Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/31/04. The contractual start date was in March 2017. The draft report began editorial review in September 2019 and was accepted for publication in May 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Morrison et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Psychosis
The term psychosis is used to refer to a mental health problem that is characterised by a change to a person’s perceptions, beliefs and/or reality testing. This may include experiencing distressing perceptions that are not generated by an external stimulus, for example hearing a voice(s) speaking when another person is not present. It may also include holding fixed and distressing beliefs that others consider unusual: out of keeping with the social or cultural background of that person and lacking rational grounds. The former is typically referred to as a hallucination and may occur in auditory, visual, tactile, gustatory or olfactory sensory domains. The latter is typically referred to as a delusional belief. With regard to delusional beliefs, various types are frequently reported, including ones that are persecutory in nature,1 ideas of reference (seeing personal meaning in innocuous objects or events) and ideas of importance/grandiosity. 2 An individual with psychosis may experience both hallucinatory experiences and delusional beliefs, and sometimes the delusional beliefs will relate to the hallucinatory experiences.
Both the International Classification of Diseases, Tenth Revision (ICD-10),3 and the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),4 recognise hallucinations and delusions to be key features of psychosis spectrum diagnoses, such as schizophrenia. Thought disorder is considered a core cognitive symptom experienced by people with psychosis, involving abnormal features and patterns of speech that can lead to difficulties in communication. 5 Symptoms of thought disorder can be divided into positive (e.g. loosening of associations and illogicality) and negative (e.g. loss of goal and poverty of speech) symptoms. 5
Hallucinations, delusions and thought disorder are commonly referred to as positive symptoms of psychosis, as they are considered to be an additional perceptual or cognitive experience that are an addition to the person’s mental state. Alongside the positive symptoms of psychosis, negative symptoms may occur, which are considered to be the loss or absence of personal functions or characteristics. 6 In the DSM-5,4 negative symptoms are referred to as blunted affect (reduced emotional expressiveness), alogia (reduced/changed ability to have social conversations), asociality (decreased social contact), avolition (reduced energy/drive) and anhedonia (reduced pleasure from activities). 4,7 Negative symptoms, although considered a feature of psychosis and schizophrenia spectrum diagnoses, are also considered to be transdiagnostic and within DSM-54 they feature across diagnoses including bipolar disorder, major depressive disorder and autism spectrum disorder. 8 Prevalence rates of negative symptoms vary, often owing to differing definitions,9 from 41% to 57%,10,11 with social withdrawal and emotional withdrawal most commonly reported in one study. 10
Psychotic experiences, such as hallucinatory experiences and delusional beliefs, have been shown to exist on a continuum, with psychosis-like experiences being reported by a significant proportion of the general population. 12,13 However, in the general population psychosis-like experiences may be less concerning for the individual experiencing them; they may be fleeting or transitory and not necessitate help-seeking or require support from services. 12–16 In addition, the symptoms of psychosis feature as part of other mental health conditions, including depression with psychotic features,17 bipolar disorder18 and autism. 19 The early stages of psychosis are sometimes referred to as the prodromal period, which tends to occur for 2–5 years before the onset of psychosis. 20,21 However, the term prodromal can be applied only once psychosis has been confirmed (i.e. retrospectively). A prospective approach is the identification of people who meet the criteria for an ultra high-risk (UHR) mental state. These criteria are commonly applied when state (attenuated psychotic symptoms or brief limited intermittent psychotic symptoms) and/or trait factors (family history) for psychosis are present in the presence of a drop in function, indicating that development of psychosis is a risk, but is not inevitable. 22
A person experiencing psychosis may receive either an ICD-103 or a DSM-54 diagnosis, such as schizophrenia, schizoaffective disorder, schizophreniform disorder, delusional disorder, non-organic psychosis unspecified or a mood disorder with psychotic features. The DSM-54 criteria for a diagnosis of schizophrenia require the person to be experiencing two of the following: delusions, hallucinations, disorganised speech, grossly disorganised or catatonic behaviour, or negative symptoms such as affective flattening or paucity of thought or speech for at least 6 months (although this can be at an attenuated level, so long as active psychotic symptoms have been present for 1 month). 4 In addition, these experiences must result in a deterioration in social, academic or vocational work and self-care functioning. In other instances, the term psychosis may be used more as an umbrella term, when a person may not have received a diagnosis but is experiencing symptoms that characterise psychosis, as outlined above. In the context of a first episode of psychosis (FEP) and the early intervention in psychosis service philosophy of embracing diagnostic uncertainty in the early phases of psychosis, the term psychosis is often applied in the absence of a schizophrenia spectrum diagnosis.
First episode of psychosis in adolescence
FEP typically occurs in young adults. For those with a schizophrenia diagnosis, the age at onset is usually between 15 and 35 years. 23 However, data from community services for FEP in Australia,24 Finland25 and the UK26 indicate that the median age at onset lies between 22 and 23 years, with an interquartile range (IQR) of 19–27 years. When psychosis occurs before the age of 18 years the term ‘early-onset psychosis’ (EOP) is typically used in the literature. Epidemiological data regarding the prevalence of EOP are limited, and up-to-date estimates are required. 23,27,28 The limited data are partly because of challenges in the collection of epidemiological data on this population, including misdiagnosis,27 reliance on retrospective data and the absence of effective community-based systems for recording incidence from which accurate data can be obtained. 23 However, estimates from available data indicate that the prevalence of early-onset schizophrenia spectrum diagnoses is 1.6 to 1.9 per 100,000 children and adolescents. 29–31 A useful data source to estimate the prevalence of psychosis in adolescents is the records of services that provide treatment for FEP. Although now somewhat outdated, information from the Early Psychosis Prevention and Intervention Centre (EPPIC) in Melbourne, Australia, which was collected between 1993 and 2000, suggests that just under one-fifth of those accepted into the service were aged ≤ 18 years. 24 Those in the service who were aged ≤ 18 years received a range of diagnoses, as follows: schizophrenia (39.3%), schizophreniform disorder (32.5%), bipolar I (9.4%), other psychoses (13.7%) and schizoaffective disorder (5.1%). Although we have some indication of the prevalence of schizophrenia spectrum diagnoses in adolescence, data on the epidemiology of more broadly defined psychosis, in which diagnostic uncertainty is embraced, are lacking. Kirkbride et al. 32 used data from ÆSOP (Aetiology and Ethnicity in Schizophrenia and Other Psychoses), a large population-based case–control study in three English sites, to examine variability in the incidence of psychotic illnesses across age, sex, ethnicity and site. They applied a broad diagnostic approach to their inclusion criteria, and the 568 people examined in this study included people with individual psychotic symptoms and those who met criteria for DSM-5 psychotic illnesses and schizophrenia subclasses. They found that the incidence rate of psychoses in women peaked between 16 and 19 years of age (in contrast to between 20 and 24 years for men). 32 In another study, Boeing et al. 33 examined data from child and adolescent mental health services, hospital case registers and admission and discharge data from the Scottish Executive in three areas of Central Scotland (covering a population of approximately 1.75 million people). Similarly to Kirkbride et al. 32 a broad diagnostic approach was taken to inclusion in the study, that is participants were eligible if they had ever been in contact with mental health services for a psychotic illness prior to their 18th birthday. The authors found a 3-year prevalence of 5.9 incidences of EOP per 100,000 people in the general population. 33 Boeing et al. 33 report that young people interviewed in their study were experiencing high levels of difficulty in both symptoms and functioning, as well as high levels of unmet clinical and social needs, suggesting the need for a national planning framework to ensure the delivery of assertive and integrative care across mental health, social care and educational/vocational services for young people with psychosis.
A key feature of early intervention in psychosis (EIP) is embracing uncertainty about diagnosis in the early stage of psychosis. 34–36 For this reason, inclusion criteria for EIP services have not specified a schizophrenia spectrum diagnosis. 37 It has been argued that the early course of psychosis can be characterised by changing symptoms and that application of a schizophrenia spectrum diagnosis is difficult unless the person has been unwell for some time. 37 Meta-analysis of reliability of prospective diagnostic stability of FEP diagnoses demonstrates that both schizophrenia spectrum psychoses and affective spectrum psychoses have high prospective stability. 38 However, other FEP diagnoses have low diagnostic stability. 38 The validity of schizophrenia spectrum diagnoses in adolescence has been questioned and, although research indicates that a schizophrenia diagnosis made in adolescence is usually stable, other diagnoses, such as schizoaffective disorder and atypical psychoses, have been shown to have poor predictive validity. 38
Given the risk of stigma and discrimination related to psychiatric diagnoses, concern over false-positive diagnoses has been raised. 14 There is a recognition that embracing diagnostic uncertainty may result in some service users later being given conditions other than psychosis, such as personality disorders or post-traumatic stress disorder. However, McGorry et al. 36 argue that EIP services should ensure sufficient specialisation of staff to address the common needs of FEP service users and that EIP services should transcend traditional diagnostic barriers, reflecting the clinical reality of comorbidity and evolution of symptoms. 36 Although historically embracing diagnostic uncertainty has been part of EIP guidance and policy, there is a challenge for services in ensuring that this is carried out appropriately, that is not misattributing organic psychosis or drug-induced or subclinical experience of psychotic phenomena. 14 Marhawa et al. 39 note that EIP service users very frequently experience comorbid mental health problems and/or are in the process of normal developmental, educational or vocational transitions at the time of presenting to services. This combination of factors can make it challenging to assess service users’ needs while embracing diagnostic uncertainty,40 as do the realities of service provision in the context of limited resources and service boundaries and transitions. Harm caused by stigma, discrimination and the adverse effects of antipsychotic (AP) medication has led to concern that inaccurate inclusion of young people in EIP, who may later go on to be diagnosed with a mental health problem other than psychosis, is a risk of adopting a diagnostic uncertainty approach to identifying FEP. 14 This complexity may be compounded in young people, in whom the prevalence of unusual perceptual experiences is high, and differentiating psychosis from other conditions that are neurodevelopmental, organic or mental health-related may be difficult. 14,40
Personal, social and economic costs of psychosis in adolescence
Adolescence is a time of significant biological, cognitive and social development. People who develop psychosis in adolescence generally experience poorer long-term outcomes than those who develop psychosis as an adult. 28,41 In particular, the probability of full remission is lower, and the long-term outcomes poorer, among those who receive a schizophrenia diagnosis before the age of 18 years. 41,42 A systematic review of 21 studies of long-term functional outcomes among people who develop psychosis in adolescence found that schizophrenia diagnoses were associated with a significantly higher rate of poor outcomes than those with other psychotic disorders. 41 In addition, 50–60% of people with early-onset schizophrenia diagnoses had poor outcomes. 41 Premorbid difficulties and developmental delays are considered to be important predictors of long-term outcomes for those who develop psychosis in adolescence, as demonstrated by findings from two systematic reviews41,43 and one non-systematic review. 44 The most recent and comprehensive review, published by Diaz-Cadeja et al. ,43 utilised outcome data from longitudinal studies of participants with a Diagnostic and Statistical Manual of Mental Disorders (DSM) (DSM-III, DSM-III-R, DSM-IV, DSM-IV-TR) or International Classification of Diseases (ICD) (ICD-9 or ICD-10) diagnosis of schizophrenia or other psychotic diagnoses that was made before the age of 18 years. A number of factors were found to be associated with a poorer outcome across clinical, cognitive and/or biological outcomes, including premorbid difficulties, higher symptom severity at baseline (in particular negative symptoms) and a longer duration of untreated psychosis (DUP). 43 Of concern, there is some indication that those who develop psychosis in adolescence may have longer DUPs. 24 The higher prevalence of negative symptoms and premorbid difficulties seen in those who develop psychosis in adolescence leaves young people at a greater risk of poor long-term outcomes. 43,45
People with psychosis often face public stigma and discrimination, and in adult psychosis populations the incidence of anticipated, experienced and internalised stigma has been shown to be high. 46,47 Many young people report that their experiences have been made worse by stigma and the negative stereotypes of psychosis that are reported by the media. 45 Indeed, research has indicated that young people who meet UHR criteria, even before the onset of psychosis, report internalised stigma that is associated with depression. 48
The risk of suicide and suicide attempts may be higher in adolescents with psychosis in young people with other mental health conditions. In one Swedish cohort,49 the long-term risk of suicide was 4.5% and the risk of attempting suicide was 25%. In the USA, data gathered from 102 young people admitted to an adolescent inpatient unit between 2003 and 2006 showed that, overall, those with psychosis had twice as many suicide attempts as those with other mental health conditions. 50
Adolescent-onset psychosis and schizophrenia are associated with significant economic costs. Young people with psychosis and schizophrenia accounted for 25% of adolescent psychiatric inpatient admissions in England and Wales between 1998 and 2004. 51
Although the evidence base for the personal and social costs points to worse outcomes for those who develop psychosis in adolescence than for those who develop psychosis in adulthood, there is some evidence to counter this. In a long-term follow-up of service users with a schizophrenia spectrum diagnosis who received care from the EPPIC early intervention service, those who developed psychosis in adolescence had significantly better global, social, occupational and community functioning and less severe positive symptoms than those who developed psychosis in adulthood. 52
Service structure for the treatment of a first episode of psychosis in adolescence
In the UK, any adolescent aged ≥ 14 years who develops a FEP is eligible to receive treatment from EIP services. However, young people with psychosis may also receive care from child and adolescent mental health services (CAMHS) both in the community and in psychiatric inpatient settings.
Early intervention in psychosis
The early treatment of psychosis is considered as essential for recovery, and research indicates that those who receive support from an EIP service have better outcomes than those who receive standard care. 53 As noted, this may be particularly indicated for young people, in whom the long-term effects of a delayed DUP on functioning are indicated to be higher.
Early intervention in psychosis services were first established in the UK in 2001, with the aim of providing holistic, evidence-based, biopsychosocial care for people aged 14–35 years with a FEP and their families. 53 For this reason, EIP services are a key provider of care for young people with a FEP. The structure of EIP services has been influenced by the World Health Organization (WHO)-endorsed consensus statement from the International Early Psychosis Association, which set standards and goals for the early intervention, detection and treatment of psychosis. 54 The objectives of the early psychosis declaration (EPD) were to increase access and care for people with FEP, raise awareness of the importance of early intervention, promote recovery, engage families and offer them support, and train primary care practitioners to both recognise psychosis and understand the importance of early detection of psychosis. The EPD sets out the values and ethos of EIP services, which are to promote recovery and hope by focusing on personal, social, educational and employment outcomes, reducing stigma and respecting the strengths of service users and their families.
Working with families is an essential component of EIP,54 and the National Institute for Health and Care Excellence (NICE) clinical guideline [CG number 155 (CG155)]55 explicitly recommends family intervention (FI) to be delivered for all families of young people with psychosis. Unfortunately, the rate of delivery of FI is often low and, in the UK, an audit of EIP services found that 69% of families had not been offered FI in the first 6 months following acceptance into EIP services, and, where FI was offered, there was a relatively low uptake of 38%. 56 A systematic review57 of the literature on the barriers to and facilitators of implementing FI suggests that a number of factors may influence families’ interest in FI, including whether or not they identify as a caregiver, the relevance of the support offered to their needs, a desire to keep their family experiences private and the offer of FI being made after their family member’s psychosis had resolved. 57 The review also highlights that families require flexibility in FI in regard to the length, location and type (individual FI vs. group) of delivery. Furthermore, the review highlights that specific family needs should be considered, such as working hours and diversity in language and ethnicity. The same review reported that for EIP service staff a key concern was access to appropriate training, qualifications, supervision and resources to deliver FI. 57
Duration of untreated psychosis is associated with a number of functional outcomes,58 with positive symptoms58 and suicidality59 and reducing DUP as priorities for EIP services. An analysis of care pathways to EIP services found that people who received mental health support from CAMHS in the first instance had a substantially longer DUP and longer delays in access to EIP services than people who had received support initially from psychiatric hospitals or home-based treatment teams. 60 This indicates that an effective interface between EIP services and CAMHS is required to minimise treatment delay and avoid the adverse affect of a long DUP on prognosis for adolescences with psychosis.
Since the inception of EIP services in the UK, a number of guidance documents have been produced to inform EIP service delivery and practice. A total of 14 policy documents that were produced by the Department of Health and Social Care, the NICE and the Initiative to Reduce the Impact of Schizophrenia network were published between 2001 and 2016. 34 Thematic analysis of these documents indicates that the values set out by EPD are maintained as central to the delivery of EIP services; the core themes across these 14 policy documents are ethical practice (respect for informed consent, privacy and confidentiality), inclusivity (equal access to the service and reducing stigma and discrimination), being patient and family centred and providing appropriate recovery-orientated treatment (avoiding overuse of diagnosis, shared decision-making, responsibility and cost-effectiveness). 34
In 2016, an audit of EIP services reported that there were 56 EIP services established across England. 56 In 2015, the National Collaborating Centre for Mental Health was commissioned by NICE and NHS England to develop a guide to the introduction of access and waiting time standards for EIP services. 37 The access and waiting time standards apply to people aged 14–65 years with a FEP. Eight standards have been set out for EIP services, which include allocation and engagement of people with confirmed or suspected FEP within a 2-week period of referral, offer of cognitive–behavioural therapy for psychosis (CBTp), FI and educational and support programmes for carers, supported employment programmes, physical health assessments and interventions, and clozapine (Clozaril®, Mylan Pharmaceuticals Ltd, Canonsburg, PA, USA) prescribing. The 2016 Healthcare Quality Improvement Partnership audit56 of the access and waiting time standards found that the proportion of people with FEP who had access to the NICE standards was low across many of the domains, but that it was particularly low in relation to engagement and assessment within 2 weeks, access to physical health assessments and interventions, and access to psychological interventions (PIs). In addition, the audit highlighted that there was considerable variation across the country in what was offered to service users.
Joint working between early intervention in psychosis services and child and adolescent mental health services
Child and adolescent mental health services are commissioned to provide care for young people up to the age of 18 years, and this includes young people with psychosis. 61 The NICE CG (CG155)55 recommends that primary care services make an urgent referral to either CAMHS (up to the age of 17 years) or an EIP service (aged ≥ 14 years) that has a consultant psychiatrist with training in child and adolescent mental health. The literature on the prevalence of psychosis cases in CAMHS is limited, so estimates are difficult to obtain. However, in 2011, audit data from Rethink (London, UK)62 indicated that just under half of the CAMHS teams who completed the survey were working with young people with psychosis who were aged < 18 years. CAMHS is structured in four tiers, with increasing complexity and specialism at each tier, as follows: non-mental health specialists working in the community with children (tier 1), CAMHS specialists in community primary care settings with children who experience mild mental health difficulties (tier 2), CAMHS specialists in multidisciplinary community child psychiatry outpatient settings (tier 3) and highly specialised CAMHS clinicians working in outpatient teams, day units or inpatient settings (tier 4).
UK policy for the treatment of psychosis stresses the importance of good communication between EIP services and other service providers, such as CAMHS, in ensuring appropriate assessment. 55 Examples of effective joint working include the provision of CAMHS psychiatry and prescribing for EIP service users aged ≤ 16 years; provision of EIP psychiatry for young people transitioning from CAMHS to adult mental health services (AMHS); input into CAMHS by EIP service team members, including training and attendance at CAMHS team meetings; and employment of FI therapists to work across CAMHS and EIP services. 62 Research conducted with UK primary care trusts (PCTs) and Strategic Health Authorities suggests that joint working is most effective when CAMHS and EIP service staff engage in joint training/education to align their philosophies, and that senior support from PCTs and Strategic Health Authorities is required to facilitate effective working. 62 Although there is evidence of effective working, a report published by Rethink62 found that 67% of EIP staff reported that they did not have training to work with 14- to 16-year-olds and 64% did not have training to work with 16- to 18-year-olds, and half of the EIP teams reported that CAMHS was not a source for identification of psychosis.
There is evidence to indicate that some young people may experience the transition from CAMHS to AMHS, such as EIP services, as an abrupt change that feels poorly planned. 63,64 Research has found that young people report largely negative experiences of the transition from CAMHS to AMHS, citing factors including feeling underprepared for the change in care provider, feeling abandoned by services and being unhappy with the quality of care received. 63–65 A recent Education Policy Institute report66 obtained data from 47 out of 60 CAMHS in England on their current waiting times for assessment and treatment. In 2017–18 the average median waiting time for treatment was 60 days. Waiting times varied substantially across the country, with the 10 CAMHS teams with the longest median waiting times to treatment reporting figures of 82–188 days in 2017–18. The authors concluded that there is still a postcode lottery in the access to time treatment from CAMHS. 66 Specifically, in relation to EIP services, several challenges have been reported in relation to joint CAMHS/EIP service working, including different philosophies,67 role confusion68 and different funding sources. 69 Although having separate EIP services and CAMHS is common across the UK, it has been argued that a unified service should be offered for young people up to the age of 25 years given the high risk of mental health issues starting between the ages of 15 and 25 years. 69 There are examples of such services developing in the UK, such as Forward-Thinking Birmingham (Birmingham, UK), which is an integrated service covering the age range 0–15 years including EIP.
Interventions for the treatment of a first episode of psychosis in adolescence
Access to evidence-based interventions for adolescents with psychosis is paramount. To date, the mainstay of research and treatment for psychosis in adolescence has been AP medication, with a limited number of studies of psychological treatments for people with psychosis who are under the age of 25 years. To understand the efficacy and safety of both pharmacological and psychological interventions, a number of helpful systematic reviews have been published that shall be considered here, along with updates to the literature following publication of these reviews. 70,71
Pharmacological treatment
Antipsychotic medication prescribing for young people with psychosis is increasing72 and, typically, AP medication is the main treatment available for adolescents with psychosis. 71 In the UK, NICE CG (CG155)55 recommends that for children and young people with FEP oral AP medication should be offered, and that the decision about the choice of AP medication should be made in collaboration with the young person and their parents/carers. 55
A number of systematic reviews and meta-analyses on the topic of AP treatment of psychosis in young people have been published. 70,71 In relation to current treatment practice in the UK, the NICE CG (CG155) was informed by the systematic review and meta-analysis published by Stafford et al. 71 This review identified 19 studies of AP medication, seven of which were placebo-controlled trials and 12 of which were head-to-head trials. A meta-analysis was conducted on the seven placebo-controlled trials, with a total sample size of 1094 participants and a mean age of 15.5 years (range 15.4–20.0 years). The drugs that were compared with placebo were quetiapine (Seroquel®; AstraZeneca UK Ltd, Cambridge, UK), aripiprazole (Abilify®; Actavis Ltd, Dublin, Ireland), risperidone (Risperdal®; McGregor Cory Ltd, Bracknell, UK), paliperidone (Invega®; Janssen: Pharmaceutical Companies of Johnson & Johnson, Beerse, Belgium; and Cilag AG, Schaffhausen, Switzerland), amisulpride (Solian®; Delpharm Dijon, Quetigny, France), olanzapine (Zyprexa®; Eli Lilly and Company, Indianapolis, IN, USA) and haloperidol (Haldol®; Ortho McNeil, Raritan, NJ, USA). Results of the meta-analysis showed small benefits of AP medication over placebo for positive and negative symptoms, depression and psychosocial functioning and large effects for global symptoms. However, data quality across the studies was considered poor and the authors highlight that the placebo groups also improved substantially.
A more recent systematic review and network meta-analysis identified 28 randomised controlled trials (RCTs) of AP medication for children or adolescents with a diagnosis of schizophrenia, schizoaffective disorder or schizophreniform disorder published from 1976 to 2017. 70 The total number of participants across the 28 RCTs was 3003, with a median age of 14.41 years (range 7.7–18.3 years). In total, the studies included in the network meta-analysis provided data on 17 AP medications but the most frequently studied were haloperidol, risperidone and olanzapine. Results of the pairwise meta-analyses revealed that, compared with placebo, the following AP medications were significantly more effective in reducing overall symptoms: olanzapine, risperidone, lurasidone (Latuda®; AndersonBrecon Ltd, Hereford, UK), aripiprazole, quetiapine, paliperidone and asenapine (Sycrest®; Schering-Plough Labo N.V., Heist-Op-Den-Berg, Belgium). Results of the network meta-analysis indicated that clozapine was superior to all other AP medication for total, positive and negative symptoms. After clozapine, olanzapine and risperidone had the greatest effect sizes of –0.74 and –0.62, respectively. However, as olanzapine was associated with the highest weight gain, the authors recommended that it should be avoided in children and young people. Network meta-analysis revealed that haloperidol, trifluoperazine (Stelazine®; Mylan NV, Canonsburg, PA, USA), loxapine (Loxapine succinate®; Mylan) and ziprasidone (Geodon®; Pfizer Inc., New York, NY, USA) were not more effective than placebo, and the authors recommended that these drugs should not be used in children and adolescents. Findings from this systematic review and network meta-analysis suggest benefits of AP medication over placebo (except for haloperidol, trifluperazine, loxapine and ziprasidone) and provide useful data to inform decision-making regarding the selection of AP medication and their relative superiority in comparison with each other. However, it should be noted that network meta-analysis relies on the use of indirect as well as direct evidence, and for clozapine the majority of the evidence was indirect. Krause et al. 70 also note limitations to the quality of data, with 50% of the RCTs providing inadequate information about the randomisation procedures and 75% not providing adequate information about allocation concealment. It should also be noted that inspection of the included studies indicates that 7 out of the 28 studies did not use either DSM or ICD-10 diagnostic criteria in application of clinical diagnoses; rather, the studies report that the diagnostic criterion was clinical diagnosis. This may draw into question the homogeneity of the participants included in the studies and the generalisability of the findings.
A review of the literature indicates that since publication of the systematic review and network meta-analysis by Krause et al. 70 two further trials of AP medication for young people with psychosis have been conducted. 73,74 The Tolerability and Efficacy of Antipsychotics (TEA) trial,74 conducted in Denmark, was a head-to-head, double-blind RCT comparing the safety and efficacy of quetiapine-extended release (ER) with aripiprazole in 12- to 17-year-olds with an ICD-10 schizophrenia spectrum diagnosis. The total sample size, across seven university clinics, was 133 (55 allocated to quetiapine-ER and 58 to aripiprazole). The primary outcome was a positive Positive and Negative Syndrome Scale (PANSS) score at 12 weeks, and the results of the trial indicated that there was no significant difference between medications in the primary outcome. However, in both groups positive PANSS scores significantly reduced from baseline to 12 weeks, by approximately 5 points, which is a modest reduction in PANSS score given that the minimum clinically important difference is thought to be about 15 points. 75 Quetiapine-ER and aripiprazole had differing adverse effect profiles. The Quetiapine-ER group had worse metabolic outcomes than aripirazole, with greater weight gain in the quetiapine-ER group. Furthermore, an analysis of the homeostatic model of insulin resistance favoured aripiprazole. However, there were significantly more cases of akathisia and sedation in the aripiprazole group. The number of adverse events (AEs) was high in both groups. In the quetiapine-ER group, the following percentages of AEs were reported: 79% experienced tremor, 92% had increased duration of sleep, 78% experienced orthostatic dizziness, 80% experienced depression, 69% experienced tension/inner unrest, 76% experienced failing memory and 87% experienced weight gain. In the aripiprazole group, 91% experienced tremor, 71% had increased duration of sleep, 81% experienced orthostatic dizziness, 77% experienced depression, 88% experienced tension/inner unrest, 77% experienced failing memory and 68% experienced weight gain. The results of the trial contribute to the relatively limited literature on head-to-head comparisons of AP medication for adolescents with FEP. However, concern has been expressed about the quality of this trial, including the limitations on the conclusions that can be drawn regarding the efficacy and safety given that one-third of participants were prescribed AP medication in addition to those being studied. 76 In the same year as the TEA trial, Correll et al. 73 published a double-blind, placebo-controlled, randomised withdrawal-design trial of oral aripiprazole maintenance treatment. In this study, all 201 participants were cross-titrated to oral aripiprazole and were stabilised on this medication for 17–21 weeks before randomisation in a 2 : 1 ratio to either oral aripiprazole or placebo. The results showed that treatment with oral aripiprazole was associated with a significantly longer time to exacerbation of symptoms of psychosis than treatment with placebo. Interestingly, the rate of serious treatment-emergent adverse effects (TEAEs) and discontinuation because of TEAEs was lower in the medication arm, although not significantly so. 73
Adverse effects of pharmacological treatment
Antipsychotic medications are associated with a wide range of adverse effects, which include metabolic effects (i.e. weight gain), cardiovascular effects, hyperprolactinaemia, antimuscarinic side effects (dry mouth, blurred vision and cognitive impairment), sexual dysfunction and movement disorders. 77 The adverse effects of AP medication are associated with increased stigma, physical morbidity and mortality, poor adherence to medication and reduced quality of life. 77 A systematic review78 of the effects of AP medication on brain volume in adult populations concluded that some of the structural brain changes found in people with a schizophrenia diagnosis may be the result of AP medication. When considering the risk of adverse effects of AP medication for young people, there are biological differences between adolescents and adults that are important to consider. 79 Adolescence represents a time of social and educational change. Medication side effects may affect important educational milestones. The stigma of taking an AP medication may be challenging for a young person’s relationships. Compared with adults, young people may be more susceptible to the adverse effects of AP medication,80 particularly in relation to weight gain. 81 A study by Correll et al. 82 showed that 12 weeks of AP treatment in children and adolescents who had less than 1 week’s prior AP exposure was associated with significant rates of obesity and new-onset categorical glucose and lipid abnormalities. For example, a weight gain of > 7% occurred in 56% of participants taking quetiapine and in 84% of participants taking olanzapine. 82 Krause et al. 70 make an explicit recommendation that this medication should not be used in young people. Moreover, there is an indication that weight gain is associated with high rates of medication discontinuation. 71 In 2016, the NICE CG (CG155)55 was updated to include specific recommendations in relation to olanzapine, advising clinicians who are choosing between olanzapine and other AP medications to discuss with the young person and their parents or carers the increased likelihood of greater weight gain with olanzapine, and that this is likely to happen soon after starting treatment. 55
Summary of pharmacological treatment
Although there is some evidence to suggest benefits of AP medication over placebo for young people with psychosis,70,71 the quality of the evidence is low. 71 Head-to-head comparisons are limited and inferences are often indirect, which limits the extent to which comparisons can be drawn between AP medication. Study durations are short, which means that the long-term adverse effects of AP medication in young people are unknown. Furthermore, the evidence base for AP medication is significantly limited compared with the larger body of adult-derived data, the risks and benefits may be less favourable for young people and the additional benefit of AP medication over placebo is small. 71
Psychological intervention
Psychological interventions for young people with psychosis are recommended in the UK NICE guideline (GC155);55 specifically, cognitive–behavioural therapy (CBT) and FI. The current guidelines suggest that CBT and FI should be offered in conjunction with AP medication. However, if a young person and their parents wish to try PI alone, the NICE CG (CG155) suggests that the care team should advise that PIs are more effective when delivered in conjunction with AP medication, but offer individual CBT and FI if the young person and family wish to pursue that option. In the next sections we will review both CBT and FI, outlining the approaches, the evidence base and potential adverse effects.
Cognitive–behavioural therapy
Cognitive models suggest that the way that we interpret events has consequences for how we feel and behave, and that interpretations are often maintained by thinking biases and behavioural responses. Cognitive models of psychosis and psychotic experiences suggest that it is the way that people interpret and respond to psychotic phenomena that accounts for distress and disability, rather than the psychotic experiences themselves. 83–85 Key elements of CBT include a shared individualised formulation of the problem that can include reflection on life events that may contribute to the development and/or maintenance of psychosis (such as trauma and deprivation), evaluating unhelpful thoughts and conducting behavioural experiments. 83 Morrison et al. 83 place an emphasis on the importance of a strong therapeutic relationship, normalising information, collaboration between client and therapist and therapy being based on the client’s problem list and idiosyncratic goals. Brabban et al. 86 suggest 10 key ethical considerations for CBT to ensure that it is recovery orientated: (1) collaboration, (2) use of everyday language, (3) acknowledging the historical and developmental context of the client’s difficulties (i.e. adverse life experiences) so as not to minimise the impact of these, (4) evaluating rather than challenging beliefs, (5) applying caution with use of the stress-vulnerability model of psychosis and schizophrenia, (6) validating the client’s experience using a psychological formulation, (7) delivering hope to the client, (8) offering informed choice about engaging with CBT, (9) ensuring that CBT training is extensive and specialist and (10) ensuring that there is access to continued supervision.
Family intervention
As noted above, a FEP often occurs in adolescence, which is a time when young people are typically living with family members. For this reason, families often play a key role in supporting a person who is experiencing psychosis, and the level of support may be greater in early psychosis and when people are younger in age. FI is considered to be a psychoeducational intervention based on the behavioural family therapy and cognitive behavioural approaches. 87 This approach includes an assessment of family understanding and appraisals of presenting difficulties; sharing the emerging psychological formulation and agreeing a problem list and FI goals; psychoeducation to develop a common understanding, including normalising and recovery-orientated information on presenting difficulties; problem-solving; communication skills training; and relapse prevention planning. 88 Families are often given between-session tasks and are encouraged to hold family meetings to support skills practice. 87 Family members are also given information about local services and signposted to support for themselves, where appropriate. The NICE CG (CG155) recommends that FI should be recovery focused; offer support, education, problem-solving and crisis management; and be delivered over 10 sessions to the family and young person (assuming that inclusion of the young person is practical). 55
Efficacy and safety of psychological intervention for adolescents with psychosis
Although there is an established evidence base for CBT in adult psychosis populations,89–92 the availability of studies to determine its efficacy in children and young people is limited. 71,93 In adult populations, meta-analyses show CBT to have small to moderate effects when delivered in combination with AP medication. 89–92 In relation to FI, NICE conducted a review of the literature for FI for adults with psychosis and found that FI reduced the risk of relapse, the risk of hospital admission during treatment and the severity of symptoms both during and up to 24 months following the intervention. 92 To inform the NICE CG (CG155),55 Stafford et al. 71 conducted a systematic review of PIs for the treatment of psychosis and schizophrenia in children, adolescents and young adults. Searching the literature up to July 2013, Stafford et al. 71 identified eight trials of PI, of which seven included a treatment arm with CBT or FI (one trial was of movement therapy vs. dance therapy). However, no trials of PI (i.e. CBT or FI) carried out exclusively in an under-18 population were identified; rather, the studies included in the review included participants up to the age of 25 years (mean age 23.2 years, range 15–24 years). Meta-analysis of the data from these studies indicates no evidence of treatment effects on symptoms and low-quality evidence for the combination of CBT and FI on the number of days to relapse. In addition to the limited evidence base, the quality of studies in under-25-year-olds was questionable, with a risk of detection bias from inadequate concealment of allocation across all studies and four trials at high risk of selective reporting. 71
Since the publication of the review by Stafford et al. ,71 a further review has been produced by Anagnostopoulou et al. 93 that included studies of PIs published up to 2017. The aim of the review was to evaluate the efficacy of PIs for young people with EOP (before the age of 18 years) in relation to positive and negative symptoms, cognitive functioning and psychosocial functioning. The authors included papers in which the study was a RCT and the participants were aged 12–18 years and had received a schizophrenia spectrum diagnosis. The review identified one controlled trial of CBT versus FI94 and one RCT of a psychoeducational group intervention for adolescents with psychosis and their families,95 both in a strictly under-18-year-old population.
In their pilot controlled trial of CBT versus FI versus treatment as usual (TAU), Browning et al. 94 recruited 30 adolescents with psychosis who had been admitted to a psychiatric inpatient unit and allocated them to receive CBT and TAU (n = 10), FI and TAU (n = 10) or TAU only (n = 10). TAU included, as a minimum, medication (although the authors do not specify whether this was AP medication or other mental health medications), a nursing care plan and group-based activities on the unit. CBT was adapted for young people by delivering shorter sessions twice per week. Participants allocated to receive CBT could access 10 sessions. FI comprised five 1 hour-long sessions and was delivered over 4–10 weeks. The study conducted by Browning et al. 94 provides data to suggest that it may be feasible to recruit young people to a study comparing PIs. However, there was a high risk of selection bias owing to the non-randomised design and the numbers recruited were too small to make any inferences regarding efficacy, although there was an encouraging signal from the data that participants in the PIs reported greater satisfaction ratings than those receiving TAU alone.
A secondary analysis of the SoCRATES trial,96 which recruited people with psychosis who had a recent first or second inpatient admission, found that participants under the age of 21 years who had supportive counselling showed significantly greater improvements at 3 months on the PANSS positive, PANSS general and the PSYRATS (Psychotic Symptoms Rating Scales) delusions subscales than those who received CBT or TAU. 97 In addition, among those under the age of 21 years, the therapist-rated therapeutic alliance was significantly poorer in the CBT group than in the supportive counselling group. 97 The authors concluded that PIs for people with early psychosis may need to consider age-related factors. 97 Although these findings contribute to the literature, it should be noted that this is a secondary analysis of the SoCRATES study, which was not designed to evaluate these treatments specifically in young people with psychosis, and so the conclusions that can be drawn are limited.
In their RCT of a group-based psychoeducational intervention for young people with psychosis and their families, Calvo et al. 95 recruited 55 young people and their families and randomly allocated them to either a psychoeducational problem-solving group arm (n = 27) or a time-matched control arm (n = 28). At the end of the intervention, young people in the psychoeducational intervention arm had significantly fewer visits to accident and emergency and lower negative symptom scores than those in the control arm; however, these results were not sustained at 2-year follow-up. 95 The results of this study are a signal that, as with adult populations, FI with adolescent populations and their families may be effective in reducing relapse. However, to our knowledge, the study by Calvo et al. 95 represents the only trial of FI in an under-18-year-old population, and any inferences that can be drawn from this study with a small number of participants is limited.
Overall, there is a paucity of evidence from which conclusions can be drawn about the efficacy of CBT and FI for psychosis in children and young people. In producing the UK NICE CG (CG155),55 the guideline development group considered if there were grounds for recommending that treatment with PIs in young people should be any different from that in adults. Given the paucity of evidence for CBT and FI in young people, the guideline development group utilised the data from the much larger adult evidence base to make the recommendation to offer CBT and FI in conjunction with AP medication. CBT trials have been criticised for poor reporting of adverse effects98 and data on the safety of PIs for adolescents are lacking. There clearly remains an important need to address the question of efficacy and safety of CBT and FI for this population.
Psychological intervention in the absence of antipsychotic medication
It has been argued that evidence from meta-analyses of adult psychosis studies demonstrates that the superiority of AP medication over placebo has been overestimated and that the adverse effects of AP medication have been underestimated. 99 For this reason, there has been a growing interest in the efficacy and safety of PIs in the absence of AP medication. A systematic review and meta-analysis of psychosocial treatment with a time-limited postponement of AP medication versus initial AP treatment found that psychosocial treatment may be effective in the absence of AP medication, with psychosocial treatment having a small–medium effect size advantage over AP medication. 100 Although the findings from Bola et al. 100 suggest that it is feasible to conduct research into psychosocial interventions in the (short-term) absence of AP medication, the quality of the studies is limited, with only one RCT, and the psychosocial interventions evaluated varied across the studies and represented treatment programmes rather than specific PIs, such as CBT or FI.
In relation to CBT, Morrison et al. 101 demonstrated that it was feasible and safe to recruit adults who chose not to take AP medication to a RCT of CBT and TAU versus TAU alone. Data from this trial showed that CBT may be effective in reducing symptoms in adults who choose not to take AP medication, particularly in participants under 21 years old. However, the trial was not definitive and conclusions about the efficacy of CBT in this population are limited. The COMPARE (Cognitive behaviour therapy Or Medication for Psychosis – A Randomised Evaluation) trial,102 a three-arm RCT for people aged ≥ 16 years with FEP, allocated participants to AP monotherapy, CBT monotherapy or a combination of both treatments. The results showed that over the 12-month follow-up period the PANSS scores were significantly lower in the combined treatment group than in the CBT monotherapy group, but there was no significant difference between AP medication and CBT, or between combined treatment and AP medication. 102 Participants who received CBT monotherapy had fewer non-neurological side effects and less weight gain than those in the AP monotherapy and combined treatment groups, which may indicate that CBT monotherapy leads to fewer adverse effects. 102 However, although one-fifth of participants in the COMPARE trial (15/75) were aged 16–18 years, there are no head-to-head studies of PI versus AP medication in young people and, therefore, no data on which to draw conclusions about the clinical effectiveness and cost-effectiveness of PI versus AP medication versus a combined treatment in young people with psychosis. 102 The COMPARE trial also did not include FI as a component of the PI.
Summary
In summary, when the UK NICE CG (CG155)55 was produced it was concluded that the available evidence, including that from adult populations, was sufficiently strong to recommend a combination of AP medication, CBT and FI as treatment for young people with psychosis. 88 However, for young people with psychosis, the risk-to-benefit ratio of AP medication appears less favourable, and research is required to establish the potential for psychological treatments alone and in combination with AP medication in this population. Consequently, CG15555 recommends research to determine the clinical effectiveness and cost-effectiveness of psychological treatment alone, compared with AP medication and compared with psychological treatment and AP medication combined. 55
Rationale for the research
In response to the NICE guideline research recommendation, the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme put out a commissioned call to answer the question of how feasible it is to conduct a study to examine the effectiveness of PI, AP medication or a combination of the two in adolescents with a FEP. Managing Adolescence first-episode Psychosis; a feasibility Study (MAPS) was commissioned by the HTA programme to answer the research question from this commissioned call.
Chapter 2 Methods
Used with permission from Morrison et al. 103 This is an Open Access article under the CC BY-NC-ND 4.0 license (https://creativecommons.org/licenses/by-nc-nd/4.0/).
To answer the question of feasibility we employed both quantitative and qualitative research methods.
Objectives
We had six specific objectives across the main trial and qualitative work to enable a strong understanding of the feasibility of running a definitive trial, as follows:
-
to identify the willingness of clinicians to refer to the trial, the proportion of young people referred who are eligible and are willing to consent to the study, and the proportion of participants who comply with the treatment allocation
-
to assess the rate of attendance at follow-up assessments
-
to identify characteristics of trial participants (to clarify selection criteria)
-
to determine how feasible and acceptable the interventions are for participants, parents and clinicians, and the appropriateness of our treatment protocols
-
to assess the suitability of our randomisation and blinding procedures
-
to determine the relevance and validity of the measures to decide their acceptability for use in a definitive trial.
We also aimed to estimate ranges of sample size parameters, finalise treatment manuals and outcome measures, determine training/supervision requirements for research assistants (RAs) and therapists, and assess the possibility for economies for scale.
Role of the funding source
MAPS (both main trial and qualitative studies) was funded by the NIHR HTA programme following a commissioned call (15/31/04). The call specified the interventions, population, setting, comparator, study design and important outcomes.
Approval
MAPS (both main trial and qualitative studies) was approved by the North West – Greater Manchester East Research Ethics Committee (REC) on 6 February 2017 (reference 16/NW/0893). The trial was also prospectively registered on the International Standard Randomised Controlled Trial Number (ISRCTN) clinical trial registry (reference ISRCTN80567433) on 27 February 2017.
Patient and public involvement
Two MAPS co-applicants and one user-led researcher provided patient and public involvement (PPI), contributing substantially to numerous facets of the trial. Rory Byrne contributed to the trial participant information sheets and leaflets. Rory Byrne wrote the Plain English summary of the study for the ISRCTN. The nested qualitative study was user led, with Rory Byrne contributing to the development of the topic guide and participant information sheet, and informing the sampling approach and data analysis. Rory Byrne conducted many of the participant, family/carer and clinician interviews. Transcription, coding and analysis of qualitative interviews was led by Rory Byrne with support from Wendy Jones.
Rory Byrne and Wendy Jones attended weekly team meetings in the central site (Manchester) to provide input on processes and issues as they arose. A PPI representative sat on our independent Data Monitoring Committee (iDMC) and Trial Steering Committee (TSC) and contributed to trial oversight and recommendations.
Throughout the course of MAPS, we sought consultation and recommendations on trial processes from the Psychosis Research Unit service user reference group, all members of which have personally experienced psychosis. For example, the service user reference group provided feedback on the outcome measures used in MAPS and documents such as consent forms, participant information sheets and the trial feedback sheet.
Changes to outcomes post trial commencement
Throughout the course of the trial, there were a number of changes to the trial protocol, including changes to the trial outcomes as agreed with our iDMC and TSC. Summaries of these changes are shown in Table 1.
| Amendment number | Date of amendment | Summary of amendment | Date of REC approval | Protocol version |
|---|---|---|---|---|
| Substantial amendment 1 | 22 February 2017 | Objectives in the protocol amended to state that we will:
|
3 March 2017 | V2: 22 February 2017 |
| Substantial amendment 2 | 27 April 2017 |
|
11 May 2017 | V3: 29 March 2017 |
| Substantial amendment 3 | 22 September 2017 | Addition of an educational film about MAPS | 25 September 2017 | V3: 29 March 2017 |
| Substantial amendment 4 | 19 October 2017 |
|
19 October 2017 | V3: 29 March 2017 |
| Substantial amendment 5 | 12 March 2018 |
|
Rejected by the REC on 12 April 2018 | V3: 29 March 2017 |
| Substantial amendment 5 modified | 18 May 2018 |
|
12 June 2018 | V4: 12 March 2018 |
| Substantial amendment 6 | 31 July 2018 |
|
21 August 2018 | V5: 17 July 2018 |
Feasibility randomised controlled trial
Trial design
MAPS was a Prospective Randomised Open Blinded Evaluation (PROBE) to explore the feasibility of running a definitive trial of three treatment options for FEP in 14- to 18-year-olds. Participants were allocated in a 1 : 1 : 1 ratio to receive AP medication monotherapy, PI monotherapy or a combination of both treatments. MAPS was conducted over 27 months across seven UK sites. The recruitment window ran from 1 April 2017 to 31 October 2018. All follow-up assessments were finalised by 31 May 2019. The current REC-approved trial protocol is available at www.journalslibrary.nihr.ac.uk/programmes/hta/153104/#/.
Settings
MAPS was conducted within NHS secondary care mental health services, namely EIP services and CAMHS. The seven UK sites were Manchester (central site); Birmingham; Lancashire; Oxfordshire and Buckinghamshire; Norfolk and Suffolk; Northumberland, Tyne and Wear; and Sussex. The study started with four sites: Manchester, Sussex and Oxford all commenced recruitment on 1 April 2017 and Lancashire commenced recruitment on 8 June 2017. The remaining three sites were added in 2018 to determine the feasibility of replicating effective recruitment approaches from the initial four sites. Norfolk and Suffolk commenced recruitment on 9 May 2018, Birmingham on 31 May 2018 and Northumberland on 20 June 2018.
MAPS sites differ considerably in their characteristics, including the levels of urbanisation and deprivation. For example, the 2015 English Indices of Deprivation104 rank Birmingham and Greater Manchester third and fifth, respectively, in having the highest proportions of neighbourhoods that are in the most deprived 10% of areas nationally, whereas Oxfordshire and Buckinghamshire have some of the lowest proportions (35th and 38th, respectively, out of 39 Local Enterprise Partnerships).
Participants
Sixty-one participants were recruited from EIP services and CAMHS in six sites: Oxfordshire and Buckinghamshire (n = 25 randomisations), Manchester (n = 21), Lancashire (n = 9), Sussex (n = 4), Birmingham (n = 1) and Northumberland, Tyne and Wear (n = 1). No participants were recruited in Norfolk and Suffolk.
Inclusion and exclusion criteria
We included young people who met all of the following criteria:
-
aged 14–18 years (up until their 19th birthday)
-
presented with FEP (defined as being within 1 year of presenting to services with psychosis)
-
under the care of an EIP service or/and CAMHS
-
scored ≥ 4 points on the PANSS105 for delusions and/or hallucinations at the baseline assessment to ensure current symptoms of psychosis
-
met the ICD-10 criteria for schizophrenia, schizoaffective disorder or delusional disorder (as diagnosed by the treating consultant) or met the entry criteria for an EIP service
-
able to provide written, informed consent and if under 16 years old have a parent/guardian willing to provide additional consent for the MAPS team to contact the young person (for ethics reasons).
To ensure that our sample was representative of young people with FEP as a primary problem, we excluded those who:
-
had a diagnosis of ICD-10 organic psychosis
-
had a moderate/severe learning disability
-
had primary alcohol/substance dependence
-
were non-English speaking (to ensure that participants were able to engage in assessments and therapy)
-
scored ≥ 5 points on the conceptual disorganisation item of the PANSS (as above)
-
presented with immediate risk to themselves or others at the time of referral
-
had received AP medication or structured PI within the last 3 months (to ensure treatment naivety).
Exclusion did not include comorbid diagnoses, such as autism spectrum disorders, personality disorders or use of substances (e.g. cannabis or alcohol) (unless this was a primary alcohol/substance-dependence diagnosis, as outlined above).
Data collection
Potential participants were informed of MAPS by a member of their care team and, if interested, were asked for a verbal agreement for basic referral details to be provided to the research team and for a member of the research team to contact them. For people under 16 years of age, the MAPS protocol required the care team member to ask the young person’s parent/guardian to provide verbal consent to be contacted by the research team initially. If verbal consent was provided, the care team member shared with the RA basic referral information for the young person and contact details for the parent. The RA would then contact the young person (aged 16–18 years) or parent (for under-16-year-olds) to describe the study briefly and send them a participant information sheet. Each young person or parent was given at least 24 hours to consider the participant information sheet. If interested, RAs would arrange to meet the young person or parent at their preferred venue [including the individual’s home, school/college, mental health services, general practitioner (GP) surgeries or youth offending services]. If there were any concerns about the individual’s home environment or risk to others, the initial meeting would take place at an NHS site.
At the initial appointment, the RA discussed the participant information sheet with the young person or parent and asked them to reflect on the information, ask questions and raise concerns to ensure that the information was understood. Once the RA and individual were satisfied that all of the information had been provided and understood, they would complete the parent/guardian consent to approach form (parents of under-16-year-olds) or the participant consent form (16- to 18-year-olds). The RA read out each statement on the form to the individual, checked their understanding and asked individuals to sign their initials next to each statement if they agreed with it. Finally, the RA and young person/parent provided their signatures and the date of consent at the bottom of the form. For individuals under 16 years old, once the parent/guardian had provided consent for their child to be contacted about MAPS, the RA could meet the young person to complete the consent form, as described above. All participants, regardless of their age, provided written informed consent to enter the trial.
Following the consent appointment, the RA completed the baseline assessment. RAs were advised to minimise participant burden, that is ideally keep appointments to a maximum of 1.5 hours, avoid multiple appointments for any one time point and prioritise participant choice. It was recognised that for some participants the assessment battery may not be complete in full should the participant choose to decline an assessment or opt for a single appointment. Participants completed the PANSS first, before completing other self-report and physical health measures. Each participant was given a personalised crisis card with details of their care co-ordinator, GP and other crisis support numbers (e.g. Samaritans, Ewell, UK). All participants who attended baseline assessments received £10 for their time and contribution to the research.
We designed a follow-up period of variable length, such that participants recruited in the first 16 months were offered assessments at 3, 6 and 12 months post baseline, and those recruited thereafter were offered assessments up to the end of treatment (6 months). RAs contacted participants or their parents (with the participants’ consent) by telephone to arrange assessments. RAs confirmed and documented ongoing consent with participants prior to completing any measures. RAs began with the PANSS before moving on to the other measures. Participants were given another copy of the personalised crisis card and were compensated £10 at each follow-up appointment.
The RAs telephoned participants at 1.5, 4.5 and 9 months to maintain ongoing contact and engagement with the follow-up appointments, and ascertain any changes in contact details (no clinical outcome data were collected). RAs posted £5 gift cards to participants for their choice of shop after completion of these telephone calls.
Outcomes
Primary outcomes
Given that MAPS was a feasibility study, our key outcomes for informing a definitive trial were referral and recruitment rates, therapy attendance and medication adherence (including discontinuation rates), and completion of follow-up appointments and questionnaires.
Our iDMC, TSC and funder agreed a three-stage progression criterion to determine how successfully each outcome was achieved and help make recommendations about proceeding to a definitive trial. Recruitment, retention and adherence to PI and AP medication were evaluated and determined as meeting one of three success levels:
-
‘green’ – indicating that progression to a definitive trial is possible without needing to substantially change design or delivery
-
‘amber’ – indicating a need for more resources and/or new ideas for recruiting and retaining participants and supporting treatment adherence
-
‘red’ – indicating that a definitive trial may not be economically viable.
Recruitment success was measured by determining the proportion of the target sample size achieved: green criterion ≥ 80%, amber criterion 79–60% and red < 60%. Retention success was measured by the proportion of participants who completed an end-of-treatment PANSS assessment: green criterion ≥ 80%, amber criterion 79–60% and red < 60%. Satisfactory delivery of adherent therapy was determined by the proportion of participants allocated to PI who received six or more sessions of CBT: green criterion ≥ 80%, amber criterion 79–60% and red criterion < 60%. Satisfactory delivery of AP medication was determined by the proportion of participants receiving an AP medicine for ≥ 6 consecutive weeks: green criterion ≥ 80%, amber criterion 79–60% and red criterion < 60%. It should be noted that we included an AP dose below the limits in the British National Formulary (BNF) given that this is frequently the approach for young people owing to the drugs being licensed for adults.
Secondary outcomes
The RAs collected secondary outcomes from participants via semistructured interviews and self-report measures at each appointment to assess their acceptability and usefulness for inclusion in a definitive trial, rather than assessing the efficacy or safety of treatments. RAs completed the measures in a prioritised order that was agreed by the chief investigator. The measures are described below in order of priority.
Symptoms of psychosis
Symptoms of psychosis, as assessed by the total score on the PANSS,106 are our provisional choice of primary outcome measure for a definitive trial, although quantitative and qualitative data from this trial will help inform the final decision. The PANSS is a semistructured assessment administered by interview that assesses the severity of symptoms experienced by people with psychosis. PANSS score is a commonly used primary outcome measure in studies of treatment for psychosis spectrum difficulties, which enables comparability with other relevant trials and inclusion of our results in systematic reviews and meta-analyses, as appropriate. The PANSS has 30 items that are scored from 1 (‘absent’) to 7 (‘extreme’). Seven items pertain to positive symptoms (e.g. delusions and hallucinations), seven to negative symptoms (e.g. blunted affect and social withdrawal) and 16 to general psychopathology (e.g. anxiety and depression). We used the five-factor PANSS model developed by van der Gaag et al. ,107 which divides the items into five subscales: positive symptoms, negative symptoms, disorganisation, excitement and emotional distress. This model has been found to be more stable and represent a more complex factor model than those previously published.
Demographic information
We developed a brief demographic questionnaire that participants were asked to complete at each assessment. The questionnaire required participants to select statements that best characterised their gender, highest level of education, current employment status, marital status, living arrangements, ethnicity and religion/belief; participants were also asked to report their date of birth.
Recovery
We used the shortened 15-item version of the Questionnaire about the Process of Recovery (QPR),105 which was developed collaboratively with service users to assess recovery from psychosis. Participants respond to the statements (such as ‘I feel that my life has a purpose’) on a five-point scale from strongly disagree to strongly agree, according to their experiences in the last 7 days.
Duration of untreated psychosis
At the baseline appointment only, RAs completed a semistructured interview with participants to identify when symptoms of psychosis began and when they first sought help. DUP was operationalised as the number of months between the emergence of symptoms and the date participants received CBTp/AP medication from their clinical service or were randomised into the trial (if they were treatment naive).
Anxiety and depression
The Hospital Anxiety and Depression Scale (HADS)108 is a reliable, valid measure of anxiety and depression symptoms over the past 7 days. Statements include ‘I feel tense or wound up’ and ‘I feel cheerful’. Participants rate the frequency/intensity of their symptoms using one of four multiple-choice response options for each of the 14 statements. Anxiety and depression scores are calculated separately.
Social and educational/occupational functioning
The First Episode Social Functioning Scale (FESFS)109 was developed for use with people with FEP. It has good reliability, validity and sensitivity to change. The FESFS assesses nine areas of functioning: ‘Living skills’, ‘Interacting with people’, ‘Friends and activities’, ‘Intimacy’, ‘Family’, ‘Relationships and social activities at work’, ‘Work abilities’, ‘School relationships and social activities at school’ and ‘Educational abilities’. Participants are asked to rate their perceived capability and frequency of engaging in these areas using four-point scales (e.g. from totally agree to totally disagree or from never to always). Higher scores correspond to better social and educational/occupational functioning.
Autism spectrum disorders
At the baseline appointment only, participants completed the 10-item version of the Autism Spectrum Quotient (AQ-10),110 which is recommended by NICE to measure diagnostic symptoms for autism spectrum conditions in young people. Participants respond to statements including ‘I find it difficult to work out people’s intentions’ on a four-point scale from ‘definitely agree’ to ‘definitely disagree’. The scoring instructions provide a threshold score that indicates a possible need for a specialist autism assessment.
Health economic data
We used an economic patient questionnaire that was adapted from previous studies conducted by the authors111,112 to gather information about the types of health and social care services used by participants and the frequency of their contact with these services. We also used the EuroQol-Dimensions, five-level version (EQ-5D-5L), health status questionnaire, a valid measure of health outcomes in people with a schizophrenia diagnosis. 113 Participants are asked to rate their mobility, self-care, usual activities, pain/discomfort and anxiety/depression on the present day on a five-point scale from no problems to extreme problems.
Substance use
The Alcohol Use Disorders Identification Test (AUDIT) was developed by WHO114 and is a reliable measure for people with FEP. 115 Participants respond to 10 statements about their alcohol use habits, with predefined answers scored from 0 to 40. Higher scores correspond to more severe alcohol use problems. The scale includes threshold scores for dangerous drinking habits. Participants also completed the Drug Abuse Screening Test (DAST). 116 Participants responded ‘yes/no’ to the 10 items, including ‘Are you always able to stop using drugs when you want to?’.
Dimensions of psychosis experiences
Participants completed the Specific Psychotic Experiences Questionnaire (SPEQ),117 which was developed for use with young people. It has five subscales each containing 8–15 items, which assess paranoia, hallucinations, cognitive disorganisation, grandiosity and anhedonia. Participants are asked to rate their belief in or frequency of experiences from predefined response options. Each subscale is scored separately, with higher scores corresponding to more severe symptoms.
Measurement of adverse events and potentially unwanted effects of trial participation
We used a number of methods to ensure a rigorous approach to recording and reporting adverse physical health effects, adverse and serious adverse events (SAEs), and potentially unwanted effects of trial participation throughout participants’ time in the trial, described in detail below.
Adverse effects of medication
At each assessment, RAs completed the Antipsychotic Non-Neurological Side Effects Scale (ANNSERS)118 with participants. This is a 44-item semistructured interview that determines the presence and severity of side effects associated with AP medication, which are categorised into different subscales including cardiovascular, autonomic and sleep problems. Each item represents a known side effect from AP medication and, if present, is allocated a rating of mild, moderate or severe. Owing to the blind nature of the trial, all participants were offered the ANNSERS interview regardless of allocation. Therefore, RAs were required to rate each item regardless of whether that particular side effect was attributable to AP medication, unless there was a very clear non-medication cause. For example, in the example of a skin rash, if a participant with a known skin allergy to a particular substance reported that only after coming into contact with the known allergen, a score of absent would be applied. Medically trained clinicians on the MAPS team were sought for consultation on scoring when required.
Metabolic side effects
At each assessment, RAs completed physical cardiovascular and metabolic screenings to determine any changes since baseline that may be related to AP medication. RAs measured participants’ height and weight [to calculate their body mass index (BMI)], waist circumference and blood pressure. In addition, RAs took three samples of blood for analysis of total cholesterol, low-density lipoproteins (LDLs), high-density lipoproteins (HDLs), triglycerides, prolactin, glycated haemoglobin (HbA1c) and fasting plasma glucose (FPG). The blood test results were sent to participants’ psychiatrists (or other responsible clinician) at each time point. If RAs were unable to obtain a blood sample or if the participant declined a test, participants’ psychiatrists were also notified.
Serious adverse events and adverse events
Consistent with Health Research Authority (HRA) guidelines, we determined all of the following as SAEs:
-
death
-
an event that is life-threatening
-
an event that requires hospitalisation or prolongation of existing hospitalisation
-
an event that results in persistent or significant disability or incapacity
-
an event that consists of a congenital anomaly or birth defect
-
an event that is otherwise considered medically significant by the investigator.
In addition to these, in the MAPS protocol we list serious violent incidents and formal complaints about treatment. In addition to SAEs, we also recorded AEs as determined as any untoward medical occurrence in a participant, including occurrences that are not necessarily caused by or related to the treatment (e.g. self-harm).
Research assistants and therapists reported AEs and suspected SAEs to the chief investigator. Both the chief investigator and the chairperson of the iDMC reviewed all SAE to determine if they were related to the trial proceedings. Any SAEs that were considered to be related to trial procedures were reported to the REC and the participant’s NHS trust. To ensure independent scrutiny of AEs and SAEs, both the iDMC and the TSC reviewed details of all SAEs at each meeting.
In addition to report of an AE or SAE from a research team member, an independent, non-blinded researcher screened all participants’ medical records to find any SAEs that may not have been reported.
Potential adverse effects of trial participation
We assessed this using a measure developed in our HTA FOCUS (Focusing on Clozapine Unresponsive Symptoms) trial. 111,119 The optional self-report measure was given to participants at point of exit from the trial or at point of withdrawal for those who left the trial. The measure contains 27 items (including ‘taking part involved too much hard work’ and ‘I did not feel listened to or believed by MAPS staff’), which participants responded to on a five-point scale from ‘not at all’ to ‘very much’. In addition, there was space for participants to describe trial participation in their own words, if wished.
Substantial deterioration
Following each participant’s 3-month follow-up, the percentage change on the PANSS was calculated using PANSS rescaling methodology. 120 If the participant’s mental health had significantly deteriorated from baseline, participants in the monotherapy arms were offered a switch to the combined treatment arm. A significant deterioration was operationalised by an involuntary admission to a psychiatric hospital or an increased rescaled PANSS score of at least 12.5%.
Diagnosis and medical record screen case report form
The participant’s electronic medical records were screened for information pertaining to diagnosis, details of AP medication prescription (type, dose, start and stop dates and adherence), concomitant psychological therapies, AEs and SAEs. The medical records were screened by a member of the research team who was not blinded to allocation. This involved screening progress notes, clinical summaries, care plans and medicine management information in the participant’s medical records for any relevant AP information. The records were searched for a date when AP medication had commenced and any periods when medication was stopped/not adhered to, to determine the period of time that the participant took AP medication. The medical records were screened at baseline, and at 3, 6 and 12 months (for those who reached the 12-month follow-up) by a non-blinded member of the research team. In addition, participants’ diagnoses were considered by consultant psychiatrists from our research team (AJ, DM, MRB and RW) on the basis of vignettes based on information extracted from PANSS interviews and reviews of medical records.
Medication adherence (self-report)
In addition to medical record data pertaining to medication adherence, self-report medication adherence was collected at each follow-up. Participants were asked to confirm if they were prescribed an AP medicine and to rate compliance using a 0–100 analogue scale. Participants were given the choice to either complete this online or complete a paper version, which was returned in a sealed envelope to the trial manager (to main the single blind).
Interventions
Antipsychotic medication
Participants who were allocated to a medication arm of the trial were offered AP medication by their usual care team psychiatrist. Psychiatrists were asked to initiate AP treatment as soon as possible following randomisation, and to maintain treatment for preferably 26 weeks, but for a minimum of 12 weeks. Psychiatrists made decisions about the type and dose of AP medication in line with their usual practice, and were free to change the dose and type of AP medication throughout treatment if deemed necessary. Psychiatrists were asked to make prescribing decisions consistent with NICE CG (CG155). 55 This recommends that the choice of AP medication should be made jointly between the clinician, young person and parent/carer, including discussing the possible benefits and side effects of each drug and providing age-appropriate information; it also recommends that physical health is monitored throughout treatment. 55 Psychiatrists were given the offer of consultation from MAPS team psychiatrists regarding AP prescription.
Participants in all treatment arms were not restricted from taking up any medications or psychological therapies offered by the care team throughout their participation, as this would be unethical. To gather feasibility information on participants’ adherence to their MAPS treatment allocation, a non-blinded researcher screened participants’ medical records for information on concomitant therapies. In addition, participants provided self-report data on (1) concomitant psychotherapies and pharmacotherapies and (2) adherence to allocated treatment via our web-based platform or via paper self-report if preferred (if via paper self-report this information was returned to a non-blinded MAPS team member).
Psychological intervention
Psychological intervention was delivered by CBTp-trained psychological therapists. Therapists were employed on a band 7 NHS salary scale, which is representative of CBT delivered within the NHS by most psychological therapists. In some instances, therapist complement was increased by using local trial therapists (bands 8a–8c) in addition to the band 7 trial therapists. Participants were offered up to 26 hours of CBT over the first 6 months, plus up to four boosters over the subsequent 6 months, and up to six FI sessions. Individual CBT commenced as soon as possible post randomisation. Sessions were delivered at the participant’s preferred venue (often home or school/college) to support the accessibility and acceptability of the CBT.
The CBT was manualised121 and informed by an integrative cognitive model of psychosis. 122 Therapists took an assertive outreach approach to engaging participants, and incorporated principles from youth work and social and vocational interventions. The aims of CBT were reducing distress (particularly that arising from psychotic symptoms) and improving quality of life. The CBT emphasised formulation, normalisation, collaboration between therapist and participant, and evaluation of participants’ appraisals of and responses to psychological experiences. Therapy addressed the problems and goals agreed by the participant and therapists. Treatment targets included positive symptoms of psychosis, social issues (e.g. ‘I have improving relationships’) and/or comorbid difficulties (e.g. anxiety, depression).
Cognitive–behavioural therapy aimed to involve four phases: engagement, assessment and formulation; change strategies; longitudinal aspects; and consolidation. 119 Key milestones for CBT were as follows: first session within 1 week of randomisation; a shared list of problems and goals by session 3; a shared formulation by session 3 (this could be a maintenance formulation); one ‘out in the real world’ session by session 10; and a longitudinal formulation by session 26. Clients were encouraged to engage in between-session practice, in line with research on increasing the effectiveness of CBT. 123 Participants’ individual formulations informed the choice of specific CBT interventions from a range described in published treatment manuals. 83,124 This results in an individualised therapy that retains standardised components.
Family intervention was delivered alongside individual CBT by the same therapist. Participants were offered up to six sessions over 6 months, but did not have to use them if not desired or if participants had no regular contact with family members. FI was based on the behavioural family therapy approach:125 a psychoeducational model designed to flexibly address current concerns and problems relevant to adolescent FEP, such as supporting families with their reactions to the development of FEP and fostering hope for recovery.
The first session of FI aimed to assess the family’s understanding and appraisals of presenting problems, sharing of the initial psychological formulation and agreeing problems and goals to be addressed as a family. Further sessions involved providing psychoeducational, normalising and recovery-oriented information; facilitating problem-solving and communication skills; and developing relapse prevention plans. The participant’s care co-ordinator was invited to attend the final session to ensure continuity and support of goals and strategies following trial participation. Therapists signposted families to support services and other relevant services if needed.
Training and supervision
Therapists
Therapists received weekly CBT supervision from a central supervisor (AM or SB) to ensure fidelity to the treatment protocol, and fortnightly clinical supervision from their clinical supervisors/research site leads to ensure adherence to local NHS policy and manage site-specific clinical issues. All clinical supervisors were appropriately experienced and trained in CBTp.
Therapists received FI supervision from Anthony P Morrison, Samantha Bowe and Jo Smith to ensure fidelity to the treatment protocol. Therapists received training in FI from Jo Smith. FI sessions were recorded with participant consent and utilised for training and fidelity. This process ensured fidelity to the treatment protocol, informed supervision and allowed corrective action to be initiated if necessary. Throughout the trial, therapist FI training days were attended by trial therapists and supervisors. After each session, therapists completed a session record sheet for FI to evidence using core elements of FI from the treatment protocol.
Therapy sessions were recorded with participants’ consent. Throughout the course of the trial, therapists submitted session tapes. The central supervisor (SB) rated the tapes on the Cognitive Therapy Scale-Revised (CTS-R)126 and provided feedback. This process ensured fidelity to the treatment protocol, informed supervision and allowed corrective action to be initiated if necessary. Throughout the trial, therapist training days were attended by trial therapists, clinical supervisors, the chief investigator and site leads. Group-based CTS-R session ratings were completed.
After each session of CBT or FI, therapists completed a session record sheet to evidence using core elements of CBT or FI and the specific CBT or FI strategies used. These data were analysed to monitor fidelity to the treatment protocol, including any between-site differences in fidelity. In addition, the therapists completed an end-of-CBT and an end-of-FI questionnaire.
Research assistants
All RAs received training from the trial manager and other trial co-applicants on the protocol and conducting the outcome measures. PANSS training was delivered by the MAPS manager, who has extensive experience of PANSS training and supervision (e.g. on the HTA-funded FOCUS trial111). Following an interactive session with the manager and RAs, in which the PANSS interview was explained in detail, RAs completed role plays of the interview. RAs were also required to watch three PANSS training videos (as used in the FOCUS111 and COMPARE102 trials) and achieve an interclass correlation coefficient of ≥ 0.80, with a gold-standard rating provided by Professor Thomas Barnes (a PANSS institute-certified trainer) that was utilised for the HTA-funded FOCUS trial. 111
Research assistants also received training from Professor Peter Haddad (a consultant psychiatrist and MAPS co-investigator) in conducting the ANNSERS interview118 and physical health checks. In addition, they had training from senior clinicians in clinical risk assessment and clinical assessment skills. All RAs completed the NIHR good clinical practice training, including the ‘Informed Consent in Paediatric Research’ module.
To gain competency in venepuncture, RAs were required to attend a theoretical training course that was run in their NHS trusts by qualified practitioners. They then attended a trust venepuncture clinic, where they were supervised by a qualified practitioner. The practitioner provided written evidence that the RA was competent to practise when they were satisfied of the RA’s competency and the RA had met the trust-specific standard for competency, for example taking 10 satisfactory blood samples from clinic attendees.
Research assistants received weekly telephone supervision by the trial manager (face to face in the central site, Manchester). Supervision for all RAs followed a structured agenda of recruitment and retention; data quality and assurance (including a blind review of RA PANSS scores); and reviewing blind breaks, SAEs and withdrawals (for further discussion with the chief investigator). The supervision supported RAs to proactively problem-solve issues with recruitment and assessments; maintain high data quality standards; and systematically report blind breaks, SAEs and withdrawals. RAs also received regular clinical supervision from their site’s principal investigator, focusing on clinical assessment and risk management, procedure and time management, and ensuring adherence to local NHS policy.
Randomisation and blinding
Randomisation was independent and concealed, used randomly sized randomised permuted blocks of three and six, and stratified by (1) centre and (2) family contact (because participants without regular family contact would receive CBT only, not FI, if allocated to a PI arm). RAs carried out the randomisations via a web-based platform developed by the Centre for Healthcare Randomised Trials (CHaRT), a UK Clinical Research Collaboration registered clinical trials unit (CTU) (#7). CHaRT provided consultation on the development of the randomisation algorithms. Using the CHaRT platform ensured that investigators were unable to enrol participants to conditions or predict the allocation sequence.
Participants were randomised within 2 working days of their eligibility being confirmed at baseline. The trial manager, administrator, therapists, chief investigator and PIs were notified via e-mail of the participants’ treatment allocations, to allow monitoring of adherence to allocation and provision of supervision to therapists. Each site had a delegated staff member (either an administrator or a therapist) who sent allocation letters to participants and their care co-ordinators, psychiatrists and GPs.
The assessors (RAs) were blind to allocation outcomes until all participants’ outcome measures were completed. RAs and therapists received extensive in-house training on retaining the blind, including reading and signing a trial-specific standard operating procedure for managing and retaining blinding. The trial used numerous methods outlined in the standard operating procedure to maintain blinding, including separate offices, diaries, pigeon holes and databases for RAs and therapists; reminding participants, family members and clinicians about the blind; protocols for taking messages and secretarial support; and encrypting and password-protecting randomisation information. All accidental unblindings were reported to the trial manager and, where possible, another (blinded) RA would complete the assessment. The chief investigator, iDMC and TSC regularly monitored unblindings in each centre in case corrective action was required.
Statistical methods and analysis
Ground rules for statistical analyses
The analysis followed a statistical analysis plan that was agreed by the chief investigator and data monitoring committee. The statistical analysis plan was published on the CTU’s website prior to pre-access to data (see www.abdn.ac.uk/hsru/what-we-do/trials-unit/statistical-analysis-plans-611.php).
All main analyses were based on the intention-to-treat principle. Safety and unwanted effects were analysed based on the treatment received rather than as randomised. For these purposes, PI was defined as any dose of CBT or FI from the trial therapist. AP was defined as any dose of an AP medication prescribed by the participant’s responsible psychiatrist. All analysis took place after full recruitment and follow-up (i.e. there were no interim analyses for efficacy), although the iDMC monitored trial progress and safety issues throughout the trial’s course. All analyses were carried out in Stata version 15 (Stata®, StataCorp LP, College Station, TX, USA). 127
Sample size
Our proposed sample size was 90 participants (30 participants per treatment arm). We did not perform a formal power calculation to detect treatment differences because the analysis focus was not hypothesis testing. Our target sample size was sufficient to gain reliable information to inform sample size estimates for a larger trial128 and feasibility information about trial procedures.
Primary feasibility outcomes
Descriptive statistics and 95% confidence intervals (CIs) were used to summarise the key success indicators of the trial, including participant recruitment, checks for absence of selective recruitment of participants, baseline balance and participant flow. Appropriate summary statistics were the numbers of participants referred, eligible referrals, consenting individuals and individuals recruited to each arm; the numbers for drop-out from the allocated interventions; the numbers of withdrawals of consent; and the number who failed to provide follow-up outcome data. We also calculated the proportion of participants who received their allocated intervention versus those who did not, and the proportion of participants who moved to the combined arm owing to deterioration.
Secondary outcomes
Appropriate descriptive statistics were used to summarise baseline and follow-up data, with means [standard deviation (SD)] or medians for continuous data and frequencies and percentages for categorical variables. To inform a definitive trial, we conducted a repeated-measures analysis of the proposed primary outcome (i.e. PANSS score) and the secondary outcome (QPR score) using a mixed-effects model to account for the discrete timing of the follow-up assessments and adjust for site and baseline score. For the analysis, missing baseline data were imputed using the centre-specific mean. The focus of the analysis was on point estimates and associated 95% CIs rather than statistical significance (p-values); however, we have reported p-values. Owing to the low response rates or small number of events, the analysis was descriptive. We also assessed the correlations of each measure across all time points and the variation within the proposed outcome measure (mean and SD), to inform a sample size calculation for a future definitive trial.
We have provided summary data for treatment adherence and treatment received in each arm to describe withdrawal from the allocated intervention. We operationalised satisfactory delivery of therapy as attendance at six or more sessions of CBT. Satisfactory delivery of AP medication was operationalised as uptake of an AP medicine for at least 6 consecutive weeks (including a dose below the BNF lower limits, as this is a frequent clinical practice for young people, and AP medication are licensed for adults). We have reported descriptive statistics for the components of the PI received, including the number of sessions and milestones achieved, and completion of between-session tasks.
Missing data
Given that MAPS was a feasibility study, there was no formal analysis to account for missing data.
Qualitative studies
Design
A nested, qualitative study aimed to explore the acceptability and feasibility of the trial and associated treatment interventions among key stakeholder groups, that is trial participants (young people with experience of FEP), their parents or carers and local clinicians (particularly those with prescribing responsibility). This qualitative approach allowed us to gain valuable views of this trial and recommendations to inform the design of a potential definitive trial. Individual semistructured interviews conducted with representative samples from each group evaluated views and experiences of recruitment to the trial, potential barriers to participation and solutions, assessment processes, randomisation to trial treatments and ongoing care and outcomes. Subjective experiences of receiving trial treatments were explored with participants and parents/carers, whereas clinicians were asked to share their perspectives on the delivery of treatments; all interview groups discussed the relative benefits and adverse effects of treatments.
Semistructured topic guides were devised for each of the three stakeholder groups of this interview study, and all interviews followed the same relevant pattern. Topic guides were considered flexible and open to amendment throughout the course of this study, as it was expected that novel areas of interest may emerge, generating additional interview prompts for subsequent interviews, or that original questions may prove to be inessential and, therefore, removed. For this reason, each topic guide was subject to several minor amendments between the first and the final interviews in each treatment arm.
All of the interviews were digitally audio-recorded. Interview recordings were returned to and stored within closed and secure NHS digital network locations. All interviews were transcribed verbatim at the central site (i.e. Manchester). All names, locations, highly idiosyncratic personal details or any other potentially identifying information were removed to ensure anonymisation of the interview data.
Young people
Inclusion criteria
All young people interview participants were drawn from the larger sample of MAPS participants. All MAPS participants had been randomised to one of three treatment arms, as described above. This interview study aimed to use purposive sampling to recruit an equal number of participants from all three treatment arms. In addition, it was intended that purposive sampling would ensure a representative sample with regard to the age range, gender spectrum and ethnic diversity seen in the trial and, where possible, diversity in terms of engagement with the trial and treatments (i.e. seeking possible positive and negative perspectives equally).
Recruitment
All potential young people interview participants had provided written consent when they entered the trial for audio-recording of interviews and publication of anonymised quotations. Potential participants were not approached if (1) they had not provided informed written consent on trial entry for the recording of assessments/interviews and/or the use of direct anonymised quotations in the trial publication (approximately 29% had not), (2) it was not clinically appropriate to approach them because of significant levels of distress or risk, or (3) they declined to be contacted. By using purposive sampling of eligible participants, we aimed to achieve a balanced and representative sample across trial sites and treatment arms that would reflect the age range (14–18 years) and gender balance of the total trial sample. We also sought to represent variance in terms of positive, negative and neutral or ambivalent perspectives on all aspects of the trial. Eligible participants were approached following completion of their 6-month follow-up assessment and after qualitative researchers had sought updated clinical risk and well-being information. It was also expected that for most of the participants allocated to receive PI, the intervention would be complete when the interviews were conducted.
As described, this study intended to systematically seek variance within the interviewee sample; however, several substantial challenges in recruitment to interviews meant that purposive sampling was not wholly feasible. Ultimately, a convenience sampling approach was adopted.
In most cases, eligible participants were informed of the optional qualitative interviews by trial therapists or RAs following 6-month assessments. For a variety of reasons, including clinical concerns or practical obstacles, several otherwise eligible participants were not invited to or reminded of the qualitative interviews. All participants informed of the interview study were advised that involvement was optional, interviews were confidential, interview questions would not require sensitive disclosure, interviews could usually take place at their preferred time and location, and interviewees would receive £10 for their contribution to this study.
Procedure
Interviews were conducted by Rory Byrne (north west), Jessica Bird and Sarah Reeve (Oxford); the semistructured interview schedule was developed by Rory Byrne, Sarah Peters and Wendy Jones in consultation with the wider research team. Interview questions focused on participants’ experience of MAPS, especially issues of acceptability and feasibility, to inform a future definitive version of the same trial design. Key feasibility and acceptability factors included referral into the trial, randomisation to treatment, attending assessment meetings and acceptability/tolerability of trial treatments. Interview topic guides were used flexibly, with additional prompt questions to enhance elaboration. Interviews lasted for an average of 41.5 minutes (range 23.25–53.53 minutes).
Participants
Of the 26 young people who gave their consent to be contacted about the study, 13 took part in an interview. The remaining 13 did not respond to invitations (n = 9), accepted participation but did not ultimately meet for interview (n = 2) or declined (n = 2). Participants in the final sample were drawn from three sites (north-west, n = 8; Oxford, n = 4; Sussex, n = 1) and ranged in age from 15 to 19 years (mean 16 years). Most identified as female (10/13) and white British (9/13). Two participants were black African, one was Hungarian and one was Pakistani. Three participants were allocated to AP only, five to PI only and five received the combined intervention.
Parents and carers
Inclusion criteria
As with the young people interview study, a representative sample of 15–20 parents or carers of young people taking part in MAPS was sought. Inclusion criteria were purposefully broad to facilitate maximum variance and represent the widest possible range of perspectives relevant to trial and treatment acceptability and feasibility. Parents/carers were eligible for inclusion providing that they had recent involvement in the care or support of a young person participating in MAPS. The only exclusion criterion was the preference of young people themselves (i.e. a parent or carer was not invited to an interview if their young person did not want them to be invited).
Recruitment
Potential parent/carer participants were generally considered for inclusion at or after the 6-month follow-up stage, although a small number of people were contacted prior to this point. Potential participants were informed of the interview study directly following interviews with young people, via trial RAs or therapists, or by staff working with young people (e.g. EIP care co-ordinators). As with the young people interview procedures, potential participants were advised that interviews were optional and confidential, would not seek private content and that interviewees would receive £10. It had been intended that purposive sampling would help to capture a diverse range of views of the trial, including different randomised treatment allocation; different levels of young people’s engagement with treatments; and different family member/carer gender, age, ethnicity and relationship with the young person. However, practical obstacles in the recruitment of family/carer participants meant that the final sample was less diverse than intended. For example, for ethics reasons it was necessary for young people to agree that researchers could meet independently with their family member. Several were not agreeable to this. Parents/carers provided written informed consent prior to commencement of interviews.
Procedure
Family interviews were conducted at participants’ homes by Rory Byrne (north-west) and two researchers from Oxford. Immediately prior to interviews commencing, written informed consent was completed and the bounds of interview confidentiality were highlighted. The semistructured interview topic guide, developed by Rory Byrne, Sarah Peters and David Shiers in consultation with the wider research team, focused on the young people’s entry to MAPS, experiences of trial processes and treatments and, where applicable, family members’ own experience of involvement in treatments. The study topic guide, intended to be used flexibly, was amended at several points to add or de-emphasise specific questions per ongoing data collection. Once complete, interviews were, on average, 46.8 minutes in duration (range 25.30–68.34 minutes).
Participants
The total number of family members/carers interviewed was 18; in two cases both mother and father attended together, so a total of 16 interviews were conducted. In total, 15 out of 16 interviews were with parents (mother, n = 13; father, n = 4) and one with a grandmother. Participants were recruited from two trial sites [north-west, n = 6; Oxford, n = 10 (10 interviews, 12 individuals)]. Interviewees were aged between 37 and 71 years (mean age 49.15 years), and all were white British. In terms of trial treatment allocations, two interviews were from the AP only group, six from the PI only group and eight from the combined treatment (AP and PI group).
Clinicians
Inclusion criteria
Inclusion criteria for the clinician interviewees required participants to be qualified mental health professionals who provided care to young people with FEP in CAMHS or EIP services based in NHS trust areas in which MAPS was recruiting. There were no time constraints for these interviews; clinician perspectives were sought during the lifetime of the trial until completion of recruitment. It was initially intended that recruitment would follow a purposive sampling approach to ensure variance among the final sample, including professional discipline. However, once recruitment of young people to MAPS was under way, it became clear that the views of prescribers in particular were instrumental in many referrals of young people to the trial. Therefore, a subsequent inclusion criterion required participants to have current prescribing responsibility for young people with experience of psychosis. Subsequent purposive sampling aimed to achieve variation in clinicians’ service type and their rates of referral to MAPS.
Recruitment
Potential clinician interview participants were identified first from established communications with MAPS staff (e.g. recruitment and referral mailing lists). Additional potential participants were identified in several instances via personal recommendation from prescriber colleagues. In most cases, potential clinician participants were initially invited to interviews by e-mail communication, which was followed up with telephone calls or reminders. Most clinicians who responded to invitations went on to participate in interviews. As with other interviewees, clinicians provided written informed consent prior to interviews commencing. Clinician interviewees were not offered a financial reimbursement or reward.
Procedure
Interviews with eligible clinicians were conducted by Rory Byrne (north-west) and a researcher from the Oxford site, using a semistructured topic guide. Clinicians were asked questions that aimed to elicit views of trial treatments (AP, CBT and FI), potential barriers to or facilitators of referral of young people into the trial and potential challenges or benefits related to the trial in clinicians’ ongoing care for young people who had been referred to MAPS. The interview topic guide was refined over the course of the study, as new or saturated topics were identified through successive interviews. At completion, the average interview duration was 39.3 minutes (range 15.29–58.12 minutes).
Participants
Recruitment for clinician interviews followed a purposive sampling framework, criteria for which included the NHS service setting (CAMHS or EIP), active responsibility for young people in MAPS, age/experience, gender and ethnicity. Of approximately 50 clinicians who were invited to participate in interviews, 28 did not respond, three expressed interest but did not ultimately participate and one cancelled an interview appointment. The final sample comprised 17 clinicians, all with prescribing responsibility for 14- to 18-year-old adolescents across three sites (north-west, n = 12; Oxford, n = 4; Sussex, n = 1). Participants were predominantly consultant psychiatrists (n = 15/17, plus one advanced practitioner and one specialty doctor) working in CAMHS (n = 6) and EIP services (n = 9) (two interviewees worked in both service types), and eight had active prescribing responsibility for young people in MAPS. Participants were aged between 32 and 63 years (mean age 44.3 years) (three participants did not indicate age), and included female (n = 8) and male (n = 9), white British (n = 13), Sri Lankan (n = 1), British Iranian (n = 1), white Irish (n = 1) and white ‘other’ (n = 1) individuals.
Confidentiality and anonymity
All qualitative interviews were conducted individually and face to face to promote interpersonal engagement and ensure privacy and confidentiality. The exceptions were that several young people interviewees chose to attend with a friend, in two instances two parents attended the same interview and one young person attended their parents’ interview. All clinician interviewees attended individually; two clinician interviews were conducted by telephone.
Prior to commencement of each interview, researchers outlined the bounds of confidentiality and sought verbal confirmation of consent to proceed. All participants were assured that all feedback would be welcomed and valuable. This was highlighted particularly to encourage possible negative feedback about the trial or treatments, as it may be reasonably expected that consenting interview participants are more likely to represent those with generally positive or neutral views.
Data analysis
The interview study was conducted with leadership and ongoing involvement from individuals with personal or parental experience of psychosis spectrum difficulties. This involvement was expected to enhance attention to young person and parent/carer concerns during the conduct and analysis of all interviews. All interview data were thematically analysed. 129 The overarching aim of this analysis was to inductively code qualitative data at the manifest level (i.e. analysing only the immediate meaning of participants’ language) to produce an accessible body of coded data from which meaningful thematic representations of participants’ perspectives can be reported.
Consistent with established thematic analysis methodology,129 all transcribed interviews were initially read through in full to enhance familiarity with the data, and initial impressions of the emerging quality of the data set were recorded in memo form. All interviews then were coded systematically and iteratively within NVivo qualitative data analysis software (version 11) (QSR International, Warrington, UK). Researchers did not impose a rigid or literal ‘line-by-line’ coding approach at this stage, but sought to identify and code all sections of data that either directly answered a relevant interview question or offered additional relevant information. This led to many areas of data being coded under multiple topic areas, as many relevant aspects of this research area are inter-related (e.g. the relationship between diagnosis and treatment).
At regular scheduled points during the analysis processes, qualitative researchers met to discuss and refine coding and analysis strategies through reliability checking and establishing consensus. Periodic analysis meetings were also conducted with MAPS leads to further develop emerging thematic and conceptual outputs, and to ensure that valued insights emerging from interviews were transmitted to the trial team as a whole as quickly as possible. The substantive analysis meetings produced evolving thematic ‘maps’ or models representing the commonalities along with the diversity expressed in the views of each respective interview group. Over time, this mapping process elevated or reduced the prominence of key candidate themes until a fixed and final model of each group’s range of perspectives was agreed on.
Chapter 3 Participant baseline characteristics
Used with permission from Morrison et al. 103 This is an Open Access article under the CC BY-NC-ND 4.0 license (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Trial recruitment
Overall, there were 705 initial enquiries in relation to potential patients. Initial enquires by site are presented in Appendix 1, Table 27. Of these, 76 were referred, with the addition of 25 patients who were directly referred because they gave verbal consent to be contacted. Of the 101 patients referred, 61 were randomised (PI, n = 18; combined, n = 21; AP, n = 22). This was 68% (95% CI 57% to 77%) of the target of 90 randomisations. The initial enquiries, referral pathway and referral source by the different centres are shown in Appendix 1, Tables 27–29. Oxford and Manchester were the highest recruiters, with 25 and 21 participants randomised, respectively. Participants were recruited from six centres between April 2017 and November 2018, and were followed up until April 2019. Figure 1 shows the trajectory of recruitment from all centres. However, owing to staggered introduction of centres, not all participants were recruited during this period; Appendix 1, Figure 8, shows the trajectory of recruitment by centre.
FIGURE 1.
Recruitment over time.
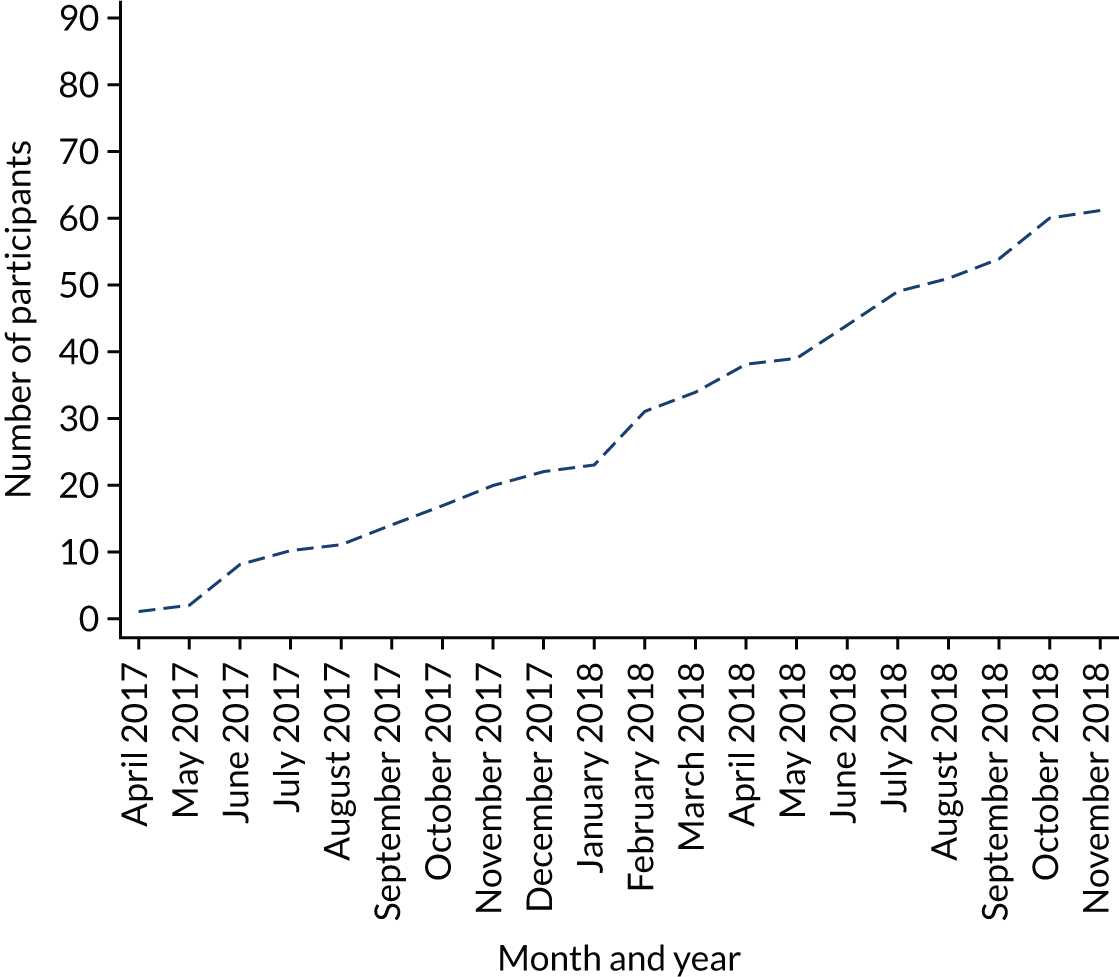
Participant flow
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is shown in Figure 2. Of the 101 referred participants, 29 were excluded; details of the reasons for exclusion are shown in Figure 2. The main reasons were declining with no reason and that the participant did not engage with the screening visit. After screening, 11 participants were excluded, the majority of whom had a score below 4 on PANSS questions P1 and P3 (see Figure 2). Across the three arms, the retention of participants for the PANSS at 6 months was 84% (95% CI 72% to 92%) (green progression zone).
FIGURE 2.
The CONSORT flow diagram. Used with permission from Morrison et al. 103 This is an Open Access article under the CC BY-NC-ND 4.0 license (https://creativecommons.org/licenses/by-nc-nd/4.0/).
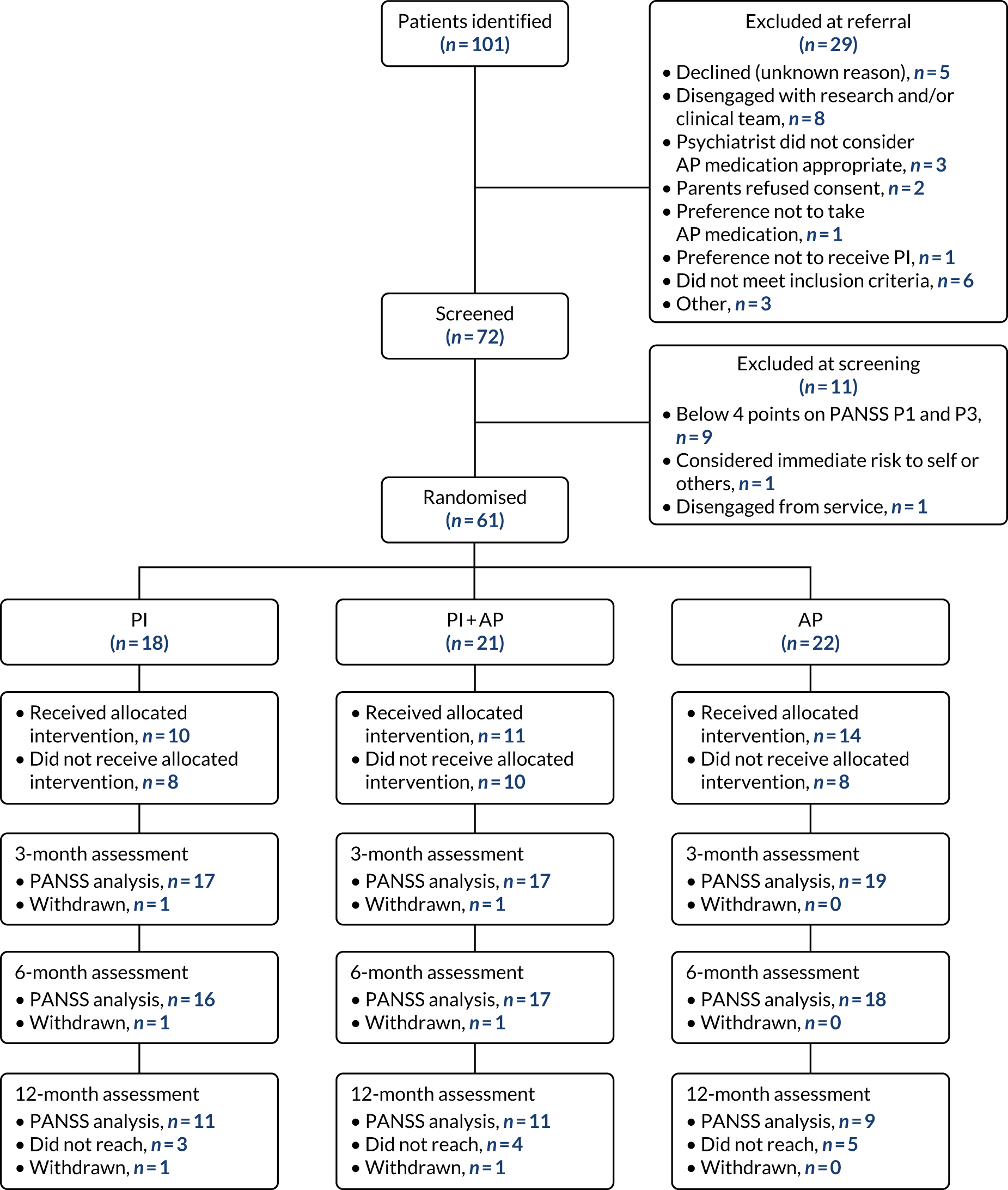
Baseline characteristics
Baseline characteristics are shown in Tables 2 and 3. Diagnoses that were allocated by trial consultant psychiatrists on the basis of PANSS vignettes and medical record data can be found in Appendix 1, Table 30. The mean age of participants across all arms was 16 years; 30 participants were male, 29 were female and two were non-binary. The mean DUP was 16.8 months in the PI arm, 13.2 months in the combined arm and 11.1 months in the AP only arm. The majority of participants were in high school, with 53 participants living with parent(s) and/or siblings. The mean PANSS total score was 72.9 in the PI arm, 75.9 in the combined arm and 74.8 in the AP arm.
| Characteristic | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| Age (years) | 18; 16.3 (1.4) | 21; 16.2 (1.3) | 22; 16.4 (1.3) |
| Gender, n (%) | |||
| Male | 8 (44.4) | 10 (47.6) | 12 (54.5) |
| Female | 10 (55.6) | 10 (47.6) | 9 (40.9) |
| Non-binary | 0 (0) | 1 (4.8) | 1 (4.5) |
| DUP (months) | 13; 16.8 (16.4) | 16; 13.2 (17.6) | 15; 11.1 (9.7) |
| Highest level of education, n (%) | |||
| Secondary | 9 (50.0) | 11 (52.4) | 11 (50.0) |
| Further | 9 (50.0) | 10 (47.6) | 11 (50.0) |
| Employment status, n (%) | |||
| College student | 7 (38.9) | 8 (38.1) | 9 (40.9) |
| High school student | 5 (27.8) | 8 (38.1) | 7 (31.8) |
| Unemployed | 4 (22.2) | 2 (9.5) | 2 (9.1) |
| Full time | 1 (5.6) | 1 (4.8) | 3 (13.6) |
| Part time | 1 (5.6) | 1 (4.8) | 1 (4.5) |
| Voluntary | 0 (0) | 1 (4.8) | 0 (0) |
| Marital status (single), n (%) | 18 (100.0) | 21 (100.0) | 22 (100.0) |
| Living arrangements, n (%) | |||
| Living with parent(s) and/or siblings | 15 (83.3) | 18 (85.7) | 20 (90.9) |
| Living in supported accommodation | 2 (11.1) | 1 (4.8) | 1 (4.5) |
| Living with other family member(s) | 1 (5.6) | 2 (9.5) | 0 (0) |
| Lives alone | 0 (0) | 0 (0) | 1 (4.5) |
| Ethnicity, n (%) | |||
| White British | 14 (77.8) | 18 (85.7) | 17 (77.3) |
| Indian | 1 (5.6) | 0 (0) | 0 (0) |
| Pakistani | 0 (0) | 1 (4.8) | 2 (9.1) |
| White Asian | 0 (0) | 1 (4.8) | 0 (0) |
| Black African | 1 (5.6) | 0 (0) | 1 (4.5) |
| Black Caribbean | 0 (0) | 1 (4.8) | 1 (4.5) |
| White Irish | 0 (0) | 0 (0) | 1 (4.5) |
| Other | 2 (11.1) | 0 (0) | 0 (0) |
| Religion, n (%) | |||
| Atheism | 7 (38.9) | 10 (47.6) | 12 (54.5) |
| Christianity | 4 (22.2) | 4 (19.0) | 4 (18.2) |
| None | 6 (33.3) | 3 (14.3) | 2 (9.1) |
| Islam | 0 (0) | 1 (4.8) | 2 (9.1) |
| Buddhism | 0 (0) | 1 (4.8) | 0 (0) |
| Other | 1 (5.6) | 2 (9.5) | 2 (9.1) |
| PANSS score | |||
| Total | 18; 72.9 (9.7) | 21; 75.9 (14.8) | 22; 74.8 (12.2) |
| Positive | 18; 21.2 (3.8) | 21; 21.2 (5.8) | 22; 22.9 (6.0) |
| Negative | 18; 15.8 (3.3) | 21; 18.5 (6.6) | 22; 17.9 (5.6) |
| Disorganised | 18; 20.1 (4.5) | 21; 21.0 (5.4) | 22; 19.9 (4.6) |
| Excitement | 18; 17.1 (3.7) | 21; 17.9 (4.9) | 22; 19.3 (4.1) |
| Emotional distress | 18; 25.2 (4.5) | 21; 24.8 (5.8) | 22; 24.7 (6.7) |
| QPR | 12; 46.1 (9.3) | 18; 42.6 (10.1) | 15; 42.1 (10.0) |
| AUDIT | 9; 7.7 (7.9) | 11; 7.4 (8.6) | 12; 6.8 (7.4) |
| DAST | 9; 1.9 (1.8) | 11; 2.5 (3.2) | 12; 1.6 (2.3) |
| AQ-10 | 8; 4.8 (2.1) | 10; 5.4 (2.1) | 11; 5.2 (1.8) |
| SPEQ | |||
| Paranoia | 8; 31.5 (12.9) | 13; 42.3 (14.8) | 14; 41.8 (14.0) |
| Hallucinations | 9; 23.4 (10.1) | 12; 27.0 (6.6) | 14; 27.4 (8.8) |
| Cognitive disorganisation | 9; 7.3 (2.9) | 12; 9.1 (1.6) | 13; 9.2 (2.6) |
| Grandiosity | 9; 6.1 (4.6) | 12; 4.9 (6.5) | 13; 3.9 (3.1) |
| Anhedonia | 9; 27.2 (9.6) | 11; 22.5 (8.7) | 13; 18.8 (9.6) |
| HADS | |||
| Anxiety | 10; 11.2 (2.6) | 13; 14.2 (2.5) | 15; 12.7 (4.0) |
| Depression | 10; 9.2 (3.8) | 13; 10.2 (4.4) | 15; 9.7 (5.1) |
| ANNSERS | |||
| Total | 8; 17.5 (10.4) | 11; 17.2 (7.1) | 12; 15.9 (6.4) |
| Number of side effects | 8; 10.6 (4.8) | 11; 11.5 (3.8) | 12; 11.2 (5.2) |
| FESFS: ability | |||
| Living skills | 9; 13.3 (1.2) | 10; 12.6 (1.6) | 12; 11.8 (2.5) |
| Interacting with people | 9; 10.6 (2.4) | 10; 10.4 (2.6) | 12; 9.2 (2.2) |
| Friends and activities | 9; 16.3 (3.9) | 10; 13.8 (2.8) | 12; 16.0 (2.8) |
| Intimacy | 9; 12.3 (2.4) | 7; 13.7 (1.6) | 10; 14.0 (3.7) |
| Family | 9; 8.6 (2.6) | 10; 8.5 (1.9) | 10; 9.1 (1.9) |
| Relationships and social activities at work | 6; 8.2 (1.6) | 7; 8.3 (1.4) | 7; 7.7 (0.8) |
| Work | 6; 9.8 (2.0) | 7; 8.6 (1.6) | 7; 9.0 (1.2) |
| School relationships and social activities at school | 6; 8.0 (1.9) | 10; 8.5 (1.3) | 8; 6.4 (1.9) |
| Educational | 6; 8.3 (1.6) | 10; 7.2 (1.7) | 7; 7.6 (1.9) |
| FESFS: frequency | |||
| Living skills | 9; 12.9 (2.3) | 10; 11.7 (2.4) | 12; 11.9 (2.4) |
| Interacting with people | 9; 11.0 (1.9) | 10; 10.2 (2.7) | 11; 9.2 (2.2) |
| Friends and activities | 9; 13.9 (4.1) | 9; 13.6 (2.5) | 12; 15.1 (2.5) |
| Intimacy | 9; 8.8 (4.1) | 9; 9.2 (4.0) | 11; 11.6 (5.5) |
| Family | 9; 9.6 (2.5) | 10; 8.7 (2.5) | 11; 8.9 (2.3) |
| Relationships and social activities at work | 6; 5.3 (3.4) | 7; 5.9 (1.9) | 7; 5.4 (1.5) |
| Work | 6; 8.5 (4.6) | 7; 8.7 (2.8) | 7; 9.1 (2.4) |
| School relationships and social activities at school | 6; 8.8 (1.7) | 10; 8.4 (1.9) | 8; 6.8 (2.4) |
| Educational | 6; 8.5 (1.4) | 10; 7.2 (2.4) | 7; 7.6 (2.4) |
| BMI | 10; 23.6 (4.5) | 13; 22.5 (3.7) | 13; 24.1 (6.4) |
| Blood pressure (mmHg) | |||
| Systolic | 10; 108.1 (11.0) | 12; 112.2 (10.1) | 10; 114.8 (11.5) |
| Diastolic | 10; 67.8 (7.8) | 12; 69.6 (9.7) | 10; 67.8 (5.7) |
| Waist circumference (cm) | 9; 80.6 (11.4) | 12; 76.3 (8.0) | 11; 78.3 (13.3) |
| FPG (mmol/l) | 5; 4.6 (0.2) | 3; 5.0 (1.1) | 6; 4.2 (0.4) |
| HbA1c | 5; 30.4 (1.1) | 2; 35.5 (2.1) | 7; 30.7 (3.7) |
| Cholesterol (mmol/l) | 6; 3.5 (0.3) | 3; 3.9 (0.4) | 7; 3.8 (0.6) |
| LDL (mmol/l) | 6; 2.0 (0.2) | 3; 2.1 (0.2) | 6; 2.1 (0.5) |
| HDL (mmol/l) | 6; 1.0 (0.3) | 3; 1.5 (0.6) | 7; 1.1 (0.5) |
| Triglycerides (mmol/l) | 6; 1.0 (0.4) | 3; 0.7 (0.2) | 7; 1.0 (0.8) |
| Prolactin (IU/ml) | 6; 163.2 (79.2) | 3; 170.3 (35.9) | 7; 268.6 (86.6) |
| Diagnosis | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| Psychosis diagnosis | |||
| No | 10 (55.6) | 5 (23.8) | 4 (18.2) |
| Yes | 8 (44.4) | 15 (71.4) | 18 (81.8) |
| Missing | 0 (0) | 1 (4.8) | 0 (0) |
| Psychosis diagnosis: type | |||
| Unspecified psychosis | 8 | 14 | 18 |
| Acute polymorphic psychotic disorder | 0 | 1 | 0 |
| Comorbid diagnosis (to psychosis) | |||
| None | 5 (62.5) | 8 (53.3) | 10 (55.6) |
| One comorbid disorder | 2 (25) | 5 (33.3) | 6 (33.3) |
| Two comorbid disorders | 1 (12.5) | 2 (13.3) | 0 (0) |
| Three comorbid disorders | 0 (0) | 0 (0) | 2 (11.1) |
| Other diagnoses for those with no psychosis diagnosis | |||
| No diagnosis recorded | 4 (40.0) | 2 (40.0) | 2 (50.0) |
| Other disorder with psychotic features | 1 (10.0) | 2 (40.0) | 2 (50.0) |
| Other disorder | 5 (50.0) | 1 (20.0) | 0 (50.0) |
Chapter 4 Outcome and results
Used with permission from Morrison et al. 103 This is an Open Access article under the CC BY-NC-ND 4.0 license (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Treatment received and adherence
Overall, in the PI arm and the combined arm, 32 out of 39 (82.1%, 95% CI 66.5% to 92.5%) participants received six or more sessions of CBT. In the combined and AP arms, 28 out of 43 (65.1%, 95% CI 49.1% to 79.0%) were exposed to AP medication for 6 consecutive weeks (Table 4).
| Indicator of adherence | n/N (%) | 95% CI (%) |
|---|---|---|
| Satisfactory delivery of adherent psychological therapya | 32/39 (82.1) | 66.5 to 92.5 |
| Satisfactory delivery of AP medicationb | 28/43 (65.1) | 49.1 to 79.0 |
Table 5 shows details of the treatment received (adherence). The treatment received (adherence) was defined in the PI arm as attending six or more sessions of CBT, in the AP arm as any exposure to AP medication for 6 consecutive weeks and in the combined arm as attending six or more sessions of CBT and any exposure to AP medication for 6 consecutive weeks. Of the participants randomised to the PI arm, 10 (55.6%) received PI exactly as allocated. In the combined arm, 11 (52.4%) participants received their allocated treatment. In the AP arm, 14 (72.3%) participants received their allocated treatment. Appendix 1, Table 31, gives reasons for crossovers.
| Adherence | Treatment arm | |||||
|---|---|---|---|---|---|---|
| PI (N = 18) | AP (N = 22) | Combined (N = 21) | ||||
| n (%) | 95% CI (%) | n (%) | 95% CI (%) | n (%) | 95% CI (%) | |
| Treatment received (adherence) | ||||||
| PI | 10 (55.6) | 30.8 to 78.5 | 0 (0) | 0 to 15.4a | 7 (33.3) | 14.6, 57.0 |
| AP | 2 (11.11) | 1.4 to 34.7 | 14 (63.66) | 40.7 to 82.8 | 1 (4.8) | 0.1 to 23.8 |
| Combined | 4 (22.2) | 6.4 to 47.6 | 2 (9.11) | 1.1 to 29.2 | 11 (52.4) | 29.8 to 74.3 |
| None | 2 (11.11) | 1.4 to 34.7 | 6 (27.3) | 10.7 to 50.2 | 2 (9.5) | 1.2 to 30.4 |
| Adherence | ||||||
| Complied with treatmenta | 14 (77.8) | 52.4 to 93.6 | 16 (72.7) | 49.8 to 89.3 | 11 (52.4) | 29.8 to 74.3 |
| Did not comply with treatment | 3 (16.7) | 3.6 to 41.4 | 1 (4.5) | 0.1 to 22.8 | 8 (38.1) | 18.1 to 61.6 |
| No treatment | 0 (0) | 0 to 18.5a | 3 (13.6) | 2.9 to 34.9 | 1 (4.8) | 0.1 to 23.8 |
| Withdrawn | 1 (5.6) | 0.1 to 27.3 | 0 (0) | 0.0 to 15.4a | 1 (4.8) | 0.1 to 23.8 |
| Unable to be captured | 0 (0) | 0.0 to 18.5a | 2 (9.1) | 1.1 to 29.1 | 0 (0) | 0.0 to 16.1a |
Table 6 provides details of the treatment received (adherence) and the crossover to a different treatment arm, with those who crossed over owing to deterioration removed. Of those who were allocated PI who crossed over into the combined treatment, one did so a result of deterioration. Of those who were allocated to the AP arm who crossed over into combined treatment, two did so as a result of deterioration.
| Treatment received (adherence) | Treatment arm | ||
|---|---|---|---|
| PI (N = 17) | AP (N = 20) | Combined (N = 21) | |
| PI | 10 (58.8) | 0 (0) | 7 (33.3) |
| AP | 2 (11.8) | 14 (70.0) | 1 (4.8) |
| Combined | 3 (17.6) | 0 (0) | 11 (52.4) |
| None | 2 (11.8) | 6 (30.0) | 2 (9.5) |
Secondary outcomes
The PANSS total and subscale scores at each time point are detailed in Table 7 along with the treatment effects for the three arms. The PANSS percentage improvement is presented in Table 8. Figure 3 shows the profile for the three treatment arms over the study period. For the comparison of PI versus AP at 3 months, the PANSS total score was lower in the PI arm (–0.39 points, 95% CI –8.39 to 7.60 points; p = 0.923). At 6 months this was the same, with a MD of –7.79 points (95% CI –16.02 to 0.45 points; p = 0.064). At 12 months, PANSS total score was lower in the AP arm, with a MD of 3.97 points (95% CI –6.80 to 14.74 points; p = 0.470). It is important to note that the number of participants with a 12-month score is low (PI, n = 11; AP, n = 9). For the PI arm versus the combined arm, at 12 months the PANSS total score was higher in the PI arm, with a MD of 5.62 points (95% CI –4.91 to 16.15 points; p = 0.295). For the AP arm versus the combined arm, the PANSS total score was higher in the combined arm (MD 1.63 points, 95% CI –10.22 to 13.49 points; p = 0.787).
| Time point | Treatment arm | Comparison | ||||
|---|---|---|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | PI vs. AP | PI vs. combined | AP vs. combined | |
| MD (95% CI) | MD (95% CI) | MD (95% CI) | ||||
| Total | ||||||
| Baseline | 18; 72.9 (9.7) | 21; 75.9 (14.8) | 22; 74.8 (12.2) | |||
| 3 months | 17; 64.9 (9.9) | 17; 64.2 (16.1) | 19; 65.6 (15.4) | –0.39 (–8.39 to 7.60) | 1.99 (–6.51 to 10.49) | 2.27 (–6.51 to 11.04) |
| 6 months | 16; 59.8 (13.7) | 17; 62.0 (15.9) | 18; 68.6 (17.3) | –7.79 (–16.02 to 0.45) | –1.31 (–9.92 to 7.30) | 6.44 (–2.44 to 15.32) |
| 12 months | 11; 61.3 (12.4) | 11; 56.2 (12.3) | 9; 55.4 (7.0) | 3.97 (–6.80 to 14.74) | 5.62 (–4.91 to 16.15) | 1.63 (–10.22 to 13.49) |
| Positive | ||||||
| Baseline | 18; 21.2 (3.8) | 21; 21.2 (5.8) | 22; 22.9 (6.0) | |||
| 3 months | 17; 19.7 (5.6) | 17; 16.1 (5.2) | 19; 18.9 (7.0) | 1.75 (–1.73 to 5.23) | 3.52 (–0.04 to 7.09) | 1.83 (–1.63 to 5.30) |
| 6 months | 16; 17.4 (6.9) | 17; 15.3 (5.9) | 18; 19.2 (6.1) | –1.01 (–4.59 to 2.57) | 2.01 (–1.61 to 5.63) | 2.88 (–0.63 to 6.39) |
| 12 months | 11; 18.6 (4.6) | 11; 13.4 (7.0) | 9; 14.0 (5.7) | 4.75 (0.10 to 9.40) | 5.16 (0.73 to 9.59) | 0.59 (–4.05 to 5.22) |
| Negative | ||||||
| Baseline | 18; 15.8 (3.3) | 21; 18.5 (6.6) | 22; 17.9 (5.6) | |||
| 3 months | 17; 14.4 (3.7) | 17; 16.8 (6.8) | 20; 16.5 (5.6) | –1.20 (–3.95 to 1.54) | –0.70 (–4.25 to 2.85) | 0.75 (–2.62 to 4.12) |
| 6 months | 16; 14.4 (5.4) | 18; 16.8 (7.4) | 18; 17.8 (5.3) | –2.09 (–4.96 to 0.79) | –1.03 (–4.55 to 2.50) | 1.31 (–2.07 to 4.70) |
| 12 months | 11; 13.7 (3.8) | 11; 14.5 (5.7) | 9; 15.1 (4.8) | –1.45 (–5.15 to 2.24) | 0.96 (–3.41 to 5.33) | 2.63 (–1.99 to 7.25) |
| Disorganised | ||||||
| Baseline | 18; 20.1 (4.5) | 21; 21.0 (5.4) | 22; 19.9 (4.6) | |||
| 3 months | 17; 18.4 (3.6) | 17; 18.4 (5.3) | 19; 18.2 (5.1) | –0.14 (–2.91 to 2.64) | 0.28 (–2.12 to 2.67) | 0.23 (–2.62 to 3.08) |
| 6 months | 16; 16.6 (3.7) | 17; 17.5 (4.3) | 18; 19.3 (6.7) | –2.57 (–5.43 to 0.28) | –0.80 (–3.24 to 1.63) | 1.55 (–1.34 to 4.44) |
| 12 months | 11; 17.8 (3.4) | 11; 16.5 (3.3) | 9; 15.1 (4.8) | 1.91 (–1.85 to 5.67) | 0.94 (–2.05 to 3.92) | –1.02 (–4.86 to 2.81) |
| Excitement | ||||||
| Baseline | 18; 17.1 (3.7) | 21; 17.9 (4.9) | 22; 19.3 (4.1) | |||
| 3 months | 17; 15.6 (3.8) | 17; 15.7 (4.5) | 20; 16.7 (5.2) | –0.21 (–2.96 to 2.54) | –0.01 (–2.90 to 2.88) | 0.17 (–2.70 to 3.04) |
| 6 months | 16; 14.8 (3.7) | 18; 16.2 (5.6) | 18; 16.6 (5.8) | –0.64 (–3.52 to 2.24) | –1.16 (–4.06 to 1.74) | –0.39 (–3.29 to 2.51) |
| 12 months | 11; 15.9 (4.8) | 11; 14.5 (4.6) | 9; 14.3 (3.0) | 1.56 (–2.12 to 5.24) | 1.55 (–2.04 to 5.15) | 0.18 (–3.71 to 4.07) |
| Emotional distress | ||||||
| Baseline | 18; 25.2 (4.5) | 21; 24.8 (5.8) | 22; 24.7 (6.7) | |||
| 3 months | 17; 21.4 (5.5) | 17; 20.3 (5.6) | 20; 21.5 (8.3) | –0.54 (–3.86 to 2.77) | 1.06 (–2.49 to 4.61) | 1.31 (–2.28 to 4.90) |
| 6 months | 16; 19.4 (5.9) | 17; 19.9 (7.2) | 18; 21.5 (8.4) | –3.08 (–6.54 to 0.38) | –0.91 (–4.52 to 2.70) | 1.77 (–1.92 to 5.46) |
| 12 months | 11; 18.9 (6.6) | 11; 16.1 (6.2) | 9; 17.1 (3.4) | 1.04 (–3.48 to 5.56) | 3.05 (–1.37 to 7.46) | 1.85 (–3.05 to 6.75) |
| Time point | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| > 25% improvement | |||
| 3 months | 7 (41.2) | 8 (47.1) | 8 (42.1) |
| 6 months | 10 (62.5) | 11 (64.7) | 5 (27.8) |
| 12 months | 6 (54.5) | 8 (72.7) | 6 (66.7) |
| > 50% improvement | |||
| 3 months | 2 (11.8) | 2 (11.8) | 3 (15.8) |
| 6 months | 5 (31.3) | 5 (29.4) | 4 (22.2) |
| 12 months | 3 (27.3) | 3 (27.3) | 3 (33.3) |
| > 75% improvement | |||
| 3 months | 0 (0) | 1 (5.9) | 0 (0) |
| 6 months | 1 (6.3) | 1 (5.9) | 0 (0) |
| 12 months | 1 (9.1) | 1 (9.1) | 0 (0) |
FIGURE 3.
Profile of PANSS total scores.
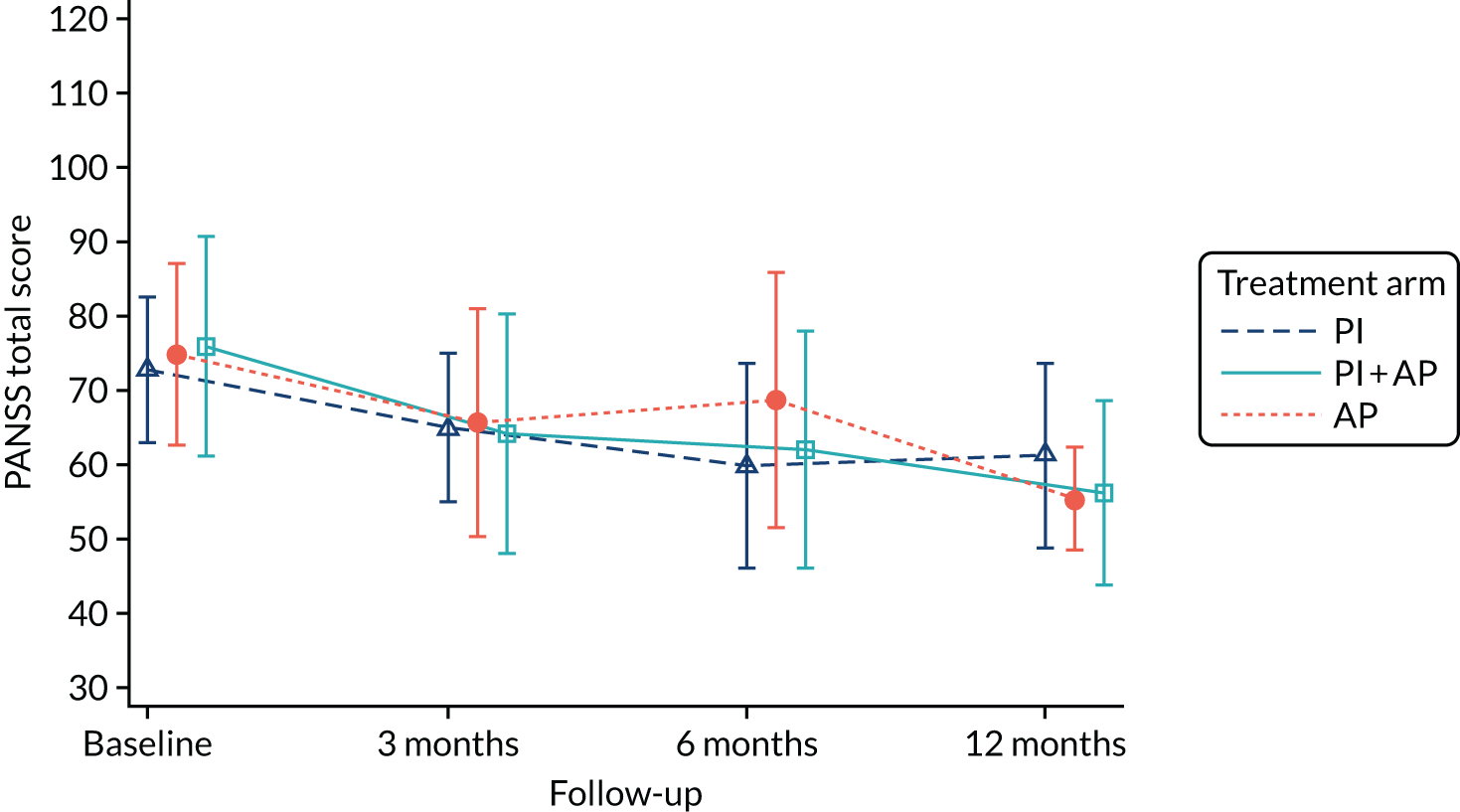
At the 3-month assessment, the number of full blind breaks was three and the number of partial blind breaks was seven. However, two participants with whom a partial break had occurred were transferred to a new and independent assessor, which meant that that five assessments were partially unblinded.
At the 6-month assessment, the number of full breaks was three but one of the affected participants was transferred to a new and independent assessor, which meant that two assessments were unblinded at the 6-month assessment. At 6 months, the number of partial breaks was seven but one affected participant was transferred to a new and independent assessor, which meant that six assessments were partially unblinded.
At the 12-month assessment, the number of full breaks was three but one affected participant was transferred to a new and independent assessor, which meant that two assessments were unblinded. At the 12-month assessment, the number of partial blind breaks was four but three affected participants were transferred to a new and independent assessor, which meant that one assessment was partially unblinded.
The QPR scores at each time point are detailed in Table 9, along with treatment effects. Figure 4 shows the profile for the three treatment arms over the study period. For the PI arm versus the AP arm, QPR scores at baseline show a difference between the PI (46.1) and the AP (42.1) arms. For PI versus AP at 6 months, the QPR score was slightly higher in the AP arm, with a MD of –2.26 (95% CI –10.24 to 5.73; p = 0.579). The QPR score was higher for the combined treatment arm than for the PI arm at 6 months (MD –2.53, 95% CI –9.47 to 4.41; p = 0.474). For the AP arm versus the combined arm at 6 months, the combined arm had a higher QPR score (MD –0.49, 95% CI –7.18 to 6.20; p = 0.886). Correlations across all time points for PANSS total and QPR scores are shown in Appendix 1, Tables 32 and 33. The other secondary outcomes are shown in Tables 10–13.
FIGURE 4.
Profile of the QPR scores.
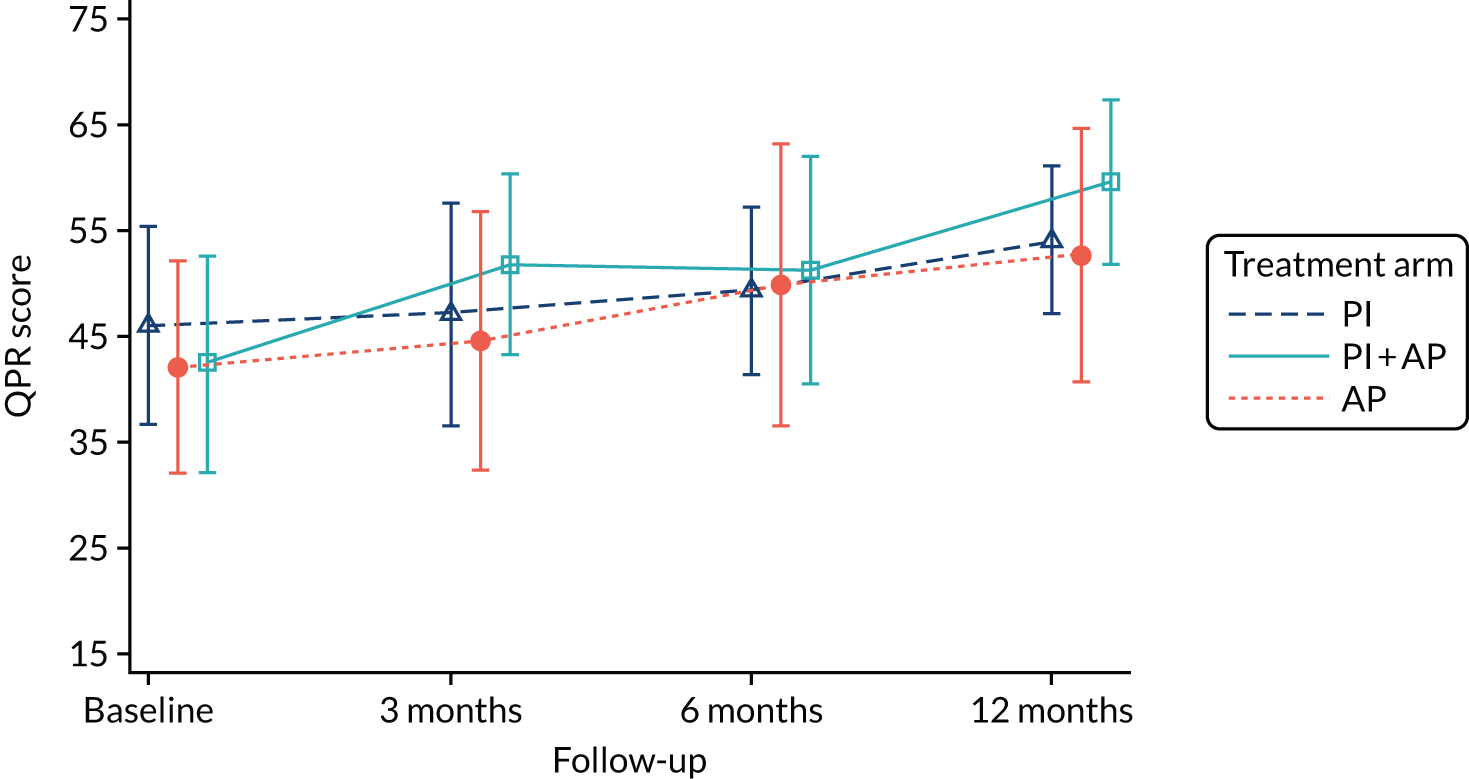
| Time point | Treatment arm | Comparison | ||||
|---|---|---|---|---|---|---|
| PI vs. AP | PI vs. combined | AP vs. combined | ||||
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | MD (95% CI) | MD (95% CI) | MD (95% CI) | |
| Baseline | 12; 46.1 (9.3) | 18; 42.6 (10.1) | 15; 42.1 (10.0) | |||
| 3 months | 11; 47.1 (10.5) | 10; 51.8 (8.6) | 13; 44.6 (12.3) | –0.61 (–8.17 to 6.94) | –6.01 (–12.69 to 0.66) | –5.98 (–13.24 to 1.29) |
| 6 months | 8; 49.4 (7.8) | 12; 51.3 (10.7) | 15; 49.9 (13.4) | –2.26 (–10.24 to 5.72) | –2.53 (–9.47 to 4.41) | –0.49 (–7.18 to 6.20) |
| 12 months | 7; 54.1 (6.9) | 9; 59.6 (7.8) | 6; 52.7 (12.0) | 2.04 (–8.06 to 12.13) | –7.83 (–15.59 to –0.07) | –10.79 (–19.99 to –1.60) |
| Time point | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| AUDIT | |||
| Baseline | 9; 7.7 (7.9) | 11; 7.4 (8.6) | 12; 6.8 (7.4) |
| 3 months | 7; 7.7 (7.7) | 9; 9.9 (10.0) | 12; 6.3 (6.3) |
| 6 months | 5; 7.6 (5.6) | 8; 6.4 (7.6) | 9; 6.1 (4.7) |
| 12 months | 4; 8.0 (5.5) | 7; 4.7 (2.9) | 6; 8.2 (6.0) |
| DAST | |||
| Baseline | 9; 1.9 (1.8) | 11; 2.5 (3.2) | 12; 1.6 (2.3) |
| 3 months | 7; 2.0 (1.8) | 10; 1.4 (2.1) | 12; 1.0 (1.2) |
| 6 months | 6; 1.7 (1.4) | 9; 2.1 (2.3) | 9; 0.8 (1.6) |
| 12 months | 4; 1.5 (1.7) | 6; 1.8 (1.8) | 6; 1.5 (2.5) |
| HADS | |||
| Anxiety | |||
| Baseline | 10; 11.2 (2.6) | 13; 14.2 (2.5) | 15; 12.7 (4.0) |
| 3 months | 10; 11.1 (3.9) | 10; 11.1 (5.4) | 13; 12.9 (4.1) |
| 6 months | 7; 10.1 (3.1) | 10; 11.8 (4.1) | 9; 11.9 (3.6) |
| 12 months | 4; 7.8 (3.9) | 7; 8.0 (4.2) | 7; 8.9 (3.2) |
| Depression | |||
| Baseline | 10; 9.2 (3.8) | 13; 10.2 (4.4) | 15; 9.7 (5.1) |
| 3 months | 10; 7.8 (4.0) | 10; 6.8 (3.0) | 13; 9.3 (5.0) |
| 6 months | 7; 6.7 (4.5) | 10; 7.3 (3.4) | 8; 10.4 (5.4) |
| 12 months | 4; 5.3 (2.2) | 7; 5.1 (4.2) | 7; 5.6 (4.9) |
| Time point | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| Paranoia | |||
| Baseline | 8; 31.5 (12.9) | 13; 42.3 (14.8) | 14; 41.8 (14.0) |
| 3 months | 9; 34.4 (15.8) | 9; 26.9 (15.0) | 11; 30.3 (21.2) |
| 6 months | 7; 24.1 (11.6) | 8; 23.6 (16.2) | 8; 31.9 (18.3) |
| 12 months | 4; 26.5 (19.2) | 6; 20.0 (9.8) | 5; 31.4 (18.5) |
| Hallucinations | |||
| Baseline | 9; 23.4 (10.1) | 12; 27.0 (6.6) | 14; 27.4 (8.8) |
| 3 months | 10; 22.1 (11.2) | 9; 15.1 (6.1) | 12; 20.9 (8.7) |
| 6 months | 6; 10.8 (9.5) | 8; 10.9 (7.9) | 9; 17.9 (11.3) |
| 12 months | 3; 11.3 (12.1) | 6; 6.8 (8.4) | 6; 16.3 (7.2) |
| Cognitive disorientation | |||
| Baseline | 9; 7.3 (2.9) | 12; 9.1 (1.6) | 13; 9.2 (2.6) |
| 3 months | 10; 6.9 (2.5) | 9; 6.2 (3.6) | 12; 8.3 (2.6) |
| 6 months | 6; 4.8 (3.6) | 6; 6.8 (3.5) | 8; 8.1 (3.3) |
| 12 months | 4; 2.8 (2.5) | 5; 3.4 (3.8) | 6; 7.8 (2.9) |
| Grandiosity | |||
| Baseline | 9; 6.1 (4.6) | 12; 4.9 (6.5) | 13; 3.9 (3.1) |
| 3 months | 9; 4.9 (6.1) | 9; 3.0 (3.0) | 11; 4.5 (6.4) |
| 6 months | 7; 3.9 (4.2) | 8; 1.3 (0.9) | 8; 5.3 (9.3) |
| 12 months | 4; 4.0 (3.7) | 6; 0.7 (1.2) | 4; 0.0 (0.0) |
| Anhedonia | |||
| Baseline | 9; 27.2 (9.6) | 11; 22.5 (8.7) | 13; 18.8 (9.6) |
| 3 months | 10; 19.3 (10.2) | 9; 22.2 (6.9) | 12; 25.9 (10.7) |
| 6 months | 7; 24.7 (7.3) | 8; 25.5 (9.2) | 9; 20.2 (12.9) |
| 12 months | 4; 14.3 (13.0) | 6; 24.3 (9.1) | 6; 25.8 (12.4) |
| Time point | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| Living skills | |||
| Baseline | 9; 13.3 (1.2) | 10; 12.6 (1.6) | 12; 11.8 (2.5) |
| 3 months | 6; 12.3 (2.0) | 6; 14.2 (1.2) | 12; 12.6 (2.2) |
| 6 months | 6; 12.3 (1.5) | 7; 12.9 (1.8) | 9; 12.6 (2.3) |
| 12 months | 4; 14.3 (1.7) | 5; 12.8 (2.3) | 7; 13.3 (2.6) |
| Interacting with people | |||
| Baseline | 9; 10.6 (2.4) | 10; 10.4 (2.6) | 12; 9.2 (2.2) |
| 3 months | 5; 12.2 (1.3) | 6; 13.2 (1.5) | 12; 10.6 (2.4) |
| 6 months | 6; 11.8 (1.9) | 6; 10.8 (2.1) | 9; 10.4 (2.1) |
| 12 months | 4; 13.3 (3.1) | 5; 11.4 (1.9) | 7; 11.6 (2.1) |
| Friends and activities | |||
| Baseline | 9; 16.3 (3.9) | 10; 13.8 (2.8) | 12; 16.0 (2.8) |
| 3 months | 5; 16.8 (1.6) | 6; 19.8 (1.8) | 11; 16.5 (3.0) |
| 6 months | 6; 19.0 (2.6) | 7; 15.7 (2.1) | 8; 17.1 (3.0) |
| 12 months | 4; 19.5 (3.1) | 5; 17.0 (3.5) | 7; 17.6 (5.1) |
| Intimacy | |||
| Baseline | 9; 12.3 (2.4) | 7; 13.7 (1.6) | 10; 14.0 (3.7) |
| 3 months | 6; 12.3 (2.9) | 6; 15.5 (2.0) | 12; 13.4 (3.5) |
| 6 months | 5; 13.4 (2.7) | 6; 15.8 (1.8) | 7; 12.4 (3.9) |
| 12 months | 4; 16.3 (2.8) | 4; 15.3 (3.3) | 6; 14.0 (2.9) |
| Family | |||
| Baseline | 9; 8.6 (2.6) | 10; 8.5 (1.9) | 10; 9.1 (1.9) |
| 3 months | 6; 9.7 (2.4) | 5; 9.6 (1.9) | 12; 8.8 (2.4) |
| 6 months | 5; 10.2 (1.3) | 7; 9.3 (1.7) | 9; 9.1 (1.8) |
| 12 months | 4; 9.8 (1.5) | 5; 9.6 (1.3) | 7; 8.7 (1.3) |
| Relationships and social activities at work | |||
| Baseline | 6; 8.2 (1.6) | 7; 8.3 (1.4) | 7; 7.7 (0.8) |
| 3 months | 2; 8.0 (1.4) | 3; 9.7 (0.6) | 5; 8.6 (1.1) |
| 6 months | 3; 9.7 (2.1) | 3; 9.3 (0.6) | 6; 8.3 (1.2) |
| 12 months | 3; 8.7 (2.5) | 2; 9.5 (0.7) | 1; 10.0 |
| Work | |||
| Baseline | 6; 9.8 (2.0) | 7; 8.6 (1.6) | 7; 9.0 (1.2) |
| 3 months | 2; 9.5 (0.7) | 3; 9.0 (1.0) | 5; 8.6 (1.1) |
| 6 months | 3; 11.0 (0.0) | 3; 9.7 (1.2) | 6; 9.3 (0.5) |
| 12 months | 3; 10.7 (1.2) | 2; 10.5 (0.7) | 1; 10.0 |
| School relationships and social activities at school | |||
| Baseline | 6; 8.0 (1.9) | 10; 8.5 (1.3) | 8; 6.4 (1.9) |
| 3 months | 4; 9.5 (2.1) | 5; 9.4 (1.1) | 9; 7.4 (1.8) |
| 6 months | 3; 8.7 (1.5) | 6; 7.5 (2.7) | 7; 7.7 (1.3) |
| 12 months | 2; 8.0 (1.4) | 5; 9.6 (1.9) | 6; 7.7 (2.5) |
| Educational | |||
| Baseline | 6; 8.3 (1.6) | 10; 7.2 (1.7) | 7; 7.6 (1.9) |
| 3 months | 4; 8.8 (1.0) | 5; 8.0 (2.9) | 10; 8.1 (1.7) |
| 6 months | 3; 8.7 (0.6) | 5; 8.4 (1.8) | 8; 7.3 (1.3) |
| 12 months | 2; 11.0 (0.0) | 5; 9.0 (1.9) | 6; 7.2 (2.9) |
| Time point | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| Living skills | |||
| Baseline | 9; 12.9 (2.3) | 10; 11.7 (2.4) | 12; 11.9 (2.4) |
| 3 months | 6; 12.5 (1.6) | 6; 14.3 (1.4) | 12; 12.3 (2.3) |
| 6 months | 6; 13.3 (2.9) | 7; 12.7 (1.6) | 9; 11.9 (2.0) |
| 12 months | 4; 13.3 (1.0) | 5; 12.2 (3.1) | 7; 12.7 (2.9) |
| Interacting with people | |||
| Baseline | 9; 11.0 (1.9) | 10; 10.2 (2.7) | 11; 9.2 (2.2) |
| 3 months | 5; 11.6 (3.2) | 6; 13.2 (1.2) | 12; 10.6 (2.2) |
| 6 months | 6; 10.7 (2.7) | 6; 11.5 (2.7) | 9; 10.8 (1.5) |
| 12 months | 4; 11.8 (4.2) | 5; 11.2 (3.3) | 7; 11.6 (2.2) |
| Friends and activities | |||
| Baseline | 9; 13.9 (4.1) | 9; 13.6 (2.5) | 12; 15.1 (2.5) |
| 3 months | 5; 16.6 (1.5) | 6; 19.7 (1.6) | 11; 16.7 (3.3) |
| 6 months | 6; 16.2 (4.5) | 6; 16.8 (1.6) | 8; 15.1 (2.4) |
| 12 months | 4; 17.0 (4.1) | 5; 16.6 (3.3) | 7; 17.3 (3.5) |
| Intimacy | |||
| Baseline | 9; 8.8 (4.1) | 9; 9.2 (4.0) | 11; 11.6 (5.5) |
| 3 months | 5; 11.2 (5.6) | 6; 9.2 (5.7) | 12; 11.0 (5.8) |
| 6 months | 5; 9.2 (6.8) | 7; 10.3 (6.3) | 9; 7.6 (4.4) |
| 12 months | 4; 11.5 (8.2) | 5; 10.0 (6.0) | 7; 11.6 (4.0) |
| Family | |||
| Baseline | 9; 9.6 (2.5) | 10; 8.7 (2.5) | 11; 8.9 (2.3) |
| 3 months | 6; 8.5 (3.2) | 5; 10.6 (1.7) | 12; 8.5 (2.4) |
| 6 months | 6; 9.2 (2.1) | 7; 9.9 (1.1) | 9; 9.3 (1.7) |
| 12 months | 4; 10.0 (1.6) | 5; 9.4 (2.1) | 7; 9.0 (1.6) |
| Relationships and social activities at work | |||
| Baseline | 6; 5.3 (3.4) | 7; 5.9 (1.9) | 7; 5.4 (1.5) |
| 3 months | 2; 7.5 (2.1) | 3; 7.7 (3.2) | 5; 6.4 (2.7) |
| 6 months | 3; 10.3 (2.1) | 3; 9.0 (2.0) | 6; 5.8 (2.0) |
| 12 months | 3; 8.3 (3.8) | 2; 9.0 (4.2) | 1; 9.0 |
| Work | |||
| Baseline | 6; 8.5 (4.6) | 7; 8.7 (2.8) | 7; 9.1 (2.4) |
| 3 months | 2; 10.0 (1.4) | 3; 9.3 (1.2) | 5; 9.6 (2.1) |
| 6 months | 3; 11.3 (0.6) | 3; 11.0 (1.7) | 6; 9.2 (2.3) |
| 12 months | 3; 10.7 (1.5) | 2; 11.5 (0.7) | 1; 11.0 |
| School relationships and social activities at school | |||
| Baseline | 6; 8.8 (1.7) | 10; 8.4 (1.9) | 8; 6.8 (2.4) |
| 3 months | 4; 9.8 (2.1) | 4; 9.5 (2.6) | 9; 7.6 (2.6) |
| 6 months | 3; 3.3 (5.8) | 6; 8.3 (1.2) | 6; 7.5 (3.5) |
| 12 months | 2; 8.5 (0.7) | 5; 9.4 (1.3) | 6; 7.8 (3.2) |
| Educational | |||
| Baseline | 6; 8.5 (1.4) | 10; 7.2 (2.4) | 7; 7.6 (2.4) |
| 3 months | 4; 9.0 (2.7) | 5; 8.4 (3.3) | 10; 8.1 (2.2) |
| 6 months | 3; 3.3 (5.8) | 6; 6.2 (2.7) | 7; 6.9 (2.3) |
| 12 months | 2; 11.5 (0.7) | 5; 7.0 (3.2) | 6; 6.5 (2.9) |
Adverse effects
The analysis of adverse effects was by treatment received (safety), which was defined as at least one dose of AP medication or at least one session of CBT. Table 14 shows the treatment received (safety).
| Treatment arm | |||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| Treatment received (safety) | |||
| PI | 9 (50.0) | 7 (33.3) | 1 (4.5) |
| Combined | 8 (44.4) | 12 (57.1) | 3 (13.6) |
| AP | 0 (0) | 0 (0) | 15 (68.2) |
| No treatment | 1 (5.6) | 1 (4.8) | 3 (13.6) |
| Unable to be captured | 0 (0) | 1 (4.8) | 0 (0) |
Table 15 shows the deterioration in the PANSS total score at 3 months by treatment received at the time of deterioration. Overall, nine participants suffered deterioration: four in the PI arm, one in the combined arm, three in the AP arm and one participant who did not receive any treatment.
| Deterioration | Treatment arm | None (N = 1) | ||
|---|---|---|---|---|
| PI (N = 4) | Combined (N = 1) | AP (N = 3) | ||
| > 12.5% | 1 | 1 | 2 | 0 |
| > 25% | 3 | 0 | 1 | 1 |
| > 50% | 0 | 0 | 0 | 0 |
Overall, by treatment received, four (23.5%) participants experienced a SAE in the PI arm, eight (34.7%) in the combination arm and two (13.3%) in the AP arm. Further details of the SAEs are provided in Table 16, as well as the AEs. Table 17 shows the potential adverse effects measure and Table 18 shows the adverse physical effects by treatment received. See Appendix 1, Tables 34–36, for details by treatment allocation.
| AE | Treatment received | ||||
|---|---|---|---|---|---|
| PI (N = 17) | Combined (N = 23) | AP (N = 15) | None (N = 5) | Unable to be captured (N = 1) | |
| SAEs | |||||
| Participants with a SAE | 4 (23.5) | 8 (34.8) | 2 (13.3) | 4 (23.5) | 0 (0) |
| Total number of SAEs | 5 | 11 | 2 | 5 | 0 |
| Participants with more than one SAE | 1 (25.0) | 2 (25.0) | 0 (0) | 1 (25.0) | 0 (0) |
| Details | |||||
| Voluntary psychiatric admission | 0 | 3 | 0 | 0 | 0 |
| Life-threatening (suicide attempt) | 1 | 1 | 0 | 1 | 0 |
| Serious violent incident | 2 | 1 | 2 | 2 | 0 |
| Admission to a general medical ward | 2 | 1 | 0 | 2 | 0 |
| Otherwise considered medically significant (overdose of medication) | 0 | 4 | 0 | 0 | 0 |
| Otherwise considered medically significant (ingested five painkillers) | 0 | 1 | 0 | 0 | 0 |
| Adverse events | |||||
| Participants with an AE | 5 (29.4) | 16 (69.6) | 13 (86.7) | 3 (60.0) | 0 (0) |
| Total number of AEs | 10 | 35 | 41 | 3 | 0 |
| Participants with more than one AE | 3 (60.0) | 9 (56.3) | 9 (69.2) | 0 (0) | 0 (0) |
| Details | |||||
| Self-harm | 6 | 12 | 7 | 3 | 0 |
| Medication side effect | 0 | 17 | 28 | 0 | 0 |
| Other AE | 4 | 5 | 6 | 0 | 0 |
| Distress reported regarding allocation | 0 | 1 | 0 | 0 | 0 |
| Deterioration in PANSS total score | |||||
| 6 months | |||||
| > 25% | 1 (6.3) | 1 (5.3) | 1 (7.7) | 1 (33.3) | 0 (0) |
| > 50% | 1 (6.3) | 1 (5.3) | 1 (14.3) | 0 (0) | 0 (0) |
| 12 months | |||||
| > 25% | 0 (0) | 1 (7.7) | 0 (0) | 0 (0) | 0 (0) |
| > 50% | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Item rated | Treatment received | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PI (N = 17) | Combined (N = 23) | AP (N = 15) | None (N = 5) | Unable to be captured (N = 1) | ||||||
| Quite a lot | Very much | Quite a lot | Very much | Quite a lot | Very much | Quite a lot | Very much | Quite a lot | Very much | |
| Number who responded | 9 (52.9) | 7 (31.8) | 5 (33.3) | 1 (20.0) | 0 (0) | |||||
| Taking part hasn’t helped me with my problems | 2 (22.2) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made my problems worse | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me feel more anxious | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part took up too much time | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part led to my mood becoming very low | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me feel more angry and irritable | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I didn’t feel ready to talk about my problems | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me think too much about bad things that have happened in the past | 0 (0) | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part meant I stopped looking after myself properly | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me feel more suspicious | 0 (0) | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part required too much energy or motivation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part increased my thoughts of killing myself | 0 (0) | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I didn’t feel listened to or believed by MAPS staff | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made my voices or visions worse | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part was making me fall out with my family or friends | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part was having a bad effect on my self-esteem | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part was making me want to harm myself | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I didn’t like or feel I could trust the MAPS team members | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I felt embarrassed talking about my problems with people I had not met before | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me have thoughts of harming other people | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part was making me feel hopeless about the future | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part meant I had to increase my medication to cope | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part involved too much hard work | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me worry that people would think badly of me because of my diagnosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me fall out with my doctor or care team | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me worry about losing control of my mind | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| My problems have improved to the point whereby I no longer feel I need helpa | 0 (0) | 0 (0) | 2 (28.6) | 1 (14.3) | 0 (0) | 1 (20.0) | 0 (0) | 1 (100.0) | 0 (0) | 0 (0) |
| Time point | Treatment received | ||||
|---|---|---|---|---|---|
| PI (N = 17) | Combined (N = 23) | AP (N = 15) | None (N = 5) | Unable to be captured (N = 1) | |
| ANNSERS total score | |||||
| Baseline | 6; 16.0 (8.6) | 12; 18.7 (8.5) | 9; 15.4 (6.8) | 4; 15.3 (6.5) | 0 (0) |
| 3 months | 5; 8.2 (4.0) | 10; 13.2 (4.9) | 9; 13.6 (6.5) | 1; 3.0 | 0 (0) |
| 6 months | 5; 7.4 (3.6) | 8; 8.0 (4.6) | 6; 13.8 (6.6) | 2; 20.5 (7.8) | 0 (0) |
| 12 months | 4; 5.5 (5.5) | 7; 4.3 (3.6) | 3; 14.3 (11.2) | 2; 12.0 (7.1) | 0 (0) |
| ANNSERS number of side effects | |||||
| Baseline | 6; 10.3 (5.3) | 12; 11.9 (3.6) | 9; 11.1 (4.8) | 4; 10.3 (6.5) | 0 (0) |
| 3 months | 5; 5.6 (2.3) | 10; 8.7 (3.0) | 9; 10.4 (5.4) | 1; 3.0 | 0 (0) |
| 6 months | 5; 5.6 (1.5) | 8; 5.9 (3.1) | 6; 10.3 (5.0) | 2; 11.0 (2.8) | 0 (0) |
| 12 months | 4; 3.5 (3.5) | 7; 3.4 (3.2) | 3; 10.7 (7.8) | 2; 9.0 (5.7) | 0 (0) |
| Weight (kg) | |||||
| Baseline | 8; 69.3 (15.8) | 13; 62.1 (11.3) | 10; 67.8 (17.9) | 4; 66.0 (22.5) | 1; 60.5 |
| 3 months | 7; 68.3 (16.1) | 11; 69.7 (11.9) | 9; 71.8 (17.5) | 2; 79.3 (32.4) | 0 (0) |
| 6 months | 6; 69.2 (13.2) | 10; 71.3 (11.7) | 7; 77.3 (19.4) | 3; 66.0 (15.6) | 0 (0) |
| 12 months | 5; 69.1 (13.4) | 6; 75.4 (8.9) | 4; 61.9 (11.8) | 2; 78.5 (31.8) | 0 (0) |
| Waist circumference (cm) | |||||
| Baseline | 7; 83.9 (13.2) | 12; 75.4 (6.3) | 9; 79.9 (14.3) | 3; 72.0 (3.5) | 1; 75.0 |
| 3 months | 6; 74.8 (26.4) | 11; 81.3 (7.1) | 9; 85.0 (16.7) | 1; 76.0 | 0 (0) |
| 6 months | 5; 78.4 (11.9) | 10; 81.0 (8.3) | 6; 85.9 (16.0) | 3; 82.0 (10.4) | 0 (0) |
| 12 months | 4; 84.3 (14.2) | 6; 82.8 (3.2) | 4; 86.0 (4.1) | 1; 77.0 | 0 (0) |
| BMI (kg/m2) | |||||
| Baseline | 8; 25.2 (4.3) | 13; 22.1 (3.5) | 10; 23.0 (4.3) | 4; 25.4 (10.8) | 1; 21.6 |
| 3 months | 7; 23.9 (4.7) | 11; 24.6 (4.2) | 9; 24.5 (4.4) | 2; 32.6 (14.9) | 0 (0) |
| 6 months | 6; 24.3 (4.3) | 10; 25.2 (4.2) | 7; 26.2 (4.8) | 3; 23.5 (2.6) | 0 (0) |
| 12 months | 5; 24.7 (4.3) | 6; 25.1 (3.7) | 4; 22.7 (3.7) | 2; 32.2 (14.6) | 0 (0) |
| Blood pressure (mmHg) | |||||
| Systolic | |||||
| Baseline | 8; 107.8 (12.0) | 12; 112.5 (9.8) | 7; 114.8 (12.0) | 4; 114.8 (10.4) | 1; 100.0 |
| 3 months | 7; 108.7 (12.2) | 11; 113.4 (10.9) | 10; 111.9 (10.5) | 2; 113.5 (7.8) | 0 (0) |
| 6 months | 6; 112.5 (13.4) | 10; 113.8 (8.0) | 7; 113.9 (13.6) | 3; 122.3 (15.6) | 0 (0) |
| 12 months | 3; 109.9 (11.0) | 5; 108.5 (7.0) | 3; 108.7 (8.5) | 2; 114.3 (6.0) | 0 (0) |
| Diastolic | |||||
| Baseline | 8; 69.0 (8.3) | 12; 68.0 (9.4) | 7; 68.5 (5.7) | 4; 66.0 (5.4) | 1; 80.0 |
| 3 months | 7; 69.6 (7.0) | 11; 70.5 (11.1) | 10; 66.2 (12.6) | 2; 66.5 (2.1) | 0 (0) |
| 6 months | 6; 71.7 (4.1) | 10; 69.5 (6.1) | 7; 64.9 (8.8) | 3; 69.7 (4.0) | 0 (0) |
| 12 months | 3; 62.6 (6.0) | 5; 65.3 (6.3) | 3; 69.1 (6.4) | 2; 65.0 (2.8) | 0 (0) |
| Fasting estimates of plasma glucose (mmol/l) | |||||
| Baseline | 2; 4.4 (0.1) | 5; 4.8 (0.8) | 5; 4.3 (0.3) | 2; 4.2 (0.8) | 0 (0) |
| 3 months | 3; 4.4 (0.2) | 2; 4.7 (0.1) | 2; 4.3 (0.6) | 0 (0) | 0 (0) |
| 6 months | 2; 5.6 (1.9) | 2; 4.5 (0.4) | 1; 5.5 | 1; 5.0 | 0 (0) |
| 12 months | 1; 3.9 | 1; 4.1 | 0 (0) | 0 (0) | 0 (0) |
| HbA1c levels (mmol/mol) | |||||
| Baseline | 3; 30.7 (1.5) | 3; 33.7 (3.5) | 6; 30.3 (3.9) | 2; 31.5 (2.1) | 0 (0) |
| 3 months | 3; 31.7 (0.6) | 2; 30.5 (2.1) | 4; 31.8 (2.2) | 0 (0) | 0 (0) |
| 6 months | 2; 29.5 (0.7) | 1; 35.0 | 2; 34.0 (4.2) | 1; 30.0 | 0 (0) |
| 12 months | 1; 40.0 | 1; 35.0 | 1; 33.0 | 0 (0) | 0 (0) |
| Lipids levels (mmol/l) | |||||
| Cholesterol | |||||
| Baseline | 3; 3.6 (0.2) | 5; 3.6 (0.5) | 6; 3.9 (0.6) | 2; 3.3 (0.1) | 0 (0) |
| 3 months | 4; 4.1 (0.3) | 2; 3.4 (0.4) | 4; 4.6 (0.4) | 0 (0) | 0 (0) |
| 6 months | 2; 3.9 (1.3) | 2; 4.5 (1.3) | 2; 3.9 (0.0) | 1; 3.1 | 0 (0) |
| 12 months | 1; 4.1 | 1; 4.0 | 1; 3.6 | 0 (0) | 0 (0) |
| LDL | |||||
| Baseline | 3; 2.1 (0.2) | 5; 2.0 (0.3) | 5; 2.2 (0.5) | 2; 1.8 (0.1) | 0 (0) |
| 3 months | 4; 2.2 (0.3) | 2; 1.8 (0.6) | 4; 2.7 (0.4) | 0 (0) | 0 (0) |
| 6 months | 2; 2.4 (0.8) | 2; 2.7 (0.8) | 2; 2.5 (0.4) | 1; 1.5 | 0 (0) |
| 12 months | 0 (0) | 1; 2.2 | 1; 1.9 | 0 (0) | 0 (0) |
| HDL | |||||
| Baseline | 3; 1.0 (0.4) | 5; 1.3 (0.6) | 6; 1.1 (0.5) | 2; 1.4 (0.1) | 0 (0) |
| 3 months | 4; 1.0 (0.2) | 2; 1.1 (0.4) | 4; 1.4 (0.3) | 0 (0) | 0 (0) |
| 6 months | 2; 0.9 (0.4) | 2; 1.1 (0.2) | 2; 1.1 (0.0) | 1; 1.2 | 0 (0) |
| 12 months | 1; 1.1 | 1; 1.6 | 1; 1.3 | 0 (0) | 0 (0) |
| Triglyceride levels | |||||
| Baseline | 3; 1.1 (0.5) | 5; 0.8 (0.3) | 6; 1.0 (0.8) | 2; 0.7 (0.1) | 0 (0) |
| 3 months | 4; 1.8 (0.3) | 2; 0.7 (0.2) | 4; 1.2 (0.5) | 0 (0) | 0 (0) |
| 6 months | 2; 1.3 (0.3) | 2; 1.8 (1.4) | 2; 1.1 (0.1) | 1; 0.8 | 0 (0) |
| 12 months | 1; 7.2 | 1; 0.6 | 1; 0.7 | 0 (0) | 0 (0) |
| Serum prolactin levels (µg/l) | |||||
| Baseline | 3; 174.3 (56.0) | 5; 173.8 (75.3) | 6; 246.7 (70.5) | 2; 249.0 (213.5) | 0 (0) |
| 3 months | 4; 138.5 (40.1) | 2; 162.0 (176.8) | 3; 503.7 (481.8) | 0 (0) | 0 (0) |
| 6 months | 2; 197.5 (20.5) | 1; 130.0 | 2; 234.0 (90.5) | 1; 336.0 | 0 (0) |
| 12 months | 1; 87.0 | 1; 150.0 | 1; 200.0 | 0 (0) | 0 (0) |
Psychological intervention
The median number of CBT sessions attended was 14 in the PI arm and 15 in the combined arm (Table 19). In the PI arm, the median number of days to the first recorded CBT session was 15 (IQR 6–44) and in the combined arm it was 14 (IQR 7–19). The number of participants for whom therapy milestones were achieved and between-session tasks were completed is shown in Table 19.
| CBT | Treatment arm | |
|---|---|---|
| PI (N = 18) | Combined (N = 21) | |
| Received CBT | 17/18 (94.4) | 20/21 (95.2) |
| Number of CBT sessions attended | ||
| 1–5 | 3 (17.6) | 2 (10.0) |
| 6–10 | 2 (11.8) | 5 (25.0) |
| 11–20 | 6 (35.3) | 12 (60.0) |
| > 20 | 6 (35.3) | 1 (5.0) |
| Overall, n; median (IQR) | 17; 14 (9, 23) | 20; 15 (9, 17) |
| Overall number of CBT sessions attended by site, n; median (IQR) | ||
| Manchester | 7; 12 (4, 29) | 7; 14 (9, 18) |
| Oxford | 6; 12 (6, 23) | 9; 15 (8, 17) |
| Other sites | 4; 18 (14, 25) | 4; 14 (17.5, 19) |
| Milestones | ||
| First session within 1 week of randomisation | 6 (35.3) | 6 (30.0) |
| Shared list of problems and goals by session 3 | 17 (100.0) | 19 (95.0) |
| Shared formulation by session 3 | 16 (94.1) | 19 (95.0) |
| Out in the world session by session 10 | 1 (5.9) | 4 (20.0) |
| Longitudinal formulation by session 26 | 10 (58.8) | 8 (40.0) |
| Between-session tasks, n; median (IQR) | ||
| Client between-session tasks set | 17; 12 (4, 23) | 20; 12 (7, 16) |
| Client between-session tasks complete | 17; 9 (2, 17) | 20; 7 (4, 10) |
| Therapist session tasks set | 17; 12 (4, 21) | 20; 12 (6, 14) |
| Therapist session tasks complete | 17; 11 (4, 19) | 20; 8 (5, 12) |
| Percentage of school- or college-based sessions, mean; SD (range) | 31.03; 37.34 (0–100) | 28.15; 38.08 (0–100) |
At the end of CBT, trial therapists were asked to indicate their perspectives about client engagement with the sessions and with between-session tasks, and the barriers to the delivery of CBT. Therapists were asked to suggest amendments to the treatment manual for a definitive trial. Nine out of 13 (69.23%) therapists who made a protocol amendment suggestion proposed that a longer therapy window would be beneficial to accommodate periods of disengagement, impact of risk on delivery of therapy, accommodate school/college exams and provide a longer engagement window. These data are presented in Table 20.
| CBT | Treatment arm | |
|---|---|---|
| PI (N = 18) | Combined (N = 21) | |
| End of therapy session | 15/17 (88.2) | 19/20 (95.0) |
| Therapists’ perspective regarding participant engagement with between-session tasks | ||
| All of the time | 2 (13.3) | 1 (5.3) |
| Most of the time | 7 (46.7) | 12 (63.2) |
| Sometimes | 6 (40.0) | 5 (26.3) |
| Never | 0 (0) | 1 (5.3) |
| Therapists’ perspective regarding client engagement | ||
| Very well | 4 (26.7) | 5 (26.3) |
| Moderately well | 7 (46.7) | 9 (47.4) |
| Not very well | 4 (26.7) | 5 (26.3) |
| Therapists’ perspective on barriers to engagement with CBT | ||
| Memory problems | 0 (0) | 2 (10.5) |
| Attention/cognitive problems | 1 (6.7) | 8 (42.1) |
| Negative symptoms | 1 (6.7) | 4 (21.1) |
| Tangential/pressured speech | 0 (0) | 0 (0) |
| Missed appointments | 4 (26.7) | 6 (31.6) |
| Difficulty researching a shared goal | 0 (0) | 1 (5.3) |
| Difficulty tolerating strong emotions in session | 2 (13.3) | 6 (31.6) |
| Trust issues/guarded | 2 (13.3) | 3 (15.8) |
| Thought disorder | 0 (0) | 1 (5.3) |
| Non-disclosure owing to minimising problems | 3 (20.0) | 4 (21.1) |
| Non-disclosure owing to fears about service response/therapist reactions | 1 (6.7) | 3 (15.8) |
| Interference from mood during the session (i.e. low mood) | 1 (6.7) | 7 (36.8) |
| Engagement issues | 5 (33.3) | 9 (47.4) |
| Unable to make use of talking therapy (i.e. monosyllabic) | 0 (0) | 5 (26.3) |
| Stressful life events | 4 (26.7) | 3 (15.8) |
| Late attendance so less session time | 0 (0) | 1 (5.3) |
| Attendance problems | 5 (33.3) | 3 (15.8) |
| Under the influence of alcohol/substances | 0 (0) | 2 (10.5) |
| Unusual experiences in session (e.g. voice hearing or persecutory beliefs) | 1 (6.7) | 4 (21.1) |
Table 21 shows the details of the FI sessions. Nine out of 18 (50.0%) participants in the PI arm, and 12 out of 21 (57.1%) participants in the combined arm, received FI. Table 21 also shows the trial therapists’ perspective regarding their clients’ engagement with between-session tasks and engagement, as well as barriers to the delivery of FI.
| FI | Treatment arm | |
|---|---|---|
| PI (N = 18) | Combined (N = 21) | |
| Received family therapy | 9/18 (50.0) | 12/21 (57.1) |
| Number of family sessions attended | ||
| 1 | 1 (11.1) | 2 (16.7) |
| > 1 | 8 (88.9) | 10 (83.3) |
| Overall, n; median (IQR) | 9; 3 (2, 5) | 12; 4 (2, 5) |
| Overall number of CBT sessions attended by site, n; median (IQR) | ||
| Manchester | 3; 2 (1, 5) | 5; 4 (3, 5) |
| Oxford | 3; 3 (2, 5) | 5; 2 (1, 5) |
| Other sites | 3; 3 (3, 7) | 2; 3.5 (2, 5) |
| Milestones | ||
| First session within 4 weeks of randomisation | 1 (11.1) | 1 (8.3) |
| End of therapy session | 8/9 (88.9) | 11/12 (91.7) |
| Therapists’ perspective regarding participant engagement with between-session tasks | ||
| All of the time | 0 (0) | 1 (9.1) |
| Most of the time | 5 (62.5) | 1 (9.1) |
| Sometimes | 3 (37.5) | 9 (81.8) |
| Therapists’ perspective regarding client engagement | ||
| Very well | 1 (12.5) | 1 (9.1) |
| Moderately well | 6 (75.0) | 5 (45.5) |
| Not very well | 1 (12.5) | 5 (45.5) |
| Therapists’ perspective on barriers to engagement | ||
| Missed appointments | 1 (12.5) | 2 (18.2) |
| Difficulty reaching a shared goal | 1 (12.5) | 2 (18.2) |
| Difficulty tolerating strong emotions in session | 2 (25.0) | 1 (9.1) |
| Trust issues/guarded | 1 (12.5) | 3 (27.3) |
| Non-disclosure owing to minimising problems | 0 (0) | 3 (27.3) |
| Non-disclosure owing to fears about service response/reaction from therapist | 0 (0) | 0 (0) |
| Engagement issues | 1 (12.5) | 1 (9.1) |
| Stressful life events | 3 (37.5) | 3 (27.3) |
| Participant unwilling to invite family members | 2 (25.0) | 1 (9.1) |
| Family members unwilling to be involved | 0 (0) | 1 (9.1) |
| Family arguments in session | 1 (12.5) | 3 (27.3) |
Fidelity to the CBT model was evaluated using seven audio-recordings of the therapy sessions (each therapist had one CTS-R tape rated). Table 22 provides descriptive statistics for the total fidelity ratings.
| Statistic | CTS-R fidelity (n = 7) |
|---|---|
| Mean (SD) | 39.71 (3.72) |
| Range | 34–46 |
| Percentage achieving a pass on the CTS-R | 85.71% |
Antipsychotic medication
For those allocated to receive AP medication, the median number of days to prescription was 18 (IQR 10–42). Fifteen out of 43 participants did not meet the AP medication success criteria, for the following reasons: participant declined AP medication (n = 3), clinician did not prescribe AP medication (n = 3), both participant and family jointly decided to decline AP medication (n = 1) and data could not be extrapolated from the medical records (n = 8).
Among those who were in receipt of AP medication the mean duration on medication was 31.5 weeks (SD 14.6 weeks, minimum 8.7 weeks and maximum 52 weeks). Fourteen participants switched from one AP medicine to another during their involvement in the study. Details of which AP medication were prescribed and their dose ranges are provided in Table 23.
| AP medication | Frequency of prescriptiona | Actual dose prescribed (mg) | Lowest dose (mg) | Highest dose (mg) |
|---|---|---|---|---|
| Aripiprazole | 21 | 19; 7.3 (2.9) | 19; 4.9 (1.5) | 20; 10.0 (5.6) |
| (5, 15) | (2, 10) | (5, 25) | ||
| Risperidone | 10 | 10; 2.0 (0.9) | 10; 1.2 (0.6) | 10; 2.7 (1.5) |
| (1, 4) | (0.5, 2) | (1, 6) | ||
| Quetiapine | 9 | 8; 173.4 (109.3) | 8; 65.6 (42.1) | 8; 281.3 (215.4) |
| (50, 312.5) | (25, 150) | (50, 600) | ||
| Olanzapine | 2 | 1; 10 | 1; 10 | 1; 10 |
| Amisulpride | 1 | 1; 75 | 1; 50 | 1; 100 |
The return rate of self-reported medication adherence was low. The number of completed measures and descriptive statistics are presented in Appendix 1, Table 37.
Health economics
EQ-5D-5L scores at all time points are shown in Appendix 1, Table 38. Scores were well balanced at baseline between the arms. Over the time period the EQ-5D-5L increased but the number of participants responding was small: [n, mean (SD)] PI arm 4, 0.763 (0.252), combined arm 6, 0.815 (0.250) and AP arm 4, 0.804 (0.133). NHS resource use at baseline and follow-up time points is shown in Appendix 1, Tables 39–42. At 12 months, one participant (5%) in each of the combined arm and the AP arm reported having attended hospital outpatient appointments that lasted for ≤ 4 hours and two participants (11%) in each of the PI arm and combined arm reported having attended an accident and emergency unit. The number of participants who reported NHS resource use was small.
Chapter 5 Qualitative results
A total of 46 semistructured interviews were carried out with clinicians with prescribing responsibility for young people who experience psychosis (n = 17); young people who participated in MAPS (n = 13); and family members of trial participants (n = 16; 18 family members). Full participant characteristics are shown in Appendix 1, Tables 43–45.
Clinician results
Three thematic areas of importance were identified from the interviews with clinicians: an overarching theme of ‘clinical decision-making’ and two subordinate thematic groupings that describe ‘professional and organisational influences’ and ‘trial factors’. These thematic areas are illustrated in Figure 5, and are discussed in detail in the following sections.
FIGURE 5.
Clinicians’ views on factors that influence referral to MAPS.
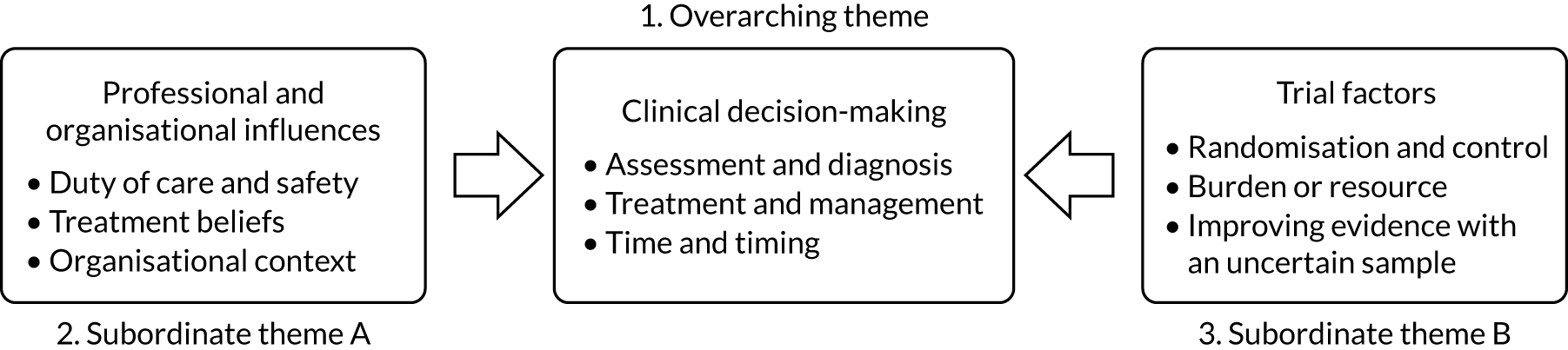
Clinical decision-making
A single overarching theme (clinical decision-making) mediated all potential referrals to MAPS. Underpinning this were two subordinate themes: (1) professional and organisational influences and (2) factors associated with the trial. The thematic structure is depicted in Figure 1, and is described with illustrative quotations from participants. Clinicians were concerned with the diagnostic assessment of FEP and consistently highlighted the need for clinical judgement in this process (partly to ensure assessment accuracy and partly to evaluate risk). Clinicians described the need for clinical judgement in determining individually appropriate treatment (weighing likely benefits and costs). Temporal factors (time and timing) were also important in their clinical decision-making, such as extended assessment periods to ensure reliable diagnosis or immediate treatment to reduce risk or distress.
Assessment and diagnosis: diagnostic uncertainty and clinical judgement
A central concern among all clinician interviewees was the importance of assessment and diagnosis of FEP when considering a referral to MAPS. They recognised that reliably identifying a FEP in adolescents is often very difficult. Along with uncertainty around distinguishing between sub threshold at-risk mental states and FEP, clinicians also highlighted the importance and complexity of assessing for neurodevelopmental difficulties, including autism spectrum features and personality, or the impact of environmental stressors among adolescents in particular:
Sometimes it can be more complicated in a child or an adolescent to make a very confident diagnosis, because you might have to watch and wait, you might have to see how symptoms develop over time, often things are not as clear-cut as they might be in somebody who is an adult, and you don’t always get textbook presentations in children, and also you have other developmental considerations to think about, such as learning, cognitive ability, there might be difficulties within the family or social settings, they might be looked-after young people living in a children’s home, you need to take all that into account so, sometimes it can be a complex assessment.
C07
Standardised psychiatric and psychological assessments for psychosis, such as the PANSS measure that was used in MAPS, were viewed as inadequate alone to resolve this diagnostic uncertainty:
I’ll see people who have experiences and are distressed by those experiences who might on a measure like PANSS might not quite make whatever the particular grade is at that PANSS in time to be part of the service but who I think very strongly that they would benefit from the service. At the same time, I might meet young people who would score on such instruments who I think are not best suited by the service and conceptualising their difficulties in that way is not the best to kind of formulate things forward.
C01
Instead, clinicians stressed the importance of using their own clinical judgement during assessment processes. Key additional dimensions central to meaningful assessment included risk, distress and severity of psychosis.
Treatment and management
There was strong consistency in clinicians’ experiences of treatment of adolescent psychosis. AP medication was commonly viewed as a necessary and helpful first-line treatment for FEP, and AP prescribing was perceived to be a key responsibility for clinicians:
If I was convinced that somebody had a psychotic illness I wouldn’t withhold antipsychotic medication to wait for therapy, you know I would see it as sort of my primary function as a psychiatrist to use the treatments that I can to try and help somebody get better, so if I thought that was appropriate I wouldn’t sort of wait to do that.
C07
Key considerations for treatment decisions included the young people’s levels of distress and risk, along with the objective severity of their symptomatology:
As the level of disturbance increases and the level of risk and the level of impairment increases, the priority is to try and reduce that risk as soon as possible, and antipsychotics can be very helpful with that.
C17
The clinical value of AP medication was nonetheless weighed against the potential serious adverse effects, particularly when prescribed to children and young people, who were viewed to be more prone to side effects. This was a further reason why such emphasis was placed on the care that is needed in conducting a reliable assessment:
You cannot just start medication based on PANSS [score]. You might realise your mistake after a year.
C05
Given that clinicians placed such importance on using their own clinical judgement to make individual treatment decisions, it was unsurprising that referring to a trial in which treatment was allocated by randomisation could be challenging:
I’ve referred a couple of people in and actually I found it quite difficult and they were kind of randomised to the opposite of what I might have chosen to do if I’d had a clinical decision, so that, that did require a degree of kind of trust.
C11
Time and timing
Clinicians also highlighted the importance of timing in assessment and treatment decisions, making it clear that, in contrast to the standardised phases of a clinical trial, in the ‘real world’ the timing of assessment and treatment decisions is far more varied. In some cases, the importance of timing in clinical practice relates to the need for urgent treatment to alleviate severe distress or risk:
It partly just really depends on the young person and where we sadly ended up last week was that her, her positive symptoms, her psychotic symptoms became far more apparent when we did the second home visit.
C03
Clinicians also discussed the value of prolonging assessment periods to ensure the reliability of a psychosis spectrum diagnosis, and subsequent treatment decisions:
There’s a degree of uncertainty when we start off about quite what might be happening for the young person so it’s not always clear-cut for me whether you know medication’s . . . something we’re going to be prescribing right at the start so you know there’s often a period of assessment before we’d be considering whether that’d be an option for someone.
C04
Professional and organisational influences
The first of the two subordinate thematic areas constructed from clinicians’ feedback encompasses the range of underlying influences that underpin and drive their clinical decision-making, which in turn have implications for referral to a trial such as MAPS.
Duty of care and safety
A dominant idea within this theme was clinicians’ own responsibility for the prescription of AP medication. Most described caution about both diagnosis and treatment of psychosis in children or adolescents, especially in relation to AP treatment:
We need to be more careful about these younger people in (a) giving a diagnosis that is as significant as psychosis and (b) using antipsychotics.
C16
However, some clinicians expressed a concern around the potential harm of not prescribing AP medication. This often related to the perceived need for immediate pharmaceutical treatment to alleviate risk or distress, but was also discussed as an important factor in long-term outlook:
If I thought they had a psychosis I wouldn’t wait to treat them, because we know that duration of untreated psychosis is you know a risk for adverse outcome.
C07
Some also expressed some concern about the delivery of psychological treatment alone to young people with psychosis. Although this concern involved the absence of AP medication, echoing concerns detailed above, it was equally grounded in direct observation of suboptimal outcomes from CBT:
I do worry that people who go for a CBT alone approach do worse, I’ve seen them a few years after they’ve finished their CBT and they want more CBT, they’ve not taken any medication, they’re frankly psychotic . . . as a clinician that can be quite distressing to see, people who’ve really avoided medication or had very very tiny bits, have never got completely better, have had grumbling psychotic symptoms, but they’ve managed them better while they’ve had the high intensity support of a CBT therapist but when that stops and the person loses that support they continue to have the symptoms.
C12
Treatment beliefs
Treatment decisions were influenced by clinicians’ prior beliefs about treatment. Clinician interviewees held a range of views on the relative benefits and risks of AP medication and psychological treatments (CBT/FI). Along with the professional caution described in relation to treatment risks, interviewees described perceived benefits of both of the treatment types offered in MAPS. For some, AP treatment was seen as essential for the most unwell young people, in part because of its perceived capacity to deliver beneficial effects quickly:
We’ve had a few under-18s who, a couple who weren’t in the trial who were very floridly psychotic and were actually admitted to paediatric wards because they were so unwell, and there was no doubt there that the appropriate thing to do was to start antipsychotic medication first.
C11
Antipsychotic medication was also seen by some to be important or even essential in the longer term to prevent relapse in young people with psychosis or after previous treatments have not helped:
I can think of a few cases where you know we’ve had very young people . . . 14 to 18 [years] who’ve been admitted to the [adolescent inpatient] unit with kind of bona fide persistent paranoid psychosis with thought disorder, negative symptoms, horrendous kind of schizophreniform illnesses, and have responded very well to for example clozapine or other antipsychotics, after other treatments have failed.
C13
Both of the psychological treatments offered within MAPS (CBT and FI) were viewed as valuable. Participants believed that they could be very helpful for dealing with a range of difficulties, such as low mood:
. . . if they are experiencing hearing voices but actually the primary problem is actually they’re struggling more with their mood or engaging with education if we were to do a psychological piece of work that’s gonna have much more of an impact than medication possibly with that.
C09
However, several clinicians voiced caution around the potentially limited capacity of individual young people to engage with psychological treatments, a concern not evident in discussions around AP medication:
. . . particularly quite young people, often that they find it quite difficult to describe their experiences or to talk about what’s going on so I’m thinking that sometimes that decision between CBT and medication is about that ability to be able to talk and think and tolerate their distress, and we have had some people who you know are very distressed and you just can’t really get them to describe their experiences, you can’t really get them to talk to you very much.
C11
The psychological treatments offered in MAPS (CBT or FI) were sometimes described as a more appropriate treatment approach than medication for certain (non-psychotic) difficulties:
It’s thinking about what actually are the specific difficulties the young person may be having for example, if they are experiencing hearing voices but actually the primary problem is actually they’re struggling more with their mood or engaging with education if we were to do a psychological piece of work that’s gonna have much more of an impact than medication possibly with that.
C09
It was evident that psychological treatment was viewed as most useful when used in combination with AP medication, as there was potential for positive interaction between the two treatments. That is, as well as providing a benefit in its own right, AP medication could enable young people to undertake and make the most use of psychological therapy:
When people are at their most unwell, that’s really difficult to even engage with and usually they’re the people who are saying you know I don’t want CBT . . . and actually medication can sometimes move them forward to the point where actually they can then engage with the CBT, in a different way.
C08
Organisational context
Clinicians represented both EIP services and CAMHS (also known as Healthy Young Minds), in which the youngest young people referred to MAPS were seen. Clinicians identified that populations attending the different services presented differently and had different needs, and moreover that the expertise and capacity within the services and the treatments available differed:
Often I guess the younger ones . . . in terms of you know their developmental stage, some of the issues they will present with might be different, and also I think sometimes in that early adolescent period it’s obviously a time when many people might have, you know, emotional symptoms anyway in the population, so sometimes kind of teasing out what represents a sort of pathological process can be, you know, the task for anyone.
C04
Differences between service settings also included important perceived variations in the diagnostic assessment expertise located in either service type. CAMHS, for example, was seen by several interviewees to offer more holistic psychological/psychiatric assessments that were more appropriate for the youngest young people:
EI [early intervention] covers a wide age range and it’s purely staffed by adult psychiatrists, so it’s difficult for me when I’ve seen say a 15-year-old and felt they were psychotic and then for an adult EI clinician to come around and say ‘well I think you they’ve got a personality disorder’, when they don’t see, bluntly they’re not experts in adolescent psychopathology and what an adolescent truly, what a generically psychologically unwell adolescent looks like because all of them are a bit dysregulated, all of them are a bit chaotic.
C15
There was, however, a perceived strength in the psychosis-specific expertise offered by EIP teams that was not always available among CAMHS clinicians:
Because a lot of the first-episode psychosis now does not sit within CAMHS . . . the experience of that within CAMHS has actually reduced, so we don’t have staff members that are used to regularly asking kids about psychotic symptoms, their experience and comfort levels with that has gone down.
C10
Something that was similar across settings was the lack of availability of PI, and accessing this type of treatment was viewed as an important organisational driver to referring young people to MAPS:
Sometimes my kind of experience of psychological therapy is coloured by often that it’s something that’s harder to deliver in as timely a manner as might be ideal and that sometimes it means that it’s coming online often much later down the line.
01
Trial factors
The second subordinate theme drawn from clinicians’ interviews concerned factors specific to MAPS. These factors interacted with the clinical influences described above, and could act as facilitators of or barriers to referral decisions.
Randomisation and control
Clinicians highlighted some concern around having less control over treatment, especially AP prescribing, if their young people were in a randomised trial:
What I’d worry about is if you came in and screened everyone who was 14 to 18 [years] with PANSS and got them all into the trial . . . there’s going to be some false positives where the psychiatrist might not want to prescribe an antipsychotic but perhaps feel pressure to because of the trial.
C06
It is important to note that clinicians recognised that MAPS did not prevent young people from accessing alternative treatment resources (e.g. psychotherapy) and did allow medication decisions to remain in prescribers’ hands, regardless of a young person’s trial allocation:
Yeah there’s certainly been people where I’ve seen them and I’ve decided not to on seeing them, even though they’re in the trial I’ve decided not to give them antipsychotics.
C16
Burden or resource
Some clinicians suggested that their involvement with MAPS involved a degree of additional workload or pressure, for example in decision-making or communication around treatment. One clinician recalled a specific incident of concern around communication with trial staff (subsequent communication of this concern ensured that further similar situations were unlikely):
Sometimes I’ve seen somebody I’m worried about and then I’ve been e-mailing and there’s not been anything and a week and a half has gone by and I’m thinking ‘if anything happens now’, I would have prescribed an antipsychotic when I saw this person, now nothing’s happened, that person has been in distress coming up to 2 weeks, they’re a bit risky.
C16
However, more frequently clinicians found that the trial offered important additional resources, especially clinical psychology (CBT/FI). Clinicians valued that the trial frequently offered access to psychological treatments far more quickly than was typical in services, and that trial-based therapists had the capacity to offer longer or more comprehensive courses of therapy:
So MAPS is great ’cos one young person who’s got shedloads of psychological therapy who we may have wished to treat with psychological therapy but without the MAPS trial we would not have been able to do that.
C01
Improving evidence with an uncertain population
Clinicians perceived that MAPS had the potential to improve the limited evidence base for treating psychosis in young people. However, some voiced uncertainty around the ultimate value of this trial’s results. It was recognised, for example, that the level of treatment resource in the trial may be unrealistic when compared with typical NHS service capacity and, therefore, study results may not be generalisable to everyday clinical settings:
The study might show that the CBT is effective, but we’re starting CBTp incredibly quickly. I think you would have to then be able to offer it very quickly if you were rolling it out as a treatment because also what you’re not testing in the study is waiting 6 months for your CBTp.
C15
The diagnostic uncertainty around young people with psychosis spectrum experiences could also affect the perceived validity of the trial’s results. Clinicians argued that if trial inclusion criteria do not adequately reflect clinician’s own diagnostic judgement then it is difficult to have confidence in generalising results from the sample included in the trial:
There will be many people in the MAPS study . . . that on reflection haven’t had first-episode psychosis . . . they might’ve met criteria but actually there’s you know there’s lots of reasons why people could meet criteria and it doesn’t mean they’re psychotic.
C16
Participants’ results
These findings are organised around the two thematic areas of importance: (1) being part of a trial, and (2) receiving treatment. Each is described in turn, supported by illustrative quotations from the young people. See Figure 6 for the thematic structure.
FIGURE 6.
Participant perspectives of MAPS.
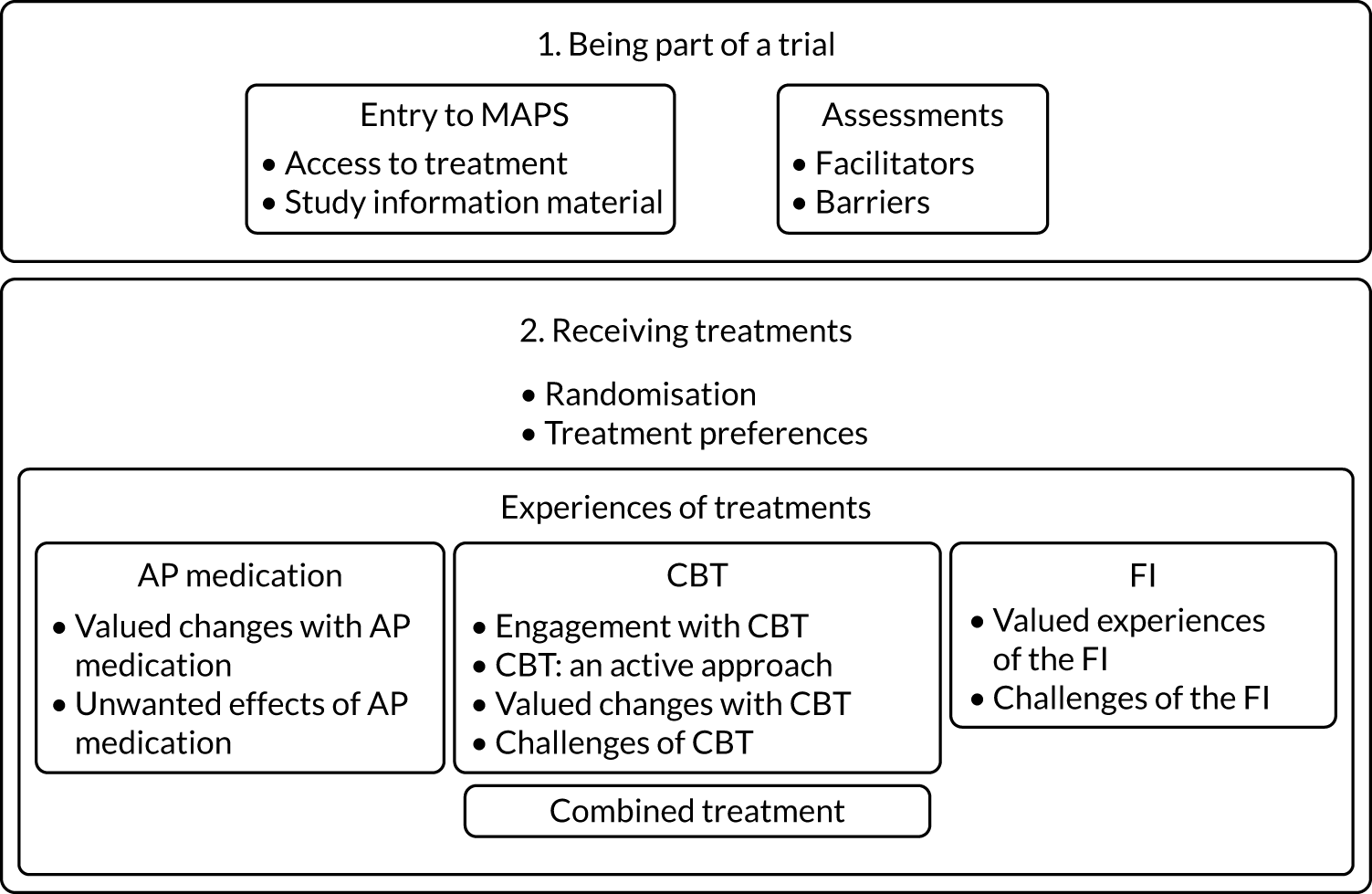
Being part of a trial
Participants described the process of becoming part of a randomised clinical trial. For these young people, randomisation to treatment had in fact been a relatively minor concern, with aspects of referral and assessment processes, along with general access to treatment, featuring more prominently in their feedback.
Entry to MAPS
Access to treatment
The over-riding motivation for taking part in the trial was continued help-seeking; that is, participants were seeking further specialist support, either in addition to local mental health services or more quickly than services could typically offer. Timely access to the psychological treatments offered in MAPS was, therefore, often seen as a primary benefit of participation:
With services like that . . . it kind of takes a while to sort of get like anything done really, but I think cos [RA] like spoke through it all and it was either medication or therapy or both so that would be like straight away like right there.
Young person (YP) 13
Study information material
Entering the trial was perceived to involve a requirement to engage with a lot of information, the key sources of which were discussions with trial staff and the study participant information sheet. Participants viewed both as trusted sources of information; however, the amount of information and technical details of the participant information sheet could be problematic, as some had difficulties with comprehension or concentration (‘I disorientate a lot’, YP04). This was seen as especially challenging for younger participants and those with learning difficulties, such as dyslexia:
When you hand me a big sheet of writing I’ll not read it, because I can’t break it down.
YP03
Several participants indicated that they had not paid much attention to study information (‘I was younger back then, so I didn’t really read them’, YP09) and had relied on family members, clinicians or trial staff to explain and retain participant information sheet details:
As long as mum knows what’s going on then she can explain it to me in a way that I will understand.
YP07
All participants had been referred from specialist mental health teams (CAMHS, EIP). A key step in this process was encounters with clinical service staff who made the referral (e.g. care co-ordinators, psychiatrists) and RAs who consented participants and conducted baseline assessments. Participants particularly valued RAs’ emphasis on choice in terms of trial entry, adherence to treatments and continued engagement with the trial:
They always said you know if at any point you don’t wanna do it, if at any point, you know they made it really clear that it really was your personal choice to do it.
YP10
Assessments
Facilitators for engagement
Participants frequently highlighted the benefit of RAs’ practical flexibility around assessment meetings, including offering a choice of meeting locations and times that suited the participant, sending appointment reminders by text message and rearranging meetings during difficult times:
[RA] was really good . . . where’s good for you to meet where’s you know, and it’s always nice when someone’s like that cos you know it shows that they actually value your time.
YP10
The interpersonal qualities that RAs demonstrated during assessments were perhaps the most frequently discussed facilitating factor identified by participants (e.g. ‘warm and welcoming’, YP03). Feeling comfortable with RAs was considered particularly important for disclosing personal information and building trust:
I was meeting [RA], and she was like so kind I could’ve like talked to her like with everything, you know.
YP01
Participants were also given a small financial token (£10) at each assessment; this was intended to help incentivise attendance and demonstrate to participants that their involvement was materially valued. Feedback from participants was understandably positive about this aspect.
Barriers to engagement
Participants also discussed aspects of assessments that could be considered barriers to engagement with RAs. Completing assessment measures, for example, was seen by some as repetitive or ‘boring’ (YP07). Participants also commonly described difficulty in ‘opening up’ (YP09) about their experiences during these assessments:
Nothing against [RA] but it’s just some things that are like quite personal you know they’re a bit difficult to talk about.
YP02
Several participants also found it hard to verbalise their concerns owing to difficulties with learning and comprehension [dyslexia (YP03), autism (YP06) and memory (YP03)]. Specific questions about use of drugs or alcohol were also viewed by one participant as unwelcome (YP09). A common cost associated with assessments was unease or physical pain related to blood sampling (optional):
I’ve never had my blood taken before . . . we did try once but yeah she missed my vein . . . so then she had to like take it out and it was just really painful.
YP08
Receiving treatments
Randomisation
The relative acceptability of treatment allocations is an essential consideration when assessing the overall feasibility and acceptability of a clinical trial. Although all of the trial participants demonstrated acceptance of MAPS treatments by consenting to be randomised, participants’ recall of their entry to the trial indicated variations in degrees of treatment acceptability. The range of views describing this variation were characterised overall by a general belief that any treatment type could potentially be helpful and, therefore, acceptable:
Didn’t know what was like going on with me and stuff like I didn’t understand like why I heard stuff so I thought . . . if it’d help then I wouldn’t mind any of them.
YP12
Treatment preferences
Although some participants’ treatment preferences prior to randomisation were based purely on expectation (what may happen), others were informed by previous experience or observation (e.g. family members’ mental health treatment). Participants often reported that CBT was preferred over AP medication, and this was commonly influenced by the perceived benefits of ‘talking to someone’, along with concerns or uncertainty about side effects of the medication:
When I looked like through the different like [medication] side effects and symptoms . . . I started like freaking out a bit, whereas I think like talking to someone’s a lot easier.
YP02
By contrast, three participants indicated a preference for medication over therapy. For one participant this was a change of view after undertaking CBT within the trial, whereas the other two participants preferred medication from the outset. Although concern about AP medication was more common, in practice most participants accepted or believed that they would have accepted it:
Although I was quite hesitant I think I always said to myself that I’d go in to the appointment and find out all the side effects, you know the possible help it could provide . . . you know never say never.
YP10
Three participants voiced a preference for the combined treatment allocation, perceiving that more treatment would offer stronger benefits. One participant’s preference for combination treatment was informed by specific prior experience:
I think they work better together . . . I’m on antidepressants and stuff as well, and them alone will not work fully, but also therapy alone doesn’t work fully because you’re not in the right frame of mind to accept therapy.
YP03
Few participants expressed any initial views on the FI element of PI, although three participants were clear that they declined or that they would have declined FI, and two of those rejected FI as they did not want to worry family members:
I don’t really like worrying anyone so bringing like family or friends along would just you know worry them a bit more.
YP02
Experiences of treatments
Subsequent experiences of the treatments received were also discussed, and interview questions aimed to discover whether each treatment had been helpful, unhelpful or harmful, and in what ways.
Antipsychotic medication
Of the nine interview participants allocated to receive AP medication during the trial, eight were prescribed AP medication and seven took them (three in the AP only arm, four in combined treatment). Both participants who did not take AP medication were in the combined arm.
Valued changes with antipsychotic medication
All seven participants who took AP medication reported benefits. Six identified both psychosis-specific and general improvements in psychological well-being. The most common psychosis-specific improvements reported by participants were reductions in auditory and visual hallucinations:
It was helpful for me to stop seeing, for me to stop seeing shadows and hearing voices.
YP05
Additional psychological benefits included reductions in unwanted cognitive intrusions, along with social anxiety or paranoia, leading to improvements in social functioning:
When you’ve got like medication that’s making you more stable, then you’re more like logical about leaving the house and stuff.
YP03
Sedation leading to improved sleep was described in a number of interviews and was considered a positive effect:
[AP] makes you a lot more like sedated, and like, doesn’t slow down your mind but it just makes you really tired so . . . I’ll have a better night’s sleep.
YP09
Unwanted effects of antipsychotic medication
All seven participants who took AP medication also reported side effects. One participant discontinued their AP medication following the onset of intolerable side effects and concerns from their mum:
My mum’s just told me to stop taking it . . . the voices got louder, and my bones started aching, a lot. I just changed my attitude where I was getting frustrated easily.
YP05
Unwanted sedative effects (e.g. ‘I felt really really tired’, YP02) were reported by most participants. Other adverse effects attributed to AP medication included physical symptoms (e.g. ‘really bad headaches’, YP09) and worsening mood (YP11). Two participants identified increased appetite and weight gain as unwanted effects; for one of those, this interacted with an ongoing difficulty with eating (YP03). The perceived stigma of taking AP medication was also mentioned as an adverse effect that had implications for interacting with others:
It’s hard like if someone’s like ‘do you wanna stay at my house’. I’m like well I’ve gotta bring my medication . . . will I have to explain what it is, will they think I’m crazy.
YP03
Cognitive–behavioural therapy
Ten participants were allocated to PI (five to PI only, five to combined treatment) and all attended CBT sessions. One participant’s course of CBT ended early after three sessions, and they requested AP medication from their psychiatrist.
Engagement with cognitive–behavioural therapy
Most participants who attended CBT sessions found it helpful to have regular opportunities to speak to a therapist about their difficulties. For some participants, the duration of their course of therapy allowed ‘a relationship’ (YP03) to form and develop, with therapists’ perceived trustworthiness and understanding being valued attributes:
I could never talk to anyone, and cos it’s kind of hard to talk to people about things that they’re never gonna understand but with [therapist] I felt like I can talk to her about anything.
YP13
The flexibility that was offered by therapists when arranging sessions was also valued, particularly therapists’ capacity to rearrange sessions and to visit participants at home. Similarly, the flexibility that therapists demonstrated within sessions allowed participants to prioritise salient topics for discussion (‘seeing and hearing things . . . anxiety . . . family . . . school’, YP08).
Although participants’ discussions of engagement were broadly positive, one participant likened their therapist to a teacher, having felt ‘spoken down’ to (YP07). Several participants described therapy as challenging, recalling difficulties with initially ‘opening up’ and sometimes feeling upset by the nature of discussions during therapy:
I think that it was good that I did that ’cos I kind of came to terms with all of it . . . but yeah while I was talking and stuff and, it was upsetting.
YP12
Cognitive–behavioural therapy: an active approach
Participants commonly experienced CBT as a more active and interactive therapeutic approach than they had expected or experienced previously. Therapists’ use of written materials and individual psychological formulations in CBT was considered particularly helpful. One participant found the process of goal-setting in therapy unhelpful, leading to frustration and ultimately disengagement (YP04). However, most participants identified goal-setting and collaborative working with their therapists as valued aspects of CBT:
I was like kind of bringing up the issues and I think she was mostly like bringing up possible like solutions almost like ways around it and things we can do about it.
YP13
Participants also recalled ‘homework’ tasks that they worked on between CBT sessions (e.g. testing appraisals around distressing experiences). In some cases homework tasks were suggested by therapists and in others the homework was devised collaboratively (e.g. YP08):
[Therapist] would always ask me to give her homework then she’d give me homework as well . . . that was really good ’cos it wasn’t just like then we’re meeting like once a week and then nothing’s happening in that week, we set each other like different tasks to do in between that week . . . it didn’t feel like homework.
YP08
Valued changes with cognitive–behavioural therapy
All but one participant who undertook CBT described resultant changes or benefits. Although some who benefited were also taking AP medication, the following changes were reported by participants who did not take AP medication or were described as specific to CBT.
Improved understanding
The most common benefit of CBT that was described by participants (8/10) was improved understanding of themselves and their psychological experiences. Participants described numerous aspects of improved understanding and how it arose. Centrally, the active process of talking to a therapist was seen to help improve self-perception:
Being able to talk about it you feel like you’re not like kind of alone anymore and yeah you feel like there’s somebody there to listen to you and you’re more understood and you understand yourself a bit more.
YP08, PI only
Some participants attributed improved understanding to more specific CBT elements. For example, many participants identified normalising their difficulties as useful. Therapists’ active normalisation of psychotic phenomena most often involved sharing information or stories of others who have had similar experiences:
Watching that [TED (Technology, Entertainment and Design) talk on voice hearing] has absolutely changed the way I view things and having that in one of the first few sessions I think allowed me to open up more because you suddenly think well it isn’t just me.
YP10
Several participants described gaining new ways of thinking about the difficulties that they had experienced and changes in how they responded to distressing thoughts or emotions:
If I was walking down the street I saw kind of everybody as a threat . . . so we decided what if I looked at them differently . . . would that change how, how bad my anxiety was and if it would have an effect on my experiences and it did . . . talking about it we were able to make links, connections and then a solution.
YP08
Participants described subsequent benefits in being able to cope with or control their psychological difficulties:
Although I still have like the symptoms and stuff and I still get it every now and then pretty bad, I have like more like control over it.
YP13
Reduced symptoms of psychosis
Four participants explicitly reported reductions in auditory or visual hallucinations, and attributed these improvements to engaging with CBT:
I used to hear my dead uncle . . . and I used to see him and everything, but that’s kind of stopped.
YP01
Several participants also identified improvements in mood-related domains, such as anxiety and anger:
I’m not getting outbreaks or blackouts as much as I used to . . . a blackout is when I get so angry that I don’t know what I’m doing.
YP06
Improved social functioning
The majority of participants who described PI as beneficial also identified improvements in social ability and functioning related to the changes detailed previously:
Like being open with people and being able to actually feel confident to get up and go out and see people . . . before I was basically completely isolated in this house apart from college.
YP13
For several participants this included feeling more able ‘to open up’ (YP10) to others, enabling further social support.
Challenges of cognitive–behavioural therapy
Although CBT was largely perceived as beneficial, and no participant described the therapy as harmful, one participant did voice scepticism about CBT leading to sustained improvements that would last beyond the duration of therapy:
There’s a way of like putting a plaster over it which I feel like therapy does for a while but, it doesn’t really stop things from happening again.
YP03
Family intervention
There was variability in participants’ engagement with the FI component of the PI, with just 6 out of 10 participants describing some level of FI experience during MAPS.
Valued experiences of family intervention
Participants who attended FI sessions with their family members described several aspects of the process as helpful. These share similarities with core features of CBT experience, but were also particular to the family context.
A chance to tell them what I’m going through
Although participants may have previously discussed their psychological difficulties with family members, such conversations were commonly limited. Therefore, family sessions served primarily to facilitate such discussions and to develop a shared perspective:
The family session was a chance for me to tell them what I’m going through and a chance for them to tell me what I’ve been doing wrong, and what I’ve been doing right.
YP06
Improved understanding
Participants highlighted the value of FI for improving family members’ understanding of participants’ difficulties, and enhancing family members’ ability to offer support with difficulties:
If I like, I’m feeling down or anything and my dad notices I guess he’ll sort of make sure he’s not, he’s not doing anything to make it worse, and he’s always kind of aware now and always like checking up on me.
YP13
Family members opening up
Family intervention sessions could also facilitate unexpected and helpful disclosures from family members about similar personal experiences of psychological distress:
It allowed me to have a conversation with my mum that actually when [therapist] left it turned out when my mum was 21 she had exactly the same thing and she didn’t see people she saw spiders . . . that then opened up a whole other world of support that I didn’t even realise was there.
YP10
Challenges of family intervention
Several participants who engaged with FI recalled that it had been difficult to begin sessions; one participant, for example, felt that their family members ‘did not understand mental health’ (YP06), whereas others felt that discussions in family sessions ‘can be a bit embarrassing’ (YP09) or ‘scary’:
To actually get that conversation started of like I hear people that aren’t there it’s, you don’t know how to break that to someone, it’s really really hard to put that into words and especially to someone that you love because what you don’t want them to do is turn around and be like you’re crazy, cos that’s just, that’s your immediate fear.
YP10
Combined treatment
Five interview participants were allocated to receive AP medication and PI, although two did not take AP medication. All three participants who continued with both treatments described the combination of treatments as helpful. Notably, AP medication was perceived to facilitate engagement with PI, with each treatment helping in different ways, but also interacting beneficially:
In my opinion it’s [combined medication and PI] the best treatment for anything, because the drugs allow you to feel in a better state of mind to accept therapy, and then when therapy is opening up things you maybe wouldn’t be comfortable opening up with the drugs help make that more comfortable, so they all work together.
YP03
Family members’ and carers’ results
Although non-related carers were eligible for interview participation, in practice interviews included family members only (parents and one grandmother); therefore, the following discussion of interview findings refers to ‘family members’ throughout. The analysis identified two themes areas of importance: (1) family role in young person’s entry to MAPS and (2) family views of treatments for psychosis. Each is described in turn and illustrated in Figure 7.
FIGURE 7.
The family perspectives of MAPS.
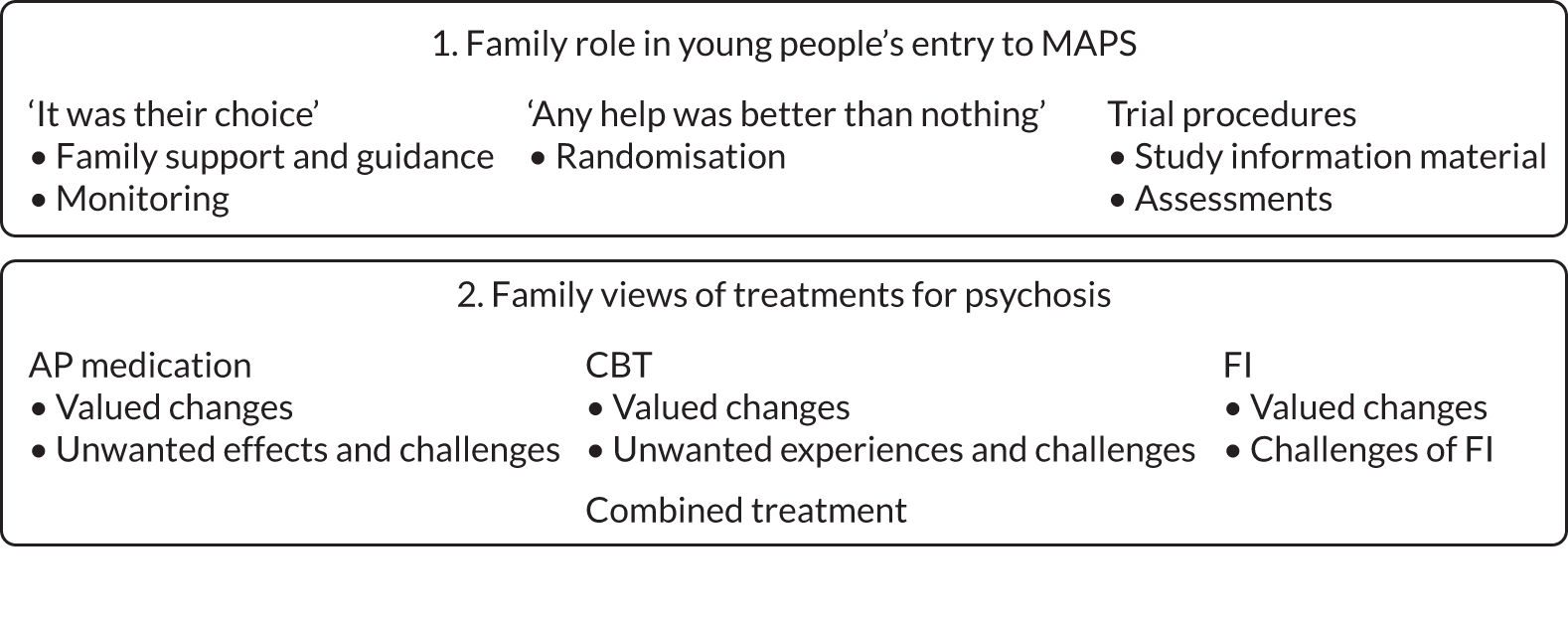
Family role in young people’s entry to MAPS
Family members did not always have an active role in the young person’s decision to take part in the trial, but were commonly motivated to support their young person’s decision and their continued engagement with trial processes and treatments, while monitoring for impacts on the young person, both positive and negative:
It was their choice
For some trial participants (14- to 15-year-olds), parental/carer assent was required for initial contact with trial staff and, therefore, entry to MAPS. Nonetheless, all family members voiced the view that the decision to enter MAPS had been the young person’s:
It was ultimately her decision . . . we just discussed which she wanted to do, what if it was one [treatment] or what if it was the other and she just said she knew she needed to have help.
F12
Family support and guidance
Some family members viewed themselves as having an active role that involved offering support and advice on starting the trial and throughout their young person’s involvement:
Yes it’s been discussed between us but it’s [young person]’s decision . . . if I can help with guidance as a parent, that’s what I’m here for.
F03
Although allowing the young person to make that initial decision, family members were mindful that there were constraints to this, which included the urgency of accessing treatment and the time limits that may have influenced entry to the trial:
I know that he didn’t feel pressurised in any way . . . I think what [psychiatrist] explained was, obviously it was an opportunity and you know he was free to take it up or not, but it was obviously time limited as well, the option to opt in was time limited but he chose to opt in so.
F11
In this example, a dad describes how he would have been willing to over-ride his son’s initial entry decision if he had seen that to be in his son’s best interest:
I think if he had have had fears about it and that we couldn’t have alleviated it I’ve gotta be honest we’d have probably pulled him from the trial purely because . . . we didn’t want him to worry about anything unnecessarily.
F09
Monitoring
An important demonstration of family support was evident from descriptions of what could be termed as ‘monitoring’, in which family members were active in assessing the impacts of trial treatments and intervening to address important concerns:
The [AP] dose that she was originally put on was too high so I tried that for 2 days and I just went back and said this isn’t my child, I need it changing, and to be fair the doctor’s worked with me and she reduced the dose.
F04
Any help was better than nothing
Family members’ own motivation to support the young person’s entry to MAPS was frequently characterised by a sense of urgency in seeking help; entry to MAPS represented a valued opportunity to access help and support that either was unavailable within local services or could be accessed more rapidly via the trial:
We thought that it would be a good idea, because I thought that she’d get more help than if she was just stayed through CAMHS.
F15
This was particularly important when family members perceived psychological therapy or ‘specialist help’ to be needed. Family members were often aware that these resources were often scarce, especially psychological support:
As soon as I heard that there was psychology involved or a possibility of psychology . . . it was like right well at least we’re gonna get a step in the right direction.
F04
Randomisation
The over-riding desire for help, any help, may explain family members’ general acceptance of randomised allocation to trial treatments:
We didn’t quite know whether it’d be the medication, if it would be the talking therapies or if it would be both . . . but we were just you know wanting something to start to just help him really.
F13
However, some family interviewees either had not been aware or made clear that randomisation occurred or had believed that trial treatments would be delivered based on individual needs:
I didn’t know it was random to be fair, yeah I thought she, she well not it’s not chosen on merit is it that’s the wrong word but you know chosen because . . .
Kind of needs-based.
On her needs yeah, yeah yeah.
F05
Two family members who did recall randomisation expressed some concern that this reduced the control around treatment:
I think the concern was obviously in terms of the, the control, you’re not sure which avenue he’s gonna be targeted down.
F11
However, more commonly participants seemed reassured that being in the trial did not prevent discontinuation or change of their young person’s treatment, or withdrawal from the trial:
I’m aware that you know it is a research study but actually if things changed, he could pull out and get the active treatment he needed at any point.
F11
Trial procedures
Along with discussions of decision-making at entry to MAPS, family members shared views relating to two additional important aspects of trial experience: study information material and assessments.
Study information material
Family members who recalled the study information material (participant information sheet) generally found it comprehensible and helpful. Some did express concern that the materials had been difficult for the young person to understand because of learning or mental health difficulties. However, they perceived that sufficient support from research staff had been in place to ensure that the young person was able to make an informed decision about entry to MAPS. There was also concern about use of the term ‘psychosis’ within the participant information sheet because of the generally negative perception of psychosis among the public and how this could have an impact on the young person:
Can a young person say to any of their peers or to anybody else, ‘I suffer from psychosis, I’m psychotic,’ no they can’t because everybody just thinks that means that they’re gonna get an axe out of their pocket and chop them up.
F06
Several family members had first seen the term ‘psychosis’ used in relation to their young person during entry to MAPS, possibly along with the provision of the study participant information sheet. CAMHS or EIP teams from which young people had been referred had not used the term, and this discrepancy was considered problematic:
I could certainly understand why someone might not want to have their child suddenly told they had psychosis based on a study, without you know, with GPs or CAMHS or however it’s done.
F07
Assessments
Family members were not typically present during assessment meetings between young people and MAPS RAs. However, several had been present or recalled comments made by the young person about these meetings. Participants often spoke very positively of the RAs who conducted assessments, referring to interpersonal and professional qualities that they perceived had ensured that the young person felt safe and comfortable:
She enjoyed it when [RA] came round . . . there were certain aspects that she didn’t want to talk about but because she was comfortable with [RA] she spoke about them freely.
F04
Family members also valued the flexibility RAs showed around arrangements for appointments, including home visits and text message reminders. A potential disruption to this positive relationship was the practice of ‘blinding’ (in which the young people were asked to conceal their treatment allocation from the RA), which one mum identified as somewhat difficult for her daughter.
Although assessment appointments were largely perceived to be acceptable and even helpful, there were suggestions that they could be overly long. Participants also reported that the nature of assessment questions could be challenging. For example, one family interview elicited concern about questions relating to suicidality, although the mum ultimately recognised that these were important and appropriate. Another negative aspect of the assessments reported by family members was blood sampling. One parent reported that the young person had considered declining entry to the trial because they feared this single aspect. Another mum attributed the young person’s recent fear of blood tests to an unpleasant experience during the trial:
Now he won’t go into the GP and get any bloods done.
F13
Family views of treatments for psychosis
Family members expressed a broad range of individual views of treatments for young people experiencing psychosis, and discussed several important areas of concern. Family members’ views of treatment were often complex and included both positive and negative reflections around the same treatments.
Antipsychotic medication
We interviewed family members of 10 young people allocated to receive AP medication, two allocated to AP alone and eight to combined treatment (one of whom was not prescribed AP medication). Given this complexity, analysis has sought to carefully isolate AP-specific features of interview feedback.
Acceptability
Antipsychotic medication, as a single treatment or part of a combination, was ultimately acceptable to all family interviewees, with several highlighting the importance for them of AP medication in reducing young people’s extreme behaviour or risk. Some were cautious about its potential to help, and viewed it as temporary or expressed concern that the young person could become permanently dependent on AP medication. All family members expressed concern about potential side effects of AP medication. However, all family members also ultimately accepted AP medication, and indeed three participants indicated that they may have withdrawn the young person from the trial if AP medication had not been prescribed:
I would have considered that him not taking antipsychotics would have put his brother and sister at risk, so I probably, if he’d happened by chance to be put on that stream I’d have said sorry no that’s not happening.
F06
Valued changes
Most participants described psychosis-specific benefits and more general improvements that they attributed directly to AP medication. These included reductions in the frequency and intensity of voice hearing, reductions in paranoia and mania, improved sleep, and feeling calmer and less angry. In several cases, improvements were described as partial or ongoing, with voices, for example, continuing at reduced intensity or frequency. Three family members recalled deteriorations when AP medication had been temporarily disrupted, and two described temporary deteriorations while young people were taking AP medication:
Soon after she had started on the medication . . . she said that the voice had told her that if she went to this place there would be a, a Stanley knife . . . I thought oh my goodness maybe she shouldn’t be on this.
F12
Unwanted effects and challenges
Family members individually discussed idiosyncratic medication side effects reported by the young people, such as tremors, anger, pacing, agitation, headaches and nausea. More frequently, AP medication was associated with weight gain and especially sedation:
[AP] slowed his whole thinking down, his whole physical being.
F13
Interviewees also described how young people’s allocation to AP medication often involved them taking on a new role to ensure continuous maintenance of AP medication. This included organising prescriptions, managing prescription delays and locating pharmacists who held the specific stock required. One mum personally administered daily doses in case her son forgot, and another helped by sourcing and posting AP medication to her son at university.
Cognitive–behavioural therapy
Fourteen out of 16 interviews were with the family member of a young person who had been allocated to receive CBT, either alone (n = 6) or in combination with AP medication (n = 8). Analysis has sought to distinguish CBT-specific aspects from more general treatment effects or changes.
Acceptability
Overall, CBT was discussed more positively than AP medication, and was more often a first preference for treatment (5/16 vs. 2/16). Perceived benefits of CBT included young people having someone to speak to about their mental health concerns, specialist therapists’ understanding of mental health concerns and CBT helping young people address historic issues, which in turn might promote longer-term recovery. However, several family members were cautious about the young person receiving CBT alone; as mentioned earlier, three family members viewed treatment without AP medication as problematic enough to consider advising the young person to withdraw from the trial. Some were concerned that CBT might not be sufficient on its own or would fail to address key concerns (e.g. risk-taking behaviours or frightening hallucinations). One mum had initially worried that having CBT (with or without AP medication) could worsen her daughter’s state of mind, based on a previous negative experience of counselling. There was also concern that CBT might be too difficult for a particularly quiet young person:
He’s you know an adolescent boy, he doesn’t really share a lot or give a lot so I knew the talking therapies would be really really difficult for him and they still prove to be difficult for him, he finds it really hard to talk.
F13
Valued changes
The most common change associated with CBT that family members identified was ‘improved understanding’. The young person’s understanding was seen to have improved, especially around ‘making sense of’ and learning to cope with distressing psychological experiences. One common aspect of ‘making sense’ identified by family members was the normalisation of psychotic phenomena and of the young person’s own self-perception that occurred during CBT:
I think it’s really good that there was somebody out there who could say actually you know what this is normal, and there’s lots of people who experience it.
F12
Improved coping was described in terms of young people’s learning coping strategies that helped them to ‘deal with’ ongoing psychotic phenomena, and being given helpful materials to keep between and after CBT sessions. Related to an improvement in coping, family members also reported relatively common improvements in young people’s capacity for social and occupational functioning. Additional changes discussed by family members also included specific reductions in psychotic experience, especially voice hearing, along with general changes in well-being, such as improved mood, motivation and confidence, and reduced stress and anxiety.
Unwanted experiences and challenges
Adverse experiences or effects of CBT were relatively uncommon, and from family members’ accounts seemed temporary. For example, one mum saw her daughter become tired and low in mood following early sessions of CBT, one mum similarly felt that her young person seemed tired because of the concentration required during CBT and another young person had found a CBT behavioural experiment (revisiting a difficult location) initially stressful. More commonly, family members discussed challenges for the young person, including general difficulty engaging with a talk therapy, in one case related to the sedative effects of AP medication, and for one young person because of a learning difficulty. Finally, an ongoing uncertainty for several family members was around the durability of benefits attributed to CBT, and the potential value of continuing therapy longer term. Indeed, four family interviews revealed degrees of disappointment around CBT ending:
She [therapist] is phenomenal, absolutely phenomenal . . . It’s gonna be such a blow to lose [therapist] . . . I suspect we probably wouldn’t be in as good a place as if the CBT were to continue even on a 2-monthly basis for the next 12 months, for example.
F09
Family intervention
Family intervention was offered to all young people receiving individual CBT, although young people did not always choose to involve family members in their therapy. During the qualitative interviews, 10 out of 14 eligible participants discussed FI. No family member discussed their initial views of FI acceptability during interviews; therefore, the following discussion relates to post-treatment views only.
Valued changes
Although family members had been involved in FI sessions (or not) to varying degrees, feedback was consistently positive and suggested that FI was highly valued. The most common perceived benefits of FI for family members were gaining an improved understanding of the young person’s psychological difficulties and improving communication with them. Both were seen to have been facilitated through FI sessions creating a space for a young person and their family to safely disclose their concerns:
We can all talk openly about it, and bring it to the surface.
F05
Family members noted that therapists had consistently sought young people’s consent before raising specific topics with family members:
I don’t think [therapist] ever spoke about anything that [young person] didn’t want her to talk about.
F03
Additional benefits of FI included normalising psychosis, being introduced to novel practical approaches that helped family members experience simulations of voice hearing, therapists allowing time for family members to raise questions of their own and sourcing helpful information or materials for family members between sessions.
Challenges of family intervention
Although FI was primarily described positively, some family members had found it very distressing to witness the young person’s disclosures during the sessions (‘I never actually knew that she was suicidal’ F04). However, in each such case family members said that they were content to tolerate such distress as part of an ultimately beneficial process:
[Young person] was amazing and needed my strength . . . it was probably one of the hardest things I’ve had to do sitting listening and I didn’t want to . . . but she didn’t want to have to go through it, and as a parent it was like ‘suck it up, deal with it’ kind of thing.
F02
An additional challenge of FI was finding extra time for sessions and between-session family activities. Finally, several family interviewees were disappointed not to have been involved in FI, as they felt such sessions would have been helpful.
Combined treatment
Acceptability
Overall, family members most frequently expressed a first preference for combined treatment (9/16), and it is noteworthy that there were no negative views of the combined allocation:
That’s the golden ticket really isn’t it.
F06
For some, this was because the combination simply meant more treatment, hence a greater likelihood of some benefit, greater benefit or a quicker treatment response:
We were so glad [young person] got both . . . I think [young person] and I both felt ‘throw everything at it’, because [young person] didn’t want to be ill.
F08
Some considered how the different treatments could work in complementary ways:
We felt well actually pills can perhaps just help for the time being but the talking therapies will really try and help him understand why he is where he is today you know, just raise his awareness about things and just help him really move on.
F13
Valued changes
Feedback about young people’s experiences of combined treatment was commonly positive. Apart from one instance of AP medication being seen to have somewhat hindered a young person’s engagement with CBT, family members generally perceived that AP medication had helped young people to engage with the psychological component:
The medication calmed her mind, helped her focus on the sessions.
F02
Four family interviews expressed the common view that, although AP medication conferred early benefits (e.g. reduced distress and improved sleep), the CBT component of combined treatment in particular was likely to confer longer-term improvements because it addresses both the historic concerns and the ongoing management of distressing experiences:
We felt well actually pills can perhaps just help for the time being but the talking therapies will really try and help him understand why he is where he is today . . . and just help him really move on.
F13
Several family members did not associate specific elements of combined treatment with specific beneficial effects, but perceived that the combination had been more helpful, more quickly, than either treatment would have been alone:
I think the medication’s taken him so far and then the CBT’s sort of boosted him the rest of the way . . . we’d have got there but I think it would’ve taken longer.
F12
Chapter 6 Discussion, conclusions and recommendations
Summary
This study aimed to obtain both the quantitative and the qualitative data required to determine the feasibility and acceptability of conducting a definitive trial to evaluate the effectiveness of psychological therapy, AP medication or a combination of the two in adolescents with FEP. To obtain these data, we conducted a feasibility PROBE trial over a 2-year period. In addition, we conducted qualitative interviews with three target stakeholder groups: prescribers (predominantly psychiatrists), trial participants and family members of trial participants. We outline each specific objective for the study and provide a summary of the learning relating to each, but first we will make some statements about the key learning from this study.
Research to inform an evidence base for the treatment of adolescent psychosis is limited. 62,63 There is a limited number of robustly conducted trials, with few head-to-head comparisons of AP medication short study duration for AP medication, no available data on the long-term adverse effects of AP medication and a paucity of evidence for PIs. 62,63,84
The MAPS trial has demonstrated that it is possible to identify, engage and retain adolescents with psychosis in a robustly conducted RCT. Findings from the feasibility trial and the qualitative interviews warrant a full-scale definitive trial. However, amendments to the trial design are required to ensure recruitment to target, to maximise value for money and generalisability and to minimise the risk of crossovers from the monotherapy arms to the combined treatment arm, and vice versa.
Key learning from the study includes costing dedicated time for an EIP psychiatrist and/or CAMHS psychiatrist to be employed to work on a definitive trial. Protected time from psychiatry at each site would facilitate their role in the assessment of a young person experiencing a FEP on entry into EIP services, addressing concerns regarding diagnostic uncertainty, clinical equipoise and suitability of AP medication for each potential participant. In addition, it would facilitate protected time for study outpatient prescribing slots, ensuring commencement of AP medication in a similar period to PI, which should also help reduce crossover from AP arms to PI monotherapy or no treatment.
Analysis of the qualitative data indicated that stakeholders consider AP medication and PI to have differing effects on outcomes and that the combined treatment may bring additional benefits, such as AP medication increasing a young person’s ability to engage in therapy. AP medication was considered to have the greatest benefit for psychiatric symptoms, and PI for a better understanding of psychological difficulties and self. Given that AP medication and PI are two distinct treatments with different mechanisms of action, this finding is understandable and it has been argued that CBT should not be seen as a quasineuroleptic by researchers and clinicians. 130 This has implications regarding selection of the most appropriate primary outcome for a definitive trial. However, in contrast, there is a signal from our quantitative data on psychiatric symptoms and user-defined recovery (often reflecting improved understanding) of little variability between the treatments on both of these outcomes; however, this should be interpreted with caution given that MAPS was not powered to evaluate the effectiveness or efficacy of these three treatments. A full discussion of these learning points is provided below, in Tables 24–26.
| Activity | Lessons learnt/challenges faced | Recommendation for a definitive trial |
|---|---|---|
| 1a Trial set-up | ||
| Identification of a local psychiatrist in EIP service/CAMHS as a site lead or as co-site lead | Referral pathways data indicate that the model taken to recruitment in Oxford improves the accuracy of referrals (with regard to eligibility) and produces a greater number of randomisations. The Oxford model was for the local EIP service or CAMHS psychiatrist to consider trial suitability as part of EIP/CAMHS initial assessment | Each site requires at least one local EIP service or CAMHS psychiatrist who is identified as a site lead, endorses that there is clinical equipoise regarding the best treatment options for FEP and has protected time for assessment of new cases into EIP services for young people in the inclusion age range. This will improve the accuracy of referrals and the number of randomisations |
| Organising excess treatment costs | Significant difficulties were experienced in obtaining ETC to cover delivery of therapy at the Birmingham site, resulting in this site initially being dropped (as it was not possible for the site to be initiated without therapist provision). This caused significant challenges to recruitment while the management team identified a new site to replace Birmingham | To facilitate a definitive trial commencing on time, ETC issues should be addressed and CBT therapists, supervisors and psychiatrists (screening assessment and prescribing appointments) should be identified as ETCs |
| EIP and CAMHS interface | The commissioning arrangements for CAMHS and EIP services were variable across the sites included in this study. In some instances, CAMHS and EIP services were commissioned by different NHS trusts and, as such, care co-ordination and prescribing responsibilities were split across these NHS trusts. This interface could result in communication delays between services regarding referral to the trial, agreement regarding FEP status and clinical equipoise regarding treatment | Prior to site selection, a scoping exercise should be carried out to better understand the interface between the CAMHS and EIP services to ensure that the interface would facilitate agreement regarding FEP status, referral to the trial and prescribing for those allocated to an AP arm |
| Integration with EIP and support from EIP leads | The best recruiting site (Oxford) had more than one lead EIP clinician identified to support the study. This facilitated conversations with team leaders regarding the study, identification of participants and navigating the variability between the study inclusion criteria and the EIP team inclusion criteria. Site leads in Oxford included representation from both psychiatry and psychology | Each site requires support from more than one clinical lead in EIP. Leads should be placed in EIP services, should have management responsibility and should ideally provide representation from both psychiatry and psychology |
| MHRA approval | In addition to REC and HRA approval, it is likely that a definitive trial would be a CTIMP and, therefore, it would require a clinical trial authorisation from the MHRA approval and a EudraCT number | Researchers for a definitive trial should become familiar with the processes required for approval from the MHRA, including the time frames for approval, costs for application for MHRA approval, etc. This will facilitate the trial starting on time |
| Utilising business intelligence for site selection | It would facilitate recruitment to target if site selection included an audit of the number of young people with a FEP accepted into EIP services in the year prior to site selection | Potential sites should obtain business intelligence data from the trust’s medical records to determine the acceptance into EIP rate for young people within the age range specified in the inclusion criteria |
| 1b Trial design/procedures | ||
| Trial design | A proportion of participants did not receive the intervention exactly as allocated for reasons related to TAU options and participant preference and choice | We make recommendations throughout this chapter to address real-world factors that may result in participants not receiving the intervention exactly as allocated. However, there are likely to be a number of factors outside the remit and scope of the research team, including patient preference and choice and what is offered as part of TAU. For this reason, we recommend a pragmatic effectiveness trial examining care pathways that will examine how these treatments work in NHS settings. If an efficacy trial was deemed to be the most appropriate approach to a definitive trial, a four-arm trial with a placebo condition may be appropriate |
| Liaison with prescribers | Qualitative research with prescribers indicates that the decision to refer to the study is sometimes influenced by treatment beliefs based on their clinical experience | Liaison with prescribers should address this issue. Prescribers should be provided with a handbook that speaks to the current evidence base regarding treatments for young people with FEP. This information should be developed in consultation with prescribers to ensure that it fully addresses their treatment beliefs and concerns. In addition, the handbook should make it clear that the prescribers retain clinical responsibility and, therefore, decision-making about AP prescription throughout the trial. Embedding the trial within the clinical team will ensure excellent communication links regarding participants and this will in turn strengthen relationships, thus addressing any concerns prescribers may have about the trial |
| Screening approach |
We collated a large number of initial enquiries through services screening their medical records for potential participants (n = 705). The conversion rate to referrals was low (n = 76) We identified, from the best recruiting sites (Oxford and Manchester), that a more effective approach to recruitment was for EIP staff to consider all new referrals into EIP services. This was a more effective approach as there was a decreased risk that the young person would have received AP medication in the past 3 months (an exclusion criterion for the trial). It was most effective in Oxford, where the screening at entry into EIP services was carried out by a psychiatrist who was able to confirm FEP status and clinical equipoise Qualitative data indicate that prescribers expressed that diagnostic uncertainty is greater with young people. They expressed that they had to balance this diagnostic uncertainty with a duty of care and decision-making regarding appropriateness of AP medication |
Ensuring confidence in the screening process is crucial to ensuring referrals into the trial. The emphasis of recruitment should be on screening all new referrals into EIP services for young people in the age range. A psychiatrist (consultant or staff grade) who is well placed professionally to determine FEP status, address issues of diagnostic uncertainty, consider clinical equipoise and discuss the option of the trial should conduct this screening |
| Selecting language/terminology used at point of referral | Qualitative data indicated that some young people and family members had not been informed (by their service) that their mental health difficulties were understood to be psychosis. For this reason, for some the first time they received this information was via the consent process into the study |
Potential participants should be made aware of how their experiences are understood by clinical services, i.e. if this is in the context of psychosis this should be discussed before a referral to the trial to ensure that the young person and their family understand what this means As suggested, we recommend that the screening is carried out by a psychiatrist who would be qualified to determine if the young person was experiencing psychosis. The psychiatrist would be well placed to facilitate discussions about psychosis with the young person and family |
| Designing participant information sheets | Qualitative interview data from both participants and family members suggest that the information sheets contained too much information or language that would be difficult for some young people to understand |
For a definitive trial, the amount of information in the participant information sheets should be reduced and different modes of communication about the trial should be considered, for example short animations that break down the various aspects of the trial Funding costs should cover producing high-quality, youth-oriented information about the trial. PPI should be central in the development of these resources |
| Inclusion criteria |
The overall recruitment rate was lower than anticipated and the rate of ineligibility at the initial enquiry stage was higher than expected Qualitative data around treatment from clinicians indicated that psychiatrists expressed the greatest concern about prescribing, or not, to the younger end of the age range. This suggests that this concern may be less prevalent in relation to older adolescents |
For a definitive trial, the upper age limit should be increased from 18 to 25 years. Current definitions of adolescence often extend to 25 years of age, e.g. the WHO’s definition of youth. This would widen the potential pool of participants and capture the full range of adolescence as defined by the WHO Increasing the upper age limit to 25 years may also prove beneficial for reducing crossovers |
| Exclusion criteria | The overall recruitment rate was lower than anticipated and the rate of ineligibility at the initial enquiry stage was higher than expected. Just under one-quarter of the 14- to 18-year-olds identified at the initial enquiry stage had taken an AP medicine within the past 3 months, making them ineligible for the trial | For a definitive trial, the exclusion criteria should be modified to match those of trials of AP medication with a similar wash out period, e.g. a maximum 2-week AP exposure to ensure washout before the drug trial started |
| Inclusion and exclusion criteria to consider clinical risk | Qualitative interviews conducted with clinicians indicated a hesitation to enter certain young people into the trial (and hence a definitive trial) owing to clinical evaluation that the young person should have an AP medicine, i.e. because of significant risk, and this could not be guaranteed because of randomisation | The inclusion and exclusion criteria should reflect these opinions and that research teams at each site include some involvement from EIP psychiatrists |
| Deterioration criteria | Overall, the deterioration rates were low. There was little difference between the monotherapy arms in the rates of deterioration. However, deterioration contributed to the number of treatment crossovers | Crossovers should be minimised in a definitive trial. Given the low rates of deterioration and the absence of evidence of difficulties associated with allocation to PI monotherapy, we propose that the deterioration criteria are removed from a definitive trial with the recognition that clinical services will be monitoring the well-being of the participants and that we would not ask them to withhold treatment should they consider a deterioration to have occurred |
| Heterogeneity of the sample | Qualitative research with prescribers indicated some concern that given the diagnostic uncertainty in the population the sample of participants would be heterogeneous. This may limit the generalisability of the results for a definitive trial |
One option to reduce heterogeneity would be the inclusion of a diagnostic checklist at the baseline assessment to determine if the sample would meet ICD-10 or DSM-V criteria for a schizophrenia spectrum diagnosis However, we highlight that this may unnecessarily stigmatise the young person by applying diagnostic labels |
| Approach to participant engagement | Flexibility regarding appointments is crucial to engagement. Qualitative data from participants and family members indicated that the assertive outreach approach taken on this study (flexibility regarding time of appointment, choice of location, text or letter reminders and flexibility regarding rearrangement of appointment) was valued | A definitive trial requires sufficient RA and therapist capacity to allow for flexibility in appointments |
| Long-term follow-up of young people with experience of FEP | Although follow-up rates were excellent at the end of treatment (6 months), the long-term follow-up of participants at 12 months was lower than at end of treatment. Youth-specific factors, including school and college exams, moving away to university, preference to book appointments via parents (who are often managing multiple appointments and commitments) and frequently changing mobile telephone numbers and/or e-mail addresses, should be considered for a definitive trial. We endeavoured to maintain contact with participants for this study between 6 and 12 months to update their contact details and provide a £5 token of appreciation | To improve follow-up rates long term a larger number of brief check-ins with a token of appreciation of trial participation should be scheduled between end of treatment and follow-up |
| Methods to maintain the blind | The rate of blind breaks was low, indicating that our approach to maintenance was successful | A definitive trial should have rigorous approaches to maintaining the blind as outlined here in Chapter 2. Qualitative feedback from participants and family members, of whom at least a minority expressed unease around maintaining the blind and felt limited in their disclosures to RAs, also suggests reconsidering the format of essential outcome measures. For example, self-report rather than interview formats could eliminate some unease around blinding, and could potentially enhance absolute accuracy of important disclosures |
| Suitability of randomisation procedures | Risk of selection bias was low and the centralised web-based platform hosted by the CTU was suitable for use in a definitive trial |
A web-based, centralised (with the CTU) platform should be used to randomise participants and reduce the risk of selection bias If the upper age limit is increased from 18 to 25 years, we recommend stratification by < 18 years or ≥ 18 years to ensure a balance of children vs. young adults |
| Activity | Lessons learnt/challenges faced | Recommendation for a definitive trial |
|---|---|---|
| Primary outcome |
The PANSS had excellent completion rates at the end of treatment, which suggests that PANSS is an acceptable assessment to young people with psychosis and an appropriate primary outcome for a definitive trial The completion rate of other secondary measures was lower. One explanation for this is that all measures other than the PANSS were deprioritised if the participant expressed that the assessment was too burdensome. For this reason, the assumption that it is not possible to collect the other secondary outcome measures should be approached with some caution and a brief self-report measure, such as the QPR, is likely to be an acceptable primary outcome measure Qualitative data from participants and family members suggest that the two monotherapies may work in different ways and have different treatment effects. PI broadly led to improved understanding and coping with psychosis and, in some instances, both participants and family members reported improved communication as a result of PI. AP medication broadly led to improved symptoms, psychological well-being and for some participants an improved ability to engage with PI. However, quantitative analysis of PANSS and QPR scores provides a signal that there is little difference between the three treatments on either the PANSS or the QPR |
Based on our data, we can recommend the PANSS as the primary outcome for a definitive trial. However, the quantitative data suggest little difference between the treatments on PANSS or the treatments on QPR; therefore, another option for the primary outcome would be the service user-defined recovery outcome measure the QPR. The QPR has the benefit of being brief, i.e. only 15 items (reducing participant burden), self-reported (the preferred method of reporting for service users) and evaluating service user-defined recovery outcomes (a priority for service users). It is also one of three mandatory patient-reported outcome measures recommended for routine use in EIP services Given the suggestion from qualitative data that AP medication and PI may have different treatment effects, we recommend that a definitive trial include a process evaluation to understand how each treatment works and consider interaction effects between treatments |
| Burden of assessments |
Our data demonstrate that there was a low response rate to the secondary outcome measures. This indicates that the number of assessment measures was burdensome for this population The burden of assessments was reflected in the qualitative data from participants. Data indicated that for some the assessment battery was repetitive or boring. In addition, difficulties with memory and difficulties with learning (e.g. dyslexia, autism spectrum disorder) can affect engagement with the questionnaires for some of the young people in this population More specifically in relation to blood tests, the qualitative data from participants indicate that for some participants there was unease about the blood tests and this is reflected in the small number of blood test results reported in the quantitative data |
The assessment battery should be reduced in quantity of measures. We propose that the essential measures to retain are PANSS (to assess psychiatric symptoms), QPR (to assess user-defined recovery) and adverse effects (to assess the comparative safety of the interventions) The low uptake of blood tests and physical health checks may limit the ability of a definitive trial to form judgements about the relative risks from those particular adverse effects in the different treatment arms. We believe that RAs may not be best placed to obtain body and blood measurements. We recommend that time is costed for the research psychiatrist to obtain the physical health measurements or another trained health-care professional, i.e. a nurse. The research psychiatrist would be well placed to complete the physical health measurements, as they are trained medical professionals with the necessary skill set for taking blood and performing physical health checks. As the prescriber they would be required to complete the checks for two-thirds of the participants before commencing them on AP medication and for the duration that they remain on AP medication. In addition, as members of the research team and the clinical team they would be familiar with all participants and would have met them as part of screening, thereby increasing the chance of engagement with the physical health checks process |
| Approach to assessment of side effects of AP medication | There were a number of challenges in relation to the measurement of side effects of AP medication. Overall completion rates were low for the ANNSERS. Baseline data suggested false positives given that participants were medication naive at baseline and yet the average number of side effects noted was between 7 and 11 at baseline. It is likely that the number of false positives is an artefact of the measure being administered by a researcher who is blind to allocation and the overlap between side effects and other conditions (e.g. fatigue, weight gain, headaches, sleep disturbance) | We recommend that side effects of AP medication be recorded via self-report methods to remove the risk of false positives. Given the feedback on burden of assessments, we recommend that the assessment of side effects should be brief. The Systematic Monitoring of Adverse events Related to TreatmentS (SMARTS)131 is an 11-item self-report measure of AP medication side effects that balances brevity with assessing the most common side effects of AP medication |
| Approach to monitoring adherence to medication | A definitive trial should consider systematically monitoring drug levels to determine medication adherence | |
| Health economics | The rates of missing data for health economics were high at follow-up. At 6 months, missing data were 36.1% for the EQ-5D-5L and 63.9% for the health economics service use questionnaire |
To answer the question of cost-effectiveness of the three treatments, health economic data would be a priority. For this reason, adjustments should be made to the design to ensure the completeness of health economic data collection To maximise health economic data we recommend that service use data are obtained by self-report methods from participants and followed up with additional details from the medical record service data. In addition, trial management supervision should include close monitoring of the completeness of the data as they are collected, so that any missing items on a form can be followed up. Use of the medical record data will require consideration of the ability of each trust’s business intelligence department to extract data from the medical record system. Ground work with business intelligence should take place early on in the trial set-up phase to identify the most streamlined method of obtaining medical data. The health economist should be included in these discussions. We recommend that these data be periodically extracted from the records throughout the trial to ensure ongoing oversight of health economic data completeness by the iDMC |
| Data extraction from medical records |
We used the electronic medical records to extract data regarding treatment received status (given that the AP prescription was made by the clinical team, not the research team, this information was on the medical records and not a study CRF), and information regarding AP prescription, diagnosis and AEs (to minimise the risk of surveillance bias) Screening the medical records was time-consuming and the task could not be performed by the RA given they were blind to allocation. Funding did not cover time for a non-blind member of the research team to screen the medical records. Therefore, obtaining these data were challenging. Business intelligence systems were not compatible to extracting the information required and, in some instances, the data in the records were limited (resulting in missing data for some variables relating to medication) |
There are a number of solutions to this challenge Time costed for a local psychiatrist to screen and prescribe could also include completion of a CRF that details diagnosis and details of medication treatment. If medication is amended or if crossovers occur, the psychiatrist could complete a CRF. The CRF would be located in the same data management system as the participant assessment measures and PI CRF. This would allow the trial management group to monitor completion rates of these data. It would allow the iDMC to review quality of these data and monitor crossover rates Medical record data would be required regarding AEs to supplement self-report (because the events may be reported to the clinical and not the research team). As part of the site selection, discussions should be held with BI at each trust to set up systems to extract details of AEs via the local systems. For example, use of the NHS Datix adverse incident and near-miss reporting system It is likely that health economic data would be required from the medical records for a definitive trial of cost-effectiveness. Discussions with BI during site selection phases should identify systems for extracting these data |
| Activity | Lessons learnt/challenges faced | Recommendations for a definitive trial |
|---|---|---|
| Ensuring swift access to treatments |
The median number of days to prescription of AP medication was 18 (IQR 10–42). In total, 27.3% of the AP monotherapy participants crossed over to ‘no treatment’ The clinical team psychiatrist made the AP prescription. We endeavoured to support the timely offer of an outpatient appointment to discuss AP medication through contact with the psychiatrist after randomisation to notify them of the outcome, but the research team was unable to control the time to initiation of AP medication. However, a trial therapist delivered PI and initiation of this was monitored by the trial through therapist supervision |
A definitive trial should mirror MAPS and employ trial therapists to deliver PI, thereby facilitating swift action to offer the treatment post randomisation Matched study procedures should be in place for both treatments (AP and PI). Locating prescribing of AP medication with a local EIP or CAMHS psychiatrist with protected MAPS outpatient time will facilitate swift access to treatment and monitoring of drug levels at follow-up. This may also improve rates of crossover from AP monotherapy to ‘no treatment’. In addition, this would reduce the crossovers from combined to PI only, as some evidence of prescribers deciding not to because participants were showing a good response to CBT by the time of outpatient appointment |
| Option of a combined treatment | Qualitative data from participants and family members indicated a general preference for as many treatment options as possible in the short term, i.e. combined treatment. There was some variation among participants and among family about the long-term value of PI or long-term risks of AP medication, so the more help the better was not always a long-term preference. Typically, clinicians preferred AP medication first but valued PI as additional help for ‘secondary’ issues, but AP medication was a short-term preference for the majority of family members and for some participants | A three-arm design that includes a combined arm should be retained for a definitive trial to provide reassurance to participants and family members that there is the potential for a loved one to be randomly allocated to a combined treatment |
| Prescribers concerns regarding the adverse effect profiles of AP medication | Clinician qualitative data indicated that, although clinicians shared a common belief in the value of AP medication, they had concern about the adverse effects of AP medication for this population |
For a definitive trial, the research team should produce a handbook for clinicians to define the parameters for prescribing for the trial. This could highlight to clinicians that a low dose of AP medication is acceptable and that this can be below BNF limits. The handbook could contain information on the current evidence base for each AP and the relative side effect profile A handbook would promote standardisation of prescribing and support psychiatry decision-making We recommend that the handbook be produced in consultation with a reference group of psychiatrists from EIP and CAMHS with expertise of working with young people |
| Participant and family perspectives on access to AP medication | The qualitative data suggest general acceptance of AP medication from participants and family members, but in some instances a preference for PI. Concerns about the side effects of AP medication were expressed. However, in some instances, both participants and family members expressed a clear preference for AP medication over PI | A three-arm design for a definitive trial would recognise the variety in treatment preferences. Any strong preferences for or against the treatment options should be discussed at screening (with the research psychiatrist) and with the RA during the informed consent process to ensure that participants are genuinely content to be randomised to a treatment. This is of importance for both recruitment and retention to a definitive trial |
| Family perspectives on the acceptability of FI | Although we did not mandate FI in this study, some family members said that they considered it a good optional extra and family members who did not receive FI reported that it would have been useful. However, participants indicated a more diverse range of important views about FI, from clear acceptability to clear refusal. This emphasises the need to continue considering FI as an optional element of PI |
For a definitive trial, FI should form part of the PI as an optional aspect of PI Standardised and detailed information should be produced for both participants and families about FI to improve decision-making about receiving FI. Information should be age appropriate, i.e. use appropriate media such as short animations |
| Feasibility of achieving CBT milestones |
There was an excellent rate of both development of a problem list/goal and sharing a formulation by session 3 Rates of ‘out in the real world’ sessions and completion of longitudinal formulations were lower The rates of first session of CBT being within 1 week of randomisation were low |
The initial training for therapists should include a focus on the implementation of real-world sessions. Training should also emphasise rapid access and this should be monitored in a definitive trial via supervision |
| Crossover from a PI monotherapy arm to a combined arm | In total, 50% of the crossovers from a PI monotherapy arm to a combined arm were a result of the participant meeting the deterioration criteria | Removal of deterioration procedures would reduce this crossover |
Trial outcomes
We collected demographic data and a range of potential primary and secondary outcomes to inform a definitive trial. The PANSS is a suitable choice of primary outcome measure for a definitive trial for a number of reasons. The completion rates of the PANSS were excellent at the end of treatment (6 months) and clearly met the prespecified progression criteria. The PANSS is a valid and reliable measure106 and it is the most commonly used primary outcome measure in psychosis trials. Therefore, use in a definitive trial, it would allow comparison of data with the wider evidence base and synthesis into systematic reviews and meta-analyses of treatments for psychosis. A review132 of 24 psychosis study outcome measures found that the PANSS was the most acceptable to service users. However, the PANSS is a clinician-rated measure and the same review of commonly used outcome measures for psychosis studies indicated that service users have a general preference for outcome measures in self-report format,132 and service users have expressed that global, recovery-oriented outcomes are as important to deliver as changes in psychiatric symptomatology. 133 Our service user-defined measure of recovery (QPR) had lower completion rates at the end of treatment; however, this may be a result of the approach to minimising participant burden, that is, when participants expressed difficulty or unease with assessment battery burden, all measures other than the PANSS were deprioritised.
At baseline and follow-up, the completion rates of secondary outcomes measures (other than the PANSS) were low, which indicated that the assessment battery should be significantly reduced for this population, with only the most essential outcomes retained. See Table 25 regarding the proposed outcomes for a definitive trial.
Limitations
Although all steps were taken to ensure a thorough approach to screening participant medical records for medication adherence data, this approach was reliant on clinicians’ records. It is possible that medication adherence may be overestimated by clinicians or by participants when reporting AP use, and in a definitive trial medication non-adherence may mask effectiveness and safety implications. We make recommendations with regard to this below.
Generally, the rate of completion of secondary outcome measures was low, including health economic measures (e.g. the EQ-5D-5L), which limits any interpretation of the appropriateness of this measure for a definitive trial.
The small sample size obtained means that we lacked statistical power and there will be considerable uncertainty regarding estimates of treatment effects. It is possible that there is also uncertainty regarding generalising from our rates of recruitment, retention and treatment adherence given the small sample size.
Specific learning points and recommendations
The specific objectives relate to the following domains:
-
trial feasibility (recruitment, retention, clarification of the selection criteria for a definitive trial, suitability of randomisation and masking procedures)
-
trial outcomes (validity and relevance of the outcome measures and estimation of plausible sample size parameters)
-
acceptability and feasibility of the treatments, including treatment manuals and staff training
-
economies of scale for a definitive trial.
In relation to recruitment, retention and acceptability of the treatments, we prespecified green/amber/red progression criteria in our study protocol as a mechanism to assess the feasibility of a definitive trial. Approval for our prespecified progression criteria was granted from both the iDMC and the study funder. Recruitment success was measured by determining the proportion of the target sample size achieved: green criterion ≥ 80%, amber criterion 79–60% and red criterion < 60% of the target sample size of 90 participants. Retention success was measured by the proportion of participants who completed an end-of-treatment PANSS assessment: green criterion ≥ 80%, amber criterion 79–60% and red criterion < 60% of participants followed up at this assessment point. Satisfactory delivery of adherent therapy was determined by the proportion of participants allocated to PI who received six or more sessions of CBT: green criterion ≥ 80%, amber criterion 79–60% and red criterion < 60% of participants having six or more sessions. Satisfactory delivery of AP medication was determined by the proportion of participants receiving an AP medicine for 6 or more consecutive weeks: green criterion ≥ 80%, amber criterion 79–60% and red criterion < 60% of participants receiving AP medication for this period of time. It should be noted that we included an AP dose below the limits in the BNF given that this is frequently the approach for young people owing to the drugs being licensed for adults.
Trial feasibility
With regard to the progression criteria, we achieved a 50/50 mix of green and amber progression zones. Overall, these results indicate that it would be possible to conduct a definitive trial, but some modifications to the trial design are required. We provide information on these modifications in full in Table 24. Recruitment was in the amber progression zone, suggesting that modifications are required to recruit to target in a definitive trial. Two out of the four original sites recruited well, with the Oxford site over-recruiting and the Manchester site recruiting to 91% of the target. Site selection is crucial to ensuring recruitment to target for a definitive trial, and a number of considerations must be made in selecting the sites, as outlined in Table 24. In addition, further refinement of the inclusion criteria could have important implications for recruitment to target. Adherence to ≥ 6 consecutive weeks of AP medication prescription for participants in an arm that contained AP prescription was in the amber zone; however, we make specific recommendations for how this could be addressed in a full-scale trial. Both retention of participants at the primary end point (end of treatment) and adherence to six or more sessions of CBT for those participants in an arm that contained CBT were in the green zone. Retention of participants at the primary end point (6 months, end of treatment) was excellent. However, retention at the 12-month follow-up was lower (27–47%); we make specific recommendations for improving this in a full-scale trial, although a substantial proportion of these missing data were because of a planned variable follow-up rate in which participants recruited within the final 3 months of recruitment between August and October 2018 (12 participants, 19.7% of the sample) were never intended to receive a 12-month assessment.
Acceptability and feasibility of treatments
Evaluation of data on adherence to treatments indicates that both PI and AP medication are acceptable treatments, and it would be feasible to deliver both in a definitive trial. Although the data indicate greater adherence to a dose of PI (six sessions or more), adherence to AP medication (6 or more consecutive weeks) was lower. However, adherence to AP medication was within the amber zone for the progression criteria, suggesting that it would be feasible to deliver in a definitive trial with some amendments as outlined in Table 26. Adherence to AP medication within our trial compares well with the general literature on AP adherence, which finds high levels of non-adherence, including in RCTs.
We obtained data from medical records to characterise the prescribing of AP medication for the sample, self-report data from trial therapists to evaluate the quality of therapy delivered (both CBT and FI), including therapy milestones and change strategies used during therapy, and CTS-R ratings for fidelity to the CBT model. In addition, end of CBT and end of FI questionnaires completed by therapists provided data on treatment manual amendments for a definitive trial.
Qualitative interviews provided an in-depth understanding of participants’ experiences of the treatments and the acceptability of the treatments to both young people and family members. Several key lessons were learnt that inform treatment delivery in a definitive trial. We present the lessons learnt in Table 26.
Economies of scale
Each site had one full-time RA costed to work on the study. As outlined in Chapter 2, the RAs were tasked with liaison and recruitment from clinical teams and research assessments including the PANSS, psychometrics and physical health checks. Possible economies of scale include dropping the physical health checks from the RA tasks and reducing the number of assessment measures. This would reduce the length and/or number of research assessment visits per follow-up. However, for a number of reasons, we recommend that for a definitive trial a full-time RA be retained for each site. First, as outlined earlier, an assertive outreach approach was highly valued by participants and it is likely that this contributed to excellent follow-up completion rates at 6-month assessment. To offer an assertive outreach approach regarding the time, location and rearrangement of appointments plus text, telephone and letter reminders (with the associated paperwork to document participant contact) flexibility is required and this would be limited in a part-time role. Second, we recommend that more check-in telephone calls are scheduled to facilitate engagement and long-term follow-up; again this would require sufficient diary capacity. Third, with improved screening methods and accuracy of screening, as well a higher age limit of 25 years, we envisage an increased number of baseline visits. To ensure swift access to entry to the trial (and, therefore, treatment) a RA would require flexibility and capacity in their diary to act quickly in response to a referral from a psychiatrist.
Conclusions
This study aimed to assess the feasibility of undertaking a definitive trial to compare AP monotherapy, PI monotherapy and a combined treatment. All of our study objectives were achieved and many important lessons for a definitive trial were learnt. The feasibility trial methods and feasibility findings can be generalised to other UK settings, given the representation of CAMHS and EIP service users. However, we cannot generalise the findings to other countries that may have different service settings for adolescent psychosis and, in particular, areas in which there are no EIP services.
Collectively, the results suggest that a definitive pragmatic effectiveness trial examining care pathways is warranted, but some amendments are required to the trial design. Improvements to the trial design include screening processes for recruitment, amendments to the inclusion/exclusion criteria, reduction of treatment crossovers and reduced participant burden (from assessment measures). Our liaison with clinical services has highlighted that EIP services and CAMHS are under considerable pressure from funding issues and increased workload, especially because of the access and waiting time standards requiring assessment, and delivery of NICE-compliant care for those taken onto the caseload within 2 weeks of referral. However, we advise that the current pressures on EIP services to respond to access and waiting time standards may help recruitment to a definitive trial, as it is clear from our qualitative studies that clinicians, parents and young people all viewed the trial as a way of accessing help as quickly as possible. Including this study, we have conducted two similar three-arm feasibility trials (one in adults102), and to inform a definitive trial we will combine these trials using a two-stage fixed-effect individual patient data meta-analysis. The analysis will focus on the effects on PANSS total and QPR total at 6 months to provide information for sample size calculations for a definitive trial. Our evaluation of the literature presented in Chapter 1 demonstrates that the research question regarding the clinical effectiveness and cost-effectiveness of AP medication versus PI versus a combined treatment remains important and warrants a definitive trial to improve the evidence base for treatments of FEP in young people.
Acknowledgements
Thank you to all of the participants who agreed to take part in the trial. This study was supported by the NIHR Clinical Research Network. We are grateful to the Psychosis Research Unit Service User Reference Group for their consultation regarding the design of the study and contribution to the developments of study-related materials. We are grateful to our independent Data Monitoring Committee (Emmanuelle Peters, Rod Taylor, Thomas RE Barnes and Carl Bateson) and our Trial Steering Committee (Graham Murray, Susanna Dodd, Rebecca Walwyn, Zak Howarth and Alison Brabban) for providing oversight of the trial. We are also grateful to the many researchers, trial therapists, psychiatrists and network staff who supported the study, including Amanda Larkin, Ann Steele, Elizabeth Murphy, Glynis Queenan, Jasper Palmier-Claus, Peter Haddad, Verity Smith, Samantha Hartley, Miriam Kirkham, Amy Langman, Ashley Louise-Teale, Sarah Reeve, Jessica Bird, Jo Clacey, Emmeline Goodby, Felicity Waite, Helena Laughton, Jana Safarikova, Laura Hancox, David Fowler, Renata Fiahlo, Laruen Wilcock, Catarina Sacadura, Rick Fraser, Samantha Fraser, Rachel Upthegrove, Ravneet Bhogal, Thomas Goodall, Robert Dudley, Fiona Padgett, Negar Khozoee, Laura McCartney, Sarah Maxwell, Jon Wilson, Leanne Groves, Peter Cairns, Roger Collin, Xavier Coll, Richard Emsley, Alison Yung, Ashley Liew, Eleanor Longden, Max Birchwood and Paul French.
Contributions of authors
Anthony P Morrison (https://orcid.org/0000-0002-4389-2091) (Professor of Clinical Psychology) planned the study, contributed to the application for funding, made substantial contributions to the design of the trial protocol and the statistical analysis plan, managed the trial as chief investigator, contributed to writing and critically read the manuscript.
Melissa Pyle (https://orcid.org/0000-0002-9561-6777) (Research Trial Manager) contributed to the application for funding, made substantial contributions to the development of the trial protocol, was responsible for the overall management of the trial and data management, and wrote the first draft of the manuscript.
Rory Byrne (https://orcid.org/0000-0001-5873-3483) (User-led Researcher) contributed to the application for funding, made substantial contributions to the design of the trial and the nested qualitative studies, qualitative data collection and analysis. He produced the first draft of Chapter 5 and critically read the manuscript.
Matthew Broome (https://orcid.org/0000-0002-6963-8884) (Professor of Psychiatry and Youth Mental Health) contributed to the application for funding, made substantial contributions to the design of the trial and protocol, and critically read the manuscript.
Daniel Freeman (https://orcid.org/0000-0002-2541-2197) (Professor of Clinical Psychology) contributed to the application for funding, made substantial contributions to the design of the trial and protocol, and critically read the manuscript.
Louise Johns (https://orcid.org/0000-0003-3355-3202) (Consultant Clinical Psychologist) made substantial contributions to the development of the trial design and protocol, and critically read the manuscript.
Anthony James (https://orcid.org/0000-0002-2742-8328) (Consultant Child and Adolescent Psychiatrist) made substantial contributions to the development of the trial design and protocol, and critically read the manuscript.
Nusrat Husain (https://orcid.org/0000-0002-9493-0721) (Professor of Psychiatry) made substantial contributions to the development of the trial design and protocol, and critically read the manuscript.
Richard Whale (https://orcid.org/0000-0003-0478-7296) (Consultant Psychiatrist) made substantial contributions to the development of the trial design and protocol, and critically read the manuscript.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (Director and Professor, The Centre for Healthcare Randomised Trials) made substantial contributions to the development of the trial design and protocol, as well as the statistical analysis plan, conducted the analysis and critically read the manuscript.
John Norrie (https://orcid.org/0000-0001-9823-9252) (Professor of Medical Statistics and Trial Methodology) contributed to the application for funding, made substantial contributions to the design of the trial and protocol and the statistical analysis plan, and critically read the manuscript.
Jemma Hudson (https://orcid.org/0000-0002-6440-6419) (Trial Statistician) made substantial contributions to the development of the trial design and protocol, as well as the statistical analysis plan, conducted the analysis and critically read the manuscript.
Sarah Peters (https://orcid.org/0000-0003-1949-3995) (Senior Lecturer in Psychology) contributed to the application for funding, made substantial contributions to the design of the trial and the nested qualitative studies the qualitative analysis, and critically read the manuscript.
Linda Davies (https://orcid.org/0000-0001-8801-3559) (Professor of Health Economics) contributed to the application for funding, made substantial contributions to the design of the trial and protocol, oversight of the health economic analysis plan, and critically read the manuscript.
Samantha Bowe (https://orcid.org/0000-0002-6675-7511) (Clinical Psychologist) made substantial contributions to the development of the trial design and protocol, and critically read the manuscript.
Jo Smith (https://orcid.org/0000-0002-0277-1680) (Professor of Early Intervention and Psychosis) made substantial contributions to the development of the trial design and protocol, and critically read the manuscript.
David Shiers (https://orcid.org/0000-0003-2531-5837) (Honorary Reader in Early Psychosis and Research Fellow at the Psychosis Research Unit) contributed to the application for funding, made substantial contributions to the design of the trial and protocol, and critically read the manuscript.
Emmeline Joyce (https://orcid.org/0000-0003-2066-0033) (Assistant Research Psychologist) made substantial contributions to the development of the trial design and protocol, and critically read the manuscript.
Wendy Jones (https://orcid.org/0000-0002-9869-2230) (User-led Researcher) made substantial contributions to the development of the trial design, the qualitative studies and the qualitative data analysis, and critically read the manuscript.
Chris Hollis (https://orcid.org/0000-0003-1083-6744) (Professor of Child & Adolescent Psychiatry) contributed to the application for funding, made substantial contributions to the development of the trial design and protocol, and critically read the manuscript.
Daniel Maughan (https://orcid.org/0000-0002-0348-6195) (Consultant Psychiatrist) made substantial contributions to the development of the trial design and protocol, and critically read the manuscript.
All authors read and approved the final manuscript.
Publications
Byrne RE, Bird JC, Reeve S, Jones W, Shiers D, Morrison AP, et al. Understanding young peoples’ and family members’ views of treatment for first episode psychosis in a randomised controlled trial (MAPS). EClinicalMedicine 2020;24:100417.
Byrne RE, Reeve S, Bird JC, Jones W, Shiers D, Morrison AP, et al. Clinicians’ views of treatment types for first episode psychosis delivered in a randomised controlled trial (MAPS). EClinicalMedicine 2020;24:100421.
Morrison AP, Pyle M, Maughan D, Johns L, Freeman D, Broome M, et al. A feasibility randomised controlled trial comparing antipsychotic medication to psychological intervention to a combined treatment in adolescents with first episode psychosis: the MAPS trial. Lancet Psychiatry 2020;7:788–800.
Data-sharing statement
Data can be obtained from the corresponding author for consideration.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Freeman D, Garety PA, Kuipers E, Fowler D, Bebbington PE. A cognitive model of persecutory delusions. Br J Clin Psychol 2002;41:331-47. https://doi.org/10.1348/014466502760387461.
- Suhail K, Cochrane R. Effect of culture and environment on the phenomenology of delusions and hallucinations. Int J Soc Psychiatry 2002;48:126-38. https://doi.org/10.1177/002076402128783181.
- World Health Organization (WHO) . The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines 1992.
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5) 2013. https://doi.org/10.1176/appi.books.9780890425596.
- Passby L, Broome M. Thought disorder. BJPsych Adv 2018;23:321-3. https://doi.org/10.1192/apt.bp.116.016071.
- Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry 1982;39:784-8. https://doi.org/10.1001/archpsyc.1982.04290070020005.
- Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull 2006;32:214-19. https://doi.org/10.1093/schbul/sbj053.
- Strauss GP, Cohen AS. A transdiagnostic review of negative symptom phenomenology and etiology. Schizophr Bull 2017;43:712-19. https://doi.org/10.1093/schbul/sbx066.
- Szkultecka-Dębek M, Walczak J, Augustyńska J, Miernik K, Stelmachowski J, Pieniążek I, et al. Epidemiology and treatment guidelines of negative symptoms in schizo-phrenia in central and eastern Europe: a literature review. Clin Pract Epidemiol Ment Health 2015;11:158-65. https://doi.org/10.2174/1745017901511010158.
- Bobes J, Arango C, Garcia-Garcia M, Rejas J. CLAMORS Study Collaborative Group . Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. J Clin Psychiatry 2010;71:280-6. https://doi.org/10.4088/JCP.08m04250yel.
- Mäkinen J, Miettunen J, Jääskeläinen E, Veijola J, Isohanni M, Koponen H. Negative symptoms and their predictors in schizophrenia within the Northern Finland 1966 Birth Cohort. Psychiatry Res 2010;178:121-5. https://doi.org/10.1016/j.psychres.2009.05.011.
- Van Os J, Hanssen M, Bijl RV, Ravelli A. Strauss (1969) revisited: a psychosis continuum in the general population?. Schizophr Res 2000;45:11-20. https://doi.org/10.1016/S0920-9964(99)00224-8.
- Johns LC, van Os J. The continuity of psychotic experiences in the general population. Clin Psychol Rev 2001;21:1125-41. https://doi.org/10.1016/S0272-7358(01)00103-9.
- Murray GK, Jones PB. Psychotic symptoms in young people without psychotic illness: mechanisms and meaning. Br J Psychiatry 2012;201:4-6. https://doi.org/10.1192/bjp.bp.111.107789.
- Van Os J. Is there a continuum of psychotic experiences in the general population?. Epidemiol Psichiatr Soc 2003;12:242-52. https://doi.org/10.1017/S1121189X00003067.
- Peters E, Ward T, Jackson M, Morgan C, Charalambides M, McGuire P, et al. Clinical, socio demographic and psychological characteristics in individuals with persistent psychotic experiences with and without a “need for care”. World Psychiatry 2016;15:41-52. https://doi.org/10.1002/wps.20301.
- Jääskeläinen E, Juola T, Korpela H, Lehtiniemi H, Nietola M, Korkeila J, et al. Epidemiology of psychotic depression – systematic review and meta-analysis. Psychol Med 2018;48:905-18. https://doi.org/10.1017/S0033291717002501.
- Smith LM, Johns LC, Mitchell R. Characterizing the experience of auditory verbal hallucinations and accompanying delusions in individuals with a diagnosis of bipolar disorder: a systematic review. Bipolar Disord 2017;19:417-33. https://doi.org/10.1111/bdi.12520.
- Pina-Camacho L, Parellada M, Kyriakopoulos M. Autism spectrum disorder and schizophrenia: boundaries and uncertainties. BJPsych Adv 2016;22:316-24. https://doi.org/10.1192/apt.bp.115.014720.
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry 2008;65:28-37. https://doi.org/10.1001/archgenpsychiatry.2007.3.
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry 2012;69:220-9. https://doi.org/10.1001/archgenpsychiatry.2011.1472.
- McGorry PD, Yung AR, Phillips LJ. The ‘close-in’ or ultra high-risk model: a safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophr Bull 2003;29:771-90. https://doi.org/10.1093/oxfordjournals.schbul.a007046.
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustün TB. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry 2007;20:359-64. https://doi.org/10.1097/YCO.0b013e32816ebc8c.
- Schimmelmann BG, Conus P, Cotton S, McGorry PD, Lambert M. Pre-treatment, baseline, and outcome differences between early-onset and adult-onset psychosis in an epidemiological cohort of 636 first-episode patients. Schizophr Res 2007;95:1-8. https://doi.org/10.1016/j.schres.2007.06.004.
- Lauronen E, Miettunen J, Veijola J, Karhu M, Jones PB, Isohanni M. Outcome and its predictors in schizophrenia within the Northern Finland 1966 Birth Cohort. Eur Psychiatry 2007;22:129-36. https://doi.org/10.1016/j.eurpsy.2006.07.001.
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 1994;344:1398-402. https://doi.org/10.1016/S0140-6736(94)90569-X.
- Orr K, Castle DJ, Murray RM, Jones PB, Susser E, van Os J, et al. The Epidemiology of Schizophrenia. Cambridge: Cambridge University Press; 2003.
- National Collaborating Centre for Mental Health . Psychosis and Schizophrenia in Children and Young People: Recognition and Management 2013.
- Hellgren L, Gillberg C, Enerskog I. Antecedents of adolescent psychoses: a population-based study of school health problems in children who develop psychosis in adolescence. J Am Acad Child Adolesc Psychiatry 1987;26:351-5. https://doi.org/10.1097/00004583-198705000-00013.
- Burd L, Kerbeshian J. A North Dakota prevalence study of schizophrenia presenting in childhood. J Am Acad Child Adolesc Psychiatry 1987;26:347-50. https://doi.org/10.1097/00004583-198705000-00012.
- Gillberg C, Remschmidt H. Schizophrenia in Children and Adolescents. Cambridge: Cambridge University Press; 2001.
- Kirkbride JB, Fearon P, Morgan C, Dazzan P, Morgan K, Tarrant J, et al. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-centre ÆSOP study. Arch Gen Psychiatry 2006;63:250-8. https://doi.org/10.1001/archpsyc.63.3.250.
- Boeing L, Murray V, Pelosi A, McCabe R, Blackwood D, Wrate R. Adolescent-onset psychosis: prevalence, needs and service provision. Br J Psychiatry 2007;190:18-26. https://doi.org/10.1192/bjp.190.1.18.
- Corsico P, Griffin-Doyle M, Singh I. What constitutes ‘good practice’ in early intervention for psychosis? Analysis of clinical guidelines. Child Adolesc Ment Health 2018;23:185-93. https://doi.org/10.1111/camh.12229.
- Spencer E, Birchwood M, McGovern D. Management of first-episode psychosis. Adv Psychiatr Treat 2001;7:133-40. https://doi.org/10.1192/apt.7.2.133.
- McGorry PD, Killackey E, Yung A. Early intervention in psychosis: concepts, evidence and future directions. World Psychiatry 2008;7:148-56. https://doi.org/10.1002/j.2051-5545.2008.tb00182.x.
- NHS England, National Collaborating Centre for Mental Health and the National Institute for Health and Care Excellence . Implementing the Early Intervention in Psychosis Access and Waiting Time Standard: Guidance (Gateway Reference 04294) 2016.
- Hollis C. Adult outcomes of child- and adolescent-onset schizophrenia: diagnostic stability and predictive validity. Am J Psychiatry 2000;157:1652-9. https://doi.org/10.1176/appi.ajp.157.10.1652.
- Marwaha S, Thompson A, Upthegrove R, Broome MR. Fifteen years on – early intervention for a new generation. Br J Psychiatry 2016;209:186-8. https://doi.org/10.1192/bjp.bp.115.170035.
- Hayes D, Kyriakopoulos M. Dilemmas in the treatment of early-onset first-episode psychosis. Ther Adv Psychopharmacol 2018;8:231-9. https://doi.org/10.1177/2045125318765725.
- Clemmensen L, Vernal DL, Steinhausen HC. A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry 2012;12. https://doi.org/10.1186/1471-244X-12-150.
- Hollis C, Rapoport J, Weinberger DR, Harrison PJ. Schizophrenia. Chichester: Blackwell Publishing Ltd; 2011.
- Díaz-Caneja CM, Pina-Camacho L, Rodríguez-Quiroga A, Fraguas D, Parellada M, Arango C. Predictors of outcome in early-onset psychosis: a systematic review. NPJ Schizophr 2015;1. https://doi.org/10.1038/npjschz.2014.5.
- Menezes NM, Milovan E. First-episode psychosis: a comparative review of diagnostic evolution and predictive variables in adolescents versus adults. Can J Psychiatry 2000;45:710-16. https://doi.org/10.1177/070674370004500803.
- Birchwood M, Bryan S, Jones-Morris MN, Kaambwa MB, Lester H, Richards MJ, et al. EDEN: Evaluating the Development and Impact of Early Intervention Services (EISs) in the West Midlands. London: National Coordinating Centre for the Service Delivery and Organisation; 2007.
- Thornicroft G, Brohan E, Rose D, Sartorius N, Leese M. INDIGO Study Group . Global pattern of experienced and anticipated discrimination against people with schizophrenia: a cross-sectional survey. Lancet 2009;373:408-15. https://doi.org/10.1016/S0140-6736(08)61817-6.
- Brohan E, Elgie R, Sartorius N, Thornicroft G. GAMIAN-Europe Study Group . Self-stigma, empowerment and perceived discrimination among people with schizophrenia in 14 European countries: the GAMIAN-Europe study. Schizophr Res 2010;122:232-8. https://doi.org/10.1016/j.schres.2010.02.1065.
- Pyle M, Stewart SL, French P, Byrne R, Patterson P, Gumley A, et al. Internalized stigma, emotional dysfunction and unusual experiences in young people at risk of psychosis. Early Interv Psychiatry 2015;9:133-40. https://doi.org/10.1111/eip.12098.
- Jarbin H, Von Knorring AL. Suicide and suicide attempts in adolescent-onset psychotic disorders. Nord J Psychiatry 2004;58:115-23. https://doi.org/10.1080/08039480410005611.
- Falcone T, Mishra L, Carlton E, Lee C, Butler RS, Janigro D, et al. Suicidal behavior in adolescents with first-episode psychosis. Clin Schizophr Relat Psychoses 2010;4:34-40. https://doi.org/10.3371/CSRP.4.1.2.
- James A, Clacey J, Seagroatt V, Goldacre M. Adolescent inpatient psychiatric admission rates and subsequent one-year mortality in England: 1998–2004. J Child Psychol Psychiatry 2010;51:1395-404. https://doi.org/10.1111/j.1469-7610.2010.02293.x.
- Amminger GP, Henry LP, Harrigan SM, Harris MG, Alvarez-Jimenez M, Herrman H, et al. Outcome in early-onset schizophrenia revisited: findings from the early psychosis prevention and intervention centre long-term follow-up study. Schizophr Res 2011;131:112-19. https://doi.org/10.1016/j.schres.2011.06.009.
- Department of Health and Social Care (DHSC) . The Mental Health Policy Implementation Guide 2001.
- Bertolote J, McGorry P. Early intervention and recovery for young people with early psychosis: consensus statement. Br J Psychiatry Suppl 2005;48:s116-9. https://doi.org/10.1192/bjp.187.48.s116.
- National Institute for Health and Care Excellence (NICE) . Psychosis and Schizophrenia in Children and Young People: Recognition and Management (Clinical Guideline CG155) 2013.
- Healthcare Quality Improvement Partnership . Early Intervention in Psychosis Audit Report 2016.
- Selick A, Durbin J, Vu N, O’Connor K, Volpe T, Lin E. Barriers and facilitators to implementing family support and education in early psychosis intervention programmes: a systematic review. Early Interv Psychiatry 2017;11:365-74. https://doi.org/10.1111/eip.12400.
- Crumlish N, Whitty P, Clarke M, Browne S, Kamali M, Gervin M, et al. Beyond the critical period: longitudinal study of 8-year outcome in first-episode non-affective psychosis. Br J Psychiatry 2009;194:18-24. https://doi.org/10.1192/bjp.bp.107.048942.
- Upthegrove R, Birchwood M, Ross K, Brunett K, McCollum R, Jones L. The evolution of depression and suicidality in first episode psychosis. Acta Psychiatr Scand 2010;122:211-18. https://doi.org/10.1111/j.1600-0447.2009.01506.x.
- Birchwood M, Connor C, Lester H, Patterson P, Freemantle N, Marshall M, et al. Reducing duration of untreated psychosis: care pathways to early intervention in psychosis services. Br J Psychiatry 2013;203:58-64. https://doi.org/10.1192/bjp.bp.112.125500.
- Department of Health and Social Care (DHSC) . National Service Framework for Children, Young People and Maternity Services: Core Standards 2004.
- Rethink . Joint Working at the Interface: Early Intervention in Psychosis and Specialist Child and Adolescent Mental Health Services 2011.
- Vyas NS, Birchwood M, Singh SP. Youth services: meeting the mental health needs of adolescents. Ir J Psychol Med 2015;32:13-9. https://doi.org/10.1017/ipm.2014.73.
- Paul M, Ford T, Kramer T, Islam Z, Harley K, Singh SP. Transfers and transitions between child and adult mental health services. Br J Psychiatry Suppl 2013;54:s36-40. https://doi.org/10.1192/bjp.bp.112.119198.
- Singh SP, Paul M, Islam Z, Weaver T, Kramer T, McLaren S, et al. Transition from CAMHS to Adult Mental Health Services (TRACK): A Study of Service Organisation, Policies, Process and User and Carer Perspectives. Report for the National Institute for Health Research Service Delivery and Organisation Programme 2010.
- Crenna-Jennings W, Hutchinson J. Access to Children and Young People’s Mental Health Services – 2018. London: Education Policy Institute; 2018.
- Singh SP, Paul M, Ford T, Kramer T, Weaver T. Transitions of care from Child and Adolescent Mental Health Services to Adult Mental Health Services (TRACK Study): a study of protocols in Greater London. BMC Health Serv Res 2008;23. https://doi.org/10.1186/1472-6963-8-135.
- England E, Lester H, Birchwood M. Collaborating to provide early-intervention services to persons in England with first-episode psychosis. Psychiatr Serv 2009;60:1484-8. https://doi.org/10.1176/ps.2009.60.11.1484.
- McGorry P. Should youth mental health become a specialty in its own right? Yes. BMJ 2009;339. https://doi.org/10.1136/bmj.b3373.
- Krause M, Zhu Y, Huhn M, Schneider-Thoma J, Bighelli I, Chaimani A, et al. Efficacy, acceptability, and tolerability of antipsychotics in children and adolescents with schizophrenia: a network meta-analysis. Eur Neuropsychopharmacol 2019;29:32-45. https://doi.org/10.1016/j.euroneuro.2018.11.1105.
- Stafford MR, Mayo-Wilson E, Loucas CE, James A, Hollis C, Birchwood M, et al. Efficacy and safety of pharmacological and psychological interventions for the treatment of psychosis and schizophrenia in children, adolescents and young adults: a systematic review and meta-analysis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0117166.
- Olfson M, King M, Schoenbaum M. Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry 2015;72:867-74. https://doi.org/10.1001/jamapsychiatry.2015.0500.
- Correll CU, Kohegyi E, Zhao C, Baker RA, McQuade R, Salzman PM, et al. Oral aripiprazole as maintenance treatment in adolescent schizophrenia: results from a 52-week, randomized, placebo-controlled withdrawal study. Am Acad Child Adolesc Psychiatry 2017;56:784-92. https://doi.org/10.1016/j.jaac.2017.06.013.
- Pagsberg AK, Jeppesen P, Klauber DG, Jensen KG, Rudå D, Stentebjerg-Olesen M, et al. Quetiapine extended release versus aripiprazole in children and adolescents with first-episode psychosis: the multicentre, double-blind, randomised tolerability and efficacy of antipsychotics (TEA) trial. Lancet Psychiatry 2017;4:605-18. https://doi.org/10.1016/S2215-0366(17)30166-9.
- Hermes ED, Sokoloff DM, Stroup TS, Rosenheck RA. Minimum clinically important difference in the Positive and Negative Syndrome Scale using data from the CATIE Schizophrenia Trial. J Clin Psychiatry 2012;73. https://doi.org/10.4088/JCP.11m07162.
- James AC, Broome MR. Antipsychotics in adolescent-onset psychosis: a work in progress. Lancet Psychiatry 2017;4:e16-e7. https://doi.org/10.1016/S2215-0366(17)30287-0.
- Haddad PM, Sharma SG. Adverse effects of atypical antipsychotics. CNS Drugs 2007;21:911-36. https://doi.org/10.2165/00023210-200721110-00004.
- Moncrieff J, Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med 2010;40:1409-22. https://doi.org/10.1017/S0033291709992297.
- Schulz E, Remschmidt H. Psychopharmacology of Depressive States in Childhood and Adolescence. New York, NY: Cambridge University Press; 2001.
- Correll CU. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. J Clin Psychiatry 2008;69:26-3. https://doi.org/10.4088/JCP.0808e24.
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19-32. https://doi.org/10.1016/j.schres.2005.07.014.
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 2009;302:1765-73. https://doi.org/10.1001/jama.2009.1549.
- Morrison A, Renton J, Dunn H, Williams S, Bentall R. Cognitive Therapy For Psychosis: A Formulation-Based Approach. London: Routledge; 2004.
- Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington P. A cognitive model of the positive symptoms of psychosis. Psychol Med 2001;31:189-95. https://doi.org/10.1017/S0033291701003312.
- Chadwick P, Birchwood M. The omnipotence of voices: a cognitive approach to auditory hallucinations. Br J Psychiatry 1994;164:190-201. https://doi.org/10.1192/bjp.164.2.190.
- Brabban A, Byrne R, Longden E, Morrison AP. The importance of human relationships, ethics and recovery-orientated values in the delivery of CBT for people with psychosis. Psychosis 2017;9:157-66. https://doi.org/10.1080/17522439.2016.1259648.
- Onwumere J, Grice S, Kuipers E. Delivering cognitive-behavioural family interventions for schizophrenia. Aust Psychol 2016;51:52-61. https://doi.org/10.1111/ap.12179.
- Pyle M, Broome MR, Joyce E, MacLennan G, Norrie J, Freeman D, et al. Study protocol for a randomised controlled trial of CBT vs antipsychotics vs both in 14-18-year-olds: Managing Adolescent first episode Psychosis: a feasibility study (MAPS). Trials 2019;20. https://doi.org/10.1186/s13063-019-3506-1.
- Jauhar S, McKenna P, Radua J, Fung E, Salvador R, Laws K. Cognitive–behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry 2014;204:20-9. https://doi.org/10.1192/bjp.bp.112.116285.
- Zimmermann G, Favrod J, Trieu V, Pomini V. The effect of cognitive behavioral treatment on the positive symptoms of schizophrenia spectrum disorders: a meta-analysis. Schizophr Res 2005;77:1-9. https://doi.org/10.1016/j.schres.2005.02.018.
- Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull 2008;34:523-37. https://doi.org/10.1093/schbul/sbm114.
- Pilling S, Bebbington P, Kuipers E, Garety P, Geddes J, Orbach G, et al. Psychological treatments in schizophrenia: I. Meta-analysis of family intervention and cognitive behaviour therapy. Psychol Med 2002;32:763-82. https://doi.org/10.1017/S0033291702005895.
- Anagnostopoulou N, Kyriakopoulos M, Alba A. Psychological interventions in psychosis in children and adolescents: a systematic review. Eur Child Adolesc Psychiatry 2019;28:735-46. https://doi.org/10.1007/s00787-018-1159-3.
- Browning S, Corrigall R, Garety P, Emsley R, Jolley S. Psychological interventions for adolescent psychosis: a pilot controlled trial in routine care. Eur Psychiatry 2013;28:423-6. https://doi.org/10.1016/j.eurpsy.2013.05.008.
- Calvo A, Moreno M, Ruiz-Sancho A, Rapado-Castro M, Moreno C, Sánchez-Gutiérrez T, et al. Intervention for adolescents with early-onset psychosis and their families: a randomized controlled trial. J Am Acad Child Adolesc Psychiatry 2014;53:688-96. https://doi.org/10.1016/j.jaac.2014.04.004.
- Lewis S, Tarrier N, Haddock G, Bentall R, Kinderman P, Kingdon D, et al. Randomised controlled trial of cognitive-behavioural therapy in early schizophrenia: acute-phase outcomes. Br J Psychiatry Suppl 2002;181:s91-7. https://doi.org/10.1192/bjp.181.43.s91.
- Haddock G, Lewis S, Bentall R, Dunn G, Drake R, Tarrier N. Influence of age on outcome of psychological treatments in first-episode psychosis. Br J Psychiatry 2006;188:250-4. https://doi.org/10.1192/bjp.188.3.250.
- Klingberg S, Herrlich J, Wiedemann G, Wölwer W, Meisner C, Engel C, et al. Adverse effects of cognitive behavioral therapy and cognitive remediation in schizophrenia: results of the treatment of negative symptoms study. J Nerv Ment Dis 2012;200:569-76. https://doi.org/10.1097/NMD.0b013e31825bfa1d.
- Morrison AP, Hutton P, Shiers D, Turkington D. Antipsychotics: is it time to introduce patient choice?. Br J Psychiatry 2012;201:83-4. https://doi.org/10.1192/bjp.bp.112.112110.
- Bola JR, Lehtinen K, Cullberg J, Ciompi L. Psychosocial treatment, antipsychotic postponement, and low dose medication strategies in first episode psychosis: a review of the literature. Psychosis 2009;1:4-18. https://doi.org/10.1080/17522430802610008.
- Morrison AP, Turkington D, Pyle M, Spencer H, Brabban A, Dunn G, et al. Cognitive therapy for people with schizophrenia spectrum disorders not taking antipsychotic drugs: a single-blind randomised controlled trial. Lancet 2014;383:1395-403. https://doi.org/10.1016/S0140-6736(13)62246-1.
- Morrison AP, Law H, Carter L, Sellers R, Emsley R, Pyle M, et al. Antipsychotic drugs versus cognitive behavioural therapy versus a combination of both in people with psychosis: a randomised controlled pilot and feasibility study. Lancet Psychiatry 2018;5:411-23. https://doi.org/10.1016/S2215-0366(18)30096-8.
- Morrison AP, Pyle M, Maughan D, Johns L, Freeman D, Broome M, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): a multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry 2020;7:788-800. https://doi.org/10.1016/S2215-0366(20)30248-0.
- Ministry of Housing, Communities and Government . English Indices of Deprivation 2015 2015. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76. https://doi.org/10.1093/schbul/13.2.261.
- van der Gaag M HT, Remijsen M, Hijman R, de Haan L, van Meijel B, . The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr Res 2006;85:280-7. https://doi.org/10.1016/j.schres.2006.03.021.
- Law H, Neil ST, Dunn G, Morrison AP. Psychometric properties of the questionnaire about the process of recovery (QPR). Schizophr Res 2014;156:184-9. https://doi.org/10.1016/j.schres.2014.04.011.
- Zigmond AS SR. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Lecomte T, Corbiere M, Ehmann T, Addington J, Abdel-Baki A, Macewan B. Development and preliminary validation of the First Episode Social Functioning Scale for early psychosis. Psychiatry Res 2014;216:412-17. https://doi.org/10.1016/j.psychres.2014.01.044.
- Allison CAB, Baron-Cohen S. Toward brief ‘red flags’ for Autism screening: the short Autism Spectrum Quotient and the Short Quantitative Checklist in 1,000 cases and 3,000 controls. J Am Acad Child Adolesc Psychiatry 2012;51:202-12. https://doi.org/10.1016/j.jaac.2011.11.003.
- Morrison AP, Pyle M, Gumley A, Schwannauer M, Turkington D, MacLennan G, et al. Cognitive-behavioural therapy for clozapine-resistant schizophrenia: the FOCUS RCT. Health Technol Assess 2019;23. https://doi.org/10.3310/hta23070.
- Camacho EM, Davies LM, Hann M, Small N, Bower P, Chew-Graham C, et al. Long-term clinical and cost-effectiveness of collaborative care (versus usual care) for people with mental-physical multimorbidity: cluster-randomised trial. Br J Psychiatry 2018;213:456-63. https://doi.org/10.1192/bjp.2018.70.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993;88:791-804. https://doi.org/10.1111/j.1360-0443.1993.tb02093.x.
- Cassidy CM, Schmidtz N, Malla A. Validation of the alcohol use disorders identification test and the drug abuse screening test in first episode psychosis. Can J Psychiatry 2008;53:26-33. https://doi.org/10.1177/070674370805300105.
- Skinner HA. The Drug Abuse Screening Test. Addict Behav 1982;7:363-71. https://doi.org/10.1016/0306-4603(82)90005-3.
- Ronald A, Sieradzka D, Cardno AG, Haworth CM, McGuire P, Freeman D. Characterization of psychotic experiences in adolescence using the specific psychotic experiences questionnaire: findings from a study of 5000 16-year-old twins. Schizophr Bull 2014;40:868-77. https://doi.org/10.1093/schbul/sbt106.
- Ohlsen RI, Williamson R, Yusufi B, Mullan J, Irving D, Mukherjee S, et al. Interrater reliability of the antipsychotic non-neurological side-effects rating scale measured in patients treated with clozapine. J Psychopharmacol 2008;22:323-9. https://doi.org/10.1177/0269881108091069.
- Pyle M, Norrie J, Schwannauer M, Kingdon D, Gumley A, Turkington D, et al. Design and protocol for the Focusing on Clozapine Unresponsive Symptoms (FOCUS) trial: a randomised controlled trial. BMC Psychiatry 2016;16. https://doi.org/10.1186/s12888-016-0983-6.
- Leucht S, Kissling W, Davis JM. The PANSS should be rescaled. Schizophr Bull 2010;36:461-2. https://doi.org/10.1093/schbul/sbq016.
- Morrison AP. A manualised treatment protocol to guide delivery of evidence-based cognitive therapy for people with distressing psychosis: learning from clinical trials. Psychosis 2017;9:271-81. https://doi.org/10.1080/17522439.2017.1295098.
- Morrison AP. The interpretation of intrusions in psychosis: an integrative cognitive approach to hallucinations and delusions. Behav Cogn Psychother 2001;29:257-76. https://doi.org/10.1017/S1352465801003010.
- Flach C, French P, Dunn G, Fowler D, Gumley AI, Birchwood M, et al. Components of therapy as mechanisms of change in cognitive therapy for people at risk of psychosis: analysis of the EDIE-2 trial. Br J Psychiatry 2015;207:123-9. https://doi.org/10.1192/bjp.bp.114.153320.
- Kingdon D, Turkington D. Cognitive Therapy of Schizophrenia: Guides to Evidence-based Practice. New York, NY: Guilford Press; 2005.
- Fadden G, Birchwood M, Jackson C, Barton K, McGorry PD, Gleeson JFM. Psychological Interventions in Early Psychosis: A Practical Treatment Handbook. Chichester: John Wiley & Sons Ltd; n.d.
- Blackburn I-M, James IA, Milne DL, Baker C, Standart S, Garland A, et al. The Revised Cognitive Therapy Scale (CTS-R): psychometric properties. Behav Cogn Psychother 2001;29:431-46. https://doi.org/10.1017/S1352465801004040.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j. 2002.384.doc.x.
- Browne RH. On the use of a pilot sample for sample size determination. Stat Med 1995;14:1933-40. https://doi.org/10.1002/sim.4780141709.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Birchwood M, Trower P. The future of cognitive-behavioural therapy for psychosis: not a quasi-neuroleptic. Br J Psychiatry 2006;188:107-8. https://doi.org/10.1192/bjp.bp.105.014985.
- Haddad PM, Fleischhacker WW, Peuskens J, Cavallaro R, Lean ME, Morozova M, et al. SMARTS (Systematic Monitoring of Adverse events Related to TreatmentS): the development of a pragmatic patient-completed checklist to assess antipsychotic drug side effects. Ther Adv Psychopharmacol 2014;4:15-21. https://doi.org/10.1177/2045125313510195.
- Crawford MJ, Robotham D, Thana L, Patterson S, Weaver T, Barber R, et al. Selecting outcome measures in mental health: the views of service users. J Ment Health 2011;20:336-46. https://doi.org/10.3109/09638237.2011.577114.
- Andresen R, Oades L, Caputi P. The experience of recovery from schizophrenia: towards an empirically validated stage model. Aust N Z J Psychiatry 2003;37:586-94. https://doi.org/10.1046/j.1440-1614.2003.01234.x.
Appendix 1
| Site | Enquiries | ||
|---|---|---|---|
| Initial: ineligible (N = 629) | Initial: referred (N = 76) | Total initial (N = 705) | |
| Manchester | 215 (30.5) | 21 (3.0) | 236 (33.5) |
| Oxford | 171 (24.3) | 30 (4.3) | 201 (28.5) |
| Sussex | 22 (3.1) | 9 (1.3) | 31 (4.4) |
| Lancashire Care | 131 (18.6) | 13 (1.8) | 144 (20.4) |
| Norfolk and Suffolk NHS Foundation Trust | 35 (5.0) | 1 (0.1) | 36 (5.1) |
| Newcastle | 21 (3.0) | 1 (0.1) | 22 (3.1) |
| Birmingham | 34 (4.8) | 1 (0.1) | 35 (5.0) |
| Site | Referred (N = 101) | Excluded at referral (N = 29) | Screened (N = 72) | Excluded at screening (N = 11) | Randomised (N = 61) | Randomised to PI arm (N = 18) | Randomised to combined arm (N = 21) | Randomised AP arm (N = 22) |
|---|---|---|---|---|---|---|---|---|
| Manchester | 34 (33.7) | 10 (34.5) | 24 (33.3) | 3 (27.3) | 21 (34.4) | 7 (38.9) | 7 (33.3) | 7 (31.8) |
| Oxford | 33 (32.7) | 7 (24.1) | 26 (36.1) | 1 (9.1) | 25 (41.0) | 7 (38.9) | 9 (42.9) | 9 (40.9) |
| Sussex | 11 (10.9) | 1 (3.4) | 10 (13.9) | 6 (54.5) | 4 (6.6) | 1 (5.6) | 2 (9.5) | 1 (4.5) |
| Lancashire Care | 20 (19.8) | 10 (34.5) | 10 (13.9) | 1 (9.1) | 9 (14.8) | 3 (16.7) | 3 (14.3) | 3 (13.6) |
| Norfolk and Suffolk NHS Foundation Trust | 1 (1.0) | 1 (3.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Newcastle | 1 (1.0) | 0 (0) | 1 (1.4) | 0 (0) | 1 (1.6) | 0 (0) | 0 (0) | 1 (4.5) |
| Birmingham | 1 (1.0) | 0 (0) | 1 (1.4) | 0 (0) | 1 (1.6) | 0 (0) | 0 (0) | 1 (4.5) |
| Referral source | All sites | Site | ||||||
|---|---|---|---|---|---|---|---|---|
| Manchester | Oxford | Sussex | Lancashire Care | Norfolk and Suffolk NHS Foundation Trust | Newcastle | Birmingham | ||
| EIP service | 83 (82.2) | 24 (70.6) | 26 (78.8) | 11 (100.0) | 19 (95.0) | 1 (100.0) | 1 (100.0) | 1 (100.0) |
| CAMHS | 11 (10.9) | 6 (17.6) | 5 (15.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| At-risk mental state service | 2 (2.0) | 1 (2.9) | 0 (0) | 0 (0) | 1 (5.0) | 0 (0) | 0 (0) | 0 (0) |
| Inpatient | 1 (1.0) | 0 (0) | 1 (3.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Joint EIP service and CAMHS | 3 (3.0) | 3 (8.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Joint CAMHS and inpatient | 1 (1.0) | 0 (0) | 1 (3.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
FIGURE 8.
Recruitment over time by centre.
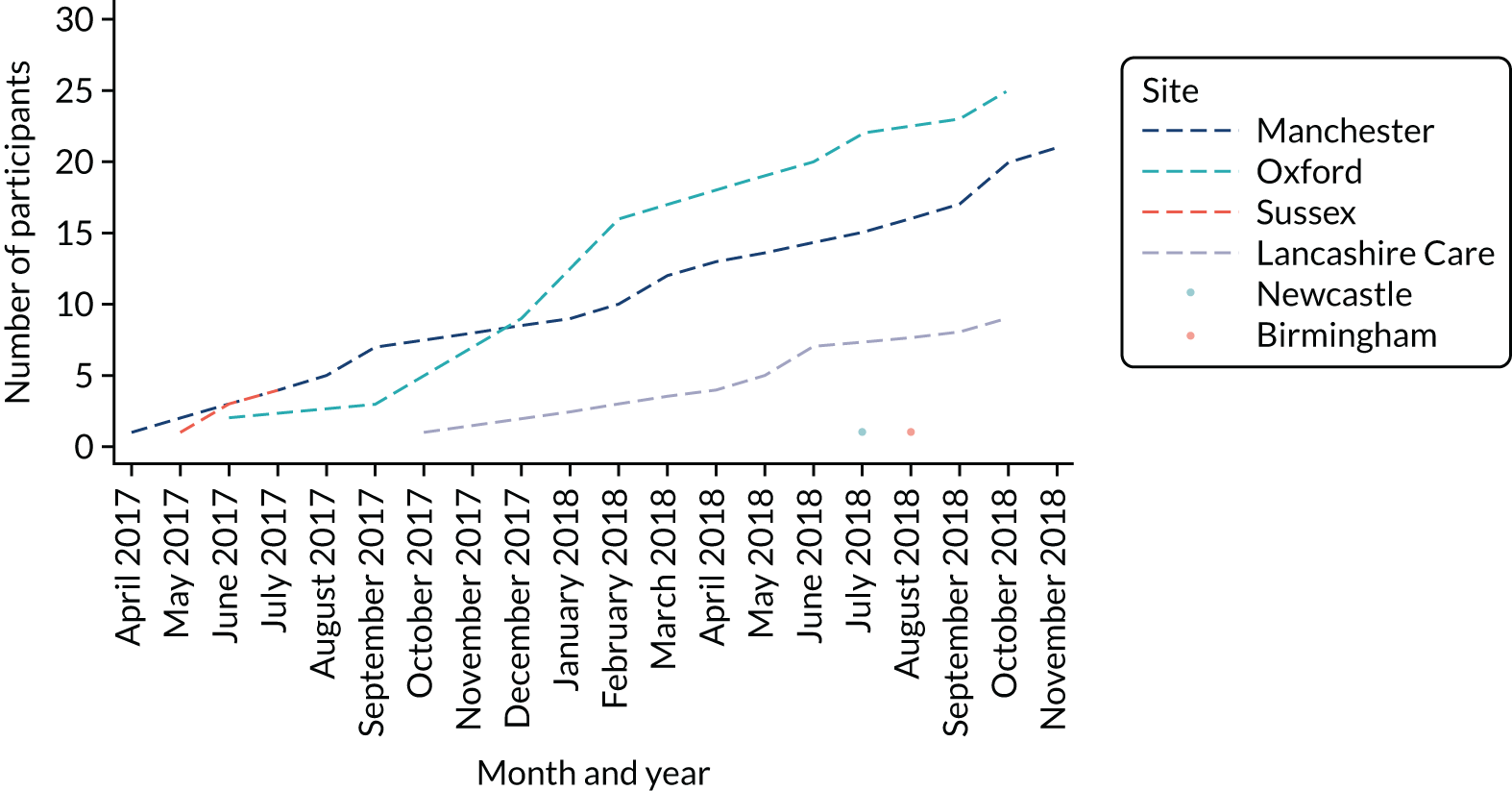
| ICD-10 diagnosis | n (%) |
|---|---|
| F20.0 (Paranoid schizophrenia) | 14 (22.9) |
| F20.81 (Schizophreniform disorder) | 5 (8.2) |
| F22.0 (Delusional disorder) | 1 (1.6) |
| F23.0 (Brief psychotic disorder) | 7 (11.5) |
| F23.2 (Acute schizophrenia-like psychotic disorder) | 2 (3.3) |
| F28.0 (Other psychotic disorder not due to a substance or known physiological condition) | 1 (1.6) |
| F29.0 (Unspecified psychosis not due to a substance or known physiological condition) | 27 (44.3) |
| Missing | 4 (6.6) |
| Reason for crossover | Treatment arm | ||
|---|---|---|---|
| PI (N = 8) | Combined (N = 10) | AP (N = 8) | |
| Unknown | 3 (37.5) | 7 (70.0) | 5 (62.5) |
| Deterioration | 1 (12.5) | 0 (0) | 2 (25.0) |
| Participant declined | 2 (25.0) | 1 (10.0) | 0 (0) |
| Clinician did not consider AP medication | 0 (0) | 1 (10.0) | 1 (12.5) |
| Participant and family declined | 0 (0) | 1 (10.0) | 0 (0) |
| Participant preference | 1 (12.5) | 0 (0) | 0 (0) |
| Voluntary admission to a psychiatric inpatient unit | 1 (12.5) | 0 (0) | 0 (0) |
| Time point | Time point | |||
|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | |
| Baseline | 1 | 0.4045 | 0.3029 | 0.3317 |
| 3 months | 0.4045 | 1 | 0.6869 | 0.1740 |
| 6 months | 0.3029 | 0.6869 | 1 | 0.2035 |
| 12 months | 0.3317 | 0.1740 | 0.2035 | 1 |
| Time point | Time point | |||
|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | |
| Baseline | 1 | 0.6824 | 0.5273 | 0.1647 |
| 3 months | 0.6824 | 1 | 0.6921 | 0.2756 |
| 6 months | 0.5273 | 0.6921 | 1 | 0.4284 |
| 12 months | 0.1647 | 0.2756 | 0.4284 | 1 |
| AE | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| SAEs | |||
| Participants with a SAE | 7 (38.9) | 4 (19.0) | 3 (13.6) |
| Number of SAEs | 10 | 5 | 3 |
| Participants with more than one SAE | 2 (28.6) | 1 (25.0) | 0 (0) |
| Details | |||
| Voluntary psychiatric admission | 1 | 1 | 1 |
| Life-threatening (suicide attempt) | 2 | 0 | 0 |
| Serious violent incident | 2 | 1 | 2 |
| Admission to a general medical ward | 2 | 1 | 0 |
| Otherwise considered medically significant (overdose of medication) | 3 | 1 | 0 |
| Otherwise considered medically significant (ingested painkillers) | 0 | 1 | 0 |
| Adverse events | |||
| Participants with an AE | 8 (44.4) | 11 (52.4) | 18 (81.8) |
| Number of AEs | 13 | 27 | 49 |
| Participants with more than one AE | 4 (50.0) | 6 (54.5) | 11 (61.1) |
| Details | |||
| Self-harm | 7 | 8 | 13 |
| Medication side effect | 0 | 15 | 30 |
| Other AE | 5 | 4 | 6 |
| Distress reported regarding allocation | 1 | 0 | 0 |
| Deterioration in PANSS total score | |||
| 6 months | |||
| > 25% | 1 (6.3) | 0 (0) | 3 (16.7) |
| > 50% | 0 (0) | 1 (5.9) | 1 (5.6) |
| 12 months | |||
| > 25% | 1 (9.1) | 0 (0) | 0 (0) |
| > 50% | 0 (0) | 0 (0) | 0 (0) |
| Adverse effect measure | Treatment arm | |||||
|---|---|---|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | ||||
| Quite a lot | Very much | Quite a lot | Very much | Quite a lot | Very much | |
| Number who responded | 8 (44.4) | 8 (38.1) | 6 (27.3) | |||
| Taking part hasn’t helped me with my problems | 2 (25.0) | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) |
| Taking part made my problems worse | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me feel more anxious | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) |
| Taking part took up too much time | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part led to my mood becoming very low | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me feel more angry and irritable | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I did not feel ready to talk about my problems | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me think too much about bad things that have happened in the past | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part meant I stopped looking after myself properly | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me feel more suspicious | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part required too much energy or motivation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part increased my thoughts of killing myself | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I did not feel listened to or believed by MAPS staff | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made my voices or visions worse | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part was making me fall out with my family or friends | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part was having a bad effect on my self-esteem | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part was making me want to harm myself | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I did not like or feel I could trust the MAPS team members | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| I felt embarrassed talking about my problems with people I had not met before | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me have thoughts of harming other people | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part was making me feel hopeless about the future | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part meant I had to increase my medication to cope | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part involved too much hard work | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me worry that people would think badly of me because of my diagnosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me fall out with my doctor or care team | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Taking part made me worry about losing control of my mind | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| My problems have improved to the point whereby I no longer feel I need helpa | 1 (12.5) | 1 (12.5) | 1 (12.5) | 0 (0) | 0 (0) | 2 (33.3) |
| Time point | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| ANNSERS total score | |||
| Baseline | 8; 17.5 (10.4) | 11; 17.2 (7.1) | 12; 15.9 (6.4) |
| 3 months | 7; 11.0 (4.3) | 8; 12.0 (5.9) | 10; 12.5 (7.0) |
| 6 months | 6; 9.0 (4.0) | 7; 6.7 (4.1) | 8; 15.5 (7.0) |
| 12 months | 5; 5.6 (4.8) | 6; 4.0 (3.9) | 5; 13.4 (8.8) |
| ANNSERS number of side effects | |||
| Baseline | 8; 10.6 (4.8) | 11; 11.5 (3.8) | 12; 11.2 (5.2) |
| 3 months | 7; 7.1 (2.7) | 8; 8.1 (3.5) | 10; 9.7 (5.6) |
| 6 months | 6; 6.3 (2.0) | 7; 5.3 (3.0) | 8; 10.5 (4.3) |
| 12 months | 5; 3.6 (3.0) | 6; 3.3 (3.5) | 5; 10.0 (6.2) |
| Weight (kg) | |||
| Baseline | 10; 66.1 (13.9) | 13; 62.8 (12.3) | 13; 68.2 (18.8) |
| 3 months | 8; 66.7 (15.2) | 9; 71.4 (12.6) | 12; 72.8 (18.1) |
| 6 months | 8; 69.8 (11.4) | 9; 72.5 (13.1) | 9; 72.8 (19.0) |
| 12 months | 6; 69.7 (11.7) | 5; 76.0 (10.4) | 6; 67.4 (19.0) |
| Waist circumference (cm) | |||
| Baseline | 9; 80.6 (11.4) | 13; 76.8 (7.8) | 11; 78.3 (13.3) |
| 3 months | 8; 83.2 (13.6) | 8; 76.0 (19.0) | 11; 82.8 (15.8) |
| 6 months | 8; 80.0 (10.6) | 8; 82.0 (9.1) | 8; 83.4 (14.3) |
| 12 months | 6; 83.2 (11.1) | 4; 83.8 (3.6) | 5; 84.2 (5.4) |
| BMI (kg/m2) | |||
| Baseline | 10; 23.6 (4.5) | 13; 22.5 (3.7) | 13; 24.1 (6.4) |
| 3 months | 8; 24.0 (4.7) | 9; 24.4 (4.3) | 12; 26.0 (6.6) |
| 6 months | 8; 24.9 (4.1) | 9; 25.0 (4.2) | 9; 25.3 (4.5) |
| 12 months | 6; 24.5 (3.7) | 5; 25.5 (4.2) | 6; 25.9 (8.7) |
| Blood pressure (kg/m2) | |||
| Systolic | |||
| Baseline | 10; 108.1 (11.0) | 12; 112.2 (10.1) | 10; 114.8 (11.5) |
| 3 months | 8; 107.3 (9.3) | 9; 115.1 (12.7) | 13; 112.4 (9.4) |
| 6 months | 8; 111.3 (11.5) | 9; 118.0 (11.1) | 9; 113.9 (12.2) |
| 12 months | 5; 106.7 (9.4) | 3; 112.8 (3.3) | 5; 110.9 (7.4) |
| Diastolic | |||
| Baseline | 10; 67.8 (7.8) | 12; 69.6 (9.7) | 10; 67.8 (5.7) |
| 3 months | 8; 69.8 (5.3) | 9; 70.7 (12.9) | 13; 66.4 (11.0) |
| 6 months | 8; 70.3 (3.4) | 9; 69.9 (6.8) | 9; 66.3 (8.3) |
| 12 months | 5; 63.2 (7.3) | 3; 66.0 (2.9) | 5; 67.5 (5.2) |
| Fasting estimates of plasma glucose levels (mmol/mol) | |||
| Baseline | 5; 4.6 (0.2) | 3; 5.0 (1.1) | 6; 4.2 (0.4) |
| 3 months | 4; 4.5 (0.2) | 1; 4.8 | 2; 4.3 (0.6) |
| 6 months | 3; 5.3 (1.4) | 1; 4.2 | 2; 5.3 (0.4) |
| 12 months | 2; 4.0 (0.1) | 0; 0 | 0; 0 |
| HbA1c levels (mmol/mol) | |||
| Baseline | 5; 30.4 (1.1) | 2; 35.5 (2.1) | 7; 30.7 (3.7) |
| 3 months | 3; 30.7 (1.5) | 2; 32.0 (0.0) | 4; 31.8 (2.2) |
| 6 months | 2; 32.0 (4.2) | 1; 30.0 | 3; 32.7 (3.8) |
| 12 months | 2; 37.5 (3.5) | 0; 0 | 1; 33.0 |
| Lipids levels (mmol/mol) | |||
| Cholesterol | |||
| Baseline | 6; 3.5 (0.3) | 3; 3.9 (0.4) | 7; 3.8 (0.6) |
| 3 months | 4; 4.0 (0.4) | 2; 3.5 (0.6) | 4; 4.6 (0.4) |
| 6 months | 3; 4.0 (1.3) | 1; 4.8 | 3; 3.6 (0.5) |
| 12 months | 2; 4.0 (0.1) | 0; 0 | 1; 3.6 |
| LDL | |||
| Baseline | 6; 2.0 (0.2) | 3; 2.1 (0.2) | 6; 2.1 (0.5) |
| 3 months | 4; 2.3 (0.3) | 2; 1.6 (0.4) | 4; 2.7 (0.4) |
| 6 months | 3; 2.4 (0.8) | 1; 2.9 | 3; 2.2 (0.7) |
| 12 months | 1; 2.2 | 0; 0 | 1; 1.9 |
| HDL | |||
| Baseline | 6; 1.0 (0.3) | 3; 1.5 (0.6) | 7; 1.1 (0.5) |
| 3 months | 4; 0.9 (0.2) | 2; 1.3 (0.1) | 4; 1.4 (0.3) |
| 6 months | 3; 0.9 (0.3) | 1; 1.2 | 3; 1.1 (0.1) |
| 12 months | 2; 1.3 (0.3) | 0; 0 | 1; 1.3 |
| Triglycerides | |||
| Baseline | 6; 1.0 (0.4) | 3; 0.7 (0.2) | 7; 1.0 (0.8) |
| 3 months | 4; 1.5 (0.5) | 2; 1.2 (0.8) | 4; 1.2 (0.5) |
| 6 months | 3; 1.6 (1.1) | 1; 1.5 | 3; 1.0 (0.2) |
| 12 months | 2; 3.9 (4.7) | 0; 0 | 1; 0.7 |
| Serum prolactin levels (µg/l) | |||
| Baseline | 6; 163.2 (79.2) | 3; 170.3 (35.9) | 7; 268.6 (86.6) |
| 3 months | 4; 170.8 (86.3) | 2; 97.5 (85.6) | 3; 503.7 (481.8) |
| 6 months | 2; 156.5 (37.5) | 1; 212.0 | 3; 268.0 (87.0) |
| 12 months | 2; 118.5 (44.5) | 0; 0 | 1; 200.0 |
| Time point | Treatment arm | |
|---|---|---|
| Combined (N = 21) | AP (N = 22) | |
| 3 months | ||
| Completed measure | 2 (9.5) | 3 (13.6) |
| Prescribed AP medication | ||
| Yes | 2 (100) | 2 (66.7) |
| No | 0 (0) | 1 (33.3) |
| Percentage of time adhered to AP medication, mean (SD); median (IQR) | 86 (9.9); 96.5 (86, 100) | 100 (0); 100 (100, 100) |
| 6 months | ||
| Completed measure | 5 (23.8) | 2 (9.1) |
| Prescribed AP medication | ||
| Yes | 3 (60.0) | 2 (100) |
| No | 2 (40.0) | 0 (0) |
| Percentage of time adhered to AP medication, mean (SD); median (IQR) | 73 (27.5); 74 (45, 100) | 92.5 (3.5); (90, 95) |
| 12 months | ||
| Completed measure | 5 (23.8) | 3 (13.6) |
| Prescribed AP medication | ||
| Yes | 4 (80.0) | 1 (33.3) |
| No | 1 (20.0) | 2 (66.7) |
| Percentage of time adhered to AP medication, mean (SD); median (IQR) | 73.8 (43.1); 92.5 (47.5, 100) | 100 (0); 100 (100, 100) |
| Time point | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| Baseline | 8; 0.686 (0.204) | 11; 0.623 (0.239) | 12; 0.620 (0.260) |
| 3 months | 5; 0.796 (0.085) | 7; 0.764 (0.179) | 12; 0.543 (0.268) |
| 6 months | 6; 0.637 (0.338) | 7; 0.868 (0.058) | 9; 0.763 (0.203) |
| 12 months | 4; 0.763 (0.252) | 6; 0.815 (0.250) | 4; 0.804 (0.133) |
| Resource use | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| Planned hospital overnight stay | |||
| No | 9 (50) | 10 (48) | 12 (55) |
| Yes | 1 (6) | 1 (5) | 1 (5) |
| Missing | 8 (44) | 10 (48) | 9 (41) |
| Attended hospital outpatient appointments that lasted for ≤ 4 hours during the last 12 months | |||
| No | 6 (33) | 6 (29) | 9 (41) |
| Yes | 4 (22) | 5 (24) | 4 (18) |
| Missing | 8 (44) | 10 (48) | 9 (41) |
| Attended hospital outpatient appointments that lasted for > 4 hours (but not overnight) during the last 12 months | |||
| No | 9 (50) | 10 (48) | 12 (55) |
| Yes | 0 (0) | 1 (5) | 1 (5) |
| Missing | 9 (50) | 10 (48) | 9 (41) |
| Attended an accident and emergency unit during the last 12 months | |||
| No | 3 (17) | 7 (33) | 6 (27) |
| Yes | 6 (33) | 4 (19) | 7 (32) |
| Do not know | 1 (6) | 0 (0) | 0 (0) |
| Missing | 8 (44) | 10 (48) | 9 (41) |
| Attended primary and community-based health services during the last 12 months | |||
| No | 0 (0) | 1 (5) | 0 (0) |
| Yes | 10 (56) | 9 (43) | 13 (59) |
| Do not know | 0 (0) | 1 (5) | 0 (0) |
| Missing | 8 (44) | 10 (48) | 9 (41) |
| GP visit | |||
| No | 0 (0) | 2 (10) | 5 (23) |
| Yes | 10 (56) | 9 (43) | 7 (32) |
| Do not know | 0 (0) | 0 (0) | 1 (5) |
| Missing | 8 (44) | 10 (48) | 9 (41) |
| GP visit at home | |||
| No | 9 (50) | 11 (52) | 13 (59) |
| Missing | 9 (50) | 10 (48) | 9 (41) |
| GP nurse visit | |||
| No | 6 (33) | 9 (43) | 10 (45) |
| Yes | 2 (11) | 2 (10) | 3 (14) |
| Missing | 10 (56) | 10 (48) | 9 (41) |
| GP nurse home visit | |||
| No | 8 (44) | 11 (52) | 12 (55) |
| Yes | 0 (0) | 0 (0) | 1 (5) |
| Missing | 10 (56) | 10 (48) | 9 (41) |
| Visited a walk-in centre | |||
| No | 7 (39) | 8 (38) | 12 (55) |
| Yes | 1 (6) | 3 (14) | 0 (0) |
| Do not know | 0 (0) | 0 (0) | 1 (5) |
| Missing | 10 (56) | 10 (48) | 9 (41) |
| Resource use | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| Planned hospital overnight stay | |||
| No | 6 (33) | 4 (19) | 13 (59) |
| Yes | 0 (0) | 2 (10) | 0 (0) |
| Missing | 12 (67) | 15 (71) | 9 (41) |
| Attended hospital outpatient appointments that lasted for ≤ 4 hours since last visit | |||
| No | 2 (11) | 4 (19) | 6 (27) |
| Yes | 4 (22) | 3 (14) | 5 (23) |
| Do not know | 0 (0) | 0 (0) | 2 (9) |
| Missing | 12 (67) | 14 (67) | 9 (41) |
| Attended hospital outpatient appointments that lasted for > 4 hours (but not overnight) since last visit | |||
| No | 6 (33) | 5 (24) | 10 (45) |
| Yes | 0 (0) | 1 (5) | 1 (5) |
| Do not know | 0 (0) | 0 (0) | 2 (9) |
| Missing | 12 (67) | 15 (71) | 9 (41) |
| Attended accident and emergency unit since last visit | |||
| No | 1 (6) | 4 (19) | 8 (36) |
| Yes | 5 (28) | 3 (14) | 4 (18) |
| Do not know | 0 (0) | 0 (0) | 1 (5) |
| Missing | 12 (67) | 14 (67) | 9 (41) |
| Attended primary and community-based health services since last visit | |||
| Yes | 6 (33) | 7 (33) | 13 (59) |
| Missing | 12 (67) | 14 (67) | 9 (41) |
| GP visit | |||
| No | 0 (0) | 2 (10) | 5 (23) |
| Yes | 6 (33) | 5 (24) | 8 (36) |
| Missing | 12 (67) | 14 (67) | 9 (41) |
| GP visit at home | |||
| No | 6 (33) | 7 (33) | 12 (55) |
| Yes | 0 (0) | 0 (0) | 1 (5) |
| Missing | 12 (67) | 14 (67) | 9 (41) |
| GP nurse visit | |||
| No | 5 (28) | 6 (29) | 7 (32) |
| Yes | 1 (6) | 1 (5) | 6 (27) |
| Missing | 12 (67) | 14 (67) | 9 (41) |
| GP nurse home visit | |||
| No | 6 (33) | 7 (33) | 11 (50) |
| Yes | 0 (0) | 0 (0) | 2 (9) |
| Missing | 12 (67) | 14 (67) | 9 (41) |
| Visited a walk-in centre | |||
| No | 4 (22) | 7 (33) | 10 (45) |
| Yes | 2 (11) | 0 (0) | 2 (9) |
| Do not know | 0 (0) | 0 (0) | 1 (5) |
| Missing | 12 (67) | 14 (67) | 9 (41) |
| Resource use | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| Planned hospital overnight stay | |||
| No | 6 (33) | 5 (24) | 9 (41) |
| Yes | 0 (0) | 1 (5) | 0 (0) |
| Do not know | 0 (0) | 1 (5) | 0 (0) |
| Missing | 12 (67) | 14 (67) | 13 (59) |
| Attended hospital outpatient appointments that lasted for ≤ 4 hours since last visit | |||
| No | 4 (22) | 5 (24) | 4 (18) |
| Yes | 2 (11) | 1 (5) | 5 (23) |
| Do not know | 0 (0) | 1 (5) | 0 (0) |
| Missing | 12 (67) | 14 (67) | 13 (59) |
| Attended hospital outpatient appointments that lasted for > 4 hours (but not overnight) since last visit | |||
| No | 6 (33) | 5 (24) | 7 (32) |
| Yes | 0 (0) | 1 (5) | 2 (9) |
| Do not know | 0 (0) | 1 (5) | 0 (0) |
| Missing | 12 (67) | 14 (67) | 13 (59) |
| Attended accident and emergency unit since last visit | |||
| No | 2 (11) | 3 (14) | 6 (27) |
| Yes | 4 (22) | 4 (19) | 3 (14) |
| Missing | 12 (67) | 14 (67) | 13 (59) |
| Attended primary and community-based health services since last visit | |||
| No | 1 (6) | 0 (0) | 0 (0) |
| Yes | 5 (28) | 6 (29) | 9 (41) |
| Do not know | 0 (0) | 1 (5) | 0 (0) |
| Missing | 12 (67) | 14 (67) | 13 (59) |
| GP visit | |||
| No | 1 (6) | 2 (10) | 2 (9) |
| Yes | 4 (22) | 5 (24) | 7 (32) |
| Missing | 13 (72) | 14 (67) | 13 (59) |
| GP visit at home | |||
| No | 5 (28) | 7 (33) | 8 (36) |
| Yes | 0 (0) | 0 (0) | 1 (5) |
| Missing | 13 (72) | 14 (67) | 13 (59) |
| GP nurse visit | |||
| No | 3 (17) | 5 (24) | 5 (23) |
| Yes | 2 (11) | 2 (10) | 4 (18) |
| Missing | 13 (72) | 14 (67) | 13 (59) |
| GP nurse home visit | |||
| No | 5 (28) | 7 (33) | 9 (41) |
| Yes | 13 (72) | 14 (67) | 13 (59) |
| Missing | 0 (0) | 0 (0) | 0 (0) |
| Visited a walk-in centre | 3 (17) | 7 (33) | 9 (41) |
| No | 2 (11) | 0 (0) | 0 (0) |
| Yes | 13 (72) | 14 (67) | 13 (59) |
| Missing | 0 (0) | 0 (0) | 0 (0) |
| Resource use | Treatment arm | ||
|---|---|---|---|
| PI (N = 18) | Combined (N = 21) | AP (N = 22) | |
| Planned hospital overnight stay | |||
| No | 4 (22) | 5 (24) | 4 (18) |
| Do not know | 0 (0) | 1 (5) | 0 (0) |
| Missing | 14 (78) | 15 (71) | 18 (82) |
| Attended hospital outpatient appointments that lasted for ≤ 4 hours since last visit | |||
| No | 4 (22) | 5 (24) | 3 (14) |
| Yes | 0 (0) | 1 (5) | 1 (5) |
| Missing | 14 (78) | 15 (71) | 18 (82) |
| Attended hospital outpatient appointments that lasted for > 4 hours (but not overnight) since last visit | |||
| No | 4 (22) | 6 (29) | 4 (18) |
| Missing | 14 (78) | 15 (71) | 18 (82) |
| Attended accident and emergency unit since last visit | |||
| No | 2 (11) | 4 (19) | 3 (14) |
| Yes | 2 (11) | 2 (10) | 0 (0) |
| Do not know | 0 (0) | 0 (0) | 1 (5) |
| Missing | 14 (78) | 15 (71) | 18 (82) |
| Attended primary and community-based health services since last visit | |||
| No | 0 (0) | 1 (5) | 0 (0) |
| Yes | 4 (22) | 5 (24) | 4 (18) |
| Missing | 14 (78) | 15 (71) | 18 (82) |
| GP visit | |||
| No | 2 (11) | 2 (10) | 1 (5) |
| Yes | 2 (11) | 4 (19) | 3 (14) |
| Missing | 14 (78) | 15 (71) | 18 (82) |
| GP visit at home | |||
| No | 4 (22) | 6 (29) | 4 (18) |
| Missing | 14 (78) | 15 (71) | 18 (82) |
| GP nurse visit | |||
| No | 3 (17) | 4 (19) | 3 (14) |
| Yes | 1 (6) | 2 (10) | 1 (5) |
| Missing | 14 (78) | 15 (71) | 18 (82) |
| GP nurse home visit | |||
| No | 4 (22) | 6 (29) | 3 (14) |
| Yes | 0 (0) | 0 (0) | 1 (5) |
| Missing | 14 (78) | 15 (71) | 18 (82) |
| Visited a walk-in centre | |||
| No | 4 (22) | 5 (24) | 4 (18) |
| Yes | 0 (0) | 1 (5) | 0 (0) |
| Missing | 14 (78) | 15 (71) | 18 (82) |
| Study ID | Characteristic | |||||
|---|---|---|---|---|---|---|
| Clinical role | Service type | Gender | Age (years) | MAPS young people under care (n) | Interview duration (minutes) | |
| C01 | Psychiatrist | CAMHS and EIP | Male | 49 | 3 | 49:43 |
| C02 | Advanced Practitioner | EIP | Male | 42 | – | 46:07 |
| C03 | Psychiatrist | CAMHS | Female | 45 | – | 34:52 |
| C04 | Psychiatrist | EIP | Female | 41 | 1 | 53:09 |
| C05 | Specialty doctor | EIP | Male | 45 | 1 | 53:04 |
| C06 | Psychiatrist | EIP | Male | 34 | 2 | 50:29 |
| C07 | Psychiatrist | CAMHS | Female | 39 | 1 | 46:40 |
| C08 | Psychiatrist | EIP | Female | 34 | – | 33:28 |
| C09 | Psychiatrist | CAMHS and EIP | Male | 36 | – | 23:07 |
| C10 | Psychiatrist | CAMHS | Female | Missing | – | 58:12 |
| C11 | Psychiatrist | EIP | Female | 50 | – | 15:29 |
| C12 | Psychiatrist | EIP | Female | Missing | 3 | 50:29 |
| C13 | Psychiatrist | EIP | Male | 32 | – | 40:26 |
| C14 | Psychiatrist | CAMHS | Male | 63 | 1 | 20:54 |
| C15 | Psychiatrist | CAMHS | Male | 32 | – | 40:32 |
| C16 | Psychiatrist | EIP | Male | 36 | 14 | 24:24 |
| C17 | Psychiatrist | CAMHS | Female | Missing | – | 26:54 |
| n = 17 | Female, n = 8; male, n = 9 | Mean = 41.3 | 39:18 | |||
| ID | Characteristic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Age (years) | Allocation | As treated | AP success criteria met | Duration of AP prescription (weeks) | PI success criteria met | Number of CBT sessions | Number of FI sessions | Interview duration (minutes) | |
| YP01 | Female | 15 | PI | PI | N/A | N/A | Yes | 11 | 0 | 40:41 |
| YP02 | Female | 15 | AP | None | No | 0 | N/A | N/A | N/A | 40:32 |
| YP03 | Female | 18 | Combined | Combined | Yes | 52.29 | Yes | 22 | 2 | 50:23 |
| YP04 | Female | 17 | PI | Combined | N/A | N/A | No | 2 | 1 | 44:04 |
| YP05 | Female | 16 | AP | AP | Yes | 19.14 | N/A | N/A | N/A | 25:34 |
| YP06 | Male | 17 | PI | PI | N/A | N/A | Yes | 24 | 5 | 37:50 |
| YP07 | Female | 16 | Combined | Combined | Yes | 31 | Yes | 17 | 1 | 23:25 |
| YP08 | Female | 18 | PI | PI | N/A | N/A | Yes | 23 | 3 | 53:53 |
| YP09 | Male | 17 | Combined | Combined | Yes | 32.86 | Yes | 17 | 5 | 41:48 |
| YP10 | Female | 19 | Combined | PI | No | 0 | Yes | 18 | 1 | 50:10 |
| YP11 | Male | 17 | AP | AP | Yes | 35.43 | N/A | N/A | N/A | 32:34 |
| YP12 | Female | 17 | Combined | PI | No | 0 | Yes | 9 | 0 | 44:42 |
| YP13 | Female | 19 | PI | PI | N/A | N/A | Yes | 22 | 3 | 53:48 |
| n = 13 | Female, n = 10; male, n = 3 | Mean = 34.14 | Mean = 16.5 | Mean = 2.1 | 41:30 | |||||
| ID | Characteristic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Age (years) | Allocation | As treated | AP success criteria met | Duration of AP prescription (weeks) | PI success criteria met | Number of CBT sessions | Number of FI sessions | Interview duration (minutes) | |
| F07 | Female and male | 39 | PI | PI | N/A | N/A | Yes | 24 | 5 | 56:22 |
| F08 | Female | 52 | Combined | Combined | Yes | 31 | Yes | 17 | 1 | 43:48 |
| F09 | Female and male | 49 and 51 | Combined | Combined | Yes | 48 | Yes | 17 | 7 | 56:14 |
| F10 | Female | 42 | PI | PI | N/A | N/A | Yes | 23 | 3 | 40:06 |
| F11 | Female | Missing | Combined | Combined | Yes | 38 | No | 1 | 0 | 1:08:34 |
| F12 | Female | 47 | Combined | Combined | Yes | 36.86 | Yes | 15 | 1 | 45:16 |
| F13 | Female | 50 | Combined | Combined | Yes | 32.86 | Yes | 17 | 5 | 53:07 |
| F14 | Female | 42 | PI | PI | N/A | N/A | Yes | 6 | 0 | 25:30 |
| F15 | Female | Missing | PI | Combined | N/A | Missing | Yes | 15 | 2 | 26:57 |
| F16 | Male | 59 | AP | AP | Yes | 37.14 | N/A | N/A | N/A | 43:34 |
| F06 | Female | Missing | AP | AP | Yes | 40 | N/A | N/A | N/A | 36:32 |
| F01 | Female | 71 | Combined | PI | No | 0 | Yes | 9 | 5 | 59:53 |
| F02 | Female | 37 | PI | Combined | N/A | Missing | Yes | 30 | 5 | 41:54 |
| F03 | Female | 50 | PI | Combined | N/A | Missing | Yes | 17 | 3 | 53:41 |
| F04 | Female | Missing | Combined | Combined | Yes | 49.14 | Yes | 14 | 4 | 48:35 |
| F05 | Male | 50 | PI | N/A | N/A | Yes | 22 | 3 | 48:07 | |
| n = 16 | Female, n = 14; male, n = 4 | Mean = 49.2 | 46:00 | |||||||
List of abbreviations
- AE
- adverse event
- AMHS
- adult mental health services
- ANNSERS
- Antipsychotic Non-Neurological Side Effects Scale
- AP
- antipsychotic
- AQ-10
- Autism Spectrum Quotient
- AUDIT
- Alcohol Use Disorders Identification Test
- BMI
- body mass index
- BNF
- British National Formulary
- CAMHS
- child and adolescent mental health services
- CBT
- cognitive–behavioural therapy
- CBTp
- cognitive–behavioural therapy for psychosis
- CG
- clinical guideline
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- COMPARE
- Cognitive behaviour therapy Or Medication for Psychosis – A Randomised Evaluation
- CONSORT
- Consolidated Standards of Reporting Trials
- CTS-R
- Cognitive Therapy Scale-Revised
- CTU
- clinical trials unit
- DAST
- Drug Abuse Screening Test
- DSM
- Diagnostic and Statistical Manual of Mental Disorders
- DSM-5
- Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- DUP
- duration of untreated psychosis
- EIP
- early intervention in psychosis
- EOP
- early-onset psychosis
- EPD
- early psychosis declaration
- EPPIC
- Early Psychosis Prevention and Intervention Centre
- EQ-5D-5L
- EuroQol five-dimension, five-level version
- ER
- extended release
- FEP
- first-episode of psychosis
- FESFS
- First Episode Social Functioning Scale
- FI
- family intervention
- FOCUS
- Focusing on Clozapine Unresponsive Symptoms
- FPG
- fasting plasma glucose
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HbA1c
- glycated haemoglobin
- HDL
- high-density lipoprotein
- HRA
- Health Research Authority
- HTA
- Health Technology Assessment
- ICD
- International Classification of Diseases
- ICD-10
- International Classification of Diseases – Tenth Revision
- iDMC
- independent Data Monitoring Committee
- IQR
- interquartile range
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LDL
- low-density lipoprotein
- MAPS
- Managing Adolescent first-episode Psychosis: a feasibility Study
- MD
- mean difference
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PANSS
- Positive and Negative Syndrome Scale
- PCT
- primary care trust
- PI
- psychological intervention
- PPI
- patient and public involvement
- PROBE
- Prospective Randomised Open Blinded Evaluation
- QPR
- Questionnaire about the Process of Recovery
- RA
- research assistant
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SPEQ
- Specific Psychotic Experiences Questionnaire
- TAU
- treatment as usual
- TEA
- Tolerability and Efficacy of Antipsychotics
- TEAE
- Treatment-emergent adverse effects
- TSC
- Trial Steering Committee
- UHR
- ultra high risk
- WHO
- World Health Organization
