Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 17/10/02. The protocol was agreed in July 2018. The assessment report began editorial review in January 2019 and was accepted for publication in October 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Tikhonova et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Background and definition of the decision problem(s)
Description of the health problem
Rheumatoid arthritis (RA) is a systemic autoimmune disease primarily causing chronic inflammation and destruction of the joints. The disease usually has a relapsing–remitting course, involving flare-ups followed by periods of low disease activity (LDA). However, for some people RA is constantly progressive and for others the disease might be short-lived. 1 Whether or not periods of remission or LDA are achieved, patients with RA need to be monitored to enable appropriate adjustments to be made to their treatment.
Aetiology, pathology and prognosis
Rheumatoid arthritis typically affects the synovial tissue of the small joints of the hands and feet. However, any synovial joint may be affected, causing swelling, stiffness and pain (synovitis), and progressive joint destruction. As RA is a systemic disease, the whole body may be affected, including the lungs, heart and eyes. Systemic symptoms may include a non-specific feeling of general illness, fatigue, systemic inflammation and depression. 2,3
The underlying reasons for the development of RA are complex and not fully understood. It is clear, however, that both genetic factors and environmental factors are involved. Genetic factors contribute an estimated two-thirds of the risk of developing RA,4 and also influence the progression and severity of the disease. 3,4 Non-genetic factors that increase the risk of developing RA include female sex (perhaps attributable to hormonal factors, with a lowered risk of developing the disease during pregnancy, with oral contraceptive use4,5 and in women who have breastfed, although this last relationship is somewhat less clear);6 regular smoking (this relationship is dose dependent,4 male smokers are particularly susceptible7 and smokers also experience more severe RA symptoms);8 dietary factors and obesity (including a high intake of red meat, salt and free fructose, and a low intake of vitamin C-containing fruits and vegetables);5,9 periodontitis;4 and advanced age. 4
For people with RA, these complex genetic and environmental factors lead to repeated activation of the innate and adaptive immune systems, leading to poor immune self-tolerance, the activation of antigen-specific T cells and B cells, and the production of antibodies associated with RA [rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP)]. These changes contribute to the destruction of the synovial joints and the other inflammatory symptoms that are seen in RA. 3 It is now known that dysregulation in the production of tumour necrosis factor-α (TNF-α) (a cell signalling protein that promotes inflammatory responses) can contribute to inflammatory disease; TNF-α is implicated in the development of many of the symptoms of RA (joint pain and destruction, fatigue and weight loss).
There is no cure for RA and there is substantial individual variation in the course of the disease. RA may be short-lived (i.e. achieving remission with no evidence of disease), relapsing–remitting (patterns of flare-ups followed by periods of improvement) or refractory despite treatment (disease continually worsening). 1 Data published in 200410 suggest that, although 10–15% of people with RA have refractory RA, and 10–15% experience full remission within 5 years of treatment, 70–80% of people with RA have relapsing–remitting disease. 10 Newer data suggest that remission rates are increasing and symptom flare-ups are decreasing, principally in the first 5 years after diagnosis. However, the majority of people with RA still experience relapsing–remitting disease. 11
Diagnosis of rheumatoid arthritis
A diagnosis of RA usually involves both laboratory tests and an assessment of clinical signs and symptoms. According to the National Institute for Health and Care Excellence (NICE) guidance on the management of RA in adults (NG100),12 initial testing should include both blood tests for RF and X-rays of the hands and feet. Additionally, C-reactive protein (CRP) testing should be considered for those with negative RF results. 12
To aid clinical diagnosis, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) have developed classification criteria for RA. These criteria attribute points based on the number of tender or swollen joints, serological tests for RF and anti-CCP antibodies and tests for acute-phase reactants [CRP and erythrocyte sedimentation rate (ESR)]. The duration of symptoms is also assessed (Table 1). A total score of ≥ 6 points (currently or previously) on the ACR/EULAR classification system, together with clinically obvious synovitis, is considered to indicate definite RA if symptoms cannot be better explained by an alternative diagnosis. 13
| Classification criteria | Score (points) |
|---|---|
| Joint distribution (score range 0–5) | |
| 1 large joint | 0 |
| > 1–10 large, asymmetric joints | 1 |
| > 1–10 large, symmetric joints | 1.5 |
| 1–3 small joints (large joints not counted) | 2 |
| 4–10 small joints (large joints not counted) | 3 |
| > 10 joints, including at least one small joint | 5 |
| Serology (score range 0–3) | |
| Negative RF and negative ACPA | 0 |
| Low positive RF or low positive ACPA | 2 |
| High positive RF or high positive ACPA | 3.5 |
| Symptom duration (score range 0–1) | |
| < 6 weeks | 0 |
| ≥ 6 weeks | 1 |
| Acute-phase reactants (score range 0–1) | |
| Normal CRP and normal ESR | 0 |
| Abnormal CRP or abnormal ESR | 0.5 |
Epidemiology
Based on estimates from 2002, there are 400,000 people in England and Wales living with RA, with 10,000 incident cases per year. 14,15 However, the figure is probably higher, with data from 2009 suggesting that, in England alone, RA affects approximately 0.8% of the population, or 580,000 adults, with 26,000 new cases diagnosed each year. 1 RA is approximately three times more common in women than in men and is less common among people with a higher educational level and people in non-manual employment. 4 The age of peak incidence in the UK is 70–79 years. 12
According to data from the British Society for Rheumatology Biologics Register in Rheumatoid Arthritis (BSRBR-RA), between 2001 and 2014 a total of 13,502 people with RA began treatment with a TNF-α inhibitor, although the real number is almost certainly higher, as not all people treated with biologics are recruited to the BSRBR-RA and not everyone consents to inclusion. 16 Consistent with RA as a whole, 76% of patients registered were female. Among those starting TNF-α inhibitor therapy the median age was 57 years [interquartile range (IQR) 49–65 years] and disease was severe, with a median Disease Activity Score in 28 joints (DAS28) of 6.5 (IQR 5.8–7.2). 16
Historically, there has been concern about geographical variation in access to TNF-α inhibitors. Although available data on this are not up to date, and despite geographical variation in service provision, differential geographical access to biological treatment for RA is no longer considered an issue. However, recent evidence suggests that the choice of specific TNF-α inhibitor in England might be influenced by age and relationship status. 17
Impact of the health problem
Rheumatoid arthritis varies greatly from person to person, but often results in substantial morbidity, impaired physical activity and poor quality of life, which leads to a reduced life expectancy (although increased mortality has been decreasing over time). 18
The disease is often multimorbid; data published in 200619 from the BSRBR-RA suggest that, among people treated with biological agents, 58% have at least one comorbid condition, most commonly hypertension, depression, peptic ulcer disease or respiratory disease. Owing to the chronic nature of RA, coupled with the high risk of comorbidities,19 a multidisciplinary team of health-care professionals and services is required for the management of the disease. 12 Support may also be sought from patient groups. RA is, therefore, associated with a substantial cost burden to the NHS. A report by the National Rheumatoid Arthritis Society (NRAS)20 published in 2010 estimates the annual cost to the NHS of RA, including the costs of drug acquisition and hospitalisation, to be nearly £700M.
Approximately one-third of people with RA stop work within 2 years of the onset of symptoms, and the number increases with time. Sickness absence is greater among people with RA than in people without RA (40 vs. 6.5 days per year). 1 The 2010 NRAS report20 estimated the total indirect cost of RA in England and Wales as a result of annual loss of productivity at over £7B. 20
Based on prices from the British National Formulary (BNF) in 2018,21 the costs to the NHS of TNF-α inhibitors per patient per year are:
-
£9187.08 for adalimumab (ADL) (Humira®; AbbVie Inc., North Chicago, IL, USA)
-
£9155.64 for golimumab (GLM) (Simponi®; Merck Sharp & Dohme Limited, Hoddesdon, UK)
-
£9326.92 for certolizumab pegol (CTZ) (Cimzia®; UCB Pharma Limited, Slough, UK) (although the cost in the first year is £10,399.42)
-
£9326.92 for etanercept (ETN) (Enbrel®; Pfizer, Sandwich, UK)
-
£8557.29 and £8394.23 for ETN biosimilars Benepali® (Biogen Biosimilars, Cambridge, MA, USA) and Erelzi® (Sandoz UK Limited, Camberley, UK), respectively
-
£5747.48 for infliximab (IFX) (Remicade®; Merck Sharp & Dohme Limited) (£7730.18 in the first year)
-
£5172.76 for IFX biosimilars Inflectra® (Pfizer, Sandwich, UK) and Remsima® (Napp Pharmaceuticals Limited, Cambridge, UK); £6957.20 in the first year
-
£5163.72 for IFX biosimilars Flixabi® (Biogen Biosimilars) and Renflexis® (Samsung Bioepis, Incheon, Republic of Korea) (£6945.05 in the first year).
Costs will vary with dosing changes or as a result of negotiated procurement discounts. It should be noted that the cost of ADL has very recently decreased, owing to the approval of ADL biosimilars [Amgevita® (Amgen, Cambridge, UK), Hulio® (Mylan/Fujifilm Kyowa Kirin, Tokyo, Japan), Imraldi® (Samsung Bioepis) and Hyrimoz® (Sandoz UK Limited)], although these costs could not be accessed and the estimated percentage uptake of these products was unclear at the time of writing. There is also a substantive wastage cost associated with biological treatments, averaging an estimated £370 per patient per year. 22 When people continue to be prescribed TNF-α inhibitors unnecessarily, there is an obvious cost implication. Unnecessary continued treatment may also lead to unnecessary side effects. Potential side effects of TNF-α inhibitors may include, but are not limited to, increased risk of viral and bacterial infections (of the respiratory tract, bladder and skin), allergic reactions, nausea and vomiting, itching and fever (Table 2). Efficient systems for monitoring responses to these treatments, and, thus, informing decisions on optimal drug dosing or on treatment discontinuation, could therefore be of benefit to the NHS.
| TNF-α inhibitor | Recommended usea | Contraindications | Very common adverse reactions | Administration | Brand namesb |
|---|---|---|---|---|---|
| ETN | In combination with MTX, for use in severe RA (i.e. DAS28 of > 5.1) or as monotherapy when MTX is contraindicated or not tolerated | Sepsis or risk of sepsis, active infections (chronic or localised) | Infections and injection site reactions | Subcutaneous injection; 50 mg weekly or 25 mg twice-weekly | Enbrel,c Erelzi, Benepali, dLifmior® and eBrenzys® |
| ADL | In combination with MTX, for use in severe RA (i.e. DAS28 of > 5.1) or as monotherapy when MTX is contraindicated or not tolerated | Active tuberculosis, other severe infections, moderate to severe heart failure | Respiratory tract infections, leucopenia, anaemia, increased lipids, headache, abdominal pain, nausea and vomiting, elevated liver enzymes, rash, musculoskeletal pain, injection site reaction | Subcutaneous injection; 40 mg every other week | Humira,c Amgevita, fCyltezo®, Imraldi, g,hSolymbic®, Hyrimoz, iHalimatoz® and Hulio |
| IFX | In combination with MTX, for use in severe RA (i.e. DAS28 of > 5.1) | Active tuberculosis, other severe infections, moderate to severe heart failure | Viral infection, headache, upper respiratory tract infection, sinusitis, abdominal pain, nausea, infusion-related reaction and pain | Intravenous infusion; 3 mg/kg at 0, 2 and 6 weeks, and then every 8 weeksj | Remicade,c Inflectra, Remsima, Flixabi, kZessly®, Renflexis land Ixifi® |
| CTZ | In combination with MTX, for use in severe RA (i.e. DAS28 of > 5.1) or as monotherapy when MTX is contraindicated or not tolerated | Active tuberculosis, other severe infections, moderate to severe heart failure | None listedm | Subcutaneous injection; 400 mg at 0, 2 and 4 weeks, and then 200 mg every 2 weeksn | Cimziac |
| GLM | In combination with MTX, for use in severe RA (i.e. DAS28 of > 5.1) | Active tuberculosis, other severe infections, moderate to severe heart failure | Upper respiratory tract infections | Subcutaneous injection; 50 mg monthlyo | cSimponi® |
Management of rheumatoid arthritis
According to the NICE guidance for RA in 201812 and the NICE RA care pathway,24 active RA in adults should be treated with the aim of achieving a target of remission or LDA (treat to target). The main aim of treatment and management of RA is, therefore, to achieve target symptom control and to prevent further damage. Monitoring of treatment response is required to enable appropriate treatment adjustments to be made.
Treatment of rheumatoid arthritis
The NICE guidance12 for RA recommends the use of disease-modifying anti-rheumatic drugs (DMARDs). Short-term (bridging) glucocorticoids might be offered prior to starting DMARDs. If control of pain and inflammation is inadequate, non-steroidal anti-inflammatory drugs (including cyclooxygenase II-selective inhibitors) are used, sometimes in combination with other analgesics. In established disease, complications and associated comorbidities are addressed and treated as appropriate. This may involve physiotherapy, occupational therapy, podiatry, psychological therapies, complementary therapies and dietary advice; patients with persistent or worsening joint damage, may be offered surgery. 12
Disease-modifying treatment may be broadly classified as conventional [conventional disease-modifying antirheumatic drugs (cDMARDs), including methotrexate, leflunomide, sulfasalazine and hydroxychloroquine], synthetic [synthetic disease-modifying antirheumatic drugs (sDMARDs), such as the Janus kinase inhibitor tofacitinib] or biologic [biologic disease-modifying antirheumatic drugs (bDMARDs), including, but not limited to, TNF-α inhibitors]. The NICE guidance for RA12 and the NICE RA care pathway24 indicate that initial DMARD treatment for adults with active RA should begin with cDMARD monotherapy, within 3 months of symptom onset if possible. If treatment targets are not met, despite dose escalation, further cDMARDs are added. 12,24
The role of tumour necrosis factor-α inhibitors in the care pathway
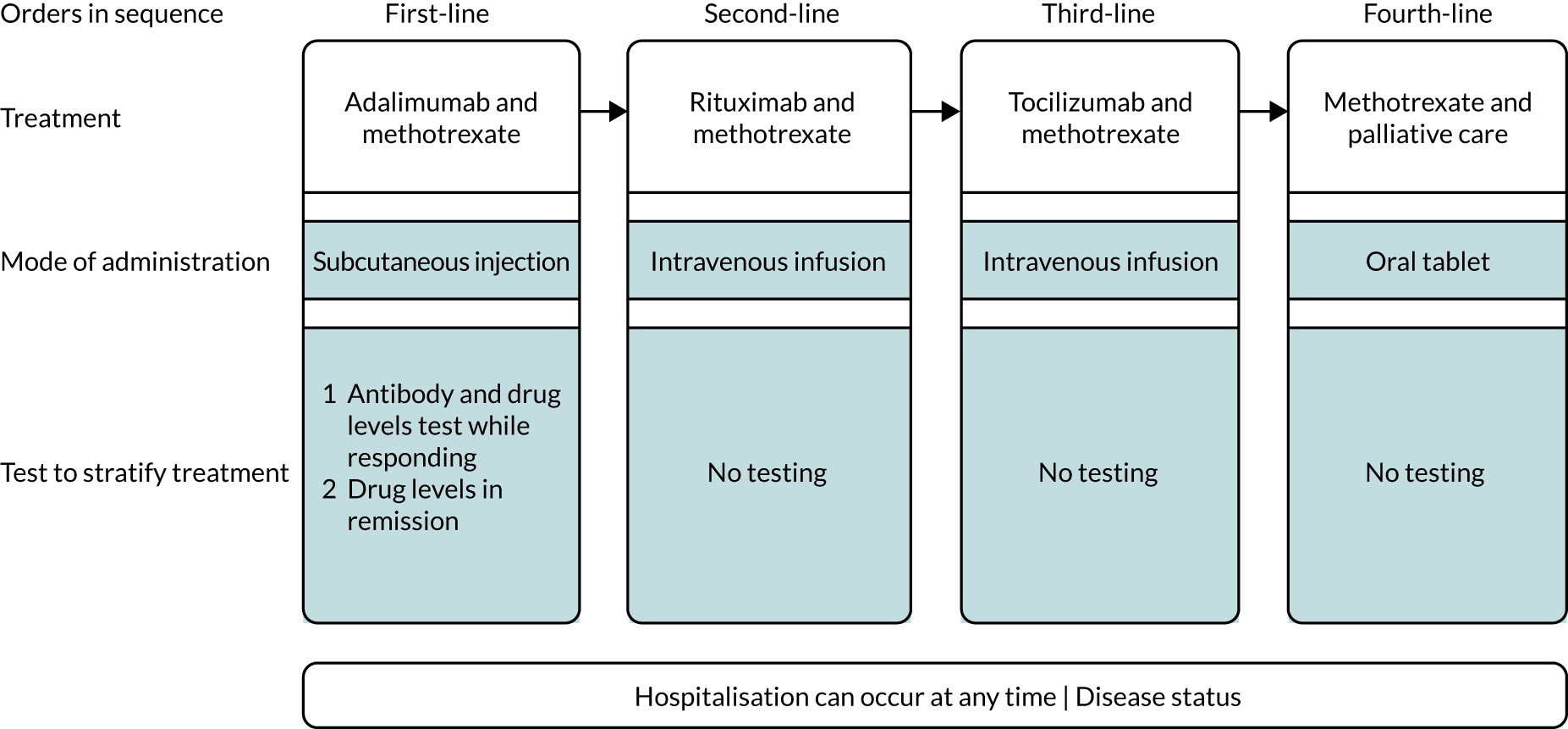
The NICE care pathway24 states that bDMARDs, including TNF-α inhibitors, should be offered only to people with severe disease that has not been controlled with cDMARDs. 12 NICE Technology Appraisal 375 (TA375)23 recommends using ADL, ETN, IFX, CTZ and GLM, in combination with methotrexate, in severe RA (i.e. DAS28 of > 5.1) that has not responded to intensive therapy with at least two cDMARDs, including methotrexate. ADL, ETN and CTZ may also be used as monotherapy for people in whom methotrexate is contraindicated or not tolerated. As part of TA375,23 NICE also makes recommendations for two other bDMARDs (tocilizumab and abatacept),23 but these interventions are outside the scope of this appraisal.
A summary of the recommended TNF-α inhibitors relevant to this report, their contraindications and very common adverse reactions, and a list of biosimilars, is provided in Table 2. The biosimilars listed in Table 2 are thought to have bioequivalence (and are also often assumed to perform similarly) to the reference/originator products. 25 It should be noted that IFX is administered by intravenous infusion in the outpatient setting, whereas the other recommended TNF-α inhibitors may be self-administered by subcutaneous injection (usually administered by patients in their own homes). TA375 recommends that treatment should start with the least expensive drug (taking into account administration costs, required dose and product price per dose). 23
Although TNF-α inhibitors have been found to be of benefit in the treatment of RA,23 some people do not respond to these treatments (primary non-responders) and others experience a loss of response (secondary non-responders). Secondary non-response may be due to changes in the disease, the development of antibodies to the TNF-α inhibitor or fluctuations in circulating drug levels.
Monitoring rheumatoid arthritis
Monitoring RA can be used to identify primary and secondary non-response, which potentially improves access to specialist services and informs treatment alteration decisions. Monitoring can also be used to guide treatment adjustments in those who have achieved treatment targets. The NICE guidance for RA12 recommends a monitoring review appointment 6 months after treatment targets are achieved, to ensure maintenance of the target. Monitoring should continue annually to assess disease activity, treatment response, functioning, impact on the patient’s quality of life, comorbidities, complications and the need for surgery, and to arrange multidisciplinary referrals. 12
Current methods for monitoring treatment response
Owing to the huge variation between individuals in the severity and course of RA, and, thus, in treatment targets, it is incredibly difficult to measure changes in the disease in a standardised way. Indeed, in clinical practice, evaluation of both treatment response and symptom flare-ups is multifaceted, and may involve assessment of a number of domains (pain, fatigue, activity level, overall physical and mental health, functioning in work and education, complications and adverse effects), in addition to measuring disease activity (using standardised scales and additional imaging).
A range of classification systems and scales have been developed to measure and monitor disease activity in RA (as well as scales that are commonly used to measure other domains, e.g. disability or activity level), such as the Health Assessment Questionnaire (HAQ). 26 Disease activity is commonly measured using clinical examination (swollen joint counts and tender joint counts), laboratory test results (e.g. CRP or ESR) or composite measures based on a combination of the above [such as DAS28,27 the Clinical Disease Activity Index (CDAI),28 the Simplified Disease Activity Index (SDAI),28 the ACR20 improvement criteria29 and the EULAR response classification system30].
In current clinical practice, the DAS28 scales and the EULAR response classification system (which is based on the DAS28) are most commonly used to monitor disease activity. The use of ultrasound is not recommended for routine monitoring of disease activity in adults with RA. 12,31
Disease Activity Score in 28 joints
There are two variations of the DAS28: the DAS28-ESR and the DAS28-CRP. 27 Both scales are composite scores that assess 28 joints (shoulder, knee, elbow, wrist, metacarpophalangeal joints 1–5, proximal interphalangeal joints 1–5, bilaterally) for swelling (SW28) and tenderness (TEN28) to touch, and also involve the patient’s self-assessment of disease activity in the past week on a scale of 0–100. Both scales additionally include blood markers of inflammation (ESR for the DAS28-ESR and CRP for the DAS28-CRP).
Overall Disease Activity Scores are calculated as follows:
Where GH is general health or patient’s global assessment of disease activity on a 100-mm visual analogue scale (VAS). A DAS28 of > 5.1 denotes severe disease activity, a score of ≤ 5.1 but > 3.2 denotes moderate disease activity (MDA), a score of ≤ 3.2 but ≥ 2.6 denotes LDA and a score of < 2.6 denotes disease remission. 32,33
European League Against Rheumatism response classification
The EULAR response classification system is based on improvement in the DAS28 from the initial measurement. 30 The EULAR system classifies improvement as ‘none’, ‘moderate’ or ‘good’. The relationship between the DAS28 and the EULAR response classifications is provided in Table 3.
| DAS28 at end point | Improvement in DAS28 of ≤ 1.2 | Improvement in DAS28 of > 0.6 and ≤ 1.2 | Improvement in DAS28 of ≤ 0.6 |
|---|---|---|---|
| ≤ 3.2 | Good | Moderate | None |
| > 3.2 and ≤ 5.1 | Moderate | Moderate | None |
| > 5.1 | Moderate | None | None |
Monitoring of response to tumour necrosis factor-α inhibitors
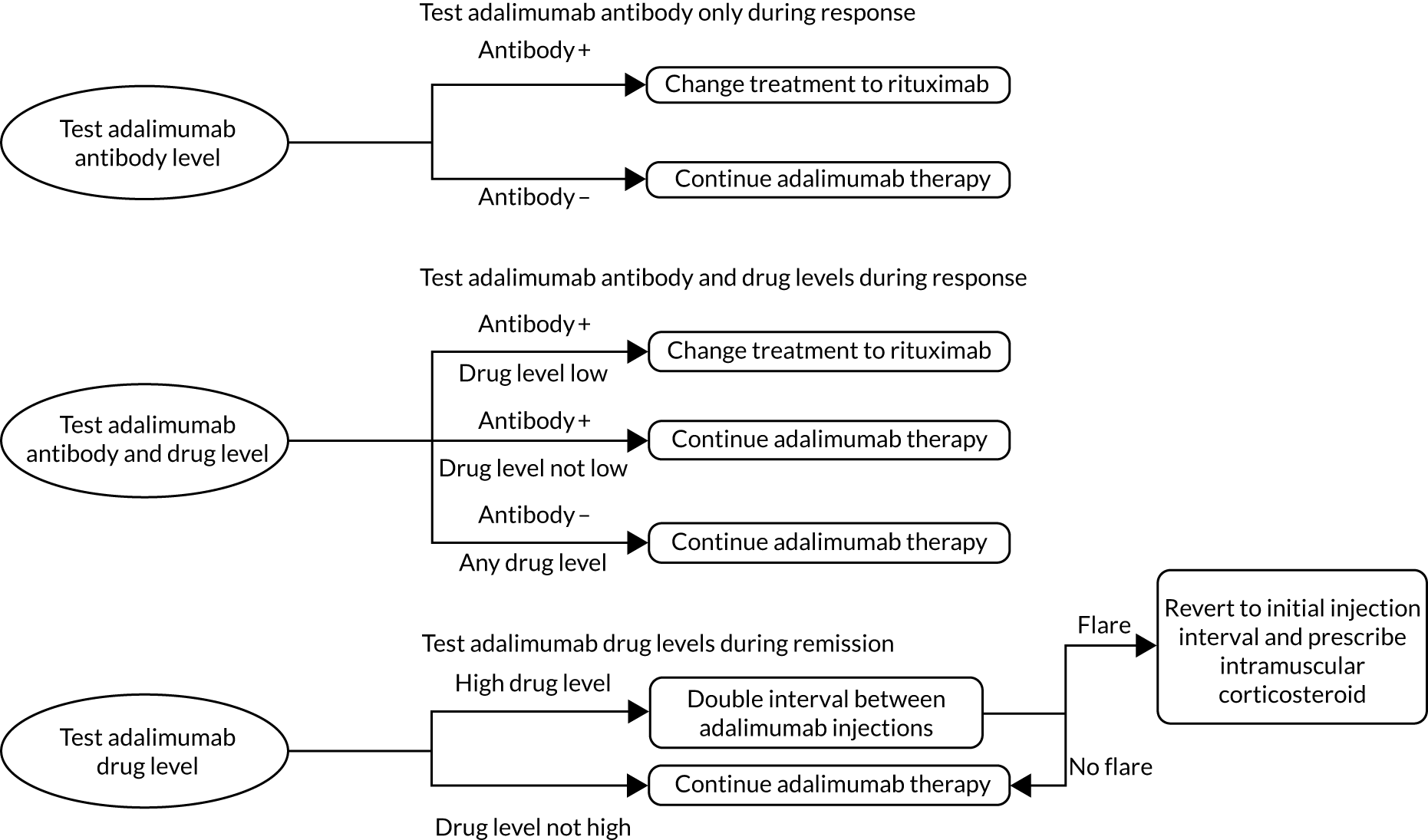
Although monitoring of response to treatment with TNF-α inhibitors typically involves the systems described above (clinical assessment, DAS28 and EULAR response criteria), there are neither gold standards nor guidelines available specifically for the monitoring of TNF-α inhibitors. More recently, biochemical enzyme-linked immunosorbent assay (ELISA) has emerged to measure blood levels of TNF-α inhibitors, or antibodies to TNF-α inhibitors, in people with RA. These testing kits and services – LISA-TRACKER (Theradiag, Croissy-Beaubourg, France), IDKmonitor (manufactured by Immundiagnostik AG, Bensheim, Germany, and distributed in the UK by BioHit Healthcare, Cheshire, UK), RIDASCREEN® (R-Biopharm, Darmstadt, Germany), MabTrack (Sanquin, Amsterdam, the Netherlands) and Promonitor kits [Progenika Biopharma SA (a Grifols–Progenika company), Derio, Spain], and ELISAs used by Sanquin Diagnostic Services (Amsterdam, the Netherlands) – might be useful for detecting primary and secondary non-response to TNF-α inhibitors and for the optimisation of dosages for those who are responding well. For those whose response to therapy has waned, the results of the tests are frequently dichotomised using a cut-off assay result; thus, people may be classified as having either therapeutic or subtherapeutic levels of the drugs, or may be classified as having clinically significant or insignificant levels of antibodies.
These tests may also elucidate reasons for treatment non-response. For example, non-adherence to TNF-α inhibitors may play a part in failure to respond to treatment. Monitoring of blood levels of TNF-α inhibitors, or antibodies to TNF-α inhibitors, can help to reveal non-adherence. In a 3-year study assessing non-adherence to ETN (using ELISAs) in people with RA, 4.1% [95% confidence interval (CI) 2.2% to 7.2%] of patients were non-adherent to treatment (non-adherence defined as serum ETN trough concentration of < 0.1 μg/ml in the absence of a valid medical reason), and 3.4% (95% CI 0.8% to 10.4%) of treatment non-responders had insufficient ETN exposure, indicative of non-adherence. 35
The administration of TNF-α inhibitor and anti-drug antibody assays most frequently occurs just before the next administration of the TNF-α inhibitor. This enables simultaneous measurement of a ‘trough’ level of the drug. The tests may be conducted concurrently, or using a reflex testing strategy, whereby the test for TNF-α-inhibitor drug levels is conducted first and the result is used to guide follow-up testing by the laboratory without a further request from the treating clinician (i.e. TNF-α inhibitor antibody testing would be conducted only if the drug was not detected in the sample).
Description of technologies under assessment
The purpose of this work is to provide NICE with the most up-to-date evidence on the clinical effectiveness and cost-effectiveness of alternative testing and monitoring approaches for assessing TNF-α inhibitor levels and antibodies to TNF-α inhibitor levels in people with RA undergoing treatment with ADL, ETN, IFX, CTZ or GLM, in the UK. There are three clinical scenarios in which the tests in the scope of this appraisal may be used: (1) remission/LDA to check whether or not continued treatment at the same dose is appropriate, (2) primary non-responders (defined as those who have little to no improvement in clinical signs and symptoms initially and as treatment continues) and (3) secondary non-responders (people with an initial response to a TNF-α inhibitor followed by loss of efficacy). Testing could help clinicians and patients to understand the reasons for a non-response or loss of response.
Summary of technologies
The technologies to be evaluated are biochemical ELISA kits and services, which are used to measure the levels of TNF-α inhibitors or antibodies to TNF-α inhibitors, typically in the period immediately before administration of the next dose (i.e. trough levels), conducted in addition to current clinical practice in the UK (i.e. clinical assessment and monitoring using a composite score, such as DAS28).
There are six companies providing different test kits or services for up to five TNF-α inhibitors or the antibodies to those TNF-α inhibitors. The test kits are summarised in Table 4. In addition to these test kits, the service provided by Sanquin Diagnostic Services (testing service using validated ELISA), covering ADL, CTZ, ETN, GLM and IFX drug levels and ETN anti-drug antibodies, will be evaluated. Further detail on these test kits and services are provided in the following sections. It should be noted that although several of the ELISAs measure the same drugs (and drug antibodies), there is significant variation between tests in their assay (detection) ranges. This means that some tests may be able to detect and quantify lower and/or higher levels of the same analyte than others.
| Technology (company) | Variations/kits | Drug/antibodies assessed |
|---|---|---|
| Promonitor ELISA kits | Promonitor-ADL-1DV (50802300DV) | Freea ADL |
| Promonitor-ANTI-ADL-1DV (50902300DV) | Freea anti-ADL antibodies | |
| Promonitor-ETN-1DV (51102300DV) | Freea ETN | |
| Promonitor-ANTI-ETN-1DV (51202300DV) | Freea anti-ETN antibodies | |
| Promonitor- IFX-1DV (50802300DV) | Freea IFX (Remicade and biosimilars) | |
| Promonitor-ANTI-IFX-1DV (50702300DV) | Freea anti-IFX antibodies | |
| Promonitor-GLM-1DV (52002300DV) | Freea GLM | |
| Promonitor-ANTI-GLM-1DV (52102300DV) | Freea anti-GLM antibodies | |
| IDKmonitor ELISA kits | IDKmonitor infliximab drug level ELISA (K9655) | Freea IFX (Remicade, Remsima and Inflectra) |
| IDKmonitor adalimumab drug level ELISA (K9657) | Freea ADL | |
| IDKmonitor etanercept drug level ELISA (K9646) | Freea ETN | |
| IDKmonitor golimumab drug level ELISA (K9656) | Freea GLM | |
| IDKmonitor infliximab free ADA ELISA (K9650) | Freea anti-IFX antibodies | |
| IDKmonitor adalimumab free ADA ELISA (K9652) | Freea anti-ADL antibodies | |
| IDKmonitor etanercept free ADA ELISA (K9653) | Freea anti-ETN antibodies | |
| IDKmonitor golimumab free ADA ELISA (K9649) | Freea anti-GLM antibodies | |
| IDKmonitor infliximab total ADA ELISA (K9654) | Totalb anti-IFX antibodies | |
| IDKmonitor adalimumab total ADA ELISA (K9651) | Totalb anti-ADL antibodies | |
| LISA-TRACKER kits | LISA-TRACKER adalimumab (LTA002) | Freea ADL |
| LISA-TRACKER certolizumab (LTC002) | Freea CTZ | |
| LISA-TRACKER etanercept (LTE002) | Freea ETN | |
| LISA-TRACKER infliximab (LTI002) | Freea IFX | |
| LISA-TRACKER golimumab (LTG002) | Freea GLM | |
| LISA-TRACKER anti-adalimumab (LTA003) | Freea anti-ADL antibodies | |
| LISA-TRACKER anti-certolizumab (LTC003) | Freea anti-CTZ antibodies | |
| LISA-TRACKER anti-infliximab (LTI003) | Freea anti-IFX antibodies | |
| LISA-TRACKER anti-etanercept (LTE003) | Freea anti-ETN antibodies | |
| LISA-TRACKER anti-golimumab (LTG003) | Freea anti-GLM antibodies | |
| LISA-TRACKER Duo adalimumab (LTA005) | Freea ADL | |
| LISA-TRACKER Duo certolizumab (LTC005) | Freea CTZ | |
| LISA-TRACKER Duo etanercept (LTE005) | Freea ETN | |
| LISA-TRACKER Duo infliximab (LTI005) | Freea IFX | |
| LISA-TRACKER Duo golimumab (LTG005) | Freea GLM | |
| RIDASCREEN | RIDASCREEN ADM monitoring (G09043) | Freea ADL |
| RIDASCREEN anti-ADM antibodies (G09044) | Freea antibodies to ADL | |
| RIDASCREEN IFX monitoring (G09041) | Freea IFX (Remicade, Remsima and Inflectra) | |
| RIDASCREEN anti-IFX antibodies (G09042) | Freea antibodies to IFX | |
| MabTrack ELISA kits | MabTrack level adalimumab M2910 | Freea ADL |
| MabTrack ADA adalimumab M2950 | Freea antibodies to ADL | |
| MabTrack level infliximab M2920 | Freea IFX (Remicade, Remsima and Inflectra) | |
| MabTrack ADA infliximab M2960 | Freea antibodies to IFX |
Promonitor
Promonitor is a portfolio of assays that measure drug levels (ETN, IFX and IFX biosimilars, ADL and GLM) and their correlating anti-drug antibodies (anti-ETN, anti-IFX, anti-ADL and anti-GLM) (see Table 4). The kits are manufactured by Progenika and distributed in the UK by Grifols–Progenika UK. They consist of strips of precoated microtitre plate (96 wells), reagents, buffers, standards, controls and ELISA cover films. The ELISAs are laboratory based and are conducted either manually or on an automated ELISA processor.
IDKmonitor ELISA kits
IDKmonitor ELISA kits are manufactured by Immundiagnostik AG and distributed in the UK by BioHit Healthcare Ltd. The 10 kits measure either levels of free TNF-α inhibitor or levels of free anti-drug antibodies, or total levels of anti-drug antibodies (free antibodies and antibodies bound to the drug) (see Table 4). The kits consist of strips of precoated microtitre plate (96 wells), reagents, buffers, standards (drug-level ELISAs only) and controls. The ELISAs are laboratory based and conducted either manually or on an automated ELISA processor.
LISA-TRACKER ELISA kits
LISA-TRACKER ELISA kits are manufactured by Theradiag. The kits measure either levels of free anti-drug antibodies or levels of free TNF-α inhibitor (see Table 4). In addition, LISA-TRACKER Duo kits (Theradiag) are available (these include assays to measure the levels of both free anti-drug antibodies and the TNF-α inhibitor). The LISA-TRACKER ELISA kits consist of precoated strips of microtitre plate (96 wells), reagents, wash buffer, standards and controls. They are laboratory-based assays that can be run simultaneously or individually, on any manual or automated standard ELISA-based processor platform.
RIDASCREEN
The RIDASCREEN ELISA kits are manufactured by R-Biopharm. The four kits are laboratory-based assays measuring either levels of free TNF-α inhibitor or levels of free anti-drug antibodies (see Table 4). The RIDASCREEN ELISAs are commercialised versions of the KU Leuven (Leuven, Belgium) in-house ELISAs, and are marketed as apDia (Turnhout, Belgium) ELISA kits in the Benelux area of Europe.
MabTrack ELISA kits and Sanquin Diagnostic Services
Sanquin is a laboratory that provides laboratory test services, including testing for TNF-α inhibitors using ELISA-based assays. The testing service, which uses validated ELISAs, is available for ETN and its correlating anti-drug antibodies, GLM drug levels and CTZ drug levels. It also provides Conformité Européenne-marked MabTrack ELISA kits for local laboratory testing for ADL and IFX levels and their correlating anti-drug antibodies (see Table 4). The MabTrack ELISA kits consist of precoated strips of microtitre plate (96 wells), reagents, wash buffer, standards or calibrators, controls and ELISA cover films.
Place of tests in the clinical pathway
Guidance from NICE (TA375)23 states that treatment with a TNF-α inhibitor should be continued only if there is a moderate initial response (using EULAR criteria) at 6 months after treatment initiation and that treatment should be withdrawn if a moderate EULAR response is not maintained. 23 NICE also provides guidance (TA195)36 on the treatment of RA after a TNF-α inhibitor has failed. The addition of ELISAs to current clinical monitoring procedures has the potential to inform decisions about treatment continuation and treatment optimisation. In addition, ELISAs may also help clinicians to understand the reasons for non-response or loss of response, inform decisions on dosing, and enable adherence to treatment to be assessed. As such, the ELISAs fall into the monitoring and review (following drug treatment) section of the NICE care pathway. 24
Identification of important subgroups
People with RA can be grouped according to three clinical scenarios: (1) primary non-response, (2) secondary non-response and (3) remission. However, with regard to particular patient characteristics, there are no subgroups for which the tests are expected to perform differently.
Current usage in the NHS
In UK clinical practice, the tests under assessment are currently not routinely used for people with RA, and are performed in two UK laboratories only [Viapath (London, UK) and Exeter Clinical Lab (Exeter, UK)]. At the Exeter Laboratory, TNF-α testing is carried out using IDKmonitor test kits, whereas LISA-TRACKER ELISAs are used at Viapath. However, these are currently used ad hoc to assist in making treatment management decisions; for example, dose adjustment rather than being used in routine monitoring strategies.
Anticipated costs associated with the use of the tests
The costs of the ELISA kits and services are detailed in Chapter 4, Assay costs provided by the manufacturer. In addition to the costs of the tests themselves, and based on a recent microcosting study,37 the following costs have been identified as being associated with the use of these tests:
-
Pretesting phase – a single outpatient appointment with a consultant rheumatologist and a follow-up appointment with a phlebotomist or clinical support worker.
-
Analysis phase – costs associated with personnel time and any additional materials required to analyse patient samples (excluding assumed costs, such as equipment costs, overhead costs, and capital costs).
-
Treatment decision stage – cost of interpretation of test results by a consultant rheumatologist, cost of a telephone discussion of the results with the patient, cost of a letter outlining results and treatment decisions.
These costs are described in further detail in Chapter 4, Processing costs.
Comparators
Comparison was made between monitoring strategies that use the index tests or services described above (in addition to current clinical practice in the UK) and current clinical practice alone (i.e. clinical assessment and monitoring using a composite score, such as DAS28, ACR response criteria or EULAR response criteria).
Outcomes
The outcomes of interest in the assessment of clinical effectiveness included:
-
test (procedural) outcomes – number of inconclusive test results and time-to-test result
-
treatment and management outcomes – number, direction and magnitude of dose changes, frequency of dose adjustments (e.g. dose reduction) as a result of monitoring, frequency of treatment switching to an alternative biologic, discontinuation of ineffective treatment
-
clinical outcomes – measures of change in disease activity, rates and duration of disease response, relapse and remission, rates of surgical intervention, rates of hospitalisation and adverse events (AEs) of treatment
-
patient-related outcomes – health-related quality of life (HRQoL).
The cost-effectiveness modelling took into account costs/resource use and patient outcomes. The main cost considerations were categorised as the costs incurred through the acquisition and administration of biologics, the costs associated with testing (drug trough levels and anti-drug antibodies) and the cost of disease management. The relevant patient outcomes that informed the economic model were the percentage of patients on tapered doses (remission), the flare rate and the rate of AEs. The economic modelling considered concurrent and reflex, single and duplicate testing, and how the frequency of testing may have an impact on cost-effectiveness.
Summary of the scope of work
In summary, this work evaluated the clinical effectiveness and cost-effectiveness of using the testing kits and services described above, in people with RA who were undergoing treatment with ADL, ETN, IFX, CTZ or GLM in England and Wales. A summary of the clinical scenarios in which each test might be used, and thus the scope of the work, is provided in Table 5.
| Clinical scenario | TNF-α inhibitor | Drug/antibody | ELISA kit | |||||
|---|---|---|---|---|---|---|---|---|
| Promonitor | IDKmonitor | LISA-TRACKER | RIDASCREEN | MabTrack | Sanquin | |||
| Response | ADL | Drug | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Antibody | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| ETN | Drug | ✗ | ✗ | ✗ | ✗ | |||
| Antibody | ✗ | ✗ | ✗ | ✗ | ||||
| IFX | Drug | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Antibody | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| GLM | Drug | ✗ | ✗ | ✗ | ✗ | |||
| Antibody | ✗ | ✗ | ✗ | |||||
| CTZ | Drug | ✗ | ✗ | |||||
| Antibody | ✗ | |||||||
| Primary non-response | ADL | Drug | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Antibody | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| ETN | Drug | ✗ | ✗ | ✗ | ✗ | |||
| Antibody | ✗ | ✗ | ✗ | ✗ | ||||
| IFX | Drug | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Antibody | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| GLM | Drug | ✗ | ✗ | ✗ | ✗ | |||
| Antibody | ✗ | ✗ | ✗ | |||||
| CTZ | Drug | ✗ | ✗ | |||||
| Antibody | ✗ | |||||||
| Secondary non-response | ADL | Drug | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Antibody | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| ETN | Drug | ✗ | ✗ | ✗ | ✗ | |||
| Antibody | ✗ | ✗ | ✗ | ✗ | ||||
| IFX | Drug | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |
| Antibody | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| GLM | Drug | ✗ | ✗ | ✗ | ✗ | |||
| Antibody | ✗ | ✗ | ✗ | |||||
| CTZ | Drug | ✗ | ✗ | |||||
| Antibody | ✗ | |||||||
As noted in Description of technologies under assessment, and as seen in Table 5, the technologies will be evaluated (1) for use during response (remission or LDA) to inform decisions regarding whether or not the same treatment should continue at the same dose, (2) to identify primary non-responders and (3) to identify and examine potential reasons for secondary non-response.
Chapter 2 Assessment of clinical effectiveness
This review assessed the clinical effectiveness of using ELISAs for measuring levels of drugs (ADL, ETN, IFX, CTZ and GLM) and/or anti-drug antibodies (anti-ADL, anti-ETN, anti-IFX, anti-CTZ and anti-GLM) for the purpose of monitoring response to those TNF-α inhibitors in people with RA. The eligible populations were people with RA who were being treated with TNF-α inhibitor therapies and had:
-
achieved treatment target (remission or LDA)
-
experienced a primary non-response
-
experienced a secondary non-response.
This report contains reference to confidential information provided as part of the NICE Diagnostic Assessment process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Methods for reviewing effectiveness
The systematic review was conducted following the Cochrane Handbook for Systematic Reviews of Intervention38 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 39 The systematic review was performed in accordance with a prespecified protocol that was registered on the international prospective register of systematic reviews (PROSPERO CRD42018105195).
Identification of studies
The following bibliographic databases were searched:
-
MEDLINE (via Ovid)
-
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
-
EMBASE (via Ovid)
-
Web of Science (via Thomson Reuters)
-
Cochrane Database of Systematic Reviews, CENTRAL (via the Cochrane Library).
In addition, searches were carried out on the following websites: ProQuest Dissertations & Theses Global, British Library EThOS, DART-Europe E-theses Portal, PROSPERO, ARIF (Aggresstive Research Intelligence Facility), Health Technology Assessment (HTA) database, DARE, CRD (Centre for Reviews and Dissemination), Open Grey, Grey Literature Report, Evidence-Based Laboratory Medicine (C-EBLM), British Society for Rheumatology, EULAR, American College of Rheumatology, Medion Grifols Diagnostics AG, Theradiag, Sanquin, R-Biopharm AG, Immunodiagnostik, Biohit Healthcare, Progenika Biopharma, ClinicalTrials.gov, World Health Organization (WHO) International Clinical Trials Registry Platform and EU Clinical Trials Register. The following resources provide coverage for ongoing trials: ClinicalTrials.gov, WHO International Clinical Trials Registry Platform and the EU Clinical Trials Register.
The search strategies were developed by an information specialist in July 2018 and were designed to be as sensitive as possible. They comprised terms for RA and terms for TNF-α inhibitors and terms for ELISA testing. No study type, language or date filters were used; studies were limited to human only (not animal studies) where appropriate. The search was conducted in late July 2018. An updated search was performed on 19 November 2018.
The full search strategies for each database are reported in Appendix 1. The search results were exported to EndNote X7 [Clarivate Analytics (formerly Thomson Reuters), Philadelphia, PA, USA] and deduplicated using automatic and manual checking.
Items included after full-text screening were forwards and backwards citation chased using Scopus (Elsevier, Amsterdam, the Netherlands) to identify additional relevant studies. The reference lists of potentially relevant systematic reviews were checked for additional relevant studies. The references lists that were submitted by industry were also checked to identify additional relevant studies.
Inclusion and exclusion criteria
The inclusion criteria for the clinical effectiveness review were as follows.
Population
The eligible population was people with RA who were receiving treatment with a TNF-α inhibitor (ADL, ETN, IFX, CTZ and GLM) and had:
-
achieved treatment target (remission or LDA) or
-
experienced a primary non-response or
-
experienced a secondary non-response.
Interventions
The ELISA kits or diagnostic services used to monitor response to TNF-α inhibitor treatments for people with RA were eligible for inclusion. These tests run on an ELISA technology platform and are used to measure drug levels (ADL, ETN, IFX, CTZ, and GLM) or their anti-drug antibodies (anti-ETN, anti-IFX, anti-ADL, anti-CTZ and anti-GLM). A serum sample is needed to perform an ELISA.
Eligible ELISAs can be run with or without automation platforms and may be used with any ELISA platform or the Triturus and SQII platforms. Each test needs to be run only once, potentially allowing for high throughput. The test should be intended for monitoring purposes to inform treatment decisions for biological therapies in people with RA.
The ELISA kits or diagnostic services shown below were included:
-
Promonitor ELISA kits –
-
Promonitor-ADL-1DV
-
Promonitor-ANTI-ADL-1DV
-
Promonitor-ETN-1DV
-
Promonitor-ANTI-ETN-1DV
-
Promonitor-GLM-1DV
-
Promonitor-ANTI-GLM
-
Promonitor- IFX-1DV
-
Promonitor-ANTI-IFX-1DV.
-
-
IDKmonitor ELISA kits –
-
IDKmonitor adalimumab drug level
-
IDKmonitor adalimumab free ADA
-
IDKmonitor adalimumab total ADA
-
IDKmonitor etanercept drug level
-
IDKmonitor etanercept free ADA
-
IDKmonitor golimumab
-
IDKmonitor golimumab free ADA
-
IDKmonitor infliximab drug level
-
IDKmonitor infliximab free ADA
-
IDKmonitor infliximab total ADA.
-
-
LISA-TRACKER ELISA kits –
-
LISA-TRACKER adalimumab (LTA002)
-
LISA-TRACKER anti-adalimumab (LTA003)
-
LISA-TRACKER Duo adalimumab (LTA005)
-
LISA-TRACKER certolizumab (LTC002)
-
LISA-TRACKER anti-certolizumab (LTC003)
-
LISA-TRACKER Duo certolizumab (LTC005)
-
LISA-TRACKER etanercept (LTE002)
-
LISA-TRACKER anti-etanercept (LTE003)
-
LISA-TRACKER Duo Etanercept (LTE005)
-
LISA-TRACKER golimumab (LTG002)
-
LISA-TRACKER anti-golimumab (LTG003)
-
LISA-TRACKER Duo golimumab (LTG005)
-
LISA-TRACKER infliximab (LTI002)
-
LISA-TRACKER anti-infliximab (LTI003)
-
LISA-TRACKER Duo infliximab (LTI005).
-
-
RIDASCREEN ELISA kits –
-
RIDASCREEN ADM monitoring (G09043)
-
RIDASCREEN anti-ADM antibodies (G09044)
-
RIDASCREEN IFX monitoring (G09041)
-
RIDASCREEN anti-IFX antibodies (G09042).
-
-
MabTrack ELISA kits –
-
MabTrack level adalimumab M2910
-
MabTrack ADA adalimumab M2950
-
MabTrack level infliximab M2920
-
MabTrack ADA infliximab M2960.
-
-
Sanquin Diagnostic Services (testing service using validated ELISAs) –
-
ADL drug levels
-
CTZ drug levels
-
ETN drug levels
-
ETN anti-drug antibodies
-
GLM drug levels
-
IFX drug levels.
-
The use of both free and total anti-drug antibody assays for these tests was assessed, depending on the availability of assessment data relating to both assays. The intervention tests were used in addition to current clinical practice (clinical assessment and monitoring using a composite score, such as the DAS28).
Comparator
The comparator was standard of care (SOC) for people with RA, in which treatment decisions are based on clinical judgements and monitoring using a composite score, such as the DAS28, without the knowledge of circulating drug levels and anti-drug antibodies by ELISA.
Outcomes
There was no restriction on when the outcomes were measured. The following outcomes were included:
-
Test (procedural) outcomes –
-
number of inconclusive test results
-
time to test result.
-
-
Treatment and management outcomes –
-
number, direction and magnitude of dose changes
-
frequency of dose adjustment (e.g. dose reduction) due to monitoring response
-
frequency of treatment switch to an alternative biologic
-
discontinuation of ineffective therapy.
-
-
Clinical outcomes –
-
change in disease activity
-
rates of disease response, relapse and remission
-
duration of response, relapse and remission
-
rates of hospitalisation
-
rates of surgical intervention
-
AEs of treatment, such as infections.
-
-
Patient-related outcomes –
-
HRQoL.
-
The primary clinical outcomes were clinical and patient-related end points, including reduction in disease activity and improvement in HRQoL. The clinically important intermediate outcomes were the change in number, direction and magnitude of TNF-α inhibitor dose; the change in frequency of dose adjustment due to monitoring response; the change in frequency of treatment switch to an alternative biologic; and the discontinuation of ineffective therapy.
Study design
Both randomised controlled trials (RCTs) and non-randomised controlled studies were included, provided that they compared therapeutic drug monitoring (TDM) using ELISA tests with SOC. Observational studies (e.g. prospective cohort studies, retrospective cohort studies and studies with a historical control) that evaluated the clinical effectiveness of the intervention tests to monitor treatment response in people with RA were included, provided that they reported any of the clinical outcomes relevant for this assessment.
Exclusions
The following types of report were excluded: editorials and opinions, case reports and reports focusing on technical aspects of the technologies only (such as technical descriptions of the testing process). Non-English studies were excluded. Studies with a sample size of ≤ 20 participants were excluded owing to inadequate statistical power. For studies that included people with RA, ankylosing spondylitis (AS) and psoriatic arthritis (PsA) to be eligible, at least 70% of the study population had to be people with RA, provided other eligibility criteria were met. In the case of studies in which < 70% of participants were people with RA, we discussed relevance with clinical experts, and we contacted the study authors to try and to obtain subgroup data for people with RA. However, these 70% criteria were subsequently relaxed to avoid a paucity of evidence. If there were multiple reports for a given study or the possibility of overlapping populations could not be excluded, the most recent or most complete report was selected.
Study selection strategy
Two reviewers independently screened the titles and abstracts (if available) of all the reports identified by the search strategy. Full-text copies of all studies deemed to be potentially relevant were obtained and two reviewers independently assessed them for inclusion. Any disagreements were resolved by consensus.
Data extraction strategy
A data extraction form was developed and piloted. One reviewer independently extracted details of the study design, participants, interventions and outcome data. The data extraction was checked by another reviewer. Disagreements were resolved by consensus.
For studies reporting clinical event outcomes, data were extracted on these as numbers of people experiencing the specified outcome. For studies reporting continuous outcomes, data were extracted as mean and standard deviation (SD). Where reported, mean differences, relative risks, odds ratios or incidence rate ratios (with 95% CIs) were extracted. Where available, results adjusted for potential confounding factors (e.g. age, gender and disease duration of RA) were extracted preferentially.
For studies in which only a subgroup of people were eligible for inclusion in the review, data were extracted and presented for this subgroup only. If some data were unclear or missing, attempts were made to contact the study authors to obtain additional data.
Critical appraisal strategy
One reviewer independently assessed the quality of included studies in terms of risk of bias. If RCTs had been identified, the Cochrane Risk of Bias tool for RCTs would have been used. 40 The Cochrane ROBINS-1 (Risk Of Bias In Non-randomised Studies - of Interventions) tool was used for non-randomised studies with adaptations as appropriate. 41 The Cochrane (ROBINS-1) tool was used to assess the quality of uncontrolled observational studies with adaptations as appropriate, although the tool was primarily designed for non-randomised controlled studies. The risk of bias of included studies was taken into account when interpreting results. The quality assessment was checked by another reviewer. Disagreements were resolved by consensus.
Methods of data synthesis
Given the clinical heterogeneity associated with the interventions, outcomes and length of follow-up, and the methodological heterogeneity identified (e.g. different study designs), quantitative synthesis was not possible and clinical effectiveness data were synthesised in a narrative fashion. Publication bias could not be investigated because quantitative synthesis was not possible.
Clinical effectiveness results
The next section provides information on the quantity of research available, including the characteristics and risk of bias of the included studies. This is then followed by the results section, in which we report the clinical effectiveness of TDM in people with RA who were treated with TNF-α inhibitors.
Quantity and quality of research available
The literature searches of bibliographic databases identified 7443 references. After initial screening of titles and abstracts, 390 were considered to be potentially relevant and were ordered for full-paper screening. In total, two studies reported in four articles42–45 were included in the systematic review of clinical effectiveness: INGEBIO (reported in three abstracts)42,43,45 and Pascual-Salcedo et al. 44 Both included studies with linked citations are presented in Appendix 2. Figure 1 shows a flow diagram outlining the screening process with reasons for exclusion of full-text papers.
FIGURE 1.
Flow diagram of the study inclusion process for the clinical effectiveness review.
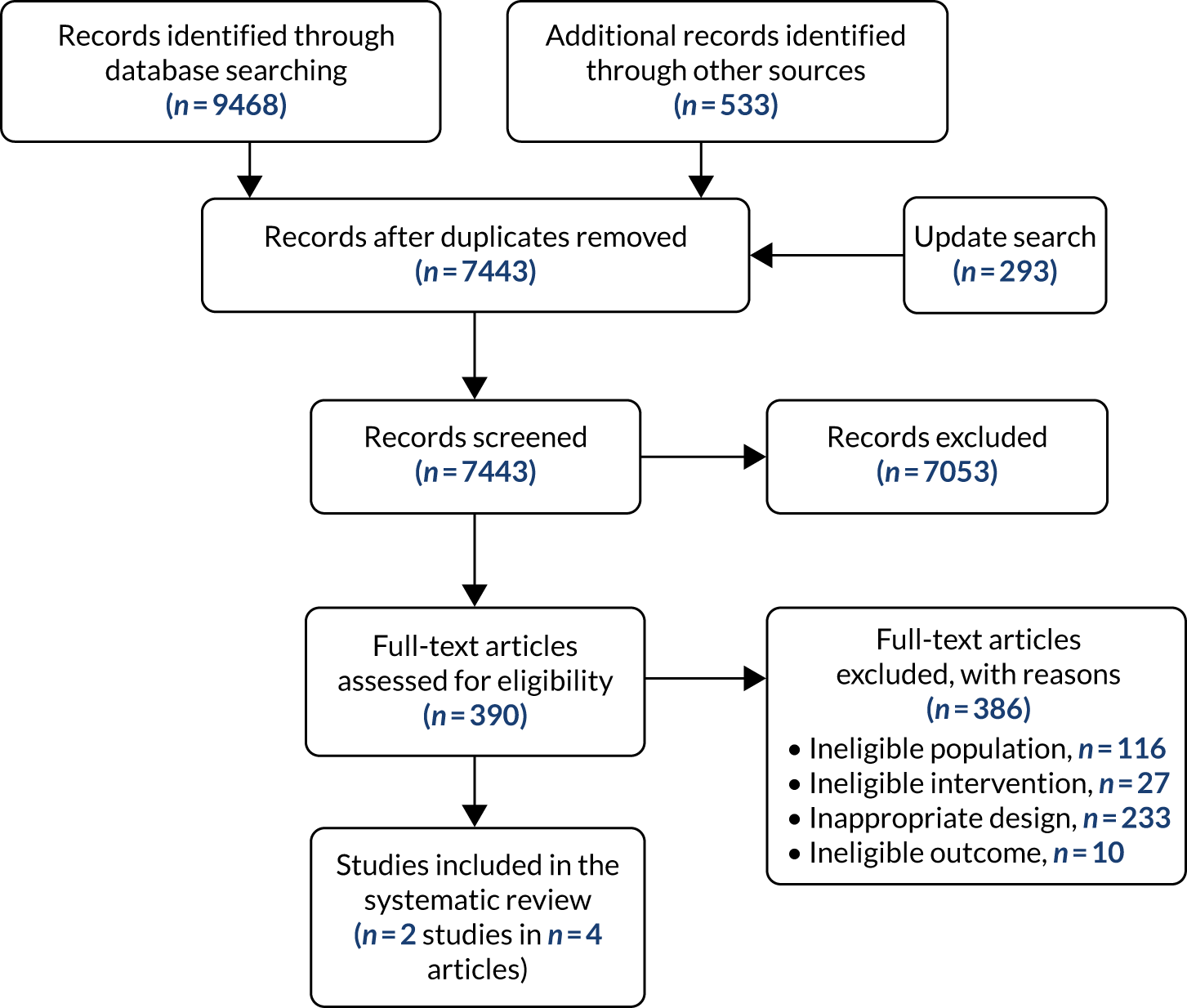
One study was reported in three abstracts, with considerable overlap in data and reporting. The paper with the most up-to-date and complete data was selected for data extraction.
Number and type of studies excluded
A list of full-text papers that were excluded along with the reasons for their exclusions is given in Appendix 2. These papers were excluded because they failed to meet one or more of the inclusion criteria in terms of the type of study design, participants, interventions or outcomes being reported.
Assessment of clinical effectiveness
Characteristics of included studies
The characteristics of the included studies are presented in Tables 6 and 7. 42–45 All studies recruited people with RA who had achieved treatment target (remission or LDA). One study, that was reported in three abstracts,42,43,45 used Promonitor ELISA kits for monitoring drug levels and/or anti-drug antibody levels. One study44 used Sanquin ELISA kits to measure drug levels and/or anti-drug antibody levels of three TNF-α inhibitors (IFX, ADL and ETN) for the treatment of RA. The type of Sanquin test kit used in this study was not reported. The two included studies were conducted in Spain. Neither study reported funding sources.
| Studya | Characteristic | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Location | Population | Sample size | Median disease duration (months) | Description of tests | Description of intervention | Description of control | Length of follow-up (months) | Number of visits | |
| Ucar et al.42 | Spain | Remission/LDA for at least 6 months | 169b | 117 | ADL/anti-ADL antibody serum levels using Promonitor-ADL and Promonitor-ANTI-ADL | 40 mg of subcutaneous ADL; TDM data released to physician | 40 mg of subcutaneous ADL; TDM data not released to physician | 18 | 8 |
| Gorostiza et al.45 | Spain | Remission/LDA for at least 6 months | 169c | 117 | ADL/anti-ADL antibody serum levels using Promonitor-ADL and Promonitor-ANTI-ADL | 40 mg of subcutaneous ADL; TDM data released to physician | 40 mg of subcutaneous ADL; physician blinded to TDM data | 34 weeks | 8 |
| Arango et al.43 | Spain | Remission/LDA | 169d | 124 | ADL/anti-ADL antibody serum levels using Promonitor-ADL and Promonitor-ANTI-ADL | 40 mg of subcutaneous ADL; TDM data released to physician | 40 mg of subcutaneous ADL; TDM data not released to physician | 18 | 8 |
| Study | Characteristic | |||||||
|---|---|---|---|---|---|---|---|---|
| Study date | Location | Study design | Population | Description of tests | Frequency of measuring | Sample size | Length of follow-up | |
| Pascual-Salcedo et al.44 | 2006–12 | Spain | Historically controlled study | Remission/LDA | Drugs: IFX, ADL, ETN; capture ELISA (Sanquin, Amsterdam) | NR | 43 | 7 years |
Non-randomised controlled studies
Three abstracts42,43,45 were identified reporting the same non-randomised controlled study (the INGEBIO study). In this trial, the results of drug and anti-drug antibodies tests were revealed to physicians in the intervention arm but not to those in the control arm. This reflected standard care in Spain, where treatment decisions are based on clinical judgements without knowledge of levels of drugs and anti-drug antibodies. Given that this was a pragmatic trial, it is likely that the findings may be generalisable to routine practice settings. For standard care in the control arm, clinicians did not follow any national guideline for the management of people with RA, as no national guidelines for monitoring in Spain were available at the time of the study. Clinicians used their best judgements to optimise treatment doses. This trial recruited a mixed population of 169 people with RA (n = 63), PsA (n = 54) or AS (n = 52) recruited from three sites in Spain. The study focused on the population who had achieved treatment target (remission or LDA) and remained clinically stable for at least 6 months.
The included abstracts reported a sample size of people with RA, ranging from 54 to 63 at baseline. The abstracts by Ucar et al. 42 and Arango et al. 43 reported results on the basis of the 18-month follow-up. The abstract by Gorostiza et al. 45 reported results based only on the 34-week follow-up. This trial reported the following relevant clinical outcomes: change in disease response, dose adjustment due to monitoring response (e.g. proportion of participants tapered) and participants’ HRQoL outcomes.
The median duration of disease at baseline among participants in the three abstracts42,43,45 ranged from 117 months to 124 months. All participants were treated with 40 mg of ADL (via subcutaneous injection). ADL and anti-ADL antibody levels were measured using Promonitor-ADL and Promonitor-ANTI-ADL. The frequency of testing in this trial was once every 2–3 months, with a total of eight visits during the trial period (details were not provided).
Observational study
One observational study reported by Pascual-Salcedo et al. 44 assessed the clinical effectiveness of using ELISA for monitoring response to TNF-α inhibitors in people with RA. The study recruited people who had achieved treatment target (remission or LDA) and had a historical control. The observational study measured levels of drug and/or anti-drug antibody in participants who were treated with ADL, ETN and IFX. This observational study reported the following relevant clinical outcomes: change in disease activity, and change in direction and magnitude of therapeutic dose.
In this study Sanquin ELISA kits were used to measure levels of three TNF-α inhibitors (IFX, ETN and ADL). The sample size was 43. The study measured the TNF-α inhibitor drug levels only. It was unclear whether or not drug trough levels were assessed in the study.
The included studies did not report other outcomes, such as the number of inconclusive results, time to result, frequency of treatment switch to an alternative biologic, rates of hospitalisation and rates of surgical interventions.
No studies in which participants were treated with CTZ and GLM were identified. No studies that reported on the use of ELISA testing in people with RA receiving biosimilar products were identified. No relevant studies (including both controlled trials and observational studies) that assessed other eligible ELISA kits, including IDKmonitor, LISA-TRACKER, RIDASCREEN and MabTrack, were identified.
Baseline characteristics of included studies
Baseline characteristics of included studies are presented in Tables 8 and 9. The mean age of participants enrolled in the INGEBIO study (according to Ucar et al. 42) was 53.59 years, but the mean age of participants in the observational study was not reported. The mean duration of RA was 17 years in the observational study.
| Study | Mean age (years) | Definition of remission | Definition of LDA | Proportion male (%) | Proportion with remission at baseline (%) | Proportion with LDA at baseline (%) | Total, n | Median disease duration (months) | Mean time on biologic (years) | Co-therapies | TNF-α inhibitor received | Dose manipulation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ucar et al.42 | 53.59a | NR | NR | NR | 70.0 (IG, 73.4; CG, 83.3)b | 30.0 (IG, 26.6; CG, 16.7)b | 169 | 117.0 | NR | Methotrexate | 40 mg of subcutaneous ADL | Dose tapering; physicians alter dose based on their judgement |
| Gorostiza et al.45 | NR | NR | NR | NR | 70.0 (IG, 73.4; CG, 83.3)b | 30.0 (IG, 26.6; CG, 16.7)b | 169 | 117.0 | NR | NR | 40 mg of subcutaneous ADL | Dose tapering; physicians alter dose based on their judgement |
| Arango et al.43 | NR | NR | NR | NR | 67.3 (IG, 71.4; CG, 82.7)b | 32.7 (IG, 28.6; CG, 17.3)b | 169 | 124.0 | NR | NR | 40 mg of subcutaneous ADL | Dose tapering; physicians alter dose based on their judgement |
| Study | Mean age (years) | Sample size, n | Definition of remission (DAS28) | Definition of LDA (DAS28) | Definition of flare (DAS28) | Proportion male (%) | Mean disease duration (years) | Mean time on biologic (years) | Co-therapies | TNF-α inhibitor received | Dose manipulation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pascual-Salcedo et al.44 | NR | 43 | < 3.2a | < 3.2a | NR | NR | 17.52 (SD 9.38) | 5.85 (SD 1.33) | NR | ADL, ETN and IFX (doses NR) | Optimisation strategy (adjusting drug dose according to clinical activity) |
The definition of remission/LDA was described as a DAS28 of < 3.2 in the observational study, but was not reported in the non-randomised controlled study (see Tables 8 and 9). Both studies included in the systematic review used one or more TNF-α inhibitors (ADL, IFX or ETN) for the treatment of RA. The mean treatment duration for participants receiving TNF-α inhibitors was 6 years in the observational study but was not reported in the non-RCT.
Only methotrexate was reported as a co-therapy in the non-RCT, whereas no co-therapies were reported in the observational study.
Ongoing study
We identified one ongoing RCT that met the inclusion criteria for this systematic review of clinical effectiveness: the Norwegian Drug Monitoring (NOR-DRUM) study. 46 Study characteristics are summarised in Appendix 3. Enrolment in the NOR-DRUM study commenced in March 2017, with an expected primary completion date of March 2020 and study completion date of March 2022.
The aim of this trial is to assess the clinical effectiveness of TDM in participants who are starting IFX and in participants who are on maintenance IFX therapy. The type of ELISA testing is not reported. The target recruitment for this study is 600 people with RA or other immunological inflammatory diseases.
The intervention of this trial will be TDM with a treatment algorithm based on measurement of serum levels of drug and anti-drug antibodies. The control group is standard care, in which clinicians will make treatment decisions without the knowledge of drug levels or status of anti-drug antibodies.
The major primary outcomes are the proportion of participants in remission and the proportion of participants experiencing sustained disease control without disease worsening. Secondary outcomes of interest include time to sustained remission, occurrence of drug discontinuation, health utility [EuroQol-5 Dimensions (EQ-5D)], HRQoL [Short Form Questionnaire-36 items (SF-36)], time to disease worsening and clinical efficacy outcomes assessed by composite disease activity scores.
Risk of bias of the included studies
The risk of bias of the included studies was assessed using the Cochrane (ROBINS-1) tool for non-randomised studies. The Cochrane (ROBINS-1) tool was also used to assess the quality of the observational study with adaptations as appropriate, although the tool was primarily designed for non-randomised controlled studies. The following domains relating to risk of bias were assessed for each individual study: confounding, selection, group classification, co-interventions, missing data, outcome measurement and selective outcome reporting. The quality assessments on the basis of all relevant domains for each study and of specific outcomes are presented in Appendix 4. Tables 10 and 11 present the quality assessment of the included studies.
| Study | Confounding (differential prognosis between groups) | Selection | Group classification | Co-intervention | Missing data | Outcome measurement | Selective outcome reporting | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Arango et al.43 | Serious | Low | Low | NI | Serious | Moderate | Low | Serious |
| Gorostiza et al.45 | Serious | Low | Low | NI | Serious | Moderate | Low | Serious |
| Ucar et al.42 | Serious | Low | Low | NI | NI | Moderate | Low | Serious |
| Study | Confounding | Selection | Group classification | Co-intervention | Missing data | Outcome measurement | Selective outcome reporting | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|
| Pascual-Salcedo et al.44 | Moderate | Moderate | Moderate | NI | NI | Moderate | Low | Moderate |
Table 10 details the quality assessment of the non-randomised controlled study (the INGEBIO study). 42,43,45 This non-randomised controlled study was judged to be at serious risk of bias. There was an issue of baseline imbalance in the proportions of participants in remission or with LDA between the intervention group and the control group: at baseline 73.4% of participants were in remission in the intervention group, compared with 83.3% of participants in the control group. The remaining participants (i.e. 26.6% of participants in the intervention group and 16.7% of participants in the control group) had achieved LDA at baseline. Furthermore, there was a lack of adjustment for baseline imbalance in this variable in the analysis of clinical outcomes. These deficiencies resulted in a serious risk of bias associated with the findings.
Table 12 presents the attrition rates for each outcome of the non-randomised controlled study (the INGEBIO study). 42,43,45 As seen in Table 12, attrition rates for three outcomes (proportion of participants who remained in remission, proportion of participants who changed from LDA to remission and proportion of participants who received dose tapering) were high, ranging from 10.3% to 30.8%, which can lead to attrition bias. Furthermore, attrition rates for these outcomes were unbalanced between the intervention group and the control group.
| Outcome | Baseline population (n) | Follow-up population (n) | Per cent attrition | ||||
|---|---|---|---|---|---|---|---|
| IG | CG | IG | CG | IG | CG | Overall | |
| Disease flare | 109 | 60 | Unclear | Unclear | Indeterminate | Indeterminate | Indeterminate |
| Per cent remaining in remission | 109 | 60 | 71 | 46 | 34.9 [(109–71)/109] | 23.3 [(60–46)/60] | 30.8 [(169–117)/169] |
| Per cent change from LDA to remission | 29 | 10 | 28 | 7 | 3.5 [(29–28)/29] | 30.0 [(10–7)/10] | 10.3 [(39–35)/39] |
| ADL tapering | 109 | 60 | 98 | 52 | 10.1 [(109–98)/109] | 13.3 [(60–52)/60] | 11.2 [(169–150)/169] |
| HRQoL | 109 | 60 | Unclear | Unclear | Indeterminate | Indeterminate | Indeterminate |
Table 11 presents the quality assessment of the observational study. 44 The study had a historical control group and was judged to be at moderate risk of bias because there was non-contemporaneous control bias as a result of the use of historical control. It should be noted that the same group of participants were assessed during the first period (i.e. the historical control, before TDM was introduced) and the second period (after TDM was introduced). Attrition rates are shown in Table 13.
| Outcomes | Baseline population (n) | Follow-up population (n) | Per cent attrition |
|---|---|---|---|
| Mean DAS scores | 43 | NI | Indeterminate |
| Weekly mean drug dose | 43 | NI | Indeterminate |
| Mean interval of drug administration | 43 | NI | Indeterminate |
Overall, the non-randomised controlled study42,43,45 was judged to be at serious risk of bias whereas the observational study was judged to be at moderate risk of bias.
Results of clinical effectiveness
Non-randomised controlled trial
Three included abstracts42,43,45 reported the same non-randomised controlled trial (the INGEBIO study). This trial recruited participants who had achieved treatment target (remission or LDA) and had remained clinically stable for at least 6 months.
This trial recruited a mixed population of 169 participants, including 63 people with RA. The results of the total mixed population were reported in the review, as the authors were not able to provide the results for the cohort of 63 people with RA (the study was not powered to detect a meaningful difference between the intervention and the control group for the cohort of people with RA only). The three cohorts of participants who had different conditions (RA, PsA and AS) may have different treatment responses to TNF-α inhibitor therapies. Therefore, there was limited generalisability of the findings from this mixed population to the RA population. At baseline, the median trough level of ADL was 5.3 mg/l in the intervention group and 5.5 mg/l in the control group. The included abstracts were judged to be at serious risk of bias. Tables 14 and 15 present the results of this non-randomised controlled study.
| Study | Population | Intervention group (n) | Control group (n) | Length of follow-up (months) | Outcome measure | Relative measurea |
|---|---|---|---|---|---|---|
| Ucar et al.42 | Remission/LDA | 109 | 60 | 18 | Number experiencing a disease flare (n) | IG = 69, CG = 53 |
| IRR (disease flare) (95% CI) | 0.7252 (95% CI 0.4997 to 1.0578)b | |||||
| Rate of flare | CG: 0.639 flares per patient-year; IG: 0.463 flares per patient-year | |||||
| Rate difference (disease flare) (95% CI) | –0.176 (95% CI –0.379 to 0.0289)b | |||||
| Gorostiza et al.45 | Remission/LDA | 109 | 60 | 18c (reported 34-week follow-up data) | Percentage that remained in remissiond | CG: 69.6% (32/46); IG: 76.1% (54/71) |
| Change from LDA to remissionb,e | CG: 28.6% (2/7); IG: 35.7% (10/28) | |||||
| Arango et al.43 | Remission/LDA | 98 | 52 | 18 | Proportion taperedb (%) | CG: 34.6% (18/52); IG: 35.7% (35/98) |
| Rate of flare | CG: 0.639 flares per patient-year; IG: 0.463 flares per patient-year | |||||
| Rate differenceb (95% CI) | –0.176 (95% CI –0.379 to 0.0289) | |||||
| IRRf (95% CI) | 0.7252 (95% CI 0.4997 to 1.0578) | |||||
| Median time to first flare | CG: 136.5 days; IG: 145 days |
| Study | Population | IG (n) | CG (n) | Length of follow-up (months) | Outcome measure | Relative measurea | p-value (IG vs. CG) at visit 2 | p-value (IG vs. CG) at visit 3 |
|---|---|---|---|---|---|---|---|---|
| Ucar et al.42 | Remission/LDA | 109 | 60 | 18 | Health-related quality of life (EQ-5D-5L) | Higher in IG throughout follow-upb | 0.001 | 0.035 |
| Arango et al.43 | Remission/LDA | 98 | 52 | 18 | Health-related quality of life (EQ-5D-5L) | Higher in IG throughout follow-upb | 0.001 | 0.035 |
Change in disease response
The abstract by Ucar et al. 42 reported that, at the18-month follow-up, the number of participants who had experienced a disease flare in the intervention and control groups was 69 and 53, respectively. In this study, a disease flare was defined as an increase in DAS28 of > 1.2 or > 0.6 if the DAS28 was ≥ 3.2 following the criteria validated in the study by van der Maas et al. 47 As seen in Table 14, the rate of flares per patient-year was 0.463 in the intervention group and 0.639 in the control group, with a rate difference of –0.176 (95% CI –0.379 to 0.0289). 42,43 There was a non-significant reduction in the risk of flare in the intervention group compared with the control group [incidence rate ratio (IRR) 0.7252, 95% CI 0.4997 to 1.0578]. The median time to the first flare was 145 days in the intervention group and 136.5 days in the control group.
The number of participants who remained in remission at the 18-month follow-up was not reported by Ucar et al. 42 However, the abstract by Gorostiza et al. 45 reported that, at the 34-week follow-up, 76.1% (54/71) of participants in the intervention group remained in remission, compared with 69.6% (32/46) in the control group. This analysis did not use an intention-to-treat (ITT) approach. The ITT analysis showed that 67.5% (54/80) of participants in the intervention group and 64.0% (32/50) in the control group remained in remission, with a difference in proportion of 3.5% (95% CI –13.3% to 20.3%; p = 0.68).
This abstract45 further reported that, among participants with LDA at baseline, 35.7% (10/28) in the intervention group and 28.6% (2/7) in the control group were in remission at the 34-week follow-up. Again, this analysis did not use an ITT approach. The ITT analysis showed that, among those participants with LDA at baseline, 34.5% (10/29) in the intervention group and 20% (2/10) in the control group were in remission at the 34-week follow-up.
Dose adjustment due to monitoring response
The abstract by Arango et al. 43 reported that ADL dose was tapered in 35 participants in the intervention group (35.7%) and in 18 participants in the control group (34.6%). The results appeared to be generally similar between the intervention and control groups.
Health-related quality of life
Table 15 presents the results of the HRQoL outcomes. Both Ucar et al. 42 and Arango et al. 43 reported the outcomes of participants’ HRQoL [EuroQol-5 Dimensions five-level version (EQ-5D-5L)]. The results showed that participants’ HRQoL (EQ-5D-5L) outcome measures were higher in the intervention group than in the control group at all visits (further details were not reported). However, statistically significant results were observed only at visit 2 (p = 0.001) and visit 3 (p = 0.035).
In summary, this non-randomised controlled trial (the INGEBIO study) found a non-significant reduction in risk of flare in the intervention group compared with the control group. Participants’ HRQoL measures were higher in the intervention group than in the control group at all visits, with statistically significant results being observed at two visits. However, given that this trial was judged to be at serious risk of bias, it may have compromised the reliability of the findings.
Observational study
The observational study by Pascual-Salcedo et al. 44 evaluated the effect of using ELISA for monitoring response to TNF-α inhibitors in people with RA. The study included participants who had achieved treatment target (remission or LDA). The study had a historical control and was judged to be at moderate risk of bias.
Change in disease activity
The observational study44 evaluated the effect of TDM, based on serum trough drug levels, in RA and SpA patients during the follow-up of 7 years. The study did not report relevant information on the duration of remission/LDA. 44 The sample size was 43 participants. Table 16 presents the results of changes in disease activity.
| Study | Study design | Populations (e.g. remission) | Sample size (n) | Missing data (at follow-up) | Length of follow-up | Outcome measure | Findings (mean DAS28) |
|---|---|---|---|---|---|---|---|
| Pascual-Salcedo et al.44 | Historically controlled study | Remission/LDA | 43 | NR | 7 years | Mean DAS28 |
First period: 2.51 (SD 0.85) Second period:a 2.31 (SD 0.52); p = 0.061 |
The study by Pascual-Salcedo et al. 44 had a historical control (i.e. the first period, before TDM was introduced). Participants had mean DAS28 of 2.51 (SD 0.85) during the historical control period. When compared with the historical control period, there was a non-significant reduction in the mean DAS28 (2.31, SD 0.52) at the 7-year follow-up during the second period, after the introduction of TDM (p = 0.061).
Overall, this study44 found that TDM was associated with a non-significant reduction in mean DAS28 at the 7-year follow-up compared with the historical control. It should be noted that the study data were judged to be at moderate risk of bias, which compromises the reliability of the findings.
Change in direction and magnitude of therapeutic dose
Table 17 presents the results of a change in the direction and magnitude of the therapeutic dose. It should be noted that the results from the study by Pascual-Salcedo et al. 44 on the change in therapeutic dose were presented for the mixed population (including 43 people with RA and 45 people with PsA). Therefore, there was limited generalisability of findings from this mixed population to the target RA population.
| Study | Study design | Population (e.g. remission) | Sample size (n) | Missing data (at follow-up) | Length of follow-up | Outcome measure | Findings |
|---|---|---|---|---|---|---|---|
| Pascual-Salcedo et al.44 | Historically controlled study | Remission/LDA | 43 | NR | 7 years | Weekly mean dose per person by drug (first vs. second period)a |
|
| Mean interval of administration by drug (weeks) (first vs. second period)a |
|
Pascual-Salcedo et al. 44 reported that, compared with the historical control period (i.e. the first period, during which TDM was not used), there were statistically significant reductions in the weekly mean dose per participant of each drug during the second period, following the introduction of TDM, as follows:
-
IFX, from 0.51 (SD 0.14) mg/kg/week to 0.42 (SD 0.12) mg/kg/week (p < 0.001).
-
ADL, from 19.19 (SD 3.72) mg/week to 15.52 (SD 4.8) mg/week (p < 0.001).
-
ETN, from 42.09 (SD 13.25) mg/week to 35.04 (SD 13.37) mg/week (p = 0.009).
The findings44 further showed that, compared with the historical control period, there was a statistically significant increase in the mean interval between administrations of each drug during the second period, as follows:
-
IFX, from 8.52 (SD 1.43) weeks to 9.7 (SD 1.44) weeks (p < 0.001).
-
ADL, from 2.19 (SD 0.58) weeks to 2.95 (SD 1.58) weeks (p = 0.07).
-
ETN, from 1.09 (SD 0.27) weeks to 1.61 (SD 0.91) weeks (p = 0.004).
Overall, the limited data from the observational study showed that TDM for optimisation of TNF-α inhibitor therapy was associated with reductions in therapeutic dose of TNF-α inhibitors in people with RA who had achieved remission or LDA. This would be expected to lead to a cost saving that was associated with TDM. However, the reliability of findings may be compromised by the poor quality of the data.
Discussion
This systematic review has identified two studies (reported in four publications)42–45 that evaluated the effect of TDM on clinical outcomes in people with RA who had achieved remission or LDA. Three articles42,43,45 reported the same non-randomised controlled trial (the INGEBIO study). The remaining study was an observational study that evaluated the impact of TDM.
Both studies recruited people with RA who had achieved remission or LDA. The non-randomised controlled trial (INGEBIO)42,43,45 used Promonitor ELISA kits to monitor levels of drugs and/or anti-drug antibody levels. The observational study44 used Sanquin ELISA kits to measure drug levels and/or anti-drug antibody levels. It was unclear whether or not these tests were performed at the centralised testing service. The included study measured levels of drug and/or anti-drug antibody in participants who were being treated with ADL, ETN and/or IFX. No studies in participants treated with CTZ or GLM were identified. No studies evaluating eligible ELISA kits, including IDKmonitor, LISA-TRACKER, RIDASCREEN and MabTrack, were identified.
Comparative controlled evidence
Three abstracts42,43,45 reporting the same non-randomised controlled trial were identified. The INGEBIO study focused on the population with RA who had achieved treatment target (remission or LDA). In this trial, levels of ADL and anti-ADL antibody were measured using Promonitor-ADL and Promonitor-ANTI-ADL. This trial recruited a mixed population of 169 participants, including a cohort of 63 people with RA. The results of the total mixed population were reported in the review, as the authors were not able to provide the results for the subgroup of people with RA.
This non-randomised controlled trial (the INGEBIO study) found a non-significant reduction in the risk of flare in the intervention group compared with the control group. In particular, participants’ HRQoL outcomes were higher in the intervention group than in the control group at all visits, with statistically significant results being observed at two visits. However, as the trial was judged to be at serious risk of bias, the results should be interpreted with caution. Ideally, randomisation of participants is required to minimise the risk of bias of study findings.
Evidence from observational studies
We identified one observational study that evaluated the effect of TDM on clinical outcomes in people with RA who had achieved remission or LDA. The study44 had a historical control.
Change in disease activity
The observational study reported by Pascual-Salcedo et al. 44 evaluated the effect of TDM on the change in disease activities after 2–7 years’ follow-up, with a sample size of 43 participants. The study focused on people who had achieved remission or LDA. Overall, the finding from the historically controlled study44 showed that TDM was associated with a non-significant reduction in mean DAS28 at 7-year follow-up compared with the historical control (before TDM was introduced). It should be noted that the data were judged to be at moderate risk of bias, which compromised the reliability of the findings.
Change in direction and magnitude of therapeutic dose
The observational study44 evaluated the outcomes of changes in direction and magnitude of therapeutic dose in people with RA who had achieved remission or LDA. The sample size was 43.
Overall, the limited data from the observational study showed that the use of TDM to optimise TNF-α inhibitor therapies was associated with reductions in the therapeutic dose of TNF-α inhibitors in people with RA who had achieved remission or LDA. This would be expected to lead to cost savings associated with TDM. Statistically significant results may also be clinically significant. However, the reliability of the findings may be compromised by the poor quality of the data.
Reliability of the findings
The non-randomised controlled study42,43,45 was judged to be at serious risk of bias. In this trial, there was an issue of baseline imbalance in disease severity between the intervention and the control groups. Furthermore, this imbalance was not adjusted for in the analysis of clinical outcomes. Attrition rates were higher for some outcomes, which can lead to attrition bias. These deficiencies resulted in the findings being at serious risk of bias. Therefore, the results should be interpreted with caution.
The historically controlled observational study44 was judged to be at moderate risk of bias because the control was non-contemporaneous, although it should be noted that the same group of participants were assessed during the first period (the historical control, before TDM was introduced) and the second period (after TDM was implemented). However, the sample size was small. Therefore, the overall poor quality of included studies compromises the reliability of the findings.
Generalisability of the findings
Given that both studies were conducted in Spain, the findings from these studies may have limited generalisability to the UK setting owing to variations in clinical practice and health policies between the two countries. Furthermore, the findings from the non-randomised controlled trial (the INGEBIO study42,43,45) and the results of changes in the therapeutic dose from the study by Pascual-Salcedo et al. 44 were presented for a mixed population. Therefore, there was limited generalisability of findings from the mixed population (including RA, PsA and/or AS) to the target RA population.
Implications for future research
We identified one ongoing Norwegian multicentre RCT (the NOR-DRUM study)46 evaluating the effect of TDM in people with RA in remission compared with standard care. This ongoing trial will provide further useful data on the impact of TDM in the target population.
Further controlled trials (especially RCTs) are required to assess the impact of using Promonitor ELISA tests for monitoring TNF-α inhibitor therapies in people with RA who have achieved remission or LDA.
No studies that assessed other eligible ELISA kits, including IDKmonitor, LISA-TRACKER, RIDASCREEN and MabTrack, were identified.
Therefore, future large RCTs are required to assess the impact of using ELISA for monitoring TNF-α inhibitor therapies in people with RA who have achieved remission or LDA. More robust evidence is also needed to evaluate the impact of using Sanquin tests to monitor TNF-α inhibitor therapies in this population.
Future RCTs are warranted to evaluate the clinical effectiveness of using ELISA tests to monitor TNF-α inhibitor therapies in people with RA who have experienced a primary non-response or a secondary non-response.
No studies among patients who were being treated with CTZ and GLM were identified. Future RCTs are required to assess the clinical effectiveness of using ELISA to monitor such TNF-α inhibitor therapies in the target populations.
Conclusions
Limited data regarding the clinical effectiveness of TDM in the target populations were identified. One non-randomised trial that compared TDM with standard care (the INGEBIO study) had serious limitations in relation to the NICE scope:48 only one-third of the participants had RA; many of the analyses were not by ITT; follow-up was only 18 months; there was no explicit algorithm to guide clinicians how to change treatment in response to the results of testing (e.g. tapering); and the study was reported in three abstracts only. In addition, we identified one observational study, but this was of limited value in informing whether or not ELISA-based monitoring is clinically effective.
Chapter 3 Systematic review of cost-effectiveness evidence
Objectives
The objectives of the systematic review of economic evaluations were to:
-
gain insights into the key drivers of the cost-effectiveness of TNF-α testing
-
get an overview of the alternative modelling approaches that have been adopted to evaluate the use of TDM in people with RA
-
provide a summary of the findings of previous relevant cost–utility, cost-effectiveness and cost–benefit studies.
Methods
Identification of studies
The following bibliographic databases were searched: MEDLINE (via Ovid), MEDLINE In-Process & Other Non-Indexed Citations (via Ovid), EMBASE (Ovid), Web of Science (via Clarivate Analytics), NHS Economic Evaluation Database (NHS EED) and HTA (the Cochrane Library) and EconLit (EBSCOhost). In addition, searches were carried out on the following websites: Health Utilities Database (HUD) [School of Health and Related Research (ScHARR)] (URL: www.scharrhud.org/; accessed 7 August 2018), Health Economics Research Centre (HERC) (Oxford) (URL: www.herc.ox.ac.uk/publications; accessed 7 August 2018), EQ-5D (EuroQol) (URL: https://euroqol.org/search-for-eq-5d-publications/; accessed 7 August 2018), Cost-effectiveness Analysis Registry (URL: https://cevr.tuftsmedicalcenter.org/databases/cea-registry; accessed 7 August 2018) and International Society for Pharmacoeconomics and Outcomes Research (ISPOR) (URL: www.ispor.org/; accessed 7 August 2018).
The searches were developed and run by an information specialist (SR) in July 2018 and updated in November 2018. They comprised terms for RA, terms for TNF-α inhibitors and terms for ELISA testing. Search filters were used to limit the searches to cost-effectiveness studies. No date or language limits were used.
Separate searches were also carried out for appropriate costs and health utilities using a variety of search terms and filters. These searches were carried out in several iterations to look for different aspects of costs and health utilities for RA and ELISA tests, as needed.
The full search strategies for each database, for cost-effectiveness, and one example iteration of the utility searches are provided in Appendix 1. The database search results were exported to and deduplicated using EndNote X7. Deduplication was also performed by manual checking. Screening was carried out independently by two reviewers. Disagreements between reviewers were resolved by consensus. All of the references that were considered for inclusion by either reviewer at the title and abstract stage were included for full-text screening.
Eligibility criteria
Studies eligible for inclusion in the systematic review were selected according to the inclusion and exclusion criteria outlined in a population, intervention, comparator, outcome (PICO) template. The inclusion criteria for population, interventions and comparator were as described in Chapter 2, Population, Interventions and Comparator. The following types of economic evaluation were included: cost–utility, cost-effectiveness, cost–benefit, cost–consequences and cost-minimisation analyses. Systematic reviews of economic studies were also considered.
Data extraction
Study characteristics and results were extracted and summarised by one reviewer (MRH). The evidence was assessed using narrative synthesis supported by summary data extraction tables.
Results
Figure 7 (see Appendix 5) shows a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the systematic review. 39 After deduplication, 214 records were identified. All records were screened on title and abstract, and 29 citations were screened at full text. In addition to the records identified from searches of electronic sources, a PhD (Doctor of Philosophy) thesis by Gavan17 that met inclusion criteria for the cost-effectiveness systematic review was brought to the attention of the External Assessment Group (EAG).
Five studies (reported in 11 publications) were eligible for inclusion (Table 18): three studies were model-based economic evaluations. Of these, two were reported in abstract format only: one was a non-randomised controlled trial (INGEBIO42,43,45) and one was an observational study. 44 The authors of the abstracts were contacted and provided two poster presentations reporting outcomes of the INGEBIO study. These sources are not included in the PRISMA flow diagram.
| First author(s) | Type of reference | Type of study | Source(s) |
|---|---|---|---|
| Arango, Ucar, Gorotzila | Abstract | Non-randomised controlled trial | Arango et al.,43 Ucar et al.42 and Gorostiza et al.45 |
| Krieckaert | Full text | Model | Krieckaert et al.,51,52 Krieckaert et al.,51,53 and Krieckaert et al.53 |
| Pascual-Salcedo | Abstract | Observational | Pascual-Salcedo et al.44 |
| Laine | Full text | Model | Laine et al.54 |
| Gavan | PhD thesis | Model | Gavan17 |
The characteristics of the included studies are given in Tables 49 and 50 (see Appendix 6).
Non-model-based studies
The two studies, by Ucar et al. 42 (INGEBIO) and Pascual-Salcedo et al. ,44 were reported as abstracts. Ucar et al. 42 investigated the impact of monitoring levels of ADL and anti-ADL antibody in people with RA, PsA and AS on the annual direct costs to the health system and health outcomes compared with conventional practice in Spain. The economic analysis reported in Ucar et al. 42 was based on clinical outcomes from a pragmatic, non-randomised, non-inferiority study. Trough levels of ADL and anti-ADL antibody were measured with Promonitor-ADL and Promonitor-ANTI-ADL. Physicians were not obliged to adhere to any therapeutic algorithm when making treatment decisions for participants in the intervention group. In the control group, treatment decisions were based on clinical judgement only. A total of 169 people were recruited, of whom 63 (37.3%) had RA (30 in the intervention group and 33 in the control group). Ucar et al. 42 reported the result for all participants and did not report results by subgroup (disease categories).Therefore, it is difficult to generalise the results to people with RA. The mean cost of ADL (Humira®) treatment per patient-year and the mean quality-adjusted life-years (QALYs) accrued over the observation period in the intervention and control arms were estimated. The authors reported, compared with the control group, those in the intervention group had better quality of life and lower risk of flares, and incurred lower treatment costs. The average ADL acquisition cost per patient-year was €10,664.54 and €9856.45 in the control and the intervention arms, respectively (–€808.08, 8% savings); the results were reported for the total (mixed) population. Given that the study was available in abstract form only, it was not clear how the mean QALYs were calculated.
The Pascual-Salcedo et al. 44 study aimed to compare the clinical and economic impact of TDM, based on trough serum drug levels, in people with RA and spondyloarthritis (SpA) in remission or with LDA. This was an observational study of routine clinical practice. The study included a total of 88 participants (RA, n = 43; SpA, n = 45) who were treated with three TNF-α inhibitors (IFX, n = 31; ADL; n = 29; and ETN, n = 28). Participants were followed for 7 years (2006–12). Drug levels were measured using ELISA tests. No further information on the test was given in the abstract. For each participant two time periods were examined: before and after the introduction of TNF-α drug monitoring (2006–9 and 2010–12, respectively). All participants in this study had stable clinical activity in both time periods. Pascual-Salcedo et al. 44 reported the monthly value of spared drug to be €91.62 per participant treated with IFX (assuming a mean participant weight of 70 kg), €324 per participant on ADL and €257 per participant on ETN.
Model-based studies
Three model-based economic evaluations were identified in the systematic review (see Appendix 6,Table 50). All were conducted in Europe (the Netherlands, Finland and the UK).
Krieckaert et al.53
Krieckaert et al. 53 conducted a cost–utility study that investigated the measurement of ADL levels in people with RA. ADL levels were measured using in-house ELISA (Sanquin) for 3 years in a cohort of 272 ADL-treated participants with RA recruited at the Department of Rheumatology, Jan van Breemen Institute, Amsterdam. 55 These participants were compared with a cohort of 1034 participants from the Utrecht Rheumatoid Arthritis Cohort (URAC), who received other treatments based on clinical judgement. The clinical characteristics of these participants are not clearly discernible from the cited references. Participants in the intervention cohort were tested after 4, 16, 28, 40 and 52 weeks of treatment and every 6 months thereafter. However, in the economic analysis, the authors modelled ELISA testing at 28 weeks only. After 3 years, 76 of a total 272 participants (28%) developed anti-ADL antibodies; 51 (67%) participants developed these during the first 28 weeks of treatment. Over the course of the study, participants with measurable antibody levels were 3.6 times less likely [hazard ratio (HR) 3.6, 95% CI 1.8 to 7.2; p < 0.001] to revert to minimal disease activity (defined as a DAS28 of < 3.2) and 7.1 times less likely (HR 7.1, 95% CI 2.1 to 23.4; p < 0.001) to enter sustained remission (DAS28 of < 2.6). Clinical outcomes from Bartelds et al. 55 are summarised in Table 19.
| Antibody titre (AU/ml) | Drug level (mg/l), median (IQR) | Treatment discontinuation, n (%) | Disease activity, n (%) | Sustained remission, n (%) |
|---|---|---|---|---|
| Undetectable (n = 196) | 12 (9–16) | 28 (14) | Minimal: n = 95 (48) | 67 (34) |
| 13–100 (n = 45) | 5 (3–9) | 29 (38) | Minimal: n = 10 (22) | 3 (7) |
| > 100 (n = 31) | 0 (0–3) |
Of note, this study55 was excluded from the clinical effectiveness systematic review because it did not meet the inclusion criteria for the population (the population was treatment naïve and had active disease). As this paper was excluded at the first-screening stage (titles and abstracts), it was not included in the list of excluded studies (see Appendix 2).
Krieckaert et al. 53 modelled a treatment algorithm (see figure 1 in Krieckaert et al. 53) based on TDM in RA patients. The authors used a Markov model with 3-month cycles and a time horizon of 3 years using microsimulation for analysis. The analysis was performed probabilistically. Discounting was applied at 4% for costs and 1.5% for utilities, following the Dutch national guidelines. 56 Results were reported from both health-care and societal perspectives. The Markov model health states were based on categorisation of DAS28 as below:
-
remission (DAS28 of < 2.6)
-
LDA (DAS28 of ≥ 2.6 and < 3.2)
-
MDA (DAS28 of ≥ 3.2 and ≤ 5.1)
-
high disease activity (HDA) (DAS28 of > 5.1).
Transition probabilities were estimated using a regression function that was derived from the URAC cohort outcome data. 55 Costs included direct medical and productivity costs. Utility was calculated based on the EQ-5D classification outcomes recorded in the URAC study.
Testing with ELISA kits was cost-saving from both the societal and the health-care perspective (Table 20). The test-based treatment strategy resulted in lower costs (due to the reduction in the treatment cost) and greater QALYs.
| Perspective | Costs | QALYs | ICER | ||
|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||
| Societal | €15,466,869 | €18,028,517 | 591.65 | 587.81 | −€646,266 |
| Health-care provider | €13,607,067 | €16,153,357 | 591.65 | 587.81 | −€666,541 |
A probabilistic sensitivity analysis around the base-case scenario predicted that ELISA testing would dominate usual care in 72% of scenarios. Scenario sensitivity analyses around, for example, the drug level cut-offs used, or the definitions of a good EULAR response, showed that ELISA testing is generally cost-saving, although some scenarios reported loss of QALYs.
Laine et al.54
Laine and colleagues54 conducted a cost-effectiveness study in Finland. The intervention involved assessment of drug and anti-drug antibody levels in people with RA who were treated with ADL or IFX. The data on drug and anti-drug antibody levels were taken from the clinical sample registry of United Medix Laboratories Ltd (Helsinki, Finland), which included 486 and 1137 samples from participants on ADL and IFX, respectively. Drug levels were measured using ELISA, whereas antibody levels were assessed using radioimmunoassay. All measurements of antibody and ADL levels were outsourced to Sanquin Diagnostic Services. Approximately half of the measurements of IFX levels were undertaken by the United Medix Laboratories using the Promonitor test kit.
Clinical management decisions based on the test results followed the algorithm proposed by Vincent et al. 57 (see figure 1 in Vincent et al. 57). Possible treatment decisions included switching to another TNF-α inhibitor or switching to a bDMARD with a different mechanism of action.
The economic impact of clinical decision-making compared with management by clinical judgement only and without testing was modelled in a short-term (3–6 months) scenario with 100 hypothetical non-responders. The outcome measures were the change in the probability of undergoing periods of suboptimal treatment and the cost-effectiveness of routine monitoring compared with clinical judgement only. An inappropriate clinical decision was defined to lead to ineffective treatment for at least 3–6 months. The authors justified this time period by basing it on the typical follow-up visit frequencies of people with RA who were treated with biologics in Finland (no data sources were provided). This meant that all of the participants in the control arm experienced a 3-month delay in receiving appropriate treatment. This delay was estimated to cost €1471 per month, which included the estimated monthly cost of subcutaneous TNF-α inhibitor (€1140); the cost of a laboratory visit, both travel cost and lost working and leisure time (€17.40); the cost of the possible standard safety-related laboratory tests (€6.80); the cost of a follow-up visit to an outpatient specialist clinic, both travel cost and lost working and leisure time (€66.60); and specialist visits (€240.60). Long-term efficacy-related costs were not modelled. The cost of resource use was valued using national unit costs adjusted for inflation to the year 2013. 58
The authors proposed a Markov model with 6-month cycles and a 3-year time horizon. Health states were defined as ‘first TNF-α blocker’, ‘second biological (TNF-α blocker or non-TNF drug)’ and ‘quitting biologics’.
The model predicted that, over the 3-year period, in the intervention arm, 40% of participants on ADL and 50% of participants on IFX would need drug modification. Based on a hypothetical cohort of 100 participants, the cost of testing was estimated to amount maximally to €20,000 (€200 × 100 participants). Dividing the cost of the test by the cost per month of non-optimised treatment will then indicate the threshold number of person-months of suboptimal treatment that correspond with testing being considered cost-effective. Laine et al. 54 reported that the routine measurement of both drug and antibody levels would be cost-saving compared with the non-testing scenario, assuming that a minimum of 2.5% or 5% of patients are treated non-optimally for 6 or 3 months, respectively.
Gavan17
Gavan17 evaluated the cost-effectiveness of using ELISA testing to monitor people with RA who are treated with ADL. In total, 12 different ELISA-based strategies were compared with the current practice in England (i.e. no TNF-α testing) (see Table 51 in Appendix 7). These strategies were a combination of using monitoring tests during response to the drug and after remission. The author considered a frequency of testing of every 3 or 6 months in responders to therapy. Among patients in remission, testing was considered after 2 and 3 years’ remission.
In Gavan,17 four lines of treatment were modelled, as shown in Figure 2.
A discrete-event simulation modelling approach was used. The following competing events were considered: time to death, ADL failure, rituximab (Rituxan®; Roche, Basel, Switzerland) failure, tocilizumab failure, time to development of antibodies against ADL, remission, EULAR response and HAQ progression. The model simulated 20,000 hypothetical patients, representative of the population with RA in England, using summary attributes of patients from the BSRBR-RA.
One of the test strategies considered in Gavan17 was monitoring drug and antibody levels in participants who were responding to treatment to avoid the harm associated with secondary non-response. Another possible test strategy was dose adjustment in patients in remission, informed by the results of TNF-α testing. Figure 3 shows the algorithm used by Gavan17 for management decisions in participants who had TDM performed.
Utilities were calculated by mapping the HAQ score from the BSRBR-RA, by using a quadratic mapping algorithm estimated previously for the NICE TA195 by Malottki et al. 59 Costs included the costs of treatment, hospitalisations and testing. Quantities of resource utilisation were derived from published sources34,37 and unit costs were taken from the NHS Reference Costs 2015–1660 and the BNF. 21
Based on the 12 strategies that were modelled (see Appendix 7, Table 51), Gavan17 concluded that routine use of ADL testing was cost-effective compared with current practice, but was unlikely to be cost-effective relative to dose reduction (without testing) for people in remission (strategy 11). Compared with current practice, strategies 1–6 and strategy 8 were estimated to be cost-effective. Strategies 9 and 10 were estimated to be less costly, but produced a lower QALY gain than current practice. Strategy 7 was dominated by current practice, that is current practice was associated with lower costs and a higher QALY gain than strategy 7. In the incremental analysis, all but three strategies (strategies 1, 3 and 11) were shown to be dominated or extendedly dominated by another strategy, that is that another strategy or combination of strategies was cheaper and produced more QALYs. Of the three remaining strategies, strategies 1 (testing levels of ADL and anti-ADL antibodies every 3 months) and 3 (testing levels of ADL and anti-ADL antibodies every 3 months, testing ADL level in patients in remission after 2 years) were not cost-effective compared with strategy 11 (no testing, halving ADL dose in all patients after responding for 2 years, irrespective of their drug level) at a willingness to pay of £20,000–30,000 per QALY gained: the incremental cost-effectiveness ratio (ICER) for strategy 1 versus 11 was £38,575 per QALY and the ICER for strategy 3 versus 11 was £37,043 per QALY. Given that strategy 11 consists of dose reduction after 2 years for people in remission, the analysis of the chosen strategies suggests that ADL testing may not be cost-effective compared with dose reduction alone.
Quality of identified cost–utility studies
Table 52 (see Appendix 8) shows the results of assessing the included studies against the CHEC checklist. 49 The methodological quality of the included modelling studies, assessed using the Philips checklist,50 is addressed in Table 53.
Discussion
A systematic literature search performed in July 2018 and updated in November 2018 identified five publications that were relevant to the decision problem, with two of these available in abstract format only. Furthermore, only two (out of six) TNF-α testing kits from the NICE scope48 (Promonitor and Sanquin) and three (out of five) TNF-α inhibitors (ADL, ETN and IFX) were considered in the selected studies (Table 21).
| TNF-α inhibitor | Drug or antibody | Promonitor | IDKmonitor | LISA-TRACKER | RIDASCREEN | Sanquin |
|---|---|---|---|---|---|---|
| ADL | Drug | ✓a | ✗ | ✗ | ✗ | ✓b |
| Antibody | ✓c | ✗d | ✗ | ✗ | ✓e | |
| ETN | Drug | ✓f | ✗ | ✗ | ✗ | |
| Antibody | ✗ | ✗ | ✗ | ✗ | ||
| IFX | Drug | ✓f | ✗ | ✗ | ✗ | ✗ |
| Antibody | ✗ | ✗d | ✗ | ✗ | ✓f | |
| GLM | Drug | ✗ | ✗ | ✗ | ✗ | |
| Antibody | ✗ | ✗ | ✗ | |||
| CTZ | Drug | ✗ | ✗ | |||
| Antibody | ✗ |
Both Krieckaert et al. 53 and Laine et al. 54 concluded that TDM was cost-saving compared with standard care, based on follow-up periods of up to 3 years. Krieckaert et al. 53 reported a formal cost-per-QALY analysis in which TDM dominated standard care in the base-case scenario in 72% of simulations. The ICERs are arguably somewhat meaningless given the small incremental QALYs involved. In a range of sensitivity analyses, a net loss of QALYs with respect to the intervention was associated with drug-level cut-off points, the use of EULAR good response as an outcome or the use of biologicals other than TNF-α inhibitors. With regard to UK clinical practice, Krieckaert et al. 53 modelled testing at the 28th week from treatment initiation and considered dose reduction by prolongation of the interval between drug administrations in responders with high levels of ADL (> 12 mg/l). However, in the UK there are variations in when treatment decisions in people with RA who are treated with biologics are made. In responders to TNF-α inhibitors, decisions could either be made 9–12 months after treatment initiation or be adjusted approximately 2 years after the initiation of biological therapy. However, in non-responders, testing may be considered earlier to detect whether non-response to biologics is due to low drug levels or the presence of anti-drug antibodies.
Laine et al. 54 did not report a cost per QALY analysis, although they attempted to analyse the frequency and cost impact of non-testing with regard to inappropriate treatment decisions (e.g. continuation of ineffective therapy). It was assumed that participants in the routine practice arm would typically experience 3 months’ delay in receiving optimal treatment compared with participants in the intervention arm. This was justified based on the typical follow-up intervals of participants in Finland. Of note, in Finland anti-drug antibody levels of at least 30 AU/ml (arbitrary unit/ml) rather than 12 AU/ml are considered clinically significant.
Both the INGEBIO study43 (n = 169) and Pascual-Salcedo et al. 44 (n = 88) recruited mixed populations in which, respectively, only 37% and 50% of participants had a diagnosis of RA. In addition, only limited details of the input parameters and analysis were provided, specifically:
-
No details of utility values or incremental QALY outcomes were provided.
-
The studies did not consider specific test-based treatment algorithms.
-
Pascual-Salcedo et al. 44 did not specify which ELISA kits were used in their study.
Furthermore, the allocation of participants to groups in the INGEBIO study was site dependent and physicians were not obliged to follow any particular algorithm with regard to treatment. However, the statistical analysis plan was not documented and the assumption of independence of observations may not be appropriate. Therefore, the statistical significance of the reported results may be insecure.
The recent study by Gavan17 perhaps most closely matches the decision problem. In this study,17 modelling was based on patient data from the BSRBR-RA register, which is the main source of evidence on the use of biologics in people with RA in the UK. Furthermore, the research questions addressed in Gavan17 are most relevant to the decision problem considered in this report. However, Gavan17 did not consider any specific test kit and only ADL treatment was modelled as the first line.
Gavan17 considered three research questions, namely:
-
What is the existing economic evidence for stratified medicine in RA?
-
How are decisions on treatment with biological therapies for patients with RA in current practice made in England?
-
Are treatment decisions stratified by levels of ADL and anti-ADL antibody in patients with RA in England a relatively cost-effective use of health-care resources?
Research questions 1 and 2 have, to some extent, also been addressed by the searches and consultations for this review. However, Gavan17 considered any strategy involving biomarker testing to stratify decisions regarding treatment with any pharmacological therapy, whereas the current review focuses on the use of ELISA to monitor response to TNF-α inhibitor treatment. Research question 3 aligns closely with the decision problem for this appraisal. Although Gavan17 pointed out that there was a high degree of decision uncertainty and reported an expected value of perfect information estimate of £7M, the decision uncertainty was based around the cost of testing and test accuracy.
Based on these searches, it is clear that further exploration of this question, including de novo modelling, would be appropriate. Sufficient prior evidence with regard to the entirety of the decision problem and/or UK populations on which to base decision making clearly does not exist, especially given discrepancies in the conclusions of the studies presented. Of these studies, only Gavan17 could be considered to be of sufficient quality; however, no evidence was identified with regard to the use of test kits for either CTZ or GLM treatments, and no studies evaluating IDKmonitor ELISA kits, LISA-TRACKER ELISA kits, RIDASCREEN ELISA kits or MabTrack ELISA kits were identified.
Conclusions
The results of the cost-effectiveness systematic review that was conducted in this study show that there is limited evidence on the cost-effectiveness of TDM in people with RA. Despite a comprehensive search of the literature, only five studies were identified. Two (out of five) TNF-α testing kits from the NICE scope (Promonitor and Sanquin) and three (out of five) TNF-α inhibitors (ADL, ETN and IFX) have been assessed in the selected studies.
Two out of the five identified studies were reported in abstract format only and, therefore, limited detail was reported.
Chapter 4 Independent economic assessment
The assessment of whether the economic analysis conducted by the EAG meets the NICE Reference Case requirements is summarised in Table 81, Appendix 25.
Methods
Summary of available evidence
The treatments and ELISA kits that were used in the studies that were included in the clinical effectiveness systematic review are shown in Table 22.
| TNF-α inhibitor | Drug/antibody | ELISA test kit | ||||
|---|---|---|---|---|---|---|
| Promonitor | IDKmonitor | LISA-TRACKER | RIDASCREEN | Sanquin | ||
| ADL | Drug | ✓a | ✗ | ✗ | ✗ | ✓b |
| Antibody | ✓a | ✗c | ✗ | ✗ | ✓b | |
| ETN | Drug | ✗ | ✗ | ✗ | ✓b | |
| Antibody | ✗ | ✗ | ✗ | ✓b | ||
| IFX | Drug | ✗ | ✗ | ✗ | ✗ | ✓b |
| Antibody | ✗ | ✗c | ✗ | ✗ | ✓b | |
| GLM | Drug | ✗ | ✗ | ✗ | ✗ | |
| Antibody | ✗ | ✗ | ✗ | |||
| CTZ | Drug | ✗ | ✗ | |||
| Antibody | ✗ | |||||
The clinical evidence identified in the systematic review was limited:
-
No studies related to IDKmonitor, LISA-TRACKER and RIDASCREEN test kits were identified.
-
No studies investigating the use of TDM in RA patients treated with two drugs from the NICE scope, the TNF-α inhibitors GLM and CTZ, were found.
In addition, it was not clear whether originator products or their biosimilars were used in the selected studies, and the type of testing, concurrent or reflex, was not reported. Furthermore, in Pascual-Salcedo et al. 44 it was not clear which type of test kits (MabTrack or those developed by Sanquin) were used by Sanquin Diagnostic Services to measure drug and antibody levels (see Table 22). Finally, no studies on TNF-α testing in primary and secondary non-responders were found.
Two studies included in the review, the non-randomised controlled trial INGEBIO42,43,45 and a historical control study reported by Pascual-Salcedo et al. ,44 considered people in remission or with LDA at baseline. The study populations were mixed, with the proportion of participants with RA being only 37% and 49%, respectively, in the INGEBIO study and the study by Pascual-Salcedo et al. 44 The patient population considered in the latter was relatively small (43 patients), whereas the former was a larger study with 169 study participants.
The only head-to-head trial identified in the review, the INGEBIO study, investigated the clinical and economic effects of TDM in patients treated with ADL. In this study, physicians were not obliged to follow any specific test-based therapeutic algorithm but could use testing to alter the treatment dose in participants from the intervention arm. The longest average follow-up, 530.8 days and 544.6 days in the intervention and control arms, respectively, was reported by Arango et al. 43 Some of the aggregate clinical outcomes from the INGEBIO study are shown in Table 23.
| Outcome | Ucar et al.42 | Arango et al.43 | ||
|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |
| Proportion of patients with tapered dose (%) | 35.8 | 36.7 | 35.7 | 34.6 |
| Rate of flares (per patient-year)a | 0.463 | 0.639 | 0.463 | 0.639 |
| Mean duration of remission (Ucar et al.42) or remission/LDA (Arango et al.43) (days) | 344 | 329 | 460.2 | 475.2 |
| Mean follow-up (days) | 499 | 505 | 530.8 | 544.6 |
Search for additional clinical effectiveness evidence
Studies that were identified by the searches conducted for the clinical effectiveness systematic review but not considered eligible for inclusion (e.g. studies reporting correlations between drug/antibody levels and therapeutic outcomes, and/or studies reporting drug/antibody levels before and after dose reductions only) were used to inform the model where appropriate.
Owing to the lack of RCT evidence on the effectiveness of the tests that are defined within the NICE scope,48 an additional systematic literature review was conducted to identify RCTs that evaluated any tests used to monitor TNF-α inhibitor treatment in people with RA. The aim of this search was to identify any evidence on the effectiveness of any strategies of treatment monitoring that could be used to inform scenario analyses for modelling.
Searches were carried out in MEDLINE, MEDLINE In-Process, EMBASE, the Cochrane Library and Web of Science. Searches were limited to RCTs and were carried out in October 2018. The search strategy and inclusion criteria are provided in Appendix 9. A total of 1418 hits were identified and were independently screened by two reviewers using the inclusion criteria shown in Appendix 9, Table 54. No relevant papers were identified.
Economic analyses
The outcomes from the only head-to-head trial that was identified in the systematic review, the INGEBIO study, were utilised in all analyses reported here. In the INGEBIO study, both mean time in remission and mean time in remission or LDA were estimated in patients from the intervention and control arms. Therefore, two separate scenario analyses (scenario 1 and scenario 2) based on alternative health state descriptions were conducted: the health states considered in scenario 1 were ‘remission’ and ‘LDA/active disease’ and the health states modelled in scenario 2 were ‘remission/LDA’ and ‘active disease’. The duration of the complementary health states (‘LDA/active disease’ in scenario 1 and ‘active disease’ in scenario 2) was estimated using the duration of follow-up.
Modelling approach
The choice of the modelling approach was primarily driven by the availability and quality of the evidence that was identified in the clinical effectiveness systematic review; other factors included the multifactorial nature of decisions to adjust treatments in people with RA34 and the recent changes in the biologics market, which contributed to the uncertainty in the prices of the TNF-α inhibitors and their uptake in the UK.
The biologics market is likely to increase in complexity over the coming months and years as more originator biological medicines lose patent exclusivity and additional biosimilar medicines come to the market. 61 The patent for ADL (Humira) expired on 16 October 2018 (when this assessment was carried out). New medications with similar active properties (‘biosimilar’ versions) are likely to become available in the NHS at the end of 2018 (see Table 2). The following ADL biosimilars have already been approved for use in the UK but have not yet launched (as of 30 November 2018): Amgevita, Hulio, Hyrimoz and Imraldi. According to the Regional Medicines Optimisation Committee Briefing,62 at least two further biosimilars are expected to become available in the UK during 2019: Cyltezo (from Boehringer Ingelheim) and another will be brought to the market by Fresenius Kabi (Bad Homburg, Germany).
The NHS has established a working group to provide an oversight of implementing the use of best-value ADL using a commissioning framework that was launched in September 2017. 63 The framework, authored by the NHS’s Medicines, Diagnostics, Personalised Medicine Policy Team, proposes that ‘at least 90% of new patients be prescribed the best value biological medicine’ within 3 months of the launch of a biosimilar for a given reference product, and that 80% of existing patients be prescribed the ‘best value medicine within 12 months of a biosimilar launch’. 63
With regard to the current uptake of biosimilars in the UK, according to the Medicines Optimisation Dashboard data published by NHS England (September 2018 release),64 92% of people prescribed IFX and 85% of those prescribed ETN are taking biosimilars. However, there are regional variations in the uptake of biosimilars. 64 In the Royal Devon & Exeter NHS Foundation Trust, people with RA who have been prescribed IFX or ETN are usually given their biosimilars, whereas biosimilars for ADL have become available only recently; patients prescribed GLM or CTZ are treated mostly with the originator products (Dr Haigh, Royal Devon and Exeter NHS Foundation Trust, Exeter, November 2018, personal communication). In the Greater Manchester area, the biosimilar Amgevita is soon to be used for patients who are prescribed ADL; patients who are prescribed IFX are usually given its biosimilars, Inflectra or Remsima; and a biosimilar Benepali is used in some patients who are prescribed ETN (Dr Jani, University of Manchester, personal communication, November 2018).
Although the NICE guidance23 recommends that people with RA receive treatment with the TNF-α inhibitor with the lowest acquisition and administration costs, in practice other non-cost factors, such as patient and hospital characteristics, and changes in regional rheumatology clinical guidelines, may influence the choice of treatment. 17
Analyses conducted
Threshold and cost–utility analyses based on a decision tree model (described in Model structure) were conducted to estimate the economic outcomes of adding TDM to SOC for RA patients who were treated with TNF-α inhibitors.
Threshold analyses
In the threshold analyses, the cost of TNF-α testing at which adding TDM to SOC would result in zero net monetary benefit (NMB) was estimated, as described below.
The NMB represents the value of an intervention in monetary terms when a willingness-to-pay (WTP) threshold for a unit of benefit (e.g. QALY) is known. It is estimated by first assuming a WTP threshold (e.g. £20,000 or £30,000 per QALY gained) and then calculating the NMB as follows:
where incremental costs and incremental benefits represent incremental costs and QALYs for the health technologies under consideration.
In this study, NMB was estimated for a range of acquisition costs of ADL (from £1000 to £9187 per patient-year) at the WTP thresholds of £20,000 and £30,000 per QALY gained, which are the thresholds usually considered by NICE. In the threshold analyses, the costs of drug acquisition and administration and the costs associated with disease management were included; the latter comprised the costs of managing flares and AEs, and the costs of managing different health states. QALYs were estimated from the rates of flares and AEs, and the average duration of remission and LDA/active disease health states (for the analysis based on data from Ucar et al. 42) or remission/LDA and active disease health states (for the analysis based on data from Arango et al. 43) in patients from the intervention and control arms.
In the threshold analyses, the cost of TNF-α testing per patient-year, under which the test-based treatment strategy has zero NMB, was estimated in the following way:
where the total cost of testing comprises the costs associated with testing patient blood samples to monitor trough drug and antibody levels. The ICER threshold represents the NICE WTP of £20,000 or £30,000 per QALY gained, and Δcosts and ΔQALYs are incremental costs and QALYs across the intervention and control arms.
The costs incurred in each arm were estimated as follows:
For scenario 1 (with ‘remission’ and ‘LDA/active disease’ health states), QALYs were derived in the following way:
For scenario 2, QALYs were estimated from the duration of ‘remission/LDA’ and ‘active disease’ health states and their corresponding utilities.
Cost-effectiveness analyses
In addition to the threshold analyses, cost–utility analyses were conducted in which ICERs were estimated using the list prices of biologics and the cost of TNF-α testing; the latter was based on the prices of the Promonitor test kits (provided by Grifols–Progenika), and the other costs associated with TNF-α testing37 and clinical advice.
Model structure
A diagram of the decision tree model that was used in the threshold and cost–utility analyses is presented in Figure 4.
FIGURE 4.
Model diagram.
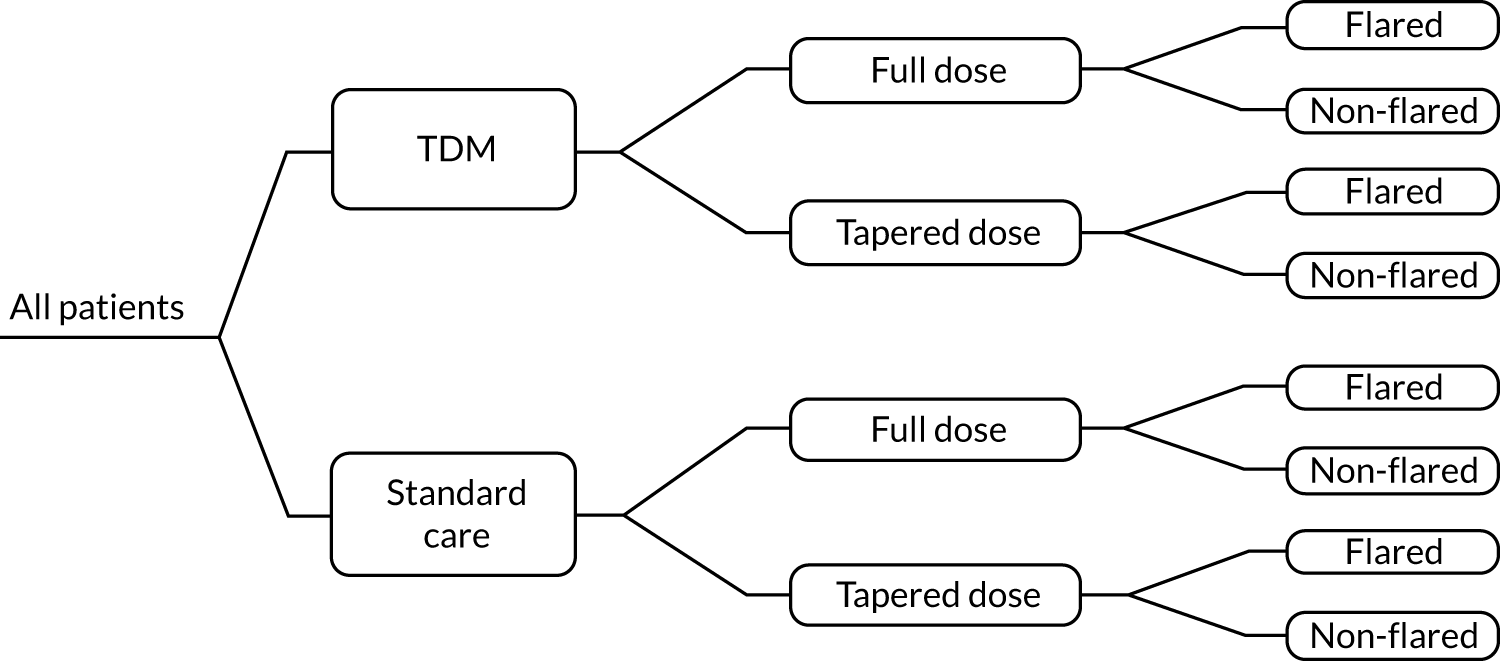
As shown in Table 23, approximately one-third of patients in the treatment and comparator arms of the INGEBIO study had their ADL dose tapered; flares were observed in patients from both arms. The effect of flares on costs and QALYs was modelled following Gavan. 17 Figure 5 (adapted from Gavan17) illustrates the cost and QALY profile depending on whether or not the dose is tapered. The figure shows changes in the acquisition cost and QALYs due to flares over time. Note that, for the sake of clarity, the other components of the total costs and QALYs considered in our analyses are not depicted here.
FIGURE 5.
(a) Acquisition cost and (b) QALY change owing to flare in tapered and non-tapered patients. a, Change in QALYs owing to flare. T1 – t0 is the time on tapered dose, t2 – t1 is the duration of flare and qf is the disutility of flare. Adapted from Gavan. 17
As shown in Figure 5, all patients had their drug levels tested at t0, resulting in tapering of the dose in some patients (see Figure 5a). It was assumed that a proportion (p) of patients on tapered doses experienced flare at t1, prompting treatment to revert to the original dose, whereas in the remainder (1 – p) the dose remained the same (i.e. tapered). In those patients who flared, the disutility of flare (qf) was applied for the duration of flare (t2– t1) (see Figure 5a). In non-tapered patients (see Figure 5b), the acquisition cost was based on the cost of the full dose; it was assumed that non-tapered patients do not experience flares.
In clinical practice, flares have been observed in patients receiving full and reduced doses of the biologics, with an increased risk of flares in tapered patients. 65 In the economic analysis, however, the occurrence of flares was modelled in all patients regardless of their treatment dose (see Figure 5a) given that the flare rates reported in the INGEBIO study were not stratified by dose.
The estimates of the mean time to the first flare were used to model the time when the dose in flared patients was restored to the full dose indefinitely (which affected the drug acquisition costs and wastage), whereas the flare rates were used to estimate the cost of flare management and the reduction in QALYs due to occurrence of flares in the treatment and control arms. It was also assumed that flares could occur in any health state. 66
Population
The modelled population comprised patients in remission or LDA. The baseline characteristics of participants in the INGEBIO study are presented in Table 24 along with the characteristics of RA patients from the BSRBR-RA database67 who responded to biological treatment.
| Study | Number of RA patients | Mean age (years) | Proportion female | Disease duration (years) | Treatment history | Concomitant treatments | Disease state |
|---|---|---|---|---|---|---|---|
| The INGEBIO study | Mixed population: 63 people with RA (out of the total 169 participants) | 53.6a | 42%a | Median = 10 | NR | MTX:b 76.7% | 77% of people in remission, 23% of people with LDA (at baseline) |
| BSRBR-RA data for respondersc | 10,186 | 56 | 76.3% | Mean = 13 (years at the time of initiation of first biologic) | Mean = 3.90 (previous DMARDs) | NRd | 30.6%: good responders |
As shown in Table 24, patients in the INGEBIO study were slightly younger, on average, than patients from the BSRBR-RA database, and were considerably less likely to be female.
Subgroups
People with RA can be grouped according to three clinical scenarios: primary non-response, secondary non-response and remission. However, with regard to particular characteristics, there are no subgroups for which the clinical effectiveness of TDM is expected to significantly vary; therefore, no subgroup analyses were considered in this assessment.
Interventions and comparators
Owing to the paucity of data, not all test kits specified in the NICE scope could be evaluated in this study. In particular, no economic analyses relevant to IDKmonitor, LISA-TRACKER, RIDASCREEN, MabTrack ELISA kits and those used by Sanquin Diagnostic Services were conducted. The only test kits considered were Promonitor assays for measuring trough ADL and antibody levels (see Table 22).
The comparator was SOC, in which treatment decisions were based on clinical judgements and other measures (such as DAS28), that is without the use of TDM.
Perspective, time horizon and discounting
The costs and resource use were considered from the perspective of the NHS and Personal Social Services. 69 Cost and health outcomes were not extrapolated into the future because the lack of long-term evidence means that external validation of extrapolated outcomes would not be feasible; therefore, no discounting was applied to estimated costs and QALYs.
The time horizon was defined by the observational period in the INGEBIO study, namely 505 days and 544.6 days for the analyses based on Ucar et al. 42 and Arango et al. ,43 respectively. The comparator arm, as reported in Ucar et al. 42 and Arango et al. ,43 had slightly longer follow-ups; therefore, the mean duration of follow-up in patients from the comparator arm was used as the time horizon in the economic analyses based on these sources. The estimates of the mean duration of remission (scenario 1) and remission/LDA (scenario 2) for the intervention arm were not adjusted to account for such a difference given that the Kaplan–Meier estimates for time in remission were not available to the EAG; therefore, it is possible that the cost-effectiveness of the intervention under consideration was underestimated. However, owing to a small difference (of about 1–2%) in the length of follow-up periods between the treatment and the comparator arm, this simplifying assumption is likely to have only a small impact on the results.
Considerations in the development of the independent economic assessment
Flares
The concept of flare remains challenging to understand, as there are no generally recognised definitions of or well-validated measures for flare in RA. 70 Nevertheless, patients, clinicians and scientists commonly resort to this term to refer to episodes of worsening disease activity, which includes a range of symptoms of different duration and magnitude. 71
Several different RA flare criteria have been used in clinical research. For instance, van der Maas et al. 47 identified six previously published DAS28-based flare criteria, and Markusse et al. 72 reported three criteria (Table 25).
| Type of flare | DAS28 | ||
|---|---|---|---|
| Current | Previous | Increase | |
| van der Maas et al.47 | |||
| 1 | Any | NA | > 1.2 |
| > 5.1 | NA | > 0.6 | |
| 2 | Any | NA | > 1.2 |
| ≥ 3.2 | NA | > 0.6 | |
| 3 | Any | NA | > 0.6 |
| > 3.2 | NA | Any | |
| 4 | Any | NA | > 1.2 |
| 5 | > 3.2 | NA | Any |
| 6 | > 2.6 | NA | Any |
| Markusse et al.72 | |||
| A | > 2.4 | Any | ≥ 0.6 |
| Minor B | > 2.4 | ≤ 2.4 | < 0.6 |
| Major Ba | > 2.4 | ≤ 2.4 | ≥ 0.6 |
Smolen et al. 66 compared RA patients treated with ETN recruited in the PRESERVE trial who did or did not have flares. In this trial, a disease flare was defined as either loss of LDA, with or without a change in DAS28 of 0.6, or relapse (DAS28 of > 5.1 or DAS28 of > 3.2 at two or more consecutive time points).
In the INGEBIO study, a flare was defined as an increase in DAS28 of > 1.2 or an increase in DAS28 of > 0.6 if the current DAS28 was ≥ 3.2.
Duration of flare
Substantial heterogeneity in the duration of flare has been reported70 and observed in clinical practice. A flare may last from 2–3 days up to 2–3 months, depending on severity (Dr Jani, personal communication). The duration of flare was estimated in the dynamic cohort in the Brigham Rheumatoid Arthritis Sequential Study (BRASS),70 which included 1105 people with established RA who had received usual care at the Brigham and Women’s Hospital in Boston70 (Table 26).
| Duration (days) | Flare duration of | ||
|---|---|---|---|
| < 7 | 7–13 | ≥ 14 | |
| Proportion of patients (%) | 57 | 14 | 30 |
The estimate of 7 days was adopted in the primary analyses based on Ucar et al. 42 and Arango et al. ;43 this was consistent with the estimate used in NICE TA375. 23 The impact on the results of a longer duration of flare, 19 days, was evaluated in scenario analyses; this represents a weighted average of the estimates reported in the BRASS study70 (see Table 26) and those provided by Dr Jani (personal communication).
Time to the first flare
Arango et al. 43 and Ucar et al. 42 reported the median time to the first flare that was observed in the intervention and control arms of the INGEBIO study; however, according to the NICE Guide to the Methods of Technology Appraisal,69 mean estimates should be utilised in economic analyses of health interventions. The mean time to the first flare in the intervention and control arms was calculated from Kaplan–Meier curves for the time to the first flare in the INGEBIO study, sourced from a poster presentation by Ucar and colleagues at the Annual European Congress of Rheumatology EULAR 2017 (Ucar and Osakidetza, personal communication, September 2018), by using the area under the curve approach.
The Kaplan–Meier estimates were available for 300 days (see Figure 8, Appendix 10) and were extrapolated for the duration of follow-up reported in Ucar et al. 42 and Arango et al. 43 (see Table 23). Given that the proportion of participants who were on a tapered dose in the intervention and control arms levelled at around 240 days after dose tapering, it was assumed that these proportions remained the same until the end of the observational periods in the INGEBIO study and, therefore, no parametric model fitting was performed. The estimated mean time to the first flare was 208.07 days and 189.32 days in the intervention and control arms, respectively. These values were used in both scenario 1 (based on Ucar et al. 42) and scenario 2 (based on Arango et al. 43).
Flare rate
Treatment arm-specific flare rates (per patient-year) were reported in both Ucar et al. 42 and Arango et al. 43 (see Table 23), and were the same in both sources despite the fact that the abstracts reported outcomes for different follow-up periods. These estimates were utilised in the primary and exploratory analyses.
Serious adverse events
When modelling the effect of AEs on patient’s HRQoL and costs, the approach used in TA37523 was adopted: it was assumed that only serious adverse events (SAEs) (serious infections in particular) would carry a significant cost and disutility burden. 23 This assumption was considered appropriate by the EAG’s clinical advisors.
Rate of serious adverse events
One study from the clinical effectiveness systematic review, Senabre Gallego et al. ,73 reported the rate of AEs experienced by patients who were treated with TNF-α inhibitor therapies. This study recruited 39 participants with RA who had achieved remission. The findings showed that one participant (3%) had septic arthritis (serious infectious arthritis) that was associated with TNF-α inhibitor therapies (ADL or ETN) in the 1-year follow-up period (see Table 55, Appendix 11).
Given that the evidence on SAEs in the population of interest was limited, additional searches were conducted. Lahiri and Dixon74 indicated that there was a time-dependent increase in the risk of serious infections in people with RA who were treated with biologics, with the maximum risk in the first 6 months of biological therapy and a gradual decline thereafter. The authors argued that this time-dependent decrease in the risk of serious infections can be attributed both to ‘depletion of susceptibles’ (i.e. high-risk participants dropping out of the TNF-α inhibitor cohort because of death, stopping therapy or loss to follow-up), which accounted for two-thirds of the observed difference, and to reduction in the inherent infection risk resulting from an improvement in patient’s functional status and a decrease in the dose of glucocorticoid.
According to Bruce et al. ,75 the risk of Pneumocystis jirovecii pneumonia in people from the BSRBR-RA register who were treated with TNF-α inhibitors was low, with an incidence rate of 2 (95% CI 1.2 to 3.3) events per 10,000 person-years of follow-up (see Table 55, Appendix 11); the rate of tuberculosis was higher among those treated with ADL (144 events per 100,000 person-years) and IFX (136 events per 100 000 person-years) than among those treated with ETN (39 events per 100,000 person-years) (see Table 55 and Dixon et al. 76).
The rate of SAEs reported in Burmester et al. 77 was 4.7 per 100 patient-years (see Table 55, Appendix 11). This estimate was derived from 15,132 people with RA who were exposed to ADL in 28 global clinical trials. A SAE was defined as a fatal or immediately life-threatening event; an event necessitating hospitalisation or prolonging hospitalisation; an event resulting in persistent or significant disability/incapacity or congenital anomaly; or an event necessitating medical or surgical intervention to prevent a serious outcome. At baseline, participants considered in this study had a mean age of 53.5 years and a mean disease duration of 9.1 years; 78.8% were female, 16.5% had a treatment duration > 2 years and 10.9% had a treatment duration > 5 years.
The rate of serious infection that was adopted in TA375,23 35 out of 1000 patients, was based on Singh et al. 78 and was assumed to be independent of the bDMARDs used (i.e. all biological therapies were assumed to have similar safety profiles).
Consultation with clinical advisors confirmed that serious infections in people with RA from the population of interest are relatively rare.
In this study, the modelled AE rate for people who were receiving a full dose of biologics, three events per 100 patient-years, was adopted from Senabre Gallego et al. 73 The AE rate in tapered patients was estimated using an odds ratio (OR) for serious infections in people who were treated with low-dose biologics compared with people who were receiving the standard dose79 (see Appendix 12). The resulting AE rate in tapered patients was two events per 100 patient-years.
Duration of serious adverse events
In TA375,23 a serious infection in RA patients was assumed to persist for 28 days, on average. This estimate was adopted in all analyses reported here.
Model parameters
The major model assumptions in the primary analyses based on Ucar et al. 42 (scenario 1) and Arango et al. 43 (scenario 2) were as follows:
-
Adalimumab dose tapering is implemented by increasing the interval between doses from 2 to 3 weeks.
-
Dose is tapered in a proportion of people in each arm at the start of simulation.
-
The full dose of ADL is restored indefinitely in all people who are on tapered doses when they experience the first flare.
The model assumptions for the primary analyses are shown in Table 27.
| Assumption | Estimate/strategy | Source | Relevant table/sections |
|---|---|---|---|
| Dose tapering strategy | Spacing: from 40 mg of ADL every 2 weeks to 40 mg of ADL every 3 weeks | First dose reduction in the Exeter Biologic Clinic recommendations (Dr Haigh, Royal Devon & Exeter NHS Foundation Trust, personal communication, November 2018) | Dose tapering and Appendix 13 |
| Proportion of patients on tapered dose | |||
| Scenario 1 (with mean duration of remission) | |||
| Intervention | 35.8% | Ucar et al.42 | Table 23 |
| Control | 36.7% | Ucar et al.42 | |
| Scenario 2 (with mean duration of remission/LDA) | |||
| Intervention | 35.7% | Arango et al.43 | Table 23 |
| Control | 34.6% | Arango et al.43 | |
| Proportion of flared patients in whom the full dose is restored (%) | 100% | Exeter Biologic Clinic recommendations for biologic dose reduction | Appendix 13 |
| Mean duration of remission or remission/LDA (days) | |||
| Scenario 1 (with mean duration of remission) | |||
| Intervention | 344 | Ucar et al.42 | Table 23 |
| Control | 329 | Ucar et al.42 | |
| Scenario 2 (with mean duration of remission/LDA) | |||
| Intervention | 460.2 | Arango et al.43 | Table 23 |
| Control | 475.2 | Arango et al.43 | |
| Time horizon (days)a | |||
| Scenario 1 | 505 | Ucar et al.42 | Table 23 |
| Scenario 2 | 544.6 | Arango et al.43 | |
| Acquisition costs (per patient-year): Humira | |||
| Full doseb | £9187 | BNF21 | Treatment costs |
| Tapered dosec | £6125 | Exeter Biologic Clinic recommendations, BNF21 | Appendix 13 |
| Flared patientsd | £9187 | Exeter Biologic Clinic recommendations, BNF21 | Appendix 13 |
| Treatment wastage on the full dose (per patient-year) | £370 | Clinical advice | Treatment wastage |
| Administration cost for Humira (ADL) (per patient-year) | £0 | Clinical advice | Drug administration |
| Cost of flare managemente | £423 per flare | Cost of diagnostic investigations (Maravic et al.80) | Cost of managing flares |
| £68 per month | Monthly cost of treatment excluding TNF-α inhibitors (Maravic et al.80) | Cost of managing flares | |
| Cost of managing health statesf | |||
| Barbieri et al.,82 Radner et al.,83 NHS Reference Costs 2017–1884 | Cost of managing different health states | ||
| Remission | £902 | ||
| Remission/LDA | £1089 | ||
| LDA/active disease | £1483 | ||
| Active disease | £1827 | ||
| Cost of managing AEs (per infection)g | £1622 | TA37523 | Cost of managing adverse events |
| Health state utilitiesh | |||
| Remission | 0.718 | Estimated from HAQ scores for different HAQ bands reported by Radner et al.83 | Health state utility values |
| Remission/LDA | 0.665 | ||
| LDA/active disease | 0.568 | ||
| Active disease | 0.483 | ||
| Disutility of flare | 0.140 | Estimated from Markusse et al.72 | Disutility of flare |
| Disutility of AEs | 0.156 | Oppong et al.85 | Disutility of serious adverse events |
| Flare rate in scenarios 1 and 2 (per patient-year) | |||
| Intervention | 0.463 | Ucar et al.,42 Arango et al.43 | Table 23 |
| Control | 0.639 | Ucar et al.,42 Arango et al.43 | |
| Mean time to the first flare (days) | |||
| Intervention | 208.07 | Derived from Kaplan–Meier estimates of the time to the first flare (Ucar and Osakidetza, personal communication, September 2018) | Time to the first flare |
| Control | 189.32 | ||
| Flare duration (days) | 7 | TA37523 | Duration of flare |
| Rate of AEs | |||
| Patients on full ADL dose | 3/100 patient-years | Senabre Gallego et al.73 | Rate of serious adverse events |
| Patients on reduced ADL dosei | 2/100 patient-years | Estimated from Singh et al.79 | Rate of serious adverse events |
| Duration of AE (days) | 28 | TA37523 | Duration of serious adverse events |
| Number of tests (per year) | 1 | Clinical advice | Frequency of testing |
| Cost of TNF-α testing | |||
| Phase 1 (pre-testing), with initial phlebotomy appointment | £107.83 | Processing costs | |
| Phase 2 (analysis of samples): single testing | |||
| Promonitor kit for drug level | £8.80 | Assay costs provided by the manufacturers | |
| Promonitor kit for antibody level | £8.80 | Assay costs provided by the manufacturers | |
| Other costs (cost per sample) | £1.23 | Processing costs | |
| Phase 3 (treatment decision) | £9.76 | Processing costs | |
| Sample transport cost (within the UK) | £4.00 | Cost of sample transport | |
Utilities for the mixed-disease population in the INGEBIO study were assumed to be the same as those for the population of people with RA since no evidence on HRQoL directly relevant to the population considered in INGEBIO has been identified. Mortality associated with RA was not modelled because of the short-term time horizon of approximately 18 months adopted in this study.
Resources and costs
Costs considered in the economic evaluation included the cost of testing, and treatment and health-care costs. Unit costs were obtained from the BNF,21 NHS Reference Costs,84 documents provided by test manufacturers and published and unpublished sources.
Parameters specific to the threshold analyses
Given that the patent for the ADL originator product (Humira) expired in October 2018 and the true costs of the ADL biosimilars to the NHS were not known to the EAG at the time of writing, in the threshold analyses the annual acquisition cost was varied from £1000 to £9187 per patient-year. The latter represents the annual cost of ADL (Humira), assuming a dose of 40 mg every 2 weeks delivered by subcutaneous injection using a prefilled pen and the NHS indicative price from the BNF21 (Table 28).
| TNF-α inhibitor | Dosing regimen | Cost (per dose) | Cost (per year) | Additional cost in year 1 |
|---|---|---|---|---|
| ADL | ||||
| Humiraa | 40 mg every 2 weeks. In non-responsive patients, dose may be increased to 40 mg per week | £352.14 | £9187.08 | |
| Amgevita | NR | |||
| Cyltezo | NR | |||
| Imraldi | NR | |||
| Solymbicb | NR | |||
| Hyrimoz | NR | |||
| Halimatoz | NR | |||
| ETN | ||||
| Enbrela | 50 mg per week | £178.75 (25 mg/0.5 ml) | £9326.92 | |
| Benepali/Brenzys | £164 | £8557.29 | ||
| Erelzi | £160.88 | £8394.23 | ||
| Lifmior | NR | |||
| CTZ | ||||
| Cimziaa | Loading dose: 400 mg at weeks 0, 2, and 4. Maintenance dose: 200 mg every 2 weeksc | £357.50 | £9326.92 | £1072.50d |
| GLM | ||||
| Simponia | 50 mg once per month, on the same date each monthe | £762.97 | £9155.64f | |
| IFX | ||||
| Remicadea | 3 mg/kg at week 0, 2 and 4 and then 3 mg/kg every 8 weeksg | £419.62 per vial (100 mg of powder for concentrate for solution for infusion vials), two or three vials per administration | £5747.48 (assuming no vial wastage), £8210.69 (assuming full vial wastage) | £1982.70 |
| Inflectra or Remsimah | £377.66 (100 mg of powder for concentrate for solution for infusion vials) | £5172.76 (assuming no vial wastage), £7389.66 (assuming full vial wastage) | £1784.44 | |
| Flixabi or Renflexis | £377.00 (100 mg of powder for concentrate for solution for infusion vials) | £5163.72 (assuming no vial wastage), £7376.75 (assuming full vial wastage) | £1781.33 | |
| Zessly | NR | |||
| Ixifi | NR | |||
Parameters specific to the cost-effectiveness analyses
In the cost–utility analyses, ICERs were estimated using the list prices of the TNF-α inhibitors (in accordance with NICE guidelines69) and the costs of testing based on the costs of Promonitor assays (provided by Grifols–Progenika), other testing costs outlined in the study conducted by Jani et al. 37 and clinical advice (see Table 28 and Cost of testing for more details).
The primary analyses were conducted for the list price of the ADL originator product, Humira®, whereas, in the exploratory analyses for other TNF-α inhibitors described in Exploratory analyses: etanercept or infliximab and Promonitor, the list prices for the ETN originator product (Enbrel®) and its biosimilar Erelzi® and IFX biosimilars Flixabi® or Renflexis® were utilised.
Conversion to Great British pounds
Where conversion from other currencies to Great British pounds (GBP) was required, International Monetary Fund purchasing power parity (PPP) was used to convert within the year (e.g. from 2001 euro to 2001 GBP), after which inflation was applied. The Campbell and Cochrane Economic Methods Group–EPPI-Centre (Evidence for Policy and Practice Information and Coordinating Centre) Cost Converter was used for the PPP conversion. 86
Inflation to 2017–18 prices
Unit costs were inflated to 2017–18 prices by inflating to 2015–16 prices using the Hospital and Community Health Services (HCHS) pay and prices index,81 and then to 2017–18 prices using the average increase in the index for the previous 3 years (from 2013–14 to 2015–16), with the average rate of 1.1% per annum (see Appendix 14).
Treatment costs
Annual acquisition costs of the TNF-α inhibitors from the NICE scope48 estimated using their list prices and assuming adherence to standard dosing regimen for each drug are shown in Table 28.
The estimated costs of treatment with ADL, ETN, GLM and CTZ were based on the price of the solution for injection in prefilled pens given that these biologics are administered subcutaneously and can be self-administered. Consultation with clinical experts confirmed that all of the TNF-α inhibitors considered in this study, except IFX, are usually self-administered by people with RA at home.
Consistent with acquisition cost calculations in TA375,23 the cost per annum of IFX was estimated using a patient average weight of 70 kg. IFX is administered intravenously (the cost of intravenous administration is described in Drug administration).
As reported in TA375,23 the manufacturers of GLM provided the 100-mg dose at the same price as the 50-mg dose under a patient access scheme arrangement. This discount would not affect the annual cost presented in Table 28, as that is based on the assumption that the average patient weight is < 100 kg.
The acquisition costs of the cheapest available pens for each drug are equivalent to the cost of the cheapest available dose. Therefore, the annual acquisition costs for the self-administration route are equivalent to those for biologics administered during outpatient visits.
Of note, the estimates for the additional acquisition costs for the first year (see Table 28) are presented for information only. They were not used in any analyses given that the population in this assessment are people experienced in biologics.
According to EULAR recommendations for the management of RA with sDMARDs and bDMARDs,66 tapering of biologics should be considered in people who are in persistent remission after having tapered glucocorticoids, especially if this treatment is combined with a conventional sDMARD. In this context, tapering means reduction of the dose, for example reducing ETN from 50 mg/week to 25 mg/week,87 or increasing the interval between applications (‘spacing’), for example increasing the interval between ADL injections from 1 week to 10 days, as in the Exeter Biologic Clinic recommendations (see Appendix 13).
The EAG is aware that there is no gold standard on how dose tapering should be carried out. Studies evaluating dose tapering have used different approaches. In clinical practice, dose tapering varies extensively depending on the clinical opinion; for example, according to the Exeter Biologic Clinic recommendations (see Appendix 13), when tapering the ADL dose the dose should be reduced by one-third to 40 mg every 3 weeks and reduced further at 3 months to 40 mg every 4 weeks in people with LDA or remission. However, it may not be a representative strategy because of variations in clinical practice.
In the primary analyses, the assumption of reducing the dose by one-third (the first dose reduction in the Exeter Biologic Clinic recommendations; see Appendix 13) was implemented (see Table 27), while the assumption of halving the dose (the second dose reduction described in Appendix 13) was explored in sensitivity analyses (see Table 39).
The dose-tapering strategy suggested in the Exeter Biologic Clinic recommendations (see Appendix 13) is spacing; therefore, when this tapering strategy is used, there is no wastage of the self-administered drugs resulting from partial use of the dose in the prefilled injection pens. Clinical advice indicated that wastage of IFX owing to partial use of vials is usually avoided (Dr Haigh, Royal Devon & Exeter NHS Foundation Trust, November 2018, personal communication).
In the primary analyses, however, wastage of £370 per patient-year was incorporated (see Table 27). This estimate was based on a survey conducted at the Royal Devon & Exeter NHS Foundation Trust (Dr Haigh, Royal Devon and Exeter NHS Foundation Trust, Exeter, December 2018, personal communication) and was derived from data on 119 people with RA who were treated with biologics, and included missed doses and oversupply (defined as delivery of treatment even if > 4 weeks’ supply was available at home). It was assumed that, on average, £370 per patient-year would be wasted in people who were on the full dose of ADL, whereas in people who were on tapered doses wastage would be reduced in proportion to the reduction in treatment dose. In scenario analyses considering other biologics (see Exploratory analyses: etanercept or infliximab and Promonitor), the treatment wastage was also assumed to be proportional to the drug acquisition cost. The effect on the outcome of the no-wastage assumption was explored in sensitivity analyses (see Table 39).
As stated above, ADL, ETN, GLM and CTZ are usually self-administered via subcutaneous injection using a prefilled pen. In this scenario, there is no administration cost for delivery. Alternatively, these drugs may be administered by a district nurse. The average administration cost that was assumed in TA37523 (which was based on an estimate reported in TA24788) was £2.61 (cost year 2012). Given that this cost is quite low and that self-administration of the drugs listed above is very common in clinical practice in England, the effect of the assumption that subcutaneous administration would be performed by a nurse was not evaluated.
The administration cost for IFX is considerably higher as it is administered intravenously over a 2-hour period. Patients may be pretreated with, for example, antihistamine, hydrocortisone and/or paracetamol, and the infusion rate may be slowed in order to decrease the risk of infusion-related reactions, especially if such reactions have occurred previously. 89 Patients are observed for at least 1–2 hours post infusion for acute infusion-related reactions. Based on clinical advice, IFX is typically administered in outpatient settings.
In DG22,90 the administration cost for IFX was estimated to be £287.93 per infusion (2014 prices). In a more recent technology appraisal, TA329,91 the cost was estimated to be £297 per administration (2015 prices). 91
Grant Smith (Specialist Pharmacist, Royal Devon & Exeter NHS Foundation Trust, December 2018, personal communication) advised us that in the Royal Devon & Exeter NHS Foundation Trust the cost of IFX administration is based on Healthcare Resource Groups (HRGs) for inflammatory bowel disease without interventions, with complications and comorbidities scores depending on patient type. The relevant HRGs from the NHS Reference Costs (2017–18)84 are shown in Table 29.
| Currency code | Currency description | Number of FCEs | National average unit cost |
|---|---|---|---|
| FD02E | Inflammatory Bowel Disease without Interventions, with CC score 5+ | 254 | £317 |
| FD02F | Inflammatory Bowel Disease without Interventions, with CC score 3–4 | 1496 | £287 |
| FD02G | Inflammatory Bowel Disease without Interventions, with CC score 1–2 | 15,187 | £282 |
| FD02H | Inflammatory Bowel Disease without Interventions, with CC score 0 | 81,985 | £283 |
The weighted-average administration cost of £283 per administration (estimated across the unit costs for the HRG codes presented in Table 29) was adopted in scenario analyses considering people with RA who were treated with IFX (see Table 41).
Cost of testing
The costs of testing comprised the cost of the test kits, the staff time to perform a test, the cost of the testing service and the cost of sample transport. Based on the information provided by the companies and on clinical opinion, it was anticipated that minimal additional training would be required by health-care staff to use any of the testing kits that were considered in this assessment. Therefore, training costs were assumed to be negligible and were not considered in the model.
Dr McDonald advised us that laboratories that conduct TNF-α testing have previously negotiated arrangements with the manufacturers of bDMARDs to cover the cost of biological monitoring, including assays and personnel costs (Dr McDonald, Royal Devon & Exeter NHS Foundation Trust, Exeter, December 2018, personal communication). However, based on advice from Dr Jani (University of Manchester, November 2018, personal communication), that might vary by geographical area and may be relevant to certain biologics only (e.g. newer biosimilars).
The cost of reflex and concurrent testing for each assay were derived from information request documents submitted by the manufacturers of the test kits (see Appendix 15, Table 59).
In addition to assay costs, the cost of testing also includes processing costs, such as administration and laboratory personnel time; these costs were reported by Jani et al. 37 (see Appendix 16). In this study, the cost of concurrent testing of drug and antibody levels in patients who were treated with ADL and tested using Promonitor kits was estimated. The study was an audit of practice in north-west England in which the direct medical costs associated with providing the test were estimated from the NHS perspective. The costs were determined from the point of a patient who was established on treatment (for ≥ 3 months) presenting to a clinic, to the results being fed back to the clinician to inform a treatment decision.
Jani et al. 37 assumed that during the pre-testing phase (see phase 1 in Appendix 16, Table 60), one outpatient appointment with a consultant rheumatologist is required to discuss the need for testing, followed by an appointment with a phlebotomist or a clinical support worker to obtain trough blood levels. This study reported that additional costs that were associated with laboratory personnel time processing samples would be incurred during the testing phase (see phase 2 in Appendix 16, Table 60). However, it was assumed that most hospital laboratories would have the necessary room requirements and would stock standard equipment that was needed to perform ELISA, and the following items of resource use were, therefore, excluded:
-
equipment costs of centrifuge systems
-
ELISA readers
-
pipettes
-
personal protective equipment
-
phlebotomy equipment costs
-
overhead costs
-
capital costs.
In addition, the treatment decision stage (see phase 3 in Appendix 16, Table 60) would require interpretation of results by a consultant rheumatologist, discussion of the results with patients via a telephone call and, finally, a letter outlining the results and treatment decision.
The mean cost per patient per test reported in Jani et al. 37 was £152.52 (2015 prices) if 40 samples were tested simultaneously; this included the cost of the test kits. The pre-testing phase incurred the highest costs, which were driven by the cost of a phlebotomy appointment to acquire trough blood samples, which constituted 67% of the total cost; labour accounted for 10% and consumables for 23% of the total cost.
One of the minor cost components that was considered by Jani et al. 37 was ‘transport, receipt and storage of sample’, which was £2.22 (2015 prices) per batch of 40 samples (see Table 60).
Blood samples are received at the Exeter Clinical Laboratory (Royal Devon & Exeter NHS Foundation Trust) as small parcels via Royal Mail (London, UK), and it is extremely unlikely that samples would be sent to Sanquin Diagnostic Services in the Netherlands, as the transportation cost would be higher than that within the UK; moreover, sending samples abroad would lead to a longer turnaround time and take expertise out of the NHS (Dr McDonald, December 2018, personal communication).
According to the Royal Mail,92 postage costs are £4 per parcel shipped within the UK and £10 per parcel shipped to Sanquin Diagnostic Services. Based on clinical advice, it was assumed that blood samples would be posted to a laboratory within the UK and, therefore, the postage of £4 per parcel was applied.
Rosas et al. 93 reported the total number of drug and anti-drug antibody monitoring tests in RA patients who were in remission over a 2-year period (94 tests in 45 patients), which is approximately one test per patient per year. Dr Jani confirmed that in England TNF-α testing would be conducted once per year in people who are in remission/under routine follow-up; however, if tapering is performed based on drug level, a clinician would typically check the drug level at least every 6 months to ensure that the level has not dropped too low. Therefore, in the primary analyses, one TNF-α test per patient per year was assumed, while 6-monthly testing was modelled in sensitivity analyses (see Table 39).
Dr McDonald (Exeter Clinical Laboratory, Royal Devon & Exeter NHS Foundation Trust November 2018, personal communication) advised that TNF-α testing for blood and antibody levels is usually carried out concurrently; blood samples that are sent to the Exeter Clinical Laboratory are kept frozen for 1 month, and the likelihood of performing antibody testing (‘reflex testing’) 1 month after testing the trough level is extremely low.
In this unlikely scenario in which reflex testing is performed, an additional phlebotomy appointment would not be required (assuming that storage of blood samples is a common practice at test laboratories). Hence, the cost difference between reflex and concurrent testing would be defined by the proportion of patients with low or undetectable drug levels (for whom antibody testing would be requested), and the cost of telephone calls to the laboratory to request antibody testing. To estimate the cost difference between reflex and concurrent testing, the proportion of people with low drug levels was derived from Chen et al. 94 and Laine et al. 54
The authors of the former study94 investigated the impact of ADL dose-halving on therapeutic responses and drug levels in people with RA. Trough serum ADL levels were determined at baseline and at week 24 of dose-halving therapy using a sandwich ELISA (Progenika Biopharma). The minimal detectable ADL level was 0.002 mg/ml. In this study, 3 out of 64 (4.7%) participants who developed ADL antibodies at week 24 of dose-halving had very low drug levels. In these participants, trough ADL levels markedly declined to very low levels (from 2.28 mg/ml, 1.92 mg/ml and 2.21 mg/ml at baseline to, respectively, 0.024 mg/ml, 0.024 mg/ml and 0.004 mg/ml at week 24 of dose-halving).
Laine et al. 54 reported low drug levels (< 5 µg/ml) in 35.8% of people with RA who were treated with ADL from the clinical sample registry of United Medix Laboratories Ltd (Helsinki, Finland). All of the samples included in the database had been sent to the laboratory on a clinical basis (i.e. none of the samples was from clinical studies). Drug levels were measured by Sanquin Diagnostic Services.
However, there is no universal agreement of what to consider a low drug level in people with RA who are treated with biologics (Dr McDonald, personal communication). Therefore, estimates for the proportion of people with low drug levels of 4.7%94 and 35.8%54 were adopted as the lower and upper bounds in scenario analyses for reflex testing.
In Jani et al. ,37 a telephone call to discuss a treatment decision with a patient was assumed to take, on average, 5.3 minutes at a cost of £3.47. Dr McDonald (personal communication) confirmed that this would also be a reasonable cost estimate for a telephone call to a laboratory to request additional testing on stored blood.
The costs of carrying out ELISA using Promonitor kits are shown in Appendix 17, Table 61. The estimates were derived assuming single or duplicate, reflex or concurrent testing with or without a phlebotomy appointment.
Single testing incurs a lower cost than duplicate testing, but it is less precise. Therefore, duplicate testing was selected in the base-case analysis that was conducted by Jani et al;37 however, single testing is more common in the UK (Dr McDonald, personal communication). For this reason, this approach was adopted in the primary analyses and duplicate testing was modelled in scenarios.
In the primary analyses, the cost of concurrent testing using Promonitor test kits was calculated following Jani et al. ,37 that is assuming that a phlebotomy appointment to collect a trough sample would be needed (see Table 61). Scenario analyses excluding this cost were also conducted.
Cost of managing different health states
Based on published literature, active disease in people with RA is more costly to manage than disease in people in remission or LDA. The major health-care costs (apart from drug acquisition costs) relate to joint replacement surgeries, hospital stays and doctor appointments. 82
A range of classification systems and scales have been developed to measure and monitor disease activity in patients with RA, and scales commonly used to measure other domains, such as disability or activity level (e.g. HAQ), are also administered. 26 Functional capacity measured with the HAQ was found to be the strongest predictor of costs. 95 Therefore, direct medical costs for hospitalisations, joint replacements and the number of outpatient visits were included by HAQ-dependency, as explained below.
Barbieri et al. 82 reported resource utilisation in people with RA treated with IFX, stratified by four HAQ bands (see Table 62, Appendix 18). These estimates were used by the authors to calculate the costs of managing people with RA beyond the first year of therapy, and were based on data from the Norfolk Arthritis Register (NOAR). The NOAR cohort includes 1236 adults who had swelling of at least two joints that had persisted for > 4 weeks. This study reported that, on average, the number of outpatient visits, hospital days and the proportion of patients undergoing joint replacement surgery increased substantially with HAQ score (see Table 62, Appendix 18).
Average cost of an inpatient day, outpatient appointment and joint replacement surgery derived from the relevant HRG codes from the NHS Reference Costs 2017–1884 are shown in Table 30 (the derivation of the cost of surgery for RA is explained in Cost of joint replacement surgery).
| Parameter | Cost | Source |
|---|---|---|
| Outpatient attendance rheumatology | £146 | NHS Reference Costs 2017–18 |
| Inpatient day | £413 | NHS Reference Costs 2017–18: elective inpatient excess bed-day for inflammatory, spine, joint or connective tissue disorders, with CC score of 0–2 (HD23 J) |
| Joint replacement surgery | £5222 | NHS Reference Costs 2017–18: weighted average over currencies for hip and knee procedures for non-trauma – HN12 – HN14 and HN22 – HN24 |
Mean HAQ scores for different levels of disease activity (remission, LDA, MDA and HDA) in people with RA were estimated by Radner et al. 83 (Table 31): the mean HAQ score based on the SDAI was 0.39, the mean HAQ score for LDA was 0.72 and the MDA and HDA were characterised by a mean HAQ score of 1.24.
| Index | Remission | LDA | MDA/HDA | |||
|---|---|---|---|---|---|---|
| Mean score | SD | Mean score | SD | Mean score | SD | |
| SDAI | 0.39 | 0.58 | 0.72 | 0.68 | 1.24 | 0.75 |
| CDAI | 0.38 | 0.56 | 0.75 | 0.70 | 1.23 | 0.74 |
| DAS28 | 0.46 | 0.62 | 0.60 | 0.66 | 1.24 | 0.74 |
Using this classification and the cost estimates shown in Appendix 18, Table 62, the costs for managing remission (for scenario 1) and active disease (for scenario 2) were calculated from the corresponding probability density functions for HAQ scores weighted by the health management costs for different HAQ scores, whereas the costs of managing mixed-health states (LDA/active disease and remission/LDA) were derived from joint probability density functions for the relevant HAQ scores (see Appendix 18). The resulting average annual costs for managing remission, remission/LDA, LDA/active disease and active disease health states in people with RA were £902, £1089, £1483 and £1827, respectively.
Cost of joint replacement surgery
The weighted average cost of joint replacement surgery was £522284 per surgery, which was estimated from HRGs relevant to hip and knee procedures for non-trauma across all clinical codes (HN12–HN14 and HN22–HN24, respectively).
Burn et al. 96 investigated hospital reimbursement for total knee replacement (TKR) and total hip replacement (THR) surgeries in NHS England between 1997 and 2014. Primary reimbursement for TKR and THR was approximately £6000 per surgery (2016/17 prices), whereas revision surgeries were approximately £8000 per surgery. These estimates were derived from the NHS primary care records of 21,128 people with osteoarthritis or RA. The authors reported on the downward trend in the costs of TKR and THR.
The average cost of joint replacement surgery in people with RA in the Royal Devon & Exeter NHS Foundation Trust is £5061.80 (standard error £5153) (see Appendix 19). This estimate was based on 15 surgeries that were conducted between April 2017 and September 2018. Of note, this estimate is slightly lower than those from the NHS Reference Costs 2017–1884 (see Table 30 and Burn et al. 96). This might be a result of the trend in the cost of surgery reported by Burn et al. 96 However, the sample size was very low and, therefore, this estimate may not be representative of the average cost of surgery in the RA patient population in the UK.
In all analyses, the annual costs of managing different health states were derived from the average cost of joint replacement surgery based on the HRGs from the NHS Reference Costs 2017–1884 (£5222 per joint replacement surgery; see Table 30).
In the analyses presented here, it was assumed, based on clinical advice, that surgery may be performed anywhere in the treatment pathway; however, the EAG is aware that older people are more likely to require surgery for RA.
Cost of managing flares
The cost of managing flares is another important consideration that needs to be parameterised in the model. A study by Maravic et al. 80 estimated the costs associated with managing flares in people with RA in a French setting. This study focused on investigational costs and treatment costs; rheumatology appointments were not considered (see Table 64, Appendix 20).
The costs of diagnostic investigations per flare and the monthly cost of treatment (excluding bDMARDs)80 were converted to GBP based on PPP and inflated to 2017–18 prices using the HCHS pay and price index, resulting in costs of £423 and £68 for diagnostic investigations (per flare) and monthly treatment, respectively (see Table 27).
Cost of managing adverse events
In TA375,23 the weighted average cost of serious infection in RA patients was estimated to be £1479, based on relevant NHS costs97 and weighted by inpatient activity. Conservatively, HRG costs without complications and contraindications were used. This cost, inflated to 2017–18 prices using the HCHS pay and price index (£1622 per infection), was assumed in all analyses (see Table 27).
Health-related quality of life
A review of HRQoL studies was conducted to inform the selection of utilities for the economic analysis. Health-state utilities, as well as disutilities for flares and SAEs (such as severe infections), that were used in the analyses are described below.
Health state utility values
The abstracts reporting the INGEBIO study provided results on the average duration of either remission42 or remission/LDA43 in both the intervention and the control arms. However, none of the sources contain any definitions of remission.
A definition of remission was provided in Krieckaert et al. 53 In this study, health states were based on the categorisation of DAS28 as below:
-
remission – DAS28 of < 2.6
-
LDA – 2.6 ≤ DAS28 of < 3.2
-
MDA – 3.2 ≤ DAS28 of ≤ 5.1
-
HDA – DAS28 of > 5.1.
The DAS28 comprises four components: counts of tender joints and counts of swollen joints (both performed by a clinician), the visual analogue scale (VAS) score of the patient’s global health and the laboratory parameter ESR. It has been shown, however, that CRP is more accurate as an indicator of inflammation than ESR, and it is also more sensitive to short-term changes. 98 A modification of the DAS28, the DAS28-CRP,99 that includes the level of CRP instead of ESR was used in Bykerk et al. 70 to define the disease activity types below:
-
severe – DAS28-CRP of > 5.1
-
moderate –3.2 ≤ DAS28-CRP of ≤ 5.1
-
low –2.6 ≤ DAS28-CRP of < 3.2
-
remission – DAS28-CRP of < 2.6.
In the study conducted by Bartelds et al. ,55 remission was defined as a DAS28 of < 2.6 at all consecutive measurements after a certain time point, with a minimum of two scores of < 2.6 in the case of participants who discontinued treatment prematurely.
In Barnabe et al. ,100 sustained remission was defined as DAS28 of ≤ 2.6 for more than 1 year, whereas non-sustained remission was defined as a DAS28 of ≤ 2.6 for less than 1 year.
In TA375,23 non-responders, moderate responders and good responders were defined according to the EULAR response criteria (see Table 3).
There are several composite scores to assess disease activity in RA. The definitions of the disease states (i.e. remission, LDA, MDA and HDA) according to the SDAI, the CDAI and the DAS28 from Aletaha et al. 101 are presented in Table 32.
| Index | Remission | LDA | MDA/HDA |
|---|---|---|---|
| CDAI | ≤ 2.8 | ≤ 10 | ≤ 22 |
| SDAI | ≤ 3.3 | ≤ 11 | ≤ 26 |
| DAS28 | < 2.6 | < 3.2 | < 5.1 |
Radner et al. ,83 at the Medical University of Vienna in Austria, collected data on clinical and laboratory characteristics (including CRP, ESR, the number of swollen and tender joints, pain by VAS, patient’s global assessment of disease activity, evaluator’s global assessment of disease activity and physical function by HAQ) from 356 consecutive people with RA at routine clinic visits (every 3–4 months). In total, 716 visits were documented, with a median of two clinic visits per person (ranging from one to four clinic visits). 83 At baseline, 87 participants (24.4%) were in remission, 150 (42.1%) in LDA, 103 (28.9%) in MDA and 16 (4.5%) in HDA, as defined according to SDAI. Owing to the small number of participants in the HDA group, the MDA and HDA groups were combined in further analyses. The differences in functional disability measured by the HAQ scores at three levels of disease activity were evident, and similar conclusions were reached during a sensitivity analysis, when the disease states were assessed according to CDAI and DAS28 (see Table 31). Unless stated otherwise, in the remainder of this article, health states are assumed to be defined by SDAI.
The EAG is aware of several algorithms for converting the HAQ score to utility in RA, and that the estimates of utilities may vary when different mapping algorithms are used. 102 In TA375,23 a comparison of published relationships between utility and HAQ was conducted (see figure 115 in Stevenson et al. 34). Three of the eight compared studies reported data from the UK. Of these three studies, Bansback et al. 103 included data for UK and Canadian patients, and Kobelt et al. 104 included data for patients in the UK and Sweden; therefore, these were not considered relevant for the purposes of this analysis. Hurst et al. 105 included people with RA in Scotland only. Malottki et al. 59 used the data set from Hurst et al. 105 to estimate the coefficients of their mapping equation; therefore, there is little difference between their estimates, despite different algorithms being used.
Throughout this monograph, the EQ-5D utility values were mapped from the HAQ scores using the same formula as in Malottki et al. :59
where a = 0.804, b1 = 0.203 and b2 = 0.045.
Hernández Alava et al. 106 argued that pain should be included as an explanatory variable when estimating QALYs from HAQ scores in people with RA. This approach was used in TA375. 23 However, the estimates presented in this article were obtained without pain scores because the EAG did not have access to patient-level data. Table 33 presents the EQ-5D utility values mapped from the HAQ scores at three levels of disease activity from Radner et al. 83
| Index | Remission | LDA | MDA/HDA | |||
|---|---|---|---|---|---|---|
| Mean score | Range | Mean score | Range | Mean score | SD | |
| SDAI | 0.718 | 0.565–0.804 | 0.635 | 0.432–0.796 | 0.483 | 0.222–0.694 |
| CDAIa | 0.720 | 0.573–0.804 | 0.626 | 0.415–0.794 | 0.486 | 0.229–0.694 |
| DAS28a | 0.701 | 0.532–0.804 | 0.666 | 0.477–0.804 | 0.483 | 0.226–0.691 |
Ucar et al. 42 reported the mean duration of remission in the intervention and control arms. In the economic analysis based on this source, the EQ-5D utility value for remission, 0.718, was applied. The utility value of 0.568 used for a mixed-disease state (LDA/active disease) was approximated by the average of the estimates for LDA and MDA/HDA weighted by the proportion of patients in each health state from Radner et al. 83 Of note, when the weighted average of HAQ scores was computed instead and mapped to EQ-5D, the utility value was very similar, 0.571.
As the health states in Arango et al. 43 (remission/LDA and active disease) were defined differently from those in Ucar et al. ,42 in analyses based on the former source,43 the EQ-5D utility score of 0.483 for MDA/HDA was used as the utility value for active disease health state, and the weighted average of the estimates for remission and LDA, 0.665, was used to approximate the utility value for the mixed-health state. When the alternative approach (described above) was used, the resulting utility value was 0.666.
Health state utility values (HSUVs) obtained from HAQ scores reported in Stevenson et al. 34 (as described in the following section were assumed in scenario analyses.
In TA375,23 the model was based on a EULAR response category (good/moderate/none) to be consistent with the NICE guidance on biologics in RA36 and to align more closely with UK clinical practice in terms of the assessment of response to therapies. The HAQ scores were estimated from the BSRBR-RA database,67 which contains values measured at 6-month intervals for up to 3 years for all people with RA on the register. The analysis conducted in TA37523 was restricted to those with the full set of baseline characteristics and at least two additional HAQ measurements while on bDMARDs. The database included data from 10,186 patients. Of these, 2417, 5492 and 2277 were classed as EULAR good responders, moderate responders and non-responders, respectively (see Table 3).
Figure 6 shows the HAQ trajectory in people with RA treated with bDMARDs. It was observed that the mean HAQ scores for patients with good, moderate or no response (according to the EULAR response criteria shown in Table 3) decreased during the first 6 months after the start of biological therapy (when the magnitude of decrease grows with the level of EULAR response), stabilised at around 6 months and remained rather flat over the remaining 2.5 years of measurement.
FIGURE 6.
Mean HAQ score by EULAR response category for patients receiving biologics. Reproduced from Stevenson et al. 34 Contains information licensed under the Non-Commercial Government Licence v2.0, which is available at www.nationalarchives.gov.uk/doc/non-commercial-government-licence/version/2/.
The HAQ scores that were measured after 6 months of therapy with biologics for all three categories of responders were mapped to EQ-5D utilities, which elicited the values shown in Table 34.
| Type of patient | Number of patients in the BSRBR-RA dataset | HAQ score | Utility |
|---|---|---|---|
| Non-responder | 2277 | 1.95 | 0.237 |
| Moderate responder | 5492 | 1.7 | 0.329 |
| Good responder | 2417 | 1.2 | 0.496 |
The utility for the remission health state was based on the utility value for good responders (0.496, see Table 34), whereas the utility for the LDA/active disease health state was estimated as the average of utility values for moderate responders and non-responders weighted by their proportions in the BSRBR-RA database, resulting in the utility value of 0.302. These HSUVs were used in sensitivity analyses.
Disutility of flare
The values of utility losses owing to flares were obtained from the Dutch multicentre clinical study ‘BeSt’,72 which involved 508 participants who were treated to target for 10 years to achieve a DAS28 of, at most, 2.4 (follow-up data that suffice to establish presence or absence of a flare during at least a single visit were available for only 480 patients). 72 The BeSt study72 considered three types of flares, which were named as ‘A’, ‘minor B’ and ‘major B’ (where ‘major B’ is a subcategory of ‘A’), with the number of occurrences of each (observed during a total of 11,458 rheumatology visits of all patients) shown in Appendix 21, Figure 10, and the definitions, frequencies and HAQ scores of each described in Table 35. The mean HAQ score of patients with no flare at a visit was estimated as 0.53 (SD 0.56).
| Type of flare | DAS28 | HAQ score | Utility scorea | Disutility | |||||
|---|---|---|---|---|---|---|---|---|---|
| Current | Previous | Increase | Mean | SD | Mean – SD | Mean | Mean + SD | ||
| Ab | > 2.4 | Any | ≥ 0.6 | 1.04 | 0.63 | 0.339 | 0.544 | 0.713 | –0.140 |
| Minor Bc | > 2.4 | ≤ 2.4 | < 0.6 | 0.85 | 0.55 | 0.432 | 0.599 | 0.739 | –0.085 |
| Major Bd | > 2.4 | ≤ 2.4 | ≥ 0.6 | 0.96 | 0.60 | 0.378 | 0.568 | 0.725 | –0.116 |
Functional mobility of patients with these types of flares was measured using HAQ scores (mean and SD values are also included in Table 35). The loss of QALYs was computed as the difference between the mapped HSUVs of patients with each type of flare and the mapped HSUVs of patients in the absence of flares. The estimated disutility values are shown in Table 35.
Disutility of serious adverse events
People with RA have increased susceptibility to serious infections owing to the features of RA, comorbidity and immunosuppressive treatment. 107 It has been shown that TNF-α inhibitors increase the risk of serious infection up to two-fold. 108 The EuroQol-5 Dimensions three-level version (EQ-5D-3L) disutility value for England of 0.156 over 4 weeks (equivalent to the loss of QALYs of 0.012) that is associated with severe infections was reported in the observational study ‘Genomics to combat Resistance against Antibiotics in Community-acquired lower respiratory tract infections (LRTI) in Europe’ (GRACE) of the management of patients with acute cough/LRTI in primary care. 85 Data were collected in 13 European countries (including England and Wales) from adults (aged ≥ 18 years) who reported to their primary care clinicians with cough and LRTI. 85 EQ-5D-3L scores were generated using the country-specific UK value set, in which the original data were collected from non-institutionalised adults in England, Scotland and Wales (with a total of 2997 participants) between August and December 1993.
The effect of SAEs on costs and QALYs was modelled in the primary analyses. It should be noted, however, that in the analyses, assuming that TDM affects the duration of remission/LDA and the rates of flares and AEs, there is a risk of double-counting the effect of flares and AEs on HRQoL given that it is possible that the disutilities have already been incorporated into health-state utility values.
Consistency between utility values
As shown in Gülfe et al. ,109 there may be discrepancies between utility values that are measured in different countries (in our case Spain, Austria, the Netherlands and the UK), which may occur owing to differences in distinct preference sets for those countries. Figure 11 (see Appendix 21) shows EQ-5D-3L scores obtained using British and Swedish preference sets for people with established RA being treated with TNF-α inhibitors.
The population considered in the INGEBIO study was mixed. This trial recruited 169 people, 63 with RA (37.3%), 54 with PsA (32%) and 52 with AS (30.8%). Gülfe et al. 110 also studied a mixed population, with two (RA and PsA) out of the three diseases the same as in the INGEBIO study; the third disease was SpA, which is usually considered as a phenotypically heterogeneous disease with PsA and AS as its best-studied manifestations. 111 One of the aims of this study110 was to analyse trends in health utilities in people diagnosed with three types of arthritis: 2554 people with RA (who constituted 68.8% of the total population), 574 with PsA (15.5%) and 586 with SpA (15.8%), who started treatment with TNF-α inhibitors. Data for the period from May 2002 to December 2008 were obtained from the Southern Sweden Arthritis Treatment Group register, which was set up in 2002 and collects health utility data from routine clinical follow-up. Treatment courses are classified as first, second or third or further TNF-α inhibitor. Among the three subpopulations, people with RA were typically older, had tried more DMARDs, were more often treated with a concomitant DMARD and were more often female than the other populations. Figure 12 (see Appendix 21) shows similar response patterns in people with RA, PsA and SpA at 6 months after the start of the first TNF-α inhibitor treatment course. 110
In Arango et al. ,43 19 patients who discontinued treatment were excluded from the analysis, although those patients were included in the ITT analysis reported in Ucar et al. 42 As shown in Gülfe et al. ,110 RA patients who terminated therapy for any reason had demonstrated lower utility gain by the time of withdrawal, which is illustrated in Appendix 21, Figure 13.
Although the use of all available data increases the generalisability of the study, it may also lead to lower utility estimates than when using data for only those participants for whom complete follow-up information is available (see Figure 14), as incomplete records may be a result of, for example, withdrawals from treatment owing to adverse effects of the intervention.
Of note, the utility values reported in this section were not used in the economic analyses.
Mortality
Although there is evidence of an association between HAQ improvement and reduced mortality risk, the impact of TNF-α testing on mortality was not considered owing to the short-term time horizon adopted in this study and the relatively small difference in the mean duration of remission42 and remission/LDA43 across the treatment arms in INGEBIO.
Checking the model for wiring errors
The model written in Microsoft Excel® 2013 (Microsoft Corporation, Redmond, WA, USA) was checked in the following way: all calculations were performed by one person and were checked by another person.
Results
Primary analyses: adalimumab (Humira) and Promonitor
Threshold analyses
Threshold analyses were conducted for both Ucar et al. 42 and Arango et al. (Table 36). 43
| ICER threshold (£) | Threshold values for the cost of testing for different ADL acquisition costs (per patient-year) (£) | |||
|---|---|---|---|---|
| Scenario 1 (based on Ucar et al.42) | Scenario 2 (based on Arango et al.43) | |||
| 1000 | 9187 | 1000 | 9187 | |
| 20,000 | 197 | 225 | –28 | 18 |
| 30,000 | 246 | 274 | –73 | –28 |
The results suggest that, if the outcomes reported in Ucar et al. 42 are used, then, under the list price of Humira, the cost of testing per patient would need to be less than £225 per year in order for TDM to be judged as a cost-effective option at the thresholds of £20,000 per QALY gained; for the threshold of £30,000 per QALY gained, the cost of testing should be below £274 per patient-year. For the lower bound, with the annual acquisition cost of £1000 per patient-year, the corresponding threshold values for the cost of testing were £197 and £246 per patient-year.
For the outcomes reported in Arango et al. 43 and at the list price of Humira, the cost of testing should not exceed £18 per year to be considered as cost-effective at the threshold of £20,000 per QALY gained. However, the other threshold values obtained for outcomes reported in this source were negative (see Table 36). This means that, when using the trial results as presented in Arango et al. ,43 there are no (positive) values of the cost of testing at which it would be a cost-effective option at £30,000 per QALY gained, as well as for the lower ADL acquisition cost of £1000 per patient-year.
The qualitatively different results obtained in the threshold analyses can be explained by the difference in the mean duration of remission42 and remission/LDA43 between the control and the intervention arms. As reported in Arango et al. ,43 patients from the control group were in remission/LDA for longer, on average, than patients in the intervention group (475.2 days vs. 460.2 days), whereas Ucar et al. 42 reported a longer duration of remission in patients in the intervention group than in the control group (344 days vs. 329 days).
The results of the threshold analyses are inconclusive for two reasons: they are inconsistent and they are based on very small and uncertain differences in outcomes, with the incremental QALYs of < 0.01.
Cost-effectiveness analyses
As in the threshold analyses, economic results were obtained for outcomes from both reports of the INGEBIO study. 42,43 The incremental costs and QALYs for testing versus SOC (Table 37) were estimated assuming that:
-
patients are treated with Humira and are tested regularly using Promonitor assays
-
the frequency of testing is one test per patient per year
-
testing of drug and antibody levels is carried out concurrently (single dilution) at a UK laboratory
-
the other testing costs are as reported in Jani et al. 37
| Intervention | Control | Intervention vs. control | |
|---|---|---|---|
| Scenario 1 (based on Ucar et al.42) | |||
| Costs (£) | |||
| Drug acquisition | 12,078 | 12,120 | –42 |
| Drug administration | 0 | 0 | 0 |
| Drug wastage | 486 | 488 | –2 |
| Cost of managing health states | 1503 | 1527 | –24 |
| Cost of flare management | 281 | 388 | –107 |
| Cost of managing AEs | 64 | 64 | 0 |
| Cost of phlebotomy appointment | 162 | 0 | 162 |
| Other costs of testing | 45 | 0 | 45 |
| Cost of sample transport | 6 | 0 | 6 |
| Total costs (mean) | 14,625 | 14,587 | 38 |
| QALYs | |||
| Remission | 0.676 | 0.647 | 0.029 |
| LDA/active disease | 0.250 | 0.274 | –0.023 |
| Flares | –0.002 | –0.002 | 0.001 |
| AEs | 0.000 | 0.000 | 0.000 |
| Total QALYs (mean) | 0.924 | 0.918 | 0.007 |
| ICER (cost per QALY gained) | £5575 | ||
| Scenario 2 (based on Arango et al.43) | |||
| Costs (£) | |||
| Drug acquisition | 13,075 | 13,149 | –74 |
| Drug administration | 0 | 0 | 0 |
| Drug wastage | 527 | 530 | –3 |
| Cost of managing health states | 1794 | 1764 | 30 |
| Cost of flare management | 303 | 418 | –115 |
| Cost of managing AEs | 69 | 70 | 0 |
| Cost of phlebotomy appointment | 162 | 0 | 162 |
| Other costs of testing | 45 | 0 | 45 |
| Cost of sample transport | 6 | 0 | 6 |
| Total costs (mean) | 15,981 | 15,930 | 51 |
| QALYs | |||
| Remission/LDA | 0.838 | 0.865 | –0.027 |
| Active disease | 0.112 | 0.092 | 0.020 |
| Flares | –0.002 | –0.003 | 0.001 |
| AEs | –0.001 | –0.001 | 0.000 |
| Total QALYs (mean) | 0.947 | 0.954 | –0.007 |
| ICER (cost per QALY gained) | SOC dominant | ||
As shown in Table 37, the major cost components in both the intervention and the control arms were the drug acquisition costs and the costs of managing health states, whereas the incremental costs were mostly driven by the cost of the initial phlebotomy appointment and the cost of managing flares. The incremental QALYs, defined primarily by QALYs accrued in different health states, were very small (of the order < 0.01). The ICER in scenario 1 (based on Ucar et al. 42) was £5575 per QALY gained, whereas in scenario 2 (based on Arango et al. 43) the results suggest that SOC dominated the intervention.
The results of the cost–utility analyses are inconclusive: using data from Ucar et al. 42 and Arango et al. 43 produced qualitatively different results, which were based on very small and uncertain differences in outcomes (with incremental QALYs of < 0.01).
Sensitivity analyses: adalimumab (Humira) and Promonitor
A number of sensitivity analyses were undertaken to explore the impact of parametric and structural uncertainty on the cost-effectiveness outcomes reported in Table 37.
One-way deterministic sensitivity analyses
Uncertainty in some of the parameters that were used to estimate the ICERs in scenario 1 and scenario 2 (detailed in Table 37) was evaluated in one-way deterministic sensitivity analyses (Table 38).
| Parameter | Assumption | Incremental costs | Incremental QALYs | ICER | Source |
|---|---|---|---|---|---|
| Percentage of people in whom the biologic was tapered | +20% in the intervention arm and −20% in the control arm | –£193 | –0.007 | £28,570 | Arango et al.43 |
| Flare rate | −20% in the intervention arm and +20% in the control arm | –£93 | –0.006 | £15,867 | Arango et al.43 |
| Time in remission/LDA | +10% in the intervention arm and −10% in the control arm of the differential time in remission/LDA | £52 | –0.006 | SOC dominant | Arango et al.43 |
| Costs of managing health states | −20% | £45 | –0.007 | SOC dominant | Arango et al.,43 Barbieri et al.82 and Radner et al.83 |
In the analysis, assuming a 20% increase and 20% decrease in the proportion of patients on tapered doses in the intervention and control arms, respectively, the intervention was less costly and less effective, with the ICER of £28,570 per QALY gained located in the south-west quadrant of the cost-effectiveness plane.
Reducing the flare rate in the intervention arm by 20% and increasing it by the same amount in the control arm resulted in negative incremental costs and QALYs (see Table 38), with an ICER of £15,867 per QALY gained.
When the costs of managing health states were reduced by 20%, SOC was dominant.
The same outcome was obtained when the time in remission/LDA in the intervention and control arms was varied by +10% and –10% of the differential time in remission/LDA across the treatment arms, respectively.
Probabilistic sensitivity analyses
Probabilistic sensitivity analyses were not conducted because of time constraints and the lack of clarity as to which model assumptions would be most relevant to the NHS, owing to a substantial variation in clinical practice with respect to disease management in people with RA, as well as uncertainty in the TNF-α testing strategies (given that therapeutic monitoring for RA is not currently part of NHS practice). These variations were explored in numerous clinically relevant scenario analyses detailed in the following sections.
Scenario analyses
Impact of therapeutic drug monitoring on flare rate only
In the sensitivity analyses, assuming that TNF-α monitoring affects the rate of flares only in patients treated with biologics (as in Gavan17), the ICERs in scenario 142 and scenario 243 were £95,070 and £29,599 per QALY, respectively (see Table 39).
When this assumption was implemented in exploratory analyses for the other TNF-α inhibitors (see Exploratory analyses: etanercept or infliximab and Promonitor and Table 41), ICERs were either very close to £30,000 per QALY gained or well above this cost-effectiveness threshold.
Impact of the cost of the initial phlebotomy appointment
Scenario analyses were conducted that assumed that trough samples are taken at the time of existing doctor appointments (i.e. a phlebotomy appointment would not be required). The costs for reflex or concurrent, single or duplicate testing implemented in these analyses are shown in Table 61. In scenarios with reflex testing, it was assumed that the proportion of patients who would need to undergo antibody testing was either 4.7% or 35.8% (see Reflex versus concurrent testing).
When the cost of phlebotomy appointments was implemented together with the other assumptions on testing (as described above), the ICERs were under £20,000 per QALY gained in all analyses for Ucar et al. ,42 whereas SOC dominated the intervention in the analyses parameterised from Arango et al. 43 (Table 39).
However, when this cost was excluded, TDM dominated SOC in all analyses based on Ucar et al. ,42 whereas the intervention was less costly and produced fewer QALYs than SOC in all analyses for Arango et al. 43 (see Table 39), with ICERs of under £20,000 per QALY gained.
| Sensitivity analysis | Assumptions | Scenario 1 (based on Ucar et al.,42) | Scenario 2 (based on Arango et al.,43) | Source/relevant sections | ||||
|---|---|---|---|---|---|---|---|---|
| Incremental costs | Incremental QALYs | ICER | Incremental costs | Incremental QALYs | ICER | |||
| Impact of TDM on flares only | Only flares contribute to incremental costs and QALYs | £62 | 0.001 | £95,070 | £21 | 0.001 | £29,599 | Scenario C (see Gavan17) |
| Cost of testing | ||||||||
| Duplicate concurrent testing with initial phlebotomy appointment | See Table 62 | £64 | 0.007 | £9405 | £77 | –0.007 | SOC dominant | Jani et al.37 (see Cost of testing) |
| Duplicate reflex testing without initial phlebotomy appointment, 35.8% of patients with LDLa,b | See Table 62 | –£114 | 0.007 | TDM dominant | –£101 | –0.007 | £14,929 | Jani et al.37 (see Cost of testing) |
| Duplicate reflex testing with initial phlebotomy appointment, 35.8% of patients with LDLb | See Table 62 | £48 | 0.007 | £7037 | £61 | –0.007 | SOC dominant | Jani et al.37 (see Cost of testing) |
| Single reflex testing without initial phlebotomy appointment, 35.8% of patients with LDLa,b | See Table 62 | –£132 | 0.007 | TDM dominant | –£119 | –0.007 | £17,547 | Jani et al.37 (see Cost of testing) |
| Single reflex testing with initial appointment, 35.8% of patients with LDLb | See Table 62 | £30 | 0.007 | £4436 | £43 | –0.007 | SOC dominant | Jani et al.37 (see Cost of testing) |
| Duplicate concurrent testing without initial phlebotomy appointmenta | See Table 62 | –£98 | 0.007 | TDM dominant | –£85 | –0.007 | £12,544 | Jani et al.37 (see Cost of testing) |
| Duplicate reflex testing without initial phlebotomy appointment, 4.7% of patients with LDLa,c | See Table 62 | –£124 | 0.007 | TDM dominant | –£111 | –0.007 | £16,465 | Jani et al.37 (see Cost of testing) |
| Duplicate reflex testing with initial phlebotomy appointment, 4.7% of patients with LDLa,c | See Table 62 | £38 | 0.007 | £5511 | £50 | –0.007 | SOC dominant | Jani et al.37 (see Cost of testing) |
| Single concurrent testing without initial phlebotomy appointmenta | See Table 62 | –£124 | 0.007 | TDM dominant | –£111 | –0.007 | £16,401 | Jani et al.37 (see Cost of testing) |
| Single reflex testing without initial phlebotomy appointment, 4.7% of patients with LDLa,c | See Table 62 | –£138 | 0.007 | TDM dominant | –£125 | –0.007 | £18,484 | Jani et al.37 (see Cost of testing) |
| Single reflex testing with initial appointment, 4.7% of patients with LDLc | See Table 62 | £24 | 0.007 | £3506 | £37 | –0.007 | SOC dominant | Jani et al.37 (see Cost of testing) |
| Proportion of flared patients on tapered dose in whom full dose is restored | 55% | £76 | 0.007 | £11,107 | £62 | –0.007 | SOC dominant | Bykerk et al.70 |
| 0% | £122 | 0.007 | £17,872 | £75 | –0.007 | SOC dominant | Assumption | |
| Frequency of testing (tests per patient-year) | 2 | £250 | 0.007 | £36,756 | £263 | –0.007 | SOC dominant | Rosas et al.93 and clinical advice (see Frequency of testing) |
| Tapering strategy | Spacing: reduction of ADL dose to 40 mg every 4 weeks | £16 | 0.007 | £2369 | £12 | –0.007 | SOC dominant | Second dose reduction in the Exeter Biologic Clinic recommendations (see Appendix 13) |
| Treatment wastage | No wastage | £40 | 0.007 | £5823 | £54 | –0.007 | SOC dominant | Assumption |
| Flare duration (days) | 19 | £31 | 0.008 | £3966 | £44 | –0.006 | SOC dominant | Weighted average based on Bykerk et al.70 and clinical advice |
| Utilitiesd | £38 | 0.009 | £4406 | £51 | –0.007 | SOC dominant | Estimated from HAQ scores reported in Stevenson et al.34 (figure 110) see Health state utility values estimated from the Health Assessment Questionnaire by European League Against Rheumatism response category | |
| Disutility of flare | 0.085 | £38 | 0.007 | £5793 | £51 | –0.007 | SOC dominant | Minor B type of utility (Table 35, see Disutility of flare) |
| 0.116 | £38 | 0.007 | £5668 | £51 | –0.007 | SOC dominant | Major B type of utility (see Table 35, see Disutility of flare) | |
Proportion of flared patients on tapered doses, whose treatment dose would be restored to full
A US study70 reported statistics on flare that showed that at least 45% of treatment strategies for coping with flares did not involve a dose increase or any other change of medication. Dr Haigh (our clinical advisor) (Royal Devon & Exeter NHS Foundation Trust, Exeter, 2018, personal communication) confirmed that in about only two-thirds of all flared patients on tapered doses would the dose be switched back to full.
Therefore, the effect of the flare management strategy outlined in Bykerk et al. ,70 that is the assumption that in only 55% of flared patients would the dose of ADL be fully restored, was evaluated. Another assumption, that all patients who flared while on tapered doses would stay on the same dose,112,113 was also tested. The resulting ICERs were under £20,000 per QALY gained in the analyses for scenario 1,42 whereas SOC dominated TDM in the analyses for scenario 2 (see Table 39). 43
The number of tumour necrosis factor alpha tests per patient-year
Under the assumption of 6-monthly testing, SOC was dominant in scenario 2 and the ICER in scenario 1 was £36,756 per QALY gained (see Table 39).
Discounts for the price of Humira®
One-way deterministic sensitivity analyses were conducted based on data from Ucar et al. 42 and Arango et al. ,43 in which the Humira acquisition cost was reduced by 20–80% (Table 40).
| Humira® acquisition cost discount | Intervention vs. control | ICER (£) | |
|---|---|---|---|
| Incremental costs (£) | Incremental QALYs | ||
| Ucar et al.42 | |||
| 20% | 47 | 0.007 | 6857 |
| 40% | 55 | 0.007 | 8139 |
| 60% | 64 | 0.007 | 9421 |
| 80% | 73 | 0.007 | 10,703 |
| Arango et al.43 | |||
| 20% | 66 | −0.007 | SOC dominant |
| 40% | 81 | −0.007 | SOC dominant |
| 60% | 97 | −0.007 | SOC dominant |
| 80% | 112 | −0.007 | SOC dominant |
Regardless of the assumed reduction in the ADL acquisition cost, SOC was estimated to dominate the intervention when data from Arango et al. 43 were used, whereas the ICERs in the analyses based on Ucar et al. 42 were under £20,000 per QALY gained (see Table 40).
Discounts for the price of Promonitor assays
The costs for the Promonitor test kits assumed in the economic analyses are shown in Appendix 15. Grifols–Progenika also offers price discounts, which depend on the uptake of testing, single or duplicate testing, concurrent or reflex, with different number of tests per year. Therefore, additional cost–utility analyses for the levels of discounts proposed by the company were also conducted (the results are not reported here).
Other scenario analyses
The other sensitivity analyses conducted are listed below:
-
tapering strategy of dose halving (see Dose tapering)
-
cost of treatment wastage assumed to be zero (see Treatment wastage)
-
mean flare duration of 19 days (see Duration of flare)
-
health-state utilities estimated from TA375 (see Health state utility values estimated from the Health Assessment Questionnaire by European League Against Rheumatism response category)
-
disutilities for major B and minor B flares as defined by Markusse et al. 72 (see Disutility of flare).
The resulting ICERs were under £20,000 per QALY gained in all analyses for scenario 1 (based on Ucar et al. 42), whereas SOC dominated the intervention in all analyses for scenario 2 (parameterised from Arango et al. 43) (see Table 39).
Exploratory analyses: etanercept or infliximab and Promonitor
The cost-effectiveness of TNF-α testing in RA patients treated with the ETN originator product (Enbrel) or its biosimilar (Erelzi), or IFX biosimilars (Flixabi or Renflexis), using Promonitor test kits was evaluated in exploratory analyses. Information on the actual costs to the NHS of these TNF-α inhibitors was not available to the EAG at the time of writing and, therefore, the list prices of the biologics were assumed.
Based on the list prices (see Table 28), Enbrel has the highest acquisition cost per patient-year among the TNF-α inhibitors that are administered subcutaneously, whereas Erelzi has the lowest cost. Therefore, by considering these two treatments, we covered the whole spectrum of acquisition costs of the TNF-α treatments with subcutaneous route of administration. Flixabi and Renflexis have the lowest acquisition cost among the treatments administered intravenously (see Table 28). However, these biologics incur substantial administration costs (as described in Drug administration) and, therefore, it was important to evaluate the impact of intravenous administration on the cost-effectiveness of TDM.
In these exploratory analyses, the clinical effectiveness of TDM in RA patients who were receiving the TNF-α inhibitors (including their biosimilars) was assumed to be the same, as was the performance of the Promonitor assays when measuring drug and antibody levels for different biologics; these simplified assumptions were made owing to lack of evidence. Therefore, the clinical outcomes from Ucar et al. 42 and Arango et al. 43 were adopted with all model assumptions, except the acquisition and administration costs and the cost of treatment wastage, as shown in Table 27. The results are presented in Table 41.
| Treatment | Scenario 1 (based on Ucar et al.42) assuming that TDM affects | Scenario 2 (based on Arango et al.43) assuming that TDM affects | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rates of flares and AEs, and the duration of remission | Rates of flares only | Rates of flares and AEs, and the duration of remission/LDA | Rates of flares only | |||||||||
| Incremental costs (£) | Incremental QALYs | ICER (£) | Incremental costs | Incremental QALYs | ICER (£) | Incremental costs (£) | Incremental QALYs | ICER | Incremental costs (£) | Incremental QALYs | ICER (£) | |
| ETN | ||||||||||||
| Enbrela | 37 | 0.007 | 5477 | £61 | 0.001 | 94,052 | 50 | –0.007 | SOC dominant | 20 | 0.001 | 27,944 |
| Erelzi | 42 | 0.007 | 6128 | £66 | 0.001 | 100,845 | 57 | –0.007 | SOC dominant | 27 | 0.001 | 38,981 |
| IFXb | ||||||||||||
| Flixabi or Renflexis (assuming no vial wastage) | 49 | 0.007 | 7144 | £73 | 0.001 | 111,450 | 69 | –0.007 | SOC dominant | 40 | 0.001 | 56,212 |
As in the previous analyses, the outcomes were dependent on the evidence used for model parameterisation: SOC was dominant when the clinical outcomes were taken from Arango et al. ,43 whereas the results based on Ucar et al. 42 signified that the intervention was likely to be cost-effective, with ICERs well under £20,000 per QALY gained.
Importantly, when assuming that TDM solely affects flare rate, the ICER for Enbrel was slightly under £30,000 per QALY in the analysis using the data from Arango et al. ,43 whereas in all other analyses ICERs exceeded this threshold significantly (see Table 41).
Other scenario analyses considered but not conducted owing to a lack of clinical data were analyses of testing in the context of primary or secondary non-response, and analyses for non-responders who did not adhere to treatment with the biological therapies, including switching to intravenously administered IFX.
Consideration of a publication by l’Ami et al.
An addendum was produced in response to a request from the NICE technical team for an exploratory analysis that considered a scenario in which the drug dose in the standard care arm was not reduced (or reduced less than in the intervention arm). This was requested because, during scoping for the appraisal, the stakeholders indicated that dose reductions are currently not part of routine care in large parts of the UK. The NICE technical team requested that the EAG consider using data from l’Ami et al. 114
The study was identified in the searches for the clinical effectiveness systematic review but did not meet the inclusion criteria specified in the protocol, and was excluded on comparator because the physicians in the control arm had knowledge of drug and anti-drug antibody levels to make their judgements (see Appendix 22).
Analyses based on additional evidence provided by Grifols–Progenika
After the original EAG’s report had been submitted to NICE, Grifols–Progenika provided additional evidence from INGEBIO on the average number of days in remission for the same follow-up period as in Arango et al. 43 Analyses based on this evidence were conducted by the EAG (see Appendix 23). The results suggest that the intervention dominated SOC.
Exploratory analyses based on the INGEBIO full study report provided by Grifols–Progenika
Exploratory analyses considering additional evidence, the INGEBIO full study report provided by Grifols–Progenika, were conducted (see Appendix 24).
When the company’s modelling approach was used, depending on the model assumptions, the intervention was either dominant or cost-effective at the threshold of £20,000 per QALY gained (see Tables 73 and 74). However, when the updated EAG model was utilised, results varied from the intervention being dominant to ICERs exceeding £160,000 per QALY gained, located in the north-east quadrant of the cost-effectiveness plane (see Tables 77–80).
Discussion
The results of the primary, sensitivity and exploratory analyses suggest that the cost-effectiveness of TDM versus SOC in RA patients receiving TNF-α inhibitors is highly uncertain. Data from two reports of the same study (INGEBIO) produced inconsistent conclusions on the cost-effectiveness of Promonitor ELISA testing in RA patients in remission or LDA, receiving ADL treatment.
In the primary cost–utility analyses (assuming one test per year carried out concurrently at a UK laboratory, with one phlebotomy appointment per test), SOC was found to be dominant based on the longer follow-up,43 whereas using data for the shorter follow-up42 produced the ICER of £5575 per QALY gained.
The intervention dominated SOC in scenario analyses that excluded the cost of phlebotomy appointments, which were based on Ucar et al. ,42 and was likely to be cost-effective in those sensitivity analyses parameterised from Arango et al. 43 When the cost of phlebotomy appointments was factored in, the intervention was either dominated by SOC or likely to be cost-effective, depending on the data source used. 42,43
Under the assumption of 6-monthly testing, SOC dominated the intervention in the analysis based on Arango et al. 43 and the ICER for Ucar et al. 42 was £36,756 per QALY gained.
When assuming that the rate of flares alone is affected as a consequence of monitoring, the ICERs were £95,070 and £29,599 per QALY gained depending on the data source used (Ucar et al. 42 or Arango et al. ,43 respectively). In the former scenario, TDM was highly unlikely to be cost-effective, whereas in the latter the ICER of TDM was only slightly under the WTP of £30,000 per QALY gained.
In the majority of other sensitivity analyses conducted, ICERs were under £20,000 per QALY gained when estimated from Ucar et al. ,42 whereas SOC dominated the intervention in all analyses parameterised from Arango et al. 43
In the exploratory analyses based on the INGEBIO full study report, the outcomes were also inconsistent and varied from the intervention being dominant to ICERs exceeding £160,000 per QALY gained.
Therefore, based on available evidence, the economic results are inconclusive and suggest that there is considerable uncertainty in the cost-effectiveness of TDM in RA patients in England and Wales.
Chapter 5 Discussion
Statement of the principal findings
Clinical effectiveness
Two studies (reported in four publications42–45) were included in the systematic review of the evaluation of using ELISA tests for TDM on clinical outcomes in RA patients who had achieved either remission or LDA, or experienced a primary or a secondary non-response. Three articles42,43,45 reported the same non-randomised controlled trial (the INGEBIO study). The remaining study44 was observational. The non-randomised controlled study42,43,45 was judged to be at a serious risk of bias. The observational study44 had a historical control and was judged to be at a moderate risk of bias. However, the study design should be taken into consideration in interpreting the risk-of-bias assessment (non-randomised controlled study vs. observational study).
The INGEBIO study used Promonitor ELISA kits to monitor levels of drug and anti-drug antibody, whereas the study by Pascual-Salcedo et al. 44 used Sanquin ELISA kits. Drug and anti-drug antibody levels were measured in RA patients treated with ADL, ETN or IFX. No studies in people being treated with CTZ or GLM were identified. No studies evaluating eligible ELISA kits, including IDKmonitor, LISA-TRACKER, RIDASCREEN and MabTrack, were found. Both studies (INGEBIO and Pascual-Salcedo et al. 44) included individuals in remission, with the INGEBIO study also including individuals with LDA (at baseline).
Comparative controlled evidence
Three articles42,43,45 reported the same non-RCT (the INGEBIO study), which focused on the population who had achieved treatment target (remission or LDA). In this trial, ADL and anti-ADL antibody levels were measured using Promonitor-ADL and Promonitor-ANTI-ADL (Grifols–Progenika) ELISA kits. TDM results were revealed to physicians in the intervention arm but not to physicians in the control arm. This reflected standard care in Spain, where treatment decisions are based on clinical judgements without knowledge of levels of drug and anti-drug antibodies in patients. The INGEBIO study recruited a mixed population of 169 people, including a cohort of 63 people with RA. The results of the total mixed population were reported in the review, as the authors were not able to separately provide the results for the cohort of people with RA. The three cohorts with different conditions (RA, PsA and AS) may have different treatment responses to TNF-α inhibitor therapies. Therefore, there was limited generalisability of findings from this mixed population to the target RA population.
The findings from this trial42 showed that, at 18-month follow-up, the rate of flares per patient-year was 0.463 for the intervention group and 0.639 for the control group, with a rate difference of –0.176 (95% CI –0.379 to 0.0289). There was a non-significant reduction in risk of flare in the intervention group compared with the control group (IRR 0.7252, 95% CI 0.4997 to 1.0578). The median time to the first flare was 145 days for participants in the intervention group and 136.5 days for participants in the control group. This trial42 also presented the results of HRQoL outcomes. The results showed that HRQoL (EQ-5D-5L) measures were higher in the intervention group than in the control group at all visits. However, statistically significant results were observed only at visit 2 (p = 0.001) and visit 3 (p = 0.035). Further details of results for this outcome were not reported.
Overall, the findings from this non-randomised controlled trial (the INGEBIO study) showed that there was a non-significant reduction in the risk of flare in the intervention group (i.e. when treatment decisions were made on the basis of the results of TDM) compared with the control group (i.e. standard care, when treatment decisions were based on clinical judgements without knowledge of patient’s drug and anti-drug antibody levels). HRQoL outcomes were higher in the intervention group than in the control group at all visits, with statistically significant results being observed at two visits. However, the trial was judged to be at a serious risk of bias due to potential attrition bias and baseline imbalance in disease severity between the two groups. Therefore, the results should be interpreted with caution.
Evidence from observational studies
One observational study44 was identified that evaluated the effect of TDM on clinical outcomes in people with RA who had achieved either remission or LDA, or had experienced a primary or secondary non-response.
Change in disease activity
The observational study44 evaluated the effect of TDM on change in disease activity during the follow-up of 7 years, with a sample size of 43 individuals. The study focused on participants who had achieved remission or LDA, and examined two different time periods: before and after the introduction of TDM. The study showed a non-significant reduction in the mean DAS28 following the implementation of TDM at 7-year follow-up [pre-TDM mean of 2.51 (SD 0.85) vs. TDM mean of 2.31 (SD 0.52); p = 0.061].
Overall, the finding from this historically controlled study showed that TDM was associated with a non-significant reduction in mean DAS28 at 7-year follow-up compared with the historical control period. It should be noted that the data were judged to be at a moderate risk of bias, which compromises the reliability of the findings.
Change in direction and magnitude of therapeutic dose
The observational study44 evaluated the outcome of changes in the direction and magnitude of dose in people with RA who had achieved remission or LDA.
The findings from the study demonstrated that, compared with the historical control period without TDM, there were statistically significant reductions in the weekly mean dose per patient of each TNF-α inhibitor (AFX, ADL and ETN) during the second period, following the introduction of TDM. The findings from this study further showed that, compared with the historical control, there were statistically significant increases in the mean interval of administration of each TNF-α inhibitor during the second period.
Overall, the limited data from this observational study showed that TDM for optimisation of TNF-α inhibitor therapies was associated with reductions in therapeutic dose of TNF-α inhibitors in people with RA who had achieved remission or LDA. This would be expected to lead to cost-saving associated with TDM; however, the reliability of the findings may be compromised by the poor quality of the study.
Cost-effectiveness
Despite substantial weaknesses in the evidence identified in the clinical effectiveness systematic review, a relatively simple decision tree model was constructed and threshold and cost–utility analyses were carried out to estimate the health and economic outcomes of adding TDM to standard care in RA patients treated with TNF-α inhibitors. Data from two reports of the same study (INGEBIO) produced inconsistent results on the cost-effectiveness of Promonitor testing in people receiving ADL who are in remission or LDA: the intervention was either cost-effective or dominated by SOC depending on the data source used. 42,43 Similarly, the results of the threshold analyses conducted for these evidence sources were inconsistent, with both positive and negative threshold values for the cost of testing per patient-year at which the NMB of monitoring is equal to zero.
Various clinically relevant sensitivity analyses were conducted to explore the effect of structural and parametric uncertainties on the economic outcomes. Probabilistic sensitivity analyses were not feasible because of time constraints and a substantial variation in clinical practice with respect to treatment, drug dose tapering, flare management and uncertainty in TNF-α testing strategies in people with RA, which made the specification of the base-case scenario very difficult. The effect of such variation on the economic outcomes was evaluated in one-way deterministic sensitivity analyses and numerous scenario analyses. Of the sensitivity analyses conducted, only one assuming that the rate of flares alone would change as a consequence of TDM produced qualitatively different results. However, the cost-effectiveness outcomes based on two reports from the INGEBIO study were still inconsistent: an ICER estimated for Ucar et al. 42 was well above £30,000 per QALY gained, whereas data from Arango et al. 43 resulted in an ICER slightly under this threshold.
The fact that the results from both threshold and cost–utility analyses were similar (i.e. highly uncertain) highlights further the uncertainty in the evidence base, which the economic analysis for this appraisal may only serve to amplify. Therefore, based on the available evidence, no firm conclusions about the cost-effectiveness of TDM in RA patients were possible.
Strengths and limitations of the assessment
Clinical effectiveness
Extensive literature searches were conducted with an attempt to maximise the retrieval of potentially relevant studies for the systematic review of clinical effectiveness. These included electronic searches of a variety of bibliographic databases, as well as the screening of clinical trial registers and conference proceedings to identify unpublished studies. The search strategy did not restrict by study design. The review process followed the recommended methods to assess the potential for error and bias. The quality of included studies was assessed and accounted for when interpreting the review results. Appropriate synthesis methods were employed by taking into account the heterogeneity of study characteristics.
In terms of limitations, only studies in English were included; therefore, some potentially relevant non-English language studies may have been missed. There was scarce evidence relating to the clinical effectiveness of TDM on clinical outcomes in people with RA who had experienced a primary or secondary non-response. No studies that assessed ELISA kits, including IDKmonitor, LISA-TRACKER, RIDASCREEN and MabTrack, were identified. There was considerable clinical heterogeneity associated with interventions, outcomes and length of follow-up between included studies. It was not possible to investigate publication bias because quantitative synthesis was not possible in this systematic review owing to considerable clinical heterogeneity.
Cost-effectiveness
A systematic review of published economic evaluations of using ELISA tests relative to the alternatives and standard care was undertaken to help inform the type and structure of the decision model. The review uncovered limited evidence on the cost-effectiveness of TDM in people with RA. Despite a comprehensive search of the literature, only two studies were identified. Two (out of five) TNF-α testing kits from the NICE scope (Promonitor and Sanquin) and three (out of five) TNF-α inhibitors (ADL, ETN and IFX) were assessed in the selected studies. The systematic review was also limited by the fact that the INGEBIO study, which was the only source of clinical effectiveness evidence used in the economic analysis, was reported only in abstract form.
Only in the INGEBIO study, selected in the clinical effectiveness systematic review, was a test-based treatment compared with usual care. In this study, however, physicians were not obliged to follow any test-based treatment algorithm but could use testing to alter doses, based on their judgement, in patients who were in the intervention arm. Moreover, the reported outcomes were not directly relevant to the NHS, given that the study was conducted in Spain. Therefore, an additional systematic literature review to identify RCTs evaluating any tests used to monitor TNF-α inhibitor treatment of people with RA was conducted to support the economic assessment. However, no relevant sources were identified.
Owing to the limited evidence available on clinical effectiveness of TNF-α monitoring in people with RA, the multifactorial nature of decisions to adjust treatments in people with RA34 and the recent changes in the biologics market, which contributed to uncertainty in the prices of the TNF-α inhibitors and their uptake in the UK, threshold analyses were conducted to address the decision problem. In these analyses, the cost of measuring the drug concentrations and anti-drug antibody levels at which addition of TNF-α testing to usual practice is likely to have zero NMB was estimated in RA patients treated with ADL for a range of annual acquisition costs. The estimates obtained under the WTP thresholds of £20,000 and £30,000 per QALY gained were compared with those derived from literature and provided to the EAG by our external advisors.
The most important limitations of the economic analysis that was undertaken in this study are described below.
The major challenge in this assessment was the limited evidence on clinical effectiveness, HRQoL and costs associated with test-based treatment strategies. Owing to the paucity of data, not all test kits, TNF-α inhibitors or populations specified in the NICE scope were considered in the economic analysis. In particular, no economic evaluations relevant to IDKmonitor ELISA kits, LISA-TRACKER ELISA kits, RIDASCREEN ELISA kits and MabTrack ELISA kits, the TNF-α inhibitors ETN, CTZ and GLM or primary or secondary non-responders were conducted.
Several test–based treatment algorithms have been proposed and used by physicians in the UK, for example the Exeter Biologic Clinic recommendations for biologic dose reduction and the NHS Greater Glasgow and Clyde recommendations on biologic drug monitoring (see Appendix 13). However, to our knowledge, there is no unified treatment algorithm based on TNF-α testing. Importantly, in the INGEBIO study (conducted in Spain), clinicians were not expected to follow any test-based strategy when making treatment decisions based on test results. Therefore, it is unclear whether or not and to what extent the economic outcomes based on this study are relevant to clinical practice in England.
To our knowledge, there are no unified recommendations on managing flares in people with RA. To address this limitation, several sensitivity analyses informed by literature and based on clinical expert advice were carried out. It is not clear, however, which of those analyses is most relevant to the NHS.
In clinical practice, flares have been observed in tapered and not tapered patients, with an increased risk of flares in patients who are on reduced doses of biologics. However, in the economic analysis flares were modelled in all patients, as the reported flare rates in the intervention and control arms of the INGEBIO study were not stratified by dose. This is an important limitation of the study.
The time horizon of the analysis was defined by the observational period in the INGEBIO trial, which was conducted for 18 months. Costs and health outcomes were not extrapolated into the future, given that external validation of extrapolated outcomes would not be feasible owing to the lack of long-term clinical studies. Furthermore, given the multifactorial nature of treatment decisions in people with RA, long-term extrapolation of the costs and health outcomes would be prone to even greater uncertainties, which would not be possible to quantify given substantial limitations in the evidence base.
Owing to limited reporting, it is not clear to what extent selection bias in the INGEBIO study (which was a non-randomised trial) could have influenced the results of the economic analysis.
In this study, as in many other economic evaluations in RA, health state utility values were estimated from HAQ scores using published regression functions. These functions have demonstrated a relatively strong correlation between the HAQ and several HRQoL instruments. The EAG adopted this approach as the evidence on HRQoL from the INGEBIO study was limited; however, it is recognised that the HAQ is a functional measure and does not capture the full impact of RA on patients’ quality of life.
Utility values estimated from HRQoL data for people with RA were applied based on clinical outputs from the INGEBIO study, which included a mixed population of people with RA, PsA and AS. Given that people with RA are usually older and are more likely to be female than people with PsA or AS, the utility values for people with RA that were used in the economic analysis are probably lower than those for the mixed population (given that men tend to value health states more highly than women, and the same applies to younger versus older people). 115 Therefore, the ICERs for the intervention versus SOC may have been overestimated.
As the rate of AEs was not reported in the INGEBIO study, the impact of AEs was modelled using evidence from other studies, which is a limitation of this analysis. However, based on clinical advice and published literature on AEs in people with RA who were treated with biologics, those AEs that carry a significant cost and disutility burden are relatively rare.
Finally, limited evidence was identified for the UK setting in this study on the following: utilities, based on EQ-5D scores, directly relevant to flared patients; patients experiencing SAEs; and people with remission, LDA or active disease health status. Therefore, utilities were derived from HAQ scores that were estimated in studies conducted in people with RA in non-UK settings; however, it should be noted that utilities were estimated by mapping to EQ-5D outcomes using UK tariffs.
Uncertainties
Clinical effectiveness
In this assessment, limited data were identified that evaluated the clinical effectiveness of using ELISA tests for monitoring the response to TNF-α inhibitors in people with RA who had either achieved remission or LDA or experienced a primary or secondary non-response. Limited data were identified for people who had experienced a primary or secondary non-response. In particular, no RCTs were identified that evaluated patient-related outcomes and disease activities that were associated with using ELISA for TDM in the target populations.
The non-randomised controlled study42,43,45 was judged to be at a serious risk of bias. The historical controlled observational study was judged to be at a moderate risk of bias. In the non-randomised controlled trial (the INGEBIO study), there was baseline imbalance in disease severity between the intervention and the control groups. Furthermore, there was a lack of adjusting for this variable in the analysis of clinical outcomes. There were high attrition rates for some outcomes, which could lead to attrition bias. The study by Pascual-Salcedo et al. 44 was associated with non-contemporaneous control bias due to the use of a historical control. Given the poor quality of the included studies, the potential role of ELISA in terms of its clinical impact on monitoring the response to TNF-α inhibitors in the target populations remains unclear.
Cost-effectiveness
Given that there is neither a gold standard nor guidelines available to monitor the TNF-α inhibitors considered in this assessment, economic analyses of test-based treatment strategies with biologics represent a substantial challenge.
Owing to data limitations and the lack of clarity in regard to test-based treatment strategies, the EAG deliberately refrained from data-intensive modelling approaches, which would be impossible to implement without making strong assumptions that were not supported by evidence.
The studies that were identified in the clinical effectiveness systematic review and were used to inform the model structure and parameters are limited by study design (e.g. none of the studies was randomised, one study was observational). In only one study (INGEBIO) was the treatment of patients based on the results of TNF-α testing compared with usual care, and this was in a mixed-disease population with only 37% of RA patients.
The EAG is aware of several test-based treatment algorithms that are used by physicians in England. However, in the only head-to-head study comparing test with no-test treatment strategies, INGEBIO (which was utilised in our economic analysis), physicians were not required to follow any therapeutic algorithm based on TDM results, but could use tests to alter doses based on their clinical judgement. However, it is unclear whether or not there are variations in clinical practice between England and Spain.
In the INGEBIO study, flare was defined as an increase in DAS28 of > 1.2, or an increase in DAS28 of > 0.6 if the current DAS28 was already ≥ 3.2. However, there is substantial variation in the definitions of flare used in studies and clinical practice. To address this uncertainty, the effect of such variations was explored in sensitivity analyses by altering assumptions on the duration of flare and the effect of flares on HRQoL.
In the INGEBIO study, the rate of AEs in the intervention and control arms was not reported. Therefore, the impact of AEs on costs and QALYs was investigated by assuming AE rates from other studies.
Given that the INGEBIO study was carried out in Spain and the reported outcomes (arm-specific average acquisition costs of ADL per patient-year and QALYs accrued over the duration of the study) were not directly relevant to the NHS, some important assumptions had to be made in the analyses conducted by the EAG. In particular, it was assumed that clinical practice in England with respect to treatment decisions in RA patients given biological therapies is similar to that in Spain.
Finally, as the actual costs to the NHS of the originator ADL (Humira) and its biosimilars were not known to the EAG at the time of writing, the effect of variation in the ADL acquisition cost within the range of £1000–9187 per patient-year was examined in the threshold analyses. However, given that (1) the actual costs of the originator products and their biosimilars vary considerably across England, (2) there is also a variation in the uptake of biosimilars across the UK and (3) the proportion of people treated with biosimilars will probably increase in the near future owing to recent changes in the biologics market, it is not clear which estimates obtained in our economic analyses are most relevant to the NHS.
Generalisability of the findings
Clinical effectiveness
As the studies were conducted in Spain, the generalisability of their findings to the UK setting remains uncertain owing to variations in clinical practice and health policies between the two countries. Furthermore, the findings from the non-randomised controlled trial (INGEBIO) and the results of changes in therapeutic dose from the Pascual-Salcedo et al. 44 study were presented for mixed-disease populations (including RA, PsA and/or AS). Therefore, there was limited generalisability of findings from those populations to the target population (RA patients) considered in this assessment.
Cost-effectiveness
Outcomes from the INGEBIO study were utilised in the economic analysis for RA patients who were in remission or LDA. It was a pragmatic trial and, therefore, it is likely that the results could be generalisable to routine practice settings. However, the generalisability to the UK setting of the findings from the INGEBIO study and the economic results reported here remains uncertain because of likely variations in clinical practice between England and Spain.
Given that the findings from the mixed population in the INGEBIO study might not be generalisable to the RA population, and that the trial was judged to be at a serious risk of bias, the economic results presented here should be considered with caution.
Owing to the paucity of data, not all test kits and TNF-α inhibitors from the NICE scope could be modelled using reported clinical outcomes considered in this study, and it is not clear whether or not and to what extent the economic estimates obtained for patients treated with ADL are applicable to people treated with the other TNF-α inhibitors.
Moreover, data limitations did not allow assessment of the long-term economic impact of TNF-α testing given that TDM in RA patients is relatively new and, therefore, there are no data relevant to the long-term outcomes of test-based treatment strategies in this patient population. Given the dynamic nature of RA treatment and the limited data available, it is not known whether or not the reported clinical effects and associated incremental costs of test-based treatment decisions would persist beyond this time.
According to NHS England63 some manufacturers of originator products have offered discounts, which enhances the competitiveness of the market and potential for cost saving for the NHS. Therefore, the list prices of TNF-α inhibitors assumed in the analyses reporting ICERs (see Tables 37–39 and 41) may not adequately reflect the actual costs of the TNF-α inhibitors to the NHS.
Chapter 6 Conclusions
Implications for service provision
The findings from this assessment provide very limited evidence on the usefulness of TDM based on ELISA tests for optimising TNF-α inhibitor therapies for people with RA who had either achieved remission or LDA or experienced a primary or secondary non-response.
In relation to the clinical effectiveness, limited data were identified evaluating TDM in the target populations. One non-randomised trial that compared TDM with standard care (the INGEBIO study) had serious limitations in relation to the NICE scope: only one-third of the participants had RA, many of the analyses were not by ITT, follow-up was for only 18 months, there was no explicit algorithm for guiding clinicians in how the results of testing should change treatment (e.g. tapering) and the study was reported in only three abstracts. In addition, one observational study was identified but was of limited value in informing whether or not ELISA-based monitoring is clinically effective.
Despite these substantial weaknesses in the clinical effectiveness evidence base, a simple model was developed to estimate the cost versus utility of ELISA-based monitoring for people with RA receiving TNF-α inhibitors. The main effectiveness evidence in the model was from the poorly reported INGEBIO study, which was heavily supplemented by evidence from other studies and expert advice. The results of the economic analysis should, therefore, be viewed as exploratory and highly speculative. For example, although the INGEBIO study evaluated testing using Promonitor ELISA kits for monitoring only in patients in remission/LDA treated with Humira (ADL), with further assumptions these clinical outcomes were used to estimate the cost-effectiveness of TDM in people taking other TNF-α inhibitors, either originator products or biosimilars.
In summary, there is limited valid and applicable research evidence and much uncertainty in relation to the clinical and cost-effectiveness of using ELISA-based testing for TDM in RA; no firm conclusions regarding the implications for service provision can be drawn.
Suggested research priorities
An ongoing Norwegian multicentre RCT (the NOR-DRUM study)46 is evaluating the effect of TDM in people with RA in remission compared with standard care. This ongoing trial will provide further useful data on the impact of TDM in the target population.
Further controlled trials (especially RCTs) are required to assess the effect of using Promonitor ELISA tests to monitor TNF-α inhibitor therapies in people with RA who have achieved remission or LDA.
No studies were identified that evaluated other eligible ELISA kits, including IDKmonitor, LISA-TRACKER, RIDASCREEN and MabTrack. Therefore, future large RCTs are required to assess the effect of using those ELISA for monitoring TNF-α inhibitor therapies in people with RA who have achieved remission or LDA. More robust evidence is also required to evaluate the effect of using Sanquin tests for monitoring TNF-α inhibitor therapies in this population.
There were no studies identified for people with RA who were treated with CTZ or GLM. Future RCTs are required to assess the clinical effectiveness of using ELISA for monitoring such TNF-α inhibitors in the target populations.
No data relevant to the population of people with RA who have experienced a primary or secondary non-response were identified. Future RCTs are warranted to assess the clinical effectiveness of using ELISA for monitoring TNF-α inhibitors in those who have developed clinical inefficacy.
Limited evidence on health-care resource use and utilities, based on EQ-5D scores, directly relevant to the populations considered in this assessment was identified in this study. This warrants further research on medium-/long-term costs and health outcomes of ELISA-based monitoring in people with RA who are treated with TNF-α inhibitors.
Acknowledgements
We would like to thank Rob Anderson for his support in preparation of the protocol and in the finalisation of the report; Ruben Mujica Mota for providing comments on the report; Jo Thompson-Coon for overall support of the project; Martin Pitt for his contribution to the PenTAG (Peninsula Technology Assessment Group) independent economic assessment; Jaime Peters for her contribution to the cost-effectiveness systematic review, PenTAG independent economic assessment and for providing comments on the report; Helen Coelho for writing the background section of the report; and Louise Crathorne for support during the final stage of the project (all from the University of Exeter Medical School). We are grateful to Sean Gavan and Katherine Payne from the University of Manchester for their advice on the economic assessment of TNF-α testing; and Grant Smith, Nicola Finch, Leanne Brown, Keith Oldfield and Rob Storey from the Royal Devon & Exeter NHS Foundation Trust for their advice on costing. We acknowledge the excellent administrative support of Sue Whiffin and Jenny Lowe.
Contributions of authors
Irina A Tikhonova (https://orcid.org/0000-0003-2723-0802) (Senior Research Fellow, Health Economics) was responsible for day-to-day project management; led the health-economics and modelling; contributed to the writing of the Abstract, Plain English summary, Scientific summary and Chapters 3–6, and contributed to the Microsoft Excel model and report collation.
Huiqin Yang (https://orcid.org/0000-0003-3985-986X) (Senior Research Fellow, Systematic Review) led the clinical effectiveness, and contributed to the writing of the Abstract, Scientific summary and Chapters 2, 5 and 6.
Segun Bello (https://orcid.org/0000-0003-0665-223X) (Postdoctoral Research Associate, Systematic Review) assisted with tasks on development of the protocol for Chapter 2, screened records for the clinical effectiveness systematic review, conducted data extraction for included studies, conducted study quality assessment, and contributed to drafting and editing of Chapter 2.
Andrew Salmon (https://orcid.org/0000-0002-6390-9524) (Associate Research Fellow, Health Economics) contributed to the cost-effectiveness systematic review as the second reviewer, screened titles and abstracts for the additional systematic review supporting the PenTAG independent economic assessment, contributed to the writing of Chapters 3–4, and checked the working of the simulation model between the submission deadline and the erratum/addendum phase.
Sophie Robinson (https://orcid.org/0000-0003-0463-875X) (Information Specialist, Systematic Literature Searches) developed and conducted the literature searches for the systematic reviews of clinical effectiveness and cost-effectiveness, and for the additional systematic review for comparator tests; developed and conducted the searches for the literature review of health utilities; and contributed to the writing, editing and proofreading of the report.
Mohsen Rezaei Hemami (https://orcid.org/0000-0002-4386-2511) (Research Fellow, Health Economic Modelling) contributed to the cost-effectiveness systematic review as the first reviewer, drafted Chapter 3, and contributed to drafting Chapter 4 and the writing of the Microsoft Excel model.
Sophie Dodman (https://orcid.org/0000-0001-7541-3027) (Research Assistant, Systematic Review) conducted a review of the literature to inform costs and contributed to the writing of Chapter 4.
Andriy Kharechko (https://orcid.org/0000-0002-1977-6840) (Postdoctoral Research Associate, Health Economic Modelling) conducted a review of literature to inform utilities, and contributed to the writing of Chapter 4 and the editing of the final version of the report.
Richard C Haigh (https://orcid.org/0000-0002-0949-3417) (Consultant Rheumatologist, Rheumatology) provided clinical advice and contributed to the editing of the report.
Meghna Jani (https://orcid.org/0000-0002-1487-277X) (NIHR Academic Clinical Lecturer, Rheumatology) provided clinical advice and academic input regarding therapeutic drug monitoring, and contributed to the editing of the report.
Timothy J McDonald (https://orcid.org/0000-0003-3559-6660) (NIHR Clinical Scientist, Clinical Science) provided clinical advice and contributed to the editing of the report.
Martin Hoyle (https://orcid.org/0000-0001-5593-9777) (Associate Professor of Health Technology Assessment, Health Economics) contributed to the development of the NICE Scope and Protocol, and was the project director until 29 June 2018.
Publication
Tikhonova I, Bello S, Salmon A, Robinson S, Rezaei Hemami M, et al. Promonitor for therapeutic drug monitoring in patients with severe rheumatoid arthritis treated with adalimumab in England and Wales. Value Health 2020;23:S227.
Data-sharing statement
Data can be obtained from the corresponding author subject to them being non-confidential.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- National Audit Office . Services for People With Rheumatoid Arthritis n.d. www.nao.org.uk/report/services-for-people-with-rheumatoid-arthritis/ (accessed November 2018).
- Dickson JL, Lanyon P, Wise E. Making a Diagnosis of Rheumatoid Arthritis n.d. www.nras.org.uk/diagnosis (accessed October 2018).
- Picerno V, Ferro F, Adinolfi A, Valentini E, Tani C, Alunno A. One year in review: the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 2015;33:551-8.
- Scott IC, Steer S, Lewis CM, Cope AP. Precipitating and perpetuating factors of rheumatoid arthritis immunopathology: linking the triad of genetic predisposition, environmental risk factors and autoimmunity to disease pathogenesis. Best Pract Res Clin Rheumatol 2011;25:447-68. https://doi.org/10.1016/j.berh.2011.10.010.
- National Rheumatoid Arthritis Society . What Is the Cause of Rheumatoid Arthritis? Non-Genetic Factors n.d. www.nras.org.uk/what-is-the-cause-of-rheumatoid-arthritis-non-genetic-factors (accessed October 2018).
- Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study. Arthritis Rheum 2004;50:3458-67. https://doi.org/10.1002/art.20621.
- Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2010;69:70-81. https://doi.org/10.1136/ard.2008.096487.
- National Rheumatoid Arthritis Society . The Genetics of Rheumatoid Arthritis n.d. www.nras.org.uk/the-genetics-of-rheumatoid-arthritis (accessed December 2018).
- Philippou E, Nikiphorou E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun Rev 2018;17:1074-7. https://doi.org/10.1016/j.autrev.2018.05.009.
- James D, Young A, Kulinskaya E, Knight E, Thompson W, Ollier W, et al. Early Rheumatoid Arthritis Study Group (ERAS), UK. Orthopaedic intervention in early rheumatoid arthritis. Occurrence and predictive factors in an inception cohort of 1064 patients followed for 5 years. Rheumatology 2004;43:369-76. https://doi.org/10.1093/rheumatology/keh059.
- Raheel S, Matteson EL, Crowson CS, Myasoedova E. Improved flare and remission pattern in rheumatoid arthritis over recent decades: a population-based study. Rheumatology 2017;56:2154-61. https://doi.org/10.1093/rheumatology/kex352.
- National Institute for Health and Care Excellence (NICE) . Rheumatoid Arthritis in Adults: Management. 2018. www.nice.org.uk/guidance/ng100 (accessed October 2018).
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580-8. https://doi.org/10.1136/ard.2010.138461.
- Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology 2002;41:793-800. https://doi.org/10.1093/rheumatology/41.7.793.
- Symmons DP, Barrett EM, Bankhead CR, Scott DG, Silman AJ. The incidence of rheumatoid arthritis in the United Kingdom: results from the Norfolk Arthritis Register. Br J Rheumatol 1994;33:735-9. https://doi.org/10.1093/rheumatology/33.8.735.
- Kearsley-Fleet L, Davies R, De Cock D, Watson KD, Lunt M, Buch MH, et al. Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2018;77:1405-12. https://doi.org/10.1136/annrheumdis-2018-213378.
- Gavan S. An Economic Evaluation of a Biomarker Test to Stratify Treatment for Rheumatoid Arthritis. 2017.
- Abhishek A, Nakafero G, Kuo CF, Mallen C, Zhang W, Grainge MJ, et al. Rheumatoid arthritis and excess mortality: down but not out. A primary care cohort study using data from Clinical Practice Research Datalink. Rheumatology 2018;57:977-81. https://doi.org/10.1093/rheumatology/key013.
- Hyrich K, Symmons D, Watson K, Silman A. BSRBR Control Centre Consortium . Baseline comorbidity levels in biologic and standard DMARD treated patients with rheumatoid arthritis: results from a national patient register. Ann Rheum Dis 2006;65:895-8. https://doi.org/10.1136/ard.2005.043158.
- National Rheumatoid Arthritis Society . The Economic Burden of Rheumatoid Arthritis n.d. www.nras.org.uk/data/files/. /1_economic_burden_of_ra_final_30_3_10.pdf (accessed November 2018).
- Joint Formulary Committee . British National Formulary n.d. www.bnf.org/products/bnf-online/ (accessed December 2018).
- Brown EB, Earl S, Haigh R, Mascarenhas R. Adherence to biologic therapies in patients with inflammatory arthritis. Rheumatology 2015;54.
- National Institute of Health and Care Excellence (NICE) . Adalimumab, Etanercept, Infliximab, Certolizumab Pegol, Golimumab, Tocilizumab and Abatacept for Rheumatoid Arthritis Not Previously Treated With DMARDs or After Conventional DMARDs Only Have Failed. 2016. www.nice.org.uk/guidance/ta375 (accessed July 2019).
- National Institute of Health and Care Excellence (NICE) . NICE Pathways n.d. https://pathways.nice.org.uk/pathways/rheumatoid-arthritis (accessed December 2018).
- Cantini F, Benucci M. Focus on biosimilar etanercept – bioequivalence and interchangeability. Biologics 2018;12:87-95. https://doi.org/10.2147/BTT.S126854.
- Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ). Clin Exp Rheumatol 2005;23:S14-8.
- Prevoo ML, van’T Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44-8. https://doi.org/10.1002/art.1780380107.
- Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005;23:100-8.
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727-35. https://doi.org/10.1002/art.1780380602.
- van Gestel AM, Prevoo ML, van’T Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis . Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum 1996;39:34-40. https://doi.org/10.1002/art.1780390105.
- Simpson E, Hock E, Stevenson M, Wong R, Dracup N, Wailoo A, et al. What is the added value of ultrasound joint examination for monitoring synovitis in rheumatoid arthritis and can it be used to guide treatment decisions? A systematic review and cost-effectiveness analysis. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22200.
- National Rheumatoid Arthritis Society . The DAS28 Score n.d. www.nras.org.uk/the-das28-score (accessed November 2018).
- Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol 2005;23:93-9.
- Stevenson M, Archer R, Tosh J, Simpson E, Everson-Hock E, Stevens J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20350.
- Vogelzang EH, Hebing RCF, Nurmohamed MT, van Kuijk AWR, Kruijff JWF, l’Ami MJ, et al. Adherence to etanercept therapy in rheumatoid arthritis patients during 3 years of follow-up. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0205125.
- National Institute of Health and Care Excellence (NICE) . Adalimumab, Etanercept, Infliximab, Rituximab and Abatacept for the Treatment of Rheumatoid Arthritis After the Failure of a TNF Inhibitor 2010.
- Jani M, Gavan S, Chinoy H, Dixon WG, Harrison B, Moran A, et al. A microcosting study of immunogenicity and tumour necrosis factor alpha inhibitor drug level tests for therapeutic drug monitoring in clinical practice. Rheumatology 2016;55:2131-7. https://doi.org/10.1093/rheumatology/kew292.
- Higgins JPT, Green S. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons Ltd; 2008.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. https://doi.org/10.1136/bmj.i4919.
- Ucar E, Gorostiza I, Gomez C, Perez C, Dios J, Alvarez B, et al. Prospective, intervention, multicenter study of utility of biologic drug monitoring with respect to the efficacy and cost of adalimumab tapering in patients with rheumatic diseases: preliminary results of ingebio study. Ann Rheum Dis 2017;76. https://doi.org/10.1136/annrheumdis-2017-eular.4785.
- Arango CG, Vivar MLG, Angulo EU, Gorostiza I, Perez CE, De Dios JR, et al. Prospective, intervention, multicenter, non-inferiority study of utility of therapeutic drug monitoring with respect to the efficacy and cost of adalimumab tapering in patients with rheumatic diseases. Arthritis Rheum 2017;69.
- Pascual-Salcedo D, Plasencia C, Gonzalez Del Valle L, Lopez Casla T, Arribas F, Villalba A, et al. Therapeutic drug monitoring (TDM) in rheumatic day clinic enables to reduce pharmaceutical cost maintaining clinical efficacy. Ann Rheum Dis 2013;72. https://doi.org/10.1136/annrheumdis-2013-eular.717.
- Gorostiza I, Angulo EU, Arango CG, Perez CE, De Dios JR, Alvarez B, et al. Prospective, intervention, multicenter study of utility of biologic drug monitoring with respect to the efficacy and cost of adalimumab tapering in patients with rheumatic diseases (34-week descriptive data) [abstract]. Arthritis Rheum 2016;68:835-6.
- Haavardsholm EA. The Norwegian Drug Monitoring Study (NOR-DRUM). 2018. https://clinicaltrials.gov/ct2/show/NCT03074656 (accessed December 2018).
- van der Maas A, Lie E, Christensen R, Choy E, de Man YA, van Riel P, et al. Construct and criterion validity of several proposed DAS28-based rheumatoid arthritis flare criteria: an OMERACT cohort validation study. Ann Rheum Dis 2013;72:1800-5. https://doi.org/10.1136/annrheumdis-2012-202281.
- National Institute for Health and Care Excellence (NICE) . Therapeutic Monitoring of TNF-Alpha Inhibitors in Rheumatoid Arthritis. 2019. www.nice.org.uk/guidance/dg36/documents/final-scope (accessed February 2020).
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5. https://doi.org/10.1017/S0266462305050324.
- Philips Z, Bojke L, Sculpher M, Claxton K, Golder S. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. PharmacoEconomics 2006;24:355-71. https://doi.org/10.2165/00019053-200624040-00006.
- Krieckaert C, Nair SC, Nurmohamed MT, Van Dongen CJ, Lems WF, Lafeber FP, et al. Evaluating the cost-effectiveness of personalized treatment with adalimumab using serum drug level and anti-adalimumab antibodies in rheumatoid arthritis patients. Ann Rheum Dis 2013;71. https://doi.org/10.1136/annrheumdis-2012-eular.1832.
- Krieckaert CLM, Nair SC, Nurmohamed MT, van Dongen CJJ, Lems WF, Lafeber FPJG, et al. Evaluating the cost-effectiveness of personalized treatment with adalimumab using serum drug levels in rheumatoid arthritis patients. Arthritis Rheum-Us 2012;64.
- Krieckaert CL, Nair SC, Nurmohamed MT, van Dongen CJJ, Lems WF, Lafeber FPJG, et al. Personalised treatment using serum drug levels of adalimumab in patients with rheumatoid arthritis: an evaluation of costs and effects. Ann Rheum Dis 2015;74:361-8. https://doi.org/10.1136/annrheumdis-2013-204101.
- Laine J, Jokiranta TS, Eklund KK, Väkeväinen M, Puolakka K. Cost-effectiveness of routine measuring of serum drug concentrations and anti-drug antibodies in treatment of rheumatoid arthritis patients with TNF-α blockers. Biologics 2016;10:67-73. https://doi.org/10.2147/BTT.S96982.
- Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 2011;305:1460-8. https://doi.org/10.1001/jama.2011.406.
- Oostenbrink JB, Koopmanschap MA, Rutten FFH. Guideline for Cost-Of-Illness Study; Methods and Guideline-Rates for Economic Evaluations in Health Care. Guideline for Cost-Of-Illness Study. Amstelveen: College voor Zorgverzekeringen; 2000.
- Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis 2013;72:165-78. https://doi.org/10.1136/annrheumdis-2012-202545.
- Hujanen T, Kapiainen S, Tuominen U, Pekurinen M. Terveydenhuollon yksikkökustannukset Suomessa vuonna 2006. Työpapereita/Stakes: Helsinki: Stakes; 2008.
- Malottki K, Barton P, Tsourapas A, Uthman AO, Liu Z, Routh K, et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15140.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2015–16. 2016.
- NHS Lambeth Clinical Commissioning Group . Ensuring the Value of Clinical Commissioning Group (CCG) Commissioned Biologics Medicines – Biologics Value Tariff Agreement. n.d. www.lambethccg.nhs.uk/news-and-publications/meeting-papers/lambeth-borough-prescribing-committee/Lambeth%20Borough%20Prescribing%20Committee/Prescribing%20Rebates/Biologics%20Value%20Agreement%202017-19%20FINAL.pdf (accessed December 2018).
- NHS England . Regional Medicines Optimisation Committee Briefing Best Value Biologicals: Adalimumab Update 3 2018. www.sps.nhs.uk/wp-content/uploads/2018/07/Adalimumab-RMOC-Briefing-Final-July.pdf (accessed December 2018).
- NHS England and NHS Improvement . Commissioning Framework for Biological Medicines (Including Biosimilar Medicines). 2017. www.england.nhs.uk/wp-content/uploads/2017/09/biosimilar-medicines-commissioning-framework.pdf (accessed December 2018).
- NHS Business Services Authority . Medicines Optimisation Dashboard n.d. www.nhsbsa.nhs.uk/epact2/dashboards-and-specifications/medicines-optimisation-dashboard (accessed November 2018).
- Verhoef LM, Tweehuysen L, Hulscher ME, Fautrel B, den Broeder AA. bDMARD dose reduction in rheumatoid arthritis: a narrative review with systematic literature search. Rheumatol Ther 2017;4:1-24. https://doi.org/10.1007/s40744-017-0055-5.
- Smolen JS, Jones H, Mahgoub E, Pedersen R, Marshall L. Association between flare and radiographic progression in patients with rheumatoid arthritis [abstract]. Ann Rheum Dis 2017;76. https://doi.org/10.1136/annrheumdis-2017-eular.1854.
- University of Manchester . BSRBR-RA Database n.d. www.bsrbr.org/database/ (accessed April 2020).
- Dennison EM, Packham J, Hyrich K. The BSRBR-RA at 15 years. Rheumatology 2016;55:2093-5. https://doi.org/10.1093/rheumatology/kew053.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed December 2018).
- Bykerk VP, Shadick N, Frits M, Bingham CO, Jeffery I, Iannaccone C, et al. Flares in rheumatoid arthritis: frequency and management. A report from the BRASS registry. J Rheumatol 2014;41:227-34. https://doi.org/10.3899/jrheum.121521.
- Bingham CO, Pohl C, Woodworth TG, Hewlett SE, May JE, Rahman MU, et al. Developing a standardized definition for disease ‘flare’ in rheumatoid arthritis (OMERACT 9 Special Interest Group). J Rheumatol 2009;36:2335-41. https://doi.org/10.3899/jrheum.090369.
- Markusse IM, Dirven L, Gerards AH, van Groenendael JH, Ronday HK, Kerstens PJ, et al. Disease flares in rheumatoid arthritis are associated with joint damage progression and disability: 10-year results from the BeSt study. Arthritis Res Ther 2015;17. https://doi.org/10.1186/s13075-015-0730-2.
- Senabre Gallego JM, Rosas Gomez De Salazar J, Marco Mingot M, Naranjo A, Llinares-Tello F, Pons A, et al. Clinical activity, ultrasound assessment and drug monitoring in rheumatoid arthritis patients receiving anti-TNF-alpha therapy with extended interval of administration. Ann Rheum Dis 2017;76. https://doi.org/10.1136/annrheumdis-2017-eular.3066.
- Lahiri M, Dixon WG. Risk of infection with biologic antirheumatic therapies in patients with rheumatoid arthritis. Best Pract Res Clin Rheumatol 2015;29:290-305. https://doi.org/10.1016/j.berh.2015.05.009.
- Bruce ES, Kearsley-Fleet L, Watson KD, Symmons DP, Hyrich KL. Risk of Pneumocystis jirovecii pneumonia in patients with rheumatoid arthritis treated with inhibitors of tumour necrosis factor α: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology 2016;55:1336-7. https://doi.org/10.1093/rheumatology/kew200.
- Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A, et al. BSRBR Control Centre Consortium. Ann Rheum Dis 2010;69:522-8. https://doi.org/10.1136/ard.2009.118935.
- Burmester GR, Landewé R, Genovese MC, Friedman AW, Pfeifer ND, Varothai NA, et al. Adalimumab long-term safety: infections, vaccination response and pregnancy outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2017;76:414-17. https://doi.org/10.1136/annrheumdis-2016-209322.
- Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011;2. https://doi.org/10.1002/14651858.CD008794.pub2.
- Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet 2015;386:258-65. https://doi.org/10.1016/S0140-6736(14)61704-9.
- Maravic M, Bergé C, Daurès JP, Boissier MC. Practices for managing a flare of long-standing rheumatoid arthritis: survey among French rheumatologists. Clin Exp Rheumatol 2005;23:36-42.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Barbieri M, Wong JB, Drummond M. The cost effectiveness of infliximab for severe treatment-resistant rheumatoid arthritis in the UK. PharmacoEconomics 2005;23:607-18. https://doi.org/10.2165/00019053-200523060-00007.
- Radner H, Smolen JS, Aletaha D. Remission in rheumatoid arthritis: benefit over low disease activity in patient-reported outcomes and costs. Arthritis Res Ther 2014;16. https://doi.org/10.1186/ar4491.
- NHS Improvment . Archived Reference Costs n.d. https://improvement.nhs.uk/resources/reference-costs/ (accessed December 2018).
- Oppong R, Kaambwa B, Nuttall J, Hood K, Smith RD, Coast J. The impact of using different tariffs to value EQ-5D health state descriptions: an example from a study of acute cough/lower respiratory tract infections in seven countries. Eur J Health Econ 2013;14:197-209. https://doi.org/10.1007/s10198-011-0360-9.
- The Campbell and Cochrane Economics Methods Group (CCEMG) and the Evidence for Policy and Practice Information and Coordinating Centre (EPPI Centre) . CCEMG–EPPI-Centre Cost Converter n.d. https://eppi.ioe.ac.uk/costconversion/default.aspx (accessed December 2018).
- Smolen JS, Nash P, Durez P, Hall S, Ilivanova E, Irazoque-Palazuelos F, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013;381:918-29. https://doi.org/10.1016/S0140-6736(12)61811-X.
- National Institute for Health and Care Excellence (NICE) . Tocilizumab for the Treatment of Rheumatoid Arthritis [TA247] 2012. www.nice.org.uk/guidance/ta247 (accessed December 2018).
- Datapharm . Remicade 100mg Powder for Concentrate for Solution for Infusion. n.d. www.medicines.org.uk/emc/product/3831/smpc (accessed December 2018).
- National Institute for Health and Care Excellence (NICE) . Therapeutic Monitoring of TNF-Alpha Inhibitors in Crohn’s Disease (LISA-TRACKER ELISA Kits, IDKmonitor ELISA Kits, and Promonitor ELISA Kits) [DG22] 2016. www.nice.org.uk/guidance/dg22 (accessed December 2018).
- National Institute for Health and Care Excellence (NICE) . Infliximab, Adalimumab And Golimumab for Treating Moderately to Severely Active Ulcerative Colitis After the Failure of Conventional Therapy [TA329]. 2015. www.nice.org.uk/guidance/ta329 (accessed December 2018).
- Royal Mail . Get a Price. n.d. www.royalmail.com/price-finder/ (accessed December 2018).
- Rosas J, Llinares-Tello F, Miguel Senabre J, Santos-Soler G, Salas-Heredia E, Barber X, et al. Economic impact of decreasing adalimumab and etanercept doses and drug monitoring in patients with rheumatoid arthritis in clinical remission: preliminary study from a local biologics unit [abstract]. Arthritis Rheumatol 2015;67.
- Chen DY, Chen YM, Hsieh TY, Hung WT, Hsieh CW, Chen HH, et al. Drug trough levels predict therapeutic responses to dose reduction of adalimumab for rheumatoid arthritis patients during 24 weeks of follow-up. Rheumatology 2016;55:143-8. https://doi.org/10.1093/rheumatology/kev298.
- Kobelt G, Lindgren P, Lindroth Y, Jacobson L, Eberhardt K. Modelling the effect of function and disease activity on costs and quality of life in rheumatoid arthritis. Rheumatology 2005;44:1169-75. https://doi.org/10.1093/rheumatology/keh703.
- Burn E, Edwards CJ, Murray DW, Silman A, Cooper C, Arden NK, et al. Trends and determinants of length of stay and hospital reimbursement following knee and hip replacement: evidence from linked primary care and NHS hospital records from 1997 to 2014. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-019146.
- NHS . National Schedule of Reference Costs Year: 2010–11 NHS Trusts and PCTS Combined HRG Data 2013. https://data.gov.uk/dataset/7fa41b07-a296-47c7-b74b-40ec3a102e6a/nhs-reference-costs-2010-11 (accessed November 2018).
- Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954-60. https://doi.org/10.1136/ard.2007.084459.
- Fleischmann RM, van der Heijde D, Gardiner PV, Szumski A, Marshall L, Bananis E. DAS28-CRP and DAS28-ESR cut-offs for high disease activity in rheumatoid arthritis are not interchangeable. RMD Open 2017;3. https://doi.org/10.1136/rmdopen-2016-000382.
- Barnabe C, Thanh NX, Ohinmaa A, Homik J, Barr SG, Martin L, et al. Healthcare service utilisation costs are reduced when rheumatoid arthritis patients achieve sustained remission. Ann Rheum Dis 2013;72:1664-8. https://doi.org/10.1136/annrheumdis-2012-201918.
- Aletaha D, Funovits J, Keystone EC, Smolen JS. Disease activity early in the course of treatment predicts response to therapy after one year in rheumatoid arthritis patients. Arthritis Rheum 2007;56:3226-35. https://doi.org/10.1002/art.22943.
- Pennington B, Davis S. Mapping from the Health Assessment Questionnaire to the EQ-5D: the impact of different algorithms on cost-effectiveness results. Value Health 2014;17:762-71. https://doi.org/10.1016/j.jval.2014.11.002.
- Bansback N, Marra C, Tsuchiya A, Anis A, Guh D, Hammond T, et al. Using the health assessment questionnaire to estimate preference-based single indices in patients with rheumatoid arthritis. Arthritis Rheum 2007;57:963-71. https://doi.org/10.1002/art.22885.
- Kobelt G, Jönsson L, Lindgren P, Young A, Eberhardt K. Modeling the progression of rheumatoid arthritis: a two-country model to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheum 2002;46:2310-19. https://doi.org/10.1002/art.10471.
- Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 1997;36:551-9. https://doi.org/10.1093/rheumatology/36.5.551.
- Hernández Alava M, Wailoo A, Wolfe F, Michaud K. The relationship between EQ-5D, HAQ and pain in patients with rheumatoid arthritis. Rheumatology 2013;52:944-50. https://doi.org/10.1093/rheumatology/kes400.
- Goh L, Jewell T, Laversuch C, Samanta A. A systematic review of the influence of anti-TNF on infection rates in patients with rheumatoid arthritis. Rev Bras Reumatol 2013;53:501-15. https://doi.org/10.1016/j.rbr.2012.12.001.
- Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology 2013;52:53-61. https://doi.org/10.1093/rheumatology/kes305.
- Gülfe A, Wallman JK, Kristensen LE. EuroQol-5 dimensions utility gain according to British and Swedish preference sets in rheumatoid arthritis treated with abatacept, rituximab, tocilizumab, or tumour necrosis factor inhibitors: a prospective cohort study from southern Sweden. Arthritis Res Ther 2016;18. https://doi.org/10.1186/s13075-016-0950-0.
- Gülfe A, Kristensen LE, Saxne T, Jacobsson LT, Petersson IF, Geborek P. Rapid and sustained health utility gain in anti-tumour necrosis factor-treated inflammatory arthritis: observational data during 7 years in southern Sweden. Ann Rheum Dis 2010;69:352-7. https://doi.org/10.1136/ard.2008.103473.
- Paramarta JE, De Rycke L, Ambarus CA, Tak PP, Baeten D. Undifferentiated spondyloarthritis vs ankylosing spondylitis and psoriatic arthritis: a real-life prospective cohort study of clinical presentation and response to treatment. Rheumatology 2013;52:1873-8. https://doi.org/10.1093/rheumatology/ket239.
- Jani M, Chinoy H, Warren RB, Griffiths CE, Plant D, Fu B, et al. Clinical utility of random anti-tumor necrosis factor drug-level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. Arthritis Rheum 2015;67:2011-19. https://doi.org/10.1002/art.39169.
- Jani M, Isaacs JD, Morgan AW, Wilson AG, Plant D, Hyrich KL, et al. High frequency of antidrug antibodies and association of random drug levels with efficacy in certolizumab pegol-treated patients with rheumatoid arthritis: results from the BRAGGSS cohort. Ann Rheum Dis 2017;76:208-13. https://doi.org/10.1136/annrheumdis-2015-208849.
- l’Ami MJ, Krieckaert CL, Nurmohamed MT, van Vollenhoven RF, Rispens T, Boers M, et al. Successful reduction of overexposure in patients with rheumatoid arthritis with high serum adalimumab concentrations: an open-label, non-inferiority, randomised clinical trial. Ann Rheum Dis 2018;77:484-7. https://doi.org/10.1136/annrheumdis-2017-211781.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Jani M, Dixon WG, Lunt M, De Cock D, Isaacs JD, Morgan AW, et al. The association of biologic drug-levels with infection risk: results from the british society for rheumatology biologics register for rheumatoid arthritis. Ann Rheum Dis 2018;77:163-4. https://doi.org/10.1136/annrheumdis-2018-eular.1946.
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. London: NICE; 2014.
- NHS Greater Glasgow and Clyde . Rheumatology Biologic Drug Monitoring Recommendations. n.d. www.nhsggc.org.uk/media/246324/rheumatology-biologic-drug-monitoring-recommendations-september-2017.pdf (accessed November 2018).
- Pouw MF, Krieckaert CL, Nurmohamed MT, van der Kleij D, Aarden L, Rispens T, et al. Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis 2015;74:513-18. https://doi.org/10.1136/annrheumdis-2013-204172.
- Pascual-Salcedo D, Chamaida P, Teresa J, Del Valle GL, Prado S, Cristina D, et al. Dose-tapering of TNF inhibitors in daily rheumatology practice enables the maintenance of clinical efficacy while improving cost-effectiveness. Pharmacovigilance 2015;3. https://doi.org/10.4172/2329-6887.1000172.
Appendix 1 Literature search strategies
Enzyme-linked immunosorbent assay for tumour necrosis factor-α inhibitors in rheumatoid arthritis: clinical effectiveness searches
MEDLINE (via Ovid)
Date range searched: 1946 to week 2 July 2018.
Date searched: 20 July 2018.
Searcher: Sophie Robinson.
Hits: 1703.
Search strategy
-
(anti-TNF* or antiTNF* or (TNF* adj2 (inhibit* or block*))).tw.
-
anti* tumo?r* necrosis* factor*.tw.
-
Tumor Necrosis Factor-alpha/
-
(biologic* adj2 DMARD*).tw.
-
((antirheumati* or anti rheumati* or anti-rheumati*) adj4 biologic*).tw.
-
((disease modify* or disease-modify*) adj4 biologic*).tw.
-
exp Antibodies, Monoclonal/
-
anti* drug* antibod*.tw.
-
ADAb.tw.
-
etanercept.tw. or ETANERCEPT/
-
(tnr001 or “tnr 001” or tnr-001 or 185243-69-0).tw.
-
(ETA or ETN).tw.
-
(enbrel or erelzi or benepali or lifmior or brenzys).tw.
-
(anti-etanercept* or antietanercept* or (anti adj3 etanercept*)).tw.
-
adalimumab.tw. or ADALIMUMAB/
-
(d 2e7 or d2e7 or d-2e7 or 331731-18-1).tw.
-
(ADA or ADL or ADM).tw.
-
(humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz).tw.
-
(anti-adalimumab* or antiadalimumab* or (anti adj3 adalimumab*)).tw.
-
infliximab.tw. or INFLIXIMAB/
-
(170277-31-3 or ta650 or ta 650 or ta-650).tw.
-
(INF or IFX).tw.
-
(anti-infliximab* or antiinfliximab* or (anti adj3 infliximab*)).tw.
-
(remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi).tw.
-
Certolizumab Pegol/or certolizumab.tw.
-
(cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2).tw.
-
(CER or CZP).tw.
-
cimzia.tw.
-
(anti-certolizumab* or anticertolizumab* or (anti adj3 certolizumab*)).tw.
-
golimumab.tw.
-
(cnto 148 or cnto148 or cnto-148 or 476181-74-5).tw.
-
(GOL or GLM).tw.
-
simponi.tw.
-
(anti-golimumab* or antigolimumab* or (anti adj3 golimumab*)).tw.
-
(biologic* adj2 agent*).tw.
-
(CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5).tw.
-
(biosimilar* or (bio* adj1 similar*)).tw.
-
or/1-37
-
exp Enzyme-Linked Immunosorbent Assay/
-
(immundiagnostik* or immunodiagnostik* or immunediagnostik*).tw.
-
biohit healthcare.tw.
-
(proteomika* or *).tw.
-
(enzyme* adj3 immunoassay*).tw.
-
(enzyme* adj3 (immuno* assay* or immuno* test*)).tw.
-
ELISA*.tw.
-
(idkmonitor* or (idk adj3 monitor*) or idk-monitor*).tw.
-
((lisa adj3 tracker*) or lisa-tracker* or lisatracker*).tw.
-
(ridascreen* or (rida adj3 screen*) or rida-screen*).tw.
-
(mabtrack* or (mab adj3 track*) or mab-track*).tw.
-
sanquin.tw.
-
theradiag.tw.
-
(grifols or progenika).tw.
-
(r-biopharm or rbiopharm or r biopharm).tw.
-
((drug* or trough) adj3 (level* or concentration)).tw.
-
or/39-54
-
exp Arthritis, Rheumatoid/
-
RA.tw.
-
Rheumarthrit*.tw.
-
((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) adj4 (arthrit* or arthros* or polyarthrit* or factor*)).tw.
-
(Chronic* adj4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)).tw.
-
((Inflammat* or pain* or swell* or stiff*) adj4 (joint* or synovial*)).tw.
-
(Beauvais* adj2 disease*).tw.
-
or/56-62
-
38 and 55 and 63
-
animals/not humans/
-
64 not 65.
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Date ranged searched: 19 July 2018.
Date searched: 20 July 2018.
Searcher: Sophie Robinson.
Hits: 70.
EMBASE (via Ovid)
Date range searched: 1974 to 19 July 2018.
Date searched: 20 July 2018.
Searcher: Sophie Robinson.
Hits: 3807.
Search strategy
-
(anti-TNF* or antiTNF* or (TNF* adj2 (inhibit* or block*))).tw.
-
anti* tumo?r* necrosis* factor*.tw.
-
Tumor Necrosis Factor-alpha/
-
(biologic* adj2 DMARD*).tw.
-
((antirheumati* or anti rheumati* or anti-rheumati*) adj4 biologic*).tw.
-
((disease modify* or disease-modify*) adj4 biologic*).tw.
-
disease modifying antirheumatic drug/
-
monoclonal antibody/
-
anti* drug* antibod*.tw.
-
ADAb.tw.
-
etanercept.tw. or ETANERCEPT/
-
(tnr001 or “tnr 001” or tnr-001 or 185243-69-0).tw.
-
(ETA or ETN).tw.
-
(enbrel or erelzi or benepali or lifmior or brenzys).tw.
-
(anti-etanercept* or antietanercept* or (anti adj3 etanercept*)).tw.
-
adalimumab.tw. or ADALIMUMAB/
-
(d 2e7 or d2e7 or d-2e7 or 331731-18-1).tw.
-
(ADA or ADL or ADM).tw.
-
(humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz).tw.
-
(anti-adalimumab* or antiadalimumab* or (anti adj3 adalimumab*)).tw.
-
infliximab.tw. or INFLIXIMAB/
-
(170277-31-3 or ta650 or ta 650 or ta-650).tw.
-
(INF or IFX).tw.
-
(anti-infliximab* or antiinfliximab* or (anti adj3 infliximab*)).tw.
-
(remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi).tw.
-
Certolizumab Pegol/or certolizumab.tw.
-
(cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2).tw.
-
(CER or CZP).tw.
-
cimzia.tw.
-
(anti-certolizumab* or anticertolizumab* or (anti adj3 certolizumab*)).tw.
-
golimumab/or golimumab.tw.
-
(cnto 148 or cnto148 or cnto-148 or 476181-74-5).tw.
-
(GOL or GLM).tw.
-
simponi.tw.
-
(anti-golimumab* or antigolimumab* or (anti adj3 golimumab*)).tw.
-
(biologic* adj2 agent*).tw.
-
(CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5).tw.
-
biological product/or biosimilar agent/
-
(biosimilar* or (bio* adj1 similar*)).tw.
-
or/1-39
-
exp Enzyme-Linked Immunosorbent Assay/
-
(immundiagnostik* or immunodiagnostik* or immunediagnostik*).tw.
-
biohit healthcare.tw.
-
(proteomika* or promonitor*).tw.
-
(enzyme* adj3 immunoassay*).tw.
-
(enzyme* adj3 (immuno* assay* or immuno* test*)).tw.
-
ELISA*.tw.
-
(idkmonitor* or (idk adj3 monitor*) or idk-monitor*).tw.
-
((lisa adj3 tracker*) or lisa-tracker* or lisatracker*).tw.
-
(ridascreen* or (rida adj3 screen*) or rida-screen*).tw.
-
(mabtrack* or (mab adj3 track*) or mab-track*).tw.
-
sanquin.tw.
-
theradiag.tw.
-
(grifols or progenika).tw.
-
(r-biopharm or rbiopharm or r biopharm).tw.
-
((drug* or trough) adj3 (level* or concentration)).tw.
-
or/41-56
-
exp Arthritis, Rheumatoid/
-
RA.tw.
-
Rheumarthrit*.tw.
-
((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) adj4 (arthrit* or arthros* or polyarthrit* or factor*)).tw.
-
(Chronic* adj4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)).tw.
-
((Inflammat* or pain* or swell* or stiff*) adj4 (joint* or synovial*)).tw.
-
(Beauvais* adj2 disease*).tw.
-
or/58-64
-
40 and 57 and 65
-
(exp animal/or nonhuman/) not exp human/
-
66 not 67.
Web of Science (SCI and CPCI-S) (via Clarivate Analytics)
Date range searched: N/A.
Date searched: 20 July 2018.
Searcher: Sophie Robinson.
Hits: 3633.
Search strategy
-
#1. TS = (anti-TNF* or antiTNF* or (TNF* near/1 (inhibit* or block*))) OR TS = tumo$r* necrosis* factor* alpha OR TS = (biologic* near/1 DMARD*) OR TS = (biologic* near/3 antirheumati*) OR TS = (anti rheumati* near/3 biologic*) OR TS = (disease modify* near/3 biologic*) OR TS = anti* drug* antibod* OR TS = ADAb OR TS = anti* tumo$r* necrosis* factor* OR TS = monoclonal antibod*
-
#2. TS = etanercept OR TS = (tnr001 or tnr 001 or tnr-001 or 185243-69-0) OR TS = (ETA or ETN) OR TS = (enbrel or erelzi or benepali or lifmior or brenzys) OR TS = (anti-etanercept* or antietanercept* or anti near/2 etanercept*)
-
#3. TS = adalimumab OR TS = (d 2e7 or d2e7 or d-2e7 or 331731-18-1) OR TS = (ADA or ADL or ADM) OR TS = (humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz) OR TS = (anti-adalimumab* or antiadalimumab* or anti near/2 adalimumab*)
-
#4. TS = infliximab OR TS = (170277-31-3 or ta650 or ta 650 or ta-650) OR TS = (INF or IFX) OR TS = (anti-infliximab* or antiinfliximab* or anti near/2 infliximab*) OR TS = (remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi)
-
#5. TS = certolizumab OR TS = (cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2) OR TS = (CER or CZP) OR TS = cimzia OR TS = (anti-certolizumab* or anticertolizumab* or anti near/2 certolizumab*)
-
#6. TS = golimumab OR TS = (cnto 148 or cnto148 or cnto-148 or 476181-74-5) OR TS = (GOL or GLM) OR TS = simponi OR TS = (anti-golimumab* or antigolimumab* or anti near/2 golimumab*)
-
#7. TS = (biologic* near/1 agent*) OR TS = (CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5) OR TS = (biosimilar* or bio* similar*)
-
#8. #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
-
#9. TS = (immundiagnostik* or immunodiagnostik* or immunediagnostik*) OR TS = biohit healthcare OR TS = (proteomika* or promonitor*) OR TS = (enzyme* near/2 immunoassay*) OR TS = (enzyme* near/2 immuno* assay*) OR TS = (enzyme* near/2 immuno* test*) OR TS = ELISA*
-
#10. TS = (idkmonitor* or idk near/2 monitor* or idk-monitor*) OR TS = (lisa near/2 tracker* or lisa-tracker* or lisatracker*) OR TS = (ridascreen* or rida near/2 screen* or rida-screen*) OR TS = (mabtrack* or mab near/2 track* or mab-track*) OR TS = (sanquin or theradiag) OR TS = (grifols or progenika) OR TS = (r-biopharm or rbiopharm or r biopharm) OR TS = ((drug* or trough) near/2 (level* or concentration))
-
#11. #10 OR #9
-
#12. TS = RA OR TS = Rheumarthrit* OR TS = ((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) near/3 (arthrit* or arthros* or polyarthrit* or factor*)) OR TS = (chronic* near/3 polyarthrit*) OR TS = (chronic* near/3 poly arthrit*) OR TS = (chronic* near/3 rheumati*) OR TS = ((Inflammat* or pain* or swell* or stiff*) near/3 (joint* or synovial*)) OR TS = (Beauvais* adj2 disease*)
-
#13. #12 AND #11 AND #8 Indexes = SCI-EXPANDED, CPCI-S Timespan = 1900-2018;
Cochrane Library (via Cochrane Collaboration)
Data parameters: CDSR issue 7 of 12 July 2018 and CENTRAL issue 6 of 12 June 2018.
Date searched: 20 July 2018.
Searcher: Sophie Robinson.
Hits: 255.
Search strategy
-
#1. (anti-TNF* or antiTNF* or (TNF* near/2 (inhibit* or block*))):ti,ab,kw
-
#2. “anti* tumo*r* necrosis* factor*”:ti,ab,kw
-
#3. MeSH descriptor: [Tumor Necrosis Factor-alpha] this term only
-
#4. (biologic* near/2 DMARD*):ti,ab,kw
-
#5. ((antirheumati* or “anti rheumati*” or anti-rheumati*) near/4 biologic*):ti,ab,kw
-
#6. ((“disease modify*” or disease-modify*) near/4 biologic*):ti,ab,kw
-
#7. MeSH descriptor: [Antibodies, Monoclonal] explode all trees
-
#8. “anti* drug* antibod*”:ti,ab,kw
-
#9. ADAb:ti,ab
-
#10. etanercept:ti,ab,kw
-
#11. MeSH descriptor: [Etanercept] this term only
-
#12. (tnr001 or “tnr 001” or tnr-001 or 185243-69-0):ti,ab
-
#13. (ETA or ETN):ti,ab
-
#14. (enbrel or erelzi or benepali or lifmior or brenzys):ti,ab,kw
-
#15. (anti-etanercept* or antietanercept* or (anti near/3 etanercept*)):ti,ab,kw
-
#16. adalimumab:ti,ab,kw
-
#17. MeSH descriptor: [Adalimumab] this term only
-
#18. (“d 2e7” or d2e7 or d-2e7 or 331731-18-1):ti,ab
-
#19. (ADA or ADL or ADM):ti,ab
-
#20. (humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz):ti,ab,kw
-
#21. (anti-adalimumab* or antiadalimumab* or (anti near/3 adalimumab*)):ti,ab,kw
-
#22. infliximab:ti,ab,kw
-
#23. MeSH descriptor: [Infliximab] this term only
-
#24. (170277-31-3 or ta650 or “ta 650” or ta-650):ti,ab
-
#25. (INF or IFX):ti,ab
-
#26. (anti-infliximab* or antiinfliximab* or (anti near/3 infliximab*)):ti,ab,kw
-
#27. (remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi):ti,ab,kw
-
#28. certolizumab:ti,ab,kw
-
#29. MeSH descriptor: [Certolizumab Pegol] this term only
-
#30. (cdp870 or “cdp 870” or cdp-870 or 428863-50-7 or 1132819-27-2):ti,ab
-
#31. (CER or CZP):ti,ab
-
#32. cimzia:ti,ab,kw
-
#33. (anti-certolizumab* or anticertolizumab* or (anti near/3 certolizumab*)):ti,ab,kw
-
#34. golimumab:ti,ab,kw
-
#35. (“cnto 148” or cnto148 or cnto-148 or 476181-74-5):ti,ab
-
#36. (GOL or GLM):ti,ab
-
#37. simponi:ti,ab,kw
-
#38. (anti-golimumab* or antigolimumab* or (anti near/3 golimumab*)):ti,ab,kw
-
#39. (biologic* near/2 agent*):ti,ab,kw
-
#40. (CT-P13 or CTP13 or “CT P13” or SB2 or SB-2 or “SB 2” or SB4 or SB-4 or “SB 4” or SB-5 or SB5 or “SB 5”):ti,ab
-
#41. (biosimilar* or “bio* similar*”):ti,ab,kw
-
#42. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41
-
#43. MeSH descriptor: [Enzyme-Linked Immunosorbent Assay] explode all trees
-
#44. (immundiagnostik* or immunodiagnostik* or immunediagnostik*):ti,ab,kw
-
#45. “biohit healthcare”:ti,ab,kw
-
#46. (proteomika* or promonitor*):ti,ab,kw
-
#47. (enzyme* near/3 immunoassay*):ti,ab,kw
-
#48. (enzyme* near/3 (“immuno* assay*” or “immuno* test*”)):ti,ab,kw
-
#49. ELISA*:ti,ab,kw
-
#50. (idkmonitor* or (idk near/3 monitor*) or idk-monitor*):ti,ab,kw
-
#51. ((lisa near/3 tracker*) or lisa-tracker* or lisatracker*):ti,ab,kw
-
#52. (ridascreen* or (rida near/3 screen*) or rida-screen*):ti,ab,kw
-
#53. (mabtrack* or (mab near/3 track*) or mab-track*):ti,ab,kw
-
#54. (sanquin or theradiag):ti,ab,kw
-
#55. (grifols or progenika):ti,ab,kw
-
#56. (r-biopharm or rbiopharm or “r biopharm”):ti,ab,kw
-
#57. ((drug* or trough) near/3 (level* or concentration)):ti,ab,kw
-
#58. #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57
-
#59. MeSH descriptor: [Arthritis, Rheumatoid] explode all trees
-
#60. RA:ti,ab
-
#61. Rheumarthrit*.ti,ab,kw
-
#62. ((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) near/4 (arthrit* or arthros* or polyarthrit* or factor*)):ti,ab,kw
-
#63. (Chronic* near/4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)):ti,ab,kw
-
#64. ((Inflammat* or pain* or swell* or stiff*) near/4 (joint* or synovial*)):ti,ab,kw
-
#65. (Beauvais* near/2 disease*):ti,ab,kw
-
#66. #59 or #60 or #61 or #62 or #64 or #65
-
#67. #42 and #58 and #66
| Database | Hits |
|---|---|
| MEDLINE | 1703 |
| MEDLINE In-Process & Other Non-Indexed Citations | 70 |
| EMBASE | 3807 |
| Web of Science (SCI and SCCI) | 3633 |
| Cochrane | 255 |
| Total records | 9468 |
| Duplicates | 2851 |
| Total unique records | 6617 |
Backward citation chasing
Citation chasing yielded 42 further references (after deduplicating and checking against already screened papers), on 12 September 2018.
ELISA for TNF-α inhibitors in rheumatoid arthritis – cost-effectiveness searches
MEDLINE (via Ovid)
Date range searched: 1946 to week 2 of July 2018.
Date searched: 26 July 2018.
Searcher: Sophie Robinson.
Hits: 4.
Search strategy
-
(anti-TNF* or antiTNF* or (TNF* adj2 (inhibit* or block*))).tw.
-
anti* tumo?r* necrosis* factor*.tw.
-
Tumor Necrosis Factor-alpha/
-
(biologic* adj2 DMARD*).tw.
-
((antirheumati* or anti rheumati* or anti-rheumati*) adj4 biologic*).tw.
-
((disease modify* or disease-modify*) adj4 biologic*).tw.
-
exp Antibodies, Monoclonal/
-
anti* drug* antibod*.tw.
-
ADAb.tw.
-
etanercept.tw. or ETANERCEPT/
-
(tnr001 or “tnr 001” or tnr-001 or 185243-69-0).tw.
-
(ETA or ETN).tw.
-
(enbrel or erelzi or benepali or lifmior or brenzys).tw.
-
(anti-etanercept* or antietanercept* or (anti adj3 etanercept*)).tw.
-
adalimumab.tw. or ADALIMUMAB/
-
(d 2e7 or d2e7 or d-2e7 or 331731-18-1).tw.
-
(ADA or ADL or ADM).tw.
-
(humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz).tw.
-
19. (anti-adalimumab* or antiadalimumab* or (anti adj3 adalimumab*)).tw.
-
infliximab.tw. or INFLIXIMAB/
-
(170277-31-3 or ta650 or ta 650 or ta-650).tw.
-
(INF or IFX).tw.
-
(anti-infliximab* or antiinfliximab* or (anti adj3 infliximab*)).tw.
-
(remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi).tw.
-
Certolizumab Pegol/or certolizumab.tw.
-
(cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2).tw.
-
(CER or CZP).tw.
-
cimzia.tw.
-
(anti-certolizumab* or anticertolizumab* or (anti adj3 certolizumab*)).tw.
-
golimumab.tw.
-
(cnto 148 or cnto148 or cnto-148 or 476181-74-5).tw.
-
(GOL or GLM).tw.
-
simponi.tw.
-
(anti-golimumab* or antigolimumab* or (anti adj3 golimumab*)).tw.
-
(biologic* adj2 agent*).tw.
-
(CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5).tw.
-
(biosimilar* or (bio* adj1 similar*)).tw.
-
or/1-37
-
exp Enzyme-Linked Immunosorbent Assay/
-
(immundiagnostik* or immunodiagnostik* or immunediagnostik*).tw.
-
biohit healthcare.tw.
-
(proteomika* or promonitor*).tw.
-
(enzyme* adj3 immunoassay*).tw.
-
(enzyme* adj3 (immuno* assay* or immuno* test*)).tw.
-
ELISA*.tw.
-
(idkmonitor* or (idk adj3 monitor*) or idk-monitor*).tw.
-
((lisa adj3 tracker*) or lisa-tracker* or lisatracker*).tw.
-
(ridascreen* or (rida adj3 screen*) or rida-screen*).tw.
-
(mabtrack* or (mab adj3 track*) or mab-track*).tw.
-
sanquin.tw.
-
theradiag.tw.
-
(grifols or progenika).tw.
-
(r-biopharm or rbiopharm or r biopharm).tw.
-
((drug* or trough) adj3 (level* or concentration)).tw.
-
or/39-54
-
exp Arthritis, Rheumatoid/
-
RA.tw.
-
Rheumarthrit*.tw.
-
((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) adj4 (arthrit* or arthros* or polyarthrit* or factor*)).tw.
-
(Chronic* adj4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)).tw.
-
((Inflammat* or pain* or swell* or stiff*) adj4 (joint* or synovial*)).tw.
-
(Beauvais* adj2 disease*).tw.
-
or/56-62
-
38 and 55 and 63
-
animals/not humans/
-
64 not 65
-
Economics/
-
exp “Costs and Cost Analysis”/
-
Economics, Nursing/
-
Economics, Medical/
-
Economics, Pharmaceutical/
-
exp Economics, Hospital/
-
Economics, Dental/
-
exp “Fees and Charges”/
-
exp Budgets/
-
budget*.ti,ab,kf.
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kf.
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab./freq = 2
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf.
-
(value adj2 (money or monetary)).ti,ab,kf.
-
exp models, economic/
-
economic model*.ab,kf.
-
markov chains/
-
markov.ti,ab,kf.
-
monte carlo method/
-
monte carlo.ti,ab,kf.
-
exp Decision Theory/
-
(decision* adj2 (tree* or analy* or model*)).ti,ab,kf.
-
or/67-88
-
66 and 89.
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Date range searched: 25 July 2018.
Date searched: 25 July 2018.
Searcher: Sophie Robinson.
Hits: 1.
Search strategy
-
(anti-TNF* or antiTNF* or (TNF* adj2 (inhibit* or block*))).tw.
-
anti* tumo?r* necrosis* factor*.tw.
-
(biologic* adj2 DMARD*).tw.
-
((antirheumati* or anti rheumati* or anti-rheumati*) adj4 biologic*).tw.
-
((disease modify* or disease-modify*) adj4 biologic*).tw.
-
anti* drug* antibod*.tw.
-
ADAb.tw.
-
etanercept.tw.
-
(tnr001 or “tnr 001” or tnr-001 or 185243-69-0).tw.
-
(ETA or ETN).tw.
-
(enbrel or erelzi or benepali or lifmior or brenzys).tw.
-
(anti-etanercept* or antietanercept* or (anti adj3 etanercept*)).tw.
-
adalimumab.tw.
-
(d 2e7 or d2e7 or d-2e7 or 331731-18-1).tw.
-
(ADA or ADL or ADM).tw.
-
(humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz).tw.
-
(anti-adalimumab* or antiadalimumab* or (anti adj3 adalimumab*)).tw.
-
infliximab.tw.
-
(170277-31-3 or ta650 or ta 650 or ta-650).tw.
-
(INF or IFX).tw.
-
(anti-infliximab* or antiinfliximab* or (anti adj3 infliximab*)).tw.
-
(remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi).tw.
-
certolizumab.tw.
-
(cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2).tw.
-
(CER or CZP).tw.
-
cimzia.tw.
-
(anti-certolizumab* or anticertolizumab* or (anti adj3 certolizumab*)).tw.
-
golimumab.tw.
-
(cnto 148 or cnto148 or cnto-148 or 476181-74-5).tw.
-
(GOL or GLM).tw.
-
simponi.tw.
-
(anti-golimumab* or antigolimumab* or (anti adj3 golimumab*)).tw.
-
(biologic* adj2 agent*).tw.
-
(CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5).tw.
-
(biosimilar* or (bio* adj1 similar*)).tw.
-
or/1-35
-
(immundiagnostik* or immunodiagnostik* or immunediagnostik*).tw.
-
biohit healthcare.tw.
-
(proteomika* or promonitor*).tw.
-
(enzyme* adj3 immunoassay*).tw.
-
(enzyme* adj3 (immuno* assay* or immuno* test*)).tw.
-
ELISA*.tw.
-
(idkmonitor* or (idk adj3 monitor*) or idk-monitor*).tw.
-
((lisa adj3 tracker*) or lisa-tracker* or lisatracker*).tw.
-
(ridascreen* or (rida adj3 screen*) or rida-screen*).tw.
-
(mabtrack* or (mab adj3 track*) or mab-track*).tw.
-
sanquin.tw.
-
theradiag.tw.
-
(grifols or progenika).tw.
-
(r-biopharm or rbiopharm or r biopharm).tw.
-
((drug* or trough) adj3 (level* or concentration)).tw.
-
or/37-51
-
RA.tw.
-
Rheumarthrit*.tw.
-
((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) adj4 (arthrit* or arthros* or polyarthrit* or factor*)).tw.
-
(Chronic* adj4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)).tw.
-
((Inflammat* or pain* or swell* or stiff*) adj4 (joint* or synovial*)).tw.
-
(Beauvais* adj2 disease*).tw.
-
or/53-58
-
36 and 52 and 59
-
budget*.ti,ab,kf.
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kf.
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab./freq = 2
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf.
-
(value adj2 (money or monetary)).ti,ab,kf.
-
economic model*.ab,kf.
-
markov.ti,ab,kf.
-
monte carlo.ti,ab,kf.
-
(decision* adj2 (tree* or analy* or model*)).ti,ab,kf.
-
or/61-69
-
60 and 70.
EMBASE (via Ovid)
Date range searched: 1974 to 25 July 2018.
Date searched: 26 July 2018.
Searcher: Sophie Robinson.
Hits: 102.
Search strategy
-
(anti-TNF* or antiTNF* or (TNF* adj2 (inhibit* or block*))).tw.
-
anti* tumo?r* necrosis* factor*.tw.
-
Tumor Necrosis Factor-alpha/
-
(biologic* adj2 DMARD*).tw.
-
((antirheumati* or anti rheumati* or anti-rheumati*) adj4 biologic*).tw.
-
((disease modify* or disease-modify*) adj4 biologic*).tw.
-
disease modifying antirheumatic drug/
-
monoclonal antibody/
-
anti* drug* antibod*.tw.
-
ADAb.tw.
-
etanercept.tw. or ETANERCEPT/
-
(tnr001 or “tnr 001” or tnr-001 or 185243-69-0).tw.
-
(ETA or ETN).tw.
-
(enbrel or erelzi or benepali or lifmior or brenzys).tw.
-
(anti-etanercept* or antietanercept* or (anti adj3 etanercept*)).tw.
-
adalimumab.tw. or ADALIMUMAB/
-
(d 2e7 or d2e7 or d-2e7 or 331731-18-1).tw.
-
(ADA or ADL or ADM).tw.
-
(humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz).tw.
-
(anti-adalimumab* or antiadalimumab* or (anti adj3 adalimumab*)).tw.
-
infliximab.tw. or INFLIXIMAB/
-
(170277-31-3 or ta650 or ta 650 or ta-650).tw.
-
(INF or IFX).tw.
-
(anti-infliximab* or antiinfliximab* or (anti adj3 infliximab*)).tw.
-
(remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi).tw.
-
Certolizumab Pegol/or certolizumab.tw.
-
(cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2).tw.
-
(CER or CZP).tw.
-
cimzia.tw.
-
(anti-certolizumab* or anticertolizumab* or (anti adj3 certolizumab*)).tw.
-
golimumab/or golimumab.tw.
-
(cnto 148 or cnto148 or cnto-148 or 476181-74-5).tw.
-
(GOL or GLM).tw.
-
simponi.tw.
-
(anti-golimumab* or antigolimumab* or (anti adj3 golimumab*)).tw.
-
(biologic* adj2 agent*).tw.
-
(CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5).tw.
-
biological product/or biosimilar agent/
-
(biosimilar* or (bio* adj1 similar*)).tw.
-
or/1-39
-
exp Enzyme-Linked Immunosorbent Assay/
-
(immundiagnostik* or immunodiagnostik* or immunediagnostik*).tw.
-
biohit healthcare.tw.
-
(proteomika* or promonitor*).tw.
-
(enzyme* adj3 immunoassay*).tw.
-
(enzyme* adj3 (immuno* assay* or immuno* test*)).tw.
-
ELISA*.tw.
-
(idkmonitor* or (idk adj3 monitor*) or idk-monitor*).tw.
-
((lisa adj3 tracker*) or lisa-tracker* or lisatracker*).tw.
-
(ridascreen* or (rida adj3 screen*) or rida-screen*).tw.
-
(mabtrack* or (mab adj3 track*) or mab-track*).tw.
-
sanquin.tw.
-
theradiag.tw.
-
(grifols or progenika).tw.
-
(r-biopharm or rbiopharm or r biopharm).tw.
-
((drug* or trough) adj3 (level* or concentration)).tw.
-
or/41-56
-
exp Arthritis, Rheumatoid/
-
RA.tw.
-
Rheumarthrit*.tw.
-
((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) adj4 (arthrit* or arthros* or polyarthrit* or factor*)).tw.
-
(Chronic* adj4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)).tw.
-
((Inflammat* or pain* or swell* or stiff*) adj4 (joint* or synovial*)).tw.
-
(Beauvais* adj2 disease*).tw.
-
or/58-64
-
40 and 57 and 65
-
(exp animal/or nonhuman/) not exp human/
-
66 not 67
-
Economics/
-
Cost/
-
exp Health Economics/
-
Budget/
-
budget*.ti,ab,kw.
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kw.
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab./freq = 2
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kw.
-
(value adj2 (money or monetary)).ti,ab,kw.
-
Statistical Model/
-
economic model*.ab,kw.
-
Probability/
-
markov.ti,ab,kw.
-
monte carlo method/
-
monte carlo.ti,ab,kw.
-
Decision Theory/
-
Decision Tree/
-
(decision* adj2 (tree* or analy* or model*)).ti,ab,kw.
-
or/69-86
-
68 and 87.
Web of Science (SCI and CPCI-S) (via Clarivate Analytics)
Date range searched: N/A.
Date searched: 2 July 2018.
Searcher: Sophie Robinson.
Hits: 63.
Search strategy
-
#1. TS = (anti-TNF* or antiTNF* or (TNF* near/1 (inhibit* or block*))) OR TS = tumo$r* necrosis* factor* alpha OR TS = (biologic* near/1 DMARD*) OR TS = (biologic* near/3 antirheumati*) OR TS = (anti rheumati* near/3 biologic*) OR TS = (disease modify* near/3 biologic*) OR TS = anti* drug* antibod* OR TS = ADAb OR TS = anti* tumo$r* necrosis* factor* OR TS = monoclonal antibod*
-
#2. TS = etanercept OR TS = (tnr001 or tnr 001 or tnr-001 or 185243-69-0) OR TS = (ETA or ETN) OR TS = (enbrel or erelzi or benepali or lifmior or brenzys) OR TS = (anti-etanercept* or antietanercept* or anti near/2 etanercept*)
-
#3. TS = adalimumab OR TS = (d 2e7 or d2e7 or d-2e7 or 331731-18-1) OR TS = (ADA or ADL or ADM) OR TS = (humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz) OR TS = (anti-adalimumab* or antiadalimumab* or anti near/2 adalimumab*)
-
#4. TS = infliximab OR TS = (170277-31-3 or ta650 or ta 650 or ta-650) OR TS = (INF or IFX) OR TS = (anti-infliximab* or antiinfliximab* or anti near/2 infliximab*) OR TS = (remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi)
-
#5. TS = certolizumab OR TS = (cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2) OR TS = (CER or CZP) OR TS = cimzia OR TS = (anti-certolizumab* or anticertolizumab* or anti near/2 certolizumab*)
-
#6. TS = golimumab OR TS = (cnto 148 or cnto148 or cnto-148 or 476181-74-5) OR TS = (GOL or GLM) OR TS = simponi OR TS = (anti-golimumab* or antigolimumab* or anti near/2 golimumab*)
-
#7. TS = (biologic* near/1 agent*) OR TS = (CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5) OR TS = (biosimilar* or bio* similar*)
-
#8. #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
-
#9. TS = (immundiagnostik* or immunodiagnostik* or immunediagnostik*) OR TS = biohit healthcare OR TS = (proteomika* or promonitor*) OR TS = (enzyme* near/2 immunoassay*) OR TS = (enzyme* near/2 immuno* assay*) OR TS = (enzyme* near/2 immuno* test*) OR TS = ELISA*
-
#10. TS = (idkmonitor* or idk near/2 monitor* or idk-monitor*) OR TS = (lisa near/2 tracker* or lisa-tracker* or lisatracker*) OR TS = (ridascreen* or rida near/2 screen* or rida-screen*) OR TS = (mabtrack* or mab near/2 track* or mab-track*) OR TS = (sanquin or theradiag) OR TS = (grifols or progenika) OR TS = (r-biopharm or rbiopharm or r biopharm) OR TS = ((drug* or trough) near/2 (level* or concentration))
-
#11. #10 OR #9
-
#12. TS = RA OR TS = Rheumarthrit* OR TS = ((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) near/3 (arthrit* or arthros* or polyarthrit* or factor*)) OR TS = (chronic* near/3 polyarthrit*) OR TS = (chronic* near/3 poly arthrit*) OR TS = (chronic* near/3 rheumati*) OR TS = ((Inflammat* or pain* or swell* or stiff*) near/3 (joint* or synovial*)) OR TS = (Beauvais* adj2 disease*)
-
#13. #12 AND #11 AND #8
-
14. TS = ((pharmacoeconomic* or socioeconomics or economic* or pric* or cost* or cba or cea or cua or “health utilit*” or “value for money”))
-
15. #14 and #15.
Database: NHS Economic Evaluation Database (via the Cochrane Library)
Data parameters: Issue 2 of 4 April 2015.
Date searched: 26 July 2018.
Searcher: Sophie Robinson.
Hits: 0.
Search strategy
-
#1. (anti-TNF* or antiTNF* or (TNF* near/2 (inhibit* or block*))):ti,ab,kw
-
#2. “anti* tumo*r* necrosis* factor*”:ti,ab,kw
-
#3. MeSH descriptor: [Tumor Necrosis Factor-alpha] this term only
-
#4. (biologic* near/2 DMARD*):ti,ab,kw
-
#5. ((antirheumati* or “anti rheumati*” or anti-rheumati*) near/4 biologic*):ti,ab,kw
-
#6. ((“disease modify*” or disease-modify*) near/4 biologic*):ti,ab,kw
-
#7. MeSH descriptor: [Antibodies, Monoclonal] explode all trees
-
#8. “anti* drug* antibod*”:ti,ab,kw
-
#9. ADAb:ti,ab
-
#10. etanercept:ti,ab,kw
-
#11. MeSH descriptor: [Etanercept] this term only
-
#12. (tnr001 or “tnr 001” or tnr-001 or 185243-69-0):ti,ab
-
#13. (ETA or ETN):ti,ab
-
#14. (enbrel or erelzi or benepali or lifmior or brenzys):ti,ab,kw
-
#15. (anti-etanercept* or antietanercept* or (anti near/3 etanercept*)):ti,ab,kw
-
#16. adalimumab:ti,ab,kw
-
#17. MeSH descriptor: [Adalimumab] this term only
-
#18. (“d 2e7” or d2e7 or d-2e7 or 331731-18-1):ti,ab
-
#19. (ADA or ADL or ADM):ti,ab
-
#20. (humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz):ti,ab,kw
-
#21. (anti-adalimumab* or antiadalimumab* or (anti near/3 adalimumab*)):ti,ab,kw
-
#22. infliximab:ti,ab,kw
-
#23. MeSH descriptor: [Infliximab] this term only
-
#24. (170277-31-3 or ta650 or “ta 650” or ta-650):ti,ab
-
#25. (INF or IFX):ti,ab
-
#26. (anti-infliximab* or antiinfliximab* or (anti near/3 infliximab*)):ti,ab,kw
-
#27. (remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi):ti,ab,kw
-
#28. certolizumab:ti,ab,kw
-
#29. MeSH descriptor: [Certolizumab Pegol] this term only
-
#30. (cdp870 or “cdp 870” or cdp-870 or 428863-50-7 or 1132819-27-2):ti,ab
-
#31. (CER or CZP):ti,ab
-
#32. cimzia:ti,ab,kw
-
#33. (anti-certolizumab* or anticertolizumab* or (anti near/3 certolizumab*)):ti,ab,kw
-
#34. golimumab:ti,ab,kw
-
#35. (“cnto 148” or cnto148 or cnto-148 or 476181-74-5):ti,ab
-
#36. (GOL or GLM):ti,ab
-
#37. simponi:ti,ab,kw
-
#38. (anti-golimumab* or antigolimumab* or (anti near/3 golimumab*)):ti,ab,kw
-
#39. (biologic* near/2 agent*):ti,ab,kw
-
#40. (CT-P13 or CTP13 or “CT P13” or SB2 or SB-2 or “SB 2” or SB4 or SB-4 or “SB 4” or SB-5 or SB5 or “SB 5”):ti,ab
-
#41. (biosimilar* or “bio* similar*”):ti,ab,kw
-
#42. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41
-
#43. MeSH descriptor: [Enzyme-Linked Immunosorbent Assay] explode all trees
-
#44. (immundiagnostik* or immunodiagnostik* or immunediagnostik*):ti,ab,kw
-
#45. “biohit healthcare”:ti,ab,kw
-
#46. (proteomika* or promonitor*):ti,ab,kw
-
#47. (enzyme* near/3 immunoassay*):ti,ab,kw
-
#48. (enzyme* near/3 (“immuno* assay*” or “immuno* test*”)):ti,ab,kw
-
#49. ELISA*:ti,ab,kw
-
#50. (idkmonitor* or (idk near/3 monitor*) or idk-monitor*):ti,ab,kw
-
#51. ((lisa near/3 tracker*) or lisa-tracker* or lisatracker*):ti,ab,kw
-
#52. (ridascreen* or (rida near/3 screen*) or rida-screen*):ti,ab,kw
-
#53. (mabtrack* or (mab near/3 track*) or mab-track*):ti,ab,kw
-
#54. (sanquin or theradiag):ti,ab,kw
-
#55. (grifols or progenika):ti,ab,kw
-
#56. (r-biopharm or rbiopharm or “r biopharm”):ti,ab,kw
-
#57. ((drug* or trough) near/3 (level* or concentration)):ti,ab,kw
-
#58. #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57
-
#59. MeSH descriptor: [Arthritis, Rheumatoid] explode all trees
-
#60. RA:ti,ab
-
#61. Rheumarthrit*.ti,ab,kw
-
#62. ((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) near/4 (arthrit* or arthros* or polyarthrit* or factor*)):ti,ab,kw
-
#63. (Chronic* near/4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)):ti,ab,kw
-
#64. ((Inflammat* or pain* or swell* or stiff*) near/4 (joint* or synovial*)):ti,ab,kw
-
#65. (Beauvais* near/2 disease*):ti,ab,kw
-
#66. #59 or #60 or #61 or #62 or #64 or #65
-
#67. #42 and #58 and #66.
EconLit (via EBSCOhost)
Date range searched: N/A.
Date searched: 2 July 2018.
Searcher: Sophie Robinson.
Hits: 56.
Search strategy
-
#1. TX Rheumarthrit*
-
#2. TX ((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) N4 (arthrit* or arthros* or polyarthrit* or factor*))
-
#3. TX ((Chronic* N4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*))
-
#4. TX ((Inflammat* or pain* or swell* or stiff*) N4 (joint* or synovial*))
-
#5. S1 OR S2 OR S3 OR S4.
| Database | Hits |
|---|---|
| MEDLINE | 5 |
| MEDLINE In-Process & Other Non-Indexed Citations | 1 |
| EMBASE | 102 |
| Web of Science (SCI and SCCI) | 63 |
| Cochrane – HTA and NHS EED | 0 |
| EconLit | 56 |
| Total records | 227 |
| Duplicates | 13 |
| Total unique records | 214 |
ELISA for TNF-α inhibitors in rheumatoid arthritis: utilities searches
MEDLINE (via Ovid)
Date range searched: 1946 to week 3 July 2018.
Date searched: 30 July 2018.
Searcher: Sophie Robinson.
Hits: 136.
Search strategy
-
(anti-TNF* or antiTNF* or (TNF* adj2 (inhibit* or block*))).tw.
-
anti* tumo?r* necrosis* factor*.tw.
-
Tumor Necrosis Factor-alpha/
-
(biologic* adj2 DMARD*).tw.
-
((antirheumati* or anti rheumati* or anti-rheumati*) adj4 biologic*).tw.
-
((disease modify* or disease-modify*) adj4 biologic*).tw.
-
exp Antibodies, Monoclonal/
-
anti* drug* antibod*.tw.
-
ADAb.tw.
-
etanercept.tw. or ETANERCEPT/
-
(tnr001 or “tnr 001” or tnr-001 or 185243-69-0).tw.
-
(ETA or ETN).tw.
-
(enbrel or erelzi or benepali or lifmior or brenzys).tw.
-
(anti-etanercept* or antietanercept* or (anti adj3 etanercept*)).tw.
-
adalimumab.tw. or ADALIMUMAB/
-
(d 2e7 or d2e7 or d-2e7 or 331731-18-1).tw.
-
(ADA or ADL or ADM).tw.
-
(humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz).tw.
-
(anti-adalimumab* or antiadalimumab* or (anti adj3 adalimumab*)).tw.
-
infliximab.tw. or INFLIXIMAB/
-
(170277-31-3 or ta650 or ta 650 or ta-650).tw.
-
(INF or IFX).tw.
-
(anti-infliximab* or antiinfliximab* or (anti adj3 infliximab*)).tw.
-
(remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi).tw.
-
Certolizumab Pegol/or certolizumab.tw.
-
(cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2).tw.
-
(CER or CZP).tw.
-
cimzia.tw.
-
(anti-certolizumab* or anticertolizumab* or (anti adj3 certolizumab*)).tw.
-
golimumab.tw.
-
(cnto 148 or cnto148 or cnto-148 or 476181-74-5).tw.
-
(GOL or GLM).tw.
-
simponi.tw.
-
(anti-golimumab* or antigolimumab* or (anti adj3 golimumab*)).tw.
-
(biologic* adj2 agent*).tw.
-
(CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5).tw.
-
(biosimilar* or (bio* adj1 similar*)).tw.
-
or/1-37
-
exp Enzyme-Linked Immunosorbent Assay/
-
(immundiagnostik* or immunodiagnostik* or immunediagnostik*).tw.
-
biohit healthcare.tw.
-
(proteomika* or promonitor*).tw.
-
(enzyme* adj3 immunoassay*).tw.
-
(enzyme* adj3 (immuno* assay* or immuno* test*)).tw.
-
ELISA*.tw.
-
(idkmonitor* or (idk adj3 monitor*) or idk-monitor*).tw.
-
((lisa adj3 tracker*) or lisa-tracker* or lisatracker*).tw.
-
(ridascreen* or (rida adj3 screen*) or rida-screen*).tw.
-
(mabtrack* or (mab adj3 track*) or mab-track*).tw.
-
sanquin.tw.
-
theradiag.tw.
-
(grifols or progenika).tw.
-
(r-biopharm or rbiopharm or r biopharm).tw.
-
((drug* or trough) adj3 (level* or concentration)).tw.
-
or/39-54
-
exp Arthritis, Rheumatoid/
-
RA.tw.
-
Rheumarthrit*.tw.
-
((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) adj4 (arthrit* or arthros* or polyarthrit* or factor*)).tw.
-
(Chronic* adj4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)).tw.
-
((Inflammat* or pain* or swell* or stiff*) adj4 (joint* or synovial*)).tw.
-
(Beauvais* adj2 disease*).tw.
-
or/56-62
-
38 and 55 and 63
-
animals/not humans/
-
64 not 65
-
“Value of Life”/
-
Quality of Life/
-
quality of life.ti,kf.
-
((instrument or instruments) adj3 quality of life).ab.
-
Quality-Adjusted Life Years/
-
quality adjusted life.ti,ab,kf.
-
(qaly* or qald* or qale* or qtime* or life year or life years).ti,ab,kf.
-
disability adjusted life.ti,ab,kf.
-
daly*.ti,ab,kf.
-
(sf36 or sf 36 or short form 36 or shortform 36 or short form36 or shortform36 or sf thirtysix or sfthirtysix or sfthirty six or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab,kf.
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six or shortform6 or short form6).ti,ab,kf.
-
(sf8 or sf 8 or sf eight or sfeight or shortform 8 or shortform 8 or shortform8 or short form8 or shortform eight or short form eight).ti,ab,kf.
-
(sf12 or sf 12 or short form 12 or shortform 12 or short form12 or shortform12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,kf.
-
(sf16 or sf 16 or short form 16 or shortform 16 or short form16 or shortform16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab,kf.
-
(sf20 or sf 20 or short form 20 or shortform 20 or short form20 or shortform20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,kf.
-
(hql or hqol or h qol or hrqol or hr qol).ti,ab,kf.
-
(hye or hyes).ti,ab,kf.
-
(health* adj2 year* adj2 equivalent*).ti,ab,kf.
-
(pqol or qls).ti,ab,kf.
-
(quality of wellbeing or quality of well being or index of wellbeing or index of well being or qwb).ti,ab,kf.
-
nottingham health profile*.ti,ab,kf.
-
sickness impact profile.ti,ab,kf.
-
exp health status indicators/
-
(health adj3 (utilit* or status)).ti,ab,kf.
-
(utilit* adj3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or weight)).ti,ab,kf.
-
(preference* adj3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or instrument or instruments)).ti,ab,kf.
-
disutilit*.ti,ab,kf.
-
rosser.ti,ab,kf.
-
willingness to pay.ti,ab,kf.
-
standard gamble*.ti,ab,kf.
-
(time trade off or time tradeoff).ti,ab,kf.
-
tto.ti,ab,kf.
-
(hui or hui1 or hui2 or hui3).ti,ab,kf.
-
(eq or euroqol or euro qol or eq5d or eq 5d or euroqual or euro qual).ti,ab,kf.
-
duke health profile.ti,ab,kf.
-
functional status questionnaire.ti,ab,kf.
-
dartmouth coop functional health assessment*.ti,ab,kf.
-
or/67-103
-
66 and 104.
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Date range searched: 27 July 2018.
Date searched: 30 July 2018.
Searcher: Sophie Robinson.
Hits: 2.
Search strategy
-
(anti-TNF* or antiTNF* or (TNF* adj2 (inhibit* or block*))).tw.
-
anti* tumo?r* necrosis* factor*.tw.
-
(biologic* adj2 DMARD*).tw.
-
((antirheumati* or anti rheumati* or anti-rheumati*) adj4 biologic*).tw.
-
((disease modify* or disease-modify*) adj4 biologic*).tw.
-
anti* drug* antibod*.tw.
-
ADAb.tw.
-
etanercept.tw.
-
(tnr001 or “tnr 001” or tnr-001 or 185243-69-0).tw.
-
(ETA or ETN).tw.
-
(enbrel or erelzi or benepali or lifmior or brenzys).tw.
-
(anti-etanercept* or antietanercept* or (anti adj3 etanercept*)).tw.
-
adalimumab.tw.
-
(d 2e7 or d2e7 or d-2e7 or 331731-18-1).tw.
-
(ADA or ADL or ADM).tw.
-
(humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz).tw.
-
(anti-adalimumab* or antiadalimumab* or (anti adj3 adalimumab*)).tw.
-
infliximab.tw.
-
(170277-31-3 or ta650 or ta 650 or ta-650).tw.
-
(INF or IFX).tw.
-
(anti-infliximab* or antiinfliximab* or (anti adj3 infliximab*)).tw.
-
(remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi).tw.
-
certolizumab.tw.
-
(cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2).tw.
-
(CER or CZP).tw.
-
cimzia.tw.
-
(anti-certolizumab* or anticertolizumab* or (anti adj3 certolizumab*)).tw.
-
golimumab.tw.
-
(cnto 148 or cnto148 or cnto-148 or 476181-74-5).tw.
-
(GOL or GLM).tw.
-
simponi.tw.
-
(anti-golimumab* or antigolimumab* or (anti adj3 golimumab*)).tw.
-
(biologic* adj2 agent*).tw.
-
(CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5).tw.
-
(biosimilar* or (bio* adj1 similar*)).tw.
-
or/1-35
-
(immundiagnostik* or immunodiagnostik* or immunediagnostik*).tw.
-
biohit healthcare.tw.
-
(proteomika* or promonitor*).tw.
-
(enzyme* adj3 immunoassay*).tw.
-
(enzyme* adj3 (immuno* assay* or immuno* test*)).tw.
-
ELISA*.tw.
-
(idkmonitor* or (idk adj3 monitor*) or idk-monitor*).tw.
-
((lisa adj3 tracker*) or lisa-tracker* or lisatracker*).tw.
-
(ridascreen* or (rida adj3 screen*) or rida-screen*).tw.
-
(mabtrack* or (mab adj3 track*) or mab-track*).tw.
-
sanquin.tw.
-
theradiag.tw.
-
(grifols or progenika).tw.
-
(r-biopharm or rbiopharm or r biopharm).tw.
-
((drug* or trough) adj3 (level* or concentration)).tw.
-
or/37-51
-
RA.tw.
-
Rheumarthrit*.tw.
-
((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) adj4 (arthrit* or arthros* or polyarthrit* or factor*)).tw.
-
(Chronic* adj4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)).tw.
-
((Inflammat* or pain* or swell* or stiff*) adj4 (joint* or synovial*)).tw.
-
(Beauvais* adj2 disease*).tw.
-
or/53-58
-
36 and 52 and 59
-
Quality of life.ti,kf.
-
((instrument or instruments) adj3 quality of life).ab.
-
quality adjusted life.ti,ab,kf.
-
(qaly* or qald* or qale* or qtime* or life year or life years).ti,ab,kf.
-
disability adjusted life.ti,ab,kf.
-
daly*.ti,ab,kf.
-
(sf36 or sf 36 or short form 36 or shortform 36 or short form36 or shortform36 or sf thirtysix or sfthirtysix or sfthirty six or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab,kf.
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six or shortform6 or short form6).ti,ab,kf.
-
(sf8 or sf 8 or sf eight or sfeight or shortform 8 or shortform 8 or shortform8 or short form8 or shortform eight or short form eight).ti,ab,kf.
-
(sf12 or sf 12 or short form 12 or shortform 12 or short form12 or shortform12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,kf.
-
(sf16 or sf 16 or short form 16 or shortform 16 or short form16 or shortform16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab,kf.
-
(sf20 or sf 20 or short form 20 or shortform 20 or short form20 or shortform20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,kf.
-
(hql or hqol or h qol or hrqol or hr qol).ti,ab,kf.
-
(hye or hyes).ti,ab,kf.
-
(health* adj2 year* adj2 equivalent*).ti,ab,kf.
-
(pqol or qls).ti,ab,kf.
-
(quality of wellbeing or quality of well being or index of wellbeing or index of well being or qwb).ti,ab,kf.
-
nottingham health profile*.ti,ab,kf.
-
sickness impact profile.ti,ab,kf.
-
(health adj3 (utilit* or status)).ti,ab,kf.
-
(utilit* adj3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or weight)).ti,ab,kf.
-
(preference* adj3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or instrument or instruments)).ti,ab,kf.
-
disutilit*.ti,ab,kf.
-
rosser.ti,ab,kf.
-
willingness to pay.ti,ab,kf.
-
standard gamble*.ti,ab,kf.
-
(time trade off or time tradeoff).ti,ab,kf.
-
tto.ti,ab,kf.
-
(hui or hui1 or hui2 or hui3).ti,ab,kf.
-
(eq or euroqol or euro qol or eq5d or eq 5d or euroqual or euro qual).ti,ab,kf.
-
duke health profile.ti,ab,kf.
-
functional status questionnaire.ti,ab,kf.
-
dartmouth coop functional health assessment*.ti,ab,kf.
-
or/61-101
-
60 and 102.
EMBASE (via Ovid)
Date range searched: 1974 to 27 July 2018.
Date searched: 30 July 2018.
Searcher: Sophie Robinson.
Hits: 64.
Search strategy
-
(anti-TNF* or antiTNF* or (TNF* adj2 (inhibit* or block*))).tw.
-
anti* tumo?r* necrosis* factor*.tw.
-
Tumor Necrosis Factor-alpha/
-
(biologic* adj2 DMARD*).tw.
-
((antirheumati* or anti rheumati* or anti-rheumati*) adj4 biologic*).tw.
-
((disease modify* or disease-modify*) adj4 biologic*).tw.
-
disease modifying antirheumatic drug/
-
monoclonal antibody/
-
anti* drug* antibod*.tw.
-
ADAb.tw.
-
etanercept.tw. or ETANERCEPT/
-
(tnr001 or “tnr 001” or tnr-001 or 185243-69-0).tw.
-
(ETA or ETN).tw.
-
(enbrel or erelzi or benepali or lifmior or brenzys).tw.
-
(anti-etanercept* or antietanercept* or (anti adj3 etanercept*)).tw.
-
adalimumab.tw. or ADALIMUMAB/
-
(d 2e7 or d2e7 or d-2e7 or 331731-18-1).tw.
-
(ADA or ADL or ADM).tw.
-
(humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz).tw.
-
(anti-adalimumab* or antiadalimumab* or (anti adj3 adalimumab*)).tw.
-
infliximab.tw. or INFLIXIMAB/
-
(170277-31-3 or ta650 or ta 650 or ta-650).tw.
-
(INF or IFX).tw.
-
(anti-infliximab* or antiinfliximab* or (anti adj3 infliximab*)).tw.
-
(remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi).tw.
-
Certolizumab Pegol/or certolizumab.tw.
-
(cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2).tw.
-
(CER or CZP).tw.
-
cimzia.tw.
-
(anti-certolizumab* or anticertolizumab* or (anti adj3 certolizumab*)).tw.
-
golimumab/or golimumab.tw.
-
(cnto 148 or cnto148 or cnto-148 or 476181-74-5).tw.
-
(GOL or GLM).tw.
-
simponi.tw.
-
(anti-golimumab* or antigolimumab* or (anti adj3 golimumab*)).tw.
-
(biologic* adj2 agent*).tw.
-
(CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5).tw.
-
biological product/or biosimilar agent/
-
(biosimilar* or (bio* adj1 similar*)).tw.
-
or/1-39
-
exp Enzyme-Linked Immunosorbent Assay/
-
(immundiagnostik* or immunodiagnostik* or immunediagnostik*).tw.
-
biohit healthcare.tw.
-
(proteomika* or promonitor*).tw.
-
(enzyme* adj3 immunoassay*).tw.
-
(enzyme* adj3 (immuno* assay* or immuno* test*)).tw.
-
ELISA*.tw.
-
(idkmonitor* or (idk adj3 monitor*) or idk-monitor*).tw.
-
((lisa adj3 tracker*) or lisa-tracker* or lisatracker*).tw.
-
(ridascreen* or (rida adj3 screen*) or rida-screen*).tw.
-
(mabtrack* or (mab adj3 track*) or mab-track*).tw.
-
sanquin.tw.
-
theradiag.tw.
-
(grifols or progenika).tw.
-
(r-biopharm or rbiopharm or r biopharm).tw.
-
((drug* or trough) adj3 (level* or concentration)).tw.
-
or/41-56
-
exp Arthritis, Rheumatoid/
-
RA.tw.
-
Rheumarthrit*.tw.
-
((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) adj4 (arthrit* or arthros* or polyarthrit* or factor*)).tw.
-
(Chronic* adj4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)).tw.
-
((Inflammat* or pain* or swell* or stiff*) adj4 (joint* or synovial*)).tw.
-
(Beauvais* adj2 disease*).tw.
-
or/58-64
-
40 and 57 and 65
-
(exp animal/or nonhuman/) not exp human/
-
66 not 67 69. Socioeconomics/
-
exp Quality of Life/
-
quality of life.ti,kw.
-
((instrument or instruments) adj3 quality of life).ab.
-
Quality-Adjusted Life Year/
-
quality adjusted life.ti,ab,kw.
-
(qaly* or qald* or qale* or qtime* or life year or life years).ti,ab,kw.
-
disability adjusted life.ti,ab,kw.
-
daly*.ti,ab,kw.
-
(sf36 or sf 36 or short form 36 or shortform 36 or short form36 or shortform36 or sf thirtysix or sfthirtysix or sfthirty six or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab,kw.
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six or shortform6 or short form6).ti,ab,kw.
-
(sf8 or sf 8 or sf eight or sfeight or shortform 8 or shortform 8 or shortform8 or short form8 or shortform eight or short form eight).ti,ab,kw.
-
(sf12 or sf 12 or short form 12 or shortform 12 or short form12 or shortform12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab,kw.
-
(sf16 or sf 16 or short form 16 or shortform 16 or short form16 or shortform16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab,kw.
-
(sf20 or sf 20 or short form 20 or shortform 20 or short form20 or shortform20 or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab,kw.
-
(hql or hqol or h qol or hrqol or hr qol).ti,ab,kw.
-
(hye or hyes).ti,ab,kw.
-
(health* adj2 year* adj2 equivalent*).ti,ab,kw.
-
(pqol or qls).ti,ab,kw.
-
(quality of wellbeing or quality of well being or index of wellbeing or index of well being or qwb).ti,ab,kw.
-
nottingham health profile*.ti,ab,kw.
-
nottingham health profile/
-
sickness impact profile.ti,ab,kw.
-
sickness impact profile/
-
health status indicator/
-
(health adj3 (utilit* or status)).ti,ab,kw.
-
(utilit* adj3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or weight)).ti,ab,kw.
-
(preference* adj3 (valu* or measur* or health or life or estimat* or elicit* or disease or score* or instrument or instruments)).ti,ab,kw.
-
disutilit*.ti,ab,kw.
-
rosser.ti,ab,kw.
-
willingness to pay.ti,ab,kw.
-
standard gamble*.ti,ab,kw.
-
(time trade off or time tradeoff).ti,ab,kw.
-
tto.ti,ab,kw.
-
(hui or hui1 or hui2 or hui3).ti,ab,kw.
-
(eq or euroqol or euro qol or eq5d or eq 5d or euroqual or euro qual).ti,ab,kw.
-
duke health profile.ti,ab,kw.
-
functional status questionnaire.ti,ab,kw.
-
dartmouth coop functional health assessment*.ti,ab,kw.
-
or/67-107
-
68 and 108.
Database: NHS EED (SCI-EXPANDED, CPCI-S) (via Cochrane Library)
Date range searched: Issue 2 of 4 April 2015.
Date searched: 30 July 2018.
Searcher: Sophie Robinson.
Hits: 0.
Search strategy
-
#1. (anti-TNF* or antiTNF* or (TNF* near/2 (inhibit* or block*))):ti,ab,kw
-
#2. “anti* tumo*r* necrosis* factor*”:ti,ab,kw
-
#3. MeSH descriptor: [Tumor Necrosis Factor-alpha] this term only
-
#4. (biologic* near/2 DMARD*):ti,ab,kw
-
#5. ((antirheumati* or “anti rheumati*” or anti-rheumati*) near/4 biologic*):ti,ab,kw
-
#6. ((“disease modify*” or disease-modify*) near/4 biologic*):ti,ab,kw
-
#7. MeSH descriptor: [Antibodies, Monoclonal] explode all trees
-
#8. “anti* drug* antibod*”:ti,ab,kw
-
#9. ADAb:ti,ab
-
#10. etanercept:ti,ab,kw
-
#11. MeSH descriptor: [Etanercept] this term only
-
#12. (tnr001 or “tnr 001” or tnr-001 or 185243-69-0):ti,ab
-
#13. (ETA or ETN):ti,ab
-
#14. (enbrel or erelzi or benepali or lifmior or brenzys):ti,ab,kw
-
#15. (anti-etanercept* or antietanercept* or (anti near/3 etanercept*)):ti,ab,kw
-
#16. adalimumab:ti,ab,kw
-
#17. MeSH descriptor: [Adalimumab] this term only
-
#18. (“d 2e7” or d2e7 or d-2e7 or 331731-18-1):ti,ab
-
#19. (ADA or ADL or ADM):ti,ab
-
#20. (humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz):ti,ab,kw
-
#21. (anti-adalimumab* or antiadalimumab* or (anti near/3 adalimumab*)):ti,ab,kw
-
#22. infliximab:ti,ab,kw
-
#23. MeSH descriptor: [Infliximab] this term only
-
#24. (170277-31-3 or ta650 or “ta 650” or ta-650):ti,ab
-
#25. (INF or IFX):ti,ab
-
#26. (anti-infliximab* or antiinfliximab* or (anti near/3 infliximab*)):ti,ab,kw
-
#27. (remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi):ti,ab,kw
-
#28. certolizumab:ti,ab,kw
-
#29. MeSH descriptor: [Certolizumab Pegol] this term only
-
#30. (cdp870 or “cdp 870” or cdp-870 or 428863-50-7 or 1132819-27-2):ti,ab
-
#31. (CER or CZP):ti,ab
-
#32. cimzia:ti,ab,kw
-
#33. (anti-certolizumab* or anticertolizumab* or (anti near/3 certolizumab*)):ti,ab,kw
-
#34. golimumab:ti,ab,kw
-
#35. (“cnto 148” or cnto148 or cnto-148 or 476181-74-5):ti,ab
-
#36. (GOL or GLM):ti,ab
-
#37. simponi:ti,ab,kw
-
#38. (anti-golimumab* or antigolimumab* or (anti near/3 golimumab*)):ti,ab,kw
-
#39. (biologic* near/2 agent*):ti,ab,kw
-
#40. (CT-P13 or CTP13 or “CT P13” or SB2 or SB-2 or “SB 2” or SB4 or SB-4 or “SB 4” or SB-5 or SB5 or “SB 5”):ti,ab
-
#41. (biosimilar* or “bio* similar*”):ti,ab,kw
-
#42. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41
-
#43. MeSH descriptor: [Enzyme-Linked Immunosorbent Assay] explode all trees
-
#44. (immundiagnostik* or immunodiagnostik* or immunediagnostik*):ti,ab,kw
-
#45. ‘biohit healthcare’:ti,ab,kw
-
#46. (proteomika* or promonitor*):ti,ab,kw
-
#47. (enzyme* near/3 immunoassay*):ti,ab,kw
-
#48. (enzyme* near/3 (“immuno* assay*” or “immuno* test*”)):ti,ab,kw
-
#49. ELISA*:ti,ab,kw
-
#50. (idkmonitor* or (idk near/3 monitor*) or idk-monitor*):ti,ab,kw
-
#51. ((lisa near/3 tracker*) or lisa-tracker* or lisatracker*):ti,ab,kw
-
#52. (ridascreen* or (rida near/3 screen*) or rida-screen*):ti,ab,kw
-
#53. (mabtrack* or (mab near/3 track*) or mab-track*):ti,ab,kw
-
#54. (sanquin or theradiag):ti,ab,kw
-
#55. (grifols or progenika):ti,ab,kw
-
#56. (r-biopharm or rbiopharm or “r biopharm”):ti,ab,kw
-
#57. ((drug* or trough) near/3 (level* or concentration)):ti,ab,kw
-
#58. #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57
-
#59. MeSH descriptor: [Arthritis, Rheumatoid] explode all trees
-
#60. RA:ti,ab
-
#61. Rheumarthrit*.ti,ab,kw
-
#62. ((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) near/4 (arthrit* or arthros* or polyarthrit* or factor*)):ti,ab,kw
-
#63. (Chronic* near/4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)):ti,ab,kw
-
#64. ((Inflammat* or pain* or swell* or stiff*) near/4 (joint* or synovial*)):ti,ab,kw
-
#65. (Beauvais* near/2 disease*):ti,ab,kw
-
#66. #59 or #60 or #61 or #62 or #64 or #65
-
#67. #42 and #58 and #66.
Database: Web of Science (SCI and CPCI-S) (via Thomson Reuters)
Date range searched: N/A.
Date searched: 30 July 2018.
Searcher: Sophie Robinson.
Hits: 187.
Search strategy
-
#1. TS = (anti-TNF* or antiTNF* or (TNF* near/1 (inhibit* or block*))) OR TS = tumo$r* necrosis* factor* alpha OR TS = (biologic* near/1 DMARD*) OR TS = (biologic* near/3 antirheumati*) OR TS = (anti rheumati* near/3 biologic*) OR TS = (disease modify* near/3 biologic*) OR TS = anti* drug* antibod* OR TS = ADAb OR TS = anti* tumo$r* necrosis* factor* OR TS = monoclonal antibod*
-
#2. TS = etanercept OR TS = (tnr001 or tnr 001 or tnr-001 or 185243-69-0) OR TS = (ETA or ETN) OR TS = (enbrel or erelzi or benepali or lifmior or brenzys) OR TS = (anti-etanercept* or antietanercept* or anti near/2 etanercept*)
-
#3. TS = adalimumab OR TS = (d 2e7 or d2e7 or d-2e7 or 331731-18-1) OR TS = (ADA or ADL or ADM) OR TS = (humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz) OR TS = (anti-adalimumab* or antiadalimumab* or anti near/2 adalimumab*)
-
#4. TS = infliximab OR TS = (170277-31-3 or ta650 or ta 650 or ta-650) OR TS = (INF or IFX) OR TS = (anti-infliximab* or antiinfliximab* or anti near/2 infliximab*) OR TS = (remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi)
-
#5. TS = certolizumab OR TS = (cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2) OR TS = (CER or CZP) OR TS = cimzia OR TS = (anti-certolizumab* or anticertolizumab* or anti near/2 certolizumab*)
-
#6. TS = golimumab OR TS = (cnto 148 or cnto148 or cnto-148 or 476181-74-5) OR TS = (GOL or GLM) OR TS = simponi OR TS = (anti-golimumab* or antigolimumab* or anti near/2 golimumab*)
-
#7. TS = (biologic* near/1 agent*) OR TS = (CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5) OR TS = (biosimilar* or bio* similar*)
-
#8. #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
-
#9. TS = (immundiagnostik* or immunodiagnostik* or immunediagnostik*) OR TS = biohit healthcare OR TS = (proteomika* or promonitor*) OR TS = (enzyme* near/2 immunoassay*) OR TS = (enzyme* near/2 immuno* assay*) OR TS = (enzyme* near/2 immuno* test*) OR TS = ELISA*
-
#10. TS = (idkmonitor* or idk near/2 monitor* or idk-monitor*) OR TS = (lisa near/2 tracker* or lisa-tracker* or lisatracker*) OR TS = (ridascreen* or rida near/2 screen* or rida-screen*) OR TS = (mabtrack* or mab near/2 track* or mab-track*) OR TS = (sanquin or theradiag) OR TS = (grifols or progenika) OR TS = (r-biopharm or rbiopharm or r biopharm) OR TS = ((drug* or trough) near/2 (level* or concentration))
-
#11. #10 OR #9
-
#12. TS = RA OR TS = Rheumarthrit* OR TS = ((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) near/3 (arthrit* or arthros* or polyarthrit* or factor*)) OR TS = (chronic* near/3 polyarthrit*) OR TS = (chronic* near/3 poly arthrit*) OR TS = (chronic* near/3 rheumati*) OR TS = ((Inflammat* or pain* or swell* or stiff*) near/3 (joint* or synovial*)) OR TS = (Beauvais* adj2 disease*)
-
13. #12 AND #11 AND #8
-
14. TS = (quality of life OR quality adjusted life OR qaly* OR qald* OR qale* OR qtime* OR life year OR life years OR disability adjusted life OR daly* OR sf36 OR sf 36 OR short form 36 OR shortform 36 OR short form36 OR shortform36 OR sf6 OR sf 6 OR short form 6 OR sf6d OR sf 6d OR short form 6d OR sf8 OR sf 8 OR short form 8 OR sf12 OR sf 12 OR short form 12 OR sf16 OR sf 16 OR sf20 OR sf 20 OR short form 20 OR hql OR hqol OR h qol OR hrqol OR hr qol OR hye OR hyes OR healthy year equivalent* OR healthy years equivalent* OR pqol OR qls OR quality of well being OR index of wellbeing OR qwb OR nottingham health profile* OR sickness impact profile OR health utilit* OR health status OR disutilit* OR rosser OR willingness to pay OR standard gamble* OR time trade off OR time tradeoff OR tto OR hui OR hui1 OR hui2 OR hui3 OR euroqol OR euro qol OR eq5d OR eq 5d OR euroqual OR euro qual OR duke health profile OR functional status questionnaire OR dartmouth coop functional health assessment* OR (utilit* AND (valu* OR measur* OR health OR life OR estimat* OR elicit* OR disease OR score* OR weight)) OR (preference* AND (valu* OR measur* OR health OR life OR estimat* OR elicit* OR disease OR score* OR instrument OR instruments)))
-
15. #14 and #15.
School of Health and Related Research Health Utilities Database
Date searched: 30 July 2018.
Searcher: Sophie Robinson.
Hits: 33.
HERC Oxford
Date searched: 30 July 2018.
Searcher: Sophie Robinson.
Hits: 1.
EuroQol-5 Dimensions
Date searched: 30 July 2018.
Searcher: Sophie Robinson.
Hits: 174.
Cost-effectiveness Analysis Registry
Date searched: 30 July 2018.
Searcher: Sophie Robinson.
Hits: 103.
| Database | Hits |
|---|---|
| MEDLINE | 136 |
| MEDLINE In-Process & Other Non-Indexed Citations | 2 |
| EMBASE | 64 |
| Cochrane – NHS EED | 0 |
| Web of Science | 187 |
| ScHARR HUD | 33 |
| HERC Oxford | 1 |
| EQ-5D – EuroQol | 174 |
| Cost-effectiveness Analysis Registry | 103 |
| Total records | 700 |
| Duplicates | 70 |
| Total unique records | 630 |
Appendix 2 Included and excluded studies
| Authors | Source | Title | Article type | Contributed data |
|---|---|---|---|---|
| Non-randomised controlled studies | ||||
| Arango CG, Vivar MLG, Angulo EU, Gorostiza I, Perez CE, De Dios JR, Alvarez B, Escribano AR, Stoye C, Vasques M, Otano JB, Escobar A, Trancho Z, Del Agua AR, Del Rio L, Jorquera C, Martinez A, Nagore D43 | Arthritis and Rheumatology | Prospective, intervention, multicentre, non-inferiority study of utility of therapeutic drug monitoring with respect to the efficacy and cost of adalimumab tapering in patients with rheumatic diseases | Conference abstract | Yes |
| Gorostiza I, Angulo EU, Arango CG, Perez CE, De Dios JR, Alvarez B, Escribano AR, Stoye C, Vasques M, Otano JB, Escobar A, Trancho Z, Del Agua AR, Del Rio L, Martinez A, Nagore D45 | Arthritis and Rheumatology | Prospective, intervention, multicentre study of utility of biologic drug monitoring with respect to the efficacy and cost of adalimumab tapering in patients with rheumatic diseases (34-week descriptive data) | Conference abstract | Yes |
| Ucar E, Gorostiza I, Gomez C, Perez CE, Dios JR, Alvarez B, Ruibal A, Stoye C, Vasques M, Belzunegui J, Escobar A, Trancho Z, Ruiz del Agua A, Martinez A, Jorquera C, Nagore D42 | Annals of the Rheumatic Diseases | Prospective, intervention, multicentre study of utility of biologic drug monitoring with respect to the efficacy and cost of adalimumab tapering in patients with rheumatic diseases: preliminary results of ingebio study | Conference abstract | Yes |
| Observational study | ||||
| Pascual-Salcedo D, Plasencia C, Gonzalez del Valle L, Lopez Casla T, Arribas F, Villalba A, Bonilla G, Lopez Granados E, Martin Mola E, Balsa A44 | Annals of the Rheumatic Diseases | Therapeutic drug monitoring (TDM) in rheumatic day clinic enables to reduce pharmaceutical cost maintaining clinical efficacy | Conference | Yes |
| Authors | Source | Title | Reasons for exclusion |
|---|---|---|---|
| Alcobendas R, Rodriguez-Vidal A, Pascual-Salcedo D, Murias S, Remesal A, Diego C, Merino R | Clinical and Experimental Rheumatology | Monitoring serum etanercept levels in juvenile idiopathic arthritis: a pilot study | Population |
| Alcocer P, Plasencia C, Pascual D, Garcia Carazo S, Franco KN, Cagijas D, Lojo L, Bonilla G, Nuno L, Villalba A, Lopez Casla MT, Balsa A, Martin Mola E | Annals of the Rheumatic Diseases | Imnunogenicity and clinical practice in patients treated with anti-TNF therapy | Population |
| Alessandri C, Scrivo R, Spinelli FR, Ceccarelli F, Magrini L, Priori R, Valesini G | Autoimmunity, Part B Novel Applications of Basic Research | Autoantibody production in anti-TNF-alpha-treated patients | Design |
| Ametzazurra A, Rivera N, Balsa A, Arreba MP, Ruiz E, Plasencia C, Ortiz J, Pascual-Salcedo D, Munoz MC, De Aysa C, Allande MJ, Torres N, Hernandez AM, Recalde X, Martinez A, Nagore D | Annals of the Rheumatic Diseases | Point-of-care monitoring of anti-infliximab antibodies in patients treated with the reference infliximab or CT-P13 in routine clinical practice | Population |
| Ancuta C, Pomirleanu C, Belibou C, Maxim R, Petrariu L, Strugariu G, Chirieac R | Annals of the Rheumatic Diseases | Clinical outcomes of immunogenicity in rheumatoid arthritis patients under anti-TNF biologics: results from an observational study | Population |
| Ancuta C, Pomirleanu C, Maxim R, Ancuta E, Iordache C, Dascalu C, Chirieac R | Revista De Chimie | Clinical relevance of rituximab immunogenicity in rheumatoid arthritis: a pilot study | Intervention |
| Arstikyte I, Kapleryte G, Butrimiene I, Venalis A | BioMed Research International | Influence of immunogenicity on the efficacy of long-term treatment with TNF-alpha blockers in rheumatoid arthritis and spondyloarthritis patients | Population |
| Avdeeva AS, Aleksandrova EN, Novikov AA, Karateev DE, Luchihina EL, Cherkasova MV, Nasonov EL | Annals of the Rheumatic Diseases | Association of clinical efficacy with serum level of adalimumab (ADA) and anti-adalimumab antibody levels in patients with early rheumatoid arthritis (RA) | Population |
| Awni W, Pilari S, Ahmed G, Noertersheuser P | Arthritis and Rheumatism | The effect of methotrexate on adalimumab pharmacokinetics: pooled analysis of adalimumab pharmacokinetics in patients with rheumatoid arthritis after subcutaneous administration | Design |
| Bader LI, Solberg SM, Kaada SH, Bolstad N, Warren DJ, Gavasso S, Gjesdal CG, Vedeler CA | Scandinavian Journal of Immunology | Assays for infliximab drug levels and antibodies: a matter of scales and categories | Design |
| Balsa A, Sanmarti R, Rosas J, Castro SG, Cabez A, Martin V, Montoro M | Arthritis and Rheumatology | Immunogenicity of anti-TNF therapies in patients with inflammatory rheumatic diseases and secondary failure: a multicentre study of 570 patients | Outcome |
| Balsa A, Sanmarti R, Rosas J, Martin V, Cabez A, Gomez S, Montoro M | Rheumatology | Drug immunogenicity in patients with inflammatory arthritis and secondary failure to tumour necrosis factor inhibitor therapies: the REASON study | Outcome |
| Bandres Ciga S, Salvatierra J, Lopez-Sidro M, Garcia-Sanchez A, Duran R, Vives F, Raya-Alvarez E | Journal of Clinical Rheumatology | An examination of the mechanisms involved in secondary clinical failure to adalimumab or etanercept in inflammatory arthropathies | Design |
| Bandres Ciga S, Salvatierra Ossorio J, Lopez-Sidro M, Garcia Sanchez A, Duran Ogalla R, Vives Montero F, Raya-Alvarez E | Annals of the Rheumatic Diseases | The utility of the mechanistic model in inflammatory arthropaties with secondary clinical failure to adalimumab, but not to etanercept | Design |
| Bantleon FI, Krauchi S, Schuster TB, Schneider M, Abel Buhlmann A | Journal of Crohn’s and Colitis | Quantum blue® adalimumab: development of the first point of care rapid test for therapeutic drug monitoring of serum adalimumab levels | Design |
| Bantleon FI, Krauchi S, Schuster TB, Schneider M, Weber JM | Annals of the Rheumatic Diseases | Quantum blue adalimumab: evaluation of a point of care rapid test for therapeutic drug monitoring of serum adalimumab levels | Design |
| Baos S, Plasencia C, Ramiro S, Moral R, Diez J, Martin-Mola E, Balsa A | Arthritis and Rheumatism | Effect on rheumatoid factor and anti-cyclic citrullinated peptide antibodies levels of treatment with infliximab and adalimumab in patients with rheumatoid arthritis | Design |
| Barlow NL, Mohammed P, Berg JD | Clinical Chemistry and Laboratory Medicine | Clinical study of serum trough infliximab concentrations and anti-infliximab antibodies in a cohort of gastroenterology and rheumatology patients | Design |
| Barlow NL, Mohammed P, Berg JD | Annals of Clinical Biochemistry | Serum trough infliximab and anti-infliximab antibodies in a cohort of gastroenterology and rheumatology patients’ infliximab therapeutic drug monitoring | Design |
| Bartelds GM, de Groot E, Nurmohamed MT, Hart MH, van Eede PH, Wijbrandts CA, Crusius JB, Dijkmans BA, Tak PP, Aarden L, Wolbink GJ | Arthritis Research & Therapy | Surprising negative association between IgG1 allotype disparity and anti-adalimumab formation: a cohort study | Design |
| Bartelds GM, Krieckaert CL, Nurmohamed MT, Van Schouwenburg P, Dijkmans BA, Wolbink GJ | Arthritis and Rheumatism | Immunogenicity in a 3-year follow-up cohort of adalimumab treated rheumatoid arthritis patients | Design |
| Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, Dijkmans BA, Aarden L, Wolbink GJ | Journal of the American Medical Association | Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up | Population |
| Bartelds GM, Wolbink GJ, Stapel S, Aarden L, Lems WF, Dijkmans BAC, Nurmohamed MT | Annals of the Rheumatic Diseases | High levels of human anti-human antibodies to adalimumab in a patient not responding to adalimumab treatment | Design |
| Bastida C, Ruiz V, Pascal M, Yague J, Sanmarti R, Soy D | British Journal of Clinical Pharmacology | Is there potential for therapeutic drug monitoring of biologic agents in rheumatoid arthritis? | Design |
| Bender NK, Heilig CE, Droll B, Wohlgemuth J, Armbruster FP, Heilig B | Rheumatology International | Immunogenicity, efficacy and adverse events of adalimumab in RA patients | Design |
| Bendtzen K | Arthritis and Rheumatism | Is there a need for immunopharmacologic guidance of anti-tumour necrosis factor therapies? | Design |
| Bendtzen K. | Immunotherapy | Anti-TNF-alpha biotherapies: perspectives for evidence-based personalised medicine | Design |
| Bendtzen K | Discovery Medicine | Personalised medicine: theranostics (therapeutics diagnostics) essential for rational use of tumour necrosis factor-alpha antagonists | Design |
| Benucci M, Damiani A, Li Gobbi F, Bandinelli F, Infantino M, Grossi V, Manfredi M, Noguier G, Meacci F | Biologics | Correlation between HLA haplotypes and the development of antidrug antibodies in a cohort of patients with rheumatic diseases | Design |
| Benucci M, Gobbi FL, Meacci F, Manfredi M, Infantino M, Severino M, Testi S, Sarzi-Puttini P, Ricci C, Atzeni F | Biologics: Targets and Therapy | Antidrug antibodies against TNF-blocking agents: Correlations between disease activity, hypersensitivity reactions, and different classes of immunoglobulins | Design |
| Benucci M, Infantino M, Manfredi M, Olivito B, Sarzi-Puttini P, Atzeni F | Annals of the Rheumatic Diseases | Antidrug-antibodies but not IgG-4 antibodies against TNF blockers influence the activity of anti-TNF drugs in rheumatoid arthritis | Design |
| Benucci M, Li Gobbi F, Meacci F, Manfredi M, Infantino M, Severino M, Testi S, Sarzi-Puttini P, Ricci C, Atzeni F | Biologics | Antidrug antibodies against TNF-blocking agents: correlations between disease activity, hypersensitivity reactions, and different classes of immunoglobulins | Design |
| Berthold E, Mansson B, Gullstrand B, Geborek P, Saxne T, Bengtsson AA, Kahn R | Scandinavian Journal of Rheumatology | Tumour necrosis factor–alpha/etanercept complexes in serum predict long-term efficacy of etanercept treatment in seronegative rheumatoid arthritis | Design |
| Bingham CO, Ince A, Haraoui B, Keystone EC, Chon Y, Baumgartner S | Current Medical Research and Opinion | Effectiveness and safety of etanercept in subjects with RA who have failed infliximab therapy: 16-week, open-label, observational study | Design |
| Bogas P, Plasencia C, Pascual-Salcedo D, Bonilla G, Moral E, Tornero C, Nuno L, Villalba A, Peiteado D, Martinez A, Hernandez B, Balsa A | Annals of the Rheumatic Diseases | Discontinuation of first biologic therapy in rheumatoid arthritis: main causes and correlation between secondary inefficacy and development of immunogenicity | Design |
| Bogas P, Plasencia C, Pascual-Salcedo D, Bonilla G, Moral E, Tornero C, Nuno L, Villalba A, Peiteado D, Martinez A, Hernandez B, Balsa A | Annals of the Rheumatic Diseases | Influence of immunogenicity to the first TNF-I therapy on response to the second biologic agent in RA patients | Design |
| Bogas P, Plasencia-Rodriguez C, Balsa A, Pascual-Salcedo D, Bonilla G, Coro EM, Tornero C, Nuno L, Peiteado D, Martinez A, Hernandez B | Arthritis and Rheumatology | Influence of immunogenicity to the first anti-TNF therapy on response to the second biologic agent in RA patients | Design |
| Braun-Moscovici Y, Ben Horin S, Dagan A, Toledano K, Markovits D, Saffouri A, Beshara R, Rozin A, Nahir MA, Chowers Y, Balbir-Gurman A | Annals of the Rheumatic Diseases | The input of measuring of infliximab and adalimumab levels and levels of antibodies to these drugs in the management of patients with autoimmune diseases treated with anti TNF monoclonal antibodies | Design |
| Cao F, Cao HL, Cao XC | International Journal of Clinical Pharmacology and Therapeutics | A review of six methods for monitoring infliximab concentrations and antibodies to infliximab | Design |
| Casal M, Ramsey M, Moreland LW, Fernandez C | Arthritis and Rheumatology | A cytometric assay for monitoring adalimumab immunogenicity and drug concentrations can distinguish anti-adalimumab antibodies from interference | Design |
| Casteele NV, Buurman DJ, Sturkenboom MGG, Kleibeuker JH, Vermeire S, Rispens T, van der Kleij D, Gils A, Dijkstra G | Alimentary Pharmacology & Therapeutics | Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays | Design |
| Cates MJ | Rheumatology | Anti-tumour necrosis factor a drug levels and antidrug antibodies in guiding clinical decision making in rheumatology: a draft algorithm and illustrative cases | Design |
| Chamaida PR, Pascual-Salcedo D, Bonilla M, Villalba A, Lopez-Casla M, Peiteado D, Garcia-Carazo S, Ramiro S, Franco K, Cajigas D, Martin-Mola E, Balsa A | Annals of the Rheumatic Diseases | The early infliximab levels monitoring can predict the development of antidrug antibodies in a cohort of rheumatoid arthritis patients treated with infliximab | Design |
| Chasseuil E, Mulleman D, Aubourg A, Lecomte T, Paintaud G, Ternant D | Fundamental and Clinical Pharmacology | Determination of infliximab cut-off concentrations predicting presence or absence of antibodies towards infliximab (ATI) in chronic inflammatory diseases | Design |
| Chatzidionysiou K | Scandinavian Journal of Rheumatology | Optimising biological treatments for rheumatoid arthritis | Design |
| Chen DY, Chen YM, Tsai WC, Tseng JC, Chen YH, Hsieh CW, Hung WT, Lan JL | Annals of the Rheumatic Diseases | Significant associations of antidrug antibody levels with serum drug trough levels and therapeutic response of adalimumab and etanercept treatment in rheumatoid arthritis | Population |
| Chen DY, Chen YM, Hung WT, Chen HH, Hsieh CW, Chen YH, Huang WN, Hsieh TY | Annals of the Rheumatic Diseases | Immunogenicity, drug trough levels and therapeutic response in patients with rheumatoid arthritis or ankylosing spondylitis after 24-week golimumab treatment | Population |
| Chen DY, Chen YM, Hsieh TY, Hung WT, Hsieh CW, Chen HH, Tang KT, Lan JL | Rheumatology | Drug trough levels predict therapeutic responses to dose reduction of adalimumab for rheumatoid arthritis patients during 24 weeks of follow-up | Intervention |
| Chighizola CB, Favalli EG, Meroni PL | Clinical Reviews in Allergy & Immunology | Novel mechanisms of action of the biologicals in rheumatic diseases | Design |
| Chollet-Martin S, Nicaise-Roland P, De Chaisemartin L, Grootenboer-Mignot S, Hayem G, Pelletier AL, Amiot A, Descamps V, Bouhnik Y, Meyer O | Annals of the Rheumatic Diseases | Simultaneous determination of anti-infliximab antibodies and residual infliximab levels to monitor anti-TNF therapy | Outcome |
| Chow V, Kaliyaperumal A, Zhang N, Miller J, Mytych D, Starcevic Manning M, Wala I, Wang H, Krishnan E | Journal of Crohn’s and Colitis | Development of antidrug antibodies among those treated with adalimumab and ABP 501 and its impact on serum drug concentration in randomised controlled studies | Design |
| Clair EWS, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A, Keystone EC | Arthritis & Rheumatism | The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis – Results from ATTRACT, a multicentre, randomised, double-blind, placebo-controlled trial | Population |
| Cludts I, Spinelli FR, Morello F, Hockley J, Valesini G, Wadhwa M | Cytokine | Anti-therapeutic antibodies and their clinical impact in patients treated with the TNF antagonist adalimumab. [Reprint in Cytokine 2018;101:70–77] | Population |
| Cludts I, Spinelli FR, Morello F, Hockley J, Valesini G, Wadhwa M | Cytokine | ‘Anti-therapeutic antibodies and their clinical impact in patients treated with the TNF antagonist adalimumab’. [Reprint in Cytokine 2017;96:16–23] | Population |
| Collet-Brose J, Couble PJ, Deehan MR, Nelson RJ, Ferlin WG, Lory S | Journal of Immunology Research | Evaluation of multiple immunoassay technology platforms to select the antidrug antibody assay exhibiting the most appropriate drug and target tolerance | Design |
| Cosan F, Cetin EA, Gazioglu SB, Yazici A, Yilmazer B, Cefle A, Deniz G | Clinical and Experimental Rheumatology | How could be used the autoantibodies against anti-TNF agents in clinical practice? Two years follow-up study | Population |
| Daien CI, Daien V, Parussini E, Dupuy AM, Combe B, Morel J | Journal of Rheumatology | Etanercept concentration in patients with rheumatoid arthritis and its potential influence on treatment decisions: a pilot study | Design |
| Damen CWN, Schellens JHM, Beijnen JH | Human Antibodies | Bioanalytical methods for the quantification of therapeutic monoclonal antibodies and their application in clinical pharmacokinetic studies | Design |
| Darrouzain F, Bian SM, Desvignes C, Bris C, Watier H, Paintaud G, de Vries A | Therapeutic Drug Monitoring | Immunoassays for measuring serum concentrations of monoclonal antibodies and anti-biopharmaceutical antibodies in patients | Design |
| den Broeder AA, van Herwaarden N, van den Bemt BJF | Current Opinion in Rheumatology | Therapeutic drug monitoring of biologicals in rheumatoid arthritis: a disconnect between beliefs and facts | Design |
| Denarie D, Rinaudo M, Thomas T, Paul S, Marotte H | Annals of the Rheumatic Diseases | Longitudinal study of serum TNF alpha levels, infliximab, and antibodies to infliximab in rheumatoid arthritis | Population |
| Denarie D, Rnaudo M, Thomas T, Paul S, Marotte H | Annals of the Rheumatic Diseases | Methotrexate reduced TNF bioactivity by anti-infliximab antibody prevention in rheumatoid arthritis patients treated with infliximab | Design |
| Denarie D, Rinaudo-Gaujous M, Thomas T, Paul S, Marotte H | Mediators of Inflammation | Methotrexate reduced TNF bioactivity in rheumatoid arthritis patients treated with infliximab | Design |
| Dervieux T, Weinblatt ME, Kivitz A, Kremer JM | Annals of the Rheumatic Diseases | Methotrexate polyglutamation in relation to infliximab pharmacokinetics in rheumatoid arthritis | Design |
| Diana M, Iliuta M, Gainaru C, Apetrei N, Luca G, Groseanu L, Saulescu I, Constantinescu C, Bojinca V, Borangiu A, Balanescu A, Predeteanu D, Ionescu R, Opris D | Annals of the Rheumatic Diseases | Clinical utility of measuring drug and antidrug antibody concentration of biologic agents in rheumatoid arthritis patients with moderate and high disease activity | Design |
| Doghanji F, Ataman S, Ozdemirel AE, Seckin RB, Yalcin AP, Bavbek S | Arthritis and Rheumatology | Relationship between immunogenicity, hypersensitivity reactions and skin tests against infliximab, etanercept and adalimumab in patients with rheumatoid arthritis and ankylosing spondylitis | Design |
| Drynda S, Beuermann R, Kekow J | Annals of the Rheumatic Diseases | Determination of antidrug antibodies in long-term treatment of rheumatoid arthritis patients with etanercept | Design |
| Drynda S, Kekow J | Zeitschrift für Rheumatologie | Determination of TNF alpha blocker serum levels and anti drug antibodies during long term treatment of rheumatoid arthritis patients and their association with clinical outcome and selected biomarkers | Design |
| Drynda S, Kekow J | Arthritis and Rheumatology | Clinical relevance of etanercept levels and anti-etanercept antibodies in long-term treatment of rheumatoid arthritis patients | Design |
| Drynda S, Kekow J | Annals of the Rheumatic Diseases | Clinical importance of antidrug and serum drug level testing in rheumatoid arthritis patients treated with etanercept | Population |
| Ducourau E, Mulleman D, Paintaud G, Lin DCM, Lauferon F, Ternant D, Watier H, Goupille P | Arthritis Research & Therapy | Antibodies towards infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases | Population |
| Ducourau E, Ternant D, Corondan A, Legoff B, Perdriger A, Devauchelle V, Solau-Gervais E | Arthritis and Rheumatism | Body surface area, erythrocyte sedimentation rate, methotrexate and antibodies to infliximab influence the pharmacokinetics of infliximab in rheumatoid arthritis | Population |
| Ducourau E, Ternant D, Mulleman D, Mammou S, Lin DCM, Watier H, Paintaud G | Arthritis and Rheumatism | Antibodies towards infliximab are associated with poor infliximab maintenance and low infliximab concentrations | Design |
| Duftner C, Dejaco C, Kullich W, Klauser A, Goldberger C, Falkenbach A, Schirmer M | Annals of the Rheumatic Diseases | Preferential type 1 chemokine receptors and cytokine production of CD28(-) T cells in ankylosing spondylitis | Design |
| Edrees AF, Misra SN, Abdou NI | Clinical & Experimental Rheumatology | Anti-tumour necrosis factor (TNF) therapy in rheumatoid arthritis: correlation of TNF-alpha serum level with clinical response and benefit from changing dose or frequency of infliximab infusions | Population |
| Emery P, Burmester GR, Naredo E, Zhou Y, Hojnik M, Conaghan PG | BMJ Open | Design of a phase IV randomised, double-blind, placebo-controlled trial assessing the ImPact of Residual Inflammation Detected via Imaging Techniques, Drug Levels and Patient Characteristics on the Outcome of Dose TaperIng of Adalimumab in Clinical Remission Rheumatoid ArThritis (RA) patients (PREDICTRA) | Design |
| Emi Aikawa N, De Carvalho JF, Artur Almeida Silva C, Bonfa E | Clinical Reviews in Allergy and Immunology | Immunogenicity of anti-TNF-alpha agents in autoimmune diseases | Design |
| Eng GP | Danish Medical Journal | Optimising biological treatment in rheumatoid arthritis with the aid of therapeutic drug monitoring | Design |
| Eng GP, Bouchelouche P, Bartels EM, Bliddal H, Bendtzen K, Stoltenberg M | PLOS ONE | Antidrug antibodies, drug levels, interleukin-6 and soluble TNF receptors in rheumatoid arthritis patients during the first 6 months of treatment with adalimumab or infliximab: a descriptive cohort study | Design |
| Eriksson C, Lind P, Nystrand M, Moverare R | Allergy: European Journal of Allergy and Clinical Immunology | A new automated antidrug antibody screening assay with high sensitivity and drug tolerance | Design |
| Fabris M, Pistis C, Zabotti A, Picco L, Curcio F, Tonutti E, De Vita S | Drug Metabolism Letters | The detection of anti-adalimumab antibodies in a series of inflammatory polyarthritis: three ELISA methods compared | Design |
| Fogdell-Hahn A | Scandinavian Journal of Immunology | Antidrug antibodies: B cell immunity against therapy | Design |
| Funk R, Shakhnovich V, Van Haandel L, Becker ML | Arthritis and Rheumatology | Infliximab use in JIA and uveitis: does methotrexate help or hinder? | Population |
| Furst DE, Wallis R, Broder M, Beenhouwer DO | Seminars in Arthritis & Rheumatism | Tumour necrosis factor antagonists: different kinetics and/or mechanisms of action may explain differences in the risk for developing granulomatous infection | Design |
| Gainaru C, Diana M, Iliuta M, Luca G, Apetrei N, Constantinescu C, Groseanu L, Bojinca V, Saulescu I, Borangiu A, Balanescu A, Predeteanu D, Ionescu R, Opris D | Annals of the Rheumatic Diseases | Infliximab vs. etanercept: the importance of immunogenicity and serum drug monitoring in clinical practice | Population |
| Garces S, Antunes M, Benito-Garcia E, Canas-Silva J, Aarden L, Demengeot J | Annals of the Rheumatic Diseases | A preliminary algorithm introducing immunogenicity assessment in the management of RA patients receiving biotechnological therapies | Design |
| Garces S, Canas-da-Silva J, Aarden L, Demengeot J | Annals of the Rheumatic Diseases | New algorithm to approach RA patients receiving biologic therapies: introducing immunogenicity assessment in the eular guidelines | Design |
| Garces S, Demengeot J, Da Silva JC, Aarden L | Arthritis and Rheumatism | Bridging ELISA as a screening assay to monitor immunogenicity in routine clinical practice | Design |
| Garces S, Demengeot J, Wolbink GJ, Aarden L, Benito-Garcia E | Arthritis and Rheumatism | The immunogenicity of infliximab, adalimumab and etanercept in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, Crohn’s disease and ulcerative colitis – a quantitative and a qualitative review | Design |
| Garces S, Demengeot J, Benito-Garcia E | Annals of the Rheumatic Diseases | Clinical impact of immunogenicity of infliximab, adalimumab and etanercept: a systematic review of the literature with a meta-analysis | Design |
| Garces S, Demengeot J, Canas-da-Silva J, Aarden L | Annals of the Rheumatic Diseases | Bridging ELISA as a secreening assay to monitor immunogenicity in routine clinical practice | Design |
| Garces S, Demengeot J, Benito-Garcia E | Annals of the Rheumatic Diseases | The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis | Design |
| Garces S, Freitas J, Canas-Silva J, Aarden L, Demengeot J | Annals of the Rheumatic Diseases | The impact of immunogenicity on drug safety profile | Design |
| Garcia Ruiz de Morales JM, Pascual-Salcedo D, Tello FL, Mendez LV | Medicina Clinica | Anti-tumour necrosis factor drug therapy: the usefulness of monitoring drug levels and antidrug antibodies in clinical practice | Design |
| Gavan S, Payne K, Barton A | Annals of the Rheumatic Diseases | A systematic review and bivariate meta-analysis of studies that measured adalimumab drug levels by elisa to detect treatment response in rheumatoid arthritis | Design |
| Gavan S, Payne K, Barton A | Value in Health | Measuring adalimumab drug levels by ELISA to detect treatment response in rheumatoid arthritis: a systematic review and bivariate meta-analysis | Design |
| Genovese MC, Ogata A, Nomura A, Bao M, Hitraya E, Lacey S, Burmester G | Arthritis and Rheumatology | Immunogenicity of subcutaneous and intravenous tocilizumab as monotherapy or in combination with DMARDS | Intervention |
| Gil Candel M, Iniesta Navalon C, Onteniente Candela M, Rentero Redondo L, Caballero Requejo C, Salar Valverde N, Gallego Munoz C | European Journal of Hospital Pharmacy | Study of the prevalence of immunogenicity in patients treated with anti-tumour necrosis factor monoclonal antibodies | Population |
| Gladman DD | Arthritis & Rheumatology | Clinical utility of random anti-tumour necrosis factor drug-level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis | Design |
| Glintborg B, Kringelbach T, Hogdall E, Sorensen IJ, Jensen DV, Loft AG, Hendricks O, Jensen Hansen IM, Bolstad N, Gron K, Eng G, Enevold C, Nielsen CH, Warren D, Goll G, Gehin J, Johansen JS, Hetland ML | Annals of the Rheumatic Diseases | Non-medical switch from originator to biosimilar infliximab among patients with inflammatory rheumatic disease - impact on s-infliximab and antidrug-antibodies. Results from the national Danish rheumatologic biobank and the DANBIO registry | Design |
| Goll GL, Jorgensen KK, Sexton J, Olsen IC, Bolstad N, Lorentzen M,Haavardsholm EA, Mork C, Jahnsen J, Kvien TK | Arthritis and Rheumatology | Long-term safety and efficacy of biosimilar infliximab (CT-P13) after switching from originator infliximab: results from the 26-week open label extension of a randomised Norwegian trial | Design |
| Goll GL, Olsen IC, Jorgensen KK, Lorentzen M, Bolstad N, Haavardsholm EA, Lundin KEA, Mork C, Jahnsen J, Kvien TK | Arthritis and Rheumatology | Biosimilar infliximab (CT-P13) is not inferior to originator infliximab: results from a 52-week randomised switch trial in Norway | Design |
| Goll GL, Olsen IC, Bolstad N, Jorgensen KK, Lorentzen M, Mork C, Jahnsen J, Haavardsholm EA, Kvien TK | Annals of the Rheumatic Diseases | Disease worsening and safety in patients switching from originator infliximab to biosimilar infliximab (CT-P13) in the nor-switch study: explorative analysis of RA patients | Design |
| Goll GL, Olsen IC, Lundin KEA, Jorgensen KK, Lorentzen M, Klaasen RA, Warren DJ, Mork C, Jahnsen J, Haavardsholm EA, Kvien TK, Bolstad N | Annals of the Rheumatic Diseases | Immunogenicity in patients switching from stable originator infliximab treatment to CT-P13: analyses across six diseases from the 52-week randomised nor-switch study | Design |
| Golovics PA, Vegh Z, Rutka M, Gecse K, Balint A, Farkas K, Banai J, Bene L, Gasztonyi B, Kristof T, Lakatos L, Miheller P, Palatka K, Patai A, Salamon A, Szamosi T, Szepes Z, Toth GT, Vincze A, Biro E, Lovasz B, Kurti Z, Nagy F, Molnar T, Lakatos P | Journal of Crohn’s and Colitis | Predicting short and medium-term efficacy of the biosimilar infliximab: trough levels/do antidrug antibody’s or clinical/biochemical markers play a more important role? | Design |
| Gorovits B, Baltrukonis DJ, Bhattacharya I, Birchler MA, Finco D, Sikkema D, Vincent MS, Lula S, Marshall L, Hickling TP | Clinical and Experimental Immunology | Immunoassay methods used in clinical studies for the detection of antidrug antibodies to adalimumab and infliximab | Design |
| Gudbrandsdottir S, Bliddal H, Petri A, Terslev L, Danneskiold-Samsoe B, Bjornhart B, Bendtzen K, Muller K | Scandinavian Journal of Rheumatology | Plasma TNF binding capacity profiles during treatment with etanercept in rheumatoid arthritis | Design |
| Guirgis M, Favre dit Jeanfavre M, Benaim C, Perreau M, Michetti P, Maillard M, Zufferey P | Arthritis and Rheumatology | Comparison of infliximab immunogenicity in inflammatory arthritis versus inflammatory bowel disease patients in routine clinical practice | Design |
| Hammer HB, Bolstad N, Warren DJ, Goll G | Annals of the Rheumatic Diseases | Patients with low serum adalimumab concentrations display poor ultrasonographic response to treatment; results of a follow-up study of patients with rheumatoid arthritis | Design |
| Haraoui B, Cameron L, Ouellet M, White B | Journal of Rheumatology | Anti-infliximab antibodies in patients with rheumatoid arthritis who require higher doses of infliximab to achieve or maintain a clinical response | Design |
| Hart MH, de Vrieze H, Wouters D, Wolbink GJ, Killestein J, de Groot ER, Aarden LA, Rispens T | Journal of Immunological Methods | Differential effect of drug interference in immunogenicity assays | Population |
| Hayashi S, Suzuki K, Yoshimoto K, Takeshita M, Kurasawa T, Yamaoka K, Takeuchi T | Rheumatology & Therapy | Early prognostic factors associated with the efficacy of infliximab treatment for patients with rheumatoid arthritis with inadequate response to methotrexate | Design |
| Hernandez D, Valor L, de da Torre I, Martinez L, Nieto JC, del Rio T, Naredo E, Gonzalez C, Lopez-Longo J, Montoro M, Monteagudo I, Carreno L | Annals of the Rheumatic Diseases | Establishing cut-off of infliximab levels and anti-infliximab antibodies by commercial elisa in patients with rheumatoid arthritis | Design |
| Herold M, Boso L, Haueis T, Klotz W, Zangerl G | Annals of the Rheumatic Diseases | No need to detect antidrug antibodies in patients treated with TNF inhibitors | Design |
| Hetland ML | Danish Medical Bulletin | Modern treatment strategies in rheumatoid arthritis: impact on, and predictors of, disease activity and disease course | Design |
| Ho D, Valtanen S, Havana M, Kroger L, Eklund K, Jokiranta S | Annals of the Rheumatic Diseases | Real-life infliximab and adalimumab trough level and antidrug antibody measurements in rheumatology: the finnish experience | Design |
| Hock BD, Stamp LK, Hayman MW, Keating PE, Helms ET, Barclay ML | Therapeutic Drug Monitoring | Development of an ELISA-based competitive binding assay for the analysis of drug concentration and antidrug antibody levels in patients receiving adalimumab or infliximab | Design |
| Hock B, O’Donnell JL, Liu J, Keating P, Spellerberg M, Stamp L, Barclay M | Annals of the Rheumatic Diseases | Antidrug antibodies: assay performance in patients treated with anti-TNF biodrugs | Design |
| Hornshoj-Sorensen C, Brock B, Tarp U, Pfeiffer-Jensen M | Annals of the Rheumatic Diseases | The time window to determine trough values of etanercept is important in personalised medicine regime independently of methotrexate coadministration | Design |
| Hoshino M, Yoshio T, Onishi S, Minota S | Modern Rheumatology | Influence of antibodies against infliximab and etanercept on the treatment effectiveness of these agents in Japanese patients with rheumatoid arthritis | Design |
| Hoxha A, Calligaro A, Tonello M, Carletto A, Paolazzi G, Bortolotti R, Felicetti M, Ramonda R, Del Ross T, Grava C, Boaretto M, Favaro M, Teghil V, Ruffatti A, Punzi L | Annals of the Rheumatic Diseases | Clinical significance of anti-adalimumab antibodies in rheumatoid arthritis, ankylosing spondilitis and psoriasic arthritis | Design |
| Inciarte-Mundo J, Hernandez MV, Cabrera S, Ruiz-Esquide V, Ramirez J, Canete JD, Yague J, Sanmarti R | Arthritis and Rheumatism | Immunogenicity induced by tumor necrosis factor antagonists in chronic inflammatory arthropathies: retrospective study in clinical practice conditions | Design |
| Inciarte-Mundo J, Hernandez MV, Cabrera-Villalba S, Ramirez J, Cuervo A, Ruiz-Esquide V, Gonzalez Navarro A, Yague J, Canete JD, Sanmarti R | Arthritis and Rheumatology | Calprotectin serum levels reflect residual inflammatory activity in patients with rheumatoid arthritis and psoriatic arthritis on clinical remission or low disease activity undergoing TNF-antagonists therapy | Design |
| Inciarte-Mundo J, Hernandez M, Ruiz-Esquide V, Ramirez J, Cuervo A, Cabrera-Villalba S, Pascal M, Yague J, Canete J, Sanmarti R | Annals of the Rheumatic Diseases | Prediction of flare in rheumatoid arthritis and psoriatic arthritis patients with low disease activity receiving TNF inhibitors: role of calprotectin and drug trough serum levels. A one-year prospective cohort study | Intervention |
| Inciarte-Mundo J, Ramirez J, Ruiz-Esquide V, Hernandez MV, Camacho O, Cabrera-Villalba S, Cuervo A, Pascal M, Yague J, Canete JD, Sanmarti R | Arthritis and Rheumatology | Calprotectin and TNF antagonist serum trough levels identify active ultrasound synovitis in rheumatoid arthritis and psoriatic arthritis patients in remission or low disease activity | Design |
| Inciarte-Mundo J, Ramirez J, Hernandez MV, Ruiz-Esquide V, Cuervo A, Cabrera-Villalba SR, Pascal M, Yague J, Canete JD, Sanmarti R | Arthritis Research & Therapy | Calprotectin and TNF trough serum levels identify power Doppler ultrasound synovitis in rheumatoid arthritis and psoriatic arthritis patients in remission or with low disease activity | Design |
| Inciarte-Mundo J, Ramirez Garcia J, Estrada Alarcon P, Garcia Manrique M, Gonzalez Navarro A, Saura C, Narvaez J, Rodriguez-Moreno J, Gomez-Centeno A, Yague J, Canete J, Sanmarti R | Annals of the Rheumatic Diseases | Drug serum levels of TNF antagonists do not correlate with subclinical synovitis by ultrasound in patients with rheumatoid arthritis and psoriatic arthritis in clinical remission or low disease activity | Design |
| Ishikawa Y, Fujii T, Kondoh-Ishikawa S, Hashimoto M, Furu M, Ito H, Imura Y, Nakashima R, Yukawa N, Yoshifuji H, Ohmura K, Mimori T | Annals of the Rheumatic Diseases | Immunogenicity is associated with lupus-like autoimmunity in rheumatoid arthritis patients treated with infliximab | Design |
| Ishikawa Y, Fujii T, Kondo-Ishikawa S, Hashimoto M, Furu M, Ito H, Imura Y, Yukawa N, Yoshifuji H, Ohmura K, Mimori T | Annals of the Rheumatic Diseases | Type I interferon plays a key role in immunogenicity and lupus-like autoimmunity in patients with rheumatoid arthritis treated by infliximab | Design |
| Ishikawa Y, Fujii T, Ishikawa SK, Yukawa N, Hashimoto M, Furu M, Ito H, Ohmura K, Mimori T | PLOS ONE | Immunogenicity and lupus-like autoantibody production can be linked to each other along with type I interferon production in patients with rheumatoid arthritis treated with infliximab: a retrospective study of a single center cohort | Design |
| Isomaki P, Vinograi V, Peltomaki J, Sokka-Isler T, Mali M, Vidqvist KL, Haapala AM, Korpela M, Makinen H | Annals of the Rheumatic Diseases | Therapeutic drug monitoring in arthritis patients receiving infliximab in daily clinical practice | Design |
| Jamnitski A, Nurmohamed MT, Hart MM, Dijkmans BA, Aarden L, Wolbink GJ | Arthritis and Rheumatism | Patients not responding to etanercept obtain lower trough etanercept concentrations compared to responding patients | Design |
| Jani M, Barton A, Warren RB, Griffiths CEM, Chinoy H | Rheumatology (United Kingdom) | The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases | Design |
| Jani M, Chinoy H, Warren RB, Fu B, Griffiths CE, Morgan AW, Wilson G, Hyrich KL, Isaacs JD, Barton A | Annals of the Rheumatic Diseases | Influence of immunogenicity and drug levels on the efficacy of long-term treatment of rheumatoid arthritis with adalimumab and etanercept: a UK-based prospective study | Design |
| Jani M, Chinoy H, Warren RB, Fu B, Griffiths CE, Morgan AW, Wilson G, Hyrich KL, Isaacs JD, Plant D, Barton A | Arthritis and Rheumatology | Clinical utility of random anti-TNF drug level testing and measurement of antidrug antibodies on long-term treatment response in rheumatoid arthritis | Design |
| Jani M, Chinoy H, Isaacs J, Morgan AW, Wilson A, Hyrich KL, Plant D, Barton A | Arthritis & Rheumatology | Clinical utility and factors associated with certolizumab pegol drug levels and antidrug antibodies in the long-term treatment of rheumatoid arthritis | Population |
| Jani M, Chinoy H, Warren RB, Griffiths CE, Plant D, Fu B, Morgan AW, Wilson AG, Isaacs JD, Hyrich K, Barton A | Arthritis & Rheumatology | Clinical utility of random anti-tumor necrosis factor drug-level testing and measurement of antidrug antibodies on the long-term treatment response in rheumatoid arthritis. [Erratum appears in Arthritis Rheum 2015;67:3096] | Design |
| Jani M, Chinoy H, Warren RB, Griffiths CE, Plant D, Morgan AW, Wilson AG, Hyrich KL, Isaacs J, Barton A | Lancet | Clinical utility of random anti-tumor necrosis factor drug testing and measurement of antidrug antibodies on long-term treatment response in rheumatoid arthritis | Design |
| Jani M, Chinoy H, Warren RB, Griffiths CEM, Plant D, Fu B, Morgan AW, Wilson AG, Isaacs JD, Hyrich KL, Barton A | Rheumatology | Clinical utility of random anti-TNF drug level testing and measurement of antidrug antibodies on long-term treatment response in rheumatoid arthritis | Design |
| Jani M, Dixon WG, Lunt M, De Cock D, Isaacs JD, Morgan AW, Wilson AG, Plant D, Watson K, Barton A, Hyrich K | Annals of the Rheumatic Diseases | The association of biologic drug-levels with infection risk: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis | Population |
| Jani M, Isaacs J, Morgan AW, Wilson AG, Plant D, Hyrich K, Chinoy H, Barton A | Rheumatology | High frequency of antidrug antibodies and correlation of low random drug levels with lack of efficacy in certolizumab pegol-treated patients with rheumatoid arthritis | Design |
| Jani M, Isaacs JD, Morgan AW, Wilson AG, Plant D, Hyrich KL, Chinoy H, Barton A | Rheumatology | Detection of antidrug antibodies using a bridging ELISA compared with radioimmunoassay in adalimumab-treated rheumatoid arthritis patients with random drug levels | Design |
| Jani M, Isaacs JD, Morgan AW, Wilson AG, Plant D, Hyrich KL, Chinoy H, Barton A, BRAGGSS | Annals of the Rheumatic Diseases | High frequency of antidrug antibodies and association of random drug levels with efficacy in certolizumab pegol-treated patients with rheumatoid arthritis: results from the BRAGGSS cohort | Design |
| Jimenez E, Garcia M, De Guadiana LG, Conesa P, Hernando A, De Bejar A, Pedregosa J, Vilchez JA, Garcia I, Albaladejo MD | Clinical Chemistry and Laboratory Medicine | Comparison of two different immunoassays to measure levels of infliximab and autoantibodies | Design |
| Jochems A, Martinez-Feito A, Plasencia C, Hernandez-Breijo B, Mezcua A, Villalba A, Monjo I, Nozal P, Balsa A, Pascual-Salcedo MD | Annals of the Rheumatic Diseases | Optimal circulating adalimumab levels range associated with good clinical response in rheumatoid arthritis patients | Design |
| Jorgensen KK, Goll GL, Sexton J, Olsen IC, Bolstad N, Lundin KE, Berset IP, Haavardsholm EA, Mork C, Kvien TK, Jahnsen J | Journal of Crohn’s and Colitis | Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: explorative subgroup analyses in IBD from the NOR-SWITCH EXTENSION trial | Population |
| Jose PD, Antonio Juan VA, Irene GG, Pablo PC, Carlos RR, Africa DBA, Ana HH, Martin Enrique JS, Iris MG, Henar GL, Ruben MT, Dolores Maria AO | Clinical Chemistry and Laboratory Medicine | Comparison of determination of adalimumab levels between two enzyme immunoassays (Promonitor and Sanquin) | Design |
| Jung SM, Lee JH, Lee J, Suh YS, Koh JH, Min HK, Lee JY, Kwok SK, Park KS, Park SH, Ju JH | International Journal of Rheumatic Diseases | Immunogenicity of anti-TNF therapy in Korean patients with RA and AS | Design |
| Jurado T, Plasencia C, Martin S, Navarro R, Bonilla G, Villalba A, Ramiro S, Jochems A Balsa A, Pascual-Salcedo D | Annals of the Rheumatic Diseases | Comparison of golimumab levels detected by two different enzyme-linked immunosorbent assays: Promonitor vs. Sanquin | Design |
| Jurado T, Plasencia C, Martinez-Feito A, Navarro-Compan V, Olariaga E, Diego C, Martin-Mola E, Balsa A, Pascual-Salcedo D | Annals of the Rheumatic Diseases | Low levels of infliximab at early stages predict the loss of drug levels and the clinical response at one year of treatment in patients with rheumatoid arthritis | Design |
| Jurado T, Plasencia-Rodriguez C, Martinez A, Navarro-Compan V, Olariaga-Merida E, Peiteado D, Villalba A, Bonilla G Diego C, Balsa A, Pascual-Salcedo D | Arthritis and Rheumatology | Infliximab low levels at early stages predict the loss of drug levels and the clinical response at one year of treatment in patients with rheumatoid arthritis | Design |
| Kadar G, Czibula A, Szalay B, Nagy K, Pusztai A, Balog A, Monostori E, Vasarhelyi B, Szekanecz Z, Kovacs L | Annals of the Rheumatic Diseases | Predictors of disease course after the discontinuation of biologic therapy in rheumatoid arthritis patients with long-term remission | Design |
| Kalden JR, Schulze-Koops H | Nature Reviews Rheumatology | Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment | Design |
| Kameda H | Nippon Rinsho – Japanese Journal of Clinical Medicine | [TNF inhibitors] | Design |
| Kameda H | Clinical Calcium | [Diagnosis and treatment of rheumatoid arthritis: towards the best practice. The best practice for TNF inhibitors] | Design |
| Kay J, Chopra A, Chandrashekara S, Olakkengil DJ, Bhojani KS, Bhatia G, Rathi G, Thomas M, Maroli S, Thomson ES, Shneyer L, Wyand MS | Annals of the Rheumatic Diseases | A phase 3, randomised, double-blind, active comparator study of the efficacy and safety of BOW015, a biosimilar infliximab, in patients with active rheumatoid arthritis on stable methotrexate doses | Design |
| Keating P, Hock B, Barclay M, Stamp L, Spellerberg M, O’Donnell J | European Journal of Immunology | Application of an ELISA based competitive binding assay to measure concentration of anti-TNF biologics and neutralising antidrug antibodies in the clinical laboratory | Design |
| Keiserman M, Codreanu C, Handa R, Xibille-Friedmann D, Mysler E, Briceno F, Akar S | Expert Review of Clinical Immunology | The effect of antidrug antibodies on the sustainable efficacy of biologic therapies in rheumatoid arthritis: practical consequences | Design |
| Kekow J, Drynda S | Arthritis and Rheumatology | Long persistence of antidrug antibodies in adalimumab treated RA patients | Design |
| Kiely PD | Rheumatology | Biologic efficacy optimization – a step towards personalised medicine | Design |
| Kim JS, Kim SH, Kwon B, Hong S | Expert Review of Clinical Immunology | Comparison of immunogenicity test methods used in clinical studies of infliximab and its biosimilar (CT-P13) | Design |
| Kneepkens EL, Pascual-Salcedo D, Plasencia C, Krieckaert CLM, van der Kleij D, Nurmohamed MT, Lopez-Casla MT, Rispens T, Wolbink G | Arthritis and Rheumatism | Golimumab levels, antibodies and clinical response in rheumatoid arthritis patients at 28 week of follow-up | Design |
| Kneepkens EL, Plasencia C, Krieckaert CL, Pascual-Salcedo D, van der Kleij D, Nurmohamed MT, Lopez-Casla MT, Wieringa R, Rispens T, Wolbink G | Annals of the Rheumatic Diseases | Golimumab trough levels, antidruganti-drug antibodies and clinical response in patients with rheumatoid arthritis treated in daily clinical practice | Population |
| Kneepkens EL, Pouw MF, Wolbink GJ, Schaap T, Nurmohamed MT, de Vries A, Rispens T, Bloem K | British Journal of Clinical Pharmacology | Dried blood spots from finger prick facilitate therapeutic drug monitoring of adalimumab and anti-adalimumab in patients with inflammatory diseases | Design |
| Kneepkens EL, an den Oever IA, Plasencia C, Salcedo Pascual D, Lopez-Casla MT, van der Kleij D, Nurmohamed MT, Rispens T, Balsa A, Wolbink GJ | Annals of the Rheumatic Diseases | Tocilizumab levels are associated with clinical response in patients with rheumatoid arthritis | Intervention |
| Kneepkens EL, Wei JCC, Nurmohamed MT, Yeo KJ, Chen CY, van der Horst-Bruinsma IE, van der Kleij D, Rispens T, Wolbink G, Krieckaert CLM | Annals of the Rheumatic Diseases | Immunogenicity, adalimumab levels and clinical response in ankylosing spondylitis patients during 24 weeks of follow-up | Population |
| Kneepkens E, van den Oever IAM, Plasencia CH, Pascual-Salcedo D, de Vries A, Hart M, Nurmohamed MT, Balsa A, Rispens T, Wolbink G | Scandinavian Journal of Rheumatology | Serum tocilizumab trough concentration can be used to monitor systemic IL-6 receptor blockade in patients with rheumatoid arthritis: a prospective observational cohort study | Intervention |
| Koyama Y, Otal T, Miura T | Annals of the Rheumatic Diseases | Analysis of patients with detectable trough serum levels of infliximab revealed significant predictors associated with non-response to actual infliximab in rheumatoid arthritis | Design |
| Kozmar A | Biochemia Medica | The role of laboratories in optimizing biological therapy | Population |
| Krieckaert C, Rispens T, Wolbink G | Current Opinion in Rheumatology | Immunogenicity of biological therapeutics: from assay to patient | Design |
| Krieckaert C, Vogelzang E, Pouw M, Nurmohamed M, Wolbink G | Annals of the Rheumatic Diseases | Adalimumab serum concentrations in patients with rheumatoid arthritis or psoriatic arthritis taking concomitant DMARD therapy | Population |
| Kuang B, King L, Wang HF | Bioanalysis | Therapeutic monoclonal antibody concentration monitoring: free or total? | Design |
| Laine J, Jokiranta TS, Eklund KK, Vakevainen M, Puolakka K | Biologics | Cost-effectiveness of routine measuring of serum drug concentrations and antidrug antibodies in treatment of rheumatoid arthritis patients with TNF-alpha blockers | Design |
| l’Ami MJ, Krieckaert CL, Nurmohamed MT, van Vollenhoven RF, Rispens T, Boers M, Wolbink GJ | Annals of the Rheumatic Diseases | Successful reduction of overexposure in patients with rheumatoid arthritis with high serum adalimumab concentrations: an open-label, non-inferiority, randomised clinical trial | Population |
| Langguth D, Wong P, Bowling A, Bagga H, Freeman D, Ford E | Arthritis & Rheumatology | Serum trough levels of adalimumab inversely correlate with disease activity in patients with inflammatory arthritis | Population |
| Leow Y, Youssef P, Richards B | International Journal of Rheumatic Diseases | Correlation of adalimumab trough level with disease activity in patients with inflammatory arthritis | Population |
| Leu JH, Xu Z, Hu C, Mendelsohn A, Ford J, Davis HM, Zhou H | Arthritis and Rheumatism | Importance of steady-state trough concentrations after intravenous golimumab with concomitant methotrexate in patients with active rheumatoid arthritis | Design |
| Li MH, Li HZ, Gao K, Wang MY, An WQ, Zhu YR, Ding L, Wang L, Gu JL, Zuo GL, Sun L | Journal of Immunological Methods | A simple and cost-effective assay for measuring antidrug antibody in human patients treated with adalimumab | Design |
| Llinares-Tello F, Rosas-Gomez de Salazar J, Senabre-Gallego JM, Santos-Soler G, Santos-Ramirez C, Salas-Heredia E, Barber-Valles X, Molina-Garcia J, AIRE-MB Group | Rheumatology International | Practical application of acid dissociation in monitoring patients treated with adalimumab | Design |
| Llinares-Tello F, Rosas-Gomez de Salazar J, Senabre-Gallego JM, Santos-Soler G, Santos-Ramirez C, Salas-Heredia E, Molina-Garcia J, AIRE-MB Group | Clinical Chemistry and Laboratory Medicine | Analytical and clinical evaluation of a new immunoassay for therapeutic drug monitoring of etanercept | Design |
| Llinares-Tello F, Rosas J, de la Torre I, Valor L, Senabre JM, Barber X, Hernandez D, Carreno L, Santos-Soler G, Salas E, Santos-Ramirez C, Sanchez-Barrioluengo M, Molina-Garcia J | Annals of the Rheumatic Diseases | Comparative study of both versions of an immunoassay commercialised for therapeutic drug monitoring of adalimumab | Design |
| Llinares-Tello F, Rosas J, de la Torre I, Valor L, Barber X, Senabre JM, el Grupo AIRE-MB, HUGM | Reumatologia Clinica | Comparative study of both versions of an immunoassay commercialised for therapeutic drug monitoring of adalimumab in rheumatoid arthritis | Design |
| Llinares-Tello F, Rosas J, Senabre-Gallego JM, Molina J, Salas E, Santos-Soler G, Santos Ramirez C, Ortega R, Barber X, Pons A, Cano C, Lorente M, Sanchez-Barrioluengo M | Annals of the Rheumatic Diseases | Usefulness of the acid dissociation in inmunogenicity detection in patients in treatment with anti-TNF drugs | Design |
| Llinares-Tello F, Rosas J, Senabre-Gallego JM, Santos-Soler G, Santos-Ramirez C, Salas-Heredia E, Barber X, Molina J, Cano C, Pons A | Arthritis and Rheumatology | Implementation of an acid dissociation procedure for immunogenicity detection in patients treated with anti-TNF drugs | Design |
| Llinares-Tello F, Rosas-Gomez de Salazar J, Senabre-Gallego JM, Santos-Soler G, Santos-Ramirez C, Salas-Heredia E, Barber-Valles X, Molina-Garcia J, AIRE-MB Group | Rheumatology International | Practical application of acid dissociation in monitoring patients treated with adalimumab. [Erratum appears in Rheumatol Int 2014;34:1709] | Design |
| Lopatnikova JA, Golikova EA, Shkaruba NS, Sizikov AE, Sennikov SV | Scandinavian Journal of Rheumatology | Analysis of the levels of tumour necrosis factor (TNF), autoantibodies to TNF, and soluble TNF receptors in patients with rheumatoid arthritis | Design |
| Lopez-Casla MT, Pascual-Salcedo D, Plasencia C, Alcozer P, Garcia-Carazo S, Bonilla G, Villalba A, Peiteado D, Arribas F, Martin-Mola E, Balsa A | Annals of the Rheumatic Diseases | The infliximab dose increase is not correlated with clinical improvement in RA patients | Intervention |
| Lopez-Rodriguez R, Martinez A, Plasencia C, Jochems A, Pascual-Salcedo D, Balsa A, Gonzalez A | Annals of the Rheumatic Diseases | Increased frequency of antidrug antibodies in patients carrying compatible IgG1 allotypes and treated with anti-TNF antibodies | Design |
| Lukina G, Sigidin Y, Alexandrova E, Novikov A, Aronova E, Kanonirova M, Glukhova S, Nasonov E | Annals of the Rheumatic Diseases | Clinical significance of antibodies to infliximad in rheumatoid arthritis (RA) patients | Population |
| Maggi E, Vultaggio A, Matucci A | Expert Review of Clinical Immunology | Acute infusion reactions induced by monoclonal antibody therapy | Design |
| Maid P, Real R, Pedersen R, Shen Q, Hidalgo R | Journal of Clinical Rheumatology | Incidence of antibodies in patients with rheumatoid arthritis from Argentina treated with adalimumab, etanercept, or infliximab in a real-world setting | Design |
| Maneiro JR, Salgado E, Gomez-Reino JJ | JAMA Internal Medicine | Immunogenicity of monoclonal antibodies against tumor necrosis factor used in chronic immune-mediated Inflammatory conditions: systematic review and meta-analysis | Design |
| Marinari B, Botti E, Bavetta M, Spallone G, Zangrilli A, Talamonti M, Richetta A, Chimenti S, Costanzo A | Drug Development Research | Detection of adalimumab and anti-adalimumab levels by ELISA: clinical considerations | Population |
| Marotte H, Maslinski W, Miossec P | Arthritis Research & Therapy | Circulating tumour necrosis factor-alpha bioactivity in rheumatoid arthritis patients treated with infliximab: link to clinical response | Population |
| Marotte H, Rinaudo M, Paul S, Fautrel B | Annals of the Rheumatic Diseases | No prediction of relapse by TNF blocker concentrations or detection of antibodies against anti-TNF: data from Strass study | Design |
| Marotte H, Rinaudo-Gaujous M, Paul S, Fautrel B | Arthritis & Rheumatology | TNF blocker concentrations or detection of antibodies against anti-TNF before a tapering process are not predictive to relapse | Design |
| Marsman A, L’Ami M, Kneepkens E, Kienhorst L, Nurmohamed M, Krieckaert C, Wolbink G | Annals of the Rheumatic Diseases | Patient reported reasons for refraining from participation in dose reduction studies with biologics | Design |
| Martelli L, Olivera P, Roblin X, Attar A, Peyrin-Biroulet L | Journal of Gastroenterology | Cost-effectiveness of drug monitoring of anti-TNF therapy in inflammatory bowel disease and rheumatoid arthritis: a systematic review | Design |
| Martin S, del Agua AR, Torres N, Pascual-Salcedo D, Plasencia C, Jurado T, Ruiz Arguello B, Martinez A, Navarro R, Nagore D | Arthritis & Rheumatology | Validation and comparison study of immunoassays for the measurement of golimumab and antibodies to golimumab in rheumatic patients | Design |
| Martinez Estupinan LP, Valor L, Hernandez D, Naredo E, Montoro M, Nieto-Gonzalez JC, Mata-Martinez C, Ovallez-Bonilla J, Serrano-Benavente B, Gonzalez-Fernandez C, Lopez-Longo J, Monteagudo I, Carreno-Perez L | Annals of the Rheumatic Diseases | Relation between serum infliximab levels and changes of rheumatoid factor and antibodies to citrullinated peptides levels in patients with rheumatoid arthritis | Population |
| Martinez-Estupinan L, Hernandez-Florez D, Janta I, Ovalles-Bonilla JG, Nieto JC, Gonzalez-Fernandez CM, del Rio T, Monteagudo I, Lopez-Longo FJ, Naredo E, Valor L | Clinical & Experimental Rheumatology | An exploratory study to determine whether infliximab modifies levels of rheumatoid factor and antibodies to cyclic citrullinated peptides in rheumatoid arthritis patients | Design |
| Martinez-Feito A, Bravo Gallego LY, Hernandez-Breijo B, Plasencia C, Jochems A, Gonzalez MA, Monjo I, Peiteado D, Bonilla G, Nozal P, Balsa A, Pascual-Salcedo D | Annals of the Rheumatic Diseases | Clinical relevance of detecting anti-adalimumab antibodies with a drug-tolerant assay | Design |
| Martinez-Feito A, Plasencia C, Villalba A, Jurado T, Mezcua A, Martin-Mola E, Bonilla G, Balsa A, Pascual-Salcedo D | Annals of the Rheumatic Diseases | Effect of methotrexate in the presence of drug and the appearance of antibodies against TNF inhibitors in patients with rheumatoid arthritis | Design |
| Martin-Lopez M, Carmona L, Balsa A, Calvo-Alen J, Sanmarti R, Tornero J, Rosas J | Rheumatology International | Serum drug levels of biologic agents in the management of rheumatoid arthritis and spondyloarthritis: a systematic review | Design |
| Matsuura Y, Narazaki M, Nishide M, Kato Y, Yorifuji H, Hirano T, Shima Y, Tanaka T, Ogata A, Kumanogoh A | Arthritis & Rheumatology | Optimization of treatment intervals of tocilizumab and golimumab by measuring serum trough levels in rheumatoid arthritis patients | Design |
| Petroni A, Matucci G, Nencini F, Pratesi S, Maggi E, Vultaggio A | Allergy: European Journal of Allergy and Clinical Immunology | Anti-infliximab antibodies production and clinical consequences: adverse reactions and loss of response | Population |
| Matucci A, Vultaggio A, Nencini F, Pratesi S, Rossi O, Parronchi P, Romagnani S, Maggi E | Allergy: European Journal of Allergy and Clinical Immunology | Adverse reactions to biological agents: role of anti-infliximab antibodies and analysis of potential risk factors | Design |
| Mazilu D, Gainaru C, Apetrei N, Luca G, Gudu T, Peltea A, Constantinescu C, Saulescu I, Bojinca V, Balanescu A, Predeteanu D, Ionescu R, Opris D | International Journal of Rheumatic Diseases | Methotrexate and infliximab immunogenicity | Design |
| Mazilu D, Opris D, Gainaru C, Iliuta M, Apetrei N, Luca G, Borangiu A, Gudu T, Peltea A, Groseanu L, Constantinescu C, Saulescu I, Bojinca V, Balanescu A, Predeteanu D, Ionescu R | BioMed Research International | Monitoring drug and antidrug levels: a rational approach in rheumatoid arthritis patients treated with biologic agents who experience inadequate response while being on a stable biologic treatment | Population |
| Mazilu D, Opris D, Iachim E, Deaconu C, Saulescu I, Borangiu A, Grosanu L, Constantinescu C, Balanescu A, Predeteanu D, Ionescu R | Arthritis & Rheumatology | Time to first signs of loss of response in rheumatoid arthritis patients treated with time to first signs of loss of response in rheumatoid arthritis patients treated with anti-TNF agents: correlations with serum drug level, immunogenicity and csDMARD association | Design |
| Medina F, Plasencia C, Goupille P, Ternant D, Bals A, Mulleman D | Therapeutic Drug Monitoring | Current practice for therapeutic drug monitoring of biopharmaceuticals in rheumatoid arthritis | Design |
| Meric JC, Mulleman D, Paintaud G, Ducourau E, Magdelaine-Beuzelin C, Valat JP, Goupille P | Arthritis and Rheumatism | Infliximab concentration monitoring improves the control of disease activity in rheumatoid arthritis | Design |
| Meroni PL, Valentini G, Ayala F, Cattaneo A, Valesini G | Autoimmunity Reviews | New strategies to address the pharmacodynamics and pharmacokinetics of tumor necrosis factor (TNF) inhibitors: a systematic analysis | Design |
| Mieke P, Charlotte K, Michael N, Margreet H, Henk TV, Desiree VDK, Lucien, Theo R, Gertjan W | Clinical Chemistry and Laboratory Medicine | Measurement of anti-TNF drugs levels is the key to optimal, personalised and cost-effective treatment | Population |
| Mistretta VI, Cavalier E, Collette J, Lutteri L, Chapelle JP | Revue Medicale de Liege | Interest of monoclonal antibodies in the biomedical laboratory analysis | Design |
| Mochizuki T, Momohara S, Ikari K, Okamoto H, Kobayashi S, Tsukahara S, Iwamoto T, Kawamura K, Saito S, Tomatsu T | Modern Rheumatology | The serum concentration of infliximab in cases of autologous blood donation for patients with rheumatoid arthritis | Design |
| Mok CC, Fong B, Ho LY, To CH | Annals of the Rheumatic Diseases | Serum levels of the anti-TNF biologics correlate with clinical efficacy in patients with inflammatory arthritis | Design |
| Mok CC, Fong LS, Ho LY, To CH | Arthritis & Rheumatology | Serum levels of the anti-TNF biologics correlate with clinical efficacy in patients with inflammatory arthritis | Design |
| Mok CC, Tsai WC, Chen DY, Wei JC | Expert Opinion on Biological Therapy | Immunogenicity of anti-TNF biologic agents in the treatment of rheumatoid arthritis | Design |
| Mok CC, van der Kleij D, Wolbink G | Annals of the Rheumatic Diseases | Antidrug antibodies, drug levels and clinical efficacy of the anti-TNF biologics in rheumatic diseases | Population |
| Mok CC, van der Kleij D, Wolbink G | Clinical Rheumatology | Drug levels, antidrug antibodies, and clinical efficacy of the anti-TNFα biologics in rheumatic diseases | Population |
| Moots RJ, Xavier R, Mok CC, Rahman MU, Tsai WC, Al Maini M, Pavelka K, Mahgoub E, Kotak S, Korth-Bradley J, Pedersen R, Mele L, Shen Q, Vlahos B | Arthritis & Rheumatology | Incidence of antibodies in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab in a real-world setting | Population |
| Moots RJ, Xavier RM, Mok CC, Rahman MU, Tsai WC, Al-Maini MH, Pavelka K, Mahgoub E, Kotak S, Korth-Bradley J, Pedersen R, Mele L, Shen Q, Vlahos B | PLOS ONE | The impact of antidrug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: results from a multinational, real-world clinical practice, non-interventional study. [Erratum appears in PLOS ONE 2017;12:e0179308] | Population |
| Mori S | Modern Rheumatology | A relationship between pharmacokinetics (PK) and the efficacy of infliximab for patients with rheumatoid arthritis: characterization of infliximab-resistant cases and PK-based modified therapy | Design |
| Mori S, Ueki Y | Modern Rheumatology | Primary lack of efficacy of infliximab therapy for rheumatoid arthritis: pharmacokinetic characterization and assessment of switching to tocilizumab | Design |
| Mulleman D, Ducourau E, Paintaud G, Ternant D, Watier H, Goupille P | Joint Bone Spine | Should anti-TNF-alpha drug levels and/or antidrug antibodies be assayed in patients treated for rheumatoid arthritis? | Design |
| Mulleman D, Lin DCM, Ducourau E, Emond P, Ternant D, Magdelaine-Beuzelin C, Valat JP, Paintaud G, Goupille P | Therapeutic Drug Monitoring | Trough infliximab concentrations predict efficacy and sustained control of disease activity in rheumatoid arthritis | Design |
| Mulleman D, Meric JC, Paintaud G, Ducourau E, Magdelaine-Beuzelin C, Valat JP, Goupille P | Arthritis Research and Therapy | Infliximab concentration monitoring improves the control of disease activity in rheumatoid arthritis | Population |
| Nedovic J, Stamenkovic B, Stojanovic S, Zivkovic V | Annals of the Rheumatic Diseases | Does concentration of antibodies to etanercept and adalimumab correlates with parameters of disease activity in patients with rheumatoid arthritis? | Population |
| Nishida K, Hashizume K, Kadota Y, Natsumeda M, Nakahara R, Saito T, Kanazawa T, Ezawa K, Ozaki T | Modern Rheumatology | Time-concentration profile of serum etanercept in Japanese patients with rheumatoid arthritis after treatment discontinuation before orthopaedic surgery | Design |
| Nunes A, Garces S, Vieira A, Demangeot J, Freitas J | Journal of Crohn’s and Colitis | Infliximab trough levels and anti-infliximab antibodies in rheumatoid arthritis and in IBD patients – a comparison from a single center | Population |
| O’Donnell J, Liu J, Keating P, Hock B, Spellerberg M, Barclay M, Stamp L | Internal Medicine Journal | Antidrug antibodies (ADA): assay performance in patients treated for inflammatory bowel and rheumatic disease with biodrugs, adalimumab and infliximab | Design |
| Ogric M, Tercelj M, Praprotnik S, Tomsic M, Bozic B, Sodin-Semrl S, Cucnik S | Immunologic Research | Detection of adalimumab and anti-adalimumab antibodies in patients with rheumatoid arthritis: a comprehensive overview of methodology pitfalls and benefits | Design |
| Ometto F, Beggio M, Friso L, Astorri D, Raffeiner B, Botsios C, Bernardi L, Padoan R, Punzi L, Ghiraldello A, Doria A | Annals of the Rheumatic Diseases | Anti-etanercept antibodies and etanercept leves levels in rheumatoid arthritis patients treated with low and full-dose etanercept in DAS28 remission | Design |
| Opris D, Borangiu A, Gudu T, Mazilu D, Balanescu A, Saulescu I, Ionescu R | Annals of the Rheumatic Diseases | Does serum drug level correlates with ultrasound evaluation in patients with rheumatoid arthritis treated with TNF antagonists? | Design |
| Opris D, Diana M, Gainaru C, Iliuta M, Groseanu L, Saulescu I, Constantinescu C, Bojinca V, Balanescu A, Predeteanu D, Ionescu R | Annals of the Rheumatic Diseases | Serum drug level and anti-citrullinated peptide antibodies as biomarkers that predict EULAR response in rheumatoid arthritis – a new step to personalised medicine | Intervention |
| Opris D, Mazilu D, Bojinca V, Balanescu A, Borangiu A, Ionescu R | Clinical and Experimental Rheumatology | Adalimumab serum drug level correlates to clinical response in patients with rheumatoid arthritis | Population |
| Opris D, Mazilu D, Bojinca V, Saulescu I, Balanescu A, Ionescu RM | Clinical and Experimental Rheumatology | Secondary failure to etanercept in rheumatoid arthritis patients–the role of immunogenicity, characteristics and evolution of the disease | Population |
| Opris D, Mazilu D, Ionescu R | Clinical and Experimental Rheumatology | Clinical response in rheumatoid arthritis patients with anti-infliximab antibodies | Population |
| Padulles A, Padulles N, Lloberas-Blanch N, Juanola X, Narvaez FJ, Leiva E, Cobo S, Bas J, Climent J, Carrere M, Colom H | European Journal of Hospital Pharmacy | Evaluation of a population pharmacokinetic model of infliximab in rheumatoid arthritis for prediction of individual dosage requirements | Design |
| Palaparthy R, Schmitt S, Rehman MI, Cai CH, Wang K, Von Richter O | Journal of Crohn’s and Colitis | Incidence and impact of immunogenicity in a randomised, double-blind phase III study comparing a proposed infliximab biosimilar (PF-06438179/GP1111) with reference infliximab | Population |
| Paredes B, Plasencia C, Pascual-Salcedo D, Monjo I, Pieren A, Moral E, Tornero C, Bonilla G, Nuno L, Villalba A, Peiteado D, Ramiro S, Jurado T, Diez J, Martin-Mola E, Balsa A | Annals of the Rheumatic Diseases | Influence of optimization of biological therapies on immunogenicity in a cohort of rheumatoid arthritis with low disease activity | Intervention |
| Partridge MA, Purushothama S, Elango C, Lu YM | Journal of Immunology Research | Emerging technologies and generic assays for the detection of antidrug antibodies | Design |
| Pecoraro V, De Santis E, Melegari A, Trenti T | Autoimmunity Reviews | The impact of immunogenicity of TNF-alpha inhibitors in autoimmune inflammatory disease. A systematic review and meta-analysis | Design |
| Perdriger A. | Biologics | Infliximab in the treatment of rheumatoid arthritis | Design |
| Petroni G, Pratesi S, Nencini F, Milla M, Maggi E, Matucci A, Vultaggio A | Allergy: European Journal of Allergy and Clinical Immunology | The onset of anti-infliximab antibodies occurs after the first drug infusions and their high levels are related to adverse reactions | Population |
| Pieren A, Pascual-Salcedo D, Aguado P, Bonilla G, De Miguel E, Monjo I, Nuno L, Peiteado D, Villalba A, Coro EM, Tornero C, Bogas P, Balsa A, Plasencia-Rodriguez C | Arthritis & Rheumatology | Flare incidence and predictive factors in a population of patients with rheumatoid arthritis under optimised treatment with adalimumab and infliximab | Population |
| Plasencia C, Jurado T, Villalba A, Peitedado D, Casla MT, Nuno L, Bonilla MG, Martinez-Feito A, Martin-Mola E, Pascual-Salcedo D, Balsa A | Frontiers in Medicine | Effect of infliximab dose increase in rheumatoid arthritis. at different trough concentrations: a cohort study in clinical practice conditions | Population |
| Plasencia C, Pascual-Salcedo D, Alcozer P, Garcia-Carazo S, Franco KN, Cajigas D, Bonilla G, Lojo L, Nuno L, Villalba A, Peiteado D, Arribas F, Lopez-Casla MT, Martin-Mola E, Balsa A | Annals of the Rheumatic Diseases | Etanercept serum trough levels are correlated with clinical activity in rheumatoid arthritis patients with long-term treatment with etanercept | Population |
| Plasencia-Rodriguez C, Pascual-Salcedo D, Bonilla MG, Villalba A, Peiteado D, Nuno L, Aguado P, Jurado T, Martin-Mola E, Balsa A | Arthritis and Rheumatology | The monitoring of infliximab levels at early stages can predict the development of anti-infliximab antibodies in a cohort of rheumatoid arthritis patients treated with infliximab | Outcome |
| Plasencia-Rodriguez C, Pascual-Salcedo MD, Bonilla G, Navarro-Compan V, Martinez-Feito A, Diego C, Villalba A, Peiteado D, Nuno L, Martin-Mola E, Balsa A | Annals of the Rheumatic Diseases | Influence of drug levels during the first anti-TNF therapy on the clinical response to a second biologic in rheumatoid arthritis patients | Intervention |
| Pouw MF, Krieckaert CL, Nurmohamed MT, Rispens T, Aarden L, Wolbink G | Annals of the Rheumatic Diseases | Adalimumab trough level in blood corresponding with clinical response | Population |
| Pouw MF, Krieckaert CL, Nurmohamed MT, van der Kleij D, Aarden L, Rispens T, Wolbink G | Annals of the Rheumatic Diseases | Key findings towards optimising adalimumab treatment: the concentration-effect curve | Intervention |
| Pouw MF, Mulleman D, Nurmohamed MT, Rispens T, Paintaud G, Wolbink G, Ternant D | Arthritis & Rheumatology | Adalimumab concentration at 16 weeks of treatment is associated with treatment discontinuation within one year | Intervention |
| Prado MS, Bendtzen K, Andrade LEC | Expert Opinion on Drug Metabolism and Toxicology | Biological anti-TNF drugs: immunogenicity underlying treatment failure and adverse events | Design |
| Puig L | Journal of the European Academy of Dermatology and Venereology | Defining effective approaches to the reduction or elimination of biologic therapy immunogenicity and loss of response | Population |
| Quesada-Masachs E, Alvarez-de la Sierra D, Garcia Prat M, Pujol-Borrell R, Martinez Gallo M, Modesto Caballero C, Marin Sanchez AM | Annals of the Rheumatic Diseases | Prospective analysis of the immunogenic response in JIA patients (paediatric and adult) on antiTNF treatment | Population |
| Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FHJ, Enevold C, van Riel PL, Bendtzen K | Annals of the Rheumatic Diseases | Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis | Population |
| Reyes-Beltran B, Delgado G | Journal of Immunotoxicology | Antidrug antibodies in Colombian patients with rheumatoid arthritis treated with Enbrel vs. Etanar – preliminary report | Population |
| Rodriguez-Muguruza S, Martinez-Morillo M, Sanint J, Quirant B, Teniente A, Prior A, Riveros-Frutos A, Holgado S, Mateo ML, Olive A, Canellas J, Tena X | Arthritis & Rheumatology | Tocilizumab serum levels and antidrug antibodies and its relationship with disease activity in rheumatic diseases | Intervention |
| Roland PN, Mignot SG, Bruns A, Hurtado M, Palazzo E, Hayem G, Dieude P, Meyer O, Martin SC | Arthritis Research and Therapy | Antibodies to mutated citrullinated vimentin for diagnosing rheumatoid arthritis in anti-CCP-negative patients and for monitoring infliximab therapy | Intervention |
| Rosas JL, Linares F, de la Torre I, Valor L, Barber X, Santos-Ramirez C, Hernandez D, Senabre JM, Carreno L, Santos-Soler G, Salas E, Sanchez-Barrioluengo M, Molina-Garcia J | Annals of the Rheumatic Diseases | Clinical usefulness of serum level of adalimumab, in patients with rheumatoid arthritis | Outcome |
| Rosas J, Llinares-Tello F, Senabre JM, Santos-Ramirez C, Santos-Soler G, Salas E, Barber X, Sanchez-Barrioluengo M, Molina-Garcia J, Llahi N, Cano C | Annals of the Rheumatic Diseases | Evaluation of anti-TNF levels and anti-TNF antibodies in rheumatic diseases treated with infliximab and adalimumab; results from a local registry | Population |
| Rosas Gomez de Salazar J, Llinares-Tello F, Senabre-Gallego JM, Santos-Soler G, Santos-Ramirez C, Salas-Heredia E, Barber-Valles X | Arthritis and Rheumatism | Evaluation of anti-tumor necrosis factor levels and anti-tumor necrosis factor antibodies in rheumatic diseases treated with infliximab and adalimumab; preliminary results from a local registry | Population |
| Rosas J, Llinares-Tello F, de la Torre I, Santos-Ramirez C, Senabre-Gallego JM, Valor L, Barber-Valles X, Hernandez-Florez D, Santos-Soler G, Salas-Heredia E, Carreno L, AIRE-MB Group | Clinical and Experimental Rheumatology | Clinical relevance of monitoring serum levels of adalimumab in patients with rheumatoid arthritis in daily practice | Design |
| Rosas J, Llinares-Tello F, Martin S, Senabre-Gallego JM, Salas E, Oliver S, Santos-Soler G, Santos-Ramirez C, Barber-Valles X, Pons A, Cano C, Lorente M | Annals of the Rheumatic Diseases | Evaluation of serum level of golimumab and antibodies anti-golimumab in patients with rheumatic diseases: results from a local registry | Population |
| Rosas J, Llinares-Tello F, Senabre JM, Santos-Soler G, Salas-Heredia E, Barber X, Pons A, Cano C, Lorente M, Molina J | Arthritis & Rheumatology | Economic impact of decreasing adalimumab and etanercept doses and drug monitoring in patients with rheumatoid arthritis in clinical remission: preliminary study from a local biologics unit | Intervention |
| Ruiz-Arguello B, Maguregui A, del Agua AR, Pascual-Salcedo D, Jurado T, Plasencia C, Balsa A, Llinares-Tello F, Rosas J, Torres N, Martinez A, Nagore D | Arthritis & Rheumatology | Antibodies to infliximab in remicade-treated rheumatic patients show identical reactivity towards biosimilars | Outcome |
| Ruiz-Arguello MB, Maguregui A, del Agua AR, Pascual-Salcedo D, Martinez-Feito A, Jurado T, Plasencia C, Balsa A, Llinares-Tello F, Rosas J, Torres N, Martinez A, Nagore D | Annals of the Rheumatic Diseases | Antibodies to infliximab in Remicade-treated rheumatic patients show identical reactivity towards biosimilars | Outcome |
| Ruiz-Esquide V, Bastida C, Pascal M, Yague J, Soy D, Sanmarti R | Annals of the Rheumatic Diseases | Therapeutic drug monitoring on rheumatoid arthritis patients with reduced doses of intravenous tocilizumab | Intervention |
| Ruiz-Esquide V, Gonzalez-Navarro A, Yague J, Inciarte-Mundo J, Hernandez MV, Ramirez J, Cabrera-Villalba S, Canete JD, Sanmarti R | Arthritis and Rheumatology | Tocilizumab serum trough levels and its relationship with disease activity and drug dosage in rheumatoid arthritis patients | Intervention |
| Ruiz-Esquide V, Gonzalez-Navarro A, Yague J, Ramirez J, Hernandez MV, Cabrera-Villalba S, Inciarte-Mundo J, Canete JDD, Sanmarti R | Annals of the Rheumatic Diseases | Serum levels of tocilizumab and its relationship with disease activity and drug dosage in patients with rheumatoid arthritis | Intervention |
| Ruiz-Esquide V, Zufferey P, Inciarte-Mundo J, Yague J, Hernandez MV, Ramirez J, Berner J, Pascal M, Cuervo A, Canete JD, Sanmarti R | Arthritis & Rheumatology | Tocilizumab serum trough levels and disease activity in rheumatoid arthritis | Intervention |
| Ruiz-Esquide V, Zufferey P, Yague J, Berner J, Inciarte-Mundo J, Gonzalez-Navarro A, Hernandez V, Ramirez J, Cuervo A, Canete J, Sanmarti R | Annals of the Rheumatic Diseases | Relationship between clinical remission and serum levels of tocilizumab in the treatment of rheumatoid arthritis | Intervention |
| Rutgeerts P, Vermeire S, Van Assche G | Gut | Predicting the response to infliximab from trough serum levels | Population |
| Saito T, Nishida K, Hashizume K, Nakahara R, Kanazawa T, Kadota Y, Ozaki T | International Journal of Rheumatic Diseases | Time-concentration profile of etanercept in the serum from Japanese rheumatoid arthritis patients after discontinuation before orthopaedic surgery | Population |
| Sanmarti R, Inciarte J, Estrada Alarcon P, Garcia Manrique M, Gonzalez Navarro A, Narvaez J, Rodriguez-Moreno J, Gomez-Centeno A, Yague J | Annals of the Rheumatic Diseases | Immunogenicity of anti-TNF antagonists in patients with rheumatoid arthritis or polyarticular psoriatic arthritis in clinical remission or low disease activity: the Inmunoremar study | Outcome |
| Sanmarti R, Inciarte J, Estrada Alarcon P, Garcia Manrique M, Narvaez J, Rodriguez J, Gomez Centeno T, Pascal M, Yague J | Annals of the Rheumatic Diseases | Serum levels of TNF antagonists in rheumatoid arthritis: can we establish an optimal cut-off to identify patients in remission or low disease activity? | Design |
| Sanmarti R, Inciarte-Mundo J, Estrada-Alarcon P, Garcia-Manrique M, Narvaez J, Rodriguez-Moreno J, Gomez-Centeno A, Pascal M, Yague J | Annals of the Rheumatic Diseases | Towards optimal cut-off trough levels of adalimumab and etanercept for a good therapeutic response in rheumatoid arthritis. Results of the INMUNOREMAR study | Outcomes |
| Sanmarti R, Inciarte-Mundo J, Estrada-Alarcon P, Garcia-Manrique M, Narvaez J, Gomez-Centeno A, Rodriguez-Moreno J, Pascal M, Yague J | Annals of the Rheumatic Diseases | Immunogenicity of TNF inhibitors in patients with rheumatoid arthritis or polyarticular psoriatic arthritis in clinical remission or low disease activity: a one-year multicentre prospective study (the INMUNOREMAR study) | Design |
| Sato M, Takemura M, Tani T, Ohashi T | Annals of the Rheumatic Diseases | Can infliximab efficacy be predicted based on blood concentration at the fourth dose? | Design |
| Senabre Gallego JM, Rosas Gomez de Salazar J, Marco Mingot M, Naranjo A, Llinares-Tello F, Pons A, Barber-Valles X, Santos-Soler G, Salas-Heredia E, Cano C, Lorente M, Garcia Gomez JA, Molina J | Annals of the Rheumatic Diseases | Clinical activity, ultrasound assessment and drug monitoring in rheumatoid arthritis patients receiving anti-TNF-alpha therapy with extended interval of administration | Intervention |
| Schmitz EMH, Benoy-De Keuster S, Meier AJL, Scharnhorst V, Traksel RAM, Broeren MAC, Derijks LJJ | Clinical Rheumatology | Therapeutic drug monitoring (TDM) as a tool in the switch from infliximab innovator to biosimilar in rheumatic patients: results of a 12-month observational prospective cohort study | Population |
| Schmitz EMH, Boekema PJ, Straathof JWA, van Renswouw DC, Brunsveld L, Scharnhorst V, van de Poll MEC, Broeren MAC, Derijks LJJ | Alimentary Pharmacology & Therapeutics | Switching from infliximab innovator to biosimilar in patients with inflammatory bowel disease: a 12-month multicentre observational prospective cohort study | Population |
| Schmitz EMH, van de Kerkhof D, Hamann D, van Dongen JLJ, Kuijper PHM, Brunsveld L, Scharnhorst V, Broeren MAC | Clinical Chemistry and Laboratory Medicine | Therapeutic drug monitoring of infliximab: performance evaluation of three commercial ELISA kits | Design |
| Schuster T, Keller E, Krauchi S, Bantleon F, Weber J, Schneider M | Journal of Crohn’s and Colitis | Performance of the BUHLMANN Quantum Blue Infliximab point-of-care assay dedicated for therapeutic drug monitoring of serum infliximab trough levels | Design |
| Secchiero P, Corallini F, Castellino G, Bortoluzzi A, Caruso L, Bugatti S, Bosco R, Montecucco M, Trotta F | Journal of Rheumatology | Baseline serum concentrations of TRAIL in early rheumatoid arthritis: relationship with response to disease-modifying antirheumatic drugs | Design |
| Senturk T, Cildag S, Akdam I, Gultekin B | International Journal of Rheumatic Diseases | Anti-TNF induced autoimmunity | Design |
| Senturk T, Cildag S, Akdam I, Gultekin B | Clinical and Experimental Rheumatology | Anti-TNF induced autoimmunity | Design |
| Sigaux J, Hamze M, Daien C, Morel J, Krzysiek R, Pallardy M, Maillere B, Mariette X, Miceli-Richard C | Annals of the Rheumatic Diseases | The lack of antidrug antibodies among patients treated with tocilizumab: a clue to good efficacy profiles when used as monotherapy? | Intervention |
| Sigaux J, Hamze M, Daien C, Morel J, Krzysiek R, Pallardy M, Maillere B, Mariette X, Miceli-Richard C | Joint, Bone, Spine: Revue du Rhumatisme | Immunogenicity of tocilizumab in patients with rheumatoid arthritis | Intervention |
| Siljehult F, Arlestig L, Eriksson C, Rantapaa-Dahlqvist S | Scandinavian Journal of Rheumatology | Concentrations of infliximab and antidrug antibodies in relation to clinical response in patients with rheumatoid arthritis | Population |
| Smolen JS, Mostafa N, Huang X, Noertersheuser P, Klunder B, Chen K, Kalabic J, Sainsbury I, Oerlemans R, Florentinus S, Burmester GR | Arthritis and Rheumatology | The value of adalimumab trough levels and clinical assessments in predicting clinical response in patients with established rheumatoid arthritis and an inadequate response to methotrexate | Population |
| Sorrentino D, Nguyen V, Henderson C, Bankole A | Inflammatory Bowel Diseases | Therapeutic drug monitoring and clinical outcomes in immune mediated diseases: the missing link | Design |
| Spinelli FR, Valesini G | Clinical and Experimental Rheumatology | Immunogenicity of anti-tumor necrosis factor drugs in rheumatic diseases | Design |
| Spirchez M, Samasca G, Bolba C, Miu N | Pediatric Rheumatology | Serum tumor necrosis factor alpha increased during remission with Etanercept | Population |
| St Clair EW, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A, Keystone EC | Arthritis & Rheumatism | The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicentre, randomised, double-blind, placebo-controlled trial | Population |
| Stamp LK, Barclay M | Rheumatology | Therapeutic drug monitoring in rheumatic diseases: utile or futile? | Design |
| Strand V, Balsa A, Al-Saleh J, Barile-Fabris L, Horiuchi T, Takeuchi T, Lula S, Hawes C, Kola B, Marshall L | Biodrugs | Immunogenicity of biologics in chronic inflammatory diseases: a systematic review | Design |
| Stubenrauch K, Wessels U, Birnboeck H, Ramirez F, Jahreis A, Schleypen J | Clinical Therapeutics | Subset analysis of patients experiencing clinical events of a potentially immunogenic nature in the pivotal clinical trials of tocilizumab for rheumatoid arthritis: evaluation of an antidrug antibody ELISA using clinical adverse event-driven immunogenicity testing | Intervention |
| Svenson M, Geborek P, Saxne T, Bendtzen K | Rheumatology | Monitoring patients treated with anti-TNF-alpha biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies | Design |
| Takeuchi T, Miyasaka N, Inoue K, Abe T, Koike T | Modern Rheumatology | Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study | Population |
| Takeuchi T, Miyasaka N, Tatsuki Y, Yano T, Yoshinari T, Abe T, Koike T | Annals of the Rheumatic Diseases | Baseline tumour necrosis factor alpha levels predict the necessity for dose escalation of infliximab therapy in patients with rheumatoid arthritis | Design |
| Takeuchi T, Miyasaka N, Tatsuki Y, Yano T, Yoshinari T, Abe T, Koike T | Annals of the Rheumatic Diseases | Inhibition of plasma IL-6 in addition to maintenance of an efficacious trough level of infliximab associated with clinical remission in patients with rheumatoid arthritis: analysis of the RISING Study | Population |
| Takeuchi T, Miyasaka N, Inui T, Yano T, Yoshinari T, Abe T, Koike T | Annals of the Rheumatic Diseases | Both high titre of RF/ACPA at baseline is closely linked with high level of baseline plasma TNF level which resulted in low drug level and low clinical response in infliximab treatment in RA patients: post-hoc analysis of a double-blind clinical study (RISING study) | Design |
| Takeuchi T, Miyasaka N, Inui T, Yano T, Yoshinari T, Abe T, Koike T | Arthritis Research and Therapy | High titres of both rheumatoid factor and anti-CCP antibodies at baseline in patients with rheumatoid arthritis are associated with increased circulating baseline TNF level, low drug levels, and reduced clinical responses: a post hoc analysis of the RISING study | Population |
| Takeuchi T, Tatsuki Y, Yano T, Yoshinari T, Miyasaka N, Abe T, Koike T | Arthritis and Rheumatism | Clinical efficacy of infliximab is maximised when both circulating TNF and IL-6 are suppressed in the treatment of rheumatoid arthritis results from the RISING study | Population |
| Terenzi R, Guiducci S, Nacci F, Romano E, Manetti M, Peruzzi F, Bruni C, Bartoli F, Matucci-Cerinic M | Annals of the Rheumatic Diseases | Soluble FAS/FASL levels in rheumatoid arthritis patients treated with infliximab and adalimumab | Population |
| Jurado T, Plasencia-Rodriguez C, Martinez-Feito A, Navarro-Compan V, Rispens T, Vries A, Bloem K, Olariaga EM, Diego C, Villalba A, Peiteado D, Nuno L, Bonilla MG, Balsa A, Pascual-Salcedo D | The Open Rheumatology Journal | Predictive value of serum infliximab levels at induction phase in rheumatoid arthritis patients | Population |
| Ternant D, Ducourau E, Fuzibet P, Vignault C, Watier H, Lequerre T, Le Loet X, Vittecoq O, Goupille P, Mulleman D, Paintaud G | British Journal of Clinical Pharmacology | Pharmacokinetics and concentration–effect relationship of adalimumab in rheumatoid arthritis | Design |
| Ternant D, Fuzibet P, Ducourau E, Vittecoq O, Lequerre T, Goupille P, Mulleman D, Paintaud G | Fundamental and Clinical Pharmacology | Adalimumab pharmacokinetics and concentration–effect relationship in rheumatoid arthritis | Design |
| Thomas SS, Borazan N, Barroso N, Duan L, Taroumian S, Kretzmann B, Bardales R, Elashoff D, Vangala S, Furst DE | BioDrugs | Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis | Design |
| Tian X, Su Y, He D, Zhang Z, Zhang F | International Journal of Rheumatic Diseases | A prospective open-label study comparing immunogenicity and clinical efficacy of etanercept and infliximab in Chinese patients with RA or AS | Population |
| Tornero C, Plasencia C, Pascual D, Jurado T, Monjo I, Paredes MB, Moral E, Pieren A, Nuno L, Bonilla G, Peitedo D, Mola EM, Balsa A | Annals of the Rheumatic Diseases | Tapering strategy in patients with rheumatoid arthritis receiving tocilizumab | Intervention |
| Tornero Marin C, Plasencia C, Pascual Salcedo D, Jurado T, Paredes MB, Monjo I, Moral E, Pieren A, Bonilla Hernan G, Peiteado D, Bogas P, Nuno L, Villalba Yllan A, Martin Mola E, Balsa Criado A | Annals of the Rheumatic Diseases | Tocilizumab serum trough levels correlate with clinical activity in rheumatoid arthritis | Intervention |
| Tweehuysen L, van den Bemt BJF, van Ingen IL, de Jong AJL, van der Laan WH, van den Hoogen FHJ, den Broeder AA | Arthritis and Rheumatology | Clinical and immunogenicity outcomes after switching treatment from innovator infliximab to biosimilar infliximab in rheumatic diseases in daily clinical practice | Population |
| Tweehuysen L, van den Ende C, Beeren F, Been E, van den Hoogen F, den Broeder A | Annals of the Rheumatic Diseases | Prediction of successful dose reduction or discontinuation of biologics in patients with rheumatoid arthritis: a systematic review | Design |
| Tweehuysen L, van den Ende C, Beeren F, Been E, van den Hoogen F, den Broeder A | Arthritis and Rheumatology | No strong evidence supporting predictors for successful dose reduction or discontinuation of a biologic in rheumatoid arthritis: a systematic review | Design |
| Tweehuysen L, van den Ende C, Beeren F, Been E, van den Hoogen F, den Broeder A | Arthritis & Rheumatology | Little evidence for usefulness of biomarkers for predicting successful dose reduction or discontinuation of a biologic agent in rheumatoid arthritis: a systematic review | Design |
| Valor L, Hernandez-Florez D, de la Torre I, del Rio T, Nieto JC, Gonzalez C, Lopez-Longo FJ, Monteagudo I, Llinares F, Rosas J, Garrido J, Naredo E, Carreno L | Clinical and Experimental Rheumatology | Investigating the link between disease activity and infliximab serum levels in rheumatoid arthritis patients | Design |
| Valor L, Hernandez-Florez D, de la Torre I, Llinares F, Rosas J, Yague J, Garrido J, Naredo E | Clinical and Experimental Rheumatology | Agreement in assessment of infliximab and adalimumab levels in rheumatoid arthritis: interlaboratory and interassay comparison | Design |
| Valor L, Hernandez Florez D, de la Torre I, Llinares F, Rosas J, Yaque J, Naredo E, Gonzalez C, Lopez-Longo J, Monteagudo I, Montoro M, Carreno Perez L | Annals of the Rheumatic Diseases | Infliximab and adalimumab levels and antidrug antibodies detection in patients with rheumatoid arthritis (RA): an interlaboratory comparison using a commercial ELISA assay | Design |
| van Bezooijen JS, Koch BC, van Doorn MB, Prens EP, an Gelder T, Schreurs MW | Therapeutic Drug Monitoring | Comparison of three assays to quantify infliximab, adalimumab, and etanercept serum concentrations | Design |
| van den Bemt B, den Broeder AA, Wolbink GJ, Hekster YA, van Riel Pl, Benraad B, van den Hoogen FHJ | Pharmacy World & Science | Predictive value of infliximab serum trough levels for response in patients with rheumatoid arthritis | Population |
| van den Bemt BJ, den Broeder AA, Snijders GF, Hekster YA, van Riel PL, Benraad B, Wolbink GJ, van den Hoogen FH | Annals of the Rheumatic Diseases | Sustained effect after lowering high-dose infliximab in patients with rheumatoid arthritis: a prospective dose titration study | Population |
| van den Bemt BJ, den Broeder AA, Wolbink G, Hekster YA, van Riel PL, Benraad B, dan den Hoogen FH | BMC Musculoskeletal Disorders | Anti-infliximab antibodies are already detectable in most patients with rheumatoid arthritis halfway through an infusioncycle: an open-label pharmacokinetic cohort study | Population |
| van den Bemt BJ, den Broeder AA, Wolbink G, van den Maas A, Hekster YA, van Riel Pl, Benraad HB, van den Hoogen, FHJ | British Journal of Clinical Pharmacology | The combined use of disease activity and infliximab serum trough concentrations for early prediction of (non-) response to infliximab in rheumatoid arthritis | Population |
| van den Bemt BJF, den Broeder AA | Pharmaceutisch Weekblad | Therapeutic drug monitoring of tumour necrosis factor inhibitors in rheumatoid arthritis. [Dutch] | Design |
| van der Laken CJ, Voskuyl AE, Roos JC, Stigter van Walsum M, de Groot ER, Wolbink G, Dijkmans BA, Aarden LA | Annals of the Rheumatic Diseases | Imaging and serum analysis of immune complex formation of radiolabelled infliximab and anti-infliximab in responders and non-responders to therapy for rheumatoid arthritis. [Reprint in Ned Tijdschr Geneeskd 2008;152:1672–7] | Design |
| van der Linden MPM, Batstra MR, Bakker-Jonges LE, Detert J, Bastian H, Scherer HU, Toes REM, Burmester GR, Mjaavatten MD, Kvien TK, Huizinga TWJ, van der Helm-van Mil AHM | Arthritis and Rheumatism | Towards a data-driven evaluation of the 2010 American College of Rheumatology/European League Against Rheumatism criteria for rheumatoid arthritis: is it sensible to look at levels of rheumatoid factor? | Population |
| van der Maas A, Den Broeder AA, Wolbink GJ, van den Hoogen FHJ, Van Riel PLCM, van den Bemt BJF | Arthritis and Rheumatism | Prevalence and persistence of low infliximab serum trough levels in RA patients with low disease activity in daily clinical practice | Design |
| van der Maas A, van den Bemt BJ, Wolbink G, van den Hoogen FH, van Riel PL, den Broeder AA | BMC Musculoskeletal Disorders | Low infliximab serum trough levels and anti-infliximab antibodies are prevalent in rheumatoid arthritis patients treated with infliximab in daily clinical practice: results of an observational cohort study | Population |
| van der Maas A, van den Bemt B, van den Hoogen F, van Riel P, ven Broeder A | Annals of the Rheumatic Diseases | Can baseline (anti-)infliximab serum trough levels predict successful down-titration or discontinuation of infliximab in rheumatoid arthritis patients with long term low disease activity? | Design |
| van Der Maas A, van den Bemt B, van der Hoogen F, Van Riel P, Den Broeder A | International Journal of Clinical Pharmacy | Baseline (anti-)infliximab serum trough levels do not predict successful down-titration or cessation of infliximab in rheumatoid arthritis patients with long term low disease activity | Design |
| van Hensbergen Y, Te Velthuis H | Annals of the Rheumatic Diseases | Ready to use CE-IVD smart ELISA kits from Sanquin for infliximab and adalimumab levels correlate with the golden standard and can be used for optimisation of personalised treatment in RA patients | Design |
| van Herwaarden N, Bouman CA, van der Maas A, van Vollenhoven RF, Bijlsma JW, van den Hoogen FH, den Broeder AA, van den Bemt BJ | Annals of the Rheumatic Diseases | Adalimumab and etanercept serum (anti)drug levels are not predictive for successful dose reduction or discontinuation in rheumatoid arthritis | Outcome |
| van Herwaarden N, van ven Bemt BJF, Wientjes MHM, Kramers C, ven Broeder AA | Expert Opinion On Drug Metabolism & Toxicology | Clinical utility of therapeutic drug monitoring in biological disease modifying anti-rheumatic drug treatment of rheumatic disorders: a systematic narrative review | Design |
| van Schouwenburg PA, Bartelds GM, Hart MH, Aarden L, Wolbink GJ, Wouters D | Journal of Immunological Methods | A novel method for the detection of antibodies to adalimumab in the presence of drug reveals ‘hidden’ immunogenicity in rheumatoid arthritis patients | Design |
| van Schouwenburg PA, Krieckaert CL, Rispens T, Aarden L, Wolbink GJ, Wouters D | Annals of the Rheumatic Diseases | Long-term measurement of anti-adalimumab using pH-shift-anti-idiotype antigen binding test shows predictive value and transient antibody formation | Design |
| van Schouwenburg PA, Rispens T, Wolbink GJ | Nature Reviews Rheumatology | Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis | Design |
| Van Stappen T, Lu J, Geukens N, Spasic D, Delport F, Zali N, Kolmel Y, Rameil S, Lammertyn J, Vande Casteele N, Gils A | United European Gastroenterology Journal | Point-of-care assays for rapid quantification of infliximab | Design |
| Van Stappen T, Vande Casteele N, Van Assche G, Ferrante M, Vermeire S, Gils A | Gut | Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial | Population |
| Verdet M, Guillou C, Potier ML, Hiron M, Jouen F, Boyer O, Lequerre T, Vittecoq O | Arthritis and Rheumatism | Immunogenicity of infliximab is related to reduction of frequency of infliximab administration in rheumatoid arthritis and spondyloarthritis patients | Population |
| Verdet M, Guillou C, Golinski ML, Hiron M, Jouen F, Boyer O, Lequerre T, Vittecoq O | Annals of the Rheumatic Diseases | Prolonging between-infusions interval is associated with positivity to anti-infliximab antibodies in rheumatoid arthritis and spondyloarthritis patients | Population |
| Villalba A, Plasencia C, Peiteado D, Nuno L, Bonilla G, Lojo L, Pascual D, del Moral R, Lopez Casla MT, Balsa A, Martin Mola E | Annals of the Rheumatic Diseases | Influence of immunogenicity of anti-TNF therapy in RA patients with a long-term treatment with infliximab or adalimumab | Population |
| Villalba Yllan A, Navarro Compan MV, Plasencia Rodriguez C, Peiteado Lopez D, Bonilla Hernan G, Nuno Nuno L, Pascual-Salcedo D, Olariaga E, Balsa Criado A, Martin Mola E | Annals of the Rheumatic Diseases | Influence of body mass index (BMI) on serum levels of infliximab in patients with rheumatoid arthritis (RA) | Design |
| Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C | Annals of the Rheumatic Diseases | Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective | Design |
| Vincent FB, Pavy S, Krzysiek R, Lequerre T, Sellam J, Taoufik Y, Mariette X, Miceli-Richard C | Joint Bone Spine | Effect of serum anti-tumour necrosis factor (TNF) drug trough concentrations and antidrug antibodies (ADAb) to further anti-TNF short-term effectiveness after switching in rheumatoid arthritis and axial spondyloarthritis | Design |
| Vogelzang E, Hebing R, Nurmohamed M, L’Ami M, Krieckaert C, Wolbink G | Annals of the Rheumatic Diseases | Assessing adherence of RA patients treated with etanercept using etanercept serum trough concentrations and patient self-report | Design |
| Vogelzang EH, Pouw MF, Nurmohamed M, Kneepkens EL, Rispens T, Wolbink GJ, Krieckaert CLM | Annals of the Rheumatic Diseases | Adalimumab trough concentrations in patients with rheumatoid arthritis and psoriatic arthritis treated with concomitant disease-modifying antirheumatic drugs | Population |
| Vogelzang E, Kneepkens E, Nurmohamed M, van Kuijk A, Rispens T, Wolbink G, Krieckaert C | Annals of the Rheumatic Diseases | A diminished clinical response at 28 and 52 weeks of adalimumab treatment in patients with psoriatic arthritis is associated with antidrug antibodies | Population |
| Westerlund J, Jokiranta TS | Scandinavian Journal of Rheumatology | Monitoring of TNF-alpha blockers infliximab and adalimumab by measuring trough level concentrations and antidrug antibodies | Design |
| Wolbink G, Goupille P, Sandborn W, Marotte H, Mulleman D, Ternant D, Paul S, de Longueville M, Vande Casteele N, Zamacona M, O’Brien C, Kvien TK, Kavanaugh AF | Arthritis and Rheumatology | Association between plasma certolizumab pegol concentration and improvement in disease activity in rheumatoid arthritis and Crohn’s disease | Population |
| Wolbink GJ, Aarden LA, Dijkmans BAC | Current Opinion in Rheumatology | Dealing with immunogenicity of biologicals: assessment and clinical relevance | Design |
| Wolbink GJ, Voskuyl AE, Lems WF, de Groot E, Nurmohamed MT, Tak PP, Dijkmans BA, Aarden L | Annals of the Rheumatic Diseases | Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis | Population |
| Wolbink GJ, Vis M, Lems W, Voskuyl AE, de Groot E, Nurmohamed MT, Stapel S, Tak PP, Aarden L,Dijkmans B | Arthritis & Rheumatism | Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis | Population |
| Wong P, Bowling A, Ford E, Freeman D, Bagga H, Langguth D | Internal Medicine Journal | Serum trough levels of adalimumab and infliximab inversely correlate with disease activity in patients with inflammatory arthritis | Population |
| Wu C, Wang S, Xian P, Yang L, Chen Y, Mo X | BioMed Research International | Effect of anti-TNF antibodies on clinical response in rheumatoid arthritis patients: a meta-analysis | Design |
| Zanker M, Becher G, Arbach O, Maurer M, Stuhlmuller B, Schafer A, Strohner P, Brand, J | Clinical and Experimental Rheumatology | Improved adalimumab dose decision with comprehensive diagnostics data | Population |
| Zufferey P, Jeanfavre MFD, Dumusc A, Benaim C, Perreau M, So AK | Arthritis and Rheumatology | Is it possible to predict which patients treated with biologic agents for rheumatic diseases will develop antidrug antibodies? | Design |
Appendix 3 Norwegian Drug Monitoring study (NOR-DRUM)
| Characteristic | Description |
|---|---|
| Study title | A NORwegian multicentre randomised controlled trial assessing the effectiveness of tailoring infliximab treatment by therapeutic DRUg Monitoring – the NOR-DRUM study |
| Study objectives |
|
| Immunological inflammatory diseases enrolled |
|
| Intervention arm | TDMa |
| Comparator arm | Standard careb |
| N (expected) | 600 participants |
| Start date | 1 March 2017 |
| Estimated primary completion date | 1 March 2020 |
| Estimated study completion date | 1 March 2022 |
| Outcomes | Primary:
|
Secondary:
|
|
| Eligibility criteria | NOR-DRUM A:
|
Appendix 4 Quality assessment
Quality assessment on the basis of specific outcomes
Table 48 presents the risk-of-bias assessment on the basis of specific outcomes: clinical disease activity (disease flare, remission and change in disease activity), proportion of patients receiving dose tapering, HRQoL and treatment dose-related outcomes. For each specific outcome, the following bias domains were assessed: bias due to confounding, bias in selection of participants into the study, bias in measurement of interventions, bias due to departures from intended interventions, bias due to missing data, bias in taking measurements and bias in selection of the reported result.
In terms of outcome-specific assessments, both the clinical activity outcome (disease flare, remission and change in disease activity) and the HRQoL outcome were judged to be at serious risk of bias given that there was serious risk of bias in the domain of bias due to confounding. For both outcomes, there was low to moderate risk of bias for the remaining bias domains: bias in selection of participants into the study, bias in measurement of interventions, bias in taking measurements and bias in selection of the reported results.
Regarding dose-related outcomes and the proportion of patients receiving dose tapering, both outcomes were judged to be at moderate risk of bias because there was moderate risk of bias for two bias domains (bias in taking measurements and bias due to confounding). For both outcomes, there was low risk of bias for the remaining bias domains: bias in selection of participants into the study, bias in measurement of interventions and bias in selection of the reported results.
| Domain | Outcome | |||
|---|---|---|---|---|
| Clinical activity (disease flare, remission and change in disease activity) | Proportion of patients receiving tapered dose | HRQoL | Dose-related outcomes | |
| Bias due to confounding | Moderate to serious | Moderate | Serious | Moderate |
| Bias in selection of participants into the study | Low to moderate | Low | Low | Moderate |
| Bias in measurement of interventions | Low to moderate | Low | Low | Moderate |
| Bias due to departures from the intended interventions | NI | NI | NI | NI |
| Bias due to missing data | Serious | NI | Serious | NI |
| Bias in taking measurements | Moderate | Moderate | Moderate | Moderate |
| Bias in selection of the reported result | Low | Low | Low | Low |
| Overall risk of bias | Moderate to serious | Moderate | Serious | Moderate |
Quality assessment of individual studies41
Based on Sterne et al. 41 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/.
The Risk Of Bias In Non-randomised Studies – of Interventions (ROBINS-I) assessment tool (version for cohort-type studies)
Arango et al. 201743
Version 19 September 2016.
ROBINS-I tool (stage I): at protocol stage
Specify the review question.
| Participants | Remission/primary non-responders/secondary non-responders |
| Experimental intervention | Therapeutic drug monitoring |
| Comparator | Standard care |
| Outcomes | 13 outcomes: inconclusive results, time to results, dose changes, dose adjustment, treatment switch, discontinuation, changes in disease activity, rate of disease response, relapse and remission, hospitalisation, rates of surgical intervention, adverse effects, health-related quality of life |
List the confounding domains relevant to all or most studies.
| From protocol: time of testing, testing method (e.g. reflex vs. concurrent) |
| Others (suggested): drug dose/levels, disease stage at enrolment, time of assessment for response/follow-up, type of drug manipulation (e.g. optimisation or tapering) |
List co-interventions that could be different between intervention groups and that could affect outcomes.
| Methotrexate, other DMARDs, combination or monotherapy |
ROBINS-I tool (stage II): for each study
Specify a target randomised trial specific to the study.
| Design | Individually randomised✓/Cluster randomised/Matched (e.g. cross-over) |
| Participants | Adult patient treated with ADL (40 mg subcutaneously) who remained clinically stable for at least 6 months |
| Experimental intervention | Adjustment of ADL frequency (tapering) plus therapeutic drug monitoring (TDM) data revealed to physicians |
| Comparator | Adjustment of ADL frequency (tapering), physicians blinded to TDM data |
Is your aim for this study . . .?
| ≤ | to assess the effect of assignment to intervention |
| ✓ | to assess the effect of starting and adhering to intervention |
Specify the outcome
Specify which outcome is being assessed for risk of bias (typically from among those earmarked for the summary of findings table). Specify whether this is a proposed benefit or harm of intervention.
| Proportion of patients tapered (benefit), rate of flare (harm) |
Specify the numerical result being assessed
In the case of multiple alternative analyses being presented, specify the numeric result (e.g. RR 1.52, 95% CI 0.83 to 2.77) and/or a reference (e.g. to a table, figure or paragraph) that uniquely defines the result being assessed.
| Proportion tapered: 34.6% (control group), 35.7% (intervention group) |
Preliminary consideration of confounders
Complete a row for each important confounding domain (i) listed in the review protocol and (ii) relevant to the setting of this particular study, or which the study authors identified as potentially important.
‘Important’ confounding domains are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention. ‘Validity’ refers to whether the confounding variable or variables fully measure the domain, while ‘reliability’ refers to the precision of the measurement (more measurement error means less reliability).
| (i) Confounding domains listed in the review protocol | ||||
|---|---|---|---|---|
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?a | Is the confounding domain measured validly and reliably by this variable (or these variables)? | Optional: is failure to adjust for this variable (alone) expected to favour the experimental intervention or the comparator? |
| Yes/no/no information | Favour experimental/favour comparator/no information | |||
| Disease stage (proportion in remission/LDA) | No | Yes |
Expected to favour control group (28.6% IG had LDA vs. 17.3% of CG) |
|
| Time of assessment for response | No | No information | No information, but likely to be unimportant. Measurement believed to be carried out at similar time points (at eight scheduled visits over 18 months) | |
| Serum adalimumab levels | No | Yes | NA – serum ADL levels 5.76 mg/l in the CG and 5.04 mg/l in IG | |
| Serum anti-adalimumab antibody levels | No | No information | No information | |
| (ii) Additional confounding domains relevant to the setting of this particular study, or which the study authors identified as important | ||||
|---|---|---|---|---|
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?a | Is the confounding domain measured validly and reliably by this variable (or these variables)? | Optional: is failure to adjust for this variable (alone) expected to favour the experimental intervention or the comparator? |
| Yes/no/no information | Favour experimental/favour comparator/no information | |||
Preliminary consideration of co-interventions
Complete a row for each important co-intervention (i) listed in the review protocol and (ii) relevant to the setting of this particular study, or which the study authors identified as important.
‘Important’ co-interventions are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention.
| (i) Co-interventions listed in the review protocol | ||
|---|---|---|
| Co-intervention | Is there evidence that controlling for this co-intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co-intervention likely to favour outcomes in the experimental intervention or the comparator |
| Favour experimental/favour comparator/no information | ||
| Favour experimental/favour comparator/no information | ||
| Favour experimental/favour comparator/no information | ||
| Favour experimental/favour comparator/no information | ||
| (ii) Additional co-interventions relevant to the setting of this particular study, or which the study authors identified as important | ||
|---|---|---|
| Co-intervention | Is there evidence that controlling for this co-intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co-intervention likely to favour outcomes in the experimental intervention or the comparator |
| Methotrexate and other DMARDs | No | Favour experimental/favour comparator/no information ✓ |
Risk-of-bias assessment
Responses underlined are potential markers for low risk of bias. Where questions relate only to sign posts to other questions, no formatting is used.
| Signalling questions | Description | Response options |
|---|---|---|
| Bias due to confounding | ||
|
0.7 Is there potential for confounding of the effect of intervention in this study? If N/PN to 1.1: the study can be considered to be at low risk of bias due to confounding and no further signalling questions need be considered |
Yes, differential baseline LDA rates and no information on co-intervention | Y/PY ✓/PN/N |
| If Y/PY to 1.1: determine whether there is a need to assess time-varying confounding: | ||
|
1.2. Was the analysis based on splitting participants’ follow up time according to intervention received? If N/PN, answer questions relating to baseline confounding (1.4 to 1.6) If Y/PY, go to question 1.3 |
NA/Y/PY/PN/N ✓/NI | |
|
1.3. Were intervention discontinuations or switches likely to be related to factors that are prognostic for the outcome? If N/PN, answer questions relating to baseline confounding (1.4 to 1.6) If Y/PY, answer questions relating to both baseline and time-varying confounding (1.7 and 1.8) |
NA/Y/PY/PN/N/NI | |
| Questions relating to baseline confounding only | ||
| 1.4. Did the authors use an appropriate analysis method that controlled for all the important confounding domains? | NA/Y/PY/PN/N ✓/NI | |
| 1.5. If Y/PY to 1.4: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | NA ✓/Y/PY/PN/N/NI | |
| 1.6. Did the authors control for any post-intervention variables that could have been affected by the intervention? | NA ✓/Y/PY/PN/N/NI | |
| Questions relating to baseline and time-varying confounding | ||
| 1.7. Did the authors use an appropriate analysis method that controlled for all the important confounding domains and for time-varying confounding? | NA/Y/PY/PN/N ✓/NI | |
| 1.8. If Y/PY to 1.7: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | NA ✓/Y/PY/PN/N/NI | |
| Risk of bias judgement | Low/Moderate ✓/Serious/Critical/NI | |
| Optional: What is the predicted direction of bias due to confounding? | Favours experimental/favours comparator/unpredictable | |
| Bias in selection of participants into the study | |
|---|---|
|
2.1. Was selection of participants into the study (or into the analysis) based on participant characteristics observed after the start of intervention? If N/PN to 2.1: go to 2.4 |
Y/PY/PN/N✓/NI |
|
2.2. If Y/PY to 2.1: Were the post-intervention variables that influenced selection likely to be associated with intervention? 2.3 If Y/PY to 2.2: Were the post-intervention variables that influenced selection likely to be influenced by the outcome or a cause of the outcome? |
NA/Y/PY/PN/N/NI NA/Y/PY/PN/N/NI |
| 2.4. Do start of follow-up and start of intervention coincide for most participants? | Y/PY✓/PN/N/NI |
| 2.5. If Y/PY to 2.2 and 2.3, or N/PN to 2.4: Were adjustment techniques used that are likely to correct for the presence of selection biases? | NA ✓/Y/PY/PN/N/NI |
| Risk of bias judgement | Low ✓/moderate/serious/critical/NI |
| Optional: What is the predicted direction of bias due to selection of participants into the study? | Favours experimental/favours comparator/towards null/away from null/unpredictable |
| Bias in classification of interventions | |
| 3.1 Were intervention groups clearly defined? | Y ✓/PY/PN/N/NI |
| 3.2 Was the information used to define intervention groups recorded at the start of the intervention? | Y✓/PY/PN/N/NI |
| 3.3 Could classification of intervention status have been affected by knowledge of the outcome or risk of the outcome? | Y/PY/PN/N✓/NI |
| Risk of bias judgement | Low ✓/moderate/serious/critical/NI |
| Optional: What is the predicted direction of bias due to classification of interventions? | Favours experimental/favours comparator/towards null/away from null/unpredictable |
| Bias due to deviations from intended interventions | |
| If your aim for this study is to assess the effect of assignment to intervention, answer questions 4.1 and 4.2 | |
| 4.1. Were there deviations from the intended intervention beyond what would be expected in usual practice? | Y/PY/PN/N/NI |
| 4.2. If Y/PY to 4.1: Were these deviations from intended intervention unbalanced between groups and likely to have affected the outcome? | NA ✓/Y/PY/PN/N/NI |
| If your aim for this study is to assess the effect of starting and adhering to intervention, answer questions 4.3 to 4.6 | |
| 4.3. Were important co-interventions balanced across intervention groups? | Y/PY/PN/N/NI ✓ |
| 4.4. Was the intervention implemented successfully for most participants? | Y ✓/PY/PN/N/NI |
| 4.5. Did study participants adhere to the assigned intervention regimen? | Y/PY ✓/PN/N/NI |
| 4.6. If N/PN to 4.3, 4.4 or 4.5: Was an appropriate analysis used to estimate the effect of starting and adhering to the intervention? | NA ✓/Y/PY/PN/N/NI |
| Risk of bias judgement | |
| Optional: What is the predicted direction of bias due to deviations from the intended interventions? | |
| Bias due to missing data | |
| 5.1 Were outcome data available for all, or nearly all, participants? | Y/PY/PN/N/NI ✓ |
| 5.2 Were participants excluded due to missing data on intervention status? | Y/PY/PN/N/NI ✓ |
| 5.3 Were participants excluded due to missing data on other variables needed for the analysis? | Y/PY/PN/N/NI ✓ |
| 5.4 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Are the proportion of participants and reasons for missing data similar across interventions? | NA ✓/Y/PY/PN/N/NI |
| 5.5 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Is there evidence that results were robust to the presence of missing data? | NA ✓/Y/PY/PN/N/NI |
| Risk of bias judgement | Low/moderate/serious/critical/NI ✓ |
| Optional: What is the predicted direction of bias due to missing data? | Favours experimental/favours comparator/towards null/away from null/unpredictable |
| Bias in measurement of outcomes | |
| 6.1 Could the outcome measure have been influenced by knowledge of the intervention received? | Y/PY/PN ✓/N/NI |
| 6.2 Were outcome assessors aware of the intervention received by study participants? | Y ✓/PY/PN/N/NI |
| 6.3 Were the methods of outcome assessment comparable across intervention groups? | Y/PY/PN/N/NI ✓ |
| 6.4 Were any systematic errors in measurement of the outcome related to intervention received? | Y/PY/PN/N/NI ✓ |
| Risk of bias judgement | Low/moderate ✓/serious/critical/NI |
| Optional: What is the predicted direction of bias due to measurement of outcomes? | Favours experimental/favours comparator/towards null/away from null/unpredictable |
| Bias in selection of the reported result | |
| Is the reported effect estimate likely to be selected, on the basis of the results, from . . . | |
| 7.1 . . . multiple outcome measurements within the outcome domain? | Y/PY/PN/N✓/NI |
| 7.2 . . . multiple analyses of the intervention–outcome relationship? | Y/PY/PN/N✓/NI |
| 7.3 . . . different subgroups? | Y/PY/PN/N✓/NI |
| Risk of bias judgement | Low ✓/moderate/serious/critical/NI |
| Optional: What is the predicted direction of bias due to selection of the reported result? | Favours experimental/favours comparator/towards null/away from null/unpredictable |
| Overall bias | |
| Risk of bias judgement | Low/moderate ✓/serious/critical/NI |
| Optional: What is the overall predicted direction of bias for this outcome? | Favours experimental/favours comparator/towards null/away from null/unpredictable |
The Risk Of Bias In Non-randomised Studies – of Interventions (ROBINS-I) assessment tool41
Gorostiza et al. 201645
Version 19 September 2016.
ROBINS-I tool (stage I): at protocol stage
Specify the review question.
| Participants | Remission/primary non-responders/secondary non-responders |
| Experimental intervention | Therapeutic drug monitoring |
| Comparator | Standard care |
| Outcomes | 13 outcomes; inconclusive results, time to results, dose changes, dose adjustment, treatment switch, discontinuation, changes in disease activity, rate of disease response, relapse and remission, hospitalisation, rates of surgical intervention, adverse effects, health-related quality of life |
List the confounding domains relevant to all or most studies.
| From protocol; time of testing, testing method (e.g. reflex vs. concurrent) |
| Others (suggested); drug dose/levels, disease stage at enrolment, time of assessment for response/follow-up, type of drug manipulation (e.g. optimisation or tapering) |
List co-interventions that could be different between intervention groups and that could impact on outcomes.
| Methotrexate, other DMARDs, combination or monotherapy |
ROBINS-I tool (stage II): for each study
Specify a target randomised trial specific to the study.
| Design | Individually randomised ✓/Cluster randomised/Matched (e.g. cross-over) |
| Participants | Patients treated with adalimumab (40 mg subcutaneously) who remained clinically stable for at least 6 months |
| Experimental intervention | Biological monitoring data were released to physicians |
| Comparator | Physicians were blinded to biological monitoring data |
Is your aim for this study . . . ?
| ≤ | to assess the effect of assignment to intervention |
| ✓ | to assess the effect of starting and adhering to intervention |
Specify the outcome
Specify which outcome is being assessed for risk of bias (typically from among those earmarked for the Summary of Findings table). Specify whether this is a proposed benefit or harm of intervention.
| Proportion remaining in remission (benefit) |
Specify the numerical result being assessed
In case of multiple alternative analyses being presented, specify the numeric result (e.g. RR 1.52, 95% CI 0.83 to 2.77) and/or a reference (e.g. to a table, figure or paragraph) that uniquely defines the result being assessed.
| Promotion remaining in remission = 69.6% (control group), 76.1% (intervention group) |
Preliminary consideration of confounders.
Complete a row for each important confounding domain (i) listed in the review protocol and (ii) relevant to the setting of this particular study, or which the study authors identified as potentially important.
‘Important’ confounding domains are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention. ‘Validity’ refers to whether the confounding variable or variables fully measure the domain, while ‘reliability’ refers to the precision of the measurement (more measurement error means less reliability).
| (i) Confounding domains listed in the review protocol | ||||
|---|---|---|---|---|
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?a | Is the confounding domain measured validly and reliably by this variable (or these variables)? | Optional: is failure to adjust for this variable (alone) expected to favour the experimental intervention or the comparator? |
| Yes/No/No information | Favour experimental/Favour comparator/No information | |||
| Disease stage (proportion in remission/LDA) | No | Yes | Expected to favour control group (26.6% IG had LDA vs. 16.7% of CG) | |
| Time of assessment for response | No | No information | No information but likely to be unimportant. Measurement believed to be carried out at similar time points (at eight scheduled visits over 18 months) | |
| Serum adalimumab levels | No | Yes | NA – serum ADL levels 5.5mg/L in the CG and 5.3mg/L in IG | |
| Serum anti-adalimumab antibody levels | No | No information | No information | |
| (ii) Additional confounding domains relevant to the setting of this particular study, or which the study authors identified as important | ||||
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?a | Is the confounding domain measured validly and reliably by this variable (or these variables)? | Optional: is failure to adjust for this variable (alone) expected to favour the experimental intervention or the comparator? |
| Yes/No/No information | Favour experimental/Favour comparator/No information | |||
Preliminary consideration of co-interventions
Complete a row for each important co-intervention (i) listed in the review protocol; and (ii) relevant to the setting of this particular study, or which the study authors identified as important.
‘Important’ co-interventions are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention.
| (i) Co-interventions listed in the review protocol | ||
|---|---|---|
| Co-intervention | Is there evidence that controlling for this co-intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co-intervention likely to favour outcomes in the experimental intervention or the comparator |
| Favour experimental/favour comparator/no information | ||
| (ii) Additional co-interventions relevant to the setting of this particular study, or which the study authors identified as important | ||
| Co-intervention | Is there evidence that controlling for this co-intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co-intervention likely to favour outcomes in the experimental intervention or the comparator |
| Methotrexate and other DMARDs | No | Favour experimental/favour comparator/no information ✓ |
Risk-of-bias assessment
Responses underlined are potential markers for low risk of bias. Where questions relate only to sign posts to other questions, no formatting is used.
| Signalling questions | Description | Response options | |
|---|---|---|---|
| Bias due to confounding | |||
|
0.7 Is there potential for confounding of the effect of intervention in this study? If N/PN to 1.1: the study can be considered to be at low risk of bias due to confounding and no further signalling questions need be considered |
Yes, differential baseline LDA rates and no information on co-intervention | Y/PY ✓/PN/N | |
| If Y/PY to 1.1: determine whether there is a need to assess time-varying confounding: | |||
|
1.2. Was the analysis based on splitting participants’ follow up time according to intervention received? If N/PN, answer questions relating to baseline confounding (1.4 to 1.6) If Y/PY, go to question 1.3 |
NA/Y/PY/PN/N ✓/NI | ||
|
1.3. Were intervention discontinuations or switches likely to be related to factors that are prognostic for the outcome? If N/PN, answer questions relating to baseline confounding (1.4 to 1.6) If Y/PY, answer questions relating to both baseline and time-varying confounding (1.7 and 1.8) |
NA/Y/PY/PN/N/NI | ||
| Questions relating to baseline confounding only | |
|---|---|
| 1.4. Did the authors use an appropriate analysis method that controlled for all the important confounding domains? | NA/Y/PY/PN/N ✓/NI |
| 1.5. If Y/PY to 1.4: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | NA ✓/Y/PY/PN/N/NI |
| 1.6. Did the authors control for any post-intervention variables that could have been affected by the intervention? | NA ✓/Y/PY/PN/N/NI |
| Questions relating to baseline and time-varying confounding | |
| 1.7. Did the authors use an appropriate analysis method that controlled for all the important confounding domains and for time-varying confounding? | NA/Y/PY/PN/N ✓/NI |
| 1.8. If Y/PY to 1.7: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | NA ✓/Y/PY/PN/N/NI |
| Risk of bias judgement | Low/moderate ✓/serious/critical/NI |
| Optional: What is the predicted direction of bias due to confounding? | Favours experimental/favours comparator/unpredictable |
| Bias in selection of participants into the study | |
|
2.1. Was selection of participants into the study (or into the analysis) based on participant characteristics observed after the start of intervention? If N/PN to 2.1: go to 2.4 |
Y/PY/PN/N ✓/NI |
|
2.2. If Y/PY to 2.1: Were the post-intervention variables that influenced selection likely to be associated with intervention? 2.3 If Y/PY to 2.2: Were the post-intervention variables that influenced selection likely to be influenced by the outcome or a cause of the outcome? |
NA/Y/PY/PN/N/NI NA/Y/PY/PN/N/NI |
| 2.4. Do start of follow-up and start of intervention coincide for most participants? | Y/PY ✓/PN/N/NI |
| 2.5. If Y/PY to 2.2 and 2.3, or N/PN to 2.4: Were adjustment techniques used that are likely to correct for the presence of selection biases? | NA ✓/Y/PY/PN/N/NI |
| Risk of bias judgement | Low ✓/moderate/serious/critical/NI |
| Optional: What is the predicted direction of bias due to selection of participants into the study? | Favours experimental/favours comparator/towards null/away from null/unpredictable |
| Bias in classification of interventions | ||
|---|---|---|
| 3.1 Were intervention groups clearly defined? | TDM data were released only to intervention group | Y ✓/PY/PN/N/NI |
| 3.2 Was the information used to define intervention groups recorded at the start of the intervention? | Y ✓/PY/PN/N/NI | |
| 3.3 Could classification of intervention status have been affected by knowledge of the outcome or risk of the outcome? | Y/PY/PN/N ✓/NI | |
| Risk of bias judgement | Low ✓/moderate/serious/critical/NI | |
| Optional: What is the predicted direction of bias due to classification of interventions? | Favours experimental/favours comparator/towards null/away from null/unpredictable | |
| Bias due to deviations from intended interventions | ||
|---|---|---|
| If your aim for this study is to assess the effect of assignment to intervention, answer questions 4.1 and 4.2 | ||
| 4.1. Were there deviations from the intended intervention beyond what would be expected in usual practice? | Y/PY/PN/N/NI | |
| 4.2. If Y/PY to 4.1: Were these deviations from intended intervention unbalanced between groups and likely to have affected the outcome? | ✓ NA/Y/PY/PN/N/NI | |
| If your aim for this study is to assess the effect of starting and adhering to intervention, answer questions 4.3 to 4.6 | ||
| 4.3. Were important co-interventions balanced across intervention groups? | Y/PY/PN/N/NI ✓ | |
| 4.4. Was the intervention implemented successfully for most participants? | Y ✓/PY/PN/N/NI | |
| 4.5. Did study participants adhere to the assigned intervention regimen? | Y/PY ✓/PN/N/NI | |
| 4.6. If N/PN to 4.3, 4.4 or 4.5: Was an appropriate analysis used to estimate the effect of starting and adhering to the intervention? | NA ✓/Y/PY/PN/N/NI | |
| Risk of bias judgement | ||
| Optional: What is the predicted direction of bias due to deviations from the intended interventions? | ||
| Bias due to missing data | ||
| 5.1 Were outcome data available for all, or nearly all, participants? | Y/PY/PN/N/NI ✓ | |
| 5.2 Were participants excluded due to missing data on intervention status? | Y/PY/PN/N/NI ✓ | |
| 5.3 Were participants excluded due to missing data on other variables needed for the analysis? | Y/PY/PN/N/NI ✓ | |
| 5.4 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Are the proportion of participants and reasons for missing data similar across interventions? | NA ✓/Y/PY/PN/N/NI | |
| 5.5 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Is there evidence that results were robust to the presence of missing data? | NA ✓/Y/PY/PN/N/NI | |
| Risk of bias judgement | Low/Moderate/Serious/Critical/NI ✓ | |
| Optional: What is the predicted direction of bias due to missing data? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable | |
| Bias in measurement of outcomes | ||
| 6.1 Could the outcome measure have been influenced by knowledge of the intervention received? | Y/PY/PN ✓/N/NI | |
| 6.2 Were outcome assessors aware of the intervention received by study participants? | Y ✓/PY/PN/N/NI | |
| 6.3 Were the methods of outcome assessment comparable across intervention groups? | Y/PY/PN/N/NI ✓ | |
| 6.4 Were any systematic errors in measurement of the outcome related to intervention received? | Y/PY/PN/N/NI ✓ | |
| Risk of bias judgement | Low/Moderate ✓/Serious/Critical/NI | |
| Optional: What is the predicted direction of bias due to measurement of outcomes? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable | |
| Bias in selection of the reported result | ||
| Is the reported effect estimate likely to be selected, on the basis of the results, from . . . | ||
| 7.1 . . . multiple outcome measurements within the outcome domain? | Y/PY/PN/N ✓/NI | |
| 7.2 . . . multiple analyses of the intervention–outcome relationship? | Y/PY/PN/N ✓/NI | |
| 7.3 . . . different subgroups? | Y/PY/PN/N ✓/NI | |
| Risk of bias judgement | Low ✓/Moderate/Serious/Critical/NI | |
| Optional: What is the predicted direction of bias due to selection of the reported result? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable | |
| Overall bias | ||
| Risk of bias judgement | Low/Moderate ✓/Serious/Critical/NI | |
| Optional: What is the overall predicted direction of bias for this outcome? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable | |
The Risk Of Bias In Non-randomised Studies – of Interventions (ROBINS-I) assessment tool (version for cohort-type studies)41
Pascual-Salcedo et al. 201344
Version 19 September 2016.
ROBINS-I tool (stage I): at protocol stage
Specify the review question.
| Participants | Remission/primary non-responders/secondary non-responders |
| Experimental intervention | Therapeutic drug monitoring |
| Comparator | Standard care |
| Outcomes | 13 outcomes; inconclusive results, time to results, dose changes, dose adjustment, treatment switch, discontinuation, changes in disease activity, rate of disease response, relapse and remission, hospitalisation, rates of surgical intervention, adverse effects, health-related quality of life |
List the confounding domains relevant to all or most studies.
|
From protocol; time of testing, testing method (e.g. reflex vs. concurrent) Others (suggested); drug dose/levels, disease stage at enrolment, time of assessment for response/follow-up, type of drug manipulation (e.g. optimisation or tapering) |
List co-interventions that could be different between intervention groups and that could impact on outcomes.
| Methotrexate, other DMARDs, combination or monotherapy |
ROBINS-I tool (Stage II): For each study
Specify a target randomised trial specific to the study.
| Design | Individually randomised/Cluster randomised/Matched (e.g. cross-over) |
| Participants | RA patients in remission or LDA |
| Experimental intervention | Down-titration or cessation of infliximab, adalimumab, etanercept plus therapeutic monitoring period |
| Comparator | Down-titration or cessation of infliximab, adalimumab, etanercept, prior to therapeutic monitoring period |
Is your aim for this study . . . ?
| ≤ | to assess the effect of assignment to intervention |
| ✓ | to assess the effect of starting and adhering to intervention |
Specify the outcome
Specify which outcome is being assessed for risk of bias (typically from among those earmarked for the Summary of Findings table). Specify whether this is a proposed benefit or harm of intervention.
| Mean DAS28 (harmful), weekly mean dose (lower better), interval of administration (higher better) |
Specify the numerical result being assessed
In case of multiple alternative analyses being presented, specify the numeric result (e.g. RR 1.52, 95% CI 0.83 to 2.77) and/or a reference (e.g. to a table, figure or paragraph) that uniquely defines the result being assessed.
| Mean DAS28; first period: 2.51 ± 0.85 vs. second period: 2.31 ± 0.52 |
Preliminary consideration of confounders
Complete a row for each important confounding domain (i) listed in the review protocol and (ii) relevant to the setting of this particular study, or which the study authors identified as potentially important.
‘Important’ confounding domains are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention. ‘Validity’ refers to whether the confounding variable or variables fully measure the domain, while ‘reliability’ refers to the precision of the measurement (more measurement error means less reliability).
| (i) Confounding domains listed in the review protocol | ||||
|---|---|---|---|---|
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?a | Is the confounding domain measured validly and reliably by this variable (or these variables)? | Optional: is failure to adjust for this variable (alone) expected to favour the experimental intervention or the comparator? |
| Yes/no/no information | Favour experimental/Favour comparator/No information | |||
| Disease stage (proportion in remission/LDA) | No | No information | No information | |
| Time of assessment for response | No | No information | No information | |
| Serum adalimumab levels | No | No information | No information | |
| Serum anti-adalimumab antibody levels | No | No information | No information | |
| (ii) Additional confounding domains relevant to the setting of this particular study, or which the study authors identified as important | ||||
|---|---|---|---|---|
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?a | Is the confounding domain measured validly and reliably by this variable (or these variables)? | Optional: is failure to adjust for this variable (alone) expected to favour the experimental intervention or the comparator? |
| Yes/no/no information | Favour experimental/favour comparator/no information | |||
Preliminary consideration of co-interventions
Complete a row for each important co-intervention (i) listed in the review protocol and (ii) relevant to the setting of this particular study, or which the study authors identified as important.
‘Important’ co-interventions are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention.
| (i) Co-interventions listed in the review protocol | ||
|---|---|---|
| Co-intervention | Is there evidence that controlling for this co-intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co-intervention likely to favour outcomes in the experimental intervention or the comparator |
| Favour experimental/favour comparator/no information | ||
| (ii) Additional co-interventions relevant to the setting of this particular study, or which the study authors identified as important | ||
| Co-intervention | Is there evidence that controlling for this co-intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co-intervention likely to favour outcomes in the experimental intervention or the comparator |
| Methotrexate, other DMARDs, combination or monotherapy | Not carried out/no information | Favour experimental/favour comparator/no information ✓ |
Risk-of-bias assessment
Responses underlined are potential markers for low risk of bias. Where questions relate only to sign posts to other questions, no formatting is used.
| Signalling questions | Description | Response options |
|---|---|---|
| Bias due to confounding | ||
|
0.7 Is there potential for confounding of the effect of intervention in this study? If N/PN to 1.1: the study can be considered to be at low risk of bias due to confounding and no further signalling questions need be considered |
Yes, differential baseline LDA rates and no information on co-intervention | Y/PY ✓/PN/N |
| If Y/PY to 1.1: determine whether there is a need to assess time-varying confounding: | ||
|
1.2. Was the analysis based on splitting participants’ follow up time according to intervention received? If N/PN, answer questions relating to baseline confounding (1.4 to 1.6) If Y/PY, go to question 1.3 |
NA/Y/PY/PN/N ✓/NI | |
|
1.3. Were intervention discontinuations or switches likely to be related to factors that are prognostic for the outcome? If N/PN, answer questions relating to baseline confounding (1.4 to 1.6) If Y/PY, answer questions relating to both baseline and time-varying confounding (1.7 and 1.8) |
NA/Y/PY/PN/N/NI | |
| Questions relating to baseline confounding only | |
|---|---|
| 1.4. Did the authors use an appropriate analysis method that controlled for all the important confounding domains? | NA/Y/PY/PN ✓/N/NI |
| 1.5. If Y/PY to 1.4: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | ✓ NA/Y/PY/PN/N/NI |
| 1.6. Did the authors control for any post-intervention variables that could have been affected by the intervention? | NA/Y/PY/PN/N ✓/NI |
| Questions relating to baseline and time-varying confounding | |
| 1.7. Did the authors use an appropriate analysis method that controlled for all the important confounding domains and for time-varying confounding? | NA/Y/PY/PN ✓/N/NI |
| 1.8. If Y/PY to 1.7: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | ✓ NA/Y/PY/PN/N/NI |
| Risk of bias judgement | Low/moderate ✓/serious/critical/NI |
| Optional: What is the predicted direction of bias due to confounding? | Favours experimental/favours comparator/unpredictable |
| Bias in selection of participants into the study | ||
|---|---|---|
|
2.1. Was selection of participants into the study (or into the analysis) based on participant characteristics observed after the start of intervention? If N/PN to 2.1: go to 2.4 |
‘. . . a total of 88 patients (43 RA and 45 SpA), treated with three TNF inhibitors . . . were included . . . | Y/PY/PN/N ✓/NI |
|
2.2. If Y/PY to 2.1: Were the post-intervention variables that influenced selection likely to be associated with intervention? 2.3 If Y/PY to 2.2: Were the post-intervention variables that influenced selection likely to be influenced by the outcome or a cause of the outcome? |
✓ NA/Y/PY/PN/N/NI ✓ NA/Y/PY/PN/N/NI |
|
| 2.4. Do start of follow-up and start of intervention coincide for most participants? | Y/PY/PN/N/NI ✓ | |
| 2.5. If Y/PY to 2.2 and 2.3, or N/PN to 2.4: Were adjustment techniques used that are likely to correct for the presence of selection biases? | ✓ NA/Y/PY/PN/N/NI | |
| Risk of bias judgement | Low/moderate ✓/serious/critical/NI | |
| Optional: What is the predicted direction of bias due to selection of participants into the study? | Favours experimental/favours comparator/towards null/away from null/unpredictable | |
| Bias in classification of interventions | |
|---|---|
| 3.1 Were intervention groups clearly defined? | Y ✓/PY/PN/N/NI |
| 3.2 Was the information used to define intervention groups recorded at the start of the intervention? | Y/PY/PN/N ✓/NI |
| 3.3 Could classification of intervention status have been affected by knowledge of the outcome or risk of the outcome? | Y/PY/PN/N ✓/NI |
| Risk of bias judgement | Low/moderate ✓/serious/critical/NI |
| Optional: What is the predicted direction of bias due to classification of interventions? | Favours experimental/favours comparator/towards null/away from null/unpredictable |
| Bias due to deviations from intended interventions | |
|---|---|
| If your aim for this study is to assess the effect of assignment to intervention, answer questions 4.1 and 4.2 | Not applicable |
| 4.1. Were there deviations from the intended intervention beyond what would be expected in usual practice? | Y/PY/PN/N/NI |
| 4.2. If Y/PY to 4.1: Were these deviations from intended intervention unbalanced between groups and likely to have affected the outcome? | NA/Y/PY/PN/N/NI |
| If your aim for this study is to assess the effect of starting and adhering to intervention, answer questions 4.3 to 4.6 | |
| 4.3. Were important co-interventions balanced across intervention groups? | Y/PY/PN/N/NI ✓ |
| 4.4. Was the intervention implemented successfully for most participants? | Y/PY ✓/PN/N/NI |
| 4.5. Did study participants adhere to the assigned intervention regimen? | Y/PY/PN/N/NI ✓ |
| 4.6. If N/PN to 4.3, 4.4 or 4.5: Was an appropriate analysis used to estimate the effect of starting and adhering to the intervention? | ✓ NA/Y/PY/PN/N/NI |
| Risk of bias judgement | NI |
| Optional: What is the predicted direction of bias due to deviations from the intended interventions? | |
| Bias due to missing data | ||
|---|---|---|
| 5.1 Were outcome data available for all, or nearly all, participants? | Results reported were basically means (SD); difficult to determine | Y/PY/PN/N/NI ✓ |
| 5.2 Were participants excluded due to missing data on intervention status? | Y/PY/PN/N/NI ✓ | |
| 5.3 Were participants excluded due to missing data on other variables needed for the analysis? | Y/PY/PN/N/NI ✓ | |
| 5.4 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Are the proportion of participants and reasons for missing data similar across interventions? | ✓ NA/Y/PY/PN/N/NI | |
| 5.5 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Is there evidence that results were robust to the presence of missing data? | ✓ NA/Y/PY/PN/N/NI | |
| Risk of bias judgement | Low/moderate/serious/critical/NI ✓ | |
| Optional: What is the predicted direction of bias due to missing data? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable | |
| Bias in measurement of outcomes | |
|---|---|
| 6.1 Could the outcome measure have been influenced by knowledge of the intervention received? | Y/PY/PN ✓/N/NI |
| 6.2 Were outcome assessors aware of the intervention received by study participants? | Y ✓/PY/PN/N/NI |
| 6.3 Were the methods of outcome assessment comparable across intervention groups? | Y/PY ✓/PN/N/NI |
| 6.4 Were any systematic errors in measurement of the outcome related to intervention received? | Y/PY/PN ✓/N/NI |
| Risk of bias judgement | Low/moderate ✓/serious/critical/NI |
| Optional: What is the predicted direction of bias due to measurement of outcomes? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable |
| Bias in selection of the reported result | |
|---|---|
| Is the reported effect estimate likely to be selected, on the basis of the results, from... | |
| 7.1 . . . multiple outcome measurements within the outcome domain? | Y/PY/PN/N ✓/NI |
| 7.2 . . . multiple analyses of the intervention–outcome relationship? | Y/PY/PN/N ✓/NI |
| 7.3 . . . different subgroups? | Y/PY/PN/N ✓/NI |
| Risk of bias judgement | Low ✓/moderate/serious/critical/NI |
| Optional: What is the predicted direction of bias due to selection of the reported result? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable |
| Overall bias | |
|---|---|
| Risk of bias judgement | Low/moderate ✓/serious/critical/NI |
| Optional: What is the overall predicted direction of bias for this outcome? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable |
The Risk Of Bias In Non-randomised Studies – of Interventions (ROBINS-I) assessment tool (version for cohort-type studies)41
Ucar et al. 201742
Version 19 September 2016.
ROBINS-I tool (stage I): at protocol stage
Specify the review question.
| Participants | Remission/primary non-responders/secondary non-responders |
| Experimental intervention | Therapeutic drug monitoring |
| Comparator | Standard care |
| Outcomes | 13 outcomes; inconclusive results, time to results, dose changes, dose adjustment, treatment switch, discontinuation, changes in disease activity, rate of disease response, relapse and remission, hospitalisation, rates of surgical intervention, adverse effects, health-related quality of life |
List the confounding domains relevant to all or most studies.
|
From protocol; time of testing, testing method (e.g. reflex vs. concurrent) Others (suggested); drug dose/levels, disease stage at enrolment, time of assessment for response/follow-up, type of drug manipulation (e.g. optimisation or tapering) |
List co-interventions that could be different between intervention groups and that could impact on outcomes.
| Methotrexate, other DMARDs, combination or monotherapy |
ROBINS-I tool (Stage II): For each study
Specify a target randomised trial specific to the study.
| Design | Individually randomised ✓/Cluster randomised/Matched (e.g. cross-over) |
| Participants | Patients treated with adalimumab (40 mg subcutaneous) who remained clinically stable for at least six months |
| Experimental intervention | Biological monitoring data were released to physicians |
| Comparator | Physicians were blinded to biological monitoring data |
Is your aim for this study . . .?
| ≤ | to assess the effect of assignment to intervention |
| ✓ | to assess the effect of starting and adhering to intervention |
Specify the outcome
Specify which outcome is being assessed for risk of bias (typically from among those earmarked for the Summary of Findings table). Specify whether this is a proposed benefit or harm of intervention.
| Disease flare (harm) |
Specify the numerical result being assessed
In case of multiple alternative analyses being presented, specify the numeric result (e.g. RR 1.52, 95% CI 0.83 to 2.77) and/or a reference (e.g. to a table, figure or paragraph) that uniquely defines the result being assessed.
| IRR = 0.7252 (95% CI 0.49997 to 1.0578) |
Preliminary consideration of confounders
Complete a row for each important confounding domain (i) listed in the review protocol; and (ii) relevant to the setting of this particular study, or which the study authors identified as potentially important.
‘Important’ confounding domains are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention. ‘Validity’ refers to whether the confounding variable or variables fully measure the domain, while ‘reliability’ refers to the precision of the measurement (more measurement error means less reliability).
| (i) Confounding domains listed in the review protocol | ||||
|---|---|---|---|---|
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?a | Is the confounding domain measured validly and reliably by this variable (or these variables)? | Optional: is failure to adjust for this variable (alone) expected to favour the experimental intervention or the comparator? |
| Yes/no/no information | Favour experimental/Favour comparator/No information | |||
| Disease stage (proportion in remission/LDA) | No | Yes | Expected to favour control group (26.6% IG had LDA vs. 16.7% of CG) | |
| Time of assessment for response | No | No information |
No information But likely to be unimportant. Measurement believed to be carried out at similar time points (at scheduled visits) |
|
| Serum adalimumab levels | No | Yes | NA – serum ADL levels 5.5 mg/l in the CG and 5.3 mg/l in IG | |
| Serum anti-adalimumab antibody levels | No | No information | No information | |
| (ii) Additional confounding domains relevant to the setting of this particular study, or which the study authors identified as important | ||||
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?a | Is the confounding domain measured validly and reliably by this variable (or these variables)? | Optional: is failure to adjust for this variable (alone) expected to favour the experimental intervention or the comparator? |
| Yes/no/no information | Favour experimental/Favour comparator/No information | |||
Preliminary consideration of co-interventions
Complete a row for each important co-intervention (i) listed in the review protocol; and (ii) relevant to the setting of this particular study, or which the study authors identified as important.
‘Important’ co-interventions are those for which, in the context of this study, adjustment is expected to lead to a clinically important change in the estimated effect of the intervention.
| (i) Co-interventions listed in the review protocol | ||
|---|---|---|
| Co-intervention | Is there evidence that controlling for this co-intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co-intervention likely to favour outcomes in the experimental intervention or the comparator? |
| Favour experimental/Favour comparator/No information | ||
| (ii) Additional co-interventions relevant to the setting of this particular study, or which the study authors identified as important | ||
| Co-intervention | Is there evidence that controlling for this co-intervention was unnecessary (e.g. because it was not administered)? | Is presence of this co-intervention likely to favour outcomes in the experimental intervention or the comparator? |
| Methotrexate and other DMARDs | No | Favour experimental/Favour comparator/No information ✓ |
Risk-of-bias assessment
Responses underlined are potential markers for low risk of bias. Where questions relate only to sign posts to other questions, no formatting is used.
| Signalling questions | Description | Response options |
|---|---|---|
| Bias due to confounding | ||
|
0.7 Is there potential for confounding of the effect of intervention in this study? If N/PN to 1.1: the study can be considered to be at low risk of bias due to confounding and no further signalling questions need be considered |
Yes, differential baseline LDA rates and no information on co-intervention | Y/PY ✓/PN/N |
| If Y/PY to 1.1: determine whether there is a need to assess time-varying confounding: | ||
|
1.2. Was the analysis based on splitting participants’ follow-up time according to intervention received? If N/PN, answer questions relating to baseline confounding (1.4 to 1.6) If Y/PY, go to question 1.3. |
NA/Y/PY/PN/N ✓/NI | |
|
1.3. Were intervention discontinuations or switches likely to be related to factors that are prognostic for the outcome? If N/PN, answer questions relating to baseline confounding (1.4 to 1.6) If Y/PY, answer questions relating to both baseline and time-varying confounding (1.7 and 1.8) |
NA/Y/PY/PN/N/NI | |
| Questions relating to baseline confounding only | |
|---|---|
| 1.4. Did the authors use an appropriate analysis method that controlled for all the important confounding domains? | NA/Y/PY/PN/N ✓/NI |
| 1.5. If Y/PY to 1.4: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | NA ✓/Y/PY/PN/N/NI |
| 1.6. Did the authors control for any post-intervention variables that could have been affected by the intervention? | NA ✓/Y/PY/PN/N/NI |
| Questions relating to baseline and time-varying confounding | |
| 1.7. Did the authors use an appropriate analysis method that controlled for all the important confounding domains and for time-varying confounding? | NA/Y/PY/PN/N ✓/NI |
| 1.8. If Y/PY to 1.7: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | NA ✓/Y/PY/PN/N/NI |
| Risk of bias judgement | Low/moderate ✓/serious/critical/NI |
| Optional: What is the predicted direction of bias due to confounding? | Favours experimental/Favours comparator/Unpredictable |
| Bias in selection of participants into the study | |
|---|---|
|
2.1. Was selection of participants into the study (or into the analysis) based on participant characteristics observed after the start of intervention? If N/PN to 2.1: go to 2.4 |
Y/PY/PN/N ✓/NI |
|
2.2. If Y/PY to 2.1: Were the post-intervention variables that influenced selection likely to be associated with intervention? 2.3 If Y/PY to 2.2: Were the post-intervention variables that influenced selection likely to be influenced by the outcome or a cause of the outcome? |
NA/Y/PY/PN/N/NI NA/Y/PY/PN/N/NI |
| 2.4. Do start of follow-up and start of intervention coincide for most participants? | Y/PY ✓/PN/N/NI |
| 2.5. If Y/PY to 2.2 and 2.3, or N/PN to 2.4: Were adjustment techniques used that are likely to correct for the presence of selection biases? | NA ✓/Y/PY/PN/N/NI |
| Risk of bias judgement | Low ✓/moderate/serious/critical/NI |
| Optional: What is the predicted direction of bias due to selection of participants into the study? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable |
| Bias in classification of interventions | ||
|---|---|---|
| 3.1 Were intervention groups clearly defined? | TDM data were released only to intervention group | Y ✓/PY/PN/N/NI |
| 3.2 Was the information used to define intervention groups recorded at the start of the intervention? | Y ✓/PY/PN/N/NI | |
| 3.3 Could classification of intervention status have been affected by knowledge of the outcome or risk of the outcome? | Y/PY/PN/N ✓/NI | |
| Risk of bias judgement | Low ✓/moderate/serious/critical/NI | |
| Optional: What is the predicted direction of bias due to classification of interventions? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable | |
| Bias due to deviations from intended interventions | |
|---|---|
| If your aim for this study is to assess the effect of assignment to intervention, answer questions 4.1 and 4.2 | |
| 4.1. Were there deviations from the intended intervention beyond what would be expected in usual practice? | Y/PY/PN/N/NI |
| 4.2. If Y/PY to 4.1: Were these deviations from intended intervention unbalanced between groups and likely to have affected the outcome? | NA ✓/Y/PY/PN/N/NI |
| If your aim for this study is to assess the effect of starting and adhering to intervention, answer questions 4.3 to 4.6 | |
| 4.3. Were important co-interventions balanced across intervention groups? | Y/PY/PN/N/NI ✓ |
| 4.4. Was the intervention implemented successfully for most participants? | Y ✓/PY/PN/N/NI |
| 4.5. Did study participants adhere to the assigned intervention regimen? | Y/PY ✓/PN/N/NI |
| 4.6. If N/PN to 4.3, 4.4 or 4.5: Was an appropriate analysis used to estimate the effect of starting and adhering to the intervention? | NA ✓/Y/PY/PN/N/NI |
| Risk of bias judgement | NI |
| Optional: What is the predicted direction of bias due to deviations from the intended interventions? | |
| Bias due to missing data | |
|---|---|
| 5.1 Were outcome data available for all, or nearly all, participants? | Y/PY/PN/N/NI ✓ |
| 5.2 Were participants excluded due to missing data on intervention status? | Y/PY/PN/N/NI ✓ |
| 5.3 Were participants excluded due to missing data on other variables needed for the analysis? | Y/PY/PN/N/NI ✓ |
| 5.4 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Are the proportion of participants and reasons for missing data similar across interventions? | NA ✓/Y/PY/PN/N/NI |
| 5.5 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Is there evidence that results were robust to the presence of missing data? | NA ✓/Y/PY/PN/N/NI |
| Risk of bias judgement | Low/Moderate/Serious/Critical/NI ✓ |
| Optional: What is the predicted direction of bias due to missing data? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable |
| Bias in measurement of outcomes | |
|---|---|
| 6.1 Could the outcome measure have been influenced by knowledge of the intervention received? | Y/PY/PN ✓/N/NI |
| 6.2 Were outcome assessors aware of the intervention received by study participants? | Y ✓/PY/PN/N/NI |
| 6.3 Were the methods of outcome assessment comparable across intervention groups? | Y/PY/PN/N/NI ✓ |
| 6.4 Were any systematic errors in measurement of the outcome related to intervention received? | Y/PY/PN/N/NI ✓ |
| Risk of bias judgement | Low/Moderate ✓/Serious/Critical/NI |
| Optional: What is the predicted direction of bias due to measurement of outcomes? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable |
| Bias in selection of the reported result | |
|---|---|
| Is the reported effect estimate likely to be selected, on the basis of the results, from . . . | |
| 7.1 . . . multiple outcome measurements within the outcome domain? | Y/PY/PN/N ✓/NI |
| 7.2 . . . multiple analyses of the intervention–outcome relationship? | Y/PY/PN/N ✓/NI |
| 7.3 . . . different subgroups? | Y/PY/PN/N ✓/NI |
| Risk of bias judgement | Low ✓/Moderate/Serious/Critical/NI |
| Optional: What is the predicted direction of bias due to selection of the reported result? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable |
| Overall bias | |
|---|---|
| Risk of bias judgement | Low/Moderate ✓/Serious/Critical/NI |
| Optional: What is the overall predicted direction of bias for this outcome? | Favours experimental/Favours comparator/Towards null/Away from null/Unpredictable |
Appendix 5 The PRISMA flow diagram for the cost-effectiveness systematic review
FIGURE 7.
The PRISMA flow diagram: a description of the study inclusion process for the cost-effectiveness systematic review.
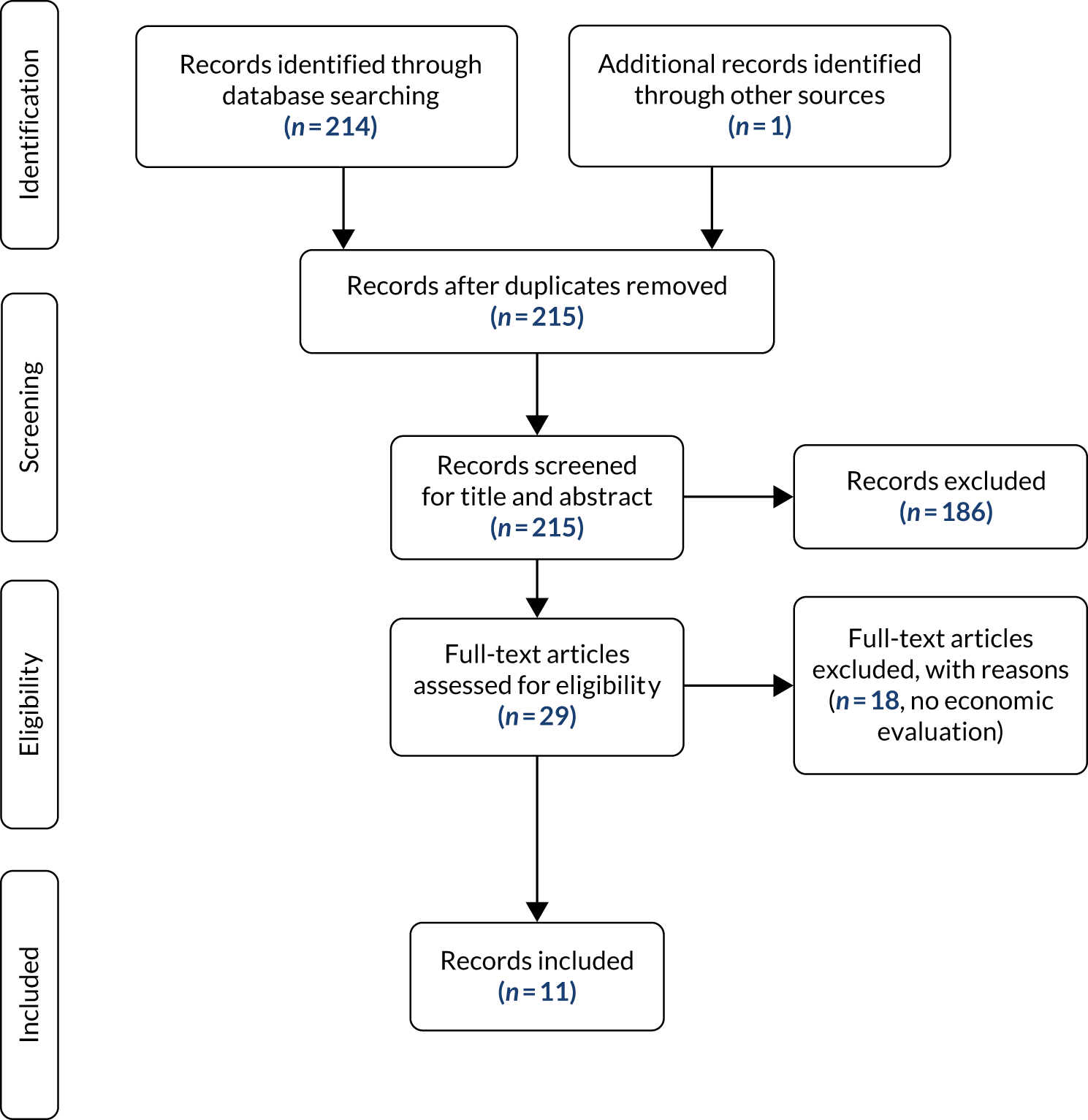
Appendix 6 Studies selected in the cost-effectiveness systematic review
| Study | Population | Setting | Test | TNF-α inhibitor | Study design | N | Time frame | Outcome | Cost measures | Results | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arango et al.43 (INGEBIO) | People with RA, PsA and AS who were treated with ADL who remained clinically stable for at least 6 months | Clinic, Spain | Trough ADL and anti-ADL antibody measured by Promonitor-ADA and Promonitor anti-ADL (Progenika) at eight time points | ADL | Non-randomised controlled trial | 109 participants in IG and 60 in CG, of whom 30 and 33 people had RA, respectively | 18 months | DAS28, BASDAI, BASFI and HAQ-DI, days with active disease | Average cost of ADL per patient-year | Mean QALYs were 1.145 and 1.076 during follow-up period per person in IG and CG, respectively. The average cost of Humira (ADL) per patient-year was €10,664.54 vs. €9856.45 (-€808.08, 8% savings) in the CG and IG, respectively (the results reported for the mixed population) | Data are reported for all participants and are not reported by disease subgroupPeople with RA have better quality of life, lower risk of flares and incur lower treatment costs if management is complemented with ELISA testing |
| Pascual-Salcedo et al.44 | People with RA and SpA in remission or LDA who were being treated with IFX, ADL and ETN | University Hospital, Spain | Drug levels by capture ELISA | ADL, IFX and ETN | Historical controlled cohort | 43 participants with RA | 7 years (between 2006 and 2012) | DAS28 | Monthly amount of spared drug per person | Decrease in drug use: €91.62 per person for IFX (70 kg of mean weight), €324 per person for ADL, €257 per person for ETN | Data are reported for all participants and are not reported by subgroup. QALYs were not reported |
| Study | Population | Perspective | Setting | Test | TNF-α inhibitor | Model structure | Time frame | Effectiveness and costs parameters | Type of study | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Krieckaert et al.53 | Cohort of 272 people with RA who were treated with ADL for 3 years,a and data on direct medical costs and HRQoL from the URAC study group (n = 1034) | Societal, health care | Clinic, the Netherlands | Sanquin, drug level | ADL | Markov | 3-year time horizon with 3-month cycles | Direct medical and productivity costs, utilities | Cost–utility |
The authors compared a cohort of people monitored by ELISA testing with a cohort from the URAC study at 28 weeks after starting treatment with ADL. Markov states were defined by DAS28 categorisation Result: substantial reduction in cost of medication, small change in efficacy of treatment for ELISA testing |
| Laine et al.54 | Cohort of people who were treated with ADL (n = 486) and IFX (n = 1137)b | Health care | Clinic, Finland | Sanquin, Promonitor, immunoassay, drug level and antibody | ADL and IFX | Markov | 3-year time horizon with 6-month cycles | Decreasing proportion of people on non-optimal treatment; costs of test and non-optimal treatment | Cost-effectiveness |
Economic impact of clinical decision-making was modelled in a short-term (3–6 months) scenario with 100 hypothetical patients for ADL and IFX. ELISA testing was performed in non-responders Result: using ELISA test was cost-saving |
| Gavan17 | People with RA in England (BSRBR-RA) | NHS and PSS | England | ELISA tests: no specific ELISA test stated | ADL | Discrete-event simulation | Lifetime | Costs of treatment, hospitalisation and testing | Cost–utility |
ELISA monitoring was investigated for use during response and in remission for dose adjustment Result: ELISA testing is not likely to be cost-effective |
Appendix 7 Treatment and testing strategies considered in Gavan17
| Strategy | Type of testing strategy | Description |
|---|---|---|
| Current practice | Not applicable | Usual care for people with RA with no testing of anti-ADL antibody or drug level |
| Strategy 1 | Monitoring | Anti-ADL antibody and drug level testing every 3 months |
| Strategy 2 | Monitoring | Anti-ADL antibody and drug level testing every 6 months |
| Strategy 3 | Monitoring and dose reduction | Anti-ADL antibody and drug level testing every 3 months, drug level test in remission after 2 years |
| Strategy 4 | Monitoring and dose reduction | Anti-ADL antibody and drug level testing every 3 months, drug level test in remission after 3 years |
| Strategy 5 | Dose reduction | Drug level test in remission after 2 years |
| Strategy 6 | Dose reduction | Drug level test in remission after 3 years |
| Strategy 7 | Monitoring | Anti-ADL antibody testing every 3 months only |
| Strategy 8 | Monitoring | Anti-ADL antibody testing every 6 months only |
| Strategy 9 | Monitoring and dose reduction | Anti-ADL antibody testing every 3 months only, drug level test in remission after 2 years |
| Strategy 10 | Monitoring and dose reduction | Anti-ADL antibody testing every 3 months only, drug level test in remission after 3 years |
| Strategy 11 | Not applicable | No testing. Just half dose in remission after 2 years |
| Strategy 12 | Not applicable | No testing. Just half dose in remission after 3 years |
Appendix 8 Quality appraisal of cost–utility studies
| Item | CHEC-list | Ucar et al.42 | Pascual-Salcedo et al.44 | Krieckaert et al.53 | Laine et al.54 | Gavan17 |
|---|---|---|---|---|---|---|
| 1 | Is the study population clearly described? | Y | Y | Y | N | Y |
| 2 | Are competing alternatives clearly described? | Y | Y | Y | Y | Y |
| 3 | Is a well-defined research question posed in answerable form? | Y | Y | Y | Y | Y |
| 4 | Is the economic study design appropriate to the stated objective? | Y | Y | Y | N | Y |
| 5 | Is the chosen time horizon appropriate in order to include relevant costs and consequences? | Y | N | Y | Y | Y |
| 6 | Is the actual perspective chosen appropriate? | N | N | Y | Y | Y |
| 7 | Are all important and relevant costs for each alternative identified? | N | N | N | N | Y |
| 8 | Are all costs measured appropriately in physical units? | N | N | N | N | Y |
| 9 | Are costs valued appropriately? | N | N | N | N | Y |
| 10 | Are all important and relevant outcomes for each alternative identified? | Y | N | Y | N | Y |
| 11 | Are all outcomes measured appropriately? | N | N | Y | Y | Y |
| 12 | Are outcomes valued appropriately? | N | N | Y | Y | Y |
| 13 | Is an incremental analysis of costs and outcomes of alternatives performed? | N | N | Y | N | Y |
| 14 | Are all future costs and outcomes discounted appropriately? | N | N | Y | N | Y |
| 15 | Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | N | N | Y | N | Y |
| 16 | Do the conclusions follow from the data reported? | Y | Y | Y | Y | Y |
| 17 | Does the study discuss the generalisability of the results to other settings and patient/client groups? | N | N | N | Y | Y |
| 18 | Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | N | N | N | N | Y |
| 19 | Are ethical and distributional issues discussed appropriately? | N | N | Y | Y | Y |
| Element | Krieckaert et al.53 | Laine et al.54 | Gavan17 |
|---|---|---|---|
| Structure (S) | |||
| S1: statement of decision problem/objective | Yes | Yes | Yes |
| S2: statement of scope/perspective | Yes | Yes | Yes |
| S3: rationale for structure | No | Yes | Yes |
| S4: structural assumptions | No | No | Yes |
| S5: strategies/comparators | Yes | Yes | Yes |
| S6: model type | Yes | Yes | Yes |
| S7: time horizon | Yes | Yes | Yes |
| S8: disease states/pathways | Yes | No | Yes |
| S9: cycle length | Yes | Yes | NA |
| Data (D) | |||
| D1: data identification | Yes | Yes | Yes |
| D2: pre-model data analysis | No | No | No |
| D2a: baseline data | Yes | Yes | Yes |
| D2b: treatment effects | No | No | Yes |
| D2c: quality-of-life weights (utilities) | Yes | No | Yes |
| D3: data incorporation | No | No | No |
| D4: Assessment of uncertainty | |||
| D4a: methodological | No | No | No |
| D4b: structural | No | No | No |
| D4c: heterogeneity | Yes | No | Yes |
| D4d: parameter | Yes | No | Yes |
| Consistency HAD | |||
| C1: internal consistency | Yes | Yes | Yes |
| C2: external consistency | No | No | No |
Appendix 9 Search strategy for the additional search for clinical effectiveness evidence
MEDLINE (via Ovid)
Date range searched: 1946 to week 3
October 2018.
Date searched: 25 October 2018.
Searcher: Sophie Robinson.
Hits: 1418.
Search strategy
-
(anti-TNF* or antiTNF* or (TNF* adj2 (inhibit* or block*))).tw.
-
anti* tumo?r* necrosis* factor*.tw.
-
Tumor Necrosis Factor-alpha/
-
(biologic* adj2 DMARD*).tw.
-
((antirheumati* or anti rheumati* or anti-rheumati*) adj4 biologic*).tw.
-
((disease modify * ordisease-modify*) adj4 biologic*).tw.
-
exp Antibodies, Monoclonal/
-
anti* drug* antibod*.tw.
-
ADAb.tw.
-
etanercept.tw. or ETANERCEPT/
-
(tnr001 or “tnr 001” or tnr-001 or 185243–69–0).tw.
-
(ETA or ETN).tw.
-
(enbrel or erelzi or benepali or lifmior or brenzys).tw.
-
(anti-etanercept* or antietanercept* or (anti adj3 etanercept*)).tw.
-
adalimumab.tw. or ADALIMUMAB/
-
(d 2e7 or d2e7 or d-2e7 or 331731-18-1).tw.
-
(ADA or ADL or ADM).tw.
-
(humira or amgevita or cyltezo or imraldi or solymbic or hyrimoz or halimatoz).tw.
-
(anti-adalimumab* or antiadalimumab* or (anti adj3 adalimumab*)).tw.
-
infliximab.tw. or INFLIXIMAB/
-
(170277-31-3 or ta650 or ta 650 or ta-650).tw.
-
(INF or IFX).tw.
-
(anti-infliximab* or antiinfliximab* or (anti adj3 infliximab*)).tw.
-
(remicade or inflectra or remsima or flixabi or zessly or renflexis or ixifi).tw.
-
Certolizumab Pegol/or certolizumab.tw.
-
(cdp870 or cdp 870 or cdp-870 or 428863-50-7 or 1132819-27-2).tw.
-
(CER or CZP).tw.
-
cimzia.tw.
-
(anti-certolizumab* or anticertolizumab* or (anti adj3 certolizumab*)).tw.
-
golimumab.tw.
-
(cnto 148 or cnto148 or cnto-148 or 476181-74-5).tw.
-
(GOL or GLM).tw.
-
simponi.tw.
-
(anti-golimumab* or antigolimumab* or (anti adj3 golimumab*)).tw.
-
(biologic* adj2 agent*).tw.
-
(CT-P13 or CTP13 or CT P13 or SB2 or SB-2 or SB 2 or SB4 or SB-4 or SB 4 or SB-5 or SB5 or SB 5).tw.
-
(biosimilar* or (bio* adj1 similar*)).tw.
-
or/1-37
-
exp Arthritis, Rheumatoid/
-
RA.tw.
-
Rheumarthrit*.tw.
-
((Rheumatoid* or rheumatic* or inflammat* or idiopathic* or deforman*) adj4 (arthrit* or arthros* or polyarthrit* or factor*)).tw.
-
(Chronic* adj4 (polyarthrit* or poly arthrit* or poly-arthrit* or rheumati*)).tw.
-
((Inflammat* or pain* or swell* or stiff*) adj4 (joint* or synovial*)).tw.
-
(Beauvais* adj2 disease*).tw.
-
or/39-45
-
Radioimmunoassay/
-
(radioimmuno* or radio immuno* or radio-immuno*).tw.
-
RIA.tw.
-
reporter* gene* assay*.tw.
-
RGA.tw.
-
(semi* fluid* phase* adj3 enzyme* immuno*).tw.
-
EIA.tw.
-
((homogenous* or homogeneous*) adj1 mobilit* shift* assay*).tw.
-
HMSA.tw.
-
(Biomonitor* or iLite or Euro Diagnostica* or Wieslab or Svar).tw.
-
(ARUP or Q-ETA or EURIA).tw.
-
(Matriks* Biotek* or Shikari*).tw.
-
(Prometheus* or Anser*).tw.
-
or/47-59
-
38 and 46 and 60
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomised.ab.
-
placebo.ab.
-
clinical trials as topic.sh.
-
randomly.ab.
-
trial.ti.
-
or/62-68
-
exp animals/not humans.sh.
-
69 not 70.
| Criteria | Specification |
|---|---|
| Population | As for the clinical effectiveness systematic review (see Population) |
| Interventions | Any test outside of the scope for monitoring patients receiving TNF-α inhibitors (ADL, ETN, IFX, CTZ and GLM) |
| Comparator | Current practice (i.e. no testing) |
| Outcomes | As for the clinical effectiveness systematic review (see Outcomes) |
| Study design | RCT |
Appendix 10 Time to the first flare estimates from the INGEBIO study
FIGURE 8.
Kaplan–Meier estimates from the INGEBIO study. BDM, biological drug monitoring. Adapted from the poster presentation by Ucar and colleagues at the Annual European Congress of Rheumatology EULAR 2017 (Ucar and Osakidetza, personal communication, September 2018).
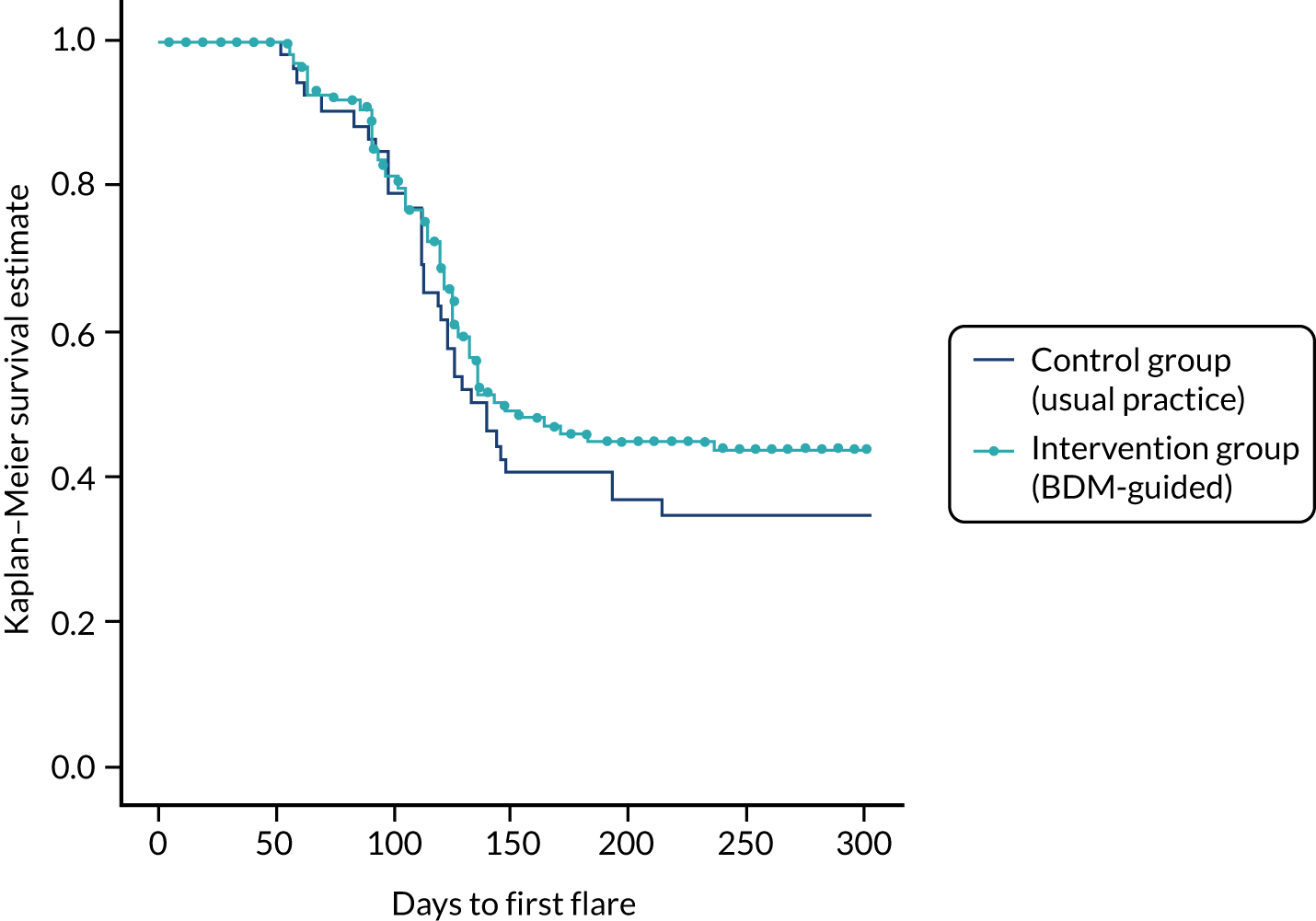
Appendix 11 Rates of serious adverse events
| Source | Type of serious infections | Population | Estimate rate of SAEs |
|---|---|---|---|
| Singh et al.79 (systematic review) | Serious infection mostly included infections associated with death, hospitalisation or the use of intravenous antibiotics | 4788 TNF-α inhibitor-experienced people with RA who were recruited to 11 RCTs during 2005–13, with mean RA duration of 10.8 years | 19/1000 |
| TA37523 (based on a systematic review by Singh et al.78) | Serious infections included opportunistic infections as well as bacterial infections in most studies | Adults (aged ≥ 16 years) with any disease (except HIV/AIDS) included in studies of any of the nine biologics: abatacept (Orencia; Bristol-Myers Squibb Pharmaceuticals Limited, Uxbridge, UK), ADL (Humira), anakinra (Kineret®; Swedish Orphan Biovitrum Ltd, Great Abington, UK), CTZ (Cimzia), etanercept (Enbrel), golimumab (Simponi), IFX (Remicade), rituximab (Rituxan or MabThera®; Roche Products Limited, Welwyn Garden City, UK) and tocilizumab (Actemra®; F. Hoffman-La Roche Ltd, Basel, Switzerland) | 35/1000 |
| Senabre Gallego et al.73 | Septic arthritis | 39 people in clinical remission | One patient (out of 39) discontinued treatment owing to the AE (study follow-up: 12 months) |
| Dixon et al.76 | Tuberculosis | People with RA from the BSRBR-RA treated with ADL, ETN or IFX |
|
| Bruce at al.75 | Pneumocystis jirovecii pneumonia | People with RA from the BSRBR-RA treated with TNF-α inhibitors | 2.0/10,000 person-years (95% CI 1.2 to 3.3 person-years) |
| Burmester et al.77 | SAE (defined as fatal or immediately life-threatening; required hospitalisation or prolonged hospitalisation; resulted in persistent or significant disability/incapacity, congenital anomaly or required medical or surgical intervention to prevent a serious outcome) | 15,132 people with RA exposed to ADL in 28 global clinical trials | 4.7 per 100 person-years |
| aJani et al.116 | In the high-level dose group: lower (34%) and upper (16%) respiratory tract infections, urinary tract infections (15%) and skin infections, including shingles (8%) | People from the BSRBR-RA (safety data) and the Biologics in RA Genetics & Genomics Syndicate (serological samples) |
Appendix 12 Odds ratios for serious infections from Singh et al.79
Odds ratios for serious infections in people who were treated with low-dose biologics compared with people who received the standard dose (Table 56) were reported in Singh et al. 79 OR estimates of 0.71 [95% credible interval (CrI) 0.5 to 1.01] and 0.7 (95% CrI 0.27 to 1.68) for consistency and inconsistency models, respectively, were obtained from a Bayesian network meta-analysis of the risk of serious infections in people with RA.
Appendix 13 Recommendations for biologic dose reduction
The Exeter Biologic Clinic recommendations for biologic dose reduction
Patient selection
-
Biologic treatment > 2 years and sustained LDA or clinical remission (DAS28 of < 2.6 ± ultrasound scanning remission) or Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) & Pain VAS of < 4.
-
No radiographic progression.
Strategy for biologic dose reduction
-
Clinical assessment:
-
DAS28 of < 2.6 or LDA ± ultrasound scanning remission
-
BASDAI & Pain VAS score of < 4 – expect to be much less than 4 and > 50% improvement from pre biologic
-
-
Reduce biologic drug by one-third (Table 57).
-
Follow-up at 3 months (plus advice line).
-
If flares, retreat at full dose.
-
If LDA or remission, review every 6 months and consider further reduction (see Table 57).
| Biologic drug | First dose reduction | Second dose reduction |
|---|---|---|
| ADL | 40 mg every 3 weeks | 40 mg every 4 weeks |
| ETN | 50 mg every 10 days | 50 mg every 14 days |
| CTZ | 200 mg every 3 weeks | 200 mg every 4 weeks |
| GLM | 50/100 mg every 6 weeks | 50/100 mg every 8 weeks |
| IFX IV | 2 mg/kg every 8 weeks/per infusion | 2 mg/kg every 12 weeks/per infusion |
Recommendations by NHS Greater Glasgow and Clyde
These recommendations are available on the NHS Greater Glasgow and Clyde website. 118
Appendix 14 Hospital and Community Health Services pay and price inflation indices
| Year | Pay and prices (%) |
|---|---|
| 2000–1 | 4.2 |
| 2001–2 | 5.1 |
| 2002–3 | 3.5 |
| 2003–4 | 5.2 |
| 2004–5 | 3.3 |
| 2005–6 | 3.7 |
| 2006–7 | 3.7 |
| 2007–8 | 2.9 |
| 2008–9 | 3.9 |
| 2009–10 | 0.6 |
| 2010–11 | 3.0 |
| 2011–12 | 2.1 |
| 2012–13 | 1.7 |
| 2013–14 | 1.1 |
| 2014–15 | 0.9 |
| 2015–16 | 1.3 |
Appendix 15 Assay costs
| Test | Number of wells | Number of controls (per assay) | Singlet testing of patient samples | Duplicate testing of patient samples | Sources and comments | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number of samples analysed (per assay) | Cost per assay | Cost (per sample) | Number of samples analysed (per assay) | Cost (per assay) | Cost per sample | ||||
| IDK Monitora | |||||||||
| Drug level monitoring | 96 | Two controls, six standards (tested once/in duplicate) | 88/80 | £855.00 | £9.72/£10.69 | 40 | £855.00 | £21.38 |
|
| Anti-drug antibody monitoring | 96 | Two controls, one standard (tested once/in duplicate) | 93/90 | £775.00 | £8.33/£8.61 | 45 | £775.00 | £17.22 |
|
| Promonitorb | |||||||||
| Drug level monitoring | 96 | Two controls, six standards (tested once/in duplicate) | 88/80 | £700.00 | £7.95/£8.75 | 40 | £700.00 | £17.50 |
|
| Anti-drug antibody monitoring | 96 | Two controls, six standards (tested once/in duplicate) | 88/80 | £700.00 | £7.95/£8.75 | 40 | £700.00 | £17.50 |
|
| RIDASCREENa | |||||||||
| Drug level monitoring | 96 | Two controls, six standards (tested once/in duplicate) | 88/80 | £565.00 | £6.42/£7.06 | 40 | £565.00 | £14.13 |
|
| Anti-drug antibody monitoring | 96 | Two controls, six standards (tested once/in duplicate) | 88/80 | £775.00 | £8.81/£9.69 | 40 | £775.00 | £19.38 |
|
| LISA-TRACKERc | |||||||||
| Drug level monitoring | 48d | One control, five standards (tested once/in duplicate) | 42/36 | £836.77 | £19.92/£23.24 | 24 | £836.77 | £34.87 |
|
| Anti-drug antibody monitoring | 48d | One control, five standards (tested once/in duplicate | 42/36 | £836.77 | £19.92/£23.24 | 24 | £836.77 | £34.87 |
|
| MabTracka | |||||||||
| Drug level monitoring | 96 | Two controls, six standards (tested once/in duplicate) | 88/80 | €1259.50 | €14.31/€15.74 | 40 | €1259.50 | €31.49 |
|
| Anti-drug antibody monitoring | 96 | Two controls, two standards (tested once/in duplicate) | 92/88 | €847.90 | €9.21/€9.64 | 44 | €847.90 | €19.27 |
|
| Sanquin Diagnostics | |||||||||
| ADL/IFX drug monitoring | NR | NR | Eighte | €50 | €6.25 | Four | €50 | €12.50 |
|
| ADL/IFX antibody monitoring | NR | NR | Eighte | €50 | €6.25 | Four | €50 | €12.50 |
|
| CTZ/GLM/ETN drug monitoring | NR | NR | Eighte | €90 | €11.25 | Four | €90 | €22.50 |
|
| CTZ/GLM/ETN antibody monitoring | NR | NR | Eighte | €90 | €11.25 | Four | €90 | €22.50 |
|
Appendix 16 Microcosting study by Jani et al.37
| Type of resource use | Cost (£) |
|---|---|
| Phase 1: pre-testing | |
| Outpatient appointment for discussion about the need for test | 2.35 |
| Clerical staff (to book the appointment and send out a letter to a patient) | 1.15 |
| Appointment for trough blood levels | 102 |
| Phase 2: analysis of samples | |
| Receipt and labelling of samples: central specimen reception | 2.22 |
| Data entry of patient information to laboratory system | 2.22 |
| Sample preparation: extraction of serum from blood | 2.22 |
| Transport, receipt and storage of sample: immunology laboratory | 2.22 |
| Preparation of reagents (wash solution, setting up assay and conjugate) | 3.20 |
| ELISA kit | 700 |
| Pipette tips for ELISAs | 6.00 |
| Semi-deep well plates for ELISAs | 2.20 |
| Troughs for ELISAs | 1.00 |
| Retrieval of patient/IQC samples from storage | 2.13 |
| Checking and sorting samples to match worklist | 2.13 |
| Pipetting samples onto ELISA plate | 4.26 |
| Pipetting calibrators, IQC samples, and incubation of samples | 2.13 |
| Washing ELISA plate and addition of conjugate | 2.13 |
| Washing ELISA plate and addition of substrate | 2.13 |
| Addition of stop solution | 1.06 |
| ELISA plate reading and printing of results | 2.13 |
| Technical validation involving a review of internal quality control | 1.06 |
| Results transcribed to worksheet | 1.06 |
| Data entry of results to patient’s record in laboratory system | 2.13 |
| Transcribed results/data entry reviewed by a second independent biomedical scientist | 1.06 |
| Clinical authorisation using reference range/delta check failure results | 2.54 |
| Hard-copy report sent to the clinician | 2.11 |
| Phase 3: treatment decision | |
| Interpretation of results by rheumatologist | 3.92 |
| Discussion with a patient (telephone call) | 3.47 |
| Letter with results and decision | 2.16 |
| Total costs (best case to worst case scenario) | 152.52 (147.68–159.24) |
Appendix 17 Single and duplicate, and concurrent and reflex testing strategies
| Strategy | Phlebotomy appointment (yes/no) | Proportion of patients tested (%) | Cost of a telephone call (£) | Postage (£) | Total cost (per patient) (£) | |||
|---|---|---|---|---|---|---|---|---|
| Initiala | Additionalb | Trough level | Ab level | Duplicate | Single | |||
| Concurrent | Yes | No | 100 | 100 | NA | 4 | 159.06 | 141.66c |
| Reflex | Yes | No | 100 | 4.7d | 3.47 | 4 | 141.38 | 132.27 |
| Reflex | Yes | No | 100 | 35.8e | 3.47 | 4 | 148.31 | 136.49 |
| Concurrent | No | No | 100 | 100 | NA | 4 | 51.23 | 33.83 |
| Reflex | No | No | 100 | 4.7d | 3.47 | 4 | 33.54 | 24.43 |
| Reflex | No | No | 100 | 35.8e | 3.47 | 4 | 40.47 | 28.66 |
Appendix 18 Estimation of the costs of managing different health states
FIGURE 9.
Density functions and disease management costs for different HAQ bands. (a) Distribution of HAQ scores for remission; (b) distribution of HAQ scores for LDA; (c) distribution of HAQ scores for MDA/HDA; and (d) annual health management costs for different HAQ bands.
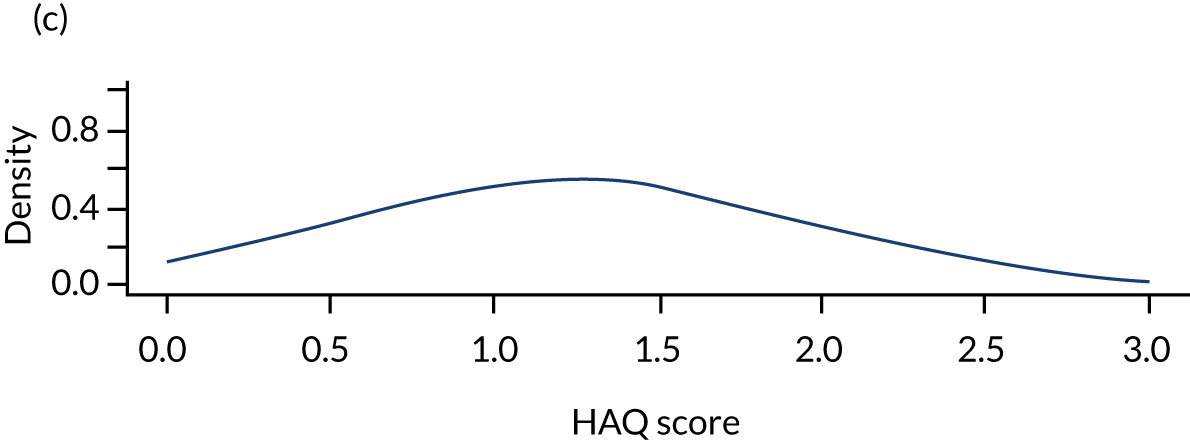
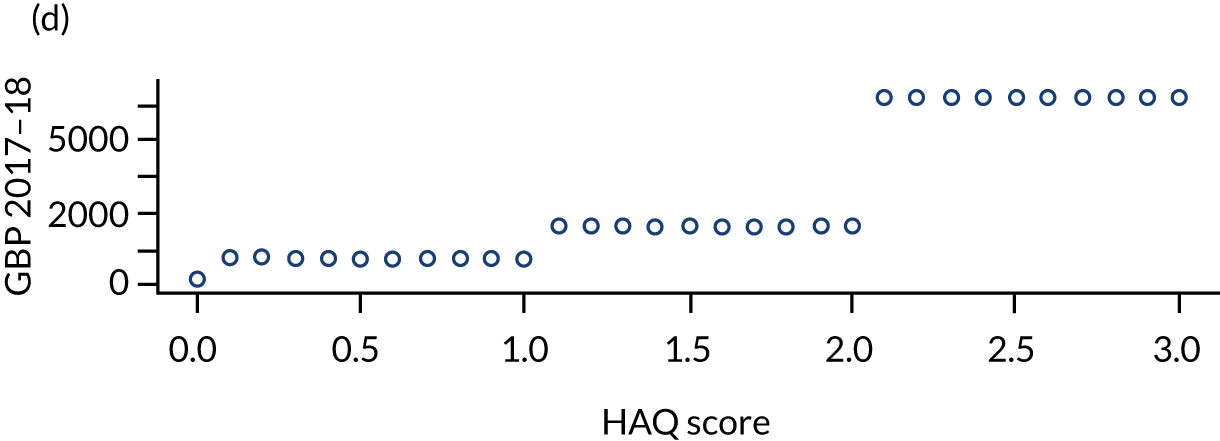
| HAQ | Hospital days (per 6 months)a | Number of outpatient visits (per 6 months)a | Proportion of patients who had joint replacement (% in 6 months)a | Total costs (per year)b |
|---|---|---|---|---|
| HAQ = 0 | 0.2 | 0.6 | 0.3 | £214 |
| 0 < HAQ ≤ 1 | 0.5 | 1 | 0.8 | £789 |
| 1 < HAQ ≤ 2 | 1.2 | 1.5 | 2.3 | £1669 |
| 2 < HAQ ≤ 3 | 5.1 | 2.1 | 4 | £5244 |
Appendix 19 Average cost of joint replacement surgery in the Royal Devon & Exeter NHS Foundation Trust
Estimates related to the cost of surgery in RA patients in Royal Devon & Exeter NHS Foundation Trust are shown in Table 63. International Classification of Diseases, Tenth Revision (ICD-10), codes included any codes from categories M05 or M06 or code M08.0 in conjunction with the Operating Procedure Codes Supplement (OPCS) procedure codes, which could be any from the following categories: W37, W38, W39, W40, W41, W42, W43, W44, W45, W46, W47, W48, W49, W54, W58, W93, W94, W95, W96, W97, W98, O06, O07, O08, O18, O21, O22, O23, O24, O25, O26 and O32. Those categories are all relevant to joint replacement surgeries. The time period considered was April 2017 to September 2018. These data were provided to the EAG by Nicola Finch, Leanne Brown, Keith Oldfield and Rob Storey from the Royal Devon & Exeter NHS Foundation Trust.
| Specialty group description | POD group description | ICD-10 (diagnostic) | OPCS (procedure) | Actual cost (£) | Episode count (n) | Average cost (per episode) (£) |
|---|---|---|---|---|---|---|
| Orthopaedics | Inpatients | M0596 Seropositive rheumatoid arthritis, unspecified | W401 Primary total prosthetic replacement of knee joint using cement | 7418.75 | 2 | 3709.37 |
| Orthopaedics | Inpatients | M0645 Inflammatory polyarthropathy | W371 Primary total prosthetic replacement of hip joint using cement | 4613.91 | 1 | 6242.08 |
| Orthopaedics | Inpatients | M0690 Rheumatoid arthritis, unspecified | O211 Primary total prosthetic replacement of elbow joint using cement | 6351.26 | 1 | 6351.26 |
| Orthopaedics | Inpatients | M0691 Rheumatoid arthritis, unspecified | O071 Primary hybrid prosthetic replacement of shoulder joint using cemented glenoid component | 4037.03 | 1 | 4037.03 |
| Orthopaedics | Inpatients | M0694 Rheumatoid arthritis, unspecified | W541 Primary prosthetic replacement of articulation of bone NEC | 5388.32 | 1 | 5388.32 |
| Orthopaedics | Inpatients | M0696 Rheumatoid arthritis, unspecified | W401 Primary total prosthetic replacement of knee joint using cement | 33,273.88 | 5 | 6654.78 |
| Orthopaedics | Inpatients | M0697 Rheumatoid arthritis, unspecified | O321 Osteotomy/ies (e.g. Scarf and Akin) for Hallux Valgus correction with or without internal fixation and soft tissue correction | 2076.00 | 1 | 2076.00 |
| Orthopaedics | Inpatients | M0699 Rheumatoid arthritis, unspecified | W371 Primary total prosthetic replacement of hip joint using cement | 4590.18 | 1 | 4590.18 |
| Plastic and reconstructive surgery | Inpatients | M0694 Rheumatoid arthritis, unspecified | W541 Primary prosthetic replacement of articulation of bone NEC | 6857.30 | 2 | 3428.65 |
| Total | 74,606.62 | 15 | 5061.80 |
Appendix 20 Cost of managing flares reported in Maravic et al.80
Maravic et al. 80 investigated the costs associated with managing flares in people with RA. This study used a survey method to collect data regarding rheumatology practice for managing a hypothetical case of a flare-up in an individual with a 10-year history of RA in a French setting. A survey questionnaire was completed by 917 practising rheumatologists. Over 80% of the respondents recommended measuring laboratory inflammation parameters, complete blood cell counts, liver enzymes and serum creatinine, and using radiographs (hands, anteroposterior cervical spine view, wrists and knees); 50–70% recommended additional cervical spine incidences, elbow and chest radiographs, and bone absorptiometry. The main recommended treatments were adding TNF-α inhibitor therapy (24%) or another DMARD (10%), increasing the methotrexate dosage (24%) and substituting leflunomide for methotrexate. Most respondents suggested continuing the glucocorticoid at the same dosage (61%) or a higher dosage (36%). Analgesics and non-steroidal anti-inflammatory drugs were recommended by 65% and 41% of respondents, respectively, and rehabilitation therapy was recommended by 83% of respondents.
This study focused on investigational costs and treatment costs; rheumatology appointments were not considered. Only the total costs of various types of tests and treatments were reported (Table 64).
| Cost component | Mean cost (€) |
|---|---|
| Diagnostic investigations | |
| Laboratory tests | 80 |
| Other tests | 276 |
| Total | 356 |
| Treatment for 1 month | |
| DMARDs (n = 884) | 724 |
| Glucocorticoids (n = 901) | 11 |
| Analgesics (n = 588) | 17 |
| Anti-inflammatory drugs (n = 348) | 14 |
| Other treatments (n = 130) | 6 |
| Total | 746 |
| Combined total | 1105 |
Of note, DMARDs include synthetic drugs (sDMARDs), such as methotrexate and leflunomide, and biological drugs, including the TNF-α inhibitors considered in this appraisal. The sDMARDs are relatively inexpensive compared with bDMARDs. 21
Note that the costs of managing flares estimated from those reported in Maravic et al. 80 and used in the model (£423 for the cost of diagnostic investigations per flare and £68 for monthly treatment costs excluding the cost of TNF-α inhibitors) were approximate owing to incomplete reporting of cost components as well as rounding and/or typos in table 4 in Maravic et al. 80 Varying these estimates by ± 10% did not change the economic outcomes qualitatively.
Appendix 21 Utilities
FIGURE 10.
Frequencies of three types of flares. Reported in Markusse et al. 72 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
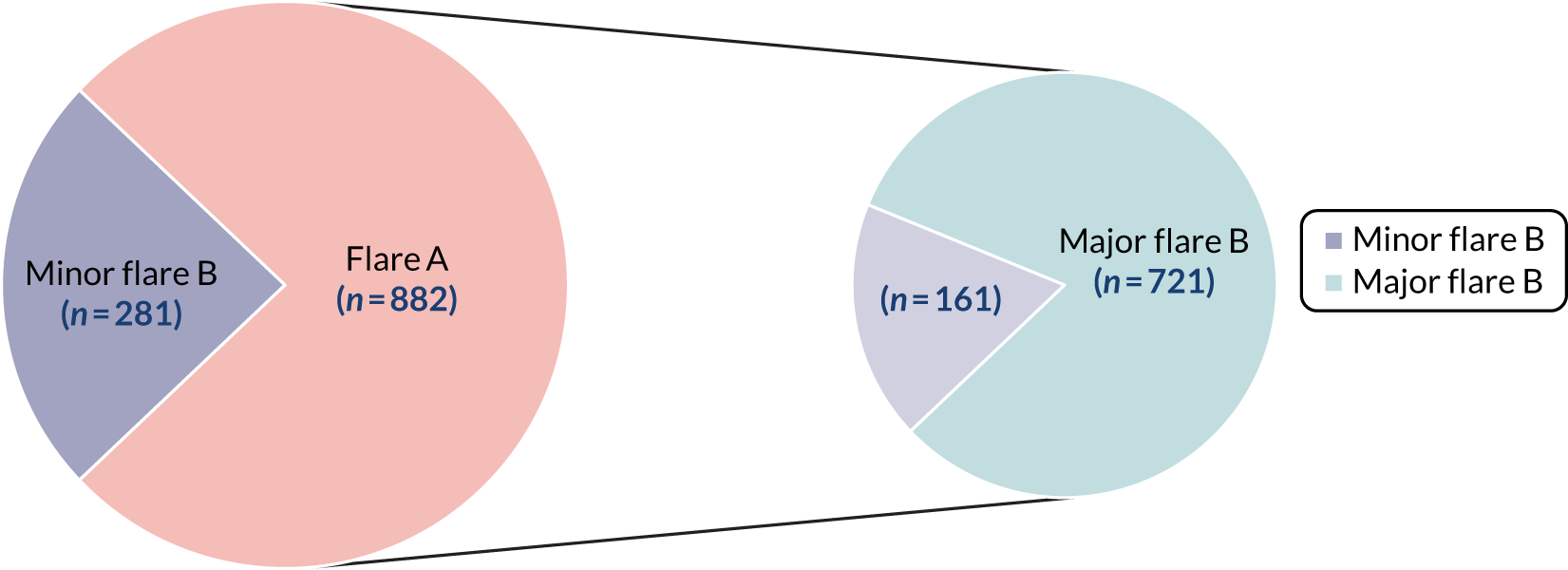
FIGURE 11.
The EQ-5D utility scores according to British (UK) and Swedish (SE) preference sets for patients with established rheumatoid arthritis treated with TNF-α inhibitors. Adapted from Gülfe et al. 109 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
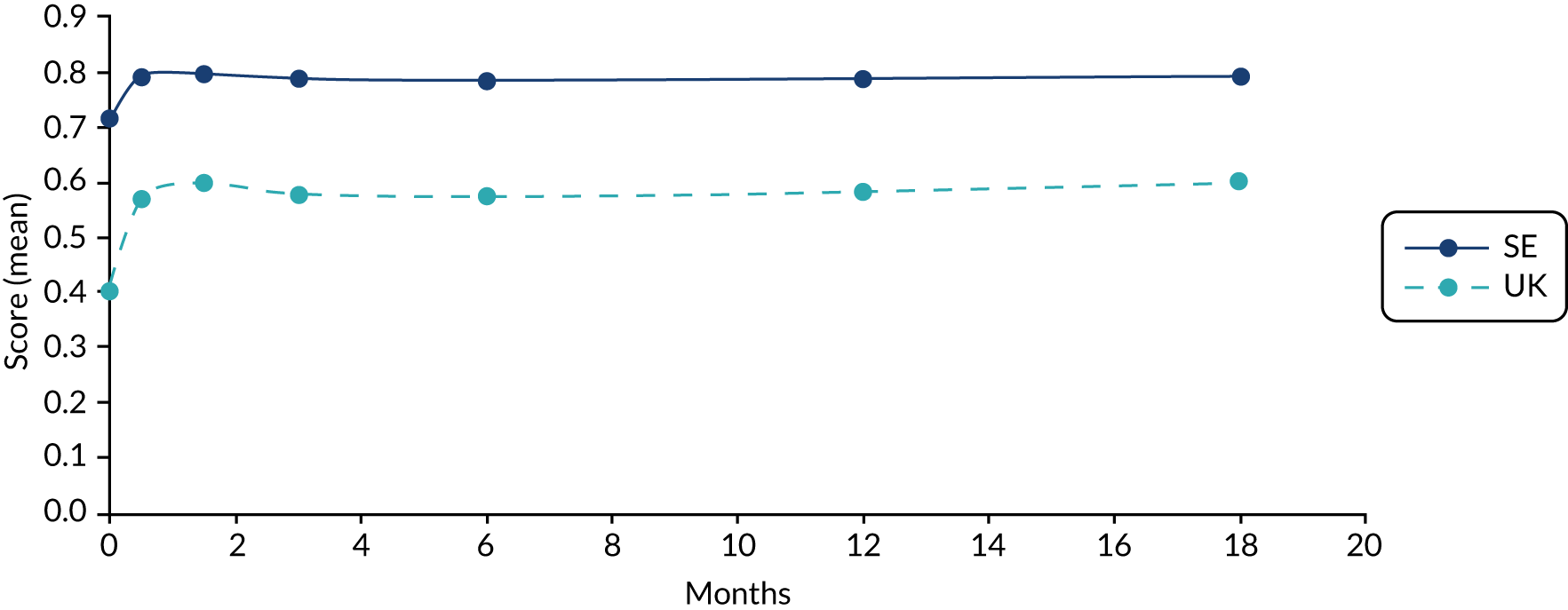
FIGURE 12.
The EQ-5D during the first course of treatment with TNF-α inhibitors in 2002–08 for people with RA, PsA, and SpA in Sweden. Adapted from Gülfe et al. 110
FIGURE 14.
The EQ-5D for people with RA (all participants vs. participants with complete data) during the first- and second-line treatment with anti-TNF-α therapies. Adapted from Gülfe et al. 110
Appendix 22 Consideration of l’Ami et al.114
This addendum was produced in response to a request from the NICE technical team for an exploratory analysis considering a scenario in which the drug dose in the standard care arm is not reduced [or reduced less than in the intervention (TDM) arm]. This was requested because, during scoping for the appraisal, the stakeholders indicated that dose reductions are currently not part of routine care in large parts of the UK. The NICE technical team requested that the EAG considered using data from l’Ami et al. 114
The study was identified in the searches for the clinical effectiveness systematic review but did not meet the inclusion criteria specified in the protocol, and was excluded on comparator because the physicians in the control arm had knowledge of drug and anti-drug antibody levels to make their judgements.
The EAG reviewed the study by l’Ami et al. 114 to assess whether or not the requested analysis could be conducted based on data reported in this source. Although demonstrating the potential benefit of TDM, the study assessed the concentration–response relationship. The intervention described in the study was ADL dose-interval prolongation and not TDM. The EAG considered there to be limited value in conducting a sensitivity analysis using the data from l’Ami et al. 114 owing to uncertainty. This was a result of the following factors: the median ADL dose at week 28 being comparable with that at baseline in both groups, and the small sample size (of approximately 50 participants).
A brief summary of the study by l’Ami et al. 114 is presented below for information.
Study characteristics
L’Ami et al. 114 reported clinical outcomes of a 28-week, open-label, randomised, parallel-group, non-inferiority trial that was performed in the Netherlands. Trough serum concentrations of ADL were determined in people with RA who had been treated with 40 mg of ADL every other week for at least 28 weeks and were not indicated for adjustment of ADL treatment, discontinuation or a scheduled surgery in the next 6 months (factors indicative of a population stable on treatment). Participants were randomly assigned (1 : 1) to 40 mg of ADL every 3 weeks (prolongation group) or to 40 mg of ADL every 2 weeks (continuation group). The study population was followed up for 28 weeks. The primary outcome was the change in DAS28-ESR after 28 weeks. A change in DAS28 of ≥ 0.6 was considered clinically relevant. Clinical and laboratory assessments were scheduled at baseline and at 12 and 28 weeks (visits 1, 2 and 3, respectively) and AEs were monitored during follow-up. Trough serum concentrations of ADL measured by ELISA were previously described in Pouw et al. 119 The study was partially funded by the Dutch Arthritis Foundation (Amsterdam, the Netherlands).
Baseline characteristics of patients
In total, 147 participants were screened and 55 (37%) had an ADL concentration > 8 µg/ml. Of the 55 participants, 54 were randomised and 53 completed follow-up. 114 The majority of participants were female (93% and 96% in the prolongation and continuation groups, respectively). The mean age of study participants was 60 years in the prolongation group and 58 years in the continuation group, and the median disease duration was 11 years in both groups. Concomitant treatment included methotrexate in most cases (> 90%) and prednisolone in some cases. The mean baseline DAS28-ESR was 2.0 (SD ± 0.8) and 1.6 (SD ± 0.7) in the prolongation group and continuation group, respectively. Median treatment duration with ADL was 6 years and 5.5 years in the prolongation group and the continuation group, respectively. Mean DAS-28 ESR score at baseline was < 2.6, which denoted disease remission. 32,33,114 Participant baseline characteristics are summarised in Table 65.
| Characteristic | Prolongation groupa | Continuation groupb |
|---|---|---|
| N | 27 | 27 |
| Age (years), mean (±SD) | 60 (± 10) | 58 (± 13) |
| Female, n (%) | 25 (93) | 26 (96) |
| BMI (kg/m2), mean (± SD) | 24.8 (± 5.0) | 23.8 (± 4.3) |
| Prior biologic, n (%) | 4 (15) | 3 (11) |
| ADL treatment duration (years), median (IQR) | 6.0 (2.9–8.0) | 5.5 (1.8–8.3) |
| MTX use, n (%) | 26 (96) | 25 (93) |
| MTX dose (mg per week), median (IQR) | 20 (15–21) | 15 (10–20) |
| Disease duration (years), median (IQR) | 11 (8–18) | 11 (6–19) |
| DAS28-ESR, mean (± SD) | 2.0 (± 0.8) | 1.6 (± 0.7) |
Disease Activity Score in 28 joints
Mean DAS28 at baseline was 2.0 (SD ± 0.8) and 1.6 (SD ± 0.7) in the prolongation group and the continuation group, respectively. The difference between groups was calculated as –0.400 (95% CI –0.811 to 0.010; p = 0.056). The mean change in DAS28 after 28 weeks was –0.14 (SD ± 0.61) in the interval prolongation group and 0.30 (± 0.52) in the continuation group. The difference in mean change in DAS28 was 0.44 (95% CI 0.12 to 0.76; p = 0.01) in favour of the prolongation group. Seven patients (26%) in the prolongation group and 10 patients (37%) in the continuation group had an increase in DAS28 of ≥ 0.6 points after 28 weeks (p = 0.56). 114 Summary results are presented in Table 66.
| Outcome | Prolongation groupa | Continuation groupb | Mean difference (95% CI) prolongation groupa vs. continuation groupb | ||
|---|---|---|---|---|---|
| Baseline | Week 28 | Baseline | Week 28 | ||
| DAS28, mean (±SD) | 2.0 (± 0.8) (n = 27) | 1.9 (± 0.7) (n = 27) | 1.6 (± 0.7) (n = 27) | 2.0 (± 0.9) (n = 27) | |
| DAS28 difference: baseline vs. week 28, mean (± SD) | –0.14 (± 0.61) (n = 27) | 0.30 (± 0.52) (n = 24c) | 0.44 (–0.76 to –0.12); p = 0.01 | ||
| ADL concentration (µg/ml), mean ± SD | 10.6 ± 2.5 (n = 26) | 6.6 ± 2.0 (n = 26) | 10.4 ± 2.4 (n = 23) | 9.3 ± 3.0 (n = 23) | 2.6 (1.2 to 4.1); p = 0.001 |
Adalimumab concentrations
In both groups, mean ADL concentration decreased (see Table 66); the mean difference between the groups at week 28 was 2.6 µg/ml (95% CI 1.2 µg/ml to 4.1 µg/ml; p = 0.001). In the prolongation group, the concentration decreased below 5 µg/ml over 28 weeks’ follow-up in seven participants; DAS28 increased by ≥ 0.6 in one participant, in whom, as a result, the dose of ADL was changed back to the standard dose. 114
Adverse events
A total of 16 AEs were reported: two in the prolongation group and 14 in the continuation group. Respiratory tract infections were the most common AE (occurring in three participants in the continuation group and two participants in the prolongation group). No SAEs were reported. 114
Study conclusions
The authors concluded that in people with RA treated with ADL in whom trough serum concentrations are > 8 µg/ml, the dosing interval can be increased to once every 3 weeks without an increase in disease activity. In most participants, the ADL concentration remained above 5 µg/ml (the concentration needed to block TNF-α). In the few patients in whom ADL concentrations decreased slightly below this level, it had no clinical consequences in the 28 weeks thereafter. 114
Appendix 23 Sensitivity analyses for scenario 2 based on Arango et al.43 and additional information provided by Grifols–Progenika
Grifols provided additional evidence on the average number of days in remission for the same follow-up period as in Arango et al. ,43 that is the follow-up of 530.8 days and 544.6 days for the intervention and control arms, respectively (Table 67).
| Group | Mean | N | SD | Sum | Median |
|---|---|---|---|---|---|
| Control | 360.00 | 52 | 226.181 | 18,720 | 401.00 |
| Intervention | 362.22 | 98 | 213.997 | 35,498 | 437.50 |
| Total | 361.45 | 150 | 217.542 | 54,218 | 431.00 |
A scenario analysis was conducted using these data and applying the relevant health state utility values and disease management costs for the remission and LDA/active disease health states.
Adalimumab and Promonitor: threshold analyses
The results of the threshold analyses are presented in Table 68.
| ICER threshold | Threshold value for annual acquisition cost of ADL of | |
|---|---|---|
| £1000 | £9187 | |
| £20,000 | £107 | £153 |
| £30,000 | £118 | £164 |
Using the new data provided by the manufacturer (see Table 67) and the list price of the originator ADL (Humira), testing would need to be cheaper than £153 and £164 per patient-year in order for TDM to be judged as cost-effective at the WTP thresholds of £20,000 and £30,000 per QALY gained, respectively. For the annual treatment cost of £1000 per patient, the corresponding threshold costs were £107 and £118 per patient-year.
Adalimumab and Promonitor: a cost–utility analysis
Cost-effectiveness outcomes for intervention versus SOC are shown in Table 69.
| Intervention | Control | Intervention vs. control | |
|---|---|---|---|
| Costs (£) | |||
| Drug acquisition | 13,075 | 13,149 | –74 |
| Drug administration | 0 | 0 | 0 |
| Drug wastage | 527 | 530 | –3 |
| Cost of managing health states | 1635 | 1639 | –4 |
| Cost of flare management | 303 | 418 | –115 |
| Cost of managing AEs | 69 | 70 | 0 |
| Cost of phlebotomy appointment | 162 | 0 | 162 |
| Other costs of testing | 45 | 0 | 45 |
| Cost of sample transport | 6 | 0 | 6 |
| Total costs | 15,822 | 15,805 | 17 |
| QALYs | |||
| Remission | 0.712 | 0.708 | 0.004 |
| LDA/active disease | 0.284 | 0.287 | –0.003 |
| Flares | –0.002 | –0.003 | 0.001 |
| AEs | –0.001 | –0.001 | 0.000 |
| Total QALYs | 0.993 | 0.992 | 0.002 |
| ICER | 10,453 | ||
The ICER was estimated at £10,453 per QALY gained, with an incremental cost of £17 and an incremental QALY gain of 0.002. In this additional analysis, the cost-effectiveness remains considerably uncertain. The results are based on very small differences in outcomes (incremental QALY gain of < 0.01).
Appendix 24 Exploratory analyses using the INGEBIO full study report
The analyses reported in this appendix were conducted in response to a request from the NICE technical team for an exploratory analysis considering additional evidence (the INGEBIO full study report) submitted by the manufacturer of Promonitor test kits (Grifols), and comments received from the NICE committee members and the company.
In Clinical effectiveness evidence, additional evidence from the INGEBIO full study report and the manuscript by Pascual-Salcedo et al. 120 are presented. In Additional analyses conducted by the External Assessment Group, additional analyses conducted by the EAG are described:
-
The cost–utility analysis carried out by Grifols was replicated using costs relevant to the NHS (Cost–utility analysis from the INGEBIO full study report adapted to the UK setting).
-
The original EAG’s model was updated using evidence from the INGEBIO full study report (Amended External Assessment Group’s model).
This paper was not identified by our searches as the journal was not indexed.
Clinical effectiveness evidence
Additional data from the INGEBIO full report
Objective
To evaluate whether or not the difference in the cumulative incidence of persistent disease flares with a duration of > 3 months between the Promonitor test group and the standard care group does not exceed the non-inferiority margin of 20% after 18 months of treatment.
Results
Relative risk = (confidential information has been removed).
| Groups | Outcome | Total | |
|---|---|---|---|
| Persistent flare | No persistent flare | ||
| Intervention | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Control | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Total | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
Conclusion
Confidential information has been removed.
Additional data from Pascual-Salcedo et al.120
Objective
To assess whether or not the clinical activity remains stable after dose tapering of TNF-α inhibitors in patients with low disease activity.
Design
Observational study.
The following were key differences between the paper by Pascual-Salcedo et al. 120 and the abstract by Pascual-Salcedo et al. :44
-
The first period reported in the paper by Pascual-Salcedo et al. 120 was 2007–9. However, the first period reported in the abstract by Pascual-Salcedo et al. 44 was 2006–9.
-
The paper by Pascual-Salcedo et al. 120 reported that their analyses included those patients who received dose-tapering during the second period. However, this was not clearly stated in the abstract by Pascual-Salcedo et al. 44
-
The number of patients included in the paper by Pascual-Salcedo et al. 120 was 77 patients (36 RA patients and 41 SpA patients). However, the number of patients included in the abstract by Pascual-Salcedo et al. 44 was 88 patients (43 RA patients and 45 SpA patients).
Comparison
Standard care (first period) versus therapeutic drug monitoring (TDM) and dose-reduction (second period).
The results are shown in Table 71.
| Outcome | First period (2007–9) | Second period (2010–12) | p-value |
|---|---|---|---|
| DAS28 (n = 36 RA patients), mean (± SD) | 2.28 (± 0.47) | 2.37 (± 0.50) | 0.20 |
| Serum trough drug level | |||
| IFX (n = 29), mean (± SD) | 3.2 (± 2.5) µg/ml | 1.8 (± 1.5) µg/ml | < 0.0001 |
| ADA (n = 27), mean (± SD) | 5.5 (± 2.8) µg/ml | 3.1 (± 2.1) µg/ml | < 0.0001 |
| ETN (n = 21), mean (± SD) | 1.8 (± 1.1) µg/ml | 1.3 (± 0.8) µg/ml | < 0.05 |
| Interval of drug administration | |||
| IFX (n = 29), mean (± SD) | 8.7 (± 1.4) weeks | 9.85 (± 1.5) weeks | < 0.001 |
| ADL (n = 27), mean (± SD) | 2.3 (± 0.63) weeks | 3.1 (± 1.02) weeks | < 0.0001 |
| ETN (n = 21), mean (± SD) | 1.4 (± 0.56) weeks | 2.16 (± 1.57) weeks | < 0.05 |
Additional analyses conducted by the External Assessment Group
Cost–utility analysis from the INGEBIO full study report adapted to the UK setting
Assumptions
Costs
The additional analysis based on the INGEBIO full study report included the same cost components as the company’s analysis. Those were:
-
drug acquisition costs
-
the costs of hospital admissions and visits to specialists
-
the cost of TDM and other (non-TDM) testing
Of note, the company did not take into consideration the cost of surgery for RA; however, based on the clinical evidence from the INGEBIO study report, these costs were similar in the intervention and control arms and, therefore, were not included in the additional analysis conducted by the EAG.
We derived the incremental drug acquisition cost in GBP (estimated as the cost of treatment in the intervention arm minus the cost in the control arm) using the formula:
The mean incremental cost of treatment was (confidential information has been removed) per person per 18-month follow-up period (INGEBIO report). However, the company wrote that the difference was significantly lower in patients with RA: it was (confidential information has been removed) per person per 18-month follow-up. We examined the effect of this on the cost-effectiveness outcomes in sensitivity analyses (see Table 74).
The cost of a 40 mg/ml vial of ADL in the company’s analysis was (confidential information has been removed); the corresponding cost in GBP obtained from the BNF was £352.14 per vial. 21
The costs of hospital admissions and visits to specialists, and the costs of other (non-TDM) tests were estimated from the frequency of resource use in the INGEBIO study and the NHS Reference Costs (Table 72). 84
| Resource | Unit cost (£)a | Frequency of resource useb | |
|---|---|---|---|
| Intervention | Control | ||
| Inpatient day (HD23 J) | 413 | Confidential information has been removed | Confidential information has been removed |
| Outpatient attendance rheumatology | 146 | Confidential information has been removed | Confidential information has been removed |
The cost of non-TDM testing was (confidential information has been removed), with the incremental cost of (confidential information has been removed) per 18 months (INGEBIO full study report).
The costs of visits to the intensive care unit were (confidential information has been removed).
The other assumptions in the EAG’s analysis were as follows:
-
The frequency of drug-level testing was (confidential information has been removed) tests per patient per year [based on the mean number of (confidential information has been removed)] tests per follow-up period of 18 months (INGEBIO report).
-
The costs of the test kits were as provided by the manufacturer (see Table 59).
-
The other costs of TDM testing were as in Jani et al. 37 (see Table 60).
-
Reflex testing of drug and antibody levels (singlet dilution) was carried out at a UK laboratory and (confidential information has been removed)% of patients would need to undergo antibody testing (as in the INGEBIO report).
-
An initial phlebotomy appointment (for collection of a trough sample for drug-level testing) was required, as in Jani et al. 37
-
The cost of administration of ADL was zero.
-
Treatment wastage was assumed.
Quality-adjusted life-years
The EQ-5L-5D and QALY estimates provided in the INGEBIO full study report are confidential.
Given that (1) the intervention group had a (confidential information has been removed) when compared with the control group, (2) patients from both groups had (confidential information has been removed) and (3) this study had a relatively small patient population (which might explain, at least partially, the irregular variation of the utility values over the follow-up period as shown on p. 46 of the INGEBIO full study report), the EAG believes that the actual incremental QALYs are likely to be lower than the company’s estimate.
The incremental QALYs, estimated by Grifols using a Spanish utility tariff, was assumed in the EAG’s additional analysis, which is a limitation of this analysis.
Results
The outcomes of the base-case analysis are given in Table 73.
| Intervention group | Control group | Intervention vs. control | |
|---|---|---|---|
| QALYs (per 18 months)a | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Acquisition costs | Confidential information has been removed | ||
| Other costs | £1643 | £906 | £737 |
| Total costs (per 18 months) | –£386 | ||
| ICER (cost per QALY gained) | Intervention dominant | ||
The mean incremental cost over the 18-month period was –£386 (which corresponds to –£257 per year) and, therefore, the intervention dominated standard care.
When the incremental cost of drug acquisition estimated by the company for the RA patient subpopulation (confidential information has been removed per person per 18-month follow-up) was assumed, the mean incremental cost per 18 months was £419 (£280 per year), and the ICER was about £5000 per QALY gained. The outcomes of sensitivity analyses are provided in Table 74.
| Sensitivity analysis | Overall patient population (£) | Subpopulation of RA patientsa (£) | ||
|---|---|---|---|---|
| ΔCosts | ICER | ΔCosts | ICER | |
| Frequency of testing (tests per year) | ||||
| 1 | –624 | TDM dominant | –87 | TDM dominant |
| 2 | –491 | TDM dominant | 46 | 800 |
| Duplicate concurrent testing with phlebotomy appointment | –159 | TDM dominant | 378 | 6629 |
| Duplicate reflex testing with phlebotomy appointment, 35.8% of patients with LDL | –199 | TDM dominant | 337 | 5919 |
| Duplicate reflex testing with phlebotomy appointment, (confidential information has been removed)% of patients with LDL | –225 | TDM dominant | 316 | 5542 |
| Single reflex testing with phlebotomy appointment, 35.8% of patients with LDL | –244 | TDM dominant | 293 | 5140 |
| Single reflex testing with phlebotomy appointment, (confidential information has been removed)% of patients with LDL | –257 | TDM dominant | 280 | 4904 |
| Single reflex testing without phlebotomy appointment, (confidential information has been removed)% of patients with LDL | –663 | TDM dominant | –126 | TDM dominant |
| Single reflex testing without phlebotomy appointment, 35.8% of patients with LDL | –649 | TDM dominant | –112 | TDM dominant |
| Duplicate reflex testing without phlebotomy appointment, (confidential information has been removed)% of patients with LDL | –626 | TDM dominant | –90 | TDM dominant |
| Single concurrent testing without phlebotomy appointment | –630 | TDM dominant | –93 | TDM dominant |
| Duplicate reflex testing without phlebotomy appointment, 35.8% of patients with LDL | –605 | TDM dominant | –68 | TDM dominant |
| Duplicate concurrent testing without phlebotomy appointment | –564 | TDM dominant | –28 | TDM dominant |
Amended External Assessment Group’s model
Assumptions
Assumptions from the company’s analysis included in the INGEBIO full report and those used by the EAG in the original cost–utility analyses are reported in Table 75; assumptions in the amended model are shown in bold.
| Assumption | INGEBIO full study report | EAG’s original analyses | ||||
|---|---|---|---|---|---|---|
| Ucar et al.42 | Arango et al.43 | |||||
| Intervention (n = 97) | Control (n = 52) | Intervention (n = 109) | Control (n = 60) | Intervention (n = 98) | Control (n = 52) | |
| Duration of follow-up (days) | 530.8 | 544.6 | 499 | 505 | 530.8 | 544.6 |
| Duration of remission (days) | 362.2 | 360 | 344 | 329 | N/A | N/A |
| Time to the first flare (days) | Confidential information has been removed | Confidential information has been removed | 208.07 | 189.32 | 208.07 | 189.32 |
| The rate of flares per patient per year | Confidential information has been removed | Confidential information has been removed | 0.463 | 0.639 | 0.463 | 0.639 |
| Number of tests (per year) | Confidential information has been removed | N/A | 1 | N/A | 1 | N/A |
| Utilities | Estimated from EQ-5D-5L data using the Spanish tariff | Estimated by mapping HAQ scores to EQ-5D-3L using UK tariff | ||||
| Initial phlebotomy appointment | Not costed | Costed as in Jani et al. 37 | ||||
| Single or duplicate | Not stated but likely single (given test kit price) | Single | ||||
| Concurrent or reflex | Reflex assuming (confidential information has been removed)% of patients with LDL | Concurrent | ||||
| Wastage | Not modelled | £370 per person on full dose per year | ||||
| Flare type | Persistent flares (see Table 77) | Type A flares (see Table 77) | ||||
| Flare duration | 3 months a | 7 days | ||||
| Tapering dose | NR | Two-thirds of the full dose | ||||
| % of flared patients in whom full ADL dose is restored | NR | 100% | ||||
| Type of flare | DAS28 | ||
|---|---|---|---|
| Current | Previous | Increase | |
| EAG’s analysesa | |||
| Type A flare | > 2.4 | Any | ≥ 0.6 |
| Company’s analysis | |||
| Persistent flare | > 1.2 | ||
| or | |||
| ≥ 3.2 | > 0.6 | ||
Results of the cost–utility analyses
Results of the cost–utility analysis (using the EAG’s updated model) are shown in Table 77.
| Intervention arm | Control arm | Intervention vs. control | |
|---|---|---|---|
| Scenario 1 (with mean duration of remission): intervention – 362.2 days, control – 360 daysa | |||
| Total costs (mean) | £16,170 | £15,714 | £457 |
| QALYs (mean) | 0.972 | 0.963 | 0.009 |
| ICER (cost/QALY gained) | £51,929 | ||
| Scenario 2 (with mean duration of remission/LDA): intervention – (confidential information has been removed) days, control – (confidential information has been removed) daysa | |||
| Total costs (mean) | £16,316 | £15,839 | £477 |
| QALYs (mean) | 0.929 | 0.926 | 0.004 |
| ICER (cost/QALY gained) | £125,272 | ||
Cost-effectiveness results under different discounts for ADL (Humira) are given in Table 78.
| ADL discount (%) | Intervention arm (£) | Control arm (£) | ΔCosts (£) | ΔQALYs | ICER (£) |
|---|---|---|---|---|---|
| Scenario 1 with mean duration of remission: in the intervention group of 362.2 days and in the control group of 360 daysa | |||||
| 20% | 13,510 | 13,024 | 486 | 0.009 | 55,249 |
| 40% | 10,850 | 10,334 | 515 | 0.009 | 58,568 |
| 60% | 8189 | 7645 | 544 | 0.009 | 61,888 |
| 80% | 5529 | 4955 | 574 | 0.009 | 65,207 |
| Scenario 2 with mean duration of remission/LDA in the intervention group of (confidential information has been removed) days and in the control of (confidential information has been removed) daysa | |||||
| 20% | 13,655 | 13,149 | 506 | 0.004 | 132,942 |
| 40% | 10,995 | 10,460 | 535 | 0.004 | 140,613 |
| 60% | 8335 | 7770 | 564 | 0.004 | 148,283 |
| 80% | 5674 | 5080 | 594 | 0.004 | 155,954 |
The results of other sensitivity analyses are presented in Tables 79 and 80.
| Sensitivity analysis | Assumptions | Results | Source | ||
|---|---|---|---|---|---|
| ΔCosts (£) | ΔQALYs | ICER (£) | |||
| Impact of flares only (health states and AEs are not included) | Only flares contribute to incremental costs and QALYs | 461 | 0.008 | 58,452 | Scenario C in Gavan17 |
| Tapering strategy | Spacing ADL dose to 40 mg every 4 weeks | 384 | 0.009 | 43,631 | Appendix 13 |
| Treatment wastage | No wastage | 462 | 0.009 | 52,572 | Clinical advice |
| Proportion of flared patients in whom full dose is restored | 55% | 499 | 0.009 | 56,760 | Bykerk et al.70 |
| 0% | 551 | 0.009 | 62,665 | Assumption | |
| Frequency of testing (tests/year) | 1 | –94 | 0.009 | TDM dominant | Clinical advice |
| 2 | 106 | 0.009 | 12,035 | Clinical advice | |
| Duplicate concurrent testing with phlebotomy appointment | 604 | 0.009 | 68,693 | Clinical advice | |
| Duplicate reflex testing with phlebotomy appointment, 35.8% of patients with LDLb | 544 | 0.009 | 61,795 | Clinical advice | |
| Single reflex testing with phlebotomy appointment, 35.8% of patients with LDLb | 477 | 0.009 | 54,220 | Clinical advice | |
| Single reflex testing without phlebotomy appointment,c (confidential information has been removed) % of patients with LDL | –151 | 0.009 | TDM dominant | Clinical advice | |
| Single reflex testing without phlebotomy appointment,c 35.8% of patients with LDLb | –131 | 0.009 | TDM dominant | Clinical advice | |
| Duplicate reflex testing without phlebotomy appointment,c (confidential information has been removed)% of patients with LDL | –97 | 0.009 | TDM dominant | Clinical advice | |
| Single concurrent testing without phlebotomy appointmentc | –102 | 0.009 | TDM dominant | Clinical advice | |
| Duplicate reflex testing without phlebotomy appointment,c 35.8% of patients with LDLb | –65 | 0.009 | TDM dominant | Clinical advice | |
| Duplicate concurrent testing without phlebotomy appointmentc | –4 | 0.009 | TDM dominant | Clinical advice | |
| Sensitivity analysis | Assumptions | Results | Source | ||
|---|---|---|---|---|---|
| ΔCosts (£) | ΔQALYs | ICER (£) | |||
| Tapering strategy | Spacing ADL dose to 40 mg every 4 weeks | 404 | 0.004 | 106,095 | Exeter Biologic Clinic recommendations (see Appendix 13) |
| Treatment wastage | No wastage | 482 | 0.004 | 126,756 | Clinical advice |
| Proportion of flared patients in whom full dose is restored | 55% | 519 | 0.004 | 136,466 | Bykerk et al.70 and clinical advice |
| 0% | 571 | 0.004 | 150,161 | ||
| Frequency of testing (tests/year) | 1 | –73 | 0.004 | TDM dominant | Clinical advice |
| 2 | 126 | 0.004 | 33,082 | ||
| Duplicate concurrent testing with phlebotomy appointment | 624 | 0.004 | 164,009 | Clinical advice | |
| Duplicate reflex testing with phlebotomy appointment, 35.8% of patients with LDLb | 564 | 0.004 | 148,070 | Clinical advice | |
| Single reflex testing with phlebotomy appointment, 35.8% of patients with LDLb | 497 | 0.004 | 130,564 | Clinical advice | |
| Single reflex testing without phlebotomy appointment,c (confidential information has been removed)% of patients with LDL | –131 | 0.004 | TDM dominant | Clinical advice | |
| Single reflex testing without phlebotomy appointment,c 35.8% of patients with LDLb | –111 | 0.004 | TDM dominant | Clinical advice | |
| Duplicate reflex testing without phlebotomy appointment,c (confidential information has been removed)% of patients with LDL | –77 | 0.004 | TDM dominant | Clinical advice | |
| Single concurrent testing without phlebotomy appointmentc | –82 | 0.004 | TDM dominant | Clinical advice | |
| Duplicate reflex testing without phlebotomy appointment,c 35.8% of patients with LDLb | –45 | 0.004 | TDM dominant | Clinical advice | |
| Duplicate concurrent testing without phlebotomy appointmentc | 16 | 0.004 | 4230 | Clinical advice | |
Appendix 25 National Institute for Health and Care Excellence reference case
| Element of health technology assessment | Reference case | Met/not met | Notes |
|---|---|---|---|
| Defining the decision problem | The scope developed by NICE | Yes | |
| Comparator(s) | As listed in the scope developed by NICE | Yes | |
| Perspective on outcomes | All direct health effects, whether for patients or, when relevant, carers | Yes | |
| Perspective on costs | NHS and PSS | Yes | |
| Type of economic evaluation | Cost–utility analysis with fully incremental analysis | Yes | |
| Time horizon | Long enough to reflect all important differences in costs or outcomes between the technologies being compared | No | The time horizon was 18 months (owing to limitations in the clinical effectiveness evidence base) |
| Synthesis of evidence on health effects | Based on systematic review | No | The analyses were based on the only relevant head-to-head study identified in the systematic review |
| Measuring and valuing health effects | Health effects should be expressed in QALYs. The EQ-5D is the preferred measure of HRQoL in adults | Yes | |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | Yes | |
| Source of preference data for valuation of changes in HRQoL | Representative sample of the UK population | Yes | |
| Equity considerations | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes | |
| Evidence on resource use and costs | Costs should relate to NHS and PSS resources and should be valued using the prices relevant to the NHS and PSS | No | All costs, except the cost of managing flares, were relevant to the NHS. The cost of flare management was sources from a study conducted in France |
| Discounting | The same annual rate for both costs and health effects (currently 3.5%) | No | Discounting was not applied because of the 18-month time horizon adopted in this study |
Glossary
- Antibody
- Protein produced by B lymphocytes in response to a foreign molecule or invading organism (Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. 3rd edn. New York, NY: W. W. Norton & Company; 1994).
- Assay range
- The lowest and highest values within which an assay can detect and quantify the target entity. There will be evidence of acceptable reliability and validity of the test within this range. [Cox KL, Devanarayan V, Kriauciunas A, Manetta J, Montrose C, Sittampalam S. Immunoassay Methods. In Sittampalam GS, Grossman A, Brimacombe K, et al., editors. Assay Guidance Manual. Bethesda, MD: Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2019. URL: www.ncbi.nlm.nih.gov/books/NBK92434/ (accessed July 2020).]
- Bioequivalence
- In the case of two (or more) drugs with identical active ingredients, similar bioavailability, equivalent physiological activity and, thus, interchangeability. Biosimilar drugs demonstrate bioequivalence to an originator product. See Bioequivalence [World Health Organization. Key Resources. URL: https://extranet.who.int/prequal/content/bioequivalence-0 (accessed August 2019).]
- Biosimilar
- A biological medicine that is highly similar to another biological medicine already licensed for use. It is a biological medicine that has been shown not to have any clinically meaningful differences from the originator biological medicine in terms of quality, safety and efficacy [NHS England and NHS Improvement. What is a Biosimilar Medicine? URL: www.england.nhs.uk/publication/what-is-a-biosimilar-medicine/ (accessed August 2019).]
- Brand name
- The name given to a pharmaceutical product by the manufacturer; for example, Valium is the originator brand name (also called trade name) for diazepam. The use of this name is reserved exclusively to its owner, in contrast to the generic name, which is diazepam. Brand names may also be used for generic products: they are then often called ‘branded generics’. These brand names are different from innovator brand names. [World Health Organization. Generic medicines. WHO Drug Information 2016;30:370–5. URL: www.who.int/medicines/publications/druginformation/WHO_DI_30-3_GenericMedicines.pdf?ua=1 (accessed August 2019).]
List of abbreviations
- ACR
- American College of Rheumatology
- ADL
- adalimumab
- AE
- adverse event
- AS
- ankylosing spondylitis
- AU
- arbitrary unit
- BASDAI
- Bath Ankylosing Spondylitis Disease Activity Index
- bDMARD
- biologic disease-modifying antirheumatic drug
- BNF
- British National Formulary
- BRASS
- Brigham Rheumatoid Arthritis Sequential Study
- BSRBR-RA
- British Society for Rheumatology Biologics Register in Rheumatoid Arthritis
- CCP
- cyclic citrullinated peptide
- CDAI
- Clinical Disease Activity Index
- cDMARD
- conventional disease-modifying antirheumatic drug
- CHEC
- Consensus on Health Economic Criteria
- CI
- confidence interval
- CrI
- credible interval
- CRP
- C-reactive protein
- CTZ
- certolizumab pegol
- DAS28
- Disease Activity Score in 28 joints
- DMARD
- disease-modifying antirheumatic drugs
- EAG
- External Assessment Group
- ELISA
- enzyme-linked immunosorbent assay
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ESR
- erythrocyte sedimentation rate
- ETN
- etanercept
- EULAR
- European League Against Rheumatism
- GBP
- Great British pounds
- GLM
- golimumab
- HAQ
- Health Assessment Questionnaire
- HCHS
- Hospital and Community Health Services
- HDA
- high disease activity
- HERC
- Health Economics Research Centre
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HSUV
- health state utility value
- HTA
- Health Technology Assessment
- HUD
- Health Utilities Database
- ICD-10
- International Classification of Diseases and Related Health Problems, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- IFX
- infliximab
- IQR
- interquartile range
- IRR
- incidence rate ratio
- ITT
- intention to treat
- LDA
- low disease activity
- LRTI
- lower respiratory tract infection
- MDA
- moderate disease activity
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NOAR
- Norfolk Arthritis Register
- NOR-DRUM
- Norwegian Drug Monitoring
- NRAS
- National Rheumatoid Arthritis Society
- OR
- odds ratio
- PPP
- purchasing power parity
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PsA
- psoriatic arthritis
- QALY
- quality-adjusted life-year
- RA
- rheumatoid arthritis
- RCT
- randomised controlled trial
- RF
- rheumatoid factor
- ROBINS-1
- Risk Of Bias In Non-randomised Studies - of Interventions
- SAE
- serious adverse event
- ScHARR
- School of Health and Related Research
- SD
- standard deviation
- SDAI
- Simplified Disease Activity Index
- sDMARD
- synthetic disease-modifying anti-rheumatic drug
- SOC
- standard of care
- SpA
- spondyloarthritis
- TDM
- therapeutic drug monitoring
- THR
- total hip replacement
- TKR
- total knee replacement
- TNF
- tumour necrosis factor
- URAC
- Utrecht Rheumatoid Arthritis Cohort
- VAS
- visual analogue scale
- WHO
- World Health Organization
- WTP
- willingness to pay
This monograph is based on the Diagnostic Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Diagnostic Advisory Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.