Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/221/02. The contractual start date was in June 2016. The draft report began editorial review in November 2018 and was accepted for publication in July 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Richardson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
In the UK, over 800,000 people are living with dementia1 and it is estimated that global prevalence of dementia will be 65.7 million by 2030. 2 In England and Wales, the number of people living with dementia will increase by 57% between 2016 and 2040, increasing future care demands substantially. 3 Dementia is a debilitating condition predominantly affecting older people, resulting in progressive decline in cognitive and daily function. People living with dementia have higher rates of hospital admission and readmission, worse health outcomes and higher rates of mortality than people without dementia. 4
Many neuropsychiatric symptoms are also associated with dementia, including aggression, agitation, depression, apathy and sleep disturbance, with nearly all patients developing some neuropsychiatric symptoms during the course of the disease. 5 There are currently no curative treatments for dementia, available treatment options generally manage symptoms. 6 As the disease progresses, disability increases and independence declines, leading to increased use of health and social services. Neuropsychiatric symptoms can cause great distress to family members and carers, contributing hugely to carer burden, stress and poor health,7 and increases the rate of institutionalisation. 8
Sleep disturbance in people with dementia
Sleep disturbance can include insomnia, fragmented night-time sleep, night-time wandering and excessive daytime sleeping. The incidence of sleep disturbance in the older population is common, with many older adults reporting insomnia. 9
Studies show that chronic insomnia predicts poor health, with better health leading to improved sleep patterns,9 and that disturbed sleep is actually a rarity in older adults who are healthy. 10 Reasons for age-related decline in sleep duration and quality include physical and psychiatric illness. 11,12 Therefore, it is unsurprising that in people living with dementia, sleep disturbance is high, with around 60% of people living with dementia affected. 13,14
Sleep disturbance in Alzheimer’s disease is correlated with further cognitive dysfunction, suggesting that treatment of sleep disturbance could be a strategy to improve cognition in patients. 15 Poor sleep impacts greatly on the quality of life (QoL) of both the patient and their informal carer. 16,17 Furthermore, frequent night-time waking can cause further risks to the individual, including wandering outside (which contributes to significant reductions in carer sleep and increases carer burden),18–20 and increases the rate of care home admissions for people living with dementia. 21 Sleep disturbance is highly prevalent in people living in care homes, and residents with dementia are reported to have more sleep disturbance then those without dementia. 22 In many cases, this leads to pharmacological interventions being commonly used to manage sleep disturbance in community, hospital and care home settings. 23
Pharmacological treatment of sleep disturbance: benzodiazepines and Z-drugs
There are several pharmacological treatments available for the management of sleep disturbance in the older population, including hypnotic benzodiazepines (BZDs) and Z-drugs (e.g. zolpidem, zaleplon and zopiclone), hormones (melatonin), antidepressants [typically low-dose tricyclic antidepressant (TCAs)] and antihistamines. The most widely used medications for sleep disturbances in older people are BZDs, low-dose TCAs and Z-drugs. 24
Low-dose TCAs have sedation effects due to their pharmacological effects of blocking histamine-1 receptors and are used for promoting sleep, but also in pain management and at higher doses for treating depression. 25 BZDs are long acting and act to enhance the effect of gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter, causing sedation and thus promoting sleep. 26 BZDs are not only given for sleep disturbance, but are also prescribed for a wide range of conditions, including anxiety and agitation. 27 BZDs are associated with a range of adverse side effects, including cognitive impairment, daytime sedation, tolerance, dependence and falls. 28–31 Z-drugs are a newer generation of BZD-like drugs, prescribed exclusively for sleep disturbance. Z-drugs were introduced in the late 1980s, also targeting GABA receptors (similarly to BZDs), to enhance GABA transmission, but with shorter half-lives and claiming to have fewer side effects. 32
However, there is increasing emerging evidence of adverse effects. 33 The National Institute for Health and Care Excellence Guidance on the Use of Zalepon, Zolpidem and Zopiclone for the Short-Term Management of Insomnia recommends that non-pharmacological approaches should be considered first, but that short-acting BZD or Z-drugs can be used for up to 4 weeks, if appropriate. 34 There is no explicit recommendation in these guidelines for use in people living with dementia. Updated guidelines35 for hypnotics discuss the growing evidence of increased risk of cognitive impairment associated with hypnotic use, but do not specifically address if they are safe for use in people living with dementia. There are no recommendations for pharmacological interventions for sleep disturbance in National Institute for Health and Care Excellence dementia guidelines. A Cochrane systematic review in 2016 concluded that there was a distinct lack of evidence to help guide drug treatment of sleep problems in people living with dementia. 36
Adverse effects of hypnotic use in the older population
Benzodiazepines and Z-drugs can improve sleep quality, but clinically relevant adverse events are a cause for concern. 37 Risks in people living with dementia have been largely unexplored, but findings from the older population suggest that the consequences of any side effects in people living with dementia could be more severe.
Falls and fractures
It is estimated that 30% of adults aged > 65 years living in the community have at least one fall each year38 and this is higher for people living with dementia, for whom it is estimated that 50–80% have a fall each year. 39,40 A recent study showed that, over an average of 2.5 years, one-third of people living with dementia had a fall, leading to hospitalisation. 41
Falls can cause a loss of confidence, potentially resulting in a decline of activity, which can cause further instability and negatively impact QoL. 42 Additionally, fall-related injuries can have a huge impact on subsequent health recovery, leading to a loss of independence, reduced QoL, and are a prominent cause of death and care home admission. Furthermore, fractures, particularly hip fractures, are a significant economic burden to the NHS. 43–46
There are several risk factors for falls, including medications. BZDs are associated with a 20% increased risk of falls in older people. 47–49 A study of > 9000 patients suffering from Alzheimer’s disease and age- and sex-matched controls reported a hazard ratio (HR) of 1.9 [95% confidence interval (CI) 1.0 to 3.6] for BZD prescription and risk of hip fracture,50 but did not disaggregate Z-drugs. Z-drugs are claimed to have fewer adverse effects, including fewer falls;51 however, recent studies have shown that Z-drug use is also associated with an increased risk of falls52,53 and fractures,54–59 particularly with new Z-drug use. 57,59 A recent systematic review and meta-analysis of 14 studies confirmed these risks, in which Z-drug use was associated with a statistically significant increased risk of fractures, with an odds ratio (OR) of 1.63 (95% CI 1.42 to 1.87) reported. 60 This same study did not, however, find a statistically significant increased risk of falls from Z-drug use. Similarly, a meta-analysis found that Z-drug use was significantly associated with increased risk of hip fracture [risk ratio (RR) 1.90, 95% CI 1.68 to 2.13]. 61 These studies have provided key evidence that there is an association between falls and injuries, with hypnotic use particularly in the older population. However, there has been little evidence of the specific effects of hypnotic use in people living with dementia. 62
Infections
A meta-analysis of randomised controlled trials (RCTs), identified through searches of published sources and US Food and Drug Administration records, found a 1.4- to twofold increased risk of infection in adults exposed to Z-drugs. 63 Some observational studies have similar findings. 64–67 The reason for this finding is unknown, but it has been speculated that hypnotics may impair immune surveillance or the clearing of oral secretions during sleep. 63,68 Infection is more common in people living with dementia than in older persons without dementia, and infection in people living with dementia is a leading cause of mortality, hospitalisation and high cost burden. 4,69
Mortality
Evidence from studies investigating the association of Z-drug or BZD use with mortality is conflicting,70–72 with reported associations possibly due to confounding. 73 A US study found that crude mortality rates were higher in people living with dementia taking hypnotics, but the association was not statistically tested. 74 Recently, a study of 31,140 community-dwelling persons with Alzheimer’s disease in Finland reported a HR of 1.59 (95% CI 1.35 to 1.88) for mortality after BZD use. 75 The use of Z-drugs, however, was not associated with increased risk of death (HR 1.06, 95% CI 0.83 to 1.35). The study was unable to adjust for sleep disturbance or anxiety.
Cerebrovascular events
People living with dementia have a higher risk of stroke than people without dementia. 76 Recently, BZD use has been associated with a 21% (95% CI 4% to 40%) increased risk of ischaemic, but not haemorrhagic, stroke in 45,050 individuals with Alzheimer’s disease in Finland. 77 The study also reported similar stroke risk for Z-drugs. However, the study was unable to adjust for anxiety, sleep disturbance or dementia severity, and only compared individuals with Alzheimer’s disease and not specifically those with sleep disturbance. There has also been a report of an increased risk of ischaemic stroke associated with zolpidem. 78
Other possible harms
Adverse behavioural symptoms have been reported with the use of Z-drugs in the older population, including driving, walking, preparing and eating food, and making telephone calls when asleep. 79 Other symptoms reported include hallucinations, parasomnia and amnesia. 80 Behaviours such as these could be particularly detrimental for people living with dementia, given their vulnerable cognitive state and increased risk of falls. 39
Potential benefits of hypnotic use
Cognitive functioning
Sleep is crucial for cognitive performance, memory consolidation and mood regulation. 81,82 Sleep loss is associated with reduced cognitive performance. 83,84 Furthermore, observational and experimental studies suggest that poor sleep is a risk factor for cognitive decline and dementia. 85 The underlying mechanisms are unknown, but this suggests that improving sleep quality could be an intervention strategy for dementia, potentially through sedative hypnotics. However, there is evidence that sedative hypnotic use is associated with cognitive decline, with a stronger association observed with long-term exposure,86–89 although results are conflicting. 90,91 A recent systematic review and meta-analysis suggests that BZD use is associated with increased dementia risk; however, observational studies cannot determine whether the association is a causal effect or due to unmeasured confounders. 92,93 Evidence is emerging that Z-drug use is also associated with dementia risk. 94,95 Despite these findings, there is a lack of studies addressing whether or not sleep drugs are safe for individuals already suffering with significant cognitive decline. 96 It is therefore important to understand if increasing sleep in people living with dementia through pharmacological interventions can maintain cognitive performance.
Quality of life
Sleep disturbance can have a detrimental impact on QoL and health-related quality of life (HRQoL). 97,98 Insomnia has been independently associated with worsened HRQoL to a similar extent as some chronic conditions, including congestive heart failure. 99 Insomnia and sleep disturbance are common in the older population and are associated with decline in QoL. 100 The QoL of carers is also severely reduced when there is a sleep disturbance. 19 Use of hypnotics could be a strategy to increase sleep and thus improve QoL. There have been studies, however, to suggest that use of hypnotics can have a negative impact. In older community-dwelling adults requiring pharmacological treatment for insomnia, hypnotics are also associated with reduced HRQoL. 101 A study of 10,430 older women identified that use of sleeping medications was associated with lower QoL scores in vitality, social functioning, general mental health and bodily pain. 102
Improving sleep
In dementia, for which sleep disturbance is common, improving sleep could be a valuable intervention strategy to improve cognitive performance. Hypnotics are widely prescribed in this population in an attempt to increase sleep. However, there is increasing evidence that the use of sedating hypnotics in the general population does not improve sleep, cognitive performance or general health, calling into doubt their clinical effectiveness. 103,104
Study rationale
Sleep disturbance is a common problem in the older population and research suggests that over half of people living with dementia have sleep disturbance. 13 Sleep disturbance affects not only the individual, but also their carer, subsequently leading to a decline in their health and QoL. 17
Studies suggest that pharmacological therapy for sleep disturbance in the older population can lead to increased rates of falls, fractures and infections. Conversely, untreated sleep disturbance, particularly insomnia, can further impair cognitive function and daily functioning, increase dementia risk and lead to worsening of other health problems. 85,105 The potential harms of BZD and Z-drugs, such as increased rates of falls, fractures and infections, could lead to higher rates of hospitalisation, adding additional financial burden for health systems. 106 Furthermore, adverse events quicken the time to institutionalisation, increasing social care requirements. 8,107
In 2016, the NHS spent £6M on Z-drugs with 6.53 million prescriptions dispensed in England,108 and prescriptions of Z-drugs have almost doubled in the UK between 2000 and 2015. 109,110 The proportion of prescribed Z-drugs used specifically by people living with dementia is unknown,108 but hypnotic prescriptions are common in people living with dementia. 111 Guidance for sleep management in dementia is limited, with little evidence of the safety and efficacy of pharmacological interventions, specifically for people living with dementia. 112 Indeed, a recent review identified a clear absence of RCTs addressing the use and effectiveness of Z-drugs for sleep disturbance in dementia. 36 Identification of safe and effective sleep medications for people living with dementia remains an unsolved challenge. 113
Together, the personal and societal impact of sleep disturbance for people living with dementia makes it an important priority to quantify the benefits and harms of current pharmacological treatments used in this patient group. This study was specifically designed to evaluate the management of sleep disturbance in people living with dementia, and to provide critical evidence on which clinicians, patients, family members and carers can base care decisions. If significant adverse events are detected when using Z-drugs, this will additionally motivate the development of alternative treatments, both pharmacological and person centred, to improve sleep and outcomes for patients.
Chapter 2 Aims and objectives
Aims
The broad aims of this study were to:
-
estimate the harms of using Z-drugs for the management of sleep disturbance in people living with dementia, using UK primary care records (primary care study)
-
explore the impact of Z-drugs on cognition and QoL for people living with dementia and their carers, using repurposed clinical study data sets (clinical cohort studies).
Objectives
Primary care study
-
To estimate the effects of first prescription of Z-drugs in people living with dementia with sleep disturbance compared with alternative treatments and no treatment. Patient outcomes included:
-
incidence of falls and factures
-
mortality
-
incidence of infection
-
incidence of cerebrovascular events, including ischaemic stroke and venous thromboembolism
-
incidence of behavioural and psychological symptoms
-
additional medication use, including sedatives, antipsychotics, antidepressants and antibiotics
-
health-care utilisation [general practitioner (GP) visits and hospital admissions].
-
-
To validate recorded dementia diagnosis and sleep disturbance codes through a GP questionnaire.
Clinical cohort studies
-
To repurpose existing clinical data sets to estimate the impact of concurrent use of Z-drugs on patient- and carer-reported outcomes, including:
-
QoL for patients and carers
-
functional ability
-
cognitive function and sleep disturbance in people with dementia.
-
Chapter 3 Methods
In this chapter we describe separately the methods for the primary care study and each of the clinical cohort studies.
Study registrations
This work was funded by the National Institute for Health Research (NIHR) under its Health Technology Assessment (HTA) programme (reference 14/221/02) and was conducted in accordance with protocol version 1.2 published on 30 January 2018.
The primary care study protocol is registered with the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance [reference European Union electronic Register of Post-Authorisation Studies (EU PAS) 18006] and approved by the Independent Scientific Advisory Committee for Clinical Practice Research Datalink (CPRD) research (reference ISAC 16_181).
Primary care study
Study design
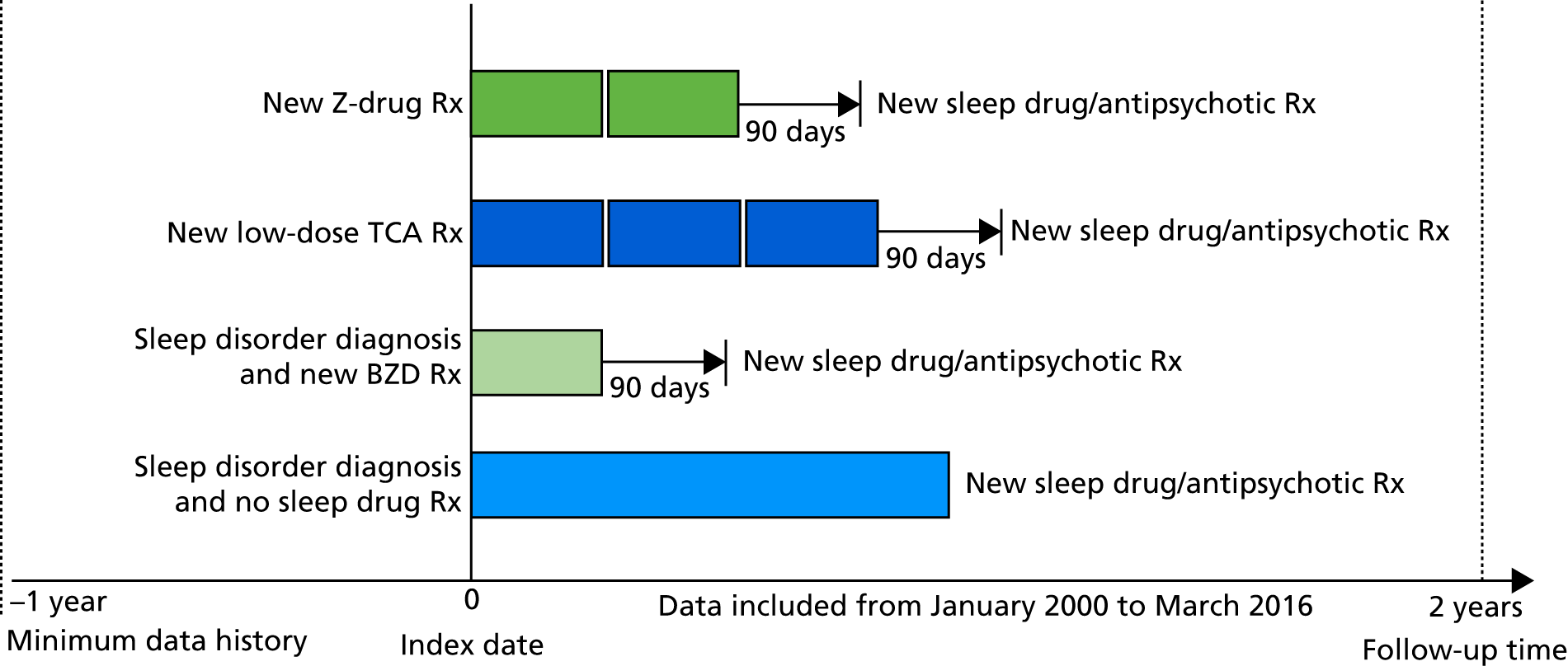
This was an inception cohort study of people with dementia and sleep disturbance, using data from a UK primary care database. The study design is summarised in Figure 1. Briefly, people living with dementia in cohorts prescribed various treatments were followed for the rate of adverse events from their first prescription for sleep disturbance in dementia (index date), until they had a different sleep drug or antipsychotic prescribed, left their general practice, died or at 2 years’ follow-up (censor date).
FIGURE 1.
Schematic of study design for the primary care cohort study of people living with dementia (n = 6809). The four cohorts of patients were followed for at most 2 years or until March 2016. Follow-up for the cohort diagnosed with sleep disturbance but not prescribed sleep medications ended when they received a first sleep medication or antipsychotic prescription. The figure displays theoretical patterns of prescriptions for the cohorts defined by a first Z-drug, BZD or low-dose TCA prescription. Here, follow-up ended if patients had a first sleep medication or antipsychotic prescription, or if it had been > 90 days since their last prescription for a Z-drug, BZD or low-dose TCA, respectively. Rx, prescription.
Setting
This study was undertaken using data extracted from the CPRD. The CPRD includes anonymised diagnosis, referral and prescribing records for > 11.3 million patients from 674 primary care practices across the UK, and is broadly representative of the UK population in terms of age, sex and ethnicity. 114 CPRD data have been widely used for pharmacoepidemiology applications. 115 The diagnosis information recorded in the CPRD is entered as computer-recorded Read codes. 116 Access to ‘free text’ entered by the GP is not available because of patient anonymity issues.
We used linked data from the CPRD to Hospital Episode Statistics (HES) data version 14, the Office for National Statistics (ONS) mortality data and Index of Multiple Deprivation (IMD) data. HES provides records of all diagnoses made during a hospital admission in England, details on the patient’s demographics and place of residence. Diagnoses during the hospital stay are recorded using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). ONS data include the date and cause of death in England, with cause of death coded according to ICD-10. The IMD is a weighted sum of a number of government indicators, including housing, employment, income, education, living environment and crime levels for each general practice’s neighbourhood. 117
The HES and ONS data sets were linked to CPRD patients by NHS number, date of birth, sex and postcode. The vast majority (92%) of eligible CPRD patients matched to HES, matched on all four characteristics, whereas a further 8% were matched on all but postcode.
Eligibility criteria
Patients were included if they satisfied all the following criteria:
-
Their general practice was in England.
-
They were diagnosed with dementia, defined as the first of a code for dementia or prescription of a cognitive enhancer (memantine, donepezil, rivastigmine or galantamine), occurring after 1 January 2000. The year 2000 onwards was chosen to exclude historical primary care records when recording was less reliable,114 and linked mortality and hospital records were only available since 1998.
-
They were aged ≥ 55 years when diagnosed with dementia.
-
There was evidence of a sleep disorder, defined as the first primary care record for sleep disorder diagnosis, symptom or referral (see Appendix 1, Table 27, for Read codes), or prescription of a Z-drug, low-dose TCA or melatonin, on or after the dementia diagnosis date and before 31 March 2016 (this first sleep disturbance date defined the ‘index date’).
-
Their records contained at least 3 months of good-quality data before the dementia diagnosis and at least 12 months of data before the index date.
Record of a dementia diagnosis was identified by Read codes in the CPRD (see Appendix 1, Table 26 for Read codes) or ICD-10 codes F00-F03, G30, G31.0 or G31.1 in HES.
Patients were excluded if there was:
-
uncertainty regarding the timing of dementia diagnosis, specifically Read codes for a dementia annual review, history of dementia, assessment of psychotic and behavioural symptoms of dementia, or antipsychotic drug therapy for dementia, prior to meeting our dementia diagnosis definition
-
diagnosis of severe mental illness or Down syndrome prior to dementia diagnosis (see Appendix 1, Table 28 for Read codes)
-
diagnosis of sleep apnoea, sleep-related respiratory failure or alcohol abuse prior to the index date (see Appendix 1, Table 29 for Read codes)
-
diagnosis of neuropathic pain in the 12 months prior to the index date (see Appendix 1, Table 30 for Read codes)
-
a prescription of sedatives, TCAs or BZDs in the 12 months prior to the index date
-
a prescription of multiple sleep medications on the index date
-
a prescription of a new antipsychotic, other sedative or other TCA on the index date
-
no linkage possible between CPRD and HES data.
Patient selection validation
To validate the accuracy of the coding on which our patient selection was based, a GP questionnaire-based validation study was conducted. 118 In collaboration with CPRD validation services we developed a questionnaire to send to GPs, requesting confirmation of the dementia diagnosis and sleep disturbance status of their patient. The questionnaire was sent to the GPs of 106 randomly selected patients who were still registered with their GP in 2017. The patients selected represented those identified as having sleep disturbance via various Read codes, who were or were not prescribed sleep medications. The questionnaire (see Report Supplementary Material 1) asked GPs if the patient had been diagnosed with dementia and the date of diagnosis. It also asked whether or not the patient had a record of a sleep disturbance since being diagnosed with dementia and, if so, on what date. We reported the number of questionnaires returned and the proportions of patients, with their dementia and sleep disturbance confirmed by the GP according to their first medication for sleep disturbance. When the records of dementia or sleep disturbance were not confirmed by GPs, we explored possible causes. We also performed a sensitivity analysis restricted to patients with sleep codes with better validity.
Exposures
The primary exposure of interest was treatment with Z-drugs; secondary exposures were treatment with other medications used for sleep disturbance. The CPRD contains detailed information on all medications prescribed in primary care, including the date of each prescription, drug name, dose, quantity and frequency. We extracted the details of all prescriptions for medications for sleep disturbance for patients in our cohort from their index date up until 31 March 2016.
Sleep disturbance medications were defined according to the World Health Organization (WHO)’s Anatomical Therapeutic Chemical (ATC) Classification System as:
-
Z-drugs (N05CF)
-
BZDs (N05BA, N05CD)
-
melatonin (N05CH)
-
low-dose TCA or related (N06AA09, amitriptyline at ≤ 25 mg/day; N06AX05 trazodone at ≤ 50 mg/day).
Patients were classified according to their prescription class on the index date, or to a ‘no prescription for sleep disturbance’ group. Patients in the ‘no prescription for sleep disturbance’ group who then went on to be prescribed any of the medications for sleep disturbance and still met the study eligibility criteria, were then assigned a second index date as the date of this first prescription.
To test for possible dose–response relationships, we determined the cumulative number of prescribed defined daily doses (DDDs) of Z-drugs. A DDD is defined as the assumed average maintenance dose per day for a drug based on its main indication in adults, using the DDD values assigned by the WHO Collaborating Centre for Drug Statistics Methodology (URL: www.whocc.no/atc_ddd_index; accessed 28 November 2019). For each prescription, we multiplied the number of tablets by the dose strength in milligrams and converted this into the number of DDDs, using the values assigned by the WHO. We then summed individual prescriptions to determine the cumulative number of prescribed DDDs, regardless of gaps in use. If the quantity of tablets prescribed was missing, we assumed that one per day (28 tablets/prescription) was prescribed. Prescribing instructions for Z-drugs were also categorised according to whether or not instructions stated pro re nata (PRN) (i.e. use as needed).
The Z-drugs historically prescribed in the UK are zopiclone, zolpidem and zaleplon. The WHO considers the DDD of zopiclone to be 7.5 mg per day, and zolpidem and zaleplon to be 10 mg per day. These values are consistent with the British National Formulary (BNF) prescribing guidelines for the recommended daily dose in adults with insomnia. 119,120 For use in the elderly, the BNF recommends using half these daily doses.
Outcome variables
The selected outcome variables were based on adverse effects of medications used to treat sleep disorders identified from previous studies or priorities identified by carers, family members and health-care professionals with direct experience of sleep disturbance in patients.
The 16 outcomes assessed were:
-
incident fracture in any location
-
incident hip fracture
-
incident forearm/wrist/hand fracture
-
incident fall
-
mortality
-
incident acute bacterial infection
-
incident urinary tract infection (UTI) or acute lower respiratory tract infection (LRTI)
-
ischaemic stroke/transient ischaemic attack (TIA)
-
venous thromboembolism
-
incident agitation or psychosis (including symptoms of hallucinations, delusions or aggression)
-
additional use of sedatives and other sleep medications (BNF version 69 subsection 4.1)120
-
additional use of antipsychotics (BNF version 69 subsection 4.2.1)120
-
additional use of antidepressants (BNF version 69 subsection 4.3)120
-
additional use of antibiotics (BNF version 69 subsection 5.1)120
-
health-care utilisation of number of GP visits
-
health-care utilisation of number of hospital admissions.
Patients with each outcome were identified using the first mention of a relevant Read code in the CPRD, or ICD-10 code in HES or as a cause of death [Part 1(a) or 1(b)] on the death certificate. See Appendix 1, Tables 31–38, for a list of the codes to identify the outcomes. Read code lists were drawn, when applicable, from Quality and Outcomes Framework business rules, published studies, keyword searches and UK GP experience within the study team.
Confounding variables
Potentially confounding variables were coded from the CPRD (unless otherwise stated) at the index date. Covariates were selected that are potentially linked to sleep disturbance, dementia or sedative use and at least one of the outcomes, as well as the availability, completeness and reliability of data within CPRD.
Demographic factors
Age, sex, year, care home residence (yes/no/unknown) practice-level IMD quintile, Strategic Health Authority region of England, index date, ethnicity (white/other/unknown).
Health behaviours
Smoking (current smoker, ex-smoker, non-smoker, missing), alcohol use (yes/no/missing), body mass index (BMI) (most recent value in last 5 years categorised as < 18.5, 18.5–24.9, 25–29.9, ≥ 30 kg/m2 or missing), last systolic blood pressure (most recent value in last 5 years categorised as < 110, 110–119, 120–139, 140–159, ≥ 160 mmHg or missing).
Immunisations in last 12 months
Influenza, pneumonia.
Dementia subtype and proxies for dementia severity
Dementia subtype (Alzheimer’s dementia, vascular dementia, mixed/other dementia, unspecified dementia), time since dementia diagnosis, cognitive enhancers in last 90 days, antipsychotic use in last 365 days, history of agitation/psychosis in dementia, end-of-life care (record of palliative care or end-of-life plans having been discussed).
Proxies for sleep disturbance severity
Sleep disturbance diagnosis before dementia diagnosis, previous Z-drug prescription (prior to 12 months before index date), previous BZD prescription (prior to 12 months before index date).
Comorbidities
Osteoporosis, other musculoskeletal conditions, depression, depression symptoms, anxiety, anxiety symptoms, Parkinson’s disease, urinary incontinence, age-related macular degeneration (ARMD), glaucoma, cataract, other visual impairment, diabetes, hyperlipidaemia, hypertension, heart attack, heart failure, atrial fibrillation, ischaemic stroke/TIA, angina, venous thromboembolism, osteoarthritis, rheumatoid arthritis, migraine/headache, back/neck pain, cancer and chronic obstructive pulmonary disease.
Medical history in last 12 months
Number of GP visits (0–3, 4–5, 6–8, 9–13 or 14–77), hospital admissions (0, 1 or ≥ 2 using data from HES), a fall, a fracture, LRTI/UTI, dizziness/unsteadiness and faints/syncope.
Concurrent medication use
Any prescription in the last 90 days of selective serotonin reuptake inhibitors (SSRIs), non-SSRIs or TCA antidepressants, other sedatives/hypnotics, antipsychotics, antihistamines, analgesics, antiepileptic drugs, anticoagulants, antiplatelets, cardiac glycosides, diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, beta-blockers, lipid-regulating drugs, non-steroidal anti-inflammatory drugs (NSAIDs), bisphosphonates, diabetes drugs, inhaled corticosteroids, calcium/vitamin D and any prescription in the last 30 days for antibiotics.
Data from HES were additionally used to supplement the coding of a history of falls, fractures, LRTI/UTI, ischaemic stroke/TIA, agitation/psychosis, venous thromboembolism, dementia subtype, ethnicity and care home residence.
Statistical methods
We described the characteristics, comorbidities and concurrent medications of the patients in the cohort, using summary statistics according to their initial treatment for sleep disturbance. Using a backwards stepwise procedure retaining a p-value of < 0.15, we developed a multinomial regression model estimating the association between patient characteristics at index date and treatment choice for sleep disturbance.
The primary statistical analysis comprised a series of survival analyses to assess the association between sleep disturbance medication and adverse outcomes. Patients entered the analysis on their index date and were followed until the earliest of:
-
the date they left the general practice
-
the last general practice data extraction date
-
their date of death
-
90 days after their last prescription for their medication for sleep disturbance
-
the date of an additional ‘medication for sleep disturbance’ or other sedative prescription
-
the date of a new antipsychotic prescription (for those not prescribed antipsychotics in the year before the index date)
-
2 years after the index date
-
31 March 2016.
Patients were excluded from the analysis of each outcome depending on their history of that outcome. For the fall and fracture outcomes, we excluded patients with a recorded fall or fracture in the 32 days before index date, because of the chance of repeated coding of the same event. 121 For the infections and antibiotics outcomes, we excluded those with an infection or prescription for a medication for infection in the previous 30 days. For the ischaemic stroke/TIA and venous thromboembolism outcomes, we excluded those with a history of these in the previous 30 days. For the analysis of incident agitation/psychosis, we excluded patients who already had a record of agitation or psychosis. For the antidepressant and antipsychotic outcomes, we excluded patients with a prescription for these in the 12 months before the index date.
We used Cox regression models to estimate the HR for the effect of sleep disturbance medication class compared with no prescription on each binary outcome. Sleep medication exposure was modelled as time varying, such that patients at the index date are included for analysis in the ‘no treatment’ group until initiation of their first treatment, and re-enter the study at time 0 as exposed thereafter to avoid immortal time bias and reduce channelling bias. CIs and p-values were calculated using robust standard errors, accounting for the correlation due to some patients appearing twice in the analysis. We checked the proportional hazards assumption using Schoenfeld residuals. 122
As the number of GP visits and hospital admissions was overdispersed (i.e. the variation was greater than the mean), we used negative binomial regression to calculate incidence rate ratios (IRRs) for the effect of sleep disturbance medication class on number of GP visits and hospital admissions in the 2 years after the index date. For the analysis of GP visits, patients were censored only at the first of either death, leaving the general practice, 31 March 2016 or 2-year follow-up. For the analysis of hospital admissions, patients were censored only at the first of either death, 31 March 2016 or 2-year follow-up.
The primary analysis reported associations relative to no sleep disturbance medication use, and secondary analysis reported associations for the effect of BZDs and low-dose TCAs compared with Z-drug use. Estimates are provided, adjusted for age and sex, and adjusted for all potential confounders listed above and age2 to allow non-linear effects with age. A quadratic association between age seemed an appropriate parametrisation for most outcomes by examining the association between age in 10 equal categories and the outcome. We performed a sensitivity analysis to this by instead modelling age using fractional polynomials and restricted cubic splines (with five knots). Owing to a small number of events for forearm fractures we adjusted for other eye conditions (ARMD, retinal disorders and glaucoma combined), region combined into only five areas (South, East, Midlands, North and London) and did not adjust for ethnicity or systolic blood pressure.
For statistically significant associations with Z-drugs, interactions between Z-drug and age and sex were tested. In addition, the absolute risks of adverse events and number needed to harm (NNH) were estimated using a standard formula for NNH in time-to-event analysis. 123
We also carried out analyses of Z-drug exposure according to both time-varying cumulative DDDs (categorised as 0, 1–13, 14–27, 28–41 and ≥ 42 DDDs) and by Z-drug dosing instructions on the index date (PRN or not).
Stata® version 14 (StataCorp LP, College Station, TX, USA) was used for data management and statistical analysis. Owing to examining 16 outcomes, the chance of finding a statistically significant association by chance alone had increased. To address this, we used a stricter threshold instead of the traditional p-value threshold of 0.05. We used the Benjamini–Hochberg procedure to estimate this critical p-value threshold in order to control rate of the false discovery rate (i.e. the proportion of rejected null hypotheses that are incorrect rejections) at < 5%. 124 This is less conservative than the Bonferroni correction,125 which simply divides the classical p-value threshold of 0.05 by the number of hypotheses tested (so our critical threshold would be 0.05/16 = 0.003). To control the false discovery rate, the Benjamini–Hochberg procedure depends on the distribution of p-values resulting from the tests, as well as the number of tests performed.
Missing data
We expected incomplete recording of variables, such as smoking and BMI. 126 In the primary analyses, patients with missing covariate data were coded in a missing data category. For covariates with at least 10% of patients with missing data, we summarised the characteristics of those with and without missing data and performed sensitivity analyses, including restricting to patients with and without the missing variable. 127 Finally, we estimated a single imputation model predicting the missing variables using the covariates in Table 2 and in Appendix 2, Table 39. For missing BMI data, we additionally used the most recent previously recorded BMI record to impute missing BMI value. We repeated our main analyses using the imputed values.
Sensitivity analyses
We performed various sensitivity analyses of our main analysis. First, based on the findings of the validation study, we repeated our main analysis with an amended definition of sleep disturbance that included diagnoses of sleep disturbance only when not accompanied by a record for a ‘satisfactory’ sleep pattern. Second, to examine the impact of the source of data used to identify outcomes, we restricted our main analysis to only those outcomes recorded in the CPRD. Third, we compared adverse events in those who initiated Z-drugs compared with those who had recently discontinued them. From the Z-drug cohort we created a further ‘discontinued Z-drug’ cohort, who after a 90-day period with no Z-drug prescriptions were still eligible for entry to the study. We compared the rates of adverse events in the ‘discontinued Z-drug’ to the ‘Z-drug’ cohort.
Protocol changes
The following changes to the analysis plan were made since our protocol:
-
We additionally censored patients at their first antipsychotic prescription. Patients initiated on Z-drugs or BZDs were more likely to then start antipsychotics. We additionally censored on first antipsychotic prescription to increase the likelihood that effects observed were due to the Z-drug use and not other sedating medications.
-
In our protocol, we aimed to examine incident ischaemic stroke/TIA and incident venous thromboembolism and to exclude anyone with a history of these conditions. However, we found more patients had these histories than expected, so instead we analysed recurrent or incident ischaemic stroke/TIA and venous thromboembolism, and only excluded patients with a history of these in the last 30 days. Findings when excluding all patients with a history of these conditions are available from the authors.
-
We made minor changes to the list of potential confounders. We omitted the following prespecified covariates in the analysis due to balance across treatment groups and rare occurrence: phobia, motor neuron disease, human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) and multiple sclerosis. We omitted epilepsy and prescription for medications for Parkinson’s disease and diabetes, due to collinearity with already included covariates of prescription for antiepileptic medications, diagnosis of Parkinson’s disease and diabetes, respectively. Finally, we omitted depression severity and depression duration, as depression diagnosis, depression symptoms, anxiety diagnosis and anxiety symptoms were considered sufficient. Subsequent to the protocol, we have also included the potential confounders of recent prescription for calcium or vitamin D and end-of-life care, based on discussions with our health-care professional advisory panel.
-
We omitted a sensitivity analysis using instrumental variables as a result of not finding a sufficiently strong instrument, such that using it would bring greater uncertainty and risk of bias than the main analysis (see Report Supplementary Material 2 for further details), but instead completed an additional analysis of those patients who discontinued Z-drugs.
A priori power calculation
We performed this a priori power calculation before we had access to HES data and made assumptions as to what the HES data would contain; hence the estimates do not match the final numbers included. There were 32,961 patients with sleep disturbance post dementia diagnosis in the CPRD (July 2017 version). Of these, 10,554 patients met study eligibility criteria and a further 6117 patients were eligible for HES linkage (HES version 14). Preliminary analysis suggested that on the index date, 2546 patients received a Z-drug, 68 patients received a melatonin, 255 patients received a BZD, 1745 patients received a low-dose TCA antidepressant and 1503 patients received none of the above treatments. We estimated that an additional 50% of dementia patients would be identified via HES (HES set 14 feasibility). Thus, when comparing 3819 Z-drug patients with 2254 patients with no treatment for a common outcome over 2 years (e.g. falls at 36% per year) we can detect a HR of 1.07 with 90% power (two-sample test, p < 0.05). For rare outcomes, such as hip fractures at 1.7% per year, we can detect a HR of 1.54 with 90% power.
Clinical cohort studies selection
The second phase of the study utilised data from RCT and cohort studies to estimate how patient-reported outcomes are affected by sleep medication, which cannot be addressed in data extracted from the primary care CPRD database. The following describes the process of identifying clinical cohort studies to examine the outcomes of cognition, neuropsychiatric symptoms, sleep disturbance, disability or QoL of the people living with dementia or their carer, and also the overarching methods applicable to the three clinical cohort studies performed.
Potential studies
We identified several RCT and cohort studies for which data were obtained and descriptive analysis performed to determine their appropriateness for use in this study. Studies were required to have:
-
a validated dementia diagnosis for participants
-
specific assessment of sleep disturbance for all participants
-
at least two assessments per participant, with assessments separated by ≤ 1 year
-
at least one key primary outcome (cognition, neuropsychiatric symptoms, sleep disturbance, disability or QoL of the person living with dementia or their carer) included
-
systematic assessment of participant medication use, with a documented protocol
-
a well-characterised sample, recruited from a defined population with clear inclusion and exclusion criteria
-
sufficient sample size (in particular sufficient numbers of participants taking Z-drugs).
Data sources explored, with at least one primary outcome measure, are described in Table 1. Those which were not deemed suitable for use in our study were not explored further from initial descriptive analysis. Three data sources that fulfilled the required criteria underwent analysis of patient-reported outcomes. These data sources were:
-
the Resource Use and Disease Course in Dementia – Nursing Homes (REDIC) Norwegian observational longitudinal study
-
the University of Washington’s National Alzheimer’s Coordinating Centre (NACC) clinical data set
-
the Improving Well-being and Health for People with Dementia (WHELD) RCT based in UK nursing homes.
| Database/study name | Study type | Number of participants with dementia | Included in our study |
|---|---|---|---|
| REDIC (nursing homes cohort only) | Observational cohort data to understand socioeconomic consequences of dementia in Norway | 678 | Yes: good number of participants taking Z-drugs (126 at baseline plus 193 during follow-up) |
| NACC | Observational cohort data – standardised clinical and neuropathological research data | 17,055 | Yes: good number of participants taking Z-drugs (373 at baseline plus 72 during follow-up) |
| WHELD128 | A RCT of a training intervention in 69 care homes, UK | 926 | Yes: good number of participants taking Z-drugs (123 at baseline plus 27 at 9 months) |
| CALM-AD129 | A pharmacological RCT of donepezil and risperidone over 12 weeks, UK | 272 | No: low Z-drug exposure |
| MAGD130 | A RCT of memantine for agitation in Alzheimer’s dementia, UK | 153 | No: low Z-drug exposure |
| ADCS131 | Harmonised data from nine US drug or dietary supplement RCTs, USA | 2609 | No: low Z-drug exposure |
| DOMINO-AD (UK)132 | A RCT of donepezil and memantine for moderate to severe Alzheimer’s disease | Aimed to recruit 800 | No: low Z-drug exposure |
The REDIC study
Setting
The REDIC study is a longitudinal cohort study of people admitted to nursing homes in Norway. The REDIC study is fully described elsewhere,133 but in brief the study began in 2012 and recruited 696 patients at admission to one of 47 nursing homes across four Norwegian counties.
Baseline assessments were made within 1 month of admission to the nursing home and then every 6 months thereafter. Comprehensive REDIC study assessments included questions on sleep disturbance, medication use and measures of cognitive function [recorded by the Mini Mental State Examination (MMSE) and Severe Impairment Battery (SIB-8)], QoL [measured by the Quality of Life in Late-Stage Dementia (QUALID) scale and EuroQol-5 Dimensions (EQ-5D)], neuropsychiatric symptoms [measured using the 12-item Neuropsychiatric Inventory – Nursing Home (NPI-NH)] and depression [measured using the Cornell Scale for Depression in Dementia (CSDD)]. These are described in detail in Outcome variables.
We received data from the REDIC study team for assessments up to October 2016, including up to five assessments per participant. Assessments were conducted and supplied in Norwegian, with translation kindly provided by Professor Sverre Burgh, the REDIC study chief investigator.
Participant inclusion and exclusion criteria
Participants were eligible for inclusion in the REDIC study if they had an expected stay in the nursing home for > 4 weeks and were excluded if their life expectancy was < 6 weeks. Participants were included in the Z-drug Evaluation in Dementia (ZED) study if they had a diagnosis of dementia or mild cognitive impairment (MCI) on admission. These diagnoses were made and recorded by the REDIC study team on the first visit after admission to the care home.
Medication exposures
Regularly used medication was recorded at each wave, coded according to WHO ATC code and dose, for up to 18 medications per participant. We coded the presence or absence of three classes of hypnotics at each visit, based on the following codes: Z-drug (Z) – ATC N05CF; BZD – ATC N05BA, N03AE or N05CD; and antipsychotic – ATC N05A.
Outcome variables
Cognitive function
The MMSE and a short eight-item version of the SIB-8 were administered at each wave. 134,135 The MMSE is a well-known and widely used test of cognitive function across several domains, which is scored from 0 to 30 based on the participant successfully answering questions or performing tasks under instruction. The MMSE has good test–retest and inter-rater reliability,136–139 and performs fairly well in classifying those with dementia and MCI. 140 The SIB-8 aims to measure cognitive function specifically in more advanced Alzheimer’s disease and is scored from 0 to 16 based on participants’ responses to eight comparatively simple tasks. The SIB-8 has been found to accurately measure progression in advanced Alzheimer’s disease and is able to accurately classify dementia stage. 141,142 Both MMSE and SIB-8 are scored with higher scores reflecting better cognitive function.
Dementia severity was measured at each visit by the Clinical Dementia Rating (CDR). 143 In contrast to MMSE and SIB-8, the CDR is scored based on semistructured interview with informants and participants to judge cognitive ability rather than participants’ correct responses to specific instructions. The CDR is scored in two different ways. First, measured as a Clinical Dementia Rating scored according to standard algorithm (CDR-global) (0 ‘no dementia’, 0.5 ‘minimal dementia’, 1 ‘mild’, 2 ‘moderate’ and 3 ‘severe’ dementia), according to the standard algorithm. Second, the CDR is often scored as Clinical Dementia Rating – Sum of Boxes (CDR-SOB), which is a simple sum of the level of impairment (scored as 0, 0.5, 1, 2 or 3, as above) in each of the six domains (memory, orientation, judgement and problem-solving, community affairs, home and hobbies, and personal care). CDR-SOB scores range from 0 to 18, with 18 reflecting the maximum level of impairment. 144 The CDR and CDR-SOB have both been reported to have good content and criterion validity, and good inter-rater reliability. 145–147
Quality of life and disability
Quality of life is measured by the QUALID scale and the EQ-5D. 148,149 QUALID is scored from 11 to 55 based on observations of 11 aspects of patient mood and behaviour in the week before assessment, and is scored such that a lower score is reflective of higher QoL. Although not extensively examined, QUALID has been reported to have good test–retest and inter-rater reliability. 148 The EQ-5D used is the standard assessment of QoL, including a score based on five questions, covering mood and function, as well as a visual analogue scale (VAS) reflecting a single subjective assessment of health scored from 0 to 100. EQ-5D is scored according to country-specific weights; there is no Norwegian-specific EQ-5D scoring and so at the recommendation of the REDIC study investigators the Danish weights are used. 150 These were scored using the EQ-5D command for Stata. 151 The EQ-5D was completed by participants on around one-quarter of occasions, otherwise by care staff. As so few participant scores are recorded, and participant and staff ratings are likely not to be comparable, we used only staff-rated EQ-5D and VAS scores in analysis. Although the EQ-5D is used extensively to measure QoL in Europe and has good validity and reliability, when specifically used in people living with dementia, ceiling effects have been reported and issues in differences across proxy ratings. 152
Disability is measured by the observer-rated Lawton Physical Self-Maintenance Scale. 153 This scale rates disability from 1 to 5 within six domains of toileting, feeding, dressing, grooming, physical ambulation and bathing, and hence has total scores ranging from 7 to 35. Higher scores reflect greater disability.
Confounders
Sleep disturbance
The NPI-NH was administered at each visit. 154 This includes a screening question on the presence of sleep disturbance, and if this is endorsed then questions on the frequency of sleep disturbance, severity and the extent to which this is occupationally disruptive for the caregiver. The ‘occupationally disruptive’ question is analogous to the ‘carer distress’ question on the original Neuropsychiatric Inventory (NPI). Measures of severity and frequency of sleep disturbance are multiplied together to give an overall sleep disturbance score (scored from 0 to 12). The occupational disturbance question is scored separately on a Likert scale (scored from 0 to 5). If the screening question on sleep disturbance is not endorsed then the occupational disruption and severity questions are all scored as zero.
In addition, three questions on sleep are asked at each visit as part of the CSDD. 155 Informants are asked to evaluate, over the course of the previous week, if the patient has had ‘difficulty falling asleep’, ‘multiple awakenings during sleep’ or ‘early morning awakening’. Each symptom can be scored as 0 (absent), 1 (mild/moderate) or 2 (severe).
Anxiety and agitation/aggression
As well as sleep disturbance, hypnotics and antipsychotics are used to manage anxiety, or agitated or aggressive behaviour in people with dementia. As with sleep disturbance, these are measured in REDIC study using the NPI-NH [severity, frequency and occupational disruption (distress) associated with anxiety and agitation/aggression] and as items on the CSDD (two questions: severity of agitation and severity of anxiety), each of these scored as for sleep disturbance (see Sleep disturbance).
Disability and other covariates
Disability is measured by the observer-rated Lawton Physical Self-Maintenance Scale. 153 Participant age at admission was coded in decades as < 70, 70–79, 80–89 or ≥ 90 years. Educational attainment is coded in terms of years in education as 0–6, 7 (the modal group in this population), 8–12 and ≥ 13 years.
Recoding of outcome measures for analysis
Sleep, anxiety and agitation
The outcome measures of sleep disturbance, QoL, other psychiatric symptoms and cognitive function are also likely to be predictors of future use of medications and so also take the role of time-varying covariates in this analysis.
The distribution of each neuropsychiatric outcome and the Spearman’s rank-order correlations between pairs of neuropsychiatric outcomes are shown in Appendix 3, Table 41. Each outcome, with the exception of anxiety (as measured by CSDD), is endorsed in < 50% of assessments, but there are reasonable numbers of participants who are reported to experience these symptoms in the mild/moderate and severe range. There are very strong correlations between NPI-NH reports of ‘total’ (severity by frequency) and ‘distress’ caused by each of sleep, anxiety and agitation, such that the ‘total’ and ‘distress’ items can be considered collinear. For this reason, because of the relatively high missing value rate, and for comparison with other studies using NPI-NH that do not use the distress variables, the NPI ‘distress’ variables are not included in any further analysis.
The diagonal of Appendix 3, Table 41, shows the auto-correlation of each measure, that is the correlation of each measure with its previous value. These show that although there is some correlation between each measure, there is also considerable fluctuation between waves.
There are some pairwise correlations between other measurements of neuropsychiatric symptoms as expected, in particular when questions aimed to measure similar constructs. A factor analysis of the NPI-NH and CSDD questions on sleep, agitation and anxiety suggested a three-factor solution, with factors corresponding to sleep, agitation and anxiety.
These factor scores are taken forward into future analysis; their distributions are shown in Appendix 4, Figures 26–28.
Cognitive measures
Appendix 4, Figures 23–25, show the trajectories of continuous measures across visits, conditional on the total number of visits completed. The correlation between MMSE and SIB-8 is 0.80, with both measures having a substantial proportion of missing or 0 values (around 20% of occasions in both cases) when participants either did not undertake the assessment or got none of the items correct. These are all coded as 0 for analysis. SIB-8 has a stronger ceiling effect than MMSE, with 10% of SIB-8 scores taking the maximum possible value (16/16) compared with only one maximum possible MMSE score of 30. However, both measures have strong floor effects, caused by a significant proportion of participants being unable to complete any element, hence scoring zero. For this reason, the CDR-SOB was also introduced as a continuous cognitive measure. The CDR is subjectively measured and is successfully completed in 99% of visits, with only 7% of observations taking the maximum or minimum value. CDR-SOB is correlated with MMSE (Spearman’s r = 0.68) and SIB-8 (Spearman’s r = 0.59).
Statistical methods
The sample is described with respect to distribution of exposures, outcomes and covariates at baseline, stratified by dementia severity. The number of participants completing each visit is described and the predictors of dropout at subsequent assessment are estimated using logistic regression. The distribution of each outcome with respect to age, visit number, total number of visits completed, CDR and concurrent Z-drug use is then described using data from all pooled together.
Dynamics of medication use
The number of occasions on which Z-drugs, BZDs and antipsychotics are continued, started or stopped between successive visits is shown, along with their co-occurrence. Separate logistic regression models are then estimated to identify predictors of stopping or starting hypnotics between visits. For each hypnotic, stopping is defined as there being no use at the current visit, estimated among those with reported use at the previous visit, whereas starting is defined as use at the current visit estimated among those with no use at the previous visit. Prior values of exposures, outcome measures and all covariates are used as predictors.
Association between hypnotics and outcomes
Three different analyses are used to explore the association between the use of hypnotics (Z-drugs, BZDs and antipsychotics) and each outcome measure. Each addresses a slightly different question and so provides a different perspective on the links.
Change in outcome measure with pattern of medication use between visits
First, each pair of successive visits for each participant is considered as a separate observation, with these recorded as ‘previous’ and ‘current’ visits. The change in use of each hypnotic between the visits is coded as ‘no use’, ‘starting use’, ‘stopping use’ or ‘continuous use’, depending on the medication status at the first (previous) and second (current) visit of the pair. The level of each outcome measure at previous and current visit is also recorded.
The mean average of each outcome is plotted at the previous and current visit, stratified by the change in each hypnotic use between visits. This gives a visual indication of whether stopping, starting or continuing to use each hypnotic affects the change in outcome measures compared with people who do not report the hypnotic at each visit.
A regression model is then used to estimate the association between pattern of hypnotic use and change in each measure, adjusting for participant age, their baseline cognitive function and visit number. One participant can contribute up to four such observations (visits 1 to 2, 2 to 3, 3 to 4 or 4 to 5), and so clustered standard errors for regression coefficients are used to account for multiple observations per participant.
Marginal structural models for time-varying covariates
The models above estimate the rate of change in outcome measures with change in hypnotic use, but do not adjust for prior values of time-varying covariates of cognitive function or neuropsychiatric symptoms, which are shown to predict later use of medication and may also affect change scores.
To control for time-varying covariates, inverse probability of treatment weights (IPTW) are generated using logistic regression models, estimating the probability of treatment at each visit conditional on previous treatment and previous values of all covariates. Following the method of marginal structural models, these models are used to generate weights reflecting the inverse probability of observed treatment at the current visit, and these weights are applied to simple linear regression models estimating the effect of current hypnotic use on change in outcome measures between waves.
Weights calculated in this way aim to balance values of potential confounders across treatment groups, so that any observed differences in current outcome values can be attributed to the treatment of interest. However, some residual differences in prior values remain (as can be seen in Appendix 3, Figures 3–12, right-hand panels) and so change scores are used as the outcome in regression models as opposed to mean differences at the current assessment. Hence, these IPTW weighted models reflect the effect of hypnotic use on change in outcome measures, accounting for differences in prior treatment and prior of outcome. Standard errors are also corrected for multiple observations per participant.
Fixed-effects regression models
Each of the approaches above considers only pairs of successive observations. So, finally, a longitudinal analysis using fixed-effects models was also conducted. This model compares, for each participant, the values of outcome measures on those occasions when hypnotics are used to the values of outcomes when they are not used. As the comparison is made within participants only, only those participants whose hypnotic use status changes can be included. In addition, this model automatically accounts for any measured or unmeasured covariate, so long as its value does not change within participants. However, it is not possible to correct for time-varying covariates that are also outcome measures. Three fixed-effects models are estimated for each combination of hypnotic and outcome. The first includes all participants, the second includes only those participants with no hypnotic use at all at their baseline visit and the third includes only those participants with minimal or mild dementia (CDR-global score of ≤ 1) at baseline.
The NACC data set
Setting
The NACC data set includes standardised longitudinal patient-level data from Alzheimer’s disease centres (ADCs) across the USA. Data include clinical evaluations and records of medication use at the time of assessment. Patients are assessed annually following referral to an ADC, with the first patient entering the study in 2005, and up to 12 assessments recorded for some participants. Full details of the NACC data set and the uniform data set that is collected at each assessment are found in successive publications describing the evolution of the resource over the years and the NACC website (URL: www.alz.washington.edu/; accessed 6 December 2019). The NACC data set is widely used for dementia research and has been previously used for looking at the impact of medication use on different outcomes among people with MCI or dementia. 156 We requested data from every assessment of all participants included in the NACC data set, with an updated data extraction supplied to us in August 2018. This data set included 131,354 visits among 38,249 unique participants.
Participant inclusion and exclusion criteria
For the current analysis, we included only participants with a study diagnosis of dementia at any time and included only their assessments from the point at which they are first recorded as having dementia. Whether or not the participant meets clinical criteria for dementia is recorded at each assessment. This is made according to clinical judgement or by specific criteria, depending on the version of assessment. In analysis we included only those visits when there is a clinical diagnosis of dementia recorded in the study (NACC coded data set variable ‘demented’ takes the value of ‘1’). This reduced data set included 43,286 annual visits among 17,055 unique participants.
Medication exposures
At each annual visit, the medications each participant had used in the 2 weeks before the visit were recorded. Participants were asked to bring medication to the NACC assessment or a detailed list of their medications. When this was not available, it was followed up by a telephone call following the assessment.
Outcome variables
Cognitive measures
At each assessment, a battery of cognitive tests and psychiatric assessments was conducted. Cognitive tests included the MMSE, the Trail Making Test, from which we calculated a delta trail time (trail B time minus trail A time) and an animal naming test, whereby the patient is asked to name as many distinct animals as possible in 60 seconds. MMSE measures general cognitive ability, delta trail time measures attention and task switching, whereas the animal naming test measures language ability and executive function. The Trail Making Test is reported to have good inter-rater reliability. 157
Further cognitive tests, including the Montreal Cognitive Assessment, were included in later assessments (the third version of the uniform data set), but are not present for the majority of participants and so are not used in the current analysis.
As in the REDIC study, cognitive tests that rely on communication or patients following specific instruction have floor effects or are missing for many severely impaired patients. Hence, the CDR scale was also included: CDR-global was used to stratify patients for descriptive analysis and CDR-SOB was used as an outcome measure. This measure is present for all participants at all visits.
Neuropsychiatric assessments
Neuropsychiatric evaluations included the NPI, although in contrast to the REDIC study only the severity of each NPI item is recorded, as opposed to the ‘severity’, ‘frequency ‘and ‘distress’ variables. Hence, relevant neuropsychiatric symptom, sleep disturbance, anxiety and agitation are assessed at each visit on a 0–3 scale, with 0 corresponding to absence of the symptom, 1 mild, 2 moderate and 3 severe.
To capture the overall burden of neuropsychiatric symptoms, the total NPI score excluding sleep is also included as an outcome measure.
As with cognitive function, more specific items on sleep disturbance are included only in later versions of the NACC data set assessment and so are not included in the current analysis.
Depression
Although there is no available direct measure of QoL, the short form of the Geriatric Depression Scale (GDS) is included in the NACC data set. 158 This includes questions on life satisfaction, helplessness, hopelessness and enjoyment of life that may be considered proxies for a QoL measure, and so GDS is included here as an additional outcome measure. Note, in contrast to many depression measures the short-form GDS does not directly assess sleep disturbance and so should not be directly confounded with measures of sleep. The GDS includes 15 binary items and so is scored from 0 to 15, with higher values indicating more depressive symptoms.
Disability
Disability was measured by 10 questions on the extent to which the participant needed help with each of 10 different activities over the 4 weeks preceding each assessment. The items assessed are writing cheques or paying bills; dealing with taxes or business affairs; shopping; playing games or working on a hobby; heating water, making coffee or turning off the stove; preparing a meal; keeping track of current events; paying attention to a TV show, book or magazine; remembering dates; and travelling.
Each is scored as 0 (no help needed), 1 (does by self but with difficulty), 2 (requires assistance) or 3 (dependent); hence, the total disability scale is scored from 0 to 30. Missing values are omitted at the item level and the total is rescaled if there are fewer than five missing items. If there ≥ 5 out of 10 items missing then the disability score is set as missing.
Statistical methods
Our analysis of the NACC data set follows closely the analysis of the REDIC study data set. First, the characteristics of participants are described stratified by the CDR-global score and the distribution of the total number of study visits with dementia for each participant is shown.
Prevalence and dynamics of medication use
The number of occasions on which each medication of interest at baseline and at subsequent visits is used is described, as well as the dynamics of medication use (number of occasions on which medications are started/stopped between waves). The distribution of each outcome by Z-drug use, CDR, age and change over study visits is shown graphically (see Figures 23–32).
Predictors of starting and continuing medication use
Logistic regression models are then estimated for the predictors of medication use at each wave. This model includes the effects of age, sex, visit number, educational attainment of each participant, along with lagged value of cognitive function (measured by MMSE), NPI sleep, NPI excluding sleep and GDS. As lagged values of covariates are included, only data from the second visit onwards are included in these models. Separate models are estimated for continuing medication use (report of drug among those reporting use at the previous wave) and starting use (reporting of drug among those not reporting use at the previous wave).
Effect of medication use on outcomes
First, the changes in outcome measures between waves are then described and plotted, stratified by whether each of Z-drugs, BZDs or antipsychotics are started, stopped, continue or are absent altogether between waves. These associations between change in outcome measures and change in medication use status are estimated using a linear regression model, adjusting for age group, baseline cognitive function and visit number, with clustered standard errors to account for multiple records per person.
Second, a marginal structural model is used to estimate the effect of each medication, with IPTW used to correct for differences in prior values of outcome measures and exposures between exposed and unexposed groups.
Finally, fixed-effects longitudinal models are estimated, modelling the association between each medication use and each outcome, hence automatically accounting for any between-patient effect (thereby automatically controlling for both measured and unmeasured fixed covariates), although not accounting for time-varying covariates. The following specific models were estimated, mapping those estimated using the REDIC data set:
-
model 1: effect of each drug on each outcome, among all participants with no adjustment for time varying covariates
-
model 2: as model 1 but including only participants with no hypnotic use at baseline
-
model 3: as model 1 but including only participants with a CDR-global score of < 2 (i.e. include only those with mild or minimal dementia at baseline).
Software
Clustered linear models and weighted linear models are estimated using the ‘survey’ package and fixed-effects linear models are estimated using the ‘plm’ package version 1.6-6 (linear models for panel data) for R statistical software version 3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria). The data set was cleaned and coded using R as well as Stata. All other analysis was conducted using R, with the ‘stargazer’, ‘dplyr’ and ‘ggplot2’ packages for data manipulation, managing outputs and plotting, respectively.
The WHELD trial
Setting
This study was undertaken using anonymised data from the WHELD cluster randomised controlled two-arm trial, covering 69 care homes in England. 128,159 The RCT was carried out between January 2013 and September 2015, with a primary aim to determine whether or not an optimised staff training intervention improved the QoL and mental health of people with dementia living in nursing homes.
Participant inclusion and exclusion criteria
Care homes and their residents were eligible for inclusion in the WHELD RCT if the:
-
care home has > 60% of residents with dementia
-
care home was not receiving special support from their local authority
-
care home met the five CQC care home quality standard checks
-
resident had a diagnosis of dementia or had a score of ≥ 1 on the CDR.
Care homes and their residents were excluded from the WHELD RCT if:
-
the care home had insufficient staffing resources or anticipated major change during the study period
-
the care home was involved in other research or undergoing a systematic programme of service improvement
-
consent or advice from a consultee could not be obtained for the resident.
A total of 971 participants met the inclusion criteria, with 504 participants randomised to receive treatment as usual and 467 to receive the WHELD intervention between January 2013 and April 2014. Follow-up assessments were available for 553 participants 9 months later and non-completion was mainly due to mortality.
Participants were not included in the ZED analysis if it was not possible to record their medication usage at baseline or had a reported diagnosis of severe mental illness (schizophrenia or bipolar disorder) at any assessment.
Medication exposures
Antipsychotic and other psychotropic drugs taken at each assessment were classified according to the BNF. 120 Drugs were coded as non-BZD hypnotics, BZDs, antidepressants, carbamazapine, sodium valproate, other anticonvulsants, memantine, cholinesterase inhibitors, barbiturates, clomethiazole, nuspirone and others.
Outcome variables
The following outcomes were assessed prior to randomisation and after 9 months of the intervention.
Patient quality of life
Measured using the QUALID. Lower scores indicate better QoL.
Neuropsychiatric symptoms (excluding sleep)
The NPI-NH was used to assess neuropsychiatric symptoms in people with dementia. 154 The NPI-NH assesses a broad range of symptoms, including delusions, hallucinations, agitation, depression/dysphoria, anxiety, euphoria/elation, apathy/indifference, disinhibition, irritability, aberrant motor behaviours, night-time behaviours and appetite/eating change. Severity scores (1–3) multiplied by frequency score (1–4) were given for each item, resulting in a score ranging from 0 to 12, with a higher score indicating more severe symptoms. The question on sleep disturbance was excluded to give a total maximum score of 132.
Sleep disturbance
The NPI night-time behaviours question ‘Does the patient awaken you during the night, rise too early in the morning, or take excessive naps during the day?’ was used to assess sleep disturbance in people living with dementia.
Confounding variables
The following confounding variables were considered at baseline: age, sex, ethnicity (white or not), marital status (single/widowed, married/long-term partner, divorced/separated), CDR (mild, moderate, severe), sleep disturbance (a NPI-NH sleep score > 0), Abbey Pain Scale score (0–2 = none, 3–7 = mild, ≥ 8 = moderate/severe), comorbidity (depression, anxiety, respiratory illness, gastrointestinal illness, cardiovascular condition, endocrine illness, musculoskeletal disorder and nervous system illness) and co-medication use (BZD, meprobamate/buspirone, clomethiazole, antidepressant, antipsychotic cholinesterase inhibitor and memantine).
Statistical methods
Care home participant characteristics were described for those who were and were not taking Z-drugs at baseline. Negative binomial regression was used to estimate IRRs for the association between Z-drug use at baseline and QUALID, NPI-NH excluding sleep and NPI-NH sleep scores at baseline. Logistic regression was used to estimate ORs for the association between baseline Z-drug use and mortality by 9-month follow-up. Linear regression models were used to estimate the mean decline in QUALID, NPI-NH excluding sleep and NPI-NH sleep scores from baseline to 9-month follow-up for Z-drug use compared with no use at baseline, as well as for continuing, starting or stopping Z-drug use between baseline and 9-month follow-up compared with no use. All associations are presented both unadjusted and adjusted for the covariates listed above. For the analysis comparing changes of Z-drug use across the 9-month follow-up, changes in the use of antidepressants, memantine, antipsychotics and BZDs were also adjusted for.
Chapter 4 Study management
Patient and public involvement
Patient and public involvement (PPI) was an important part of the ZED study. In this section, we detail the different ways in which PPI members contributed to the development and progression of the study during its course.
There was early engagement of PPI participants during the protocol design process to highlight important outcomes to investigate, based on their personal experiences of dementia and sleep disturbance, including its management. PPI members were recruited from Inspire, a PPI group based at the Norfolk and Suffolk NHS Foundation Trust. PPI members included older people with an interest and experience relevant to dementia research. A PPI meeting attended by six panel members was conducted in June 2016 during the initial study stages, from which two members were invited to be part of the annual Study Steering Group meetings and thus allow the continued contribution of PPI members during the study. Feedback from this initial meeting influenced protocol design of the CPRD work package. For example, PPI members helped prioritise the outcomes to be investigated. Their main priority was falls, due to the risk of fractures. They were also keen to see comparisons of Z-drugs with other, alternative treatments. They were keen to see the effect of using Z-drugs at different doses, for different durations and when using them sparingly, rather than every day. There were three Study Steering Group meetings during the study, of which one had a member of PPI present. Additionally, an update was communicated by e-mail in 2017 to keep all PPI participants involved in the study as it progressed. A second and final meeting took place in June 2018 to discuss the results of the study and interpretations from a PPI perspective. We also asked our PPI team for their advice on dissemination activities and publication strategies.
Health-care professional advisory panel
Hypnotic medication is widely prescribed in different health-care settings; in order to inform on hypnotic prescribing experience across different health services we had a second public group, a health-care professional advisory panel consisting of a mental health pharmacist, a clinical doctor specialising in older person and dementia care, a community nurse and a GP. An initial meeting took place in June 2016, during protocol development stages, when we sought advice on sleep disturbance coding in primary care and personal experiences of prescribing hypnotics in people living with dementia. A second meeting took place in April 2018 to discuss the final results of the study, when we sought advice on interpretations of the data and on which health-care professionals to target during our dissemination activities.
Patient and public involvement and health-care professional panel reflections
Although our study was based on secondary data analysis, we found the PPI and health-care professional input vital. Listening to the various different experiences described by carers in the PPI panel whose family member had sleep disturbance and the various approaches used in different settings to manage sleep disturbance described by the health-care professional panel helped us to focus our analyses and interpret the findings.
The PPI members highlighted the importance of the care setting and who the primary carers were in the management of sleep disturbance and the outcomes. This encouraged us to code, as best we could, whether or not the person living with dementia was in a care home.
The PPI members discussed comorbidities and the impact if, for example, the person living with dementia, also had a UTI. Hence, we were careful to consider this and include as a potential confounder in our analyses. The meetings also helped us refine our coding lists for the CPRD, especially regarding different terms for ‘sleep disturbance’.
Although the outcomes we could examine were limited by the NIHR commissioned call, it was still valuable for PPI members to discuss the relative importance of these and see if any others needed to be added. Owing to time constraints, personnel changes and changes within the PPI group, we had limited ability for the PPI members to contribute to the statistical analysis and interpret the results as they became available. However, the dissemination meeting was very important to help understand the importance of downplaying the association between Z-drugs and mortality, emphasising the needs of the carer and the person living with dementia together, and of clearly communicating absolute risks and risk differences alongside the HRs of outcomes when reporting the findings.
Study management
A Study Steering Group met regularly throughout the study to provide oversight and expertise. Study Steering Group meetings were organised throughout the study; PPI representatives were also invited to take part in these meetings to encourage a public perspective on study plans and progression.
Ethics approval
The primary care study was approved by the Independent Scientific Advisory Committee for CPRD for CPRD research (reference 16_181). No further ethics approval was required for the analysis of the data. The CPRD group has obtained ethics approval from a Multicentre Research Ethics Committee for all purely observational research using CPRD data.
The Regional Ethics Committee for Medical Research in South-Eastern Norway approved the REDIC study (reference 2011/1738a). The patient’s capacity to consent to participation in the study was considered by the nursing home staff, including the physician. Written consent for participation was obtained from all participants with the capacity to consent. For participants lacking the capacity to consent, their next of kin gave consent on behalf of the patients. The next of kin gave written consent for their own participation in the study, as they provided information about themselves.
All participants or their legally authorised representatives signed informed consent prior to participation in the NACC study. The institutional review board overseeing each ADC approved local procedures.
Ethics approval for the WHELD RCT was obtained from the South-Central Oxford Research Ethics Committee C (reference 11/SC0066). The trial is registered as a clinical trial (ISRCTN40313497). Consent for nursing home involvement was obtained from the management of the homes. If residents lacked capacity, informed consent was obtained through the involvement of a nominated or personal consultee who represented the residents’ interests and wishes in accordance with the Mental Capacity Act. 160
Funding
The NIHR HTA programme (reference 14/221/02) funded the Z-drugs in dementia (ZED) study.
Sponsorship
The University of East Anglia was the study sponsor.
Chapter 5 Primary care study results
Study cohort selection
The CPRD database contained data from 17.1 million patients in the UK in July 2017, of whom 8.3 million were from English practices with linkage to HES data. Of these, 15,842 patients were diagnosed with dementia at age ≥ 55 years between 1 January 2000 and 31 March 2016, and met our definition of having a sleep disturbance on or after the dementia diagnosis (Figure 2). We excluded 8972 patients from analysis who did not meet our inclusion criteria, as further described in Figure 2. As there was an insufficient number of patients (n = 61) first prescribed melatonin to analyse the outcomes for this drug, the patients prescribed melatonin were excluded, leaving 6809 patients in the study cohort for analysis. Of the included patients, 2952 patients were prescribed a Z-drug on the index date, 1898 patients were prescribed a low-dose TCA, 308 patients were prescribed a BZD and 1651 patients were not prescribed sedative medication.
FIGURE 2.
Selection of patients for the primary care study cohort.
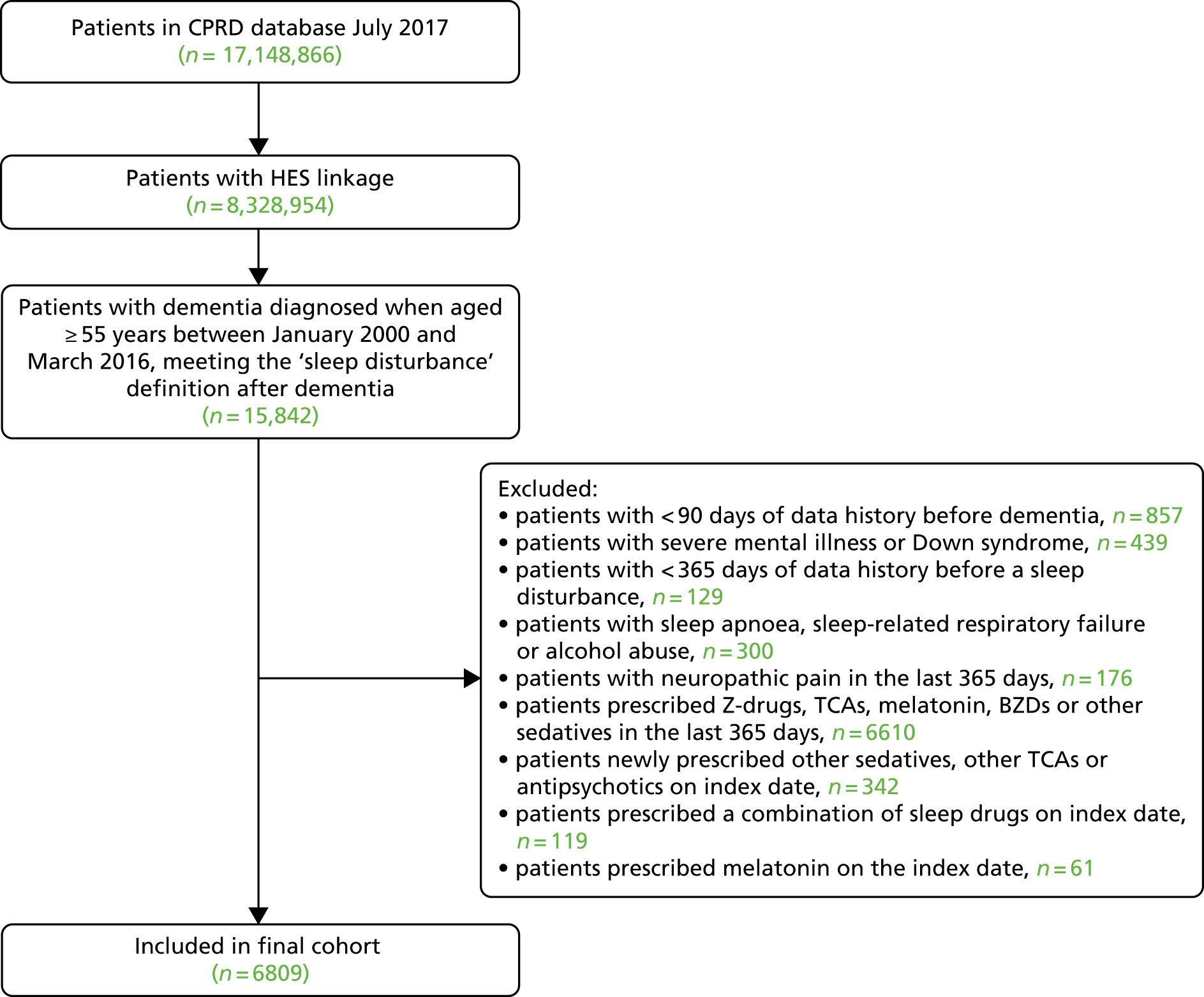
Patient characteristics
The mean age of included patients at index date was 83 [standard deviation (SD) 7.5] years and 4127 (61%) of the patients were women. General practices were located across England and patients had been registered with their general practice for a median of 20 [interquartile range (IQR) 12–33] years before their first recorded sleep disturbance in dementia. As per the study design, 100% of patients in the BZD and ‘no sleep medication’ cohorts had a diagnosis of sleep disturbance on their index date. A total of 572 (19%) and 73 (4%) of the Z-drug and low-dose TCA cohorts had a diagnosis of sleep disturbance on the day of their index date. Further patient characteristics, comorbidities and concomitant medications can be found in Table 2 and in Appendix 2, Table 39.
| Characteristic | First sleep disturbance treatment | |||
|---|---|---|---|---|
| Z-drug (N = 2952) | Low-dose TCA (N = 1898) | BZD (N = 308) | No drug (N = 1651) | |
| Female, n (%) | 1723 (58) | 1193 (63) | 175 (57) | 1036 (63) |
| Age (years), mean (SD) | 83.0 (7.7) | 82.3 (7.3) | 82.9 (7.7) | 83.2 (7.1) |
| White ethnicity, n (%) | 2725 (92) | 1782 (94) | 265 (86) | 1503 (91) |
| Ethnicity missing | 165 (6) | 86 (5) | 37 (12) | 111 (7) |
| Care home residence, n (%) | 694 (24) | 409 (22) | 69 (22) | 393 (24) |
| Residence missing | 1059 (36) | 727 (38) | 113 (37) | 439 (27) |
| Index year, median (IQR) | 2009 (2006–12) | 2010 (2007–13) | 2007 (2004–10) | 2008 (2005–12) |
| General practice region (England), n (%) | ||||
| North | 692 (23) | 517 (27) | 68 (22) | 442 (27) |
| Midlands | 463 (16) | 221 (12) | 39 (13) | 330 (20) |
| East | 326 (11) | 179 (9) | 49 (16) | 247 (15) |
| South | 1184 (40) | 847 (45) | 134 (44) | 503 (30) |
| London | 287 (10) | 134 (7) | 18 (6) | 129 (8) |
| General practice area IMD quintile, mean (SD) | 3.2 (1.4) | 3.1 (1.4) | 3.1 (1.4) | 3.5 (1.4) |
| Health behaviours | ||||
| Smoking status, n (%) | ||||
| Non-smoker | 1947 (66) | 1233 (65) | 203 (66) | 1101 (67) |
| Ex-smoker | 635 (22) | 472 (25) | 58 (19) | 374 (23) |
| Current smoker | 257 (9) | 147 (8) | 31 (10) | 129 (8) |
| Missing | 113 (4) | 46 (2) | 16 (5) | 47 (3) |
| Alcohol user, n (%) | 605 (20) | 428 (23) | 54 (18) | 440 (27) |
| BMI (kg/m2), mean (SD) | 24.7 (4.8) | 25.0 (4.8) | 24.8 (4.7) | 24.5 (4.6) |
| BMI missing, n (%) | 932 (32) | 511 (27) | 111 (36) | 435 (26) |
| Systolic blood pressure (mmHg), mean (SD) | 134.0 (19.2) | 134.4 (17.9) | 134.9 (20.6) | 133.8 (19.1) |
| Blood pressure missing, n (%) | 81 (3) | 44 (2) | 18 (6) | 26 (2) |
Examination of predictors of first sleep disturbance medication revealed small differences between the groups (see Appendix 2, Table 40). Patients had a greater chance of being prescribed Z-drugs if they were a man, had a history of fractures or hospitalisation, or had recent antipsychotic or analgesic use. Those prescribed BZDs were more likely to be men, have osteoporosis, have a history of agitation or psychosis, and have recent antiepileptic and NSAID use. Patients prescribed low-dose TCA had fewer falls and were more likely to have neuropathic, back and/or neck pain or headaches, and recent NSAIDs, antiplatelets and analgesic use, but less SSRI or other antidepressant use. Those not prescribed sleep drugs were more likely to be from a deprived neighbourhood, have a falls history, have more frequent GP visits and have a history of urinary incontinence and of insomnia before dementia, but not to have a history of prior BZD or Z-drug use. Low-dose TCAs and Z-drugs were more likely to be prescribed in recent years (2010), whereas BZDs were more likely to be prescribed in earlier years (2007).
Validation study
A total of 56 (53%) GPs completed our validation questionnaire. The GPs confirmed a diagnosis of dementia for 54 (96%) of the patients (Table 3). The dementia diagnosis date recorded by the GP was very similar to the date we recorded (74% were within 1 month). Sleep disturbance was confirmed for fewer patients, with 35 (63%) patients confirming sleep disturbance.
| Questionnaire result | Sleep disturbance treatment | Total (N = 106) | ||||||
|---|---|---|---|---|---|---|---|---|
| Z-drug (N = 46) | Low-dose TCA (N = 32) | No drug (N = 28) | ||||||
| n | % | n | % | n | % | n | % | |
| Valid questionnaires returned | 22 | 48 | 15 | 47 | 19 | 68 | 56 | 53 |
| Dementia verified by GP | 22 | 100 | 14 | 93 | 18 | 95 | 54 | 96 |
| Sleep disturbance verified by GP | 18 | 82 | 9 | 60 | 8 | 42 | 35 | 63 |
This varied by which drug cohort the patient was in, such that 82%, 60% and 42% of the patients in the Z-drug, low-dose TCA and ‘no sleep drug’ cohort were identified by the GP as having sleep disturbance, respectively.
Further inspection of the patient records revealed that additional information recorded when certain insomnia and sleep disturbance Read codes were entered resulted in the GP software providing a ‘pop-up screen’ asking for the GP to record the patients’ ‘sleep pattern’. For 11 of the 13 patients (85%) with a sleep disturbance code and for whom the GP did not report a sleep disturbance, the record of sleep disturbance also occurred alongside a record of ‘satisfactory’ ‘sleep pattern’ on the pop-up screen (Table 4).
| ‘Satisfactory’ sleep pattern coded on index date | Number of patients | Number of patients (%) with sleep disturbance confirmed |
|---|---|---|
| Yes | 17 | 6 (35) |
| No | 12 | 10 (83) |
| Total | 29 | 16 (55) |
Owing to the validation study findings, we performed a sensitivity analysis with an amended definition of sleep disturbance that excludes Read codes accompanied by a record of ‘satisfactory sleep pattern’ on the GP pop-up screen. We also advise caution interpreting any risks estimated with low-dose TCAs, as we could not confirm that sleep disturbance was the indication for many of these prescriptions, and people living with dementia with chronic pain may have different risks of the adverse outcomes than those with sleep disturbance.
Patient follow-up
A total of 137, 44 and 135 patients from the ‘no sleep disturbance treatment’ group subsequently met the criteria for the Z-drug, low-dose TCA and BZD groups, respectively. Hence, each of these patients occur in the analysis twice.
Patients were followed for a median of 3.2 (IQR 2.3–9.1) months. The main reason for censoring was patients having no further Z-drug, BZD, or low-dose TCA prescriptions for 90 days, from each cohort, respectively (Table 5). The second most common reason for censoring was because of a new sedating medication being prescribed. This resulted in a median follow-up for each cohort of 3.0 (IQR 1.8–5.3), 3.2 (IQR 3.0–8.7), 3.0 (IQR 2.0–5.0) and 8.9 (IQR 2.8–23.9) months for the Z-drug, low-dose TCA, BZD and no sleep disturbance treatments groups, respectively.
| Censoring reason | Sleep disturbance treatment at index datea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Z-drug (N = 3089) | Low-dose TCA (N = 1942) | BZD (N = 443) | No drug (N = 1651) | Discontinued Z-drug (N = 1274) | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Death | 351 | 11 | 192 | 10 | 64 | 14 | 261 | 16 | 179 | 14 |
| Transferred out | 311 | 10 | 165 | 8 | 33 | 7 | 269 | 16 | 209 | 16 |
| Last practice data extraction | 69 | 2 | 85 | 4 | 7 | 2 | 106 | 6 | 95 | 7 |
| New sedative drug | 906 | 29 | 396 | 20 | 119 | 27 | 527 | 32 | 510 | 40 |
| Z-drug | 0 | 0 | 70 | 4 | 40 | 9 | 149 | 9 | 280 | 22 |
| Low-dose TCA | 119 | 4 | 0 | 0 | 17 | 4 | 46 | 3 | 30 | 2 |
| BZD | 440 | 14 | 167 | 9 | 0 | 0 | 161 | 10 | 115 | 9 |
| Melatonin | 11 | 0 | < 5 | 0 | < 5 | 0 | 5 | 0 | < 5 | 0 |
| Other sedative | 35 | 1 | 17 | 1 | 6 | 1 | 24 | 1 | 15 | 1 |
| Antipsychotic | 305 | 10 | 142 | 7 | 55 | 12 | 149 | 9 | 69 | 5 |
| No prescription for 90 days | 1306 | 42 | 921 | 47 | 193 | 44 | 0 | 0 | 0 | 0 |
| End of study (2 years) | 123 | 4 | 143 | 7 | 23 | 5 | 411 | 25 | 213 | 17 |
| End of study (31 March 2016) | 23 | 1 | 40 | 2 | < 5 | 1 | 77 | 5 | 68 | 5 |
For the sensitivity analysis, of the 3089 patients prescribed Z-drugs, 1274 of them stopped receiving prescriptions and met the inclusion criteria to be included in a ‘discontinued Z-drugs’ cohort. Their median follow-up was 9.1 (IQR 3.0–22.1) months and the most common reason for censoring was being prescribed a new sedating medication. A total of only 280 (22%) were censored as a result of receiving another Z-drug prescription.
First prescription for sleep disturbance
The first sleep disturbance prescriptions issued to the cohort are described in Table 6. Of the 3089 patients receiving Z-drugs, the majority (95%) were prescribed zopiclone. The most common daily dose prescribed of zopiclone was 3.75 mg (80%). The most common low-dose TCA prescribed was amitriptyline (57%) and the most common BZD prescribed was temazepam (64%).
| First prescription | Number of patients | Per cent within class |
|---|---|---|
| Z-drug | ||
| Zaleplon | 12 | 0.4 |
| Zolpidem | 138 | 4.5 |
| Zopiclone | 2939 | 95.1 |
| Low-dose TCA | ||
| Amitriptyline | 1116 | 57.5 |
| Trazodone | 826 | 42.5 |
| BZD | ||
| Diazepam | 54 | 12.2 |
| Lorazepam | 50 | 11.3 |
| Lormetazepam | 13 | 2.9 |
| Midazolam | 14 | 3.2 |
| Nitrazepam | 21 | 4.7 |
| Oxazepam | 6 | 1.4 |
| Temazepam | 285 | 64.3 |
During the median 3.0 (IQR 1.8–5.3) months’ follow-up for the cohort initiated on Z-drugs, 1484 (48%), 429 (14%), 212 (7%) and 964 (31%) go on to receive none, one, two and three or more further Z-drug prescriptions.
Fractures, falls and mortality
A total of 368 patients experienced a fracture during follow-up, with around half of these being hip fractures. A total of 1078 patients reported a fall to their GP or were admitted to hospital with a recorded fall. Overall, 883 patients died during follow-up.
Table 7 displays the incidence rates and HRs for fractures, falls and mortality for the different sleep disturbance treatments, relative to no prescription for sleep disturbance, adjusted for age and sex, and adjusted for all potential confounders. Generally, there was little difference between the full adjustment for confounders and adjustment for just age and sex. The incidence of fractures in patients receiving Z-drugs was 11.3 per 100 person-years. In patients receiving BZDs, low-dose TCA or no prescriptions for sleep disturbance, the incidence of fractures was 12.0, 10.2 and 7.3 per 100 person-years, respectively. After adjustment for potential confounders, prescription of Z-drugs was associated with a greater incidence of fractures, with a HR of 1.40 (95% CI 1.01 to 1.94).
| Outcome and sleep medication | Patients (n) | Incidence/100 PYs | Age, sex adjusted | Adjusteda | |||
|---|---|---|---|---|---|---|---|
| Exposed | With outcome | HR | 95% CI | HR | 95% CI | ||
| Fracture | |||||||
| No sleep drug | 1636 | 108 | 7.3 | 1.00 | 1.00 | ||
| Low-dose TCA | 1913 | 105 | 10.2 | 1.29 | 0.98 to 1.70 | 1.12 | 0.80 to 1.58 |
| BZD | 433 | 20 | 12.0 | 1.41 | 0.87 to 2.29 | 1.34 | 0.69 to 2.61 |
| Z-drug | 2997 | 135 | 11.3 | 1.39 | 1.06 to 1.82 | 1.40 | 1.01 to 1.94 |
| Hip fracture | |||||||
| No sleep drug | 1636 | 47 | 3.1 | 1.00 | 1.00 | ||
| Low-dose TCA | 1913 | 56 | 5.3 | 1.59 | 1.06 to 2.38 | 1.53 | 0.95 to 2.48 |
| BZD | 433 | 12 | 7.1 | 2.01 | 1.03 to 3.93 | 2.07 | 0.72 to 5.97 |
| Z-drug | 2997 | 66 | 5.4 | 1.53 | 1.02 to 2.27 | 1.59 | 1.00 to 2.53 |
| Forearm/wrist/hand fracture | |||||||
| No sleep drug | 1636 | 18 | 1.2 | 1.00 | 1.00 | ||
| Low-dose TCA | 1913 | 16 | 1.5 | 1.11 | 0.57 to 2.19 | 0.99 | 0.35 to 2.79 |
| BZD | 433 | < 5 | N/A | N/A | |||
| Z-drug | 2997 | 27 | 2.2 | 1.83 | 0.94 to 3.56 | 1.29 | 0.53 to 3.15 |
| Fall | |||||||
| No sleep drug | 1506 | 328 | 26.7 | 1.00 | 1.00 | ||
| Low-dose TCA | 1864 | 286 | 30.4 | 0.99 | 0.85 to 1.05 | 0.84 | 0.69 to 1.02 |
| BZD | 412 | 65 | 43.9 | 1.32 | 1.00 to 1.73 | 1.05 | 0.75 to 1.47 |
| Z-drug | 2888 | 399 | 37.8 | 1.11 | 0.96 to 1.30 | 1.05 | 0.87 to 1.25 |
| Mortality | |||||||
| No sleep drug | 1651 | 266 | 16.9 | 1.00 | 1.00 | ||
| Low-dose TCA | 1942 | 196 | 18.1 | 1.00 | 0.83 to 1.21 | 0.89 | 0.72 to 1.11 |
| BZD | 443 | 66 | 36.7 | 1.80 | 1.35 to 2.39 | 1.38 | 0.96 to 1.96 |
| Z-drug | 3089 | 355 | 27.8 | 1.43 | 1.21 to 1.69 | 1.34 | 1.10 to 1.64 |
The incidence of hip fractures was 5.4 per 100 person-years in those prescribed Z-drugs compared with 3.1 per 100 person-years in those not prescribed medication for sleep disturbance. After adjustment for confounders there was a trend towards an increased risk of hip fracture with Z-drugs (HR 1.59, 95% CI 1.00 to 2.53), but this did not reach statistical significance (p = 0.052).
There were few reported fractures of the forearm, wrist or hand, with 2.2 per 100 person-years for the Z-drug group and 1.2 per 100-person years for the no sleep medication group. There was not a statistically significant association between Z-drug use and forearm fractures when adjusted for age and sex, or all potential confounders.
The incidence of falls was 37.8 per 100 person-years in those prescribed Z-drugs compared with 26.7 per 100 person-years for those not prescribed medication for sleep disturbance. There was no statistically significant difference in the risk of falls with Z-drug use when adjusted for age and sex, or all potential confounders.
The mortality rate was 27.8 per 100-person years in the Z-drug group compared with 16.9 per 100 person-years in the no sleep medication group. After adjustment for potential confounders, Z-drug prescription was associated with a 34% (95% CI 10% to 64%) increased risk of mortality.
Generally, the risks of fractures, falls and mortality for Z-drugs were similar to those of BZDs (see Appendix 5, Table 55). Risk of hip fracture with low-dose TCA appeared similar to that of Z-drugs; however, there was no evidence of increased risk of overall fractures, falls or mortality with low-dose TCA.
There was no evidence that the reported HRs varied over time and there was no evidence of an interaction between Z-drug use and either age, sex, mortality or fractures. Using different parametrisations for age had little impact on the findings (see Appendix 5, Table 56).
Absolute risks
The absolute annual risks of fractures, hip fractures and mortality in the unexposed group were 7.4%, 3.2% and 15.4%, respectively. These equate to annual risks of 10.2%, 5.0% and 20.1% for fractures, hip fractures and mortality when taking Z-drugs if our estimated risks are causal. This is equivalent to a NNH of 36, 54 and 21, and extra cases per 1000 treated of 28, 18 and 47, respectively.
Dose–response
When examining outcomes by cumulative Z-drug dose, we found that fracture risk increased with cumulative exposure to Z-drugs (Table 8). During periods when cumulative prescriptions totalled < 28 DDDs of Z-drugs, equivalent to 56 days at the recommended half-dose in the elderly, there were no excess risks of fractures. For 42–55 prescribed DDDs the HR for a fracture increased to 2.81 (95% CI 1.47 to 5.37).
| Outcome and DDD of Z-drug | Age, sex adjusted | Test for trend | Fully adjusteda | Test for trend | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Fracture | ||||||
| 0 | 1.00 | 0.001 | 1.00 | 0.002 | ||
| 1–13 | 0.74 | 0.39 to 1.41 | 0.69 | 0.36 to 1.36 | ||
| 14–27 | 0.93 | 0.56 to 1.53 | 0.91 | 0.53 to 1.54 | ||
| 28–41 | 1.90 | 1.15 to 3.11 | 1.96 | 1.15 to 3.33 | ||
| 42–55 | 2.78 | 1.54 to 5.04 | 2.81 | 1.47 to 5.37 | ||
| ≥ 56 | 1.48 | 1.07 to 2.05 | 1.53 | 1.04 to 2.25 | ||
| Hip fracture | ||||||
| 0 | 1.00 | 0.001 | 1.00 | 0.003 | ||
| 1–13 | 0.83 | 0.36 to 1.88 | 0.80 | 0.35 to 1.84 | ||
| 14–27 | 0.58 | 0.26 to 1.29 | 0.60 | 0.26 to 1.35 | ||
| 28–41 | 1.83 | 0.91 to 3.65 | 1.98 | 0.97 to 4.01 | ||
| 42–55 | 3.30 | 1.50 to 7.24 | 3.33 | 1.41 to 7.91 | ||
| ≥ 56 | 1.90 | 1.18 to 3.06 | 2.03 | 1.15 to 3.60 | ||
| Forearm fracture | ||||||
| 0 | 1.00 | 0.005 | 1.00 | 0.16 | ||
| 1–27 | 0.61 | 0.17 to 2.26 | 0.47 | 0.12 to 1.86 | ||
| 28–41 | 4.12 | 1.40 to 12.06 | 4.15 | 1.21 to 14.24 | ||
| 42–55 | 2.27 | 0.42 to 12.15 | 1.89 | 0.27 to 13.22 | ||
| ≥ 56 | 2.08 | 1.02 to 4.26 | 1.37 | 0.49 to 3.80 | ||
| Fall | ||||||
| 0 | 1.00 | 0.14 | 1.00 | 0.49 | ||
| 1–13 | 0.93 | 0.69 to 1.27 | 0.83 | 0.60 to 1.14 | ||
| 14–27 | 1.11 | 0.87 to 1.42 | 1.07 | 0.83 to 1.39 | ||
| 28–41 | 1.49 | 1.12 to 1.98 | 1.43 | 1.06 to 1.94 | ||
| 42–55 | 1.70 | 1.17 to 2.45 | 1.59 | 1.08 to 2.34 | ||
| ≥ 56 | 1.02 | 0.82 to 1.25 | 0.94 | 0.74 to 1.19 | ||
| Mortality | ||||||
| 0 | 1.00 | 0.001 | 1.00 | 0.11 | ||
| 1–13 | 1.30 | 0.91 to 1.87 | 1.34 | 0.91 to 1.97 | ||
| 14–27 | 1.65 | 1.23 to 2.22 | 1.68 | 1.23 to 2.30 | ||
| 28–41 | 1.33 | 0.93 to 1.91 | 1.32 | 0.90 to 1.92 | ||
| 42–55 | 1.75 | 1.14 to 2.67 | 1.64 | 1.05 to 2.57 | ||
| ≥ 56 | 1.36 | 1.12 to 1.67 | 1.21 | 0.96 to 1.53 | ||
The picture was similar for hip fractures, with no excess risk when prescribed < 28 DDDs of Z-drugs, rising to a HR of 3.33 (95% CI 1.41 to 7.91) when cumulatively prescribed 42–55 DDDs. There were few forearm, wrist or hand fracture outcomes, but there was evidence of a similar pattern to that of fractures with no risk before 28 DDDs and greater risks after. There was evidence of increased falls risks after > 28 DDDs of Z-drugs had been prescribed, but this association disappeared after 56 DDDs were prescribed. The risk of mortality was generally fairly similar, regardless of cumulative exposure to Z-drugs. In addition, we found no evidence of differences in risk according to whether or not the first Z-drug prescription was PRN (see Appendix 5, Table 57).
Infections, cardiovascular and agitation or psychosis outcomes
A total of 1104 patients were reported to have an acute bacterial infection during follow-up, with 86% of these being a UTI or LRTI. Whereas, 188 patients experienced an ischaemic stroke or TIA, 72 patients reported venous thromboembolism and 340 patients were recorded as having incident agitation or psychosis during follow-up.
Table 9 displays the incidence and HRs for infections, cardiovascular and agitation or psychosis outcomes for the different sleep disturbance treatment, relative to no prescription for sleep disturbance, adjusted for age and sex, and adjusted for all potential confounders. Although the incidence of acute bacterial infections was greater at 47.7 compared with 34.7 per 100 person-years in patients receiving Z-drugs compared with no sleep disturbance medications, this association was not statistically significant when adjusted for potential confounders. There was also no statistically significant difference between Z-drug prescription and the rate of UTI and LRTI.
| Outcome and sleep medication | Patients (n) | Incidence/100 PYs | Age, sex adjusted | Fully adjusteda | |||
|---|---|---|---|---|---|---|---|
| Exposed | With outcome | HR | 95% CI | HR | 95% CI | ||
| Acute bacterial infection | |||||||
| No sleep drug | 1303 | 374 | 34.7 | 1.00 | 1.00 | ||
| Low-dose TCA | 1423 | 273 | 37.8 | 1.02 | 0.87 to 1.19 | 1.00 | 0.83 to 1.21 |
| BZD | 334 | 49 | 38.6 | 0.92 | 0.68 to 1.24 | 0.84 | 0.59 to 1.21 |
| Z-drug | 2267 | 408 | 47.7 | 1.17 | 1.01 to 1.36 | 1.09 | 0.92 to 1.29 |
| UTI/LRTI | |||||||
| No sleep drug | 1303 | 328 | 29.5 | 1.00 | 1.00 | ||
| Low-dose TCA | 1423 | 226 | 31.3 | 0.96 | 0.81 to 1.14 | 0.96 | 0.78 to 1.18 |
| BZD | 334 | 41 | 31.5 | 0.90 | 0.65 to 1.25 | 0.85 | 0.58 to 1.26 |
| Z-drug | 2267 | 354 | 40.2 | 1.16 | 0.99 to 1.36 | 1.10 | 0.92 to 1.32 |
| Ischaemic stroke/TIA | |||||||
| No sleep drug | 1640 | 64 | 4.2 | 1.00 | 1.00 | ||
| Low-dose TCA | 1933 | 50 | 4.7 | 1.03 | 0.71 to 1.49 | 1.20 | 0.75 to 1.92 |
| BZD | 438 | 15 | 8.7 | 1.81 | 1.01 to 3.26 | 1.56 | 0.69 to 3.51 |
| Z-drug | 3045 | 80 | 6.5 | 1.45 | 1.02 to 2.07 | 1.33 | 0.85 to 2.07 |
| Venous thromboembolism | |||||||
| No sleep drug | 1648 | 22 | 1.4 | 1.00 | 1.00 | ||
| Low-dose TCA | 1940 | 24 | 2.2 | 1.53 | 0.88 to 2.67 | 1.23 | 0.56 to 2.69 |
| BZD | 442 | < 5 | N/A | N/A | |||
| Z-drug | 3074 | 26 | 2.1 | 1.53 | 0.86 to 2.72 | 1.66 | 0.69 to 3.98 |
| Incident agitation/psychosis | |||||||
| No sleep drug | 1282 | 79 | 6.4 | 1.00 | 1.00 | ||
| Low-dose TCA | 1633 | 85 | 9.7 | 1.36 | 0.99 to 1.05 | 1.43 | 0.96 to 2.13 |
| BZD | 313 | 36 | 30.5 | 3.89 | 2.54 to 5.96 | 5.61 | 3.14 to 10.01 |
| Z-drug | 2574 | 140 | 13.6 | 1.59 | 1.20 to 2.11 | 1.71 | 1.21 to 2.42 |
Although the incidence of ischaemic stroke or TIA was greater at 6.5 compared with 4.2 per 100 person-years in patients receiving Z-drugs compared with no sleep disturbance medications, this association was not statistically significant when adjusted for potential confounders.
There were few venous thromboembolism events and, again, we did not detect a statistically significant difference in their rate between patients prescribed Z-drugs and those not prescribed medications for sleep disturbance.
The incidence of agitation or psychosis in patients prescribed Z-drugs was 13.6 per 100 person-years, compared with 6.4 per 100 person-years for those not prescribed medication for sleep disturbance. After adjustment for potential confounders, Z-drug prescription was associated with a HR of 1.71 (95% CI 1.21 to 2.42) for agitation or psychosis. There was no evidence that this association varied by age or sex.
There was no association between low-dose TCA and any of the infection, cardiovascular, or agitation or psychosis outcomes. There was a greater incidence of ischaemic stroke or TIA in those prescribed BZDs, but this difference failed to reach statistical significance. BZD prescription was, however, associated with an increased rate of agitation or psychosis.
Dose–response
When examining outcomes by cumulative Z-drug dose, we found some suggestion of increased acute bacterial infections with cumulative exposure to Z-drugs (Table 10); however, this was only during periods when cumulative prescriptions totalled 28–41 DDDs and not for > 42 DDDs. There was no evidence of increased risks of ischaemic stroke, TIA, or venous thromboembolism with cumulative Z-drug use. However, the incidence of agitation or psychosis increased with greater cumulative Z-drug prescriptions, such that for 42–55 prescribed DDDs, the HR increased to 2.55 (95% CI 1.31 to 4.98). In addition, we found no evidence of differences in risk according to whether or not the first Z-drug prescription was PRN (see Appendix 5, Table 57).
| Outcome and DDD of Z-drug | Age, sex adjusted | Test for trend | Fully adjusteda | Test for trend | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Acute bacterial infection | ||||||
| 0 | 1.00 | 0.002 | 1.00 | 0.04 | ||
| 1–13 | 0.82 | 0.60 to 1.14 | 0.77 | 0.55 to 1.08 | ||
| 14–27 | 0.93 | 0.71 to 1.21 | 0.89 | 0.68 to 1.17 | ||
| 28–41 | 1.44 | 1.09 to 1.90 | 1.38 | 1.04 to 1.84 | ||
| 42–55 | 1.18 | 0.78 to 1.78 | 1.14 | 0.74 to 1.74 | ||
| ≥ 56 | 1.27 | 1.06 to 1.53 | 1.17 | 0.95 to 1.44 | ||
| UTI/LRTI | ||||||
| 0 | 1.00 | 0.01 | 1.00 | 0.12 | ||
| 1–13 | 0.87 | 0.61 to 1.24 | 0.84 | 0.58 to 1.21 | ||
| 14–27 | 0.96 | 0.72 to 1.28 | 0.96 | 0.71 to 1.29 | ||
| 28–41 | 1.46 | 1.08 to 1.98 | 1.43 | 1.04 to 1.95 | ||
| 42–55 | 1.12 | 0.71 to 1.78 | 1.09 | 0.68 to 1.75 | ||
| ≥ 56 | 1.22 | 1.00 to 1.49 | 1.13 | 0.90 to 1.41 | ||
| Ischaemic stroke/TIA | ||||||
| 0 | 1.00 | 0.005 | 1.00 | 0.11 | ||
| 1–13 | 0.71 | 0.29 to 1.74 | 1.02 | 0.39 to 2.71 | ||
| 14–27 | 1.02 | 0.54 to 1.92 | 1.09 | 0.55 to 2.15 | ||
| 28–41 | 1.77 | 0.92 to 3.39 | 1.63 | 0.79 to 3.36 | ||
| 42–55 | 0.91 | 0.27 to 3.06 | 0.80 | 0.22 to 2.86 | ||
| ≥ 56 | 1.69 | 1.14 to 2.51 | 1.48 | 0.90 to 2.42 | ||
| Venous thromboembolism | ||||||
| 0 | 1.00 | 0.26 | 1.00 | 0.47 | ||
| 1–13 | 2.08 | 0.64 to 6.76 | 1.74 | 0.55 to 5.56 | ||
| 14–27 | 1.83 | 0.64 to 5.22 | 1.78 | 0.50 to 6.32 | ||
| 28–41 | 1.30 | 0.27 to 6.22 | 1.58 | 0.29 to 8.50 | ||
| ≥ 42 | 1.44 | 0.72 to 2.85 | 1.52 | 0.53 to 4.40 | ||
| Incident agitation/psychosis | ||||||
| 0 | 1.00 | 0.001 | 1.00 | 0.008 | ||
| 1–13 | 1.09 | 0.64 to 1.87 | 1.38 | 0.76 to 2.48 | ||
| 14–27 | 1.45 | 0.95 to 2.21 | 1.72 | 1.08 to 2.74 | ||
| 28–41 | 1.42 | 0.83 to 2.43 | 1.58 | 0.89 to 2.80 | ||
| 42–55 | 2.31 | 1.24 to 4.32 | 2.55 | 1.31 to 4.98 | ||
| ≥ 56 | 1.74 | 1.20 to 2.52 | 1.70 | 1.09 to 2.64 | ||
Additional medications
A total of 1124 (16%) patients were prescribed a different class of sleep medication during follow-up (Table 11). We did not detect a statistically significant increased rate of new medications for sleep disturbance in the Z-drug or BZD groups. Those prescribed low-dose TCA were 79% (95% CI 65% to 97%) less likely to be prescribed another medication for sleep disturbance. The additional sleep medications prescribed are described in Table 5.
| Outcome and sleep drug | Patients exposed (n) | Number of outcomes | Incidence/100 PYs | Age, sex adjusted | Fully adjusteda | ||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| Additional sleep medication | |||||||
| No sleep drug | 1651 | 336 | 21.4 | 1.00 | 1.00 | ||
| Low-dose TCA | 1942 | 258 | 23.8 | 0.89 | 0.75 to 1.05 | 0.79 | 0.65 to 0.97 |
| BZD | 443 | 45 | 25.0 | 0.76 | 0.56 to 1.05 | 0.63 | 0.43 to 0.93 |
| Z-drug | 3089 | 485 | 38.0 | 1.26 | 1.08 to 1.46 | 1.13 | 0.95 to 1.35 |
| Incident antipsychotic prescription | |||||||
| No sleep drug | 1291 | 175 | 13.8 | 1.00 | 1.00 | ||
| Low-dose TCA | 1618 | 164 | 19.1 | 1.13 | 0.90 to 1.40 | 1.17 | 0.90 to 1.53 |
| BZD | 375 | 66 | 59.5 | 2.37 | 1.75 to 3.21 | 2.23 | 1.50 to 3.32 |
| Z-drug | 2402 | 355 | 39.5 | 2.03 | 1.67 to 2.47 | 2.01 | 1.60 to 2.52 |
| Incident antidepressant prescription | |||||||
| No sleep drug | 1207 | 113 | 10.3 | 1.00 | 1.00 | ||
| BZD | 326 | 31 | 24.4 | 1.75 | 1.17 to 2.64 | 1.77 | 1.02 to 3.09 |
| Z-drug | 2177 | 236 | 28.3 | 2.04 | 1.61 to 2.57 | 2.05 | 1.56 to 2.68 |
| Incident antibiotic prescription | |||||||
| No sleep drug | 1364 | 616 | 69.8 | 1.00 | 1.00 | ||
| Low-dose TCA | 1552 | 562 | 93.8 | 1.21 | 1.08 to 1.36 | 1.13 | 0.99 to 1.29 |
| BZD | 357 | 116 | 120.8 | 1.38 | 1.12 to 1.70 | 1.35 | 1.06 to 1.71 |
| Z-drug | 2430 | 818 | 116.4 | 1.39 | 1.25 to 1.55 | 1.26 | 1.12 to 1.42 |
However, Z-drug users were more likely to be prescribed a new antipsychotic (HR 2.01, 95% CI 1.60 to 2.52), antidepressant (HR 2.05, 95% CI 1.56 to 2.68) and antibiotic during follow-up (HR 1.26, 95% CI 1.12 to 1.42). There was no evidence that these associations varied by age or sex. BZD users had similar increased rates of antipsychotic, antidepressant and antibiotic prescribing to the Z-drug users (see Appendix 5, Table 58). No increased rates of antipsychotic or antibiotic prescriptions were detected for low-dose TCA users.
Health-care utilisation
A total of 133,673 GP visits were recorded by the patients in the 2 years following the index date (before 31 March 2016 and before leaving the practice), equivalent to a mean number of visits of 15.9 (SD 20.4). Patients prescribed Z-drugs visited their GP 922 times per 100 person-years, compared with those not prescribed medications for sleep disturbance visiting their GP 783 times per 100 person-years (Table 12). After adjusting for potential confounders, Z-drug users visited their GP on average 14% (95% CI 8% to 19%) more frequently over the next 2 years.
| Outcome and sleep drug | Patients exposed (n) | Number of outcomes | Incidence/100 PYs | Age, sex adjusted | Fully adjusteda | ||
|---|---|---|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | ||||
| Number of GP visits | |||||||
| No sleep drug | 1651 | 25,610 | 783.2 | 1.00 | 1.00 | ||
| Low-dose TCA | 1942 | 33,530 | 882.1 | 1.22 | 1.15 to 1.30 | 1.01 | 0.96 to 1.07 |
| BZD | 443 | 6806 | 791.4 | 1.12 | 1.02 to 1.23 | 1.05 | 0.97 to 1.14 |
| Z-drug | 3089 | 47,259 | 922.5 | 1.24 | 1.17 to 1.31 | 1.14 | 1.08 to 1.19 |
| Number of hospital admissions | |||||||
| No sleep drug | 1651 | 2484 | 99.7 | 1.00 | 1.00 | ||
| Low-dose TCA | 1942 | 2991 | 105.2 | 1.08 | 0.97 to 1.19 | 1.04 | 0.95 to 1.15 |
| BZD | 443 | 614 | 98.9 | 1.00 | 0.87 to 1.16 | 0.97 | 0.84 to 1.13 |
| Z-drug | 3089 | 4836 | 112.3 | 1.15 | 1.05 to 1.26 | 1.12 | 1.03 to 1.21 |
A total of 12,850 hospital admissions were recorded by the patients in the 2 years following the index date and before 31 March 2016, equivalent to a mean number of admissions of 1.5 (SD 2.3). Patients prescribed Z-drugs were admitted to hospital 112 times per 100 person-years, compared with 98 times per 100 person-years for those not prescribed medications for sleep disturbance. After adjusting for potential confounders, Z-drug users were admitted to hospital on average 12% (95% CI 3% to 21%) more frequently over the next 2 years.
There was no evidence that these associations varied by age or sex. We also did not detect an increased frequency of GP visits or hospital admission among BZD and low-dose TCA users.
Multiple testing
Owing to the number of outcomes tested, we would expect some associations to be statistically significant by chance alone. For the association between Z-drug prescription and the 16 outcomes, we estimated a critical threshold of p < 0.02 to define statistical significance, such that the false discovery rate was < 5%. Of the 16 outcomes tested, the outcomes of mortality, agitation or psychosis, antipsychotics, antidepressants, antibiotics, GP visits and hospital admissions, all had associations with Z-drug use with p < 0.02 and, hence, can be considered statistically significant after accounting for the multiple tests performed. However, the observed association with fractures, with p = 0.045, would not be considered statistically significant and has an increased chance of being a false discovery. However, multiple testing procedures vary as to how they define statistical significance and assume that all outcomes are independent events, which may not be the case here. Regardless, with a p-value so close to 0.05 for fractures, it would not pass any multiple testing correction. Our findings need interpretation within this context (i.e. the association between Z-drug prescription and fractures could be a chance finding due to many outcomes tested). The fracture finding needs to be considered in the context of its consistency with findings for other outcomes (e.g. specific fractures, falls), consistency within cumulative dose–response relationships, as well as evidence from other studies.
Sensitivity analysis: missing data
Data were missing for smoking status (for 3% of patients), alcohol use (for 9% of patients), recent BMI (for 32% of patients), systolic blood pressure (for 2% of patients), care home status (for 34% of patients) and quantity of tablets prescribed (for < 0.01% of those prescribed Z-drugs). Below, we explore the consequences of missing data for BMI and care home status, as these variables are missing for > 10% of patients.
Body mass index values were not missing completely at random. Those without a BMI measurement in the last 5 years were more likely to be older, to be living in a care home, to visit their GP less often, to have fewer comorbidities, fewer prescriptions and immunisations, to have had dementia for longer with a history of agitation or psychosis, and to be from a less deprived area. Recent BMI was also more likely to be recorded in the no sleep drug cohort. Associations between Z-drug use and the adverse events varied in the patients with and without missing BMI data. For example, the HR for fractures adjusted only for age and sex was 1.13 (95% CI 0.71 to 1.79) and was 1.56 (95% CI 1.12 to 2.18) in those with and without missing BMI data.
Care home status was also not missing completely at random. Patients missing their care home status were more likely to be younger, male and to have fewer comorbidities and prescriptions, except for more lipid-lowering medications and inhaled corticosteroids. Again, care home status was more likely to be recorded in the no sleep drug cohort. Associations between Z-drug use and the adverse events varied in the patients with and without missing care home data. For example, the HR for fractures adjusted only for age and sex was 1.35 (95% CI 0.99 to 1.83) and was 1.73 (95% CI 0.98 to 3.06) in those with and without care home status recorded.
A single imputation model recoded 429 (15%) of those with missing care home status as being in a care home, and the rest as not. Subsequently, a single imputation model including information from BMI values more than 5 years ago, recategorised 26 (1%), 1598 (69%), 594 (26%), and 87 (4%) patients with recent BMI missing into values of < 18.5, 18.5–24.9, 25–29.9, and ≥ 30 kg/m2, respectively. Adjustment for all the covariates included the imputed BMI and care home variables, had little effect on estimates (see Appendix 5, Tables 59 and 60).
Sensitivity analysis: sleep disturbance definition
When excluding diagnoses of sleep disturbance in the GP records if they were accompanied by record of a ‘satisfactory’ sleep pattern, only 924 (56%) of the patients in the no sleep medication cohort met this criterion. These 924 patients were more likely to have a recent fracture, dizziness, faint, hospitalisation, agitation or psychosis, antipsychotic prescription and to be from less deprived neighbourhoods, and were less likely to have urinary incontinence, a history of insomnia, to drink alcohol or be in a care home, than those with record of satisfactory sleep disturbance. Comparing to this group generally decreased the associations with Z-drug use and the various outcomes (see Appendix 5, Tables 61 and 62). The HR between Z-drug use and fracture, hip fracture and mortality reduced to 1.34 (95% CI 0.90 to 1.99), 1.33 (95% CI 0.77 to 2.32) and 1.09 (95% CI 0.86 to 1.38), respectively.
Sensitivity analysis: Clinical Practice Research Datalink records only
Appendix 5, Table 63, displays the main analyses when the adverse event outcomes are ascertained only through the CPRD. In general, omission of the HES- and ONS-linked data resulted in fewer outcomes, particularly for the Z-drug group than the no sleep medication group. Consequently, associations between Z-drug use and the outcomes were attenuated closer to the null.
Our main analysis included 368 incident fractures, yet restricting outcome ascertainment to CPRD only reduced this to 262 fractures (71%). The number of fractures in the Z-drug group reduced by 67% and in the no sleep medication group by 77%, and this deceased the resulting adjusted HR from 1.40 to 1.27. Similarly, the number of incident hip fractures reduced to 61% of that in the main analysis in the Z-drug group and to 74% in the no sleep medication group, leading to a reduced adjusted HR from 1.59 to 1.38. Besides incident agitation or psychosis, for which only 9% fewer outcomes occur when restricting to CPRD, all the other adverse events have a reduction of around 28–45% in the number of outcomes recorded.
Sensitivity analysis: discontinuing Z-drugs
The 1274 patients who discontinued Z-drugs generally experienced fewer adverse events in the preceding period than the 3089 patients who were initiated on Z-drugs. Those initiating Z-drugs had higher rates of falls, mortality, infections and agitation or psychosis than those who had just discontinued Z-drugs (see Appendix 5, Table 64). Although the rates of fractures were lower when discontinuing Z-drugs, these differences did not reach statistical significance. Rates of new antipsychotic, antidepressant and antibiotic prescribing were lower in those discontinuing Z-drugs.
Chapter 6 Clinical cohort studies results
The REDIC study results
Participant characteristics
Of the 696 participants recruited to the REDIC study, 17 participants had no dementia or MCI at baseline and one participant did not provide enough information at their baseline assessment to be included in the current analysis. Therefore, these participants were excluded from the current analysis, leaving 95 participants with MCI and 583 participants with dementia at baseline.
The number with usable assessments at each wave, along with the prevalence of use of each hypnotic reported at each wave, is shown in Appendix 3, Table 42. Forty-two per cent of the sample gave assessments at visit 5 (2 years after baseline). The rate of Z-drug and BZD use was high, with around 20% of participants reporting Z-drug use, 20% reporting BZDs and up to 20% reporting antipsychotics at each visit. Antidepressants were also widely used (30–40% of participants at each wave), with lower numbers reporting antihistamines or antiepileptics. The distribution of key outcome measures and dementia severity at baseline stratified by Z-drug, BZD and antipsychotic use is shown in Table 13.
| Participant characteristics | Dementia severity, n (%) | ||||
|---|---|---|---|---|---|
| All | Very mild | Mild | Moderate | Severe | |
| Sex (female) | 415 (64.4) | 49 (65.3) | 108 (65.1) | 187 (68.8) | 71 (54.2) |
| Marital status | |||||
| Unmarried | 57 (8.9) | 7 (9.5) | 15 (9.1) | 24 (8.9) | 11 (8.5) |
| Married | 194 (30.5) | 19 (25.7) | 45 (27.4) | 80 (29.7) | 50 (38.5) |
| Widowed or divorced | 386 (60.6) | 48 (64.9) | 104 (63.4) | 165 (61.3) | 69 (53.1) |
| Years of education | |||||
| 0–6 | 25 (3.9) | 2 (2.7) | 7 (4.2) | 12 (4.4) | 4 (3.1) |
| 7 | 212 (32.9) | 21 (28) | 51 (30.7) | 102 (37.5) | 38 (29) |
| 8–12 | 216 (33.5) | 34 (45.3) | 65 (39.2) | 84 (30.9) | 33 (25.2) |
| ≥ 13 | 191 (29.7) | 18 (24) | 43 (25.9) | 74 (27.2) | 56 (42.7) |
| Age group (years) | |||||
| < 69 | 26 (4) | 1 (1.3) | 10 (6) | 10 (3.7) | 5 (3.8) |
| 70–79 | 115 (17.9) | 8 (10.7) | 24 (14.5) | 50 (18.4) | 33 (25.2) |
| 80–89 | 318 (49.4) | 38 (50.7) | 92 (55.4) | 130 (47.8) | 58 (44.3) |
| ≥ 90 | 185 (28.7) | 28 (37.3) | 40 (24.1) | 82 (30.1) | 35 (26.7) |
| Medication use | |||||
| Z-drug | 120 (18.6) | 26 (34.7) | 32 (19.3) | 49 (18.0) | 13 (9.9) |
| BZD | 101 (15.7) | 23 (30.7) | 25 (15.1) | 30 (11) | 23 (17.6) |
| Antipsychotics | 83 (12.9) | 7 (9.3) | 18 (10.8) | 35 (12.9) | 23 (17.6) |
| Antihistamines | 24 (3.7) | 3 (4) | 6 (3.6) | 9 (3.3) | 6 (4.6) |
| Antidepressants | 190 (29.5) | 24 (32) | 43 (25.9) | 81 (29.8) | 42 (32.1) |
| Antiepileptics | 33 (5.1) | 5 (6.7) | 8 (4.8) | 8 (2.9) | 12 (9.2) |
| Indicationsa | |||||
| Anxiety | 214 (33.4) | 21 (28) | 43 (25.9) | 91 (33.6) | 59 (46.1) |
| Agitation/aggression | 196 (30.7) | 6 (8) | 34 (20.7) | 87 (32.1) | 69 (53.9) |
| Sleep disturbance | 183 (28.7) | 13 (17.6) | 37 (22.4) | 84 (31.1) | 49 (38) |
The vast majority of those who left the study between waves had died, but some had refused to continue, were withdrawn by their nursing home, moved home or had moved to a nursing home not participating in the REDIC study.
Dynamics of hypnotic use throughout the study
The ability to estimate the effect of hypnotics is ultimately derived from within-person comparisons of hypnotic use compared with no hypnotic use. Hence, the number of transitions between hypnotic use and no hypnotic use within individuals between visits is crucial. The number of such transitions is shown for Z-drugs, BZDs and antipsychotics in Table 14, between each of the 1540 pairs of consecutive visits in the REDIC study data set. There is a positive correlation between use of pairs of medications (e.g. out of 421 occasions where Z-drugs were reported, BZDs were also reported on 126 occasions) (Table 15).
| Medication | No use at previous visit, n (%) | With use at previous visit, n (%) | ||||
|---|---|---|---|---|---|---|
| Total | No use | Starting | Total | Stopping | Continuous use | |
| Z-drugs | 1259 (100) | 1175 (93) | 84 (7) | 281 (100) | 70 (25) | 211 (75) |
| BZDs | 1224 (100) | 1121 (92) | 93 (8) | 316 (100) | 76 (24) | 240 (76) |
| Antipsychotics | 1295 (100) | 1214 (94) | 81 (6) | 245 (100) | 61 (25) | 184 (75) |
| Z-drugs | BZDs | Antipsychotics | |
|---|---|---|---|
| Z-drugs | 421 | 126 | 78 |
| BZDs | 2.0 (1.6 to 2.6) | 440 | 122 |
| Antipsychotics | 1.3 (0.95 to 1.7) | 2.6 (2.0 to 3.4) | 349 |
Predictors of dropout and mortality
A logistic regression model was applied with drop-out at the next wave as the outcome, and hypnotic use, outcomes and demographics covariates as predictors (see Appendix 3, Table 43). The vast majority of dropouts from the study were due to death and so the different forms of outcome were not modelled individually. Clustered standard errors were used to account for multiple data points per individual contributing to this analysis.
After adjusting for covariates, those who used Z-drugs were less likely to complete the next assessment (OR 1.47, 95% CI 1.03 to 2.09), although those using BZDs were more likely. Antipsychotic use did not predict subsequent loss to follow-up independently of disability and cognitive function (as measured with CDR-global). Those who were more severely disabled or older were more likely to be lost, but net of this there was no clear link between dropout and cognitive function. There was little association between neuropsychiatric symptoms and subsequent loss to follow-up.
Predictors of hypnotic use
Tables 14 and 15 show that medication use at a previous wave is an extremely strong predictor of medication use at the current wave. Hence, for each medication of interest two logistic models were estimated, one for stopping and another for starting, conditional on participant characteristics and lagged values of time-varying covariates. Clustered standard errors were used to account for multiple data points per individual contributing to this analysis.
Table 16 shows the independent predictors of starting (current use conditional on no prior use) and Appendix 3, Table 44, shows the factors predicting continuing medication use (current use conditional on prior use) estimated using these models, which are also used to identify confounding variables to develop the marginal structural model analysis below.
| Participant characteristics | Starting Z-drugs | Starting BZDs | Starting antipsychotics | |||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age (years) | ||||||
| < 70 | 0.59 | 0.14 to 2.46 | 1.37 | 0.47 to 4.00 | 0.82 | 0.30 to 3.78 |
| 70–79 | Ref | Ref | Ref | |||
| 80–89 | 1.29 | 0.66 to 2.54 | 0.97 | 0.51 to 1.85 | 0.51* | 0.25 to 1.25 |
| ≥ 90 | 0.76 | 0.34 to 1.73 | 0.75 | 0.33 to 1.67 | 0.46 | 0.23 to 1.39 |
| Dementia severity | ||||||
| Minimal | 5.31*** | 2.15 to 13.12 | 3.09* | 1.30 to 7.34 | 0.37 | 0.22 to 2.75 |
| Mild | 1.19 | 0.49 to 2.91 | 1.47 | 0.70 to 3.11 | 0.53 | 0.37 to 2.23 |
| Moderate | 1.50 | 0.73 to 3.09 | 0.86 | 0.44 to 1.67 | 0.51* | 0.28 to 1.18 |
| Severe | Ref | Ref | Ref | |||
| Sex (female) | 1.46 | 0.85 to 2.52 | 1.07 | 0.63 to 1.81 | 1.18 | 0.47 to 2.03 |
| Marital status | ||||||
| Unmarried | Ref | Ref | Ref | |||
| Married | 3.92 | 0.90 to 17.06 | 1.54 | 0.59 to 3.97 | 0.51 | 0.2 to 1.59 |
| Widowed/divorced | 4.74* | 1.07 to 20.92 | 1.44 | 0.54 to 3.87 | 0.64 | 0.25 to 1.71 |
| Hypnotic use | ||||||
| BZDs | 1.22 | 0.67 to 2.21 | 0.99 | 0.28 to 1.28 | ||
| Z-drugs | 1.64 | 0.92 to 2.91 | 1.20 | 0.52 to 2.37 | ||
| Antipsychotics | 1.45 | 0.76 to 2.77 | 0.54 | 0.25 to 1.17 | ||
| Neuropsychiatric symptoms | ||||||
| Sleep | 1.86*** | 1.43 to 2.42 | 1.05 | 0.76 to 1.44 | 1.14 | 0.92 to 1.76 |
| Anxiety | 1.63* | 1.08 to 2.44 | 1.59* | 1.10 to 2.30 | 1.59* | 1.52 to 3.67 |
| Agitation | 0.63 | 0.37 to 1.08 | 1.55 | 0.97 to 2.48 | 1.22 | 0.89 to 2.21 |
| Disability (Lawton Physical Self-Maintenance Scale), per unit | 1 | 0.95 to 1.05 | 0.99 | 0.94 to 1.04 | 1.01 | 0.93 to 1.08 |
| Years in education | ||||||
| < 7 | 2.95 | 0.95 to 9.19 | 0.91 | 0.27 to 3.09 | 0.65 | 0.13 to 3.71 |
| 7 | 1.05 | 0.53 to 2.09 | 0.62 | 0.33 to 1.17 | 0.91 | 0.37 to 1.65 |
| 8–11 | 1.39 | 0.73 to 2.65 | 0.69 | 0.38 to 1.28 | 0.84 | 0.30 to 1.33 |
| ≥ 12 | Ref | Ref | Ref | |||
| Type of admission | ||||||
| Long stay | Ref | Ref | Ref | |||
| Short stay | 0.96 | 0.49 to 1.87 | 1.80 | 1.00 to 3.27 | 0.78 | 0.40 to 2.10 |
| Nursing | 1.83 | 0.55 to 6.03 | 0.69 | 0.16 to 2.91 | 0.71 | 0.13 to 3.89 |
Z-drugs and BZDs are more commonly started among those with less severe dementia, more strikingly so for Z-drugs than for BZDs. Conversely, antipsychotics are more commonly started among those with more severe dementia. Prior use of Z-drugs predicts new use of BZDs. Prior levels of neuropsychiatric symptoms independently predict new use of hypnotics, in particular prior sleep disturbance predicts new Z-drug and BZD use, anxiety predicts new use of all three classes. However, a considerable number of participants started Z-drugs without having any prior sleep disturbance, anxiety or agitation recorded in any previous assessment (not shown).
There were fewer clear predictors of continuing hypnotic use (equivalently of stopping use) between visits. In particular, levels of neuropsychiatric symptoms and dementia severity were not significantly linked to use of any hypnotic if there was use at a previous wave.
Dynamics of outcome measures and estimating the effect of hypnotic use on outcome measures
Figures 13–22 in Appendix 3 describe how each outcome measure changes between waves and vary with age, CDR stage and with concurrent Z-drug use. The left-hand panels of Figures 3–12 show how outcome measures change with changing hypnotic use status. The right-hand panels of Figures 3–12 show the association between current hypnotic use and outcome measures, with weights applied to each observation such that levels of previous hypnotic use, cognitive function and neuropsychiatric variables are balanced between those currently using each medication and those not using each medication. Thus, these graphs show the effect of current medication use controlling for key patient characteristics, in particular those characteristics that predict new or continuing medication use at the previous wave. The difference in current mean levels of outcomes between groups can then be interpreted as the effect of the medication, although when weights do not cause prior levels to perfectly coincide, comparing the change over time between groups may better estimate the effect.
Cognitive measures
With respect to cognitive outcomes, Appendix 3, Figures 3–5 (SIB-8, MMSE and CDR-SOB), shows that those who use Z-drugs tend to have better cognitive function than average, those who use antipsychotics have worse cognitive function than average, but different patterns of changing status are not consistently associated with different levels of decline in cognitive function between assessments. That is, rates of decline between assessments do not appear to be systematically different between those who use no hypnotics, those who start or stop between assessments or those who use hypnotics at both assessments.
Table 17 quantifies these associations, while adjusting for age, the specific visit at which the cognitive assessment is made and baseline cognitive function. There is some evidence for more decline in SIB-8 score among those starting BZDs compared with no use, and some worsening by CDR-SOB among those starting antipsychotics compared with no use. However, these changes are not reflected in other cognitive scores and are not seen among those starting Z-drugs compared with those with no use. When cognitive measures are combined there is no evidence for any additional decline with starting or continuing Z-drug use.
| Exposure | Measure, β (95% CI) | |||
|---|---|---|---|---|
| MMSE | CDR-SOB | SIB-8 | Disability scale | |
| Z-drug | ||||
| No use | Ref | Ref | Ref | Ref |
| Starting | 0.01 (–1.44 to 1.46) | –0.17 (–0.88 to 0.55) | –0.58 (–1.72 to 0.55) | –0.05 (–0.72 to 0.61) |
| Stopping | –0.51 (–2.04 to 1.01) | 0.53 (–0.18 to 1.24) | –0.94 (–2.26 to 0.37) | 0.53 (–0.38 to 1.44) |
| Continuing | 0.65 (–0.06 to 1.36) | –0.14 (–0.51 to 0.24) | –0.13 (–0.68 to 0.42) | –0.54** (–0.94 to –0.15) |
| BZD | ||||
| No use | Ref | Ref | Ref | Ref |
| Starting | –0.36 (–1.83 to 1.11) | 0.78** (0.20 to 1.37) | 0.60 (–0.25 to 1.45) | 0.02 (–0.61 to 0.65) |
| Stopping | 0.82 (–0.37 to 2.01) | –0.37 (–0.99 to 0.25) | 0.40 (–0.67 to 1.47) | –0.12 (–0.89 to 0.65) |
| Continuing | 0.44 (–0.20 to 1.08) | –0.07 (–0.43 to 0.29) | 0.20 (–0.30 to 0.70) | –0.30 (–0.67 to 0.08) |
| Antipsychotic | ||||
| No use | Ref | Ref | Ref | Ref |
| Starting | –1.39** (–2.41 to –0.36) | 0.33 (–0.25 to 0.91) | –0.49 (–1.35 to 0.37) | 0.91 (–0.05 to 1.86) |
| Stopping | –0.42 (–1.79 to 0.94) | 0.36 (–0.27 to 0.98) | –0.03 (–0.96 to 0.90) | 0.11 (–0.60 to 0.82) |
| Continuing | 0.05 (–0.63 to 0.74) | –0.06 (–0.44 to 0.33) | 0.20 (–0.39 to 0.79) | 0.33 (–0.10 to 0.77) |
Appendix 3, Figures 3–5 and Table 45 (first column), shows the change in cognitive function between waves for Z-drug users compared with non-users, using IPTW to balance the prior cognitive function and neuropsychiatric symptoms between groups. Again, these show no effect of any hypnotic on cognitive function. There is some suggestion that the change in CDR-SOB scores is around 0.5 points higher for those using hypnotics, but this is only statistically significant for BZDs and there is no effect on any other cognitive measure. Fixed-effects linear models (see Appendix 3, Table 45) comparing occasions with hypnotic use with those without within each participant yield similar results in the whole cohort, and when restricted to those not using any hypnotics at baseline and among those with mild dementia at baseline.
Sleep disturbance
As seen in Cognitive measures, participants with a sleep disturbance are more likely to start Z-drugs at the next wave than any other group. This is reflected in Appendix 3, Figure 6, which shows that those who start Z-drugs have higher prior values of sleep disturbance than each of the other groups. There is a subsequent decline in sleep disturbance among this group compared with those who do not use Z-drugs; however, when corrected for baseline sleep disturbance using IPTW weighting, this effect is no longer apparent. In fact, there is some evidence for an increase in sleep disturbance associated with Z-drug use. Fixed-effects models show no effect of Z-drugs on sleep after adjusting for between-patient differences.
There are no significant associations between antipsychotics, BZD use and sleep disturbance in any of the models estimated (see Appendix 3, Table 46).
Anxiety and agitation
Those using BZDs and antipsychotics, or those starting or stopping between waves, had higher levels of anxiety and agitation than those who did not use either drug (see Appendix 3, Figures 7 and 8). There was no such association with Z-drugs. There was also was no significant difference in the change in anxiety or agitation scores in any group compared with those not using hypnotics, except for an apparent decline in agitation among those continuously using antipsychotics compared with each other group. This difference was also seen using IPTW models, along with an increase in anxiety among antipsychotic users compared with those not using antipsychotics and an increase in agitation scores among BZD users (see Appendix 3, Table 46).
Quality of life and disability
Although few associations were statistically significant, use of any hypnotic medication appears associated with lower QoL scores when measured by VAS (see Appendix 3, Figure 9), EQ-5D questions (see Appendix 3, Figure 10) or the QUALID scale (see Appendix 3, Figure 11). This finding is consistent across models (see Appendix 3, Table 45).
When patterns of starting or stopping are considered, there is some evidence that starting Z-drugs is associated with lower QoL, but it is not statistically significant. Both stopping and starting antipsychotics was associated with worse QoL compared with no use at either wave (see Appendix 3, Table 47).
Disability is correlated with CDR-SOB (Spearman’s r = 0.52) and the effect of hypnotics on disability follows a similar pattern. Those using antipsychotics are more disabled and there is some evidence that those starting antipsychotics become more disabled than those who do not use them, although this is not statistically significant except under the fixed-effect model including all participants (see Appendix 3, Figure 12 and Table 17). There is no evidence for any impact of Z-drug use on disability.
The NACC data set results
Participant characteristics
The distribution of the characteristics of the 17,055 people living with dementia on study entry (defined as their first assessment with a record of dementia) stratified by baseline CDR-global is shown in Table 18. The median year of joining the study is 2009 and the median age is 76 (range 21–110, IQR 67–82) years. Fifty-two per cent of the cohort were female. There is little association between age at study entry and dementia stage at entry. Although most participants join the study at dementia stage ≤ 1, 14% joined at CDR 2 (moderate dementia) and 8% at CDR 3 (severe dementia).
| Participant characteristics | Dementia severity (CDR stage) | |||
|---|---|---|---|---|
| Minimal (CDR < 1) | Mild (CDR = 1) | Moderate (CDR = 2) | Severe (CDR = 3) | |
| n | 6070 | 7315 | 2371 | 1299 |
| Year, median (IQR) | 2010 (2007–2013) | 2009 (2007–2012) | 2008 (2006–2011) | 2008 (2006–2011) |
| Female, n (%) | 3064 (50) | 3768 (52) | 1334 (56) | 755 (58) |
| Age (years), median (IQR) | 74 (67–81) | 76 (67–82) | 76 (67–83) | 76 (66–83) |
| Drug use, n (%) | ||||
| Z-drug | 146 (2) | 149 (2) | 39 (2) | 39 (3) |
| BZD | 401 (7) | 492 (7) | 194 (8) | 189 (15) |
| Antipsychotic | 163 (3) | 466 (6) | 382 (16) | 443 (35) |
| Antidepressant | 2122 (35) | 2983 (41) | 1049 (45) | 588 (47) |
| Medication data missing | 57 (1) | 57 (1) | 35 (1) | 39 (3) |
| Cognitive function | ||||
| Delta trail time, mean (SD) | 133.9 (76.0) | 155.7 (73.6) | 170.0 (59.9) | 130.0 (94.6) |
| Delta trail time data missing, n (%) | 1245 (21) | 2905 (40) | 1756 (74) | 1242 (96) |
| Animal fluency, median (IQR) | 12 (9–16) | 10 (7–13) | 6 (3–9) | 2 (0–4) |
| Animal fluency data missing, n (%) | 374 (6) | 618 (8) | 627 (26) | 934 (72) |
| MMSE, median (IQR) | 25 (22–27) | 22 (18–25) | 14 (9–19) | 0 (0–7) |
| MMSE data missing, n (%) | 953 (16) | 1016 (14) | 269 (11) | 163 (13) |
| CDR-SOB, median (IQR) | 3 (2–3.5) | 5.5 (4.5–6.5) | 11 (10–12) | 18 (16–18) |
| CDR-SOB data missing, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Neuropsychiatric symptoms | ||||
| Sleep disturbance, n (%) | 1526 (26) | 2282 (32) | 876 (38) | 473 (41) |
| Sleep disturbance data missing, n (%) | 226 (4) | 270 (4) | 92 (4) | 158 (12) |
| Anxiety, n (%) | 1940 (33) | 3006 (43) | 1110 (49) | 517 (45) |
| Anxiety data missing, n (%) | 218 (4) | 262 (4) | 91 (4) | 155 (12) |
| Agitation, n (%) | 1593 (27) | 2544 (36) | 1079 (47) | 610 (53) |
| Agitation data missing, n (%) | 216 (4) | 257 (4) | 89 (4) | 155 (12) |
| NPI excluding sleep, mean (SD) | 3.47 (3.64) | 5.12 (4.57) | 6.98 (5.33) | 7.60 (5.95) |
| NPI excluding sleep data missing, n (%) | 226 (4) | 270 (4) | 92 (4) | 158 (12) |
| Other outcomes | ||||
| GDS, median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 2 (1–5) |
| GDS data missing, n (%) | 330 (5) | 687 (9) | 579 (24) | 999 (77) |
| Disability, median (IQR) | 7 (4–12) | 16 (11–21) | 26 (22–29) | 30 (29–30) |
| Disability data missing, n (%) | 195 (3) | 190 (3) | 63 (3) | 61 (5) |
The reported prevalence of Z-drug and BZD use is far lower in NACC data set than in the REDIC study cohort. Only 2–3% of participants report Z-drug use, whereas 7–15% report BZD use, with highest levels of use among those with severe dementia. Antipsychotic use increases markedly with dementia severity, from 3% (CDR < 1) to 34% (CDR = 3). A substantial proportion of participants at all stages (35–45%) report use of an antidepressant. On fewer than 2% of visits the medication use form was not completed (3% among CDR 3 patients).
Neuropsychiatric symptoms are reported relatively frequently at all stages. Some sleep disturbance is reported for 26–41% of participants across groups; anxiety and agitation have similar numbers. These assessments are present for 96% of participants in CDR stages 0.5–2 and 88% at CDR stage 3. The average total NPI symptom score excluding sleep disturbance increases from 3.5 to 7.6 with dementia severity.
Average disability score increases with dementia severity, such that almost all of those with severe dementia score 29 or 30 out of 30 on the disability scale. In contrast, the level of depression is generally low (median GDS 2/10, IQR 1–4), but in severe dementia 77% of participants are not able to complete the GDS.
The number of participants completing each annual visit is shown in Appendix 4, Table 48. Only 9889 (59%) participants complete more than one visit, 4020 (24%) of participants complete visit 4, whereas only 838 (5%) complete visit 7. Prevalence of Z-drugs does not change markedly with visit number, whereas antipsychotics become more common at later visits.
Dynamics of hypnotic use
The numbers of participants starting and stopping Z-drugs, BZDs and antipsychotics between pairs of consecutive visits are shown in Appendix 4, Table 49. Only 1% of visits report new Z-drug use, although this still corresponds to 219 instances of new Z-drug use, which is a reasonable number from which to estimate changes in outcome measures. Of those who use Z-drugs and return medication data at a subsequent visit, 51% still report Z-drug use. For antipsychotics, the rate of new use (6%) and continuing use (79% of antipsychotic users still use antipsychotics at the next wave) is higher.
Predictors of starting or stopping hypnotics between waves
Table 19 and Appendix 4, Table 50, show the independent effects of cognitive function, neuropsychiatric variables and demographic factors at each visit on the use of hypnotics at the subsequent visit, conditional on current hypnotic use.
| Factor | Starting drug at next wave, β (95% CI) | ||
|---|---|---|---|
| Z-drug | BZD | Antipsychotic | |
| Drug use | |||
| Z-drug | 2.31*** (1.62 to 3.30) | 1.10 (0.73 to 1.67) | |
| BZD | 1.97** (1.30 to 2.99) | 1.76*** (1.46 to 2.12) | |
| Antipsychotic | 1.48 (0.96 to 2.28) | 1.61*** (1.33 to 1.96) | |
| Education (per year) | 0.97 (0.92 to 1.01) | 1.03*** (1.01 to 1.06) | 1.01 (0.99 to 1.03) |
| Sex (female) | 1.11 (0.83 to 1.48) | 1.06 (0.92 to 1.23) | 0.81*** (0.71 to 0.91) |
| CDR | |||
| Minimal | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Mild | 0.85 (0.60 to 1.21) | 1.25* (1.02 to 1.52) | 2.22*** (1.85 to 2.67) |
| Moderate | 1.10 (0.73 to 1.65) | 1.94*** (1.56 to 2.41) | 4.63*** (3.81 to 5.62) |
| Severe | 0.83 (0.50 to 1.37) | 2.17*** (1.70 to 2.78) | 4.02*** (3.20 to 5.04) |
| Age (years) | |||
| < 61 | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 61–70 | 1.44 (0.87 to 2.38) | 0.76** (0.62 to 0.93) | 0.82* (0.69 to 0.98) |
| 71–80 | 1.22 (0.75 to 2.00) | 0.58*** (0.48 to 0.70) | 0.73*** (0.62 to 0.86) |
| 81–90 | 1.06 (0.62 to 1.81) | 0.46*** (0.36 to 0.58) | 0.58*** (0.47 to 0.70) |
| ≥ 90 | 1.38 (0.48 to 3.92) | 0.46** (0.27 to 0.79) | 0.51** (0.33 to 0.80) |
| Sleep | |||
| None | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Mild | 1.43 (0.98 to 2.09) | 1.21* (1.01 to 1.45) | 1.15 (0.99 to 1.34) |
| Moderate | 1.89** (1.28 to 2.80) | 1.13 (0.92 to 1.38) | 1.13 (0.95 to 1.33) |
| Severe | 2.08** (1.20 to 3.59) | 1.09 (0.79 to 1.50) | 1.02 (0.78 to 1.32) |
| Anxiety | |||
| None | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Mild | 0.74 (0.52 to 1.07) | 1.29** (1.09 to 1.53) | 1.18* (1.03 to 1.36) |
| Moderate | 0.76 (0.49 to 1.17) | 1.81*** (1.50 to 2.18) | 1.47*** (1.25 to 1.72) |
| Severe | 1.00 (0.53 to 1.88) | 1.74*** (1.26 to 2.40) | 1.86*** (1.44 to 2.40) |
| Agitation | |||
| None | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Mild | 1.07 (0.76 to 1.51) | 1.08 (0.90 to 1.28) | 1.49*** (1.30 to 1.71) |
| Moderate | 0.59* (0.36 to 0.97) | 1.36** (1.12 to 1.66) | 1.73*** (1.47 to 2.03) |
| Severe | 1.04 (0.55 to 1.95) | 1.67*** (1.24 to 2.26) | 2.77*** (2.16 to 3.54) |
Among those not using Z-drugs, sleep disturbance did predict new use of Z-drugs at the next wave, but was only slightly linked to new BZD use and was not independently linked to new antipsychotic use. Conversely, anxiety and agitation predicted new use of antipsychotics and BZDs at the subsequent visit. Cognitive function did not predict new use of Z-drugs, but those with more severe dementia were more likely to start BZDs and antipsychotics (see Table 19). Independently of cognitive function and symptoms, older people with dementia were less likely to start BZD or antipsychotics, but there was no age effect with Z-drugs.
Among those using hypnotics there were very few variables that were able to predict which participants continued to report hypnotics at the next visit (see Appendix 4, Table 50). Older people and those with more severe cognitive impairment appeared less likely to continue BZDs, but, crucially, current levels of psychiatric symptoms did not predict whether or not current medications were continued.
Predictors of dropout in the NACC data set
As in the REDIC study, medication use did not strongly predict whether or not each participant completed a subsequent assessment, but older and more severely impaired participants were more likely to drop out. Psychiatric symptoms also did not predict dropout. Note, we do not distinguish dropout through leaving the study, death or censoring and so this should not be interpreted as an assessment of the risk of hypnotics on any outcome.
Distribution and dynamics of outcome measures
Appendix 4, Figures 23–32, describes how each outcome measure changes between waves and varies with age, CDR stage and with concurrent Z-drug use. Delta trail time does not appear to work well among those with severe cognitive impairment. The score is missing for the vast majority of participants with CDR stage 3, and for those who do return a score it is often at the maximum allowed time for both trails; hence the information provided by this measure is likely to be extremely limited.
Association between each outcome and starting or stopping hypnotic medication
Cognitive outcomes
The change in cognitive function with changing use of hypnotics is shown in Appendix 4, Figure 33–42. There appears to be little association between Z-drug use and cognitive function, as measured by any cognitive outcome. Those who use antipsychotics and BZDs have lower cognitive function than those who do not, and starting both antipsychotics and BZDs appears to be linked to more decline across waves. When accounting for prior use of medication, neuropsychiatric symptoms and cognition these patterns remain.
Table 20 quantifies the association between change in cognitive outcomes and patterns of hypnotic use, adjusting for age, baseline cognitive function and the visit number. Those starting BZDs or antipsychotics have an associated decline in cognitive function across all measures, except delta trail time. Stopping antipsychotics is associated with improvements in CDR-SOB and animal fluency compared with those who did not use antipsychotics, but not in MMSE or delta trail time, but there is no significant improvement associated with stopping BZDs.
| Medication | β (95% CI) | |||
|---|---|---|---|---|
| CDR-SOB | MMSE | Animal fluency | Delta trail time | |
| Z-drug | ||||
| No use | 0 (Ref) | 0 (Ref) | 0 (Ref) | 0 (Ref) |
| Starting | 0.06 (–0.36 to 0.48) | 0.34 (–0.26 to 0.93) | 0.21 (–0.41 to 0.83) | –4.69 (–20.96 to 11.58) |
| Stopping | –0.03 (–0.38 to 0.33) | 0.35 (–0.44 to 1.15) | 0.56 (–0.11 to 1.22) | –11.56 (–25.68 to 2.56) |
| Continuing | –0.64*** (–0.95 to –0.34) | 0.45 (–0.28 to 1.19) | 0.65* (0.13 to 1.17) | 0.31 (–11.66 to 12.29) |
| BZD | ||||
| No use | 0 (Ref) | 0 (Ref) | 0 (Ref) | 0 (Ref) |
| Starting | 1.01*** (0.78 to 1.23) | –1.24*** (–1.71 to –0.78) | –0.49* (–0.88 to –0.10) | –3.29 (–14.11 to 7.53) |
| Stopping | 0.02 (–0.21 to 0.26) | 0.11 (–0.44 to 0.65) | 0.07 (–0.37 to 0.51) | –4.11 (–14.58 to 6.35) |
| Continuing | –0.39*** (–0.53 to –0.24) | 0.67*** (0.34 to 0.99) | 0.48*** (0.23 to 0.72) | –2.19 (–7.70 to 3.31) |
| Antipsychotic | ||||
| No use | 0 (Ref) | 0 (Ref) | 0 (Ref) | 0 (Ref) |
| Starting | 1.58*** (1.39 to 1.76) | –2.02*** (–2.41 to –1.62) | –0.94*** (–1.29 to –0.60) | 1.49 (–8.09 to 11.06) |
| Stopping | –0.26* (–0.49 to –0.02) | –0.24 (–0.86 to 0.38) | 0.74** (0.25 to 1.23) | –12.34 (–25.88 to 1.20) |
| Continuing | –0.10 (–0.22 to 0.02) | 0.06 (–0.23 to 0.35) | 0.11 (–0.13 to 0.35) | –9.27** (–14.94 to –3.61) |
Those who continue Z-drugs or BZDs appear to have better cognitive function than those who did not use Z-drugs or BZDs at all.
This pattern of effects is confirmed using the marginal structural model approach (see Appendix 4, Table 51), whereby use of BZDs and antipsychotics is associated with significantly worse cognition after weighting to balance users and non-users across previous CDR, previous medication use and previous levels of neuropsychiatric symptoms. There is no evidence for any effect of Z-drugs on cognitive outcomes.
Neuropsychiatric outcomes
Despite the strong association between sleep disturbance and subsequent Z-drug use, there is little association between starting Z-drugs and change in sleep disturbance between waves (Table 21). There is a borderline significant increase in agitation associated with starting Z-drugs and some evidence for less sleep disturbance association with stopping Z-drugs.
| Medication | NPI score, β (95% CI) | |||
|---|---|---|---|---|
| Sleep | Agitation | Anxiety | Total (excluding sleep) | |
| Z-drug | ||||
| No use | 0 (Ref) | 0 (Ref) | 0 (Ref) | 0 (Ref) |
| Starting | 0.06 (–0.11 to 0.23) | 0.18* (0.04 to 0.32) | 0.09 (–0.04 to 0.23) | 0.17 (–0.53 to 0.87) |
| Stopping | –0.12 (–0.26 to 0.02) | –0.06 (–0.19 to 0.06) | –0.07 (–0.20 to 0.06) | –0.41 (–0.98 to 0.16) |
| Continuing | –0.01 (–0.12 to 0.11) | 0.03 (–0.08 to 0.14) | 0.01 (–0.10 to 0.12) | 0.13 (–0.33 to 0.59) |
| BZD | ||||
| No use | 0 (Ref) | 0 (Ref) | 0 (Ref) | 0 (Ref) |
| Starting | 0.04 (–0.05 to 0.12) | 0.10* (0.02 to 0.19) | 0.10* (0.02 to 0.17) | 0.55** (0.18 to 0.93) |
| Stopping | –0.11* (–0.20 to –0.02) | –0.11* (–0.20 to –0.02) | –0.15*** (–0.24 to –0.06) | –0.97*** (–1.38 to –0.56) |
| Continuing | –0.05* (–0.10 to –0.00) | –0.04 (–0.09 to 0.01) | –0.07** (–0.12 to –0.02) | –0.33** (–0.55 to –0.11) |
| Antipsychotic | ||||
| No use | 0 (Ref) | 0 (Ref) | 0 (Ref) | 0 (Ref) |
| Starting | 0.11** (0.04 to 0.18) | 0.16*** (0.09 to 0.23) | 0.004 (–0.06 to 0.07) | 0.77*** (0.45 to 1.09) |
| Stopping | –0.10 (–0.19 to 0.00) | –0.18*** (–0.28 to –0.08) | –0.22*** (–0.31 to –0.13) | –1.05*** (–1.50 to –0.59) |
| Continuing | –0.05* (–0.09 to –0.01) | –0.12*** (–0.16 to –0.08) | –0.10*** (–0.14 to –0.06) | –0.85*** (–1.05 to –0.64) |
Similar patterns are seen for reports of anxiety and agitation, whereby those starting, stopping or continuing any of Z-drugs, BZDs or antipsychotics have higher symptom levels that those who do not use the medication at all, with a slight decline in symptoms associated with stopping medications and a significant association between medication use and symptoms remaining once prior levels of cognitive function, medication use and symptoms are taken into account.
Depression and disability
As in the REDIC study, the disability measure among people with dementia is closely correlated with cognitive function, and the patterns of associations with medication use match the associations between medication use and cognitive measures.
The GDS does not appear to be linked to severity of impairment, and there is no evidence of any association between changes in depressive status and changes in use of Z-drugs, antipsychotics or BZDs (Table 22).
| Medication status | β (95% CI) | |
|---|---|---|
| GDS | Disability | |
| Z-drugs | ||
| No use | 0 (Ref) | 0 (Ref) |
| Starting | –0.28 (–0.68 to 0.13) | –0.55 (–1.23 to 0.13) |
| Stopping | –0.08 (–0.52 to 0.37) | –0.61 (–1.30 to 0.09) |
| Continuing | –0.01 (–0.31 to 0.29) | –0.65* (–1.29 to –0.01) |
| BZDs | ||
| No use | 0 (Ref) | 0 (Ref) |
| Starting | 0.05 (–0.21 to 0.30) | 0.48** (0.14 to 0.83) |
| Stopping | –0.24 (–0.52 to 0.04) | –0.15 (–0.59 to 0.30) |
| Continuing | –0.07 (–0.24 to 0.10) | –0.64*** (–0.90 to –0.38) |
| Antipsychotics | ||
| No use | 0 (Ref) | 0 (Ref) |
| Starting | 0.15 (–0.07 to 0.37) | 0.81*** (0.51 to 1.11) |
| Stopping | –0.11 (–0.52 to 0.31) | –1.24*** (–1.59 to –0.88) |
| Continuing | –0.02 (–0.19 to 0.15) | –1.48*** (–1.65 to –1.31) |
Fixed-effects models
Each of the results discussed above using marginal structural models is also reflected in the fixed-effects models (see Appendix 4, Tables 52–54). There is a strong, consistent association seen between worse cognitive function and greater disability with concurrent BZD and antipsychotic use, but no association with Z-drug use. Depression is not linked to the use of any hypnotics, except for a positive association with antipsychotics among those with no hypnotic use on study entry, whereas other neuropsychiatric symptoms are consistently positively associated with use of hypnotics, as above.
The WHELD trial results
Participant characteristics
After excluding 21 participants with missing medication data and 24 participants with a severe mental illness diagnosis, the 926 participants in the WHELD trial with dementia were mainly women (n = 659, 71%) and the mean age at study entry was 85.3 (SD 8.8) years. A total of 123 (13%) participants reported Z-drug use at baseline and a further 27 (3%) participants reported Z-drug use at 9-month follow-up. Those participants taking Z-drugs were more likely to have a partner, have a nervous system illness and be taking concurrent BZDs, memantine or antipsychotics (Table 23).
| Baseline characteristic | Z-drug (N = 123) | No Z-drug (N = 803) |
|---|---|---|
| Female, n (%) | 74 (60) | 585 (73) |
| Age (years), mean (SD) | 83.9 (9.6) | 85.6 (8.6) |
| White ethnicity, n (%) | 116 (94) | 761 (95) |
| Marital status, n (%)a | ||
| Single/widowed | 71 (58) | 578 (72) |
| Married/partner | 42 (34) | 162 (20) |
| Divorced/separated | 8 (7) | 54 (7) |
| CDR, n (%) | ||
| Mild dementia | 17 (14) | 174 (22) |
| Moderate dementia | 45 (37) | 294 (937) |
| Severe dementia | 61 (50) | 335 (42) |
| NPI-NH score (excluding sleep), median (IQR) | 17 (7–34) | 9 (3–20) |
| Any sleep disturbance, n (%) | 34 (28) | 162 (20) |
| Pain, n (%) | ||
| Mild | 34 (28) | 232 (29) |
| Moderate/severe | 13 (11) | 48 (6) |
| Comorbidity, n (%) | ||
| Depression | 12 (10) | 99 (12) |
| Anxiety | 4 (3) | 29 (4) |
| Respiratory illness | 42 (34) | 271 (34) |
| Gastrointestinal illness | 34 (28) | 209 (26) |
| Cardiovascular condition | 24 (20) | 129 (16) |
| Endocrine illness | 18 (15) | 97 (12) |
| Musculoskeletal disorder | 29 (24) | 169 (21) |
| Nervous system illness | 15 (12) | 43 (5) |
| Medication use, n (%) | ||
| BZD | 31 (25) | 98 (12) |
| Meprobamate/buspirone | 2 (2) | 7 (1) |
| Clomethiazole | 1 (1) | 11 (1) |
| Antidepressant | 56 (46) | 357 (45) |
| Antipsychotic | 36 (29) | 106 (13) |
| Cholinesterase inhibitor | 30 (24) | 170 (21) |
| Memantine | 34 (28) | 68 (9) |
Cross-sectional analysis
The results of the cross-sectional analyses comparing Z-drug use at baseline and baseline QoL and sleep scores are shown in Table 24.
| Scale | Baseline | Mean (SD) | IRR (95% CI) | IRRa (95% CI) | |
|---|---|---|---|---|---|
| Z-drug | n | ||||
| NPI-NH excluding sleep | No | 803 | 14.1 (15.1) | 1.00 | 1.00 |
| Yes | 123 | 21.6 (17.6) | 1.53 (1.23 to 1.90) | 1.24 (1.00 to 1.54) | |
| NPI-NH sleep | No | 803 | 1.0 (2.5) | 1.00 | 1.00 |
| Yes | 123 | 1.6 (3.0) | 1.57 (0.84 to 2.90) | 1.42 (0.71 to 2.85) | |
| QUALID | No | 803 | 21.3 (7.3) | 1.00 | 1.00 |
| Yes | 122 | 22.9 (8.0) | 1.07 (1.01 to 1.14) | 0.96 (0.92 to 1.01) | |
At baseline, the mean QUALID scores for those taking Z-drugs or not at baseline were 22.9 (SD 8.0) and 21.3 (SD 7.3), respectively. Z-drug use was not associated with any difference in QUALID scores at baseline (RR 0.96, 95% CI 0.92 to 1.01, adjusted for confounders).
At baseline, the mean NPI-NH sleep scores for those taking Z-drugs or not at baseline were 1.6 (SD 3.0) and 1.0 (SD 2.5), respectively. There was no significant difference in sleep disturbance at baseline in Z-drug users (RR 1.42, 95% CI 0.71 to 2.85, adjusted for confounders).
At baseline, the mean NPI-NH score (excluding sleep) for those taking Z-drugs and not taking Z-drugs at baseline were 21.6 (SD 17.6) and 14.1 (SD 15.1). Greater neuropsychiatric symptoms were reported in those taking Z-drugs after adjusting for confounders (RR 1.24, 95% CI 1.00 to 1.54).
Mortality analysis
A total of 20 (16%) and 177 (22%) participants taking and not taking Z-drugs, respectively, at baseline died during follow-up. Baseline Z-drug use was not associated with mortality after adjusting for confounders (OR 0.66, 95% CI 0.38 to 1.15).
Longitudinal analyses
A total of 627 people living with dementia completed the 9-month follow-up interviews. The mean increase in QUALID, NPI-NH sleep score and NPI-NH excluding sleep over the 9 months’ follow-up was 0.25 (SD 7.67), 0.11 (SD 0.60) and 0.17 (SD 16.59), respectively. Z-drug use at baseline was not significantly associated with any greater increase in either score, with adjusted additional average increases in QUALID, NPI-NH sleep, and NPI-NH excluding sleep scores of 1.43 (95% CI –0.33 to 3.19), 0.08 (95% CI –0.07 to 0.22) and 0.60 (95% CI –3.26 to 4.46), respectively (Table 25).
| Scale | Baseline | Mean change (SD) | Beta (95% CI) | Betaa (95% CI) | |
|---|---|---|---|---|---|
| Z-drug | n | ||||
| NPI-NH excluding sleep | No | 539 | 0.33 (16.28) | 0.00 | 0.00 |
| Yes | 88 | –0.80 (18.45) | –1.13 (–4.87 to 2.62) | 0.60 (–3.26 to 4.46) | |
| NPI-NH sleep | No | 539 | 0.10 (0.59) | 0.00 | 0.00 |
| Yes | 88 | 0.16 (0.66) | 0.06 (–0.08 to 0.19) | 0.08 (–0.07 to 0.22) | |
| QUALID | No | 525 | 0.20 (7.42) | 0.00 | 0.00 |
| Yes | 85 | 0.52 (9.09) | 0.31 (–1.45 to 2.07) | 1.43 (–0.33 to 3.19) | |
Considering changes in medication use between baseline and 9-month follow-up, 26 (4%) reported Z-drug use at baseline but not at 9 months (‘stopping’), 62 (10%) participants reported Z-drug use at both times (‘continuing’) and 25 (4%) reported Z-drug use at 9 months but not at baseline (‘starting’). Although those starting, stopping and continuing Z-drugs reported, on average, increased NPI sleep scores over the 9-month follow-up, none were statistically different from the increase for those not using Z-drugs (see Appendix 6,Table 65). Although those initiating Z-drugs saw a decline in their NPI score (excluding sleep), this was not statistically different from those not using Z-drugs. Although those stopping Z-drugs reported a worsening in their QoL over the 9-month follow-up, this was not statistically different from those not using Z-drugs.
Chapter 7 Discussion
Summary of the main findings
Primary care study
By examining linked primary care and hospital admission data we found evidence of an increased risk of fractures and, in particular, hip fractures, in people with dementia taking Z-drugs. The risk also increased with cumulative exposure to Z-drugs, suggesting a causal relationship. However, multiple outcomes were examined, increasing the risk of false-positive findings. We observed a greater mortality rate in people living with dementia who were prescribed Z-drugs; however, the association did not vary significantly by cumulative exposure to Z-drugs, suggesting that this finding may be due to reverse causation (i.e. that patients in later stage dementia were more probably prescribed Z-drugs).
We did not detect a significantly increased risk of stroke, infection or venous thromboembolism with Z-drug use. We observed that people living with dementia who were prescribed Z-drugs were more likely to be further prescribed antipsychotics, antidepressants and antibiotics. People living with dementia who were prescribed Z-drugs also visited their GP more frequently and were more often admitted to hospital in the next 2 years.
Our study was not sufficiently powered to examine adverse events of BZDs, but the risk estimates for Z-drugs and fractures and mortality were generally similar to those for BZDs.
We did not find any associations between the use of low-dose TCAs and adverse events; however, these findings should be interpreted with caution, as we were unable to distinguish whether patients had been prescribed low-dose TCAs for sleep disturbance or for chronic pain. Patients with chronic or neuropathic pain may be at a lower risk of fractures than patients with sleep disturbance.
Clinical cohort studies
In cohort studies of people with dementia in Norway (the REDIC study) and the USA (the NACC data set), we observed no impact of Z-drug use on cognition, neuropsychiatric symptoms, disability or QoL. There was some suggestion of an association between recurrent Z-drug use and lesser declines in cognition and disability; however, we believe that this is more likely to reflect the people living with dementia who are stable on their Z-drugs and not progressing onto using alternative medications, such as antipsychotics or BZDs. In a cohort study of UK care home residents with dementia (the WHELD trial), we observed greater neuropsychiatric symptoms in those taking Z-drugs at baseline, but Z-drug use at baseline was not associated with greater improvement in neuropsychiatric symptoms over the following 9 months. We also observed no greater mortality risk in those taking Z-drugs.
Strengths and limitations of the study
Primary care study
The primary care study is the first study to examine a range of adverse events for Z-drug use in people with dementia. We used data from a large number of patients in a population-representative primary care database linked to hospital records, which allowed detailed analysis of the prescriptions of Z-drugs and patient-relevant outcomes. We designed the study to aim to minimise possible sources of bias and consequently this reduced the number of patients and follow-up in the study, reducing our statistical power to detect adverse events. We shall discuss the strengths and limitations in the context of the main sources of bias. 161
Selection bias
We followed patients from their first Z-drug prescription in dementia, thus allowing for the examination of early events associated with the drug and for the evaluation of risks that may change over time. 162,163 People living with dementia already using Z-drugs when diagnosed with dementia were excluded as they may bias associations due to being ‘survivors’ of the early period of pharmacotherapy and having differing characteristics of persistence with their medication. We also selected a cohort of people living with dementia taking Z-drugs, but no other sedative medications, except for antipsychotics. We ceased follow-up when a new sedative medication or antipsychotic was prescribed, hence enabling greater confidence that any effects seen were attributable to the Z-drug, but this reduced statistical power. Having said that, we applied far fewer exclusion criteria than RCTs164 and, as such, our findings are generalisable to most of the population with dementia and sleep disturbance. Our selection of patients with dementia was demonstrated to be valid, with 96% of GPs confirming the dementia diagnosis, similar to other studies using UK primary care data. 165 We are likely to have underestimated the numbers of dementia patients with sleep disturbance, but not prescribed a Z-drug, as many of these patients may not have their sleep disturbance reported to their GP. However, as our capture of dementia patients who were prescribed Z-drugs is likely to be fairly accurate, it is of less concern that we are under-representing the comparator group. In fact, those patients in the comparison group with sleep disturbance recorded by the GP are more likely to represent the more severe cases of sleep disturbance and therefore are more comparable to the group that was prescribed Z-drugs.
Validity of exposures
Although the recording of prescriptions issued in primary care may be accurate, we lack data on medications prescribed in secondary care and obtained elsewhere. However, the majority of Z-drug prescribing should be in primary care. We captured only whether or not medications were prescribed and if they were dispensed or taken. We may have underestimated the effect of Z-drug use on the adverse events, if many patients prescribed Z-drugs had not taken them. As our adverse event risks were observed only in those with repeat prescriptions of Z-drugs, these findings suggest that patients returning for further prescriptions were more likely to have taken them.
We were able to compare the risks of Z-drug use with those of BZDs. However, ensuring that the BZDs were prescribed for sleep disturbance limited the number of eligible patients and therefore our power to detect differences between BZDs and Z-drugs. As per our protocol, we also examined the medication class of low-dose TCAs; however, we advise caution in interpreting these findings as we were unable to determine the indication for these medications and sleep disturbance was confirmed in only 60% of cases in the validation study. The Z-drugs prescribed historically in the UK are zopiclone, zolpidem and zaleplon, but with zopiclone by far in the majority and zaleplon now discontinued. Although comparisons between specific Z-drugs might be of interest, there is little evidence to suggest that their effects are different and there were insufficient numbers to be able to make this comparison.
Validity of the outcomes
Patients with diagnoses recorded in the CPRD have generally been shown to have the condition. 166,167 Validity studies report around 88–100% of patients coded as having hip fracture, UTI, respiratory tract infection, venous thromboembolism and psychosis in UK primary care records databases were clinically confirmed. 166,168,169 Rates are slightly lower for stroke, where 66–76% of reported ischaemic strokes or TIAs are confirmed. 170–172 However, the degree of under-reporting in the CPRD has not often been examined. The validity of our outcomes was improved through linkage to HES and ONS. This reduced bias was due to not solely relying on the GP who probably wrote the Z-drug prescription to accurately record the outcomes. Including HES and ONS data increased our number of outcome events by approximately 46%. The number of additional events was greater in the Z-drug group than in the no sleep medication group, representing substantial bias if we had relied on the CPRD data alone (e.g. the HR for Z-drug use and fractures decreased from 1.40 to 1.27 when restricting to only the CPRD data). Other studies found that pneumonia rates increased substantially when HES linkage was included. 173
Our recording of mortality is likely to be highly accurate as this was based on ONS records, which were also very similar to that of primary care recorded death dates. We probably underestimated forearm, wrist and hand fracture incidence, as some may have been reported to accident and emergency departments without hospital admission and may not have made it onto the primary care record. Similarly, falls are probably under-reported. GP records of falls may under-represent all falls that occur in the older population, but more accurately represent ‘injurious falls requiring medical attention’. 121 However, they may be under-reported as a result of their recording being in the GP’s free text, or because the medical consequence of the fall (e.g. fracture) has been recorded preferentially over the fall.
Some infections may be undetected, but we concentrated on acute bacterial ones for which medical attention would usually have been sought. We attempted to record incident behavioural and psychological symptoms of dementia by including any record of agitation, psychosis, hallucinations, delusions or aggression, but these are probably under-reported and poorly defined. They also may represent late coding of the indication for the Z-drug or BZD of interest. Hence, we express cautious interpreting of the agitation or psychosis outcome.
Although some outcomes were potentially under-reported, we do not expect substantial bias in our risk estimates due to outcome validity. Owing to the inclusion of linked HES and ONS data, we do not expect differential recording of outcomes among those prescribed Z-drugs or not. However, we examined multiple outcomes, which increased the risk of false-positive findings.
Confounding
We reduced the impact of confounding by indication by comparing adverse events to people living with dementia with recorded sleep disturbance. However, sleep disturbance was challenging to identify within the electronic primary care record. Only 42% of our control group with no sleep disturbance had sleep disturbance confirmed in our validation study. The issue of the additional pop-up screen recording the patient’s sleep pattern added confusion to the often contradictory diagnosis of sleep disturbance. Discussion with health-care professionals identified a mixture of opinion that the patient has no sleep disturbance when a ‘satisfactory’ sleep pattern was entered, or that the GP may just want to remove the pop-up screen on sleep patterns by clicking the first option (of ‘satisfactory’). We remain uncertain whether or not these patients have a sleep disturbance, but 35% and 83% of sleep disturbance was confirmed when accompanied and not accompanied, respectively, by a ‘satisfactory’ sleep pattern in the validation study. We performed a sensitivity analysis excluding control patients with a satisfactory sleep pattern and our effects were reduced. We are not aware of any study that has previously investigated the reliability of sleep disorder records in primary care, therefore our validation study strengthens future work on sleep disturbance using primary care data. 118
Although the detailed primary care database allowed adjustment for a wide range of potential confounders, there is the possibility of residual confounding, the most important of which is that we were unable to measure dementia severity. Although we adjusted for duration since the dementia diagnosis, prescription of dementia medications and antipsychotics, history of agitation or psychosis and end-of-life care, there is likely to be residual confounding by dementia severity for the mortality outcome. We were also lacking information on the severity of the sleep disturbance. As more severe sleep disturbances may involve longer wandering around at night and hence greater risk of fractures, there may be residual confounding by sleep disturbance severity for the fracture outcomes. Studies have suggested that dementia severity is unrelated to fracture risk,174 but residual confounding by stage of dementia cannot be ruled out. We also had no data on genetic information, environmental factors and cohabiting status, which have been shown to be important in falls risks in people living with dementia. 41
Missing data
Missing data for covariates were generally low. Missing data occurring more frequently for covariates of BMI and care home, had minimal effects on the associations reported in the sensitivity analyses.
Clinical cohort studies
Generally, there were few studies reporting the outcomes of interest with sufficient Z-drug use in people living with dementia. However, the three included cohort studies were of a large size, with frequent follow-up and detailed outcome recording. Our results varied, and this could reflect differences in the prescribing of Z-drugs across the USA, UK and Norway, and the settings of nursing homes or the community. We were limited by not having a record of the outcome measurements just before the Z-drug was first prescribed. As each study measured outcomes at fixed intervals, this did not necessarily coincide with the initiation of Z-drugs or the start of a sleep disturbance. We were limited by which outcome measures each study recorded. With NPI, it is not obvious whether a respondent is reporting on the level of symptoms that are currently experienced given current treatment, or those that they believe would be experienced if the patient was not treated. Hence, these findings should be interpreted with caution.
Although in the REDIC study and the NACC data set our analysis was strengthened by using marginal structural models to balance users and non-users on prior covariates, the extent of balance was not always perfect and could not be improved using different models for estimating weights. However, balance was very good for prior CDR, suggesting that our findings are not driven by underlying differences in dementia severity. Perhaps more importantly, this approach is valid for causal inference only if the assumption of exchangeability is met, that is after balancing over the covariates included in our model there is no longer any indirect path from the exposure in question to the outcome measure. 175 In our case it is difficult to be certain of this and, as the exact timing of medication initiation is not known, it is impossible to know whether the outcome preceded each exposure or vice versa.
Interpretation of the study in light of previous research
Fractures
In the primary care study, we found evidence of a 40% greater risk of fractures with Z-drug use in people living with dementia, which is lower than that reported in studies in the older population. A systematic review, including a subgroup analysis of the findings from five observational studies in the older population, estimated a pooled relative risk for fractures of 1.73 (95% CI 1.31 to 2.27) with zolpidem use. 176 However, none of these studies accounted for sleep disturbance, which is the indication for zolpidem. A further systematic review and meta-analysis investigated Z-drugs in general and risks for falls and fractures from 14 eligible observational studies. 60 They estimated an OR of 1.70 (95% CI 1.36 to 2.12) for Z-drugs and fractures in the older population, but noted substantial heterogeneity. 60
Although several studies have examined the risk of fractures associated with Z-drug use in the older population, studies targeting people living with dementia have been limited. 36 A recent Cochrane systematic review concluded a distinct lack of evidence to help guide drug treatment of sleep problems in dementia,36 despite being widely prescribed to this large patient group. 177 To our knowledge, no studies have previously examined the risk of general fractures specifically in people living with dementia taking Z-drugs.
Current BZD use was not found to be associated with fractures in a study of 8036 people living with dementia in London. 41 We observed similar rates of fractures among new BZD and new Z-drug use. Other studies in the older population that compare BZDs with Z-drugs for fracture risk, report varying results, often dependent on the type of BZD. 59,178
We found evidence of a 59% greater risk of hip fractures with Z-drug use in people living with dementia. A systematic review and meta-analysis pooled the findings from six observational studies in older adults and estimated a 90% (95% CI 68% to 113%) greater risk of hip fracture with Z-drug use. 61 However, studies suggest that the risk is lower in people living with dementia. 57,179 A Finnish study of 67,072 community-dwelling people diagnosed with Alzheimer’s disease reported no association between any history of BZD or Z-drug use in the 5 years before study entry and incident hip fracture (HR 1.03, 95% CI 0.97 to 1.09); however, this exposure definition may not be sufficiently specific and would not necessarily cover medication use immediately prior to most of the incident hip fractures (i.e. was measured only at baseline and not updated during follow-up). 179 A study of 15,528 US nursing home residents estimated an OR for non-BZD hypnotic drug use and hip fracture in residents with moderate to severe cognitive impairment of 1.43 (95% CI 1.15 to 1.77), which was lower than in the residents with no or MCI (OR 1.86, 95% CI 1.56 to 2.21). 57
We observed that hip fracture risk increased with greater cumulative Z-drug exposure, but then tapered after 56 DDDs. This is consistent with an initial expression then down-regulation of GABA receptors,180,181 or a depletion of susceptible patients among long-term users. 182 Other studies in the older population found similar findings, but with risks tapering over shorter periods, than we did in dementia. 59,183 This perhaps represents delays getting the prescription dispensed or many people living with dementia not taking their prescribed Z-drugs during the first month in our sample, or a slower effect of Z-drugs in dementia. We found no effect in the first 14 days, contrary to other studies in the older population. 54,56,57 This could be partly explained if some patients receiving only one prescription did not take the Z-drugs. Excluding those with only one Z-drug prescription had little effect on our cumulative Z-drug prescription findings (results available from the authors).
We observed evidence of a greater risk of hip fractures for those prescribed BZDs than Z-drugs, but numbers of hip fractures were small and this could represent residual confounding by dementia severity. Studies differ on whether they find those prescribed BZDs or Z-drugs at greater risk of hip fracture,55,56,58,184 but generally suggest greater risks with long-acting BZDs than with Z-drugs. 56,185
Evidence is lacking to indicate that Z-drugs increase the risk of osteoporosis,186 in contrast to other psychotropic drugs (e.g. antidepressants and antipsychotics). 187 Although studies suggest that long-term sleep disturbance may increase osteoporosis risk though disrupting the rhythm of bone turnover. 188,189 The mechanism by which Z-drugs increase fracture risk is probably due to their effects on gait and balance, although this is contradictory to our findings on falls outcomes (see Falls). 59,186 A trial randomised 25 adults to 5-mg zolpidem or placebo 10 minutes before scheduled sleep and asked them to perform 10 tandem walks on a beam on night-time awakening. 190 The NNH was estimated as one tandem walk failure for every 1.7 (95% CI 1.4 to 2.0) older adults treated with zolpidem. The trial authors concluded that the ‘use of nonbenzodiazepine hypnotic medications may have greater consequences for health and safety than previously recognized’. 190
In a comprehensive review of the evidence for major adverse outcomes of BZDs and Z-drugs, there was compelling evidence to establish a causal connection between BZD or Z-drug use and only motor vehicle accidents, falls and fractures. 186 Our study suggests that the increased risk of fractures extends to those people living with dementia taking Z-drugs.
Falls
Counterintuitive to the fractures findings in the primary care study, we did not find an increased falls risk with Z-drug use. Similarly, current BZD use was also not found to be associated with falls in a study of 8036 people living with dementia in London, once accounting for differences in ethnicity, living environment and disability. 41 A systematic review published in 2008, reported modest associations (RRs 1.2–1.3) between BZDs and falls in nursing home residents with dementia,191 and BZDs are consistently associated with around a 20% increased falls risk in older people. 48
Z-drugs were originally claimed to cause fewer falls,51 but a recent meta-analysis estimated an OR for Z-drug use and falls of 2.40 (95% CI 0.92 to 6.27) when pooling the findings from three observational studies in adult populations. 60 However, there was considerable heterogeneity (97%) between the studies and reasons for this were cited as difficulty recording falls, particularly in patients with multimorbidity, or recall bias in the studies relying on self-reported falls. Among 4450 US community-dwelling older men enrolled in the Osteoporotic Fractures in Men study, Z-drug use was associated with a 37% (95 CI 9% to 71%) greater risk of a self-reported fall. 192
Falls are thought be the main mechanism whereby increased fractures and injury occur in Z-drug users resulting from the sedation effect. Falls also account for 90% of hip fractures. 193 A study of older people in Taiwan found that high doses of Z-drugs were associated with an increased risk of fall-related injuries requiring hospitalisation (OR 1.24, 95% CI 1.05 to 1.48), so the lack of association between Z-drug use and falls in our study might be considered surprising. 53
To gain a potential clinical explanation we discussed this finding with our health-care advisory panel, who suggested that a fracture would be coded in electronic records preferentially over falls as the cause for their referral or reason for admission to hospital. This does suggest that in such cases, if a fracture is a direct result of a fall, it could be missed in our analysis due to our reliance on the complete recording of all required coding during contact with a GP or hospital. Alternatively, although the rate of falls may have been similar between groups, it is possible that the nature of the falls may have been more severe or of greater physical impact in patients taking Z-drugs.
Mortality
We observed a 34% increased mortality rate in people living with dementia prescribed Z-drugs, using primary care data; however, no association in UK nursing homes (the WHELD trial). Other studies have been conflicting on the effect of hypnotic use on mortality. A study of 31,140 community-dwelling people with Alzheimer’s disease in Finland, found that BZD use was associated with increased risk of mortality (HR 1.59, 95% CI 1.35 to 1.88), but not Z-drug use (HR 1.06, 95% CI 0.83 to 1.35), after accounting for psychotropic drug use, comorbidities, socioeconomic status, hip fractures and stroke. 75 In a US study,74 crude mortality rates were higher in people living with dementia taking hypnotics, but the association was not tested statistically.
A systematic review and meta-analysis examining the effect of hypnotics on mortality estimated a HR of 1.73 (95% CI 0.95 to 3.16) when pooling the findings from five observational studies on Z-drug use and mortality risk in adults. 71 However, there was considerable heterogeneity (I2 = 97%), with three studies reporting no effect and two with HRs > 3. Two of these studies used similar UK primary data to our study. One matched 2435 Z-drug initiators to two non-initiators on age, sex and general practice in the UK adult population during 1998–2001, and reported a HR for mortality of 3.32 (95% CI 3.19 to 3.45), adjusted for age, sex, comorbidities and co-medications, with a fairly constant risk across cumulative Z-drug prescriptions, similar to our study. 194 A further study of 4964 UK patients with pneumonia showed that patients were not at a greater mortality risk if taking zopiclone (HR 1.11, 95% CI 0.77 to 1.60). 64 Studies on Z-drug or BZD use and mortality have been conflicting,64,70–72,194 and reported associations may simply stem from confounding. 73 A large database study of US adults observed an increased mortality rate in 1.25 million BZD initiators matched to 1.25 million patients not initiating BZDs, but not once accounting for comorbidities, concomitant medications and health-care utilisation via propensity score matching (HR 0.89, 95% CI 0.85 to 0.94 in those aged > 65 years). 195
We found the same risk of mortality regardless of cumulative Z-drug exposure from our dose–response analysis, suggesting that the relationship may not be causal between Z-drug use and mortality risk in this case. We suspect that our findings are at least partially due to residual confounding by indication, whereby the cohort of patients on Z-drugs are already at increased risk of death. 73 However, it could also be speculated that the increased risk of hip fractures in this group drives an increased mortality rate, given that older adults have a five- to eightfold increased risk for all-cause mortality during the first 3 months after a hip fracture. 196
Infections
In the primary care study we did not find a statistically significant increased risk of infections in Z-drug users (HR 1.10, 95% CI 0.92 to 1.32 for UTI and LRTI). However, there was a suggestion of an increased infection risk after 28 DDDs of Z-drugs were prescribed, and we observed a greater rate of antibiotic prescribing. A Finnish study of community-dwelling adults with Alzheimer’s disease found that BZD use was associated with increased pneumonia rates and also found a very similar Z-drug use estimate to our study (HR 1.10, 95% CI 0.84 to 1.44). 67
A meta-analysis pooled data from published or Food and Drug Administration RCTs on 2432 participants randomised to eszopiclone and on 1626 participants randomised to zolpidem, estimated RRs of 1.48 (95% CI 1.25 to 1.74) and 1.99 (95% CI 1.21 to 3.26) for infections, respectively. 63 Observational studies in the adult population vary according to whether or not they detect an increased risk of infection with Z-drug use. A UK study using similar primary care to our study did not detect a significant association between Z-drug prescription and influenza-like illness-related pneumonia (HR 1.18, 95% CI 0.89 to 1.56). 66 A study in Taiwan found that patients using zolpidem with cumulative DDDs of 1–28, 29–84 and > 84 had HRs of 1.67 (95% CI 1.32 to 2.11), 1.91 (95% CI 1.47 to 2.49) and 1.62 (95% CI 1.32 to 1.98), respectively, compared with patients who did not use zolpidem during a 3-year follow-up. 65 However, there is probably residual confounding due to accounting only for a comorbidity score and few co-medications. A later case–control study in Taiwan in adults with chronic kidney disease found no difference in Z-drug use at the time of pneumonia (OR 1.07, 95% CI 0.80 to 1.44). 197
The mechanism whereby Z-drug use may increase infection is uncertain. It has been speculated that BZDs may suppress immune surveillance, which is supported by animal study findings. 63 Alternatively, Z-drugs could impair the clearing of oral secretions through inhibiting the swallowing of saliva. 68 Another possibility is that Z-drugs may increase gastro-oesophageal reflux events during sleep by relaxing the lower oesophageal sphincter. 198
Our study and the supporting literature suggest that if there is a greater risk of acute infections with Z-drug use in people living with dementia, then it is likely to be small and our study was inadequately powered to detect it.
Cardiovascular outcomes
In the primary care study we did not find a statistically significant increased risk of ischaemic stroke (HR 1.33, 95% CI 0.85 to 2.07) or venous thromboembolism (HR 1.66, 95% CI 0.69 to 3.98) to people living with dementia prescribed Z-drugs. However, there was a suggestion of an increased stroke risk after 28 DDDs of Z-drugs were prescribed. A study of 45,050 community-dwelling persons with Alzheimer’s disease in Finland reported that BZD or Z-drug prescription was associated with a 21% (95% CI 4% to 40%) increased risk of stroke, and this risk did not vary when stratified by BZD or Z-drug use. 77 However, residual confounding is possible as they were unable to account for sleep disturbance or dementia severity. A case–control study of adults in Taiwan found that exposure to zolpidem was associated with increased risks of ischaemic stroke (OR 1.37, 95% CI 1.30 to 1.44), increasing further with greater exposure to zolpidem. 78 They also found that regardless of whether or not a person presented with a sleep disorder, the risk of stroke was increased with zolpidem use (OR 1.37 without a sleep disorder and 1.41 with a sleep disorder), suggesting that the increased risk was due to drug use and not the sleep disturbance itself.
Mechanisms for Z-drugs causing increased stroke risk are uncertain, but speculated to relate to a decrease in local cerebral blood flow. 77 However, there is evidence that sleep disturbances increase stroke risk,199 suggesting that residual confounding by indication could underlie reported associations. We are not aware of any studies examining Z-drug use and risk of venous thromboembolism, although sleep disturbance has been observed to be a risk factor. 200
Prescriptions and health-care utilisation
In the primary care study, we found that people living with dementia who were prescribed Z-drugs were more likely to be initiated on antipsychotics and antidepressants. In line with our findings, a study in Taiwan reported that Z-drug users aged > 65 years were more frequently exposed to antipsychotics (OR 1.31, 95% CI 1.14 to 1.50), antidepressants (OR 1.45, 95% CI 1.28 to 1.65) and opioids (OR 2.38, 95% CI 2.07 to 2.73). 53 We also found that people living with dementia who were prescribed Z-drugs had more GP and hospital visits. This could reflect the increased risk of fractures in these patients, which would drive increased service use. The study in Taiwan also found that older adults who were prescribed Z-drugs were at an increased risk of fall-related injuries necessitating hospitalisation (OR 1.24, 95% CI 1.05 to 1.48). 53
Cognition, quality of life and sleep disturbance
In the clinical cohort studies, we found no evidence that Z-drug use impacts significantly on cognitive function or QoL. This differs from other studies in older adults with insomnia, which reported that hypnotic use is associated with reduced HRQoL. 101,102 Greater cognitive declines have been reported in people living with dementia taking BZDs;96 however, to the best of our knowledge, no other studies have examined the impact of Z-drugs on cognition in people living with dementia.
Estimating the effect of Z-drug use on sleep disturbance was challenging using the observational data sets, given that sleep scores were not measured immediately prior to Z-drug initiation; however, we observed no impact of Z-drug use on sleep scores. This is in line with other studies, suggesting that the use of sedating hypnotics in the general population does not improve sleep, human performance or general health, calling into doubt their effectiveness. 103,104
Chapter 8 Conclusions
The evidence suggests an increased risk of fractures in people living with dementia and taking Z-drugs, which is cause for concern. However, our study is limited by being observational with possible residual confounding and the many outcomes examined increases the chances of false-positive findings. In the past, Z-drugs have been thought of as a safer alternative to BZDs for the management of sleep disturbance, and their usage has been increasing with drives to decrease antipsychotic and BZD prescribing. 110 One meta-analysis reported a pooled estimate of a 92% increased risk of fractures with zolpidem use (RR 1.92, 95% CI 1.65 to 2.24),176 exceeding the risk reported with BZD use in some studies. 55,58 Another meta-analysis similarly found that Z-drugs were associated with higher risk of hip fracture (RR 1.90, 95% CI 1.68 to 2.13) than BZD use (RR 1.52, 95% CI 1.37 to 1.68). 61 The evidence suggests that Z-drugs may not be a safer alternative to BZDs, particularly in the context of fractures, and that they may need to be used with similar caution.
Furthermore, previous studies have mostly targeted the older population, whereas our study specifically focuses the use of Z-drugs in people living with dementia, for whom fracture rate risk is already higher, particularly hip fractures. 201,202 There has previously been little evidence of the specific effects in people living with dementia; our study has provided targeted information for this vulnerable and growing patient group, which will be valuable to both patients and carers but also health-care professionals. 62
Clinical implications
It is important to remember that the population with dementia who are taking Z-drugs is likely to include people with more severe dementia than the population taking no medication for sleep disturbance, so we cannot assume that the effects we observe are entirely causal. With that in mind, we estimate that the absolute annual risks of fractures and hip fractures in the unexposed group are 7.4% and 3.2%, respectively. These equate to annual risks of 10.2% and 5.0% for fractures and hip fractures, respectively, when taking Z-drugs. If causal, this is equivalent to 36 and 56 people living with dementia being treated with Z-drugs before a fracture or hip fracture occurs, respectively.
The consequences of hip fracture are particularly serious for older people with dementia. In the UK in 2011 hip fracture cases cost around £1B per year,203 with UK hospital costs alone estimated to be £14,163 and £2139 in the first and second year following hip fracture in 2012–13. 204 In England, 648,465 people have dementia, of whom 436,416 (67.3%) are diagnosed. 205 Using an estimate of the prevalence of Z-drug use in people living with dementia in Scotland of 9% in 2011, assumes 39,277 people diagnosed with dementia are taking Z-drugs per year. 206 The additional annual hospital cost to the NHS of hip fractures for people with a diagnosis of dementia who take Z-drugs is likely to be in the order of £11.5M, assuming £16,302 per hip fracture for the additional 1.8% of the exposed population. The wider NHS and societal costs are likely to be much higher.
The individual decision to prescribe a sleep drug includes balancing many complex and often competing risks and benefits for patients and carers. Our estimated increased risks for fractures were also comparatively small for the individual patient, so patients would need to be reviewed on a case-by-case basis to assess whether or not their risks outweigh their individual potential benefits from Z-drugs. Further research is also required to confirm whether or not an increased risk of fractures, hip fracture and potentially mortality should be considered when prescribing Z-drugs. Our findings suggest that when pharmacological management of sleep disturbance is initiated, fracture risk management plans may need to be implemented and monitored to mitigate potential adverse health outcomes.
Implications for further research
Further research is needed to confirm our findings of an increased fracture risk with Z-drug use in people living with dementia. Research is needed to better understand whether or not Z-drugs increase the risk of falls, and to understand the mechanism and type of falls, for example if the risk varies by time of day (e.g. is it through drowsiness on waking in the morning, or falls from bed or getting to the bathroom at night). In addition, studies should examine whether or not Z-drugs contribute by any other mechanism to increase fracture risk (e.g. via increasing the risk of osteoporosis). Although further well-designed observational studies may provide additional evidence, RCTs are needed to obtain a clear assessment of potential risks. A pragmatic cluster trial that includes de-prescribing of Z-drugs as part of a multifaceted intervention, delivered in community settings, would be feasible and could include detailed falls reporting. Our findings suggest that ideally patients should be de-prescribed Z-drugs soon after starting them, to avoid them developing tolerance to the Z-drugs. Such trials should also consider the informal carer burden associated with any intervention, as this is often overlooked. 207
Our study highlights the lack of existing studies that routinely record outcome information on the carer(s) of people living with dementia. It was an aim in our original protocol to examine carer outcomes, but we found very few sufficiently large studies that recorded them. In balancing the risks and benefits of treatments for sleep disturbance, information on any potential benefits to carers is a key piece in the decision-making that we are missing. Other research has suggested that informal carers, in general, find medication management for people living with dementia challenging. 208 Particular challenges include monitoring for adverse events and deciding whether or not to administer an ‘as required’ medication, such as a hypnotic. More research is needed on carer outcomes to properly be able to weigh up the risks and benefits of Z-drugs in people living with dementia.
Further research is needed into alternative non-pharmacological therapies and whether or not they may be appropriate as first-line treatments for sleep disturbance in dementia. 209 Various studies have examined artificial light therapy for people living with dementia, but a recent Cochrane review concluded they had no effect on sleep activity. 210 Further studies should pursue activity programmes that include outdoor light, as some have demonstrated positive benefits on sleep. A daily structured activity programme offered outdoors was successful in increasing sleep duration in nursing home residents with dementia. 211 In addition, a multidimensional intervention including sunlight exposure, increased activities and improved sleep hygiene was successful in reducing daytime napping and decreased night-time awakenings in care home residents. 212 Programmes with individualised social activities have demonstrated improved sleep outcomes in people living with dementia, whereas other interventions have had mixed findings. 213 However, the previous studies have been criticised for their inconsistency and for not building on each other. 213 Replication is needed in order to be more confident about the evidence base. Previous studies have also highlighted the need for a personalised approach to sleep disturbance interventions. 213 Research into multicomponent personalised therapies that combine light therapy and activity components may be fruitful. 214 In addition, more research is needed to better understand the differing types of sleep disturbance in dementia and their underlying mechanisms in order to better target possible treatments. 215,216
This study highlights important issues for sleep disturbance research, using UK patient electronic medical records. To our knowledge, no previous studies have investigated the validity of sleep disturbance coding in UK primary care data. However, our validation study identified that only 55% of people living with dementia coded with a diagnosis or symptom of sleep disturbance had this diagnosis confirmed by their GP, highlighting some anomalies. We also found that sleep disturbance diagnoses were unlikely to occur within the primary care Read codes. For example, Z-drugs are prescribed exclusively for sleep disturbance, but only 18% of people living with dementia prescribed a Z-drug had a code for sleep disturbance on the date of the Z-drug prescription. From conversations with our health-care professional panel it seems that, due to time constraints during GP appointments, often only the primary conditions are coded. It could also potentially be an issue of ‘diagnostic overshadowing’, whereby the fact that the patient has a diagnosis of dementia is taking priority over the recording of other relevant diagnoses. 217 This information is important for future epidemiological research relying on sleep disturbance Read coding in medical records; without free text it can be problematic to confirm or gain further insight into sleep disturbance issues in patients.
Another limitation of existing standardised primary care data sets for dementia research is that they lack information on the severity of dementia or the cognitive state of the people living with dementia. Further linkages of large primary care databases with secondary care memory clinics and dementia services would be beneficial to advance research in dementia.
We found that considerable bias would have affected our risk estimates if we had not included linkage to hospital admissions data. We recommend that research on the effects of medication using UK primary care data additionally uses linked data sets when possible. 218
Acknowledgements
There are many who contributed to this research project; we would like to thank to following people for their valuable participation.
We are grateful to the patients who participated in the clinical trial and cohort study data sets, which we subsequently repurposed for our study.
The NACC database is funded by the National Institute on Aging/National Institutes of Health grant U01 AG016976. The NACC data are contributed by the National Institute on Aging-funded ADCs: P30 AG019610 [principal investigator (PI) Eric Reiman, Doctor of Medicine (MD)], P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 [PI Allan Levey, MD, Doctor of Philosophy (PhD)], P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, Doctor of Psychology), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, Fellowship of the Royal College of Physicians of London), P50 AG005681 (PI John Morris, MD) and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Robert Howard is supported by the University College London Hospitals NIHR Biomedical Research Centre.
We are grateful to the GPs who completed the GP questionnaires as part of the validation study and to Emma Boyle (Medicines and Healthcare products Regulatory Agency) for her support facilitating the GP questionnaire.
We also thank our PPI and health-care professional advisory group members for their valuable contributions to the ZED study, including Kate Massey, Peter Richmond, Lesley Evans, Heather Edwards, Tris Jackson, Mandi Bowhill, Amander Wellings, Rebecca Harmston, Jay Foden, Maureen Tilford, Daisy Lo, Magda Turczyn and Liz Yaxley. Kate Massey was a member of the Study Steering Committee in a PPI advisory role, as well as a member of the ZED PPI Advisory Team. Their knowledge and insights based on real-life experiences provided critical information and contribution throughout the study. We would also like to thank the PPI group co-ordinator, Rhianna Broadway (Inspire), and the HCP group co-ordinator, Sarah Housden, for their support during the study.
We thank those who provided data for the clinical cohort studies, including those that were kindly offered but we were not able to use and, in particular, to Dr Sverre Bergh (Innlandet Hospital Trust, Norway) who facilitated access to the REDIC study data.
We additionally would like to thank members of our Steering Group Committee for their support and guidance throughout the study.
Disclaimers
This study is based in part on data from the CPRD, obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. However, the interpretation and conclusions contained in this report are those of the authors alone.
Contributions of authors
Kathryn Richardson (https://orcid.org/0000-0002-0741-8413) (Research Fellow in Statistics, University of East Anglia) was a co-applicant, co-developed and co-wrote the funding application. Led the statistical design and analysis of the primary care study and the WHELD trial data set, and was responsible for writing and revising this report.
George M Savva (https://orcid.org/0000-0001-9190-124X) (Institute Statistician, Quadram Institute, formerly Senior Lecturer in Applied Statistics, University of East Anglia) was chief investigator for the study in the period June 2016–September 2017. He co-developed the study concept and led the study design and the funding application, led the statistical design and analysis for the clinical data sets of the REDIC study and NACC, and contributed to writing and revising this report.
Penelope J Boyd (https://orcid.org/0000-0003-4786-1230) (Senior Research Associate, University of East Anglia) was the research assistant from October 2017 to July 2018. She contributed to PPI and health-care professional activity, dissemination and literature review, and co-ordinated and contributed to writing and revising this report.
Clare Aldus (https://orcid.org/0000-0002-0197-2755) (Research Fellow, University of East Anglia) was study manager from October 2017 to July 2018. She managed the research and administrative team, governance, impact and dissemination, and contributed to writing and revising this report.
Ian Maidment (https://orcid.org/0000-0003-4152-9704) (Reader in Clinical Pharmacy, Aston University) was a co-applicant and pharmacy lead. He provided expertise in dementia and medication optimisation, contributed to study design and development, interpretation of the data, and contributed to writing and revising this report.
Eduwin Pakpahan (https://orcid.org/0000-0002-0058-1808) (Research Associate in Medical Statistics, Newcastle University) was the statistician responsible for the clinical cohort studies from November 2016 to September 2017. He contributed to interpretation of the data, and contributed to writing and revising this report.
Yoon K Loke (https://orcid.org/0000-0001-9109-2307) (Professor of Medicine and Pharmacology, University of East Anglia) was a co-applicant and provided pharmacological and epidemiological support, contributed to study design, primary care coding and interpretation of the data, and contributed to writing and revising this report.
Antony Arthur (https://orcid.org/0000-0001-8617-5714) (Professor of Nursing Science, University of East Anglia) was a co-applicant, provided expertise in older person care, contributed to study design, and contributed to writing and revising this report.
Nicholas Steel (https://orcid.org/0000-0003-1528-140X) (Clinical Professor in Public Health, University of East Anglia) was a co-applicant and provided CPRD and statistical support. He contributed to study design, primary care coding, interpretation of the data, and contributed to writing and revising this report.
Clive Ballard (https://orcid.org/0000-0003-0022-5632) (Executive Dean of Medicine, University of Exeter) was a co-applicant and provided expertise in dementia research. He provided the WHELD RCT data set for analysis, contributed to study design and interpretation of the data, and contributed to writing and revising this report.
Robert Howard (https://orcid.org/0000-0002-3071-2338) (Professor of Old Age Psychiatry, University College London) was a co-applicant and provided expertise in pharmacology and dementia. He contributed to study design, primary care coding and interpretation of the data, and contributed to writing and revising this report.
Chris Fox (https://orcid.org/0000-0001-9480-5704) (Professor in Psychiatry, University of East Anglia) was chief investigator for the study in the period October 2017–July 2018. He was a co-applicant involved in study development, study design, interpretation of data and drafting this report.
Publication
Richardson K, Loke YK, Fox C, Maidment I, Howard R, Steel N, et al. Adverse effects of Z-drugs for sleep disturbance in people living with dementia: a population-based cohort study. BMC Med 2020;18:351.
Data-sharing statement
Data from the CPRD cannot be shared by the authors but are available directly from the CPRD. Full code lists corresponding to each of the covariates we included are available from the authors on request.
Data from the REDIC study are available for researchers in co-operation with the data owner, the Research Centre For Old Age Psychiatry Research – Innlandet Hospital Trust. Information is available at https://sykehuset-innlandet.no/avdelinger/alderspsykiatrisk-forskningssenter (accessed 6 December 2019).
Data from the NACC data set are available directly from the National Alzheimer’s Coordinating Center on application.
Data from the WHELD trial are available by approval from the Trial Management Committee for the WHELD trial.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Alzheimer’s Society . Dementia UK Update 2014. www.alzheimers.org.uk/dementiauk (accessed 31 March 2019).
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and meta-analysis. Alzheimers Dement 2013;9:63-75.e2. https://doi.org/10.1016/j.jalz.2012.11.007.
- Ahmadi-Abhari S, Guzman-Castillo M, Bandosz P, Shipley MJ, Muniz-Terrera G, Singh-Manoux A, et al. Temporal trend in dementia incidence since 2002 and projections for prevalence in England and Wales to 2040: modelling study. BMJ 2017;358. https://doi.org/10.1136/bmj.j2856.
- Rao A, Suliman A, Vuik S, Aylin P, Darzi A. Outcomes of dementia: systematic review and meta-analysis of hospital administrative database studies. Arch Gerontol Geriatr 2016;66:198-204. https://doi.org/10.1016/j.archger.2016.06.008.
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002;288:1475-83. https://doi.org/10.1001/jama.288.12.1475.
- Sarah Landry CK-A . Innovating the Next Generation of Dementia and Alzheimer’s Disease Care Interventions: Addressing the Needs of Person Living With Dementia, Caregivers, and Care Providers 2017.
- Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci 2009;11:217-28.
- Afram B, Stephan A, Verbeek H, Bleijlevens MH, Suhonen R, Sutcliffe C, et al. Reasons for institutionalization of people with dementia: informal caregiver reports from 8 European countries. J Am Med Dir Assoc 2014;15:108-16. https://doi.org/10.1016/j.jamda.2013.09.012.
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep 1995;18:425-32. https://doi.org/10.1093/sleep/18.6.425.
- Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6800 persons over three years. Sleep 1999;22:366-72.
- Kamel NS, Gammack JK. Insomnia in the elderly: cause, approach, and treatment. Am J Med 2006;119:463-9. https://doi.org/10.1016/j.amjmed.2005.10.051.
- Chien MY, Chen HC. Poor sleep quality is independently associated with physical disability in older adults. J Clin Sleep Med 2015;11:225-32. https://doi.org/10.5664/jcsm.4532.
- Guarnieri B, Adorni F, Musicco M, Appollonio I, Bonanni E, Caffarra P, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord 2012;33:50-8. https://doi.org/10.1159/000335363.
- Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev 2015;19:29-38. https://doi.org/10.1016/j.smrv.2014.03.007.
- Shin HY, Han HJ, Shin DJ, Park HM, Lee YB, Park KH. Sleep problems associated with behavioral and psychological symptoms as well as cognitive functions in Alzheimer’s disease. J Clin Neurol 2014;10:203-9. https://doi.org/10.3988/jcn.2014.10.3.203.
- Gitlin LN, Hodgson N, Piersol CV, Hess E, Hauck WW. Correlates of quality of life for individuals with dementia living at home: the role of home environment, caregiver, and patient-related characteristics. Am J Geriatr Psychiatry 2014;22:587-97. https://doi.org/10.1016/j.jagp.2012.11.005.
- Lee DR, Thomas AJ. Sleep in dementia and caregiving – assessment and treatment implications: a review. Int Psychogeriatr 2011;23:190-201. https://doi.org/10.1017/S1041610210001894.
- Donaldson C, Tarrier N, Burns A. Determinants of carer stress in Alzheimer’s disease. Int J Geriatr Psychiatry 1998;13:248-56. https://doi.org/10.1002/(SICI)1099-1166(199804)13:4<248::AID-GPS770>3.0.CO;2-0.
- McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med Rev 2007;11:143-53. https://doi.org/10.1016/j.smrv.2006.09.002.
- McCurry SM, Logsdon RG, Teri L, Gibbons LE, Kukull WA, Bowen JD, et al. Characteristics of sleep disturbance in community-dwelling Alzheimer’s disease patients. J Geriatr Psychiatry Neurol 1999;12:53-9. https://doi.org/10.1177/089198879901200203.
- Pollak CP, Perlick D. Sleep problems and institutionalization of the elderly. J Geriatr Psychiatry Neurol 1991;4:204-10. https://doi.org/10.1177/089198879100400405.
- Pat-Horenczyk R, Klauber MR, Shochat T, Ancoli-Israel S. Hourly profiles of sleep and wakefulness in severely versus mild-moderately demented nursing home patients. Aging 1998;10:308-15. https://doi.org/10.1007/BF03339793.
- Conn DK, Madan R. Use of sleep-promoting medications in nursing home residents: risks versus benefits. Drugs Aging 2006;23:271-87. https://doi.org/10.2165/00002512-200623040-00001.
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015;72:136-42. https://doi.org/10.1001/jamapsychiatry.2014.1763.
- Wichniak A, Wierzbicka A, Walęcka M, Jernajczyk W. Effects of antidepressants on sleep. Curr Psychiatry Rep 2017;19. https://doi.org/10.1007/s11920-017-0816-4.
- Schwartz TL, Goradia V. Managing insomnia: an overview of insomnia and pharmacologic treatment strategies in use and on the horizon. Drugs Context 2013;2013. https://doi.org/10.7573/dic.212257.
- Ashton H. Guidelines for the rational use of benzodiazepines. When and what to use. Drugs 1994;48:25-40. https://doi.org/10.2165/00003495-199448010-00004.
- Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging 2012;29:639-58. https://doi.org/10.2165/11633250-000000000-00000.
- Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc 2000;48:682-5. https://doi.org/10.1111/j.1532-5415.2000.tb04729.x.
- Brett J, Murnion B. Management of benzodiazepine misuse and dependence. Aust Prescr 2015;38:152-5. https://doi.org/10.18773/austprescr.2015.055.
- Swift CG, Swift MR, Hamley J, Stevenson IH, Crooks J. Side-effect ‘tolerance’ in elderly long-term recipients of benzodiazepine hypnotics. Age Ageing 1984;13:335-43. https://doi.org/10.1093/ageing/13.6.335.
- Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol 2013;9:155-62. https://doi.org/10.1007/s13181-013-0292-0.
- Rodriguez JC, Dzierzewski JM, Alessi CA. Sleep problems in the elderly. Med Clin North Am 2015;99:431-9. https://doi.org/10.1016/j.mcna.2014.11.013.
- National Institute for Health and Care Excellence . Guidance on the Use of Zaleplon, Zolpidem and Zopiclone for the Short-Term Management of Insomnia 2004. www.nice.org.uk/guidance/ta77 (accessed 31 March 2019).
- National Institute for Health and Care Excellence . Hypnotics 2015. www.nice.org.uk/advice/ktt6 (accessed 31 March 2019).
- McCleery J, Cohen DA, Sharpley AL. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev 2016;11. https://doi.org/10.1002/14651858.CD009178.pub3.
- Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ 2005;331. https://doi.org/10.1136/bmj.38623.768588.47.
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;9. https://doi.org/10.1002/14651858.CD007146.pub3.
- Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: a prospective study in older people. PLOS ONE 2009;4. https://doi.org/10.1371/journal.pone.0005521.
- Eriksson S, Gustafson Y, Lundin-Olsson L. Risk factors for falls in people with and without a diagnose of dementia living in residential care facilities: a prospective study. Arch Gerontol Geriatr 2008;46:293-306. https://doi.org/10.1016/j.archger.2007.05.002.
- Sharma S, Mueller C, Stewart R, Veronese N, Vancampfort D, Koyanagi A, et al. Predictors of falls and fractures leading to hospitalization in people with dementia: a representative cohort study. J Am Med Dir Assoc 2018;19:607-12. https://doi.org/10.1016/j.jamda.2018.03.009.
- Rasinaho M, Hirvensalo M, Leinonen R, Lintunen T, Rantanen T. Motives for and barriers to physical activity among older adults with mobility limitations. J Aging Phys Act 2007;15:90-102. https://doi.org/10.1123/japa.15.1.90.
- Heruti RJ, Lusky A, Barell V, Ohry A, Adunsky A. Cognitive status at admission: does it affect the rehabilitation outcome of elderly patients with hip fracture?. Arch Phys Med Rehabil 1999;80:432-6. https://doi.org/10.1016/S0003-9993(99)90281-2.
- Tinetti ME. Prevention of falls and fall injuries in elderly persons: a research agenda. Prev Med 1994;23:756-62. https://doi.org/10.1006/pmed.1994.1130.
- Sattin RW, Lambert Huber DA, DeVito CA, Rodriguez JG, Ros A, Bacchelli S, et al. The incidence of fall injury events among the elderly in a defined population. Am J Epidemiol 1990;131:1028-37. https://doi.org/10.1093/oxfordjournals.aje.a115594.
- Scuffham P, Chaplin S, Legood R. Incidence and costs of unintentional falls in older people in the United Kingdom. J Epidemiol Community Health 2003;57:740-4. https://doi.org/10.1136/jech.57.9.740.
- Hartikainen S, Lönnroos E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci 2007;62:1172-81. https://doi.org/10.1093/gerona/62.10.1172.
- Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med 2009;169:1952-60. https://doi.org/10.1001/archinternmed.2009.357.
- Pariente A, Dartigues JF, Benichou J, Letenneur L, Moore N, Fourrier-Réglat A. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging 2008;25:61-70. https://doi.org/10.2165/00002512-200825010-00007.
- Baker NL, Cook MN, Arrighi HM, Bullock R. Hip fracture risk and subsequent mortality among Alzheimer’s disease patients in the United Kingdom, 1988–2007. Age Ageing 2011;40:49-54. https://doi.org/10.1093/ageing/afq146.
- Allain H, Bentué-Ferrer D, Polard E, Akwa Y, Patat A. Postural instability and consequent falls and hip fractures associated with use of hypnotics in the elderly: a comparative review. Drugs Aging 2005;22:749-65. https://doi.org/10.2165/00002512-200522090-00004.
- Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med 2013;8:1-6. https://doi.org/10.1002/jhm.1985.
- Yu NW, Chen PJ, Tsai HJ, Huang CW, Chiu YW, Tsay WI, et al. Association of benzodiazepine and Z-drug use with the risk of hospitalisation for fall-related injuries among older people: a nationwide nested case–control study in Taiwan. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0530-4.
- Lin FY, Chen PC, Liao CH, Hsieh YW, Sung FC. Retrospective population cohort study on hip fracture risk associated with zolpidem medication. Sleep 2014;37:673-9. https://doi.org/10.5665/sleep.3566.
- Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Zolpidem use and hip fractures in older people. J Am Geriatr Soc 2001;49:1685-90. https://doi.org/10.1111/j.1532-5415.2001.49280.x.
- Bakken MS, Engeland A, Engesæter LB, Ranhoff AH, Hunskaar S, Ruths S. Risk of hip fracture among older people using anxiolytic and hypnotic drugs: a nationwide prospective cohort study. Eur J Clin Pharmacol 2014;70:873-80. https://doi.org/10.1007/s00228-014-1684-z.
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 2013;173:754-61. https://doi.org/10.1001/jamainternmed.2013.3795.
- Kang DY, Park S, Rhee CW, Kim YJ, Choi NK, Lee J, et al. Zolpidem use and risk of fracture in elderly insomnia patients. J Prev Med Public Health 2012;45:219-26. https://doi.org/10.3961/jpmph.2012.45.4.219.
- Finkle WD, Der JS, Greenland S, Adams JL, Ridgeway G, Blaschke T, et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc 2011;59:1883-90. https://doi.org/10.1111/j.1532-5415.2011.03591.x.
- Treves N, Perlman A, Kolenberg Geron L, Asaly A, Matok I. Z-drugs and risk for falls and fractures in older adults – a systematic review and meta-analysis. Age Ageing 2018;47:201-8. https://doi.org/10.1093/ageing/afx167.
- Donnelly K, Bracchi R, Hewitt J, Routledge PA, Carter B. Benzodiazepines, Z-drugs and the risk of hip fracture: a systematic review and meta-analysis. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0174730.
- Guarnieri B, Musicco M, Caffarra P, Adorni F, Appollonio I, Arnaldi D, et al. Recommendations of the Sleep Study Group of the Italian Dementia Research Association (SINDem) on clinical assessment and management of sleep disorders in individuals with mild cognitive impairment and dementia: a clinical review. Neurol Sci 2014;35:1329-48. https://doi.org/10.1007/s10072-014-1873-7.
- Joya FL, Kripke DF, Loving RT, Dawson A, Kline LE. Meta-analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. J Clin Sleep Med 2009;5:377-83.
- Obiora E, Hubbard R, Sanders RD, Myles PR. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax 2013;68:163-70. https://doi.org/10.1136/thoraxjnl-2012-202374.
- Huang CY, Chou FH, Huang YS, Yang CJ, Su YC, Juang SY, et al. The association between zolpidem and infection in patients with sleep disturbance. J Psychiatr Res 2014;54:116-20. https://doi.org/10.1016/j.jpsychires.2014.03.017.
- Nakafero G, Sanders RD, Nguyen-Van-Tam JS, Myles PR. The association between benzodiazepines and influenza-like illness-related pneumonia and mortality: a survival analysis using UK Primary Care data. Pharmacoepidemiol Drug Saf 2016;25:1263-73. https://doi.org/10.1002/pds.4028.
- Taipale H, Tolppanen AM, Koponen M, Tanskanen A, Lavikainen P, Sund R, et al. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ 2017;189:E519-E529. https://doi.org/10.1503/cmaj.160126.
- Sato K, Nakashima T. Human adult deglutition during sleep. Ann Otol Rhinol Laryngol 2006;115:334-9. https://doi.org/10.1177/000348940611500503.
- Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry 2009;195:61-6. https://doi.org/10.1192/bjp.bp.108.055335.
- Charlson F, Degenhardt L, McLaren J, Hall W, Lynskey M. A systematic review of research examining benzodiazepine-related mortality. Pharmacoepidemiol Drug Saf 2009;18:93-103. https://doi.org/10.1002/pds.1694.
- Parsaik AK, Mascarenhas SS, Khosh-Chashm D, Hashmi A, John V, Okusaga O, et al. Mortality associated with anxiolytic and hypnotic drugs – a systematic review and meta-analysis. Aust N Z J Psychiatry 2016;50:520-33. https://doi.org/10.1177/0004867415616695.
- Palmaro A, Dupouy J, Lapeyre-Mestre M. Benzodiazepines and risk of death: results from two large cohort studies in France and UK. Eur Neuropsychopharmacol 2015;25:1566-77. https://doi.org/10.1016/j.euroneuro.2015.07.006.
- Neutel CI, Johansen HL. Association between hypnotics use and increased mortality: causation or confounding?. Eur J Clin Pharmacol 2015;71:637-42. https://doi.org/10.1007/s00228-015-1841-z.
- Kales HC, Valenstein M, Kim HM, McCarthy JF, Ganoczy D, Cunningham F, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry 2007;164:1568-76. https://doi.org/10.1176/appi.ajp.2007.06101710.
- Saarelainen L, Tolppanen AM, Koponen M, Tanskanen A, Tiihonen J, Hartikainen S, et al. Risk of death associated with new benzodiazepine use among persons with Alzheimer disease: a matched cohort study. Int J Geriatr Psychiatry 2018;33:583-90. https://doi.org/10.1002/gps.4821.
- Douglas IJ, Smeeth L. Exposure to antipsychotics and risk of stroke: self controlled case series study. BMJ 2008;337. https://doi.org/10.1136/bmj.a1227.
- Taipale H, Koponen M, Tanskanen A, Lavikainen P, Tolppanen AM, Sund R, et al. Use of benzodiazepines and related drugs is associated with a risk of stroke among persons with Alzheimer’s disease. Int Clin Psychopharmacol 2017;32:135-41. https://doi.org/10.1097/YIC.0000000000000161.
- Huang WS, Tsai CH, Lin CC, Muo CH, Sung FC, Chang YJ, et al. Relationship between zolpidem use and stroke risk: a Taiwanese population-based case-control study. J Clin Psychiatry 2013;74:e433-8. https://doi.org/10.4088/JCP.12m08181.
- Schroeck JL, Ford J, Conway EL, Kurtzhalts KE, Gee ME, Vollmer KA, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther 2016;38:2340-72. https://doi.org/10.1016/j.clinthera.2016.09.010.
- Ben-Hamou M, Marshall NS, Grunstein RR, Saini B, Fois RA. Spontaneous adverse event reports associated with zolpidem in Australia 2001–2008. J Sleep Res 2011;20:559-68. https://doi.org/10.1111/j.1365-2869.2011.00919.x.
- Maquet P. The role of sleep in learning and memory. Science 2001;294:1048-52. https://doi.org/10.1126/science.1062856.
- Stickgold R. Sleep-dependent memory consolidation. Nature 2005;437:1272-8. https://doi.org/10.1038/nature04286.
- Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep 1996;19:318-26. https://doi.org/10.1093/sleep/19.4.318.
- Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiatr Dis Treat 2007;3:553-67.
- Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry 2014;27:478-83. https://doi.org/10.1097/YCO.0000000000000106.
- Billioti de Gage S, Moride Y, Ducruet T, Kurth T, Verdoux H, Tournier M, et al. Benzodiazepine use and risk of Alzheimer’s disease: case–control study. BMJ 2014;349. https://doi.org/10.1136/bmj.g5205.
- Billioti de Gage S, Bégaud B, Bazin F, Verdoux H, Dartigues JF, Pérès K, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ 2012;345. https://doi.org/10.1136/bmj.e6231.
- Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs 2004;18:37-48. https://doi.org/10.2165/00023210-200418010-00004.
- Shash D, Kurth T, Bertrand M, Dufouil C, Barberger-Gateau P, Berr C, et al. Benzodiazepine, psychotropic medication, and dementia: a population-based cohort study. Alzheimers Dement 2016;12:604-13. https://doi.org/10.1016/j.jalz.2015.10.006.
- Zhang Y, Zhou XH, Meranus DH, Wang L, Kukull WA. Benzodiazepine use and cognitive decline in elderly with normal cognition. Alzheimer Dis Assoc Disord 2016;30:113-17. https://doi.org/10.1097/WAD.0000000000000099.
- Biétry FA, Pfeil AM, Reich O, Schwenkglenks M, Meier CR. Benzodiazepine use and risk of developing Alzheimer’s disease: a case–control study based on Swiss claims data. CNS Drugs 2017;31:245-51. https://doi.org/10.1007/s40263-016-0404-x.
- Islam MM, Iqbal U, Walther B, Atique S, Dubey NK, Nguyen PA, et al. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology 2016;47:181-91. https://doi.org/10.1159/000454881.
- Zhong G, Wang Y, Zhang Y, Zhao Y. Association between benzodiazepine use and dementia: a meta-analysis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0127836.
- Chen PL, Lee WJ, Sun WZ, Oyang YJ, Fuh JL. Risk of dementia in patients with insomnia and long-term use of hypnotics: a population-based retrospective cohort study. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0049113.
- Shih HI, Lin CC, Tu YF, Chang CM, Hsu HC, Chi CH, et al. An increased risk of reversible dementia may occur after zolpidem derivative use in the elderly population: a population-based case-control study. Medicine 2015;94. https://doi.org/10.1097/MD.0000000000000809.
- Defrancesco M, Marksteiner J, Fleischhacker WW, Blasko I. Use of benzodiazepines in alzheimer’s disease: a systematic review of literature. Int J Neuropsychopharmacol 2015;18. https://doi.org/10.1093/ijnp/pyv055.
- Zammit GK, Weiner J, Damato N, Sillup GP, McMillan CA. Quality of life in people with insomnia. Sleep 1999;22:379-85.
- Kyle SD, Espie CA, Morgan K. ‘. . . Not just a minor thing, it is something major, which stops you from functioning daily’: quality of life and daytime functioning in insomnia. Behav Sleep Med 2010;8:123-40. https://doi.org/10.1080/15402002.2010.487450.
- Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract 2002;51:229-35.
- Schubert CR, Cruickshanks KJ, Dalton DS, Klein BE, Klein R, Nondahl DM. Prevalence of sleep problems and quality of life in an older population. Sleep 2002;25:889-93.
- Stein MB, Barrett-Connor E. Quality of life in older adults receiving medications for anxiety, depression, or insomnia: findings from a community-based study. Am J Geriatr Psychiatry 2002;10:568-74. https://doi.org/10.1097/00019442-200209000-00010.
- Byles JE, Mishra GD, Harris MA, Nair K. The problems of sleep for older women: changes in health outcomes. Age Ageing 2003;32:154-63. https://doi.org/10.1093/ageing/32.2.154.
- Johnson LC, Chernik DA. Sedative-hypnotics and human performance. Psychopharmacology 1982;76:101-13. https://doi.org/10.1007/BF00435262.
- Kripke DF. Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit. F1000Res 2016;5. https://doi.org/10.12688/f1000research.8729.3.
- Hamblin JE. Insomnia: an ignored health problem. Prim Care 2007;34:659-74. https://doi.org/10.1016/j.pop.2007.05.009.
- Tamiya H, Yasunaga H, Matusi H, Fushimi K, Ogawa S, Akishita M. Hypnotics and the occurrence of bone fractures in hospitalized dementia patients: a matched case–control study using a national inpatient database. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0129366.
- Bianchetti A, Scuratti A, Zanetti O, Binetti G, Frisoni GB, Magni E, et al. Predictors of mortality and institutionalization in Alzheimer disease patients 1 year after discharge from an Alzheimer dementia unit. Dementia 1995;6:108-12. https://doi.org/10.1159/000106930.
- Health and Social Care Information Centre . Prescription Cost Analysis England – 2016 2016.
- Cohen D. Opioid prescriptions in England doubled over 12 years, study shows. BMJ 2017;358. https://doi.org/10.1136/bmj.j4249.
- Cartagena Farias JPL, McManus S, Strang J, Hickman M, Reed K, Smith N. Prescribing Patterns in Dependence Forming Medicines. London: Public Health Research Consortium; 2017.
- Høiseth G, Kristiansen KM, Kvande K, Tanum L, Lorentzen B, Refsum H. Benzodiazepines in geriatric psychiatry: what doctors report and what patients actually use. Drugs Aging 2013;30:113-18. https://doi.org/10.1007/s40266-012-0045-9.
- Montastruc F, Gardette V, Cantet C, Piau A, Lapeyre-Mestre M, Vellas B, et al. Potentially inappropriate medication use among patients with Alzheimer disease in the REAL.FR cohort: be aware of atropinic and benzodiazepine drugs!. Eur J Clin Pharmacol 2013;69:1589-97. https://doi.org/10.1007/s00228-013-1506-8.
- Kinnunen KM, Vikhanova A, Livingston G. The management of sleep disorders in dementia: an update. Curr Opin Psychiatry 2017;30:491-7. https://doi.org/10.1097/YCO.0000000000000370.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK primary care data resource. Ther Adv Drug Saf 2012;3:89-9. https://doi.org/10.1177/2042098611435911.
- Chisholm J. The Read clinical classification. BMJ 1990;300. https://doi.org/10.1136/bmj.300.6732.1092.
- GOV.UK . English Indices of Deprivation 2015 n.d. www.gov.uk/government/statistics/english-indices-of-deprivation-2015 (accessed 24 May 2018).
- Ehrenstein V, Petersen I, Smeeth L, Jick SS, Benchimol EI, Ludvigsson JF, et al. Helping everyone do better: a call for validation studies of routinely recorded health data. Clin Epidemiol 2016;8:49-51. https://doi.org/10.2147/CLEP.S104448.
- National Institute for Health and Care Excellence . Zopiclone n.d. https://bnf.nice.org.uk/drug/zopiclone.html (accessed 6 December 2019).
- Joint Formulary Committee . British National Formulary 2015.
- Gribbin J, Hubbard R, Smith C, Gladman J, Lewis S. Incidence and mortality of falls amongst older people in primary care in the United Kingdom. QJM 2009;102:477-83. https://doi.org/10.1093/qjmed/hcp064.
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515-26. https://doi.org/10.1093/biomet/81.3.515.
- Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492-5. https://doi.org/10.1136/bmj.319.7223.1492.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Soc Series B Stat Methodol 1995;57:289-300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.
- Bonferroni CE. Teoria statistica delle classi e calcolo delle probabilità. Pubblicazioni Del R Istituto Superiore Di Scienze Economiche E Commerciali Di Firenze 1936;8:3-62.
- Marston L, Carpenter JR, Walters KR, Morris RW, Nazareth I, Petersen I. Issues in multiple imputation of missing data for large general practice clinical databases. Pharmacoepidemiol Drug Saf 2010;19:618-26. https://doi.org/10.1002/pds.1934.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. https://doi.org/10.1136/bmj.b2393.
- Whitaker R, Fossey J, Ballard C, Orrell M, Moniz-Cook E, Woods RT, et al. Improving Well-being and Health for People with Dementia (WHELD): study protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-284.
- Howard RJ, Juszczak E, Ballard CG, Bentham P, Brown RG, Bullock R, et al. Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med 2007;357:1382-92. https://doi.org/10.1056/NEJMoa066583.
- Fox C, Crugel M, Maidment I, Auestad BH, Coulton S, Treloar A, et al. Efficacy of memantine for agitation in Alzheimer’s dementia: a randomised double-blind placebo controlled trial. PLoS ONE 2012;7. https://doi.org/10.1371/journal.pone.0035185.
- Schneider LS1, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11:S22-32. https://doi.org/10.1097/00002093-199700112-00004.
- Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med 2012;366:893-90. https://doi.org/10.1056/NEJMoa1106668.
- Røen I, Selbæk G, Kirkevold Ø, Engedal K, Testad I, Bergh S. Resourse Use and Disease Couse in dementia – Nursing Home (REDIC-NH), a longitudinal cohort study; design and patient characteristics at admission to Norwegian nursing homes. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2289-x.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. https://doi.org/10.1016/0022-3956(75)90026-6.
- Schmitt FA, Saxton J, Ferris SH, Mackell J, Sun Y. Evaluation of an 8-item Severe Impairment Battery (SIB-8) vs. the full SIB in moderate to severe Alzheimer’s disease patients participating in a donepezil study. Int J Clin Pract 2013;67:1050-6. https://doi.org/10.1111/ijcp.12188.
- O’Connor DW, Pollitt PA, Hyde JB, Fellows JL, Miller ND, Brook CP, et al. The reliability and validity of the Mini-Mental State in a British community survey. J Psychiatr Res 1989;23:87-96. https://doi.org/10.1016/0022-3956(89)90021-6.
- Pezzotti P, Scalmana S, Mastromattei A, Di Lallo D. Progetto Alzheimer Working Group . The accuracy of the MMSE in detecting cognitive impairment when administered by general practitioners: a prospective observational study. BMC Fam Pract 2008;9. https://doi.org/10.1186/1471-2296-9-29.
- Magni E, Binetti G, Bianchetti A, Rozzini R, Trabucchi M. Mini-Mental State Examination: a normative study in Italian elderly population. Eur J Neurol 1996;3:198-202. https://doi.org/10.1111/j.1468-1331.1996.tb00423.x.
- Fabrigoule C, Lechevallier N, Crasborn L, Dartigues JF, Orgogozo JM. Inter-rater reliability of scales and tests used to measure mild cognitive impairment by general practitioners and psychologists. Curr Med Res Opin 2003;19:603-8. https://doi.org/10.1185/030079903125002298.
- Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 2009;43:411-31. https://doi.org/10.1016/j.jpsychires.2008.04.014.
- Schmitt FA, Ashford W, Ernesto C, Saxton J, Schneider LS, Clark CM, et al. The severe impairment battery: concurrent validity and the assessment of longitudinal change in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11:51-6. https://doi.org/10.1097/00002093-199700112-00008.
- Ahn IS, Kim JH, Ku HM, Saxton J, Kim DK. Reliability and validity of the severe impairment battery (SIB) in Korean dementia patients. J Korean Med Sci 2006;21:506-17. https://doi.org/10.3346/jkms.2006.21.3.506.
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-14. https://doi.org/10.1212/wnl.43.11.2412-a.
- O’Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores. Arch Neurol 2008;65:1091-5. https://doi.org/10.1001/archneur.65.8.1091.
- Fillenbaum GG, Peterson B, Morris JC. Estimating the validity of the clinical Dementia Rating Scale: the CERAD experience consortium to establish a registry for Alzheimer’s disease. Aging 1996;8:379-85. https://doi.org/10.1007/BF03339599.
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 1997;9:173-6. https://doi.org/10.1017/S1041610297004870.
- Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology 1997;48:1508-10. https://doi.org/10.1212/wnl.48.6.1508.
- Weiner MF, Martin-Cook K, Svetlik DA, Saine K, Foster B, Fontaine CS. The quality of life in late-stage dementia (QUALID) scale. J Am Med Dir Assoc 2000;1:114-16. https://doi.org/10.1037/t00432-000.
- Williams A. Euroqol – a new facility for the measurement of health-related quality-of-life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Szende A, Oppe M, Devlin N. EQ-5D Value Sets: Inventory, Comparative Review and User Guide. Netherlands: Springer; 2007.
- Ramos-Goni JM, Rivero-Arias O. EQ5D: a command to calculate index values for the EQ-5D quality-of-life instrument. Stata J 2011;11:120-5. https://doi.org/10.1177/1536867X1101100108.
- Hounsome N, Orrell M, Edwards RT. EQ-5D as a quality of life measure in people with dementia and their carers: evidence and key issues. Value Health 2011;14:390-9. https://doi.org/10.1016/j.jval.2010.08.002.
- Lawton MP, Brody EM. Physical Self-Maintenance Scale (PSMS) – original observer-rated version. Psychopharmacol Bull 1988;24:793-4.
- Wood S, Cummings JL, Hsu MA, Barclay T, Wheatley MV, Yarema KT, et al. The use of the neuropsychiatric inventory in nursing home residents. Characterization and measurement. Am J Geriatr Psychiatry 2000;8:75-83. https://doi.org/10.1097/00019442-200002000-00010.
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry 1988;23:271-84. https://doi.org/10.1016/0006-3223(88)90038-8.
- Swami S, Cohen RA, Kairalla JA, Manini TM. Anticholinergic drug use and risk to cognitive performance in older adults with questionable cognitive impairment: a cross-sectional analysis. Drugs Aging 2016;33:809-18. https://doi.org/10.1007/s40266-016-0400-3.
- Fals-Stewart W. An interrater reliability study of the trail making test (parts a and b). Percept Mot Skills 1992;74:39-42. https://doi.org/10.2466/pms.1992.74.1.39.
- Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS). Clin Gerontol 1986;5:165-73. https://doi.org/10.1300/J018v05n01_09.
- Ballard C, Corbett A, Orrell M, Williams G, Moniz-Cook E, Romeo R, et al. Impact of person-centred care training and person-centred activities on quality of life, agitation, and antipsychotic use in people with dementia living in nursing homes: a cluster-randomised controlled trial. PLOS Med 2018;15. https://doi.org/10.1371/journal.pmed.1002500.
- Great Britain . Mental Capacity Act 2005 2005.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. https://doi.org/10.1136/bmj.i4919.
- Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915-20. https://doi.org/10.1093/aje/kwg231.
- Schneeweiss S, Patrick AR, Sturmer T, Brookhart MA, Avorn J, Maclure M, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care 2007;45:S131-42. https://doi.org/10.1097/MLR.0b013e318070c08e.
- Gill SS, Bronskill SE, Mamdani M, Sykora K, Li P, Shulman KI, et al. Representation of patients with dementia in clinical trials of donepezil. Can J Clin Pharmacol 2004;11:e274-85.
- Imfeld P, Brauchli Pernus YB, Jick SS, Meier CR. Epidemiology, co-morbidities, and medication use of patients with Alzheimer’s disease or vascular dementia in the UK. J Alzheimers Dis 2013;35:565-73. https://doi.org/10.3233/JAD-121819.
- Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract 2010;60:e128-36. https://doi.org/10.3399/bjgp10X483562.
- Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4-14. https://doi.org/10.1111/j.1365-2125.2009.03537.x.
- Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med 2007;167:935-43. https://doi.org/10.1001/archinte.167.9.935.
- Van Staa T-PLA. The quality of information recorded on a UK database of primary care records: a study of hospitalizations due to hypoglycemia and other conditions. Pharmacoepidemiol Drug Saf 1994;3:15-21. https://doi.org/10.1002/pds.2630030106.
- Ruigómez A, Martín-Merino E, Rodríguez LA. Validation of ischemic cerebrovascular diagnoses in the health improvement network (THIN). Pharmacoepidemiol Drug Saf 2010;19:579-85. https://doi.org/10.1002/pds.1919.
- Gibbs RG, Newson R, Lawrenson R, Greenhalgh RM, Davies AH. Diagnosis and initial management of stroke and transient ischemic attack across UK health regions from 1992 to 1996: experience of a national primary care database. Stroke 2001;32:1085-90. https://doi.org/10.1161/01.STR.32.5.1085.
- Derby LE, Myers MW, Jick H. Use of dexfenfluramine, fenfluramine and phentermine and the risk of stroke. Br J Clin Pharmacol 1999;47:565-9. https://doi.org/10.1046/j.1365-2125.1999.00928.x.
- Millett ER, Quint JK, De Stavola BL, Smeeth L, Thomas SL. Improved incidence estimates from linked vs. stand-alone electronic health records. J Clin Epidemiol 2016;75:66-9. https://doi.org/10.1016/j.jclinepi.2016.01.005.
- Friedman SM, Menzies IB, Bukata SV, Mendelson DA, Kates SL. Dementia and hip fractures: development of a pathogenic framework for understanding and studying risk. Geriatr Orthop Surg Rehabil 2010;1:52-6. https://doi.org/10.1177/2151458510389463.
- Williamson T, Ravani P. Marginal structural models in clinical research: when and how to use them?. Nephrol Dial Transplant 2017;32:ii84-ii90. https://doi.org/10.1093/ndt/gfw341.
- Park SM, Ryu J, Lee DR, Shin D, Yun JM, Lee J. Zolpidem use and risk of fractures: a systematic review and meta-analysis. Osteoporos Int 2016;27:2935-44. https://doi.org/10.1007/s00198-016-3605-8.
- Gustafsson M, Karlsson S, Gustafson Y, Lövheim H. Psychotropic drug use among people with dementia – a six-month follow-up study. BMC Pharmacol Toxicol 2013;14. https://doi.org/10.1186/2050-6511-14-56.
- Tang YJ, Ho SY, Chu FY, Chen HA, Yin YJ, Lee HC, et al. Is zolpidem associated with increased risk of fractures in the elderly with sleep disorders? A nationwide case cross-over study in Taiwan. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0146030.
- Tolppanen AM, Taipale H, Tanskanen A, Tiihonen J, Hartikainen S. Comparison of predictors of hip fracture and mortality after hip fracture in community-dwellers with and without Alzheimer’s disease – exposure-matched cohort study. BMC Geriatr 2016;16. https://doi.org/10.1186/s12877-016-0383-2.
- Hutchinson MA, Smith PF, Darlington CL. The behavioural and neuronal effects of the chronic administration of benzodiazepine anxiolytic and hypnotic drugs. Prog Neurobiol 1996;49:73-97. https://doi.org/10.1016/0301-0082(96)00011-1.
- Lanctôt KL, Herrmann N, Mazzotta P, Khan LR, Ingber N. GABAergic function in Alzheimer’s disease: evidence for dysfunction and potential as a therapeutic target for the treatment of behavioural and psychological symptoms of dementia. Can J Psychiatry 2004;49:439-53. https://doi.org/10.1177/070674370404900705.
- Moride Y, Abenhaim L, Yola M, Lucein A. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol 1994;47:731-7. https://doi.org/10.1016/0895-4356(94)90170-8.
- Sylvestre MP, Abrahamowicz M, Čapek R, Tamblyn R. Assessing the cumulative effects of exposure to selected benzodiazepines on the risk of fall-related injuries in the elderly. Int Psychogeriatr 2012;24:577-86. https://doi.org/10.1017/S1041610211002031.
- Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf 2010;19:1248-55. https://doi.org/10.1002/pds.2031.
- Khong TP, de Vries F, Goldenberg JS, Klungel OH, Robinson NJ, Ibáñez L, et al. Potential impact of benzodiazepine use on the rate of hip fractures in five large European countries and the United States. Calcif Tissue Int 2012;91:24-31. https://doi.org/10.1007/s00223-012-9603-8.
- Brandt J, Leong C. Benzodiazepines and Z-drugs: an updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D 2017;17:493-507. https://doi.org/10.1007/s40268-017-0207-7.
- Brown MJ, Mezuk B. Brains, bones, and aging: psychotropic medications and bone health among older adults. Curr Osteoporos Rep 2012;10:303-11. https://doi.org/10.1007/s11914-012-0121-4.
- Yen CM, Kuo CL, Lin MC, Lee CF, Lin KY, Lin CL, et al. Sleep disorders increase the risk of osteoporosis: a nationwide population-based cohort study. Sleep Med 2014;15:1339-44. https://doi.org/10.1016/j.sleep.2014.07.005.
- Swanson CM, Shea SA, Stone KL, Cauley JA, Rosen CJ, Redline S, et al. Obstructive sleep apnea and metabolic bone disease: insights into the relationship between bone and sleep. J Bone Miner Res 2015;30:199-211. https://doi.org/10.1002/jbmr.2446.
- Frey DJ, Ortega JD, Wiseman C, Farley CT, Wright KP. Influence of zolpidem and sleep inertia on balance and cognition during nighttime awakening: a randomized placebo-controlled trial. J Am Geriatr Soc 2011;59:73-81. https://doi.org/10.1111/j.1532-5415.2010.03229.x.
- Sterke CS, Verhagen AP, van Beeck EF, van der Cammen TJ. The influence of drug use on fall incidents among nursing home residents: a systematic review. Int Psychogeriatr 2008;20:890-91. https://doi.org/10.1017/S104161020800714X.
- Diem SJ, Ewing SK, Stone KL, Ancoli-Israel S, Redline S, Ensrud KE. Osteoporotic Fractures in Men (MrOS) Study Group . Use of non-benzodiazepine sedative hypnotics and risk of falls in older men. J Gerontol Geriatr Res 2014;3. https://doi.org/10.4172/2167-7182.1000158.
- Hayes WC, Myers ER, Robinovitch SN, Van Den Kroonenberg A, Courtney AC, McMahon TA. Etiology and prevention of age-related hip fractures. Bone 1996;18:77-86. https://doi.org/10.1016/8756-3282(95)00383-5.
- Weich S, Pearce HL, Croft P, Singh S, Crome I, Bashford J, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ 2014;348. https://doi.org/10.1136/bmj.g1996.
- Patorno E, Glynn RJ, Levin R, Lee MP, Huybrechts KF. Benzodiazepines and risk of all cause mortality in adults: cohort study. BMJ 2017;358. https://doi.org/10.1136/bmj.j2941.
- Haentjens P, Magaziner J, Colón-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 2010;152:380-90. https://doi.org/10.7326/0003-4819-152-6-201003160-00008.
- Wang MT, Wang YH, Chang HA, Tsai CL, Yang YS, Lin CW, et al. Benzodiazepine and Z-drug use and risk of pneumonia in patients with chronic kidney disease: a population-based nested case-control study. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0179472.
- Gagliardi GS, Shah AP, Goldstein M, Denua-Rivera S, Doghramji K, Cohen S, et al. Effect of zolpidem on the sleep arousal response to nocturnal esophageal acid exposure. Clin Gastroenterol Hepatol 2009;7:948-52. https://doi.org/10.1016/j.cgh.2009.04.026.
- Hermann DM, Bassetti CL. Role of sleep-disordered breathing and sleep-wake disturbances for stroke and stroke recovery. Neurology 2016;87:1407-16. https://doi.org/10.1212/WNL.0000000000003037.
- Chung WS, Chen YF, Lin CL, Chang SN, Hsu WH, Kao CH. Sleep disorders increase the risk of venous thromboembolism in individuals without sleep apnea: a nationwide population-based cohort study in Taiwan. Sleep Med 2015;16:168-72. https://doi.org/10.1016/j.sleep.2014.07.031.
- Melton LJ, Beard CM, Kokmen E, Atkinson EJ, O’Fallon WM. Fracture risk in patients with Alzheimer’s disease. J Am Geriatr Soc 1994;42:614-19. https://doi.org/10.1111/j.1532-5415.1994.tb06859.x.
- Sato Y, Kanoko T, Satoh K, Iwamoto J. Risk factors for hip fracture among elderly patients with Alzheimer’s disease. J Neurol Sci 2004;223:107-12. https://doi.org/10.1016/j.jns.2004.03.033.
- National Institute for Health and Care Excellence . Hip Fracture: Management 2011. www.nice.org.uk/guidance/cg124 (accessed 31 March 2019).
- Leal J, Gray AM, Prieto-Alhambra D, Arden NK, Cooper C, Javaid MK, et al. REFReSH study group . Impact of hip fracture on hospital care costs: a population-based study. Osteoporos Int 2016;27:549-58. https://doi.org/10.1007/s00198-015-3277-9.
- NHS Digital . Record Dementia Diagnoses, May 2018 2018. https://digital.nhs.uk/data-and-information/publications/statistical/recorded-dementia-diagnoses/may-2018 (accessed 31 March 2019).
- Johnson CF, Frei C, Downes N, McTaggart SA, Akram G. Benzodiazepine and z-hypnotic prescribing for older people in primary care: a cross-sectional population-based study. Br J Gen Pract 2016;66:e410-5. https://doi.org/10.3399/bjgp16X685213.
- Aston L, Hilton A, Moutela T, Shaw R, Maidment I. Exploring the evidence base for how people with dementia and their informal carers manage their medication in the community: a mixed studies review. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0638-6.
- Maidment ID, Aston L, Moutela T, Fox CG, Hilton A. A qualitative study exploring medication management in people with dementia living in the community and the potential role of the community pharmacist. Health Expect 2017;20:929-42. https://doi.org/10.1111/hex.12534.
- Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep 1999;22:1134-56. https://doi.org/10.1093/sleep/22.8.1134.
- Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev 2014;2. https://doi.org/10.1002/14651858.CD003946.pub4.
- Connell BR, Sanford JA, Lewis D. Therapeutic effects of an outdoor activity program on nursing home residents with dementia. J Hous Elderly 2007;21:194-209. https://doi.org/10.1300/J081v21n03_10.
- Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc 2005;53:803-10. https://doi.org/10.1111/j.1532-5415.2005.53251.x.
- Brown CA, Berry R, Tan MC, Khoshia A, Turlapati L, Swedlove F. A critique of the evidence base for non-pharmacological sleep interventions for persons with dementia. Dementia 2013;12:210-37. https://doi.org/10.1177/1471301211426909.
- Livingston G, Barber JA, Kinnunen KM, Webster L, Kyle SD, Cooper C, et al. DREAMS-START (Dementia RElAted Manual for Sleep; STrAtegies for RelaTives) for people with dementia and sleep disturbances: a single-blind feasibility and acceptability randomized controlled trial. Int Psychogeriatr 2019;31:251-65. https://doi.org/10.1017/S1041610218000753.
- Zhang F, Zhong R, Li S, Chang RC, Le W. The missing link between sleep disorders and age-related dementia: recent evidence and plausible mechanisms. J Neural Transm 2017;124:559-68. https://doi.org/10.1007/s00702-017-1696-9.
- Proserpio P, Arnaldi D, Nobili F, Nobili L. Integrating sleep and Alzheimer’s disease pathophysiology: hints for sleep disorders management. J Alzheimers Dis 2018;63:871-86. https://doi.org/10.3233/JAD-180041.
- Jones S, Howard L, Thornicroft G. ‘Diagnostic overshadowing’: worse physical health care for people with mental illness. Acta Psychiatr Scand 2008;118:169-71. https://doi.org/10.1111/j.1600-0447.2008.01211.x.
- McDonald L, Schultze A, Carroll R, Ramagopalan SV. Performing studies using the UK Clinical Practice Research Datalink: to link or not to link?. Eur J Epidemiol 2018;33:601-5. https://doi.org/10.1007/s10654-018-0389-5.
Appendix 1 Coding used to identify dementia and sleep disturbance diagnosis, and outcomes in the primary care study
The dementia code list is the Quality and Outcomes Framework definition, excluding alcohol-induced dementia, and is very similar to lists other validated studies have used (Table 26). 165
| MedCode | Read code | Read term |
|---|---|---|
| 33707 | E00..00 | Senile and presenile organic psychotic conditions |
| 1916 | E00..11 | Senile dementia |
| 1350 | E00..12 | Senile/presenile dementia |
| 7323 | E000.00 | Uncomplicated senile dementia |
| 15165 | E001.00 | Presenile dementia |
| 42602 | E001000 | Uncomplicated presenile dementia |
| 49513 | E001100 | Presenile dementia with delirium |
| 30032 | E001200 | Presenile dementia with paranoia |
| 27677 | E001300 | Presenile dementia with depression |
| 38438 | E001z00 | Presenile dementia NOS |
| 44674 | E002.00 | Senile dementia with depressive or paranoid features |
| 18386 | E002000 | Senile dementia with paranoia |
| 21887 | E002100 | Senile dementia with depression |
| 41089 | E002z00 | Senile dementia with depressive or paranoid features NOS |
| 37015 | E003.00 | Senile dementia with delirium |
| 19477 | E004.00 | Arteriosclerotic dementia |
| 8634 | E004.11 | Multi infarct dementia |
| 43089 | E004000 | Uncomplicated arteriosclerotic dementia |
| 56912 | E004100 | Arteriosclerotic dementia with delirium |
| 55467 | E004200 | Arteriosclerotic dementia with paranoia |
| 43292 | E004300 | Arteriosclerotic dementia with depression |
| 42279 | E004z00 | Arteriosclerotic dementia NOS |
| 15249 | E00y.00 | Other senile and presenile organic psychoses |
| 51494 | E00y.11 | Presbyophrenic psychosis |
| 2882 | E00z.00 | Senile or presenile psychoses NOS |
| 62132 | E02y100 | Drug-induced dementia |
| 25386 | E041.00 | Dementia in conditions EC |
| 7664 | Eu00.00 | [X]Dementia in Alzheimer’s disease |
| 49263 | Eu00000 | [X]Dementia in Alzheimer’s disease with early onset |
| 25704 | Eu00011 | [X]Presenile dementia, Alzheimer’s type |
| 60059 | Eu00012 | [X]Primary degen dementia, Alzheimer’s type, presenile onset |
| 61528 | Eu00013 | [X]Alzheimer’s disease type 2 |
| 38678 | Eu00100 | [X]Dementia in Alzheimer’s disease with late onset |
| 46762 | Eu00111 | [X]Alzheimer’s disease type 1 |
| 11379 | Eu00112 | [X]Senile dementia, Alzheimer’s type |
| 43346 | Eu00113 | [X]Primary degen dementia of Alzheimer’s type, senile onset |
| 30706 | Eu00200 | [X]Dementia in Alzheimer’s disease, atypical or mixed type |
| 29386 | Eu00z00 | [X]Dementia in Alzheimer’s disease, unspecified |
| 8195 | Eu00z11 | [X]Alzheimer’s dementia unspecified |
| 6578 | Eu01.00 | [X]Vascular dementia |
| 9565 | Eu01.11 | [X]Arteriosclerotic dementia |
| 46488 | Eu01000 | [X]Vascular dementia of acute onset |
| 11175 | Eu01100 | [X]Multi-infarct dementia |
| 55838 | Eu01111 | [X]Predominantly cortical dementia |
| 8934 | Eu01200 | [X]Subcortical vascular dementia |
| 31016 | Eu01300 | [X]Mixed cortical and subcortical vascular dementia |
| 55313 | Eu01y00 | [X]Other vascular dementia |
| 19393 | Eu01z00 | [X]Vascular dementia, unspecified |
| 12621 | Eu02.00 | [X]Dementia in other diseases classified elsewhere |
| 28402 | Eu02000 | [X]Dementia in Pick’s disease |
| 54106 | Eu02100 | [X]Dementia in Creutzfeldt-Jakob disease |
| 37014 | Eu02200 | [X]Dementia in Huntington’s disease |
| 9509 | Eu02300 | [X]Dementia in Parkinson’s disease |
| 26270 | Eu02500 | [X]Lewy body dementia |
| 64267 | Eu02y00 | [X]Dementia in other specified diseases classified elsewhere |
| 4693 | Eu02z00 | [X]Unspecified dementia |
| 48501 | Eu02z11 | [X]Presenile dementia NOS |
| 47619 | Eu02z12 | [X]Presenile psychosis NOS |
| 34944 | Eu02z13 | [X]Primary degenerative dementia NOS |
| 4357 | Eu02z14 | [X]Senile dementia NOS |
| 27935 | Eu02z15 | [X]Senile psychosis NOS |
| 27759 | Eu02z16 | [X]Senile dementia, depressed or paranoid type |
| 53446 | Eu04100 | [X]Delirium superimposed on dementia |
| 1917 | F110.00 | Alzheimer’s disease |
| 16797 | F110000 | Alzheimer’s disease with early onset |
| 32057 | F110100 | Alzheimer’s disease with late onset |
| 11136 | F111.00 | Pick’s disease |
| 29512 | F112.00 | Senile degeneration of brain |
| 7572 | F116.00 | Lewy body disease |
| 59122 | Fyu3000 | [X]Other Alzheimer’s disease |
Sleep disturbance
The following diagnosis and referral codes are classified according to being ‘probable’ or ‘possible’ evidence of a sleep disorder in a dementia patient (Table 27).
| MedCode | Read code | Read term | Possible or probable sleep disturbance |
|---|---|---|---|
| 21305 | 1B1B.00 | Cannot sleep – insomnia | Probable |
| 4537 | 1B1B.11 | C/O – insomnia | Probable |
| 3523 | 1B1B000 | Initial insomnia | Probable |
| 5675 | 1B1B100 | Middle insomnia | Probable |
| 4597 | 1B1B200 | Late insomnia | Probable |
| 7725 | 1B1Q.00 | Poor sleep pattern | Probable |
| 30385 | 1BN1.00 | Wanders at night | Probable |
| 94508 | 1BN2.00 | Wanders during the day and at night | Probable |
| 42847 | 1BX0.00 | Delayed onset of sleep | Probable |
| 60806 | 1BX2.00 | Sleeping pattern | Probable |
| 60974 | 1BX9.00 | Light sleep | Probable |
| 96037 | 8G9B.00 | Sleep hygiene behaviour education | Probable |
| 95887 | 8HTn.00 | Referral to sleep clinic | Probable |
| 12072 | 8Q0..00 | Sleep management | Probable |
| 107666 | 9Ngt.00 | On melatonin for sleep disorder | Probable |
| 7819 | E274.00 | Non-organic sleep disorders | Probable |
| 26546 | E274.12 | Insomnia due to nonorganic sleep disorder | Probable |
| 15515 | E274100 | Transient insomnia | Probable |
| 4023 | E274111 | Insomnia NOS | Probable |
| 16115 | E274200 | Persistent insomnia | Probable |
| 39990 | E274B00 | Repeated rapid eye movement sleep interruptions | Probable |
| 55179 | E274C00 | Other sleep stage or arousal dysfunction | Probable |
| 19514 | E274D11 | Restless sleep | Probable |
| 32987 | E274E00 | ‘Short-sleeper’ | Probable |
| 36745 | E274F00 | Inversion of sleep rhythm | Probable |
| 30626 | Eu51000 | [X]Nonorganic insomnia | Probable |
| 23923 | Eu51200 | [X]Nonorganic disorder of the sleep-wake schedule | Probable |
| 42753 | Eu51211 | [X]Psychogenic inversion of circadian rhythm | Probable |
| 101729 | Eu51213 | [X]Psychogenic inversion of sleep rhythm | Probable |
| 5921 | Fy00.00 | Disorders of initiating and maintaining sleep | Probable |
| 8997 | Fy02.00 | Disorders of the sleep-wake schedule | Probable |
| 8084 | R005.00 | [D]Sleep disturbances | Probable |
| 10349 | R005.11 | [D]Insomnia – symptom | Probable |
| 31236 | R005.12 | [D]Sleep rhythm problems | Probable |
| 750 | R005200 | [D]Insomnia NOS | Probable |
| 16447 | R005500 | [D]Sleep rhythm inversion | Probable |
| 15732 | R005600 | [D]Sleep rhythm irregular | Probable |
| 58911 | R005700 | [D]Sleep-wake rhythm non-24-hour cycle | Probable |
| 54458 | R005800 | [D]Sleep dysfunction with sleep stage disturbance | Probable |
| 22081 | Z1M..00 | Sleep and rest interventions | Probable |
| 101913 | Z1M1.00 | Disturbing sleep | Probable |
| 51397 | ZV1B100 | [V]Personal history of unhealthy sleep-wake schedule | Probable |
| 43397 | Z7CCC00 | Found wandering the streets | Possible |
| 26009 | Z7CCB00 | Wandering | Possible |
| 15407 | R005z00 | [D]Sleep dysfunction NOS | Possible |
| 41737 | R005900 | [D]Sleep dysfunction with arousal disturbance | Possible |
| 1244 | R005000 | [D]Sleep disturbance, unspecified | Possible |
| 15283 | K5A2100 | Menopausal sleeplessness | Possible |
| 53912 | Fyu5800 | [X]Other sleep disorders | Possible |
| 49601 | Fy05.00 | Nocturnal sleep-related eating disorder | Possible |
| 2329 | Fy0..00 | Sleep disorders | Possible |
| 21032 | Eu51z11 | [X]Emotional sleep disorder NOS | Possible |
| 22819 | Eu51z00 | [X]Nonorganic sleep disorder, unspecified | Possible |
| 62925 | Eu51y00 | [X]Other nonorganic sleep disorders | Possible |
| 17687 | Eu51511 | [X]Dream anxiety disorder | Possible |
| 6943 | Eu51300 | [X]Sleepwalking | Possible |
| 24894 | Eu51.00 | [X]Nonorganic sleep disorders | Possible |
| 27649 | E274z00 | Non-organic sleep disorder NOS | Possible |
| 8519 | E274y11 | Dreams | Possible |
| 43098 | E274y00 | Other non-organic sleep disorder | Possible |
| 48783 | E274D00 | Repetitive intrusions of sleep | Possible |
| 47745 | E274600 | Shifting sleep-work schedule | Possible |
| 7409 | E274500 | Jet lag syndrome | Possible |
| 36992 | E274300 | Transient hypersomnia | Possible |
| 16434 | E274000 | Unspecified non-organic sleep disorder | Possible |
| 93615 | 9Nk0.00 | Seen in sleep clinic | Possible |
| 103449 | 1F9C.00 | Eats at night | Possible |
| 9090 | 1BN..00 | Wandering | Possible |
| 8123 | 1B1O.00 | Restless | Possible |
| 56809 | 7065800 | Sleep studies | Possible |
| 4559 | 3148 | Sleep studies | Possible |
Exclusions: severe mental illness or Down syndrome
Patients will be excluded if they are diagnosed with a severe mental illness or Down syndrome before their dementia diagnosis (Table 28).
| MedCode | Read code | Read term |
|---|---|---|
| 1543 | PJ0..00 | Down’s syndrome – trisomy 21 |
| 10759 | PJ0z.00 | Down’s syndrome NOS |
| 18415 | PJ0..12 | Trisomy 21 |
| 23489 | PJ0..11 | Mongolism |
| 32010 | PJ01.00 | Trisomy 21, mosaicism |
| 42701 | PJ00.00 | Trisomy 21, meiotic nondisjunction |
| 61499 | PJ02.00 | Trisomy 21, translocation |
| 61627 | PJ0z.11 | Trisomy 21 NOS |
| 101309 | PJ02.11 | Partial trisomy 21 in Down’s syndrome |
| 107919 | PJ01.11 | Trisomy 21, mitotic nondisjunction |
| 15958 | E1...00 | Non-organic psychoses |
| 854 | E10..00 | Schizophrenic disorders |
| 32222 | E100.00 | Simple schizophrenia |
| 73295 | E100.11 | Schizophrenia simplex |
| 15733 | E100000 | Unspecified schizophrenia |
| 3984 | E100200 | Chronic schizophrenic |
| 44498 | E100400 | Acute exacerbation of chronic schizophrenia |
| 58687 | E100500 | Schizophrenia in remission |
| 53625 | E100z00 | Simple schizophrenia NOS |
| 25546 | E102.00 | Catatonic schizophrenia |
| 102427 | E102500 | Catatonic schizophrenia in remission |
| 1494 | E103.00 | Paranoid schizophrenia |
| 33383 | E103000 | Unspecified paranoid schizophrenia |
| 31362 | E103200 | Chronic paranoid schizophrenia |
| 53032 | E103400 | Acute exacerbation of chronic paranoid schizophrenia |
| 36172 | E103500 | Paranoid schizophrenia in remission |
| 9281 | E103z00 | Paranoid schizophrenia NOS |
| 576 | E104.00 | Acute schizophrenic episode |
| 96883 | E105500 | Latent schizophrenia in remission |
| 38063 | E106.00 | Residual schizophrenia |
| 2117 | E107.00 | Schizo-affective schizophrenia |
| 58862 | E107000 | Unspecified schizo-affective schizophrenia |
| 43800 | E107200 | Chronic schizo-affective schizophrenia |
| 56438 | E107500 | Schizo-affective schizophrenia in remission |
| 10575 | E107z00 | Schizo-affective schizophrenia NOS |
| 33338 | E10y000 | Atypical schizophrenia |
| 49761 | E10yz00 | Other schizophrenia NOS |
| 8407 | E10z.00 | Schizophrenia NOS |
| 14656 | E11..00 | Affective psychoses |
| 8567 | E11..11 | Bipolar psychoses |
| 2560 | E11..12 | Depressive psychoses |
| 26161 | E11..13 | Manic psychoses |
| 37070 | E110.00 | Manic disorder, single episode |
| 18909 | E110.11 | Hypomanic psychoses |
| 20110 | E110000 | Single manic episode, unspecified |
| 14728 | E110100 | Single manic episode, mild |
| 70000 | E110600 | Single manic episode in full remission |
| 36611 | E110z00 | Manic disorder, single episode NOS |
| 26227 | E111.00 | Recurrent manic episodes |
| 19967 | E111000 | Recurrent manic episodes, unspecified |
| 32295 | E111400 | Recurrent manic episodes, severe, with psychosis |
| 37178 | E111600 | Recurrent manic episodes, in full remission |
| 46415 | E111z00 | Recurrent manic episode NOS |
| 32159 | E112400 | Single major depressive episode, severe, with psychosis |
| 24171 | E113400 | Recurrent major depressive episodes, severe, with psychosis |
| 3702 | E114.00 | Bipolar affective disorder, currently manic |
| 17385 | E114.11 | Manic-depressive – now manic |
| 35738 | E114000 | Bipolar affective disorder, currently manic, unspecified |
| 36126 | E114100 | Bipolar affective disorder, currently manic, mild |
| 46434 | E114200 | Bipolar affective disorder, currently manic, moderate |
| 63784 | E114600 | Bipolar affective disorder, currently manic, full remission |
| 4677 | E115.00 | Bipolar affective disorder, currently depressed |
| 12831 | E115.11 | Manic-depressive – now depressed |
| 35734 | E115100 | Bipolar affective disorder, currently depressed, mild |
| 27890 | E115200 | Bipolar affective disorder, currently depressed, moderate |
| 35607 | E115300 | Bipolar affect disorder, now depressed, severe, no psychosis |
| 63701 | E115400 | Bipolar affect disorder, now depressed, severe with psychosis |
| 57465 | E115600 | Bipolar affective disorder, now depressed, in full remission |
| 37296 | E115z00 | Bipolar affective disorder, currently depressed, NOS |
| 31316 | E116.00 | Mixed bipolar affective disorder |
| 31535 | E116000 | Mixed bipolar affective disorder, unspecified |
| 54195 | E116400 | Mixed bipolar affective disorder, severe, with psychosis |
| 63651 | E116500 | Mixed bipolar affective disorder, partial/unspecified remission |
| 55064 | E116600 | Mixed bipolar affective disorder, in full remission |
| 63583 | E116z00 | Mixed bipolar affective disorder, NOS |
| 14784 | E117.00 | Unspecified bipolar affective disorder |
| 49763 | E117000 | Unspecified bipolar affective disorder, unspecified |
| 68647 | E117200 | Unspecified bipolar affective disorder, moderate |
| 24230 | E117600 | Unspecified bipolar affective disorder, in full remission |
| 27986 | E117z00 | Unspecified bipolar affective disorder, NOS |
| 60178 | E11y.00 | Other and unspecified manic-depressive psychoses |
| 11596 | E11y000 | Unspecified manic-depressive psychoses |
| 33426 | E11yz00 | Other and unspecified manic-depressive psychoses NOS |
| 41992 | E11z.00 | Other and unspecified affective psychoses |
| 54607 | E11z000 | Unspecified affective psychoses NOS |
| 33425 | E11zz00 | Other affective psychosis NOS |
| 4261 | E12..00 | Paranoid states |
| 14743 | E120.00 | Simple paranoid state |
| 3890 | E121.00 | Chronic paranoid psychosis |
| 14971 | E122.00 | Paraphrenia |
| 50868 | E123.11 | Folie a deux |
| 31589 | E12y.00 | Other paranoid states |
| 31455 | E12yz00 | Other paranoid states NOS |
| 12771 | E12z.00 | Paranoid psychosis NOS |
| 31984 | E13..00 | Other nonorganic psychoses |
| 20228 | E13..11 | Reactive psychoses |
| 8478 | E130.00 | Reactive depressive psychosis |
| 17770 | E130.11 | Psychotic reactive depression |
| 29937 | E131.00 | Acute hysterical psychosis |
| 7332 | E132.00 | Reactive confusion |
| 15053 | E133.00 | Acute paranoid reaction |
| 24345 | E134.00 | Psychogenic paranoid psychosis |
| 16333 | E13y.00 | Other reactive psychoses |
| 14965 | E13z.00 | Nonorganic psychosis NOS |
| 3636 | E13z.11 | Psychotic episode NOS |
| 22188 | E1z..00 | Non-organic psychosis NOS |
| 61969 | E212200 | Schizotypal personality |
| 17281 | Eu2..00 | [X]Schizophrenia, schizotypal and delusional disorders |
| 34236 | Eu20.00 | [X]Schizophrenia |
| 16764 | Eu20000 | [X]Paranoid schizophrenia |
| 35877 | Eu20213 | [X]Schizophrenic catatonia |
| 20785 | Eu20400 | [X]Post-schizophrenic depression |
| 24107 | Eu20511 | [X]Chronic undifferentiated schizophrenia |
| 35848 | Eu20600 | [X]Simple schizophrenia |
| 49420 | Eu20y00 | [X]Other schizophrenia |
| 94001 | Eu20y12 | [X]Schizophreniform disord NOS |
| 18053 | Eu20y13 | [X]Schizophrenifrm psychos NOS |
| 34966 | Eu20z00 | [X]Schizophrenia, unspecified |
| 39316 | Eu21.00 | [X]Schizotypal disorder |
| 26859 | Eu21.18 | [X]Schizotypal personality disorder |
| 28562 | Eu22.00 | [X]Persistent delusional disorders |
| 34389 | Eu22000 | [X]Delusional disorder |
| 2113 | Eu22011 | [X]Paranoid psychosis |
| 11172 | Eu22012 | [X]Paranoid state |
| 47947 | Eu22013 | [X]Paraphrenia – late |
| 4843 | Eu22015 | [X]Paranoia |
| 62405 | Eu22100 | [X]Delusional misidentification syndrome |
| 55221 | Eu22111 | [X]Capgras syndrome |
| 101720 | Eu22300 | [X]Paranoid state in remission |
| 40981 | Eu22y11 | [X]Delusional dysmorphophobia |
| 50248 | Eu22y12 | [X]Involutional paranoid state |
| 49223 | Eu22z00 | [X]Persistent delusional disorder, unspecified |
| 25019 | Eu23.00 | [X]Acute and transient psychotic disorders |
| 36720 | Eu23000 | [X]Acute polymorphic psychotic disorder without symptoms of schizophrenia |
| 21455 | Eu23012 | [X]Cycloid psychosis |
| 26143 | Eu23112 | [X]Cycloid psychosis with symptoms of schizophrenia |
| 44307 | Eu23300 | [X]Other acute predominantly delusional psychotic disorders |
| 27770 | Eu23312 | [X]Psychogenic paranoid psychosis |
| 44503 | Eu23y00 | [X]Other acute and transient psychotic disorders |
| 34168 | Eu23z00 | [X]Acute and transient psychotic disorder, unspecified |
| 31707 | Eu23z11 | [X]Brief reactive psychosis NOS |
| 29651 | Eu23z12 | [X]Reactive psychosis |
| 105606 | Eu24.11 | [X]Folie a deux |
| 11973 | Eu24.13 | [X]Induced psychotic disorder |
| 9422 | Eu25.00 | [X]Schizoaffective disorders |
| 33847 | Eu25000 | [X]Schizoaffective disorder, manic type |
| 16905 | Eu25011 | [X]Schizoaffective psychosis, manic type |
| 51903 | Eu25012 | [X]Schizophreniform psychosis, manic type |
| 11055 | Eu25100 | [X]Schizoaffective disorder, depressive type |
| 35274 | Eu25111 | [X]Schizoaffective psychosis, depressive type |
| 33693 | Eu25200 | [X]Schizoaffective disorder, mixed type |
| 37580 | Eu25212 | [X]Mixed schizophrenic and affective psychosis |
| 58532 | Eu25y00 | [X]Other schizoaffective disorders |
| 37681 | Eu25z00 | [X]Schizoaffective disorder, unspecified |
| 33410 | Eu25z11 | [X]Schizoaffective psychosis NOS |
| 101987 | Eu26.00 | [X]Nonorganic psychosis in remission |
| 30985 | Eu2y.00 | [X]Other nonorganic psychotic disorders |
| 31738 | Eu2y.11 | [X]Chronic hallucinatory psychosis |
| 11244 | Eu2z.00 | [X]Unspecified nonorganic psychosis |
| 694 | Eu2z.11 | [X]Psychosis NOS |
| 5726 | Eu3..00 | [X]Mood – affective disorders |
| 12173 | Eu30.00 | [X]Manic episode |
| 9521 | Eu30.11 | [X]Bipolar disorder, single manic episode |
| 2741 | Eu30000 | [X]Hypomania |
| 13024 | Eu30100 | [X]Mania without psychotic symptoms |
| 21065 | Eu30200 | [X]Mania with psychotic symptoms |
| 48632 | Eu30212 | [X]Mania with mood-incongruent psychotic symptoms |
| 32088 | Eu30y00 | [X]Other manic episodes |
| 44513 | Eu30z00 | [X]Manic episode, unspecified |
| 4678 | Eu30z11 | [X]Mania NOS |
| 6874 | Eu31.00 | [X]Bipolar affective disorder |
| 1531 | Eu31.11 | [X]Manic-depressive illness |
| 6710 | Eu31.12 | [X]Manic-depressive psychosis |
| 16808 | Eu31000 | [X]Bipolar affective disorder, current episode hypomanic |
| 26299 | Eu31100 | [X]Bipolar affect disorder current episode manic wout psychotic symptoms |
| 28277 | Eu31200 | [X]Bipolar affect disorder current episode manic with psychotic symptoms |
| 16562 | Eu31300 | [X]Bipolar affect disorder current episode mild or moderate depress |
| 23713 | Eu31400 | [X]Bipol affect disorder current episode severe depress, no psychotic symptoms |
| 4732 | Eu31500 | [X]Bipolar affect disorder current episode severe depress with psychotic symptoms |
| 27584 | Eu31700 | [X]Bipolar affective disorder, currently in remission |
| 103915 | Eu31900 | [X]Bipolar affective disorder type II |
| 53840 | Eu31y00 | [X]Other bipolar affective disorders |
| 73924 | Eu31y11 | [X]Bipolar II disorder |
| 51032 | Eu31y12 | [X]Recurrent manic episodes |
| 33751 | Eu31z00 | [X]Bipolar affective disorder, unspecified |
| 12099 | Eu32300 | [X]Severe depressive episode with psychotic symptoms |
| 24117 | Eu32311 | [X]Single episode of major depression and psychotic symptoms |
| 52678 | Eu32312 | [X]Single episode of psychogenic depressive psychosis |
| 24112 | Eu32313 | [X]Single episode of psychotic depression |
| 28863 | Eu32314 | [X]Single episode of reactive depressive psychosis |
| 98417 | Eu32800 | [X]Major depression, severe with psychotic symptoms |
| 29451 | Eu33213 | [X]Manic-depress psychosis, depress, no psychotic symptoms |
| 47009 | Eu33300 | [X]Recurrent depress disorder current episode severe with psychotic symptoms |
| 23731 | Eu33311 | [X]Endogenous depression with psychotic symptoms |
| 28677 | Eu33312 | [X]Manic-depress psychosis, depressed type + psychotic symptoms |
| 32941 | Eu33313 | [X]Recurrent severe episodes/major depression + psychotic symptom |
| 31757 | Eu33314 | [X]Recurrent severe episodes/psychogenic depressive psychosis |
| 16861 | Eu33315 | [X]Recurrent severe episodes of psychotic depression |
| 37764 | Eu33316 | [X]Recurrent severe episodes/reactive depressive psychosis |
| 31633 | Eu3z.11 | [X]Affective psychosis NOS |
| 28168 | Eu44.14 | [X]Hysterical psychosis |
Exclusions: sleep apnoea, sleep-related respiratory failure, or alcohol abuse
Patients will be excluded if they are diagnosed with sleep apnoea, sleep-related respiratory failure, or alcohol abuse on or before their index date (Table 29).
| MedCode | Read code | Read term |
|---|---|---|
| 8430 | 1462 | H/O: alcoholism |
| 16237 | E01..00 | Alcoholic psychoses |
| 16225 | E010.00 | Alcohol withdrawal delirium |
| 22277 | E010.11 | DTs – delirium tremens |
| 1476 | E010.12 | Delirium tremens |
| 20762 | E011.00 | Alcohol amnestic syndrome |
| 4500 | E011000 | Korsakov’s alcoholic psychosis |
| 11106 | E011100 | Korsakov’s alcoholic psychosis with peripheral neuritis |
| 18636 | E011200 | Wernicke-Korsakov syndrome |
| 41920 | E011z00 | Alcohol amnestic syndrome NOS |
| 54505 | E012.00 | Other alcoholic dementia |
| 27342 | E012.11 | Alcoholic dementia NOS |
| 37946 | E012000 | Chronic alcoholic brain syndrome |
| 25110 | E013.00 | Alcohol withdrawal hallucinosis |
| 57939 | E014.00 | Pathological alcohol intoxication |
| 20407 | E014.11 | Drunkenness – pathological |
| 30404 | E015.00 | Alcoholic paranoia |
| 33670 | E01y.00 | Other alcoholic psychosis |
| 2082 | E01y000 | Alcohol withdrawal syndrome |
| 68111 | E01yz00 | Other alcoholic psychosis NOS |
| 67651 | E01z.00 | Alcoholic psychosis NOS |
| 2084 | E23..00 | Alcohol dependence syndrome |
| 2081 | E23..11 | Alcoholism |
| 1399 | E23..12 | Alcohol problem drinking |
| 5740 | E230.00 | Acute alcoholic intoxication in alcoholism |
| 57714 | E230.11 | Alcohol dependence with acute alcoholic intoxication |
| 40530 | E230000 | Acute alcoholic intoxication, unspecified, in alcoholism |
| 56947 | E230100 | Continuous acute alcoholic intoxication in alcoholism |
| 21624 | E230200 | Episodic acute alcoholic intoxication in alcoholism |
| 59574 | E230300 | Acute alcoholic intoxication in remission, in alcoholism |
| 36296 | E230z00 | Acute alcoholic intoxication in alcoholism NOS |
| 31443 | E231.00 | Chronic alcoholism |
| 37605 | E231.11 | Dipsomania |
| 43193 | E231000 | Unspecified chronic alcoholism |
| 24064 | E231100 | Continuous chronic alcoholism |
| 26106 | E231200 | Episodic chronic alcoholism |
| 24485 | E231300 | Chronic alcoholism in remission |
| 33635 | E231z00 | Chronic alcoholism NOS |
| 6169 | E23z.00 | Alcohol dependence syndrome NOS |
| 39327 | Eu10200 | [X]Mental and behaviour disorder due to use alcohol: dependence syndrome |
| 28780 | Eu10211 | [X]Alcohol addiction |
| 5758 | Eu10212 | [X]Chronic alcoholism |
| 69691 | Eu10213 | [X]Dipsomania |
| 20514 | Eu10300 | [X]Mental and behaviour disorder due to use alcohol: withdrawal state |
| 64101 | Eu10400 | [X]Men and behaviour disorder due alcohol: withdrawal state with delirium |
| 17259 | Eu10411 | [X]Delirium tremens, alcohol induced |
| 6467 | Eu10511 | [X]Alcoholic hallucinosis |
| 65932 | Eu10512 | [X]Alcoholic jealousy |
| 30162 | Eu10513 | [X]Alcoholic paranoia |
| 17607 | Eu10514 | [X]Alcoholic psychosis NOS |
| 11670 | Eu10611 | [X]Korsakov’s psychosis, alcohol induced |
| 26323 | Eu10711 | [X]Alcoholic dementia NOS |
| 37691 | Eu10712 | [X]Chronic alcoholic brain syndrome |
| 47555 | F11x000 | Cerebral degeneration due to alcoholism |
| 36748 | F11x011 | Alcoholic encephalopathy |
| 33839 | F144000 | Cerebellar ataxia due to alcoholism |
| 2925 | F375.00 | Alcoholic polyneuropathy |
| 31742 | F394100 | Alcoholic myopathy |
| 7603 | Fy03.00 | Sleep apnoea |
| 8148 | Fy03.11 | Obstructive sleep apnoea |
| 38686 | Fy04.00 | Sleep-related respiratory failure |
| 59155 | Fy04.11 | Ondine’s curse |
| 4915 | G555.00 | Alcoholic cardiomyopathy |
| 8363 | G852300 | Oesophageal varices in alcoholic cirrhosis of the liver |
| 23779 | H5B..00 | Sleep apnoea |
| 20748 | H5B0.00 | Obstructive sleep apnoea |
| 4506 | J153.00 | Alcoholic gastritis |
| 10691 | J610.00 | Alcoholic fatty liver |
| 3216 | J611.00 | Acute alcoholic hepatitis |
| 4743 | J612.00 | Alcoholic cirrhosis of liver |
| 21713 | J612000 | Alcoholic fibrosis and sclerosis of liver |
| 7885 | J613.00 | Alcoholic liver damage unspecified |
| 17330 | J613000 | Alcoholic hepatic failure |
| 7943 | J617.00 | Alcoholic hepatitis |
| 7602 | J617000 | Chronic alcoholic hepatitis |
| 24984 | J671000 | Alcohol-induced chronic pancreatitis |
| 48539 | R005100 | [D]Insomnia with sleep apnoea |
| 36301 | R005300 | [D]Hypersomnia with sleep apnoea |
| 2506 | R005311 | [D]Sleep apnoea syndrome |
| 20438 | R005312 | [D]Syndrome sleep apnoea |
| 7123 | ZV11300 | [V]Personal history of |
Exclusions: neuropathic pain
Patients will be excluded if they are diagnosed with neuropathic pain in the 12 months on or before their index date (Table 30).
| MedCode | Read code | Read term |
|---|---|---|
| 1598 | A531.11 | Post herpetic neuralgia |
| 27403 | A531100 | Geniculate herpes zoster |
| 7331 | A531111 | Ramsay – Hunt syndrome |
| 11498 | A531200 | Postherpetic trigeminal neuralgia |
| 31709 | A531300 | Postherpetic polyneuropathy |
| 17180 | A531500 | Postzoster neuralgia |
| 10223 | A531511 | Postherpetic neuralgia |
| 28333 | C373200 | Familial neuropathic amyloid |
| 7584 | F300.00 | Post-herpetic trigeminal neuralgia |
| 1541 | F301.00 | Other specified trigeminal neuralgia |
| 4912 | F301000 | Tic douloureux |
| 6581 | F301z00 | Trigeminal neuralgia NOS |
| 18016 | F336000 | Phantom limb syndrome with pain |
| 23768 | F337.00 | Nerve root and plexus compressions in diseases EC |
| 55335 | F337000 | Nerve root and plexus compressions in neoplastic disease |
| 33604 | F337100 | Nerve root and plexus compressions in intervertion discussion disorder |
| 23699 | F337200 | Nerve root and plexus compressions in spondylosis |
| 24410 | F337300 | Nerve root and plexus compressions in other dorsopathies |
| 56272 | F374.00 | Polyneuropathy in disease EC |
| 39692 | F374400 | Polyneuropathy in herpes zoster |
| 63555 | F374z00 | Polyneuropathy in disease NOS |
| 93868 | Fyu6A00 | [X]Other mononeuropathies of upper limb |
| 72922 | Fyu6B00 | [X]Other mononeuropathies of lower limb |
| 91741 | Fyu6C00 | [X]Other specified mononeuropathies |
| 107322 | Fyu6D00 | [X]Other mononeuropathies in diseases classified elsewhere |
| 22238 | Fyu6E00 | [X]Ilio-inguinal nerve entrapment |
| 55076 | Fyu7.00 | [X]Polyneuropathies & other disorder of peripheral nervous system |
| 97449 | Fyu7000 | [X]Other hereditary and idiopathic neuropathies |
| 97479 | Fyu7100 | [X]Other inflammatory polyneuropathies |
| 97306 | Fyu7200 | [X]Other specified polyneuropathies |
| 39858 | Fyu7B00 | [X]Inflammatory polyneuropathy, unspecified |
| 35537 | Fyu7C00 | [X] Polyneuropathy, unspecified |
| 99855 | M271700 | Neuropathic foot ulcer |
| 49575 | N035.00 | Neuropathic arthropathy |
| 8710 | N035.11 | Charcot’s arthropathy |
| 36643 | N035.12 | Neuropathic arthritis |
| 37759 | N11y200 | Neuropathic spondylopathy | |
| 54992 | N242.00 | Neuralgia, neuritis and radiculitis unspecified |
| 2284 | N242000 | Neuralgia unspecified |
| 1416 | N242100 | Neuritis unspecified |
| 769 | N242200 | Radiculitis unspecified |
| 11544 | N242300 | Neuropathic pain |
| 23839 | N242z00 | Neuralgia, neuritis or radiculitis NOS |
| 41736 | N242z11 | Policeman’s disease |
| 18492 | SD72200 | Neuropathic foot blister |
Outcomes: codes for the hospital admission and death certificate data
The following ICD-10 codes were used to identify the following outcomes in the HES or ONS data:
-
fracture: S02, S12, S22, S32, S42, S52, S62, S72, S82 or S92
-
hip fracture: S72.0, S27.1 or S72.2
-
forearm/wrist/hand fracture: S52 or S62
-
fall: W0 or W1
-
acute bacterial infection: A4, B95, B96, H05.0, H60.1, K12.2, L03, J15, J18, N30.0, N30.2, N30.8, N30.9 or N39.0
-
UTI or acute LRTI: J15, J18, N30.0, N30.2, N30.8, N30.9 or N39.0
-
ischaemic stroke/TIA: G45 or I63
-
venous thromboembolism: I26, I80, I81 or I82
-
agitation/psychosis: R44, F29, R45.1, R45.4, R45.5, R45.6 or F06.0.
Outcomes: Read codes in the Clinical Practice Research Datalink
Tables 31–38 show the Read codes that were used to identify outcomes in the CPRD.
| MedCode | Read code | Read term |
|---|---|---|
| 9792 | 7K1D01E | DHS – Dynamic hip screw primary fixation of neck of femur |
| 12544 | 7K1D01F | Dynamic hip screw primary fixation of neck of femur |
| 6660 | 7K1L400 | Closed reduction of fracture of hip |
| 2225 | S30..00 | Fracture of neck of femur |
| 1994 | S30..11 | Hip fracture |
| 19387 | S302011 | Closed fracture of femur, greater trochanter |
| 8648 | S302400 | Closed fracture of femur, intertrochanteric |
| 8243 | S305.00 | Subtrochanteric fracture |
| 24276 | S30w.00 | Closed fracture of unspecified proximal femur |
| 18273 | S30y.00 | Closed fracture of neck of femur NOS |
| 10570 | S30y.11 | Hip fracture NOS |
| MedCode | Read code | Read term |
|---|---|---|
| 8885 | 7K1LJ00 | Closed reduction of fracture of thumb |
| 6942 | 7K1LL00 | Closed reduction of fracture of radius and or ulna |
| 5951 | 7K1LM00 | Closed reduction of fracture of wrist |
| 1250 | S224.11 | Elbow fracture – closed |
| 6825 | S23..00 | Fracture of radius and ulna |
| 43570 | S230.00 | Closed fracture of proximal radius and ulna |
| 7009 | S230600 | Closed fracture radius, head |
| 18299 | S234.00 | Closed fracture of radius and ulna, lower end |
| 203 | S234.11 | Wrist fracture – closed |
| 343 | S234100 | Closed Colles’ fracture |
| 1742 | S234200 | Closed fracture of the distal radius, unspecified |
| 9165 | S234300 | Closed fracture of ulna, styloid process |
| 40476 | S234500 | Closed fracture distal ulna, unspecified |
| 199 | S23B.00 | Fracture of lower end of radius |
| 137 | S23x111 | Fracture of radius NOS |
| 1073 | S23x211 | Fracture of ulna NOS |
| 909 | S23z.00 | Fracture of radius and ulna, NOS |
| 22375 | S24..00 | Fracture of carpal bone |
| 15666 | S240.00 | Closed fracture of carpal bone |
| 8056 | S242.00 | Fracture at wrist and hand level |
| 553 | S242000 | Fracture of scaphoid |
| 993 | S242200 | Fracture of other metacarpal bone |
| 25519 | S250300 | Closed fracture finger metacarpal shaft |
| 12546 | S250400 | Closed fracture finger metacarpal neck |
| 29111 | S251.00 | Open fracture of metacarpal bone(s) |
| 441 | S26..00 | Fracture of one or more phalanges of hand |
| 5260 | S26..11 | Finger fracture |
| 8302 | S260.00 | Closed fracture of one or more phalanges of hand |
| 24516 | S260D00 | Closed fracture finger proximal phalanx |
| 7500 | S262.00 | Fracture of thumb |
| 6299 | S263.00 | Fracture of other finger |
| 4582 | S26z.00 | Fracture of one or more phalanges of hand NOS |
| 18614 | S4C..00 | Fracture-dislocation or subluxation of wrist |
| 10250 | S4D..00 | Fracture-dislocation/subluxation finger/thumb |
| MedCode | Read code | Read term |
|---|---|---|
| 18962 | 7K1L500 | Closed reduction of fracture of femur |
| 6106 | 7K1L800 | Closed reduction of fracture of ankle |
| 7339 | 7K1LA00 | Closed reduction of fracture of toe |
| 6379 | 7K1LF00 | Closed reduction of fracture of humerus |
| 7428 | 7K1LG00 | Closed reduction of fracture of shoulder |
| 5526 | N331.00 | Pathological fracture |
| 30616 | N331000 | Pathological fracture of thoracic vertebra |
| 17377 | N331800 | Osteoporosis + pathological fracture lumbar vertebrae |
| 12673 | N331900 | Osteoporosis + pathological fracture thoracic vertebrae |
| 11543 | N331G00 | Collapse of lumbar vertebra |
| 45736 | N331H00 | Collapse of cervical vertebra due to osteoporosis |
| 4013 | N331L00 | Collapse of vertebra due to osteoporosis NOS |
| 11503 | N331M00 | Fragility fracture due to unspecified osteoporosis |
| 93497 | N331N00 | Fragility fracture |
| 4409 | S10..12 | Fracture of vertebra without spinal cord lesion |
| 11296 | S100.00 | Closed fracture of cervical spine |
| 39887 | S100A00 | Closed fracture axis, odontoid process |
| 3888 | S104.00 | Closed fracture lumbar vertebra |
| 8266 | S104100 | Closed fracture lumbar vertebra, wedge |
| 34403 | S10A100 | Fracture of second cervical vertebra |
| 9072 | S10B400 | Fracture of acetabulum |
| 280 | S120.00 | Closed fracture rib |
| 7831 | S120000 | Closed fracture of rib, unspecified |
| 9688 | S127.00 | Fracture of rib |
| 738 | S13..00 | Fracture or disruption of pelvis |
| 5302 | S132.00 | Closed fracture pubis |
| 7004 | S132000 | Closed fracture pelvis, single pubic ramus |
| 6667 | S132100 | Closed fracture pelvis, multiple pubic rami – stable |
| 46592 | S132200 | Closed fracture pelvis, multiple pubic rami – unstable |
| 28702 | S132z00 | Closed fracture pubis NOS |
| 28375 | S13y.00 | Closed fracture of pelvis NOS |
| 11277 | S150.00 | Multiple fractures of thoracic spine |
| 6195 | S2...00 | Fracture of upper limb |
| 5929 | S2...11 | Arm fracture |
| 483 | S20..00 | Fracture of clavicle |
| 44715 | S200000 | Closed fracture of clavicle, unspecified part |
| 29899 | S200300 | Closed fracture clavicle, lateral end |
| 1177 | S21..00 | Fracture of scapula |
| 10735 | S21..11 | Shoulder blade fracture |
| 517 | S22..00 | Fracture of humerus |
| 11222 | S220.00 | Closed fracture of the proximal humerus |
| 44721 | S220000 | Closed fracture of proximal humerus, unspecified part |
| 11313 | S220100 | Closed fracture proximal humerus, neck |
| 11044 | S220300 | Closed fracture proximal humerus, greater tuberosity |
| 6893 | S224100 | Closed fracture distal humerus, supracondylar |
| 1548 | S228.00 | Fracture of lower end of humerus |
| 10382 | S22z.00 | Fracture of humerus NOS |
| 8891 | S3...00 | Fracture of lower limb |
| 8040 | S31..00 | Other fracture of femur |
| 6868 | S310.00 | Closed fracture of femur, shaft or unspecified part |
| 37662 | S310000 | Closed fracture of femur, unspecified part |
| 12791 | S310011 | Thigh fracture NOS |
| 6320 | S312100 | Closed fracture of femoral condyle, unspecified |
| 5332 | S312300 | Closed fracture distal femur, supracondylar |
| 8646 | S314.00 | Fracture of shaft of femur |
| 8589 | S315.00 | Fracture of lower end of femur |
| 520 | S31z.00 | Fracture of femur, NOS |
| 235 | S32..00 | Fracture of patella |
| 27719 | S334.00 | Closed fracture distal tibia |
| 6839 | S339000 | Closed fracture of distal fibula |
| 78444 | S33A.00 | Fracture of tibia |
| 4304 | S33x100 | Closed fracture of fibula, unspecified part, NOS |
| 4572 | S33x200 | Closed fracture of tibia and fibula, unspecified part |
| 325 | S34..00 | Fracture of ankle |
| 7135 | S342000 | Closed fracture ankle, lateral malleolus, low |
| 7317 | S344.00 | Closed fracture ankle, bimalleolar |
| 169 | S35..11 | Metatarsal bone fracture |
| 2710 | S35..12 | Tarsal bone fracture |
| 8276 | S350.00 | Closed fracture of calcaneus |
| 3937 | S352700 | Closed fracture metatarsal |
| 35077 | S352E00 | Closed fracture metatarsal head |
| 6062 | S356.00 | Fracture of metatarsal bone |
| 2176 | S362.00 | Fracture of great toe |
| 2470 | S3z..00 | Fracture of unspecified bones |
| 358 | S3z..11 | Fracture NOS |
| 34212 | S4J2100 | Closed fracture-subluxation of pelvis |
| MedCode | Read code | Read term |
|---|---|---|
| 6008 | 16D..00 | Falls |
| 8694 | 16D1.00 | Recurrent falls |
| 46559 | 16D2.00 | Number of falls in last year |
| 98223 | 16D5.00 | Fall onto outstretched hand |
| 108062 | 16D6.00 | Fall |
| 5284 | 1B65.00 | Had a collapse |
| 1634 | 1B65.11 | Collapse – symptom |
| 10412 | 224..00 | O/E – collapsed |
| 105499 | 8CMW400 | Falls care pathway |
| 2307 | R002.00 | [D]Syncope and collapse |
| 1812 | R002300 | [D]Collapse |
| 4859 | R200.12 | [D] Geriatric fall |
| 6815 | TC...00 | Accidental falls |
| 384 | TC...11 | Fall – accidental |
| 38818 | TC42000 | Fall from chair |
| 26432 | TC42100 | Fall from bed |
| 15112 | TC5..00 | Fall on same level from slipping, tripping or stumbling |
| 18007 | TC50.00 | Fall on same level from slipping |
| 7948 | TC52.00 | Fall on same level from stumbling |
| 8730 | TCy..00 | Other falls |
| 11308 | TCyz.00 | Other accidental fall NOS |
| 6835 | TCz..00 | Accidental falls NOS |
| 7970 | U10..00 | [X]Falls |
| 43191 | U10J000 | [X]Other fall on same level, occurrence at home |
| 24776 | U10z.00 | [X]Unspecified fall |
| MedCode | Read code | Read term |
|---|---|---|
| 23640 | H0y..00 | Other specified acute respiratory infections |
| 21113 | H0z..00 | Acute respiratory infection NOS |
| 10086 | H2...00 | Pneumonia and influenza |
| 1849 | H21..00 | Lobar (pneumococcal) pneumonia |
| 12061 | H22y200 | Pneumonia – Legionella |
| 23095 | H22z.00 | Bacterial pneumonia NOS |
| 25694 | H23..00 | Pneumonia due to other specified organisms |
| 886 | H25..00 | Bronchopneumonia due to unspecified organism |
| 16287 | H25..11 | Chest infection – unspecified bronchopneumonia |
| 572 | H26..00 | Pneumonia due to unspecified organism |
| 9639 | H260.00 | Lobar pneumonia due to unspecified organism |
| 3683 | H261.00 | Basal pneumonia due to unspecified organism |
| 5324 | H28..00 | Atypical pneumonia |
| 104121 | H2B..00 | Community acquired pneumonia |
| 389 | K15..00 | Cystitis |
| 15074 | K150.00 | Acute cystitis |
| 1353 | K155.00 | Recurrent cystitis |
| 22682 | K15y000 | Cystitis cystica |
| 34645 | K15y200 | Abscess of bladder |
| 1289 | K190.00 | Urinary tract infection, site not specified |
| 1572 | K190.11 | Recurrent urinary tract infection |
| 4453 | K190100 | Pyuria, site not specified |
| 10515 | K190300 | Recurrent urinary tract infection |
| 2985 | K190311 | Recurrent UTI |
| 97002 | K190500 | Urinary tract infection |
| 104141 | K190600 | Urosepsis |
| 150 | K190z00 | Urinary tract infection, site not specified NOS |
| 2465 | K193.00 | Urethral caruncle |
| 507 | K197.00 | Haematuria |
| 19361 | K197.11 | Traumatic haematuria |
| 2784 | K197200 | Microscopic haematuria |
| 7232 | K197300 | Frank haematuria |
| 5264 | K19y300 | Pneumaturia |
| 7733 | K19y411 | Urethral bleeding |
| MedCode | Read code | Read term |
|---|---|---|
| 885 | A38..00 | Septicaemia |
| 30102 | A381000 | Septicaemia due to Staphylococcus aureus |
| 10872 | A384200 | Escherichia coli septicaemia |
| 23991 | A384211 | E. coli septicaemia |
| 33765 | A38z.00 | Septicaemia NOS |
| 2136 | A38z.11 | Sepsis |
| 3382 | A3B0.00 | Streptococcal infection |
| 1426 | A3B1.00 | Staphylococcal infection |
| 8673 | A3B1100 | Meticillin resistant staphylococcus aureus |
| 5534 | A3B2.00 | Pneumococcal infection |
| 12062 | A3B4.00 | E. coli infection |
| 8329 | A3B4.11 | E. coli infection |
| 6856 | A3B7.00 | Pseudomonas infection |
| 105405 | A3BC.00 | Infection due to ESBL producing bacteria |
| 5945 | A3By800 | Coliform bacteria |
| 104028 | A3C..00 | Sepsis |
| 104150 | A3Cy.00 | Other specified sepsis |
| 4328 | F4G0100 | Orbital cellulitis |
| 8852 | F501112 | Cellulitis, external ear |
| 4456 | K284300 | Cellulitis of scrotum |
| 4779 | M020.00 | Cellulitis and abscess of finger |
| 3527 | M020000 | Cellulitis and abscess of finger unspecified |
| 3960 | M021.00 | Cellulitis and abscess of toe |
| 3363 | M021000 | Cellulitis and abscess of toe unspecified |
| 16536 | M03..00 | Other cellulitis and abscess |
| 3998 | M030.00 | Cellulitis and abscess of face |
| 10485 | M030111 | Cellulitis and abscess of nose |
| 1874 | M032200 | Cellulitis and abscess of back |
| 14937 | M032400 | Cellulitis and abscess of umbilicus |
| 3461 | M033.00 | Cellulitis and abscess of arm |
| 1415 | M034.11 | Cellulitis and abscess of hand |
| 2914 | M034000 | Cellulitis and abscess of hand unspecified |
| 7865 | M036.00 | Cellulitis and abscess of leg excluding foot |
| 10326 | M036.11 | Cellulitis and abscess of leg |
| 25890 | M036300 | Cellulitis and abscess of lower leg |
| 680 | M036z00 | Cellulitis and abscess of leg NOS |
| 2089 | M037000 | Cellulitis and abscess of foot unspecified |
| 7328 | M037200 | Cellulitis in diabetic foot |
| 309 | M03z.00 | Cellulitis and abscess NOS |
| 4207 | M03z000 | Cellulitis NOS |
| 943 | M05..00 | Impetigo |
| 14934 | M05z.00 | Impetigo NOS |
| 6833 | M08..00 | Cutaneous cellulitis |
| 1315 | M081.00 | [X]Cellulitis of other parts of limb |
| 6368 | M085.00 | Cellulitis of leg |
| 7684 | M08B.00 | Cellulitis of foot |
| 94868 | M08C.00 | Cellulitis of toe |
| MedCode | Read code | Read term |
|---|---|---|
| 8837 | G64..00 | Cerebral arterial occlusion |
| 5363 | G64..11 | CVA – cerebral artery occlusion |
| 569 | G64..12 | Infarction – cerebral |
| 6155 | G64..13 | Stroke due to cerebral arterial occlusion |
| 16517 | G640.00 | Cerebral thrombosis |
| 36717 | G640000 | Cerebral infarction due to thrombosis of cerebral arteries |
| 3149 | G64z.00 | Cerebral infarction NOS |
| 5602 | G64z.12 | Cerebellar infarction |
| 10504 | G64z300 | Right-sided cerebral infarction |
| 504 | G65..00 | Transient cerebral ischaemia |
| 3132 | G65..11 | Drop attack |
| 1433 | G65..12 | Transient ischaemic attack |
| 1469 | G66..00 | Stroke and cerebrovascular accident unspecified |
| 1298 | G66..11 | CVA unspecified |
| 6253 | G66..12 | Stroke unspecified |
| 6116 | G66..13 | CVA – cerebrovascular accident unspecified |
| 51767 | G666.00 | Pure sensory lacunar syndrome |
| 12833 | G668.00 | Right-sided CVA |
| MedCode | Read code | Read term |
|---|---|---|
| 94552 | 8HTm.00 | Referral to deep-vein thrombosis clinic |
| 1266 | G401.00 | Pulmonary embolism |
| 9701 | G401.12 | Pulmonary embolus |
| 3576 | G801.00 | Deep-vein phlebitis and thrombophlebitis of the leg |
| 824 | G801.11 | Deep-vein thrombosis |
| 3392 | G801.13 | DVT – deep-vein thrombosis |
| 15382 | G801600 | Thrombophlebitis of the femoral vein |
| 22038 | G801D00 | Deep-vein thrombosis of lower limb |
| 100103 | G824.00 | Axillary vein thrombosis |
Appendix 2 Additional primary care patient characteristics
| Characteristic | First sleep disturbance treatment | |||
|---|---|---|---|---|
| Z-drug (N = 2952) | Low-dose TCA (N = 1898) | BZD (N = 308) | No drug (N = 1651) | |
| Dementia | ||||
| Months since dementia diagnosis, median (IQR)a | 11.6 (3.5–26.2) | 12.5 (4.2–27.2) | 11.6 (3.6–26.2) | 11.4 (4.1–24.6) |
| Dementia subtype, n (%) | ||||
| Alzheimer’s disease | 1135 (38) | 790 (42) | 116 (38) | 643 (39) |
| Vascular dementia | 800 (27) | 529 (28) | 72 (23) | 452 (27) |
| Other/mixed dementia | 309 (10) | 159 (8) | 34 (11) | 172 (10) |
| Unspecified dementia/missing | 708 (24) | 420 (22) | 86 (28) | 384 (23) |
| Anticholinesterase/memantine prescription in last 90 days, n (%) | 670 (23) | 485 (26) | 51 (17) | 313 (19) |
| Antipsychotic prescription in last 90 days, n (%) | 542 (18) | 229 (12) | 46 (15) | 282 (17) |
| Agitation/psychosis history, n (%) | 484 (16) | 292 (15) | 75 (24) | 369 (22) |
| End-of-life care, n (%) | 160 (5) | 110 (6) | 11 (4) | 73 (4) |
| Sleep disturbance, n (%) | ||||
| Sleep disturbance diagnosis pre dementia | 644 (22) | 335 (18) | 65 (21) | 532 (32) |
| History of BZD use | 718 (24) | 468 (25) | 89 (29) | 299 (18) |
| History of Z-drug use | 258 (9) | 107 (6) | 18 (6) | 90 (5) |
| Medical history in past year | ||||
| Falls, n (%) | 820 (28) | 440 (23) | 82 (27) | 473 (29) |
| Fractures, n (%) | 309 (10) | 153 (8) | 27 (9) | 117 (7) |
| Dizziness/unsteadiness, n (%) | 157 (5) | 101 (5) | 17 (6) | 108 (7) |
| Faints/syncope, n (%) | 152 (5) | 86 (5) | 26 (8) | 103 (6) |
| UTI/acute LRTI, n (%) | 766 (26) | 441 (23) | 77 (25) | 368 (22) |
| Influenza vaccination, n (%) | 2049 (69) | 1438 (76) | 220 (71) | 1185 (72) |
| Pneumonia vaccination, n (%) | 138 (5) | 75 (4) | 13 (4) | 97 (6) |
| Physician consultations, mean (SD) | 8.4 (7.3) | 8.9 (7.1) | 7.8 (6.7) | 7.6 (6.7) |
| Hospital admissions, mean (SD) | 1.2 (1.9) | 1.1 (1.7) | 1.2 (1.8) | 0.9 (2.1) |
| Comorbidities, n (%) | ||||
| Depression | 705 (24) | 500 (26) | 76 (25) | 422 (26) |
| Depression symptoms | 533 (18) | 425 (22) | 52 (17) | 311 (19) |
| Anxiety | 458 (16) | 322 (17) | 58 (19) | 269 (16) |
| Anxiety symptoms | 353 (12) | 291 (15) | 37 (12) | 214 (13) |
| Parkinson’s disease | 170 (6) | 72 (4) | 21 (7) | 103 (6) |
| Urinary incontinence | 424 (14) | 342 (18) | 59 (19) | 425 (26) |
| Benign prostatic hyperplasia | 310 (11) | 177 (9) | 36 (12) | 156 (9) |
| Asthma | 292 (10) | 212 (11) | 26 (8) | 143 (9) |
| Cancer | 627 (21) | 399 (21) | 64 (21) | 290 (18) |
| COPD | 223 (8) | 181 (10) | 23 (7) | 120 (7) |
| Osteoporosis | 332 (11) | 265 (14) | 39 (13) | 186 (11) |
| Other musculoskeletal conditions | 363 (12) | 265 (14) | 51 (17) | 211 (13) |
| Osteoarthritis/rheumatoid arthritis | 1160 (39) | 855 (45) | 116 (38) | 664 (40) |
| Other joint conditions | 2426 (82) | 1604 (85) | 245 (80) | 1383 (84) |
| Headache/migraine | 578 (20) | 489 (26) | 44 (14) | 318 (19) |
| Back/neck pain | 1588 (54) | 1198 (63) | 150 (49) | 876 (53) |
| ARMD | 167 (6) | 130 (7) | 25 (8) | 100 (6) |
| Cataract | 832 (28) | 585 (31) | 84 (27) | 479 (29) |
| Glaucoma | 288 (10) | 205 (11) | 26 (8) | 158 (10) |
| Retinal disorder | 248 (8) | 176 (9) | 28 (9) | 122 (7) |
| Diabetes | 432 (15) | 302 (16) | 44 (14) | 218 (13) |
| Hyperlipidaemia | 380 (13) | 296 (16) | 40 (13) | 247 (15) |
| Hypertension | 1521 (52) | 1082 (57) | 151 (49) | 902 (55) |
| Stroke/TIA | 644 (22) | 404 (21) | 65 (21) | 353 (21) |
| Myocardial infarction | 247 (8) | 159 (8) | 29 (9) | 157 (10) |
| Heart failure | 254 (9) | 138 (7) | 27 (9) | 168 (10) |
| Atrial fibrillation | 454 (15) | 273 (14) | 46 (15) | 235 (14) |
| Angina | 435 (15) | 279 (15) | 44 (14) | 278 (17) |
| Venous thromboembolism | 189 (6) | 139 (7) | 24 (8) | 103 (6) |
| Prescriptions in last 90 days | ||||
| SSRI | 610 (21) | 341 (18) | 50 (16) | 298 (18) |
| Other antidepressant | 183 (6) | 93 (5) | 15 (5) | 122 (7) |
| Antiepileptic | 174 (6) | 135 (7) | 40 (13) | 91 (6) |
| Analgesic | 1249 (42) | 1049 (55) | 114 (37) | 590 (36) |
| Inhaled corticosteroid | 124 (4) | 108 (6) | 14 (5) | 71 (4) |
| Lipid-regulating medication | 986 (33) | 712 (38) | 88 (29) | 532 (32) |
| Diuretic | 917 (31) | 583 (31) | 90 (29) | 520 (31) |
| Beta-blocker | 527 (18) | 327 (17) | 51 (17) | 270 (16) |
| ACE inhibitor | 574 (19) | 437 (23) | 62 (20) | 322 (20) |
| Angiotensin II receptor antagonist | 193 (7) | 157 (8) | 19 (6) | 109 (7) |
| Calcium channel blocker | 526 (18) | 389 (20) | 38 (12) | 295 (18) |
| Anticoagulant | 149 (5) | 121 (6) | 12 (4) | 84 (5) |
| Antiplatelet | 1296 (44) | 814 (43) | 121 (39) | 753 (46) |
| Cardiac glycoside | 234 (8) | 122 (6) | 26 (8) | 124 (8) |
| NSAID | 209 (7) | 223 (12) | 32 (10) | 96 (6) |
| Bisphosphonate | 278 (9) | 248 (13) | 24 (8) | 143 (9) |
| Calcium/vitamin D | 514 (17) | 398 (21) | 44 (14) | 282 (17) |
| Antibiotic (in last 30 days) | 622 (21) | 376 (20) | 56 (18) | 287 (17) |
| Characteristic | Z-drug | Low-dose TCA | BZD | |||
|---|---|---|---|---|---|---|
| ORa (95% CI) | p-value | ORa (95% CI) | p-value | ORa (95% CI) | p-value | |
| Women | 0.85 (0.73 to 0.98) | 0.02 | 1.00 (0.85 to 1.17) | 0.99 | 0.75 (0.58 to 0.96) | 0.02 |
| Age (years), per year | 1.00 (0.99 to 1.01) | 0.41 | 0.99 (0.98 to 1.00) | 0.07 | 1.01 (0.99 to 1.02) | 0.56 |
| Care home resident | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.07 (0.90 to 1.27) | 0.45 | 1.02 (0.84 to 1.24) | 0.83 | 0.83 (0.61 to 1.12) | 0.23 |
| Missing | 1.33 (1.15 to 1.55) | < 0.01 | 1.40 (1.18 to 1.65) | < 0.01 | 1.23 (0.94 to 1.60) | 0.13 |
| Index date (per year) | 1.05 (1.03 to 1.07) | < 0.01 | 1.07 (1.05 to 1.09) | < 0.01 | 0.96 (0.93 to 0.99) | 0.02 |
| Region | ||||||
| North East | 1.00 | 1.00 | 1.00 | |||
| North West | 2.30 (1.49 to 3.54) | < 0.01 | 1.46 (0.96 to 2.24) | 0.08 | 0.65 (0.34 to 1.24) | 0.19 |
| Yorkshire and The Humber | 2.58 (1.52 to 4.40) | < 0.01 | 1.26 (0.73 to 2.18) | 0.41 | 1.28 (0.58 to 2.80) | 0.54 |
| East Midlands | 1.25 (0.73 to 2.12) | 0.41 | 0.61 (0.34 to 1.07) | 0.09 | 0.73 (0.33 to 1.58) | 0.42 |
| West Midlands | 1.99 (1.28 to 3.10) | < 0.01 | 0.71 (0.45 to 1.11) | 0.13 | 0.49 (0.24 to 0.97) | 0.04 |
| East of England | 1.60 (1.02 to 2.52) | 0.04 | 0.69 (0.44 to 1.09) | 0.11 | 0.71 (0.36 to 1.40) | 0.33 |
| South West | 2.42 (1.55 to 3.78) | < 0.01 | 1.33 (0.86 to 2.08) | 0.20 | 1.02 (0.53 to 1.97) | 0.94 |
| South Central | 2.05 (1.30 to 3.24) | < 0.01 | 1.72 (1.09 to 2.71) | 0.02 | 0.94 (0.48 to 1.87) | 0.87 |
| London | 2.36 (1.48 to 3.78) | < 0.01 | 0.81 (0.50 to 1.31) | 0.39 | 0.48 (0.23 to 1.02) | 0.06 |
| South East Coast | 3.37 (2.10 to 5.41) | < 0.01 | 0.93 (0.57 to 1.52) | 0.78 | 1.00 (0.49 to 2.04) | 1.00 |
| IMD quintile | ||||||
| 1 | 1.00 | 1.00 | 1.00 | |||
| 2 | 0.91 (0.71 to 1.15) | 0.42 | 1.00 (0.77 to 1.29) | 0.99 | 0.75 (0.50 to 1.12) | 0.16 |
| 3 | 0.95 (0.75 to 1.20) | 0.64 | 0.72 (0.55 to 0.93) | 0.01 | 0.53 (0.35 to 0.80) | < 0.01 |
| 4 | 0.69 (0.55 to 0.85) | < 0.01 | 0.58 (0.46 to 0.74) | < 0.01 | 0.63 (0.43 to 0.90) | 0.01 |
| 5 | 0.74 (0.59 to 0.93) | 0.01 | 0.54 (0.42 to 0.70) | < 0.01 | 0.63 (0.43 to 0.92) | 0.02 |
| Smoking status | ||||||
| Non-smoker | 1.00 | 1.00 | 1.00 | |||
| Ex-smoker | 0.97 (0.82 to 1.13) | 0.66 | 1.10 (0.93 to 1.31) | 0.27 | 1.05 (0.79 to 1.39) | 0.75 |
| Current smoker | 1.26 (0.99 to 1.60) | 0.06 | 1.10 (0.83 to 1.44) | 0.51 | 1.14 (0.75 to 1.72) | 0.55 |
| Missing | 0.83 (0.53 to 1.32) | 0.44 | 0.60 (0.35 to 1.02) | 0.06 | 0.45 (0.22 to 0.95) | 0.04 |
| Alcohol user | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 0.80 (0.68 to 0.93) | < 0.01 | 0.83 (0.70 to 0.99) | 0.03 | 0.79 (0.59 to 1.05) | 0.10 |
| Missing | 1.53 (1.12 to 2.11) | 0.01 | 1.79 (1.27 to 2.53) | < 0.01 | 1.33 (0.81 to 2.18) | 0.27 |
| BMI (kg/m2) | ||||||
| < 18.5 | 1.00 | 1.00 | 1.00 | |||
| 18.5–24.9 | 1.04 (0.78 to 1.38) | 0.80 | 1.02 (0.74 to 1.41) | 0.89 | 0.64 (0.38 to 1.10) | 0.11 |
| 25–29.9 | 1.06 (0.78 to 1.43) | 0.73 | 1.17 (0.84 to 1.65) | 0.36 | 0.87 (0.49 to 1.52) | 0.62 |
| ≥ 30 | 1.30 (0.92 to 1.84) | 0.14 | 1.21 (0.82 to 1.78) | 0.34 | 0.93 (0.48 to 1.80) | 0.83 |
| Missing | 1.36 (1.01 to 1.83) | 0.05 | 1.29 (0.92 to 1.81) | 0.14 | 1.59 (0.94 to 2.68) | 0.08 |
| Systolic blood pressure (mmHg) | ||||||
| < 110 | 1.00 | 1.00 | 1.00 | |||
| 110–119 | 1.18 (0.87 to 1.60) | 0.28 | 1.50 (1.05 to 2.13) | 0.03 | 1.13 (0.68 to 1.88) | 0.64 |
| 120–139 | 1.12 (0.86 to 1.45) | 0.40 | 1.39 (1.03 to 1.88) | 0.03 | 0.80 (0.52 to 1.24) | 0.32 |
| 140–159 | 1.20 (0.92 to 1.57) | 0.17 | 1.45 (1.06 to 1.99) | 0.02 | 1.03 (0.66 to 1.62) | 0.89 |
| ≥ 160 | 1.31 (0.95 to 1.80) | 0.11 | 1.69 (1.17 to 2.45) | 0.01 | 1.20 (0.70 to 2.04) | 0.51 |
| Missing | 1.71 (0.99 to 2.93) | 0.05 | 2.87 (1.56 to 5.29) | < 0.01 | 1.86 (0.88 to 3.94) | 0.11 |
| Dementia | ||||||
| Anticholinesterase/ memantine prescription × in last 90 days | 1.29 (1.10 to 1.52) | < 0.01 | 1.26 (1.05 to 1.50) | 0.01 | 1.19 (0.88 to 1.61) | 0.26 |
| Antipsychotic prescription in last 90 days | 1.31 (1.10 to 1.56) | < 0.01 | 0.81 (0.66 to 1.00) | 0.05 | 0.78 (0.57 to 1.07) | 0.12 |
| Agitation/psychosis history | 0.64 (0.54 to 0.75) | < 0.01 | 0.72 (0.60 to 0.87) | < 0.01 | 1.35 (1.03 to 1.77) | 0.03 |
| End-of-life care | 0.89 (0.65 to 1.21) | 0.45 | 0.90 (0.64 to 1.26) | 0.53 | 1.52 (0.91 to 2.54) | 0.11 |
| Sleep disturbance | ||||||
| Sleep disturbance diagnosis pre dementia | 0.58 (0.50 to 0.68) | < 0.01 | 0.45 (0.37 to 0.54) | < 0.01 | 0.46 (0.33 to 0.64) | < 0.01 |
| History of BZD use | 1.63 (1.38 to 1.93) | < 0.01 | 1.65 (1.37 to 1.99) | < 0.01 | 1.95 (1.44 to 2.64) | < 0.01 |
| History of Z-drug use | 1.83 (1.39 to 2.40) | < 0.01 | 1.15 (0.84 to 1.58) | 0.39 | 1.21 (0.69 to 2.11) | 0.51 |
| Medical history in past year | ||||||
| Falls | 0.77 (0.66 to 0.91) | < 0.01 | 0.65 (0.54 to 0.78) | < 0.01 | 0.86 (0.65 to 1.14) | 0.30 |
| Fractures | 1.44 (1.11 to 1.86) | 0.01 | 1.08 (0.80 to 1.44) | 0.62 | 1.00 (0.63 to 1.59) | 0.99 |
| Influenza vaccination | 0.93 (0.81 to 1.07) | 0.33 | 1.17 (0.99 to 1.38) | 0.06 | 1.16 (0.90 to 1.50) | 0.24 |
| Pneumonia vaccination | 0.91 (0.69 to 1.21) | 0.53 | 0.79 (0.56 to 1.09) | 0.15 | 0.46 (0.26 to 0.83) | 0.01 |
| GP consultations | ||||||
| 0–3 | 1.00 | 1.00 | 1.00 | |||
| 4–5 | 1.00 (0.82 to 1.21) | 0.97 | 1.24 (0.99 to 1.54) | 0.06 | 1.11 (0.79 to 1.56) | 0.55 |
| 6–8 | 1.13 (0.93 to 1.36) | 0.21 | 1.37 (1.10 to 1.69) | < 0.01 | 1.27 (0.91 to 1.77) | 0.16 |
| 9–13 | 1.05 (0.86 to 1.27) | 0.63 | 1.40 (1.13 to 1.74) | < 0.01 | 1.05 (0.75 to 1.49) | 0.77 |
| 14–77 | 1.28 (1.03 to 1.59) | 0.03 | 1.57 (1.23 to 2.00) | < 0.01 | 1.15 (0.77 to 1.70) | 0.49 |
| Hospital admissions | ||||||
| 0 | 1.00 | 1.00 | 1.00 | |||
| 1 | 1.48 (1.26 to 1.74) | < 0.01 | 1.31 (1.09 to 1.56) | < 0.01 | 1.39 (1.05 to 1.84) | 0.02 |
| ≥ 2 | 1.92 (1.61 to 2.30) | < 0.01 | 1.51 (1.23 to 1.84) | < 0.01 | 1.59 (1.16 to 2.16) | < 0.01 |
| Comorbidities | ||||||
| ARMD | 0.97 (0.74 to 1.27) | 0.82 | 1.34 (1.00 to 1.78) | 0.05 | 1.42 (0.90 to 2.25) | 0.13 |
| Back/neck pain | 0.97 (0.84 to 1.11) | 0.62 | 1.17 (1.00 to 1.36) | 0.05 | 0.59 (0.46 to 0.76) | < 0.01 |
| Migraine/headache | 1.00 (0.85 to 1.18) | 0.98 | 1.27 (1.06 to 1.52) | 0.01 | 0.65 (0.45 to 0.92) | 0.02 |
| Depression symptoms | 0.83 (0.70 to 1.00) | 0.05 | 1.09 (0.90 to 1.32) | 0.37 | 0.76 (0.54 to 1.09) | 0.14 |
| Hypertension | 0.83 (0.72 to 0.95) | 0.01 | 0.93 (0.80 to 1.09) | 0.37 | 0.61 (0.48 to 0.79) | < 0.01 |
| Heart failure | 0.82 (0.65 to 1.03) | 0.08 | 0.74 (0.57 to 0.96) | 0.02 | 0.63 (0.40 to 1.00) | 0.05 |
| Osteoporosis | 0.91 (0.73 to 1.14) | 0.43 | 0.95 (0.74 to 1.21) | 0.67 | 1.62 (1.10 to 2.40) | 0.02 |
| Other joint conditions | 0.72 (0.61 to 0.85) | < 0.01 | 0.79 (0.65 to 0.96) | 0.02 | 0.31 (0.24 to 0.41) | < 0.01 |
| Other musculoskeletal conditions | 1.02 (0.84 to 1.24) | 0.85 | 1.01 (0.82 to 1.25) | 0.94 | 1.50 (1.09 to 2.08) | 0.01 |
| Parkinson’s disease | 0.89 (0.68 to 1.17) | 0.41 | 0.57 (0.41 to 0.79) | < 0.01 | 0.66 (0.39 to 1.11) | 0.12 |
| Urinary incontinence | 0.52 (0.44 to 0.61) | < 0.01 | 0.67 (0.56 to 0.81) | < 0.01 | 0.72 (0.53 to 0.98) | 0.04 |
| Prescriptions in last 90 days | ||||||
| ACE inhibitor | 1.04 (0.88 to 1.23) | 0.65 | 1.15 (0.96 to 1.38) | 0.13 | 1.39 (1.04 to 1.88) | 0.03 |
| Analgesic | 1.29 (1.13 to 1.49) | < 0.01 | 2.09 (1.79 to 2.44) | < 0.01 | 1.24 (0.97 to 1.58) | 0.09 |
| Antiepileptic | 0.97 (0.73 to 1.28) | 0.81 | 1.12 (0.83 to 1.50) | 0.46 | 3.70 (2.57 to 5.34) | < 0.01 |
| Antihistamine | 0.76 (0.53 to 1.09) | 0.13 | 0.98 (0.67 to 1.42) | 0.91 | 1.48 (0.84 to 2.61) | 0.17 |
| Beta-blocker | 1.10 (0.92 to 1.31) | 0.30 | 0.91 (0.75 to 1.10) | 0.33 | 1.18 (0.86 to 1.62) | 0.31 |
| Bisphosphonate | 0.98 (0.77 to 1.26) | 0.89 | 1.32 (1.01 to 1.71) | 0.04 | 0.83 (0.52 to 1.32) | 0.43 |
| NSAID | 1.22 (0.94 to 1.59) | 0.14 | 1.73 (1.32 to 2.27) | < 0.01 | 1.95 (1.29 to 2.94) | < 0.01 |
| SSRI | 1.17 (0.99 to 1.39) | 0.07 | 0.80 (0.66 to 0.98) | 0.03 | 0.95 (0.69 to 1.29) | 0.73 |
| Other antidepressant | 0.79 (0.61 to 1.02) | 0.08 | 0.52 (0.38 to 0.70) | < 0.01 | 0.78 (0.49 to 1.25) | 0.30 |
| Antibiotic (in last 30 days) | 1.18 (1.00 to 1.40) | 0.05 | 1.00 (0.83 to 1.21) | 0.97 | 1.01 (0.75 to 1.36) | 0.93 |
Appendix 3 Additional REDIC study analyses
| Measure | Number missing (%) | None, n (%) | Mild/moderate, n (%) | Severe, n (%) | Measure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPI | CSDD | ||||||||||||||
| Anxiety total | Agitation total | Sleep total | Anxiety distress | Agitation distress | Sleep distress | Agitation | Anxiety | Difficulty falling asleep | Frequent waking | Waking early | |||||
| NPI | |||||||||||||||
| Anxiety total | 81 (4) | 1344 (63) | 498 (23) | 295 (14) | 0.52 | ||||||||||
| Agitation total | 82 (4) | 1448 (68) | 456 (21) | 232 (11) | 0.21 | 0.55 | |||||||||
| Sleep total | 82 (4) | 1637 (77) | 322 (15) | 177 (8) | 0.17 | 0.21 | 0.46 | ||||||||
| Anxiety distress | 356 (16) | 1197 (64) | 402 (22) | 263 (14) | 0.87 | 0.25 | 0.19 | 0.53 | |||||||
| Agitation distress | 376 (17) | 1212 (66) | 297 (16) | 333 (18) | 0.23 | 0.91 | 0.23 | 0.30 | 0.64 | ||||||
| Sleep distress | 463 (21) | 1278 (73) | 252 (14) | 216 (12) | 0.17 | 0.23 | 0.91 | 0.21 | 0.26 | 0.52 | |||||
| CSDD | |||||||||||||||
| Agitation | 33 (1) | 1700 (78) | 319 (15) | 166 (8) | 0.29 | 0.41 | 0.18 | 0.33 | 0.43 | 0.21 | 0.47 | ||||
| Anxiety | 51 (2) | 787 (36) | 949 (44) | 431 (20) | 0.53 | 0.20 | 0.18 | 0.50 | 0.20 | 0.18 | 0.32 | 0.51 | |||
| Difficulty falling asleep | 59 (3) | 1826 (85) | 243 (11) | 90 (4) | 0.17 | 0.08 | 0.31 | 0.15 | 0.11 | 0.29 | 0.13 | 0.18 | 0.29 | ||
| Frequent waking | 68 (3) | 1487 (69) | 487 (23) | 176 (8) | 0.19 | 0.10 | 0.59 | 0.18 | 0.11 | 0.55 | 0.14 | 0.21 | 0.44 | 0.38 | |
| Waking early | 54 (2) | 1889 (88) | 197 (9) | 78 (4) | 0.17 | 0.10 | 0.25 | 0.15 | 0.08 | 0.23 | 0.15 | 0.17 | 0.33 | 0.38 | 0.21 |
| Visit | Assessed, n (%) | Z-drugs, n (%) | BZD, n (%) | Antipsychotics, n (%) | Antihistamines, n (%) | Antidepressants, n (%) | Antiepileptics, n (%) |
|---|---|---|---|---|---|---|---|
| 1 (baseline) | 678 (100.0) | 126 (18.6) | 107 (15.8) | 84 (12.4) | 26 (3.8) | 196 (28.9) | 37 (5.5) |
| 2 (6 months) | 496 (73.2) | 106 (21.4) | 97 (19.6) | 85 (17.1) | 23 (4.6) | 193 (38.9) | 33 (6.7) |
| 3 (12 months) | 417 (61.5) | 74 (17.7) | 93 (22.3) | 66 (15.8) | 18 (4.3) | 169 (40.5) | 26 (6.2) |
| 4 (18 months) | 341 (50.3) | 62 (18.2) | 83 (24.3) | 68 (19.9) | 19 (5.6) | 135 (39.6) | 29 (8.5) |
| 5 (24 months) | 286 (42.2) | 53 (18.5) | 60 (21.0) | 46 (16.1) | 12 (4.2) | 119 (41.6) | 24 (8.4) |
| OR (drop out at next wave) | 95% CI | |
|---|---|---|
| Hypnotic use | ||
| BZD | 0.64* | 0.44 to 0.92 |
| Z-drug | 1.47* | 1.03 to 2.09 |
| Antipsychotic | 0.88 | 0.60 to 1.29 |
| Age (years) | ||
| < 70 | 0.54 | 0.22 to 1.32 |
| 70–79 | 1 | 1.00 to 1.00 |
| 80–89 | 1.47 | 0.96 to 2.26 |
| ≥ 90 | 1.63* | 1.03 to 2.59 |
| Dementia severity | ||
| Minimal | 1.06 | 0.62 to 1.81 |
| Mild | 1.15 | 0.78 to 1.70 |
| Moderate | 0.61** | 0.43 to 0.86 |
| Severe | 1 | 1.00 to 1.00 |
| Sex: female (vs. male) | 1.13 | 0.84 to 1.53 |
| Marital status | ||
| Unmarried | 1 | 1.00 to 1.00 |
| Married | 1.01 | 0.59 to 1.73 |
| Widowed/divorced | 1 | 0.59 to 1.69 |
| Neuropsychiatric symptoms | ||
| Sleep | 1.12 | 0.96 to 1.31 |
| Anxiety | 1.22 | 0.98 to 1.51 |
| Agitation | 0.76 | 0.56 to 1.03 |
| Disability (Lawton Physical Self-Maintenance Scale), per unit | 1.12*** | 1.08 to 1.15 |
| Years in education | ||
| < 7 | 0.82 | 0.39 to 1.74 |
| 7 | 0.70* | 0.49 to 1.00 |
| 8–11 | 0.77 | 0.55 to 1.09 |
| ≥ 12 | 1 | 1.00 to 1.00 |
| Type of admission | ||
| Long stay | 1 | 1.00 to 1.00 |
| Short stay | 1.52* | 1.07 to 2.15 |
| Nursing | 1.08 | 0.59 to 1.99 |
| Continuing Z-drugs | Continuing BZDs | Continuing antipsychotics | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age (years) | ||||||
| < 70 | 3.1 | 0.24 to 40.08 | 0.99 | 0.14 to 7.12 | 2.36 | 0.41 to 13.48 |
| 70–79 | Ref | Ref | Ref | |||
| 80–89 | 0.66 | 0.28 to 1.57 | 1.26 | 0.50 to 3.17 | 0.79 | 0.32 to 1.96 |
| ≥ 90 | 1.11 | 0.37 to 3.33 | 0.71 | 0.23 to 2.19 | 0.73 | 0.22 to 2.47 |
| Dementia severity | ||||||
| Minimal | 1.75 | 0.62 to 4.98 | 1.68 | 0.47 to 6.03 | 0.57 | 0.14 to 2.33 |
| Mild | 0.93 | 0.37 to 2.37 | 0.97 | 0.32 to 2.94 | 1.91 | 0.54 to 6.79 |
| Moderate | 1.9 | 0.81 to 4.48 | 1.06 | 0.43 to 2.57 | 1.24 | 0.52 to 2.96 |
| Severe | Ref | Ref | Ref | |||
| Sex: female (vs. male) | 0.9 | 0.34 to 2.37 | 0.93 | 0.45 to 1.92 | 0.54 | 0.23 to 1.26 |
| Marital status | ||||||
| Unmarried | Ref | Ref | Ref | |||
| Married | 1.02 | 0.21 to 4.92 | 0.3 | 0.06 to 1.46 | 1.38 | 0.48 to 3.93 |
| Widowed/divorced | 0.91 | 0.19 to 4.29 | 0.4 | 0.10 to 1.68 | 0.61 | 0.23 to 1.62 |
| Hypnotic use | ||||||
| BZDs | 2.02 | 0.97 to 4.20 | 1.19 | 0.58 to 2.43 | ||
| Z-drugs | 1.49 | 0.72 to 3.11 | 1.19 | 0.47 to 2.98 | ||
| Antipsychotics | 0.8 | 0.38 to 1.68 | 1.79 | 0.81 to 3.97 | ||
| Neuropsychiatric symptoms | ||||||
| Sleep | 0.88 | 0.58 to 1.34 | 0.88 | 0.60 to 1.29 | 0.89 | 0.59 to 1.34 |
| Anxiety | 0.98 | 0.59 to 1.61 | 0.87 | 0.50 to 1.50 | 1.15 | 0.66 to 1.98 |
| Agitation | 0.75 | 0.35 to 1.61 | 1.31 | 0.70 to 2.47 | 0.95 | 0.46 to 1.96 |
| Disability (Lawton Physical Self-Maintenance Scale), per unit | 0.95 | 0.88 to 1.03 | 1.03 | 0.96 to 1.12 | 0.97 | 0.89 to 1.06 |
| Years in education | ||||||
| < 7 | 3.92 | 0.55 to 28.12 | 1.66 | 0.20 to 14.06 | 0.35 | 0.04 to 3.18 |
| 7 | 1.67 | 0.63 to 4.42 | 0.32** | 0.13 to 0.76 | 0.65 | 0.25 to 1.64 |
| 8–11 | 1.8 | 0.78 to 4.20 | 0.85 | 0.37 to 1.95 | 0.5 | 0.22 to 1.17 |
| ≥ 12 | Ref | Ref | Ref | |||
| Type of admission | ||||||
| Long stay | Ref | Ref | Ref | |||
| Short stay | 0.81 | 0.32 to 2.03 | 0.56 | 0.26 to 1.22 | 1.31 | 0.49 to 3.49 |
| Nursing | 1.59 | 0.14 to 17.81 | 0.46 | 0.12 to 1.75 | 0.66 | 0.02 to 20.89 |
| Outcome | Exposure | Estimate of effect, β (95% CI) | |||
|---|---|---|---|---|---|
| Marginal structural model | Fixed effects model 1 (all participants) | Fixed effects model 2 (new users) | Fixed effects model 3 (mild dementia) | ||
| Sleep disturbance | Z-drugs | 0.20 (–0.02 to 0.43) | –0.01 (–0.15 to 0.13) | –0.13 (–0.38 to 0.11) | 0.25 (–0.02 to 0.52) |
| BZDs | –0.08 (–0.43 to 0.28) | –0.01 (–0.15 to 0.12) | –0.02 (–0.24 to 0.20) | 0.03 (–0.25 to 0.32) | |
| Antipsychotics | 0.05 (–0.21 to 0.31) | –0.08 (–0.24 to 0.08) | –0.10 (–0.35 to 0.14) | 0.21 (–0.28 to 0.70) | |
| Agitation | Z-drugs | 0.01 (–0.17 to 0.19) | 0.07 (–0.01 to 0.16) | 0.25*** (0.11 to 0.40) | 0.11 (–0.05 to 0.28) |
| BZDs | 0.11* (0.00 to 0.21) | –0.02 (–0.11 to 0.06) | 0.04 (–0.08 to 0.17) | 0.07 (–0.09 to 0.24) | |
| Antipsychotics | 0.13* (0.01 to 0.26) | 0.07 (–0.02 to 0.16) | 0.13 (–0.02 to 0.27) | 0.00 (–0.29 to 0.29) | |
| Anxiety | Z-drugs | 0.04 (–0.09 to 0.17) | 0.01 (–0.09 to 0.12) | 0.00 (–0.18 to 0.18) | 0.03 (–0.17 to 0.24) |
| BZDs | 0.09 (–0.06 to 0.24) | 0.04 (–0.06 to 0.14) | 0.05 (–0.10 to 0.21) | 0.13 (–0.08 to 0.35) | |
| Antipsychotics | 0.32** (0.12 to 0.51) | 0.11 (0.00 to 0.23) | 0.21* (0.03 to 0.39) | 0.22 (–0.15 to 0.59) | |
| QUALID | Z-drugs | 0.18 (–1.46 to 1.82) | 1.01* (0.06 to 1.96) | 0.44 (–1.09 to 1.97) | 0.74 (–1.03 to 2.51) |
| BZDs | 1.04 (–0.48 to 2.56) | 0.49 (–0.43 to 1.42) | –0.47 (–1.85 to 0.91) | 1.91* (0.18 to 3.65) | |
| Antipsychotics | 1.63* (0.30 to 2.95) | 1.21* (0.20 to 2.23) | 1.20 (–0.27 to 2.66) | 2.52 (–0.51 to 5.54) | |
| EQ-5D (staff rated) | Z-drugs | –0.02 (–0.07 to 0.03) | –0.02 (–0.06 to 0.03) | –0.02 (–0.10 to 0.06) | –0.05 (–0.15 to 0.04) |
| BZDs | –0.02 (–0.07 to 0.04) | 0.00 (–0.05 to 0.05) | 0.05 (–0.02 to 0.12) | 0.00 (–0.09 to 0.09) | |
| Antipsychotics | –0.06 (–0.16 to 0.04) | –0.04 (–0.09 to 0.01) | –0.03 (–0.11 to 0.04) | –0.13 (–0.26 to 0.01) | |
| VAS (staff rated) | Z-drugs | –5.37 (–12.76 to 2.03) | –6.76* (–12.73 to –0.79) | –3.88 (–14.80 to 7.04) | –1.11 (–14.62 to 12.41) |
| BZDs | –4.33 (–15.73 to 7.06) | –2.00 (–8.58 to 4.59) | –1.74 (–12.08 to 8.59) | 1.18 (–12.52 to 14.87) | |
| Antipsychotics | –5.91 (–13.72 to 1.89) | 1.16 (–5.56 to 7.89) | –1.79 (–12.09 to 8.51) | 2.82 (–14.78 to 20.42) | |
| MMSE | Z-drugs | –0.43 (–1.91 to 1.05) | 0.10 (–0.78 to 0.99) | –0.16 (–1.63 to 1.30) | –1.20 (–3.50 to 1.10) |
| BZDs | 0.28 (–1.06 to 1.61) | –0.55 (–1.42 to 0.32) | –0.82 (–2.16 to 0.51) | 0.85 (–1.45 to 3.15) | |
| Antipsychotics | –0.44 (–1.56 to 0.68) | –0.33 (–1.29 to 0.62) | –0.11 (–1.53 to 1.30) | –0.91 (–4.91 to 3.10) | |
| SIB-8 | Z-drugs | –0.84 (–2.03 to 0.35) | 0.33 (–0.38 to 1.04) | 0.16 (–1.04 to 1.37) | –0.14 (–1.87 to 1.59) |
| BZDs | 0.43 (–0.55 to 1.41) | –0.14 (–0.84 to 0.56) | 0.20 (–0.90 to 1.30) | 0.37 (–1.36 to 2.09) | |
| Antipsychotics | –0.01 (–0.91 to 0.90) | 0.05 (–0.71 to 0.81) | –0.27 (–1.44 to 0.90) | –0.39 (–3.40 to 2.61) | |
| CDR | Z-drugs | 0.51 (–0.16 to 1.18) | –0.10 (–0.53 to 0.34) | 0.66 (–0.07 to 1.39) | –0.44 (–1.29 to 0.41) |
| BZDs | 0.69* (0.12 to 1.26) | 0.52* (0.09 to 0.95) | 0.71* (0.05 to 1.37) | 0.39 (–0.45 to 1.23) | |
| Antipsychotics | 0.53 (–0.19 to 1.25) | 0.41 (–0.06 to 0.88) | 0.31 (–0.40 to 1.01) | 0.61 (–0.85 to 2.07) | |
| Disability | Z-drugs | –0.07 (–0.75 to 0.60) | –0.17 (–0.69 to 0.35) | 0.29 (–0.60 to 1.18) | –0.52 (–1.53 to 0.49) |
| BZDs | 0.08 (–0.60 to 0.75) | –0.11 (–0.62 to 0.40) | –0.07 (–0.88 to 0.74) | 0.26 (–0.75 to 1.27) | |
| Antipsychotics | 0.50 (–0.32 to 1.33) | 0.74** (0.18 to 1.31) | 0.56 (–0.30 to 1.42) | 1.24 (–0.52 to 3.00) | |
| Exposure | Pattern | β (95% CI) | ||
|---|---|---|---|---|
| Agitation | Sleep | Anxiety | ||
| Z-drugs | No use | Ref | Ref | Ref |
| Starting | 0.09 (–0.06 to 0.25) | –0.27 (–0.58 to 0.03) | 0.02 (–0.16 to 0.20) | |
| Stopping | –0.02 (–0.14 to 0.10) | –0.10 (–0.35 to 0.16) | 0.03 (–0.13 to 0.20) | |
| Continuing | 0.05 (–0.02 to 0.12) | –0.03 (–0.14 to 0.08) | 0.00 (–0.09 to 0.08) | |
| BZDs | No use | Ref | Ref | Ref |
| Starting | 0.04 (–0.13 to 0.21) | 0.16 (–0.11 to 0.44) | 0.01 (–0.18 to 0.19) | |
| Stopping | 0.01 (–0.14 to 0.16) | 0.24 (–0.02 to 0.50) | 0.00 (–0.20 to 0.19) | |
| Continuing | –0.01 (–0.08 to 0.05) | –0.01 (–0.13 to 0.11) | 0.05 (–0.04 to 0.14) | |
| Antipsychotics | No use | Ref | Ref | Ref |
| Starting | 0.15 (–0.06 to 0.36) | 0.02 (–0.27 to 0.31) | 0.22 (–0.02 to 0.46) | |
| Stopping | 0.15 (–0.03 to 0.32) | 0.15 (–0.08 to 0.38) | 0.12 (–0.10 to 0.34) | |
| Continuing | –0.10* (–0.19 to –0.01) | –0.10 (–0.24 to 0.05) | –0.01 (–0.13 to 0.12) | |
| Exposure | β (95% CI) | ||
|---|---|---|---|
| VAS | QUALIDa | EQ-5D (staff) | |
| Z-drugs | |||
| No use | Ref | Ref | Ref |
| Starting | –7.13 (–16.88 to 2.61) | 0.59 (–1.07 to 2.26) | –0.01 (–0.08 to 0.06) |
| Stopping | 4.02 (–3.11 to 11.15) | –0.60 (–2.35 to 1.15) | 0.06 (–0.01 to 0.13) |
| Continuing | 4.04 (–0.56 to 8.64) | –0.17 (–0.94 to 0.60) | 0.03 (–0.01 to 0.08) |
| BZDs | |||
| No use | Ref | Ref | Ref |
| Starting | –0.67 (–12.81 to 11.48) | 0.83 (–0.66 to 2.31) | 0.05 (–0.02 to 0.12) |
| Stopping | 7.38 (–7.13 to 21.89) | –0.97 (–2.60 to 0.66) | 0.09* (0.01 to 0.16) |
| Continuing | –1.07 (–6.52 to 4.39) | –0.23 (–0.94 to 0.48) | 0.03 (0.00 to 0.07) |
| Antipsychotics | |||
| No use | Ref | Ref | Ref |
| Starting | –9.95* (–19.75 to –0.14) | 1.66 (–0.19 to 3.50) | –0.03 (–0.10 to 0.05) |
| Stopping | –15.44*** (–24.03 to –6.85) | 0.42 (–1.66 to 2.51) | –0.03 (–0.12 to 0.05) |
| Continuing | –5.96* (–11.74 to –0.17) | –0.01 (–0.98 to 0.96) | –0.01 (–0.05 to 0.04) |
FIGURE 3.
Association between changing hypnotic use status between previous and current visit on SIB-8 scores (higher scores represent better cognitive function) at previous and current visit (a, c and e). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (b, d and f). Data from the REDIC-NH study. AP, antipsychotic.
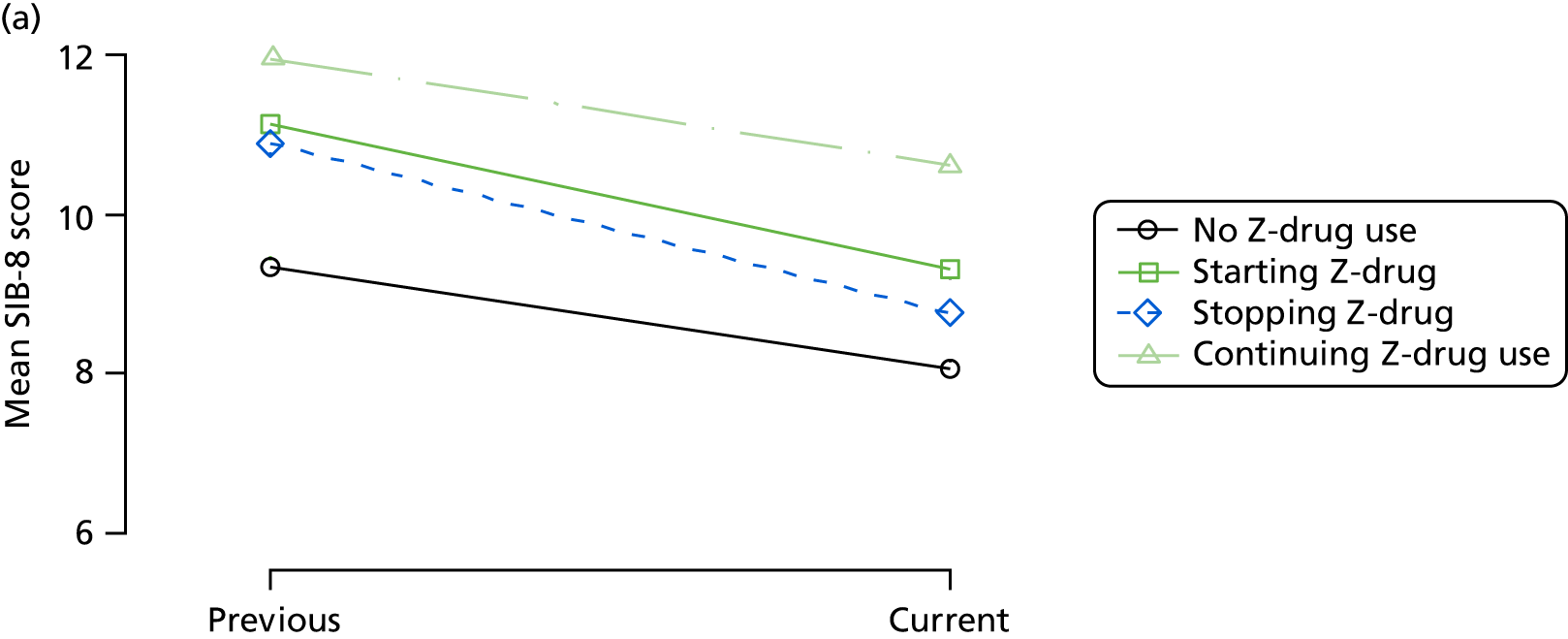
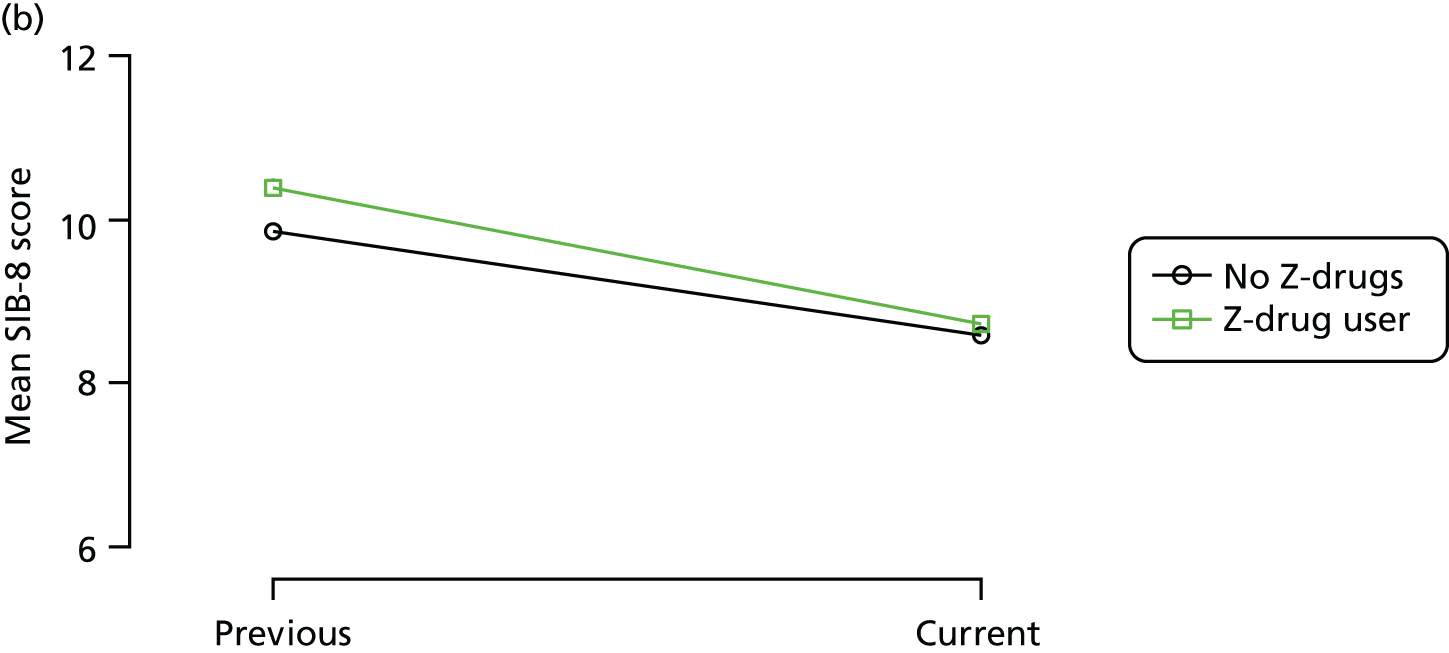
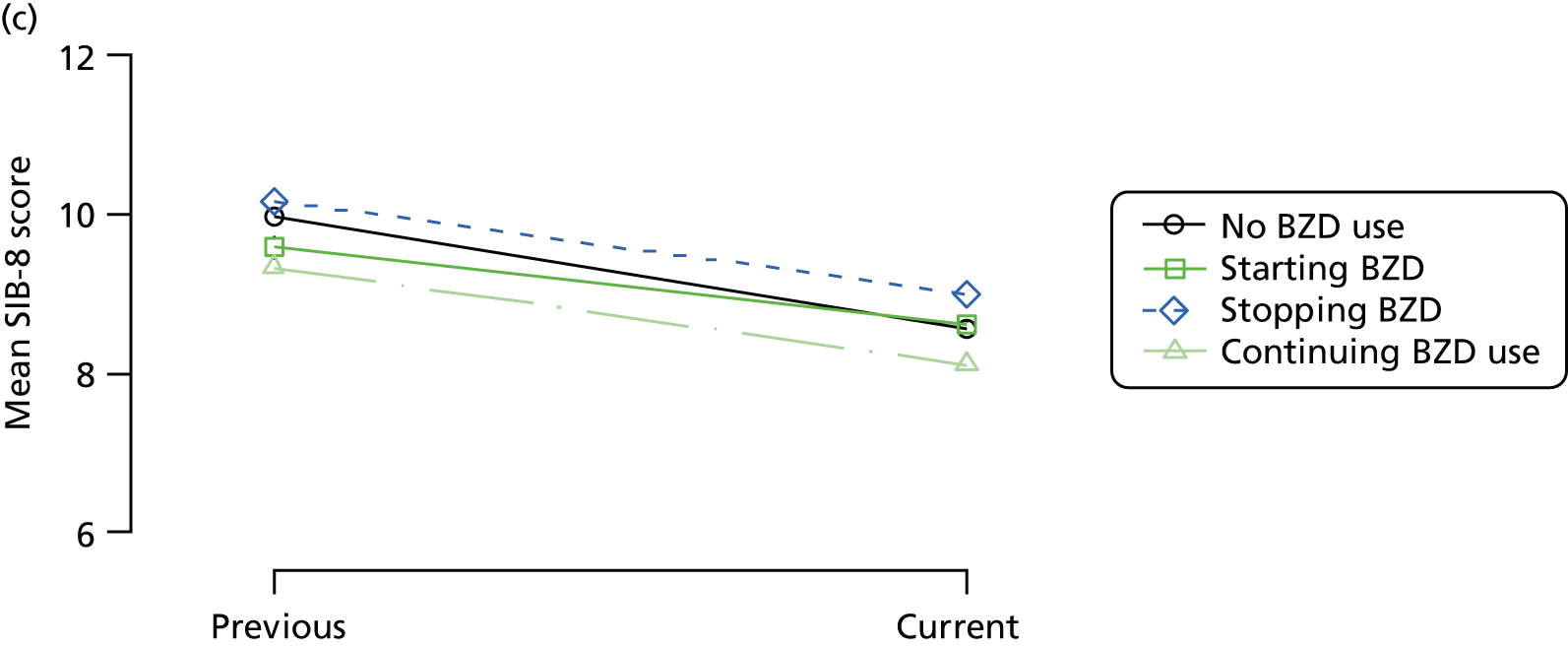
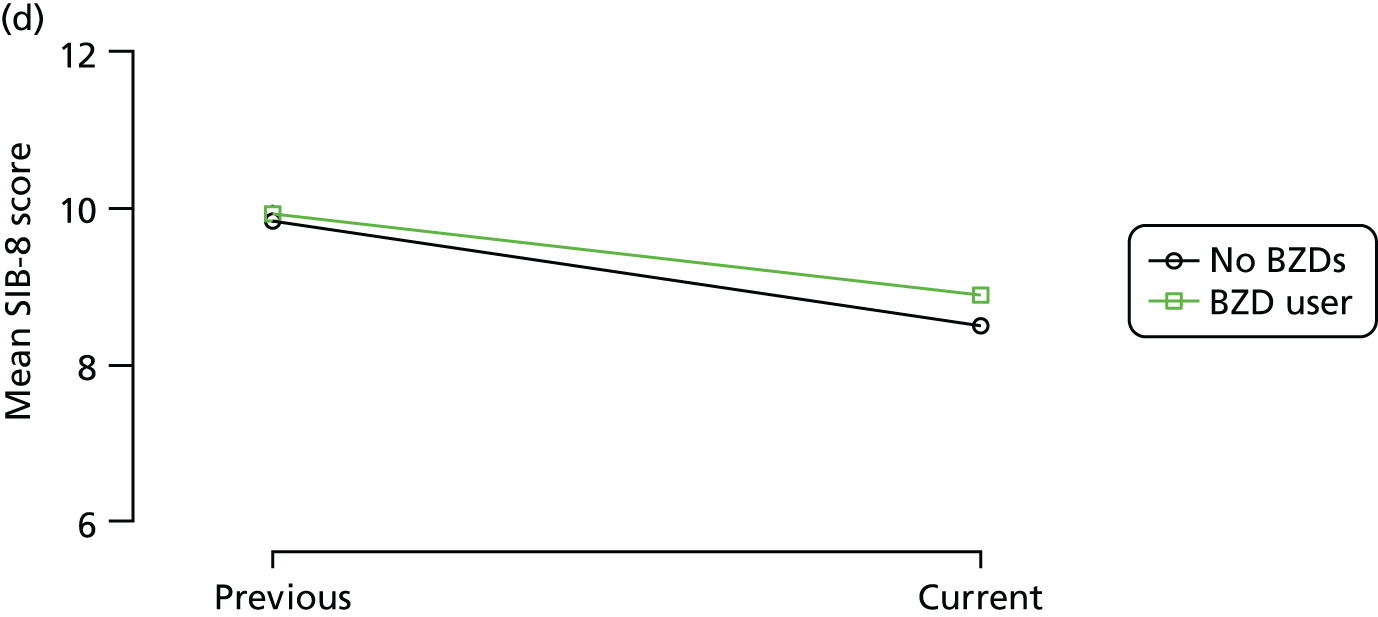
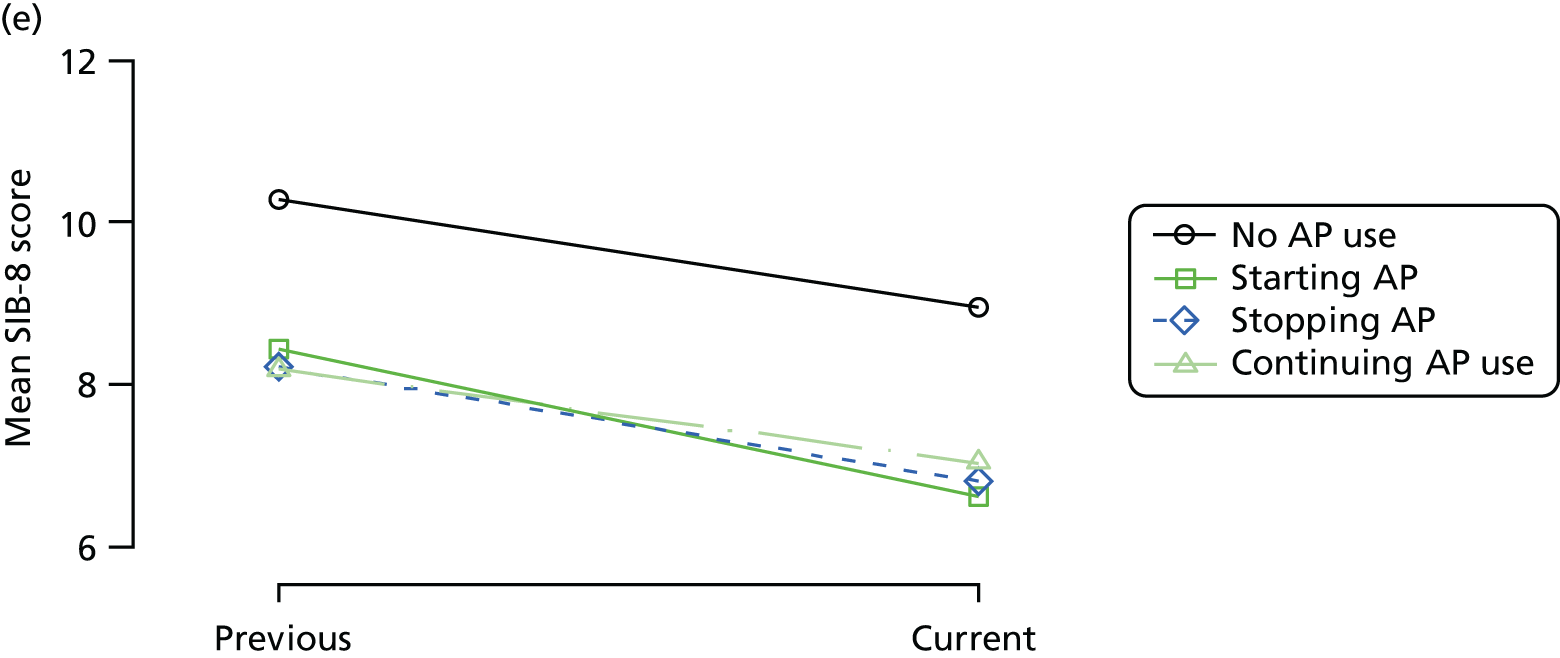
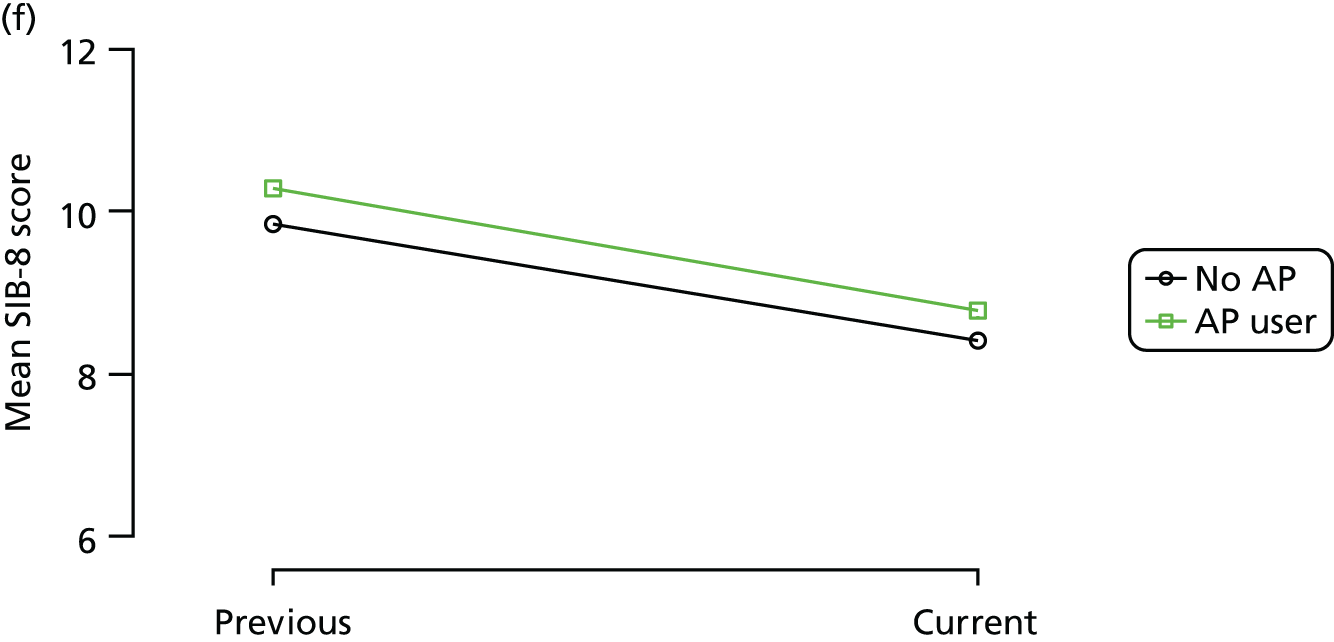
FIGURE 4.
Association between changing hypnotic use status between previous and current visit on MMSE scores (higher scores represent better cognitive function) at previous and current visit (a, c and e). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (b, d and f). Data from the REDIC-NH study. AP, antipsychotic.
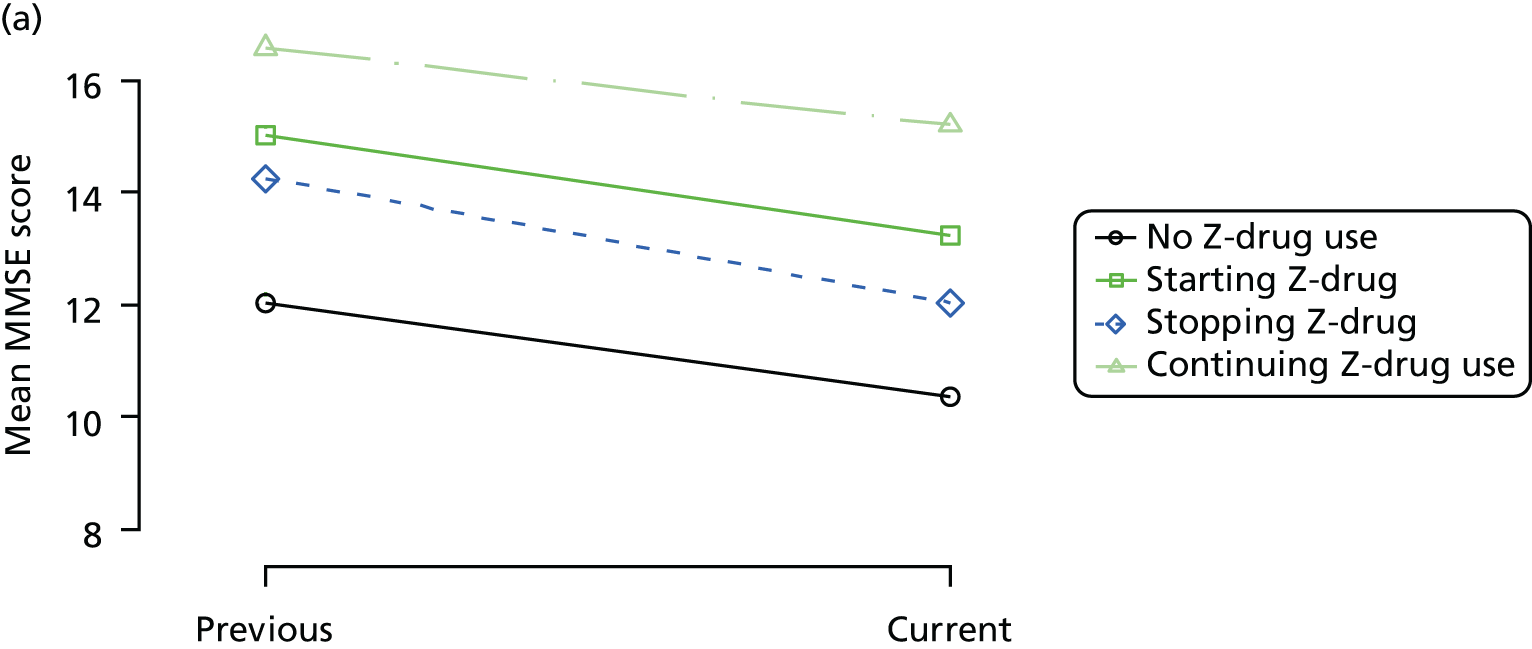
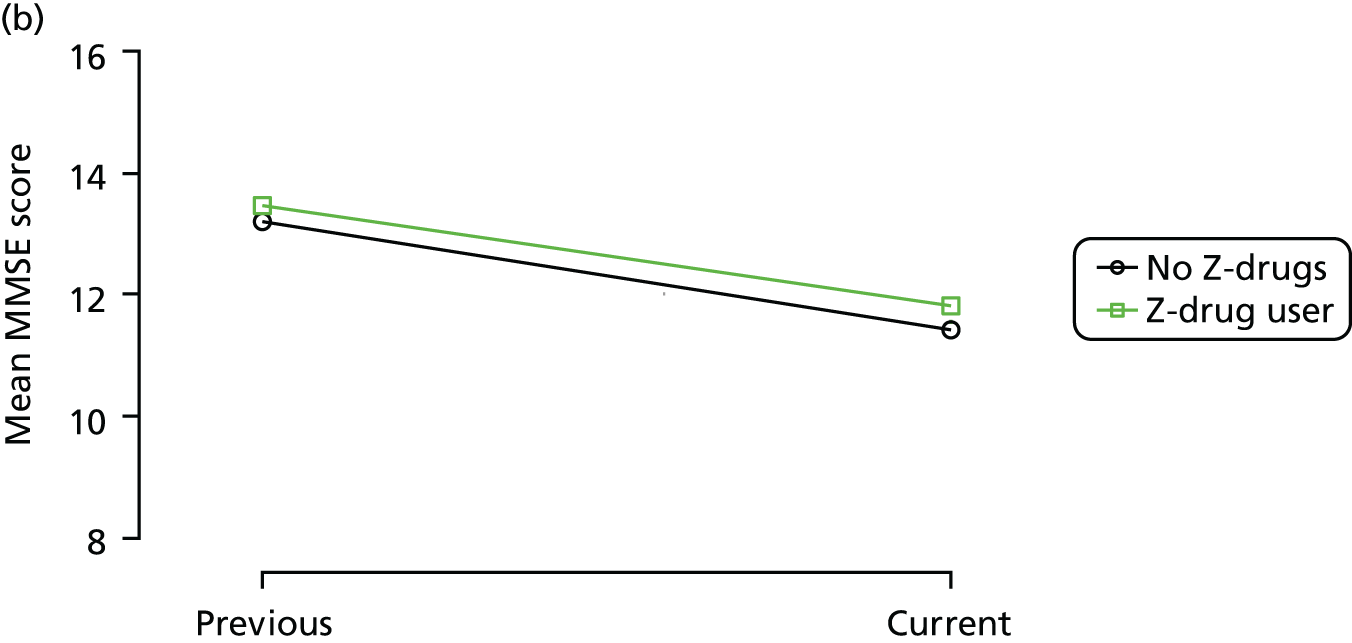
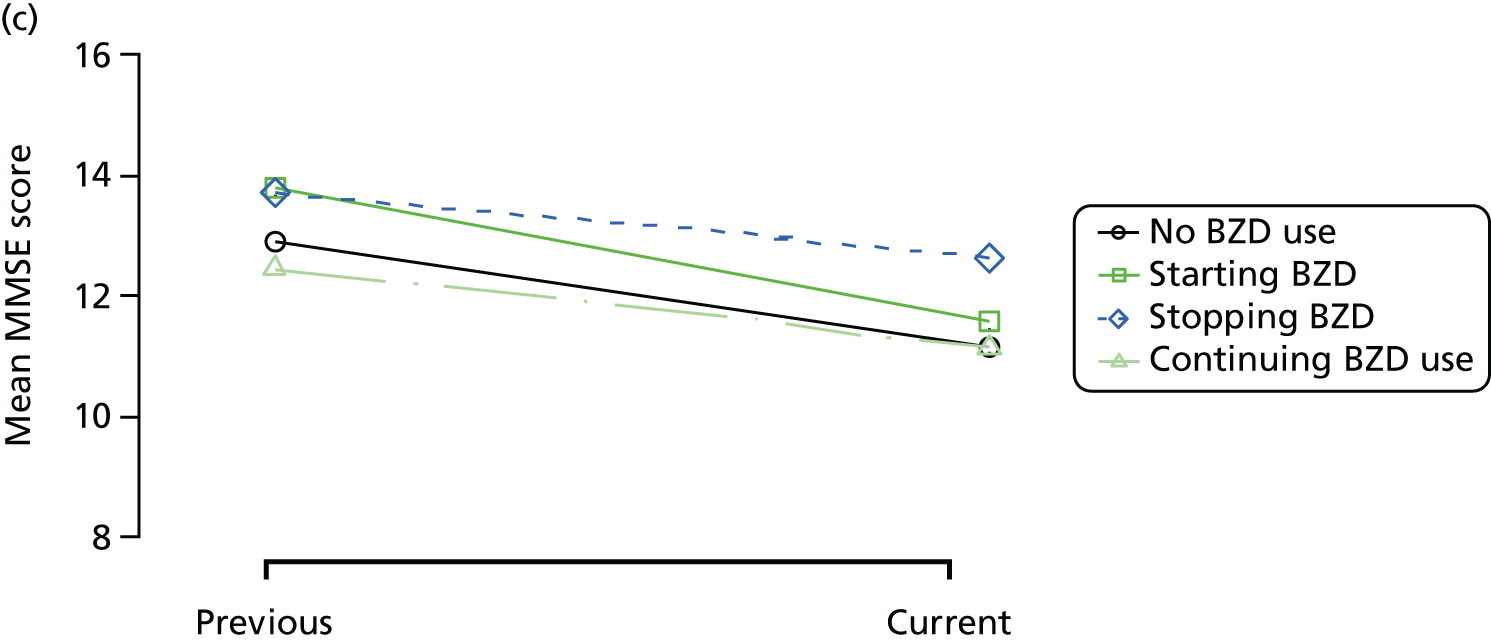
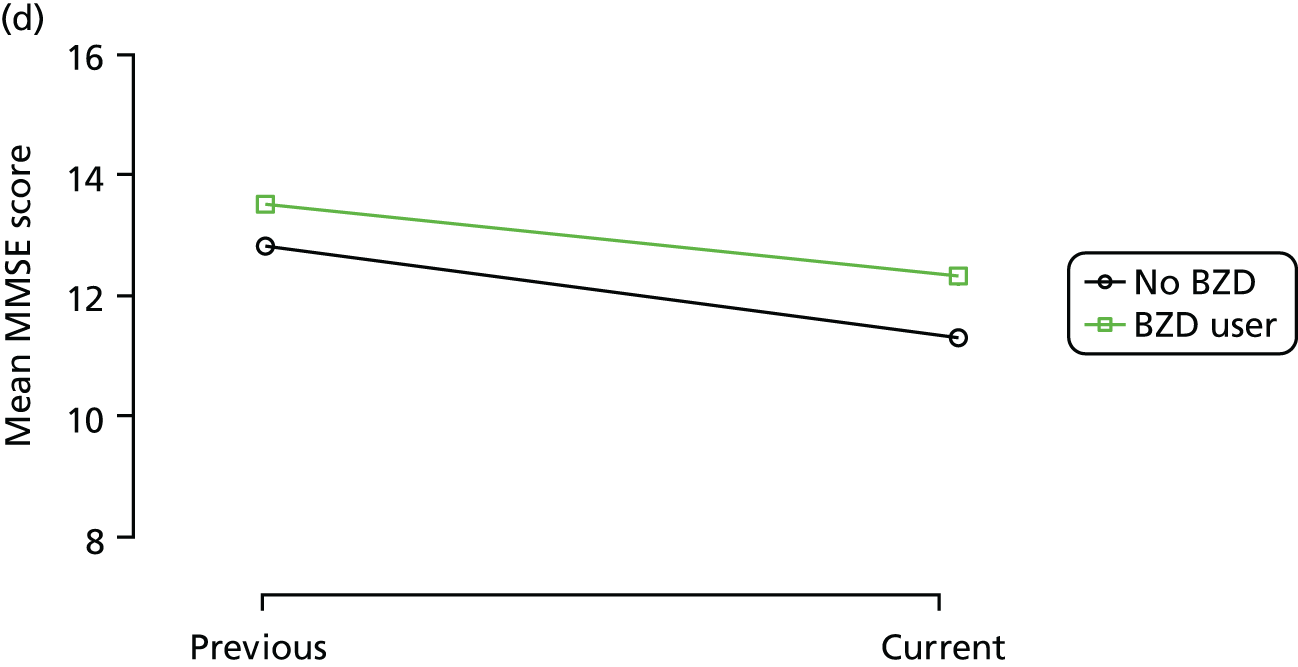
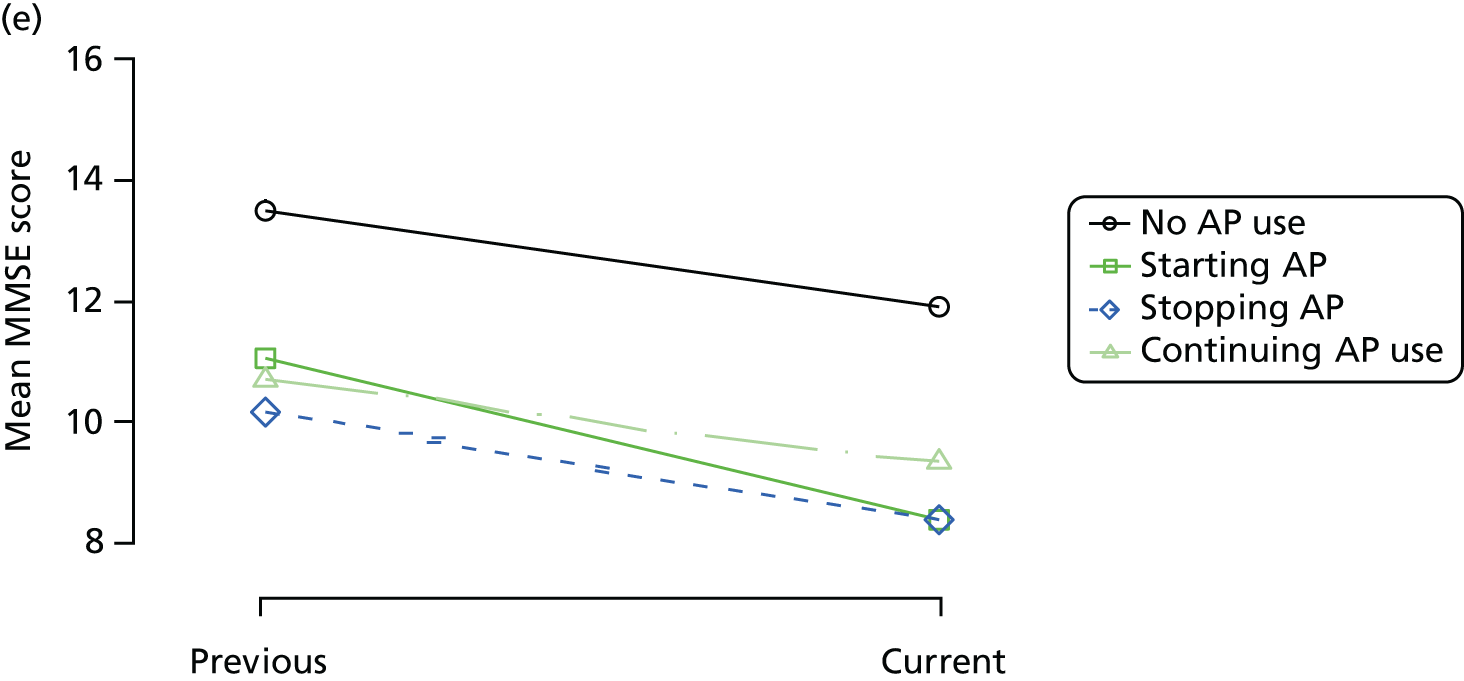
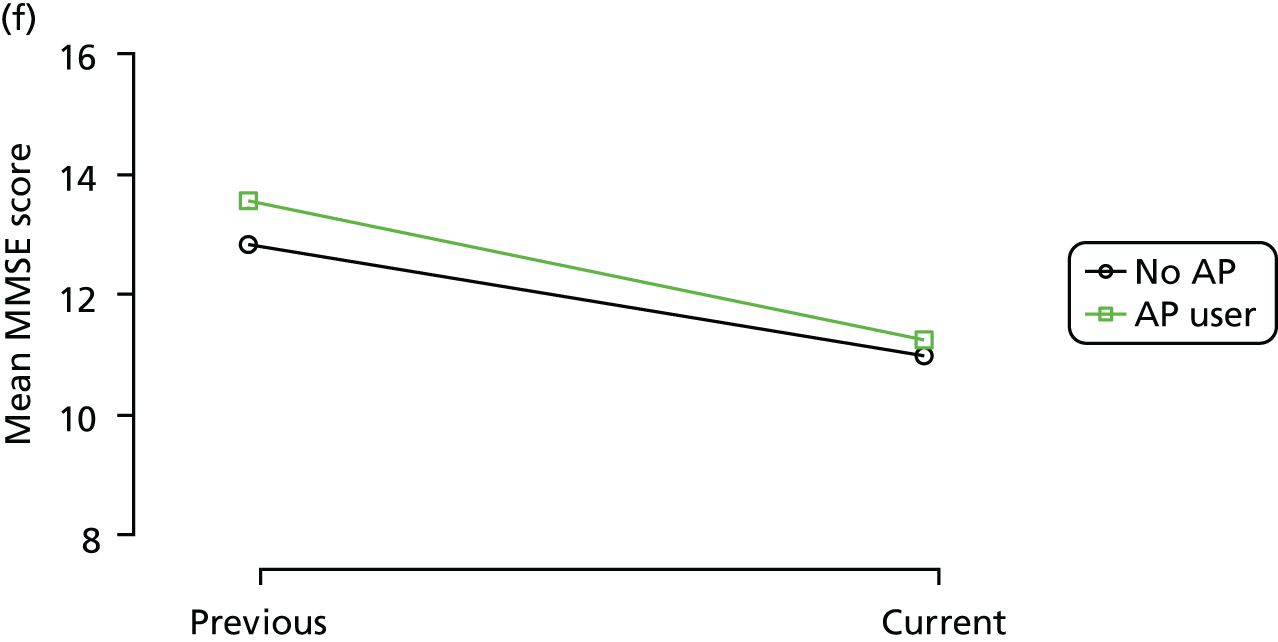
FIGURE 5.
Association between changing hypnotic use status between previous and current visit on mean CDR-SOB scores (high scores represent more cognitive impairment) at previous and current visit (a, c and e). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (b, d and f). Data from the REDIC-NH study. AP, antipsychotic.
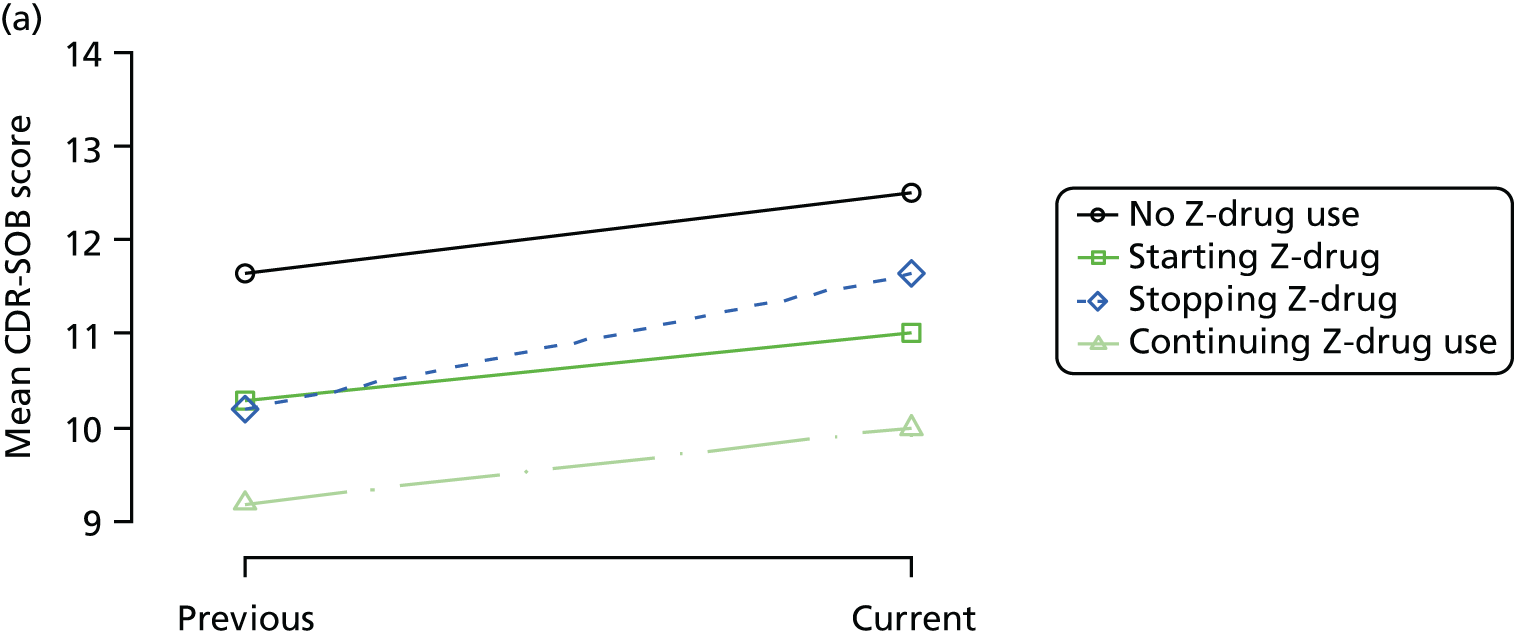
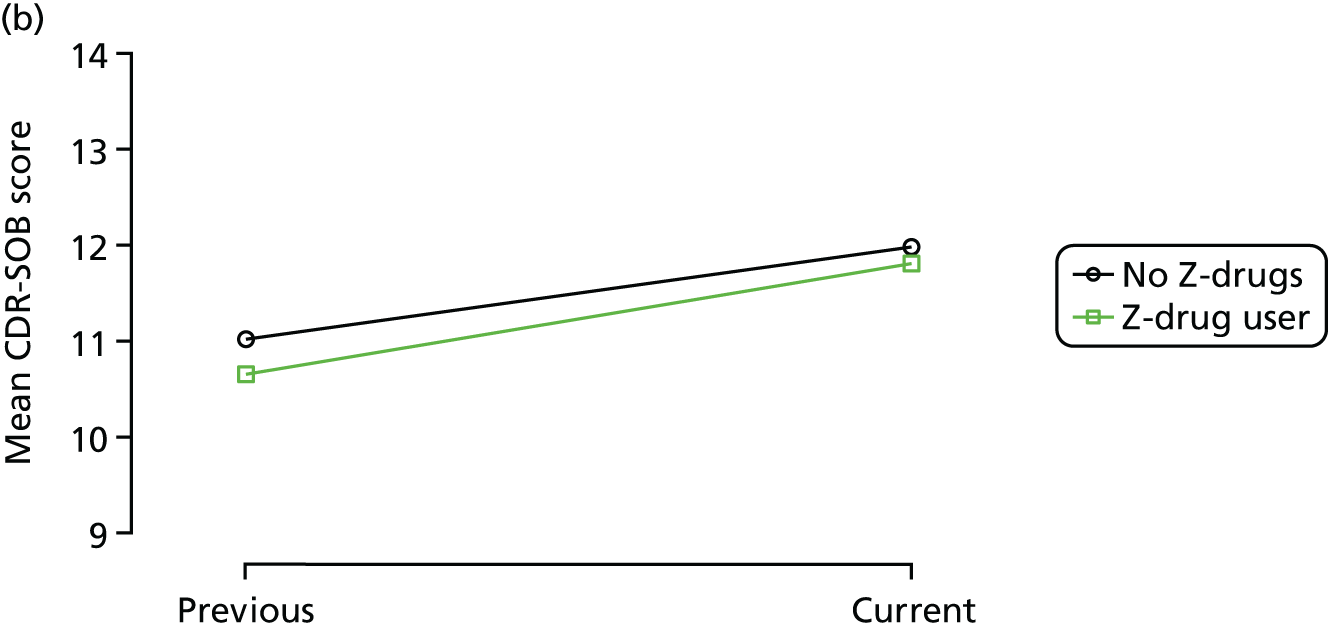
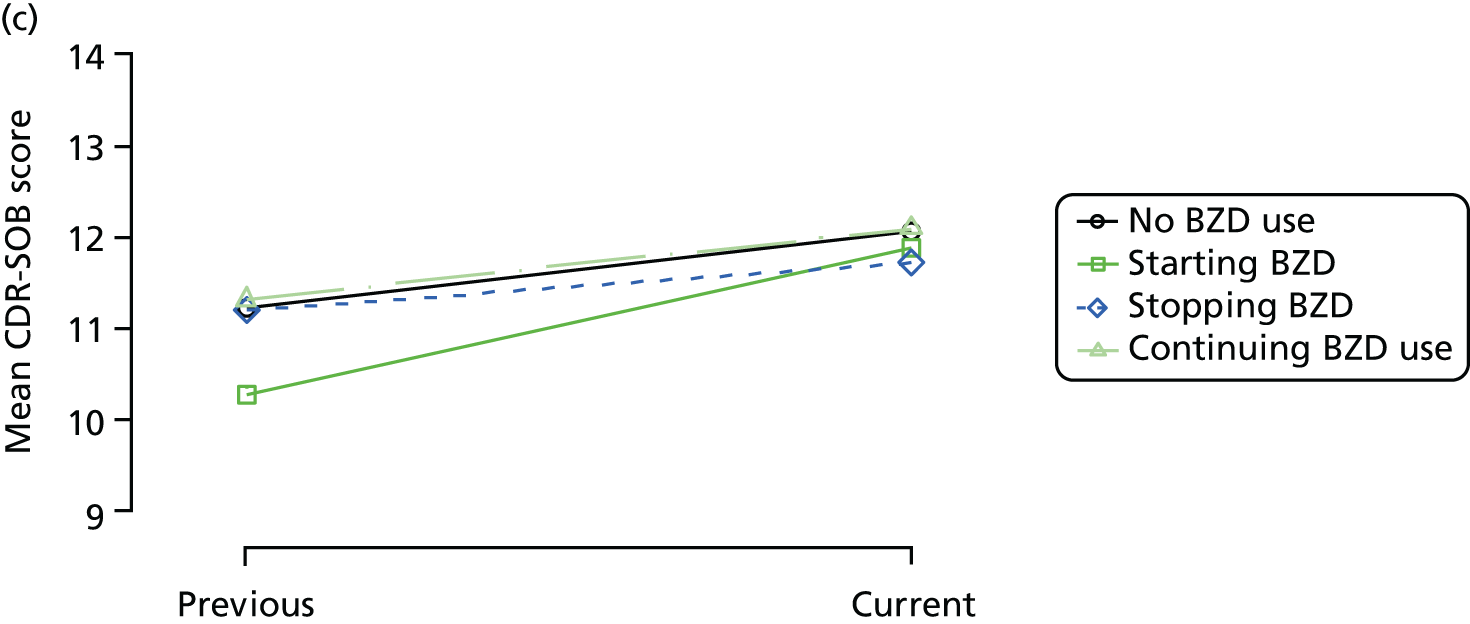
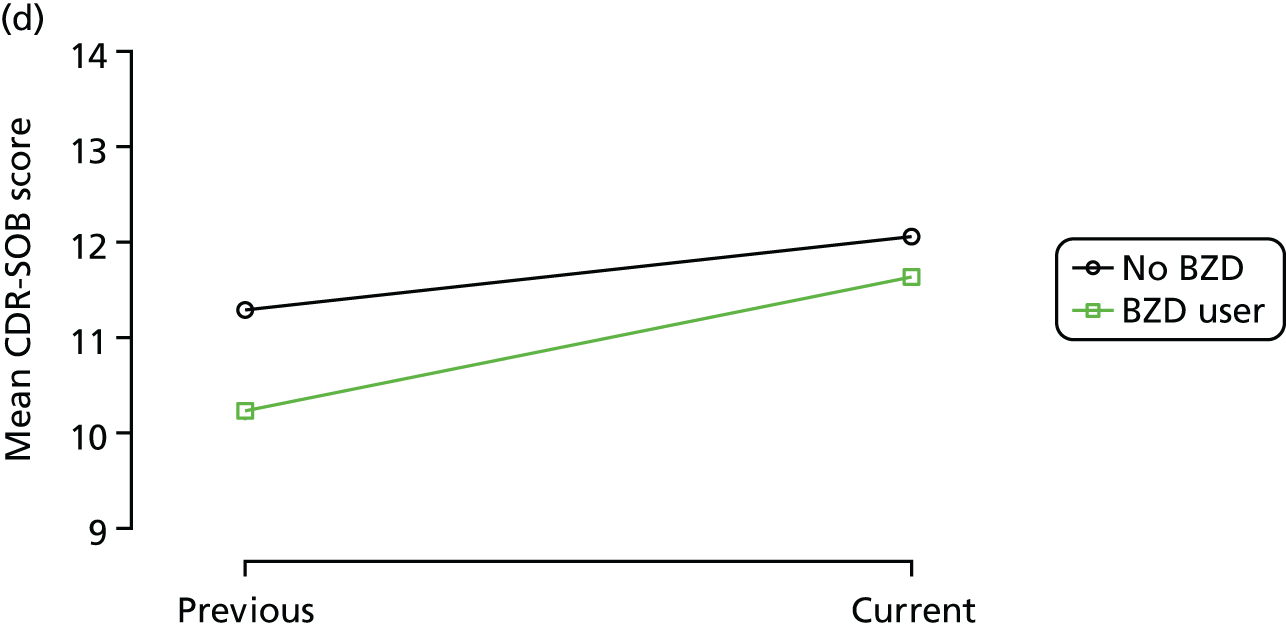
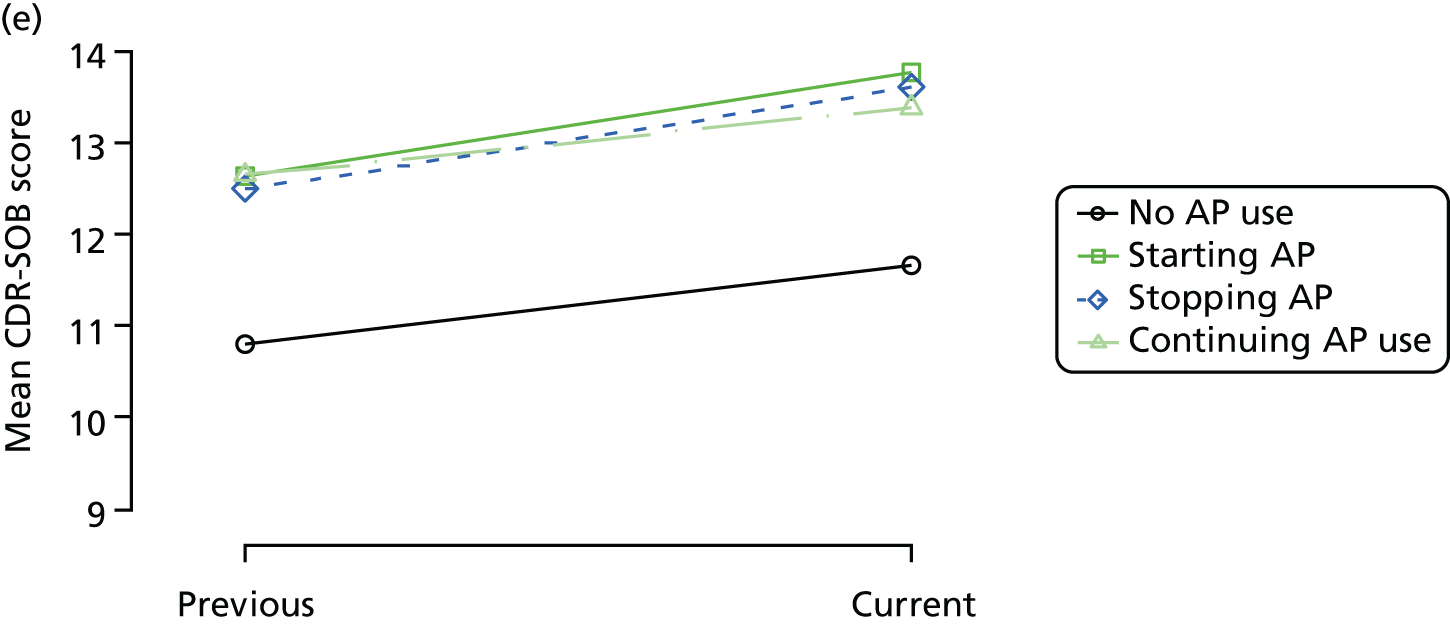
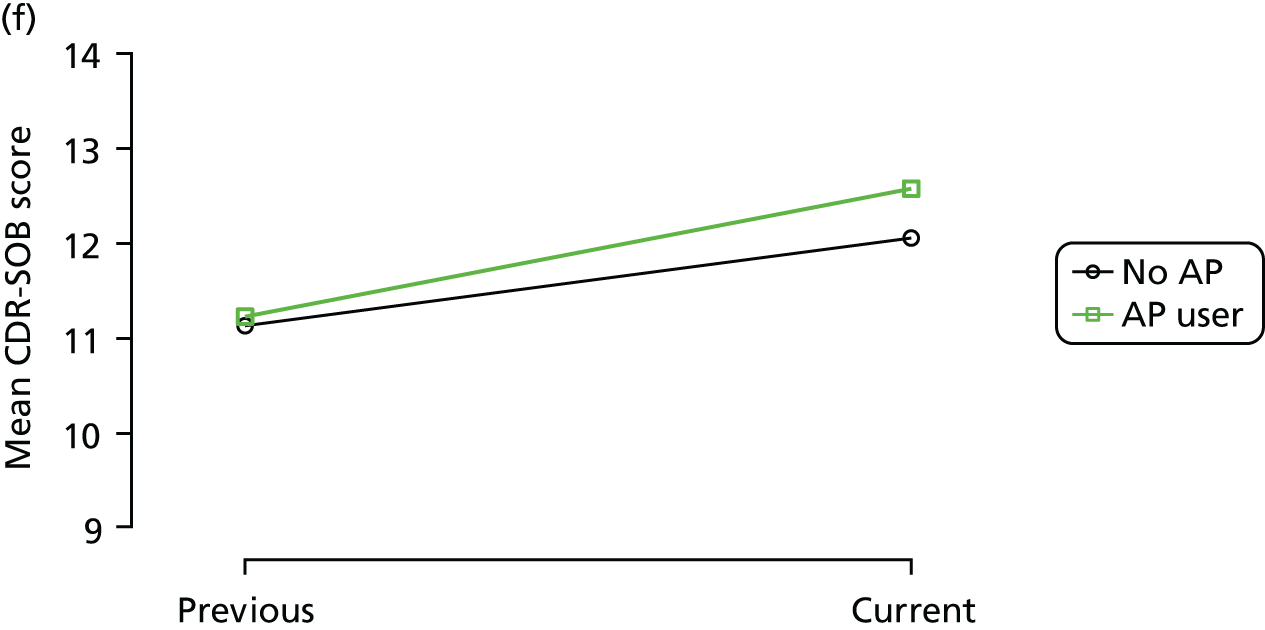
FIGURE 6.
Association between changing hypnotic use status between previous and current visit on sleep disturbance scores at previous and current visit (a, c and e). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (b, d and f). Data from the REDIC-NH study. AP, antipsychotic.
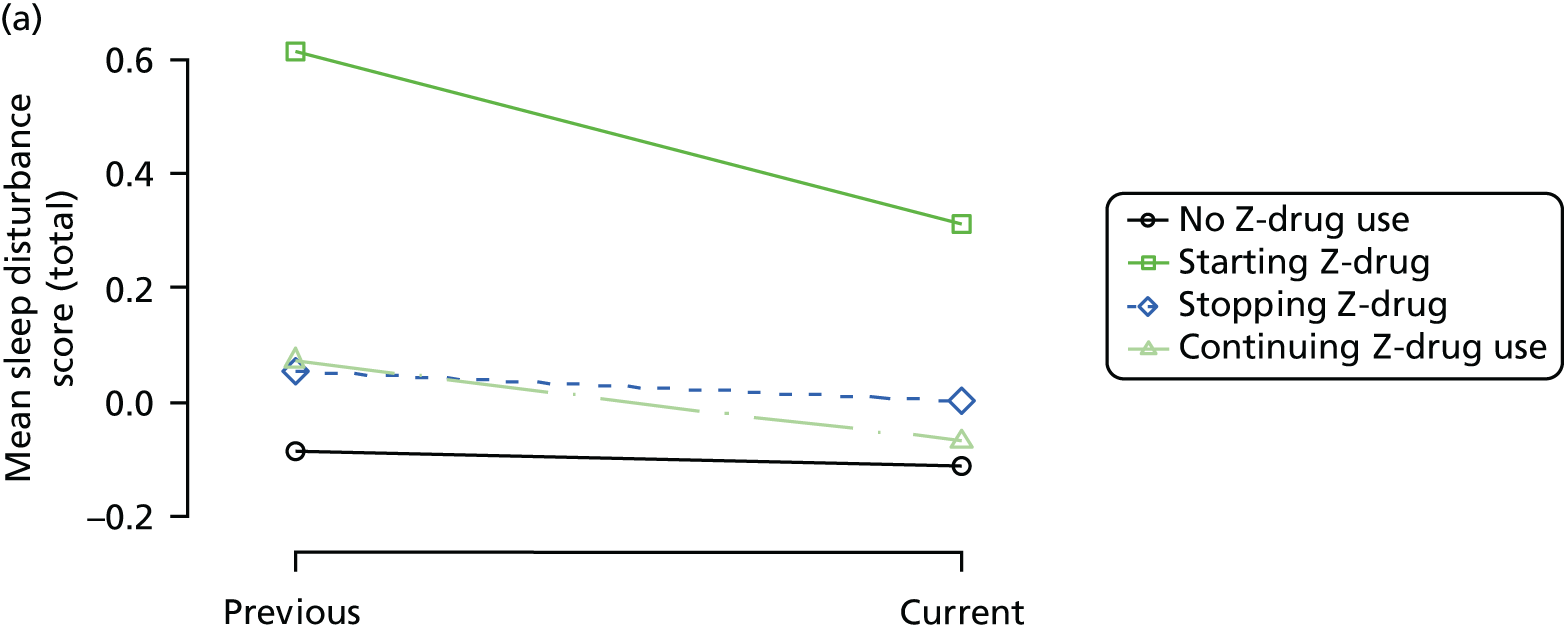
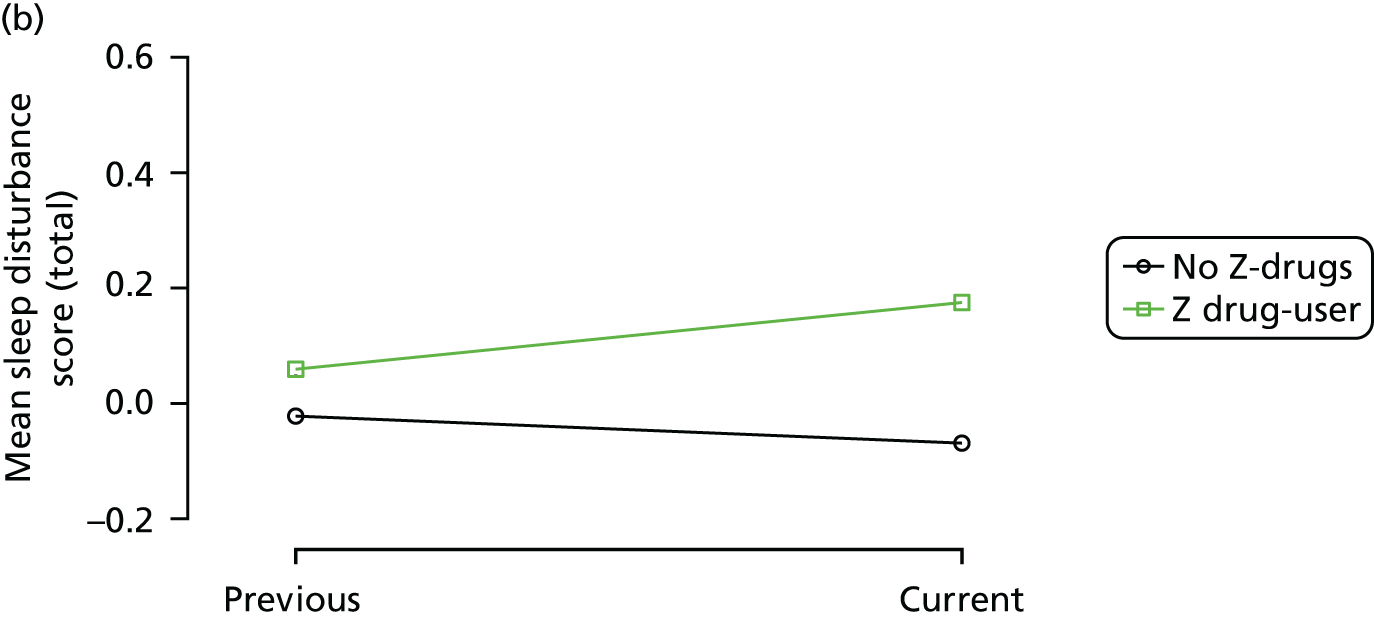
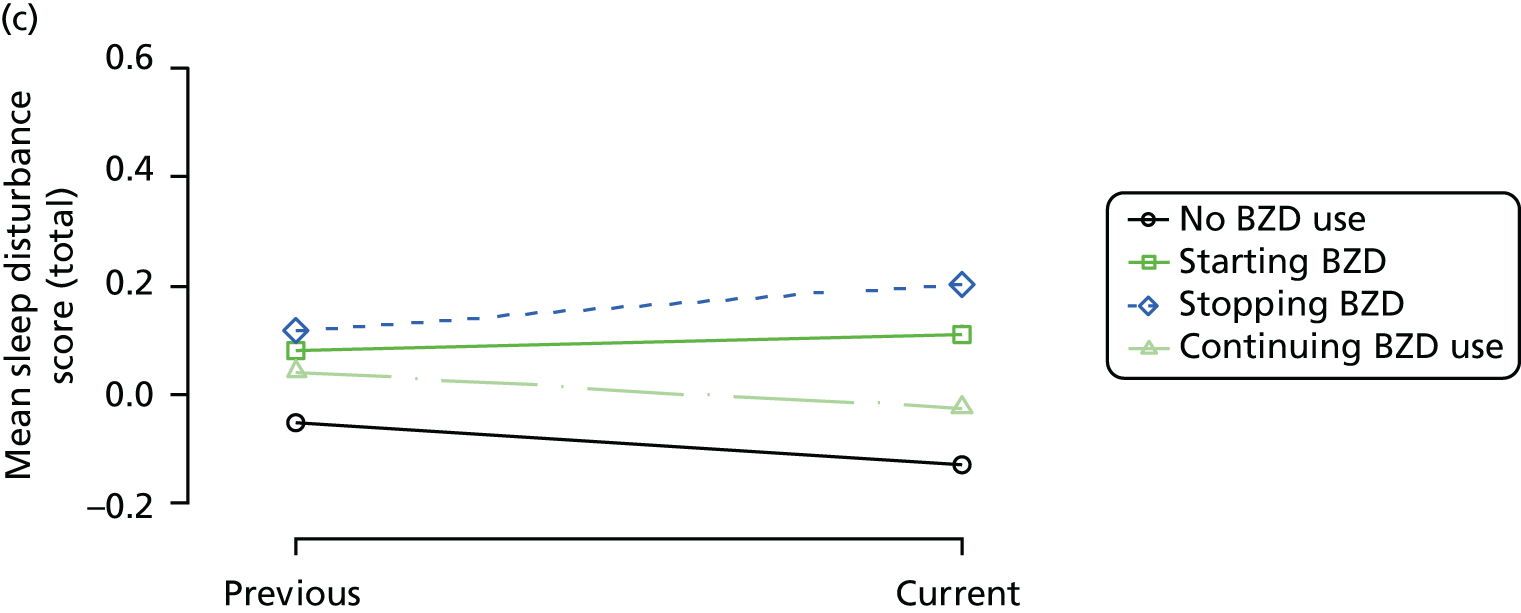
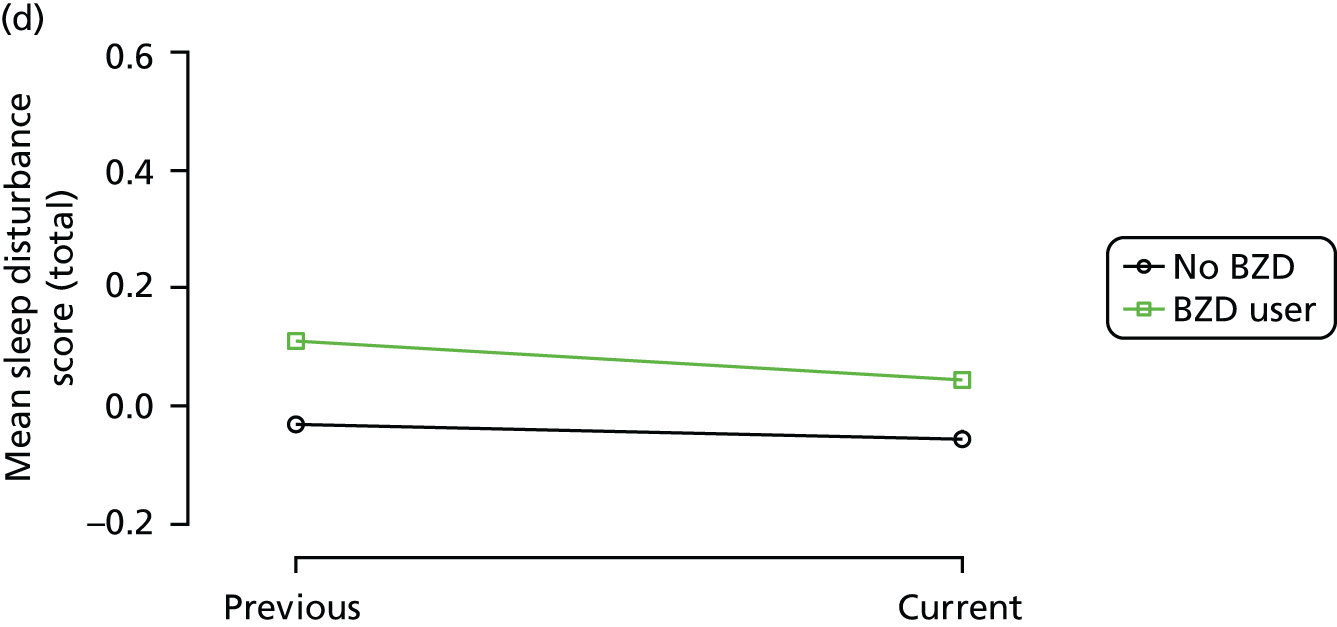
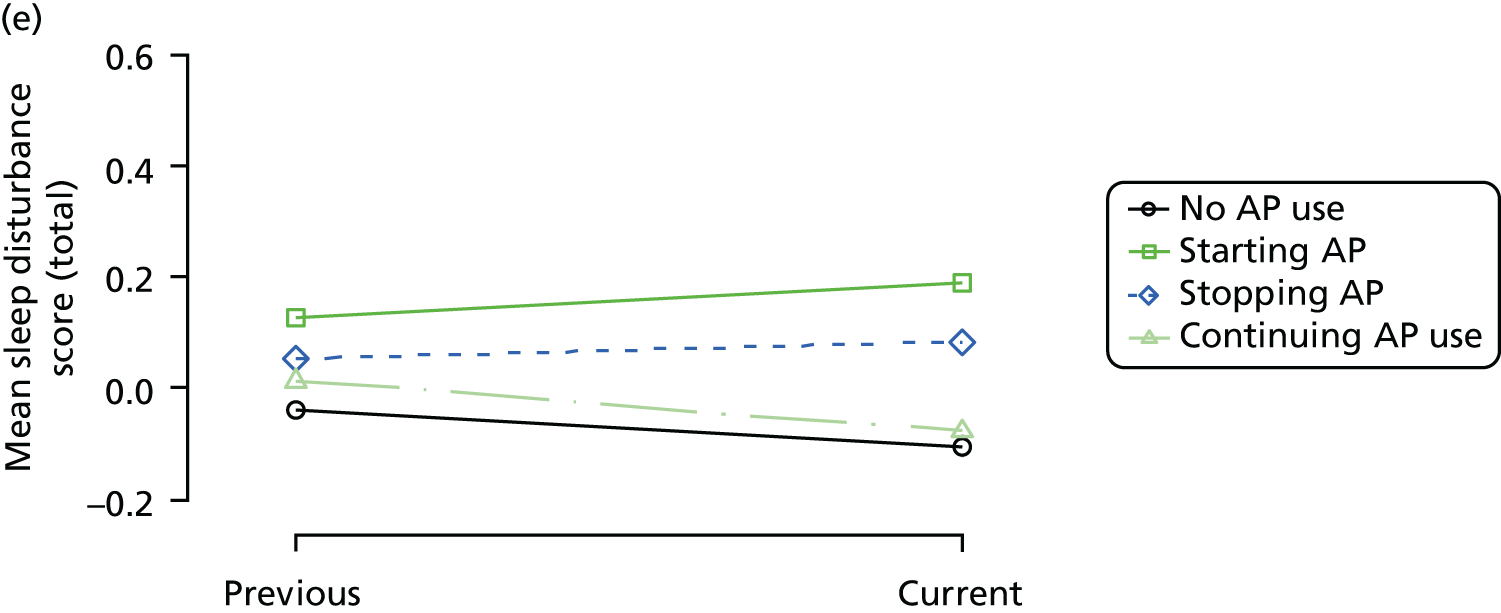
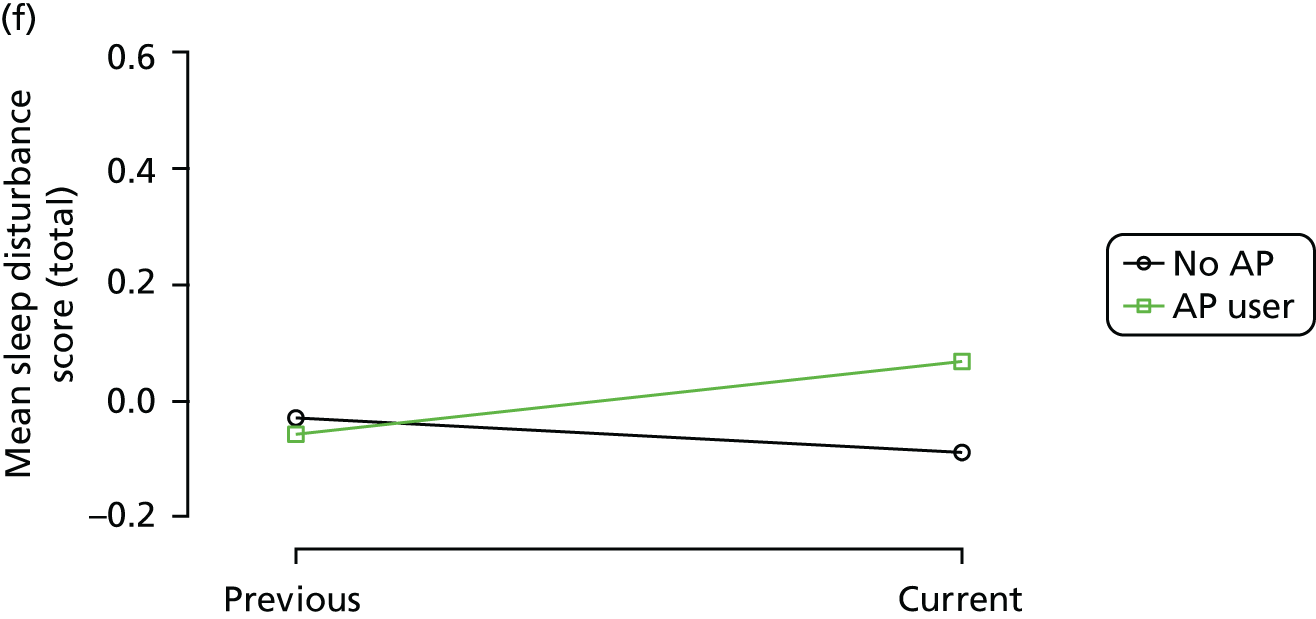
FIGURE 7.
Association between changing hypnotic use status between previous and current visit on mean agitation scores at previous and current visit (a, c and e). Mean scores stratified by current hypnotic use, with inverse probability weights to account for differences in prior predictors of hypnotic use (b, d and f). Data from the REDIC-NH study. AP, antipsychotic.
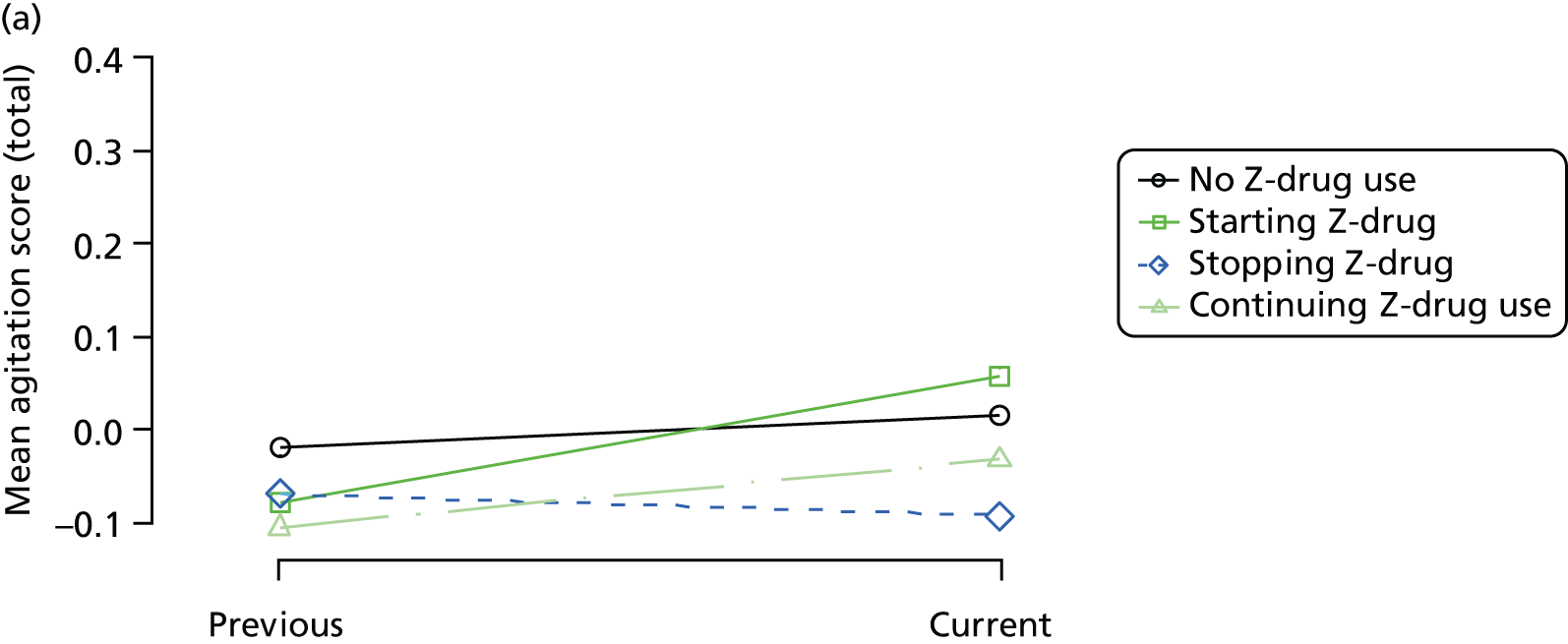
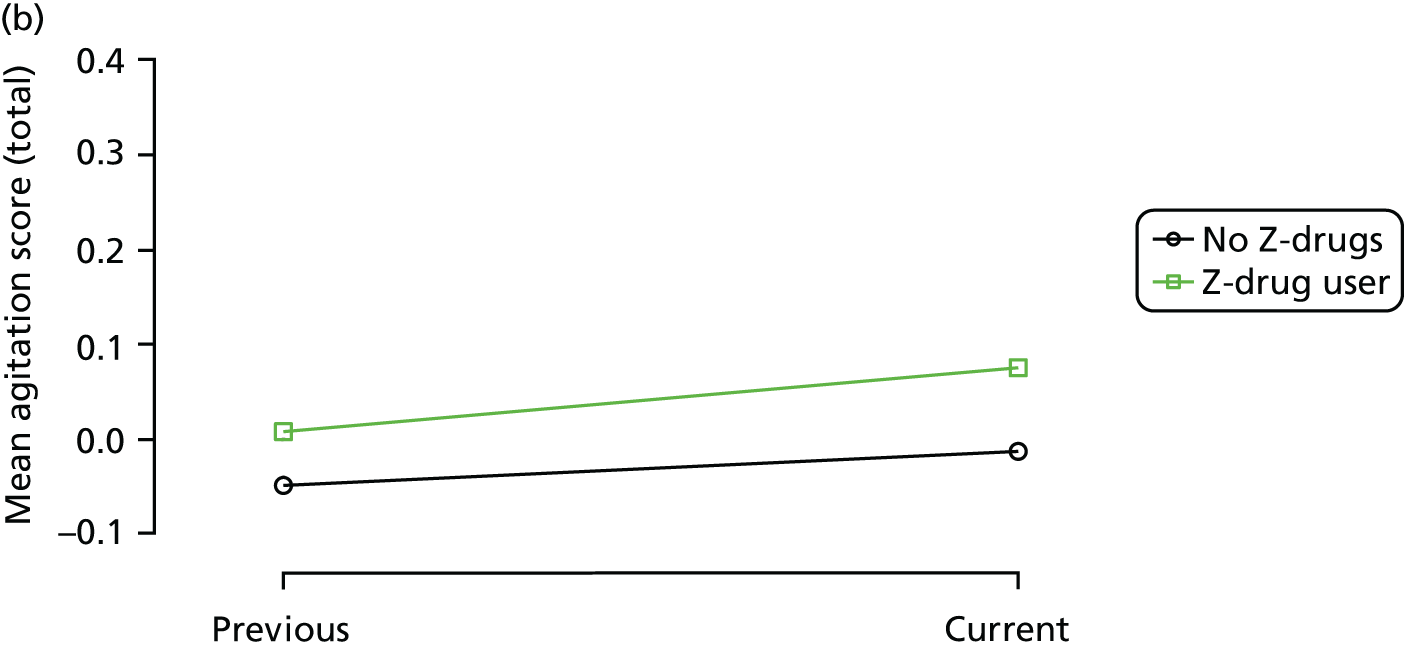
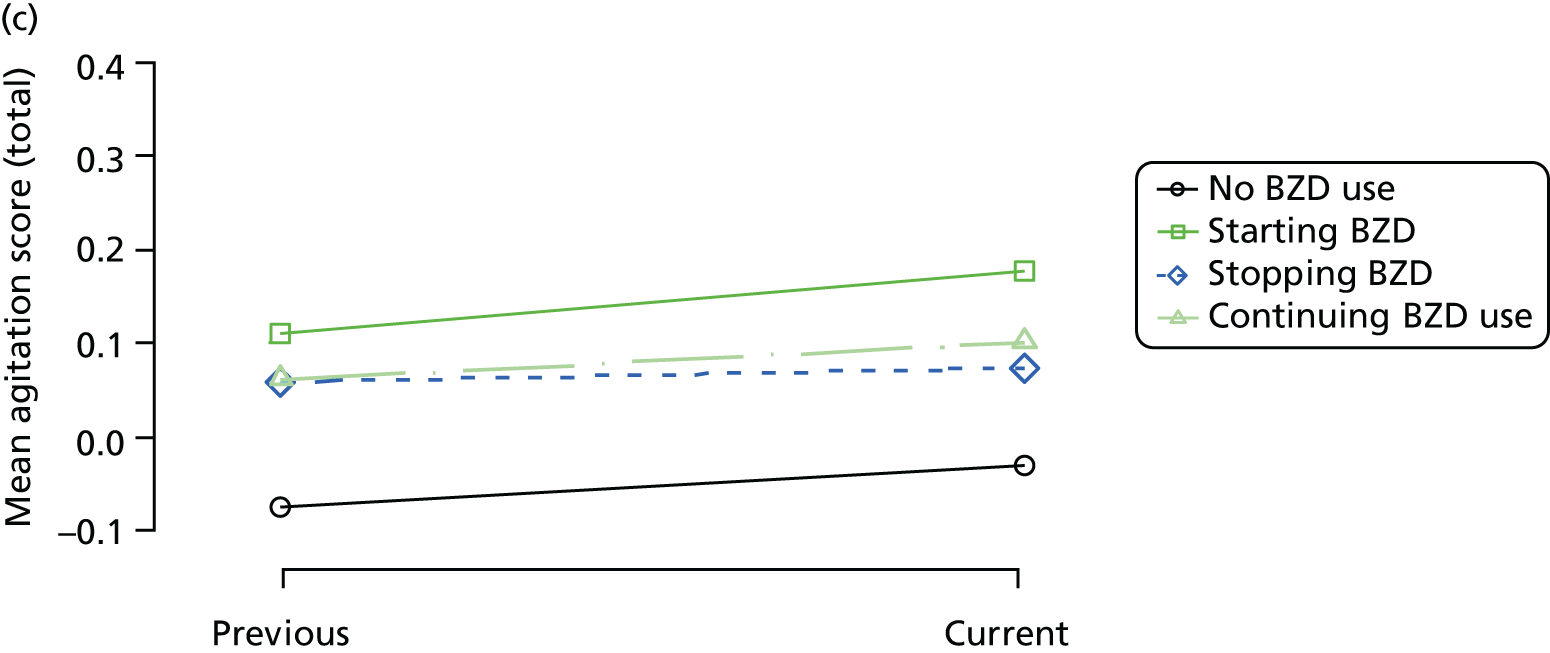
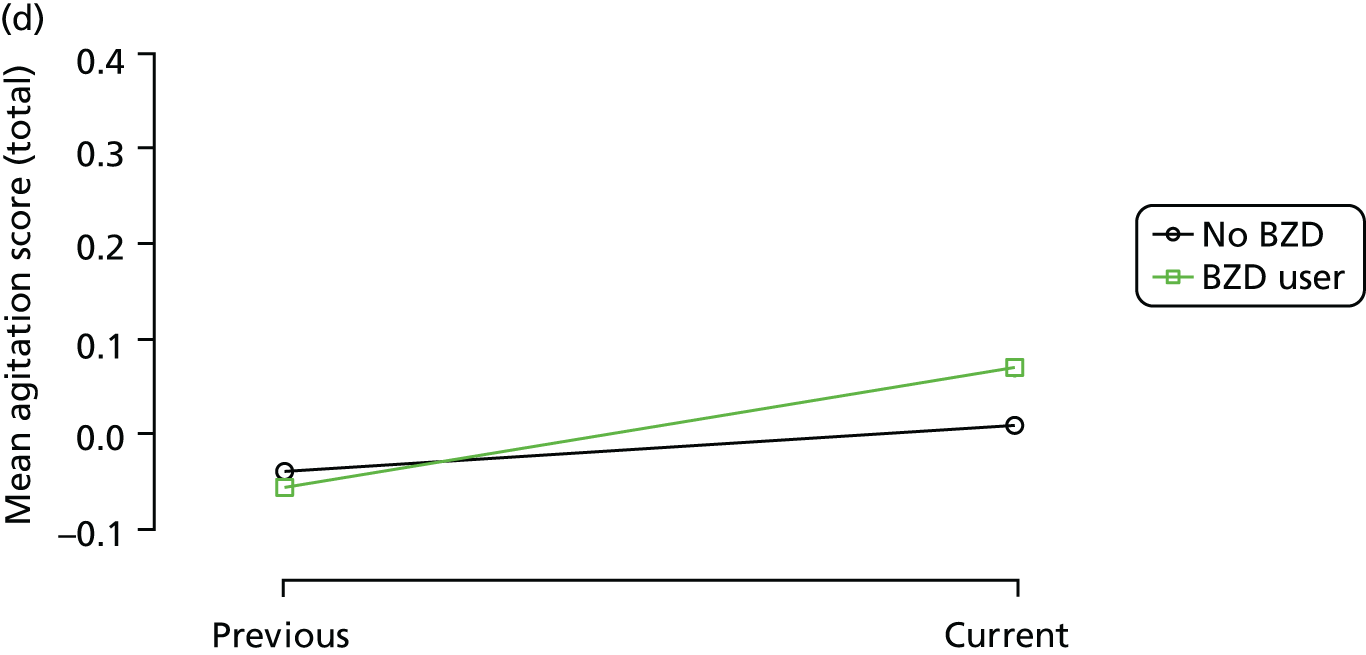
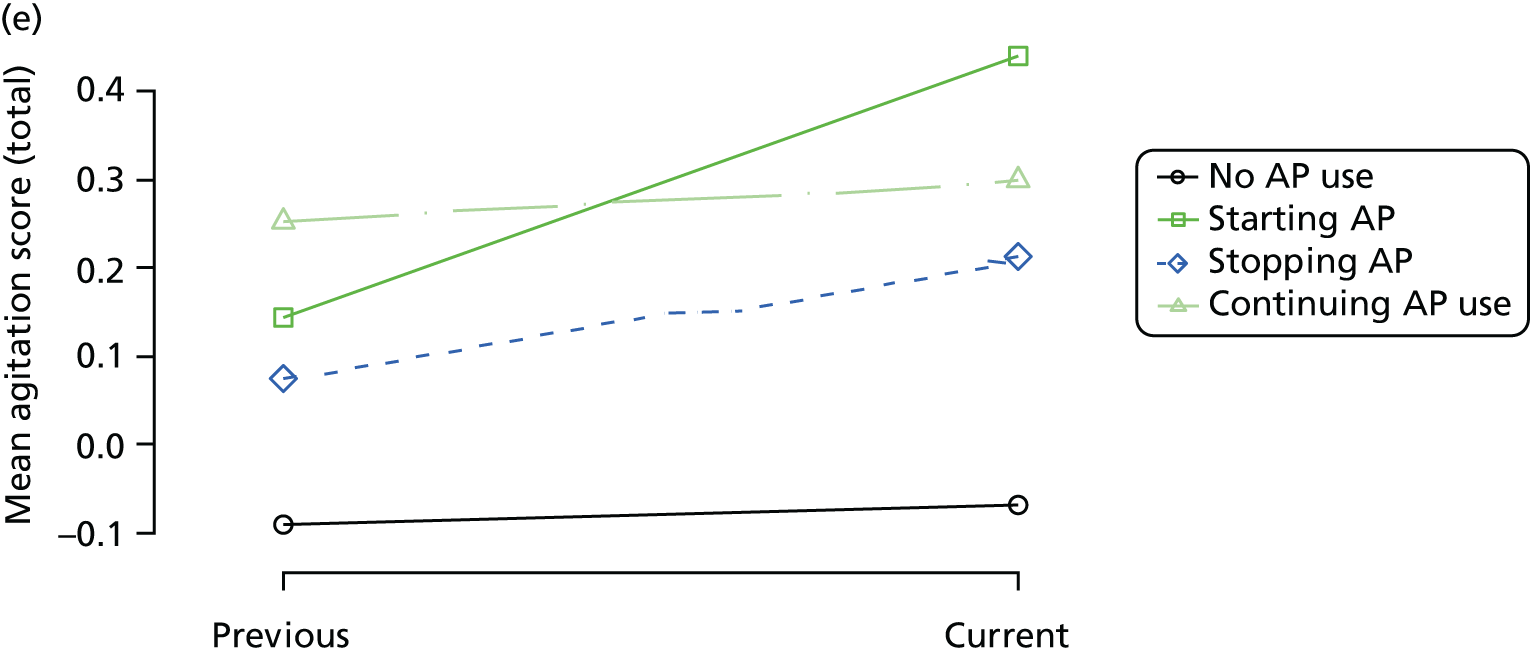
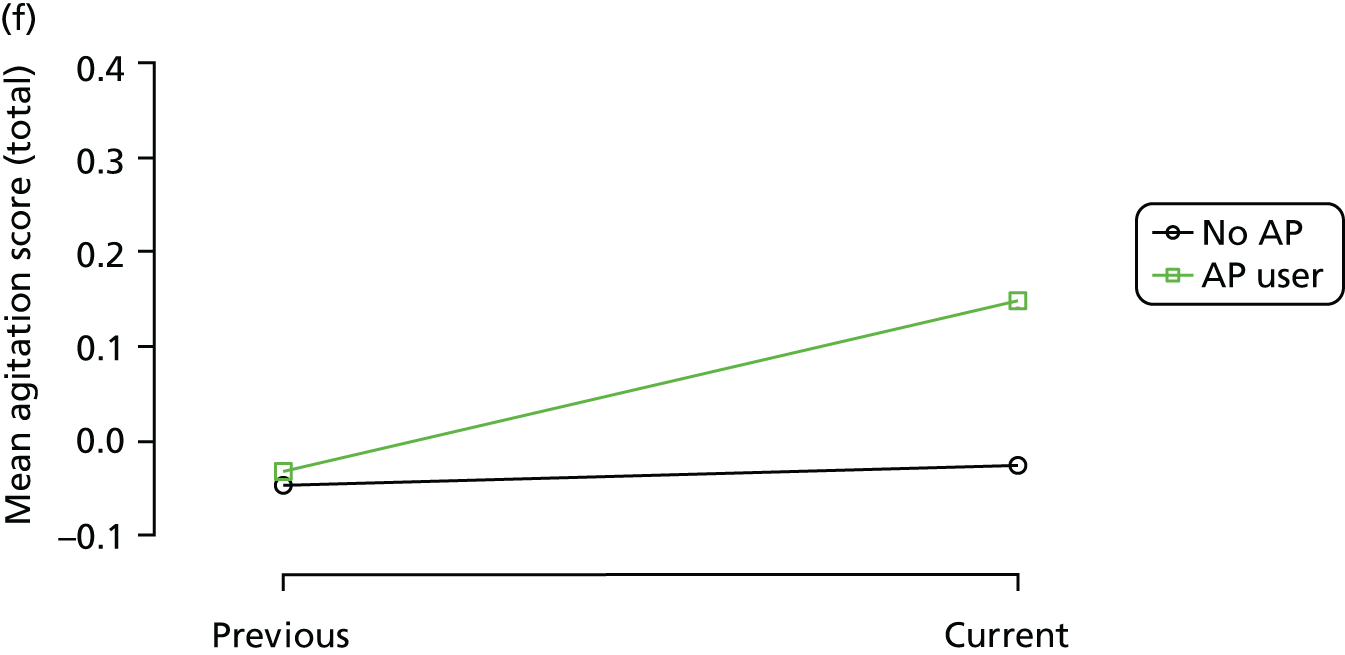
FIGURE 8.
Association between changing hypnotic use status between previous and current visit on mean anxiety scores at previous and current visit (a, c and e). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (b, d and f). Data from the REDIC-NH study. AP, antipsychotic.
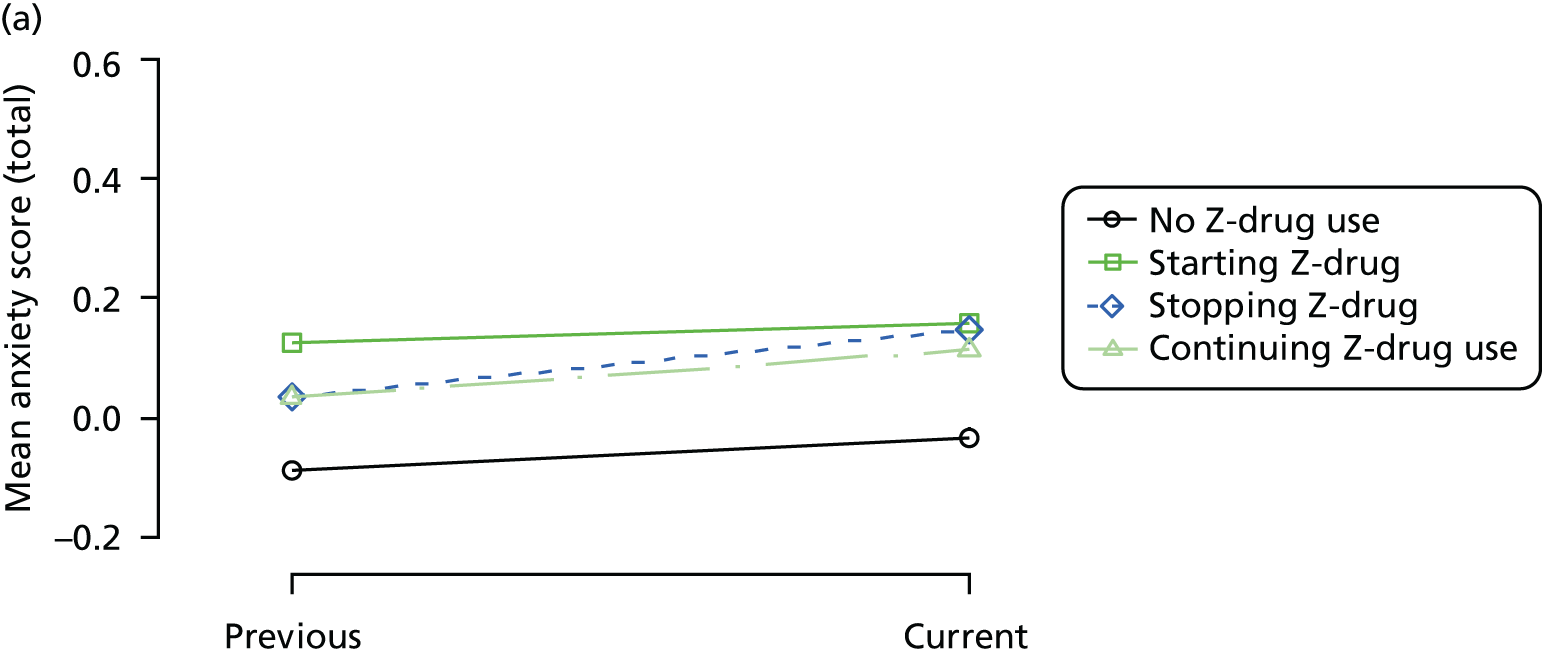
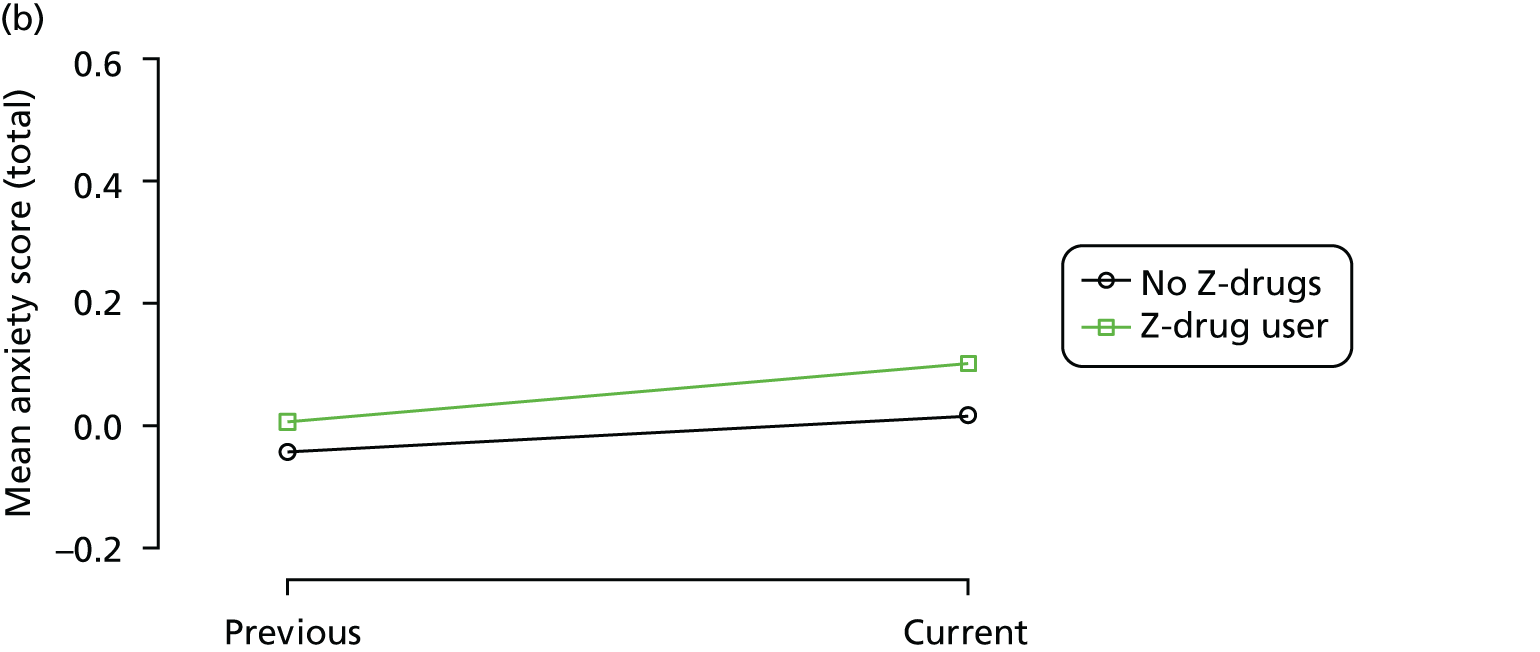
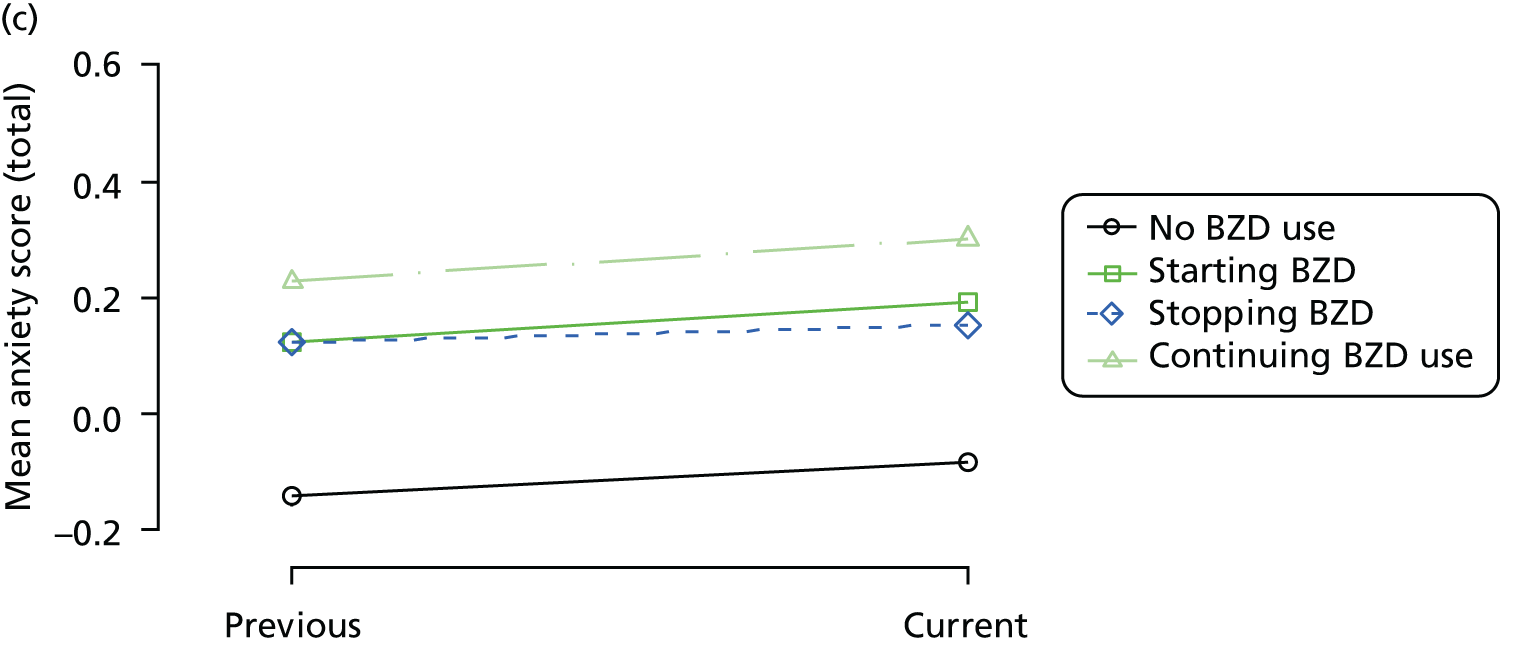
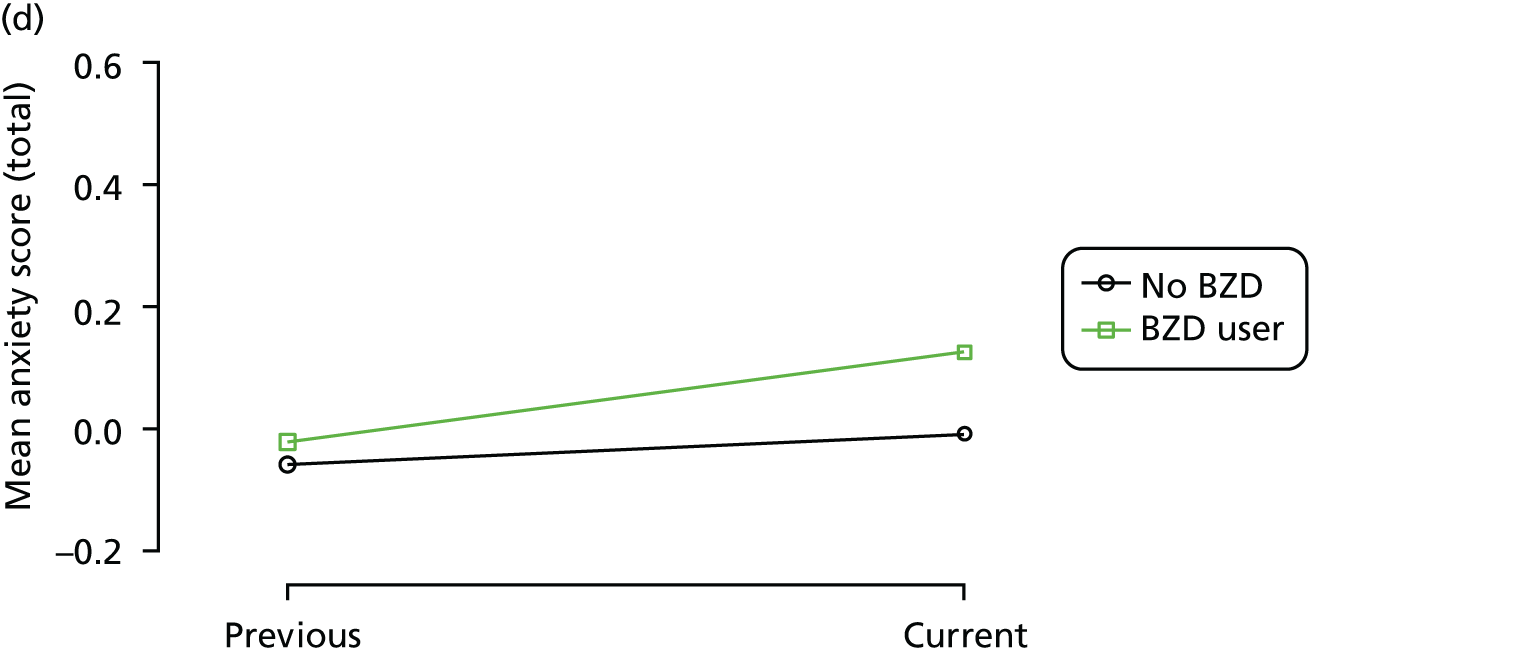
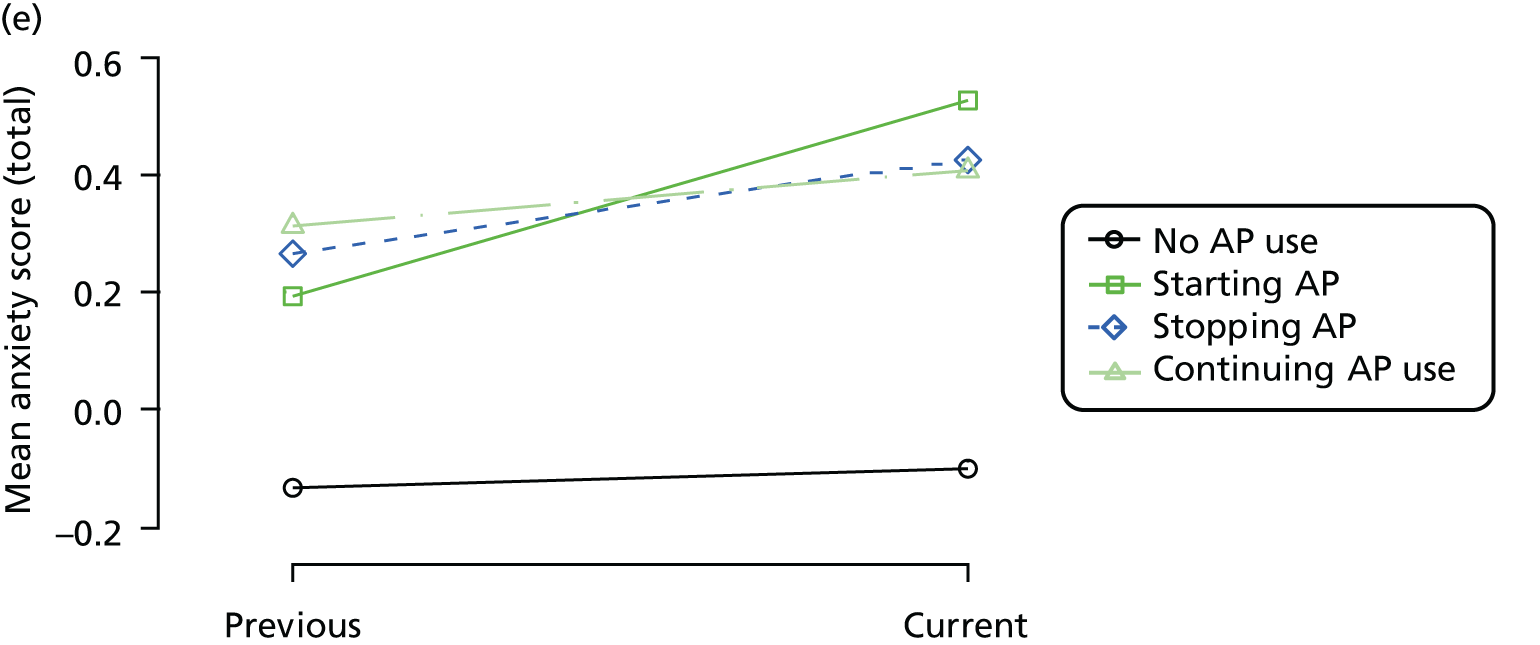
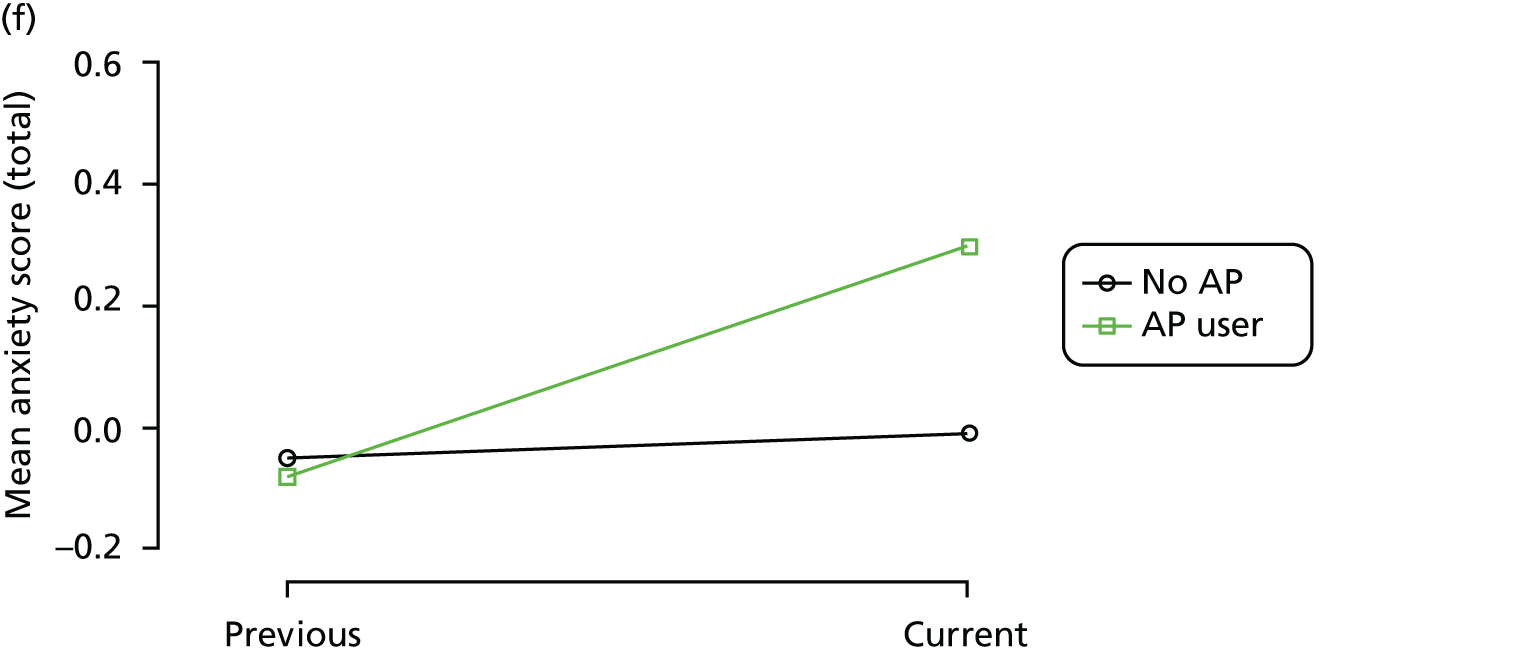
FIGURE 9.
Association between changing hypnotic use status between previous and current visit on VAS scores (higher scores represent better QoL) at previous and current visit (a, c and e). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (b, d and f). Data from the REDIC-NH study. AP, antipsychotic.
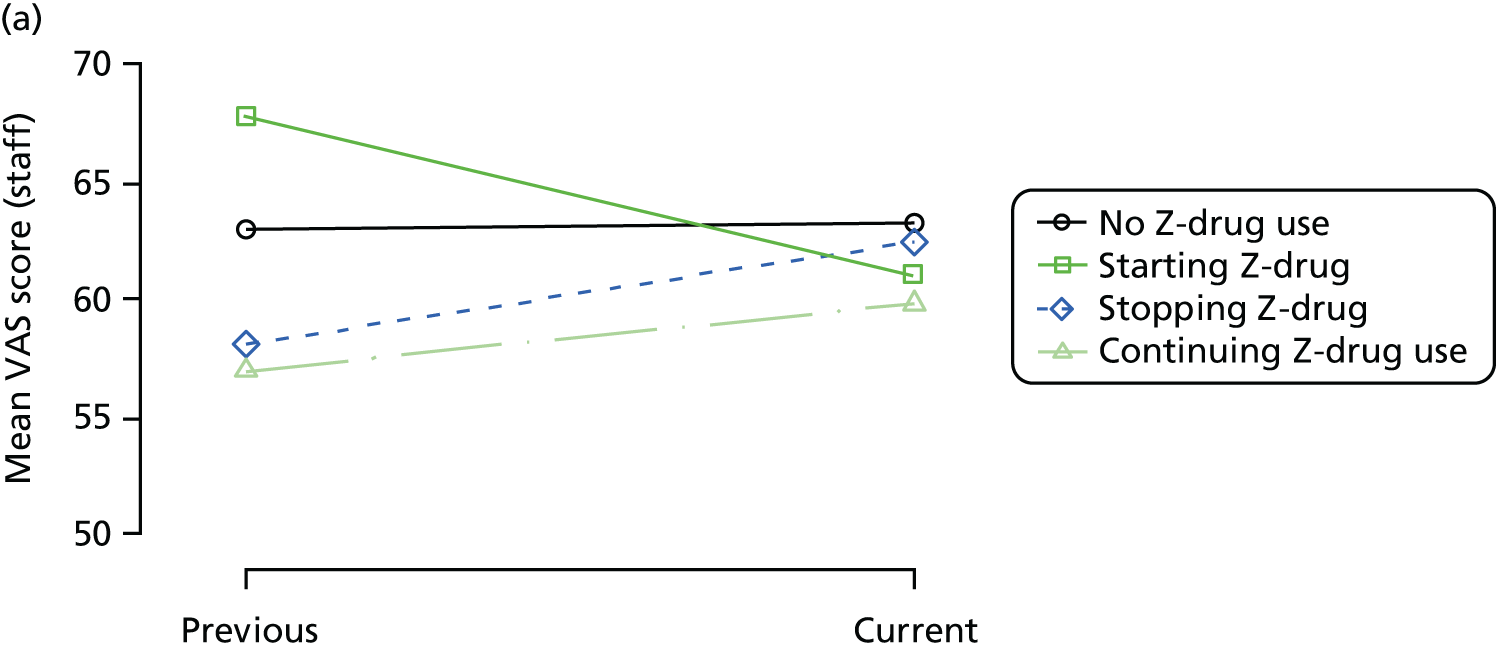
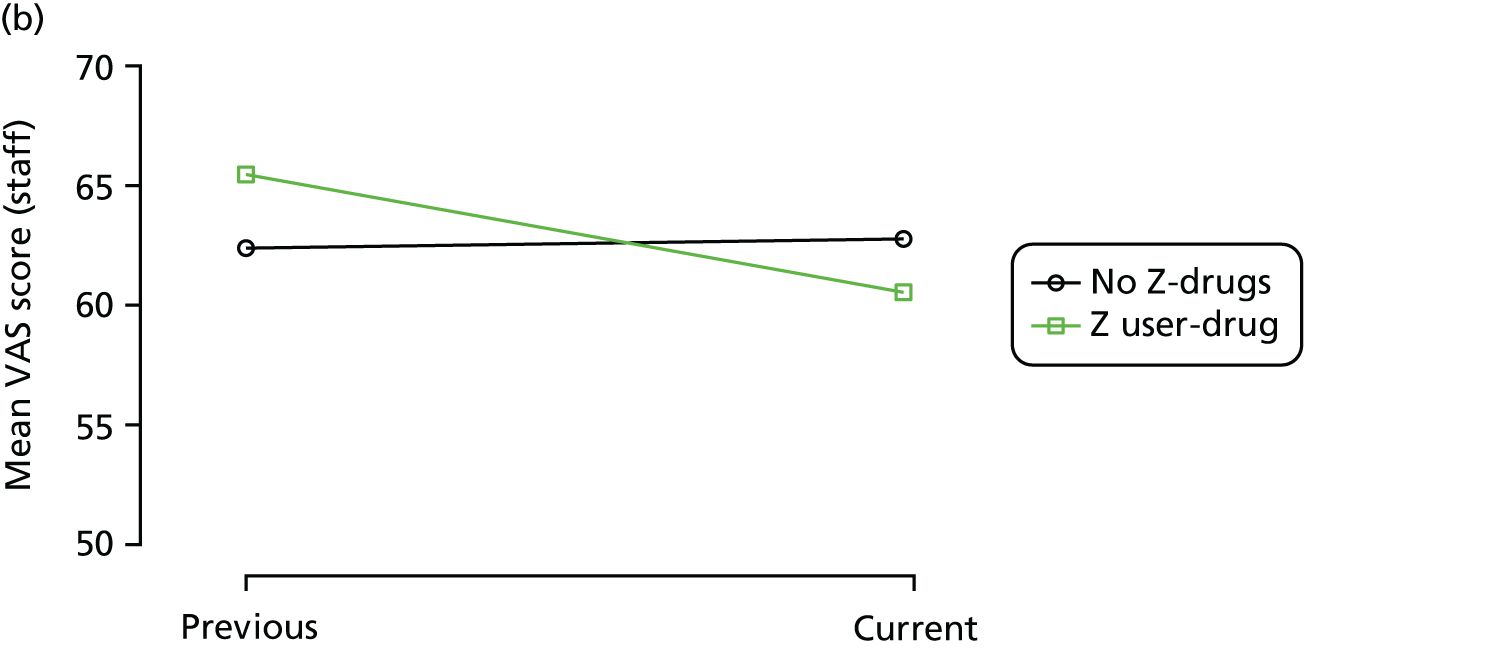
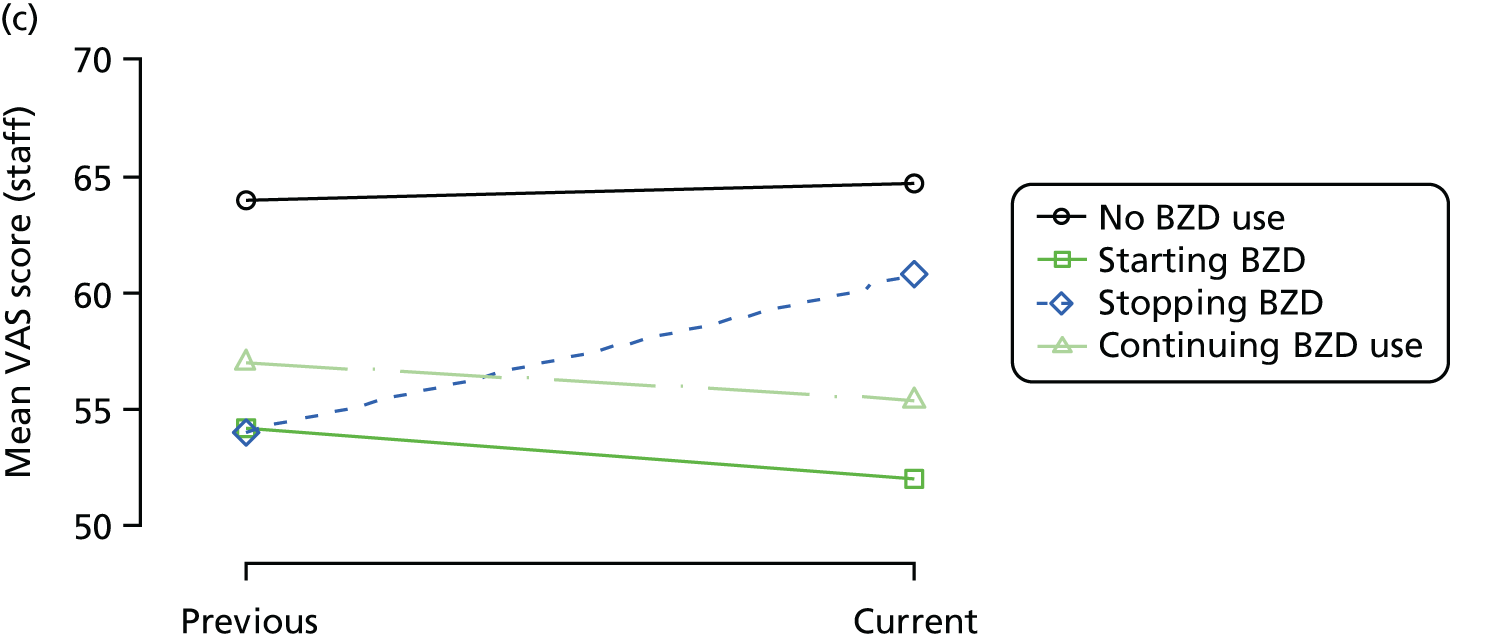
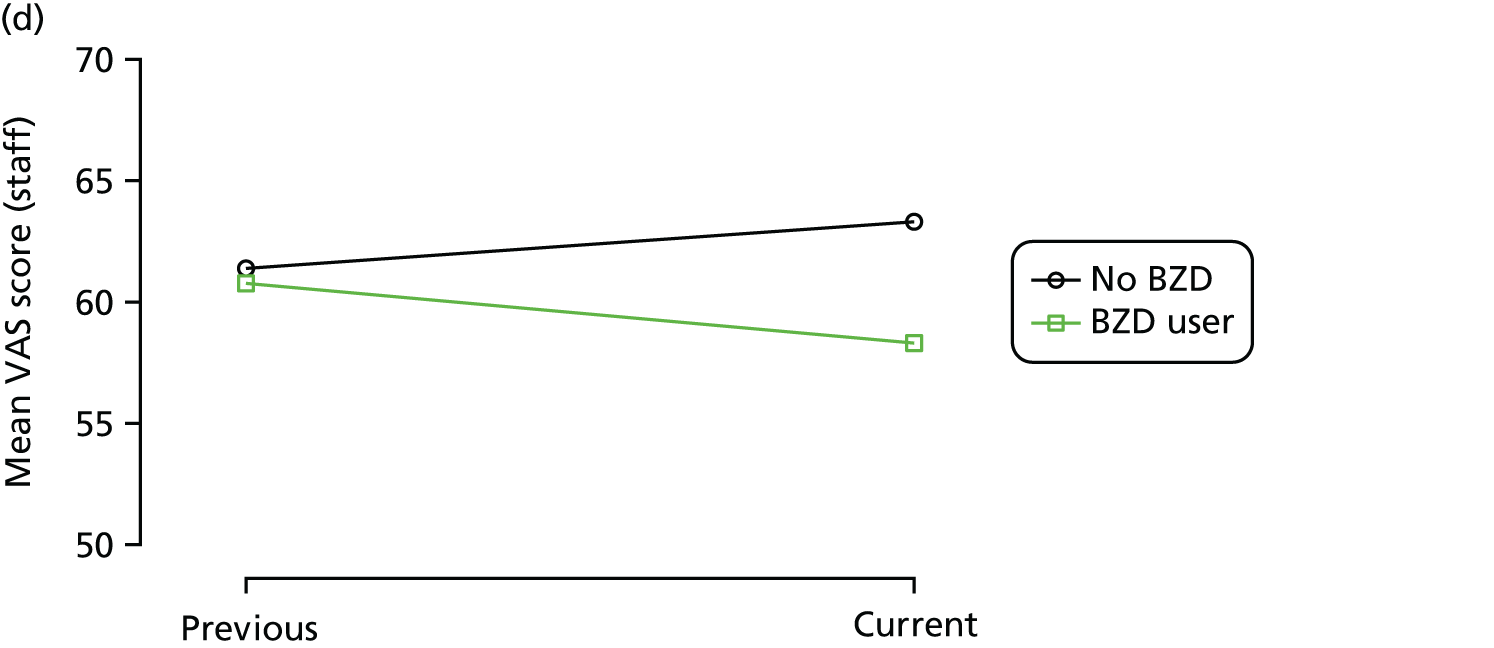
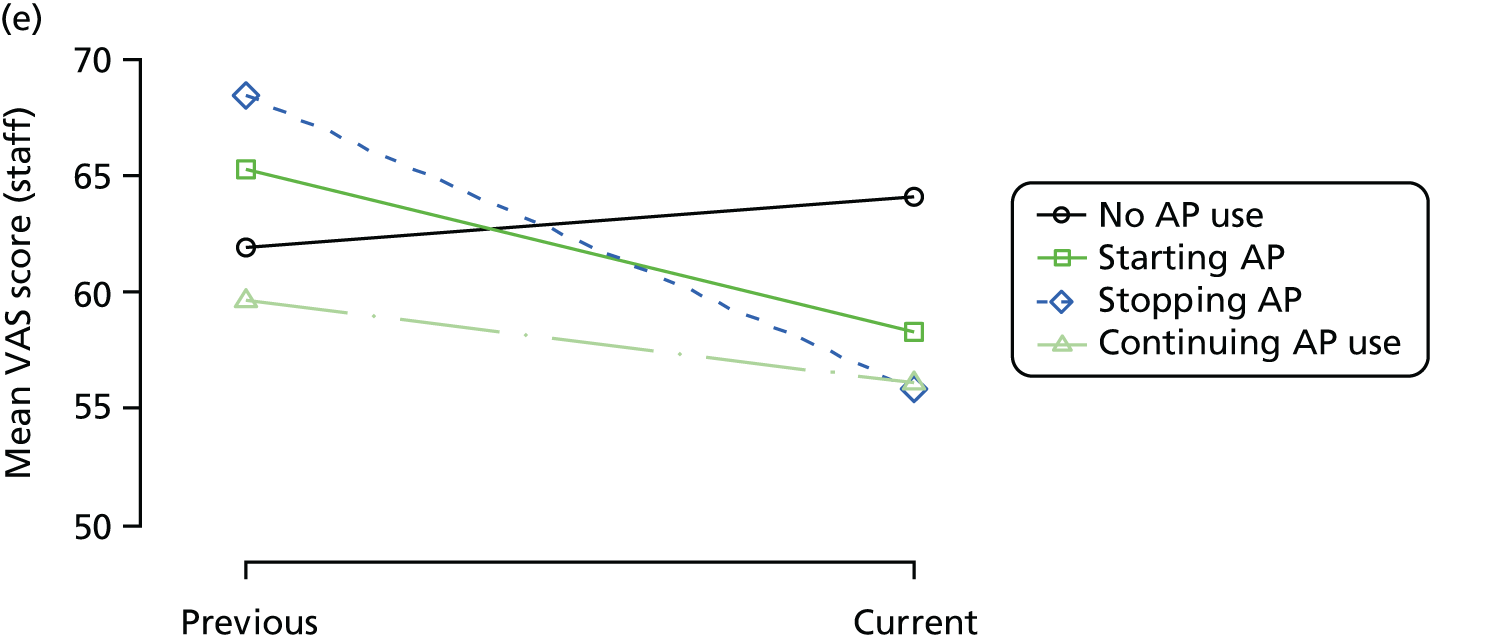
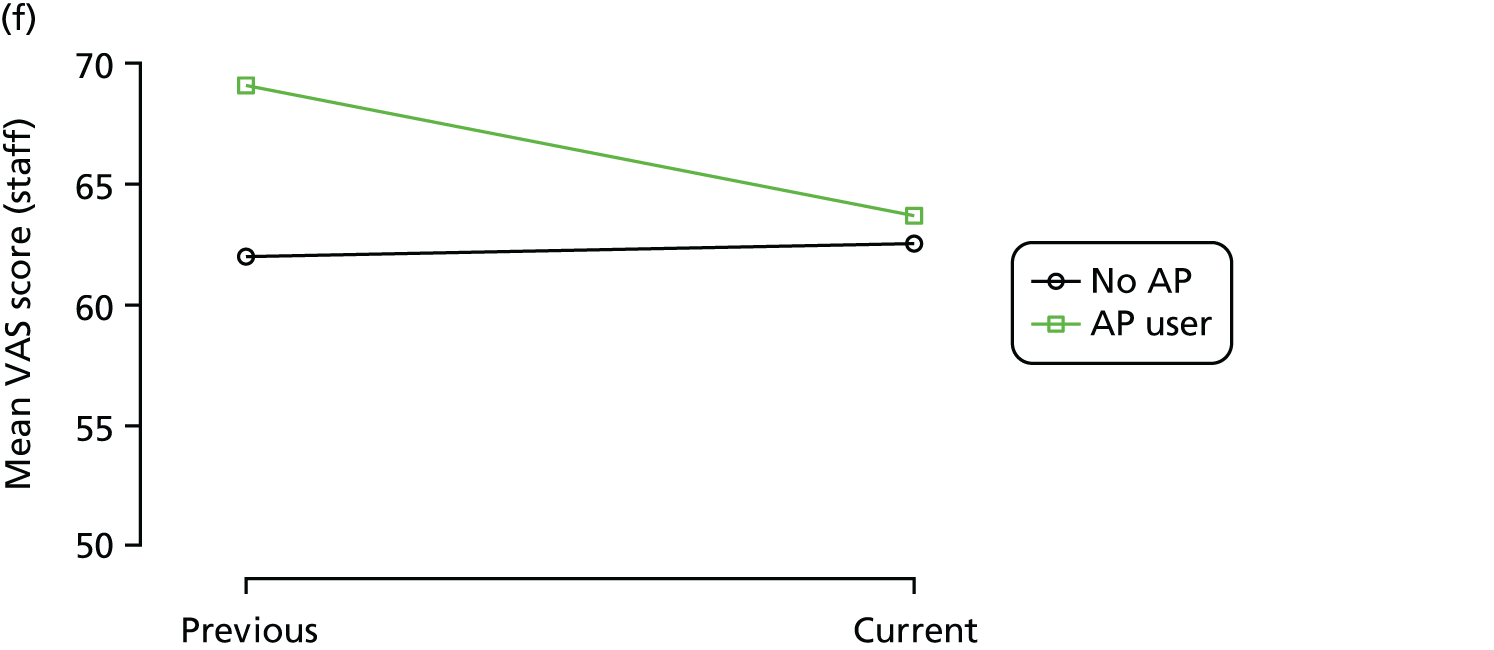
FIGURE 10.
Association between changing hypnotic use status between previous and current visit on EQ-5D scores (higher scores represent better QoL) at previous and current visit (a, c and e). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (b, d and f). Data from the REDIC-NH study. AP, antipsychotic.
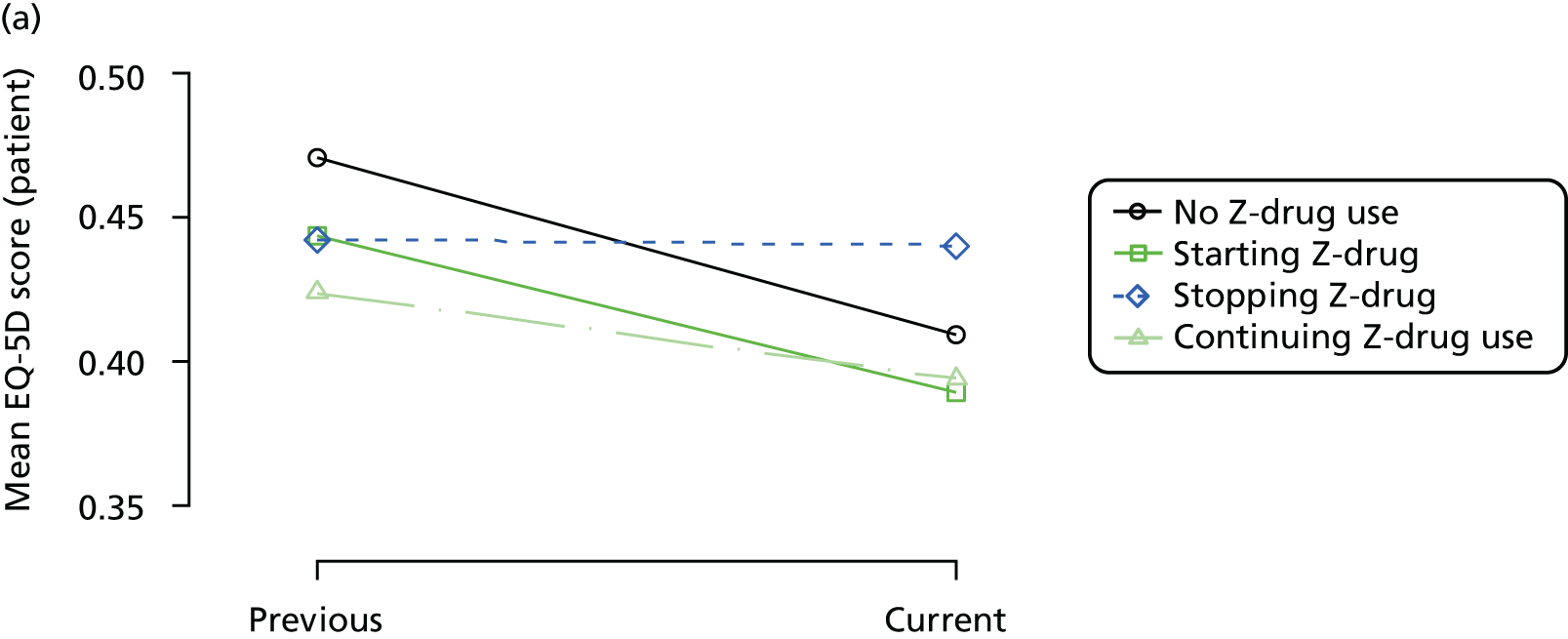
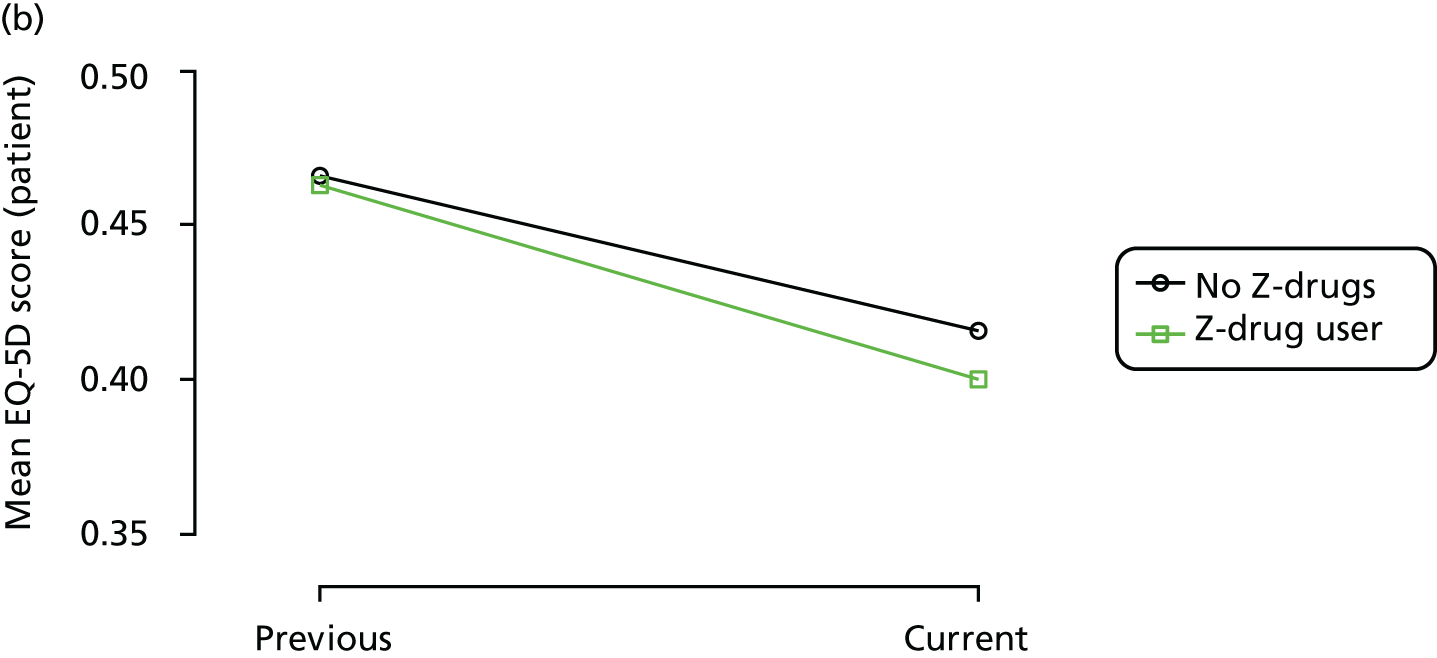
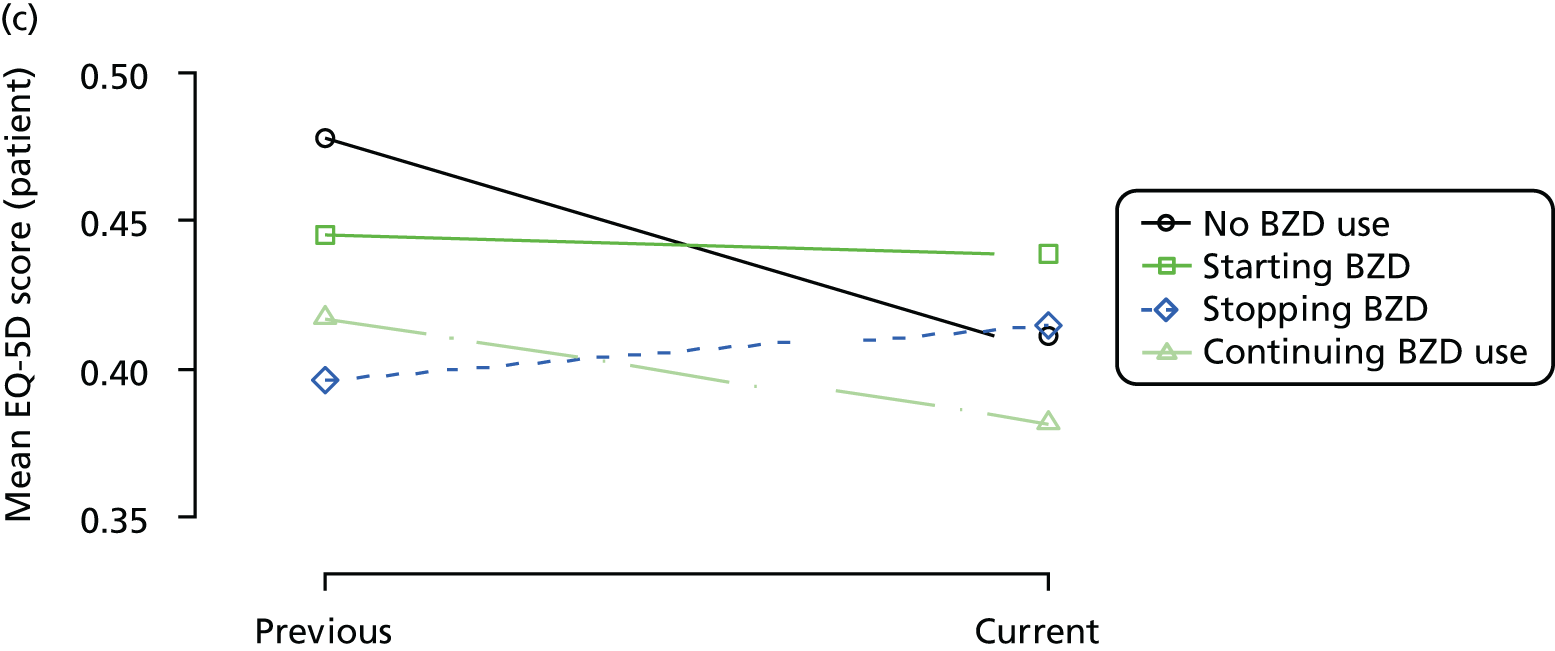
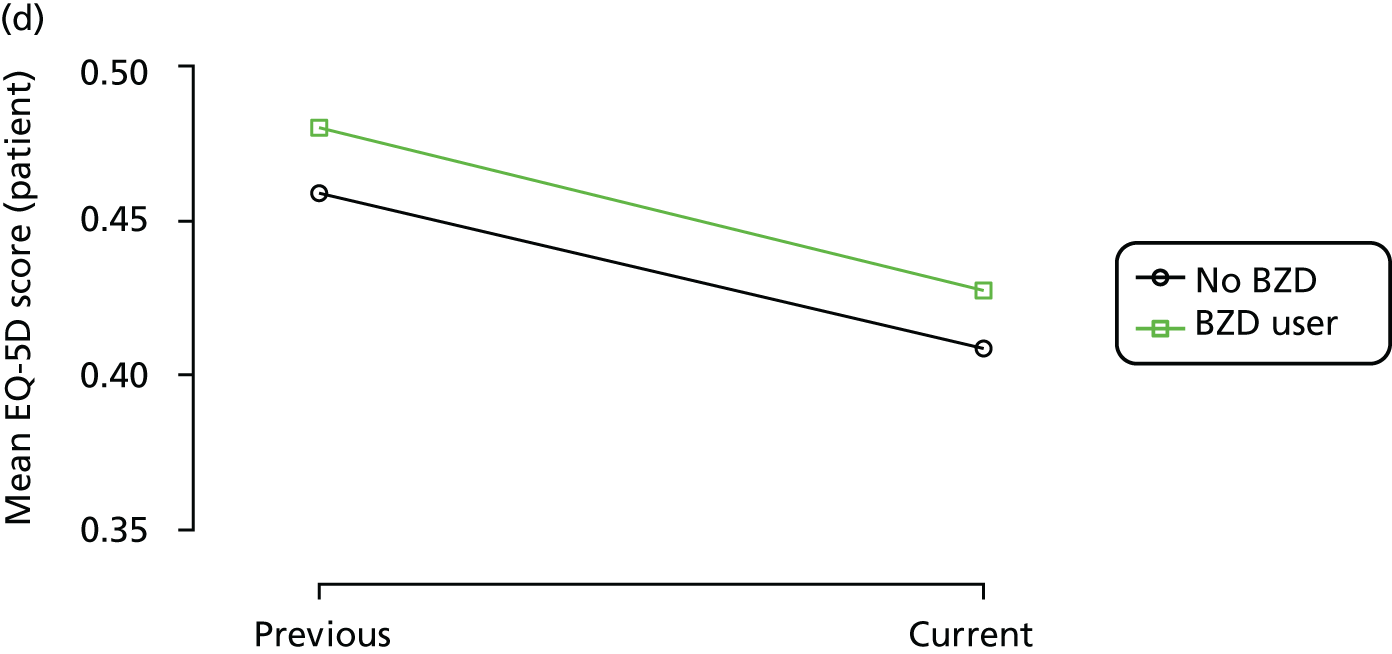
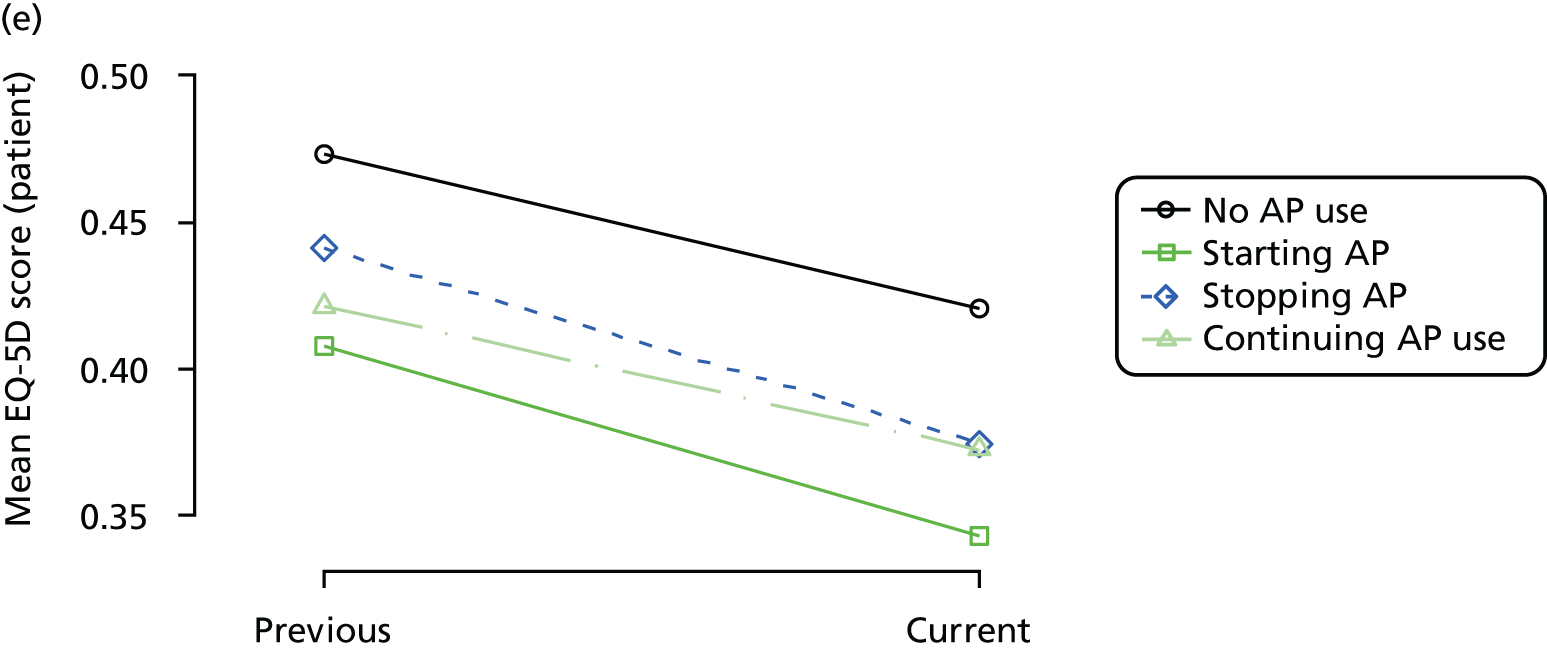
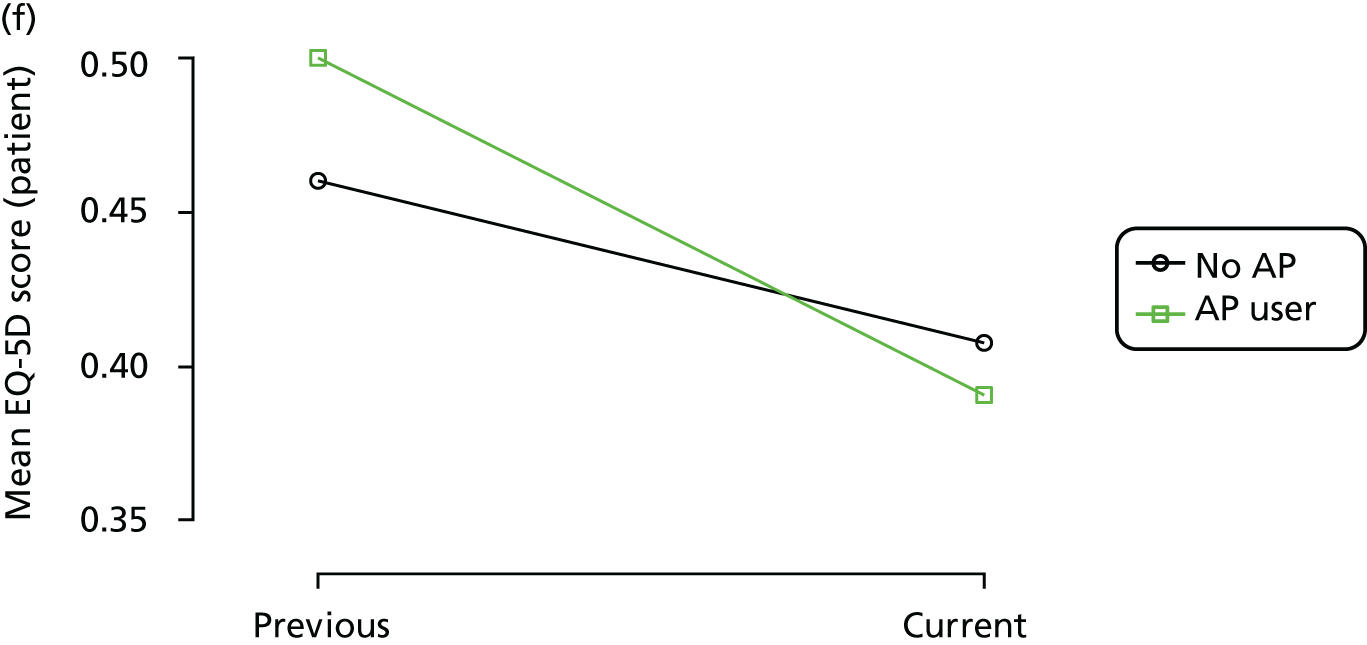
FIGURE 11.
Association between changing hypnotic use status between previous and current visit on QUALID scores (higher scores represent lower QoL) at previous and current visit (a, c and e). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (b, d and f). Data from the REDIC-NH study. AP, antipsychotic.
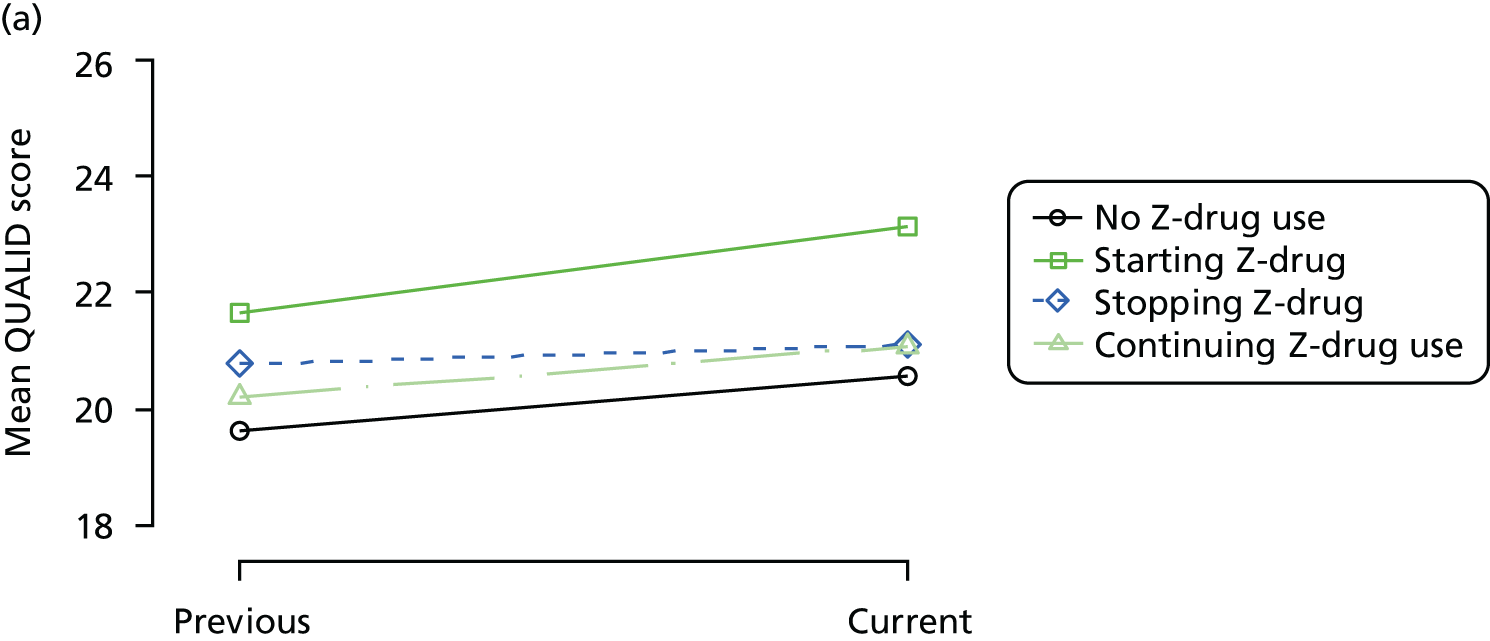
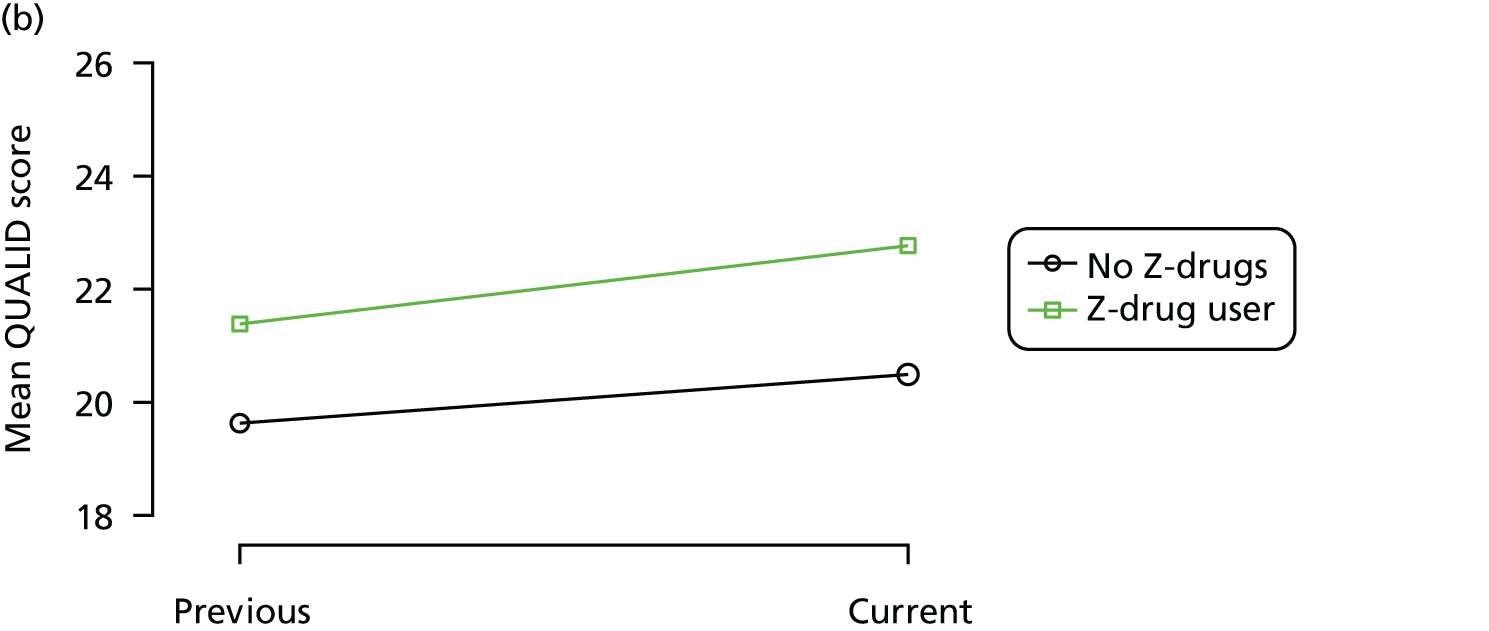
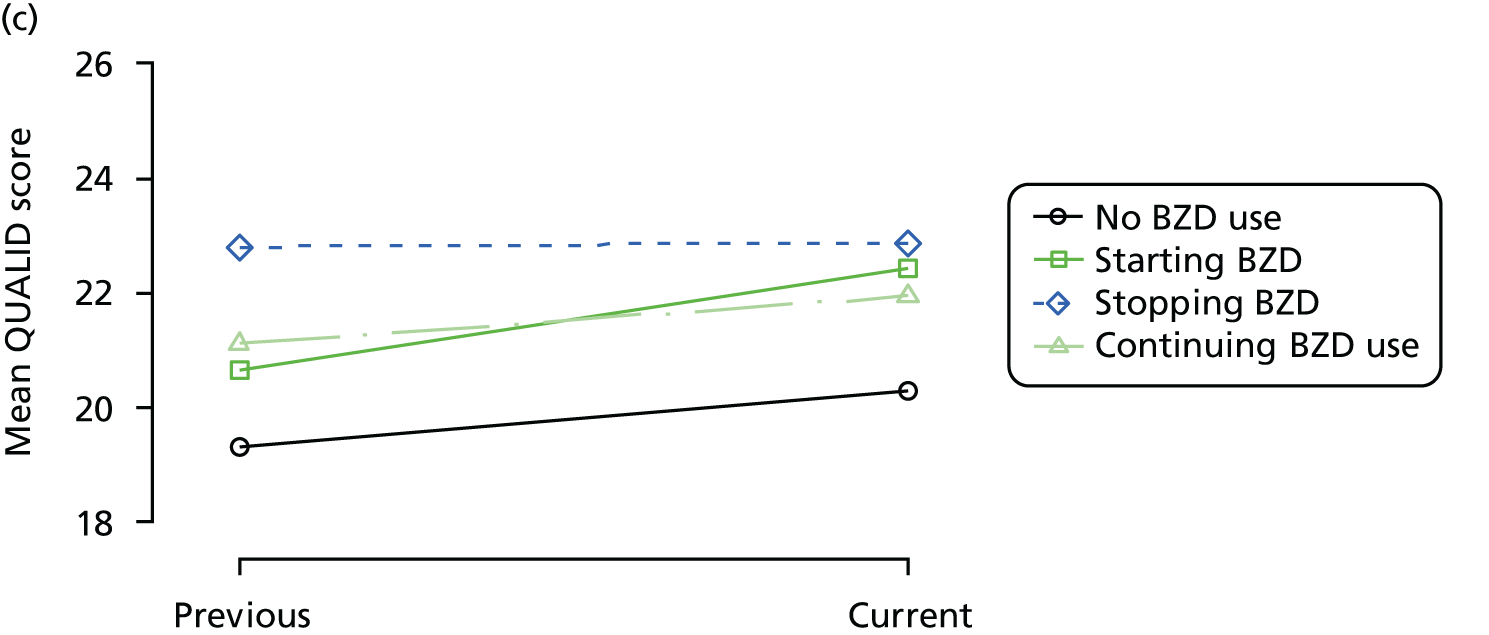
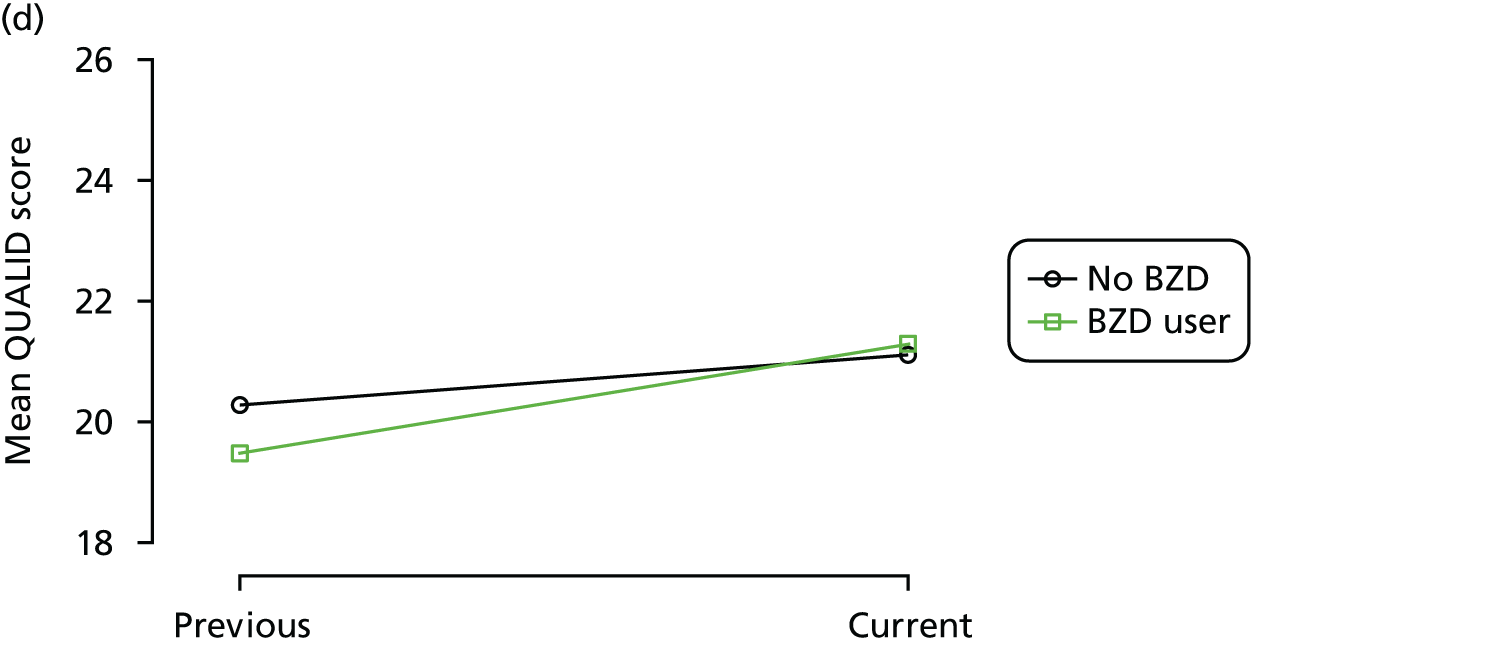
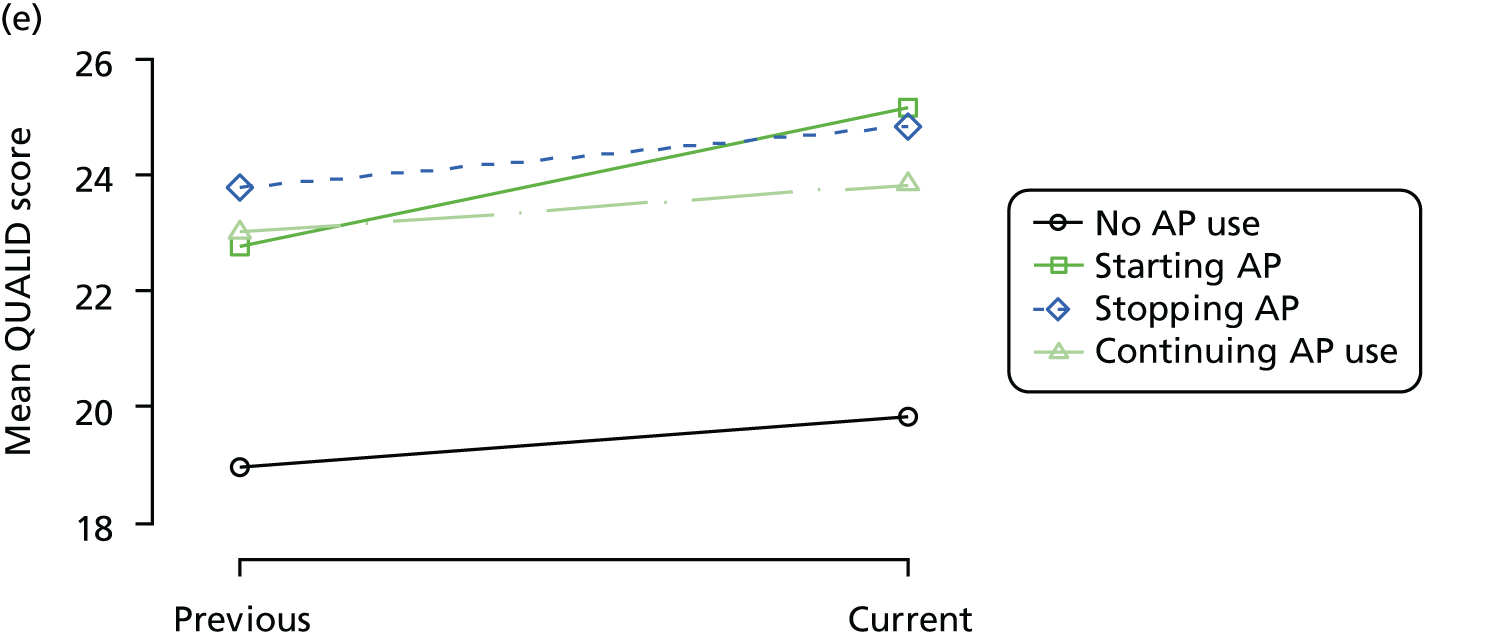
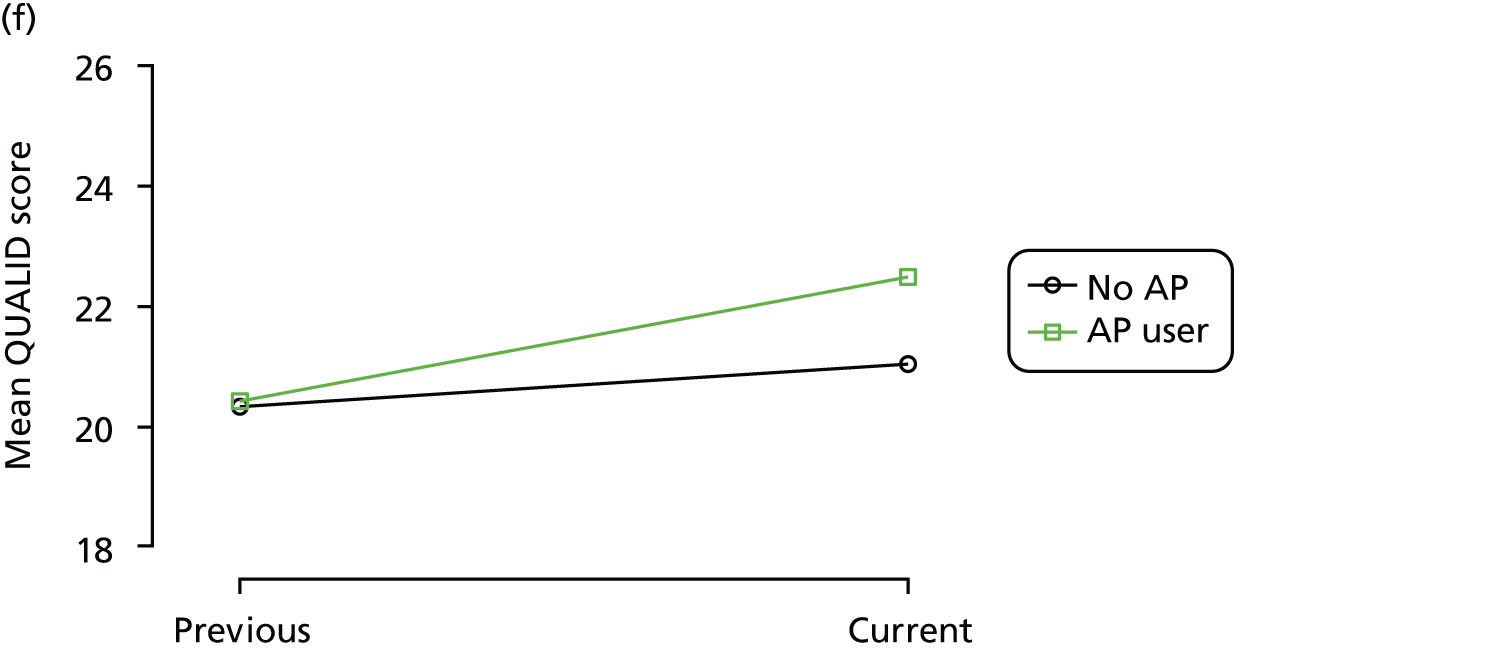
FIGURE 12.
Association between changing hypnotic use status between previous and current visit on disability scores (higher scores represent more disability) at previous and current visit (a, c and e). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (b, d and f). Data from the REDIC-NH study. AP, antipsychotic.
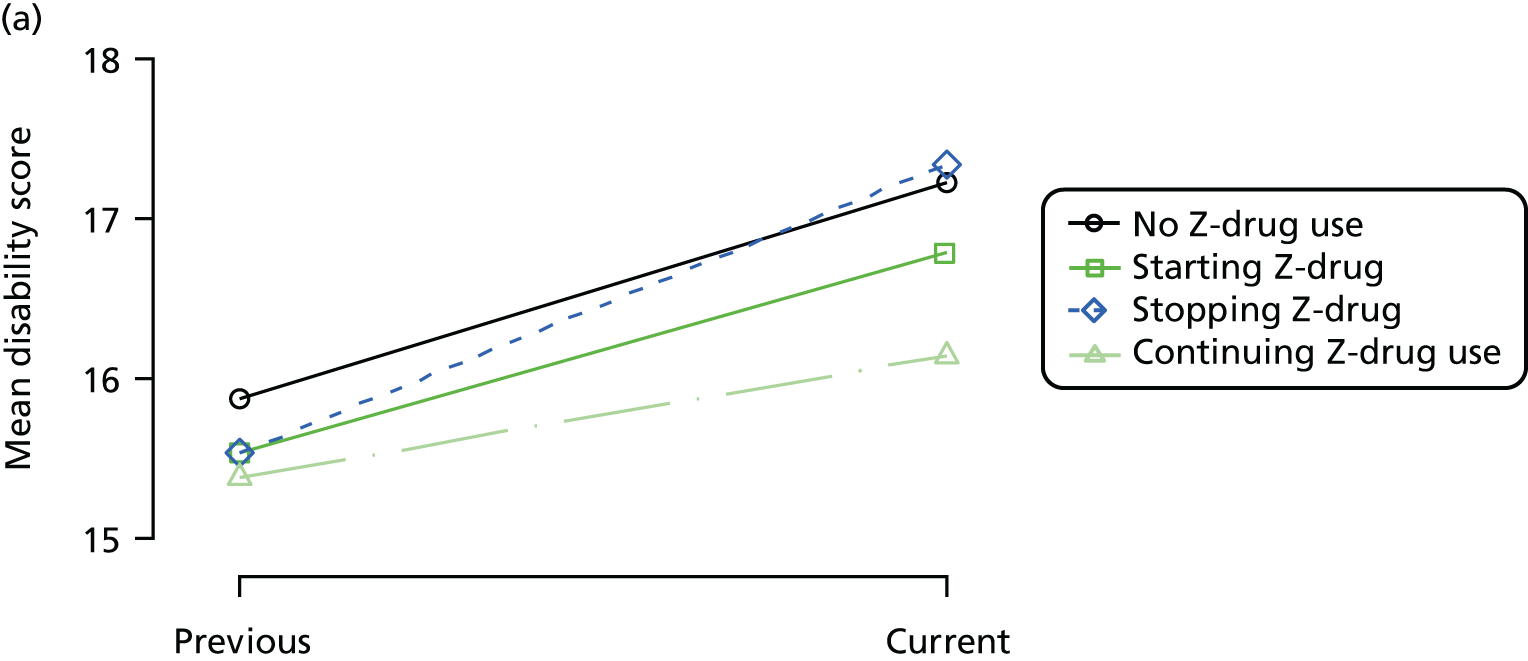
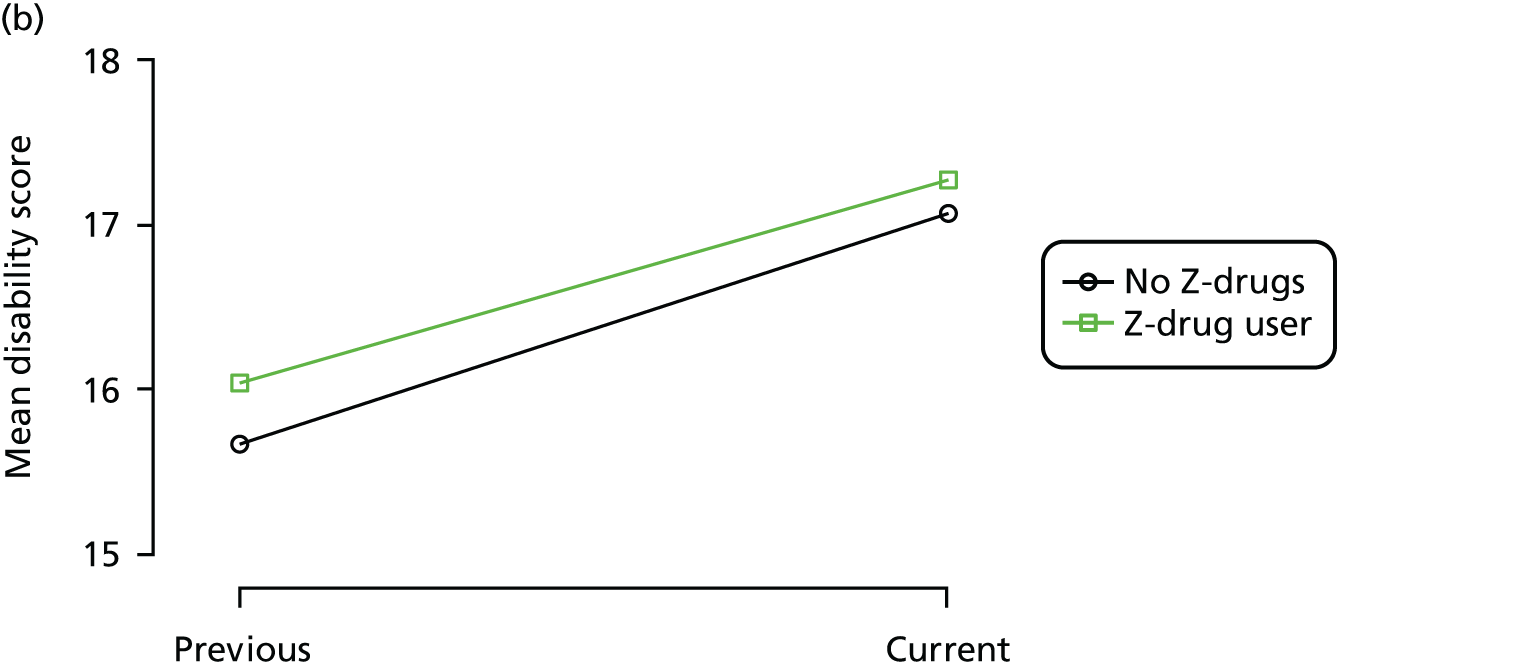
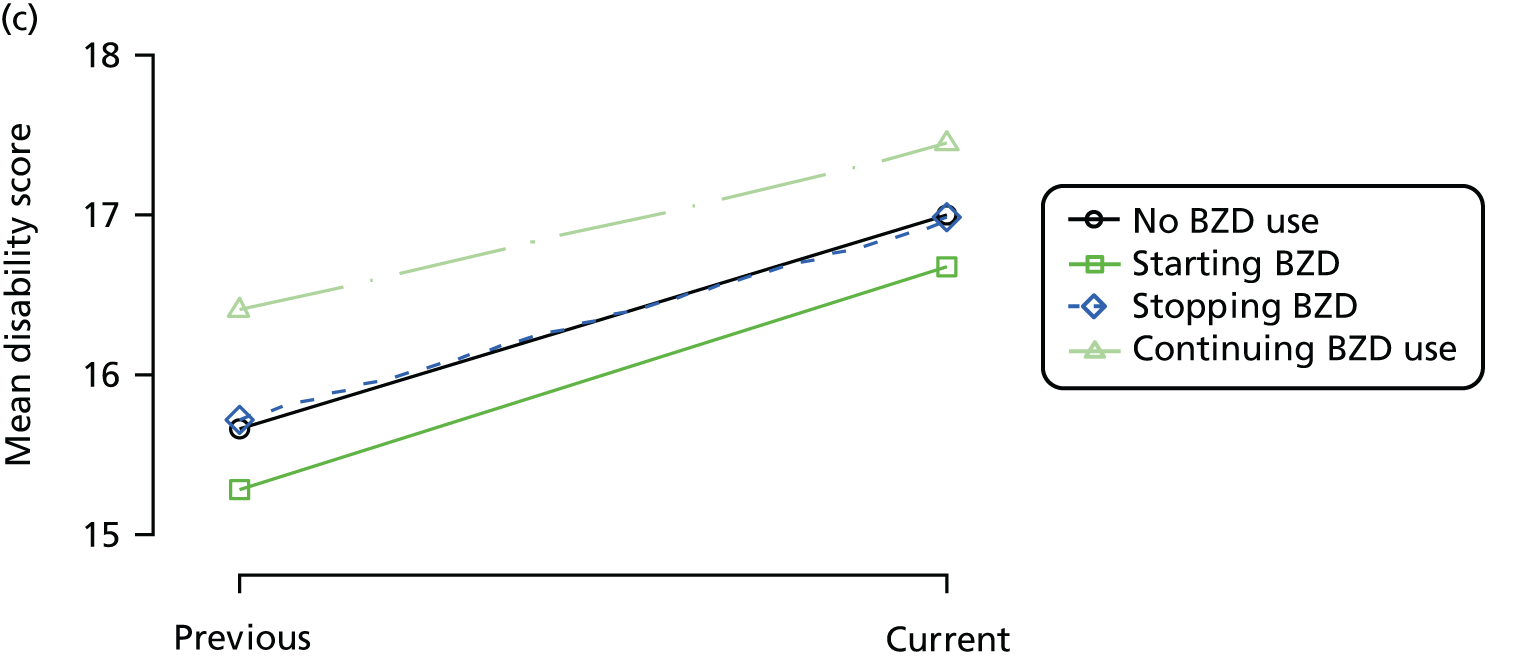
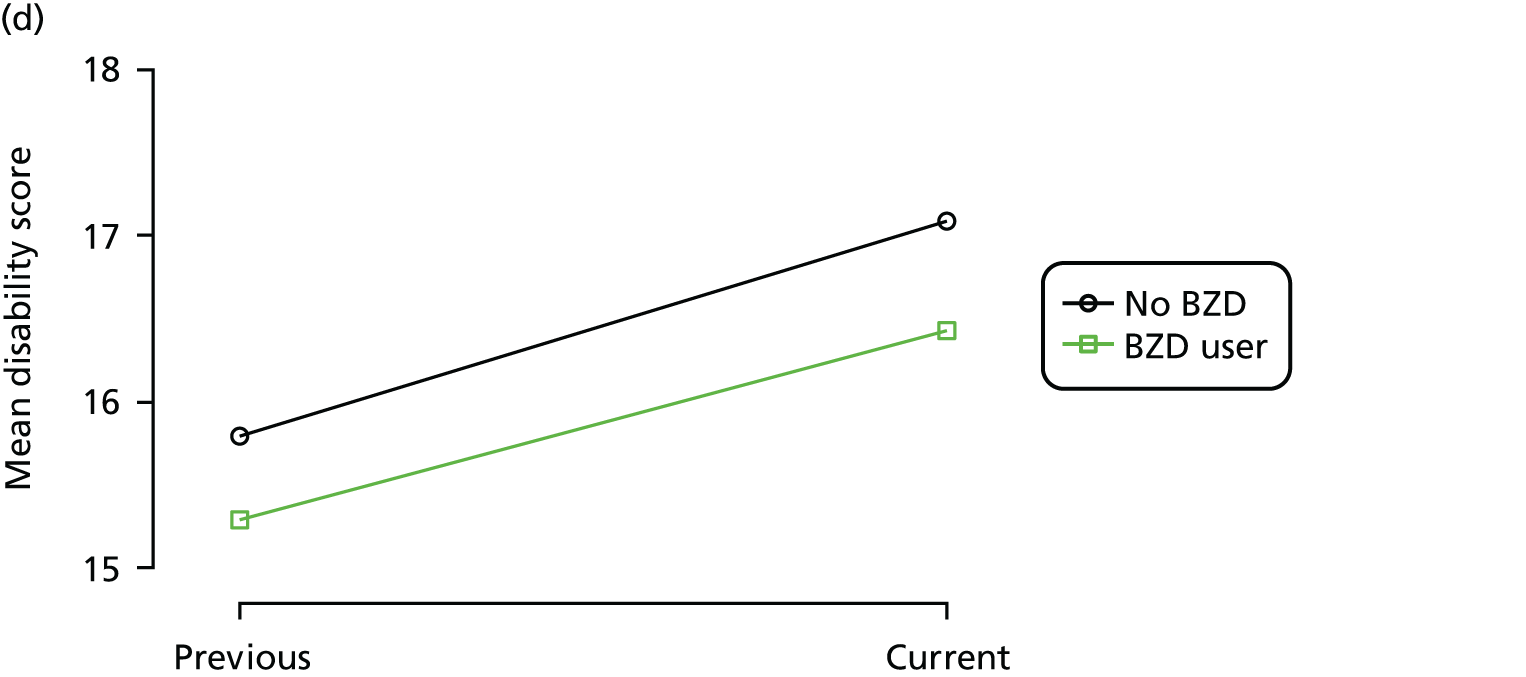
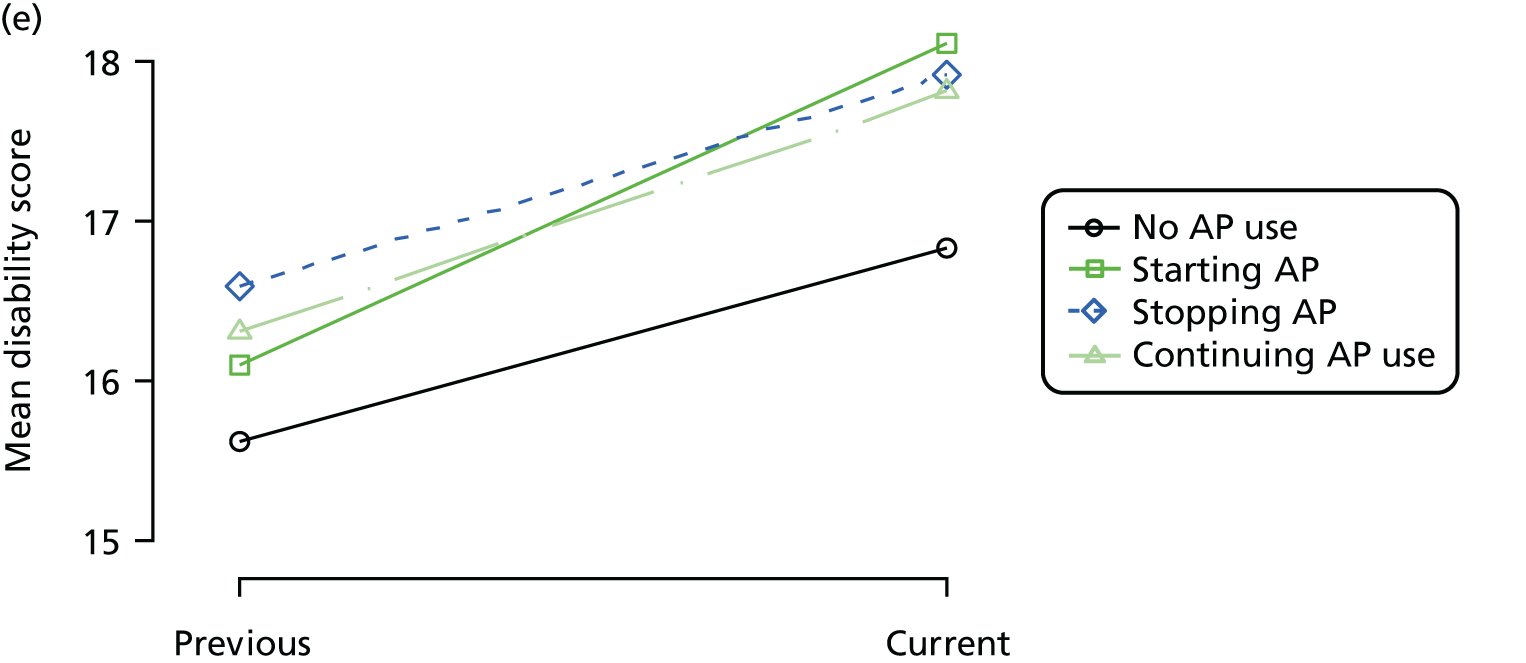
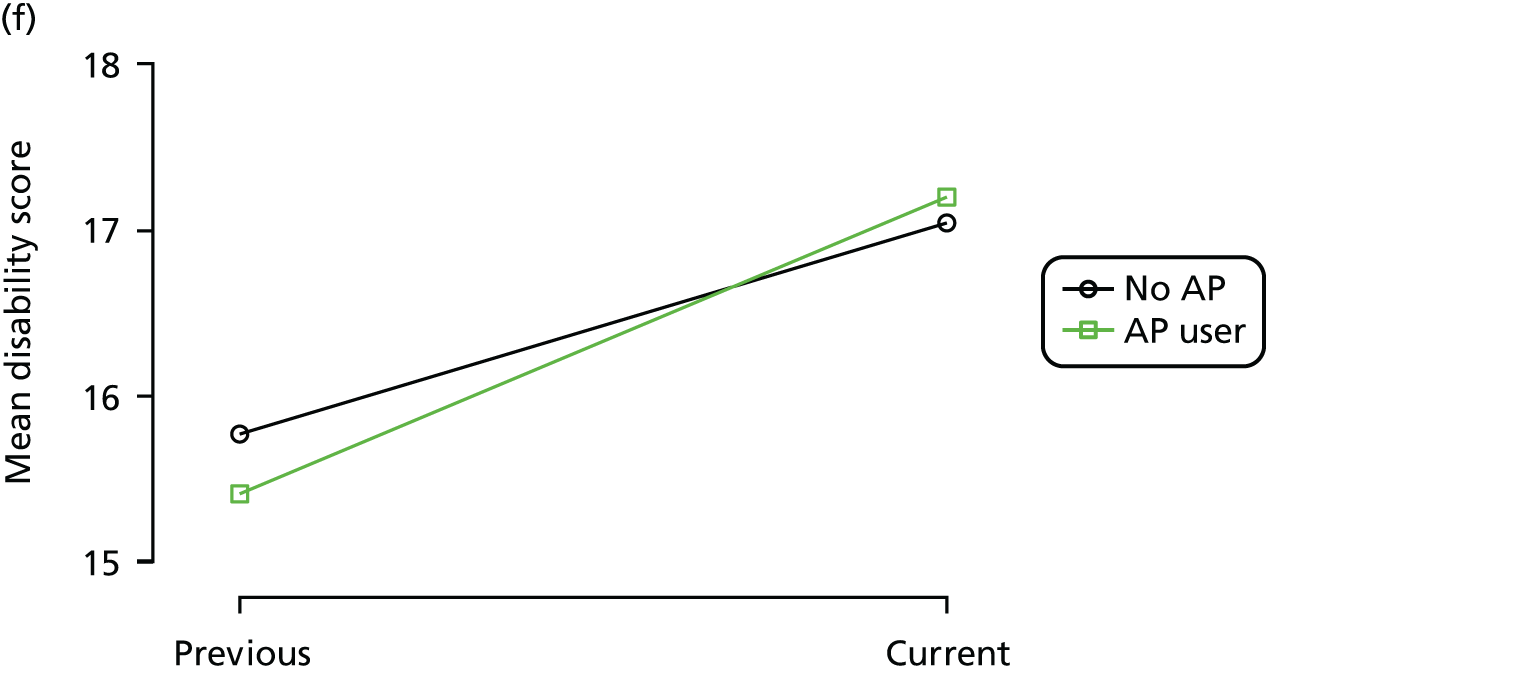
FIGURE 13.
(a) Distribution of SIB-8 with respect to dementia severity; (b) distribution of SIB-8 with respect to Z-drug use; (c) mean score over REDIC visit, stratified by age group; and (d) mean score over REDIC visit, stratified by number of visits completed.
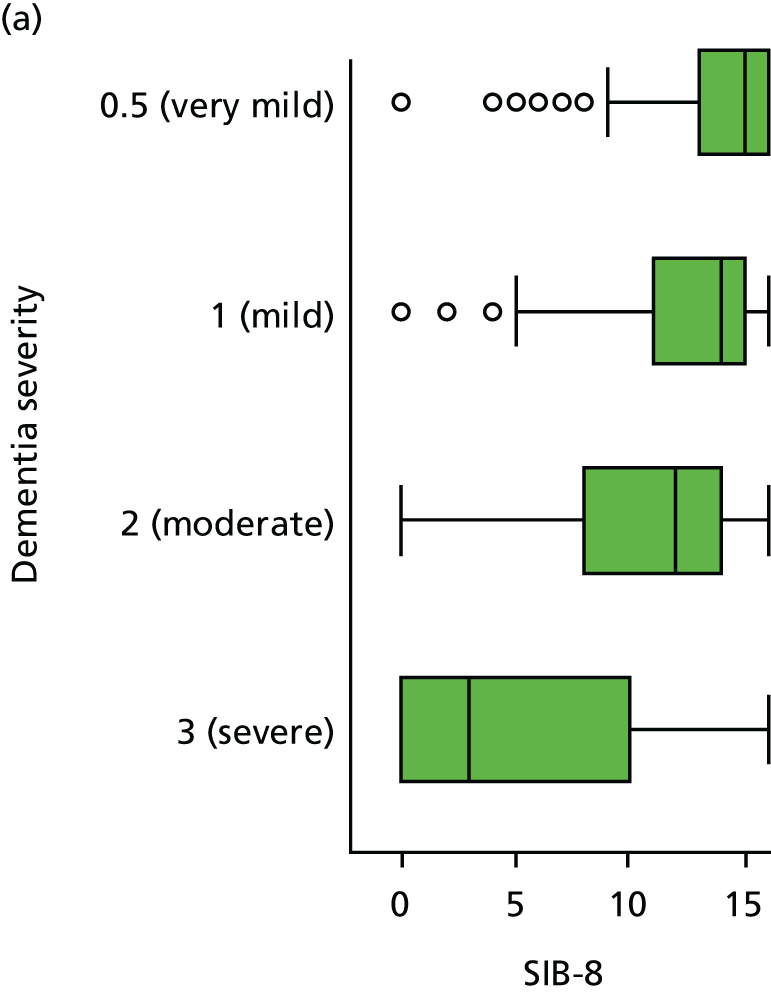
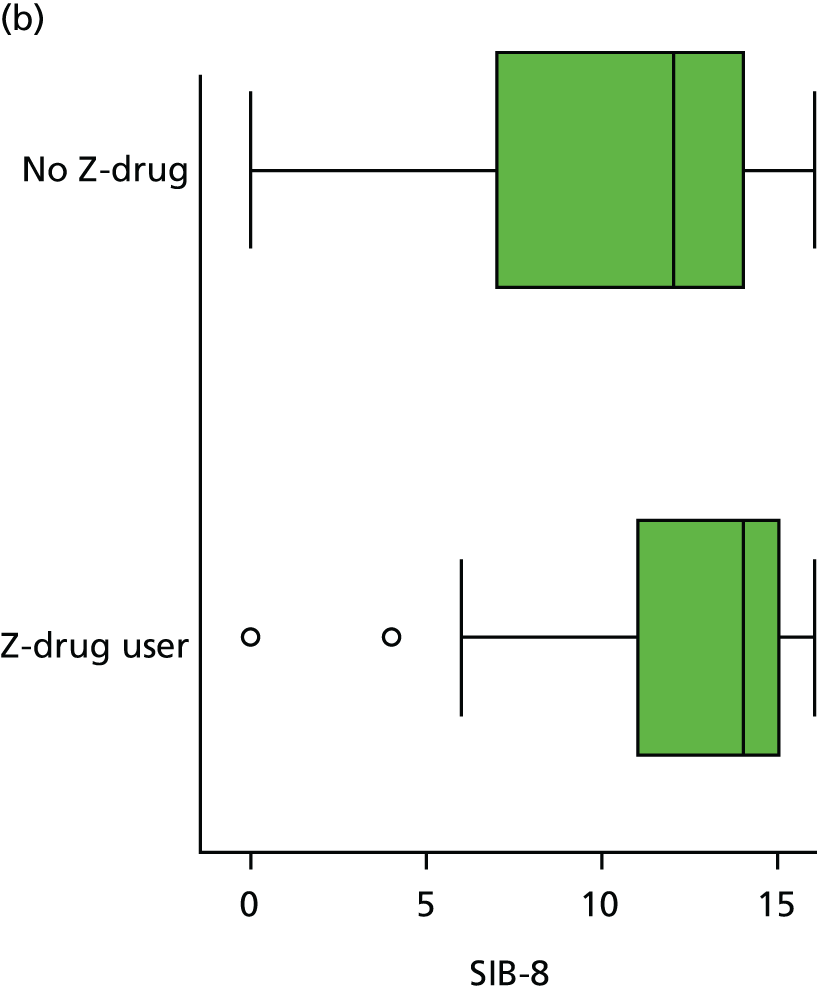
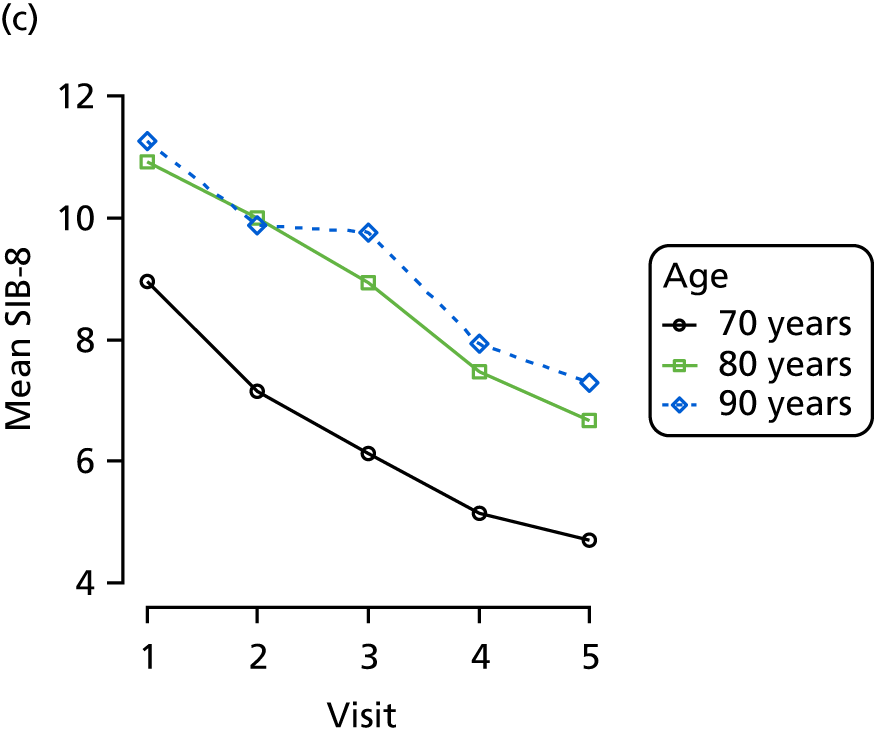
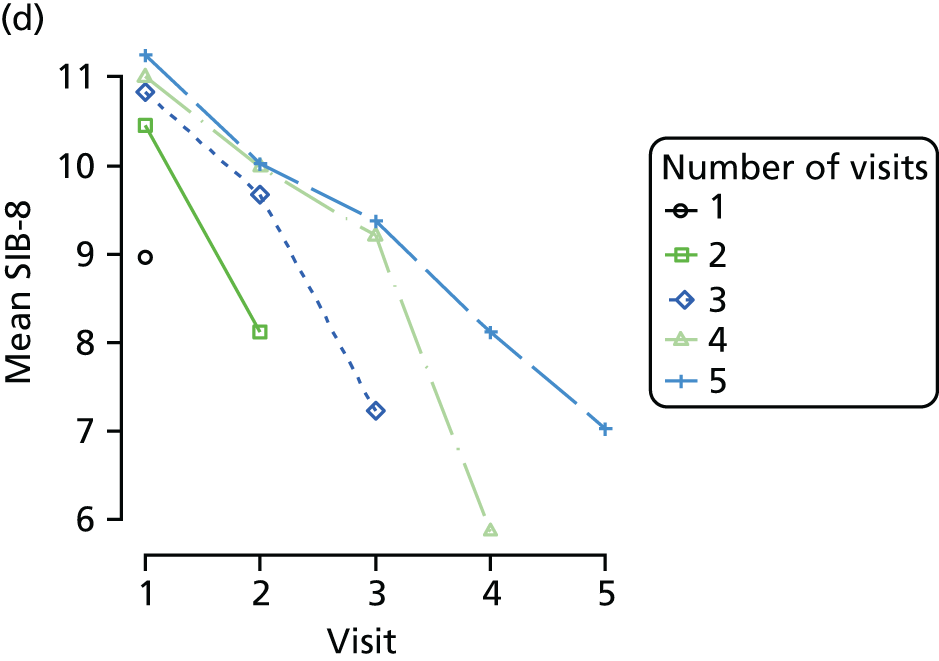
FIGURE 14.
(a) Distribution of MMSE with respect to dementia severity; (b) distribution of MMSE with respect to Z-drug use; (c) mean score over REDIC visit, stratified by age group; and (d) mean score over REDIC visit, stratified by number of visits completed.
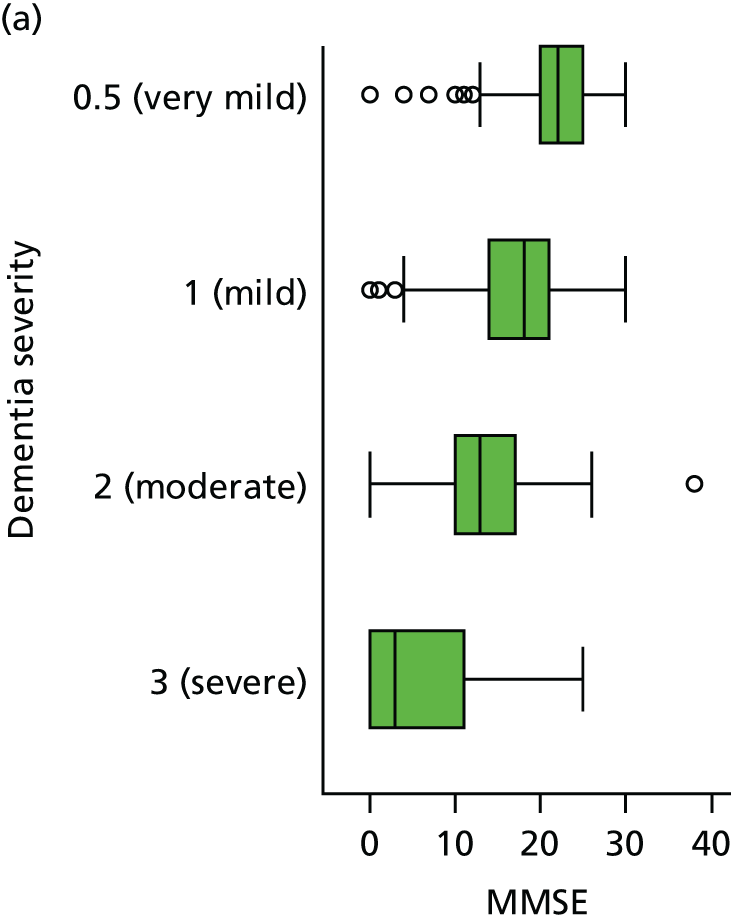
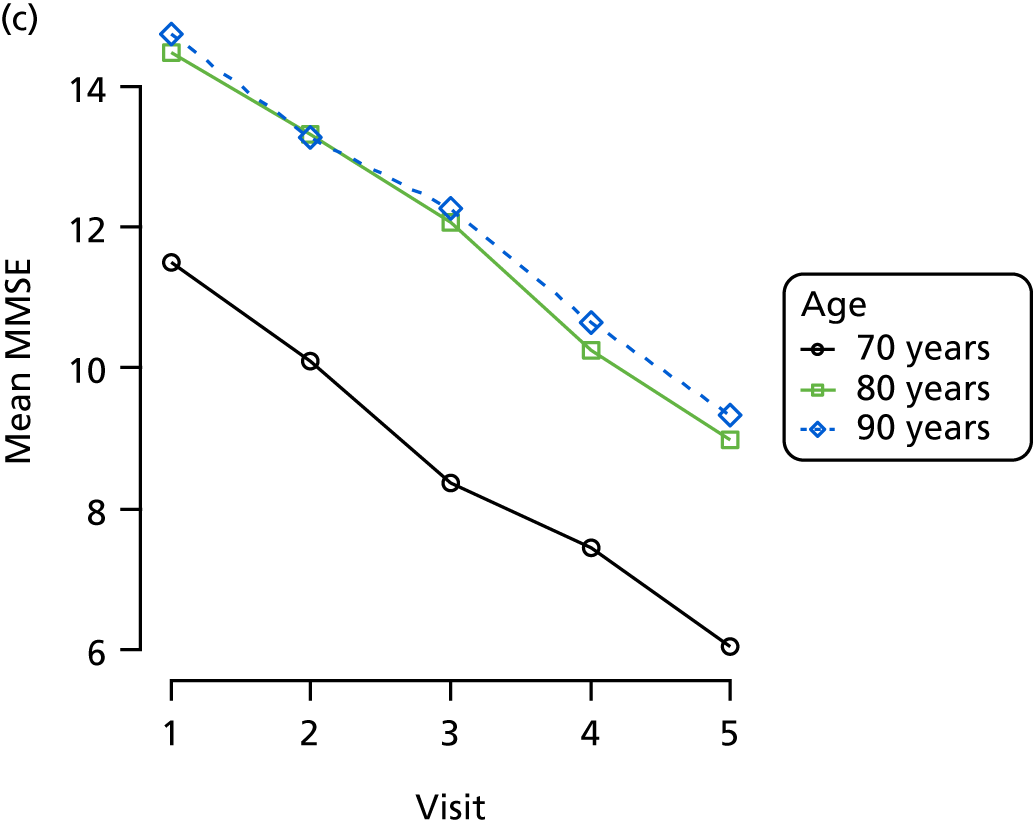
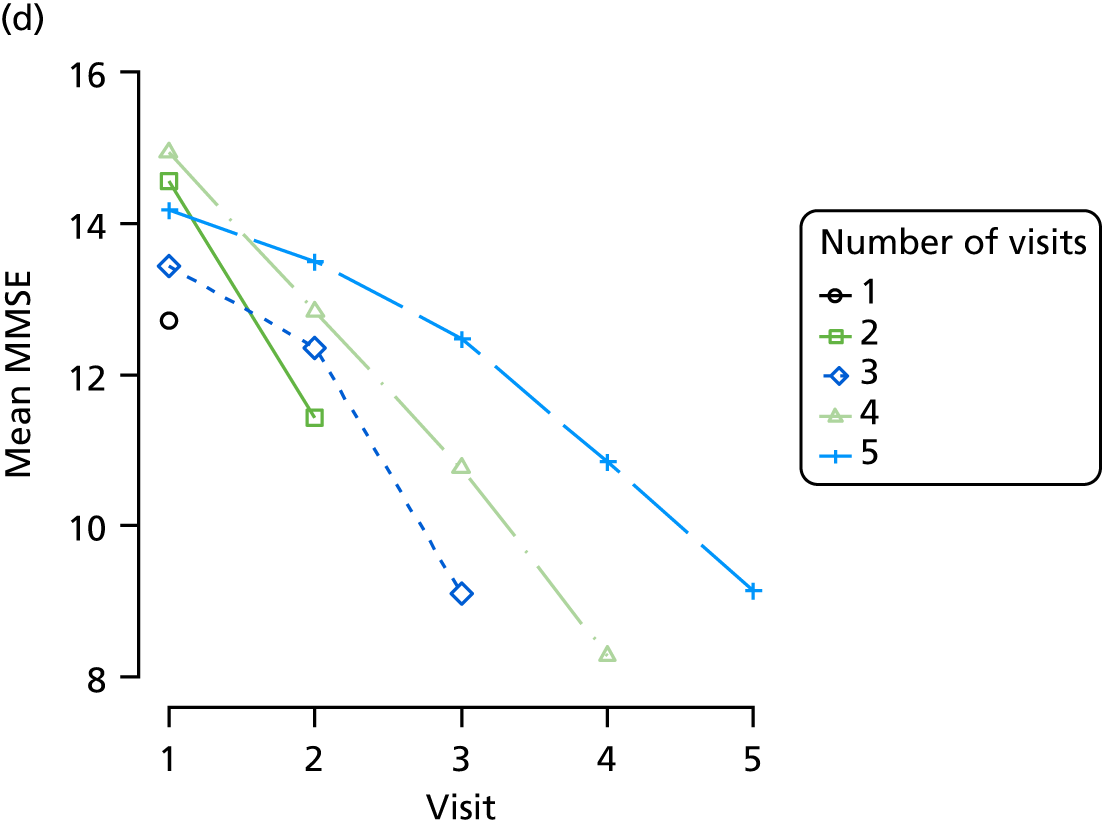
FIGURE 15.
(a) Distribution of CDR-SOB with respect to dementia severity; (b) distribution of CDR-SOB with respect to dementia and Z-drug; (c) mean score over REDIC visit, stratified by age group; and (d) mean score over REDIC visit, stratified number of visits completed.
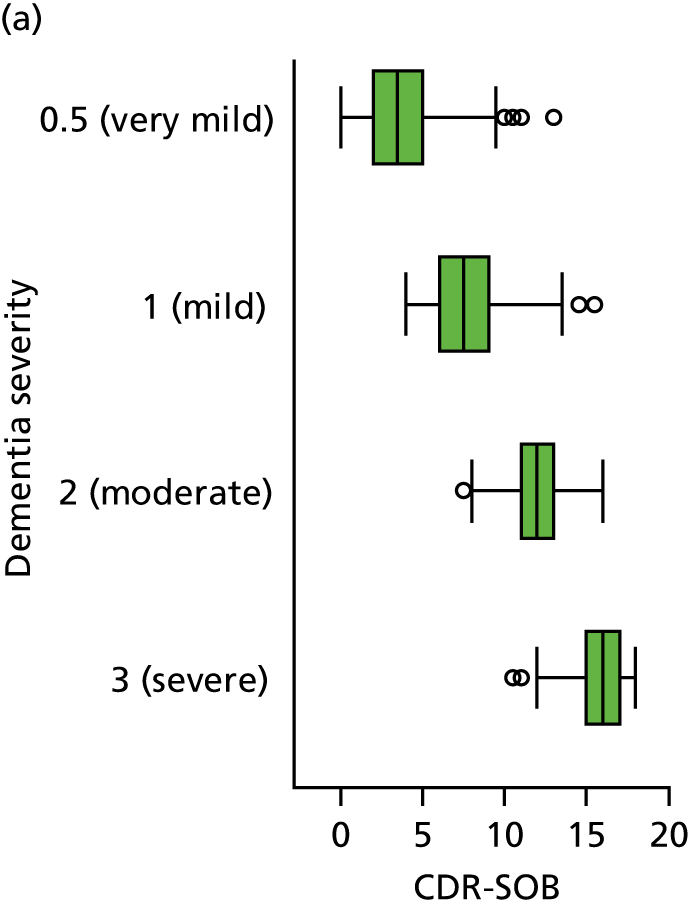
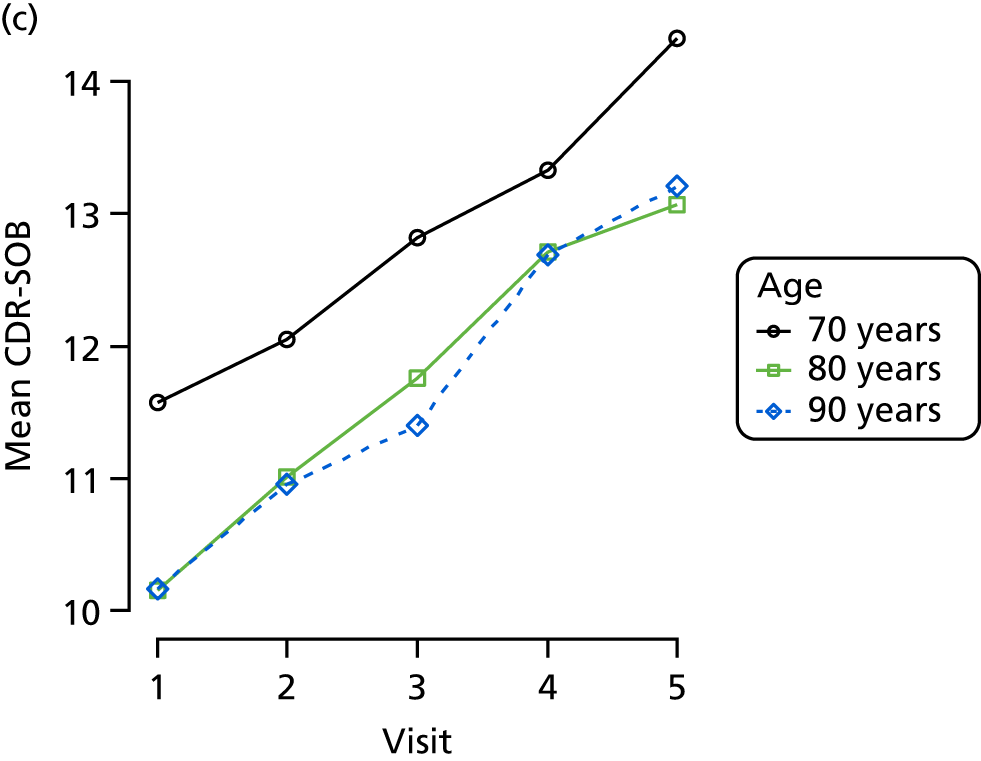
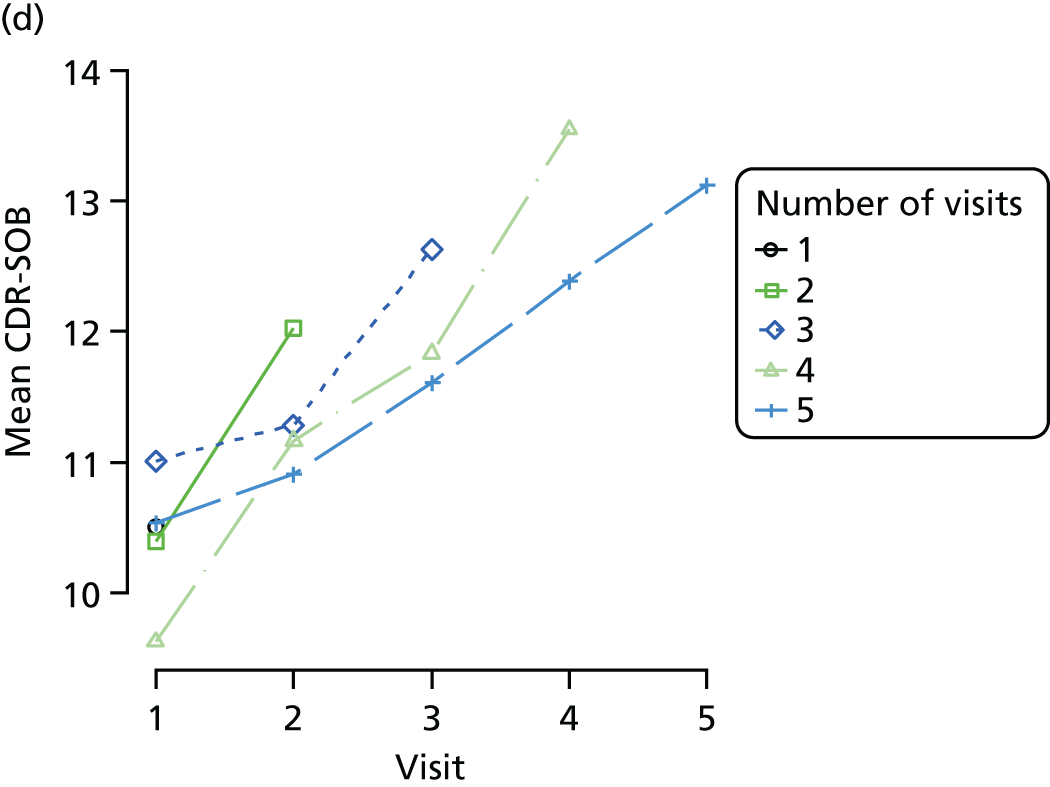
FIGURE 16.
(a) Distribution of sleep disturbance with respect to dementia severity; (b) distribution of sleep disturbance with respect to Z-drug use; (c) mean score over REDIC visit, stratified by age group; and (d) mean score over REDIC visit, stratified by number of visits completed.
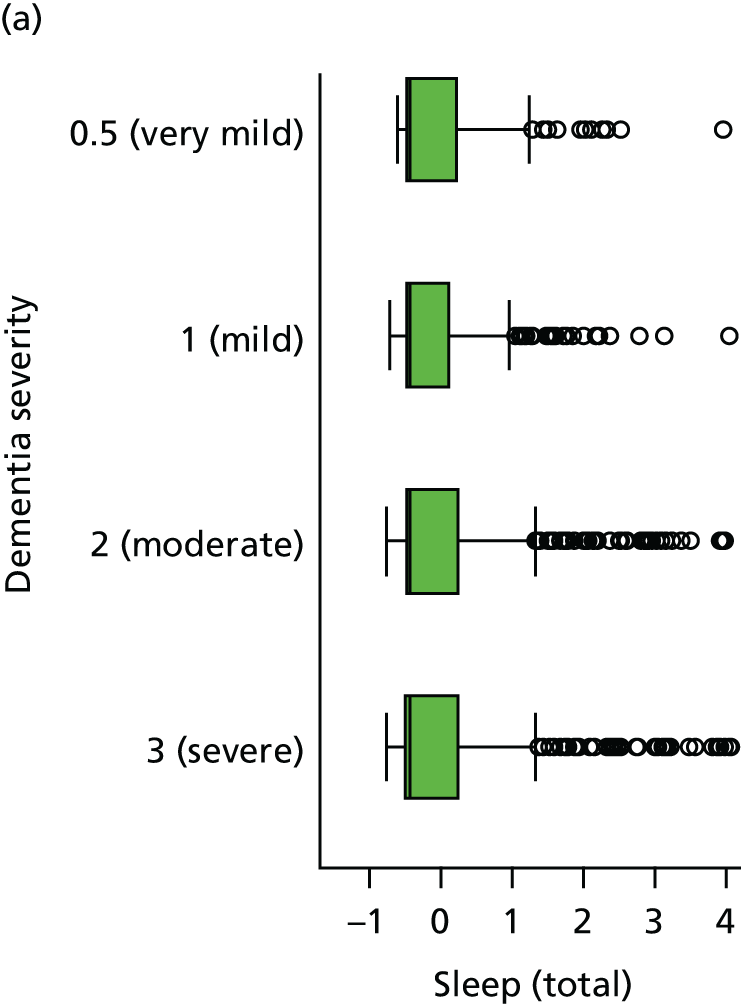
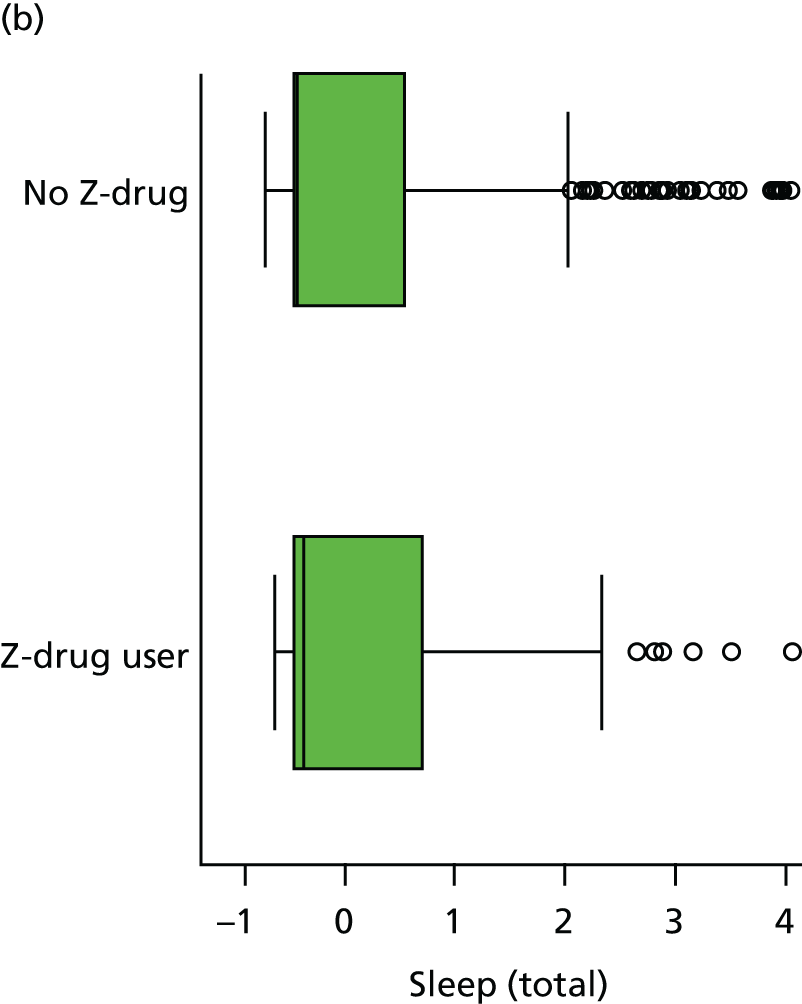
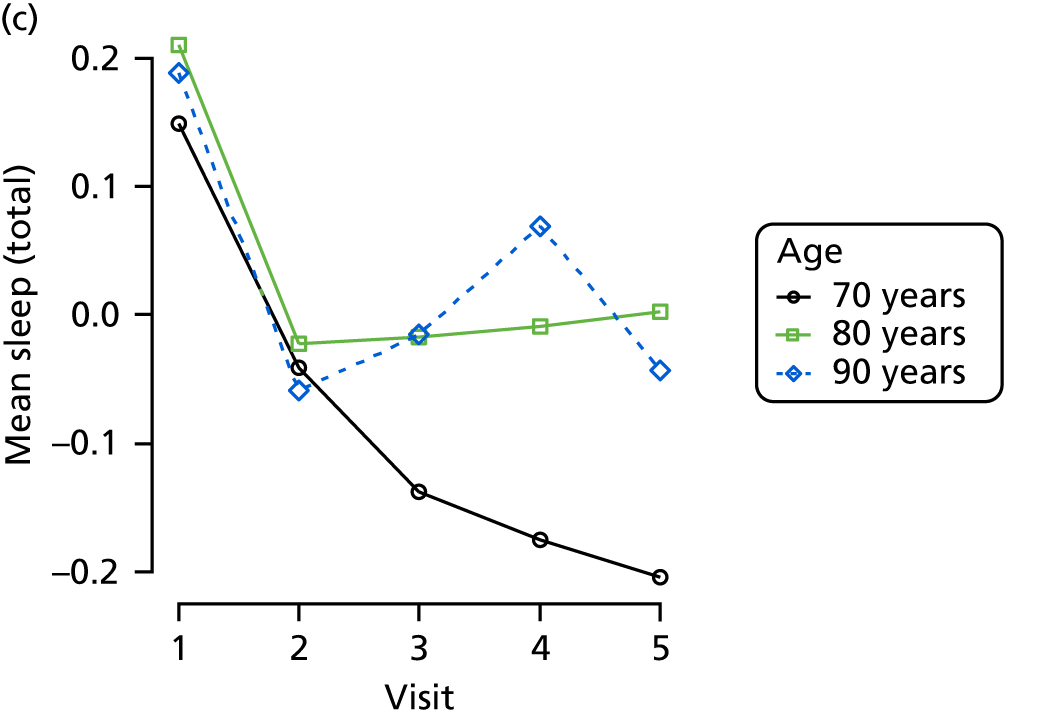
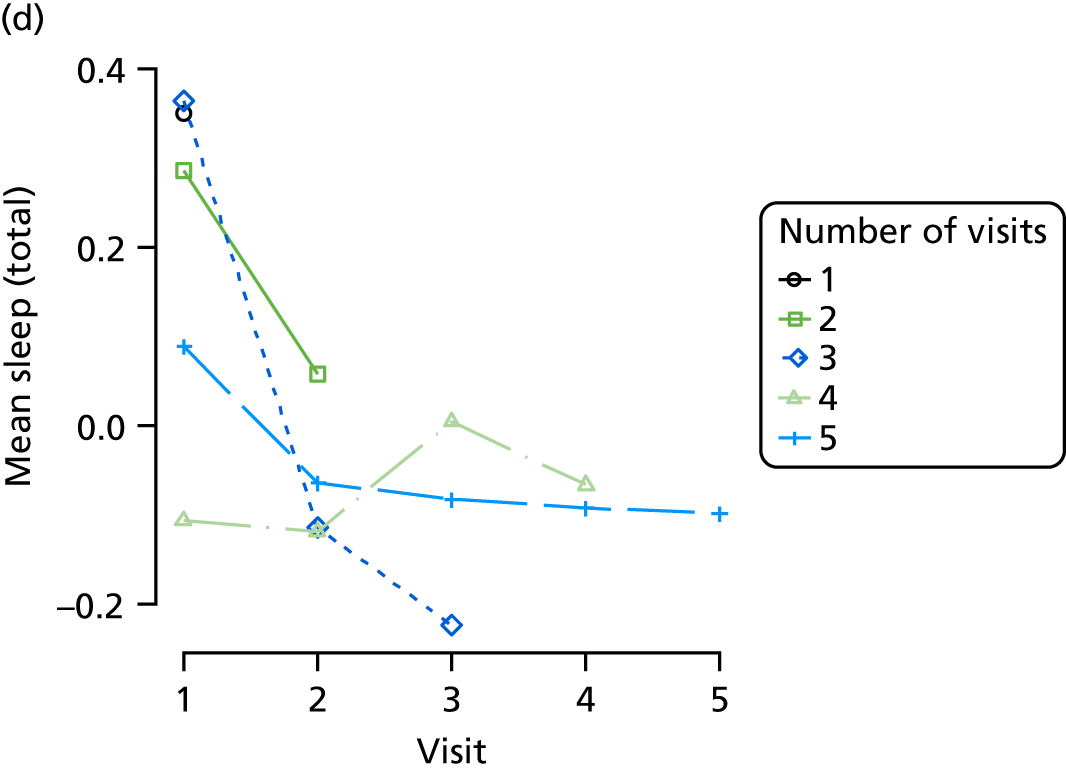
FIGURE 17.
(a) Distribution of agitation with respect to dementia severity; (b) distribution of agitation with respect to Z-drug use; (c) mean score over REDIC visit, stratified by age group; and (d) mean score over REDIC visit, stratified by number of visits completed.
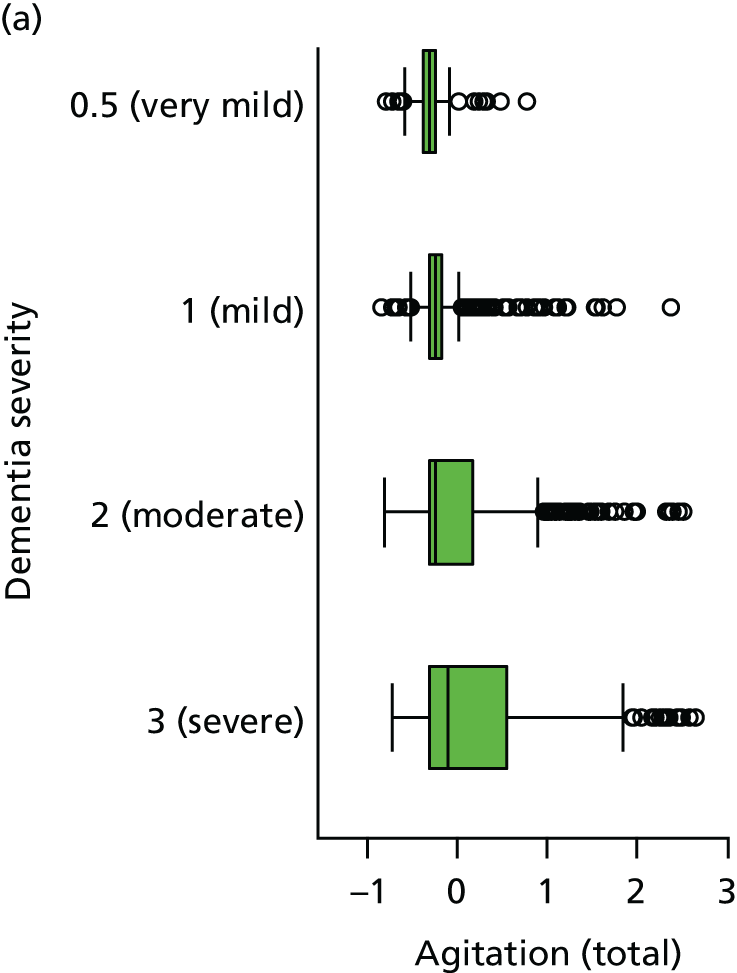
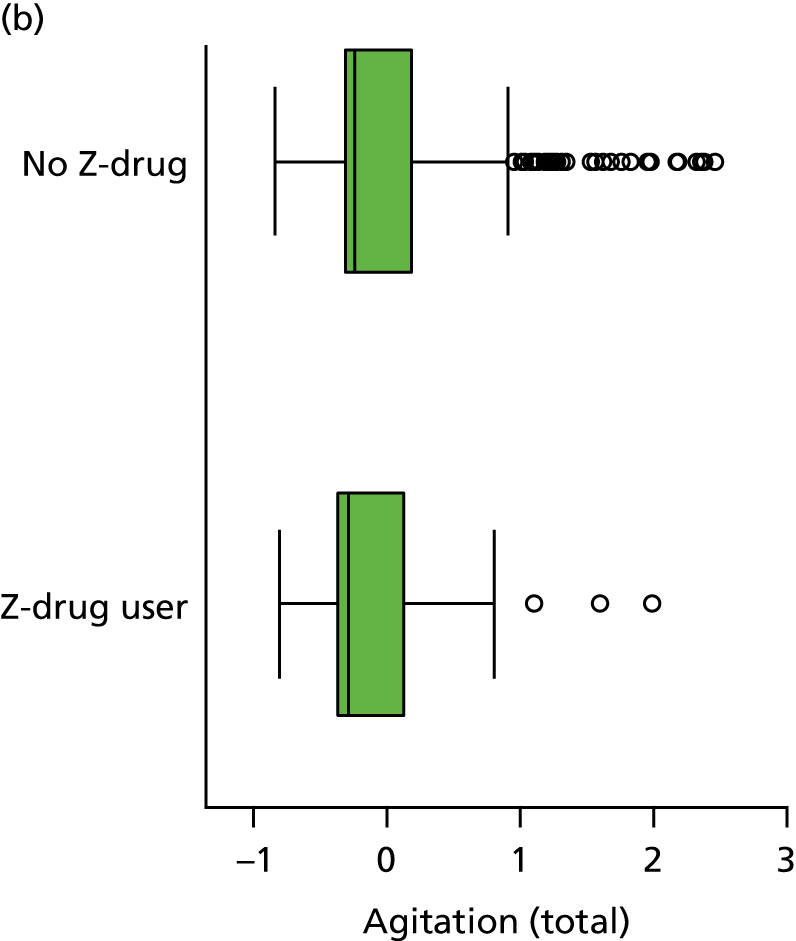
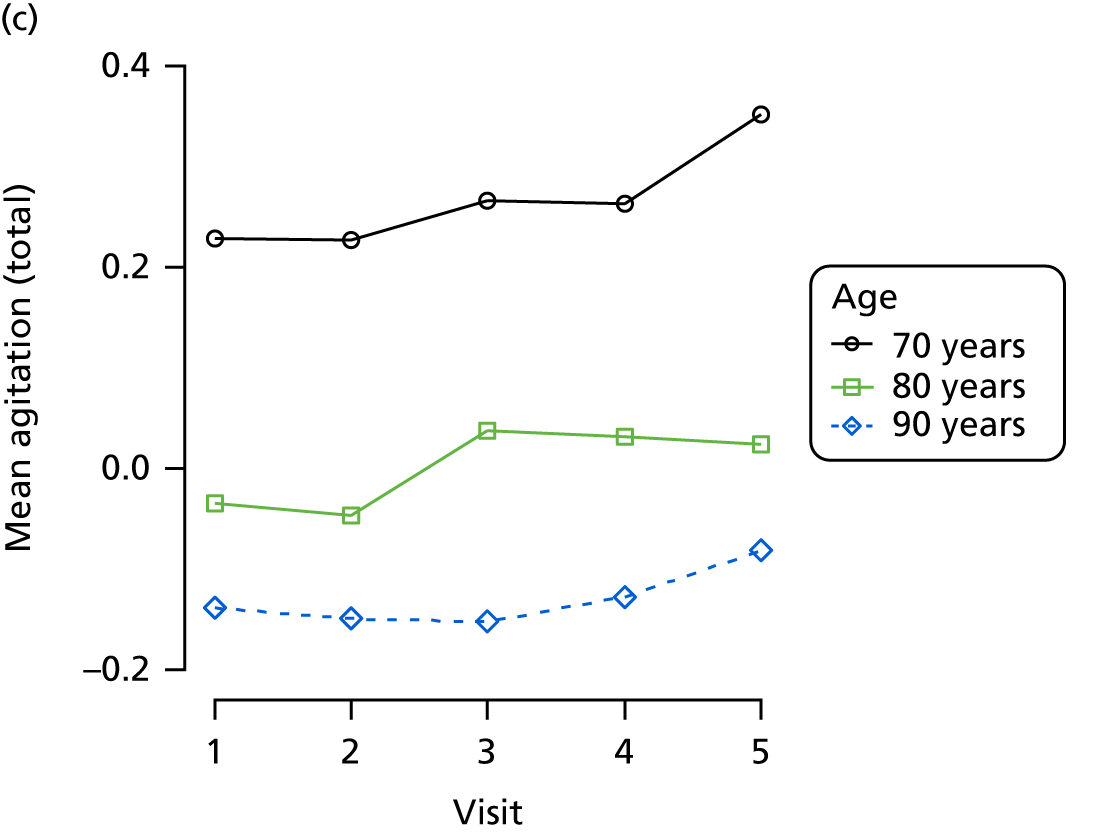
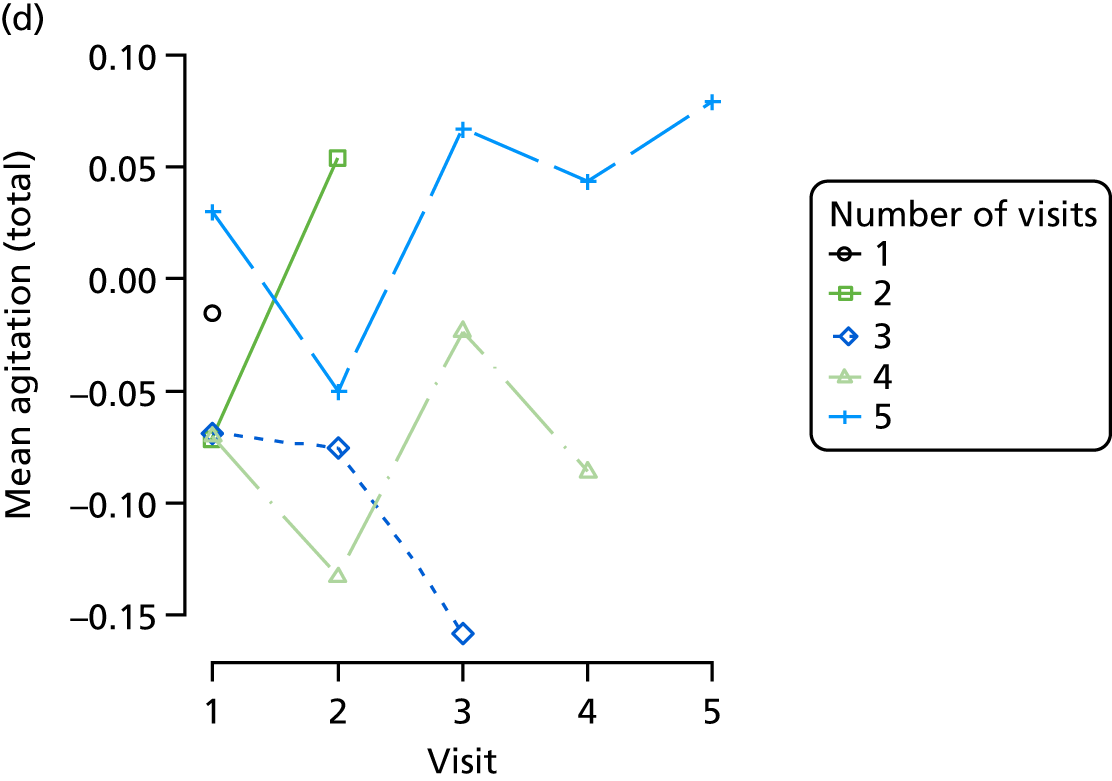
FIGURE 18.
(a) Distribution of anxiety with respect to dementia severity; (b) distribution of anxiety with respect to Z-drug use; (c) mean score over REDIC visit, stratified by age group; and (d) mean score over REDIC visit, stratified by number of visits completed.
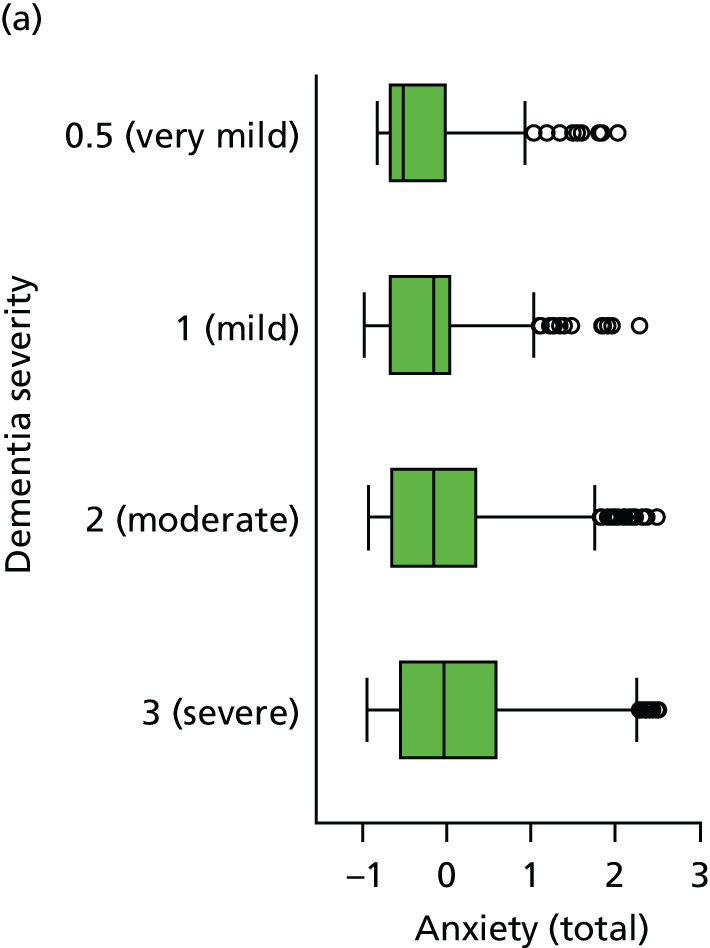
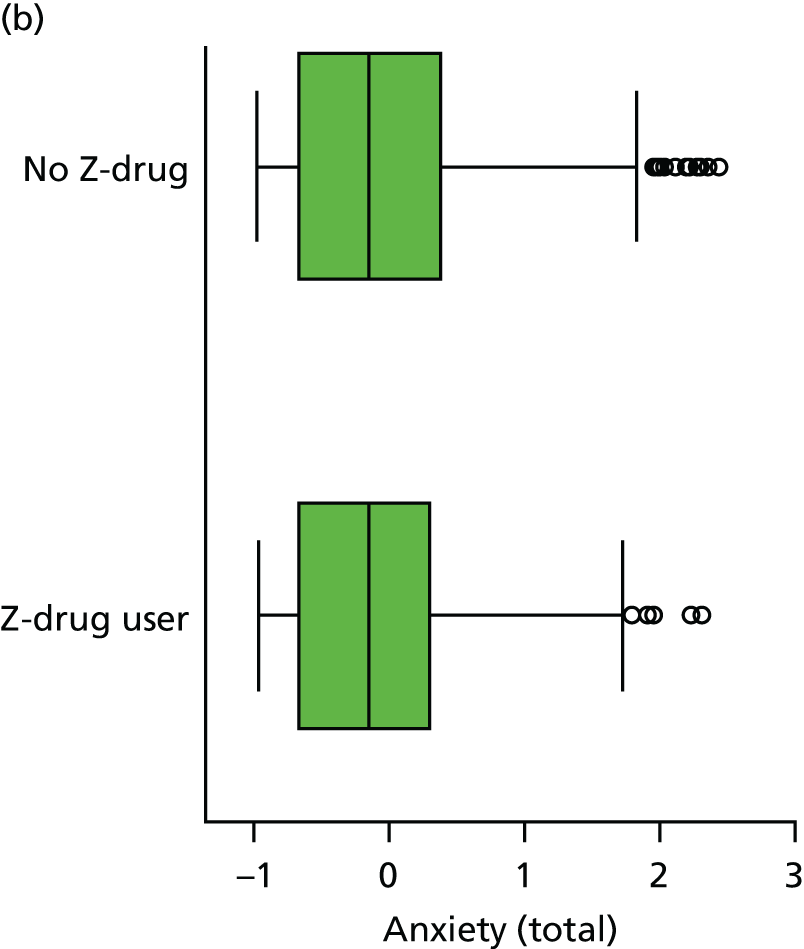
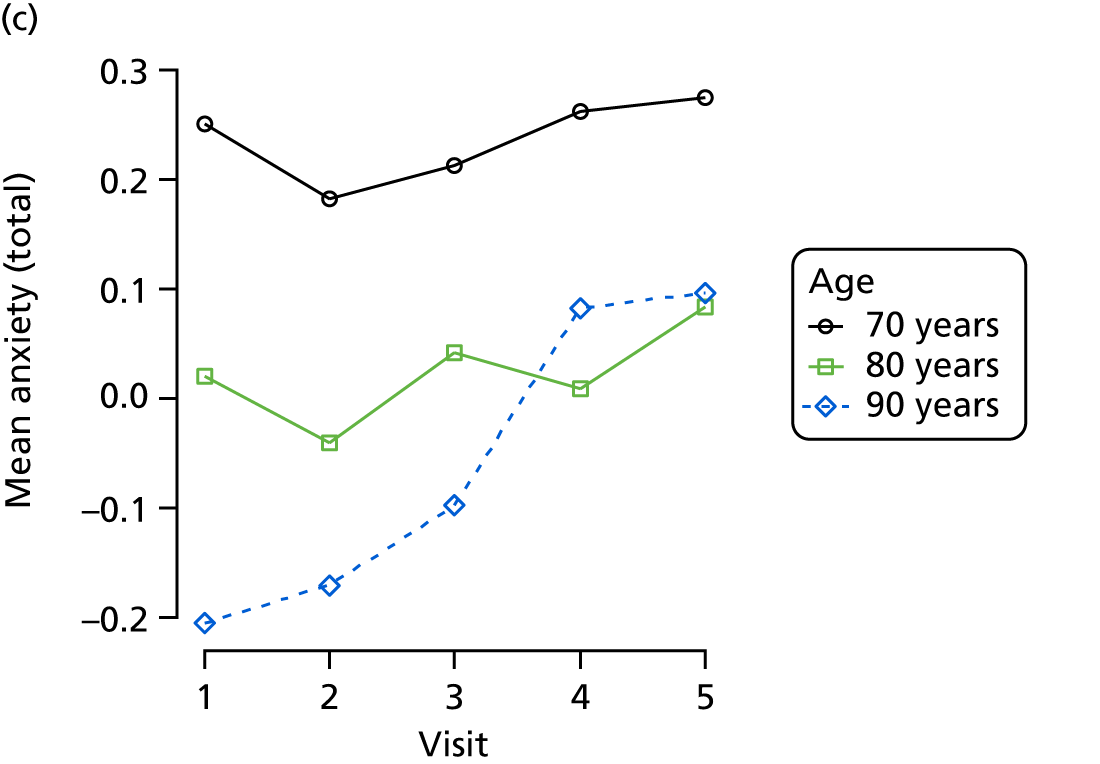
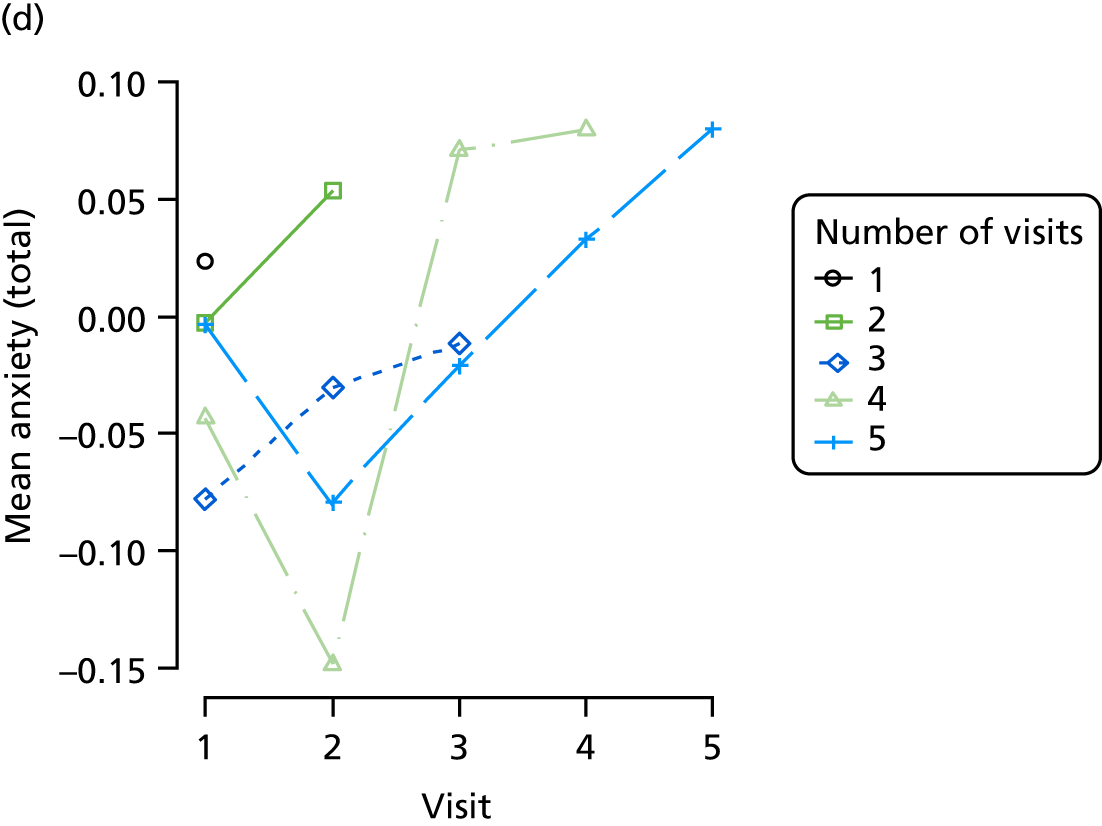
FIGURE 19.
(a) Distribution of VAS with respect to dementia severity; (b) distribution of VAS with respect to Z-drug use; (c) mean score over REDIC visit, stratified by age group; and (d) mean score over REDIC visit, stratified by number of visits completed.
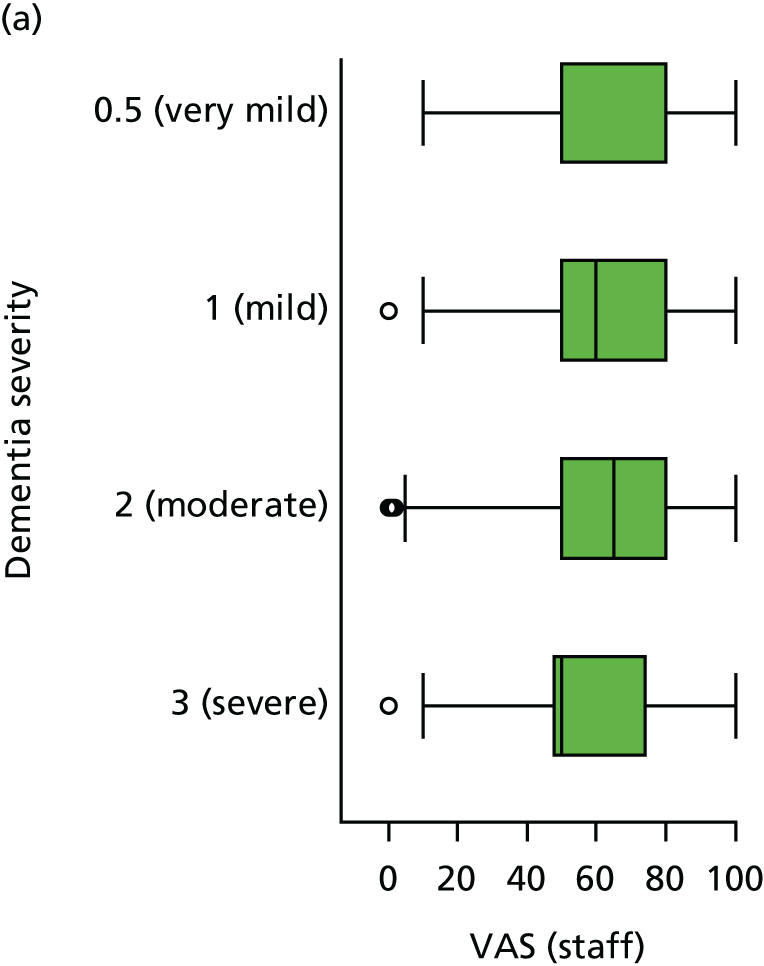
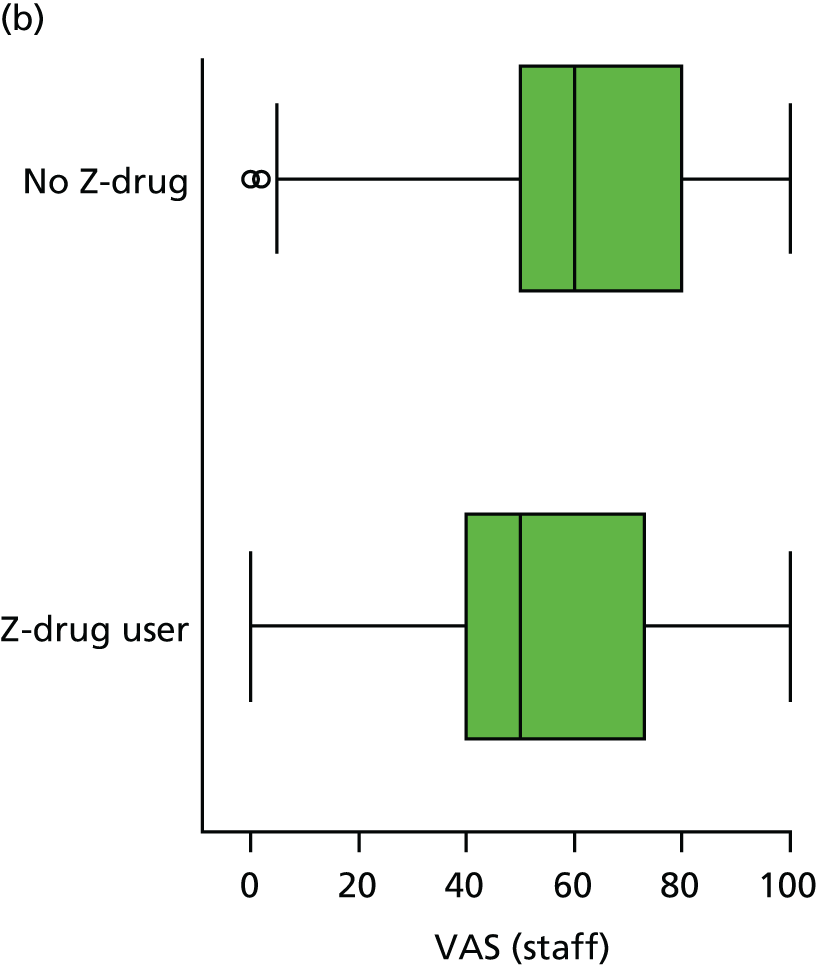
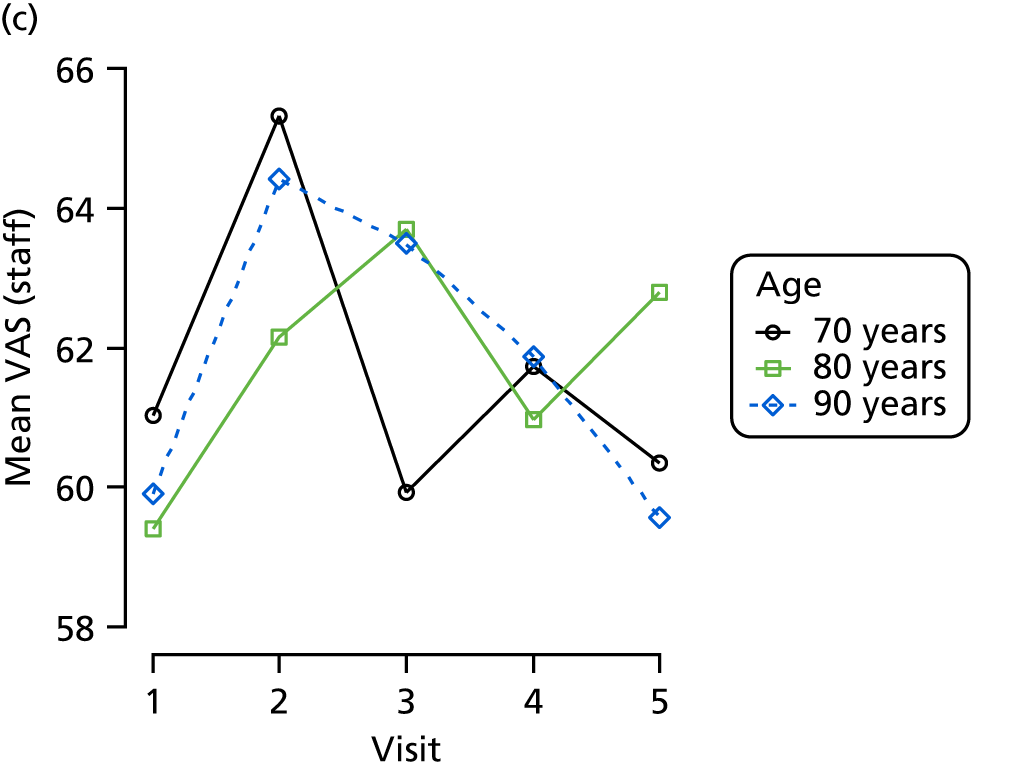
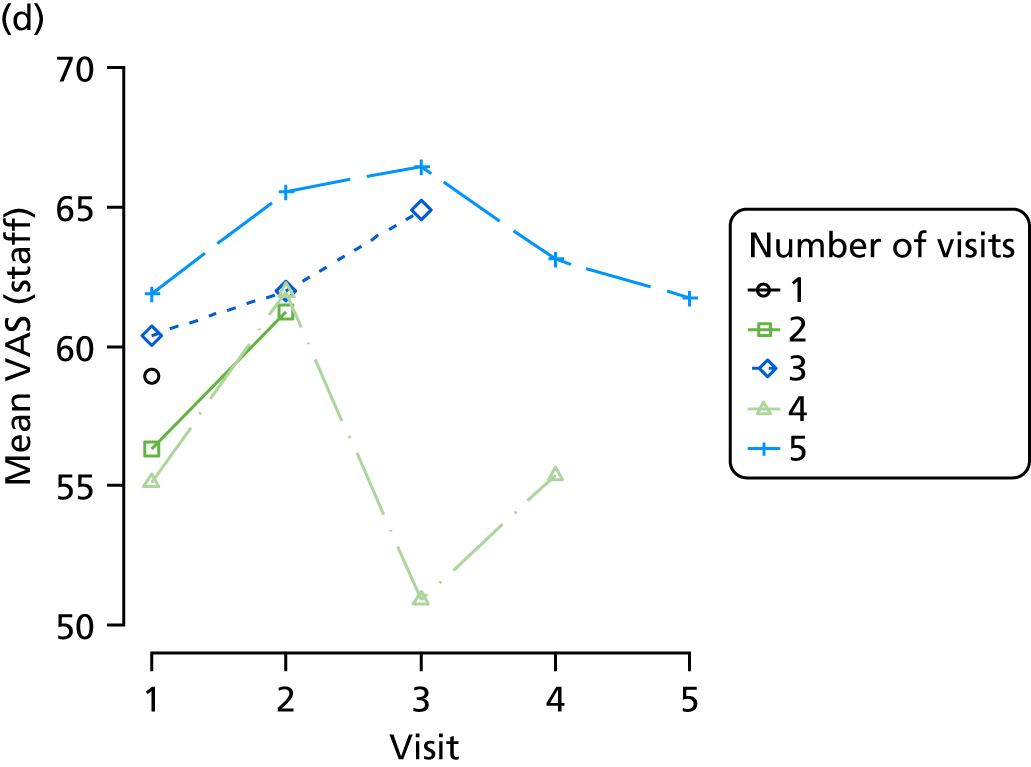
FIGURE 20.
(a) Distribution of EQ-5D with respect to dementia severity; (b) distribution of EQ-5D with respect to Z-drug; (c) mean score over REDIC visit, stratified by age group; and (d) mean score over REDIC visit, stratified by number of visits completed.
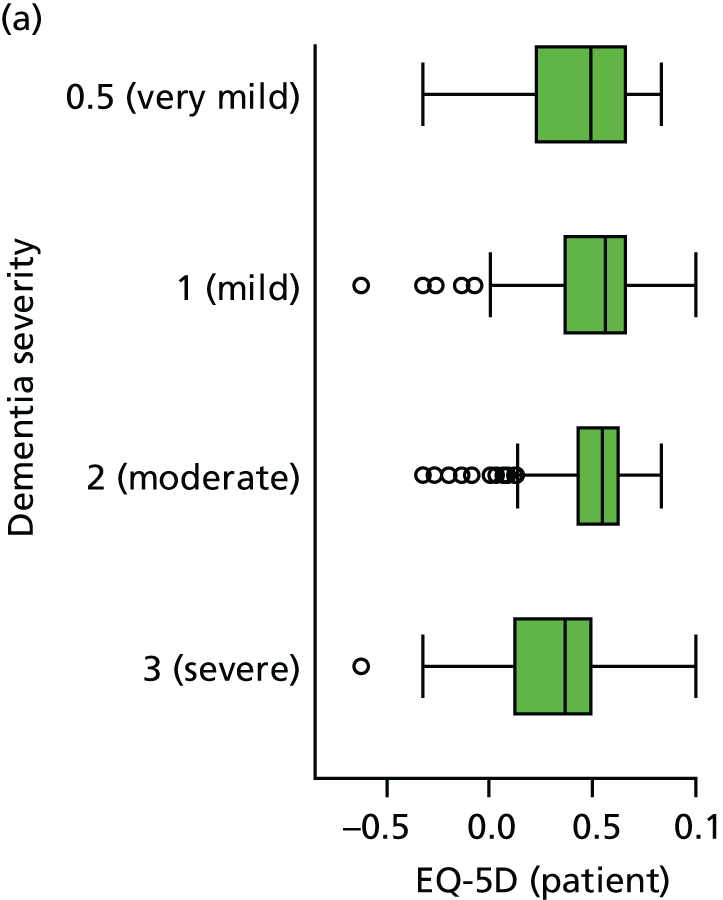
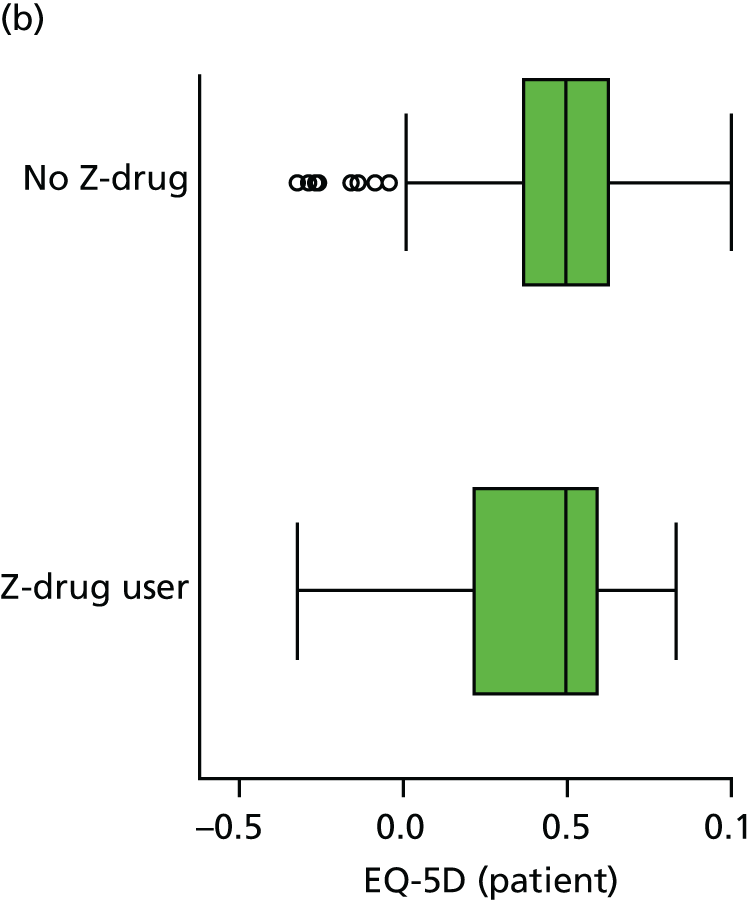
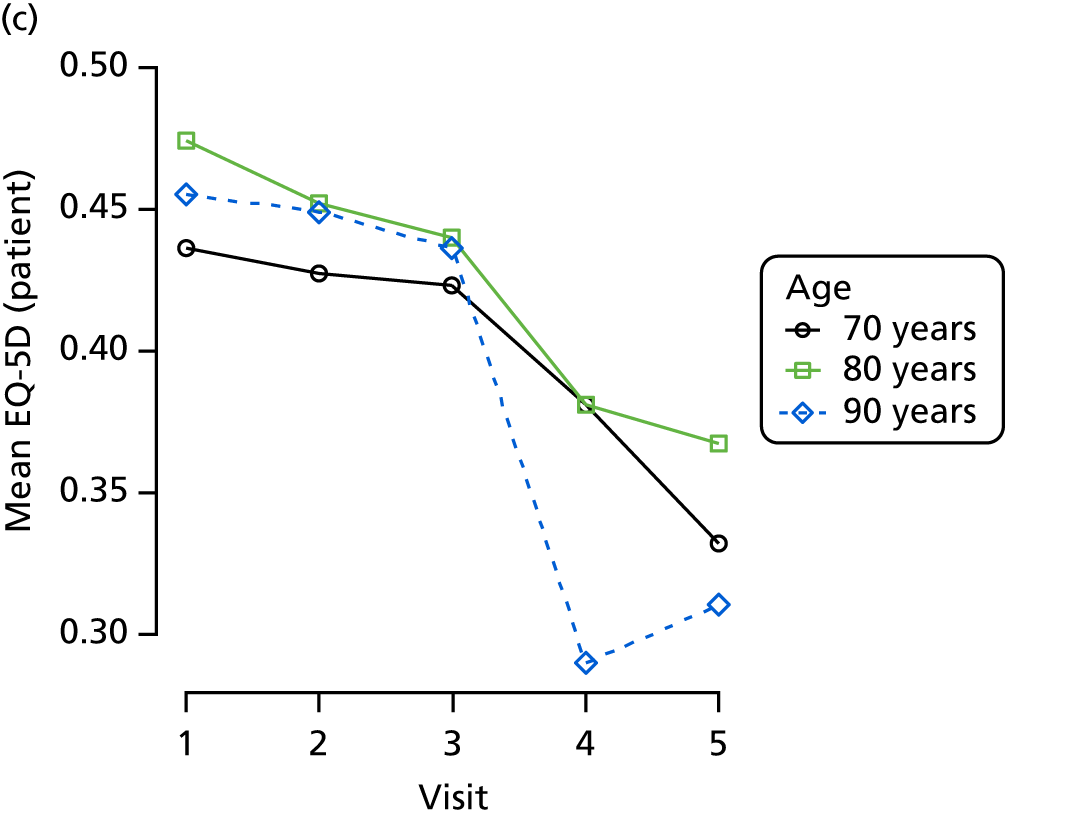
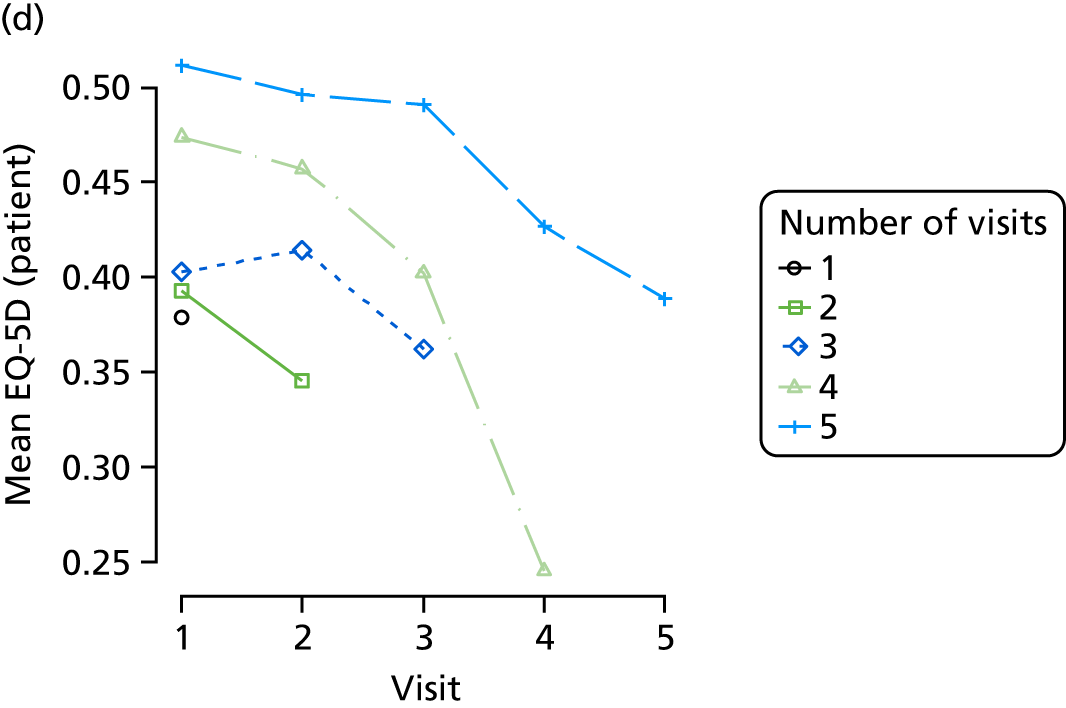
FIGURE 21.
(a) Distribution of QUALID with respect to dementia severity; (b) distribution of QUALID with respect to Z-drug use; (c) mean score over REDIC visit, stratified by age group; and (d) mean score over REDIC visit, stratified by number of visits completed.
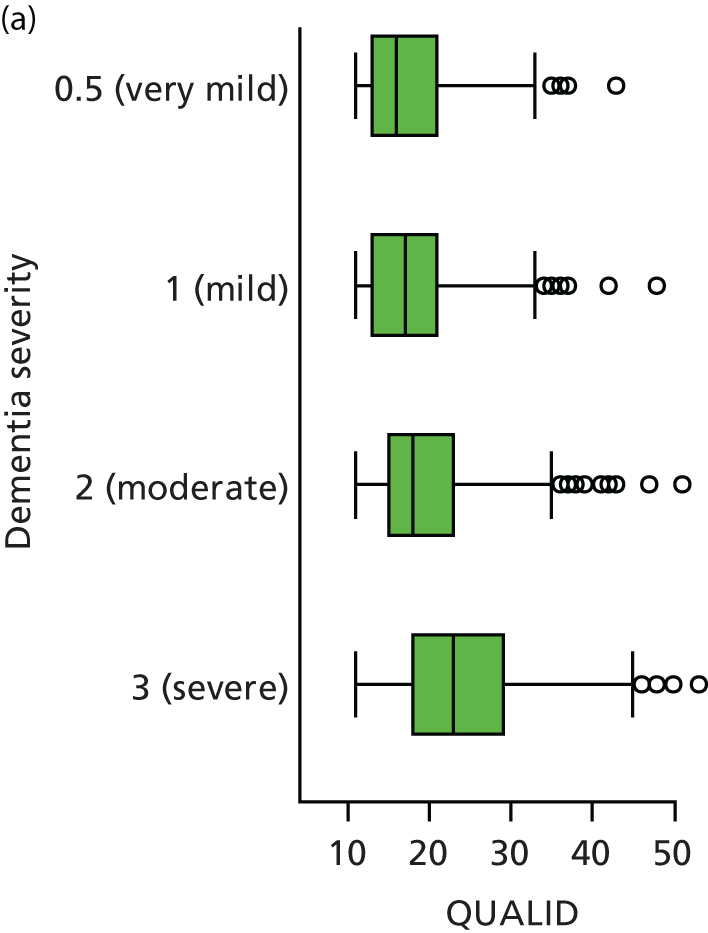
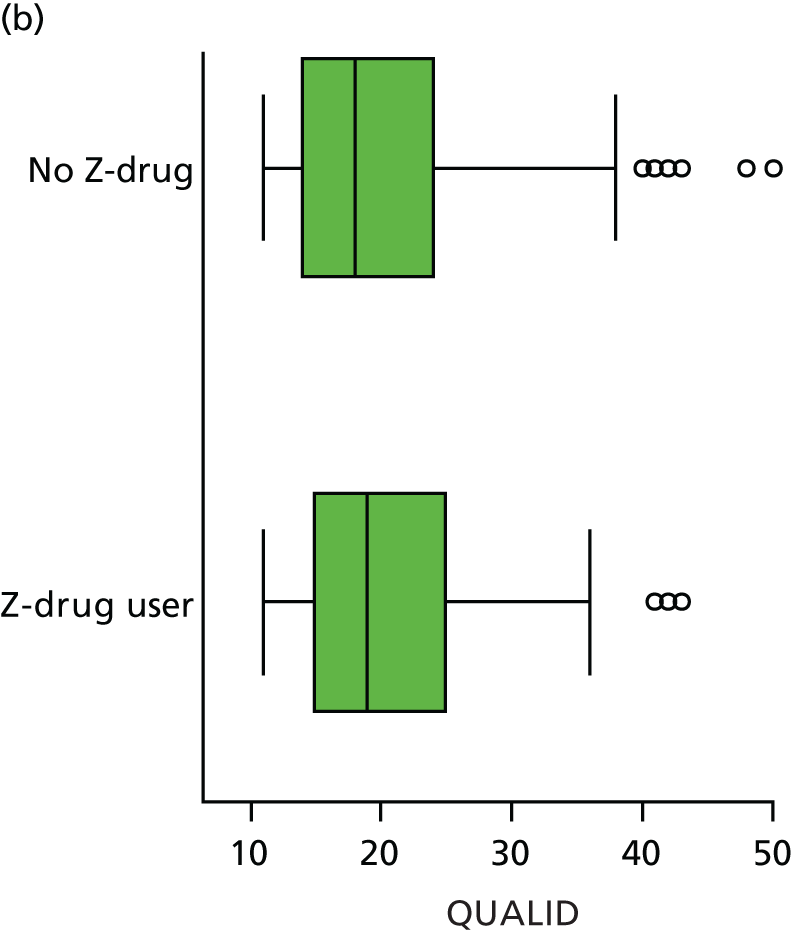
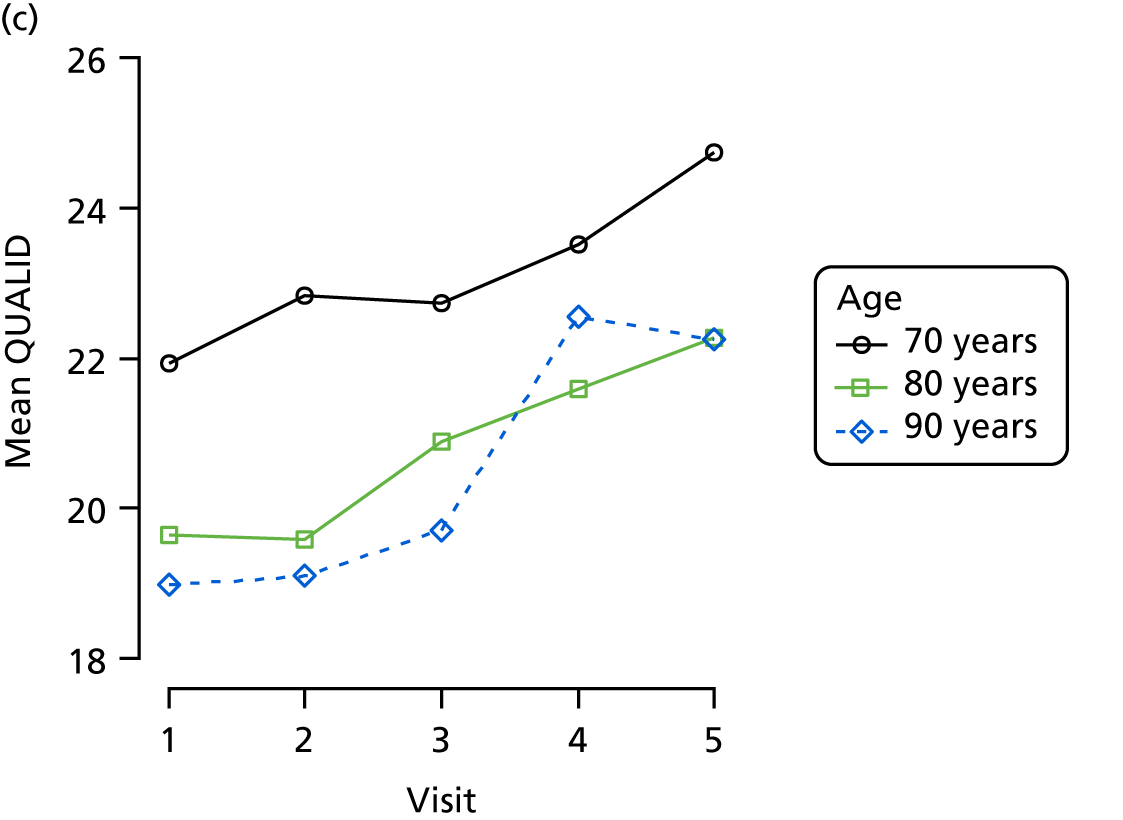
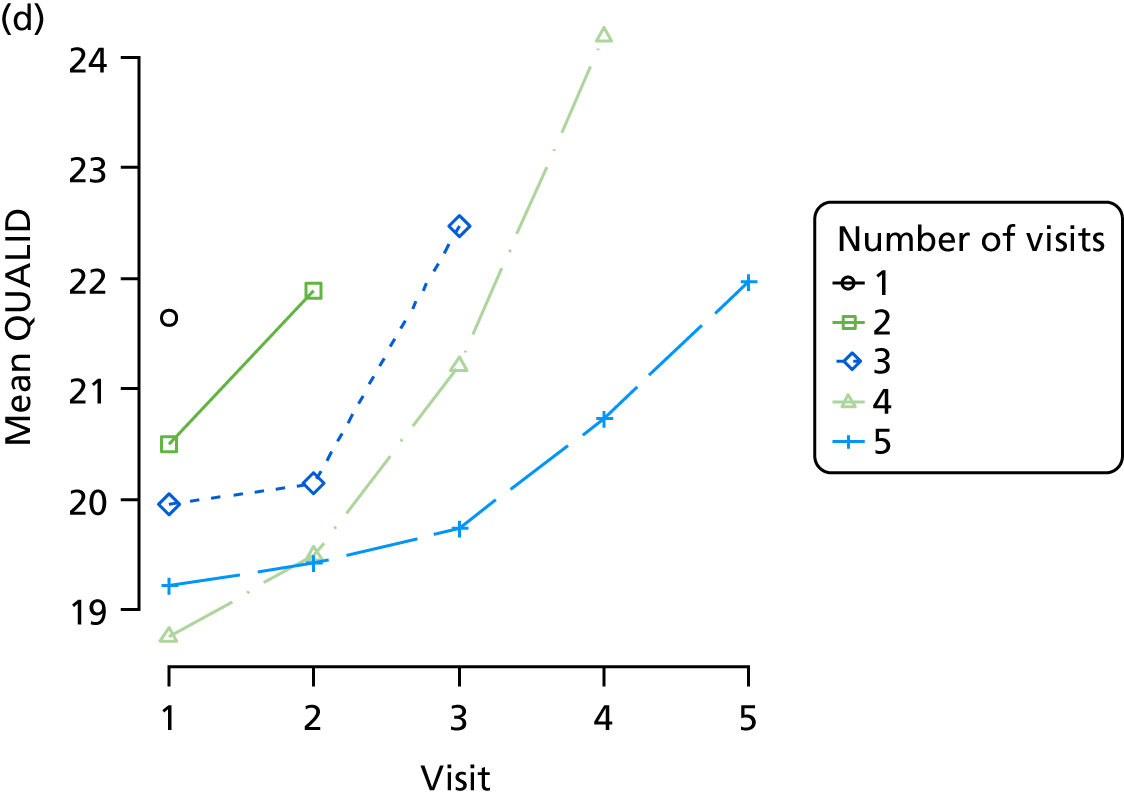
FIGURE 22.
(a) Distribution of disability (Lawton Physical Self-Maintenance Scale) with respect to dementia severity; (b) distribution of disability (Lawton Physical Self-Maintenance Scale) with respect to Z-drug use; (c) mean score over REDIC visit, stratified by age group; and (d) mean score over REDIC visit, stratified by number of visits completed.
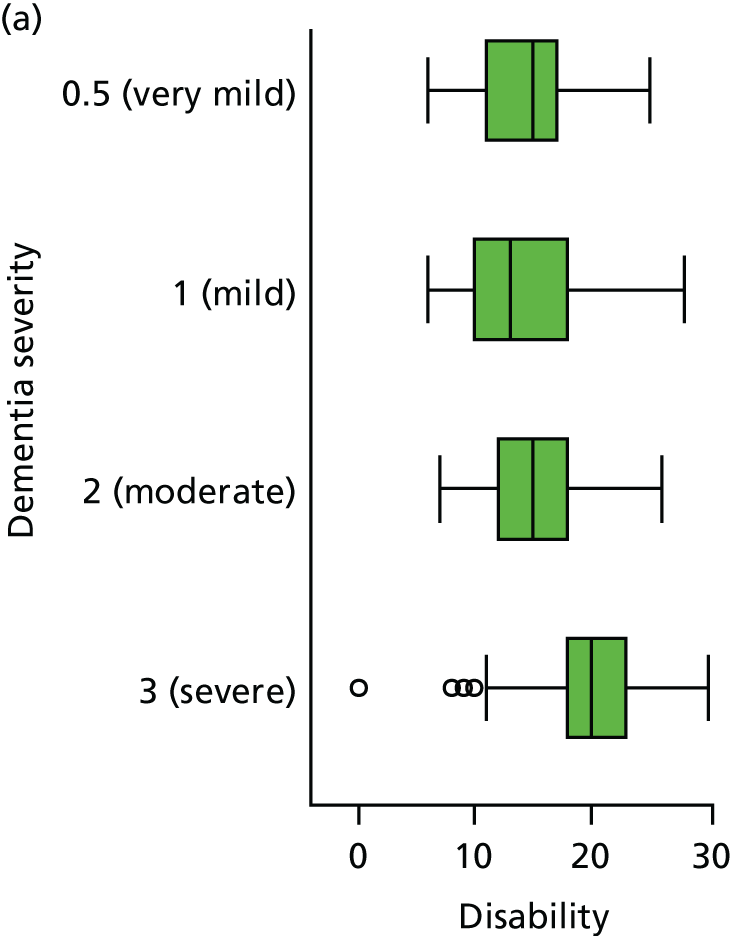
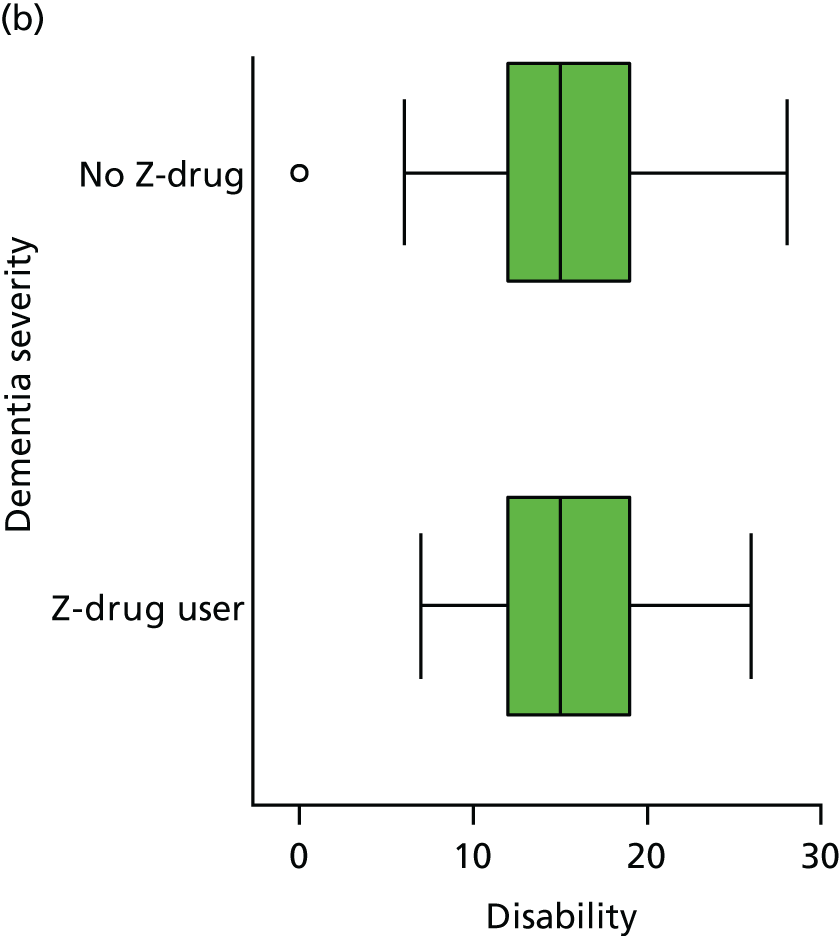
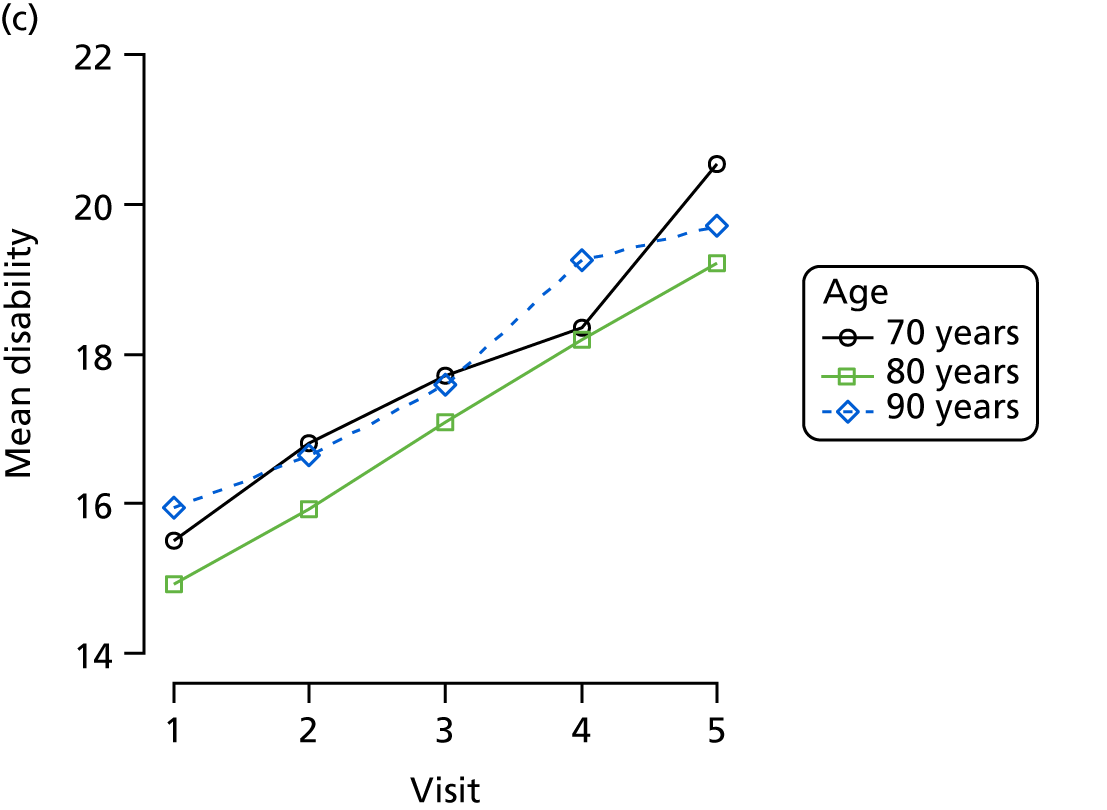
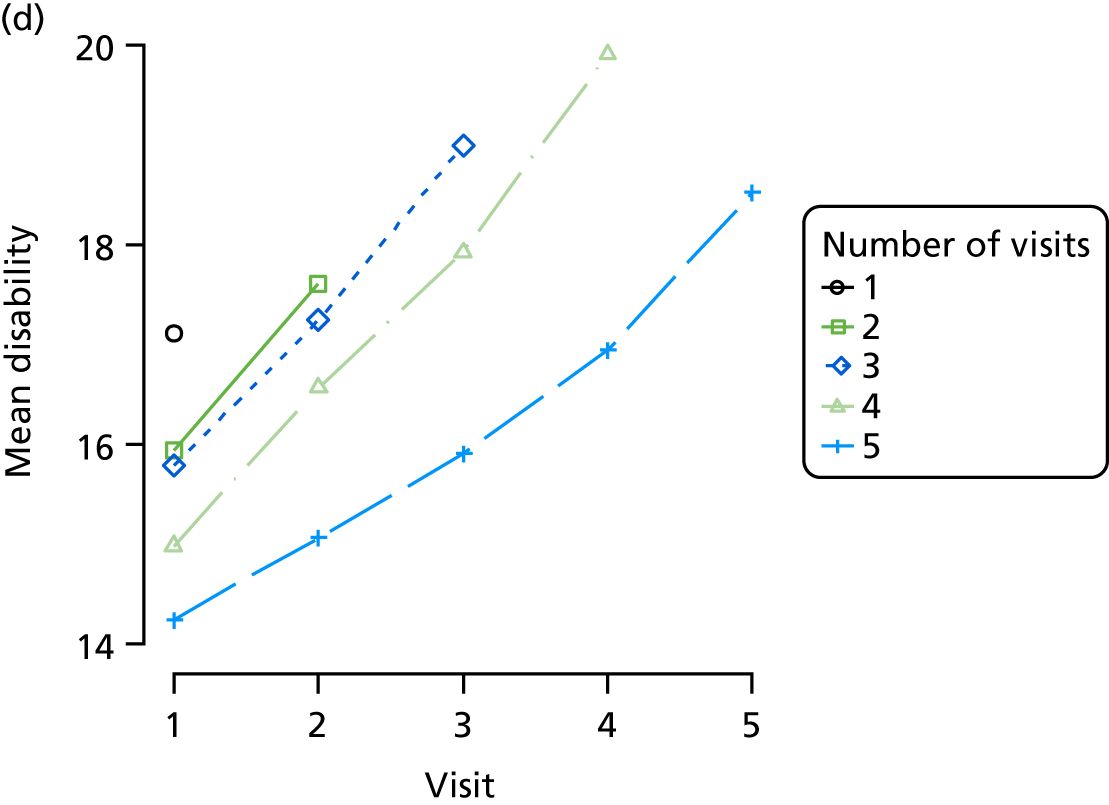
Appendix 4 Additional NACC study analyses
| Visit number | Visits, n (%) | Z-drugs, n (%) | BZDs, n (%) | Antipsychotics, n (%) | Antidepressants, n (%) |
|---|---|---|---|---|---|
| 1 | 16,862 (100) | 373 (2) | 1276 (8) | 1454 (9) | 6742 (40) |
| 2 | 9890 (59) | 198 (2) | 776 (8) | 1070 (11) | 4397 (44) |
| 3 | 6375 (38) | 121 (2) | 557 (9) | 845 (13) | 3011 (47) |
| 4 | 4020 (24) | 75 (2) | 371 (9) | 637 (16) | 1980 (49) |
| 5 | 2441 (14) | 42 (2) | 265 (11) | 420 (17) | 1212 (50) |
| 6 | 1469 (9) | 26 (2) | 164 (11) | 275 (19) | 741 (50) |
| 7 | 838 (5) | 12 (1) | 86 (10) | 167 (20) | 388 (46) |
| 8 | 437 (3) | 8 (2) | 39 (9) | 87 (20) | 206 (47) |
| 9 | 238 (1) | 2 (1) | 23 (10) | 50 (21) | 116 (49) |
| 10 | 111 (1) | 1 (1) | 15 (14) | 28 (25) | 51 (46) |
| 11 | 51 (0.3) | 1 (2) | 8 (16) | 11 (22) | 26 (51) |
| 12 | 19 (0.1) | 0 (0) | 5 (26) | 6 (32) | 13 (68) |
| No use at previous wave | Use at previous wave | |||||
|---|---|---|---|---|---|---|
| Total, N (%) | No use, n (%) | Starting, n (%) | Total, N (%) | Stopping, n (%) | Continued, n (%) | |
| Z-drugs | 25,289 (100) | 25,070 (99) | 219 (1) | 522 (100) | 257 (49) | 265 (51) |
| BZDs | 23,851 (100) | 22,898 (96) | 953 (4) | 1960 (100) | 615 (31) | 1345 (69) |
| Antipsychotics | 23,102 (100) | 21,658 (94) | 1444 (6) | 2709 (100) | 572 (21) | 2137 (79) |
| Factor | Level | Continuing Z-drugs, OR (95% CI) | Continuing BZDs, OR (95% CI) | Continuing antipsychotics, OR (95% CI) |
|---|---|---|---|---|
| Drug use | Z-drug use | 0.72 (0.43 to 1.22) | 0.73 (0.38 to 1.08) | |
| BZD use | 0.93 (0.53 to 1.64) | 1.01 (0.75 to 1.27) | ||
| AP use | 1.26 (0.72 to 2.21) | 1.48** (1.13 to 1.94) | ||
| Education | Per year | 0.96 (0.92 to 1.00) | 0.99 (0.96 to 1.02) | 0.99 (0.96 to 1.02) |
| Sex | Female | 1.00 (0.65 to 1.52) | 0.86 (0.68 to 1.08) | 0.81* (0.63 to 0.98) |
| CDR | Minimal | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Mild | 1.41 (0.88 to 2.25) | 0.92 (0.70 to 1.22) | 1.30 (0.76 to 1.84) | |
| Moderate | 0.72 (0.39 to 1.34) | 0.73 (0.53 to 1.02) | 1.44 (0.83 to 2.05) | |
| Severe | 0.87 (0.46 to 1.67) | 0.63** (0.45 to 0.88) | 1.08 (0.64 to 1.52) | |
| Age (years) | < 61 | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 61–70 | 0.81 (0.43 to 1.52) | 0.70* (0.50 to 0.99) | 1.05 (0.71 to 1.38) | |
| 71–80 | 0.80 (0.43 to 1.46) | 0.55*** (0.40 to 0.77) | 0.98 (0.68 to 1.29) | |
| 81–90 | 0.70 (0.36 to 1.36) | 0.60** (0.41 to 0.88) | 0.93 (0.61 to 1.25) | |
| ≥ 90 | 0.87 (0.27 to 2.78) | 0.62 (0.25 to 1.52) | 1.29 (–0.005 to 2.58) | |
| Sleep | None | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Mild | 0.97 (0.60 to 1.57) | 0.78 (0.58 to 1.04) | 1.21 (0.87 to 1.55) | |
| Moderate | 0.85 (0.49 to 1.47) | 1.15 (0.85 to 1.57) | 1.04 (0.75 to 1.34) | |
| Severe | 0.58 (0.28 to 1.22) | 1.11 (0.72 to 1.73) | 1.00 (0.61 to 1.39) | |
| Anxiety | None | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Mild | 1.23 (0.77 to 1.98) | 0.98 (0.77 to 1.26) | 1.06 (0.78 to 1.33) | |
| Moderate | 0.93 (0.54 to 1.62) | 0.86 (0.65 to 1.14) | 0.85 (0.61 to 1.09) | |
| Severe | 1.59 (0.55 to 4.59) | 0.92 (0.60 to 1.40) | 0.95 (0.56 to 1.35) | |
| Agitation | None | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Mild | 0.76 (0.47 to 1.21) | 1.20 (0.93 to 1.56) | 1.07 (0.81 to 1.32) | |
| Moderate | 0.76 (0.42 to 1.37) | 1.03 (0.76 to 1.40) | 1.50** (1.07 to 1.93) | |
| Severe | 0.92 (0.35 to 2.39) | 1.18 (0.77 to 1.81) | 1.30 (0.78 to 1.81) |
| Outcome measure | β (95% CI) | ||
|---|---|---|---|
| Z-drug | BZD | Antipsychotic | |
| CDR-SOB | 0.21 (–0.25 to 0.67) | 1.37*** (1.10 to 1.65) | 1.98*** (1.73 to 2.23) |
| MMSE | 0.17 (–0.40 to 0.75) | –1.26*** (–1.76 to –0.77) | –1.81*** (–2.27 to –1.36) |
| Animal fluency | 0.25 (–0.42 to 0.92) | –0.47* (–0.88 to –0.06) | –0.92*** (–1.30 to –0.53) |
| Delta trail time | –5.14 (–23.14 to 12.85) | –0.87 (–11.34 to 9.60) | 1.85 (–8.61 to 12.30) |
| Sleep | 0.23** (0.08 to 0.38) | 0.11** (0.03 to 0.18) | 0.18*** (0.11 to 0.24) |
| Agitation | 0.14* (0.00 to 0.28) | 0.19*** (0.12 to 0.27) | 0.35*** (0.28 to 0.42) |
| Anxiety | 0.05 (–0.08 to 0.18) | 0.24*** (0.16 to 0.31) | 0.18*** (0.12 to 0.25) |
| NPI (excluding sleep) | 0.13 (–0.56 to 0.82) | 1.16*** (0.81 to 1.51) | 1.62*** (1.29 to 1.95) |
| GDS | –0.29 (–0.72 to 0.13) | 0.00 (–0.25 to 0.26) | 0.18 (–0.05 to 0.41) |
| Disability | –0.19 (–0.93 to 0.56) | 1.27*** (0.81 to 1.72) | 2.25*** (1.77 to 2.72) |
FIGURE 23.
(a) Distribution of disability with respect to dementia severity; (b) distribution of disability with respect to Z-drug use; (c) mean score over NACC visit, stratified by age group; and (d) mean score over NACC visit, stratified by number of visits completed.
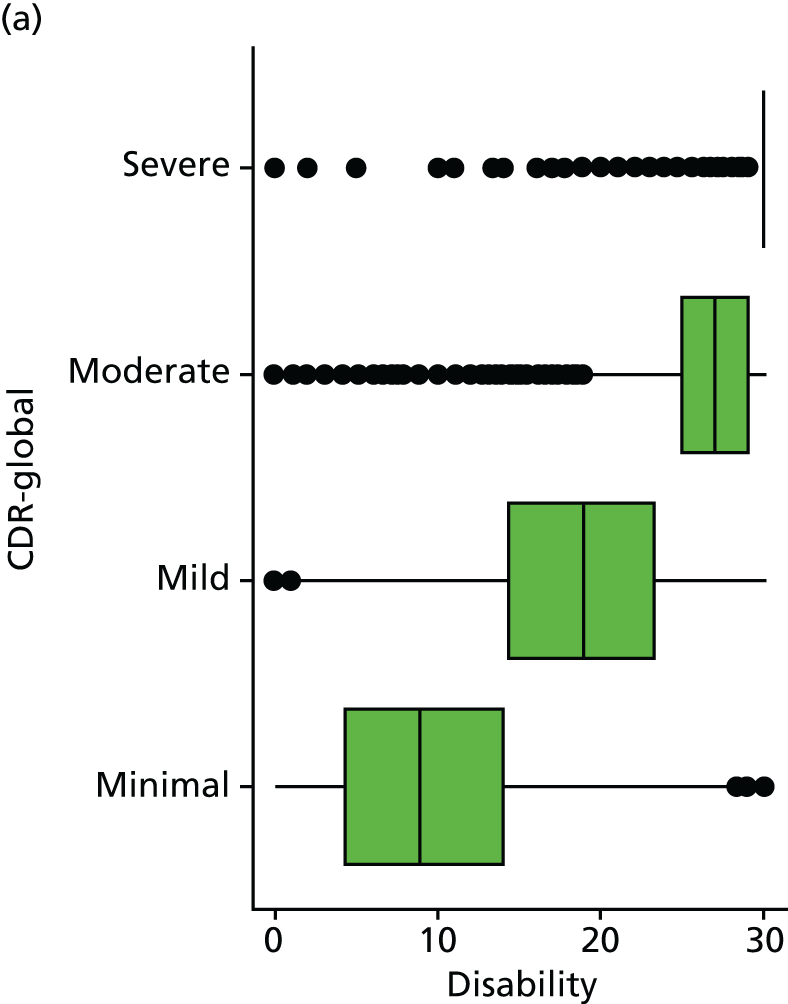
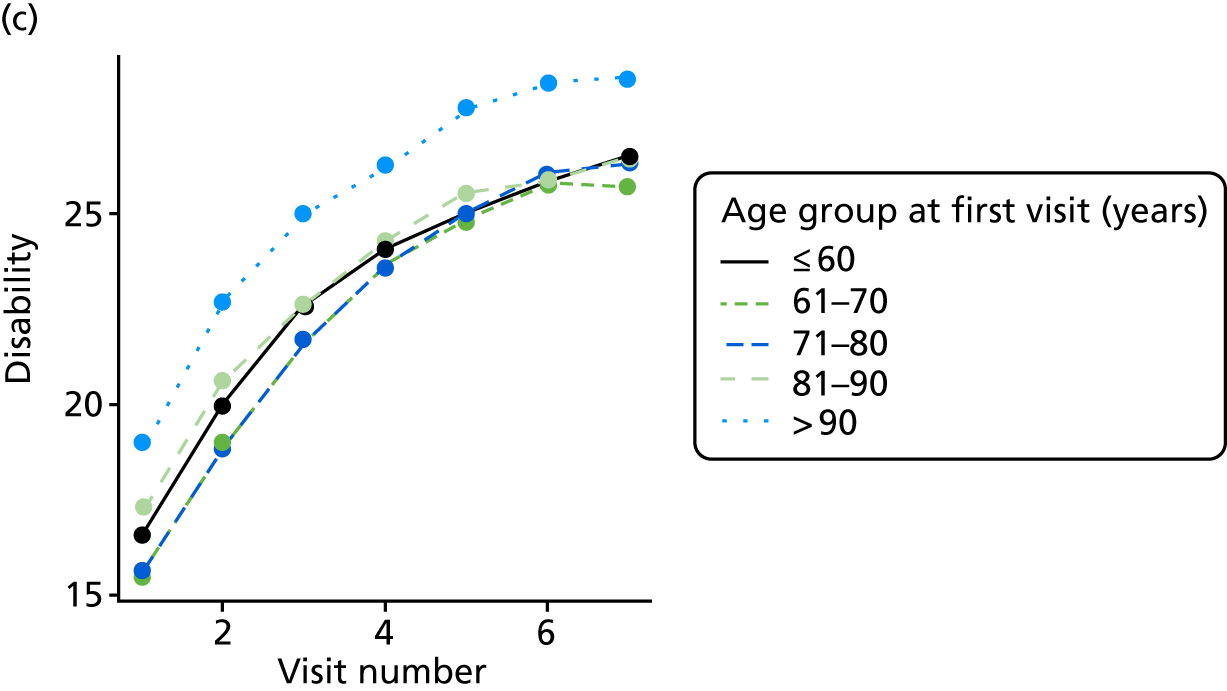
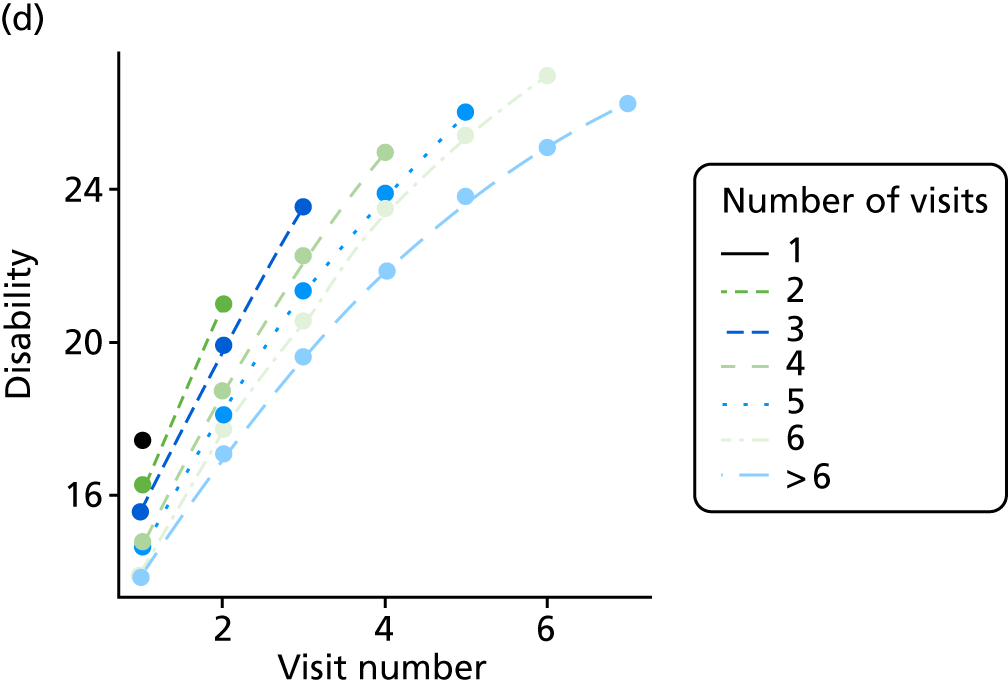
FIGURE 24.
(a) Distribution of animal fluency with respect to dementia severity; (b) distribution of animal fluency with respect to Z-drug use; (c) mean score over NACC visit, stratified by age group; and (d) mean score over NACC visit, stratified by number of visits completed.
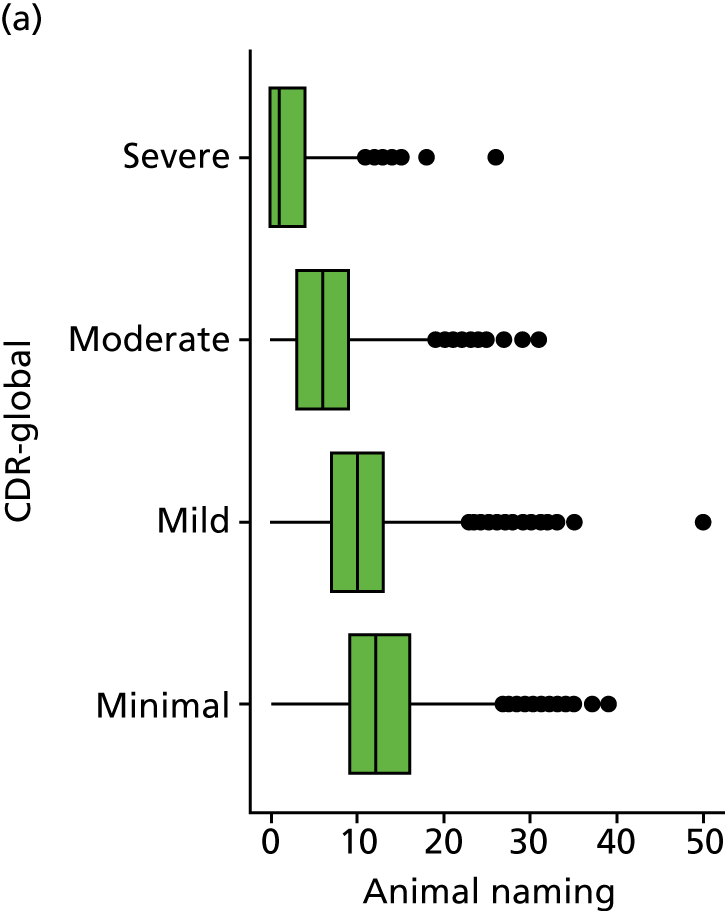
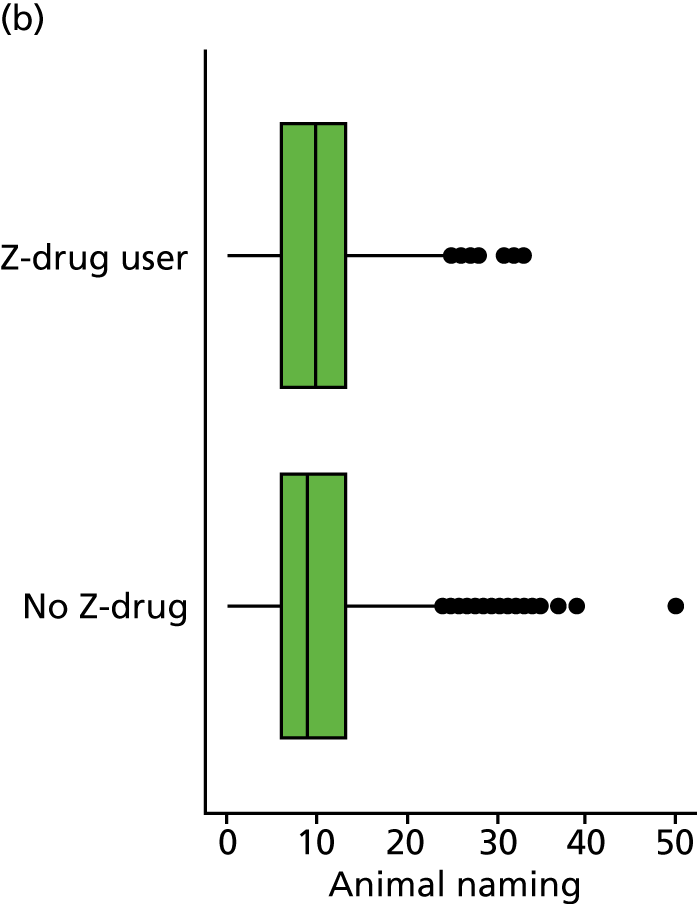
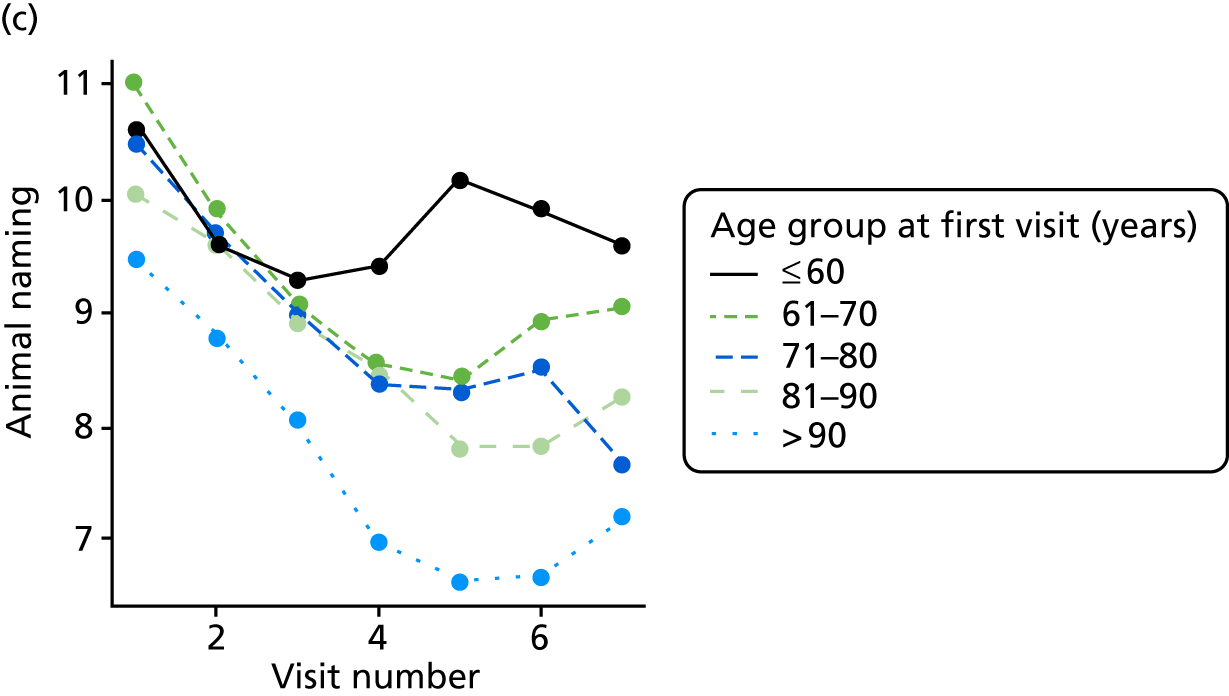
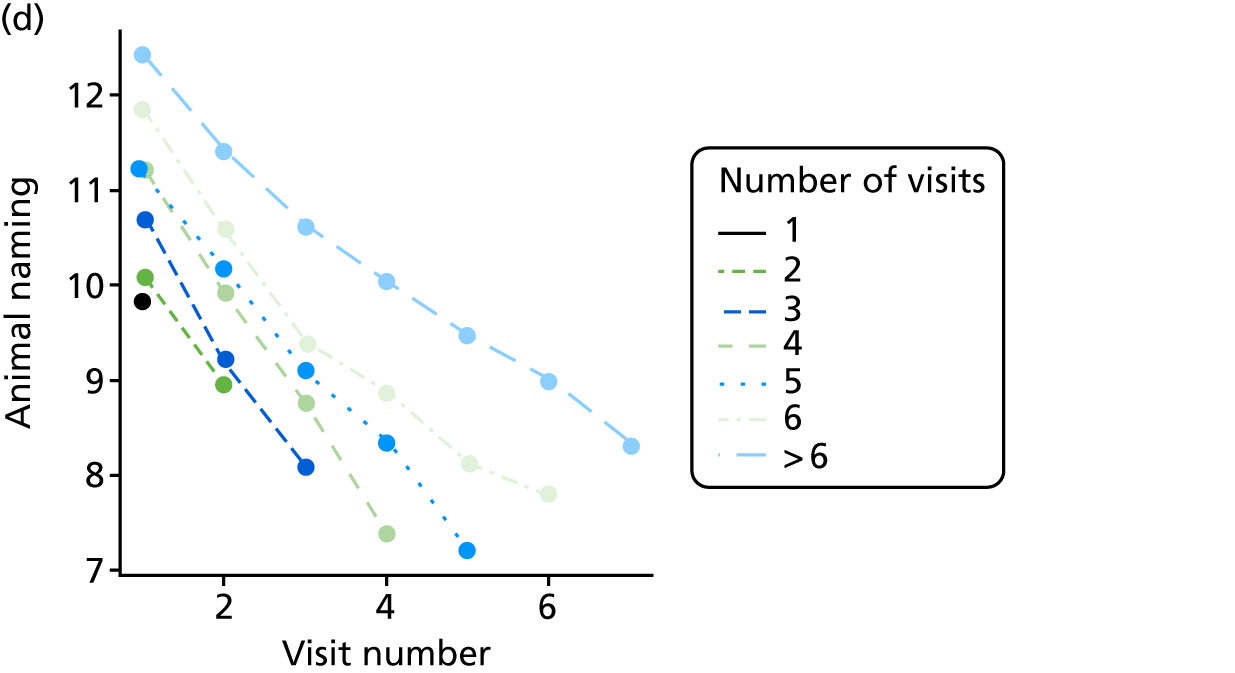
FIGURE 25.
(a) Distribution of CDR-SOB with respect to dementia severity; (b) distribution of CDR-SOB with respect to Z-drug use; (c) mean score over NACC visit, stratified by age group; and (d) mean score over NACC visit, stratified by number of visits completed.
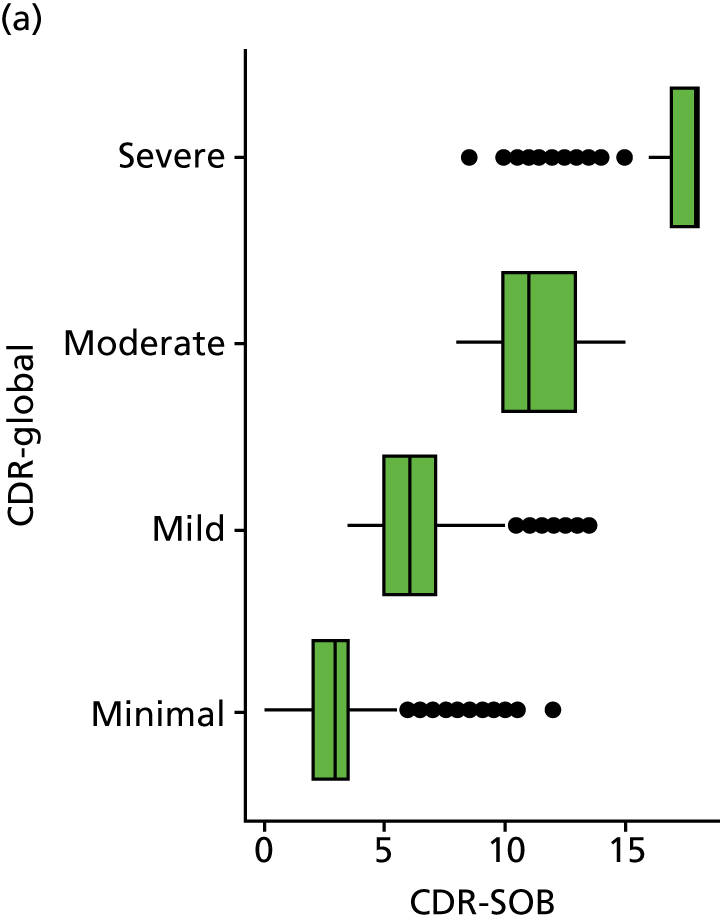
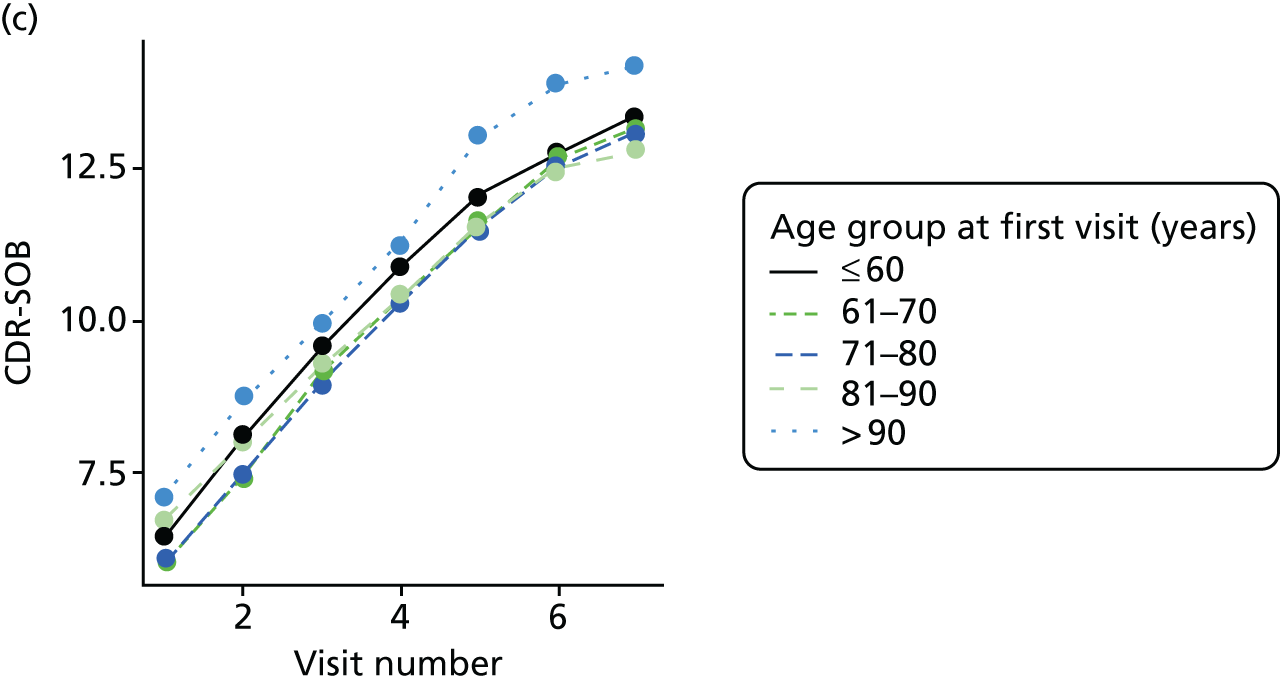
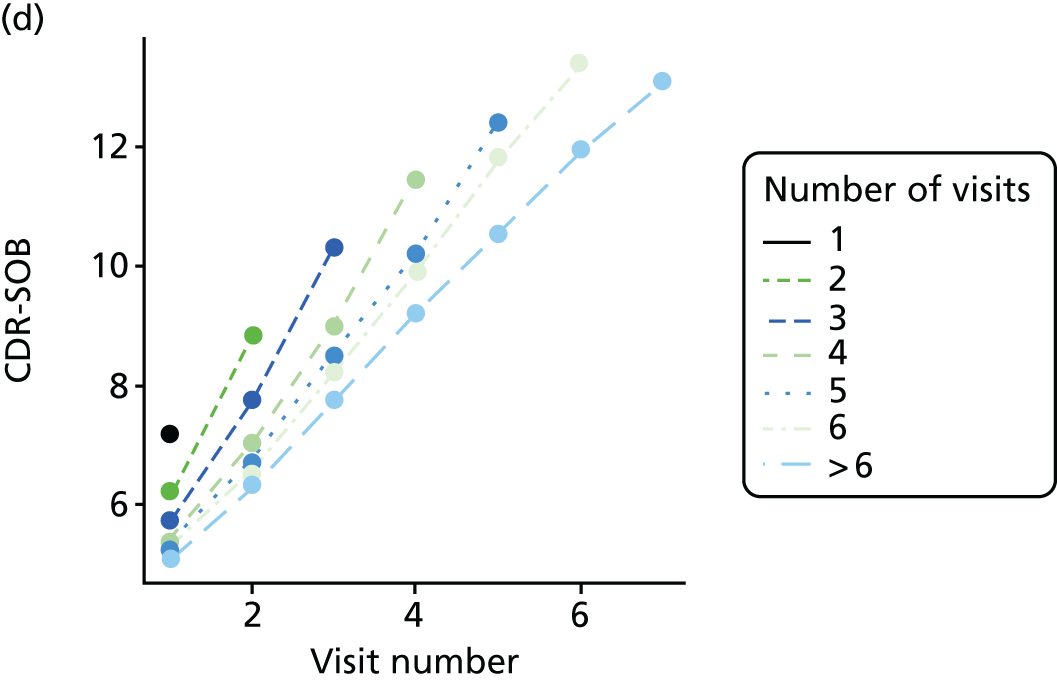
FIGURE 26.
(a) Distribution of delta trail time with respect to dementia severity; (b) distribution of delta trail time with respect to Z-drug use; (c) mean score over NACC visit, stratified by age group; and (d) mean score over NACC visit, stratified by number of visits completed.
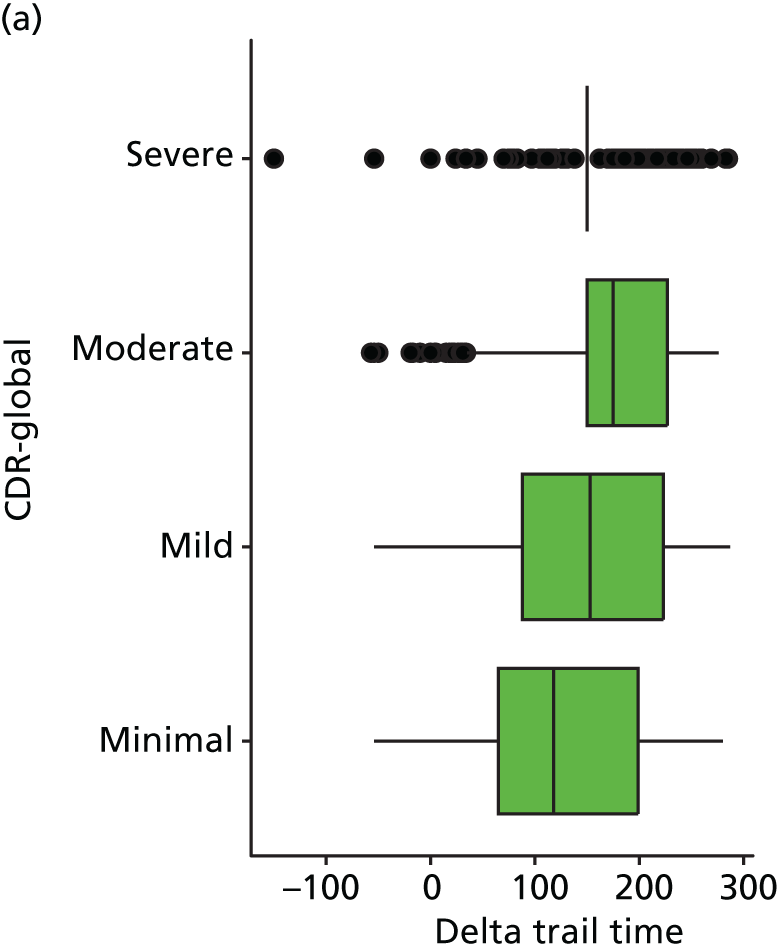
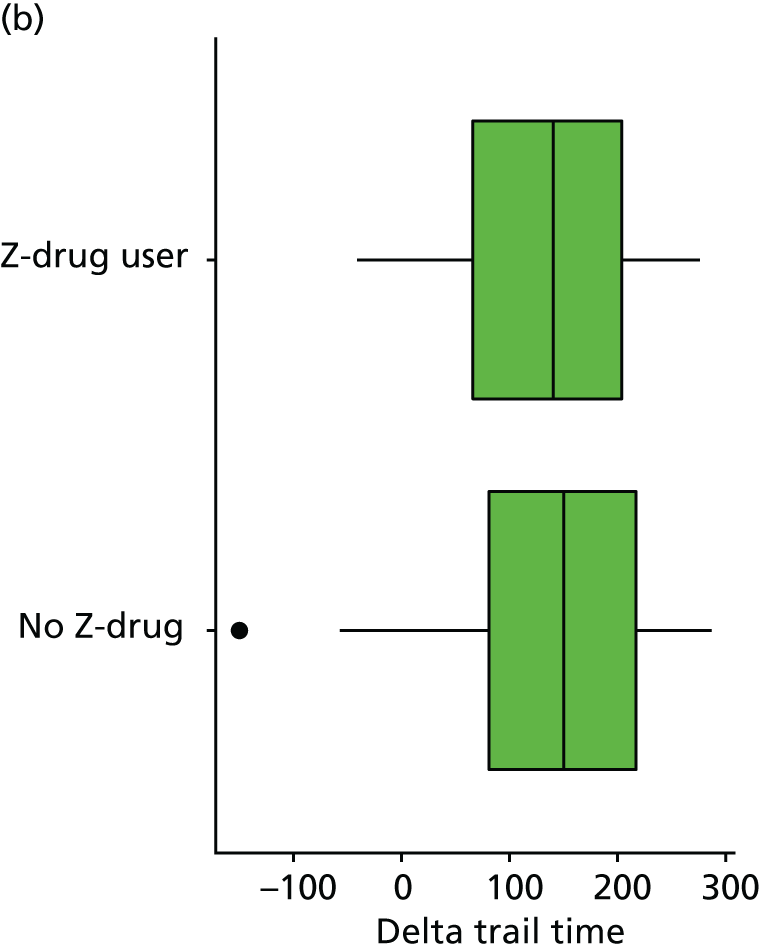
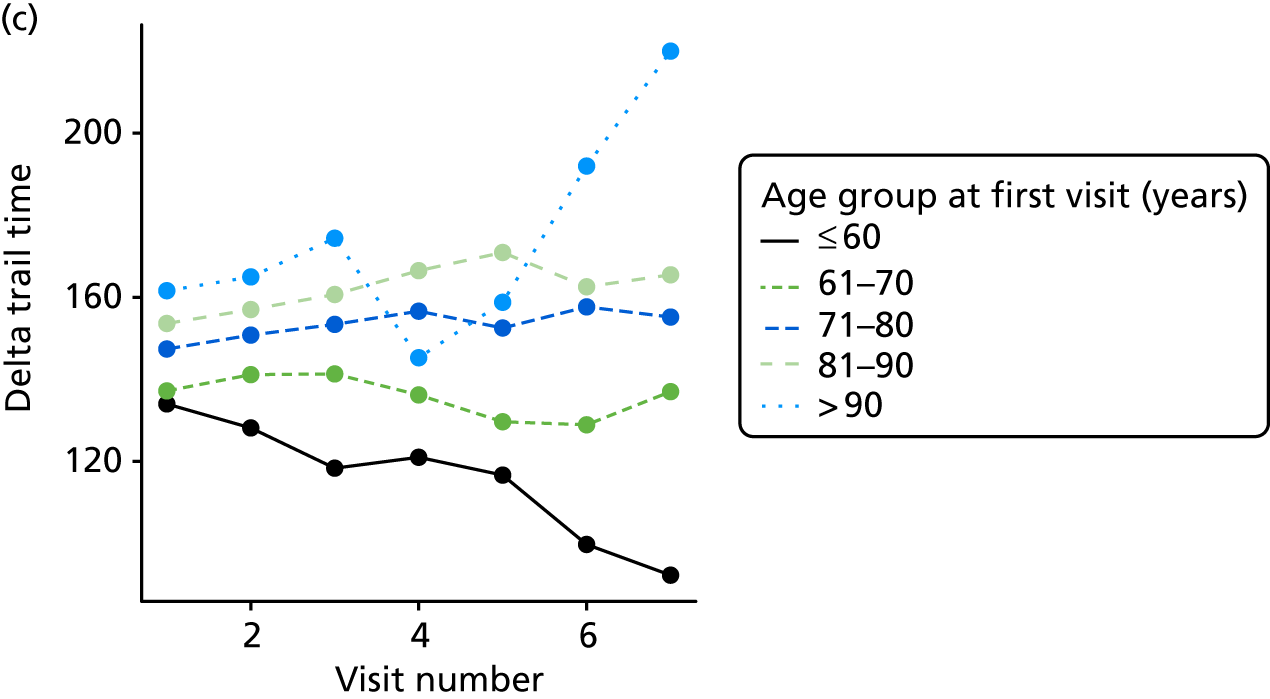
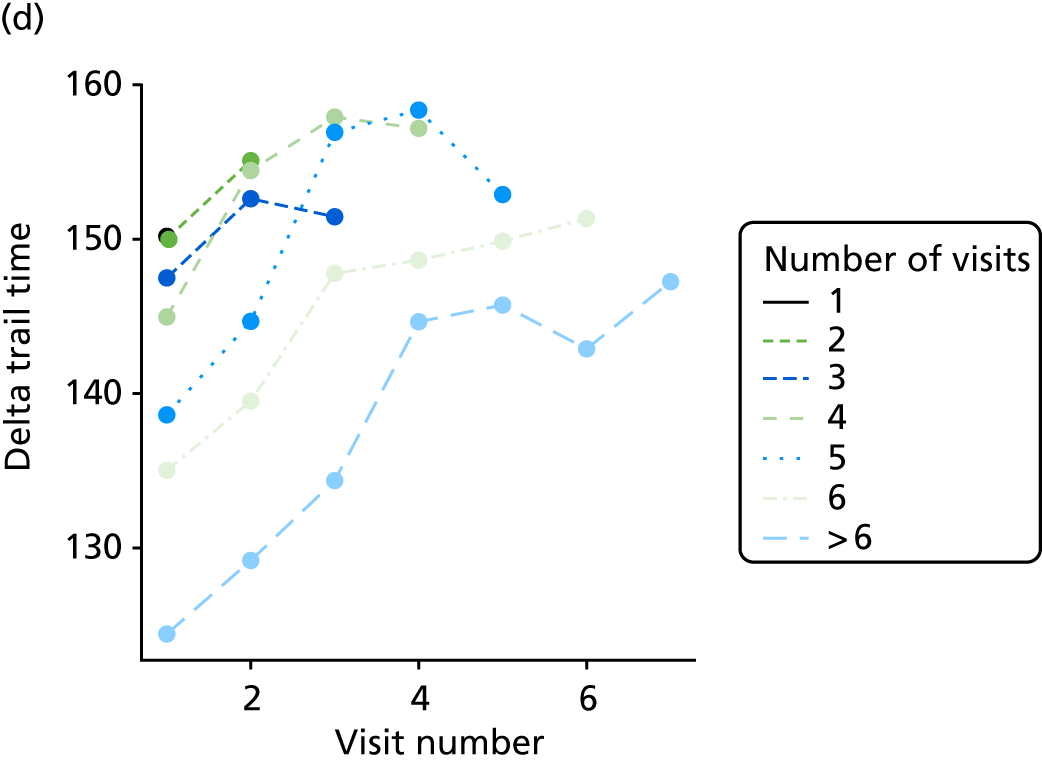
FIGURE 27.
(a) Distribution of GDS with respect to dementia severity; (b) distribution of GDS with respect to Z-drug use; (c) mean score over NACC visit, stratified by age group; and (d) mean score over NACC visit, stratified by number of visits completed.
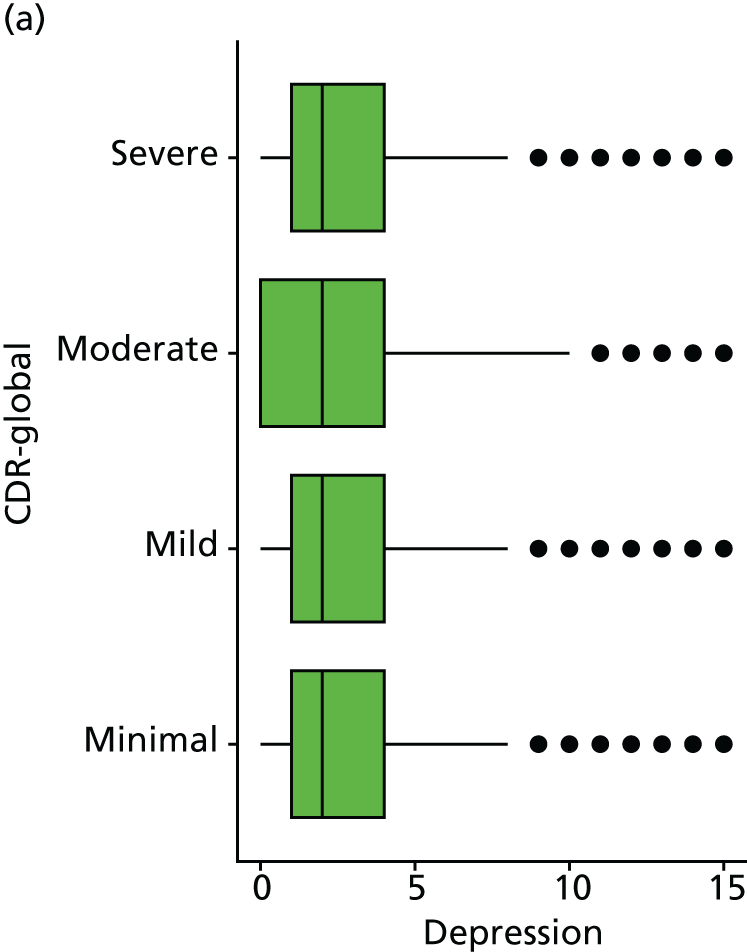
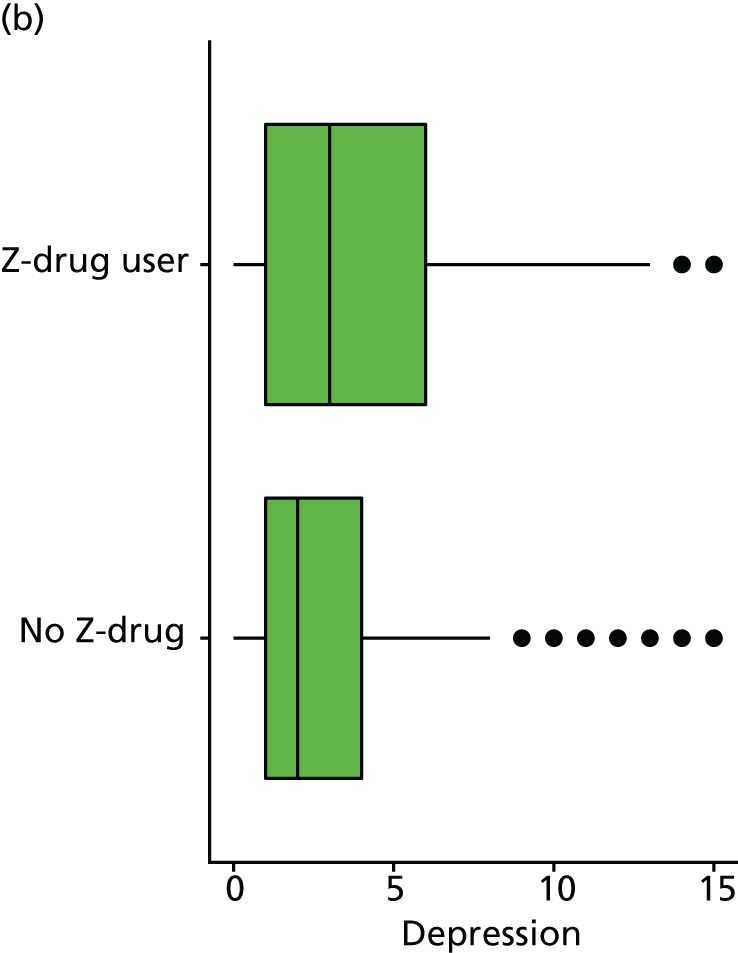
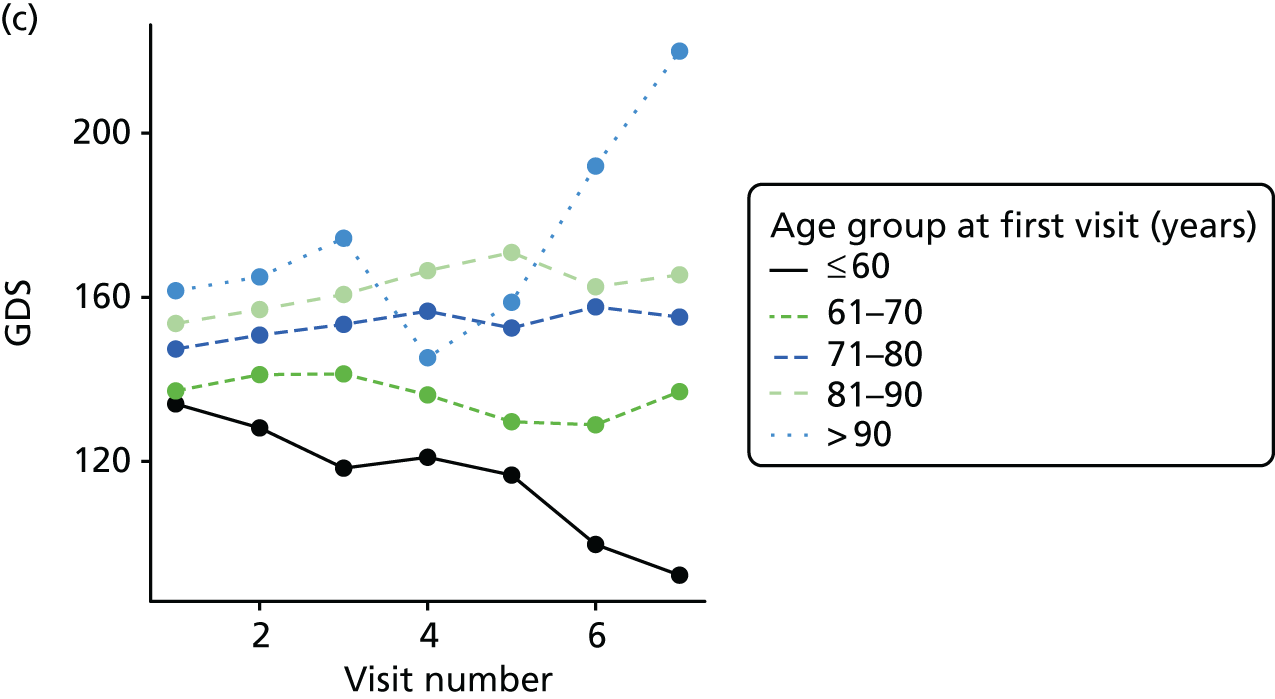
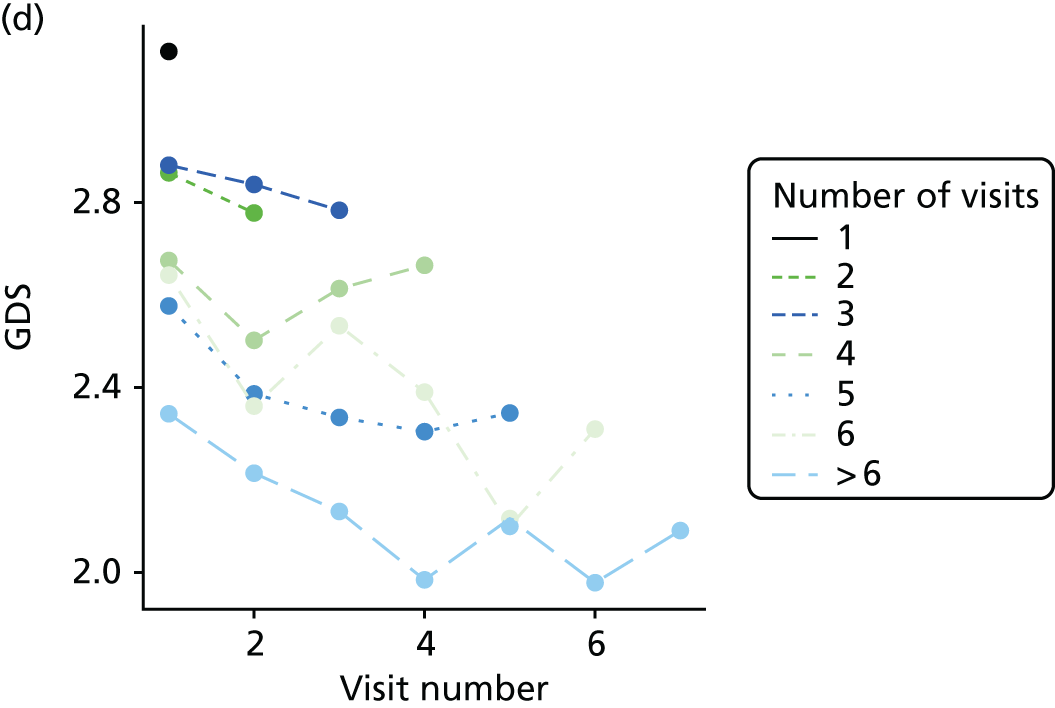
FIGURE 28.
(a) Distribution of MMSE with respect to dementia severity; (b) distribution of MMSE with respect to Z-drug use; (c) mean score over NACC visit, stratified by age group; and (d) mean score over NACC visit, stratified by number of visits completed.
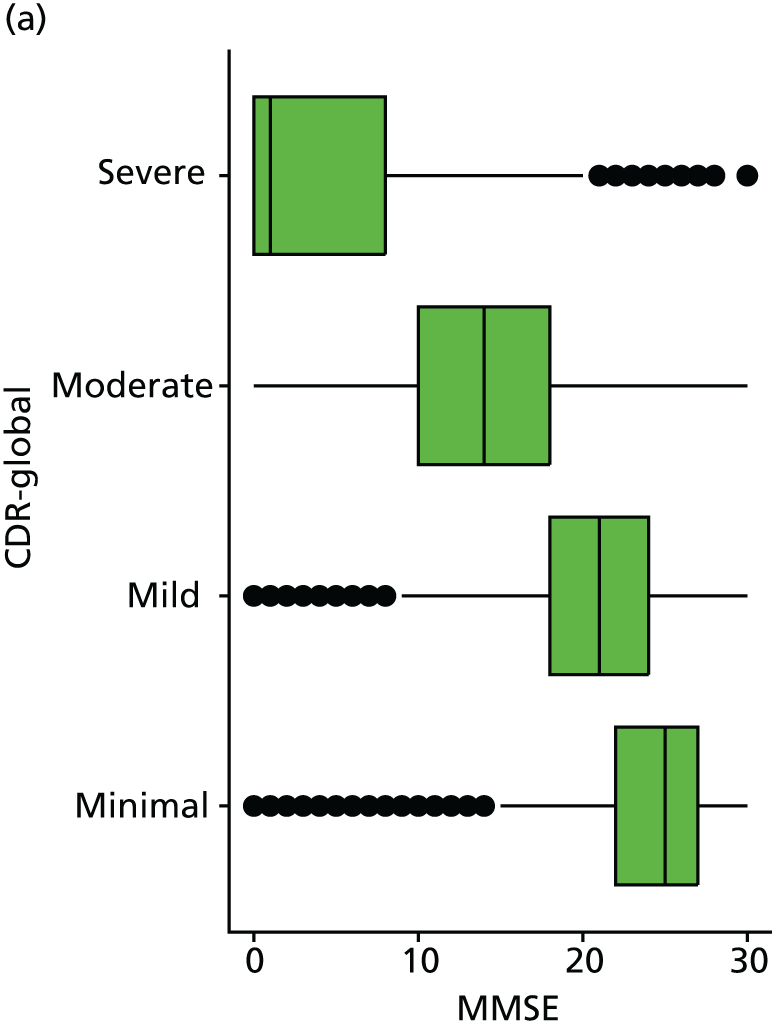
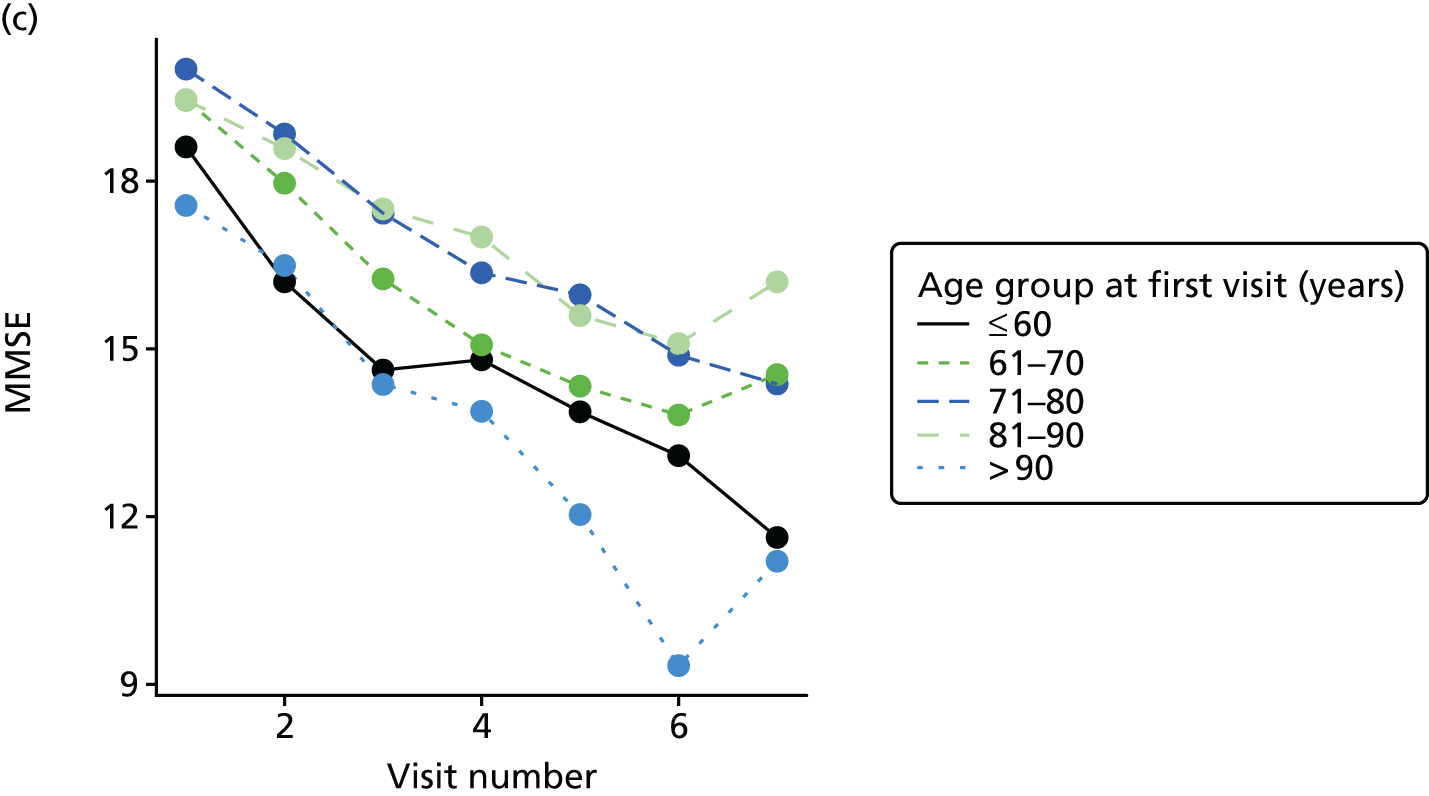
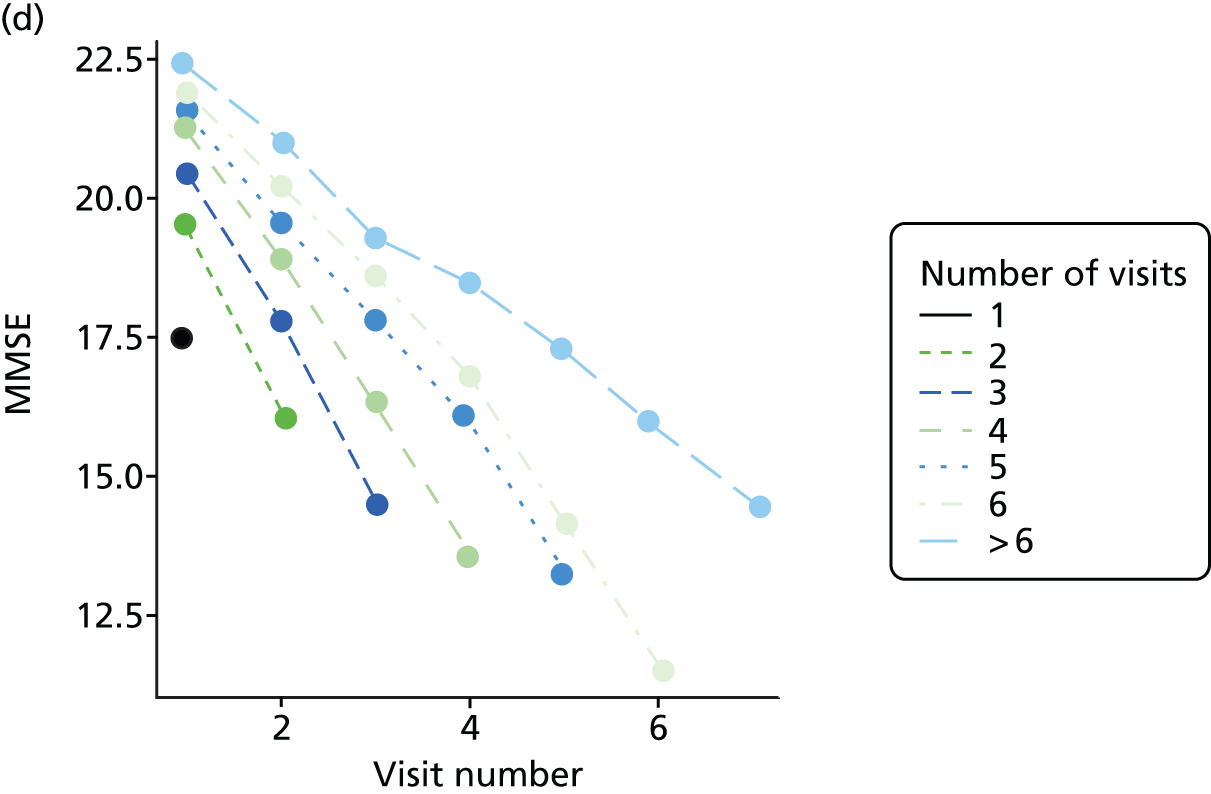
FIGURE 29.
(a) Distribution of agitation with respect to dementia severity; (b) distribution of agitation with respect to Z-drug use; (c) mean score over NACC visit, stratified by age group; and (d) mean score over NACC visit, stratified by number of visits completed.
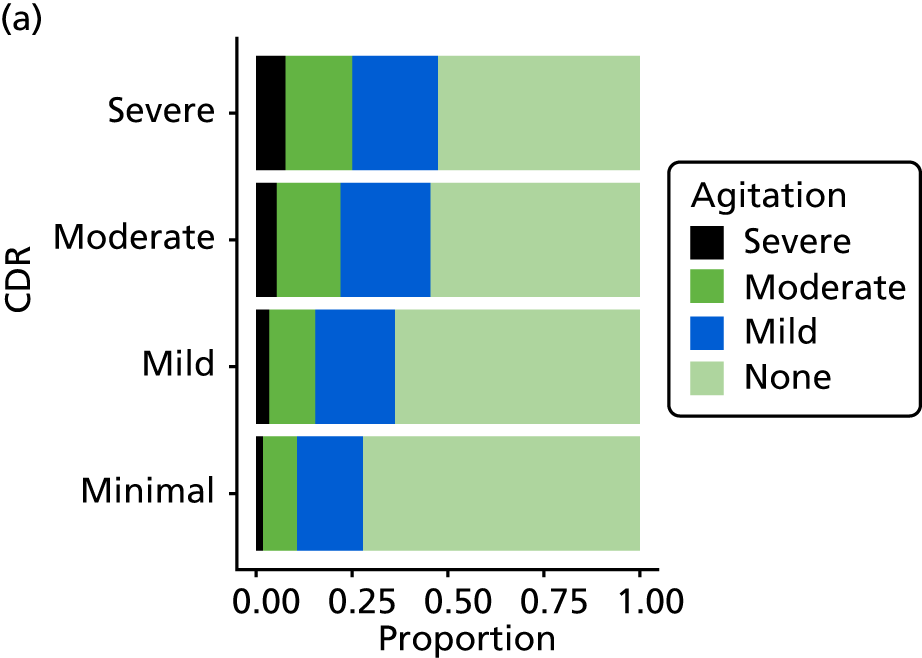
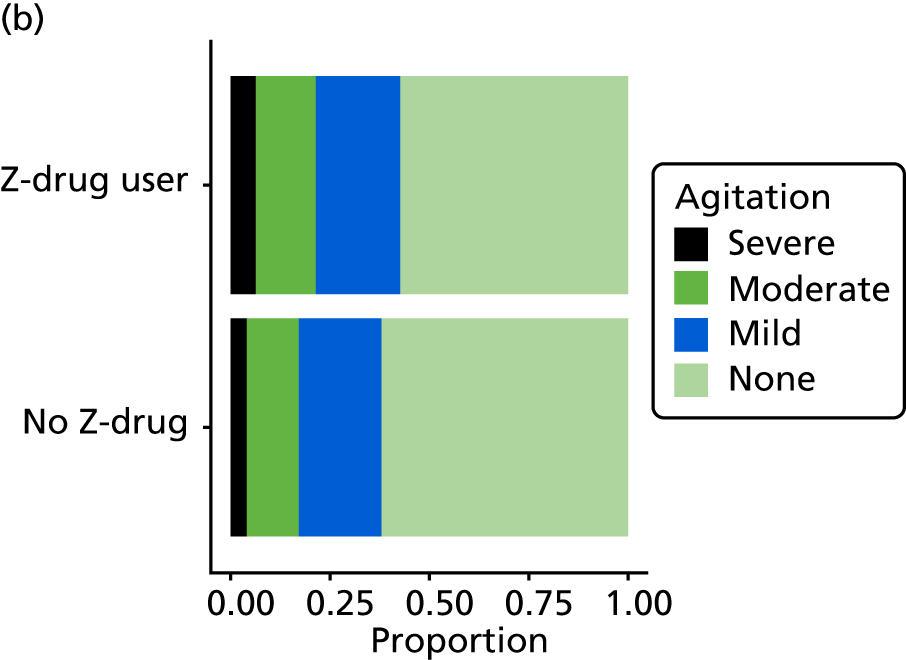
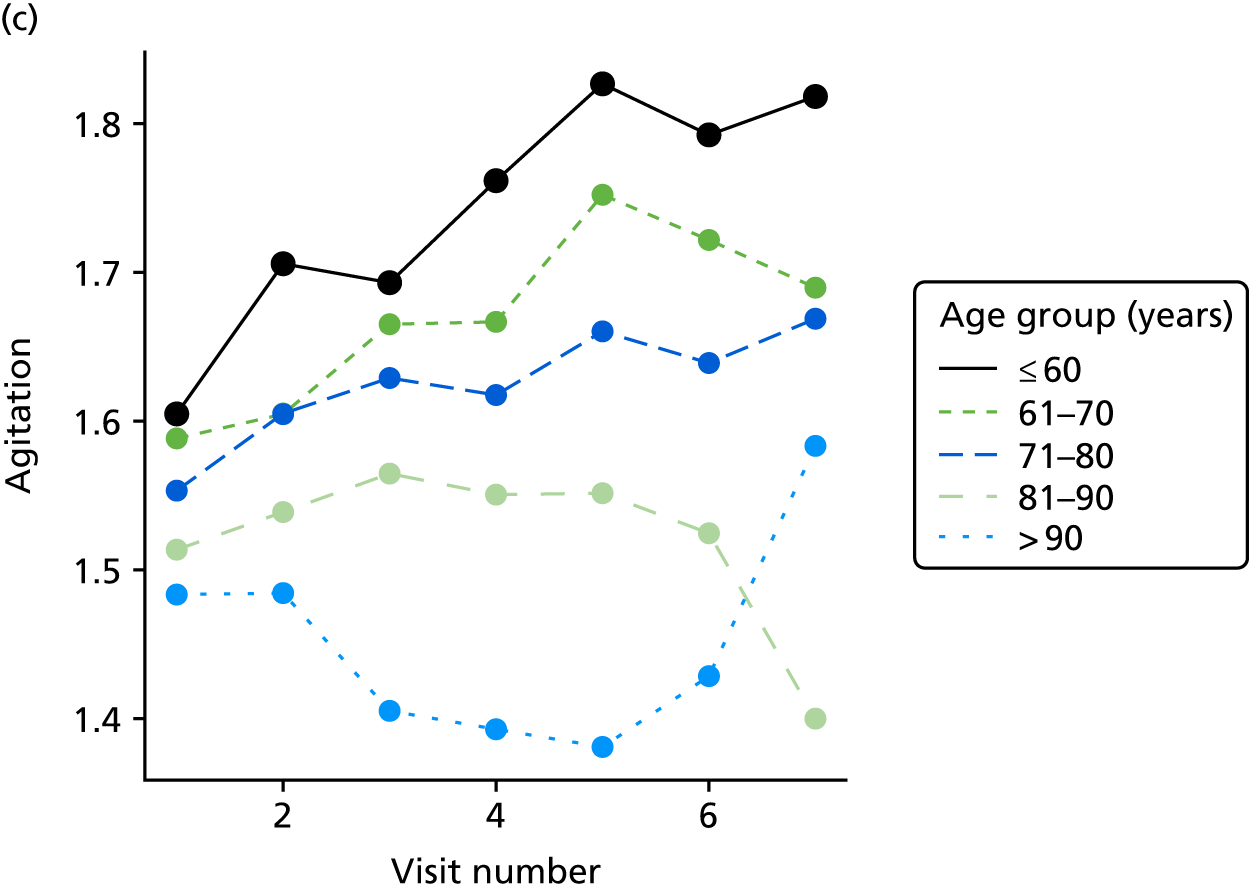
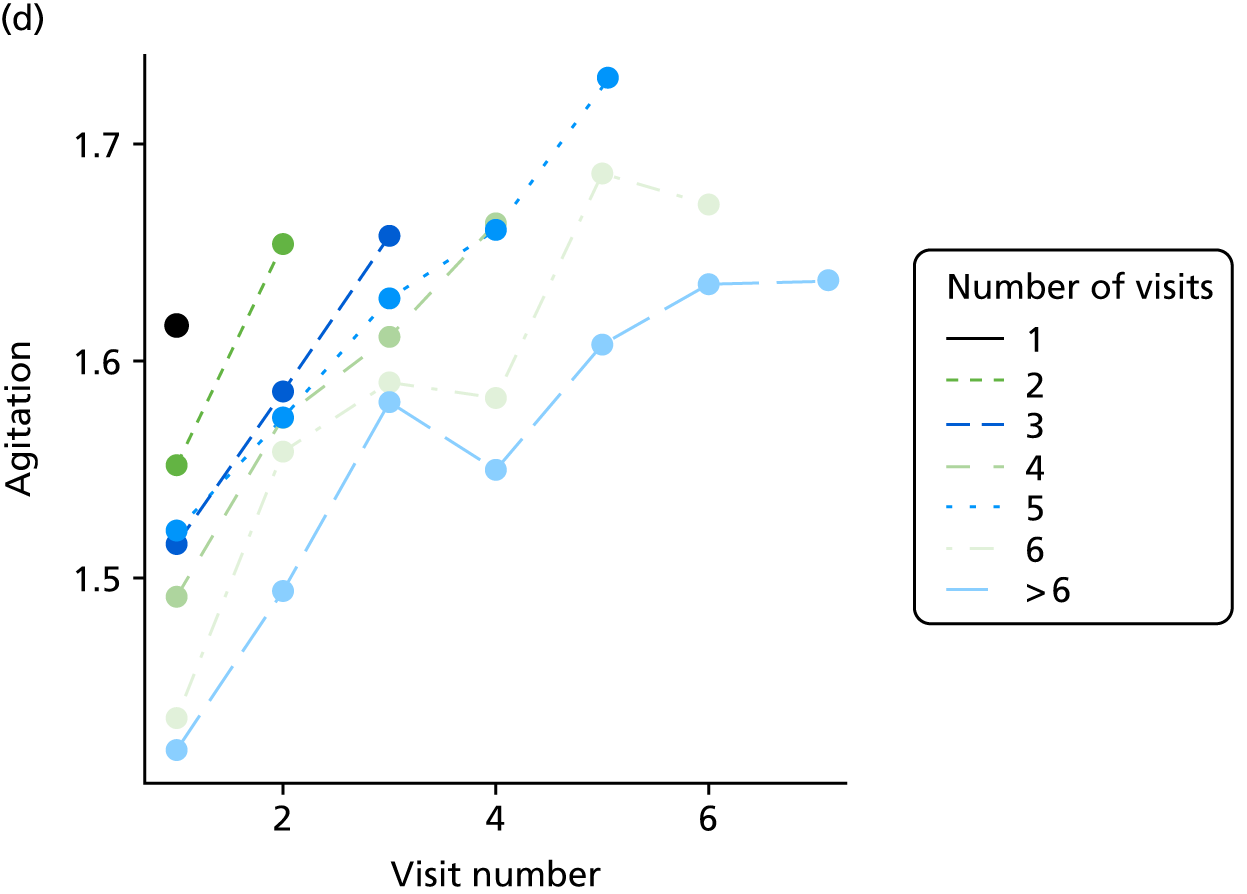
FIGURE 30.
(a) Distribution of anxiety with respect to dementia severity; (b) distribution of anxiety with respect to Z-drug use; (c) mean score over NACC visit, stratified by age group; and (d) mean score over NACC visit, stratified by number of visits completed.
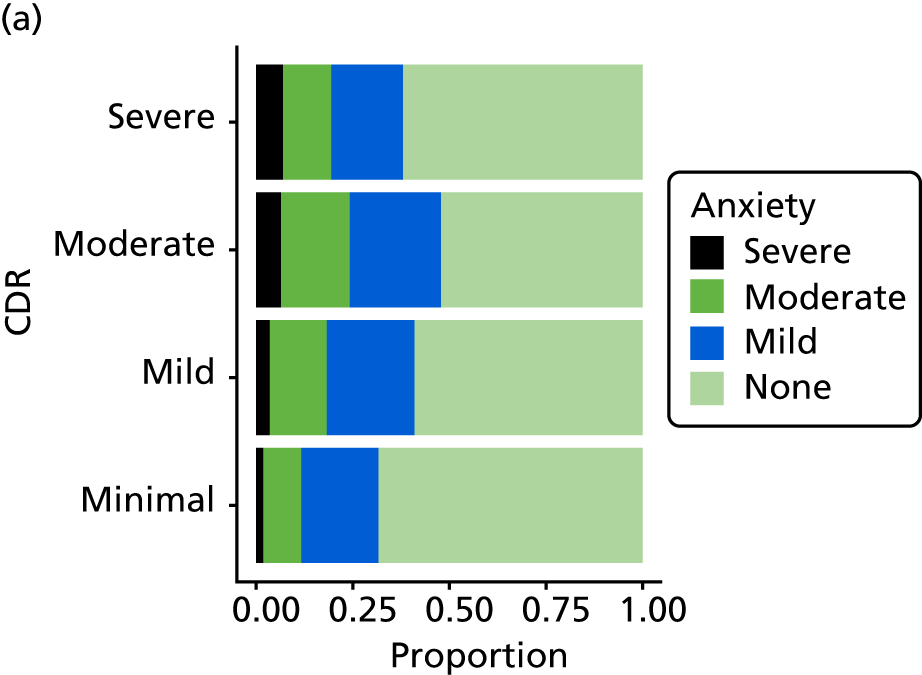
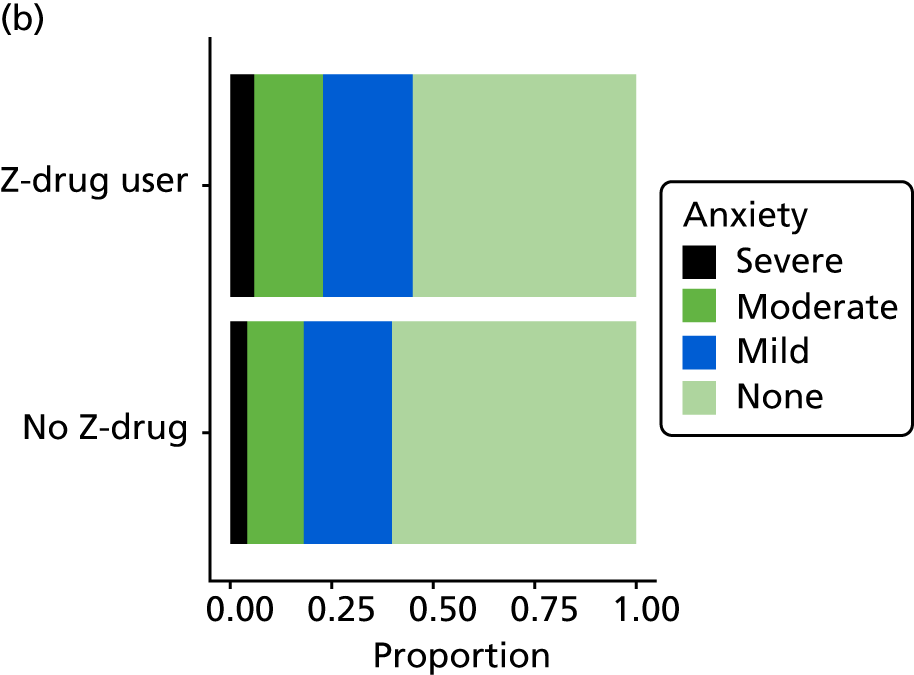
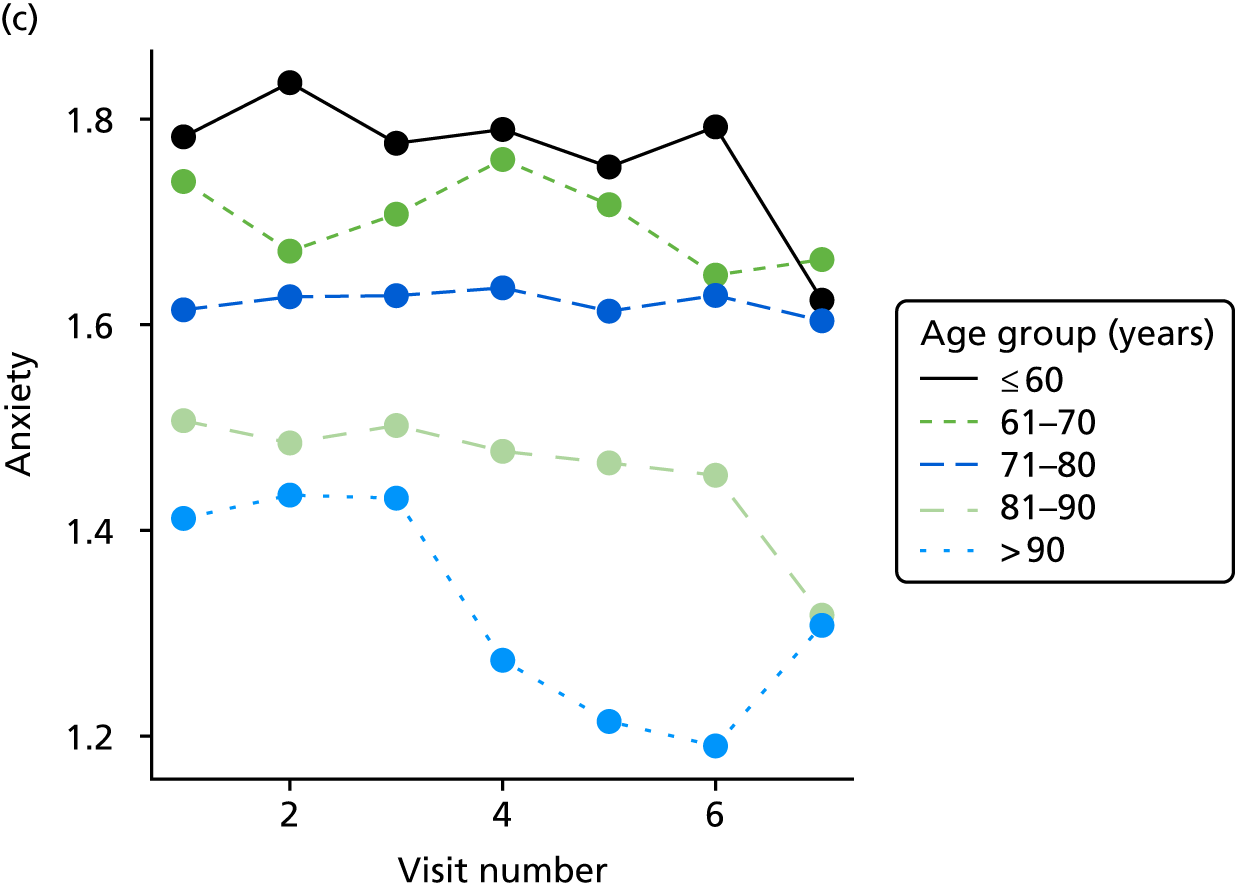
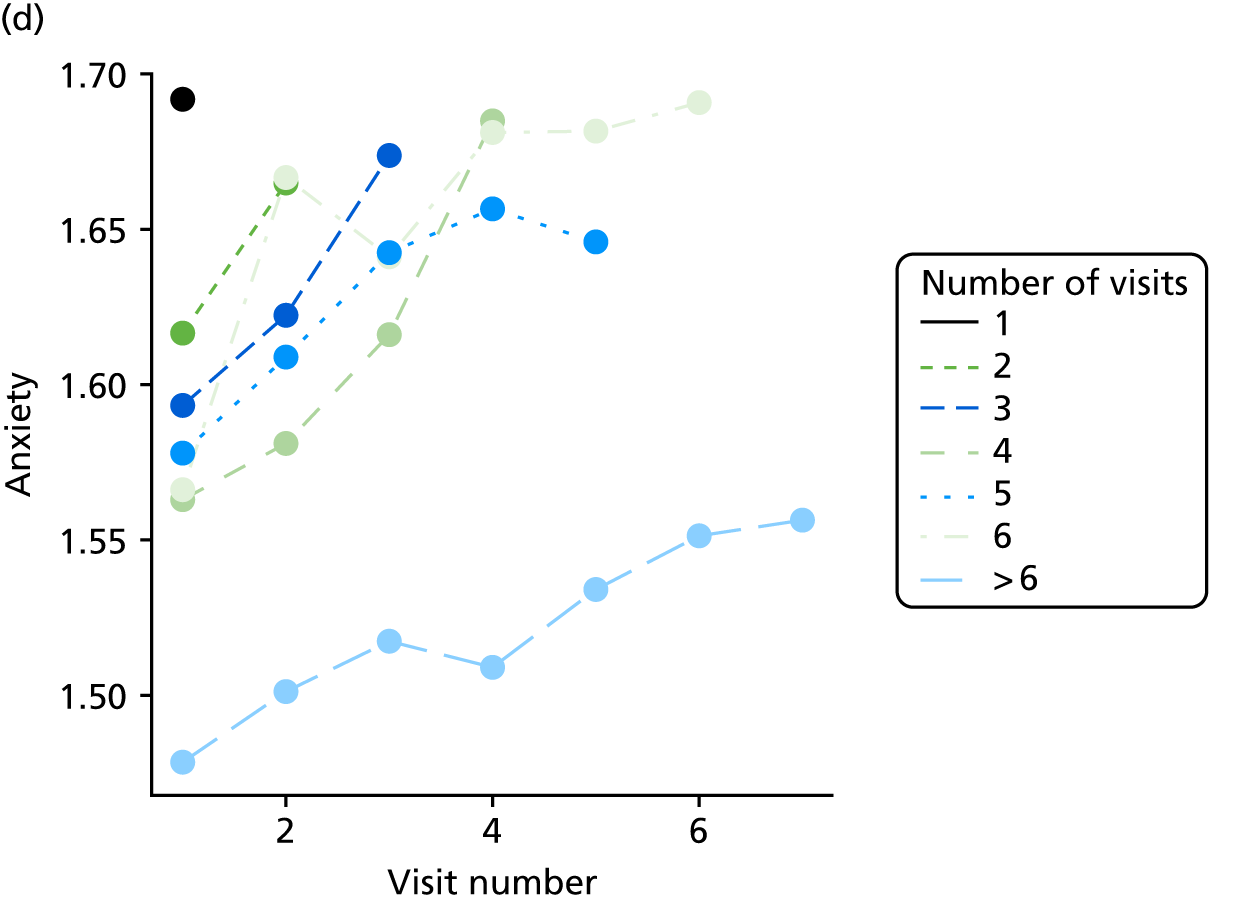
FIGURE 31.
(a) Distribution of NPI (excluding sleep) with respect to dementia severity; (b) distribution of NPI (excluding sleep) with respect to Z-drug use; (c) mean score over NACC visit, stratified by age group; and (d) mean score over NACC visit, stratified by number of visits completed.
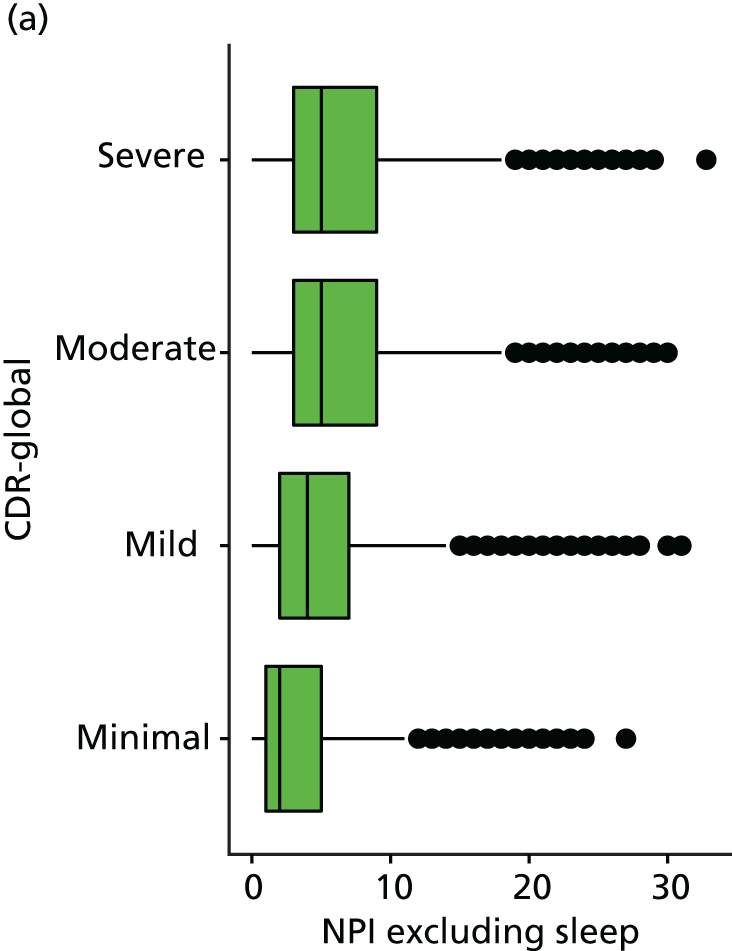
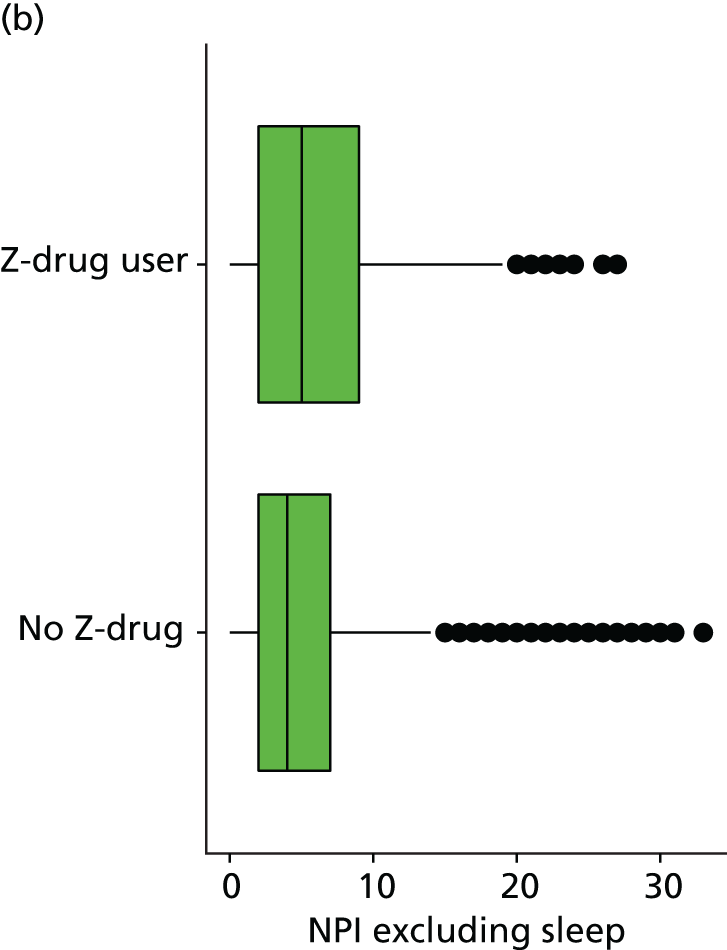
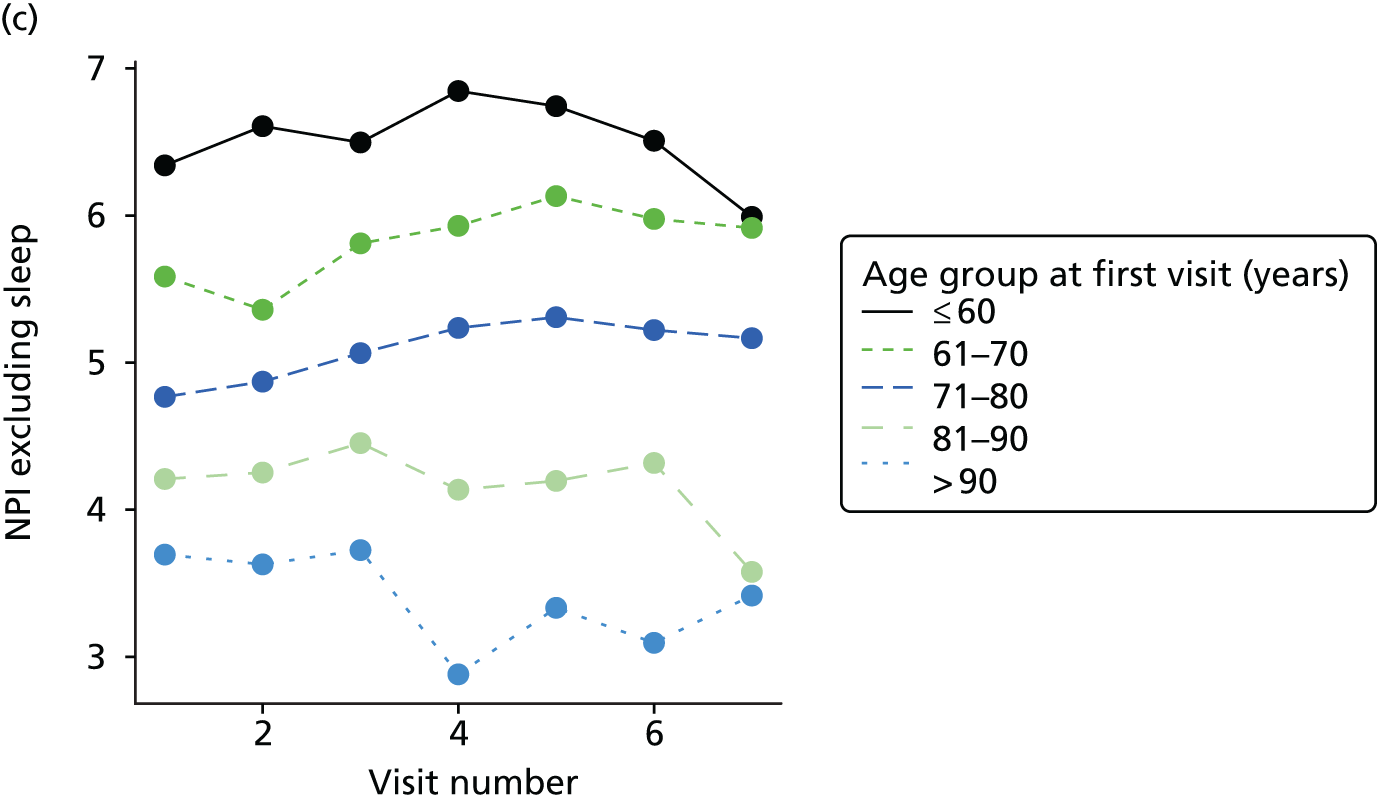
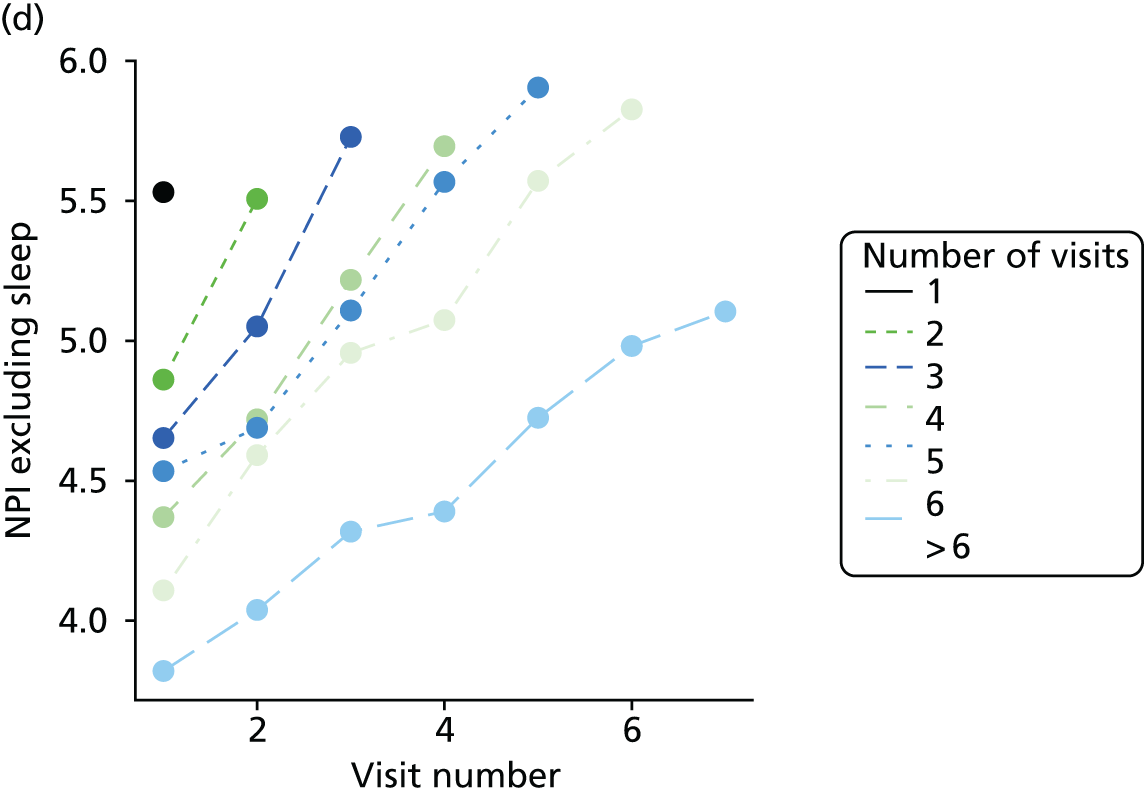
FIGURE 32.
(a) Distribution of sleep disturbance with respect to dementia severity; (b) distribution of sleep disturbance with respect to Z-drug use; (c) mean score over NACC visit, stratified by age group; and (d) mean score over NACC visit, stratified by number of visits completed.
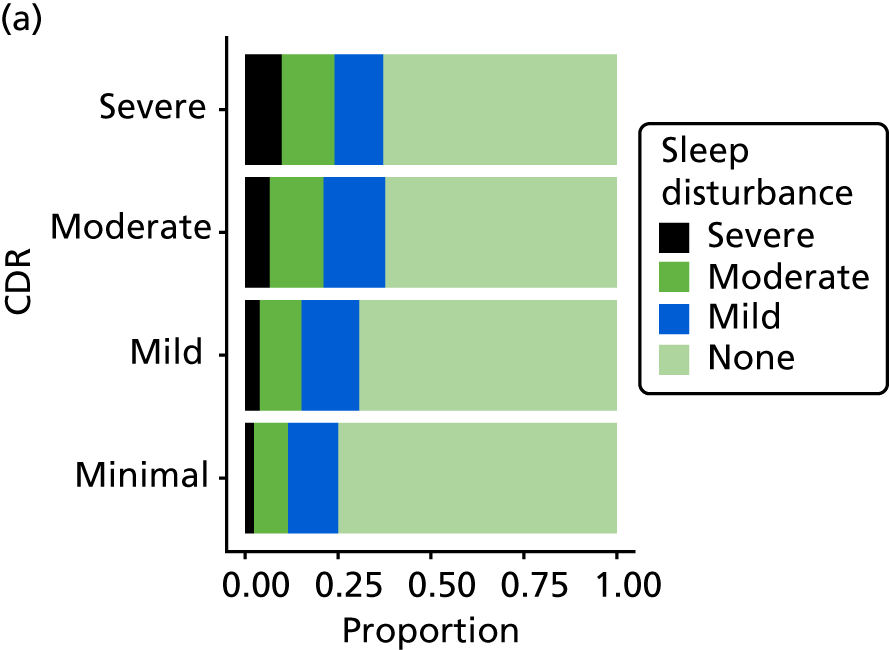
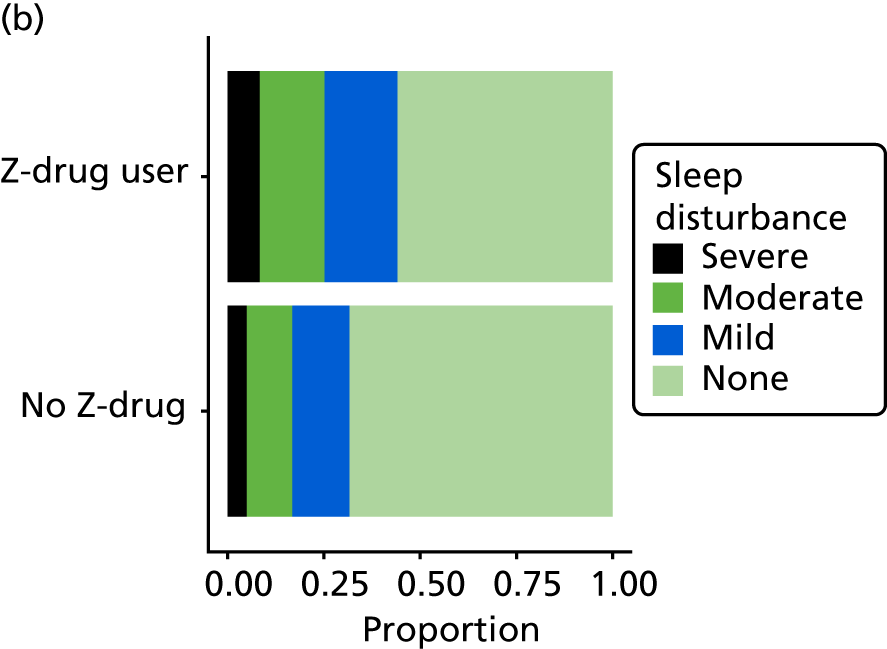
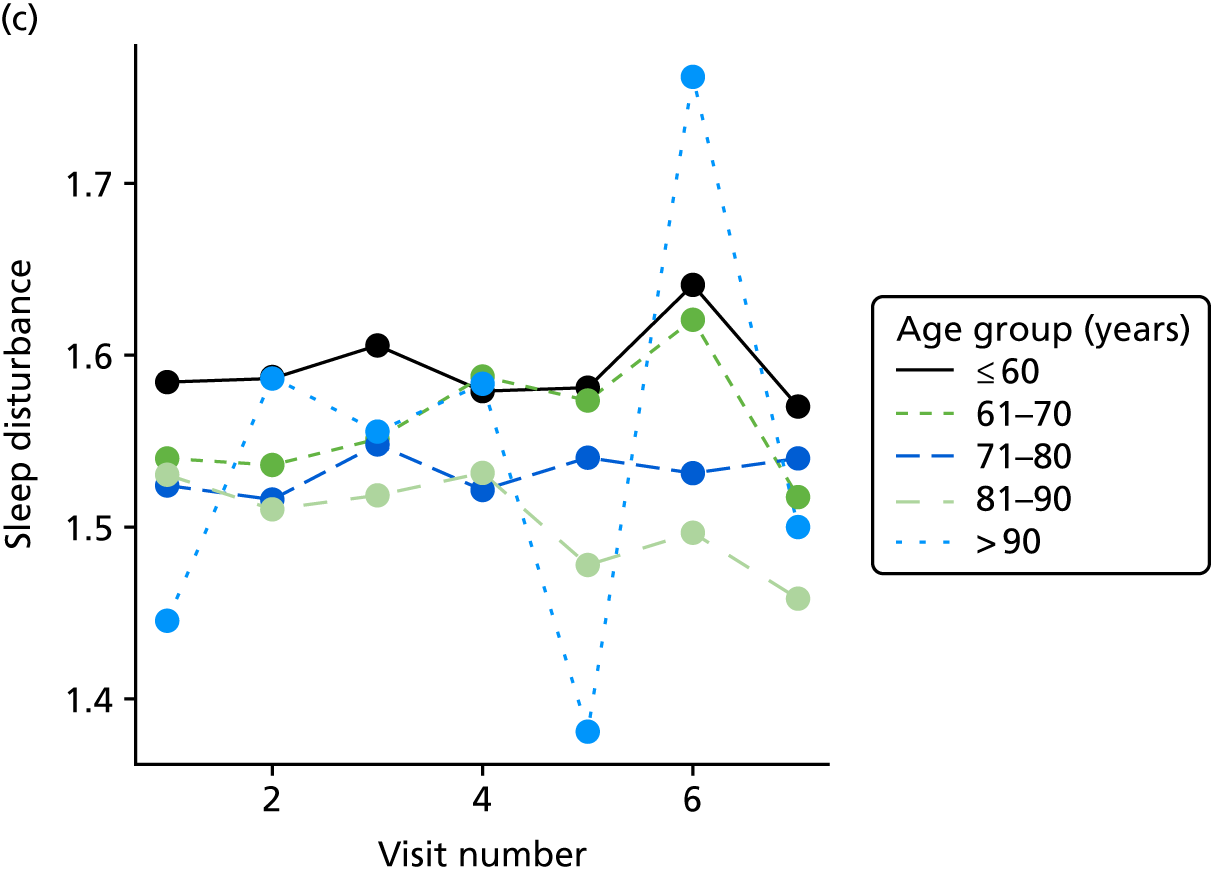
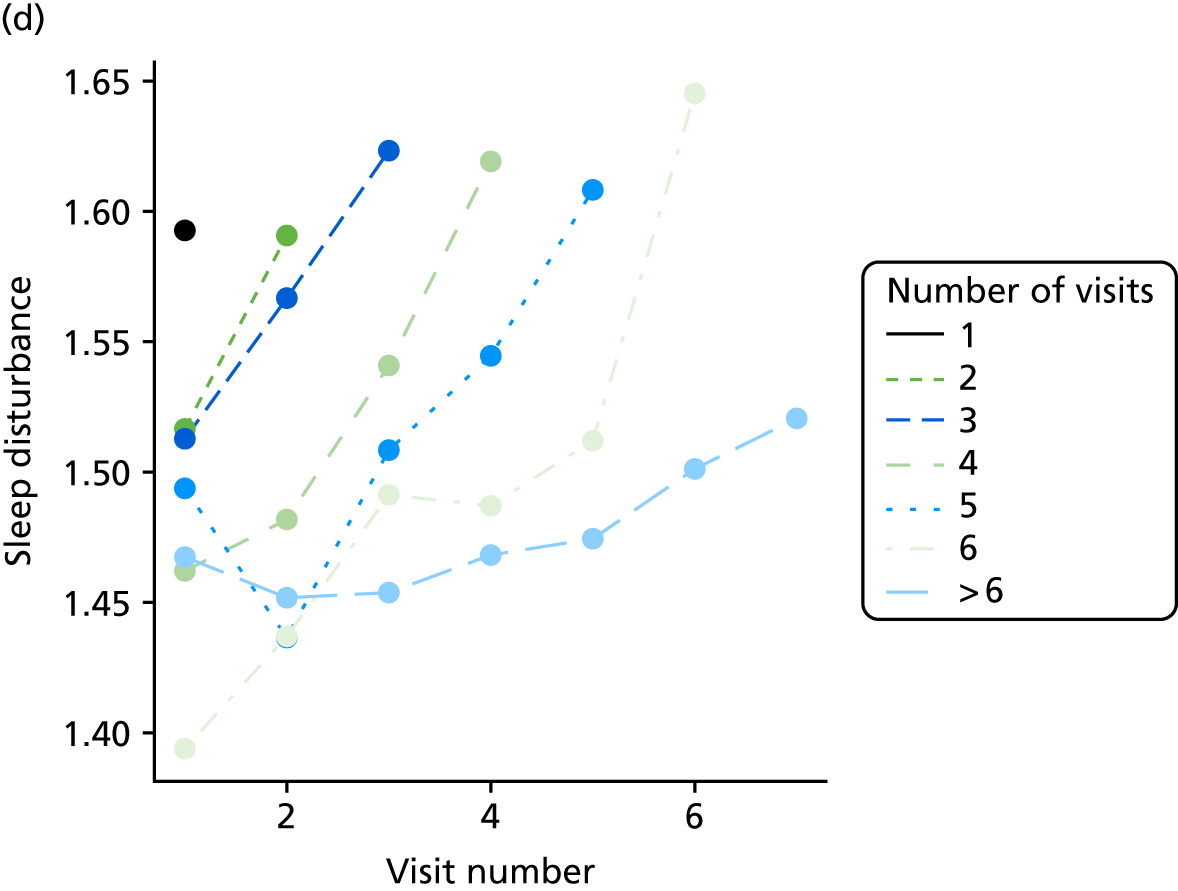
FIGURE 33.
Association between changing hypnotic use status between previous and current visit on depression (measured by GDS, higher scores represent more depressive symptoms) scores at previous and current visit (a–c). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (d–f). (a) Z-drug; (b) BZD; and (c) antipsychotics. Data from the NACC data set.
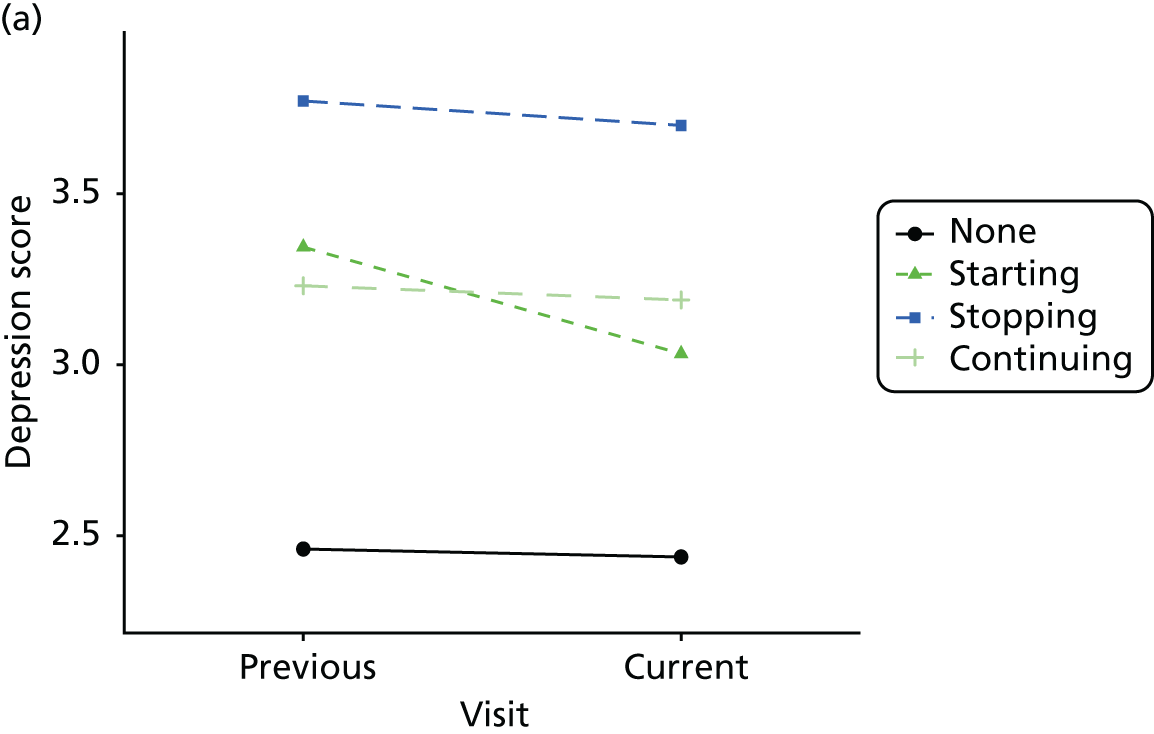
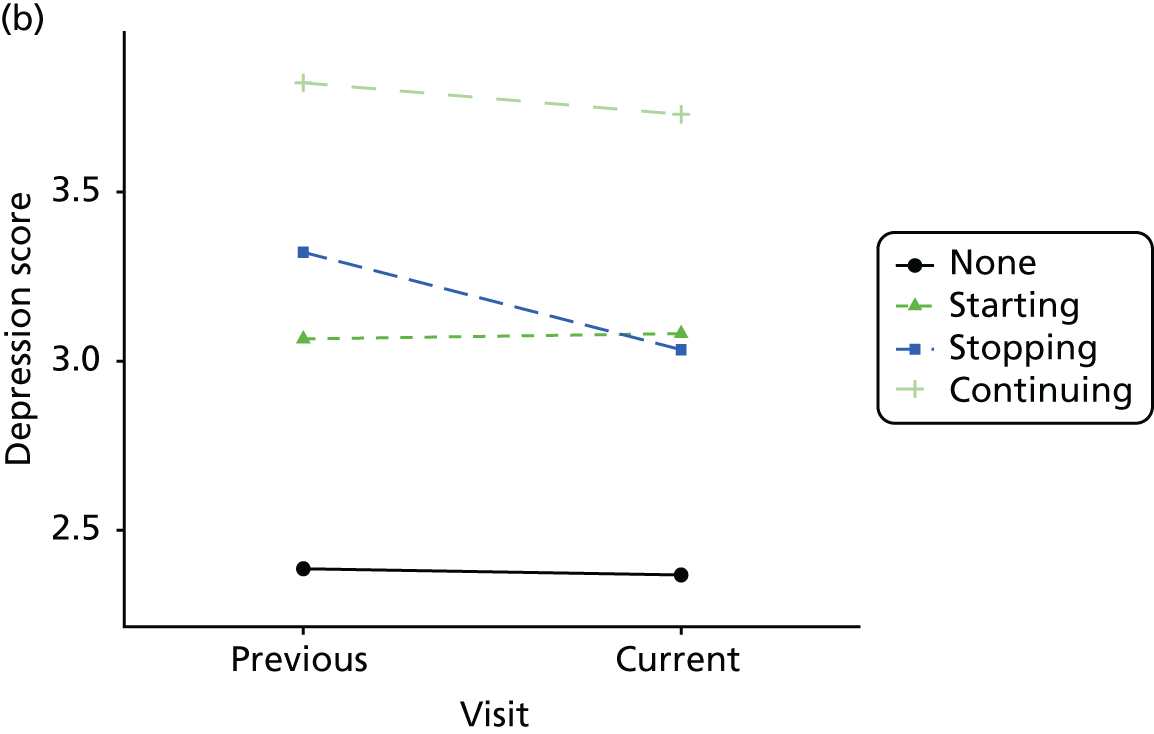
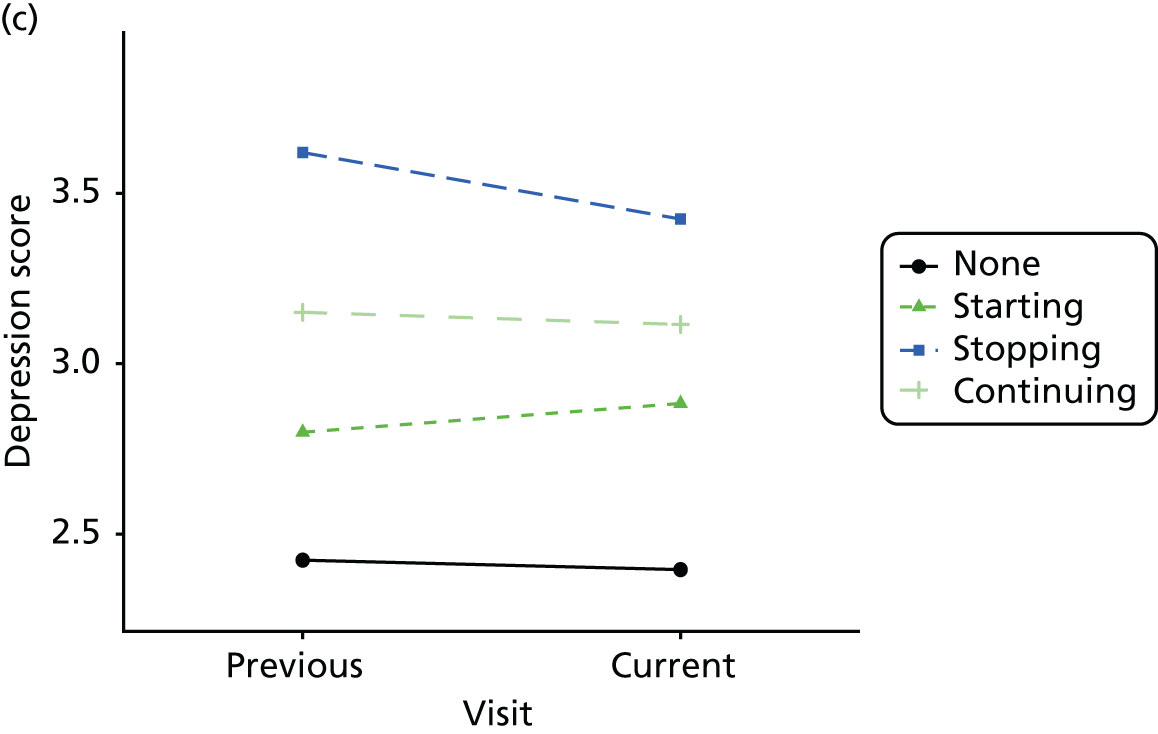
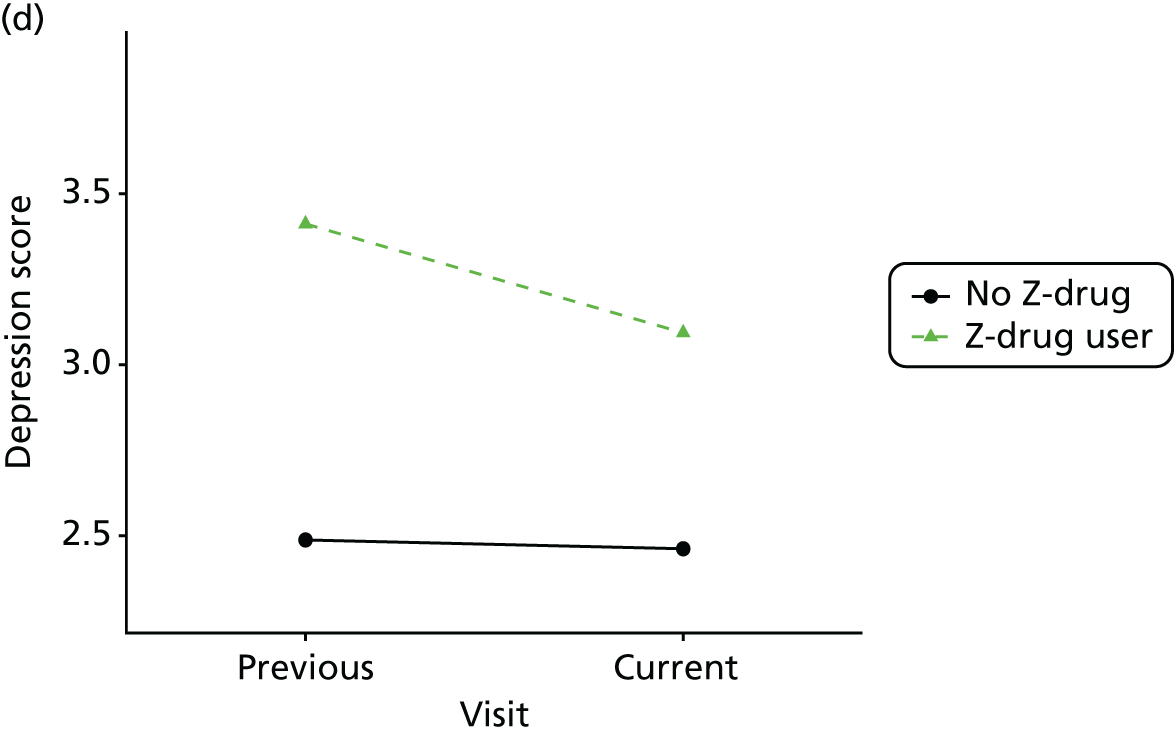
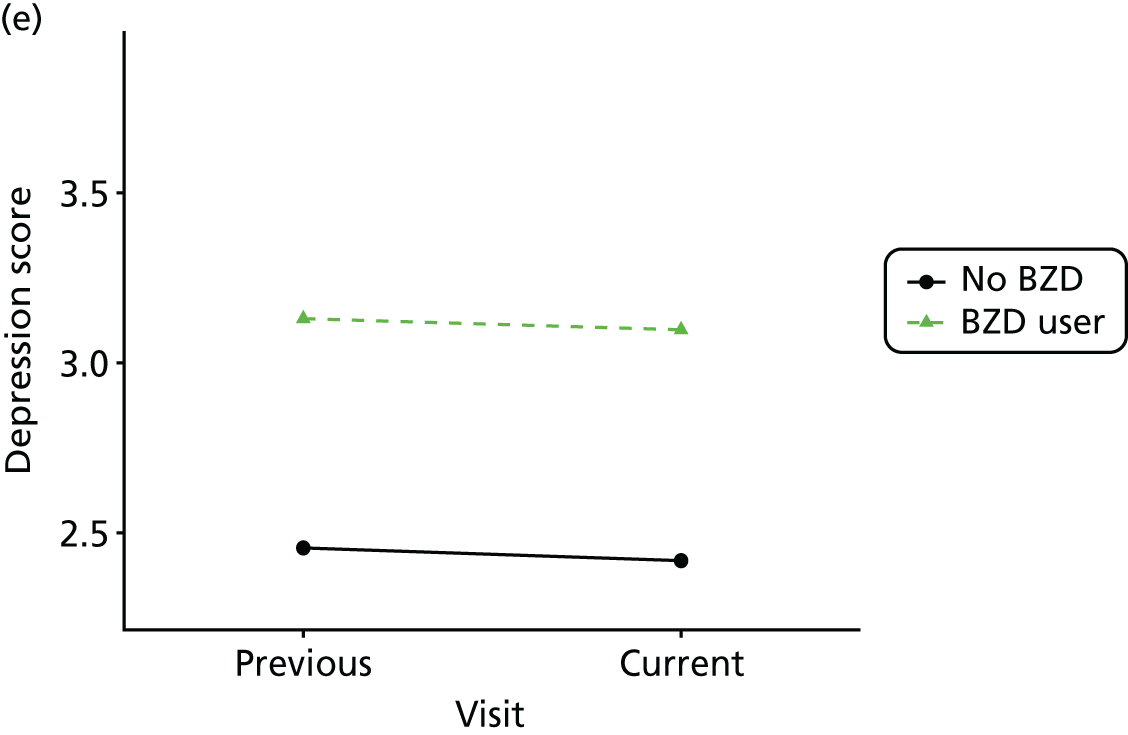
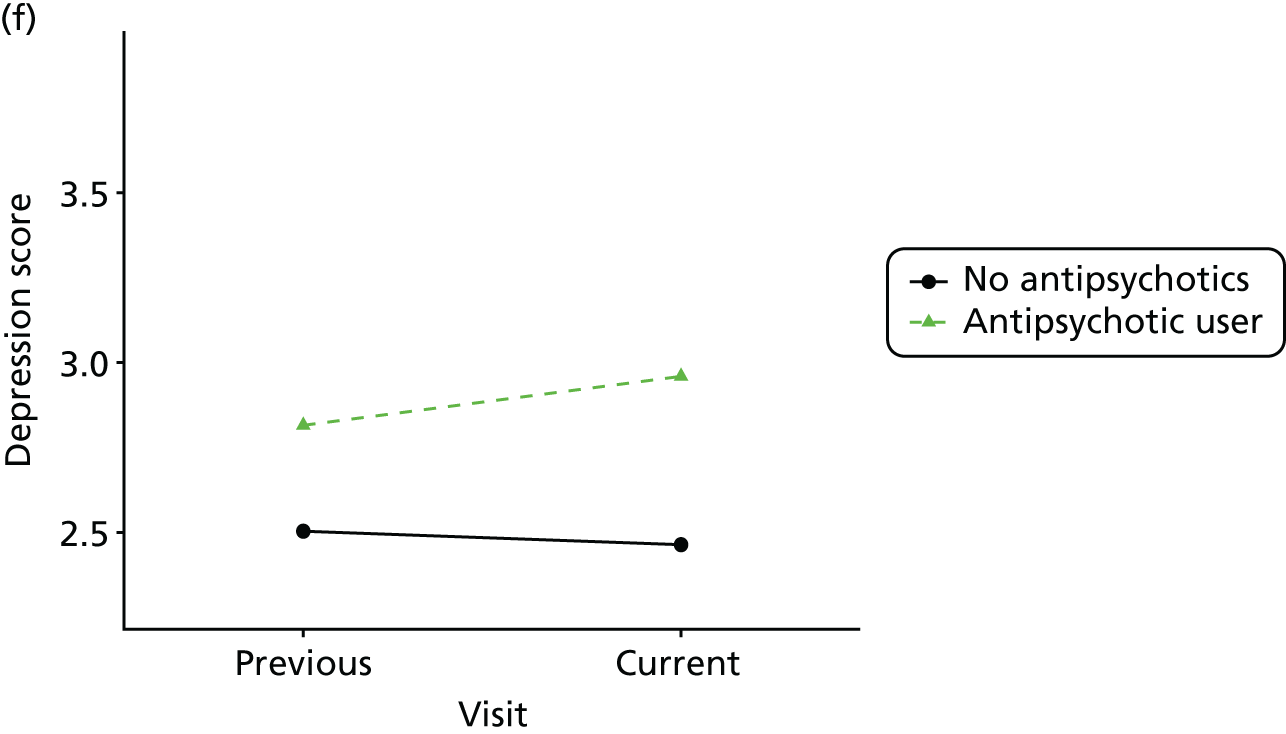
FIGURE 34.
Association between changing hypnotic use status between previous and current visit on MMSE scores (higher scores represent better cognitive function) at previous and current visit (a–c). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (d–f). (a) Z-drug; (b) BZD; and (c) antipsychotics. Data from the NACC data set.
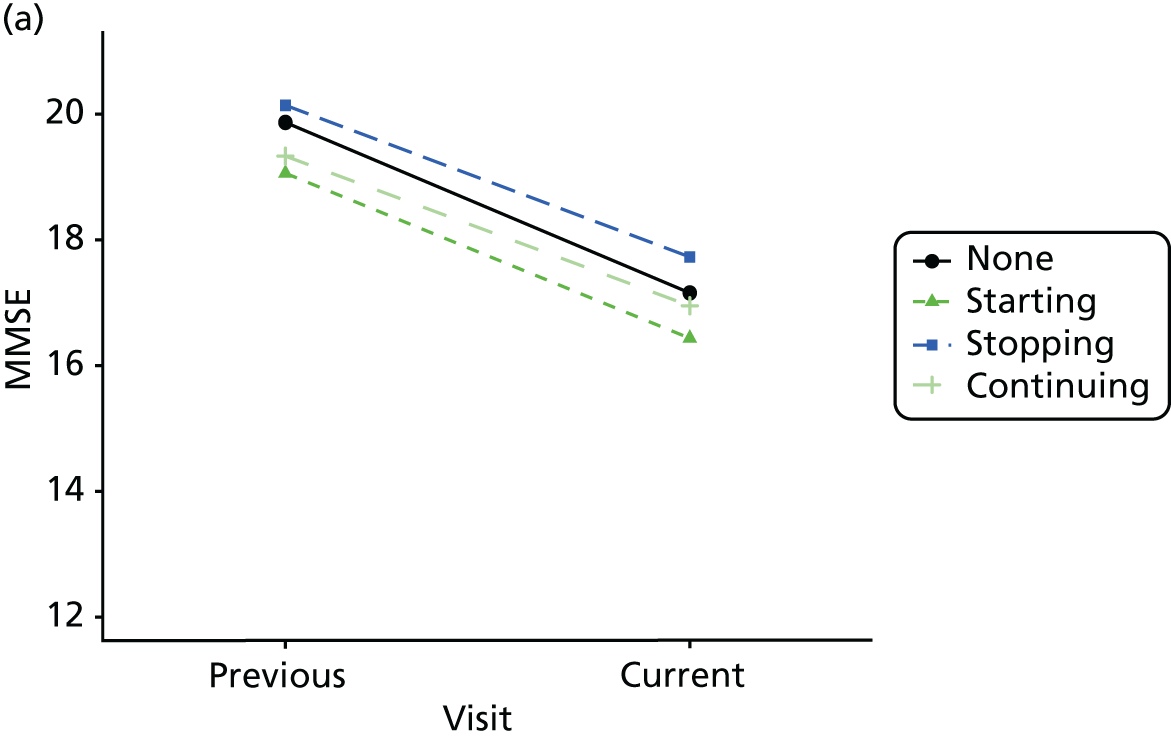
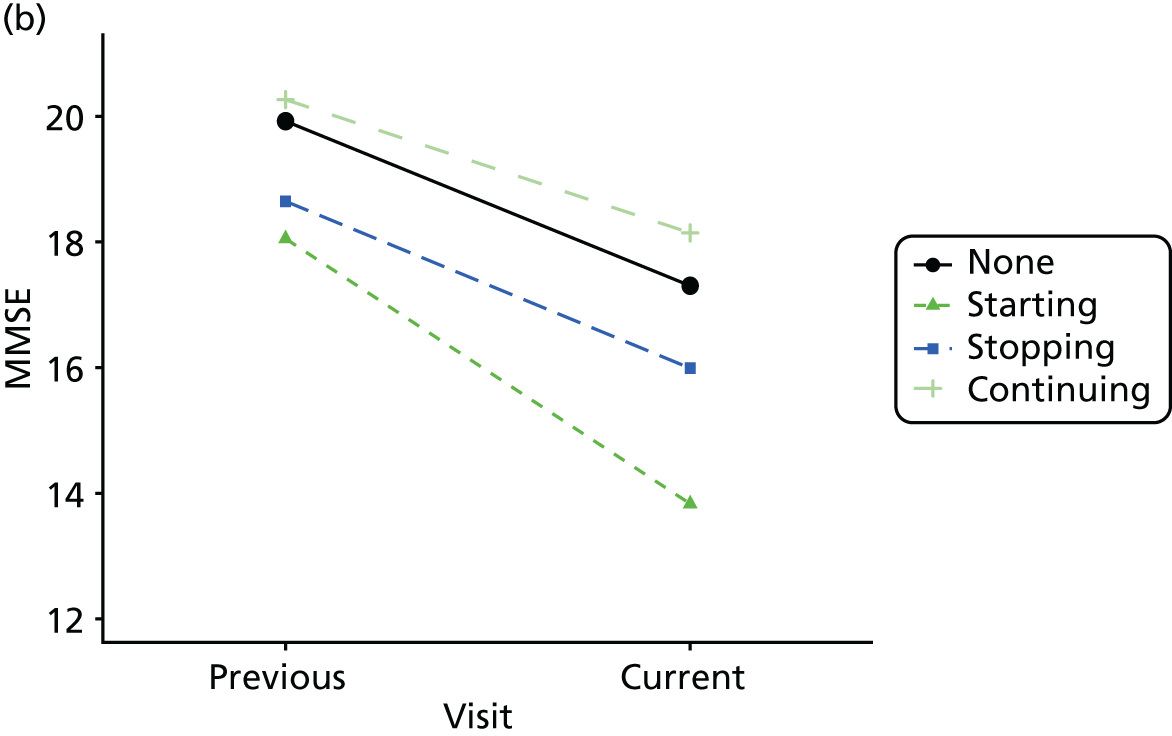
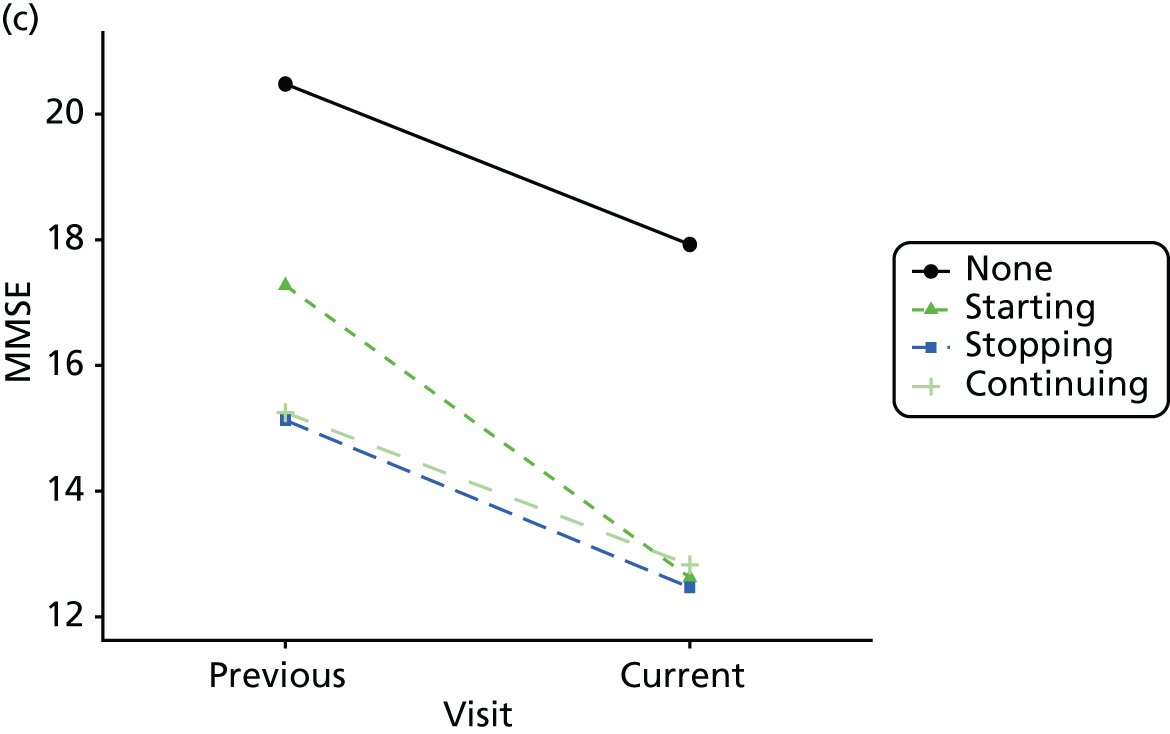
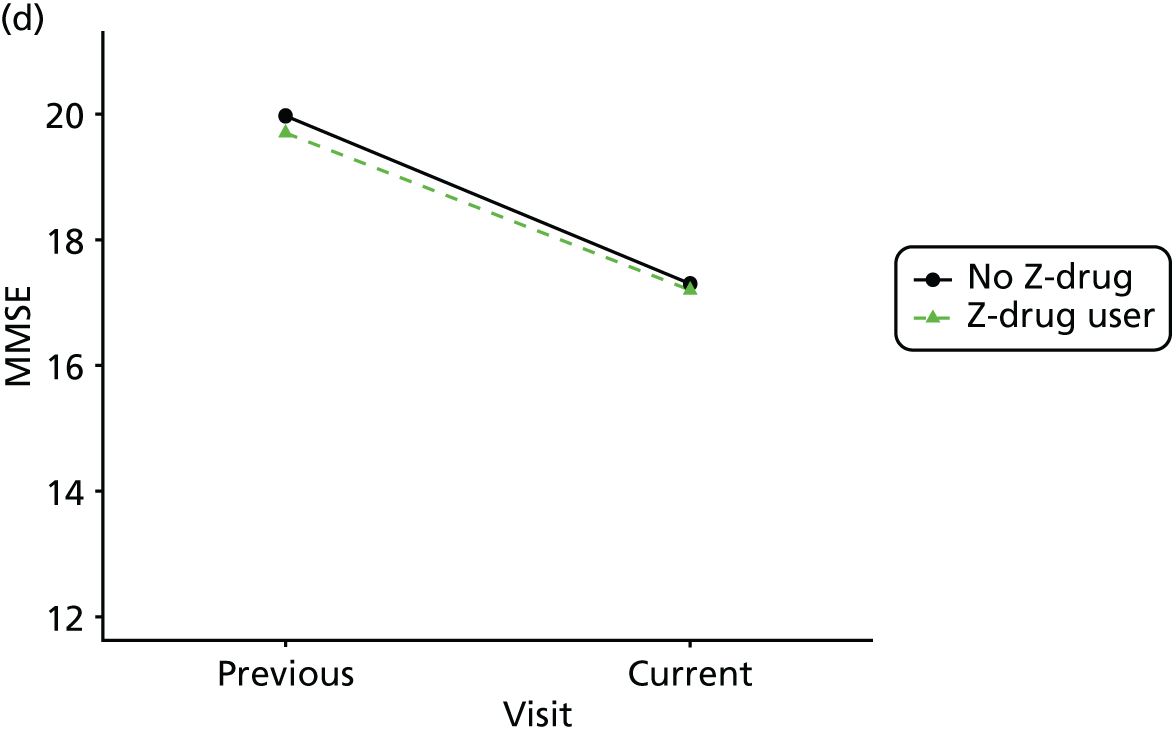
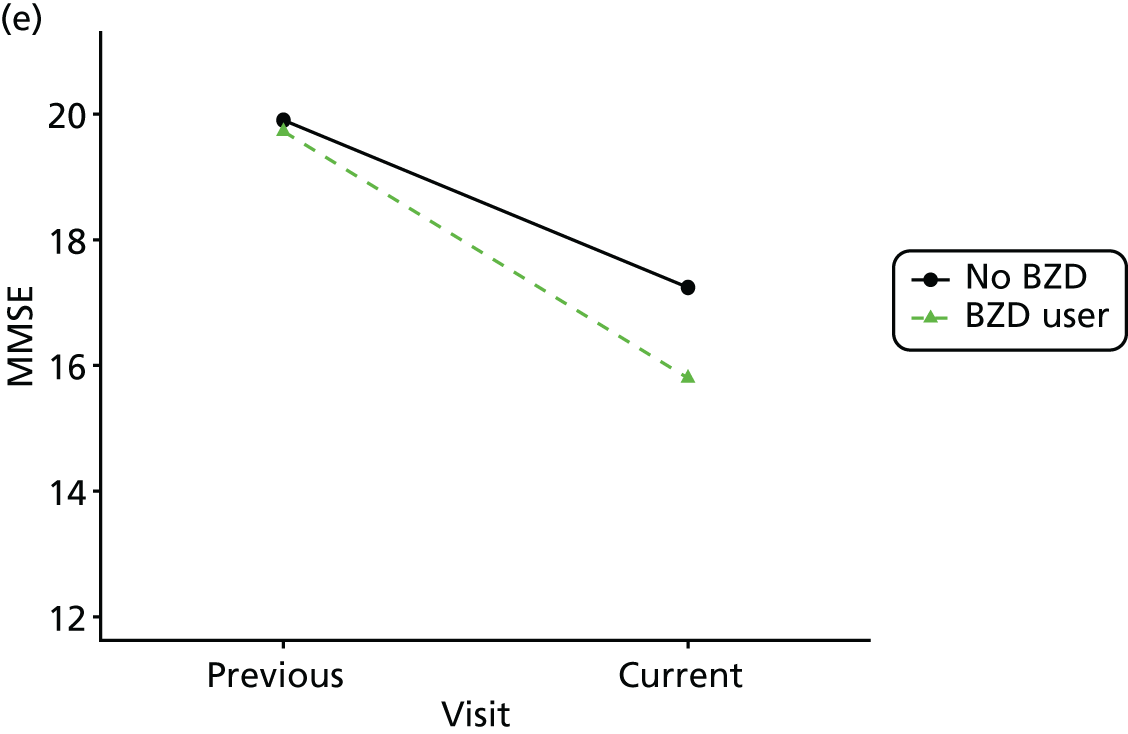
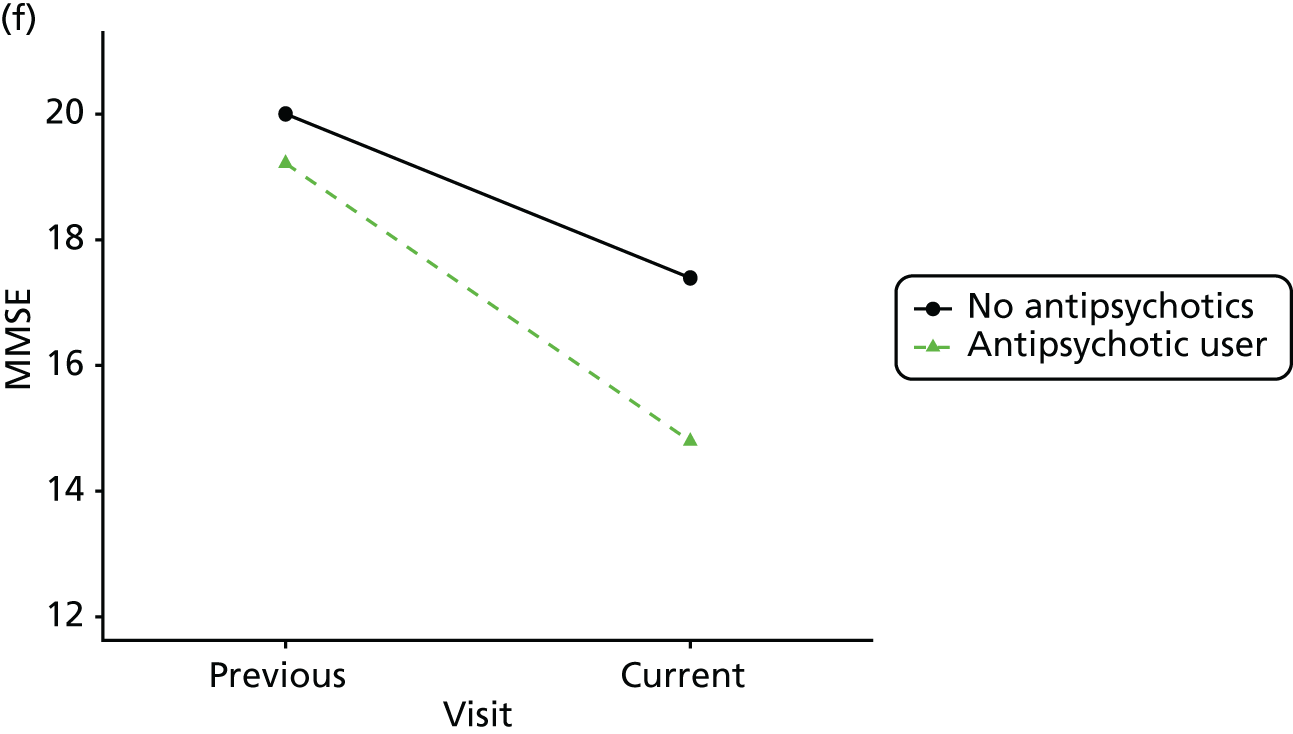
FIGURE 35.
Association between changing hypnotic use status between previous and current visit on anxiety scores at previous and current visit (a–c). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (d–f). (a) Z-drug; (b) BZD; and (c) antipsychotics. Data from the NACC data set.
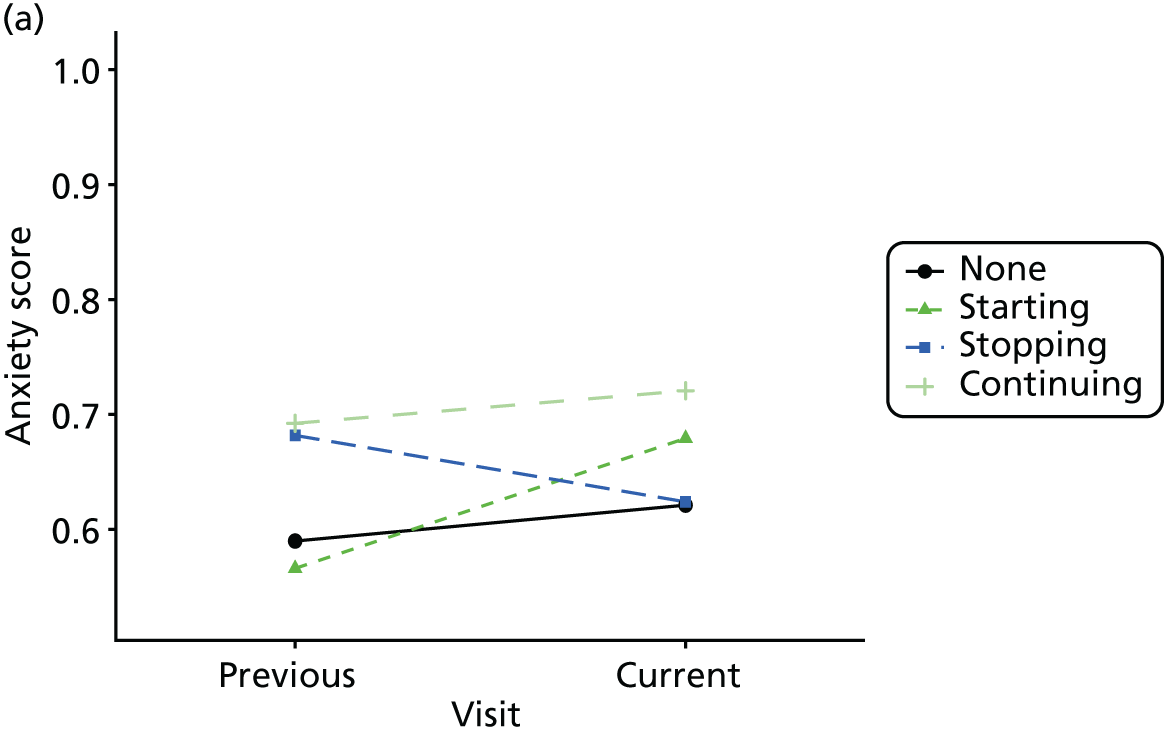
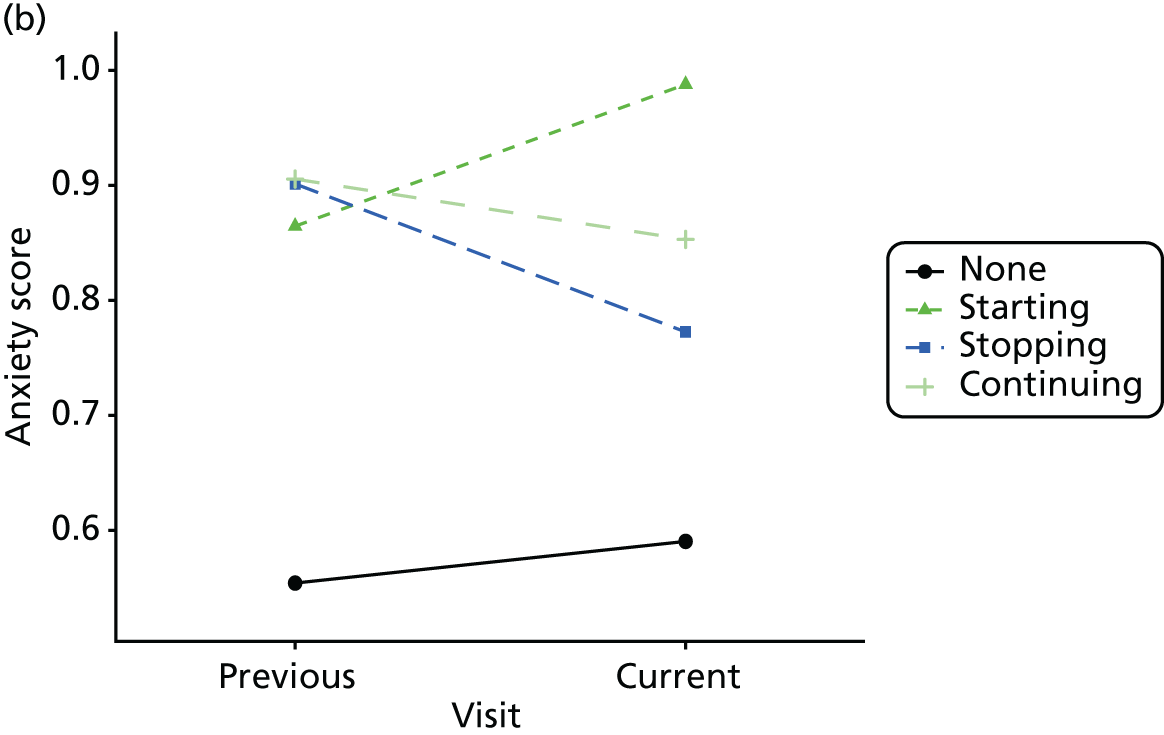
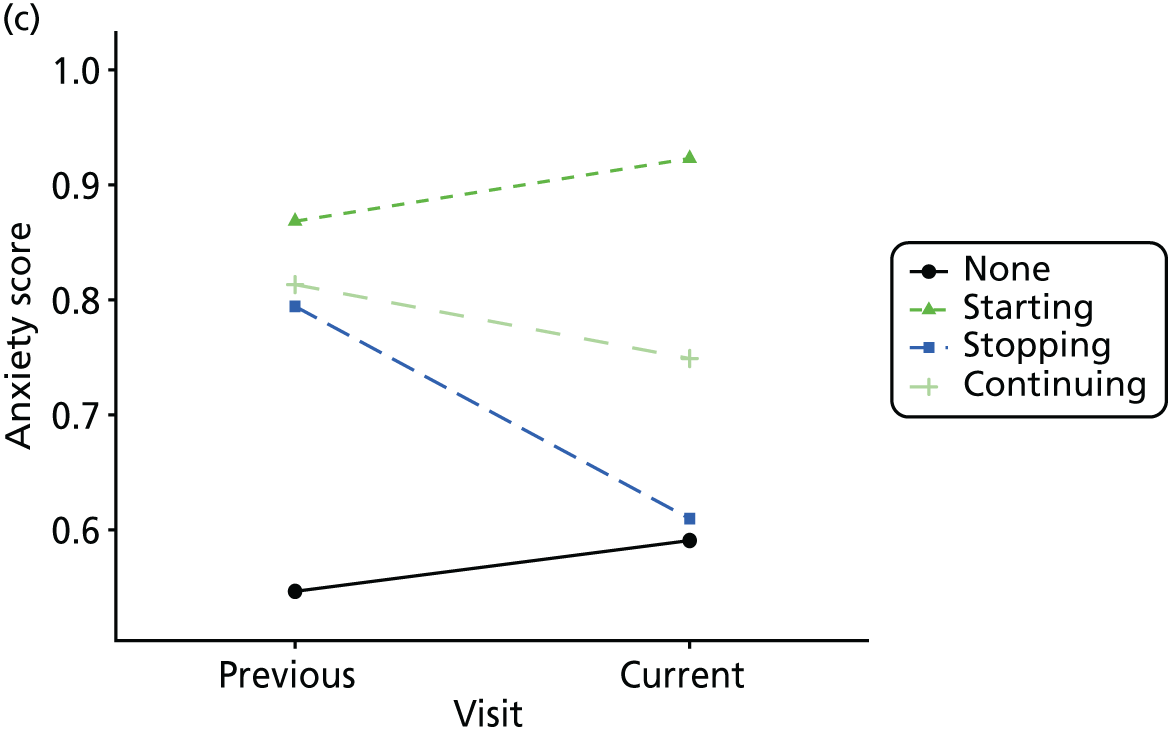
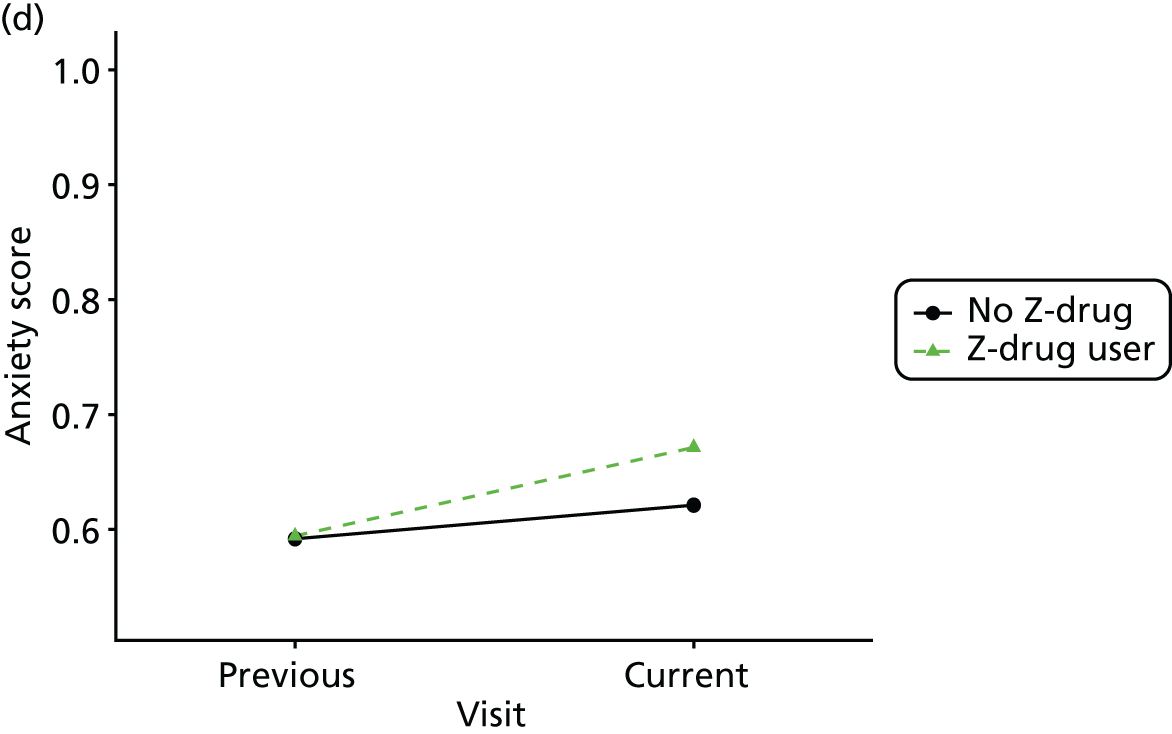
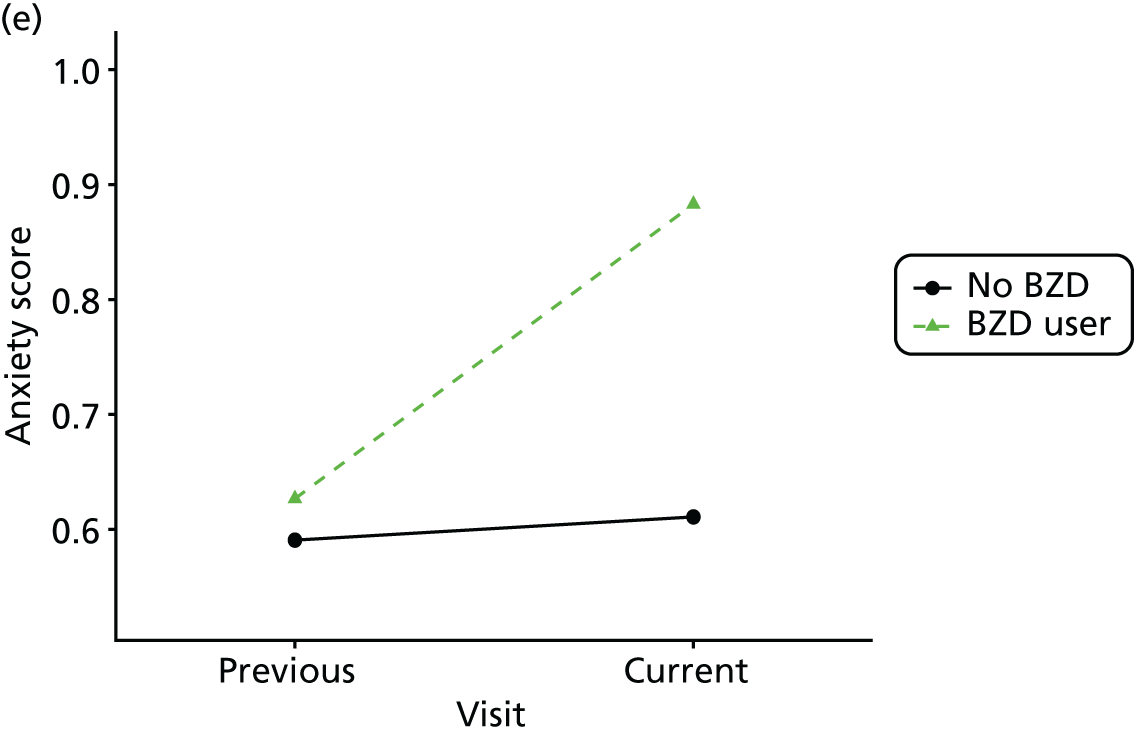
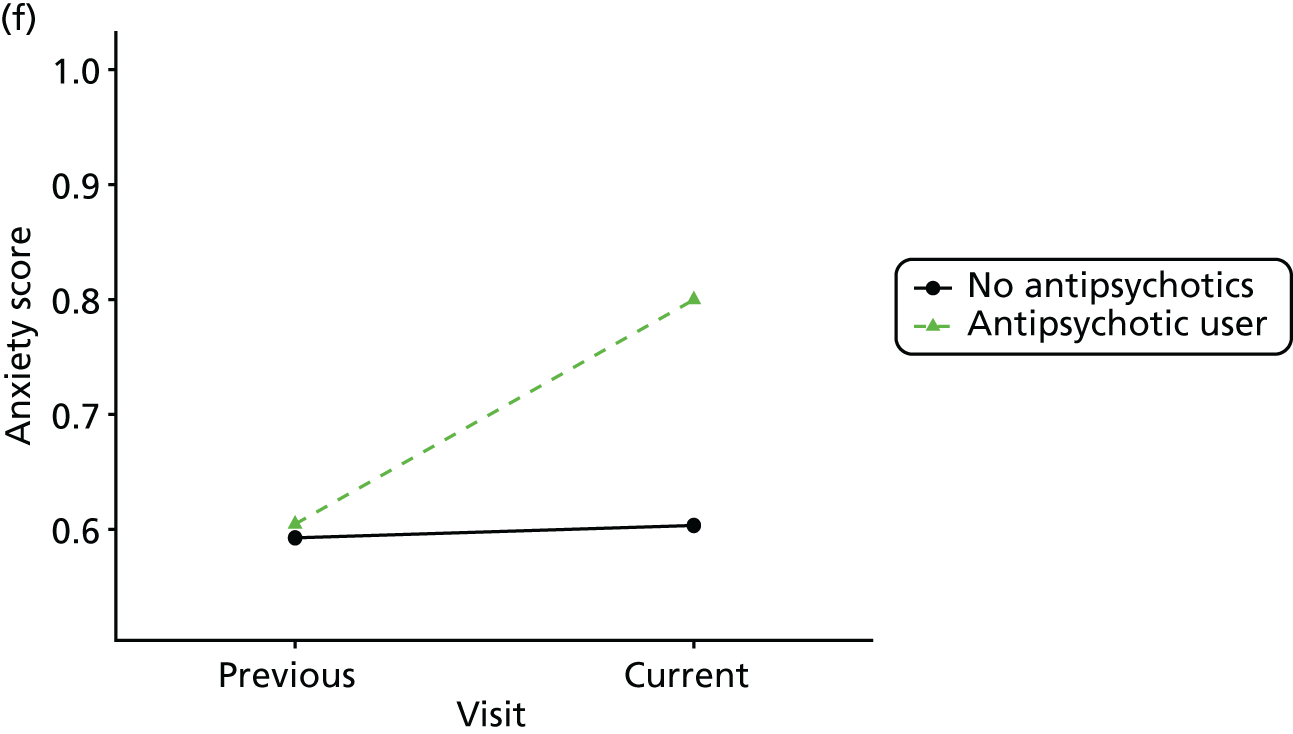
FIGURE 36.
Association between changing hypnotic use status between previous and current visit on agitation scores at previous and current visit (a–c). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (d–f). (a) Z-drug; (b) BZD; and (c) antipsychotics. Data from the NACC data set.
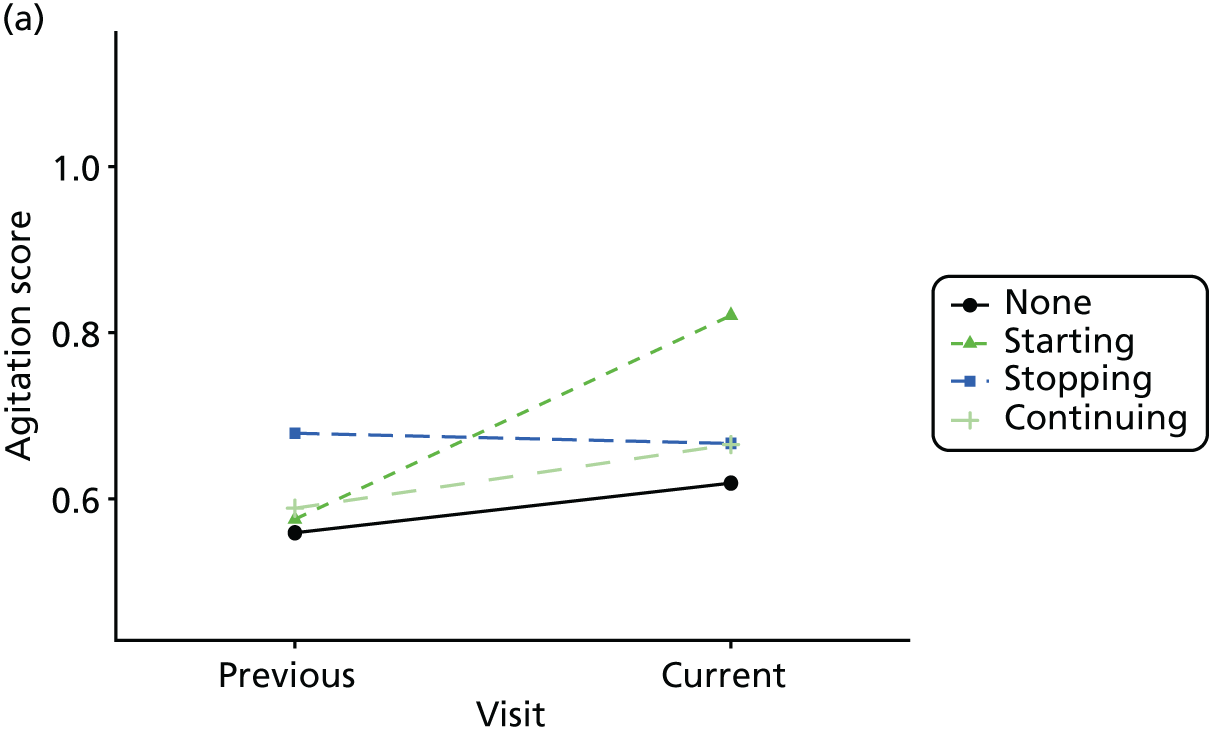
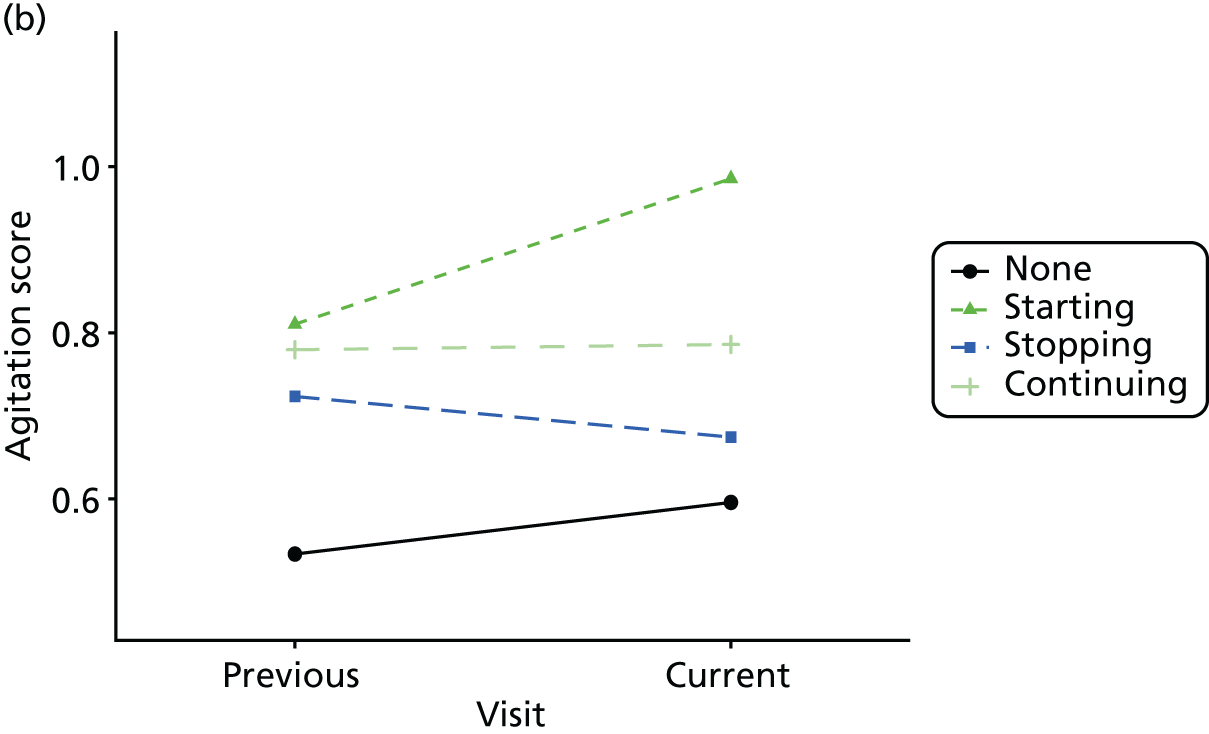
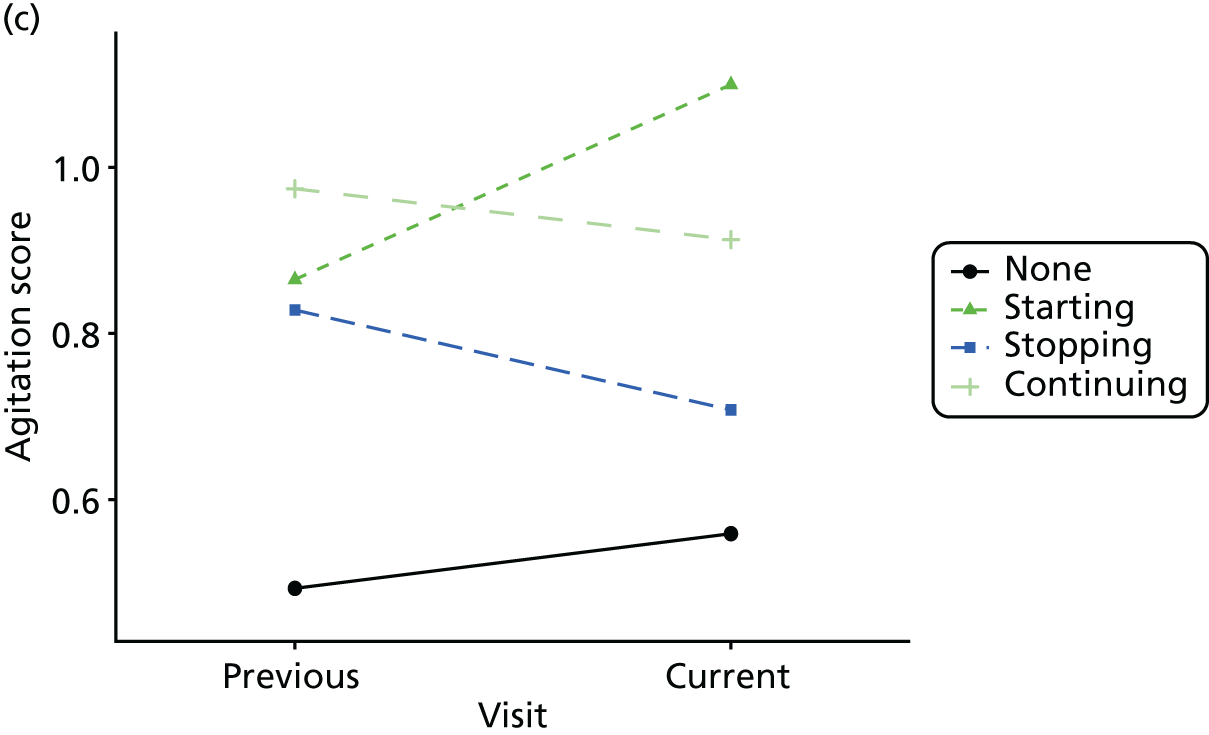
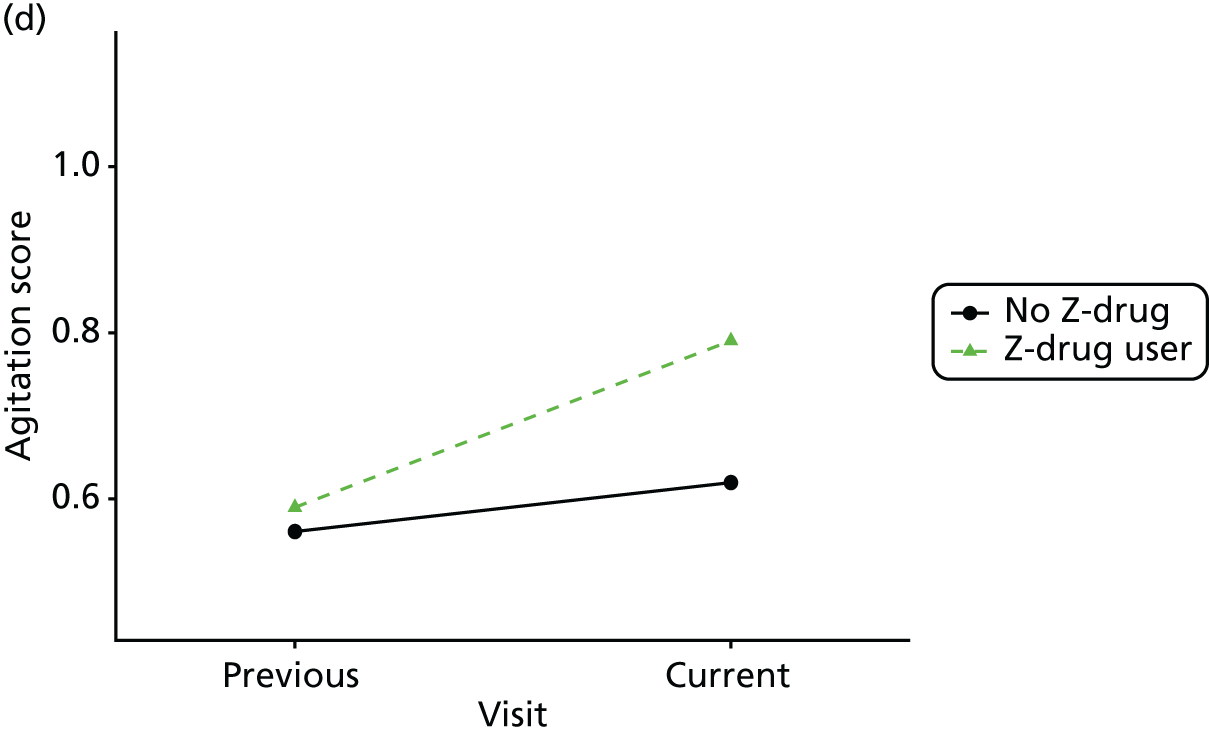
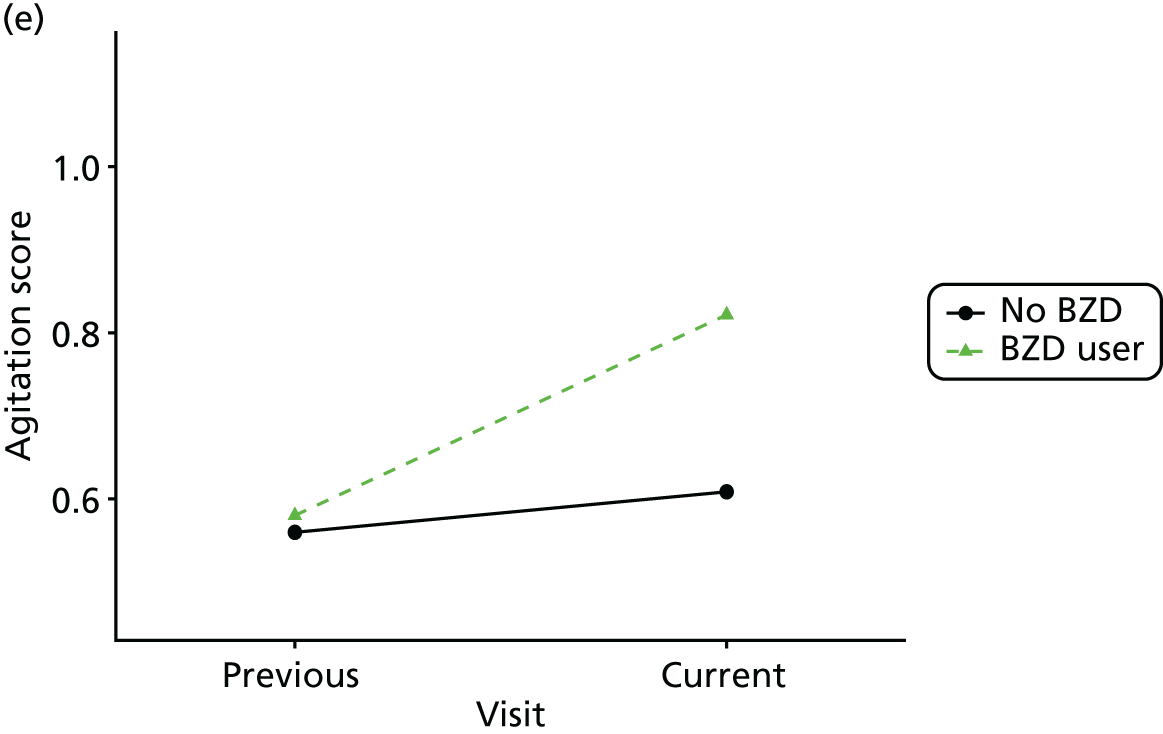
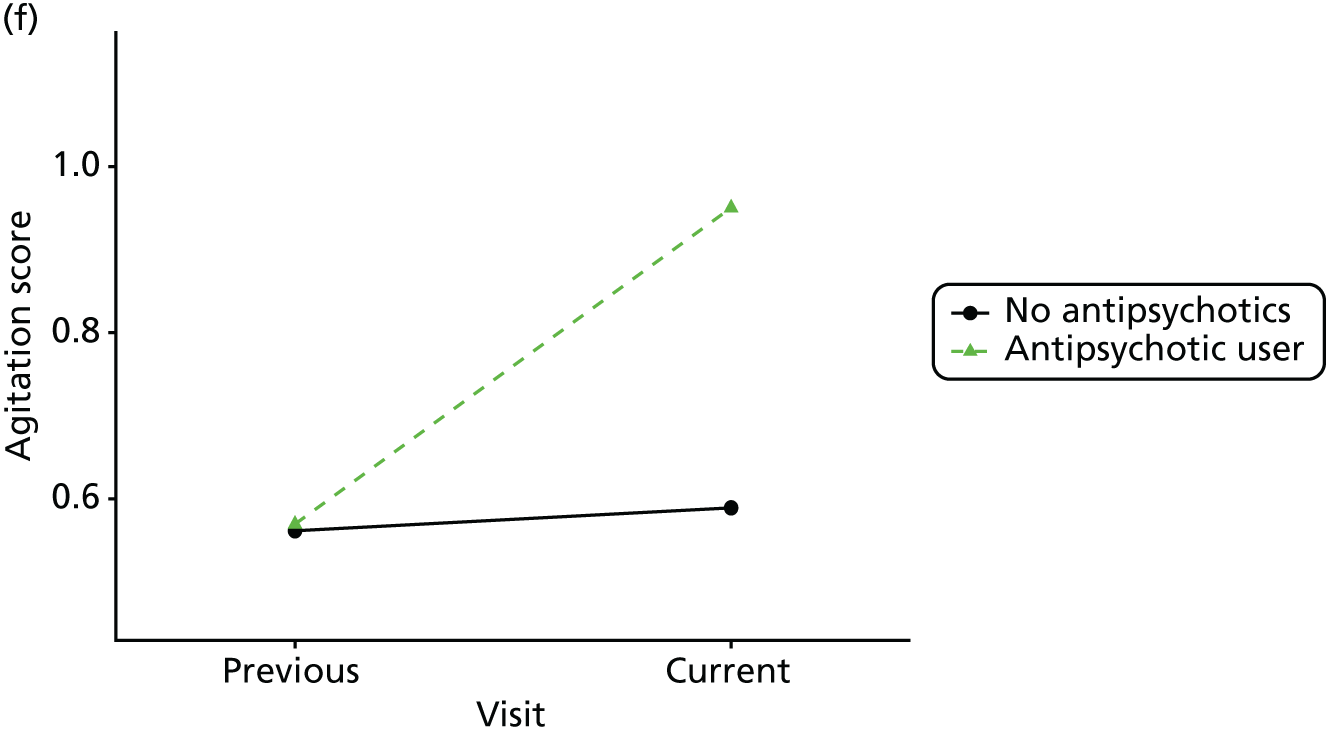
FIGURE 37.
Association between changing hypnotic use status between previous and current visit on NPI (excluding sleep question) scores at previous and current visit (a–c). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (d–f). (a) Z-drug; (b) BZD; and (c) antipsychotics. Data from the NACC data set.
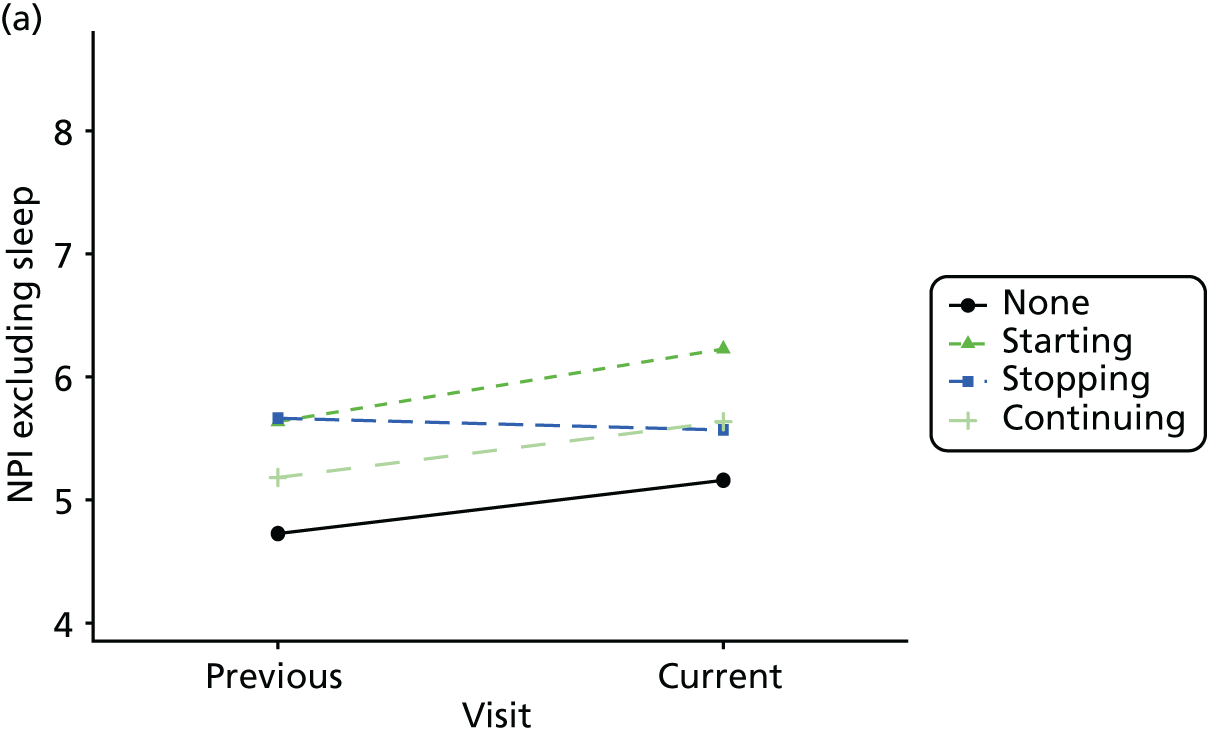
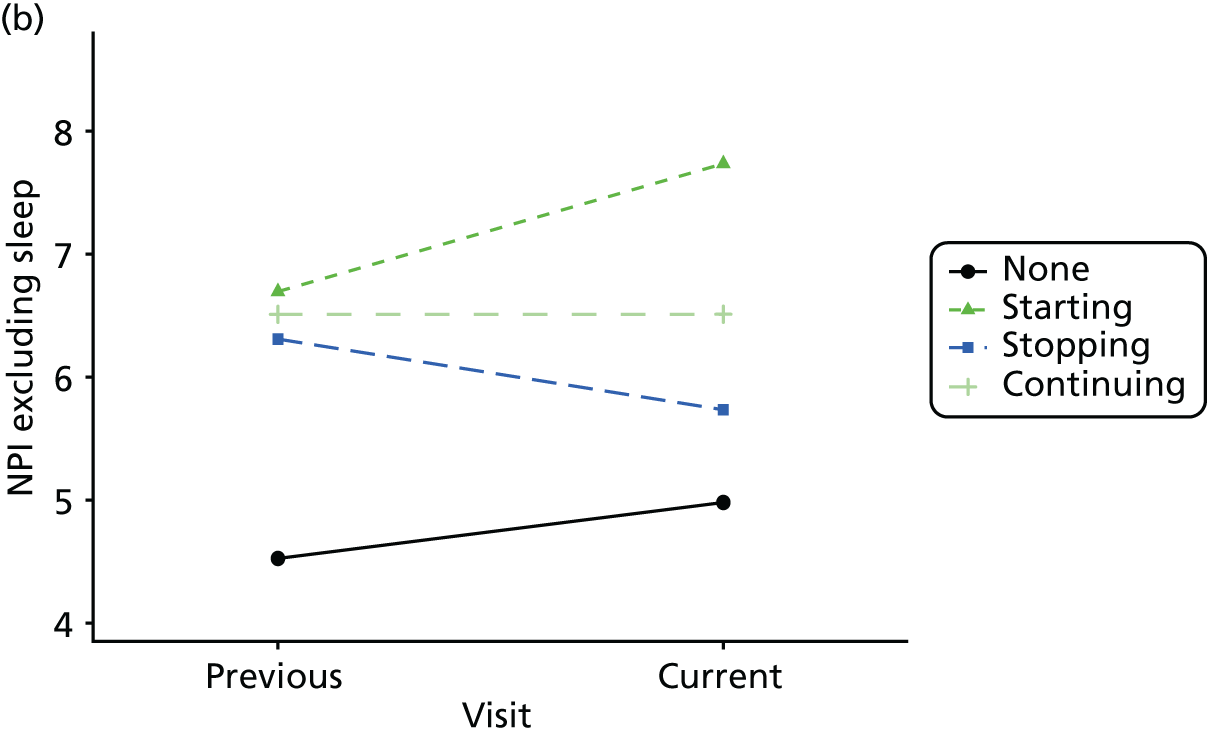
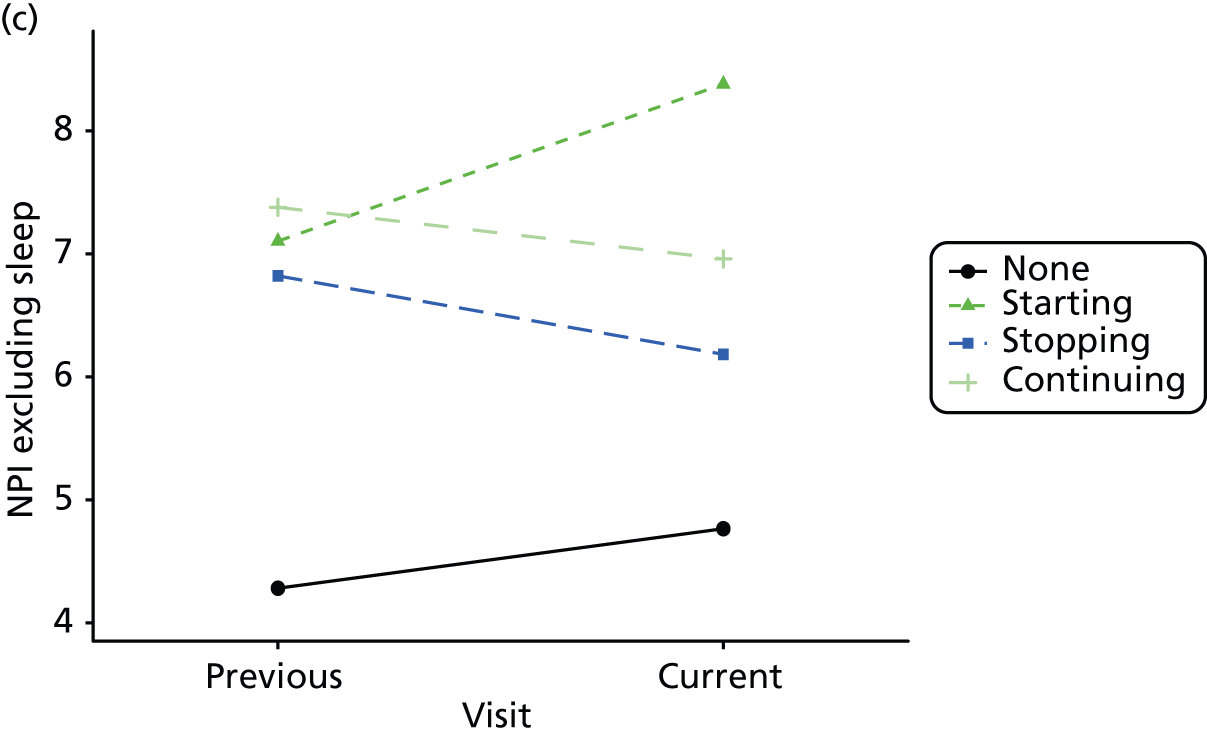
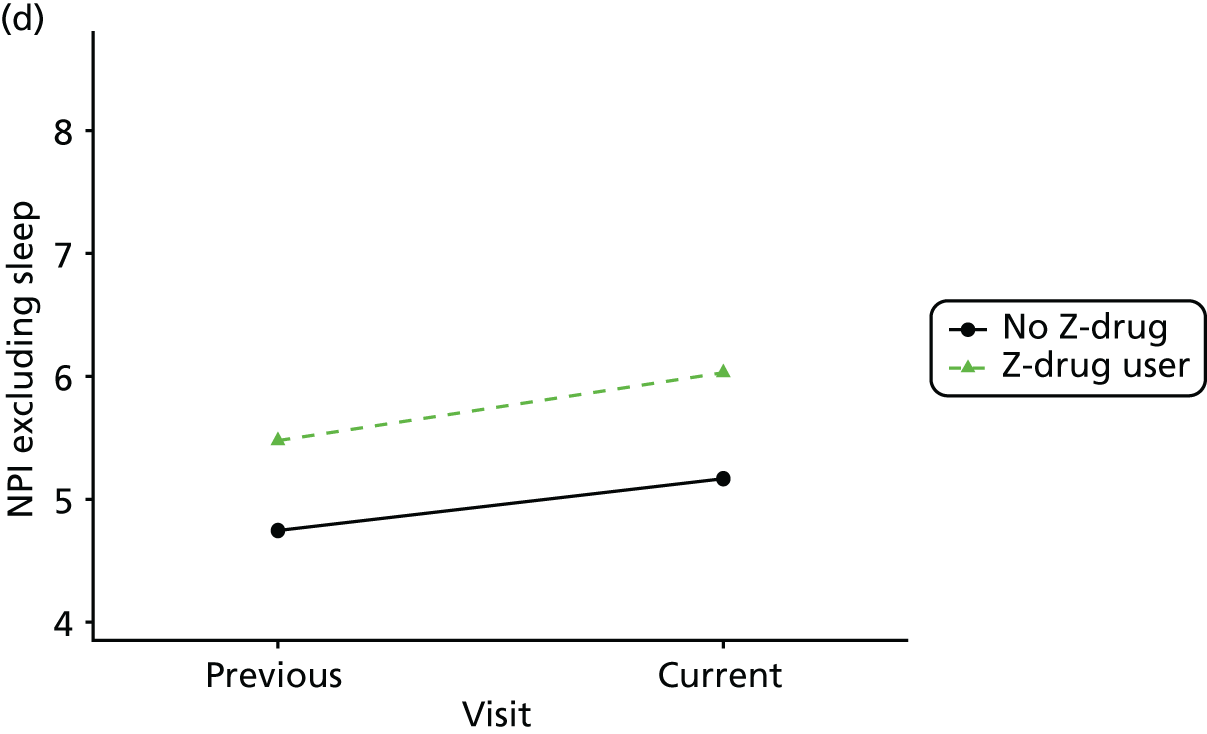
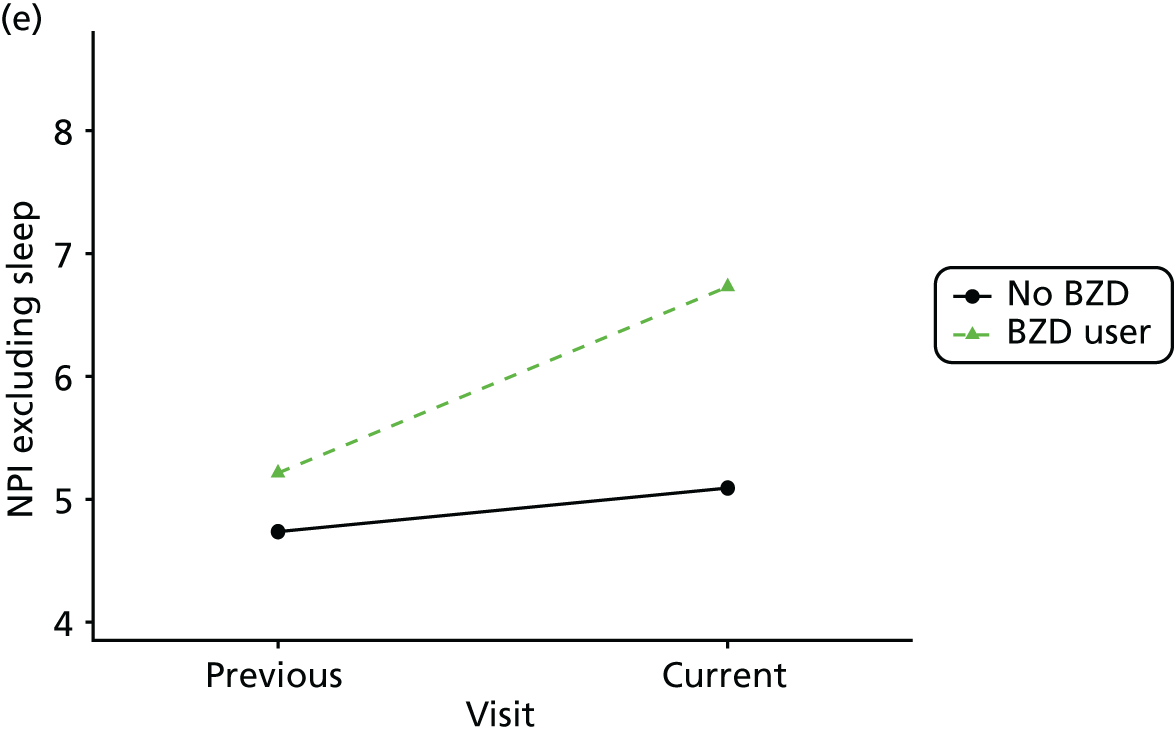
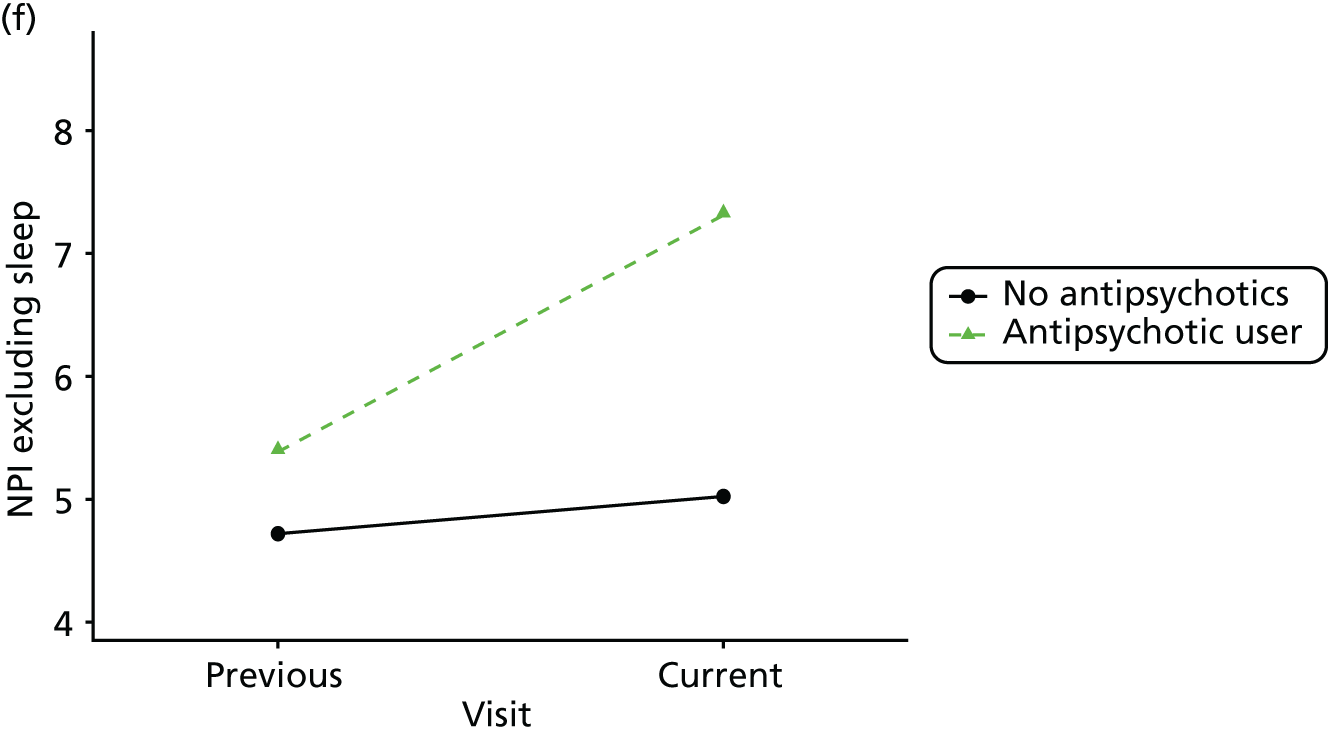
FIGURE 38.
Association between changing hypnotic use status between previous and current visit on NPI sleep disturbance scores at previous and current visit (a–c). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (d–f). (a) Z-drug; (b) BZD; and (c) antipsychotics. Data from the NACC data set.
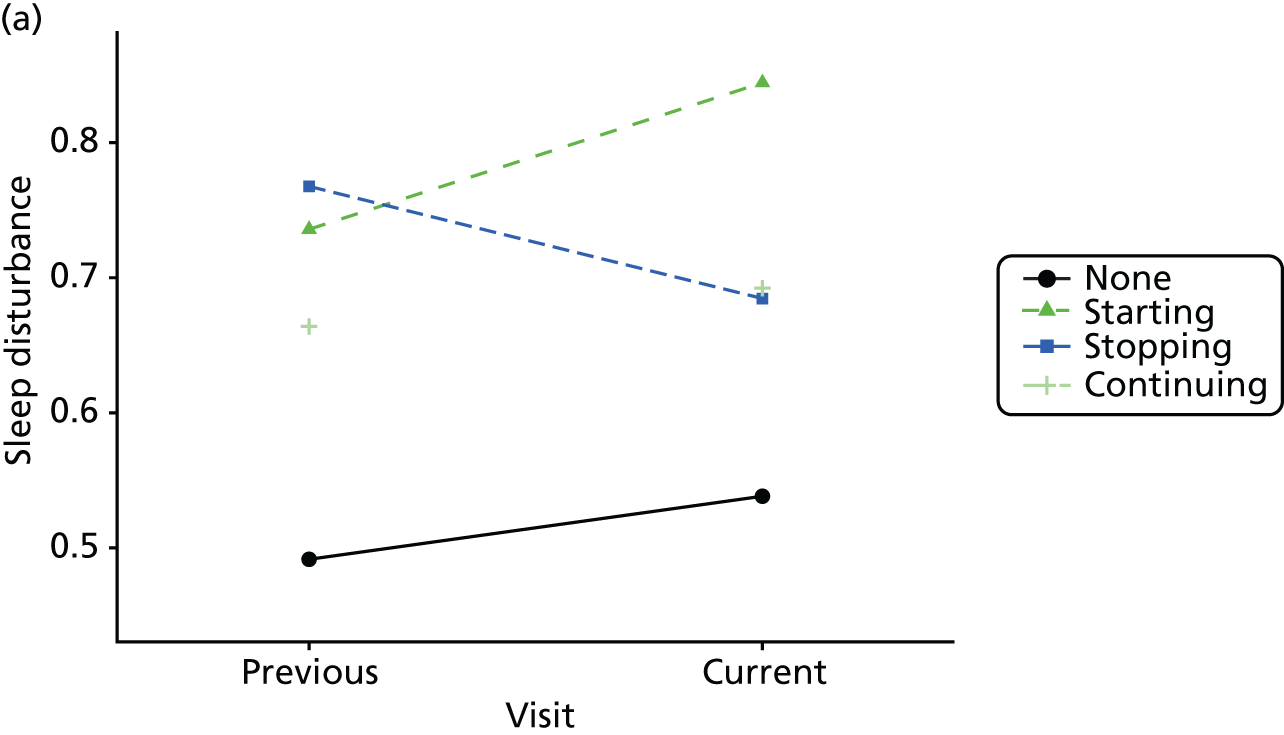
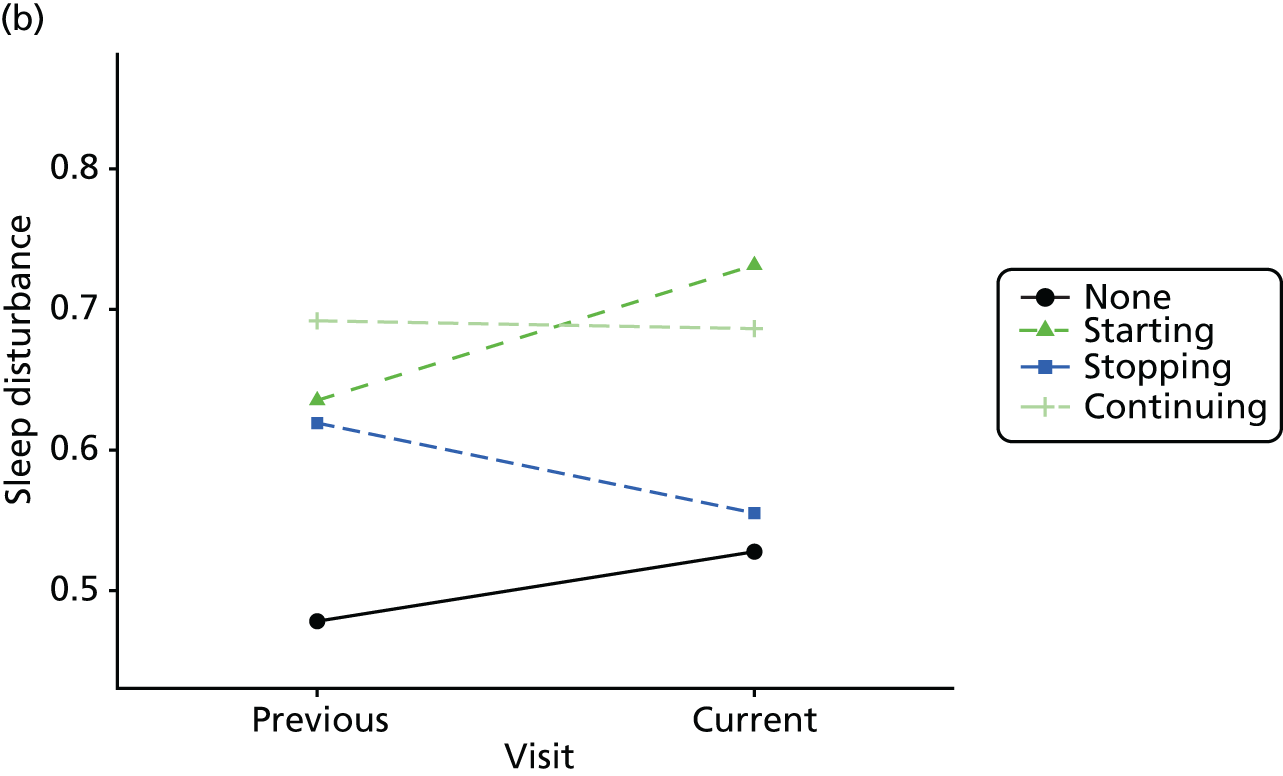
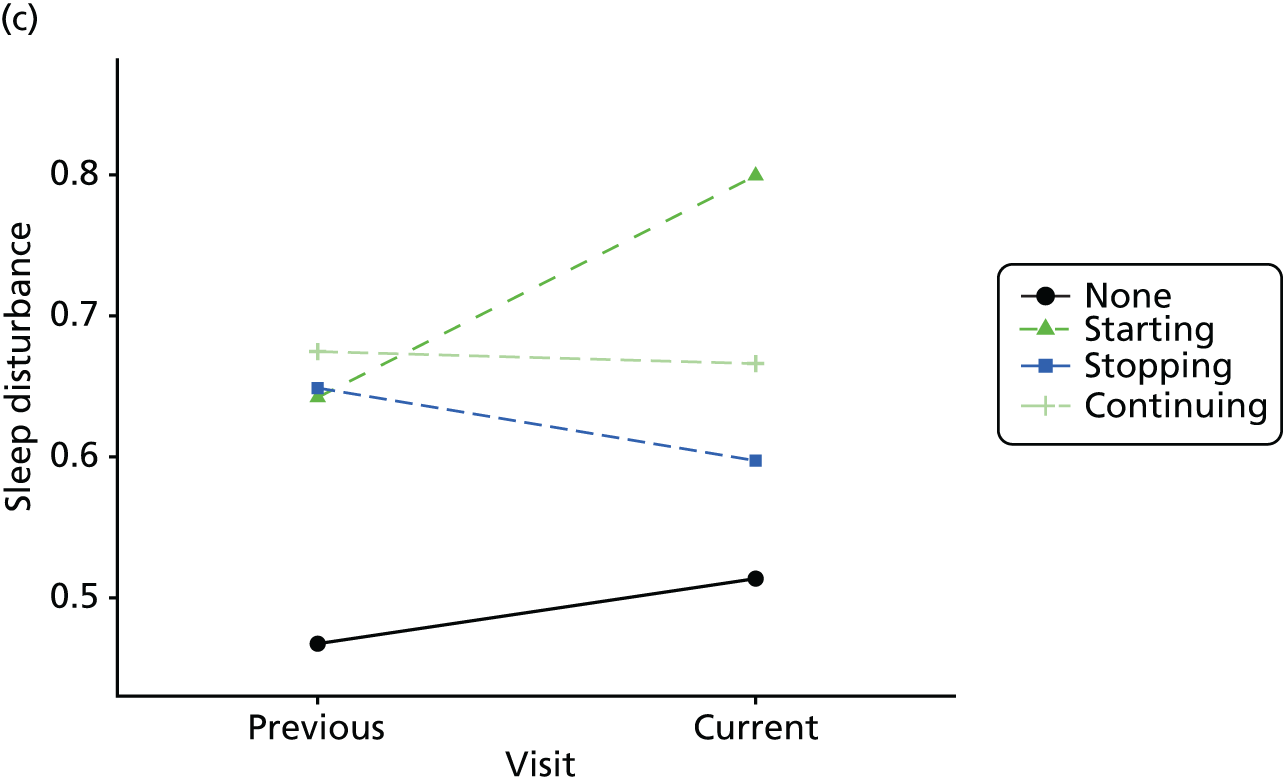
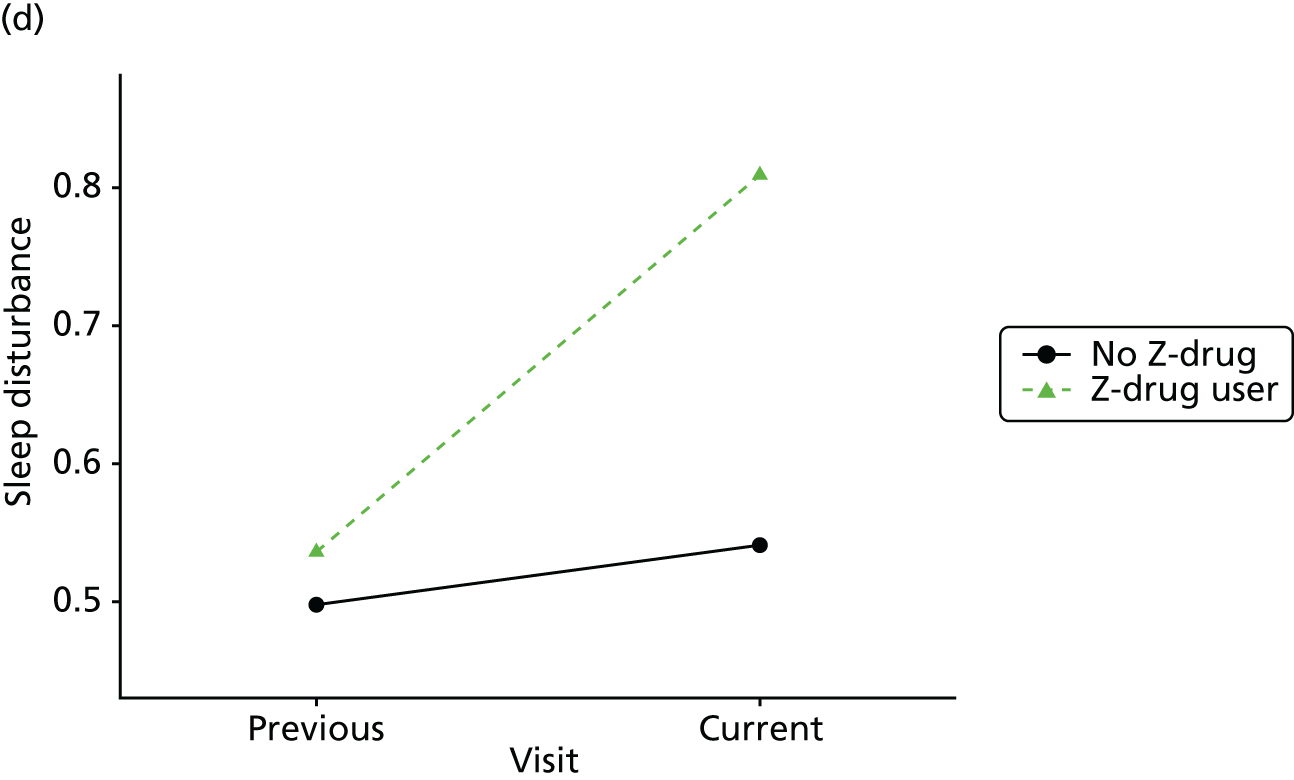
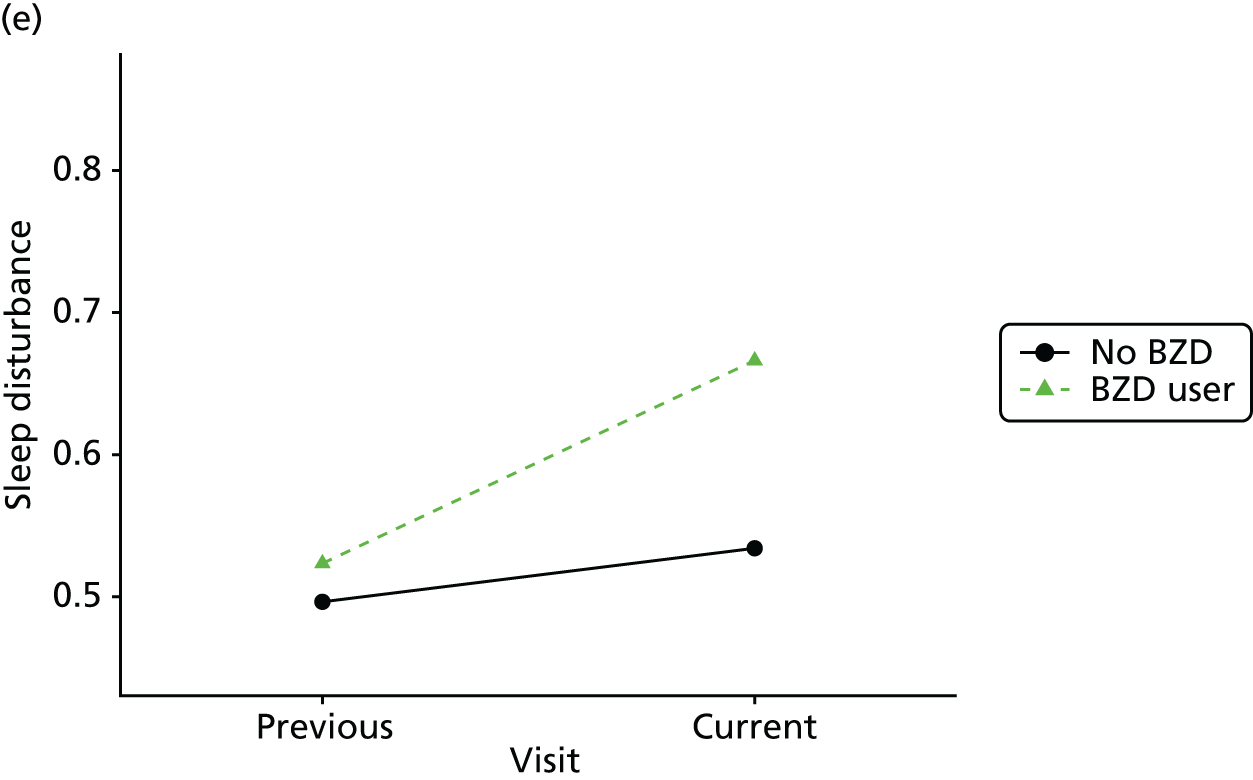
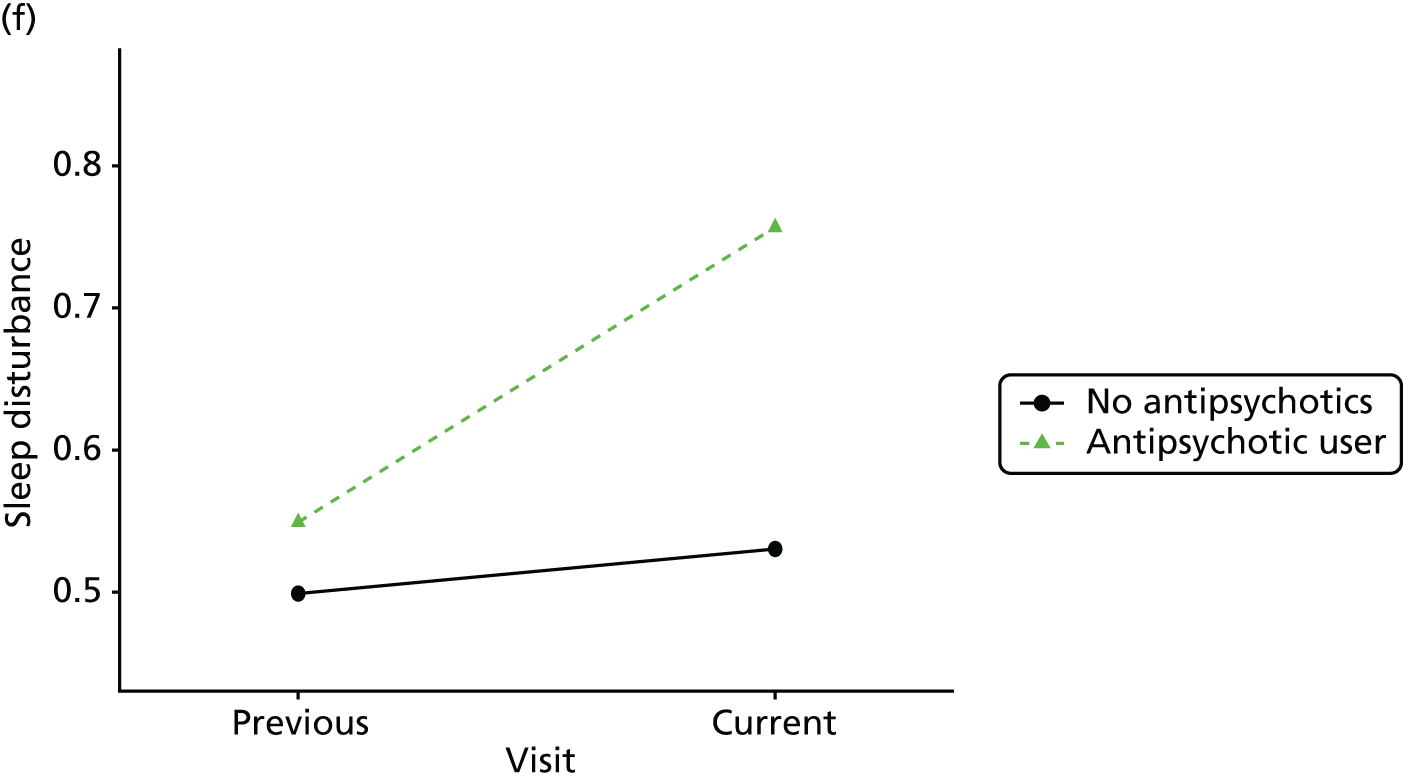
FIGURE 39.
Association between changing hypnotic use status between previous and current visit on animal fluency scores (higher scores represent better cognitive function) at previous and current visit (a–c). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (d–f). (a) Z-drug; (b) BZD; and (c) antipsychotics. Data from the NACC data set.
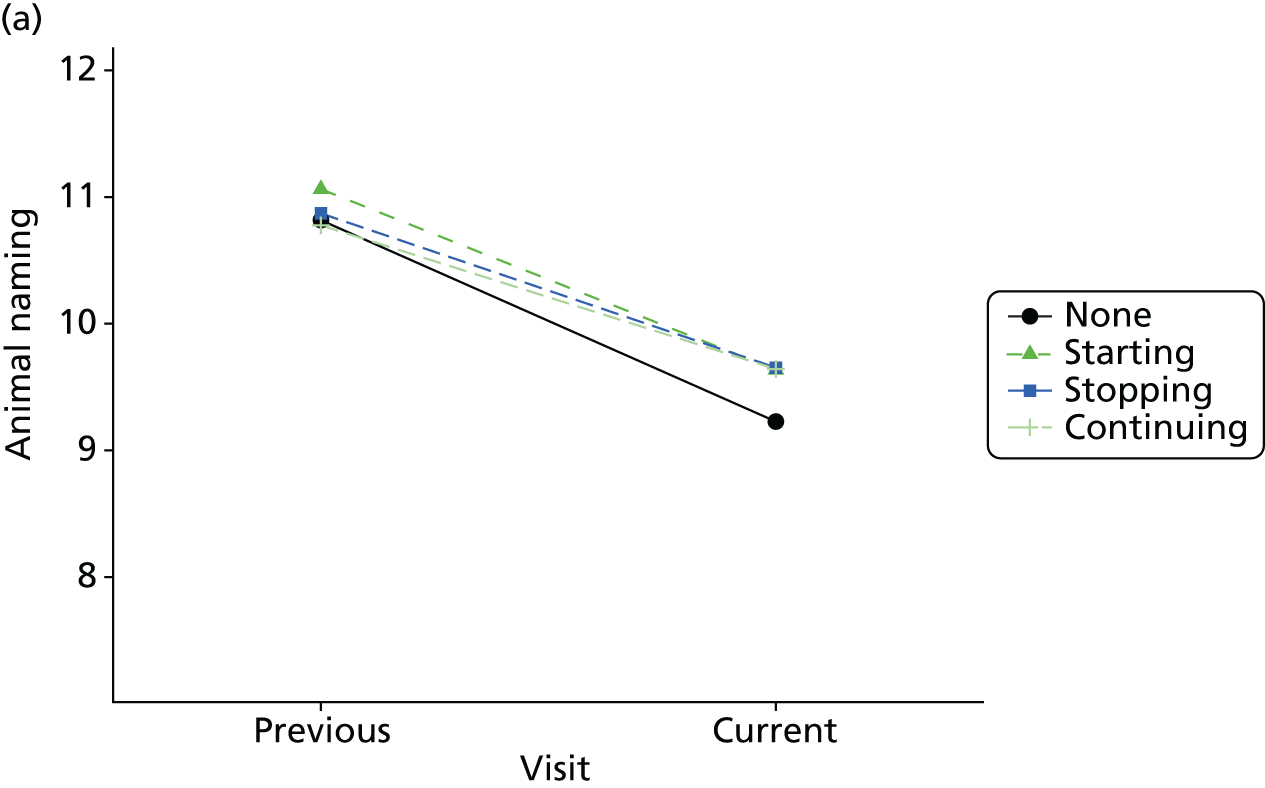
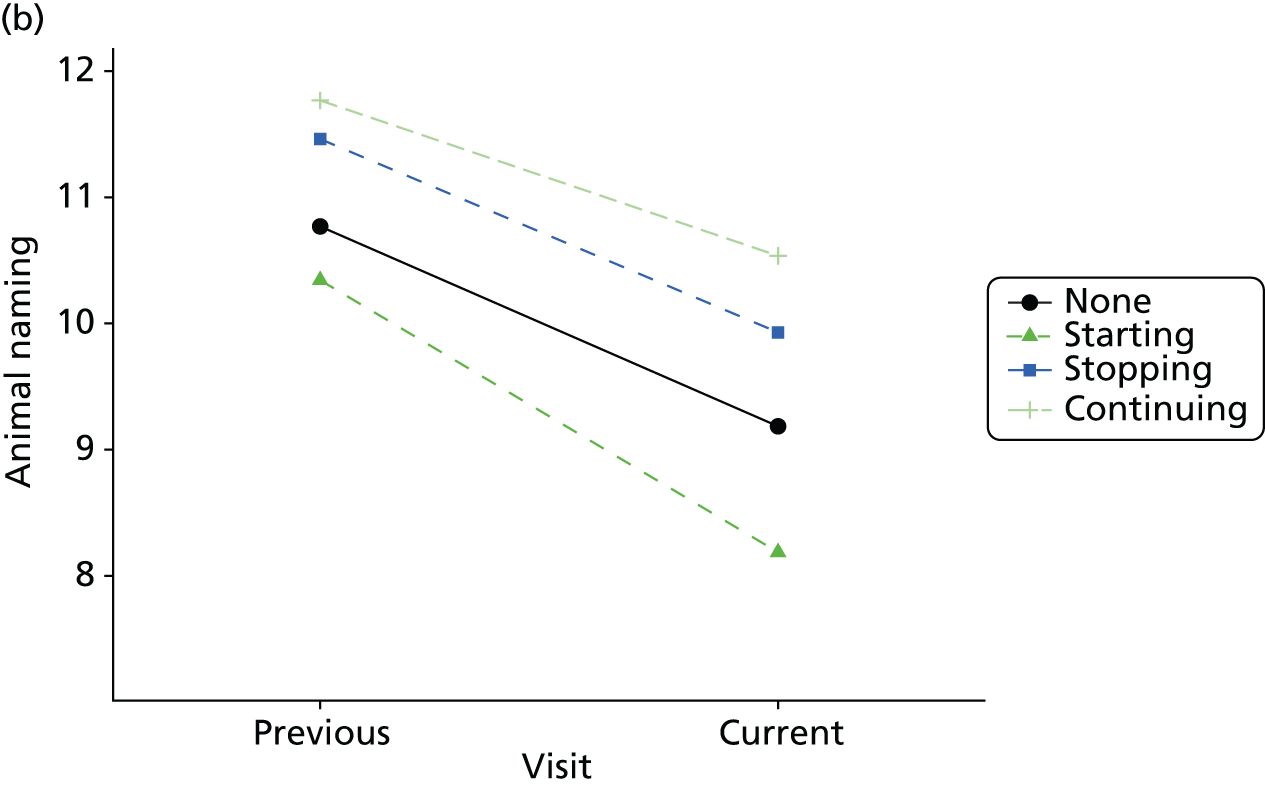
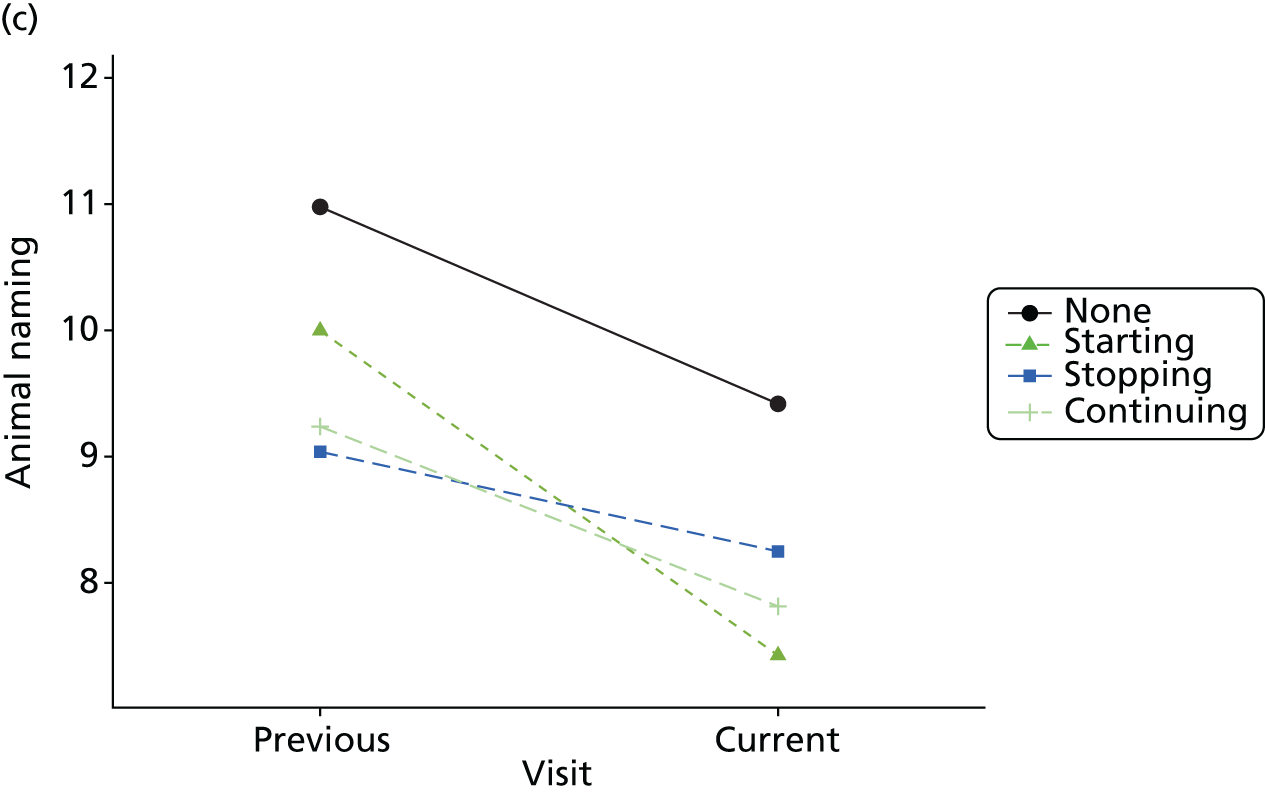
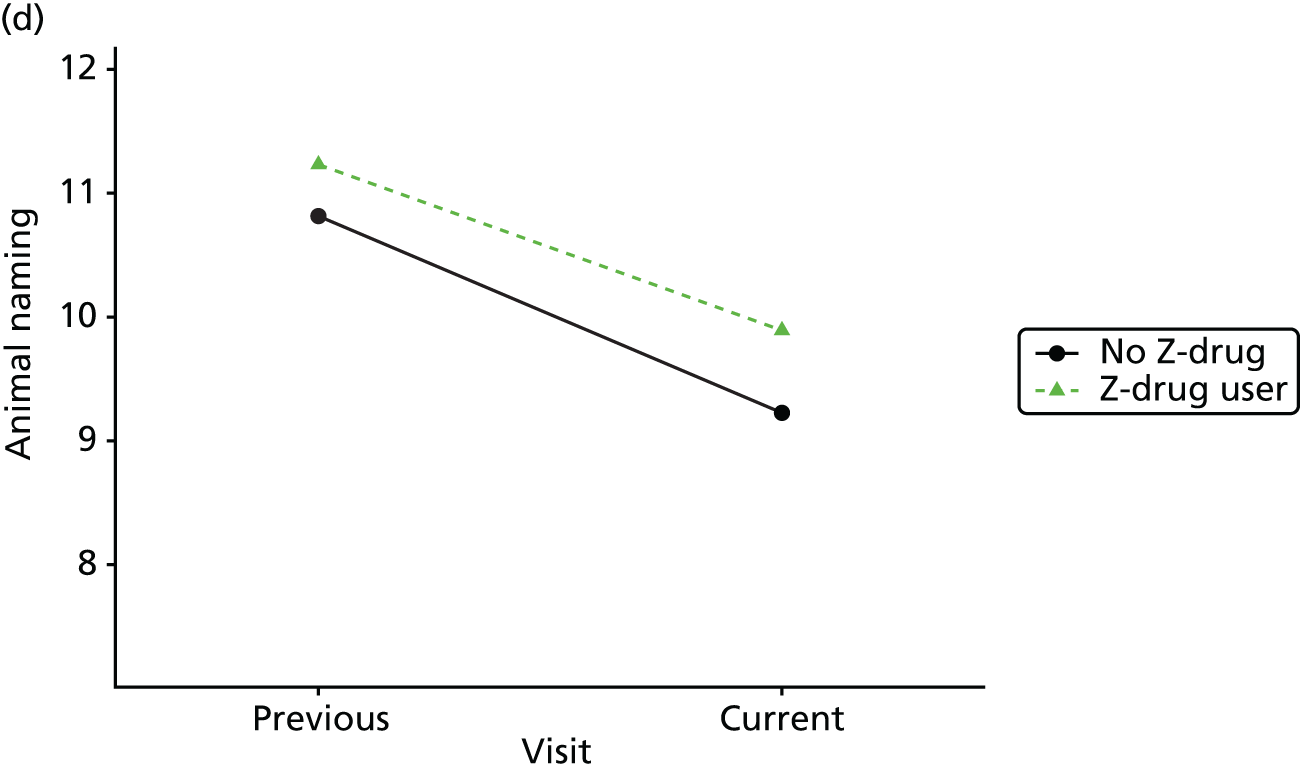
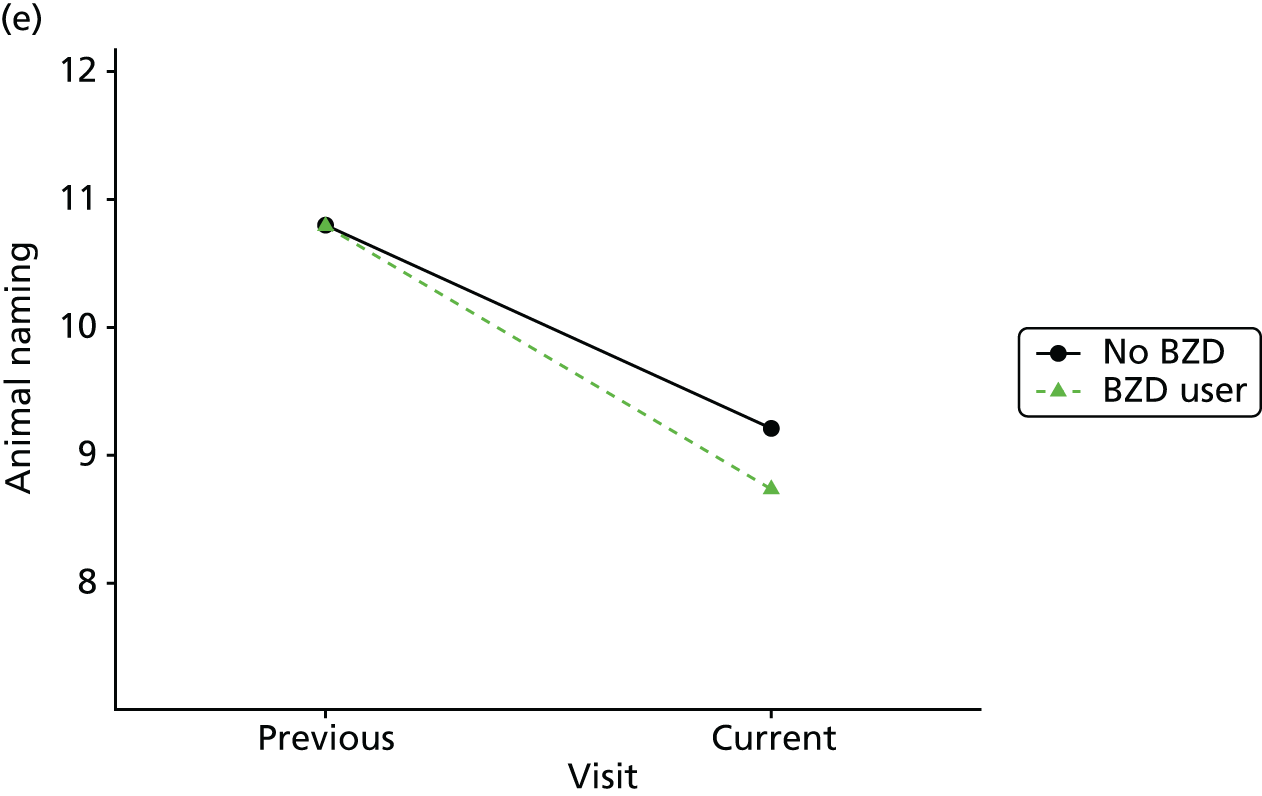
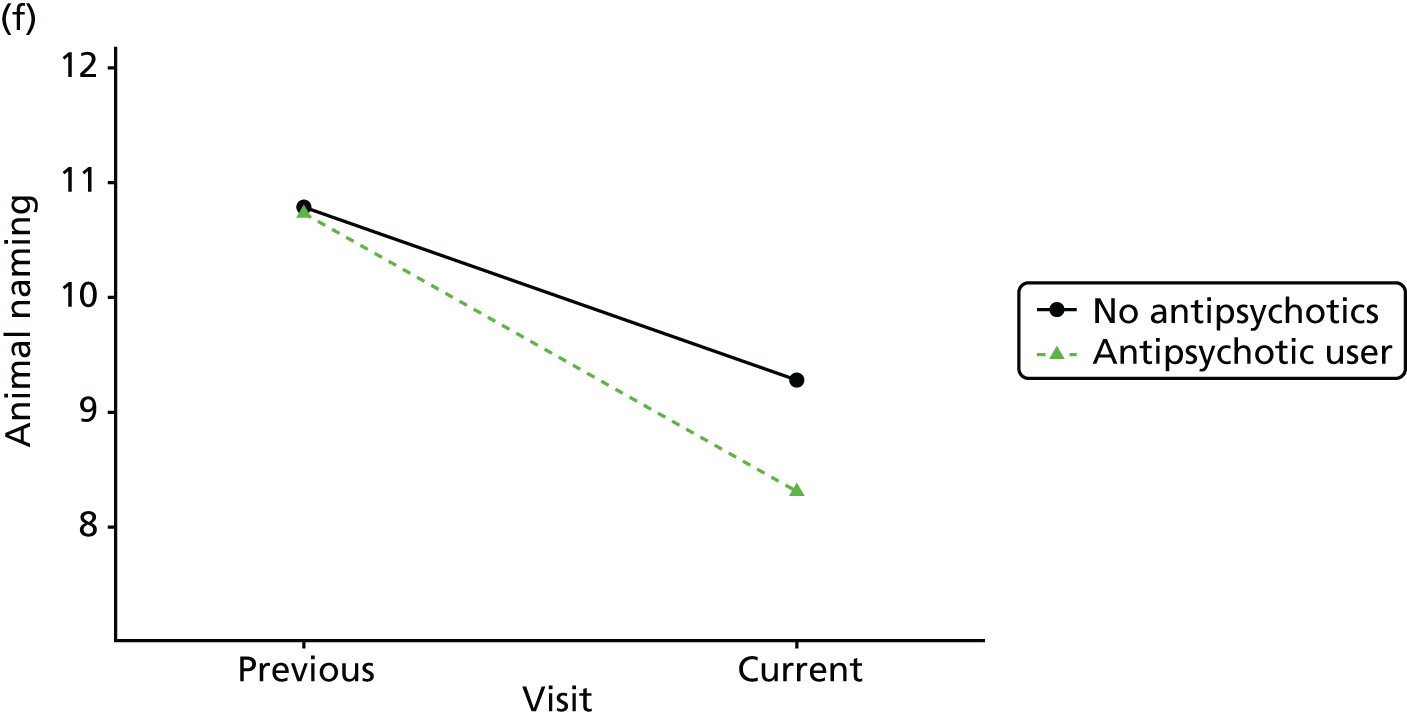
FIGURE 40.
Association between changing hypnotic use status between previous and current visit on CDR-SOB scores (higher scores represent more cognitive impairment) at previous and current visit (a–c). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (d–f). (a) Z-drug; (b) BZD; and (c) antipsychotics. Data from the NACC data set.
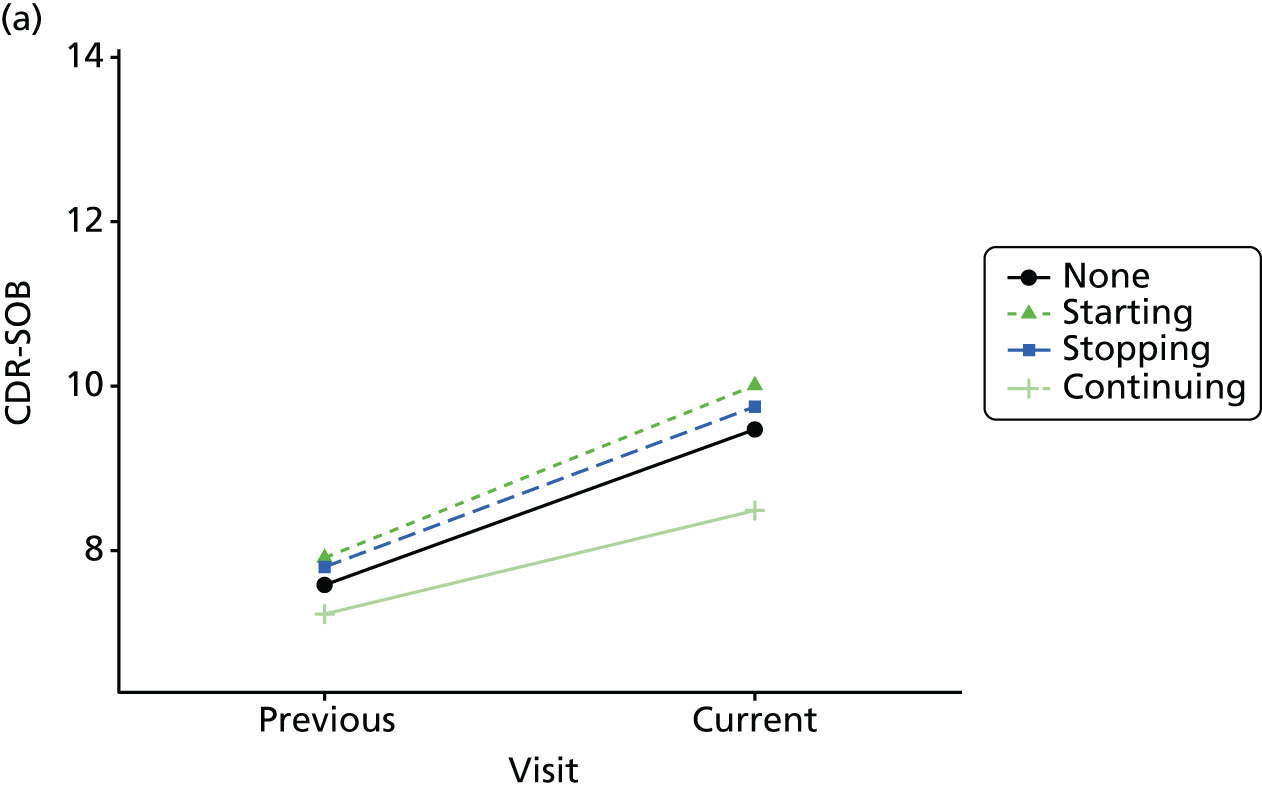
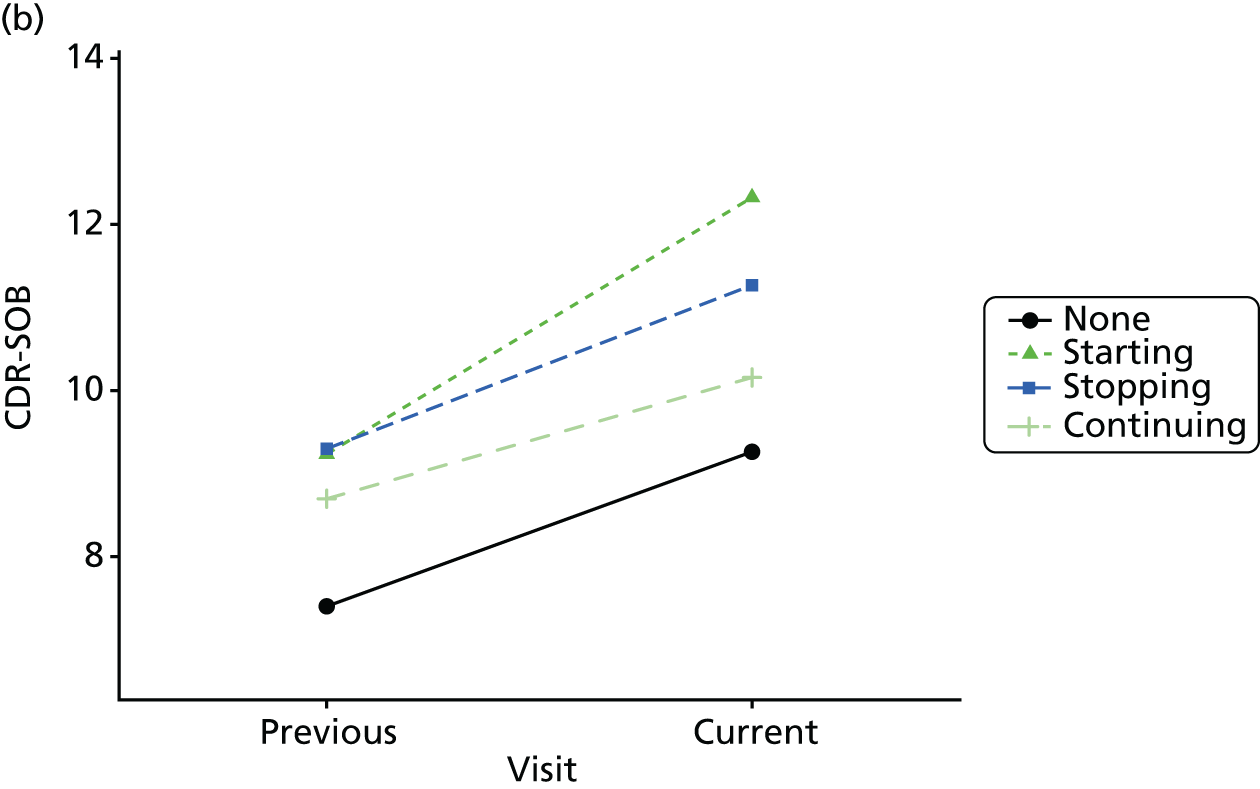
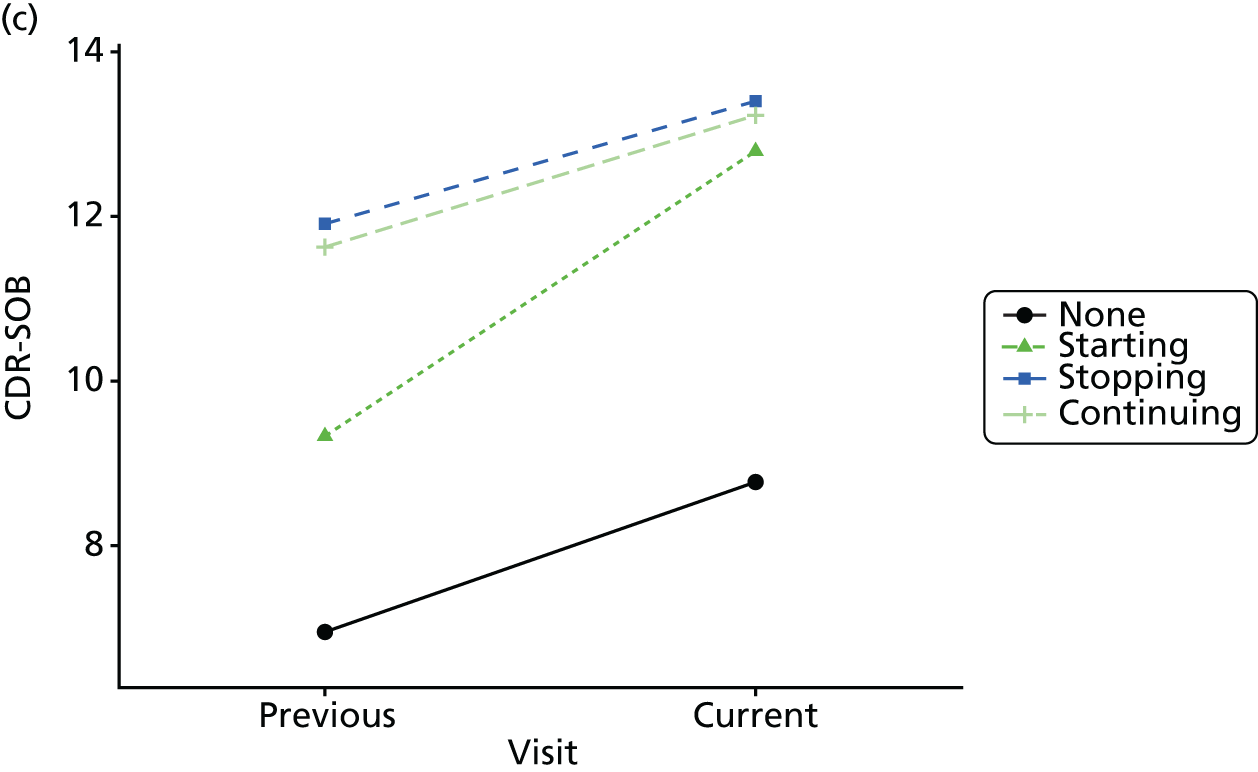
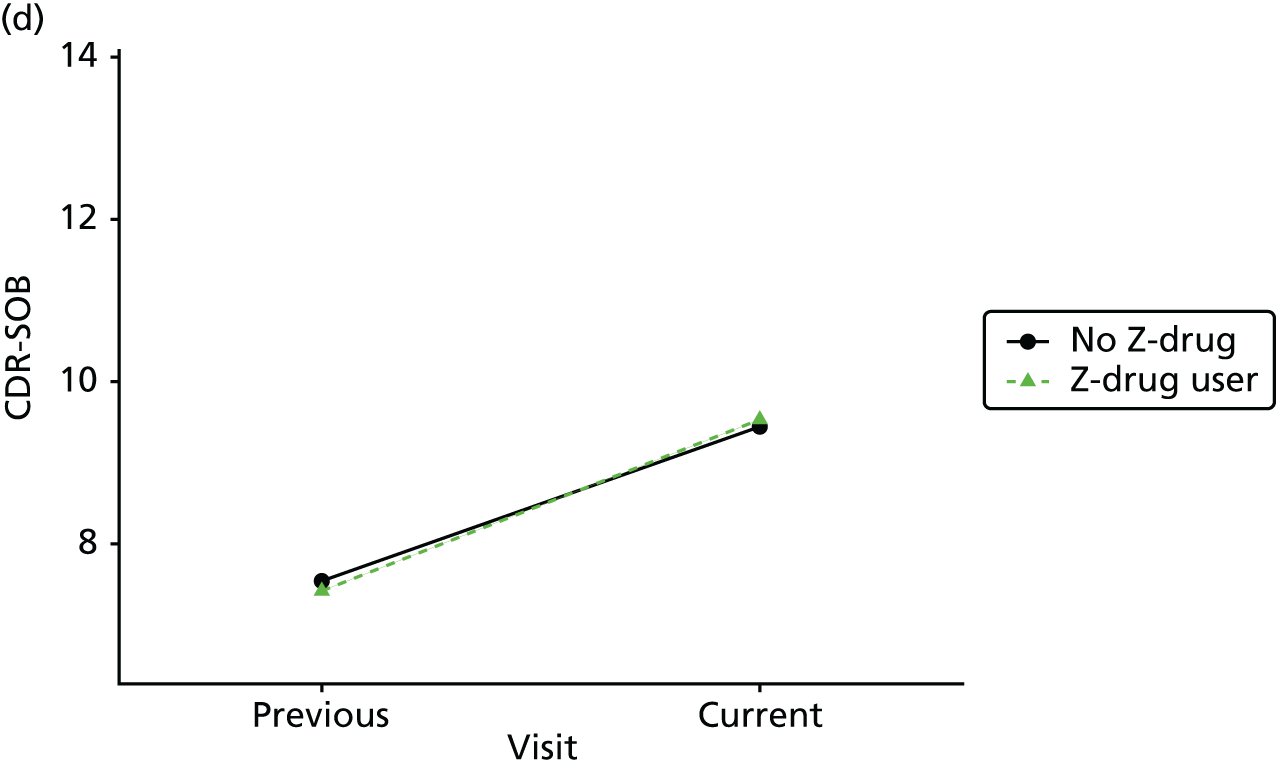
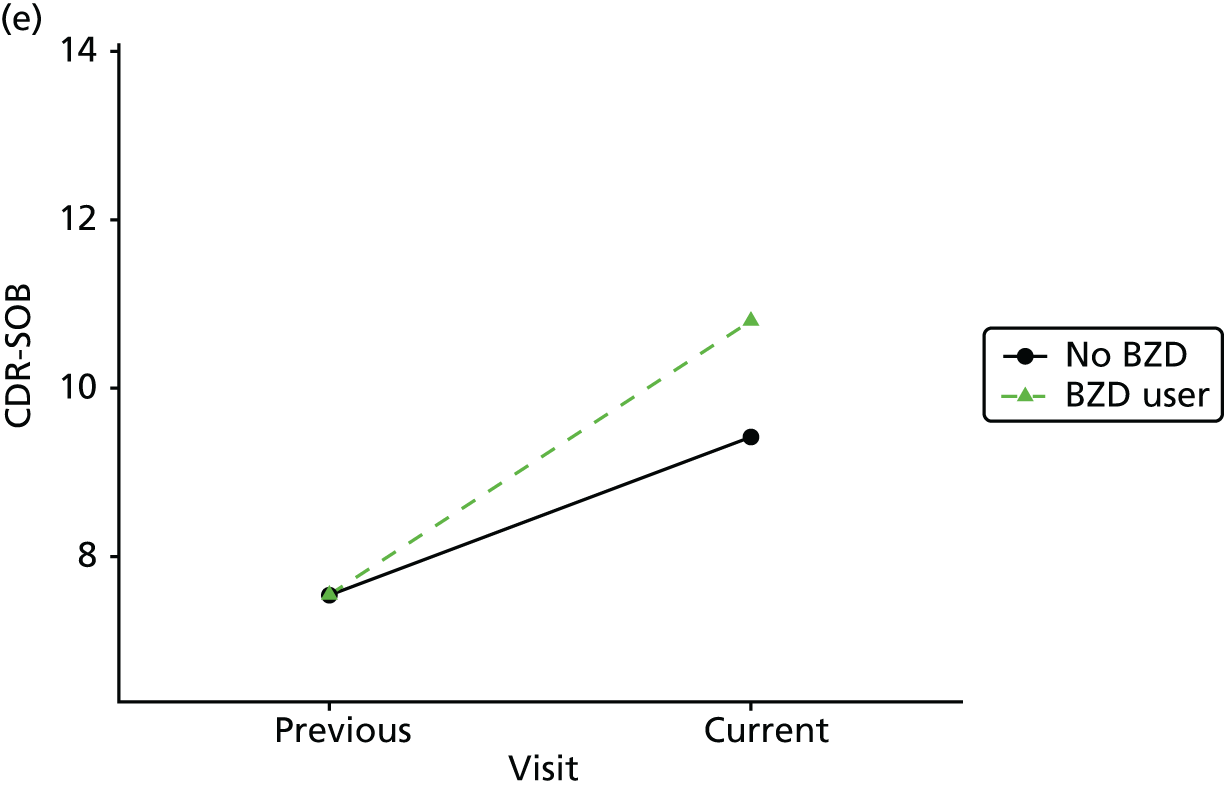
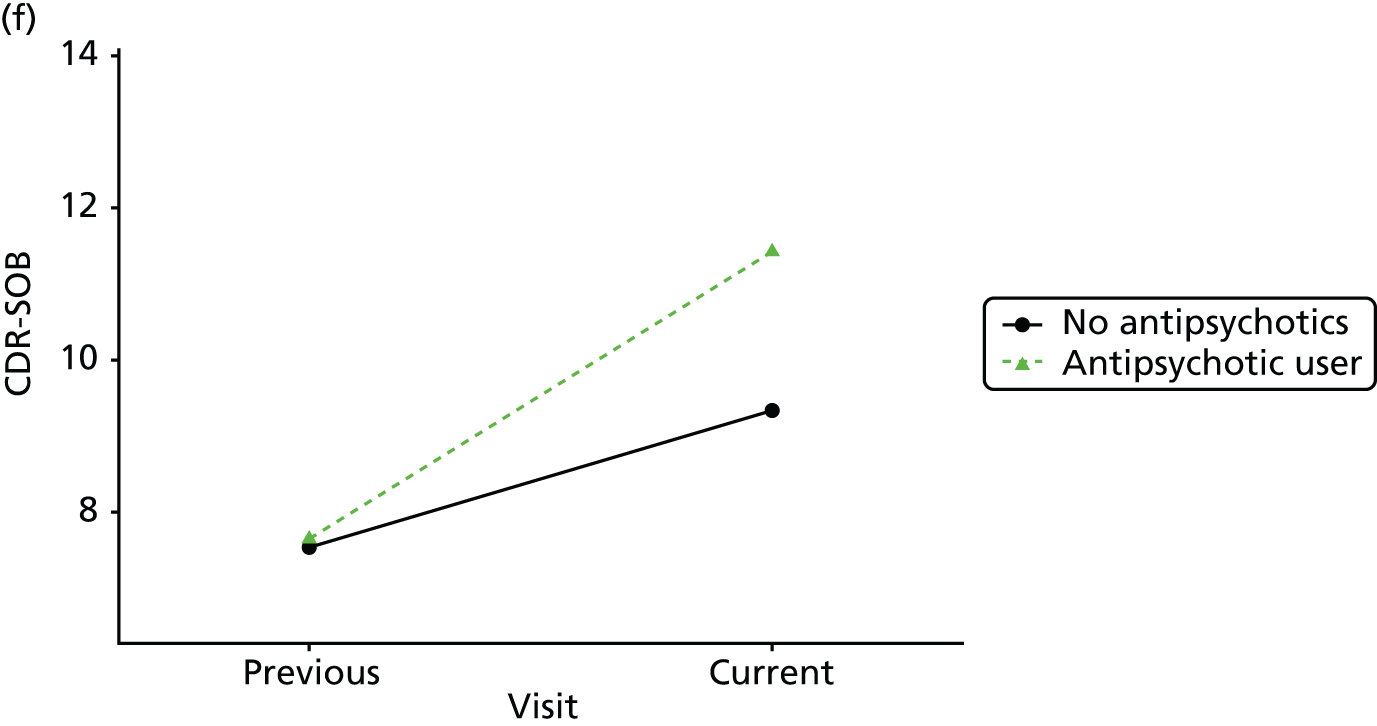
FIGURE 41.
Association between changing hypnotic use status between previous and current visit on delta trail time scores (higher scores represent more cognitive impairment) at previous and current visit (a–c). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (d–f). (a) Z-drug; (b) BZD; and (c) antipsychotics. Data from the NACC data set.
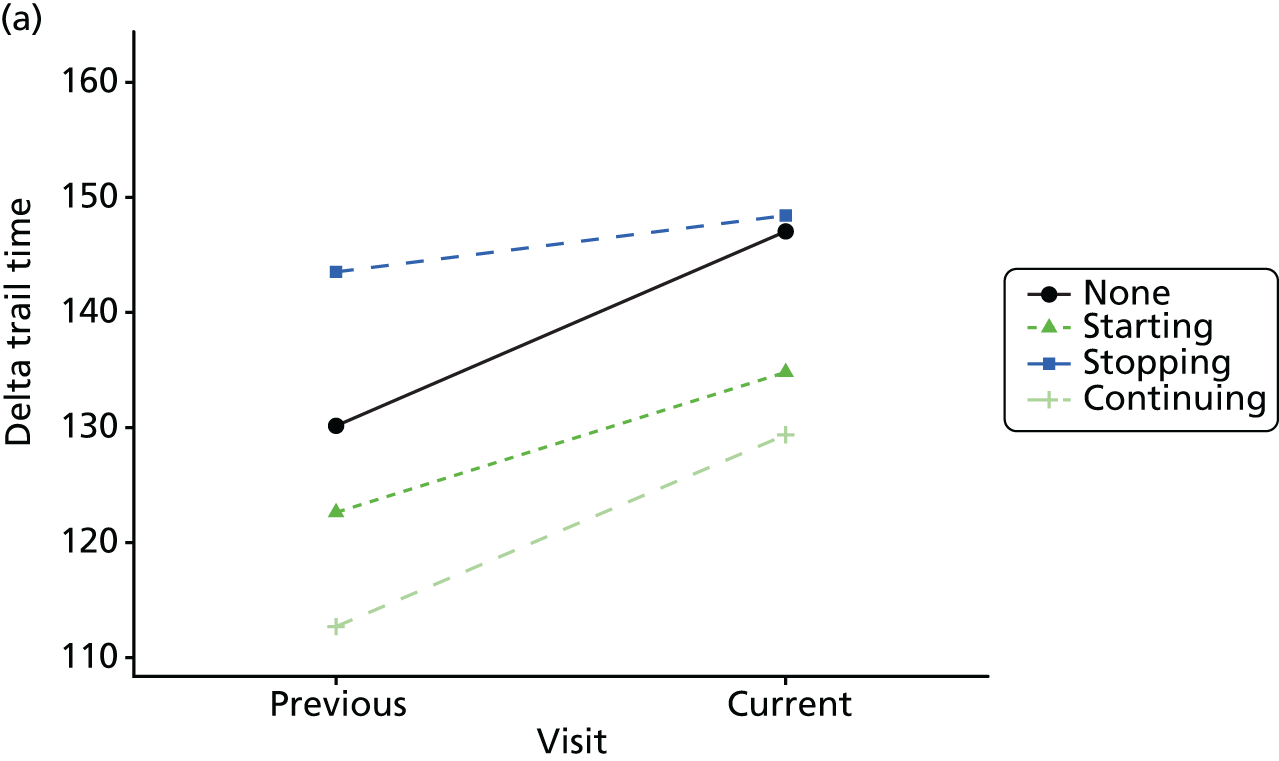
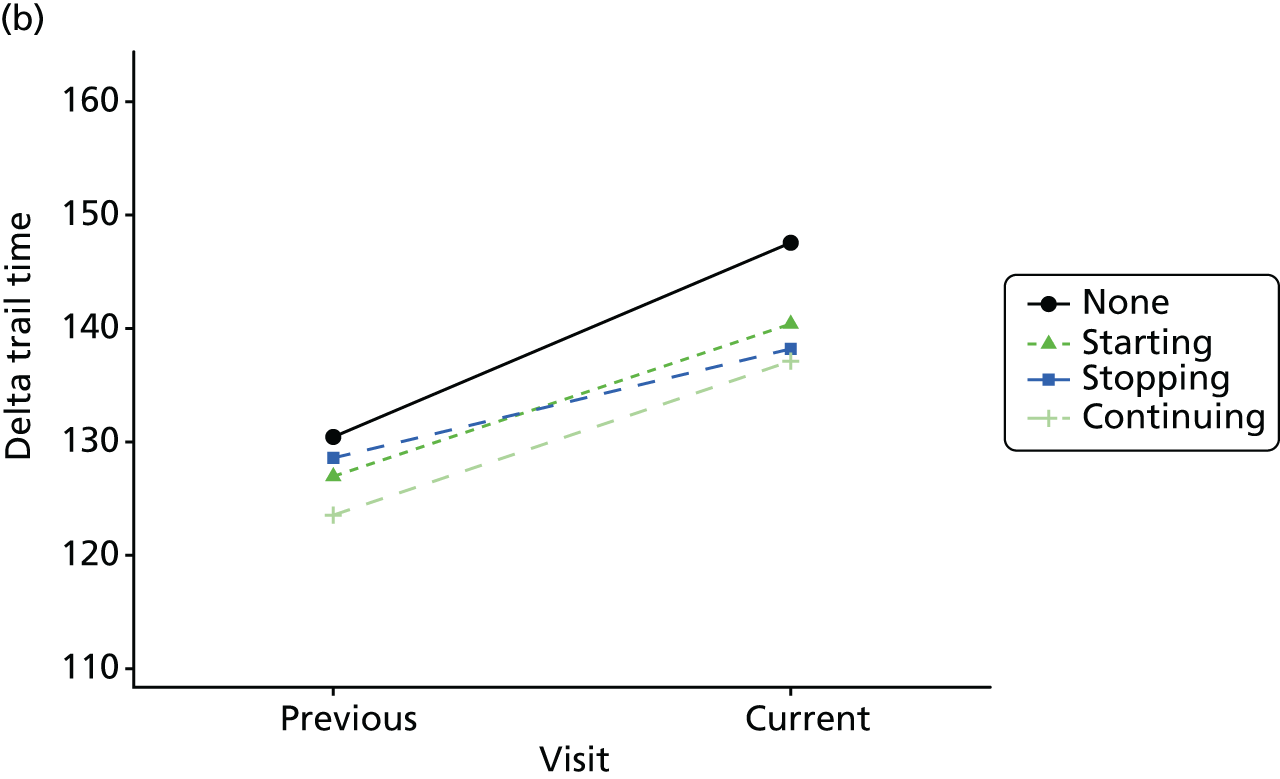
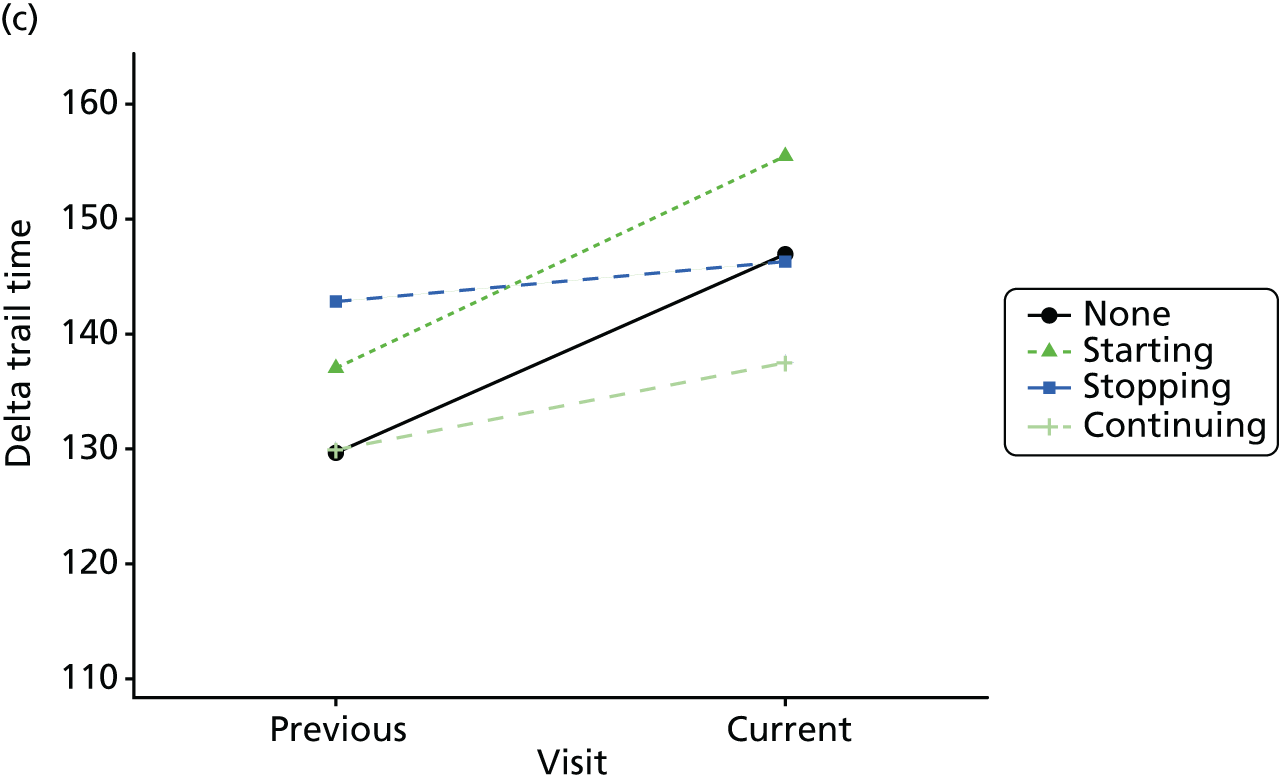
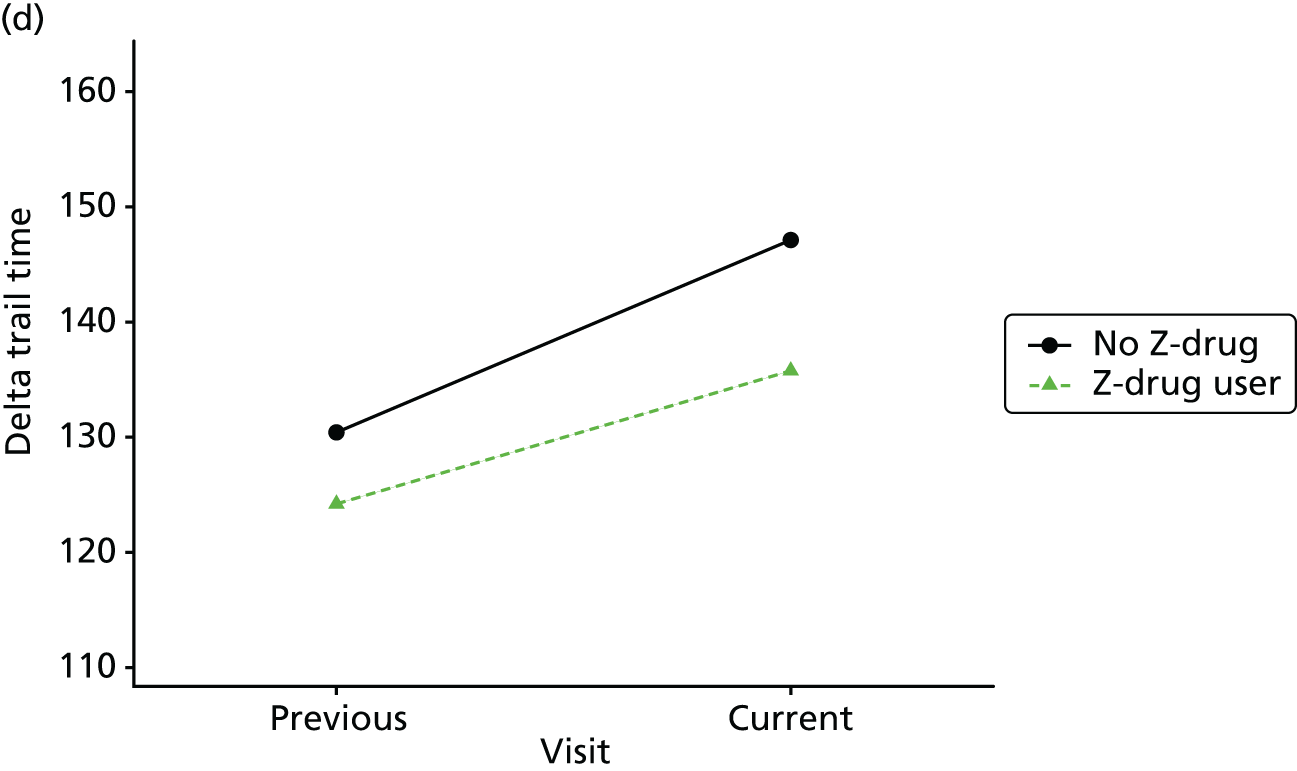
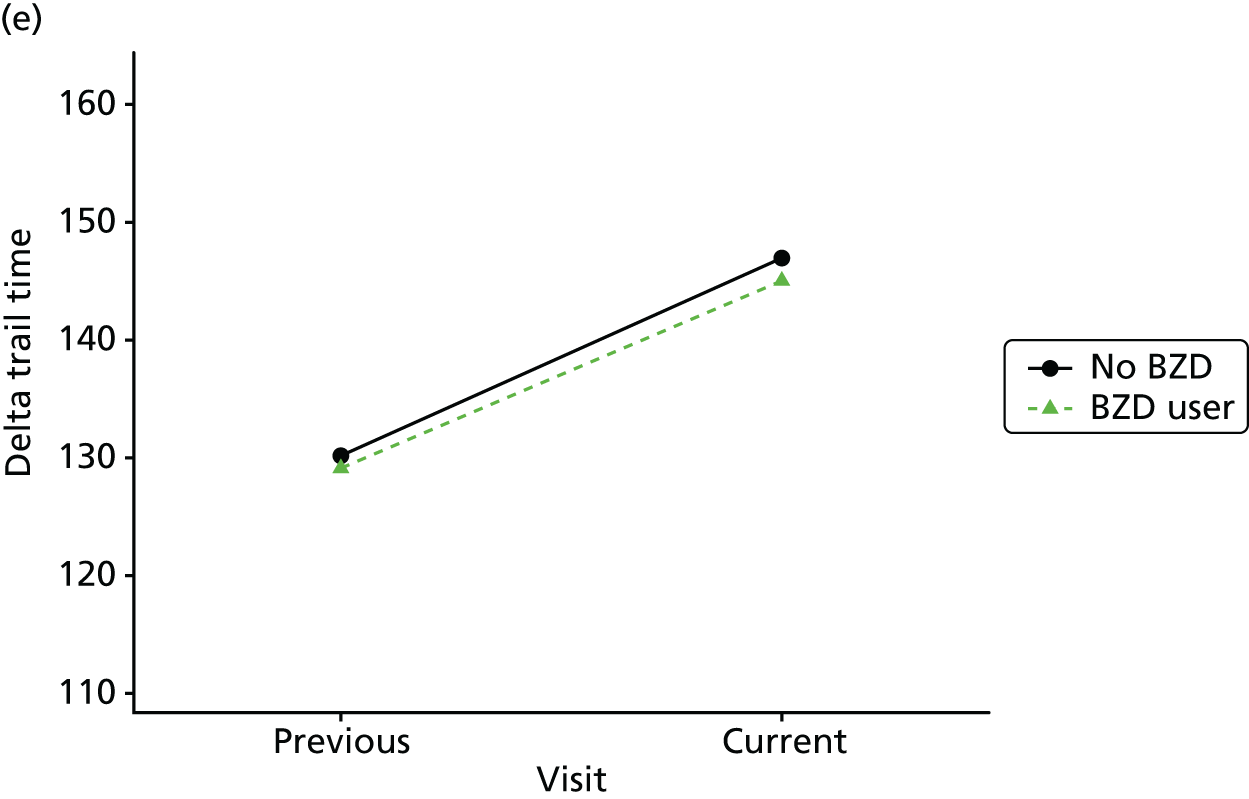
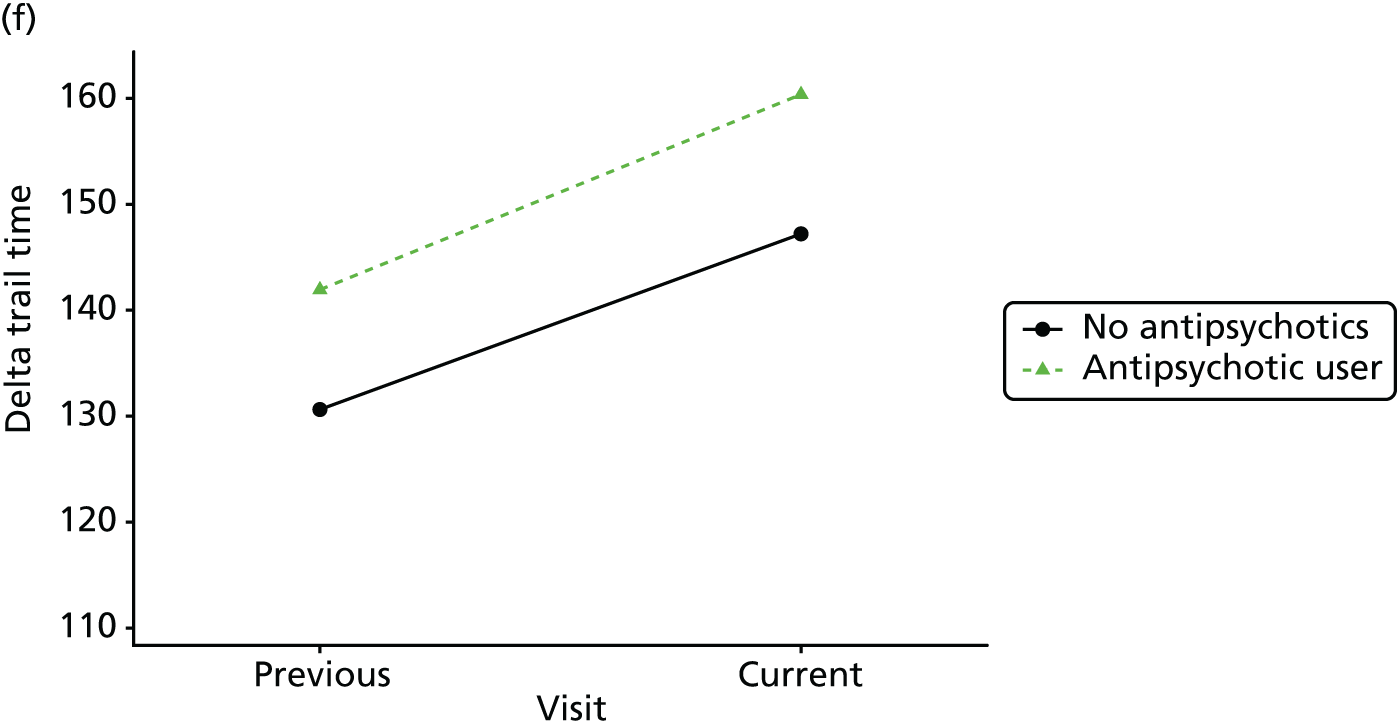
FIGURE 42.
Association between changing hypnotic use status between previous and current visit on disability scores (higher scores represent more disability) at previous and current visit (a–c). Mean scores stratified by current hypnotic use, with inverse probability weights used to account for differences in prior predictors of hypnotic use (d–f). (a) Z-drug; (b) BZD; and (c) antipsychotics. Data from the NACC data set.
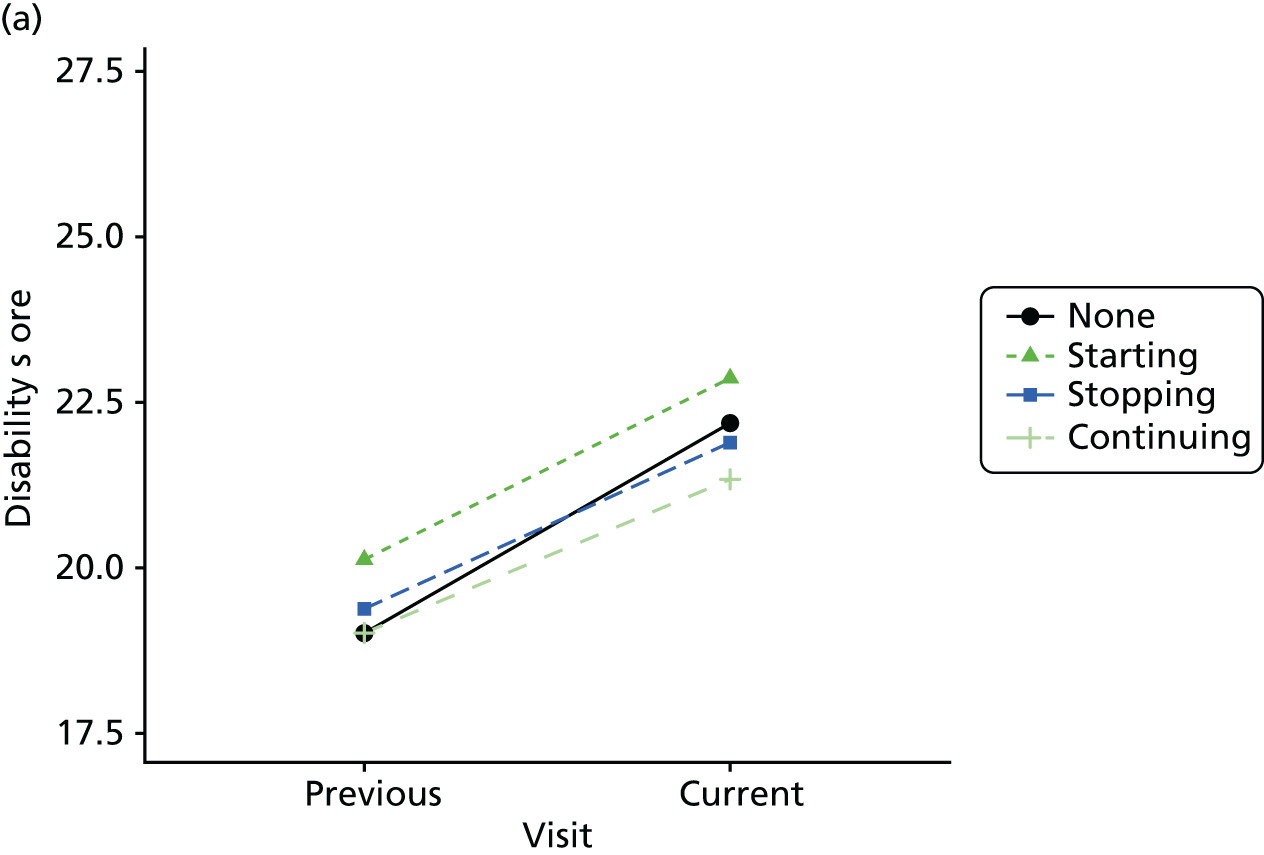
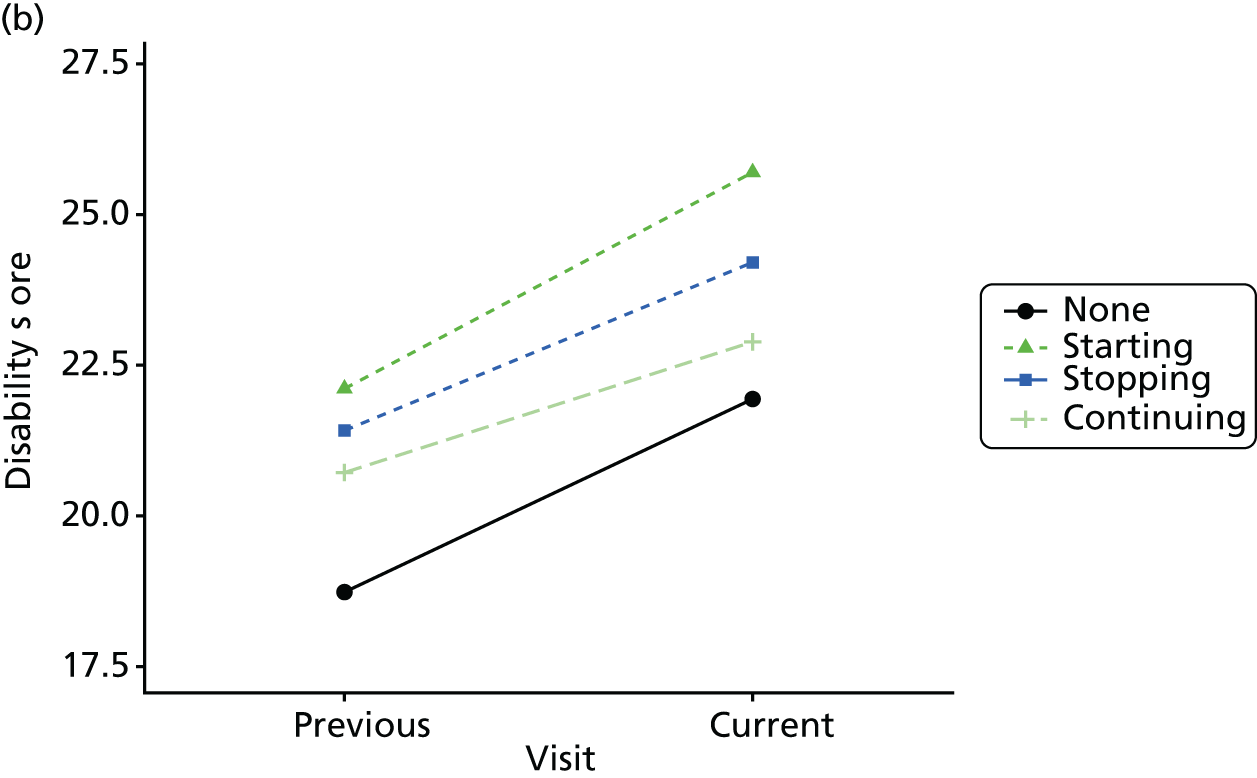
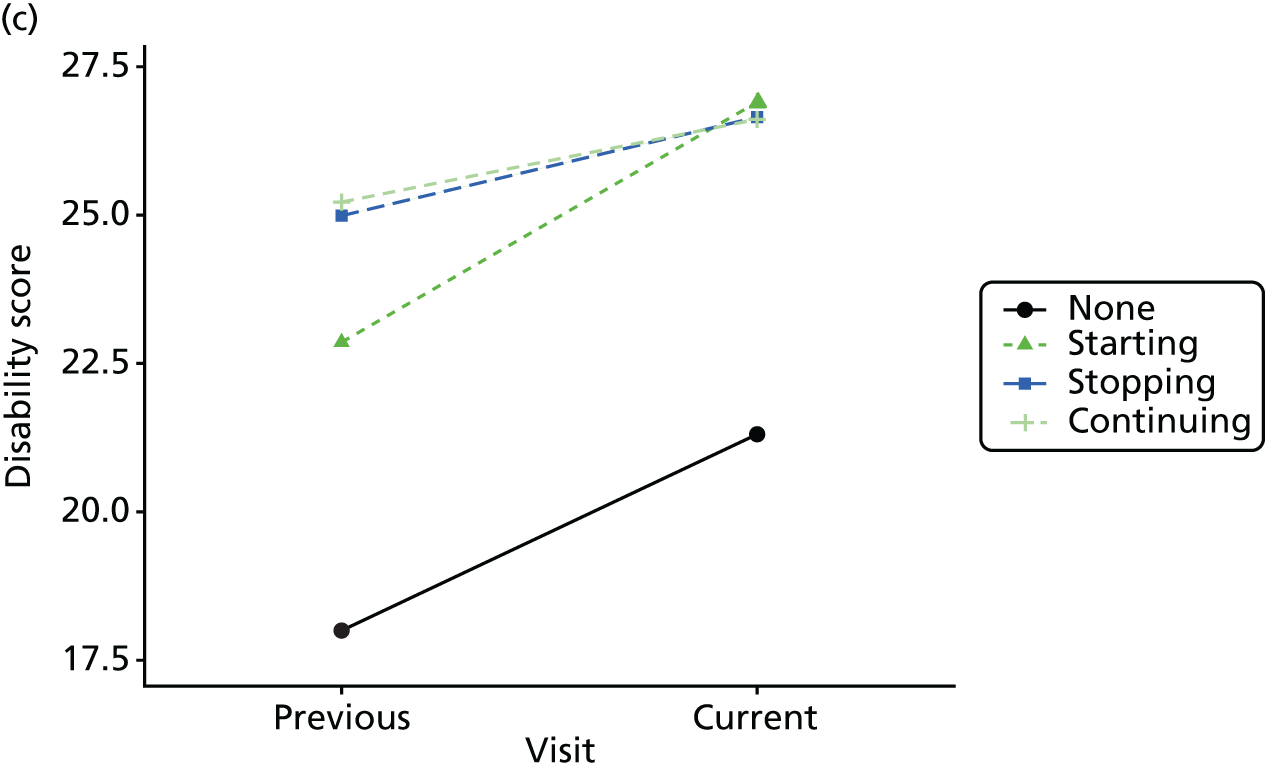
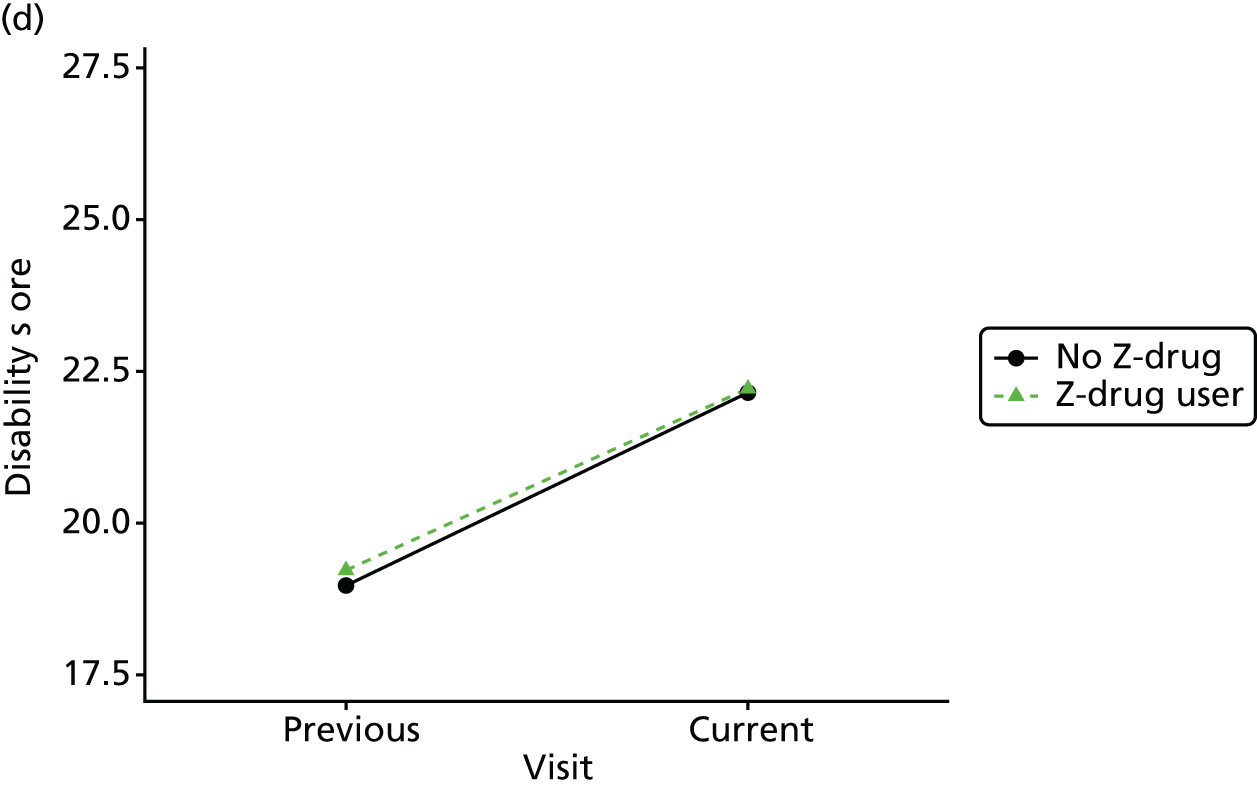
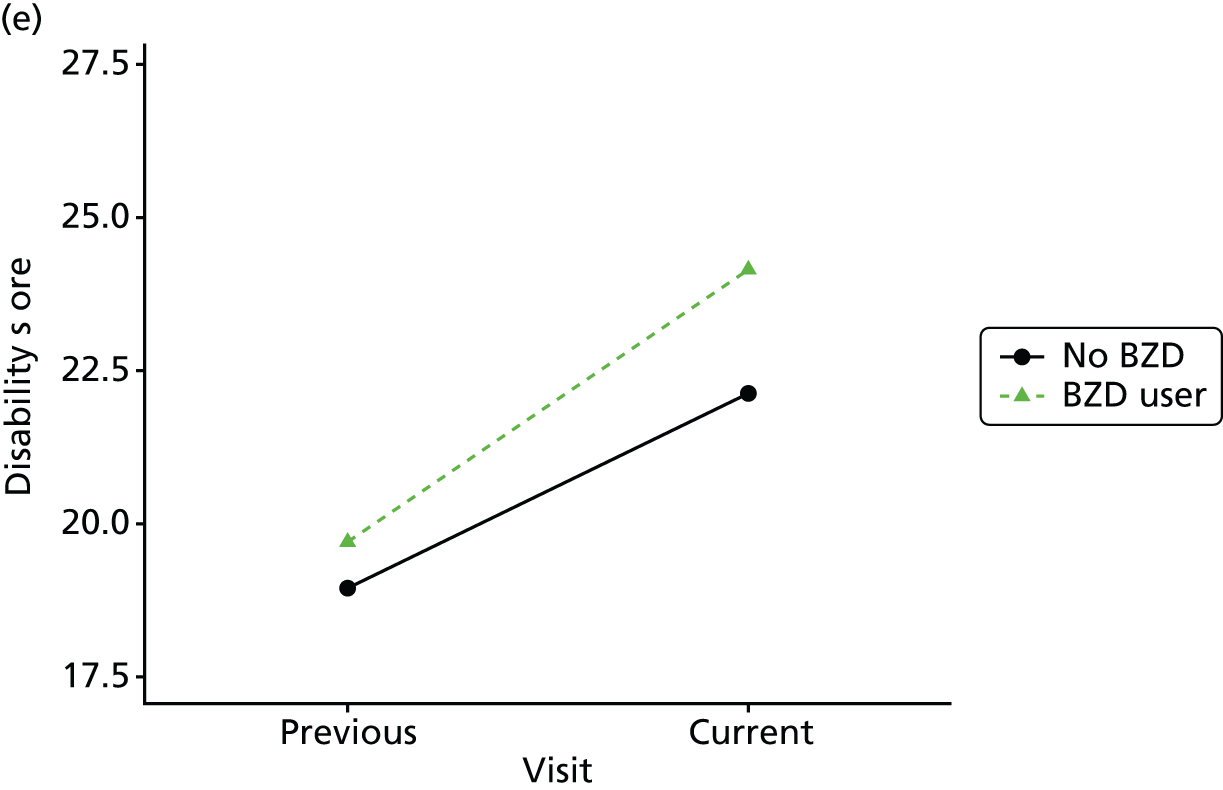
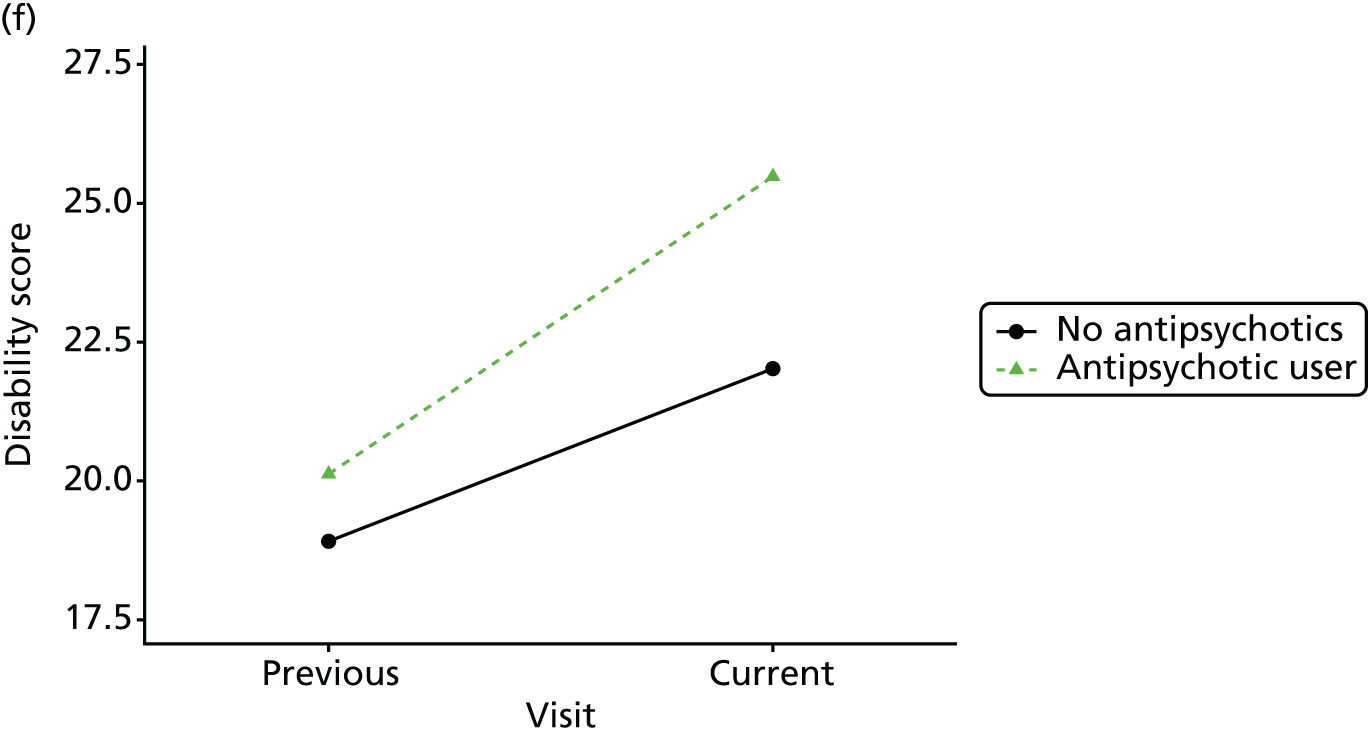
| Subgroup and medication | Outcome, β (95% CI) | |||
|---|---|---|---|---|
| CDR-SOB | MMSE | Animal naming | Delta trail time | |
| All participants | ||||
| Z-drugs | –0.02 (–0.29 to 0.26) | 0.02 (–0.54 to 0.59) | –0.15 (–0.58 to 0.29) | 2.25 (–7.96 to 12.45) |
| BZDs | 1.01*** (0.86 to 1.15) | –1.43*** (–1.77 to –1.08) | –0.65*** (–0.91 to –0.39) | 4.44 (–2.22 to 11.09) |
| Antipsychotics | 1.87*** (1.74 to 1.99) | –1.98*** (–2.27 to –1.69) | –1.19*** (–1.42 to –0.95) | 3.00 (–3.64 to 9.64) |
| New users only | ||||
| Z-drugs | –0.30 (–0.86 to 0.27) | 0.79 (–0.34 to 1.93) | –0.10 (–0.99 to 0.79) | 5.02 (–17.18 to 27.23) |
| BZDs | 1.46*** (1.20 to 1.72) | –1.88*** (–2.52 to –1.24) | –0.58* (–1.09 to –0.07) | –8.75 (–23.25 to 5.75) |
| Antipsychotics | 2.17*** (1.97 to 2.37) | –2.58*** (–3.04 to –2.11) | –1.21*** (–1.58 to –0.84) | –4.14 (–16.02 to 7.74) |
| Mild/minimal dementia | ||||
| Z-drugs | 0.04 (–0.29 to 0.37) | 0.15 (–0.50 to 0.81) | –0.08 (–0.55 to 0.40) | –0.44 (–11.03 to 10.16) |
| BZDs | 1.11*** (0.94 to 1.28) | –1.55*** (–1.94 to –1.16) | –0.72*** (–1.00 to –0.43) | 1.81 (–5.17 to 8.78) |
| Antipsychotics | 2.12*** (1.97 to 2.27) | –2.44*** (–2.78 to –2.09) | –1.27*** (–1.54 to –1.01) | 1.78 (–5.21 to 8.78) |
| Subgroup and medication | Outcome, β (95% CI) | |||
|---|---|---|---|---|
| Sleep | Agitation | Anxiety | NPI excluding sleep | |
| All participants | ||||
| Z-drugs | 0.18*** (0.09 to 0.27) | 0.08 (–0.003 to 0.17) | 0.04 (–0.05 to 0.12) | 0.25 (–0.15 to 0.65) |
| BZDs | 0.08*** (0.03 to 0.13) | 0.15*** (0.10 to 0.19) | 0.11*** (0.06 to 0.15) | 0.88*** (0.66 to 1.09) |
| Antipsychotics | 0.10*** (0.06 to 0.14) | 0.25*** (0.21 to 0.29) | 0.10*** (0.06 to 0.14) | 1.28*** (1.10 to 1.46) |
| New users only | ||||
| Z-drugs | 0.28** (0.10 to 0.45) | 0.31*** (0.14 to 0.48) | 0.08 (–0.09 to 0.24) | 0.74 (–0.05 to 1.53) |
| BZDs | 0.12** (0.04 to 0.20) | 0.22*** (0.15 to 0.30) | 0.15*** (0.07 to 0.23) | 1.34*** (0.97 to 1.71) |
| Antipsychotics | 0.12*** (0.06 to 0.19) | 0.30*** (0.24 to 0.36) | 0.07* (0.01 to 0.13) | 1.56*** (1.28 to 1.84) |
| Mild/minimal dementia at baseline | ||||
| Z-drugs | 0.16** (0.06 to 0.26) | 0.04 (–0.05 to 0.14) | 0.03 (–0.07 to 0.13) | 0.17 (–0.27 to 0.61) |
| BZDs | 0.10*** (0.04 to 0.15) | 0.10*** (0.05 to 0.15) | 0.10*** (0.05 to 0.15) | 0.83*** (0.59 to 1.06) |
| Antipsychotics | 0.12*** (0.08 to 0.17) | 0.26*** (0.21 to 0.30) | 0.10*** (0.05 to 0.14) | 1.36*** (1.16 to 1.57) |
| Subgroup and medication | Outcome, β (95% CI) | |
|---|---|---|
| Disability | Depression | |
| All participants | ||
| Z-drugs | 0.09 (–0.43 to 0.61) | –0.13 (–0.39 to 0.13) |
| BZDs | 0.68*** (0.40 to 0.96) | 0.16 (–0.01 to 0.32) |
| Antipsychotics | 1.58*** (1.35 to 1.82) | 0.14 (–0.01 to 0.28) |
| New users only (no Z-drug, BZD or antipsychotic use at baseline) | ||
| Z-drugs | –0.12 (–1.20 to 0.97) | –0.44 (–0.93 to 0.06) |
| BZDs | 0.89*** (0.39 to 1.40) | –0.13 (–0.42 to 0.16) |
| Antipsychotics | 1.37*** (0.98 to 1.75) | 0.44*** (0.22 to 0.66) |
| Mild/minimal dementia at baseline | ||
| Z-drugs | 0.17 (–0.44 to 0.79) | –0.07 (–0.35 to 0.20) |
| BZDs | 0.85*** (0.53 to 1.18) | 0.13 (–0.04 to 0.30) |
| Antipsychotics | 1.73*** (1.44 to 2.01) | 0.18* (0.02 to 0.34) |
Appendix 5 Additional primary care study analyses
Further primary care study results tables, as referred to in the text (Tables 55–64).
| Outcome and sleep drug | Patients (n) | Incidence/100 PYs | Age, sex adjusted | Fully adjusteda | |||
|---|---|---|---|---|---|---|---|
| Exposed | With outcome | HR | 95% CI | HR | 95% CI | ||
| Fracture | |||||||
| Z-drug | 2997 | 135 | 11.3 | 1.00 | 1.00 | ||
| Low-dose TCA | 1913 | 105 | 10.2 | 0.94 | 0.73 to 1.22 | 0.91 | 0.64 to 1.23 |
| BZD | 433 | 20 | 12.0 | 1.05 | 0.66 to 1.68 | 0.92 | 0.53 to 1.62 |
| Hip fracture | |||||||
| Z-drug | 2997 | 66 | 5.4 | 1.00 | 1.00 | ||
| Low-dose TCA | 1913 | 56 | 5.3 | 1.05 | 0.73 to 1.51 | 1.09 | 0.70 to 1.70 |
| BZD | 433 | 12 | 7.1 | 1.30 | 0.71 to 2.41 | 1.16 | 0.51 to 2.65 |
| Forearm fracture | |||||||
| Z-drug | 2997 | 27 | 2.2 | 1.00 | 1.00 | ||
| Low-dose TCA | 1913 | 16 | 1.5 | 0.71 | 0.37 to 1.36 | 0.89 | 0.43 to 1.82 |
| BZD | 433 | < 5 | N/A | N/A | |||
| Fall | |||||||
| Z-drug | 2888 | 399 | 37.8 | 1.00 | 1.00 | ||
| Low-dose TCA | 1864 | 286 | 30.4 | 0.90 | 0.77 to 1.05 | 0.86 | 0.73 to 1.02 |
| BZD | 412 | 65 | 43.9 | 1.16 | 0.89 to 1.50 | 1.15 | 0.87 to 1.54 |
| Mortality | |||||||
| Z-drug | 3089 | 355 | 27.8 | 1.00 | 1.00 | ||
| Low-dose TCA | 1942 | 196 | 18.1 | 0.74 | 0.62 to 0.88 | 0.75 | 0.62 to 0.92 |
| BZD | 443 | 66 | 36.7 | 1.31 | 1.00 to 1.73 | 1.22 | 0.90 to 1.65 |
| Acute bacterial infection | |||||||
| Z-drug | 2267 | 408 | 47.7 | 1.00 | 1.00 | ||
| Low-dose TCA | 1423 | 273 | 37.8 | 0.87 | 0.75 to 1.02 | 0.91 | 0.76 to 1.08 |
| BZD | 334 | 49 | 38.6 | 0.81 | 0.60 to 1.09 | 0.85 | 0.62 to 1.17 |
| UTI or acute LRTI | |||||||
| Z-drug | 2267 | 354 | 40.2 | 1.00 | 1.00 | ||
| Low-dose TCA | 1423 | 226 | 31.3 | 0.85 | 0.71 to 1.00 | 0.89 | 0.74 to 1.07 |
| BZD | 334 | 41 | 31.5 | 0.79 | 0.57 to 1.10 | 0.83 | 0.58 to 1.19 |
| Ischaemic stroke/TIA | |||||||
| Z-drug | 3045 | 80 | 6.5 | 1.00 | 1.00 | ||
| Low-dose TCA | 1933 | 50 | 4.7 | 0.77 | 0.54 to 1.11 | 0.74 | 0.49 to 1.12 |
| BZD | 438 | 15 | 8.7 | 1.35 | 0.78 to 2.34 | 1.50 | 0.77 to 2.91 |
| Venous thromboembolism | |||||||
| Z-drug | 3074 | 26 | 2.1 | 1.00 | 1.00 | ||
| Low-dose TCA | 1940 | 24 | 2.2 | 1.16 | 0.66 to 2.05 | 1.02 | 0.50 to 2.06 |
| BZD | 442 | < 5 | N/A | N/A | |||
| Incident agitation/psychosis | |||||||
| Z-drug | 2574 | 140 | 13.6 | 1.00 | 1.00 | ||
| Low-dose TCA | 1633 | 85 | 9.7 | 0.78 | 0.60 to 1.02 | 0.84 | 0.63 to 1.13 |
| BZD | 313 | 36 | 30.5 | 2.19 | 1.53 to 3.13 | 1.86 | 1.21 to 2.87 |
| Outcome and sleep medication | Age parametrisation | |||
|---|---|---|---|---|
| Restricted cubic splines | Fractional polynomials | |||
| HRa | 95% CI | HRa | 95% CI | |
| Fracture | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.12 | 0.80 to 1.58 | 1.12 | 0.80 to 1.58 |
| BZD | 1.37 | 0.70 to 2.66 | 1.34 | 0.69 to 2.61 |
| Z-drug | 1.42 | 1.02 to 1.98 | 1.42 | 1.02 to 1.97 |
| Hip fracture | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.54 | 0.95 to 2.50 | 1.54 | 0.95 to 2.49 |
| BZD | 2.10 | 0.70 to 6.27 | 2.11 | 0.72 to 6.12 |
| Z-drug | 1.61 | 1.01 to 2.56 | 1.60 | 1.00 to 2.55 |
| Forearm/wrist/hand fracture | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.03 | 0.36 to 2.96 | 0.99 | 0.35 to 2.80 |
| Z-drug | 1.37 | 0.58 to 3.25 | 1.32 | 0.55 to 3.17 |
| Fall | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.84 | 0.69 to 1.02 | 0.84 | 0.69 to 1.02 |
| BZD | 1.05 | 0.75 to 1.48 | 1.05 | 0.75 to 1.47 |
| Z-drug | 1.05 | 0.87 to 1.25 | 1.05 | 0.87 to 1.25 |
| Mortality | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.89 | 0.72 to 1.11 | 0.89 | 0.72 to 1.11 |
| BZD | 1.39 | 0.98 to 1.99 | 1.38 | 0.97 to 1.97 |
| Z-drug | 1.33 | 1.09 to 1.63 | 1.34 | 1.10 to 1.64 |
| Acute bacterial infection | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.00 | 0.83 to 1.21 | 1.00 | 0.83 to 1.21 |
| BZD | 0.84 | 0.59 to 1.21 | 0.84 | 0.59 to 1.21 |
| Z-drug | 1.09 | 0.92 to 1.29 | 1.09 | 0.92 to 1.29 |
| UTI or acute LRTI | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.97 | 0.79 to 1.19 | 0.96 | 0.78 to 1.18 |
| BZD | 0.86 | 0.58 to 1.28 | 0.85 | 0.58 to 1.26 |
| Z-drug | 1.10 | 0.92 to 1.32 | 1.10 | 0.92 to 1.32 |
| Ischaemic stroke/TIA | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.24 | 0.78 to 1.99 | 1.21 | 0.76 to 1.93 |
| BZD | 1.56 | 0.70 to 3.46 | 1.56 | 0.69 to 3.52 |
| Z-drug | 1.35 | 0.86 to 2.10 | 1.33 | 0.85 to 2.08 |
| Venous thromboembolism | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.23 | 0.56 to 2.69 | 1.26 | 0.57 to 2.76 |
| Z-drug | 1.71 | 0.69 to 4.24 | 1.68 | 0.70 to 4.05 |
| Incident agitation/psychosis | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.43 | 0.96 to 2.13 | 1.43 | 0.96 to 2.13 |
| BZD | 5.66 | 3.13 to 10.26 | 5.60 | 3.15 to 9.98 |
| Z-drug | 1.72 | 1.21 to 2.44 | 1.71 | 1.21 to 2.42 |
| Outcome and sleep medication | Patients (n) | Age, sex adjusted | Fully adjusteda | Test for equality | |||
|---|---|---|---|---|---|---|---|
| Exposed | With outcome | HR | 95% CI | HR | 95% CI | ||
| Fracture | |||||||
| No sleep drug | 1636 | 108 | 1.00 | 1.00 | 0.88 | ||
| Z-drug PRN | 488 | 24 | 1.62 | 1.03 to 2.56 | 1.47 | 0.86 to 2.50 | |
| Z-drug not PRN | 1848 | 79 | 1.36 | 1.00 to 1.86 | 1.41 | 0.98 to 2.03 | |
| Hip fracture | |||||||
| No sleep drug | 1636 | 47 | 1.00 | 1.00 | 0.45 | ||
| Z-drug PRN | 488 | 13 | 1.98 | 1.05 to 3.73 | 2.18 | 1.00 to 4.75 | |
| Z-drug not PRN | 1848 | 38 | 1.48 | 0.93 to 2.33 | 1.63 | 0.96 to 2.77 | |
| Forearm fracture | |||||||
| No sleep drug | 1636 | 18 | 1.00 | 1.00 | 0.36 | ||
| Z-drug PRN | 488 | < 5 | 1.34 | 0.39 to 4.63 | 1.16 | 0.24 to 5.64 | |
| Z-drug not PRN | 1848 | 22 | 2.49 | 1.24 to 4.99 | 2.42 | 0.92 to 6.31 | |
| Fall | |||||||
| No sleep drug | 1506 | 328 | 1.00 | 1.00 | 0.65 | ||
| Z-drug PRN | 461 | 62 | 1.19 | 0.90 to 1.57 | 1.10 | 0.82 to 1.48 | |
| Z-drug not PRN | 1786 | 240 | 1.11 | 0.94 to 1.33 | 1.03 | 0.84 to 1.26 | |
| Mortality | |||||||
| No sleep drug | 1651 | 266 | 1.00 | 1.00 | 0.57 | ||
| Z-drug PRN | 499 | 51 | 1.47 | 1.21 to 1.79 | 1.31 | 1.06 to 1.63 | |
| Z-drug not PRN | 1894 | 184 | 1.33 | 1.18 to 1.51 | 1.24 | 1.08 to 1.42 | |
| Acute bacterial infection | |||||||
| No sleep drug | 1303 | 374 | 1.00 | 1.00 | 0.48 | ||
| Z-drug PRN | 354 | 61 | 1.23 | 0.93 to 1.62 | 1.15 | 0.85 to 1.56 | |
| Z-drug not PRN | 1429 | 235 | 1.10 | 0.92 to 1.30 | 1.03 | 0.85 to 1.24 | |
| UTI or acute LRTI | |||||||
| No sleep drug | 1303 | 328 | 1.00 | 1.00 | 0.45 | ||
| Z-drug PRN | 354 | 53 | 1.24 | 0.92 to 1.66 | 1.17 | 0.84 to 1.62 | |
| Z-drug not PRN | 1429 | 199 | 1.08 | 0.90 to 1.30 | 1.03 | 0.84 to 1.26 | |
| Ischaemic stroke/TIA | |||||||
| No sleep drug | 1640 | 64 | 1.00 | 1.00 | 0.62 | ||
| Z-drug PRN | 487 | 12 | 1.53 | 0.81 to 2.89 | 1.76 | 0.85 to 3.66 | |
| Z-drug not PRN | 1877 | 50 | 1.48 | 1.00 to 2.19 | 1.46 | 0.86 to 2.46 | |
| Venous thromboembolism | |||||||
| No sleep drug | 1648 | 22 | 1.00 | 1.00 | 0.58 | ||
| Z-drug PRN | 498 | < 5 | 1.24 | 0.36 to 4.20 | 1.44 | 0.29 to 7.02 | |
| Z-drug not PRN | 1889 | 18 | 1.69 | 0.91 to 3.16 | 2.18 | 0.81 to 5.85 | |
| Incident agitation/psychosis | |||||||
| No sleep drug | 1282 | 79 | 1.00 | 1.00 | 0.06 | ||
| Z-drug PRN | 408 | 13 | 1.01 | 0.56 to 1.82 | 0.92 | 0.47 to 1.81 | |
| Z-drug not PRN | 1576 | 90 | 1.69 | 1.24 to 2.31 | 1.80 | 1.23 to 2.63 | |
| Outcome and sleep drug | Patients exposed (n) | Number of outcomes | Incidence/100 PYs | Age, sex adjusted | Fully adjusteda | ||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| Additional sleep medication | |||||||
| Z-drug | 3089 | 485 | 38.0 | 1.00 | 1.00 | ||
| Low-dose TCA | 1942 | 258 | 23.8 | 0.69 | 0.59 to 0.80 | 0.65 | 0.55 to 0.77 |
| BZD | 443 | 45 | 25.0 | 0.66 | 0.48 to 0.89 | 0.59 | 0.42 to 0.83 |
| Incident antipsychotic prescription | |||||||
| Z-drug | 2402 | 355 | 39.5 | 1.00 | 1.00 | ||
| Low-dose TCA | 1618 | 164 | 19.1 | 0.55 | 0.46 to 0.67 | 0.56 | 0.46 to 0.70 |
| BZD | 375 | 66 | 59.5 | 1.25 | 0.95 to 1.64 | 1.12 | 0.84 to 1.51 |
| Incident antidepressant prescription | |||||||
| Z-drug | 2177 | 236 | 28.3 | 1.00 | 1.00 | ||
| BZD | 326 | 31 | 24.4 | 0.86 | 0.59 to 1.26 | 1.09 | 0.71 to 1.68 |
| Incident antibiotic prescription | |||||||
| Z-drug | 2430 | 818 | 116.4 | 1.00 | 1.00 | ||
| Low-dose TCA | 1552 | 562 | 93.8 | 0.88 | 0.97 to 1.21 | 0.87 | 0.77 to 0.98 |
| BZD | 357 | 116 | 120.8 | 1.00 | 0.82 to 1.23 | 1.04 | 0.83 to 1.30 |
| Number of GP visitsb | |||||||
| Z-drug | 3089 | 47,259 | 922.5 | 1.00 | 1.00 | ||
| Low-dose TCA | 1942 | 33,530 | 882.1 | 0.97 | 0.92 to 1.02 | 0.91 | 0.87 to 0.95 |
| BZD | 443 | 6806 | 791.4 | 0.91 | 0.82 to 1.00 | 0.98 | 0.90 to 1.06 |
| Number of hospital admissionsb | |||||||
| Z-drug | 3089 | 4836 | 112.3 | 1.00 | 1.00 | ||
| Low-dose TCA | 1942 | 2991 | 105.2 | 0.94 | 0.85 to 1.03 | 0.96 | 0.88 to 1.05 |
| BZD | 443 | 614 | 98.9 | 0.88 | 0.76 to 1.02 | 0.92 | 0.79 to 1.06 |
| Outcome and sleep medication | Approach to missing BMI and care home data | |||
|---|---|---|---|---|
| Missing category | Imputation | |||
| HRa | 95% CI | HRa | 95% CI | |
| Fracture | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.12 | 0.80 to 1.58 | 1.08 | 0.77 to 1.52 |
| BZD | 1.34 | 0.69 to 2.61 | 1.38 | 0.72 to 2.65 |
| Z-drug | 1.40 | 1.01 to 1.94 | 1.39 | 1.00 to 1.93 |
| Hip fracture | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.53 | 0.95 to 2.48 | 1.46 | 0.91 to 2.36 |
| BZD | 2.07 | 0.72 to 5.97 | 1.97 | 0.68 to 5.67 |
| Z-drug | 1.59 | 1.00 to 2.53 | 1.56 | 0.98 to 2.48 |
| Forearm fracture | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.99 | 0.35 to 2.79 | 0.96 | 0.34 to 2.73 |
| Z-drug | 1.29 | 0.53 to 3.15 | 1.29 | 0.54 to 3.09 |
| Fall | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.84 | 0.69 to 1.02 | 0.83 | 0.68 to 1.00 |
| BZD | 1.05 | 0.75 to 1.47 | 1.04 | 0.74 to 1.45 |
| Z-drug | 1.05 | 0.87 to 1.25 | 1.04 | 0.87 to 1.24 |
| Mortality | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.89 | 0.72 to 1.11 | 0.92 | 0.74 to 1.14 |
| BZD | 1.38 | 0.96 to 1.96 | 1.46 | 1.04 to 2.05 |
| Z-drug | 1.34 | 1.10 to 1.64 | 1.35 | 1.11 to 1.65 |
| Acute bacterial infection | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.00 | 0.83 to 1.21 | 1.00 | 0.83 to 1.21 |
| BZD | 0.84 | 0.59 to 1.21 | 0.86 | 0.60 to 1.22 |
| Z-drug | 1.09 | 0.92 to 1.29 | 1.09 | 0.93 to 1.29 |
| UTI or acute LRTI | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.96 | 0.78 to 1.18 | 0.96 | 0.79 to 1.18 |
| BZD | 0.85 | 0.58 to 1.26 | 0.86 | 0.58 to 1.27 |
| Z-drug | 1.10 | 0.92 to 1.32 | 1.10 | 0.92 to 1.32 |
| Ischaemic stroke/TIA | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.20 | 0.75 to 1.92 | 1.19 | 0.74 to 1.91 |
| BZD | 1.56 | 0.69 to 3.51 | 1.54 | 0.67 to 3.54 |
| Z-drug | 1.33 | 0.85 to 2.07 | 1.33 | 0.85 to 2.08 |
| Venous thromboembolism | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.23 | 0.56 to 2.69 | 1.27 | 0.59 to 2.72 |
| Z-drug | 1.66 | 0.69 to 3.98 | 1.67 | 0.67 to 4.13 |
| Incident agitation/psychosis | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.43 | 0.96 to 2.13 | 1.39 | 0.93 to 2.06 |
| BZD | 5.61 | 3.14 to 10.01 | 5.38 | 2.99 to 9.68 |
| Z-drug | 1.71 | 1.21 to 2.42 | 1.71 | 1.22 to 2.42 |
| Outcome and sleep drug | Approach to missing BMI and care home data | |||
|---|---|---|---|---|
| Missing category | Imputation | |||
| HRa | 95% CI | HRa | 95% CI | |
| Additional sleep medication | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.79 | 0.65 to 0.97 | 0.79 | 0.65 to 0.96 |
| BZD | 0.63 | 0.43 to 0.93 | 0.63 | 0.43 to 0.92 |
| Z-drug | 1.13 | 0.95 to 1.35 | 1.14 | 0.95 to 1.36 |
| Incident antipsychotic prescription | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.17 | 0.90 to 1.53 | 1.17 | 0.90 to 1.53 |
| BZD | 2.23 | 1.50 to 3.32 | 2.19 | 1.47 to 3.27 |
| Z-drug | 2.01 | 1.60 to 2.52 | 2.00 | 1.59 to 2.51 |
| Incident antidepressant prescription | ||||
| No sleep drug | 1.00 | 1.00 | ||
| BZD | 1.77 | 1.02 to 3.09 | 1.73 | 1.00 to 2.98 |
| Z-drug | 2.05 | 1.56 to 2.68 | 2.05 | 1.56 to 2.68 |
| Incident antibiotic prescription | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.13 | 0.99 to 1.29 | 1.14 | 0.99 to 1.30 |
| BZD | 1.35 | 1.06 to 1.71 | 1.36 | 1.07 to 1.72 |
| Z-drug | 1.26 | 1.12 to 1.42 | 1.26 | 1.12 to 1.43 |
| Number of GP visitsb | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.01 | 0.96 to 1.07 | 1.01 | 0.96 to 1.07 |
| BZD | 1.05 | 0.97 to 1.14 | 1.05 | 0.97 to 1.14 |
| Z-drug | 1.14 | 1.08 to 1.19 | 1.14 | 1.09 to 1.20 |
| Number of hospital admissionsb | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.04 | 0.95 to 1.15 | 1.03 | 0.94 to 1.14 |
| BZD | 0.97 | 0.84 to 1.13 | 0.95 | 0.82 to 1.11 |
| Z-drug | 1.12 | 1.03 to 1.21 | 1.11 | 1.02 to 1.21 |
| Outcome and sleep medication | Age, sex adjusted | Fully adjusteda | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Fracture | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.22 | 0.88 to 1.70 | 1.00 | 0.66 to 1.52 |
| BZD | 1.31 | 0.76 to 2.26 | 1.62 | 0.70 to 3.75 |
| Z-drug | 1.32 | 0.95 to 1.84 | 1.34 | 0.90 to 1.99 |
| Hip fracture | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.35 | 0.85 to 2.14 | 1.17 | 0.64 to 2.14 |
| Z-drug | 1.28 | 0.81 to 2.02 | 1.33 | 0.77 to 2.32 |
| Forearm/wrist/hand fracture | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.86 | 0.39 to 1.89 | 0.94 | 0.30 to 2.90 |
| Z-drug | 1.45 | 0.65 to 3.23 | 1.24 | 0.43 to 3.60 |
| Fall | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.83 | 0.69 to 1.00 | 0.72 | 0.58 to 0.90 |
| BZD | 1.06 | 0.79 to 1.44 | 0.88 | 0.60 to 1.30 |
| Z-drug | 0.90 | 0.75 to 1.07 | 0.87 | 0.71 to 1.07 |
| Mortality | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.87 | 0.70 to 1.08 | 0.78 | 0.61 to 1.01 |
| BZD | 1.51 | 1.09 to 2.08 | 0.98 | 0.64 to 1.52 |
| Z-drug | 1.23 | 1.00 to 1.51 | 1.09 | 0.86 to 1.38 |
| Acute bacterial infection | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.91 | 0.76 to 1.10 | 0.92 | 0.74 to 1.15 |
| BZD | 0.78 | 0.56 to 1.10 | 0.68 | 0.43 to 1.06 |
| Z-drug | 1.06 | 0.89 to 1.27 | 0.98 | 0.80 to 1.19 |
| UTI or acute LRTI | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.86 | 0.70 to 1.05 | 0.86 | 0.68 to 1.09 |
| BZD | 0.82 | 0.57 to 1.17 | 0.76 | 0.46 to 1.23 |
| Z-drug | 1.04 | 0.86 to 1.26 | 0.97 | 0.79 to 1.19 |
| Ischaemic stroke/TIA | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.99 | 0.63 to 1.56 | 1.11 | 0.64 to 1.93 |
| BZD | 1.66 | 0.84 to 3.28 | 1.38 | 0.22 to 8.71 |
| Z-drug | 1.42 | 0.91 to 2.22 | 1.17 | 0.68 to 2.03 |
| Venous thromboembolism | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.16 | 0.62 to 2.18 | 0.93 | 0.39 to 2.17 |
| Z-drug | 1.16 | 0.60 to 2.22 | 1.37 | 0.61 to 3.10 |
| Incident agitation/psychosis | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.89 | 0.63 to 1.26 | 1.04 | 0.68 to 1.58 |
| BZD | 2.64 | 1.65 to 4.21 | 4.11 | 2.15 to 7.84 |
| Z-drug | 1.06 | 0.77 to 1.45 | 1.10 | 0.77 to 1.56 |
| Outcome and sleep drug | Age, sex adjusted | Fully adjusteda | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Additional sleep medication | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.58 | 0.48 to 0.69 | 0.56 | 0.46 to 0.69 |
| BZD | 0.50 | 0.36 to 0.70 | 0.45 | 0.30 to 0.66 |
| Z-drug | 0.82 | 0.70 to 0.96 | 0.80 | 0.67 to 0.96 |
| Incident antipsychotic prescription | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 0.76 | 0.60 to 0.98 | 0.75 | 0.57 to 1.00 |
| BZD | 1.45 | 1.04 to 2.03 | 1.16 | 0.76 to 1.79 |
| Z-drug | 1.39 | 1.11 to 1.74 | 1.34 | 1.05 to 1.73 |
| Incident antidepressant prescription | ||||
| No sleep drug | 1.00 | 1.00 | ||
| BZD | 1.33 | 0.86 to 2.05 | 1.47 | 0.74 to 2.92 |
| Z-drug | 1.47 | 1.12 to 1.93 | 1.69 | 1.25 to 2.30 |
| Incident antibiotic prescription | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.02 | 0.89 to 1.17 | 1.02 | 0.87 to 1.19 |
| BZD | 1.20 | 0.96 to 1.52 | 1.29 | 0.99 to 1.70 |
| Z-drug | 1.17 | 1.03 to 1.34 | 1.13 | 0.98 to 1.30 |
| Number of GP visitsb | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.09 | 1.01 to 1.17 | 0.98 | 0.92 to 1.04 |
| BZD | 1.05 | 0.94 to 1.16 | 1.01 | 0.92 to 1.11 |
| Z-drug | 1.14 | 1.06 to 1.21 | 1.09 | 1.03 to 1.15 |
| Number of hospital admissionsb | ||||
| No sleep drug | 1.00 | 1.00 | ||
| Low-dose TCA | 1.00 | 0.90 to 1.12 | 0.97 | 0.87 to 1.08 |
| BZD | 0.91 | 0.79 to 1.06 | 0.91 | 0.78 to 1.07 |
| Z-drug | 1.07 | 0.97 to 1.18 | 1.04 | 0.95 to 1.14 |
| Outcome and sleep medication | Patients with outcome in CPRD (n) | Per cent of main analysis outcomes | Age, sex adjusted | Fully adjusteda | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Fracture | ||||||
| No sleep drug | 83 | 77 | 1.00 | 1.00 | ||
| Low-dose TCA | 76 | 72 | 1.25 | 0.91 to 1.73 | 1.07 | 0.72 to 1.60 |
| BZD | 13 | 65 | 1.21 | 0.68 to 2.18 | 1.05 | 0.44 to 2.51 |
| Z-drug | 90 | 67 | 1.23 | 0.89 to 1.68 | 1.27 | 0.87 to 1.87 |
| Hip fracture | ||||||
| No sleep drug | 35 | 74 | 1.00 | 1.00 | ||
| Low-dose TCA | 37 | 66 | 1.46 | 0.91 to 2.35 | 1.38 | 0.78 to 2.44 |
| BZD | 7 | 58 | 1.71 | 0.74 to 3.94 | 2.35 | 0.54 to 10.11 |
| Z-drug | 40 | 61 | 1.38 | 0.85 to 2.23 | 1.38 | 0.75 to 2.52 |
| Forearm fracture | ||||||
| No sleep drug | 13 | 72 | 1.00 | 1.00 | ||
| Low-dose TCA | 11 | 69 | 1.04 | 0.45 to 2.41 | 0.87 | 0.22 to 3.51 |
| Z-drug | 20 | 74 | 1.82 | 0.82 to 4.07 | 1.63 | 0.56 to 4.75 |
| Fall | ||||||
| No sleep drug | 245 | 75 | 1.00 | 1.00 | ||
| Low-dose TCA | 183 | 64 | 0.84 | 0.70 to 1.03 | 0.77 | 0.61 to 0.96 |
| BZD | 46 | 71 | 1.26 | 0.91 to 1.74 | 1.09 | 0.74 to 1.62 |
| Z-drug | 269 | 67 | 1.01 | 0.84 to 1.21 | 1.03 | 0.83 to 1.27 |
| Acute bacterial infection | ||||||
| No sleep drug | 239 | 64 | 1.00 | 1.00 | ||
| Low-dose TCA | 165 | 60 | 0.97 | 0.79 to 1.19 | 0.99 | 0.77 to 1.27 |
| BZD | 37 | 76 | 1.11 | 0.78 to 1.58 | 1.01 | 0.67 to 1.54 |
| Z-drug | 243 | 60 | 1.13 | 0.93 to 1.36 | 1.14 | 0.91 to 1.42 |
| UTI or acute LRTI | ||||||
| No sleep drug | 206 | 63 | 1.00 | 1.00 | ||
| Low-dose TCA | 127 | 56 | 0.86 | 0.69 to 1.07 | 0.90 | 0.69 to 1.17 |
| BZD | 30 | 73 | 1.07 | 0.73 to 1.58 | 1.00 | 0.63 to 1.60 |
| Z-drug | 184 | 52 | 0.98 | 0.80 to 1.21 | 1.01 | 0.79 to 1.28 |
| Ischaemic stroke/TIA | ||||||
| No sleep drug | 47 | 73 | 1.00 | 1.00 | ||
| Low-dose TCA | 37 | 74 | 1.02 | 0.66 to 1.58 | 1.17 | 0.67 to 2.06 |
| BZD | 12 | 80 | 1.92 | 0.98 to 3.74 | 2.88 | 1.10 to 7.52 |
| Z-drug | 52 | 65 | 1.21 | 0.79 to 1.85 | 1.22 | 0.72 to 2.08 |
| Venous thromboembolism | ||||||
| No sleep drug | 14 | 64 | 1.00 | 1.00 | ||
| Low-dose TCA | 12 | 50 | 1.32 | 0.61 to 2.86 | 1.12 | 0.29 to 4.34 |
| Z-drug | 14 | 54 | 1.18 | 0.56 to 2.49 | 1.06 | 0.36 to 3.12 |
| Incident agitation/psychosis | ||||||
| No sleep drug | 74 | 94 | 1.00 | 1.00 | ||
| Low-dose TCA | 77 | 91 | 1.30 | 0.93 to 1.81 | 1.51 | 1.01 to 2.27 |
| BZD | 34 | 94 | 3.88 | 2.50 to 6.02 | 5.76 | 3.22 to 10.28 |
| Z-drug | 126 | 90 | 1.53 | 1.14 to 2.06 | 1.77 | 1.24 to 2.54 |
| Outcome and sleep drug | Patients (n) | Incidence/100 PYs | Age, sex adjusted | Fully adjusteda | |||
|---|---|---|---|---|---|---|---|
| Exposed | With outcome | HR | 95% CI | HR | 95% CI | ||
| Fracture | |||||||
| Discontinued Z-drug | 1265 | 80 | 8.2 | 1.00 | 1.00 | ||
| New Z-drug | 2997 | 135 | 11.3 | 1.24 | 0.94 to 1.65 | 1.31 | 0.94 to 1.84 |
| Hip fracture | |||||||
| Discontinued Z-drug | 1265 | 45 | 4.5 | 1.00 | 1.00 | ||
| New Z-drug | 2997 | 66 | 5.4 | 1.13 | 0.77 to 1.65 | 1.01 | 0.63 to 1.62 |
| Forearm fracture | |||||||
| Discontinued Z-drug | 1265 | 17 | 1.7 | 1.00 | 1.00 | ||
| New Z-drug | 2997 | 27 | 2.2 | 1.24 | 0.65 to 2.39 | 1.60 | 0.75 to 3.41 |
| Fall | |||||||
| Discontinued Z-drug | 1236 | 221 | 25.7 | 1.00 | 1.00 | ||
| New Z-drug | 2888 | 399 | 37.8 | 1.25 | 1.06 to 1.47 | 1.30 | 1.06 to 1.58 |
| Mortality | |||||||
| Discontinued Z-drug | 1274 | 182 | 17.8 | 1.00 | 1.00 | ||
| New Z-drug | 3089 | 355 | 27.8 | 1.47 | 1.23 to 1.77 | 1.45 | 1.17 to 1.81 |
| Acute bacterial infection | |||||||
| Discontinued Z-drug | 1029 | 251 | 35.5 | 1.00 | 1.00 | ||
| New Z-drug | 2267 | 408 | 47.7 | 1.21 | 1.03 to 1.42 | 1.34 | 1.11 to 1.63 |
| UTI or acute LRTI | |||||||
| Discontinued Z-drug | 1029 | 211 | 28.9 | 1.00 | 1.00 | ||
| New Z-drug | 2267 | 354 | 40.0 | 1.24 | 1.04 to 1.48 | 1.38 | 1.12 to 1.70 |
| Ischaemic stroke/TIA | |||||||
| Discontinued Z-drug | 1268 | 43 | 4.3 | 1.00 | 1.00 | ||
| New Z-drug | 3045 | 80 | 6.5 | 1.44 | 0.97 to 2.12 | 1.23 | 0.76 to 1.97 |
| Venous thromboembolism | |||||||
| Discontinued Z-drug | 1273 | 12 | 1.2 | 1.00 | 1.00 | ||
| New Z-drug | 3074 | 26 | 2.1 | 1.68 | 0.83 to 3.41 | 2.71 | 0.88 to 8.31 |
| Incident agitation/psychosis | |||||||
| Discontinued Z-drug | 1039 | 41 | 4.9 | 1.00 | 1.00 | ||
| New Z-drug | 2574 | 140 | 13.6 | 2.26 | 1.60 to 3.19 | 2.16 | 1.47 to 3.17 |
| Incident antipsychotic prescription | |||||||
| Discontinued Z-drug | 1274 | 107 | 13.8 | 1.00 | 1.00 | ||
| New Z-drug | 2402 | 355 | 39.5 | 2.09 | 1.59 to 2.75 | 2.66 | 2.09 to 3.40 |
| Incident antidepressant prescription | |||||||
| Discontinued Z-drug | 825 | 66 | 10.6 | 1.00 | 1.00 | ||
| New Z-drug | 2177 | 236 | 28.3 | 2.09 | 1.59 to 2.75 | 2.20 | 1.62 to 2.99 |
| Incident antibiotic prescription | |||||||
| Discontinued Z-drug | 1087 | 483 | 88.8 | 1.00 | 1.00 | ||
| New Z-drug | 2430 | 818 | 116.4 | 1.14 | 1.02 to 1.27 | 1.21 | 1.07 to 1.37 |
Appendix 6 Additional WHELD trial analyses
| Scale | Z-drug | ||||
|---|---|---|---|---|---|
| Pattern | n | Mean change (SD) | Beta (95% CI) | Betaa (95% CI) | |
| NPI-NH excluding sleep | None | 514 | 0.44 (16.06) | 0.00 | 0.00 |
| Stopping | 26 | 0.65 (17.02) | 0.22 (–6.34 to 6.77) | 1.46 (–5.49 to 8.40) | |
| Starting | 25 | –1.84 (20.44) | –2.28 (–8.96 to 4.40) | –2.52 (–10.07 to 5.03) | |
| Continuing | 62 | –1.40 (19.12) | –1.84 (–6.23 to 2.55) | 0.09 (–4.51 to 4.69) | |
| NPI-NH sleep | None | 514 | 0.10 (0.59) | 0.00 | 0.00 |
| Stopping | 26 | 0.27 (0.53) | 0.17 (–0.07 to 0.41) | 0.11 (–0.14 to 0.37) | |
| Starting | 25 | 0.16 (0.62) | 0.06 (–0.18 to 0.30) | 0.05 (–0.23 to 0.33) | |
| Continuing | 62 | 0.11 (0.70) | 0.01 (–0.15 to 0.17) | 0.07 (–0.10 to 0.24) | |
| QUALID | None | 502 | 0.22 (7.34) | 0.00 | 0.00 |
| Stopping | 26 | 1.88 (6.26) | 1.66 (–1.37 to 4.70) | 2.28 (–0.79 to 5.34) | |
| Starting | 23 | –0.17 (9.16) | –0.40 (–3.61 to 2.82) | –0.33 (–3.79 to 3.13) | |
| Continuing | 59 | –0.09 (10.07) | –0.31 (–2.38 to 1.77) | 0.86 (–1.23 to 2.95) | |
List of abbreviations
- ADC
- Alzheimer’s disease centre
- ARMD
- age-related macular degeneration
- ATC
- Anatomical Therapeutic Chemical
- BNF
- British National Formulary
- BMI
- body mass index
- BZD
- benzodiazepine
- CDR
- Clinical Dementia Rating
- CDR-global
- Clinical Dementia Rating scored according to standard algorithm
- CDR-SOB
- Clinical Dementia Rating – Sum of Boxes
- CI
- confidence interval
- CPRD
- Clinical Practice Research Datalink
- CSDD
- Cornell Scale for Depression in Dementia
- DDD
- defined daily dose
- EQ-5D
- EuroQol-5 Dimensions
- GABA
- gamma-aminobutyric acid
- GDS
- Geriatric Depression Scale
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- IMD
- Index of Multiple Deprivation
- IPTW
- inverse probability of treatment weights
- IQR
- interquartile range
- IRR
- incidence rate ratio
- LRTI
- lower respiratory tract infection
- MCI
- mild cognitive impairment
- MD
- Doctor of Medicine
- MMSE
- Mini Mental State Examination
- NACC
- National Alzheimer’s Coordinating Centre
- NIHR
- National Institute for Health Research
- NNH
- number needed to harm
- NPI
- Neuropsychiatric Inventory
- NPI-NH
- Neuropsychiatric Inventory – Nursing Home
- NSAID
- non-steroidal anti-inflammatory drug
- ONS
- Office for National Statistics
- OR
- odds ratio
- PhD
- Doctor of Philosophy
- PI
- principal investigator
- PPI
- patient and public involvement
- PRN
- pro re nata
- QoL
- quality of life
- QUALID
- Quality of Life in Late-Stage Dementia
- RCT
- randomised controlled trial
- REDIC
- Resource Use and Disease Course in Dementia – Nursing Homes
- RR
- risk ratio
- SD
- standard deviation
- SIB-8
- Severe Impairment Battery
- SSRI
- selective serotonin reuptake inhibitor
- TCA
- tricyclic antidepressant
- TIA
- transient ischaemic attack
- UTI
- urinary tract infection
- VAS
- visual analogue scale
- WHELD
- Improving Well-being and Health for People with Dementia
- WHO
- World Health Organization
- ZED
- Z-drug Evaluation in Dementia
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25010).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
