Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/155/05. The contractual start date was in April 2016. The draft report began editorial review in January 2020 and was accepted for publication in July 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by McRobbie et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Material throughout the report has been adapted from the trial protocol [see NIHR Journals Library, www.journalslibrary.nihr.ac.uk/programmes/hta/1315505/#/ (accessed September 2020)].
Around 70% of smokers who quit in the short term return to smoking within 1 year. 1 The UK government invests £84.3M annually to fund stop smoking services, not including the cost of smoking cessation medicines. The initial self-reported 4-week quit rate in smokers who engage in such treatment is around 50%;2 however, in the longer term, the ubiquitous relapse substantially reduces the impact of these initiatives. As the health benefits of stopping smoking are primarily realised with long-term abstinence, relapse reduces the public health benefit of investment in smoking cessation interventions.
Preventing relapse to smoking has proven difficult. Two behavioural relapse prevention strategies have been formally evaluated: (1) a ‘skills-based’ approach that focuses on teaching clients to identify relapse situations and put in place coping strategies,3 and (2) extending the duration of the initial treatment with maintenance sessions to provide ongoing support. A systematic review from the Cochrane Collaboration4 identified 81 relevant studies and concluded that, despite the good intuitive validity of these approaches, they showed no significant benefit. Another systematic review5 arrived at the same conclusion. The lack of benefit may be because clients do not learn the cognitive–behavioural skills or may not practise them, or the skills themselves may not be helpful for preventing relapse. 6
The Cochrane review4 also identified 13 studies that examined the extended use of stop smoking pharmacotherapy. Extended use of varenicline (Champix®, Pfizer) (6 months vs. the standard 3 months; two studies) was associated with a small increase in 1-year abstinence rates [risk ratio (RR) = 1.23; 95% confidence interval (CI) 1.08 to 1.41], but no benefit was found for extended use of bupropion (Zyban®, GlaxoSmithKline). Insufficient studies have evaluated effects of extended nicotine replacement treatment (NRT). Most successful quitters seem to have limited interest in continued use of currently licensed smoking cessation medicines over an extended period7,8 and long-term use of these medicines also has substantial financial implications.
In 2014, the National Institute for Health Research commissioned a trial to further address this issue. The project was co-funded with the National Health and Medical Research Council, Canberra, ACT, Australia. The commissioned call specified a four-arm trial, with a control, a behavioural intervention, a pharmacological intervention and a combination of the two interventions, to be conducted in England and Australia.
Our team identified two interventions that met these specifications and appeared worth evaluating.
Regarding behavioural support, extended support requiring ex-smokers to attend treatment sessions or maintain telephone contact is ineffective. 4 This is most likely because successful quitters may not see the need to put effort into such contacts when they are not smoking and when they have lapsed to smoking may believe that there is no benefit in making contact or feel embarrassed to renew it. For example, in a trial where the provision of support relied on smokers taking the initiative to telephone the service when they felt in danger of lapsing or following a lapse, very few clients used the offer. 9
Information technology (IT), in particular web-based resources and text messaging, offers a more convenient way of providing ongoing support and is more consistent with the preference of many smokers to quit without using professional help. 10 An online Structured Planning and Prompting Protocol (S3P), which provides tailored advice following online assessments with a particular focus on developing strategies for tempting situations in a form that helps ensure that they will be remembered when needed, reduced relapse rates between 1 and 24 weeks (from 71% to 61%). 11 It is delivered online and can potentially be enhanced by mobile phone text messages. Texting interventions are inexpensive and can be easily disseminated on a large scale. The use of ongoing text-based contact to prevent relapse was piloted in 202 stop smoking service clients who were abstinent 4 weeks after their quit date. 8 Unlike invitations to attend sessions or call their advisors, the texting intervention was well received (i.e. 70% of recent ex-smokers gave an overall score for helpfulness of the messages of 4 or 5 on a 5-point scale) and the retention rates were higher than with face-to-face approaches or reactive telephone-based approaches. We aimed to evaluate an intervention combining the online coaching programme with text messaging.
Regarding pharmacological support, we discussed problems relating to persuading ex-smokers to continue using medications that they felt they did not need any more and the financial implications of such long-term treatments. An alternative approach is to provide ex-smokers with medications to be used ‘in an emergency’. Fast-acting NRT, such as nicotine mouth spray or lozenges, seems well suited to this purpose. Another relatively new option is electronic cigarettes (e-cigarettes). E-Cigarettes have become popular among smokers12,13 and there is increasing evidence supporting their effectiveness for smoking cessation. 14,15 Some data are also emerging showing that e-cigarette use may help prevent relapse. 16 In the pharmacological arm of the study, we aimed to evaluate the provision of ‘emergency supplies’ of clients’ choice of fast-acting NRT or an e-cigarette.
Chapter 2 Methods
Original and curtailed trial design
This was an individually randomised factorial trial that included two interventions and usual care. The original plan was to recruit 1400 participants and follow them over 12 months. Owing to circumstances described below, the trial was curtailed with 236 participants randomised and the follow-up period was reduced to 6 months.
Changes to trial design/protocol (trial curtailment)
The trial encountered serious delays. The commencement of the trial was delayed as a result of the failure of the contracted IT consultants to deliver a version of the S3P programme, which, among other improvements and updates to the version trialled earlier, was to include data management capabilities (e.g. programing and scheduling of follow-up surveys, dynamic lists of scheduled calls for telephone interviewers, reminder e-mails). Unexpected technical issues and loss of staff with expertise were the primary reasons for this failure. The work had to be abandoned in April 2017, at which time we moved quickly to make necessary adaptations to the earlier version to the extent these were possible and to program the data management aspects in REDCap® version 7.0.19 (Vanderbilt University Medical Centre, Nashville, TN, USA).
Integrating these two systems presented additional technical challenges. The integrated system was finally implemented in Australia in August 2017. UK regulations, however, did not allow the use of a system based abroad, and the integrated system had to be fully replicated and installed on a UK server. Experts responsible for implementing the Australian installation were not allowed direct access to it. The work proved difficult for local IT personnel and took a further 6 months to complete. During these delays, the English stop smoking services were subjected to substantial changes, which emerged as the next major problem for the study because of effects on client recruitment. Service management moved from the NHS to local councils that commissioned private providers. The stop smoking service throughput shrunk by > 60% and few services remained that were able to contribute to such studies.
Recruitment in Australia also proved difficult. The original plan was to recruit participants solely from the quitline in the state of Victoria. However, the number of participants it referred was smaller than anticipated; therefore, to help boost numbers we sought to recruit more widely and the quitline from Tasmania agreed to take part. Despite this, referrals remained low. To boost recruitment in England, recruitment methods were expanded to include smokers quitting through the Stoptober campaign. In Australia, ex-smokers were approached via targeted Facebook (Facebook, Inc., Menlo Park, CA, USA; www.facebook.com) adverts and through St Vincent’s Hospital Melbourne, Fitzroy, VIC, Australia, recruiting patients who were discharged following a period of abstinence in hospital. The Australian site also expanded its recruitment window from 21–45 days post quit date to 7–100 days post quit date. Despite these measures, the recruitment rate remained slow in both countries. After consultation with funders, the decision was made to curtail the study. Recruitment was stopped in January 2019 and the decision was made to complete follow-up at 6 months rather than 12 months. Saliva sample collection, which had originally been planned to validate self-reports of abstinence at 12 months, and cost-effectiveness analyses could not be carried out.
See Appendix 1, Tables 25 and 26, for details of changes to the protocol.
Inclusion/exclusion criteria
Inclusion criteria
-
Originally, participants were people in England who had quit smoking with stop smoking services and people in Australia who had quit smoking with quitlines and who were abstinent from smoking for at least 2 weeks and no more than 45 days at the point of recruitment. Later, participants from the Stoptober campaign were added in England (see Appendix 1, Table 25) and participants in Australia recruited via Facebook or from St Vincent’s Hospital Melbourne. In Australia the abstinence criterion was extended to at least 1 week and no more than 100 days post quit date at the point of recruitment (see Appendix 1, Table 26).
-
Participants had to be willing to use a nicotine product or an online behavioural support tool if allocated to it.
-
Participants had to be aged ≥ 18 years.
-
Participants had to own a mobile phone.
-
Participants had to have access to the internet.
-
Participants had to be able to read, write and understand English.
Exclusion criteria
Potential participants were excluded if they were:
-
enrolled in other smoking cessation/relapse prevention research
-
currently using e-cigarettes or oral NRT and planning to use it for longer than 3 months.
Recruitment and setting
The original plan was to recruit 1400 participants (700 participants in England and 700 participants in Australia). In total, 236 participants were randomised between September 2017 and January 2019. Two participants withdrew and requested that their data not be used, resulting in a total of 234 participants (131 participants in Australia and 103 participants in England).
In England, participants were originally recruited from six stop smoking services: Tower Hamlets, City of London, Leicester, Medway, Birkenhead and Durham. Later, those who had quit using the Stoptober 2018 campaign were also invited.
Participants in Australia were originally recruited from quitlines in Victoria and Tasmania. From March 2018, participants were recruited via Facebook advertising and in the final months participants were recruited from among patients discharged from St Vincent’s Hospital Melbourne.
Interventions were delivered remotely (i.e. online and/or by telephone/e-mail/text messages) and participants did not have any face-to-face contact. Follow-up was completed online or by telephone calls conducted by researchers at the Health and Lifestyle Research Unit (London, UK) and Cancer Council Victoria (Melbourne, VIC, Australia).
Study procedures
Potential participants from stop smoking services, Stoptober, quitlines and St Vincent’s Hospital Melbourne were informed of the study by service staff and, if interested, were given written information and referred to the research team. Potential participants from Facebook visited the study Facebook page and the associated study website where they self-referred.
Potential participants were contacted by e-mail as soon as possible after referral. Those relapsing back to smoking before being reached were not invited to take part.
Participants were screened for eligibility online or by telephone, depending on their preference. Informed consent was also provided either online or verbally. Consenting participants completed a baseline survey (see Measures) and were randomised into one of four study arms (see Randomisation). Following this, the allocated treatment was initiated (see Interventions).
All participants were contacted by telephone approximately 1 week later to check that all was well, including that they had begun receiving text messages or received their chosen product. Any difficulties with the intervention were discussed and resolved.
Interventions
Participants who, at randomisation, were using stop smoking medications obtained from their service (e.g. NRT or varenicline) were encouraged to complete the course regardless of treatment allocation. In this report, we refer to these medications as ‘base medication’, unless they were similar fast-acting nicotine products as those we offered in the nicotine product intervention. ‘Base medication’ is defined as nicotine patches, varenicline or bupropion.
Common to all interventions was the provision of text messages for up to 6 months post quit date. These text messages included reinforcers of milestones, general motivational messages and some general hints. Non-S3P messages were non-interactive, untailored and of lower frequency than the text messages provided in the S3P condition, which are described in more detail below.
Usual-care arm
Participants received a text messaging programme without any personalisation or reference to the specific strategies focused on in the S3P intervention. There was a maximum of 33 messages sent over 6 months and participants could request to stop the messages at any time. The usual-care static text messages were not expected to have a clinically significant relapse prevention effect and, therefore, this arm is considered for the purpose of analysis to have received no intervention. All non-S3P arms received these messages in addition to any post-treatment care provided by stop smoking services that delivered the initial stop smoking interventions. Such interventions include an invitation to contact services if experiencing difficulties or lapses, although these are very rarely acted on and would be present across randomisation groups.
Nicotine product arm
Participants were given a choice of oral nicotine products: Nicorette® 1-mg nicotine mouth spray (Johnson & Johnson, Brunswick, NJ, USA), NiQuitin® 4-mg nicotine minis (Omega Pharma Ltd, London, UK) or branded Nicabate® 4-mg nicotine minis (GlaxoSmithKline Australia Pty Ltd, Melbourne, VIC, Australia). In Australia, additional choice of 1.5-mg nicotine minis was offered as well. A refillable e-cigarette (Innokin®, Endura T18E, Innokin Technology, Shenzen, China) with a choice of menthol- or tobacco-flavoured liquid, containing 11 mg/ml of nicotine, was also offered. Participants chose one product to use as a coping strategy if they found themselves at risk of relapse. The products were mailed to participants along with instructions on their use.
The initial supply comprised an e-cigarette starter kit (including the refillable device, a USB charger, a spare battery, a pack of five coils and four 10-ml bottles of e-liquid, with a total cost of £32.75), two bottles of nicotine mouth spray (with a total cost of £17.95) or six tubs of minis (with a total cost of £19.58).
In Australia, nicotine e-liquid is available only on prescription, therefore, if a participant requested an e-cigarette, a local physician was asked by the study team to provide a prescription before the starter kit was sent to the participant.
Participants were offered further supply via an e-mail/text approximately 4 weeks later. Those taking up the offer received four bottles of e-liquid, two bottles of mouth spray or six tubs of minis. Participants were able to switch to a different product for their second supply.
In England, participants who enquired about further supplies were asked to buy the products themselves. To enable extended e-cigarette use in Australia, a note was sent with the second supply enabling participants to purchase further e-liquid and coils at a website managed by the study team via appropriate prescriptions.
The participant received a telephone call approximately 1 week after randomisation to check product receipt and to discuss product use as a strategy for coping with present or anticipated temptations to smoke.
Participants also received static text messages as per the usual-care study arm (see Usual-care arm).
S3P arm
Participants were asked to complete a web-based assessment [QuitCoach, www.quitcoach.org.au (accessed September 2020)], which generates a three- or four-page letter of personalised advice. The advice includes a list of priority activities, with the prioritisation based on assessment responses. The letter could be viewed on screen in HTML or PDF format and printed if desired. It could be retrieved from the study website for later reference until such time as a new assessment was commenced. New assessments were not mandatory. Participants received reminder e-mails, but could complete the assessments at any desired time by returning to the study website. The resultant advice would then reflect their current situation and relevant progress since their previous assessment. In addition, participants could use a structured tool (Problem Planner) for generating ‘if–then’ statements (i.e. implementation intentions17). Such self-generated statements link problematic situations (e.g. after dinner) with desired behavioural responses (e.g. staying inside and playing with the kids) and are designed to ensure that the appropriate response is triggered whenever the problematic situation is encountered. The Problem Planner could also be modified as frequently as desired.
The tailored advice contained separate strategies for those using and those not using a nicotine product, and provided general advice about countering more stable residual beliefs about the value of smoking. In addition, the advice provided suggestions for monitoring the ongoing benefits of having quit smoking and taking appropriate rewards for reaching milestones.
The web-based intervention was augmented by a series of interactive text messages [QuitTxt, www.quitcoach.org.au/QuitTextInformation.aspx (accessed September 2020)] that were provided for up to 6 months post quit date. These text messages further encouraged use of ‘if–then’ statements, provided motivational messages and gave some more generic advice. Unlike the non-S3P text messages, these text messages were tailored to baseline survey responses and to responses from the most recent QuitCoach assessment, particularly to measures of difficulty staying abstinent (e.g. evidence of a recent slip-up or frequent ongoing temptations to smoke). This primarily determined whether participants received the standard stream of 55 messages or an augmented stream of 72 messages. In addition, there was the potential to send two special streams of messages:
-
The ‘OFFMEDS’ stream was a timed or user-triggered module designed to be sent either when people reported going off their base medication (i.e. bupropion, varenicline or nicotine patches) or at around the time they indicated at baseline they would do so. Going off base medication was reported via either a QuitCoach assessment or an incoming short message service command (‘OFFMEDS’). The OFFMEDS stream consisted of 17 messages and ran concurrently with the underlying standard or augmented stream.
-
A ‘RELAPSE’ stream was sent immediately on notification of a relapse, following either an incoming ‘RELAPSE’ command or a QuitCoach assessment. The aim of this stream (12 messages) was to encourage immediate recommitment to the quit attempt. Once completed, the underlying messages resumed with the augmented stream.
The text messaging program was able to respond to a variety of requests for additional help from the user. The commands were listed on a one-page PDF instruction form that was e-mailed to the user immediately following randomisation. In addition to ‘OFFMEDS’ and ‘RELAPSE’, these took the form of ‘emergency help’ messages in response to commands such as ‘SOCIAL’ or ‘STRESS’. These were designed to provide help in different forms of tempting situations via an immediate message suggesting a strategy and a follow-up message 30-minutes later to encourage reflection. Any participant-requested additional messages were on top of the above frequencies.
Two interventions arm
Participants received both interventions described above (i.e. the S3P and a nicotine product). The S3P advice and text messages were modified to include references to nicotine products as a relapse prevention strategy.
Follow-up
Follow-up was originally planned for 3, 6 and 12 months post quit date to record smoking status and other measures (see Measures); however, because of the trial curtailment the 12-month follow-up was not completed. Participants had the option to complete the follow-ups over the telephone or online. Participants in England received £10 for completing each of these questionnaires and participants in Australia received AU$20. The final follow-ups took place between March 2018 and July 2019.
Measures
At baseline we collected the following:
-
demographic details, smoking characteristics (e.g. the Heaviness of Smoking Index from when they were smoking),18 previous quit attempts, previous use of stop smoking medications and e-cigarettes, and medical history (e.g. screening for depression, measures of perceived stress and affect)
-
information regarding the current quit attempt (e.g. type of support/medicines used), frequency and strength of cravings, extent of slip-ups (if any), plans on how long to continue use of base medication, self-efficacy for maintenance, perceived challenges and number of smokers in social network
-
quality-of-life data [measured by the EuroQol-5 Dimensions (EQ-5D)]19
-
health Service Use Questionnaire (HSUQ) data.
At all follow-up contacts we collected the following:
-
self-reported smoking status and cigarette consumption
-
lapse/relapse details for those relapsing (including number of lapses, when, where, reasons for relapse, how many cigarettes at first lapse, how soon after first cigarette was full relapse)
-
strategies used by the participant to prevent relapse
-
details regarding cravings to smoke
-
details regarding use of any, including non-allocated, smoking cessation/relapse prevention treatments
-
details regarding use of allocated interventions and ratings of the allocated interventions, including helpfulness (3-month follow-up only)
-
details of any adverse events (AEs).
We also collected data regarding the use of the S3P, including the number of assessments completed, the number of text messages received, which message streams were received, and if participants requested ‘emergency help’ messages or stopped the text messages.
At 12-month follow-up we had planned to readminister the EQ-5D and HSUQ and collect saliva samples for cotinine analysis; however, because of the issues described above, follow-up was terminated at 6 months and so this could not be done. The baseline EQ-5D and HSUQ data were therefore not analysed as there were no accompanying follow-up data.
The curtailed schedule of measurements is shown in Table 1.
| Measure/procedure | Time point: post quit date | |||
|---|---|---|---|---|
| ≈ 4 weeks | 8 weeks | 3 months | 6 months | |
| Demographics | ✓ | |||
| Smoking history | ✓ | |||
| Detail of current quit attempt | ✓ | |||
| Randomisation and post-randomisation telephone call | ✓ | |||
| Smoking status/cigarette consumption | ✓ | ✓ | ✓ | |
| Slip-ups and cravings | ✓ | ✓ | ✓ | |
| Use of non-allocated products/interventions | ✓ | ✓ | ✓ | |
| Use and feedback of S3P (allocated cases only) | ✓ | |||
| Use of allocated nicotine products (allocated cases only) | ✓ | ✓ | ✓ | |
| Ratings of interventions | ✓ | ✓ | ||
| EMA (substudy participants only; see Substudies) | ✓ | ✓ | ||
| Qualitative interviews (substudy participants only; see Substudies) | ✓ | ✓ | ||
| HSUQ | ✓ | |||
| Quality-of-life measures | ✓ | |||
| AEs | ✓ | ✓ | ||
Adverse events and serious adverse events
Participants were asked in the follow-up surveys about any AEs and serious adverse events (SAEs) experienced. In those allocated to the nicotine product arms [i.e. nicotine product study (NIC) arm and NIC + S3P arm], the following AEs were deemed to be related and expected in the study protocol: nausea, throat/mouth irritation and sleep disturbance.
Substudies
Qualitative substudy
The original plan was to recruit a subsample of participants at each follow-up for qualitative interviews (n = 160 in total, split equally between the two countries and four arms) using quota sampling to include participants who had lapsed/relapsed/maintained abstinence. Owing to the issues described earlier, only 94 participants were recruited at the 3- and 6-month follow-ups (see Figure 2). Therefore, a far greater proportion of the total sample than originally planned also participated in the qualitative substudy.
The qualitative study was conducted and reported in accordance with COnsolidated criteria for REporting Qualitative research criteria. 20 Data on the feasibility, acceptability, use and perceived impact of study interventions were collected from abstainers, lapsers and relapsers in the four trial arms from both countries. A topic guide (see Appendix 2) was developed by the research team and the non-trial-related questions about relapse and relapse prevention were piloted in advance with two people who were former smokers and not connected to the study. The topic guide explored how lapses influence relapse, triggers and context of lapses and relapses, barriers to and facilitators of maintaining abstinence, and views in England and Australia on cessation and relapse prevention support.
Trial participants were advised on recruitment into the main study that they might be invited into substudies. Participants were asked at each follow-up whether or not they were willing to participate in the qualitative study. Those who agreed were grouped into relapsers (defined as smoking on at least 7 consecutive days since recruitment into the main study), lapsers (defined as those reporting any lapses, even a puff of a cigarette, but not relapsed) and complete abstainers. Within these categories, participants were selected based on filtering characteristics (e.g. age and gender distributions, arm of trial and outcome status) and invited by e-mail, text and telephone to a telephone interview with a postdoctoral research associate (CE) who is trained in qualitative research methods. Those who agreed to take part gave verbal informed consent and a convenient time to conduct the interview was agreed. The interviews took approximately 30 minutes and were conducted by telephone using Skype™ (Microsoft Corporation, Redmond, WA, USA). The researcher introduced herself and explained that she was seeking to learn more about the participant’s experience of taking part in the relapse prevention trial and the process of relapse. The researcher was open with participants about her status as an ex-smoker if asked. Written field notes were taken throughout the interviews to clarify unanswered and unclear responses at the end of the interview, and also to help with contextualisation during analysis. The researcher had no prior contact with participants and no counselling was provided during the interviews. Interviews were recorded and transcribed. Participants received £20 (or AU$40). Those who participated after the 3-month survey (or refused to participate) were not subsequently asked to participate at 6 months.
Ecological momentary assessment substudy
We planned to recruit a subset of 50 participants from each arm (i.e. 200 participants in total, split between the two countries) to take part in 3 weeks of ecological momentary assessment (EMA) monitoring,21 which included detailed monitoring of the use and relationship to cravings and slip-ups of the two interventions, using a handheld electronic diary. However, because of the issues described in Changes to trial design/protocol (trial curtailment), only 79 participants were recruited (37 participants in England and 42 participants in Australia).
The monitoring took place immediately following the cessation of base medication, when applicable. (For most participants this occurred approximately 4–8 weeks after randomisation and 8–12 weeks post quit date.) During monitoring, participants were asked to log every time they used a study nicotine product (if allocated to use), any lapses that occurred and to respond to randomly scheduled prompts (four or five per day). In addition, participants were asked to complete a daily morning and evening report.
The EMA device administered multiple types of questions across various assessments. The assessments included baseline data, logging of cravings and/or slip-up cigarettes, detailed questions about a subsample of these situations, and daily reports of mood and overall coping. The detailed questions included an assessment of the participant’s current state (e.g. mood, withdrawal severity, craving, etc.), as well as contextual and situational details (e.g. where the participant is, who they are with, what they are doing, etc.), the trigger of the event (e.g. bad mood, smoking cues, etc.) and the use of any behavioural coping strategies during the event. To avoid overburdening participants with assessments, only a subset of reported events were sampled for full assessment. The device logged the time and date of events.
Participants were invited to participate in the EMA study during the 1 week post randomisation call for the main study. At 4–8 weeks post randomisation, those who had expressed an interest were consented and trained on EMA procedures via telephone. The participants were mailed the device with an instruction booklet.
Participants were contacted during the first 3 days of EMA monitoring to ensure that they understood, and were following procedures and received further EMA training, as necessary. At the end of EMA monitoring, the devices were posted back in pre-paid envelopes. Participants received £60 (or AU$120) for completing the study.
Data management
Data collection and entry
Non-identifiable participant data were collected using the server on which the S3P intervention was run (for baseline data) and REDCap (for screening and follow-up data). All data were kept in accordance with good clinical practice and data protection requirements.
Data quality
The English site checked completed electronic surveys on a weekly basis for anomalies and raised/resolved queries with the participant concerned. Once data collection was complete and data were cleaned, the English and Australian data sets were merged.
Sample size
In our original sample size calculation, we expected that 70% of participants would relapse by 12 months in usual care, that each relapse prevention intervention would reduce the rate to 58% and that the combination of the two interventions would result in a further reduced 48% relapse rate. Assuming no interaction and comparisons between those who received (two arms) and did not receive (two arms) each intervention individually, 257 participants were needed per arm to detect this difference (90% power, alpha = 0.025, two-sided). We aimed to recruit 300 participants in each arm, with an additional 50 participants per arm for the EMA study. However, with our reduced sample size of 234, we used an alternative prespecified approach that compared the number of interventions [i.e. none (usual care), one (S3P or NIC), or two (NIC + S3P)]. Using a one-tailed alpha of 0.05, the sample size afforded 78% power to detect the differences in relapse rates, as estimated above, while avoiding multiple testing. This approach allows for utilising the information that the trial generated, despite the limited power of the reduced sample, so that study data can contribute to any future meta-analyses.
Randomisation
Participants were randomised and stratified by country in permuted blocks of random size, automatically via pre-programed lists generated by the study statistician using Stata® version 15 (StataCorp LP, College Station, TX, USA). The randomisation list was programed into the server, on which the baseline survey was run by the study programer who had no involvement in the recruitment of participants. At the end of the baseline survey, the next unused entry on the list was selected and the participant was randomised accordingly. Subsequent on-screen prompts and questions were specific to each randomised condition.
Treatment blinding
Researchers and participants were blind to allocation until the point of randomisation. Researchers conducting follow-up calls were not informed of the participants’ treatment allocation in their call lists, and questions establishing relapse/abstinence outcomes were asked before condition-specific questions that could reveal the allocation. The trial statistician did not see the trial data (apart from recruitment updates) until data lock took place and remained blinded to participant allocation until analysis was complete.
Statistical methods
Main study
Changes from planned analysis
The planned analysis was changed because of trial curtailment. [See NIHR Journals Library, www.journalslibrary.nihr.ac.uk/programmes/hta/1315505/#/ (accessed September 2020), for the statistical analysis plan, which includes details of the planned and curtailed trial analyses.] The revised analyses were all prespecified and agreed by the independent Data Monitoring and Ethics Committee (DMEC) prior to data download and analysis.
General analysis principles
The planned and actual main analysis for each outcome used the intention-to-treat principle, meaning that all randomised participants were included in the analysis in the treatment group to which they were randomised. Participants with missing abstinence outcomes were considered to be smoking (i.e. relapsed) as per the Russell Standard. 22
In the curtailed study, the key study outcomes were analysed using a logistic regression where relapse status is regressed onto an ordinal predictor that codes the number of active interventions assigned (i.e. none, one or two), adjusted for the stratifier (i.e. country). A one-sided p-value was used for the analysis of the primary outcome and the significance level was set at 5%.
Withdrawn participants
Participants who did not wish to be followed up were withdrawn from the study. Unless participants requested otherwise, data collected up to the point of their withdrawal were used in the study analysis and the participants were assumed to be smoking at later follow-ups.
Qualitative substudy
Transcribed interviews were indexed and imported into NVivo 12 Pro (QSR International, Warrington, UK) for systematic analysis. The initial coding frame was based on the interview topic guides and new codes were added as they emerged from the data during the coding process. Coded data were then analysed using the ‘framework’ method. This involves examining key themes from the interviews organised through ‘charting’ (see Appendix 3 for an example). This allowed for investigation of participants’ views on relapse prevention interventions by treatment group. Interviewing stopped once a sense of thematic exhaustion and variability across the framework had accrued. What has been referred to as ‘theoretical saturation’23 occurred in the present study when significantly novel information relevant to the progression of thematic development and theorising ceased to emerge from the interview transcripts. 24,25 To enhance the validity of qualitative findings, two researchers were involved in all data analysis (CE and AM), and preliminary analyses were presented to the then UK Centre for Tobacco & Alcohol Studies (Nottingham, UK) Tobacco and Nicotine Group (current smokers or recent ex-smokers) for feedback.
Ecological momentary assessment substudy
The EMA substudy was designed to provide data on the use of interventions and relationship to cravings and lapses for participants in the usual-care and intervention arms at around 8–12 weeks post quit date. Although the reduced sample size did not allow for some of our originally planned analyses, we could conduct some exploratory analyses. Specifically, to explore the hypothetical mechanisms through which treatments can prevent relapse, we used the EMA data to examine group differences in the correlates of lapse episodes and the immediate consequences of lapses, including self-efficacy and use of coping strategies. As each participant could contribute multiple lapse episodes, multilevel models were used to account for autocorrelation. This analysis plan is based on similar work that explores differences in lapse episodes between active and placebo patches. 26
To be eligible for this analysis, participants needed to report at least one lapse in real time during monitoring. In total, 54 participants (i.e. 68.4% of all the EMA substudy participants) reported at least one lapse event in real time and were eligible for inclusion (usual care, n = 16; S3P, n = 10; NIC, n = 15; NIC + S3P, n = 13).
Chapter 3 Outcomes
Original primary outcome
The original primary outcome was relapse rate at 12 months post quit date. Relapse was defined as self-report of smoking on at least 7 consecutive days reported at any follow-up time point, or any cigarettes smoked (even just a puff) in the last month, biochemically validated.
Original secondary outcomes
Original secondary outcomes were sustained abstinence using different criteria to the primary outcome and different assumptions about missing cases; point prevalence and shorter-term period prevalence outcomes; sustained reduction in cigarette consumption; evaluations of likely mechanisms of effect, focusing on strategies that were encouraged and participant perceptions of effect (e.g. participant ratings and data from EMA/qualitative substudies); dose–response effects (testing whether or not the dose of the interventions, or extent of compliance, is associated with relapse); cost-effectiveness of the different strategies; the effects of intervention components (e.g. on relapse rates, participant ratings, etc.) by country and on people from different socioeconomic groups, ethnic groups, gender and prior smoking habits (including those who stopped smoking using different forms of medication); and rates of AEs/SAEs reported in people who use a study nicotine product compared with those who do not, and by type of product used.
Owing to the issues described in Changes to trial design/protocol (trial curtailment), the study outcomes were curtailed. All revised outcomes were prespecified and agreed by the independent DMEC prior to data download and analysis.
Curtailed primary outcome
The curtailed primary outcome was relapse rate at 6 months post quit date. Relapse was defined as self-report of smoking on at least 7 consecutive days reported at either follow-up time point, or any cigarettes smoked (even just a puff) in the last month at 6 months. Participants lost to follow-up were included as smokers.
Sensitivity analyses for primary outcome
To assess the robustness of the results, a series of sensitivity analyses were conducted, for example multiple imputation by chained equation27 and complete-case analysis (where we excluded cases with missing outcomes). To build the multiple imputation model, we explored differences in baseline measures between participants with complete and missing outcome measures.
Per-protocol analysis was conducted to exclude those participants who never accessed/used their allocated intervention. Initiation was coded as having tried the allocated product in the NIC and NIC + S3P arms, having read at least one text for the usual-care arm, and having completed at least one assessment for the S3P and NIC + S3P arms. Missing data were counted as not initiated treatment.
Curtailed secondary outcomes
The curtailed secondary outcomes were as follows:
-
abstinence from smoking using alternative definitions: sustained abstinence (Russell Standard, defined as self-report of not smoking more than five cigarettes since 2 weeks post quit date) and point prevalence abstinence at 3 and 6 months [defined as self-report of no smoking (not even a puff) in the past 7 days]
-
adherence to and ratings of the interventions by participants, and coping strategies used
-
characteristics of and reactions to lapses across the four groups explored in the EMA/qualitative substudies
-
rates of AEs/SAEs reported in people who use a study nicotine product compared with those who do not.
Statistical software
All analyses were carried out using Stata software.
Public and patient involvement
Members of the UK Centre for Tobacco & Alcohol Studies Public Engagement Panel and a member of the New Nicotine Alliance charity (London, UK) provided feedback on the study design and participant documents, and helped to inform the decision about which e-cigarette products to use. The Trial Steering Committee (TSC) included members of the public and contributed to decisions made regarding trial progress and curtailment.
Trial committees
In England, a TSC and DMEC were convened every 6–12 months. A Trial Management Group also met at regular intervals throughout the study. Appendix 1, Table 27, lists the trial committee members.
Quality control and quality assurance
In England, a risk assessment was carried out in conjunction with the study sponsor and Barts Clinical Trials Unit (CTU), now King’s CTU, which was used as a basis for the study monitoring plan. During the recruitment phase, a monitor from the co-ordinating site carried out 6-monthly monitoring at the English site. The Barts CTU was responsible for oversight of the monitoring process and overall audit of the trial.
Approvals
The study was sponsored by the Queen Mary University of London Joint Management Research Office, London, UK, and the Cancer Council Victoria Melbourne, VIC, Australia.
Ethics approval was obtained from the National Research Ethics Service Committee London – Camden and Islington on 15 November 2016 (reference 16/LO/1771) in England, and the Cancer Council Victoria Human Research Ethics Committee (HREC) (project number HREC 1606) on 15 August 2016 and the St Vincent’s Hospital Melbourne HREC (reference number HREC 092/18) on 6 August 2018 in Australia.
In Australia, the study was also notified to the Therapeutic Goods Administration (Clinical Trial Notification number CT-2016-CTN-02901-1).
Chapter 4 Results
Throughout this chapter, characteristics and descriptive statistics are shown according to the four original study arms, but the analysis of relapse and abstinence is presented in three groups (i.e. no intervention, one intervention or two interventions) as per the prespecified analysis plan for the curtailed trial.
Participant flow
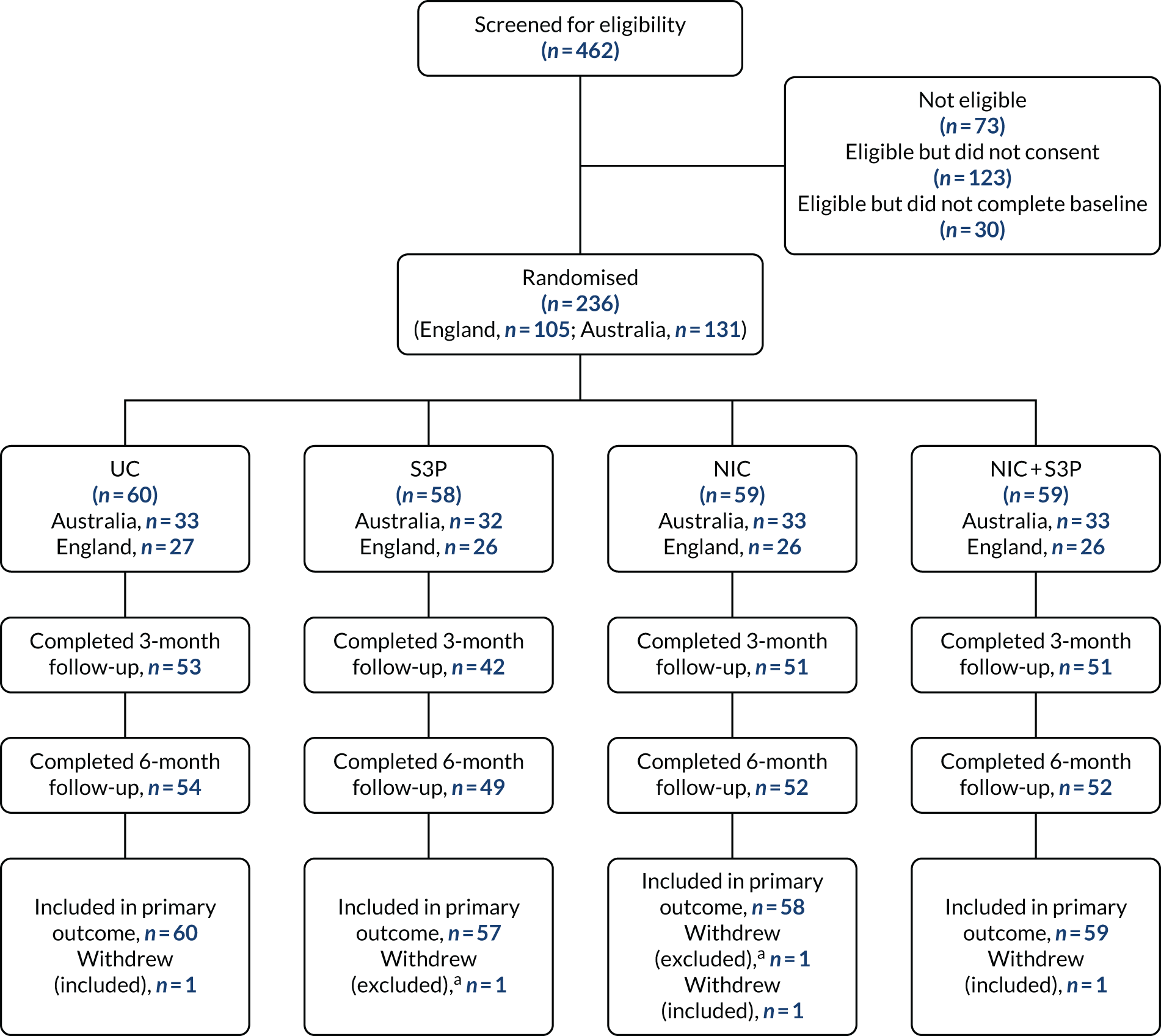
Figure 1 shows the flow of participants through the trial.
FIGURE 1.
Main study flow diagram: relapse prevention trial. a, Participant did not want their data to be used in the study.
Sample characteristics
Table 2 shows participant characteristics. The sample comprised largely middle-aged smokers classified as ‘medium’ on the Heaviness of Smoking Index. Less than half were in full employment and 28% reported a history of mental illness.
| Baseline characteristic | Study arm | |||
|---|---|---|---|---|
| Usual care (N = 60) | NIC (N = 58) | S3P (N = 57) | NIC + S3P (N = 59) | |
| Age (years) (n = 234a), median (IQR) | 44 (34.5–55.5) | 46.5 (37–57) | 44 (35–56) | 43 (30–56) |
| Female, n (%) | 31 (51.7) | 26 (44.8) | 23 (40.3) | 32 (54.2) |
| Partner smokes: yes, n (%) | 7 (11.7) | 3 (5.2) | 8 (14.0) | 11 (18.6) |
| Mental health condition: yes,b n (%) | 23 (38.3) | 16 (27.6) | 14 (24.6) | 12 (20.3) |
| In full-time employment, n (%) | 24 (40.0) | 20 (34.5) | 24 (42.1) | 25 (42.4) |
| Receiving benefits, n (%) | 34 (56.7) | 32 (55.2) | 34 (59.7) | 29 (49.2) |
| Heaviness of Smoking Index, n (%) | ||||
| Low | 7 (11.7) | 8 (13.8) | 9 (15.8) | 7 (11.9) |
| Medium | 42 (70.0) | 44 (75.9) | 34 (59.7) | 43 (72.9) |
| High | 11 (18.3) | 6 (10.3) | 14 (24.6) | 9 (15.3) |
| Using base medication, n (%) | 31 (51.7) | 24 (41.4) | 26 (45.6) | 34 (57.6) |
| Ethnicity, n (%) | ||||
| Australian born (non-aboriginal) | 27 (81.8) | 24 (72.7) | 26 (81.3) | 27 (81.8) |
| White British | 21 (77.8) | 22 (88.0) | 19 (76.0) | 22 (84.6) |
| Country, n (%) | ||||
| Australia (n = 131) | 33 (55.0) | 33 (56.9) | 32 (56.1) | 33 (55.9) |
| England (n = 103) | 27 (45.0) | 25 (43.1) | 25 (43.9) | 26 (44.1) |
At the time of recruitment, 49.1% of all participants reported using a base medication (69.9% and 32.8% of participants in England and Australia, respectively).
Overall, the follow-up rate was 88.5% at 6 months. This rate was similar in Australia (87.8%) and England (89.3%). Follow-up rates were also similar across arms (usual care, 90%; NIC, 90%; S3P, 86%; NIC + S3P, 88%).
Table 3 shows the missing data across primary and secondary outcomes, and Table 4 provides data on the differences in baseline characteristics between participants who provided primary outcome data and those who did not.
| Outcome | Study arm, n (%) | |||
|---|---|---|---|---|
| Usual care (N = 60) | NIC (N = 58) | S3P (N = 57) | NIC + S3P (N = 59) | |
| Relapse at 6 months post quit date | 6 (10) | 6 (10.3) | 8 (14.0) | 7 (11.9) |
| Point prevalence abstinence at 3 months post quit date | 7 (11.7) | 7 (12.1) | 16 (28.1) | 8 (13.6) |
| Point prevalence abstinence at 6 months post quit date | 6 (10.0) | 6 (10.3) | 8 (14.0) | 7 (11.9) |
| Sustained abstinence at 6 months post quit date | 6 (10.0) | 6 (10.3) | 8 (14.0) | 7 (11.9) |
| Baseline characteristic | Data | |
|---|---|---|
| Complete (N = 207) | Missing (N = 27) | |
| Age (years), na (median) [IQR] | 199 (46) [33–57] | 25 (39) [33–46] |
| Gender, n (%) | ||
| Female | 103 (92.0) | 9 (8.0) |
| Male | 104 (85.3) | 18 (14.8) |
| Partner smokes, n (%) | ||
| Yes | 27 (93.1) | 2 (6.9) |
| No | 96 (85.7) | 16 (14.3) |
| No spouse/partner | 84 (90.3) | 9 (9.7) |
| Mental health condition, n (%)b | ||
| Yes | 62 (95.4) | 3 (4.6) |
| No | 139 (86.3) | 22 (13.7) |
| Prefer not to answer | 6 (75.0) | 2 (25.0) |
| Employment status, n (%) | ||
| Full time | 79 (85.0) | 14 (15.0) |
| Part time | 32 (86.5) | 5 (13.5) |
| Neither | 96 (92.3) | 8 (7.7) |
| Receiving benefits, n (%) | ||
| Yes | 115 (89.2) | 14 (10.9) |
| No | 92 (87.6) | 13 (12.4) |
| Heaviness of Smoking Index, n (%) | ||
| Low | 28 (90.3) | 3 (9.7) |
| Medium | 144 (88.3) | 10 (11.7) |
| High | 35 (87.5) | 5 (12.5) |
| Using base medication, n (%) | ||
| Yes | 99 (86.1) | 16 (13.9) |
| No | 108 (90.8) | 11 (9.2) |
| Ethnicity: Australia, n (%) | n = 115a | n = 16a |
| Australian born (non-aboriginal) | 90 (86.5) | 14 (13.5) |
| Other | 25 (92.6) | 2 (7.4) |
| Ethnicity: England, n (%) | n = 92a | n = 11a |
| White | 74 (88.1) | 10 (11.9) |
| Other | 18 (94.7) | 1 (5.3) |
| Country, n (%) | ||
| Australia | 115 (87.8) | 16 (12.2) |
| England | 92 (89.3) | 11 (10.7) |
Relapse rates
Relapse to smoking was somewhat higher in usual care than in the individual or combined interventions, but the combined intervention did not show any higher efficacy compared with single interventions. The overall difference was not significant (p = 0.11) (Table 5).
| Study arm | n (%) relapsed |
|---|---|
| Usual care (no intervention) | 36 (60.0) |
| NIC or S3P (one intervention) | 50 (43.5) |
| NIC | 26 (44.8) |
| S3P | 24 (42.1) |
| NIC + S3P (two interventions) | 29 (49.2) |
The results of the sensitivity analyses of the primary outcome tallied with the main analyses (Table 6).
| Type of analysis | n | Likelihood of relapse for each additional intervention received, RR (95% CI) |
|---|---|---|
| Primary analysis | 234 | 0.88 (0.73 to 1.07) |
| Complete cases | 207 | 0.84 (0.67 to 1.06) |
| Multiple imputationa | 234 | 0.87 (0.69 to 1.1) |
| Participants who initiated treatmentb | 206 | 0.83 (0.65 to 1.07) |
To provide more descriptive information, we looked at the relative risks for reducing relapse in each of the arms using the usual-care arm as the reference. These were 0.75 (95% CI 0.53 to 1.06), 0.70 (95% CI 0.48 to 1.00) and 0.83 (95% CI 0.60 to 1.14) for the NIC, S3P and NIC + S3P arms, respectively.
Sustained and 7-day point prevalence abstinence rates
Table 7 shows sustained abstinence rates, as defined by the Russell Standard (i.e. allows up to five lapses), and point prevalence abstinence rates (i.e. no smoking over the past 7 days). There were no significant differences between the study arms.
| Outcome | Study arm, n (%) [95% CI] | Likelihood of abstinence for each additional intervention received, RR (95% CI) | ||
|---|---|---|---|---|
| No intervention (usual care) (n = 60) | One intervention (NIC or S3P) (NIC, n = 58; S3P, n = 57; N = 115) | Two interventions (NIC + S3P) (n = 59) | ||
| 7-day abstinence at 3 months | 37 (61.7) [49–73] | 77 (67.0) [60–75] | 38 (64.4) [52 to 76] | 1.03 (0.91 to 1.17) |
| NIC: 42 (72.4) [60 to 82] | ||||
| S3P: 35 (61.4) [48–73] | ||||
| 7-day abstinence at 6 months | 36 (60.0) [47–71] | 75 (65.2) [56–73] | 36 (61.0) [48 to 72] | 1.01 (0.89 to 1.16) |
| NIC: 35 (60.3) [48 to 72] | ||||
| S3P: 40 (70.2) [57 to 81] | ||||
| Sustained abstinence at 6 months | 25 (41.7) [30–54] | 63 (54.8) [46 to 64] | 30 (50.9) [38 to 63] | 1.09 (0.92 to 1.29) |
| NIC: 31 (53.5) [41 to 66] | ||||
| S3P: 32 (56.1) [43–68] | ||||
Treatment adherence and use of allocated treatments
Nicotine product selection by arm and by country is shown in Tables 8 and 9, respectively. More participants chose e-cigarette than NRT (63.4% vs. 36.6%) [chi-square(1) = 8.04; p = 0.005].
| Product chosena | Study arm, n (%) | Total (N = 112), n (%) | |
|---|---|---|---|
| NIC (N = 55) | NIC + S3P (N = 57) | ||
| E-cigarette | 31 (56.4) | 40 (70.2) | 71 (63.4) |
| NRT | 24 (43.6) | 17 (29.8) | 41 (36.6) |
| Product chosena | Country, n (%) | |
|---|---|---|
| Australia (N = 62) | England (N = 50) | |
| E-cigarette | 44 (71.0) | 27 (54.0) |
| NRT | 18 (29.0) | 23 (46.0) |
In Australia, 71.0% of participants chose e-cigarettes and 29.0% chose NRT [χ2(1) = 10.9; p = 0.001]. In England, there was no significant difference in the number of participants choosing e-cigarette and the number of participants choosing NRT (54.0% vs. 46.0%) [χ2(1) = 0.3; p = 0.57)].
Of those who chose NRT, nicotine minis and mouth spray were selected with similar frequency within NIC arms and countries.
Table 10 shows the use of and adherence to the study nicotine products. At 3 months, > 50% of participants in the NIC and NIC + S3P study arms reported using their product at least occasionally. At 6 months, this applied to 40% of participants in these arms. Among participants who chose e-cigarette, 26.8% were using them daily at 6 months, whereas 17.1% of participants who used NRTs were using it daily at 6 months.
| Measure of use/adherence | Study arm | Total (N = 117) | |
|---|---|---|---|
| NIC (N = 58) | NIC + S3P (N = 59) | ||
| NIC product use at 3 months post quit, n (%)a,b | |||
| Never used | 4 (6.9) | 7 (11.9) | 11 (9.4) |
| Triedc | 9 (15.5) | 7 (11.9) | 16 (13.7) |
| Used but now stoppedd | 7 (12.1) | 4 (6.8) | 11 (9.4) |
| Using some days | 10 (17.2) | 16 (27.1) | 26 (22.2) |
| Using daily | 20 (34.5) | 16 (27.1) | 36 (30.8) |
| NIC product at 6 months post quit, n (%)a,b | |||
| Not using | 30 (51.7) | 27 (45.8) | 57 (48.7) |
| Using some days | 5 (8.6) | 11 (18.6) | 16 (13.7) |
| Using daily | 16 (27.6) | 11 (18.6) | 27 (23.1) |
| Using, frequency unknown | 1 (1.7) | 3 (5.1) | 4 (3.4) |
| Daily use at 6 months by product, n/N (%)b,e | |||
| E-cigarette | 10/31 (32.3) | 9/40 (22.5) | 19/71 (26.8) |
| NRT | 5/24 (20.8) | 2/17 (11.8) | 7/41 (17.1) |
Most participants (n = 66, 58.9%) requested and were sent a second supply of nicotine product.
With regard to continued use of any oral nicotine products in the non-NIC arms, at 6 months the proportions were 16 participants (26.7%) and 13 participants (22.8%) in the usual-care and S3P arms, respectively. In the usual-care arm, 15 (25.0%) participants were using oral NRT and 2 (3.3%) participants were using e-cigarettes (one participant was using both oral NRT and e-cigarette). In the S3P arm, 12 (21.1%) participants were using oral NRT and 1 (1.8%) participant was using e-cigarettes.
Table 11 shows the use of and adherence to the S3P. Most participants allocated to the S3P intervention completed one QuitCoach assessment only.
| Measure of use/adherence | Study arm, n (%) | |
|---|---|---|
| S3P (N = 57) | NIC + S3P (N = 59) | |
| Number of QuitCoach assessments completed | ||
| Zero | 7 (12.3) | 9 (15.3) |
| One | 35 (61.4) | 37 (62.7) |
| Two | 9 (15.8) | 7 (11.9) |
| Three | 4 (7.0) | 3 (5.1) |
| Five | 1 (1.8) | 3 (5.1) |
| Six | 1 (1.8) | 0 (0) |
| Did you read the QuitCoach advice?a | ||
| Yes, I studied it carefully | 12 (21.1) | 19 (32.2) |
| Yes, but only quickly | 19 (33.3) | 26 (44.1) |
| No | 8 (14.0) | 6 (10.2) |
| Did you use the Problem Planner?a | ||
| Yes, quite a lot | 0 (0) | 1 (1.7) |
| Yes, a bit | 14 (24.6) | 22 (37.3) |
| No | 17 (29.8) | 23 (39.0) |
At 3 months, most responders in the S3P arms reported having read the advice generated by the intervention, with the majority responding ‘yes, but only quickly’. Only one-third of participants in the S3P arms reported having used the Problem Planner (see Table 11).
Table 12 shows the use of the text messages. Most responders reported reading all/most of the text messages sent. Roughly the same small proportion of participants (12% maximum) requested to stop the text messages across the study arms, although this was least in the NIC arm (5.2%).
| Measure of use/adherence | Study arm, n (%) | |||
|---|---|---|---|---|
| Usual care (N = 60) | NIC (N = 58) | S3P (N = 57) | NIC + S3P (N = 59) | |
| Read most/all text messages | 35 (58.3) | 38 (65.5) | 28 (49.1) | 36 (61.0) |
| Requested to stop text messages | 6 (10.0) | 3 (5.2) | 6 (10.5) | 7 (11.9) |
A feature of the S3P arms was the interactive element of the text messages. Only one participant (in the S3P arm) used the interactive text message commands, sending the following: ‘STRESS’ (four times), ‘SOCIAL’ (twice), ‘MISSING SMOKING’ (twice) and ‘TEMPTATION’ (twice).
Ratings of the S3P intervention
Participant ratings of the S3P intervention at 3 and 6 months are shown in Tables 13 and 14.
| QuitCoach rating | Study arm, n (%) | |
|---|---|---|
| S3P | NIC + S3P | |
| 3 months post quit date | 28 | 43 |
| Very useful | 5 (17.9) | 10 (23.3) |
| Somewhat useful | 10 (35.7) | 19 (44.2) |
| Neither | 9 (32.1) | 11 (25.6) |
| Somewhat useless | 4 (14.3) | 3 (7.0) |
| Very useless | 0 (0) | 0 (0) |
| 6 months post quit date | 48 | 52 |
| Very useful | 15 (31.3) | 12 (23.1) |
| Somewhat useful | 12 (25.0) | 24 (46.2) |
| Neither | 13 (27.1) | 11 (21.2) |
| Somewhat useless | 3 (6.3) | 1 (1.9) |
| Very useless | 5 (10.4) | 4 (7.7) |
| Response to question | Study arm, n (%) | |
|---|---|---|
| S3P (N = 28) | NIC + S3P (N = 43) | |
| Yes | 6 (21.4) | 14 (32.6) |
| Not sure | 17 (60.7) | 19 (44.2) |
| No | 5 (17.9) | 10 (23.3) |
At 3 months, the most common rating for the S3P was ‘somewhat useful’, with a median rating of 2 [interquartile range (IQR) 2–3] in both the S3P arm and the NIC + S3P arm. At 6 months, ratings seemed to improve in the S3P arm (median rating 1, IQR 1–3), while remaining the same in the NIC + S3P arm (median rating 2, IQR 2–3). Most participants considered the intervention useful, but only one-quarter of responders said that they would visit QuitCoach again.
Table 15 shows the ratings of the text messages. Despite different contents and intensity of the text messages, and the tailoring and interactive features of messages in the S3P condition, ratings were similar across the four study arms.
| Text message rating | Study arm, n (%) | |||
|---|---|---|---|---|
| Usual care | NIC | S3P | NIC + S3P | |
| 3 months post quit date | 48 | 50 | 38 | 50 |
| Very useful | 13 (27.1) | 17 (34.0) | 11 (29.0) | 15 (30.0) |
| Somewhat useful | 15 (31.3) | 25 (50.0) | 14 (36.8) | 20 (40.0) |
| Neither | 5 (10.4) | 1 (2.0) | 6 (15.8) | 10 (20.0) |
| Somewhat useless | 7 (14.6) | 3 (6.0) | 3 (7.9) | 3 (6.0) |
| Very useless | 8 (16.7) | 4 (8.0) | 4 (10.5) | 2 (4.0) |
| 6 months post quit date | 53 | 52 | 48 | 52 |
| Very useful | 16 (30.2) | 21 (40.4) | 12 (25.0) | 13 (25.0) |
| Somewhat useful | 19 (35.9) | 17 (32.7) | 14 (29.2) | 21 (40.4) |
| Neither | 5 (9.4) | 5 (9.6) | 9 (18.8) | 8 (15.4) |
| Somewhat useless | 6 (11.3) | 4 (7.7) | 4 (8.3) | 3 (5.8) |
| Very useless | 7 (13.2) | 5 (9.6) | 9 (18.8) | 7 (13.5) |
Use of S3P recommended strategies
Behavioural strategies specifically recommended in the S3P intervention were used across all four study arms (Tables 16–18). The S3P arms did not consistently report more use of the recommended strategies than the non-S3P arms.
| Strategy | Study arm, n/N (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Usual care | NIC | S3P | NIC + S3P | |||||
| 3 months | 6 months | 3 months | 6 months | 3 months | 6 months | 3 months | 6 months | |
| Remind myself of reasons for quitting (n = 191, n = 201)a | 46/51 (90.2) | 47/52 (90.4) | 40/50 (80.0) | 47/50 (94.0) | 38/39 (97.4) | 45/48 (93.8) | 43/51 (84.3) | 4/51 5 (88.2) |
| Distracted myself by doing something else (n = 191, n = 201)a | 44/51 (86.3) | 42/52 (80.8) | 38/50 (76.0) | 35/50 (70.0) | 33/39 (84.6) | 39/48 (81.3) | 41/51 (80.4) | 42/51 (82.4) |
| Put in place a plan I had for resisting (n = 191, n = 200)a | 19/51 (37.3) | 18/51 (35.3) | 22/50 (44.0) | 19/50 (38.0) | 10/39 (25.6) | 15/48 (31.3) | 18/51 (35.3) | 20/51 (39.2) |
| Just tried to ignore it (n = 190, n = 200)a | 42/50 (84.0) | 39/51 (76.5) | 44/50 (88.0) | 42/50 (84.0) | 31/39 (79.5) | 43/48 (89.6) | 38/51 (74.5) | 38/51 (74.5) |
| Just waited until the craving went away (n = 190, n = 200)a | 34/50 (68.0) | 33/51 (64.7) | 38/50 (76.0) | 37/50 (74.0) | 28/39 (71.8) | 34/48 (70.8) | 35/51 (68.6) | 34/51 (66.7) |
| Told myself that I am beating my addiction (n = 190, n = 201)a | 31/50 (62.0) | 31/52 (59.6) | 36/50 (72.0) | 34/50 (68.0) | 23/39 (59.0) | 32/48 (66.7) | 34/51 (66.7) | 36/51 (70.6) |
| Some other strategy (n = 191, n = 200)a | 19/51 (37.3) | 12/51 (23.5) | 15/50 (30.0) | 13/50 (26.0) | 8/39 (20.5) | 11/48 (22.9) | 12/51 (23.5) | 13/51 (25.5) |
| Made a list of reasons for quitting at 3 months post quit date | Study arm, n (%) | |||
|---|---|---|---|---|
| Usual care (N = 50) | NIC (N = 50) | S3P (N = 39) | NIC + S3P (N = 51) | |
| Yes, and I remind myself | 15 (30.0) | 17 (34.0) | 15 (39) | 12 (23.5) |
| Yes, but I never look at it | 5 (10.0) | 12 (24.0) | 11 (28.2) | 12 (23.5) |
| No | 30 (60.0) | 21 (42.0) | 13 (33.3) | 27 (52.9) |
| Rewards given | Study arm, n (%) | |||
|---|---|---|---|---|
| Usual care | NIC | S3P | NIC + S3P | |
| 3 months post quit date | 24/50 (48.0) | 32/50 (64.0) | 22/39 (56.4) | 30/51 (58.8) |
| 6 months post quit date | 19/36 (52.8) | 18/30 (60.0) | 16/33 (48.5) | 16/35 (45.7) |
The S3P intervention included a suggestion for participants to make a list of reasons for quitting and advised participants to consult their list when tempted to smoke. Somewhat surprisingly, participants in the usual-care and NIC arms reported doing this to a similar extent as those who received the S3P intervention (see Table 17).
The S3P intervention also encouraged participants to give themselves rewards for achieving milestones at 3 and 6 months. This was also carried out as frequently in non-S3P study arms as in S3P study arms (see Table 18).
Adverse events
At 3 months, the proportion of participants reporting AEs was higher in the usual-care arm than in the intervention arms (25% in the usual-care arm vs. 12.1%, 15.8% and 10.2% for the NIC, S3P and NIC + S3P arms, respectively) (Table 19).
| AE | Study arm, n (%) | Total, n (%) (N = 234) | |||
|---|---|---|---|---|---|
| Usual care (N = 60) | NIC (N = 58) | S3P (N = 57) | NIC + S3P (N = 59) | ||
| Yes to an AEa | 15 (25.0) | 7 (12.1) | 9 (15.8) | 6 (10.2) | 37 (15.8) |
| Yes to a servere AE | 5 (8.3) | 3 (5.2) | 4 (7.0) | 2 (3.4) | 14 (6.0) |
| AE listed, n (%)b | |||||
| Anaemia vitamin B12 deficiency | 1 | 1 | |||
| Anorexia nervosa | 1 | 1 | |||
| Anxiety | 3 | 1 | 4 | ||
| Bursitis | 1 | 1 | |||
| Cough | 2 | 1 | 1 | 4 | |
| Depressed mood | 1 | 1 | |||
| Depression | 1 | 1 | |||
| Diabetes mellitus | 1 | 1 | |||
| Diarrhoea | 1 | 1 | |||
| Disturbance in attention | 1 | 1 | |||
| Dysphagia | 1 | 1 | |||
| Dyspnoea | 4 | 1 | 1 | 6 | |
| Eczema | 1 | 1 | |||
| Epilepsy | 1 | 1 | |||
| Fatigue | 1 | 1 | 2 | ||
| Folate deficiency | 1 | 1 | |||
| Gastritis | 1 | 1 | |||
| Headache | 3 | 3 | |||
| Hernia | 1 | 1 | |||
| Hypermobility syndrome | 1 | 1 | |||
| Immunodeficiency | 1 | 1 | |||
| Influenza | 1 | 1 | |||
| Insomnia | 1 | 2 | 3 | ||
| Lower respiratory tract infection | 1 | 1 | |||
| Mental disorder | 1 | 1 | |||
| Micturition disorder | 1 | 1 | |||
| Mouth ulceration | 1 | 1 | |||
| Multiple sclerosis | 1 | 1 | |||
| Oropharyngeal pain | 1 | 1 | |||
| Palpitations | 1 | 1 | |||
| Paraesthesia | 1 | 1 | |||
| Pertussis | 1 | 1 | |||
| Respiration abnormal | 1 | 1 | |||
| Restlessness | 1 | 1 | |||
| Rhinorrhoea | 1 | 1 | |||
| Sciatica | 1 | 1 | 2 | ||
| Sleep disorder | 1 | 1 | |||
| Weight increased | 1 | 1 | 2 | 2 | 6 |
At 6 months, the numbers of participants reporting AEs were similar across all study arms (Table 20).
| AE | Study arm, n (%) | Total, n (%) (N = 234) | |||
|---|---|---|---|---|---|
| Usual care (N = 60) | NIC (N = 58) | S3P (N = 57) | NIC + S3P (N = 59) | ||
| Yes to an AEa | 12 (20.0) | 11 (19) | 10 (17.5) | 12 (20.3) | 45 (19.2) |
| Yes to a severe AE, n (%) | 3 (5.0) | 1 (1.7) | 2 (3.5) | 3 (5.1) | 14 (3.9) |
| AEs listedb | |||||
| Anaemia vitamin B12 deficiency | 1 | 1 | |||
| Anorexia nervosa | 1 | 1 | |||
| Anxiety | 1 | 1 | 2 | ||
| Atrial fibrillation | 1 | 1 | |||
| Back pain | 1 | 1 | 2 | ||
| Blood cholesterol increase | 1 | 1 | |||
| Blood pressure increase | 1 | 1 | 2 | ||
| Bursitis | 1 | 1 | |||
| Chest discomfort | 1 | 1 | |||
| Cholelithiasis | 1 | 1 | |||
| Chronic obstructive pulmonary disease | 1 | 2 | 1 | 4 | |
| Cough | 2 | 2 | |||
| Depression | 1 | 1 | |||
| Dyspnoea | 2 | 1 | 3 | ||
| Eczema | 1 | 1 | |||
| Endometrial hyperplasia | 1 | 1 | |||
| Epilepsy | 1 | 1 | |||
| Fatigue | 2 | 2 | |||
| Folate deficiency | 1 | 1 | |||
| Frequent bowel movement | 1 | 1 | |||
| Gingivitis | 1 | 1 | |||
| Headache | 1 | 1 | 2 | ||
| Increased umbilical hernia | 1 | 1 | |||
| Injury | 1 | 1 | |||
| Insomnia | 1 | 1 | |||
| Intervertebral disc degeneration | 1 | 1 | |||
| Lower respiratory tract infection | 1 | 1 | |||
| Malaise | 1 | 1 | |||
| Mental disorder | 1 | 1 | |||
| Mood altered | 1 | 1 | |||
| Parkinson’s disease | 1 | 1 | |||
| Renal cyst | 1 | 1 | |||
| Rhinorrhoea | 1 | 1 | 2 | ||
| Sleep apnoea syndrome | 1 | 1 | |||
| Surgery | 1 | 1 | |||
| Thyroid gland disorder | 1 | 1 | |||
| Vitamin D deficiency | 1 | 1 | |||
| von Willebrand disease | 1 | 1 | |||
| Weight increased | 1 | 1 | 2 | 1 | 5 |
Severe AEs were uncommon (reported by 6.0% of participants at 3 months and 3.9% of participants at 6 months) (see Tables 19 and 20).
There were eight SAEs reported across six participants [arm surgery and an incarcerated umbilical hernia in the usual-care arm; anorexia and a car accident in the S3P arm; and gallstones, a mastectomy with breast reconstruction (which later resulting in emergency breast reconstruction removal) and tarsometatarsal fusion in the NIC + S3P arm]. None of the events was deemed related to study procedures.
Participation in the qualitative and ecological momentary assessment substudies
The qualitative and EMA substudies were originally planned to involve only a small subsample of a large trial, with identical sampling across study arms. In the curtailed study, a much larger proportion of the sample took part in qualitative interviews (n = 94, 40%) and about one-third of participants (n = 79) took part in the EMA study. Of these participants, 48 participated in both substudies.
Relapse rates were lower among those participating in the substudies (qualitative substudy, 1.7%; EMA substudy, 10.4%; both substudies, 16.5%) than among those who did not participate (51.3%).
The results of these substudies are presented in Chapters 5 and 6.
Chapter 5 Qualitative substudy results
All participants were asked at each follow-up whether or not they were willing to participate in the qualitative substudy; however, at 6-month’ follow-up, those who had participated in the qualitative substudy at 3 months were not asked again. Of those invited, no participants refused outright to take part in the qualitative substudy, and of those who took part no one dropped out or withdrew. However, an interview was stopped early by the researcher because of a participant becoming upset when describing the reason for their relapse. Figure 2 shows recruitment into the qualitative substudy.
FIGURE 2.
Qualitative substudy flow diagram: relapse prevention trial. UC, usual care.
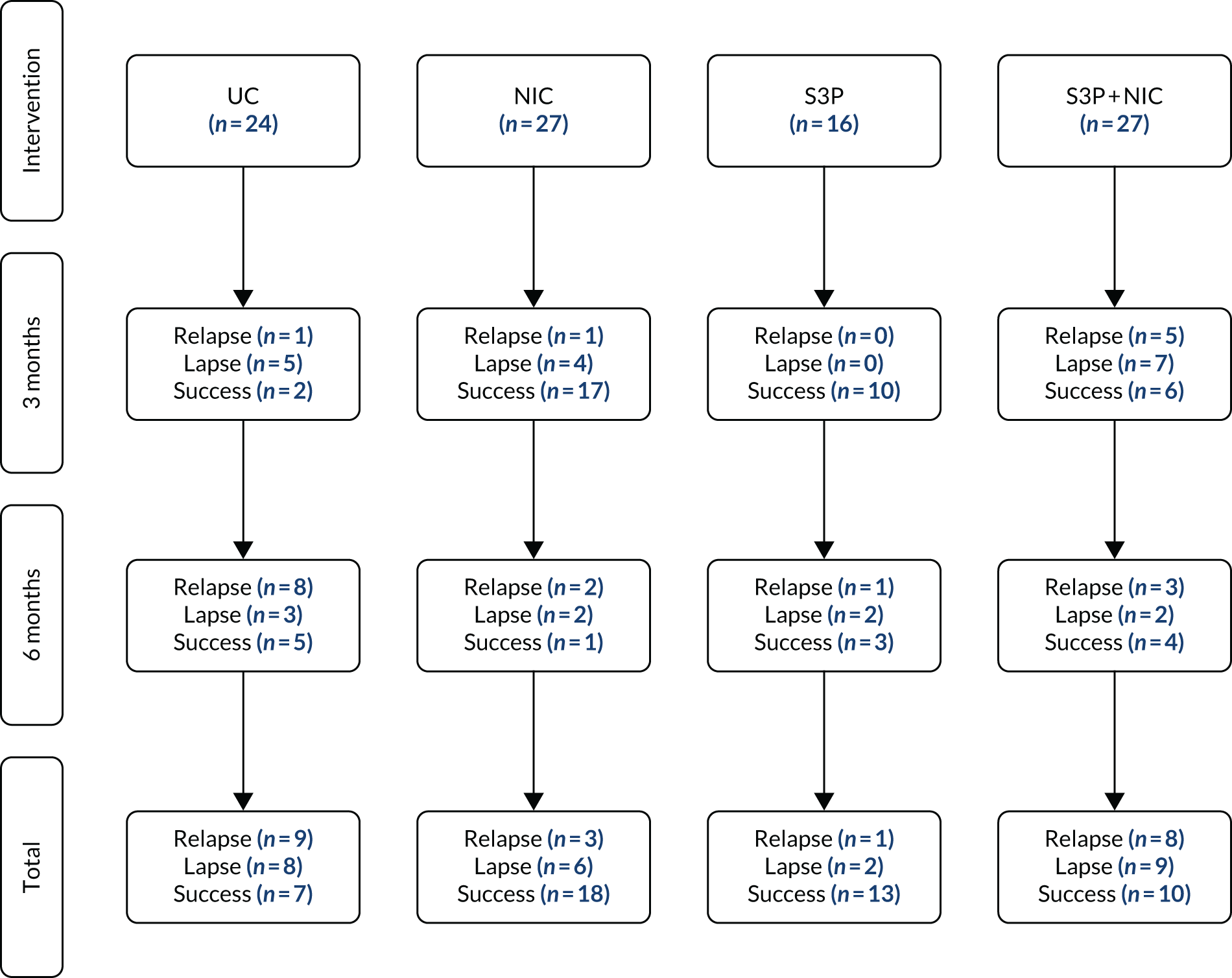
Qualitative research participants were divided fairly equally across the trial arms, although fewer participants (17% of qualitative research participants) were from the S3P arm. Around half (48/94, 51%) of the qualitative subjects were abstinent smokers [just over one-quarter of participants (25/94, 27%) were lapsers and just under one-quarter of participants (21/94, 22%) had relapsed].
Participant characteristics
Table 21 gives the characteristics of the qualitative sample across the trial arms. Approximately 60% were from Australia, just over half (55%) were female and 30% were aged between 51 and 60 years. Just over one-third of the sample had completed only secondary education, 54% were not working and 60% were in receipt of benefits.
| Characteristic | Study arm, n (%) | Total (N = 94), n (%) | |||
|---|---|---|---|---|---|
| Usual care (N = 24) | NIC (N = 27) | S3P (N = 16) | NIC + S3P (N = 27) | ||
| Country | |||||
| Australia | 18 (75.0) | 14 (51.9) | 10 (62.5) | 14 (51.9) | 56 (59.6) |
| England | 6 (25.0) | 13 (48.1) | 6 (37.5) | 13 (48.1) | 38 (40.4) |
| Gender | |||||
| Male | 10 (41.7) | 14 (51.9) | 9 (56.3) | 9 (33.3) | 42 (44.7) |
| Female | 14 (58.3) | 13 (48.1) | 7 (43.7) | 18 (66.7) | 52 (55.3) |
| Age (years) | |||||
| 18–30 | 3 (12.5) | 5 (18.5) | 4 (25.0) | 7 (25.9) | 19 (20.2) |
| 31–40 | 7 (29.2) | 4 (14.8) | 3 (18.7) | 6 (22.2) | 20 (21.3) |
| 41–50 | 5 (20.8) | 7 (25.9) | 2 (12.5) | 4 (14.8) | 18 (19.1) |
| 51–60 | 7 (29.2) | 10 (37.0) | 5 (31.3) | 6 (22.2) | 28 (29.8) |
| 61–70+ | 2 (8.3) | 1 (3.7) | 2 (12.5) | 4 (14.8) | 9 (9.6) |
| Level of education | |||||
| Primary school | 0 (0) | 1 (3.7) | 1 (6.25) | 1 (3.7) | 3 (3.2) |
| Some secondary | 3 (12.5) | 1 (3.7) | 0 (0) | 0 (0) | 4 (4.3) |
| Completed secondary | 6 (25.0) | 13 (48.1) | 4 (25.0) | 10 (37.0) | 33 (35.1) |
| Some tertiary | 6 (25.0) | 3 (11.1) | 4 (25.0) | 5 (18.5) | 18 (19.1) |
| Completed tertiary | 4 (16.7) | 4 (14.8) | 4 (25.0) | 4 (14.8) | 16 (17.0) |
| Further education/diploma | 2 (8.3) | 2 (7.4) | 1 (6.25) | 4 (14.8) | 9 (9.6) |
| Higher education | 1 (4.2) | 2 (7.4) | 2 (12.5) | 3 (11.1) | 8 (8.5) |
| Does not wish to answer | 2 (8.3) | 1 (3.7) | 0 (0) | 0 (0) | 3 (3.2) |
| Employment status | |||||
| Working full time | 5 (20.8) | 9 (33.3) | 3 (18.8) | 7 (25.9) | 24 (25.5) |
| Working part time | 6 (25.0) | 3 (11.1) | 5 (31.2) | 5 (18.5) | 19 (20.2) |
| Neither | 13 (54.2) | 15 (55.6) | 8 (50.0) | 15 (55.5) | 51 (54.3) |
| Receiving benefits | 17 (70.8) | 17 (62.9) | 11 (68.8) | 11 (40.7) | 56 (59.6) |
In the below sections, when giving illustrative quotes, we list whether participants were abstinent, lapsed or relapsed (according to the definitions given earlier), their trial arm, whether they were interviewed at the 3- or 6-month follow-up, their gender, their age (in years) and their country of origin.
Summary of key findings
Analysis identified the following themes (see italicised phrases) as most persistent across participants’ accounts:
-
Adversity in participants’ lives, which was interweaved with their smoking and relapse experiences.
-
Many participants reported using multiple study and non-study relapse prevention strategies to cope with urges to smoke, there was differential engagement with study interventions and additional benefit was derived for some participants from the study surveys and the EMA substudy.
-
The acceptability, use and impact of study interventions varied across study arms. For example, in relation to the NIC arms, the products offered had different perceived strengths and weaknesses and there were concerns around e-cigarette safety, particularly in Australia. In addition, there was a desire to taper nicotine content over time. The S3P advice and strategies were useful when accessed, but difficulties with access were commonly reported and a preference was expressed for an easier method of use, specifically a mobile phone application (app) rather than a web-based program. In addition, text messages were a helpful reinforcement and could be improved if their content and timing were tailored to individual circumstances, but there were some reports that text messages triggered urges to smoke.
-
The battle to overcome craving played a key role in relapse.
-
Participants had differential responses to lapses and relapse.
It should be noted that the trial was complex and involved a number of different processes, including baseline, 3- and 6-month surveys; differential text messages according to study arm and user interactions; choice of product in the NIC arm and the option to switch product if desired; postal delivery of product/leaflet; 1-week follow-up call (all arms); an option for further supplies, if desired; prompts to use the S3P, which varied according to participants’ assessment responses online; and the EMA substudy. In addition, some participants continued to use their base medications [i.e. those used in their quit attempt (e.g. varenicline and nicotine patches)] during the study period. Furthermore, especially when the inclusion criteria were extended in Australia to include participants who had quit for 1 week, there was potential for the participants to confuse elements of any support they had received for their quit attempt with the relapse prevention interventions. Some participants struggled to distinguish the research elements (such as the surveys and EMA substudy) from the study interventions, and at times to disentangle their acute cessation treatment from the relapse prevention interventions. These reported instances should be borne in mind when reading further.
Adversity in participants’ lives
A very strong theme running through the majority of the interviews, which provided an important context, was the difficult circumstances of participants’ lives. Many participants discussed lapses or relapses in the context of people close to them dying of diseases (often smoking related) and, on occasion, suicides. In addition, participants mentioned mental health issues (e.g. depression and bipolar disorder) and their use of smoking to combat loneliness, stigma and blame. Occasionally, participants referred to relationship problems, such as divorce. Frequently, participants referred to their own physical health with descriptions of smoking-related diseases, such as heart and vascular disease and lung disease, and on a few occasions struggling with abstaining from other substances, such as alcohol or marijuana. Many had been smoking for a long time and sometimes at very high daily cigarette consumption:
I was smoking 80 ciggies a day, look where I am a year later I’m on five or six.
Relapsed/S3P/6-month follow-up/female/51 years/England
In addition, participants reported having been trying to quit for a long time (e.g. ‘it’s 25 years I’ve been trying to quit’) and talked about how they had lost periods of their lives because of smoking:
I’ve lost 15 years to smoking.
Abstinent/NIC + S3P/3-month follow-up/female/31 years/Australia
Multiple study and non-study relapse prevention strategies
The majority of participants reported using different motivational, behavioural and pharmacological strategies for different situations and at different time points in the study period:
The vaporiser was useful for that earlier part and the Nicabates now I find are pretty useful.
Abstinent/NIC + S3P/3-month follow-up/female/59 years/Australia
Some tools were study interventions, others were non-study methods and many participants used a combination of strategies, including both study and non-study methods, simultaneously. It was commonly recognised that different strategies were likely to work for different people:
What works for one mightn’t work for another.
Abstinent/NIC/6-month follow-up/male/62 years/Australia
Not everyone fits the same mould obviously I know that but erm there’s not a one thing fits all you know, their approach to their recovery is different and different things will work obviously, I can’t say one thing works even for me type-thing, you know I can’t say anything constructive on that really, I’m still learning myself.
Relapsed/usual care/6-month follow-up/female/33 years/Australia
In terms of motivational strategies for relapse prevention, participants drew attention to the importance of having a clear understanding of their reasons for quitting, planning in advance and setting goals, self-positioning as a non-smoker or ex-smoker and having a strong sense of willpower, commitment and determination. Willpower was frequently perceived as the most important element, regardless of support strategies:
And I don’t think anybody’s in a position to, the support is great but I don’t think you can, I don’t think one can stop for anybody, if that makes sense, it’s a personal thing.
Abstinent/NIC/3-month follow-up/male/57 years/England
In relation to behavioural strategies, the importance of rewarding oneself for abstinence and changing routines (e.g. tidying up, going shopping or not drinking coffee in the morning, or at all) were highlighted. Distraction as a strategy was commonly reported in the form of walks, going to the gym, keeping busy, drinking water, using non-nicotine-containing gum and mints, breathing techniques and meditation:
Like every morning that was my favourite cigarette, that was the one I enjoyed, the rest of them during the day were just habit, nicotine addiction, but the only one that I really really enjoyed was my first one with my cup of coffee so now I don’t have my cup of coffee first thing when I wake up I wait.
Abstinent/NIC/3-month follow-up/male/54 years/England
Some participants mentioned noting down urges and feelings (e.g. stress) in a booklet, or recording days quit and money saved on a calendar. Some used the strategy of confronting risky situations for relapse (e.g. socialising with alcohol), whereas others felt that it was important to avoid such situations, particularly at the beginning of quitting. Indeed, reducing alcohol consumption, or stopping altogether, was mentioned by some as crucial to them staying quit:
Originally I didn’t go out to places where smoking or alcohol was going to be.
Relapsed/NIC + S3P/6-month follow-up/male/62 years/Australia
Smoke-free environments and a sense of social stigma towards smoking were mentioned as important elements in staying quit.
Several participants were still using non-study medications, such as nicotine patches or varenicline, when they joined, which they carried on using throughout the study period, sometimes in addition to the study interventions:
I just went on the Champix and it blocks your cravings and I found that a lot better, [ . . . ] and I have your e-cig [e-cigarette] and the e-ciggie [e-cigarette] is always there in case you do fall, and I think that’s a good idea.
Abstinent/NIC + S3P/3-month follow-up/female/47 years/England
I had the lozenges as part of my support from the doctors, NHS quit smoking service, she gave me loads of those so I’ve had them left over.
Lapsed/NIC + S3P/3-month follow-up/male/29 years/England
A sense of feeling supported was highlighted as important in staying quit:
That positive feeling you know that they’re with you and they want you to succeed.
Abstinent/NIC + S3P/6-month follow-up/female/61 years/Australia
For example, the role of social support from partners, family and friends (or for some a lack of support) was highlighted by many as an important motivation to maintaining abstinence. For some, this was having people close to them who were quitting at the same time. For others, having people to offer support when needed provided enough motivation:
But addiction is pretty strong, so you need to have a wing man that can be very strict with you if they need to, someone that you really trust.
Lapsed/S3P/6-month follow-up/female/35 years/Australia
Others discussed more structured support from their general practitioner, quitlines and mobile phone apps as being helpful in addition to or instead of the study interventions:
They gave me what they called an emotional backpack.
Abstinent/usual care/3-month follow-up/female/39 years/Australia
I’ve been using an app since I stopped smoking it’s not the NHS one because I didn’t like the NHS one it’s another one called QuitNow which I found really motivating and I still do when I look at it I can see how many days it’s been since I stopped smoking and how many cigarettes I’ve avoided and how much lunch money I’ve saved erm yeah so that’s sort of enough of a motivation rather than having those text messages.
Abstinent/NIC/3-month follow-up/female/46 years/England
For several participants, it was difficult to distinguish between all the methods that they had used and state, definitively, the single strategy that worked for them:
It’s not just one of them, it’s all of them.
Abstinent/S3P/3-month follow-up/female/37 years/England
The strategies used were part of an ‘overall package’ or a ‘concerted effort’ to make use of all the tools available to them, without which many of those who were abstinent imagined they might have relapsed:
The texts, the QuitCoach and the nicotine substitute or in my case I had the e-cigarette down to no nicotine erm but it was just the comforting fact that I’ve spent 30 years with a cigarette in my hand and now I have something else in my hand so that is the whole package . . . I think if someone tried to give up or tried an e-cigarette or that Nicabate or whatever it is erm without other support I don’t think that it’d work.
Abstinent/NIC + S3P/6-month follow-up/male/62 years/Australia
Surveys
Some participants perceived the screening, baseline and follow-up surveys as an intervention in themselves, or at least partly, suggesting a possible ‘Hawthorne’ or screening effect:28
I think maybe the survey a bit sooner might’ve helped me too [ . . . ] If I had the survey at maybe 6-week intervals to start with.
Lapsed/NIC/3-month follow-up/female/60 years/Australia
Others referred to completion of the surveys as part of the bundle of interventions, without which they imagined that they would not have been able to stay quit. Some suggested that providing responses to the survey served as:
. . . more of a reminder to keep giving up.
Abstinent/NIC + S3P/6-month follow-up/male/62 years/Australia
Substudy: ecological momentary assessment
Although we did not specifically ask about the EMA substudy, some participants spontaneously mentioned the device during the interview. Like the surveys, many participants perceived the EMA substudy to be an intervention rather than a data collection tool. The device was described as helping participants realise what their triggers were by asking questions about thoughts of smoking or temptations in certain situations. It helped them to identify feelings related to the temptation to smoke, and to identify how urges might be related to certain situations, specific times and places, which they could then change or avoid:
It made me realise what things were triggering me off erm because it would say well have you had a thought about having a smoke within that time or were you tempted and I would say actually yeah I was and then I’d think back to when, when did I feel tempted and why did I feel tempted and I think that helped it to actually erm realise what things to avoid.
Lapsed/usual care/3-month follow-up/female/38 years/Australia
The questionnaire with the handheld device you know with the phone thingy, and that helped as well [ . . . ] It’s in your mind not to smoke because you’re taking part in that and that’s another reason not to do it because it kind of switches your mind away from it so that’s helped as well.
Abstinent/NIC/3-month follow-up/male/51 years/England
Some participants also suggested that having to add lapses to the device acted as a deterrent to smoking. Some participants partly attributed their ability to stay quit to the EMA:
And what do you think lies behind success?
A good support base so that’s from my advisor my partner, and obviously the motivation with the EMA I’m doing as well.
I became dependent on it actually I kind of looked at it as I didn’t want to have to put relapse on it I didn’t want to you know disappoint this electronic thing.
Lapsed/usual care/3-month follow-up/female/38 years/Australia
However, others referred to how, after the initial benefits of using the EMA, the device served as a constant reminder that they could not smoke, which subsequently initiated intense cravings. These cravings, however, returned to ‘normal’ after the device was returned.
Acceptability, use and impact of study interventions
In this section we discuss feasibility, acceptability, barriers to and facilitators of the interventions in the different arms of the trial. For the combined arm (i.e. NIC + S3P) we discuss any synergistic or other effects of having both interventions [note that comments on the individual interventions are discussed within the specific intervention sections (i.e. NIC or S3P)]. As text messages were sent in all arms, these are discussed separately, including the enhanced messages alongside the S3P.
Usual-care arm
A minority of participants mentioned not relying on any specific strategy other than contact with the study team and the study texts, and some even mentioned explicitly that they were against any other type of support:
I’m anti that type of thing I’m a person where I just do everything on my own terms so I’ve not used anything.
Abstinent/usual care/3-month follow-up/female/39 years/Australia
A few participants reported that they would have appreciated the opportunity to have selected study products, but they did not have the opportunity as they were allocated to the control arm of the trial:
Well personally when the choice was made at the start, I would’ve preferred to have been on the inhalers, because I’ve been on them before and they’re what helped me give up a couple of years ago, and I didn’t have the chance for that this time, so giving up this time wasn’t as successful.
Relapsed/usual care/3-month follow-up/male/48 years/Australia
As discussed above, however, many participants in the usual-care arm reported using a range of behavioural, pharmacological and motivational strategies to stay quit:
I got it [e-cigarette] about the same time I started the programme, yeah it’s been over 3 months. I’m going to get the patches the nicotine patches and I use the inhalator, is that what it’s called, what you put the cartridge in, I use that as well, and I try to stay away from alcohol as well.
Relapsed/usual care/3-month follow-up/female/40 years/England
A mixture of things, some of it was like in Victoria we have like a helpline to quit smoking, some of it was using a vape pen, that was good too, and some of it was, I don’t know motivation because I have a young daughter now so I had a bit more motivation to quit now than I had before.
Abstinent/usual care/3-month follow-up/male/34 years/Australia
Nicotine product arms
Product choice: acceptability
The process of choosing a suitable product was explored. Some participants talked about how they had tried several products during previous quit attempts and had developed a sense of which products had ‘not worked’ for them (e.g. because of taste or side effects), which had informed their choice of study product:
I had the patches before and they made me really ill, yeah they didn’t agree with me, but I think the spray’s really good.
Abstinent/NIC/3-month follow-up/male/46 years/England
There had been a lot more prior experience with different nicotine replacement products and so an e-cigarette was often chosen because it was the most novel product on offer:
The other options I’d already tried and they had not worked.
Abstinent/NIC + S3P/3-month follow-up/25 years/England
Others talked about trying one product then switching to another because it was not suitable. Many described ‘trying’ and ‘finding’ different products until an acceptable strategy was found:
Just to try different things for me, I tried everything else and I had the chewing gums you know from the stop smoking service, the chewies you know I thought were OK, they did help me, and I thought I’ll try the minis and they were helpful, and then I thought I’d try the spray since it’s being offered like, I’m really happy with the spray, it does help a lot.
Abstinent/NIC/3-month follow-up/male/54 years/England
Palatability of products was a key criterion, particularly for NRT products, and this differed across participants, with the same product being unacceptable to some and preferred by others, again emphasising that there is no ‘one size fits all’ solution. For example, some considered the taste of the spray as repulsive, particularly when taken alongside alcohol; however, using the spray when drinking alcohol was acceptable for others. The mint taste of the lozenges was disliked by a few, with participants commenting, ‘I’m not a minty person’ or ‘vile’ and ‘nasty’, whereas others described the taste as acceptable, ‘more palatable’, or ‘quite refreshing’. The flavours of the study e-cigarette (i.e. tobacco and menthol) were broadly acceptable; however, several participants commented that a wider range of flavours would be preferred. For example, some participants disliked the taste of the tobacco flavour as they had not smoked for 5 weeks and did not want to be reminded of the taste of tobacco. Others, however, reported a preference for tobacco-flavoured e-liquid. Some participants bought their preferred flavour separately and added it to the study e-cigarette. However, it was acknowledged that tobacco cigarettes do not taste very nice either. The barrel of the e-cigarette was described as too small for those who used the e-cigarette frequently and there were some concerns expressed about the e-cigarette unexpectedly running out of battery charge.
Some participants described physical reactions (e.g. hiccups, heartburn and a sore throat), particularly to the spray and lozenges, which deterred them from using the product or led them to try other products. Physical reactions or oral surgery had sometimes led some participants to try vaping. Others described these factors as ‘not being able to tolerate’, ‘just didn’t get on with it’ or physically not being able to use the products correctly, which ruled them out:
They told me the options then I said OK I’ll try the spray but I couldn’t open my mouth wide enough to spray it onto the inside of my cheek, I kept getting it on my lips, it was unpleasant and it was not something that I’d look forward to doing so I didn’t use that.
Abstinent/NIC + S3P/3-month follow-up/female/69 years/England
Speed of action and strength were other important factors influencing participants’ choice of NRT product, and these product characteristics often seemed inter-related. For example, the effects of the spray were described as ‘fast’ and giving ‘a very immediate hit of nicotine’, which was good for some participants, but for others it was ‘too intense’, ‘aggressive’ and ‘compulsive’. A participant described the nicotine patches received from their general practitioner as ‘not very much help’, as they provided only a ‘maintenance dose’ of nicotine. This was unacceptable to this participant, as they wanted to be able to ‘alter the frequency or the strength of the stuff [nicotine]’ and for this reason had selected the spray for ‘more immediacy and control basically’. Many alluded to a need to ‘control’ nicotine intake, but views differed on which products facilitated better control, with some believing the slower release of nicotine through products such as lozenges was more ‘controllable’ and ‘more easy to control’ than the spray. E-cigarettes simulated smoking in certain ways for some users, but they did not give the same ‘kick’ or ‘hit’ as smoking, which in a few cases led to overuse:
I was smoking more the e-cigarette than a cigarette constantly, because I wasn’t getting that hit.
Relapsed/usual care/6-month follow-up/male/50 years/England
For others, the ‘throat hit’ was key and reportedly ‘missing’ from the spray. Occasionally, however, the throat hit was too strong, with some participants commenting on ‘burning the back of the throat’. Sometimes negative characteristics such as this were perceived as being helpful, but deterred sustained use:
You’re not as encouraged to use it [spray] because of the crazy taste that it leaves in your mouth for so long [ . . . ] and I know the concept of it is not to make it so desirable so you’re replacing smoking with anything else, but eventually the desire is to not have it.
Lapsed/NIC + S3P/3-month follow-up/male/29 years/England
Price was another factor. For example, e-cigarettes had been too expensive for some participants to buy for themselves and, therefore, they selected it as part of the study, viewing them as really valuable. Price was also a factor in selection of and continuing use of NRT products. For others, the comparable price to cigarettes led them to lapse:
Oh I can’t be bothered to keep buying these they’re the same price anyway so I might as well have a cigarette and do something that I enjoy.
Lapsed/NIC/6-month follow-up/female/23 years/England
Some participants appreciated how the lozenges were discrete and very different from smoking, as they lasted longer or were minimally disruptive (e.g. not having to go outside):
It wouldn’t interrupt what I was doing.
Abstinent/NIC + S3P/3-month follow-up/female/33 years/England
In some cases the NRT product was chosen because it prevented smoking (e.g. by putting a tablet or gum in the mouth, one then could not smoke). A few participants could comprehensively list all the pros and cons of different products they had tried or used, underscoring the extent to which smokers will go to understand and get the right type of support. Some described how they did not want to use products such as an e-cigarette because they were reproducing or ‘emulating’ the hand-to-mouth actions associated with smoking and for others this was precisely the reason why they preferred such products. In the latter category, participants often regarded e-cigarettes as very similar to smoking and considered it a ‘cleaner’ way to consume nicotine. However, others stated that they had expected the e-cigarette to be similar to smoking, but found that the effects were not the same. For some participants it was acceptable if vaping was not exactly the same as smoking, as they had not sought to completely replicate the tobacco cigarette smoking experience:
It’s not like a cigarette where you have a cigarette and you smoke a cigarette, with the e-cigarette you have one or two puffs, it’s completely different from smoking.
Abstinent/NIC + S3P/3-month follow-up/male/51 years/England
One attractive characteristic of products was the ability to reduce nicotine intake ‘to wean down that dose’ over time. This could be done by, for example, reducing the number of lozenges taken. However, more commonly this was noted in relation to e-cigarette use. Common to both countries were buying non-study nicotine separately and ‘diluting’ or over time gradually reducing the amount of nicotine in the e-cigarette liquid to zero. The e-cigarette could most easily be tailored to individual needs by varying nicotine content and using different flavours. For example, one participant described it as:
. . . one of the greatest tools [ . . . ] start diluting the nicotine and change the flavours and gradually cut down yourself.
Relapsed/NIC + S3P/3-month follow-up/female/56 years/Australia
Rejection of all study products
There were concerns about the long-term health risks of vaping ‘in 5 years’ time find out oh shit it actually was worse than smoking’. One participant assumed that the products were not harmful because they were given to them by the study team:
Because I was really worried that it would be [harmful], that that it is actually doing my lungs harm, I’m assuming it’s not.
Abstinent/NIC/3-month follow-up/female/54 years/Australia
Worries emanated largely, but not exclusively, from participants in Australia. A few participants, again predominantly from Australia, felt that the research team could have provided detailed literature on the safety and risks of e-cigarettes, which would have helped them to decide on whether or not to use that product, particularly for first-time use:
If you give me like a brochure that says like this is the effects this is the risks these are the risks of doing this compared to the risks of smoking which is obviously like backed by research and whatever and then compared to none, compared to nothing compared to fresh air and then also compared to walking in the city [ . . . ] all those things should be clear for me to make a decision on a product that I’m willing to take.
Lapsed/NIC + S3P/3-month follow-up/male/24 years/Australia
Some participants rejected all the study products on offer. One of the main concerns was that they did not feel that they needed such support:
I’m an all or nothing person, you know I’m either smoking cigarettes or I’m not smoking.
Abstinent/NIC + S3P/3-month follow-up/Female/33 years/England
Likewise, participants felt that the support ‘would just lead me back to smoking eventually’.
Others rejected the products because they did not want to become ‘hooked’ or dependent on something else, stating that they want to avoid ‘developing another addiction after stopping smoking’ (abstinent/NIC/3-month follow-up/female/38 years/England), and this was mostly, but not exclusively, in relation to e-cigarettes. Common concerns included ‘substituting one addiction for another addiction’, or ‘replacing a harm with a harm’. Another participant likened use of an e-cigarette as a tool to a ‘vegetarian eating vegetarian sausages’, which to them was ‘pointless’ as it was at odds with their idea of what it means to stay quit:
For me I prefer to work with the head stuff, for me I prefer to do the talking to myself stuff than substituting with things like that or substituting with an e-cigarette, I want to get rid of the habit I don’t want to substitute.
Lapsed/NIC/6-month follow-up/female/60 years/Australia
Other concerns raised were by those who had stopped all nicotine use when they were enrolled into the relapse prevention study and did not want to start using nicotine again, as they felt that it increased the risk of relapse:
I’ve pushed myself to the ultimate limit and I’m trying not to use the liquid mist I was given by the prevention team because I don’t want to have the nicotine in my body any more, because if I feel like I’ve got nicotine in my body I’m more likely to relapse than if I don’t take it [ . . . ] I haven’t used it [nicotine mouth spray] to be honest, it hasn’t been that bad, and because obviously I’m trying to keep nicotine out of my system I’ll only use it if I desperately need.
Abstinent/NIC + S3P/3-month follow-up/male/25 years/England
In a similar vein, many commented that offering nicotine-free e-cigarettes as a study option would have been beneficial. Some participants believed that a non-nicotine e-cigarette would help them to cope with any persistent behavioural habits related to smoking, such as providing the hand-to-mouth and exhalation of vapour actions, but without the nicotine:
So I got one [nicotine-free e-liquid], but I took the nicotine ones just as a backup in case I ever do relapse, because if I did relapse, this is how I was thinking, if I had the oil-free ones if I’d been on nicotine it wouldn’t work, I’d have to go back to the nicotine one and reduce myself off it again, you know what I mean, but I was thinking that you could throw no-nicotine ones in as a choice for people who’ve been on Champix.
Abstinent/NIC + S3P/3-month follow-up/female/47 years/England
It was clear that there was widespread misunderstanding about nicotine for the purposes of relapse prevention:
Like it’s defeating the object really . . . when you’re doing the nicotine one it’s still like you’re smoking if you know what I mean because it’s still nicotine isn’t it.
Abstinent/NIC + S3P/3-month follow-up/female/51 years/England
There was little understanding among the majority of participants of the role of nicotine in helping people to stop smoking, with participants perceiving that the use of nicotine could ‘elevate’ the risk of relapsing to smoking. Subsequently, there was high resistance to its continued use among some participants. A few participants, however, clearly understood the role that the products were meant to play in a quit smoking attempt, for example:
What really works for me is to try and separate out the chemical addiction from the actual behaviour of smoking yeah because that way you directly displace the smoking with just the, it’s almost like reducing down to the addiction let’s just deal with the addiction.
Abstinent/NIC/3-month follow-up/male/59 years/England
In addition to a wider range of flavours, as discussed above, some participants mentioned that the study could have benefited from offering a wider range of NRT products, such as options for gum and patches. These participants perceived that the gum was more palatable than the spray and the chewing action element of the gum was viewed as:
. . . partly a replacement for the ritualistic aspect of smoking.
Abstinent/NIC/3-month follow-up/male/59 years/England
Use and impact
In line with the study advice, when the NIC products were used by participants, this was mostly to help with urges to smoke so that they ‘don’t feel tempted to smoke’. How they were used to achieve this, however, differed.
Some described more frequent and compulsive use throughout the day in a planned way to maintain nicotine levels and reduce cravings occurring:
I give myself two squirts [spray] every 2 hours.
Abstinent/NIC/3-month follow-up/male/54 years/England
I take one or two puffs [e-cigarette] once an hour.
Abstinent/NIC/3-month follow-up/male/54 years/England
These users either began use straight away or started the NIC when they came off their base cessation medications:
I’ve only started using it the e-cigarette in the, in the last week of the 3-month period, I was using patches and I started using the e-cigarette just to get used to it and once I came off the patches I’ve been using it consistently and whenever I used to smoke I use the e-cigarette now.
Abstinent/NIC/6-month follow-up/male/31 years/Australia
For some e-cigarette users there were concerns about transferring their dependence:
But now I’m just addicted to it, the e-cigarette, I don’t even think about cigarettes nowadays, it’s a nicotine thing.
Lapsed/NIC + S3P/3-month follow-up/female/39 years/England
One participant was concerned that they would have to stop using their e-cigarette soon (because of visiting family members who would not like it) and were very concerned about how they would manage (anticipating failure).
Other participants talked about using the NIC product only in certain situations and at certain times, such as at night, after meals, walking the dog, with alcohol, when stressed and when socialising, mainly as these times were when urges to smoke were greatest. Some would use the product regularly in these situations:
I’d grab it [e-cigarette] and I’d go and have two big puffs outside and then I would just go back inside.
Relapsed/NIC + S3P/3-month follow-up/male/33 years/Australia
Sometimes it would be used in anticipation of craving rather than waiting for the craving:
. . . about 10/half 10 I might start thinking ooh I haven’t had a ciggie [cigarette] yet and that’s when I’ll get the spray.
Lapsed/NIC + S3P/3-month follow-up/female/33 years/England
These participants stated that they needed several minutes for the nicotine to kick in:
If you can anticipate a craving and you need 5 or 7 minutes for that to work.
Lapsed/NIC/3-month follow-up/male/34 years/Australia
Other participants, however, would carry around the NIC product all the time, but use it only in vulnerable situations and only if they felt a great need. These participants were storing the NIC for ‘emergencies’ and without the intention of using, but it was there as a ‘fallback’ or ‘safety net’ or for use when they felt ‘desperate’. These participants often placed greater faith in their motivational beliefs for stopping, drawing on ‘mind over matter’. For example, one participant mentioned that they knew that it was good to have the NIC products available, even if not using them, to ‘feel more secure’, as they were concerned that because they had nicotine in their ‘system’ for the past 15 years they did not know when they might be overcome with urges. Use in social situations was commonly reported, with some participants commenting that they did not want to be ‘left out’ while others are smoking. Many reported a benefit of the e-cigarette was that it enabled them to continue to socialise with tobacco cigarette smokers. Therefore, these participants did not avoid such difficult situations and embraced them by using their NIC product, for example:
I’ve got the second method as well which is the e-cigarette and that has been very helpful for the other situation which I was managing with the lozenges but erm, which is sort of easier to manage with the e-cigarette and that is I’ve got two friends who smoke so seeing them it’s easier if I have an e-cigarette because erm yeah I’m not tempted to ask them for a cigarette.
Lapsed/NIC/3-month follow-up/female/57 years/Australia
When I was in the situation where I did go out with my girlfriends and they were smoking I would grab a lozenge out of my bag and have that instead of the cigarettes.
Abstinent/NIC + S3P/6-month follow-up/female/25 years/Australia
It was clear that, for some participants, different NIC products were used for different reasons, purposes and situations. One participant talked about using the e-cigarette in social situations and nicotine gum (a non-study product) while at work in a bar where they were not permitted to use the e-cigarette.
In relation to impact, generally the different products worked well in combating and ‘killing the craving’, and for some participants there did not appear to be a prior expectation that they would work:
And they did actually stop some of the cravings.
Relapsed/NIC + S3P/6-month follow-up/male/43 years/England
The spray is excellent it stops the craving almost immediately.
Abstinent/NIC/3-month follow-up/male/54 years/England
Without that I probably would’ve failed, that was there every time I had the urge I could go and reach for one of those little lozenges.
Lapsed/NIC + S3P/3-month follow-up/female/48 years/England
A combination of NIC products was sometimes reported to combat craving as well:
So I was already using patches and I found the lozenges just augmented it when I needed it [ . . . ] I took the patch off at night time to sleep, and first thing in the morning I put a patch on and I put a lozenge in my mouth, it’s for an instant thing.
Relapsed/NIC + S3P/6-month follow-up/female/69 years/Australia
Yes I think from a scale of zero to 10 the lozenges is like a six but the main thing is your own self-control and when you combine the lozenges and the e-cigarette it’s like eight yeah.
Lapsed/NIC/3-month follow-up/male/34 years/Australia
Many of those who had lapsed talked about how they used the NIC products to help them get back to quitting and hence did not relapse fully. Others talked about how they relapsed when they stopped NIC use:
Then I thought to myself I can’t go on forever using this so I had to come off all kinds of nicotine, which I did, and I was able to get away from any kind of nicotine for a good few weeks.
Relapsed/usual care/6-month follow-up/male/50 years/England
For a few participants, the use of the products in some situations was associated with improved well-being or mental health, for example being:
. . . able to hang around with people while they were smoking and be social, it improved my mood to be honest.
Abstinent/NIC/6 month follow-up/male/25 years/Australia
For others, it was the ability to have something to do with one’s hands and mouth that seemed to be the most important aspect:
Because in the past I’ve tried quitting but found it boring without using my hands, so because I’ve got used to my mouth being with a cigarette so I didn’t have that in the past and I’ve ended up relapsing, and the time with the e-cigarette it felt like I had something to do with my hands and something to inhale, yeah so that helps me but it’s not something that I intend to use permanently.
Abstinent/S3P/3-month follow-up/female/37 years/England
Reducing nicotine
As stated above, reducing nicotine intake was a common desire which for several participants was implemented somewhat regimentally:
I used the vaping method to reduce, so what I did was erm I started off with 10% nicotine level then I’d say almost weekly or say fortnightly would reduce that, and within 2 months I was down to zero nicotine in the vape, erm and then I kept vaping for another 2 months, and I haven’t vaped for 2 months or so.
Abstinent/NIC + S3P/6-month follow-up/male/62 years/Australia
A few participants who had relapsed described how they liked using the e-cigarette, but stopped using it following relapse. However, many who relapsed mentioned continuing to use the e-cigarette, which they believed helped to reduce the amount they were smoking.
S3P arm
Acceptability, barriers and facilitators
The ability to access QuitCoach of their own accord was a benefit for some:
It sort of gives you your own choice as to whether you want to use like you log in or not log in as much as you want.
Lapsed/NIC + S3P/3-month follow-up/female/33 years/England
However, this was also acknowledged as a barrier, as they needed more reminders, prompts or persuasion to log in. It was even suggested that logging in a number of times in the first few weeks should be ‘mandatory’ to get into the habit of using it. Some found having to log in to the study website as bothersome in terms of remembering their username and password every time they wanted to access QuitCoach, especially after not using it for a while. This deterred many participants from using it either once or after the first access:
I can honestly say that I haven’t even been to it.
Relapsed/NIC + S3P/3-month follow-up/female/56 years/Australia
I have to go into my emails find my username find my password then go into the website.
Lapsed/NIC + S3P/3-month follow-up/male/24 years/Australia
A few suggested that an ability to request a text message with login details would have been beneficial. Likewise, a reminder text message to log in with the details included would have been helpful:
See I’m not a very tech-savvy person.
Lapsed/NIC + S3P/6-month follow-up/female/37 years/Australia
I’m not into computers.
Relapsed/NIC + S3P/3-month follow-up/female/56 years/Australia
Other barriers were technical issues logging in or issues with internet connection (e.g. from living somewhere remote). However, a few participants remarked that, once their username and password were set, then logging into QuitCoach:
. . . wasn’t hard work.
Abstinent/QuitCoach/3-month follow-up/female/37 years/England
Some participants tried to access QuitCoach on their mobile phone and found it very clustered. To this end, many stated they would have preferred an app instead:
But if it was something like an app where maybe you can incorporate the texts into it and then it just becomes like an erm how do you say it like a pop-up kind of thing yeah a reminder or something and some distracting games because that’s one of the things that happens when you’re craving I’m guessing you need to distract your mind.
Lapsed/NIC + S3P/3-month follow-up/male/24 years/Australia
Other participants found QuitCoach to be ‘mind-boggling’ and ‘long-winded’, with repetitive questions that ‘go on for a while’ after initial use, which had stopped them from returning to it:
. . . asks the same question over and over again.
Abstinent/NIC + S3P/6-month follow-up/male/62 years/Australia
Some suggested that it was too much of a ‘commitment’ to get QuitCoach going:
Investment to get it up and going, erm and I don’t know if I gained that much value.
Lapsed/NIC + S3P/3-month follow-up/male/29 years/England
For example, a parent with a young child mentioned appreciating the option to use QuitCoach, but had little extra time to go through it because her child slept for about 20 minutes only at a time. Those who accessed QuitCoach minimally (i.e. once) talked about how they may have engaged more if the information provided was more ‘bitesize’. As mentioned above, a preference was expressed by many for an app, and suggested improvements included a better user interface and gamification for distraction from urges to smoke. Some participants described the QuitCoach website as ‘laid out well’, whereas others remarked that the user interface could be improved.
Some participants commented that it was difficult to provide an accurate answer to some of the questions in the assessment because of the prespecified responses available:
I wasn’t able to give a definitive answer and I had to just choose one because it didn’t match up with my thoughts.
Abstinent/NIC + S3P/3-month follow-up/female/69 years/England
A few participants felt they did not need to use the online intervention at the time as they were:
. . . trying to do it without being online because I’m not online all the time, so I need to look at it in the real world, in the context of that rather than going onto a website.
Relapsed/NIC + S3P/6-month follow-up/female/56 years/Australia
Other reasons for not using QuitCoach included already using non-study quitting apps with comparable functionality, lack of incentivisation to stick with it, not liking to read materials online and failure to capture attention:
I’m old school so I find it hard reading materials online.
Lapsed/NIC + S3P/6-month follow-up/female/age unknown/Australia
Some participants preferred to speak with someone face to face and, therefore, did not take up the opportunity to use QuitCoach. Others did not engage with QuitCoach because they viewed it as a ‘robot’ or ‘algorithm’ and it could not replace human interaction with a health professional:
What I really need is to talk to a doctor and to get real medical advice and to like you know, talk face to face with someone that’s a professional, I kind of just palmed it off a little bit.
Lapsed/NIC + S3P/3-month follow-up/male/25 years/Australia
It was possible, as mentioned previously, that some participants were confusing the various study tools or interventions, such as the follow-up surveys, with QuitCoach. Additionally, many participants in the S3P arms did acknowledge the utility and helpfulness of the text messages, which were more tailored in the S3P arms:
I never thought about it [QuitCoach] because I was busy reading the text messages.
Abstinent/NIC/3-month follow-up/male/24 years/England
Use and impact
Some participants who had already abstained for some time reported having minimal urges and did not feel a need to use the QuitCoach, or they were combating the urges with other tools. However, for some, having to learn and use behavioural strategies recommended by QuitCoach was taken as an indication that they were not coping.
On the whole, however, participants described QuitCoach as ‘helpful’, ‘positive’ and ‘clear and concise’. QuitCoach reportedly provided ‘good advice’ that served to ‘recap’, ‘reinforce’, ‘encourage’ and ‘remind’ them to stay quit. For example, QuitCoach asked questions such as what plans were in place ‘when something goes wrong’, which helped participants to consider and potentially prepare for such scenarios. QuitCoach was perceived by some as providing ‘emotional’ support that could be accessed whenever needed, which was not something that a NIC product or existing stop smoking services provided:
It’s like a friend or a sister, it’s really good.
Abstinent/S3P/3-month follow-up/female/37 years/England
How QuitCoach was used, like NIC products, again varied considerably. Some participants described how QuitCoach was particularly helpful at the beginning:
Particularly useful at the start when you sort of like struggling.
Abstinent/S3P/6-month follow-up/male/21 years/Australia
Some participants found the ‘scientific content’ to be beneficial to motivation and even wanted more of it:
It really made you think at the start about all those triggers and how your individual habit works, and how the addiction is in your individual life [ . . . ] that mixture of scientific information about what was happening in my body and my brain together with yeah having a bit of a motivational kind of boost was really beneficial to have that together.
Lapsed/S3P/6-month follow-up/female/35 years/Australia
Some participants did not feel a need to return to it after the first use:
There’s things that I might not have thought of before that now I’ve got in my head already . . . that it makes you consider these things in advance.
Abstinent/NIC + S3P/3-month follow-up/female/33 years/England
Other participants used QuitCoach only when experiencing urges or for anticipation of urges. Some participants actively used QuitCoach online when they had strong urges to smoke:
And if I ever have one of the urges which are unbearable and I can go to the QuitCoach and fill in an assessment if I feel I can’t handle the urge and see what I can do to combat the urge.
Abstinent/NIC + S3P/3-month follow-up/male/25 years/England
QuitCoach generated a personalised report based on the assessment responses, which some participants repeatedly used for reference by either printing out materials or screenshotting advice for reference when needed (e.g. ‘I can reread it’). The personalised advice was found to be helpful in terms of identifying and planning for situations that participants themselves had not identified (e.g. to ‘point out things that people might not be aware of’). It helped to identify ‘things to watch out for’ and ‘times and places’ at which they felt themselves most vulnerable to relapse (e.g. drinking alcohol or stress). QuitCoach helped to plan for such situations with something written down and, therefore, ‘referenceable’ and ‘concrete’. Participants who read their personalised advice liked how ‘in depth’ it was and, in particular, the ‘accuracy’, with comments including that it was the ‘best advice’ they had received to date and:
. . . it knows how you think, it’s so intelligent It made me think of situations that might bother me before I’m stood in them you know so yeah it was definitely a helpful tool even if you don’t look at it again after you’ve filled it in, it’s the fact that it makes you consider these things in advance.
Abstinent/NIC + S3P/3-month follow-up/female/33 years/England
Do you know the beginning when you have to do this huge questionnaire and it gives you sort of like an elaborate step by step plan for you that was awesome, that was the best advice I ever got [ . . . ] it had the tools it tells look you could be doing this you could doing a list you could do this do that, it was all tailored for me you know based on the answers that I gave and I thought that was very very useful.
Abstinent/NIC + S3P/3-month follow-up/female/48 years/England
The tailored advice, and the ability to update this advice subsequently when circumstances changed, was valued as ‘really good’, as a participant observed:
It altered it to fit the changes that had gone on.
Abstinent/NIC + S3P/3-month follow-up/female/33 years/England
QuitCoach was therefore helpful for participants to track their progression:
Just going back to it I was able to see how much has changed and [ . . . ] maybe it gave me a sense of like development.
Abstinent/S3P/6-month follow-up/male/21 years/Australia
Similar to NIC products, participants described an intention to use QuitCoach only in an emergency situation when ‘desperate’ and the urges to smoke were ‘unbearable’, so that they could seek advice on how to ‘combat the urge’. These participants acknowledged that it was useful to know that QuitCoach was there if they ever needed it.
Strengths of QuitCoach included that it was interactive, not too formal, that it could be used as a distraction from urges to smoke and that it was accompanied by tailored text messages (see NIC + S3P arm).
For some participants, QuitCoach had been an adjunct used in conjunction with non-study strategies, whereas others reported using nicotine products, such as the e-cigarette, with and without regular use of QuitCoach.
NIC + S3P arm
Many of the views expressed in the combined arm about the individual products (i.e. NIC or S3P) mirrored those in the single-product arms and, therefore, will not be repeated here. Instead, we focus on comments about the synergy between the NIC and S3P interventions.
Participants varied in the extent to which they used the two interventions, with some predominantly using NIC, relying more on the NIC product alongside the tailored text messages, with minimal access to the QuitCoach aspect of the S3P intervention. Other participants predominantly used the QuitCoach more frequently because of their beliefs about nicotine and substituting smoking with NRT or an e-cigarette. When participants made use of a NIC product alongside regular use of QuitCoach, they generally reported using the NIC product to prevent urges from occurring or as a ‘go-to’ in an emergency to avert a lapse turning into a full relapse. QuitCoach appeared to help guide one participant in this situation:
But I think the QuitCoach yeah when I had a temptation to smoke when I was on holiday, and I had the e-cigarette.
Lapsed/NIC + S3P/3-month follow-up/female/39 years/England
Some participants acknowledged that QuitCoach alone could not ‘override’ physical urges to smoke, which they felt the NIC product could help with, as it ‘softens the edges’:
They were both helpful and even the e-cigarette although with the QuitCoach it gives you things to watch out for and with the e-cigarette that’s my go-to let’s say I’ve had a bad day at work I’ll pick the e-cigarette over buying a packet of cigarettes, that’s pretty helpful because erm because if I bought cigarettes god knows what would happen, and an e-cigarette will do what it needs to in the case of an emergency sort of thing.
Relapsed/NIC + S3P/6-month follow-up/male/33 years/Australia
Study text messages (all arms)
Non-tailored and non-interactive texts in the usual-care and NIC arms
There were mixed views on the helpfulness of the standard stream of text messages for those in the usual-care and NIC arms of the trial, ranging from encouragement and reinforcement to stay quit (not necessarily the content, just the fact that the text messages kept coming in) through to annoyance and triggering thoughts and urges to smoke when they were not thinking about cigarettes. Some participants expressed a ‘neutral’ stance towards the text messages or mixed views (e.g. ‘60% very good’), with some text messages being considered as helpful (e.g. at some times of the day or on some days) and other text messages being considered as less motivating (e.g. on the other days).
Some participants reported that the texts helped them to cope with urges, describing them as ‘positive’, ‘extremely helpful’, a ‘wake-up call’, ‘brilliant’, ‘an invisible friend’, ‘a pat on the back’ and a ‘back-up’, with the ‘right kind of tone’. Some noted that this was very different from the lack of support from family members who did not expect them to succeed in quitting for so long. One participant reported how the text messages had prevented a lapse going into a full-blown relapse on this occasion:
I’ve stopped for months and months and I’ve had a go and thought oh this won’t hurt me and then it does, it’s very easy to fall back into it but I found this time with the messages I’ve been getting, I’ve been getting text messages which I think are now down to about once a week, and I just found reflecting on those really helpful to get it into my head.
Lapsed/NIC/6-month follow-up/female/60 years/Australia
Conversely, others reported that the texts triggered urges or reminded them of smoking, for example ‘the texts make me think of a cigarette’ and ‘made the cravings worse’. If they were not tailored for participants who had relapsed, then the text messages that were encouraging people to stay quit were unhelpful and inappropriate.
Participants remarked that the texts were helpful only at the beginning only, but then became monotonous, with some participants mentioning that they stopped reading the texts following relapse to smoking, as they kept reminding them:
. . . how bad I am for smoking again.
Relapsed/NIC/3-month follow-up/female/33 years/Australia
Many participants responded that they would like the text messages to be tailored to their own circumstances with regard to timing and content, or in relation to their own needs or circumstances, or other forms of support.
Responses to the timing of the text messages varied. For example, some participants appreciated the text message first thing in the morning to remind them not to have a cigarette and keep strong (e.g. ‘it just starts your day off in a positive way’), whereas others found it annoying, as it would remind them of smoking:
Would remind me first thing in the morning because I’d be waking up and it’d already be on my phone and that would be the first thing I see when I wake up in the morning, going I’m trying to quit cigarettes and this is right in my face.
Abstinent/NIC + S3P/3-month follow-up/female/31 years/Australia
Participants felt that the frequency was just right and when they started to reduce the frequency they reported ‘missing them’. Others felt that the frequency of the text messages was right initially, but should have been reduced more quickly. Participants would have liked to receive text messages at times when they themselves experienced urges to smoke, such as scheduled breaks at work, mornings and after food or, for those on shifts, for when they were awake. This would be possible if participants were asked when their urges were greatest or how frequently they would prefer to receive the messages. Some participants wanted to see the timings of the text messages vary (‘ad hoc’) rather than always coming in at the same time of day.
The scientific content of the text messages, such as the health benefits, was well received, and many participants commented that they would like to have seen more of these text messages, particularly messages explaining how their body (e.g. their lungs) would have improved after abstaining for different lengths of time:
I wanted information about the body what’s happening in my body in the first you know 24 hours the first day second day and I wanted all the nitty-gritty about the body and what’s going on but I didn’t get that [ . . . ] actually give me like little facts about erm like you know where is it if I can look at my actual tobacco the actual facts of the tobacco you know like the packet it’s got you know this BDE [1,3-Butadiene] is found in large amounts of tobacco smoke you know and just erm but how my body is responding in recovery you know when I stop smoking like your hair your skin your organs what’s going on with your organs and giving me hope I needed to hear I needed to hear hope for the future with that like I’m scared I mean honestly I’m scared I’m going to have lung cancer you know.
Relapsed/usual care/3-month follow-up/female/47 years/Australia
Another participant described how they did not socialise in bars and, therefore, the texts relating to coping in those situations had not been relevant. Some participants appreciated the text messages on the amount of money they were saving by staying quit, but others found these annoying, having succeeded several times at stopping smoking previously for periods of time and so they already knew all the reasons why they were stopping. Other participants wanted the text messages to relate to the support they were using. That is, those not using NIC products were unhappy with texts encouraging them to use NRT or e-cigarettes when in vulnerable social situations, as they were irrelevant (they also thought people using these products would not need encouragement to use them). These participants, instead, preferred more motivational messages on the positives of not being a smoker or negatives of being a smoker:
So maybe that’s what these constant texts is giving you, it’s reminding you of the good things and not the bad things, because when you stop smoking, for me it’s all about what I’m missing out on [ . . . ] so those texts give me a or gave me or continue to give me that someone cares, someone yeah knows what I’m going through, or something, does that make sense.
Abstinent/NIC/3-month follow-up/female/54 years/Australia
Reasons for stopping the text messages included that the messages were lost among the hundreds of other text messages a participant received in a day and a participant feeling that they needed to speak to someone. A minority of participants reported them to be ‘worthless’, ‘irrelevant, ‘a bit too much’ and ‘over the top’. Suggestions for improvement included making them interactive:
I pretty much ignored a lot of them [ . . . ] I think the only way it might work is if people had the option to actually send a message when they felt like it and then get one back telling them not to do it.
Abstinent/NIC/6-month follow-up/female/44 years/Australia
Participants described scrolling through texts as and when needed.
Tailored and interactive text messages from S3P and NIC + S3P arms
Varied views on the tailored and interactive text messages as part of the S3P arms were also apparent. Many participants suggested that the tailored texts were ‘reassuring’, ‘reinforcing’, ‘brilliant’ and made ‘good points’ about which ‘strategies’ to use in different situations. Others remarked how the text messages received in the morning helped them to prepare for or become ‘more mindful’ of how they were going to ‘deal with the day’. Although ‘impersonal’, some participants described how the text messages helped them to remember what they had accomplished in managing to stay quit, which had been a ‘good feeling’, and the ‘recognition’ had also been appreciated. Having the texts on a mobile phone to refer to whenever needed, for example when struggling during an urge, was also helpful for many. The tailored text messages were described as important by a few participants who did not have support to quit and stay quit from others around them (e.g. the text messages were ‘a mental boost’ and an ‘invisible friend’). The text messages helped them to feel less alone, were a ‘constant reminder’, ‘encouraging’ and a ‘positive reinforcement’, which was helpful:
They’re great because they actually reinforce hey there’s someone looking out for you, you know and you need to do this and you need to do that, it’s like a positive reinforcement, it’s like somebody giving me a yeah you can do this, they come randomly, they come at whatever time, so it’s random out of the blue to say hey stay strong, keep going, so I think they’re great, especially as I said I was on my own and I thought of it as a positive reinforcement randomly sent to me, it’s excellent.
Relapsed/NIC + S3P/3-month follow-up/female/56 years/Australia
I think that [QuitCoach] together with the text messages was a good combination rather than one or the other, I think they went well together you know, I don’t think it would’ve been as effective just having one.
Lapsed/S3P/6-month follow-up/female/35 years/Australia
Some participants mentioned rereading the study text messages to remind themselves why they were quitting and to stay quit, particularly during an urge. Others described taking screenshots of or referring to the text messages when struggling during an urge to remind themselves about their motivations for quitting:
I think that every time I’ve wanted to smoke or had an urge to smoke I’ve just reminded myself why I quit, and I had a look back over the texts that had been sent to me, and I had a look back over those and they helped, it’s just reminding myself why I quit more than anything.
Abstinent/NIC + S3P/3-month follow-up/male/24 years/England
The tailored text messages were described as ‘thorough’ and ‘helpful and motivational’. After coming off Champix and redoing an assessment, one participant described the times when the tailored text messages were received as ‘more varied’, ‘unexpected’ and ‘random’, which was a welcome change. Again, a few participants commented that it would be better if the tailored and interactive text messages could focus less on products if they were not using them and focus on motivational messages. Specific references to a nicotine product the participant was not using may have been due to participant error when they were online, the participant failing to notify the system of having stopped using a product or a coding error. An illustration is provided in the below example:
It texts me as if I am a vaper, if that makes sense, it texts me saying erm take a moment to think about how much better your life is since you quit smoking and started vaping and things like that but obviously I don’t vape, I think out of the whole time I’ve been using it I’ve only had about four texts that obviously include vaping.
Abstinent/NIC + S3P/3-month follow-up/male/24 years/England
Complaints such as this were rare, as the system was programmed to send only relevant messages that were based on participant preferences.
Some participants mentioned that the frequency of the text messages could be increased and that they had ‘tailed off’ too early. For others, a few months of receiving the text messages was enough:
With the text messages they kind of tailed off too early, the frequency to begin with was really really great and really worked for me, but then the movement to greater frequency, I’m sure some people would find it annoying but it’s almost better to be an annoyance than having too few text messages and for someone to not have it at the right moment.
Lapsed/NIC + S3P/3-month follow-up/male/29 years/England
Some participants were more negative about the text messages. As in other trial arms, the messages frequently reminded participants of smoking and triggered urges to smoke:
It makes me think of smoking when I’m not actually thinking about smoking.
Abstinent/S3P/3-month follow-up/male/69 years/Australia
However, a few participants found the reminder of trying to quit smoking beneficial. Again, the first text message of the day was welcomed by some, but annoying to others, even when it was acknowledged that the text messages had helped with urges to smoke in the morning. Although helpful, receiving text messages later in the day, at other trigger points, such as teatime and after dinner, would have been appreciated:
Instead of throwing them all at the person first thing in the morning.
Abstinent/NIC + S3P/3-month follow-up/female/50 years/England
A few participants remarked that they no longer opened the text messages after a certain period of time:
After a while you know what they are so you don’t open them up any more.
Lapsed/NIC + S3P/3-month follow-up/male/24 years/Australia
However, they appreciated receiving them, acknowledging that perhaps ‘subconsciously’ they were modifying their behaviour:
It just reminds you about cigarettes and stuff you know what I mean [ . . . ] Yeah like I mean you’re not thinking about nothing and then all of a sudden you get a text message saying blah blah blah you know from the QuitText and you think woah I wasn’t thinking about cigarettes and then you just made me.
Abstinent/NIC + S3P/3-month follow-up/female/51 years/England
I wasn’t thinking about cigarettes and stuff like that, well they’d sent me the e-cigarette but I didn’t think about it, but every time I saw those text messages I thought about it, I don’t know why but in my normal routine I feel like those messages remind me of cigarettes.
Lapsed/NIC + S3P/3-month follow-up/female/39 years/England
Some participants also felt that the constancy of the messages suggested that they were wanting them to slip-up:
Have you had a fag and not told us and I’m thinking that is something that I don’t want to hear I’m trying to pack in smoking here, it seemed as if they were egging me into lapsing because it was the norm . . . should I have a fag because they want me to lapse-type thing.
Abstinent/NIC + S3P/3-month follow-up/female/50 years/England
Others reported not accessing the study website often, as they had appreciated the text messages more. For example, a participant remarked that they initially logged in to access QuitCoach out of ‘curiosity’, but did not use it because it reminded them of cigarettes and they did not want to be reminded. In addition, the participant felt that the tailored text messages had been sufficient. Many participants expressed a clear preference for face-to-face contact rather than tailored text messages, as some felt that they would disregard text messages more easily.
Suggestions for improvements to the tailored text messages included more personalisation, and one participant remarked that they would prefer a shift in terminology from ‘quitting smoking’ to ‘stopped smoking’, as ‘quitting’ sounds as if you are still doing it. Some participants remarked that the frequency of the tailored text messages had been too much; for example, one participant commented, ‘slightly irritated by my phone going all the time’. Many participants wanted to be able to nominate how many text messages they would receive. Another noted that the text messages were:
. . . just platitudes, they aren’t telling me anything that I didn’t know, they might be useful for some people but for me they really irritated me something stupid [laughs] [ . . . ] to be honest all I can think about the messages is that they irritated me.
Abstinent/NIC + S3P/3-month follow-up/female/48 years/England
In addition, one participant stopped the text messages before going on holiday, perceiving (inaccurately) that there might be additional charges for receiving text messages abroad.
Battling to overcome craving
Craving was a key concern for most interviewees. When asked about when they started to become aware of urges, most frequently it was in the context of quitting. Indeed, one participant stated that they only really became aware of these when they stopped smoking, as usually urges were indulged. Many participants described how it felt to have urges or cravings, with some having to be constantly on their toes:
Early on I would say I was getting quite a few, but yeah it was always quite reassuring, but it is a daily battle I guess, not so much now but earlier on it was an ongoing thing that you’d have to be on your toes and be aware of possible things that could be a trigger or erm yeah, without even knowing what’s a trigger for you.
Lapsed/NIC + S3P/3-month follow-up/female/30 years/Australia
Some participants would often describe a conversation in the brain as ‘a battle’ that played out in different ways. However, one participant said that the voices changed to encouragement when trying not to relapse:
This constant sort of heckling in the brain about needing to satisfy particular sort of, yeah a particular craving for what you know is a cigarette, it’s not food it’s actually a cigarette.
Relapsed/usual care/3-month follow-up/male/52 years/Australia
Oh look I think a person you know has to be more strong-willed in their efforts to quit if you know what I mean, and not fall for the voice in the head [ . . . ] it seems that all of a sudden it has a grip on you. Craving gets really really strong sometimes it seems overpowering, you know, and you just buckle under pressure sometimes I think.
Lapsed/NIC/6-month follow-up/male/28 years/Australia
When asked how long urges to smoke lasted, answers varied from ‘a couple of minutes’ to ‘a good 10 minutes’. Participants described how sometimes they gave in to the urges, as they were completely overwhelming:
. . . when I was dying for a, like actually dying for a smoke.
Relapsed/usual care/6-month follow-up/male/54 years/Australia
To the extent that they would:
. . . go down the street and bum one off someone.
Relapsed/usual care/6-month follow-up/male/54 years/Australia
Sometimes participants differentiated among various types of urges:
. . . there’s so many different types of cravings and yeah urges to smoke.
Relapsed/usual care/3-month follow-up/female/47 years/Australia
For example, one participant differentiated physical urges from emotional urges when they were going through a difficult time:
It was just a pressure building and I just couldn’t cope.
Relapsed/NIC + S3P/6-month follow-up/female/69 years/Australia
It sort of like starts off as like a tightness in my chest type-thing, from there I’m like I need a smoke, my mind won’t stop thinking about it where it’s like erm obsessive thoughts about it almost and then if I can’t get it I get really agitated you know I get the tightness in the chest and things start happening.
Relapsed/usual care/6-month follow-up/female/33 years/Australia
The first wave I found it so easy, physical cravings were taken care of with patches and lozenges, I really really wanted to quit and still do, but yeah and it was really the second wave the cravings it was kind of sort of more emotional more than anything else.
Relapsed/NIC + S3P/6-month follow-up/female/69 years/Australia
So at that point I didn’t care about stopping any more, my sort of erm feeling of having almost like no resources was stronger than anything else at that time.
Lapsed/NIC/3-month follow-up/female/57 years/Australia
Some abstainers and lapsers reported that they were still getting urges, even after some time, but for others that battle lessened over time:
I’m always feeling tempted to smoke.
Lapsed/usual care/3-month follow-up/female/38 years/Australia
But yeah that went away over time and now it’s not even a thought that’s in my head like . . . It’s totally gone.
Lapsed/NIC + S3P/3-month follow-up/female/30 years/Australia
Differential responses to lapses and relapse
There were differences between participants in terms of how they interpreted lapses to smoking. In addition, regardless of how they interpreted lapses, frequently lapses and relapses were not defined as they are in academia, with a slip-up (i.e. smoking even a few puffs of a cigarette) being referred to as a ‘relapse’.
For some abstainers, a lapse would be regarded as a failure and likely to progress to a full-blown return to smoking. Some suggested that ‘you’re either a smoker or not’ and therefore a lapse was the equivalent of relapse and there was no distinction between the two. Lapsing was therefore to be avoided at all costs. In these instances, a lapse was grounds for having to start a new quit attempt:
I just keep telling myself a lot of the time that if I even have one cigarette I have to start again and that’s a very strong motivator for me.
Abstinent/usual care/3-month follow-up/female/39 years/Australia
I know that if you have one puff that can just get you back in [ . . . ] I would say the reason I’ve been more successful this time is because it happened to me before, that they sucked me back in.
Abstinent/NIC/3-month follow-up/female/54 years/Australia
The minute you have a ciggie it opens that up in your head and then once you’ve done that you’re just back to square one, so never having that first cigarette is the biggest thing and remember there’s no such thing as just one.
Abstinent/NIC/3-month follow-up/male/54 years/England
For these participants who believed a lapse was a personal failure, then the lapse could progress to relapse:
I had a cigarette and I stopped again [ . . . ] I had the temptation but I was like fighting with the temptation that I shouldn’t be smoking I shouldn’t be smoking, and I kept telling myself that, reminding myself, and then eventually I said one more won’t hurt me, and after a week I think I took another fag, and from there it was frequent.
Relapsed/usual care/6-month follow-up/male/50 years/England
Sometimes the physiological response to a lapse meant that more cigarettes would follow. A few alluded, to being ‘gripped’ by urges to smoke more after one puff, referring to it as a recurring ‘cycle’ that ‘sucked you in’ or was ‘easy to fall back in to’.
For others, there was a sense that learning the best strategies takes time and comes with experience (e.g. ‘slipping isn’t failing’). Among many, a lapse was viewed as a transitional learning experience and for these participants the progression to relapse was less likely. Many participants reported how they were more likely to experiment with alternative coping strategies in the future, which may lead to the learning of more effective coping responses in high-risk situations. Indeed, for these participants, relapse prevention appeared to be an iterative process, as they talked about learning from the situations and strategies used during past lapses to succeed. Abstainers, in particular, conceived of relapse as a dynamic process of learning strategies and techniques to cope with temptation and cravings over successive quit attempts, of expanding and honing their capabilities over time and making use of existing resources available to them to achieve this.
For these participants, a lapse was differentiated from relapse. More specifically, for these participants a lapse or a slip was not a failure, ‘it’s not falling off the wagon’, but rather an opportunity to experiment and learn about what is required for them and in their particular circumstances to stay quit:
Relapse for me, it doesn’t mean that I’ve failed, it means that I’ve tried and I’ll have to try harder [ . . . ] relapse prevention I think is about how to control my urges, it just makes me more confident if I manage to do it, so I also think it links to someone’s self-confidence, if you know what I mean, the more confident you are in thinking that you’ll achieve your goal they more successful you become.
Abstinent/NIC/3-month follow-up/female/38 years/England
Your success will probably be built on failures it doesn’t matter yeah [ . . . ] it’s just part of the process so actually it’s getting to know how your addiction works and getting to know how you overcome it [ . . . ] just go straight back to giving up.
Abstinent/NIC/3-month follow-up/male/59 years/England
For those who viewed a lapse as a learning experience, their successful coping was described as having been built on by learning from previous quit attempts. Lapses were positioned as part of the learning process to the extent that they help one know what to do to recover and return to abstinence:
Don’t beat yourself up because you might’ve slipped up once in the week so what, don’t beat yourself up about it, if you slip-up every day that’s a different thing, well that’s what I’m doing, I didn’t beat myself up about it, I didn’t go oh well I’ve blown it I’ve got back on it.
Lapsed/NIC/3-month follow-up/female/60 years/Australia
It’s just a setback, you haven’t failed, it’s just a setback and start again but you know what you’ve learnt in the last few months and just apply them again, you can do it, [ . . . ] it’s easier to go back to ciggies than it is to stay stopped, I’d just say you know just try and do it again and keep on going, use these cigarette things where they help you and you’ll do it, eventually you’ll do it.
Abstinent/NIC/3-month follow-up/male/54 years/England
Keep persisting and if you fall off the band wagon just get back on again and [laughs] yeah it’s like that saying if you fall off the horse just get back on and try again. I mean it took me you know like three attempts just to finally stop.
Abstinent/NIC + S3P/3-month follow-up/female/33 years/England
Some participants described how they had learnt which triggers lead to smoking, which situations to avoid, or which attitude or approach was required through previous lapsing experiences, which enabled them to have more ‘control’ over urges to smoke:
When I have done it in the past, it’s more about how I’ve approached, like if I go into it with oh it doesn’t really matter type-thing and I’m really blasé about it then I find I’m just like one more packet won’t hurt me, if I’m not strict with myself it turns back into full-blown relapse where I’m smoking all the time [ . . . ] I find that I’m a lot more strict with myself, yeah if I go into it very blasé I find that I lose control.
Relapsed/usual care/6-month follow-up/female/33 years/Australia
Many participants reported that lapses were particularly common in situations involving alcohol consumption. Alcohol and smoking were frequently described as going together ‘hand in hand’, and several participants reported difficulty in refraining from smoking while consuming alcohol:
It’s mostly just the nights out when there’s alcohol involved that’s when I relapse.
Lapsed/NIC + S3P/3-month follow-up/male/24 years/Australia
There was some discussion of ‘permissive’ lapses. 29 These were lapses that they decided to allow:
I’ve just thought that I’ll decide to have a smoke today.
Lapsed/S3P/6-month follow-up/female/35 years/Australia
Alternatively, ‘permissive’ lapses were lapses that participants felt were somehow under their control:
I felt that it was my own decision and I was kind of empowered.
Lapsed/S3P/6-month follow-up/female/35 years/Australia
Permissive lapses were described as ‘disappointing’, ‘upsetting’ and ‘annoying’, although they had been ‘justifiable’, as some participants reported how they ‘still felt quite in control of that decision’ to smoke, as they were not buying cigarettes or not carrying cigarettes around all the time, and therefore did not consider themselves to be a smoker:
A little bit disappointed in myself I guess, but I still felt quite in control of that decision, so I guess I was able to justify to myself, you know like it’s OK I’ll just have one, and I wouldn’t consider myself to be a smoker again, I haven’t bought a packet of cigarettes, I don’t carry them with me all the time you know. It doesn’t matter if I just have one, because that’s not going to ruin my whole plan of quitting so I was still quite focussed on the end goal of not becoming a full-on smoker again.
Lapsed/S3P/6-month follow-up/female/35 years/Australia
Following the lapse, participants would want to ‘move on’ and go back to quitting. However, serial lapses were common among these participants. Alcohol was, again, frequently involved in the occurrence of these permissive lapses. Previous quit attempts had equipped participants with knowledge about how to cope with temptations to smoke while socialising and drinking alcohol:
There were times when I hadn’t smoked for 2 years, and you just drop your guard, you say save me some of that cigarette, and the next minute you buy 20 then, so it’s the old adage isn’t it, if you’re socialising with alcohol, or even sometimes just socialising, it’s funny, you could be in a good mood or sometimes if something really goes wrong or you’ve had a bad day you know, I think if you’re not as strong as I am now, when I was weaker if something, if I got bad news, your first thought would be I need a cigarette, whereas now I know that if I buy a packet of cigarettes I’m not helping myself.
Lapsed/NIC/3-month follow-up/male/44 years/England
Despite determination to stay quit, it was apparent that some participants felt that a lack of support and negative affect (such as stress, loneliness or boredom) or unexpected life events (such as a relationship ending or loss of a loved one) contributed to lapses, which in some cases were followed by prolonged periods of returning to smoking. Having been smokers for a long period of time contributed to this, as smoking had been the response to such occasions over a period of time and unlearning this was difficult. However, there were also a few reports of lapse progressing to relapse while relaxed on holiday, for example, which for some had also been partly because of the low cost of cigarettes.
Chapter 6 Ecological momentary assessment substudy results
Table 22 shows the characteristics of participants recruited to the EMA substudy. Approximately 53% of EMA participants were recruited from Australia. The sample comprised largely middle-aged smokers, and around half (52%) were female, 25% were in full-time employment and 68% were in receipt of benefits. Recruitment varied across the arms, with 46% of the target usual-care participants enrolled, whereas only 28%, 40% and 44% of the target participants were enrolled from the S3P, NIC and NIC + S3P arms, respectively.
| Baseline characteristic | Study arm | |||
|---|---|---|---|---|
| Usual care (N = 23) | NIC (N = 20) | S3P (N = 14) | NIC + S3P (N = 22) | |
| Age (years), median (IQR) | 47 (34–57) | 45 (33–53) | 45 (32–62) | 48 (31–56) |
| Female, n (%) | 13 (56.5) | 8 (40.0) | 4 (28.6) | 16 (72.7) |
| Partner smokes: yes, n (%) | 4 (17.4) | 0 (0) | 2 (14.3) | 5 (22.7) |
| Mental health condition: yes, n (%) | 11 (47.8) | 6 (30.0) | 3 (21.4) | 8 (36.4) |
| In full-time employment, n (%) | 6 (26.1) | 6 (30.0) | 4 (28.6) | 4 (18.2) |
| Receiving benefits, n (%) | 14 (60.9) | 15 (75.0) | 11 (78.6) | 14 (63.6) |
| Heaviness of Smoking Index, n (%) | ||||
| Low | 5 (21.7) | 4 (20.0) | 1 (7.1) | 0 (0) |
| Medium | 15 (65.2) | 15 (75.0) | 11 (78.6) | 19 (86.4) |
| High | 3 (13.0) | 1 (5.0) | 2 (14.3) | 3 (13.6) |
| Using base medication, n (%) | 12 (52.2) | 9 (45.0) | 8 (57.1) | 14 (63.6) |
| Ethnicity, n (%) | ||||
| Australian born (non-aboriginal) | 11 (84.6) | 9 (75.0) | 5 (83.3) | 10 (90.9) |
| White British | 8 (80.0) | 8 (80.0) | 8 (100.0) | 8 (72.7) |
| Country, n (%) | ||||
| Australia (n = 42) | 13 (56.5) | 12 (60.0) | 6 (42.9) | 11 (50.0) |
| England (n = 37) | 10 (43.5) | 8 (40.0) | 8 (57.1) | 11 (50.0) |
Table 23 shows the smoking status of the EMA participants at the approximate time they were recruited into the substudy.
| Smoking statusa | Study arm, n (%) | |||
|---|---|---|---|---|
| Usual care (N = 23) | NIC (N = 20) | S3P (N = 13)b | NIC + S3P (N = 22) | |
| Abstainer (not a puff since baseline survey) | 16 (69.6) | 12 (60.0) | 10 (77.0) | 17 (77.3) |
| Lapser (smoked, but not ≥ 7 consecutive days since baseline survey) | 5 (21.7) | 8 (40) | 3 (23.0) | 5 (22.7) |
| Relapser (≥ 7 days of continuous smoking since baseline survey) | 2 (8.7) | 0 (0) | 0 (0) | 0 (0) |
Participants were asked to report every lapse they experienced during monitoring in real time. Overall, 54 participants reported a total of 227 lapse episodes during monitoring (i.e. an average of 4.2 lapses per lapser). A total of 68.4% of all participants in the EMA substudy therefore experienced one or more lapse episodes during the 3 weeks of monitoring. The number of lapse episodes across the four treatment groups was 86 in the usual-care arm, 62 in the S3P arm, 36 in the NIC arm and 43 in the S3P + NIC arm.
Among 78 participants (one EMA substudy participant had missing data; see Table 23) classified as abstainers, lapsers and relapsers when joining the EMA substudy, 33 (60% of the participants initially categorised as abstainers), 18 (85.71% of the lapsers) and 2 (100% of the relapsers) reported a lapse in real time during the EMA monitoring.
Nine participants who joined the EMA substudy as ‘non-relapsers’ (n = 76) experienced five or more lapses during the 3-week monitoring period.
The antecedents and immediate consequences of lapse episodes, by treatment group, are shown in Table 24. Of the 227 smoking lapses reported in real time by participants, 168 events (usual care, n = 69; S3P, n = 51; NIC, n = 19; NIC + S3P, n = 29) included contextual data and therefore could be used to explore correlates of lapses. These lapses were reported by 39 of the 54 lapsers (usual care, n = 12; S3P, n = 5; NIC, n = 13; NIC + S3P, n = 9).
| Lapse episode | Study arm | |||
|---|---|---|---|---|
| Usual care (n = 69) | NIC (n = 19) | S3P (n = 51) | NIC + S3P (n = 29) | |
| Mean (SD) number of lapses | 5.8 (12.00) | 1.5 (0.66) | 10.2 (18.41) | 3.2 (3.87) |
| Mean (SE) craving before lapse | 39.93 (6.95) | 44.59 (9.74) | 18.95 (8.41) | 56.52 (8.91) |
| Concentrationa before lapse, mean (SE) | 37.54 (6.69) | 29.64 (8.83) | 14.12 (8.21) | 30.20 (8.35) |
| Felt like abandoning quit attempt after lapse,b mean (SE) | 59.04 (6.58) | 65.41 (8.92) | 97.60c (7.90) | 30.04c (7.91) |
None of the intervention groups differed significantly from the usual-care group in terms of the level of craving reported during lapses. As users of high-dose nicotine patches have been shown to be more likely to lapse under situations that involve low levels of craving,26 we examined the frequency of lapses that occurred in situations with little or no craving. Overall, approximately one-third (32.7%) of lapses occurred in low-craving situations (defined as events where craving was rated as ≤ 10 on a scale of 0–100 single-item craving measure). Surprisingly, participants in the S3P group were significantly more likely than those in the usual-care group to report lapses during low craving intensity (66.9% vs. 16.1%; p < 0.05) and neither the NIC (25.7%) nor the NIC + S3P (25.2%) group differed from the usual-care group. Participants in the intervention groups did not differ from those in the usual-care group in levels of concentration immediately before a lapse episode (see Table 24).
Previous studies have reported that the use of coping mechanisms during a cessation attempt is associated with better treatment outcomes. We examined the use of coping mechanisms reported during lapse events. Overall, participants reported using coping mechanisms – either behavioural or cognitive – in less than half (43.2%) of lapse events (usual care, 51.3%; S3P, 23.1%; NIC, 51.5%; NIC + S3P, 34.4%).
Following a lapse, participants in the S3P group were significantly more likely to feel like abandoning their quit attempt than participants in the usual-care group (see Table 24). Conversely, participants in the NIC + S3P group reported being significantly less likely to feel like abandoning their quit attempt than participants in the usual-care group.
Chapter 7 Health economics
As described earlier, the health economics analysis could not be carried out, as 12-month data were not collected in the curtailed trial. However, it is estimated that the development of the S3P intervention for the trial cost approximately £9000.
Although no 12-month data are available, the EQ-5D was administered at baseline and the data are presented in Table 25. There was some variation in the dimension scores by study arm, but subjective ratings of participants’ overall health at baseline (visual analogue scale scores) were similar and the majority of participants were in good health.
| Measure | Study arm | |||
|---|---|---|---|---|
| Usual care (N = 60) | NIC (N = 58) | S3P (N = 57) | NIC + S3P (N = 59) | |
| VAS score, median (IQR) | 69 (50–80) | 70 (65–80) | 70 (60–81) | 70 (58–84) |
| Mobility, n (%) | ||||
| No problems | 40 (66.7) | 44 (75.9) | 41 (71.9) | 44 (74.6) |
| Slight problems | 11 (18.3) | 9 (15.5) | 9 (15.8) | 5 (8.5) |
| Moderate problems | 4 (6.7) | 4 (6.9) | 4 (7.0) | 8 (13.6) |
| Severe problems | 5 (8.3) | 1 (1.7) | 3 (5.3) | 2 (3.4) |
| Self-care, n (%) | ||||
| No problems | 50 (83.3) | 53 (91.4) | 51 (89.5) | 55 (93.2) |
| Slight problems | 4 (6.7) | 4 (6.9) | 2 (3.5) | 2 (3.4) |
| Moderate problems | 5 (8.3) | 1 (1.7) | 3 (5.3) | 1 (1.7) |
| Severe problems | 1 (1.7) | 0 (0) | 2 (1.8) | 1 (1.7) |
| Usual activities, n (%) | ||||
| No problems | 38 (63.3) | 40 (69.0) | 40 (70.2) | 48 (81.4) |
| Slight problems | 14 (23.3) | 13 (22.4) | 9 (15.8) | 5 (8.5) |
| Moderate problems | 2 (3.3) | 5 (8.6) | 2 (3.5) | 4 (6.8) |
| Severe problems | 3 (5.0) | 0 (0) | 6 (10.5)c | 2 (3.4) |
| Pain/discomfort, n (%) | ||||
| No problems | 27 (45.0) | 34 (58.6) | 28 (49.1) | 29 (49.2) |
| Slight problems | 14 (23.3) | 12 (20.7) | 15 (26.3) | 16 (27.1) |
| Moderate problems | 10 (16.7) | 10 (17.2) | 12 (21.1) | 11 (18.6) |
| Severe problems | 6 (10.0) | 2 (3.5) | 2 (3.5) | 2 (3.4) |
| Extreme pain/discomfort | 3 (5.0) | 0 (0) | 0 (0) | 1 (1.7) |
| Anxiety/depression, n (%) | ||||
| No problems | 23 (38.3) | 27 (46.6) | 26 (45.6) | 26 (44.1) |
| Slight problems | 15 (25.0) | 15 (25.9) | 18 (31.6) | 18 (30.5) |
| Moderate problems | 14 (23.3) | 12 (20.7) | 11 (19.3) | 9 (15.3) |
| Severe problems | 6 (10.0) | 3 (5.2) | 2 (3.5) | 4 (6.8) |
| Extremely anxious/depressed | 2 (3.3) | 1 (1.7) | 0 (0) | 2 (3.4) |
Chapter 8 Discussion
The curtailed trial lacked power to detect effects that the interventions could reasonably be expected to have, but it nevertheless generated outcome data that can contribute to future meta-analyses, as well as novel data on ex-smokers’ reactions to different relapse prevention interventions.
Acceptability of the interventions
Initiation of allocated interventions was high, suggesting that both interventions are acceptable to recent ex-smokers. The qualitative study suggests that both interventions were perceived as sensible and helpful. Both interventions also obtained generally positive ratings. These are encouraging findings, although, as noted below, these need to be taken into account with the findings that NRT/e-cigarettes were more likely to be used than QuitCoach, which most people used only once. Some relapse prevention interventions examined in the past seem to have generated little enthusiasm and there was a concern that smokers who have recently quit smoking may feel that they have achieved their objective and have little interest in trying to prevent any theoretical lapses. The positive ratings could have been in part because some of the study participants were still in the early stages of smoking cessation rather than abstinent for at least 4 weeks, as originally planned. This, however, concerns only a minority of participants in Australia, and treatment ratings were positive at both trial sites. Overall, we can conclude that most participants found their allocated intervention of some utility, but there was variation.
Nicotine products
Regarding nicotine products, e-cigarettes were selected more often than NRT in Australia (71% vs. 29%, respectively), but the difference was not significant in England (54% vs. 46%, respectively). This was unexpected in the English site, as e-cigarettes are more popular among UK smokers than NRT. 12,13 Given that nicotine-containing e-cigarettes are banned from general sale in Australia, we expected that they would be a popular choice there, as this study provided a way to access these products. It is possible that smokers in England who attend stop smoking services are less interested in vaping, or are more affected by media scares concerning e-cigarettes than smokers in Australia, where there is no vaping and, therefore, media scares are less prominent. Nevertheless, concerns around the safety of vaping were more likely to be voiced by participants in Australia in the qualitative research.
The intervention consisted of recommending opportunistic use when at risk of relapse and to use as much as needed to prevent a return to smoking. At the 3-month follow-up, we found that 53% of participants used their products at least occasionally (31% used daily). At the 6-month follow-up, we found that 40% of participants used their products at least occasionally (23% used daily). Of those still using their study product at least occasionally, 74% were using an e-cigarette and 26% were using NRT at 3 months, and the equivalent values at 6 months were 76% and 23% (one person used both). This shows a higher extended use of e-cigarettes than of NRT, but by a smaller margin than noted in a recent randomised trial15 (the trial, however, included nicotine patches as opposed to the fast-acting NRT that was used here). Faster-acting NRT products generate higher rates of extended use than nicotine patches. 30,31
Regarding product safety, there were no SAEs related to trial medications. The most common AE reported was weight gain, which is a common side effect of quitting smoking. 32 Indeed, it is notable that those in the groups provided with nicotine products were less likely to report AEs, suggesting that a proportion of AEs are due to abstinence from nicotine. However, as the differences were not significant this possibility should be treated with caution.
Behavioural interventions
Behavioural interventions included a series of practical recommendations for implementing behavioural and cognitive coping strategies imparted via the online QuitCoach and Problem Planner. The intervention was further accompanied by an intensive texting schedule that was tailored to participants’ responses to an online assessment and included an option for interactive messaging.
Although the comprehensive package received overall positive ratings, both for the online and for the text messaging components, its utilisation in terms of site visits, assessments and use of interactive texting was low. Most participants completed only a single QuitCoach assessment, typically immediately after joining the study (i.e. on the same website visit), and only one-third of participants reported using the Problem Planner. Only one participant used the interactive feature of the text messaging, indicating that participants either did not see a need for it or were not aware of it. Future work may need to focus on encouraging re-engagement with such tools. Most participants indicated that they read the advice that the programme generated for them ‘quickly’. The qualitative study offers some insights into the reasons for this. Clients needed usernames and passwords to visit the site, which represented a significant barrier to some of them. The extensive text and long questionnaires may have also limited engagement with the program. Some of these features were because of the trial components, but future iterations of the program may look at simplifying access to the information, as well as looking at ways to present the content differently. The revised version of QuitCoach that was being programed for the study (but ultimately was not delivered by our IT consultants) would have provided the capacity to break the assessment and advice into smaller chunks. Use of a version with capacity to break the advice up and provide summary versions, as well as the full advice, may better meet the varying needs of participants.
We hoped that the detailed follow-up questionnaires would identify coping strategies that the behavioural intervention imparted (i.e. strategies used by participants in the S3P and NIC + S3P study arms but not by participants in the other two arms) and highlight advice that was most popular, suggesting components that were particularly practicable and useful. Of the six listed strategies, five were used by the majority of participants at both the 3- and 6-month follow-ups. The most popular advice was reminding oneself of reasons for quitting, used by some 90% of participants. Some 80% used distraction. The most demanding feature that was the focus of the intervention (i.e. putting in place a pre-prepared plan for resisting temptations) was used by approximately one-third of participants. However, unexpectedly, the proportion of those who reported using each coping strategy when tempted to smoke was practically identical in the non-S3P groups (i.e. usual care and NIC only). This included the use of a pre-prepared plan. More relevant than whether or not planning occurred, however, may be the quality of planning, something which is more difficult to quantify. Recognising that most ex-smokers engage in some form of planning,33 the intention of the S3P intervention was primarily to increase the quality of existing planning strategies and to help ensure that they were remembered and acted on at times they were needed. At the intermediate follow-ups (i.e. at 3 and 6 months), we could not ask participants in all study arms about whether or not they had formed strategies in terms of ‘if–then’ plans (implementation intentions). This would have made little sense to those in the non-S3P arms, but more importantly risked exposing these participants to a key idea underlying the S3P intervention. The EMA substudy also found no differences in coping strategies used by different study arms when faced with lapse situations. Overall, these findings could suggest that the S3P intervention had limited effects on the actual behavioural responses to temptations to smoke. However, given the trend for this intervention to reduce relapse, it may have supported people in another way (e.g. promoting recovery from a lapse) that we have not investigated in this report.
Process of relapse
Approximately half of the participants maintained sustained abstinence at 6 months. Of those taking part in the EMA substudy, which monitored lapses in real time, two-thirds experienced a lapse during the 3 weeks of monitoring. This included lapses in participants who were classified as having lapsed or relapsed already (n = 20) and also 33 participants who were, until the EMA monitoring, classified as sustained abstainers. A good proportion of lapses happened when craving levels were relatively low, and many lapses made participants feel like abandoning their quit attempt. There was no clear signal of any intervention effects on these variables.
The EMA substudy found few significant differences between the usual-care group and the intervention group. A significant difference was observed in the experience of low-craving lapses. As noted earlier, it has previously been reported that users of high-dose nicotine patches are more likely to lapse under situations that involve low levels of craving. 26 Here, however, participants in the S3P group were significantly more likely than those in the usual-care group to report lapses during low craving intensity. This finding is difficult to explain, as participants in the S3P group were provided additional information about coping with craving and avoiding lapses, including the use of implementation intentions. However, it may be that those allocated to S3P were more likely to recover from a lapse, which is also something the content focuses on. This and other differences between groups need to be explored in future, using more adequately powered studies, before firm conclusions can be drawn.
Some lessons from the trial tribulations
The trial faced serious difficulties in integrating a proprietary online programme with the data collection requirements of a randomised controlled trial, which led to significant delays in recruitment. Future trials using such interventions need to consider the vulnerability of specific programs and apps to the demands of data management. Even more importantly, trials that involve international collaborations need to research relevant data protection and IT security requirements, as these can generate major hurdles.
Another issue that can serve as a warning to future projects was difficulties in recruitment. Recruiting recent quitters from quitlines proved much more challenging than expected, and we expected it to be hard. The main problems included (1) marked drop-offs in the use of quitline services before recruitment; (2) reluctance to enrol in a new intervention study after succeeding with support already provided; (3) unwillingness to participate in a trial that could result in being assigned to a pharmacotherapy intervention; and (4) greater difficulties experienced by quitline specialists in contacting callers on their last scheduled call. In England, over the past few years, the throughput of the stop smoking services has shrunk by > 60% from their inception, and the services have been relocated from NHS to local councils, which commission private providers to deliver stop smoking treatments. The reorganisation and the change of service focus meant that only a few services remained that could contribute to research. Future studies, at least those conducted in England, that involve smoking cessation may need to identify recruitment venues other than stop smoking services. Planning for recruitment in other UK countries may have also been a useful strategy. However, this planning needs to take place early, as gaining regulatory approvals takes time.
In Australia, we attempted to recruit other quitlines. However, we were unsuccessful, largely as a result of one arm of the study involving vaping products. Nevertheless, even if we had been able to recruit all quitlines, recruitment would have still been below what we needed. In Australia, we conclude that the required strategy of recruiting ex-smokers after successfully quitting for 1 month is not feasible, at least from services that had already provided a quite intensive programme of help.
In Australia, we tested two alternative methods of recruitment: (1) use of social media and (2) recruiting from hospitals where patients are not allowed to smoke as inpatients. Our conclusion is that social media are probably a useful recruitment source, but their use can be complicated when there are complex inclusion criteria for the trial, as there were in our case. Furthermore, social media are not likely to be a major approach for service delivery, although promoting effective programmes on social media is likely to draw some traffic and may be able to do this at a reasonable cost.
With regard to recruiting from hospital inpatients, overall, initial recruitment in Australia relied heavily on manual screening processes of patients’ smoking status and receiving referrals from other health professionals within the hospital. This process was later further automated, but is something to consider early on in the set up process.
Common to both countries was a greater than expected intention to use medications or vaping devices for extended periods (i.e. > 3 months), therefore rendering participants ineligible for this trial.
These issues highlight the need for pilot phases within large trials with clear stop/go criteria.
Study limitations
The key study limitation is the curtailed sample size that limited the power that the trial had to detect intervention effects. However, study outcomes can still contribute to future meta-analyses. Related to study curtailment, the follow-up period was shortened from 12 to 6 months. As the efficacy of smoking cessation interventions typically declines over time, the hint we detected that the single interventions may show some efficacy needs to be interpreted with caution. Another consequence of the trial curtailment is the lack of biochemical validation. This means that the abstinence rates are likely to have been overestimated overall and, in particular, in the intervention groups that received more input and may have felt a stronger obligation to report success. It is reassuring that the NIC + S3P group reported somewhat higher relapse than the NIC and S3P groups, but the numerical advantage of all intervention groups compared with usual care could still reflect different response expectations.
The trial curtailment also meant that the planned health economics analyses could not be carried out, as follow-up data collection for the EQ-5D and HSUQ was planned only at 12 months.
The trial was originally designed to test the interventions following formal stop smoking treatment from either the English stop smoking services or Australian quitlines. The addition of recruitment via Facebook, St Vincent’s Hospital Melbourne and Stoptober meant that 34% of participants had not received any formal stop smoking support. Furthermore, extending the eligibility window meant that some participants entered the trial after only 1 week quit in Australia, whereas others joined after 3 months quit. This variation reduces the focus on relapse happening from around 1 month, as originally planned. However, the notion that relapse prevention only begins at 4 weeks of abstinence is arbitrary. It can be argued that from the moment that a person stops smoking they require strategies that help prevent them from relapsing to smoking.
Another feature of the trial that warrants additional caution is in assessing relapse rates overall, in particular the interpretation of findings for the control group. The qualitative and EMA substudies were originally planned to involve only a small subsample of a large trial, with identical sampling across study arms. In the curtailed study, the qualitative aspects of the project are relatively more important. A much larger proportion of the sample took part in qualitative interviews, approximately 40%, and approximately one-third of participants took part in the EMA substudy. We were also unable to balance the proportions of lapsers, relapsers and abstainers within or between the study arms, and those participating in these substudies were less likely to have relapsed than those who did not. A potential issue that this created is that qualitative interviews suggested that EMA data collection represented a perceived intervention, and the interviews themselves could have had this impact too. The EMA substudy and qualitative interviews could have provided motivational input, perhaps more memorable than online instructions because of their interactive nature, and could have even have imparted or reinforced coping strategies by focusing on their details during surveys and EMA monitoring. Such input could, in theory, have contributed to the lack of differences in the use of coping strategies between study arms, as discussed above. It could have also boosted abstinence rates, especially in the control group, and therefore have diluted any intervention effects. However, given the self-selection for these substudies, it is not possible to make any definitive conclusions regarding these effects.
The overall 6-month follow-up rate was 88.5%, which is relatively high for trials of this type. Achieving higher follow-up rates is difficult, as smokers who are unsuccessful tend to feel embarrassed and avoid contact. It is reassuring that follow-up rates across the four study arms were similar, as were the follow-up rates in the two countries. Nevertheless, incomplete data represent a limitation.
Generalisability
The majority of participants (66%) received structured stop smoking support before joining the trial. Therefore, the results may not be generalisable to populations who quit unaided or without formal support. This is particularly true of participants in the nicotine product arms, as e-cigarettes are readily available in England and are widely used without any structured support as an effective tool to quit smoking. 34,35
Recommendations for research
The efficacy of fast-acting nicotine products provided for use in emergencies and accompanied by text message reminders for relapse prevention may deserve further exploration. Online training programmes have good intuitive validity and should also be further examined. An intermediate aim could be to develop delivery modes that ensure that participants recall the advice and act on it. Once this has been established, evaluating the intervention in a simplified package (e.g. as a mobile phone app) would be the natural next step. Another recommendation is that future trials should aim to initiate relapse prevention interventions around the time of quitting. Delaying the offer until later in a successful quit attempt risks a reduced likelihood of treatment uptake. That said, it should also be available for those who have quit and seek extra help as well, but this is likely to represent only a minority (largely self-quitters who find themselves in trouble). There may also be utility in testing these interventions in those who are hospitalised and unable to smoke. Our experience suggests that this will be best done if the relapse prevention interventions can be initiated while hospitalised, and so advice and/or medications can be provided before the person is discharged. Future studies should also consider using simpler designs, and plan for the challenges faced when integrating trial data management systems into existing programs or apps. Furthermore, international collaborations involving such systems will need to factor in data protection and IT security requirements.
Conclusion
Both interventions were well received and safe. The effects of the combined interventions did not surpass those of each intervention alone. There was a trend in favour of single interventions reducing relapse, but it did not reach statistical significance and there are reasons to interpret the trend with caution.
The EMA substudy showed that two-thirds of participants experienced a lapse, and around one-third of these lapses happened when craving levels were relatively low. For many, lapses made participants feel like abandoning their quit attempt.
Adherence to nicotine products was high, with e-cigarettes preferred to medicinal nicotine products. Behavioural advice was appreciated, but did not seem to affect behaviour. Nonetheless, the findings of this curtailed trial suggest that these interventions may have promise, but more likely as alternative interventions, as there is no evidence they work additively or synergistically.
Acknowledgements
We would like to thank the following:
-
all trial participants
-
St Vincent’s Hospital Melbourne, the stop smoking services and the quitlines that referred participants
-
TSC and DMEC members, particularly the chairpersons, Dr Nicola Lindson and Dr Jamie Hartman-Boyce, and the lay member, Mr Dan Griffin
-
National Institute for Health Research research project managers, Hazel Church, Zoe Hurworth and Roderick Delanougerede
-
all of the team at the Health and Lifestyle Research Unit and Cancer Council Victoria, past and present, who worked on the trial, in particular Krista Murray, Tessa Letcher and Laura Miller
-
Mr Steve Parrott, Professor Alan Brennan and Professor Petra Meier for their contribution to the health economics aspect of the original study design
-
Tim Albion for his support and guidance with REDCap
-
the Barts CTU (now King’s CTU), in particular Benoit Aigret and Jonathan Croft for their support throughout the trial
-
Public Health England for providing the funds to purchase the nicotine products in England.
Dedication
In March 2020, while this report was being revised, James Balmford died suddenly after a short illness. He made a large contribution to the study and is greatly missed.
Contributions of authors
Hayden J McRobbie (https://orcid.org/0000-0002-7777-1845) (Professor in Public Health Interventions) led on the original grant application, co-designed the trial, co-wrote the statistical analysis plan, trained staff, interpreted the study findings and co-led on the drafting of the report.
Anna Phillips-Waller (https://orcid.org/0000-0001-6856-6439) (Research Manager) managed the English trial site, co-wrote the statistical analysis plan, trained staff, contributed to data collection, interpreted the study findings and co-led on the drafting of the report.
Catherine El Zerbi (https://orcid.org/0000-0003-4041-2379) (Postdoctoral Research Associate) conducted the qualitative interviews, co-wrote the statistical analysis plan, interpreted the study findings and assisted with the drafting of the report.
Ann McNeill (https://orcid.org/0000-0002-6223-4000) (Professor of Tobacco Addiction) led the qualitative substudy, co-wrote the statistical analysis plan, interpreted the study findings and assisted with the drafting of the report.
Peter Hajek (https://orcid.org/0000-0001-9160-4296) (Professor of Clinical Psychology) contributed to trial design, co-wrote the statistical analysis plan, interpreted the study findings and co-led on the drafting of the report.
Francesca Pesola (https://orcid.org/0000-0002-2054-7930) (Research Fellow) co-wrote the statistical analysis plan, analysed the study data and assisted with the drafting of the report.
James Balmford (https://orcid.org/0000-0003-1423-4361) (Research Fellow) assisted with the development, refinement and maintenance of the S3P intervention and electronic data management system, co-wrote the statistical analysis plan, interpreted the study findings and assisted with the drafting of the report.
Stuart G Ferguson (https://orcid.org/0000-0001-7378-3497) (Associate Professor) led the EMA substudy, co-wrote the statistical analysis plan, interpreted the study findings and assisted with the drafting of the report.
Lin Li (https://orcid.org/0000-0002-4764-1679) (Postdoctoral Research Fellow) managed the Australian trial site, co-wrote the statistical analysis plan, trained staff, contributed to data collection, interpreted the study findings and assisted with the drafting of the report.
Sarah Lewis (https://orcid.org/0000-0001-5308-6619) (Professor of Medical Statistics) co-wrote the statistical analysis plan, provided statistical oversight and assisted with the drafting of the report.
Ryan J Courtney (https://orcid.org/0000-0003-1339-3221) (Senior Research Fellow) co-wrote the statistical analysis plan, interpreted the study findings and assisted with the drafting of the report.
Coral Gartner (https://orcid.org/0000-0002-6651-8035) (Associate Professor) assisted with the study set-up in Australia, interpreted the study findings and assisted with the drafting of the report.
Linda Bauld (https://orcid.org/0000-0001-7411-4260) (Bruce and John Usher Professor of Public Health) assisted with the drafting of the report.
Ron Borland (https://orcid.org/0000-0003-0059-178X) (Professor of Psychology, Health Behaviour) led the Australian study site, co-wrote the original grant application, co-designed the trial, co-wrote the statistical analysis plan, trained staff, interpreted the study findings and assisted with the drafting of the report.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Stapleton J. Cigarette smoking prevalence, cessation and relapse. Stat Method Med Res 1998;7:187-203. https://doi.org/10.1177/096228029800700206.
- NHS Digital . Statistics on NHS Stop Smoking Services in England April 2019 to June 2019 2019. https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-nhs-stop-smoking-services-in-england/april-2019-to-june-2019 (accessed September 2020).
- Marlatt GA, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York, NY: Guilford Press; 2005.
- Livingstone-Banks J, Norris E, Hartmann-Boyce J, West R, Jarvis M, Chubb E, et al. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev 2019;2019. https://doi.org/10.1002/14651858.CD003999.pub6.
- Agboola S, McNeill A, Coleman T, Leonardi Bee J. A systematic review of the effectiveness of smoking relapse prevention interventions for abstinent smokers. Addiction 2010;105:1362-80. https://doi.org/10.1111/j.1360-0443.2010.02996.x.
- Segan C, Borland R. Does extended telephone callback counselling prevent smoking relapse?. Health Educ Res 2011;26:336-47. https://doi.org/10.1093/her/cyr009.
- Turner J, McNeill A, Coleman T, Bee JL, Agboola S. Feasibility of offering nicotine replacement therapy as a relapse prevention treatment in routine smoking cessation services. BMC Health Serv Res 2013;13. https://doi.org/10.1186/1472-6963-13-38.
- Snuggs S, McRobbie H, Myers K, Schmocker F, Goddard J, Hajek P. Using text messaging to prevent relapse to smoking: intervention development, practicability and client reactions. Addiction 2012;107:39-44. https://doi.org/10.1111/j.1360-0443.2012.04084.x.
- Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev 2009;1. https://doi.org/10.1002/14651858.CD003999.pub3.
- Smith AL, Carter SM, Dunlop SM, Freeman B, Chapman S. The views and experiences of smokers who quit smoking unassisted. A systematic review of the qualitative evidence. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0127144.
- Borland R, Balmford J, Swift E. Effects of encouraging rapid implementation and/or structured planning of quit attempts on smoking cessation outcomes: a randomized controlled trial. Ann Behav Med 2015;49:732-42. https://doi.org/10.1007/s12160-015-9706-3.
- Brown J, West R, Beard E, Michie S, Shahab L, McNeill A. Prevalence and characteristics of e-cigarette users in Great Britain: Findings from a general population survey of smokers. Addict Behav 2014;39:1120-5. https://doi.org/10.1016/j.addbeh.2014.03.009.
- Action on Smoking and Health . Use of Electronic Cigarettes (Vapourisers) Among Adults in Great Britain 2015. https://ash.org.uk/information-and-resources/fact-sheets/statistical/use-of-e-cigarettes-among-adults-in-great-britain-2019/ (accessed September 2020).
- Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD010216.pub3.
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019;380:629-37. https://doi.org/10.1056/NEJMoa1808779.
- McNeill A, Driezen P, Hitchman SC, Cummings KM, Fong JT, Borland R. Indicators of cigarette smoking dependence and relapse in former smokers who vape compared with those who do not: findings from the 2016 International Tobacco Control Four Country Smoking and Vaping Survey. Addiction 2019;114:49-60. https://doi.org/10.1111/add.14722.
- Gollwitzer PM. Implementation intentions: strong effects of simple plans. Am Psychol 1999;54. https://doi.org/10.1037/0003-066X.54.7.493.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86:1119-27. https://doi.org/10.1111/j.1360-0443.1991.tb01879.x.
- The Euroqol Group . EQ-5D-5L User Guide: Basic Information on How to Use the EQ-5D-5L Instrument 2013.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. https://doi.org/10.1093/intqhc/mzm042.
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol 2008;4:1-32. https://doi.org/10.1146/annurev.clinpsy.3.022806.091415.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. https://doi.org/10.1111/j.1360-0443.2004.00995.x.
- Glaser B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. New Brunswick, NJ: Aldine Transaction; 1967.
- Higginbotham N, Albrecht G, Connor L. Health Social Science: A Transdisciplinary and Complexity Perspective. Docklands, VIC: Oxford University Press; 2001.
- Patton MQ. Qualitative Evaluation and Research Methods. New York, NY: SAGE Publications Ltd; 1990.
- Ferguson SG, Shiffman S. Effect of high-dose nicotine patch on the characteristics of lapse episodes. Health Psychol 2010;29:358-66. https://doi.org/10.1037/a0019367.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol 2014;67:267-77. https://doi.org/10.1016/j.jclinepi.2013.08.015.
- Notley C, Ward E, Dawkins L, Holland R, Jakes S. Vaping as an alternative to smoking relapse following brief lapse. Drug Alcohol Rev 2019;38:68-75. https://doi.org/10.1111/dar.12876.
- West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology 2000;149:198-202. https://doi.org/10.1007/s002130000382.
- Hajek P, McRobbie H, Gillison F. Dependence potential of nicotine replacement treatments: effects of product type, patient characteristics, and cost to user. Prev Med 2007;44:230-4. https://doi.org/10.1016/j.ypmed.2006.10.005.
- Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ 2012;345. https://doi.org/10.1136/bmj.e4439.
- Balmford J, Swift E, Borland R. Reported planning before and after quitting and quit success: retrospective data from the ITC 4-Country Survey. Psychol Addict Behav 2014;28:899-906. https://doi.org/10.1037/a0035711.
- Beard E, Jackson SE, West R, Kuipers MAG, Brown J. Population-level predictors of changes in success rates of smoking quit attempts in England: a time series analysis. Addiction 2020;115:315-25. https://doi.org/10.1111/add.14837.
- Jackson SE, Kotz D, West R, Brown J. Moderators of real-world effectiveness of smoking cessation aids: a population study. Addiction 2019;114:1627-38. https://doi.org/10.1111/add.14656.
Appendix 1 Supplementary tables
| Approved versiona | Date | Summary |
|---|---|---|
| 3.0 | 2 September 2016 | Original approved version |
| 4.0 | 9 February 2017 | Clarification and rephrasing of sections, minor changes to simplify trial procedures, correction of minor errors, and changes to web app and data management processes as a result of the issues experienced with QuitCoach |
| 4.1 | 8 August 2018 | Updated sponsor representative contact and addition of Stoptober campaign for participant recruitment |
| Approved version | Date of approval | Summary |
|---|---|---|
| 17-9-10 version | 15 August 2016 | First approved version by CCV HREC |
| 17-10-2 version | 11 October 2017 | Amendment to widen the eligibility window for participating to 7–100 days quit, and to post adverts on social media to supplement recruitment through quitlines |
| 18-4-17 version | 6 August 2018 (HREC/18/SVHM/155) | Seeking approval to recruit participants from St Vincent’s Hospital Melbourne |
| 19-M-7 version | 5 June 2019 | Amendment proposal to reduce the study follow-up duration to 6 months and adjust reimbursement accordingly |
| Name | Role | Committee |
|---|---|---|
| Dr Nicola Lindson | Chairperson (independent) | TSC |
| Professor Marcus Munafò | Member (independent) | TSC |
| Ms Jo Locker | Member (independent) | TSC |
| Mr Dan Griffin | Public member (independent) | TSC |
| Dr Sue Cooper | Member (independent) | TSC |
| Dr Jamie Hartman-Boyce | Chairperson (independent) | DMEC |
| Dr Lynne Dawkins | Member (independent) | DMEC |
| Dr Margaret MacDougall | Member (independent) | DMEC |
| Mrs Anna Phillips-Waller | England – study manager | TMG |
| Professor Hayden McRobbie | England – co-investigator | TMG |
| Professor Peter Hajek | England – chief investigator | TMG |
| Professor Ann McNeill | England – co-investigator | TMG |
| Professor Sarah Lewis | Senior study statistician | TMG |
| Mr Benoit Aigret | Head of Barts CTU (now King’s CTU) | TMG |
| Professor Ron Borland | Australia – chief investigator | TMG |
| Dr Lin Li | Australia – study manager | TMG |
Appendix 2 Topic guide
Qualitative substudy semistructured interview questions
Questions will differ depending on smoking/abstinence status, intervention condition and country. Below is a comprehensive list of the questions that will be tailored in accordance with the above criteria. Probes will be used throughout to elicit maximum information. We will have access to baseline and follow-up data on smoking history, lapses, relapses, etc., for the purposes of reference only.
-
Preamble on format and approximate length of time (45 minutes maximum).
-
Emphasise confidentiality.
-
Emphasise that there are no right or wrong answers.
-
Warm up conversation and test sound on audio-recorder with a question: how is it going? (Based on the response to this question, we will reallocate to lapse/relapse/abstainer.)
-
Clarify when the participant’s last cigarette was, including just a puff of a cigarette.
Below, the questions marked with * refer to the intervention questions for appropriate arm of the study.
Take notes and return to unanswered and unclear responses at the end of interview.
Questions for lapsers (adapted in line with any responses to the warm up)
Lapsers: a puff in the last 2 weeks but not smoked daily for at least 7 consecutive days, including participants who have been serial lapsers.
-
Have you had any cigarettes since (see REDCap for recruitment date), including just a puff of a cigarette?
-
Can you tell me about any cigarettes you have smoked in the last couple of weeks? First, when did you last smoke? How many and how often in the last 2 weeks? Did you have just a few puffs or the whole cigarette? (If relapse – switch, if abstinent – switch.) If multiple: was this one period of smoking or were they separated by cigarettes resisted? Now thinking about the first cigarette (in the last bout of smoking), what triggered that cigarette?
-
Did you do anything to try to resist? If yes, what?
-
Had you been in a similar situation before and successfully resisted? If yes, what was different this time?
-
How many cigarettes did you smoke before you recovered and resumed abstinence?
-
What did you do to resume your quit attempt? What thoughts did you have?
-
Had you tried previously to resume abstinence? How did it go?
-
Explore relationship to use of interventions below as appropriate.*
-
Explore relationship between intervention and identified triggers/situations as appropriate.*
-
How did you feel after smoking it? (Relate to intervention questions below as appropriate.)
-
Have you smoked any further cigarettes since that lapse? Can you remember the interval between your first lapse and second?
-
Have you been supported or influenced by family members, friends and other smokers to quit smoking?
-
Have you felt tempted to smoke further cigarettes since then? What strategies have you used to stop yourself smoking? (Relate to intervention questions below as appropriate.*)
-
How has this lapse experience differed from any previous quit attempts and lapses. If so, why was it different?
Lapse situations
-
Was there anything you could have done differently?
-
Did you anticipate that you might lapse in advance?
Permissive lapsing
-
How confident were you that you would be able to recover from that lapse?
-
Which, to you, is the most important benefit of quitting?
-
Looking back, hopefully it will help other people, but can you recall the occasion of your first lapse, and then what happened the second time? Did you know you would become a smoker again?
Questions for relapsers (adapted in line with any responses to the warm up)
Relapsers: smoking daily for at least 7 consecutive days, including participants who were lapsing and then relapsed subsequently, as well as participants who were abstinent and then relapsed.
-
Have you had any cigarettes since (see REDCap for recruitment date), including just a puff of a cigarette?
-
When was the last time you smoked? (Within 2 weeks?)
-
When did you first lapse? Can you say a bit about what you think might have led to this situation? Explore the situation leading up to the relapse, was it a lapse initially or a full-blown relapse in one go? [Use questions above, as appropriate, if initially lapses (particularly in relation to triggers, interval between lapses, and between lapses and relapse, plus any differences with previous quit attempts and full-blown relapses).]
-
Could you talk a little bit about resuming smoking and how you feel about this? Have you previously tried to remain abstinent from smoking and how do you feel about not being able to remain abstinent? (Explore timing of relapse in relation to use of interventions.*)
-
Prior to the relapse had you been using any effective coping strategies, for example when you had an urge for a cigarette? Had you been using any coping strategies and, if yes, which strategy did you find to be most effective? Why did you think this strategy was particularly helpful? Were there any strategies you found to be ineffective and, if yes, could you say why you think this strategy failed? (Relate to intervention questions below as appropriate.*)
-
What, if anything, might have prevented you from relapsing? (Relate to intervention questions below as appropriate.*)
-
How confident were you feeling about staying stopped in the run-up to your relapse?
-
Have you been supported or influenced by family members, friends and other smokers to quit smoking?
-
Will you be trying to quit again soon? (Explore interest in using the interventions if so, and explore if this is different from previous relapses and why.)
-
How did you feel before relapsing? And now?
-
How do you think you would feel if you successfully quit smoking?
Questions for abstainers (adapted in line with any responses to the warm up)
Abstainers: previously lapsed, or indeed relapsed, but subsequently abstinent in the last week.
Abstinence at 12 months: not a single puff in the last month at 12-month follow-up.
Continuous abstinence: not smoking a puff at each follow-up at 12 months.
-
Just to check, have you smoked any cigarettes at all since the start of our study?
-
What do you think lies behind your success on this quit attempt, and why? What are the main strategies/methods you have been using to help you stay quit? When did you start using the strategy/method?
-
How often, if at all, have you had to make special efforts to resist smoking? Have you been supported or influenced by family members, friends and other smokers to quit smoking?
-
Which, if any, strategies did you adopt? Which did you find to be the most successful?
-
Explore use of interventions as below.* (Probe individual strategies and relate to intervention questions below as appropriate.)
-
How do you feel since quitting smoking?
*Specific questions on interventions specific to the 3-month follow-up
Use knowledge of follow-up data to explore answers.
All (including usual care)
-
Check length of time using base medications (from follow-up data). Need to know if they were using at time of lapse? If using any Fast Acting Nicotine (FAN), did you use any to try to control the urge?
-
Can you recall receiving text messages? (Check follow-up data for the 1-week call to see if participant received and found useful the interactive texts. If available, skip.)
-
View on messages (e.g. which types of messages were most helpful and why)? Frequency of messages OK? (Probe milestone reinforcers, motivational messages, hints, etc.).
-
Suggestions for improvements?
-
Did you use any other products to help you stop smoking since (date)? If yes, explore why, what, how frequently, appeal, etc. [Specific questions for specific products (e.g. e-cigarette/NRT).]
S3P
-
How often did you use the QuitCoach programme and for how long? Overall, how useful was this and what did you find useful? Explore when/why they accessed it, frequency, etc., which aspects used?
-
How easy did you find it to complete the web-based assessment?
-
What do you remember about the tailored advice you were given about staying stopped? Did you find it to be helpful/relevant on every occasion? How could it be improved?
-
Do you recall the structured tool that helped you to generate statements about strategies that you could use when the urge or temptation to smoke occurred? Did you generate any such statements? Did you use your strategies? How could this tool be improved?
-
How useful did you find the follow-up call about how to use the S3P materials? How could it be improved? (Only for participants who were contactable for follow-up call.)
-
Check self-reported frequency of accessing the S3P online.
-
Link usage to lapse/relapse timing and whether or not still using and why?
-
Further suggestions for improvements?
Nicotine products
-
Check data about product chosen: I believe that you chose (see REDCap for e-cigarette/NRT product), is this correct?
-
Why did you choose this product? Probe: had you used this product before, or a similar product?
-
What is your overall impression of it?
-
(For current use of product check REDCap follow-up data.) How is it helping you?
-
Do you enjoy using it or do you find it a chore?
-
Are there any aspects of use you do not like?
-
How often did you use the smoking replacement product and for how long? Did you find it to be helpful/relevant on every occasion? Overall, how useful did you find it and why?
-
What do you recall of the materials we provided explaining how to use the product? (Check follow-up data for the 1-week call to see if participant received and found useful the interactive texts. If available, skip.)
-
Did products arrive on time, OK, etc.?
-
Did you use the product at all after receiving it? Probe frequency/quantity/any difficulties using it/appeal?
-
(Only for participants who were contactable for follow-up call; see REDCap. ) How useful did you find the follow-up call about how to use the smoking replacement product? How could it be improved?
-
How do you access the product if still using?
-
For lapsers and relapsers: were you using the product when you lapsed/relapsed?
-
Did you continue to use it after the lapse/relapse? If you did not use it again, why not?
-
Are you still using now?
-
Suggestions for improvement (e.g. would it have been helpful to have been provided with free products for longer)?
S3P plus nicotine product (combine questions above)
-
To what extent, if any, do you think combining these two strategies worked well in helping to quit smoking? If you think they worked well together, could you say how? If you think the combination did not work well, could you describe why?
E-Cigarettes
-
What do you understand about the relevant risk between smoking and nicotine?
-
Why are you tapering the nicotine (if relevant)?
Final questions
-
Do you have any thoughts as to how we could improve our materials/product selection/provision, etc.? When, by whom, where, etc.? If favourable, do you think our intervention should be given to all those who have stopped smoking after using the stop smoking services or quitline?
-
Do you think getting this help at around 1 month quit was a good time, or would you have liked it at around the time you stopped?
-
Strategies around confrontation and avoidance of situations identified by participants as high risk? Ask what their views are.
-
Confidence about staying quit in the future and perceived barriers to staying quit.
Appendix 3 Example coding frame for ‘lapses’
Definitions
-
Slip-up.
-
Serial lapses.
-
Full-blown relapse.
-
Permissive lapses.
-
Anticipating lapses.
Context
-
Settings.
-
Stress.
-
Loneliness.
-
Boredom.
-
Alcohol.
-
Adverse situations.
Interpretations
-
Failure because of own ability.
-
Failure because of context.
-
Learning experiences.
-
Unlearning previous patterns.
-
A test of resolve.
-
Confidence.
-
Shame and guilt.
Responses
-
Physiological sensations.
-
Recovering and resuming.
-
Learned techniques for coping with cravings.
-
Persistence.
-
Relapse.
List of abbreviations
- AE
- adverse event
- app
- application
- CI
- confidence interval
- CTU
- Clinical Trials Unit
- DMEC
- Data Monitoring and Ethics Committee
- e-cigarette
- electronic cigarette
- EMA
- ecological momentary assessment
- EQ-5D
- EuroQol-5 Dimensions
- HREC
- Human Research Ethics Committee
- HSUQ
- Health Service Use Questionnaire
- IQR
- interquartile range
- IT
- information technology
- NIC
- nicotine product study
- NRT
- nicotine replacement treatment
- S3P
- Structured Planning and Prompting Protocol
- SAE
- serious adverse event
- TSC
- Trial Steering Committee
