Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/25/20. The contractual start date was in January 2015. The draft report began editorial review in January 2019 and was accepted for publication in November 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Taylor et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
As described elsewhere,1 evidence-based guidelines recommend both aerobic training and strength training for improving health markers and quality of life among those people with common chronic metabolic conditions1–5 and musculoskeletal conditions. 6 To prevent or reduce depression, mostly aerobic exercise is recommended. 7 Public health guidelines of 150 minutes of moderate activity per week (accumulated in bouts of ≥ 10 minutes) or 75 minutes of vigorous activity per week are met by only 66% of men and 58% of women aged ≥ 19 years in England,8 which is similar to results from the Scottish Health Survey 20149 (68% men; 59% women), based on self-report data from a national representative survey. The guidelines also highlight the importance of reducing sedentary behaviour and regularly doing bouts of resistance exercise. Physical inactivity, based on data from 2013–14, collected by Clinical Commissioning Groups in the UK, costs the NHS £455M. 10
Even small increases in physical activity (PA) and reduced sedentary time, especially among the least active,10 are likely to provide health benefits. 11,12 Patients with obesity, hypertension, type 2 diabetes, osteoarthritis and depression do less PA than the general population2 and report greater barriers to increased PA.
Exercise referral schemes in the UK
A variety of approaches have been explored to promote PA within primary care, such as referring patients to ‘exercise on prescription’ [i.e. an exercise referral scheme (ERS)]. As described previously,1 in the UK, ERSs have been popular, with an estimated 600 schemes involving up to 100,000 patients per year in 2008. 13 There is currently no single model for ERSs in the UK, but they mainly involve referral to a programme (e.g. 10–12 weeks) of structured, supervised exercise at an exercise facility (e.g. gym or leisure centre) or a counselling (and signposting) approach to support patients to engage in a variety of types of PA. 13 ERSs operate diversely to accommodate patient choice and local availability of facilities, the common goal being to reduce the risk of long-term metabolic, musculoskeletal and mental health conditions due to physical inactivity.
Evidence from a meta-analysis of eight randomised controlled trials (RCTs) involving 5190 participants eligible for ERSs14 indicated only a small increase in the proportion of participants who achieved 90–150 minutes of PA of at least moderate intensity per week, compared with no exercise control, at the 6- to 12-month follow-up among at-risk individuals. However, uncertainty remains regarding the effects for patients with specific medical conditions, no study assessed long-term PA objectively and many of the eight studies reviewed had relatively small sample sizes.
A review15 reported that the average ERS uptake (attendance at the first ERS session) ranged from 66% in observational studies to 81% in RCTs, and average levels of ‘adherence’ ranged from 49% in observational studies to 43% in RCTs. Predictors of uptake and adherence have been explored; women were more likely than men to begin an ERS, but less likely to adhere to it, and older people were more likely to begin and adhere to an ERS. 15 As an example of a large observational retrospective study,16 of 6894 participants who had attended an ERS over 6 years, 37.8% (n = 2608) dropped out within 6 weeks and 50.03% (n = 3449) dropped out by the (final) 12th week, and males (p < 0.001) and older people (p < 0.001) were more likely to adhere than females and younger people, respectively. ERSs may help patients to become familiar with medical conditions17 and target key processes of behaviour change. However, the following features of an ERS may reduce uptake and adherence: inconvenience, cost, limited sustainable PA support (e.g. for 10 weeks) and low appeal for structured exercise and/or the medical model (i.e. ‘exercise on prescription’), which may in some schemes do little to provide autonomous support or empower patients to develop self-determined behaviour to manage chronic medical conditions. 13,18,19
It therefore appears that additional support may be needed that is accessible and is low cost, can be tailored to support a wide range of individual needs and empowers patients to develop and use self-regulatory skills (e.g. self-monitoring, goal-setting) to self-manage their chronic conditions. In one study, training for ERS staff to foster self-determined behaviour increased PA more than when the ERS was delivered by untrained staff at 3 and 6 months. 20 Similarly, training of ERS staff in behaviour change techniques and motivational interviewing led to small additional changes in self-reported PA after 12 months17 compared with no ERS. Challenges in training staff across a wide geographical area across Wales and monitoring intervention fidelity were noted.
Intervention technologies to promote physical activity
To address the challenges with face-to-face promotion of PA noted above and to encompass the growing use and availability of new technologies, a wide variety of online and mobile support has been developed and used to promote PA.
As described previously,1 there is growing evidence on the effectiveness of technology-based interventions for promotion of PA. 21,22 Studies include a wide range of interventions (from quite simple self-monitoring to interventions with multiple complex behaviour change components), targeted at different clinical groups with different baseline levels of PA, with various PA outcomes reported (very few using objective measures), and with mostly short-term follow-ups. In addition, some comparisons are between intervention versus no intervention and others are versus human contact, although none reports on the effects of adding web-based support to complement face-to-face support provided by ERSs. The impact of web-based and technology interventions on increasing PA is small to moderate (effect size ≤ 0.4). However, there is evidence from more rigorous studies that interventions with more behaviour change components and ones targeting less active populations are more effective. 21,22 A systematic review23 highlighted the importance of maximising sustained engagement in web-based interventions for enhancing change in the target behaviour. A recent study24 highlighted that self-monitoring of PA and tailored feedback were important to increase engagement, and periodic communications helped to maintain participant engagement.
The LifeGuide© (version 1.0.7.30, University of Southampton, Southampton, UK; www.LifeGuideonline.org/; accessed 25 February 2020) platform has been extensively used to develop and evaluate the acceptability and impact of online behaviour change and self-management interventions with a variety of clinical groups, including in primary care. 25–27 As an example, when adding online LifeGuide support to face-to-face support there was a greater lasting reduction in obesity than with face-to-face dietetic advice alone. 28 The LifeGuide platform provides a researcher-led tool to develop theory-driven interventions and evidence of the effectiveness of techniques. 29,30 It also provides the opportunity to capture intervention engagement and assess the utility of different behaviour change components.
The potential for e-coachER
Following iterative development work and user group testing and involvement, drawing on some online modules used in other LifeGuide interventions, for example in secondary prevention of coronary heart disease,25 we developed a bespoke intervention. We called the intervention ‘e-coachER’ and it was designed to support patients with chronic physical and mental health conditions who have been referred from primary care to an ERS to receive face-to-face support. 1 The overarching aim of the e-coachER intervention was to facilitate long-term PA by promotion of evidence-based self-regulatory skills and to encourage interaction with others (including the ERS professional, family and friends), and founded on self-determination theory31 to build a sense of competence in managing PA, autonomy or control over PA, and connection or relatedness with others. We wanted to encourage uptake and adherence to the ERS but if that was not acceptable or feasible for participants then we offered support to find alternative ways to be physically active in a way that may support their needs as someone who was inactive. It was also important that the intervention could be scaled up to promote PA for patients with obesity, hypertension, type 2 diabetes, osteoarthritis and risk of depression at probably low cost32,33 and also potentially make it available for patients with other chronic medical conditions (e.g. low back pain, heart disease and cancer).
Developing the e-coachER intervention
The intervention development included the piloting of the welcome pack and development of an initial version of e-coachER, built on wide-ranging experiences from the development of other self-management interventions using the LifeGuide platform34 and beta testing over 7 months. Co-applicants and researchers then provided feedback on a time-truncated version, and ERS users provided feedback on a real-time version for 5 months before the website was locked for the RCT.
The development of all components of the e-coachER intervention followed a logic model as shown in Figure 1. 1
FIGURE 1.
Logic model for the e-coachER intervention. BCT, behaviour change technique; MVPA, moderate and vigorous physical activity. Reproduced with permission from Ingram et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.
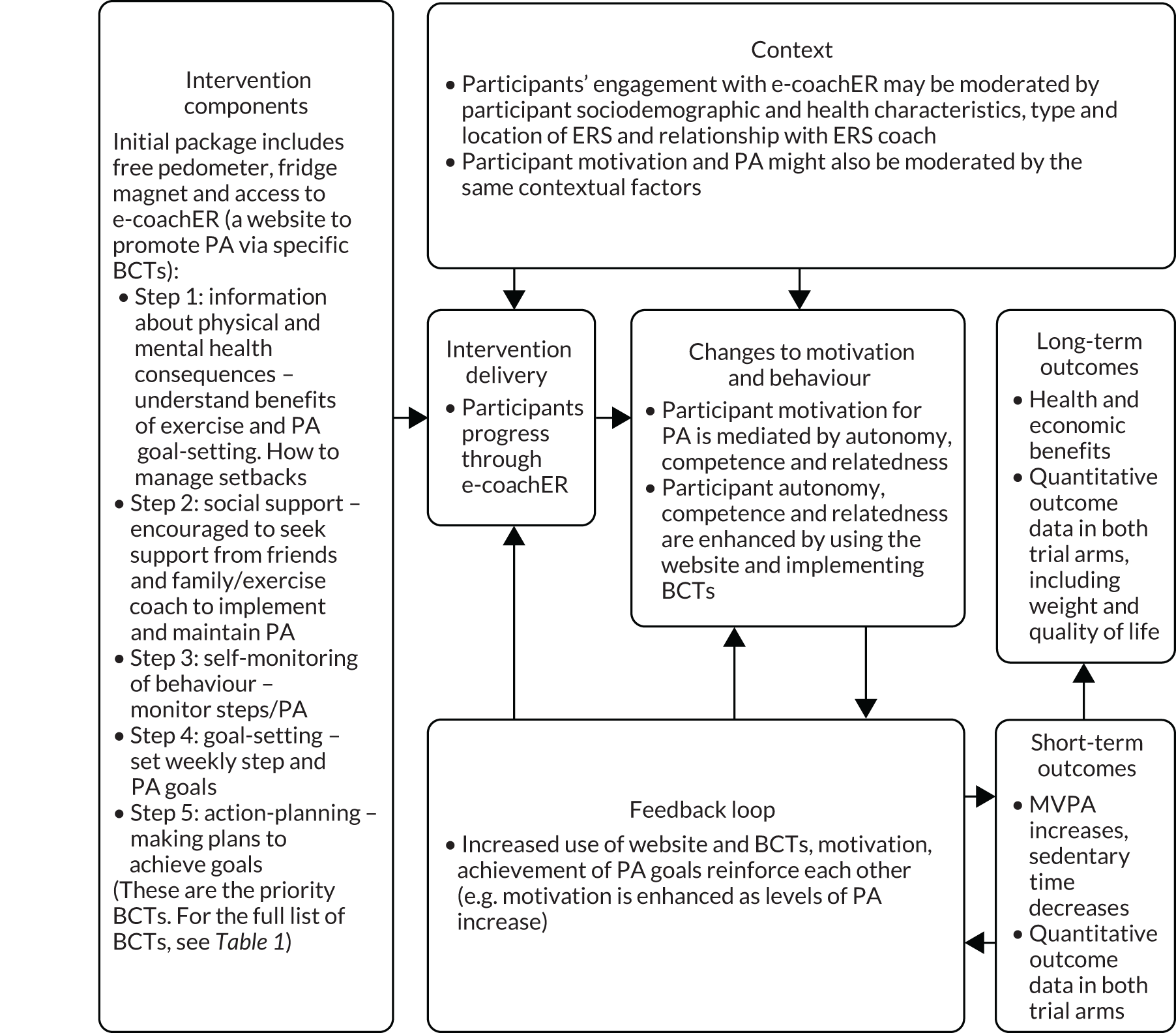
The key components of e-coachER include the following:
-
On allocation to the intervention, all participants received a ‘welcome pack’ (Figure 2) that contained a user guide and the participant’s unique user log-in and registration details to access the e-coachER website, a simple pedometer (step counter) with instruction sheet and a fridge magnet with tear-off sheets to record daily PA (complete with trial branding and e-coachER helpline number). Participants were encouraged to use the pedometer and the PA record sheets for self-monitoring and goal-setting in conjunction with the website.
-
The e-coachER support system, hosted on the LifeGuide platform, provided support through seven ‘steps to health’ lasting approximately 5–10 minutes each, as shown in Table 1.
-
The steps were designed to do the following: encourage participants to think about the benefits of PA (motivation); seek support from an ERS practitioner, friends/family and the internet (support/relatedness); set progressive goals; self-monitor PA with a pedometer and upload step counts or minutes of moderate and vigorous physical activity (MVPA) (self-regulation, building confidence/autonomy); and find ways to increase PA more sustainably in the context of day-to-day life and deal with setbacks (building confidence). The sequential content, objectives and how this was implemented were mapped against a taxonomy for behaviour change techniques shown in Table 1. 35 The website content is illustrated in Appendix 1.
-
Participants were encouraged to use e-coachER support as an interactive tool by using pre-set or personally set reminders to upload step counts or minutes of MVPA, and messages of encouragement. A lack of engagement (e.g. not reviewing a goal by entering step counts 1 week later, or not signing into the website for 1, 2 and 4 weeks) triggered reminder e-mails. Participants were reminded by prompts to review goals the next day.
-
An avatar was used throughout the content to avoid having to represent a range of individual characteristics, such as age, gender and ethnicity. The avatar delivered brief narratives to normalise and support behaviour change and encourage the use of e-coachER. Automatic and user-defined e-mails generated by the LifeGuide system promoted ongoing use of functions such as recording weekly PA and goal-setting. Participants were provided with links to reputable generic websites for further information about the chronic conditions of interest and lifestyle, links to other websites and apps (applications) for self-monitoring health behaviour and health, as well as modifiable listings of local opportunities to engage in PA.
-
The only webpages that were not ‘locked’ after the initial participant began the intervention were pages for the respective recruitment sites that displayed links to the following websites: (1) local community opportunities for engaging in PA, (2) disease-specific (e.g. Diabetes UK, Royal College of Psychiatrists) informational sites about the role of PA in preventing and managing the condition, and (3) sites about other methods to optimise ways to be physically active (e.g. more sophisticated technologies to self-monitor).
-
Our aim was to maximise accessibility and use. Therefore, a local researcher provided technical support if requested, but did not support behaviour change directly. If participants did not register on the e-coachER website within the first few weeks, they were followed up with a telephone call to offer support to use e-coachER. If technical support to resolve any user operational issues with the website (e.g. re-issuing passwords) was needed, participants were referred to a centralised technician within the LifeGuide team for further support.
Trial aim and objectives
The overarching aim was to determine if e-coachER online support combined with usual ERS provided a clinically effective and cost-effective approach to supporting increases in PA in people referred to an ERS with a range of chronic conditions.
The specific objectives were to:
-
determine whether or not there is an increase in the total weekly minutes of MVPA at 12 months post randomisation in the intervention group compared with the control group
-
determine whether or not there is an increase in the proportion of participants in the intervention group compared with the control group who:
-
take up the opportunity to attend an initial consultation with an exercise practitioner
-
maintain objectively assessed PA at 4 and 12 months post randomisation
-
maintain self-reported PA at 4 and 12 months post randomisation
-
have improved health-related quality of life at 4 and 12 months post randomisation
-
-
quantify the additional costs of delivering the intervention, and determine the differences in health utilisation and costs between the intervention and control groups at 12 months post randomisation
-
assess the cost-effectiveness of the intervention compared with control at 12 months post randomisation [incremental cost per quality-adjusted life-year (QALY)] using a previously developed decision model to estimate future costs and benefits
-
quantitatively and qualitatively explore whether or not the impact of the intervention is moderated by medical condition, age, gender, socioeconomic status, information technology (IT) literacy or ERS characteristics
-
quantitatively and qualitatively explore the mechanisms through which the intervention may have an impact on the outcomes, through rigorous mixed-methods process evaluation and mediation analyses (if appropriate).
Chapter 2 Methods
Study design
This was a multicentre, parallel, two-arm RCT with participant allocation to usual ERS alone (control) or usual ERS plus web-based behavioural support (intervention) with parallel economic and mixed-methods process evaluations. 1 The trial design is summarised in Figure 3, from Ingram et al. 1
FIGURE 3.
Participant pathway. Reproduced with permission from Ingram et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original text.
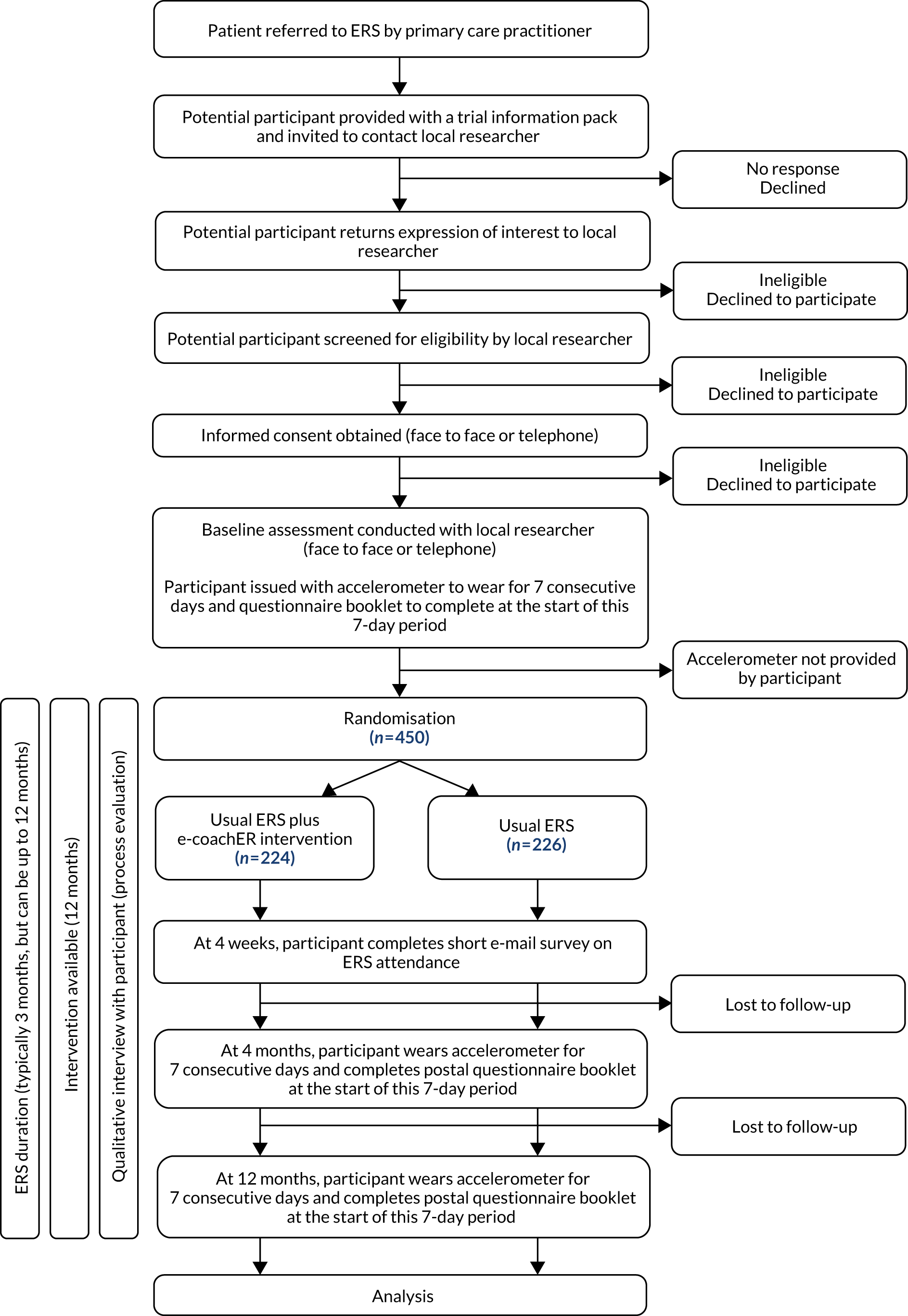
Recruitment to the trial took place over a 21-month period (July 2015 to March 2017) in three areas in the UK: Greater Glasgow, West Midlands and South West England (including Plymouth, Cornwall and Mid Devon). The majority of participants lived in urban locations. Further information about the characteristics of the cities involved and the respective ERSs to which participants were referred is given in Ingram et al. 1
Ethics approval and research governance
Ethics approval for the study was granted by North West Preston NHS Research Ethics Committee (REC) in May 2015 (reference 15/NW/0347). Approval for activity at non-NHS sites was obtained from the same REC for the following ERSs: Everyone Active (Plymouth), Teignbridge District Council (Cornwall), Tempus Leisure (Cornwall), Be Active Plus (West Midlands) and Live Active (Glasgow) in December 2015, and Docspot (West Midlands) in November 2016.
NHS Research and Development approval was granted by the Royal Devon and Exeter NHS Foundation Trust Shared Management Service for the Plymouth site (July 2015); NIHR Clinical Research Network (CRN) West Midlands for the Birmingham site (July 2015); and the NHS Greater Glasgow and Clyde Health Board for the Glasgow site (January 2016).
Prior to commencing recruitment, the trial was registered with the International Standard Randomised Controlled Trial Number Register (ISRCTN) under reference number 16900744 and NIHR CRN Portfolio 19047. A summary of the changes made to the original protocol is given later in this chapter.
Patient and public involvement
Aim
The aim was to involve public representatives throughout the study to ensure that the intervention and trial methods were appropriate for the target population.
Methods
The target trial population was composed of patients with one or more physical and mental health conditions; there was no single patient support group or user group that could be invited to contribute as patient and public involvement (PPI) representatives. Hence, PPI representatives with diverse clinical conditions were sought from an ERS provider in Plymouth. Others involved in the delivery of ERSs as managers or practitioners were also consulted to ensure that the methods and intervention were aligned with the usual ERS, especially in the recruitment locations.
There was comprehensive PPI in intervention development. PPI representatives provided critical feedback in informal focus groups on the e-coachER website and registration processes, and on the use of the pedometer and the fridge magnet (with recording strips) as a motivational tool in the e-coachER support package.
Patient and public involvement representatives with experience of ERSs also contributed to the study via their membership of the e-coachER Project Management Group and Trial Steering Committee (TSC).
Results
Notable benefits included having access to PPI representatives’ perspectives on (1) participant-facing documents, such as the e-coachER invitation materials and participant newsletter, (2) usability of the e-coachER intervention package and (3) suggestions to overcome barriers to recruitment.
Discussion
One PPI representative was influential with regard to including a ‘personal message’ from an ERS user in the periodic participant newsletter, the aim being to convey the importance and value of taking part in the study from the perspective of someone who has been referred to an ERS. There were no negative aspects of the PPI activities undertaken in the study.
Reflections
Being a resident of Plymouth, one PPI representative was able to attend all of the meetings held at the chief investigator’s institution in person. Face-to-face contact with the PPI representative meant that a rapport was more readily generated than would have been possible if teleconferencing had been used.
The PPI representative on the TSC provided a welcome contribution to the various discussions on the problems faced with recruitment in the pilot phase of the trial. He took a keen interest in the wider issues faced by the trial team, such as ERS provision in the UK, national guidelines for PA and the choice of cut-off points for accelerometer-derived data that informed the primary outcome. The PPI representative provided a ‘real-life’ perspective on such matters, as he saw them.
Participants
The study population was composed of patients who had been referred to an ERS administrator, or were about to be referred, mostly by a general practitioner (GP), and occasionally by a nurse to a local ERS for a programme of support to increase PA.
Patients were eligible for the study if they were aged between 16 and 74 years, inclusive, were contactable via e-mail, had some experience of using the internet and had one or more of the following conditions:
-
obesity [i.e. a body mass index (BMI) of 30–40 kg/m2]
-
a diagnosis of hypertension
-
prediabetes
-
type 2 diabetes
-
lower limb osteoarthritis
-
current or recent history of treatment for depression
-
categorised as ‘inactive’ (i.e. 0 hours per week of physical exercise and in a sedentary occupation) or ‘moderately inactive’ (i.e. some activity but < 1 hour per week and in a sedentary occupation, or 0 hours per week of physical exercise and in a standing occupation) according to the General Practice Physical Activity Questionnaire (GPPAQ). 36
Patients were excluded for the following reasons:
-
They did not meet the eligibility criteria for their local ERS.
-
They had an unstable, severe and enduring mental health problem.
-
They were being treated for an alcohol or drug addiction that may have limited their involvement with the study.
-
They were unable to use written materials in English, unless they had a designated family member or friend to act as translator.
Intervention
All participants had been offered usual ERS. Participants allocated to the intervention group were additionally offered access the e-coachER web-based support package. Development and delivery of the intervention is fully described in Chapter 1, Developing the e-coachER intervention.
Usual care
There is currently no single model for ERSs in the UK, but the predominant mode of delivery involves referral to a programme (e.g. 10–12 weeks) of structured, supervised exercise at an exercise facility (e.g. gym or leisure centre) or a counselling approach to support patients to engage in a variety of types of PA. 13 ERSs operate diversely to accommodate patient choice and local availability of facilities, the common goal being to reduce the risk of long-term metabolic, musculoskeletal and mental health conditions as a result of physical inactivity. The three participating sites were selected from different regions of the UK (different ERS providers) to provide diversity of approach; the schemes are described in Ingram et al. 1
Recruitment procedures
Patients were identified as potentially eligible for the trial in a number of ways.
-
By health-care professionals in primary care at the point of being actively referred to an ERS or having been opportunistically found to be eligible for an ERS at a consultation with the primary care practitioner.
-
Via a search of patient databases at the participating GP practices (conducted by the local NIHR Primary Care Research Network team).
-
Via patient self-referral to the GP arising from community-based publicity for the trial.
-
By the ERS programme administrator on receipt of an ERS referral form from a GP practice.
-
By exercise advisors at the ERS service at enrolment on the ERS. With the patient’s consent, the exercise advisor provided the local researcher with the patient’s contact details for the purposes of the trial.
Potentially eligible patients were approached by the primary care practitioner or the local researcher, depending on how the patient had been identified. Some patients self-referred to the local researcher in response to publicity campaigns. These various means of identification and approach were designed to accommodate the variation in usual-care referral pathways to ERSs across the participating sites and individual GP practices.
Amenable patients were offered a study-specific participant information sheet (see Report Supplementary Material 1) by post, by e-mail or by hand (the route used largely depended on the preference of the participating GP practice or ERS service). Interested patients were asked to communicate their expression of interest to the local researcher via a prepaid reply slip, by telephone or by e-mail. On receipt of an expression of interest, the local researcher contacted the potential participant by telephone to discuss the trial, confirm eligibility and seek informed consent.
Informed consent
Informed, written consent (see Report Supplementary Material 2) was obtained prior to undertaking the baseline assessment, either over the telephone or at a face-to-face visit, depending largely on the patient’s preference but also on the availability of suitable venues, such as the GP practice. The original completed informed consent forms were held securely at the site and a copy was given to the participant.
In the case of telephone consent, the researcher was required to sign and date the informed consent form, but the participant was not required to sign or date their copy.
Patients who were deemed to be ineligible for inclusion in the study were informed of this outcome.
Randomisation, concealment and blinding
On receipt of the baseline accelerometer at the Peninsula Clinical Trials Unit (PenCTU) (after 1 week of wear), participants were randomised to usual ERS or usual ERS plus access to e-coachER in a 1 : 1 ratio, stratified by site with minimisation by participant’s perception of main medical referral reason (i.e. weight loss, diabetes control, reduce blood pressure, manage lower limb osteoarthritis symptoms, manage low mood/depression) and by self-reported IT literacy level on a visual analogue scale (i.e. lower or higher confidence).
To maintain concealment, the minimisation algorithm retained a stochastic element.
Randomisation was conducted by means of a secure, password-protected web-based system created and managed by the clinical trials unit (CTU).
Blinding of participants was not possible, given the nature of the intervention. Given that the primary outcome was an objective measure of PA recorded by accelerometer, and the secondary outcomes were assessed by a participant self-completed questionnaire, the risk of assessor bias was considered to be negligible in this study. However, to minimise any potential bias, the statistical analysis was kept blinded and the code for group allocation was not broken until the primary and secondary analyses had been completed. The ERS practitioners would not have been aware of trial participants’ treatment allocations.
Process evaluation: qualitative interviews
Participants who had engaged with the e-coachER website (i.e. logged on to the website, as a minimum) were invited to participate in one or more qualitative interviews to inform the process evaluation (see Chapter 4, Qualitative process evaluation).
Data collection and management
Data were collected and stored in accordance with the Data Protection Act 199837/General Data Protection Regulation 2016. 38
Participant numbering
Following receipt of an expression of interest, each patient was allocated a unique identification number and was then identified in all study-related documentation by this number and their initials. A record of names, addresses, telephone numbers and e-mail addresses linked to participants’ identification numbers was stored securely on the study database at the CTU for administrative purposes only.
Data collection
Data were recorded on study-specific paper-based case report forms (CRFs) by the local researcher, and participants completed a paper-based questionnaire booklet comprising validated and non-validated self-report outcome measures.
Accelerometers [GENEActiv™ Original accelerometer (version 3.0_09.02.2015), Active Insights Kimbolton, UK; www.geneactiv.org/ (accessed 26 February 2020)] were configured for use prior to being issued to participants by the local researcher at baseline and the CTU thereafter, using GENEActiv software. A recording window of 10 days, starting at midnight of the day of issue and recording at 75 Hz, was pre-set, thus accounting for transits in the post while optimising the battery life of the device.
Accelerometers received by the CTU following 1 week of wear by the participant were physically cleaned with liquid detergent in accordance with the manufacturer’s instructions before data were downloaded and linked to the participant identification number (see Accelerometer data processing). Accelerometers were then issued to other participants in the trial as required.
Data on participants’ uptake of the ERS were collected via participant self-report at 4 weeks post baseline and 4 months post randomisation, and via the ERS service provider.
Recording and reporting of non-serious adverse events (AEs) in this study were not required. Serious adverse events (SAEs) were reported to the CTU via the self-report questionnaire booklet administered at 4 and 12 months, but were also reported to the CTU or local researcher by the participant (or relative) outside these time points, until the end of follow-up. SAEs were categorised using the Medical Dictionary for Regulatory Activities (MedDRA) Terminology Systems Organ Classification List Internationally Agreed Order Version 19.0, March 2016 (www.meddra.org/sites/default/files/guidance/file/intguide_19_0_english.pdf; accessed 26 February 2020).
All persons authorised to collect and record study data at each site were listed on the study site delegation logs, which were signed by the principal investigator.
Data processing
Accelerometer data processing
Accelerometer data were downloaded using the GENEActiv and analysed in software R (The R Foundation for Statistical Computing, Vienna, Austria) using package GGIR version 1.2–8 (https://cran.r-project.org/web/packages/GGIR/index.html). 39 GGIR performs autocalibration with the reference of local gravity. 40 Raw acceleration data are used to compute Euclidean norm minus one (ENMO in mg). 41 Data were analysed from the first to the final midnight using 5-second epochs. Participants were included in the main analysis if they achieved a minimum of 16 hours of wear-time for a minimum of 4 days (including at least 1 weekend day). Non-wear was detected if the standard deviation (SD) of two axes was < 13 mg with a range of < 50 mg in windows of 60 minutes. Time spent in activity intensities was established using published thresholds. 42
Computed variables included average daily MVPA accumulated in any 5-second epochs. Ten-minute bouts of MVPA were detected when at least 80% of a 10-minute period was above the MVPA intensity threshold. 43 Total time accumulated in bouted and unbouted MVPA minutes was calculated by multiplying the average daily values by 7 to represent 1 full week.
Diurnal activity and nocturnal periods of inactivity were also estimated to determine if the intervention had an effect on reducing daytime inactivity and increasing sleep. Sleep duration was established using sustained nocturnal inactivity bouts. An inactivity bout occurred when no change in arm angle > 5° was observed for at least 5 minutes. 44 Diurnal inactivity represents the sustained inactivity bouts (> 5 minutes) that occur in the day,43 which includes naps, but omits other inactivity that results in measurable movement. 45 However, it is likely that some misclassification of this inactivity may occur. 43
To explore if different ways of processing accelerometer data influenced the findings, four additional wear-time criteria were calculated:
-
≥ 16 hours over any 4 days (irrespective of weekday/weekend day)
-
≥ 10 hours for 4 days (including at least 1 weekend day)
-
≥ 10 hours over any 4 days (irrespective of weekday/weekend day)
-
a minimum wear criterion of 1 day for 10 hours but with individuals weighted by the number of valid days with ≥ 10 hours of wear.
Case report forms and questionnaire booklets
Original CRFs and questionnaire booklets were posted to the CTU, with copies of the CRF retained at the site. All data were double-entered by CTU staff into a password-protected Structured Query Language Server database and encrypted using Secure Sockets Layer (version V3, QuoVadis Global SSL ICA G3, QuoVadis Online Limited, Lincolnshire, UK). Double-entered data were compared for discrepancies using a stored procedure, and discrepant data were verified using the original CRF. Incomplete, incoherent, unreadable or other problem data in the CRF pages were queried with site staff by staff at the CTU during data entry to ensure a complete and valid data set. Self-reported data in the questionnaire booklet were not queried with participants.
The CTU staff completed further validation of data items and performed logical data checks after data collection had been completed. After all data-cleaning duties had been performed at the CTU, the final export of anonymous data was transferred to the statistician and health economist for analysis.
Baseline assessment
Consented participants attended a baseline assessment with the local researcher. This assessment was conducted over the telephone or in person at a suitable venue in the community.
Demographic data were collected (i.e. age, gender, BMI, blood pressure, ethnic group, relationship status, domestic residence status, smoking status, employment status, education status, GPPAQ score, internet use capability, requirement for translator for trial purposes and clinical condition).
Information technology literacy level was determined using a visual analogue scale for self-reported ‘confidence using the internet’, where 1 = not at all confident and 10 = totally confident. Arbitrarily, scores of 1–5 were set to indicate a low literacy level and scores of 6–10 indicated a high literacy level, for the purposes of stratification.
‘Clinical condition’ was the participant’s perception of the reason for referral to the ERS; where more than one medical condition was stated, the participant ranked these in order of importance and the most important reason was used as a stratification variable.
The participant was issued with the wrist-worn waterproof accelerometer to wear constantly for 1 whole week (day and night), and a self-report questionnaire booklet to complete at the beginning of the week-long period. At the face-to-face baseline assessments, the accelerometer was fitted by the local researcher; at telephone visits, the accelerometer (and self-report questionnaire booklet) were posted to participants. All participants were provided with a bespoke guidance sheet for wearing the accelerometer (see Report Supplementary Material 3), including instructions to wear the accelerometer on the wrist of the non-dominant hand (i.e. the hand not favoured for writing). After 1 week of wear, participants were required to post the accelerometer and completed questionnaire to the CTU in a preaddressed padded envelope provided. A prepaid postal service was used so as not to incur costs to the participant.
The measures collected at baseline are summarised in Table 2.
| Measure | Baseline | 4 weeks post baseline | 4 months post randomisation | 12 months post randomisation | On completion of participants’ ERS programme at site |
|---|---|---|---|---|---|
| Demographics | ✗ | ||||
| Engagement with the ERS: self-reported | ✗ | ✗ | |||
| Engagement with the ERS: ERS service provider’s record | ✗ | ||||
| Accelerometer-recorded MVPA | ✗ | ✗ | ✗ | ||
| Self-reported MVPA | ✗ | ✗ | ✗ | ||
| Self-reported health and social care resource use | ✗ | ✗ | ✗ | ||
| Self-reported quality-of-life measure (EQ-5D-5L) | ✗ | ✗ | ✗ | ||
| Self-reported Hospital Anxiety and Depression Scale | ✗ | ✗ | ✗ | ||
| Self-reported process evaluation outcomes (confidence to be physically active; importance of being physically active, perceived frequency and availability of support; perceived autonomy over choices; involvement in self-monitoring and action-planning PA) | ✗ | ✗ | ✗ | ||
| Qualitative interviews as part of the process evaluation focusing on participants’ experiences with the ERS and the intervention (optional for participants) | ✗ | ||||
| Engagement with e-coachER (captured from the LifeGuide platform) | ✗ | ||||
Follow-up assessments
The measures collected at follow-up are summarised in Table 2.
At 4 weeks post baseline, a short survey on initial uptake of the ERS was administered via e-mail.
At 4 and 12 months post randomisation, participants were sent an accelerometer (along with the guidance sheet on how to wear it), a questionnaire booklet, and a pre-addressed, prepaid envelope to return the items to the CTU.
To maximise data completeness at follow-up assessments, participants were sent standard letters from the CTU: (1) 7 days before delivery of the accelerometer, (2) 3 days into the 10-day recording window as a prompt to begin wearing the accelerometer (if not already doing so) and (3) at 3 and 5 weeks after issue as a reminder to post the accelerometer to the CTU (for those participants who had not already done so). If a participant had not sent the accelerometer back to the CTU after 6 weeks, the local researcher telephoned the participant to remind them to return the device. Participants who returned the accelerometer to the CTU were provided with a £20 voucher for an online store as a token ‘thank you’ to maximise response rates.
Once a participant’s involvement in the ERS had concluded, the ERS service providers completed a simple pro forma to confirm whether or not the participant attended an appointment with the exercise specialist and, if so, how many appointments were available to the participant.
Measures
Primary outcome measure
The primary outcome was the number of weekly minutes of MVPA, in ≥ 10-minute bouts, measured objectively using the GENEActiv Original accelerometer,46 over 1 week at 12 months post randomisation, compared with the control group. To be included, participants had to have provided activity recorded over 4 days, including a weekend day, for at least 16 hours per day.
Secondary outcome measures
-
Total weekly minutes of MVPA in ≥ 10-minute bouts, measured objectively using an accelerometer, over 1 week at 4 months.
-
Achievement of at least 150 minutes of MVPA, measured objectively by accelerometer, over 1 week at 4 and 12 months.
-
Self-reported achievement of at least 150 minutes of MVPA over 1 week using the 7-day recall of PA47 (7-day Physical Activity Recall questionnaire) at 4 and 12 months.
-
Self-reported weekly minutes of MVPA at 4 and 12 months.
-
Average daily hours of sedentary behaviour measured objectively using an accelerometer over 1 week at 4 and 12 months.
-
Self-reported average daily hours of sleep over 1 week at 4 and 12 months.
-
Self-reported health-related quality of life, assessed using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L),48 at 4 and 12 months.
-
Self-reported symptoms of anxiety and depression, assessed using the Hospital Anxiety and Depression Scale (HADS),49 at 4 and 12 months.
-
Uptake of the ERS according to the attendance records held by the ERS service provider, with imputed participant-reported attendance at 4 weeks and/or 4 months where the ERS service data are missing.
-
Adherence to PA using a composite measure to describe the proportion of participants in each arm of the trial who achieved at least 150 minutes of MVPA in bouts of at least 10 minutes at 4 months and were still doing so at 12 months.
Self-reported survey process measures
The following survey items were adapted from existing measures or originally developed, using processes to be reported elsewhere in more detail, and were used as process measures:
-
importance and confidence to be physically active (single items)
-
perceived competence in being regularly physically active (four items)
-
autonomous in decisions about PA (four items)
-
availability of support (three items)
-
frequency of support (three items)
-
action-planning (five items)
-
self-monitoring (two items).
The respective measures were not validated but exploratory analysis, to be reported in more detail elsewhere, indicated that Cronbach’s alpha coefficients of all scales were ≥ 0.77, using data from participants included in the primary analysis.
In the intervention group, we measured engagement with e-coachER. This included whether or not the participant visited the website at least once and whether or not they reached a stage of the online support to indicate that they have set and reviewed at least one PA goal [i.e. step 5 – users make their specific, measurable, achievable, relevant, time-bound (SMART) activity plan and then review their step goal and SMART activity goal].
Sample size
In the absence of a published minimally important difference for MVPA, assuming a ‘small’ to ‘moderate’ standardised effect size of 0.35, we estimated that 413 participants were required, with 88% power and a two-sided alpha of 5%, assuming 20% attrition, or 90% power at a two-sided alpha of 5% allowing for 16% attrition [using ‘sampsi’ in Stata® version 14 (StataCorp LP, College Station, TX, USA)]. Given that the intervention was delivered at the level of the individual participant, clustering was not factored into the sample size calculation. Based on the baseline SD for MVPA total weekly minutes in ≥ 10-minute bouts of 104–113,50 an effect size of 0.35 corresponds to a between-group difference of 36–39 minutes of MVPA per week.
Statistical analysis
Analyses were in accordance with International Conference on Harmonisation guideline 9 (ICH-9) statistical guidelines51 for clinical trials and updated Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines52,53 for non-drug trials. All primary and secondary analyses were undertaken and reported in accordance with a prespecified detailed statistical analysis plan that was agreed with the Project Management Group, TSC and Data Monitoring Committee (DMC).
Following data lock by the CTU data manager, the statistician undertook primary analyses blinded to group (i.e. randomised groups were coded ‘A’ or ‘B’). Following the blinded presentation of the primary results to the Project Management Group and agreed interpretation of the results, the groups were unblinded.
Descriptive analyses
A summary of baseline characteristics and baseline outcome values in the intervention and control groups was undertaken and between-group equivalence was assessed descriptively. Because differences between randomised groups at baseline could have occurred by chance, no formal significance testing will be conducted.
Interim analysis
No interim inferential analysis was planned and none was conducted.
Inferential analyses
Definition of comparison groups
Intention to treat (ITT), complete case: groups according to original randomised allocation in participants with complete data (i.e. meeting required accelerometer wear-time) at 12-month follow-up.
Intention to treat, imputed: groups according to original randomised allocation in all participants.
Per protocol [complier-average casual effect (CACE)]: ITT complete-case participants who have completed step 5 – making your activity plans.
Primary analysis
The primary analysis using a linear model (continuous outcomes – using Stata ‘regress’) or logistic model (binary outcomes – using Stata ‘logistic’ command) compared primary and secondary outcomes between groups according to the principle of intention to treat (i.e. according to original randomised allocation) in participants with complete outcomes at 12 months. This adjusted for baseline outcome values and stratification (by site) and minimisation variables (participant’s perception of main medical reason for referral to the ERS and IT literacy level). Given that age and gender are known to be predictive of PA, these baseline characteristics were also added to the adjusted model.
Given the overdispersion and high frequency of the primary outcome, the primary mixed-effects model was found to be a poor fit. Therefore, alternative post hoc regression models were explored for the analysis of the primary outcome. These included a log-transformed mixed-effects model (with a constant added), a mixed-effect model (with outliers removed), negative binomial models and zero-inflated binomial models.
Secondary analyses
Secondary analyses were undertaken to compare groups at follow-up across all follow-up points, using a mixed-model repeated-measures approach (using Stata ‘xtmixed’ command). Secondary per-protocol analysis using a CACE approach (using Stata ‘ivregress’ command) was undertaken to examine the impact of adherence to the intervention on primary and secondary outcomes at 12 months post randomisation.
Subgroup analyses
The primary analysis model was extended to fit interaction terms to explore possible subgroup differences in intervention effect in stratification and minimisation variables for the primary outcome at 12 months post randomisation. Given the relatively low power for testing interactions, these results were to be considered as exploratory only.
Sensitivity analysis to test the effects of different ways of processing accelerometer data
Sensitivity analysis was undertaken using four additional wear-time criteria:
-
≥ 16 hours over any 4 days (irrespective of weekday/weekend day)
-
≥ 10 hours for 4 days (including at least 1 weekend day)
-
≥ 10 hours over any 4 days (irrespective of weekday/weekend day)
-
a minimum wear criterion of 1 day for 10 hours but with individuals weighted by the number of valid days with ≥ 10 hours of wear.
Handling of missing data
Missingness was defined as those participants with the absence of data at follow-up for one or more outcomes. Given that the proportion of patients with missing accelerometry data out of the total number of participants who fulfil the criteria of includable PA data (n = 243) was small (i.e. 0–10 participants or < 5%), no imputation was undertaken for the primary outcome or any of the derived secondary outcomes. For EQ-5D-5L and HADS, missing data were replaced using multiple imputation using the covariates of age, gender, reason for referral and confidence in IT, and assuming that unobserved measurements were missing at random (using Stata ‘mi’ command). Using the same primary analysis model as described above, between-group outcomes were compared in ITT complete-case and imputed data sets for primary and secondary outcomes at 12 months post randomisation.
Adverse events
Safety data and AEs were listed descriptively by group and include details of the event and the likely relatedness to either treatment.
Data presentation
Results were reported as between-group mean differences with 95% confidence intervals (CIs); global p-values were provided with regard to categorical explanatory variables. The threshold for determining significant effects was p < 0.05. No adjustment of p-values was made to account for multiple testing, although the implications of multiple testing were considered when evaluating the results of the analyses.
Model checking and validation
All analyses were undertaken using Stata version 14.2.
Stata coding for the primary analysis was prepared independently and the analyses were cross-checked.
Checks were undertaken to assess the robustness of models, including an assessment of model residual normality and heteroscedasticity.
Changes to the project protocol
Primary outcome measure and sample size
The original protocol featured an internal pilot. During the internal pilot phase, 180 participants were to be recruited over 3 months to provide sufficient information to justify progression to a main trial. Progression from the internal pilot to the main trial was dependent on recruitment rate and engagement with the intervention according to the scenarios in Table 3. In the main trial, an additional 1220 participants were to be recruited, giving a total of 1400 participants (recruited over 16 months).
| Criteria | Scenario 3 | Scenario 2 | Scenario 1 |
|---|---|---|---|
| Percentage of internal pilot sample size target (180 participants) recruited (%) | < 65 | 65–79 | ≥ 80 |
| Intervention engagement (% participants who access e-coachER at least once) | < 65 | 65–79 | ≥ 80 |
| Proposed action | No progression | Discuss with TSC and funder about progression and resources needed to achieve target | Proceed to full trial |
The recruitment rate during the internal pilot phase was lower than expected as a result of limitations on the time that primary care practitioners had available to approach potential participants, a delayed start at one of the research sites, poor uptake when patients were approached via a postal mailshot and a high ineligibility rate among patients who were identified via a primary care database.
In response to poor recruitment, the following strategies to increase recruitment were introduced in November 2015:
-
The inclusion criterion for BMI was aligned with the ERS entry (upper BMI limit for the trial was originally 35 kg/m2 and was raised to 40 kg/m2), and prediabetes was included as an inclusion criterion.
-
Provision was made for the Birmingham and Plymouth sites to recruit participants via the ERS service (a strategy already in place at the Glasgow site), in addition to recruitment via primary care.
-
Incentive payments to participants (for returning an accelerometer) were increased from a maximum of £40 per participant (£10 at baseline, £10 at 4 months and £20 at 12 months) to a maximum of £60 per participant (£20 at each of the aforementioned time points).
Having implemented these measures, the conditions for progression in terms of recruitment rate and engagement with the intervention were not met by the end of the internal pilot phase, despite a 4-month extension period. A ‘recovery plan’ was developed in collaboration with the funders, based on amending the choice of primary outcome.
The original primary outcome was achievement of at least 150 minutes of MVPA measured objectively by accelerometer over 1 week at 12 months. This outcome was based on the findings of a systematic review of ERSs14,54 demonstrating that trials had primarily reported their outcomes according to percentage of participants reaching the NICE guidelines for PA level (i.e. 150 minutes of MVPA per week). We estimated that recruiting 700 participants per group would allow us to detect a difference at 12-month follow-up of at least 10% (intervention group 53% vs. control group 43%), assuming an attrition rate of 20% and small effect of clustering (intracluster correlation coefficient 0.006) at 90% power and 5% alpha. Thus, the original sample size was 1400 participants to be recruited over a 16-month period.
From the outset, the TSC and DMC had recommended that this dichotomous primary outcome measure be replaced with a continuous variable (i.e. total weekly minutes of MVPA). This was because:
-
A continuous primary outcome measure would be more relevant in this study population, in terms of detecting a small but clinically significant increase in minutes of MVPA.
-
Based on sample size calculations, this would offer greater statistical power than to the categorical assessment of whether or not participants reach a threshold of 150 minutes of MVPA. This would therefore afford a reduction in sample size.
The TSC and funders agreed to these changes (in August 2016) and the original sample size was reduced in accordance with this new primary outcome measure and revised sample size calculation, from 1400 to 413 participants (to be recruited over 21 months). A similar reduction in sample size was incorporated into the qualitative component of the process evaluation work.
The primary outcome was subsequently specifically defined as total weekly minutes of MVPA in ≥ 10-minute bouts recorded objectively by accelerometer over 1 week at 12 months, in participants with activity recorded for at least 16 hours per day on at least 4 days, including 1 weekend day.
Capture of exercise referral scheme attendance data
Initially, uptake of the ERS was solely self-reported, captured via an e-mailed survey at approximately 4 weeks and a postal questionnaire at 4 months. Owing to poor compliance (especially at 4 weeks), data were sought from the ERS service providers, in addition to the self-reported data. Participants consented to the ERS service sharing their attendance data for the purposes of the trial.
Omission of Short Form questionnaire-12 items data analysis
Owing to an error in the compilation of the participant self-report questionnaire booklet, the Short Form questionnaire-12 items (SF-12) data collected could not be analysed in this study. We had intended to administer the SF-12 version 2 at each of the study time points but it transpired that a number of the response options for SF-12 version 1, instead of SF-12 version 2, had been printed in the questionnaire booklet in error.
Chapter 3 Trial results: quantitative results
Participant recruitment
A total of 450 participants were recruited (randomised) to the trial over a 20-month period (3 September 2016 to 10 April 2017).
Table 4 shows the number (%) of participants who entered the trial by referral source (i.e. by mailout from the GP or opportunistically in primary care, at the point of initial contact with the ERS providers, or by word of mouth or community advertisement) across the different sites.
| Referral source | Site, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| Plymouth | Birmingham | Glasgow | ||
| ERS | 38 (25) | 109 (71) | 141 (100) | 288 (64) |
| Primary care | 102 (66) | 45 (29) | 0 (0) | 147 (33) |
| Self-referral | 15 (10) | 0 (0) | 0 (0) | 15 (3) |
| Total | 155 (100) | 154 (100) | 141 (100) | 450 (100) |
Flow of participants in the trial
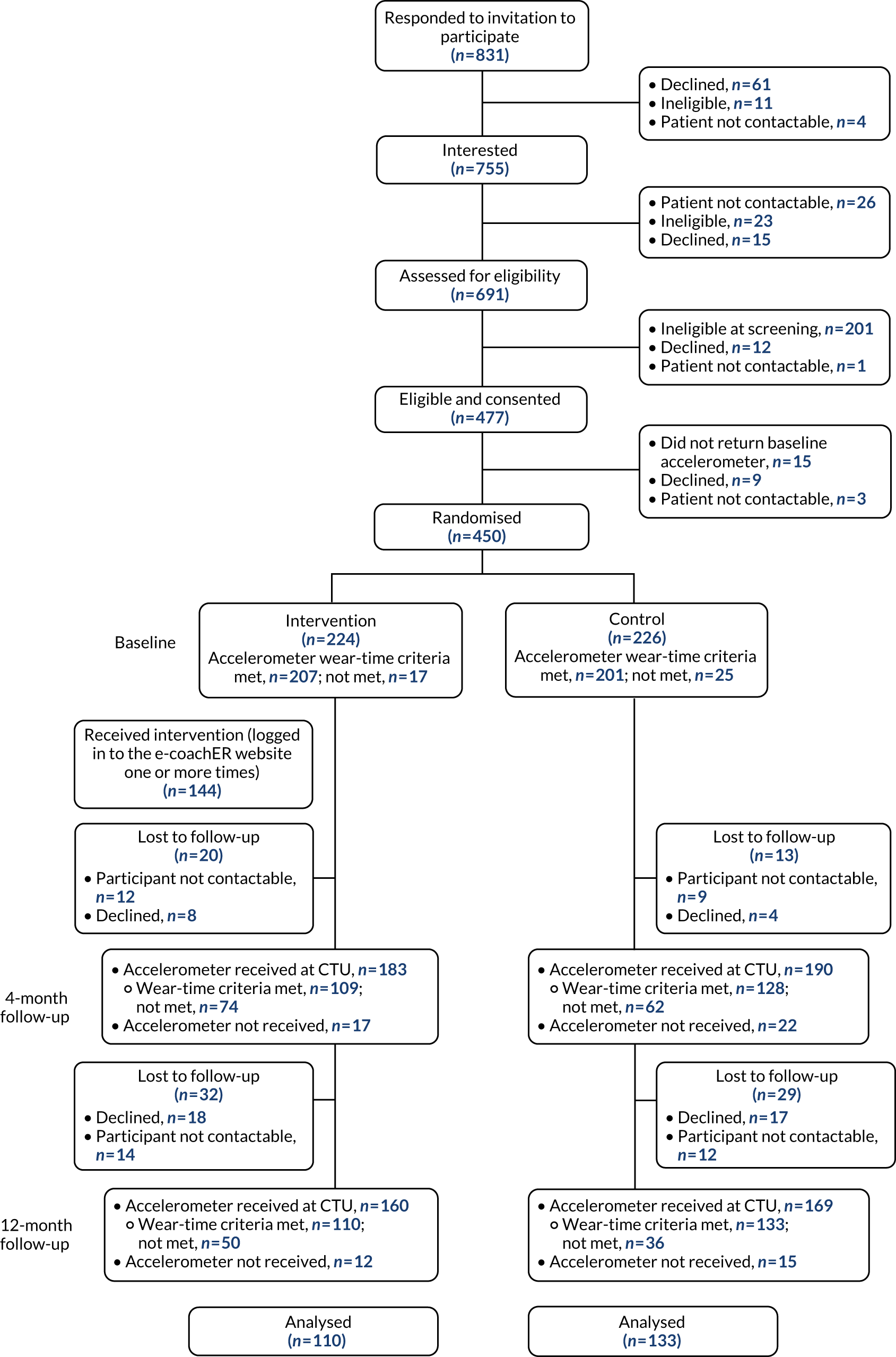
Figure 4 shows the flow of participants through the trial. Of those expressing an interest in participating, 477 (63%) individuals were eligible. The main reasons for ineligibility were BMI being too high (n = 104, 14%), being too active according to the GPPAQ (n = 47, 6%) and clinical condition of interest not being present (n = 26, 3%). An additional 27 individuals (4%) could not be contacted following their expression of interest. A detailed CONSORT flow diagram is given in Appendix 2.
FIGURE 4.
Participant flow.
Baseline comparability
The baseline characteristics of the whole sample (n = 450) and those who were included in the primary analysis (n = 232) are shown in Tables 5 and 6, respectively. The groups were well balanced.
| Variable | Control group | Intervention group | Both groups |
|---|---|---|---|
| N | 226 | 224 | 450 |
| Gender, n male (%) | 84 (37) | 76 (34) | 160 (36) |
| Age (years), mean (SD) [range] | 51 (14) [18–75] | 50 (13) [20–73] | 50 (12) [18–75] |
| BMI (kg/m2), mean (SD) [range] | 32.5 (4.4) [18.8–40.5] | 32.7 (4.5) [18.8–40.4] | 32.6 (4.4) [18.8–40.5] |
| Requirement for translator for trial purposes, n (%) | 3 (1.3) | 3 (1.3) | 6 (1.3) |
| GPPAQ score, n (%) | |||
| 2 (inactive) | 144 (63.7) | 149 (66.5) | 293 (65.1) |
| 3 (moderately inactive) | 82 (36.3) | 75 (33.5) | 157 (34.9) |
| Participant’s perception of any medical reason(s) for referral to ERS – prevalence, n (%) | |||
| Prediabetes | 8 (4.0) | 15 (7.7) | 23 (5.8) |
| Type 2 diabetes | 47 (20.8) | 42 (18.8) | 89 (19.8) |
| Osteoarthritis | 64 (28.3) | 45 (20.1) | 109 (24.2) |
| Weight loss | 182 (80.5) | 182 (81.3) | 364 (80.9) |
| Low mood | 122 (54.0) | 121 (54.0) | 243 (54.0) |
| High blood pressure | 79 (35.0) | 68 (30.4) | 147 (32.7) |
| Ethnicity, n (%) | |||
| White | 195 (86.3) | 179 (79.9) | 374 (83.1) |
| Black Caribbean | 3 (1.3) | 8 (3.6) | 11 (2.4) |
| Black African | 3 (1.3) | 6 (2.7) | 9 (2.0) |
| Black other | 1 (0.4) | 4 (1.8) | 5 (1.1) |
| Indian | 4 (1.8) | 12 (5.4) | 16 (3.6) |
| Pakistani | 7 (3.1) | 4 (1.8) | 11 (2.4) |
| Bangladeshi | 1 (0.4) | 0 (0) | 1 (0.2) |
| Chinese | 0 (0) | 0 (0) | 0 (0) |
| Other | 13 (5.8) | 10 (4.5) | 23 (5.1) |
| Relationship status, n (%) | |||
| Single | 78 (34.5) | 78 (34.8) | 156 (34.7) |
| Married | 97 (42.9) | 110 (49.1) | 207 (46.0) |
| Civil partnership | 5 (2.2) | 2 (0.9) | 7 (1.6) |
| Divorced or dissolved civil partnership | 35 (15.5) | 25 (11.2) | 60 (13.3) |
| Widowed or surviving civil partnership | 11 (4.9) | 9 (4.0) | 20 (4.4) |
| Domestic residence status (live with), n (%) | |||
| Live alone | 59 (26.1) | 48 (21.4) | 107 (23.8) |
| Partner | 120 (53.1) | 130 (58.0) | 250 (55.6) |
| Parent | 11 (4.9) | 13 (5.8) | 24 (5.3) |
| Child aged < 18 years | 66 (29.2) | 67 (29.9) | 133 (29.6) |
| Child aged ≥ 18 years | 39 (17.3) | 53 (23.7) | 92 (20.4) |
| Other family | 10 (4.4) | 8 (3.6) | 18 (4.0) |
| Non-family | 9 (4.0) | 12 (5.4) | 21 (4.7) |
| Education status, n (%) | |||
| No qualifications | 52 (23.0) | 29 (12.9) | 81 (18.0) |
| GCSEs | 146 (64.6) | 162 (72.3) | 308 (68.4) |
| A level | 71 (31.4) | 96 (42.9) | 167 (37.1) |
| First degree | 36 (15.9) | 54 (24.1) | 90 (20.0) |
| Higher degree | 22 (9.7) | 20 (8.9) | 42 (9.3) |
| Other | 108 (47.8) | 104 (46.4) | 212 (47.1) |
| Smoking status, n (%) | |||
| Smoker | 34 (15.0) | 32 (14.3) | 66 (14.7) |
| Ex-smoker | 90 (39.8) | 89 (39.7) | 179 (39.8) |
| Never smoked | 102 (45.1) | 103 (46.0) | 205 (45.6) |
| IT literacy level, n (%) | |||
| Low | 36 (16) | 35 (16) | 72 (16) |
| High | 190 (84) | 189 (84) | 379 (84) |
| Site, n (%) | |||
| Birmingham | 78 (34) | 76 (34) | 154 (34) |
| Glasgow | 69 (31) | 72 (32) | 141 (31) |
| Plymouth | 79 (35) | 76 (34) | 155 (35) |
| Participant-reported main reason for referral, n (%) | |||
| High blood pressure | 19 (8) | 18 (8) | 37 (8) |
| Low mood | 42 (18) | 42 (19) | 84 (19) |
| Osteoarthritis | 27 (12) | 26 (12) | 53 (12) |
| Type 2 diabetes and prediabetes | 24 (11) | 25 (12) | 49 (11) |
| Weight loss | 114 (50) | 113 (50) | 227 (50) |
| Variable | Control group | Intervention group | Both groups |
|---|---|---|---|
| N | 124 | 108 | 232 |
| Gender, n male (%) | 44.0 (35.5) | 36.0 (33.3) | 80 (34.4) |
| Age (years), mean (SD) [range] | 52.1 (13.4) [18.0–74.7] | 49.9 (12.9) [20.6–72.9] | 51.1 (13.2) [18.0–74.7] |
| BMI (kg/m2), mean (SD) [range] | 32.3 (4.3) [18.8–40.5] | 32.6 (4.9) [18.8–40.4] | 32.4 (4.6) [18.8–40.5] |
| Requirement for translator for trial purposes, n (%) | 0 (0.0) | 3 (2.8) | 3 (1.3) |
| GPPAQ score, n (%) | |||
| 2 (inactive) | 82 (66.1) | 70 (64.8) | 152 (65.5) |
| 3 (moderately inactive) | 42 (33.9) | 38 (35.2) | 80 (34.5) |
| Participant’s perception of any medical reason(s) for referral to ERS – prevalence, n (%) | |||
| Prediabetes | 4 (3.7) | 4 (4.3) | 8 (4.0) |
| Type 2 diabetes | 26 (21.0) | 15 (13.9) | 41 (17.7) |
| Osteoarthritis | 40 (32.3) | 22 (20.4) | 62 (26.7) |
| Weight loss | 100 (80.6) | 84 (77.8) | 184 (79.3) |
| Low mood | 65 (52.4) | 57 (52.8) | 122 (52.6) |
| High blood pressure | 47 (37.9) | 38 (35.2) | 85 (36.6) |
| Ethnicity, n (%) | |||
| White | 109 (87.9) | 95 (88.0) | 204 (87.9) |
| Black Caribbean | 2 (1.6) | 3 (2.8) | 5 (2.2) |
| Black African | 2 (1.6) | 3 (2.8) | 5 (2.2) |
| Black other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Indian | 1 (0.8) | 3 (2.8) | 4 (1.7) |
| Pakistani | 4 (3.2) | 0 (0.0) | 4 (1.7) |
| Bangladeshi | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Chinese | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 6 (4.8) | 4 (3.7) | 10 (4.3) |
| Relationship status, n (%) | |||
| Single | 40 (32.3) | 37 (34.3) | 77 (33.2) |
| Married | 57 (46.0) | 50 (46.3) | 107 (46.1) |
| Civil partnership | 3 (2.4) | 2 (1.9) | 5 (2.2) |
| Divorced or dissolved civil partnership | 19 (15.3) | 13 (12.0) | 32 (13.8) |
| Widowed or surviving civil partnership | 5 (4.0) | 6 (5.6) | 11 (4.7) |
| Domestic residence status (live with), n (%) | |||
| Live alone | 30 (24.2) | 29 (26.9) | 59 (25.4) |
| Partner | 69 (55.6) | 58 (53.7) | 127 (54.7) |
| Parent | 6 (4.8) | 6 (5.6) | 12 (5.2) |
| Child aged < 18 years | 39 (31.5) | 26 (24.1) | 65 (28.0) |
| Child aged ≥ 18 years | 19 (15.3) | 25 (23.1) | 44 (19.0) |
| Other family | 4 (3.2) | 3 (2.8) | 7 (3.0) |
| Non-family | 3 (2.4) | 6 (5.6) | 9 (3.9) |
| Education status, n (%) | |||
| No qualifications | 29 (23.4) | 11 (10.2) | 40 (17.2) |
| GCSEs | 84 (67.7) | 83 (76.9) | 167 (72.0) |
| A level | 39 (31.5) | 50 (46.3) | 89 (38.4) |
| First degree | 18 (14.5) | 28 (25.9) | 46 (19.8) |
| Higher degree | 16 (12.9) | 9 (8.3) | 25 (10.8) |
| Other | 59 (47.6) | 49 (45.4) | 108 (46.6) |
| Smoking status, n (%) | |||
| Smoker | 13 (10.5) | 12 (11.1) | 25 (10.8) |
| Ex-smoker | 52 (41.9) | 51 (47.2) | 103 (44.4) |
| Never smoked | 59 (47.6) | 45 (41.7) | 104 (44.8) |
| IT literacy level, n (%) | |||
| Low | 17 (13.7) | 14 (13.0) | 31 (13.4) |
| High | 107 (86.3) | 94 (87.0) | 201 (86.6) |
| Site, n (%) | |||
| Birmingham | 43 (34.7) | 34 (31.5) | 77 (33.2) |
| Glasgow | 29 (23.4) | 34 (31.5) | 63 (27.2) |
| Plymouth | 52 (41.9) | 40 (37.0) | 92 (39.7) |
| Participant-reported main reason for referral, n (%) | |||
| High blood pressure | 10 (8.1) | 10 (9.3) | 20 (8.6) |
| Low mood | 15 (12.1) | 22 (20.4) | 37 (15.9) |
| Osteoarthritis | 18 (14.5) | 13 (12.0) | 31 (13.4) |
| Type 2 diabetes and prediabetes | 14 (11.3) | 10 (9.3) | 24 (10.3) |
| Weight loss | 67 (54.0) | 53 (49.1) | 120 (51.7) |
Study attrition
Loss to follow-up
A total of 450 participants were randomised. A total of 94 participants (21% of those randomised) were lost to follow-up: 47 (10%) participants declined to participate further and 47 (10%) participants were non-contactable (see Appendix 2).
There were no differences in age, BMI, gender, IT literacy and educational attainment between participants who were included in the primary analysis and those who were not.
Accelerometer and questionnaire booklet return rates
Accelerometer and questionnaire booklet return rates are given in Table 7. Receipt of the baseline accelerometer at the CTU was a prerequisite for randomisation; therefore, there is a 100% return rate for this accelerometer.
| Time point | Sent to participant (n) | Returned to CTU, n (% of sent) | ||||
|---|---|---|---|---|---|---|
| Intervention | Control | Total | Intervention | Control | Total | |
| Accelerometer | ||||||
| Baseline | 224 | 226 | 450 | 224 (100) | 226 (100) | 450 (100) |
| 4 months | 203 | 213 | 417 | 183 (90) | 190 (89) | 373 (89) |
| 12 months | 172 | 184 | 356 | 160 (93) | 169 (92) | 329 (92) |
| Questionnaire | ||||||
| Baseline | 224 | 226 | 450 | 220 (98) | 220 (97) | 440 (98) |
| 4 months | 204 | 213 | 417 | 166 (81) | 183 (86) | 349 (84) |
| 12 months | 172 | 184 | 356 | 155 (90) | 170 (92) | 325 (91) |
At 12 months, 329 participants returned the accelerometer (return rate of 92%). The wear-time criteria were met by 243 participants, this being 54% of those randomised (Table 8). At this time point, 325 participants returned the questionnaire booklet (return rate of 91%), that is 72% of those randomised.
| Number of valid weekdays | Number of valid weekend days | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | Total | |
| 0 | 181 | 181 | ||||
| 1 | 3 | 2 | 2 | 7 | ||
| 2 | 1 | 4 | 5 | |||
| 3 | 7 | 6 | 5 | 18 | ||
| 4 | 6 | 10 | 31 | 47 | ||
| 5 | 5 | 30 | 77 | 16 | 2 | 130 |
| 6 | 2 | 49 | 7 | 58 | ||
| 7 | 4 | 4 | ||||
| Total | 203 | 50 | 172 | 23 | 2 | 450 |
In Table 8, purple shading denotes the number of participants meeting the wear-time criteria. A day was ‘valid’ when the accelerometer was worn for ≥ 16 hours on that day. Participants who did not meet the ‘valid’ criterion (n = 181) were 94 participants lost to follow-up prior to the 12-month time point, 60 participants who returned an accelerometer at 12 months but failed to meet the wear-time criteria and 27 participants who remained in follow-up but did not return the accelerometer at 12 months.
Losses to follow-up and non-compliance in returning the accelerometer were balanced across the two trial groups. Failure to meet the wear-time threshold was less consistent across the two trial groups.
Intervention engagement
Table 9 shows the level of engagement in the various steps offered online.
| Stage started | Summary of content | Number (% of 224 in intervention group) |
|---|---|---|
| Did not register | 81 (36) | |
| Step 1 | Quiz on benefits of PA | 144 (64) |
| Step 2 | Support to get active | 133 (59) |
| Step 3 | Encourage self-monitoring of steps | 107 (48) |
| Step 4 | Setting SMART step-count goals for next week | 99 (44) |
| Step 5 | Setting SMART goals for any PA for next week | 96 (43) |
| Goal review | Review goal and personalised feedback | 81 (36) |
| Step 6 | Ways to achieve goals/overcoming barriers (optional) | N/A |
| Step 7 | Overcoming setbacks (optional) | N/A |
The sample was evenly split, with 36% of participants not registering and logging in to e-coachER and 36% progressing through the support to record at least one goal review (i.e. having set a PA goal review, the participant logged back in about 1 week later to record PA against the goal and obtain feedback on the PA achieved against the goal set). Participants were routinely ‘locked out’ of accessing the web-based support to ensure that they did not complete it in one or two visits to the website, and then reminded by e-mail after 1 week to log in and continue with the steps and later record their PA in minutes, set goals and review them. Reaching step 5 involved > 4 weeks of intervention engagement.
Among all participants allocated to the intervention group, the mean number of goal reviews was 2.5 (SD 4.5), with a range of 0–52. Among the 144 participants who registered for e-coachER, they logged in for a mean and median number of times of 14.1 (SD 16.7) and 6, respectively, with a range of 1–101. Of these participants, 81 (36%) completed a goal review; the mean and median number of reviews was 14.4 (SD 13.8) and 4.5, respectively, with a range of 1–52.
Table 10 shows the mean, SD and median time spent during the respective stage (session) for the 144 participants who registered or 81 participants who completed at least one goal review. These data come with the limitation that participants may have left their browser open after some sessions rather than logging off, which leads to an overestimation of time spent. ‘n’ refers to the number of visits used to estimate the descriptive data for the time in sessions 1–5, the number of visits when a goal was first set and the number of sessions when a goal was reviewed.
| Status | Mean (minutes) | SD (minutes) | Median (minutes) | n |
|---|---|---|---|---|
| Visited steps 1–5 (from 144 participants who registered) | 9.53 | 9.68 | 6.45 | 539 |
| Goal-setting initial session (from 91 participants who set a goal) | 7.81 | 7.54 | 5.92 | 91 |
| Goal review session (from 81 participants doing ≥ 1 goal review) | 4.75 | 5.25 | 3.18 | 1034 |
| Total | 6.47 | 7.45 | 4.08 | 1664 |
On the basis that participants spent approximately 6 minutes logged in for steps (sessions) 1–5, and for 3 minutes for each goal review, for those 144 participants who registered, the total mean and median time that participants spent on steps 1–5 was 24.1 (SD 5.9) minutes and 30 minutes respectively.
For those 81 participants who completed at least one goal review, the overall mean and median time that participants spent doing goal reviews was 43.3 (SD 37.3) minutes and 21 minutes respectively. The 144 participants who registered spent a total mean and median time of 48.4 (SD 41.9) minutes and 36 minutes respectively. The range was 6–186 minutes.
Descriptive data for the primary and secondary outcomes by group and time
The descriptive statistics for the primary and secondary outcomes at baseline and 4- and 12-month follow-up are shown in Table 11 for all participants who provided data.
| Variable | Baseline | 4-month follow-up | 12-month follow-up | |||
|---|---|---|---|---|---|---|
| Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | |
| Primary outcome | ||||||
| Total weekly minutes of MVPA in ≥ 10-minute bouts,a n (mean); SD | 201 (30.2); 105.8 | 207 (31.8); 53.7 | 128 (30.9); 64.5 | 109 (38.4); 74.5 | 133 (18.7); 37.6 | 110 (35.4); 78.3 |
| Secondary outcomes | ||||||
| Average daily minutes of MVPA,a n (mean); SD | 201 (45.6); 35.6 | 207 (53.1); 35.9 | 128 (46.3); 37.8 | 109 (58.3); 35.9 | 133 (42.6); 30.1 | 110 (51.9); 36.6 |
| Weekly achievement of at least 150 minutes of MVPA in ≥ 10-minute bouts,a n/N (%) | 8/201 (4) | 9/207 (4) | 2/128 (2) | 7/109 (6) | 3/133 (2) | 6/110 (5) |
| Weekly achievement of at least 150 minutes of MVPA,a n/N (%) | 149/201 (74) | 178/207 (86) | 98/128 (76) | 99/109 (91) | 99/133 (74) | 93/110 (85) |
| Weekly achievement of at least 150 minutes of MVPA self-reported, n/N (%) | 83/220 (37) | 77/220 (48) | 94/183 (51) | 88/166 (53) | 76/170 (45) | 76/154 (49) |
| Average daily diurnal inactivity (hours),a n (mean); SD | 199 (1.7); 1.1 | 205 (1.5); 1.1 | 125 (1.4); 1.1 | 109 (1.4); 0.9 | 99 (1.4); 1.0 | 78 (1.5); 1.0 |
| Average daily sleep (hours),a n (mean); SD | 199 (6.8); 1.5 | 205 (6.9); 1.2 | 125 (6.7); 1.3 | 109 (6.7); 1.4 | 128 (6.8); 1.5 | 110 (7.0); 1.5 |
| EQ-5D-5L (Devlin values), n (mean); SD | 216 (0.74); 0.24 | 215 (0.76); 0.23 | 162 (0.72); 0.26 | 148 (0.76); 0.25 | 158 (0.72); 0.26 | 138 (0.73); 0.27 |
| HADS-D, n (mean); SD | 217 (7.6); 4.5 | 214 (7.4); 4.7 | 164 (7.4); 4.8 | 147 (6.0); 4.7 | 156 (7.1); 4.8 | 139 (6.3); 5.1 |
| HADS-A, n (mean); SD | 217 (8.7); 4.6 | 214 (8.6); 5.1 | 164 (8.5); 4.8 | 146 (7.5); 5.0 | 156 (8.4); 4.8 | 139 (7.6); 5.2 |
The groups were well balanced at baseline. Only 4% of participants achieved at least 150 minutes of accelerometer-recorded MVPA (in ≥ 10-minute bouts) over 1 week at baseline and the average weekly minutes of MVPA (not in 10-minute bouts) was 49 minutes, which reflects our success in recruiting inactive or moderately inactive participants with chronic conditions. In contrast, 80% achieved 150 minutes of accelerometer-recorded MVPA without regard for ≥ 10-minute bouts at baseline. Cassidy et al. 55 have also shown lower levels of accelerometer-recorded MVPA minutes when data are processed using ≥ 10-minute bouts compared with bouts of at least 1 minute. This proportion drops to 36% for self-reported MVPA, reflecting the way that the 7-day Physical Activity Recall questionnaire (7-D PAR) measure focuses on only discrete bouts of memorable MVPA. There were also no baseline differences between groups for the EQ-5D-5L and the two HADS scales.
Descriptive data for PA and participant-reported outcomes are also shown in Table 11 for those providing data at baseline, 4 and 12 months by trial arm, without controlling for baseline or other covariates. Qualitatively, the intervention group was more active than the control group at 4 and 12 months for all primary and secondary outcomes. The intervention group also qualitatively had higher well-being (EQ-5D-5L) and lower depression and anxiety scores than the control group at 4 and 12 months.
Primary outcomes
The primary outcome, ITT complete-case analysis showed a weak indicative effect in favour of the intervention group in the primary outcome at 12 months (mean difference 11.8 weekly minutes of MVPA, 95% CI –2.1 to 26.0 minutes; p = 0.10) (Table 12). A plot of the repeated-measures model estimates for the primary outcome over time for the intervention and control groups is shown in Appendix 3. Although the alternative model p-values varied somewhat, a similar pattern of results was seen across alternative post hoc models. In interpreting these results, it is important to recognise the limitations of all these models: lack of fit of the predefined primary and post hoc models, and the need to assume data as counts with both negative binomial and zero-inflated binomial models.
| Primary outcome | Primary analysis | Secondary analysis | |
|---|---|---|---|
| ITT complete-case comparison at 12 months, n, coefficienta (95% CI); p-value | ITT imputed comparison at 12 months | Complete-case comparison at all follow-up points, p-value for interaction between intervention effect and time | |
| Total weekly minutes of MVPA in ≥ 10-minute bouts | 232, 11.8 (–2.1 to 26.0); 0.10b | Not calculated | 0.63 |
| 223, 2.5 (–5.8 to 10.7); 0.55c | |||
| 232, 1.2 (0.8 to 1.5); 0.27d | |||
| 232, RR 1.90 (0.90 to 4.00); 0.09e | |||
| 232, RR 1.59 (1.13 to 2.25); 0.01f | |||
The results of the CACE analysis for the primary outcome were consistent with the ITT results (Table 13). In other words, when controlling for whether or not intervention participants completed a prespecified ‘dose’ of the intervention [i.e. they completed a goal review (reached step 5)], this made no difference to the findings, but qualitatively the difference between the intervention and control groups did appear to be larger (in favour of the intervention).
| Variable | Between-group difference, n, mean difference (weekly minutes) (95% CI); p-value |
|---|---|
| Total weekly minutes of MVPA in ≥ 10-minute bouts | 232, 22.9 (–3.4 to 47.8); 0.09 |
As Table 14 shows, there was no evidence of any interactions between stratification variables and age and gender with the intervention effect for the primary outcome at 12 months. The ITT complete-case model was adjusted for stratification variables age, gender and baseline scores, and random effects for site and fulfil the criteria of includable PA data.
| Variable | Interaction p-value | Subgroup coefficient (95% CI) |
|---|---|---|
| Age | 0.10 | –0.9 (–1.2 to 0.2)a |
| Gender | 0.91 | |
| Male | 16.7 (–5.2 to 38.7) | |
| Female | 10.3 (–7.8 to 17.9) | |
| Trial site | 0.20 | |
| Plymouth | 4.3 (–15.2 to 23.9) | |
| Birmingham | 19.4 (–9.8 to 48.8) | |
| Glasgow | 9.0 (–9.8 to 27.8) | |
| Participant’s perception of main medical referral reason | 0.33 | |
| Control diabetes | 11.9 (–0.1 to 24.1) | |
| Weight loss | 7.3 (–9.5 to 24.2) | |
| Lower blood pressure | 20.6 (–5.9 to 27.2) | |
| Manage lower limb osteoarthritis symptoms | 21.1 (–8.1 to 32.2) | |
| Manage mood/depression | 25.1 (–32.4 to 82.7) | |
| IT literacy level | 0.59 | |
| Lower confidence | 2.3 (–6.4 to 11.0) | |
| Higher confidence | 13.5 (–2.2 to 29.2) |
Table 15 shows the descriptive data for participants included in the primary analysis (n = 232). Data shown for the 4-month assessment are from participants who were included in the primary analysis and also had complete data at 4 months. The intervention group had qualitatively greater mean weekly minutes of MVPA than the control group at baseline.
| Variable | Baseline | 4 months | 12 months | |||
|---|---|---|---|---|---|---|
| Control group | Intervention group | Control group | Intervention group | Control group | Intervention group | |
| MVPA minutes (in ≥ 10-minute bouts), n, mean (SD) | 124, 22.6 (60.0) | 108, 33.5 (53.8) | 95, 31.7 (67.3) | 77, 41.8 (82.4) | 124, 17.6 (35.4) | 108, 36.1 (78.9) |
An analysis of complete data available at both baseline and 4 months (Table 16) shows that the control group significantly increased MVPA up to 4 months, whereas the intervention group did not. Between baseline and 12 months, there were no changes in either group, although qualitatively the control group had reduced their MVPA.
| Variable | Mean difference, n, mean (SD) [95% CI]; p-value | |
|---|---|---|
| Control group | Intervention group | |
| Baseline vs. month 4 | 118, 8.2 (32.1) [2.4 to 14.1]; 0.006 | 105, 6.4 (68.1) [–6.7 to 19.6]; 0.334 |
| Baseline vs. month 12 | 124, –5.0 (52.2) [–14.3 to 4.3]; 0.288 | 108, 2.6 (74.5) [–11.6 to 16.8]; 0.721 |
Secondary outcomes
Results of the complete-case imputed ITT and repeated-measures analyses (including both 4- and 12-month follow-ups) for the PA secondary outcomes appeared consistent with the analysis for the primary outcome (see Table 12). As Table 17 shows, there were no significant between-group differences in ITT complete-case analyses for any of the secondary outcomes at 12 months.
| Variable | Primary analysis | Secondary analysis | |
|---|---|---|---|
| ITT complete-case comparison at 12 months, n, coefficienta (95% CI); p-value | ITT imputed comparison at 12 months, n, coefficienta (95% CI); p-value | Complete-case comparison at all follow-up points, p-value (for interaction between intervention effect and time) | |
| Average daily minutes of accelerometer-recorded MVPA | 232, 1.9 (–3.8 to 7.7); 0.51 | Not calculated | 0.68 |
| Weekly achievement of ≥ 150 minutes of accelerometer-recorded MVPA in ≥ 10-minute bouts | 232, OR 3.80 (0.16 to 20.92); 0.12 | Not calculated | 0.03 |
| Weekly achievement of ≥ 150 minutes of accelerometer-recorded MVPA | 232, OR 1.67 (0.82 to 3.42); 0.16 | Not calculated | 0.23 |
| Weekly achievement of ≥ 150 minutes of self-reported MVPA | 324, OR 1.23 (0.79 to 1.90); 0.36 | 450, OR 1.55 (0.99 to 2.42); 0.05 | 0.39 |
| Average daily diurnal inactivity | 226, 0.6 (0.5 to 0.7); < 0.0001 | Not calculated | 0.66 |
| Average daily sleep | 226, 0.3 (–0.1 to 0.6); 0.11 | Not calculated | 0.18 |
| ERS attendance | 446, OR 1.13 (0.72 to 1.79); 0.58 | 450, OR 1.09 (0.70 to 1.71); 0.70 | – |
| EQ-5D-5L Devlin measure | 290, 0.00 (–0.4 to 0.05); 0.89 | 450, 0.01 (–0.03 to 0.04); 0.70 | 0.99 |
| HADS-D | 289, –0.2 (–1.0 to 0.6); 0.44 | 450, –0.2 (–0.9 to 0.6); 0.63 | 0.02 |
| HADS-A | 289, –0.5 (–1.2 to 0.2); 0.20 | 450, –0.2 (–1.0 to 0.5); 0.52 | 0.05 |
The ITT imputed comparison at 12 months showed that the intervention group had a greater proportion of participants achieving ≥ 150 minutes of self-reported MVPA (p = 0.05) than the control group. In the repeated-measures analyses (including both 4- and 12-month follow-ups), a greater proportion of intervention participants achieved ≥ 150 minutes of accelerometer-recorded MVPA (in ≥ 10-minute bouts) than control group participants. As Table 11 shows, 2% and 6% of the participants achieved this at 4 months in the control and intervention groups, respectively, and 2% and 5% at 12 months, respectively.
There were no significant between-group differences in the ITT complete-case or imputed comparison analyses for EQ-5D-5L scores or for the HADS anxiety and depression scores at 12 months. In complete-case repeated-measures analyses (including both 4- and 12-month follow-ups), the intervention participants reported lower depression (p < 0.05) and anxiety (p = 0.05) scores than the control group.
Exercise referral scheme uptake was derived from records held by the ERS service provider, with imputed participant-reported attendance at 4 weeks and/or 4 months where the ERS service data were missing. Data were not available via any of the three sources for four participants (control group, n = 3; intervention group, n = 1), resulting in 223 participants in each group with ERS uptake data. A total of 173 participants (78%) in the control group attended the ERS at least once, compared with 167 participants (75%) in the intervention group.
Adverse events
In total, 42 SAEs were reported among 35 participants (see Appendix 4), which were all deemed to be either ‘not related’ or ‘unlikely to be related’ to the trial. In the control group there were 26 SAEs among 21 participants and in the intervention group there were 16 SAEs among 14 participants. One SAE was reported as a life-threatening event (asthma attack) and all other SAEs were hospitalisations. SAEs were consistent with the patient population.
Chapter 4 Mixed-methods process evaluation
This chapter presents the findings from both qualitative and quantitative methods and brings the two together to help understand and explain the findings from the main analyses presented in Chapter 3.
Introduction
The overarching aim of the e-coachER trial was to determine whether or not adding support to usual ERS would be more clinically effective and cost-effective than usual ERS alone for supporting increases in PA in inactive patients referred to an ERS with a range of chronic conditions. The qualitative part of this chapter will explore participants’ and e-coachER researchers’ views and experiences of the support package and how it did or did not contribute to changes in PA. The quantitative part of the chapter will seek to understand if specific survey process measures were changed by the intervention, compared with usual ERS, as predicted from our logic model (Figure 5). Finally, we explore whether or not changes in the process outcomes mediated intervention effects on the primary outcome.
FIGURE 5.
The e-coachER logic model, adapted to show relationship with qualitative research objectives. Reproduced with permission from Ingram et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original.
Previous research has quantitatively and qualitatively explored the barriers to and facilitators19,56 of engaging in ERS, and the moderators of ERS engagement. 15,57,58 The e-coachER intervention was designed to help overcome many of the reported barriers to, and enhance the use of facilitators of, attending ERSs, becoming physically active in other ways, or both, through this primary care-based intervention. 59
The e-coachER intervention is theoretically underpinned by self-determination theory,31 which asserts that all humans possess three innate basic psychological needs (i.e. autonomy, competence and relatedness). When met, these needs foster intrinsic motivation resulting in personal growth and satisfaction. A range of behaviour change techniques35 based on self-determination theory were employed within e-coachER’s seven ‘steps to health’ (see Table 1), so this process evaluation seeks to establish whether or not the intervention influenced basic psychological needs and behaviour change processes.
A mixed-methods process evaluation seeks to best understand how participants engaged in the intervention (and trial methods) and what the consequences were with respect to our logic model.
Qualitative process evaluation
The logic model shown in Figure 1 has been adapted to show the causal pathways proposed to contribute to behaviour change and intervention outcomes and the objectives for the qualitative work (see Figure 5).
Aims and objectives
The qualitative interviews primarily aimed to explore how participants experienced and engaged with the e-coachER intervention.
The objectives were to explore:
-
whether the impact of the intervention is moderated by medical condition, age, gender, socioeconomic status, IT literacy or ERS characteristics
-
the mechanisms through which the intervention may have an impact on the outcomes.
The implementation and delivery of the trial and recommendations for future research in this area were also explored with participants and with the trial research assistants, who provided their views on e-coachER and their role in the study.
Methods
Recruitment
Semistructured interviews were originally planned with a purposeful sampling framework (considering gender, age, underlying health condition and trial site). The trial sample size was subsequently reduced with a corresponding reduction in the number of interview participants and a change to focus the sampling from across the three sites. All participants who had logged on to the website (at least once) were approached by the CTU team to take part in a qualitative interview. Those expressing an interest were then telephoned or e-mailed by the researcher (NC or RT), who explained the interview purpose and process. Participants were invited to take part in an initial interview and to give permission to be contacted for follow-up telephone interviews (up to three were planned) over the course of the intervention period. Participants provided informed consent to participate in the qualitative interview (in addition to prior consent to take part in the RCT). As most interviews were carried out by telephone, the informed consent form for the qualitative interview component was read point by point to the participant and signed by the researcher. Interviews were recorded, transcribed verbatim and anonymised.
Interviews with researchers at each research site were carried out following the completion of recruitment.
Interviews
Interviews with participants
Interviews were conducted at different stages of participation in the trial as it was anticipated that not everyone allocated to the intervention would progress through all of the steps to health. Each individual was invited to participate in an interview and further follow-up interviews, which enabled interviews to be conducted over the whole duration of the e-coachER intervention period (with online support available for up to 12 months).
The interview topic guide for participants (see Appendix 5) focused on each of the steps to health. Participants were also asked for their views on the welcome pack, the overall online support and the pedometer. Participants were asked to identify if and how they thought e-coachER provided support for ERSs, and for maintaining PA over and above attending their ERS. Participants were also asked to put forward any suggestions to improve or modify e-coachER.
The topic guide was also designed to gauge the participant’s development of self-regulatory skills (e.g. self-monitoring, goal-setting) and the extent to which the intervention enhanced a sense of autonomy (control), competence and relatedness. However, these terms were not used explicitly; instead, the researchers used their knowledge of the guiding principles of the e-coachER intervention to prompt the participant to expand on their responses to the broader topic guide questions.
Interviews with research assistants
The interview topic guide (see Appendix 5) focused on exploring issues surrounding the delivery of the trial, including recruitment and an exploration of processes that worked well, and what and where improvements could be made.
Analysis procedure
Interviews were audio-recorded, transcribed verbatim and anonymised, with any personal data or ways of identifying participants removed. Transcripts were imported into NVivo 11 (QSR International, Warrington, UK) for data management and coded by one researcher (Rohini Terry) following an initial period of coding by Nigel Charles. A thematic analysis was performed to identify key findings, initially focusing on ‘top-level’ themes reflected in the logic model (see Figure 5). Additional in-depth analysis took place to further explore the data. Rohini Terry, Jeffery Lambert and Sarah Dean discussed the emerging codes and themes, and a consensus about these was reached. More in-depth analysis was also undertaken, and this will be fully reported in a forthcoming manuscript as the main publication arising from the qualitative research. Results are reported to (1) address the objectives and (2) give the findings emergent from the data.
Results
Participant sample and interviews
Of the 144 participants approached for interview, 36 participants (25%) expressed an initial interest in taking part. Thereafter, six participants who had initially expressed an interest did not respond to the invitation for interview. It was not possible to make contact with a further three participants. Hence, 27 participants (18%) who had logged on to e-coachER at least once were recruited to take part in the qualitative interview. Nineteen participants completed a single interview. Seven participants completed more than one interview several weeks apart, with one participant completing four interviews, three participants completing three interviews and three participants completing two interviews. Therefore, 38 interviews were carried out in total, each lasting between 16 and 80 minutes; the average interview length was approximately 48 minutes. In total, 11 participants from Plymouth, nine participants from Birmingham and six participants from Glasgow took part in a qualitative interview. Participants had logged on to e-coachER at least once. The majority had progressed to the point that they were automatically locked out of the website for the first time (i.e. prior to step 2). At the time of the initial interview being conducted, participants may not have engaged with or progressed through all of the steps to health. Interviewing at these points, and as participants progressed through the steps, was intentional as it was important to obtain the experiences of those who did not progress as well as those who did. Interviews were also conducted with three research assistants, one from each site.
Table 18 provides a summary of interviewed participant demographics: gender, health condition, age, confidence using internet/IT, geographical location and access to IT facilities.
| Characteristic | Category | |
|---|---|---|
| Gender (n) | Female | 20 |
| Male | 6 | |
| Health condition (n) | Weight loss only | 5 |
| Weight loss plus other morbidities but not low mood | 4 | |
| Weight loss and low mood only | 7 | |
| Low mood only | 2 | |
| Low mood and other morbidity but not weight loss | 1 | |
| Weight loss plus low mood and other morbidities | 5 | |
| No low mood, not weight loss, other physiological conditions | 2 | |
| Age range (years) | Female | 28–69 |
| Male | 39–72 | |
| Confidence using internet/IT (n) | Low | 0 |
| High | 26 | |
| Participants at each research site (n) | Plymouth | 11 |
| Birmingham | 9 | |
| Glasgow | 6 | |
| Access to IT facilities (n) | Home/work access/mobile | 22 |
| Mobile not home access | 3 | |
| Public only, not mobile access | 1 |
Exploring the extent to which the impact of the intervention is moderated by medical condition, age, gender, socioeconomic status, information technology literacy or exercise referral scheme characteristics
A number of factors were identified as possibly affecting participant engagement with e-coachER and/or the impact of the intervention. These reasons included ill health, issues related to time and other issues specific to the individuals’ circumstances.
In some cases, illness and comorbidities (e.g. stroke) made it harder for participants to fully engage with e-coachER:
I have put some goals in there, erm, but as I think I hope you are aware, erm, I had a stroke a while ago, about 10 weeks ago, erm, so I’m being a bit slow, a bit cautious at the moment and I’m not going to, I can’t think of the word, erm, I’m not making my goals too high.
P09
For some participants, other unexpected life events made it difficult to engage with or experience benefits from e-coachER:
Yeah I mean, again, I had the motivation to do it it’s just I’ve had things going on that have forced my hand into stopping. But now I have, I do genuinely have the intention to restart and keep it up definitely . . . I have an illness and a loss in the family . . . It’s stopped everything in all honesty . . . Everything in my life . . . Has been ground to a halt virtually.
P08
By contrast, other participants found that, if their health condition had a negative impact on their motivation or ability to exercise, e-coachER provided reassurance and fostered their sense of competence:
In a way that’s saying look you haven’t achieved everything that you said you were going to that’s fine, but there are reasons for it and do you know what, that’s OK. If you’re too sore that you couldn’t do it. If you’re ill you couldn’t do it. You know if you were in a particularly low mood that day like if you’re in a low mood ‘here’s a little bit of information for you’ and that little bit of information on the low mood, I suffer from depression so the low mood one is really part of it.
P05
Access to IT facilities was generally good, and participants had high levels of confidence using the internet. A number of participants reported IT-related difficulties that may have an impact on e-coachER engagement and use and some felt that other apps or similar support provided more in terms of functionality:
I didn’t feel it was that user friendly especially ’cos I had used, sort of, [a commercially available app] and things like that in the past. And as I was comparing the two, sort of, the design of it I didn’t find e-coachER that great just the white and the green. I also was using the [a commercially available app] and I found that one a lot better because I was able to keep track of exactly what I was doing in a lot more of an easier way than that.
P22
Exploring the mechanisms through which the intervention may have an impact on the outcomes
Most participants found that e-coachER was an easy-to-understand, flexible and supportive resource:
. . . it’s [the e-coachER website] given me support as well and there’s always, as I say, sometimes there’s information there to look at. . . . it’s changed my attitude to exercising because, as I say, I was not very eager to do it but now I’ve done it and I’m enjoying it and I have enjoyed it from the start because it’s just I’ve seen the results basically and that’s really given me a boost.
P02
Most reported the package, or aspects of it, to be of some utility, for example acting as a prompt to increase PA:
I suppose really it is a reminder to actually do it.
P13
The e-coachER intervention was also described as providing an ‘incentive’ (P02), ‘a bit of a lift’ (P09) or a ‘pat on the back’ (P11) and that simply having access to e-coachER was motivating:
My initial issue was the motivation and confidence that were missing to go and get started, which e-coachER has been very instrumental in and then once I was started now I’ve got that and I’ve got the benchmark of doing this.
P16
The goal-setting aspects of the e-coachER support package, particularly in conjunction with the pedometer and setting step-count goals, using either the e-coachER website or the tear-off slips on the fridge magnet, were particularly important components of the package. They acted as a catalyst in helping participants consider new ways to increase their PA:
. . . fascinating . . . Erm, well just really interesting to find out what I do every day and if I do, do something different what impact that has, what the result of that is and it does encourage me to think, erm, right I’ll get up and go and do whatever, anything, erm, and it makes me think in a different way about doing things.
P09
A, erm, and it makes me think in a different way about doing things because, for instance, my doctor’s is, err, a bus ride, it’s awkward for me to say to my family ‘can you take me?’, I’m sort of now starting to think since I’ve got a bit more energy, my, erm, my legs are working better if you like, I’m starting to think along the lines of, well I can catch a bus and then walk the rest of the way, I don’t need somebody to take me and it will, that will certainly increase my steps . . .
P09
Although the aim of achieving or exceeding step goals was motivating, the desire to avoid not meeting these goals was also motivating and participants would set their goals to avoid feelings of failure and/or guilt, with one participant using paper-based goal-setting to avoid setting goals online with e-coachER:
Yeah, about, like, I say, like, you keep them realistic don’t you? So you don’t, you don’t set yourself up to fail.
P04
I haven’t as yet put anything down on e-coachER, simply because I put down on a piece of paper what I hope to do on a given week and failed miserably to do them so I thought I didn’t want to start to put them down, erm, until I knew that they were reasonable goals that could be achieved, not pipe dreams that have got no chance of being achieved.
P01
Participants generally felt that e-coachER provided a reminder to think about being more physically active, for example from the fridge magnet with tear-off PA-recording strips and use of the pedometer:
Every day I, well at the end of every day, I look at the pedometer and say ‘oh OK you’ve exceeded your goal’.
P04
It’s just it’s another tool that you can use to, you know, either monitor or encourage or, you know, everyone works differently I suppose and I didn’t, what I needed was something concrete, goal-wise, to do and to achieve and like I say, you know, once I’d set that goal then and if I don’t reach it well shame on me kind of thing.
P16
However, it became clear these aspects of the intervention were often used only initially and then some people switched to using their phones or other devices to record activity:
I would say so, even though I’m not doing it on the, erm, website now ’cos I monitor my step counts through my phone.
P15
Some participants felt that the e-coachER package was instrumental in helping to ameliorate any feelings of guilt experienced when participants perceived their efforts to increase PA were in some way unsatisfactory. For example, the reiteration that failing to meet set goals is normal and something that should be expected, which occurred later in the steps to health (the ‘slips, trips and falls’), was particularly helpful for some:
Being someone who suffers from depression, I understand about triggers and setbacks . . . But you know this part of the system is explaining to you that it’s OK, don’t worry about it, there are always going to be times in your life where you’re not going to be able to do this and you shouldn’t beat yourself up too badly about it.
P01
Participants reported that being able to choose their preferred level of engagement with e-coachER was a valuable feature and allowed participants autonomy over their exercise choices:
Oh, [I would] definitely recommend it. Like I say, I know that I haven’t engaged with it as well as I could have but the point still stands that it was instrumental in that motivation at the beginning of going and doing it and doing exercise referral and setting those goals and trying to maintain achieving those goals each day and then obviously upping them and it’s definitely worth it.
P16
Use of e-coachER also had the potential to help participants to develop or expand social networks conducive to augmenting PA:
It gives you different tips and tells you who to ask and different sites to get in touch with. I went to an osteoporosis meeting and they do an exercise thing and you can actually become a teacher with a qualification eventually but, like I say, I can’t do anything like that at the moment. . . . they give monthly talks and they have someone come to visit to give a talk who works and specialises in osteoporosis and . . . and they do an exercise group once a week. . . . so it’s quite good really.
P06
However, many participants felt that they already had an adequate social support network:
I’ve already got quite a good support network, so I just utilised my work colleagues.
P14
Improvements to e-coachER
Many participants felt that e-coachER could offer more in terms of functionality and that it did not provide as much information or as many features as could readily be obtained from other ‘app’-based packages. For example, some participants felt that the package was not interesting or did not provide enough information and some were disappointed by these shortcomings. Some felt that e-coachER did not work in the way that they had expected, or were frustrated by being locked out of the website rather than being able to progress on to the next stage when they were ready to do so:
I think you know, erm, the website is useful, erm, albeit frustrating at times but once . . . I get past step 7 I might have a different view about that because I won’t be locked out then or I would think not, I don’t know.
P12
Others found that the e-coachER website was ‘not that inspiring’ (P12) or ‘drab’ (P03) and one participant referred to it as ‘death by PowerPoint’ (P04). A number of participants described the website or aspects of it as ‘confusing’ (P02), ‘annoying and irritating’ (P07) or not ‘smart’ enough (P04). Participants went on to offer suggestions for improvements to e-coachER, which are summarised in Table 19.
| Topic | Summary of problem and/or suggestions for improvements | Participant quotations |
|---|---|---|
| Welcome pack | The lack of language options in the information pack and the lack of options regarding disabilities was highlighted | I don’t see any foreign language on it. In terms of diversity I don’t see any Arabic, Chinese, Punjabi erm . . . Whatever so you can have this leaflet in X, Y or ZP04 |
| Although straightforward in terms of ease of understanding and clarity, some felt that it did not look interesting or engaging | User manual should be made more visual . . . It didn’t inspire me to turn the page . . . It is I mean it’s not a huge criticism when I say that, I don’t mean that as it perhaps sounds it’s not a huge criticism, it is a bit dull . . . I mean I read it because I had to but I didn’t enjoy it, erm, and you know, I wonder if how many people are skipping over itP05 | |
| . . . I suppose really it’s got to be generic but . . . if you’re a woman I would like it, sort of, a little bit more interesting just . . . it just may be needed to be a little bit more eye-catching I think I’m trying to sayP18 | ||
| Website design, logging on, access and navigation, and lock-out | Although many found that the e-coachER website was easy to navigate and use, many found the layout of the package and being logged out at certain predefined points frustrating and found navigation difficult and restrictive | . . . I thought it would be very helpful if you could see what every stage was and then go back to where you were rather than having the situation where you’re in a stage and you can’t go onto the next one. . . . but at the moment of course you can’t move on without being locked out . . . with the website, you know, I’m not technically in control of what’s on the other end so it’s sort of going in and having a good look round and seeing what you can and can’t doP01 |
| More tailored website was suggested (e.g. could be split up into different categories of condition for referral) | I think at the front end of the e-coachER it might be a wider remit and then take it out from the tree stem and then branch off so that if it’s obesity you can look at different goals, if its diabetes again and if its mental health but it’s splitting them up from each otherP04 | |
| A minority of participants experienced IT-related problems, logging on, passwords, accessing e-coachER, etc. These were not difficult to resolve and logging on was straightforward for most. Some commented that logging on could have been made easier as participants had to go through the original e-mail link. Some felt that further developments regarding mobile access would have improved e-coachER. Some differences in the experience of e-coachER using different platforms [e.g. one participant reported not being able to access links using an iPad (Apple Inc., Cupertino, CA, USA)] | So I think again that would be something to say if you are having trouble if you use I don’t know iPads, Mac [Apple Inc., Cupertino, CA, USA] other things just click on the link and it will take you to itP04 | |
| It was suggested that ‘well you’ve got to do it through Microsoft [Microsoft Corporation, Redmond, WA, USA], you’ve got to do it through this, you’ve got to do it through that’ . . . I don’t know if it would actually work with a MacP04 | ||
| Stage 1 quiz | Many felt that the quiz did not offer new information | No, I mean I’m not putting myself up as a genius but some of it is at quite simplistic levelP03 |
| Erm, going through the quiz saying, you know, ‘did you realise that’, ah, but you know ‘if you did this’, it did feel rather high school-ishP05 | ||
| Stage 2 links to finding support | Some found links for external and additional support out of date or irrelevant. Although some found the links thought-provoking, few directly benefited from using these links | Some of the information that I was looking at was actually dated for last year and all the classes weren’t up to dateP18 |
| Pedometer | The pedometer did not always tally with other devices | The pedometer seemed to just do its own thing. It stopped logging them properly . . . again the next day it was the same sort of thingP06 |
| Some found that the pedometer was difficult to open; some found that it was difficult to wear | The other thing I’ve found quite tricky was getting it placed properly . . . Sometimes it was a bit uncomfortableP04 | |
| I am using my own [fitness tracker] anyway just purely because I think I said to you before with the pedometer it suggests that you put it on your waistband well women and maybe for men I don’t know, don’t always wear things that are suitable for the pedometer and so it would be easier if you could use whatever other means you have, if you have thatP24 | ||
| Entering step count onto e-coachER website | Inputting steps on the website required participants to round up or round down, which may negate the message perceived by many that ‘every little bit helps’. Some felt that the step count should accurately reflect the number of steps achieved | I think it would be quite useful if you could put in the actual steps you’ve done rather than, erm, rounding it to the 500. Because I, well this morning, I put mine in and I’m a very honest person and if I’ve only done 3200 I’m not going to put in 3500. I’m going to put in 3000 but that in itself can be a little bit, erm, disappointing because you think well actually I’ve done a bit more than thatP09 |
| Reminder e-mails | Did not factor highly as a necessary component of e-coachER; some found that these reminders were unhelpful or did not reflect their actual engagement | Erm, well I’ve been logging in every few days but, erm, apparently I kept getting e-mails to say, erm, where have I been and ‘it’s been 4 weeks since we heard from you’P06 |
| The next day I’ll get one ‘oh we noticed you haven’t logged in for 1 week’ and what would help is perhaps when one sets one’s goals that a date was inserted so there’s no argument about thatP03 | ||
| Later steps: setting goals and dealing with setbacks | Later steps used less frequently. Those who engaged with the later stages of e-coachER found that goals were not always saved | What I found frustrating . . . it completely wiped what I’d put the week before . . . for me once I get into the routine I can generally do the same activities just adding more in and virtually review them but you have to input absolutely everything again plus the extra stuff that you were doing that week. And I don’t I think, you know, it wouldn’t take much for that information to pull through and you just amend it. Rather than rewrite it each week and if you’re going to set up completely new goals that’s absolutely fine but actually, you know, you should be able to, it would be better to pull it through and amend it . . .P14 |
| One participant suggested that support regarding ‘slips, trips and falls’ may be better placed at an earlier step | ||
| Goal-setting sections could be more ‘inspirational’ | ||
| That message coming at the end if you like although I know it’s not the end of e-coachER but it’s the end of the steps. If it was in earlier . . .P13 | ||
| It’s good to have inspirational stuff to help you along . . . It wasn’t inspirational in any way the [web]site, you know, because you can have goals and you can have inspiration, sort of, pushing you towards the goalsP07 | ||
| General | Lack of interaction with health professional or ‘real person’ | Yes, yeah maybe, I don’t know, 2 or 3 weeks or even 4 weeks down the line, you know a conversation with somebody just even if it’s just to see how they’re getting on. ‘How are you getting on, how are you finding it?’ . . . if somebody is struggling and they’ve thought ‘oh no I can’t do this’ in the early days then you would have picked them up already, do you get what I mean? . . . Just to have somebody there as well as on the computer sort of thing, have a bit more of an input earlier on that’s great. As much as we are embracing the technology of life and all the rest of it, there is nothing more valuable than a voice and like talking to youP21 |
e-coachER as a support for exercise referral scheme use and uptake or as an alternative to exercise referral schemes
The aim of the e-coachER package was to support and enhance the uptake of ERSs:
And I think what . . . e-coachER did was it acted like a prompt. So, I suppose in terms of my own personality I don’t like to let people or anybody down so if I had an appointment with the practitioner I would make sure I got there or try my damnedest you know what I mean.
P19
One participant described a lack of support from the ERS and that e-coachER was able to mitigate the effects of this:
Because with the exercise referral scheme . . . I had to do things myself, if that makes sense and I went and I had my induction, she, there was, there was no come back to say ‘how are you getting on, how are you finding things?’. The person that did the induction with me hasn’t been in touch with me again, whereas at least with the e-coachER I can refer back to it. I can look at it at my leisure and go back and be inspired again. Whereas I had, I’ve not had that contact with the person at the gym.
P21
Although the combined use of ERS and e-coachER was noted, a number of participants reported that they found the ERS difficult to commit to, perhaps more so than other self-selected activities, whereas others expressed a lack of interest in the types of exercise being offered. There were other difficulties for some participants in accessing the facilities or services being offered by the ERS. These included geographical location, transport issues, gym costs, the participants’ working patterns or problems with ERS oversubscription and delays in the referral process or staffing levels. In addition, other barriers to ERS attendance stemmed from unforeseen individual circumstances (e.g. bereavement, injury and other health issues). e-coachER was therefore an important alternative or seen as separate to ERS:
It just feels like they’re two different things because I don’t always see my instructor all that much and I don’t know what, how that works.
P18
Trial research assistants’ experiences and role in recruiting and supporting participants through e-coachER
Research assistants’ suggestions for improvements to trial recruitment processes
Researchers suggested that a more direct approach to recruitment would improve rates, for example (1) using more refined criteria for identifying possible participants and (2) using study researchers to carry out this task:
They looked at the databases and contacted like hundreds of patients who they thought were eligible for e-coachER and they had a really poor return. I think to be cost-effective we’re going to have to be very clear and definite about finding the right criteria. [. . .] So I feel that that’s going to be a big way forward is the clinical research network or somebody in the research field is going to be able to, have to go into practices and have access to the databases and pull, going, doing that directly rather than asking the GP to spend part of his valuable few moments that he has with patients trying to encourage them to take part in a research study. That’s not going to happen, they just, although they are willing to do it they just don’t have time to do it.
RA02
Broadly, simplifying the referral and paperwork, streamlining the referral system, contacting participants via the ERS rather than via the GP, or contacting the participants directly about the study after the ERS practitioner had sought permission to pass on contact details to the researcher were found to be helpful:
We had approval to go straight to the exercise referrer who was receiving the exercise referral from the GP and then ask the staff there to help us identify the people that would be suitable for our study looking at our study criteria and then approach them to either get, give consent for the patient to pass on their telephone details to me as the RA [research assistant]. And then I would either approach them directly or she would give them out the patient, the trial information pack and then the patient could contact me if they were interested in taking part and that was really successful.
RA01
Similarly, at another research site, opportunistic sampling via general practice was slow. Engaging the ERS team to help gave little opportunity for researcher–participant interaction, but a significant improvement in recruitment rates:
So opportunistic recruitment didn’t work well at any point for us but the ERS, using the ERS system mailout out to potential participants, that worked really well. I mean we really, we kind of went from way behind to meeting our recruitment targets from the ERS, so they were really helpful.
RA03
Opportunistic recruitment it was very slow and it was also, I was constantly, kind of, chasing and reminding the GPs. Whereas with the ERS, it was one of the data team who was sending out the packs and she, you know, she just had to input the data and it was really a small job for her just to send out a letter to any potential participants. So, it was really, it worked much better, it was much more fluid.
RA03
Research assistants’ suggestions for improvements to the e-coachER package
As with the study participants, researchers felt that there was some scope to refine e-coachER in terms of functionality:
I guess I mean there was a couple of people that had said that the website should have been a little more, I don’t know, maybe all singing all dancing sort of thing. I think the competition is now with all these devices, apps and things that are out there, a lot of people were maybe, well not a lot, a couple of people had flagged up that they were disappointed with how basic the website is. On the flipside of that, it does need to be basic because a lot of people aren’t computer literate but I guess that’s a downside with the competition now from all the apps and so many things that are out there that it maybe does look less appealing.
RA02
Research assistants’ views of participants’ attitude towards the trial
The researchers felt that most participants were already highly motivated to take part in something (the research) that would help them to make lifestyle changes and increase PA. Although it was less clear that they had understood why they had been referred to an ERS, there was some confusion regarding being contacted about the research study and being referred to an ERS:
I think they were all quite positive really. There were obviously some people who just weren’t interested but the main thing that came out when I’ve been looking into it, there was a lot of confusion over what they’d actually been referred to. So there was a lot of the times I had to explain that they’d been referred by their GP and that it was the [exercise referral] scheme and I had to explain what that was but, generally, once they kind of knew what we were doing in conjunction with that, they were very positive . . . I think they were all interested in doing something that helped them and a couple of them were even, you know, I think also the voucher, obviously that was a nice bonus to have but yeah they were all very positive about doing it.
RA03
One further finding relating to participant experiences of being in the research trial arises from both the research assistant interviews and from a final follow-up interview with one of the participants, who remarked that just having the interview was ‘motivational’ (P13) as it was:
. . . another reminder that I need to go back to e-coachER to keep it there so you’re you [the research interviewer] are part of the motivation.
P13
The research assistants also noted that participants using e-coachER often described the importance of social support, and at times either explicitly or implicitly mentioned the support obtained from the researchers (including the qualitative interviewers). Two of the researchers commented:
Again, it wasn’t something that we have discussions with because it wasn’t part of my role, however, I did get some e-mails from a lot of people who just felt that they wanted to tell someone that they were logging their steps. Although they didn’t have to, a few participants have done that, it was a very important aspect to them.
RA02
A lot of participants said it was nice to have me on the phone to contact, so it’s not just online. . . . I think a lot of the people when they fill in the website they phoned me after to say why they haven’t been as active . . . this week because, this month because they’d been, you know, had an injury or they’d been busy and I think it was just that, that kind of option to explain that they don’t get via the website.
RA03
Discussion
The qualitative component of the process evaluation helped to develop a better understanding of the participants’ experience of and engagement with e-coachER and to explore how factors both related and unrelated to the e-coachER contents affected participants’ engagement with ERSs and the e-coachER website itself. A further element was to understand the experiences of the researchers working on the e-coachER trial. The findings are also of relevance beyond the e-coachER RCT, in particular regarding the role of support, self-monitoring, goal-setting and feedback from IT support tools. Interviews with members of the trial site teams involved in recruitment provided further information regarding trial implementation and delivery.
The e-coachER intervention went through substantial initial piloting with public and patient input prior to going live within the trial and some specific features of the intervention were designed for a purpose. For example, the lock-out feature of the intervention was aimed to prevent participants going through all seven ‘steps to health’ in the same session, which would leave less reason to return to the website at a later date. It was inevitable that some participants found this frustrating or worthy of comment, such as the useful suggestion that relapse management (‘slips, trips and falls’) would have been helpful at an earlier step. Strategies that prevent existing, or developing, competencies from being undermined were an important feature of e-coachER’s design and the ‘slips, trips and falls’ step was instrumental in helping participants to develop and maintain competence rather than experience failure. However, potential opportunities to develop competence by engaging in these later steps were often not realised as participants found these stages less ‘user friendly’ than earlier steps or were not able to proceed onto these steps when they wanted to, having been automatically locked out in the earlier stages. Being locked out of the package may have provoked annoyance and disengagement and may have a negative impact on the control/autonomy dimension of self-determination theory. It should be noted that these findings arise from the relatively few participants interviewed who reached these end steps.
We also designed the intervention to engage with participants with a range of levels of IT literacy. However, for those interviewed it was clear that most were competent IT users, meaning that, for some participants, e-coachER provided insufficient content and functionality. Similarly, information provided in e-coachER was designed to be straightforward and accessible for all, for example the quiz, sometimes described as a ‘useful reminder’, seemed to serve as a prompt to remind participants what they ‘should’ be doing. Thus, although little of the information was considered to be new, the reiteration appeared to be valuable and some felt that the links provided useful information but others found that it was too basic, perhaps offering insufficient challenge to allow the development of a sense of competence around using e-coachER. It is clear that although some participants appreciated the information provided, there is scope to add further levels of information and advice as this would both allow tailoring for specific conditions and help to create a sense of progression (of information detail) within e-coachER. It is possible that the efficacy of the intervention may vary depending on the condition for which the participant was referred, and many had multiple comorbidities. More tailored information, which could be selected if relevant, may have added to the functionality of e-coachER and provided further opportunities for the user to develop feelings of competence regarding undertaking more PA with their underlying health condition. In the future, the intervention could be refined to include more explicit statements about the intervention design features (e.g. the purpose of the lock-out, that the pedometer is only a basic monitoring tool) as well as provide more optional levels of information content related to specific health conditions.
Interviewed participants identified two main ‘active ingredients’ of e-coachER. The first was the skills training and opportunity to set goals and to monitor progress by using the pedometer. This encouraged participants to reach or exceed their personal goals. Goal-setting and self-monitoring have been found to facilitate the ability to initiate and maintain behaviour change. 29 The participants who were interviewed did seem to set their goals realistically and they were broadly SMART; they ‘set their pace’ and mentioned how setting and attempting to meet these goals were positive aspects of the intervention and participants particularly valued the step-count activities. Donnachie et al. 60 also described how pedometers can provide tangible evidence of progress and demonstrate enhanced competence, with the device being seen as an ‘ally’ to meeting goals. It was clear that participants appreciated receiving the pedometer (and fridge magnet) and recent research has shown that providing primary care patients with a pedometer by mail is effective in increasing PA,61 so this finding was not surprising. However, although most participants were very enthusiastic about obtaining feedback from the pedometer, some were disappointed by the quality of the device and wondered if the step count was accurate. Whether or not these limitations acted as barriers to engagement with the e-coachER or the PA (or both) is unclear, although previous research suggested that mistrust of monitoring equipment may be detrimental. 59
Second, the provision of support, for example by providing a virtual ‘reminder’ or ‘pat on the back’, was regarded as an important aspect of e-coachER. These features provided opportunities for social interaction and/or the support of social interaction where social interaction had been in some way lacking. The e-coachER intervention, grounded in self-determination theory, sought to build a sense of connection or relatedness with others. Throughout the interviews, participants referred to the importance of social support and how this was met to varying degrees through e-coachER. Step 2 of e-coachER encouraged participants to seek social support, highlighting opportunities for joining groups, communities, discussion forums, etc. Although designed to be specific to the participant’s geographical area, some felt that these were not relevant to their needs or could be improved in other ways. For other participants, however, e-coachER did help to facilitate an open discussion about health within the participants’ social network and provide them with an initial starting point or ‘trigger’ to try new things. The concept of competence refers to an individual’s need to master tasks or to learn new or different skills,31 and some participants described how e-coachER helped them to develop the confidence to engage more with PA.
In general, most participants reported at least some benefits of using e-coachER; participants often felt that its use was ‘motivating’ but when asked to explain more they were less clear in identifying which specific aspects had this motivating effect. A qualitative study evaluating the effects of a walking intervention found that many participants reported benefits of trial participation, even when objective quantitative measures of PA did not increase. 59 Although satisfied with the e-coachER website providing a ‘starting point’ for their plans to increase PA, many found the limitations inherent in the e-coachER package to be frustrating. For example, it is possible that the e-coachER prompts (if inappropriately timed) undermined intrinsic motivation and this may have been compounded by a reduced sense of control arising from being locked out of e-coachER for predefined periods of time.
A number of participants felt that components of e-coachER were limited or could be improved, but they often described the package as a valuable resource. This may be because participants were already motivated to make changes and e-coachER provided additional support to do this. Many of the participants had actively sought to attend ERSs and described a pre-existing motivation or readiness to change. 62 Those who reported few benefits from e-coachER may not have been at a stage where they were ready to make changes and it is also possible that, over the duration of the engagement with e-coachER, participants progressed non-linearly through stages of readiness to change. None of the participants explicitly stated that they were not motivated to increase their PA and most were positive about their involvement in the trial. They felt that they were supported by an intervention that was designed to help them to achieve greater levels of PA and felt that e-coachER consolidated, directed and focused their desire to make changes.
Strengths and limitations
Interviewing participants at different stages of engagement with the intervention, and the follow-up interviews with seven participants, allowed us to tap into experiences at particular stages of e-coachER and, for those giving repeat interviews, how perceptions may have changed as engagement progressed.
The present findings were prepared blind to overall trial findings before being presented at a trial management meeting. This strengthens the findings from the qualitative research as the main trial results did not influence the interpretation of the qualitative findings.
One limitation is that the extent to which the interviews and the support provided by the research assistants may have affected the participants’ interaction with e-coachER is unclear. However, most of those interviewed had only one interview (n = 19), and these contacts were relatively early on in the intervention period. Furthermore, we could find no obvious differentiation between interviewees who had one interview and those who had multiple interviews regarding their comments about engagement with the other intervention components. Moreover, given the absence of a difference in the primary outcome and most other outcomes in the trial, it is unlikely that a few additional interviews had any influence on the findings.
It is also unclear whether or not the participants’ appreciation of being in the ‘additional’ intervention group affected their responses to the interview questions and to e-coachER as well. Some were apologetic about being critical of the intervention components and participants may have felt obliged to modify their criticism.
None of the 16% of the total e-coachER study sample (n = 450) who were classified as having low IT literacy (as a stratification variable) was interviewed and this may have limited our ability to understand how the web-based support helped or did not help these participants. This is a limitation but does not necessarily mean that we interviewed only those with very strong IT skills.
We did not interview any participants who were not in the e-coachER group of the trial about their experiences of ERSs. Therefore, we do not know how trial control group participants felt about not being selected for the intervention group or about their experiences of ERSs. A further limitation is that we did not interview any participants at the end of their time in the trial, for example at about 12 months post enrolment, to explore their experiences over the longer term and whether or not e-coachER had helped them maintain PA levels. Interviewing at this time point may have provided a clearer picture of the extent to which any behaviour change achieved during interactions with e-coachER were sustained. However, undertaking such late-stage interviews could have compounded the co-intervention effects already mentioned and resources were not available to conduct such interviews after the primary end point of the main trial.
Conclusions
There has been considerable literature on the barriers to and facilitators of ERSs, so the qualitative work focused mainly on if and how the e-coachER intervention complemented usual ERSs. The e-coachER intervention was acceptable and was positively experienced by many of the interviewed participants, and enhanced competence, autonomy and relatedness for many, but not all of the interviewed participants, with several areas for enhancement and augmentation identified. Engaging in self-monitoring and progressive goal-setting helped to build a sense of competence to increase PA. In doing so, participants appreciated the opportunity to make personal decisions about the types of activities they engaged in and how often. The website encouraged participants to get personal support from the ERS and this worked for some but not others, for example those who had other competing demands on their time. We were not able to identify specific examples in the interviews of how the website had brought people together to share PA experiences. There were positive and negative experiences of e-coachER but these were not necessarily of equal impact. For example, finding e-coachER a useful reminder to exercise may have more positive impact than the dislike of the web page layout or colour scheme. Instead of focusing only on individual positive and negative experiences of e-coachER itself, it is perhaps more important to place the findings from the interview study into the wider context. This was a complex intervention and, as such, may be more or less effective for individuals depending on their morbidities or comorbidities, complex personal circumstances or whether or not they were ready to increase PA.
For future trials, it is important to ensure that recruitment and referral pathways are as direct, targeted and straightforward as possible (i.e. not dependent on busy general practices). Future trials could be resourced sufficiently to allow any parallel qualitative study to be balanced across trial groups.
Quantitative process evaluation
Aims and objectives of the quantitative process evaluation
Within our logic model, we expected that the e-coachER support package would more favourably influence some key theoretical components (i.e. a sense of competence, autonomy and relatedness, and heighten value or importance attached to the behaviour) and behaviour change processes (i.e. action-planning, self-monitoring, enlisting social support) known to be involved in health behaviour change.
The objectives were to explore whether or not:
-
the e-coachER intervention led to more favourable process outcomes compared with usual ERS alone at 4 and 12 months
-
any changes in the process outcomes between baseline and 4 months (during which intervention engagement was expected to predominantly take place) mediated any intervention effects on the primary outcome [i.e. minutes of accelerometer-recorded MVPA (in bouts of ≥ 10 minutes) at 12 months].
Methods
The survey used to capture the process outcomes was described in Chapter 2. Briefly, items were derived from extensive reviews of the literature to ensure that they matched our expected changes within our logic model but also were fit for purpose within a RCT. In other words, they had to make sense for a responder whether or not they did any PA and they had to have some sensitivity to identify change. In response to PPI input, we also had to maximise the participant completion rates so we ensured that the questions were easy to interpret and the response format was clear. The items and respective scales are supplied in Report Supplementary Material 4.
Questions about importance and confidence are single items using an 11-point scale. The remaining items were chosen to represent specific constructs and create composite scores. Confirmatory factor analysis revealed support for adding survey items to assess: perceived competence in being regularly physically active (four items), autonomous in decisions about PA (four items), availability of support (three items), frequency of support (three items), action-planning (five items) and self-monitoring (two items). The Cronbach’s alpha coefficient of all factors were found to be in excess of 0.77, indicating good internal consistency of each. Composite scores were calculated and used in the analysis.
Using a model adjusted for age, gender, stratification variables and baseline scores, and random effects for centre, with only participants included in the primary analysis (i.e. based on complete accelerometer data and had completed the respective survey items), we compared each of the eight process outcomes by group at both 4 and 12 months. We restricted the analysis to outcome data deemed valid, with no more than 200 minutes spent in 10-minute bouts of MVPA, as per the primary analysis.
The size and significance of any mediating effects were evaluated through the product of coefficients method. 63 This was performed irrespective of the results from the main analysis, as mediation may still be possible without having detected a significant effect of the intervention on the primary outcome. 64 Referring to the causal diagram in Figure 6, the coefficient, a, for the intervention effect on process measures in path A was derived from the mixed model of changes in process measures regressed on the intervention, adjusted for age, gender, stratification variables and random effects for centre. Utilising the same adjustment variables, the coefficient, b, for the change in process measures on the primary outcome in path B was obtained by modelling the outcome on the process measure change, also adjusting for the effect of the intervention. The coefficient of the mediating effect was, therefore, calculated as the product a × b. The CIs were calculated using the Sobel test,65 dividing the coefficient product by the estimated standard error used:
FIGURE 6.
A priori path model for testing mediation effects.
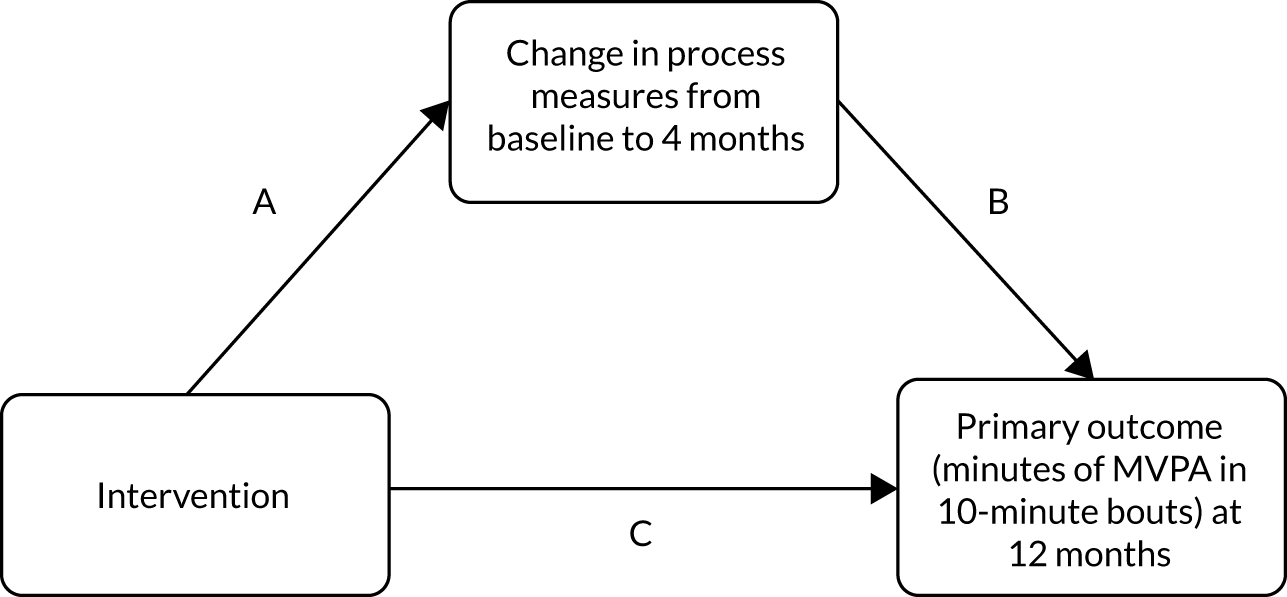
Results
Descriptive data are shown in Table 20 for each of the eight outcomes at baseline and 4 and 12 months, by trial group.
| Mediators | Baseline | Month 4 | Month 12 | |||
|---|---|---|---|---|---|---|
| Control, n, mean (SD) | Intervention, n, mean (SD) | Control, n, mean (SD) | Intervention, n, mean (SD) | Control, n, mean (SD) | Intervention, n, mean (SD) | |
| 1. Importance | 121, 5.49 (2.90) | 96, 5.58 (2.58) | 117, 6.53 (2.76) | 95, 7.55 (2.22) | 122, 6.34 (2.77) | 100, 7.14 (2.55) |
| 2. Confidence | 121, 5.60 (3.10) | 97, 6.06 (2.73) | 117, 5.56 (3.28) | 95, 6.72 (2.82) | 122, 5.44 (3.28) | 100, 6.07 (2.94) |
| 3. Competence | 123, 13.14 (3.65) | 97, 13.74 (3.46) | 113, 12.69 (3.92) | 93, 14.27 (3.64) | 118, 12.51 (3.94) | 99, 13.40 (4.09) |
| 4. Autonomy | 121, 14.26 (3.48) | 98, 14.54 (3.18) | 116, 14.69 (3.64) | 93, 15.31 (3.31) | 121, 14.53 (3.45) | 96, 15.32 (3.41) |
| 5. Support availability | 122, 9.89 (3.39) | 97, 10.47 (2.93) | 115, 9.77 (3.38) | 94, 10.80 (2.87) | 121, 9.69 (3.30) | 97, 10.36 (3.18) |
| 6. Support frequency | 122, 7.01 (3.50) | 99, 7.61 (3.17) | 116, 7.58 (3.62) | 94, 8.03 (3.41) | 120, 6.97 (3.62) | 100, 7.70 (3.38) |
| 7. Use of action-planning | 117, 12.99 (5.25) | 97, 13.13 (5.03) | 114, 16.10 (5.00) | 92, 17.09 (4.67) | 120, 14.84 (5.19) | 97, 15.88 (4.91) |
| 8. Use of self-monitoring | 121, 5.17 (2.16) | 98, 5.70 (1.97) | 115, 6.60 (2.02) | 94, 7.36 (2.03) | 121, 6.32 (1.95) | 99, 6.70 (2.09) |
Analysis of the intervention effect on the process outcomes among participants meeting the minimum accelerometer wear-times as specified for the primary analysis indicated that intervention participants reported greater change in beliefs from baseline to 4 months for PA beliefs about importance, confidence and competence than the control group (Table 21). By 12 months, there was no longer evidence of differences in change in beliefs between the intervention group and the control group.
| Mediators | Month 4 | Month 12 | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Coefficient | p-value | 95% CI | n | Coefficient | p-value | 95% CI | |
| 1. Importance | 208 | 0.888 | 0.013 | 0.184 to 1.592 | 218 | 0.705 | 0.098 | –0.129 to 1.540 |
| 2. Confidence | 209 | 1.041 | 0.007 | 0.287 to 1.794 | 219 | 0.393 | 0.332 | –0.401 to 1.187 |
| 3. Competence | 205 | 1.214 | 0.024 | 0.158 to 2.269 | 216 | 0.571 | 0.337 | –0.593 to 1.735 |
| 4. Autonomy | 207 | 0.51 | 0.306 | –0.466 to 1.486 | 216 | 0.495 | 0.335 | –0.511 to 1.500 |
| 5. Support availability | 208 | 0.399 | 0.318 | –0.384 to 1.182 | 216 | 0.026 | 0.951 | –0.818 to 0.870 |
| 6. Support frequency | 211 | –0.072 | 0.889 | –1.090 to 0.945 | 220 | 0.115 | 0.831 | –0.947 to 1.178 |
| 7. Use of action-planning | 200 | 1.249 | 0.18 | –0.575 to 3.073 | 210 | 1.042 | 0.239 | –0.694 to 2.777 |
| 8. Use of self-monitoring | 209 | 0.293 | 0.45 | –0.468 to 1.054 | 218 | –0.102 | 0.774 | –0.798 to 0.594 |
Figure 6 shows the a priori model to be tested in the mediation analysis.
Table 22 shows the analysis of mediating effects of change in the process outcomes (from baseline to 4 months) on the primary outcome at 12 months, using only participants included in the primary analysis. There were no significant mediation effects. Despite there being significant intervention effects on change in beliefs about importance, confidence and competence at 4 months, these changes in beliefs did not translate into increases in MVPA minutes at 12 months. A possible explanation is that the intervention effects on the primary outcome were insufficient to fully test the mediating effects, especially given the distributions, with many participants recording zero for minutes accumulated in bouts of ≥ 10 minutes. Another explanation may be that the 10-point scales used to assess importance and confidence were more sensitive to identify change than a 5-point scale used for the other outcomes.
| Mediators | n | Path A | Path B | Mediated effect | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (SE) | p-value | Coefficient (SE) | p-value | Coefficient (SE) | 95% CI | p-value | ||
| 1. Importance | 204 | 0.919 (0.365) | 0.012 | 2.483 (0.924) | 0.007 | 2.282 (1.242) | –0.152 to 4.716 | 0.066 |
| 2. Confidence | 205 | 1.056 (0.392) | 0.007 | 1.433 (0.832) | 0.085 | 1.513 (1.043) | –0.531 to 3.557 | 0.147 |
| 3. Competence | 201 | 1.139 (0.547) | 0.037 | 0.267 (0.614) | 0.664 | 0.304 (0.714) | –1.095 to 1.704 | 0.670 |
| 4. Autonomy | 203 | 0.478 (0.508) | 0.347 | 0.437 (0.679) | 0.520 | 0.209 (0.393) | –0.561 to 0.979 | 0.595 |
| 5. Support availability | 204 | 0.419 (0.407) | 0.303 | –0.104 (0.820) | 0.899 | –0.044 (0.346) | –0.722 to 0.635 | 0.899 |
| 6. Support frequency | 207 | –0.065 (0.527) | 0.903 | 1.568 (0.598) | 0.009 | –0.102 (0.827) | –1.723 to 1.519 | 0.902 |
| 7. Use of action-planning | 196 | 1.198 (0.938) | 0.201 | 1.012 (0.362) | 0.005 | 1.212 (1.044) | –0.834 to 3.259 | 0.246 |
| 8. Use of self-monitoring | 205 | 0.235 (0.389) | 0.545 | 2.533 (0.828) | 0.002 | 0.595 (1.004) | –1.373 to 2.563 | 0.553 |
Nevertheless, according to analysis of path B in the mediation diagram (see Figure 6), the primary outcome did appear to be sensitive to changes (from baseline to 4 months) in importance, the frequency of support, action-planning and self-monitoring. However, in all but importance, there were insufficient changes to assume a mediating effect in the process measures between baseline and 4 months. With regards to importance, although paths A and B were individually significant, the combined change in this process measure and its effect on the primary outcome according to the Sobel test was insufficient to produce a significant mediating effect.
Summary
Our mixed-methods approach to understanding if and how the intervention worked for some participants provided interesting insights into engagement with a novel technology-based support system and how that complemented the available support from usual ERSs. The qualitative and quantitative approaches were conducted independently. The interviews highlighted the role of developing self-monitoring and SMART goal-setting skills to increase confidence, which featured strongly in being physically active on a regular basis. The survey process outcomes confirmed that the intervention increased importance and confidence up to 4 months but the effects (compared with usual ERSs) had dissipated by 12 months.
The qualitative interviews were mostly not able to follow intervention participants for long. The lack of intervention effects on changes in other process outcomes (e.g. action-planning and self-monitoring) was surprising given that 36% of the participants reached step 5 (completed a goal review). Some participants continued to complete goal reviews for up to 12 months, but it would appear that an insufficient number of participants did so, and most had stopped completing goal reviews long before completing the 4-month follow-up assessment. This was also despite periodic reminders from the online system to log in.
The interviews provided a little information on how e-coachER had prompted intervention participants to find and use social support to increase PA (e.g. support to get active – step 2). Although designed to be specific to the participant’s geographical area, some felt that the e-coachER links to other PA opportunities were not relevant to their needs. For other participants, however, e-coachER did help to facilitate an open discussion about health within the participants’ social networks. It is possible that simply talking about being in the e-coachER study (i.e. an opportunity to connect) would have been done by participants in both of the trial groups and few actually got around to joining groups or setting plans to exercise with others. Hence, this may explain why the intervention had no effects on the process outcomes, namely identifying and using social support.
In this pragmatic trial, the aim was to determine if there were intervention effects in addition to usual ERSs. An alternative explanation to null intervention effects on some of the process outcomes is that usual ERSs have sufficient positive effects on PA beliefs to make additional effects unlikely. The Glasgow ERS differed from the other ERSs involved in the trial, in that the exercise professional supported participants with behaviour change counselling and signposting to different PA options. In this sense, the Glasgow ERS may have been expected to build a sense of autonomy and the e-coachER support may not have added much. Further analysis is needed to explore differences in intervention effects on process outcomes by site, although site did not interact with the intervention effects on the primary outcome. Health interventions, including ERSs, are notorious for having short-term effects on health behaviour that dissipate with time. Given that ERSs can provide barriers to engagement and sometimes may not promote a range of sustainable PA options, we designed e-coachER to have more lasting appeal and to develop self-regulatory skills and the intrinsic motivation to be physically active for managing chronic conditions, above and beyond usual ERSs. Some key processes did change as a result of e-coachER engagement in the short term but these were not sustained, which is consistent with the absence of any mediating effects of change in these measures on intervention effects on the objective primary outcome of MVPA.
Chapter 5 Economic evaluation
Introduction
This chapter reports the economic analysis of an augmented ERS with web-based behavioural support (e-coachER) versus ERS alone. Comparing the costs and consequences of alternative approaches to promoting health care is key to facilitating efficient allocation of resources. 66
Although economic evidence on ERSs exists (mostly compared with usual care), it is unclear;17 little is known about the value for money of an augmented ERS with online behavioural support. A review of other reviews (n = 3) conducted by Pavey et al. 54 found one study67 showing ERSs to be cost-effective, another one68 reporting mixed findings and a third69 that found limited evidence of effectiveness but higher cost. In an analysis of 21 economic evaluation studies published by NICE from 2006 to 2010, Owen et al. 70 found ERSs to be cost-effective at the NICE threshold of £20,000 to £30,000 per QALY. In a systematic review of economic evaluations, Vijay et al. 71 reported three studies showing a cost per disability-adjusted life-year (DALY)/QALY estimate below £10,000 for exercise prescriptions.
The most recent NICE guideline development on ERSs identified two key gaps in knowledge. 72 First, there is paucity of cost-effectiveness evidence on alternative models of ERS. Second, information on the cost-effectiveness of ERSs for people with comorbidities is lacking. We are aware of an ongoing multicountry, multicentre RCT examining the cost-effectiveness of enhanced ERSs plus self-management strategies compared with ERS alone among inactive patients. 73
This chapter addresses the gaps in the literature by estimating the cost-effectiveness of e-coachER compared with ERSs alone, in adults with range of chronic conditions. The analysis uses a 1-year time horizon (from baseline to 12 months post randomisation) and is conducted from the viewpoint of the NHS, Personal Social Services and patients. The base-case analysis covered NHS and Personal Social Services perspectives.
Methods
The intervention and control population are as defined in Chapter 2. In line with the clinical effectiveness analysis, the samples for base-case analyses are participants who provided valid accelerometry data. Sensitivity analysis explored the impact of using the whole sample.
Measurement and valuation of cost
Total costs were expressed per participant and calculated by multiplying resource use with each relevant unit cost and summing across the range of resource use possibilities across the 12 months of the study.
Resource use, including those ‘in kind’, associated with both intervention and control was identified through discussions with the management team of the trial. Following this, resources were measured and valued without research-driven resource use. The range of resource use covered (1) set-up and design of the intervention, (2) delivery of the intervention including handbooks, pedometers, guide for using the LifeGuide platform, technical support and maintenance of the website, (3) consultation provided by an exercise specialist and staff support to participants, (4) primary and secondary health service use: GP, nurse, social worker, care worker, physiotherapist consultations (both home and practice visits), prescriptions, hospital admissions, accident and emergency (A&E) visits, and other (e.g. podiatrist visits); and (5) time and money expenses borne by participants in relation to participation in the intervention (e.g. time spent on web platform), visit to exercise specialist and PA (e.g. membership fees for gym or sports club). Data on resource use were collected using the trial administrative records, key informant interviews (e.g. trial manager), review of trial management records and participants’ questionnaires at baseline, 4 and 12 months.
Resources were valued using national tariffs (e.g. Unit Costs of Health and Social Care 2017,74 NHS Reference Costs 2015 to 2016,75 Annual Survey of Hours and Earnings76) to increase generalisability. In the absence of available national costs, unit costs came from trial administrative records. Appendix 6 provides details of unit costs. The unit cost of capital costs (i.e. pedometer) was calculated pro rata (costs were spread over their expected lifetime) because use can occur beyond the time period of this analysis. 77 Costs were expressed in 2017/18 Great British pounds, using the Hospital and Community Health Service (HCHS) inflation index where appropriate (PSSRU 2017). 74 No discounting was used as the time horizon of the analysis is 1 year.
Measurement and valuation of outcomes
Two types of outcomes were used for estimating cost-effectiveness: physical units (PA indicator) and QALYs. The PA measure is the primary outcome measure of the trial (as described in Chapter 2): total weekly minutes of MVPA in ≥ 10-minute bouts, recorded objectively by accelerometer. QALYs were estimated by converting EQ-5D-5L utilities using the area under the curve method. EQ-5D-5L questionnaires were completed by trial participants at baseline and 4 and 12 months. In line with the 2018 NICE recommendation,66 utility weight based on the crosswalk function78 was used to assign utility weights. Sensitivity analysis explored the impact of using other valuation sets.
Methods of analysis
Missing data analysis
Multiple imputation was employed to replace missing values as it incorporates uncertainty around imputed estimates. Imputed values are drawn from a regression model fitted for each variable with missing data. Multiple imputation by chained equations was used and not multivariate normal imputation as the variables with missing data include binary and categorical variables and these are not suitable with the multivariate normal imputation.
Five imputations were used. 79 The standard approach was followed in building the imputation model as we ensured that the imputation model matched the model used for the analysis while including the predictors of missingness as possible. In addition, the dependent variable was included in the imputation model to ensure that the imputed values have the same relationship to the dependent variable as the observed values. 80
As the purpose of the multiple imputations was to replace missing values for raw data to allow the generation of derived variables, a point estimate was required, not its variance per se. In line with Rubin’s rules,81 we derived the overall point estimate for the imputations by averaging the estimates of the multiply imputed data. For categorical data, the overall point estimate was rounded up to the nearest decimal point as relevant. The mean of multiple imputations is an unbiased estimate of the missing value, and their contributions to increased variance in subsequent analysis viewed as an estimate of the added uncertainty caused by data missingness. 81
Incremental analyses
The within-trial analyses were twofold:
-
Incremental cost–utility analysis – this was the primary analysis of the economic evaluation and used QALY as the effectiveness measure. The outcome of the analysis was cost per QALY.
-
Incremental cost-effectiveness analysis – analysis generated cost per change in MVPA minutes based on accelerometer data indicated in Chapter 3.
Descriptive statistics based on mean and SD were conducted for the cost components and quality-of-life measure. Estimations of the outcomes used regression models fitted separately for costs, QALYs and MVPA minutes. Details of the estimation of MVPA minutes is presented in Chapter 2. Generalised linear models fitted with gamma and binomial 1 (equivalent to beta regression) distributional families were fitted for the costs and QALYs analyses, respectively. 82,83 The modified Park test was used to select the appropriate distributional family. 82 Regression models adjusted for covariates, as recommended, including baseline QALYs (as appropriate) and potential correlates of the dependent variables age, gender, ethnicity and health condition. Model specification was tested using the link test. All analyses accounted for cluster effect (based on site) through clustered standard errors. Sample means and incremental values for costs and QALYs were estimated using the margins method82 to improve the precision of estimates. The estimation of standard error and CIs also accounted for the cluster design. 84
Uncertainty in estimations was analysed using deterministic analyses to examine the impact of (1) using the whole sample (all randomised people) for analysis, (2) changing perspective of analysis to include costs incurred by participants, (3) excluding costs of health and social service use, (4) using different value sets85 to estimate quality of life and (5) complete-case analysis (excluding missing data). In addition, subgroup analyses were conducted to explore whether or not the cost-effectiveness of the intervention differed across different types of disease groups reported as the reason for referral to the ERS (hypertension, low mood, type 2 diabetes, weight loss and osteoarthritis).
Probabilistic uncertainty was assessed through non-parametric bootstrapping (n = 2000 replications). To ensure that the observations within the resampled clusters are independent in each bootstrap replication, the bootstrap estimation was fitted with unique identifiers inter and intra clusters. 61 Based on the bootstrap samples, cost-effectiveness planes (CEPs) and cost-effectiveness acceptability curves (CEACs) were constructed using the bsceaprogs program code. 82
Results
Summary statistics (unadjusted for baseline differences) on costs to both providers and participants, and quality of life are provided in Table 23 (see Appendices 7–9). At 12 months, the cost to providers per participant was higher in the intervention group (£1730) than in the control group (£1385). The biggest component of the cost borne by providers was health and social service use cost (91%) and the least was cost associated with the provision of support to participants (< 1%, £0.60 per participant). In terms of cost to participants, the pattern was different as participants in the control group incurred an average cost of £298, compared with £255 in the intervention group. The vast majority of this cost was because of cost related to participation in PA (69%). Fees paid for child/dependant care during the exercise specialist visit were the smallest part (< 1%, £0.20 per participant). The intervention group experienced higher quality of life at 12 months with a QALY of 0.662 (Table 23).
| Costs and quality of life | Mean (SD) | |
|---|---|---|
| Control (n = 133) | Intervention (n = 110) | |
| Cost to providers at 12 months (£) | ||
| Total costs to providers | 1385 (2177) | 1730 (1707) |
| Set-up cost | 0 | 182 (0) |
| Delivery cost | 0 | 29 (0) |
| Cost of participants’ consultation with exercise specialist | 30 (45) | 29 (45) |
| Cost of attendance at ERS centre | 3.1 (4) | 2.5 (3) |
| Number of ERS attendancesa | 11 (12) | 7 (9) |
| Cost associated with general support to participants | 0.3 (1) | 1 (2) |
| Costs of health and social service use | 1352 (2180) | 1487 (1691) |
| Cost to participants at 12 months (£) | ||
| Total costs to participants | 298 (1252) | 255 (347) |
| Travel cost to visit the exercise specialist | 13 (23) | 14 (29) |
| Time cost in consultation with exercise specialist | 43 (64) | 42 (66) |
| Fees paid for child/dependant care during exercise specialist visit | 0.3 (2) | 0.1 (1) |
| Other expenses related to exercise specialist visit | 2 (8) | 4 (20) |
| Time cost using the e-coachER web platform | 0 | 10 (12) |
| Cost of attendance to ERS centre | 27 (34) | 18 (26) |
| Costs related to PA participation | 213 (1253) | 168 (311) |
| Quality of life | ||
| QALY (1 year) | 0.637 (0.245) | 0.662 (0.261) |
| EQ-5D-5L index (baseline) | 0.656 (0.240) | 0.663 (0.261) |
| EQ-5D-5L index (4 months) | 0.642 (0.257) | 0.672 (0.279) |
| EQ-5D-5L index (12 months) | 0.620 (0.286) | 0.641 (0.305) |
Table 24 shows the main results based on regression estimates that adjusted for baseline differences. Consistent with the pattern observed in the unadjusted estimates, the average cost per participant was £1355 (95% CI £701 to £2008) and £1793 (95% CI £1635 to £1952) in the control and intervention groups respectively. This represents an additional cost of £439 (95% CI –£182 to £1060) in the intervention group, although the difference is not statistically significant. The intervention led to a weak indicative effect on total weekly minutes of MVPA in bouts of ≥ 10 minutes (mean difference 11.8 minutes, 95% CI –2.1 to 26.0 minutes), compared with the control group. In terms of quality-of-life outcome, the intervention group (mean 0.663, 95% CI 0.625 to 0.701) had more QALYs than the control group (mean 0.637, 95% CI 0.585 to 0.688) (see Table 24). The difference in QALYs (0.026, 95% CI 0.013 to 0.040) between the two groups was statistically significant. The cost–utility ratio shows that, compared with the control group, the intervention cost an additional £16,885 per QALY. This is below the NICE threshold of £20,000–30,000 per QALY.
| Costs and effects | Control | Intervention | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| Within-trial analysis, costs and effects over 12 months | ||||
| Total cost per participant (£) | 1355 | 701 to 2008 | 1793 | 1635 to 1952 |
| Incremental cost (£) | – | 439 | –182 to 1060 | |
| Total QALYs per participant | 0.637 | 0.585 to 0.688 | 0.663 | 0.625 to 0.701 |
| Incremental QALYs | – | 0.026 | 0.013 to 0.040 | |
| Incremental total weekly minutes of MVPA in bouts of ≥ 10 minutes | – | 11.8 | –2.1 to 26 | |
| Within-trial analysis, incremental cost-effectiveness ratio/utility ratio at 12 months | ||||
| Cost per additional QALY (£) | – | 16,885 | ||
| Cost per additional minute of MVPA in a bout of ≥ 10 minutes (£) | – | 37.20 | ||
Table 25 shows the results of the deterministic sensitivity analyses. The base-case finding was robust to deterministic sensitivity analyses with a few exceptions. The intervention was found to be more expensive but produced more QALYs. Excluding health and social service use costs, or changing the perspective of analysis, improved the cost-effectiveness of e-coachER, with incremental cost-effectiveness ratios (ICERs) ranging between £9000 and £15,885. Using estimates based on the whole sample (all participants who were randomised) was decisionally significant. It produced a worse cost-effectiveness ratio, with the intervention becoming not cost-effective (£43,900 per QALY).
| Parameter | Incremental cost (£) | Incremental QALY | ICER (£) | ||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||
| Base case | 439 | 124 to 754 | 0.026 | 0.013 to 0.040 | 16,885 |
| Whole sample (all participants who were randomised) | 439 | 124 to 754 | 0.010 | –0.005 to 0.024 | 43,900 |
| Changing cost perspective (both participants and NHS and PSS costs) | 413 | –266 to 1093 | 0.026 | 0.013 to 0.040 | 15,885 |
| Excluding all health and social care use cost (cost directly related to intervention) | 234 | 216 to 251 | 0.026 | 0.013 to 0.040 | 9000 |
| Complete-case analysis (exclude missing data) | 529 | –166 to 1223 | 0.035 | 0.010 to 0.059 | 15,114 |
| Changing the values for measuring QALYs (based on EQ-5D-5L value set from Devlin et al.85) | 439 | –182 to 1060 | 0.030 | 0.022 to 0.039 | 14,633 |
| Participants with hypertension (n = 37) | –46 | –1260 to 1168 | 0.009 | –0.020 to 0.038 | Intervention dominates control |
| Participants with low mood (n = 84) | 989 | 637 to 1341 | –0.056 | –0.098 to –0.014 | Control dominates intervention (less expensive and more effective) |
| People with type 2 diabetes (n = 49) | 655 | –187 to 1497 | 0.044 | –0.021 to 0.110 | 14,886 |
| Participants who were overweight (n = 227) | 350 | –44 to 745 | 0.019 | –0.035 to 0.072 | 18,421 |
| Participants with osteoarthritis (n = 53) | 223 | –781 to 1228 | 0.018 | –0.016 to 0.052 | 12,389 |
Compared with the base-case findings, subgroup analysis showed the intervention to be more cost-effective in groups that reported that hypertension (dominates control) or osteoarthritis (ICER £12,389 per QALY) or type 2 diabetes (ICER £14,886 per QALY) was the primary reason for referral. Among individuals who reported that being overweight was the primary reason for referral, e-coachER was still cost-effective but at a higher ICER value (£18,421 per QALY). In the group that reported that low mood was the primary reason for referral, e-coachER was more expensive (additional cost of £989 per participant) and less beneficial (fewer QALYs of –0.056 per participant) than the control.
Figure 7 shows the cost-effectiveness plane for the intervention compared with the control. The majority of the cloud of points (representing mean differences in costs and QALYs) are located in the north-east quadrant of the plane. This indicates that the intervention has high likelihood of generating more QALYs but at higher costs. The probability of the intervention being cost-effective (compared with control) at multiple willingness to pay per QALY values is presented in Figure 8. At £10,000 per QALY, the intervention has about 30% chance of being cost-effective compared with the control. The likelihood of cost-effectiveness nearly doubles (51%) at the £20,000-per-QALY threshold and increases further to 63% at the £30,000 threshold.
FIGURE 7.
Cost-effectiveness plane for intervention vs. control at 12 months.
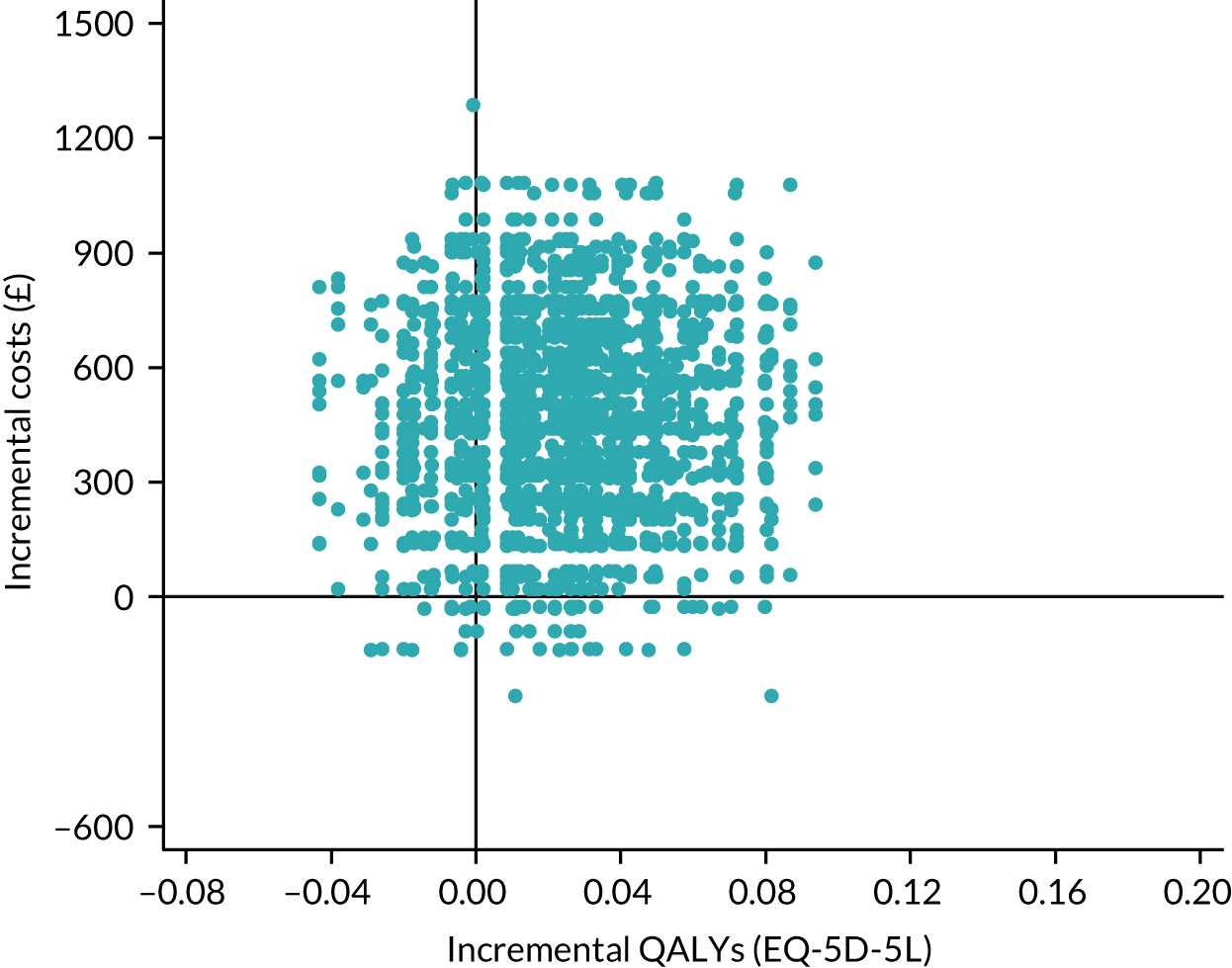
FIGURE 8.
Cost-effectiveness acceptability curve showing the probability of within-trial cost-effectiveness for intervention vs. control at different willingness to pay per QALY thresholds.
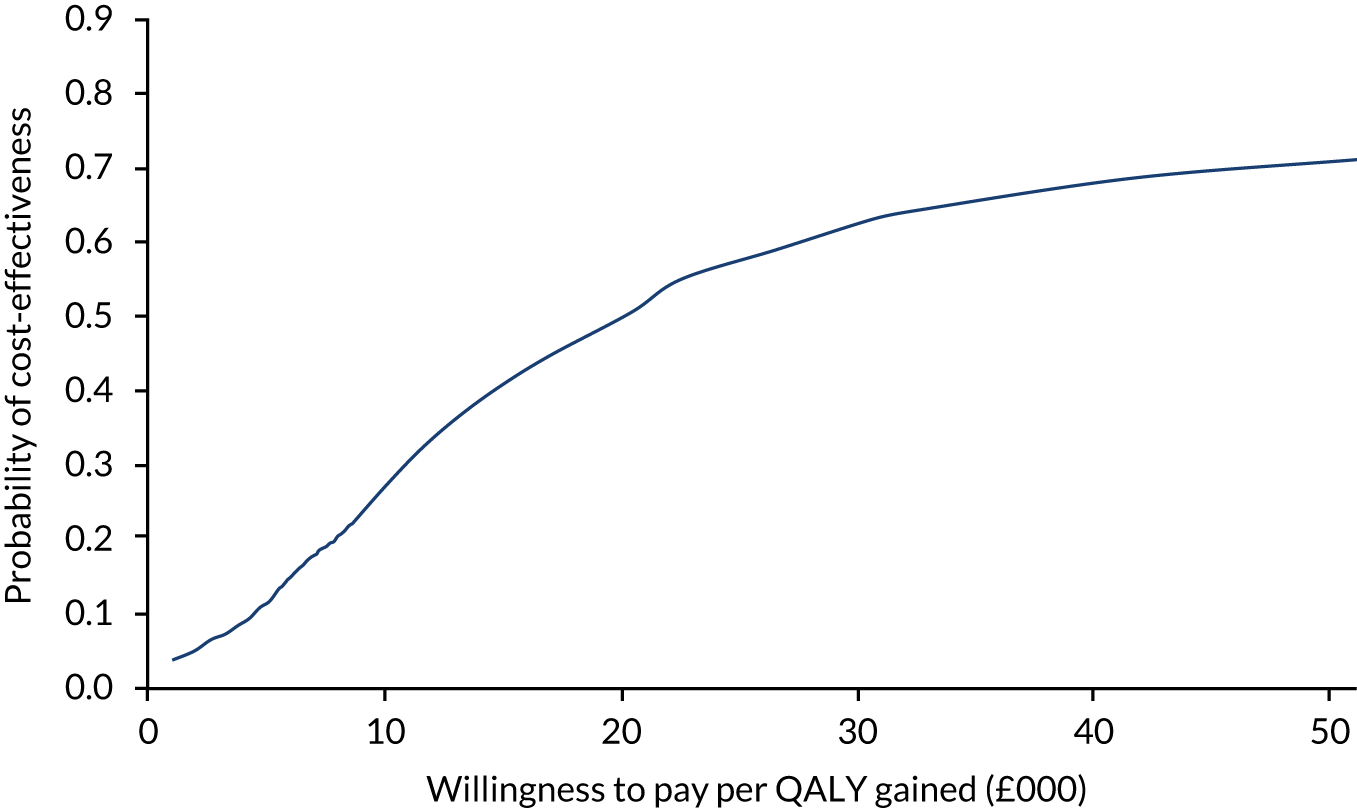
Discussion
This study shows that providing online behavioural support for participants of ERSs cost providers £1793 per person. Although the intervention costs £439 more than offering the ERS alone, it leads to better quality-of-life outcomes (0.026 more QALYs per person) and increased participation in PA (12 more weekly minutes of MVPA in ≥ 10-minute bouts per person). Although the differences were mostly not statistically significant, it is important to note that this is not sufficient proof of no significant effect, as the clinical trial was not powered to identify the changes in the economic outcomes (cost and QALY), particularly given the small number of observations in the subgroup analysis. Compared with the control, the intervention cost an additional £37 to gain a 1-minute increase in weekly MVPA (in ≥ 10-minute bouts) and £16,885 per QALY gain per person. Based on the NICE threshold of £20,000–30,000 per QALY, e-coachER could be considered more cost-effective than ERS alone. If decision-makers are willing to pay £30,000 for 1 QALY, e-coachER has a 63% probability of being cost-effective. The findings were robust to sensitivity analysis, including when the cost of participants was added to total costs of the programmes. Exploratory subgroup analysis found that the intervention was cost-effective among participants who reported that hypertension, osteoarthritis, type 2 diabetes or weight problems (i.e. being overweight) was the primary reason for referral but was not cost-effective among those who reported that low mood was the primary reason.
The novelty of e-coachER, its comparison with usual ERSs and the disease-specific population makes it complicated to relate the findings here meaningfully to the existing economic literature on ERSs. We identified one comparable study. Murphy et al. ,17 a pragmatic RCT, assessed the cost-effectiveness of the Wales National Exercise Referral Scheme (NERS), a 16-week programme including motivational interviewing, goal-setting and relapse prevention compared with usual care. Participants were inactive people with depression and/or coronary heart disease risk. The NERS intervention was shown to be cost-effective at 12 months, with a cost-per-QALY estimate of £12,111 and 89% likelihood at the £30,000-per-QALY threshold.
Our analysis found that costs associated with health and social services constitute the largest proportion of total costs associated with the intervention. Although the impact on cost-effectiveness was not decisionally insignificant, an important consideration is why the intervention group had higher health service use cost (mean cost of £135) than the control group. Further exploration shows that in the first 4 months of the trial, on average, the intervention group had a health service cost of £819 per participant and the control group had a health service cost of £639 per participant (see Appendix 9). In terms of the subcomponents of health service use cost collected in this study (n = 13), the intervention had higher costs in all except two [A&E visits and hospital visits (outpatients)]. The reverse pattern was, however, observed from 5 to 12 months – the control group had higher health service costs than the intervention group (£713 vs. £668). Similarly, the control group had higher costs for all subcost components apart from prescriptions and care worker visits. A systematic review on injury consequences of PA found that, although relatively minor, increased activity could lead to adverse health consequences. 86 However, for older adults, improved participation reduces the risk of fall-related injuries. 87 Future studies are required to further investigate the impact of adverse effects on the cost-effectiveness of PA programmes.
This study feeds into a limited evidence base around the efficiency of different models of ERS. To the best of our knowledge, this is the first study to demonstrate the cost-effectiveness of online support for an ERS and in a population with common chronic conditions. The strengths of this study include the use of robust clinical effectiveness data from a large multicentre trial, rigorous analyses to determine the impact variant measures of quality of life and participant perspective, subgroup analysis among different disease groups, comprehensive coverage of health service and social care use cost. A key limitation, however, is the short-term perspective of the analysis herein. Modelling of the long-term costs and effects is important where benefits and costs extend beyond the end of a trial. It is of particular relevance to this trial, where the benefits or costs could be experienced in the future too (as resource savings from reduced disease and, therefore, benefits in terms of increased quality of life). We expect that the impact of the long-term effects may have underestimated the cost-effectiveness of e-coachER.
There is currently limited national public health guidance on the implementation of ERSs enhanced with online support for participants. The results herein show that based on the NICE threshold of £20,000–30,000 per QALY, e-coachER could be considered more cost-effective than ERS alone. To strengthen the economic case, future studies are recommended to examine the long-term cost-effectiveness of the e-coachER and ascertain the impact of the trajectory of activity levels on future health service and future quality of life.
Chapter 6 Discussion
Summary of findings
The 450 trial participants were 64% female (n = 290), had an average age of 50 years and had an average BMI of 32.6 kg/m2; 65% and 35% were classified as inactive (n = 293) and moderately inactive (n = 157), respectively, and 50% reported that weight loss was the primary reason for referral (n = 225), followed by low mood (n = 85, 19%), osteoarthritis (n = 54, 12%), diabetes or prediabetes (n = 49, 11%) and high blood pressure (n = 36, 8%). Participants also noted that weight loss (n = 364, 81%), low mood (n = 243, 54%), high blood pressure (n = 149, 33%), diabetes or prediabetes (n = 117, 26%) and osteoarthritis (n = 108, 24%) may have been one of the reasons for referral.
The e-coachER support package was developed, which included mailing participants a pedometer, a fridge magnet with attached tear-off strips to record daily PA and a bespoke website to overcome barriers to increase daily PA for some people who are not willing or able to engage in an ERS. The e-coachER intervention demonstrated a mixed level of engagement. About one-third of participants did not register online and about one-third completed what we thought would be an adequate ‘dose’ of at least five of the seven steps, involving at least setting a PA goal and reviewing it 1 week later.
The results show that, compared with usual ERSs, the e-coachER intervention group had slightly more, but not significantly more, accelerometer-recorded minutes of MVPA (recorded in bouts of ≥ 10 minutes) at 12 months. The pattern was similar when considering the level of intervention engagement, with a slightly larger difference in favour of the intervention group. Applying the same approach as in the primary analysis, there were no between-group differences at 12 months in any of the other accelerometer-derived or self-reported MVPA outcomes, with one exception. The intervention group had significantly more daytime sedentary time (accumulated in blocks of ≥ 5 minutes) at 12 months. In ITT imputed comparison at 12 months, the intervention group was more likely than the control group to self-report that they had achieved 150 minutes of weekly MVPA (odds ratio 1.55, 95% CI 0.99 to 2.42; p = 0.05). The intervention also had no effect on ERS attendance (78% vs. 75% in control and intervention, respectively), or EQ-5D-5L or HADS scores at 12 months, compared with the control group. In ITT imputed comparison at 12 months, the intervention group had lower HADS depression and anxiety scores than the control group. The proportion of participants attending the ERS was comparable to findings from a review15 that reported that the average ERS uptake was 81% in RCTs.
Economic evaluation
Over the 12-month follow-up, the average cost per participant was £1355 (95% CI £701 to £2008) and £1793 (95% CI £1635 to £1952) in the control and intervention groups respectively. Compared with the control group, the intervention group incurred an additional mean cost of £439 (95% CI –£182 to £1060) but generated more mean QALYs (0.026, 95% CI 0.013 to 0.040), with an incremental cost-effectiveness ratio of an additional £16,885 per QALY.
Although insignificant, an important consideration is why the intervention group had higher health service use cost (mean cost of £135) than the control group given the intervention was effective and led to increases in both PA and health-related quality of life. The difference in the cost, which was not statistically significant, was observed irrespective of the central measure of tendency, mean or median (see Appendix 9). Further exploration shows that in the 4 months of the trial, on average, the intervention group had a health service cost of £819 and the control group had a cost of £639. In terms of the subcomponents of health service use cost collected in this study (n = 13), the intervention group had higher costs in all except two components [A&E visits and hospital visits (outpatients)]. The reverse pattern was, however, observed from 5 to 12 months, with the control group qualitatively having greater health service costs (£713 vs. £668). Similarly, the control group had higher costs for all subcost components apart from prescriptions and care worker visits.
Effectiveness of and engagement in the intervention
One of the criticisms of e- and m-health interventions is that engagement is not appealing to enough people and can be rather short-lived. 23 The 9-month work in developing the intervention, building on other effective LifeGuide interventions and with public and patient involvement, aimed to maximise engagement to theoretically have the greatest impact on MVPA outcomes at 12-month follow-up. Automated periodic e-mails were sent to intervention participants up to 12 months. The intervention included evidence-based and theory-driven components as well as a pragmatic approach to enhance engagement. Pedometers and recording sheets attached to fridge magnets were provided in the initial introductory pack as basic tools for self-monitoring PA and setting and reviewing SMART goals. These may have been sufficient to get some of the 36% of intervention participants who never registered to think about behavioural self-regulatory processes to increase PA for managing their chronic condition(s). Attending the ERS may also have provided sufficient support to become more physically active, without the use of the pedometer or web-based support. Other participants may also have regarded the pedometer as rather basic and decided to use a more sophisticated app on their smartphone.
The fact that 64% of participants did register online and completed their first step, with 36% going on for over 4 weeks to complete a goal review, provided us with some assurance that the e-coachER intervention was acceptable for a reasonable proportion of participants. There is evidence from previous research with LifeGuide interventions that even limited online engagement can be effective and there is no clear ‘adequate dose’ to optimise behavioural change. Our CACE analysis confirmed that completing our prespecified intervention engagement level (step 5) did not lead to significantly greater 12-month objectively recorded minutes of MVPA, recorded in ≥ 10-minute bouts, compared with the control group, although the between-group differences were qualitatively greater (22.9 vs. 11.8 minutes).
Our logic model predicted that e-coachER engagement would strengthen various beliefs that would in turn translate into increases in MVPA, compared with usual ERS support. Among only the participants included in the primary analysis, the intervention did increase the following compared with the control group: perceived importance of doing at least 30 minutes of moderate-intensity PA (e.g. brisk walk) on at least 5 days per week, confidence in achieving at least 30 minutes of moderate-intensity PA (e.g. brisk walk) on at least 5 days per week and perceived competence in being regularly physically active at 4 months (but not 12 months). Changes (from baseline to 4 months) in these process outcomes did not mediate changes in the primary outcome at 12 months.
In the qualitative part of our process evaluation, 12% of intervention participants were interviewed. Overall, e-coachER was acceptable and positively experienced and did ‘do what it said on the tin’ in terms of enhancing autonomy, competence and relatedness for many participants. Inevitably, because the web-based support was available to support participants with a range of levels of IT literacy and chronic conditions, some of the content did not appeal to everyone. That said, the idea that the support aimed to facilitate engagement with the self-monitoring process and then encourage them to move on to using more sophisticated devices to self-monitor PA for example, was overlooked by some participants, but not all. Similarly, we tried to help participants to find any source of social support to support increases in PA and a preferred form of PA that they could enjoy, whether or not that involved the ERS, and that overarching intent ‘may have been lost’ by some of those interviewed. The user guide sent to intervention participants initially could have spelled out some aims of the support more explicitly and if this had been understood then some comments from participant interviews may not have been made. That said, some interviewees did note that they had a strong IT background and acknowledged that their comments were personal and acknowledged the need to appeal to those with a lower level of IT literacy than themselves.
Strengths and limitations
Sample characteristics
We believe this to be the first study to recruit inactive participants with chronic conditions into a trial involving ERSs, and then follow them up at 12 months to assess objectively measured PA. We had planned to conduct separate analyses for different chronic conditions to explore the possibility of establishing an evidence-based case for disease-specific ERS pathways, but the study findings have clearly confirmed the extent of multimorbidity. Nevertheless, when considering the participant-reported primary reason for referral, in sensitivity analysis there was no interaction between this reason and the overall intervention effects on the 12-month MVPA primary outcome.
During the course of the study, we recalculated the sample size to recruit 430 participants (decreased from original funding application sample size of 900), allowing for 20% attrition for the primary outcome at 12 months. We actually recruited 450 participants, but, partly because we set a very rigorous primary outcome threshold (wear-time of at least 4 days per week, including 1 weekend day, with data from at least 16 hours per day), the primary analysis was based on data from 232 participants. Further analysis explored if more lenient thresholds, which resulted in more participants being involved in the analysis, influenced the findings. The overall findings remained consistent.
Exercise referral scheme context
The e-coachER intervention was designed with considerable public and patient input to ensure that it would support patients with a wide range of IT expertise. We categorised 16% of trial participants as having low IT literacy and further analysis revealed that IT literacy did not have an impact on the primary analysis.
Exercise referral schemes are delivered in various formats across the UK and we were keen to ensure that the trial produced findings with good generalisability. In other words, we wanted to know if adding the e-coachER intervention improves long-term levels of MVPA in patients with chronic conditions. The three sites in which recruitment took place offered quite different types of ERS, which have been described elsewhere. 1 For example, in Glasgow the ERS involves initial contact with an ERS practitioner, who provides some behavioural support to increase MVPA and also helps to signpost participants to preferred and appropriate PA opportunities, with following consultations available at 6 and 12 months. In contrast, the schemes in Birmingham and the South West are more traditional ERSs with support provided by an ERS professional in an exercise facility. Within the trial, we examined the impact of site on the primary analysis and found no different effects of the intervention across sites.
A further strength of the study was the use of an evidence-based, theory-driven intervention that allowed us to identify website usage. The LifeGuide system provided data on number of visits (and duration) to the website and which steps were completed. Our detailed mixed-methods process evaluation allowed us to collate this information and explore the impact of engagement on cognitive and behaviour processes we had proposed within our logic model. In turn, we were able to explore if changes in these process outcomes mediated changes in the primary outcome.
Accelerometer measures at 12 months
To our knowledge, this is the first trial of an ERS to objectively assess MVPA at follow-up. Only one previous trial has involved a 12-month follow-up to assess the long-term effects of an ERS intervention on PA and that was based on self-reported measures. Owing to the very low levels of MVPA in the present sample, relative to other studies, new challenges have appeared for data analysis. We prespecified our primary outcome and the research team, TSC and DMC have been involved in discussions about the most appropriate approach to data analysis. The statistical analysis plan was agreed and signed before seeing the data. In our primary analysis, we present various scenarios from extensive exploratory modelling, and also sensitivity analysis using different thresholds for analysing raw accelerometer data. These analyses, and those of the self-reported MVPA data, will provide valuable insights into how best to examine within-trial PA data. At the time of writing, we understand that national and international guidelines for completing MVPA weekly minutes are expected to no longer mention the need to accumulate MVPA in at least 10-minute bouts. Our analyses provide a broad range of findings that will contribute to the understanding of past and future evidence and research.
The accelerometer findings were broadly similar to self-report measures in terms of between-group differences, but levels of activity were strongly influenced by the way the data were collected and processed. Only participants classified as inactive or moderately inactive according to the GPPAQ were included in the study but, at baseline, self-reported data revealed that 36% met the 150 minutes per week MVPA guideline, and accelerometer data revealed that 80% met the guideline from non-bouted activity and only 4% met it from activity recorded in bouts of ≥ 10 minutes. Others have also shown lower levels of accelerometer-recorded MVPA minutes when data are processed using ≥ 10-minute bouts compared with bouts of at least 1 minute. 55 Current national and international guidelines do not fully reflect this variability because of measurement method.
Given the overdispersion and high frequency of zero counts, the primary statistical model was found to poorly fit and post hoc analysis models were therefore also explored. For example, for the primary outcome of total weekly minutes of MVPA in ≥ 10-minute bouts, 142 out of 243 (58%) participants at 12 months had zero scores and the SD (60.0) was more than two times the mean value (26.2). It is important to recognise the limitations of all these models. These include lack of fit of the models and the need to assume data as counts for some models. However, reassuringly, the interpretation of the impact of the intervention on primary outcome analysis was insensitive to the choice of statistical model.
Although the effectiveness findings show only a weak indicative effect on MVPA at 12-month follow-up, these changes were found to have a 63% probability of being cost-effective based on a UK threshold of £30,000 per QALY.
The mixed-methods process evaluation indicated that the intervention was generally acceptable and of value to patients with a variety of physical and mental health conditions. The intervention resulted in changes in some but not all behaviour change processes that we designed the intervention to change. Notably, the perception of importance of being physically active was greater as a result of the intervention compared with the control group, at both 4 and 12 months. Step 1 in the intervention involved a quiz about the benefits of PA for health generally and also specific chronic conditions. In other steps, we encouraged participants to feel the broader value of being active for both physical and mental well-being. By supporting change in MVPA it is likely that intervention participants learned to place greater value and importance on being physically active, but improvements in confidence to be physically active and a perception of importance of being more physically active did not mediate any effects of the intervention on MVPA at 12 months.
Implications for health care
Offering e-coachER support had only small but non-significant effects on objectively recorded MVPA compared with usual ERSs at 12 months. The cost–utility ratio shows that when compared with the control, the intervention cost an additional £16,885 per QALY and has a 63% probability of being cost-effective for increasing MVPA, compared with usual ERS.
As a result of usual ERS alone (i.e. in the control group), across the sites there was only weak evidence of a change in accelerometer-recorded MVPA at 4 months, but none at 12 months, albeit without comparison with no ERS. If anything, there were small but non-significant reductions in MVPA among only those engaging in usual ERS. The rationale for conducting the present study was that we offered an additional ‘package’ of support (e-coachER) aimed at developing self-determined PA alongside usual ERSs or instead of usual ERSs. If shown to add additional benefit, our intervention would be available to primary care professionals to offer to inactive patients, with a range of physical and mental health conditions, at the time of making a referral to a local ERS.
Providing web-based behavioural support to participants of ERSs offers an additional strategy to augment usual ERSs to promote PA. There are a few small aspects of the intervention that we would change based on participant feedback, but the intervention could also be extended to be suitable for use by patients with other chronic conditions (e.g. cancer). There was reasonable engagement in e-coachER support for participants with a range of confidence in using IT, indicating that, if implemented, it would have low costs and moderate value in promoting PA in addition to usual ERSs. Our process evaluation indicated that there were changes in some of the measures that we collected to assess key components of the logic model. For example, the intervention led to improvements up to 4 months in confidence and competence in doing PA, perceiving the importance of PA, a sense of availability of support, action-planning and self-monitoring compared with the control group. But these intervention effects were sustained at 12 months only for perceived importance of PA. In our exploration of whether or not any of the changes between 0 and 4 months mediated any intervention effects on the primary outcome (accelerometer-recorded minutes of MVPA in bouts of ≥ 10 minutes) at 12 months, there was no evidence that this was the case. Our ability to detect these mediation effects may have been limited by the overall intervention effects on MVPA at 12 months.
The present study found that 36% of participants did not log into the online e-coachER support, but did receive a pedometer and a fridge magnet to record MVPA. We will further analyse the data to determine if this was sufficient to increase MVPA compared with usual ERSs. Other evidence suggests that providing primary care patients with a pedometer to self-monitor PA is effective in changing objectively recorded MVPA. 61 It would be relatively easy to provide patients with a pedometer at the same time as referring them to an ERS.
The LifeGuide platform has been used to deliver evidence-based and theory-driven interventions online to support change in a wide range of health behaviours. One of its strengths is the ability to capture intervention engagement to help understand fidelity issues and add to the literature on how people change as a result of e-health interventions. Although LifeGuide-delivered interventions do these things well and have been shown to be effective in supporting change in a range of health behaviours and weight loss, other more sophisticated technological innovations are rapidly taking over. Indeed, feedback captured within our process evaluation noted that the e-coachER support was rather unsophisticated, and typing step counts or minutes of MVPA accumulated in the past week into a website and getting feedback on whether or not goals had been achieved can instead undoubtedly be done on a range of more sophisticated devices that are embracing digital technology and artificial intelligence. For these reasons, the LifeGuide platform will no longer support digital interventions from 2021.
We hoped that the intervention would encourage participants to go to the local ERS that they had been referred to but this did not happen. Almost 25% of referred participants did not attend any sessions.
Future research implications
Previous research has compared usual ERSs with an enhanced ERS, involving additional exercise practitioner training, but showed no additional effect20 on PA. The present trial provides no clear support for adding a web-based support package to usual ERSs, to increase long-term MVPA. Our process evaluation revealed that some improvements to the web support could be made, such as mobile options with smartphone apps for self-monitoring and goal-setting.
The modest engagement in the online e-coachER support suggests that work is needed to understand which factors influenced intervention engagement and how best to further develop low-cost and scalable support to increase ERS uptake and maintenance of PA. Once this has been done, further research could examine the effects of a modified e-coachER-type intervention for participants with chronic conditions involved in the present study and others (e.g. with cancer, back pain and in cardiac rehabilitation).
The e-coachER study has provided a rich data set, which offers the chance to explore additional questions including the following:
-
What were the characteristics of participants that predicted changes in 4- and 12-month PA?
-
How did different measures of MVPA (i.e. self-report and accelerometer derived) influence the findings, beyond what we present here?
-
What other aspects of intervention engagement (derived from the LifeGuide platform) were used, and did any influence changes in process and behavioural outcomes?
-
Among subsets of the sample (e.g. those with low mood), what changes in quality of life, depression and anxiety occurred as a result of the intervention versus usual ERSs?
Conclusions
With modest engagement in the evidence-based and theory-driven e-coachER intervention, which was captured by the web-based system, the intervention effects on a rigorously defined, objectively assessed, PA primary outcome at 12 months were only small and not significant, and, because of a smaller sample size than intended, should be treated with caution.
In the cost-effectiveness analyses, the cost–utility ratio shows that, compared with an ERS alone, ERS plus the e-coachER intervention cost an additional £16,885 per QALY and has a 63% probability of being cost-effective based on the UK threshold of £30,000 per QALY.
Acknowledgements
We would like to thank the external members of the TSC [Sharon Simpson (chairperson), Mark Kelson and Charlie Foster] and the DMC [Paul Aveyard (chairperson), Anne Haase and Richard Morris] for their advice and support.
We thank the Research Design Service South West, especially Andy Barton, for assisting with the finding application.
We are grateful to the participants, GPs and exercise professionals who supported the study, giving their time so generously and sharing their experience with us. Likewise, we are grateful to the practice managers and administrative staff at all of the collaborating practices. We thank the ERS managers and employees who provided valuable assistance to us throughout the study.
We would like to thank a number of people who helped towards the successful completion of the study, especially Hayley O’Connell, who worked as a researcher for the study. We would like to thank Melvyn Hillsdon and Brad Metcalf, who provided valuable input into the analysis of accelerometer data. We would also like to thank Lucy Cartwright and Liz Ford, who provided administrative support to the trial. We thank the Plymouth City Public Health team for ensuring that participants in the study were eligible for a subsidy when attending the ERS.
We would like to thank Ray Jones for his insights into digital interventions, and Jane Vickery, who supported the PenCTU trial management team. In addition, within the PenCTU, thanks go to Elliot Carter, who led on developing and maintaining the study database, and Brian Wainman and Mark Warner for data management.
The research programme of Lucy Yardley and Mary Steele is partly supported by the NIHR Southampton Biomedical Research Centre.
Sarah Dean is partly funded by the SouthWest Peninsula Applied Research Collaboration.
Contributions of authors
Professor Adrian H Taylor (https://orcid.org/0000-0003-2701-9468) (Professor of Health Services Research) conceived the idea for the study with co-applicants: Professor Rod S Taylor (https://orcid.org/0000-0002-3043-6011) (Professor of Health Services Research, Statistician), Dr Nana Anokye (https://orcid.org/0000-0003-3615-344X) (Senior Lecturer in Health Economics), Professor Sarah Dean (https://orcid.org/0000-0002-3682-5149) (Professor in Psychology Applied to Rehabilitation and Health), Professor Kate Jolly (https://orcid.org/0000-0002-6224-2115) (Professor of Public Health and Primary Care), Professor Nanette Mutrie (https://orcid.org/0000-0002-5018-6398) (Director of Physical Activity for Health Research Centre), Professor Lucy Yardley (https://orcid.org/0000-0002-3853-883X) (Professor of Health Psychology), Professor Colin Greaves (https://orcid.org/0000-0003-4425-2691) (Professor of Psychology Applied to Health), Professor John Campbell (https://orcid.org/0000-0002-6752-3493) (Professor of General Practice and Primary Care), Mr Ben Jane (https://orcid.org/0000-0002-0774-2942) (Senior Lecturer, Exercise and Public Health), Dr Jo Erwin (https://orcid.org/0000-0002-9648-4825) (Research Associate), Professor Paul Little (https://orcid.org/0000-0003-3664-1873) (Professor of Primary Care Research within Medicine) and Professor Anthony Woolf (https://orcid.org/0000-0001-8482-8056) (Consultant Rheumatologist, Honorary Professor of Rheumatology).
Professor Adrian H Taylor, all co-applicants listed above, Dr Wendy M Ingram (https://orcid.org/0000-0003-0182-9462) (Clinical Trials Manager) and Mr Douglas Webb (https://orcid.org/0000-0001-6818-1838) (Assistant Clinical Trials Manager) contributed to the final study design and development of the protocol.
Professor Adrian H Taylor, Dr Jeffrey Lambert (https://orcid.org/0000-0003-4774-9054) (Research Associate in Primary Care) and Dr Mary Steele (https://orcid.org/0000-0003-2595-3855) (Research Fellow) developed the web support using LifeGuide and, with Ms Jennie King (https://orcid.org/0000-0001-7571-6829) (Research Assistant), led the patient and public input into testing and feedback.
Professor Sarah Dean developed the process evaluation plan with Dr Jeffrey Lambert, Professor Colin Greaves, Mr Nigel Charles (Associate Research Fellow) and Dr Rohini Terry (https://orcid.org/0000-0003-4188-8504) (Research Fellow). Professor Sarah Dean led the analysis and reporting of the qualitative analysis for the process evaluation, in collaboration with Professor Colin Greaves, Mr Nigel Charles, Dr Rohini Terry and Professor John Campbell (https://orcid.org/0000-0002-6752-3493).
Dr Nana Anokye provided the health economics evaluation plan and conducted and reported the economic evaluation in accordance with the health economics evaluation plan.
Professor Rod S Taylor provided the statistical analysis plan and conducted and reported the statistical analysis in accordance with the statistical analysis plan.
Dr Adam Streeter (https://orcid.org/0000-0001-9921-2369) (Research Fellow in Medical Statistics) provided additional support to the statistical analysis (primarily the quantitative process evaluation).
Mr Ben Jones (https://orcid.org/0000-0001-7374-1107) (Research Assistant in Medical Statistics) assisted with statistical data analysis.
Dr Lisa Price (https://orcid.org/0000-0001-9478-3488) (Lecturer in Physical Activity and Health) was responsible for accelerometer data processing, advising and reporting on accelerometer-derived measures.
Dr Ben Ainsworth (https://orcid.org/0000-0002-5098-1092) (Senior Research Fellow) developed the web support and processed intervention engagement data on the LifeGuide platform.
Professor Adrian H Taylor, Professor Kate Jolly and Professor Nanette Mutrie were principal investigators, assisted by Dr Chloe McAdam (https://orcid.org/0000-0002-7574-7489) (Research and Knowledge Exchange Co-ordinator at the Physical Activity Health Research Centre) at the Glasgow site.
Ms Jennie King was the lead research assistant and Ms Lucy Hughes (https://orcid.org/0000-0002-6015-0356) was the research assistant at the Birmingham site.
Dr Wendy Ingram and Mr Douglas Webb (https://orcid.org/0000-0001-6818-1838) were the trial managers.
Mr Chris Cavanagh was the PPI representative.
All authors critically revised successive drafts of the manuscript and approved the final version.
Publications
Ingram W, Webb D, Taylor RS, Anokye N, Yardley L, Jolly K, et al. Multicentred randomised controlled trial of an augmented exercise referral scheme using web-based behavioural support in individuals with metabolic, musculoskeletal and mental health conditions: protocol for the e-coachER trial. BMJ Open 2018;8:e022382.
Taylor AH, Taylor RS, Ingram W, Dean S, Jolly K, Mutrie N, et al. A randomised controlled trial of an augmented exercise referral scheme using web-based behavioural support for inactive adults with chronic health conditions: the e-coachER trial. Br J Sports Med 2020; in press.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review and appropriate agreements being in place.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Ingram W, Webb D, Taylor RS, Anokye N, Yardley L, Jolly K, et al. Multicentred randomised controlled trial of an augmented exercise referral scheme using web-based behavioural support in individuals with metabolic, musculoskeletal and mental health conditions: protocol for the e-coachER trial. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-022382.
- Department of Health and Social Care (DHSC) . Start Active, Stay Active: A Report On Physical Activity From The Four Home Countries’ Chief Medical Officers 2011.
- National Institute for Health and Care Excellence (NICE) . Obesity: Guidance on the Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children 2010.
- National Institute for Health and Care Excellence (NICE) . Hypertension: Clinical Management of Primary Hypertension in Adults 2011.
- National Institute for Health and Care Excellence (NICE) . Type 2 Diabetes: The Management of Type 2 Diabetes 2008.
- National Institute for Health and Care Excellence (NICE) . Osteoarthritis: The Care and Management of Osteoarthritis in Adults 2008.
- National Institute for Health and Care Excellence (NICE) . Depression: The Treatment and Management of Depression in Adults 2009.
- Scholes S. Health Survey for England 2016 Physical Activity in Adults. Leeds: NHS Digital; 2017.
- Gill V, Campbell-Jack D, Hinchliffe S, Bromley C. The Scottish Health Survey 2014:260-1. www.gov.scot/publications/scottish-health-survey-2014-volume-1-main-report/ (accessed 24 March 2020).
- Public Health England . Physical Inactivity: Economic Costs to NHS Clinical Commissioning Groups 2016.
- Bouchard C, Blair SN, Katzmarzyk PT. Less sitting, more physical activity, or higher fitness?. Mayo Clin Proc 2015;90:1533-40. https://doi.org/10.1016/j.mayocp.2015.08.005.
- Warburton DE, Bredin SS. Reflections on physical activity and health: what should we recommend?. Can J Cardiol 2016;32:495-504. https://doi.org/10.1016/j.cjca.2016.01.024.
- British Heart Foundation National Centre for Physical Activity and Health . A Toolkit for the Design, Implementation – Evaluation of Exercise Referral Schemes 2010.
- Pavey TG, Taylor AH, Fox KR, Hillsdon M, Anokye N, Campbell JL, et al. Effect of exercise referral schemes in primary care on physical activity and improving health outcomes: systematic review and meta-analysis. BMJ 2011;343. https://doi.org/10.1136/bmj.d6462.
- Pavey T, Taylor A, Hillsdon M, Fox K, Campbell J, Foster C, et al. Levels and predictors of exercise referral scheme uptake and adherence: a systematic review. J Epidemiol Community Health 2012;66:737-44. https://doi.org/10.1136/jech-2011-200354.
- Kelly MC, Rae GC, Walker D, Partington S, Dodd-Reynolds CJ, Caplan N. Retrospective cohort study of the South Tyneside Exercise Referral Scheme 2009-14: predictors of dropout and barriers to adherence. J Public Health 2017;39:e257-64. https://doi.org/10.1093/pubmed/fdw122.
- Murphy SM, Edwards RT, Williams N, Raisanen L, Moore G, Linck P, et al. An evaluation of the effectiveness and cost effectiveness of the National Exercise Referral Scheme in Wales, UK: a randomised controlled trial of a public health policy initiative. J Epidemiol Community Health 2012;66:745-53. https://doi.org/10.1136/jech-2011-200689.
- Rouse PC, Ntoumanis N, Duda JL, Jolly K, Williams GC. In the beginning: role of autonomy support on the motivation, mental health and intentions of participants entering an exercise referral scheme. Psychol Health 2011;26:729-49. https://doi.org/10.1080/08870446.2010.492454.
- Morgan F, Battersby A, Weightman AL, Searchfield L, Turley R, Morgan H, et al. Adherence to exercise referral schemes by participants - what do providers and commissioners need to know? A systematic review of barriers and facilitators. BMC Public Health 2016;16. https://doi.org/10.1186/s12889-016-2882-7.
- Duda JL, Williams GC, Ntoumanis N, Daley A, Eves FF, Mutrie N, et al. Effects of a standard provision versus an autonomy supportive exercise referral programme on physical activity, quality of life and well-being indicators: a cluster randomised controlled trial. Int J Behav Nutr Phys Act 2014;11. https://doi.org/10.1186/1479-5868-11-10.
- Joseph RP, Durant NH, Benitez TJ, Pekmezi DW. Internet-based physical activity interventions. Am J Lifestyle Med 2014;8:42-68. https://doi.org/10.1177/1559827613498059.
- Devi R, Singh SJ, Powell J, Fulton EA, Igbinedion E, Rees K. Internet-based interventions for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev 2015;12. https://doi.org/10.1002/14651858.CD009386.pub2.
- Davies CA, Spence JC, Vandelanotte C, Caperchione CM, Mummery WK. Meta-analysis of internet-delivered interventions to increase physical activity levels. Int J Behav Nutr Phys Act 2012;9. https://doi.org/10.1186/1479-5868-9-52.
- Morrison LG, Hargood C, Lin SX, Dennison L, Joseph J, Hughes S, et al. Understanding usage of a hybrid website and smartphone app for weight management: a mixed-methods study. J Med Internet Res 2014;16. https://doi.org/10.2196/jmir.3579.
- Lloyd S, Dennison L, Morrison L, Phillips D, Michie S, Murray E, et al. Losing weight online with POWeR: a randomised controlled trial of a web-based behavioural intervention in a community setting. Lancet 2013;382. https://doi.org/10.1016/S0140-6736(13)62487-3.
- Williams S, Yardley L, Wills GB. A qualitative case study of LifeGuide: users’ experiences of software for developing internet-based behaviour change interventions. Health Informatics J 2013;19:61-75. https://doi.org/10.1177/1460458212458915.
- Yardley L, Morrison LG, Andreou P, Joseph J, Little P. Understanding reactions to an internet-delivered health-care intervention: accommodating user preferences for information provision. BMC Med Inform Decis Mak 2010;10. https://doi.org/10.1186/1472-6947-10-52.
- Little P, Stuart B, Hobbs FR, Kelly J, Smith ER, Bradbury KJ, et al. An internet-based intervention with brief nurse support to manage obesity in primary care (POWeR+): a pragmatic, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:821-8. https://doi.org/10.1016/S2213-8587(16)30099-7.
- Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-119.
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690-701. https://doi.org/10.1037/a0016136.
- Deci EL, Ryan RM. Handbook of Self-determination Research. New York, NY: University of Rochester Press; 2002.
- Anokye NK, Trueman P, Green C, Pavey TG, Hillsdon M, Taylor RS. The cost-effectiveness of exercise referral schemes. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-954.
- Benaissa M, Malik B, Kanakis A, Wright NP. Tele-healthcare for diabetes management: a low cost automatic approach. Conf Proc IEEE Eng Med Biol Soc 2012;2012:1290-3. https://doi.org/10.1109/EMBC.2012.6346174.
- Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res 2015;17. https://doi.org/10.2196/jmir.4055.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Ahmad S, Harris T, Limb E, Kerry S, Victor C, Ekelund U, et al. Evaluation of reliability and validity of the General Practice Physical Activity Questionnaire (GPPAQ) in 60–74 year old primary care patients. BMC Fam Pract 2015;16. https://doi.org/10.1186/s12875-015-0324-8.
- Great Britain . Data Protection Act 1998. 1998. www.legislation.gov.uk/ukpga/1998/29/contents (accessed March 2020).
- Official Journal of the European Union . General Data Protection Regulation 2016. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32016R0679 (accessed 20 March 2020).
- van Hees V FZ, Zhao J, Heywood J, Mirkes E, Sabia S, Migueles J. GGIR: Raw Accelerometer Data Analysis R Package Version 12-8 2018. https://CRANR-projectorgpackage=GGIR (accessed 30 November 2018).
- van Hees VT, Fang Z, Langford J, Assah F, Mohammad A, da Silva IC, et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Appl Physiol 2014;117:738-44. https://doi.org/10.1152/japplphysiol.00421.2014.
- Hildebrand M, van Hees VT, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc 2014;46:1816-24. https://doi.org/10.1249/MSS.0000000000000289.
- Esliger DW, Rowlands AV, Hurst TL, Catt M, Murray P, Eston RG. Validation of the GENEA Accelerometer. Med Sci Sports Exerc 2011;43:1085-93. https://doi.org/10.1249/MSS.0b013e31820513be.
- Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, Beaumont RN, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun 2019;10. https://doi.org/10.1038/s41467-018-08259-7.
- van Hees VT, Sabia S, Anderson KN, Denton SJ, Oliver J, Catt M, et al. A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0142533.
- Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, Beaumont RN, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides new insights into circadian rhythms in humans and links to disease. Nat Comm 2019;10. https://doi.org/10.1101/303941.
- Powell C, Carson BP, Dowd KP, Donnelly AE. Simultaneous validation of five activity monitors for use in adult populations. Scand J Med Sci Sports 2017;27:1881-92. https://doi.org/10.1111/sms.12813.
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985;122:794-80. https://doi.org/10.1093/oxfordjournals.aje.a114163.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Harris T, Kerry SM, Victor CR, Ekelund U, Woodcock A, Iliffe S, et al. A primary care nurse-delivered walking intervention in older adults: PACE (pedometer accelerometer consultation evaluation)-Lift cluster randomised controlled trial. PLOS Med 2015;12. https://doi.org/10.1371/journal.pmed.1001783.
- International Conference on Harmonisation . ICH 1998, ICH Topic E 9: Statistical Principles for Clinical Trials, European Agency of Medicines 1998.
- Boutron I, Moher D, Altman D, Schulz K, Ravaud P. CONSORT Group . Extending the CONSORT statement to randomized trials of non-pharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. https://doi.org/10.7326/0003-4819-148-4-200802190-00008.
- Schulz K, Altman D, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals Inter Med 2010;152:1-15. https://doi.org/10.7326/0003-4819-152-11-201006010-00232.
- Pavey TG, Anokye N, Taylor AH, Trueman P, Moxham T, Fox KR, et al. The clinical effectiveness and cost-effectiveness of exercise referral schemes: a systematic review and economic evaluation. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15440.
- Cassidy S, Fuller H, Chau J, Catt M, Bauman A, Trenell MI. Accelerometer-derived physical activity in those with cardio-metabolic disease compared to healthy adults: a UK Biobank study of 52,556 participants. Acta Diabetol 2018;55:975-9. https://doi.org/10.1007/s00592-018-1161-8.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process Evaluation of Complex Interventions: Medical Research Council Guidance. London: MRC Population Health Science Research Network; 2014.
- Hanson CL, Allin LJ, Ellis JG, Dodd-Reynolds CJ. An evaluation of the efficacy of the exercise on referral scheme in Northumberland, UK: association with physical activity and predictors of engagement. A naturalistic observation study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002849.
- Tobi P, Estacio EV, Yu G, Renton A, Foster N. Who stays, who drops out? Biosocial predictors of longer-term adherence in participants attending an exercise referral scheme in the UK. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-347.
- Normansell R, Smith J, Victor C, Cook DG, Kerry S, Iliffe S, et al. Numbers are not the whole story: a qualitative exploration of barriers and facilitators to increased physical activity in a primary care based walking intervention. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-1272.
- Donnachie C, Wyke S, Mutrie N, Hunt K. ‘It’s like a personal motivator that you carried around wi’ you’: utilising self-determination theory to understand men’s experiences of using pedometers to increase physical activity in a weight management programme. Int J Behav Nutr Phys Act 2017;14. https://doi.org/10.1186/s12966-017-0505-z.
- Harris T, Kerry S, Victor C, Iliffe S, Ussher M, Fox-Rushby J, et al. A pedometer-based walking intervention in 45- to 75-year-olds, with and without practice nurse support: the PACE-UP three-arm cluster RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22370.
- Spencer L, Adams TB, Malone S, Roy L, Yost E. Applying the transtheoretical model to exercise: a systematic and comprehensive review of the literature. Health Promot Pract 2006;7:428-43. https://doi.org/10.1177/1524839905278900.
- Alwin DF, Hauser RM. The decomposition of effects in path analysis. Am Sociol Rev 1975;40:37-4. https://doi.org/10.2307/2094445.
- Cerin E, Mackinnon DP. A commentary on current practice in mediating variable analyses in behavioural nutrition and physical activity. Public Health Nutr 2009;12:1182-8. https://doi.org/10.1017/S1368980008003649.
- Sobel M. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol 1982;13. https://doi.org/10.2307/270723.
- National Institute for Health and Care Excellence (NICE) . NICE Process and Methods Guides. Developing NICE Guidelines: The Manual 2015.
- Sørensen JB, Skovgaard T, Puggaard L. Exercise on prescription in general practice: a systematic review. Scand J Prim Health Care 2006;24:69-74. https://doi.org/10.1080/02813430600700027.
- National Institute for Health and Care Excellence (NICE) . NICE Process and Methods Guides. A Rapid Review of the Effectiveness of ERS to Promote Physical Activity in Adults 2006.
- Williams NH, Hendry M, France B, Lewis R, Wilkinson C. Effectiveness of exercise-referral schemes to promote physical activity in adults: systematic review. Br J Gen Pract 2007;57:979-86. https://doi.org/10.3399/096016407782604866.
- Owen L, Morgan A, Fischer A, Ellis S, Hoy A, Kelly MP. The cost-effectiveness of public health interventions. J Public Health 2012;34:37-45. https://doi.org/10.1093/pubmed/fdr075.
- Vijay GC, Wilson EC, Suhrcke M, Hardeman W, Sutton S. VBI Programme Team . Are brief interventions to increase physical activity cost-effective? A systematic review. Br J Sports Med 2016;50:408-17. https://doi.org/10.1136/bjsports-2015-094655.
- National Institute for Health and Care Excellence (NICE) . Physical Activity: Exercise Referral Schemes 2014.
- Deidda M, Coll-Planas L, Giné-Garriga M, Guerra-Balic M, Roqué I, Figuls M, et al. Cost-effectiveness of exercise referral schemes enhanced by self-management strategies to battle sedentary behaviour in older adults: protocol for an economic evaluation alongside the SITLESS three-armed pragmatic randomised controlled trial. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-022266.
- Curtis LA. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015 to 2016 2016.
- Office for National Statistics (ONS) . Annual Survey of Hours and Earnings 2017 2017.
- Walker D, Kumaranayake L. Allowing for differential timing in cost analyses: discounting and annualization. Health Policy Plan 2002;17:112-18. https://doi.org/10.1093/heapol/17.1.112.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Allison PD. Multiple imputation for missing data: a cautionary tale. Sociol Methods Res 2000;28:301-9. https://doi.org/10.1177/0049124100028003003.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. https://doi.org/10.1136/bmj.b2393.
- Rubin DB. Inference and missing data. Biometrika 1976;63:581-92. https://doi.org/10.1093/biomet/63.3.581.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Basu A, Manca A. Regression estimators for generic health-related quality of life and quality-adjusted life years. Med Decis Making 2012;32:56-69. https://doi.org/10.1177/0272989X11416988.
- Graubard BI, Korn EL. Analyzing health surveys for cancer-related objectives. J Natl Cancer Inst 1999;91:1005-16. https://doi.org/10.1093/jnci/91.12.1005.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- BC Injury Research and Prevention Unit . The Injury Consequences of Promoting Physical Activity: An Evidence Review 2013.
- Physical Activity Guidelines for Americans. Washington, DC: US Department of Health and Human Services; 2018.
- Curtis LA. Unit Costs of Health and Social Care 2010. Canterbury PSSRU: University of Kent; 2010.
- Meng H, Friedberg F, Castora-Binkley M. Cost-effectiveness of chronic fatigue self-management versus usual care: a pilot randomized controlled trial. BMC Fam Pract 2014;15. https://doi.org/10.1186/s12875-014-0184-7.
Appendix 1 Illustrative screenshots from the e-coachER website
Appendix 2 The CONSORT flow diagram (detailed)
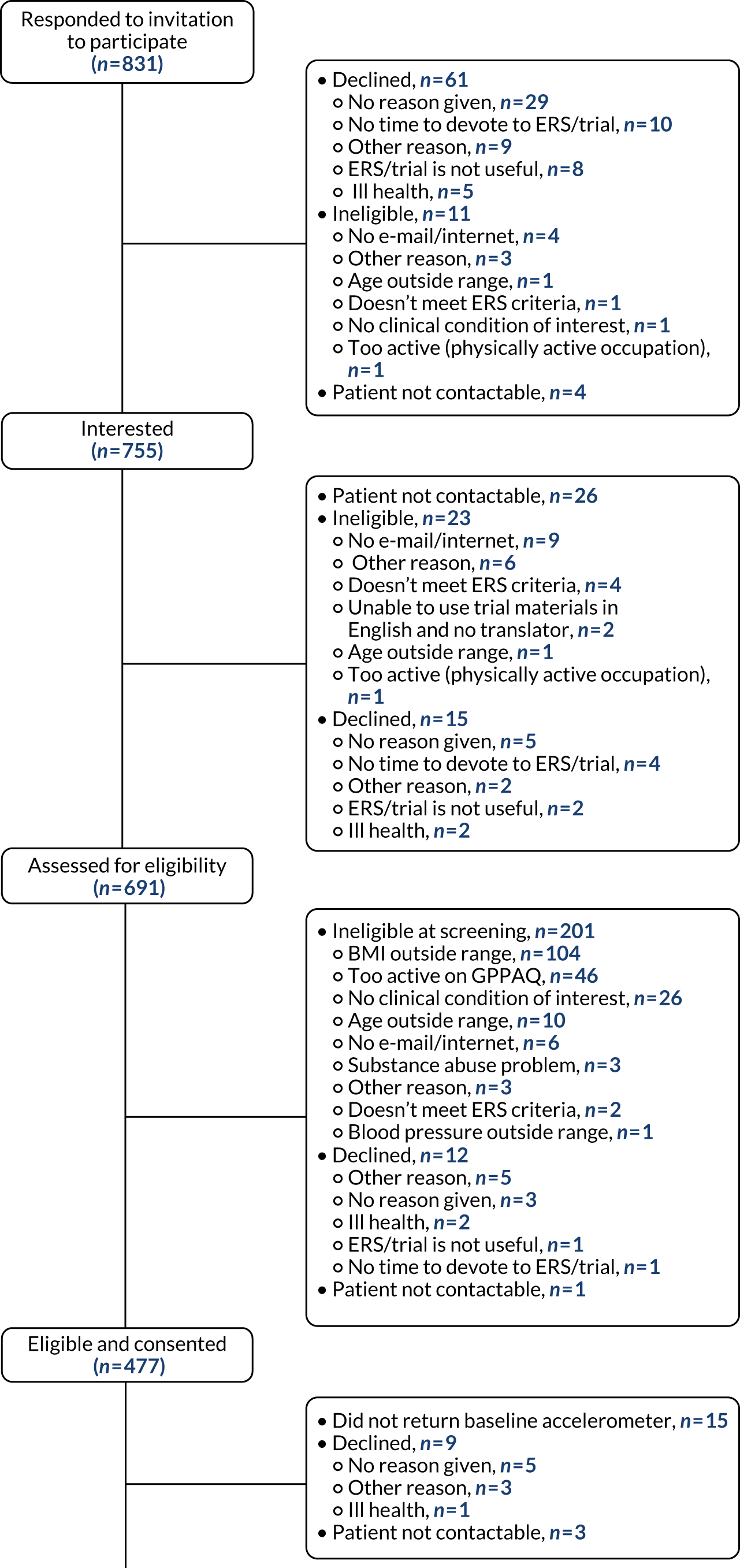
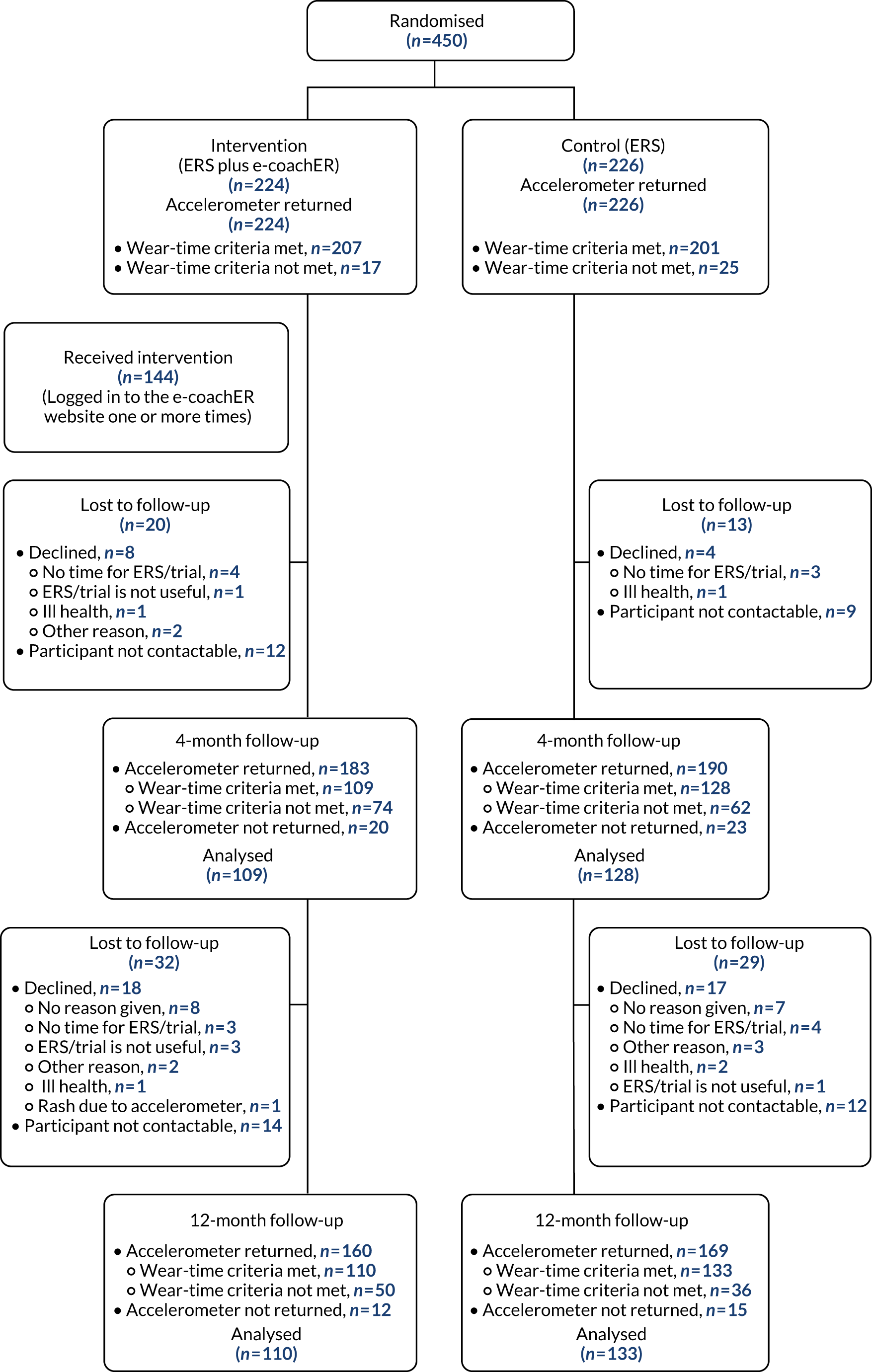
Appendix 3 Estimates for repeated-measures model for the primary outcome
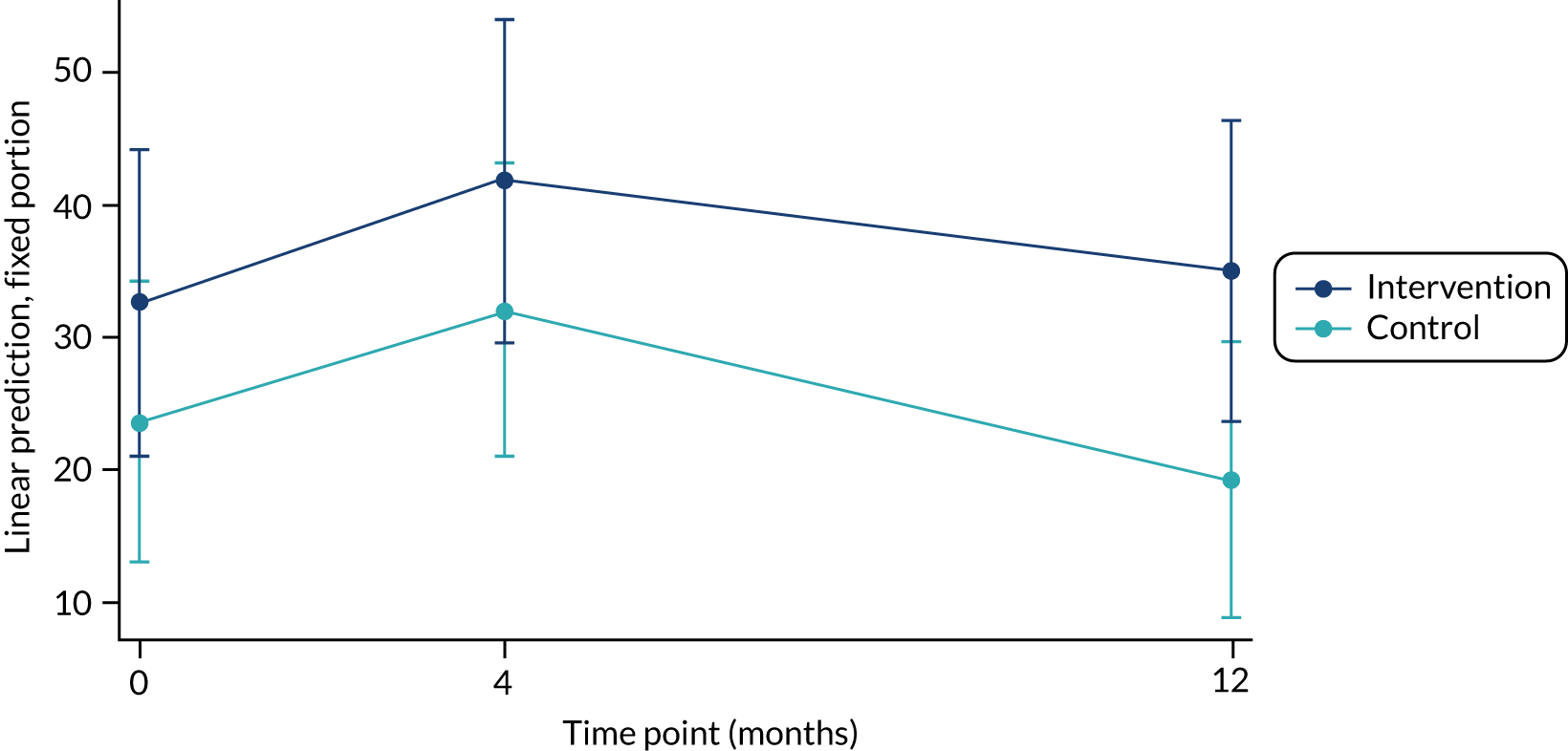
Appendix 4 Serious adverse events
Serious adverse events reported in the control group
Serious AEs were hospitalisations, with the exception of SAE010029/001 (Morton’s neuroma), which was categorised as ‘persistent/significant disability/incapacity’.
| Site | SAE log number | Outcome | Relationship of the event to the study processes | MedDRA organ system | Summary description of event |
|---|---|---|---|---|---|
| 03 | SAE030003/002 | Recovered with sequelae | Not related | Neoplasms (2) | Diagnosed with chronic myeloid leukaemia |
| 03 | SAE030210/002 | Recovered | Not related | Neoplasms (2) | Prolonged hospitalisation caused by recurrence of breast cancer |
| 03 | SAE030184/001 | Ongoing | Unlikely | Psychiatric (7) | Inpatient stay on mental health ward |
| 03 | SAE030210/001 | Recovered | Not related | Psychiatric (7) | Hospitalised for depression |
| 01 | SAE010029/001 | Ongoing | Not related | Nervous system (8) | Morton’s neuroma |
| 01 | SAE010081/001 | Recovered | Unlikely | Respiratory (13) | Treated in hospital for fluid on the lungs |
| 03 | SAE030157/001 | Recovered | Not related | Gastrointestinal (14) | Varices of gastrointestinal tract. Prolonged inpatient stay caused by major organ system involvement |
| 02 | SAE020261/001 | Recovered | Not related | Musculoskeletal (17) | Admitted to hospital because unable to walk |
| 02 | SAE020194/001 | Recovered | Not related | Pregnancy (19) | Childbirth and postnatal inpatient stay |
| 03 | SAE030157/002 | Recovered | Not related | Investigations (23) | Admitted to hospital with symptoms of meningitis |
| 02 | SAE020193/001 | Recovered with sequelae | Not related | Investigations (23) | Collapse. No diagnosis made |
| 01 | SAE010007/001 | Recovered | Not related | Surgical/medical (25) | Planned admission for femorodistal bypass (peripheral vascular disease), subsequent infection/abscess behind knee |
| 03 | SAE030003/001 | Recovered | Not related | Surgical/medical (25) | Hospitalised for treatment of boils in groin |
| 01 | SAE010139/001 | Recovered | Not related | Surgical/medical (25) | Planned hospital admission for bunion removal |
| 01 | SAE010139/002 | Recovered | Not related | Surgical/medical (25) | Planned hospital admission for bunion removal |
| 03 | SAE030065/001 | Recovered | Unlikely | Surgical/medical (25) | Hospital admission for treatment for diverticular bleeding |
| 02 | SAE020342/001 | Recovered | Not related | Surgical/medical (25) | Injury to foot led to planned admission for partial amputation of left great toe |
| 01 | SAE010088/001 | Recovered | Not related | Surgical/medical (25) | Planned hospital admission for right hip replacement |
| 03 | SAE030171/001 | Recovered | Not related | Surgical/medical (25) | Planned hospitalisation for total hip replacement |
| 01 | SAE010098/001 | Recovered | Not related | Surgical/medical (25) | Planned hospital admission for total knee replacement |
| 02 | SAE020111/001 | Recovered | Not related | Surgical/medical (25) | Preplanned hospitalisation for knee surgery because of osteoarthritis |
| 02 | SAE020195/001 | Recovered with sequelae | Not related | Surgical/medical (25) | Planned admission for total right knee replacement |
| 02 | SAE020195/002 | Recovered with sequelae | Not related | Surgical/medical (25) | Planned admission following infected right knee joint – continuing physiotherapy and using crutches |
| 02 | SAE020313/001 | Recovered | Not related | Surgical/medical (25) | Hospitalisation for emergency operation on knee following a number of falls and pre-existing weakness in knee |
| 01 | SAE010063/001 | Recovered | Not related | Surgical/medical (25) | Hospital admission for surgical repair of bulging disc in lower back |
| 02 | SAE020185/001 | Recovered | Not related | Surgical/medical (25) | Hospitalised because of complications from type 2 diabetes and heart failure |
Serious adverse events reported in the intervention group
Serious AEs were hospitalisations, with the exception of SAE010011/001 (asthma attack), which was categorised as a life-threatening event.
| Site | SAE log number | Outcome | Relationship of the event to the study processes | MedDRA organ system | Summary description of event |
|---|---|---|---|---|---|
| 01 | SAE010104/001 | Recovered | Not related | Cardiac (11) | Admitted to hospital with abnormal ECG. Diagnosis: paroxysmal atrial fibrillation |
| 03 | SAE030240/001 | Recovered | Not related | Cardiac (11) | Hospitalised because of a heart attack |
| 03 | SAE030134/001 | Recovered | Unlikely | Vascular (12) | Hospitalised because of minor stroke |
| 01 | SAE010011/001 | Recovered | Not related | Respiratory (13) | Asthma attack |
| 01 | SAE010119/001 | Recovered | Not related | Investigations (23) | Hospital admission for suspected meningitis. No formal diagnosis made. Symptoms attributed to adverse effects of prescription medication |
| 01 | SAE010159/001 | Recovered | Not related | Investigations (23) | Fall resulting in fracture of left radius. Admitted for investigations of reasons for the fall |
| 01 | SAE010160/002 | Recovered | Not related | Surgical/medical (25) | Admitted to hospital following fall with fracture to right ankle and trauma to right knee |
| 01 | SAE010115/002 | Recovered | Not related | Surgical/medical (25) | Preplanned hospital admission for abdominal surgery |
| 03 | SAE030078/001 | Recovered | Not related | Surgical/medical (25) | Planned hospitalisation for operation on right ankle |
| 02 | SAE020290/001 | Recovered | Not related | Surgical/medical (25) | Admitted to hospital for 1 day (day case) because of osteoarthritis |
| 01 | SAE010201/001 | Recovered | Not related | Surgical/medical (25) | Planned hospital admission for tendon surgery on hand related to rheumatoid arthritis |
| 03 | SAE030049/001 | Recovered | Unlikely | Surgical/medical (25) | Hospital admission for treatment of rheumatoid arthritis flare-up |
| 02 | SAE020354/001 | Recovered | Not related | Surgical/medical (25) | Inpatient stay for removal of Bartholin’s cyst |
| 01 | SAE010115/001 | Recovered | Not related | Surgical/medical (25) | Planned hospital admission for knee replacement |
| 01 | SAE010160/001 | Recovered | Not related | Surgical/medical (25) | Planned admission for partial right knee replacement |
| 03 | SAE030102/001 | Recovered | Not related | Surgical/medical (25) | Hospitalised for surgery on both knees, as treatment for long-standing osteoarthritis |
Appendix 5 Participant telephone interview topic guide
Participant telephone interview schedule
Preamble script
Thank you very much for agreeing to participate in this phone interview in order to help us understand what it has been like to be part of the e-coachER study, we really appreciate your time.
Just to recap on the information we sent you, the purpose of this interview is to understand your experience of e-coachER and any impact it may have had so that we can learn for future development. Please say anything you wish, we want to hear all types of feedback and are keen to hear your views on how things might be done differently to improve the study.
The interview will take around 45 minutes and will be audio-taped to ensure that we do not miss anything. All information you provide will be anonymised; if we use any quotes from you we will not give your name but use a false name.
Before we begin do you have any questions about doing the interview?
Are you therefore willing to give consent to do this interview . . . thank you.
When we are ready to start I will switch the recorder on, say your name and the date; is that OK?
OK so the recorder is now going on . . .
Take verbal consent
Background
Can you begin by telling me about why you were prescribed the exercise referral scheme?
How did you hear about e-coachER?
Have you been referred to an exercise centre? Which one? Has a programme been devised for you?
You should have received a welcome pack in the post – what did you think of the welcome pack?
How did you find the user guide?
Is there anything you’d recommend we changed about the user guide?
Did register on e-coachER website? If no:
It is not a problem that you decided not to visit the website; but we are keen to find out your reasons so we can change things for the better in the future . . . so please will you tell me a little bit more about why you did not go to the website? Were there any technical barriers/problems . . . was something else putting you off . . . Now conclude interview
In general what did you think of the website? (Prompt – what were your first impressions? We will go through it in more detail in a minute)
So did you register? How was it to do this? . . . is there anything that could be changed to help make registering easier?
There was also an e-coachER facilitator to help you with the technology . . . did you make use of this? . . . in what ways was this helpful? Tell me more about the help you received . . . or would have liked to have been given?
In Step 1 there was a quiz
Do you remember doing this?
What did you think about the quiz?
What were its key messages about the benefits of activity for someone with your condition?
In Step 2 you were encouraged to find support to get physically active. Can you tell me more about how you used this part of e-coachER?
Did you involve family or friends?
In Step 2 you were also introduced to the ‘Links’ pages on the website which gives information about local exercise referral schemes and other local support for becoming physically active. These pages also provided general information about becoming physically active.
Did you use the links?
What did you think about the information provided?
Were the links page useful?
What links were most helpful?
How did you use this support?
In Step 3 you were asked to use the pedometer to count your steps. This is the little device you wear on your belt.
How did you get on with using this?
In what ways was it useful for you to use the pedometer?
In what ways was the pedometer difficult to use?
Is there anything else you’d like to say about the pedometer, anything we should do differently?
In Step 4 you were asked to set step count goals.
What did you understand about the purpose/usefulness of setting these goals?
Tell me more about your goal-setting:
Was it easy to set step count goals that were . . . specific . . . achievable . . . realistic . . .?
In what ways was the pedometer helpful for achieving your step count goals?
. . . it was useful (for measuring/seeing progress)?
. . . it was not helpful because?
In the welcome pack there was a fridge magnet with tear off strips to record your steps.
Did you use these?
Did you put them up on your fridge (or elsewhere)?
How have you used these strips to record your steps?
How have you found these strips useful or not useful?
In Step 5 you were asked to make some physical activity plans.
Did you use this step to make plans for moderate physical activity?
In what ways was it easy or hard to set weekly goals?
How did you find the advice about setting SMART goals helpful or unhelpful?
In what ways was it easy or hard to keep to a weekly goal?
In what ways was it easy or hard to review your weekly goals?
In what ways was reviewing your step goals helpful or unhelpful?
In this step there was some advice on other opportunities to be physically active, for example, travel, leisure time, household chores.
Did you find this advice helpful or useful?
What did you think about the progress graph? . . .
What did you think about the personalised feedback? . . . was the praise . . . encouragement helpful?
What was it like not to achieve your goals?
To what extent have you used e-coachER to set yourself new step goals each week?
In Step 6 you were asked about finding ways to help you achieve your physical activity plans. Dealing with the influences in your environment on your physical activity.
Did you use this part of the website? How helpful did you find the advice?
Please tell me a little more about what you did?
Did you make any changes, for example to your daily routine in order to meet your goals?
What did you find most motivating?
Did you make use of the motivational messages/text/e-mails?
In Step 7 you were asked to identify any barriers or obstacles to carrying on with your physical activity plans. . . . how did you get on with this task?
Were you able to identify any causes of stopping your activity programme? (e.g. something to do with your health condition . . . holidays . . . sickness . . . change at work/caring, etc.)
In what way have you found it easy – or not – to challenge negative thoughts about not doing your planned physical activity?
Do you feel you have learned how to plan and avoid lapses in physical activity in the future?
I would like to ask you some more general questions about e-coachER.
How relevant was it for you?
Overall, how did it help you to set and manage your own goals to increase your physical activity?
To what extent did it provide you with new information?
How well were you able to engage with e-coachER?
How easy was e-coachER to navigate? (e.g. layout of ‘steps to health’/main menu, goals)
How was the general tone of the website? (Was the language appropriate? Was it supportive? Were the success stories relevant/helpful?)
What did you think about the structure/look of the website? (e.g. font size, colour, length of sessions, ability to unlock sessions after set time period).
When did you use e-coachER – where were you?/what were you doing?
What was the most useful aspect of the e-coachER support package?
Is there anything else that we have not talked about that you would like to discuss about e-coachER?
Did using e-coachER support you in the ERS?
Was e-coachER useful on its own?
Thank participant for their time, etc.
Interview topic guide for e-coachER study research assistants
Thank you very much for taking part in an interview to talk about your experiences of working on e-coachER. The aim of the interview is hear your views and experiences of what worked well and what could be done differently if a full trial were to be carried out. Additionally, it is hoped that your experiences and views can help to develop a clearer understanding of ‘general’ issues related to the research process, particularly recruitment of patients to a trial.
| Topic | Specific questions and prompts |
|---|---|
| Background | Can you begin by telling me about your role in the e-coachER trial? |
| Recruitment/your role in supporting patients through e-coachER | How have you been involved in recruiting participants? Has your role changed during the study? |
| After addressing the earlier recruitment issues, can you tell me about things that worked well in the recruitment process? Anything that did not work so well, even in the later stages of recruitment? | |
| What further improvements do you feel could be made to the recruitment process? | |
| Is there anything else that you feel is important to consider in relation to recruitment to e-coachER? More generally, to a trial? | |
| Could you tell me about the role you played in supporting participants after they had logged on and begun using e-coachER | |
| About the patients | How do you feel patients have felt about (or reacted to) being invited to participate in the study? |
| What do you feel are the main reasons why patients agreed to participate in e-coachER? In a trial? | |
| What do you feel have been the main barriers for patients to participating in e-coachER? (Also in relation to invite, reply slips, registration process, logging on?) | |
| What do we know about patients opening the information pack and reading the contents? What else could be done to encourage this (for e-coachER, more generally)? | |
| After the changes had been made to the protocol, do you feel that anything could/has put participants off (i) opening info pack (ii) returning the reply slips? How can these issues be resolved? | |
| What do we know about patients who are signing up for the study but not logging on to e-coachER? What might have put them off? | |
| Have you had any feedback from patients about the benefits of e-coachER support/participating in a trial? | |
| Is there anything else you feel is important in relation to what patients have told you about e-coachER/participating in the study? Anything that could have been done differently? | |
| About primary care/ERS | Which primary care/ERS staff were involved in recruitment? How did they recruit to e-coachER? |
| Can you tell me about any feedback you have had from primary care/ERS staff about in the study? | |
| Can you tell me about any specific issues faced by different primary care and ERS staff groups when recruiting to e-coachER? | |
| Finishing up | What do you feel is the most beneficial aspect of e-coachER for participants? Least beneficial aspect of the package? |
| What do you feel are the main ‘lessons learned’ from working on e-coachER, in general and for a larger e-coachER trial? | |
| Anything else you feel is important for future recruiting to a trial (i) generally, (ii) for e-coachER? |
Appendix 6 Economic evaluation: overview of unit costs
| Type of resource | Unit cost adjusted to 2017/18 prices as appropriate (£) | Source |
|---|---|---|
| GP visit at practice (9.22 minutes) | 37 | PSSRU 201774 |
| Nurse visit at practice (15.5 minutes) | 11 | PSSRU 201774 |
| A&E visit (weighted average accounting for activity) | 158 | NHS Reference Costs 2015 to 2016 75 |
| Social worker visit (30 minutes) | 29.50 | PSSRU 201774 |
| Prescriptions | 8 | PSSRU 201774 |
| Hospital visit (as outpatient) (weighted average accounting for activity) | 137 | PSSRU 201774 |
| GP home visit | 135 | PSSRU 201088 |
| Nurse home visit | 23 | PSSRU 201088 |
| Physiotherapy appointment (1-hour appointment) | 33 | PSSRU 201774 |
| Care worker/advisor visit (30 minutes) | 13 | PSSRU 201774 |
| Hospital visit (as day case) (weighted average accounting for activity) | 727 | PSSRU 201774 |
| Hospital visit (as inpatient) (weighted average accounting for activity) | 1478 | NHS Reference Costs 2015 to 2016 75 |
| Mental health support (DBT assessment/counsellor) | 9 | PSSRU 201774 |
| Dentist | 127 | PSSRU 201774 |
| Occupational therapist (1 hour) | 35 | PSSRU 201774 |
| Psychiatrist (1 hour) | 108 | PSSRU 201774 |
| Psychologist (1 hour) | 53 | PSSRU 201774 |
| Podiatrist (1 hour) | 33 | PSSRU 201774 |
| Ambulance service | 119 | PSSRU 201774 |
| Fitness instructor | 7.46 | www.payscale.com/research/UK/Job=Fitness_Instructor/Hourly_Rate (accessed 7 May 2018) |
| Wage rate per hour for participantsa | Employed (18–21 years, 8.77; 22–29 years, 12.76; 30–39 years, 16.83; 40–49 years, 18.29; 50–59 years, 17.69; ≥ 60 years, 15.69) | ONS (2017)76 |
| Not employed (18–21 years, 4.39; 22–29 years, 6.38; 30–39 years, 8.42; 40–49 years, 9.15; 50–59 years, 8.85; ≥ 60 years, 7.85) |
Appendix 7 Economic evaluation: resource use associated with the set-up cost of the intervention
Design was included as these activities may happen in the future following further learning from this trial.
| Activity | Resource: discussions with chief investigator and trial manager, review of trial records, diaries and routine administrative records | Total quantity | Cost per participant (unit cost values are omitted to maintain confidentiality) (£) |
|---|---|---|---|
| Design | |||
| Designing of intervention (welcome pack contents) | Time (in hours) spent by: | ||
| Assistant trial manager | 20 | 8.90 | |
| Professor | 20 | ||
| PPI representative (in kind) | 20 | ||
| Designing of website | Time (in hours) spent by: | ||
| Technical specialists | 960 | 172.01 | |
| Professor | 48 | ||
| Publicity campaigns/identification of participants | |||
| Community-based publicity campaigns/identification of participants | Time (in hours) spent by: | ||
| PPI representatives (in kind) | 21 | 8.21 | |
| Research assistants | 84 | ||
| Centre administrators | 9 | ||
| Money costs of travel: | |||
| Car trips | 150 miles | 0.30 | |
| Advertising: | |||
| Posters | 150 posters | 10.00 | |
| Newspaper advert | 3 adverts | 3.33 | |
| Total cost per participant | 180.91 | ||
Appendix 8 Economic evaluation: resource use associated with the delivery cost of the intervention
| Activity | Resource (from trial records) | Total quantity of resource items | Unit cost (from invoice and trial records) (£) | Cost per patient (£) |
|---|---|---|---|---|
| Handbooks for participants | Number of handbooks | 225 | 0.04 | 0.04 |
| Welcome pack boxes | Number of welcome pack unit boxes | 225 | 0.28 | 0.28 |
| Pedometer | Number of pedometers | 225 | 4.05 | 4.05 |
| Recording sheets for weekly PA activity (a fridge magnet) | Number of sheets | 225 | 1.01 | 1.01 |
| Guide for using e-coachER website | Number of website guides | 225 | 0.04 | 0.04 |
| Postage/packaging of welcome pack | Number of postage packs | 225 | 10.33 | 10.33 |
| Maintenance/technical support (for participants) of e-coachER website | Time spent (in hours) by: | |||
| Technical specialist | 128 | n/a | 13.43 | |
| Research assistants | 16 | |||
| Total cost per participant | 29.17 | |||
Appendix 9 Economic evaluation: costs of health and social service use
| Cost | Cost (£), mean (SD) | Which group had higher cost? | ||
|---|---|---|---|---|
| Whole sample (n = 243) | Control (n = 133) | e-coachER intervention (n = 110) | ||
| Costs at 4 months | ||||
| Cost of GP practice visit | 85 (89) | 84.5 (96) | 85 (80) | Intervention |
| Cost of nurse practice visit | 15 (33) | 13 (18) | 17 (44) | Intervention |
| Cost of A&E visit | 31 (57) | 37 (64) | 25 (47) | Control |
| Cost of social worker visit | 6 (61) | 3 (26) | 10 (85) | Intervention |
| Cost of prescriptions | 33 (68) | 32 (86) | 35 (38) | Intervention |
| Cost of hospital (outpatients) | 133 (184) | 136 (189) | 130 (178) | Control |
| Cost of GP home visit | 3 (14) | 2 (14) | 3 (15) | Intervention |
| Cost of nurse home visit | 3 (13) | 2 (14) | 3 (13) | Intervention |
| Cost of physiotherapist visit | 27 (75) | 22 (44) | 32 (101) | Intervention |
| Cost of care worker visit | 3 (17) | 0.6 (4) | 5 (25) | Intervention |
| Cost of hospital day case | 185 (375) | 158 (351) | 218 (402) | Intervention |
| Cost of hospital (inpatient) | 190 (595) | 144 (442) | 245 (738) | Intervention |
| Costs of other health service | 8 (57) | 4 (34) | 12 (77) | Intervention |
| Total cost at 4 months | 721 (952) | 639 (842) | 819 (1065) | Intervention |
| Costs at 5–12 months | ||||
| Cost of GP practice visit | 111 (139) | 114 (170) | 108 (89) | Control |
| Cost of nurse practice visit | 16 (18) | 16 (21) | 15 (14) | Control |
| Cost of A&E visit | 24 (65) | 27 (74) | 20 (53) | Control |
| Cost of social worker visit | 2 (11) | 3 (14) | 1 (6) | Control |
| Cost of prescriptions | 43 (189) | 28 (31) | 60 (279) | Intervention |
| Cost of hospital (outpatients) | 132 (221) | 136 (250) | 128 (180) | Control |
| Cost of GP home visit | 1 (9) | 2 (12) | 0.5 (4) | Control |
| Cost of nurse home visit | 1 (12) | 0.6 (4) | 2 (18) | Intervention |
| Cost of physiotherapist visit | 24 (62) | 24 (64) | 23 (60) | Control |
| Cost of care worker visit | 5 (29) | 4 (20) | 6 (37) | Intervention |
| Cost of hospital day case | 204 (885) | 224 (1119) | 180 (472) | Control |
| Cost of hospital (inpatient) | 128 (528) | 131 (573) | 124 (469) | Control |
| Costs of other health service | 2 (19) | 3 (24) | 1 (11) | Control |
| Total cost (5–12 months after the study) | 692 (1432) | 713 (1761) | 668 (891) | Control |
| Total costs at 12 months | 1413 (1971) | 1352 (2180) | 1487 (1691) | Intervention |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- BMI
- body mass index
- CACE
- complier-average causal effect
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CRN
- Clinical Research Network
- CTU
- clinical trials unit
- DMC
- Data Monitoring Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ERS
- exercise referral scheme
- GP
- general practitioner
- GPPAQ
- General Practice Physical Activity Questionnaire
- HADS
- Hospital Anxiety and Depression Scale
- ICER
- incremental cost-effectiveness ratio
- IT
- information technology
- ITT
- intention to treat
- MedDRA
- Medical Dictionary for Regulatory Activities
- MVPA
- moderate and vigorous physical activity
- PA
- physical activity
- PenCTU
- Peninsula Clinical Trials Unit
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SMART
- specific, measurable, achievable, relevant, time-bound
- TSC
- Trial Steering Committee
Notes
Supplementary material can be found on the NIHR Journals Library report page (http://doi.org/10.3310/hta24630).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
