Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/36/02. The contractual start date was in December 2015. The draft report began editorial review in October 2018 and was accepted for publication in March 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Robinson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
There are 536,000 fragility fractures per year in the UK. 1 Reducing the burden of fragility fractures is, thus, a key health priority in the NHS. Osteoporosis is a silent disease of the skeleton that causes bone fragility and increases the risk of fracture.
Over one-quarter of people with osteoporosis have moderate or severe chronic kidney disease (CKD). 2 It is speculated that these numbers will increase as a result of improved detection and diagnosis, and sociodemographic changes. CKD stage is based on estimated glomerular filtration rate (eGFR), with eGFR values separated into six categories: stage 1 (normal), an eGFR of > 90 ml/minute/1.73m2; stage 2 (mild CKD), an eGFR of between 60 and 90 ml/minute/1.73m2; stage 3A (moderate CKD), an eGFR of between 45 and 60 ml/minute/1.73m2; stage 3B (moderate CKD), an eGFR of between 30 and 45 ml/minute/1.73m2; stage 4 (severe CKD), an eGFR of between 15 and 30 ml/minute/1.73m2; and stage 5 (end-stage CKD), an eGFR of < 15 ml/minute/1.73m2. Formulae to calculate the eGFR based on serum creatinine are increasingly popular. According to routinely collected data from biochemistry tests in NHS settings, the proportion of UK patients tested for serum creatinine for whom eGFR measurements were estimated increased by almost 30% between 2004 and 2009. This led to a dramatic rise in the number of patients diagnosed with CKD. 3
Chronic kidney disease has been shown to predict low bone mass, which is a proxy for bone fragility, due to accelerated bone loss. 4 It also predicts fracture risk, with a doubled risk in patients with stage 3A CKD,5 a 2.5- to threefold increased risk in patients with stage 3B CKD6 and a fourfold higher fracture incidence in patients with stage 4 CKD7 or in renal replacement therapy for end-stage renal failure. 8
Although effective therapies exist to reduce the risk of fracture in postmenopausal women and men with osteoporosis, the use of first-line anti-osteoporosis therapies (bisphosphonates) is restricted in patients with CKD. There are concerns that bisphosphonates raise the risk of worsening kidney function and other adverse events that are already increased in patients with CKD, such as severe hypocalcaemia,9 upper gastrointestinal events and acute kidney injury. As the biological mechanism by which CKD weakens bone differs from osteoporosis, it is uncertain whether or not bisphosphonates will have a similar beneficial effect in reducing fracture rates in CKD patients. 10 Efficacy data for bisphosphonates is scarce in CKD, as few patients with moderate or severe CKD were included in pivotal trials. For example, only 301 (of 4496) participants with an eGFR of < 30 ml/minute/1.73m2 (i.e. CKD stages 4 and 5) were recruited in the risedronate arms of nine randomised controlled trials (RCTs). 11 We have previously shown that the participants of these trials with CKD were healthier and had fewer comorbidities than patients with CKD in real-life settings. 12
About 40–45% of patients with end-stage renal disease,13,14 and an unknown proportion of those with stage 4 CKD, may suffer adynamic bone disease, which is a marked reduction in bone resorption activity. 15 As bisphosphonates are anti-resorptive therapies that also reduce this activity, bisphosphonate therapy in this setting could increase rather than decrease fracture risk. A recent systematic review of 13 trials and 9850 participants suggested that bisphosphonates could improve bone mineral density (BMD) as a proxy for bone strength in kidney transplant recipients, but their effects on fracture risk remained unclear. 16
Thus, despite good safety data from randomised trials of risedronate11 and alendronate,17 the National Institute for Health and Care Excellence (NICE) guidelines18,19 do not support the use of bisphosphonates in patients with eGFR under 35 (moderate to severe CKD). Drug regulators do not recommend alendronate20 for patients with an eGFR of < 35 ml/minute/1.732, or ibandronate21 or risedronate22 for patients with an eGFR of < 30 ml/minute/1.732, mainly because of a lack of evidence, rather than evidence demonstrating worse outcomes. 20 Alternative therapies, such as subcutaneous denosumab (Prolia®; Amgen Inc. Thousand Oaks, CA, USA), have been proposed and used in recent years, but reports of severe and even life-threatening hypocalcaemia after denosumab injection in patients with moderate to severe CKD23–26 have limited the use of this therapy in this population.
This patient group is thus left with a very high fracture risk that is effectively untreatable. The most recent (2017) Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines27 do not rule out the use of bisphosphonates in patients with moderate to severe CKD, despite acknowledging the limited evidence available, but ask clinicians to use bisphosphonates with caution. The decision to treat with bisphosphonates should be made taking into account potential CKD progression, the severity of any biochemical abnormalities, fracture risk, and bone and mineral disorders related to CKD. 28
As the use of bisphosphonates is not supported for patients with CKD, new and more expensive treatments may be used instead. However, many CKD patients who are at a high risk of fracture are not offered any treatment. Bisphosphonates were contraindicated in patients with an eGFR of < 30 ml/minute/1.732, based on the information from the relatively few patients with an eGFR of < 45 ml/minute/1.732 included in the pivotal RCTs of bisphosphonates, a lack of data on the safety of bisphosphonates for patients with stage 3B or higher CKD, and information on the adverse events induced by intravenous zoledronate. Zoledronate is the most powerful of all bisphosphonates and its pharmacokinetics means it reaches a much higher maximal concentration in the blood than other bisphosphonates. Safety data on zoledronate are, therefore, unlikely to be generalisable to other bisphosphonates.
There is an urgent need for data on the risks and benefits of bisphosphonates for patients with moderate to severe CKD. Before embarking on a RCT, which is the gold-standard design to answer these questions, it is prudent to fully explore existing resources, given the concerns of randomising patients to treatments that are, formally, not recommended, or that may even be contraindicated.
Aims and objectives
We planned this study to answer a commissioned call from the National Institute for Health Research (NIHR), with the aim to improve our understanding of the risks and benefits of bisphosphonate therapy in patients with stage 3B+ CKD (i.e. those with an eGFR of < 45 ml/minute/1.732). We proposed four work packages:
-
Work package (WP) 1 – to study the association between bisphosphonate use and CKD progression, defined as stage progression or the requirement of dialysis or transplantation (primary outcome). We also aimed to study the association between bisphosphonate use and the annualised rates of renal function loss, measured as the loss in eGFR over time (secondary outcome).
-
WP2 – to study the association between bisphosphonate use and fragility-related clinical (symptomatic) fractures.
-
WP3 – to study the association between bisphosphonate use and severe adverse events: hypocalcaemia or hypophosphataemia necessitating hospital admission, acute kidney injury necessitating hospital admission and upper gastrointestinal events necessitating hospitalisation or recorded in primary care records (symptomatic oesophagitis, ulcer, perforation or upper gastrointestinal bleeding).
-
WP4 – to study the association between bisphosphonate use and changes in BMD over time, measured as the annualised percentage change in BMD compared with baseline BMD. Change in femoral neck BMD was the primary outcome; changes in lumbar spine and total hip BMD were secondary outcomes.
Structure of the report
Chapter 2 describes the data sources, defines the main exposure, gives the operational definition used to identify bisphosphonate use and describes the statistical method of propensity score matching used to account for confounding by indication.
The specific statistical methods and results of each WP are reported in Chapter 3 (WP1), Chapter 4 (WP2), Chapter 5 (WP3) and Chapter 6 (WP4).
We synthesise and discuss our findings, discuss the strengths and limitations of the study and interpret the data in Chapter 7.
Chapter 2 Methods
This chapter describes the data sources used for this research: the UK Clinical Practice Research Datalink (CPRD) GOLD; this was linked to the Office for National Statistics (ONS) mortality data, the Index of Multiple Deprivation (IMD) and the English Hospital Episode Statistics (HES) for WPs 1–3 and to the Odense University Hospital Databases (OUHD) from Denmark for WP4. The study sample and target population are also defined in this chapter.
Data sources
Clinical Practice Research Datalink GOLD
The CPRD GOLD data were used to define the patients who were eligible for the study cohorts in WPs 1–3 and characterise them in detail for adjustments in further modelling of the proposed outcomes. CPRD GOLD contains anonymised, routinely collected electronic health records for a UK-representative sample of > 11 million current and historic patients registered in 674 participating practices that use the Vision GP electronic medical records software (In Practice Systems Ltd, London, UK). 29
The CPRD GOLD covers all four countries in the UK: England, Northern Ireland, Scotland and Wales. However, this study was restricted to patients registered in 375 English practices, representing 58% of the CPRD GOLD practices, for whom linked patient data collected in other data sets were also available. 29
Data were linked to hospital admission data in HES, mortality data from the ONS and deprivation area codes based on the IMD. These data sets are provided by CPRD fully anonymised and linkable to each other through a patient identification. For HES and ONS data, CPRD also produces a match rank value that evaluates the likelihood of linkage, based on an eight-step algorithm. Although CPRD provides data for patients with a match rank of between 1 and 5, we included only links with a match rank of 1 or 2 to improve data quality. CPRD also provides a HES general patient identification to identify and track individuals, as they may change practice during their lifetime.
Electronic health records in CPRD GOLD comprise clinical, referral and immunisation events; biochemistry results; and prescription records from primary care:
-
General practitioners (GPs) and primary care health professionals use the hierarchical coding system of Read Codes, initially developed by GP Dr James Read in the early 1980s. 30 Read Codes are used to record clinical, referral, test and immunisation events in primary care electronic medical records. Read Codes have been revised and expanded several times, but maintain their original hierarchical structure. Each character represents a more detailed, granular description of a clinical term, event or measurement. Read Codes are mapped individually to medCODES (Medical CRF Online Documentation and Evaluation System) in CPRD GOLD.
-
Biochemistry results are reported in CPRD GOLD with associated Read Codes or medCODES and/or with CPRD-specific entity types.
-
Prescription information in CPRD GOLD includes the product name, an associated product number (prodcode), the pharmaceutical substance, its strength and its British National Formulary code. Quantity and dosage are often incompletely recorded in CPRD GOLD as they are not mandatory fields in the Vision software. A CPRD-developed algorithm was used to calculate the numeric daily dose for each prescription in this study.
Hospital Episode Statistics
The HES data contain administrative and clinical details recorded for each hospital admission episode in England, including NHS hospitals and those in the independent sector that provide services commissioned by the NHS. At the time of writing, HES included admitted patient care data from 1997, outpatient data from 2003, accident and emergency data from 2007, diagnostic imaging data from 2012, and patient-reported outcome measures from 2009.
All data recorded in HES are submitted by contributing hospitals for reimbursement purposes. HES is an administrative data set, but has been used extensively for research purposes in recent years, including previous NIHR-funded work31 resulting in high-impact publications. 32 Validation studies have demonstrated that the health outcomes recorded in HES have high validity, including two of our chosen study outcomes: acute kidney injury and end-stage renal disease. 33,34
For each hospital admission recorded in HES, information is available on hospital diagnoses and procedures, administrative details (e.g. date of admission and discharge) and basic sociodemographic details (e.g. the region where the practice is located and the patient’s ethnic background). We used the admitted patient care records in HES, as these inpatient records had sufficient clinical detail for our study. They contain coded information on the primary reason for admission, secondary or concomitant diagnoses (comorbidity) and the procedure undertaken. The primary diagnosis and comorbidities are recorded using the International Classification of Diseases, Tenth Edition (ICD-10). 35 Inpatient procedures are coded using the Office of Population Censuses and Surveys – Classification of Interventions and Procedures version 4 (OPCS-4). 36
Odense University Hospital Databases
We used OUHD for WP4 because there is no equivalent data framework in the UK. Some existing UK national data sources have incomplete and non-comprehensive BMD codification, such as CPRD GOLD. Others, such as existing nationally representative cohorts like the Hertfordshire and Chingford cohorts, are underpowered for studying bisphosphonate use in a relatively rare group of patients (here, those with a diagnosis of CKD) for whom the drug is to be used with caution.
The OUHD holds BMD data measured using dual-energy X-ray absorptiometry (DXA) for the whole of the Funen region, the second largest island of Denmark. It includes 36,024 individuals examined between 1990 and 2015. The Odense University Hospital performs a standard panel of blood tests during routine assessment for osteoporosis that includes serum creatinine, from which eGFR can be calculated. This panel is also performed by the local general practices in the region. The general practices and Odense University Hospital use the same clinical biochemistry laboratory. Biochemistry values (including serum creatinine tests) and pharmacy drug dispensations can be tracked back to 1995.
Pharmacy dispensations are recorded using Anatomic Therapeutic Classification codes, and ICD-10 codes are used for diagnoses. The Odense Patient Data Exploratory Network provides a unique platform for linking clinical and biochemistry data and is an approved Statistics Denmark institutional partner for linking to national data on pharmacy dispensations and comorbid conditions. The facility has previously been used to link clinical biochemistry to fracture outcomes in the context of thyroid status and fractures. 37 Ad hoc extraction and linking to renal function from the Odense University Hospital clinical biochemistry database were done as part of this study using a similar approach.
In OUHD, > 10,000 patients, 30% of those examined, were recommended osteoporosis treatment. Alendronate is the first-line osteoporosis treatment in this setting. In Denmark, reimbursement for alendronate and other bisphosphonates requires a DXA assessment or a radiograph-verified spine or hip fracture.
UK Renal Registry
In our initial protocol (version 1.0), we planned to use the UK Renal Registry (UKRR) to obtain detailed information on relevant aspects of renal disease and treatment for end-stage renal disease. The UKRR collects data from the specialised treatment centres that treat most patients with end-stage renal disease.
The advantages of linking UKRR to the readily available CPRD GOLD–HES data set were obvious:
-
Access to this external data set would allow us to validate the diagnosis of end-stage renal disease and initiation of renal replacement therapies recorded in primary (CPRD GOLD) and secondary (HES) care records.
-
The UKRR contains more detailed and probably higher-quality information on dates and types of renal replacement therapies offered than CPRD GOLD–HES.
However, despite the early and full involvement of the UKRR in this study, we encountered difficulties that made it impossible to obtain approval for linkage by NHS Digital as a trusted third party to finalise this project on time. Nine months passed between the submission of our first application for linkage to NHS Digital and our decision to abandon this approach.
The steps followed and the difficulties encountered were as follows:
-
After some discussion, it was deemed necessary to apply for approval from the Confidentiality Advisory Group (CAG) and the Health Research Authority, through a Research Ethics Committee. We applied for both and were approved in March 2017 (reference numbers 17/CAG/0032 and 17/SC/0073).
-
We then needed to submit a Data Access Request Service (DARS) application to the Independent Group Advising on the Release of Data (IGARD) committee. CPRD offered to lead the application process and prepare the first draft as they had more experience, although the University of Oxford was the only data controller.
-
In August 2017 the first application was submitted. The lead applicant was changed from CPRD to the principal investigator of the study, as the CPRD information governance team had limited time available to track every step of the application. In November 2017, CPRD was added to the application as data controllers and the lead applicant changed back to the CPRD information governance team.
-
Once CPRD became the lead applicant, conversations and any modifications to the DARS application were in the hands of the CPRD and NHS Digital teams. It was not clear to us from this point onwards what other steps were needed to help progress the application, except for a CAG application from CPRD. The barriers were also unclear. We offered our assistance, but were assured that no other contributions were needed from us for the rest of the process.
-
The DARS application was deemed ready for submission by NHS Digital in May 2018. However, the implementation of the new European General Data Protection Regulation (GDPR)38 on 25 May 2018 required modifications to the application, causing delays. IGARD reviewed our DARS application and requested further amendments from CPRD and amendments to our fair processing notice to account for the new GDPR regulations.
-
We were informed by NHS Digital that they would prioritise our application and aim to obtain the UKRR data by the end of September 2018. We thus asked for a second extension to our contract with the NIHR Health Technology Assessment (HTA) programme in August 2018. However, we were then informed that a new version of the data-sharing framework contract was needed between the University of Oxford and NHS Digital before we could receive the linked data. Although our legal team tried to speed up the process, we felt that there was insufficient time to finalise this new contract and obtain the data by the end of September 2018.
For these reasons and to minimise an unnecessary delay in reporting our findings, we thus withdrew our extension submission and DARS application. In this report, as agreed with the HTA programme, we conducted all analyses using CPRD GOLD with linked HES data only. Data were extracted from the CPRD GOLD data set’s March 2016 release, with linkage to HES, ONS and IMD using linkage set 11.
Target population and statistical analyses
The target population of this study was patients with an eGFR of < 45 ml/minute/1.73 m2 who were aged ≥ 40 years when biochemistry testing was done, in the period 1997–2014. The date of the first eGFR measurement of < 45 ml/minute/1.73 m2 in primary care (CPRD GOLD) was defined as the baseline date for all patients.
Patients were excluded if any of the following criteria applied:
-
CPRD GOLD participants with no possible linkage to HES.
-
Less than 1 year of run-in up-to-standard data available in CPRD GOLD before the index or baseline date (i.e. eGFR or creatinine measurement).
-
Use of any anti-osteoporosis medications in the year before index (except calcium and/or vitamin D supplements).
-
Use of any anti-osteoporosis medications other than bisphosphonates at any point (except calcium and/or vitamin D supplements; see Report Supplementary Material 3 for the code list).
We needed at least 1 year of CPRD GOLD data before baseline dates to ascertain whether or not an exclusion criterion applied to a patient. We also used this information in our propensity score modelling to minimise confounding by indication [e.g. age, sex, fracture history and body mass index (BMI)] by adjusting.
Main exposure: bisphosphonate use
Users of bisphosphonates were identified from primary care prescription records in CPRD for WPs 1–3 and from pharmacy dispensations data in the Danish Prescription Registers, linked as part of OUHD, for WP4. We included all bisphosphonates prescribed in primary care in the study period, of which alendronate, risedronate and ibandronate were the most common. Report Supplementary Material 1 lists the product names, corresponding British National Formulary codes and equivalent Prodcodes.
The data sources included information on drug prescriptions (CPRD GOLD) and dispensing (OUHD) instead of drug consumption. A prescription’s duration does not always reflect the true number of days over which the prescribed drug was used. Assumptions were made to account for non-adherence and non-compliance to define episodes of continuous exposure.
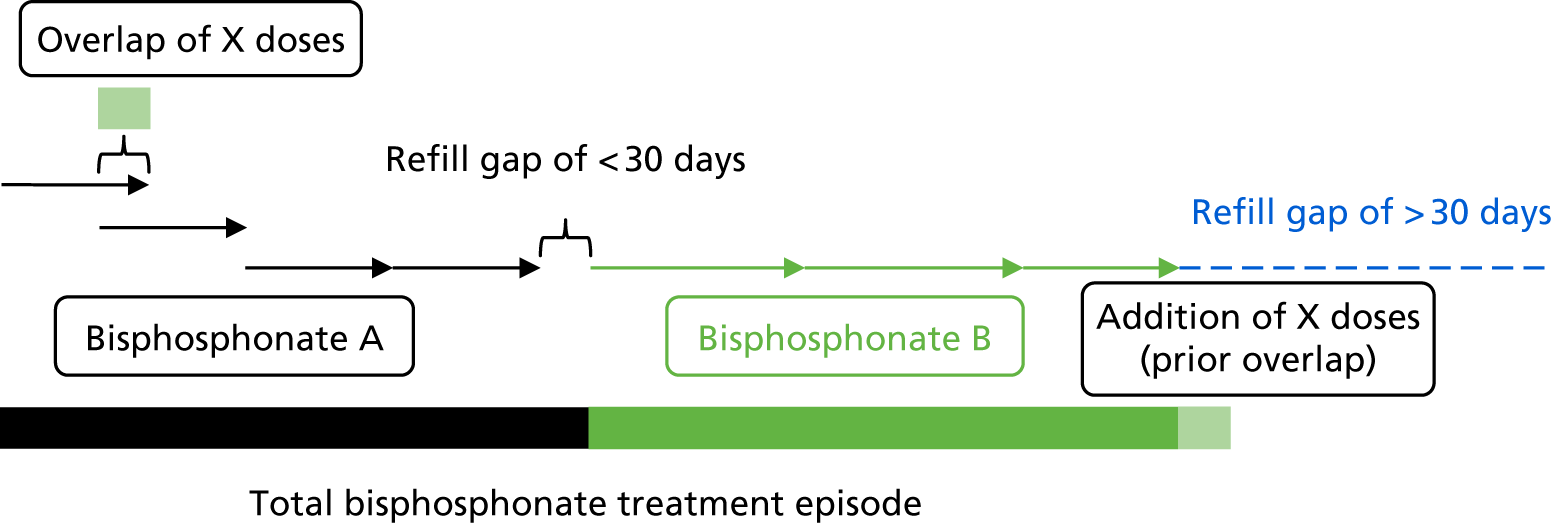
We assumed that any overlap between two prescriptions of the same bisphosphonate represented early collection of a repeat prescription. Any overlapping days between two prescriptions of the same drug were added to the end of the period covered by the two prescriptions. To define periods of continuous use of a study drug, any two prescriptions of the same drug were concatenated if the gap between the end of the first and the start of the second was < 30 days. Continuous use is illustrated in Figure 1.
FIGURE 1.
Construction of treatment episodes based on prescription data.
Immortal time bias
Immortal time bias is a common issue in pharmacoepidemiology. It appears when a study’s event of interest cannot occur for a certain time span because of the study design and/or the data analysis methods used. 39 In this study, immortal time could arise when the definition of drug use involved a delay or wait period during which follow-up time was accrued. For example, immortal time could arise when a participant waited for a prescription or drug dispensation after being discharged from hospital, when their discharge date represented the start of follow-up. 40
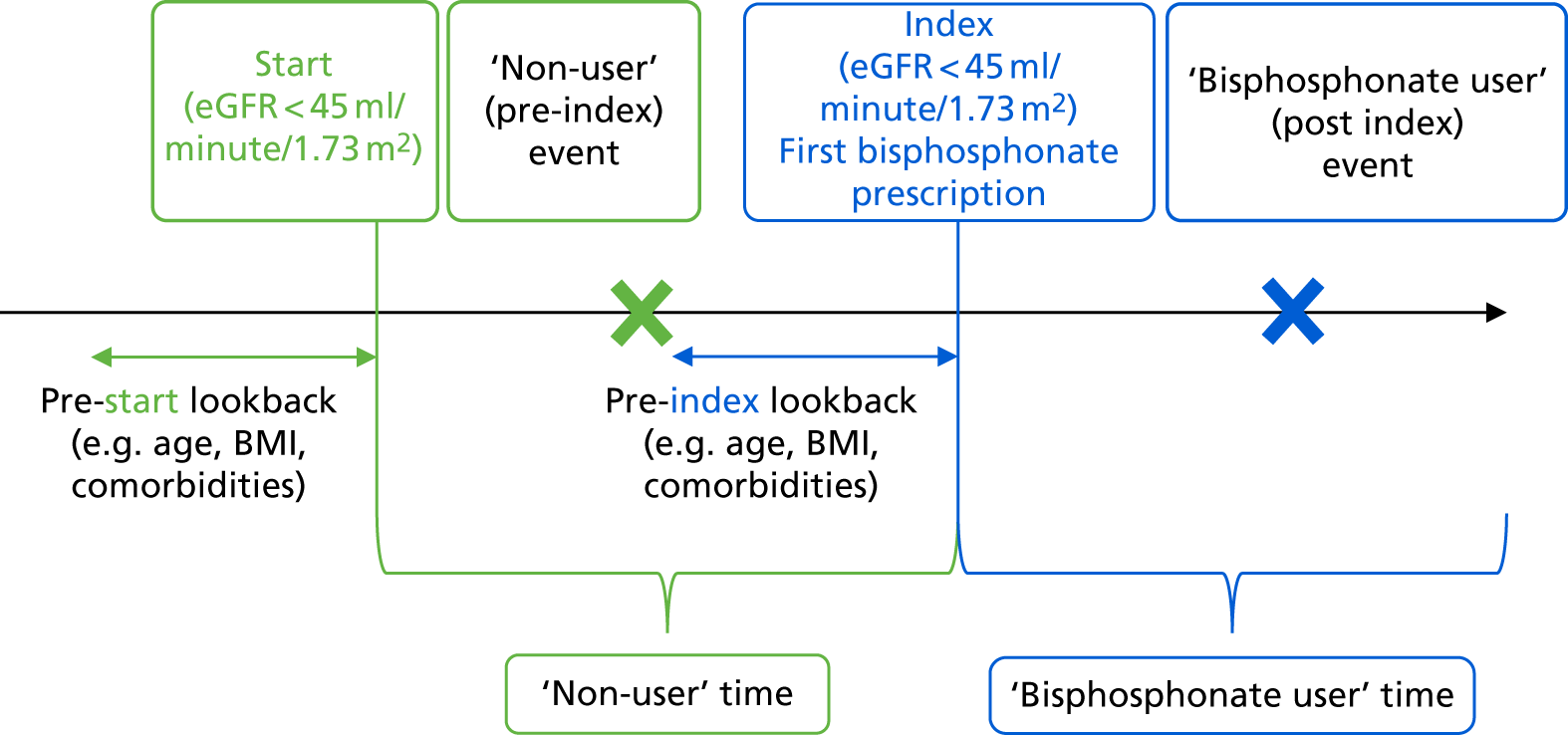
We used time-varying exposures, reclassifying the time before the first prescription of bisphosphonate as non-user person-years and classifying the time after the first prescription of bisphosphonate together with a recent eGFR of < 45 ml/minute/1.73 m2 before that prescription as bisphosphonate user person-years. We have extensive expertise using this method,41–43 which is the most efficient at avoiding immortal time bias in pharmacoepidemiological studies. 44 Figure 2 demonstrates our implementation of time-varying exposures in our data management process.
FIGURE 2.
Time-varying exposure to avoid immortal time bias.
Estimated glomerular filtration rate measurement
Formulae to calculate eGFR based on serum creatinine are increasingly popular. According to routinely collected data on biochemistry tests in NHS settings, the number and proportion of UK patients tested for serum creatinine for whom eGFR measurements were calculated increased by almost 30% between 2004 and 2009. 3 Automated laboratory reporting of eGFR has also been increasingly used in UK primary care since 2006. 45 We used recorded eGFR from CPRD GOLD when available. When an automated laboratory report of eGFR was not available but serum creatinine data were recorded, we calculated eGFR using the Chronic Kidney Disease – Epidemiology Collaboration (CKD-EPI) formula. 46 We chose this formula after consulting two renal epidemiologists.
Propensity score methods
The chosen study design is a propensity score-matched ‘real-world’ new-user cohort study. This is a standard pharmacoepidemiological design used to assess the intended (benefits) and unintended (risks) effects of drugs in drug safety observational research. Propensity scores are defined as the probability that a patient will receive the drug of interest (here, bisphosphonates) according to their baseline sociodemographic and clinical characteristics. Propensity scores have been proposed as a valid method for minimising confounding by indication in the absence of residual (unobserved) confounding.
Multivariable logistic regression equations were used to calculate one propensity score for each of the study outcomes of interest,47 as described in later chapters. A separate model was built for each of the outcomes, as the confounders and risk factors for different events might have differed. Prespecified predictors for each of these outcomes were included in each of the models. 48
The created propensity scores were used to match bisphosphonate users with comparable non-users using calliper matching, with a maximum calliper width of 0.02 standard deviations (SDs). Bisphosphonate non-users were thus only eligible to be matched if their propensity score fell within a bandwidth of 0.02 SDs of a bisphosphonate user’s propensity score. This method is one of the most efficient for minimising confounding by indication in pharmacoepidemiological studies. 49 Propensity score calliper matching typically excludes participants with extremely high or low risks for the outcome, if those extreme risk values are not seen in both intervention and comparator participant groups.
The imbalance in each of the listed confounders was assessed before and after matching using the absolute standardised mean difference (ASMD). An ASMD of < 0.1 was considered acceptable, based on previous literature. 50
Selection of confounders for inclusion in the propensity score equations
Previous methodological research has suggested that propensity scores should include true confounders (variables associated with the probability of treatment and the probability of the event of interest) and risk factors for the study outcomes. 51 We created a matrix of variables for inclusion in each of the propensity score equations created for each of the WPs. The list of variables to be included was defined as follows:
-
A list of all potential confounders and risk factors for each of the study outcomes was created by a postdoctoral epidemiologist based on a literature review and the availability of information recorded in the CPRD GOLD–HES data set.
-
The list was circulated to two clinician scientists, a GP and a rheumatologist, who added any potentially relevant variables not included in the list.
-
The amended list was sent to both clinician scientists, who independently highlighted the variables associated with each of the outcomes of interest.
-
Discrepancies in the highlighted lists were resolved in a consensus meeting involving the postdoctoral epidemiologist and both clinicians. The final lists of variables included in each of the propensity score equations are detailed in Table 1.
| Outcome events | WP1: CKD progression | WP2: symptomatic fracture | WP3: adverse events | WP4: change in DXA-measured hip BMD |
|---|---|---|---|---|
| Sociodemographic and/or clinical factors | ||||
| Age | ✗ | ✗ | ✗ | ✗ |
| Sex | ✗ | ✗ | ✗ | ✗ |
| Socioeconomic status | ✗ | ✗ | ✗ | ✗ |
| Height, weight, and BMI | ✗ | ✗ | ✗ | ✗ |
| Smoking status | ✗ | ✗ | ✗ | |
| Alcohol drinking | ✗ | ✗ | ✗ | |
| Number of GP visits/hospital visits in the previous year | ✗ | ✗ | ✗ | ✗ |
| BMD | ✗ | |||
| Comorbidities | ||||
| Charlson Comorbidity Index 5-year score | ✗ | ✗ | ✗ | |
| Type 2 diabetes mellitus | ✗ | ✗ | ✗ | ✗ |
| Cancer | ✗ | ✗ | ✗ | ✗ |
| CKD | ✗ | ✗ | ✗ | ✗ |
| Rheumatoid arthritis | ✗ | ✗ | ✗ | ✗ |
| Dementia | ✗ | ✗ | ✗ | ✗ |
| History of cardiovascular disease | ✗ | ✗ | ✗ | ✗ |
| History of fracture | ✗ | ✗ | ✗ | ✗ |
| Previous jaw osteonecrosis or rickets | ✗ | ✗ | ||
| Previous thromboembolic events | ✗ | ✗ | ✗ | ✗ |
| Varicose veins | ✗ | ✗ | ✗ | |
| Kidney function (eGFR) | ✗ | ✗ | ✗ | ✗ |
| Peptic ulcer disease | ✗ | |||
| Renal disease | ✗ | |||
| Hypertension | ✗ | ✗ | ✗ | ✗ |
| Hyperlipidaemia | ✗ | ✗ | ✗ | |
| Liver disease | ✗ | ✗ | ✗ | ✗ |
| Charlson Comorbidity Index | ✗ | ✗ | ✗ | ✗ |
| Cerebrovascular disease | ✗ | ✗ | ✗ | ✗ |
| Peripheral vascular disease | ✗ | ✗ | ✗ | ✗ |
| Hyperthyroidism | ✗ | ✗ | ✗ | |
| Co-medications | ||||
| Number of prescriptions in the previous year | ✗ | ✗ | ✗ | |
| Non-steroidal anti-inflammatory use | ✗ | ✗ | ✗ | ✗ |
| Hormone replacement therapy or contraceptives | ✗ | ✗ | ✗ | ✗ |
| Calcium or vitamin D supplements | ✗ | ✗ | ✗ | ✗ |
| Bisphosphonate use for > 1 year before index date | ✗ | ✗ | ✗ | ✗ |
| Systemic corticosteroids | ✗ | ✗ | ✗ | ✗ |
| Oral glucocorticoids | ✗ | ✗ | ✗ | ✗ |
| Oral anticoagulants | ✗ | ✗ | ✗ | ✗ |
| Proton pump inhibitors | ✗ | ✗ | ✗ | ✗ |
| Low-molecular-weight heparins | ✗ | ✗ | ✗ | ✗ |
| Aromatase inhibitors | ✗ | ✗ | ✗ | ✗ |
| Antidepressants | ✗ | ✗ | ✗ | ✗ |
| Statins/lipid-lowering drugs | ✗ | ✗ | ✗ | ✗ |
| Hypnotics | ✗ | ✗ | ✗ | ✗ |
| Antiepileptics | ✗ | ✗ | ✗ | ✗ |
| Diuretics | ✗ | ✗ | ✗ | ✗ |
| Beta blockers | ✗ | ✗ | ✗ | ✗ |
| ACE inhibitors | ✗ | ✗ | ✗ | ✗ |
| Calcium channel blockers | ✗ | ✗ | ✗ | ✗ |
| Other antihypertensives | ✗ | ✗ | ✗ | ✗ |
| Digoxin | ✗ | ✗ | ✗ | ✗ |
| Antihypertensives | ✗ | |||
| Antiarrhythmic agents | ✗ | ✗ | ✗ | ✗ |
| Oral antidiabetic drugs | ✗ | ✗ | ✗ | ✗ |
| Insulin | ✗ | ✗ | ✗ | ✗ |
The code lists of the included confounders can be found in Report Supplementary Material 4.
Missing data
We defined clinical characteristics according to consultation behaviour in primary care medical records. No consultation equates to the absence of a health problem, and no prescription equates to no drug use. This assumption is valid as, in the UK, GPs serve as the gatekeepers to authorise further medical services and are obliged to hold full records of their patients’ medical histories. However, some characteristics, such as smoking, alcohol drinking and BMI values, can be missing from these records.
We imputed this missing information for the propensity score (logistic) models using multiple imputation by chained equations (MICE) methods. As MICE assumes ‘at random’ missingness, we processed the data as follows:
-
We fitted logistic regression models using the whole data set to look for predictors of missingness for a specific variable (e.g. BMI). If such predictors were present in the data, then we assumed that missingness was not completely at random.
-
In the subset of patients with complete data for the same variable (e.g. BMI), we ran a different regression model (linear regression in this example) to look for predictors of that variable’s values. If we also identified predictors of such values, then we assumed that the data were ‘missing at random’.
-
We used the identified predictors of missingness (step 1) and values (step 2) in our MICE for imputation models.
-
As these steps confirmed the assumption of ‘at random’ missingness, we used MICE to impute missing values in each of the 10 imputed data sets. For each imputed data set, two propensity score-matched cohorts (bisphosphonate users and comparable non-users) were identified and analysed per study outcome.
-
Treatment estimates from each of the 10 imputed data sets were combined using Rubin’s rules to obtain an overall outcome estimate. 52
Study outcomes
Detailed descriptions of each outcome are given in each work package chapter (see Chapters 4–7). In brief, the study outcomes were as follows:
-
CKD progression –
-
based on stage progression (changing to a worse CKD stage as defined by the KDOQI guidelines) or requiring haemodialysis or transplantation, as recorded in HES (primary outcome)
-
based on the change in eGFR over time (secondary outcome).
-
-
Clinical (symptomatic) fractures of osteoporotic sites (all but face, skull, fingers or toes), ascertained using prespecified Read code lists in CPRD GOLD.
-
Acute kidney injury, identified using ICD-10 codes recorded in HES.
-
Hospitalisation for hypocalcaemia or hypophosphataemia, identified using ICD-10 codes recorded in HES.
-
Upper gastrointestinal events, identified using ICD-10 codes recorded in HES and Read codes recorded in CPRD GOLD.
-
Change (percentage from baseline) in BMD over time –
-
in the femoral neck (primary outcome)
-
in the total hip and lumbar spine (secondary outcomes).
-
The follow-up windows are described in each WP chapter; see Chapters 4–7.
Data analysis
Detailed descriptions of the data analysis used in each WP are given in the relevant chapters. Analyses were conducted using R version 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria) or Stata® version 15.1 (StataCorp LP, College Station, TX, USA), unless otherwise mentioned.
Ethics and scientific approval
No additional ethics approval was required as this study used anonymised routinely collected data from the UK’s CPRD GOLD with linked HES data and the Danish OUDH. To access these data sets, we submitted an application to and obtained approval from the Independent Scientific Advisory Committee (ISAC) for CPRD GOLD/HES (WPs 1–3) (ISAC protocol number 15_153R2) and the Region of Southern Denmark (reference number 15/37999), the health trust accountable to the Data Protection Agency for oversight of research data at Odense University Hospital under the Danish Health Act (WP4).
Chapter 3 Work package 1: the association between the use of bisphosphonates and the progression of kidney disease
Introduction
Bisphosphonates and renal toxicity: mechanistic studies
Bisphosphonates are contraindicated in patients with stage 4+ CKD who have an eGFR of < 35 ml/minute/1.73 m2, because of a scarcity of risk–benefit data for these patients and a number of spontaneous reports suggesting nephrotoxicity.
Animal studies have identified a dose-dependent association between the administration of bisphosphonates and renal injury, identified by urinary (malate dehydrogenase)53 and serum (urea, nitrogen and creatinine) biomarkers. 54 Mice exposed to high doses of pamidronate expressed histological changes such as focal cellular necrosis, increased cellular vesicles and loss of tubular cell brush borders. 55 Similar effects have been reported for other bisphosphonates, including zoledronate and ibandronate. 56 Single-dose infusions of zoledronate or ibandronate in mice caused renal proximal tubular damage with loss of brush borders, cytoplasmic swelling, and cell necrosis. 57
Numerous human case reports of renal failure, acute kidney injury and nephrotic syndrome58 following the use of a bisphosphonate have been published. Subsequent renal biopsies have found focal segmental glomerulosclerosis (FSGS), collapsing FSGS, and toxic acute tubular necrosis. 59–65 As expected, these spontaneous reports differ in sample size (reporting between one and seven patients) and scientific value. Some of the reports have claimed clinical and histological improvement after the bisphosphonate therapy was discontinued. 66
Evidence of renal toxicity from clinical trials
Data from RCTs are, in general, reassuring, suggesting that bisphosphonates are safe for use in fracture prevention for patients with normal renal function. 67,68 However, studies of high-dose bisphosphonate treatment for indications other than fracture prevention (e.g. treatment of bone cancer and metastasis) have been inconsistent. A Phase III trial of high-dose monthly intravenous pamidronate for multiple myeloma found no adverse renal effects in 203 treated participants compared with 189 placebo-exposed participants. 69 However, renal safety concerns have been reported in oncology RCTs of zoledronic acid. A Phase III RCT comparing zoledronic acid and pamidronate for multiple myeloma had to adjust treatment regimens and still found high incidences of nephrotoxicity of 9.3% and 8.1%, respectively. 70 Another trial in patients with bone metastases found a noticeable (although not statistically significant) 4.2% absolute risk increase in participants treated with zoledronic (10.9%) compared with placebo (6.7%). 71
Most of the participants in RCTs have a normal baseline renal function. A recent meta-analysis16 showed that none of the seven identified studies investigating the effect of bisphosphonate use in patients with stages 3–5 CKD or who required dialysis or kidney transplant reported a statistically significant difference between bisphosphonate users and non-users. However, the included studies generally contained low numbers of participants and were not powered to test for statistically significant differences in safety events such as eGFR stage change. The meta-analysis may not have been able to capture renal toxicity.
Work package 1 aimed to assess the association between bisphosphonate use and CKD progression (worsening) in real-world users of bisphosphonates with moderate to severe (stage 3B+) CKD.
Methods
Comparing Clinical Practice Research Datalink and Hospital Episode Statistics reporting of dialysis and transplant
As we were unable to acquire UKRR data, we cross-validated the recording of renal replacement therapy (dialysis or transplant) in CPRD GOLD and HES. HES was used as the gold standard for comparison, as previous linkage of HES to the UKRR found good validity and completeness of recording of both dialysis and kidney transplant in HES data, with quality improving further in recent years. 34
The positive predictive value and sensitivity of dialysis and transplant recording in CPRD GOLD and HES were calculated and compared. The number of events for which dates in CPRD GOLD and HES differed by less than 3 months (90 days) was also calculated.
Study participants and exposure
A new-user cohort analysis was conducted using data from CPRD GOLD linked to HES.
Patients recorded in CPRD GOLD who were aged > 40 years and had at least two eGFR measurements within 1 year of < 45 ml/minute/1.73 m2 were eligible. Patients were excluded if they had received any bisphosphonate prescription in the year before their first eGFR measurement of < 45 ml/minute/1.73 m2, had previously used other (non-bisphosphonate) anti-osteoporosis therapies, were missing IMD data or had no follow-up eGFR measurements.
All participants initially joined the study unexposed to bisphosphonate on their second eGFR measurement of < 45 ml/minute/1.73 m2. Participants could contribute to both exposed and unexposed time. They were followed up until one of the following occurred:
-
the end of enrolment in the database (due to moving out or death)
-
(exposed participants only) stopping treatment > 210 days, made up of the 30 days of the last prescription and a washout period of 180 days; they would not have any repeated prescription within 6 months
-
(exposed participants only) switching treatment to another osteoporosis medication and no repeated prescription of bisphosphonates within the next 6 months
-
incident recorded renal event, as defined under Study outcomes.
Exposure
The exposure of interest was bisphosphonate use. At first bisphosphonate use, a participant’s most recent eGFR measurement was assessed. Those with an eGFR of < 45 ml/minute/1.73 m2 moved to the exposed category. Those with a bisphosphonate prescription and an eGFR of ≥ 45 ml/minute/1.73 m2 were censored at the time of starting bisphosphonate therapy.
Bisphosphonate prescriptions were concatenated to create treatment (exposure) episodes as described in Chapter 2, Main exposure: bisphosphonate use. Exposed patients were censored 210 days (30 days to account for the last prescription, plus a 6-month washout period) after the last bisphosphonate prescription if this was earlier than their censor time, as specified in the previous section.
Study outcomes
The outcomes of interest were as follows:
-
primary (per-protocol) analysis – CKD stage worsening based on follow-up eGFR or start (first session or surgery) of renal replacement therapy
-
secondary (per-protocol) analysis – annualised eGFR change based on all follow-up eGFR measurements
-
post hoc analyses –
-
CKD stage worsening based on follow-up eGFR measurements
-
CKD stage change based on eGFR (compared with stable CKD)
-
increase (worsening) in CKD stage
-
decrease (improvement) in CKD stage.
-
-
As described in Chapter 2, Study outcomes, the outcome follow-up period ran from the baseline (second eGFR measurement of < 45 ml/minute/1.73 m2 within 1 year) or index date (first bisphosphonate treatment) until the earliest occurrence of any of the following:
-
end of enrolment in the database (due to moving out, death or the practice stopping follow-up)
-
therapy cessation (last prescription before a 6-month prescription gap) + 210 days, made up of the 30 days of the last prescription and a washout period of 180 days
-
switching to (or adding) another anti-osteoporosis medication
-
a dialysis, transplant or CKD stage-change event
-
10 years follow-up.
Chronic kidney disease stage was measured using eGFR records from CPRD GOLD. Dialysis and transplant events were identified in HES using ICD-10 and OPCS-4 codes, respectively. If a patient had more than one eGFR record within a calendar year, we used the latest one to represent the eGFR records for that follow-up year.
Code lists for dialysis and transplant outcomes are presented in Report Supplementary Material 2.
Statistical analysis
We conducted a stratified propensity score analysis to account for potential imbalance in the years of follow-up between bisphosphonate users and non-users. The stratification of years of follow-up was grouped into four categories: 1–2 years, 3–4 years, 5–6 years and 7–10 years. Each user was propensity matched with up to five non-users, all with the same follow-up period, based on 46 covariates (see Chapter 2, Propensity score methods). Balance before and after matching for each confounder was assessed using the ASMD with a cut-off point of 0.1.
The crude and age-sex specific incidence rates [and 95% confidence intervals (CIs)] of each of the outcomes were estimated separately in the propensity score-matched cohorts for bisphosphonate users and non-users per 1000 person-years. Rates were calculated assuming a Poisson distribution. Average incidence rates were used to derive the absolute increase in risk and to calculate the number needed to harm in the primary analysis at 1, 3 and 5 years of treatment. 72 Kaplan–Meier plots were used to depict the predicted cumulative probability of each of the study end points according to bisphosphonate use.
Initially, Cox proportional hazards models were used to compute the hazard ratio (HR) (and 95% CIs) for mortality according to drug use. These were fitted for the propensity score-matched cohorts of each imputation and combined using Rubin’s rules. 52 The assumption of proportionality was checked graphically using c-loglog plots. The model for mortality showed a reduced risk of death in patients using bisphosphonates (HR 0.67, 95% CI 0.62 to 0.73). Therefore, competing risks analyses, both Fine and Gray and cause-specific hazards, were calculated. Because the results were similar, only Fine and Gray subdistribution hazard ratios (sHRs) are reported. Cumulative incidence function plots are shown in Appendix 1, Figure 25.
A post hoc multivariable analysis was undertaken on the full data set, as requested by the Steering Committee, using all of the covariates included in the propensity score. No difference in mortality was found in this model, and hence Cox proportional hazards were used.
The rate of annual eGFR change was estimated using the slopes of a mixed-effects model with linear splines and an interaction between bisphosphonate use and time. Fractional polynomial models were used to identify the best-fitting shape of the non-linear association between the predictor (time) and the outcome (eGFR), based on the deviation chi-squared tests. An examination of the best-fitting fractional polynomial model was then used to identify the year when the linear rate diverges. This approach allowed us to interpret the rate easily as suggested by Tilling et al. 73 The models were fitted using the fp package in Stata version 15.
Sensitivity analyses
We tested for predefined interactions between the use of bisphosphonates and sex or history of previous fracture. Stratified analyses were reported if the interactions were significant. We did not test for an interaction with CKD starting stage. As a participant starting at CKD stage 5 cannot decrease in stage, the outcome would be highly associated with the interaction term. A Fine and Gray competing risk model was run to assess the competing risk of death. 74
One of the Bradford Hill causality criteria is that an observed association follows a gradient. 75 We tested the associations between bisphosphonate use and each of the outcomes by categorising bisphosphonate users into quartiles using their medication possession ratios (MPRs). The MPR was calculated as the number of defined daily doses prescribed over the total number of days of follow-up. Fine and Gray competing risk models were used to compare the sHR for each of the MPR quartiles with those of matched non-users. A mantel extension test was used to test for trend in the MPR quartiles.
A post hoc array analysis was conducted to measure the potential impact of unmeasured confounding, as recommended by Schneeweiss,76 based on the competing risk analyses.
Results
Agreement between Clinical Practice Research Datalink and Hospital Episode Statistics reporting of dialysis and transplant
The agreement between CPRD GOLD and HES is reported in Table 2. The positive predictive value of CPRD GOLD reporting of dialysis in comparison with HES was high, at 80%. However the sensitivity of dialysis reporting was only 33%, suggesting that events would be lost if CPRD data were used alone. The positive predictive value of transplant reporting was also high, at 86%, with an acceptable sensitivity of 72%.
| CPRD | Dialysis (n) | Transplant (n) | ||
|---|---|---|---|---|
| HES: yes | HES: no | HES: yes | HES: no | |
| Yes | 1539 | 385 | 469 | 75 |
| No | 3084 | 222,043 | 182 | 226,325 |
When a 3-month window was used, the number of dialysis events in agreement between CPRD GOLD and HES dropped to 856 (55.6%), and the number of transplant events dropped to 440 (93.8%), again suggesting that CPRD GOLD and HES differ in their reporting of the numbers of, and the times and dates of initiation of, dialysis events.
Identifying Clinical Practice Research Datalink GOLD patients during data extraction
We identified 217,405 CPRD GOLD patients who had an index eGFR of ≤ 45 ml/minute/1.73 m2 and were therefore eligible. As seen in Figure 3, patients were excluded if they had only one eGFR measurement of < 45 ml/minute/1.73 m2, had only eGFR measurements before 1997, had an eGFR of exactly 45 ml/minute/1.73 m2, were aged < 40 years at the time of their first eGFR measurement of < 45 ml/minute/1.73 m2, had used bisphosphonates in the year before the index eGFR measurement of < 45 ml/minute/1.73 m2, had previously used any other anti-osteoporotic drug, had an adverse event of interest before study entry or had a missing IMD value. Exposed patients were also excluded if their most recent eGFR measurement before bisphosphonate exposure was ≥ 45 ml/minute/1.73 m2.
FIGURE 3.
Summary of identification of CPRD GOLD participants using inclusion and exclusion criteria for renal and safety analyses. a, To avoid immortal time bias, unexposed participants could also contribute to exposed time. See Figure 2 for detailed explanations.
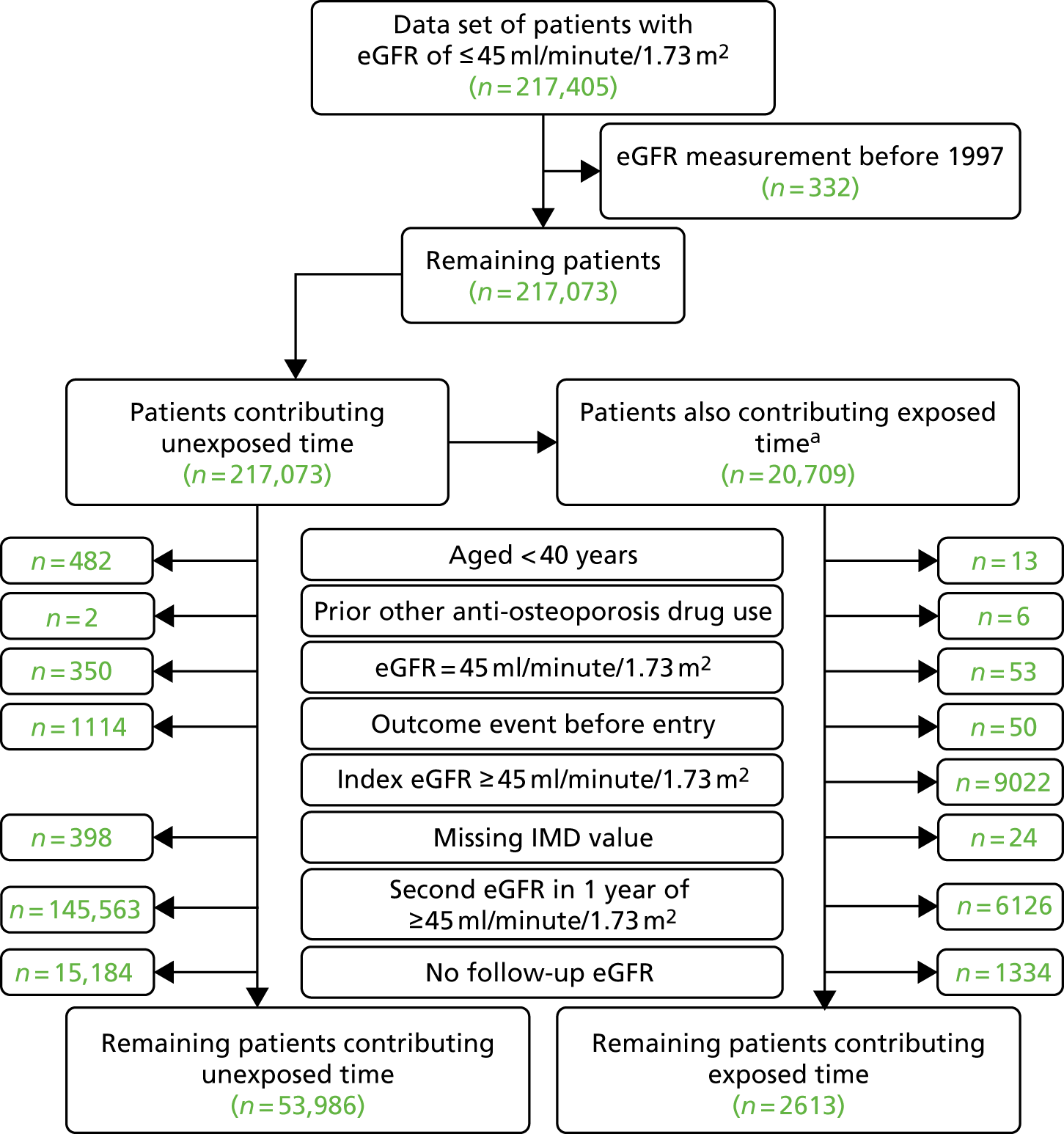
Time unexposed to bisphosphonates was contributed by 53,986 participants, with 2613 participants also contributing time as exposed to bisphosphonates.
Propensity score matching
Table 3 shows sociodemographic, clinical and prescription characteristics for the exposed and unexposed cohorts before and after propensity score matching. Before matching, participants exposed to bisphosphonates were older (80.6 years vs. 77.6 years), more likely to be female (77.2% vs. 56.9%), less likely to smoke (8.6% vs. 11.1%), less likely to drink alcohol (65.3% vs. 69.0%), had a lower eGFR [28.7% were in the highest category (40–44.9 ml/minute/1.73 m2) compared with 40.1% of unexposed participants], more likely to have been admitted to hospital in the previous year (60.9% vs. 38.2%), had a higher Charlson Comorbidity Index score (36.8% vs. 14.9% with an index 3+) and had more polypharmacy (97.3% vs. 83.8% on more than three concomitant medications) than unexposed participants. They were also more likely to have a recorded diagnosis of CKD (52.9% vs. 15.3%), have a history of non-hip fracture (22.1% vs. 3.9%) and use calcium supplements (29.9% vs. 7.4%) or bisphosphonates (5.2% vs. 1.8%) more than 1 year before their index date.
| Category | Before matching, n (%) | After matching, n (%) | ||
|---|---|---|---|---|
| Unexposed | Exposed | Unexposed | Exposed | |
| Number | 53,986 | 2613 | 8931 | 2447 |
| Age (years), mean (SD) | 77.6 (9.8) | 80.6 (8.8) | 80.3 (9.1) | 80.4 (8.8) |
| Sex (male) | 23,280 (43.1) | 595 (22.8) | 2669 (29.9) | 584 (23.9) |
| IMD quintiles | ||||
| 1 – least deprived | 11,949 (22.1) | 637 (24.4) | 2127 (23.8) | 587 (24.0) |
| 2 | 12,649 (23.4) | 620 (23.7) | 2089 (23.4) | 585 (23.9) |
| 3 | 11,539 (21.4) | 550 (21.0) | 1881 (21.1) | 517 (21.1) |
| 4 | 10,771 (20.0) | 480 (18.4) | 1654 (18.5) | 452 (18.5) |
| 5 – most deprived | 7078 (13.1) | 326 (12.5) | 1180 (13.2) | 306 (12.5) |
| BMI (kg/m2), mean (SD) | 27.6 (5.5) | 26.7 (5.2) | 26.9 (5.4) | 26.8 (5.3) |
| Smoking category | ||||
| No | 28,093 (52.0) | 1423 (54.5) | 4784 (53.6) | 1329 (54.3) |
| Ex | 19,910 (36.9) | 966 (37.0) | 3295 (36.9) | 906 (37.0) |
| Yes | 5983 (11.1) | 224 (8.6) | 852 (9.5) | 212 (8.7) |
| Drinking category | ||||
| No | 14,621 (27.1) | 781 (29.9) | 2663 (29.8) | 734 (30.0) |
| Ex | 2117 (3.9) | 126 (4.8) | 400 (4.5) | 118 (4.8) |
| Yes | 37,248 (69.0) | 1706 (65.3) | 5868 (65.7) | 1595 (65.2) |
| eGFR category (ml/minute/1.73 m2) | ||||
| 0–4.9 | 67 (0.1) | 1 (0.0) | 5 (0.1) | 1 (0.0) |
| 5–9.9 | 362 (0.7) | 16 (0.6) | 60 (0.7) | 16 (0.7) |
| 10–14.9 | 616 (1.1) | 29 (1.1) | 116 (1.3) | 29 (1.2) |
| 15–19.9 | 1278 (2.4) | 81 (3.1) | 263 (2.9) | 73 (3.0) |
| 20–24.9 | 2541 (4.7) | 164 (6.3) | 537 (6.0) | 149 (6.1) |
| 25–29.9 | 4665 (8.6) | 327 (12.5) | 996 (11.2) | 299 (12.2) |
| 30–34.9 | 8417 (15.6) | 542 (20.7) | 1709 (19.1) | 498 (20.4) |
| 35–39.9 | 14,415 (26.7) | 704 (26.9) | 2378 (26.6) | 657 (26.8) |
| 40–44.9 | 21,625 (40.1) | 749 (28.7) | 2867 (32.1) | 725 (29.6) |
| Number of hospital visits | ||||
| 0 | 33,367 (61.8) | 1022 (39.1) | 4066 (45.5) | 988 (40.4) |
| 1 | 10,968 (20.3) | 737 (28.2) | 2368 (26.5) | 683 (27.9) |
| 2 | 4971 (9.2) | 399 (15.3) | 1170 (13.1) | 367 (15.0) |
| 3–5 | 3876 (7.2) | 371 (14.2) | 1078 (12.1) | 340 (13.9) |
| ≥ 6 | 804 (1.5) | 84 (3.2) | 249 (2.8) | 69 (2.8) |
| Charlson Comorbidity Index score | ||||
| 0 | 30,485 (56.5) | 682 (26.1) | 3107 (34.8) | 671 (27.4) |
| 1 | 7723 (14.3) | 364 (13.9) | 1366 (15.3) | 350 (14.3) |
| 2 | 7719 (14.3) | 604 (23.1) | 1905 (21.3) | 565 (23.1) |
| 3–5 | 6468 (12.0) | 746 (28.5) | 2001 (22.4) | 666 (27.2) |
| ≥ 6 | 1591 (2.9) | 217 (8.3) | 552 (6.2) | 195 (8.0) |
| Rheumatoid arthritis | 532 (1.0) | 148 (5.7) | 171 (1.9) | 136 (5.6) |
| Varices | 3323 (6.2) | 316 (12.1) | 886 (9.9) | 281 (11.5) |
| Deep-vein thrombosis | 1895 (3.5) | 113 (4.3) | 388 (4.3) | 108 (4.4) |
| Type 2 diabetes mellitus | 6172 (11.4) | 404 (15.5) | 1304 (14.6) | 371 (15.2) |
| Dementia | 944 (1.7) | 79 (3.0) | 263 (2.9) | 74 (3.0) |
| CKD | 8245 (15.3) | 1381 (52.9) | 3479 (39.0) | 1225 (50.1) |
| Cerebrovascular disease | 4193 (7.8) | 320 (12.2) | 976 (10.9) | 299 (12.2) |
| Peripheral vascular disease | 1246 (2.3) | 88 (3.4) | 266 (3.0) | 80 (3.3) |
| Hypertension | 23,630 (43.8) | 1518 (58.1) | 4628 (51.8) | 1391 (56.8) |
| Hyperlipidaemia | 5788 (10.7) | 459 (17.6) | 1289 (14.4) | 415 (17.0) |
| Liver disease | 224 (0.4) | 32 (1.2) | 73 (0.8) | 28 (1.1) |
| Peptic ulcer | 816 (1.5) | 65 (2.5) | 187 (2.1) | 58 (2.4) |
| Osteomalacia/rickets | 15 (0.0) | 2 (0.1) | 5 (0.1) | 2 (0.1) |
| Cancer | 5415 (10.0) | 454 (17.4) | 1374 (15.4) | 416 (17.0) |
| Hip fracture | 440 (0.8) | 33 (1.3) | 125 (1.4) | 32 (1.3) |
| Non-hip fracture | 2088 (3.9) | 577 (22.1) | 1070 (12.0) | 482 (19.7) |
| Number of prescriptions | ||||
| 0 | 1527 (2.9) | 4 (0.2) | 72 (0.9) | 4 (0.2) |
| 1–3 | 6880 (13.3) | 61 (2.5) | 360 (4.3) | 61 (2.7) |
| 4–6 | 12,453 (24.0) | 230 (9.4) | 1104 (13.3) | 226 (9.9) |
| 7–9 | 12,408 (23.9) | 468 (19.2) | 1763 (21.2) | 451 (19.7) |
| 10–12 | 9019 (17.4) | 548 (22.5) | 1906 (22.9) | 519 (22.7) |
| 13 | 9524 (18.4) | 1129 (46.3) | 3125 (37.5) | 1026 (44.9) |
| Hormone replacement therapy | 2979 (5.5) | 244 (9.3) | 714 (8.0) | 228 (9.3) |
| Contraceptive | 38 (0.1) | 1 (0.0) | 5 (0.1) | 1 (0.0) |
| Calcium supplements | 3996 (7.4) | 782 (29.9) | 1818 (20.4) | 680 (27.8) |
| Bisphosphonate use more than 1 year before index date | 996 (1.8) | 136 (5.2) | 486 (5.4) | 122 (5.0) |
| Steroids | 9194 (17.0) | 1311 (50.2) | 3509 (39.3) | 1179 (48.2) |
| Anticoagulants | 7078 (13.1) | 486 (18.6) | 1567 (17.5) | 455 (18.6) |
| Heparin | 432 (0.8) | 61 (2.3) | 160 (1.8) | 51 (2.1) |
| Aromatase inhibitors | 265 (0.5) | 58 (2.2) | 122 (1.4) | 45 (1.8) |
| NSAIDs | 30,095 (55.7) | 1873 (71.7) | 5862 (65.6) | 1737 (71.0) |
| Proton pump inhibitors | 19,596 (36.3) | 1593 (61.0) | 4795 (53.7) | 1465 (59.9) |
| Anxiols/sedatives/hypnotics | 10,488 (19.4) | 726 (27.8) | 2429 (27.2) | 668 (27.3) |
| Antidepressants | 14,578 (27.0) | 1118 (42.8) | 3386 (37.9) | 1032 (42.2) |
| Statins | 21,548 (39.9) | 1402 (53.7) | 4368 (48.9) | 1293 (52.8) |
| Calcium channel blockers | 23,668 (43.8) | 1507 (57.7) | 4683 (52.4) | 1392 (56.9) |
| ACE inhibitors | 32,246 (59.7) | 1907 (73.0) | 6142 (68.8) | 1773 (72.5) |
| Antiepileptics | 2251 (4.2) | 207 (7.9) | 631 (7.1) | 192 (7.8) |
| Diuretics | 38,403 (71.1) | 2164 (82.8) | 7139 (79.9) | 2023 (82.7) |
| Beta blockers | 25,175 (46.6) | 1395 (53.4) | 4461 (49.9) | 1302 (53.2) |
| Antidiabetics | 9507 (17.6) | 443 (17.0) | 1567 (17.5) | 420 (17.2) |
| Insulin | 2359 (4.4) | 127 (4.9) | 421 (4.7) | 120 (4.9) |
| Digoxin | 5839 (10.8) | 319 (12.2) | 1152 (12.9) | 310 (12.7) |
| Antihypertensives | 8608 (15.9) | 588 (22.5) | 1770 (19.8) | 548 (22.4) |
| Antiarrythmics | 7010 (13.0) | 447 (17.1) | 1418 (15.9) | 417 (17.0) |
After matching, the remaining participants in both cohorts were more similar, with some remaining differences. Exposed participants started with a lower eGFR (29.6% had an eGFR of ≥ 40 ml/minute/1.73 m2 vs. 32.1%), more hospital visits (40.4% vs. 45.5% with zero hospital visits) and a higher Charlson Comorbidity Index score (27.4 vs. 34.8 with a score of 0) than unexposed participants. They were more likely than unexposed participants to have diagnosed CKD (50.1% vs. 39.0%), a history of non-hip fracture (19.7% vs. 12.0%), more prescriptions (44.9% with ≥ 13 vs. 37.5% with < 13 prescriptions) and to receive calcium supplements (27.8% vs. 20.4%) and steroids (48.2% vs. 39.3%).
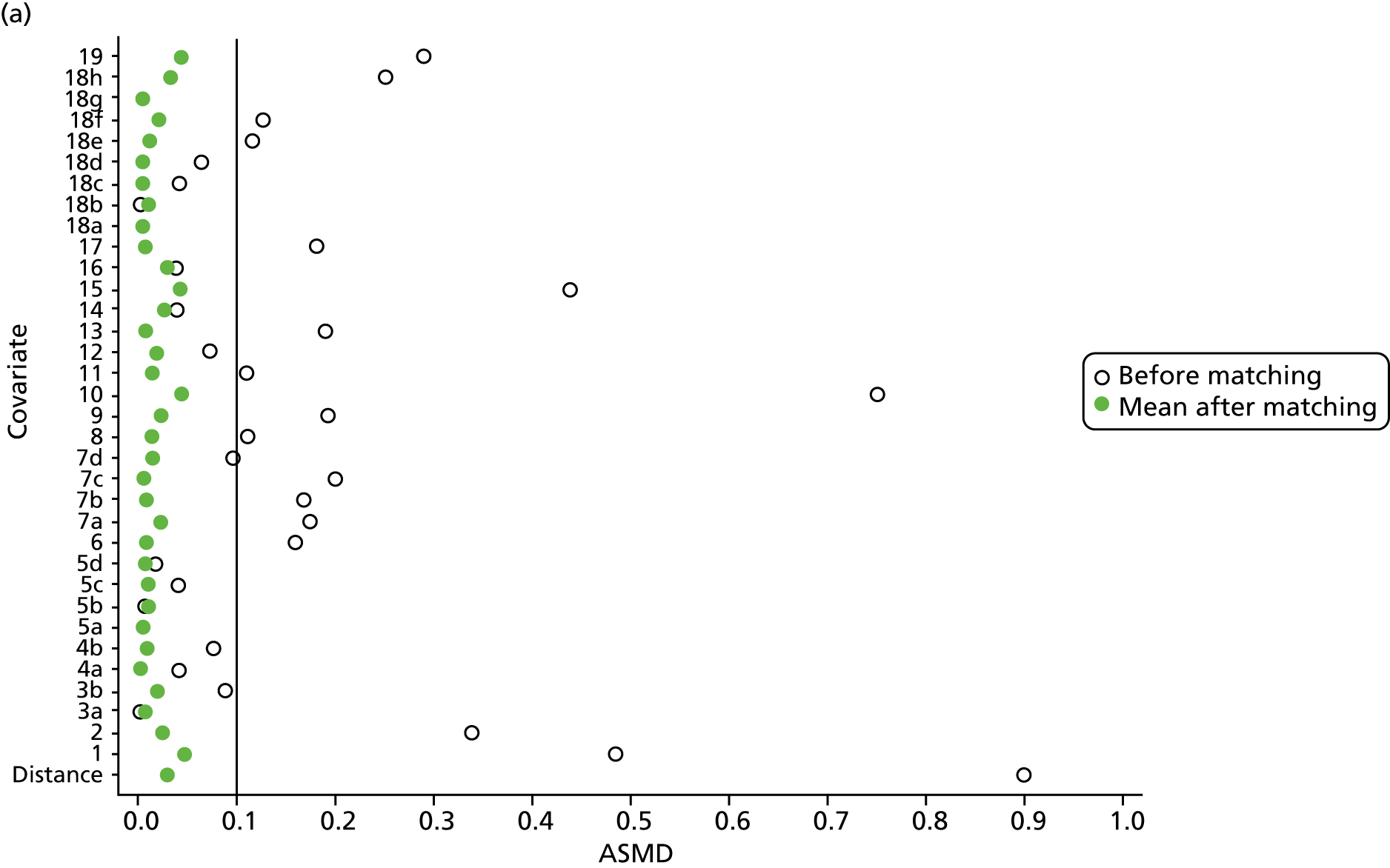
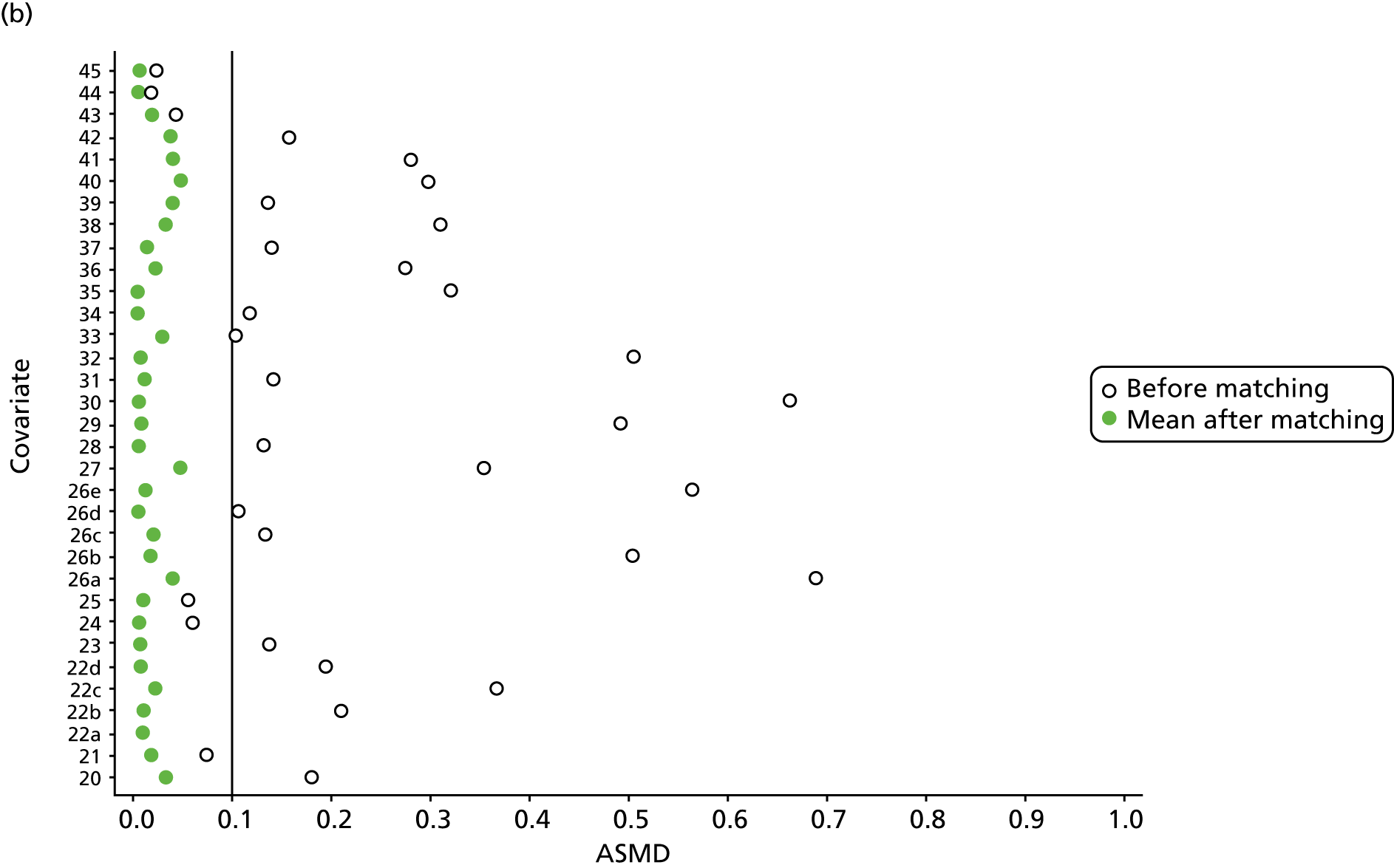
However, these differences were not great enough to influence the balance between the matched cohorts. Histograms show that the overlap in propensity score values was much improved after matching (see Appendix 1, Figure 24). Figure 4 shows ASMD values of < 0.1 for all covariates after matching, suggesting good balance.
FIGURE 4.
The ASMD of each covariate included in the propensity score matching for renal and safety outcomes before and after matching. (a) 1, Sex; 2, age; 3a, ex-smoker; 3b, current smoker; 4a, ex-drinker; 4b, current drinker; 5a, IMD quintile 2; 5b, IMD quintile 3; 5c, IMD quintile 4; 5d, IMD quintile 5; 6, BMI; 7a, 1 hospital visit in the previous year; 7b, 2 hospital visits in the previous year; 7c, 3–5 hospital visits in the previous year; 7d, ≥ 6 hospital visits in the previous year; 8, type 2 diabetes mellitus; 9, cancer; 10, CKD; 11, antiarrhythmic agents; 12, dementia; 13, cardiovascular disease; 14, hip fracture; 15, non-hip fracture; 16, deep-vein thrombosis; 17, varices; 18a, start eGFR value of 5–9.9 ml/minute/1.73 m2; 18b, start eGFR value of 10–14.9 ml/minute/1.73 m2; 18c, start eGFR value of 15–19.9 ml/minute/1.73 m2; 18d, start eGFR value of 20–24.9 ml/minute/1.73 m2; 18e, start eGFR value of 25–29.9 ml/minute/1.73 m2; 18f, start eGFR value of 30–34.9 ml/minute/1.73 m2; 18g, start eGFR value of 35–39.9 ml/minute/1.73 m2; 18h, start eGFR value of 40–44.9 ml/minute/1.73 m2; 19, hypertension; and (b) 20, hyperlipidaemia; 21, liver disease; 22a, 5-year Charlson Comorbidity Index score of 1; 22b, 5-year Charlson Comorbidity Index score of 2; 22c, 5-year Charlson Comorbidity Index score of 3–5; 22d, 5-year Charlson Comorbidity Index score of ≥ 6; 23, cerebrovascular disease; 24, peripheral vascular disease; 25, hyperthyroidism; 26a, 1–3 prescriptions in the previous year; 26b, 4–6 prescriptions in the previous year; 26c, 7–9 prescriptions in the previous year; 26d, 10–12 prescriptions in the previous year; 26e, ≥ 13 prescriptions in the previous year; 27, NSAIDs; 28, hormone replacement therapy; 29, calcium supplements; 30, steroids; 31, anticoagulants; 32, proton-pump inhibitors; 33, heparins; 34, aromatase inhibitors; 35, antidepressants; 36, statins; 37, antiepileptics; 38, diuretics; 39, beta blockers; 40, ACE inhibitors; 41, calcium channel blockers; 42, antihypertensives; 43, digoxin; 44, antidiabetics; 45, insulin. ACE, angiotensin-converting enzyme; NSAID, non-steroidal anti-inflammatory drug.
Chronic kidney disease category changes
In the primary analysis of CKD stage worsening, bisphosphonate users were more likely to progress to a worse CKD stage (i.e. move to a higher stage) than non-users (22.8% vs. 21.5%). The number and percentage of participants changing CKD stage are shown in Table 4.
| Baseline CKD stage | Value | First change to a worse stage of CKD | |||||
|---|---|---|---|---|---|---|---|
| Bisphosphonate user | Bisphosphonate non-user | ||||||
| 3b | 4 | 5 | 3b | 4 | 5 | ||
| 3b | n | 1370 | 481 | 12 | 5257 | 1579 | 73 |
| % | 73.4 | 25.2 | 0.6 | 76.1 | 22.9 | 1.1 | |
| 4 | n | 0 | 473 | 65 | 0 | 1569 | 268 |
| % | 0 | 87.9 | 12.1 | 0 | 85.4 | 14.6 | |
| 5 | n | 0 | 0 | 46 | 0 | 0 | 185 |
| % | 0 | 0 | 100 | 0 | 0 | 100 | |
In a post hoc analysis that also included improvements in CKD stage, 38.0% of non-users of bisphosphonate saw an improvement in their CKD after their first eGFR measurement of < 45 ml/minute/1.73 m2, with an decrease in stage. Only 35.1% of bisphosphonate users improved their CKD severity (Table 5). The most common change in stage was a decrease (improvement) by one stage from the starting stage, seen in 31.3% of bisphosphonate users and 34.2% of non-users.
| Baseline category | Baseline CKD stage | Value | First change to a better or worse stage of CKD | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3a | 3b | 4 | 5 | |||
| Unexposed | 3b | n | < 5 | 219 | 2316 | 2831 | 1473 | 69 |
| % | < 0.1 | 3.2 | 33.5 | 41.0 | 21.3 | 1.0 | ||
| 4 | n | 0 | 14 | 82 | 697 | 788 | 256 | |
| % | 0 | 0.8 | 4.5 | 38.0 | 42.9 | 13.9 | ||
| 5 | n | < 5 | < 5 | 9 | 8 | 37 | 125 | |
| % | < 2.7 | < 2.7 | 4.9 | 4.3 | 20 | 67.6 | ||
| Exposed | 3b | n | 0 | 58 | 534 | 800 | 460 | 11 |
| % | 0 | 3.1 | 28.7 | 42.9 | 24.7 | 0.6 | ||
| 4 | n | 0 | < 5 | 21 | 218 | 236 | 60 | |
| % | 0 | 3.9 | 40.5 | 43.9 | 11.2 | |||
| 5 | n | 0 | < 5 | < 5 | 8 | 13 | 21 | |
| % | 0 | 17.4 | 28.3 | 45.7 | ||||
Incidence rates of chronic kidney disease progression
In the primary analysis, participants exposed to bisphosphonates had a similar incidence rate of CKD stage worsening or starting renal replacement treatment (dialysis or transplant) to those not exposed to bisphosphonates, with incidence rates of 89.07 (95% CI 82.06 to 96.67) per 1000 person-years and 85.64 (95% CI 81.97 to 89.47) per 1000 person-years, respectively.
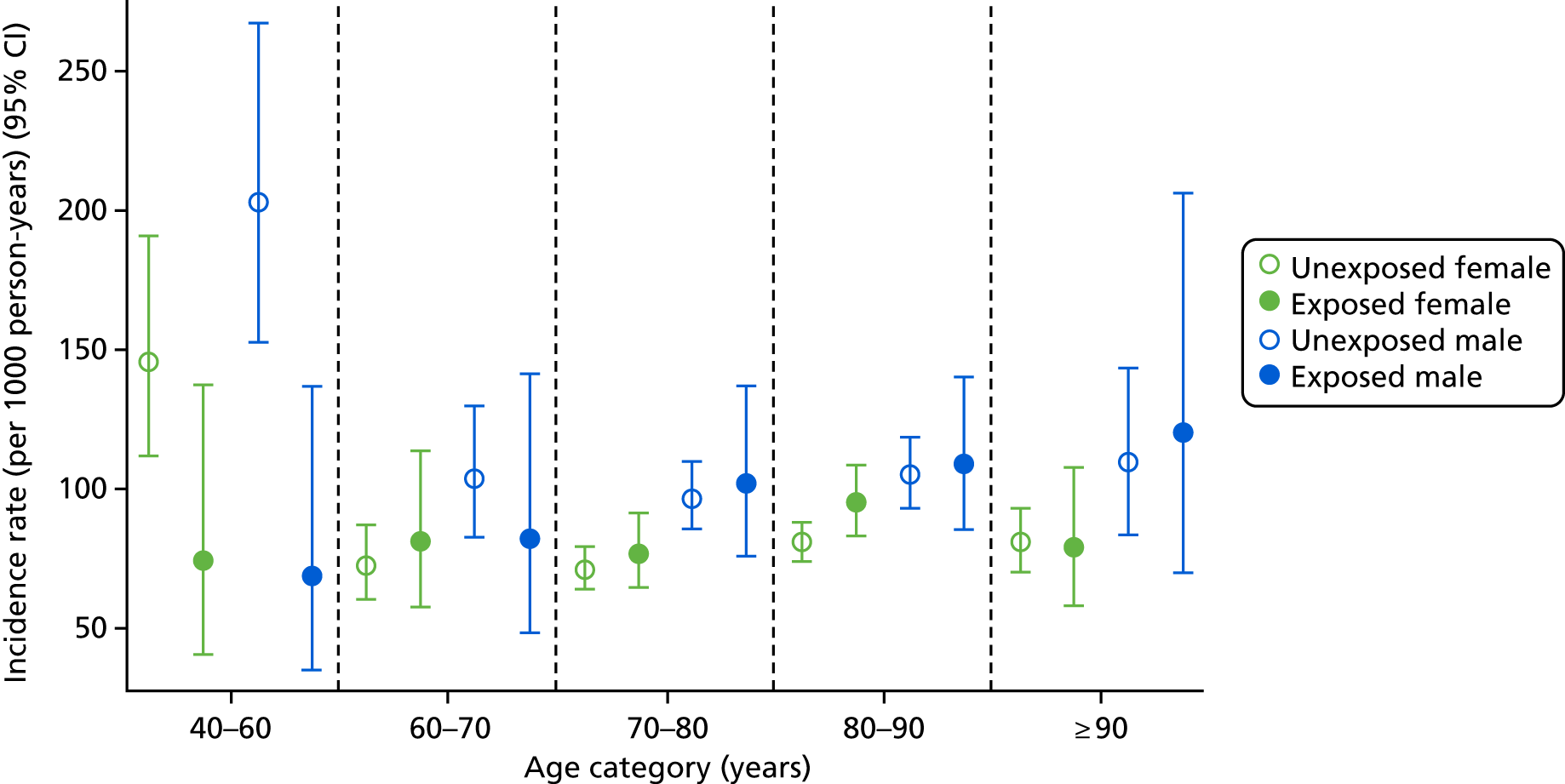
Age- and sex-specific incidence rates, stratified by bisphosphonate use, are shown in Figure 5. Men had a higher incidence of CKD worsening than women, with incidence rates of 100.82 (95% CI 85.38 to 119.06) per 1000 person-years for exposed men and 85.86 (95% CI 78.15 to 94.33) per 1000 person-years for exposed women.
FIGURE 5.
Male and female incidence rates of end-stage renal failure treatment or CKD stage worsening per 1000 person-years, stratified by exposure to bisphosphonate and age.
In the first post hoc analysis of CKD worsening based on eGFR measures alone, bisphosphonate users had a similar incidence rate to non-users [85.75 (95% CI 78.90 to 93.19) vs. 81.10 (95% CI 77.55 to 84.81) per 1000 person-years], agreeing with the results of the primary analysis. Age- and sex-specific incidence rates, stratified by bisphosphonate use, are shown in Figure 6. Few participants aged 40–60 years had an eGFR of < 45 ml/minute/1.73 m2, resulting in wide CIs for this group. However, those aged > 60 years showed increased incidences of CKD worsening. For example, participants aged 60–70 years had an incidence rate of 78.38 (95% CI 58.52 to 104.98) per 1000 person-years for bisphosphonate users and 74.5 (95% CI 68.66 to 80.84) per 1000 person-years for non-users, whereas those aged > 90 years had an incidence rate of 86.14 (95% CI 65.81 to 112.75) per 1000 person-years for bisphosphonate users and 85.86 (95% CI 75.5 to 97.65) per 1000 person-years for non-users.
FIGURE 6.
Male and female incidence rates of CKD worsening, compared with participants who did not worsen in CKD stage, per 1000 person-years, categorised by exposure to bisphosphonate and age.
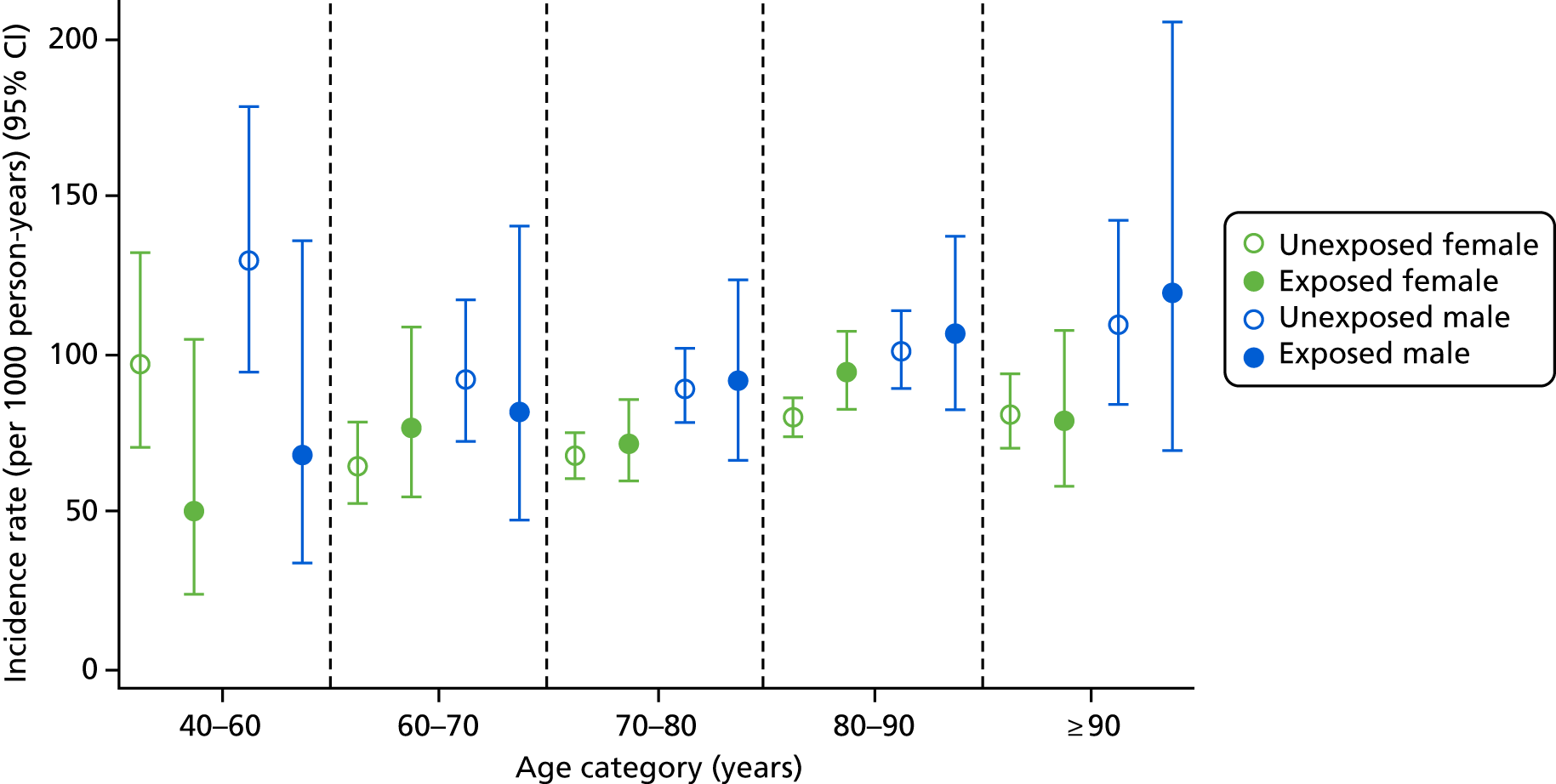
Men were more likely to experience worsening CKD, with incidence rates of 97.09 (95% CI 89.78 to 105.00) and 75.10 (95% CI 71.12 to 79.31) per 1000 person-years for unexposed men and women, respectively.
In a post hoc analysis focused on CKD improvement (i.e. reclassification to a less severe stage based on eGFR measures), improving CKD was slightly more frequent in participants who had not been exposed to bisphosphonates [incidence rate 246.83 (95% CI 238.66 to 255.28) per 1000 person-years] than bisphosphonate users [incidence rate 224.01 (95% CI 209.52 to 239.50) per 1000 person-years]. The age-, sex- and exposure-specific incidence rates for CKD stage improvement are shown in Figure 7.
FIGURE 7.
Male and female incidence rates of CKD improvement per 1000 person-years, stratified by exposure to bisphosphonate, age and sex.
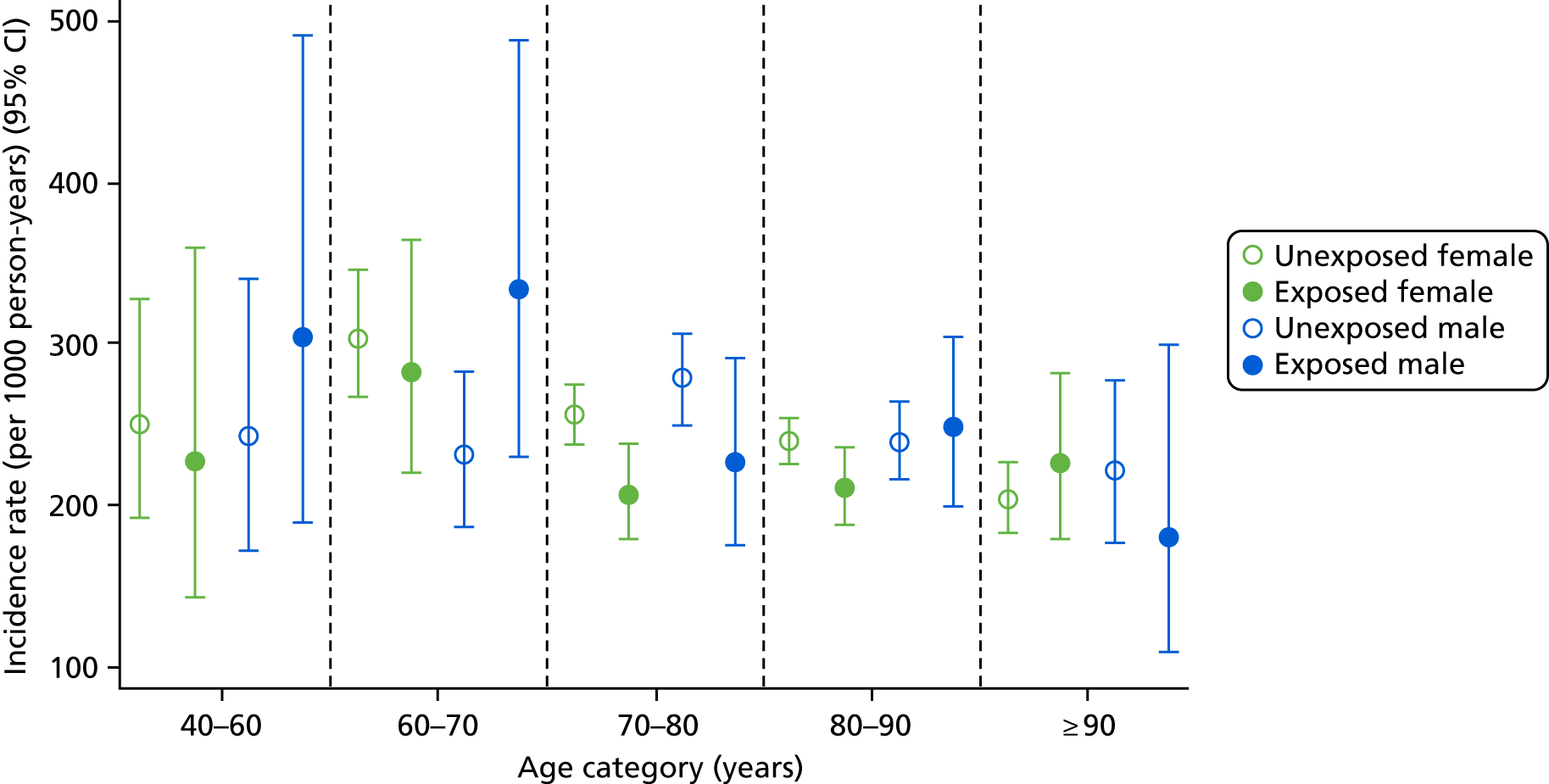
Improvement in men and women was similar, with relatively static incidence rates of improvement regardless of age and sex. Overall, men had a slightly higher rate of improvement than women [exposed men: incidence rate 246.92 (95% CI 215.41 to 283.05) per 1000 person-years; exposed women: incidence rate 217.64 (95% CI 201.57 to 234.99) per 1000 person-years]. The lowest rate of improvement was found in those aged > 90 years, with an incidence rate of 208.22 (95% CI 188.45 to 230.07) per 1000 person-years for unexposed participants. However, the incidence rates for participants exposed to bisphosphonate were constant across the following age groups: 70–80 years, 80–90 years and > 90 years; they were 211.7 (95% CI 187.2 to 239.4), 218.85 (95% CI 198.2 to 241.66) and 217.96 (95% CI 177.48 to 267.68) per 1000 person-years, respectively.
Fine and Gray competing risks model
In the primary analysis, there were 576 events in the propensity-matched bisphosphonate user cohort: 36 end-stage renal failure events and 540 eGFR-based CKD stage-worsening events. The matched unexposed cohort reported 1996 events: 192 end-stage renal failure and 1804 eGFR-based CKD stage-worsening events. Cumulative incidence function plots are depicted in Figure 8.
FIGURE 8.
Cumulative incidence plots of (a) CKD stage worsening or treatment for end-stage renal failure; and (b) only CKD stage worsening, stratified by bisphosphonate use or non-use.
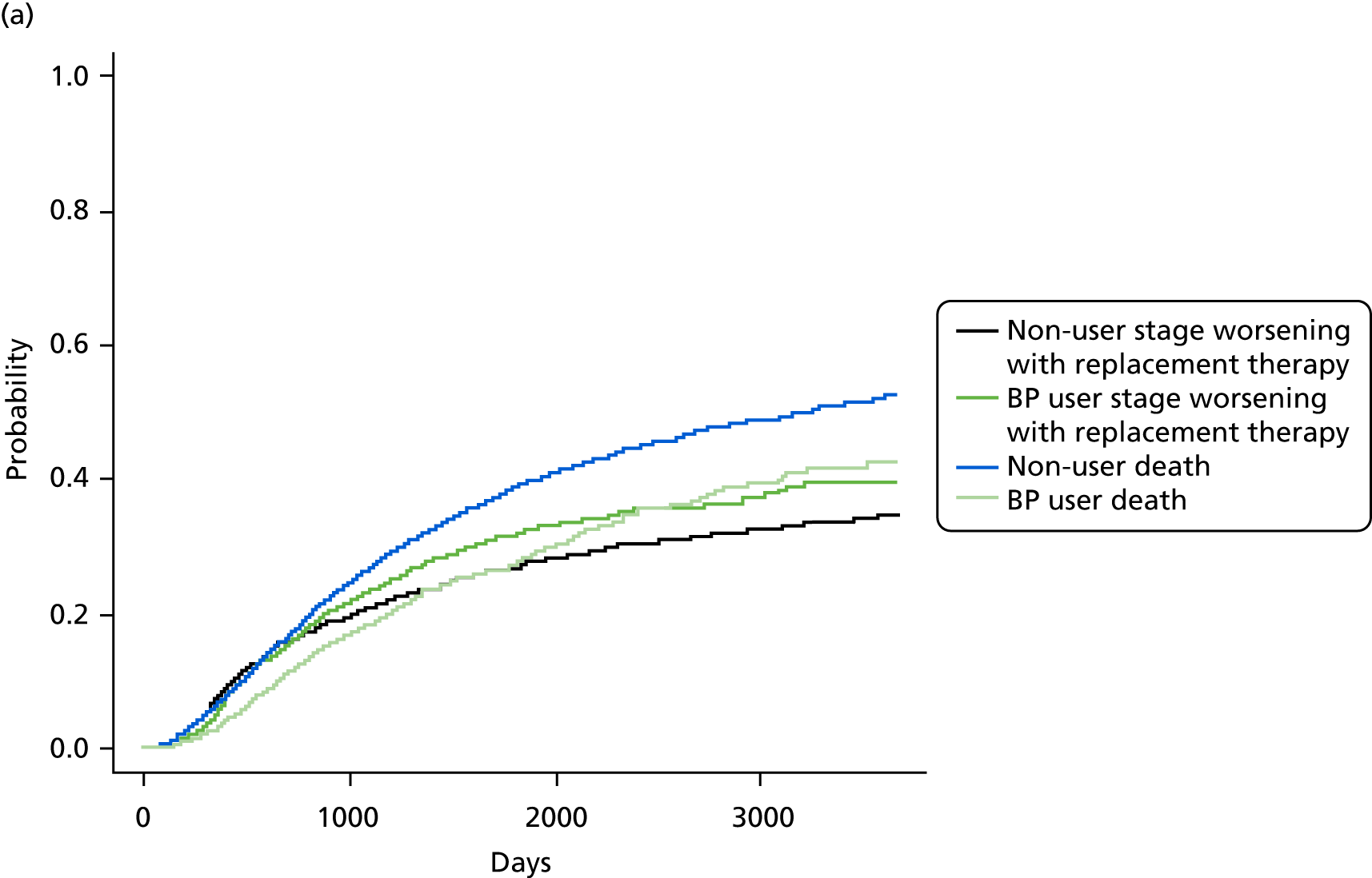
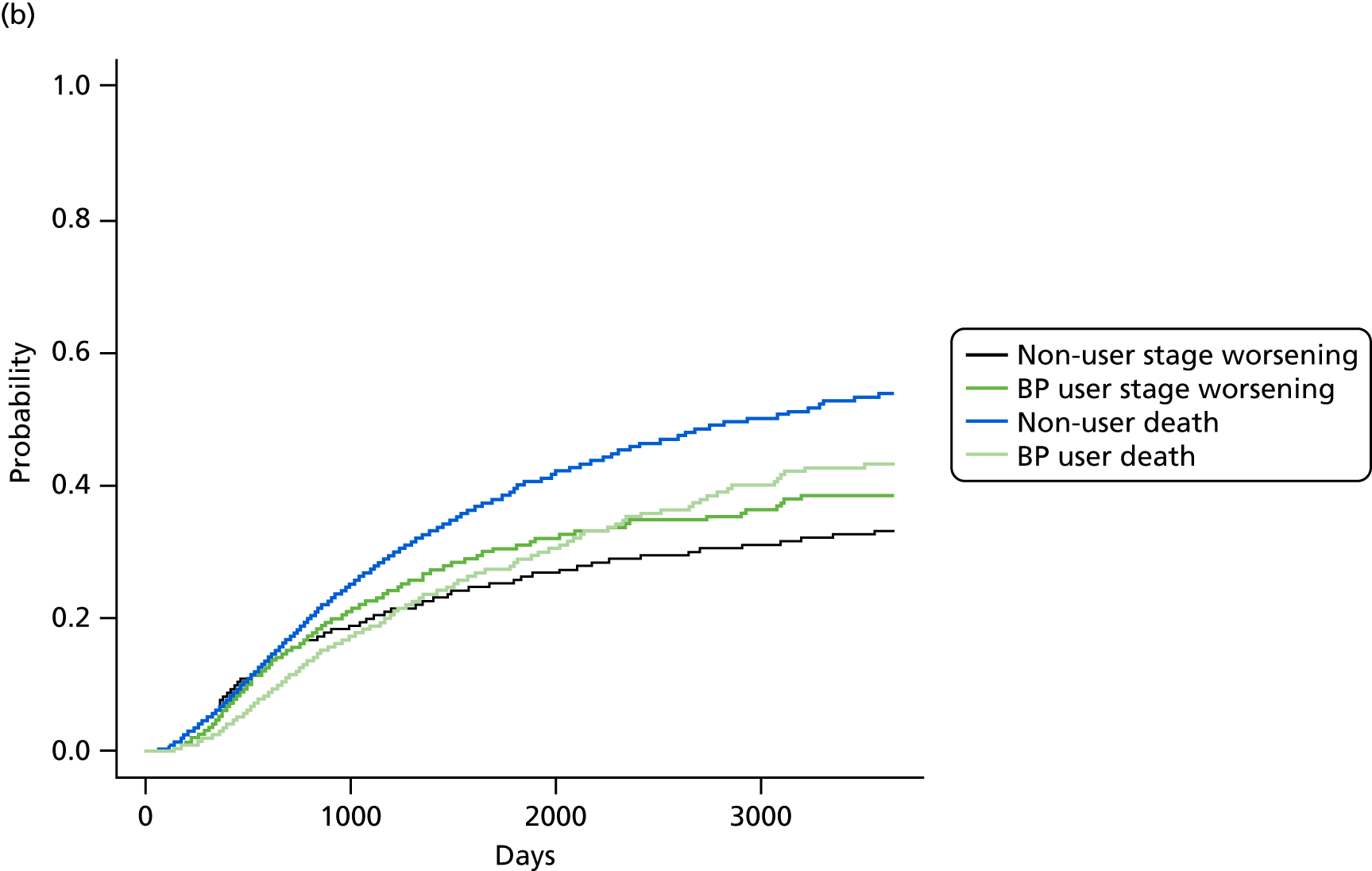
Bisphosphonate use was associated with an increased risk of CKD progression, measured by stage worsening or receiving renal replacement therapy (sHR 1.12, 95% CI 1.02 to 1.24). The results of the post hoc sensitivity analyses are detailed in Table 6.
| Outcome | Number of exposed events | Number of unexposed events | HR | 95% CI |
|---|---|---|---|---|
| End-stage renal failure or CKD stage worsening | 576 | 1996 | 1.12 | 1.02 to 1.24 |
| CKD stage worsening | 559 | 1908 | 1.14 | 1.03 to 1.27 |
| CKD stage change | ||||
| Stable | 1057 | 3698 | Ref | Ref |
| Worsening | 534 | 1783 | 1.11 | 1.00 to 1.23 |
| Improvement | 858 | 3473 | 0.93 | 0.86 to 1.01 |
The first post hoc analysis defined CKD progression as only stage worsening. It gave results similar to the primary analysis, with 559 bisphosphonate users and 1908 non-users in the propensity-matched cohort changing to a worse eGFR-based stage (sHR 1.14, 95% CI 1.03 to 1.27).
The second post hoc analysis used participants with stable CKD (no change in stage, neither improving nor worsening, during follow-up) as the reference group. Bisphosphonate users were at a higher risk of worsening CKD (sHR 1.11, 95% CI 1.0 to 1.23). There was a borderline significant reduction in their probability of improving: sHR 0.93 (95% CI 0.86 to 1.01).
See Figure 8 for the cumulative incidence plots of the primary and first post hoc analyses. Similar plots of the second post hoc analysis are shown in Appendix 1, Figure 25.
The cumulative incidence function of CKD stage worsening or treatment for end-stage renal failure gave a number needed to harm of 40.8 for a 3-year bisphosphonate treatment regimen, and of 20.7 for a 5-year bisphosphonate treatment regimen.
Interactions
Interactions were identified with history of fracture (p = 0.03) and sex (p = 0.04). Participants with a history of fracture were more likely to experience CKD stage worsening when exposed to bisphosphonates (sHR 1.32, 95% CI 1.05 to 1.66) than those without a history of fracture (sHR 1.10, 95% CI 0.99 to 1.22). These CIs overlap, suggesting a non-significant difference without clinical relevance between the participants with and those without a history of fracture who experienced improved CKD. Women were also more likely to experience stage change than men after matching within sex, with sHRs of 1.24 (95% CI 1.11 to 1.38) and 1.00 (95% CI 0.83 to 1.21) for women and men, respectively.
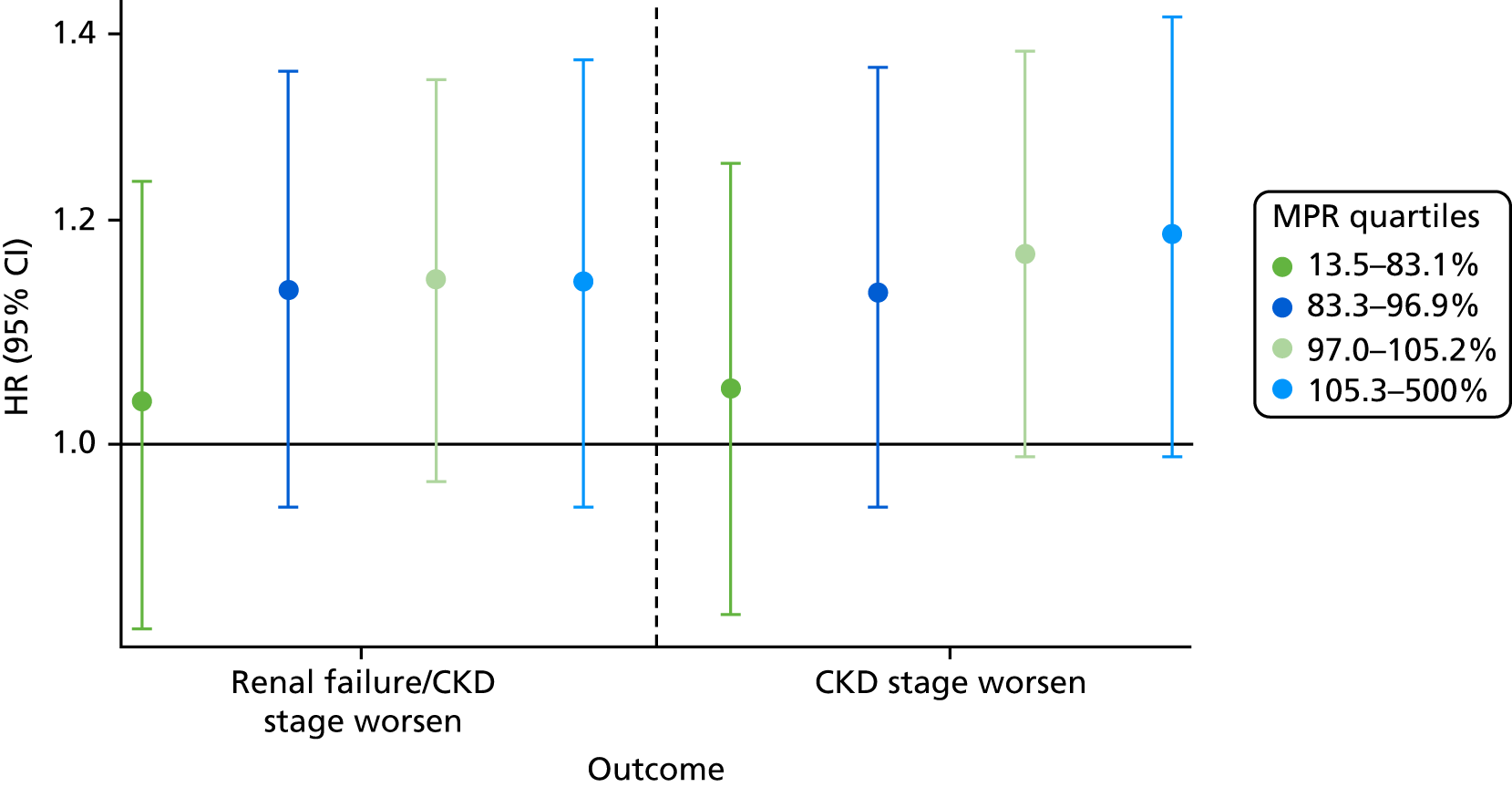
Medication possession ratio
When the exposed participants were split into their MPR quartiles, there was little difference between the quartiles, with all CIs overlapping (Figure 9). Participants in the highest quartile were at increased risk of worsening CKD. The participants who were least likely to worsen in CKD stage were those in the lowest quartile, i.e., the participants least adherent to their prescribed bisphosphonate treatment. The Mantel extension test detected no difference between MPRs for renal failure/stage worsening (p-value 0.38) but a borderline significant difference (p = 0.07) for CKD progression based on eGFR stage change alone.
FIGURE 9.
Hazard ratios of each outcome, split into MPR quartiles.
Multivariable analysis
As requested by the Steering Committee, a multivariable analysis was used as a post hoc sensitivity analysis in place of propensity score matching. Bisphosphonate use was associated with an 18% increase in the risk of the primary outcome of CKD worsening, defined by stage change or end-stage renal disease (adjusted HR 1.18, 95% CI 1.08 to 1.29). The full results of this multivariable analysis are shown in Table 7.
| Covariate | Renal failure or CKD stage worsening | CKD stage worsening |
|---|---|---|
| Bisphosphonate use | 1.18 (1.08 to 1.29) | 1.22 (1.12 to 1.33) |
| Sex (female) | 0.77 (0.75 to 0.80) | 0.78 (0.75 to 0.80) |
| Age per year | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.00) |
| Smoker | ||
| Non-smoker | Ref | Ref |
| Ex-smoker | 1.05 (1.01 to 1.10) | 1.05 (1.00 to 1.10) |
| Current smoker | 1.19 (1.11 to 1.27) | 1.20 (1.12 to 1.29) |
| Alcohol | ||
| Non-drinker | Ref | Ref |
| Ex-drinker | 0.99 (0.90 to 1.08) | 0.99 (0.89 to 1.09) |
| Current drinker | 0.94 (0.90 to 0.99) | 0.94 (0.90 to 0.98) |
| IMD | ||
| 1 – least deprived | Ref | Ref |
| 2 | 0.97 (0.93 to 1.02) | 0.98 (0.93 to 1.02) |
| 3 | 1.05 (1.00 to 1.10) | 1.05 (1.00 to 1.11) |
| 4 | 1.01 (0.96 to 1.06) | 1.02 (0.97 to 1.07) |
| 5 – most deprived | 1.11 (1.05 to 1.17) | 1.11 (1.05 to 1.18) |
| BMI (kg/m2) per 1 unit | 1.00 (1.00 to 1.01) | 1.00 (1.00 to 1.01) |
| Baseline eGFR (ml/minute/1.73 m2) | ||
| 0.1–4.9 | 6.85 (5.09 to 9.22) | Not possible |
| 5–9.9 | 5.46 (4.72 to 6.30) | Not possible |
| 10–14.9 | 2.31 (2.01 to 2.67) | Not possible |
| 15–19.9 | 4.09 (3.76 to 4.45) | 3.74 (3.43 to 4.09) |
| 20–24.9 | 1.66 (1.53 to 1.79) | 1.60 (1.48 to 1.74) |
| 25–29.9 | 0.72 (0.66 to 0.78) | 0.66 (0.60 to 0.72) |
| 30–34.9 | 3.99 (3.82 to 4.16) | 4.02 (3.85 to 4.19) |
| 35–39.9 | 1.89 (1.81 to 1.97) | 1.89 (1.82 to 1.97) |
| 40–44.9 | Ref | Ref |
| Number of hospital visits | ||
| 0 | Ref | Ref |
| 1 | 1.04 (1.00 to 1.08) | 1.04 (1.00 to 1.09) |
| 2 | 1.08 (1.02 to 1.14) | 1.10 (1.04 to 1.17) |
| 3–5 | 1.12 (1.05 to 1.19) | 1.13 (1.06 to 1.20) |
| ≥ 6 | 1.31 (1.16 to 1.49) | 1.28 (1.12 to 1.47) |
| 5-year Charlson score | ||
| 0 | Ref | Ref |
| 1 | 1.09 (1.04 to 1.15) | 1.09 (1.04 to 1.14) |
| 2 | 1.07 (1.01 to 1.13) | 1.06 (1.00 to 1.12) |
| 3–5 | 1.15 (1.08 to 1.23) | 1.15 (1.08 to 1.22) |
| ≥ 6 | 1.34 (1.21 to 1.48) | 1.33 (1.20 to 1.48) |
| Type 2 diabetes mellitus | 0.91 (0.85 to 0.96) | 0.90 (0.84 to 0.96) |
| Cancer | 0.98 (0.93 to 1.04) | 0.98 (0.93 to 1.04) |
| CKD | 0.96 (0.91 to 1.02) | 0.95 (0.90 to 1.00) |
| Antiarrhythmic agents | 0.92 (0.87 to 0.96) | 0.93 (0.88 to 0.97) |
| Dementia | 0.97 (0.84 to 1.13) | 0.98 (0.85 to 1.14) |
| Cardiovascular disease | 0.97 (0.93 to 1.02) | 0.98 (0.93 to 1.03) |
| Hip fracture | 1.23 (1.02 to 1.49) | 1.21 (1.00 to 1.46) |
| Non-hip fracture | 0.97 (0.90 to 1.06) | 0.97 (0.89 to 1.05) |
| Deep-vein thrombosis | 1.01 (0.92 to 1.11) | 1.02 (0.92 to 1.12) |
| Varices | 0.98 (0.91 to 1.05) | 0.99 (0.92 to 1.06) |
| Hypertension | 0.92 (0.89 to 0.95) | 0.92 (0.89 to 0.96) |
| Hyperlipidaemia | 0.95 (0.90 to 1.00) | 0.95 (0.90 to 1.00) |
| Liver disease | 0.93 (0.73 to 1.19) | 0.89 (0.69 to 1.15) |
| Cerebrovascular disease | 0.97 (0.90 to 1.04) | 0.96 (0.90 to 1.04) |
| Peripheral vascular disease | 1.03 (0.92 to 1.14) | 1.03 (0.92 to 1.15) |
| Hyperthyroidism | 0.89 (0.69 to 1.17) | 0.88 (0.67 to 1.16) |
| Number of prescriptions | ||
| 0 | Ref | Ref |
| 1–3 | 0.90 (0.81 to 0.99) | 0.94 (0.84 to 1.04) |
| 4–6 | 0.86 (0.78 to 0.95) | 0.89 (0.80 to 0.99) |
| 7–9 | 0.89 (0.80 to 0.99) | 0.92 (0.83 to 1.03) |
| 10–12 | 0.90 (0.80 to 1.00) | 0.93 (0.83 to 1.04) |
| ≥ 13 | 0.93 (0.83 to 1.04) | 0.97 (0.86 to 1.09) |
| NSAID | 0.89 (0.86 to 0.92) | 0.89 (0.86 to 0.92) |
| Hormone replacement therapy | 0.81 (0.75 to 0.88) | 0.82 (0.76 to 0.89) |
| Calcium supplements | 1.12 (1.05 to 1.19) | 1.10 (1.02 to 1.17) |
| Steroids | 0.97 (0.93 to 1.01) | 0.97 (0.92 to 1.01) |
| Anticoagulants | 1.00 (0.94 to 1.06) | 1.00 (0.94 to 1.06) |
| Proton-pump inhibitors | 0.94 (0.90 to 0.97) | 0.94 (0.91 to 0.98) |
| Heparins | 1.21 (1.00 to 1.46) | 1.21 (0.99 to 1.47) |
| Aromatase inhibitors | 0.98 (0.76 to 1.26) | 1.00 (0.78 to 1.30) |
| Antidepressants | 0.96 (0.92 to 0.99) | 0.96 (0.92 to 0.99) |
| Statins | 0.92 (0.89 to 0.96) | 0.91 (0.87 to 0.94) |
| Antiepileptics | 0.93 (0.86 to 1.02) | 0.94 (0.86 to 1.02) |
| Diuretics | 1.03 (0.99 to 1.07) | 1.02 (0.98 to 1.06) |
| Beta blockers | 0.95 (0.92 to 0.98) | 0.95 (0.91 to 0.98) |
| ACE inhibitors | 1.09 (1.04 to 1.13) | 1.08 (1.04 to 1.13) |
| Calcium channel blockers | 1.12 (1.08 to 1.16) | 1.11 (1.08 to 1.16) |
| Antihypertensives | 1.07 (1.02 to 1.12) | 1.06 (1.01 to 1.11) |
| Digoxin | 1.10 (1.04 to 1.17) | 1.10 (1.04 to 1.17) |
| Antidiabetics | 1.44 (1.37 to 1.52) | 1.49 (1.41 to 1.57) |
| Insulin | 1.12 (1.04 to 1.20) | 1.11 (1.03 to 1.20) |
Covariates associated with an increased risk included the following:
-
smoking –
-
HR 1.19 (95% CI 1.11 to 1.27) for current smokers
-
HR 1.05 (95% CI 1.01 to 1.10) for ex-smokers
-
-
socioeconomic deprivation –
-
HR 1.05 (95% CI 1.00 to 1.10) for IMD quartiles 3 and 4
-
HR 1.11 (95% CI 1.05 to 1.17) for IMD quartile 5
-
-
having a lower eGFR at baseline –
-
HR 6.85 (95% CI 5.09 to 9.22) for an eGFR of 0.1–4.9 ml/minute/1.73 m2
-
HR 5.46 (95% CI 4.72 to 6.30) for an eGFR of 5–9.9 ml/minute/1.73 m2
-
HR 2.31 (95% CI 2.01 to 2.67) for an eGFR of 10–14.9 ml/minute/1.73 m2
-
HR 4.09 (95% CI 3.76 to 4.45) for an eGFR of 15–19.9 ml/minute/1.73 m2
-
HR 1.66 (95% CI 1.53 to 1.79) for an eGFR of 20–24.9 ml/minute/1.73 m2
-
HR 3.99 (95% CI 3.82 to 4.16) for an eGFR of 30–34.9 ml/minute/1.73 m2
-
HR 1.89 (95% CI 1.81 to 1.97) for an eGFR of 35–39.9 ml/minute/1.73 m2
-
-
high health resource use, as measured by hospital episodes in the year before index –
-
HR 1.04 (95% CI 1.00 to 1.08) for those with one admission
-
HR 1.08 (95% CI 1.02 to 1.14) for those with two admissions
-
HR 1.12 (95% CI 1.05 to 1.19) for those with three to five admissions
-
HR 1.31 (95% CI 1.16 to 1.49) for those with six or more admissions
-
-
comorbidity to as measured by the Charlson Comorbidity Index score –
-
HR 1.09 (95% CI 1.04 to 1.15) for a score of 1
-
HR 1.07 (95% CI 1.01 to 1.13) for a score of 2
-
HR 1.15 (95% CI 1.08 to 1.23) for a score of 3–5
-
HR 1.34 (95% CI 1.21 to 1.48) for a score of ≥ 6
-
-
history of hip fracture – HR 1.23 (95% CI 1.02 to 1.49)
-
treatment in the year before index with –
-
calcium supplements: HR 1.12 (95% CI 1.05 to 1.19)
-
heparins: HR 1.21 (95% CI 1.00 to 1.46)
-
angiotensin-converting enzyme (ACE) inhibitors: HR 1.09 (95% CI 1.04 to 1.13)
-
calcium channel blockers: HR 1.12 (95% CI 1.08 to 1.16)
-
other antihypertensives: HR 1.07 (95% CI 1.02 to 1.12)
-
digoxin: HR 1.10 (95% CI 1.04 to 1.17)
-
antidiabetics: HR 1.44 (95% CI 1.37 to 1.52)
-
insulin: HR 1.12 (95% CI 1.04 to 1.20).
-
Covariates protective against CKD stage worsening or end-stage renal therapy included the following:
-
being female – HR 0.77 (95% CI 0.75 to 0.80)
-
currently drinking alcohol – HR 0.94 (95% CI 0.90 to 0.99)
-
having type 2 diabetes mellitus – HR 0.91 (95% CI 0.85 to 0.96)
-
having hypertension – HR 0.92 (95% CI 0.89 to 0.95)
-
using antiarrhythmic agents – HR 0.92 (95% CI 0.87 to 0.96)
-
using non-steroidal anti-inflammatory drugs (NSAIDs) – HR 0.89 (95% CI 0.86 to 0.92)
-
using hormone replacement therapy – HR 0.81 (95% CI 0.75 to 0.88)
-
using proton-pump inhibitors – HR 0.94 (95% CI 0.90 to 0.97)
-
using antidepressants – HR 0.96 (95% CI 0.92 to 0.99)
-
using statins – HR 0.92 (95% CI 0.89 to 0.96)
-
using beta blockers – HR 0.95 (95% CI 0.92 to 0.98).
Similar results were found in a sensitivity analysis that defined CKD progression as only a worsening in the eGFR-based CKD stage (see Table 7).
Array sensitivity analysis
We conducted post hoc analyses to measure the effect of potential unmeasured confounding on the results. Array analysis combines a number of values for an expected difference in a particular unmeasured variable’s prevalence and that variable’s association with the study outcome to adjust the main (estimated) treatment effect size. We considered two potential unobserved confounders: osteoporosis, which has a prevalence of 30% in bisphosphonate non-users, and an albumin-to-creatine ratio (ACR) of > 3.3, which has a prevalence of 21.5% in bisphosphonate non-users. For both confounders, we considered a range of prevalences in bisphosphonate users, 15% to 80%.
In the first array, we evaluated the potential effect of under-recording osteoporosis diagnoses on CKD worsening, from no effect (HR 1.0) to a threefold excess risk (HR 3.0). The results are shown in Figure 10a, where bright green, turquoise and blue represent scenarios in which the observed effect would be explained by the given unobserved confounder. Only combinations of high prevalence and extreme hazard ratios (e.g. a prevalence of 40% and HR of 3.0, or a prevalence of 35% and HR of 5.0 or a prevalence of 80% and HR of 1.25) attenuated the association between bisphosphonate use and CKD progression after adjusting for this otherwise completely unobserved variable. This simulates the potential effect of unmeasured diagnoses of osteoporosis, which is often not well recorded in electronic medical records.
FIGURE 10.
Array sensitivity analysis of the effect of an unobserved confounder at a range of prevalences in users of bisphosphonates, when the confounder’s prevalence in non-users of bisphosphonates is (a) 30% and (b) 21.5%. Bright green, blue and turquoise (HR < 1) indicate scenarios in which the observed effect would be explained by the unobserved confounder.
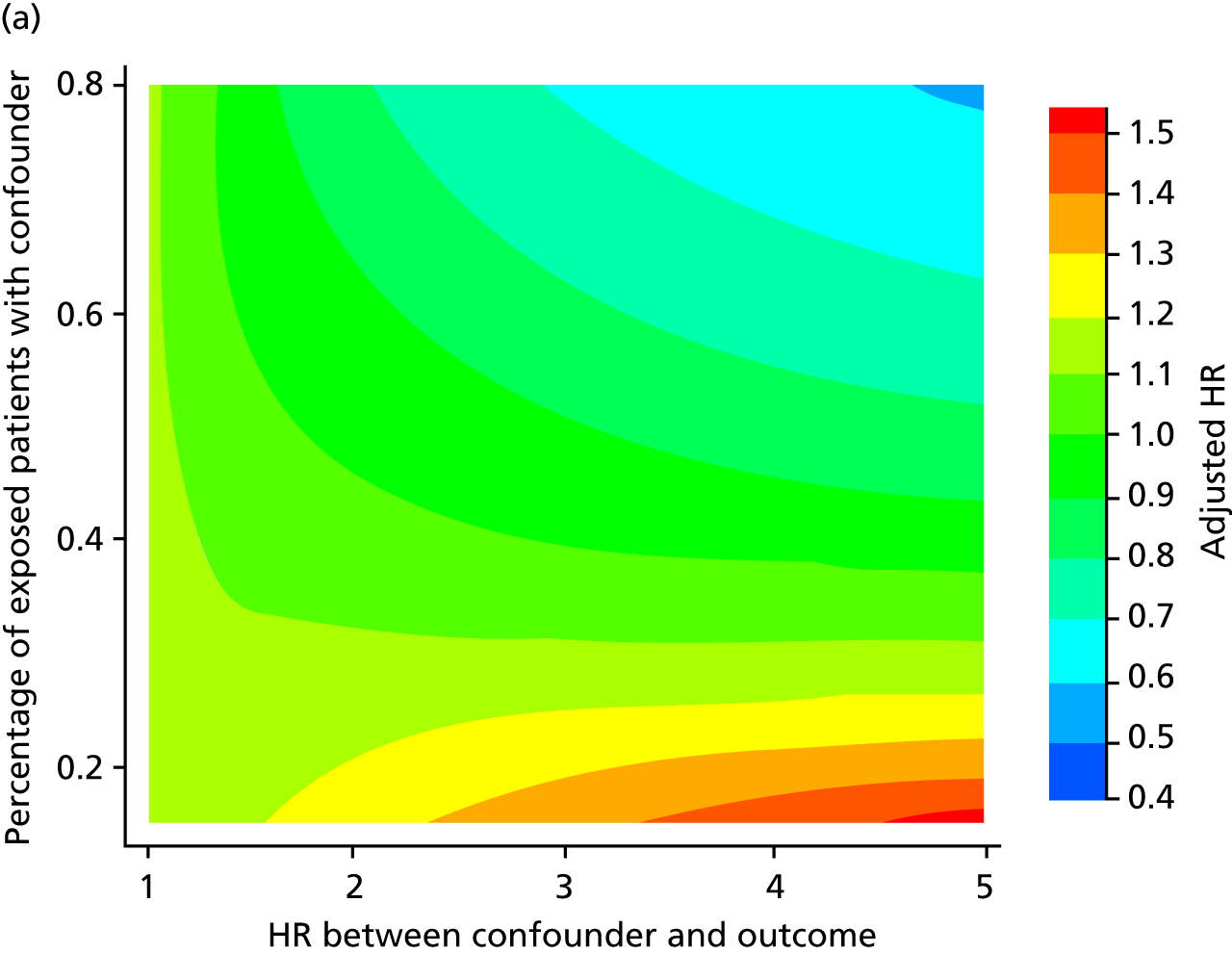
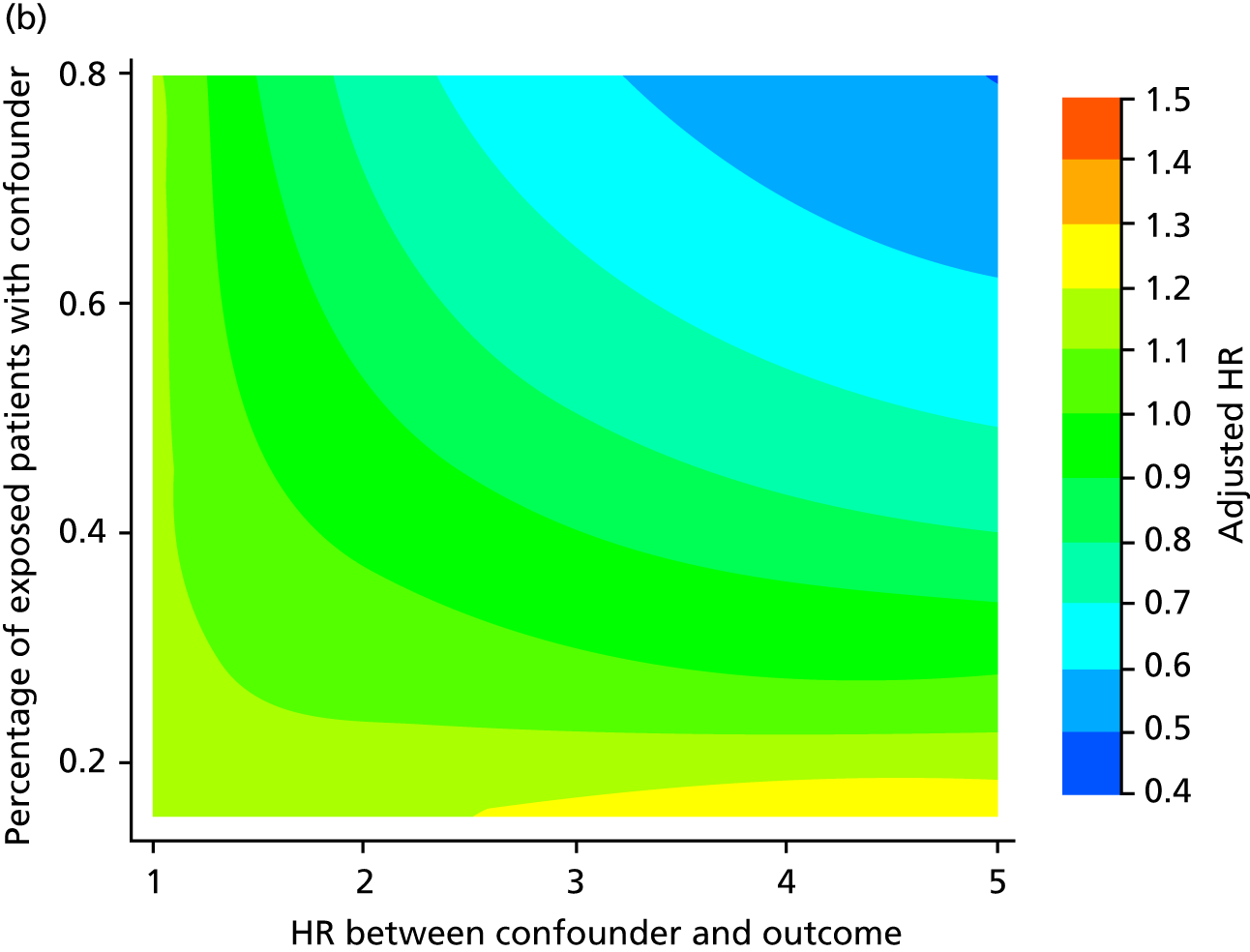
In the second array, we evaluated the potential impact of an unmeasured ACR of > 3.3 on CKD worsening, from no effect (HR 1.0) to a fivefold excess risk (HR 5.0). The results (see Figure 10b) again suggest that only extreme situations would result in a null association between bisphosphonate use and CKD progression, such as when > 30% of the exposed participants have an ACR of > 3.3 with a HR of 5.0, or when the HR is 1.5 but 50% of the exposed participants have the confounder. Unmeasured ACR of > 3.3 has a prevalence of 21.5% and HR of 2.68 for CKD progression. Therefore, the results would be nullified only if > 35% of bisphosphonate users have an ACR of > 3.3. We believe these to be unlikely yet not impossible clinical scenarios.
Random-effects modelling
Table 8 shows the distribution of annual eGFR for bisphosphonate users and matched non-users. Overall, we found no evidence of a large change in repeat annual eGFR over the available 10-year period.
| Time point | Bisphosphonate users | Propensity score-matched non-users | ||
|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Baseline | 34.66 (7.46) | 36.00 (30.40–40.37) | 34.95 (7.76) | 36.83 (30.69–41) |
| 1 year | 36.02 (10.98) | 36.00 (29.13–42.24) | 36.65 (10.83) | 37.22 (30.35–43.00) |
| 2 years | 36.46 (11.49) | 36.00 (29.41–42.98) | 37.17 (11.69) | 37.34 (29.89–44.30) |
| 3 years | 35.89 (12.25) | 35.26 (27.69–43.00) | 37.19 (12.07) | 37.21 (29.75–44.72) |
| 4 years | 36.54 (12.80) | 36.00 (28.00–44.62) | 37.46 (12.62) | 37.22 (29.44–45.14) |
| 5 years | 36.71 (12.89) | 36.00 (28.57–44.71) | 37.30 (13.32) | 37.00 (29.00–45.10) |
| 6 years | 37.87 (12.95) | 38.00 (29.00–45.00) | 37.36 (13.86) | 37.00 (28.12–46.00) |
| 7 years | 38.93 (13.29) | 39.00 (31.00–48.00) | 37.11 (13.68) | 36.64 (27.86–46.00) |
| 8 years | 38.99 (13.26) | 39.13 (29.47–49.00) | 36.99 (14.08) | 36.64 (27.99–46.00) |
| 9 years | 37.71 (16.27) | 37.26 (27.53–45.15) | 36.18 (14.40) | 35.00 (27.00–46.00) |
| 10 years | 33.69 (12.71) | 34.00 (25.65–47.64) | 35.76 (14.12) | 35.00 (26.00–44.40) |
The best-fitting model based on the fractional polynomial models suggested that the rate of eGFR change was non-linear and was best represented with a power of –2. An examination of the best model suggested that the shape of the annual rate of change in eGFR changed at 2 years. Mixed models with two linear rates before and after 2 years were fitted. Figure 11 shows that the mean eGFR increased at the rate of 3.29 (95% CI 3.22 to 3.37) ml/minute/1.73 m2 per year for non-users and 1.29 (95% CI 1.14 to 1.44) ml/minute/1.73 m2 per year for users, and then slowly declined with slopes of –0.70 (95% CI –0.79 to –0.62) per year for non-users and –0.38 (95% CI –0.54 to –0.22) per year for users.
FIGURE 11.
Mean eGFR over 10 years for bisphosphonate users and non-users.
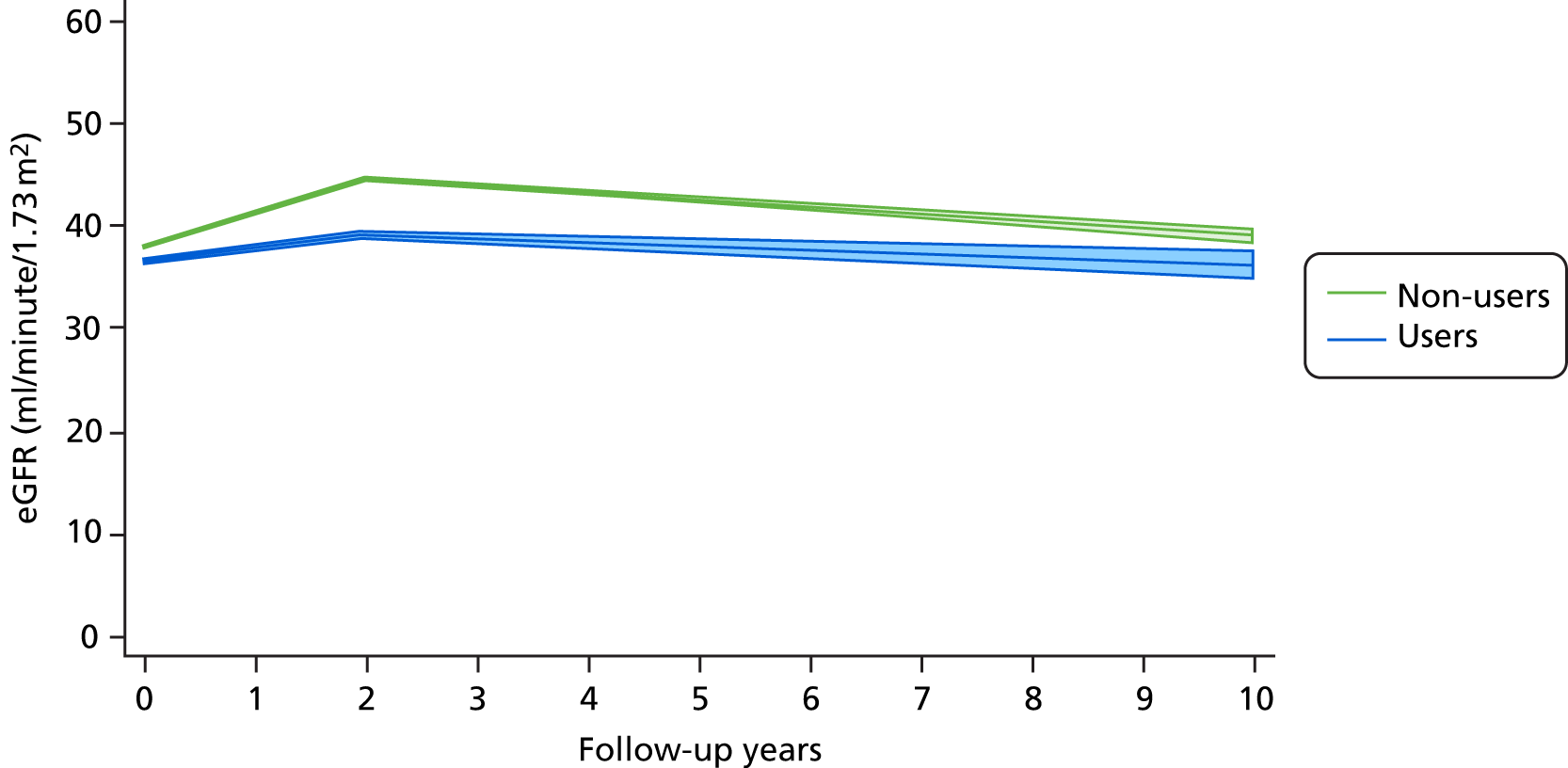
Random-effects models do not censor patients at the time point they exit from the outcome at-risk window. Instead, these models assume that missingness is not informative. This assumption is likely to be violated, particularly in cases where data are missing due to death. In addition, there was heterogeneity in the individual eGFR patterns. The median rate of eGFRs changes in the first 2 years for non-users and users were –0.13 [minimum –9.28, maximum 11.59; interquartile range (IQR) –2.73 to 2.50] and –0.08 (minimum –5.10, maximum 6.74; IQR –1.29 to 1.78). The median rates of changes for the remaining follow-up years were –0.13 (minimum –9.28, maximum 11.59; IQR –2.73 to 2.50) for non-users and 0.46 (minimum –11.51, maximum 7.97; IQR –2.26 to 2.57) for users. These imply that the results in this section should be interpreted with caution.
Chapter 4 Work package 2: the relationship between bisphosphonate use and incident symptomatic (clinical) osteoporotic fractures
Introduction
Large multicentre clinical trials have consistently shown that oral bisphosphonates reduce the risk of incident clinical non-vertebral and vertebral fractures in women. 77 In men, trials have demonstrated commensurate increases in BMD and reductions in vertebral and potentially non-vertebral fractures. 78 Post hoc analyses of trial results have not found differences in BMD or antifracture efficacy by renal function. 79 However, participants in these trials may not be generalisable to the general population treated with oral bisphosphonates.
There are limitations when translating efficacy from oral bisphosphonate clinical trials to effectiveness in real-world settings. 12 A major limitation of trials for oral bisphosphonates is that they exclude participants with impaired renal function, defined as a creatinine level of > 144 µmol/l for alendronate,80 > 1.1× the upper limit of normal for risedronate11 and > 212 µmol/l for oral ibandronate,81 excluding participants with CKD stage 5. 79 The fracture efficacy of oral alendronate in real-world settings has been demonstrated in patients with hip fracture, with consistent reductions in fracture risk using an ecological interrupted time-series analysis. 82 However, the effect of patients’ renal function on fracture efficacy has not been examined.
The aetiopathophysiology of increased bone fragility differs in patients with more severe CKD from those with postmenopausal osteoporosis. 83 The reduced renal clearance of bisphosphonates in CKD patients may be associated with reduced efficacy due to the accumulation of metabolites in bone tissue. A meta-analysis of bisphosphonate therapy in patients with CKD found that bisphosphonates reduced non-vertebral fracture risk, although few events were observed. However, conflict associations between bisphosphonates and vertebral fracture risk were also identified. 16
We aimed to assess the association between bisphosphonate use and risk of hip (primary outcome) and osteoporotic fractures in patients with CKD.
Methods
Participants and follow-up period
A new-user cohort analysis was conducted. Patients aged > 40 years with at least one eGFR measurement of < 45 ml/minute/1.73 m2 recorded in the CPRD were eligible. Patients were excluded if they had used bisphosphonates in the year before their first eGFR measurement of < 45 ml/minute/1.73 m2, used other non-bisphosphonate anti-osteoporotic therapy any time previously, or were missing IMD information. The process of deriving the code lists for each drug is described in Chapter 2, Main exposure: bisphosphonate use.
All participants initially joined as unexposed to bisphosphonates. Participants could contribute both exposed and unexposed time (see Chapter 2, Immortal time bias). Unexposed participants were followed up from their index date (first eGFR measurement of < 45 ml/minute/1.73 m2) until the earliest occurrence of one of the following: end of enrolment in the database due to migration, transfer out or death, or a newly recorded fracture. When applicable, participants switched to the exposure cohort (time-varying exposure) at the date of their first bisphosphonate prescription after their index date. On a participant’s first bisphosphonate use within the observation time after the index date, their most recent eGFR measurement was assessed. Those with an eGFR of < 45 ml/minute/1.73 m2 moved to the exposed category. Those with a bisphosphonate prescription and an eGFR of ≥ 45 ml/minute/1.73 m2 were censored at this point and contributed unexposed time only before their prescription.
Bisphosphonate users were followed from the start of their bisphosphonate therapy until the earliest occurrence of one of the following:
-
end of enrolment in the database (due to moving out or death)
-
stopping therapy (last prescription before a 6-month prescription gap) plus 210 days, made up of 30 days of the last prescription and a washout period of 180 days
-
switching to (or having added) another anti-osteoporosis medication
-
10-year follow-up
-
incident recorded fracture, as defined below.
Study exposure
The exposure of interest was bisphosphonate use, as identified in primary care prescriptions recorded in CPRD data. Prescriptions of bisphosphonates (same or other types within the same drug class) were concatenated to create treatment episodes (see Chapter 2, Immortal time bias). Each bisphosphonate user was then propensity matched with up to five non-users based on the prespecified confounders described in Chapter 2, Propensity score methods. Balance before and after matching for each characteristic was assessed using the ASMD with a cut-off point of 0.1.
Outcomes
The outcome of interest was clinical (symptomatic) fracture as recorded in CPRD primary care records. Hip fracture was chosen as the primary outcome, as this is the most widely validated type of fracture in CPRD. Non-hip fracture (all locations except hip, face, digit and skull fractures) and osteoporotic fracture (all locations except face, digit and skull fractures) were used as secondary outcomes. Face, digit and skull fractures were not analysed as they are usually trauma related, not osteoporotic or fragility related. Code lists for these outcomes were prespecified using previous research and validation studies within the CPRD (see Report Supplementary Material 2). These lists were updated to include the most recent version of the Read coding system.
Statistical analysis
The crude and age- and sex-specific incidence rates (and 95% CIs) of each of the outcomes were estimated separately in the propensity score-matched cohorts for bisphosphonate users and non-users per 1000 person-years. Rates were calculated assuming a Poisson distribution. Kaplan–Meier plots were used to depict the predicted cumulative probability of each of the study end points, stratified by bisphosphonate use.
Cox proportional hazards models were fitted for the propensity score-matched cohorts to estimate HRs and 95% CIs for each of the outcomes, according to bisphosphonate use. Models were computed for each imputation and combined using Rubin’s rules. 52 The assumption of proportionality was checked graphically using c-loglog plots.
As requested by the Steering Committee, a multivariable analysis was undertaken on the full data set using all covariates included in the propensity score.
Sensitivity analyses
Predefined interactions with the use of bisphosphonates were tested for sex, history of previous Read-coded fracture and baseline CKD stage as defined by index eGFR. Stratified analyses are reported for any interactions with a p-value of < 0.1.
To test whether or not the observed associations between bisphosphonate use and each of the outcomes followed a gradient, bisphosphonate users were further categorised into MPR quartiles, defined as the number of defined daily doses prescribed over the total number of days of follow-up. Cox proportional hazards models were used to compare the HRs for each of the MPR categories when compared with matched non-users.
Post hoc analysis
Given the counterintuitive results detailed below, a member of the Steering Committee with a long track record in similar observational analyses of drug effectiveness suggested a method to check for the presence of unresolved confounding. Pivotal RCTs have shown that bisphosphonates need between 6 months and 1 year to have any antifracture efficacy. 80,84 This was used as a test for residual confounding: any association between bisphosphonate use and fracture risk in the first 6–12 months of treatment reflected baseline risk, not treatment effects. We fitted the Cox survival analyses after restricting the follow-up time to the first 180 days and to the first year of treatment to test for this confounding.
We conducted a sensitivity array analysis looking at the effect of unmeasured confounding, as recommended by Schneeweiss. 76
Results
Target cohort and study participants
We identified 217,405 CPRD patients who had an eGFR of ≤ 45 ml/minute/1.73 m2 and were therefore eligible. Figure 12 shows that patients were excluded if they had eGFR measurements only before 1997, their eGFR was exactly 45 ml/minute/1.73 m2, they were aged < 40 years at their first eGFR measurement of < 45 ml/minute/1.73 m2, they had used bisphosphonates in the year before the eGFR measurement of < 45 ml/minute/1.73 m2, they had previously used any other anti-osteoporotic drugs, they had experienced an adverse event of interest before study entry or their IMD information was missing. Time unexposed to bisphosphonates was contributed by 215,617 participants, of whom 11,567 participants also contributed time as bisphosphonate users.
FIGURE 12.
Summary of identification of eligible participants in CPRD GOLD for investigating the relationship between bisphosphonate use and osteoporotic fracture in patients with CKD, as per inclusion/exclusion criteria.
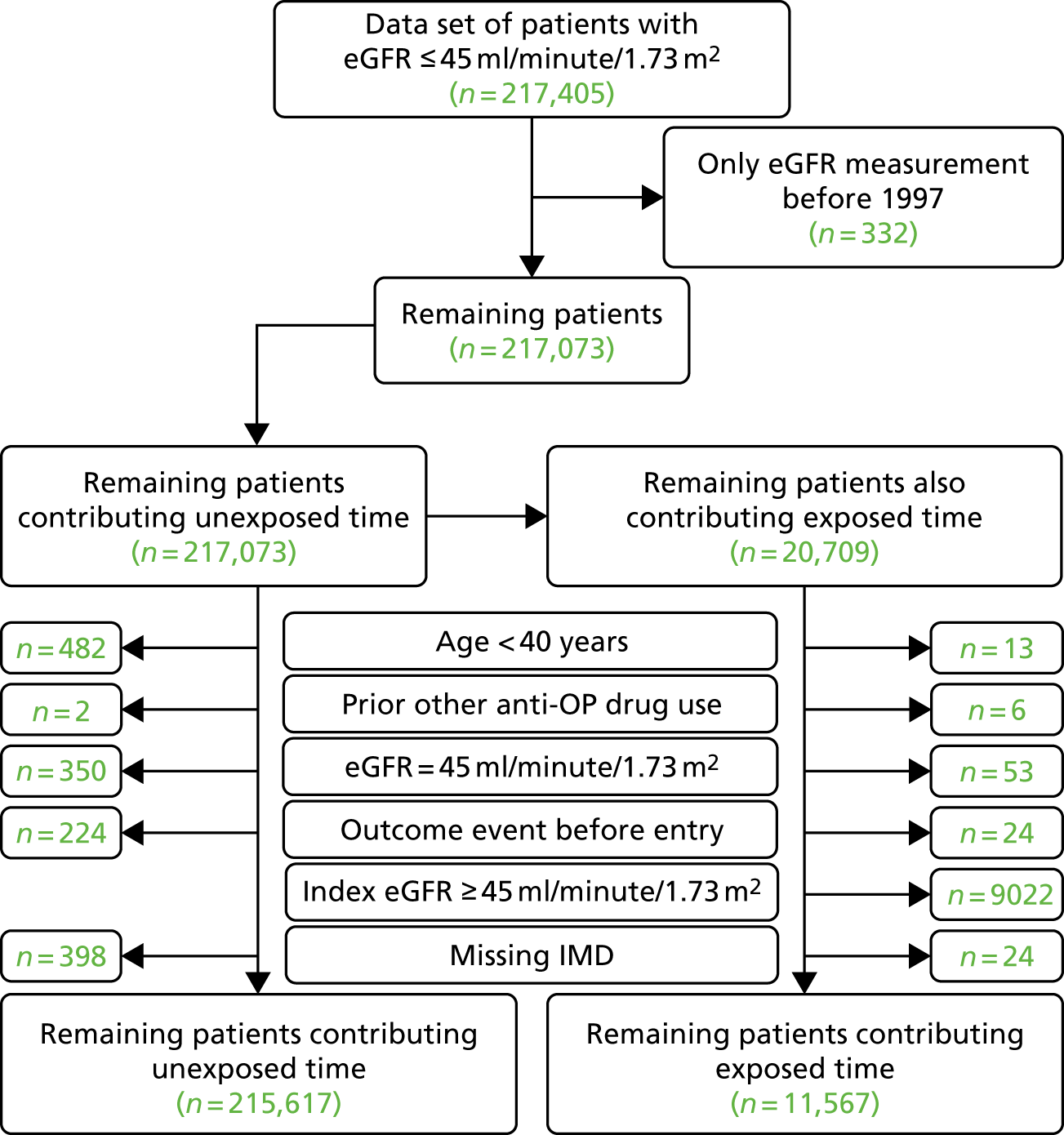
Propensity score matching
Table 9 shows the sociodemographic and clinical characteristics of bisphosphonate users and non-users before and after propensity score matching. Before matching, bisphosphonate users were more likely than non-users to be female (80.4% vs. 58.6%), have a higher Charlson Comorbidity Index score (29.5% vs. 14.9% with a 3+ index), have had one or more hospital admissions in the previous year (60.4% vs. 36.5%), and had ≥ 10 prescriptions in the previous 12 months (61.9% vs. 38.5%). Bisphosphonate users were also more likely than non-users to have diagnosed CKD (45.8% vs. 16.5%), have a history of non-hip fracture (24.3% vs. 4.9%), use systemic glucocorticoids (44.5% vs. 19.2%) and have previously used either calcium supplements (29.5%) in the year before the index date or bisphosphonates more than 1 year before the index date (6.6% vs. 2.5%).
| Characteristic | Before matching, n (%) | After matching, n (%) | ||
|---|---|---|---|---|
| Unexposed | Exposed | Unexposed | Exposed | |
| Number | 215,617 | 11,567 | 43,999 | 11,118 |
| Age (years), mean (SD) | 78.1 (10.1) | 82.0 (8.8) | 80.9 (9.3) | 81.8 (8.9) |
| Sex (female) | 126,347 (58.6) | 9,301 (80.4) | 33,176 (75.4) | 8864 (79.7) |
| IMD quintiles | ||||
| 1 – least deprived | 47,935 (22.2) | 686 (23.2) | 9952 (22.6) | 2566 (23.1) |
| 2 | 50,849 (23.6) | 2720 (23.5) | 10,322 (23.5) | 2628 (23.6) |
| 3 | 45,815 (21.2) | 2462 (21.3) | 9390 (21.3) | 2363 (21.3) |
| 4 | 42,522 (19.7) | 2187 (18.9) | 8525 (19.4) | 2113 (19.0) |
| 5 – most deprived | 28,496 (13.2) | 1512 (13.1) | 5810 (13.2) | 1448 (13.0) |
| BMI (kg/m2), mean (SD)a | 27.7 (5.6) | 26.5 (5.4) | 26.5 (5.4) | 26.3 (5.3) |
| Smoking categoryb | ||||
| No | 88,732 (41.2) | 5975 (51.7) | 24,784 (56.3) | 6367 (57.3) |
| Ex | 62,942 (29.2) | 3635 (31.4) | 15,267 (34.7) | 3838 (34.5) |
| Yes | 19,071 (8.8) | 822 (7.1) | 3948 (9.0) | 913 (8.2) |
| Drinking categoryc | ||||
| No | 35,178 (16.3) | 2603 (22.5) | 13,364 (30.4) | 3501 (31.5) |
| Ex | 5597 (2.6) | 407 (3.5) | 1962 (4.5) | 529 (4.8) |
| Yes | 95,366 (44.2) | 5295 (45.8) | 28,673 (65.2) | 7088 (63.8) |
| eGFR category (ml/minute/1.73 m2) | ||||
| 0–4.9 | 500 (0.2) | 19 (0.2) | 80 (0.2) | 19 (0.2) |
| 5–9.9 | 1669 (0.8) | 89 (0.8) | 354 (0.8) | 85 (0.8) |
| 10–14.9 | 2162 (1.0) | 112 (1.0) | 435 (1.0) | 109 (1.0) |
| 15–19.9 | 3644 (1.7) | 253 (2.2) | 897 (2.0) | 238 (2.1) |
| 20–24.9 | 6413 (3.0) | 557 (4.8) | 1782 (4.1) | 522 (4.7) |
| 25–29.9 | 11,569 (5.4) | 1076 (9.3) | 3548 (8.1) | 998 (9.0) |
| 30–34.9 | 21,437 (9.9) | 1985 (17.2) | 6491 (14.8) | 1861 (16.7) |
| 35–39.9 | 44,788 (20.8) | 3038 (26.3) | 10,988 (25.0) | 2909 (26.2) |
| 40–44.9 | 123,435 (57.2) | 4438 (38.4) | 19,424 (44.1) | 4377 (39.4) |
| Number of hospital visits | ||||
| 0 | 136,907 (63.5) | 4578 (39.6) | 20,325 (46.2) | 4514 (40.6) |
| 1 | 42,034 (19.5) | 3239 (28.0) | 11,516 (26.2) | 3075 (27.7) |
| 2 | 18,398 (8.5) | 1822 (15.8) | 5945 (13.5) | 1720 (15.5) |
| 3–5 | 14,194 (6.6) | 1520 (13.1) | 4869 (11.1) | 1415 (12.7) |
| ≥ 6 | 4084 (1.9) | 408 (3.5) | 1344 (3.1) | 394 (3.5) |
| Charlson Comorbidity Index score | ||||
| 0 | 120,169 (55.7) | 3871 (33.5) | 17,567 (39.9) | 3827 (34.4) |
| 1 | 30,272 (14.0) | 1618 (14.0) | 6681 (15.2) | 1584 (14.2) |
| 2 | 33,055 (15.3) | 2675 (23.1) | 9340 (21.2) | 2532 (22.8) |
| 3–5 | 25,702 (11.9) | 2678 (23.2) | 8239 (18.7) | 2498 (22.5) |
| ≥ 6 | 6419 (3.0) | 725 (6.3) | 2172 (4.9) | 677 (6.1) |
| Rheumatoid arthritis | 2669 (1.2) | 511 (4.4) | 1335 (3.0) | 468 (4.2) |
| Varices | 15,312 (7.1) | 1252 (10.8) | 4169 (9.5) | 1165 (10.5) |
| Deep-vein thrombosis | 8000 (3.7) | 425 (3.7) | 1680 (3.8) | 412 (3.7) |
| Type 2 diabetes mellitus | 24,407 (11.3) | 1380 (11.9) | 4964 (11.3) | 1330 (12.0) |
| Dementia | 6670 (3.1) | 490 (4.2) | 1785 (4.1) | 469 (4.2) |
| Diagnosed CKD | 35,483 (16.5) | 5295 (45.8) | 15,087 (34.3) | 4872 (43.8) |
| Cerebrovascular disease | 18,083 (8.4) | 1368 (11.8) | 4660 (10.6) | 1285 (11.6) |
| Peripheral vascular disease | 4787 (2.2) | 367 (3.2) | 1166 (2.7) | 347 (3.1) |
| Hypertension | 96,797 (44.9) | 6479 (56.0) | 22,587 (51.3) | 6128 (55.1) |
| Hyperlipidaemia | 24,703 (11.5) | 1745 (15.1) | 6018 (13.7) | 1645 (14.8) |
| Liver disease | 1241 (0.6) | 93 (0.8) | 318 (0.7) | 90 (0.8) |
| Peptic ulcer | 3535 (1.6) | 308 (2.7) | 1038 (2.4) | 286 (2.6) |
| Osteomalacia/rickets | 54 (0.0) | 9 (0.1) | 21 (0.0) | 8 (0.1) |
| Cancer | 27,016 (12.5) | 2054 (17.8) | 7195 (16.4) | 1964 (17.7) |
| Hip fracture | 2582 (1.2) | 210 (1.8) | 767 (1.7) | 207 (1.9) |
| Non-hip fracture | 10,553 (4.9) | 2806 (24.3) | 6311 (14.3) | 2464 (22.2) |
| Number of prescriptions | ||||
| 0 | 5412 (2.5) | 98 (0.8) | 487 (1.1) | 98 (0.9) |
| 1–3 | 29,359 (13.6) | 541 (4.7) | 2846 (6.5) | 538 (4.8) |
| 4–6 | 49,951 (23.2) | 1495 (12.9) | 6807 (15.5) | 1472 (13.2) |
| 7–9 | 47,895 (22.2) | 2277 (19.7) | 9316 (21.2) | 2212 (19.9) |
| 10–12 | 34,845 (16.2) | 2304 (19.9) | 8737 (19.9) | 2224 (20.0) |
| ≥ 13 | 48,155 (22.3) | 4852 (42.0) | 15,806 (35.9) | 4574 (20.0) |
| Hormone replacement therapy | 14,366 (6.7) | 949 (8.2) | 3478 (7.9) | 912 (8.2) |
| Contraceptive | 224 (0.1) | 9 (0.1) | 26 (0.1) | 9 (0.1) |
| Calcium supplements | 19,705 (9.1) | 3372 (29.2) | 9558 (21.7) | 3114 (28.0) |
| Bisphosphonates more than 1 year before baseline | 5494 (2.5) | 760 (6.6) | 2740 (6.2) | 718 (6.5) |
| Steroids | 41,451 (19.2) | 5149 (44.5) | 16,535 (37.6) | 4832 (43.5) |
| Anticoagulants | 26,714 (12.4) | 1829 (15.8) | 6523 (14.8) | 1745 (15.7) |
| Heparin | 2457 (1.1) | 257 (2.2) | 796 (1.8) | 237 (2.1) |
| Aromatase inhibitors | 1651 (0.8) | 297 (2.6) | 800 (1.8) | 274 (2.5) |
| NSAIDs | 127,469 (59.1) | 8098 (70.0) | 28,086 (63.8) | 7754 (69.7) |
| Proton-pump inhibitors | 83,680 (38.8) | 6582 (56.9) | 22,697 (51.6) | 6242 (56.1) |
| Anxiols/sedatives/hypnotics | 45,761 (21.2) | 3292 (28.5) | 11,694 (26.6) | 3145 (28.3) |
| Antidepressants | 84,371 (39.1) | 5433 (47.0) | 16,821 (38.2) | 4636 (41.7) |
| Statins | 90,970 (42.2) | 6185 (53.5) | 19,231 (43.7) | 5167 (46.5) |
| Calcium channel blockers | 120,034 (55.7) | 7536 (65.2) | 21,811 (49.6) | 5883 (52.9) |
| ACE inhibitors | 10,854 (5.0) | 925 (8.0) | 27,042 (61.5) | 7177 (64.6) |
| Antiepileptics | 148,421 (68.8) | 9298 (80.4) | 3014 (6.9) | 877 (7.9) |
| Diuretics | 97,489 (45.2) | 5843 (50.5) | 33,921 (77.1) | 8888 (79.9) |
| Beta blockers | 33,340 (15.5) | 1570 (13.6) | 21,129 (48.0) | 5570 (50.1) |
| Antidiabetics | 7730 (3.6) | 420 (3.6) | 5957 (13.5) | 1525 (13.7) |
| Insulin | 22,197 (10.3) | 1368 (11.8) | 1558 (3.5) | 403 (3.6) |
| Digoxin | 31,169 (14.5) | 2223 (19.2) | 5916 (13.4) | 1296 (11.7) |
| Antihypertensives | 27,296 (12.7) | 1774 (15.3) | 7607 (17.3) | 2098 (18.9) |
| Antiarrhythmic agents | 14,366 (6.7) | 949 (8.2) | 6378 (14.5) | 1687 (15.2) |
After matching, bisphosphonate users and non-users were similar in most characteristics. Some key differences remained. Users had more hospital visits, a higher Charlson Comorbidity Index score and a slightly lower eGFR than non-users. Users were more likely than non-users to have a history of diagnosed CKD (43.8% vs. 34.3%), hypertension (55.1% vs. 51.3%) and non-hip fracture (22.2% vs. 14.3%). They were also more likely than non-users to receive calcium supplements (28.0% vs. 21.7%), steroids (43.5% vs. 37.6%), NSAIDs (69.7% vs. 63.8%) and proton-pump inhibitors (56.1% vs. 51.6%).
The differences were considered acceptable as per the prespecified balance diagnostics after propensity score matching, as all characteristics had an ASMD of < 0.1 (Figure 13). Histograms also showed a much better overlap of propensity scores between bisphosphonate users and non-users after matching (see Appendix 2, Figure 26).
FIGURE 13.
The ASMD of each covariate included in propensity score matching for assessing the association between bisphosphonate use and osteoporotic fracture, before and after matching. (a) 1, Age; 2, sex; 3a, ex-smoker; 3b, current smoker; 4a, ex-drinker; 4b, current drinker; 5a, IMD quintile 2; 5b, IMD quintile 3; 5c, IMD quintile 4; 5d, IMD quintile 5; 6, BMI; 7a, start eGFR value of 5–9.9 ml/minute/1.73 m2; 7b, start eGFR value of 10–14.9 ml/minute/1.73 m2; 7c, start eGFR value of 15–19.9 ml/minute/1.73 m2; 7d, start eGFR value of 20–24.9 ml/minute/1.73 m2; 7e, start eGFR value of 25–29.9 ml/minute/1.73 m2; 7f, start eGFR value of 30–34.9 ml/minute/1.73 m2; 7g, start eGFR value of 35–39.9 ml/minute/1.73 m2; 7h, start eGFR value of 40–44.9 ml/minute/1.73 m2; 8a, 1 hospital visit in the previous year; 8b, 2 hospital visits in the previous year; 8c, 3–5 hospital visits in the previous year; 8d, ≥ 6 hospital visits in the previous year; 9a, 5-year Charlson Comorbidity Index score of 1; 9b, 5-year Charlson Comorbidity Index score of 2; 9c, 5-year Charlson Comorbidity Index score of 3–5; 9d, 5-year Charlson Comorbidity Index score of ≥ 6; 10, history of rheumatoid arthritis; 11, varices; 12, deep-vein thrombosis; 13, hip fracture; 14, non-hip fracture; 15, dementia; 16, type 2 diabetes mellitus; and (b): 17, chronic renal disease; 18, cerebrovascular disease; 19, peripheral vascular disease; 20, hypertension; 21, liver disease; 22, peptic ulcer; 23, cardiovascular disease; 24, renal disease; 25, hyperthyroidism; 26, cancer; 27, hyperlipidaemia; 28a, 1–3 prescriptions in the previous year; 28b, 4–6 prescriptions in the previous year; 28c, 7–9 prescriptions in the previous year; 28d, 10–12 prescriptions in the previous year; 28e, ≥ 13 prescriptions in the previous year; 29, history of osteomalacia or rickets; 30, prior prescription of calcium supplements; 31, steroids; 32, anticoagulants; 33, heparins; 34, hormone replacement therapy; 35, proton-pump inhibitors; 36, aromatase inhibitors; 37, anxiolytics/sedatives/hypnotic; 38, antidepressant; 39, statins; 40, calcium channel blockers; 41, ACE inhibitors; 42, antiepileptics; 43, diuretics; 44, beta blockers; 45, antihypertensives; 46, antidiabetics; 47, antiarrhythmic agents; 48, insulin.
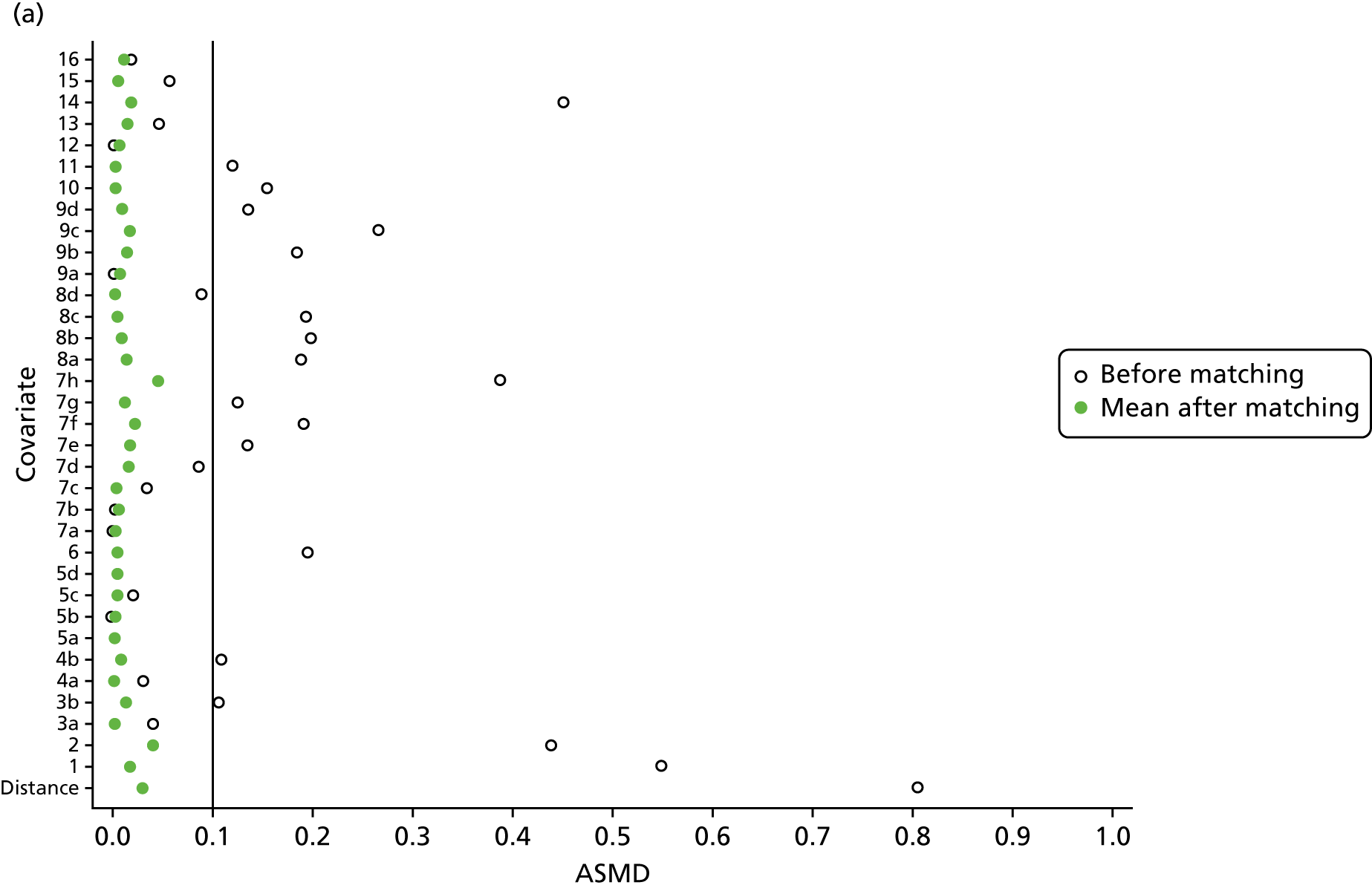
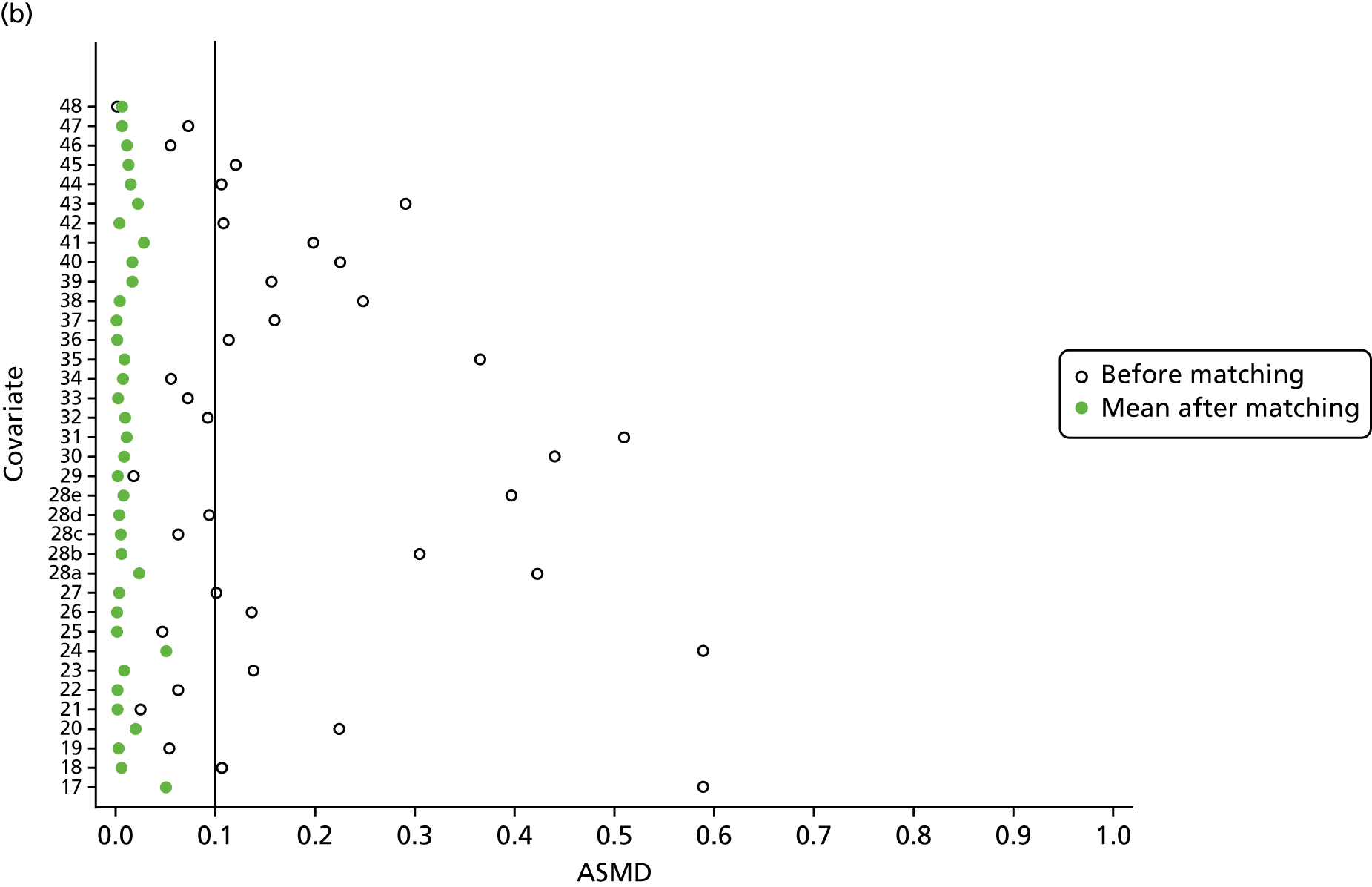
Hip fracture
Bisphosphonate users had an increased rate of hip fracture compared with non-users, with incidence rates of 20.70 (95% CI 20.14 to 21.28) per 1000 person-years and 16.41 (95% CI 16.2 to 16.62) per 1000 person-years, respectively. As expected, hip fracture rates increased dramatically with age. For example, exposed participants aged 40–60 years had an incidence rate of 4.03 (95% CI 2.82 to 5.77) per 1000 person-years, whereas exposed participants aged > 90 years had an incidence rate of 37.59 (95% CI 35.46 to 39.86) per 1000 person-years. Women had a greater hip fracture risk than men. For example, exposed women had an incidence rate of 21.71 (95% CI 21.09 to 22.37) per 1000 person-years and exposed men an incidence rate of 15.77 (95% CI 14.61 to 17.03) per 1000 person-years. Incidence rates stratified by age, sex and bisphosphonate use are shown in Figure 14.
FIGURE 14.
Incidence rates per 1000 person-years of hip fractures, stratified by age, sex and bisphosphonate exposure.
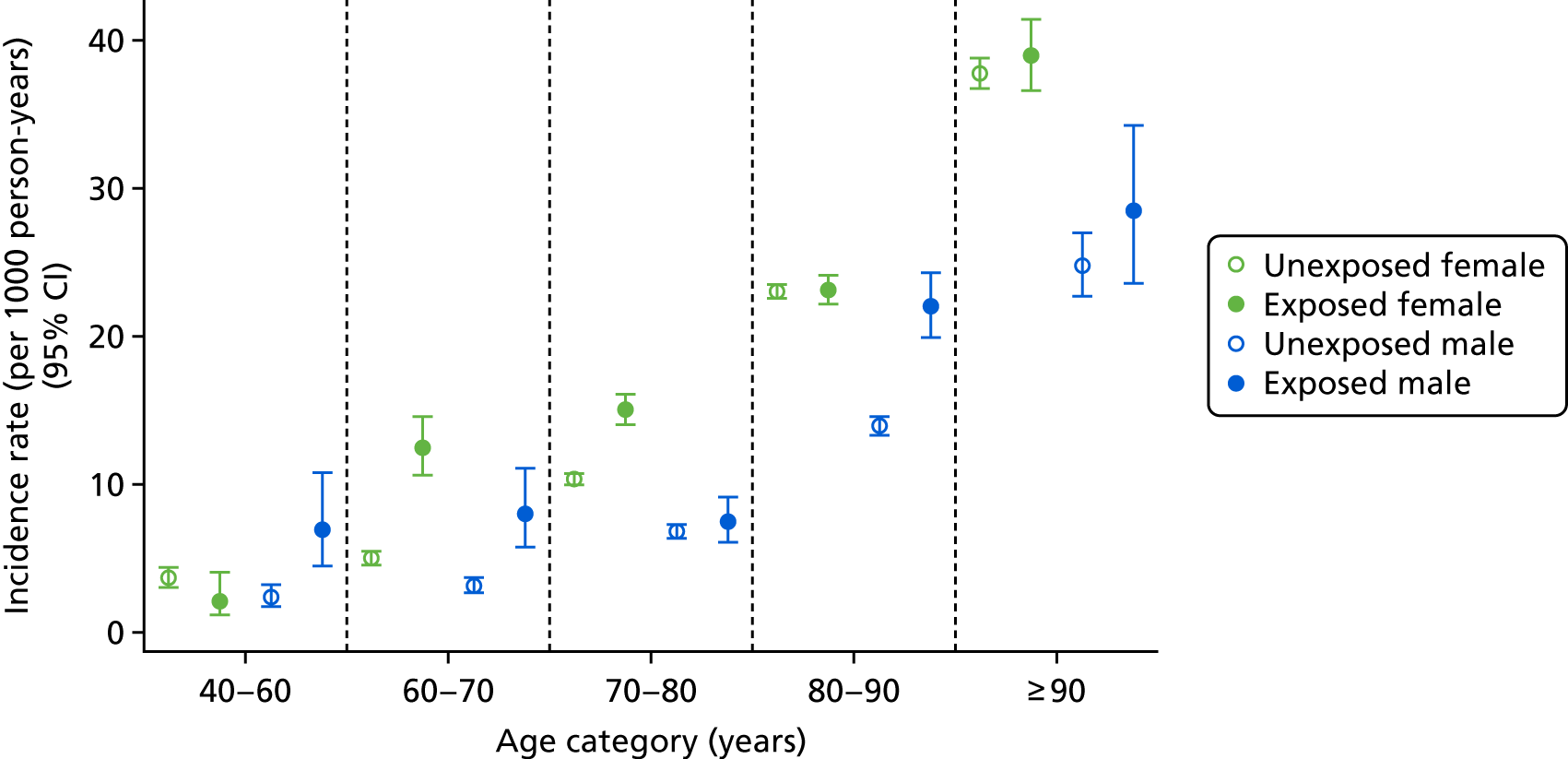
Non-hip fracture
During follow-up, 4339 participants sustained a non-hip fracture (919 bisphosphonate users and 3420 matched non-users) of whom 3832 were women and 507 men. Non-hip fractures occurred at all ages and occurred at an earlier age than hip fractures. For example, 69 non-hip fractures were identified in participants aged 40–60 years at index date, 262 in those aged 60–70 years, 1118 in those aged 70–80 years, 2147 in those aged 80–90 years and 743 in those aged > 90 years who entered the study.
Bisphosphonate users had a higher rate of non-hip fracture than non-users, with incidence rates of 39.4 (95% CI 38.6 to 40.21) per 1000 person-years and 24.73 (95% CI 24.47 to 25.00) per 1000 person-years, respectively. Few participants in the younger age groups were exposed to bisphosphonates, and the incidence rates of non-hip fractures increased with age. For example, exposed participants aged 40–60 years had an incidence rate of 13.16 (95% CI 10.75 to 16.10) per 1000 person-years, whereas those aged > 90 years had an incidence rate of 54.86 (95% CI 52.23 to 57.61) per 1000 person-years. Women had a higher non-hip fracture risk than men. For example, female bisphosphonate users had an incidence rate of 42.71 (95% CI 41.8 to 43.65) per 1000 person-years, whereas male users had an incidence rate of 23.78 (95% CI 22.33 to 25.33) per 1000 person-years. Non-hip fractures had a higher incidence rate than hip fractures in general. Figure 15 shows the age-, sex- and exposure-specific incidence rates and CIs.
FIGURE 15.
Incidence rates of non-hip fractures, stratified by age, sex and exposure to bisphosphonates.
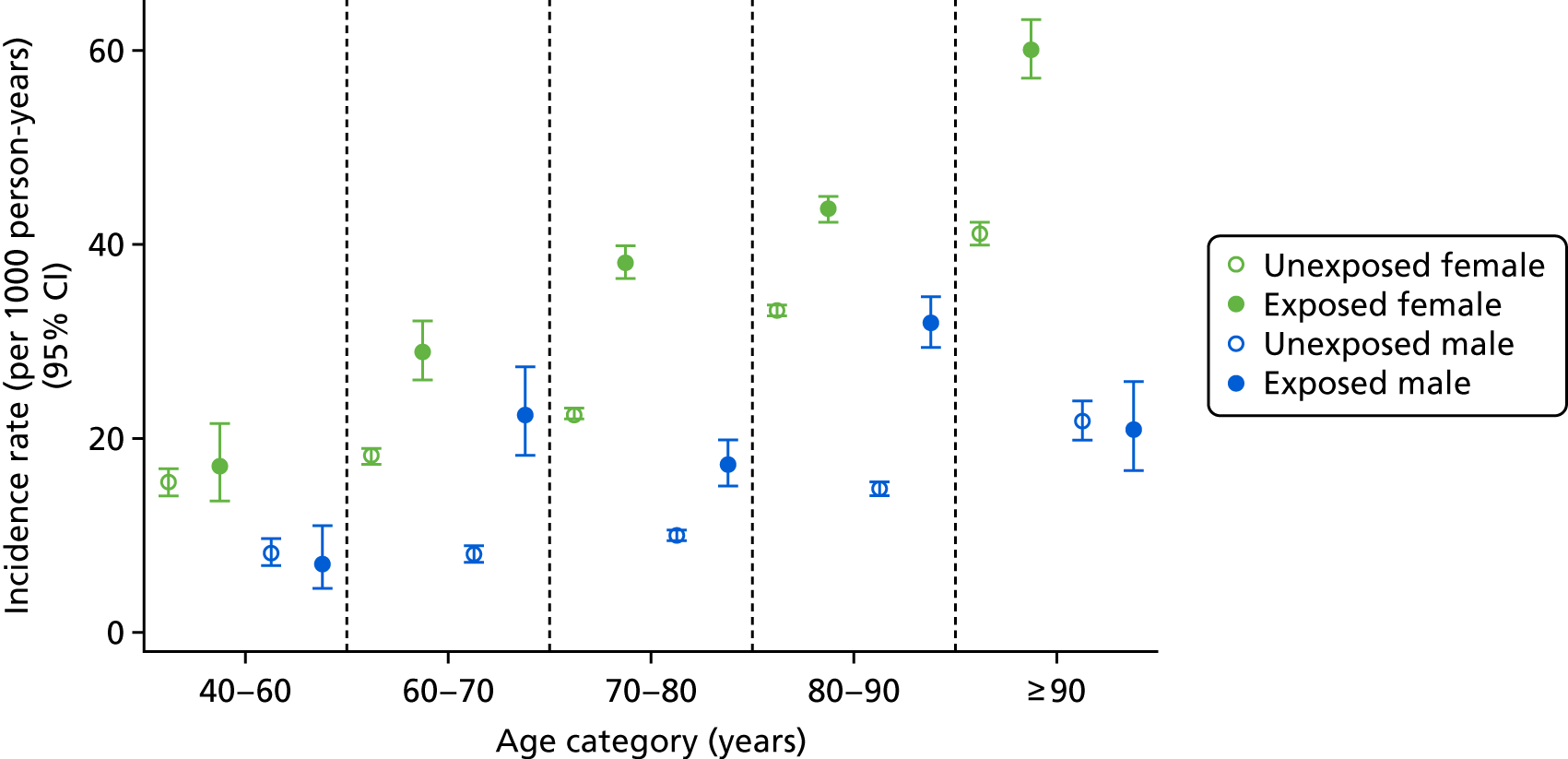
Osteoporotic fracture
We identified 4465 osteoporotic fractures, which was the largest number of events, as this is a more inclusive fracture category; 855 fractures were in bisphosphonate users and 3610 were in non-users (3157 in women and 453 in men). Osteoporotic fractures occurred at all ages, with 45 in participants aged 40–60 years at cohort entry, 179 in those aged 60–70 years, 1015 in those aged 70–80 years, 2303 in those aged 80–90 years and 923 in those aged > 90 years at index date.
Bisphosphonate users had a higher rate of osteoporotic fracture than non-users, with incidence rates of 36.16 (95% CI 35.4 to 36.93) per 1000 person-years and 25.95 (95% CI 25.68 to 26.22) per 1000 person-years, respectively. Few participants in the younger age groups used bisphosphonates, and the incidence rates of osteoporotic fractures increased with age. For example, exposed participants aged 40–60 years had an incidence rate of 13.06 (95% CI 10.67 to 15.98) per 1000 person-years, whereas exposed participants aged > 90 years had an incidence rate of 59.98 (95% CI 57.23 to 62.87) per 1000 person-years. Women had a higher osteoporotic fracture risk than men. For example, female bisphosphonate users had an incidence rate of 38.8 (95% CI 37.94 to 39.68) per 1000 person-years, whereas male users had an incidence rate of 23.54 (95% CI 22.1 to 25.07) per 1000 person-years. Figure 16 shows incidence rates stratified by age, sex and exposure to bisphosphonates.
FIGURE 16.
Incidence rates per 1000 person-years of osteoporotic fractures, stratified by age, sex and exposure to bisphosphonates.
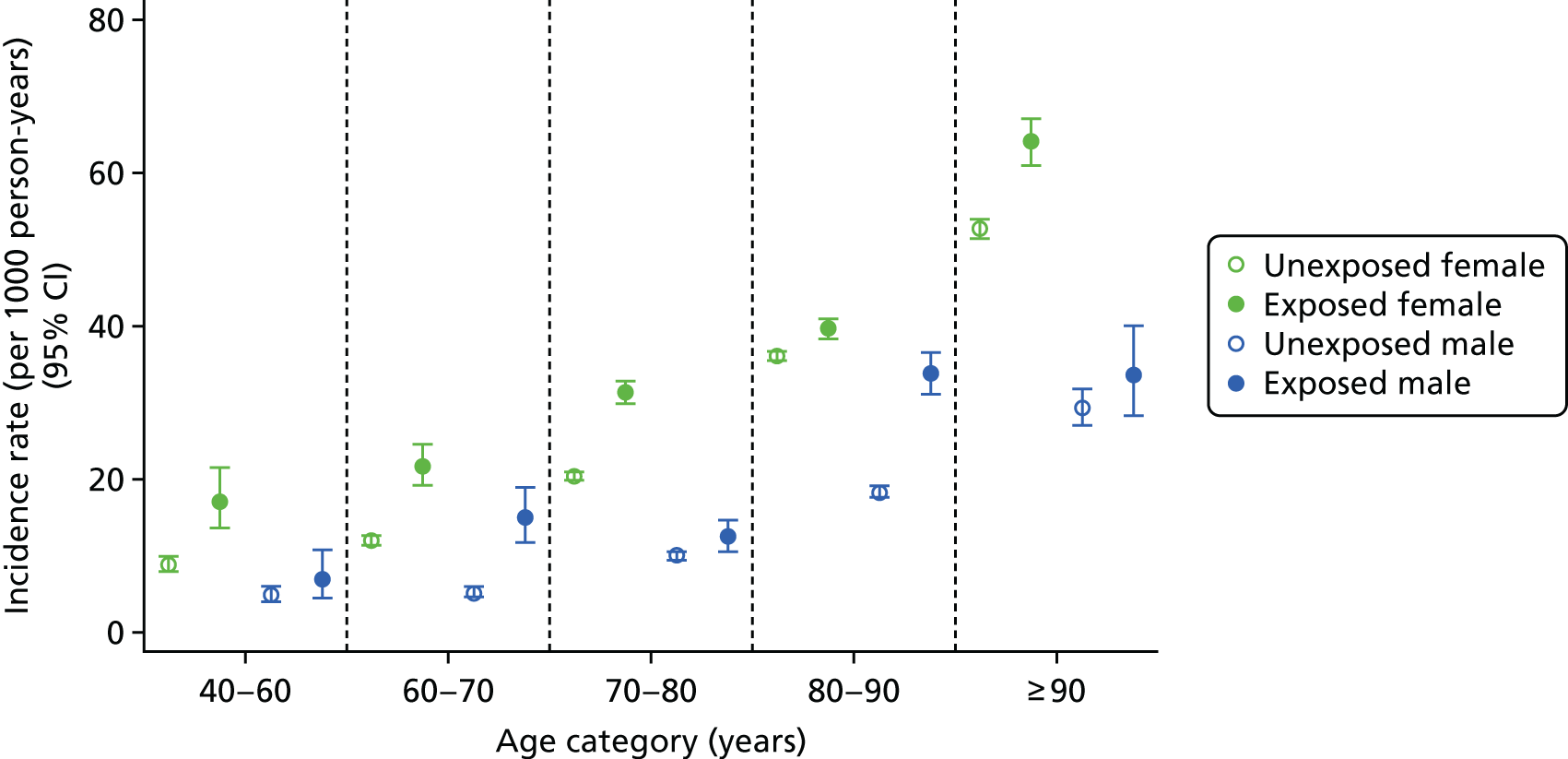
Cox proportional hazards model
Figure 17 shows Kaplan–Meier plots for hip, non-hip and osteoporotic fractures, stratified by bisphosphonate use. The assumption of proportionality was met for each of the models (data are not shown). Bisphosphonate users had a greater risk of all three outcomes than non-users, with HRs of 1.25 (95% CI 1.13 to 1.39), 1.58 (95% CI 1.46 to 1.70) and 1.38 (95% CI 1.27 to 1.50) for hip, non-hip and osteoporotic fractures, respectively.
FIGURE 17.
Kaplan–Meier survival curves of hip, non-hip and osteoporotic fractures. (a) Hip fracture; (b) non-hip fracture; and (c) osteoporotic fracture. Survival curves show the probability of hip, non-hip and osteoporotic fracture for bisphosphonate users (BPUSE = 1) and non-users (BPUSE = 0).
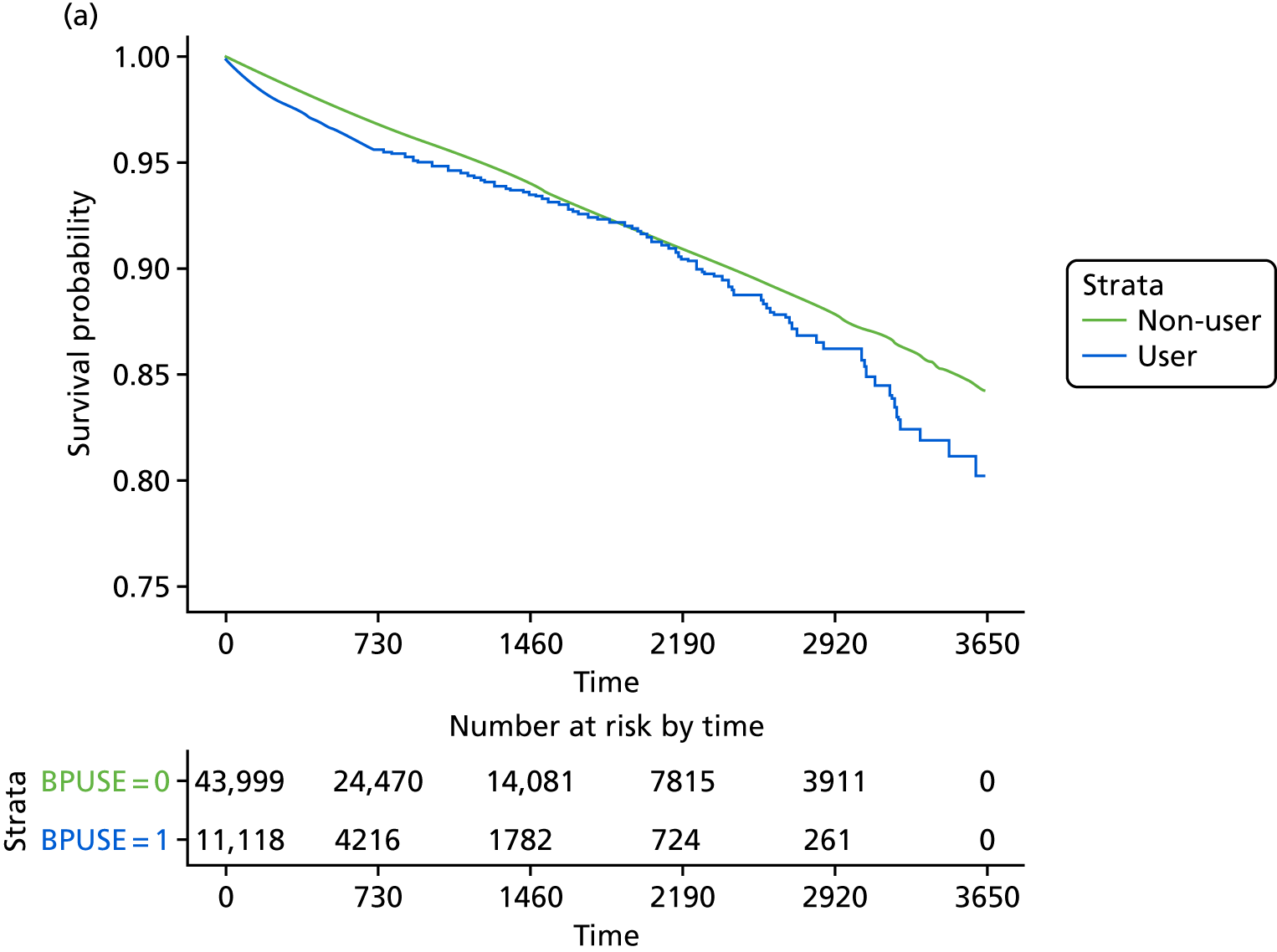
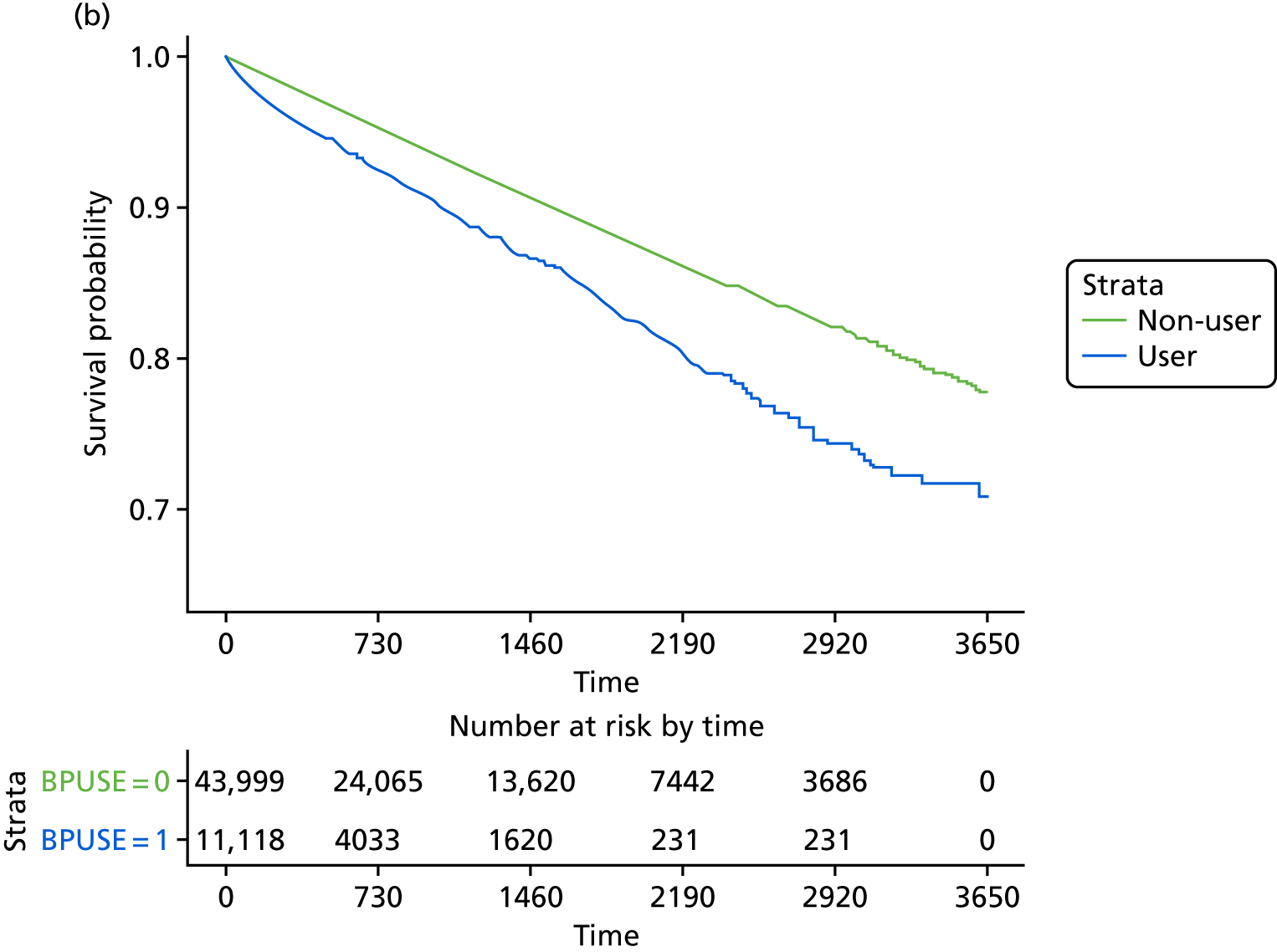
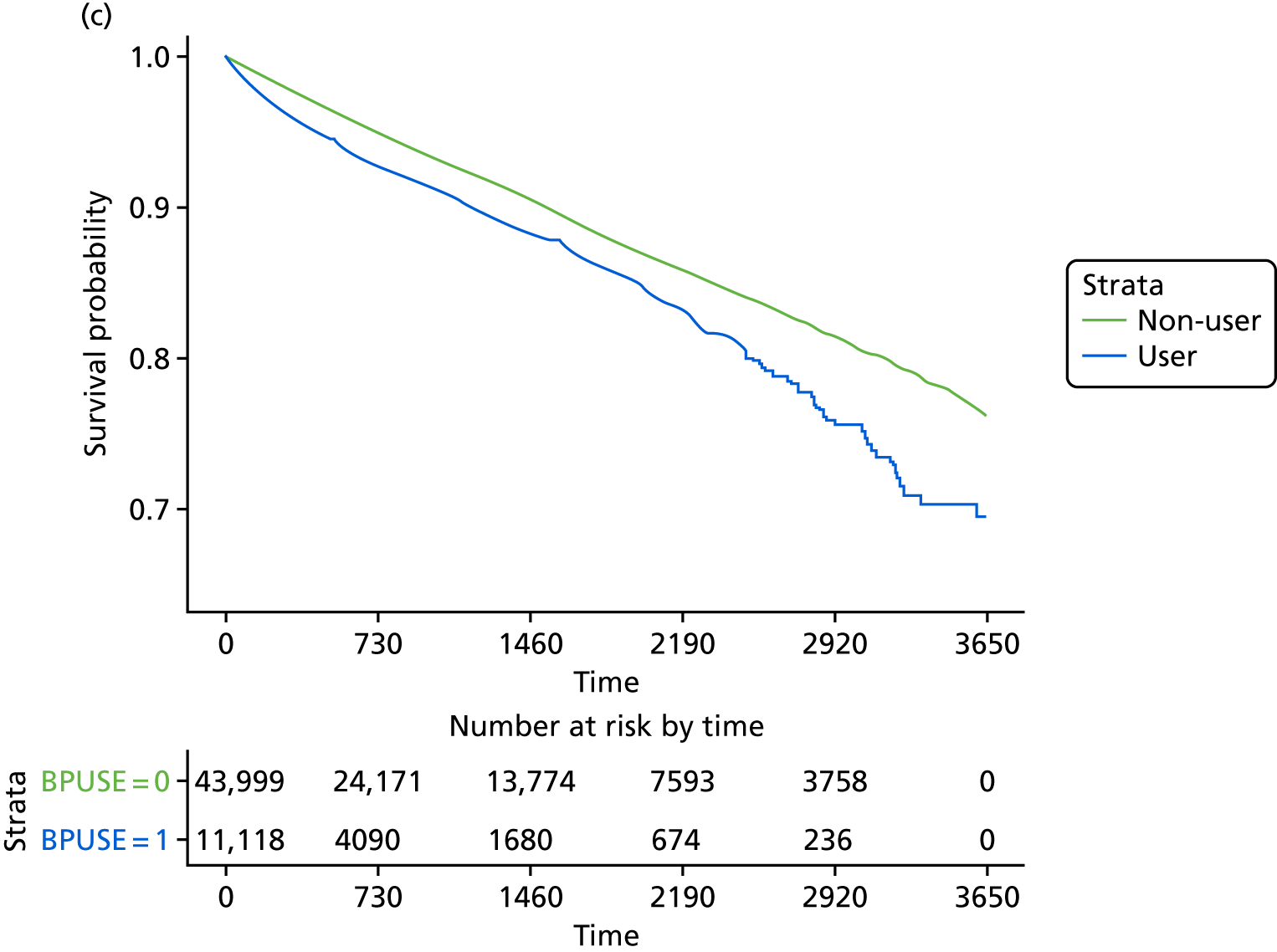
An interaction term between exposure to bisphosphonates and sex was significant in the Cox models of hip fracture (p = 0.01), non-hip fracture (p < 0.001) and osteoporotic fracture (p < 0.001). Sex-stratified Cox hazard models showed that the excess risk of fracture associated with bisphosphonate use was higher among men than women (Table 10). However, the CIs of the HRs for hip fractures for men (HR 1.46, 95% CI 1.11 to 1.91) and women (HR 1.19, 95% CI 1.07 to 1.32) overlapped.
| Outcome | Number of fractures in bisphosphonate users | Number of fractures in non-users | HR | 95% CI |
|---|---|---|---|---|
| Female | ||||
| Hip fractures | 443 | 2084 | 1.19 | 1.07 to 1.32 |
| Non-hip fractures | 817 | 3130 | 1.50 | 1.39 to 1.62 |
| Osteoporotic fractures | 759 | 3286 | 1.31 | 1.21 to 1.42 |
| Male | ||||
| Hip fractures | 65 | 294 | 1.46 | 1.11 to 1.91 |
| Non-hip fractures | 95 | 333 | 1.86 | 1.48 to 2.35 |
| Osteoporotic fractures | 95 | 382 | 1.66 | 1.32 to 2.08 |
There was no evidence that a history of fracture changed bisphosphonate users’ risk of developing hip (interaction term, p = 0.17), non-hip (p = 0.56) or osteoporotic (p = 0.28) fractures. Likewise, baseline CKD stage did not modify the observed associations between bisphosphonate use and hip (interaction term, p = 0.52), non-hip (p = 0.22) or osteoporotic (p = 0.58) fractures.
Post hoc analysis: restricted time windows
Table 11 shows the results of the restricted time window analysis. The proportionality assumption was met for all models (data are not shown). The baseline fracture rate was higher for bisphosphonate users than non-users. For example, users had a hip fracture rate of 34.78 (95% CI 33.19 to 36.44) per 1000 person-years, whereas non-users had a hip fracture rate of 18.20 (95% CI 17.61 to 18.81) per 1000 person-years.
| Fracture location | Category | 180 days | 360 days | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Event (n) | Fracture rate (per 1000 person-years) (95% CI) | HR (95% CI) | Event (n) | Fracture rate (per 1000 person-years) (95% CI) | HR (95% CI) | Event (n) | Fracture rate (per 1000 person-years) (95% CI) | HR (95% CI) | ||
| Hip | Unexposed | 358 | 18.2 (17.61 to 18.81) | 1.91 (1.57 to 2.33) | 618 | 16.85 (16.44 to 17.28) | 1.68 (1.43 to 1.98) | 2321 | 16.41 (16.2 to 16.62) | 1.25 (1.13 to 1.39) |
| Exposed | 176 | 34.78 (33.19 to 36.44) | 268 | 28.33 (27.28 to 29.42) | 504 | 20.7 (20.14 to 21.28) | ||||
| Non-hip | Unexposed | 519 | 26.46 (25.75 to 27.18) | 2.18 (1.88 to 2.54) | 928 | 25.42 (24.91 to 25.94) | 1.85 (1.65 to 2.07) | 3420 | 24.73 (24.47 to 25) | 1.58 (1.46 to 1.70) |
| Exposed | 290 | 57.64 (55.58 to 59.78) | 440 | 46.96 (45.6 to 48.37) | 919 | 39.4 (38.6 to 40.21) | ||||
| Osteoporotic | Unexposed | 552 | 28.11 (27.38 to 28.86) | 1.96 (1.67 to 2.30) | 971 | 26.57 (26.05 to 27.11) | 1.76 (1.54 to 2.01) | 3610 | 25.95 (25.68 to 26.22) | 1.38 (1.27 to 1.50) |
| Exposed | 277 | 55.09 (53.08 to 57.18) | 439 | 46.75 (45.39 to 48.15) | 855 | 36.16 (35.4 to 36.93) | ||||
The excess risk associated with bisphosphonate use peaked in the first 180 days, and then declined slightly when the analysis was restricted to 360 days. The risk declined further when the analysis was extended to 10 years of follow-up, as per the primary analysis. This pattern was observed for all three fracture outcomes (see Table 11).
These findings suggest an excess baseline risk that is not attributable to bisphosphonates, as these do not affect fracture risk for at least 6 months after therapy begins. Instead, these findings suggest the presence of residual confounding by indication, possibly related to unobserved confounders such as BMD or other unavailable proxies for bone strength or fracture risk.
Medication possession ratios
Four MPR quartiles were calculated for fracture outcomes, as adherence to bisphosphonates was, in general, found to be high (median 97%) in this population. The quartiles contained MPRs of 2–88%, 88–97%, 97–110% and > 110%. The risk of fracture for each quartile is shown in Figure 18.
FIGURE 18.
The HRs of each fracture (hip, non-hip and osteoporotic) outcome, split by MPR quartiles.
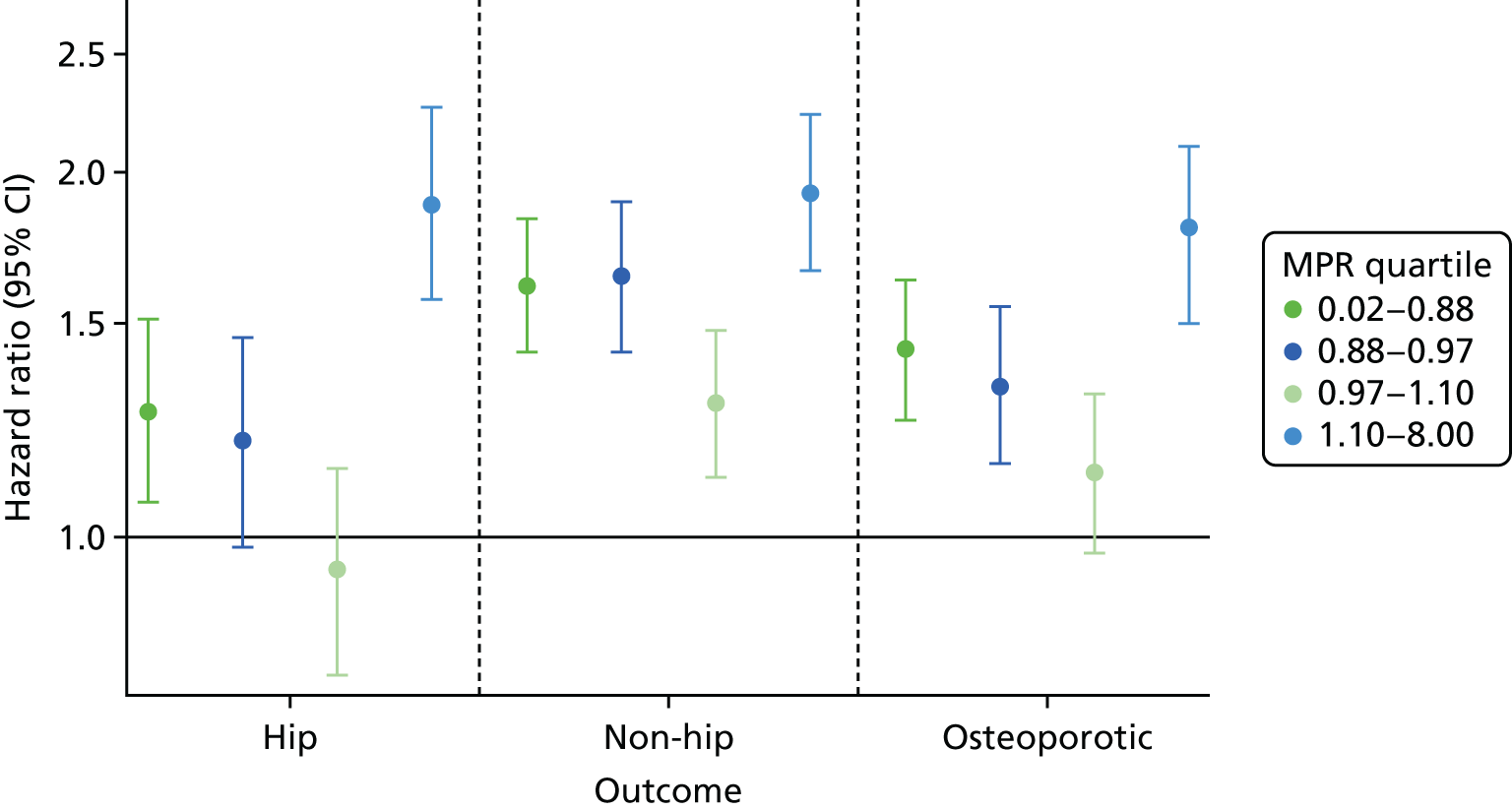
Participants in the highest quartile were at increased risk of hip fracture (HR 1.88, 95% CI 1.57 to 2.26). They also had a higher risk of non-hip (HR 1.92, 95% CI 1.66 to 2.23) and osteoporotic (HR 1.80, 95% CI 1.50 to 2.10) fracture, although the CIs for non-hip and osteoporotic fractures overlapped with other quartiles in each outcome. In contrast, the third quartile (97–110% MPR) had the lowest point estimates, although the CIs overlapped with those of the first and second quartiles. Bisphosphonate users with a MPR of between 97 and 100% had HRs of 0.94 (95% CI 0.77 to 1.14), 1.29 (95% CI 1.12 to 1.48) and 1.13 (95% CI 0.97 to 1.31) for hip, non-hip and osteoporotic fractures, respectively.
Multivariable model
As requested by the Steering Committee, a sensitivity analysis of the multivariable models on the full data set (before propensity matching) of 11,567 exposed and 215,617 unexposed participants is shown in Table 12. As an interaction between sex and bisphosphonate use was identified in the propensity score analysis, an interaction between sex and bisphosphonate use was also included in the multivariable model.
| Category | Fracture, HR (95% CI) | ||
|---|---|---|---|
| Hip | Non-hip | Osteoporotic | |
| Male | Ref | Ref | Ref |
| Male bisphosphonate user | 1.47 (1.15 to 1.88) | 1.83 (1.49 to 2.24) | 1.63 (1.33 to 2.00) |
| Female | 1.61 (1.53 to 1.69) | 2.25 (2.16 to 2.35) | 2.00 (1.92 to 2.08) |
| Female bisphosphonate user | 1.04 (0.63 to 1.73) | 1.30 (0.86 to 1.97) | 1.14 (0.75 to 1.73) |
| Age (per year increase) | 1.08 (1.07 to 1.08) | 1.03 (1.03 to 1.03) | 1.06 (1.06 to 1.06) |
| BMI (kg/m2) per 1-unit increase | 0.94 (0.93 to 0.94) | 0.98 (0.97 to 0.98) | 0.96 (0.95 to 0.96) |
| Smoking status | |||
| Non-smoker | Ref | Ref | Ref |
| Ex-smoker | 1.10 (1.05 to 1.16) | 1.04 (1.00 to 1.08) | 1.08 (1.04 to 1.12) |
| Current smoker | 1.48 (1.38 to 1.60) | 1.08 (1.01 to 1.15) | 1.27 (1.20 to 1.35) |
| Drinking status | |||
| Non | Ref | Ref | Ref |
| Ex | 0.99 (0.88 to 1.12) | 1.06 (0.96 to 1.17) | 1.01 (0.92 to 1.11) |
| Current | 0.90 (0.85 to 0.95) | 1.02 (0.98 to 1.06) | 0.95 (0.91 to 0.99) |
| IMD quintile | |||
| 1 – least deprived | Ref | Ref | Ref |
| 2 | 1.02 (0.96 to 1.08) | 0.98 (0.94 to 1.03) | 1.00 (0.95 to 1.04) |
| 3 | 1.04 (0.98 to 1.1) | 0.98 (0.93 to 1.03) | 1.02 (0.97 to 1.07) |
| 4 | 1.06 (0.99 to 1.12) | 1.03 (0.99 to 1.09) | 1.05 (1.00 to 1.10) |
| 5 – most deprived | 1.13 (1.05 to 1.21) | 1.01 (0.95 to 1.06) | 1.09 (1.04 to 1.15) |
| Baseline eGFR score (ml/minute/1.73 m2) | |||
| 0.1–4.9 | Ref | Ref | Ref |
| 5–9.9 | 1.07 (0.58 to 1.97) | 0.98 (0.63 to 1.55) | 0.78 (0.50 to 1.22) |
| 10–14.9 | 0.90 (0.50 to 1.62) | 0.85 (0.55 to 1.33) | 0.69 (0.45 to 1.06) |
| 15–19.9 | 0.95 (0.54 to 1.66) | 0.79 (0.52 to 1.21) | 0.72 (0.48 to 1.08) |
| 20–24.9 | 0.88 (0.50 to 1.53) | 0.85 (0.57 to 1.27) | 0.73 (0.49 to 1.08) |
| 25–29.9 | 0.78 (0.45 to 1.35) | 0.78 (0.52 to 1.16) | 0.64 (0.44 to 0.95) |
| 30–34.9 | 0.73 (0.42 to 1.26) | 0.77 (0.51 to 1.14) | 0.62 (0.42 to 0.91) |
| 35–39.9 | 0.66 (0.38 to 1.14) | 0.76 (0.51 to 1.12) | 0.57 (0.39 to 0.84) |
| 40–44.9 | 0.61 (0.35 to 1.05) | 0.74 (0.50 to 1.10) | 0.55 (0.37 to 0.81) |
| Number of hospital visits | |||
| 0 | Ref | Ref | Ref |
| 1 | 1.07 (1.02 to 1.13) | 1.08 (1.04 to 1.12) | 1.05 (1.01 to 1.09) |
| 2 | 1.11 (1.03 to 1.19) | 1.11 (1.05 to 1.18) | 1.09 (1.03 to 1.15) |
| 3–5 | 1.21 (1.11 to 1.32) | 1.19 (1.11 to 1.28) | 1.15 (1.07 to 1.23) |
| ≥ 6 | 1.41 (1.17 to 1.70) | 1.32 (1.14 to 1.52) | 1.35 (1.16 to 1.56) |
| 5-year Charlson Comorbidity Index score | |||
| 0 | Ref | Ref | Ref |
| 1 | 1.03 (0.97 to 1.09) | 0.99 (0.94 to 1.04) | 1.04 (0.99 to 1.09) |
| 2 | 0.99 (0.93 to 1.06) | 0.96 (0.91 to 1.02) | 1.00 (0.95 to 1.06) |
| 3–5 | 1.01 (0.92 to 1.09) | 1.04 (0.98 to 1.11) | 1.04 (0.98 to 1.11) |
| ≥ 6 | 1.13 (0.97 to 1.32) | 1.12 (0.99 to 1.25) | 1.21 (1.07 to 1.36) |
| Rheumatoid arthritis | 1.09 (0.91 to 1.31) | 1.02 (0.89 to 1.17) | 1.06 (0.92 to 1.22) |
| Varices | 1.08 (1.00 to 1.16) | 1.04 (0.98 to 1.11) | 1.05 (0.99 to 1.11) |
| Deep-vein thrombosis | 1.12 (0.99 to 1.25) | 1.08 (0.98 to 1.18) | 1.04 (0.95 to 1.13) |
| History of hip fracture | 1.38 (1.21 to 1.57) | 1.40 (1.24 to 1.57) | 1.33 (1.19 to 1.48) |
| History of non-hip fracture | 1.34 (1.24 to 1.44) | 1.73 (1.63 to 1.82) | 1.47 (1.39 to 1.56) |
| Dementia | 1.75 (1.60 to 1.93) | 1.20 (1.09 to 1.32) | 1.52 (1.40 to 1.65) |
| Type 2 diabetes mellitus | 0.95 (0.87 to 1.04) | 0.93 (0.87 to 0.99) | 0.91 (0.85 to 0.98) |
| Chronic renal disease | 0.99 (0.92 to 1.06) | 1.02 (0.96 to 1.07) | 0.98 (0.93 to 1.04) |
| Cerebrovascular disease | 1.20 (1.11 to 1.30) | 1.04 (0.97 to 1.11) | 1.07 (1.00 to 1.15) |
| Peripheral vascular disease | 1.10 (0.96 to 1.27) | 0.96 (0.85 to 1.08) | 1.00 (0.89 to 1.13) |
| Hypertension | 0.95 (0.91 to 0.99) | 1.01 (0.98 to 1.05) | 0.98 (0.95 to 1.02) |
| Liver disease | 1.67 (1.26 to 2.23) | 1.39 (1.12 to 1.72) | 1.75 (1.42 to 2.16) |
| Peptic ulcer | 0.94 (0.79 to 1.10) | 0.94 (0.82 to 1.07) | 0.94 (0.83 to 1.07) |
| Cardiovascular disease | 0.96 (0.90 to 1.02) | 0.96 (0.91 to 1.01) | 0.99 (0.94 to 1.04) |
| Hyperthyroidism | 1.10 (0.86 to 1.41) | 0.88 (0.71 to 1.09) | 0.97 (0.79 to 1.20) |
| Cancer | 0.96 (0.90 to 1.03) | 1.01 (0.96 to 1.07) | 1.00 (0.95 to 1.06) |
| Hyperlipidaemia | 0.87 (0.81 to 0.94) | 0.94 (0.89 to 0.99) | 0.94 (0.89 to 1.00) |
| Prescriptions in the previous year | |||
| 0 | Ref | Ref | Ref |
| 1–3 | 0.85 (0.75 to 0.97) | 0.86 (0.77 to 0.96) | 0.88 (0.80 to 0.98) |
| 4–6 | 0.85 (0.75 to 0.96) | 0.90 (0.81 to 1.00) | 0.90 (0.81 to 1.00) |
| 7–9 | 0.88 (0.78 to 1.01) | 0.91 (0.82 to 1.02) | 0.92 (0.83 to 1.03) |
| 10–12 | 0.87 (0.76 to 0.99) | 0.97 (0.87 to 1.09) | 0.94 (0.84 to 1.05) |
| ≥ 13 | 0.99 (0.86 to 1.14) | 1.05 (0.93 to 1.18) | 1.03 (0.92 to 1.16) |
| Calcium supplements | 0.98 (0.91 to 1.04) | 1.16 (1.10 to 1.22) | 1.04 (0.98 to 1.09) |
| Steroids | 1.03 (0.98 to 1.09) | 1.01 (0.97 to 1.06) | 1.02 (0.98 to 1.06) |
| Anticoagulants | 1.08 (1.01 to 1.15) | 1.05 (0.99 to 1.11) | 1.12 (1.06 to 1.18) |
| Heparin | 0.95 (0.74 to 1.22) | 0.99 (0.83 to 1.20) | 0.93 (0.76 to 1.13) |
| Hormone replacement therapy | 0.79 (0.71 to 0.87) | 0.88 (0.83 to 0.94) | 0.86 (0.80 to 0.93) |
| Proton-pump inhibitors | 0.99 (0.95 to 1.04) | 1.05 (1.01 to 1.08) | 1.01 (0.97 to 1.04) |
| Aromatase inhibitors | 1.14 (0.91 to 1.44) | 1.19 (1.01 to 1.41) | 1.09 (0.91 to 1.30) |
| Anxiols/sedatives/hypnotics | 1.01 (0.96 to 1.06) | 1.12 (1.07 to 1.16) | 1.05 (1.01 to 1.09) |
| Antidepressants | 1.12 (1.07 to 1.17) | 1.13 (1.09 to 1.17) | 1.13 (1.09 to 1.18) |
| Statins | 0.91 (0.87 to 0.96) | 1.00 (0.96 to 1.04) | 0.93 (0.89 to 0.97) |
| Calcium channel blockers | 1.03 (0.98 to 1.07) | 0.99 (0.96 to 1.03) | 1.00 (0.97 to 1.04) |
| ACE inhibitors | 0.98 (0.93 to 1.02) | 1.01 (0.97 to 1.05) | 0.96 (0.92 to 0.99) |
| Antiepileptics | 1.20 (1.09 to 1.32) | 1.32 (1.23 to 1.41) | 1.22 (1.14 to 1.32) |
| Diuretics | 1.11 (1.06 to 1.17) | 0.98 (0.94 to 1.02) | 1.05 (1.01 to 1.10) |
| Beta blockers | 0.92 (0.88 to 0.96) | 0.87 (0.84 to 0.9) | 0.91 (0.88 to 0.94) |
| Antihypertensive | 0.98 (0.92 to 1.04) | 0.98 (0.94 to 1.03) | 0.98 (0.94 to 1.03) |
| Antidiabetics | 1.24 (1.15 to 1.34) | 1.18 (1.12 to 1.26) | 1.22 (1.15 to 1.29) |
| Antiarrhythmic agents | 1.05 (0.99 to 1.12) | 1.13 (1.07 to 1.18) | 1.09 (1.04 to 1.14) |
| Insulin | 1.41 (1.25 to 1.59) | 1.26 (1.15 to 1.38) | 1.27 (1.15 to 1.40) |
These results show a great difference between male and female bisphosphonate users than the stratified analysis after propensity score matching. Other significant interactions for each fracture outcome are in bold in Table 12. Other covariates that were found to increase the risk of fracture for all locations included (HRs and 95% CIs for hip fractures are reported) age per year (HR 1.08, 95% CI 1.07, 1.08); smoking status (non-smoker: reference, ex-smoker: HR 1.10, 95% CI 1.05 to 1.16; current smoker: HR 1.48, 95% CI 1.38 to 1.60); number of hospital visits in the previous year (none: reference, one: HR 1.07, 95% CI 1.02 to 1.13; two: HR 1.11, 95% CI 1.03 to 1.19; three to five: HR 1.21, 95% CI 1.11 to 1.32; six or more: HR 1.41, 95% CI 1.17 to 1.70); a history of hip fracture (HR 1.38, 95% CI 1.21 to 1.57), non-hip fracture (HR 1.34, 95% CI 1.24 to 1.44), dementia (HR 1.75, 95% CI 1.60 to 1.93) or liver disease (HR 1.67, 95% CI 1.26 to 2.23); and prior use of statins (HR 1.12, 95% CI 1.07 to 1.17), antiepileptics (HR 1.20, 95% CI 1.09 to 1.32), antidiabetics (HR 1.24, 95% CI 1.15 to 1.34) or insulin (HR 1.41, 95% CI 1.25 to 1.59). The following variables were associated with a reduction in fracture risk: BMI per unit increase (HR 0.94, 95% CI 0.93 to 0.94); having one to three prescriptions in the previous year, (compared with no prescriptions) (HR 0.85, 95% CI 0.75 to 0.97); and having prior use of hormone replacement therapy (HR 0.79, 95% CI 0.71 to 0.87) or beta blockers (HR 0.92, 95% CI 0.88 to 0.96). HRs were similar for other fracture locations for all of these aforementioned covariates except dementia, which had a HR of 1.20 (95% CI 1.09 to 1.32) for non-hip fractures and 1.52 (95% CI 1.40 to 1.65) for osteoporotic fractures.
The risk of hip fracture was also increased by being in the lowest IMD quintile (HR 1.13), having a history of varices (HR 1.08) or cerebrovascular disease (HR 1.20), and having taken anticoagulants (HR 1.08) or diuretics (HR 1.11). The risk of hip fracture was reduced by currently drinking alcohol (HR 0.90), having a history of hypertension (HR 0.95) or hyperlipidaemia (HR 0.87), having four to six prescriptions in the previous year (HR 0.85) or taking calcium channel blockers (HR 0.91).
The risk of non-hip fracture was also increased by having previously taken calcium supplements (HR 1.16), proton-pump inhibitors (HR 1.05), anxiols/sedatives/hypnotics (HR 1.19), antidepressants (HR 1.12), or antiarrhythmic agents (HR 1.13). The risk was reduced if the patient had a history of hyperlipidaemia (HR 0.94).
The risk of osteoporotic fracture was also increased by being in the fourth or fifth quintile for IMD quintile 4 (HR 1.05) or 5 and (HR 1.09) respectively, having a history of cerebrovascular disease (HR 1.07) and previously using history of use of anticoagulants (HR 1.12), antidepressants (HR 1.05) or diuretics (HR 1.05). The risk of osteoporotic fracture was reduced by currently drinking (HR 0.95), having an eGFR of > 25 ml/minute/1.73 m2 at baseline (HRs 0.55–0.64) and previously using calcium channel blockers (HR 0.93) or proton-pump inhibitors (HR 0.96).
Array sensitivity analysis
We conducted a final post hoc analysis to measure the impact of potential unmeasured confounding on our results. BMD (i.e. osteoporosis) is not recorded in CPRD, so is likely to be imbalanced between the exposed and unexposed cohorts. As BMD is a known independent risk factor for hip fracture, we analysed its effect on the observed estimates. We used the unadjusted HR for hip fracture from the primary analysis (HR 1.19) and an estimated prevalence of low BMD in bisphosphonate non-users with CKD stage 3B+ of 34.8% (based on the data used for WP4; see Chapter 6, Participants and baseline characteristics). We then simulated a range of prevalence values in bisphosphonate users, from 30% to 80%. We also simulated a range of potential effects of low BMD on hip fracture risk, from no effect (HR 1.0) to a fivefold excess risk (HR 5.0).
The results are shown in Figure 19, with blue areas showing scenarios in which adjustment for BMD would attenuate the observed risk. Clinically plausible combinations of 60% osteoporosis prevalence in bisphosphonate users (as seen in the Danish bisphosphonate users cohort in WP4) and a HR of ≥ 2.2 would result in a null association between bisphosphonate use and hip fracture risk after adjustment for currently unavailable BMD values. A stronger association (HR > 4 between confounder and outcome) would even be compatible with a protective effect (adjusted HR < 1).
FIGURE 19.
Array sensitivity analysis of the possible effect of an unobserved confounder (BMD) on fracture risk at a range of prevalences in users of bisphosphonates. The confounder’s prevalence in non-users of bisphosphonates is 38%. Blue and turquoise (HR < 1) indicate scenarios in which the observed effect would be explained by the unobserved confounder.
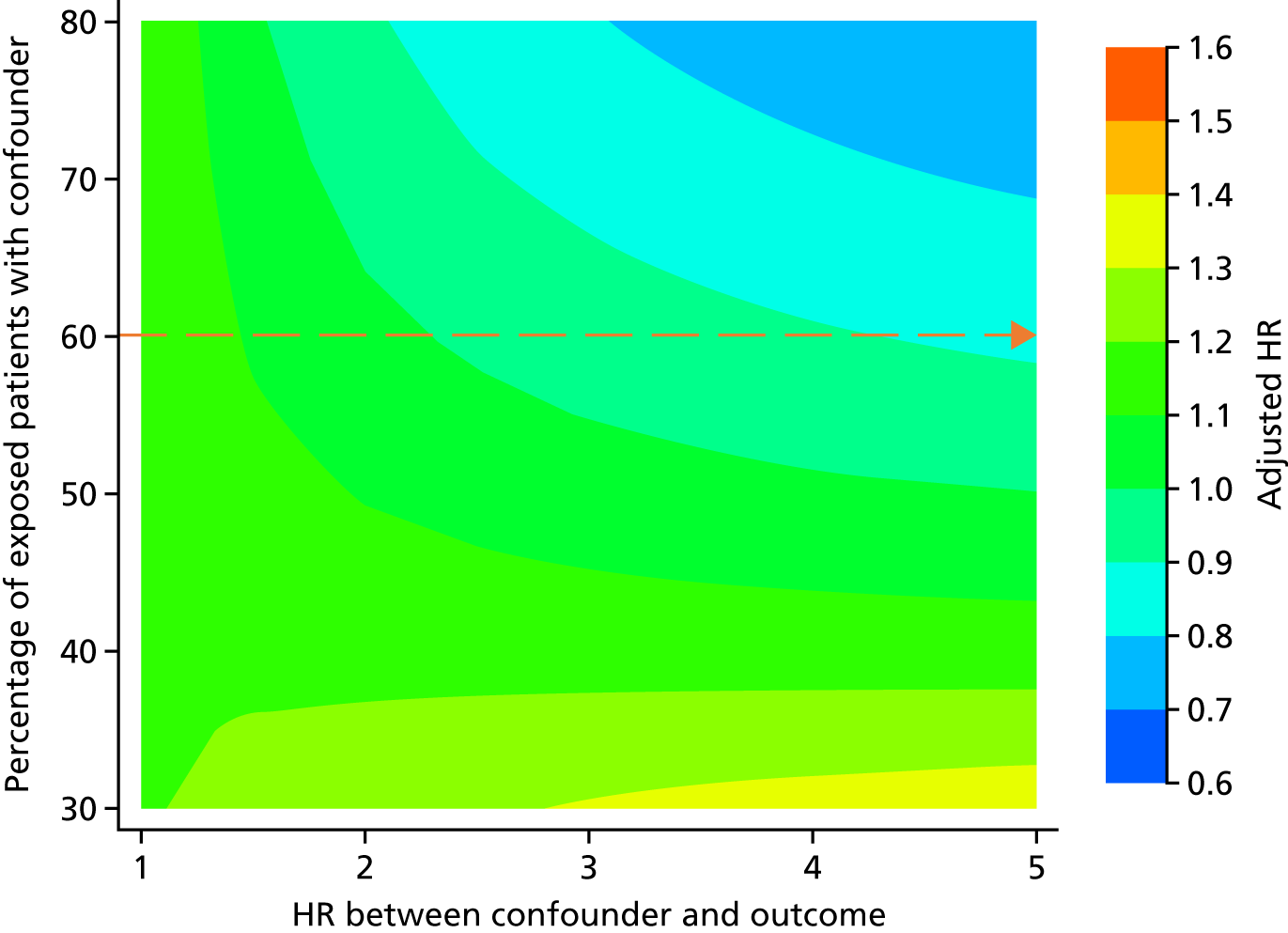
Chapter 5 Work package 3: the risk of adverse events among users of bisphosphonates, compared with matched non-users
Introduction
Published reviews85,86 have identified safety concerns relating to the use of bisphosphonates.
Regulators have highlighted acute kidney injury as a potential risk of bisphosphonates,87,88 and some cases of acute kidney injury have been reported. 63,66,89 This is an obvious concern for patients with underlying moderate to severe CKD. The population-based incidence of acute kidney injury has been estimated at 209 cases per 1 million person-years. 90 A recent nationwide cohort study estimated that almost 13% of hip fracture patients experience acute kidney injury, with a worse prognosis for those with pre-existing stage 3+ CKD. 91
Gastrointestinal events have also been identified as a potential risk, mostly associated with the use of oral bisphosphonates. The pivotal RCT for oral alendronate [the Fracture Intervention Trial (FIT)]92 showed that 11% of the participants allocated to the active treatment developed upper gastrointestinal events, with 1.6% developing serious events including perforation, ulcer or bleeding. A population-based cohort study93 identified CKD as an independent risk factor for gastrointestinal events, but a post hoc analysis of FIT did not find a higher risk of gastrointestinal events among participants with stage 3 CKD. 17
Hypocalcaemia and hypophosphataemia are known effects of bisphosphonates. The HORIZON (The Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly) RCT67 of intravenous zoledronic acid for fracture reported a 0.2% incidence of hypocalcaemia. Eight per cent of cancer patients given a high-dose infusion of intravenous zoledronic acid developed hypocalcaemia that required medical attention. 9 Experts have also raised concerns that bisphosphonates could cause hypophosphataemia. 94 This is particularly worrying for CKD patients, as data from the UKRR95 have demonstrated that hypocalcaemia and hypophosphataemia are frequent in end-stage renal disease, with a prevalence of 11% and 12% among patients on haemodialysis, respectively.
This work package aimed to estimate the risk of these adverse events in patients with moderate to severe CKD who are exposed to bisphosphonates, compared with unexposed patients.
Methods
Participants and exposure
This work package used the same propensity score-matched cohort as WP1, as adverse events could occur any time after starting bisphosphonates. The eligibility criteria for inclusion in these cohorts are presented in Chapter 3, Study participants and exposure. To ensure similar length of eGFR follow-up periods between bisphosphonate users and non-users, we conducted stratified propensity score matching. Each bisphosphonate user was matched with up to five non-users who were followed up over the same duration. Bisphosphonate use was introduced as a time-varying exposure, allowing unexposed participants to become exposed after being included as a non-user.
The outcome follow-up window was censored at the earliest occurrence of any of the following:
-
the date of the last CPRD data update available
-
stopping bisphosphonate treatment
-
switching treatment to other non-bisphosphonate anti-osteoporosis medication
-
death, migration, or transfer out of the area
-
10 years of follow-up
-
the occurrence of the outcome of interest (different date for each study outcome).
Outcome definitions
We conducted a literature review of validation studies using CPRD or HES data. Validated lists of codes were found for acute kidney injury in HES33 and gastrointestinal events in CPRD. 96 No validated code lists for hypocalcaemia or hypophosphataemia were identified. We therefore created our own code list, following the steps outlined in Chapter 3, Study outcomes. The final code lists used to identify each of these three outcomes are reported in Report Supplementary Material 2.
Statistical analysis
As stated in Chapter 2, Missing data, 10 imputed data sets were used after MICE to minimise the impact of missing information on smoking status, drinking category and BMI. Propensity score-matched cohorts were identified within each of these data sets. All of the treatment effect estimates presented were pooled after running analyses on each of the 10 imputations, using Rubin’s rule. 52 The incidence rates presented are based on the first imputed data set.
For each of the three outcomes, crude and age- and sex-stratified incidence rates and 95% CIs are reported per 1000 person-years. The Poisson distribution was used to estimate the incidence rate. Cumulative incidence function plots were used to depict the probability of the death and the outcome, stratified by bisphosphonate use.
Subdistribution HRs and 95% CIs of each of the study outcomes were estimated using Fine and Gray competing risk models as there was an increased risk of death.
Two sensitivity (subgroup) analyses were prespecified:
-
Interactions of bisphosphonate use with sex, previous history of fracture 1 year before the start date, and baseline CKD stage were tested, introducing multiplicative terms into the Fine and Gray models. When a significant interaction was found, stratified sHRs were reported.
-
Adherence to bisphosphonate use was estimated using MPRs. SHRs stratified by MPR quartile (compared with bisphosphonate non-use) were computed using Fine and Gray competing risk models.
Results
Cohort creation
The WP1 cohort was used. In short, for the first imputed data set, there were 2447 exposed participants matched with 8931 unexposed participants. After matching, propensity score balance was achieved for all covariates.
Incidence rate of safety outcomes
Acute kidney injury
We identified 480 acute kidney injury events in the first imputation of the CPRD propensity score-matched cohorts. Unexposed participants had a similar incidence rate of 15.23 (95% CI 13.8 to 16.8) per 1000 person-years to bisphosphonate users, who had an incidence rate of 12.02 (95% CI 9.66 to 14.94) per 1000 person-years. Men had a higher incidence of acute kidney injury than women, with rates of 23.02 (95% CI 19.77 to 26.82) per 1000 person-years for unexposed men and 12.29 (95% CI 10.81 to 13.97) per 1000 person-years for unexposed women. However, women had more events (290 events) than men (190 events). Age- and sex-stratified incidence rates are shown in Figure 20.
FIGURE 20.
Incidence rates per 1000 person-years of acute kidney injury, stratified by age, sex and exposure to bisphosphonates.
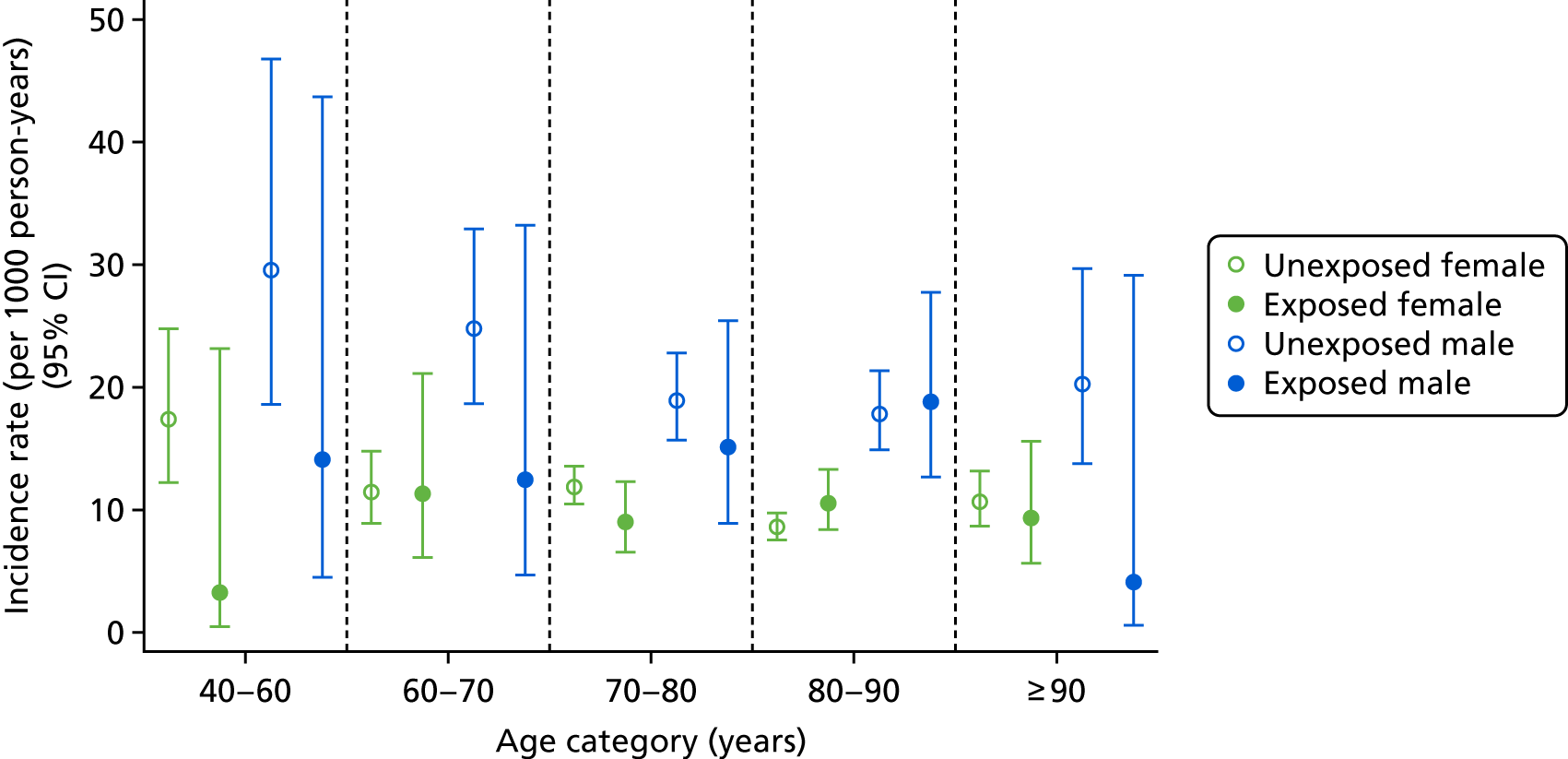
Gastrointestinal events
Gastrointestinal events occurred 205 times, 168 in unexposed participants and 37 in bisphosphonate users. Users and non-users had similar rates of events [5.45 (95% CI 3.95 to 7.52) vs. 6.39 (95% CI 5.49 to 7.43) per 1000 person-years]. Men had higher rates of gastrointestinal events (9.03, 95% CI 5.24 to 15.55 per 1000 person-years) than women (4.48, 95% CI 3.01 to 6.69 per 1000 person-years). The age groups had similar rates of gastrointestinal events. Only five events occurred in the group aged 40–60 years, of which four occurred in non-users. Figure 21 shows the incidence rates stratified by age, sex and exposure to bisphosphonates.
FIGURE 21.
Incidence rates per 1000 person-years of gastrointestinal events, stratified by age, sex and exposure to bisphosphonates.
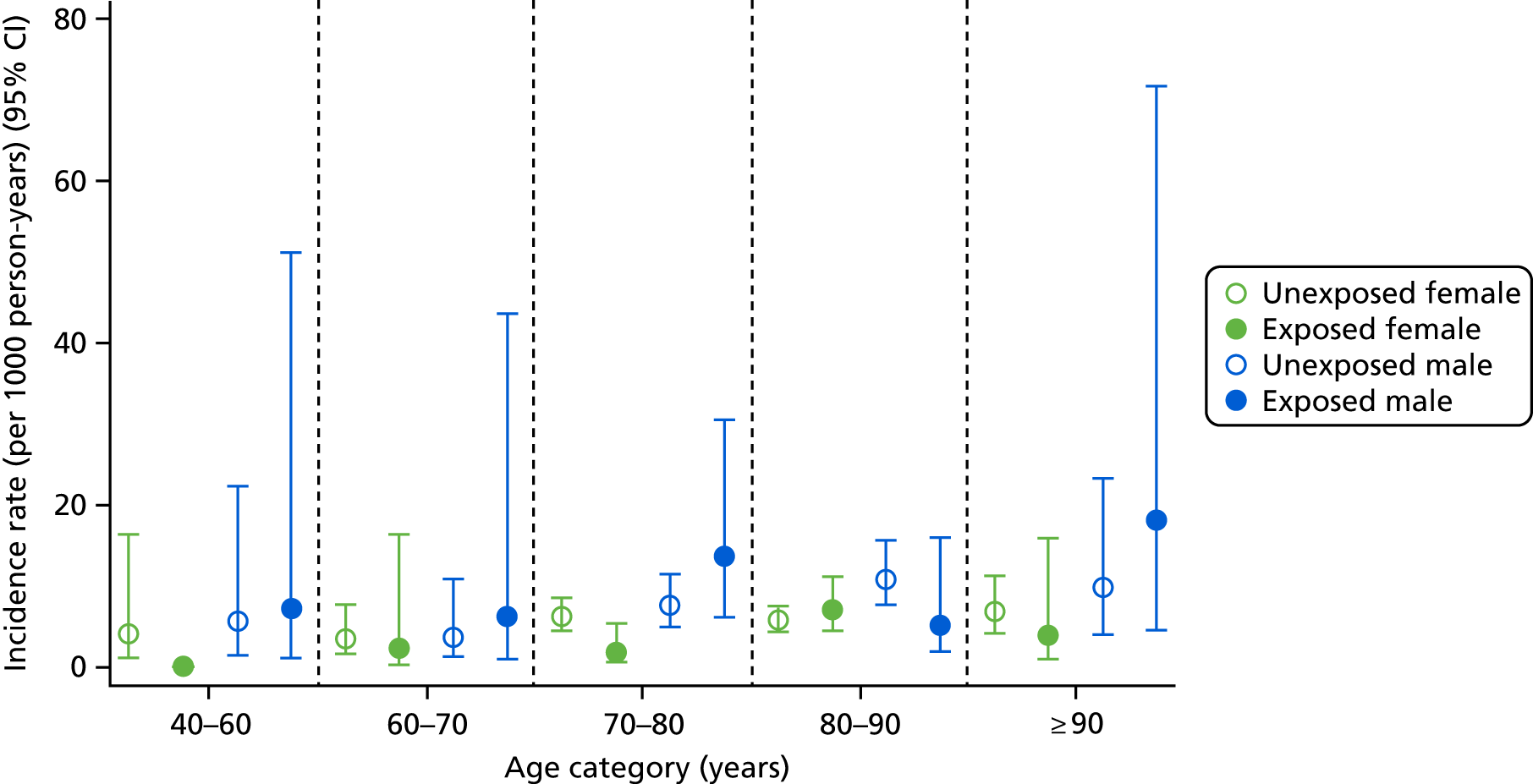
Hypocalcaemia
Thirty-one hypocalcaemia events occurred in the propensity score-matched cohort, of which two occurred in bisphosphonate users. Incidence rates were 0.29 (95% CI 0.07 to 1.17) per 1000 person-years for bisphosphonate users and 1.09 (95% CI 0.76 to 1.57) per 1000 person-years for non-users. Owing to the lack of events in the exposed population, age- and sex-stratified incidence rates were not calculated.
Hypophosphataemia
Only seven hypophosphataemia events were recorded in the unmatched cohort, all in unexposed participants. This was too few events to assess the risk of hypophosphataemia in this cohort.
Fine and Gray competing risks models
Bisphosphonate use was not associated with acute kidney injury (sHR 0.86, 95% CI 0.66 to 1.10), gastrointestinal events (sHR 0.96, 95% CI 0.66 to 1.40) or hypocalcaemia (sHR 0.33, 95% CI 0.08 to 1.45) (Table 13). Figure 22 shows the cumulative incidence plots for acute kidney injury, gastrointestinal events and hypocalcaemia.
| Outcome | Number of events in bisphosphonate users | Number of events in bisphosphonate non-users | HR | 95% CI |
|---|---|---|---|---|
| Acute kidney injury | 80 | 402 | 0.86 | 0.66 to 1.10 |
| Gastrointestinal events | 37 | 160 | 0.96 | 0.66 to 1.40 |
| Hypocalcaemia | 2 | 26 | 0.33 | 0.08 to 1.45 |
FIGURE 22.
Cumulative incidence function plots for (a) AKI; (b) GIE; and (c) hypocalcaemia. AKI, acute kidney injury; GIE, gastrointestinal event.
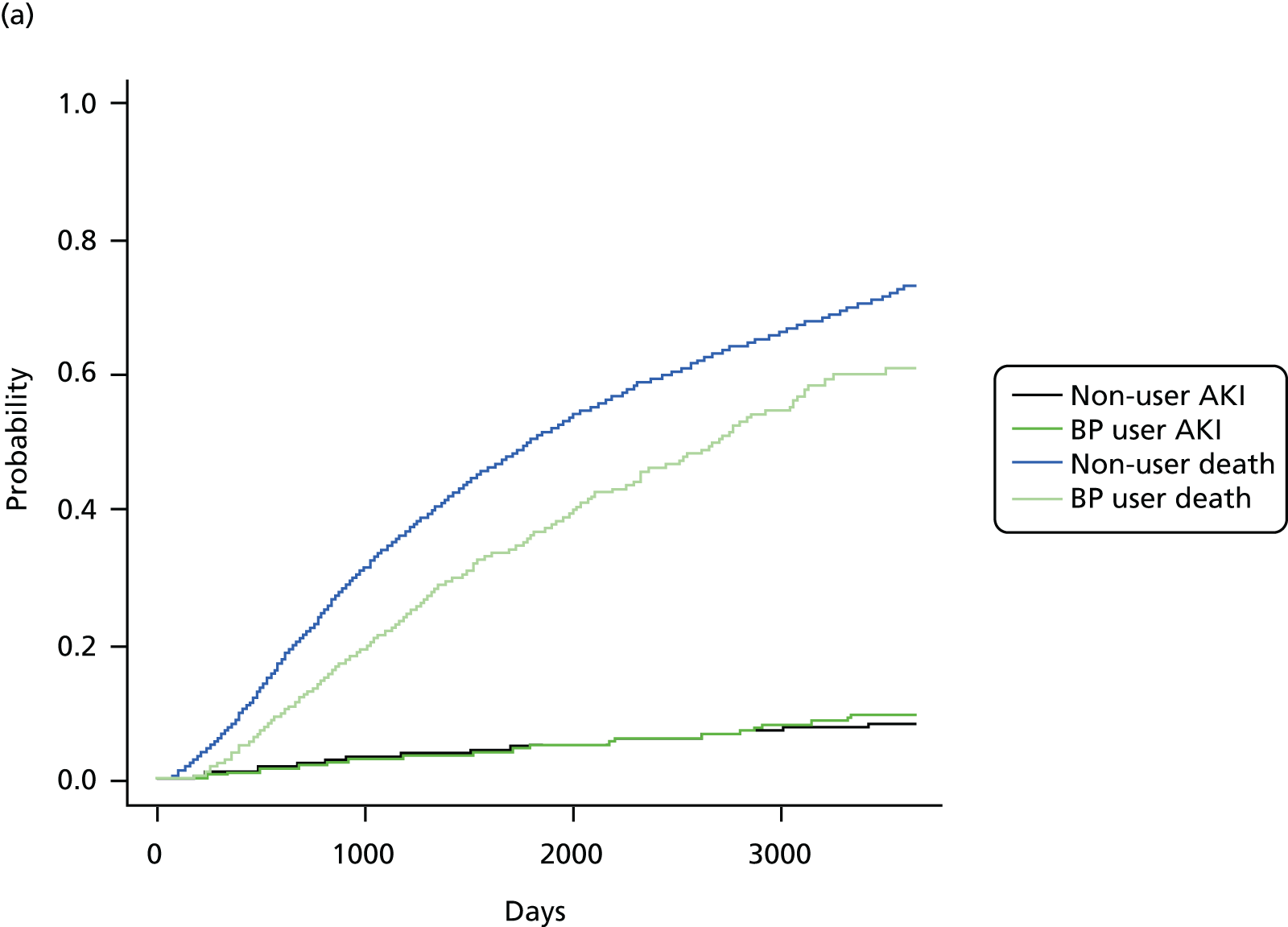
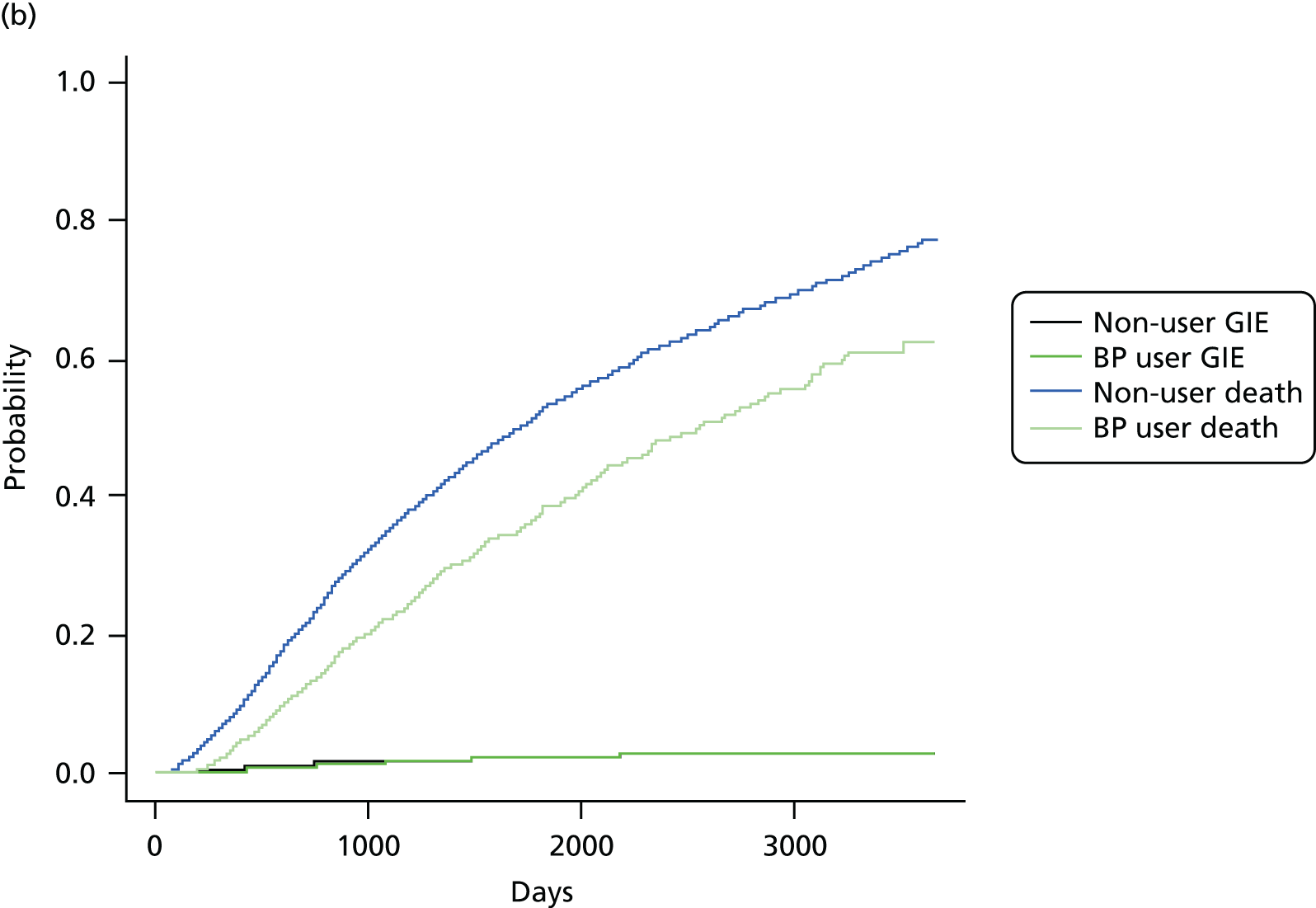
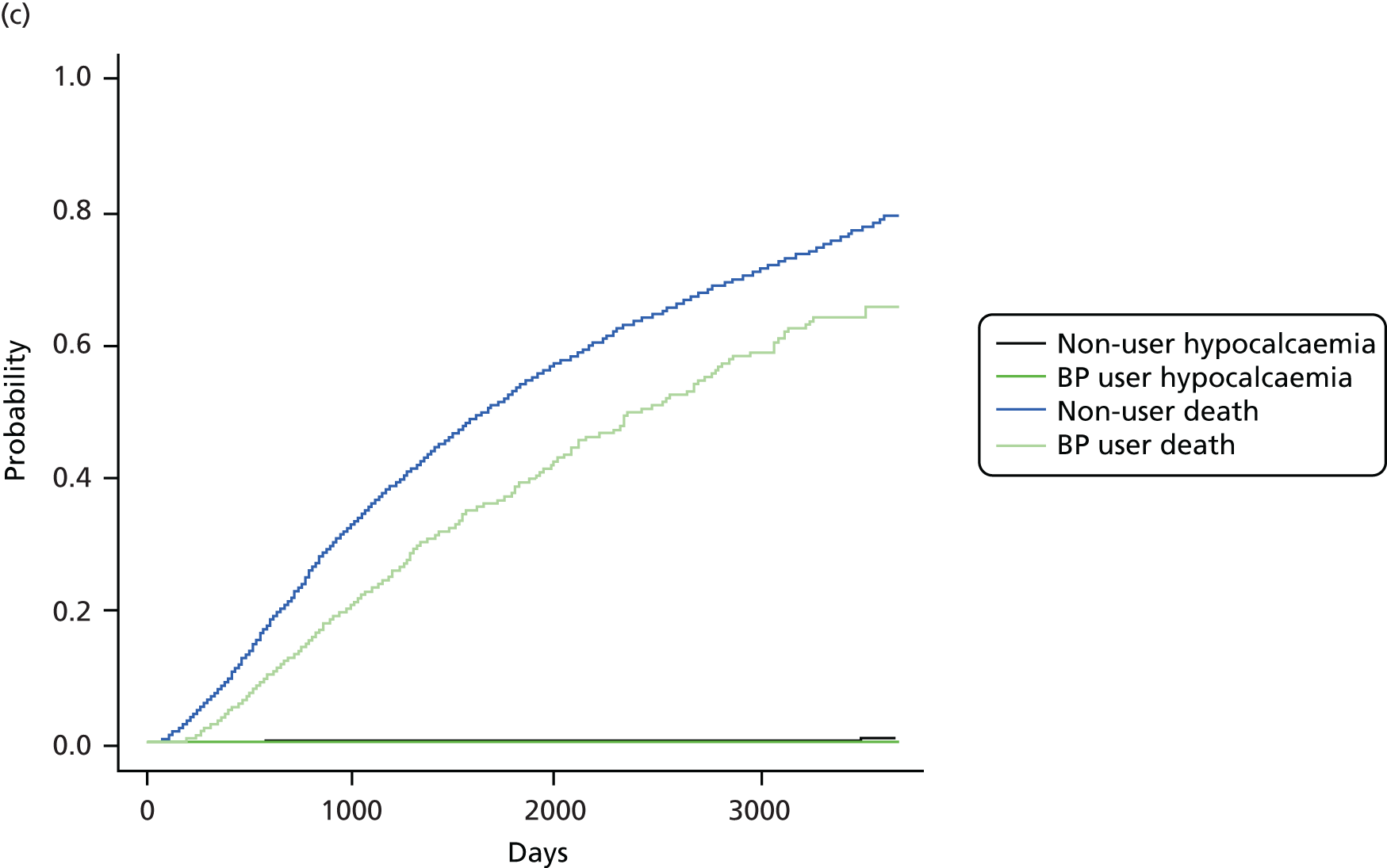
Interactions
Interaction tests identified no significant interactions between history of fracture and bisphosphonate use on risk of acute kidney injury (p = 0.95) or gastrointestinal events (p = 0.19). There were too few hypocalcaemia events to test for stratified history of fracture interactions.
No significant sex-specific associations were identified for bisphosphonate users or non-users on risks of acute kidney injury (p = 0.28) or gastrointestinal events (p = 0.57). There were too few hypocalcaemia events to test for stratified sex for bisphosphonate users. There were also no significant differences in the risks of acute kidney injury (p = 0.95) between bisphosphonate users and non-users who started with different baseline CKD stages. Too few gastrointestinal and hypocalcaemia events occurred to test for stratified baseline CKD stages for bisphosphonate users.
Medication possession ratios
The same MPRs were identified for safety outcomes as in WP1 (see Chapter 3, Medication possession ratio). All HRs for each MPR are reported in Figure 23.
FIGURE 23.
The HRs of each safety outcome split by the MPR quartile.
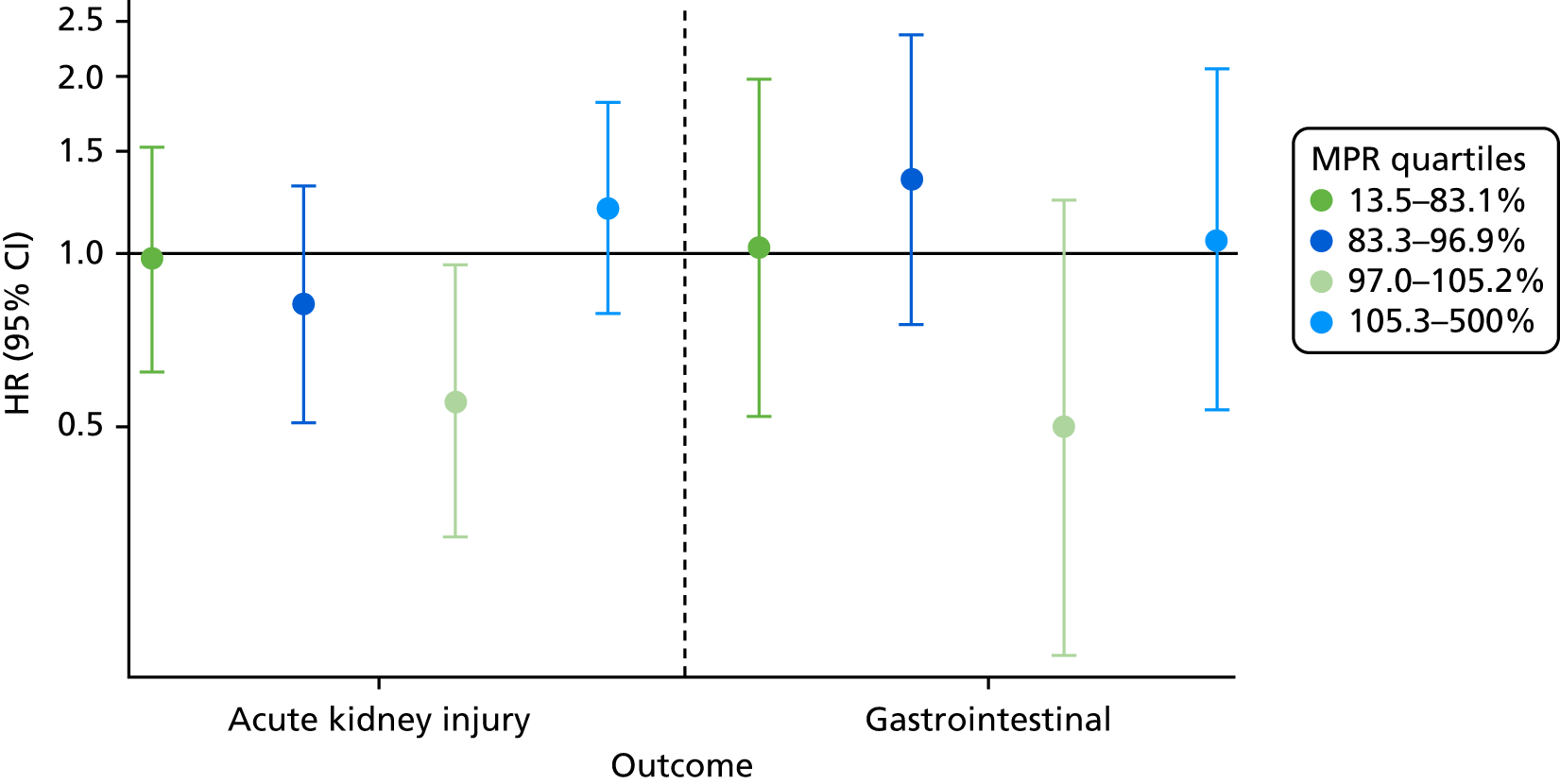
Bisphosphonate users with a MPR of between 97% and 105.2% had an approximately 45% reduced risk of acute kidney injury (sHR 0.55, 95% CI 0.32 to 0.95). These participants had a mean age of 81.1 years and spent an average of 3 years at risk of acute kidney injury. They also had the lowest risk of gastrointestinal events (sHR 0.50, 95% CI 0.20 to 1.23), although this was not a statistically significant result.
The participants at highest risk of acute kidney injury were those with a MPR of ≥ 105.3% (sHR 1.19, 95% CI 0.78 to 1.82). They had a mean age of 82.3 years and spent an average of 2.3 years at risk of acute kidney injury.
Medication possession ratios for hypocalcaemia were not calculated because fewer than five hypocalcaemia events occurred in bisphosphonate users of the matched cohort.
Multivariable model
Table 14 shows the HRs and 95% CIs included in the full multivariable model.
| Category | Acute kidney injury | Gastrointestinal events | Hypocalcaemia |
|---|---|---|---|
| Bisphosphonate use | 0.84 (0.66 to 1.05) | 1.00 (0.71 to 1.41) | 0.28 (0.07 to 1.17) |
| Sex (female) | 0.64 (0.59 to 0.70) | 0.73 (0.65 to 0.83) | 1.02 (0.71 to 1.46) |
| Age (per year increase) | 1.01 (1.01 to 1.01) | 1.02 (1.01 to 1.02) | 0.98 (0.96 to 1.00) |
| Smoker | |||
| Non | Ref | Ref | Ref |
| Ex | 1.16 (1.06 to 1.27) | 1.11 (0.96 to 1.29) | 1.03 (0.66 to 1.59) |
| Current | 1.31 (1.13 to 1.52) | 1.53 (1.28 to 1.84) | 1.49 (0.90 to 2.45) |
| Alcohol | |||
| Non | Ref | Ref | Ref |
| Ex | 1.10 (0.89 to 1.36) | 1.16 (0.8 to 1.68) | 0.73 (0.25 to 2.15) |
| Current | 0.89 (0.81 to 0.97) | 1.00 (0.86 to 1.16) | 0.67 (0.42 to 1.07) |
| IMD score | |||
| 1 – least deprived | Ref | Ref | Ref |
| 2 | 1.11 (1.00 to 1.25) | 1.05 (0.90 to 1.23) | 0.66 (0.42 to 1.06) |
| 3 | 1.14 (1.02 to 1.28) | 0.94 (0.80 to 1.11) | 0.73 (0.46 to 1.16) |
| 4 | 1.15 (1.02 to 1.29) | 1.07 (0.91 to 1.27) | 0.79 (0.50 to 1.26) |
| 5 – most deprived | 1.06 (0.93 to 1.21) | 1.03 (0.85 to 1.25) | 0.67 (0.39 to 1.15) |
| BMI (kg/m2) per 1-unit increase | 1.02 (1.01 to 1.03) | 1.01 (0.99 to 1.02) | 1.00 (0.97 to 1.04) |
| Baseline eGFR score (ml/minute/1.73 m2) | |||
| 0.1–4.9 | Ref | Ref | Ref |
| 5–9.9 | 1.12 (0.54 to 2.29) | 2.05 (0.26 to 16.04) | 2.34 (0.28 to 19.7) |
| 10–14.9 | 1.22 (0.61 to 2.44) | 1.95 (0.26 to 14.66) | 1.76 (0.21 to 15.05) |
| 15–19.9 | 1.14 (0.58 to 2.26) | 1.52 (0.21 to 11.24) | 0.88 (0.10 to 7.69) |
| 20–24.9 | 0.86 (0.44 to 1.69) | 1.8 (0.25 to 13.04) | 0.70 (0.08 to 5.89) |
| 25–29.9 | 0.70 (0.36 to 1.38) | 1.47 (0.20 to 10.63) | 0.93 (0.12 to 7.39) |
| 30–34.9 | 0.45 (0.23 to 0.88) | 1.47 (0.20 to 10.54) | 0.67 (0.08 to 5.27) |
| 35–39.9 | 0.36 (0.19 to 0.71) | 1.25 (0.17 to 9.00) | 0.49 (0.06 to 3.86) |
| 40–44.9 | 0.27 (0.14 to 0.53) | 1.12 (0.16 to 8.03) | 0.33 (0.04 to 2.59) |
| Hospital visits | |||
| 0 | Ref | Ref | Ref |
| 1 | 1.10 (1.00 to 1.21) | 0.97 (0.84 to 1.12) | 0.87 (0.55 to 1.37) |
| 2 | 1.06 (0.93 to 1.21) | 0.91 (0.74 to 1.12) | 1.17 (0.66 to 2.06) |
| 3–5 | 1.34 (1.17 to 1.53) | 1.17 (0.94 to 1.46) | 1.81 (1.05 to 3.09) |
| ≥ 6 | 1.63 (1.27 to 2.11) | 0.86 (0.50 to 1.47) | 3.25 (1.53 to 6.93) |
| 5-year Charlson Comorbidity Index score | |||
| 0 | Ref | Ref | Ref |
| 1 | 1.07 (0.95 to 1.20) | 1.05 (0.89 to 1.24) | 1.1 (0.66 to 1.83) |
| 2 | 1.00 (0.88 to 1.13) | 1.05 (0.87 to 1.27) | 1.02 (0.60 to 1.75) |
| 3–5 | 1.22 (1.06 to 1.40) | 1.15 (0.93 to 1.44) | 1.31 (0.72 to 2.38) |
| ≥ 6 | 1.35 (1.09 to 1.67) | 1.44 (0.99 to 2.08) | 1.27 (0.49 to 3.30) |
| Type 2 diabetes mellitus | 0.97 (0.85 to 1.11) | 0.97 (0.77 to 1.21) | 0.84 (0.44 to 1.60) |
| Cancer | 1.16 (1.02 to 1.31) | 0.92 (0.76 to 1.13) | 1.91 (1.17 to 3.12) |
| CKD | 1.14 (1.01 to 1.28) | 0.71 (0.58 to 0.87) | 0.94 (0.56 to 1.57) |
| Antiarrhythmic agents | 0.82 (0.72 to 0.92) | 0.80 (0.67 to 0.95) | 0.41 (0.20 to 0.82) |
| Dementia | 1.26 (0.90 to 1.77) | 0.43 (0.20 to 0.90) | 2.71 (0.97 to 7.63) |
| Cardiovascular disease | 0.90 (0.80 to 1.00) | 1.05 (0.89 to 1.23) | 0.67 (0.39 to 1.15) |
| Hip fracture | 0.47 (0.24 to 0.95) | 0.72 (0.34 to 1.52) | 1.90 (0.46 to 7.93) |
| Non-hip fracture | 1.23 (1.02 to 1.48) | 1.15 (0.88 to 1.51) | 1.34 (0.64 to 2.78) |
| Deep-vein thrombosis | 1.16 (0.94 to 1.42) | 1.44 (1.09 to 1.91) | 0.72 (0.24 to 2.13) |
| Varices | 1.13 (0.97 to 1.32) | 0.98 (0.78 to 1.23) | 0.84 (0.39 to 1.80) |
| Hypertension | 0.97 (0.89 to 1.05) | 0.95 (0.85 to 1.08) | 0.74 (0.51 to 1.06) |
| Hyperlipidaemia | 0.94 (0.83 to 1.07) | 0.97 (0.80 to 1.17) | 1.39 (0.84 to 2.31) |
| Liver disease | 0.67 (0.36 to 1.25) | 1.48 (0.73 to 2.98) | 4.04 (1.25 to 13.06) |
| Cerebral vascular disease | 1.20 (1.02 to 1.40) | 1.07 (0.85 to 1.34) | 1.28 (0.59 to 2.78) |
| Peripheral vascular disease | 1.04 (0.81 to 1.32) | 1.19 (0.84 to 1.68) | 1.44 (0.49 to 4.20) |
| Hyperthyroidism | 0.97 (0.52 to 1.81) | 0.76 (0.28 to 2.02) | 1.75 (0.24 to 12.63) |
| Prescription category | |||
| 0 | Ref | Ref | Ref |
| 1–3 | 0.65 (0.51 to 0.81) | 0.93 (0.65 to 1.35) | 0.42 (0.18 to 0.97) |
| 4–6 | 0.68 (0.54 to 0.85) | 1.06 (0.74 to 1.52) | 0.54 (0.24 to 1.20) |
| 7–9 | 0.64 (0.50 to 0.80) | 1.04 (0.72 to 1.52) | 0.40 (0.17 to 0.94) |
| 10–12 | 0.67 (0.52 to 0.85) | 1.03 (0.70 to 1.53) | 0.56 (0.23 to 1.35) |
| ≥ 13 | 0.77 (0.59 to 0.99) | 1.06 (0.70 to 1.59) | 0.41 (0.16 to 1.06) |
| NSAIDs | 0.85 (0.79 to 0.92) | 1.05 (0.93 to 1.18) | 0.96 (0.68 to 1.35) |
| Hormone replacement therapy | 0.90 (0.74 to 1.09) | 0.83 (0.62 to 1.12) | 0.85 (0.42 to 1.68) |
| Calcium supplements | 0.94 (0.81 to 1.10) | 1.20 (0.97 to 1.49) | 1.51 (0.90 to 2.54) |
| Steroids | 1.09 (0.98 to 1.20) | 0.99 (0.85 to 1.15) | 1.26 (0.84 to 1.89) |
| Anticoagulants | 1.00 (0.88 to 1.14) | 1.03 (0.85 to 1.25) | 0.84 (0.44 to 1.60) |
| Proton-pump inhibitors | 1.10 (1.01 to 1.20) | 1.00 (0.89 to 1.13) | 1.34 (0.94 to 1.90) |
| Heparins | 1.52 (1.03 to 2.24) | 0.72 (0.32 to 1.61) | 2.24 (0.53 to 9.52) |
| Aromatase inhibitors | 0.57 (0.26 to 1.28) | 1.05 (0.43 to 2.54) | 1.16 (0.16 to 8.62) |
| Antidepressants | 1.08 (0.99 to 1.18) | 1.08 (0.95 to 1.23) | 1.27 (0.87 to 1.83) |
| Statins | 1.00 (0.91 to 1.09) | 0.86 (0.75 to 0.98) | 0.97 (0.66 to 1.44) |
| Antiepileptics | 1.22 (1.03 to 1.46) | 1.03 (0.77 to 1.38) | 0.37 (0.12 to 1.18) |
| Diuretics | 0.98 (0.89 to 1.07) | 1.11 (0.97 to 1.28) | 0.91 (0.61 to 1.35) |
| Beta blockers | 1.02 (0.94 to 1.11) | 1.14 (1.01 to 1.29) | 0.93 (0.65 to 1.33) |
| ACE inhibitors | 1.14 (1.04 to 1.25) | 0.93 (0.82 to 1.05) | 1.28 (0.87 to 1.90) |
| Calcium channel blockers | 1.10 (1.01 to 1.19) | 0.95 (0.84 to 1.07) | 1.34 (0.93 to 1.92) |
| Antihypertensives | 0.90 (0.81 to 1.00) | 0.88 (0.75 to 1.03) | 0.90 (0.57 to 1.40) |
| Digoxin | 1.30 (1.13 to 1.48) | 1.26 (1.04 to 1.53) | 1.04 (0.52 to 2.07) |
| Antidiabetics | 1.51 (1.35 to 1.69) | 1.10 (0.92 to 1.33) | 0.97 (0.57 to 1.65) |
| Insulin | 1.26 (1.08 to 1.47) | 0.99 (0.74 to 1.33) | 1.21 (0.62 to 2.39) |
The risk of acute kidney injury was increased by being a current (HR 1.31, 95% CI 1.13 to 1.52) or ex-smoker (HR 1.16, 95% CI 1.06 to 1.27); having an IMD in the second (HR 1.11, 95% CI 1.00 to 1.25), third (HR 1.14, 95% CI 1.02 to 1.28) or fourth (HR 1.15, 95% CI 1.02 to 1.29) quartile; having an increased BMI (HR 1.02, 95% CI 1.01 to 1.03); having previously visited a hospital, whether only once (HR 1.10, 95% CI 1.00 to 1.21), three or four times (HR 1.34, 95% CI 1.17 to 1.53) or six or more times (HR 1.63, 95% CI 1.27 to 2.11); having a Charlson Comorbidity Index score of between 3 and 5 (HR 1.22, 95% CI 1.06 to 1.4) or > 6 (HR 1.35, 95% CI 1.09 to 1.67); having a history of CKD (HR 1.14, 95% CI 1.01 to 1.28), non-hip fracture (HR 1.23, 95% CI 1.02 to 1.48), cerebrovascular disease (HR 1.20, 95% CI 1.02 to 1.40) or cancer (HR 1.16, 95% CI 1.02 to 1.31); and having previously been prescribed heparin (HR 1.52, 95% CI 1.03 to 2.24), proton-pump inhibitors (HR 1.10, 95% CI 1.01 to 1.20), ACE inhibitors (HR 1.14, 95% CI 1.04 to 1.25), antiepileptics (HR 1.22, 95% CI 1.03 to 1.46), calcium channel blockers (HR 1.10, 95% CI 1.01 to 1.19), antidiabetics (HR 1.51, 95% CI 1.35 to 1.69), digoxin (HR 1.30, 95% CI 1.13 to 1.48) or insulin (HR 1.26, 95% CI 1.08 to 1.47).
The risk of acute kidney injury was reduced by being female (HR 0.64, 95% CI 0.59 to 0.70), currently drinking alcohol (HR 0.89, 95% CI 0.81 to 0.97), having an eGFR of > 30 ml/minute/1.73 m2 [HR 0.45 (95% CI 0.23 to 0.88) for an eGFR of 30–14.9 ml/minute/1.73 m2 and HR 0.27 (95% CI 0.14 to 0.53) for an eGFR of 40–44.9 ml/minute/1.73 m2], having a history of hip fracture (HR 0.47, 95% CI 0.24 to 0.95), being prescribed one to three prescriptions in the previous year (HR 0.65, 95% CI 0.51 to 0.81), being prescribed ≥ 13 prescriptions in the previous year (HR 0.77, 95% CI 0.59 to 0.99) and currently taking NSAIDs (HR 0.85, 95% CI 0.79 to 0.92).
The risk of gastrointestinal events was increased by being older (HR 1.02, 95% CI 1.01 to 1.02 per year), a current smoker (HR 1.53, 95% CI 1.28 to 1.84) and having previously been prescribed beta blockers (HR 1.14, 95% CI 1.01 to 1.29) or digoxin (HR 1.26, 95% CI 1.04 to 1.53).
The risk of gastrointestinal events was reduced by being female (HR 0.73, 95% CI 0.65 to 0.83), having a history of antiarrhythmics (HR 0.80, 95% CI 0.67 to 0.95), dementia (HR 0.43, 95% CI 0.20 to 0.90) or CKD (HR 0.71, 95% CI 0.58 to 0.87).
The risk of hypocalcaemia was increased by visiting a hospital three to five (HR 1.81, 95% CI 1.05 to 3.09) or six or more (HR 3.25, 95% CI 1.53 to 6.93) times in the previous year and having a history of liver disease (HR 4.04, 95% CI 1.25 to 13.06) or cancer (HR 1.91, 95% CI 1.17 to 3.12).
The risk of hypocalcaemia was reduced by having been prescribed one to three or seven to nine prescriptions in the previous year.
Post hoc analysis: participants who survived the first 30 days
A post hoc analysis of patients who survived the first 30 days after cohort entry found similar results to those of the full model (Table 15). Bisphosphonate use, again, did not affect the risk of acute kidney injury (HR 0.86, 95% CI 0.70 to 1.06), gastrointestinal events (HR 1.03, 95% CI 0.77 to 1.38) or hypocalcaemia (HR 0.89, 95% CI 0.48 to 1.66). The proportionality assumption was met after participants who did not survive the first 30 days were excluded.
| Outcome | Number of exposed events | Number of unexposed events | HR | 95% CI |
|---|---|---|---|---|
| Acute kidney injury | 123 | 631 | 0.86 | 0.70 to 1.06 |
| Gastrointestinal events | 64 | 291 | 1.03 | 0.77 to 1.38 |
| Hypocalcaemia | 12 | 64 | 0.89 | 0.48 to 1.66 |
Chapter 6 Work package 4: bisphosphonate use and bone mineral density
Introduction
Bone mineral density is a recognised key determinant of future fracture risk in postmenopausal women97 and patients with CKD. 98 The relationship between changes in BMD during treatment with oral bisphosphonates and fracture risk is less clear. Clinical trial participants with higher BMD gains on oral bisphosphonate therapy had a significantly lower risk of fracture than participants with no change or a reduction in BMD. 99 BMD is thus an accepted surrogate marker in bridging studies for anti-osteoporosis treatments where efficacy has already been demonstrated in postmenopausal women. Such strategies have been followed to test different dosing regimens100 and to study the efficacy in other populations, such as men101 and patients with glucocorticoid-associated osteoporosis. 102
However, early BMD changes during therapy are difficult to interpret as a result of regression to the mean. 103 Most patients who lose BMD in the first year of alendronate therapy have significant increases in the second year. The relationship between BMD change and fracture risk in postmenopausal osteoporosis versus bone fragility in CKD may differ given the different aetiopathology of bone fragility in CKD,104 with particular reference to potential adynamic bone disease. Treatment-related BMD may actually increase fracture risk. For example, fluoride therapy results in increased BMD, which is associated with a paradoxical increased risk of fracture. 105 Patients with CKD also have vascular calcification, which artificially inflates spinal BMD, which, in turn, can affect interpretation of their BMD. 106
We studied the association between bisphosphonate use and changes in BMD over time, focusing on femoral neck BMD as the primary outcome and analysing total hip and lumbar spine BMD as secondary outcomes.
Methods
Study design and data source
We conducted a risk-set (incidence density) sampling propensity score-matched cohort study using routinely collected data from OUHD.
The OUHD holds DXA-measured BMD data for the whole of the Funen region, the third-largest island of Denmark, collected between 1990 and February 2015.
Serum creatinine is part of the routine panel of blood tests performed at the Odense University Hospital as part of osteoporosis care. Primary care practices and hospitals in this Danish region all use the same clinical biochemistry laboratory, making all biochemistry values (including serum creatinine tests) available for this study. We were able to calculate the eGFR for participants in this cohort using the CKD-EPI formula, as done for the CPRD data set in WP1 (see Chapter 2, Estimated glomerular filtration rate measurement).
Pharmacy drug dispensations of primary and secondary care prescriptions can be tracked back to 1995 from the Danish Prescriptions Register and linked to OUHD using unique national patient identifiers. Pharmacy dispensations are recorded using Anatomic Therapeutic Classification codes.
The Odense Patient Data Exploratory Network provides a unique platform for linking clinical, dispensation and biochemistry data. It is an approved Statistics Denmark institutional partner for linking to diagnoses and comorbidity recorded in Danish hospital records. The Network has previously been used to link clinical biochemistry to fracture outcomes in numerous studies. 107–111
Study participants
All patients registered in OUHD who were aged ≥ 40 years at the time of biochemistry testing, had at least one eGFR measurement of < 45 ml/minute/1.73 m2 (stage 3B CKD), had at least 2 years of follow-up data available and had at least two DXA BMD measurements recorded 2 or more years apart were eligible for inclusion.
Previous users of any anti-osteoporosis medication (except calcium and vitamin D supplements) in the year before eGFR testing and those with > 1 year between the closest renal measurement (eGFR) and DXA scan (BMD measurement) were excluded.
The first dispensation of a bisphosphonate (therapy initiation) was considered the index date for bisphosphonate users. Participants were risk set-matched with replacement to non-users on ± 5 years of the year of birth at index date. For every bisphosphonate user, up to five non-users at index date were randomly selected from a large pool of non-users. Selection bias was thus minimised while preserving statistical power, as initial non-users could become bisphosphonate users and be included later.
Exposure, outcome and confounders
Bisphosphonate use, defined by pharmacy dispensations in the Danish Prescriptions Register, was the main exposure. Users of bisphosphonates were identified using prespecified lists of Anatomic Therapeutic Classification codes (see Report Supplementary Material 1 for the code list). Like prescriptions, dispensation data do not necessarily reflect treatment duration. Treatment episodes were created using a previously validated algorithm that accounts for non-adherence (or non-compliance) and defines treatment episodes of continuous exposure. Any overlapping prescriptions of the same drug were interpreted as early collection of a repeat prescription. Any overlapping days between two prescriptions of the same drug were added to the end of the period covered by the two prescriptions. Any two prescriptions of the same drug were concatenated if the gap between the end of the first and the start of the second was < 30 days.
The primary study outcome for WP4 was annualised femoral neck BMD percentage change, relative to the previous or baseline BMD, up to 3 years after bisphosphonate use (or non-use), measured using a DXA scan. If more than two measurements were available, the last DXA scan measurement 2–3 years from the index date was considered. Any measurements within 1 year of each other were dismissed, as they were unlikely to provide clinically relevant information.
Secondary outcomes were similarly defined as annualised BMD percentage changes, measured at the lumbar spine and total hip. If participants had serial spine measurements, we used the vertebrae that were still assessable at the last recorded visit to calculate the rate of change.
A sensitivity analysis used DXA scan measures taken 3–5.5 years after the index date.
Potential confounders were pre identified using clinical knowledge and a literature review (see Chapter 2). Confounders were measured at baseline and included age, sex, BMI, baseline eGFR, fracture history, comorbidities (Charlson Comorbidity Index, renal disease and diabetes mellitus), hospital visits in the previous year and concomitant drug use (number of Anatomic Therapeutic Classifications in the year before index, aromatase inhibitors, antihypertensives, antidepressants and antidiabetics). As systemic steroids have an effect on BMD and are commonly used in certain renal conditions, we accounted for these drugs in a more granular fashion. We calculated the number of defined daily doses in the previous year and stratified into quartiles.
Statistical analysis
In a primary analysis, propensity scores were estimated using multivariable logistic regression, including the confounders listed in the previous section. Interactions between age and BMI, as well as age and baseline eGFR, were added to the propensity score equation to improve the balance of the baseline characteristics. The calculated propensity scores were used to match each bisphosphonate user with up to five comparable non-users, with a calliper width of, at most, 0.02 SDs. Balance before and after matching was checked using the ASMD. As in WPs 1–3, an ASMD of ≤ 0.10 was considered acceptable and representative of good balance.
Linear regression modelling was used to estimate average differences (expressed as beta coefficient and 95% CIs) in the annualised BMD change (per cent vs. index measure) at the femoral neck (primary outcome), total hip and lumbar spine (secondary outcomes).
Two post hoc analyses were conducted to deal with the low number of bisphosphonate users eligible for inclusion in the final analyses:
-
A linear regression model including all eligible bisphosphonate users and non-users and adjusted for the previously calculated propensity scores was fitted.
-
A multivariable linear regression model, adjusted for the same confounders included in the propensity score logistic equations, was fitted to model annualised percentage BMD change according to bisphosphonate use, with 95% CIs.
All analyses were conducted using R. Statistical syntax was programmed at Oxford using mock data extracted from the analytical data set. These programmes were run ‘in situ’ by a statistician at Odense, Denmark. Final results were checked by two researchers (DPA and BA).
Results
Participants and baseline characteristics
We identified 36,024 patients in the linked OUHD BMD database who were eligible for the study, including 12,544 (34.8%) identified as bisphosphonate users within the study period. Of the 12,544 bisphosphonate users, we excluded 233 (1.9%) who were aged < 40 years at the time of therapy initiation, 1323 (10.5%) who did not have an eGFR measurement recorded in the previous year and 10,586 (84.4%) who had an eGFR value of ≥ 45 ml/minute/1.73 m2. Of the remaining 402 eligible bisphosphonate users, one-third (132/402, 32.8%) had no DXA measures in the year before therapy initiation. Therefore, 270 bisphosphonate users were eligible for the study. Of these, 35 (13.0%) died during follow-up, 93 (34.4%) discontinued bisphosphonate therapy before having a repeat BMD measure and 142 (52.6%) did not have a repeat BMD measure between 1 and 3.5 years after the first scan after therapy started. Therefore, 71 bisphosphonate users were included in the study.
In parallel, 23,713 non-users were initially available for comparison. Of these, 1228 (5.2%) died during follow-up. Only 1609 (6.8%) had repeat BMD measures between 1 and 3.5 years after their index DXA and were eligible for the analysis.
Baseline characteristics for all 270 eligible bisphosphonate users and the final sample of 71 are reported in Table 16. As expected, participants included in the final analyses were younger (79 vs. 82 years, on average), healthier (54.9% vs. 52.6% with a Charlson Comorbidity Index score of 0) and more likely to use systemic corticosteroids (40.8% vs. 36.3%), statins (42.3% vs. 39.3%) or antihypertensives (81.7% vs. 73.3%) than those excluded.
| Characteristic | User | |
|---|---|---|
| Eligible | Included | |
| Unique patients (n) | 270 | 71 |
| eGFR (ml/minute/1.73 m2), mean (SD) | 37.2 (7.2) | 37.9 (8.0) |
| Dead within 3 years of first bisphosphonate, n (%) | 35 (13.0) | 0 (0.0) |
| Age (years), median (IQR) | 82 (76–87) | 79 (70–83) |
| Sex (female), n (%) | 268 (99.3) | 71 (100.0) |
| Index BMD (g/cm2),a mean (SD) | ||
| Total hip | 0.657 (0.123) | 0.678 (0.117) |
| Femoral neck | 0.551 (0.110) | 0.560 (0.106) |
| Spine | 0.791 (0.165) | 0.782 (0.166) |
| Fractures, n (%) | ||
| Hip | 21 (7.8) | 5 (7.0) |
| Non-hip other | 21 (7.8) | 5 (7.0) |
| Non-hip osteoporotic | 28 (10.4) | 6 (8.5) |
| Charlson Comorbidity Index score, n (%) | ||
| 0 | 142 (52.6) | 39 (54.9) |
| 1 | 64 (23.7) | 18 (25.4) |
| 2 | 29 (10.7) | 7 (9.9) |
| ≥ 3 | 35 (13.0) | 7 (9.9) |
| Concomitant drugs, n (%) | ||
| Systemic corticosteroids | 98 (36.3) | 29 (40.8) |
| Anticoagulants | 163 (60.4) | 35 (49.3) |
| Statins | 106 (39.3) | 30 (42.3) |
| Antidiabetics | 38 (14.1) | 7 (9.9) |
| Antihypertensives | 198 (73.3) | 58 (81.7) |
Table 17 reports baseline characteristics for the included participants, stratified by bisphosphonate use. Both groups contained only women and had an average age of 79–80 years. As expected, bisphosphonate users had a much lower index BMD (0.56 vs. 0.62 g/cm2 at femoral neck), were more likely to use systemic corticosteroids (40.8% vs. 32.8) and had a much higher prevalence of previous fracture (7.0% vs. 1.7% for hip fracture history) than non-users. Low bone mass, prevention of glucocorticoid-induced osteoporosis and secondary fracture prevention are the most probable indicators of bisphosphonates in patients with moderate to severe CKD. All of these variables and the confounders listed in previous sections were adjusted for using propensity scores and multivariable regression modelling.
| Characteristic | Before matching | After matching | ||
|---|---|---|---|---|
| Users | Non-users | Users | Non-users | |
| Patients (n) | 71 | 1492a | 40 | 142 |
| eGFR (ml/minute/1.73 m2), mean (SD) | 37.9 (8.0) | 36.1 (7.7) | 37.1 (9.1) | 38.0 (6.7) |
| Age (years), median (IQR) | 79 (70–83) | 80 (76–85) | 79 (75–82) | 79 (73–84) |
| Sex, n (%) | ||||
| Female | 71 (100) | 1492 (100) | 40 (100.0) | 142 (100.0) |
| Male | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| BMD (g/cm2) | ||||
| Total hip | 0.678 (0.117) | 0.754 (0.144) | 0.663 (0.119) | 0.738 (0.134) |
| Femoral neck | 0.560 (0.106) | 0.627 (0.121) | 0.547 (0.104) | 0.617 (0.104) |
| Spine | 0.782 (0.166) | 0.877 (0.171) | 0.806 (0.146) | 0.881 (0.176) |
| Fractures, n (%) | ||||
| Hip | 5 (7.0) | 28 (1.9) | 5 (12.5) | n < 5 |
| Non-hip other | 5 (7.0) | 99 (6.6) | n < 5 | n < 5 |
| Non-hip osteoporotic | 6 (8.5) | 89 (6.0) | n < 5 | 6 (4.2) |
| Charlson Comorbidity Index score, n (%) | ||||
| 0 | 39 (54.9) | 778 (52.1) | 27 (67.5) | 95 (66.9) |
| 1 | 18 (25.4) | 322 (21.6) | 8 (20.0) | 23 (16.2) |
| 2 | 7 (9.9) | 213 (14.3) | n < 5 | 9 (6.3) |
| ≥ 3 | 7 (9.9) | 179 (12.0) | n < 5 | 8 (5.6) |
| Drugs, n (%) | ||||
| Systemic corticosteroids | 29 (40.8) | 493 (33.0) | 11 (27.5) | 51 (35.9) |
| Anticoagulants | 35 (49.3) | 818 (54.8) | 18 (45.0) | 61 (43.0) |
| Statins | 30 (42.3) | 522 (35.0) | 17 (42.5) | 51 (35.9) |
| Antidiabetics | 7 (9.9) | 316 (21.2) | 5 (12.5) | 26 (18.3) |
| Antihypertensives | 58 (81.7) | 1331 (75.8) | 32 (80.0) | 112 (78.9) |
| Additional diagnoses, n (%) | ||||
| Diabetes mellitus (uncomplicated) | 5 (7.0) | 171 (11.5) | n < 5 | 16 (11.3) |
| Diabetes mellitus with complications | n < 5 | 30 (2.0) | n < 5 | n < 5 |
| Dementia | n < 5 | 40 (2.7) | n < 5 | n < 5 |
| Cardiovascular disease | n < 5 | 17 (1.1) | 0 (0) | n < 5 |
| Hypertension | 16 (22.5) | 328 (22.0) | 7 (17.5) | 26 (18.3) |
| COPD | 8 (11.3) | 143 (9.6) | n < 5 | 14 (9.9) |
| Heart failure | 17 (23.9) | 383 (25.7) | 8 (20.0) | 27 (19.0) |
| Renal transplant | 0 (0.0) | 6 (0.4) | 0 (0) | 0 (0) |
| Renal dialysis | 0 (0.0) | 20 (1.3) | 0 (0) | 0 (0) |
Propensity score matching
Before matching, the two groups had little overlap in their propensity scores, with medians of 0.49 (IQR 0.17–0.84) for the bisphosphonate users and < 0.001 (IQR < 0.001–0.003) for the non-users. After matching, the medians of the propensity scores were more similar: 0.19 (IQR 0.08–0.43) in the bisphosphonate users and 0.13 (IQR 0.04–0.43) in the non-users.
Of the 71 bisphosphonate users, 40 were matched to 142 non-users. Matching improved balance and reduced differences to acceptable limits (ASMD < 0.1) for most of the assessed participant characteristics included in the model (see Appendix 3, Figure 27). However, relevant differences, for users and non-users, respectively, remained for key confounders, including baseline (index) femoral neck BMD (0.55 vs. 0.62 g/cm2), use of systemic glucocorticoids (27.5% vs. 35.9%) and previous hip fracture history (12.5% vs. < 3.5%). Charlson Comorbidity Index score, polypharmacy, number of hospital visits, history of key comorbid conditions (complicated diabetes mellitus, hypertension, chronic heart failure and chronic obstructive pulmonary disease) and previous use of antiepileptics or antidiabetic therapies also still had unacceptable imbalances (ASMD > 0.1) after matching.
Matching excluded > 91% of the potentially eligible bisphosphonate non-users.
Annualised bone mineral density changes over time
In the propensity-matched cohorts (prespecified primary analysis), bisphosphonate non-users lost, on average, 1.59% of their baseline femoral neck BMD per year, whereas bisphosphonate users increased their BMD by 1.07% per year of therapy. The average percentage BMD changes in the propensity score-matched cohort are detailed in Table 18. In a linear regression model, after propensity score matching, the mean difference in percentage annual change in femoral neck BMD between bisphosphonate users and non-users favoured the users by 2.65% (95% CI 1.32% to 3.99%) per year of bisphosphonate exposure.
| Location of BMD measure | Analysis | Non-user | User | Mean difference (%) (95% CI) | ||
|---|---|---|---|---|---|---|
| n | BMD change (g/cm2) | n | BMD change (g/cm2) | |||
| Femoral neck | Propensity score matched | 142 | –1.59 | 40 | 1.07 | 2.65 (1.32 to 3.99) |
| Multivariable | 1492 | –1.67 | 71 | 0.63 | 2.14 (1.22 to 3.05) | |
| Propensity score adjusted | 1492 | –1.67 | 71 | 0.63 | 2.15 (0.97 to 3.34) | |
| Spine | Propensity score matched | 142 | 0.34 | 40 | 3.36 | 3.01 (1.74 to 4.28) |
| Multivariable | 1492 | 0.65 | 71 | 3.98 | 2.14 (1.22 to 3.05) | |
| Propensity score adjusted | 1492 | 0.65 | 71 | 3.98 | 2.87 (1.62 to 4.12) | |
| Total hip | Propensity score matched | 142 | –1.16 | 40 | 0.95 | 2.12 (0.98 to 3.25) |
| Multivariable | 1492 | –2.14 | 71 | 0.82 | 2.29 (1.46 to 3.11) | |
| Propensity score adjusted | 1492 | –2.14 | 71 | 0.82 | 1.91 (0.82 to 3.00) | |
In the analyses including the full cohorts, bisphosphonate non-users lost, on average, 1.67% of their baseline femoral neck BMD per year, whereas bisphosphonate users gained, on average, 0.63% per year on treatment. The results of linear regression modelling supported the findings from the propensity-matched analysis, with a multivariable-adjusted beta coefficient (mean difference between groups) of 2.14% (95% CI 1.22% to 3.05%) and a propensity score-adjusted beta of 2.15% (95% CI 0.97% to 3.34%) per year.
In the analysis of secondary outcomes, BMD at the spine increased among bisphosphonate users by 3.98% per year for the full cohort and by 3.36% in the propensity-matched cohort. Bisphosphonate non-users in the full cohort increased their BMD by 0.65% per year and those in the matched cohort increased their BMD by 0.34% per year. The mean difference in percentage BMD change was, again, in favour of bisphosphonate users, with betas of 3.01% (95% CI 1.74% to 4.28%) per year for the propensity score-matched analysis, 2.14% (95% CI 1.22% to 3.05%) for the multivariable analysis and 2.87% (95% CI 1.62% to 4.12%) for the propensity score-adjusted analysis.
Total hip BMD improved by an average of 0.82% per year for the full cohort of bisphosphonate users and by 0.95% for the matched cohort. Bisphosphonate non-users in the full cohort lost, on average, –2.14% BMD per year and those in the propensity-matched cohort lost –1.16% per year. The mean difference was 2.12 (95% CI 0.98% to 3.25%) per year in favour of bisphosphonate users in the propensity score-matched model, 2.29 (95% CI 1.46% to 3.11%) for the multivariable model and 1.91% (95% CI 0.82% to 3.00%) for the propensity-adjusted model.
The prespecified sensitivity analysis using DXA scan measures taken between 3 and 5.5 years after index date could not be performed as only one bisphosphonate user and 12 non-users had valid follow-up data in this time window.
Chapter 7 Discussion and interpretation
Work package 1: the association between bisphosphonate use and the progression of kidney disease
Key results
Our primary analysis included > 2400 bisphosphonate users and > 8900 comparable (propensity score-matched) non-users with stage 3B+ CKD. A post hoc sensitivity analysis using multivariable regression included > 2600 bisphosphonate users and almost 54,000 non-users with similarly severe renal disease.
As expected, bisphosphonate users and non-users had differing baseline eGFR, comorbidity, fracture history and concomitant drug use, including systemic steroids. Propensity score matching resolved relevant imbalances in prespecified observed confounders. Disease progression (worsening) was common, and seen slightly more often in bisphosphonate users (almost 23%) than non-users (about 21%). Most participants who experienced worsening progressed by one stage in CKD severity, with few participants reaching end-stage renal disease. The rates of progression were higher in bisphosphonate users (89 per 1000 person-years overall) than matched non-users (86 per 1000 person-years) and increased with age (particularly for those aged > 70 years) and for males.
Cox survival models confirmed an association between bisphosphonate use and a 12% increased risk of CKD progression. Post hoc and sensitivity analyses confirmed these findings and showed that bisphosphonate therapy was also related to borderline significant 7% decrease in the probability of CKD improvement, shown by reversion to a less severe stage. Relevant interactions were identified with sex and fracture history, with a stronger effect among women (24% excess risk) and in those with a previous fracture (32% increased risk). A prespecified additional analysis of the association between adherence to bisphosphonate therapy and CKD worsening demonstrated a gradient in risk, with those fully adherent (100% compliance) to bisphosphonates having the most marked excess risk, which was ≈60% higher than matched non-users, and those with lower compliance (< 85%) having a more moderate increase in risk, which was ≈35% higher than matched non-users.
Multivariable regression modelling confirmed an 18% increase in risk of CKD progression associated with bisphosphonate use and confirmed the effect of known clinical risk factors for CKD progression, including age, smoking, comorbidity, cardiovascular disease and diabetes mellitus.
A prespecified secondary analysis of continuous change in renal function (eGFR) was conducted. Fitted random-effects modelling suggested stable renal function over 10 years, on average. However, large variations around the mean curves were observed in individual trajectories of eGFR change over time. The mean curves obtained in the random-effects model probably did not well represent all individual bisphosphonate users and non-users. The findings obtained in the random-effects models should therefore be interpreted with caution.
Limitations and strengths
Our study has limitations. We used observational data to study the safety of bisphosphonates, which are licensed medications for the treatment of osteoporosis and bone fragility. Differences are expected between patients who are prescribed bisphosphonates and those who are not. We used both propensity score matching and multivariable regression adjustment to minimise or adjust for any imbalance in patient characteristics between the two groups. Observational routinely collected data are commonly used by medicines regulators both nationally [by the Medicines and Healthcare products Regulatory Agency (MHRA)] and internationally [by the European Medicines Agency (EMA)] as part of post-marketing drug surveillance. Published EMA-endorsed methodological guidelines recognise the strengths and limitations of the analysis methods used here. 113 The adjustment methods used minimise confounding by indication related to the observed variables, but do not account for any imbalance in unavailable confounders. Missing data imputation was used to impute large proportions of missing data in the lifestyle risk factors of BMI, smoking status and alcohol drinking. Such methods assume missingness at random, which cannot be tested for statistically. There might have been imbalance in these risk factors if missing factors were related to the study outcomes.
Misclassifications in the exposure (bisphosphonate use) and study outcome (CKD worsening) are possible. Primary care prescriptions are not necessarily equivalent to the actual consumption or use of the prescribed therapies. A study114 conducted by our group demonstrated a 20% primary non-adherence to alendronic acid in similar primary care data linked to prescription and pharmacy dispensation records from Spain. Similarly, eGFR renal function measures based on serum creatinine and formulae like the CKD-EPI used in our study have been criticised and might not be accurate in certain patient subgroups. 115 Our random-effects analyses showed large fluctuations in the trajectories of eGFR change over time, both individually and at the cohort level. Random-effects models cannot accurately model such fluctuations.
Our study also has major strengths. To our knowledge, this is the biggest cohort study of the association between bisphosphonate use and the worsening or progression of renal disease in patients with moderate to severe CKD. Our study population included > 11,000 participants with stage 3B+ CKD, comprising 2447 bisphosphonate users and 8931 matched non-users, who were followed up for up to 10 years.
New-user cohort designs, like the one used here, are among the best currently available to study the safety of medicines under real-world practice conditions. By excluding prevalent users and starting follow-up of drug users at the time of their first prescription, we avoided selection bias resulting from the depletion of susceptibles. 116 Unlike more efficient designs, such as case–control studies, this cohort design allowed us to calculate absolute risks, which are essential for therapeutic risk–benefit evaluation. Similar real-world analyses based on routinely collected data from actual practice have been shown to accurately replicate RCT findings. 117
We conducted sensitivity analyses to identify the effects of our analytical methods on our study results. They consistently confirmed an almost 20% increase in the risk of CKD progression associated with bisphosphonate use, with narrow CIs. The potential trend of increasing point estimates or risk with increasing compliance to therapy suggests a causal explanation for the observed associations. Further research is needed to confirm the existence of a dose–response relationship.
The chosen data sources, CPRD linked to HES, are highly representative of the UK population and NHS practice. Our findings should thus be generalisable to the wider community of patients affected by stage 3B+ CKD in the UK.
Findings in context
As there are few data on the renal safety of bisphosphonates in patients with moderate to severe CKD, these drugs are not supported in patients with stage 4+ CKD. Large, placebo-controlled RCTs of bisphosphonates for treating osteoporosis have shown a good renal safety profile. Unfortunately, these RCTs excluded participants with known CKD, including only a few participants with unknown CKD. 11,16 All bisphosphonates are thus contraindicated for use by patients with an eGFR of < 30 ml/minute/1.73 m2, and some are even contraindicated for those with an eGFR of < 35 ml/minute/1.73 m2. 20–22
Official regulatory adverse event reporting systems have identified safety signals about renal toxicity associated with bisphosphonates. The US Food and Drug Administration’s Adverse Event Reporting System published a report including 72 cases of renal failure associated with intravenous zoledronate. 87 Serum creatinine increased from a baseline average of 1.7 mg/dl to 6.5 mg/dl after a zoledronate infusion, then dropped back to 2.7 mg/dl after drug discontinuation. Similar data have also been published by the French Adverse Effect Reporting Database. 88 Although weak in study design, spontaneous reporting is useful for regulatory authorities as it allows otherwise unexpected adverse effects to be identified and safety to be studied in populations who are under-represented in, or excluded from, pivotal RCTs.
Four small RCTs118–121 including 373 participants with CKD reported renal adverse events associated with bisphosphonate treatment, compared with placebo, in participants with stages 3–5 CKD. Three of these trials were conducted in kidney transplant recipients118–120 and the other was a pilot study in stages 3–4 CKD. 121 Although none of these trials found an excess risk of CKD worsening, they were small and short in duration. A post hoc analysis of a large RCT including 1332 women with stage 3 CKD and 31 women with stage 4 CKD is the biggest potential source of data on the renal safety of bisphosphonates in moderate to severe CKD to date. Unfortunately, the published article17 did not report the effect of treatment on CKD progression or other renal events.
Our finding of a 12% excess risk of CKD progression (worsening) with bisphosphonate use agrees with the spontaneous report data described above, not the RCT findings. These differences may have arisen from unresolved bias due to confounding in our analysis or the low external validity of the RCT results. The available RCT data are scarce and from small, short-term studies. The participants of these trials were also not representative of the general population of patients with stage 3B+ CKD in the community. Unresolved confounding is always a possibility in observational research. Propensity score matching and multivariable regression modelling are common strategies for reducing the confounding related to observed variables. We also used array sensitivity analyses to measure the impact of potential unknown confounders on our findings. The results were reassuring, as only unlikely clinical scenarios combining high prevalence and strong effect(s) of residual confounder(s) would attenuate the observed effect.
Conclusions
In a propensity-matched new-user cohort analysis of > 11,000 NHS patients with stage 3B+ CKD, we found a 12% excess risk of renal disease worsening by one or more stages associated with bisphosphonate use. The observed effect increased with better compliance to these therapies and was highest in women and in those with a previous fracture history.
Post hoc analyses using alternative analytical methods suggested a similar but slightly higher association of up to 18% excess risk. They also showed a relationship between bisphosphonate use and a borderline significant 7% reduction in the probability of CKD improvement to a less severe stage in this population. A bias analysis suggested that our findings are unlikely to be explained by an alternative unknown confounder.
Work package 2: the relationship between bisphosphonates and incident symptomatic (clinical) osteoporotic fractures
Key results
We demonstrated an unexpected higher fracture risk at hip and non-hip fracture sites in CKD patients exposed to bisphosphonates than in bisphosphonate non-users. A causal association between fracture risk and bisphosphonate use or a spurious association from residual bias may have driven this result.
The major strengths of this analysis are its large sample size and real-world setting. The fracture end-point analyses were based on > 11,000 bisphosphonate users, propensity score-matched with almost 44,000 non-users with similar observed characteristics. All participants had moderate to severe CKD as per our inclusion criteria, almost two-thirds with stage 3B, around one-third with stage 4 and 1% with stage 5 CKD. In comparison, a recent meta-analysis16 of bisphosphonate trials included just 1030 participants with CKD. In a post hoc multivariable analysis requested by the Steering Committee, we included all participants in both cohorts: 215,617 bisphosphonate non-users and 11,567 users.
Clinical trials of bisphosphonates have excluded participants with comorbidities, recruiting a healthier sample than the real-world community of patients with CKD and reducing the generalisability of their findings. 12 This is, in part, reflected by the high fracture risk of participants in the primary analysis of our propensity-matched cohorts, with hip fracture rates of 2.1/100 and 1.6/100 person-years among bisphosphonate users and non-users, respectively, and an additional 3.9/100 and 2.5/100 person-years for non-hip osteoporotic fractures. The risk was always higher in women than in men and increased with age, particularly for hip fractures, which agrees with the literature.
Fracture incidence rates were either similar (with overlapping CIs) or higher in bisphosphonate users than matched non-users. Bisphosphonate users had Cox-derived increases in their risk of hip (25%), non-hip (58%) and overall osteoporotic fractures (almost 40%) over 10 years of follow-up, compared with non-users. The excess risk was more noticeable in men, who had an almost 50% higher risk of hip fracture, a 90% higher risk of non-hip fracture and a 70% higher risk of overall osteoporotic fracture. No significant interactions were found for age, previous fracture history or baseline renal function.
These counterintuitive increases in risk were highest in the first 6 months of bisphosphonate therapy, with risk then declining. For example, hip fracture risk almost doubled in the first 6 months after therapy started (compared with no therapy), then declined to a 25% excess risk over 10 years of treatment. This result does not agree with previous knowledge, such as efficacy data from pivotal RCTs67,68 that found no bone effects in the first 6 months of bisphosphonate treatment. This pattern of higher imminent fracture risk in those exposed to bisphosphonates than in those unexposed suggests an imbalance in time-dependent determinants of fracture risk, such as time since a fragility fracture, onset of specific comorbidities and specific medications, rather than time-dependent risk factors for fracture such as parenteral history of hip fracture. 122 Analyses of the effect of compliance on fracture risk did not demonstrate a clear dose–response pattern. For example, participants in the third quartile of adherence (97% to 110% compliance) had a lower risk than those in the lowest quartile (< 88%) for all three outcomes. This lack of a dose–response effect suggests that something other than causality is the most probable explanation for the observed increased risk, such as unresolved bias.
Post hoc multivariable regression models agreed with the primary analysis and confirmed known risk factors for fracture, including age, smoking status, comorbidity, fracture history, dementia, liver disease, type 2 diabetes mellitus and insulin treatment. Our data also confirmed the protective, antifracture effects of adiposity, better baseline renal function and previous use of hormone replacement therapy.
Limitations and strengths
In the absence of clear guidelines for starting bisphosphonates in patients with CKD, patient and physician characteristics influence exposure to bisphosphonates. Propensity score matching is currently the best method for minimising imbalance in these determinants. Confounding by indication is the key barrier to observational comparative effectiveness research, as the risk of the key preventable outcome (here fracture) drives the need or indication for treatment. Extreme confounding cannot be corrected without information on the key drivers of treatment. Age and sex, two key determinants of fracture risk, were both balanced in this analysis. No interaction was found with previously reported fracture history, which is another key determinant of fracture risk. 123
At least one key factor for decision-making is BMD, which is not routinely recorded in CPRD or HES. BMD could have been predicted to some degree before conducting the analyses. Alternatively, we could have identified surrogates or proxies in the data that were at least partially co-related to BMD, such as BMI, smoking status or the use of treatments that induce bone loss, such as glucocorticoids. The findings suggested the presence of strong unresolved confounding, as low BMD was more common in bisphosphonate users than in matched non-users. The array analysis found that a 55–60% osteoporosis prevalence in users (which was similar to the prevalence in similar participants in WP4), combined with a HR of > 2.2 for fracture, would negate the increased risk of fracture in bisphosphonate users.
The association observed was probably not causal, but driven by bias. Most of the increase in fracture happened in the first 6 months of exposure, when bisphosphonates are known to have no effect on fracture risk. As we found no dose–response association, higher cumulative exposure as a result of better compliance did not result in a higher risk of fracture. The observed effect also disagrees with results from the literature and WP4 on the effects of bisphosphonates on bone strength. We found a positive association between the use of bisphosphonates and a gain in BMD over time when using a smaller but much more granular data set that included repeat BMD measures in WP4.
Information bias due to misclassification of exposure (drug use) and outcome (fracture) was possible, and has been discussed in Work package 1: the association between bisphosphonate use and the progression of kidney disease, Limitations and strengths. However fractures, particularly hip fractures, have previously been validated in CPRD and found to be highly accurately coded. 124
This study also has strengths. This cohort analysis included > 11,000 bisphosphonate users and > 44,000 non-users. Long-term follow-up for up to 10 years of bisphosphonate treatment was available, which is much longer than the duration of existing RCTs on the use of these drugs. The chosen study outcome is one that has been previously validated in CPRD. Fractures, particularly hip fractures, are known to be well recorded in CPRD records, and we have extensive expertise in using these data sources to study the epidemiology of osteoporotic fractures nationally. 125,126 Our multivariable models confirmed the effect of previously described risk factors for fracture, including baseline eGFR.
Findings in context
A large body of evidence has demonstrated the antifracture efficacy of bisphosphonates in RCTs,84,127 systematic reviews of RCTs128 and observational real-world data. 82,129,130 However, observational studies comparing fracture rates between bisphosphonate users and non-users have found an excess risk of fracture in patients treated with these therapies. For example, a case–control study131 of > 10,000 elderly women found a 30% increased fracture risk in participants exposed to bisphosphonates for ≥ 3 years. A cohort study132 of primary fracture prevention found no reduction in risk. Although these two studies focused on different populations, they also did not have BMD measurements, which is one of the key indications for treatment.
Unfortunately, the evidence is more sparse and of worse quality in patients with bone fragility associated with CKD, sometimes called chronic kidney disease–mineral and bone disorder (CKD-MBD). A 2017 systematic review16 identified six RCTs of bisphosphonate versus placebo that included participants with CKD at different stages and used fracture as a key outcome. Although a promising treatment effect size was seen, suggesting a 30–40% relative risk reduction in vertebral fracture risk, it was not statistically significant, with the upper limit of the 95% CI reaching 1.65 (95% CI 0.30 to 1.65).
Conclusions
In this large cohort study with long-term follow-up, we could not demonstrate the expected antifracture effectiveness of bisphosphonates in participants with stage 3B+ CKD, despite matching on potential confounders. We found an increased risk of hip and non-hip fractures associated with bisphosphonate use in these patients, mainly in the first few months of treatment. The lack of plausible temporality (most of the increased risk happened too early in the exposure for the treatment to have had an effect) or dose–response gradient (no association between compliance and fracture risk) suggest that unresolved confounding is the most probable explanation for our counterintuitive results, and that any beneficial effect of bisphosphonate was insufficient to overcome this bias.
Work package 4 found a positive effect of bisphosphonate on BMD in patients with moderate to severe CKD (see Chapter 6). However, despite the use of advanced pharmacoepidemiological analyses, we have not confirmed the antifracture efficacy of bisphosphonates. Instead, our bias analyses suggested that unobserved BMD could explain the observed association between bisphosphonate use and fracture risk. It is, therefore, improbable that other similar observational analyses will be able to establish the clinical effectiveness of bisphosphonates compared with non-use in moderate to severe CKD. A RCT with fracture as the primary end point is needed to determine the antifracture efficacy of bisphosphonates in patients with stage 3B+ CKD.
Work package 3: the risk of adverse events among users of bisphosphonates, compared with matched non-users
Key results
Our propensity score-matched cohorts included > 2400 bisphosphonate users and 8900 non-users with stage 3B+ CKD, of similar baseline characteristics for all measured confounders, and with up to 10 years of follow-up available. We identified 480 cases of severe acute kidney injury leading to hospital admission, equivalent to incidence rates of around 12 to 15 per 1000 person-years, with similar rates in bisphosphonate users and non-users. We identified 205 gastrointestinal events, with incidence rates of 5.5 to 6 per 1000 person-years in both users and non-users. Hypocalcaemia (incidence rate of 0.3 to 1.1 per 1000 person-years) and hypophosphataemia (only seven cases identified) leading to hospital admission were rare events, with similar rates in bisphosphonate users and matched non-users.
Survival analyses confirmed these findings, with similar HRs for the exposed and unexposed propensity-matched cohorts for all of the outcomes.
A prespecified interaction was found with previous fracture history. Although no difference in risk was noted among those with no previous fracture, a 50% excess risk of acute kidney injury was associated with the use of bisphosphonates in secondary prevention in those with a previous fracture history. The dose–effect analyses found no gradient, with no clear trend towards an increased risk with higher cumulative exposure for any of the outcomes.
Post hoc multivariable adjustment obtained consistent findings, with no excess risk of acute kidney injury, gastrointestinal events or hypocalcaemia associated with bisphosphonate use in the full cohort. Predictors of three outcomes were identified:
-
A higher risk of acute kidney injury was seen with older age, smoking status, higher BMI, lower baseline eGFR, more hospital visits in the previous year, higher comorbidity, a history of dementia, type 2 diabetes mellitus, cerebrovascular disease or cancer, and use of anticoagulants, heparin, proton-pump inhibitors, ACE inhibitors, antiepileptics, digoxin or antidiabetic therapies, including insulin.
-
A higher risk of gastrointestinal events was seen with older age, smoking, alcohol drinking, socioeconomic deprivation, more hospital visits, a higher Charlson Comorbidity Index score, a previous history of liver disease or hyperthyroidism and use of NSAIDs, diuretics, beta blockers, antidiabetics or digoxin.
-
A higher risk of hypocalcaemia was seen with smoking, more hospital visits in the previous year, a history of hyperthyroidism or cancer history and previous use of calcium supplements. The hypocalcaemia models may have been overfitted as a result of the low number of events.
Limitations and strengths
This safety analysis has some limitations. Despite the use of state-of-the-art methods to adjust for confounding by indication, unresolved confounding may have remained and could partially explain the findings. However, we believe that the granularity of the data used and the extensive list of variables included in the propensity score and multivariable models was sufficient to account for imbalances in key confounders. Propensity matching reduced the observed differences in baseline characteristics between bisphosphonate users and non-users. Post hoc multivariable analyses confirmed the robustness of these findings. No dose–response gradient was seen, suggesting that there is no association between higher cumulative use of bisphosphonates and the risk of any of the proposed outcomes.
Hypophosphataemia could not be analysed as only seven cases were identified in among > 11,000 participants. With such a low incidence, and thus a low public health impact, any potential increase in the risk of hypophosphatemia is a low priority. Even if a relative increase in risk existed, the absolute excess risk would be small and the number needed to harm very high. Furthermore, the used ICD-10 codes for phosphorus alterations are non-specific and could result in misclassification of the study outcome hypophosphataemia.
As in previous chapters, misclassification of the exposure (drug use) was possible and has already been discussed. Severe acute kidney injury has previously been validated and shown to be accurately coded in English hospital records (HES). 33 Gastrointestinal events are also well recorded and have recently been validated by our group. 133 Hypocalcaemia and hypophosphataemia coding have not been validated, to our knowledge, and their coding quality may have been lower than that of the validated events.
This study also has strengths. The large size of the study population (> 11,000 participants in the propensity-matched analysis), the choice of study design (new-user cohort with propensity matching) and the length of follow-up available (up to 10 years) should have led to highly accurate results. The use of routinely collected NHS primary and secondary care data make the findings generalisable to the wider population of UK patients with stage 3B+ CKD.
Findings in context
As in WP1, there are few data on the safety of bisphosphonates for patients with CKD in general, or related to acute kidney injury, gastrointestinal events, hypocalcaemia or hypophosphataemia.
According to a recent systematic review and meta-analysis,16 only four RCTs, including 373 participants, have reported on the renal safety (including acute kidney injury) of bisphosphonates in CKD stage 3+ patients. Although these trials did not identify any safety signals, a number of spontaneous reports have raised concerns on the potential association between acute kidney injury and bisphosphonate use,134,135 including oral bisphosphonate. 136 Although sparse, the observational data available are also reassuring. Only one large study,137 including > 120,000 elderly patients, has been published to date on the association between bisphosphonate use and acute kidney injury risk in people with previous CKD. This cohort analysis found no excess risk of acute kidney risk among users of bisphosphonates, compared with non-users. 137 These findings from RCTs and cohort studies agree with our results and suggest no excess risk of acute kidney injury associated with bisphosphonate use by patients with underlying stage 3+ CKD. Our finding of an excess risk among participants with a previous fracture history and concomitant stage 3+ CKD have not been replicated elsewhere and should be externally validated in other data sets.
To our knowledge, two placebo-controlled trials118,121 have reported on the gastrointestinal safety of bisphosphonates in CKD: a pilot RCT of patients with stage 3–4 CKD121 and a small RCT in kidney transplant patients. 118 Neither trial found an increase in any of the reported gastrointestinal events in the treatment (bisphosphonate) arm compared with the placebo group. However, studies including more healthy populations have found an excess risk of gastrointestinal events related to the use of bisphosphonates. For example, the FIT pivotal RCT17 of alendronate found an increased risk of upper gastrointestinal events in the treatment arm, with no difference between those with and those without CKD. Observational studies have also found an excess risk of upper and lower gastrointestinal events among bisphosphonate users, with an even higher risk among those with pre-existing CKD. 93 A 2018 propensity-matched population-based cohort study,138 including participants with stage 3+ CKD from Spain, reported a 53% excess risk of gastrointestinal events among bisphosphonate users. Our finding of no excess risk of gastrointestinal events with bisphosphonate use in stage 3B+ CKD therefore agrees with the two smaller RCTs,118,121 but not with the bigger pivotal RCT17 or observational studies. 93 It remains unclear whether or not bisphosphonates lead to an increased risk of gastrointestinal events, and whether or not any resulting outcomes are particularly severe or common in people with CKD. More research is needed on the impact of bisphosphonates on gastrointestinal health.
Hypocalcaemia and hypophosphataemia are highly prevalent in severe CKD and are a key component of CKD-MBD. According to the recent CKD guidelines from KDOQI,139 CKD-MBD is defined as a disorder characterised by the presence of any of the following in CKD patients: calcium, phosphorus, parathyroid or vitamin D abnormalities; bone turnover, mineralisation or strength abnormalities; or extraskeletal calcification. At least one RCT120 conducted in patients with severe CKD requiring kidney transplant has reported an increased risk of hypocalcaemia associated with bisphosphonate (pamidronate) treatment. The only re-analysis17 of pivotal RCTs of bisphosphonates in participants with CKD excluded those with baseline abnormal serum calcium or phosphate levels and did not report on hypocalcaemia. Case reports140,141 have suggested an association between bisphosphonate use and symptomatic hypocalcaemia, but bigger, better-designed cohort studies have failed to demonstrate this. Instead, they have confirmed an asymptomatic reduction in serum calcium following the initiation of bisphosphonate therapy, but with no clinical consequences. 142 Our findings are in line with the previously mentioned trial120 and with this cohort study. 142 A 2018 study138 of participants with stage 3B+ CKD in Spain also found no association between bisphosphonate use and symptomatic hypocalcaemia.
To our knowledge, and according to a recent systematic review,16 no placebo-controlled RCTs of bisphosphonates involving CKD patients have reported on hypophosphataemia. We found low rates of symptomatic hypophosphataemia that did not allow for comparison between bisphosphonate users and non-users. Similar, and even lower, rates were observed in the aforementioned Spanish cohort analysis. 138
Conclusions
Our results showed that bisphosphonates were not associated with an increased risk of acute kidney injury, gastrointestinal events, hypocalcaemia or hypophosphataemia in participants with stage 3B+ CKD. No excess risk of any of these undesired events was seen, even in participants with long-term follow-up or complete (≥ 100%) compliance with the drugs.
These findings must be interpreted in the context of the results of an increased risk of CKD progression in WP1 to fully understand the safety profile of bisphosphonates in patients with moderate to severe CKD. The results from WPs 2 and 4 must also be taken into account, to better inform the risks and benefits expected from bisphosphonate therapy in these patients.
Work package 4: bisphosphonate use and bone mineral density
Key results
We used region-wide population-based data from Funen, Denmark, for WP4. Despite the size of the source data set, only 71 participants were identified with stage 3B+ CKD who were given bisphosphonates, continued the treatment for at least 1 year and had at least two recorded BMD measurements. Only 1609 records of repeat BMD measurements from around 200 eligible participants not using bisphosphonates were available. Propensity score matching led to the exclusion of almost half of the users and > 90% of the non-users. Although matching helped to reduce confounding, imbalance remained when bisphosphonate users and matched non-users were compared, including key confounders such as baseline BMD, fracture history and use of systemic steroids. The small sample demonstrates the low likelihood of Danish patients with stage 3B+ CKD being prescribed bisphosphonates, reflecting the safety concerns and lack of evidence of efficacy that led to this study.
The primary propensity-matched cohort analysis identified a significant, clinically relevant effect, with a 1.1% femoral neck BMD gain per year in bisphosphonate users, compared with a –1.1% bone loss at the same site per year in matched non-users. This is equivalent to a 2.2% difference in BMD change per year in favour of bisphosphonate users. The full cohort multivariable and propensity-adjustment analyses found consistent results, with a 2.1% and 2.2% difference per year favouring bisphosphonate use, respectively.
Consistent results were found for the secondary outcomes. Lumbar spine and total hip BMD improved by 3.4% and 1.0% per year, respectively, in bisphosphonate users, compared with 0.3% and –1.2% (bone loss) per year in matched non-users, respectively. This equated to a significant 3.0% per year average difference in the lumbar spine BMD and a 2.1% difference in the total hip BMD, both favouring bisphosphonate users. Multivariable and propensity-adjusted models found similar results.
Limitations and strengths
This cohort analysis has limitations. The eligibility criteria excluded most of the records in the database. Although this was not surprising, as bisphosphonates are to be used with caution in patients with stage 3 CKD and are contraindicated in stages 4+, it is possible that the generalisability of the findings might have been compromised. The need for repeat DXA testing to obtain BMD change over time, our outcome, excluded more than half of the potentially eligible bisphosphonate users. These patients may have differed from the included participants, as BMD monitoring during treatment is not routine practice in actual care conditions. Current evidence suggests that routine monitoring of BMD within 3 years of starting bisphosphonates in postmenopausal women is clinically unhelpful143 and may contribute to a lack of follow-up densitometry in an unexpectedly large proportion of those treated. Trials of bisphosphonates have shown a significant attenuation of BMD gain after the first 12 months of therapy. 80,144
The overlap between bisphosphonate users and non-users was small, excluding almost half of the bisphosphonate users and > 90% of the non-users when propensity score matching was applied. However, the multivariable and propensity-adjustment models that include the full cohort gave reassuringly similar estimates to the propensity-matched analysis.
Only women were included in these analyses. The findings are thus limited to the female population.
As previously discussed, misclassification of the exposure (bisphosphonate use) is always a possibility, and non-adherence is more probable in actual practice than in RCT settings. However, the data used for these analyses identified drug use based on linked pharmacy dispensation records from the Danish Prescription Registry, thereby minimising the issue of primary non-adherence. The study outcome (BMD change over time) was probably more accurate in this work package than in WPs 1–3, as it did not rely on diagnostic coding.
This analysis also has some strengths. The data set used has high granularity and quality, and comprises information from a number of linked data sources. It is a unique data source for research as it includes comorbidity, hospital visits, biochemistry, drug use, BMD testing and related questionnaires. 145 The data set also includes most of the population of a whole region of Denmark, thus maximising the external validity of the findings.
Findings in context
The observed differences in femoral neck BMD in exposed versus unexposed participants over 1 year were similar to those observed in trials of postmenopausal women. 80 However, our bisphosphonate users had only half the increase in BMD seen in the trial’s treatment arm after 1 year of therapy, where the increase in femoral neck BMD was 2% with alendronate. 80 The difference in BMD at the spine in our bisphosphonate users was also smaller than that seen in the trial, in which there was a 4.2% increase after 1 year. It is difficult to compare our unexposed CKD participants with those in the placebo arm of the trial as the trial participants who received the placebo also received calcium and vitamin D supplements.
Early BMD changes during therapy are difficult to interpret because of regression to the mean,103 with most patients who lose BMD in the first year of alendronate therapy going on to have significant increases in BMD in the second year. The relationship between BMD change and fracture risk may differ in postmenopausal osteoporosis and bone fragility in CKD as they have different aetiopathology, with the potential involvement of adynamic bone disease in bone fragility in CKD. 104 The disconnect between treatment-related BMD increases and fracture risk is exemplified in trials of fluoride therapy, in which increased BMD was associated with a paradoxical increased risk of fracture. 105 The artefactual effects of vascular calcification on spinal BMD further complicate the interpretation of BMD in patients with CKD. 106
Conclusions
We demonstrated that bisphosphonate therapy had a positive effect on BMD in participants with stage 3B+ CKD. BMD is the best surrogate available for bone strength in clinical practice, and RCTs using BMD as a primary outcome have been accepted by regulators as bridge studies to extend the indication of bisphosphonate therapies for men101 and for the prevention or treatment of steroid-induced osteoporosis. 146
We found that BMD improved more (by 2% to 3% per year, depending on skeletal site) in bisphosphonate users than in non-users. In fact, BMD decreased over time when participants were off bisphosphonate therapy, by about 1% per year in the femoral neck and total hip. The result was an average difference of 2.7%, 3% and 2.1% BMD change per year in the femoral neck, lumbar spine and total hip, respectively, all in favour of bisphosphonate users. These findings were not modified by age, sex (the analysis included only women), previous fracture history or baseline renal function.
Conclusions: clinical implications and future research recommendations
Risk–benefit of bisphosphonates in moderate to severe chronic kidney disease
Our findings from WPs 1 and 3 suggest that bisphosphonates could slightly accelerate the progression (worsening) of chronic kidney failure in patients with stage 3B+ CKD. The data obtained from WP1 demonstrated a 12–18% excess risk of stage worsening among users of bisphosphonates compared with non-users. The risk increase appeared higher in women and in those with a previous fracture history. Unfortunately, it is in this particular scenario – secondary fracture prevention in those with a substantially higher absolute fracture risk – that a benefit from bisphosphonate therapy is likely to be observed and, hence, bisphosphonates are likely to be recommended in patients with moderate to severe CKD. Given the relatively high rates of CKD progression seen in this particular population, the absolute risk increase is high; therefore, the overall number needed to harm is relatively low at 41 and 31 for the usually recommended 3- or 5-year treatment regime, respectively.
The results from WP3 are more reassuring, with no increase in severe unwanted effects related to bisphosphonate use (acute kidney injury, gastrointestinal events, hypocalcaemia or hypophosphataemia leading to hospital admission).
We studied the antifracture and BMD benefits of bisphosphonates in participants with stage 3B+ CKD in WPs 2 and 4, respectively. WP2 failed to demonstrate the expected fracture protective effectiveness seen in previous RCTs in postmenopausal women, in men and in those with glucocorticoid-induced osteoporosis. Sensitivity and post hoc analyses suggest that routinely collected observational data are unlikely to be useful for assessing the antifracture effectiveness of bisphosphonates, given the lack of sufficient detail on key variables such as BMD and other potential unmeasured confounders. WP4 used more granular data from Denmark and found a positive effect of bisphosphonates on BMD in participants with moderate to severe CKD. Similar improvements were seen in femoral neck (primary outcome), lumbar spine and total hip BMD for bisphosphonate users, compared with non-users, but were smaller than those observed in treated participants in the pivotal RCTs for bisphosphonates in postmenopausal women147 and in men. 101 These improvements result in clinically relevant fracture risk reduction in postmenopausal osteoporosis, with numbers needed to treat of around 12 for preventing any clinical fracture, 46 for preventing a hip fracture in patients with a previous fracture and 66 for preventing a hip fracture in patients without a previous fracture. 78,84
These results support the potential benefit of bisphosphonate therapy in bone mineral disease associated with moderate to severe (stage 3B+) CKD, but raise concerns on the renal toxicity of these drugs in this particular patient group. Given the combination of risk and benefit estimates above, bisphosphonates should continue to be used with caution in these patients, and treatment decisions should be discussed with patients and all physicians involved in their care. The results from our multivariable models might be useful for identifying patients at high risk of fracture (the preventable event) and flagging those at high risk of renal toxicity due to CKD progression or acute kidney injury. Unfortunately, many risk factors are predictors of both fracture and renal disease progression, making it difficult for patients and physicians to decide on ideal candidates for treatment.
The data should also inform regulatory decision-making. Current small product characteristics either contraindicate or advise the cautious use of bisphosphonates in patients with an eGFR of < 35 or < 30 ml/minute/1.73 m2, depending on the bisphosphonate. Our data suggest that these drugs should also be used with caution in the larger group of patients with stage 3B CKD, who have an eGFR of 30–45 ml/minute/1.73 m2. These findings will be reported to national (MHRA) and European (EMA) regulators to maximise the public health impact of our research.
Future research recommendations
There are few current alternatives for the treatment of bone mineral disease in CKD. Denosumab is the only anti-osteoporosis therapy not formally contraindicated in patients with even severe CKD. However, the product label states a ‘precaution for use’, suggesting that calcium levels be monitored in patients with severe renal impairment (creatinine clearance of < 30 ml/minute). Post-marketing safety data have suggested an increased risk of severe hypocalcaemia among denosumab users with stage 4+ CKD. 148
The high fracture risk seen in patients with CKD in this and previous studies and the complex risk–benefit assessment of the only available therapies suggest that more data are needed to fully understand the best possible management of bone mineral disease in patients with moderate to severe CKD. Future research should take four steps:
-
These findings should be replicated in a different population.
-
Observational, real-world data on the potential renal toxicity of bisphosphonates in stage 3A CKD is urgently needed. Such data would inform the potential lower limit of renal function that is associated with excess toxicity. Thousands of patients with stage 3A CKD currently receive bisphosphonates. Potential deleterious effects on their renal function must be established.
-
Real-world studies on the comparative safety of denosumab versus bisphosphonates in stage 3+ CKD are warranted. For regulators to make a decision about the potential risk–benefit of both drugs in stage 3B CKD, we need more information about the effects of bisphosphonates and denosumab in this patient group.
-
Pending safety data, a RCT comparing the antifracture efficacy of bisphosphonates and denosumab should be considered. As shown in WP2, it is improbable that observational data will be able to establish the antifracture effectiveness of anti-osteoporosis therapies in patients with moderate to severe CKD. Once safety has been established, a RCT comparing possible alternative bone-specific therapies would provide key information on the potential benefit of these treatments.
Acknowledgements
This study was approved by the ISAC (protocol number 15_ 53R2A). It was based, in part, on data from CPRD obtained under licence from the UK MHRA. The data were provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone.
Patient and public involvement
Two patient and public involvement co-applicants participated in the study as co-investigators and attended the co-investigator teleconferences to monitor the study progress.
The PPI co-applicants will be key in the dissemination of the study. A plain English summary of the results will be written with their help for charity websites and publications. We will also disseminate the results to patient societies with their help. A meeting with relevant charities and patient organisations will take place in 2019 to discuss the results of the study.
We acknowledge English language editing by Dr Jennifer A de Beyer of the Centre for Statistics in Medicine, University of Oxford.
Contributions of authors
Danielle E Robinson (Statistician) contributed to the acquisition, analysis and interpretation of data.
M Sanni Ali (Assistant Professor in Epidemiology and Senior Researcher in Pharmacoepidemiology Methods) contributed to the design of the work and the acquisition, analysis and interpretation of data.
Victoria Y Strauss (Senior Medical Statistician and Senior Research Associate in Pharmaco- and Device Epidemiology) contributed to the acquisition, analysis and interpretation of data.
Leena Elhussein (Statistician) was responsible for the statistical analyses and reviewed the manuscript for scientific content.
Bo Abrahamsen (Clinical Professor and Consultant Endocrinologist) contributed to the acquisition, analysis and interpretation of data.
Nigel K Arden (Professor in Rheumatic Disease) contributed to the interpretation of data.
Yoav Ben-Shlomo (Professor of Clinical Epidemiology) contributed to the interpretation of data.
Fergus Caskey (Consultant Senior Lecturer) contributed to the interpretation of data.
Cyrus Cooper (Professor of Rheumatology and Honorary Consultant Rheumatologist) contributed to the interpretation of data.
Daniel Dedman (Research Scientist) contributed to the interpretation of data.
Antonella Delmestri (Senior Database Manager) contributed to the acquisition, analysis and interpretation of data.
Andrew Judge (Professor of Translational Statistics) contributed to the interpretation of data.
Muhammad Kassim Javaid (Associate Professor and University Lecturer in Metabolic Bone Disease) contributed to the conception and design of the work and the acquisition, analysis and interpretation of data.
Daniel Prieto-Alhambra (Professor in Pharmaco- and Device Epidemiology) contributed to the conception and design of the work and the acquisition, analysis and interpretation of data.
All authors contributed to the revision of this work and approved the final version.
Data-sharing statement
The data used in this study were obtained from the Clinical Practice Research Datalink (CPRD), Hospital Episodes Statistics (HES) and the Danish Odense University Hospital Databases (OUHD). The agreements in place for use of this data do not permit further distribution or sharing. Requests for the relevant data sets should be made directly to CPRD, HES, and the OUHD. Further information can be obtained from the corresponding author.
Publications
Sanni Ali M, Ernst M, Robinson DE, Caskey F, Arden NK, Ben-Shlomo Y, et al. Alendronate use and bone mineral density gains in women with moderate-severe (stages 3B–5) chronic kidney disease: an open cohort multivariable and propensity score analysis from Funen, Denmark. Arch Osteoporos 2020;15:81.
Robinson DE, Sanni Ali M, Pallares N, Tebé C, Elhussein L, Abrahamsen B, et al. Safety of oral bisphosphonates in moderate-to-severe chronic kidney disease: a bi-national cohort analysis. J Bone Mineral Res 2021; in press.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 2013;8. https://doi.org/10.1007/s11657-013-0137-0.
- Lubwama R, Nguyen A, Modi A, Chirovsky D, Miller PD. Prevalence of renal impairment among osteoporotic women in the USA, NHANES 2005–2008: is treatment with bisphosphonates an option?. Osteoporos Int 2014;25:1607-15. https://doi.org/10.1007/s00198-014-2645-1.
- Gifford FJ, Methven S, Boag DE, Spalding EM, Macgregor MS. Chronic kidney disease prevalence and secular trends in a UK population: the impact of MDRD and CKD-EPI formulae. QJM 2011;104:1045-53. https://doi.org/10.1093/qjmed/hcr122.
- Jamal SA, Swan VJ, Brown JP, Hanley DA, Prior JC, Papaioannou A, et al. Kidney function and rate of bone loss at the hip and spine: the Canadian Multicentre Osteoporosis Study. Am J Kidney Dis 2010;55:291-9. https://doi.org/10.1053/j.ajkd.2009.10.049.
- Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 2006;17:3223-32. https://doi.org/10.1681/ASN.2005111194.
- Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, et al. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 2007;167:133-9. https://doi.org/10.1001/archinte.167.2.133.
- Dooley AC, Weiss NS, Kestenbaum B. Increased risk of hip fracture among men with CKD. Am J Kidney Dis 2008;51:38-44. https://doi.org/10.1053/j.ajkd.2007.08.019.
- Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 2000;58:396-9. https://doi.org/10.1046/j.1523-1755.2000.00178.x.
- Chennuru S, Koduri J, Baumann MA. Risk factors for symptomatic hypocalcaemia complicating treatment with zoledronic acid. Intern Med J 2008;38:635-7. https://doi.org/10.1111/j.1445-5994.2007.01580.x.
- Miller PD. Fragility fractures in chronic kidney disease: an opinion-based approach. Cleve Clin J Med 2009;76:715-23. https://doi.org/10.3949/ccjm.76a.08108.
- Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res 2005;20:2105-15. https://doi.org/10.1359/JBMR.050817.
- Reyes C, Pottegård A, Schwarz P, Javaid MK, Van Staa TP, Cooper C, et al. Real-life and RCT participants: alendronate users versus FITs’ trial eligibility criterion. Calcif Tissue Int 2016;99:243-9. https://doi.org/10.1007/s00223-016-0141-7.
- Couttenye MM, D’Haese PC, Deng JT, Van Hoof VO, Verpooten GA, De Broe ME. High prevalence of adynamic bone disease diagnosed by biochemical markers in a wide sample of the European CAPD population. Nephrol Dial Transplant 1997;12:2144-50. https://doi.org/10.1093/ndt/12.10.2144.
- Monier-Faugere MC, Mawad H, Qi Q, Friedler RM, Malluche HH. High prevalence of low bone turnover and occurrence of osteomalacia after kidney transplantation. J Am Soc Nephrol 2000;11:1093-9.
- Ott SM. Bone histomorphometry in renal osteodystrophy. Semin Nephrol 2009;29:122-32. https://doi.org/10.1016/j.semnephrol.2009.01.005.
- Wilson LM, Rebholz CM, Jirru E, Liu MC, Zhang A, Gayleard J, et al. Benefits and harms of osteoporosis medications in patients with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2017;166:649-58. https://doi.org/10.7326/M16-2752.
- Jamal SA, Bauer DC, Ensrud KE, Cauley JA, Hochberg M, Ishani A, et al. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. J Bone Miner Res 2007;22:503-8. https://doi.org/10.1359/jbmr.070112.
- National Institute for Health and Care Excellence . Raloxifene for the Primary Prevention of Osteoporotic Fragility Fractures in Postmenopausal Women n.d. www.nice.org.uk/guidance/ta160 (accessed 12 July 2012).
- National Institute for Health and Care Excellence . Raloxifene and Teriparatide for the Secondary Prevention of Osteoporotic Fragility Fractures in Postmenopausal Women n.d. www.nice.org.uk/guidance/ta161 (accessed 12 July 2019).
- Electronic Medicines Compendium . Fosamax Once Weekly 70 mg Tablets: Summary of Product Characteristics n.d. www.medicines.org.uk/emc/product/1281/smpc (accessed April 2018).
- Electronic Medicines Compendium . Bonviva 150 mg Film-Coated Tablets: Summary of Product Characteristics n.d. www.medicines.org.uk/emc/product/9383/smpc (accessed April 2018).
- Electronic Medicines Compendium . Actonel 30 mg Film-Coated Tablets: Summary of Product Characteristics n.d. www.medicines.org.uk/emc/product/3836/smpc (accessed April 2018).
- Saleem S, Patel S, Ahmed A, Saleem N. Denosumab causing severe, refractory hypocalcaemia in a patient with chronic kidney disease. BMJ Case Rep 2018;2018. https://doi.org/10.1136/bcr-2017-224068.
- Salim SA, Nair LR, Thomas L, Garla V, Palabindala V, Agarwal M, et al. Denosumab-associated severe hypocalcemia in a patient with chronic kidney disease. Am J Med Sci 2018;355:506-9. https://doi.org/10.1016/j.amjms.2017.09.008.
- Kostine M, Mehsen-Cetre N, Bannwarth B. Denosumab-induced severe hypocalcemia in a patient with Paget’s disease of bone and impaired renal function. Therapie 2017;72:383-5. https://doi.org/10.1016/j.therap.2016.07.003.
- Huynh AL, Baker ST, Stewardson AJ, Johnson DF. Denosumab-associated hypocalcaemia: incidence, severity and patient characteristics in a tertiary hospital setting. Pharmacoepidemiol Drug Saf 2016;25:1274-8. https://doi.org/10.1002/pds.4045.
- Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, Gutiérrez OM, et al. KDOQI US commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Am J Kidney Dis 2017;70:737-51. https://doi.org/10.1053/j.ajkd.2017.07.019.
- Nitta K, Yajima A, Tsuchiya K. Management of osteoporosis in chronic kidney disease. Intern Med 2017;56:3271-6. https://doi.org/10.2169/internalmedicine.8618-16.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- Benson T. The history of the Read Codes: the inaugural James Read Memorial Lecture 2011. Inform Prim Care 2011;19:173-82. https://doi.org/10.14236/jhi.v19i3.811.
- Judge A, Javaid MK, Leal J, Hawley S, Drew S, Sheard S, et al. Models of care for the delivery of secondary fracture prevention after hip fracture: a health service cost, clinical outcomes and cost-effectiveness study within a region of England. Health Serv Deliv Res 2016;4. https://doi.org/10.3310/hsdr04280.
- Kynaston-Pearson F, Ashmore AM, Malak TT, Rombach I, Taylor A, Beard D, et al. Primary hip replacement prostheses and their evidence base: systematic review of literature. BMJ 2013;347. https://doi.org/10.1136/bmj.f6956.
- Tomlinson LA, Riding AM, Payne RA, Abel GA, Tomson CR, Wilkinson IB, et al. The accuracy of diagnostic coding for acute kidney injury in England – a single centre study. BMC Nephrol 2013;14. https://doi.org/10.1186/1471-2369-14-58.
- Fotheringham J, Fogarty D, Jacques R, El Nahas M, Campbell M. Chapter 13 The linkage of incident renal replacement therapy patients in England (2002–2006) to hospital episodes and national mortality data: improved demography and hospitalisation data in patients undergoing renal replacement therapy. Nephron Clin Pract 2012;120:c247-60. https://doi.org/10.1159/000342857.
- World Health Organization . International Classification of Diseases n.d. www.who.int/classifications/icd/icdonlineversions/en/ (accessed 17 September 2019).
- NHS Digital . NHS Classifications OPCS-4 n.d. https://isd.digital.nhs.uk/trud3/user/guest/group/0/pack/10 (accessed 17 September 2019).
- Laulund AS, Nybo M, Brix TH, Abrahamsen B, Jørgensen HL, Hegedüs L. Duration of thyroid dysfunction correlates with all-cause mortality. the OPENTHYRO Register Cohort. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0110437.
- European Union . Regulation (EU) 2016 679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons With Regard to the Processing of Personal Data and on the Free Movement of Such Data, and Repealing Directive 95 46 EC (General Data Protection Regulation) (OJ L 119 4.5.2016 P. 1–88) 2016.
- Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008;167:492-9. https://doi.org/10.1093/aje/kwm324.
- Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010;340. https://doi.org/10.1136/bmj.b5087.
- Prieto-Alhambra D, Javaid MK, Judge A, Maskell J, Cooper C, Arden NK. COASt Study Group . Hormone replacement therapy and mid-term implant survival following knee or hip arthroplasty for osteoarthritis: a population-based cohort study. Ann Rheum Dis 2015;74:557-63. https://doi.org/10.1136/annrheumdis-2013-204043.
- Vestergaard P, Prieto-Alhambra D, Javaid MK, Cooper C. Fractures in users of antidepressants and anxiolytics and sedatives: effects of age and dose. Osteoporos Int 2013;24:671-80. https://doi.org/10.1007/s00198-012-2043-5.
- Prieto-Alhambra D, Lalmohamed A, Abrahamsen B, Arden NK, de Boer A, Vestergaard P, et al. Oral bisphosphonate use and total knee/hip implant survival: validation of results in an external population-based cohort. Arthritis Rheum 2014;66:3233-40. https://doi.org/10.1002/art.38789.
- Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol 2005;162:1016-23. https://doi.org/10.1093/aje/kwi307.
- Klebe B, Farmer C, Cooley R, de Lusignan S, Middleton R, O’Donoghue D, et al. Kidney disease management in UK primary care: guidelines, incentives and information technology. Fam Pract 2007;24:330-5. https://doi.org/10.1093/fampra/cmm026.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
- Williamson EJ, Forbes A. Introduction to propensity scores. Respirology 2014;19:625-35. https://doi.org/10.1111/resp.12312.
- Westreich D, Cole SR, Funk MJ, Brookhart MA, Stürmer T. The role of the c-statistic in variable selection for propensity score models. Pharmacoepidemiol Drug Saf 2011;20:317-20. https://doi.org/10.1002/pds.2074.
- Austin PC. The performance of different propensity-score methods for estimating relative risks. J Clin Epidemiol 2008;61:537-45. https://doi.org/10.1016/j.jclinepi.2007.07.011.
- Nguyen TL, Collins GS, Spence J, Daurès JP, Devereaux PJ, Landais P, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol 2017;17. https://doi.org/10.1186/s12874-017-0338-0.
- Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149-56. https://doi.org/10.1093/aje/kwj149.
- Leyrat C, Seaman SR, White IR, Douglas I, Smeeth L, Kim J, et al. Propensity score analysis with partially observed covariates: how should multiple imputation be used?. Stat Methods Med Res 2017. https://doi.org/10.1177/0962280217713032.
- Green JR, Seltenmeyer Y, Jaeggi KA, Widler L. Renal tolerability profile of novel, potent bisphosphonates in two short-term rat models. Pharmacol Toxicol 1997;80:225-30. https://doi.org/10.1111/j.1600-0773.1997.tb01964.x.
- Cal JC, Daley-Yates PT. Disposition and nephrotoxicity of 3-amino-1-hydroxypropylidene-1, 1-bisphosphonate (APD), in rats and mice. Toxicology 1990;65:179-97. https://doi.org/10.1016/0300-483X(90)90088-X.
- Braun JP, Rico AG, Benard P, Burgat-Sacaze V, Eghbali B, Godfrain JC. Urinary gamma-glutamyl transferase in renal toxicology of the rat. Bases of its use and significance in acute mercurial nephritis. Toxicology 1978;11:73-82. https://doi.org/10.1016/S0300-483X(78)90539-5.
- Bauss F, Russell RG. Ibandronate in osteoporosis: preclinical data and rationale for intermittent dosing. Osteoporos Int 2004;15:423-33. https://doi.org/10.1007/s00198-004-1612-7.
- Pfister T, Atzpodien E, Bohrmann B, Bauss F. Acute renal effects of intravenous bisphosphonates in the rat. Basic Clin Pharmacol Toxicol 2005;97:374-81. https://doi.org/10.1111/j.1742-7843.2005.pto_160.x.
- Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 2001;12:1164-72.
- Barri YM, Munshi NC, Sukumalchantra S, Abulezz SR, Bonsib SM, Wallach J, et al. Podocyte injury associated glomerulopathies induced by pamidronate. Kidney Int 2004;65:634-41. https://doi.org/10.1111/j.1523-1755.2004.00426.x.
- Desikan R, Veksler Y, Raza S, Stokes B, Sabir T, Li ZJ, et al. Nephrotic proteinuria associated with high-dose pamidronate in multiple myeloma. Br J Haematol 2002;119:496-9. https://doi.org/10.1046/j.1365-2141.2002.03826.x.
- Shreedhara M, Fenves AZ, Benavides D, Stone MJ. Reversibility of pamidronate-associated glomerulosclerosis. Proc 2007;20:249-53. https://doi.org/10.1080/08998280.2007.11928298.
- Markowitz GS, Fine PL, D’Agati VD. Nephrotic syndrome after treatment with pamidronate. Am J Kidney Dis 2002;39:1118-22. https://doi.org/10.1053/ajkd.2002.32797.
- Kunin M, Kopolovic J, Avigdor A, Holtzman EJ. Collapsing glomerulopathy induced by long-term treatment with standard-dose pamidronate in a myeloma patient. Nephrol Dial Transplant 2004;19:723-6. https://doi.org/10.1093/ndt/gfg567.
- Lockridge L, Papac RJ, Perazella MA. Pamidronate-associated nephrotoxicity in a patient with Langerhans’s histiocytosis. Am J Kidney Dis 2002;40. https://doi.org/10.1053/ajkd.2002.33933.
- Nasr SH, Preddie DC, Markowitz GS, Appel GB, D’Agati VD. Multiple myeloma, nephrotic syndrome and crystalloid inclusions in podocytes. Kidney Int 2006;69:616-20. https://doi.org/10.1038/sj.ki.5000144.
- Bodmer M, Amico P, Mihatsch MJ, Haschke M, Kummer O, Krahenbuhl S, et al. Focal segmental glomerulosclerosis associated with long-term treatment with zoledronate in a myeloma patient. Nephrol Dial Transplant 2007;22:2366-70. https://doi.org/10.1093/ndt/gfm209.
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356:1809-22. https://doi.org/10.1056/NEJMoa067312.
- Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007;357:1799-809. https://doi.org/10.1056/NEJMoa074941.
- Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med 1996;334:488-93. https://doi.org/10.1056/NEJM199602223340802.
- Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer 2000;88:1082-90. https://doi.org/10.1002/(SICI)1097-0142(20000301)88:5<1082::AID-CNCR20>3.0.CO;2-Z.
- Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial – the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol 2003;21:3150-7. https://doi.org/10.1200/JCO.2003.04.105.
- Nunn AJ, Mwaba P, Chintu C, Mwinga A, Darbyshire JH, Zumla A, et al. Collaboration. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ 2008;337. https://doi.org/10.1136/bmj.a257.
- Tilling K, Macdonald-Wallis C, Lawlor DA, Hughes RA, Howe LD. Modelling childhood growth using fractional polynomials and linear splines. Ann Nutr Metab 2014;65:129-38. https://doi.org/10.1159/000362695.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. https://doi.org/10.1080/01621459.1999.10474144.
- Hill AB. The environment and disease: association or causation?. Proc R Soc Med 1965;58:295-300. https://doi.org/10.1177/003591576505800503.
- Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 2006;15:291-303. https://doi.org/10.1002/pds.1200.
- Freemantle N, Cooper C, Diez-Perez A, Gitlin M, Radcliffe H, Shepherd S, et al. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int 2013;24:209-17. https://doi.org/10.1007/s00198-012-2068-9.
- Nayak S, Greenspan SL. Osteoporosis treatment efficacy for men: a systematic review and meta-analysis. J Am Geriatr Soc 2017;65:490-5. https://doi.org/10.1111/jgs.14668.
- Miller PD, Jamal SA, Evenepoel P, Eastell R, Boonen S. Renal safety in patients treated with bisphosphonates for osteoporosis: a review. J Bone Miner Res 2013;28:2049-59. https://doi.org/10.1002/jbmr.2058.
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348:1535-41. https://doi.org/10.1016/S0140-6736(96)07088-2.
- Chesnut CH, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004;19:1241-9. https://doi.org/10.1359/JBMR.040325.
- Hawley S, Leal J, Delmestri A, Prieto-Alhambra D, Arden NK, Cooper C, et al. Anti-osteoporosis medication prescriptions and incidence of subsequent fracture among primary hip fracture patients in England and Wales: an interrupted time-series analysis. J Bone Miner Res 2016;31:2008-15. https://doi.org/10.1002/jbmr.2882.
- Pimentel A, Ureña-Torres P, Zillikens MC, Bover J, Cohen-Solal M. Fractures in patients with CKD-diagnosis, treatment, and prevention: a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int 2017;92:1343-55. https://doi.org/10.1016/j.kint.2017.07.021.
- Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 2000;85:4118-24. https://doi.org/10.1210/jcem.85.11.6953.
- Suresh E, Pazianas M, Abrahamsen B. Safety issues with bisphosphonate therapy for osteoporosis. Rheumatology 2014;53:19-31. https://doi.org/10.1093/rheumatology/ket236.
- Lewiecki EM. Safety of long-term bisphosphonate therapy for the management of osteoporosis. Drugs 2011;71:791-814. https://doi.org/10.2165/11585470-000000000-00000.
- Chang JT, Green L, Beitz J. Renal failure with the use of zoledronic acid. N Engl J Med 2003;349:1676-9. https://doi.org/10.1056/NEJM200310233491721.
- Munier A, Gras V, Andrejak M, Bernard N, Jean-Pastor MJ, Gautier S, et al. Zoledronic acid and renal toxicity: data from French adverse effect reporting database. Ann Pharmacother 2005;39:1194-7. https://doi.org/10.1345/aph.1E589.
- Banerjee D, Asif A, Striker L, Preston RA, Bourgoignie JJ, Roth D. Short-term, high-dose pamidronate-induced acute tubular necrosis: the postulated mechanisms of bisphosphonate nephrotoxicity. Am J Kidney Dis 2003;41. https://doi.org/10.1016/S0272-6386(03)00214-2.
- Liaño F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 1996;50:811-18. https://doi.org/10.1038/ki.1996.380.
- Pedersen AB, Christiansen CF, Gammelager H, Kahlert J, Sørensen HT. Risk of acute renal failure and mortality after surgery for a fracture of the hip: a population-based cohort study. Bone Joint J 2016;98–B:1112-18. https://doi.org/10.1302/0301-620X.98B8.37497.
- Bauer DC, Black D, Ensrud K, Thompson D, Hochberg M, Nevitt M, et al. Upper gastrointestinal tract safety profile of alendronate: the fracture intervention trial. Arch Intern Med 2000;160:517-25. https://doi.org/10.1001/archinte.160.4.517.
- Peng YL, Hu HY, Luo JC, Hou MC, Lin HC, Lee FY. Alendronate, a bisphosphonate, increased upper and lower gastrointestinal bleeding: risk factor analysis from a nationwide population-based study. Osteoporos Int 2014;25:1617-23. https://doi.org/10.1007/s00198-014-2647-z.
- Recker RR, Lewiecki EM, Miller PD, Reiffel J. Safety of bisphosphonates in the treatment of osteoporosis. Am J Med 2009;122:22-3. https://doi.org/10.1016/j.amjmed.2008.12.004.
- UK Renal Registry . Front & Back Matter. Nephron Clinical Practice 2013;125:1-4. https://doi.org/10.1159/000362374.
- Nuffield Department of Orthopaedics, Rhuematology and Musculoskeletal Sciences . European Program of Post-Authorization Safety Studies for Protelos® Osseor® through EU-ADR Alliance n.d. www.ndorms.ox.ac.uk/research-groups/Musculoskeletal-Pharmacoepidemiology/ongoing-projects/european-program-of-post-authorisation-safety-studies-for-protelos-r-osseor-r-through-eu-adr-alliance (accessed November 2018).
- Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 1993;341:72-5. https://doi.org/10.1016/0140-6736(93)92555-8.
- West SL, Lok CE, Langsetmo L, Cheung AM, Szabo E, Pearce D, et al. Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res 2015;30:913-19. https://doi.org/10.1002/jbmr.2406.
- Hochberg MC, Ross PD, Black D, Cummings SR, Genant HK, Nevitt MC, et al. Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum 1999;42:1246-54. https://doi.org/10.1002/1529-0131(199906)42:6<1246::AID-ANR22>3.0.CO;2-U.
- Schnitzer T, Bone HG, Crepaldi G, Adami S, McClung M, Kiel D, et al. Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group. Aging 2000;12:1-12. https://doi.org/10.1007/BF03339822.
- Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med 2000;343:604-10. https://doi.org/10.1056/NEJM200008313430902.
- Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 1998;339:292-9. https://doi.org/10.1056/NEJM199807303390502.
- Cummings SR, Palermo L, Browner W, Marcus R, Wallace R, Pearson J, et al. Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. Fracture Intervention Trial Research Group. JAMA 2000;283:1318-21. https://doi.org/10.1001/jama.283.10.1318.
- Iwasaki Y, Kazama JJ, Fukagawa M. Molecular abnormalities underlying bone fragility in chronic kidney disease. Biomed Res Int 2017;2017. https://doi.org/10.1155/2017/3485785.
- Riggs BL, Hodgson SF, O’Fallon WM, Chao EY, Wahner HW, Muhs JM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990;322:802-9. https://doi.org/10.1056/NEJM199003223221203.
- Kinsella S, Murphy K, Breen M, O’Neill S, McLaughlin P, Coyle J, et al. Comparison of single CT scan assessment of bone mineral density, vascular calcification and fat mass with standard clinical measurements in renal transplant subjects: the ABC HeART study. BMC Nephrol 2015;16. https://doi.org/10.1186/s12882-015-0182-6.
- Abrahamsen B, Jørgensen HL, Laulund AS, Nybo M, Brix TH, Hegedüs L. Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures-the OPENTHYRO register cohort. J Bone Miner Res 2014;29:2040-50. https://doi.org/10.1002/jbmr.2244.
- Abrahamsen B, Jørgensen HL, Laulund AS, Nybo M, Bauer DC, Brix TH, et al. The excess risk of major osteoporotic fractures in hypothyroidism is driven by cumulative hyperthyroid as opposed to hypothyroid time: an observational register-based time-resolved cohort analysis. J Bone Miner Res 2015;30:898-905. https://doi.org/10.1002/jbmr.2416.
- Händel MN, Frederiksen P, Cohen A, Cooper C, Heitmann BL, Abrahamsen B. Neonatal vitamin D status from archived dried blood spots and future risk of fractures in childhood: results from the D-tect study, a population-based case-cohort study. Am J Clin Nutr 2017;106:155-61. https://doi.org/10.3945/ajcn.116.145599.
- Praetorius K, Madsen CM, Abrahamsen B, Jørgensen HL, Lauritzen JB, Laulund AS. Low levels of hemoglobin at admission are associated with increased 30-day mortality in patients with hip fracture. Geriatr Orthop Surg Rehabil 2016;7:115-20. https://doi.org/10.1177/2151458516647989.
- Rubin KH, Glintborg D, Nybo M, Andersen M, Abrahamsen B. Fracture risk is decreased in women with polycystic ovary syndrome: a register-based and population-based cohort study. J Bone Miner Res 2016;31:709-17. https://doi.org/10.1002/jbmr.2737.
- Sanni Ali M, Ernst M, Robinson DE, Caskey F, Arden NK, Ben-Shlomo Y, et al. Alendronate use and bone mineral density gains in women with moderate-severe (stages 3B–5) chronic kidney disease: an open cohort multivariable and propensity score analysis from Funen, Denmark. Arch Osteoporos 2020;15. https://doi.org/10.1007/s11657-020-00746-z.
- The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) . Guide on Methodological Standards in Pharmacoepidemiology (Revision 5). EMA 95098 2010 n.d. www.encepp.eu/standards_and_guidances/documents/ENCePPGuideofMethStandardsinPE_Rev5.pdf (accessed November 2018).
- Prieto-Alhambra D, Elorza-Ricart JM, Hermosilla E, Rodriguez-Ruiz J, Mendez-Boo L, Medina-Peralta M. Primary care prescriptions and subsequent pharmacy dispensing: a population-based study. Pharmacoepidemiol Drug Saf 2014;23.
- McDonald HI, Shaw C, Thomas SL, Mansfield KE, Tomlinson LA, Nitsch D. Methodological challenges when carrying out research on CKD and AKI using routine electronic health records. Kidney Int 2016;90:943-9. https://doi.org/10.1016/j.kint.2016.04.010.
- Moride Y, Abenhaim L, Yola M, Lucein A. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol 1994;47:731-7. https://doi.org/10.1016/0895-4356(94)90170-8.
- Patorno E, Goldfine AB, Schneeweiss S, Everett BM, Glynn RJ, Liu J, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. BMJ 2018;360. https://doi.org/10.1136/bmj.k119.
- Smerud KT, Dolgos S, Olsen IC, Åsberg A, Sagedal S, Reisæter AV, et al. A 1-year randomized, double-blind, placebo-controlled study of intravenous ibandronate on bone loss following renal transplantation. Am J Transplant 2012;12:3316-25. https://doi.org/10.1111/j.1600-6143.2012.04233.x.
- Torregrosa JV, Fuster D, Gentil MA, Marcen R, Guirado L, Zarraga S, et al. Open-label trial: effect of weekly risedronate immediately after transplantation in kidney recipients. Transplantation 2010;89:1476-81. https://doi.org/10.1097/TP.0b013e3181dc13d0.
- Walsh SB, Altmann P, Pattison J, Wilkie M, Yaqoob MM, Dudley C, et al. Effect of pamidronate on bone loss after kidney transplantation: a randomized trial. Am J Kidney Dis 2009;53:856-65. https://doi.org/10.1053/j.ajkd.2008.11.036.
- Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Effect of alendronate on vascular calcification in CKD stages 3 and 4: a pilot randomized controlled trial. Am J Kidney Dis 2010;56:57-68. https://doi.org/10.1053/j.ajkd.2009.12.039.
- Johansson H, Siggeirsdottir K, Harvey NC, Oden A, Gudnason V, McCloskey E, et al. Imminent risk of fracture after fracture. Osteoporos Int 2017;28:775-80. https://doi.org/10.1007/s00198-016-3868-0.
- Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008;19:385-97. https://doi.org/10.1007/s00198-007-0543-5.
- Van Staa TP, Abenhaim L, Cooper C, Zhang B, Leufkens HG. The use of a large pharmacoepidemiological database to study exposure to oral corticosteroids and risk of fractures: validation of study population and results. Pharmacoepidemiol Drug Saf 2000;9:359-66. https://doi.org/10.1002/1099-1557(200009/10)9:5<359::AID-PDS507>3.0.CO;2-E.
- Prieto-Alhambra D, Javaid MK, Judge A, Maskell J, Kiran A, de Vries F, et al. Fracture risk before and after total hip replacement in patients with osteoarthritis: potential benefits of bisphosphonate use. Arthritis Rheum 2011;63:992-1001. https://doi.org/10.1002/art.30214.
- Prieto-Alhambra D, Javaid MK, Maskell J, Judge A, Nevitt M, Cooper C, et al. Changes in hip fracture rate before and after total knee replacement due to osteoarthritis: a population-based cohort study. Ann Rheum Dis 2011;70:134-8. https://doi.org/10.1136/ard.2010.131110.
- Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000;11:83-91. https://doi.org/10.1007/s001980050010.
- Davis S, Martyn-St James M, Sanderson J, Stevens J, Goka E, Rawdin A, et al. A systematic review and economic evaluation of bisphosphonates for the prevention of fragility fractures. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20780.
- Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016;353. https://doi.org/10.1136/bmj.i3365.
- Prieto-Alhambra D, Javaid MK, Judge A, Maskell J, Kiran A, Cooper C, et al. Bisphosphonate use and risk of post-operative fracture among patients undergoing a total knee replacement for knee osteoarthritis: a propensity score analysis. Osteoporos Int 2011;22:1555-71. https://doi.org/10.1007/s00198-010-1368-1.
- Erviti J, Alonso A, Gorricho J, Lopez A. Oral bisphosphonates may not decrease hip fracture risk in elderly Spanish women: a nested case-control study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002084.
- Real J, Galindo G, Galván L, Lafarga MA, Rodrigo MD, Ortega M. Use of oral bisphosphonates in primary prevention of fractures in postmenopausal women: a population-based cohort study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0118178.
- He Y, Reyes C, Lapi F, Simonetti M, Rijnbeek P, Van der Lei J, et al. A Multi-Database, Multinational Validation Study of Cardiovascular Death, Venous Thromboembolic Events and Gastrointestinal Diseases in the EU-ADR Alliance n.d.
- Edwards BJ, Usmani S, Raisch DW, McKoy JM, Samaras AT, Belknap SM, et al. Acute kidney injury and bisphosphonate use in cancer: a report from the research on adverse drug events and reports (RADAR) project. J Oncol Pract 2013;9:101-6. https://doi.org/10.1200/JOP.2011.000486.
- Komada T, Morishita Y, Kitamura M, Iwazu K, Numata A, Kobayashi T, et al. Acute kidney injury in a patient with nephrotic syndrome due to focal segmental glomerular nephritis induced by a single oral administration of high-dose bisphosphonate (minodronate). Intern Med 2013;52:1383-7. https://doi.org/10.2169/internalmedicine.52.0094.
- Peña de la Vega L, Fervenza FC, Lager D, Habermann T, Leung N. Acute granulomatous interstitial nephritis secondary to bisphosphonate alendronate sodium. Ren Fail 2005;27:485-9. https://doi.org/10.1081/JDI-65397.
- Shih AW, Weir MA, Clemens KK, Yao Z, Gomes T, Mamdani MM, et al. Oral bisphosphonate use in the elderly is not associated with acute kidney injury. Kidney Int 2012;82:903-8. https://doi.org/10.1038/ki.2012.227.
- Ali M, Robinson D, Pallares N, Tebe C, Cooper C, Abrahamsen B, et al. The effect of oral bisphosphonates on acute kidney injury, gastrointestinal events and hypocalcaemia in patients with chronic kidney disease. Pharmacoepidemiol Drug Saf 2018;27.
- Moe SM, Drüeke TB, Block GA, Cannata-Andía JB, Elder GJ, Fukagawa M, et al. Kidney Disease: Improving Global Outcomes CKDMBDWG. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009;188:S1-130. https://doi.org/10.1038/ki.2009.188.
- Kreutle V, Blum C, Meier C, Past M, Müller B, Schütz P, et al. Bisphosphonate induced hypocalcaemia – report of six cases and review of the literature. Swiss Med Wkly 2014;144. https://doi.org/10.4414/smw.2014.13979.
- Do WS, Park JK, Park MI, Kim HS, Kim SH, Lee DH. Bisphosphonate-induced severe hypocalcemia – a case report. J Bone Metab 2012;19:139-45. https://doi.org/10.11005/jbm.2012.19.2.139.
- Vouri SM, Alvarez CA, Blaszczyk AT. Effects of oral bisphosphonate therapy on serum calcium in elderly veterans with poor kidney function. Am J Geriatr Pharmacother 2012;10:178-84. https://doi.org/10.1016/j.amjopharm.2012.04.001.
- Bell KJ, Hayen A, Macaskill P, Irwig L, Craig JC, Ensrud K, et al. Value of routine monitoring of bone mineral density after starting bisphosphonate treatment: secondary analysis of trial data. BMJ 2009;338. https://doi.org/10.1136/bmj.b2266.
- Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999;282:1344-52. https://doi.org/10.1001/jama.282.14.1344.
- Rubin KH, Abrahamsen B, Hermann AP, Bech M, Gram J, Brixen K. Prevalence of risk factors for fractures and use of DXA scanning in Danish women. A regional population-based study. Osteoporos Int 2011;22:1401-9. https://doi.org/10.1007/s00198-010-1348-5.
- Eastell R, Devogelaer JP, Peel NF, Chines AA, Bax DE, Sacco-Gibson N, et al. Prevention of bone loss with risedronate in glucocorticoid-treated rheumatoid arthritis patients. Osteoporos Int 2000;11:331-7. https://doi.org/10.1007/s001980070122.
- Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995;333:1437-43. https://doi.org/10.1056/NEJM199511303332201.
- Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012;27:1471-9. https://doi.org/10.1002/jbmr.1613.
- Alarkawi D, Bliuc D, Pallares N, Tebe C, Cooper C, Caskey F, et al. Oral bisphosphonate use and all-cause mortality in patients with advanced (stage IIIB+) chronic kidney disease: a propensity score analysis. Pharmacoepidemiol Drug Saf 2018;27.
Appendix 1 Additional results from work package 1
FIGURE 24.
Histograms of the propensity scores for analysing renal failure and safety outcomes for the exposed and unexposed participants (a) before and (b) after matching for imputation 1.
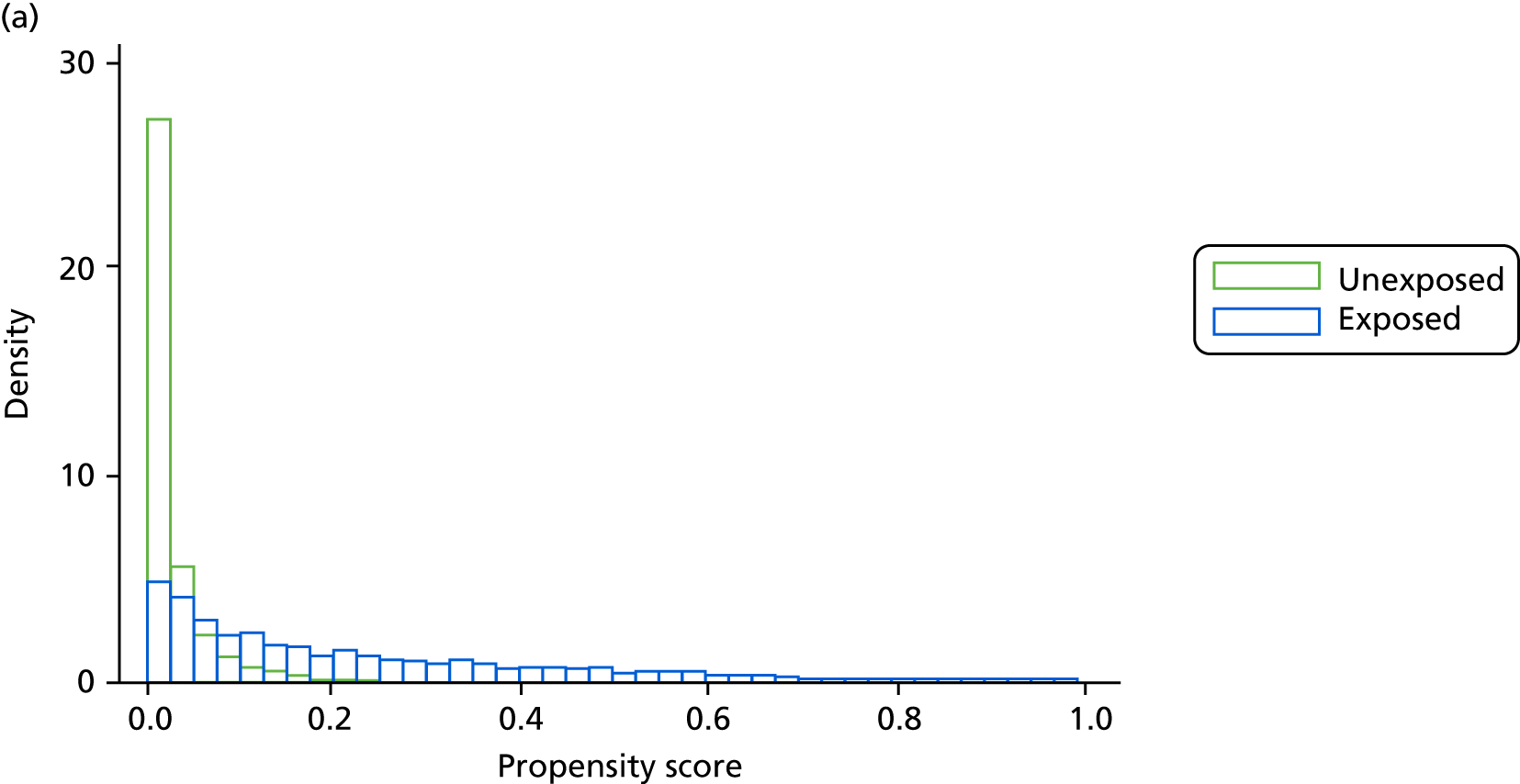
FIGURE 25.
Cumulative incidence function plot of CKD stage change versus stable: worsening and improving.
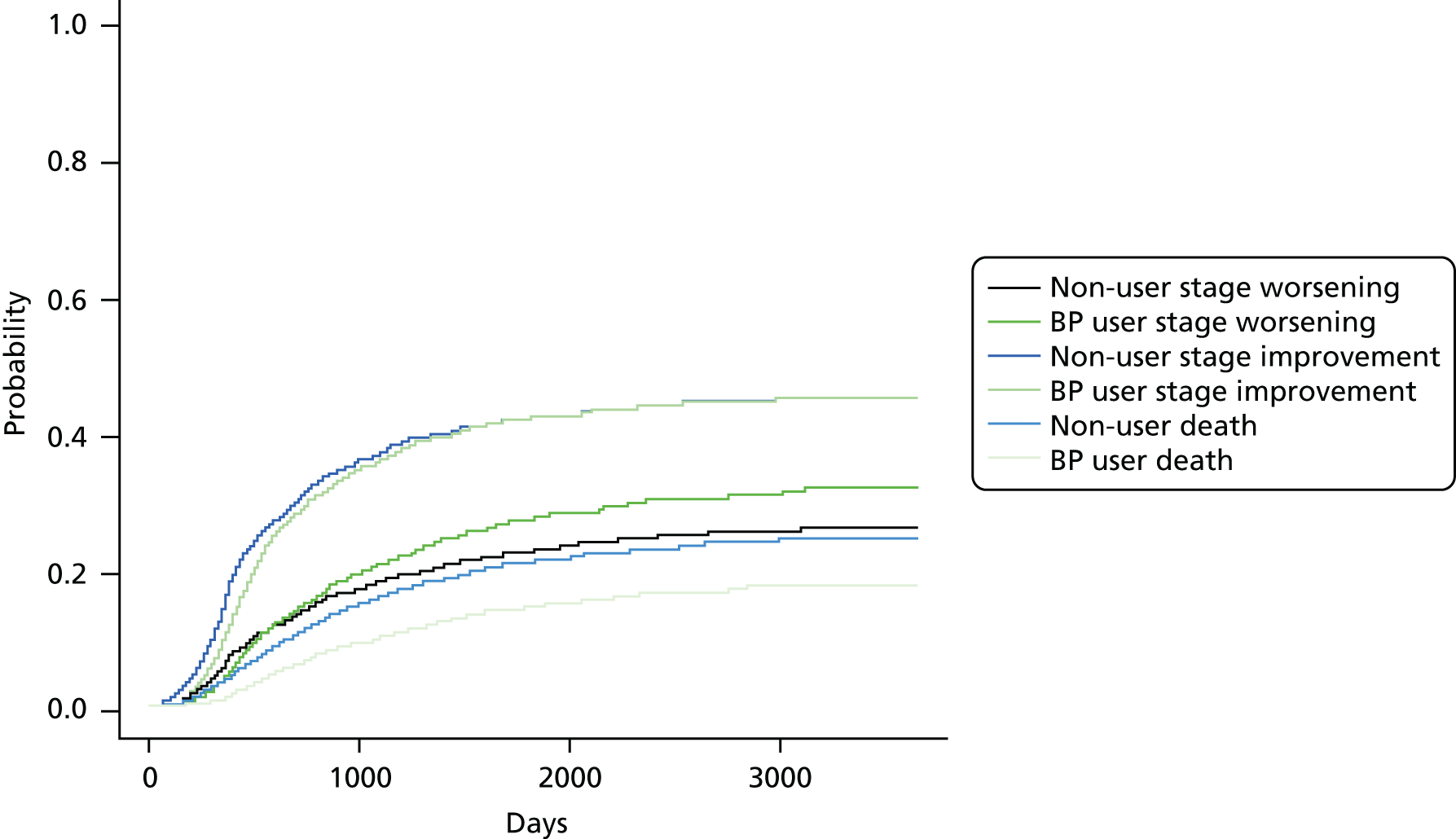
Appendix 2 Additional results from work package 2
FIGURE 26.
Histograms of the propensity scores for analysis of fracture outcomes for the exposed and unexposed participants (a) before and (b) after matching for imputation 1.
Appendix 3 Additional results from work package 4
FIGURE 27.
The ASMD of each covariate included in the propensity score matching for renal and safety outcomes before and after matching. 1, Age; 2a, Charlson Comorbidity Index score of 0; 2b, Charlson Comorbidity Index score of 1; 2c ,Charlson Comorbidity Index score of 2; 2d, Charlson Comorbidity Index score of 3; 2e, Charlson Comorbidity Index score of 4; 2f, Charlson Comorbidity Index score of 5; 2g, Charlson Comorbidity Index score of ≥ 6; 3, eGFR; 4a, two prescriptions in the previous year; 4b, three prescriptions in the previous year; 4c, four or more prescriptions in the previous year; 5, BMI; 6a, two hospital visits; 7, history of hip fracture; 8, history of non-osteoporotic fracture; 9, history of non-hip osteoporotic fracture; 10, diagnosis of rheumatoid arthritis; 11, uncomplicated diabetes mellitus; 12, complicated diabetes mellitus; 13, dementia; 14, cerebrovascular disease; 15, peripheral vascular disease; 16, hypertension; 17, mild liver disease; 18, renal disease; 19, chronic heart failure; 20, chronic obstructive pulmonary disease; 21, cancer; 22, treatment with oral contraceptives; 23, anti-osteoporotics; 24, defined daily dose of systemic steroids; 25, treatment with anticoagulants; 26, proton-pump inhibitors; 27, anti-anxiety medication; 28, selective serotonin reuptake inhibitors; 29, statins; 30, ACE inhibitors; 31, antiepileptics; 32, diuretics; 33, beta blockers; 34, antidiabetics 35, antihypertensives.
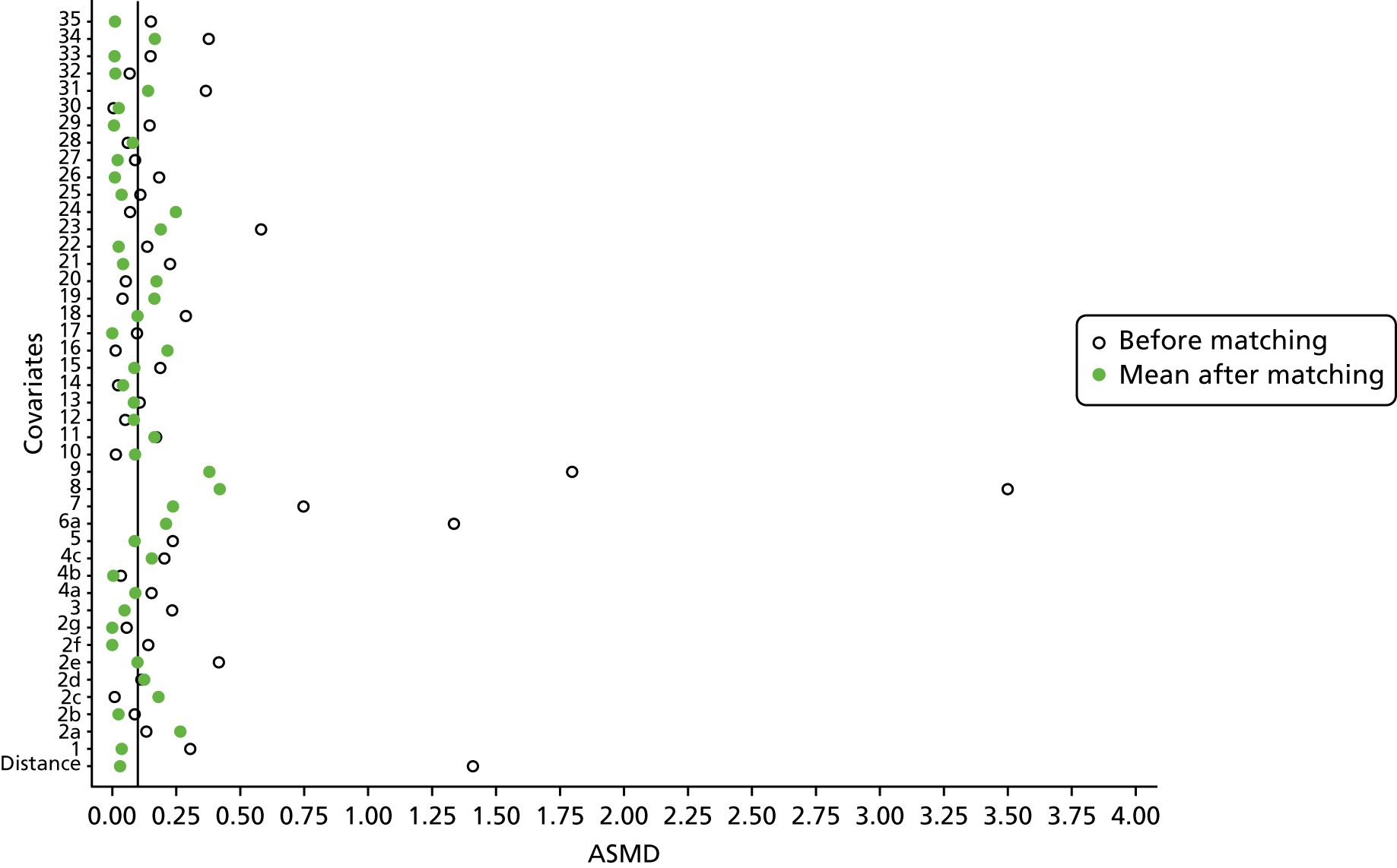
FIGURE 28.
Average hip BMD change per month for bisphosphonate (a) non-users and (b) users. Patients can contribute to multiple time points.
FIGURE 29.
Average spine BMD change per month for bisphosphonate (a) non-users and (b) users. Patients can contribute to multiple time points.
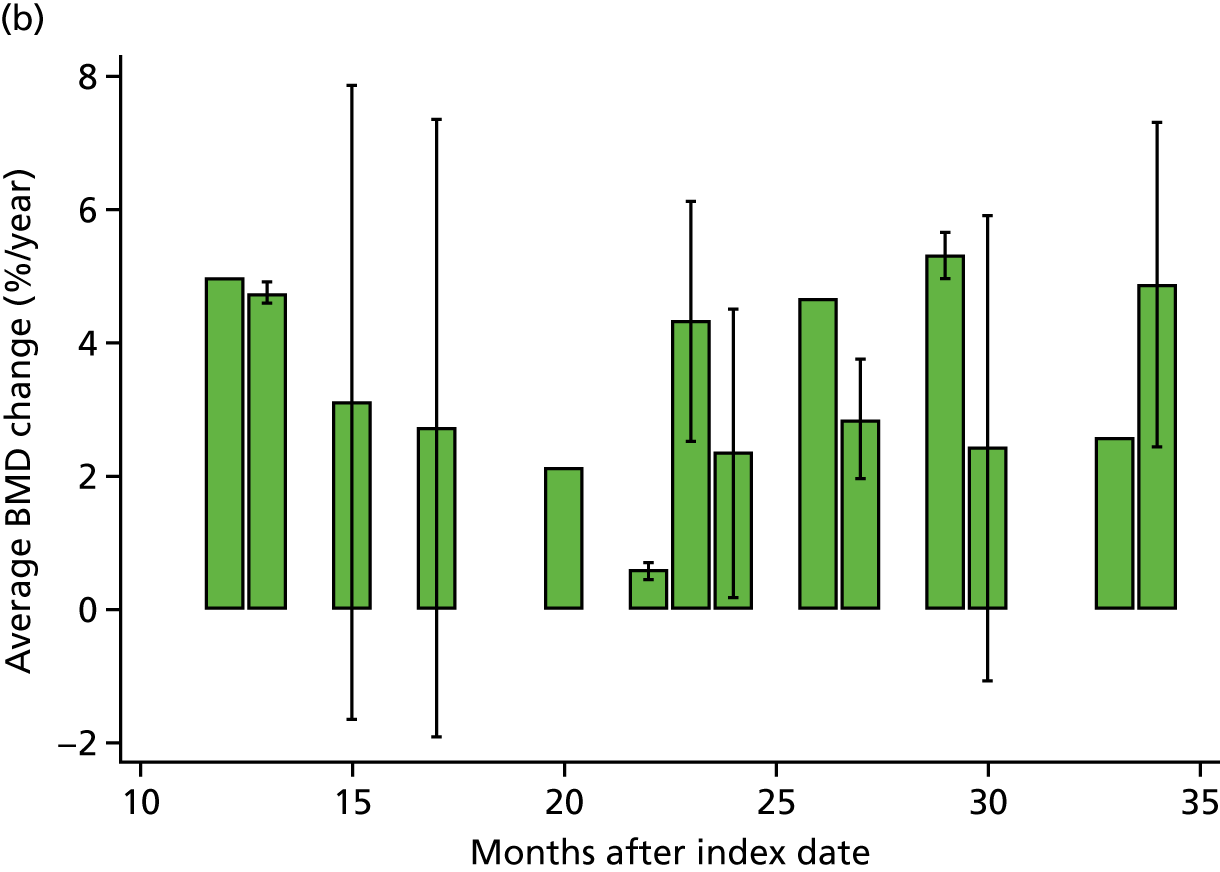
List of abbreviations
- ACE
- angiotensin-converting enzyme
- ACR
- albumin-to-creatinine ratio
- ASMD
- absolute standardised mean difference
- BMD
- bone mineral density
- BMI
- body mass index
- CAG
- Confidentiality Advisory Group
- CI
- confidence interval
- CKD
- chronic kidney disease
- CKD-EPI
- Chronic Kidney Disease – Epidemiology Collaboration
- CKD-MBD
- chronic kidney disease–mineral and bone disorder
- CPRD
- Clinical Practice Research Datalink
- DARS
- Data Access Request Service
- DXA
- dual-energy X-ray absorptiometry
- eGFR
- estimated glomerular filtration rate
- EMA
- European Medicines Agency
- FIT
- Fracture Intervention Trial
- FSGS
- focal segmental glomerulosclerosis
- GDPR
- General Data Protection Regulation
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICD-10
- International Classification of Diseases, Tenth Edition
- IGARD
- Independent Group Advising on the Release of Data
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- IR
- incidence rate
- ISAC
- Independent Scientific Advisory Committee
- KDOQI
- Kidney Disease Outcomes Quality Initiative
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MICE
- multiple imputation by chained equations
- MPR
- medication possession ratio
- NIHR
- National Institute for Health Research
- NSAID
- non-steroidal anti-inflammatory drug
- ONS
- Office for National Statistics
- OPCS-4
- Office of Population Censuses and Surveys – Classification of Interventions and Procedures version 4
- OUHD
- Odense University Hospital Databases
- RCT
- randomised controlled trial
- SD
- standard deviation
- sHR
- subdistribution hazard ratio
- UKRR
- UK Renal Registry
- WP
- work package
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hta25170).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
