None | 22% | 77 (13) | |
| Employmentc | Employed | 61% | 328 (55) |
| Unemployed | 4% | 27 (5) | |
| Retired | 14% | 143 (24) | |
| Student | 9% | 13 (2) | |
| Other | 11% | 86 (14) | |
| Self-reported dental healthf | Very poor | 1% | 11 (2) |
| Poor | 6% | 45 (8) | |
| Fair | 21% | 201 (34) | |
| Good | 47% | 260 (44) | |
| Very good | 24% | 80 (13) | |
| Self-reported general healthc | Very poor | 1% | 11 (2) |
| Poor | 4% | 44 (7) | |
| Fair | 13% | 175 (29) | |
| Good | 34% | 268 (45) | |
| Very good | 47% | 99 (17) | |
| Dental health categoryg | Good | N/A | 340 (57) |
| Moderate | 221 (37) | ||
| Poor | 36 (6) | ||
| Experiences of dental care | |||
| Registered with dental practice | Yes | NRh | 576 (96) |
| No | 17 (3) | ||
| Do not know | 4 (1) | ||
| Usual method of payment for dental carei | Co-charge/mix | 45% | 276 (46) |
| NHS | 23% | 110 (18) | |
| Out of pocket | 131 (22) | ||
| Treatment plan | 62 (10) | ||
| Insurance | 6 (1) | ||
| Any private treatment | 27% | 199 (33) | |
| Never | 2% | 2 (0) | |
| Other | 3% | 10 (2) | |
| Time since last check-upi | < 6 months | 450 (75) | |
| 6–12 months | 106 (18) | ||
| < 1 year | 69% | 556 (93) | |
| 1–2 years | 10% | 20 (3) | |
| 2–5 years | 8% | 8 (1) | |
| > 5 years | 12% | 9 (2) | |
| Never | 1% | 4 (1) | |
Respondents are generally in good dental health, with only 6% of the sample assigned to the poorest segment of dental health in the DCE design. The smaller sample in this segment means that the DCE has less power to detect significant preferences for the worst levels of dental health outcomes (bleeding very often and advanced decay).
Preferences for dental care
The results of the error components model describing preferences estimated from the effects coded DCE and calculated WTP tariffs for mapping to the trial interventions and outcomes are reported in Table 22.
| Attribute level | Preference coefficient | WTP | ||
|---|---|---|---|---|
| Coefficient | SD | Coefficient | 95% CI | |
| ASC | 1.484*** | 1.675*** | £252.67 | £210.65 to £294.69 |
| 24-month recalla,b | –0.423*** | –£71.95b | –£86.33 to –£57.57 | |
| Annual recallb | –0.015 | –£2.61b | –£15.10 to £9.88 | |
| 6-month recallb | 0.348*** | £59.32b | £46.35 to £72.29 | |
| Risk-based recallb,c | 0.089** | £15.24c | £2.91 to £27.57 | |
| Bleed nevera | 0.208*** | £35.37 | £16.36 to £54.37 | |
| Bleed hardly ever | 0.099** | £16.82 | £1.05 to £32.58 | |
| Bleed occasionally | 0.089** | £15.23 | £1.55 to £28.91 | |
| Bleed fairly often | –0.127** | –£21.70 | –£40.60 to –£2.79 | |
| Bleed very often | –0.268*** | –£45.71 | –£79.85 to –£11.58 | |
| Decay nonea | 0.565*** | £96.21 | £77.57 to £114.84 | |
| Decay early | 0.058* | £9.91 | –£1.91 to £21.74 | |
| Decay moderate | –0.241*** | –£41.02 | –£53.08 to –£28.95 | |
| Decay advanced | –0.382*** | –£65.11 | –£83.70 to –£46.51 | |
| Annual cost | –0.006*** | |||
| Number of observations | 14,328 | |||
| Number of respondents | 597 | |||
| Log-likelihood (null) | –4578 | |||
| Log-likelihood (model) | –3856 | |||
| AIC | 7739 | |||
| BIC | 7837 | |||
| AIC/N | 0.54 | |||
| BIC/N | 0.55 | |||
The ASC is both positive and statistically significant. This indicates that the general population prefers any dental check-up package to none. The SD of the constant is also significant, suggesting that there is significant preference heterogeneity among the sample for this parameter. This indicates that the strength of preference for opting into a dental check-up package varies across the sample. Overall, 57% (339/597) of respondents always opted in, compared with only 6% (35/597) who always opted out. Preferences for dental recall frequency, decay, bleeding on brushing, and cost are as expected. Respondents prefer to have more frequent recalls, prefer to avoid dental decay, prefer no bleeding gums and prefer cheaper dental care packages. The WTP estimates appear to have good face validity, and findings are in line with those expected after completion of the preparatory qualitative work. Additionally, the WTP tariffs obtained for bleeding gums closely match those obtained in a previously conducted DCE for the IQuaD study,101 indicating a high level of benefit transferability.
The WTP tariffs can be interpreted as follows: the estimated tariffs are the general population’s WTP for each attribute level (e.g. 6-month recall) controlling for variation in all other attributes included in the DCE. In terms of understanding the impact of the WTP results for mapping to the trial outcomes, the difference in WTP across levels within an attribute represent the general population’s value for moving from one state of the world to another. Consider dental decay: the DCE results show that the general population is willing to pay, on average, £161.32 per year, over a 4-year period, to avoid moving from a state of no dental decay (WTP tariff: +£96.21) to a state of advanced dental decay (WTP tariff: –£65.11).
Discrete choice experiment subgroup analyses
Subgroup analyses are conducted by using interaction effects to examine the impact of subgroup membership (gender, region, smoking status, employment status, income, experienced check-up frequency and experience of dental decay) on preferences. In general, subgroup membership influences overall preference patterns (as indicated by significant likelihood ratio tests). Many of the interaction effects are expected and serve to verify the face validity of the analysis results. For example, those who are in employment and have higher incomes are less sensitive to changes in the cost attribute. Those with experience of dental decay have stronger preferences to keep their teeth decay free and avoid more advanced levels of decay. Those who regularly attend the dentist every 6 months are more likely to prefer 6-month recalls, and are more likely to accept more expensive dental care packages. Smoking status and region do not appear to have any meaningful impact on preferences for any of the individual attribute levels; however, female participants in the sample placed less emphasis on differences in dental decay than the sample as a whole. Full details of subgroup analysis results are provided in Appendix 2, Table 58.
Total monetary measures of benefit (discrete choice experiment results mapped to trial outcomes)
The WTP tariffs obtained from the DCE analysis are mapped to the corresponding services received in the trial and observed trial outcomes, to enable a CBA to be completed. Total benefits (complete-case data) across randomised arms and strata are reported in Table 23. The impact of plausible alternative assumptions for mapping DCE results to trial outcomes (as described in Chapter 3) is highlighted in light blue.
| Number | Description | Eligible | Ineligible | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk based | 6 month | 24 month | Risk based | 6 month | ||||||||||||
| Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | ||
| WTP tariffs mapped to individual trial outcomes (including sensitivity analyses)a | ||||||||||||||||
| 1. | Constant | £948 | £0 | 217 | £948 | £0 | 215 | £948 | £0 | 216 | £948 | £0 | 861 | £948 | £0 | 863 |
| Recall | ||||||||||||||||
| 2. | Recall ITT | –£122 | £198 | 201 | –£56 | £206 | 201 | –£236 | £211 | 203 | –£30 | £195 | 800 | £1 | £175 | 804 |
| 3. | SA1 recall PPb | £54 | £0 | 217 | £211 | £0 | 215 | –£256 | £0 | 216 | £54 | £0 | 861 | £211 | £0 | 863 |
| 4. | Bleeding on brushing | £71 | £57 | 147 | £61 | £55 | 154 | £71 | £47 | 151 | £67 | £54 | 589 | £67 | £58 | 601 |
| Caries | ||||||||||||||||
| 5. | Untreated caries + fillings assume moderatec,d | –£140 | £66 | 186 | –£131 | £65 | 178 | –£138 | £73 | 175 | –£149 | £56 | 767 | –£144 | £66 | 757 |
| 6. | SA2 untreated caries onlye | –£127 | £87 | 141 | –£110 | £87 | 133 | –£120 | £95 | 138 | –£130 | £88 | 607 | –£123 | £99 | 599 |
| 7. | SA3 untreated + fillings assume extensived,f | –£186 | £82 | 186 | –£186 | £87 | 178 | –£189 | £89 | 175 | –£203 | £68 | 767 | –£198 | £79 | 757 |
| WTP for health outcomes | ||||||||||||||||
| 8. (4 + 5) | Health (bleeding on brushing + untreated caries + fillings assume moderate) | –£71 | £84 | 140 | –£65 | £86 | 142 | –£68 | £91 | 137 | –£83 | £81 | 561 | –£78 | £86 | 568 |
| 9. (4 + 6) | Health (bleeding on brushing + untreated caries only) | –£58 | £100 | 126 | –£45 | £99 | 128 | –£53 | £106 | 126 | –£62 | £104 | 517 | –£57 | £108 | 517 |
| 10. (4 + 7) | Health (bleeding on brushing + untreated caries + fillings assume extensive) | –£109 | £98 | 140 | –£114 | £103 | 142 | –£113 | £106 | 137 | –£131 | £90 | 561 | –£127 | £95 | 568 |
| WTP overall | ||||||||||||||||
| 11. (1 + 2 + 4 + 5) | Total WTP ITT (bleeding + untreated caries + fillings assume moderate) | £780 | £207 | 137 | £871 | £207 | 140 | £659 | £219 | 135 | £870 | £181 | 550 | £902 | £186 | 553 |
| 12. (1 + 2 + 4 + 6) | Total WTP ITT (bleeding on brushing + untreated caries only) | £794 | £212 | 125 | £901 | £213 | 126 | £661 | £224 | 124 | £893 | £190 | 506 | £929 | £194 | 504 |
| 13. (1 + 2 + 4 + 7) | Total WTP ITT (bleeding on brushing + untreated caries + fillings assume extensive) | £742 | £207 | 137 | £822 | £209 | 140 | £614 | £207 | 135 | £822 | £175 | 550 | £854 | £186 | 553 |
Including WTP for health benefit (caries experience + bleeding on brushing) only in the definition of total benefits (see Table 23, WTP for health outcomes, analysis number 8) shows little evidence of difference across groups in either stratum. This is based on attaching WTP to avoid caries to both fillings received and untreated caries as measured at the final trial outcome assessment appointment. Amending the definition of caries applied for the mapping of WTP to trial outcomes (i.e. including or excluding fillings from the measurement of caries experience) impacts on mean WTP, but does not substantially alter conclusions across the randomised arms in either stratum.
However, substantial cross-group differences are observed when expanding the valuation space to include WTP for the service received (and all the perceived benefits associated with more frequent check-ups). Applying the high valuation of more frequent check-ups to the number of check-ups received in the trial generates substantial differences in WTP across the randomised arms from this wider, societal perspective.
Economic evaluation results
This section combines costs and benefits data, using multiple imputation of missing data, under three evaluation frameworks: (1) cost per QALY gained, (2) INB (intervention receipt + dental health benefits) and (3) INB (dental health outcomes: bleeding gums and caries experience).
Economic evaluation results for risk-based versus 6-month recall (whole trial population)
Table 24 reports the results and Figures 15–17 illustrate the joint uncertainty in costs and benefits for the comparison of risk-based versus 6-month recall using the data set pooled across strata. The figures illustrate joint uncertainty in costs and benefits using scatterplots of the cost-effectiveness (benefit) plane and cost-effectiveness (benefit) acceptability curves for each analysis framework. The preferred recall strategy is highlighted in each of the analyses in Table 24 for ease of reading.
For the comparison of risk-based versus 6-month recall, there was no evidence of a difference in total costs to the dental budget, QALYs gained or dental health outcomes. On the balance of probabilities, 6-month recall has a greater chance of being the most efficient strategy when considering costs from only a dental health budget perspective. There is substantial uncertainty in this conclusion for the CUA and cost per monetary valuation of dental outcomes, with a much higher chance of net benefit associated with 6-month recall when adopting a societal approach to benefit valuation in the CBA. The results for the CUA and CBA remain robust to the range of deterministic sensitivity analyses undertaken. The results of the incremental cost per unit of (monetary) health benefit (i.e. framework 3) are generally robust, with two exceptions:
-
Including participant costs of attending dental appointments (i.e. the opportunity cost of time and travel, and the co-charge payments incurred) reduces the cost-effectiveness case for 6-monthly recalls. In this scenario, there is no evidence to suggest that 6-month recall is an efficient use of resources when society is only willing to pay for health benefits; however, broadening the costing perspective does not change the findings from the CBA that 6-monthly recalls have the greatest probability of maximising societal net benefit.
-
The most efficient recall strategy when measuring benefits in terms of WTP for dental health (bleeding and caries) outcomes is sensitive to the UK region analysed. Considering data from Scotland (fee for service) only suggests a substantially higher chance that risk-based recall is associated with positive net (dental health) benefits, whereas in England (treatment banding), 6-month recall is the most likely net beneficial strategy. There are two reasons for these divergent region-specific results. First, in England there are more band 2 treatments (specifically fillings) provided in the risk-based group (ineligible stratum only), generating higher costs relative to 6-month recall than in Scotland. Second, the larger number of fillings is also counted on the benefits side (as a negative) in the WTP calculation in England; therefore, the combined effect of these drivers is a substantial difference in the probability of each strategy being an efficient use of resources across the regions (simultaneously accounting for the joint uncertainty in both costs and dental health benefits).
| Analysis | Incremental cost: mean difference, risk based vs. 6 month (95% CI) | Framework 1: CUA | Framework 2: CBA | Framework 3: WTP (dental outcomes) | Probability of being the most efficient recall strategy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Incremental QALY: mean difference, risk based vs. 6 month (95% CI) | ICER (£/QALY) | Incremental benefits: mean difference, risk based vs. 6 month (95% CI) | INB | Incremental benefits: mean difference, risk based vs. 6 month (95% CI) | INB | Recall strategy | CUA (@ £20,000 per QALY) | CBA (@ benefit/cost = 1) | WTP (dental outcomes) (@ benefit/cost = 1) | ||
| Costing perspective: NHS dental | +£1.82 (–£6.66 to +£10.30) | –0.006 (–0.042 to +0.031) | Dominated | –£39.39 (–£58.40 to –£20.38) | –£41.21 | –£4.05 (–£11.76 to +£3.66) | –£5.87 | Risk based | 30% | 0% | 17% |
| 6 month | 70% | 100% | 83% | ||||||||
| Costing perspective: NHS all | –£1.32 (–£19.22 to +£16.58) | –0.006 (–0.042 to +0.031) | £220 | –£39.39 (–£58.40 to –£20.38) | –£38.07 | –£4.05 (–£11.76 to +£3.66) | –£2.73 | Risk based | 31% | 0% | 34% |
| 6 month | 69% | 100% | 66% | ||||||||
| Costing perspective: NHS + participant-incurred costs | –£13.02 (–£68.09 to +£42.05) | –0.006 (–0.042 to +0.031) | £2170 | –£39.39 (–£58.40 to –£20.38) | –£26.37 | –£4.05 (–£11.76 to +£3.66) | +£8.97 | Risk based | 32% | 22% | 69% |
| 6 month | 68% | 78% | 31% | ||||||||
| Costing perspective: direct costs to NHS and participants of attending NHS dental care | –£2.87 (–£20.31 to +£14.57) | –0.006 (–0.042 to +0.031) | £478 | –£39.39 (–£58.40 to –£20.38) | –£36.52 | –£4.05 (–£11.76 to +£3.66) | –£1.18 | Risk based | 30% | 0% | 51% |
| 6 month | 70% | 100% | 49% | ||||||||
| Map WTP for caries outcome to untreated caries only | +£1.82 (–£6.66 to +£10.30) | –0.006 (–0.042 to +0.031) | Dominated | –£41.79 (–£61.10 to –£22.49) | –£43.61 | –£6.45 (–£17.07 to +£4.17) | –£8.27 | Risk based | 30% | 0% | 12% |
| 6 month | 70% | 100% | 88% | ||||||||
| WTP mapped to trial outcomes using per protocol analysis | +£1.82 (–£6.66 to +£10.30) | –0.006 (–0.042 to +0.031) | Dominated | –£171.66 (–£159.92 to –£183.41) | –£176.48 | –£6.45 (–£17.07 to +£4.17) | –£8.27 | Risk based | 30% | 0% | 17% |
| 6 month | 70% | 100% | 83% | ||||||||
| Scotland only | –£3.57 (–£12.61 to +£5.48) | –0.008 (–0.057 to +0.041) | £446 | –£65.54 (–£99.29 to –£31.78) | –£61.97 | +£6.52 (–£10.72 to +£23.77) | +£10.09 | Risk based | 37% | 0% | 91% |
| 6 month | 63% | 100% | 9% | ||||||||
| England only | +£11.42 (–£3.91 to +£26.74) | –0.003 (–0.058 to +0.052) | Dominated | –£23.18 (–£48.03 to +£1.67) | –£34.60 | –£13.21 (–£23.95 to –£2.46) | –£24.63 | Risk based | 35% | 0% | 1% |
| 6 month | 65% | 100% | 99% | ||||||||
| Undiscounted costs and benefits | +£1.74 (–£7.23 to +£10.70) | –0.007 (–0.064 to +0.050) | Dominated | –£11.83 (–£17.12 to –£6.54) | –£13.57 | –£1.15 (–3.58 to +£1.28) | –£2.89 | Risk based | 31% | 0% | 25% |
| 6 month | 69% | 100% | 75% | ||||||||
| Complete-case analysisa |
CUA: –£1.48 (–£13.01 to +£10.05) CBA: –£7.00 (–£20.90 to +£6.89) WTP (dental): –£7.00 (–£20.90 to +£6.89) |
–0.022 (–0.067 to + 0.020) | £67.27 | –£53.43 (–£83.25 to –£23.61) | –£46.00 | –£4.42 (–£14.19 to +£5.35) | +£2.58 | Risk based | 25% | 0% | 62% |
| 6 month | 75% | 100% | 38% | ||||||||
FIGURE 15.
Risk-based vs. 6-month recall (CUA): (a) scatterplot of incremental costs and incremental QALYs; and (b) CEAC.
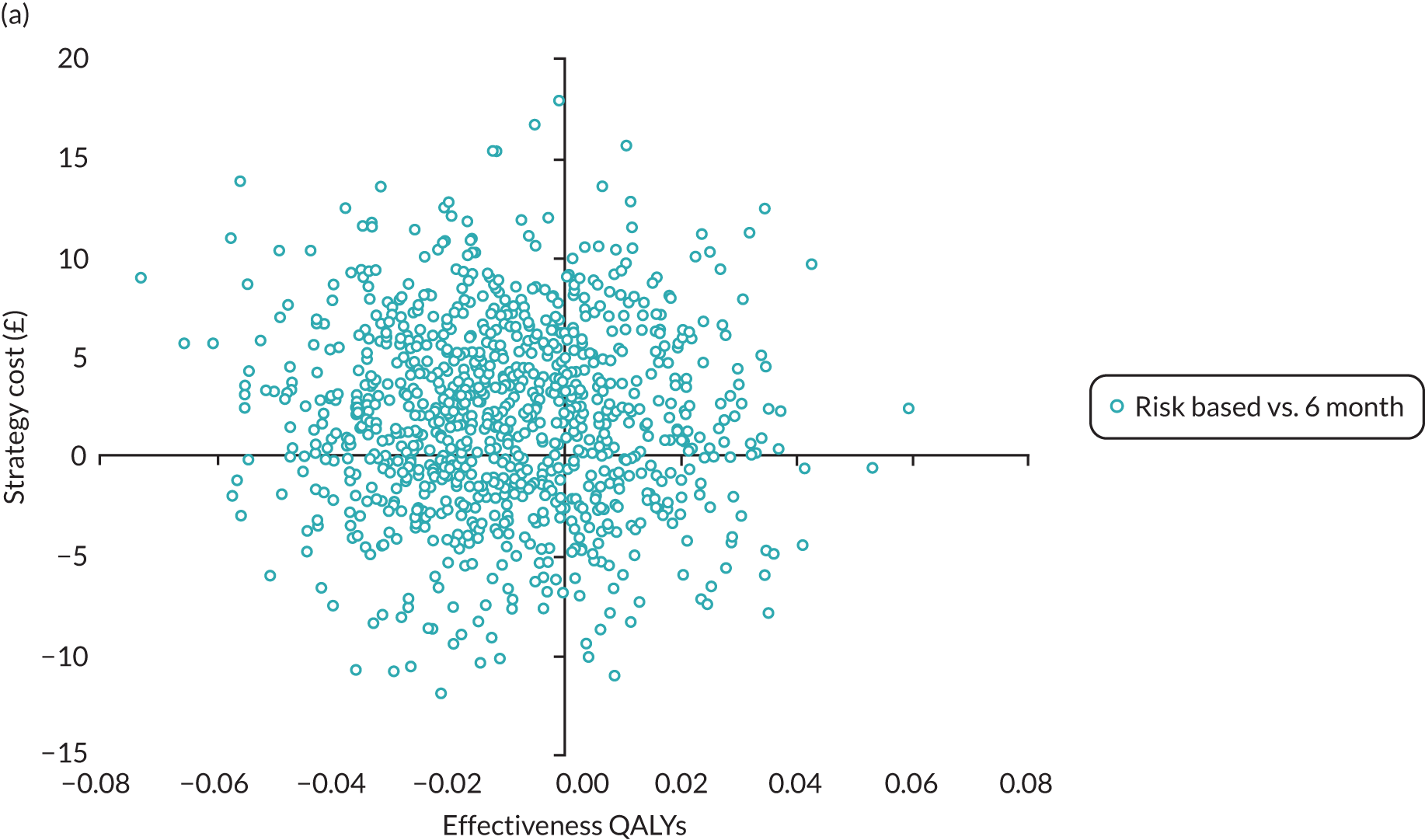
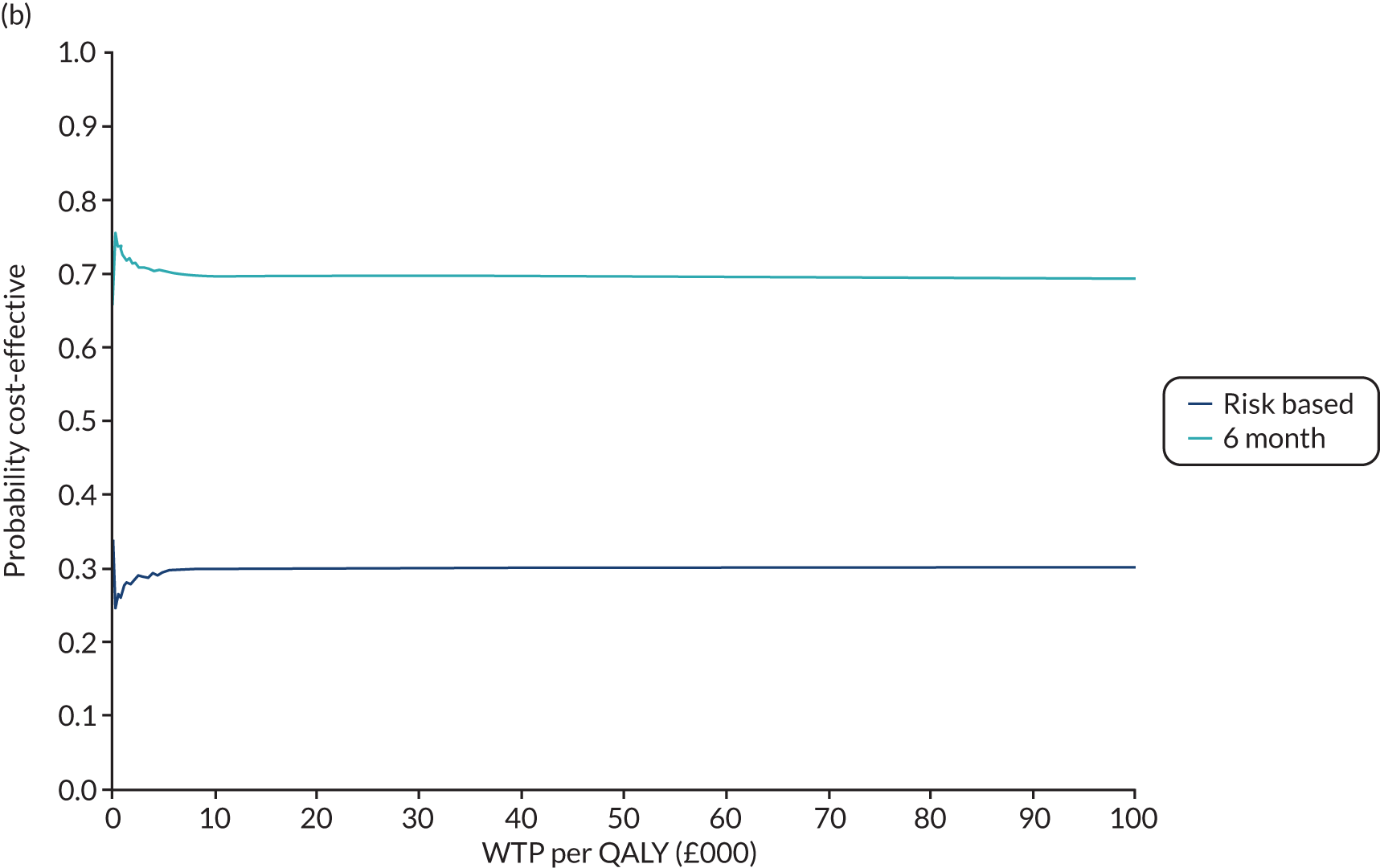
FIGURE 16.
Risk-based vs. 6-month recall (CBA): (a) scatterplot of incremental costs and incremental benefits (WTP); and (b) CBAC. NB, net benefit.
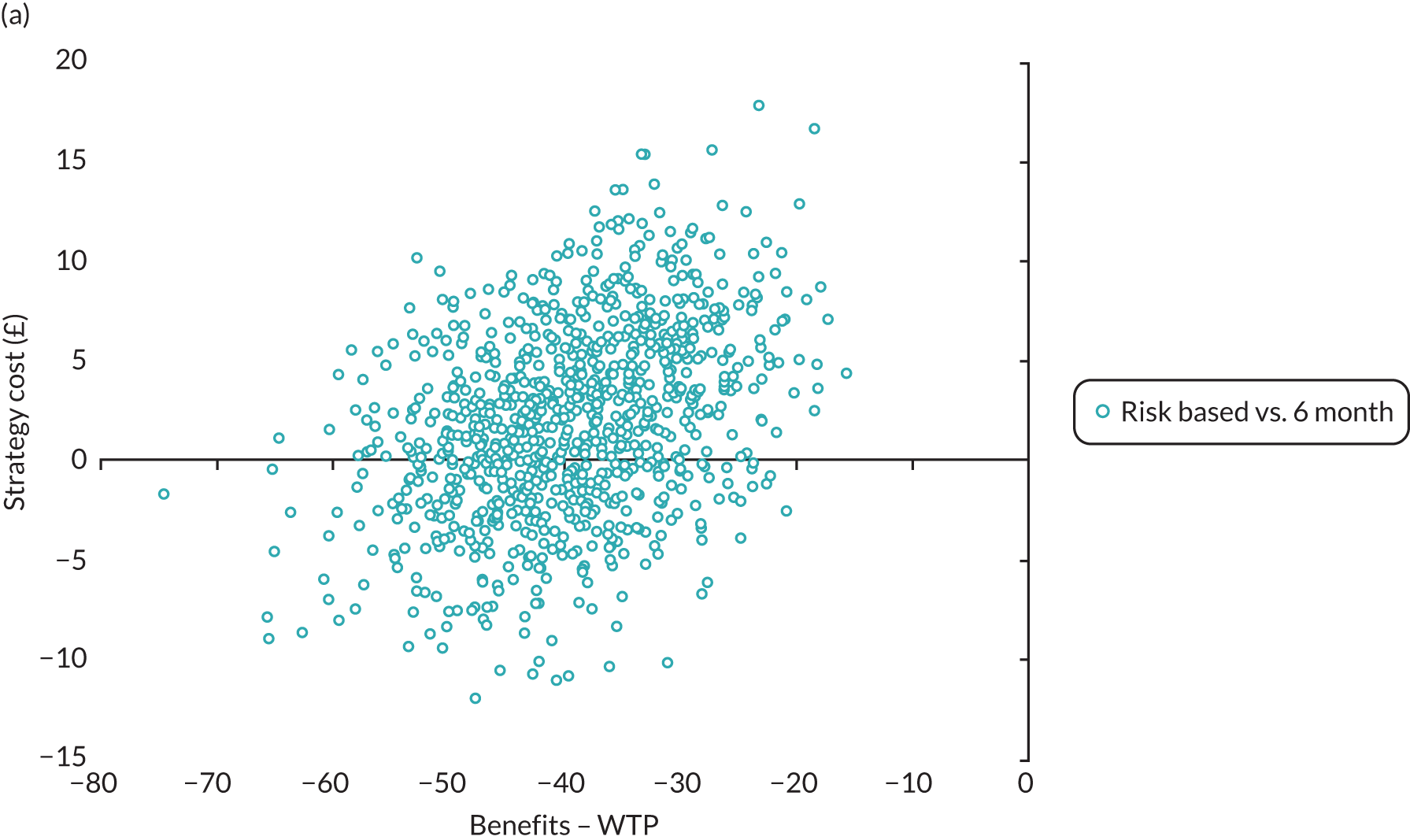
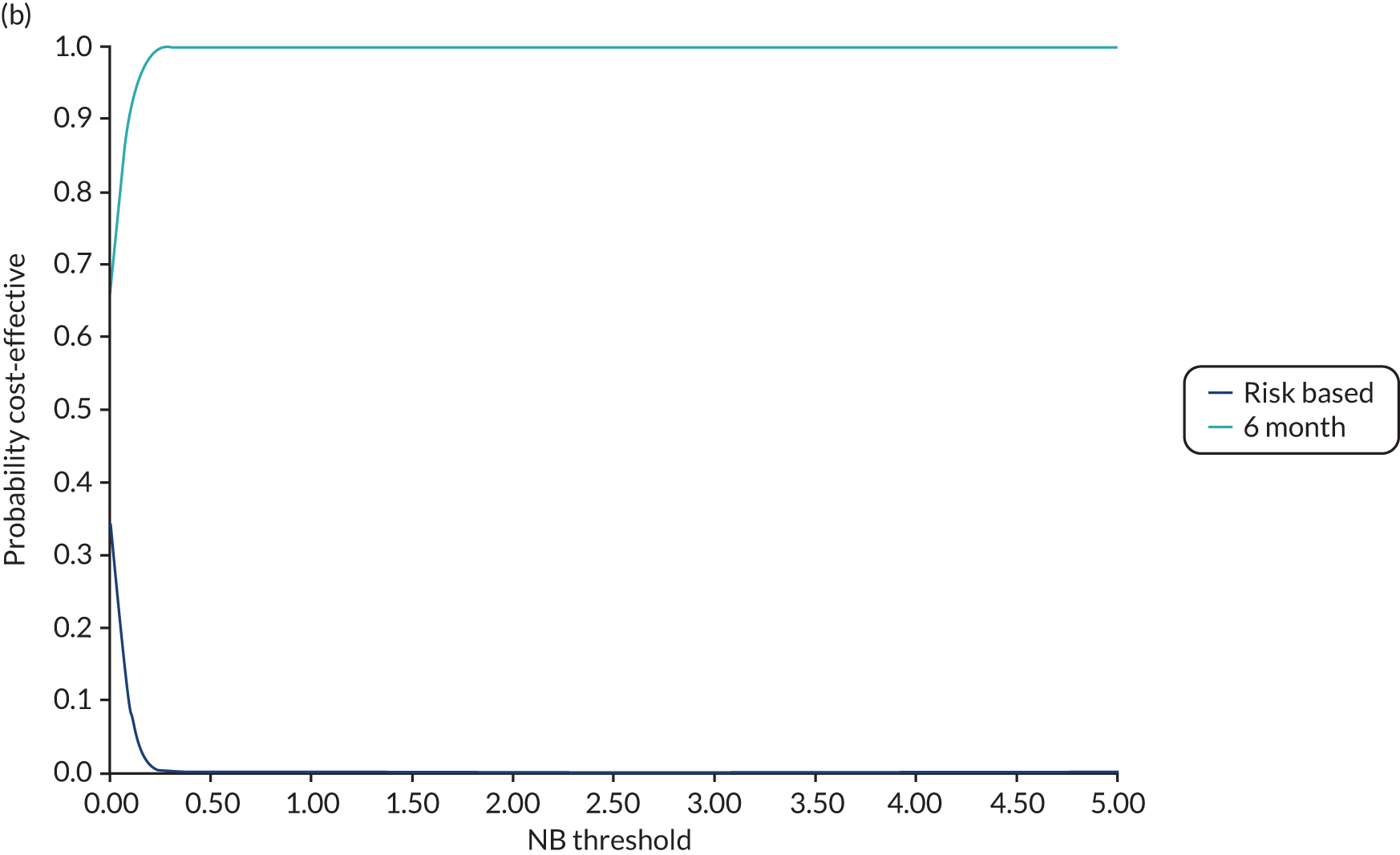
FIGURE 17.
Risk-based vs. 6-month recall (WTP for dental health outcomes): (a) scatterplot of incremental costs and incremental benefits (WTP); and (b) CBAC. NB, net benefit.
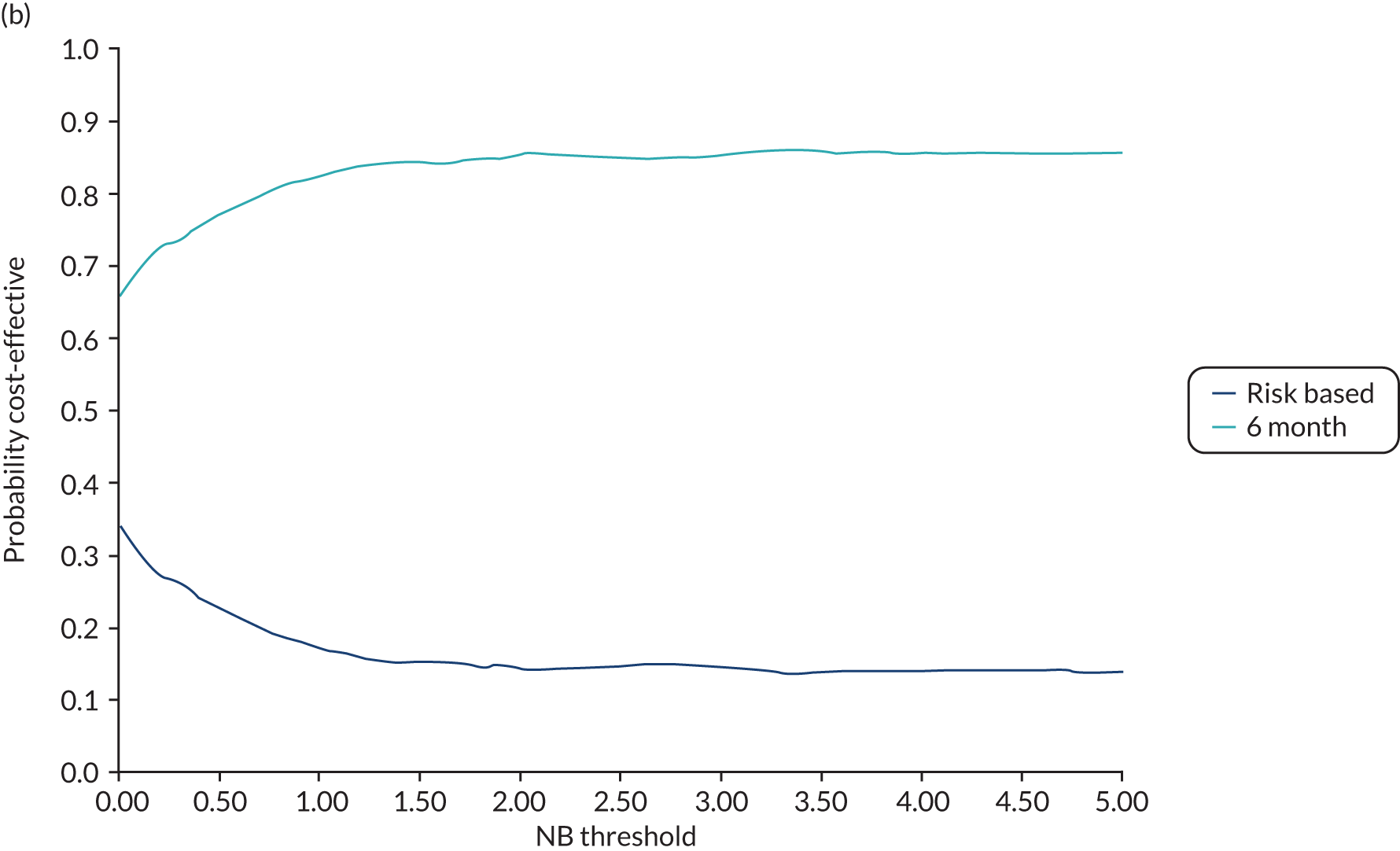
Economic evaluation results for risk-based versus 6-month versus 24-month recall (stratum eligible for 24-month recall)
Table 25 and Figures 18–20 report the corresponding findings from the three-way comparison of risk-based versus 6-month versus 24-month recall for those deemed eligible for 24-month recall.
| Analysis | Comparison | Incremental cost, mean difference (95% CI) | Framework 1: CUA | Framework 2: CBA | Framework 3: WTP (dental outcomes) | Probability of being the most efficient recall strategya | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incremental QALY, mean difference (95% CI) | ICER | Incremental benefits, mean difference (95% CI) | INB | Incremental benefits, mean difference (95% CI) | INB | Recall strategy | CUA P (C/E) @ £20,000 | CBA P (C/B) @ BCR = 1 | WTP (dental health) P (C/B) @ BCR = 1a | |||
| Costing perspective: NHS dental | 24 month vs. risk based | –£6.46 (–£18.03 to +£5.11) | +0.007 (–0.102 to +0.117) | Dominant | –£109.22 (–£151.34 to –£67.09) | –£102.76 | +£0.57 (–£18.28 to +£19.43) | £7.03 | Risk based | 16% | 0% | 12% |
| 24 month vs. 6 month | –£12.32 (–£30.94 to +£6.29) | +0.021 (–0.052 to +0.095) | Dominant | –£172.43 (–£241.82 to –£103.04) | –£160.11 | –£1.22 (–£20.62 to +£18.18) | £11.10 | 6 month | 25% | 100% | 3% | |
| Risk based vs. 6 month | –£5.86 (–£21.85 to +£10.12) | +0.014 (–0.082 to +0.110) | Dominant | –£63.21 (–£110.24 to –£16.18) | –£57.35 | –£1.80 (–£19.72 to +£16.13) | £4.06 | 24 month | 59% | 0% | 86% | |
| Costing perspective: NHS all | 24 month vs. risk based | –£3.61 (–£40.11 to +£32.89) | +0.007 (–0.102 to +0.117) | Dominant | –£109.22 (–£151.34 to –£67.09) | –£105.61 | +£0.57 (–£18.28 to +£19.43) | £4.18 | Risk based | 17% | 0% | 20% |
| 24 month vs. 6 month | –£9.33 (–£50.44 to +£31.77) | +0.021 (–0.052 to +0.095) | Dominant | –£172.43 (–£241.82 to –£103.04) | –£163.10 | –£1.22 (–£20.62 to +£18.18) | £8.11 | 6 month | 25% | 100% | 10% | |
| Risk based vs. 6 month | –£5.73 (–£43.81 to +£32.35) | +0.014 (–0.082 to +0.110) | Dominant | –£63.21 (–£110.24 to –£16.18) | –£57.48 | –£1.80 (–£19.72 to +£16.13) | £3.93 | 24 month | 59% | 0% | 70% | |
| Costing perspective: NHS + participant–incurred costs | 24 month vs. risk based | –£37.92 (–£178.37 to +£102.53) | +0.007 (–0.102 to +0.117) | Dominant | –£109.22 (–£151.34 to –£67.09) | –£71.30 | +£0.57 (–£18.28 to +£19.43) | £38.49 | Risk based | 17% | 6% | 26% |
| 24 month vs. 6 month | –£25.22 (–£126.33 to +£75.89) | +0.021 (–0.052 to +0.095) | Dominant | –£172.43 (–£241.82 to –£103.04) | –£147.21 | –£1.22 (–£20.62 to +£18.18) | £24.00 | 6 month | 25% | 94% | 9% | |
| Risk based vs. 6 month | +£12.70 (–£120.90 to +£146.31) | +0.014 (–0.082 to +0.110) | £907 | –£63.21 (–£110.24 to –£16.18) | –£75.91 | –£1.80 (–£19.72 to +£16.13) | –£14.50 | 24 month | 59% | 0% | 65% | |
| Costing perspective: direct costs to NHS and participants of attending NHS dental care | 24 month vs. risk based | –£41.84 (–£64.87 to –£18.82) | +0.007 (–0.102 to +0.117) | Dominant | –£109.22 (–£151.34 to –£67.09) | –£67.38 | +£0.57 (–£18.28 to +£19.43) | £42.41 | Risk based | 16% | 1% | 1% |
| 24 month vs. 6 month | –£57.82 (–£106.55 to –£9.09) | +0.021 (–0.052 to +0.095) | Dominant | –£172.43 (–£241.82 to –£103.04) | –£114.61 | –£1.22 (–£20.62 to +£18.18) | £56.60 | 6 month | 23% | 99% | 0% | |
| Risk based vs. 6 month | –£15.98 (–£58.60 to +£26.64) | +0.014 (–0.082 to +0.110) | Dominant | –£63.21 (–£110.24 to –£16.18) | –£47.23 | –£1.80 (–£19.72 to +£16.13) | £14.18 | 24 month | 62% | 0% | 99% | |
| Map WTP for caries outcome to untreated caries only | 24 month vs. risk based | –£6.46 (–£18.03 to +£5.11) | +0.007 (–0.102 to +0.117) | Dominant | –£108.03 (–£153.00 to –£63.07) | –£101.57 | +£1.76 (–£23.33 to +£26.84) | £8.22 | Risk based | 17% | 0% | 8% |
| 24 month vs. 6 month | –£12.32 (–£30.94 to +£6.29) | +0.021 (–0.052 to +0.095) | Dominant | –£174.98 (–£248.18 to –£101.78) | –£162.66 | –£3.78 (–£27.71 to +£20.16) | £8.54 | 6 month | 26% | 100% | 5% | |
| Risk based vs. 6 month | –£5.86 (–£21.85 to +£10.12) | +0.014 (–0.082 to +0.110) | Dominant | –£66.95 (–£118.77 to –£15.13) | –£61.09 | –£5.53 (–£32.39 to +£21.23) | £0.33 | 24 month | 57% | 0% | 88% | |
| WTP mapped to trial outcomes using per protocol analysis | 24 month vs. risk based | –£6.46 (–£18.03 to +£5.11) | +0.007 (–0.102 to +0.117) | Dominant | –£304.53 (–£345.87 to –£263.20) | –£298.07 | +£0.57 (–£18.28 to +£19.43) | £7.03 | Risk based | 16% | 0% | 12% |
| 24 month vs. 6 month | –£12.32 (–£30.94 to +£6.29) | +0.021 (–0.052 to +0.095) | Dominant | –£468.59(–£511.45 to –£425.72) | –£456.27 | –£1.22 (–£20.62 to +£18.18) | £11.10 | 6 month | 25% | 100% | 3% | |
| Risk based vs. 6 month | –£5.86 (–£21.85 to +£10.12) | +0.014 (–0.082 to +0.110) | Dominant | –£164.06 (–£196.36 to –£131.76) | –£158.20 | –£1.80 (–£19.72 to +£16.13) | £4.06 | 24 month | 59% | 0% | 86% | |
| Scotland only | 24 month vs. risk based | –£9.98 (–£17.44 to –£2.51) | +0.000 (–0.163 to +0.163) | Dominant | –£126.67 (–£184.99 to –£68.35) | –£116.69 | –£11.92 (–£55.69 to +£31.86) | –£1.94 | Risk based | 34% | 0% | 30% |
| 24 month vs. 6 month | –£16.27 (–34.02 to +£1.48) | –0.000 (–0.095 to +0.095) | £162,700 | –£229.56 (–£332.09 to –£127.03) | –£213.29 | +£6.34 (–£33.22 to +£45.89) | £22.61 | 6 month | 43% | 100% | 0% | |
| Risk based vs. 6 month | –£6.30 (–£25.79 to +£13.20) | –0.001 (–0.154 to +0.153) | £6,300 | –£102.89 (–£196.35 to –£9.44) | –£96.59 | +£18.26 (–£24.47 to +£60.98) | £24.56 | 24 month | 23% | 0% | 70% | |
| England only | 24 month vs. risk based | –£9.62 (–£40.96 to +£21.72) | +0.048 (–0.098 to +0.194) | –£131.30 (–£190.82 to –£71.79) | –£121.68 | +£12.51 (–£15.94 to +£40.95) | £22.13 | Risk based | 5% | 22% | 10% | |
| 24 month vs. 6 month | –£15.67 (–£47.99 to +£16.64) | +0.063 (–0.064 to +0.190) | Dominant | –£170.20 (–£269.40 to –£70.99) | –£154.53 | –£4.14 (–£36.90 to +£28.63) | £11.53 | 6 month | 10% | 78% | 14% | |
| Risk based vs. 6 month | –£6.05 (–£31.95 to +£19.85) | +0.015 (–0.156 to +0.187) | Dominant | –£38.89 (–£111.66 to +£33.88) | –£32.84 | –£16.64 (–£48.90 to +£15.62) | –£10.59 | 24 month | 86% | 0% | 76% | |
| Undiscounted costs and benefits | 24 month vs. risk based | –£6.43 (–£18.23 to +£5.38) | +0.023 (–0.071 to +0.118) | Dominant | –£31.38 (–£43.57 to –£19.19) | –£24.95 | +£0.91 (–£3.81 to +£5.62) | +£7.34 | Risk based | 17% | 16% | 15% |
| 24 month vs. 6 month | –£13.97 (–£33.15 to +£5.20) | +0.002 (–0.089 to +0.094) | Dominant | –£48.81 (–£68.23 to –£29.40) | –£34.84 | +£1.21 (–£4.53 to +£6.95) | +£15.18 | 6 month | 29% | 84% | 2% | |
| Risk based vs. 6 month | –£7.55 (–£24.51 to £9.42) | –0.021 (–0.109 to +0.067) | £359.52 | –£17.43 (–£30.76 to –£4.10) | –£9.88 | +£0.30 (–£5.19 to +£5.79) | +£7.85 | 24 month | 54% | 0% | 83% | |
| Complete–case analysisb | 24 month vs. risk based |
CUA:–£10.58 (–£31.51 to +£10.35) CBA: –£15.23 (–£36.23 to +£5.77) WTP (dental):–£15.23 (–£36.23 to +£5.77) |
+0.036 (–0.101 to +0.173) | Dominant | –£111.63 (–£171.69 to –£51.56) | –£96.40 | +£4.95 (–£19.78 to +£29.67) | +£20.18 | Risk based | 6% | 0% | 11% |
| 24 month vs. 6 month |
CUA: –£14.49 (–£36.47 to +£7.50) CBA: –£26.45 (–£59.45 to +£6.54) WTP (dental): –£26.45 (–£59.45 to +£6.54) |
–0.048 (–0.152 to +0.056) | £301.88 | –£218.40 (–£305.18 to –£131.62) | –£191.95 | –£3.02 (–£30.36 to +£24.32) | +£23.43 | 6 month | 76% | 100% | 8% | |
| Risk based vs. 6 month |
CUA: –£3.91 (–£25.54 to +£17.73) CBA: –£11.22 (–£45.81 to + 23.36) WTP (dental): –£26.45 (–£59.45 to +£6.54) |
–0.084 (–0.210 to +0.042) | £46.45 | –£106.77 (–£173.03 to –£40.52) | –£95.55 | –£7.97 (–£31.22 to +£15.28) | +£18.48 | 24 month | 17% | 0% | 81% | |
FIGURE 18.
Eligible for 24-month recall: (a) scatterplot of incremental costs and incremental QALYs; and (b) CEAC, for risk-based vs. 6-month recall (CUA).
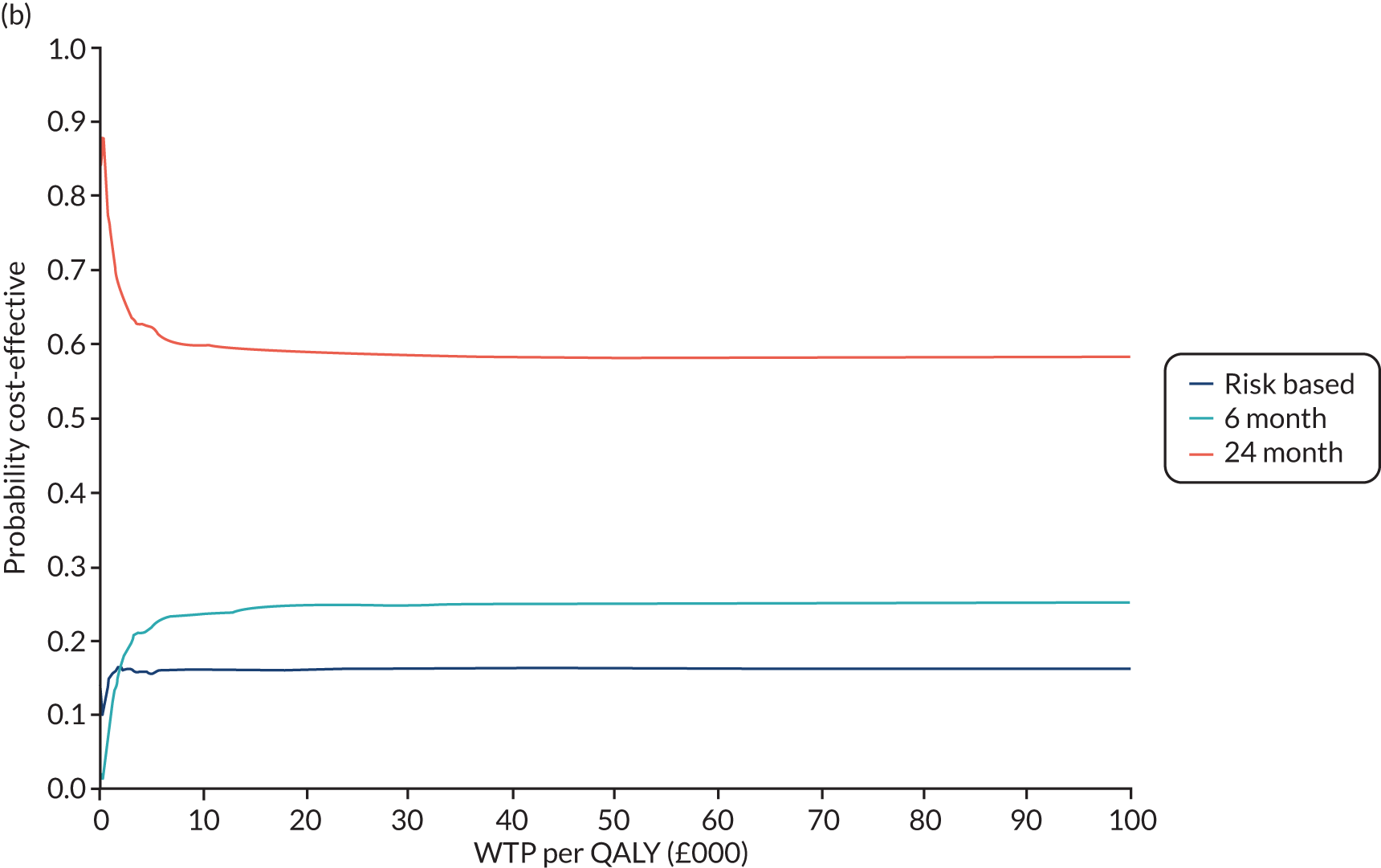
FIGURE 19.
Eligible for 24-month recall: (a) scatterplot of incremental costs and incremental benefits (WTP); and (b) CBAC, for risk-based vs. 6-month recall (CBA). NB, net benefit.
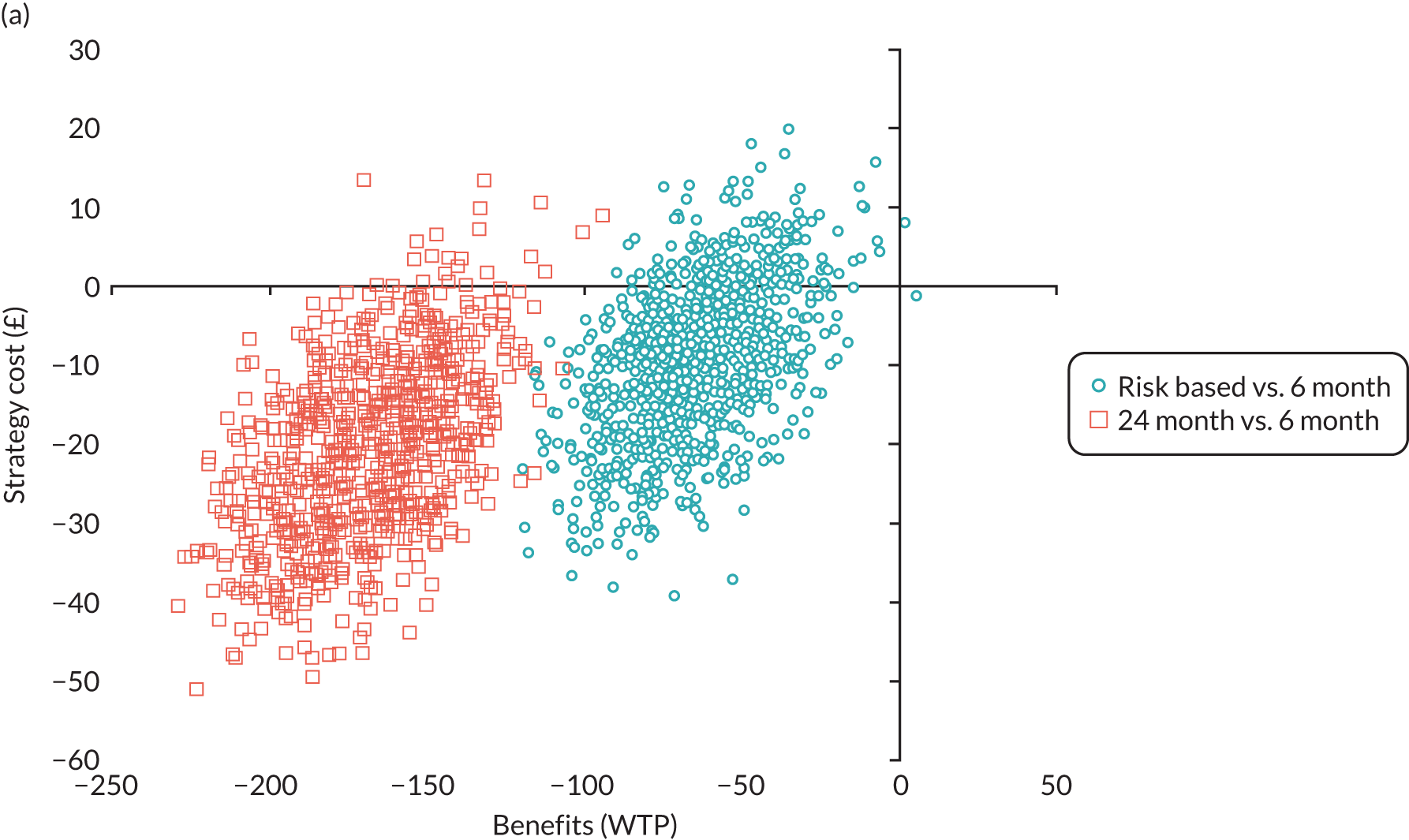
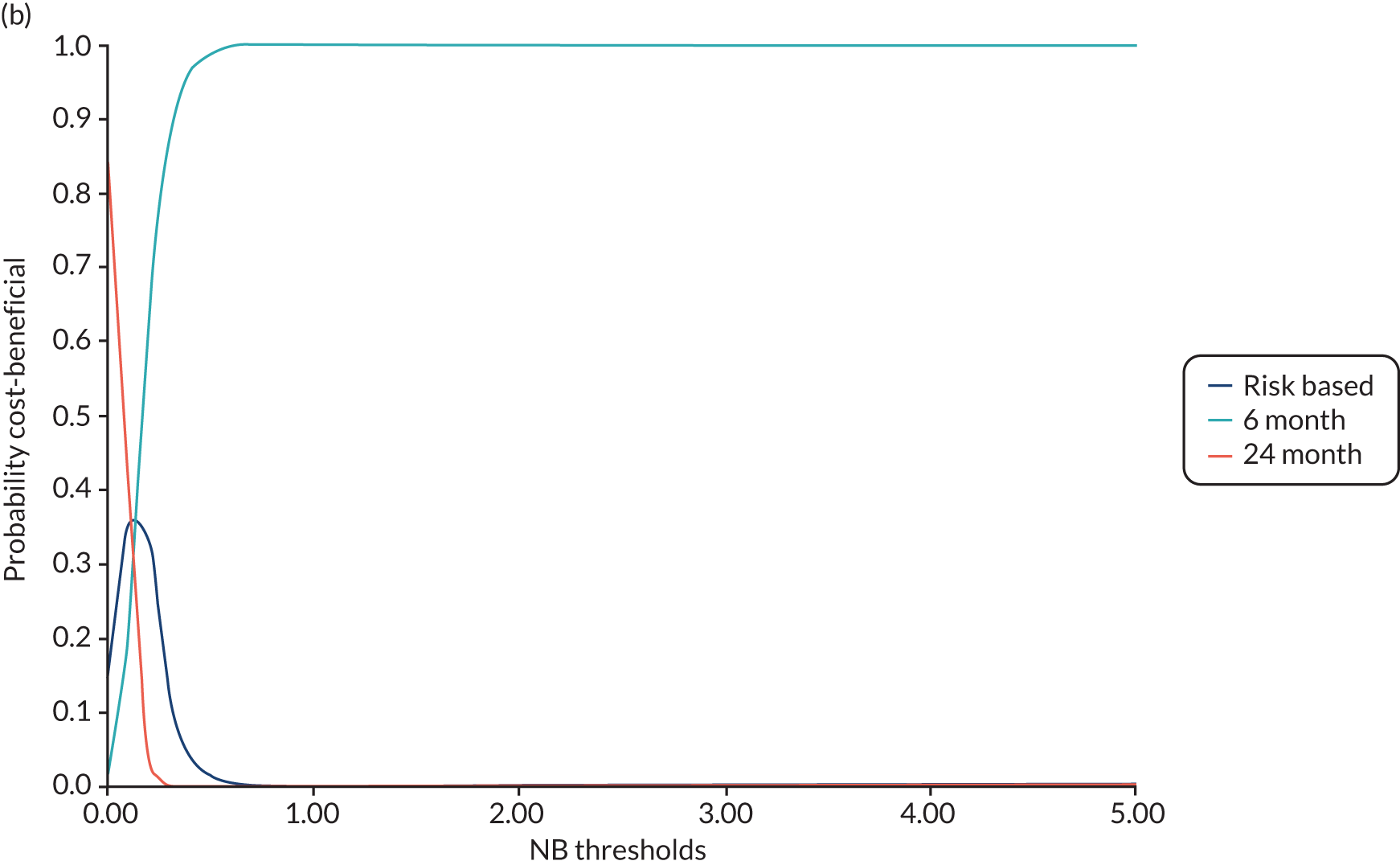
FIGURE 20.
Eligible for 24-month recall: (a) scatterplot of incremental costs and incremental benefits (WTP); and (b) CBAC, for risk-based vs. 6-month recall (WTP for dental health outcomes). NB, net benefit.
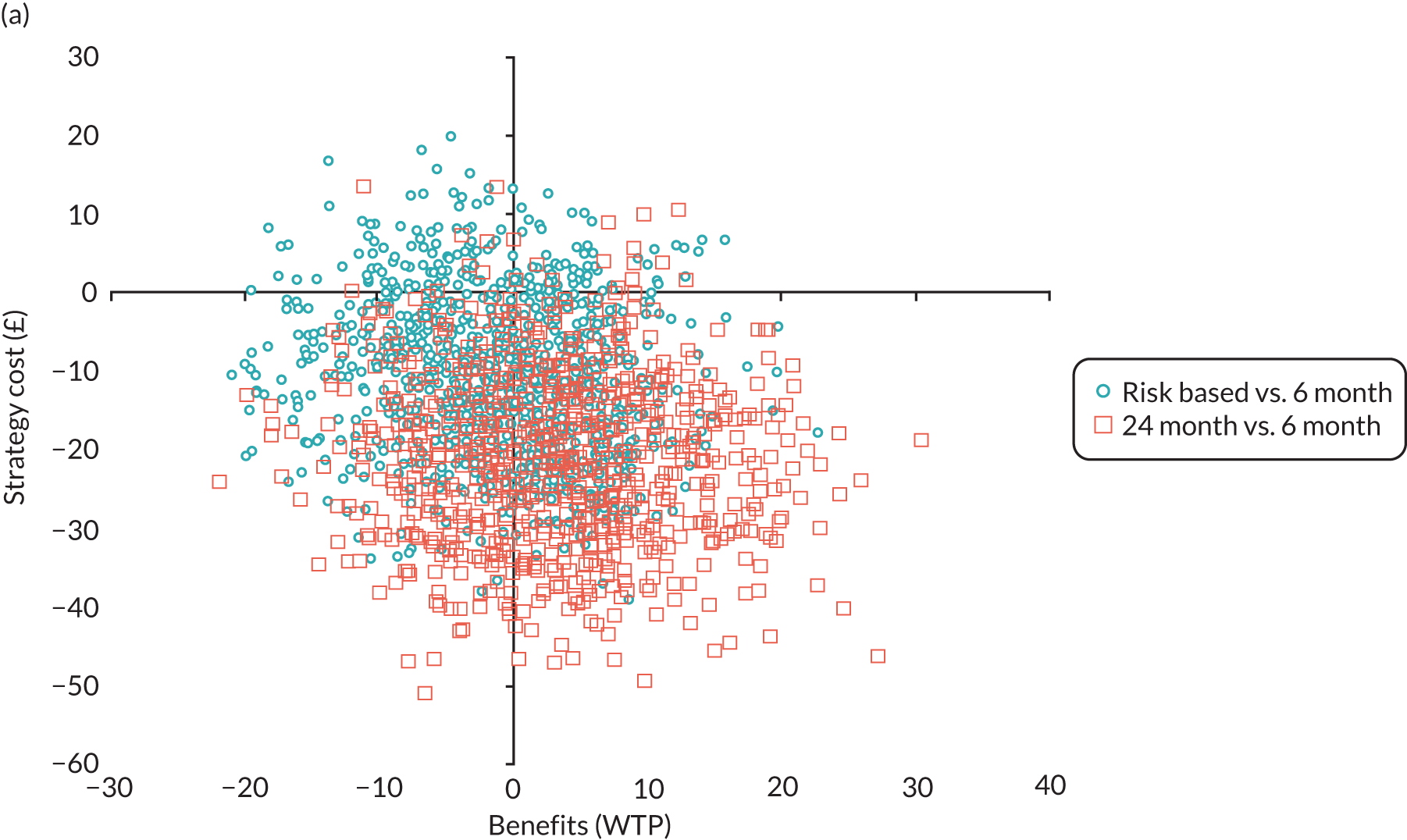
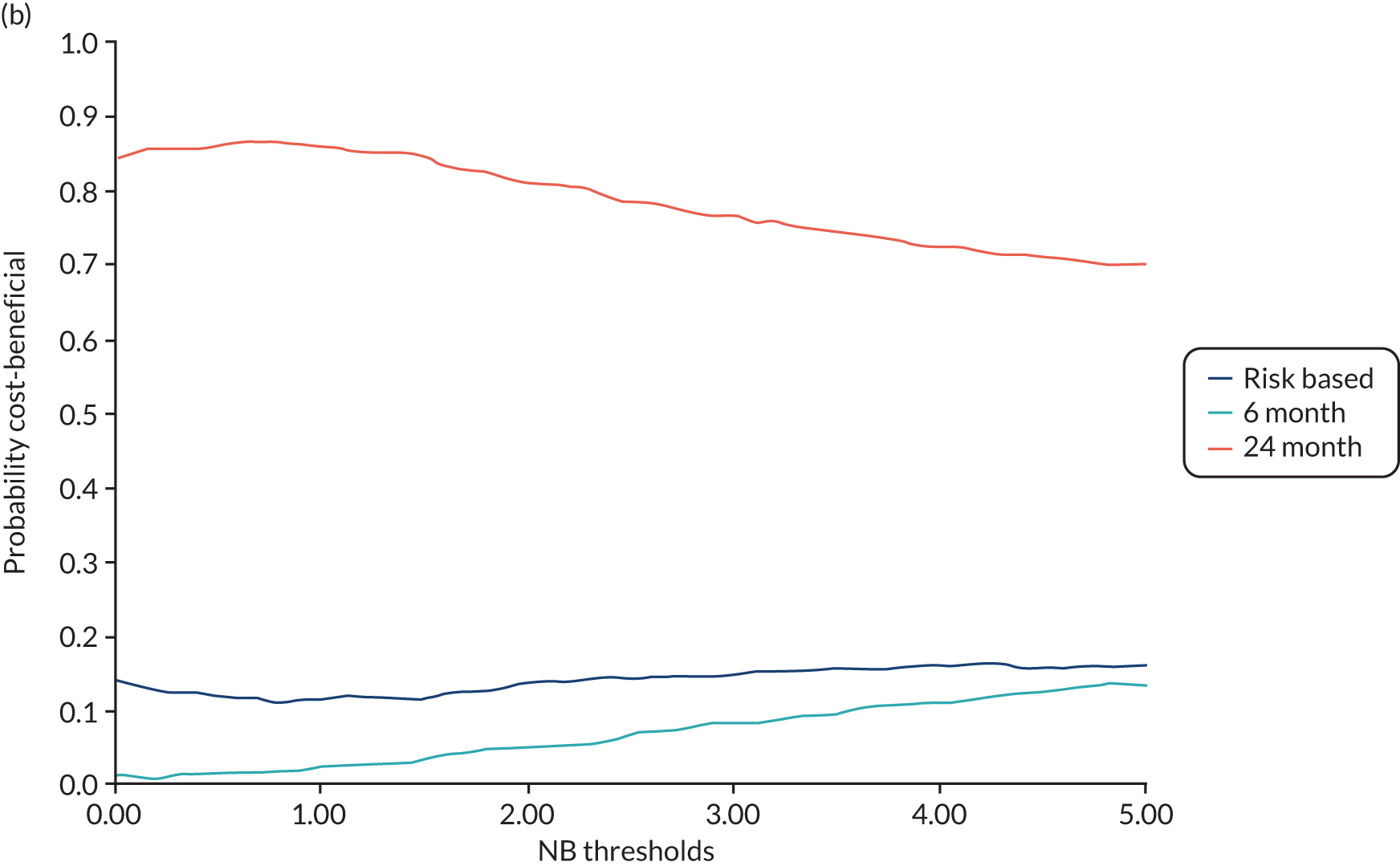
For the analysis of trial data for those eligible for the 24-month recall, the 24-month interval is the least costly interval overall, and is significantly less costly than 6-month recall. For the CUA and WTP for dental outcomes frameworks, there is a 59% and a 86% probability that 24-month recall is the most efficient strategy when considering costs to the dental budget, driven by the potential for cost-savings without adversely affecting health outcomes (generic or dental). However, as DCE respondents highly value more frequent check-ups, the wider societal measure of benefit (CBA) remains favourable to 6-month recall.
In the eligible stratum, analysis frameworks focused on generic health (CUA) and dental health (WTP for dental outcomes) and both indicate that 24-month recall is the most likely recall strategy to deliver an efficient use of resources. The conclusion remains robust to the range of scenario analyses undertaken; however, broadening the scope of benefit valuation to include society’s WTP for services received, the optimal treatment decision would remain 6-month recalls. For the CBA, sensitivity analyses remain robust to this conclusion.
In summary, for those eligible for longer recall intervals, 24-month recall is, on average, the least costly interval for both the NHS and patients, and delivers significant cost savings when combining all costs of attending dental appointments (NHS and patient incurred). These cost savings are achieved with no meaningful differences in generic or dental health outcomes. From these perspectives, 24-month recall is the most likely strategy to generate positive net benefit; however, the general population places a significant value on more frequent dental checks, despite their additional cost and lack of clinical benefit. Taking a more holistic view of benefits, 6-month recall generates the greatest net benefits despite the potential for additional patient and NHS costs.
Chapter 6 Discussion and conclusions
The INTERVAL trial involving regular adult NHS dental attenders has shown that a variable risk-based recall interval is not detrimental to oral health and is acceptable to patients and dentists with the potential for cost savings. Over a 4-year period, we found no difference in oral health between patient participants allocated to a 6-month or a variable risk-based interval. Nor did we find a difference between the intervals of 24-month, 6-month and risk-based recall for the 30% of adults considered suitable to be recalled at 24 months by their dentist. However, people greatly value and are willing to pay for frequent dental check-ups.
To our knowledge, this is the first national, multicentre, pragmatic RCT in a primary care setting to evaluate the clinical and patient-centred outcomes as well as the cost–benefit of different recall intervals. The INTERVAL trial investigated the implementation of a risk-variable approach to recall as recommended in the NICE guideline on dental recall. 60 The guideline takes account of the effect of dental checks on people’s well-being, general health and preventative habits; caries incidence and avoiding restorations; periodontal health and avoiding tooth loss; and avoiding pain and anxiety. It aims to improve or maintain patients’ quality of life and reduce morbidity associated with oral and dental disease. This guideline was initially published in 2004 and most recently reviewed in 2018, confirming that there was no emerging evidence to change the recommendations. Challenges to assumed routines of dental practice, such as the 6-month dental recall and the benefit of regular scale and polish, were voiced as early as 1970. 2,124 The mantra of a 6-month recall has been in existence for decades, and trying to establish the scientific basis for a 6-month or a variable risk-based recall interval was the reason for this trial. Assessment of the cost-effectiveness of different recall intervals was also considered important given the associated patient and NHS costs. Contemporary health care supports a patient-centred, appropriate, preventative and compassionate approach, and a dental recall visit is the opportunity for oral disease to be diagnosed early and for preventative advice and therapy to be provided. The aim of this RCT in primary care dental practice was to provide evidence for the benefit or harm of dental check-ups at different recall intervals on maintaining oral health.
The primary clinical outcome, gingival bleeding on probing, is a measure of gingivitis, a recognised precursor of periodontitis, caused by plaque retention, and is reversible with effective plaque removal. This outcome was chosen because it is an indicator of general oral health status, measurable and responsive to changes in oral self-care behaviour leading to either an improvement or a deterioration. At follow-up, on average, 35% of sites were bleeding on probing, which is similar to values reported in other studies, including the IQuaD study. 21,119 The prevalence of gingivitis confirmed that the trial interventions had the opportunity to have an impact on maintaining oral health, and that we could detect a beneficial or harmful effect. The intervention in INTERVAL was a check-up or recall appointment, which would include oral assessment, disease diagnosis and preventative/oral self-care advice, therefore, with the potential to have an impact on maintaining oral health. Around half of the participants had generalised gingivitis, as defined by the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions (> 30% sites bleeding) and only around 10% had clinical gingival health. Our findings challenge the usefulness of the new classification, since it does not seem to be sensitive enough to distinguish diseased from non-diseased individuals in a healthy sample.
At the 4-year follow-up, for adults allocated by their dentist to the 24-month eligible stratum (therefore, at low risk and suitable for a dental recall of 24 months), there was no evidence of a clinically meaningful or statistically significant difference in gingival bleeding on probing between those randomised to a recall interval of 24 months, risk based or 6 months (24-month vs. 6-month recall –0.91, 95% CI –5.02 to 3.20, p = 0.66; risk-based vs. 6-month recall –0.98, 95% CI –5.05 to 3.09, p = 0.64; 24-month vs. risk-based recall 0.07, –3.99 to 4.12, p = 0.97). Similarly, there was no evidence of a difference between the overall group of participants randomised to a variable risk-based or 6-month recall (0.78 95% CI –1.17 to 2.72; p = 0.43).
We are confident in the finding of no clinical benefit of 6-month recall over a variable risk-based interval, including for those assessed by the dentist as being eligible because of low risk for a 24-month recall, because the adjusted mean bleeding difference between interventions was < 1% and the 95% CIs were small enough to exclude the prespecified clinically important difference in bleeding of 7.5% and 4.5%. INTERVAL did not find a difference in patient-reported generic or oral health-related quality of life between any group, and the participants were satisfied being allocated to a recall interval based on risk.
No evidence of a difference was found in any of the secondary clinical outcomes measured between the three recall intervals for those eligible for 24-month recall or the overall 6-month and risk-based recall groups. The clinical secondary outcomes were periodontal probing depth, caries on coronal surfaces measured at three levels (i.e. initial, moderate and extensive) and the presence of root surface caries, calculus, dental treatment. Patient-centred outcomes included patient anxiety, patient satisfaction with care and patient oral health knowledge, attitudes and behaviours. The range of outcomes measured in INTERVAL was comprehensive, encompassing the important and relevant clinical and patient-centred outcomes. The absence of evidence of a difference between the three recall strategies, therefore, indicates that a variable risk-based recall interval can be supported as it is not detrimental to oral health.
All participants were assessed by their dentists at trial entry to determine whether or not they were suitable for a 24-month recall, and the 30% of participants considered to be eligible were randomised to a 24-month, risk-based or 6-month recall interval. All other participants were randomised to be recalled at a variable interval determined by risk or at 6 months. Dental practices were asked to recall their participants in keeping with their randomised allocation; however, this could be changed if the dentist considered it necessary or if requested by the patient participant because of concerns about their oral health. The pragmatic nature of the trial meant that scheduling of dental appointments and, therefore, the interval between them varied, as they do in routine dental practice because of either practice factors or patient factors. Prior to and since the publication of the NICE dental recall guidelines,60 common practice is to provide a scale and polish or PI at the same time as a dental recall visit. The instruction to participants was not to alter the frequency of this treatment and not to delay in making an appointment if they needed to see the dentist. However, towards the end of the trial follow-up period, dentists were instructed to provide any planned scale and polish following the collection of clinical outcomes by blinded assessors.
At the start of INTERVAL, dentists’ attitudes towards a risk-based approach to recall interval varied. Prior to randomising, all participant dentists were provided with training in the process of the NICE allocation of a variable recall interval. In addition to face-to-face training, it included an interactive online resource that dentists were asked to review as required and repeat annually. The online training included clinical scenarios to demonstrate the range of factors that should be assessed and considered prior to deciding the risk-based recall interval. It was designed to embed the process by taking the clinician through the stages of consideration of modifying factors of risk, integrating this with clinical information and making a clinical judgement of a suitable recall interval, discussing this with the patient and reviewing the interval at subsequent check-ups. The online training was designed to be compliant with CPD requirements and, after completion, a form could be printed to record verifiable CPD.
Oral health-related quality of life was assessed using the validated OHIP-14 measure, which has been widely used as a measure of OHRQoL. Participants deemed eligible to be allocated to a 24-month recall had, on average, a better OHRQoL score than those deemed ineligible. Participants deemed ineligible were also more likely to identify themselves as regular attenders. This suggests that dentists reliably assessed patients’ risks and had done so already, which is in line with their confidence to assess patients’ risks. Similarly, dentists reported that they intended to schedule appointments every 12 months for participants allocated to the risk-based arm in the eligible for 24-month recall stratum, and every 9 months in the ineligible for 24-month recall stratum. National routine data suggest that, in the NHS, the most frequent interval between check-up visits is 9 months, and this reflects the experience of the participants randomised to 6-month visits.
At 4 years, there was a clear separation in the number of check-ups received by the 30% of participants in the 24-month recall stratum: those randomised to the 24-month recall interval had half the number of check-ups of those in the 6-month interval group, and those allocated to a risk-based interval experienced a frequency between the two (24 month = 2.4, 6 month = 4.3 and risk based = 3.4 check-ups). There was a twofold difference in the number of check-up visits between groups of participants who were considered eligible for a 24-month recall. For participants randomised to a risk-based interval who had been considered suitable for a 24-month recall, the decision made by the dentist when applying the risk assessment framework was to allocate an interval of around 12 months. This was closer to the actual recall attendance of the 6-month group than those randomised to be seen at 24-month intervals.
For the 70% of INTERVAL participants who at baseline were considered not eligible for a 24-month recall interval, compared with those who were eligible, both the 6-month and risk-based groups were seen more frequently than the corresponding groups. Having the eligible for 24-month recall stratum allowed us to demonstrate the ability of dentists to assess risk as evidenced by the different number of check-ups for the risk-based groups between strata. At baseline the mean OHRQoL (OHIP-14) score was higher in the ineligible group, indicating that the dentists reliably judged these patients to be at higher risk when scheduling check-ups more frequently. The difference in the number of check-ups during the 4 years was small, 0.3 from routine claims data. National routine data suggest that, in the NHS, the most frequent interval between check-up visits is 9 months, and this reflects the experience of the participants randomised to 6-month visits. From the practice data and the monitoring of allocated recall interval, dentists intended to schedule appointments according to group; however, as INTERVAL was a pragmatic trial, the patient participants were able to amend visit dates in accordance with their need for a convenient appointment time.
The economic analysis was conducted using different perspectives of benefits (QALYs, WTP for dental health outcomes, WTP for dental recall and associated outcomes) and costs (NHS dental, sourced from the routine claims data; NHS dental and other services, such as primary and secondary medical care; and societal, including NHS and participant perspective costs). The preferred perspective depends on normative views of what benefits should be maximised with the NHS dental budget and what costs should be minimised.
The comparison of risk-based recall with 6-month recall is based on data across both trial strata, to which patients who are ineligible for 24-month recall contribute the majority of data. Using evaluation frameworks that maximise generic or dental health, there is substantial uncertainty regarding the most efficient recall strategy. In general, 6-month recall has a higher probability of cost-effectiveness, but the certainty around this conclusion tends to decrease as a wider perspective of participant-incurred costs is included. The results are sensitive to country-level subgroups, with risk-based recall having a higher probability of cost-effectiveness in a Scottish setting. As the general population places a high value on 6-month recall, expanding the valuation space to include all societal benefits (i.e. WTP for different recall intervals) leads to a high probability that 6-month recall has the greatest net benefits in a societal CBA framework. This conclusion is robust to sensitivity analyses undertaken.
Only participants who were deemed eligible for 24-month recall were randomised to the three-way comparison of 6-month versus risk-based versus 24-month recall, and so were more likely to be deemed at low risk of dental problems. The 24-month recall strategy is the least costly strategy to the NHS and generates significant cost savings compared with both 6-month and risk-based recall when also considering the opportunity cost of time and travel, and patient co-charges associated with attending the dental practice more regularly. When adopting either a generic or a dental health maximisation perspective, 24-month recall is likely to be the most efficient strategy; however, when taking a broader perspective of benefits, and incorporating the general population’s valuation of recall intervals, these cost savings are offset by the perceived benefit of more frequent recall. In all cases where a wider perspective of benefits is included in the CBA, 6-month recall remains the strategy with the greatest likelihood of positive net benefits and this conclusion is robust to sensitivity analyses undertaken.
In summary, the recall strategy generating the greatest value for money is dependent on eligibility for 24-month recall and the decision-maker’s views on the scope of costs and benefits that should be valued in the economic evaluation.
Framework 1 (maximising generic health benefit) used the results of the CUA to assess the most cost-effective strategy in terms of maximising generic health outcomes (i.e. EQ-5D-3L-based QALYs). There was substantial uncertainty surrounding the optimal recall strategy across all analyses undertaken. This is a result of concerns regarding the QALY’s sensitivity to capture any potential benefits of dental care interventions. The CEACs and scatterplots of the cost-effectiveness plane illustrate the residual uncertainty, rendering it difficult to draw clear cost-effectiveness conclusions using this metric; for example, in the combined analysis across both trial strata, no strategy achieved a probability of cost-effectiveness > 70% at a threshold value of society’s WTP for a QALY gain of £20,000. The probability of cost-effectiveness was higher for the 24-month recall strategy in the analysis restricted to the eligible for 24-month recall stratum because of the potential for cost savings from longer recall intervals.
Framework 2 (maximising societal well-being), taking the broadest perspective of benefits, including all components of value to the general population (incorporating both health and non-health sources of utility), generates a high probability that 6-month recalls are net beneficial. This finding is consistent across the full range of sensitivity analyses undertaken. This conclusion is influenced by the high value that the general population attaches to the 6-month recall service attribute in the DCE.
The DCE provided important information on the valuation of dental health outcomes; however, it is unclear why the general population places such a high value on service provision, controlling for health outcomes. The high WTP values attached to more frequent dental recall services was the main driver of results favouring 6-month recall in the wider-perspective CBA. Policy-makers may require further insights into the source of value attached to interventions in order to make informed decisions using a CBA framework, and further research is required to identify these sources of value. Some hypotheses for the high valuation of 6-month check-ups might include:
-
Status quo bias, where respondents place a high value on services they usually receive.
-
Omitted variable bias may occur if respondents place a value on some perceived outcomes that were not included in the DCE, such as increased likelihood of detection of oral cancers, or an assumption that check-ups come with scale and polish and associated aesthetic benefits.
-
Supplier-induced demand where the population feel that they need to see the dentist every 6 months because that is the expert advice they have received over a long period of time (even if it is not evidence based).
-
Reassurance provided by a consultation with a health professional.
Further work is required to more completely understand what it is that respondents explicitly value about 6-month recalls.
Framework 3 (maximising dental health benefits) evaluated the most efficient dental recall strategy in terms of maximising dental health benefit (i.e. through the DCE valuation of bleeding and caries outcomes). For the group deemed eligible for 24-month recalls, differences in costs to the total NHS dental budget (across the UK) are not statistically significantly different across the randomised arms; however, substantial cost savings can be achieved from longer recall intervals when considering the combined cost burden to both patients and the NHS. These savings can be achieved without adversely affecting dental health outcomes. A 24-month recall has the greatest probability of positive net dental health benefit, ranging between 65% and 99% across the full range of sensitivity analyses conducted.
For the trial population as a whole (including both eligible and ineligible for 24-month recall strata), there is substantial uncertainty regarding the most efficient strategy to maximise dental health benefit. Risk-based recalls were more likely to generate positive net dental health benefit in Scotland than in England, and when a wider perspective of the costing analysis was considered.
Comparison with other randomised clinical trials and studies
The Cochrane systematic review of dental recall, which was updated in 2013,72 reported insufficient evidence to determine the effect of different recall intervals, with only one study included. 125 The trial population of the only included study125 comprised children or adolescents aged 3, 16 or 18 years at trial entry. Wang et al. 125 included 185 patients and compared recall intervals of 12 months and 24 months over a 24-month follow-up period for the outcomes caries, as measured by decayed, missing and filled surfaces of primary teeth (dmfs) increment, and decayed, missing and filled surfaces of permanent teeth (DMFS) increment, and total time taken for examination and treatment. The authors of the Cochrane review concluded that a very low-quality body of evidence from one RCT was insufficient to reach any conclusions regarding the potential beneficial and harmful effects of varying recall intervals between dental check-ups. The Cochrane review Recall intervals for oral health in primary care patients was updated in 2020 by authors of the INTERVAL trial monograph, incorporating results from this study. 126 The updated search of 17 January 2020 identified the INTERVAL trial as the only new study eligible to be included in the updated review and the only trial to include patient participants > 18 years at trial entry. Given that different outcomes were reported in the two studies included in the updated Cochrane review, it was not possible to synthesise the data. The forest plots from the INTERVAL trial data are included to present the treatment effects across trial arm comparisons for the INTERVAL trial-reported outcomes (Figures 21–23).
FIGURE 21.
Forest plot for (a) the continuous outcomes; and (b) the outcome prevalence of extensive caries: risk-based vs. 6-month recall. M–H, Mantel–Haenszel.
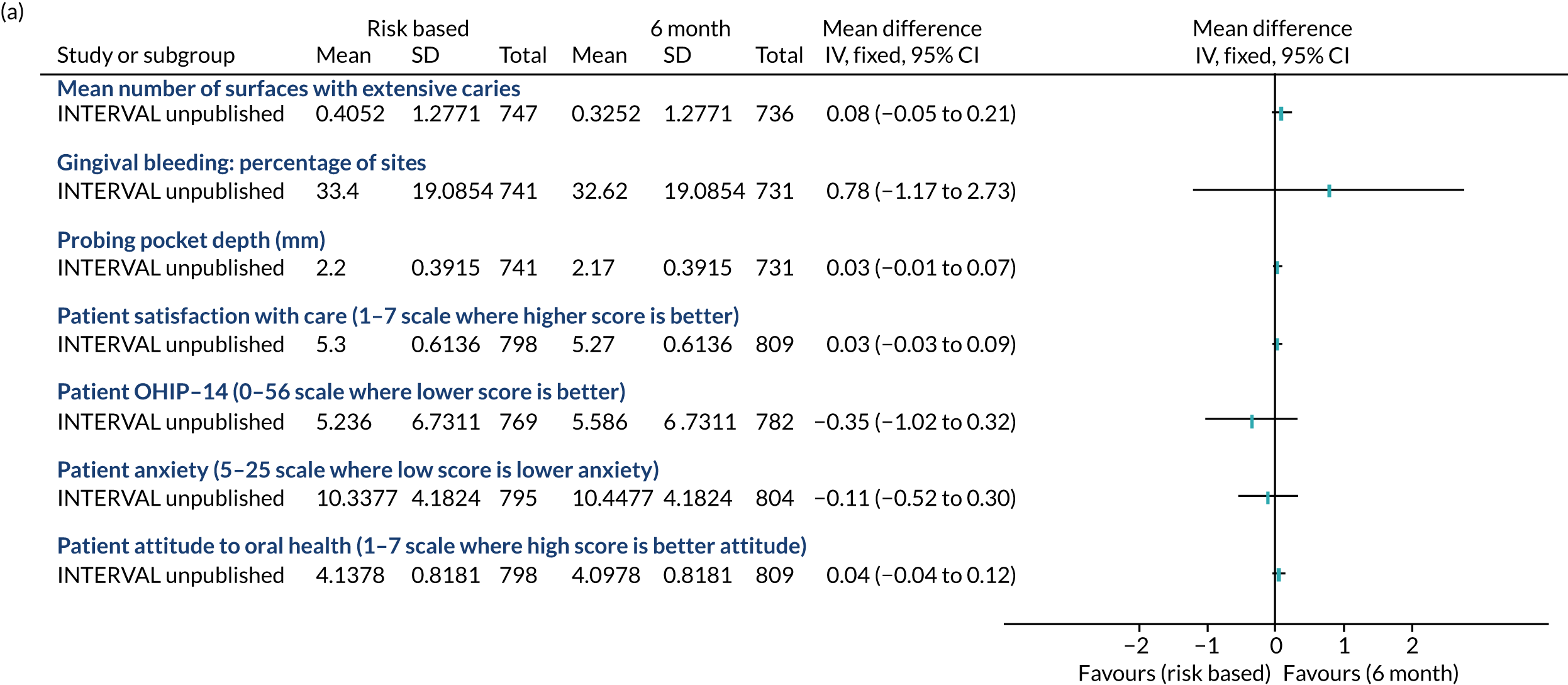

FIGURE 22.
Forest plot for (a) the continuous outcomes; and (b) the outcome prevalence of extensive caries: 24-month vs. 6-month recall.
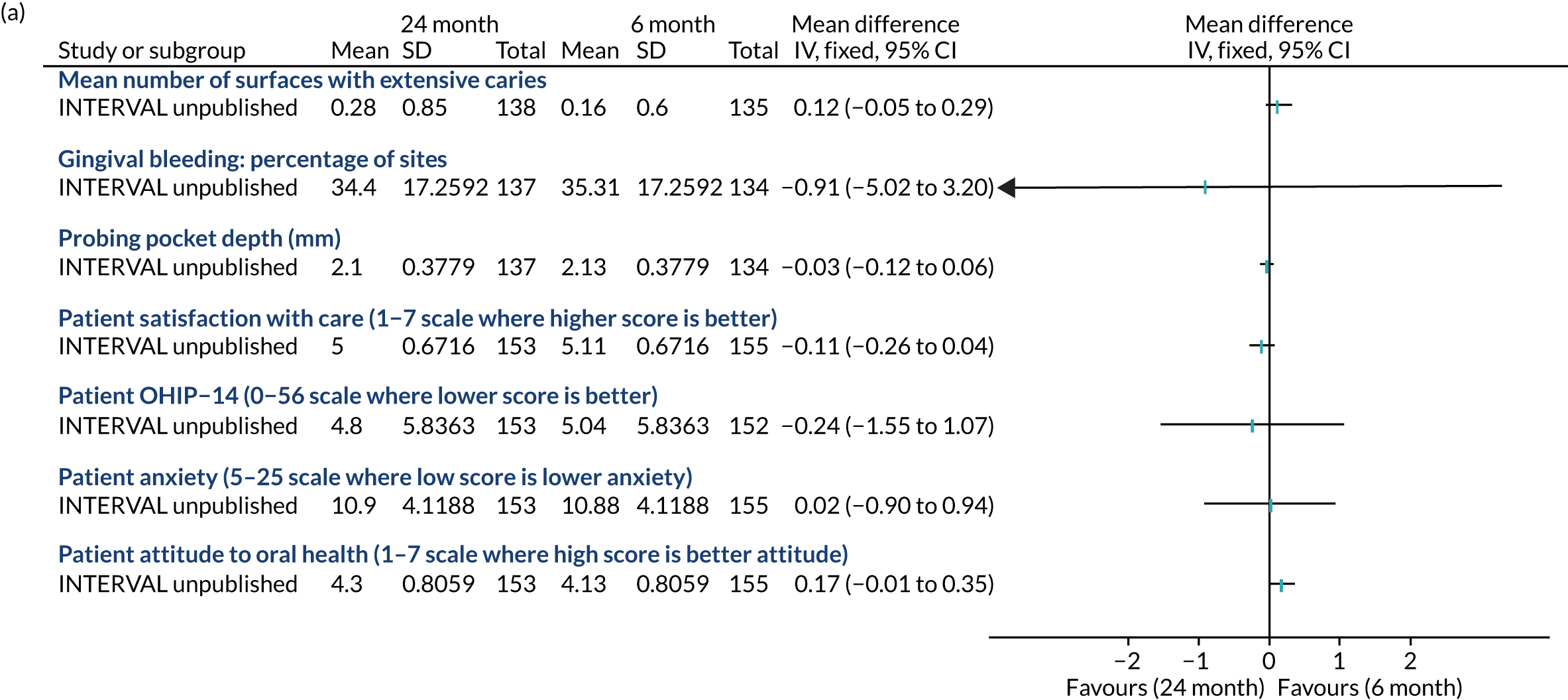
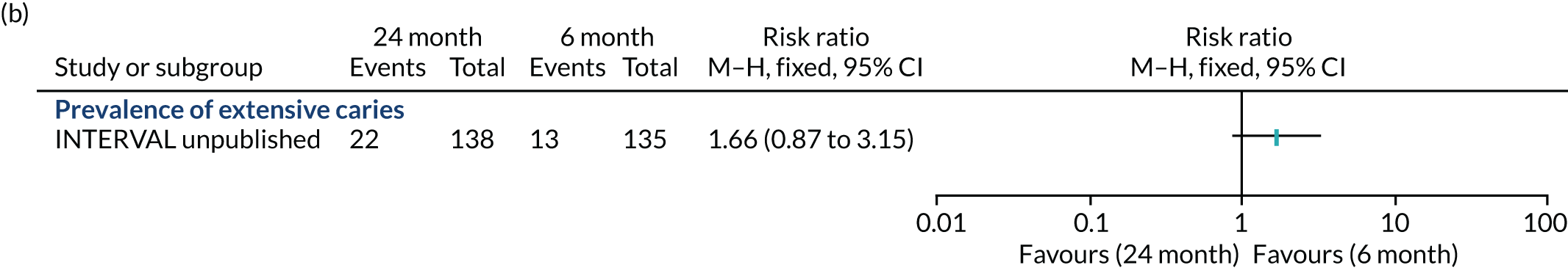
FIGURE 23.
Forest plot for (a) the continuous outcomes; and (b) the outcome prevalence of extensive caries: risk-based vs. 24-month recall. M–H, Mantel–Haenszel.
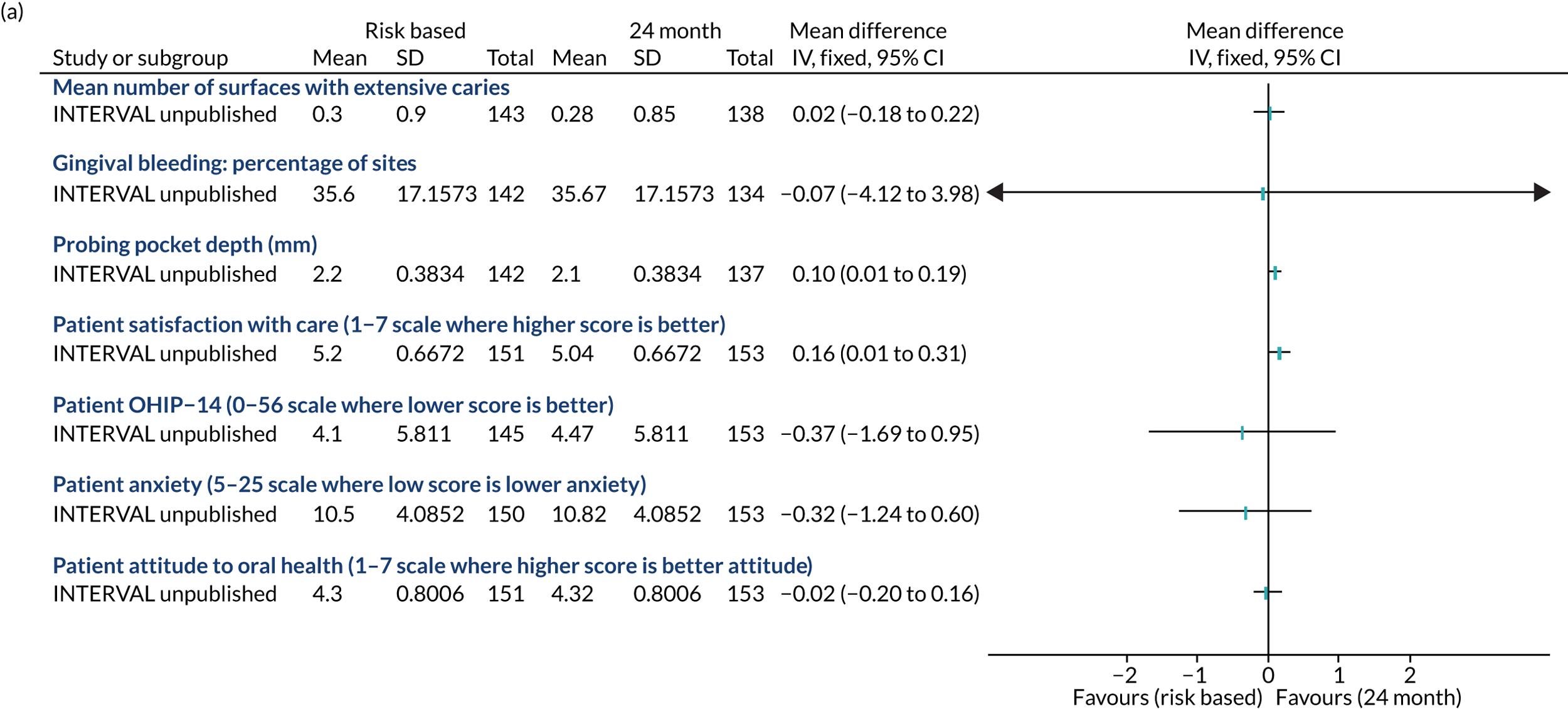
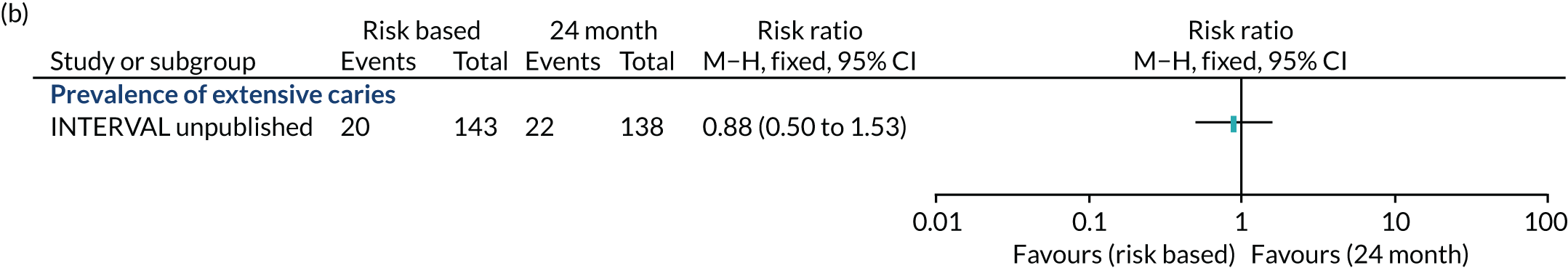
To our knowledge, this is the first RCT to fully integrate costs and benefits of different dental recall intervals and, therefore, provides the best available evidence on the short-term cost-effectiveness of different recall strategies proposed in the NICE guidance. 127 The NICE guidance was informed by a HTA report that developed a lifetime decision model to assess the cost-effectiveness of 3-, 6-, 12-, 18-, 24- and 36-month recall from a UK NHS perspective. 71 The report found that the incremental cost per decayed, missing, filled (DMF)-free tooth gained increased as recall intervals were narrowed; however, given that there is no evidence or precedence regarding society’s WTP for a DMF-free tooth gained, the results are difficult to interpret. Furthermore, the scope of costs was limited, and included only check-ups and treatment for decay, omitting any other dental treatments that may be triggered by recall. Finally, the results are of limited value to current decision-making as the payment system for dentistry has been updated in England and Wales, since this review was carried out, to the current banding system. To our knowledge, there are no other studies directly relevant to the cost-effectiveness of routine dental checks or oral health reviews in a UK setting.
Strengths
The INTERVAL trial was a pragmatic trial in primary care dental practice designed to provide evidence for the clinical and cost-effectiveness of variable recall intervals. The traditional view and ‘mantra’ of 6-month check-ups still exists among the majority of patients and dental professionals despite the introduction of recommendations decades ago for a risk-based and variable approach to reviewing patients. 3 The design of INTERVAL was complex and unique in order to answer the commissioning brief. A strength of the design was being able to compare three recall intervals and demonstrate the ability of dentists to distinguish between high- and low-risk routine dental attenders. INTERVAL included outcomes that were relevant and important to patients, clinicians and policy-makers. The design enabled and ensured that the most robust measures were collected including independent clinical assessments and access to national routine data.
Surprisingly, there have been few high-quality studies comparing different recall intervals, whether fixed or variable, according to risk. This is at a time when NHS resources are constrained, and there are demands for realistic and patient-centred care across health care. The published studies evaluating different recall intervals have involved few providers and most are in specialist settings that evaluate the impact of complex interventions usually directed at preventing caries in high-risk children. The only other trial undertaken was in a population of children and adolescents who received regular dental care in a public dental clinic in Norway from one dentist and one dental hygienist. 125 The trial inclusion criteria were less pragmatic than in INTERVAL, with individuals considered at high risk of dental caries excluded from the study. The commissioned call that INTERVAL answered presented a complex question that required a novel trial design. The strength of the design was to include two strata to avoid allocating participants to a 24-month recall for whom it was not clinically appropriate. A misinterpretation of INTERVAL may come about if the design is not understood. A significant strength is that dentists in INTERVAL applied their professional and clinical judgement before allocating a patient participant to the stratum that included randomisation to 24-month recall.
A strength of INTERVAL was that it was a pragmatic trial allowing the dentists and patient participants to respond to changes in circumstance. Changes in circumstance could have been logistics for allocating a recall appointment, or not attending as scheduled because of holidays, or responses to change in clinical condition. We predicted that the participants allocated to a 6-month recall would be most likely to attend at 9 months, thereby taking into account the added duration of any course of treatment following the previous check-up date and adjustments for the logistics or general life. A strength of INTERVAL is that dentists did implement a different recall interval for the patient participants randomised to the risk-based group in both strata of the trial. This overcame a potential risk at the outset that we might not observe a difference in the number of check-ups experienced; however, a surprising observation was that the time interval for the risk-based group was so close to the 6-month recall group. In addition, for patients whom dentists felt were suitable not to be seen, unless needed, for 24 months, dentists allocated a risk-based recall interval in between 6 months and 24 months, which was, however, not closer to the latter. A further strength of INTERVAL is that because of its pragmatic nature it has demonstrated that dentists can accurately and reliably assess risk as shown by the difference in the recall behaviours of the two strata.
The strengths of the INTERVAL trial include the recruitment and retention of a large number of centres (n = 51) and, to our knowledge, is the first dental trial to involve all the nations of the UK. As the nations operate in different contractual systems, INTERVAL provides insight to the potential impact that the system has on clinical decision-making and practice systems. The dental practices represent a wide range of geographical locations including remote and rural settings, and a range of characteristics including the number of dentists (average three), and whether or not they were a training practice, or employed at least one hygienist (70%). INTERVAL was not powered to detect differences across contractual systems and the numbers from Northern Ireland are too small to make any conclusions, although there appears to be a difference in the determination of risk-based recall interval for dentists in England and Scotland.
Recruitment and retention in trials is a frequent challenge, but an achievement for the INTERVAL trial was the recruitment of 2372 of the potentially eligible patients, and their retention with provision of data at 4 years from 1765 participants with 69% providing clinical data. This may be as a result of the participants being routine dental attenders and, hence, interested in dental health, and it was possibly their first invitation to participate in research. The randomised groups in both strata were balanced/similar at baseline and the reasons for loss to follow-up were related to inability of the practice to contact its patients; therefore, we were confident in the robustness of the results regardless of the missing data. The dental behaviour and clinical characteristics of the participants mean that the findings of the INTERVAL trial are generalisable to regular dental attenders across the UK in the NHS, and, therefore, also in similar third-party funding systems. Applying a risk-based determination of recall interval is advocated in many dental health-care systems and hence the findings of INTERVAL have relevance internationally.
The INTERVAL trial found no evidence of a difference in oral health from a risk-based recall interval compared with the traditional 6-month check-up. This also applied to patient participants whom the dentist assessed as low risk and suitable for a check-up at 24 months. For this group, the INTERVAL trial found no evidence of a difference between 24-month, risk-based or 6-month recall. The results were robust to sensitivity analyses exploring the impact of missing data.
The pragmatic design of the trial did not prevent fidelity to the interventions with separation in the frequency of dental check-ups between groups. We have evidence of intervention fidelity though monitoring intended check-up appointments. Dentists participated in training and were asked to revisit the e-learning tool, although compliance was variable. Conducting the trial in a primary care NHS setting with a design that involved minimal requirements for the dental practices, blind outcome assessment and access to routine data are strengths. The process undertaken to inform the determination of a risk-based recall was not independently observed (i.e. information collection, assessment and consultation with the patient); however, we believe the dentists performed this intervention as instructed in a way with which they were confident. The evidence from the dentist experience questionnaire supports the view that dentists who randomised participants to the 24-month stratum increased in confidence and were more likely to adopt/maintain that behaviour. In contrast to those who did not, their concern/anxiety increased by the end of the trial.
There was no evidence that different recall intervals made a difference to measures of patient quality of life, anxiety and satisfaction with care. The strength of INTERVAL was that these measures were collected annually during the 4 years of the trial. The reaction of some to them being considered suitable to have a recall interval of 24 months was an indication of confidence in their ability to maintain their oral health.
The health economic analysis is based on best practice methodology for conducting economic evaluation alongside RCTs. The analysis provides the only evidence that is relevant to decision-making in the UK NHS regarding the most efficient dental recall interval, and is based on the current, most up-to-date payment systems across the UK regions. The analysis is novel in implementing a CBA considering both WTP for dental health outcomes and wider societal benefits. The use of a DCE to capture the value placed on both the trial interventions and important dental health outcomes by the general population is a distinct advantage and overcomes the problems with interpreting economic evaluation results from other studies. The approach ensures consistency of methodology with recent economic evaluations of routine dental interventions (e.g. the IQuaD study101). A further advantage of CBA is the simultaneous valuation of multiple dental health outcomes within a single outcome measure (i.e. bleeding gums and dental decay), representing an important advantage over more traditional, single outcome measure cost-effectiveness studies (e.g. decayed teeth only) typically conducted in dentistry.
In terms of the within-trial analysis, we used best practice methodology, incorporating the most advanced recommendations for analysis with the appropriate use of missing data models to minimise the potential for bias.
Limitations
The INTERVAL trial experienced challenges throughout its course, some of which were anticipated and others of which could not have been predicted. Recruitment of dentists and participants was a challenge which was overcome, but this led to a considerable delay in the trial. The lack of research-experienced practices and the fact that the dentists were responsible for recruiting and obtaining consent from patient participants had an impact on our ability to reliably predict recruitment. INTERVAL was the first in a series of trials that the research team have been involved with, and experience has shown that a weakness was not having the research nurses supporting practices in person at dedicated recruitment sessions to overcome this. We did not collect information on the total number of routine patients approached as practices operated a process that complemented their practice management system; therefore, the reasons why patients chose not to participate in INTERVAL are unknown.
We experienced delays in analysis due to challenges encountered obtaining routine data. The process and mechanisms for establishing permission for access to routine data did not exist for England and Wales. Retrieving the routine data delayed analysis; the reasons for this included there not being a system for information retrieval or sharing of dental data at NHS services in England and Northern Ireland prior to INTERVAL.
A weakness was not collecting information on what factors were considered to inform the risk-based interval and the interaction with the patient to both determine risk and negotiate the interval. A possible weakness was the choice of the primary outcome as gingival bleeding on probing for which calibration is not possible. The rationale for this decision was that it is a measurable and modifiable outcome that indicates oral health in general. INTERVAL evaluated different recall intervals on maintaining oral health and no difference in this disease measure would reflect the absence of either improvement or deterioration. The other clinical outcomes were recorded, but the team’s experience and previous research confirmed that the progression of caries and periodontal disease was going to be too slow in this population.
INTERVAL had a drop-out rate of 25–30% in the questionnaire data and 30–37% in attendance at appointments, a higher value than expected at the design stage of the trial. However, the 95% CIs for risk-based versus 6-month recall and risk-based versus 24-month recall precluded our predefined clinical minimally important differences in gingival bleeding of 4.5% and 7.5%, respectively. In addition, the drop-out rates were balanced between the arms, and sensitivity analyses using multiple imputation showed that the results were robust.
The analysis is based on 4-year outcomes from the trial. It is important to extrapolate trial results over a longer-term time horizon in order to fully capture all the costs and outcomes associated with different interventions. This was outwith the scope of the current study. There is no evidence from the trial to suggest differences in caries experience or bleeding across the different recall intervals, and it is therefore unlikely that a decision model would lead to different conclusions regarding the most efficient allocation of resources. There is a lack of good-quality information in dentistry to inform decision analysis models, for example estimates of long-term baseline transition probabilities to determine the life course of a tooth through different stages (well, decayed, root canal treatment required, missing). Further research is urgently required to bridge this gap in the evidence base and determine the economic value of long-term caries prevention.
There are different payment systems across the different UK regions. Dental check-ups are free of charge in the fee-for-service system in Scotland, there are patient co-payments in the Northern Ireland fee-for-service system, whereas England and Wales adopt a treatment banding approach. The method of payment and the cross-region variation raise three specific issues for the economic evaluation:
-
The payment for dental care services is not necessarily a true reflection of the opportunity cost of the resource required to deliver dental services. Owing to the practical barriers to conducting a detailed micro-costing exercise, and the vast number of different possible treatments in primary dental practice, we were not able to directly elicit opportunity costs in this study.
-
The different payment systems across the regions, and the process of submitting dental claims means that the resource use data collected from the practices varies substantially, which limits comparability. The quality of the data, in particular the detailed treatment information (e.g. check-ups and fillings) obtained from the routine claims data, could also potentially vary across countries.
-
The different systems may induce different provider and consumer behaviours, and different regional-specific incentives make direct comparability of results across the regions difficult. However, results are unlikely to be biased at the average UK level because the proportion of practices randomising to each group was approximately equal across the different regions.
Clinical assessment
Overcoming the practical challenge of arranging the final clinical assessment visits for participants at 51 dental practices across the four nations of the UK relied on trust, respect and partnership working between the research team and the general dental practices involved. Patient participants volunteered around 30 minutes of their time for the clinical assessment by one of the blinded assessment teams. The attendance of 1624 participants for their clinical assessments demonstrates their interest, commitment and the value placed on seeking evidence for routine oral health care and the role of practice-based dental research. The robust training undertaken prior to data collection and throughout the assessment period ensured consistency between assessors. The fact that calibration was not possible does not limit the findings, and we believe that no more could have been done to ensure reliable measures.
Patient and public involvement and engagement
Prior to the start of the trial, advice on the design and conduct of the study was sought from members of the Public Partnership Groups and from similar patient groups in other parts of the UK sourced under guidance from INVOLVE.
These independent public partnership groups comprise volunteers who work in partnership with NHS Tayside and aim to provide a conduit for the views of people about their local services.
Patient advisors were a valuable resource at the outset of the trial and they helped to ensure good conduct and patient-friendly practice throughout the duration of the trial. Patient advisors were involved with the trial design and provided invaluable feedback on trial recruitment and communication strategies. Patient advisors also contributed to the content and layout of the trial invitation, trial newsletters and the design of patient participant questionnaires. This ensured that trial participants could understand and easily complete these materials. This input was obtained remotely and from individuals. In future studies, we would recommend working with a group with some face-to-face interaction.
As quality of life was a primary outcome of the trial, patient advisors’ input to the proposed questionnaire design was essential. Qualitative work with patients was carried out to ensure that the outcome measures were patient-centred.
Lay representatives on the TSC and TMC actively contributed to trial oversight, processes and procedures, including helping to interpret the trial findings, preparation of the monograph, and assisting with the review of the Plain English summary.
The results were discussed with the four Chief Dental Officers of the UK to enable early consideration of the implications for policy and practice.
Generalisability
The INTERVAL trial was designed pragmatically to investigate the effectiveness and cost–benefits of three different recall intervals. The 51 recruiting dental practices were situated in England, Wales, Northern Ireland and Scotland. On average, participating dentists had been qualified for 16 years and had an approximate total list size of 5000 patients. The average number of dentists in their practices was three and approximately 70% of practices employed at least one hygienist.
As at 30 September 2018, 94% of the adult population in Scotland were registered with an NHS dentist. Of these, 67% had attended their dentist at least once in the previous 2 years. The Adult Dental Health Survey 2009114 reported that over half of the population in England, Wales and Northern Ireland had attended a dental practice within the last 3 years. The INTERVAL trial recruited 2372 patient participants who were regular attenders and had attended the dentist at least once in the previous 2 years, with 43% recruited in England and Wales, 7% in Northern Ireland and 50% in Scotland. 128
At baseline, the average age of participants was 45 years, 55% were female, 15% identified as smokers and 55% used manual toothbrushes.
We are confident that the practices and participants recruited are a true representation of adults who attend NHS dental practices across the UK.
Recommendation for research
Further research is required to:
-
enhance and support the communication of a variable risk-based recall interval to patients and the dental team, and the co-development of risk assessment tools
-
develop recall interval quality management and improvement tools
-
understand the role of risk-based recall and risk management of adults who are irregular dental attenders
-
better understand public perceptions of the value of 6-month fixed-period recall intervals to help elucidate barriers to and facilitators of change
-
inform methods for long-term decision modelling in dentistry utilising long-term dental cohort data where possible
-
better utilise routine claims data sets, including tooth-level treatment data, to create cohorts of data to follow and predict individuals’ health and to understand and improve care pathways in dentistry.
Acknowledgements
Recruitment sites
We would like to thank the staff and participants of the following dental practices:
Aesthetics Dental & Implant Surgery, Amblecote Dental Care, Anita Belbin Dental Surgery, Bayview Dental Practice, Bridge Dental Centre, Brightons & Polmont Dental Practice, Brunswick Dental Practice, Bute Dental Surgery, Castle House Dental Practice, Chong Kwan Dental Centre, Church Road Dental Care, Colin C Yule & Associates, Collegiate Dental Practice, Cottam’s Dental, Implant & Orthodontic Practice, Craigmillar Dental Centre, Discovery Dental Care, Dunbar Dental Practice, Eastside Dental Practice, Green Lane Dental Surgery, Hazel House Dental Surgery, Horizon Dental Clinic (Blyth), Horizon Dental Clinic (Monkseaton), Kelvingrove Dental Care Ltd, Kingsway Dental Practice, Lochshell Dental Clinic, Loughside Dental Practice, M&S Dental Care, Montgomery Street Dental Care, Montrose Dental Practice, Mount Florida Dental, My Dental Care, N13 Dental Clinic, Nairn Dental Clinic, Number One Surgery, Park View Family Dental, PCK Wee Dental Surgery, PG Lawson & Associates, Platt & Common, Roseville Dental Practice, Salmon Lane Dental Care, Shotley Bridge Dental Care, Six Gables Dental Practice, Smiledent Dental Practice, Southside Dental Care, St Leonard’s Dental Practice, Walmley Dental Practice, Windsor Dental Practice (Manchester), Windsor Dental Practice (Salford), Woodside Dental Practice and Young Smile Dental Care.
Independent members of the Trial Steering Committee
Robert Elford, Trevor Johnson, Edwina Kidd (former chairperson), the late James Steele (chairperson) and Elizabeth Treasure (chairperson).
Members of the Trial Management Committee
Tony Anderson, Debbie Bonetti, Trevor Burke, Philip Dolan, Mark Forrest, Ronald Gorter, Richard Herbert, Penny Hodge, Dirk Mettes, Wendy McCombes, Ian Needleman, Margaret Ross and Debbie White.
Independent members of the Data Monitoring Committee
Martin Chalkley, Chris Deery and Simon Gates (chairperson).
Ethics approval
Ethics approval for the trial was given by the Fife and Forth Valley Research Ethics Committee on 13 January 2009 and 20 September 2011 (Research Ethics Committee reference 09/SO501/1).
The trial was registered with the ISRCTN (reference number ISRCTN95933794).
Funding
The University of Dundee agreed to act as sponsor for the research and the study was adopted by the Scottish Dental Practice Based Research Network and the relevant local research networks in England, Wales and Northern Ireland.
Service support was provided by a central subvention from the NIHR and the local primary care trusts and health boards.
Contributions of authors
Jan E Clarkson (https://orcid.org/0000-0001-5940-2926) (Professor and Joint Chief Investigator) contributed to the conception and design of the trial, the conduct of the trial, the recruitment of dentists, the interpretation of the results, and the drafting, editing and approval of the monograph.
Nigel B Pitts (https://orcid.org/0000-0001-6184-4213) (Professor and Joint Chief Investigator) contributed to the conception and design of the trial, the conduct of the trial, the recruitment of dentists, the interpretation of results, and the drafting, editing and approval of the monograph.
Beatriz Goulao (https://orcid.org/0000-0003-1490-7183) (Statistician) developed the statistical analysis plan, led the statistical analyses, contributed to the interpretation of the data, made a significant contribution to the drafting of Chapter 4, and reviewed and approved the monograph.
Dwayne Boyers (https://orcid.org/0000-0002-9786-8118) (Health Economist) conducted the economic analysis, contributed to the interpretation of the data, drafted Chapters 3 and 5, and reviewed and approved the monograph.
Craig R Ramsay (https://orcid.org/0000-0003-4043-7349) (Professor) contributed to the conception and design of the trial, the conduct of the trial, oversaw trial methods, the interpretation of the results, and the drafting, editing and approval of the monograph.
Ruth Floate (https://orcid.org/0000-0003-2424-4643) (Trial Manager) was responsible for the day-to-day management of the trial, contributed to the interpretation of results, and the drafting and editing of the monograph.
Hazel J Braid (https://orcid.org/0000-0002-2062-8546) (Trial Administrator) was responsible for the day-to-day management of the trial, contributed to the interpretation of results, and the drafting and editing of the monograph.
Patrick A Fee (https://orcid.org/0000-0002-7188-3416) (Clinical Research Fellow) was responsible for the conduct of the trial, contributed to the collection of clinical outcomes, the interpretation of results, and the drafting and editing of the monograph.
Fiona S Ord (https://orcid.org/0000-0002-9901-7739) (Research Hygienist) was responsible for the recruitment of participants, contributed to the collection of clinical outcomes, and contributed to the drafting and editing of the monograph.
Helen V Worthington (https://orcid.org/0000-0002-4851-7469) (Professor and Triallist) contributed to the design of the trial, the interpretation of results and reviewing and approving the monograph.
Marjon van der Pol (https://orcid.org/0000-0003-0636-1184) (Professor) contributed to the conception and design of the health economics analysis, oversaw health economic methods, and contributed to editing the monograph.
Linda Young (https://orcid.org/0000-0002-6004-3374) (Research Manager and Economist) contributed to the design of the trial, the interpretation of results, and drafting and editing of the monograph.
Ruth Freeman (https://orcid.org/0000-0002-8733-1253) (Professor) contributed to the design of the trial, the interpretation of results, and reviewing and approving the monograph.
Jill Gouick (https://orcid.org/0000-0002-1456-8241) (Research Dental Nurse) was responsible for the collection of clinical outcomes, and contributed to reviewing and approving the monograph.
Gerald M Humphris (https://orcid.org/0000-0002-4601-8834) (Professor) contributed to the design of the trial, the interpretation of results, and reviewing and approving the monograph.
Fiona E Mitchell (https://orcid.org/0000-0002-7922-0676) (Research Dental Nurse) was responsible for the collection of clinical outcomes, and contributed to reviewing and approving the monograph.
Alison M McDonald (https://orcid.org/0000-0002-0256-2889) (Senior Trials Manager) was responsible for overseeing management of the trial and contributed to the interpretation of data, and to reviewing and approving the monograph.
John DT Norrie (https://orcid.org/0000-0001-9823-9252) (Professor and Triallist) contributed to the design of the trial, the interpretation of the data, and reviewing and approving the monograph.
Kirsty Sim (https://orcid.org/0000-0002-2365-5013) (Research Hygienist) was responsible for the collection of clinical outcomes, and contributed to reviewing and approving the monograph.
Gail Douglas (https://orcid.org/0000-0002-0531-3909) (Professor) contributed to the design of the trial, the interpretation of results, and reviewing and approving the monograph.
David Ricketts (https://orcid.org/0000-0002-9851-1778) (Professor) contributed to the design of the trial, the interpretation of results, and reviewing and approving the monograph.
Publication
Clarkson JE, Pitts NB, Bonetti D, Boyers D, Braid H, Elford R, et al. INTERVAL (investigation of NICE technologies for enabling risk-variable-adjusted-length) dental recalls trial: a multicentre randomised controlled trial investigating the best dental recall interval for optimum, cost-effective maintenance of oral health in dentate adults attending dental primary care. BMC Oral Health 2018;18:135.
Data-sharing statement
All available data can be obtained by contacting the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Clarkson JE, Pitts NB, Bonetti D, Boyers D, Braid H, Elford R, et al. INTERVAL (investigation of NICE technologies for enabling risk-variable-adjusted-length) dental recalls trial: a multicentre randomised controlled trial investigating the best dental recall interval for optimum, cost-effective maintenance of oral health in dentate adults attending dental primary care. BMC Oral Health 2018;18. https://doi.org/10.1186/s12903-018-0587-2.
- Sheiham A. Is there a scientific basis for six-monthly dental examinations?. Lancet 1977;310:442-4. https://doi.org/10.1016/S0140-6736(77)90620-1.
- Clarkson JE, Amaechi BT, Ngo H, Bonetti D. Recall, reassessment and monitoring. Monogr Oral Sci 2009;21:188-98. https://doi.org/10.1159/000224223.
- Frame PS, Sawai R, Bowen WH, Meyerowitz C. Preventive dentistry: practitioners’ recommendations for low-risk patients compared with scientific evidence and practice guidelines. Am J Prev Med 2000;18:159-62. https://doi.org/10.1016/S0749-3797(99)00138-5.
- Kay EJ. How often should we go to the dentist?. BMJ 1999;319:204-5. https://doi.org/10.1136/bmj.319.7204.204.
- Patel S, Bay RC, Glick M. A systematic review of dental recall intervals and incidence of dental caries. J Am Dent Assoc 2010;141:527-39. https://doi.org/10.14219/jada.archive.2010.0225.
- Gussy MG, Bracksley S, Boxall A. How Often Should You Have Dental Visits?. Deakin, ACT: Deeble Institute; 2013.
- Fauchard P, Lindsay L. The Surgeon Dentist: Or, Treatise on the Teeth. In Which is Seen the Means Used to Keep Them Clean and Healthy, of Beautifying Them, of Repairing Their Loss and Remedies for their Diseases and Those of the Gums and for Accidents Which May Befall the Other Parts in Their Vicinity. With Observations and Reflexions on Several Special Cases. London: Butterworth & Co; 1946.
- Sheiham A. Routine check-ups. Br Dent J 2000;189. https://doi.org/10.1038/sj.bdj.4800718a.
- Routine six-monthly checks for dental disease?. Drug Ther Bull 1985;23:69-72.
- Elderton RJ. Routine six-monthly checks for dental disease?. Br Dent J 1985;159. https://doi.org/10.1038/sj.bdj.4805706.
- Lock S. Getting the balance right. Br Med J 1986;292:428-9. https://doi.org/10.1136/bmj.292.6518.428.
- Perlus J. Determining recall frequency. A controversial issue. Ont Dent 1994;71:31-5.
- Renson T. The six-monthly dental examination. Dent Update 1977;4:421-3.
- Renson T. The professor, the newspaper and the six-monthly check-up. Prim Dent Care 2000;7. https://doi.org/10.1308/135576100322694141.
- Sheiham A. Is the six-monthly dental examination generally necessary?. Br Dent J 1980;148. https://doi.org/10.1038/sj.bdj.4804393.
- Taylor G. Modernising NHS dentistry-implementing the NHS plan. Community Dent Health 2000;17:207-9.
- Pitts NB. NHS Dentistry: Options for Change in context – a personal overview of a landmark document and what it could mean for the future of dental services. Br Dent J 2003;195:631-5. https://doi.org/10.1038/sj.bdj.4810779.
- Tan EH, Batchelor P, Sheiham A. A reassessment of recall frequency intervals for screening in low caries incidence populations. Int Dent J 2006;56:277-82. https://doi.org/10.1111/j.1875-595x.2006.tb00101.x.
- Leake JL, Birch S, Main PA, Ho E. Is regular visiting associated with lower costs? Analyzing service utilization patterns in the first nations population in Canada. J Public Health Dent 2006;66:116-22. https://doi.org/10.1111/j.1752-7325.2006.tb02566.x.
- Trombelli L, Farina R, Silva CO, Tatakis DN. Plaque-induced gingivitis: case definition and diagnostic considerations. J Periodontol 2018;89:46-73. https://doi.org/10.1002/JPER.17-0576.
- Papapanou PN. Epidemiology of periodontal diseases: an update. J Int Acad Periodontol 1999;1.
- Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodont 2018;45:S162-70.
- Schätzle M, Löe H, Bürgin W, Ånerud Å, Boysen H, Lang NP. Clinical course of chronic periodontitis. Der klinische Verlauf chronischer Parodontitis: I. Die Rolle der Gingivitis. L’évolution clinique de la parodontite chronique. I. Le rôle de la gingivite. J Clin 2003;30:887-901. https://doi.org/10.1034/j.1600-051X.2003.00414.x.
- Lang NP, Adler R, Joss A, Nyman S. Absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol 1990;17:714-21. https://doi.org/10.1111/j.1600-051x.1990.tb01059.x.
- Loe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol 1965;36:177-87. https://doi.org/10.1902/jop.1965.36.3.177.
- Theilade E. The non-specific theory in microbial etiology of inflammatory periodontal diseases. J Clin Periodontol 1986;13:905-11. https://doi.org/10.1111/j.1600-051x.1986.tb01425.x.
- Ekstrand KR, Bruun G, Bruun M. Plaque and gingival status as indicators for caries progression on approximal surfaces. Caries Res 1998;32:41-5. https://doi.org/10.1159/000016428.
- Linden GJ, Lyons A, Scannapieco FA. Periodontal systemic associations: review of the evidence. J Clin Periodontol 2013;40:8-19. https://doi.org/10.1111/jcpe.12064.
- Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Clin Periodontol 2013;40:51-69. https://doi.org/10.1111/jcpe.12060.
- Chapple IL, Genco R. Working group 2 of joint EFP/AAP workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol 2013;40:106-12. https://doi.org/10.1111/jcpe.12077.
- Tonetti MS, Van Dyke TE. working group 1 of the joint EFP/AAP workshop*. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 2013;84:24-9. https://doi.org/10.1902/jop.2013.1340019.
- Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol 2017;44:456-62. https://doi.org/10.1111/jcpe.12732.
- Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000 2012;60:15-39. https://doi.org/10.1111/j.1600-0757.2011.00425.x.
- Albandar JM. Periodontal diseases in North America. Periodontol 2000 2002;29:31-69. https://doi.org/10.1034/j.1600-0757.2002.290103.x.
- Corbet EF. Periodontal diseases in Asians. J Int Acad Periodontol 2006;8.
- Sheiham A, Smales FC, Cushing AM, Cowell CR. Changes in periodontal health in a cohort of British workers over a 14-year period. Br Dent J 1986;160:125-7. https://doi.org/10.1038/sj.bdj.4805788.
- Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and metaregression. J Dent 2014;93:1045-53. https://doi.org/10.1177/0022034514552491.
- Petersen PE. Sociobehavioural risk factors in dental caries – international perspectives. Community Dent Oral Epidemiol 2005;33:274-9. https://doi.org/10.1111/j.1600-0528.2005.00235.x.
- Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Primers 2017;3:1-16. https://doi.org/10.1038/nrdp.2017.30.
- Mejàre I, Källest l C, Stenlund H. Incidence and progression of approximal caries from 11 to 22 years of age in Sweden: a prospective radiographic study. Caries Res 1999;33:93-100. https://doi.org/10.1159/000016502.
- Pine CM, ten Bosch JJ. Dynamics of and diagnostic methods for detecting small carious lesions. Caries Res 1996;30:381-8. https://doi.org/10.1159/000262348.
- Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ 2005;83:661-9.
- Broadbent JM, Thomson WM, Poulton R. Trajectory patterns of dental caries experience in the permanent dentition to the fourth decade of life. J Dent Res 2008;87:69-72. https://doi.org/10.1177/154405910808700112.
- Bignozzi I, Crea A, Capri D, Littarru C, Lajolo C, Tatakis DN. Root caries: a periodontal perspective. J Periodontal Res 2014;49:143-63. https://doi.org/10.1111/jre.12094.
- Pretty IA, Ellwood RP. The caries continuum: opportunities to detect, treat and monitor the re-mineralization of early caries lesions. J Dent 2013;41:12-21. https://doi.org/10.1016/j.jdent.2010.04.003.
- Takahashi N, Nyvad B. Ecological hypothesis of dentin and root caries. Caries Res 2016;50:422-31. https://doi.org/10.1159/000447309.
- Featherstone JD. Fluoride, remineralization and root caries. Am J Dent 1994;7:271-4.
- Slade GD, Gansky SA, Spencer AJ. Two-year incidence of tooth loss among South Australians aged 60+ years. Community Dent Oral Epidemiol 1997;25:429-37. https://doi.org/10.1111/j.1600-0528.1997.tb01734.x.
- Fure S, Zickert I. Incidence of tooth loss and dental caries in 60-, 70- and 80-year-old Swedish individuals. Community Dent Oral Epidemiol 1997;25:137-42. https://doi.org/10.1111/j.1600-0528.1997.tb00911.x.
- Slade GD, Spencer AJ, Locker D, Hunt RJ, Strauss RP, Beck JD. Variations in the social impact of oral conditions among older adults in South Australia, Ontario, and North Carolina. J Dent Res 1996;75:1439-50. https://doi.org/10.1177/00220345960750070301.
- Slade GD, Spencer AJ, Roberts-Thomson K. Tooth loss and chewing capacity among older adults in Adelaide. Aust N Z J Public Health 1996;20:76-82. https://doi.org/10.1111/j.1467-842X.1996.tb01341.x.
- Curzon ME, Preston AJ. Risk groups: nursing bottle caries/caries in the elderly. Caries Res 2004;38:24-33. https://doi.org/10.1159/000074359.
- Lamster IB, Asadourian L, Del Carmen T, Friedman PK. The aging mouth: differentiating normal aging from disease. Periodontol 2000 2016;72:96-107. https://doi.org/10.1111/prd.12131.
- Gluzman R, Katz RV, Frey BJ, McGowan R. Prevention of root caries: a literature review of primary and secondary preventive agents. Spec Care Dentist 2013;33:133-40. https://doi.org/10.1111/j.1754-4505.2012.00318.x.
- Tonetti MS, Chapple ILC, Jepsen S, Sanz M. Primary and secondary prevention of periodontal and peri-implant diseases. J Clin 2015;42:S1-4. https://doi.org/10.1111/jcpe.12382.
- Axelsson P, Nyström B, Lindhe J. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol 2004;31:749-57. https://doi.org/10.1111/j.1600-051X.2004.00563.x.
- Kay E, Locker D. A systematic review of the effectiveness of health promotion aimed at improving oral health. Community Dent Health 1998;15:132-44.
- Soldani FA, Lamont T, Jones K, Young L, Walsh T, Lala R, et al. One-to-one oral hygiene advice provided in a dental setting for oral health. Cochrane Database Syst Rev 2018;10. https://doi.org/10.1002/14651858.CD007447.pub2.
- National Collaborating Centre for Acute Care (UK) . Dental Recall: Recall Interval Between Routine Dental Examinations 2004.
- Scottish Dental Clinical Effectiveness Programme . Oral Health Assessment and Review: Dental Clinical Guidance 2012.
- Richards D. Are risk-based dental recalls risky?. Evid Based Dent 2018;19:98-9. https://doi.org/10.1038/sj.ebd.6401353.
- Burt BA. Dentistry, Dental Practice and the Community. St Louis, MO: Elsevier; 2005.
- Deep P. Screening for common oral diseases. J Can Dent Assoc 2000;66.
- Mettes TG, van der Sanden WJ, Mulder J, Wensing M, Grol RP, Plasschaert AJ. Predictors of recall assignment decisions by general dental practitioners performing routine oral examinations. Eur J Oral Sci 2006;114:396-402. https://doi.org/10.1111/j.1600-0722.2006.00396.x.
- Conway DI, Purkayastha M, Chestnutt IG. The changing epidemiology of oral cancer: definitions, trends, and risk factors. Br Dent J 2018;225:867-73. https://doi.org/10.1038/sj.bdj.2018.922.
- Purkayastha M, McMahon AD, Gibson J, Conway DI. Is detecting oral cancer in general dental practices a realistic expectation? A population-based study using population linked data in Scotland. Br Dent J 2018;225:241-6. https://doi.org/10.1038/sj.bdj.2018.544.
- Brocklehurst PR, Speight PM. Screening for mouth cancer: the pros and cons of a national programme. Br Dent J 2018;225:815-19. https://doi.org/10.1038/sj.bdj.2018.918.
- Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol 2008;44:10-22. https://doi.org/10.1016/j.oraloncology.2007.06.011.
- Tomar SL. There is weak evidence that a single, universal dental recall interval schedule reduces caries incidence. J Evid Based Dent Pract 2011;11:89-91. https://doi.org/10.1016/j.jebdp.2011.03.006.
- Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al. The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation. Health Technol Assess 2003;7. https://doi.org/10.3310/hta7070.
- Riley P, Worthington HV, Clarkson JE, Beirne PV. Recall intervals for oral health in primary care patients. Cochrane Database Syst Rev 2013;12. https://doi.org/10.1002/14651858.CD004346.pub4.
- Dixon J. The international conference on harmonization good clinical practice guideline. Qual Assur 1998;6:65-74. https://doi.org/10.1080/105294199277860.
- Department of Health and Social Care (DHSC) . Research Governance Framework for Health and Social Care: Second Edition 2005.
- Scottish Executive Health Department . Research Governance Framework for Health and Community Care 2006.
- NIHR . Good Clinical Practice n.d. www.nihr.ac.uk/health-and-care-professionals/learning-and-support/good-clinical-practice.htm (accessed November 2013).
- Hefti AF, Preshaw PM. Examiner alignment and assessment in clinical periodontal research. Periodontol 2012;59:41-60. https://doi.org/10.1111/j.1600-0757.2011.00436.x.
- Ismail AI, Stookey G. Rationale and Evidence for the International Caries Detection and Assessment System (ICDAS II). Indianapolis, IN: Proceedings of the 7th Indiana Conference; 2005.
- Chesters RK, Pitts NB, Matuliene G, Kvedariene A, Huntington E, Bendinskaite R, et al. An abbreviated caries clinical trial design validated over 24 months. J Dent Res 2002;81:637-40. https://doi.org/10.1177/154405910208100912.
- NICE . Dental Recall – Recall Interval Between Routine Dental Examinations n.d. www.nice.org.uk/guidance/cg19/evidence/full-guideline-pdf-193348909 (accessed October 2020).
- Slade GD. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol 1997;25:284-90. https://doi.org/10.1111/j.1600-0528.1997.tb00941.x.
- Humphris GM, Morrison T, Lindsay SJ. The Modified Dental Anxiety Scale: validation and United Kingdom norms. Community Dent Health 1995;12:143-50.
- Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol 1967;38:610-16. https://doi.org/10.1902/jop.1967.38.6.610.
- Humphris GM, Freeman R, Campbell J, Tuutti H, D’Souza V. Further evidence for the reliability and validity of the Modified Dental Anxiety Scale. Int Dent J 2000;50:367-70. https://doi.org/10.1111/j.1875-595x.2000.tb00570.x.
- Bandura A. Health promotion from the perspective of social cognitive theory. Psychology Health 1998;13:623-49. https://doi.org/10.1080/08870449808407422.
- Gollwitzer PM. Implementation intentions – strong effects of simple plans. Am Psychologist 1999;54:493-50. https://doi.org/10.1037/0003-066X.54.7.493.
- Clarkson JE, Young L, Ramsay CR, Bonner BC, Bonetti D. How to influence patient oral hygiene behavior effectively. J Dent Res 2009;88:933-7. https://doi.org/10.1177/0022034509345627.
- Worthington HV, Clarkson JE, Bryan G, Beirne PV. Routine scale and polish for periodontal health in adults. Cochrane Database Syst Rev 2013;2013. https://doi.org/10.1002/14651858.CD004625.pub4.
- Clarkson JE. The Effectiveness of Enhanced Oral Health Advice and Instruction Upon Patient Oral Hygiene, Knowledge, and Self-Reported Behaviour 2005.
- Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al. Contamination in trials of educational interventions. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11430.
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Sullivan TR, White IR, Salter AB, Ryan P, Lee KJ. Should multiple imputation be the method of choice for handling missing data in randomized trials?. Stat Methods Med Res 2018;27:2610-26. https://doi.org/10.1177/0962280216683570.
- Kastenbom L, Falsen A, Larsson P, Sunnegårdh-Grönberg K, Davidson T. Costs and health-related quality of life in relation to caries. BMC Oral Health 2019;19. https://doi.org/10.1186/s12903-019-0874-6.
- Bala MV, Zarkin GA. Are QALYs an appropriate measure for valuing morbidity in acute diseases?. Health Econ 2000;9:177-80. https://doi.org/10.1002/(SICI)1099-1050(200003)9:2<177::AID-HEC497>3.0.CO;2-2.
- Birch S, Ismail AI. Patient preferences and the measurement of utilities in the evaluation of dental technologies. J Dent Res 2002;81:446-50. https://doi.org/10.1177/154405910208100702.
- Statistics for Wales . NHS Dental Statistics in Wales: 2016–17 2017.
- NHS Digital . A Guide to NHS Dental Publications 2017.
- Business Services Authority . NHS Payments to Dentists n.d. www.nhsbsa.nhs.uk/dental-data/nhs-payments-dentists (accessed 19 July 2019).
- Department of Health and Social Care . NHS Dental Services in England. An Independent Review Led by Professor Jimmy Steele 2009. https://webarchive.nationalarchives.gov.uk/20130124050405/www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_101180.pdf (accessed 1 June 2019).
- Lord J, Longworth L, Singh J, Onyimadu O, Fricke J, Bayliss S, et al. Oral Health Guidance – Economic Analysis of Oral Health Promotion Approaches for Dental Teams. Birmingham: Birmingham and Brunel Consortium External Assessment Centre; 2015.
- Ramsay CR, Clarkson JE, Duncan A, Lamont TJ, Heasman PA, Boyers D, et al. Improving the Quality of Dentistry (IQuaD): a cluster factorial randomised controlled trial comparing the effectiveness and cost–benefit of oral hygiene advice and/or periodontal instrumentation with routine care for the prevention and management of periodontal disease in dentate adults attending dental primary care. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22380.
- Campbell and Cochrane Economics Methods Group (CCEMG) and the Evidence for Policy and Practice Information and Coordinating Centre (EPPI-Centre) . CCEMG – EPPI-Centre Cost Converter v.1.4 n.d. https://eppi.ioe.ac.uk/costconversion/ (accessed April 2019).
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 201718 2018. https://improvement.nhs.uk/resources/reference-costs/#rc1718 (accessed July 2019).
- Thompson S, Wordsworth S. An Annotated Cost Questionnaire for Completion by Patients. Heru Discussion Paper No (03 01) 2001. www.abdn.ac.uk/heru/documents/BP/HERU_Discussion_paper_03-01.pdf (accessed February 2019).
- Office for National Statistics . Annual Survey of Hours and Earnings: 2017 Provisional and 2016 Revised Results n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2017provisionaland2016revisedresults (accessed September 2019).
- HM Revenue & Customs . Travel – Mileage and Fuel Rates and Allowances n.d. www.gov.uk/government/publications/rates-and-allowances-travel-mileage-and-fuel-allowances/travel-mileage-and-fuel-rates-and-allowances (accessed May 2019).
- NHS Pay Review Body, Cope J . NHS Pay Review Body Twenty-Ninth Report 2016 2016.
- Department for Transport . Transport Analysis Guidance (TAG) Data Book 2017.
- Health Services Research Unit . Statistical Analysis of Costs – Stata Programs n.d. www.uphs.upenn.edu/dgimhsr/stat-cstanal.htm (accessed July 2019).
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Gomes M, Díaz-Ordaz K, Grieve R, Kenward MG. Multiple imputation methods for handling missing data in cost-effectiveness analyses that use data from hierarchical studies: an application to cluster randomized trials. Med Decis Making 2013;33:1051-63. https://doi.org/10.1177/0272989X13492203.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: Wiley; 2009.
- Steele J, O’Sullivan I. Executive Summary: Adult Dental Health Survey 2009. Leeds: NHS Digital; 2011.
- McFadden D, Zarembka P. Frontiers in Econometrics. New York, NY: Academic Press; 1973.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Brand J, van Buuren S, le Cessie S, van den Hout W. Combining multiple imputation and bootstrap in the analysis of cost-effectiveness trial data. Stat Med 2019;38:210-20. https://doi.org/10.1002/sim.7956.
- Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 2018;45:68-77. https://doi.org/10.1111/jcpe.12940.
- Office for National Statistics . Census Data Catalogue 2011. www.ons.gov.uk/census/2011census/2011censusdata/2011censusdatacatalogue (accessed June 2019).
- Office for National Statistics . Adult Smoking Habits in Great Britain, 2013 n.d. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/compendium/opinionsandlifestylesurvey/2015-03-19/adultsmokinghabitsingreatbritain2013 (accessed 1 June 2019).
- UK Government Department for Work and Pensions . Family Resources Survey 2016 17 n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/692771/family-resources-survey-2016-17.pdf (accessed 1 June 2019).
- Kirsch J, Tchorz J, Hellwig E, Tauböck TT, Attin T, Hannig C. Decision criteria for replacement of fillings: a retrospective study. Clin Exp Dent Res 2016;2:121-8. https://doi.org/10.1002/cre2.30.
- Hettiarachchi RM, Kularatna S, Downes MJ, Byrnes J, Kroon J, Lalloo R, et al. The cost-effectiveness of oral health interventions: a systematic review of cost-utility analyses. Community Dent Oral Epidemiol 2018;46:118-24. https://doi.org/10.1111/cdoe.12336.
- Wang N, Marstrander P, Holst D, Ovrum L, Dahle T. Extending recall intervals – effect on resource consumption and dental health. Community Dent Oral Epidemiol 1992;20:122-4. https://doi.org/10.1111/j.1600-0528.1992.tb01544.x.
- Fee PA, Riley P, Worthington HV, Clarkson JE, Boyers D, Beirne PV. Recall intervals for oral health in primary care patients. Cochrane Database Syst Rev 2020;10.
- Beirne P. Dental Recall: Recall Interval Between Routine Dental Examinations. London: National Collaborating Centre for Acute Care UK; 2004.
- Information Services Scotland . Dental Statistics – NHS Registration and Participation – Statistics up to 30 September 2018 2019.
Appendix 1 Clinical effectiveness outcomes
| Outcomes | Questions | Scoring |
|---|---|---|
| Behaviour | Section 2 Q1, Q3, Q9, Q11 | Sum of the questionsa |
| Knowledge | Section 2 Q2, Q4, Q10, Q12 | Sum of the questionsa |
| Attitude | Section 3 Q1 to Q7 | Mean of all questions |
| PBC | Section 3 Q8 to Q11 | Mean of all questions |
| Satisfaction with care | Section 3 Q12 to Q23 | Mean of all questions |
| Outcomes | Description | Questions | Scoring |
|---|---|---|---|
| Attitude to the 6-month interval | Attitude to the 6-month interval. Higher scores represent a positive attitude towards the 6-month interval | Q1, Q4, Q5, Q7, Q8, Q9, Q10, Q12, Q13, Q15, Q16, Q18 | Mean of all questions |
| Attitude to the 24-month interval | Attitude to the 24-month interval. Higher scores represent a positive attitude towards the 24-month interval | Q3, Q14, Q17, Q19 | Mean of all questions |
| General attitude | General attitude to the patient’s ability to maintain self-care. Higher scores represent a positive attitude | Q2, Q6, Q11 | Mean of all questions |
| PBC | Perceived ability to assess risk. Higher scores represent greater PBC | Q20, Q21 (a) to (c) | Mean of all questions |
| Centre name | Strata | Total | |
|---|---|---|---|
| Ineligible 24-month recall | Eligible 24-month recall | ||
| Six Gables Dental Practice, Cardiff | 6 | 7 | 13 |
| Craigmillar Dental Centre, Edinburgh | 1 | 8 | 9 |
| Colin C Yule & Associates, Forfar | 1 | 59 | 60 |
| Roseville Dental Practice, West Midlands | 2 | 19 | 21 |
| Cottam’s Dental, Implant & Orthodontic Practice, Birmingham | 26 | 1 | 27 |
| Walmley Dental Practice, Sutton Coldfield | 18 | 12 | 30 |
| Amblecote Dental Care, West Midlands | 48 | 14 | 62 |
| M&S Dental Care, Fort William | 3 | 44 | 47 |
| Southside Dental Care, Edinburgh | 0 | 54 | 54 |
| Kingsway Dental Practice, Dundee | 38 | 38 | 76 |
| Brightons Dental Surgery, Falkirk | 14 | 38 | 52 |
| Montrose Dental Care, Montrose | 5 | 47 | 52 |
| Bayview Dental Practice, Banff | 6 | 16 | 22 |
| Young Smile Dental Care, Alford | 38 | 17 | 55 |
| Mount Florida Dental Care, Glasgow | 40 | 15 | 55 |
| Horizon Dental Clinic, Monkseaton | 0 | 6 | 6 |
| Horizon Dental Clinic, Blyth | 0 | 4 | 4 |
| Green Lane Dental, Birmingham | 23 | 40 | 63 |
| Aesthetics Dental and Implant Surgery, Birmingham | 16 | 23 | 39 |
| Platt & Common, Stirling | 3 | 6 | 9 |
| Chong Kwan Dental Centre, Dunfermline | 0 | 79 | 79 |
| Brunswick Dental Practice, Newcastle | 4 | 75 | 79 |
| Hazel House Dental Surgery, Inverness | 11 | 5 | 16 |
| Castle House Dental Practice, Inverness | 10 | 30 | 40 |
| PCK Wee Dental Surgery, London | 1 | 110 | 111 |
| Family Dental Care, London | 1 | 5 | 6 |
| Smiledent Dental Practice, London | 4 | 33 | 37 |
| Ballynahinch Dental Care, Belfast | 47 | 2 | 49 |
| Loughside Dental Practice, Belfast | 30 | 22 | 52 |
| Church Road Dental Care, Belfast | 11 | 41 | 52 |
| Anita Belbin Dental Surgery, Glasgow | 2 | 50 | 52 |
| N13 Dental Clinic, London | 3 | 51 | 54 |
| Mr I Lightfoot, Newcastle | 0 | 28 | 28 |
| Shotley Bridge Dental Care, Newcastle | 6 | 44 | 50 |
| Discovery Dental Care, Dundee | 28 | 26 | 54 |
| Nairn Dental Clinic, Inverness | 15 | 41 | 56 |
| My Dental Care, London | 5 | 35 | 40 |
| Salmon Lane Dental Care, London | 3 | 49 | 52 |
| Eastside Dental Practice, London | 15 | 52 | 67 |
| Bridge Dental Centre, London | 4 | 52 | 56 |
| Park View Family Dental, Newcastle | 16 | 130 | 146 |
| Dunbar Dental Practice, Dunbar | 67 | 28 | 95 |
| St Leonards Dental Practice, Glasgow | 3 | 49 | 52 |
| Woodside Dental Practice, Glasgow | 4 | 11 | 15 |
| Lochshell Dental Clinic, Wick | 21 | 11 | 32 |
| Bute Dental Surgery, Bute | 14 | 39 | 53 |
| Windsor Dental Practice, Salford | 4 | 19 | 23 |
| Windsor Dental Practice, Manchester | 4 | 1 | 5 |
| Kelvingrove Dental Care Ltd, Glasgow | 0 | 100 | 100 |
| Montgomery Street Dental Practice, Edinburgh | 20 | 33 | 53 |
| Collegiate Dental Practice, Manchester | 7 | 5 | 12 |
| Characteristic | Eligible 24-month recall | Ineligible 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Randomised, n (%) | 111 (100.0) | 117 (100.0) | 116 (100.0) | 419 (100.0) | 425 (100.0) |
| Baseline questionnaire returned, n (%) | 102 (91.9) | 109 (93.2) | 108 (93.1) | 391 (93.3) | 387 (91.1) |
| Age, mean (SD), n | 42.4 (14.6), 111 | 47.2 (15.4), 117 | 46.6 (14.7), 116 | 49.1 (13.4), 419 | 49.9 (14.3), 425 |
| Female participants, n (%) | 70 (63.1) | 66 (56.4) | 66 (56.9) | 245 (58.5) | 248 (58.4) |
| Regular smoker, n (%) | 19 (17.1) | 18 (15.4) | 17 (14.7) | 88 (21.0) | 60 (14.1) |
| Time since previous visit to dentist, n (%) | |||||
| < 1 year | 96 (86.5) | 96 (82.1) | 100 (86.2) | 370 (88.3) | 366 (86.1) |
| 1–2 years | 4 (3.6) | 13 (11.1) | 7 (6.0) | 18 (4.3) | 16 (3.8) |
| > 2 years | 1 (0.9) | – | 1 (0.9) | – | – |
| Patient status, n (%) | |||||
| NHS | 89 (80.2) | 97 (82.9) | 93 (80.2) | 347 (82.8) | 325 (76.5) |
| Private | 2 (1.8) | 5 (4.3) | 2 (1.7) | 12 (2.9) | 13 (3.1) |
| Combination | 4 (3.6) | 2 (1.7) | 6 (5.2) | 17 (4.1) | 29 (6.8) |
| Type of toothbrush, n (%) | |||||
| Manual | 65 (58.6) | 76 (65.0) | 60 (51.7) | 232 (55.4) | 234 (55.1) |
| Electric | 35 (31.5) | 32 (27.4) | 48 (41.4) | 158 (37.7) | 150 (35.3) |
| Regular attender: self-report, n (%) | 92 (82.9) | 102 (87.2) | 101 (87.1) | 373 (89.0) | 359 (84.5) |
| Difficulty travelling to dentist, mean (SD), n | 6.5 (1.2), 101 | 6.6 (1.0), 109 | 6.4 (1.2), 107 | 6.4 (1.1), 389 | 6.3 (1.2), 383 |
| Characteristic | Eligible 24-month recall | Ineligible 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Randomised, n (%) | 75 (100.0) | 69 (100.0) | 72 (100.0) | 410 (100.0) | 405 (100.0) |
| Baseline questionnaire returned, n (%) | 62 (82.7) | 61 (88.4) | 64 (88.9) | 391 (95.4) | 386 (95.3) |
| Age, mean (SD), n | 43.9 (14.6), 75 | 39.8 (14.3), 69 | 40.0 (13.1), 72 | 49.8 (14.6), 410 | 50.7 (16.1), 405 |
| Female participants, n (%) | 45 (60.0) | 40 (58.0) | 43 (59.7) | 236 (57.6) | 221 (54.6) |
| Regular smokers, n (%) | 9 (12.0) | 7 (10.1) | 12 (16.7) | 53 (12.9) | 65 (16.0) |
| Time since previous visit to dentist, n (%) | |||||
| < 1 year | 54 (72.0) | 47 (68.1) | 51 (70.8) | 342 (83.4) | 345 (85.2) |
| 1–2 years | 7 (9.3) | 10 (14.5) | 9 (12.5) | 41 (10.0) | 35 (8.6) |
| > 2 years | – | 4 (5.8) | 2 (2.8) | 4 (1.0) | – |
| Patient status, n (%) | |||||
| NHS | 53 (70.7) | 48 (69.6) | 54 (75.0) | 328 (80.0) | 330 (81.5) |
| Private | 2 (2.7) | 5 (7.2) | 2 (2.8) | 17 (4.1) | 15 (3.7) |
| Combination | 5 (6.7) | 4 (5.8) | 4 (5.6) | 34 (8.3) | 24 (5.9) |
| Type of toothbrush, n (%) | |||||
| Manual | 39 (52.0) | 47 (68.1) | 44 (61.1) | 238 (58.0) | 244 (60.2) |
| Electric | 22 (29.3) | 13 (18.8) | 20 (27.8) | 151 (36.8) | 136 (33.6) |
| Regular attender: self-report, n (%) | 52 (69.3) | 49 (71.0) | 54 (75.0) | 340 (82.9) | 348 (85.9) |
| Difficulty travelling to dentist, mean (SD), n | 6.3 (1.2), 61 | 6.1 (1.6), 61 | 6.3 (1.2), 64 | 6.3 (1.3), 388 | 6.3 (1.2),3 78 |
| Characteristic | Eligible 24-month recall | Ineligible 24-month recall | |||
|---|---|---|---|---|---|
| Risk-based | 24 month | 6 month | Risk based | 6 month | |
| Randomised, n (%) | 31 (100.0) | 30 (100.0) | 27 (100.0) | 32 (100.0) | 33 (100.0) |
| Baseline questionnaire returned, n (%) | 17 (54.8) | 16 (53.3) | 15 (55.6) | 28 (87.5) | 30 (90.9) |
| Age, mean (SD), n | 45.2 (18.3), 31 | 42.4 (14.5), 30 | 39.7 (14.6), 27 | 46.7 (17.8), 32 | 44.8 (16.6), 33 |
| Female participants, n (%) | 13 (41.9) | 9 (30.0) | 12 (44.4) | 17 (53.1) | 22 (66.7) |
| Regular smokers, n (%) | 4 (12.9) | 2 (6.7) | 3 (11.1) | 4 (12.5) | 5 (15.2) |
| Time since previous visit to dentist, n (%) | |||||
| < 1 year | 15 (48.4) | 14 (46.7) | 14 (51.9) | 25 (78.1) | 30 (90.9) |
| 1–2 years | 2 (6.5) | 1 (3.3) | – | 3 (9.4) | – |
| > 2 years | – | – | 1 (3.7) | – | – |
| Patient status, n (%) | |||||
| NHS | 10 (32.3) | 9 (30.0) | 8 (29.6) | 20 (62.5) | 22 (66.7) |
| Private | 4 (12.9) | 2 (6.7) | 3 (11.1) | 4 (12.5) | 4 (12.1) |
| Combination | 3 (9.7) | 2 (6.7) | 4 (14.8) | 3 (9.4) | 3 (9.1) |
| Type of toothbrush, n (%) | |||||
| Manual | 10 (32.3) | 8 (26.7) | 9 (33.3) | 19 (59.4) | 18 (54.5) |
| Electric | 7 (22.6) | 8 (26.7) | 6 (22.2) | 9 (28.1) | 12 (36.4) |
| Regular attender: self-report, n (%) | 14 (45.2) | 12 (40.0) | 13 (48.1) | 27 (84.4) | 28 (84.8) |
| Difficulty travelling to dentist, mean (SD), n | 6.0 (1.3), 17 | 6.9 (0.3), 16 | 6.5 (1.2), 15 | 6.2 (1.5), 28 | 6.4 (1.1), 30 |
| Oral health-related behaviour or knowledge | Eligible 24-month recall | Ineligible 24-month recall | |||
|---|---|---|---|---|---|
| Risk based (N = 111) | 24 month (N = 117) | 6 month (N = 116) | Risk based (N = 419) | 6 month (N = 425) | |
| Frequency of brushing, n (%) | |||||
| Twice a day | 66 (59.5) | 75 (64.1) | 76 (65.5) | 272 (64.9) | 270 (63.5) |
| More than twice a day | 14 (12.6) | 9 (7.7) | 6 (5.2) | 42 (10.0) | 43 (10.1) |
| How often should you be brushing? n (%) | |||||
| Twice a day | 81 (73.0) | 85 (72.6) | 89 (76.7) | 271 (64.7) | 281 (66.1) |
| More than twice a day | 19 (17.1) | 20 (17.1) | 18 (15.5) | 109 (26.0) | 95 (22.4) |
| Duration of brushing, n (%) | |||||
| 2 minutes | 49 (44.1) | 58 (49.6) | 49 (42.2) | 183 (43.7) | 178 (41.9) |
| > 2 minutes | 16 (14.4) | 16 (13.7) | 19 (16.4) | 71 (16.9) | 61 (14.4) |
| How long should you brush for? n (%) | |||||
| 2 minutes | 68 (61.3) | 66 (56.4) | 70 (60.3) | 242 (57.8) | 246 (57.9) |
| > 2 minutes | 24 (21.6) | 29 (24.8) | 29 (25.0) | 109 (26.0) | 95 (22.4) |
| Spit not rinse after brushing, n (%) | 51 (45.9) | 44 (37.6) | 47 (40.5) | 140 (33.4) | 126 (29.6) |
| Should spit not rinse after brushing, n (%) | 59 (53.2) | 59 (50.4) | 57 (49.1) | 161 (38.4) | 154 (36.2) |
| Oral health-related behaviour or knowledge | Eligible 24-month recall | Ineligible 24-month recall | |||
|---|---|---|---|---|---|
| Risk based (N = 75) | 24 month (N = 69) | 6 month (N = 72) | Risk based (N = 410) | 6 month (N = 405) | |
| Frequency of brushing, n (%) | |||||
| Twice a day | 45 (60.0) | 40 (58.0) | 48 (66.7) | 271 (66.1) | 294 (72.6) |
| More than twice a day | 8 (10.7) | 5 (7.2) | 1 (1.4) | 39 (9.5) | 34 (8.4) |
| How often should you be brushing? n (%) | |||||
| Twice a day | 49 (65.3) | 56 (81.2) | 54 (75.0) | 312 (76.1) | 304 (75.1) |
| More than twice a day | 11 (14.7) | 5 (7.2) | 7 (9.7) | 65 (15.9) | 66 (16.3) |
| Duration of brushing, n (%) | |||||
| 2 minutes | 32 (42.7) | 34 (49.3) | 24 (33.3) | 164 (40.0) | 191 (47.2) |
| > 2 minutes | 8 (10.7) | 9 (13.0) | 13 (18.1) | 91 (22.2) | 67 (16.5) |
| How long should you brush for? n (%) | |||||
| 2 minutes | 42 (56.0) | 42 (60.9) | 45 (62.5) | 222 (54.1) | 226 (55.8) |
| > 2 minutes | 14 (18.7) | 12 (17.4) | 13 (18.1) | 114 (27.8) | 99 (24.4) |
| Spit not rinse after brushing, n (%) | 8 (10.7) | 6 (8.7) | 13 (18.1) | 59 (14.4) | 49 (12.1) |
| Should spit not rinse after brushing, n (%) | 8 (10.7) | 9 (13.0) | 14 (19.4) | 75 (18.3) | 74 (18.3) |
| Oral health-related behaviour or knowledge | Eligible 24-month recall | Ineligible 24-month recall | |||
|---|---|---|---|---|---|
| Risk based (N = 31) | 24 month (N = 30) | 6 month (N = 27) | Risk based (N = 32) | 6 month (N = 33) | |
| Frequency of brushing, n (%) | |||||
| Twice a day | 12 (38.7) | 13 (43.3) | 8 (29.6) | 17 (53.1) | 23 (69.7) |
| More than twice a day | 1 (3.2) | 1 (3.3) | 1 (3.7) | 1 (3.1) | 3 (9.1) |
| How often should you be brushing? n (%) | |||||
| Twice a day | 14 (45.2) | 14 (46.7) | 13 (48.1) | 25 (78.1) | 18 (54.5) |
| More than twice a day | 1 (3.2) | 2 (6.7) | 0 (0) | 3 (9.4) | 12 (36.4) |
| Duration of brushing, n (%) | |||||
| 2 minutes | 6 (19.4) | 7 (23.3) | 6 (22.2) | 15 (46.9) | 14 (42.4) |
| > 2 minutes | 2 (6.5) | 4 (13.3) | 4 (14.8) | 1 (3.1) | 4 (12.1) |
| How long should you brush for? n (%) | |||||
| 2 minutes | 7 (22.6) | 9 (30.0) | 10 (37.0) | 18 (56.3) | 17 (51.5) |
| > 2 minutes | 3 (9.7) | 4 (13.3) | 3 (11.1) | 6 (18.8) | 9 (27.3) |
| Spit not rinse after brushing, n (%) | 5 (16.1) | 3 (10.0) | 4 (14.8) | 14 (43.8) | 15 (45.5) |
| Should spit not rinse after brushing, n (%) | 6 (19.4) | 5 (16.7) | 4 (14.8) | 14 (43.8) | 18 (54.5) |
| Patient-reported outcomes | Eligible 24-month recall, mean (SD), count unless otherwise stated | Ineligible 24-month recall, mean (SD), count unless otherwise stated | |||
|---|---|---|---|---|---|
| Risk based (n = 111) | 24 month (n = 117) | 6 month (n = 116) | Risk based (n = 419) | 6 month (n = 425) | |
| Baseline questionnaire returned | 102 (91.9) | 109 (93.2) | 108 (93.1) | 391 (93.3) | 387 (91.1) |
| Attitude | 4.2 (0.8), 101 | 4.3 (0.9), 109 | 4.2 (0.7), 108 | 4.1 (0.9), 388 | 4.1 (0.9), 383 |
| PBC | 5.3 (1.3), 100 | 5.2 (1.4), 109 | 5.1 (1.4), 107 | 5.0 (1.5), 362 | 5.1 (1.4), 357 |
| Satisfaction | 5.1 (0.8), 99 | 5.2 (0.7), 107 | 5.1 (0.7), 107 | 5.2 (0.6), 310 | 5.2 (0.7), 313 |
| Anxiety | 10.0 (4.4), 98 | 10.4 (4.4), 109 | 10.4 (4.8), 107 | 10.1 (4.6), 387 | 9.9 (4.6), 384 |
| OHIP-14 | 4.4 (7.4), 99 | 5.5 (7.0), 107 | 4.5 (5.5), 104 | 5.5 (6.4), 374 | 5.9 (7.3), 375 |
| Patient-reported outcomes | Eligible 24-month recall, mean (SD), count unless otherwise stated | Ineligible 24-month recall, mean (SD), count unless otherwise stated | |||
|---|---|---|---|---|---|
| Risk based (n = 75) | 24 month (n = 69) | 6 month (n = 72) | Risk based (n = 410) | 6 month (n = 405) | |
| Baseline questionnaire returned | 62 (82.7) | 61 (88.4) | 64 (88.9) | 391 (95.4) | 386 (95.3) |
| Attitude | 4.2 (0.8), 61 | 4.3 (0.8), 61 | 4.0 (0.7), 64 | 4.0 (0.8), 390 | 4.0 (0.9), 382 |
| PBC | 5.3 (1.3), 59 | 5.3 (1.4), 61 | 5.1 (1.5), 64 | 5.0 (1.5), 388 | 5.1 (1.5), 382 |
| Satisfaction | 5.3 (0.8), 32 | 5.3 (0.8), 30 | 5.4 (0.7), 36 | 5.4 (0.6), 365 | 5.3 (0.6), 358 |
| Anxiety | 10.8 (4.5), 62 | 11.4 (4.2), 61 | 11.0 (3.7), 64 | 10.1 (4.5), 386 | 10.2 (4.5), 384 |
| OHIP-14 | 4.6 (6.0), 60 | 4.1 (5.9), 60 | 3.9 (5.1), 63 | 6.1 (7.5), 376 | 6.2 (8.1), 374 |
| Patient-reported outcomes | Eligible 24-month recall, mean (SD), count unless otherwise stated | Ineligible 24-month recall, mean (SD), count unless otherwise stated | |||
|---|---|---|---|---|---|
| Risk based (n = 31) | 24 month (n = 30) | 6 month (n = 27) | Risk based (n = 32) | 6 month (n = 33) | |
| Baseline questionnaire returned | 17 (54.8) | 16 (53.3) | 15 (55.6) | 28 (87.5) | 30 (90.9) |
| Attitude | 4.0 (0.5), 16 | 3.8 (0.9), 16 | 3.9 (0.5), 15 | 3.9 (0.6), 28 | 3.8 (0.7), 30 |
| PBC | 5.2 (1.8), 16 | 4.7 (1.9), 16 | 5.0 (1.4), 15 | 4.6 (1.6), 28 | 4.7 (1.6), 30 |
| Satisfaction | 5.4 (0.6), 16 | 5.1 (0.9), 16 | 5.2 (0.6), 15 | 5.4 (0.6), 28 | 5.4 (0.5), 30 |
| Anxiety | 11.1 (6.3), 16 | 9.7 (5.2), 16 | 9.8 (5.1), 14 | 11.5 (4.4), 28 | 11.0 (4.7), 30 |
| OHIP-14 | 4.8 (8.5), 16 | 2.1 (2.5), 16 | 6.0 (11.5), 15 | 5.3 (6.8), 28 | 6.1 (6.6), 29 |
Description of the intervention by country
| Number of check-ups, n (%) | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based (N = 78) | 24 month (N = 76) | 6 month (N = 75) | Risk based (N = 295) | 6 month (N = 308) | |
| 0 | 1 (1.3) | 8 (9.8) | 0 (0.0) | 1 (0.3) | 0 (0.0) |
| 1 | 14 (17.7) | 31 (37.8) | 3 (3.9) | 10 (3.3) | 4 (1.3) |
| 2 | 23 (29.1) | 11 (13.4) | 5 (6.5) | 37 (12.3) | 23 (7.3) |
| 3 or more | 40 (50.6) | 26 (31.7) | 67 (87.0) | 247 (82.3) | 281 (89.8) |
| Missing, n (%) | 1 (1.3) | 6 (7.3) | 2 (2.6) | 5 (1.7) | 5 (1.6) |
| Number of check-ups, mean (SD), n | 3.0 (1.8), 78 | 2.3 (2.1), 76 | 4.3 (1.5), 75 | 3.9 (1.5), 295 | 4.4 (1.5), 308 |
| Number of check-ups, n (%) | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based (N = 45) | 24 month (N = 37) | 6 month (N = 266) | Risk based (N = 266) | 6 month (N = 249) | |
| 1 | 1 (2.0) | 11 (27.5) | 3 (6.5) | 4 (1.4) | 3 (1.1) |
| 2 | 2 (4.0) | 9 (22.5) | 4 (8.7) | 7 (2.5) | 7 (2.6) |
| 3 or more | 42 (84.0) | 17 (42.5) | 35 (76.1) | 255 (89.8) | 239 (89.5) |
| Missing | 5 (10.0) | 3 (7.5) | 4 (8.7) | 18 (6.3) | 18 (6.7) |
| Number of check-ups mean (SD), n | 4.5 (1.8), 45 | 3.1 (2.6), 37 | 6.5 (5.9), 42 | 6.2 (2.4), 266 | 6.5 (2.0), 249 |
| Number of check-ups, n (%) | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based (N = 14) | 24 month (N = 15) | 6 month (N = 11) | Risk based (N = 21) | 6 month (N = 21) | |
| 1 | 1 (2.0) | 11 (27.5) | 3 (6.5) | 4 (1.4) | 3 (1.1) |
| 2 | 2 (4.0) | 9 (22.5) | 4 (8.7) | 7 (2.5) | 7 (2.6) |
| 3 or more | 42 (84.0) | 17 (42.5) | 35 (76.1) | 255 (89.8) | 239 (89.5) |
| Missing | 5 (10.0) | 3 (7.5) | 4 (8.7) | 18 (6.3) | 18 (6.7) |
| Number of check-ups mean (SD), n | 4.5 (1.8), 14 | 2.1 (1.5), 15 | 5.4 (2.2), 11 | 5.2 (1.5), 21 | 6.6 (1.2), 21 |
Follow-up demographic characteristics
| Characteristic | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Regular smokers, n (%) | 19 (13.1) | 16 (10.3) | 17 (11.2) | 109 (17.1) | 98 (15.3) |
| Missing | 1 (0.7) | 1 (0.6) | 1 (0.7) | 5 (0.8) | 1 (0.2) |
| Time since previous visit to dentist, n (%) | |||||
| < 1 year | 122 (84.1) | 97 (62.6) | 143 (94.1) | 600 (94.0) | 629 (98.3) |
| 1–2 years | 21 (14.5) | 56 (36.1) | 8 (5.3) | 31 (4.9) | 9 (1.4) |
| Missing | 2 (1.4) | 2 (1.3) | 1 (0.7) | 7 (1.1) | 2 (0.3) |
| Patient status, n (%) | |||||
| NHS | 69 (47.6) | 60 (38.7) | 91 (59.9) | 410 (64.3) | 431 (67.3) |
| Private | 5 (3.4) | 3 (1.9) | 2 (1.3) | 15 (2.4) | 11 (1.7) |
| Combination | 8 (5.5) | 9 (5.8) | 10 (6.6) | 61 (9.6) | 65 (10.2) |
| Missing | 63 (43.4) | 83 (53.5) | 49 (32.2) | 152 (23.8) | 133 (20.8) |
| Type of toothbrush, n (%) | |||||
| Manual | 88 (60.7) | 99 (63.9) | 89 (58.6) | 348 (54.5) | 383 (59.8) |
| Electric | 55 (37.9) | 54 (34.8) | 58 (38.2) | 268 (42.0) | 240 (37.5) |
| Toothbrush not used | – | – | 1 (0.7) | – | – |
| Both | 1 (0.7) | 1 (0.6) | 3 (2.0) | 16 (2.5) | 14 (2.2) |
| Missing | 1 (0.7) | 1 (0.6) | 1 (0.7) | 6 (0.9) | 3 (0.5) |
| Regular attender: self-report, n (%) | 132 (91.0) | 131 (84.5) | 142 (93.4) | 601 (94.2) | 615 (96.1) |
| Missing | 1 (0.7) | 2 (1.3) | 1 (0.7) | 5 (0.8) | 1 (0.2) |
| Difficulty travelling to dentist, mean (SD), n | 6.2 (1.3), 144 | 6.5 (1.0), 154 | 6.4 (1.2), 151 | 6.3 (1.2), 775 | 6.3 (1.3), 786 |
| Replied to year 4 questionnaire, n (%) | 145 (100.0) | 155 (100.0) | 152 (100.0) | 638 (100.0) | 640 (100.0) |
| Characteristic | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Questionnaire returned, n (%) | 135 (100.0) | 144 (100.0) | 142 (100.0) | 610 (100.0) | 619 (100.0) |
| Smoked in the last 12 months, n (%) | 12 (8.9) | 16 (11.1) | 20 (14.1) | 96 (15.7) | 76 (12.3) |
| Missing | 1 (0.7) | – | – | 6 (1.0) | 3 (0.5) |
| Time since previous visit to dentist, n (%) | |||||
| < 1 year | 120 (88.9) | 105 (72.9) | 136 (95.8) | 588 (96.4) | 598 (96.6) |
| 1–2 years | 14 (10.4) | 32 (22.2) | 6 (4.2) | 18 (3.0) | 17 (2.7) |
| > 2 years | – | 6 (4.2) | – | 2 (0.3) | 1 (0.2) |
| Missing | 1 (0.7) | 1 (0.7) | – | 2 (0.3) | 3 (0.5) |
| Patient status, n (%) | |||||
| NHS | 84 (62.2) | 73 (50.7) | 89 (62.7) | 415 (68.0) | 425 (68.7) |
| Private | 3 (2.2) | 6 (4.2) | 3 (2.1) | 10 (1.6) | 9 (1.5) |
| Combination | 12 (8.9) | 9 (6.3) | 15 (10.6) | 72 (11.8) | 63 (10.2) |
| Missing | 36 (26.7) | 56 (38.9) | 35 (24.6) | 113 (18.5) | 122 (19.7) |
| Type of toothbrush, n (%) | |||||
| Manual | 67 (49.6) | 88 (61.1) | 83 (58.5) | 316 (51.8) | 347 (56.1) |
| Electric | 59 (43.7) | 47 (32.6) | 52 (36.6) | 231 (37.9) | 213 (34.4) |
| Toothbrush not used | 1 (0.7) | – | – | 1 (0.2) | – |
| Toothbrush | 7 (5.2) | 8 (5.6) | 7 (4.9) | 57 (9.3) | 56 (9.0) |
| Missing | 1 (0.7) | 1 (0.7) | – | 5 (0.8) | 3 (0.5) |
| Regular attender: self-report, n (%) | 123 (91.1) | 124 (86.1) | 136 (95.8) | 584 (95.7) | 588 (95.0) |
| Missing | 1 (0.7) | – | – | 2 (0.3) | 3 (0.5) |
| Difficulty travelling to dentist mean (SD), n | 6.4 (1.2), 134 | 6.5 (0.9), 143 | 6.3 (1.2), 141 | 6.3 (1.2), 742 | 6.3 (1.2), 751 |
| Characteristic | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Questionnaire returned, n (%) | 134 (100.0) | 131 (100.0) | 134 (100.0) | 571 (100.0) | 584 (100.0) |
| Smoked in the last 12 months, n (%) | 13 (9.7) | 14 (10.7) | 13 (9.7) | 72 (12.6) | 64 (11.0) |
| Missing | 1 (0.7) | – | 1 (0.7) | 4 (0.7) | 2 (0.3) |
| Time since previous visit to dentist, n (%) | |||||
| < 1 year | 117 (87.3) | 92 (70.2) | 123 (91.8) | 545 (95.4) | 569 (97.4) |
| 1–2 years | 14 (10.4) | 36 (27.5) | 8 (6.0) | 21 (3.7) | 14 (2.4) |
| > 2 years | 2 (1.5) | 3 (2.3) | 1 (0.7) | 3 (0.5) | – |
| Missing | 1 (0.7) | – | 2 (1.5) | 2 (0.4) | 1 (0.2) |
| Patient status, n (%) | |||||
| NHS | 90 (67.2) | 81 (61.8) | 96 (71.6) | 407 (71.3) | 432 (74.0) |
| Private | 4 (3.0) | 4 (3.1) | 4 (3.0) | 10 (1.8) | 19 (3.3) |
| Combination | 18 (13.4) | 14 (10.7) | 17 (12.7) | 65 (11.4) | 62 (10.6) |
| Missing | 22 (16.4) | 32 (24.4) | 17 (12.7) | 89 (15.6) | 71 (12.2) |
| Type of toothbrush, n (%) | |||||
| Manual | 68 (50.7) | 77 (58.8) | 66 (49.3) | 304 (53.2) | 316 (54.1) |
| Electric | 58 (43.3) | 46 (35.1) | 58 (43.3) | 227 (39.8) | 234 (40.1) |
| Toothbrush not used | 1 (0.7) | – | – | – | 1 (0.2) |
| Both | 7 (5.2) | 6 (4.6) | 9 (6.7) | 38 (6.7) | 33 (5.7) |
| Missing | – | 2 (1.5) | 1 (0.7) | 2 (0.4) | – |
| Regular attender: self-report, n (%) | 119 (88.8) | 108 (82.4) | 129 (96.3) | 543 (95.1) | 558 (95.5) |
| Missing | – | 1 (0.8) | 1 (0.7) | 3 (0.5) | 3 (0.5) |
| Difficulty travelling to dentist mean (SD), n | 6.4 (1.2), 134 | 6.6 (0.9), 131 | 6.3 (1.2), 133 | 6.4 (1.1), 703 | 6.3 (1.2), 715 |
Follow-up behaviour and knowledge related to oral health
| Behaviours/knowledge | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Responded | 145 (100.0) | 155 (100.0) | 152 (100.0) | 638 (100.0) | 640 (100.0) |
| Frequency of brushing | |||||
| Once a day | 20 (13.8) | 32 (20.6) | 36 (23.7) | 112 (17.6) | 100 (15.6) |
| Twice a day | 99 (68.3) | 110 (71.0) | 104 (68.4) | 449 (70.4) | 461 (72.0) |
| More than twice a day | 22 (15.2) | 11 (7.1) | 7 (4.6) | 69 (10.8) | 63 (9.8) |
| Missing | 2 (1.4) | 1 (0.6) | 3 (2.0) | 4 (0.6) | 10 (1.6) |
| How often should you be brushing? | |||||
| Once a day | 1 (0.7) | 3 (1.9) | 3 (2.0) | 10 (1.6) | 5 (0.8) |
| Twice a day | 111 (76.6) | 125 (80.6) | 118 (77.6) | 481 (75.4) | 483 (75.5) |
| More than twice a day | 31 (21.4) | 25 (16.1) | 28 (18.4) | 138 (21.6) | 143 (22.3) |
| Missing | 2 (1.4) | 2 (1.3) | 2 (1.3) | 9 (1.4) | 9 (1.4) |
| Duration of brushing | |||||
| 1–2 minutes | 44 (30.3) | 48 (31.0) | 48 (31.6) | 195 (30.6) | 208 (32.5) |
| 2 minutes | 73 (50.3) | 75 (48.4) | 71 (46.7) | 304 (47.6) | 315 (49.2) |
| > 2 minutes | 19 (13.1) | 24 (15.5) | 23 (15.1) | 112 (17.6) | 86 (13.4) |
| Missing | 2 (1.4) | 3 (1.9) | 3 (2.0) | 6 (0.9) | 8 (1.3) |
| How long should you brush for? | |||||
| 1–2 minutes | 15 (10.3) | 12 (7.7) | 15 (9.9) | 65 (10.2) | 69 (10.8) |
| 2 minutes | 97 (66.9) | 104 (67.1) | 102 (67.1) | 394 (61.8) | 401 (62.7) |
| > 2 minutes | 29 (20.0) | 36 (23.2) | 32 (21.1) | 164 (25.7) | 156 (24.4) |
| Missing | 3 (2.1) | 2 (1.3) | 2 (1.3) | 10 (1.6) | 11 (1.7) |
| After brushing | |||||
| Rinse with water | 56 (38.6) | 78 (50.3) | 69 (45.4) | 306 (48.0) | 308 (48.1) |
| Rinse with mouthwash | 20 (13.8) | 18 (11.6) | 18 (11.8) | 98 (15.4) | 98 (15.3) |
| Spit not rinse | 67 (46.2) | 57 (36.8) | 61 (40.1) | 224 (35.1) | 222 (34.7) |
| Missing | 2 (1.4) | 2 (1.3) | 4 (2.6) | 10 (1.6) | 12 (1.9) |
| After brushing should you . . . ? | |||||
| Rinse with water | 29 (20.0) | 40 (25.8) | 39 (25.7) | 200 (31.3) | 210 (32.8) |
| Rinse with mouthwash | 33 (22.8) | 35 (22.6) | 30 (19.7) | 145 (22.7) | 157 (24.5) |
| Spit not rinse | 79 (54.5) | 76 (49.0) | 79 (52.0) | 276 (43.3) | 261 (40.8) |
| Missing | 4 (2.8) | 4 (2.6) | 4 (2.6) | 17 (2.7) | 12 (1.9) |
| Frequency of flossing | |||||
| At least once a week | 33 (22.8) | 20 (12.9) | 27 (17.8) | 128 (20.1) | 128 (20.0) |
| At least once a month | 13 (9.0) | 17 (11.0) | 17 (11.2) | 86 (13.5) | 76 (11.9) |
| Never | 35 (24.1) | 51 (32.9) | 48 (31.6) | 188 (29.5) | 176 (27.5) |
| Missing | 41 (28.3) | 42 (27.1) | 40 (26.3) | 114 (17.9) | 119 (18.6) |
| How often should you floss? | |||||
| At least once a week | 8 (5.5) | 6 (3.9) | 5 (3.3) | 24 (3.8) | 14 (2.2) |
| At least once a month | 15 (10.3) | 29 (18.7) | 29 (19.1) | 103 (16.1) | 88 (13.8) |
| Never | 75 (51.7) | 69 (44.5) | 68 (44.7) | 353 (55.3) | 374 (58.4) |
| Missing | 44 (30.3) | 44 (28.4) | 45 (29.6) | 137 (21.5) | 139 (21.7) |
| Frequency of using interdental brushes | |||||
| At least once a week | 10 (6.9) | 7 (4.5) | 8 (5.3) | 73 (11.4) | 60 (9.4) |
| At least once a month | 4 (2.8) | 8 (5.2) | 14 (9.2) | 49 (7.7) | 43 (6.7) |
| Never | 78 (53.8) | 86 (55.5) | 73 (48.0) | 291 (45.6) | 308 (48.1) |
| Missing | 45 (31.0) | 41 (26.5) | 46 (30.3) | 134 (21.0) | 133 (20.8) |
| How often should you use interdental brushes? | |||||
| At least once a week | 5 (3.4) | 10 (6.5) | 3 (2.0) | 29 (4.5) | 24 (3.8) |
| At least once a month | 18 (12.4) | 14 (9.0) | 18 (11.8) | 79 (12.4) | 61 (9.5) |
| Never | 19 (13.1) | 17 (11.0) | 17 (11.2) | 95 (14.9) | 101 (15.8) |
| Missing | 98 (67.6) | 104 (67.1) | 106 (69.7) | 401 (62.9) | 411 (64.2) |
| Behaviours/knowledge | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Responded | 135 (100.0) | 144 (100.0) | 142 (100.0) | 610 (100.0) | 619 (100.0) |
| Frequency of brushing | |||||
| Once a day | 24 (17.8) | 37 (25.7) | 27 (19.0) | 103 (16.9) | 99 (16.0) |
| Twice a day | 95 (70.4) | 96 (66.7) | 105 (73.9) | 443 (72.6) | 443 (71.6) |
| More than twice a day | 15 (11.1) | 10 (6.9) | 9 (6.3) | 55 (9.0) | 64 (10.3) |
| Missing | 1 (0.7) | 0 (0.0) | 0 (0.0) | 3 (0.5) | 5 (0.8) |
| How often should you be brushing? | |||||
| Once a day | 1 (0.7) | 1 (0.7) | 1 (0.7) | 11 (1.8) | 8 (1.3) |
| Twice a day | 106 (78.5) | 120 (83.3) | 118 (83.1) | 455 (74.6) | 471 (76.1) |
| More than twice a day | 27 (20.0) | 22 (15.3) | 23 (16.2) | 137 (22.5) | 130 (21.0) |
| Missing | 1 (0.7) | 1 (0.7) | 0 (0.0) | 7 (1.1) | 9 (1.5) |
| Duration of brushing | |||||
| 1–2 minutes | 41 (30.4) | 45 (31.3) | 43 (30.3) | 178 (29.2) | 210 (33.9) |
| 2 minutes | 72 (53.3) | 74 (51.4) | 73 (51.4) | 323 (53.0) | 304 (49.1) |
| > 2 minutes | 16 (11.9) | 22 (15.3) | 22 (15.5) | 88 (14.4) | 83 (13.4) |
| Missing | 1 (0.7) | 0 (0.0) | 1 (0.7) | 3 (0.5) | 2 (0.3) |
| How long should you brush for? | |||||
| 1–2 minutes | 9 (6.7) | 13 (9.0) | 10 (7.0) | 59 (9.7) | 68 (11.0) |
| 2 minutes | 93 (68.9) | 105 (72.9) | 98 (69.0) | 401 (65.7) | 409 (66.1) |
| > 2 minutes | 31 (23.0) | 24 (16.7) | 34 (23.9) | 138 (22.6) | 135 (21.8) |
| Missing | 1 (0.7) | 2 (1.4) | 0 (0.0) | 10 (1.6) | 6 (1.0) |
| After brushing | |||||
| Rinse with water | 49 (36.3) | 64 (44.4) | 46 (32.4) | 264 (43.3) | 275 (44.4) |
| Rinse with mouthwash | 19 (14.1) | 15 (10.4) | 19 (13.4) | 100 (16.4) | 90 (14.5) |
| Spit not rinse | 66 (48.9) | 65 (45.1) | 74 (52.1) | 239 (39.2) | 247 (39.9) |
| Missing | 1 (0.7) | 0 (0.0) | 3 (2.1) | 7 (1.1) | 7 (1.1) |
| After brushing should you . . .? | |||||
| Rinse with water | 19 (14.1) | 33 (22.9) | 31 (21.8) | 159 (26.1) | 171 (27.6) |
| Rinse with mouthwash | 27 (20.0) | 27 (18.8) | 29 (20.4) | 143 (23.4) | 130 (21.0) |
| Spit not rinse | 85 (63.0) | 83 (57.6) | 78 (54.9) | 292 (47.9) | 297 (48.0) |
| Missing | 4 (3.0) | 1 (0.7) | 4 (2.8) | 16 (2.6) | 21 (3.4) |
| Behaviours/knowledge | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Responded | 134 (100.0) | 131 (100.0) | 134 (100.0) | 571 (100.0) | 584 (100.0) |
| Frequency of brushing | |||||
| Once a day | 22 (16.4) | 29 (22.1) | 29 (21.6) | 98 (17.2) | 88 (15.1) |
| Twice a day | 100 (74.6) | 91 (69.5) | 99 (73.9) | 414 (72.5) | 438 (75.0) |
| More than twice a day | 11 (8.2) | 10 (7.6) | 5 (3.7) | 53 (9.3) | 50 (8.6) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.4) | 2 (0.3) |
| How often should you be brushing? | |||||
| Once a day | 1 (0.7) | 1 (0.8) | 1 (0.7) | 6 (1.1) | 9 (1.5) |
| Twice a day | 111 (82.8) | 108 (82.4) | 110 (82.1) | 448 (78.5) | 457 (78.3) |
| More than twice a day | 22 (16.4) | 22 (16.8) | 22 (16.4) | 112 (19.6) | 115 (19.7) |
| Missing | 0 (0.0) | 0 (0.0) | 1 (0.7) | 5 (0.9) | 2 (0.3) |
| Duration of brushing | |||||
| 1–2 minutes | 44 (32.8) | 33 (25.2) | 42 (31.3) | 175 (30.6) | 187 (32.0) |
| 2 minutes | 68 (50.7) | 76 (58.0) | 68 (50.7) | 296 (51.8) | 316 (54.1) |
| > 2 minutes | 17 (12.7) | 18 (13.7) | 23 (17.2) | 83 (14.5) | 65 (11.1) |
| Missing | 0 (0.0) | 1 (0.8) | 1 (0.7) | 4 (0.7) | 2 (0.3) |
| How long should you brush for? | |||||
| 1–2 minutes | 14 (10.4) | 6 (4.6) | 4 (3.0) | 59 (10.3) | 73 (12.5) |
| 2 minutes | 93 (69.4) | 95 (72.5) | 100 (74.6) | 384 (67.3) | 396 (67.8) |
| > 2 minutes | 26 (19.4) | 30 (22.9) | 29 (21.6) | 121 (21.2) | 111 (19.0) |
| Missing | 0 (0.0) | 0 (0.0) | 1 (0.7) | 4 (0.7) | 4 (0.7) |
| After brushing | |||||
| Rinse with water | 38 (28.4) | 59 (45.0) | 44 (32.8) | 236 (41.3) | 263 (45.0) |
| Rinse with mouthwash | 21 (15.7) | 13 (9.9) | 13 (9.7) | 76 (13.3) | 72 (12.3) |
| Spit not rinse | 74 (55.2) | 59 (45.0) | 75 (56.0) | 254 (44.5) | 243 (41.6) |
| Rinse with both water and mouthwash | 1 (0.7) | 0 (0.0) | 2 (1.5) | 2 (0.4) | 1 (0.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (0.5) | 5 (0.9) |
| After brushing should you . . .? | |||||
| Rinse with water | 22 (16.4) | 34 (26.0) | 33 (24.6) | 140 (24.5) | 162 (27.7) |
| Rinse with mouthwash | 26 (19.4) | 21 (16.0) | 20 (14.9) | 112 (19.6) | 121 (20.7) |
| Spit not rinse | 84 (62.7) | 76 (58.0) | 81 (60.4) | 309 (54.1) | 289 (49.5) |
| Missing | 2 (1.5) | 0 (0.0) | 0 (0.0) | 10 (1.8) | 12 (2.1) |
| Behaviours/knowledge | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Responded | 151 (100.0) | 153 (100.0) | 156 (100.0) | 650 (100.0) | 655 (100.0) |
| Frequency of brushing | |||||
| Once a day | 27 (17.9) | 31 (20.3) | 32 (20.5) | 112 (17.2) | 94 (14.4) |
| Twice a day | 103 (68.2) | 103 (67.3) | 112 (71.8) | 463 (71.2) | 491 (75.0) |
| More than twice a day | 21 (13.9) | 16 (10.5) | 8 (5.1) | 66 (10.2) | 62 (9.5) |
| Missing | 0 (0) | 0 (0) | 1 (0.6) | 4 (0.6) | 2 (0.3) |
| How often should you be brushing? | |||||
| Once a day | 3 (2.0) | 3 (2.0) | 1 (0.6) | 6 (0.9) | 6 (0.9) |
| Twice a day | 121 (80.1) | 123 (80.4) | 125 (80.1) | 512 (78.8) | 515 (78.6) |
| More than twice a day | 26 (17.2) | 27 (17.6) | 27 (17.3) | 129 (19.8) | 128 (19.5) |
| Missing | 1 (0.7) | 0 (0) | 2 (1.3) | 3 (0.5) | 4 (0.6) |
| Duration of brushing | |||||
| 1–2 minutes | 52 (34.4) | 51 (33.3) | 44 (28.2) | 192 (29.5) | 210 (32.1) |
| 2 minutes | 79 (52.3) | 76 (49.7) | 82 (52.6) | 318 (48.9) | 341 (52.1) |
| > 2 minutes | 17 (11.3) | 23 (15.0) | 28 (17.9) | 114 (17.5) | 78 (11.9) |
| Missing | 0 (0) | 1 (0.7) | 1 (0.6) | 5 (0.8) | 4 (0.6) |
| How long should you brush for? | |||||
| 1–2 minutes | 19 (12.6) | 9 (5.9) | 10 (6.4) | 66 (10.2) | 56 (8.5) |
| 2 minutes | 104 (68.9) | 113 (73.9) | 106 (67.9) | 432 (66.5) | 452 (69.0) |
| > 2 minutes | 24 (15.9) | 30 (19.6) | 39 (25.0) | 142 (21.8) | 137 (20.9) |
| Missing | 3 (2.0) | 1 (0.7) | 1 (0.6) | 8 (1.2) | 6 (0.9) |
| After brushing | |||||
| Rinse with water | 49 (32.5) | 63 (41.2) | 55 (35.3) | 261 (40.2) | 264 (40.3) |
| Rinse with mouthwash | 22 (14.6) | 13 (8.5) | 11 (7.1) | 91 (14.0) | 76 (11.6) |
| Spit not rinse | 80 (53.0) | 74 (48.4) | 87 (55.8) | 276 (42.5) | 300 (45.8) |
| Rinse with both water and mouthwash | 0 (0) | 0 (0) | 2 (1.3) | 13 (2.0) | 12 (1.8) |
| Missing | 0 (0) | 3 (2.0) | 1 (0.6) | 9 (1.4) | 3 (0.5) |
| After brushing should you . . .? | |||||
| Rinse with water | 30 (19.9) | 29 (19.0) | 31 (19.9) | 158 (24.3) | 166 (25.3) |
| Rinse with mouthwash | 27 (17.9) | 26 (17.0) | 23 (14.7) | 124 (19.1) | 119 (18.2) |
| Spit not rinse | 91 (60.3) | 92 (60.1) | 98 (62.8) | 344 (52.9) | 346 (52.8) |
| Rinse with both water and mouthwash | 0 (0) | 2 (1.3) | 1 (0.6) | 6 (0.9) | 6 (0.9) |
| Missing | 3 (2.0) | 4 (2.6) | 3 (1.9) | 18 (2.8) | 18 (2.7) |
Clinical outcomes
The following tables and graphs present the clinical outcomes at 4 years for the overall sample.
| Clinical outcome, mean (SD), n | Risk based | 6 month |
|---|---|---|
| Percentage of sites bleeding | 33.8 (21.6), 741 | 33.3 (22.0), 731 |
| Calculus | 36.7 (27.5), 746 | 37.9 (27.3), 733 |
| Mean pocket depth (mm) | 2.2 (0.5), 736 | 2.2 (0.4), 727 |
| Number of teeth | 23.9 (4.7), 749 | 23.5 (5.0), 737 |
| Caries category, n (%) | ||
| Sound surfaces | 4 (0.5) | 8 (1.1) |
| Initial lesions | 122 (16.3) | 140 (19.0) |
| Moderate lesions | 491 (65.6) | 463 (62.8) |
| Extensive caries or treatment needed | 130 (17.4) | 120 (16.3) |
| Missing | 2 (0.3) | 6 (0.8) |
| Maximum caries score per mouth, n (%) | ||
| 1 | 4 (0.5) | 10 (1.4) |
| 2 | 118 (15.8) | 130 (17.6) |
| 3 | 167 (22.3) | 171 (23.2) |
| 4 | 324 (43.3) | 292 (39.6) |
| 5 | 110 (14.7) | 102 (13.8) |
| 6 | 20 (2.7) | 18 (2.4) |
| Missing | 2 (0.3) | 6 (0.8) |
| Root caries, n (%) | ||
| Yes | 146 (19.5) | 154 (20.9) |
| No | 509 (68.0) | 481 (65.3) |
| Missing | 94 (12.6) | 102 (13.8) |
Clinical outcomes by country
Scotland
| Clinical outcome, mean (SD), n | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Attended/randomised | 79/111 | 82/117 | 77/116 | 300/419 | 313/425 |
| Bleeding score | 30.9 (18.0), 78 | 30.9 (19.9), 81 | 32.5 (20.8), 76 | 33.6 (22.9), 295 | 37.2 (22.8), 310 |
| Calculus | 40.3 (27.4), 78 | 45.9 (28.3), 82 | 43.8 (25.0), 76 | 32.2 (26.0), 299 | 36.0 (26.9), 312 |
| Mean pocket depth (mm) | 2.2 (0.5), 78 | 2.2 (0.4), 81 | 2.1 (0.4), 75 | 2.1 (0.4), 293 | 2.2 (0.4), 309 |
| Number of teeth | 23.6 (4.8), 79 | 22.8 (5.2), 82 | 24.0 (4.1), 77 | 23.5 (4.9), 300 | 22.7 (5.3), 313 |
| Caries category, n (%) | |||||
| Sound surfaces | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.0) | 1 (0.3) |
| Initial lesions | 12 (15.2) | 14 (17.1) | 13 (16.9) | 53 (17.7) | 49 (15.7) |
| Moderate lesions | 57 (72.2) | 53 (64.6) | 56 (72.7) | 186 (62.0) | 199 (63.6) |
| Extensive caries or treatment needed | 9 (11.4) | 15 (18.3) | 7 (9.1) | 58 (19.3) | 62 (19.8) |
| Missing | 1 (1.3) | 0 (0.0) | 1 (1.3) | 0 (0.0) | 2 (0.6) |
| Root caries, n (%) | |||||
| Yes | 26 (18.2) | 21 (15.2) | 17 (12.6) | 120 (19.8) | 137 (22.8) |
| No | 96 (67.1) | 100 (72.5) | 96 (71.1) | 413 (68.2) | 385 (64.0) |
| Missing | 21 (14.7) | 17 (12.3) | 22 (16.3) | 73 (12.0) | 80 (13.3) |
England
| Clinical outcome, mean (SD), n | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Attended/randomised | 50/75 | 40/69 | 46/72 | 284/410 | 267/405 |
| Percentage of sites bleeding | 43.4 (18.0), 50 | 39.7 (19.6), 40 | 39.4 (22.8), 46 | 32.2 (21.4), 282 | 27.6 (20.1), 265 |
| Calculus | 26.1 (22.3), 50 | 25.5 (25.8), 40 | 27.9 (22.3), 45 | 42.7 (29.1), 283 | 40.4 (28.9), 266 |
| Mean pocket depth (mm) | 2.2 (0.4), 50 | 2.1 (0.2), 40 | 2.2 (0.5), 46 | 2.2 (0.5), 279 | 2.2 (0.5), 263 |
| Number of teeth | 25.5 (4.1), 50 | 25.5 (2.1), 40 | 25.8 (3.8), 46 | 24.3 (4.4), 284 | 23.8 (5.0), 267 |
| Caries category, n (%) | |||||
| Sound surfaces | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (2.6) |
| Initial lesions | 9 (18.0) | 11 (27.5) | 15 (32.6) | 43 (15.1) | 51 (19.1) |
| Moderate lesions | 29 (58.0) | 24 (60.0) | 27 (58.7) | 191 (67.3) | 165 (61.8) |
| Extensive caries or treatment needed | 10 (20.0) | 5 (12.5) | 3 (6.5) | 50 (17.6) | 42 (15.7) |
| Missing | 1 (2.0) | 0 (0.0) | 1 (2.2) | 0 (0.0) | 2 (0.7) |
| Root caries, n (%) | |||||
| Yes | 26 (18.2) | 21 (15.2) | 17 (12.6) | 120 (19.8) | 137 (22.8) |
| No | 96 (67.1) | 100 (72.5) | 96 (71.1) | 413 (68.2) | 385 (64.0) |
| Missing | 21 (14.7) | 17 (12.3) | 22 (16.3) | 73 (12.0) | 80 (13.3) |
Northern Ireland
| Clinical outcome, mean (SD), n | Eligible for 24-month recall | Ineligible for 24-month recall | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Attended/randomised | 14/31 | 16/30 | 12/27 | 22/32 | 22/33 |
| Percentage of sites bleeding | 34.1 (21.4), 14 | 39.0 (19.5), 16 | 40.5 (21.3), 12 | 46.6 (18.8), 22 | 33.5 (21.7), 22 |
| Calculus | 27.9 (21.4), 14 | 30.7 (21.6), 16 | 32.4 (23.0), 12 | 37.4 (22.2), 22 | 37.1 (26.3), 22 |
| Mean pocket depth (mm) | 2.1 (0.7), 14 | 1.9 (0.2), 16 | 2.0 (0.2), 12 | 2.0 (0.2), 22 | 2.0 (0.3), 22 |
| Number of teeth | 22.5 (6.4), 14 | 26.1 (2.0), 16 | 26.2 (2.8), 12 | 23.0 (4.0), 22 | 24.5 (3.4), 22 |
| Caries category, n (%) | |||||
| Sound surfaces | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Initial lesions | 1 (7.1) | 3 (18.8) | 5 (41.7) | 4 (18.2) | 7 (31.8) |
| Moderate lesions | 12 (85.7) | 10 (62.5) | 4 (33.3) | 16 (72.7) | 12 (54.5) |
| Extensive caries or treatment needed | 1 (7.1) | 2 (12.5) | 3 (25.0) | 2 (9.1) | 3 (13.6) |
| Root caries, n (%) | |||||
| Yes | 26 (18.2) | 21 (15.2) | 17 (12.6) | 120 (19.8) | 137 (22.8) |
| No | 96 (67.1) | 100 (72.5) | 96 (71.1) | 413 (68.2) | 385 (64.0) |
| Missing | 21 (14.7) | 17 (12.3) | 22 (16.3) | 73 (12.0) | 80 (13.3) |
Subgroup analyses
Interaction coefficients for bleeding
| Characteristic | Eligible for 24-month recall (24 month vs. 6 month), mean difference (99% CI); p-value | Overall (risk based vs. 6 month), mean difference (99% CI); p-value |
|---|---|---|
| Age | ||
| < 45 years | 3.50 (–4.59 to 11.58); 0.27 | 0.53 (–3.80 to 4.86); 0.75 |
| 45–64 years | –9.23 (–21.01 to 2.55); 0.04 | –1.18 (–6.89 to 4.52); 0.59 |
| ≥ 65 years | –0.22 (–19.86 to 19.43); 0.98 | 4.47 (–3.28 to 12.23); 0.14 |
| Country | ||
| Scotland | –2.02 (–9.37 to 5.33); 0.48 | –3.36 (–6.90 to 0.18); 0.01 |
| England | 3.47 (–9.00 to 15.94); 0.47 | 8.34 (3.11 to 13.57); < 0.001 |
| Northern Ireland | 1.94 (–17.16 to 21.05); 0.79 | 9.63 (–2.53 to 21.79); 0.04 |
| Do you pay for treatment? | ||
| Yes | –0.90 (–10.69 to 8.88); 0.81 | –1.35 (–6.06 to 3.35); 0.46 |
| No | 0.35 (–11.69 to 12.39); 0.94 | 2.96 (–2.65 to 8.57); 0.17 |
Interaction coefficients for caries
| Characteristic | Eligible for 24-month recall (24 month vs. 6 month), odds ratio (99% CI); p-value | Overall (risk based vs 6 month), odds ratio (99% CI); p-value |
|---|---|---|
| Age | ||
| < 45 years | 1.14 (0.37 to 3.53); 0.77 | 1.57 (0.89 to 2.79); 0.04 |
| 45–64 years | 0.91 (0.16 to 5.07); 0.89 | 0.70 (0.32 to 1.51); 0.23 |
| ≥ 65 years | 4.27 (0.20 to 88.96); 0.22 | 0.74 (0.24 to 2.28); 0.49 |
| Country | ||
| Scotland | 0.96 (0.30 to 3.02); 0.92 | 0.88 (0.53 to 1.45); 0.51 |
| England | 1.52 (0.26 to 8.87); 0.54 | 1.89 (0.91 to 3.91); 0.03 |
| Northern Ireland | 2.89 (0.21 to 39.89); 0.30 | 3.98 (0.76 to 20.69); 0.03 |
| Do you pay for treatment? | ||
| Yes | 1.23 (0.30 to 4.98); 0.71 | 1.53 (0.78 to 3.00); 0.11 |
| No | 1.04 (0.19 to 5.84); 0.95 | 0.77 (0.35 to 1.70); 0.39 |
Patient-reported outcomes
Oral Health Impact Profile-14
| Time point | Eligible for 24-month recall, mean (SD), n | Ineligible for 24-month recall, mean (SD), n | |||
|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | |
| Baseline | 4.5 (7.0), 175 | 4.7 (6.4), 183 | 4.4 (6.1), 182 | 5.8 (6.9), 778 | 6.1 (7.7), 778 |
| Year 1 | 3.7 (5.4), 141 | 4.4 (5.6), 152 | 4.8 (6.4), 145 | 5.6 (7.6), 617 | 5.4 (7.4), 623 |
| Year 2 | 4.4 (5.7), 128 | 4.6 (6.4), 143 | 5.2 (7.1), 137 | 5.6 (7.4), 585 | 5.1 (7.3), 599 |
| Year 3 | 3.9 (5.3), 131 | 4.6 (5.6), 129 | 4.1 (5.5), 129 | 5.4 (6.9), 551 | 5.7 (8.1), 566 |
| Year 4 | 4.1 (5.7), 145 | 4.8 (6.4), 153 | 4.8 (6.2), 152 | 5.5 (6.8), 624 | 5.8 (8.3), 630 |
Attitude and satisfaction with care
| Patient-reported outcome | Time point | Eligible for 24-month recall, mean (SD), n | Ineligible for 24-month recall, mean (SD), n | |||
|---|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | ||
| Attitude | Baseline | 4.2 (0.8), 178 | 4.3 (0.9), 186 | 4.1 (0.7), 187 | 4.1 (0.9), 806 | 4.0 (0.9), 795 |
| Year 1 | 4.4 (0.8), 143 | 4.2 (1.0), 154 | 4.2 (0.8), 150 | 4.1 (0.9), 635 | 4.1 (0.8), 635 | |
| Year 2 | 4.2 (0.8), 134 | 4.2 (0.9), 144 | 4.2 (0.8), 142 | 4.1 (0.9), 608 | 4.1 (0.8), 616 | |
| Year 3 | 4.2 (0.7), 134 | 4.2 (0.8), 131 | 4.0 (0.8), 134 | 4.1 (0.9), 570 | 4.0 (0.8), 583 | |
| Year 4 | 4.3 (0.9), 151 | 4.3 (0.9), 153 | 4.1 (0.8), 155 | 4.1 (0.9), 647 | 4.1 (0.8), 654 | |
| Satisfaction with care | Baseline | 5.2 (0.8), 147 | 5.2 (0.8), 153 | 5.2 (0.7), 158 | 5.3 (0.6), 703 | 5.3 (0.6), 701 |
| Year 1 | 5.1 (0.7), 143 | 5.0 (0.8), 153 | 5.1 (0.8), 150 | 5.1 (0.7), 620 | 5.2 (0.7), 623 | |
| Year 2 | 5.1 (0.7), 134 | 4.9 (0.7), 144 | 5.1 (0.6), 142 | 5.2 (0.7), 609 | 5.2 (0.7), 616 | |
| Year 3 | 5.0 (0.7), 134 | 5.0 (0.7), 131 | 5.1 (0.7), 134 | 5.2 (0.6), 570 | 5.2 (0.7), 583 | |
| Year 4 | 5.2 (0.7), 151 | 5.0 (0.7), 153 | 5.1 (0.7), 155 | 5.3 (0.6), 647 | 5.2 (0.6), 654 | |
Anxiety
| Anxiety measure | Time point | Eligible for 24-month recall, mean (SD), n | Ineligible for 24-month recall, mean (SD), n | |||
|---|---|---|---|---|---|---|
| Risk based | 24 month | 6 month | Risk based | 6 month | ||
| Overall anxiety (score from 5 to 25), mean (SD), n | Baseline | 10.4 (5), 176 | 10.7 (4), 186 | 10.5 (4), 185 | 10.2 (5), 801 | 10.1 (5), 798 |
| Year 1 | 10.2 (4), 145 | 10.7 (5), 154 | 10.5 (4), 150 | 10.4 (4), 635 | 10.3 (4), 637 | |
| Year 2 | 10.1 (4), 133 | 10.5 (5), 144 | 10.4 (4), 142 | 10.3 (5), 608 | 10.3 (4), 617 | |
| Year 3 | 10.6 (5), 134 | 10.9 (5), 131 | 10.7 (4), 134 | 10.4 (5), 568 | 10.4 (5), 582 | |
| Year 4 | 10.5 (5), 150 | 10.9 (5), 153 | 11.0 (5), 155 | 10.3 (5), 645 | 10.5 (5), 649 | |
| High-anxiety patients (score ≥ 19), n (%) | Baseline | 12 (6.8) | 12 (6.5) | 13 (7.0) | 53 (6.6) | 53 (6.6) |
| Year 1 | 9 (6.2) | 13 (8.4) | 10 (6.7) | 46 (7.2) | 38 (6.0) | |
| Year 2 | 7 (5.3) | 12 (8.3) | 10 (7.0) | 42 (6.9) | 40 (6.5) | |
| Year 3 | 13 (9.7) | 11 (8.4) | 6 (4.5) | 38 (6.7) | 45 (7.7) | |
| Year 4 | 9 (6.0) | 12 (7.8) | 11 (7.1) | 40 (6.2) | 46 (7.1) | |
Dentist belief questionnaire
| Dentist belief | Baseline, mean (SD), n | Follow-up, mean (SD), n | Change follow-up–baseline, mean (SD), n | |||
|---|---|---|---|---|---|---|
| Deemed at least one patient eligible for 24-month recall | Deemed all ineligible for 24-month recall | Deemed at least one patient eligible for 24-month recall | Deemed all ineligible for 24-month recall | Deemed at least one patient eligible for 24-month recall | Deemed all ineligible for 24-month recall | |
| Attitude regarding a 24-month recall | 3.7 (1.2), 40 | 4.3 (1.1), 6 | 4.4 (1.2), 43 | 4.4 (1.5), 6 | 0.7 (1.1), 40 | 0.1 (1.4), 6 |
| Attitude regarding a 6-month recall | 3.6 (1.0), 40 | 4.5 (0.5), 6 | 3.9 (1.2), 43 | 4.1 (0.6), 6 | 0.3 (0.9), 40 | –0.4 (0.7), 6 |
| General attitude | 4.5 (1.1), 40 | 4.7 (0.7), 6 | 3.9 (1.1), 43 | 4.6 (1.0), 6 | –0.6 (1.4), 40 | –0.2 (1.2), 6 |
| PBC risk | 4.0 (0.9), 40 | 4.9 (0.6), 6 | 4.1 (1.1), 43 | 3.9 (1.1), 6 | 0.1 (1.1), 40 | –1.0 (1.1), 6 |
Appendix 2 Health economics outcomes
Discrete choice experiment subgroup analyses
| Attribute level | Base case | Female | Scotland | Smoker | Employed | Low income | Pay private | 6-month check up | Decay experience | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main effects | ||||||||||||||||||
| Recall (12 month) | –0.015 | –0.017 | 0.002 | –0.038 | –0.017 | –0.019 | –0.010 | –0.042 | –0.016 | |||||||||
| Recall (6 month) | 0.348 | *** | 0.351 | *** | 0.359 | *** | 0.373 | *** | 0.351 | *** | 0.335 | *** | 0.350 | *** | 0.282 | *** | 0.331 | *** |
| Recall (risk based) | 0.089 | ** | 0.090 | ** | 0.088 | ** | 0.079 | * | 0.097 | *** | 0.094 | ** | 0.086 | ** | 0.108 | ** | 0.087 | ** |
| Bleed hardly ever | 0.099 | ** | 0.100 | ** | 0.080 | 0.066 | 0.107 | ** | 0.096 | ** | 0.113 | ** | 0.014 | 0.109 | * | |||
| Bleed occasional | 0.089 | ** | 0.091 | ** | 0.072 | 0.075 | 0.099 | ** | 0.087 | ** | 0.081 | * | 0.076 | 0.091 | * | |||
| Bleed fairly often | –0.127 | ** | –0.125 | ** | –0.142 | ** | –0.149 | ** | –0.129 | ** | –0.127 | ** | –0.135 | ** | –0.213 | *** | –0.153 | ** |
| Bleed very often | –0.268 | *** | –0.277 | *** | –0.272 | ** | –0.238 | ** | –0.297 | *** | –0.257 | ** | –0.279 | ** | –0.103 | –0.258 | * | |
| Early decay | 0.058 | * | 0.059 | * | 0.050 | 0.054 | 0.057 | 0.046 | 0.070 | * | –0.010 | 0.008 | ||||||
| Moderate decay | –0.241 | *** | –0.239 | *** | –0.245 | *** | –0.192 | *** | –0.238 | *** | –0.252 | *** | –0.270 | *** | –0.278 | *** | –0.230 | *** |
| Advanced decay | –0.382 | *** | –0.384 | *** | –0.382 | *** | –0.379 | *** | –0.400 | *** | –0.400 | *** | –0.399 | *** | –0.408 | *** | –0.295 | *** |
| Annual cost | –0.006 | *** | –0.006 | *** | –0.006 | *** | –0.006 | *** | –0.006 | *** | –0.006 | *** | –0.005 | *** | –0.007 | *** | –0.005 | *** |
| ASC | 1.484 | *** | 1.482 | *** | 1.446 | *** | 1.490 | *** | 1.513 | *** | 1.465 | *** | 1.565 | *** | 1.203 | *** | 1.412 | *** |
| Interaction terms | ||||||||||||||||||
| Recall (12 month) | 0.075 | ** | 0.040 | –0.042 | 0.009 | –0.036 | 0.005 | 0.036 | < 0.001 | |||||||||
| Recall (6 month) | 0.010 | 0.025 | 0.043 | –0.010 | –0.058 | 0.003 | 0.096 | * | 0.043 | |||||||||
| Recall (risk based) | –0.046 | –0.008 | –0.017 | –0.035 | 0.021 | –0.010 | –0.021 | 0.010 | ||||||||||
| Bleed hardly ever | –0.045 | –0.045 | –0.067 | –0.036 | –0.030 | 0.044 | 0.121 | * | –0.026 | |||||||||
| Bleed occasional | –0.043 | –0.046 | –0.036 | –0.063 | –0.049 | –0.030 | 0.025 | –0.009 | ||||||||||
| Bleed fairly often | 0.105 | * | –0.030 | –0.049 | –0.003 | –0.042 | –0.018 | 0.109 | 0.033 | |||||||||
| Bleed very often | 0.053 | –0.013 | 0.106 | 0.159 | 0.164 | –0.028 | –0.236 | * | –0.023 | |||||||||
| Early decay | –0.076 | ** | –0.010 | –0.014 | 0.041 | –0.055 | 0.027 | 0.101 | ** | 0.091 | ** | |||||||
| Moderate decay | 0.065 | * | –0.003 | 0.085 | ** | –0.011 | 0.003 | –0.111 | *** | 0.043 | –0.058 | |||||||
| Advanced decay | 0.132 | *** | –0.003 | –0.004 | 0.074 | –0.098 | * | –0.055 | 0.032 | –0.173 | *** | |||||||
| Annual cost | < 0.001 | > –0.001 | < 0.001 | 0.001 | *** | –0.001 | *** | 0.002 | *** | 0.002 | *** | –0.001 | *** | |||||
| ASC | –0.125 | –0.111 | –0.015 | –0.056 | –0.240 | *** | 0.200 | ** | 0.428 | *** | 0.147 | |||||||
| LR tests | ||||||||||||||||||
| Log-likelihood | –3856 | –3839 | –3849 | –3850 | –3845 | –3835 | –3821 | –3829 | –3840 | |||||||||
| AIC | 7739 | 7730 | 7751 | 7752 | 7742 | 7722 | 7694 | 7711 | 7733 | |||||||||
| BIC | 7837 | 7926 | 7948 | 7949 | 7939 | 7919 | 7891 | 7907 | 7930 | |||||||||
| LR test (chi sq) | 34.92 | 13.61 | 12.12 | 22.05 | 42.59 | 70.14 | 53.91 | 31.80 | ||||||||||
| LR test (p-value) | < 0.001 | 0.001 | 0.002 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||||||||
| Variable | M = 0 | M = 1 | M = 2 | M = 3 | M = 4 | M = 5 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| WTP | ||||||||||||||||||
| WTP (constant) | 2372 | £948 | £0 | 2372 | £947.51 | £0.00 | 2372 | £947.51 | £0.00 | 2372 | £947.51 | £0.00 | 2372 | £947.51 | £0.00 | 2372 | £947.51 | £0.00 |
| WTP (recall) | 2209 | –£48 | £203 | 2358 | –£47.84 | £202.77 | 2358 | –£50.49 | £203.65 | 2358 | –£47.18 | £202.67 | 2358 | –£49.38 | £202.16 | 2358 | –£50.22 | £203.15 |
| WTP (bleeding) | 1642 | £67 | £55 | 2357 | £65.78 | £56.34 | 2357 | £65.00 | £57.57 | 2357 | £66.26 | £57.47 | 2357 | £66.09 | £56.82 | 2357 | £65.62 | £55.80 |
| WTP (caries) | 2063 | –£144 | £63 | 2371 | –£142.97 | £64.49 | 2371 | –£143.24 | £63.89 | 2371 | –£143.07 | £64.85 | 2371 | –£142.31 | £64.49 | 2371 | –£143.41 | £63.20 |
| EQ-5D-3L utilities | ||||||||||||||||||
| Utility (baseline) | 1606 | 0.905 | 0.198 | 2359 | 0.909 | 0.190 | 2359 | 0.909 | 0.193 | 2359 | 0.908 | 0.191 | 2359 | 0.905 | 0.202 | 2359 | 0.909 | 0.193 |
| Utility (1 year) | 1670 | 0.889 | 0.203 | 2358 | 0.894 | 0.202 | 2358 | 0.891 | 0.202 | 2358 | 0.889 | 0.204 | 2358 | 0.889 | 0.207 | 2358 | 0.890 | 0.206 |
| Utility (2 year) | 1618 | 0.850 | 0.210 | 2358 | 0.849 | 0.215 | 2358 | 0.848 | 0.213 | 2358 | 0.849 | 0.216 | 2358 | 0.846 | 0.217 | 2358 | 0.852 | 0.208 |
| Utility (3 year) | 1525 | 0.819 | 0.208 | 2358 | 0.821 | 0.209 | 2358 | 0.821 | 0.209 | 2358 | 0.818 | 0.211 | 2358 | 0.821 | 0.207 | 2358 | 0.822 | 0.206 |
| Utility (4 year) | 1735 | 0.784 | 0.210 | 2358 | 0.783 | 0.211 | 2358 | 0.783 | 0.214 | 2358 | 0.782 | 0.212 | 2358 | 0.780 | 0.215 | 2358 | 0.784 | 0.211 |
| NHS costs | ||||||||||||||||||
| NHS dental | 2209 | £113.13 | £173.29 | 2358 | £112.94 | £173.69 | 2358 | £111.88 | £170.19 | 2358 | £114.03 | £174.73 | 2358 | £112.57 | £172.41 | 2358 | £112.53 | £172.08 |
| NHS GP | 990 | £29.94 | £62.65 | 2357 | £32.30 | £64.99 | 2357 | £32.25 | £65.54 | 2357 | £33.38 | £70.81 | 2357 | £31.24 | £63.35 | 2357 | £30.65 | £65.64 |
| NHS inpatient | 1153 | £8.32 | £75.18 | 2357 | £7.87 | £73.19 | 2357 | £8.60 | £75.86 | 2357 | £9.32 | £79.52 | 2357 | £9.17 | £78.29 | 2357 | £9.00 | £78.08 |
| NHS outpatient | 1003 | £7.29 | £56.29 | 2357 | £9.04 | £66.76 | 2357 | £9.86 | £68.83 | 2357 | £8.13 | £57.22 | 2357 | £7.28 | £57.46 | 2357 | £9.04 | £63.73 |
| NHS accident and emergency | 1003 | £0.90 | £10.80 | 2357 | £1.03 | £11.46 | 2357 | £1.50 | £13.92 | 2357 | £1.09 | £11.84 | 2357 | £1.32 | £13.07 | 2357 | £0.92 | £10.87 |
| Participant | ||||||||||||||||||
| Time and travel | 2128 | £92.22 | £50.58 | 2358 | £89.74 | £51.55 | 2358 | £89.40 | £51.61 | 2358 | £89.82 | £51.46 | 2358 | £89.35 | £51.35 | 2358 | £89.02 | £51.25 |
| Patient co-charges | 2209 | £98.88 | £120.69 | 2358 | £100.07 | £122.59 | 2358 | £98.93 | £119.80 | 2358 | £99.32 | £120.35 | 2358 | £98.89 | £121.08 | 2358 | £98.59 | £119.94 |
| Private dental | 1022 | £84.25 | £660.22 | 2357 | £109.04 | £849.88 | 2357 | £63.28 | £499.06 | 2357 | £84.98 | £728.30 | 2357 | £66.04 | £625.52 | 2357 | £66.34 | £531.18 |
| Manual toothbrush | 747 | £20.09 | £9.90 | 2357 | £19.46 | £10.14 | 2357 | £19.76 | £10.07 | 2357 | £19.68 | £10.03 | 2357 | £19.51 | £9.99 | 2357 | £19.34 | £10.03 |
| Electric toothbrush | 526 | £38.61 | £55.08 | 2357 | £37.02 | £53.56 | 2357 | £35.55 | £50.66 | 2357 | £35.34 | £51.21 | 2357 | £36.49 | £54.15 | 2357 | £34.06 | £48.40 |
| Replacement heads | 572 | £15.40 | £11.09 | 2357 | £13.88 | £11.15 | 2357 | £13.86 | £11.25 | 2357 | £13.50 | £10.84 | 2357 | £13.82 | £11.12 | 2357 | £13.85 | £11.17 |
List of abbreviations
- AIC
- Akaike information criterion
- ASC
- alternative specific constant
- BSO
- Business Services Organisation
- CBA
- cost–benefit analysis
- CBAC
- cost–benefit acceptability curve
- CEAC
- cost-effectiveness acceptability curve
- ChaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CPD
- continuing professional development
- CUA
- cost–utility analysis
- DCE
- discrete choice experiment
- DMC
- Data Monitoring Committee
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GDS
- general dental service
- GP
- general practitioner
- HTA
- Health Technology Assessment
- ICDAS
- International Caries Detection and Assessment System
- ICER
- incremental cost-effectiveness ratio
- INB
- incremental net benefit
- INTERVAL
- Investigation of NICE Technologies for Enabling Risk-Variable-Adjusted-Length
- IQuaD
- Improving the Quality of Dentistry
- ISD
- Information Services Division
- MDAS
- Modified Dental Anxiety Scale
- NHSBSA
- NHS Business Services Authority
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OHIP-14
- Oral Health Impact Profile-14
- OHRQoL
- oral health-related quality of life
- PAD
- patient attendance data
- PBC
- perceived behavioural control
- PCRF
- patient case report form
- PI
- periodontal instrumentation
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SDCEP
- Scottish Dental Clinical Effectiveness Programme
- TOD
- Trial Office in Dundee
- TMC
- Trial Management Committee
- TSC
- Trial Steering Committee
- UDA
- unit of dental activity
- UNC15
- University of North Carolina-15
- WTP
- willingness to pay
