Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/57/23. The contractual start date was in November 2012. The draft report began editorial review in May 2018 and was accepted for publication in May 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Pickard et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction: background and objectives
Material from Stephenson et al. 1 Open urethroplasty versus endoscopic urethrotomy – clarifying the management of men with recurrent urethral stricture (the OPEN trial): study protocol for a randomised controlled trial. Trials 2015;16:600; Whybrow et al. 2 Equipoise across the patient population: optimising recruitment to a randomised controlled trial. Trials 2017;18:140; and Whybrow et al. 3 How men manage bulbar urethral stricture by concealing urinary symptoms. Qual Health Res 2015;25:1435–42 have been used within this report under Creative Commons Licenses.
Scientific background
Urethral stricture disease
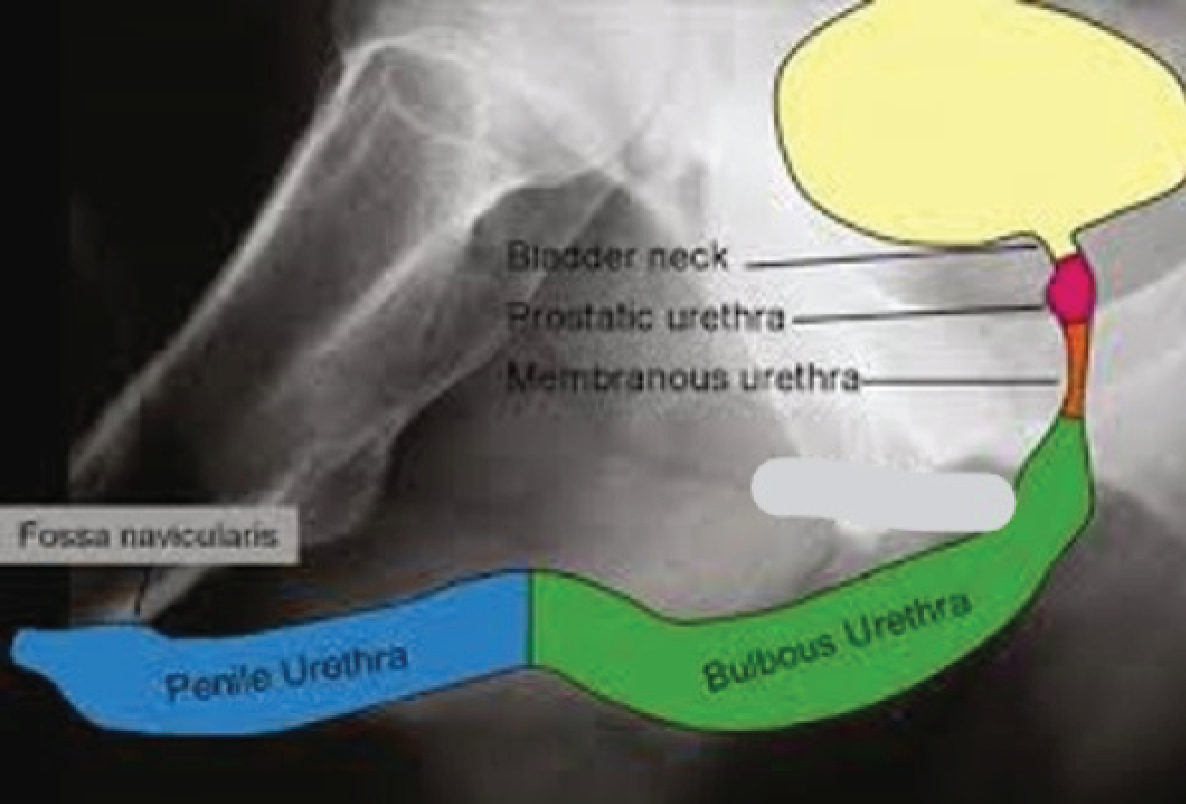
The male urethra is an epithelium-lined tube enveloped by smooth muscle erectile tissue (corpus spongiosum) and an outer layer of striated muscle (bulbospongiosus). It runs from the bladder outlet (neck), through the prostate gland, perineum and the ventral (under) aspect of the penis, opening at the fossa navicularis at the penile tip (external urethral meatus). It is anatomically divided (from proximal to distal) into prostatic, membranous, bulbous and penile segments (Figure 1).
FIGURE 1.
Pictorial representation of male urethra. This figure was published in Surgery, Vol. 35, Watkin and Patel,4 The diagnosis and management of acquired urethral stricture disease, pp. 313–23, © Elsevier 2017. Reproduced with permission.
It functions as an active conduit to void urine and thereby empty the urinary bladder when desired. 5 It is also the conduit for ejaculation and emission of semen, which enters the urethra from ejaculatory ducts running through the prostate.
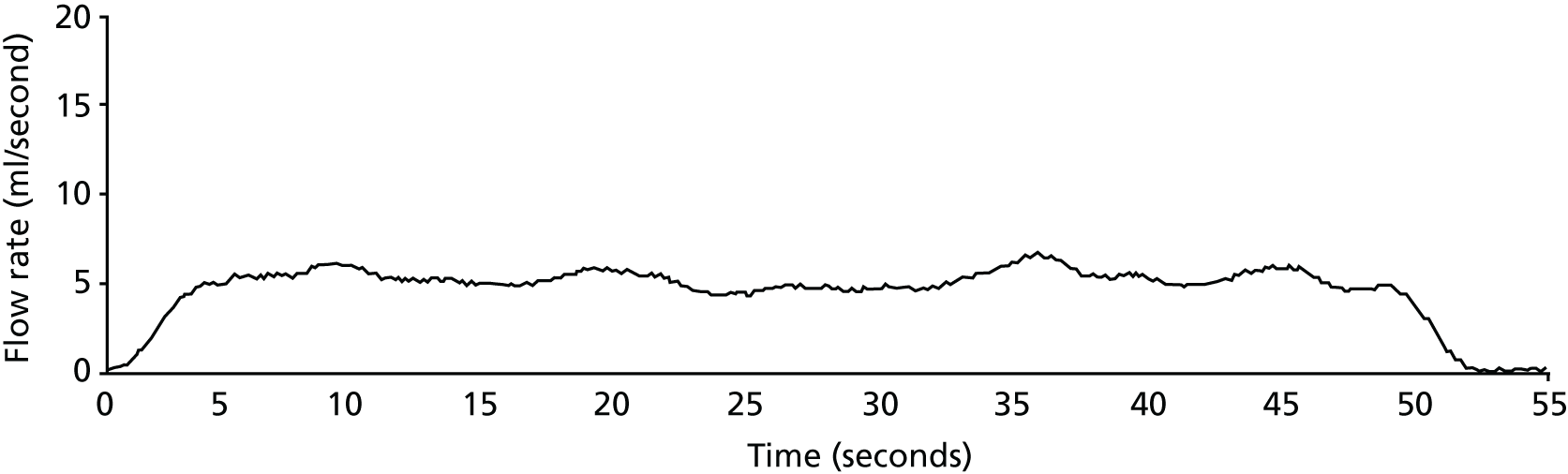
Urethral stricture is caused by annular scar formation in the epithelium and underlying smooth muscle layers, resulting in narrowing of the lumen, loss of propulsive muscle action and restriction of urine flow. Published estimates of the prevalence of urethral stricture disease among adult men derived from hospital activity registries in the USA include 0.6%6 and 0.9%. 7 Urethral stricture disease (OPCS Classification of Interventions and Procedures version 3 – character primary diagnosis code N35) resulted in > 17,000 admissions of men to NHS hospitals in England during 2016–17. 8 Men notice a reduction in the strength of urinary stream, prolonged voiding time and slow emptying of the urethra, often with dribbling of urine after micturition. When these symptoms become intolerable men seek medical help and are generally assessed by a urology specialist. Stricture site and length are evaluated by performing either a telescopic (urethroscopy) or radiological (urethrography) examination of the urethra or both (Figure 2). Severity of urinary obstruction is assessed by measurement of maximum urinary flow rate (Qmax) and residual urine after voiding (Figure 3). Most strictures are located in the bulbar segment of the urethra between the pelvic floor and penoscrotal junction. The cause of stricture formation is most often unknown, but it may result from previous injury or infection (urethritis).
FIGURE 2.
Ascending urethrogram showing 2-cm bulbar urethral stricture. Courtesy of Nick Watkin, St George’s University Hospitals NHS Foundation Trust.
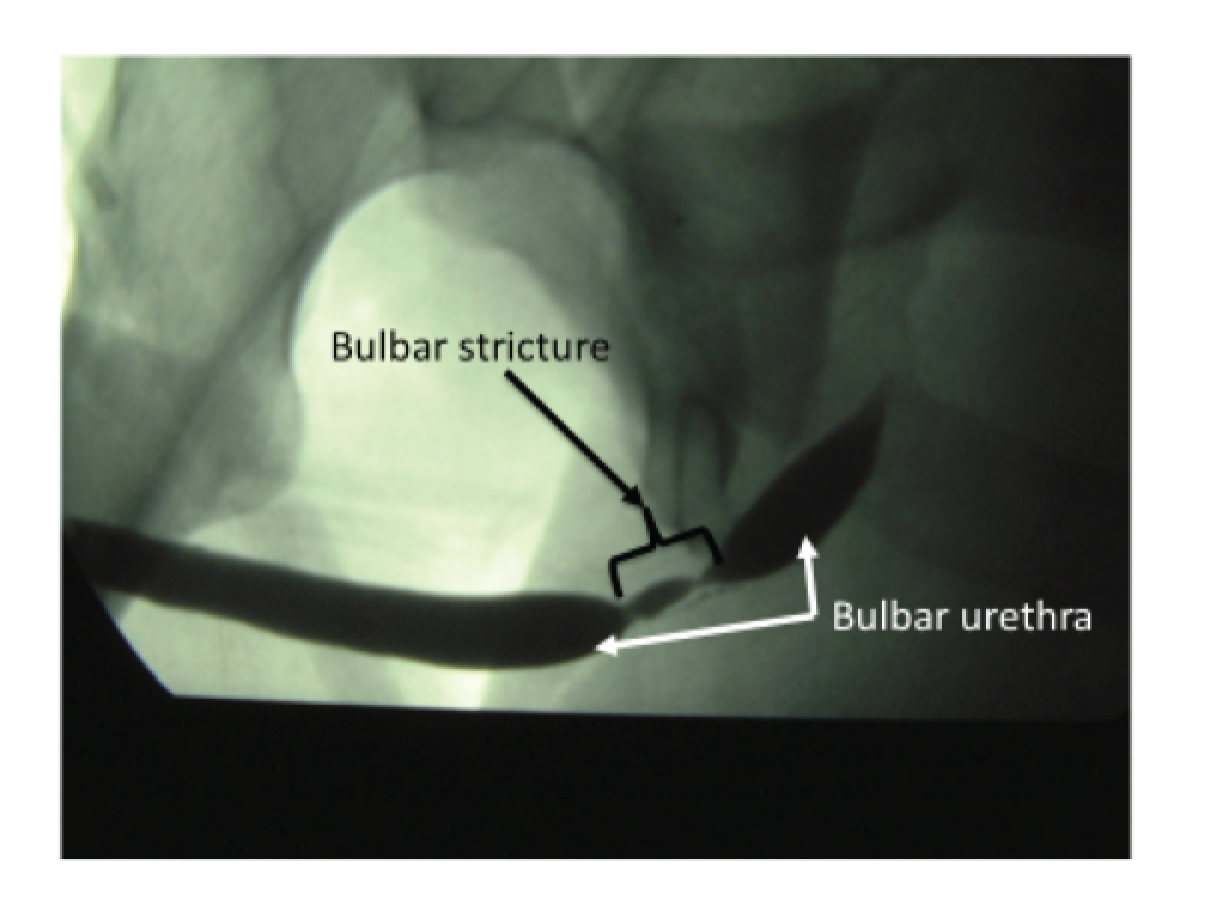
FIGURE 3.
Uroflowmetry record of man with bulbar urethral stricture. The flow is slow up to a maximum of 6 ml/second (normal ≥ 15 ml/second) and prolonged. Courtesy of Alison Bray, Newcastle upon Tyne Hospitals NHS Foundation Trust.
Treatment options for bulbar urethral stricture
Men with bulbar urethral stricture have two management options: (1) endoluminal treatment, in which the stricture is disrupted from within the urethral lumen (urethrotomy or dilatation); or (2) open surgery (urethroplasty), in which the stricture is approached through an incision in the perineal skin and excised or bridged by interposition of a healthy tissue graft. Unless there are complicating factors, men with a first stricture occurrence generally choose to undergo an endoscopic urethrotomy, as it is a straightforward procedure and can be carried out under the same anaesthetic following diagnostic urethroscopy. A review of case series suggested that following this first urethrotomy the rate of recurrence at 2 years was 40% and 60% at 8 years. 9 For those men whose stricture recurs, the choice is between further urethrotomy (or simple dilatation for short flimsy strictures) and open urethroplasty. Urethroplasty is a more complex procedure, but case series suggest a lower stricture recurrence rate of around 15% at 2 years and 20% at 6 years. 10–12 Management discussions between urologists and men with recurrent bulbar stricture centre around the choice between these two strategies: (1) planning and carrying out urethroplasty surgery with a view to longer-term freedom from recurrent stricture, or (2) further urethrotomy repeated as needed at stricture recurrence. The relative efficiency of these two strategies for the management of recurrent bulbar urethral stricture in men is the focus of this trial.
Interventions under study
The experimental intervention strategy is open urethroplasty. 13 The bulbar urethra is approached through a longitudinal incision in the perineal skin behind the undersurface of the scrotum. The bulbar urethra is dissected free and the strictured segment incised in either the dorsal or ventral surface. The area of the stricture is then either removed and the cut ends rejoined (anastomotic urethroplasty) or augmented by insertion of a tissue graft. 14 The tissue graft (typical dimensions 5 × 2 cm) is harvested from the mucosa of the mouth. The urethra is then retubularised and the repaired area protected by passage of a urethral catheter to drain urine. Urethroplasty requires a general anaesthetic, takes 2–3 hours to perform and is followed by a 2-week period of urethral catheterisation postoperatively. Median hospital stay in the UK NHS is 2 days. 8
The control intervention strategy is endoscopic urethrotomy (also known as direct visual internal urethrotomy, internal urethrotomy or optical urethrotomy). 15 This procedure involves passing an endoscope under direct vision down to the distal end of the stricture. 16 The narrowed area is then progressively incised from distal to proximal with a straight longitudinal cut, either using a steel blade (‘cold knife’) mounted on the endoscope17 or under endoscopic control with a diathermy needle or laser fibre (‘hot knife’). Once the lumen has been incised sufficiently to restore a normal calibre (approximately 6 mm diameter) the endoscope is withdrawn and a urethral catheter passed to tamponade bleeding from the cut area and provide reliable urine drainage during the postoperative period. If the stricture is short and flimsy, simple dilatation with the endoscope or graduated dilators may suffice without incising the urethral mucosa and underlying corpus spongiosum. Endoscopic urethrotomy requires a general anaesthetic and takes approximately 45 minutes to perform. The median hospital stay in the UK NHS is 1 day. 8 The urethral catheter is typically removed 24–48 hours postoperatively. Men having repeated urethrotomy may be offered training for a programme of intermittent self-dilatation, using an appropriately sized soft plastic catheter. This adjunctive intervention appears to lessen the risk of recurrence over the subsequent 12 months. 18
Both procedures have a similar spectrum of complications, predominantly bleeding from the wound site and urinary infection. If an oral mucosal graft is used to augment the urethra, then there are specific complications related to the donor site in the mouth.
Current management guidance
Evidence for effectiveness of optical urethrotomy and open urethroplasty for treatment of bulbar urethral stricture has been summarised in four systematic reviews. 13,15,19,20 The Cochrane review,19 up to June 2012, found no randomised trials comparing outcomes after urethrotomy and urethroplasty and could not make any conclusion regarding comparative effectiveness. An updated Cochrane search performed to August 2017 found no more recent relevant completed randomised controlled trials (RCTs) or any other RCTs in progress. Reviews underpinning published guidelines, sponsored jointly by the Société Internationale d’Urologié, the International Consultation on Urologic Disease13,15 and by the American Urological Association,20 included non-randomised studies, predominantly of retrospective cohort design. Guideline formulation subsequent to these reviews was predominantly based on expert opinion and panel consensus. Guidance from the Société Internationale d’Urologié15 suggests that optical urethrotomy is an appropriate management option for initial treatment of bulbar urethral stricture, with an overall long-term success rate of about 50%. The guidance also states that the first recurrence of bulbar stricture could be treated with urethrotomy as long as no adverse factors, such as recurrence at < 3 months post surgery, are present. Success rates in terms of up to 2 years freedom from recurrence in the case series varied from 53% to 95%. Buckley et al. 15 recommended that urethrotomy should not be used for second or subsequent recurrences. Urethroplasty with scar excision and primary mucosal anastomosis or use of an interposition graft of oral mucosa was recommended for complex or recurrent bulbar stricture. 13 The more recent systematic review and guideline from the American Urological Association also did not find any robust evidence on which to base clinical practice recommendations. 20 Wessells et al. 20 suggested that both optical urethrotomy and urethroplasty were appropriate initial procedures for men with bulbar urethral stricture. For those men with recurrent stricture, they considered that only urethroplasty would potentially give long-term symptom control. Further recurrence after repeat urethrotomy was regarded as highly likely and therefore should be considered only as a palliative management option. There is little published qualitative research on patient experience of living with and managing the condition. Patients do appear most concerned regarding the impact of their urinary symptoms, particularly difficulty voiding and post-micturition dribbling, on their daily activities. 21 Agreement between patients and specialist clinicians regarding ranking of importance of particular symptoms is poor. 21
Evidence regarding choice of urethrotomy and urethroplasty
Guidance from two professional urologist organisations, the Société Internationale d’Urologie and the American Urological Association, is clear that open urethroplasty is the recommended option for men with recurrent bulbar stricture. However, this recommendation is based on low-level evidence and expert opinion. Both guideline panels were predominantly made up of urologists specialising in urethroplasty, without general physician or patient representation. Registry studies suggest that optical urethrotomy is more frequently used than urethroplasty for recurrent strictures. 22 Case series from a number of health-care systems also suggest that repeated urethrotomy is preferred in practice for recurrent bulbar strictures,23–26 with urethroplasty reserved for complex strictures. Reasons for the more frequent choice of urethrotomy for treatment of recurrent bulbar stricture may include patient and clinician preference, restricted availability of urethroplasty, health service organisational issues and lower cost to the patient and health-care provider. Information regarding management of men with urethral stricture disease and costs in the UK, derived from NHS England hospital activity and tariff data8 from April 2016 to March 2017, showed a total cost of £17.8M, comprising 742 urethroplasty procedures [OPCS Classification of Interventions and Procedures version 4 (OPCS-4) M73.6], with a mean tariff cost of £4157 (Healthcare Resource Group code LB29A), 5074 endoscopic urethrotomy procedures (OPCS-4 M76.3) and 5838 male urethral dilatation procedures (OPCS-4 M76.4/M79.2), both with a mean tariff cost of £1468 (Healthcare Resource Group code LB55A). A number of studies have made cost comparisons between the two procedures accounting for procedure costs derived from personally funded health-care provision and risk of recurrence derived from cohort studies. A UK-based cost comparison suggested that a strategy of initial urethrotomy followed by urethroplasty at first recurrence was least costly. 27 US decision-analytic models, using cost minimisation28 and cost-effectiveness29 methodology, suggest that initial urethrotomy (providing a success rate of > 40%) followed by urethroplasty on recurrence is the most efficient treatment strategy for men with bulbar urethral stricture, although utility weights were not used and parameter estimates were from non-comparative studies.
Summary with implications for trial design
Decisions for men and their clinicians regarding how to best manage recurrent bulbar stricture continue to be based on low-level evidence with no robust comparative studies of clinical effectiveness or cost-effectiveness. A strategy of urethrotomy repeated as necessary has the attraction of being a straightforward, widely available procedure practised by 95% of urologists. 24 It is characterised by a short hospital stay and rapid recovery. It does not appear to be a curative procedure, meaning that multiple subsequent interventions may be needed during the man’s lifetime. A strategy of open urethroplasty, on the other hand, requires longer operating time and the need for specific expertise, restricting availability. However, it appears to be associated with lower recurrence rate and longer duration of symptom relief. At present, men with recurrent bulbar stricture are guided by clinician experience and preference, together with their own past experience of interventions and their individual values and preferences. Decisions by health-care providers regarding investment in, and provision of, specialist urethroplasty services are also made difficult by this evidence gap.
Aims and objectives
This trial aimed to determine whether or not a strategy of urethroplasty is superior to one of urethrotomy for alleviation of urinary symptoms in men with recurrent bulbar stricture over 24 months and whether or not it is cost-effective for the UK NHS. The trial hypothesis was that the difference in the control of voiding symptoms over 24 months after randomisation was > 10%. To achieve these aims we set the following objectives.
Primary objectives
-
Determine the relative impact on symptoms over 24 months.
-
Determine the incremental cost per quality-adjusted life-year (QALY) over 24 months.
Secondary objectives
-
Determine the relative rate of need for reintervention.
-
Determine the relative change in Qmax at 24 months.
-
Establish the safety profile of each procedure.
-
Model the incremental cost per QALY of the most effective treatment over 10 years.
-
Qualitatively assess the views of men with urethral stricture and their clinicians regarding the disease, the available treatment interventions and participation in the trial.
-
Determine the factors that men trade-off in deciding between the two strategies.
Chapter 2 Methods
This chapter covers trial design and methods, statistical analysis and governance. Details of the methods and findings of the parallel qualitative study and economic evaluation are provided in Chapters 4 and 5, respectively.
Summary of study design
We designed an open-label, patient-randomised, parallel-group superiority trial comparing an experimental strategy of open urethroplasty against a control strategy of endoscopic urethrotomy in men with recurrent bulbar stricture, primarily in terms of symptom control over 24 months. Both strategies are in routine use in the UK NHS. For urethroplasty, there is a need for specialist referral for further assessment and counselling regarding harvesting of an oral mucosal graft. Because of this complex and variable care pathway, we chose a pragmatic design without blinding of clinicians or participants. Clinical trials unit staff entering and managing trial data were blinded to participant allocation. Men in both groups received standard perioperative care, including consent for the surgical intervention that they were allocated to and/or underwent. Inclusion criteria were made as broad as possible within the constraint of subjects having to be able and willing to undergo either procedure and agreeing to random allocation. The trial was set in the UK – England, Wales and Scotland – recruiting participants from NHS hospitals providing operative urological procedures for men with urethral stricture.
Sites
From 18 February 2013 to 5 March 2015 we progressively established 53 research sites (38 recruited at least one participant), all being NHS secondary care providers affiliated to the National Institute for Health Research (NIHR) Clinical Research Network in England and equivalent organisations in Scotland and Wales, which agreed to host the study locally. We initially concentrated on sites that could undertake both urethrotomy and urethroplasty. Subsequently, we set up sites that offered only urethrotomy. Those sites required delineation of routine referral pathways to a previously established trial site that offered urethroplasty (Table 1).
| Study intervention and outcome data collection component | Visit 1: initial screen | Visit 2 | Visit 3: intervention (according to site processes) | Visit 4: 3-month clinical follow-upa | Postal: 6 monthsa | Postal: 9 monthsa | Remote: 12 months follow-upa | Postal: 18 monthsa | Visit 5: 24-month follow-upa | Postal: 24 months after surgery | End of study (November 2017) | Pre and post reintervention | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consent | Baseline | Randomisation | ||||||||||||
| Eligibility checklist | ✓ | |||||||||||||
| Trial discussed and patient information sheet provided | ✓ | |||||||||||||
| Informed consent | ✓ | |||||||||||||
| USS-PROMa | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Resource use questionnaire | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Further intervention questionnaire | ✓ | ✓ | ||||||||||||
| Uroflowmetry | ✓ | ✓ | ✓ | |||||||||||
| Randomisation | ✓ | |||||||||||||
| Process of care | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| AEs | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
Additionally, four participant identification centres were opened between August and September 2014 to support recruitment to the trial.
Trial management
The central trial office was established at the Newcastle Clinical Trials Unit (NCTU), Newcastle University, Newcastle upon Tyne, UK. The NCTU was responsible for obtaining approvals, trial registration, trial management and organising the collection of outcome measures. The health economic evaluation and qualitative research teams were also based in Newcastle. The randomisation service, database construction and management, and statistical analysis were based in the Centre for Healthcare Randomised Trials (CHaRT) at the University of Aberdeen, Aberdeen, UK.
Participants
Adult men with a history of urethral stricture disease having previously undergone at least one intervention for bulbar urethral stricture were identified at the time of clinic presentation with recurrent symptoms and from health-care records at each site. Clinicians were encouraged, through the NIHR Clinical Research Network, local and national meetings, and relevant professional organisations, including the British Association of Urological Surgeons (BAUS) and the British Association of Genito-urethral Surgeons, to introduce the study to men under their care. Patients were approached and introduced to the study by clinical staff at site. If men were interested, an eligibility check was carried out by local research staff at each site according to the following criteria:
Inclusion criteria
-
Men aged ≥ 16 years.
-
Stricture located predominantly in the bulbar urethra.
-
Undergone at least one previous intervention for bulbar urethral stricture.
-
Clinician and patient agreement that intervention was required.
-
Suitable for general or regional anaesthesia of up to 3 hours’ duration.
-
Willingness to have a catheterisation period of up to 2 weeks.
Exclusion criteria
-
Current perineal sepsis and/or urethrocutaneous fistula.
-
No previous intervention for bulbar stricture.
-
Inability to adhere to the trial protocol.
-
Previous participation in the study.
Consent procedures
Men who were eligible and in provisional agreement for participation were seen by local research staff and given trial information. Trial eligibility was checked for each potential subject using information from the prospective participant and from his clinical record. Sites kept a screening log documenting non-identifiable information and reasons for non-participation. The right to refuse to participate without giving reasons was respected.
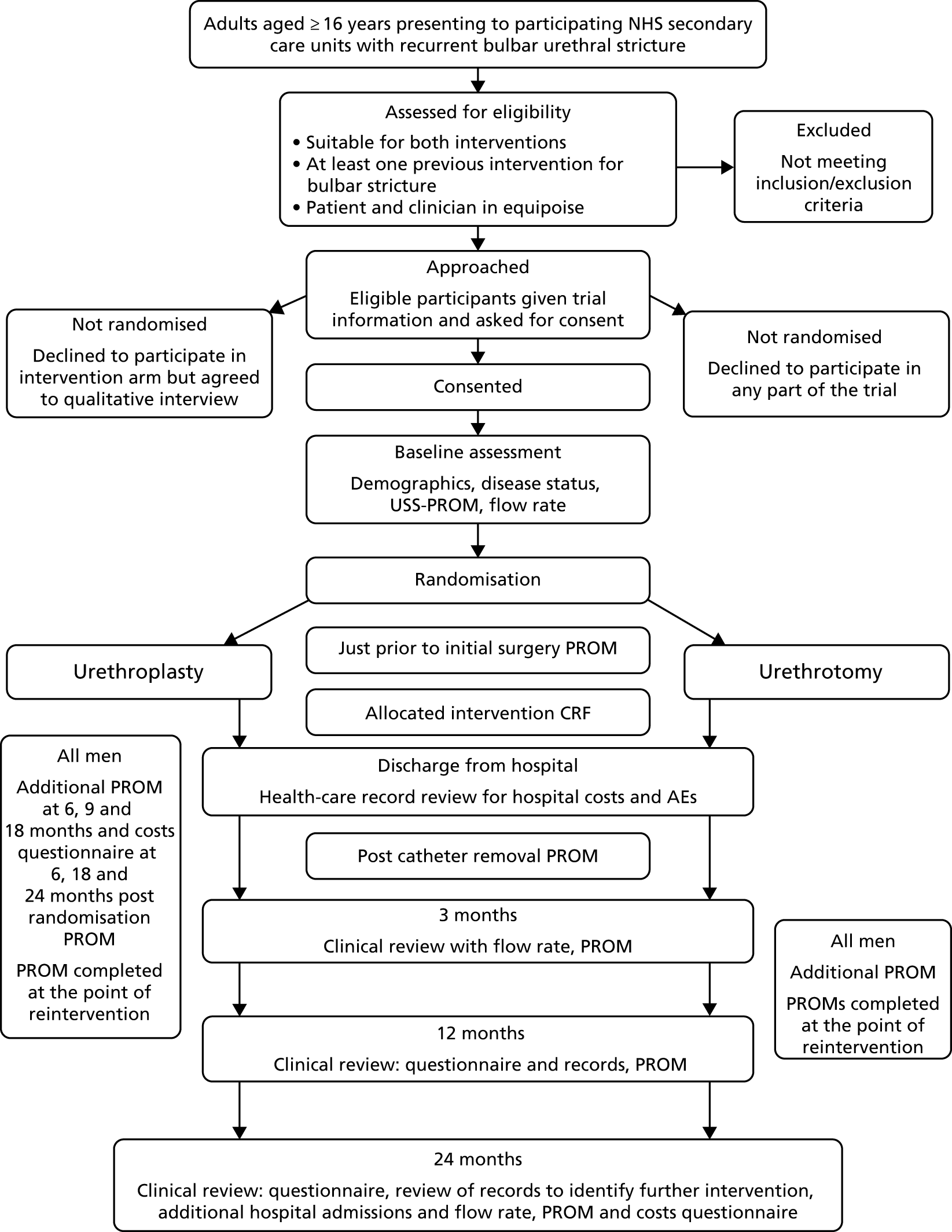
Following a period of at least 48 hours post provision of trial information, interested and eligible patients were then contacted using their preferred means of communication (surface mail, e-mail, telephone or text) and reviewed by local research staff to explain fully the purpose and conduct of the study, including the need for randomisation to allocate them to either procedure. If they agreed to take part they gave written consent to be randomised to either open urethroplasty or endoscopic urethrotomy by signing and dating the study consent form, which was witnessed and dated by a member of the research team with documented, delegated responsibility to do so. The timing of randomisation was usually at the time of trial consent, provided that there was agreement between patient and clinician that intervention for recurrent bulbar stricture was required. Standard local arrangements concerning preoperative assessment, hospital admission, consent for surgery, conduct of surgery and after care continued unaffected by study participation. Men eligible for the study who were not willing to consider randomisation were asked to consent to being approached by a qualitative researcher, regarding participation in an interview-based study. Urologists participating in trial recruitment were also asked to give expressions of interest in participating in this qualitative study. The recruitment timetable is shown in Figure 4.
FIGURE 4.
Chart showing flow of participants through the trial. AE, adverse event; CRF, case report form; PROM, patient-reported outcome measure; USS-PROM, urethral stricture surgery – patient-reported outcome measure.
Randomisation
Participant allocation
Consented men were allocated to urethroplasty or urethrotomy using a centralised, automated, randomisation application hosted by CHaRT and accessed by telephone or through the internet. The algorithm allocated participants to urethroplasty or urethrotomy in a 1 : 1 ratio, with recruitment site and time since last procedure (< 12 months or ≥ 12 months) as minimisation covariates. The final allocation algorithm was set by a statistician not involved in trial planning or analysis and included a random component. Participants were informed of their allocated treatment group immediately following randomisation.
Progress on study
The schedule of events for trial participants is shown in Table 1. Baseline data were collected by site research staff just prior to randomisation to minimise any biases that might result from knowledge of the allocated intervention. We aimed for participants to undergo their allocated procedure as soon as possible and preferably no longer than 12 weeks after randomisation, subject to participant and clinician preference and health-care provider service constraints. At 3, 12 and 24 months post intervention, research staff at site contacted participants for a follow-up to complete case report forms (CRFs), face to face or by telephone, with supplementation by health-care record review.
Participant expenses
Expenses incurred by participants as a result of extra attendances outside standard local NHS care were reimbursed. Participants were given £25 thank-you gift vouchers to cover any unforeseen additional costs at the time of randomisation, 24 months after randomisation and on receipt of a completed end-of-trial questionnaire in December 2017.
Withdrawal
Consented participants remained on study unless they withdrew their consent or trial staff deemed that further participation by an individual was not appropriate. Participants who declined their allocated procedure after randomisation were kept on study, including those men who underwent the alternative procedure and those men who did not undergo an intervention. The reason for withdrawal was recorded if the participant agreed.
Patient and public involvement
Prior to funding application, we discussed the rationale of the trial with a patient with urethral stricture who had experienced both urethrotomy and urethroplasty. He agreed to be a co-investigator and reviewed the funding application from a patient perspective. Subsequently, he reviewed and commented on trial documents relevant to patients prior to submission for ethics approval. He contributed to discussions at trial management meetings, particularly at the start of recruitment and at the end of the study. As part of governance of the trial, we recruited a member of the public through the Northern Regional Public Involvement in Medical Research Network to act as a lay member of the Trial Steering Committee (TSC).He attended and contributed to TSC meetings throughout the trial, offering very helpful lay input into trial oversight and management. Both individuals critically appraised the final report from a lay perspective.
Outcome measurement
Participant-reported outcomes for the OPEN trial were collected through a specific trial questionnaire completed by participants. The questionnaire, urethral stricture surgery – patient-reported outcome measure (USS-PROM), has been validated in this patient group in English,30 German,31 Turkish,32 Russian,33 Spanish34 and Portuguese. 35 It comprised six questions on voiding symptoms and their impact on daily activities, self-rating of urine flow strength (using a pictorial guide) and the EuroQol-5 Dimensions, five-level version (EQ-5D-5L), questionnaire measuring health status. For the purposes of this trial, a single-item question, overall satisfaction with sexual function, was added from the validated International Index of Erectile Function questionnaire. 36 Participant completion of trial questionnaires at baseline, prior to allocated intervention and 1 week after catheter removal following intervention were prompted and overseen by local research staff at site. Subsequently, participants were automatically sent a letter enclosing a questionnaire and instruction for completion at 3, 6, 9, 12 and 24 months following the date of allocated intervention and at 18 and 24 months after the date of randomisation. A final questionnaire was sent out to all randomised participants for completion at the end of the study in November 2017. Participants also had the option to complete the questionnaires online with automatic upload to the trial database, or by telephone call with trial management staff. Need for completion was prompted by letter from the trial office at NCTU. We scheduled additional collections of outcome measures just prior to and at 1 month subsequent to any further surgical procedure for bulbar urethral stricture.
Primary effectiveness outcome
The primary outcome for the OPEN trial was the area under the curve (AUC) of the urinary voiding symptom score component of the USS-PROM, repeatedly measured over 24 months following randomisation. The voiding symptom measure comprised six questions each scored from 0 (symptom not present) to 4 (symptom present all of the time), giving a range of total score of 0–24. The completed questionnaires were returned to the trial office at NCTU for data entry.
Secondary effectiveness outcomes
Patient reported
The USS-PROM questionnaire also included measurements assessing the outcomes of urine stream strength, impact of urinary symptoms on daily activity and overall health status. The single-item visual scale of urinary stream strength was scored from 1 (strong stream) to 4 (weak stream). The question regarding impact of urinary symptoms on daily activities was scored from 0 (not at all) to 3 (a lot). The USS-PROM questionnaire measured health status using the EQ-5D-5L questionnaire. 37 Each domain was scored 0 (no problem) to 4 (extreme or incapacitating problem), giving a total ranging from 0 to 20. This measure was accompanied by the EQ-5D visual analogue scale (VAS), on which participants rated their overall health between 0 (the worst health imaginable) and 100 (the best health imaginable). An additional single-item question, overall satisfaction with sexual function, was scored from 1 (very dissatisfied) to 5 (very satisfied). 36 All measures included in the USS-PROM were self-completed by participants at the same multiple time points as the primary outcome.
Clinical
Maximum urinary flow rate was measured for each participant by asking them to void at least 150 ml of urine into a commercial, calibrated uroflowmeter available at site. This measurement was scheduled to take place at baseline, at 3 months post intervention, and between the 12 and 24 months’ post-intervention visits. An increase in Qmax of ≥ 10 ml/second compared with baseline was categorised as a successful outcome. 38 If participants underwent a further intervention for bulbar urethral stricture subsequent to the allocated trial procedure (excluding self-dilatation), this was documented at the 3-, 12- or 24-month follow-up, completed by research staff at site. Additionally, site staff were asked to complete a reintervention CRF. Participants were asked to provide information regarding any further interventions for recurrent bulbar stricture in the further intervention questionnaire sent to all participants at 24 months post surgery and at the end of the study (November 2017). Recurrence of bulbar stricture but without a planned or completed further intervention was recorded in the 24-month follow-up CRF, using information from symptom report, urine flow rate and documentation in the health-care record. We considered that a stricture was likely to have recurred if at least one of the following conditions were met during the 24 months after intervention: a reintervention had occurred or was scheduled, the Qmax had deteriorated to the preintervention value or the voiding score had deteriorated to baseline value.
Harms
Harms arising from trial participation, principally related to the procedure undergone, were collected through CRFs completed by research staff at site at the time of, and shortly after, the trial intervention, at 3, 12 and 24 months after intervention, and at the time of, and shortly after, reintervention. They were categorised as being expected adverse events (AEs), as listed in the trial protocol, or unexpected AEs. The trial office were notified of any AE deemed serious [i.e. a serious adverse event (SAE)] via an e-mail alert originating from the CHaRT database, triggered by data entry at site. The SAEs were then adjudicated by the chief investigator in discussion with other members of the trial team and, when required, by the site principal investigator (PI), to decide if the SAE was indeed serious and whether it was related or unrelated to participation in the OPEN trial.
Primary cost-effectiveness outcome
The primary outcome of the health economic evaluation was cost–utility, assessed as the incremental cost per QALY at 24 months after randomisation. Health-care costs were calculated from hospital visits recorded by research staff at site on CRFs completed at 3 and 24 months, and from use of primary care services as reported by participants on a patient’s cost questionnaire completed at 6 and 12 months after allocated intervention and at 18 and 24 months after randomisation. Patient costs were collected through a questionnaire completed at 6 months after allocated intervention. Responses to the EQ-5D-5L health status questionnaire were transformed using UK population tariffs39 to produce a health state utility score for each participant.
Secondary cost-effectiveness outcome
To complement the primary cost–utility analysis, the health economic evaluation included a time trade-off (TTO) experiment to better understand choices made by men with urethral stricture and assess the likely short-term disutility of the procedures that would not be measured at the EQ-5D-5L time points, and also a Markov model to project observed differences in outcome and their impact on cost-effectiveness over a 10-year time horizon. The health economic evaluation carried out as part of the OPEN trial is fully described in Chapter 5.
Data collection
Summary
Data from trial CRFs were entered by research staff at site through a password-protected portal onto the internet-based data management system developed, set up, hosted and maintained by CHaRT. Participant-completed questionnaires from catheter removal post intervention onwards were collated at the central trial office and entered into the trial database by NCTU staff, whereas patient-reported outcomes at baseline and just prior to intervention were entered at site by research staff. Staff in the NCTU trial office in Newcastle worked closely with the local research teams at site and the trial team at CHaRT in Aberdeen to ensure that data were complete and accurate. We made concerted efforts to chase and complete any missing data entries through contact with the sites. Participants’ details were stored securely in the CHaRT study database under the guidelines of the Data Protection Act 1998. 40 Participants were allocated an individual specific trial number and all data, other than personal data, were identified only by this unique study number. Data collected during the course of the research were kept strictly confidential and accessed only by members of the trial team.
Trial events
The schedule of events for the OPEN trial is shown in Table 1.
Screening
After identification by clinical staff, men potentially eligible for the trial and who were willing to consider participating were introduced to research staff at site. General demographics and eligibility were checked and anonymised data entered on a screening log. Trial Information was provided and potential participants were given at least 48 hours to consider participation in the study. Following review, at a mutually convenient time, those men who wanted to take part, who fulfilled the entry criteria and who understood the rationale and conduct of the trial provided written consent witnessed by research staff at site with delegated approval to do so. The participant and local research staff then completed baseline data collection.
Randomisation
Randomisation was performed as close as possible to the date of consent (normally immediately thereafter) and the participant and clinical staff responsible for their care informed. For participants allocated to urethroplasty, recruited at urethrotomy-only sites, onward referral to the appropriate specialist centre was organised by the responsible clinician and the trial office informed. Details of baseline assessment and allocated intervention were entered on the web-based data management system baseline and randomisation CRF.
Intervention
Research staff at site liaised with the clinical team caring for the individual participant regarding arrangements for undergoing the allocated intervention (urethroplasty or urethrotomy). The protocol anticipated a maximum 12-week delay between randomisation and surgery. A more extended time awaiting allocated intervention was nonetheless allowed. This resulted from uncertainty regarding assessment required prior to anaesthesia and surgery, clinical prioritisation, need for onward referral for some men allocated to urethroplasty and time waiting for surgery. Just prior to intervention, participants were asked to again complete the trial questionnaire. Research staff at site recorded details of the intervention given, together with any AEs during surgery, the subsequent postoperative hospital stay and during the period of postoperative catheterisation in the allocated intervention CRF. Participants completed a further trial questionnaire 1 week after catheter removal, which was generally performed in hospital.
Follow-up
When possible, the 3-monthly trial follow-up visits carried out by research staff at site coincided with routine or extra clinical visits. If appropriate, the trial participant reviews could be completed by telephone in conjunction with the review of health-care records. Local research staff recorded any deviations in standard care, such as reinterventions for bulbar urethral stricture, AEs, unscheduled outpatient visits and any hospital stays. Owing to slower than planned recruitment, longer than anticipated waiting times for surgery and the need to complete the trial within the funded period, a final review of all participants was arranged at the end of the study during November and December 2017, 24 months after the date of the last randomisation. This consisted of participant completion of a trial questionnaire and a further intervention questionnaire. Uroflowmetry was carried out at baseline and at 3 and 24 months after surgery.
Data handling and record keeping
Data were recorded by site staff on electronic CRFs in a bespoke electronic database within a software package designed and maintained by CHaRT in Aberdeen. In certain cases, data were initially recorded on paper CRFs prior to transfer to the study database. The database was accessed by research staff at site through a password-protected portal unique to that site. Participant questionnaires returned by post to the trial office in NCTU were entered by NCTU staff who remained blind to participant allocation. Participants had the option of completing the questionnaire online with immediate electronic transfer to the database. Participants who chose this option at baseline were sent the web address to the participant area and a unique login identifier for that participant to gain access to the area. Once logged in, the participant would then be able to complete the questionnaire for the relevant time point. Participant data collected under a unique identifier were kept confidential and accessed only by members of the trial team. Extensive efforts were made to ensure completion and collection of trial questionnaires at each time point. Regular checks were carried out and missing data pursued with research and clinical staff at site and with participants through their preferred means of communication. Up to three reminders to complete questionnaires were sent at each scheduled time point. Essential data will be retained for a period of at least 10 years following close of study, in line with sponsor policy and the latest European Directive on Good Clinical Practice (GCP) (2005/28/EC). Data were handled, digitalised and stored in accordance with the Data Protection Act 1998. 40
Details of study interventions
General
Study design was pragmatic, in that, apart from randomised allocation of intervention and outcome data collection, standard care pathways for each procedure at individual sites were followed. These included type of anaesthesia, use and regimen of antibiotic prophylaxis, surgical instrumentation, closure or non-closure of oral mucosal graft donor site, duration of postoperative urethral catheterisation and clinical follow-up schedule, including use of investigations to detect stricture recurrence. The interventions were funded by the NHS in accordance with local contracting mechanisms. The NHS excess treatment costs were approved by the sponsor (Newcastle upon Tyne Hospitals NHS Foundation Trust, Freeman Hospital, Newcastle upon Tyne, UK) and the host NHS organisation at each participating site.
Urethroplasty (experimental)
For urethroplasty, men were positioned supine on the operating table with hips and knees held in an abducted and flexed position by suitable leg supports. A longitudinal skin incision was made in the perineum above the anus and towards the base of the scrotum. The bulbar urethra was localised and mobilised from its attachments. The stricture segment was incised longitudinally on the dorsal or ventral surface according to surgeon preference. The surgeon then decided if the stricture could be excised with or without transection of the corpus spongiosum and a primary anastomosis made (typically, proximal strictures < 2 cm in length with limited fibrosis) or if an oral mucosal graft should be placed without stricture excision in an augmented repair (typically, fibrotic strictures ≥ 2 cm in length). The oral mucosal graft was harvested from the inner cheek, defatted and sutured to the cut urethral edges, then stabilised against the corpora cavernosa if dorsally placed or the corpus spongiosum if ventrally placed. A 16-French silicone Foley urethral catheter was then placed and left in situ for free drainage of urine for a postoperative period of approximately 2 weeks. Once recovered, the patient was discharged home to return at a planned later date for a urethrogram to check that there was no leakage from the area of repair, catheter removal and trial of voiding. The median stay from NHS England hospital activity data at the time of the trial was 2 days. 8 Clinical follow-up was usually by wound and symptom review at 3 months and urinary flow rate measured at 3 and 24 months post intervention.
Endoscopic urethrotomy (control)
For urethrotomy, men were positioned supine on the operating table with legs supported in an abducted and flexed position. The endoscope (Sachse urethrotome) was passed retrogradely through the urethral lumen under direct vision until the distal end of the stricture segment was encountered. A fine-calibre guidewire was placed through the stricture into the bladder to aid incision planning. The stricture was progressively incised longitudinally under vision using the steel blade (‘cold knife’) mounted on the endoscope until healthy mucosa signalling the proximal end of the stricture was reached. Alternatively, a diathermy needle mounted on the endoscope or a laser fibre passed through the endoscope was used to make the incision (‘hot knife’). For short flimsy strictures, dilatation with the endoscope or graduated dilators could be used rather than formal urethrotomy. A 16-French silicone Foley catheter was then placed for free drainage of urine during the postoperative period. It was typically removed at 24–48 hours postoperatively, with a trial of voiding prior to discharge or following hospital reattendance after discharge. Follow-up was by outpatient review and urinary flow measurement 3 and 24 months postoperatively. According to patient and clinician decision, a standardised programme of intermittent self-dilatation could be initiated 1 week after catheter removal, as this can delay time to recurrence. 18
Delivery of interventions
All procedures were carried out by accredited consultant urologists or senior trainees in urology. Competency in performing endoscopic urethrotomy is a mandatory component of training as urologists and is regularly performed by approximately 95% of practising urologists. 24 Urethroplasty is a specialised technique requiring extra training. Specialist surgeons with recognised expertise working in specific UK centres were identified through BAUS and acted as PIs for the trial at these sites. The precise technique of urethroplasty used for each participant was decided by the operating surgeon. Details of each intervention were recorded by research staff at site on a CRF.
Changes to study design
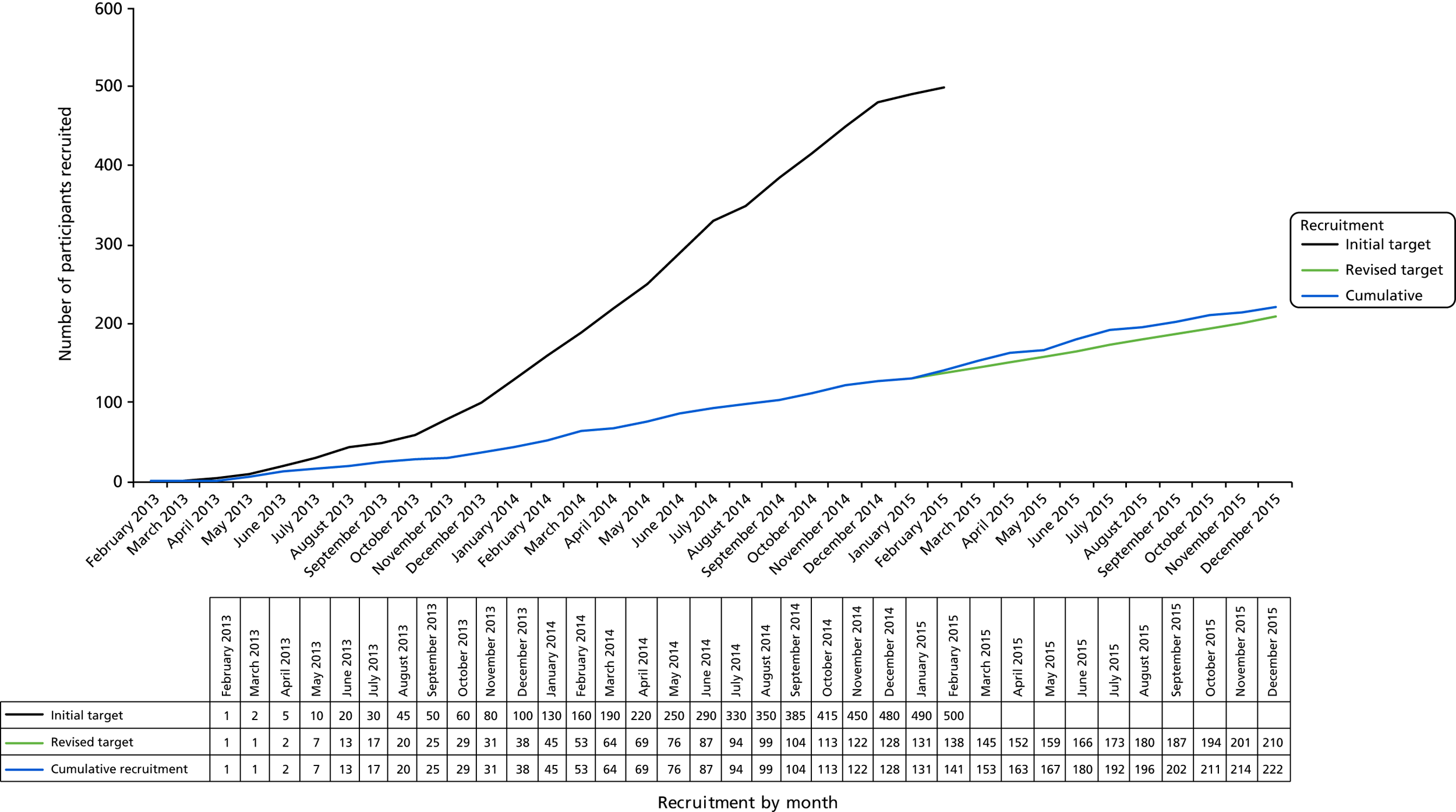
Changes made to the protocol during the trial are listed in Table 2. Owing to slow recruitment, the trial protocol was adjusted over the period October 2014 to February 2015. The sample size required to detect a 10% difference in voiding symptom score was recalculated using primary outcome data collected up to September 2014, without unblinding of allocated groups. This gave a revised target recruitment of 210 men. To take into account the prolonged recruitment period and differing waiting times for surgery, we reprofiled timing of collection of the primary outcome, anchoring the timing of participant completion of trial questionnaire completed during the last 6 months of the study to the date of randomisation, rather than the date of allocated intervention. In line with these changes, the end of recruitment period was put back to December 2015 and the end of study to December 2017.
| Description | Protocol version | Date |
|---|---|---|
|
Justification of sample size for the secondary outcome of need for further intervention in case of poor recruitment (104 rather than 500 men) Inclusion of two extra trial questionnaire completions (just prior to the procedure and at 1 week after catheter removal) Addition of validated single-item global sexual function question to the PROM |
1.1 | 15 November 2012 |
|
Protocol contacts page updated Emergency contact details updated Removal of SF-12 and clarification of the time points that the resource use questionnaires will be completed Clarification of what participant data will be stored on the trial database AE reporting had been written according to the regulations for CTIMP studies. As trial is not a CTIMP, amended with guidance from the National Research Ethics Service website |
1.2 | 15 February 2013 |
|
Amended protocol appendix (with no changes to the main protocol) to include the second qualitative phase, a TTO evaluation Addition of site monitoring centrally when possible Amended protocol and consent form to ask site to fax a copy of the consent form to the central trial office |
1.3 | 12 January 2014 |
|
Changes to members of the health economics team Change of trial manager Typographical changes |
1.4 | 20 February 2014 |
|
Addition of online advertisement URL: www.trialreach.com, which feeds information to URL: www.patient.co.uk. Basic trial information to be advertised online Addition of video demonstrating a model trial consultation on the OPEN trial electronic CRF website Clarification in the protocol and patient information sheet that there is a limit to the amount of patient travel expenses that can be claimed: £25 per visit, when the visit occurs outside routine care Length of archiving altered to 10 years in line with the clinical trial agreement and sponsor practice Alteration of the number of recruiting sites and locations within the UK |
1.5 | 16 July 2014 |
| Change to end of recruitment date from 31 October 2014 to 27 February 2015 to reflect the delayed opening of the study | 1.6 | 22 October 2014 |
|
Change to the length of recruitment period. Recruitment to close 31 December 2015. End of follow-up therefore December 2017 Reduction in the sample size required from 500 to 210 Change of the timing of 18- and 24-month post-surgery questionnaires to be 18 and 24 months after randomisation to mitigate bias from different waiting times for surgery Addition of two secondary objectives: (1) looking at the symptom control and quality of life over the study period and (2) need for reintervention over the study period (median time from intervention) Participant follow-up by questionnaires will be increased with two additional time points: 24 months after surgery and at the end of study (December 2017) Introduction of a reintervention questionnaire for participants to complete at 24 months post surgery and at the end of study (December 2017) Addition of a third ‘thank you’ voucher at trial end Update to the TTO substudy protocol (see Appendix 1) Change to trial manager contact details Typographical changes |
1.7 | 17 February 2015 |
|
Clarification of study procedures and analysis for participants who did not receive their randomised allocated intervention Update to SAE reporting procedure (introduction of paper SAE form) Administrative changes |
1.8 | 3 October 2016 |
Sample size calculation
We aimed to detect at least a 0.1 (10%) difference in the AUC of the voiding symptom score calculated from the plotting of repeated measurements over 24 months for both trial groups and using a 0–1 utility scale. This conservatively assumed a standard deviation (SD) score of < 0.33 based on the finding of a SD of 0.15 in a previous shorter-term study among men undergoing bulbar urethroplasty. 30 For 90% power this would require 500 men to be randomised. Following the trial feasibility phase, it was clear that this sample size was not achievable in a fundable time frame. We therefore recalculated the SD of the symptom score in August 2014, using trial data collected from the first 69 men randomised who had completed at least one score following intervention (220 measurements in total), while maintaining blinding of allocated intervention. This recalculation gave a SD of 0.165, which reduced to 0.15 when adjusted for baseline score and trial site. Using these data we updated our sample size estimate assuming a reduced SD of ≤ 0.21. This indicated a requirement of 170 men to be randomised to have 90% power for detection of a 10% difference at a two-sided 5% level. We inflated the figure to 210 to allow an up to 19% loss to follow-up rate. When interpreting actual trial data we found results more straightforward to consider by scaling the AUC to the USS-PROM scale minimum and maximum over 24 months, in which a score of 0 indicated a complete lack of symptoms over the trial and a score of 24 indicated full symptoms throughout the trial. Rescaling the AUC did not change the assumptions of the sample calculation.
Statistical analysis
Primary outcome
The primary analysis was based on the intention-to-treat (ITT) principle, with participant groups compared according to randomly allocated intervention using available data in a complete-case ‘modified’ ITT analysis. Details of the planned analyses were documented prior to the end of the trial in a statistical analysis plan (SAP) [see www.journalslibrary.nihr.ac.uk/programmes/hta/105723# (accessed 26 June 2019)].
Additional planned analyses using a per-protocol definition and inclusion of more men enabled by data imputation in a further ITT sensitivity analysis were detailed in an additional SAP [see URL: www.journalslibrary.nihr.ac.uk/programmes/hta/105723#/ (accessed 26 June 2019)].
Baseline and follow-up data were summarised using mean (SD) or median [interquartile range (IQR)] when appropriate for continuous variables. Discrete variables were summarised with numbers and percentages. Treatment effects were presented with 95% confidence intervals (CIs).
The primary outcome measure, AUC for the repeated measurement of voiding symptom score contained in the USS-PROM trial questionnaire over 24 months following randomisation, was analysed using linear regression adjusted for the minimisation covariates of site and stricture severity, defined as the time duration between the last previous intervention for bulbar stricture and the date of randomisation (< 12 months or ≥ 12 months). Voiding score was measured at baseline; prior to intervention; 1 week after catheter removal; 3, 6, 9 and 12 months after intervention; and 18 and 24 months after randomisation. Additionally, participants were asked to complete the trial questionnaire 24 months after intervention and at the end of study (November 2017). Trial questionnaires were also completed before and after any reintervention for stricture recurrence. The AUC was constructed using the trapezoidal rule, which assumes a constant increment (or decrement) in score between two points when outcome is measured. The AUC was divided by total duration of the trial for each participant up to 24 months post randomisation to facilitate interpretation. The original sample size calculation introduced the AUC in its usual format, 0–1 utility; however, in our results we decided to multiply the scale by 24 in order to present it in the same scale as the voiding score (0–24) and therefore facilitate interpretation.
For the primary analysis, all participants who had surgery and completed at least three voiding scores, comprising one baseline measure prior to trial intervention, one early measure up to 12 months after intervention and one later measure up to 24 months post randomisation, were included. Participants who did not have a late measure but had returned an end of study or a 24 months post intervention measure were included if this measure was collected within 6 months after their 24 months post randomisation time point. For the two participants without a baseline or preintervention measure, we used imputation based on the mean score observed at that site.
Sensitivity analyses detailed in the additional SAP were conducted to assess the robustness of the primary treatment effect estimate. This was done by relaxing and tightening the minimum number of measures needed to be included in the analysis. When available, all observed data were used across all time points. However, for many participants, data were missing at various time points. The assumptions of the data inclusion for the primary analysis and proposed sensitivity strategies are outlined below for each group of measurement time points.
Baseline
If either of the baseline and prior to surgery measurements were missing but the other was available, we assumed a constant score between these two time points and imputed one with the other. If both were missing, we imputed the site mean for each time point to allow calculation of the AUC for the primary analysis.
Early
If the 1 week after catheter removal measure was missing we did not impute a value; in these circumstances the AUC calculation was made between the baseline and first available early measure. As the 1 week after catheter removal measure could occur at any real time point throughout the trial, we used real time (in months) to incorporate this into the relevant time section of the AUC. Only one of the 3-, 6-, 9- and 12-month post-surgery measures was required. The AUC calculation used the notional time, in weeks, between the last available time point prior to 3 months and the first of these time points. If one or two time points were missing, we did not impute a value for those missing time points, but assumed constant increment (or decrement) in score between those points where outcomes were measured.
Late
Only one of the 18- and 24-month post-randomisation time points was required to calculate the AUC. If the 18-month time point was missing but the 24-month time point was measured, we did not impute a value for 18 months. Rather, the AUC calculation used the notional time (in months) between the last available measurement prior to 18 months and assumed constant increment (or decrement) in score between these two time points. If the 18-month time point was measured but the 24-month time point was missing, we carried the 18-month measurement forward to 24 months to allow calculation of the AUC. If there was a 24-month post-intervention or end of study measurement closer in time to the 24-month post-randomisation measure than the 18-month time point, we used that rather than the 18-month measure.
Accounting for occurrence of reintervention
For participants who underwent reintervention and submitted outcome measures prior to or following this intervention, we incorporated these into the relevant time section of the AUC by inserting the extra observations between notional time point measures. When reintervention clashed with a scheduled outcome measurement, the reintervention reported measurements were used. If a reintervention took place but the associated outcome measures were missing, we used index intervention outcome data for that participant. We assessed the appropriateness of this assumption empirically with observed data.
Secondary outcomes
The following secondary outcomes were recorded:
-
difference in condition-specific quality-of-life trajectory, measured from 0 to 3 by the AUC for the single item on the USS-PROM
-
difference in global sexual functioning trajectory, measured from 1 to 5 by the AUC for the single-item male sexual satisfaction score from Index of Erectile Function questionnaire
-
difference in generic quality-of-life trajectory, measured by the AUC for the EQ-5D-5L total score based on responses to 5-dimension items and using UK population valuations (0 death to 1 full health)39 and VAS score (0 worse possible health state to 100 best possible health state)
-
difference in rate of improvement of Qmax, measured at baseline, 3 months, and between 12 and 24 months with an increase in Qmax ≥ 10 ml/second from baseline taken to signify a successful outcome
-
difference in rate of need for further intervention, recorded from the clinical record for those participants returning to the care of their original specialist with recurrent stricture, by patient questionnaire for participants seeking care elsewhere and checked by the local trial research staff at the final 24-month assessment.
Secondary outcomes were analysed using generalised linear models appropriate for the distribution of the outcome, with adjustment for minimisation and baseline variables as appropriate. Reintervention was analysed as a time-to-event outcome using Cox regression and adjusting for minimisation variables and centre. Hazard ratios and 95% CI were calculated with the model. Kaplan–Meier curves were generated. Participants were included in the analysis using the observation time available until database closure (at least 2 years and up to 4 years). Box regression with multiple failure time data used the Andersen–Gill model.
For assessment of the primary outcome, missing follow-up data were estimated in sensitivity analysis using multiple imputation models for participants who had missing time points. We explored differences between responders and non-responders to inform our missing data model. We calculated an AUC for each imputation and combined these using Rubin’s rules under a missing at random assumption. 41,42 We also explored, using pattern mixture models, imputation of a range of values estimated from observed data using different missing not at random scenarios. Measures of the primary outcome collected at 24 months post intervention and at the end of the study were also included, when applicable, as a sensitivity analysis of the calculation of the AUC.
There were no planned or requested interim outcome analyses. The analyses were performed in Stata® version 14 (StataCorp LP, College Station, TX, USA).
Subgroup analysis
Subgroup analyses explored the possible modification of treatment effect by clinically important factors: time since last procedure (< 12 months or ≥ 12 months) as a global measure of stricture severity, age, stricture length and number of previous interventions. This was done by including treatment-by-factor interactions in the model and they were classified as exploratory analyses. No adjustment of the significance level was applied and findings should not be considered definitive but require replication.
Adverse events
An AE may be defined as any untoward medical occurrence in a subject to whom a study intervention or procedure has been administered, including occurrences which are not necessarily caused by or related to that intervention.
For the purposes of this trial:
-
all AEs were recorded at time of initial or reintervention surgery, and at 3, 12 and 24 months after initial intervention, and categorised by trial staff according to expectedness, relatedness, severity and, for postoperative complications, according to the Clavien–Dindo classification. 43
Please refer to the protocol [see www.journalslibrary.nihr.ac.uk/programmes/hta/105723/#/documentation (accessed 26 June 2019)] for more information about AE classification and reporting.
Qualitative substudy
Qualitative work was undertaken to establish factors determining willingness of patients and support of their clinicians to consider participation. Timely and successful completion of the planned qualitative study first established that the aims of the trial were important to men eligible to participate, given the troublesome and chronic nature of their symptoms, and to both general and specialist clinicians; these findings reinforced the rationale and need for the trial. As part of this work, we found that men eligible for inclusion were most likely to be willing to participate when their symptoms had first recurred and this was the point at which they expressed most uncertainty as to which option would be best for them as individuals. Both general and specialist clinicians were also very supportive of the aims of the trial given the uncertainty of guidance on best treatment, but expressed concerns regarding delivery of balanced information to men eligible for participation. To assist men eligible for participation in making a decision about participation, appropriate written guidance and an example video were provided, supported by personal contact from the trial team.
Trial progress and monitoring
The study initially set out to progressively build to a target of 500 participants over 24 months. This included an initial 12-month feasibility study, during which recruitment and patient adherence to the intervention were evaluated. Feasibility of recruitment was analysed after 9 months of active recruitment (trial month 12) and reported in August 2014 to the TSC and the funder, with an additional safety report reviewed by the Data Monitoring Committee (DMC). Recruitment continued to be monitored by the Trial Management Group (TMG) through returns to the randomisation website. The funder and TSC requested a recovery plan to mitigate the slow recruitment and ensure that the trial was completed over a fundable period of time. The recovery plan, which principally involved a re-estimation of required sample size using early observed data to 210 randomised participants, opening of additional sites and the recruitment window increasing from 24 months to 35 months, was submitted and approved by the TSC and funder in December 2014.
Sources of bias
To allow randomisation, both the eligible participant and the responsible clinician needed to be sufficiently uncertain whether the experimental or control strategy was best for management of the individual’s recurrent urethral stricture. Given the lack of high-level evidence as to which was the more effective intervention, trial information was provided illustrating the uncertainty and the need for a definitive trial. This aimed to ensure that any selection bias in terms of differing characteristics of men with recurrent bulbar urethral stricture willing to be randomised compared with those men who were eligible but not willing to participate was minimised. As far as possible and within the limits of data protection legislation, we recorded reasons for declining randomisation, but patients were free to decline participation and randomisation without giving a reason.
Trial literature for men eligible for participation or who were participating in the trial included the following: the OPEN trial participant information sheet and consent form v1.5 (main trial), the OPEN trial participant information sheet and consent form interview study v1.3, the OPEN trial website synopsis v1.1, the OPEN trial patient end of study questionnaire letter v1.0, the OPEN trial patient questionnaire letter v1.2, the OPEN trial patient invitation letter main study v1.0, the OPEN trial contact card v1.0 and participant flowchart v1.0. These documents are available at www.journalslibrary.nihr.ac.uk/programmes/hta/105723#/ (accessed 26 June 2019).
Definition and end of study
The end of study, defined as the 24-month post-randomisation follow-up for the last recruited participant, was originally planned for 28 February 2017. Owing to slow recruitment, an extension was granted in December 2014 by the funder and approved by the TSC to a new end date of 31 January 2018. A further extension to 30 April 2018 was approved in December 2017 by the funder and TSC in consultation with the DMC to allow more time to consider the primary result of the trial in the light of additional analyses prior to report submission. Active participation in the trial ended on 23 December 2017.
Compliance and withdrawal
Outcome data were collected remotely whenever feasible by participant completion of the trial questionnaires. Local research staff made use of planned routine clinical visits to check completion of trial documentation, with reference to the trial database and participants’ health-care record. Adherence to the allocated group (urethroplasty or urethrotomy) was checked by completion of an intervention CRF at the time of surgery. Reasons why participants chose not to have their allocated intervention but underwent the alternative procedure instead or who did not have any intervention were recorded. These participants continued to complete trial questionnaires and trial visits according to protocol to allow them to be included in the complete-case ITT primary analysis. The trial statistician monitored attrition rate against the anticipated maximum of 19% and reported to the TMG, TSC and DMC as appropriate.
Data monitoring, quality control and assurance
Quality control was maintained through adherence to standard operating procedures governing the work of sponsor (Research and Development Directorate, Newcastle upon Tyne Hospitals NHS Foundation Trust), NCTU, CHaRT and local research teams, and in accordance with the study protocol, the principles of GCP, research governance and clinical trial regulations. An independent DMC was set up comprising one methodologist, one clinician not connected to the trial and one statistician (chairperson). The purpose of this committee was to monitor efficacy and safety end points. It operated in accordance with written terms of reference linked to the DMC’s lessons, ethics, statistics charter. 44 Only the DMC and the trial statistician preparing reports to the DMC had access, prior to completion of the trial, to data separated by allocated group. The DMC met at the start and completion and four times during the study. The DMC meetings were also attended by the trial statistician.
A TSC was established to provide overall supervision of the trial. The TSC consisted of an independent clinician who acted as chairperson, two further independent clinicians, an independent statistician, a lay representative and the chief investigator. Other members of the TMG attended as required or as requested by the chairperson. The committee met approximately every 6 months during recruitment and annually thereafter for the duration of the trial.
Monitoring of study conduct and collected data followed a written monitoring plan, informed by a risk assessment and agreed with sponsor. It was performed by a combination of central review and site monitoring visits to ensure that the study was conducted in accordance with GCP. Study site monitoring was undertaken by appropriately trained members of the NCTU. The main areas of focus were consent, eligibility, SAEs and completeness of the investigator site file at each site and the trial master file held at NCTU. Audit of data entry using a random 10% sample of trial questionnaires showed a 3% error rate, below the threshold of 5% that would have triggered a full audit.
Ethics and governance
The Newcastle upon Tyne Hospitals NHS Foundation Trust Research and Development Directorate sponsored the trial (reference 6332). Favourable ethics opinion for the trial was obtained on 16 October 2012 from the NHS Research Ethics Service Committee North East – Newcastle and North Tyneside 1 (reference 12/NE/0343) and subsequent research and development and Caldicott Guardian approvals were granted by each participating site. Approval was sought and obtained for all substantive protocol amendments (see Table 2).
Trial registration and protocol availability
The trial was registered as ISRCTN98009168 on 29 November 2012 and in the UK NIHR Portfolio (reference 13507). The latest version (1.8) of the full protocol is available at www.journalslibrary.nihr.ac.uk/programmes/hta/105723/#/ (accessed 14 February 2017) and a published version is also available. 1
Chapter 3 Results
Recruitment
The trial recruited from 38 NHS hospital sites across the UK (England, Scotland and Wales). The first participant was randomised on 27 February 2013 and the last on 23 December 2015. The planned recruitment window was extended by 12 months to 35 months to allow the opening of further sites and to achieve the revised recruitment target (Figure 5). Participants were identified when attending hospital urology clinics. The recruitment strategy was first to open specialist sites where both urethroplasty and urethrotomy were carried out and subsequently to open general urology sites where only urethrotomy was provided, with urethroplasty accessed by referral to a previously established specialist site. Overall, 222 participants were recruited and randomised [58 (26%) from general sites and 164 (74%) from specialist sites] (Table 3).
FIGURE 5.
Planned and actual recruitment trajectory for the OPEN trial.
| Site of recruitmenta | Number of participants randomised at site |
|---|---|
| Freeman Hospital, Newcastle upon Tyne | 21 |
|
1 |
|
5 |
| St George’s Hospital, London | 12 |
|
3 |
|
2 |
| University College London Hospitals NHS Foundation Trust, London | 4 |
|
1 |
|
9 |
|
6 |
|
6 |
|
2 |
| Russells Hall Hospital, West Midlands | 17 |
| Queen Alexandra Hospital, Portsmouth | 11 |
| St Richard’s Hospital, Chichester | 4 |
| Manchester Royal Infirmary, Manchester | 0 |
|
5 |
| St James’s University Hospital, Leeds | 3 |
|
3 |
| Weston General Hospital, Weston-super-Mare | 2 |
|
2 |
|
3 |
|
1 |
|
1 |
|
2 |
| Addenbrooke’s Hospital, Cambridge | 31 |
| Royal Hallamshire Hospital, Sheffield | 1 |
| Aintree University Hospital, Liverpool | 8 |
| Stepping Hill Hospital, Stockport | 2 |
|
1 |
| Kent and Canterbury Hospital, Canterbury | 13 |
| Southampton General Hospital, Southampton | 12 |
| Aberdeen Royal Infirmary, Aberdeen | 5 |
| Sunderland Royal Hospital, Sunderland | 9 |
| Guy’s Hospital, London | 5 |
| Charing Cross Hospital, London | 1 |
| Princess of Wales Hospital, Bridgend | 2 |
|
1 |
| Western General Hospital, Edinburgh | 5 |
| Total randomised | 222 |
Participant flow
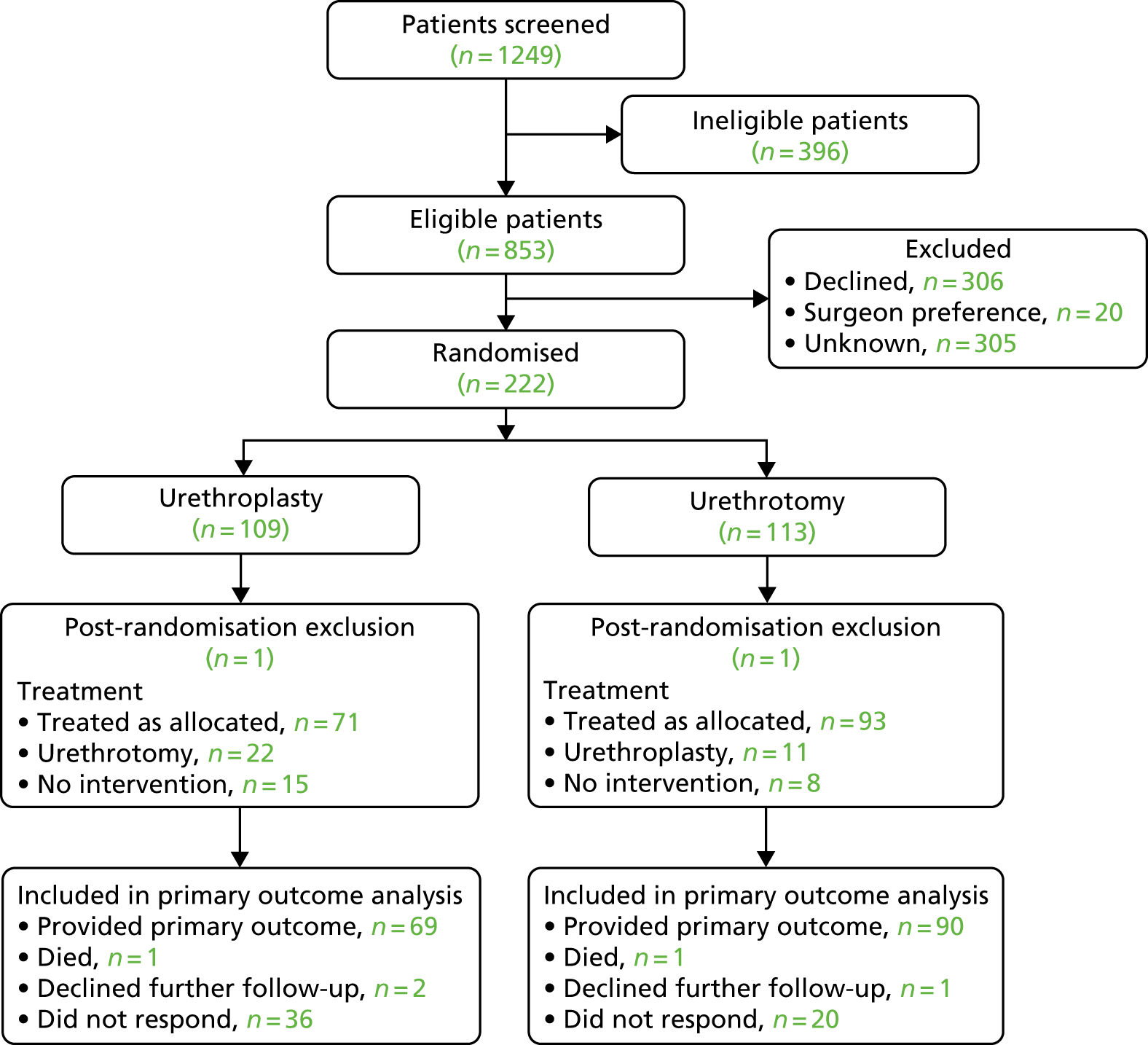
The flow of participants enrolled in the study is shown in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 6). A total of 1249 men were identified by study sites (83% of the target of 1500 men) and screened for eligibility. Of these men, 1027 (82%) were either deemed ineligible to take part by local research staff or declined to participate in the trial (Tables 4 and 5). Following completion of written consent and collection of baseline data, 222 participants (106% of revised target) were randomised, with 109 men allocated to open urethroplasty and 113 men to endoscopic urethrotomy.
FIGURE 6.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram showing progress of participants through the study. Reprinted from European Urology, vol. 78, Goulao B, Carnell S, Shen S, MacLennan G, Norrie J, Cook J, et al. , Surgical treatment for recurrent bulbar urethral stricture: a randomised open-label superiority trial of open urethroplasty versus endoscopic urethrotomy (the OPEN trial), pp. 572–80,45 Copyright 2020, with permission from Elsevier.
| Participants approached | Total (N = 1249; 100.0%), n (%) |
|---|---|
| Ineligible | 396 (32) |
| Age < 16 years | 4 (0.3) |
| Stricture in penile urethra | 66 (5.3) |
| No previous intervention for stricture | 101 (8.1) |
| Intervention not required | 65 (5.2) |
| Unwilling to have 2-week catheterisation | 1 (0.1) |
| Unable to give consent | 13 (1.0) |
| Perineal sepsis or fistula | 7 (0.6) |
| Previous participation in the OPEN trial | 5 (0.4) |
| Unable to have 3-hour anaesthetic | 22 (1.8) |
| Inability to adhere to trial protocol | 11 (0.9) |
| Ineligible, no reason stated | 101 (8.1) |
| Patient declined participation | 306 (24.5) |
| Preference for open urethroplasty | 185 (14.8), 60% of those men who declined |
| Preference for endoscopic urethrotomy | 79 (6.3), 26% of those men who declined |
| Potential adverse effects of urethroplasty | 6 (0.5) |
| Potential adverse effects of urethrotomy | 1 (0.1) |
| Need for urethrogram for urethroplasty | 2 (0.2) |
| Unable to fulfil protocol commitments | 19 (1.5), 6% of those men who declined |
| Patient did not attend follow-up | 14 (1.1) |
| Surgeon preference | 20 (1.6), 7% of those men who declined |
| Unknown | 305 (24.4) |
| Reason for withdrawal | Intervention (n) | Total | |
|---|---|---|---|
| Urethroplasty (N = 109) | Endoscopic urethrotomy (N = 113) | ||
| Withdrawal by participant | 5 | 5 | |
| No longer requires treatment | 1 | 1 | |
| Withdrawn by clinician | 1 | 1 | |
| Participant did not accept allocation and wished to have alternative (crossover) | 1 | 1 | 1 |
| Participant referred to specialist site where urologist was not available to perform procedure | 2 | 2 | |
| Participant not suitable for surgery | 1 | 1 | |
| Participant deceased | 1 | 1 | 2 |
| No reason recorded/given | 1 | 3 | 4 |
| Total | 12 | 6 | 18 |
Numbers analysed
Primary analysis
In the primary complete-case ITT analysis we included 159 (72%) of the randomised participants using the criteria of having completed primary outcome questionnaires on a minimum of three occasions: at baseline, during the first year after intervention and at 18–24 months after randomisation. This was 94% of the pre-set target of 170 participants providing data on the primary outcome in our revised sample size calculation. The total of 159 men comprised 69 men (63% of those men randomised) allocated to the urethroplasty group and 90 men (81% of those men randomised) allocated to urethrotomy. A total of 25 men allocated to urethroplasty had urethrotomy and a further 15 men had no intervention. From the urethrotomy group, 11 men had urethroplasty and 8 men did not have any intervention. Accordingly, 19 men (17%) allocated to urethrotomy did not receive urethrotomy and 40 men (37%) allocated to urethroplasty did not receive urethroplasty. All participants who ‘crossed over’ or did not have any intervention but who remained in active follow-up and provided sufficient completed trial questionnaires were analysed according to allocated group (complete-case ITT analysis). Reasons for exclusion from the primary analysis are detailed in Table 6.
| Intervention (n) | ||
|---|---|---|
| Urethroplasty (N = 108) | Urethrotomy (N = 112) | |
| Included | ||
| All three required questionnaires received | 69 | 90 |
| Excluded | ||
| At least one required questionnaire missing | 39 | 22 |
| Intervention performed in those men excluded | ||
| Allocated intervention | 17 | 13 |
| Alternative intervention (crossover) | 7 | 1 |
| No intervention performed | 15 | 8 |
Of 222 randomised participants, 109 were allocated to the urethroplasty group and 113 allocated to the urethrotomy group. Two randomised participants, one from each group, were excluded soon after randomisation because further assessment showed them to be ineligible and they were classified as post-randomisation withdrawals, excluding them from the primary analysis. One man (allocated to urethroplasty) was unfit for prolonged anaesthesia and one (allocated to urethrotomy) had a stricture that, following urethrography, was deemed unsuitable for endoscopic management. Accordingly, at baseline, there were 108 participants in the urethroplasty group and 112 in the urethrotomy group. Baseline characteristics were well balanced between the two groups (Tables 7 and 8). Twenty-two participants allocated to urethroplasty underwent urethrotomy and 15 participants did not have either procedure. In the urethrotomy group, 11 men underwent urethroplasty and eight men no procedure. These protocol deviations and their reasons are given in Tables 9 and 10. The median time between randomisations and interventions was 92 days, compared with 47.5 days for urethrotomy (Table 11). The rate of trial questionnaire return by each group for each time point is given in Table 12. The attrition rate for inclusion in the primary analysis was 30 of 109 (27%) men in the urethroplasty group, 23 of 113 (20%) men in the urethrotomy group and 53 of 222 (24%) men in the randomised population as a whole.
| Variable | Urethroplasty (N = 108) | Number of participants providing data | Urethrotomy (N = 112) | Number of participants providing data |
|---|---|---|---|---|
| Age (years), mean (SD) | 49.4 (14.3) | 108 | 48.5 (15.4) | 112 |
| Length of stricture (cm), mean (SD) | 2.0 (1.4) | 67 | 1.7 (1.1) | 63 |
| Duration of disease (years), mean (SD) | 7.3 (9.7) | 78 | 9.9 (11.7) | 80 |
| Previous interventions, mean (SD) | 1.9 (2.0) | 108 | 1.8 (1.7) | 112 |
| Time (months) since last intervention, n (%) | ||||
| < 12 | 36 (33.3) | 36 (32.1) | ||
| ≥ 12 | 72 (66.7) | 76 (67.9) | ||
| Predominant site of stricture in bulbar urethra, n (%) | ||||
| Proximal | 30 (27.8) | 24 (21.4) | ||
| Mid | 34 (31.5) | 41 (36.6) | ||
| Distal | 17 (15.7) | 17 (15.2) | ||
| Unknown | 6 (5.6) | 14 (12.5) | ||
| Missing | 21 (19.4) | 16 (14.3) | ||
| Cause of stricture, n (%) | ||||
| Unknown | 76 (70.4) | 81 (72.3) | ||
| Trauma | 11 (10.2) | 11 (9.8) | ||
| Infection | 5 (4.6) | 6 (5.4) | ||
| Other | 12 (11.1) | 7 (6.3) | ||
| Missing | 4 (3.7) | 7 (6.3) | ||
| Use of intermittent self-dilatation, n (%) | ||||
| Never | 60 (55.6) | 66 (58.9) | ||
| Previously | 25 (23.1) | 31 (27.7) | ||
| Currently | 23 (21.3) | 14 (12.5) | ||
| Missing | 0 (0) | 1 (0.9) | ||
| Qmax (ml/second), mean (SD) | 10.0 (6.0) | 83 | 9.7 (5.2) | 90 |
| Urethrogram performed, n (%) | 70 (64.8) | 62 (55.4) | ||
| Urethroscopy performed, n (%) | 34 (31.5) | 42 (37.5) | ||
| Patient-reported symptoms measure | Urethroplasty (N = 108) | Number of participants providing data | Urethrotomy (N = 112) | Number of participants providing data |
|---|---|---|---|---|
| USS-PROM | ||||
| Total voiding score (0 = no symptoms, 24 = symptoms all the time), mean (SD) | 13.4 (4.5) | 105 | 13.2 (4.7) | 109 |
| Impact of urinary symptoms on daily activities (0 = none, 3 = a lot), median (P25–P75) | 2.0 (1.0–3.0) | 107 | 2.0 (1.0–3.0) | 110 |
| Satisfaction with sexual function (1 = very satisfied, 5 = very dissatisfied), median (P25–P75) | 2.0 (1.0–3.0) | 97 | 2.0 (1.0–3.0) | 100 |
| Urinary stream picture score (1 = strong, 4 = weak), median (P25–P75) | 3.0 (3.0–4.0) | 103 | 3.0 (3.0–4.0) | 108 |
| Voiding score component questionsa | ||||
| Delay before starting to urinate, median (P25–P75) | 2.0 (1.0–3.0) | 105 | 2.0 (1.0–3.0) | 110 |
| Poor strength of urinary stream, median (P25–P75) | 3.0 (3.0–4.0) | 105 | 3.0 (3.0–4.0) | 109 |
| Having to strain before urinating, median (P25–P75) | 2.0 (1.0–3.0) | 105 | 2.0 (1.0–3.0) | 110 |
| Intermittent urinary stream, median (P25–P75) | 2.0 (1.0–3.0) | 104 | 2.0 (1.0–3.0) | 110 |
| Feeling of incomplete bladder emptying, median (P25–P75) | 2.0 (1.0–3.0) | 105 | 2.0 (1.0–3.0) | 110 |
| Post-micturition dribbling, median (P25–P75) | 2.0 (1.0–3.0) | 105 | 2.0 (1.0–3.0) | 110 |
| Overall health status on VAS, rating from 0 (worst possible health) to 100 (best health imaginable), mean (SD) | 72.4 (19.8) | 104 | 76.7 (17.3) | 105 |
| Allocated intervention | Intervention, n (%) | |
|---|---|---|
| Urethroplasty (N = 108) | Urethrotomy (N = 112) | |
| Urethroplasty | 71 (66) | 11 (9.8) |
|
9 (8.3) | 4 (3.6) |
|
9 (8.3) | 1 (0.89) |
|
7 (6.5) | 2 (1.8) |
|
45 (42) | 4 (3.6) |
|
1 (0.93) | 0 (0) |
| Urethrotomy | 22 (20) | 93 (83.0) |
|
15 (14) | 90 (80) |
|
0 (0) | 1 (0.89) |
|
7 (6.5) | 2 (1.8) |
| Commenced regimen of intermittent self-dilatation | 9 (8.3)a | 29 (26) |
| No intervention performed | 15 (13.9) | 8 (7.1) |
| Reason | Intervention, n (%) | |
|---|---|---|
| Urethroplasty (N = 108) | Urethrotomy (N = 112) | |
| Reason for crossing over | ||
| Patient decision | 16 (14.8) | 8 (7.1) |
| Patient unsuitable/ineligible | 6 (5.6) | 0 (0) |
| Unknown | 0 (0) | 3 (2.7) |
| Reasons for not having surgery | ||
| Unknown | 15 (13.9) | 8 (7.1) |
| Allocated intervention | Number of participants providing data | Median (IQR) days |
|---|---|---|
| Urethroplasty (N = 108) | 93 | 90 (53–157) |
| Urethrotomy (N = 112) | 104 | 47.5 (28–88) |
| Study time point | Intervention (n) | |
|---|---|---|
| Urethroplasty (N = 108) | Urethrotomy (N = 112) | |
| Baseline | 107 (99.1) | 110 (98.2) |
| Pre intervention | 107 (99.1) | 110 (98.2) |
| 1 week post intervention | 56 (51.9) | 70 (62.5) |
| 3 months post intervention | 73 (67.6) | 88 (78.6) |
| 6 months post intervention | 61 (56.5) | 78 (69.6) |
| 9 months post intervention | 65 (60.2) | 82 (73.2) |
| 12 months post intervention | 66 (61.1) | 81 (72.3) |
| 18 months post randomisation | 64 (59.3) | 82 (73.2) |
| 24 months post randomisation | 58 (53.7) | 58 (51.8) |
| 24 months post intervention | 51 (47.2) | 57 (50.9) |
| End of study | 51 (47.2) | 57 (50.9) |
Outcomes
Primary
Area under the curve for USS-PROM total voiding score
Figure 7 shows the mean total USS-PROM voiding score for each randomised group by time point. The mean AUC of multiple (at least three) voiding score measurements on a scale from 0 (no symptoms) to 24 (worst symptoms) over the 24 months after randomisation was 7.4 (SD 3.8) in the urethroplasty group and 7.8 (SD 4.2) in the urethrotomy group (a mean difference of –0.36, 95% CI –1.74 to 1.02; p = 0.60 in favour of the urethroplasty group). To assess whether or not the primary outcome was biased by the exclusion of participants with fewer than the required three measures, we undertook as sensitivity analyses an ITT calculation using the predefined methods of data imputation to replace missing values and a per-protocol analysis using data only from participants eligible for the primary analysis who underwent the intervention to which they were randomly allocated. The results of these three analyses are presented in Table 13. The estimate of the primary outcome was robust to sensitivity analyses using pattern-mixture models for missing data for all but unrealistic scenarios (e.g. missing observations in one arm of the trial staying at baseline levels for all 24 months, whereas in the other arm all missing observations were consistent with observed scores).
FIGURE 7.
Mean (standard error of the mean) for total voiding score at each nominal time point. AI, after intervention; AR, after randomisation.
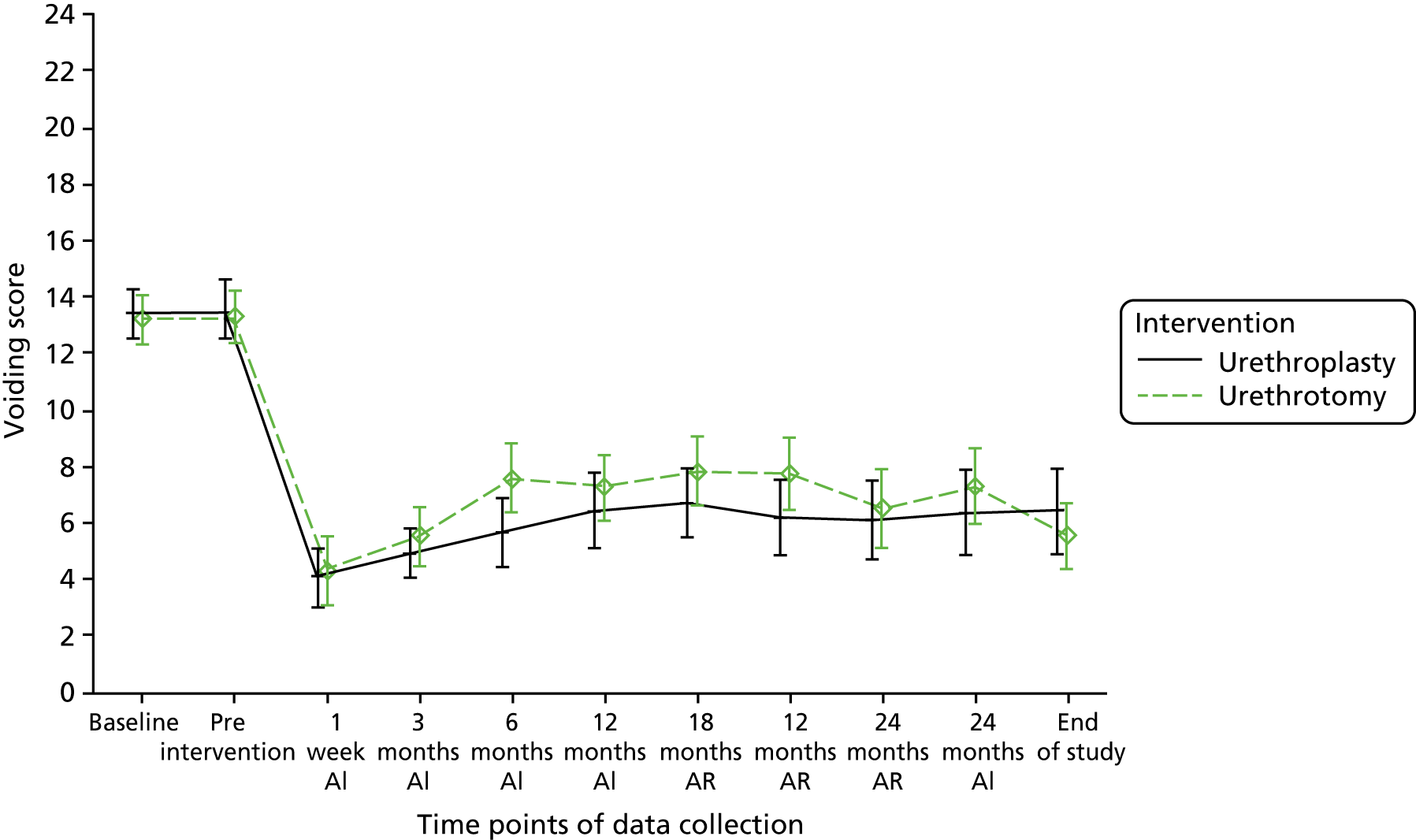
| Analysis | AUC (primary outcome) in each sensitivity analysis | ||
|---|---|---|---|
| Urethroplasty (N = 108), mean (SD); n | Urethrotomy (N = 112), mean (SD); n | Effect size (95% CI); p-value | |
| Complete-case ITT analysis (primary analysis) | 7.4 (3.8); 69 | 7.8 (4.2); 90 | –0.36 (–1.74 to 1.02); 0.60 |
| Sensitivity analysis with multiple imputation (ITT) | 7.7 (3.9); 93 | 7.9 (4.2); 104 | –0.33 (–1.74 to 1.09); 0.64 |
| Per protocol (underwent allocated intervention) | 6.8 (2.9); 54 | 7.8 (4.2); 80 | –1.02 (–2.12 to 0.07); 0.07 |
Subgroup analyses
We identified number of previous interventions, length of stricture, time since last intervention prior to randomisation and age as risk factors with potential to increase the risk of failure to control symptoms. Figure 8 presents subgroup analyses for the AUC of the voiding score. Table 14 presents the interaction term for each subgroup. The results show no evidence that the treatment effect of the different subgroups varies significantly from the main effect.
FIGURE 8.
Subgroup analyses for the USS-PROM voiding score AUC.
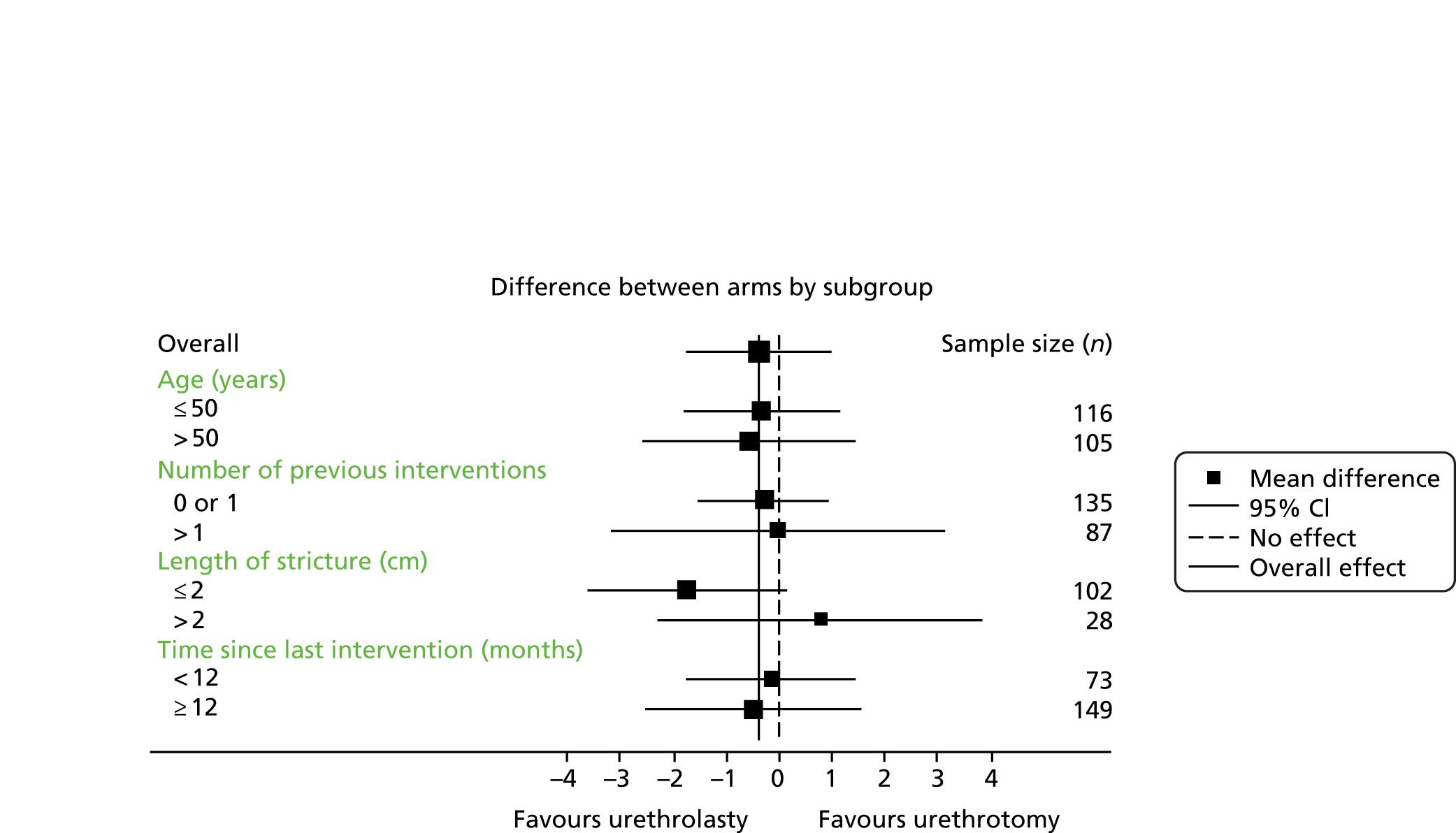
| Interaction term | Effect size (95% CI); p-value |
|---|---|
| Overall | –0.36 (–1.74 to 1.02); 0.60 |
| Time since last intervention | –0.36 (–3.19 to 2.46); 0.79 |
| Length of stricture | 2.54 (–1.42 to 6.50); 0.20 |
| Number of previous interventions | 0.56 (–2.89 to 4.02); 0.74 |
| Age | –0.25 (–2.58 to 2.08); 0.83 |
Secondary outcomes
Area under the curve for impact of urinary symptoms on daily activities: assessing condition-specific quality of life
Sufficient data were available to calculate this outcome for 69 participants in the urethroplasty group and 90 participants in the urethrotomy group. The urethroplasty group had a mean score at 24 months of 1.1 (SD 0.8), compared with 1.0 (SD 0.7) in the urethrotomy group. The mean difference between treatments adjusting for minimisation variables was 0.06 (95% CI –0.19 to 0.30).
Area under the curve for satisfaction with sexual function
Sufficient data were available to calculate this outcome for 63 participants in the urethroplasty group and 87 participants in the urethrotomy group. The urethroplasty group had a mean AUC of 2.9 (SD 1.2), compared with a mean of 2.5 (SD 1.2) in the urethrotomy group. The mean difference between treatments adjusting for minimisation variables was 0.35 (95% CI –0.06 to 0.75).
Reintervention
In total, 44 participants had at least one reintervention and there were 52 reinterventions overall (Table 15). During the trial observation period for each participant (up to 4 years), 15 (16%) men in the urethroplasty group required a reintervention at a median of 474 (IQR 399–577) days after initial surgery, compared with 29 (28%) men at a median of 308 (IQR 211–448) days for men allocated to the urethrotomy group. The hazard ratio for time until first reintervention was 0.52 (95% CI 0.31 to 0.89; p = 0.02) (Figure 9), representing a 48% lower risk of reintervention with urethroplasty. Calculation including multiple events gave a similar hazard ratio of 0.49 (95% CI 0.30 to 0.82). A secondary analysis involving only men who underwent the allocated intervention (per protocol) showed a hazard ratio for time to reintervention of 0.28 (95% CI 0.15 to 0.55; p < 0.001) (see Figure 9). Subgroup analyses showed no evidence of heterogeneity around the ITT treatment effect (Figure 10). For men undergoing urethrotomy, commencement of intermittent self-dilatation did not lengthen the time to recurrence. Median time until first reintervention was 384 (IQR 214–555) days for 21 participants who did not commence dilatation and 308 (IQR 171–399) days for seven participants who did.
| Intervention, n (%) | ||
|---|---|---|
| Urethroplasty | Urethrotomy | |
| Total participants randomised | 108 | 112 |
| Participants with data available | 93 | 104 |
| Number of reinterventions received | ||
| 0 | 78 (83.9) | 75 (72.1) |
| 1 | 13 (14.0) | 26 (25.0) |
| 2 | 1 (1.1) | 2 (1.9) |
| 3 | 1 (1.1) | 0 (0) |
| 4 | 0 (0) | 1 (1.0) |
FIGURE 9.
Hazard curves for reintervention by randomised or treatment received group up to 4 years after initial intervention. (a) Analysis of participants who had surgery according to their randomised allocation (modified ITT); and (b) restricted to men who underwent procedure allocated at randomisation (per protocol). Reprinted from European Urology, vol. 78, Goulao B, Carnell S, Shen S, MacLennan G, Norrie J, Cook J, et al. , Surgical treatment for recurrent bulbar urethral stricture: a randomised open-label superiority trial of open urethroplasty versus endoscopic urethrotomy (the OPEN trial), pp. 572–80,45 Copyright 2020, with permission from Elsevier.
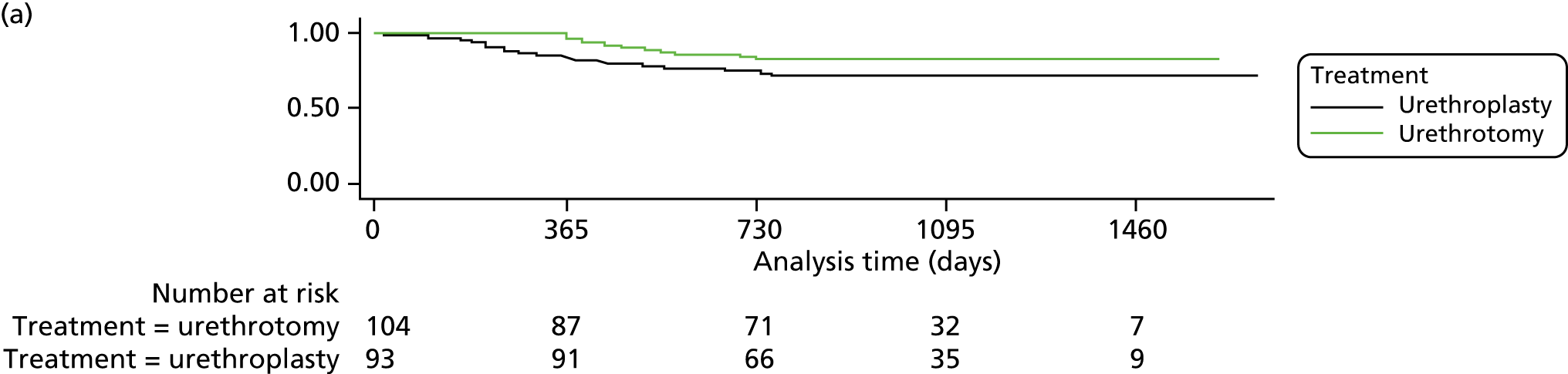
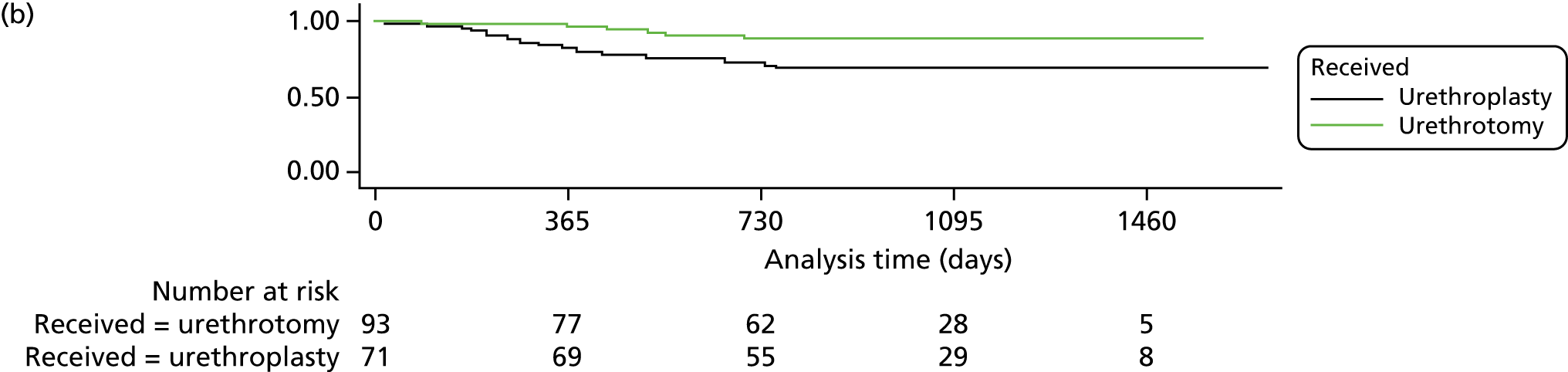
FIGURE 10.
Subgroup analyses for time to reintervention. a, Upper limit of the CI truncated to fit the graph (95% CI 0.19 to 28.4).
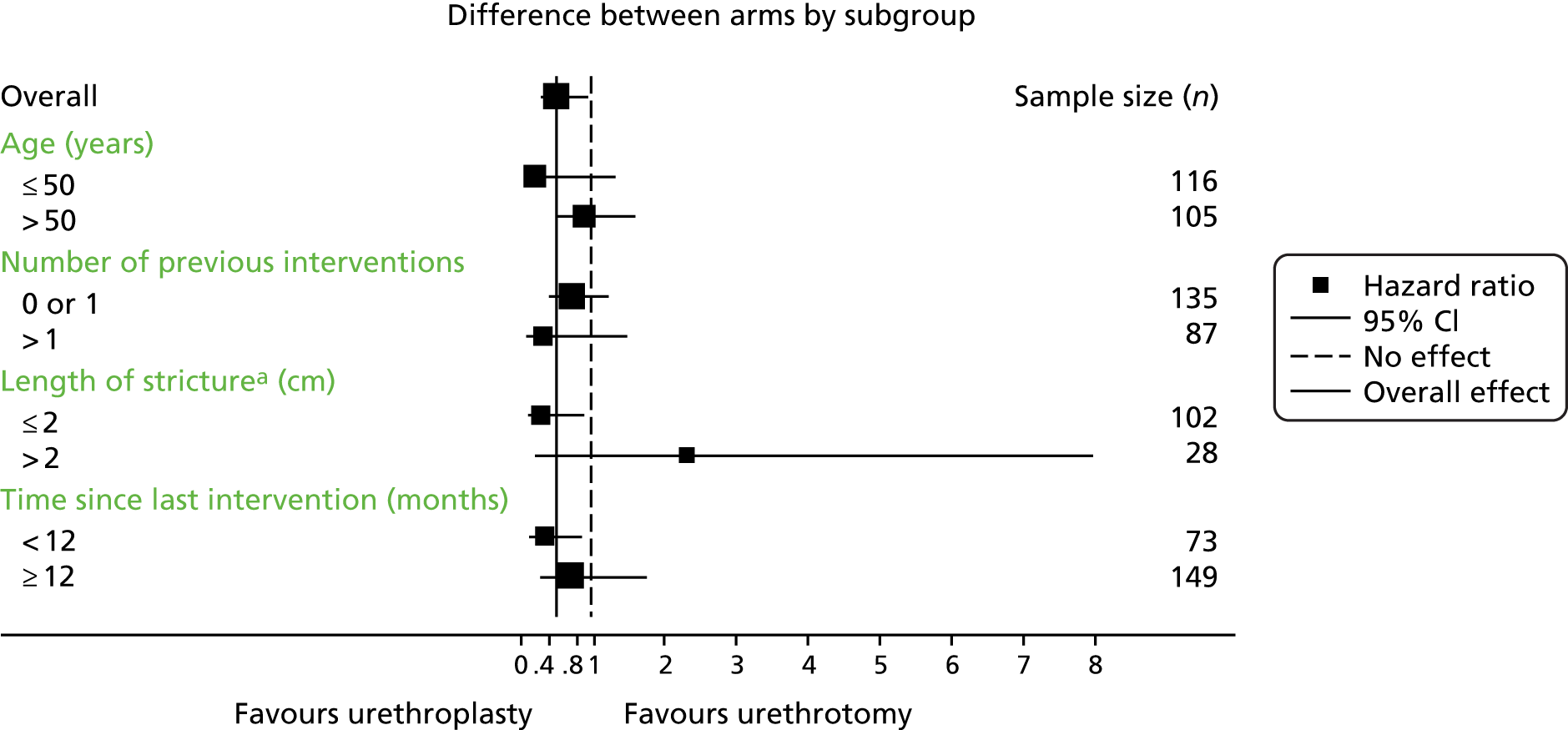
Improvement in maximum flow rate
A successful outcome in terms of Qmax was defined as an increase of ≥ 10 ml/second from baseline at 3 months, and between 12 and 24 months. Data allowing calculation of this outcome were available from 64 men in the urethroplasty group and from 68 men in the urethrotomy group at 3 months. Participants in the urethroplasty group had twice the odds of experiencing an improvement ≥ 10ml/second in their maximum flow rate at 3 months compared with participants in the urethrotomy arm. Data were available from 44 participants in the urethroplasty group and from 63 participants in the urethrotomy group at 12 or 24 months. At these time points, participants in the urethroplasty arm had 2.64 times greater odds of experiencing an improvement of ≥ 10ml/second in their maximum flow rate (Table 16).
| Maximum flow rate | Urethroplasty (N = 93) | Number of participants providing data | Urethrotomy (N = 104) | Number of participants providing data | Odds ratio (95% CI); p-valuea (urethroplasty vs. urethrotomy) |
|---|---|---|---|---|---|
| Flow rate baseline, mean (SD) | 13.2 (15.5) | 75 | 10.5 (6.7) | 89 | |
| Flow rate at 3 months, mean (SD) | 22.3 (11.4) | 64 | 17.6 (11.1) | 68 | |
| Flow rate at 12 months, mean (SD) | 22.3 (12.2) | 41 | 16.8 (11.5) | 55 | |
| Flow rate at 24 months, mean (SD) | 21.7 (13.7) | 29 | 19.1 (11.8) | 49 | |
| Improved at 3 months from baseline,b n (%) | 2.08 (1.05 to 4.12); 0.035 | ||||
| Not improved | 28 (30.1) | 42 (40.4) | |||
| Improved | 29 (31.2) | 21 (20.2) | |||
| Missing | 36 (38.7) | 41 (39.4) | |||
| Improved at 12 or 24 months from baseline,b n (%) | 2.64 (1.14 to 6.15); 0.024 | ||||
| Not improved | 26 (28.0) | 50 (48.1) | |||
| Improved | 18 (19.4) | 13 (12.5) | |||
| Missing | 49 (52.7) | 41 (39.4) | |||
Recurrence
Stricture recurrence was observed in 19 (20%) participants in the urethroplasty group and 39 (38%) participants in the urethrotomy group. The difference was significant in favour of urethroplasty, with a mean odds ratio of 0.43 (95% CI 0.24 to 0.76) (Table 17).
| Intervention, n (%) | Odds ratio (95% CI); p-value (urethroplasty vs. urethrotomy) | ||
|---|---|---|---|
| Urethroplasty (N = 93) | Urethrotomy (N = 104) | ||
| Any recurrence | 19 (20.4) | 39 (37.5) | 0.43 (0.24 to 0.76); < 0.001 |
| Reintervention | 15 (16.1) | 29 (27.9) | |
| Stricture recurrence at 12 months | 4 (4.3) | 14 (13.5) | |
| Stricture recurrence at 24 months | 6 (6.5) | 8 (7.7) | |
| Deterioration in flow rate | 5 (5.4) | 7 (6.7) | |
| Deterioration in voiding score | 1 (1.1) | 1 (1.0) | |
Urinary stream picture score
Median stream score was lower at 24 months post randomisation than at baseline in both randomised groups. A total of 24 out of 59 (41%) participants reported an improvement of at least two grades in their urinary stream score in the urethroplasty group compared with 18 out of 59 (31%) participants in the urethrotomy group. There was a significant association between self-reported urinary stream improvement at 24 months and improved Qmax at 24 months (n = 66; p = 0.02).
Health status: EuroQol-5 Dimensions
Participants’ rating of their state of health at 24 months on the EuroQol-5 Dimensions (EQ-5D) VAS from 0 (worst health imaginable) to 100 (best health imaginable) was a mean of 81.9 (SD 19.5) in the urethroplasty group and a mean of 79.4 (SD 17.5) in the urethrotomy group.
Absolute changes in outcomes between baseline and 24 months
Changes in the absolute values of participant-reported outcomes between baseline and 24 months were similar between the urethroplasty and urethrotomy groups (Table 18).
| USS-PROM domain | Intervention | |||||||
|---|---|---|---|---|---|---|---|---|
| Urethroplasty (N = 108) | Urethrotomy (N = 112) | |||||||
| Baseline (USS-PROM score) | n | 24 months (USS-PROM score) | n | Baseline (USS-PROM score) | n | 24 months (USS-PROM score) | n | |
| Total voiding score (0 = no symptoms to 24 = symptoms all the time), mean (SD) | 13.4 (4.5) | 104 | 6.0 (5.5) | 58 | 13.2 (4.7) | 109 | 6.4 (5.3) | 59 |
| Delay before starting to urinate (0–4), median (P25–P75) | 2.0 (1.0–3.0) | 105 | 0.0 (0.0–1.0) | 58 | 2.0 (1.0–3.0) | 110 | 1.0 (0.0–2.0) | 59 |
| Poor strength of urinary stream (0–4), median (P25–P75) | 3.0 (3.0–4.0) | 105 | 1.0 (0.0–2.0) | 58 | 3.0 (3.0–4.0) | 109 | 1.0 (0.0–2.0) | 59 |
| Having to strain before urinating (0–4), median (P25–P75) | 2.0 (1.0–3.0) | 105 | 0.0 (0.0–1.0) | 58 | 2.0 (1.0–3.0) | 110 | 1.0 (0.0–1.0) | 59 |
| Intermittent urinary stream (0–4), median (P25–P75) | 2.0 (1.0–3.0) | 104 | 1.0 (0.0–1.0) | 58 | 2.0 (1.0–3.0) | 110 | 1.0 (0.0–1.0) | 59 |
| Feeling of incomplete bladder emptying (0–4), median (P25–P75) | 2.0 (1.0–3.0) | 105 | 1.0 (0.0–2.0) | 58 | 2.0 (1.0–3.0) | 110 | 1.0 (0.0–2.0) | 59 |
| Post-micturition dribbling (0–4), median (P25–P75) | 2.0 (1.0–3.0) | 105 | 1.0 (0.0–2.0) | 58 | 2.0 (1.0–3.0) | 110 | 1.0 (0.0–2.0) | 59 |
| Impact of urinary symptoms (0 = no impact to 3 = a lot of impact), median (P25–P75) | 2.0 (1.0–3.0) | 107 | 0.0 (0.0–1.0) | 58 | 2.0 (1.0–3.0) | 110 | 1.0 (0.0–1.0) | 59 |
| Satisfaction with sexual function (0 = very satisfied to 4 = very dissatisfied), median (P25–P75) | 3.0 (2.0–4.0) | 97 | 1.0 (0.0–3.0) | 53 | 3.0 (2.0–4.0) | 100 | 1.0 (0.0–2.0) | 56 |
| Urine stream strength picture (1 = weak to 5 = strong), median (P25–P75) | 3.0 (3.0–4.0) | 103 | 2.0 (1.0–3.0) | 58 | 3.0 (3.0–4.0) | 108 | 2.0 (2.0–3.0) | 58 |
| Overall health state (0 = worst health imaginable to 100 = best health imaginable), mean (SD) | 72 (20) | 104 | 81.9 (19.5) | 58 | 77 (17) | 105 | 79.4 (17.5) | 59 |
Adverse events
At least one AE was reported for 89 of the trial participants (81 participants had one AE, seven participants had two AEs and one participant had three AEs). The reported AEs and SAEs are tabulated according to allocated group and treatment received (Tables 19–22). Postoperative complications categorised by the Clavien–Dindo grade are also tabulated by allocated group and intervention received (see Table 21).
| AE | Intervention, n (%) | |
|---|---|---|
| Urethroplasty | Urethrotomy | |
| Participants undergoing an intervention (allocated group) | (N = 101) | (N = 104) |
| AEs during the perioperative period | ||
| Mouth pain | 12 (11.9) | 4 (3.8) |
| Wound infection | 4 (4.0) | 0 (0) |
| Bladder ‘spasm’ requiring treatment | 2 (2.0) | 1 (1.0) |
| Urinary tract infection | 3 (3.0) | 0 (0) |
| Initial failed trial without catheter | 0 (0) | 1 (1.0) |
| AEs during follow-up | ||
| Erectile dysfunction | 5 (5.0) | 2 (1.9) |
| Mouth pain | 2 (2.0) | 2 (1.9) |
| Urinary tract infection | 5 (5.0) | 6 (5.8) |
| New urinary symptom | 9 (8.9) | 5 (4.8) |
| Wound infection | 0 (0) | 2 (1.9) |
| Wound pain | 5 (5.0) | 1 (1.0) |
| Numb testicles | 2 (2.0) | 0 (0) |
| Issues related to sexual climax | 2 (2.0) | 0 (0) |
| Othera | 1 (1.0) | 3 (2.9) |
| Erectile dysfunction and wound infection | 1 (1.0) | 0 (0) |
| Erectile dysfunction and wound pain | 1 (1.0) | 0 (0) |
| Wound infection, urinary tract infection and fistula | 1 (1.0) | 0 (0) |
| Participants undergoing an intervention (treatment received) | (N = 90) | (N = 115) |
| AEs during the perioperative period | ||
| Mouth pain | 14 (15.6) | 2 (1.7) |
| Wound infection | 4 (4.4) | 0 (0) |
| Bladder ‘spasm’ requiring treatment | 2 (2.2) | 1 (0.9) |
| Urinary tract infection | 3 (3.3) | 0 (0) |
| Initial failed trial without catheter | 0 (0) | 1 (0.9) |
| AEs during follow-up | ||
| Erectile dysfunction | 4 (4.4) | 3 (2.6) |
| Mouth pain | 4 (4.4) | 0 (0) |
| Urinary tract infection | 5 (5.6) | 6 (5.2) |
| New urinary symptom | 8 (8.9) | 6 (5.2) |
| Wound infection | 1 (1.1) | 1 (0.9) |
| Wound pain | 5 (5.6) | 1 (0.9) |
| Numb testicles | 2 (2.2) | 0 (0) |
| Issues related to sexual climax | 2 (2.2) | 0 (0) |
| Other | 1 (1.1) | 3 (2.6) |
| Erectile dysfunction and wound infection | 1 (1.1) | 0 (0) |
| Erectile dysfunction and wound pain | 1 (1.1) | 0 (0) |
| Wound infection, urinary tract infection and fistula | 1 (1.1) | 0 (0) |
| Total | 58 | 25 |
| SAE | Intervention, n (%) | |
|---|---|---|
| Urethroplasty | Urethrotomy | |
| Participants undergoing an intervention (allocated group) | (N = 98) | (N = 104) |
| Related | 0 (0) | 2 (1.9) |
| Expected | 3 (3.1) | 2 (1.9) |
| SAEs | ||
| Urinary tract infection | 3 (3.1) | 1 (1.0) |
| New urinary symptom | 1 (1.0) | 0 (0) |
| Wound infection | 1 (1.0) | 1 (1.0) |
| Wound pain | 1 (1.0) | 0 (0) |
| Haematuria | 2 (2.0) | 0 (0) |
| Readmission to hospital | 3 (3.1) | 1 (1.0) |
| Diverticular perforation | 0 (0) | 1 (1.0) |
| Death | 0 (0) | 1 (1.0) |
| Othera | 2 (2.0) | 2 (1.9) |
| Wound infection and fistula | 1 (1.0) | 0 (0) |
| Participants undergoing an intervention (treatment received) | (N = 84) | (N = 118) |
| Related | 0 (0) | 2 (1.7) |
| Expected | 3 (3.6) | 2 (1.7) |
| SAEs | ||
| Urinary tract infection | 3 (3.6) | 1 (0.8) |
| New urinary symptom | 1 (1.2) | 1 (0.8) |
| Wound infection | 1 (1.2) | 1 (0.8) |
| Wound pain | 1 (1.2) | 0 (0) |
| Haematuria | 1 (1.2) | 1 (0.8) |
| Readmission to hospital | 0 (0) | 4 (3.4) |
| Diverticular perforation | 0 (0) | 1 (0.8) |
| Deathb | 0 (0) | 1 (0.8) |
| Other | 1 (1.2) | 3 (2.5) |
| Wound infection and fistula | 1 (1.2) | 0 (0) |
| Clavien–Dindo classification | Intervention, n (%) | |
|---|---|---|
| Urethroplasty | Urethrotomy | |
| Allocated group undergoing either interventiona | (N = 98) | (N = 104) |
| Clavien classification | ||
| Grade I | 6 (6.1) | 4 (3.8) |
| Grade II | 3 (3.1) | 2 (1.9) |
| Grade III-a | 4 (4.1) | 0 (0) |
| Grade III-b | 1 (1.0) | 0 (0) |
| Grade IV | 0 (0) | 1 (1.0) |
| Grade V | 0 (0) | 1 (1.0) |
| Participants undergoing an intervention (treatment received)b | (N = 84) | (N = 118) |
| Clavien classification | ||
| Grade I | 4 (4.8) | 6 (5.1) |
| Grade II | 3 (3.6) | 2 (1.7) |
| Grade III-a | 1 (1.2) | 3 (2.5) |
| Grade III-b | 1 (1.2) | 0 (0) |
| Grade IV | 0 (0) | 1 (0.8) |
| Grade V | 0 (0) | 1 (0.8) |
| Pseudonym | Age (years) | Site | Agreed to randomisation |
|---|---|---|---|
| Archie | 34 | Greentown | Agreed |
| Alexander | 39 | Greentown | Agreed |
| Raymon | 36 | Greentown | Agreed |
| Aydan | 47 | Greentown | Agreed |
| Darren | 39 | Blacktown | Agreed |
| Jake | 31 | Blacktown | Agreed |
| James | 44 | Blacktown | Agreed |
| Terry | 48 | Goldtown | Agreed |
| Elliot | 62 | Silvertown | Agreed |
| Carl | 25 | Bronzetown | Declined |
| Miles | 70 | Blacktown | Declined |
| Asif | 26 | Blacktown | Declined |
| Richard | 45 | Blacktown | Declined |
| Taylor | 34 | Blacktown | Declined |
| Stuart | 44 | Bluetown | Declined |
| Lucas | 27 | Goldtown | Declined |
| Jeremy | 27 | Goldtown | Declined |
| Ben | 25 | Silvertown | Declined |
| Michael | 35 | Silvertown | Declined |
Mouth pain was the most common perioperative AE and was more frequent in the urethroplasty group, according to both randomised allocation and treatment received.
Serious adverse events
During the trial 22 participants were reported to have experienced at least one SAE (17 participants suffered one SAE, three participants suffered two SAEs and two participants suffered three SAEs).
Chapter 4 Qualitative substudy
Introduction
About half of all RCTs face difficulties in achieving target recruitment. 47 Surgical RCTs can be particularly challenging, as they involve clinical uncertainty about the relative impact of procedurally different options. In addition, clinicians and patients may have preferences for one type of surgery, informed by past experience and expectations, among other factors. Owing to the complex target population, necessity for multiple sites and relatively different treatment arms, it was anticipated that the OPEN trial would face recruitment challenges. A recent review of qualitative research within and alongside trials shows the range of issues that qualitative work can elucidate, including issues around trial design, conduct and processes. 48 Embedded qualitative work has been shown to have great potential in helping to improve recruitment and trial design. 49–51 Qualitative research can be used to highlight organisational barriers, reveal recruiters’ discomforts, improve information provision52,53 and to understand patients’ perspectives and decision-making. 54–57 Patient and clinician interviews have been used to evaluate trial acceptability as well as inform outcome measures. 58,59
This chapter reports on the qualitative process evaluation that was conducted as part of the OPEN trial feasibility phase. This research was conducted during the first 10 months of OPEN trial recruitment (February–December 2013). It had three objectives, to:
-
explore the factors most important to men with bulbar urethral stricture in differentiating between treatments and agreeing to trial participation
-
explore the factors most important to clinicians in recommending treatments and approaching men eligible for the trial about participation
-
contribute to the feasibility assessment by identifying potential barriers to participation and thereby informing recruitment strategy and design.
Methods
Following ethics approval (reference 12/NE/0343), the qualitative team conducted interviews with two groups of participants: (1) men eligible to participate (declined or accepted randomisation) and (2) urological practitioners (general or specialists, active recruiters or non-recruiters).
Interviews with men eligible for participation
Interviews with trial-eligible men were designed to explore their priorities in seeking surgical intervention and deciding whether or not to participate in the OPEN trial (interview schedules can be found in Appendices 2 and 3). To be eligible for the OPEN trial, and therefore for the qualitative study, men had to have a diagnosed urethral stricture and be able to undergo both endoscopic management by urethrotomy and reconstructive surgery by urethroplasty. Participating sites were encouraged to ask men if they would be willing to participate in an interview, regardless of their decision to participate in the trial. Men were asked to sign a consent form, separate from trial participation, and the site research nurse then passed the men’s contact details onto the qualitative team. All men who were willing to be interviewed were then contacted by the researcher (PW) and offered an interview either face to face or by telephone. For face-to-face interviews the researcher either travelled to the participant’s home or preferred location or offered them travel expenses to come to where the qualitative team was based. It was anticipated that interviews with men with bulbar urethral stricture may raise potentially sensitive issues, such as erectile function and urination problems. The researcher (who was male) was provided with guidance and support in how to prepare for, and manage, potentially sensitive topic areas.
Interviews with clinicians
Interviews with urologists were designed to explore clinicians’ opinions regarding the management of urethral stricture, their sense of equipoise between the trial groups and the acceptability of approaching men about being randomised (interview schedule can be found in Appendix 4). In discussion with the chief investigator and trial team, it was decided that qualitative interviews would be conducted with a broad range of urological practitioners. Criterion sampling was used to include both specialist and general urologists, including those urologists who were not recruiting to the OPEN trial. General urologists are those who perform urethrotomy but would have to refer men elsewhere to receive an urethroplasty procedure. Specialist urologists are those who can perform both procedures. To be eligible for an interview, urologists needed to be currently practising and routinely treating men with urethral stricture, but were not necessarily contributing to the OPEN trial recruitment. The sampling and recruitment of clinicians for interviewing involved a mixture of snowballing and contacting practitioners from a BAUS database. Surgeons were contacted by the researcher either by telephone or by e-mail. Potential interviewees were sent a consent form and information about qualitative substudy. All those clinicians approached were reassured of their anonymity and that their interview data would be managed independently of the OPEN trial team. When a clinician was willing to be interviewed, the researcher arranged for the consent form to be signed and the interview to be conducted either face to face or by telephone. All interviews were audio-recorded, except for one that the clinician requested not be. In this case, a full description of the interview was written by the researcher and discussed with the qualitative research team before informing the analysis.
Data management and analysis
Qualitative analysis is an interpretive process that benefits from interdisciplinary working. Throughout the qualitative substudy, results and findings were discussed with the CI and presented to PIs at the OPEN trial investigators meeting in Newcastle upon Tyne (August 2013). All interviews were audio-recorded, transcribed verbatim, checked and edited to ensure respondents’ anonymity. Analysis was conducted in accordance with the standard procedures of rigorous qualitative analysis (Rapley60) and aimed to identify, explore and refine emergent patterns. Procedures from first-generation grounded theory – coding, constant comparison, memoing61 – were used, alongside tables,62 diagrams and mapping. 63 Proprietary software (NVivo version 10; QSR International, Warrington, UK) was used to support the management and retrieval of data. 64 Data collection and analysis occurred concurrently, so that issues raised in earlier phases of fieldwork were explored in subsequent ones. Data collection and analysis were also supported by discussion in trial meetings and regular qualitative data clinic sessions, which included health professionals and social scientists from different clinical and academic backgrounds.
Results
The qualitative substudy was conducted in the first 10 months of recruitment (between February and December 2013). During this period, 38 men had agreed to randomisation at 10 different sites. The lead site had recruited 10 of the men (26% of eligible men approached at this site). The qualitative substudy conducted a total of 34 interviews during this time, 19 with men eligible for participation in the trial and 15 with clinicians.
By the end of the qualitative substudy, 25% (40/159) of the men screened for the OPEN trial had said they would be willing to be interviewed. Of these, 19 men were eligible for participation in the OPEN trial and were available for an interview. These participants had been recruited from five sites across the UK. The age of participants ranged from 25 to 70 years (median age 36 years). Table 22 shows the details of the men who were interviewed. All respondents had a diagnosed bulbar urethral stricture and had received at least one previous urethrotomy. The interviews took place between February and November 2013. Most men opted to be interviewed by telephone, which was found to be beneficial as some respondents said they were more comfortable discussing embarrassing issues by telephone.
To recruit clinicians to the substudy the researchers used (with permission) records from the BAUS database to contact 47 practising urological surgeons. Twenty clinicians responded and 15 agreed to an interview, all based at different UK hospitals. The 15 clinicians interviewed included those clinicians who were actively recruiting to the OPEN trial (n = 9), those considering involvement (n = 4) and those clinicians who were not involved (n = 2). The number of years of urological experience varied from 4 to 30. At the point of being interviewed, some clinicians were approaching men about the OPEN trial and could reflect on their experience of recruitment and how the trial was integrated with their standard treatment practices.
The qualitative findings are divided into five sections. The first focuses on the context of men’s experiences of urethral stricture and how this shaped their decision-making in regards to surgery. The second section looks at men’s accounts of either declining study participation owing to a preference for one of the procedures or accepting randomisation. The third section focuses on general urologists’ views of the OPEN trial and their expectations about recruitment practices. The fourth section deals with specialist urologists’ expectations. The final section discusses findings relevant to the organisation of standard care pathway and its alignment with trial recruitment.
Interviews with men
The interviews with men revealed a number of common themes in the experience of living with symptoms of a stricture and seeking medical treatment. The men’s accounts revealed some of the practical issues that they faced on a daily basis. Understanding their experiences provides an insight into their help-seeking and priorities in treatment decision-making.
Symptoms
Men repeatedly described their frustration at having a recurrent sense of urgency while not being able to empty their bladder. They struggled to describe the physical sensation giving rise to notions of there being a ‘blockage’ and of something ‘being wrong’. Some of the men described the sensation as ‘burning’, although for most this was ‘uncomfortable’ or a ‘dull ache’, rather than painful. Nonetheless, the sensation of a full bladder was upsetting and frustrating, causing significant distress. One described it as ‘horrible . . . it’s like a torture’ (Terry) and another noted:
Imagine like all the time needing to go to the toilet and eh, not being able to basically, that’s what it was and that was horrific.
Ben
Periods of retention were sporadic and could last for hours. Sitting in a hot bath, urethral massage or trying to relax were tactics these men tried to help themselves void. Several participants described trying to structure their work and life so they could carry out symptom management without others knowing. When symptoms occurred, it was typical to stop activities and head home. Such interruption of daily life was described as causing the most distress. Nocturia (waking up in order to pass urine) was common and caused distress because of the interruption to sleep. One participant described having not slept properly in years:
Before the operation I could be waking up anything to six or seven times in 4 hours to go to the toilet. Being constantly tired, it’s horrible, it’s so draining.
Carl
Nocturia and frequency could be managed privately at home, but during the day many of the men found that symptoms interrupted their work. Several of the men described disruption to their work or not being able to concentrate:
I am doing [ . . . ] a lot of technical work and things like, you know, I keep on going to the toilet it’s really a pain. It just, yeah, especially when I am doing very intricate things in the workplace I get a bit nervous and I get urgency to go to the toilet like it’s really [sighs and stops].
Ben
The fact that sensations of urgency could arise unexpectedly meant that men often felt anxious and uneasy. Men were particularly concerned about social embarrassment and reported a fear of being ‘caught out’. The fact that this was ‘always in the back of your mind’ illustrates the wider pervasiveness of symptoms beyond their actual occurrence.
Concealment and routines of self-management
Underpinning the general discomfort described above was the desire to conceal symptoms from others. Most of the men felt that urethral stricture was a condition that could not be shared publicly. Several men described avoiding telling even their closest family about their condition:
No, no I didn’t tell anybody. No, nobody could have noticed it not unless I let somebody know. It’s not something you want to shout about!
Miles
Taylor described how only his wife knew and that he had pretended to his children that his regular hospital visits were for knee treatments:
It’s funny you’ve got a little secret that you can’t tell anybody. Because I can sit there and talk to [ . . . ] people about a lot of health-related issues, but this is the one thing that I won’t let anybody know about and I wouldn’t talk about.
Taylor
The desire for privacy is understandable but can mean missing the potential therapeutic benefit of talking to others about their illness. This is evident in how many of the men described concealing their illness and treatment from friends or family:
I think if I told my family, they’ll just make a fuss out of things. No, I kept it quiet. [Pause] I didn’t have any support from friends and family because I didn’t inform anyone.
Alex
Management and concealment of symptoms was achieved through planning and routines. Men described maintaining the appearance of normality through planning daily activities around toilet access:
[I am] so self-conscious about it if it was the case of we’re going out to go and do the food shop I would start getting ready half hour before obviously get your shoes on and do whatever you need to do but it was a case of like first thing go to the toilet then I’d go and do something and it would be go back to the toilet and go and do something else and then go back, I would go to the toilet about five times in half an hour and go out every half hour I would have to go to the toilet again.
Carl
A number of tactics were described as being part of a routine to help voiding before going out or meeting others, such as taking a hot shower or using a catheter tube for self-dilatation. Some men also said they used tissue or cloth in the underwear to disguise urine leakage (post-micturition dribble).
Another aspect of planning and routine employed to manage symptoms was restricting fluid intake. Some men said that they preferred to feel thirsty rather than risk urgency. Ben had been living with the stricture for a relatively short amount of time and said that he was starting to avoid liquids in order to both sleep better and keep working:
I am supposed to be drinking a lot of water [according to my doctor] but recently I’ve been pretty bad but it’s only because I am absolutely sick and tired of going to the toilet at night! If I have a lot of work to do I tend to drink less so I don’t have to go, you know what I mean? Rather than take me from my work. Probably it’s not the best like in terms of, you know, my health ‘cause my doctors tell me I have to drink loads of water but, you know, sometimes it’s just not practical.
Ben
For those men who had lived with the condition for decades, tactics for managing their stricture had become second nature. Miles, who was 70 years of age, had experienced stricture symptoms most of his adult life and described developing tactics to avoid people’s offers of alcoholic drinks and having become accustomed to not drinking alcohol.
Routines of self-management allow men with urethral stricture to continue their lives without friends or colleagues having to know about their urinary problems. Most of the men were worried that others would notice the time they spent in the toilet or at a urinal. Public toilets were a particular ‘danger zone’ where urinary problems might be exposed. Bars were discussed by several of the men as somewhere they might be ‘caught out’ because of the combination of drinking and socialising:
There’s times when I’ve been out and I think ‘shit I can’t go to piss! I can’t go to the toilet! I can’t do this: I’ve got to go!’ and everyone says ‘what are you doing?’ and I say ‘oh, I’m just nipping off somewhere’ and then just go home.
Terry
Most of the participants described avoiding or limiting social interactions in order to conceal their condition, reflecting how urinary symptoms can significantly impact upon a person’s social life.
Help-seeking
Although the symptoms and concealment caused distress, most of the men said that they had delayed for some time before seeking help. Throughout the interviews it was common for the men to downplay the severity of their symptoms as ‘not life-threatening’ and say that they preferred not to ‘make a fuss’:
Obviously, it’s affecting my life in a lot of ways at the moment . . . obviously my marriage, sexually, the inconvenience, pain, and everything else that I’m going through. So apart from that you know you’ve just got to get on with things I suppose. Well, I mean it is it’s a lot but there’s a lot of people worse off than I am that’s the way I look at it.
Terry
The men interviewed said that they preferred to ‘get on with it’ and tolerate symptoms rather than seek curative treatment. This reluctance to present symptoms corresponds to previous research showing that men often delay in seeking medical treatment. 65 Other men described getting used to the symptoms and having a different sense of what was normal urination and toileting behaviour. This was often because of a gradual onset of symptoms and becoming accustomed to low flow rates:
The symptoms possibly started very more back but I never realised. ‘Cos how would you realise? You know, for never been known what is a normal passing flow and what is not normal [ . . . ] being patient or being an ordinary person? You would never know that this [was] part of some sort of symptoms.
Asif
Previous research suggests that traditional notions of male identity, such as being tough, resilient and independent, can undermine men’s health-seeking and acknowledgement of a problem. 65 In the case of urethral stricture, such delays are likely to be exacerbated by the concealment and self-management of symptoms.
Men’s management of urethral stricture is inextricably related to the desire to conceal symptoms from others through routines of self-management. The tendency to hide urinary problems raises issues not just of unseen emotional burdens on these men, but also of significant delays in seeking medical help and advice. Many of the men interviewed described seeking medical advice only once self-management and concealment were no longer tenable. As discussed in the following section, this has implications for men’s decision-making and treatment preferences.
Preference for urethrotomy
Men were supportive of the OPEN trial and wanted to be able to contribute to research for the benefit of future patients. This type of altruism and wanting to ‘give back’ is common in patients’ accounts of trial participation. 57 Despite wanting to help, 10 of the men we interviewed declined randomisation due to an over-riding preference for one of the procedures; all had prior experience of urethrotomy.
Those men who expressed a preference for urethrotomy said it was to avoid wearing a catheter, taking time off work or undergoing the ‘serious’ operation necessary for urethroplasty:
You’re going to be in hospital for a couple of days you have the catheter in for over 10 days or what not. It’s kind of, no it’s freaked me out a little bit. I’m quite happy with urethrotomy.
Carl
The shorter recovery period and minimum disruption to work and social life were typical reasons given for opting to have a repeat urethrotomy. The desire to conceal their condition from others contributed to the preference for fast treatment with minimum recovery time.
Most men perceived a trade-off between repeated treatments (urethrotomy) and the possibility of a permanent solution (urethroplasty). Men who chose to have an urethrotomy sometimes included the possibility of further treatment within their decision-making. Although urethral stricture is a burden on these men’s lives, it is relatively benign, meaning that men’s decision-making can concern long-term management rather than seeking a curative solution. This means that these men could reasonably opt for the ‘short-term solution’ of urethrotomy and potentially delay curative treatments. As one man outlined, ‘The operation I’ve already had, I’m quite happy with that until it’s really necessary to move on’. Delaying what they saw as a more serious operation (urethroplasty) until ‘moving on’ is necessary or unavoidable was a common justification for having a preference for urethrotomy:
I don’t think I could go through that operation unless anything drastic happened [yeah] where I really feel, you know, the pain was getting too much.
Richard
Men who declined trial participation and opted to have a repeat urethrotomy said they were willing to risk having only short-term relief from their stricture symptoms rather than commit to the time and recovery trajectory of the more complex urethroplasty. The fact that men would be able to have a urethroplasty at a later date was an important aspect of choosing to have a repeat urethrotomy:
Nobody really fancies surgery but [the clinician] says I should have [urethroplasty] done and I said ‘well I will next time and I will make the time for it’. I’ll have to because if you add up all the time I’ve had for [urethrotomy] that I’ve lost I could have been sorted by now.
Raymond
Preference for urethroplasty
Other interviewees declined randomisation because of an over-riding preference for urethroplasty. The common account of this decision was seeking a curative solution because the recurring symptoms, following a previous urethrotomy, were no longer tolerable. These men felt unwilling to accept the possibility of being randomised to a repeat urethrotomy. As one man explained, ‘but it’s like I’ve already had it [urethrotomy] done, you don’t want to go through that again’ (Jeremy). There was no threshold number of previous urethrotomies that shaped this decision. Some men were unwilling to have a second whereas others were willing to receive a third or fourth.
The need to travel and referral to specialist sites were key factors in a preference for urethroplasty. Some men described that they had already actively sought an alternative to urethrotomy at the point when they were approached about the OPEN trial. They felt that they had already made a practical commitment to reconstructive surgery by seeking referral to a specialist and, in some cases, already arranging for time off work for the recovery period. These men saw themselves as being at a point at which urethrotomy was not an acceptable treatment option and this could be understood as being past a point of equipoise:
I don’t see the sense in sitting here in 6 months’ time going in for regular dilation because, you know, there has to come an end to it somewhere. So that’s the route I went down. And I said [to the consultant], ‘look you know, I’m very happy to help in any way that I can [with the trial] but eh I’d rather go for the sort of bigger op on the basis that there’s a good chance that will sort it out and make life more comfortable for a longer period of time’. And that’s where I’m at.
Elliot
Men’s accounts of treatment preferences often included aspects of temporality in their decision-making. This can also be seen in the interviews with men who agreed to be randomised.
Accepting randomisation
The nine men interviewed who had accepted randomisation described being at a point at which the differences in cost and benefits of each treatment were negligible. Three of the men expressed a weak preference but were still willing to ‘help out’. The other six said that they were undecided as to which treatment would be best for them at that point in time. These men can be understood as being at a point of equipoise as they were fully informed and had balanced the comparative costs and benefits of the two alternative treatments. The following quote is an example of being equally poised in the desires for a shorter recovery time and the possibility of a curative solution:
[With urethroplasty] I’m not so worried about the catheter. I would take the time off and just sit that out [ . . . ] But it’s, obviously, [worrying] that you will make a full recovery afterwards and you won’t be having to look after your wounds forever, essentially. That’s the worry on that. [Consultant] got a very good reputation for this sort of thing. So, it’s not this actual procedure that bothers me, it’s the recovery afterwards and affecting your ability in later life. [ . . . ] As for concerns about the urethrotomy, my main concern with that is that I don’t want to keep having that every 2 years. It was a relatively painless operation. I was in and out during the same day. But I don’t want to keep going back every 2 years or so to have that repeatedly done.
Archie
This man decided to have his treatment randomised and is a good example of a point of equipoise within the OPEN trial. Having had two previous urethrotomies, he was willing to have another but also ready to try the more invasive alternative. Archie describes being worried both about recovering from urethroplasty and needing repeat surgery following urethrotomy, which are the uncertainties underpinning the OPEN trial. This balance of factors illustrates the necessary uncertainty for accepting randomisation. It is also the uncertainty that clinicians needed to allow for in order to recruit to the OPEN trial.
The relevance of being at the right point in time could be seen in the accounts of men who accepted randomisation. For example, Jake went on to say that if there was to be a ‘next time’, he would no longer be indifferent:
Would you feel disappointed at all if you were randomised to the urethrotomy?
I’m not disappointed at this stage, no. I think, obviously, the next time, I would probably be heavily in favour of the other.
The recurrent theme of temporality for decision-making and preferences suggests that there is a particular window of opportunity in which men with recurrent bulbar urethral stricture are in equipoise and willing to accept randomisation.
Throughout men’s accounts there was a common theme of a perceived trade-off between potentially worsening symptoms and commitment to ‘serious surgery’. Men balanced the immediate inconvenience of a long recovery period with better chances of a curative solution. Table 23 illustrates how treatment decisions were closely related to men’s perception of the severity and manageability of their symptoms. Those men with an over-riding preference understood their own urethral stricture symptoms to be at a particular point: either too slight to consider a serious operation or too severe not to.
| Decision | Preference | Symptoms | Operation |
|---|---|---|---|
| Declined randomisation | Preference for urethrotomy | Symptom recurrence and severity is tolerable | Symptom recurrence sufficiently tolerable to not want to endure serious operation and recovery time |
| Preference for urethroplasty | Symptom recurrence and severity no longer tolerable. Patient desires a permanent solution | Desire for long-term solution over-rides immediate symptom relief. Unwilling to risk further recurrence | |
| Accepted randomisation | No preference | Symptoms tolerable but considering serious operation | Willing to commit to recovery time. An additional repeated urethrotomy is also acceptable |
The results highlight how the decision to accept or decline randomisation was made in the context of an overall trajectory of treatment and worsening symptoms. Generally, the longer a man has had urethral stricture, the more likely it is that his symptoms will return and the more willing he will be to commit the time necessary for the curative solution anticipated from urethroplasty. Men who felt that their symptoms were tolerable were less likely to commit to the recovery and more likely to opt for urethrotomy. There is a window within men’s treatment pathway in which they are willing to receive either treatment. Recruiters could allow for uncertainty by answering concerns about the acceptability of symptom recurrence and the commitment needed for reconstructive surgery.
Clinician interviews
To place these interviews in context, it is important to note that there are two types of clinicians participating in the trial: specialist urologists and general urologists. Specialist urological surgeons are able to offer both of the OPEN trial treatment options as they are trained to carry out urethroplasty, whereas general urologists are able to deliver only urethrotomy and have to refer patients to a specialist if they feel urethroplasty is required. Regardless of their participation in the trial, the clinicians who were interviewed were generally supportive of OPEN trial, with one exception. The exception, a general urologist who asked not to be audio-recorded, said that they felt that the OPEN trial was a promotion of urethroplasty and therefore not to the benefit of general urologists. All other clinicians supported the OPEN trial and felt that there existed a genuine uncertainty in the management of urethral stricture patients:
We know that urethroplasty lasts for quite a while but we don’t know really how they compare in the same patients or group, because classically they’ve been different lots of patients.
Specialist urologist 3
A less experienced specialist had decided not to participate in the OPEN trial because it would mean reducing the opportunity to practise urethroplasty and potentially having their ‘learning curve’ contained within the study results:
The last thing I want, from a personal perspective, is to see a great little stricture and think ‘perfect, that will do nicely’ only to have him randomised to a [urethrotomy] – do you see what I mean? . . . Obviously we all support research but I don’t think [we are a] busy centre and do I want my result – my learning curve results in a trial?
Specialist urologist 3
Despite general support for the study, when asked about their expectations or experience of recruiting to the OPEN trial, most clinicians felt that it already was, or would be, difficult. Some of the general urologists felt concerned that they would see only a small number of eligible patients at their clinic. However, the most prominent concern about recruitment was dealing with men’s preferences.
General urologists
Most general urologists anticipated that eligible patients would prefer urethrotomy and that this preference would be a barrier to recruitment. Their account focused on the ‘easy’ option that patients would ‘obviously’ prefer:
Most patients are going to opt for a urethrotomy and not a urethroplasty but you can either have daycase operation and go home with a catheter for a day or two or you can have a major procedure and be in hospital for a few days and I think it’s just going to be hard, why would I want to be randomised? I’d rather just have the easy one.
General urologist 1
These expectations were related to ideas about the average types of eligible patient. General urologists described men with recurrent stricture as being older or with relatively minor symptoms. These men were typically treated by repeated urethrotomies and self-management using the adjunctive technique of intermittent self-dilatation. They felt that putting these patients forward for randomisation would be challenging:
I think the work [of the OPEN trial] needs doing, it’s a good study to do. I think we will have a few issues with people opting to have an operation that we may not have recommended in the normal situation.
General urologist 3
Although eligible, general urologists felt that randomising these patients proved, or would prove, difficult when at odds with their routine clinical practice. This difficulty can be tied to the clinician’s feeling around the severity of the problem:
If somebody has a very simple urethral stricture and then you will think oh why should I subject this person to an open procedure rather than just an optical urethrotomy.
General urologist 4
The following quote also suggests a blurred line between what the ‘old guys’ want and what the general urologist is comfortable offering:
There are few guys, old guys, who really don’t want to have an urethroplasty that keep getting urethrotomy. You know most of them, after I have seen them, will go with the urethrotomy and then send him home generally.
General urologist 5
The perceived expectations of the ‘older patients’ or those with ‘relatively minor symptoms’ underlie the clinician’s temptation to be selective in identifying potential participants:
I think we do treat the different age groups slightly differently so the young guys are more likely to go for a urethroplasty [ . . . ] whereas your elderly guy, you’re trying to avoid operations, [ . . . ] I think there is a lot of individual basis that we are going to make these decisions on.
General urologist 3
Uneven representation of the treatment arms can be seen to be an extension of what the clinicians felt their patients wanted or needed. The perceived expectation of the ‘typical’ patient with recurrent stricture led to a tendency to recommend urethrotomy:
Patients who would like to take the chance [with urethroplasty] if you tell them that with the urethrotomy there is a certain chance that you’ll be fine you won’t need the reconstruction so they might go for that.
General urologist 1
The findings therefore highlight a preference, a potential source of selection bias among general urologists (Table 24). However, it is important to note how this preference is underpinned by consideration of patients who tend to be older or with relatively minor symptoms.
| Clinician | Patient population | Clinicians’ expectation | Recruitment practice |
|---|---|---|---|
| General urologist | Older, history of self-management | Men will opt for urethrotomy | Older patients excluded, reluctance to refer |
| Specialist urologist | Younger, complex referrals | Men will opt for urethroplasty | Reluctance to suggest repeat urethrotomy |
Specialist urological surgeons
Specialist urologists anticipated the opposite: that few patients would be willing to consider another urethrotomy and that over-riding preference for urethroplasty would be a barrier to recruitment (see Table 24):
I would imagine for somebody who’s had two urethrotomies, of average – of typical age, probably 75% of them definitely are happy to proceed to the urethroplasty.
Specialist urologist 5
Specialists see a large proportion of referrals with either severe or complicated strictures referred specifically with the intention of discussing urethroplasty as a treatment option:
One of [my] concerns with the OPEN trial is that the guys I’m seeing are generally those who have been referred to me with a view to doing an urethroplasty.
Specialist urologist 1
Those men who had received such referrals found it hard ‘selling’ the trial to patients and felt compelled to guide them towards urethroplasty, especially patients with severe or complex strictures. Here a specialist describes an encounter with a man who has been tolerating severe symptoms:
He said ‘oh that’s about normal!’ I said, ‘Well if I told you that was less than 10% of the flow of a normal person and that you’re leaving behind more than half a bladder full of urine, how would you react to that?’ And he said, ‘oh that’s terrible!’ I said ‘Yes, well you really should have an [urethroplasty] operation!’
Specialist urologist 2
In this case, despite the patient’s eligibility and uncertainty, the surgeon could not remain neutral and felt compelled to recommend urethroplasty. Another specialist outlined how, with some men, he felt he could not offer the trial:
I think the hardest thing which I find is to er, try and explain, and get someone whose had say five urethrotomies, whose chances of a urethrotomy actually working is zero . . . and then trying to get him to consider having a urethrotomy done when it’s not something that I would advise to a friend or a relative . . . I find it very difficult and in fact I don’t really bother trying to get them into the OPEN trial.
Specialist urologist 3
Such accounts are evidence of a lack of clinical equipoise at the limits of the eligibility criteria. 53 Many specialists described difficulty in staying neutral. Even if they would not explicitly recommend urethroplasty, they reported producing language and terminology representing treatment arms unevenly:
If you say to them you can have a more complicated operation you’ve got a 95% chance of being cured versus we can keep doing this [urethrotomy] every couple of years and you’ll end up urethral cripple, most of them will take the option [of urethroplasty].
Specialist urologist 6
The eligibility criteria for the OPEN trial had no upper age limit. Neither was the severity of the stricture symptoms a reason for exclusion. However, interviews revealed these factors as potential grounds for selectivity. Previous nested qualitative studies have highlighted clinician ‘selection bias’ as a distinct barrier to recruitment. 53 Similarly, we found that, despite supporting the trial, general and specialist urological surgeons found, or felt that they would find, discussing the trial with certain types of patient a struggle, or they struggled to represent the arms of the trial evenly. It is important to note that these differences in clinicians’ expectations of men’s preferences reflect their relative position within the overall organisation of care for urethral stricture.
The clinical organisation of referrals
Interview data from both clinicians and men were used to map the standard care pathways for bulbar stricture patients in the UK. Figure 11 is a simplistic representation of a standard patient pathway to treatment and the two potential points of recruitment. Typically, a man will present his symptoms to a general practitioner (GP) before being referred to a local urology clinic. Most men will be initially treated at a general urology clinic and receive their first urethrotomy. If the man’s stricture symptoms return, he tends to return to the same clinic, either directly or through his GP. At this point, the man is now eligible to receive either a repeat urethrotomy or a reconstructive urethroplasty, making him potentially eligible for inclusion in the OPEN trial. In standard practice, the urologist can either recommend another repeat urethrotomy or refer the man to a specialist to discuss reconstructive options. There is no precedent for how many repeated urethrotomies a man should have had prior to referral for urethroplasty; indeed, this is part of the uncertainty about how to manage the condition. If the man is interested in urethroplasty, or the clinician feels that the patient’s case is severe enough, the clinician may recommend referral to a specialist centre to discuss urethroplasty.
FIGURE 11.
Different points of recruitment to the OPEN trial.
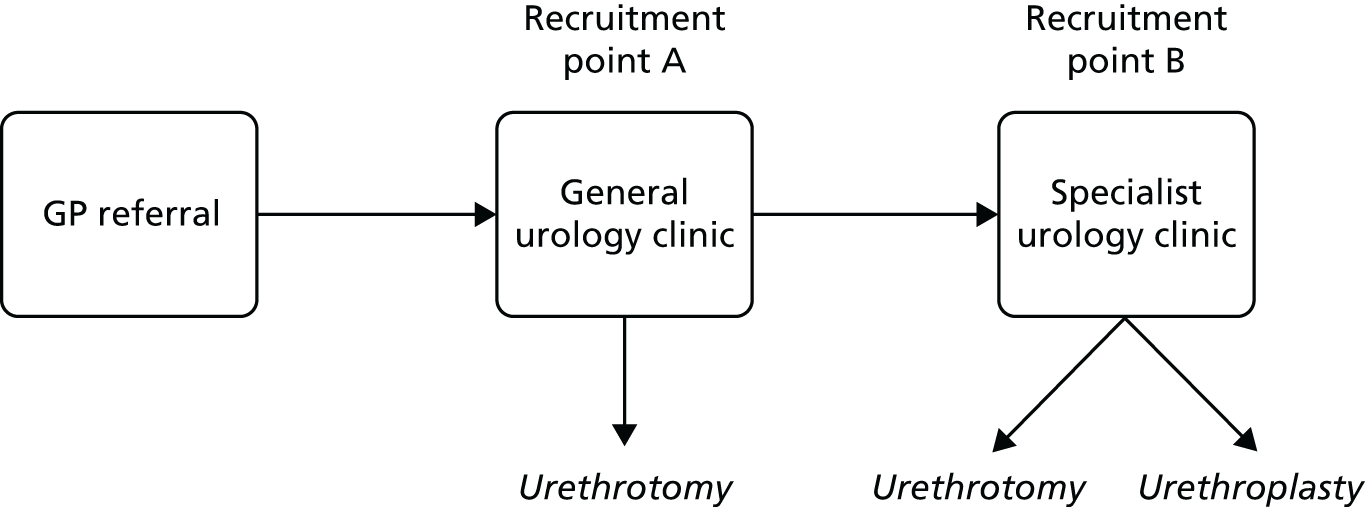
In practice, this means that younger patients and more complex cases tend to be referred to specialists more readily than older patients or those with relatively simple strictures:
You know the 18-, 20-, 30-year-olds, it just seems to me that consultants around the region acknowledge the fact that they’re facing a lifetime of self-catheterisation and they tend to get referred younger, whereas the people in the seventies or what have you, may well get pushed towards intermittent self-dilatation and referred to me only when they’ve had complications.
Specialist urologist 5
As outlined above, specialist urologists felt reluctant to randomise patients whose symptoms were severe, whereas general urologists felt that it was difficult to refer patients whose symptoms were manageable with a repeat urethrotomy. These differing perspectives correspond with the types of stricture patient they typically treat. General urologists see patients with moderate symptoms, whereas specialists see more complex and severe cases. In this way, the difficulty recruiters have in achieving clinical equipoise is not adequately explained as individual bias, but is partly a result of the relative positions of general and specialist urologists in the organisation and division of urethral stricture care.
The differences in clinicians’ expectations of patient preferences reflect their relative position within the overall organisation of care for urethral stricture. Intersite referrals were a key factor and underpinned the expectations of men’s willingness to be randomised. In particular, there was concern that men who were approached post referral would be expecting to discuss urethroplasty and therefore less willing to be randomised. Echoing some of the interviews with men, a specialist urologist said that speaking to men about randomisation would be difficult where the man was already anticipating discussion of urethroplasty, as ‘a lot of the men are already primed’ (specialist urologist 3). Specialists were concerned that men who had already been told by the referring clinician that they would be having urethroplasty would make recruitment harder:
The referring urologist says, ‘Oh, what you need is an urethroplasty, I’ll send you to [consultant’s name]’. And then I try and talk them back into randomisation! It’ll be more difficult for us. If they come to me [without preconceptions] it’ll be easier for me . . . what you need really [is] the guy who’s not quite sure what to do.
Specialist urologist 3
A few of the specialists said that they had spoken to or were planning to speak with referring centres and to try and involve them in the recruitment process and in keeping patients open to both procedures. One of the clinicians outlined that this was more feasible when there was already a good network of communication between urological sites. Related to this, a few urologists expressed concern about having to refer patients to consultants whom they did not know personally. One remarked that, had trial recruitment involved referring a patient to a clinician they did not know, they would not have agreed to become a recruiting centre.
When asked in the interview about the referral process, many of the specialist urologists said that men were not referred frequently or early enough. Some complained that the men who were referred to them from general clinics were severe or complex cases and felt that the men may have benefited from an urethroplasty sooner. On the other hand, some general urologists reported that the patients often wanted to be treated simply and locally if possible:
You know you’re talking about a 2.5-hour journey each way, you know. Plus, if it’s a consultation and surgery, that’s two stages. Some men will go for it and some won’t.
General urologist 2
Other general urologists admitted that they may try and ‘hold on to’ a patient if they could reasonably be treated locally. They were concerned that early referral, and therefore trial participation, might not be in the interest of their patients who wanted local care. General urologists also felt that referrals could potentially undermine recruitment. This is because patients would have to agree to potentially travel between sites before they could agree to be randomised. This would be particularly burdensome for patients when the nearest specialist was some distance away:
You tell [men] that in this arm of the trial, I mean this part, will be done locally but if you, for the reconstruction you might have to go to another hospital, and then that might influence. They might say ‘OK I don’t want to take part in the trial because I’d rather stay local’.
General urologist 4
Relatedly, another issue raised by some was the need for men to take time off work and inform friends and family. As discussed earlier, many men with urethral stricture prefer to conceal and self-manage the condition. Several of the clinicians anticipated that it might not be easy for patients to agree to potentially take time off work and add to the recovery time, as this would usually require some personal preparation.
Discussion
There is a growing body of literature concerning the use of qualitative studies in order to inform RCT recruitment strategies. Although the majority of this literature focuses on the activities of recruiters and the need to improve information provision to patients, qualitative research can also provide valuable insights into the experiences and opinions of the eligible patients. Patient preferences are often the primary reason for declining trial participation,57,66–68 although they are not always well understood and do not always receive detailed attention. For the purposes of this study, patient preferences were conceived as being complex and trial specific. 2 Living with bulbar urethral stricture can be a distressing experience for men. The condition can interrupt daily life, leaving them feeling frustrated, anxious and uneasy. They can attempt, through generating plans and formulating routines, to conceal symptoms from others. Although the symptoms and the concealment can cause significant distress, most of the men had delayed seeking help. Many described seeking medical advice only once symptoms, attempts at self-management and concealment were no longer tenable. Importantly, any discussion about treatment options, including taking part in the OPEN trial, happens within this broader trajectory of living with stricture.
As with many trials, patients demonstrated conditional altruism,57,69 in that they were supportive of the idea of the OPEN trial. However, given the context of an evolving symptom experience that leads to a decision to seek help, and a prior experience of at least one urethrotomy, some men had developed clear preferences for a specific treatment, which meant that they felt they could not take part. Those men with a preference for urethrotomy felt that symptom recurrence was, at this point in time, sufficiently tolerable to not want to endure what they saw as a serious operation with longer recovery time. They were also aware that in the future, if or when necessary, they could always opt for urethroplasty. In contrast, those men with a preference for urethroplasty felt that symptom recurrence was no longer tolerable and they wanted a more permanent solution, so could not risk randomisation to repeat urethrotomy. Notably, they felt that they had already made a practical commitment to reconstructive surgery by being referred to a specialist. Finally, for those men who were willing to be randomised, the difference in cost and benefits of each treatment were relatively negligible at this point in time.
Patient interviews were particularly important in understanding men’s subjective experiences and understanding of the care pathways and recruitment process. Previous research has highlighted the importance of understanding the patient pathways for recruitment50,70 and focused on understanding and improving the congruence of trial design and standard care pathways. 54 In the feasibility stage of a multicentre RCT trial, it is important to describe and evaluate the current care pathways, in order to inform early design decisions and practical choices. There is clearly a specific point in the trajectory of the experience of symptom recurrence, severity and help-seeking when men are willing to consider randomisation. Going forward, the substudy highlighted that the OPEN trial needed to maximise recruitment discussions with men at this point in their trajectories. Those discussions can be initiated only through a clinician’s willingness to approach eligible men about trial participation. The OPEN trial sought to work with clinicians to embed those recruitment discussions within specific points in the men’s treatment pathways. The standard treatment pathway for the majority of men eligible for trial participation is referral for an initial visit to a general urology clinic. At the general urology clinic, they discuss the options of receiving a repeat urethrotomy or a reconstructive urethroplasty with a general urologist. If men opt for a reconstructive urethroplasty they will then be referred to a specialist urology clinic for discussion with a specialist urologist. At the time of conducting the qualitative substudy, February–December 2013, the majority of recruitment discussions were happening at specialist centres initiated by specialist clinicians.
Multicentred trials have often encountered difficulty in achieving consistent practice across all sites. 71 Previous work has shown that qualitative research can be valuable for exploring the acceptability of a trial among clinicians. 49 Barriers need to be identified early, so they can be removed or bypassed. A number of studies have reported qualitative research embedded within RCTs as a recruitment intervention. 53,72,73 Elliott et al. 50 have analysed recruiter and patient interactions in order to understand how triallists are presenting information to patients, including the description of the trial arms and the randomisation process. 50 They suggest that recruitment is a fragile process and that clinicians may feel uncomfortable approaching patients about trial participation and therefore require training. 72
General and specialist clinicians were supportive of the OPEN trial. They felt that there was genuine uncertainty about how to manage men with bulbar urethral stricture. However, they were concerned that recruitment could be difficult. Each group of clinicians outlined a specific set of expectations about how men would react to the recruitment discussion. General urologists felt that eligible men would be more likely to want to have a repeat urethrotomy, to be treated simply and locally. They reported that they themselves might be reluctant to recommend randomisation if the man had had only one previous operation, was old or would need to travel far for an urethroplasty. In contrast, specialist urologists felt that eligible men seeing them would be more likely to want to have a urethroplasty. They reported that they personally might be reluctant to recommend randomisation if the man had complex or severe strictures. They also noted, as did some of the men in their interviews, that a man’s preference for urethroplasty is often already established prior to consulting a specialist. The men are primed before arrival: they know that they are being referred to the specialist centre specifically in order to further discuss one option, namely urethroplasty.
Shaping trial recruitment and design
While the qualitative substudy was being conducted during the first 10 months of recruitment, the OPEN trial was not recruiting to target. The findings of the qualitative work suggested specific barriers to recruitment. Clearly, some men have strong preferences, and when recruitment discussion took place with them they declined randomisation. However, some men who took part in recruitment discussions were at a specific point in the trajectory of their experience of symptom recurrence, severity and help-seeking, at which point they were willing to accept randomisation. Given the differing expectations and expressions of reluctance of approaching some men, which both general and specialist urologists raised, some men potentially eligible for the trial may not have had the opportunity to discuss potential trial participation at all.
Following the report of the substudy, the qualitative team collaborated with the chief investigator and Trial Management Group to develop implementation strategies to increase both the number and location of recruitment discussions. It was agreed that the findings would be used to develop an overall approach, as well as relevant tools to help with the initiation of new sites, the training of recruiters and the maintenance of good, consistent recruitment practices. We mapped the key problems we had identified against Powell et al. ’s74 compilation of strategies for implementing clinical innovations in health and mental health care (Table 25). This helped to inform the detail, direction and content of the strategies we suggested to shape the trial recruitment and design going forward. It was important that all discussions and support tools used with clinicians reflect the types of concerns that men were likely to have, as well as the discomfort experienced by specialists and general clinicians in approaching different types of men.
| Key problem | Implementation strategies |
|---|---|
| Clinicians have potential to be selective about the men who they decide to approach | Education strategies: develop effective educational materials; distribute educational materials; conduct educational outreach visits |
| Men have potential to develop strong treatment preference through referral process to specialist centres |
Plan strategies: build a coalition Restructure strategies: change service sites Education strategies: prepare patients/consumers to be active participants |
Enabling more recruitment discussions
Given that recruiting clinicians can be selective about the men to whom they introduce the trial, it was felt that new and existing recruiters should be provided with support that emphasises the need to offer trial participation to all potentially eligible men. We recommended that, within the OPEN trial, support should include examples of men with different treatment preferences. This should also be sensitive to the different perspectives and biases of specialist and general urologists. Thus, support for general urologists should emphasise how it can be appropriate to refer men to a specialist, even if they are older and their symptoms are relatively minor. Conversely, support for specialist urologists should emphasise how repeat urethrotomy can also be appropriate for younger men or those men with severe or frequently recurring urethral stricture.
In part, the focus needed to be on getting clinicians to explicitly reflect on, and so challenge, their expectations and feeling of discomfort about approaching some potentially eligible men. For example, to encourage recruiters to approach more potentially eligible men, the findings were used to create several illustrative vignettes of urethral stricture patients (see Appendix 5). These reflected the types of men who were seen, their concerns, previous treatment history and severity of symptoms. Early versions of the vignettes were designed to look like GP referral letters; however, feedback from clinicians suggested that these were too lengthy and not like typical referrals. Incorporating this feedback, these were revised to be short case studies with explanations of their learning outcome for recruiting clinicians. A video was also made to introduce and promote the OPEN trial. It outlined the trial and then depicted two clinical scenarios, which simulated recruitment discussions around the available treatment options and study participation. The video was publicly available and was designed to target new and potential recruiting clinicians, as well as to be a resource for men who had been informed about the study and were considering trial participation.
Enabling different recruitment discussions
Another focus was on expanding the points at which eligible men could be initially informed about and recruited to the OPEN trial. As noted above, at the time of conducting the qualitative substudy, the majority of recruitment discussion was happening at specialist centres and was undertaken by specialist clinicians. However, findings strongly suggested that recruitment discussions at specialist centres could be too late in the treatment pathway for some men, given that they could be entering those discussions already primed with an expectation that the focus of the consultation would be on one option, namely urethroplasty. However, recruitment discussions with men eligible to participate in the trial could potentially occur at two distinct points in the standard treatment pathway, at both general centres and specialist centres.
One strategy that was suggested was to focus on changing the emphasis of discussions that men have at general sites, prior to referral to specialist sites. When possible, specialist sites and the OPEN trial team could directly engage with their colleagues at general sites to reframe elements of the discussions general clinicians have with men prior to referral. Rather than suggest that the focus of the referral would be to only discuss and then receive urethroplasty, they could outline that both options would be considered. A related strategy was also suggested, to approach men earlier in the pathway by increasing the number of general urology centres participating in the OPEN trial. This could then improve access to eligible men who are yet to develop a strong preference for either procedure. However, a disadvantage of trying to approach men at general clinics is that they would see far fewer men potentially eligible for the OPEN trial than specialist sites. This meant that more general sites would be needed to recruit the same number of participants as specialist sites, albeit with the potential for a better conversion of eligible patients accepting randomisation.
Chapter 5 Health economic evaluation
Introduction
This chapter describes the economic evaluation undertaken as part of the OPEN trial, aiming to provide an analysis of relative cost-effectiveness of open urethroplasty (experimental) against endoscopic urethrotomy (control), for men with recurrent bulbar urethral stricture. It comprises a within-trial cost-effectiveness analysis and a 10-year Markov model. The within-trial analysis examined the relative efficiency of open urethroplasty compared with endoscopic urethrotomy over a 24-month period after randomisation. A sensitivity analysis to assess the robustness of the primary findings compares the interventions over a 24-month period after initial surgery. As open urethroplasty was a priori expected to be both more effective and more costly than endoscopic urethrotomy and its benefits may persist beyond 24 months, Markov modelling was conducted with a 10-year time horizon to compare the costs, QALYs gained and incremental cost per QALY of the interventions.
The study adopted the viewpoint of both the NHS and the patient and collected resource use data, which included the costs of treatments and the use of primary and secondary NHS services, as well as participants’ out-of-pocket expenses relating to the condition and its management. All costs and QALYs were appropriately discounted using the recommended discount rate (3.5% per annum) at the time of the data collection. 75 A completed Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist is reported in Appendix 6.
Within-trial analysis
Information on NHS resources used to deliver the interventions was collected on the initial intervention CRF. Effectiveness of the intervention was measured by participant completion of the EQ-5D-5L at a number of time points as part of the USS-PROM in the trial questionnaire. Participant’ use of health services and out-of-pocket costs during the follow-up period were collected from a bespoke participant costs questionnaire (PCQ). Costs collected from different time points were all converted to the same price year (2017) using the Consumer Price Index. 76
Results
Analysis of resource use and cost
Cost of the intervention
Costs associated with each of the two trial interventions were micro-costed and estimated on a per-patient basis. The resource use of the interventions included staff time and drugs associated with the performance of the procedures, single-use and reusable equipment utilised during each intervention and duration of use of theatre suites. Information on principal clinical staff present at the procedures and their job title, surgery time and length of hospital stay was obtained from the CRF. Information on additional staff required to be present during the procedures and the drugs used was obtained from study clinicians. Details of single use and reusable equipment required during the procedures and their costs were provided by NHS operating suite staff at the main site (Freeman Hospital, Newcastle upon Tyne, UK). Study clinicians were consulted throughout the micro-costing exercise.
Following the initial surgery, a further urethral catheter removal appointment of variable duration was required for every participant. This did not usually require a hospital bed and it was assumed that NHS resource use for this activity would be the same for both trial groups. The NHS resource use of catheter removal was assumed to be 10 minutes of a qualified nurse’s time in a standard treatment room. This was used to derive a uniform cost for every participant who had a catheter removal. Three participants were recorded as having an overnight stay for the catheter removal, the cost of which was added to those participants’ overall hospital stay.
Multiple imputation was performed to complete any missing data on length of hospital stay and length of operation theatre time used in the sensitivity analysis. The resource use for each trial intervention is shown in Table 26.
| Resource type | Intervention | |||
|---|---|---|---|---|
| Urethroplasty | Urethrotomy | |||
| Mean (SD) | n | Mean (SD) | n | |
| Base-case analysis | ||||
| Theatre suite time (minutes) | 159 (84.3) | 77 | 46 (47) | 85 |
| Length of hospital stay (days) | 1.34 (0.95) | 89 | 0.52 (1) | 97 |
| Sensitivity analysis with data imputation | ||||
| Theatre suite time (minutes) | 158 (81.9) | 93 | 57 (56) | 104 |
| Length of hospital stay (days) | 1.32 (0.93) | 93 | 0.55 (1) | 104 |
Unit costs associated with each resource use were summed to calculate total cost to the NHS. The unit costs were obtained from the following sources: the standard time costs of different grades of staff were based on Unit Cost of Health and Social Care 2017 documentation from Personal Social Services Research Unit;77 theatre suite costs were based on information from the Information Services Division, NHS Scotland;78 and the costs of drugs used during the procedure was obtained from manufacturers’ price lists from the NHS Dictionary of Medicines and Devices database. 79
As shown in Table 27, in general, urethroplasty had a higher procedure cost than urethrotomy, both in the base-case analysis and in the sensitivity analysis with multiple imputations. This higher cost was mainly driven by longer operating time and hospital stay for urethroplasty relative to urethrotomy.
| Resource type | Intervention | |||
|---|---|---|---|---|
| Urethroplasty | Urethrotomy | |||
| Mean (SD) (£) | n | Mean (SD) (£) | n | |
| Base-case analysis | ||||
| Theatre suite cost | 3096.09 (1639.57) | 77 | 890.35 (924.07) | 85 |
| Hospital stay cost | 423.42 (274.97) | 89 | 219.27 (157.22) | 97 |
| Total NHS intervention cost | 4892.83 (2355.48) | 74 | 1540.50 (1300.77) | 83 |
| Total NHS intervention cost including those men who did not receive surgery | 4068.20 (2827.62) | 89 | 1405.08 (1316.82) | 91 |
| Sensitivity analysis with data imputation | ||||
| Theatre suite cost | 3070.55 (1592.56) | 93 | 1106.49 (1093.39) | 104 |
| Hospital stay cost | 418.40 (269.98) | 93 | 225.16 (153.37) | 104 |
| Total NHS intervention cost | 4769.59 (2283.16) | 93 | 1831.87 (1537.73) | 104 |
| Total NHS intervention cost including those men who did not receive surgery | 4107.15 (2688.54) | 108 | 1701.02 (1555.24) | 112 |
Cost of health-care service use during follow-up
Data on resource use during the follow-up period were collected retrospectively through the bespoke PCQ. The PCQ had two parts. Part A administered at 6, 12, 18 and 24 months after initial surgery recorded information on the level of the usage of the health services and the costs of any other self-purchased health care required to manage the condition. Part B, administered once only, at 6 months after initial surgery, collected information on the time and travel costs the participant incurred while accessing health services. The role of part B was to calculate the unit costs to the participant of attending each type of health service and was combined with data from part A to derive total costs to the participant. The use of health services recorded in part A included primary (GP and nurse visits) and secondary care (outpatient visits and inpatient stays) resource use. Unit costs associated with the use of these health-care services were from the Personal Social Services Research Unit. 77 Owing to the relatively small number of patients reporting use of health-care services related to urethral stricture during the follow-up period, it was assumed that participants who did not complete a questionnaire had no urethral stricture-related health service use during the follow-up period.
Measures of resource use were combined with unit costs to provide an estimate of the total cost for each participant. All costs were adjusted to the price year 2017 and those costs that occurred in the second follow-up year were discounted by 3.5%. Details of the services used and their costs are reported in Table 28.
| NHS resource use and costs during follow-up | Intervention | |||
|---|---|---|---|---|
| Urethroplasty (N = 108) | Urethrotomy (N = 112) | |||
| Resource use, mean (SD) | Cost, mean (SD) | Resource use, mean (SD) | Cost, mean (SD) | |
| Inpatient admission | 0.06 (0.34) | 43.95 (252.67) | 0.21 (0.72) | 268.91 (2270.90) |
| Outpatient visit | 0.38 (1.02) | 40.29 (108.41) | 0.86 (1.74) | 91.18 (184.37) |
| GP visit | 0.80 (2.70) | 29.28 (100.61) | 1.04 (2.18) | 36.45 (77.22) |
| Nurse visit | 0.16 (0.61) | 1.38 (5.50) | 0.18 (0.56) | 1.92 (6.01) |
| Total NHS costs | NA | 114.90 (375.86) | NA | 398.46 (2444.35) |
| Total patient costs (time and travel costs and out-of-pocket expenses) | NA | 29.28 (135.57) | NA | 60.66 (305.92) |
As indicated by the high SD, the resource use and costs during follow-up were highly variable. This arose because the majority of participants did not report any use of health services following their initial treatment. For those men who did report the use of health services, those men randomised to the urethrotomy group used significantly more than those in the urethroplasty group.
Cost of reintervention
A total of 44 patients had reinterventions during the trial’s follow-up period (29 patients in the urethrotomy group and 15 patients in the urethroplasty group). Three patients in the urethrotomy group had more than one reintervention and two patients from the urethroplasty group had more than one reintervention. The same process of micro-costing (see Cost of the intervention) was conducted to calculate those reintervention costs (Table 29). It is worth noting that both types of procedures were used for reinterventions in both groups. The reason why urethrotomy patients used more resources on average than urethroplasty patients was that patients randomised to the urethrotomy group were more likely to have urethroplasty as their reintervention treatment; therefore, the resource use for the urethrotomy group was higher on average for those men who had reinterventions.
| Reintervention cost | Intervention | |||
|---|---|---|---|---|
| Urethroplasty | Urethrotomy | |||
| Mean (SD) (£) | n | Mean (SD) (£) | n | |
| Base-case analysis | 3896.39 (4606.26) | 9 | 4626.09 (2222.69) | 17 |
| Sensitivity analysis with data imputation | 3255.86 (3842.12) | 15 | 4674.68 (1997.89) | 29 |
Total cost
Taking the perspective of the NHS and patients and their families, total costs combining NHS resource use costs (intervention, reintervention and health service use during follow-up) and patients’ out-of-pocket costs are presented in Table 30.
| Total cost | Intervention | |||
|---|---|---|---|---|
| Urethroplasty | Urethrotomy | |||
| Mean (SD) (£) | n | Mean (SD) (£) | n | |
| Base-case analysis | ||||
| Total intervention and reintervention cost | 4331.51 (3150.60) | 89 | 2209.19 (2368.13) | 91 |
| Total NHS cost (intervention and reintervention with follow-up) | 4454.55 (3190.76) | 89 | 2657.41 (3475.67) | 91 |
| Total societal cost (NHS and patient costs) | 4479.94 (3218.47) | 89 | 2729.90 (3712.57) | 91 |
| Sensitivity analysis with data imputation | ||||
| Total intervention and reintervention cost | 4559.35 (3060.64) | 108 | 2911.43 (2712.73) | 112 |
| Total NHS cost (intervention and reintervention with follow-up) | 4674.25 (3134.93) | 108 | 3309.89 (3552.15) | 112 |
| Total societal cost (NHS and patient costs) | 4703.53 (3155.05) | 108 | 3370.55 (3755.24) | 112 |
As Table 30 shows, the cost of urethroplasty was higher over the 24 months post randomisation than that of urethrotomy. However, the magnitude of the difference in cost reduced when reintervention and follow-up costs were included.
Quality-adjusted life-years
Effectiveness was measured in terms of utility derived from responses to the EQ-5D-5L collected at baseline, immediately prior to surgery, 1 week after catheter removal, at 3, 6, 9, 12 and 24 months following surgery, at 18 and 24 months after randomisation and at the end of study. Additional EQ-5D-5L questionnaires were intended to be completed at times of participants requiring reintervention; however, very few patients submitted both pre- and post-reintervention EQ-5D-5L data. Among those participants who had both pre- and post-reintervention EQ-5D-5L data, no difference was found between the allocated intervention groups or between pre and post reintervention; therefore, these EQ-5D-5L data were not used in the calculation of total QALYs. The responses to the EQ-5D-5L questionnaire were transformed using UK population tariffs39 to produce a health state utility score for each participant in each of the treatment groups using the AUC method. 80
Given the large number of time points for EQ-5D-5L data, and to align with the primary effectiveness analysis, it was decided that, to be included in the AUC analysis without imputation, the participant must have at least three EQ-5D-5L observations with one at the start of the assessment period, one at the mid-range and one at the end, and the specific requirement was dependent on the status of the participants (whether or not they had received surgery) and the type of analysis conducted, as explained below.
For those participants who did not receive an initial surgery, to be included in the AUC analysis without imputation, they must have completed EQ-5D-5L data on the three time points: baseline, 18 months and 24 months after randomisation.
For those participants who received an initial surgery, the base-case analysis examined QALYs over the period from baseline to 24 months after randomisation; therefore, the AUC analysis required complete EQ-5D-5L data at baseline and 24 months after randomisation, and at one of the data collection points of 3, 6, 9 and 12 months following surgery and 18 months following randomisation. Given the differences in the duration of the period between randomisation and undergoing an intervention between urethroplasty and urethrotomy, we also examined QALYs over the period from the time prior to surgery to 24 months post surgery, in which case the AUC analysis required complete EQ-5D-5L data at prior to surgery and 24 months after surgery, and at one of the data collection points of 3, 6, 9 and 12 months following surgery and 18 and 24 months following randomisation.
For all calculations of QALYs the first observation used (either baseline or prior to surgery, depending on the patient groups and analysis type) was set at time point zero and the date on which the EQ-5D-5L was recorded as being completed was used to calculate the number of days from the first observation. This time dimension was initially used in the calculation of QALYs rather than the nominal time point at which the EQ-5D-5L was to be completed. In an alternative analysis, the responses were rescaled to the nominal data collection points (i.e. 730 days; note that cost data did not need to be rescaled in the same way as the recall period was predefined within the data collection tools). Additionally, multiple imputation for EQ-5D-5L at all missing time points was conducted to calculate QALYs for all participants.
A summary of the results of the EQ-5D-5L for each time point by intervention, as well as the estimates of QALYs for both the base-case analysis (QALYs at 24 months after randomisation) and the sensitivity analyses described above, is provided in Table 31 and in Figure 12.
| EQ-5D-5L | Intervention | |||
|---|---|---|---|---|
| Urethroplasty | Urethrotomy | |||
| Mean (SD) | n | Mean (SD) | n | |
| Baseline | 0.83 (0.22) | 106 | 0.87 (0.17) | 107 |
| Just before surgery | 0.84 (0.21) | 74 | 0.86 (0.16) | 96 |
| 1 week after catheter removal | 0.84 (0.17) | 53 | 0.89 (0.13) | 70 |
| 3 months after surgery | 0.88 (0.15) | 69 | 0.89 (0.19) | 84 |
| 6 months after surgery | 0.87 (0.17) | 60 | 0.87 (0.19) | 77 |
| 9 months after surgery | 0.88 (0.17) | 63 | 0.88 (0.19) | 81 |
| 12 months after surgery | 0.87 (0.21) | 63 | 0.87 (0.21) | 80 |
| 18 months after randomisation | 0.85 (0.23) | 62 | 0.87 (0.20) | 79 |
| 24 months after randomisation | 0.87 (0.25) | 58 | 0.90 (0.16) | 58 |
| 24 months after surgery | 0.87 (0.26) | 48 | 0.91 (0.13) | 59 |
| QALYs at 24 months after randomisation | 1.75 (0.40) | 55 | 1.76 (0.35) | 54 |
| QALYs at 24 months after randomisation (rescaled to 730 days) | 1.66 (0.34) | 55 | 1.70 (0.34) | 54 |
| QALYs at 24 months after surgery | 1.73 (0.54) | 44 | 1.77 (0.34) | 56 |
| QALYs at 24 months after surgery (rescaled to 730 days) | 1.42 (0.40) | 44 | 1.58 (0.30) | 56 |
| QALYs at 24 months after randomisation with imputation | 1.73 (0.32) | 108 | 1.76 (0.28) | 112 |
| QALYs at 24 months after randomisation with imputation (rescaled to 730 days) | 1.67 (0.29) | 108 | 1.72 (0.27) | 112 |
| QALYs at 24 months after surgery with imputation | 1.75 (0.37) | 108 | 1.76 (0.29) | 112 |
| QALYs at 24 months after surgery with imputation (rescaled to 730 days) | 1.67 (0.30) | 108 | 1.72 (0.26) | 112 |
FIGURE 12.
Utility values at each time point and QALYs over the trial follow-up with corresponding number of observations.
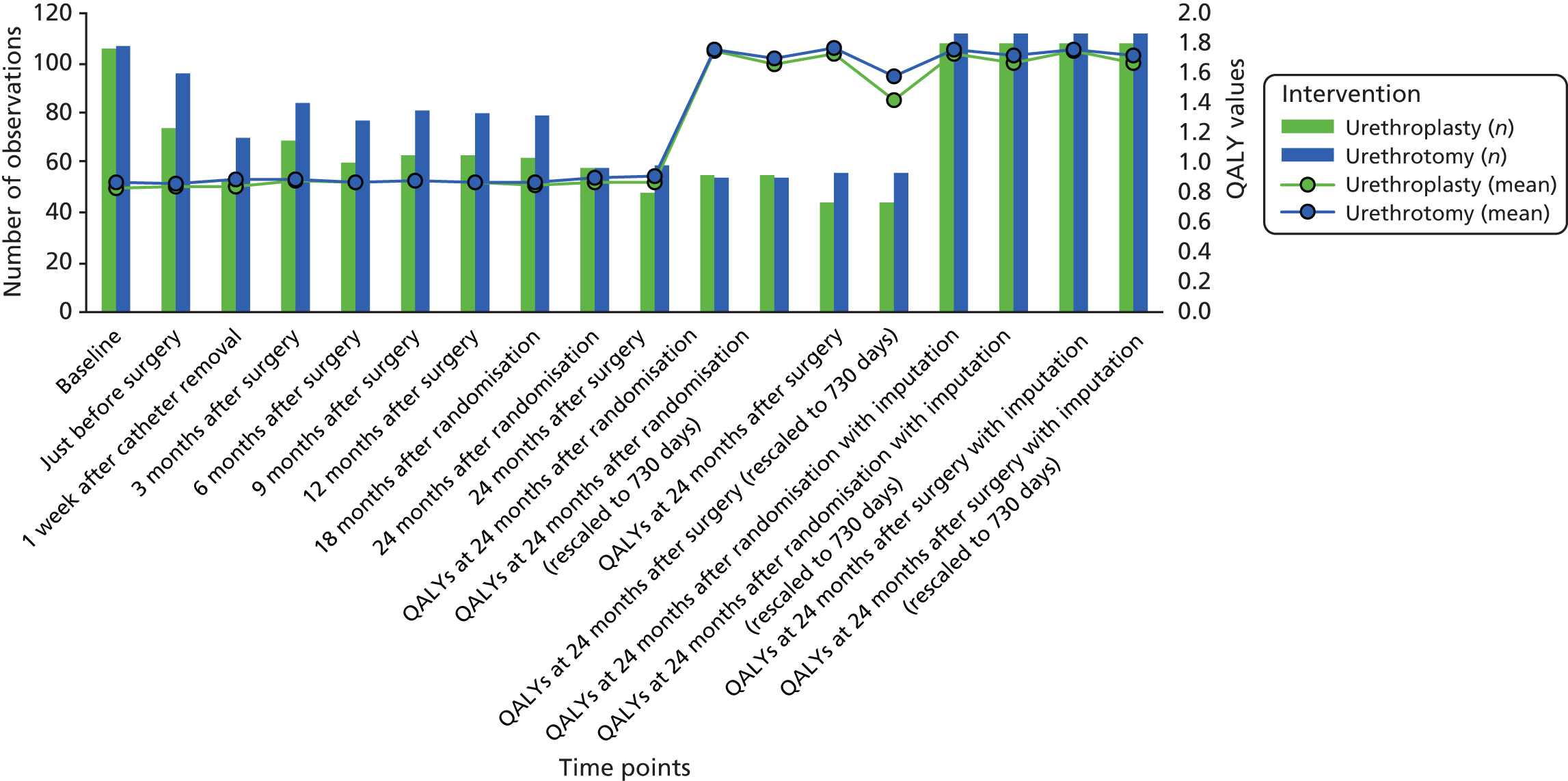
Cost–utility analysis
Method
Although a comparison of cost-effectiveness over a 24-month time horizon from baseline to end of study was intended as described in the study protocol, owing to the unpredictable nature of surgery planning at different sites, there was a considerable difference in time from baseline to having initial surgery between patients, making it an unfair comparison to use baseline as time zero to the end of study. It was, therefore, decided to examine the cost-effectiveness with different time points as time zero. In the base-case analysis, QALY gain was based on QALYs calculated from baseline to 24 months post randomisation. The sensitivity analysis examined QALY gain based on QALYs calculated from the time prior to surgery to 24 months post surgery. For both cases, QALY gain was also rescaled to the nominal time (i.e. 730 days) as additional sensitivity analyses. All of the above used complete cases only. Additionally, multiple imputation was conducted for all the missing observations and sensitivity analyses with imputation were also conducted in the same manner as complete-case analyses.
Costs used in the cost–utility analyses included initial intervention costs and subsequent follow-up costs, as well as patients’ out-of-pocket costs. Costs with imputation were used together with QALYs with imputation in the sensitivity analyses. The incremental cost-effectiveness ratio (ICER) was calculated by dividing the difference in mean costs by the difference in mean QALYs for each group. Uncertainty in parameter estimates was addressed through the application of bootstrapping and the estimation of cost-effectiveness acceptability curves, to provide both probabilistic and deterministic sensitivity analyses.
Results
The base-case analysis and all sensitivity analysis results are presented in Table 32. In the base case, urethroplasty cost more (cost difference £2148, 95% CI £689 to £3606) than urethrotomy, while generating a lower point estimate of QALY gain (QALY difference –0.01, 95% CI –0.17 to 0.14). Urethroplasty was therefore dominated by urethrotomy. This suggests that urethrotomy was more cost-effective than urethroplasty in the base-case scenario. The base-case result appears to be robust, as it is seen in all of the sensitivity analyses that urethroplasty cost more and the point estimate of effect was lower. The cost-effectiveness acceptability curve (Figure 13) and incremental cost and QALY plots (Figure 14) are presented for the base case. As seen in Figure 13, the probability of urethroplasty being cost-effective increases as the threshold for society’s willingness to pay for a QALY increases; however, urethroplasty never had more than approximately a 20% chance of being considered cost-effective over a 2-year time horizon for the range of cost per QALY thresholds considered. This is supported by the incremental cost and QALY plots (see Figure 14), in which the mean incremental cost per QALY falls in the north-east quadrant of the graph, indicating urethroplasty to cost more but to be less effective using the point estimate of benefit.
| Investigation strategy | Cost (£) mean (95% CI) | Incremental cost (£) mean (95% CI) | QALY mean (95% CI) | Incremental effect mean (95% CI) | ICER (£) | Probability (%) of each treatment strategy being cost-effective for different threshold values for society’s willingness to pay | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||||
| Base case, 24 months post randomisation | ||||||||||
| Urethroplasty (n = 46) | 4869 (4123 to 5614) | 2148 (689 to 3606) | 1.74 (1.61 to 1.86) | –0.01 (–0.17 to 0.14) | 0 | 2 | 8 | 14 | 23 | |
| Urethrotomy (n = 46) | 2721 (1444 to 3999) | 1.75 (1.65 to 1.85) | Dominant | 100 | 98 | 92 | 86 | 77 | ||
| 24 months post randomisation (rescaled QALY) | ||||||||||
| Urethroplasty (n = 46) | 4869 (4123 to 5614) | 2148 (689 to 3606) | 1.65 (1.55 to 1.76) | –0.04 (–0.18 to 0.11) | 0 | 1 | 3 | 5 | 10 | |
| Urethrotomy (n = 46) | 2721 (1444 to 3999) | 1.69 (1.59 to 1.79) | Dominant | 100 | 99 | 93 | 95 | 90 | ||
| 24 months post surgery | ||||||||||
| Urethroplasty (n = 37) | 4963 (3977 to 5949) | 1672 (–65 to 3409) | 1.73 (1.54 to 1.92) | –0.04 (–0.24 to 0.16) | 2 | 7 | 14 | 19 | 25 | |
| Urethrotomy (n = 48) | 3291 (1947 to 4636) | 1.77 (1.67 to 1.87) | Dominant | 98 | 93 | 86 | 81 | 75 | ||
| 24 months post surgery (rescaled QALY) | ||||||||||
| Urethroplasty (n = 37) | 4963 (3977 to 5949) | 1672 (–65 to 3409) | 1.42 (1.28 to 1.56) | –0.16 (–0.31 to –0.01) | 2 | 1 | 1 | 1 | 1 | |
| Urethrotomy (n = 48) | 3291 (1947 to 4636) | 1.58 (1.49 to 1.67) | Dominant | 98 | 99 | 99 | 99 | 99 | ||
| 24 months post randomisation with imputation | ||||||||||
| Urethroplasty (n = 108) | 4704 (4102 to 5305) | 1333 (410 to 2256) | 1.73 (1.67 to 1.79) | –0.03 (–0.11 to 0.05) | 0 | 0 | 2 | 4 | 9 | |
| Urethrotomy (n = 112) | 3371 (2667 to 4074) | 1.76 (1.71 to 1.81) | Dominant | 100 | 100 | 98 | 96 | 91 | ||
| 24 months post randomisation with imputation (rescaled QALY) | ||||||||||
| Urethroplasty (n = 108) | 4704 (4102 to 5305) | 1333 (410 to 2256) | 1.67 (1.62 to 1.73) | –0.05 (–0.13 to 0.02) | 0 | 0 | 0 | 1 | 2 | |
| Urethrotomy (n = 112) | 3371 (2667 to 4074) | 1.72 (1.67 to 1.77) | Dominant | 100 | 100 | 100 | 99 | 98 | ||
| 24 months post surgery with imputation | ||||||||||
| Urethroplasty (n = 108) | 4704 (4102 to 5305) | 1333 (410 to 2256) | 1.75 (1.68 to 1.82) | –0.02 (–0.10 to 0.07) | 0 | 1 | 5 | 10 | 19 | |
| Urethrotomy (n = 112) | 3371 (2667 to 4074) | 1.76 (1.71 to 1.82) | Dominant | 100 | 99 | 95 | 90 | 81 | ||
| 24 months post surgery with imputation (rescaled QALY) | ||||||||||
| Urethroplasty (n = 108) | 4704 (4102 to 5305) | 1333 (410 to 2256) | 1.67 (1.61 to 1.73) | –0.05 (–0.12 to 0.02) | 0 | 0 | 0 | 1 | 2 | |
| Urethrotomy (n = 112) | 3371 (2667 to 4074) | 1.72 (1.67 to 1.77) | Dominant | 100 | 100 | 100 | 99 | 98 | ||
FIGURE 13.
Cost-effectiveness acceptability curve (base case).
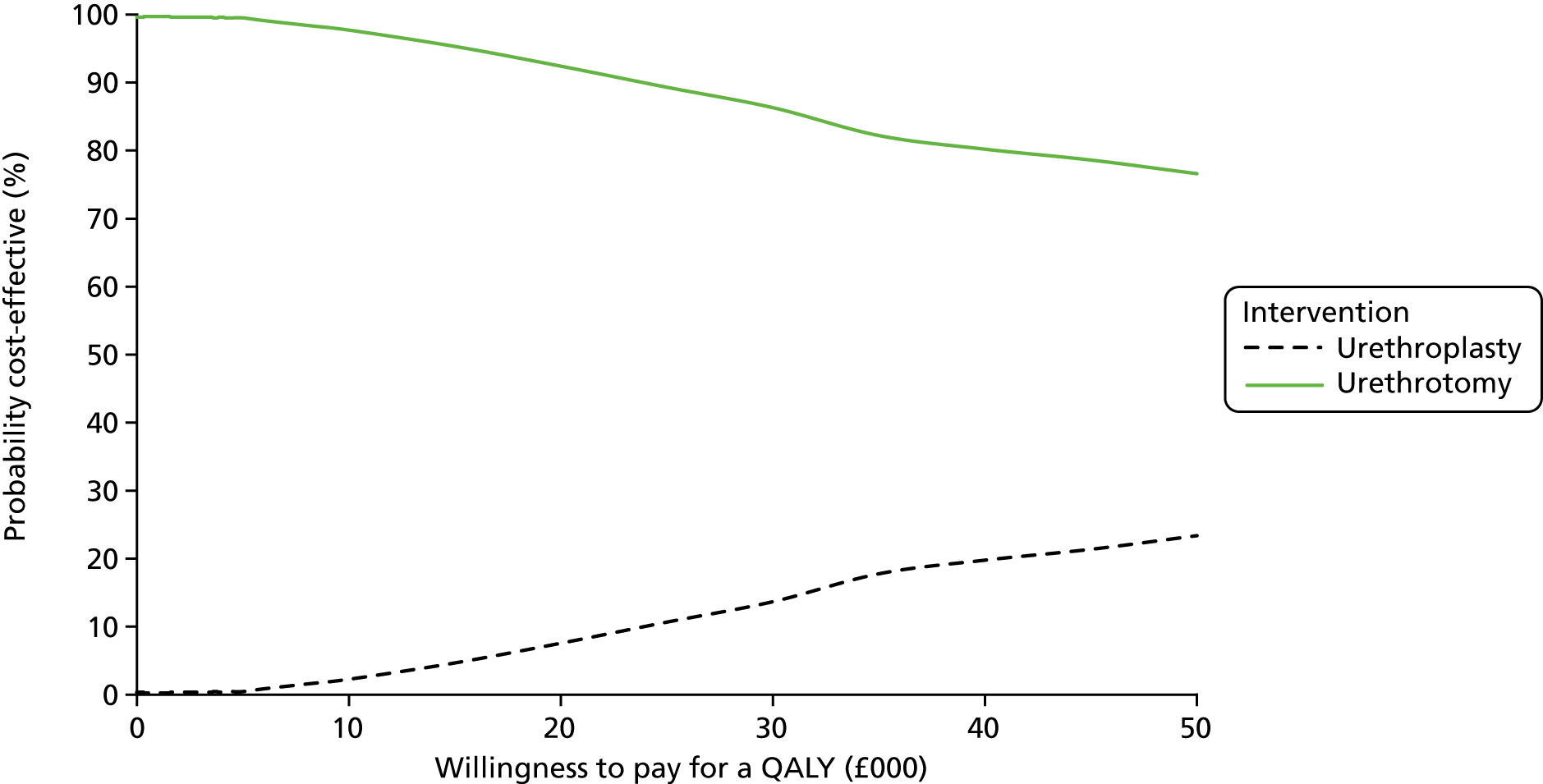
FIGURE 14.
Incremental cost and QALY plots (base case).
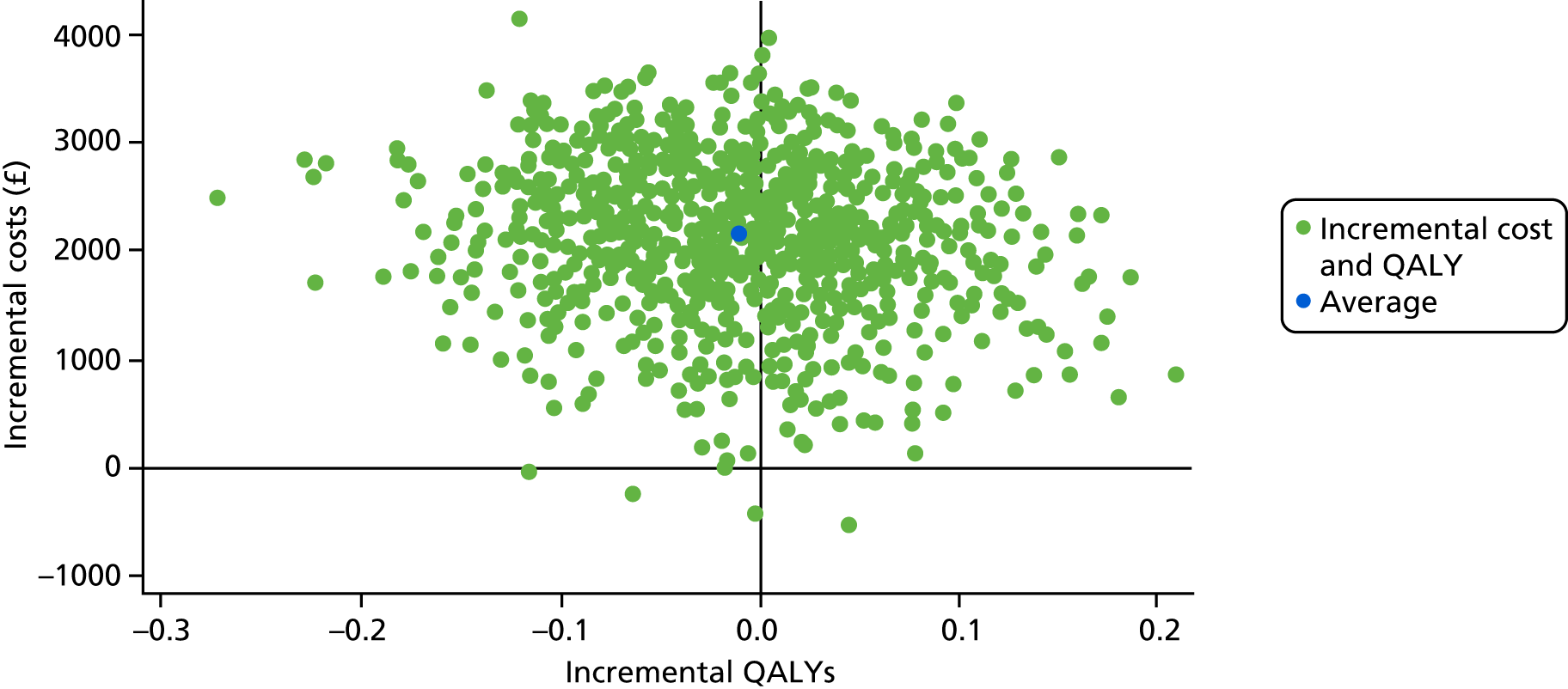
Long-term modelling
Methods
The Markov model consisted of three health states: (1) cured (symptom free), (2) symptomatic and (3) deceased. The deceased state was included to allow for general population mortality when examining the cohorts over a long-term horizon. In the model, every patient started off receiving one of the two trial interventions. They then moved through the care pathways over time, incurring costs and accruing QALYs. The care pathways followed described the process of care, disease incidence and progression. These features were linked in a logical way, defined by a set of mathematical relationships that dictated how and when an individual might move through the model. The structure of the model is shown in Figure 15. The model parameters were based on information derived from the trial and the distribution for each parameter was defined considering the mean, standard error and anticipated shape of the distribution. Model parameters included costs for each intervention and their follow-up; utility for cured (symptom-free) and symptomatic states; probability of surgery success for each intervention; probability of receiving treatment when symptomatic; and probability of the type of treatment procedure received conditional on the previous intervention. Survival analysis was used to generate forward transition probabilities of recurrence following each intervention. Table 33 describes all model parameters. Half-cycle corrections were applied in the model.
FIGURE 15.
Markov model structure.
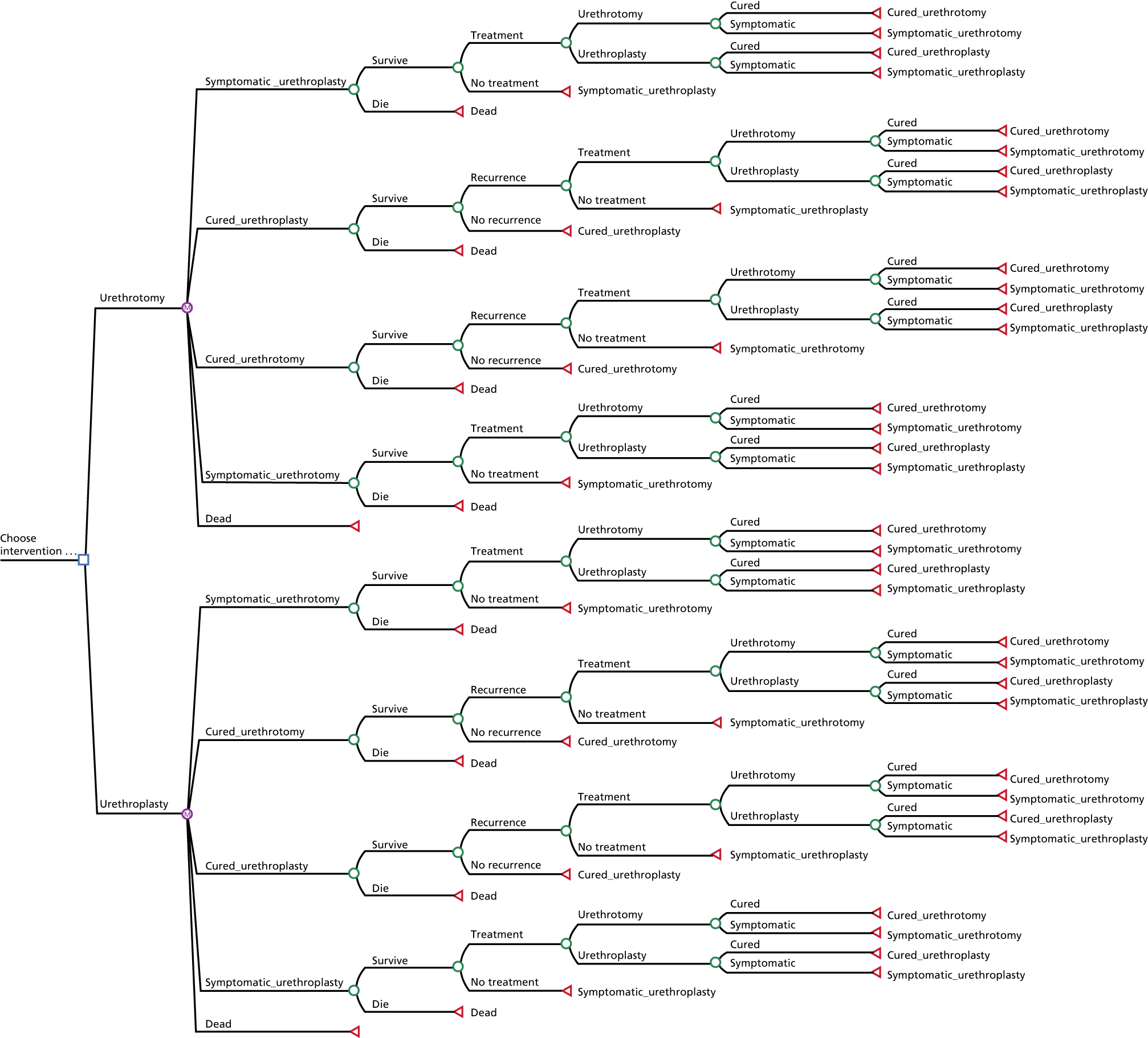
| Parameter name | Value, mean (SE) | Source and distribution |
|---|---|---|
| Cost of urethroplasty (including initial surgery with catheter removal and hospital stay) |
Base case: £5871 (£229) By treatment received: £5808 (£219) |
Based on value from the trial; gamma distribution |
| Cost of urethrotomy (including initial surgery with catheter removal and hospital stay) |
Base case: £1316 (£96) By treatment received: £1367 (£90) |
Based on value from the trial; gamma distribution |
| Utility associated with cured (symptom-free) health state |
Base case: 0.899 (0.013) By treatment received: same as base case |
Based on value from the trial; beta distribution; utility values at 3 months after surgery, of those men who showed a significant improvement in voiding scores |
| Utility associated with symptomatic health state |
Base case: 0.852 (0.014) By treatment received: same as base case |
Based on value from the trial; beta distribution; utility values at baseline |
| Cost of health service use and patient’s out-of-pocket expenses following urethroplasty |
Base case: £130 (£46) By treatment received: £141 (£45) |
Based on value from the trial; gamma distribution |
| Cost of health service use and patient’s out-of-pocket expenses following urethrotomy |
Base case: £227 (£42) By treatment received: £210 (£38) |
Based on value from the trial; gamma distribution |
| Surgery success rate for urethroplasty |
Base case: 0.95 (0.03) By treatment received: 0.94 (0.03) |
Based on value from the trial; beta distribution |
| Surgery success rate for urethrotomy |
Base case: 0.91 (0.03) By treatment received: 0.92 (0.03) |
Based on value from the trial; beta distribution |
| Probability of being treated when symptomatic |
Base case: 0.90 (0.02) By treatment received: same as base case |
Based on value from the trial; beta distribution |
| Probability of receiving urethroplasty if the last treatment is urethroplasty |
Base case: 0.12 (0.12) By treatment received: 0.11 (0.11) |
Based on value from the trial; beta distribution |
| Probability of receiving urethroplasty if the last treatment is urethrotomy |
Base case: 0.70 (0.08) By treatment received: 0.63 (0.07) |
Based on value from the trial; beta distribution |
| Probability of recurrence following urethroplasty |
Base case: 0.042 By treatment received: 0.041 |
Based on survival analysis from the trial |
| Probability of recurrence following urethrotomy |
Base case: 0.1497 By treatment received: 0.150 |
Based on survival analysis from the trial |
The base-case analysis used parameters estimated based on information from study participants who received the intervention to which they had been allocated. Probabilistic sensitivity analysis of the base-case scenario was carried out using Monte Carlo simulation, in which model inputs for each parameter were randomly selected from predefined distributions and the results recorded. This process was repeated for 10,000 iterations to produce a distribution of results from the model.
Deterministic sensitivity analyses were conducted by changing the key parameters in set ways that were determined based on information from those men who received the same intervention, regardless of whether or not it was the intervention they were allocated.
An important model parameter is the choice of the next treatment given the previous treatment the patient had. The parameters used in the model were based on observations from the trial, in which about 70% of patients would receive urethroplasty and 30% of patients would receive urethrotomy if the last treatment was urethrotomy, and about 12% of patients would receive urethroplasty and 88% of patients would receive urethrotomy if the last treatment was urethroplasty. This suggests that a large proportion of patients switch to a treatment different from their previous one every time they have a reintervention. There is no consensus on the treatment choices in reintervention and such choices are often influenced by many non-clinical factors, such as patient choice, waiting time and travel time. Given the high uncertainty around treatment choice in reintervention, deterministic sensitivity analyses also examined the impact of changing the percentages of patients switching treatments.
The costs and effects were combined in an incremental analysis, comparing different treatment strategies, and presented as the point estimates of mean costs, mean QALYs and mean incremental cost per QALY. These results were presented graphically using the incremental cost and QALY plots and a cost-effectiveness acceptability curve.
Results
In the base-case analysis, in which model parameters were based on those men who received the intervention they were allocated by randomisation, urethroplasty is unlikely to be considered cost-effective under society’s current willingness to pay for a QALY. This is also illustrated by the probabilistic sensitivity analysis in which urethroplasty has almost no chance of being considered cost-effective at any of the threshold values for society’s willingness to pay for a QALY considered (Table 34 and Figures 16 and 17).
| Treatment strategy | Cost (£) | QALY | ICER (£) | Probability (%) of each treatment strategy is cost-effective for different threshold values for society’s willingness to pay for a QALY | ||||
|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||
| Base case | ||||||||
| Urethroplasty | 8026 | 7.61 | 301,073 | 0 | 0 | 0 | 0 | 2 |
| Urethrotomy | 6553 | 7.60 | 100 | 100 | 100 | 100 | 98 | |
| Parameters based on treatment received | ||||||||
| Urethroplasty | 7987 | 7.61 | 307,328 | 0 | 0 | 0 | 0% | 1 |
| Urethrotomy | 6490 | 7.60 | 100 | 100 | 100 | 10 | 99 | |
| Always receive the same treatment at recurrence | ||||||||
| Urethroplasty | 9026 | 7.61 | 476,162 | 0 | 0 | 0 | 0 | 0 |
| Urethrotomy | 4059 | 7.60 | 100 | 100 | 100 | 100 | 100 | |
| Always receive the other treatment at recurrence | ||||||||
| Urethroplasty | 8076 | 7.61 | 263,383 | 0 | 0 | 1 | 2 | 4 |
| Urethrotomy | 7054 | 7.60 | 100 | 100 | 99 | 98 | 96 | |
FIGURE 16.
Cost-effectiveness acceptability curve (Markov model – base case).
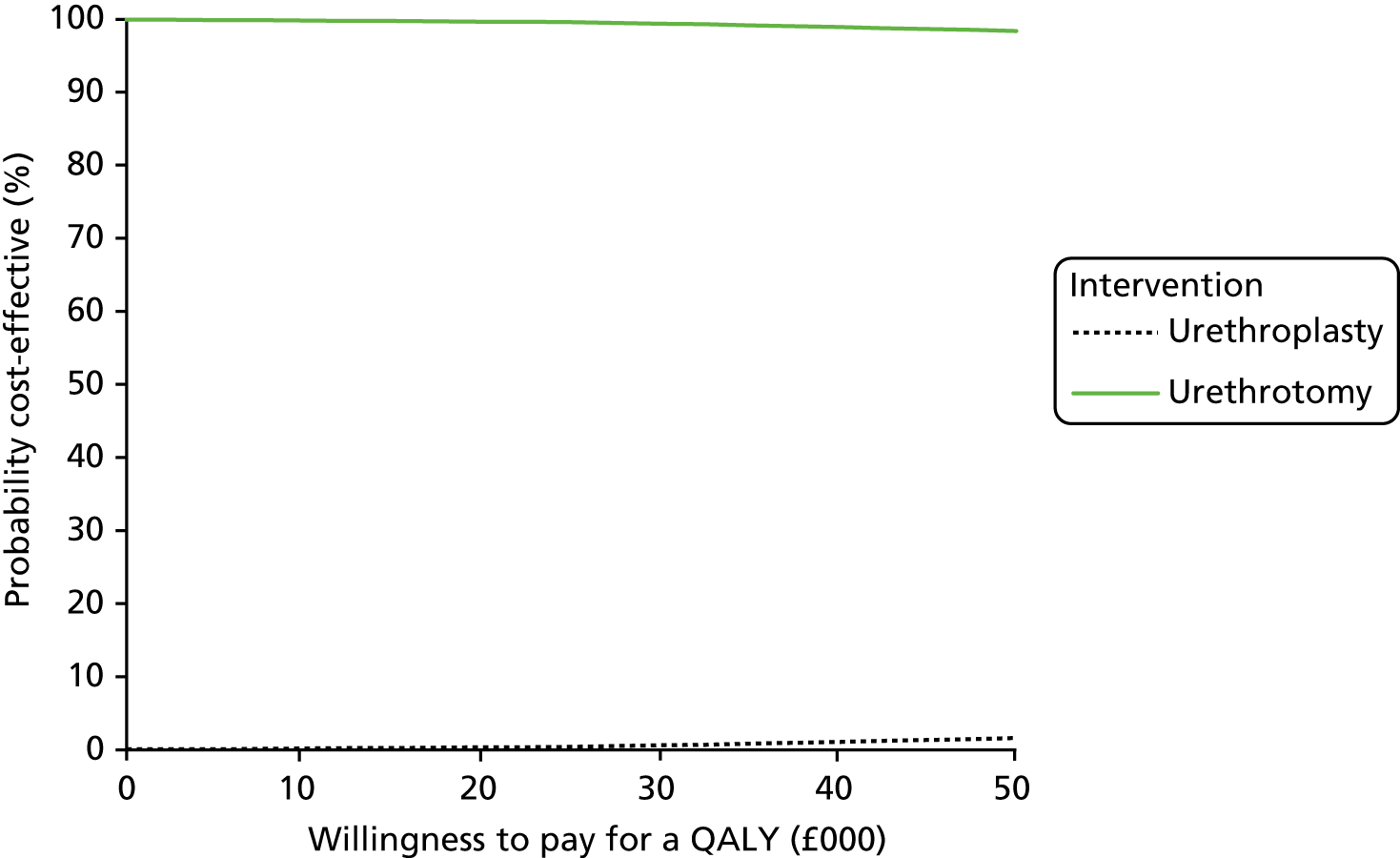
FIGURE 17.
Incremental costs and QALY plots (model base case).
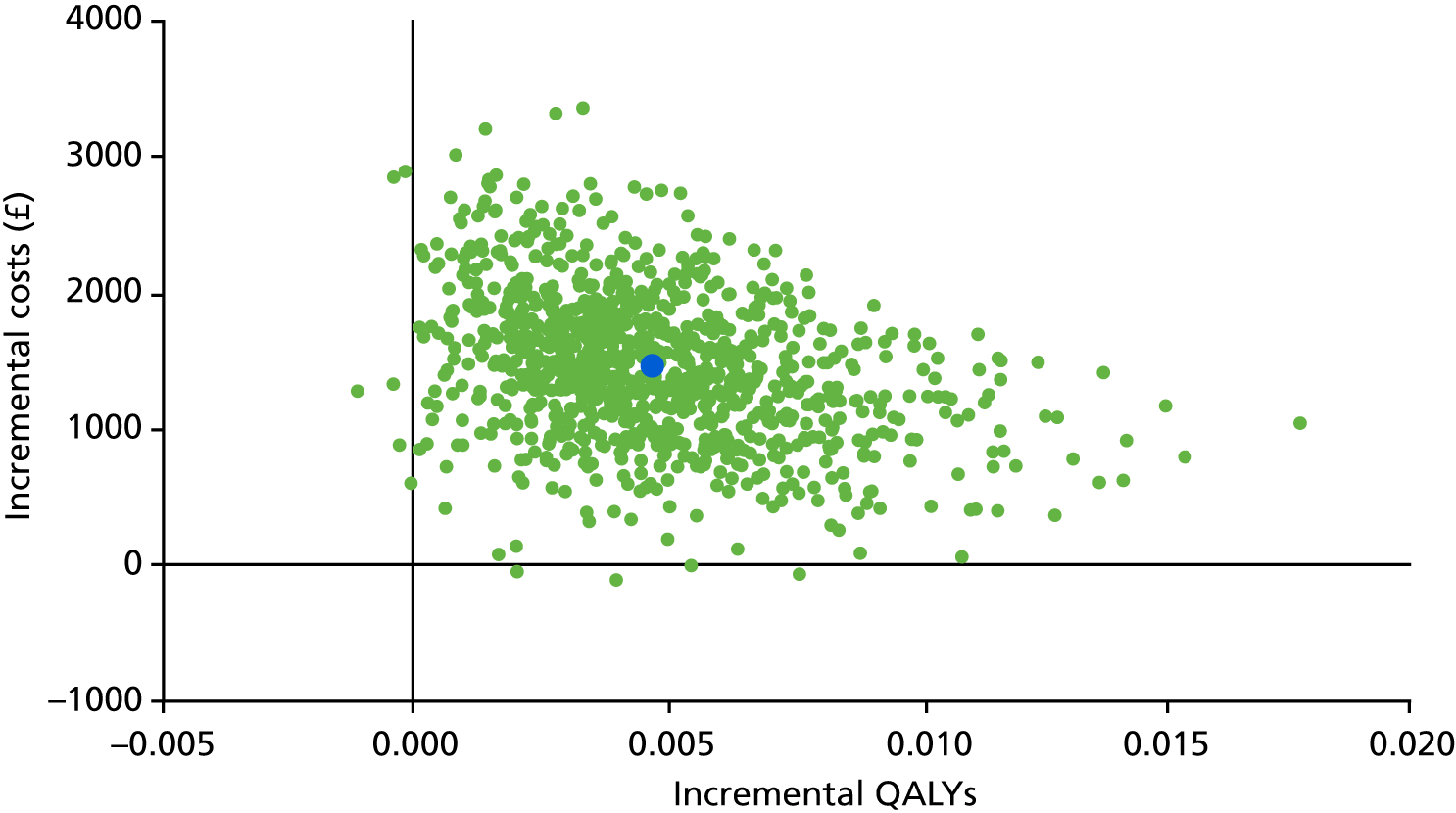
In the deterministic sensitivity analysis, in which model parameters were based on those men who received the same treatment procedure regardless of their allocated intervention group, a similar trend is observed to the base-case analysis, with urethroplasty being unlikely to be cost-effective under society’s current willingness-to-pay threshold for a QALY. The same is observed in the deterministic sensitivity analyses varying the probabilities of the choices of treatments in reintervention.
Both probabilistic and deterministic sensitivity analyses show that urethroplasty is unlikely to be cost-effective over a 10-year time horizon under a number of plausible scenarios. This is mainly driven by the higher cost of urethroplasty than urethrotomy, while both of the treatment procedures produce similar QALY gains, despite those men receiving urethroplasty having a lower chance of recurrence than those men receiving urethrotomy. It could be argued that the EQ-5D-5L may not be a sensitive measure for this group of patients and would, therefore, not capture important changes in quality of life for this condition. Additionally, decrement in quality of life following the procedures were not taken into consideration in the analysis conducted. Given the likelihood of recurrence requiring repeated treatments, this decrement could make a difference in QALY gains between the two treatments. The potential way to measure this decrement was explored using the TTO exercise.
Time trade-off exercise
A TTO exercise was conducted to estimate the short-term impact of undergoing the treatments on health-related quality of life (HRQoL). This was to complement the results of completion of the EQ-5D-5L as part of trial follow-up. The EQ-5D-5L is a measure of HRQoL on the day of completion and, as such, it does not ask about the duration of any impacts on HRQoL. Thus, we wished to use a TTO exercise to assess the important but short-term changes in health that occurred at the time of a surgery, which would otherwise be missed in the EQ-5D-5L completion schedule. The TTO exercise gathers data on the impact of impaired health by asking participants to state preferences between quality and quantity of life in hypothetical scenarios. The TTO method is mainly used to elicit utility values for chronic health states that last for a number of years; however, the two surgical procedures in this study are likely to have a short impact over days or weeks postoperatively, before a return to normal health. In these circumstances, a conventional TTO exercise may become less responsive81,82 because the exercise offers an unrealistic choice, between an impaired health state for a fixed duration and a perfect health state for a shorter duration of time, both followed by death. 83 Attempts to remedy this problem have involved using an intermediate health state, rather than directly comparing the temporary health state with perfect health and death, and this method is referred to as the ‘chained TTO’. 84 In this study, both the conventional and chained TTO exercise were used with participants who agreed to take part. The participants were randomly allocated to one of the two alternative methods of eliciting TTO valuations: the conventional and chained TTO.
Development of time trade-off materials
Prior to the TTO exercise being conducted among trial participants, extensive development and piloting of materials and processes were carried out. The TTO materials developed include a decision board, which was used as a visual aid for participants to compare health states, and written health state profiles (see Appendix 7), which described the likely side effects of the two procedures (categorised into mild, moderate and severe). These profiles were printed on A6-sized card for use with the decision board. An anchor health state was designed for the chained TTO, as an intermediate state that was worse than the six health profiles, but not as severe as death, and described a scenario containing severe pain with usual functioning impaired, but maintaining basic self-caring activities possible. A set of practice profiles was also designed based on a selection of EQ-5D-5L profiles to allow participants to become familiar with the process prior to valuing the health states relating to the study. The time horizon for the profiles to be evaluated was chosen as 14 days, and this was a balance between providing a clinically accurate prediction of the likely duration of side effects and providing participants with a clear scenario.
Three rounds of piloting were conducted with different groups of participants before finalising the materials and process: round 1 conducted with 17 male and female volunteers from Newcastle University; round 2 conducted with 15 male staff members at a participating (hospital) study site; and round 3 conducted with nine men eligible for the open trial. Piloting interviews consisted of mock TTO interviews, but also encouraged feedback on the process and materials. Feedback was collated and used iteratively to refine processes for subsequent pilots. Health-care professionals involved in the trial were consulted throughout the development process to ensure that the TTO materials were clinically accurate and understandable to a lay audience. A patient and public involvement representative was also consulted as part of the piloting process.
The TTO participants were recruited from those men who were eligible for the OPEN trial. At the point of consent to the main trial, participants were asked to indicate willingness to be contacted about a further interview study. Those men who expressed interest were posted an information pack regarding TTO, containing a response slip and post-paid envelope. On receipt of an affirmative response slip, a researcher contacted respondents to answer any further questions or arrange a time and place of the participant’s choosing. Interviews were most frequently conducted in the participant’s home and written consent was taken prior to commencing the interview.
Following the TTO interview, participants were asked to provide feedback on the interview process. Participants were asked to rate on a scale of 1 to 5 (with 1 being not difficult at all and 5 being very difficult) how difficult they found the TTO process and asked if they had any comments relating to the process. This was recorded by the researcher as free text. In addition, the interviewer recorded anything about the conduct and process of the interview that they felt to be important.
Data analysis
Stata was used to analyse data. Descriptive statistics were produced for demographic details of the sample and Tobit regressions of reported TTO values were performed, controlling for sociodemographic characteristics. The level of usual physical activity and place of residence (urban or rural) were included as variables in the analyses, as it was considered possible that these would have an impact on how participants valued health states in which their mobility was affected. An additional control variable was generated based on the consistency between utility values derived and how each participant ranked those profiles prior to the TTO exercise in order to indicate data quality. Predicted values of health state utility were estimated for each health state and compared using unpaired t-tests. Qualitative data collected were also analysed to understand the feasibility of conducting a TTO alongside a surgical RCT.
Results
Forty participants were recruited to take part in the TTO. Two participants had missing or invalid essential data and were excluded. Of the 38 participants included in the analysis, the sociodemographic details were as follows: mean age of the sample was 38 years; 84% were married; 41% had a household income > £36,400; just over half (55%) of the sample were employed and 29% were retired. The majority (71%) of participants described living in an urban area. Twenty-nine per cent of the sample reported high levels of physical activity, 47% reported medium levels and 24% reported low levels of activity.
The estimated utility values for the two types of surgery using the two TTO methods are presented in Table 35, together with significance of the difference in post-surgery utilities between the two surgeries. The majority of the urethroplasty health states had lower utility scores than the urethrotomy health states, this was significant for the severe scenario irrespective of the TTO method used. For both types of surgery, mean utility values decreased as health states became more severely impaired, which suggests face validity of the profiles.
| TTO method | Health states by severity of side effects following surgery | Intervention, utility value (95% CI) | p-value | |
|---|---|---|---|---|
| Urethrotomy | Urethroplasty | |||
| Conventional (n = 20) | Mild | 0.81 (0.72 to 0.90) | 0.79 (0.72 to 0.87) | 0.61 |
| Moderate | 0.58 (0.44 to 0.72) | 0.54 (0.43 to 0.65) | 0.48 | |
| Severe | 0.56 (0.45 to 0.68) | 0.39 (0.26 to 0.51) | < 0.001 | |
| Chained (n = 18) | Mild | 0.83 (0.74 to 0.92) | 0.83 (0.73 to 0.93) | 0.71 |
| Moderate | 0.67 (0.56 to 0.77) | 0.62 (0.54 to 0.69) | 0.43 | |
| Severe | 0.44 (0.35 to 0.53) | 0.29 (0.19 to 0.39) | 0.03 | |
The median difficulty for the TTO exercise reported by participants was 2 out of 5, which indicated that the TTO exercise was considered by most participants as reasonably easy to complete. Additional comments covered participants’ opinions on the difficulty of the interview process, as well as elaborating on their individual decision-making process. Some described a desire to avoid particular symptoms, particularly pain and anxiety. Some participants related previous life experience as informing their perception of the impact of certain symptoms; for example, one participant stated that having experienced frequent catheterisation, this was something he no longer worried about. In general, the interviewers felt that participants had understood the process reasonably well.
Discussion
Using a TTO nested in a clinical RCT allowed estimation of the short-term impact on HRQoL from side effects arising immediately following the procedures being compared.
Lower utility values were observed for urethroplasty-related health states, implying that there was a larger reduction in HRQoL immediately following the urethroplasty procedure, although the differences were seen to be statistically significant only for the severe impairment health states. Although this may be due to insufficient sample size, when taken in the context of the qualitative feedback regarding decision-making, this could be because participants were particularly averse to one or more of the symptoms described in the severe urethroplasty health state.
Only differences between utilities for each intervention were examined statistically, a more meaningful comparison would be to examine whether or not these differences were relevant from a clinical perspective. Further understanding of the short-term impact of the different treatments would offer valuable information from a policy-making perspective, as well as improving patient information on treatment choices. Combining these data with other information, such as incidence of side effects and recurrence rates, may help to support shared decision-making between patients and clinicians.
No attempt was formally made to incorporate the results of the exploratory analysis of the TTO exercise into the economic valuation. However, if the results were taken at face value and knowing something about the number of reoperations in both arms of the trial, then we might expect a more marked difference in QALYs between the two treatment groups, given that patients in the urethrotomy group are more likely to require repeated treatments than those patients in the urethroplasty group. Such marked difference is unlikely to come from the impact of the treatment itself if the findings were robust in terms of little difference found in utility between the two treatment strategies, except in the severe state which may account for only a small percentage of the patient population. Although qualitative comments were generally brief and did not constitute a valid or robust qualitative interview, they were useful to add to our understanding of people’s experience of taking part in the interviews and highlight aspects which were deemed to be difficult and could be revised for future studies. The aim of this research was to ask people to compare two states of health for varying lengths of time. People’s decision-making process appeared to be influenced by multiple factors, including their own experience of health and illness, their current health and their family situation. These data are useful in developing future TTO exercises and, in particular, will help research teams to refine their information materials, such as introductory scripts.
Conclusion
The TTO exercise has proved to be a feasible method of collecting data on short-term changes in HRQoL within a clinical trial. Although the study finding is preliminary, it suggests that both urethroplasty and urethrotomy have a negative impact on HRQoL immediately following the procedures. The likelihood of men with recurrent urethral stricture requiring repeated treatments may indicate that this important but short-lived decrement to HRQoL immediately following the procedures should be incorporated into the calculation of QALYs associated with each intervention in the longer term. Future research should aim to elicit the utilities associated with this decrement with a large sample size.
Chapter 6 Discussion
Statement and interpretation of results
This RCT directly compared clinical effectiveness and cost-effectiveness of the options of open urethroplasty and endoscopic urethrotomy to manage recurrent bulbar urethral stricture in men. To achieve this, we measured outcomes important to patients, clinicians and funders or planners of health care. Men with recurrent stricture are most concerned about their poor and prolonged voiding which threatens urinary retention, a problem they find distressing and which negatively affects their lives. 2,21 Men manage their symptoms by concealment and forbearance, acknowledging the benign nature of the underlying disease. When these management strategies become no longer tenable they seek medical help, principally from urologists. The advice they receive varies particularly depending on whether the urologist is a generalist or a specialist urethral surgeon. 2 Patients may also have established preferences based on their experience of previous interventions (predominantly urethrotomy) and their individual situation in terms of having resources to cope with the more prolonged recovery period and possible travel associated with urethroplasty.
The trial results show that they can be confident that either procedure will control their urinary symptoms and both require a short hospital stay and have a low risk of SAEs. They will then have to weigh up the contrasting aspects of the procedures confirmed by the trial results. The need for a longer period of catheterisation with urethroplasty was often highlighted as a negative by men, although recent quality improvement work suggests that this can be safely reduced to 8–10 days. 86 Wound and donor site discomfort are expected AEs of urethroplasty and when these were severe the TTO experiment, conducted in parallel with the OPEN trial, showed that a greater degree of short-term quality-of-life decrement occurred among men who underwent urethroplasty. This, together with the higher rate of, and shorter time to, recurrence associated with urethrotomy, will need to be considered in the light of preferences and values. It will be reassuring to men that both procedures were associated with greater satisfaction regarding sexual activity. Clinicians need to inform any prejudices they may hold regarding the two treatment options and present information in a balanced way, in light of the trial findings. In particular, older age, the number of previous urethrotomies and stricture severity do not appear to influence the estimate of treatment effect.
Outcomes for the procedures in the UK NHS seem equivalent across the OPEN trial sites and are similar to those outcomes in other European countries and the USA, suggesting that standards of care and surgical performance are consistent and reproducible. General urologists will need to ensure that there are efficient and workable pathways to allow men access to urethroplasty, irrespective of locality. Specialist urologists need to ensure that they have the capacity to offer a urethroplasty service and, through audit, monitor their outcome performance against the benchmark established by the OPEN trial results. All clinicians will need to ensure that men requiring further intervention for bulbar stricture have sufficient information based on evidence provided by the OPEN trial and other sources, and that the preferences and values of individual patients are elicited and taken into account during discussions around treatment decisions.
Providers of urology services can use the OPEN trial results to plan and cost the service provision required to offer men their preferred procedure. This may require the establishment of robust clinical pathways and ensuring that specialist urethroplasty services are sufficiently resourced in terms of theatre suite time and ongoing specialist surgeon availability. 87 This may also impact on training needs within the urology specialty. The greater initial cost for performance of urethroplasty seems to be partially offset over a longer time period by the lower need for reintervention.
The analyses suggest that urethroplasty is not cost-effective because it is likely to have a higher cost and does not result in a higher gain than urethrotomy. Reducing costs will improve the relative cost-effectiveness of urethroplasty. Further cost reduction for urethroplasty may be achievable by greater use of day-case surgery, which appears feasible,88 and minimisation of follow-up. 89
The optimum outcome measure for routine clinical follow-up remains uncertain. In common with routine clinical experience, we found it difficult to motivate men to adhere to a follow-up schedule while they remained symptom free. In particular, attendance for measurement of maximum flow rate was poor and deteriorated over time. We considered whether or not a single question from the USS-PROM, such as self-rating of flow strength, aided by pictorial representation might be suitable. 90 During the trial this was sensitive to change and correlated well with improvement in maximum flow rate, but only about half of the participants answered the question after 2 years of follow-up. The primary result from the OPEN trial was reassuring to patients in that both urethroplasty and urethrotomy offered rapid symptom relief, sustained over 2 years, but there was no clear winner in terms of the ‘best’ procedure. The point estimate of symptom improvement showed a slight advantage for open urethroplasty, but this was less than the pre-stated minimum important difference on which we powered the study. Moreover, the CI included the possibilities of no difference and advantage for urethrotomy. Clinicians tend to favour objective outcome assessment, such as measurement of urinary flow rate and delineation of the stricture by visual inspection or radiographic imaging. Successful outcome of surgery is most often assessed by the rate of requirement for further intervention over a specified time period91 or improvement in maximum flow rate. Improvement of ≥ 10 ml/second in measured flow rate (Qmax) at 3 months and between 12 and 24 months was more likely in the urethroplasty group than in the urethrotomy group, although the number of participants with missing data was high, suggesting that this might not be a useful measure of outcome in routine care.
The important secondary outcome of need for reintervention showed a definite advantage for open urethroplasty, which had a 48% lower rate of recurrence over a minimum of 24 months and up to 4 years of observation, greater than the minimum important difference of 30% used in a secondary power calculation, and was statistically significant. A number of studies have investigated the usefulness of adjuncts that can be used at the time of, and following, urethrotomy to increase duration of benefit and decrease rate of recurrence, and hence overcome this main drawback of endoluminal treatment of stricture.
A number of participants undertook a period of intermittent self-dilatation with a plastic catheter following urethrotomy, which does have some evidence of effectiveness to support its use. 18 The numbers were too small, however, to justify post hoc subgroup analysis in the OPEN trial. Other investigators have trialled a number of drugs injected into the strictures area at the time of urethrotomy, including steroids92 and mitomycin C,93 but there is no robust evidence of benefit. 94 Another approach is to maintain luminal patency using an internal stent, but again there is no robust evidence of effectiveness. 95 Perhaps the most appropriate technology would be to combine an effective antifibrotic drug with a drug-eluting stent; developments for application in the field of urethral stricture are awaited. 96
Minor self-limiting adverse effects, particularly mouth pain from the graft donor site, were more common after urethroplasty, but severe complications were few for either procedure. Refinements to the urethroplasty technique to help minimise technical failure continue to be explored but the current results do appear stable and equivalent between different surgeons, countries and health-care systems. 97
From a health-care funder and planner perspective, the results suggest that both procedures should be available to men seeking relief of symptoms. We did not find that any particular subgroup of men defined by age or the number of previous interventions were more or less likely to benefit from either. Inclusion of these possible confounders and others did not modify the relative treatment effect. Higher procedure cost of urethroplasty is partially mitigated by the lower requirement for retreatment and the improvement in health state was similar for the two procedures at 2 years, although QALYs may be higher at 10 years for urethroplasty because of the lower requirement for retreatment. Nevertheless, for both the within-trial and model-based analyses, there was very little likelihood that urethroplasty would be considered cost-effective at values for a QALY that might be judged acceptable.
Our difficulties in recruitment did highlight the need for explicit and efficient care pathways to enable men to access urethroplasty, including change of provider if needed. Overall, the results of the OPEN trial provide the evidence required to enable well-informed shared decision-making discussions between patients and their urologists. The results will influence guideline formulation and planning of health-care provision.
Strengths and limitations
The OPEN trial was carried out in accordance with current best practice for pragmatic surgical trials. We used a remote internet-based randomisation system with the assignment algorithm written by an independent statistician to ensure concealment of allocation. This included stratification for the most important confounder: stricture severity defined by the time since previous intervention. We identified other possible confounders from our literature review. All likely confounders were well balanced across the two groups and their inclusion in the statistical model did not influence the primary result. The nature of the interventions did not allow blinding of participants, clinicians or local research teams to allocation, although central trial staff were blinded to allocated group when possible. There was no obvious and feasible way to overcome this limitation. However, we think the repeated measure of outcome mitigates against this, given that likely demoralisation (or other) effects would dissipate over time from initial intervention. Although the primary outcome was subjective, we chose it because it was of most importance to men suffering recurrent stricture, as demonstrated by our qualitative study. 3 We also included, as secondary outcomes, objective measures favoured by clinicians and a thorough health economic evaluation useful to funders and planners of health care. The randomised study design helps to minimise the risk of bias inherent in previous cohort studies. We anticipated that, in common with other pragmatic trials of surgical procedures being undertaken at the time of the OPEN trial, recruitment would be difficult. 98 To address this, we undertook a qualitative study as part of the feasibility phase. The study found wide support for the trial among men with recurrent stricture and urologists, which motivated us to persist with the trial. In addition, the qualitative study suggested two design modifications that were carried out: first, to establish general urology practices as research sites, with the aim of introducing the trial to men before they had formed strong preferences; and, second, to provide information resources to aid clinicians in balanced recruitment discussions. This was done by writing vignettes for use at site initiation visits and by producing a video showing how beliefs and preferences among possible participants could be challenged, to ensure a balanced discussion around possible trial participation. We also used emerging data as a more certain basis for a revised sample size calculation, which was checked and approved by external monitors of trial progress, and allowed a more realistic recruitment target to be set. The final trial results validated the assumptions made in the revised sample size calculation, with a SD for the primary outcome, scaled from 0 to 1, being 0.17 compared with the 0.21 that we assumed in the revised sample size calculation. These changes resulted in an increased recruitment rate over the final 12 months of the study, enabling attainment of the revised target sample size.
In common with previous clinical studies for this condition, attrition rates were relatively high and missing data points were frequent. For the primary complete-case ITT analysis we included 68% of those men randomised, a higher attrition rate (32%) than we anticipated (19%). The results of sensitivity analyses maintaining the groups as randomised and where multiple imputation for missing data was performed were able to include 90% of those randomised. In contrast a per-protocol analysis could include only 61% of those randomised. For all three analyses the proportion of included participants was lower in the urethroplasty group, owing to a lower questionnaire return rate, and, for the per-protocol analysis, a higher rate of crossover to the alternative intervention. This might be due to the longer interval between randomisation and intervention in the urethroplasty group. Despite these procedural difficulties, all three analyses showed consistent results for the primary outcome and the rate of reintervention. There are various degrees of missing EQ-5D-5L data across the different data collection points and, although this is inevitable for a surgical trial, it may affect the study results. Sensitivity analysis using multiple imputations, however, suggested that the study results were robust.
A further limitation of the study is the imbalance in the proportion of randomised participants who received no intervention during the follow-up period (13.9% of the urethroplasty group and 7.1% of the urethrotomy group). The reasons for this imbalance are unknown, although the urethroplasty arm had longer waiting times between randomisation and intervention. Urethroplasty is usually available in specialty centres, whereas urethrotomy is more widely available. The imbalance between the two groups could also be due to the perceived intensity of the procedures. In any case, this could lead to biased estimates.
Generalisability
Baseline characteristics of the OPEN trial population were similar to recent published cohorts of men undergoing urethroplasty or urethrotomy. We found five recent studies validating translation of the USS-PROM from English into various languages. 31–35 The improvements found in voiding score were similar to the group of men allocated urethroplasty in the OPEN trial. The rate of recurrence following urethrotomy and the improvement in measured flow rate found in the urethrotomy group were also similar to the findings of recent published cohorts. 15,99 A qualitative study from the USA found that men with recurrent stricture had similar concerns, particularly fear of retention, as documented in our qualitative study. 21 It would therefore appear that the OPEN trial results are generalisable to the wider population of men suffering recurrence of bulbar urethra stricture across the world.
Future work
-
Formulate methods to incorporate short-term disutility data into the main cost-effectiveness analysis.
-
Survey pathways of care for men with urethral stricture across the UK NHS, including use of enhanced recovery after urethroplasty.
-
Use other qualitative data and quantitative trial data to validate TTO findings.
-
Establish a pragmatic and achievable follow-up schedule to allow national audit of outcomes following urethral surgery, with linkage to UK NHS Hospital Episode Statistics.
Chapter 7 Conclusions
The OPEN RCT provides unique high-level evidence of the relative benefits of open urethroplasty compared with endoscopic urethrotomy to manage recurrent bulbar urethral stricture in men. The trial showed no difference in the outcome of most importance to men with recurrent stricture, voiding symptom control, but did show a lower rate of recurrence and a higher rate of improvement in measured urinary flow rate in the urethroplasty group: outcomes that appear to be of lesser importance to patients but which are more valued by clinicians and providers of health care. This evidence will help inform discussions in the clinic and provision of impartial patient information. The current evidence on cost-effectiveness suggests that urethroplasty is highly unlikely to be considered cost-effective. However, this is mainly driven by the higher cost of urethroplasty compared with urethrotomy. Efforts to reduce the procedure cost for urethroplasty would substantially increase the likelihood that it could be cost-effective. Should that occur, then this would reinforce conclusions that the choice between urethrotomy and urethroplasty will remain preference sensitive, whereby men seeking treatment and clinicians advising them should come to a shared decision, guided by robust evidence provide by the OPEN trial and taking into account each individual’s values and preferences. Health-care planners and funders should ensure equitable and ready access to the specialist care required for urethroplasty.
Recommendations for research
-
Determine the most efficient pathway of care for men with urethral stricture, including enhanced recovery after urethroplasty.
-
Identify factors driving choice of treatment in men with bulbar urethral stricture.
-
Determine the most appropriate method of defining stricture recurrence.
-
Experiment with adjunctive treatments that may lessen risk of recurrence after urethrotomy.
-
A RCT to compare outcomes from non-transecting with transecting anastomotic urethroplasty.
Acknowledgements
We thank the patients and health-care professionals for their participation in qualitative interviews; Stewart Barclay, the patient and service user representative in the OPEN TMG; the TSC members, Roger Kockelburg (chairperson), John Matthews, Alan McNeil, Howard Kynaston and Neil Campling; DMC members, Gordon Murray (chairperson), Richard Martin and Thomas Pinkney. We also thank the following people who worked on the trial: Matthew Jackson, Research Fellow; Gladys McPherson, Data Manager; Lee Munro, Trial Manager; Rachel Stephenson, Trial Manager; Sue Tremble, Trial Manager; Robbie Brown, Trial Manager; Mark Deverill, Health Economist; Amy Collins, Project Secretary; Lavinia Miceli, Project Secretary; and Ann Payne, Project Secretary.
We thank Sarah Hill and David Mott, who contributed to the development and interviews of the TTO exercise; Joanne O’Connor and Beena David, who contributed to the interviews of the TTO exercise; and Peter Murphy and Wendy Robson for providing assistance when conducting TTO pilots in Freeman Hospital.
We thank all the volunteers and participants who took part in the TTO pilots and interviews, and the following sites and PIs for their support:
Trevor Dorkin, Freeman Hospital, Newcastle upon Tyne; Nick Watkin, St George’s Hospital, London; Anthony Mundy, University College London Hospitals NHS Foundation Trust, London; Paul Anderson, Russells Hall Hospital, West Midlands; Suzie Venn, Queen Alexandra Hospital, Portsmouth; Ian Eardley, St James’s University Hospital, Leeds; Mr David Dickerson, Weston General Hospital, Weston-super-Mare; Nikesh Thiruchelvam, Addenbrooke’s Hospital, Cambridge; Richard Inman and Chris Chapple, Royal Hallamshire Hospital, Sheffield; Andrew Baird, Aintree University Hospital, Liverpool; Andrew Sinclair, Stepping Hill Hospital, Stockport; Rajeshwar Krishnanm, Kent and Canterbury Hospital, Canterbury; Rowland Rees, Southampton General Hospital, Southampton; James N’dow, Aberdeen Royal Infirmary, Aberdeen; Bruce Montgomery, Frimley Park Hospital, Surrey; Michael Swinn, East Surrey Hospital, Surrey; Alastair Henderson and John Donohue, Maidstone Hospital, Kent; Suzie Venn, St Richard’s Hospital, Chichester; Robert Mason, Torbay Hospital, Torquay; Sanjeev Madaan, Darent Valley Hospital, Kent; Mustafa Hilmy, York Hospital, York; Vivienne Kirchin, Sunderland Royal Hospital, Sunderland; Kim Davenport, Cheltenham General Hospital, Cheltenham; John McGrath, Royal Devon Exeter, Hospital, Exeter; Tim Porter, Yeovil District Hospital, Yeovil; Ruaraidh MacDonagh and Amerdip Birring, Musgrove Park Hospital, Taunton; Ramachandran Ravi, Basildon University Hospital, Essex; Jawad Husain, Royal Albert Edward Infirmary, Wigan; Maj Shabbir, Guy’s Hospital, London; Omer Baldo, Airedale General Hospital, West Yorkshire; Sadhanshu Chitale, Whittington Hospital, London; Mary Garthwaite, James Cook University Hospital, Middlesbrough; Shalom Srirangam, Royal Blackburn Hospital, Blackburn; Liaqat Chowoo, Bedford Hospital, Bedfordshire; Tina Rashid, Charing Cross Hospital, London; Rob Skyrme and Jon Featherstone, Princess of Wales Hospital, Bridgend; Ammar Alhasso, Western General Hospital, Edinburgh; and Oleg Tatarov, University Hospital of Wales, Cardiff.
We thank the following trusts for offering participant identification centre support:
Basingstoke and Northamptonshire NHS Foundation Trust; Royal Liverpool and Broadgreen University Hospitals NHS Trust; Chelsea and Westminster NHS Foundation Trust; and Wirral University Teaching Hospital NHS Foundation Trust.
Contributions of authors
Robert Pickard (Professor of Urology) led the study and wrote the final report.
Beatriz Goulao (Reseach Fellow, Statistician) supervised by Graeme MacLennan (Professor of Medical Statistics and Director of CHaRT) performed the statistical analysis and co-wrote the final report.
Sonya Carnell (Senior Trial Manager, NCTU) and Rebecca Forbes (Trial Manager, NCTU) supported by Stephanie Currer (Trial Administrator, NCTU) and supervised by Jennifer Wilkinson (Senior Trial Manager, NCTU) managed the trial and co-wrote the final report.
Jing Shen (Senior Research Associate, Health Economist), supervised by Luke Vale (Professor of Health Economics), led the health economic evaluation and analysis, and co-wrote the final report.
John Norrie (Professor of Medical Statistics and Trial Methodology/Director of Edinburgh Clinical Trials Unit) contributed to the funding application and SAP. He commented on the final report and provided support to the statistical analysis team.
Matt Breckons (Research Associate, Qualitative Researcher), supervised by Jing Shen, carried out and wrote-up the TTO experiment.
Paul Whybrow (Research Associate, Qualitative Researcher), supervised by Tim Rapley (Professor, Medical Sociologist), carried out the qualitative research and co-wrote the final report.
Mark Forrest (Senior Information Technology Development Manager, CHaRT) managed and maintained the trial database.
Elaine McColl (Professor of Health Service Research) contributed to the funding application and protocol, and co-wrote the final report.
Daniela Andrich (Consultant Reconstructive Urological Surgeon), Anthony Mundy (Professor of Urology), James N’Dow (Professor of Urology) and Stephen Payne (Consultant Urological Surgeon) provided clinician support to the funding application, acted as PIs at key sites, and read and commentated on the final report from a clinician perspective.
Stewart Barclay (Patient Representative) contributed a patient view to the trial, including the funding application, the protocol, input to the TMG and writing the final report.
Jonathan Cook (Associate Professor) was involved in the design of the study, interpretation and commented on the report. He also advised on sensitivity analyses around the primary result.
Nick Watkin (Professor of Urology) co-wrote the funding application, contributed clinical insight to the TMG and commented on the final report.
Publications
Stephenson R, Carnell S, Johnson N, Brown R, Wilkinson J, Mundy A, et al. Open urethroplasty versus endoscopic urethrotomy – clarifying the management of men with recurrent urethral stricture (the OPEN trial): study protocol for a randomized controlled trial. Trials 2015;16:600.
Whybrow P, Rapley T, Pickard R, Hrisos S. How men manage bulbar urethral stricture by concealing urinary symptoms. Qual Health Res 2015;25:1435–42.
Whybrow P, Pickard R, Hrisos S, Rapley T. Equipoise across the patient population: optimising recruitment to a randomised controlled trial. Trials 2017;18:140.
Shen J, Breckons M, Vale L, Pickard R, for the OPEN trial investigators. Using time trade-off methods to elicit short-term utilities associated with treatments for bulbar urethral stricture. Pharmacoecon Open 2019;3:551–8.
Goulao B, Carnell S, Shen S, MacLennan G, Norrie J, Cook J, et al. Surgical treatment for recurrent bulbar urethral stricture: a randomised open-label superiority trial of open urethroplasty versus endoscopic urethrotomy (the OPEN trial). Eur Urol 2020;78:572–80.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Stephenson R, Carnell S, Johnson N, Brown R, Wilkinson J, Mundy A, et al. Open urethroplasty versus endoscopic urethrotomy – clarifying the management of men with recurrent urethral stricture (the OPEN trial): study protocol for a randomised controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-1120-4.
- Whybrow P, Pickard R, Hrisos S, Rapley T. Equipoise across the patient population: optimising recruitment to a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-016-1711-8.
- Whybrow P, Rapley T, Pickard R, Hrisos S. How men manage bulbar urethral stricture by concealing urinary symptoms. Qual Health Res 2015;25:1435-42. https://doi.org/10.1177/1049732315573208.
- Watkin N, Patel P. The diagnosis and management of acquired urethral stricture disease. Surgery 2017;35:313-23.
- Tritschler S, Roosen A, Füllhase C, Stief CG, Rübben H. Urethral stricture: etiology, investigation and treatments. Dtsch Arztebl Int 2013;110:220-6. https://doi.org/10.3238/arztebl.2013.0220.
- Santucci R, Eisenberg L. Urethrotomy has a much lower success rate than previously reported. J Urol 2010;183:1859-62. https://doi.org/10.1016/j.juro.2010.01.020.
- Anger JT, Buckley JC, Santucci RA, Elliott SP, Saigal CS. Urologic Diseases in America Project . Trends in stricture management among male Medicare beneficiaries: underuse of urethroplasty?. Urology 2011;77:481-5. https://doi.org/10.1016/j.urology.2010.05.055.
- NHS Digital . Hospital Admitted Patient Care Activity, 2016–2017 2017. https://digital.nhs.uk/ (accessed 20 March 2018).
- Pansadoro V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long-term followup. J Urol 1996;156:73-5. https://doi.org/10.1016/S0022-5347(01)65942-1.
- Barbagli G, Palminteri E, Lazzeri M, Guazzoni G, Turini D. Long-term outcome of urethroplasty after failed urethrotomy versus primary repair. J Urol 2001;165:1918-19. https://doi.org/10.1016/S0022-5347(05)66242-8.
- Kessler TM, Schreiter F, Kralidis G, Heitz M, Olianas R, Fisch M. Long-term results of surgery for urethral stricture: a statistical analysis. J Urol 2003;170:840-4. https://doi.org/10.1097/01.ju.0000080842.99332.94.
- O’Riordan A, Narahari R, Kumar V, Pickard R. Outcome of dorsal buccal graft urethroplasty for recurrent bulbar urethral strictures. BJU Int 2008;102:1148-51. https://doi.org/10.1111/j.1464-410X.2008.07763.x.
- Chapple C, Andrich D, Atala A, Barbagli G, Cavalcanti A, Kulkarni S, et al. SIU/ICUD consultation on urethral strictures: the management of anterior urethral stricture disease using substitution urethroplasty. Urology 2014;83:31-47. https://doi.org/10.1016/j.urology.2013.09.012.
- Chapple C. Surgical Techniques In Substitution Urethroplasty Using Buccal Mucosa 2011. www.youtube.com/watch?v=It3J0U6tNQs (accessed 9 May 2018).
- Buckley JC, Heyns C, Gilling P, Carney J. SIU/ICUD consultation on urethral strictures: dilation, internal urethrotomy, and stenting of male anterior urethral strictures. Urology 2014;83:18-22. https://doi.org/10.1016/j.urology.2013.08.075.
- Shergill U. Optical Urethrotomy 2011. www.youtube.com/watch?v=8YijhQEHpbs (accessed 5 May 2018).
- Sachse H. Treatment of urethral stricture: transurethral slit in view using sharp section. Fortschr Med 1974;92:12-5.
- Jackson MJ, Veeratterapillay R, Harding CK, Dorkin TJ. Intermittent self-dilatation for urethral stricture disease in males. Cochrane Database Syst Rev 2014;12. https://doi.org/10.1002/14651858.CD010258.pub2.
- Wong SS, Aboumarzouk OM, Narahari R, O’Riordan A, Pickard R. Simple urethral dilatation, endoscopic urethrotomy, and urethroplasty for urethral stricture disease in adult men. Cochrane Database Syst Rev 2012;12. https://doi.org/10.1002/14651858.CD006934.pub3.
- Wessells H, Angermeier KW, Elliott S, Gonzalez CM, Kodama R, Peterson AC, et al. Male urethral stricture: American Urological Association guideline. J Urol 2017;197:182-90. https://doi.org/10.1016/j.juro.2016.07.087.
- Breyer BN, Edwards TC, Patrick DL, Voelzke BB. Comprehensive qualitative assessment of urethral stricture disease: toward the development of a patient centered outcome measure. J Urol 2017;198:1113-18. https://doi.org/10.1016/j.juro.2017.05.077.
- Rapp DE, Chanduri K, Infusino G, Hoda ZA, Orvieto MA, Elliott SP, et al. Internet survey of management trends of urethral strictures. Urol Int 2008;80:287-91. https://doi.org/10.1159/000127343.
- Ferguson GG, Bullock TL, Anderson RE, Blalock RE, Brandes SB. Minimally invasive methods for bulbar urethral strictures: a survey of members of the American Urological Association. Urology 2011;78:701-6. https://doi.org/10.1016/j.urology.2011.02.051.
- van Leeuwen MA, Brandenburg JJ, Kok ET, Vijverberg PL, Bosch JL. Management of adult anterior urethral stricture disease: nationwide survey among urologists in the Netherlands. Eur Urol 2011;60:159-66. https://doi.org/10.1016/j.eururo.2011.03.016.
- Granieri MA, Peterson AC. The management of bulbar urethral stricture disease before referral for definitive repair: have practice patterns changed?. Urology 2014;84:946-9. https://doi.org/10.1016/j.urology.2014.06.014.
- Bullock TL, Brandes SB. Adult anterior urethral strictures: a national practice patterns survey of board certified urologists in the United States. J Urol 2007;177:685-90. https://doi.org/10.1016/j.juro.2006.09.052.
- Greenwell TJ, Castle C, Andrich DE, MacDonald JT, Nicol DL, Mundy AR. Repeat urethrotomy and dilation for the treatment of urethral stricture are neither clinically effective nor cost-effective. J Urol 2004;172:275-7. https://doi.org/10.1097/01.ju.0000132156.76403.8f.
- Rourke KF, Jordan GH. Primary urethral reconstruction: the cost minimized approach to the bulbous urethral stricture. J Urol 2005;173:1206-10. https://doi.org/10.1097/01.ju.0000154971.05286.81.
- Wright JL, Wessells H, Nathens AB, Hollingworth W. What is the most cost-effective treatment for 1 to 2-cm bulbar urethral strictures: societal approach using decision analysis. Urology 2006;67:889-93. https://doi.org/10.1016/j.urology.2005.11.003.
- Jackson MJ, Sciberras J, Mangera A, Brett A, Watkin N, N’dow JM, et al. Defining a patient-reported outcome measure for urethral stricture surgery. Eur Urol 2011;60:60-8. https://doi.org/10.1016/j.eururo.2011.03.003.
- Kluth LA, Dahlem R, Becker A, Schmid M, Soave A, Rosenbaum C, et al. Psychometric validation of a German language version of a PROM for urethral stricture surgery and preliminary testing of supplementary ED and UI constructs. World J Urol 2016;34:369-75. https://doi.org/10.1007/s00345-015-1610-8.
- Önol FF, Bindayi A, Tahra A, Basibuyuk I, Onol SY. Turkish validation of the urethral stricture surgery specific patient-reported outcome measure (USS-PROM) with supplemental assessment of erectile function and morbidity due to oral graft harvesting. Neurourol Urodyn 2017;36:2089-95. https://doi.org/10.1002/nau.23243.
- Bazaev VV, Shibaev AN, Pavlova YV. Validation of the Russian version of the questionnaire to assess the effectiveness of surgical treatment of patients with anterior urethral stricture (Patient-Reported Outcome Measure for Urethral Stricture Surgery (PROM-USS): a pilot study. Urologiia 2015;5:15-9.
- Puche-Sanz I, Martín-Way D, Flores-Martín J, Expósito-Ruiz M, Vicente-Prados J, Nogueras-Ocaña M, et al. Psychometric validation of the Spanish version of the USS-PROM questionnaire for patients who undergo anterior urethral surgery. Actas Urol Esp 2016;40:322-7. https://doi.org/10.1016/j.acuro.2016.01.002.
- Lucas ET, Koff WJ, Rosito TE, Berger M, Bortolini T, Neto BS. Assessment of satisfaction and Quality of Life using self-reported questionnaires after urethroplasty: a prospective analysis. Int Braz J Urol 2017;43:304-10. https://doi.org/10.1590/S1677-5538.IBJU.2016.0207.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. https://doi.org/10.1016/S0090-4295(97)00238-0.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Erickson BA, Breyer BN, McAninch JW. Changes in uroflowmetry maximum flow rates after urethral reconstructive surgery as a means to predict for stricture recurrence. J Urol 2011;186:1934-7. https://doi.org/10.1016/j.juro.2011.07.010.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Great Britain . Data Protection Act 1998 1998.
- White IR, Kalaitzaki E, Thompson SG. Allowing for missing outcome data and incomplete uptake of randomised interventions, with application to an Internet-based alcohol trial. Stat Med 2011;30:3192-207. https://doi.org/10.1002/sim.4360.
- Bell ML, King MT, Fairclough DL. Bias in area under the curve for longitudinal clinical trials with missing patient reported outcome data: summary measures versus summary statistics. SAGE Open 2014;4. https://doi.org/10.1177/2158244014534858.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
- Group DS. A proposed charter for clinical trial data monitoring committees: helping them to do their job well. Lancet 2005;365:711-22. https://doi.org/10.1016/S0140-6736(05)70939-9.
- Goulao B, Carnell S, Shen S, MacLennan G, Norrie J, Cook J, et al. Surgical treatment for recurrent bulbar urethral stricture: a randomised open-label superiority trial of open urethroplasty versus endoscopic urethrotomy (the OPEN trial). Eur Urol 2020;78:572-80. https://doi.org/10.1016/j.eururo.2020.06.003.
- The British Association of Urological Surgeons . Patients: Surgical Outcomes Audit. Grading of Surgical Complications n.d. www.baus.org.uk/patients/surgical_outcomes/grading_of_surgical_complications.aspx (accessed 29 November 2019).
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7. https://doi.org/10.1186/1745-6215-7-9.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002889.
- Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open 2012;2. https://doi.org/10.1136/bmjopen-2011-000496.
- Elliott D, Husbands S, Hamdy FC, Holmberg L, Donovan JL. Understanding and improving recruitment to randomised controlled trials: qualitative research approaches. Eur Urol 2017;72:789-98. https://doi.org/10.1016/j.eururo.2017.04.036.
- Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002360.
- Wade J, Donovan JL, Lane JA, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018-28. https://doi.org/10.1016/j.socscimed.2009.02.023.
- Donovan JL, de Salis I, Toerien M, Paramasivan S, Hamdy FC, Blazeby JM. The intellectual challenges and emotional consequences of equipoise contributed to the fragility of recruitment in six randomized controlled trials. J Clin Epidemiol 2014;67:912-20. https://doi.org/10.1016/j.jclinepi.2014.03.010.
- Bower P, Brueton V, Gamble C, Treweek S, Smith CT, Young B, et al. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-399.
- Kaur G, Hutchison I, Mehanna H, Williamson P, Shaw R, Tudur Smith C. Barriers to recruitment for surgical trials in head and neck oncology: a survey of trial investigators. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002625.
- Canvin K, Jacoby A. Duty, desire or indifference? A qualitative study of patient decisions about recruitment to an epilepsy treatment trial. Trials 2006;7. https://doi.org/10.1186/1745-6215-7-32.
- McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-31.
- Murdoch M, McColl E, Howel D, Deverill M, Buckley BS, Lucas M, et al. INVESTIGATE-I (INVasive Evaluation before Surgical Treatment of Incontinence Gives Added Therapeutic Effect?): study protocol for a mixed methods study to assess the feasibility of a future randomised controlled trial of the clinical utility of invasive urodynamic testing. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-169.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70. https://doi.org/10.1136/bmj.325.7367.766.
- Rapley T, Silverman D. Qualitative Research: Theory, Method and Practice. London: Sage Publications Ltd; 2010.
- Glaser BG. The constant comparative method of qualitative analysis. Social Probl 1965;12:436-45. https://doi.org/10.2307/798843.
- Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. London: Sage; 1994.
- Clarke AE. Situational Analysis: Grounded Theory After the Postmodern Turn. London: Sage; 2005.
- Weitzman EA. Analyzing qualitative data with computer software. Health Serv Res 1999;34.
- Courtenay WH. Constructions of masculinity and their influence on men’s well-being: a theory of gender and health. Soc Sci Med 2000;50:1385-401. https://doi.org/10.1016/S0277-9536(99)00390-1.
- Bower P, King M, Nazareth I, Lampe F, Sibbald B. Patient preferences in randomised controlled trials: conceptual framework and implications for research. Soc Sci Med 2005;61:685-95. https://doi.org/10.1016/j.socscimed.2004.12.010.
- Huddart RA, Hall E, Lewis R, Birtle A. on behalf of the SPARE Trial Management Group . Life and death of spare (selective bladder preservation against radical excision): reflections on why the spare trial closed. BJU Int 2010;106:753-5. https://doi.org/10.1111/j.1464-410X.2010.09537.x.
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999;52:1143-56. https://doi.org/10.1016/S0895-4356(99)00141-9.
- Locock L, Smith L. Personal benefit, or benefiting others? Deciding whether to take part in clinical trials. Clin Trials 2011;8:85-93. https://doi.org/10.1177/1740774510392257.
- Wilson C, Rooshenas L, Paramasivan S, Elliott D, Jepson M, Strong S, et al. Development of a framework to improve the process of recruitment to randomised controlled trials (RCTs): the SEAR (Screened, Eligible, Approached, Randomised) framework. Trials 2018;19. https://doi.org/10.1186/s13063-017-2413-6.
- Blencowe NS, Cook JA, Pinkney T, Rogers C, Reeves BC, Blazeby JM. Delivering successful randomized controlled trials in surgery: methods to optimize collaboration and study design. Clin Trials 2017;14:211-18. https://doi.org/10.1177/1740774516687272.
- Rooshenas l, Townsend D, Wade J, Wilson C, Blazeby J, Donovan J. Equipoise in action: a qualitative investigation across six pragmatic randomised controlled trials (RCTs). Trials 2015;16. https://doi.org/10.1186/1745-6215-16-S2-P115.
- Paramasivan S, Rogers CA, Welbourn R, Byrne JP, Salter N, Mahon D, et al. Enabling recruitment success in bariatric surgical trials: pilot phase of the By-Band-Sleeve study. Int J Obes 2017;41:1654-61. https://doi.org/10.1038/ijo.2017.153.
- Powell BJ, McMillen JC, Proctor EK, Carpenter CR, Griffey RT, Bunger AC, et al. A compilation of strategies for implementing clinical innovations in health and mental health. Med Care Res Rev 2012;69:123-57. https://doi.org/10.1177/1077558711430690.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Office for National Statistics . Consumer Prices Index 2018. www.ons.gov.uk/economy/inflationandpriceindices/timeseries/l522/mm23 (accessed April 2018).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- ISD Scotland . Theatre Services 2018. www.isdscotland.org/Health-Topics/Finance/Costs/Detailed-Tables/Theatres.asp (accessed 9 May 2018).
- NHS Business Services Authority . NHS Dictionary of Medicines and Devices Database n.d. www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/dictionary-medicines-and-devices-dmd (accessed May 2018).
- Matthews J, Altman D, Campbell M, Royston P. Analysis of serial measurements in medical research: authors’ reply. BMJ 1990;300. https://doi.org/10.1136/bmj.300.6725.680-a.
- Jansen SJ, Kievit J, Nooij MA, Stiggelbout AM. Stability of patients’ preferences for chemotherapy: the impact of experience. Med Decis Making 2001;21:295-306. https://doi.org/10.1177/0272989X0102100405.
- Locadia M, Stalmeier PF, Oort FJ, Prins MH, Sprangers MA, Bossuyt PM. A comparison of 3 valuation methods for temporary health states in patients treated with oral anticoagulants. Med Decis Making 2004;24:625-33. https://doi.org/10.1177/0272989X04271042.
- Jansen SJ, Stiggelbout AM, Wakker PP, Vliet Vlieland TP, Leer JW, Nooy MA, et al. Patients’ utilities for cancer treatments: a study of the chained procedure for the standard gamble and time tradeoff. Med Decis Making 1998;18:391-9. https://doi.org/10.1177/0272989X9801800406.
- Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ 1986;5:1-30. https://doi.org/10.1016/0167-6296(86)90020-2.
- Shen J, Breckons M, Vale L, Pickard R. Using time trade-off methods to elicit short-term utilities associated with treatments for bulbar urethral stricture. Pharmacoecon Open 2019. https://doi.org/10.1007/s41669-019-0133-4.
- Poelaert F, Oosterlinck W, Spinoit A-F, Lumen N. Duration of urethral catheterization after urethroplasty: how long is enough?. Minerva Urol Nefrol 2017;69:372-6.
- Consolo MJ, Syed KK, Robison C, McFadden J, Shalowitz DI, Brown GA, et al. Barriers to accessing urethroplasty. Rev Urol 2016;18:188-93. https://doi.org/10.3909/riu0731.
- MacDonald S, Haddad D, Choi A, Colaco M, Terlecki R. Anterior urethroplasty has transitioned to an outpatient procedure without serious rise in complications: data from the National Surgical Quality Improvement Program. Urology 2017;102:225-8. https://doi.org/10.1016/j.urology.2016.09.043.
- Belsante MJ, Zhao LC, Hudak SJ, Lotan Y, Morey AF. Cost-effectiveness of risk stratified followup after urethral reconstruction: a decision analysis. J Urol 2013;190:1292-7. https://doi.org/10.1016/j.juro.2013.04.024.
- Peeling WB. Diagnostic assessment of benign prostatic hyperplasia. Prostate Suppl 1989;2:51-68. https://doi.org/10.1002/pros.2990150507.
- Meeks JJ, Erickson BA, Granieri MA, Gonzalez CM. Stricture recurrence after urethroplasty: a systematic review. J Urol 2009;182:1266-70. https://doi.org/10.1016/j.juro.2009.06.027.
- Yıldırım ME, Kaynar M, Ozyuvali E, Badem H, Cakmak M, Kosem B, et al. The effectiveness of local steroid injection after internal urethrotomy to avoid recurrence. Arch Ital Urol Androl 2016;87:295-8. https://doi.org/10.4081/aiua.2015.4.295.
- Mazdak H, Meshki I, Ghassami F. Effect of mitomycin C on anterior urethral stricture recurrence after internal urethrotomy. Eur Urol 2007;51:1089-92. https://doi.org/10.1016/j.eururo.2006.11.038.
- Mundy AR. Adjuncts to visual internal urethrotomy to reduce the recurrence rate of anterior urethral strictures. Eur Urol 2007;51:1467-8. https://doi.org/10.1016/j.eururo.2007.02.061.
- McNamara ER, Webster GD, Peterson AC. The UroLume stent revisited: the Duke experience. Urology 2013;82:933-6. https://doi.org/10.1016/j.urology.2013.06.017.
- Zilberman M, Eberhart RC. Drug-eluting bioresorbable stents for various applications. Annu Rev Biomed Eng 2006;8:153-80. https://doi.org/10.1146/annurev.bioeng.8.013106.151418.
- Horiguchi A. Substitution urethroplasty using oral mucosa graft for male anterior urethral stricture disease: current topics and reviews. Int J Urol 2017;24:493-50. https://doi.org/10.1111/iju.13356.
- Janye D. Synopsis of the FIAT Trial – The Fistula-In-Ano Trial n.d. www.birmingham.ac.uk/Documents/college-mds/trials/bctu/fiat/FIATTrialSynopsis.pdf (accessed 11 May 2018).
- Jhanwar A, Kumar M, Sankhwar SN, Prakash G. Holmium laser vs. conventional (cold knife) direct visual internal urethrotomy for short-segment bulbar urethral stricture: outcome analysis. Can Urol Assoc J 2016;10:E161-E164. https://doi.org/10.5489/cuaj.3382.
- Brazier J, Brazier J, Ratcliffe J, Saloman J, Tsuchita A. Measuring and Valuing Health Benefits for Economic Evaluation. Oxford: Oxford University Press; 2007.
Appendix 1 Time trade-off protocol
To understand the short-term disutility of trade-offs that individuals may make in choosing between the two procedures of open urethroplasty and endoscopic urethrotomy, we will conduct a TTO exercise among a group of men eligible for trial participation and consented for this substudy, alongside the main trial.
Time trade-off theory
The TTO method is used to measure an individual’s preference for particular health states. These preferences are measured in the form of utility values which, in the health context, are an assessment of how ‘good’ or ‘bad’ a health state is. Thus, different degrees of impairment can be weighted (or given a utility value) between 0 and 1, in which 0 is assumed to be equated to ‘being dead’ and 1 is equated with ‘being in full/perfect health’.
Deriving utility values using the TTO method, health improvements are valued in terms of the amount of time an individual is prepared to spend in a worse health state in order to achieve a defined better health state. In the case of negative health consequences, disutility is measured in terms of the amount of time in perfect health an individual is prepared to sacrifice in order to avoid a defined worse health state.
Owing to the nature of the study, in which we are investigating short-term disutility, in addition to the conventional method of conducting TTO, we will also be using a chained method, and results from both methods will be compared. The conventional TTO method is commonly used to measure the utility of chronic health states in which participants typically remain in the impaired health state for the remainder of their lives. Temporary health states, however, only last for a defined period of time (e.g. days, weeks) before a return to normal health and, as a result, the conventional TTO method may become less feasible to use with temporary health states, as respondents may find it difficult to associate the severity of short-term impairment with death as a stated end point. 81,82 In the chained method, an anchoring state is created to be used as a bridge between the temporary states and death. 84
Recruitment
The TTO will be conducted among the target patient population of men with recurrent bulbar urethral strictures. Patients will be identified by NHS clinical staff (principally consultant and trainee urologists) at participating centres as part of the trial. They will either be new referrals from primary care or men already under review in urology clinics. Following screening, eligible patients will be approached for their interest in participating (being randomised to a procedure) in the main trial. For both those men who consent to randomisation and those men who decline randomisation, they will also be asked for an expression of interest in participating in a 60-minute structured interview (TTO exercise), to explore their valuation of the health states with the immediate consequences of the two alternative procedures (Table 36).
| Procedure | Common and well-understood consequences of treatment | Less common and unpleasant side effects | Rare events |
|---|---|---|---|
| Endoscopic urethrotomy |
Mild urethral bleeding Local urinary tract infection Catheter discomfort Transient discomfort on passing urine after catheter removal |
Severe bleeding requiring reintervention Systemic urinary infection (urosepsis) requiring parental antibiotics Transient (up to 12 months) erectile dysfunction |
Extravasation of urine Persistent erectile dysfunction (> 12 months) |
| Open urethroplasty |
Mild mouth pain/discomfort if oral mucosal graft harvested Perineal wound pain Catheter discomfort Localised wound infection Local urinary tract infection Delayed healing requiring prolonged period of catheterisation Transient discomfort on passing urine after catheter removal Post-micturition dribble |
Severe mouth pain if oral mucosal graft harvested Scarring in mouth after oral mucosal harvest Severe wound infection affecting deep layers Systemic urinary infection (urosepsis) requiring parental antibiotics Transient (up to 12 months) erectile dysfunction |
Fistula formation Persistent erectile dysfunction (> 12 months) |
Those men who express initial interest in participating in the TTO interview will be sent an invitation letter and the participant information sheet for this substudy. The patients will be asked to get in contact with the researchers if they wish to take part in the TTO study or require more information. If the patient fails to make contact after being sent the invitation letter and patient information sheet, we may try to make contact with them via telephone to gauge their continuing interest in the TTO study. Interviews will be arranged at a convenient time and place for the participants, and written consent will be sought before the start of the interviews.
Health state profiles
Six health state profiles will be developed which describe the health consequences and discomforts immediately following the two procedures of open urethroplasty and endoscopic urethrotomy (three profiles for each procedure, representing ‘best case’, ‘moderate case’ and ‘worse case’ profiles for each procedure). The profiles are compiled based on information from health-care professionals on the likely consequences following each procedure, as well as findings from the qualitative interviews conducted in the pilot phase of the trial, in which patients provided a more personal and plausible account of their experience. The different health consequences and discomforts following each procedure can last for varying lengths of time, ranging from a few days to 4 weeks, and for consistency we chose 14 days as the length of the time used in the TTO study to value the health states.
In addition to the health state profiles for the effects of the procedures, there will also be a ‘perfect health’ profile and an anchoring state profile describing ‘severe pain’. The anchor state profile will be used in the chained TTO81,82 in two stages: in the first stage it is evaluated as a temporary health state after which perfect health would resume, in the second stage it is evaluated as a short chronic state followed by death.
We will also use a set of practice profiles developed from the EQ-5D. These will be used to introduce the TTO method to participants at the beginning of the task, so that they become familiar with the procedure prior to completing the TTO exercise with the health states to be valued. For the chained TTO method we will use a practice profile before each of the two different stages, as the task differs slightly in each stage. Using practice profiles is common practice in TTO studies,100 to reduce confusion with the task and thus increase the validity of the utilities derived.
An A3-size decision board will be used in the TTO exercises to help make the questions understandable to participants. The profiles will be printed on A5-size cards, coloured and laminated, using a different pastel colour for each health profile. During the TTO exercise, the health profiles will be placed on the decision board and the participant will be able to visually compare the length of time for each pair of health profiles being evaluated (detailed in Interview schedule of time trade-off).
Interview schedule of time trade-off
Two methods of TTO will be conducted: the conventional method and the chained method. There is an interview schedule designed for each method. Participants will be randomly allocated to one of the methods. The general approach is similar for each method; however, the chained TTO requires an additional step. In both methods, respondents will also be asked to rank the six health profiles to be valued from the best to the worst. Additionally, sociodemographic information will be collected at the interview to aid final analysis.
The conventional method
Respondents will be offered a choice between two alternative ‘lives’. Life A, containing the less desirable health state (temporary health; hi), is measured relative to life B, which contains the best health state (perfect health; 1). The respondents will be shown on the decision board one of the health state profiles describing the health consequences post treatment for the length of time (t) as life A and the perfect health profile as life B. The time in life A will be fixed at t (14 days), whereas there is a moving slider for the length of time (between 0 and t) spent in life B. Both lives are set at the maximum length t initially. Respondents will be asked to find a point on the board with the slider pointing at a time (x) for life B, at which they are indifferent to the length of time spent in life A (t) and life B (x). This is achieved by asking respondents a series of questions for each profile while moving the slider for life B. ‘Assuming you are either in life A or life B for the specified number of days shown on the board, after which time you will die painlessly, which life would you prefer?’ If the participant has a preference for life B, we will reduce the time (x) spent in life B, and if the respondent prefers life A, we will increase the time (x) spent in life B. The above question will be repeated until a point of indifference is reached, by confirming with the respondent that ‘you consider spending t days in life A is equivalent to spending x days in life B’, then we will take a note for the length of time the respondent settles on (x). We will do the same for all six health profiles to be valued in a random order.
For analysis, the value of each health state (hi) is then calculated as:
The chained method
This method requires two stages of comparison. In the first stage, we will ask the respondents to compare each health state profile (hi) as life A with an anchor state (hj) as life B. As with the conventional method, the number of days (t) in life A will be fixed at 14 days and the number of days (x) in life B will also be initially set at 14 days. Respondents will be asked a similar question to that in the conventional method, but instead of death following the length of time spent in the health states, they would return to full health: ‘Assuming you are either in life A or life B for the specified number of days shown on the board, after which time you will return to full health, which life would you prefer?’ If the participant has a preference for life B, we will increase the time (x) spent in life B; and if the respondent prefers life A, we will reduce the time (x) spent in life B. The above question will be repeated until a point of indifference is reached, by confirming with the respondent that ‘you consider spending t days in life A is equivalent to spending x days in life B’, then we will take a note for the length of time the respondent settles on (x). We will do the same for all six health profiles to be valued in a random order.
The second stage is then carried out to compare the anchor state profile with the perfect health profile. In this stage, participants will choose between the anchor state as life A for 14 (t) days and perfect health as life B for (y) days conducted using the conventional TTO approach. We choose to leave the value as 14 days to be consistent with the rest of the valuations, despite the length of life being relatively short for use in a conventional TTO method.
For analysis, the value of the anchor state (hj) is calculated as:
The utility of each health profile is then calculated as:
Appendix 2 Interview schedule: men who accepted randomisation
Appendix 3 Interview schedule: men who declined randomisation
Appendix 4 Interview schedule: clinicians
Appendix 5 Recruitment vignettes
Recruitment vignettes that are developed from real cases and illustrate patient eligibility and good recruitment practices
Appendix 6 Consolidated Health Economic Evaluation Reporting Standards checklist
The CHEERS checklist: items to include when reporting economic evaluations of health interventions.
| Section/item | Item number | Recommendation | Reported on page number/line number |
|---|---|---|---|
| Title and abstract | |||
| Title | 1 | Identify the study as an economic evaluation or use more specific terms, such as ‘cost-effectiveness analysis’, and describe the interventions compared | Not applicable. Economic evaluation was conducted as part of a clinical trial |
| Abstract | 2 | Provide a structured summary of objectives, perspective, setting, methods (including study design and inputs), results (including base case and uncertainty analyses) and conclusions |
Page vii/lines 4–11 Page viii/lines 23–26 and 29–32 |
| Introduction | |||
| Background and objectives | 3 | Provide an explicit statement of the broader context for the study | Page 5/lines 6–22 |
| Present the study question and its relevance for health policy or practice decisions | |||
| Methods | |||
| Target population and subgroups | 4 | Describe characteristics of the base-case population and subgroups analysed, including why they were chosen | Page 7/lines 33–39 and page 9/lines 1–14 |
| Setting and location | 5 | State relevant aspects of the system(s) in which the decision(s) need(s) to be made | Page 7/lines 15–26 |
| Study perspective | 6 | Describe the perspective of the study and relate this to the costs being evaluated | Page 57/lines 13–15 |
| Comparators | 7 | Describe the interventions or strategies being compared and state why they were chosen | From page 2/line 11 to page 4/line 41 |
| Time horizon | 8 | State the time horizon(s) over which costs and consequences are being evaluated and say why appropriate | Page 57/lines 3–10 |
| Discount rate | 9 | Report the choice of discount rate(s) used for costs and outcomes and say why appropriate | Page 57/lines 13–15 |
| Choice of health outcomes | 10 | Describe what outcomes were used as the measure(s) of benefit in the evaluation and their relevance for the type of analysis performed | From page 60/line 13 to page 61/line 28 |
| Measurement of effectiveness | 11a | Single study-based estimates: describe fully the design features of the single effectiveness study and why the single study was a sufficient source of clinical effectiveness data | From page 7/line 5 to page 22/line 7 |
| 11b | Synthesis-based estimates: describe fully the methods used for identification of included studies and synthesis of clinical effectiveness data | Not applicable | |
| Measurement and valuation of preference based outcomes | 12 | If applicable, describe the population and methods used to elicit preferences for outcomes |
Page 71/line 9 Page 71/lines 32–37 |
| Estimating resources and costs | 13a | Single study-based economic evaluation: describe approaches used to estimate resource use associated with the alternative interventions. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs | Page 57/line 20 to page 60/line 12 |
| 13b | Model-based economic evaluation: describe approaches and data sources used to estimate resource use associated with model health states. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs |
Page 65/line 10 to page 66/line 5 Page 68/lines 1–5/see Table 33 |
|
| Currency, price date and conversion | 14 | Report the dates of the estimated resource quantities and unit costs. Describe methods for adjusting estimated unit costs to the year of reported costs if necessary. Describe methods for converting costs into a common currency base and the exchange rate | Page 57/lines 24 and 25 and page 23/line 4 |
| Choice of model | 15 | Describe and give reasons for the specific type of decision-analytical model used. Providing a figure to show model structure is strongly recommended |
Page 65/lines 3–10 Page 66/see Figure 15 |
| Assumptions | 16 | Describe all structural or other assumptions underpinning the decision-analytical model |
Page 65/lines 3–13 Page 68/lines 1–18 and page 69/lines 1–4 |
| Analytical methods | 17 | Describe all analytical methods supporting the evaluation. This could include methods for dealing with skewed, missing, or censored data; extrapolation methods; methods for pooling data; approaches to validate or make adjustments (such as half-cycle corrections) to a model; and methods for handling population heterogeneity and uncertainty |
Page 67/lines 1 and 2 Page 57/lines 8 and 9 Page 60/lines 13–23 and page 61/lines 1–25 |
| Results | |||
| Study parameters | 18 | Report the values, ranges, references, and, if used, probability distributions for all parameters. Report reasons or sources for distributions used to represent uncertainty where appropriate. Providing a table to show the input values is strongly recommended | Pages 67/see Table 33 |
| Incremental costs and outcomes | 19 | For each intervention, report mean values for the main categories of estimated costs and outcomes of interest, as well as mean differences between the comparator groups. If applicable, report ICERs |
Page 62/lines 3–17/see Table 32 Page 68/lines 23–43/see Table 34 |
| Characterising uncertainty | 20a | Single study-based economic evaluation: describe the effects of sampling uncertainty for the estimated incremental cost and incremental effectiveness parameters, together with the impact of methodological assumptions (such as discount rate, study perspective) | Page 62/lines 8–17 |
| 20b | Model-based economic evaluation: describe the effects on the results of uncertainty for all input parameters, and uncertainty related to the structure of the model and assumptions | Page 68/lines 26–43 | |
| Characterising heterogeneity | 21 | If applicable, report differences in costs, outcomes, or cost-effectiveness that can be explained by variations between subgroups of patients with different baseline characteristics or other observed variability in effects that are not reducible by more information | Not applicable |
| Discussion | |||
| Study findings, limitations, generalisability and current knowledge | 22 | Summarise key study findings and describe how they support the conclusions reached. Discuss limitations and the generalisability of the findings and how the findings fit with current knowledge |
Page 75/lines 41–44 Page 76/lines 35–41 Page 77/lines 33–41 |
| Other | |||
| Source of funding | 23 | Describe how the study was funded and the role of the funder in the identification, design, conduct and reporting of the analysis. Describe other non-monetary sources of support | Page xxv |
| Conflicts of interest | 24 | Describe any potential for conflict of interest of study contributors in accordance with journal policy. In the absence of a journal policy, we recommend authors comply with the International Committee of Medical Journal Editors’ recommendations | Pages iii |
Appendix 7 Health state profiles
Health state profiles used in the study
Control intervention urethrotomy health state profiles
Urethrotomy: mild
-
Discomfort in the penis and bladder from using a catheter for a few days.
-
Brief discomfort on passing urine after the catheter is removed.
-
A few drops of blood after you have finished passing urine.
-
Mild urinary tract infection giving you mild fever-like symptoms.
Urethrotomy: moderate
-
Discomfort in the penis and bladder from using a catheter for a few days.
-
Discomfort on passing urine after the catheter is removed.
-
Moderate urethral bleeding which requires you to keep the catheter in longer or have a telescopic examination under anaesthetic.
-
Serious urinary tract infection which makes you feel ill and requires you to stay in hospital overnight for antibiotics from an intravenous drip.
Urethrotomy: severe
-
Discomfort in the penis and bladder from using a catheter.
-
Severe urethral bleeding which requires you to have a telescopic examination under anaesthetic.
-
Serious urinary tract infection which makes you feel ill and requires you to stay in hospital overnight for antibiotics from an IV drip.
-
Severe pain in the penis and bladder area, requiring you to take regular painkillers.
-
Difficulty getting and maintaining a penile erection for sex.
Experimental intervention urethroplasty health state profiles
Urethroplasty: mild
-
Discomfort in the penis and bladder from using a catheter.
-
Mild mouth pain or discomfort when you eat or drink.
-
Mild urinary tract infection giving you mild fever-like symptoms.
-
Mild swelling and wound pain in the area between the testes and back passage.
Urethroplasty: moderate
-
Discomfort in the penis and bladder from using a catheter.
-
Moderate and constant mouth pain and scarring in the mouth needing regular painkillers.
-
Serious urinary tract and wound infection which makes you feel ill and requires you to stay in hospital overnight for antibiotics from an intravenous drip.
-
Moderate wound pain in the area between the testes and back passage needing regular painkillers.
Urethroplasty: severe
-
Discomfort in the penis and bladder from using a catheter.
-
Severe and constant mouth pain and scarring in the mouth needing regular painkillers.
-
Serious urinary tract and wound infection which makes you feel ill and requires you to stay in hospital overnight for antibiotics from an intravenous drip.
-
Severe wound pain in the area between the testes and back passage, needing regular painkillers.
-
Leakage of urine from the area between the testes and back passage, requiring you to wear incontinence pads.
-
Difficulty getting and maintaining a penile erection for sex.
List of abbreviations
- AE
- adverse event
- AUC
- area under the curve
- BAUS
- British Association of Urological Surgeons
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CRF
- case report form
- DMC
- Data Monitoring Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GCP
- Good Clinical Practice
- GP
- general practitioner
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- NCTU
- Newcastle Clinical Trials Unit
- NIHR
- National Institute for Health Research
- OPCS-4
- OPCS Classification of Interventions and Procedures version 4
- PCQ
- participant costs questionnaire
- PI
- principal investigator
- QALY
- quality-adjusted life-year
- Qmax
- maximum urinary flow rate
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SD
- standard deviation
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TTO
- time trade-off
- USS-PROM
- urethral stricture surgery – patient-reported outcome measure
- VAS
- visual analogue scale
