Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/50/02. The contractual start date was in January 2013. The draft report began editorial review in September 2019 and was accepted for publication in December 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2021 Gathercole et al. This work was produced by Gathercole et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 The authors
Chapter 1 Introduction
Parts of this report are reproduced with permission from Leroi et al. 1 This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Parts of this report are reproduced with permission from Howard et al. 2 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for non-commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Scientific background
There are approximately 850,000 people with dementia in the UK3,4 and an estimated 700,000 people who provide unpaid care for them. Many of these people with dementia will require accommodation in nursing or residential care homes when their illness has progressed to the point at which they can no longer live safely and independently in their own homes. It has been estimated that, over the next two decades, the number of people aged ≥ 85 years will increase by two-thirds. 5 Over half of all users of adult social care services are aged ≥ 65 years,6 and a steep rise in the numbers of people living with dementia is expected over the next few decades. The financial cost of caring for people with dementia is considerable,3 as is the social and psychological cost to unpaid caregivers (generally a relative or friend, subsequently referred to as ‘caregivers’). Caregiver breakdown is a common reason for the unplanned admission of older people (many of whom will have dementia) to permanent nursing or residential care. 7
Living Well with Dementia, the theme of the 2009 National Dementia Strategy for England,8 involves helping people with dementia to retain their independence while living in their own homes, and to maintain their quality of their life. People living with dementia who move from their own homes into institutional care often experience a loss of independence and quality of life. To minimise this possibility, the NHS and councils with adult social services responsibilities (CASSRs) in England aim to support people with dementia to live safely in their own homes for as long as possible.
Assistive technology and telecare (ATT) offer relatively new means of delivering care and support to people with social care needs by helping to manage the risks facing older people with dementia who wish to remain living independently at home. It is claimed that sensors (e.g. to detect falls, floods or the presence of gas from an unlit appliance in someone’s home), passive monitoring that uses sensors placed in a home environment to detect movement, and alerting devices to relay information from the person’s home to a remote site such as a call centre support the independence of people with social care needs,9,10 reduce the burden on caregivers11–15 and save money for CASSRs. 16 By addressing risks associated with independent living for people with dementia, it is claimed that ATT help reduce the need for community care, prevent unnecessary hospital admissions and delay or prevent admission to residential or nursing care. 12,17–19 The evidence to support such claims is limited, and based largely on qualitative evidence or uncontrolled quantitative studies. 20,21 There is, therefore, an urgent need to provide evidence to inform decisions about whether or not to provide ATT in the homes of people with dementia.
The first use of electronic ATT in the UK, in the 1990s, was to provide support for people with dementia and their caregivers. 17,22–25 Within a decade, interest in ATT has developed from a fringe interest for a handful of enthusiasts to a multimillion-pound industry commanding government support, a Department of Health and Social Care (DHSC) strategy26 and, increasingly, the use of ATT in CASSR settings as a mainstream service (see Woolham et al. 25). However, as interest in ATT has increased, the specific focus on its application for those living with dementia has diminished. 13 The performance indicators that followed the Preventative Technology Grant (given to CASSRs in 2008 by the DHSC26) were intended to promote the widest possible use of telecare. The DHSC did not, however, offer a clear indication of what this grant was supposed to ‘prevent’. Although Woolham12 has drawn attention to the cost-effectiveness of telecare for people with dementia by closely matching ATT with assessed need, thereby preventing the need for more expensive forms of care, Poole27 has argued that CASSRs should see ATT as a long-term investment, deploying it at an early stage without expecting immediate savings. This has contributed to a situation in which CASSRs have implemented ATT across several different care groups without always referring to the needs of the specific groups, such as people with dementia. The current economic situation, and a significant reduction in government CASSR funding, has led to increasing numbers of CASSRs developing an interest in ATT. Some have developed local strategies to use it, whereas others already have well-developed ATT services that can be deployed alongside, or instead of, non-institutional forms of support, often known as ‘community care’ in the UK.
Despite growing ATT use, the evidence to support its use is limited. The Whole System Demonstrator (WSD) study was funded by the DHSC in 2008 to investigate the impact and effectiveness of ATT in England. 28–35 However, individuals with dementia were not specifically included. This, together with the relative dearth of dementia-specific studies relating ATT, means that a significant gap in the evidence remains. Although there are relatively large numbers of qualitative studies, audits and service evaluations, there are few studies with sufficient rigour and appropriate design to offer any degree of generalisability21 or agreement about how ‘success’ can be measured. 36 One study25 has suggested that, when used appropriately, ATT are highly cost-effective, but limitations in design and methodology constrain the generalisability of this study’s findings. A Cochrane review in 201737 found no research evaluating assistive technology for people via a randomised controlled trial (RCT), with the exception of this Assistive Technology and Telecare to maintain Independent Living At home for people with dementia (ATTILA) trial, which was in progress at the time of publication of the Cochrane review. 37 Evidence from a well-designed trial such as ATTILA is clearly needed to guide future policy direction.
The DHSC’s ‘Building Telecare’ strategy in 200513 provided generic advice to CASSRs. As part of this strategy, a Preventative Technology Grant, which CASSRs were required to spend on developing local ATT services, was made available. England’s 2009 National Dementia Strategy8 recommended a ‘watching brief’ for emerging evidence of the impact of telecare, stating:
However, with respect to more recent innovations, this is not an area where the strategy is able at this time to make specific recommendations. Instead, central, regional and local teams should keep in touch with initiatives in the areas of housing and telecare and make appropriate commissioning decisions as data become available, for example from the Department’s large-scale field trials of telecare and assistive technology.
Aims and objectives of the trial
The ATTILA trial was designed to answer questions about the efficacy and cost-effectiveness of ATT, with relevance for those who commission and provide care for people with dementia.
The aims of the ATTILA trial were to test the following hypotheses:
-
that the application of ATT will significantly extend the time that people with dementia can continue to live independently and safely in their own homes
-
that ATT interventions are cost-effective in the management of risk and maintenance of independence for people with dementia living in their own homes
-
that provision of ATT interventions to people with dementia living at home will significantly reduce the number of incidents involving serious risks to safety and independent living, particularly those involving acute admissions to hospital
-
that ATT interventions will reduce burden and stress in family and other caregivers and increase quality of life for people with dementia.
These hypotheses were tested by the following primary and secondary objectives:
-
Primary objective – to establish whether or not ATT assessments and interventions extend the time that people with dementia can continue to live independently in their own homes and whether or not this is cost-effective.
-
Secondary objectives –
-
to establish whether or not these technologies can significantly reduce the number of incidents involving serious risks to safety and independent living, including acute admissions to hospital
-
to reduce burden and stress in family and other caregivers, and increase quality of life for people with dementia and their caregivers
-
to collect qualitative and quantitative data from people living with dementia, their paid and unpaid caregivers, and members of the NHS and CASSR teams about their experience of using these technologies.
-
Structure of the report
In Chapter 2, we provide a summary of the trial methods. In the chapters that follow, we set out the methods and results of the research, beginning with work carried out to describe the intervention (see Chapter 3). In subsequent chapters, we report on participant outcomes, cost-effectiveness, caregiver outcomes and ethnographic research with participants and caregivers. In Chapter 8, we summarise the findings, reflect on the strengths and weaknesses of the research and make recommendations for future research and practice.
Deviations from the protocol
We did not conduct one proposed cost-effectiveness analysis. It was proposed that an analysis of the change in EuroQol-5 Dimensions, five-level version (EQ-5D-5L), score over 2 years would take into account the costs of permanent care home and hospital stays of those admitted to care homes over that period. This analysis was not conducted because no outcomes and costs data were collected from caregivers when participants were permanently admitted to care during the 2-year follow-up.
Chapter 2 Methods
Trial objectives
The ATTILA trial was a pragmatic RCT comparing outcomes for people with dementia who received a full ATT package with the outcomes for people with dementia who received equivalent community services but ATT were limited to a pendant alarm, non-monitored smoke and carbon monoxide detectors, and key safes. Assistive technology is defined as ‘any item, piece of equipment, product or system, whether acquired commercially, off the shelf, modified or customised, that is used to increase, maintain, or improve functional capabilities of individuals with cognitive, physical or communication difficulties’. 22 Telecare can include many different interventions, including those aimed at delivering care and monitoring remotely. 38 For the purposes of this trial, these devices had to be provided to support challenges related to memory problems and recommended by a health or social care professional.
The primary objectives of the trial were to establish whether or not:
-
ATT assessments and interventions can extend the time that people with dementia can continue to live independently and safely in the community
-
ATT interventions are cost-effective in the management of risk and maintenance of independence in people with dementia living in their own homes.
The secondary objectives were to:
-
establish whether or not these technologies can significantly reduce the number of incidents involving serious risks to safety and independent living, including acute admissions to hospital; reduce stress in family and other caregivers; and increase quality of life for those with dementia and their caregivers
-
collect qualitative and quantitative data from people living with dementia and their formal caregivers about their experience of using these technologies.
Trial design
The ATTILA trial was a multicentre, pragmatic RCT, conducted over 260 weeks, that took place in the homes of people living with dementia who were eligible to receive a package of care. The trial compared outcomes in two groups of participants randomised to one of the two trial arms: (1) receiving an assessment of needs followed by the installation of appropriate ATT devices and response services to be deployed by the CASSR or NHS (a full ATT package) or (2) receiving an assessment of needs followed by the installation of an ATT package restricted to smoke and carbon monoxide detectors, a key safe and a pendant alarm if indicated, also arranged by the CASSR (a basic ATT package). The co-primary outcomes were time to institutionalisation and cost-effectiveness of the intervention.
The trial was not funded to source, assess for, or deploy ATT. Our approach was to work alongside CASSRs who were charged by the Department of Health and Social Care with responsibility for establishing and developing local ATT services.
Site identification and recruitment
Recruitment of local authority Adult Social Care Department (ASCD) sites to the trial was opportunistic because of anticipated difficulties in securing permissions. Sites known to have well-established telecare services were identified, as well as those located in geographical areas close to the place of employment of research team members. This also meant that participating ASCDs were geographically widely spread across England, and that all types of local authority were represented. Telephone contact was usually first made with a telecare manager in identified sites. In most cases, this person referred our request to the departmental senior management team or to the director. In some sites, repeated visits were needed to discuss the request. Several ASCDs declined to take part in the main trial, either because they did not feel that they had the resources to do so or because of lack of fit between the trial aims and the strategic priorities of the service.
Participants
Participants were people with any dementia diagnosis, or suspected dementia, who were living in the community and were from one or more of three constituencies:
-
people who sought help or support from local authority social care services in the areas that had agreed to support the trial (Barnsley, Blackburn, Blackpool, Cambridgeshire, Croydon, Lambeth, Lancashire, Nottingham, Norfolk, Oxfordshire, Southwark and Suffolk) and who met local ASCD eligibility criteria for their support
-
people who were supported by the services of the NHS and were referred to an ASCD and met local ASCD eligibility criteria
-
people who were recruited from the caseload of NHS services for older adults and referred to local social services and met local ASCD eligibility criteria.
Those referred from the NHS usually had to meet eligibility criteria for social care because this often determined if ATT could be provided.
Most participants (n = 431, 87.1%) did have a dementia diagnosis, but some of those referred by LAs had not yet had a formal diagnosis. In these cases, clinical judgement was used by the research worker and health or social care staff involved to decide whether or not the cause of the potential participant’s memory impairment was dementia. If necessary, the research worker could discuss further with the local principal investigator.
Screening for eligibility and the preliminary information visit
All trial procedures, including the initial visit and consent visit, took place in participants’ homes. At the first appointment, participants were assessed for eligibility based on the following inclusion and exclusion criteria.
Inclusion criteria
-
Had any dementia diagnosis, evidence of memory difficulties or possible dementia.
-
Had a professionally assessed need for ATT from a health or social care professional.
-
Was resident in a community.
-
Lived in a dwelling suitable for the installation of ATT.
Exclusion criteria
-
Had already received an ATT intervention (excluding non-linked smoke detector or carbon monoxide detector, key safe or pendant alarm) or ATT had been previously provided but was not used.
-
Was unlikely to comply with follow-up, for example owing to an unstable medical or psychiatric condition.
-
Was participating in another clinical trial involving an intervention for dementia.
-
Had an urgent need for a care package owing to immediate and severe risks to self or others.
-
Did not have a suitable caregiver.
-
Was living in accommodation unsuitable for the provision of ATT.
Selection and recruitment
There were several routes for participant recruitment. Most participants were referred from local authority social services, but other referring services included not-for-profit organisations providing ATT; charitable organisations such as Age UK and the Alzheimer’s Society; and NHS mental health, community care and primary care services. Those referred from the NHS had to meet eligibility criteria for social care provision.
After assessing eligibility of new referrals for both social care support and the ATTILA trial, participating services asked if a potential participant’s contact details could be made available to a named individual in the local research team. Once identified, the research worker contacted this person and arranged to visit them and a caregiver who knew them well. Those meeting the eligibility criteria had the possible benefits and risks of participation in the trial explained to them. Following this, the participant was given a general outline of three possible options: (1) taking part in the ATTILA trial with the intervention (i.e. ATT package or regular support package without ATT) decided by randomisation, (2) declining to participate in the ATTILA trial and (3) taking more time to consider their decision about whether or not to participate. Those who were interested in taking part in the trial were provided with participant and caregiver information leaflets to find out more about the trial before deciding whether or not to participate. After a full explanation of the intervention options and the manner of treatment allocation, all suitable participants were invited to take part in the randomised component of the trial. If urgent provision of support services was required, then consent was sought at that visit so that they could be immediately randomised. Otherwise, information about the trial could be left with the prospective participant and, if they required more time to consider participation, the researcher would return at a later date to take consent and subsequently randomise the participant. Consent was also obtained from the caregiver using the caregiver consent form in the trial folder. If a participant lacked capacity, a professional or personal consultee was involved to ensure that participation in the trial was in the person’s best interests (according to guidelines established in the Mental Capacity Act 2005: Code of Practice39). When appropriate, data-sharing agreements were agreed with the CASSRs and health services concerned to ensure that the transfer of personal data from the local authority to the research team was lawful. If consent was not given, the participant was not included and any personal data were removed from research team records and destroyed. Reasons why those who were potentially eligible did not consent to take part were recorded on a screening log in the ATTILA trial folder. After randomisation, assessment for ATT and provision of ATT services (within limits set by randomisation) were left entirely up to the local authority or health service operational teams concerned.
Outcome measures
The co-primary trial outcomes were (1) time to institutionalisation and (2) the cost-effectiveness of the ATT intervention. Table 1 shows the schedule of all assessment points and the measures used at each.
| Assessment | Time point | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week –1 | Eligibility and randomisation screening | Follow-up assessments | |||||||||||
| Face to face | Telephone | ||||||||||||
| Week 0 | Week 12 | Week 24 | Week 52 | Week 104 | Week 130 | Week 156 | Week 182 | Week 208 | Week 234 | Week 260 | |||
| Participant information | ✗ | ||||||||||||
| Inclusion criteria | ✗ | ||||||||||||
| ATT needs assessment at home | ✗ | ||||||||||||
| Capacity assessment | ✗ | ✗ (prior to consent) | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||
| Informed consent | ✗ | ||||||||||||
| Randomisation data | ✗ | ||||||||||||
| Inform local authority of randomisation outcome | ✗ | ||||||||||||
| Install intervention (ATT or alternatives)a | ✗ | ||||||||||||
| SMMSE | ✗ | ||||||||||||
| BADLS | ✗ | ✗ | |||||||||||
| EQ-5D-5L | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| EQ-5D-5L Proxy (carer) | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| STAI-6 (carer) | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| CES-D-10 (carer) | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| ZBI (carer) | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| SUTAQ (carer) | ✗ | ✗ | ✗ | ✗ | |||||||||
| CSRI (carer) | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| Follow-up form | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| ATT checklist | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| Adverse events | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
Time in days from randomisation to institutionalisation
This was defined as permanent transition of a participant from living in their own home to living in a nursing or residential care home or to admission to an acute care facility that results in permanent placement in a residential care or nursing home. Caregivers were asked to report the date of this transition; if necessary, health or social care results would also be consulted. Analyses were by intention to treat, with all randomised participants included in the comparison and analysed according to their randomised allocation, including those who discontinued the trial. The primary outcome of time to institutionalisation was compared between intervention and control arms using survival analysis methods. Kaplan–Meier survival curves were created for graphical representation of the time to event comparisons (see Figures 4, 5 and 7). Statistical significance was determined by the log-rank test. Analyses included all events, even those occurring after 2 years. Participants who died, withdrew from follow-up or were lost to follow-up were censored at the date of withdrawal from the trial.
Cost-effectiveness
Economic evaluation methods (see Chapter 5) included cost-effectiveness and cost–utility analyses. The evaluation considered three outcomes: days to institutionalisation, change in the EuroQol-5 Dimensions (EQ-5D) index40,41 and quality-adjusted life-years (QALYs). Cost-effectiveness analyses were conducted from two perspectives: (1) health and social care and (2) societal. Costs were calculated by attaching nationally applicable unit cost measures to health and social service use. 42,43 These data focused on ATT, health-care and other service use patterns and caregiver inputs, and were collected at baseline and at 12, 24, 52 and 104 weeks for each participant using a modified version of the Client Service Receipt Inventory (CSRI). 44 Data on caregiver time and task inputs came from the CSRI and were valued using (and comparing, in sensitivity analyses) replacement wage and opportunity cost approaches. ATT intervention costs were calculated drawing on sources including key informant interviews about the production of ATT in ATTILA trial sites, and from price data drawn from procurement contract databases of the Northern Housing Consortium (NHC). Difference-in-difference analyses of EQ-5D change, with non-parametric bootstrapping, were performed; institutionalisation-free days and QALY outcome analyses employed a combination of population-averaged generalised gamma and survival models with non-parametric bootstrapping. Incremental cost-effectiveness ratios were computed and cost-effectiveness acceptability curves (CEACs) were plotted over a range of values of willingness to pay (WTP) for each outcome.
Secondary efficacy parameters
Caregiver burden
We measured both burden associated with caregiving and levels of psychological distress among the principal caregivers of participants at baseline and at 12, 24, 52 and 104 weeks. The 22-item short version of the Zarit Burden Interview (ZBI) questions caregivers about their experiences in terms of emotional, physical and social strains or difficulties that result from their role as a caregiver. Items include topics such as feeling that one’s own health has suffered, feeling that caregiving has affected relationships with family and friends and how burdened one feels. Caregivers respond by indicating how often they experience each item and responses are scored on a five-point scale ranging from ‘never’ to ‘frequently’. Higher burden is indicated by a higher score and the combined 12 items have high reliability (alpha = 0.86). 45 We also assessed psychological distress with the 10-item Center for Epidemiological Studies Depression Scale (CES-D-10) and the State–Trait Anxiety Inventory (STAI).
Number and severity of serious adverse events
Serious adverse events (SAEs) were recorded and reported. Researchers systematically enquired about changes in participants’ health, any compromises of participant safety or changes in living circumstances between assessments. Safety was assessed by the researcher at the 12-, 24-, 52- and 104-week assessments, and then at the 130-, 156-, 182-, 208-, 234- and 260-week telephone calls to participants’ caregivers. The adverse event (AE) reporting arrangements that apply to investigational medicinal products were not applicable, or appropriate, for the ATTILA trial. The focus was to capture as complete information as possible on compromises of participant safety that might have been preventable by the use of ATT. An adapted version of the AE reporting scheme was therefore used in the ATTILA trial.
A SAE was any compromise of participant safety that:
-
resulted in death
-
was life-threatening
-
necessitated hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
necessitated the intervention of emergency services
-
resulted in admission to permanent residential care.
Assessment of preventability
The potential preventability of a SAE by the use of ATT was also assessed and categorised by the local researcher and principal investigator into one of the following five categories:
-
Not preventable with assistive technology – the event or its consequences would have been the same with or without ATT.
-
Unlikely to be preventable – the event or its consequences were unlikely to be altered by ATT.
-
Possibly preventable – it is possible that the event or its consequences might have been prevented or mitigated by ATT.
-
Likely to be preventable – it is reasonable to believe that the event or its consequences might have been prevented or mitigated by use of ATT.
-
Definitely preventable – the event or its consequences would have been prevented or mitigated by ATT.
In the analysis, ‘possibly’, ‘likely’ and ‘definitely’ preventable categories were considered as preventable. Throughout the trial, the local ASCD retained responsibility for the ATT and any other support provided, and retained full case responsibility. The trial team had a duty of care to report relevant issues if the ASCD involved were not already aware and would signpost when necessary, but it did not provide equipment, care or support to trial participants.
Quantitative and qualitative data
Data on the acceptability, applicability and reliability of ATT intervention packages were collected using the Service User Technology Acceptability Questionnaire. This questionnaire was validated using data from the WSD project. 46 We anticipated that unpaid caregivers’ experiences would provide examples of ways that their lives, well-being and caregiver roles had been enhanced and/or undermined by the use of these technologies. Longitudinal qualitative data were collected through an embedded ethnographic study with a subset of the ATTILA trial participants to observe how people with dementia and their caregivers actually used (or chose not to use) ATT in their everyday routines and built environments. This methodology allowed for the team to investigate changes in participants’ technologically enabled care practices over time, as the care needs of people with dementia became more acute.
Sample size
The two primary outcome measures were time to transition to institutional care and cost-effectiveness. It was anticipated that 50% of participants with a Bristol Activities of Daily Living Scale (BADLS)47 score of ≥ 15 would transition to institutional care after 24 months, based on observed institutionalisation rates in participants from the AD2000 (Alzheimer’s disease) cohort. 48 A reduction in the estimated 24-month transition to care home rate by 30% (i.e. 50% institutionalised at 2 years reduced to 35%) would require the involvement of 500 participants, allowing for 10% attrition due to death while still community resident. This equated to an average of 55 days more of independent home life for each participant who received ATT. The trial would therefore be powered to detect a mean institutionalisation delay of just under 8 weeks. Expert opinion suggests that 8 weeks is close to the minimum clinically important difference in delaying institutionalisation.
Trial interventions
As the trial design was pragmatic, all aspects of the intervention (ATT assessment, funding, choice of devices, or ordering and installation of devices) were determined by staff from participating LAs or telecare providers. We worked alongside these teams, which have been charged by the Department of Health and Social Care with responsibility for establishing and developing local ATT services. The trial was not funded to source, assess for or deploy ATT.
Each participant underwent an assessment with the ATT provider to determine the level of need and what services that they required. The intervention involved the installation of simple, battery-operated, standalone technologies and/or telecare (a range of devices and sensors that communicate and relay messages to an external call centre where an appropriate response is arranged). The installation and selection of the technology to be deployed was the responsibility of the LAs involved. Those in the control arm were limited to a pendant alarm, non-monitored smoke and carbon monoxide detectors, and a key safe, as recommended by the health or social care professional assessing their needs. Both arms could use additional support services, such as paid care, meals on wheels and day centres.
Randomisation
Participants were randomised in a one-to-one ratio, using telephone-based randomisation and a computerised data entry portal provided by the Oxford Clinical Trial Service Unit. Treatment allocation was via a minimised randomisation procedure stratified by the following variables to reduce the risk of chance imbalances between arms. This information was obtained by the local trial team following consent and during the screening process. Variables were as follows:
-
sex
-
age (< 65, 65–80 or > 80 years)
-
risk of wandering or leaving the home inappropriately (low, moderate or high risk)
-
safety risk in the home (low, moderate or high risk)
-
level of caregiver support available (live-in caregiver, caregiver visits at least once daily or caregiver visits less often than daily).
This stratification procedure was reviewed by the Trial Steering Committee after the first 100 randomisations.
Blinding
Blinding was not undertaken for participants or trial staff collecting data as it was not practicable or ethical to conceal allocation of ATT. The staff who entered the data were unaware of which arm a participant had been allocated to.
Patient and public involvement
The Alzheimer’s Society was involved in devising the research question and in the production of the trial materials. Two service user representatives sat on the Trial Steering Committee.
Ethics approval
The trial was conducted and designed in compliance with the principles of the Declaration of Helsinki49 and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice (ICH-GCP). 50 All researchers working on the trial received training in ICH-GCP guidelines. The integrated form for both site-specific information and research and development approval at all participating NHS sites was approved prior to recruitment at each site. Annual progress and safety reports and a final report at conclusion of the trial were submitted to the Multicentre Research Ethics Committee and the Health Technology Assessment programme within the timelines agreed.
The trial was approved by the NHS Health Research Authority National Research Ethics Committee (reference number 12/LO/186) and is registered with the International Standard Randomised Controlled Trial Number registry (ISRCTN86537017).
Sponsorship
The trial was sponsored by South London and Maudsley NHS Foundation Trust.
Chapter 3 Describing the intervention
Parts of this chapter are reproduced with permission from Forsyth et al. 51 Reprinted from Alzheimers & Dementia, 5, Forsyth K, Henderson C, Davis L, Singh Roy A, Dunk B, Curnow E, et al. , Assessment of need and practice for assistive technology and telecare for people with dementia – The ATTILA (Assistive Technology and Telecare to maintain Independent Living At home for people with dementia) trial, 420–30, 2019, with permission from Elsevier.
Introduction
A detailed exploration of the intervention under investigation is needed to give insight into the fidelity of the intervention and to allow for replication. 52 The aim, therefore, was to provide an overview of routine ATT practice and the systems in place to deliver ATT for people with dementia.
Method
We adhered to the Template for Intervention Description and Replication (TIDieR)53 checklist in describing the components of the ATTILA trial intervention, in terms of what happened; who was involved; how, where and when the intervention happened; how much ATT was provided; and whether or not it was tailored to participants.
Assistive technology and telecare delivery systems
To describe the delivery systems for ATT deployment, interviews were conducted by staff with key informants from local authority operational/commissioning teams and telecare monitoring centre managers in the seven sites from which the majority of trial participants were recruited (n = 484). Invitations were sent to 21 potential key informants, resulting in 14 interviews covering six sites (no key informants were available for interview in one site) between June and September 2016. Interviews were not recorded but written notes were taken; interviewees were also asked for supporting documentation that might help in understanding the policies and procedures in relation to ATT deployment. Data were also collected on ATT assessment and delivery processes via pro formas completed by local researchers in 2015 and via a follow-up desk-based search in 2017. Data were examined using NVivo version 11 qualitative data analysis software (QSR International, Warrington, UK). Data were first structured into five production stages within a framework analysis:54 assessment, equipment procurement/ordering, installation, call monitoring, and response to sensor activations. To identify commonalities in local systems for delivering ATT to trial participants, we took an approach based on value network role analysis. 55,56 Production inputs and processes observed in each site were mapped onto value network frameworks.
Assistive technology and telecare
The ATT intervention was defined for the purposes of the ATTILA trial as a two-stage process:
-
an ATT assessment, with subsequent ATT recommendation(s)
-
the installation of ATT devices alongside monitoring services, as appropriate.
Framework analysis
We assumed that social services departments in each ATTILA trial site had distilled local and national guidelines on best practice in ATT assessment when constructing local assessment templates. To establish a practice standard for ATT assessments in the ATTILA trial sites, ATT assessment templates and guidance were sourced from each site between August 2013 and August 2016. Sites were asked to resend documentation if there were changes during the lifetime of the trial; as a result, two sets of new documentation were submitted. Framework analysis54 to identify common assessment themes across sites was applied to this documentation, using the Model of Human Occupation Screening Tool (MOHOST). 57 The MOHOST is designed to detail people’s values, insight, interests, routines, communication, cognitive and physical skills, and physical and social environment to gain a detailed picture of an individual’s life. The resultant ATT assessment standard consisted of a set of 14 ATT assessment areas (Table 2). A 4-point scale was developed for each assessment area in the ATT assessment standard, where 4 = no risk when doing daily activity, 3 = mostly risk free when doing daily activity, 2 = some risk when doing daily activity and 1 = significant multiple risks when doing daily activity. Specific definitions were developed for rating each assessment area (see Appendix 2). ATT needs were identified when an assessment area received a rating of 1 (significant multiple risks when doing daily activity) or 2 (some risk when doing daily activity).
| Key themesb | ATTILA trial site exemplar questionsc |
|---|---|
Motivation
|
1. Insight
|
2. Values
|
|
Routines
|
3. Wandering/disorientation
|
4. Daily activity
|
|
Communication skills
|
5. Conversation
|
6. Express needs
|
|
Cognitive skills
|
7. Memory
|
8. Problem solving
|
|
Physical skills
|
9. Mobility
|
10. Grip/dexterity
|
|
Physical environment
|
11. Space
|
12. Resources
|
|
Social environment
|
13. Social support
|
14. The way an activity is completed
|
Fidelity of assistive technology and telecare assessment to assessment standard
Locally completed ATT assessments for each participant were reviewed against the ATT assessment standard to assess whether or not locally completed ATT assessments across ATTILA trial sites addressed the ATT assessment areas identified by the templates. Fidelity to this standard was determined by two trial practitioners with experience in dementia care and ATT assessment, who independently classified the content of each locally completed ATT assessment against the ATT assessment standard and assigned risk ratings. They then reviewed ratings together and resolved discrepancies.
Assistive technology and telecare taxonomy/checklist
There is no recognised taxonomy of ATT for people with dementia; therefore, a taxonomy was developed in collaboration with Trent Dementia Services Development Centre and the ‘atdementia’ initiative (www.atdementia.org.uk; accessed 13 July 2018), an independent online ATT resource. This taxonomy was then developed into two identical technology checklist forms (one for recommended ATT and one for installed ATT), which covered the following ATT functions: (1) reminder or prompting devices, (2) devices to support safety, (3) safer walking technologies, (4) communication devices, (5) devices that support meaningful use of leisure time and (6) monitoring and response information. The form also recorded data about which type of assessor had assessed for ATT (ATT assessor, health or social care professional, other), the method of assessment (in person, at home; in person, not at home; telephone assessment; using case notes; other), whether or not ATT were monitored (yes/no) and who would respond to ATT alerts (direct to responder or via a call centre). Two trial practitioners with experience in dementia care and ATT assessment collaboratively classified each device recommended in the locally completed ATT needs assessment using the technology checklist (for recommended ATT).
Assistive technology and telecare installations
Assistive technology and telecare checklist
Local trial researchers used the technology checklist (for installed ATT) during home visits at weeks 12, 24, 52 and 104.
Statistical analysis
Categorical data were summarised in percentages and numbers of observations. Correlations between count variables were tested using non-parametric methods (Kendall rank correlation coefficient, τ). The Kruskal–Wallis test was used to assess if there were statistically significant differences between multiple groups for outcomes. Freidman’s test was used to determine the significance of change over time in the count variables. In the case of categorical variables, differences between observed and expected frequencies were tested using Pearson’s chi-squared test for independence, or, alternatively, Fisher’s exact test, when the assumption of minimum expected cell count in contingency tables was not met. 58 Standardised Mini-Mental State Examination (SMMSE) scores were categorised into stages of dementia59 for the purposes of analysis (30 = no dementia, 26–29 = questionable dementia, 21–25 = mild dementia, 11–20 = moderate dementia and 0–10 = severe dementia). Effective tailoring of the intervention was described through the strength of the correlation60 between ATT needs and ATT recommendations at baseline and between ATT recommendations and ATT installation by 24 weeks. We also compared the ATT that were recommended in the needs assessment with subsequent installations for each participant in the intervention arm up to 24 weeks. Any installation after 24 weeks was considered unrelated to the baseline ATT assessment.
Results
Participants
A total of 495 participants were randomised to the ATTILA trial (intervention group, n = 248; control group, n = 247). Of these, 451 had a documented needs assessment. A total of 209 intervention group participants had documented ATT installations.
Assessment
Of the 451 documented ATT needs assessments available, 413 contained an ATT recommendation. Of the 248 participants recruited to the intervention arm, data from 209 participants were available for analysis of ATT installations.
In total, 60% of assessment responses identified an ATT need, with 4.4 ATT needs (range 0–12) identified per participant (Tables 3 and 4). The mean number of ATT needs identified varied, ranging from two to six per site (p < 0.001). The areas of concern most frequently identified as triggering the need for ATT were daily activities (93%), memory (89%) and problem-solving (83%). Health and social care professionals identified more ATT needs than ATT assessors (p = 0.047). More ATT needs were identified through in-person, at-home assessments than through telephone assessment methods (p < 0.001). There was no significant difference between ATT needs in men and women (p = 0.337). The number of ATT needs identified for each participant differed depending on the risk of wandering (p = 0.005): a medium risk of wandering was associated with more ATT needs than a low risk of wandering (p = 0.016). ATT needs varied by category of SMMSE score (p < 0.001): participants with severe dementia had more ATT needs than those with mild (p < 0.001), those with moderate (p = 0.002) or those with questionable dementia (p < 0.001).
| Site ATT assessment areas/standard | Fidelity with ATT assessments standard | ATT needs (i.e. responses rated as ‘at risk’) | ||
|---|---|---|---|---|
| n/N | % | n/N | % | |
| 1. Insight | 241/451 | 53 | 151/241 | 63 |
| 2. Values | 245/451 | 54 | 100/245 | 41 |
| 3. Wandering/disorientation | 284/451 | 63 | 219/284 | 77 |
| 4. Daily activity | 321/451 | 71 | 298/321 | 93 |
| 5. Conversation | 226/451 | 50 | 100/226 | 44 |
| 6. Express needs | 175/451 | 39 | 24/175 | 14 |
| 7. Memory | 320/451 | 71 | 284/320 | 89 |
| 8. Problem-solving | 218/451 | 48 | 181/218 | 83 |
| 9. Mobility | 335/451 | 74 | 224/335 | 67 |
| 10. Grip/dexterity | 147/451 | 33 | 18/147 | 12 |
| 11. Space | 140/451 | 31 | 47/140 | 34 |
| 12. Resources | 128/451 | 28 | 26/128 | 20 |
| 13. Social support | 325/451 | 72 | 183/325 | 56 |
| 14. The way the activity is completed | 162/451 | 36 | 118/162 | 73 |
| Total responses | 3267/6314 | 52 | 1973/3267 | 60 |
| Characteristic | Fidelity with ATT assessments standard | Number of ATT needs | ||||
|---|---|---|---|---|---|---|
| Median | Mean | % | Median | Mean | % | |
| Participant characteristic | ||||||
| Gender | ||||||
| Female | 8 | 7.67 | 62 | 4 | 4.46 | 60 |
| Male | 5 | 6.65 | 38 | 3 | 4.25 | 40 |
| p = 0.027 | p = 0.337 | |||||
| Risk of wandering | ||||||
| Low | 7 | 6.93 | 70 | 4 | 4.10 | 68 |
| Medium | 9 | 8.37 | 23 | 5 | 5.04 | 23 |
| High | 6 | 7.24 | 7 | 4 | 5.24 | 9 |
| p = 0.038 | p = 0.005 | |||||
| SMMSE score of 18 points | ||||||
| Questionable dementia (26–29) | 7 | 7.22 | 13 | 3 | 3.38 | 10 |
| Mild dementia (21–25) | 7 | 6.6 | 27 | 4 | 3.9 | 27 |
| Moderate dementia (11–20) | 7 | 7.38 | 45 | 4 | 4.27 | 44 |
| Severe dementia (0–10) | 8.5 | 7.96 | 15 | 5.5 | 5.79 | 19 |
| p = 0.309 | p < 0.000 | |||||
| Assessment characteristic | ||||||
| Assessors | ||||||
| Health and social care professionals | 8 | 7.85 | 68 | 4 | 4.66 | 67 |
| ATT assessor | 5.5 | 6.51 | 29 | 3 | 3.86 | 29 |
| p = 0.051 | p = 0.028 | |||||
| Assessment method | ||||||
| In person, at home | 10 | 9.14 | 85 | 5 | 5.06 | 82 |
| In person, not at home | 5 | 6.43 | 8 | 3 | 3.38 | 8 |
| Telephone | 2 | 3.42 | 6 | 2 | 2.71 | 9 |
| Case notes | 3 | 3.33 | 1 | 2.5 | 3 | 1 |
| p < 0.000 | p < 0.000 | |||||
| Service structure | ||||||
| Public telecare provider | 7 | 7.59 | 73 | 4 | 4.41 | 70 |
| Not-for-profit telecare provider | 6 | 6.41 | 25 | 4 | 4.31 | 28 |
| p = 0.026 | p = 1.00 | |||||
| Mean fidelity with ATT assessment standard per participant: 7.2 assessment areas addressed (range 0–13) | Mean number of responses per participant rated as an ATT need: 4.4 ATT needs (range 0–12) | |||||
Fidelity of assessment
The local ATT assessment fidelity with the ATT assessment standard was 52% (7.2 assessment areas were addressed per assessment) (see Tables 3 and 4). Of 451 ATT assessments reviewed, 99 (22%) addressed 0–2 areas of assessment. There was higher fidelity to assessment areas relating to ‘mobility’ (74%), ‘social support’ (72%), ‘daily activity’ (71%) and ‘memory’ (71%). Fidelity varied across sites: the mean number of assessment areas addressed ranged from two to 13 per site (p < 0.001), with public telecare providers addressing more assessment areas than not-for-profit telecare providers (p = 0.026). Health and social care professionals addressed more assessment areas than ATT assessors (p = 0.046). Fidelity varied across assessment methods (p < 0.001), with the in-person, at-home assessment method addressing more assessment areas than in-person, not-at-home (p = 0.003), telephone (p < 0.001) and case notes assessment methods (p = 0.003). Women had more assessment areas addressed than men (p = 0.027). More assessment areas were addressed for participants at medium risk of wandering than for participants at low risk of wandering (p = 0.028).
Assistive technology and telecare delivery system
Value networks
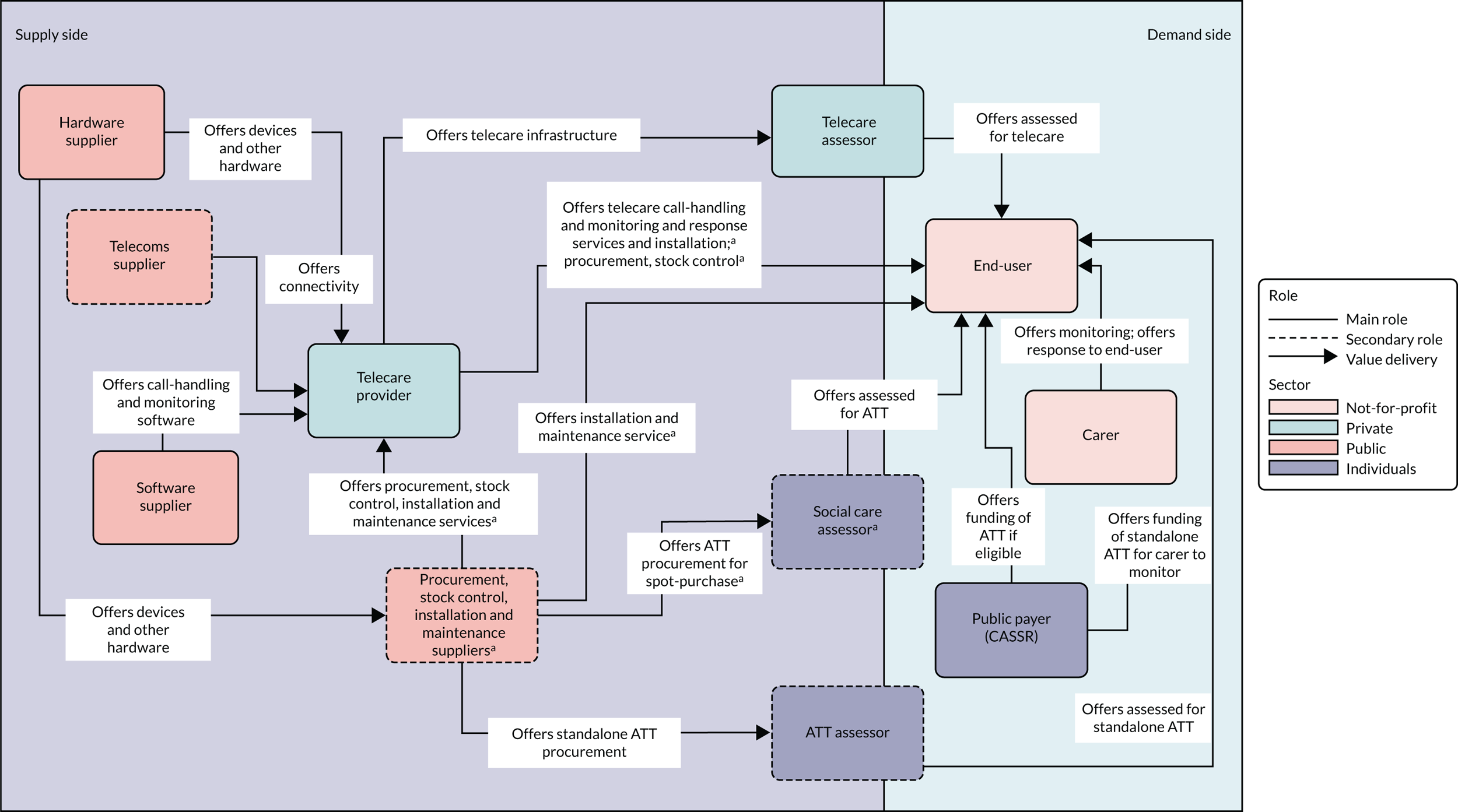
Networks delivering services (offering value) to participants in ATTILA trial sites were classified into two types (Figures 1 and 2). First were ‘public telecare provider networks’ (n = 4), for which two assessor roles were identified: the ATT assessor and the authorised (or trusted) assessor (health or social care professional). ATT assessors were employed by public agencies (NHS or CASSRs); their primary role was to assess for a full range of ATT devices [‘networked’ (monitored by a telecare call centre or caregiver) or ‘standalone’]. Authorised assessors could offer first-generation telecare (pendant-only systems) or straightforward ATT (e.g. adding on an additional sensor or providing a memo minder), depending on their level of experience and local permissions; they performed ATT assessment as a secondary role. In these networks, most or all of the ATT infrastructure for procurement, installation, stock control and maintenance of ATT devices fell to units in the CASSR. The second type of networks were ‘not-for-profit provider networks’ (n = 3). Three assessor roles were identified across these ‘not-for-profit telecare networks’. Telecare assessors working for not-for-profit telecare providers assessed for AT that was networked to providers’ call-monitoring centres. Assessment for standalone assistive technology fell to assessors in the CASSR. A ‘social care ATT assessor’ role was also identified; these assessors could assess for ATT (networked or standalone) and work with a choice of suppliers to procure and arrange the installation of ATT devices. Private companies offered combinations of procurement and stock control, installation, and maintenance services to the not-for-profit telecare providers.
FIGURE 1.
Public assessor value networks. a, Featured in some but not all networks.
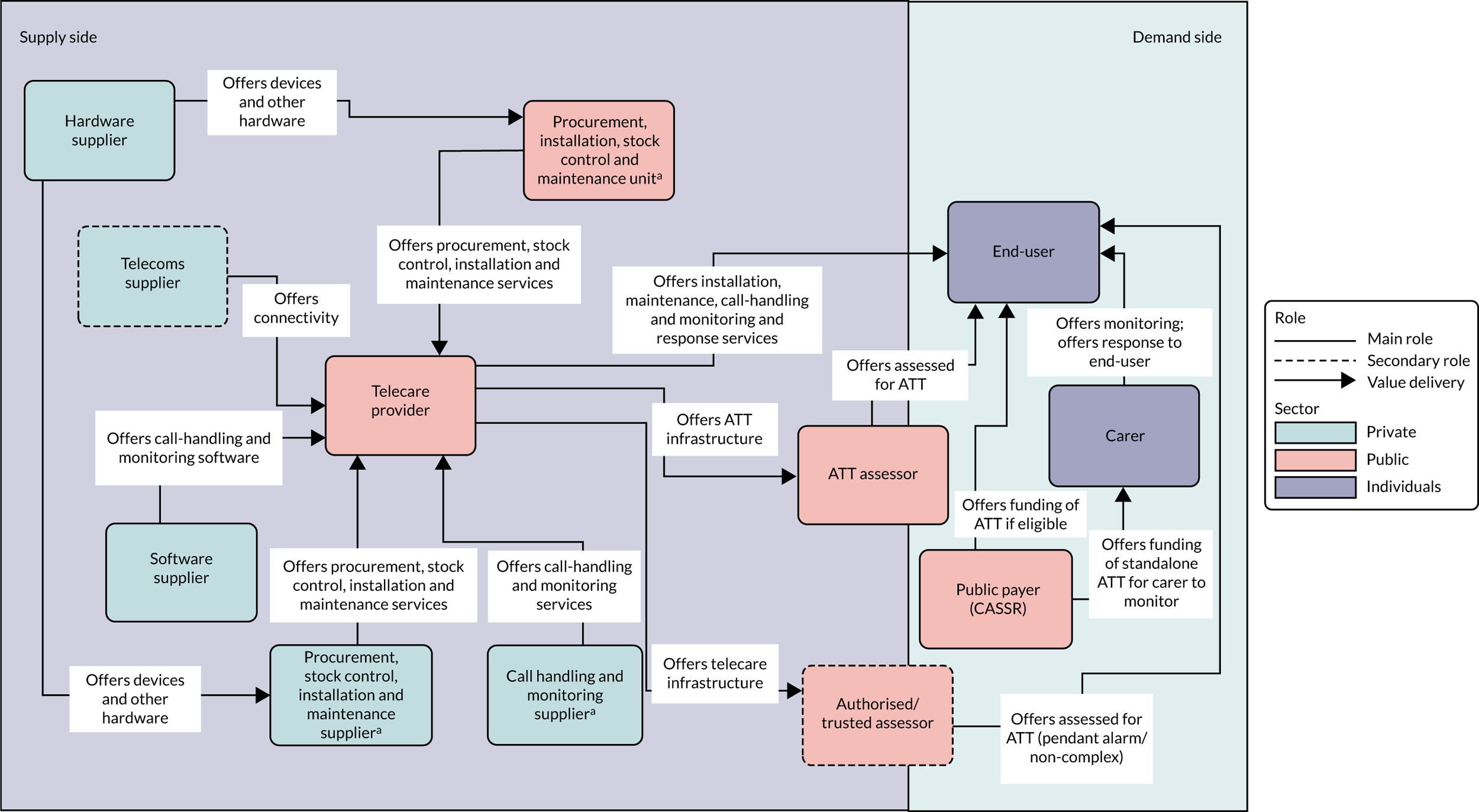
FIGURE 2.
Not-for-profit value networks. a, Featured in some but not all networks.
Assistive technology and telecare recommendations
A documented ATT recommendation was given for 413 participants, with 1090 ATT devices recommended at baseline [mean three devices (range 1–14 devices)]. For 57% (n = 235) of participants, just one or two ATT devices were recommended. The correlation between the ATT needs and the ATT recommendations identified in local ATT assessments was weak (τ = 0.242; p < 0.001). Most recommendations were for safety-related devices (59%, 644/1090), followed by reminder/prompting devices (25%, 269/1090). ATT devices required monitoring in 62% (673/1090) of recommendations, and 67% (353/526) of monitored devices with an identified responder required a formal (call centre) response.
Assistive technology and telecare installations
Frequency of assistive technology and telecare categories
By 24 weeks, a mean of 3.5 devices had been recommended for participants in the intervention arm. Of the ATT devices recommended, 53% (306/572) were not installed.
Relationship of assistive technology and telecare installations to assistive technology and telecare recommendations
A total of 62% (438/704) of the ATT devices that were installed had not been recommended in the needs assessment (Table 5). There was a moderate negative correlation between number of recommendations and number of installations per participant per ATT category (τ = –0.470; both p < 0.001).
| ATT technology checklist | ATT devices, n/N (%) | ||||
|---|---|---|---|---|---|
| Recommended | Recommended and installed by 24 weeks | Recommended but not installed | Installed by 24 weeks | Not recommended but installed | |
| Control group technology | |||||
| Pendant alarm | 44/572 (8) | 22/44 (50) | 22/44 (50) | 89/704 (13) | 67/89 (75) |
| Non-monitored smoke detector | 0/572 (0) | 0 (0) | 0 (0) | 68/704 (10) | 68/68 (100) |
| Non-monitored carbon monoxide detector | 1/572 (0) | 0/1 (0) | 1 (100) | 36/704 (5) | 36/36 (100) |
| Key safe | 18/572 (3) | 9/18 (50) | 9/18 (50) | 89/704 (13) | 80/89 (90) |
| Activity monitors assessment only | 8/572 (1) | 4/8 (50) | 4/8 (50) | 5/704 (1) | 1/5 (20) |
| Other devices | 1/572 (0) | 0/1 (0) | 1/1 (100) | 6/704 (1) | 6/6 (100) |
| Intervention group technology | |||||
| Reminder or prompting devices | |||||
| Date and time reminders | 31/572 (5) | 13/31 (42) | 18/31 (58) | 46/704 (7) | 33/46 (72) |
| Item-locator devices | 9/572 (2) | 8/9 (89) | 1/9 (11) | 11/704 (2) | 3/11 (27) |
| Medication reminders/dispensers | 56/572 (10) | 25/56 (45) | 31/56 (55) | 33/704 (5) | 8/33 (24) |
| Voice recorders and memo minders | 46/572 (8) | 27/46 (59) | 19/46 (41) | 38/704 (5) | 11/38 (29) |
| Other reminder/prompting devices | 1/572 (0) | 0/1 (0) | 1/1 (100) | 6/704 (1) | 6/6 (100) |
| Devices to promote safety | |||||
| Activity monitors – ongoing monitoring | 5/572 (1) | 1/5 (20) | 4/5 (80) | 6/704 (1) | 5/6 (83) |
| Fall detectors | 75/572 (13) | 31/75 (41) | 44/75 (59) | 53/704 (8) | 22/53 (42) |
| Continence management devices | 1/572 (0) | 1/1 (100) | 0/1 (0) | 1/704 (0) | 0/1 (0) |
| Alarm and pager units | 5/572 (1) | 2/5 (40) | 3/5 (60) | 5/704 (1) | 3/5 (60) |
| Flood detectors and water temperature monitor | 14/572 (2) | 9/14 (64) | 5/14 (36) | 11/704 (2) | 2/11 (18) |
| Gas detectors | 21/572 (4) | 8/21 (38) | 13/21 (62) | 19/704 (3) | 11/19 (58) |
| Monitored carbon monoxide detectors | 25/572 (4) | 8/25 (32) | 17/25 (68) | 22/704 (3) | 14/22 (64) |
| Monitored smoke detectors | 59/572 (10) | 39/59 (66) | 20/59 (34) | 47/704 (7) | 8/47 (17) |
| Monitored extreme temperature sensors | 26/572 (5) | 18/26 (42) | 15/26 (58) | 19/704 (3) | 8/19 (42) |
| Lighting devices | 2/572 (0) | 1/2 (50) | 1/2 (50) | 8/704 (1) | 7/8 (88) |
| Other safety and security devices | 15/572 (3) | 2/15 (13) | 13/15 (87) | 9/704 (1) | 7/9 (78) |
| Safer walking technologies | |||||
| To locate the user | 43/572 (8) | 20/43 (47) | 23/43 (53) | 28/704 (4) | 8/28 (29) |
| To alert the responder to movement | 59/572 (10) | 25/59 (42) | 34/59 (58) | 37/704 (5) | 12/37 (32) |
| Communication devices | |||||
| Intercoms | 2/572 (0) | 0/2 (0) | 2/2 (100) | 1/704 (0) | 1/1 (100) |
| Telephones | 3/572 (1) | 0/3 (0) | 3/3 (100) | 7/704 (1) | 7/7 (100) |
| Communication aids | 0/572 (0) | 0/0 (0) | 0/0 (0) | 1/704 (0) | 1/1 (100) |
| Other communication devices | 1/572 (0) | 0/1 (0) | 1/1 (100) | 0/704 (0) | 0/0 (0) |
| Devices that support meaningful use of leisure time | |||||
| Computer aids | 0/572 (0) | 0/0 (0) | 0/0 (0) | 0 (0) | 0 (0) |
| Dementia-friendly television/radio/music players | 0/572 (0) | 0/0 (0) | 0/0 (0) | 0 (0) | 0 (0) |
| Electronic photograph albums/electronic reminiscence aids | 0/572 (0) | 0/0 (0) | 0/0 (0) | 0 (0) | 0 (0) |
| Electronic games | 0/5572 (0) | 0/0 (0) | 0/0 (0) | 1/551 (0) | 1/1 (100) |
| Other devices | 1/572 (0) | 0/1 (0) | 1/1 (100) | 2/551 (0) | 2/2 (100) |
| Total | 572 | 266/572 (47) | 306/572 (53) | 704 | 438/704 (62) |
Week 12–104, assistive technology and telecare devices installed (intervention arm only)
From week 12 to week 104, 888 ATT devices were installed for 209 participants in the intervention arm, which is a mean of 4.2 devices per participant (range 1–15 devices). Of the devices installed for intervention participants (Table 6), 42% (374/888) involved the types of technology provided to control arm participants (e.g. non-monitored smoke detectors). Installations decreased over time (p = 0.031), with 79% (704/888) of ATT installed by week 24. Intervention participants’ ATT devices were most frequently installed for safety reasons (38%) or for reminder/prompting (18%). ATT assessors were most frequently identified as having assessed for the installed devices (32%), followed by health and social care professionals (20%), but 40% of assessors’ backgrounds were unknown. A total of 41% of installations followed an in-person home visit (41%), but in 42% of cases participants could not report the method of assessment. Nearly half (47%) of the ATT devices installed required monitoring; 38% of monitored devices were networked to a call centre (so that any alerts would receive an initial response from paid services).
| Week, n (%) | Total (weeks 12–104), n (%) | ||||
|---|---|---|---|---|---|
| 12 | 24 | 52 | 104 | ||
| Control group technology installed | |||||
| Basic ATT | 235 (41) | 58 (47) | 45 (52) | 36 (37) | 374 (42) |
| Intervention technology installed | |||||
| Reminder/prompting | 116 (20) | 18 (15) | 9 (10) | 17 (18) | 160 (18) |
| Safety | 220 (38) | 45 (36) | 30 (35) | 43 (44) | 338 (38) |
| Communication | 8 (1) | 1 (0) | 2 (2) | 1 (1) | 12 (2) |
| Support leisure time | 1 (0) | 2 (2) | 1 (1) | 0 (0) | 4 (0) |
| Any other devices | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total installed | 580 (100) | 124 (100) | 87 (100) | 97 (100) | 888 (100) |
| Assessor | |||||
| Health or social care professional | 126 (22) | 20(16) | 13 (15) | 17 (18) | 176 (20) |
| ATT assessor | 152 (26) | 58 (47) | 23 (26) | 45 (46) | 278 (32) |
| Other | 68 (12) | 0 (0) | 3 (4) | 4 (4) | 75 (8) |
| Unknown | 234 (40) | 46 (37) | 48 (55) | 31 (32) | 359 (40) |
| Total installed | 580 (100) | 124 (100) | 87 (100) | 97 (100) | 888 (100) |
| Assessment method | |||||
| In person, at home | 216 (37) | 70 (57) | 30 (34) | 55 (57) | 371 (41) |
| In person, not at home | 7 (1) | 3 (2) | 1 (1) | 4 (4) | 15 (2) |
| Telephone | 50 (9) | 4 (3) | 1 (1) | 4 (4) | 59 (7) |
| Using case notes | 7 (1) | 0 (0) | 0 (0) | 2 (2) | 9 (1) |
| Other | 56 (10) | 1 (1) | 2 (2) | 0 (0) | 59 (7) |
| Unknown | 244 (42) | 46 (37) | 53 (61) | 32 (33) | 375 (42) |
| Total installed | 580 (100) | 124 (100) | 87 (100) | 97 (100) | 888 (100) |
| Monitoring | |||||
| Yes | 292 (51) | 56 (45) | 32 (37) | 42 (43) | 422 (47) |
| No | 147 (25) | 45 (36) | 25 (29) | 40 (41) | 257 (29) |
| Unknown | 141 (24) | 23 (19) | 30 (34) | 15 (16) | 209 (24) |
| Total installed | 580 (100) | 124 (100) | 87 (100) | 97 (100) | 888 (100) |
| Response | |||||
| Formal services | 104 (36) | 29 (52) | 15 (47) | 14 (33) | 1622 (38) |
| Informal services | 79 (27) | 11 (20) | 8 (25) | 16 (38) | 1142 (27) |
| Mixed services | 106 (36) | 14 (25) | 8 (25) | 12 (29) | 1402 (33) |
| Unknown | 3 (1) | 2 (3) | 1 (3) | 0 (0) | 62 (2) |
| Total installed | 292 (100) | 56 (100) | 32 (100) | 42 (100) | 422 (100) |
Summary
The findings are the first to describe assistive technology for people with dementia. The components of the ATTILA trial intervention have been described, in terms of what happened, who was involved, how, where and when the intervention happened, how many devices was provided and whether or not the intervention was tailored to participants. Value networks operating in ATTILA trial sites were characterised as either public or not-for-profit telecare provider network types. The ATTILA trial intervention is summarised in Table 7, using the TIDieR format.
| TIDieR format | Current ATT practice for people with dementia |
|---|---|
| When? | |
| When did assessments, recommendation and installations happen? | Baseline (week 0): assessment and recommendations |
| Weeks 12, 24, 52 and 104: assessment and installation | |
| What? | |
| What areas of assessment, in local ATT assessments, had higher fidelity to the ATT assessment standard? | Daily activity, memory, mobility and social support |
| What areas of assessment more frequently triggered the need for ATT? | Daily activities, memory and problem-solving |
| What ATT devices were recommended more frequently in local ATT assessments? | Devices for safety issues and to remind/prompt with monitoring/formal response |
| What ATT devices were installed more frequently? | Devices for safety issues and to remind/prompt with monitoring/formal response and control arm devices (e.g. non-monitored smoke detectors) |
| How much? | |
| How much of the ATT assessment was completed? | 52% of the ATT assessment areas were completed |
| 7.2 ATT assessment areas were addressed, on average (range 0–13) | |
| How many ATT needs were present? | 4.4 ATT needs, on average (range 0–12 needs) |
| How many ATT recommendations were identified? | 3 ATT devices, on average (range 1–14 devices) |
| 57% of participants had one or two ATT devices recommended | |
| How many installations were conducted? | 4.2 ATT devices were installed, on average (range 1–15 devices) (including control arm devices) |
| 79% were installed by week 24, with a reduction in installation over time | |
| How much monitoring and response happened? | 47% of installed ATT devices required monitoring, of which 38% required a formal response |
| Who? | |
| Who were the participants? | > 80 years of age, female, widowed, white British, not living alone and had moderate dementia |
| Who were the assessors of the installed devices? | Baseline:
|
Weeks 12–104:
|
|
| Where? | |
| Where did the ATT assessment take place? | 41% of assessments were in-person, at home |
| Where did the installations take place? | Participant’s homes |
| Tailoring | |
| Were the devices tailored to the participants? | There was an expectation that ATT installations would be tailored to participants by the baseline ATT assessment; however, there was weak to moderate tailoring between (1) baseline ATT needs and ATT recommendations (τ = 0.242; p < 0.000) and (2) ATT recommendations and the ATT installed (τ = –0.470; p < 0.000); 62% of devices were installed for ATT needs that had not been identified in the assessment process, and 53% of the devices recommended as a result of assessment were not installed by week 24 |
Chapter 4 Primary outcome results
Recruitment
Eleven sites in England were opened for recruitment to the ATTILA trial. The first participant was randomised on 14 August 2013; recruitment ended on 26 October 2016. The ATTILA trial randomised over a period of 38 months, with an average recruitment rate of 13 participants per month. Yearly recruitment per site is shown in Table 8.
| Site | Year (number of participants recruited) | Total number of participants recruited | |||
|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | ||
| Croydon | 4 | 21 | 13 | 10 | 48 |
| Lambeth | 5 | 13 | 28 | 17 | 63 |
| Southwark | 3 | 21 | 21 | 26 | 71 |
| Cambridge | 2 | 49 | 37 | 51 | 139 |
| Oxford | 0 | 18 | 8 | 4 | 30 |
| Suffolk | 3 | 23 | 24 | 11 | 61 |
| Lancashire | 1 | 14 | 35 | 22 | 72 |
| Blackpool | 0 | 1 | 3 | 0 | 4 |
| Nottingham | 0 | 2 | 0 | 0 | 2 |
| Barnsley | 0 | 0 | 0 | 3 | 3 |
| Blackburn | 0 | 0 | 2 | 0 | 2 |
| Yearly total | 18 | 162 | 171 | 144 | 495 |
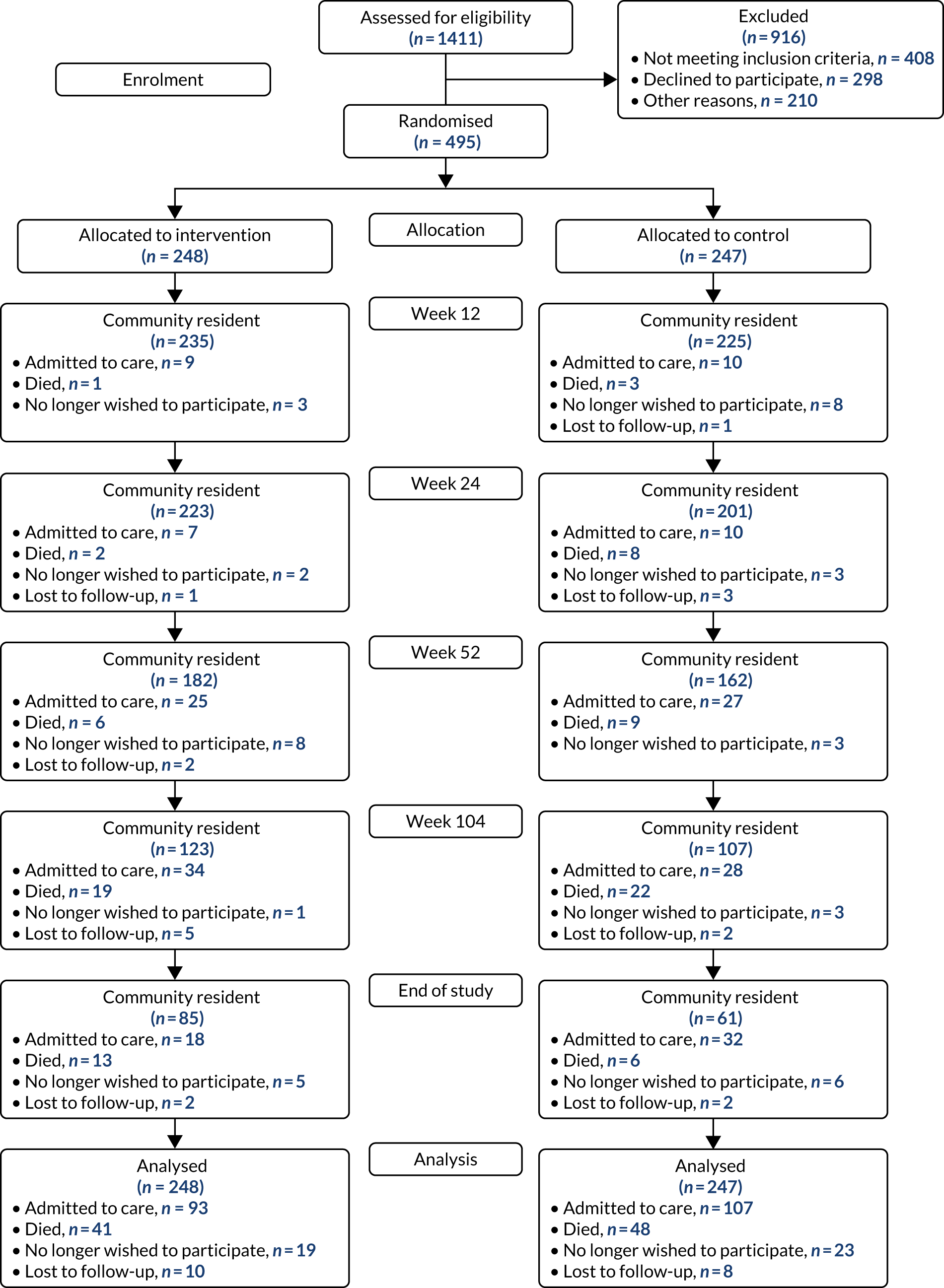
A total of 1411 people were assessed for trial eligibility; of these, 495 were randomised across the 11 sites: 248 were randomised to receive ATT and 247 were randomised to the control arm. The Consolidated Standards of Reporting Trials flow diagram of participants through the ATTILA trial is shown in Figure 3, and Table 9 shows the participant status at the end of the trial.
FIGURE 3.
The Consolidated Standards of Reporting Trials flow diagram.
| Status | Trial arm, n (%) | Total (N = 495), n (%) | |
|---|---|---|---|
| Intervention (N = 248) | Control (N = 247) | ||
| Admitted to care | 93 (37.5) | 107 (43.3) | 200 (40.4) |
| Death while a community resident | 41 (16.5) | 48 (19.4) | 89 (18.0) |
| Withdrew from further follow-up | 19 (7.7) | 23 (9.3) | 42 (8.5) |
| Lost to follow-up (unable to contact) | 10 (4.0) | 8 (3.2) | 18 (3.6) |
| Finished trial living in the community | 85 (34.3) | 61 (24.8) | 146 (29.5) |
Those people who declined to consent did so because they wanted ATT (n = 53), because they did not want ATT (n = 83) or because they did not want to participate in research (n = 162). Other reasons for being excluded were, primarily, that the researcher was unable to contact the potential participant (n = 131) or because participation was deemed inappropriate (n = 48).
During the follow-up of the ATTILA trial, in total, 200 participants were admitted to care, 89 participants died, 42 withdrew from further follow-up and 18 were lost to follow-up. This resulted in 146 participants finishing the trial living independently in the community: 85 in the intervention arm and 61 in the control arm. Relatively few participants (3.6%) were lost to follow-up, as, once randomised, every effort was made to follow up participants throughout the trial to obtain all follow-up forms and outcome assessments. Of the 18 lost to follow-up, 10 were in the intervention arm and eight were in the control arm. Once a participant was admitted to care, the follow-up was terminated and no outcome assessments were collected. Table 10 displays a comparison of the key participant characteristics of the 495 participants randomised.
| Characteristic | Trial arm | |
|---|---|---|
| Intervention (N = 248) | Control (N = 247) | |
| Age (years) | ||
| < 65, n (%) | 11 (4) | 4 (2) |
| 65–80, n (%) | 89 (36) | 93 (38) |
| > 80, n (%) | 148 (60) | 150 (61) |
| Mean (SD) | 81.0 (8.2) | 80.8 (7.4) |
| Gender, n (%) | ||
| Male | 102 (41) | 103 (42) |
| Female | 146 (59) | 144 (58) |
| Risk of wandering/leaving home inappropriately, n (%) | ||
| Low | 178 (72) | 180 (73) |
| Medium | 52 (21) | 48 (19) |
| High | 18 (7) | 19 (8) |
| Safety risks within home identified, n (%) | ||
| Low | 125 (50) | 124 (50) |
| Medium | 104 (42) | 101 (41) |
| High | 19 (8) | 22 (9) |
| Level of caregiver support, n (%) | ||
| Live in | 119 (48) | 121 (49) |
| Once daily | 60 (24) | 61 (25) |
| Less than once daily | 69 (28) | 65 (26) |
| SMMSE scorea | ||
| 0–9, n (%) | 23 (10) | 34 (15) |
| 10–19, n (%) | 79 (36) | 96 (43) |
| 20–25, n (%) | 87 (39) | 74 (33) |
| 26–30, n (%) | 32 (14) | 19 (9) |
| Mean (SD) | 18.7 (6.6) | 16.9 (6.9) |
| BADLS scoreb | ||
| 0–4, n (%) | 17 (7) | 10 (4) |
| 5–14, n (%) | 72 (31) | 64 (28) |
| 15–29, n (%) | 95 (41) | 102 (45) |
| 30–60, n (%) | 46 (20) | 49 (22) |
| Mean (SD) | 19.5 (11.3) | 20.4 (10.9) |
All characteristics appear to be reasonably well balanced across the two randomised arms. The average age was 80.9 years, 59% (290/495) were female and 48% (240/495) had a live-in caregiver. The majority of participants, 72% (358/495), were classified as being at low risk of wandering or leaving their home inappropriately. Half of the participants (249/495) were deemed to have low safety risks identified within the home. The average SMMSE score was 18.7 points in the intervention arm and 16.9 points in the control arm, so participants in the intervention arm had a slightly higher baseline SMMSE score than those in the control arm. The average BADLS score was 19.5 in the intervention arm and 20.4 in the control arm. The missingness of these data was similar between the two arms.
Primary outcomes
Time to institutionalisation
The primary analysis of admission to care was defined as a permanent transition from living in a participant’s own home to living in nursing or residential care, or admission to an acute care facility that resulted in permanent placement. The end point was compared between the intervention and control arms using survival analysis. Kaplan–Meier curves are for graphical representation of time to an event. Statistical significance was determined through log-rank test. The primary analysis was conducted according to intention to treat, and participants who have died, withdrawn from follow-up or who were lost to follow-up were censored at the date of withdrawal. The time to admission to care, split by randomised arm, is shown in Figure 4.
FIGURE 4.
Kaplan–Meier survival curve: time to admission to care by randomised arm, unadjusted analysis.
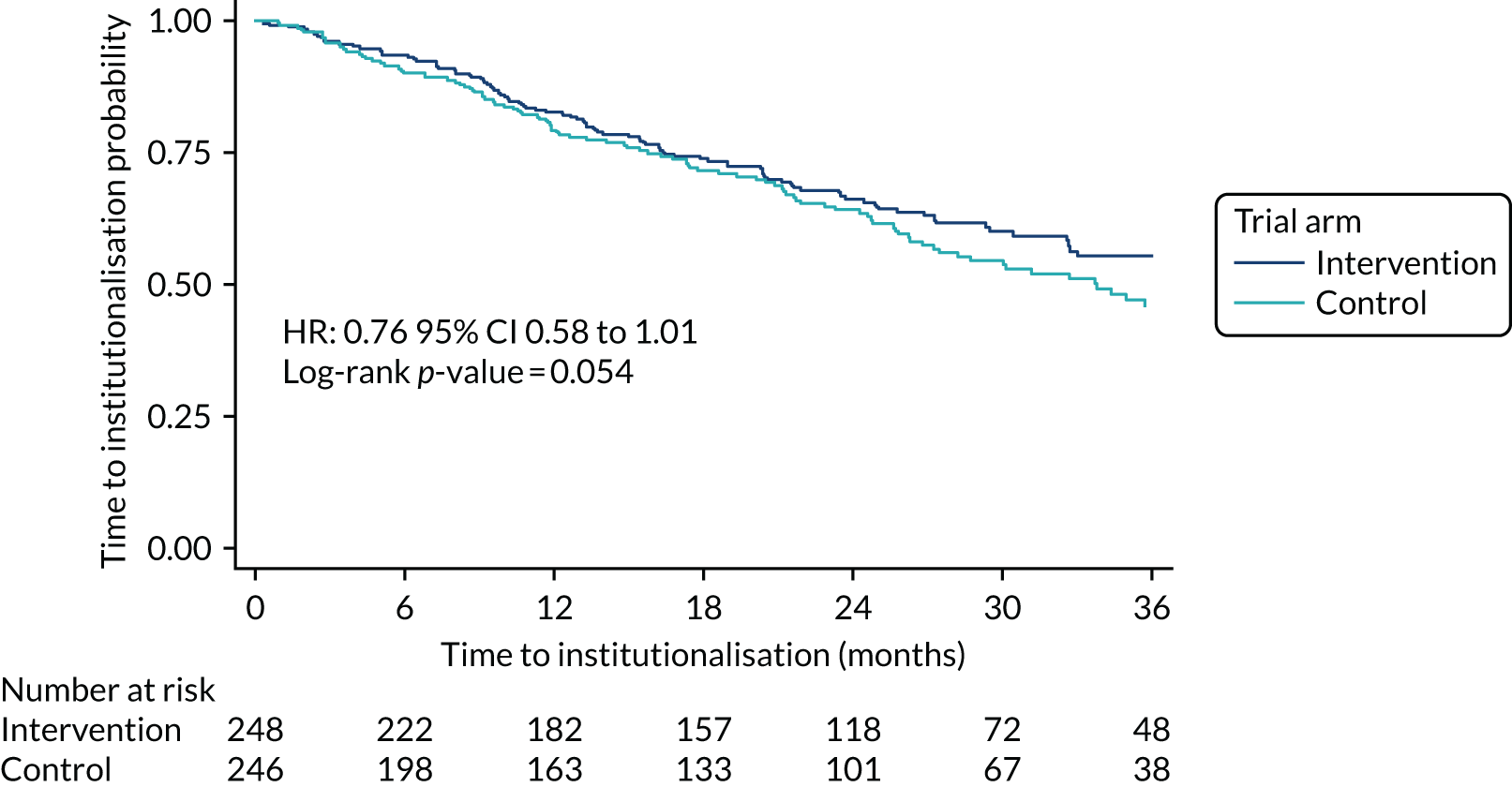
The intervention and control arms showed a similar pattern of time to admission to care over the 3-year period plotted. Comparing the ATT arm with the control arm, the hazard ratio is 0.76 [95% confidence interval (CI) 0.58 to 1.01; p = 0.054]. This unadjusted analysis showed a borderline significant difference in slowing the time to admission to care with ATT use when compared with the control. At 2 years, the admission to care rate for the ATT arm was 65.6% (95% CI 58.8% to 71.5%), compared with 63.4% (95% CI 56.3% to 69.7%) for the control arm.
The rates of admission to care can be affected by participants’ functional ability. This was measured using the BADLS. BADLS scores range from 0–60, with higher scores indicating greater impairment. Figure 5 shows the time to admission to care split by BADLS scores 0–4, 5–14, 15–29 and 30–60.
FIGURE 5.
Kaplan–Meier survival curve: time to admission to care by baseline BADLS score.
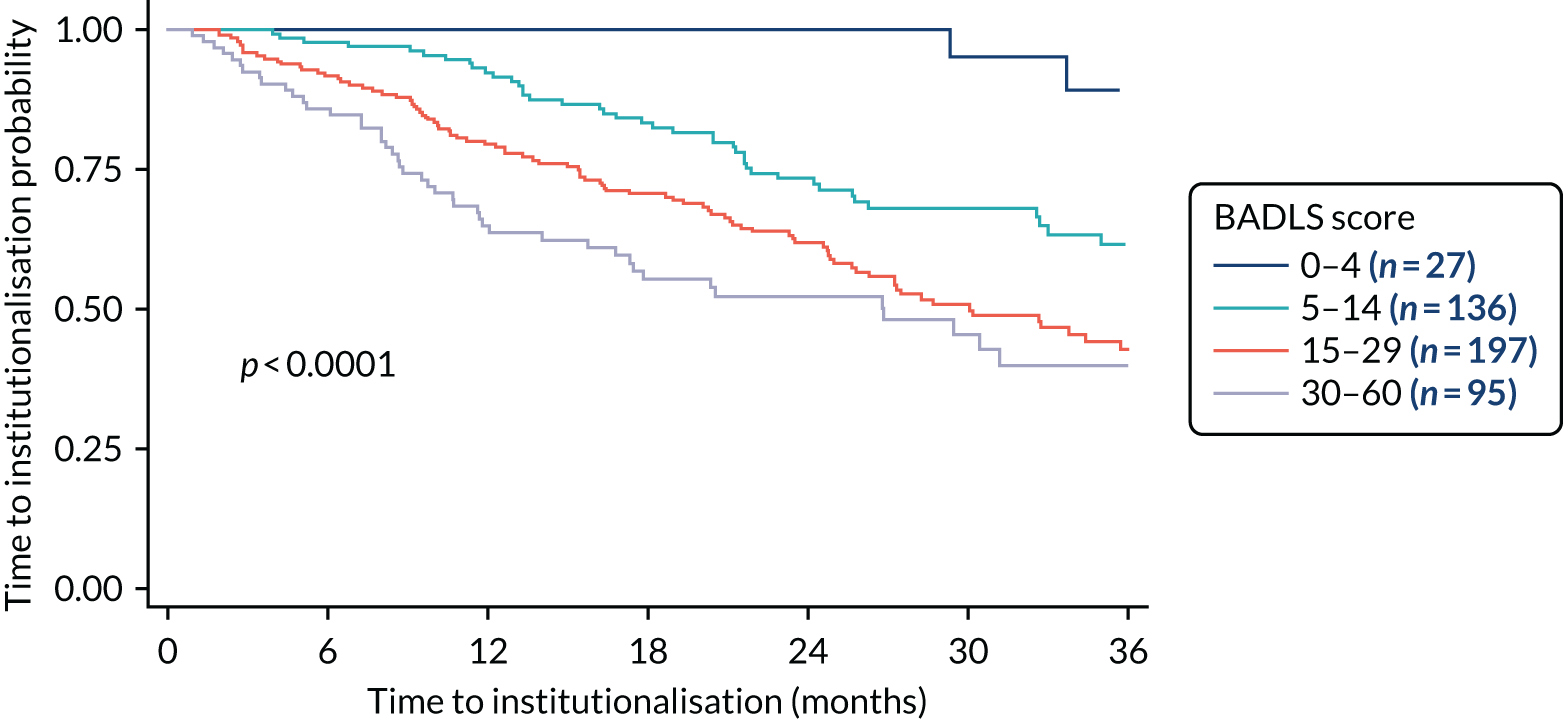
There was a highly significant difference in the time to admission to care when comparing baseline BADLS scores. Participants with a higher baseline BADLS score were more likely to be admitted to care (p < 0.0001). Baseline BADLS scores are presented in Table 10 and shows an imbalance in baseline scores. More participants in the intervention arm than in the control arm had a lower baseline BADLS score. As Figure 5 showed that participants with a higher baseline BADLS score are more likely to be admitted to care; this difference at baseline was adjusted for in the primary analysis. A forest plot split by baseline BADLS score is shown in Figure 6.
FIGURE 6.
Forest plot: admission to care by randomised arm, adjusted for baseline BADLS score. NS, not significant; O/E, observed over expected; Var., variance.
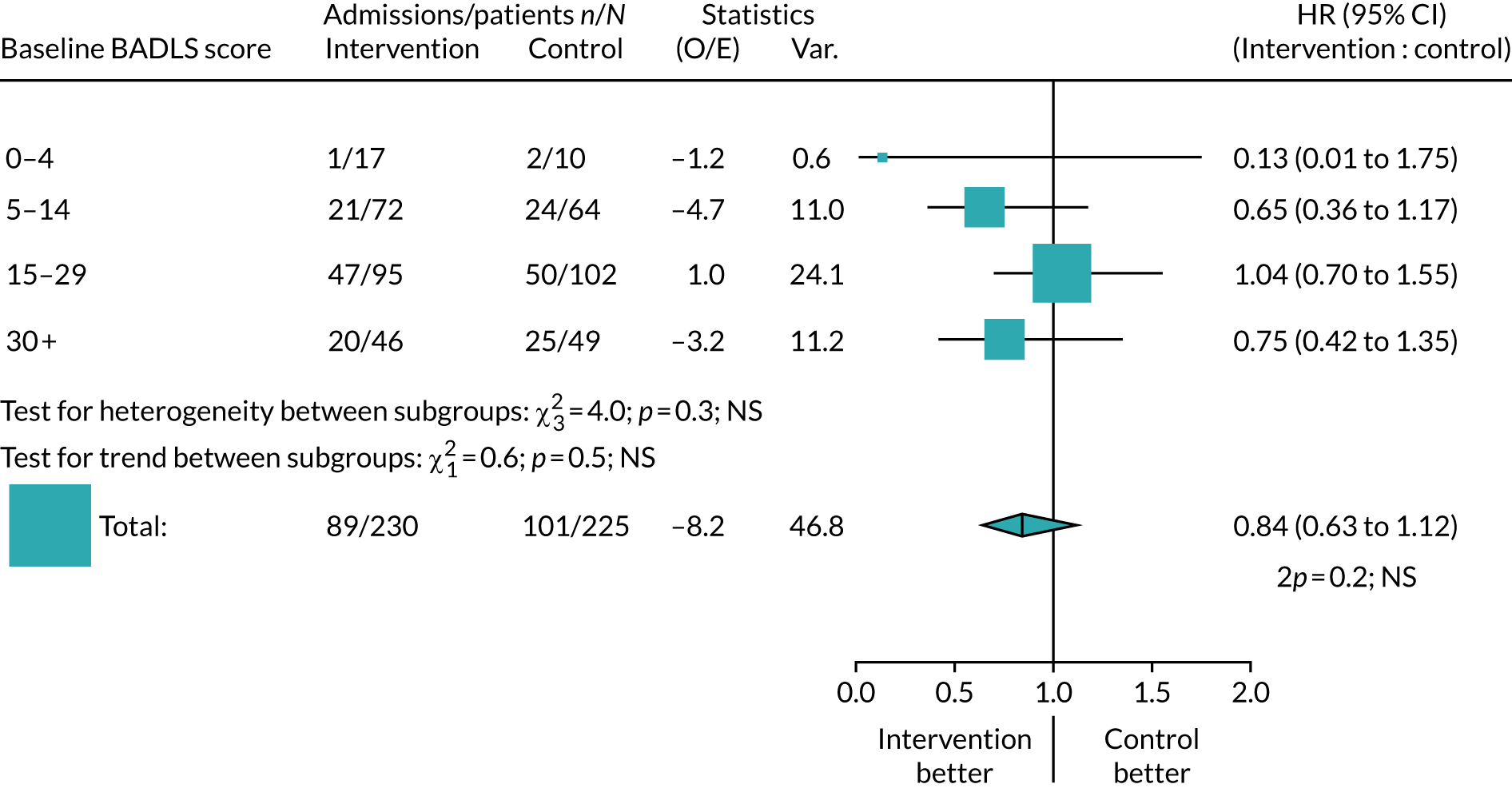
When adjusting for baseline BADLS scores, there is no significant difference in the time to admission to care between those in the intervention group and those in the control group (hazard ratio 0.84, 95% CI 0.63 to 1.12; p = 0.20).
The reasons for admission to care are usually multifactorial. To determine whether or not ATT might have helped prevent admissions to care, the reasons given for institutionalisation have been categorised as having any mention of safety, then any mention of wandering and then falls, with others classified as inability to perform activities of daily living (ADL), behaviour, other medical condition, deterioration (unspecified), caregiver health, other and unknown. This can give only an approximate classification, given the complexity of Alzheimer’s symptoms, but the breakdown of the most likely causes is given in Table 11.
| Categorised reason | Trial arm (number of participants) | Total number of participants (N = 495) | p-valuea | |
|---|---|---|---|---|
| Intervention (N = 248) | Control (N = 247) | |||
| Safety concern | 12 | 4 | 16 | 0.043 |
| Wandering | 5 | 13 | 18 | 0.054 |
| Falls | 13 | 13 | 26 | 0.990 |
| Loss of ADL | 14 | 29 | 43 | 0.016 |
| Behaviour | 8 | 10 | 18 | 0.630 |
| Other medical condition | 7 | 6 | 13 | 0.790 |
| Deterioration (unspecified) | 14 | 11 | 25 | 0.540 |
| Caregiver health | 9 | 3 | 12 | 0.081 |
| Other | 6 | 8 | 14 | 0.580 |
| Unknown | 5 | 10 | 15 | 0.190 |
| Any cause | 93 | 107 | 200 | |
The most common reason for admission to care is the inability to perform ADL. Institutionalisation for safety concerns, which might have been expected to be reduced by ATT, is actually more common in the intervention group (12 vs. 4 participants; p = 0.043). By contrast, the risk of wandering, which might, again, be mitigated by appropriate ATT, was reduced in the intervention group (5 vs. 13 participants; p = 0.054). There was also a significant reduction in the number of participants moving into residential care because of the inability to perform ADL (14 vs. 29 participants; p = 0.016). A total of 15 admissions to care were classified as being for an unknown reason.
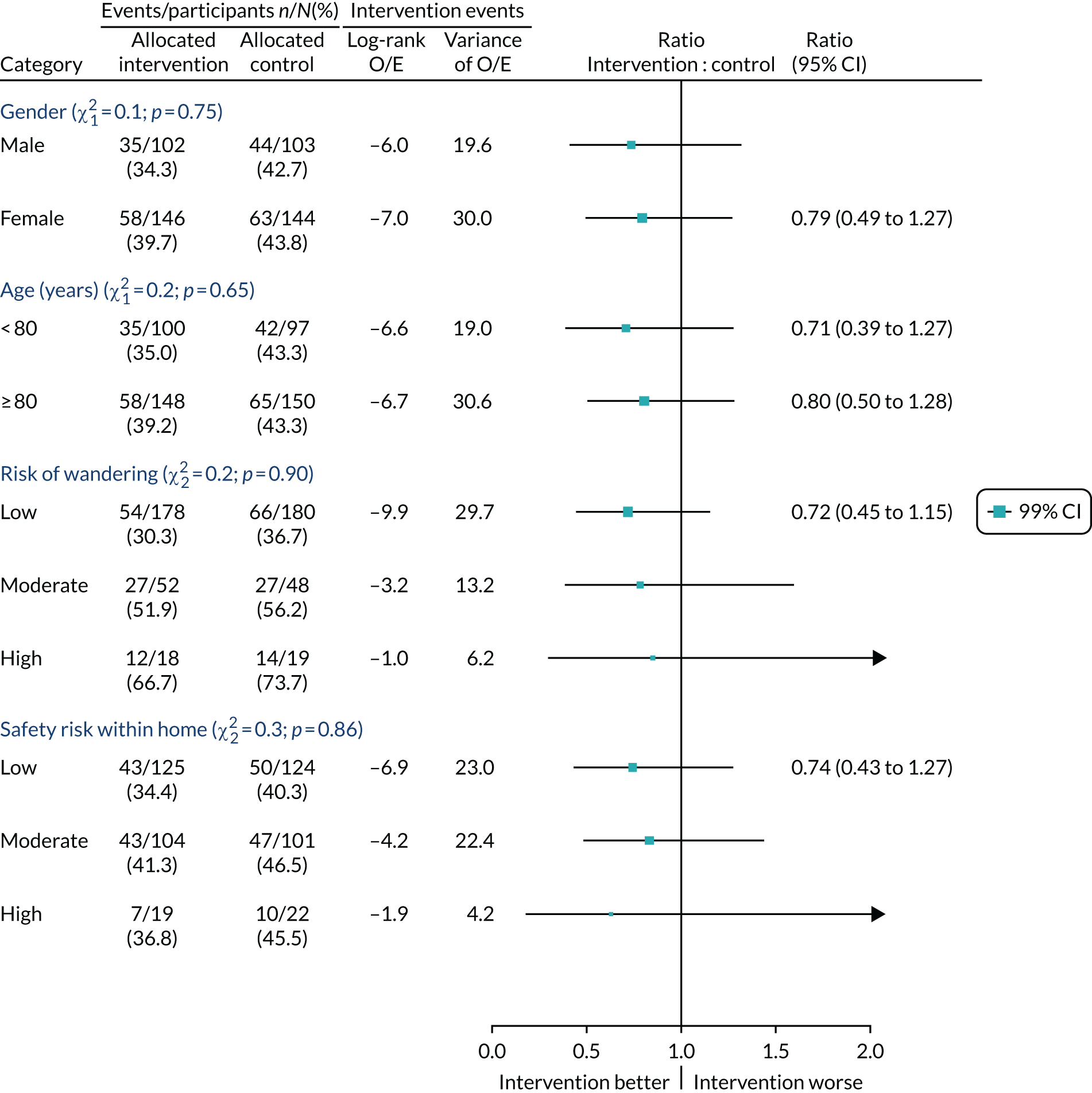
Subgroup analysis
To investigate whether or not ATT use varied by baseline characteristics, we did subgroup analyses of admission to care in the ATT group compared with admission to care in the control group by gender, age, risk of wandering from home and safety risk within the home (Figure 7). As there were no significant differences seen, there is no indication of any benefit from ATT use in any of these subgroups.
FIGURE 7.
Subgroup analyses of admission to care for the ATT group vs. the control group, by baseline characteristics. O/E, observed over expected; Var., variance.
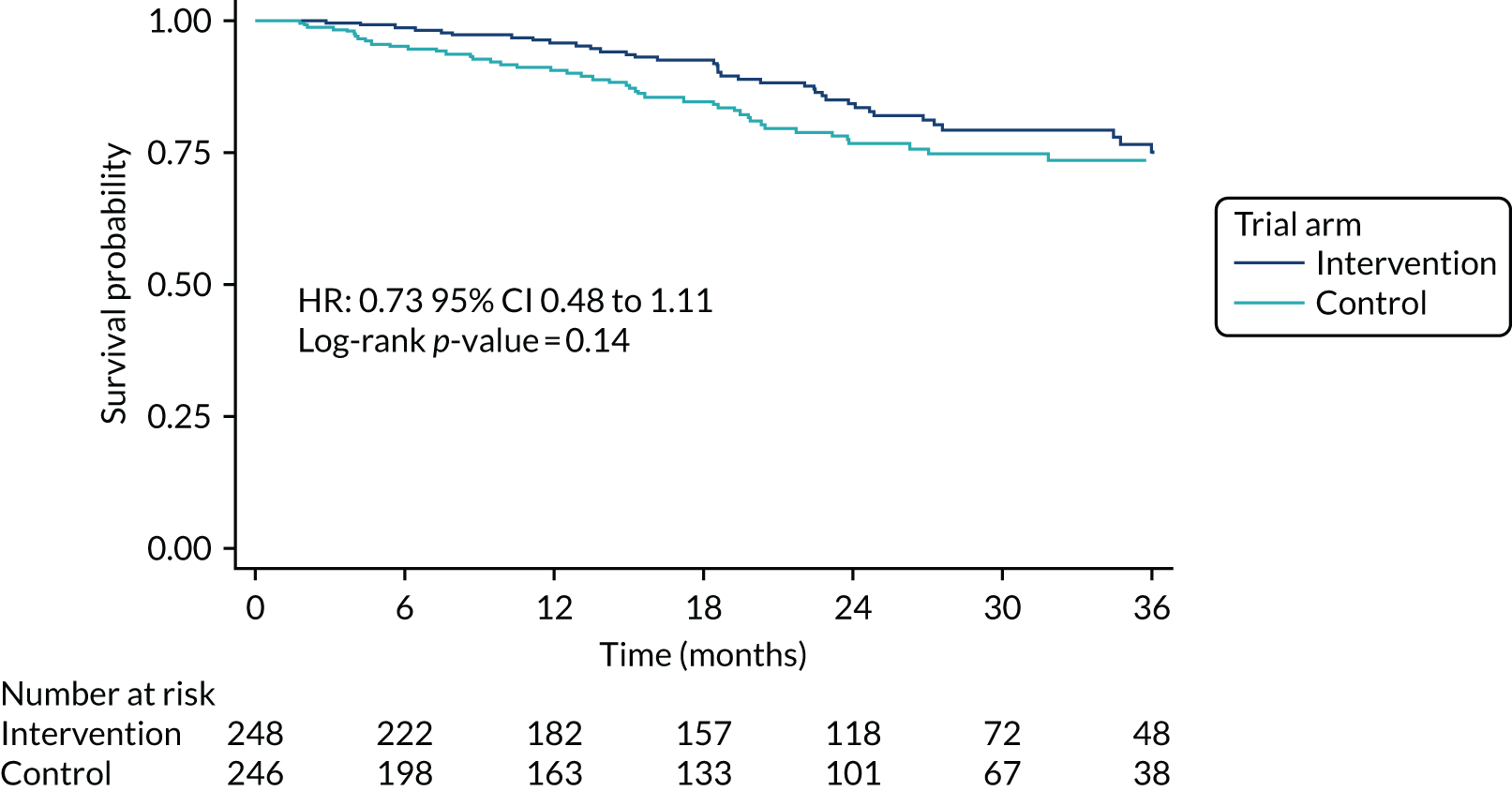
Deaths while in the community
Eighty-nine participants died while in the community. Figure 8 is the Kaplan–Meier graph of time to death while community resident and Table 12 shows the categorised reasons for cause of death. In the Kaplan–Meier analysis, participants who had been admitted to care, withdrawn from follow-up or lost to follow-up were censored at the date of withdrawal.
FIGURE 8.
Kaplan–Meier survival curve: time to death while community resident, by randomised arm.
| Cause of death | Trial arm (number of participants) | Total number of participants (N = 495) | p-valuea | |
|---|---|---|---|---|
| Intervention (N = 248) | Control (N = 247) | |||
| Health/dementia deterioration | 8 | 4 | 12 | 0.25 |
| Pneumonia/respiratory failure | 4 | 10 | 14 | 0.10 |
| Heart attack/heart failure | 3 | 8 | 11 | 0.13 |
| Stroke | 7 | 5 | 12 | 0.56 |
| Cancer | 7 | 4 | 11 | 0.36 |
| Infection | 6 | 4 | 10 | 0.53 |
| Other | 2 | 4 | 6 | 0.41 |
| Unknown | 4 | 9 | 13 | 0.16 |
| Total | 41 | 48 | 89 | |
There were no significant differences seen overall (p = 0.14) or in the grouped categories for cause of death (see Figure 8 and Table 12).
Serious adverse events
Serious adverse events have been grouped into broad categories and are summarised in Tables 13 and 14. The categories were decided on by members of the ATTILA trial team with clinical expertise and were categorised separately by two members of the team, then assessed for consistency. Any differences were discussed, and input sought from a clinical expert in the team. Raters were unaware of treatment allocation of the participants involved. Table 13 presents the counts of SAEs recorded and Table 14 presents the number of participants reporting the SAEs, as participants can report multiple SAEs. Similarly to the reasons for admissions to care, SAEs could be multifactorial. The categories are any mention of safety concerns, wandering, falls, dementia progression, behaviour, other medical condition, caregiver related, accidents, health deterioration, other and unknown.
| Categorised SAE | SAE count (n) | ||
|---|---|---|---|
| Intervention arm | Control arm | Total | |
| Safety concerns | 15 | 5 | 20 |
| Wandering | 36 | 71 | 107 |
| Falls | 182 | 187 | 369 |
| Dementia progression | 37 | 46 | 83 |
| Behaviour | 5 | 21 | 26 |
| Other medical condition | 214 | 220 | 434 |
| Caregiver related | 11 | 10 | 21 |
| Environmental/accident | 14 | 21 | 35 |
| Health deterioration | 6 | 3 | 9 |
| Other | 2 | 1 | 6 |
| Unknown | 10 | 18 | 28 |
| Total count of SAEs | 532 | 603 | 1135 |
| Categorised SAE | Number of participants | p-value | ||
|---|---|---|---|---|
| Intervention arm | Control arm | Total | ||
| Safety concerns | 13 | 5 | 18 | 0.06 |
| Wandering | 25 | 36 | 61 | 0.13 |
| Falls | 86 | 88 | 174 | 0.83 |
| Dementia progression | 37 | 43 | 80 | 0.45 |
| Behaviour | 5 | 16 | 21 | 0.01 |
| Other medical condition | 107 | 109 | 216 | 0.83 |
| Caregiver related | 11 | 10 | 21 | 0.83 |
| Environmental/accident | 13 | 15 | 28 | 0.69 |
| Health deterioration | 5 | 2 | 7 | 0.26 |
| Other | 2 | 1 | 3 | 0.57 |
| Unknown | 10 | 16 | 26 | 0.22 |
| Total | 195 | 201 | 396 | 0.45 |
Participants reported multiple SAEs. Overall, 1135 SAEs were reported from 396 participants. The most common SAE was ‘other medical condition’, which was reported by 216 participants. The second most-reported SAE was related to falls (369 falls were reported by 174 participants). Figure 9 plots the number of participants experiencing each SAE type, ordered by hierarchy of classification, with a test of significance between intervention and control participants.
FIGURE 9.
Forest plot of the incidence of SAEs. p-values from Mantel–Haenszel tests (ignoring time to event). OR, odds ratio.
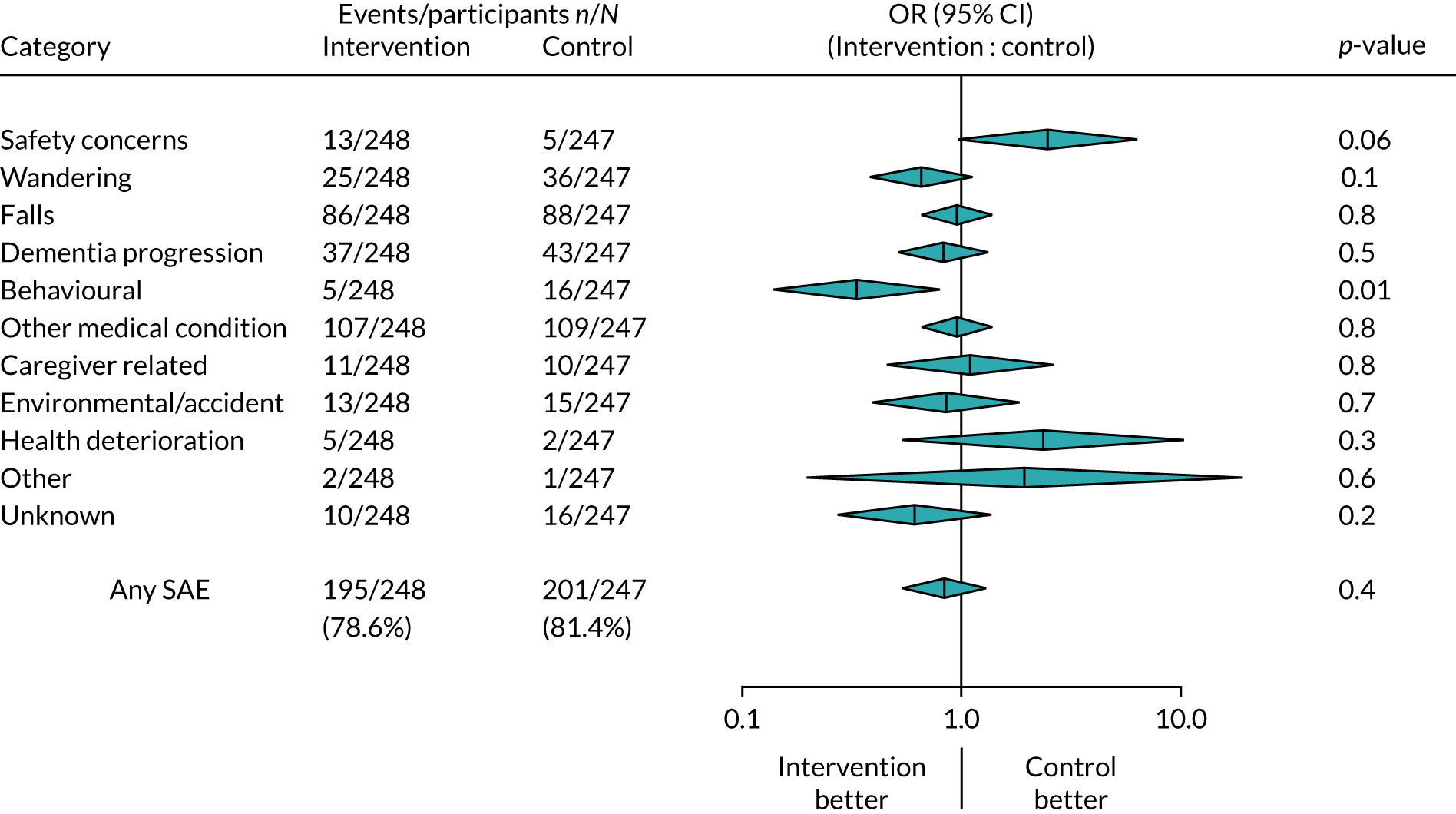
Figure 9 shows that there was a significant reduction in the number of participants experiencing behavioural-related SAEs in the intervention group, compared with the control group (p = 0.01). More participants in the intervention group than in the control group reported SAEs related to safety concerns (p = 0.06).
Chapter 5 Economic evaluation
The economic evaluation addressed the question ‘are ATT interventions cost-effective in the management of risk and maintenance of independence in people with dementia living in their own homes?’.
Methods
The economic evaluation included cost-effectiveness and cost–utility analyses.
Outcomes
The economic evaluation examined three outcomes for participants with dementia:
-
the incremental cost of community-based support per institutional day avoided (days to institutionalisation)
-
the incremental cost of change in the EQ-5D-5L index over 24, 52 and 104 weeks
-
the incremental cost per QALY over 24, 52 and 104 weeks.
Participant-rated and proxy-rated utilities were calculated from the EQ-5D-5L, valued by ‘crosswalking’ EQ-5D-5L health-state profiles to the UK value set for EuroQol-5 Dimensions, three-level version,61,62 as currently recommended by the National Institute for Health and Care Excellence (NICE). 63
Perspective
Analyses were conducted from a health and social care perspective (cost to the NHS and to CASSRs) and from a societal perspective (costs to the participant and caregiver). We assumed service costs (e.g. of community, primary and hospital health care) were borne by public payers, except in the case of home adaptations and ADL equipment, for which only items reported to have been paid for by the NHS or council were included. Societal costs included lost production, costs of providing unpaid care and out-of-pocket payments for home adaptations and ADL equipment and travel costs (restricted to dementia-related treatment and day care).
Time horizon
The participant- and caregiver-reported EQ-5D-5L and the caregiver-reported CSRI were administered alongside other measures at each assessment point (see Table 1). The CSRI covered service receipt over the previous 3 months. An annual discount rate of 3.5%64 was applied to costs and days in the community in the second year, as the time horizon was 2 years.
Costs
The analysis considered comprehensive costs of care and support to the person with dementia. Health-care, social care and societal costs (excluding direct costs of the ATT intervention) were calculated by drawing on data from the CSRI. Direct costs of the ATT intervention were calculated from the ATT technology checklist and from interview data (see Intervention costs, Valuing assessment time and Valuing the assistive technology and telecare package). Costs of health and social care service use were calculated from service use data by applying the relevant published unit costs. 42,43 Caregiver inputs were valued by taking an opportunity-costs approach in the main analyses, following methods described in Wimo et al. ,65 by valuing the lost working time of working caregivers at the national average wage66 and lost leisure time of non-working caregivers at 35% of that figure. In the case of care from others (e.g. other relatives), caregivers were assumed to be working and their time was valued at national average wage. A replacement-costs valuation (using the hourly cost of a domiciliary care worker) was applied in a sensitivity analysis. 43,67 Unit costs used in valuing resource use are reported in Appendix 3.
The costs of individual services were first aggregated to category level (hospital; primary and community health; mental health; overnight respite; community, social and day care; equipment and adaptations; mental health medications; telecare intervention; and caregiver), and then to health and social care and societal totals. Costs at category level were calculated so that if > 2% of component costs in the category were not available, the total of the category was considered missing. Total costs across categories were considered missing if any category of cost was missing.
The CSRI data did not cover the entirety of the 2-year follow-up, as caregivers were always asked to report service use over the previous 3 months. The costs of the intervening periods were estimated by carrying forward cost categories of the previous period to the interval between the 24-week point and the retrospectively recalled 3-month period ending at week 52, and then between week 52 and the retrospectively recalled 3-month period ending at week 104. The carrying-forward process for the 9-month interval in year 2 created three additional intervals running up to the 3 months prior to the 104-week assessment point. The one exception in the process of carrying forward costs was for hospital admissions and emergency department visits: data drawn from SAE reporting were used to estimate these costs. Therefore, the costs of routinely used services were assumed to be constant over the intervening periods, but emergency department and hospital admissions reflected the observed use in those periods. The costs were not carried forward to intervals when the person had been admitted to care, died, was lost to follow-up or no longer wished to participate.
Intervention costs
Proposed methods of costing the ATT intervention are described in detail in Appendix 1. We planned to describe the production of the full ATT package and to assess the feasibility of collecting data from sites to calculate the costs of the ATT intervention. Data on the delivery system for ATT were collected from local researchers via pro formas and from key informants via interviews with local authority operational/middle managers in adult services, local authority commissioners of telecare and managers in telecare provider organisations. Interviews in seven sites took place in 2016 (only sites with more than five participants were approached). We had planned to request data on ATT equipment from providers, but it proved difficult to draw up the necessary data-sharing agreements covering electronic data extraction in every site. The scope and level of detail of information on total costs and unit costs of ATT gathered to date varied considerably between sites, depending on the size of the local authority and the complexity of the local ATT market. Information from this process was used to describe, in some detail, the local actors involved in delivering ATT and the process of ATT production in each site. Based on these descriptions, the production of ATT across all sites involves the following components: assessment, procurement/purchasing, installation, call-handling (monitoring) and response. The data collected were not consistent enough to enable calculation of the costs of ATT for use in the economic evaluation. Instead, the costs of the ATT production process were built up from several sources; some components will reflect costs at a more granular level of detail than others, as described in Valuing assessment time and Valuing the assistive technology and telecare package.
Valuing assessment time
The time taken to carry out ATT assessments could vary substantially depending on the level of need and the specific devices required. Feedback from pro formas and interviews yielded a range of estimates of assessment time. The assumption based on information from interviews was that an ATT assessment took 1 hour.
The personnel conducting assessments varied depending on the site and the nature of the ATT need. Data from the ATT technology checklist were available: the checklist distinguishes between health and social care assessors and specialist ATT assessors, but researchers were not always able to determine an assessor’s qualifications. There was considerable variation in assessment personnel, depending on the local area and the sector of the assessor’s employer (see Chapter 3). The assumption made in costing assessor time was that health and social care assessors were non-specialist NHS professionals paid at (NHS) Agenda for Change band 5 and that specialist ATT assessors were specialist community occupational therapists paid at (NHS) Agenda for Change band 6. 43 Assessor costs (including on-costs, overheads and capital costs; see Appendix 3) were calculated from the relevant costs per working hour given in the Unit Costs of Health and Social Care 2017. 43
When the assessor type was unknown, the proportions of health and social care assessors and specialist ATT assessors (disregarding other/unknown assessor types) conducting assessments at the needs assessment point (see Tables 3 and 4 and Appendix 3) were used to calculate a weighted hourly rate. Only the pre-baseline ATT needs assessment was costed into the package, in line with the protocol’s definition of the intervention. ATT device data were taken from the ATT checklist data. All device types noted by the researchers at each time point were considered to be relevant costs.
Valuing the assistive technology and telecare package
Data to estimate ATT package costs were taken from several sources. Data from the NHC68 were obtained to enable valuation of components of the ATT production process. The NHC offers consortium procurement services to the UK public sector organisations that make up its membership. Members include local authorities, not-for-profit providers, housing associations and industry partners. Each consortium procurement framework remains in place for 4 years, recording contract prices paid by members for any service or product covered by the framework to supply partners in relevant industries. Two consortium frameworks that were relevant to ATT products and services were in operation during the trial period. The Assisted Living framework, and the Technology Enabled Care Services (TECS) framework that superseded it, covered telecare products and related installation and services. The TECS framework covered a broader range of ATT devices than the Assisted Living framework (not only telecare, but also telemedicine and telehealth). The TECS framework covered services such as installation and maintenance of devices, call monitoring and mobile response. The NHC supplied data from consortium framework databases on actual contract prices paid during contracts that had expired by 2017. Data were available from Assisted Living framework contracts in place in 2015 and from TECS contracts in place in 2016. The frameworks covered key elements of ATT provision relevant to purchasers in local authorities and not-for-profit agencies. The activities covered by the contracts corresponded well with three ATT production stages (as previously outlined): installation, call-handling/monitoring and response. A per-person annual cost of the installation, maintenance, call-monitoring and mobile response elements of ATT was calculated from contract prices derived from NHC framework data (see Appendix 3).
Assistive technology and telecare devices
The Consortium provided information on 2016 device prices from the TECS framework suppliers’ catalogue. Prices for some categories of devices were available as mean and median prices across that category, weighted (by volume) and including NHC discounts available to members. In other cases, only the lowest and highest prices per item were available, and it was not possible to calculate a weighted mean. In such cases, the lowest and highest costs were summarised by category and the mid-point of the range between these was used. Device costs were annuitised over 5 years at a discount rate of 3.5%. Data on the types of ATT devices installed at each assessment point (see Chapter 3) were taken from the ATT checklist; valuation of these devices was drawn from information obtained from the NHC price data, as described in the preceding section.
Missing data
A sizeable number of participants and caregivers declined to complete at least one of the follow-up assessments while continuing to participate in status checks. Missingness in the CSRI data available from people who had participated in complete assessments was very low for most variables (typically around 1%). Data on certain variables were missing at baseline because of subsequent version changes in the ATT and CSRI measures. The first version of the CSRI (subsequently revised in September 2014) did not include questions on caregivers’ time spent providing care (for this reason, 8% of baseline data on this variable were missing) and thus missing for reasons unrelated to participants’ health status.
Missing data reduction strategies were employed in certain cases. When ADL equipment/adaptations provider data were missing (fewer than 10 instances), the provider was assumed to be the local authority. For medication costs, if the dates of first use were missing, when there was information on first use and ongoing use of particular medications, dosages, units and frequencies, these dates were assigned to preceding and succeeding periods when the same medication, unit dosage and frequency were reported, as long as the dates preceded the assessment date. As a further step, the average duration over which medications were taken in contiguous periods from baseline to 24 weeks (per medication, per participant) was calculated from available duration data and applied to missing durations over these periods. The average duration of medications taken in these periods was 84 days, and < 5% had durations of ≤ 36 days, indicating long-term use. For the remaining missing durations, it was assumed that the medication had been taken over the whole of the prior period. For future evaluations, the medication question could be improved by asking whether or not the participant had been taking the medication for > 3 months, rather than asking the date of first use. No assumptions could reasonably be made on caregiver time spent providing care when this was missing because of version changes.
Compared with the expected number of responses (given the number of assessments administered), approximately 10% of EQ-5D participant-reported index scores were missing at baseline. At 12 weeks, 13% of intervention participants’ and 20% of control participants’ responses were missing; at 24 weeks, 15% of intervention and 21% of control group participants’ responses were missing; at 52 weeks, 25% intervention and 31% of control group participants’ responses were missing. At 104 weeks, 22% of intervention and 34% of control group participants’ responses were missing. The proportion of missing responses did not differ between groups at the 5% level on chi-squared tests at any time point. Of expected responses (given the number of assessments administered), proxy-completed EQ-5D index scores featured lower levels of missingness than seen in the participant-reported measure (baseline missingness: 9% intervention, 12% control; missingness at 12 weeks: 10% intervention, 9% control; missingness at 24 weeks: 8% intervention, 8% control; missingness at 52 weeks: 5% intervention, 9% control; and missingness at 104 weeks: 4% intervention, 7% control).
When EQ-5D index scores were missing, index score values were interpolated between adjacent time points. Compared with the expected number of responses (given the number of assessments administered) for the EQ-5D participant-reported index scores, after interpolation, at 12 weeks, 7% of the intervention and 14% of control group participants’ responses were missing; at 24 weeks, 12% of the intervention and 15% of control group participants’ responses were missing; and at 52 weeks, 20% of intervention and 25% of control group participants’ responses were missing. Of expected responses (given the number of assessments administered) for the proxy-completed EQ-5D index scores, after interpolation, at 12 weeks, 6% of intervention and 5% of control group participants’ responses were missing; at 24 weeks, 3% of the intervention and 6% of control group participants’ responses were missing; and at 52 weeks, 4% of the intervention and 7% of control group participants’ responses were missing.
Apart from these measures, missing costs and outcome data were not imputed. Data required for the cost-effectiveness analyses were ‘missing’ for several reasons. When the trial end point of care home admission was met, no further assessments were administered. Some participants died, there was loss to trial follow-up and some dyads decided to cease participation in the trial completely. Some dyads did not complete assessments (no measures were administered), although the dyad continued to participate in the trial status checks; this could have been for several reasons, including disagreement with allocation, burden of assessments and delays in assessments being completed. Missingness was handled in different ways, depending on the analysis. The difference-in-difference analyses were estimated by maximum likelihood (see Analyses). Cases were excluded from the analyses when the dyad had participated in no assessments over the trial and when the baseline BADLS score was missing (there were no missing data in other baseline covariates, which were stratifying variables used in the randomisation procedure). The analyses of institutionalisation-free days and QALYs employed models to explicitly manage data-censoring due to withdrawal, loss to follow-up and death.
Analyses
Descriptive analyses were produced for service and other resource use items available at each time point, presented in terms of the proportions of each treatment group using the service and the mean use of the service in each group. Group means and standard errors (SEs) were calculated for categories and totals of costs and for outcomes at each time point, as were mean differences and SEs of the difference between groups.
For the outcome days in the community until admission to a care home, the number of days was estimated in a survival analysis accelerated failure time model using the Weibull distribution, adjusting for baseline BADLS score. A further step was involved in the QALY outcome: to quality-adjust the days lived in the community, taking a population (or group-based) approach to produce a quality-adjusted survival curve, using the EQ-5D index scores at each assessment point to estimate the average utility per treatment group. 69,70 Costs were partitioned and estimated in a generalised gamma accelerated failure time model with a square root link; the probability of not being censored in each time interval was estimated by accelerated failure time models (generalised gamma model at 104 weeks and a Weibull model at 52 and 104 weeks, as the generalised gamma model did not converge), generating inverse probability weights for costs at each interval. 70–72 Cost regressions were adjusted for the treatment allocation, BADLS score and stratifying variables. Cases without a baseline BADLS score and cases for whom the dyad had never participated in assessments were excluded from the analyses. Bias-corrected bootstrapped CIs of regression estimates of cost and outcome differences were produced (25,000 resamples).
For the change in EQ-5D-5L index outcome, multilevel linear difference-in-difference models were fitted to costs and EQ-5D index scores data. The models estimated the difference in the change in scores from baseline to follow-up in the intervention group, less the difference in the change in scores from baseline to follow-up in the control group (i.e. the difference between groups in the difference between baseline and follow-up costs/outcomes in each group). Models were adjusted for stratifying variables and the three-category BADLS variable for dependency (see Chapter 4). Multilevel mixed-effects linear models were estimated by maximum likelihood, assuming that data were missing at random on the response variable. In other words, missingness was assumed to be dependent on model covariates or on previous or following responses, had they been observed, but not on the missing responses. 73,74 Cases at 52 and 104 weeks were considered available for analysis if baseline outcome/cost data and at least two follow-up data points were available; at 24 weeks, cases were considered available if baseline outcome/cost data and at least one follow-up data point were available. Bias-corrected bootstrapped CIs of the model estimates of cost and outcome differences were obtained (5000 resamples).
Cost-effectiveness analyses
Incremental cost-effectiveness ratios (ICERs) were calculated for each outcome. ICERs were calculated separately for the difference in the EQ-5D outcome at the 24-, 52- and 104-week follow-ups, and for QALY-adjusted institutionalisation-free days. The ICER was defined as the difference in mean costs incurred by the intervention and control groups (ΔC), divided by the difference in mean outcome (ΔE) between the treatment groups. The ATT intervention can be interpreted as representing value for money if the ICER is below some threshold of WTP for a unit of additional effectiveness, λ:75
A full package of ATT can be considered cost-effective if (1) the package is significantly more effective and less expensive than a basic package of ATT or (2) ATT is significantly more effective and more expensive, but the payer is willing to pay the additional cost (up to λ) to achieve the additional effectiveness or, possibly, (3) ATT is significantly less effective and less expensive, but the payer considers the sacrifice of some effectiveness worth making because of the savings that could be achieved. ATT can be considered, unambiguously, to be not cost-effective if it is both significantly less effective and more expensive.
Incremental net monetary benefit75 can be expressed as a rearrangement of the decision rule in (1):
This is the monetary value of gains in outcomes associated with the treatment at a given WTP threshold, net of (less) the additional cost of providing the treatment. 76
Cost-effectiveness acceptability curves (CEACs) were produced when the intervention strategy was more effective and costs were lower. Estimates of cost and outcome differences were obtained by non-parametric bootstrapping of regression estimates, producing 25,000 replicates in the case of the institutionalisation-free days and QALY outcomes and 5000 replicates in the case of the change in EQ-5D index outcome. The proportion of replicates for which the net monetary benefit was positive was graphed over a series of WTP values from £0 to £50,000 to produce CEACs. The current NICE WTP threshold for the adoption of new technologies is between £20,000 and £30,000 per QALY. 77
A sensitivity analysis explored the impact on societal costs of valuing time spent by caregivers in providing care to the person with dementia at replacement cost (the hourly cost of a home care worker) at 104 weeks.
Results
Sample numbers
The flow of dyads who completed assessments is given in Figure 10. As can be seen, some dyads declined to participate in full assessments involving the completion of participant-/proxy-/caregiver-reported measures, but agreed to remain in the study and provide more limited information on community residence and SAEs or AEs (by telephone follow-up). There were 412 dyads who participated in the baseline and at least one follow-up assessment. A small number (intervention, n = 11; control, n = 14) did not participate in an assessment at any point and 45 (intervention, n = 19; control, n = 26) participated only at baseline. A substantial proportion of the 12-week follow-up assessments were not conducted: 20% in the intervention group and 17% in the control group. The numbers of dyads contributing data to the cost-effectiveness analyses varied depending on the measures and the analysis; valid numbers of observations associated with each measure are presented with the results of the analyses (see Cost-effectiveness analyses). Demographic characteristics of the sample participating in full assessments at baseline are given in Appendix 4.
FIGURE 10.
The ATTILA trial flow of dyads. Delayed: researcher noted that the assessment had been delayed enough that the date of the assessment was closer to that of the next scheduled assessment than to that of the previous assessment or randomisation screening visit.
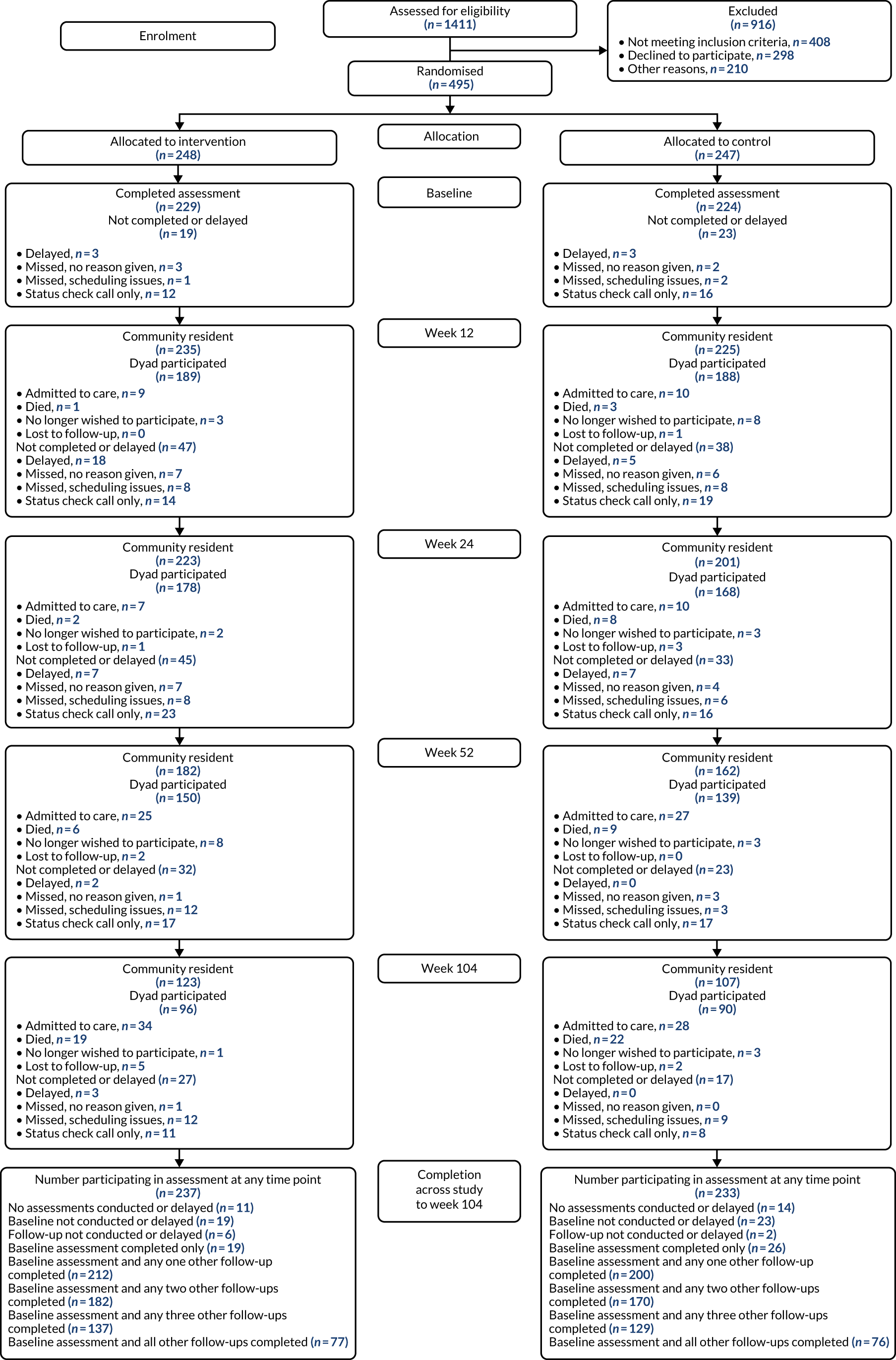
Use of care and support services
Baseline
The participant use of community health and social care services at baseline was very high (see Appendix 4): 69% of intervention and 65% of control participants had seen a general practitioner (GP) in the previous 3 months. Practice nurses were seen by 38% of participants in both groups. Thirteen per cent of intervention and 17% of control group participants had used emergency department services. In terms of inpatient stays, 10% of intervention and 16% of control group participants had had a spell in hospital prior to baseline; the mean number of inpatient days among control group participants was twice that among intervention group participants [2.39 days (SD 0.57 days) vs. 1.24 days (SD 0.35 days), respectively]. In terms of outpatient attendances, 43% of intervention and 41% of control group participants had had at least one outpatient attendance. Use of community rehabilitation professionals, particularly occupational therapists, was noticeably higher at baseline than at the other time points. This suggests that some initial ATT assessment-related visits were being reported as being community rehabilitation-related visits or that involvement in the trial in some other way stimulated referrals to these services. One-third of participants had seen a social worker or care manager over the previous 3 months and 40% of participants received home care (an average of 57 and 60 visits were received among intervention and control group participants, respectively). Day centre use was reported by approximately one-sixth of participants. Almost all caregivers reported providing care to participants over the previous 3 months. A mean of 564 hours of unpaid care was provided to participants in the intervention group, and a mean of 661 hours of unpaid care was provided to participants in the control group.
In terms of ATT devices (of any type, including those defined as ‘basic’, and rounding to whole numbers), intervention participants had three ATT devices whereas control participants had two ATT devices.
Follow-up time points
Over the follow-up assessments, the proportion reporting GP visits declined slightly, then increased to around baseline levels at 104 weeks (71% and 65% for the intervention and control groups, respectively). Practice nurses were seen by 30% of both groups at 104 weeks. About one-sixth of participants used emergency department services across all follow-ups. The numbers of home care visits and the total duration (in hours) of visits rose steadily in each group over the follow-up period. At 104 weeks, 49% of intervention participants and 56% of control participants remaining in the community received home care; these users received very substantial numbers of visits (98 and 110 visits in the intervention and control groups, respectively), indicating the receipt of multiple visits per day. Few received any other community social services, such as meals on wheels. Day centres were used by between one-quarter and one-fifth of participants at the 52- and 104-week follow-ups. As at baseline, most caregivers reported providing care to participants over the previous 3 months. At 104 weeks, caregivers had provided 656 hours of care to intervention group participants, and 777 hours of care to control group participants, over the previous 3 months. Control group participants had received somewhat more care hours than intervention group participants at all follow-ups.
In terms of ATT devices of any type, intervention group participants had three ATT devices at 12 and 24 weeks and four devices at 52 and 104 weeks; control group participants had two devices at 12 and 24 weeks and three devices at 52 and 104 weeks.
Outcomes
The mean EQ-5D participant-reported index scores (Table 15) were higher than the proxy-reported scores at all time points. The mean participant-reported baseline and week 12 scores were similar between groups, but at 24, 52 and 104 weeks, the mean scores for the intervention group were significantly lower than the scores for the control group (p < 0.05 in each case). The mean scores of the proxy-reported measures did not differ between groups.
| Outcome measure | Trial arm | Intervention – control, mean difference (95% CI) in score | p-value | |||
|---|---|---|---|---|---|---|
| Intervention | Control | |||||
| Responses received (n) | Mean (SE) score | Responses received (n) | Mean (SE) score | |||
| Baseline | Expected = 229 | Expected = 224 | ||||
| EQ-5D – participant | 208 | 0.748 (0.016) | 199 | 0.774 (0.016) | –0.026 (–0.070 to 0.018) | 0.2452 |
| EQ-5D – proxy | 208 | 0.539 (0.015) | 197 | 0.526 (0.018) | 0.014 (–0.032 to 0.060) | 0.5616 |
| Week 12 | Expected = 189 | Expected = 188 | ||||
| EQ-5D – participant | 175 | 0.734 (0.019) | 161 | 0.767 (0.017) | –0.033 (–0.084 to 0.018) | 0.2031 |
| EQ-5D – proxy | 178 | 0.551 (0.017) | 178 | 0.512 (0.019) | 0.039 (–0.011 to 0.088) | 0.1248 |
| Week 24 | Expected = 178 | Expected = 168 | ||||
| EQ-5D – participant | 157 | 0.731 (0.02) | 143 | 0.785 (0.019) | –0.054 (–0.108 to 0.001) | 0.055 |
| EQ-5D – proxy | 172 | 0.512 (0.019) | 158 | 0.517 (0.019) | –0.006 (–0.059 to 0.048) | 0.8371 |
| Week 52 | Expected = 150 | Expected = 139 | ||||
| EQ-5D – participant | 120 | 0.709 (0.023) | 104 | 0.787 (0.02) | –0.079 (–0.139 to –0.018) | 0.0112 |
| EQ-5D – proxy | 144 | 0.482 (0.023) | 129 | 0.48 (0.022) | 0.001 (–0.062 to 0.065) | 0.9643 |
| Week 104 | Expected = 96 | Expected = 90 | ||||
| EQ-5D – participant | 75 | 0.73 (0.03) | 59 | 0.818 (0.026) | –0.088 (–0.169 to –0.008) | 0.0321 |
| EQ-5D – proxy | 92 | 0.462 (0.029) | 84 | 0.429 (0.029) | 0.032 (–0.048 to 0.113) | 0.4305 |
Costs of care and support services
Baseline
Baseline costs (unadjusted for covariates) over the previous 3 months (Table 16) were similar between the groups except for hospital costs, which were significantly higher for the control group (–£518, 95% CI –£1025 to –£12; p = 0.045). The costs of ATT were £85 (SE £2) and £75 (SE £2) for the intervention and control groups, respectively. Average health and social care costs (including the intervention) were £2276 for the intervention group and £3400 for the control group. Societal costs were more than double those of health and social care in both arms.
| Cost | Trial arm | Intervention – control, mean difference (95% CI) | |||
|---|---|---|---|---|---|
| Intervention | Control | ||||
| Responses received (n) | Mean (SE) cost | Responses received (n) | Mean (SE) cost | ||
| Baseline | Expected = 229 | Expected = 224 | |||
| Hospital | 229 | 619 (130) | 223 | 1138 (225) | –518* (–1025 to –12) |
| Primary and community health | 229 | 253 (18) | 223 | 227 (18) | 26 (–25 to 77) |
| Community mental health | 227 | 62 (7) | 223 | 51 (7) | 11 (–8 to 30) |
| Respite residential/nursing | 228 | 35 (19) | 223 | 83 (40) | –48 (–135 to 39) |
| Community care | 224 | 1433 (299) | 220 | 1813 (405) | –380 (–1367 to 608) |
| Day care (any provider) | 229 | 153 (36) | 223 | 127 (23) | 27 (–58 to 111) |
| Equipment and adaptationsa | 218 | 4 (1) | 203 | 6 (1) | –2 (–5 to 2) |
| Medications | 226 | 23 (5) | 222 | 23 (5) | –1 (–14 to 12) |
| Unpaid careb | 217 | 5928 (488) | 202 | 6553 (473) | –625 (–1965 to 715) |
| Equipment and adaptations – selfc | 218 | 2 (1) | 203 | 2 (1) | 0 (–3 to 3) |
| Out of pocketd | 219 | 8 (2) | 202 | 4 (1) | 3 (–0 to 7) |
| Health and social care | 210 | 2231 (228) | 201 | 3281 (521) | –1050 (–2152 to 52) |
| Intervention | 223 | 85 (2) | 203 | 75 (2) | 11*** (5 to 16) |
| Intervention + health and social care | 205 | 2276 (228) | 189 | 3400 (550) | –1124 (–2262 to 14) |
| Societale | 208 | 8162 (540) | 200 | 9836 (680) | –1674 (–3374 to 26) |
| Intervention + societale | 203 | 8262 (546) | 188 | 9963 (715) | –1701 (–3454 to 52) |
| Week 12 | Expected = 189 | Expected = 188 | |||
| Hospital | 189 | 467 (121) | 186 | 623 (148) | –156 (–532 to 220) |
| Primary and community health | 188 | 223 (21) | 185 | 231 (23) | –8 (–70 to 54) |
| Community mental health | 186 | 36 (8) | 186 | 38 (8) | –1 (–24 to 21) |
| Respite residential/nursing | 188 | 45 (26) | 185 | 82 (31) | –38 (–117 to 41) |
| Community care | 188 | 1857 (377) | 185 | 2060 (508) | –203 (–1445 to 1038) |
| Day care | 189 | 229 (45) | 186 | 185 (39) | 44 (–72 to 160) |
| Equipment and adaptationsa | 186 | 4 (1) | 184 | 5 (2) | –1 (–6 to 3) |
| Medications | 189 | 34 (9) | 186 | 26 (5) | 8 (–12 to 28) |
| Unpaid careb | 186 | 6214 (470) | 183 | 6928 (547) | –714 (–2129 to 702) |
| Equipment and adaptations – selfc | 186 | 2 (1) | 184 | 4 (2) | –2 (–6 to 2) |
| Out of pocketd | 188 | 7 (2) | 184 | 6 (2) | 1 (–5 to 7) |
| Health and social care | 182 | 2930 (416) | 181 | 2986 (457) | –57 (–1271 to 1158) |
| Intervention | 188 | 61 (3) | 166 | 39 (3) | 21*** (13 to 29) |
| Intervention + health and social care | 181 | 2978 (418) | 161 | 3005 (501) | –27 (–1301 to 1247) |
| Societale | 182 | 9202 (620) | 180 | 10,010 (679) | –807 (–2616 to 1001) |
| Intervention + societale | 181 | 9283 (624) | 160 | 10,017 (734) | –734 (–2616 to 1148) |
| Week 24 | Expected = 178 | Expected = 168 | |||
| Hospital | 177 | 296 (73) | 168 | 848 (274) | –552* (–1098 to -7) |
| Primary and community health | 177 | 193 (20) | 168 | 215 (31) | –22 (–94 to 50) |
| Community mental health | 177 | 21 (4) | 168 | 25 (5) | –4 (–16 to 9) |
| Respite residential/nursing | 175 | 35 (21) | 166 | 37 (26) | –2 (–67 to 64) |
| Community care | 176 | 2475 (537) | 165 | 2005 (439) | 469 (–906 to 1844) |
| Day care | 177 | 230 (48) | 167 | 181 (36) | 49 (–70 to 168) |
| Equipment and adaptationsa | 176 | 7 (2) | 168 | 5 (2) | 1 (–4 to 7) |
| Medications | 177 | 26 (5) | 168 | 22 (5) | 3 (–10 to 17) |
| Unpaid careb | 175 | 6843 (575) | 168 | 7352 (592) | –510 (–2133 to 1113) |
| Equipment and adaptations – selfc | 176 | 3 (2) | 168 | 1 (1) | 2 (–1 to 6) |
| Out of pocketd | 177 | 6 (2) | 168 | 7 (3) | –2 (–8 to 5) |
| Health and social care | 173 | 3298 (560) | 162 | 3289 (531) | 9 (–1513 to 1532) |
| Intervention | 176 | 55 (3) | 157 | 43 (3) | 13** (5 to 20) |
| Intervention + health and social care | 171 | 3382 (566) | 151 | 3358 (554) | 24 (–1542 to 1591) |
| Societale | 172 | 9954 (769) | 162 | 10637 (766) | –683 (–2820 to 1454) |
| Intervention + societale | 170 | 10,032 (778) | 151 | 10,567 (797) | –535 (–2731 to 1660) |
| Week 52 | Expected = 150 | Expected = 139 | |||
| Hospital | 148 | 470 (149) | 137 | 786 (177) | –316 (–769 to 137) |
| Primary and community health | 148 | 195 (18) | 137 | 266 (32) | –71 (–143 to 0) |
| Community mental health | 148 | 28 (18) | 137 | 17 (5) | 10 (–28 to 48) |
| Respite residential/nursing | 148 | 60 (37) | 137 | 94 (45) | –34 (–148 to 80) |
| Community care | 148 | 3377 (747) | 137 | 2206 (462) | 1171 (–590 to 2933) |
| Day care | 148 | 361 (72) | 137 | 212 (44) | 149 (–21 to 318) |
| Equipment and adaptationsa | 148 | 8 (2) | 137 | 5 (2) | 3 (–3 to 9) |
| Medications | 147 | 25 (5) | 137 | 22 (6) | 2 (–13 to 17) |
| Unpaid careb | 147 | 6851 (560) | 136 | 9002 (793) | –2151* (–4040 to –262) |
| Equipment and adaptations – selfc | 148 | 6 (2) | 137 | 3 (2) | 3 (–3 to 9) |
| Out of pocketd | 147 | 6 (2) | 136 | 7 (3) | –2 (–8 to 5) |
| Health and social care | 147 | 4510 (777) | 137 | 3608 (523) | 901 (–970 to 2773) |
| Intervention | 146 | 64 (3) | 129 | 50 (3) | 15*** (6 to 23) |
| Intervention + health and social care | 143 | 4613 (797) | 129 | 3615 (548) | 998 (–947 to 2942) |
| Societale | 146 | 11,442 (927) | 136 | 12,629 (972) | –1187 (–3830 to 1457) |
| Intervention + societale | 143 | 11,492 (947) | 128 | 12,526 (1010) | –1033 (–3757 to 1690) |
| Week 104 | Expected = 96 | Expected = 90 | |||
| Hospital | 93 | 430 (186) | 89 | 767 (202) | –337 (–877 to 203) |
| Primary and community health | 93 | 227 (26) | 89 | 283 (44) | –56 (–155 to 43) |
| Community mental health | 93 | 4 (2) | 89 | 10 (3) | –6 (–14 to 2) |
| Respite residential/nursing | 93 | 3 (3) | 89 | 69 (33) | –66* (–130 to –3) |
| Community care | 93 | 4537 (1264) | 87 | 3062 (754) | 1475 (–1478 to 4427) |
| Day care | 93 | 365 (98) | 89 | 285 (70) | 80 (–159 to 319) |
| Equipment and adaptationsa | 93 | 9 (3) | 89 | 3 (1) | 7* (0 to 13) |
| Medications | 93 | 21 (6) | 89 | 18 (5) | 4 (–11 to 19) |
| Unpaid careb | 93 | 7308 (781) | 89 | 7672 (753) | –364 (–2508 to 1779) |
| Equipment and adaptations – selfc | 93 | 3 (2) | 89 | 2 (1) | 1 (–4 to 6) |
| Out of pocketd | 93 | 6 (3) | 89 | 9 (4) | –3 (–12 to 6) |
| Health and social care | 93 | 5693 (1300) | 87 | 4599 (811) | 1094 (–1978 to 4166) |
| Intervention | 93 | 63 (4) | 87 | 51 (4) | 12* (2 to 23) |
| Intervention + health and social care | 92 | 5808 (1314) | 84 | 4728 (838) | 1080 (–2060 to 4220) |
| Societale | 93 | 12,961 (1599) | 87 | 12,347 (1013) | 614 (–3178 to 4406) |
| Intervention + societale | 92 | 13,117 (1614) | 84 | 12,535 (1045) | 582 (–3291 to 4455) |
Follow-up time points
The 3-month hospital costs were higher in the control group than in the intervention group at the 24-week follow-up (–£552, 95% CI –£1098 to –£7; p = 0.047). Average health and social care costs (including the intervention) were similar to those prior to baseline at the first two follow-ups. Costs in the intervention sample remaining in the community were 36% higher at 52 weeks than at the 24-week point and 72% higher at 104 weeks than at the 24-week point; costs of controls were 8% higher at 52 weeks than at the 24-week point and 41% higher at 104 weeks than at 24 weeks. The 3-month costs of unpaid care were higher in the control group than in the intervention group at 52 weeks (–£2151, 95% CI –£4040 to –£262; p = 0.026). Societal costs at 12 weeks were approximately 13% higher than baseline in the intervention group, but had changed little in the control group. Societal costs at 52 weeks were 15% higher than at 24 weeks in the intervention group, and 18% higher than at 24 weeks in the control group. At 104 weeks, the societal costs of intervention group participants were 14% higher than at 52 weeks, whereas the societal costs of control group participants were similar to those at 52 weeks.
The costs of ATT devices varied little among the groups over the follow-ups. Unsurprisingly, the 3-month costs of the full ATT package were significantly higher than those of the basic package at each time point; however, the additional cost was low in absolute terms (ranging between £11 at baseline and £21 at week 12).
Estimated costs for intervals not covered by the CSRI are given in Appendix 5. The raw mean cumulative costs (see Appendix 6) of ATT over the 2-year follow-up were estimated to be £322 (SE £18) in the intervention group and £214 (SE £16) in the control group. The raw mean cumulative costs of ATT over the baseline and follow-up period were estimated at £408 (SE £18) in the intervention group and £288 (SE £16) in the control group (a difference of £119, 95% CI £71 to £168; p = 0.000). Cumulative costs were estimated at £19,649 (SE £3206) and £15,186 (SE £2102) in the intervention and control groups, respectively.
Cost-effectiveness analyses
Model results: outcomes and costs
Institutionalisation-free days
The regression estimate of the between-group difference in days lived in the community to 104 weeks was 7.9 days (95% CI –26.2 to 42.2 days) (Table 17). Adjusted mean days in the intervention group were 597 and 589 in the control group (approximately 85 weeks and 84 weeks, respectively). Total health and social care costs over 104 weeks were £909 lower in the intervention group, but with very wide CIs that crossed zero (95% CI –£5336 to £3345), indicating that the costs did not differ between the intervention and control groups. Adjusted mean health and social care costs per participant over 2 years were £20,616 (95% CI £16,229 to £26,708) in the intervention group and £21,252 (95% CI £17,134 to £28,435) in the control group.
| Trial arm, mean (95% CIa) | Intervention – control, mean difference (95% CIa) | Cost (£) per institutionalisation-free daya | ||
|---|---|---|---|---|
| Intervention | Control | |||
| Institutionalisation-free days (n) | 597.075 (572.464 to 620.939) | 589.177 (563.373 to 614.062) | 7.898 (–26.438 to 42.425) | – |
| Health and social care costs (£) | 20,616 (16,229 to 26,708) | 21,525 (17,134 to 28,435) | –909 (–5336 to 3345) | –909/7.898 = –115 |
| Societal costs (£) | 56,770 (50,175 to 64,584) | 60,316 (52,647 to 69,843) | –3545 (–13,914 to 6581) | –3545/7.898 = –449 |
EuroQol-5 Dimensions
Participant reported
Difference-in-difference coefficients and bootstrapped CIs are presented in Table 18. Model estimates of group means, within-group differences from baseline to follow-up and model-based SEs are presented in Table 17 and in Appendix 7, Tables 30–35.
| Time point | Difference | 95% CIa | ICERb (difference in costs/MCID) |
|---|---|---|---|
| 24 weeks | |||
| Participant reported (n = 287) | |||
| EQ-5Dc score | –0.011 | –0.052 to 0.028 | 367/–0.148 = –2475 |
| Health and social cared costs (£) | 367 | –850 to 1474 | |
| Participant reported (n = 284) | |||
| EQ-5Dc score | –0.015 | –0.056 to 0.024 | 251/–0.204 = –1231 |
| Societald costs (£) | 251 | –1164 to 2005 | |
| Proxy reported (n = 309) | |||
| EQ-5Dc score | 0.034 | –0.007 to 0.074 | 313/0.463 = 677 |
| Health and social cared costs (£) | 313 | –949 to 1313 | |
| Proxy reported (n = 308) | |||
| EQ-5Dc score | 0.033 | –0.008 to 0.073 | 110/0.448 = 246 |
| Societald costs (£) | 110 | –1569 to 1630 | |
| 52 weeks | |||
| Participant reported (n = 229) | |||
| EQ-5Dc score | –0.056 | –0.617 to 0.5 | 534/–0.004 = –9595 |
| Health and social cared costs (£) | 534 | –748 to 2082 | |
| Participant reported (n = 227) | |||
| EQ-5Dc score | –0.105 | –0.675 to 0.441 | 116/–0.008 = –1103 |
| Societald costs (£) | 116 | –1765 to 2185 | |
| Proxy reported (n = 257) | |||
| EQ-5Dc score | 0.027 | –0.015 to 0.068 | 442/0.360 = 1226 |
| Health and social cared costs (£) | 442 | –926 to 1502 | |
| Proxy reported (n = 257) | |||
| EQ-5Dc score | 0.027 | –0.015 to 0.068 | –220/0.360 = –611 |
| Societald costs (£) | –220 | –2175 to 1443 | |
| 104 weeks | |||
| Participant reported (n = 243) | |||
| EQ-5Dc score | –0.016 | –0.056 to 0.021 | 698/–0.217 = –3221 |
| Health and social cared costs (£) | 698 | –919 to 2309 | |
| Participant reported (n = 243) | |||
| EQ-5Dc score | –0.019 | –0.06 to 0.017 | 153/–0.262 = –584 |
| Societald costs (£) | 153 | –1969 to 2248 | |
| Proxy reported (n = 266) | |||
| EQ-5Dc score | 0.021 | –0.022 to 0.06 | 478/0.281 = 1699 |
| Health and social cared costs (£) | 478 | –938 to 1946 | |
| Proxy reported (n = 266) | |||
| EQ-5Dc score | 0.021 | –0.022 to 0.06 | –32/0.281 = –113 |
| Societald costs (£) | –32 | –1956 to 1908 | |
At the 24-week follow-up, the between-group difference in change from baseline EQ-5D scores was positive in both groups, but the change was larger in the control group than in the intervention group. At the 52-week follow-up, the between-group difference in change from baseline to follow-up EQ-5D scores was negative because, although changes were negative in both groups, the change was larger in the intervention than in the control group. At the 104-week follow-up, the between-group difference in change from baseline to follow-up EQ-5D scores was negative because the change was negative in the intervention group and positive in the control group. However, at each time point, the bootstrapped CIs of the difference-in-difference coefficient crossed zero, indicating no significant difference in between-group change in outcome. The difference between groups in the change in 3-month costs from baseline to follow-up was positive at each of these time points, but bootstrapped CIs of the difference-in-difference coefficient again crossed zero. Health and social care costs and societal 3-month costs were (non-significantly) higher in the intervention group than in the control group.
Proxy reported
At the 24-, 52- and 104-week follow-ups, the between-group difference in change from baseline scores (see Table 18) was positive, but CIs of the difference crossed zero, indicating no difference between groups in this outcome. At all time points, proxy-rated EQ-5D scores were lower than participant-rated scores.
The difference in average follow-up health and social care 3-month costs from baseline were (non-significantly) higher in the intervention group than in the control group. The difference in average follow-up societal 3-month costs from baseline were slightly (non-significantly) lower in the intervention group than in the control group (reflecting the larger size of the sample with proxy-reported outcomes available than on the participant-reported measure).
Quality-adjusted life-years
Results are given in Tables 19 and 20.
| Time point | Trial arm, mean (95% CIa) | Intervention – control, mean difference (95% CIa) | |
|---|---|---|---|
| Intervention | Control | ||
| 24 weeks | |||
| Costs (£) | |||
| Health and social care | 4455 (3428 to 5883) | 4964 (3672 to 6975) | –509 (–1883 to 579) |
| Societal | 14,109 (12,251 to 15,738) | 15,368 (13,083 to 18,233) | –1258 (–4680 to 1461) |
| QALY (EQ-5D-5L) | |||
| Participant reported | 0.334 (0.319 to 0.348) | 0.350 (0.336 to 0.364) | –0.016 (–0.036 to 0.003) |
| Proxy reported | 0.245 (0.231 to 0.258) | 0.234 (0.220 to 0.248) | 0.010 (–0.009 to 0.029) |
| 52 weeks | |||
| Costs (£) | |||
| Health and social care | 9363 (7296 to 11,999) | 10,122 (7843 to 13,332) | –759 (–3109 to 1430) |
| Societal | 28,180 (24,733 to 31,839) | 29,293 (25,648 to 33,414) | –1114 (–6186 to 3701) |
| QALY (EQ-5D-5L) | |||
| Participant reported | 0.680 (0.646 to 0.712) | 0.724 (0.692 to 0.754) | –0.044 (–0.088 to –0.000) |
| Proxy reported | 0.485 (0.453 to 0.516) | 0.470 (0.439 to 0.499) | 0.016 (–0.026 to 0.057) |
| 104 weeks | |||
| Costs (£) | |||
| Health and social care | 20,524 (16,109 to 26,413) | 21,602 (17,234 to 28,395) | –1078 (–5648 to 3062) |
| Societal | 56,745 (50,085 to 64,530) | 60,399 (52,804 to 69,823) | –3654 (–13,884 to 6316) |
| QALY (EQ-5D-5L) | |||
| Participant reported | 1.201 (1.127 to 1.271) | 1.306 (1.234 to 1.376) | –0.105 (–0.204 to –0.007) |
| Proxy reported | 0.828 (0.762 to 0.894) | 0.798 (0.733 to 0.861) | 0.030 (–0.058 to 0.117) |
| Time point and costs | ICER (£) intervention over controla | |
|---|---|---|
| QALYs derived from participant-reported EQ-5D | QALYs derived from proxy-reported EQ-5D | |
| 24 weeks | ||
| Health and social care | –509/–0.016 = 31,561 | –509/0.010 = –49,656 |
| Societal | –1258/–0.016 = 77,994 | –1258/0.010 = –122,711 |
| 52 weeks | ||
| Health and social care | –759/–0.044 = 17,279 | –759/0.016 = –48,861 |
| Societal | –1114/–0.044 = 25,339 | –1114/0.016 = –71,654 |
| 104 weeks | ||
| Health and social care | –1078/–0.105 = 10,237 | –1078/0.030 = –35,432 |
| Societal | –3654/–0.105 = 34,706 | –3654/0.030 = –120,125 |
Derived from the participant-rated EuroQol-5 Dimensions
At 24 weeks, in terms of QALYs derived from the participant-rated EQ-5D, the intervention group had 0.016 fewer QALYs than the control group; the CIs of the difference crossed zero, indicating that the groups did not differ on this outcome. At 52 weeks, in terms of QALYs derived from the participant-rated EQ-5D, the intervention group had 0.044 fewer QALYs than the control group; the CIs did not cross zero, although the upper confidence limit was very close to it (p = 0.0498). At 104 weeks, in terms of QALYs derived from the participant-rated EQ-5D, the intervention group had 0.105 fewer QALYs than the control group, and the CIs did not cross zero. Thus, at 52 and 104 weeks, the intervention group participants had a worse outcome on this measure than the control group participants.
Derived from the proxy-rated EuroQol-5 Dimensions
There were small gains in QALYs in the intervention group at 24, 52 and 104 weeks (of 0.01, 0.016 and 0.03, respectively), but the CIs of the differences crossed zero in each case, indicating that the groups did not differ on this outcome.
Incremental cost-effectiveness ratios and probability of cost-effectiveness
Institutionalisation-free days
At 104 weeks, the cost per institutionalisation-free day from either health and social care or societal perspectives was negative (see Table 17) because the point estimate for cost difference (from either perspective) was negative and the point estimate for institutionalisation was positive. The CEAC indicates that there is no WTP value in the range of WTP values of between £0 and £50,000 at which we could be certain that either the basic or full ATT package was better value for money (see Appendix 7, Figure 13), as the probability of cost-effectiveness did not exceed 72% (health and social care) and 80% (societal perspective) over that range.
Proxy-reported EuroQol-5 Dimensions
At the 24-week point, on the proxy-reported EQ-5D (see Table 18), the ICERs for health and social care costs and societal costs were positive because the estimates of both costs and outcomes differences were positive. The CEAC (see Appendix 7, Figure 14) indicates that the probability of cost-effectiveness on achieving a minimal clinically important difference from either perspective is approximately 90% at a WTP of £5200 and just under 95% across WTP values of between £25,700 and £50,000. We cannot, however, be confident from either perspective at the 95% level that the full ATT package strategy is more cost-effective than the basic ATT package over the range. 80 At the later time points, the ICERs from the health and social care perspective were positive (small positive differences in outcomes and costs), and from the societal perspective they were negative (small positive differences in outcomes and small negative differences in costs). At 52 weeks, the probability of cost-effectiveness (see Appendix 7, Figure 15) did not exceed 90% from either perspective over a range of WTP of between £15,000 and £50,000; at 104 weeks, the probability of cost-effectiveness did not exceed 84% from either perspective over this range (see Appendix 7, Figure 16). As with the 24-week results, we cannot be confident from either perspective at the 95% level that the full ATT package strategy is more cost-effective than the basic ATT package over this range. The probability of cost-effectiveness at WTP values of £20,000 was highest in the short term (24 weeks) and lowest in the long term (104 weeks), from both perspectives.
Participant-reported EuroQol-5 Dimensions
In the case of ICERs produced for participant-reported EQ-5D (see Table 18) the outcome was (non-significantly) worse in the intervention group and costs were (non-significantly) higher or lower in the intervention group, depending on perspective and time horizon. The CEACs have not been presented for this reason.
Quality-adjusted life-years
Derived from the proxy-rated EuroQol-5 Dimensions
At the 24-, 52- and 104-week time points, in terms of the QALYs generated from the proxy-reported EQ-5D (see Table 19), the ICERs for health and social care costs and societal costs were negative because of negative cost difference estimates and positive QALY difference estimates. The CEAC (see Appendix 7, Figure 17) indicates that the probability of cost-effectiveness was highest at the 24-week point from the health and social care perspective, but this did not exceed 90% across a range of WTP values of £0–50,000. Curves for the later time points were lower than those at 24 weeks, with the 104-week probabilities being the lowest (not exceeding 82%, from the societal perspective, over the £0–50,000 range).
Derived from the participant-rated EuroQol-5 Dimensions
The ICERs for QALYs based on the participant-reported EQ-5D were positive, but this was because the costs were slightly lower and the outcomes somewhat worse in the intervention group than in the control group. The CEAC is not presented for this reason.
Sensitivity analysis: replacement cost of unpaid care
The impact on societal costs and the ICER of valuing unpaid care at replacement cost at 104 weeks was explored in a sensitivity analysis. The costs of unpaid care and societal and intervention costs using replacement costs are presented in Appendix 7, Table 36. Cost-effectiveness analysis results are presented in Appendix 7, Tables 37–39.
Institutionalisation-free days
Valuation of caregiver time at replacement cost resulted in a smaller difference between groups in total societal costs over 104 weeks, but, as with the main results, the 95% CIs of the difference crossed zero. The ICER remained negative, in line with the main results.
EuroQol-5 Dimensions
Valuation of caregiver time at replacement cost yielded larger estimates of the difference-in-difference between groups in societal costs. For the participant-reported EQ-5D, this yielded a larger negative ICER (because the difference-in-difference in cost was non-significantly positive and the outcome was non-significantly worse in the intervention group than in the control group); for the proxy-completed EQ-5D, this also yielded a larger negative ICER (because the difference-in-difference in cost was non-significantly negative and because the outcome was non-significantly better in the intervention group than in the control group). The results were in line with the main results valuing caregiver time at opportunity cost.
Quality-adjusted life-years
As with the main results, the ICER for QALYs based on the participant-reported EQ-5D remained positive (with non-significantly lower total costs and with fewer QALYs for the intervention group than the control group), but was lower (approximately half). The ICER for QALYs based on the proxy-reported EQ-5D remained negative (with non-significantly lower total costs and with non-significantly more QALYs for the intervention group than the control group) and was lower (approximately half).
Discussion
The additional 3-month cost of a full ATT package over a basic package at each time point was modest (ranging from £11 at baseline to £21 at week 12). Although the cumulative cost estimated for the full package was significantly greater than that of the basic package (an additional £119), the average costs of ATT were modest in both the intervention and control groups (£408 and £288, respectively). Mean (unadjusted) societal costs were in the order of three times that of health and social care costs. Total health and social care and societal costs over 104 weeks, taking censoring into account, did not differ between groups. Average 3-month costs estimated in difference-in-difference analyses did not differ between groups at 24, 52 or 104 weeks. Change in proxy-rated and participant-rated EQ-5D index scores estimated in difference-in-difference analyses also did not differ between groups at those time points. For the institutionalisation-free days outcome, there was no statistical difference between the intervention and control groups in days spent in the community. Groups did not differ on proxy-reported QALYs. The mean participant-reported QALY was lower in the intervention group than in the control group at the 52- and 104-week time points. The probability of cost-effectiveness on a measure of change in proxy-rated health-related quality of life was greater in the short term than in the long term from the health and social care and societal perspectives, at a WTP of £20,000. A similar pattern was seen in proxy-rated QALYs from the health and social care perspective, but the probability of cost-effectiveness was higher in the long term from the societal perspective.
Some limitations must be acknowledged. Costs were measured by asking caregivers to retrospectively report service use, so the results could be subject to recall bias. The costs accrued during the intervals between periods with CSRI data available were estimated by carrying forward most costs, although the costs of inpatient and emergency department use were estimated from the SAE data available over these times. Costs of regularly used services were thus assumed to be constant over these intervening periods. Participant-reported EQ-5D data were missing for one-quarter of intervention group participants and for one-third of control group participants who had participated in assessments at 104 weeks. Although difference-in-difference analyses controlled for baseline covariates (stratification variables and dependency, as measured by the BADLS) that might have accounted for differences in data availability between groups on this measure, QALY analyses drew on group mean utilities at each time point and did not adjust for baseline characteristics. Therefore, the finding that the intervention group had fewer QALYs derived from participant-reported EQ-5D must be interpreted with some caution as substantial rates of missingness in that measure could be a concern. Generalisability of these findings may also be limited because all assessment data were missing from some participating dyads at the baseline and follow-up points (8% of the sample at baseline and between 16% and 19% of the community-dwelling sample still participating in the study over the follow-ups did not participate in an interview).
The study sample size was powered to detect a delay to entry to permanent care home residence of 55 days over 24 months. As can be seen from the measures of uncertainty around all point estimates of cost differences, there was substantial variability in the cost data (as might be expected). It is possible that, because the variance around the mean difference in costs was of a greater magnitude than the variance around the survival outcome, a larger sample size would have been required to detect a between-group difference in costs than in days.
The mean EQ-5D proxy-rated index scores were consistently lower than index scores derived from the participant-rated version of the measure. This pattern is in line with that reported in other studies involving people with dementia and caregiver proxy raters. 81 The EQ-5D has been reported to be more reliable than some dementia-specific quality-of-life measures in terms of agreement between caregiver proxy-rated and self-rated total scores, in a population with mild and moderate dementia participating in a psychosocial intervention. 82 The divergence we observed between proxy and participant raters’ EQ-5D and QALY outcomes may relate to selection effects, validity of the instrument when used for people with more advanced dementia and genuine differences in perspectives between raters. 81 The participant–proxy inter-rater reliability of the EQ-5D index scores from this study merits further investigation, as does the response rate of participants at different stages of dementia.
Conclusions
The results suggest that the health and social care and societal costs of a full package of ATT were not greater than those of a basic package of ATT for people with dementia. There was little evidence that the intervention influenced the length of time lived in the community over 104 weeks, improved health-related quality of life as rated by participants and proxies or increased participants’ QALYs based on quality of life as rated by proxies. The results suggest that the intervention reduced participants’ QALYs based on quality of life rated by people with dementia themselves. Generalisability of findings based on the dyad-reported outcomes data is limited by the extent of missing data at the individual outcome measure and at the assessment level.
Chapter 6 Impact of assistive technology for people with dementia on burden and psychological well-being in unpaid caregivers
People living with dementia increasingly depend on informal caregivers who assist with ADL, ensure safety, manage challenging behaviours and provide company to the cared-for person. The majority of caregivers for people with dementia are family members, and approximately 60–70% of them are women. 83 It is estimated that 11% of all caregivers in the UK provide care to someone with dementia in a home setting. 84 As caregivers are frequently middle-aged or older children of the person with dementia, many may be in part- or full-time employment, which results in difficulties in balancing work and care commitments that are likely to diminish their ability to provide care. 85 Moreover, a large number of people providing unpaid care have a long-standing illness or disability themselves. 86
These caregivers are normally untrained to deliver care and the performance of the caring role is a potential source of substantial psychosocial stress. There is some evidence showing that this stress is manifested in some measures of immunity and may also affect cognition. 87 Caregivers report worsening of their mental health and physical health due to their caring role. 88 Importantly, caregivers of people with dementia show higher levels of anxiety and depression than caregivers of other conditions. 89,90 Caring for a person with dementia has been found to adversely affect a caregiver’s financial resources through early retirement, reduced paid working hours or reliance on benefits. 91,92 The physical health of these caregivers is also adversely affected. 93–95
As this care is provided informally and is generally unfunded, the savings to the public purse are extensive. The annual cost of unpaid care in the UK has been estimated to be £11.6B. 96 One study estimated that the total societal worldwide costs of unpaid care for dementia at US$329B per year. 97 This is a rough estimate and assumes informal care time of 3.7 hours per day. It is cheaper to care for people with mild dementia at home (£26,000 per annum) than in residential care (£31,000 per year). 96
‘Carer burden’ refers to ‘the degree to which a carer’s emotional or physical health, social life or financial status had suffered as a result of caring for their relative’;98 it is often used to describe the impact of providing unpaid care. Both patient and caregiver characteristics have been found to contribute to caregiver burden, for example the type of dementia, the extent of personality change, cognitive changes and the presence of behavioural and psychiatric symptoms due to the dementia. Importantly, a systematic review99 confirmed that a person with dementia’s functional status, prevalence of behavioural disturbances and level of neuropsychiatric symptoms, such as wandering and delusions, were found to be the most burdensome characteristics for caregivers. Factors that influence the impact of caregivers include gender; age; cultural values; the nature of the relationship with the person with dementia; the amount of unpaid and paid care available; the caregiver’s physical and mental status, personality and coping strategies; and the duties of caregiving. 99
Nonetheless, it should be acknowledged that providing unpaid care may also benefit these caregivers. For example, there are some reports of caregivers gaining satisfaction by performing the caregiver role and developing a close relationship with the recipient. 100–102 To this end, interventions to support caregivers that aim to minimise the negative impacts and enhance the benefits of the caregiving relationship, both for the cared-for person and the caregiver, have been developed.
A number of psychosocial interventions are specifically directed to caregivers of people with dementia. The bulk of these involve face-to-face delivery. Some other interventions involve telephone delivery. 103 An early systematic review of 40 studies found that the content of these interventions varied a lot. 104 Approximately two-thirds of the interventions did not result in improvements in any outcome measure, but, of those that were successful, the inclusion of a social component, with or without a cognitive component, proved to be important. The small numbers in the studies as well as a number of methodological issues were noted as limitations of the review. 104 It has been reported that, in the 30 years up to 2015, > 200 interventions for caregivers have been tested in randomised trials and found to have some efficacy on caregivers’ outcomes. 105
Some of these have used information and communications technology (ICT) to facilitate collecting, capturing, storing, processing, transmitting, exchanging and presenting information and/or communication. 106,107 A 2019 systematic review108 examined the impact of ICT interventions on dementia caregivers’ outcomes. Of the six studies that used telephone interventions, three resulted in significant effects on a range of caregiver outcomes, including reducing caregivers’ depressive symptoms. Three of the four studies evaluating video interventions had an effect on a range of caregiver outcomes, as did the two computer-based interventions. The authors108 conclude that interventions that use the telephone show the most evidence to support their use. Similarly to interventions directed at the person with dementia, interventions for caregivers can be delivered to the individual, in a group setting, online or to the caregiver–cared-for person dyad. However, the evidence is as yet unclear about which is the most effective delivery method for caregiver outcomes.
An alternative to interventions that are specifically directed to caregivers are interventions that aim to remotely monitor and manage the care recipient. Commonly termed telecare, these interventions involve installing ATT devices to remotely monitor the care recipient. The devices are generally employed to continuously, automatically and remotely monitor for real-time emergencies and lifestyle changes to manage risks of living at home. Although directed at the care recipient, these may also affect caregiver outcomes by improving sleep and reducing worry and stress by preventing serious incidents such as falls, cooking accidents, or wandering. A systematic review109 explored the evidence for the use of these devices on caregiver well-being across several types of care recipient and identified seven studies, all of which were rated as having weak methodological quality. The most common types of equipment were passive sensors such as bed and door sensors, and sensors to monitor home emergencies such as flood and gas alarms. However, a tentative conclusion was that the evidence indicated that telecare exerts a positive effect on caregiver stress and strain. Only one study evaluated the impact of telecare on quality of life110 and one study investigated caregiver burden;111 neither study found evidence for a positive impact of their interventions. Two112,113 of the seven studies in the systematic review109 examined caregivers of people with dementia. Holthe112 reported on 25 caregivers of people with dementia, of whom slightly more than 50% lived with a person being cared for; Woolham113 reported on 123 caregivers. Although limitations of the studies’ methodologies were identified, they reported a positive impact on caregiver stress, suggesting a potential benefit of telecare for caregivers as well as care recipients that is deserving of further investigation.
Aims
We aimed to assess and compare the use of ATT with usual care for people with dementia on caregiver psychosocial and health outcomes, namely caregiver burden, depression and anxiety.
Methods
Design and participants
Participants were caregivers of participants recruited to the main ATTILA RCT, and, as such, were recruited and allocated to their study group according to their care recipient’s randomisation allocation.
Eligibility criteria for the recruited care recipient participants are discussed in detail in Chapter 2. They were adults, and were spouses, partners or children of the care recipient. Caregivers could be resident or non-resident with the trial participant. Caregivers remained in the trial until their care recipient left owing to death or a move to residential care.
Intervention and control conditions
Caregivers were not the direct recipient of the ATT assessment and interventions. The related care recipient received the intervention ATT assessment and installation or control package, randomly allocated as described in Chapter 2.
Recruitment
The recruitment of participants is described in Chapter 2.
Sample size
The sample size was estimated on the expected effect size of the intervention on the primary outcome for the ATT recipients. No required number of participants was identified for the caregiver sample.
Procedure
Outcome rating scales were completed by caregivers at the same time points as data collections for their care recipient: baseline (0 weeks) and 12, 24, 52 and 104 weeks. Participants completed the baseline paper outcome rating scales at home, with or without the assistance of the data collection assistants. Further assessments were mailed to participants or completed at the care recipients’ follow-up appointments.
Data collection
Outcome rating scales relevant to the assessment of caregiver outcomes are reported here.
Descriptive data and covariates
Data about the caregiver, their caring responsibilities, and their relationship to the participant were collected: (1) caregiver age; (2) frequency of caring responsibility – lives with the care recipient, visits once per day or visits fewer times than once per day; and (3) who lived with the care recipient – spouse or partner, care recipient lives alone or other. Data about the severity of the care recipient’s dementia symptoms were captured using the SMMSE. 114
Caregiver outcome data
Data were collected about carer outcomes on three scales:
-
Caregiver burden – the ZBI115 is a 22-item scale assessing the burden of caregiving. Participants respond on a five-point Likert scale ranging from 0 (never) to 4 (always). A single score is calculated by summing the responses to the 22 items. A higher score indicates greater burden, with 0–20 indicating little or no burden, 21–40 indicating mild to moderate burden, 41–60 indicating moderate to severe burden and 61–88 indicating severe burden.
-
Depression – CES-D-10: a 10-item scale. Participants respond on a four-point Likert scale ranging from 0 (rarely/none of the time) to 3 (all of the time). A single score is calculated by reverse-scoring two items and summing the 10 item scores. A score of ≥ 10 indicates depression.
-
STAI-6 – short form of the state scale of the STAI:116 a six-item scale on which participants rate anxiety symptoms on a four-point Likert scale ranging from 1 (not at all) to 4 (very much). Three items are reverse-scored, followed by a sum of items multiplied by 20/6. A single score is calculated ranging from 20–80. A ‘normal’ score is 34–36; higher scores indicate greater anxiety.
It was also planned to use the Short Form questionnaire-12 items to assess quality of life, but there were some difficulties in registering the use of this instrument; therefore, it was not deployed.
Analyses
Data analysis
We analysed the data with IBM SPSS Statistics version 25 (IBM Corporation, Armonk, NY, USA) (alpha level = 0.05). Preliminary analyses provided descriptive details for all the measures at the item or scale level, as appropriate. We explored normality of the data by visually inspecting histograms and conducting the Shapiro–Wilk test of normality. We also conducted a principal component analysis with an oblimin rotation on the ZBI. We used the Kaiser–Meyer–Olkin measure to check the suitability of the data for principal component analysis. We then used a scree plot to determine the number of factors to retain and decided to extract three components (see Appendix 7, Table 40).
Selection of cases for inclusion in analyses
There were several sources of attrition across time points, including loss to follow-up, death and institutionalisation of the care recipient. Because the attrition rate was substantially higher at the last two time points (i.e. weeks 52 and 104), reaching approximately 50% by week 104, than at the first three time points (i.e. baseline and weeks 12 and 24), we excluded the last two time points from the analyses. We conducted our analyses according to the intention-to-treat principle.
Imputation
To account for missing data across demographic variables and outcomes, we conducted multiple imputation for baseline only by including all predictors to fill the missing data. We used data from all time points (baseline, week 12 and week 24) within the same multiple imputation model. We produced 10 imputed data sets; each of the multiply imputed data sets was analysed as usual, after which the 10 sets of results produced for each analysis were combined using Rubin’s rules. 117–119
Descriptive data, randomisation and loss to follow-up analyses
Means and standard deviations (SDs) were calculated for continuous data, and frequencies and percentages were calculated for categorical data. We conducted linear mixed modelling (LMM) to analyse between-group differences, change over time (baseline, week 12, week 24) as well as interaction effects of group and time. An initial set of analyses was conducted to examine the assumption that within-participant scores are highly correlated by calculating the intraclass correlation. The second set of models included covariates. Time was entered as a fixed effect for each linear mixed model, with participants’ identification numbers as a random effect with the default variance components structure.
In addition to the main effects of group and time, the effects of the time–group interaction were examined and interpreted where a significant interaction term indicating differential treatment effectiveness was found. The decomposition of interaction effects for (1) group differences at each time point and (2) changes over time within each group individually were examined. Significant effects were investigated using pairwise comparison with the estimated marginal means. The 95% CI around the estimated marginal means on each outcome for each group were also calculated.
Results
The findings reported here consist of the first three waves of data collection in the ATTILA trial: at baseline and at 12 and 24 weeks. All LMM analyses in each section were adjusted for each of the demographic variables presented in Tables 21 and 22. The tables below (see Tables 23–26) provide the adjusted means of both groups on each outcome variable. We conducted a whole-sample analysis and two secondary subgroup analyses. The results are broken up into three sections:
| Variable | Mean | SEM | 95% CI | p-value |
|---|---|---|---|---|
| Caregiver age | 62.5 | 0.60 | 61.3 to 63.7 | |
| Control | 62.1 | 0.85 | 60.4 to 63.7 | 0.455 |
| Intervention | 63.0 | 0.85 | 61.3 to 64.6 | |
| Care recipient SMMSE score | 17.8 | 0.30 | 17.2 to 18.4 | |
| Control | 17.0 | 0.43 | 16.2 to 17.8 | 0.006 |
| Intervention | 18.6 | 0.41 | 17.8 to 19.4 |
| Variable | Caregiver visits | |
|---|---|---|
| Frequency | % | |
| Living status | ||
| Living alone | 229 | 46.3 |
| Living with spouse/partner | 195 | 39.4 |
| Other | 71 | 14.3 |
| Total | 495 | 100 |
| Caregiver status | ||
| Caregiver visits at least once per day | 121 | 24.4 |
| Caregiver visits less than once per day | 134 | 27.1 |
| Live-in caregiver | 240 | 48.5 |
| Total | 495 | 100 |
-
section 1 – all caregivers in the study
-
section 2 – caregivers who were living with the person with dementia (co-resident)
-
section 3 – caregivers who were partners of the person with dementia (spousal care partners).
Caregiver participants
Of the 495 caregivers in the trial, 354 provided data on age (control group, n = 182; intervention group, n = 172). Therefore, we imputed the remaining 141 missing data for age; SMMSE scores were also imputed. Details of caregivers’ ages, the SMMSE scores of the people with dementia and the living status and frequency of caregiver visits to the person with dementia are provided in Tables 21 and 22.
Section 1: findings for all caregivers
Caregiver burden
The ZBI was analysed as a total score, and into its three component factors following a principal component analysis. The three components were defined as:
-
component 1 – negative appraisal of the care partner role
-
component 2 – adequacy as a care partner
-
component 3 – caregiver burden and strain.
Total scores for the ZBI were not significantly different between the control and intervention groups at week 24. Furthermore, there were no significant within-group or interaction effects across all time points. Similarly, we found no significant between-group differences at week 24 for component 1, 2 or 3. There were no significant within-group or interaction effects for any of the components. Parameter estimates and adjusted mean scores for each group at each time point are presented in Tables 23 and 24.
| Time, group and interaction effects | F-value | df | p-value | |
|---|---|---|---|---|
| 1 | 2 | |||
| Total score | ||||
| Time | 0.472 | 2 | 1438503 | 0.623 |
| Group | 0.036 | 1 | 161355 | 0.849 |
| Interaction | 0.172 | 2 | 2228089 | 0.842 |
| Component 1: negative appraisal of caring | ||||
| Time | 0.127 | 2 | 645845 | 0.881 |
| Group | 0.042 | 1 | 654751 | 0.838 |
| Interaction | 0.200 | 2 | 4649804 | 0.819 |
| Component 2: adequacy as a caregiver | ||||
| Time | 1.259 | 2 | 318819 | 0.284 |
| Group | 0.144 | 1 | 37476 | 0.704 |
| Interaction | 0.653 | 2 | 50769 | 0.520 |
| Component 3: caregiver burden and strain | ||||
| Time | 1.696 | 2 | 250490 | 0.183 |
| Group | 0.030 | 1 | 272088 | 0.863 |
| Interaction | 1.657 | 2 | 578798 | 0.191 |
| Time point | Trial arm | Mean | SE | 95% CI |
|---|---|---|---|---|
| Total score | ||||
| Baseline | Control | 29.6 | 1.36 | 26.9 to 32.3 |
| Intervention | 29.3 | 1.44 | 26.4 to 32.1 | |
| Week 12 | Control | 29.7 | 1.41 | 27.0 to 32.5 |
| Intervention | 30.0 | 1.48 | 27.1 to 32.9 | |
| Week 24 | Control | 30.0 | 1.43 | 27.2 to 32.7 |
| Intervention | 29.7 | 1.48 | 26.7 to 32.6 | |
| Component 1: negative appraisal of caring | ||||
| Baseline | Control | 13.8 | 0.67 | 12.5 to 15.1 |
| Intervention | 14.0 | 0.70 | 12.6 to 15.4 | |
| Week 12 | Control | 14.3 | 0.70 | 13.0 to 15.7 |
| Intervention | 14.2 | 0.73 | 12.8 to 15.6 | |
| Week 24 | Control | 14.3 | 0.70 | 13.0 to 15.7 |
| Intervention | 14.2 | 0.73 | 12.8 to 15.6 | |
| Component 2: adequacy as a caregiver | ||||
| Baseline | Control | 3.8 | 0.25 | 3.3 to 4.3 |
| Intervention | 3.9 | 0.26 | 3.4 to 4.4 | |
| Week 12 | Control | 3.9 | 0.27 | 3.3 to 4.4 |
| Intervention | 4.1 | 0.27 | 3.5 to 4.6 | |
| Week 24 | Control | 3.9 | 0.27 | 3.3 to 4.4 |
| Intervention | 3.7 | 0.28 | 3.2 to 4.3 | |
| Component 3: caregiver burden and strain | ||||
| Baseline | Control | 7.8 | 0.503 | 6.8 to 8.8 |
| Intervention | 7.4 | 0.527 | 6.4 to 8.5 | |
| Week 12 | Control | 7.5 | 0.526 | 6.5 to 8.6 |
| Intervention | 8.0 | 0.544 | 6.9 to 9.1 | |
| Week 24 | Control | 7.7 | 0.532 | 6.6 to 8.7 |
| Intervention | 7.8 | 0.547 | 6.7 to 8.8 | |
Caregiver mood
Total scores for the CES-D-10 (depressed mood) were not significantly different between the control and intervention groups at week 24. There were no significant group or interaction effects across all time points. Similarly, total scores for the STAI-6 (anxiety) were also not significantly different between the control and intervention group at week 24. Group and interaction effects across all time points for the STAI-6 were also not statistically significant. Parameter estimates and adjusted mean scores for each group at each time point are presented in Tables 25 and 26.
| Time, group and interaction effects | F-value | df | p-value | |
|---|---|---|---|---|
| 1 | 2 | |||
| CES-D-10 | ||||
| Time | 1.726 | 2 | 935042 | 0.178 |
| Group | 0.282 | 1 | 341074 | 0.596 |
| Interaction | 0.595 | 2 | 830859 | 0.551 |
| STAI-6 | ||||
| Time | 1.110 | 2 | 4613788 | 0.329 |
| Group | 0.539 | 1 | 2187757 | 0.463 |
| Interaction | 0.713 | 2 | 896778.4 | 0.490 |
| Time point | Trial arm | Mean | SE | 95% CI |
|---|---|---|---|---|
| CES-D-10 | ||||
| Baseline | Control | 9.6 | 0.56 | 8.5 to 10.7 |
| Intervention | 8.7 | 0.59 | 7.5 to 9.8 | |
| Week 12 | Control | 9.8 | 0.59 | 8.6 to 10.9 |
| Intervention | 9.1 | 0.61 | 7.9 to 10.3 | |
| Week 24 | Control | 9.7 | 0.59 | 8.5 to 10.8 |
| Intervention | 9.3 | 0.61 | 8.1 to 10.5 | |
| STAI-6 | ||||
| Baseline | Control | 40.3 | 1.22 | 37.9 to 42.7 |
| Intervention | 39.7 | 1.28 | 37.2 to 42.2 | |
| Week 12 | Control | 39.9 | 1.30 | 37.4 to 42.5 |
| Intervention | 40.2 | 1.34 | 37.6 to 42.8 | |
| Week 24 | Control | 40.1 | 1.33 | 37.5 to 42.7 |
| Intervention | 41.2 | 1.36 | 38.5 to 43.9 | |
Section 2: spousal caregivers who were living with the person with dementia (co-resident)
The sample of caregivers in the ATTILA trial were diverse in their living arrangements with the person with dementia. Some lived in the same home as the person with dementia. Others were resident in proximity, but living separately, and some were located some distance from the person with dementia. As there is some evidence that co-location of the caregivers with the person in receipt of care implies a more involved role in caring for the person with dementia,120 this group was selected for secondary analysis, whereby effects of the telecare intervention on caregivers’ outcomes were examined only for those who were living in the same household. Details of the group in this substudy are presented in Table 27.
| Variable | Mean | SEM | 95% CI | p-value |
|---|---|---|---|---|
| Age (years) | 71.8 | 0.80 | 70.2 to 73.4 | |
| Control | 71.9 | 1.12 | 69.7 to 74.1 | 0.901 |
| Intervention | 71.7 | 1.14 | 69.5 to 73.9 | |
| SMMSE scorea | 17.4 | 0.49 | 16.5 to 18.4 | |
| Control | 17.0 | 0.68 | 15.6 to 18.3 | 0.344 |
| Intervention | 17.9 | 0.71 | 16.5 to 19.3 |
The analyses presented in the following sections mirror those conducted for all caregivers in the previous sections.
Caregiver burden
Total scores for the ZBI were not significantly different between the control and intervention groups at week 24. There were no significant group or interaction effects across all time points. Similarly, we found no significant between-group differences at week 24 for any of the three subcomponents of the ZBI. There were no significant group, time or interaction effects for any of the components. Parameter estimates and adjusted mean scores for each group at each time point are presented in Appendix 7, Tables 41 and 42.
Caregiver mood
Total scores for the CES-D-10 (depressed mood) were not significantly different between the control and intervention groups at week 24. Furthermore, there were no significant within-group or interaction effects across all time points. Similarly, total scores for the STAI-6 were also not significantly different between the control and intervention groups at week 24. Within-group and interaction effects across all time points for the STAI-6 were also not statistically significant. Parameter estimates and adjusted mean scores for each group at each time point are presented in Appendix 7, Tables 43 and 44.
Section 3: caregivers who were the spouse or partner of the person with dementia
Much of the literature on caregivers has selected spousal care partners for special attention, and there is evidence that spouses are particularly vulnerable to burden and poor psychological well-being. 121–123 Spousal caregivers of people with dementia have to live with an altered relationship with the person they elected to partner and live with as the mental faculties of the person with dementia alter. Furthermore, spousal carers may be of similar age to the care recipient and, therefore, may be experiencing physical or cognitive difficulties themselves. A secondary analysis was conducted on spousal caregivers of the person with dementia in the sample, so as to keep the relationship between the caregivers and the person with dementia constant. Details of their age and the SMMSE score of the person with dementia are provided in Table 28.
| Variable | Mean | SEM | 95% CI | p-value |
|---|---|---|---|---|
| Age | 68.6 | 0.83 | 66.9 to 70.2 | |
| Control | 68.7 | 1.14 | 66.5 to 70.9 | 0.863 |
| Intervention | 68.4 | 1.23 | 66 to 70.8 | |
| SMMSE scorea | 17.3 | 0.45 | 16.4 to 18.2 | |
| Control | 16.8 | 0.62 | 15.6 to 18 | 0.255 |
| Intervention | 17.8 | 0.65 | 16.5 to 19.1 |
Caregiver burden
In this subgroup analysis, total scores for the ZBI were not significantly different between the control and intervention groups at week 24. Furthermore, there were no significant within-group or interaction effects across all time points. Similarly, we found no significant between-group differences at week 24 for components 1, 2 or 3. There were no significant within-group or interaction effects for any of the components. Parameter estimates and adjusted mean scores for each group at each time point are presented in Appendix 7, Tables 45 and 46.
Caregiver mood
Total scores for the CES-D-10 (depressive mood) were not significantly different between the control and intervention groups at week 24. Furthermore, there were no significant within-group or interaction effects across all time points. Similarly, total scores for the STAI-6 (state anxiety) were not significantly different between the control and intervention groups at week 24. Within-group and interaction effects across all time points for the STAI-6 were also not statistically significant. Parameter estimates and adjusted mean scores for each group at each time point are presented in Appendix 7, Tables 47 and 48.
Discussion
The impact on caregivers’ health and well-being of caring for someone with dementia has led to the development of interventions to reduce the burden they face. It has also been recognised that these interventions may have a broader impact, as the alleviation of caregiver burden may reduce the likelihood of the cared-for person being institutionalised, thereby reducing social and health-care costs. In this study within the ATTILA trial, we investigated the effects of using ATT for people with dementia on the outcomes of the participants’ caregivers, namely caregiver burden and psychological well-being in the first 24 weeks following installation of the ATT intervention.
No effects of the installation of ATT compared with usual care were found on caregiver burden and its subcomponents, or depression and state anxiety. We also conducted post hoc subgroup analyses among live-in caregivers, and among caregivers who were the spouse or partner of the cared-for person, in whom we might expect poorer psychological well-being and levels of burden. Neither of these subgroup analyses revealed differences between the two groups in any of these outcomes. It is notable that the levels of caregiver burden, depression and anxiety remained stable during the course of the trial. Although this is not a non-inferiority trial, the data suggest no negative impact of receiving the ATT interventions.
One explanation for the lack of impact on these outcomes is the relatively low levels of burden, depression and state anxiety reported in the sample at baseline. 124 The mean levels of burden across both the intervention and control groups for the overall sample and the examined subgroups were in the mild to moderate range. Similarly, mean levels of depression in this sample were below the clinically relevant threshold on the CES-D-10 scale, for which a score of > 10 indicates depression. Mean scores on the STAI-6 at baseline were 9.6 and 8.7 for control and intervention group participants, respectively. These data suggest that there may have been limited scope to reduce burden, depression and anxiety among the sampled caregivers. A previous study125 indicated higher levels of depression and anxiety at baseline in its study population, and a 2016 study,126 using the same instrument for assessing depression as used in this study, found higher scores, above the clinically relevant threshold, in its sample.
Alternatively, it is possible that the effects of ATT interventions may be limited in effecting change in these outcomes, and that interventions specifically targeting caregivers’ well-being may be more effective than those aiming to support the cared-for person. Meta-analyses indicate that caregiver-directed interventions have demonstrated effectiveness in reducing burden, depression and anxiety in this population. Effective interventions include cognitive–behavioural therapy, cognitive reframing and educational interventions. 127–130 Therefore, to optimise the benefits of the installation of ATT for both the care recipient and the caregiver, it may be important to provide additional caregiver-directed support. Effective and potentially low-burden and low-cost interventions include the use of telephone- and internet-based interventions to improve caregiver outcomes. 131,132
In the current sample, the mean scores on the SMMSE indicated moderate levels of cognitive impairment in the cared-for participant sample (mean 10–19). There is some evidence indicating that the severity of the dementia is related to levels of depression and anxiety, with only severe dementia leading to caregivers having high levels of depression and anxiety,133 although this relationship has not always been supported. 134 It is possible that the level of dementia in the current study was not sufficiently severe to produce high scores at baseline in the caregivers such that they may have been reduced by the intervention.
Strengths, limitations and suggestions for future research
This study provides the first insight into the potential impact of up-to-date technological interventions for people with dementia on outcomes for their informal caregivers within 6 months of deployment. A large data set to assess impact of ATT is available; however, the extent of missing data at follow-up precluded investigation of longer-term effects of the technology on caregiver outcomes. Furthermore, loss to follow-up in the caregiver data set was non-random, introducing some degree of bias. This is because dropout among some caregivers was partly due to the care recipient moving into residential care or dying. Furthermore, power analysis was conducted on the study primary outcome (time to institutionalisation), rather than on caregivers’ outcomes. Therefore, it is possible that our analyses were statistically underpowered.
A general limitation of the trial should also be recognised, as we have reported elsewhere51 that there was limited fidelity of technology deployment in relation to the recommendations arising out of the needs assessment. This mismatch may have also contributed to the lack of any impact on caregiver burden and mood.
Future work will need to determine the minimum sample size required to detect an effect of the ATT intervention based on expected effect size for caregiver outcomes. It may well be that longer follow-up times with additional caregiver support are necessary to produce any intervention effects for caregivers’ outcomes.
Conclusions and implications for practice
This study provides a first insight into the potential impact of ATT on caregiver burden, psychological well-being and quality of life. No impact of ATT on caregiver burden, depression and anxiety was identified. As key drivers of transfer to long-term residential care, interventions aiming to specifically target these aspects of caregiver psychosocial well-being along with the deployment of ATT may be important for delaying institutionalisation and preventing the costs associated with this, both for social care services and for the individual and their family members. Evidence-based effective interventions to prevent negative impact of caregiving may include care partner-directed psychological and educational techniques, as well as ensuring that caregivers have an appropriate understanding of the role of ATT.
Chapter 7 Practices of people with dementia and caregivers using assistive technologies and telecare at home: a community-based ethnographic study
Industry, government and care service providers see ATT services as likely to enable people with dementia to continue living independently and safely in their communities. There is relatively little research to examine how people with dementia and their caregivers actually use these technologies in their everyday lives and how such experiences may affect their well-being and ability to sustain their community-based care arrangements. The little research that exists is limited in scale and quality. The ethnographic ATTILA substudy described here [referred to elsewhere as ACCOMMODATE (A Collaborative COMMunity-based ethnography Of people with Dementia using Assistive technology and Telecare at home in England)] aimed to address this empirical research gap from a subsample of ATILLA trial participants and to complement the ATTILA trial findings on the effects of participants’ technology use in their everyday routines. In-depth participant cases presented here are pseudonymised to ensure anonymity and confidentiality.
Aims and research questions
The study aimed to exemplify and examine how and why people with dementia and their caregivers used or chose not to use ATT in their lives and the ways in which ATT use affected their environments and relationships.
To address this research aim, the study team sought to answer three research questions:
-
How and why do people with dementia and their caregivers use ATT at home?
-
How does ATT fit into people’s lives and care in their homes?
-
How does ATT affect people’s lives and care in their homes?
Methods
This study used a qualitative ethnographic observational, longitudinal design to investigate how and why people with dementia in the intervention arm of the ATTILA trial used or did not use the ATT offered to them. This section describes the study design, sampling strategy, data collection and analysis methods.
Design
Ethnographic approaches have commonly relied on sustained fieldwork, whereby a researcher takes part in a group’s practices while observing them, so as to rigorously interpret how people make sense of their everyday lives and social systems. 135 The ethnographic approach used in this study, however, drew on recent multidisciplinary research in trials to design a study ‘embedded’ in the ATTILA trial136,137 to collect focused138,139 observational data on situated practices of people with dementia and their caregivers when using ATT in their everyday lives. We used these data to construct in-depth extended cases140,141 of how people with dementia and caregivers used (or chose not to use) these technologies, to help explain how and to what extent specific technologies for supporting people with dementia may be relevant in the context of their everyday places and interactions in home and community settings.
This study demonstrates how technology-enabled dementia care systems work with and for people, how they attempted to fit ATT into their lives and homes and how ATT-related processes affected their activities and care relationships.
To provide ‘trustworthy’142,143 findings and interpretations, we formulated credible, transferable, dependable, and confirmable processes for data collection and analysis through:
-
Extensive and intensive data collection from participants (see Participant observation and Analysis) and critically discussing anonymised data (credibility) findings within the study teams (credibility, dependability).
-
Noting in contextualised detail participants’ situated practices and how noting these may affect interpretation of findings to other contexts (transferability).
-
Reflecting on researcher role in the process and how this could affect interpretations. Detailed fieldnotes and accounts of the research context to allow future research to confirm, challenge or otherwise build on findings from this study (confirmability).
Ethics approval for the study was granted by the National Research Ethics Services Committee East of England, Norfolk (reference number 15/EE/0015) on 3 February 2015.
Sample
A purposive sampling strategy was used to select potential participants from the wider ATTILA trial population who were able to provide data relevant to examining care practices and specific reasons for, and ways of, their uptake (or not) of diverse technological interventions.
This strategy, therefore, had to incorporate the ATTILA trial inclusion and exclusion criteria (see Chapter 2), but also ethnographic-specific purposive sampling criteria that would provide contextually relevant and diverse types of participants’ experiences from three characteristics:
-
severity of the person’s dementia, as recorded by ATTILA trial research workers
-
type of family relationship between the caregiver and person with dementia
-
types of ATT equipment provided to the person with dementia.
Settings
To recruit participants, we collaborated with local ATTILA trial research workers, who collected data across three distinct authorities in east and south-east England: ‘Shire’, ‘Metropolitan’ and ‘Coast’. They included a mix of urban and rural populations in areas of economic wealth and areas of economic deprivation. 144 These are pseudonymised to ensure anonymity and confidentiality:
-
‘Shire’ – participants from this area lived in large villages or small market towns with independent vendors and occasional high-street shops.
-
‘Metropolitan’ – a major city divided into different districts. Trial participants lived in two adjoining districts with historical reputations of poverty. More recent gentrification is transforming these areas.
-
‘Coast’ – two counties with a seaside border, sharing service responsibilities for adult services. Participants from this area lived predominantly in large market towns or in villages near major regional city hubs.
These brief setting descriptions highlighted distinct features of the wider areas where people with dementia lived to contextualise wider relations with their environment.
Recruitment
The ethnographic team recruited prospective participants alongside three ATTILA trial research workers responsible for recruiting and collecting data from ATTILA trial participants in Metropolitan, Shire or Coast. This process to embed ethnographic recruitment in the ATTILA trial involved four steps:
-
The fieldworker (ML) collaborated with an ATTILA trial research worker to identify prospective ACCOMMODATE participants from the existing ATTILA trial sample. The fieldworker selected people based on how they aligned with the purposive sampling criteria for ACCOMMODATE. He selected participants to ensure a maximum variation across all three purposive sampling criteria.
-
The fieldworker attended the pre-arranged ATTILA trial follow-up visit with the area’s local research worker to meet with prospective participants to discuss participation in the ethnographic substudy.
-
The fieldworker sought substudy informed consent from people with dementia and their caregiver to take part in the ethnography substudy, or a consultee declaration from the caregiver for the person with dementia to participate.
-
The fieldworker continually renegotiated informed consent or consultee declaration for each subsequent monthly visit during independent fieldwork.
Participant observation
The study included nine ethnographic cases, each consisting of at least one person with dementia (n = 10) and their caregiver (n = 10). Two cases included more than one caregiver (the Campbells included two caregivers) or more than one person with dementia (the Stewarts included two people with dementia). Data collection involved up to six visits over 6 months (one per month) to the home of each person with dementia. These monthly visits lasted between 1 and 5 hours (mean 3.5 hours). A total of 208 hours of observation took place over 60 visits. Each visit involved Matthew Lariviere observing the practices of people with dementia and their caregivers, focusing particularly on whether or not and how they were using ATT and unstructured ethnographic interviews with participants to elicit their reasons for using or not using ATT.
Analysis
Initial notes from observations were written in a journal during or immediately after the visit and maps were drawn to illustrate objects and people’s places in settings. The initial notes were later transcribed and developed into field notes with any accompanying audio-recordings from conversations. Additional researcher memos identified where participants were interacting with or discussing the technologies.
We analysed each case using situational analysis of longitudinally extended cases. 8,11 The main themes identified from early memos highlighted how people with dementia and caregivers attempted to fit ATT into their everyday practices, leading to placing, replacing and displacing care and uses of spaces inside and outside the home. Focused coding was used to identify instances of these themes during different visits for each ethnographic case, which informed comparisons within and across cases and contextualised specific instances of these analytical themes. The findings are presented in the following section as extended cases that highlight everyday features seen as common to several cases. These are depicted further through indicative maps.
Findings
Each of these ethnographic cases provides in-depth representations of how participants enacted their practices with ATT in their homes. Table 29 provides descriptions of the nine ethnographic cases to highlight the diversity of the nine cases based on their case name, location, the severity of the person’s dementia, the nature of participant’s family or care relationship, and the types of assistive technologies and/or telecare devices in place. Ethnographic cases were evenly distributed across different levels of dementia severity (i.e. three mild, three moderate and three severe cases). Participants in the ethnographic substudy most commonly received a falls detector (n = 6) and door sensors (n = 4). Keysafes (n = 3) and calendar-clocks (n = 3) were also relatively common. In most cases, an adult child was caring for an older parent with dementia (n = 6). Caregivers rarely co-resided with the person with dementia under their care (n = 3).
| Case names and location | Dementia severity | Nature of relationship with caregiver(s) | ATT devices |
|---|---|---|---|
| Clydes – Coast | Moderate | Father (person with dementia) lived in his own house. Son and daughter-in-law (caregivers) lived in separate house, but they worked from an office in the front room of the father’s house | Automatic falls detector (wristband model), keysafe |
| Drapers – Coast | Mild | Mother (person with dementia) lived in her own home. Son (caregiver) lived in his own separate home, but visited her for up to 6 hours every day | Calendar-clock, bed sensors, automatic falls detector, falls alarm (wrist version; replaced pendant after first visit), keysafe |
| Stewarts – Coast | Moderate (both parents) | Mother and father (people with dementia) lived in an annex of the daughter’s house (caregiver) | Door sensors |
| Betty and Rose – Shire | Severe | Betty (caregiver) is Rose’s (person with dementia) neighbour. They each lived in their own house | Automatic falls detector (pendant), keysafe |
| Anthony and Mrs Archer – Metropolitan | Severe | Mrs Archer (person with dementia) lived in a sheltered housing flat. Anthony (caregiver; friend of family) lived his own flat in the same neighbourhood as Mrs Archer. He visited her a few days each week | GPS tracking device, calendar-clock, automatic falls sensor (pendant), cooker-timer |
| Campbells – Metropolitan | Severe | Son (caregiver) lived in mother’s (person with dementia) home | Bed sensor, door sensor/alarm, pendant alarm |
| Browns – Shire | Mild/MCI | Wife (caregiver) shared house with husband (person with dementia); daughter (caregiver) and son-in-law lived in annex | Door sensors, object finder |
| Anansis – Metropolitan | Moderate | Father (person with dementia) lived alone in a flat; daughter (caregiver) visited him regularly from her home across the city | Automatic falls detector (pendant), GPS ‘watch’ and pendant (Buddi; Buddi, Rickmansworth, UK) |
| Smiths – Shire | Mild | Father lived alone in his own house. Daughter (caregiver) lived with her family in village in another county and tended to arrange her visits to coincide with other activities, such as doctor visits. Her visits appeared more ad hoc than those in the other cases in which caregivers all lived closer to the people under their care | Wrist alarm, automatic falls detector (waist), calendar-clock (self-purchased), door sensors, networked smoke alarm, activity monitoring sensors and software (Just Checking Ltd, Lapworth, UK) |
This diverse range of characteristics across all cases supported robust interpretation of how this diversity shaped differences and similarities across the nine ethnographic cases. Three key themes were identified as relevant to understanding the ATT-relevant and care-relevant everyday practices, routines and relationships of study participants:
-
Placing technology in care.
-
Replacing care with technology.
-
Technology displacing care and everyday life. Displacing everyday life refers to how people’s everyday routines and built environments become disrupted and altered.
Placing technology in care
The theme ‘placing technology in care’ refers to instances when people with dementia and/or caregivers fit ATT products into their existing care arrangements. It addresses participants’ processes and practices in incorporating and adapting technologies into their everyday lives with varying degrees of success.
The Drapers showed initial troubles in how they placed a falls detector within their pre-existing everyday practices. The person with dementia, Violet Draper, received a neck-worn falls detector pendant, after experiencing several falls. Around the when time research visits began, she had another fall, but the alarm did not trigger. She decided not to trigger the alarm manually as she ‘did not want to be a bother’ to her son and caregiver, Thomas, or to the emergency response services. Thomas also commented that his mother frequently forgot to wear the pendant or took it off in the evening with her bed having been moved down to the sitting room (Figure 11 shows a map). After this fall, Thomas changed two elements of his mother’s care. First, he asked the local ATT provider to swap the pendant-style detector for one worn around the wrist. Second, he reminded Violet every day to ‘press the button’ if she ever fell again. A couple of months later, Violet had another much more serious fall in which she broke her leg; this time, the falls detector did not detect the fall automatically, but Violet did remember to press the falls detector button to contact first responders and Thomas.
FIGURE 11.
Map of sitting room in Violet Draper’s home.
This case highlights that both Thomas and Violet acted to make the falls detector fit into their lives. Their case illustrates the work of caregivers to instruct and to reinforce any such instruction to ensure ‘successful’ implementation of ATT that would otherwise be invisible to care workers and designers of the technology. Such work was even more important here because the falls detector did not activate automatically. This raises further questions about the different types of technologies designed to enhance the safety of people with dementia. The key distinction here is between ‘passive’ devices that automatically trigger, not requiring the user to perform any actions, and devices that require an action to be performed to activate them. It seems important to consider what use of passive devices is appropriate in the case of people with dementia, especially where they are either reluctant to trigger alarms or have memory difficulties that lead them to fail to remember to trigger a device. This case demonstrated how social connections and support were important even for enacting ‘technology-enabled’ care systems.
In contrast, the Stewarts’ case illustrates how people with dementia or caregivers may appropriately place assistive technology, yet find that other objects in the home may suitably address problems when they arise. Mary and Michael are a married couple, both of whom have dementia. Their daughter, Sally, moved them into an annex of her home to support them full time. During one fieldwork visit, Sally asked her parents for the date. Neither Michael nor Mary knew the date. They also did not appear to notice the nearby calendar-clock that had been provided to display this information but did perceive wooden calendar blocks across the room, which helped orientate them. Michael told Sally the correct date.
This case highlights the importance of appropriately placing ATT in a person’s home. Here, a wooden calendar may be seen to serve as more ‘assistive’ than the ‘formally provided’ calendar-clock. Although the calendar-clock did not disrupt or disorientate Michael or Mary, the technology did not actively facilitate them to be orientated to date and time. Someone still had to interact with the regular calendar to make it work by changing the date each day, but its familiar location and design may have more easily supported their orientation because it relied in part on older memory and was perhaps more readily recognisable than more recent digital counterparts.
The Browns’ case raises further questions about how researcher and practitioners come to define ‘use’ of ATT. Sam Brown, a person with mild cognitive impairment, had a memo minder in his house entrance. A recording of his daughter’s voice reminded him to lock the front door whenever anyone walked in front of the infrared motion sensor. Sam told the researcher that he always remembered to lock the door because of it. Sam also shared his home with his wife and with his adult daughter and son-in-law, who lived in a converted garage annex. The other household residents became annoyed with the memo minder repeatedly going off whenever they went to put on or remove their shoes and outerwear. Sam decided to turn off the recording but leave the memo minder in its place next to the front door. He insisted, and the researcher observed and confirmed, multiple times, that seeing the now-silent memo minder, still beside the door, reminded him to close and lock it when he left the house to go back home.
Such practices blur ‘use’ of ATT and its ‘non-use’. Although the person with dementia switched off this device, its co-location with him in its ‘appropriate’ place provided the prompt he needed to remember to lock up. This case, alongside others, illustrates both the importance of ‘place’ in sustaining appropriate technology-enabled care for people with cognitive impairments, and how people are able to actively accommodate technology to work in their shared spaces and their relationships with others, including their caregivers.
Technology replacing care
This theme, ‘technology replacing care’, particularly addresses how caregivers, through ATT, replaced or reconfigured their practices of caring for people with dementia.
The Clydes’ case involved Arthur Clyde, an older person with dementia, who received a falls detector from his local authority. His son and daughter-in-law, Mark and Cathy, visited his home every weekday to work from the front room of his house, which they had converted into an office for Mark’s business. Mark also used to visit his father at least 1 day over the weekend to see whether or not he remembered to heat up and eat his pre-prepared meals. However, after Arthur started to wear the falls detector around his wrist, Mark visited his father less frequently. Mark told the researcher that he had ‘peace of mind’ that the call centre would notify him if his father had a fall. Mark decided instead to telephone Arthur on Saturdays and Sundays to ask him whether or not he had eaten his meals instead of visiting to confirm this.
This case illustrates how caregivers may change their care practices for a person with dementia after they introduce ATT into their arrangements. Here, the caregiver visited his father less frequently and relied on the falls detector and telephone to monitor his father with dementia. Falls detectors and telephones reconfigure monitoring practices to be mediated through technologies rather than face-to-face interactions. Caregivers’ sense of security, often articulated as their ‘peace of mind’, was a common response across cases, including the case that follows.
In the Smiths’ case, Lauren had the local service provider install an activity monitoring system in the living room of her father’s bungalow. Lauren thought her father, Christopher Smith, frequently got up from his favourite chair to walk around the house based on activity reported on the monitoring system’s accompanying application for her tablet. Lauren told the researcher that she had ‘peace of mind’ that her father remained active even when home alone, especially as she lived in another county, distant from her father. However, the researcher rarely saw Christopher move from his chair during his visits (Figure 12 shows a map). During one visit, Lauren and the researcher noticed the dog jumping on the couch. The activity monitoring system’s motion sensors happened to be at the same height as the dog. The researcher asked Lauren whether or not she possibly monitored the dog instead of her father. She looked at the application and noticed recent activity when no one else appeared to have moved in the room except for the dog. She wondered aloud how frequently her father really left his chair.
FIGURE 12.
Map of Christopher Smith’s bungalow.
The Smiths’ case highlights how people use the devices for reassurance and peace of mind. In this case, the poorly placed product led to inaccurate information and misguided reassurance. Once this was established, the caregiver’s ‘peace of mind’ became replaced by concern as she could no longer be certain whether the activity monitoring system monitored only her father’s movement or also that of other people or animals.
In contrast to the other two cases exemplifying this theme, the case of the Campbells demonstrates how people can independently adopt ‘other technologies’, and how this will also shape their care practices. Kenneth Campbell shared his home with Lillian, his mother living with dementia. They were offered door and bed sensors, but Lillian tore out the cable of the bed sensor from under the mattress. They did not have a telephone line for the door sensor to connect with. Instead, Kenneth independently purchased and used a closed-circuit television (CCTV) system to monitor the downstairs rooms of the house, where his mother lived, through monitors in his living room upstairs.
The Campbells’ case displays marked changes in how caregivers may provide care with the addition of technologies. Notably, here, the ATT did not appear to fit into the lives of Kenneth or Lillian. Rather than reconfigure their practices or home, Kenneth adapted security equipment, namely CCTV, as a means to monitor his mother in their home. This case raises further questions about how we characterise means to monitor people with dementia in their home as appropriate yet still ensure dignity and safeguard them against harm. It also calls into question, as noted in Chapter 6, whether or not carers’ work here may have changed rather than diminished.
Technology displacing care and everyday life
The final theme, ‘technology displacing care and everyday life’, refers to cases in which people with dementia experienced their care arrangements and everyday practices as being displaced from their usual routines by ATT.
In the Rose and Betty case, Rose previously visited the local chapel in her village every Sunday for her church service. She received a lift from another neighbour to attend this service. Rose became increasingly frail, which affected her ability to move safely around in, and leave, the house. To support her living safely, she received a falls detector. However, the neighbouring family decided to stop attending the same service. Rose did not have the physical capacity to walk to the chapel herself, so she had to stop going to the local service.
Here the technology she received, a falls detector, could not facilitate her social participation in activities centrally important to her. The ATT here was not necessarily designed to facilitate these connections, but it shows how, as people with dementia encounter increased challenges in their participation in everyday activities, technology cannot facilitate and support every connection and activity in a person’s life. Other interventions from friends and family may support such inclusion for a person with dementia, but they may not sustain support for social participation indefinitely. Other participants with dementia became increasingly isolated as their dementia progressed and they experienced more acute care needs that were met in only limited ways by home monitoring.
In some cases, technology played a greater role in displacing people’s activities and care. In the Anansi case, for example, technology seemed to constrain how William Anansi could engage as he wanted with his wider community. William received a Global Positioning System (GPS) tracking system from his local council. Claire, his daughter and primary caregiver, told the researcher that she hoped this device would allow both her father to leave the house when he wished but also for her to locate him if he became lost. During one research visit, William left his flat without telling Claire. She called the call centre for the GPS tracking device, which located him in a nearby market that he frequented for his favourite Caribbean cuisine. Claire called her father on his mobile phone to tell him to return home. She also told the call centre operator to contact him through the speaker on the GPS tracking device. William initially did not answer any calls. After 10 minutes of her calling him, he answered his mobile phone and told Claire that he had had lunch. Claire again told him to return home. The call centre operator confirmed that William appeared to be on a bus on his way back to his flat.
This case illustrates how caregivers can use technologies to affect how people with dementia interact with spaces outside their home and engage with their wider community. Here, Claire tracks her father’s location through the GPS system worn on his person, with the assistance of a call centre operator to confirm his movement and changes to his location. Such practices with ATT illustrate how people can attempt to control the movements and behaviour of people with dementia, even when this may contest how they want to live their lives. Again, as noted in Chapter 6, new challenges and concerns for caregivers may be raised, rather than removed, by ATT.
Discussion
These findings illustrate how technological mediation through ATT with associated applications and screens could replace, displace and disrupt co-located, face-to-face interactions. The reported findings suggest that how policy-makers and industry imagine community care through ATT service provision may not always reflect actual practices in technology-enabled dementia care. As previous research has also suggested, people with dementia and caregivers may make ATT work for them in contingently, rather than systematically. 145–147 This study, however, highlights not only the role taken by caregivers to fit these technologies into care practices, but also how their use of ATT can change the spaces and placement of care and everyday life. It shows how people’s practices with ATT may shift dependencies in care arrangements as a result of actual or perceived changes or disruptions in care. 147,148 These findings identify limitations for ATT to enable people with dementia to ‘live independently in the community’ if they do not also have added support from caregivers to help adapt technologies to fit into their lives.
Strengths and limitations
Study findings highlighted granular details of how people with dementia and their caregivers do and could use assistive technologies. We adopted systematic measures to ensure that the findings were trustworthy. However, these findings only exemplify practices of participants taking part in a trial and agreeing to receive this intervention. It may have limited transferability to how people with dementia and caregivers may use other current or emergent technologies. Caregivers also have diverse living arrangements and familial ties and relationships, and it was not possible to sample all of these. Nonetheless, it provides critical insights into the complexity of technology-enabled dementia care to consider in current and future design and provision of such technologies. Transparent reporting of this study’s methods of data collection and analysis allows other researchers to challenge, confirm or add to understandings and implications of the findings.
Conclusions and implications
These ethnographic findings flag up unintended and unanticipated consequences for ATT implementation and uptake in ‘real-world’ community-based dementia care contexts. The ethnographic approach details how people’s use of ATT shifted over time. Nonetheless, even temporary use of ATT may have deferred more complex and more acute care crises for the person with dementia or caregiver. Transient effects or limited engagement with technology should not necessarily be interpreted, therefore, as a failure in its uptake or effect. It underlines the need to identify and map the context of ATT provision over time within the changing lives of people with dementia and their caregivers, and relative to service provider organisations, as revealed in these cases.
These findings have relevance for technology developers and providers in indicating how to consider more appropriate products and services that can support more sustainable technology-enabled care for people with dementia and their partners in care. Designers and service provider organisations should also work with caregivers and people with dementia, including charities representing these groups, such as Carers UK and the Alzheimer’s Society, to co-produce suitable technological interventions.
This study suggests that we must more fully appreciate the importance of people’s activities and relationships in continuously shaping care, including dementia care involving the implementation and uptake of technology in community settings to improve their effectiveness and sustainability.
Chapter 8 Discussion
Main outcomes
To the best of our knowledge, this study is the first RCT of ATT for people living with dementia in the community. Despite the large increase in available products and their promotion as effective, cost-saving ways of helping people with dementia remain at home, there has been a paucity of robust research on how beneficial these products and services can be. 37 The main outcomes of this trial were whether or not ATT could help keep people living at home longer and whether or not this was more cost-effective than a basic ATT package and alternative support. The answer to both questions was no: there were no significant differences found between the ATT and groups on any outcomes.
Home-based dementia care is a policy prioritised across the world,149,150 including in the UK. 151 Remaining at home to maintain a higher quality of life is preferred by people with dementia, is more cost-effective than residential care and can provide continuity and familiarity that is beneficial to those experiencing a decline in cognitive and functional abilities. 152 ATT has been marketed as an aid to keeping people with dementia safely at home by providing monitoring of movement, safety and well-being; reminders for events and medication; and social support, among other things.
There have been many studies conducted around ATT, but all have been very small and/or have had poor methodology. The results of these have often been conflicting, with some viewing ATT very positively,153 others finding no benefits154 and some even regarding it negatively, for example although surveillance can be enabling, it can also be an invasion of privacy. 8 A 2017 Cochrane Review37 found that there were no studies sufficiently robust to make a judgement and cited our study as being the only ongoing research that met their criteria. The lack of evidence to date makes the contribution of this study to the evidence base vital.
Dementia is an increasing challenge for health-care systems across the world, with 43.8 million cases in 2016;11 this is predicted to rise to 75 million by 2030. 155 With no treatment for dementia, or many of its causes, currently available, and medication to slow development showing only marginal clinical improvement,156 it is increasingly important to help people with dementia and their families to live well in the community for as long as is safe for them to do so. Caregivers provide 42% of the estimated US$604B spent globally on dementia,37 so supporting their role is also vital.
There are a number of possible reasons for the lack of efficacy shown in the current study. Previous research has suggested that ATT are often introduced when the cognitive decline is too great and the ability to adapt is too impaired for the user to incorporate it into their routines, and, at all stages of dementia, devices are seen as most useful when they are simple. 157 Aids that do not need adjusting have been considered most useful by caregivers, whereas complex interfaces and connection problems reduce the perceived utility of ATT. 158 However, other studies have found that a lot of ATT devices lack user-centred design approaches,159 and user experience can be improved by optimising specific details of a product. 160
Conclusions from Chapter 6
Evidence from the ethnographic study (see Chapter 7) may also provide some insight into the lack of effects on caregiver outcomes. The ethnographic study indicates that, although technology was deployed, how it was used by caregivers and the cared-for person in the context of their own home and lifestyle may be important in determining the effectiveness and beneficial impact of the technology on their well-being. Data from the ethnographic study reported issues with reliability, situations in which the cared-for person did not use it as instructed or as intended, and that it may not have reduced the amount of time spent caregiving, even if the caregiving was carried out remotely rather than as visits to the home.
How ethnographic findings complement ATTILA trial findings
As detailed in Chapter 4, the primary outcomes from the ATTILA trial demonstrated no significant effect of ATT on reducing mortality or on care home use by the person with dementia. Ethnographic findings illustrate how ATT provision could, nonetheless, provide participants, especially caregivers, with a sense of security or ‘peace of mind’, which they specifically valued, yet could also pose other challenges to caregivers.
These findings demonstrate the need to understand specifically how the ATT devices are incorporated into people’s space and behaviours. Appreciating the specific context is important for fully understanding how ATT came to work as an intervention. Here, the technology’s ‘effectiveness’ was influenced by participants’ social relations (e.g. instructing how to use the device) and environment (e.g. in-home object placement, other occupants). This emphasises the need for complementary studies on the effectiveness of ATT as an intervention, while also appreciating the ways in which people with dementia and caregivers make sense and work to include these devices within their care practices and other arrangements. Technology takes up space on a person or in their home; this requires people to make choices about whether or not and how it fits on people’s bodies and in domestic spaces. As the findings illustrated, these choices come packaged with value judgements about what care practices and interventions people find suitable in their everyday routines for people living with dementia in the community. People with dementia, caregivers and care workers may often contest the suitability of ATT, and extent to which they allow ATT to intervene in their lives.
The diverse range of experiences of living with dementia and caring for a person with dementia, and the wide variety of different ATT devices available through local authorities and on the consumer market, make it difficult to understand what may make ATT an appropriate and effective intervention for community-based dementia care. Individual caregivers and people with dementia may find that a specific ATT product helps them to manage their specific care responsibilities or ADL. Yet, these may be time bound, as care needs can rapidly fluctuate as a person experiences further limitations owing to their dementia progression, and there are fresh challenges for caregivers to negotiate, emphasising the need to incorporate a review process in providing ATT in dementia care.
Limitations
There was great variation across sites in the assessment and provision of ATT, and the study was not powered or intended to examine the impact of local configurations of ATT. The trial was designed to embrace the heterogeneous nature of local ATT assessment and equipment provision for people with dementia. As discussed in Chapter 3, the way in which ATT was delivered locally varied considerably: two types of value networks were found to be operating in our relatively small sample of local authority areas. A mixture of models of providing and financing ATT was encountered across trial sites. The extent to which councils and providers charged user fees varied depending on the nature of local markets and on the needs profiles of ATT users. The extent of information on locally available ATT services and charges is limited in the UK; most ATT services, including ASCDs, charge fees. 161 We did not investigate the impact of deficiencies in service information or the presence of user charges, although such barriers might have affected the uptake of ATT and reduced the use of potentially beneficial devices.
Delivery systems were investigated and described as planned (see Chapter 3 and Appendix 2), but it proved difficult to collect data from sites in a consistent manner sufficient to calculate a unit cost of ATT. The unit costs of ATT devices, installation, monitoring and response were drawn from databases of prices set by public sector procurement frameworks. This approach allowed a realistic costing of ATT services and equipment provided by the public sector (e.g. in comparison to pricing devices via telecare providers’ and online technology retailers’ websites); prices paid for ATT devices and services by private individuals might be higher, and so ATT costs, which made up a very modest proportion of participants’ total costs, might have been somewhat higher. Because control participants were also in receipt of at least a basic ATT package over the course of the study, their costs could equally be higher and differences in costs between groups little affected overall. We explored the feasibility of collecting providers’ data on ATT devices used by participants; however, as the study was not resourced to negotiate data-sharing arrangements with all the providers involved, we relied on researcher-collected technology checklist data.
The CSRI was reliant on participant recall, which may have affected the precision and size of cost estimates. Costs were measured by asking caregivers to retrospectively report service use, so the results could be subject to recall bias. Costs of the intervals between periods with CSRI data available were estimated by carrying forward most costs, although costs of inpatient and emergency department use were estimated from the SAE data available over these times. Costs of regularly used services were thus assumed to be constant over these intervening periods. Participant-reported EQ-5D data were missing for one-quarter of intervention participants and for one-third of control participants who had participated in assessments at 104 weeks. Although difference-in-difference analyses controlled for baseline covariates (stratification variables and dependency, as measured by the BADLS) that might have accounted for differences in data availability between groups on this measure, QALY analyses drew on group mean utilities at each time point and did not adjust for baseline characteristics. Therefore, the finding that the intervention group had fewer QALYs derived from participant-reported EQ-5D data must be interpreted with some caution, as substantial numbers of missing data in that measure could be a concern. Generalisability of these findings may also be limited because all assessment data were missing from some participating dyads at the baseline and follow-up points (8% of the sample at baseline and between 16% and 19% of the community-dwelling sample still participating in the study over the follow-ups did not participate in an interview).
Future research
An official national collection of data on local authority ATT provision, the size of contracts with provider partners and total expenditure and unit costs would shed light on the variety of delivery and financing models in place, and the relationship with expenditure. A study on the nature and size of the private ATT market (including internet of things and smart home applications) would shed light on the relative importance of public and private sectors in ATT provision. 161
An observational study of telecare for dementia could be designed, which could employ a larger sample than would be achievable with an experimental design, and observe longer and continuous durations of service use. Such research could also identify processes and mechanisms of implementation (procurement, clinical and care decision-making, maintenance of technologies, call centre activities, etc.) of ATT in health and care systems to support people with dementia. If routine data from health and social care providers could be linked162 to telecare providers’ data, further research could examine associations between ATT (activations and device types) and hospital and care home admissions.
Implications
This study found that a full package of ATT did not result in a significant increase in the length of time a person with dementia can remain living in the community, nor did it achieve decreases in caregiver burden, depression or anxiety. The findings of Chapter 3 suggest that providers of assessments and providers of ATT do not always work in tandem. Work to understand why local delivery systems were producing such mismatches should be conducted with local authorities, not-for-profit providers and the NHS in England. Meanwhile, the use of ATT that extend beyond basic devices (including pendant alarms) as support for people with dementia should not be unconditionally adopted and should be subject to the same rigorous evaluation as other interventions. However, ATT recommended on the basis of need and installed and used as intended might be very useful for people with dementia and their caregivers. Designers and service provider organisations should work with caregivers and people with dementia and their advocates to co-produce suitable technological interventions. Alternatives to technological solutions to difficulties should also be actively sought.
Acknowledgements
The ATTILA trial triallists group
Writing Committee
Rebecca Gathercole, Catherine Henderson, Rosie Bradley, Anna Davies, Shashivadan Hirani, Stefano Brini, Stanton Newman, Kirsty Forsyth, Matthew Lariviere, Chris Fox, Fiona Poland, Martin Knapp, Iracema Leroi, John Woolham, Richard Gray and Robert Howard.
Data Monitoring Committee
Professor Ian McKeith, Institute for Ageing and Health, Newcastle University (chairperson); Professor James Lindesay, Department of Health Sciences, Leicester University Hospitals NHS Trust; and Dr Tracey Young, School of Health and Related Research, University of Sheffield.
Trial Steering Committee
Dr Peter Bentham, The Barberry Centre, Birmingham and Solihull Mental Health NHS Foundation Trust (chairperson); Dr Louise Brown, Medical Research Council Clinical Trials Unit at University College London; and Mrs Gillian Harrison and Mrs Sue Tucker, Service User Representatives, Alzheimer’s Society.
Trial Management Group
Rebecca Gathercole, Emma Harper, Linda Kelly, Natalie Lam, Lynn Pank, Rosie Bradley and Richard Gray and Robert Howard.
Health Economics
Catherine Henderson and Martin Knapp (London School of Economics and Political Science).
Participating centres
South London and Maudsley NHS Foundation Trust: Robert Howard (principal investigator), Rebecca Gathercole, Heather Westwood, Bethany Scutt and Grace Lavelle.
Cambridge Community Services NHS Trust, Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge University Hospitals NHS Foundation Trust: John O’Brien (principal investigator), Andrew Bateman (principal investigator), Rachel Winson, Samantha Nunn and Victoria Ordonez Montano.
Oxford and Buckinghamshire Mental Health NHS Foundation Trust: Rupert McShane (principal investigator), Sarah-Jane Cellan-Jones, Marni Moran and Rowena Johns.
Norfolk and Suffolk NHS Foundation Trust: Chris Fox (principal investigator) and Emma Talbot.
Lancashire Care NHS Foundation Trust: Iracema Leroi (principal investigator) and Emma Hooper.
Nottinghamshire Healthcare NHS Foundation Trust: Dr Anand Ramakrishnan (principal investigator) and David Trevor.
South West Yorkshire Partnership NHS Foundation Trust: Seelam Kalyan (principal investigator), Lubena Mirza and Amber Hemmingway.
Contributions of authors
Rebecca Gathercole (https://orcid.org/0000-0002-6380-2655) (Trial Manager, Old Age Psychiatry) ran the trial, contributed to the paper, interpreted the data and wrote the initial draft paper.
Rosie Bradley (https://orcid.org/0000-0002-0758-4905) (Medical Statistician, Medical Statistics) analysed the survival data, contributed to the paper, interpreted the data and wrote the initial draft paper.
Emma Harper (https://orcid.org/0000-0001-5651-6258) (Clinical Trials Co-ordinator, Population Health) ran the trial.
Lucy Davies (https://orcid.org/0000-0001-6632-7215) (Medical Statistician, Medical Statistics) analysed the survival data.
Lynn Pank (https://orcid.org/0000-0001-6398-6565) (Administrative Assistant, Medical Statistics) and Natalie Lam (https://orcid.org/0000-0001-8591-444X) (Data Manager, Medical Statistics) provided data management support and data entry at the Clinical Trials Unit.
Anna Davies (https://orcid.org/0000-0003-0743-6547) (Senior Research Associate, Health Psychology) analysed the caregiver outcome data, contributed to the paper, interpreted the data and wrote the initial draft paper.
Emma Talbot (https://orcid.org/0000-0002-7614-9683) (Research Practitioner, Mental Health Research), Emma Hooper (https://orcid.org/0000-0002-4059-6035) (Research Practitioner, Mental Health Research), Rachel Winson (Research Practitioner, Mental Health Research), Bethany Scutt (https://orcid.org/0000-0003-0456-2556) (Research Worker, Old Age Psychiatry), Victoria Ordonez Montano (https://orcid.org/0000-0002-3500-1922) (Research Practitioner, Mental Health Research), Samantha Nunn (https://orcid.org/0000-0002-2853-9652) (Research Practitioner, Mental Health Research) and Grace Lavelle (https://orcid.org/0000-0003-3768-1797) (Research Worker, Old Age Psychiatry) recruited and retained participants.
Matthew Lariviere (https://orcid.org/0000-0001-6901-3115) (Research Fellow, Anthropologist of Care, Ageing and Technology) designed and analysed the ethnographic study.
Shashivadan Hirani (https://orcid.org/0000-0002-1577-8806) (Senior Lecturer, Health Services Research) and Stefano Brini (https://orcid.org/0000-0002-1909-1796) (Research Fellow, Health Services Research and Management) analysed the caregiver outcome data and contributed to the paper.
Andrew Bateman (https://orcid.org/0000-0002-2547-5921) (Clinical Manager, Neuropsychological Rehabilitation), Peter Bentham (https://orcid.org/0000-0002-6443-3353) (Consultant, Psychiatry), Alistair Burns (https://orcid.org/0000-0002-9837-0645) (Professor, Old Age Psychiatry) and Barbara Dunk (https://orcid.org/0000-0002-6363-5009) (Senior Occupational Therapist, Older Adult Memory Services) were principal investigators.
Kirsty Forsyth (https://orcid.org/0000-0002-6732-1699) (Professor, Occupational Therapy) analysed the needs assessment data and contributed to the paper.
Chris Fox (https://orcid.org/0000-0001-9480-5704) (Clinical Professor, Psychiatry) designed and analysed the ethnographic study, contributed to the paper and was a principal investigator.
Catherine Henderson (https://orcid.org/0000-0003-4340-4702) (Assistant Professorial Research Fellow, Health Policy) analysed the health economic data, contributed to the paper, interpreted the data and wrote the initial draft paper.
Martin Knapp (https://orcid.org/0000-0003-1427-0215) (Professor of Social Policy, Health Policy) analysed the health economic data and contributed to the paper.
Iracema Leroi (https://orcid.org/0000-0003-1822-3643) (Professor, Psychiatry in Ageing and Dementia) contributed to the paper and was a principal investigator.
Stanton Newman (https://orcid.org/0000-0001-6712-6079) (Professor, Health Psychology) analysed the caregiver outcome data and contributed to the paper.
John O’Brien (https://orcid.org/0000-0002-0837-5080) (Professor, Old Age Psychiatry) was a principal investigator.
Fiona Poland (https://orcid.org/0000-0003-0003-6911) (Professor, Social Research Methodology) designed and analysed the ethnographic study and contributed to the paper.
John Woolham (https://orcid.org/0000-0003-3128-7756) (Senior Research Fellow, Social Care Research) designed the trial and contributed to the paper.
Richard Gray (https://orcid.org/0000-0003-4440-574X) (Professor, Medical Statistics) designed and ran the trial, analysed the survival data, contributed to the paper, interpreted the data and wrote the initial draft paper.
Robert Howard (https://orcid.org/0000-0002-3071-2338) (Professor, Old Age Psychiatry) designed and ran the trial, contributed to the paper, was a principal investigator, interpreted the data and wrote the initial draft paper.
All authors assume responsibility for the accuracy and completeness of the data and for the overall content and integrity of the paper.
Publications
Leroi I, Woolham J, Gathercole R, Howard R, Dunk B, Fox C, et al. Does telecare prolong community living in dementia? A study protocol for a pragmatic, randomised controlled trial. Trials 2013;14:349.
Forsyth K, Henderson C, Davis L, Roy AS, Dunk B, Curnow E, et al. Assessment of need and practice for assistive technology and telecare for people with dementia – The ATTILA (Assistive Technology and Telecare to maintain Independent Living At home for people with dementia) trial. Alzheimers Dement 2019;5:420–30.
Howard R, Gathercole R, Bradley R, Harper E, Davis L, Pank L, et al. The effectiveness and cost-effectiveness of assistive technology and telecare for independent living in dementia: a randomised controlled trial. Age Ageing 2021:afaa284.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Leroi I, Woolham J, Gathercole R, Howard R, Dunk B, Fox C, et al. Does telecare prolong community living in dementia? A study protocol for a pragmatic, randomised controlled trial. Trials 2013;14.
- Howard R, Gathercole R, Bradley R, Harper E, Davis L, Pank L, et al. The effectiveness and cost-effectiveness of assistive technology and telecare for independent living in dementia: a randomised controlled trial. Age Ageing 2021.
- Knapp M, Prince M, Albanese E, Banerjee S, Dhanasiri S, Fernandez JL, et al. Dementia UK: The Full Report. A Report into the Prevalence and Economic Cost of Dementia in the UK Produced by King’s College London and the London School of Economics 2007.
- Lewis F, Karlsberg Schaffer S, Sussex J, O’Neill P, Cockcroft L. The Trajectory of Dementia in the UK – Making a Difference. London: Office of Health Economics; 2014.
- Wanless D, Forder J. Securing Good Care for Older People. Taking a Long-term View. London: The King’s Fund; 2006.
- Department of Health and Social Care . The Government’s Expenditure Plans. Departmental Report 2003 n.d. https://webarchive.nationalarchives.gov.uk/20030731053810/http://www.doh.gov.uk:80/dohreport/report2003/download.html (accessed 26 February 2020).
- Bebbington A, Darton R, Netten A. Care Homes for Older People: Volume 2. Admissions, Needs and Outcomes. The 1995/96 National Longitudinal Survey of Publicly-Funded Admissions. Canterbury: Personal Social Services Research Unit, University of Kent; 2001.
- Department of Health and Social Care . Living Well With Dementia: A National Dementia Strategy 2009.
- Woolham J, Frisby B, Quinn S, Moore A, Smart W. The Safe at Home Project. London: Hawker Publications; 2002.
- Woolham J, Frisby B, Benson S. Dementia Topics for the Millennium and Beyond. London: Hawker Publications; 2002.
- Gitlin LN, Winter L, Dennis MP. Assistive devices caregivers use and find helpful to manage problem behaviors of dementia. Gerontechnology 2010;9:408-14. https://doi.org/10.4017/gt.2010.09.03.006.00.
- Woolham J. Safe at Home: The Effectiveness of Assistive Technology in Supporting the Independence of People with Dementia: The Safe at Home Project. London: Hawker; 2006.
- Department of Health and Social Care . Building Telecare in England 2005.
- Audit Commission . Assistive Technology: Independence and Well-Being 4 2004.
- Miskelly FG. Assistive technology in elderly care. Age Ageing 2001;30:455-8. https://doi.org/10.1093/ageing/30.6.455.
- Lansley P, McCreadie C, Tinker A. Can adapting the homes of older people and providing assistive technology pay its way?. Age and Ageing 2004;33:571-6. https://doi.org/10.1093/ageing/afh190.
- Mitchell R. An Evaluation of Falkirk’s Mobile Community Alarms Service. Falkirk: Falkirk Council; 1996.
- Cash M. At Home with AT (Assistive Technology). An Evaluation of the Practical and Ethical Implications of Assistive Technology and Devices to Support People with Dementia and their Carers. Bristol: Dementia Voice; 2004.
- Doughty K, Williams G. Practical solutions for the integration of community alarms, assistive technologies and telecare. Qual Ageing Older Adults 2001;2:31-47. https://doi.org/10.1108/14717794200100006.
- Bharucha AJ, Anand V, Forlizzi J, Dew MA, Reynolds CF, Stevens S, et al. Intelligent assistive technology applications to dementia care: current capabilities, limitations, and future challenges. Am J Geriatr Psychiatry 2009;17:88-104. https://doi.org/10.1097/JGP.0b013e318187dde5.
- Barlow J. Building an Evidence Base for Successful Telecare Implementation. Updated Report of the Evidence Working Group of the Telecare Policy Collaborative Chaired by James Barlow 2006.
- Marshall M. ASTRID: A Social and Technological Response to Meeting the Needs of Individuals with Dementia and their Carers; A Guide to Using Technology Within Dementia Care. London: Hawker; 2000.
- Frisby B, Woolham J. Building a local infrastructure that supports the use of assistive technology in the care of people with dementia. Res Policy Plan 2002;20:11-24.
- Fisk MJ. Social Alarms to Telecare: Older People’s Services in Transition. Bristol: The Policy Press; 2003.
- Woolham J, Gibson G, Clarke P. Assistive technology, telecare, and dementia: some implications of current policies and guidance. Res Policy Plan 2006;24:149-64.
- Department of Health and Social Care . Transforming Adult Social Care 2009.
- Poole T. Wanless Social Care Review: Telecare and Older People. London: The King’s Fund; 2006.
- Girodano R, Clark M, Goodwin N. Perspectives on Telehealth and Telecare: Learning from the 12 Whole System Demonstrator Action Network (WSDAN) Sites. WSDAN Briefing Paper. London: The King’s Fund; 2010.
- Bower P, Cartwright M, Hirani SP, Barlow J, Hendy J, Knapp M, et al. A comprehensive evaluation of the impact of telemonitoring in patients with long-term conditions and social care needs: protocol for the whole systems demonstrator cluster randomised trial. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-184.
- Cartwright M, Hirani SP, Rixon L, Beynon M, Doll H, Bower P, et al. Effect of telehealth on quality of life and psychological outcomes over 12 months (Whole Systems Demonstrator telehealth questionnaire study): nested study of patient reported outcomes in a pragmatic, cluster randomised controlled trial. BMJ 2013;346. https://doi.org/10.1136/bmj.f653.
- Henderson C, Knapp M, Fernández JL, Beecham J, Hirani SP, Cartwright M, et al. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ 2013;346. https://doi.org/10.1136/bmj.f1035.
- Steventon A, Bardsley M, Billings J, Dixon J, Doll H, Hirani S, et al. Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ 2012;344. https://doi.org/10.1136/bmj.e3874.
- Hirani SP, Beynon M, Cartwright M, Rixon L, Doll H, Henderson C, et al. The effect of telecare on the quality of life and psychological well-being of elderly recipients of social care over a 12-month period: the Whole Systems Demonstrator cluster randomised trial. Age Ageing 2014;43:334-41. https://doi.org/10.1093/ageing/aft185.
- Steventon A, Bardsley M, Billings J, Dixon J, Doll H, Beynon M, et al. Effect of telecare on use of health and social care services: findings from the Whole Systems Demonstrator cluster randomised trial. Age Ageing 2013;42:501-8. https://doi.org/10.1093/ageing/aft008.
- Henderson C, Knapp M, Fernández JL, Beecham J, Hirani SP, Beynon M, et al. Cost-effectiveness of telecare for people with social care needs: the Whole Systems Demonstrator cluster randomised trial. Age Ageing 2014;43:794-800. https://doi.org/10.1093/ageing/afu067.
- Brownsell S. Measuring the ‘success’ of telehealth interventions. J Assistive Technol 2009;3:12-20. https://doi.org/10.1108/17549450200900030.
- Van der Roest HG, Wenborn J, Pastink C, Dröes RM, Orrell M. Assistive technology for memory support in dementia. Cochrane Database Syst Rev 2017;6. https://doi.org/10.1002/14651858.CD009627.pub2.
- Knapp M, Barlow J, Comas-Herrera A, Damant J, Freddolino P, Hamblin K, et al. The Case for Investment in Technology to Manage the Global Costs of Dementia. London: Policy Innovation Research Unit; 2015.
- Department for Constitutional Affairs . Mental Capacity Act 2005: Code of Practice 2007.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27. https://doi.org/10.1007/s11136-012-0322-4.
- NHS Improvement . Reference Cost Collection: National Schedule of Reference Costs, 2016–17 – NHS Trusts and NHS Foundation Trusts 2017.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- Beecham J, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring Mental Health Needs. London: Gaskell/Royal College of Psychiatrists; 2001.
- Leggett AN, Zarit S, Taylor A, Galvin JE. Stress and burden among caregivers of patients with Lewy body dementia. Gerontologist 2011;51:76-85. https://doi.org/10.1093/geront/gnq055.
- Hirani SP, Rixon L, Beynon M, Cartwright M, Cleanthous S, Selva A, et al. Quantifying beliefs regarding telehealth: development of the Whole Systems Demonstrator Service User Technology Acceptability Questionnaire. J Telemed Telecare 2017;23:460-9. https://doi.org/10.1177/1357633X16649531.
- Fish J, Kreutzer JS, DeLuca J, Caplan B. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer; 2011.
- Courtney C, Farrell D, Gray R, Hills R, Lynch L, Sellwood E, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet 2004;363:2105-15. https://doi.org/10.1016/S0140-6736(04)16499-4.
- World Medical Assembly . Declaration of Helsinki (1964). Br Med J 1996;313:1448-9. https://doi.org/10.1136/bmj.313.7070.1448a.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice . Code of Federal Regulations &Amp; Guidelines Vol. 1. International Committee on Harmonization 1997.
- Forsyth K, Henderson C, Davis L, Singh Roy A, Dunk B, Curnow E, et al. Assessment of need and practice for assistive technology and telecare for people with dementia – The ATTILA (Assistive Technology and Telecare to maintain Independent Living At home for people with dementia) trial. Alzheimers Dement 2019;5:420-30. https://doi.org/10.1016/j.trci.2019.07.010.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Ritchie J, Lewis J. Qualitative Research Practice : A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Ehrenhard M, Kijl B, Nieuwenhuis L. Market adoption barriers of multi-stakeholder technology: smart homes for the aging population. Technol Forecast Soc Change 2014;89:306-15. https://doi.org/10.1016/j.techfore.2014.08.002.
- Kijl B, Nieuwenhuis LJ, Huis in ‘t Veld RM, Hermens HJ, Vollenbroek-Hutten MM. Deployment of e-health services – a business model engineering strategy. J Telemed Telecare 2010;16:344-53. https://doi.org/10.1258/jtt.2010.006009.
- Parkinson SFK, Kielhofner G. User’s Manual for the Model of Human Occupation Screening Tool (MOHOST). Chicago, IL: University of Illinois; 2002.
- Siegel S. Nonparametric Statistics for the Behavioral Sciences. New York, NY: McGraw-Hill; 1956.
- Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry 2006;14:139-44. https://doi.org/10.1097/01.JGP.0000192478.82189.a8.
- Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg 2018;126:1763-8. https://doi.org/10.1213/ANE.0000000000002864.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Valuation Set for England (Updated November 2018) n.d. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed July 2019).
- HM Treasury . The Green Book: Appraisal and Evaluation in Central Government 2015.
- Wimo A, Reed CC, Dodel R, Belger M, Jones RW, Happich M, et al. The GERAS Study: a prospective observational study of costs and resource use in community dwellers with Alzheimer’s disease in three European countries – study design and baseline findings. J Alzheimers Dis 2013;36:385-99. https://doi.org/10.3233/JAD-122392.
- Office for National Statistics . Annual Survey of Hours and Earnings: 2017 Provisional Results 2017.
- Koopmanschap MA, van Exel JN, van den Berg B, Brouwer WB. An overview of methods and applications to value informal care in economic evaluations of healthcare. PharmacoEconomics 2008;26:269-80. https://doi.org/10.2165/00019053-200826040-00001.
- Northern Housing Consortium . Consortium Procurement n.d. https://consortiumprocurement.org.uk (accessed October 2018).
- Billingham LJ, Abrams KR. Simultaneous analysis of quality of life and survival data. Stat Methods Med Res 2002;11:25-48. https://doi.org/10.1191/0962280202sm269ra.
- Gray A. Applied Methods of Cost-effectiveness Analysis in Health Care. Oxford: Oxford University Press; 2011.
- Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika 2000;87:329-43. https://doi.org/10.1093/biomet/87.2.329.
- Basu A, Manning WG. Estimating lifetime or episode-of-illness costs under censoring. Health Econ 2010;19:1010-28. https://doi.org/10.1002/hec.1640.
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. College Station, TX: Stata Press; 2011.
- Allison PD. Handling Missing Data by Maximum Likelihood. Paper 312-2012. SAS Global Forum. Haverford, PA: Statistical Horizons; 2012.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30. https://doi.org/10.1002/hec.678.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013.
- Oremus M, Aguilar SC. A systematic review to assess the policy-making relevance of dementia cost-of-illness studies in the US and Canada. PharmacoEconomics 2011;29:141-56. https://doi.org/10.2165/11539450-000000000-00000.
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523-32. https://doi.org/10.1007/s11136-004-7713-0.
- Glick H. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Hounsome N, Orrell M, Edwards RT. EQ-5D as a quality of life measure in people with dementia and their carers: evidence and key issues. Value Health 2011;14:390-9. https://doi.org/10.1016/j.jval.2010.08.002.
- Aguirre E, Kang S, Hoare Z, Tudor Edwards R, Orrell M. How does the EQ-5D perform when measuring quality of life in dementia against two other dementia-specific outcome measures?. Qual Life Res 2016;25:45-9. https://doi.org/10.1007/s11136-015-1065-9.
- Alzheimer’s Research UK . Women and Dementia: A Marginalised Majority n.d. www.alzheimersresearchuk.org/about-us/our-influence/policy-work/reports/women-dementia/2015 (accessed May 2019).
- Trust C. Key Facts About Carers and the People They Care For n.d. https://carers.org/key-facts-about-carers-and-people-they-care (accessed June 2019).
- Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci 2009;11.
- NHS Digital . Personal Social Services Survey of Adult Carers in England 2016–17 n.d. https://digital.nhs.uk/data-and-information/publications/statistical/personal-social-services-survey-of-adult-carers/personal-social-services-survey-of-adult-carers-in-england-2016-17#resources (accessed June 2019).
- Allen AP, Curran EA, Duggan Á, Cryan JF, Chorcoráin AN, Dinan TG, et al. A systematic review of the psychobiological burden of informal caregiving for patients with dementia: Focus on cognitive and biological markers of chronic stress. Neurosci Biobehav Rev 2017;73:123-64. https://doi.org/10.1016/j.neubiorev.2016.12.006.
- Carers UK. State of Caring 2018 n.d. www.carersuk.org/for-professionals/policy/policy-library/state-of-caring-2018-2 (accessed June 2019).
- Mahoney R, Regan C, Katona C, Livingston G. Anxiety and depression in family caregivers of people with Alzheimer disease: the LASER-AD study. Am J Geriatr Psychiatry 2005;13:795-801. https://doi.org/10.1097/00019442-200509000-00008.
- Seeher K, Low LF, Reppermund S, Brodaty H. Predictors and outcomes for caregivers of people with mild cognitive impairment: a systematic literature review. Alzheimers Dement 2013;9:346-55. https://doi.org/10.1016/j.jalz.2012.01.012.
- Chen ML. The growing costs and burden of family caregiving of older adults: a review of paid sick leave and family leave policies. Gerontologist 2016;56:391-6. https://doi.org/10.1093/geront/gnu093.
- Moore MJ, Zhu CW, Clipp EC. Informal costs of dementia care: estimates from the National Longitudinal Caregiver Study. J Gerontol B Psychol Sci Soc Sci 2001;56:S219-28. https://doi.org/10.1093/geronb/56.4.s219.
- Dassel KB, Carr DC. Does dementia caregiving accelerate frailty? Findings from the health and retirement study. Gerontologist 2016;56:444-50. https://doi.org/10.1093/geront/gnu078.
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull 2003;129:946-72. https://doi.org/10.1037/0033-2909.129.6.946.
- Alzheimer’s Association . 2017 Alzheimer’s disease facts and figures. Alzheimer Dement 2017;13:325-73. https://doi.org/10.1016/j.jalz.2017.02.001.
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Hererra A, et al. Dementia UK: Update. London: Alzheimer’s Society; 2014.
- Wimo A, Winblad B, Jönsson L. The worldwide societal costs of dementia: estimates for 2009. Alzheimers Dement 2010;6:98-103. https://doi.org/10.1016/j.jalz.2010.01.010.
- Zarit SH, Todd PA, Zarit JM. Subjective burden of husbands and wives as caregivers: a longitudinal study. Gerontologist 1986;26:260-6. https://doi.org/10.1093/geront/26.3.260.
- Chiao CY, Wu HS, Hsiao CY. Caregiver burden for informal caregivers of patients with dementia: a systematic review. Int Nurs Rev 2015;62:340-50. https://doi.org/10.1111/inr.12194.
- Schofield H, Bloch S. Family Caregivers: Disability, Illness and Ageing. Sydney, NSW: Allen & Unwin; 1998.
- Schofield H, Murphy B, Herrman HE, Bloch S, Singh BS. Carers of people aged over 50 with physical impairment, memory loss and dementia: a comparative study. Ageing Soc 1998;18:355-69. https://doi.org/10.1017/S0144686X98006965.
- Ashworth M, Baker AH. ‘Time and space’: carers’ views about respite care. Health Soc Care Community 2000;8:50-6. https://doi.org/10.1046/j.1365-2524.2000.00221.x.
- Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry 2012;169:946-53. https://doi.org/10.1176/appi.ajp.2012.11101529.
- Cooke DD, McNally L, Mulligan KT, Harrison MJ, Newman SP. Psychosocial interventions for caregivers of people with dementia: a systematic review. Aging Ment Health 2001;5:120-35. https://doi.org/10.1080/713650019.
- Gitlin LN, Marx K, Stanley IH, Hodgson N. Translating evidence-based dementia caregiving interventions into practice: state-of-the-science and next steps. Gerontologist 2015;55:210-26. https://doi.org/10.1093/geront/gnu123.
- Lindberg B, Nilsson C, Zotterman D, Söderberg S, Skär L. Using information and communication technology in home care for communication between patients, family members, and healthcare professionals: a systematic review. Int J Telemed Appl 2013;2013. https://doi.org/10.1155/2013/461829.
- Schware R, Kenny C, Foster V, Wellenius B, Dokeniya A, Wheeler D, et al. Information and Communication Technologies: A World Bank Group Strategy. Washington, DC: World Bank Publications; 2002.
- Lucero RJ, Fehlberg EA, Patel AGM, Bjarnardottir RI, Williams R, Lee K, et al. The effects of information and communication technologies on informal caregivers of persons living with dementia: a systematic review. Alzheimers Dement 2019;5:1-12. https://doi.org/10.1016/j.trci.2018.11.003.
- Davies A, Rixon L, Newman S. Systematic review of the effects of telecare provided for a person with social care needs on outcomes for their informal carers. Health Soc Care Community 2013;21:582-97. https://doi.org/10.1111/hsc.12035.
- Alwan M, Dalal S, Mack D, Kell SW, Turner B, Leachtenauer J, et al. Impact of monitoring technology in assisted living: outcome pilot. IEEE Trans Inf Technol Biomed 2006;10:192-8. https://doi.org/10.1109/titb.2005.855552.
- Mellors S. Service User and Carer Views of Telecare in Derbyshire. Matlock: Derbyshire County Council; 2009.
- Holthe T. Enabling Technologies for People With Dementia: National Report on Results from Norway 2004. www.enableproject.org/download/Enable%20-%20National%20Report%20-%20Norway.pdf (accessed 26 February 2020).
- Woolham J. The Safe at Home Project: Using Technology to Help People with Dementia Remain Living in their Own Homes in Northampton. London: Hawker; 2005.
- Molloy DW, Alemayehu E, Roberts R. Reliability of a Standardized Mini-Mental State Examination compared with the traditional Mini-Mental State Examination. Am J Psychiatry 1991;148:102-5. https://doi.org/10.1176/ajp.148.1.102.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649-55. https://doi.org/10.1093/geront/20.6.649.
- Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol 1992;31:301-6. https://doi.org/10.1111/j.2044-8260.1992.tb00997.x.
- Little RJA, Rubin DB. The analysis of social science data with missing values. Soc Methods Res 1989;18:292-326. https://doi.org/10.1177/0049124189018002004.
- Rubin DB. The calculation of posterior distributions by data augmentation: comment: a noniterative sampling/importance resampling alternative to the data augmentation algorithm for creating a few imputations when fractions of missing information are modest: the SIR algorithm. J Am Stat Assoc 1987;82:543-46. https://doi.org/10.2307/2289460.
- Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3-15. https://doi.org/10.1177/096228029900800102.
- Neubauer S, Holle R, Menn P, Grossfeld-Schmitz M, Graesel E. Measurement of informal care time in a study of patients with dementia. Int Psychogeriatr 2008;20:1160-76. https://doi.org/10.1017/S1041610208007564.
- Joling KJ, van Marwijk HW, Veldhuijzen AE, van der Horst HE, Scheltens P, Smit F, et al. The two-year incidence of depression and anxiety disorders in spousal caregivers of persons with dementia: who is at the greatest risk?. Am J Geriatr Psychiatry 2015;23:293-30. https://doi.org/10.1016/j.jagp.2014.05.005.
- Meshefedjian G, McCusker J, Bellavance F, Baumgarten M. Factors associated with symptoms of depression among informal caregivers of demented elders in the community. Gerontologist 1998;38:247-53. https://doi.org/10.1093/geront/38.2.247.
- Schoenmakers B, Buntinx F, Delepeleire J. Factors determining the impact of care-giving on caregivers of elderly patients with dementia. A systematic literature review. Maturitas 2010;66:191-200. https://doi.org/10.1016/j.maturitas.2010.02.009.
- Jütten LH, Mark RE, Sitskoorn MM. Empathy in informal dementia caregivers and its relationship with depression, anxiety, and burden. Int J Clin Health Psychol 2019;19:12-21. https://doi.org/10.1016/j.ijchp.2018.07.004.
- Blom MM, Zarit SH, Groot Zwaaftink RB, Cuijpers P, Pot AM. Effectiveness of an Internet intervention for family caregivers of people with dementia: results of a randomized controlled trial. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0116622.
- Borsje P, Hems MA, Lucassen PL, Bor H, Koopmans RT, Pot AM. Psychological distress in informal caregivers of patients with dementia in primary care: course and determinants. Fam Pract 2016;33:374-81. https://doi.org/10.1093/fampra/cmw009.
- Sörensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist 2002;42:356-72. https://doi.org/10.1093/geront/42.3.356.
- Parker D, Mills S, Abbey J. Effectiveness of interventions that assist caregivers to support people with dementia living in the community: a systematic review. Int J Evid Based Healthc 2008;6:137-72. https://doi.org/10.1111/j.1744-1609.2008.00090.x.
- Thompson CA, Spilsbury K, Hall J, Birks Y, Barnes C, Adamson J. Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatr 2007;7. https://doi.org/10.1186/1471-2318-7-18.
- Vernooij-Dassen M, Draskovic I, McCleery J, Downs M. Cognitive reframing for carers of people with dementia. Cochrane Database Syst Rev 2011;11. https://doi.org/10.1002/14651858.CD005318.pub2.
- Lins S, Hayder-Beichel D, Rücker G, Motschall E, Antes G, Meyer G, et al. Efficacy and experiences of telephone counselling for informal carers of people with dementia. Cochrane Database Syst Rev 2014;9. https://doi.org/10.1002/14651858.CD009126.pub2.
- Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, Del Ser T, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord 2010;30:161-78. https://doi.org/10.1159/000316119.
- Kaizik C, Caga J, Camino J, O’Connor CM, McKinnon C, Oyebode JR, et al. Factors underpinning caregiver burden in frontotemporal dementia differ in spouses and their children. J Alzheimers Dis 2017;56:1109-17. https://doi.org/10.3233/JAD-160852.
- Alfakhri AS, Alshudukhi AW, Alqahtani AA, Alhumaid AM, Alhathlol OA, Almojali AI, et al. Depression among caregivers of patients with dementia. Inquiry 2018;55. https://doi.org/10.1177/0046958017750432.
- Hammersley M, Atkinson P. Ethnography: Principles in Practice. Abingdon: Routledge; 2007.
- Wenborn J, Hynes S, Moniz-Cook E, Mountain G, Poland F, King M, et al. Community occupational therapy for people with dementia and family carers (COTiD-UK) versus treatment as usual (Valuing Active Life in Dementia [VALID] programme): study protocol for a randomised controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-015-1150-y.
- Lewis SJ, Russell AJ. Being embedded: a way forward for ethnographic research. Ethnography 2011;12:398-416. https://doi.org/10.1177/1466138110393786.
- Pink S, Morgan J. Short-term ethnography: intense routes to knowing. Symb Interact 2013;36:351-61. https://doi.org/10.1002/symb.66.
- Knoblauch H. Focused ethnography. Forum Qual Social Res 2005;6. https://doi.org/10.17169/fqs-6.3.20.
- Burawoy M. The extended case method. Soc Theory 1998;16:4-33. https://doi.org/10.1111/0735-2751.00040.
- Van Velsen J, Epstein AL. The Craft of Social Anthropology. Oxford: Pergamon Press; 1979.
- Guba EG. Criteria for assessing the trustworthiness of naturalistic inquiries. Educ Commun Technol J 1981;29:75-91.
- Guba EG, Lincoln YS, Denzin NK, Lincoln YS. Handbook of Qualitative Research. Thousand Oaks, CA: SAGE Publications Inc.; 1994.
- Office for National Statistics . Towns and Cities Analysis, England and Wales, March 2016 2016.
- Gibson G, Dickinson C, Brittain K, Robinson L. Personalisation, customisation and bricolage: how people with dementia and their families make assistive technology work for them. Ageing Soc 2019;39:2502-19. https://doi.org/10.1017/S0144686X18000661.
- Greenhalgh T, Wherton J, Sugarhood P, Hinder S, Procter R, Stones R. What matters to older people with assisted living needs? A phenomenological analysis of the use and non-use of telehealth and telecare. Soc Sci Med 2013;93:86-94. https://doi.org/10.1016/j.socscimed.2013.05.036.
- Mort M, Roberts C, Pols J, Domenech M, Moser I. EFORTT investigators . Ethical implications of home telecare for older people: a framework derived from a multisited participative study. Health Expect 2015;18:438-49. https://doi.org/10.1111/hex.12109.
- Sanders C, Rogers A, Bowen R, Bower P, Hirani S, Cartwright M, et al. Exploring barriers to participation and adoption of telehealth and telecare within the Whole System Demonstrator trial: a qualitative study. BMC Health Serv Res 2012;12. https://doi.org/10.1186/1472-6963-12-220.
- Samus QM, Black BS, Bovenkamp D, Buckley M, Callahan C, Davis K, et al. Home is where the future is: the BrightFocus Foundation consensus panel on dementia care. Alzheimers Dement 2018;14:104-14. https://doi.org/10.1016/j.jalz.2017.10.006.
- von Kutzleben M, Schmid W, Halek M, Holle B, Bartholomeyczik S. Community-dwelling persons with dementia: what do they need? What do they demand? What do they do? A systematic review on the subjective experiences of persons with dementia. Aging Ment Health 2012;16:378-90. https://doi.org/10.1080/13607863.2011.614594.
- Department of Health and Social Care . Prime Minister’s Challenge on Dementia 2020: Implementation Plan 2016.
- Aminzadeh F, Dalziel WB, Molnar FJ, Garcia LJ. Symbolic meaning of relocation to a residential care facility for persons with dementia. Aging Ment Health 2009;13:487-96. https://doi.org/10.1080/13607860802607314.
- Tchalla AE, Lachal F, Cardinaud N, Saulnier I, Rialle V, Preux PM, et al. Preventing and managing indoor falls with home-based technologies in mild and moderate Alzheimer’s disease patients: pilot study in a community dwelling. Dementia and Geriatric Cognitive Disorders 2013;36:251-61. https://doi.org/10.1159/000351863.
- Brims L, Oliver K. Effectiveness of assistive technology in improving the safety of people with dementia: a systematic review and meta-analysis. Aging and Mental Health 2019;23:942-51. https://doi.org/10.1080/13607863.2018.1455805.
- Alzheimer’s Disease International . Dementia Statistics n.d. www.alz.co.uk/research/statistics (accessed July 2019).
- Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med 2008;148:379-97. https://doi.org/10.7326/0003-4819-148-5-200803040-00009.
- Holthe T, Jentoft R, Arntzen C, Thorsen K. Benefits and burdens: family caregivers’ experiences of assistive technology (AT) in everyday life with persons with young-onset dementia (YOD). Disabil Rehabil Assist Technol 2018;13:754-62. https://doi.org/10.1080/17483107.2017.1373151.
- Nauha L, Keränen NS, Kangas M, Jämsä T, Reponen J. Assistive technologies at home for people with a memory disorder. Dementia 2018;17:909-23. https://doi.org/10.1177/1471301216674816.
- Liddle J, Smith SJ. Intelligent assistive technology for people living with dementia is a rapidly growing and changing area requiring clinical consideration. Aust Occup Ther J 2017;64:510-11. https://doi.org/10.1111/1440-1630.12434.
- Megges H, Freiesleben SD, Rösch C, Knoll N, Wessel L, Peters O. User experience and clinical effectiveness with two wearable global positioning system devices in home dementia care. Alzheimers Dement 2018;4:636-44. https://doi.org/10.1016/j.trci.2018.10.002.
- Gibson G, Newton L, Pritchard G, Finch T, Brittain K, Robinson L. The provision of assistive technology products and services for people with dementia in the United Kingdom. Dementia 2016;15:681-70. https://doi.org/10.1177/1471301214532643.
- Momanyi K. Enhancing Quality in Social Care Through Economic Analysis 2018.
- Allen C, Beecham J, Netten A, Beecham J. Costing Community Care: Theory and Practice. Avebury: Ashgate; 1993.
- Beecham J. Unit Costs – Not Exactly Child’s Play. London: Department of Health and Social Care, Personal Social Services Research Unit and Dartington Social Care Research Unit; 2000.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Banks L, Barnes M. Evaluation of the East Sussex Carers’ Breaks Demonstrator Site. Brighton: University of Brighton; 2011.
- Federation of (Ophthalmic and Dispensing) Opticians (FODO) . GOS Sight Test Fees 2018. www.fodo.com/resource-categories/nhs-sight-test-fees (accessed August 2019).
- Department of Health and Social Care . General Ophthalmic Services: NHS Sight Test Fee, Increases to NHS Optical Voucher Values, Payments for Continuing Education and Training and Pre-Registration Supervisors Grant 2016.
- Department of Health and Social Care . General Ophthalmic Services – Increases to NHS Sight Test Fee, Continuing Education and Training Payment and Pre-Registration Supervisors Grant 2015.
- Romeo R, Knapp M, Banerjee S, Morris J, Baldwin R, Tarrier N, et al. Treatment and prevention of depression after surgery for hip fracture in older people: cost-effectiveness analysis. J Affect Disord 2011;128:211-19. https://doi.org/10.1016/j.jad.2010.07.026.
- Office for National Statistics (ONS) . Consumer Prices Index Including Owner occupiers’ Housing Costs (CPIH) 2019. www.ons.gov.uk/economy/inflationandpriceindices/timeseries/l522/mm23 (accessed 10 May 2019).
- Iliffe S, Wilcock J, Drennan V, Goodman C, Griffin M, Knapp M, et al. Changing practice in dementia care in the community: developing and testing evidence-based interventions, from timely diagnosis to end of life (EVIDEM). Programme Grants Appl Res 2015;3. https://doi.org/10.3310/pgfar03030.
- NHS Digital . Prescription Cost Analysis England 2017 2018.
- Automobile Association . Mileage Calculator n.d. www.theaa.com/driving/mileage-calculator.jsp (accessed 11 October 2018).
Appendix 1 Costing the intervention
The ATTILA trial: proposal for costing the assistive technology and telecare intervention
In this appendix, we set out the proposed plan of methods to calculate the costs of providing the ATT intervention, written while the trial was still in progress. The proposal begins with an overview of the trial as a whole and of the economic evaluation in particular, and lists the data collections that supported the economic evaluation. The costs that we proposed to collect are then summarised, and the methods for these data collections are outlined.
Trial overview
The ATTILA trial is evaluating the impacts of ATT on people with dementia and their carers. ATT is defined for the purposes of the trial as ‘simple, battery-operated, standalone technologies and/or telecare (a range of devices and sensors that communicate and relay messages to an external call centre where an appropriate response is arranged)’ (see Additional interviews and correspondence to support the economic evaluation: sample selection and recruitment of key informants). The trial is designed as a pragmatic RCT. Participants randomised to the intervention will receive an ATT needs assessment, followed by the installation of ATT devices and response services deployed by the host local authority, in addition to usual health and social care services. Control group participants will receive an ATT needs assessment followed by a package of ATT that is limited to smoke/carbon monoxide detectors and pendant alarms, in addition to usual health and social care services. Thirteen sites in England (Lambeth, Southwark, Croydon, Lancashire, Oxford, Suffolk, Norfolk, Cambridgeshire, Nottingham, Blackpool, West Sussex, Barnsley and Blackburn) are currently involved in the trial. As the trial design is pragmatic, all aspects of the intervention (ATT assessment, choice of devices, or ordering and installation of devices) are determined by staff from the participating local authorities or telecare providers.
Economic evaluation activities
The economic evaluation will investigate service use and costs associated with the introduction of the ATT intervention, and examine the relationship between service costs and other impacts. The co-primary trial outcomes are (1) time, in days, from randomisation to institutionalisation and (2) cost-effectiveness of the ATT intervention. The economic evaluation addresses the second outcome. The methods of the cost-effectiveness analysis described in the trial protocol (see Additional interviews and correspondence to support the economic evaluation: sample selection and recruitment of key informants) are summarised in Box 1.
Cost-effectiveness of the ATT intervention: costs related to the use of ATT, health-care and other service use patterns and (unpaid) caregiver inputs will be calculated for each participant using a modified version of the CSRI.
The cost-effectiveness analyses will be of two types, each conducted from two perspectives: (1) health and social care, and (2) societal, whereby the first type will measure costs only up to the point that a trial participant goes into a care home or hospital, and not beyond (‘community costs’), and then examine cost-effectiveness in achieving the primary outcome (days from randomisation to institutionalisation in the 2-year period). This analysis will show the incremental cost of community-based support of each additional institutional day avoided. The second analysis type will measure costs for the whole 2-year period, including costs of care home and hospital stays (‘total costs’), and then examine cost-effectiveness for which the outcome is change in EQ-5D score, over the 2-year period.
Trial data collections
Current data collections include:
-
Participants and their identified carers will be visited by study research assistants to complete a battery of questionnaires covering self-reported quality of life, acceptability of the technology, ATT in situ (using a technology checklist) and service use and costs (using the CSRI44). These visits will take place at five time points: baseline and 12, 24, 52 and 104 weeks thereafter. Questionnaire packs will be sent to the Oxford Clinical Trials Service Unit for data entry or data will be entered directly into an electronic database.
-
Data on ATT assessments are gathered by Kirsty Forsyth. Forms completed by assessors in order to recommend a package of ATT are rated against the MOHOST.
Proposed methods for calculating the costs of the assistive technology and telecare intervention
In this section, we set out options for collecting information sufficient to describe, measure and value the ATT intervention.
In the ATTILA trial, there is no funding provided alongside the trial for ATT equipment and there is no definitive list of ATT equipment to be provided across sites. ATT packages provided to ATTILA trial participants are likely to be highly heterogeneous across and between sites. It will be necessary to describe those packages to understand the relationship between participant outcomes and the intervention, but we face considerable challenges in collecting these data. Local authority budgets are under great pressure and the team is aware of the resultant constraints on frontline and senior management staff time.
We propose to calculate the ATT intervention costs as follows:163,164
-
We will describe the interventions in terms of typical resource inputs and associated activities. This will entail creating descriptions of the organisations involved in producing the ATT intervention, including details of staffing and overheads.
-
We will calculate a relevant service unit, in this case the weekly per-person cost of the intervention.
-
We will collect cost data. Several elements make up the total cost of an ATT package –
-
Costs of ATT needs assessment.
-
Assessment costs will be calculated based on attaching a per-minute staff cost (including relevant overheads) of local authority and NHS staff to the estimated length of time spent in carrying out and writing up ATT assessments, including travel time, and allowing for travel costs. Data on average length of ATT assessments will be sought from key informants. Some providers may be able to make use of routinely collected data on time spent in ATT assessment, whereas others may provide their best estimate through less formal means.
-
-
ATT service and equipment costs.
-
Costs of equipment.
-
Monitored equipment: ideally, sites will be able to provide lists of equipment used by each participant so that prices can be attached to establish their costs. The appropriate data-sharing agreements must be in place with the ATTILA trial team at KCL prior to requesting such lists. Price lists of standalone and networked equipment will be sought from the appropriate organisation or obtained from publicly available lists, where these exist.
-
Standalone equipment as (a).
-
-
Costs of ATT support.
-
Costs to the local authority. We would expect that a unit cost of a telecare package would comprise:
-
costs of installation, routine maintenance and upgrading of equipment
-
call centre infrastructure – operators’ staff costs (and on-costs), capital, premises and administrative overheads
-
-
Response-related costs.
-
Costs of any contracts with response service dedicated to telecare.
-
Other responders, for example emergency services – this information will be partly captured via CSRI (responses reported within the 3 month retrospective period). To avoid double-counting, these costs will be for the most part estimated by weighting units of service use as reported in the self-completed CSRI by unit costs from the PSSRU Unit Costs. 165
-
-
Costs to carers.
-
Carers’ time spent responding to ATT-related sensor and other alerts can be captured via the CSRI question on time spent in caring tasks such as supervision (responses reported within the 3-month retrospective period).
-
-
-
-
The exact strategy for costing these elements is dependent on the extent of available financial and activity information from sites. Ideally, key informants in each site would be able to provide budgetary/expenditure and activity data for use in calculating the unit cost of an ATT package. However it would also be acceptable to use a unit cost of a telecare package if the site has already made that calculation.
We will calculate a unit cost for the intervention.
The methods required to make this calculation again depend on the availability of financial or local authority -calculated unit cost data, of already established unit costs, and of equipment data. The total ATT package cost will be calculated by summing the costs of ATT assessment, telecare and standalone devices, and telecare support services.
The cost of the ATT assessment and of a telecare support package will be estimated at the site level; that is, all participants in that site would be considered to have incurred the same cost for these elements of the intervention. Networked and standalone devices should be available for each participant and the costs will be unique to that participant. In the case that providers cannot or decline to assemble a list of equipment provided, prices will be assigned to devices as recorded on the technology checklist administered to participants, and costs estimated on this basis.
Proposed new data collections relevant to the economic evaluation
As a first step in the proposed costing strategy, we devised a pro forma (see Appendix 2) to support a desk-based review of ATT assessment and provision processes in each site. The pro forma was devised by the economic evaluator and completed in co-operation with the researchers at Queen Margaret University, Edinburgh, the ATTILA trial project manager and the study’s local researchers, who are embedded in each site. In this pro forma are listed any relevant documents already shared by the sites with the project team, brief descriptions of the processes for ATT assessment and provision already known to the local researchers in the execution of their role and suggestions for potential local key informants (e.g. operational managers, commissioners and managers of telecare providers). We now wish to proceed with collecting information from the following personnel in participating sites:
-
Local authority operational managers – operational managers will probably have an overview of the mechanisms in place in their site for assessment and referral for ATT. These managers may also be able to advise on whether or not their local authority databases encompass telecare and standalone equipment records.
-
Local authority commissioners of telecare – it is likely that the commissioners of telecare have information on the cost of telecare services within their local authority (including situations in which all telecare provision is contracted out), they may have calculated a unit cost of telecare for their local authority, and might be able to provide equipment price lists.
-
Managers of telecare providers – it is likely that the providers of telecare have information on their own staff costs, premises, costs of installation/de-installation and maintenance if provided, also server costs and database maintenance. The managers would be able to advise on whether or not their databases contain records of the telecare equipment in place and might be able to provide equipment price lists.
Proposed activities
-
Seek NHS Research Ethics Committee permission for interviews with key informants.
-
Local researchers to provide the economic evaluator with a set of current contact details for potential key informants, with their permission:
-
local authority operational/middle managers in adult services
-
local authority commissioners of telecare
-
telecare providers managers.
-
-
Researchers from the project team to set up and carry out interviews with key informants in each site (after Research Ethics Committee approval). Contact to be made by e-mail or telephone and a list of the relevant questions sent to the potential participants, who can then decide whether or not to participate. Interviews to be carried out via site visits or over the telephone. Interviews will not be recorded but handwritten notes will be taken.
-
Project team to request relevant budget/expenditure data from key informants (local authority and telecare providers’ managers).
-
Project team to update data-sharing agreements as required prior to requesting participants’ equipment data from relevant local authority and telecare providers; project team to establish procedures for transferring these data to the project team.
Additional interviews and correspondence to support the economic evaluation: sample selection and recruitment of key informants
Recruitment targets for key informants
| Cross-reference | Participants | Numbers | Nature of contact | Time taken | Information sought |
|---|---|---|---|---|---|
| Figure 18 | Local authority commissioners of telecare | 1 per site | Face-to-face interview OR | 45 minutes maximum | Costs of telecare services within their local authority |
| Telephone interview; any follow-up enquiries by correspondence | |||||
| Figure 19 | Local authority operational managers | 1 or 2 per site | Face-to-face interview OR | In person or by telephone: 20–30 minutes maximum | Mechanisms in place for assessment and referral for ATT; systems for recording telecare and standalone equipment provision |
| Telephone interview; any follow-up enquiries by correspondence | |||||
| Table 38 | Managers of telecare providers | 1–5 per sitea | Face-to-face interview OR | In person or by telephone: 45 minutes maximum | Information on staff costs, premises, costs of installation/de-installation and maintenance if provided; server costs and database maintenance; systems for recording telecare and standalone equipment provision and prices |
| Telephone interviews; any follow-up enquiries by correspondence |
Pro formas
Appendix 2 Rating of assistive technology and telecare need
| ATT assessment standard | Rating ATT need | |||
|---|---|---|---|---|
| ATTILA trial sites’ key questions | ATTILA trial sites’ questions | MOHOST | ||
| Items (partial) | Domains | |||
| Does the person’s motivation put them at risk when doing daily activity? | Does the person’s insight put them at higher/lower risk? | Appraisal of ability | Motivation for occupation |
When doing daily activity, does this issue cause risk? If no . . . choose either:If yes . . . choose either: |
| Does what is important to the person put them at higher or lower risk? | Choices | |||
| Do the person’s routines and responsibilities put them at risk when doing daily activity? | Do the person’s routines put them at higher/lower risk? | Routines | Pattern of occupation | |
| Do the person’s responsibilities put them at higher/lower risk? | Responsibilities | |||
| Does the person’s communication skill place them at risk when doing daily activity? | Does the person’s ability to have a conversation put them at higher/lower risk? | Conversation | Communication and interaction skills | |
| Does the person’s ability to express their needs put them at higher/lower risk? | Vocal expression | |||
| Does the person’s cognitive skill place them at risk when doing daily activity? | Does memory and understanding of how to do things put the person at higher/lower risk? | Knowledge | Process skills | |
| Does the ability to problem solve put the person at higher/lower risk? | Problem-solving | |||
| Does the person’s physical skill place them at risk when doing daily activity? | Does the person’s mobility put them at higher/lower risk? | Posture and mobility | Motor skills | |
| Does the person’s grip/dexterity put them at higher/lower risk? | Strength and effort | |||
| Do the features of the physical environment put the person at risk when doing daily activity? | Does the person’s physical space put them at higher/lower risk? | Physical space | Physical environment | |
| Does the persons physical resources put them at higher/lower risk? | Physical resources | |||
| Does who is involved and how activities are completed put the person at risk when doing daily activity? | Does the support available put them at higher/lower risk? | Social groups | Social environment | |
| Does the way the person completes activity put them at higher/lower risk? | Occupational demands | |||
Motivation
| ATTILA trial sites’ questions | Score | ATT needs scale |
|---|---|---|
| Key question: Does the person’s motivation put them at risk when doing daily activity? | ||
|
Insight Does the person’s insight put them at higher/lower risk? |
4 | Accurately assesses own capacity, recognises strengths, aware of limitations. No risk when doing daily activity because the person is mostly doing activities within their ability (appropriately confident), mostly has insight to activate ATT if required, has involvement in ATT process |
| 3 | Reasonable tendency to over/underestimate own abilities, recognises some limitations. Mostly risk free when doing daily activity, the person is mostly doing activities within their ability (appropriately confident), mostly has insight to activate ATT if required, has involvement in ATT process. Difficulty understanding strengths and limitations without support | |
| 2 | Some risk when doing daily activity related to the person being overconfident (thinking they have the ability to do activity when they do not), underconfident (can do the activity but do not think they can), difficulty knowing to activate ATT owing to some lack of insight, difficulty being involved in ATT process. Does not reflect on skills, fails to realistically estimate own abilities | |
| 1 | Significant multiple risks when doing daily activity due to no insight into their lack of ability to safely do everyday activity (may appear overconfident), lacks confidence to do activities leading to risks, lack insight to activate ATT if required, not able to be involved in ATT process | |
|
Values Does what is important to the person put them at higher or lower risk? |
4 | Clear preferences and sense of what is important, motivated to work towards occupational goals. No risk when doing daily activity, the person’s skills matches what they think is really important to do, they have things that are important to them and are active, support is acceptable to them, willing to explore options |
| 3 | Mostly able to make choices, may need encouragement to set and work towards goals. Mostly risk free when doing daily activity, the person’s skills mostly match what they think is really important to do, they have some things that are important to them and are mostly active, support is marginally acceptable to them but they feel it is important to be independent, reluctantly willing to explore options, that is they do not want ugly equipment as they are house proud. | |
| 2 | Difficulties identifying what is important or setting and working towards goals, inconsistent. Some risk when doing daily activity because the person’s skills do not always match what they think is really important to do, some things are important to them but can be passive, support is marginally acceptable to them but they feel it is important to be independent, reluctantly willing to explore options, that is they do not want ugly equipment as they are house proud | |
| 1 | Cannot set goals, impulsive, chaotic, goals are unattainable or based on antisocial values. Significant multiple risks when doing daily activity because the person’s skills do not match what they think is really important to do; nothing important to them, leading to passivity; support is not acceptable to them as they feel that it is important to be independent; not willing to explore options, that is they do not want ugly equipment as they are house proud | |
Routine
| ATTILA trial questions | Score | ATTILA trial scale |
|---|---|---|
| Key question: Do the person’s routines and responsibilities put them at risk when doing daily activity? | ||
|
Wandering/disorientation Do the person’s routines put them at higher/lower risk? |
4 | Able to arrange a balanced, organised and productive routine of daily activates. No risk when doing daily routine, for example productive routine, no wandering, balance of sleeping at night and productive activity during day, up at night but able to go back to bed, calm settled routine |
| 3 | Generally able to maintain or follow an organised and productive daily schedule. Mostly risk free when doing daily routine including sporadic wandering, disturbance in day/night activity levels, getting up at night and become disoriented, kitchen routines not effective, periods of restlessness, periods of agitation/aggression | |
| 2 | Difficulty organising balanced, productive routines of daily activities without support. Some risk when doing daily routine including some wandering, disturbance in day/night activity levels, getting up at night and become disoriented, kitchen routines not effective, periods of restlessness, periods of agitation/aggression | |
| 1 | Chaotic or empty routine, unable to support responsibilities and goals, erratic routine. Significant multiple risks when doing daily routines including wandering, disturbance in day/night activity levels, getting up at night and become disoriented, kitchen routines not effective, periods of restlessness, periods of agitation/aggression | |
|
Daily activity Do the person’s responsibilities put them at higher/lower risk? |
4 | Reliably completes activities and meets the expectations related to role obligations. No risk when doing daily responsibilities, for example including managing medication, safely doing their cooking, able to safely make a hot drink/snack, safely bathe/dress |
| 3 | Copes with most responsibilities, meets most expectations, able to fulfil most role obligations. Mostly risk free when doing daily responsibilities including sporadic difficulties with managing medication, safely do their cooking, make a hot drink/snack, safely bathe/dress | |
| 2 | Difficulty being able to fulfil expectations and meet role obligations without support. Some risk related to doing daily responsibilities including difficulties managing medication, difficulty to safely do their cooking, make a hot drink/snack, difficulty to safely bathe/dress | |
| 1 | Limited ability to meet demands of activities or obligations, unable to complete role activities. Significant multiple risks doing daily responsibilities, for example cannot manage medication, cannot safely do their cooking, make a hot drink/snack, cannot safely bathe/dress | |
Communication
| ATTILA trial questions | Score | ATTILA trial scale |
|---|---|---|
| Key question: Does the person’s communication skill place them at risk when doing everyday things? | ||
|
Conversation Does the person’s ability to have a conversation put them at higher or lower risk? |
4 | Appropriately initiates, discloses and sustains conversation (clear/direct/open). Mostly risk free when doing daily activity as a result of no confabulation, able to communicate their needs, ability to use a telephone or lifeline unit without becoming disorientated in conversation |
| 3 | Generally able to use language or signing to effectively exchange information. Mostly risk free when doing daily activity owing to limited confabulation, mostly able to communicate their needs, mostly able to use a telephone or lifeline unit without becoming disorientated in conversation | |
| 2 | Difficulty initiating, disclosing or sustaining conversation (hesitant/abrupt/limited/irrelevant). Some risk when doing daily activity owing to some confabulation, only able to communicate some of their needs, some ability to use a telephone or lifeline unit without becoming disorientated in conversation | |
| 1 | Uncommunicative, disjointed, bizarre or inappropriate disclosure of information. Significant multiple risks when doing daily activity owing to confabulation, unable to communicate their needs, unable to use a telephone or lifeline unit without becoming disorientated in conversation | |
|
Express needs Does the person’s ability to express their needs put them at higher or lower risk? |
4 | Assertive, articulate, uses appropriate tone, volume and pace. Mostly risk free when doing daily activity owing to no speech impairment, no word substitutions, no stammering, adequate vocabulary |
| 3 | Vocal expression is generally appropriate in tone, volume and pace. Mostly risk free when doing daily activity owing to minimal speech impairment, minimal word substitutions, minimal stammering, mostly adequate vocabulary | |
| 2 | Difficulty with expressing self (mumbling/pressured speech/monotone). Some risk when doing daily activity owing to speech impairment, as there are word substitutions for words that sound the same, stammering, function of items described rather than the names of items, limited vocabulary | |
| 1 | Unable to express self (unclear/too quiet or loud/too fast or too passive). Significant multiple risks when doing daily activity owing to speech impairment, an inability to express their needs, incomplete sentence structure, mute, speak in another language only | |
Cognitive skills
| ATTILA trial questions | Score | ATTILA trial scale |
|---|---|---|
| Key question: Does the person’s cognitive skill place them at risk when doing everyday things? | ||
|
Memory Does memory and understanding of how to do things put the person at higher/lower risk? |
4 | Seeks and retains relevant information, know how to use tools appropriately. No risk when doing daily activity inclusive of not needing prompting, remembering to take medication, remembering to close doors/turn off taps, aware of how to use appliances, aware of how to respond to alarms |
| 3 | Generally able to seek and retain information and know how to use tools. Mostly risk free when doing daily activity inclusive of occasionally needing prompting, occasionally forgetting to take medication, occasionally forgetting to close doors/turn off taps, mostly awareness of how to use appliances, mostly aware of how to respond to alarms | |
| 2 | Difficulty knowing how to use tools, difficulty in asking for or retaining information. Some risk when doing daily activity inclusive of needing some prompting, sometimes forgetting to take medication, sometimes forgetting to close doors/turn off taps, some awareness of how to use appliances, some awareness of how to respond to alarms | |
| 1 | Unable to use knowledge/tools, does not retain information, asks repeatedly for same information. Significant multiple risks when doing daily activity inclusive of needing prompting, forgetting to take medication, forgetting to close doors/turn off taps, no awareness of how to use appliances, no awareness of how to respond to alarms | |
|
Problem-solving Does the ability to problem solve put the person at higher/lower risk? |
4 | Shows good judgement, anticipates difficulties and generates workable solutions (rational). No risk when doing daily activity inclusive of never letting in strangers, always closes doors in winter, never burns food, no cigarette burn marks, gas use always safe, no tampering with controls, heating always switched on in cold weather |
| 3 | Generally able to make decisions based on difficulties that arise. Mostly risk free when doing daily activity inclusive of rarely letting in strangers, mostly closes doors in winter, rarely burns food, one or two cigarette burn marks, gas use mostly safe, minimal tampering with controls, rarely heating not switched on in cold weather | |
| 2 | Difficulty anticipating and adapting to difficulties that arise, seeks reassurance. Some risk when doing daily activity inclusive of sometimes letting in strangers, sometimes leaving doors open in winter, sometimes burns food, some cigarette burn marks, some flooding, sometimes gas left on despite smell, sometimes tampering with controls, heating sometimes not switched on in cold weather | |
| 1 | Unable to anticipate and adapt to difficulties that arise and makes inappropriate decisions. Significant multiple risks when doing daily activity inclusive of letting in strangers, leaving doors open in winter, history of burning food, cigarette burn marks, flooding, gas left on despite smell, tampering with controls, heating not switched on in cold weather | |
Physical skills
| ATTILA trial questions | Score | ATTILA trial scale |
|---|---|---|
| Key question: Does the person’s physical skill place them at risk when doing everyday things? | ||
|
Mobility Does the person’s mobility put them at higher/lower risk? |
4 | Stable, upright, independent, flexible, good range of movement (possibly agile). No risk when doing daily activity as posture and stability adequate, walking indoors is safe, the person is safe using stairs, safe walking outdoors, walking is stable enough not to put person at risk of falls |
| 3 | Generally able to maintain posture and mobility in occupation, independently or with aids. Mostly risk free when doing daily activity as posture and stability/balance mostly adequate, walking indoors is mostly safe, mostly safe using stairs, mostly safe walking outdoors, mostly walking is stable enough not to put person at risk of falls | |
| 2 | Unsteady at times despite any aids, slow or manages with difficulty. Some risk when doing daily activity including some poor posture and instability/balance when walking indoors, some safety issues using stairs, some safety issues walking outdoors, walks with a shuffle or a stoop putting person at some risk of falls | |
| 1 | Extremely unstable, unable to reach and bend or unable to walk. Significant multiple risks when doing daily activity owing to poor posture and instability/poor balance when walking indoors, unsafe using stairs, unsafe walking outdoors, walks with a shuffle or a stoop putting person at risk of falls | |
|
Grip/dexterity Does the person’s grip/dexterity put them at higher/lower risk? |
4 | Grasps, moves and transports objects securely with adequate force/speed (possibly strong). No risk when doing daily activity as grip is adequate, hand strength is adequate, able to carry hot liquids, able to use grip when turning on/off domestic appliances, can operate ATT as required |
| 3 | Strength and effort are generally sufficient for most tasks. Mostly risk free when doing daily activity as grip is mostly adequate, hand strength mostly adequate, mostly able to carry hot liquids, mostly able to use grip when turning on/off domestic appliances, can mostly operate ATT as required | |
| 2 | Has difficulty with grasping, moving, transporting objects with adequate force and speed. Some risk when doing daily activity due poorer grip, poorer hand strength, may drop hot liquids, some challenge effectively using domestic appliances owing to poorer grip, difficulty to operate ATT owing to poorer grip and some limitation in strength | |
| 1 | Unable to grasp, move, transport objects with appropriate force and speed (weak/frail). Significant multiple risks when doing daily activity owing to poor grip, poor hand strength, drops hot liquids/burn risk, cannot effectively use domestic appliances owing to poor grip, cannot operate ATT owing to poor grip and lack of strength | |
Physical environment
| ATTILA trial questions | Score | ATTILA trial scale |
|---|---|---|
| Key question: Do the features of the physical environment put the person at risk when doing daily activity? | ||
|
Mobility Does the person’s physical space put them at higher/lower risk? |
4 | Space affords a range of opportunities, supports and stimulates valued occupations. No risk when doing daily activity in the physical space, e.g., clear access, appropriate flooring, no trailing cables, uses bolts/chains effectively, good state of repair, good lighting, safe on stairs, can access rooms |
| 3 | Space is mostly adequate, allows daily occupations to be pursued. Mostly risk free when doing daily activity in physical space, e.g. occasional low risk i.e., blocked access, rugs, cables, bolts/chains, poor state of repair, poor lighting, negotiating stairs, accessing rooms | |
| 2 | Affords a limited range of opportunities and curtails performance of valued occupations. Some risk in some aspects of the physical space when doing daily activity, e.g. including blocked access, rugs, cables, bolts/chains, poor state of repair, poor lighting, negotiating stairs, accessing rooms | |
| 1 | Space restricts opportunities and prevents performance of valued occupations. Significant multiple risks when doing activity in physical space, e.g. blocked access, rugs, cables, bolts/chains, poor state of repair, poor lighting, negotiating stairs, accessing rooms | |
|
Grip/dexterity Do the persons physical resources put them at higher/lower risk? |
4 | Enable occupational goals to be achieved with ease, equipment and tools are appropriate. No risk when using appliances/objects to do daily activity, e.g. appliances are in good repair i.e., electric fire, cookers, smoke alarm fitted, hot water not a risk for scalding, bath available and person uses safely, shower available as required |
| 3 | Generally allow occupational goals to be achieved; may present some obstacles. Mostly risk free when using appliances/objects to do daily activity, e.g. mostly appliances are in good repair, that is electric fire, cookers, smoke alarm fitted, an episode of excessively hot water/risk of scalding, only bath available and person mostly uses safely | |
| 2 | Impede ability to achieve occupational goals safely, equipment and tools are inadequate. Some risk in some aspect of using appliances/objects to do daily activity, e.g. some appliances are in disrepair and a fire risk, that is electric fire, cookers, no smoke alarms, an episode of excessively hot water/risk of scalding, only bath available and person not safe to use, no night light when needed | |
| 1 | Have major impact on ability to achieve occupational goals, lack of tools lead to high risks. Significant multiple risks when using appliances/objects to do daily activity, e.g. appliances are in disrepair and a fire risk, that is electric fire, cookers, no smoke alarms, excessively hot water/risk of scalding, only bath available and person not safe to use, no night light when needed | |
Social environment
| ATTILA trial questions | Score | ATTILA trial scale |
|---|---|---|
| Key question: Does who is involved and how activities are completed put the person at risk when doing daily activity? | ||
|
Social support Does the support available put them at higher/lower risk? |
4 | Social groups offer practical support, values and attitudes support optimal functioning. No risk due to carer(s) when doing daily activity, e.g. appropriate family support, carer’s needs being met, carer available when needed to prompt, provide emergency access or respond to an alert, accepting of a non-familiar person, clarity around who maintains ATT |
| 3 | Generally able to offer support but may be some under or over involvement. Mostly risk free due to carer(s) when doing daily activity, e.g. mostly appropriate family support, carer’s needs mostly being met, mostly carer available when needed to prompt, provide emergency access or respond to an alert, mostly accepting of a non-familiar person, mostly clarity around who maintains ATT | |
| 2 | Offer reduced support, or detracts from participation, some groups support but not others. Some risk due to carer(s) when doing daily activity, e.g. minimal family support, carer’s needs not fully being met, minimal carer availability when needed to prompt, provide emergency access or respond to an alert, minimal acceptance of a non-familiar person, lack of clarity around who maintains ATT | |
| 1 | Do not support participation due to lack of interest or inappropriate involvement. Significant multiple risks due to carer(s) when doing activity, e.g. no family support, carer’s needs not being met, no carer currently available when needed to prompt, provide emergency access or respond to an alert, no acceptance of a non-familiar person, no one to maintain ATT | |
|
The way the activity is completed Does the way the person completes activity put them at higher/lower risk? |
4 | Demands of activities match well with abilities, interests, energy and time available. No risk due to the way the activity is being done, e.g. safe when using an overhead gas grill, safe going to the toilet at night by putting light on, safe having a night-time bath, safe using stairs repeatedly in the day, evidence of wearing shoes/coat outdoors in wet weather |
| 3 | Generally consistent with abilities, interest, energy or time available, may present challenges. Mostly risk free due to the way the activity is being done, e.g. mostly safe when using an overhead gas grill instead of a toaster, mostly safe going to the toilet at night by putting light on, mostly safe having a night-time bath, mostly safe using stairs repeatedly in the day, some evidence of wearing shoes/coat outdoors in wet weather | |
| 2 | Some clear inconsistencies with abilities and interest, or energy and time available. Some risk due to the way the activity is being done, e.g. can be unsafe when using an overhead gas grill instead of a toaster, can be unsafe going to the toilet at night owing to lack of light, can be unsafe having a night-time bath when tired, can be unsafe using stairs repeatedly in the day when physically not able, minimal evidence of wearing shoes/coat outdoors in wet weather | |
| 1 | Mostly inconsistent with abilities, construction of activity is under- or over-demanding. Significant multiple risks due to the way the activity is being done, e.g. unsafely using an overhead gas grill instead of a toaster, unsafely going to the toilet at night owing to lack of light, unsafely having a night-time bath when tired, using stairs repeatedly in the day when physically not able, not wearing shoes/coat outdoors in wet weather | |
Appendix 3 Unit costs table
| Variable name | Unit cost (£, 2016/17) | Unit | Source | Notes/assumptions |
|---|---|---|---|---|
| Respite and care home use | ||||
| Private-sector residential care for older people, cost of stay | 94 | Per day | PSSRU 2017,43 table 1.2 | Includes personal living expenses |
| Local authority residential care for older people, cost of stay | 162 | Per day | PSSRU 2017,43 table 1.3 | Includes personal living expenses |
| Private sector nursing home for older people, cost of stay | 119 | Per day | PSSRU 2017,43 table 1.1 | Includes personal living expenses |
| Community health and social care services | ||||
| GP time, home visit | 88 | Per visit | PSSRU 2017,43 table 10.3b for costs; PSSRU 2013,165 table 10.3b, for ratios | No information about home visits in PSSRU 2017.43 Assumed ratio of clinic-to-home cost per minute remained the same and average duration of visit remained the same as given in PSSRU 2013.165 Assumes average home visit duration of 23.4 minutes |
| GP time, home visit | 3.7 | Per minute | PSSRU 2017,43 table 10.3b for costs; PSSRU 2013,165 table 10.3b, for ratios | No information about home visits in PSSRU 2017.43 Assumed ratio of clinic-to-home cost per minute remained the same and average duration of visit remained the same as given in PSSRU 2013165 |
| GP time, surgery | 28 | Per visit | PSSRU 2017,43 table 10.3b | No direct care staff and no qualification costs, per surgery consultation of 9.22 minutes |
| GP time, surgery | 3 | Per minute | PSSRU 2017,43 table 10.3b | No direct care staff and no qualification costs, per surgery consultation of 9.22 minutes |
| Practice nurse, face-to-face time | 0.60 | Per minute | PSSRU 2017,43 table 10.2 | Excludes qualification costs |
| Practice nurse, face-to-face time | 9.30 | Per consultation | PSSRU 2017,43 table 10.2 | Per 15.5-minute consultation. Excludes qualification costs |
| Community nursing time | 0.73 | Per minute | PSSRU 2017,43 table 10.1 | Assumes AfC band 6 |
| Community nursing time | 37 | Per contact | NHS reference costs 2016/1742 | CHS tab |
| Nurse (mental health) time | 0.73 | Per minute | PSSRU 2017,43 table 10.1 | Assumes average hourly cost of a member of a community mental health team for older people |
| Nurse (mental health) time | 44 | Per contact | PSSRU 2017,43 table 12.1 | Assumes average visit duration in community mental health teams for older people, 60 minutes |
| Consultant: psychiatrist time | 1.8 | Per minute | PSSRU 2017,43 table 15 | Excludes qualification costs |
| Consultant: psychiatrist time | 54 | Per contact | PSSRU 2017,43 table 15 | Excludes qualification costs. Assumes 30-minute visit |
| Social worker, face-to-face time | 59 | Per visit | PSSRU 2017,43 table 11.2 | Excludes qualification costs. Assumes 1 hour of client-related work |
| Social worker, face-to-face time | 0.98 | Per minute | PSSRU 2017,43 table 11.2 | Excludes qualification costs. Assumes 1 hour of client-related work |
| Physiotherapist | 0.72 | Per minute | PSSRU 2017,43 table 9 | Assumes AfC band 61 |
| Physiotherapist | 53 | Per contact | NHS reference costs 2016/1742 | CHS tab |
| NHS occupational therapist | 0.72 | Per minute | PSSRU 2017,43 table 9 | Assumes AfC band 61 |
| NHS occupational therapist | 76.73 | Per contact | NHS reference costs 2016/1742 | CHS tab |
| NHS community mental health team worker for older people with mental health problems, per team member | 44 | Per contact | PSSRU 2017,43 table 12.1 | Assumes average visit duration in community mental health teams for older people, 60 minutes |
| Dietitian | 84.85 | Per contact | NHS reference costs 2016/1742 | CHS tab |
| Home care – average of independent and social services | 0.44 | Per minute | PSSRU 2017,43 table 11.6 | Face-to-face time: average cost of private and social services costs; weighted average of weekday and weekend costs |
| Home care – average of independent and social services | 13.21 | Per contact | PSSRU 2017,43 table 11.6 | Face-to-face time: average cost of private and Social Services costs; weighted average of weekday and weekend costs. Assumes 30 minute visit |
| Cleaner | 20 | Per visit | Internet search. Assumes 2-hour visit | |
| Meals on wheels | 6 | Per meal | PSSRU 2014,166 table 8.1.1 | Uprated using HCHS Pay & Prices index43 |
| Sitting service i.e. Crossroads Care Essex (Canvey Island, UK); carer support worker | 45 | Per visit | Evaluation of the East Sussex Carers’ Breaks Demonstrator Site 167 | Cost of short break for carers of 2.5 hours. Uprated using HCHS Pay & Prices Index |
| Chiropodist | 44.02 | Per contact | NHS reference costs 2016/1742 | Activity-weighted average of podiatrist services; CHS tab |
| Optician – office | 21.31 | Per visit | Department of Health and Social Care, FODO168–170 | Cost of a sight test |
| Optician – domiciliary | 58.87 | Per visit | Department of Health, FODO168–170 | NHS domiciliary visit: ophthalmic service + sight test |
| Dentist, general dental service | 85 | Per visit | NHS reference costs 2016/1742 | CHS tab |
| Dentist, community dental service | 153.88 | Per visit | NHS reference costs 2016/1742 | CHS tab |
| Day care for older people, per session | 63 | Per session | PSSRU 2017,43 table 1.4 | |
| Day care in NHS facilities, per attendance | 132.23 | Attendance | NHS reference costs 2016/1742 CHS tab | Day care facilities regular attendances – elderly |
| Day care for people with mental health problems, per session | 34 | Per session | PSSRU 2017,43 table 2.4 | |
| Lunch club | 8 | Per session | Romeo et al.171 | Uprated using HCHS Pay & Prices index |
| Paramedic visit, see and treat and refer | 181.25 | Per attendance | NHS reference costs 2016/1742 | ASS01: see and treat or refer |
| ATT assessment | 36 | Per working hour | PSSRU 2017,43 table 9 | Assumes the following: health and social care assessor is on AfC band 5 (cost per working hour: £33); specialist ATT assessor is on AfC band 6 (cost per working hour: £43); of assessors where assessor type was known, 70% were health and social care assessors and 30% were specialist ATT assessors |
| ATT equipment | ||||
| Activity monitoring detectors | 7 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Bed and chair sensors | 11 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Clocks and time reminders | 9 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Enuresis sensors | 9 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Epilepsy sensors | 30 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| GPS devices | 21 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Networked carbon monoxide and gas monitors | 9 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Networked flood detectors | 6 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Networked smoke detector | 5 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Networked temperature detectors | 5 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| PIR movement and exit detectors | 5 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Pager units | 10 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Telecare base units and pendants | 11 | Per item | NHC68 | Mid-point of the range between minimum and maximum prices per device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Key safes | 7 | Per item | NHC68 | Mean price for device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Standalone smoke detectors | 2 | Per item | NHC68 | Mean price for device-type. Annuitised over 5 years at 3.5% discount rate; three-month cost. Uprated to 2017 prices using CPIH172 |
| Standalone carbon monoxide monitors | 4 | Per item | NHC68 | Mean price for device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Fall detectors | 5 | Per item | NHC68 | Mean price for device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Standalone gas detectors | 8 | Per item | NHC68 | Mean price for device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Medications reminders and dispensers | 11 | Per item | NHC68 | Mean price for device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Intercoms | 5 | Per item | NHC68 | Mean price for device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| Adapted telephones | 2 | Per item | NHC68 | Mean price for device type. Annuitised over 5 years at 3.5% discount rate; 3-month cost. Uprated to 2017 prices using CPIH172 |
| ATT installation | Range: 0.2–9 | Per item | NHC68 | 3-month cost. Uprated to 2017 prices using CPIH172 |
| ATT maintenance | 10 | Per ATT package | NHC68 | 3-month cost. Uprated to 2017 prices using CPIH172 |
| Call handling/monitoring and response | 40 | Per networked ATT package | NHC68 | 3-month cost. Uprated to 2017 prices using CPIH172 |
| Equipment and adaptations | ||||
| Wheelchair (average of self-/attendant propelled) | 24.00 | Per item | PSSRU 2017,43 table 7.2 | Annuitised over 5 years; 3-month cost |
| Outdoor rail | 1.35 | Per item | PSSRU 2017,43 table 7.2 | Annuitised over 10 years; 3-month cost |
| Stair/grab rail | 0.95 | Per item | PSSRU 2017,43 table 7.2 | Annuitised over 10 years; 3-month cost |
| Over-bath shower adaptation | 38.75 | Per item | PSSRU 2017,43 table 7.2 | Annuitised over 10 years; 3-month cost |
| Walk-in shower adaptation | 155.00 | Per item | PSSRU 2017,43 table 7.2 | Annuitised over 10 years; 3-month cost |
| Perching stool | 1.00 | Per item | PSSRU 2013,165 table 7.3.1 | Annuitised over 10 years; 3-month cost. Uprated using HCHS Pay & Prices index43 |
| Commode | 2.00 | Per item | PSSRU 2013,165 table 7.3.1 | Annuitised over 10 years; 3-month cost. Uprated using HCHS Pay & Prices index43 |
| Toilet frame/raised toilet seat | 4.00 | Per item | PSSRU 2013,165 table 7.3.1 | Annuitised over 10 years; 3-month cost. Uprated using HCHS Pay & Prices inflator |
| Chair/bed raisers | 4.00 | Per item | PSSRU 2013,165 table 7.3.1 | Annuitised over 10 years; 3-month cost. Uprated using HCHS Pay & Prices index43 |
| All four-wheeled and four-footed walking frames | 9.00 | Per item | PSSRU 2013,165 table 7.3.1 | Annuitised over 10 years; 3-month cost. Uprated using HCHS Pay & Prices index43 |
| Bath seat | 10.00 | Per item | PSSRU 2013,165 table 7.3.1 | Annuitised over 10 years; 3-month cost. Uprated using HCHS Pay & Prices index43 |
| Bed rail | 4.00 | Per item | PSSRU 2017,43 table 7.2 | Annuitised over 10 years; 3-month cost. Uprated using HCHS Pay & Prices index43 |
| Incontinence pads | 88.00 | Per 3-month supply | Uprated from 2011 prices using the CPIH172 to 2017 prices173 | |
| Medications | ||||
| Various | Range: 0.02–28 | Standard quantity units | Prescription Cost Analysis England 2017 174 | Central nervous system prescription medications, British National Formulary chapter 4, Hypnotics and Anxiolytics (section 1), Drugs Used in Psychoses & Related Disorders (section 2), Antidepressant Drugs (section 3), Antiepileptic Drugs (section 8), Drugs Used in Parkinsonism/Related Disorders (section 9) and Drugs for Dementia (section 11) |
| Unpaid carer costs | ||||
| National average wage – value of lost work time | 16.20 | Per hour | Annual survey of hours and earnings66 | Gross mean wage for all employee jobs, 2017 |
| National average wage – value of lost leisure time | 5.67 | Per hour | Annual survey of hours and earnings66 | 35% of gross mean wage for all employee jobs, 2017 |
| Travel costs | ||||
| Cost per mile of travel for carer (car running costs), per mile | 0.16 | Per mile | Automobile Association175 | |
| Ambulance to A&E | 247.5 | Attendance | NHS reference costs 2016/1742 | AMB tab: see and treat and convey |
| Hospital services | ||||
| A&E attendances, weighted average of admitted attendances | 221.25 | Attendance | NHS reference costs 2016/1742 | EM tab |
| A&E attendances, weighted average of non-admitted attendances | 127.56 | Attendance | NHS reference costs 2016/1742 | EM tab |
| A&E attendances, weighted average of admitted and non-admitted attendances | 148.36 | Attendance | NHS reference costs 2016/1742 | EM tab |
| Inpatients | ||||
| Subchapter AA: nervous system procedures and disorders | 477.75 | Day | NHS reference costs 2016/1742 | NEL tab |
| 295.42 | Excess day | NEL_XS tab | ||
| Subchapter AB: pain management | 563.14 | Day | NHS reference costs 2016/1742 | NEL tab |
| 507.96 | Excess day | NEL_XS tab | ||
| Subchapter BZ: eyes and periorbita procedures and disorders | 666.23 | Day | NHS reference costs 2016/1742 | NEL tab |
| 342.52 | Excess day | NEL_XS tab | ||
| Subchapter CB: Ear, nose, mouth, throat and neck disorders | 521.46 | Day | NHS reference costs 2016/1742 | NEL tab |
| 295.10 | Excess day | NEL_XS tab | ||
| Subchapter DZ: respiratory system procedures and disorders | 402.23 | Day | NHS reference costs 2016/1742 | NEL tab |
| 271.11 | Excess day | NEL_XS tab | ||
| Subchapter EB: cardiac disorders | 452.02 | Day | NHS reference costs 2016/1742 | NEL tab |
| 291.01 | Excess day | NEL_XS tab | ||
| Subchapter ED: open cardiac procedures for acquired conditions | 1368.01 | Day | NHS reference costs 2016/1742 | NEL tab |
| 399.24 | Excess day | NEL_XS tab | ||
| Subchapter EY: interventional cardiology for acquired conditions | 820.12 | Day | NHS reference costs 2016/1742 | NEL tab |
| 383.32 | Excess day | NEL_XS tab | ||
| Subchapter FD: digestive system disorders | 452.79 | Day | NHS reference costs 2016/1742 | NEL tab |
| 294.18 | Excess day | NEL_XS tab | ||
| Subchapter FE: digestive system endoscopic procedures | 517.57 | Day | NHS reference costs 2016/1742 | NEL tab |
| 313.94 | Excess day | NEL_XS tab | ||
| Subchapter FF: digestive system open and laparoscopic procedures | 825.31 | Day | NHS reference costs 2016/1742 | NEL tab |
| 342.91 | Excess day | NEL_XS tab | ||
| Subchapter GC: hepatobiliary and pancreatic system disorders | 435.85 | Day | NHS reference costs 2016/1742 | NEL tab |
| 288.25 | Excess day | NEL_XS tab | ||
| Subchapter HC: spinal procedures and disorders | 540.73 | Day | NHS reference costs 2016/1742 | NEL tab |
| 309.87 | Excess day | NEL_XS tab | ||
| Subchapter HN: orthopaedic non-trauma procedures | 731.69 | Day | NHS reference costs 2016/1742 | NEL tab |
| 325.66 | Excess day | NEL_XS tab | ||
| Subchapter HT: orthopaedic trauma procedures | 724.34 | Day | NHS reference costs 2016/1742 | REHAB tab |
| 312.97 | ||||
| Subchapter KA: endocrine system disorders | 460.64 | Day | NHS reference costs 2016/1742 | NEL tab |
| 307.37 | Excess day | NEL_XS tab | ||
| Subchapter KB: diabetic medicine | 413.93 | Day | NHS reference costs 2016/1742 | NEL tab |
| 273.36 | Excess day | NEL_XS tab | ||
| Subchapter local authority: renal procedures and disorders | 415.24 | Day | NHS reference costs 2016/1742 | NEL tab |
| 272.35 | Excess day | NEL_XS tab | ||
| Subchapter LB: urological and male reproductive system procedures and disorders | 505.19 | Day | NHS reference costs 2016/1742 | NEL tab |
| 304.76 | Excess day | NEL_XS tab | ||
| Subchapter MA: female reproductive system procedures | 1172.12 | Day | NHS reference costs 2016/1742 | NEL tab |
| 472.35 | Excess day | NEL_XS tab | ||
| Subchapter SA: haematological procedures and disorders | 549.53 | Day | NHS reference costs 2016/1742 | NEL tab |
| 349.89 | Excess day | NEL_XS tab | ||
| Subchapter VC: rehabilitation | Day | NHS reference costs 2016/1742 | NEL tab | |
| Excess day | NEL_XS tab | |||
| Subchapter WD: treatment of mental health patients by non-mental health service providers | 356.25 | Day | NHS reference costs 2016/1742 | NEL tab |
| 264.04 | Excess day | NEL_XS tab | ||
| Subchapter WH: poisoning, toxic effects, special examinations, screening and other health-care contacts | 440.99 | Day | NHS reference costs 2016/1742 | NEL tab |
| 274.22 | Excess day | NEL_XS tab | ||
| Subchapter YQ: vascular open procedures and disorders | 569.10 | Day | NHS reference costs 2016/1742 | NEL tab |
| 294.38 | Excess day | NEL_XS tab | ||
| Inpatients, weighted average across specialties | 645 | Day | NHS reference costs 2016/1742 | NEL tab |
| 299 | Excess day | NEL_XS tab | ||
| Day cases | ||||
| Subchapter AA: nervous system procedures and disorders | 563.24 | Day | NHS reference costs 2016/1742 | DC tab |
| Subchapter AB: pain management | 709.80 | Day | NHS reference costs 2016/1742 | DC tab |
| Subchapter BZ: eyes and periorbita procedures and disorders | 825.04 | Day | NHS reference costs 2016/1742 | DC tab |
| Subchapter DZ: respiratory system procedures and disorders | 606.65 | Day | NHS reference costs 2016/1742 | DC tab |
| Subchapter EY: interventional cardiology for acquired conditions | 1399.54 | Day | NHS reference costs 2016/1742 | NEL tab NEL_XS tab |
| Subchapter FE: digestive system endoscopic procedures | 539.46 | Day | NHS reference costs 2016/1742 | DC tab |
| Subchapter HD: musculoskeletal and rheumatological disorders | 386.87 | Day | NHS reference costs 2016/1742 | DC tab |
| Day-case radiotherapy | 132.69 | Day | NHS reference costs 2016/1742 | RAD tab |
| Outpatients | ||||
| Service code 101: urology | 102.88 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 104: colorectal surgery | 109.83 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 107: vascular surgery | 141.35 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 108: spinal surgery service | 141.91 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 110: trauma and orthopaedics | 109.78 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 120: ENT | 87.94 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 130: ophthalmology | 82.93 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 144: maxillo-facial surgery | 116.47 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 150: neurosurgery | 182.88 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 160: plastic surgery | 94.52 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 170: cardiothoracic surgery | 213.07 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 172: cardiac surgery | 166.49 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 173: thoracic surgery | 204.35 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 191: pain management | 127.87 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 301: gastroenterology | 138.29 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 302: endocrinology | 144.74 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 303: clinical haematology | 164.74 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 304: clinical physiology | 72.41 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 307: diabetic medicine | 141.00 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 320: cardiology | 117.34 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 324: anticoagulant service | 30.04 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 330: dermatology | 98.36 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 340: respiratory medicine | 144.26 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 361: nephrology | 148.53 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 370: medical oncology | 163.93 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 400: neurology | 149.30 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 410: rheumatology | 134.47 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 430: geriatric medicine | 194.56 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 450: dental medicine specialties | 97.03 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 502: gynaecology | 130.36 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 650: physiotherapy | 44.96 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 652: speech and language therapy | 94.88 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 653: podiatry | 41.87 | Follow-up attendance | NHS reference costs 2016/1742 | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 654: dietetics | 68.75 | Follow-up attendance | NHS reference costs 2016/17 (NHS Improvement 2017) | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 655: orthoptics | 61.02 | Follow-up attendance | NHS reference costs 2016/17 (NHS Improvement 2017) | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 658: orthotics | 115.18 | Follow-up attendance | NHS reference costs 2016/17 (NHS Improvement 2017) | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 662: optometry | 54.79 | Follow-up attendance | NHS reference costs 2016/17 (NHS Improvement 2017) | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 713: psychotherapy | 187.65 | Follow-up attendance | NHS reference costs 2016/17 (NHS Improvement 2017) | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 715: old age psychiatry | 179.66 | Follow-up attendance | NHS reference costs 2016/17 (NHS Improvement 2017) | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 800: clinical oncology (previously radiotherapy) | 126.39 | Follow-up attendance | NHS reference costs 2016/17 (NHS Improvement 2017) | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 812: diagnostic imaging | 80.65 | Follow-up attendance | NHS reference costs 2016/17 (NHS Improvement 2017) | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Service code 840: audiology | 87.04 | Follow-up attendance | NHS reference costs 2016/17 (NHS Improvement 2017) | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Weighted average across laboratory tests | 2.032 | Per test | NHS reference costs 2016/17 (NHS Improvement 2017) | DAPS tab |
| Haemodialysis | 157.83 | Per attendance | NHS reference costs 2016/17 (NHS Improvement 2017) | RENAL tab |
| Memory clinic | 406.45 | Follow-up attendance | PSSRU 2014,166 table 1.10 | Uprated using HCHS Pay & Prices index43 |
| Weighted average of follow-up attendances across service codes | 105.52 | Follow-up attendance | NHS reference costs 2016/17 (NHS Improvement 2017) | Consultant- and non-consultant-led follow-up face-to-face attendances, CL and NCL tabs |
| Subchapter AA: nervous system procedures and disorders | 171.70 | Outpatient procedure | NHS reference costs 2016/1742 | OPROC tab |
| Subchapter EY: interventional cardiology for acquired conditions | 147.80 | Outpatient procedure | NHS reference costs 2016/1742 | OPROC tab |
| Subchapter FE: digestive system endoscopic procedures | 202.45 | Outpatient procedure | NHS reference costs 2016/1742 | OPROC tab |
| Subchapter JC: skin procedures | 126.66 | Outpatient procedure | NHS reference costs 2016/1742 | OPROC tab |
| Subchapter MA: female reproductive system procedures | 177.28 | Outpatient procedure | NHS reference costs 2016/1742 | OPROC tab |
Appendix 4 Baseline demographic characteristics of the sample with dyads participating in full baseline assessments
| Characteristic | Trial arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Intervention | Control | ||
| Female | 131 (57) | 131 (58) | 262 (58) |
| Age (years) | |||
| < 65 | 11 (5) | 3 (1) | 14 (3) |
| 65–80 | 83 (36) | 89 (40) | 172 (38) |
| > 80 | 135 (59) | 132 (59) | 267 (59) |
| Risk of wandering | |||
| Low | 164 (72) | 167 (75) | 331 (73) |
| Moderate | 48 (21) | 40 (18) | 88 (19) |
| High | 17 (7) | 17 (8) | 34 (8) |
| Safety risk in the home | |||
| Low | 116 (51) | 113 (50) | 229 (51) |
| Moderate | 95 (41) | 93 (42) | 188 (42) |
| High | 18 (8) | 18 (8) | 36 (8) |
| Caregiver involvement | |||
| Visits at least once/day | 51 (22) | 54 (24) | 105 (23) |
| Visits less than once/day | 64 (28) | 56 (25) | 120 (26) |
| Live in | 114 (50) | 114 (51) | 228 (50) |
| Caregiver–participant relationship | |||
| Spouse/partner | 88 (38) | 82 (37) | 170 (38) |
| Sibling/child/child-in-law | 119 (52) | 122 (54) | 241 (53) |
| Other relative | 18 (8) | 8 (4) | 26 (6) |
| Non-relative | 4 (2) | 12 (5) | 16 (4) |
Appendix 5 Use of health, social and unpaid care over the previous 3 months
Use of health, social and unpaid care over the previous 3 months, intervention and control groups, for cases with economic data available at baseline and at the 12-, 24-, 52- and 104-week follow-ups.
| Service/item | Units | Trial arm | |||||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| Valid (n) | Users, n (%) | Mean (SE) | Valid (n) | Users, n (%) | Mean (SE) | ||
| Baseline | Expected = 229 | Expected = 224 | |||||
| Community health | |||||||
| GP | Visits | 229 | 159 (69) | 1.82 (0.13) | 223 | 146 (65) | 1.54 (0.13) |
| Practice nurse | Visits | 229 | 88 (38) | 0.94 (0.16) | 223 | 84 (38) | 0.69 (0.12) |
| Community nurse | Visits | 229 | 52 (23) | 2.76 (0.86) | 223 | 42 (19) | 1.26 (0.45) |
| Physiotherapist | Visits | 229 | 33 (14) | 1.04 (0.28) | 223 | 22 (10) | 0.23 (0.06) |
| Occupational therapist | Visits | 229 | 61 (27) | 0.44 (0.06) | 223 | 52 (23) | 0.39 (0.06) |
| Dietitian | Visits | 229 | 3 (1) | 0.02 (0.01) | 223 | 11 (5) | 0.06 (0.02) |
| Paramedic | Visits | 228 | 24 (11) | 0.13 (0.03) | 223 | 30 (13) | 0.26 (0.07) |
| Specialist nurse | Visits | 229 | 22 (10) | 0.22 (0.07) | 223 | 19 (9) | 0.23 (0.08) |
| Dentist | Visits | 229 | 55 (24) | 0.33 (0.05) | 223 | 45 (20) | 0.26 (0.04) |
| Optician | Visits | 229 | 46 (20) | 0.24 (0.04) | 223 | 43 (19) | 0.23 (0.04) |
| Chiropodist | Visits | 229 | 84 (37) | 0.60 (0.06) | 223 | 79 (35) | 0.65 (0.15) |
| Mental health | |||||||
| Mental health nurse | Visits | 229 | 78 (34) | 0.78 (0.10) | 223 | 57 (26) | 0.58 (0.10) |
| Psychiatrist | Visits | 229 | 52 (23) | 0.75 (0.38) | 223 | 47 (21) | 0.25 (0.03) |
| Psychologist | Visits | 229 | 4 (2) | 0.03 (0.02) | 223 | 6 (3) | 0.06 (0.03) |
| Mental health team | Visits | 229 | 17 (7) | 0.29 (0.08) | 223 | 11 (5) | 0.13 (0.06) |
| Community care | |||||||
| Home care | Visits | 229 | 91 (40) | 56.79 (6.79) | 223 | 96 (43) | 59.70 (6.89) |
| Home care | Hours | 229 | 91 (40) | 49.69 (11.06) | 223 | 96 (43) | 64.29 (15.09) |
| Social worker | Visits | 229 | 75 (33) | 0.51 (0.06) | 223 | 76 (34) | 0.66 (0.12) |
| Cleaner | Visits | 229 | 59 (26) | 2.90 (0.37) | 223 | 40 (18) | 2.22 (0.35) |
| Meals on wheels | Visits | 229 | 9 (4) | 1.41 (0.66) | 223 | 16 (7) | 3.45 (1.06) |
| Laundry service | Visits | 229 | 7 (3) | 0.43 (0.17) | 223 | 3 (1) | 0.14 (0.09) |
| Sitting service | Visits | 229 | 6 (3) | 0.41 (0.19) | 223 | 9 (4) | 0.33 (0.16) |
| Carer support worker | Visits | 229 | 11 (5) | 0.18 (0.12) | 222 | 12 (5) | 0.07 (0.02) |
| Day services | |||||||
| Day centre | Attendances | 229 | 38 (17) | 3.34 (0.76) | 223 | 36 (16) | 2.73 (0.53) |
| Lunch club | Attendances | 229 | 19 (8) | 0.64 (0.20) | 223 | 26 (12) | 0.99 (0.23) |
| Patient education | Attendances | 229 | 11 (5) | 0.38 (0.13) | 223 | 5 (2) | 0.26 (0.18) |
| Hospital care | |||||||
| Emergency department | Attendances | 229 | 29 (13) | 0.14 (0.03) | 223 | 39 (17) | 0.20 (0.03) |
| Inpatients services | Days | 229 | 24 (10) | 1.24 (0.35) | 223 | 36 (16) | 2.39 (0.57) |
| Day hospital services | Days | 229 | 2 (1) | 0.01 (0.01) | 223 | 2 (1) | 0.01 (0.01) |
| Outpatients services | Visits | 229 | 100 (44) | 0.95 (0.11) | 223 | 92 (41) | 1.00 (0.14) |
| Residential respite | |||||||
| Residential home | Days | 228 | 1 (0) | 0.00 (0.00) | 223 | 0 (0) | 0.00 (0.00) |
| Nursing home | Days | 228 | 5 (2) | 0.37 (0.20) | 223 | 8 (4) | 0.91 (0.43) |
| Medications | |||||||
| Any medications | Units | 225 | 144 (64) | 0.94 (0.06) | 221 | 142 (64) | 0.90 (0.06) |
| Dementia | Units | 226 | 116 (51) | 0.56 (0.04) | 221 | 115 (52) | 0.56 (0.04) |
| Mental health | Units | 225 | 71 (32) | 0.39 (0.04) | 222 | 62 (28) | 0.34 (0.04) |
| Equipment and adaptations | |||||||
| Equipment (health and social care providers) | Items | 217 | 48 (22) | 0.47 (0.07) | 202 | 51 (25) | 0.43 (0.06) |
| Unpaid care; out of pocket | |||||||
| Equipment (private) | Items | 217 | 9 (4) | 0.04 (0.01) | 202 | 10 (5) | 0.06 (0.02) |
| Travel to appointment | Trips | 219 | 111 (51) | 2.50 (0.45) | 202 | 91 (45) | 1.30 (0.21) |
| Unpaid care | Hours | 214 | 212 (99) | 563.95 (43.71) | 201 | 200 (100) | 661.00 (46.31) |
| Carer cut down work | Hours | 205 | 3 (1) | 1.00 (0.69) | 195 | 4 (2) | 1.57 (1.06) |
| Carer stopped work | Weeks | 207 | 1 (0) | 0.02 (0.02) | 199 | 2 (1) | 0.05 (0.04) |
| Unpaid care other carersa | Hours | 216 | 124 (57) | 121.63 (17.86) | 202 | 96 (48) | 87.80 (14.63) |
| Time off work other carersa | Days | 215 | 15 (7) | 0.00 (0.00) | 202 | 20 (10) | 0.01 (0.00) |
| ATT devices (including basicb) | Items | 223 | 217 (97) | 2.66 (0.10) | 203 | 187 (92) | 2.01 (0.09) |
| 12 weeks | Expected = 189 | Expected = 188 | |||||
| Community health | |||||||
| GP | Visits | 188 | 118 (63) | 1.35 (0.12) | 186 | 119 (64) | 1.62 (0.14) |
| Practice nurse | Visits | 188 | 72 (38) | 0.65 (0.09) | 186 | 72 (39) | 0.91 (0.24) |
| Community/district nurse | Visits | 188 | 36 (19) | 3.64 (1.11) | 186 | 32 (17) | 2.10 (0.77) |
| Physiotherapist | Visits | 188 | 21 (11) | 0.73 (0.22) | 186 | 20 (11) | 0.32 (0.13) |
| Occupational therapist | Visits | 188 | 24 (13) | 0.23 (0.06) | 186 | 29 (16) | 0.23 (0.05) |
| Dietitian | Visits | 188 | 3 (2) | 0.02 (0.01) | 186 | 6 (3) | 0.03 (0.01) |
| Paramedic | Visits | 188 | 18 (10) | 0.12 (0.03) | 186 | 20 (11) | 0.20 (0.06) |
| Specialist nurse | Visits | 188 | 18 (10) | 0.15 (0.04) | 186 | 15 (8) | 0.19 (0.07) |
| Dentist | Visits | 189 | 33 (17) | 0.33 (0.07) | 186 | 33 (18) | 0.21 (0.04) |
| Optician | Visits | 189 | 39 (21) | 0.24 (0.04) | 186 | 26 (14) | 0.19 (0.04) |
| Chiropodist | Visits | 189 | 77 (41) | 0.65 (0.07) | 186 | 67 (36) | 0.57 (0.07) |
| Mental health | |||||||
| Mental health nurse | Visits | 188 | 34 (18) | 0.25 (0.04) | 186 | 31 (17) | 0.41 (0.11) |
| Psychiatrist | Visits | 188 | 24 (13) | 0.26 (0.11) | 186 | 24 (13) | 0.19 (0.05) |
| Psychologist | Visits | 187 | 2 (1) | 0.04 (0.03) | 186 | 1 (1) | 0.02 (0.02) |
| Mental health team | Visits | 188 | 7 (4) | 0.21 (0.09) | 186 | 12 (6) | 0.18 (0.07) |
| Community care | |||||||
| Home care | Visits | 189 | 81 (43) | 67.14 (8.16) | 186 | 87 (47) | 66.00 (8.22) |
| Home care | Hours | 189 | 81 (43) | 65.94 (14.18) | 186 | 87 (47) | 73.21 (19.04) |
| Social worker | Visits | 188 | 41 (22) | 0.29 (0.05) | 186 | 55 (30) | 0.43 (0.06) |
| Cleaner | Visits | 189 | 54 (29) | 3.16 (0.43) | 186 | 43 (23) | 2.86 (0.47) |
| Meals on wheels | Visits | 189 | 11 (6) | 3.41 (1.19) | 186 | 12 (6) | 3.55 (1.17) |
| Laundry service | Visits | 189 | 4 (2) | 0.25 (0.12) | 186 | 6 (3) | 0.45 (0.20) |
| Sitting service | Visits | 189 | 8 (4) | 0.29 (0.12) | 186 | 5 (3) | 0.11 (0.07) |
| Carer support worker | Visits | 189 | 10 (5) | 0.12 (0.05) | 186 | 10 (5) | 0.18 (0.07) |
| Day services | |||||||
| Day centre | Attendances | 189 | 42 (22) | 4.62 (0.82) | 186 | 31 (17) | 3.42 (0.74) |
| Lunch club | Attendances | 189 | 17 (9) | 0.80 (0.27) | 186 | 19 (10) | 1.33 (0.40) |
| Patient education | Attendances | 189 | 11 (6) | 0.42 (0.17) | 186 | 7 (4) | 0.19 (0.09) |
| Hospital care | |||||||
| Emergency department | Attendances | 189 | 30 (16) | 0.20 (0.04) | 186 | 27 (15) | 0.19 (0.04) |
| Inpatients services | Days | 189 | 20 (11) | 0.76 (0.28) | 186 | 23 (12) | 1.34 (0.41) |
| Day hospital services | Days | 189 | 1 (1) | 0.19 (0.19) | 186 | 1 (1) | 0.01 (0.01) |
| Outpatients services | Visits | 189 | 74 (39) | 0.79 (0.09) | 186 | 76 (41) | 0.78 (0.09) |
| Residential respite | |||||||
| Residential home | Days | 188 | 0 (0) | 0.00 (0.00) | 185 | 3 (2) | 0.22 (0.16) |
| Nursing home | Days | 188 | 5 (3) | 0.52 (0.28) | 185 | 7 (4) | 0.59 (0.26) |
| Medications | |||||||
| Medications | Units | 187 | 122 (65) | 0.98 (0.07) | 186 | 115 (62) | 0.88 (0.07) |
| Dementia | Units | 189 | 104 (55) | 0.61 (0.04) | 186 | 97 (52) | 0.57 (0.04) |
| Mental health | Units | 187 | 58 (31) | 0.40 (0.05) | 186 | 47 (25) | 0.31 (0.04) |
| Equipment and adaptations | |||||||
| Equipment (HSC) | Items | 184 | 27 (15) | 0.28 (0.07) | 181 | 28 (15) | 0.22 (0.04) |
| Unpaid care; out of pocket | |||||||
| Equipment (private) | Items | 185 | 6 (3) | 0.03 (0.01) | 181 | 10 (6) | 0.07 (0.02) |
| Travel to appointments | Trips | 188 | 85 (45) | 2.12 (0.36) | 184 | 62 (34) | 1.01 (0.18) |
| Unpaid care | Hours | 186 | 186 (100) | 641.24 (48.85) | 182 | 182 (100) | 721.37 (50.71) |
| Carer cut down work | Hours | 174 | 1 (1) | 0.14 (0.14) | 174 | 1 (1) | 0.48 (0.48) |
| Carer stopped work | Weeks | 175 | 3 (2) | 0.09 (0.07) | 179 | 0 (0) | 0.00 (0.00) |
| Unpaid care other carersa | Hours | 186 | 99 (53) | 92.16 (13.09) | 184 | 95 (52) | 108.22 (19.21) |
| Time off work other carersa | Days | 184 | 9 (5) | 0.00 (0.00) | 183 | 15 (8) | 0.01 (0.00) |
| ATT devices (including basicb) | Items | 188 | 164 (87) | 3.33 (0.18) | 166 | 120 (72) | 1.98 (0.16) |
| Week 24 | Expected = 178 | Expected = 168 | |||||
| Community health | |||||||
| GP | Visits | 176 | 101 (57) | 1.31 (0.17) | 168 | 100 (60) | 1.23 (0.13) |
| Practice nurse | Visits | 177 | 69 (39) | 1.05 (0.25) | 168 | 57 (34) | 0.51 (0.07) |
| Community/district nurse | Visits | 177 | 30 (17) | 2.45 (0.93) | 168 | 33 (20) | 2.73 (0.90) |
| Physiotherapist | Visits | 177 | 20 (11) | 0.49 (0.14) | 168 | 17 (10) | 0.21 (0.06) |
| Occupational therapist | Visits | 177 | 18 (10) | 0.14 (0.03) | 168 | 20 (12) | 0.14 (0.03) |
| Dietitian | Visits | 177 | 4 (2) | 0.02 (0.01) | 168 | 6 (4) | 0.04 (0.01) |
| Paramedic | Visits | 177 | 12 (7) | 0.08 (0.02) | 168 | 8 (5) | 0.06 (0.02) |
| Specialist nurse | Visits | 176 | 11 (6) | 0.20 (0.09) | 168 | 15 (9) | 0.46 (0.28) |
| Dentist | Visits | 177 | 38 (21) | 0.27 (0.05) | 168 | 38 (23) | 0.30 (0.06) |
| Optician | Visits | 177 | 37 (21) | 0.26 (0.04) | 168 | 28 (17) | 0.21 (0.04) |
| Chiropodist | Visits | 177 | 65 (37) | 0.54 (0.06) | 168 | 52 (31) | 0.52 (0.08) |
| Mental health | |||||||
| Mental health nurse | Visits | 177 | 24 (14) | 0.26 (0.07) | 168 | 25 (15) | 0.26 (0.06) |
| Psychiatrist | Visits | 177 | 13 (7) | 0.08 (0.02) | 168 | 17 (10) | 0.13 (0.04) |
| Psychologist | Visits | 177 | 1 (1) | 0.02 (0.02) | 168 | 2 (1) | 0.04 (0.03) |
| Mental health team | Visits | 177 | 7 (4) | 0.11 (0.07) | 168 | 10 (6) | 0.15 (0.07) |
| Community care | |||||||
| Home care | Visits | 177 | 79 (45) | 81.98 (9.66) | 168 | 79 (47) | 71.18 (8.49) |
| Home care | Hours | 177 | 79 (45) | 88.09 (20.15) | 168 | 79 (47) | 74.56 (16.77) |
| Social worker | Visits | 177 | 29 (16) | 0.24 (0.05) | 168 | 35 (21) | 0.33 (0.06) |
| Cleaner | Visits | 177 | 47 (27) | 3.20 (0.48) | 168 | 44 (26) | 2.86 (0.42) |
| Meals on wheels | Visits | 177 | 10 (6) | 4.05 (1.37) | 168 | 11 (7) | 3.86 (1.87) |
| Laundry service | Visits | 177 | 2 (1) | 0.22 (0.16) | 168 | 8 (5) | 0.86 (0.56) |
| Sitting service | Visits | 177 | 8 (5) | 0.61 (0.23) | 168 | 7 (4) | 0.12 (0.05) |
| Carer support worker | Visits | 177 | 3 (2) | 0.01 (0.01) | 168 | 8 (5) | 0.16 (0.08) |
| Day services | |||||||
| Day centre | Attendances | 177 | 36 (20) | 4.33 (0.89) | 168 | 30 (18) | 3.32 (0.67) |
| Lunch club | Attendances | 177 | 15 (8) | 0.69 (0.21) | 168 | 20 (12) | 1.65 (0.47) |
| Patient education | Attendances | 177 | 10 (6) | 0.45 (0.16) | 168 | 12 (7) | 0.53 (0.17) |
| Hospital care | |||||||
| Emergency department | Attendances | 177 | 23 (13) | 0.17 (0.04) | 168 | 25 (15) | 0.18 (0.04) |
| Inpatients services | Days | 177 | 10 (6) | 0.44 (0.20) | 168 | 16 (10) | 1.69 (0.69) |
| Day hospital services | Days | 177 | 3 (2) | 0.02 (0.01) | 168 | 2 (1) | 0.02 (0.01) |
| Outpatients services | Visits | 177 | 62 (35) | 0.78 (0.17) | 168 | 68 (40) | 0.91 (0.17) |
| Residential respite | |||||||
| Residential home | Days | 175 | 1 (1) | 0.00 (0.00) | 166 | 1 (1) | 0.21 (0.21) |
| Nursing home | Days | 175 | 4 (2) | 0.38 (0.23) | 166 | 3 (2) | 0.13 (0.07) |
| Medications | |||||||
| Any medications | Units | 175 | 122 (70) | 1.05 (0.07) | 168 | 110 (65) | 0.88 (0.06) |
| Dementia | Units | 177 | 105 (59) | 0.65 (0.04) | 168 | 95 (57) | 0.61 (0.04) |
| Mental health | Units | 175 | 57 (33) | 0.41 (0.05) | 168 | 38 (23) | 0.27 (0.04) |
| Equipment (health and social care providers) | Items | 175 | 28 (16) | 0.25 (0.05) | 167 | 20 (12) | 0.22 (0.06) |
| Unpaid care; out-of-pocket | |||||||
| Equipment (private) | Items | 175 | 8 (5) | 0.06 (0.02) | 167 | 3 (2) | 0.02 (0.01) |
| Travel to appointments | Trips | 177 | 58 (33) | 1.91 (0.40) | 168 | 66 (39) | 1.24 (0.23) |
| Unpaid care | Hours | 175 | 173 (99) | 667.58 (53.00) | 168 | 168 (100) | 732.41 (53.61) |
| Carer cut down work | Hours | 171 | 2 (1) | 0.42 (0.42) | 159 | 0 (0) | 0.00 (0.00) |
| Carer stopped work | Weeks | 169 | 1 (1) | 0.03 (0.03) | 165 | 0 (0) | 0.00 (0.00) |
| Unpaid care other carersa | Hours | 175 | 102 (58) | 135.57 (20.81) | 167 | 91 (54) | 115.89 (19.44) |
| Time off work other carersa | Days | 175 | 20 (11) | 0.01 (0.01) | 167 | 16 (10) | 0.01 (0.00) |
| ATT devices (including basicb) | Items | 176 | 148 (84) | 2.91 (0.17) | 157 | 121 (77) | 2.28 (0.17) |
| Week 52 | Expected = 150 | Expected = 139 | |||||
| Community health | |||||||
| GP | Visits | 148 | 96 (65) | 1.41 (0.13) | 137 | 80 (58) | 1.35 (0.14) |
| Practice nurse | Visits | 148 | 64 (43) | 0.87 (0.16) | 137 | 50 (36) | 0.70 (0.13) |
| Community nurse | Visits | 148 | 31 (21) | 2.36 (0.88) | 137 | 26 (19) | 1.95 (0.77) |
| Physiotherapist | Visits | 148 | 9 (6) | 0.34 (0.15) | 137 | 9 (7) | 0.66 (0.48) |
| Occupational therapist | Visits | 148 | 17 (11) | 0.22 (0.06) | 137 | 20 (15) | 0.28 (0.07) |
| Dietitian | Visits | 148 | 4 (3) | 0.03 (0.02) | 137 | 9 (7) | 0.09 (0.03) |
| Paramedic | Visits | 148 | 15 (10) | 0.12 (0.03) | 137 | 18 (13) | 0.18 (0.05) |
| Specialist nurse | Visits | 148 | 17 (11) | 0.14 (0.04) | 137 | 16 (12) | 0.21 (0.07) |
| Dentist | Visits | 148 | 33 (22) | 0.32 (0.06) | 137 | 38 (28) | 0.40 (0.06) |
| Optician | Visits | 148 | 25 (17) | 0.18 (0.03) | 137 | 31 (23) | 0.30 (0.05) |
| Chiropodist | Visits | 148 | 61 (41) | 0.61 (0.07) | 137 | 52 (38) | 0.63 (0.08) |
| Mental health | |||||||
| Mental health nurse | Visits | 148 | 12 (8) | 0.14 (0.04) | 137 | 19 (14) | 0.25 (0.07) |
| Psychiatrist | Visits | 148 | 7 (5) | 0.05 (0.02) | 137 | 10 (7) | 0.08 (0.03) |
| Psychologist | Visits | 148 | 1 (1) | 0.01 (0.01) | 137 | 0 (0) | 0.00 (0.00) |
| Mental health team | Visits | 148 | 4 (3) | 0.18 (0.16) | 137 | 3 (2) | 0.10 (0.08) |
| Community care | |||||||
| Home care | Visits | 148 | 77 (52) | 86.19 (10.48) | 137 | 67 (49) | 92.06 (11.46) |
| Home care | Hours | 148 | 77 (52) | 122.81 (28.27) | 137 | 67 (49) | 77.20 (17.46) |
| Social worker | Visits | 148 | 29 (20) | 0.26 (0.06) | 137 | 33 (24) | 0.30 (0.05) |
| Cleaner | Visits | 148 | 38 (26) | 2.91 (0.46) | 137 | 36 (26) | 3.69 (0.81) |
| Meals on wheels | Visits | 148 | 5 (3) | 2.82 (1.26) | 137 | 12 (9) | 4.98 (1.60) |
| Laundry service | Visits | 148 | 4 (3) | 0.43 (0.22) | 137 | 5 (4) | 0.36 (0.17) |
| Sitting service | Visits | 148 | 5 (3) | 0.61 (0.36) | 137 | 9 (7) | 0.94 (0.39) |
| Carer support worker | Visits | 148 | 1 (1) | 0.00 (0.00) | 137 | 6 (4) | 0.15 (0.07) |
| Day services | |||||||
| Day centre | Attendances | 148 | 35 (24) | 5.83 (1.18) | 137 | 30 (22) | 4.54 (0.97) |
| Lunch club | Attendances | 148 | 12 (8) | 0.73 (0.22) | 137 | 16 (12) | 2.28 (1.12) |
| Patient education | Attendances | 148 | 7 (5) | 0.48 (0.23) | 137 | 7 (5) | 0.42 (0.17) |
| Hospital care | |||||||
| Emergency department | Attendances | 148 | 25 (17) | 0.18 (0.03) | 137 | 24 (18) | 0.24 (0.05) |
| Inpatients services | Days | 148 | 17 (11) | 0.86 (0.33) | 137 | 17 (12) | 1.69 (0.51) |
| Day hospital services | Days | 148 | 0 (0) | 0.00 (0.00) | 137 | 3 (2) | 0.02 (0.01) |
| Outpatients services | Visits | 148 | 52 (35) | 0.65 (0.10) | 137 | 56 (41) | 1.13 (0.31) |
| Residential respite | |||||||
| Residential home | Days | 148 | 1 (1) | 0.00 (0.00) | 137 | 4 (3) | 0.47 (0.32) |
| Nursing home | Days | 148 | 5 (3) | 0.64 (0.39) | 137 | 7 (5) | 0.61 (0.24) |
| Medications | |||||||
| Any medications | Units | 145 | 101 (70) | 0.99 (0.07) | 137 | 92 (67) | 0.93 (0.07) |
| Dementia | Units | 147 | 85 (58) | 0.63 (0.05) | 137 | 78 (57) | 0.62 (0.05) |
| Mental health | Units | 145 | 45 (31) | 0.37 (0.05) | 137 | 35 (26) | 0.31 (0.05) |
| Equipment (health and social care providers) | Items | 147 | 22 (15) | 0.24 (0.06) | 137 | 18 (13) | 0.21 (0.05) |
| Unpaid care; out of pocket | |||||||
| Equipment (private) | Items | 147 | 14 (10) | 0.11 (0.03) | 137 | 8 (6) | 0.06 (0.02) |
| Travel to appointments | Trips | 147 | 49 (33) | 2.27 (0.55) | 136 | 47 (35) | 1.11 (0.27) |
| Unpaid care | Hours | 145 | 145 (100) | 653.68 (56.15) | 135 | 135 (100) | 793.88 (59.57) |
| Carer cut down work | Hours | 146 | 1 (1) | 0.34 (0.34) | 135 | 3 (2) | 1.03 (0.71) |
| Carer stopped work | Weeks | 144 | 0 (0) | 0.00 (0.00) | 136 | 0 (0) | 0.00 (0.00) |
| Unpaid care other carersa | Hours | 147 | 84 (57) | 100.98 (17.96) | 136 | 78 (57) | 145.61 (28.77) |
| Time off work other carersa | Days | 146 | 7 (5) | 0.00 (0.00) | 134 | 16 (12) | 0.01 (0.00) |
| ATT devices (including basicb) | Items | 146 | 131 (90) | 3.70 (0.20) | 129 | 111 (86) | 2.63 (0.17) |
| Week 104 | Expected = 96 | Expected = 90 | |||||
| Community health | |||||||
| GP | Visits | 93 | 66 (71) | 1.39 (0.15) | 89 | 58 (65) | 1.55 (0.18) |
| Practice nurse | Visits | 92 | 28 (30) | 0.55 (0.14) | 89 | 28 (31) | 0.69 (0.22) |
| Community/district nurse | Visits | 93 | 20 (22) | 2.35 (1.05) | 89 | 31 (35) | 4.29 (1.59) |
| Physiotherapist | Visits | 93 | 7 (8) | 0.56 (0.31) | 89 | 11 (12) | 0.70 (0.38) |
| Occupational therapist | Visits | 93 | 8 (9) | 0.12 (0.05) | 89 | 8 (9) | 0.18 (0.07) |
| Dietitian | Visits | 93 | 2 (2) | 0.02 (0.02) | 89 | 6 (7) | 0.12 (0.05) |
| Paramedic | Visits | 93 | 10 (11) | 0.15 (0.06) | 89 | 9 (10) | 0.16 (0.06) |
| Specialist nurse | Visits | 93 | 11 (12) | 0.23 (0.09) | 89 | 8 (9) | 0.15 (0.06) |
| Dentist | Visits | 93 | 16 (17) | 0.26 (0.07) | 89 | 18 (20) | 0.29 (0.07) |
| Optician | Visits | 93 | 21 (23) | 0.27 (0.06) | 89 | 15 (17) | 0.21 (0.06) |
| Chiropodist | Visits | 93 | 37 (40) | 0.66 (0.11) | 89 | 39 (44) | 0.55 (0.07) |
| Mental health | |||||||
| Mental health nurse | Visits | 93 | 6 (6) | 0.11 (0.06) | 89 | 7 (8) | 0.11 (0.04) |
| Psychiatrist | Visits | 93 | 1 (1) | 0.01 (0.01) | 89 | 6 (7) | 0.07 (0.03) |
| Psychologist | Visits | 93 | 0 (0) | 0.00 (0.00) | 89 | 0 (0) | 0.00 (0.00) |
| Mental health team | Visits | 93 | 0 (0) | 0.00 (0.00) | 89 | 4 (4) | 0.07 (0.04) |
| Community care | |||||||
| Home care | Visits | 93 | 46 (49) | 97.81 (15.06) | 89 | 50 (56) | 110.49 (16.87) |
| Home care | Hours | 93 | 46 (49) | 169.38 (49.12) | 89 | 50 (56) | 113.33 (28.65) |
| Social worker | Visits | 93 | 11 (12) | 0.24 (0.11) | 89 | 19 (21) | 0.33 (0.08) |
| Cleaner | Visits | 93 | 32 (34) | 4.00 (0.65) | 89 | 25 (28) | 4.68 (1.59) |
| Meals on wheels | Visits | 93 | 2 (2) | 1.96 (1.38) | 89 | 8 (9) | 8.13 (4.43) |
| Laundry service | Visits | 93 | 1 (1) | 0.14 (0.14) | 89 | 3 (3) | 0.28 (0.20) |
| Sitting service | Visits | 93 | 6 (6) | 1.44 (0.64) | 89 | 7 (8) | 1.10 (0.48) |
| Carer support worker | Visits | 93 | 2 (2) | 0.29 (0.27) | 89 | 3 (3) | 0.25 (0.20) |
| Day services | |||||||
| Day centre | Attendances | 93 | 20 (22) | 7.00 (1.72) | 89 | 22 (25) | 5.46 (1.26) |
| Lunch club | Attendances | 93 | 5 (5) | 0.37 (0.20) | 89 | 8 (9) | 0.84 (0.39) |
| Patient education group | Attendances | 93 | 3 (3) | 0.18 (0.14) | 89 | 4 (4) | 0.58 (0.33) |
| Hospital care | |||||||
| Emergency department | Attendances | 93 | 8 (9) | 0.11 (0.04) | 89 | 20 (22) | 0.35 (0.08) |
| Inpatients services | Days | 93 | 6 (6) | 1.00 (0.56) | 89 | 16 (18) | 1.33 (0.44) |
| Day hospital services | Days | 93 | 1 (1) | 0.01 (0.01) | 89 | 1 (1) | 0.01 (0.01) |
| Outpatients services | Visits | 93 | 35 (38) | 0.67 (0.12) | 89 | 28 (31) | 1.17 (0.54) |
| Residential respite | |||||||
| Residential home | Days | 93 | 0 (0) | 0.00 (0.00) | 89 | 1 (1) | 0.15 (0.15) |
| Nursing home | Days | 93 | 1 (1) | 0.03 (0.03) | 89 | 4 (4) | 0.57 (0.31) |
| Medications | |||||||
| Any medications | Units | 92 | 61 (66) | 0.99 (0.09) | 89 | 63 (71) | 0.98 (0.09) |
| Dementia | Units | 93 | 50 (54) | 0.61 (0.06) | 89 | 53 (60) | 0.61 (0.05) |
| Mental health | Units | 92 | 29 (32) | 0.39 (0.07) | 89 | 25 (28) | 0.37 (0.07) |
| Equipment (health and social care providers) | Items | 92 | 10 (11) | 0.15 (0.05) | 89 | 9 (10) | 0.16 (0.05) |
| Unpaid care | |||||||
| Equipment (private) | Items | 92 | 5 (5) | 0.10 (0.05) | 89 | 3 (3) | 0.03 (0.02) |
| Travel to appointments | Trips | 93 | 35 (38) | 1.41 (0.36) | 89 | 29 (33) | 2.97 (1.02) |
| Unpaid care | Hours | 91 | 88 (97) | 656.30 (72.42) | 88 | 87 (99) | 776.74 (74.79) |
| Carer cut down work | Hours | 90 | 1 (1) | 0.56 (0.56) | 89 | 1 (1) | 0.22 (0.22) |
| Carer stopped work | Weeks | 89 | 0 (0) | 0.00 (0.00) | 89 | 0 (0) | 0.00 (0.00) |
| Unpaid care other carersa | Hours | 93 | 53 (57) | 154.89 (36.97) | 89 | 53 (60) | 118.22 (29.03) |
| Time off work other carersa | Days | 93 | 3 (3) | 0.01 (0.01) | 88 | 4 (5) | 0.00 (0.00) |
| ATT devices (including basicb) | Items | 93 | 85 (91) | 3.63 (0.24) | 87 | 72 (83) | 2.78 (0.22) |
Appendix 6 Mean cumulative costs
Mean cumulative costs (SEs): intervention costs, total health and social care and societal costs (at opportunity and replacement cost valuation of unpaid carer time) from baseline to 104 weeks (£, 2016–17).
| Cost | Trial arm | Intervention – control | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| n | Mean | SE | n | Mean | SE | Mean difference | 95% CI | |
| Expected = 229 | Expected = 224 | |||||||
| Intervention: ATT including baseline ATT costa | 223 | 408 | 18 | 203 | 288 | 16 | 119*** | 71 to 168 |
| Intervention: ATT over 104 weeks’ follow-up | 223 | 322 | 18 | 203 | 214 | 15 | 109*** | 62 to 155 |
| Health and social care | 210 | 19,232 | 3086 | 201 | 16,073 | 2189 | 3159 | –4337 to 10,655 |
| Intervention + health and social care | 205 | 19,649 | 3206 | 189 | 15,186 | 2102 | 4463 | –3209 to 12,135 |
| Societalb | 208 | 55,209 | 4404 | 200 | 58,272 | 4473 | –3064 | –15,405 to 9277 |
| Intervention + societalb | 203 | 56,000 | 4579 | 188 | 53,378 | 4441 | 2622 | –9953 to 15,196 |
| Sensitivity analysis | ||||||||
| Societalc | 208 | 128,935 | 8862 | 200 | 139,524 | 10,008 | –10,589 | –36,816 to 15,638 |
| Intervention + societalc | 203 | 129,845 | 9163 | 188 | 125,952 | 10,026 | 3893 | –22,756 to 30,543 |
Appendix 7 Economic evaluation and caregiver data supplementary tables and figures
| Costs | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up period | Follow-up – baseline | ||||
| Mean | SE | Mean | SE | Mean | 95% CI | |
| Health and social care | N = 287 | |||||
| Intervention | 2295 | 250 | 2785 | 384 | 490 | –97 to 1078 |
| Control | 2541 | 356 | 2665 | 434 | 124 | –917 to 1164 |
| Societal | N = 284 | |||||
| Intervention | 8152 | 545 | 8978 | 553 | 825 | –172 to 1823 |
| Control | 8558 | 614 | 9133 | 640 | 574 | –730 to 1878 |
| Costs | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up period | Follow-up – baseline | ||||
| Mean | SE | Mean | SE | Mean | 95% CI | |
| EQ-5D-5L-participant | N = 287 | |||||
| Intervention | 0.746 | 0.017 | 0.747 | 0.017 | 0.001 | –0.027 to 0.028 |
| Control | 0.792 | 0.020 | 0.804 | 0.017 | 0.012 | –0.017 to 0.04 |
| EQ-5D-5L-proxy | N = 309 | |||||
| Intervention | 0.546 | 0.014 | 0.546 | 0.013 | 0.000 | –0.026 to 0.026 |
| Control | 0.565 | 0.016 | 0.531 | 0.016 | –0.034 | –0.066 to –0.002 |
| Costs | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up period | Follow-up – baseline | ||||
| Mean | SE | Mean | SE | Mean | 95% CI | |
| Health and social cared | N = 229 | |||||
| Intervention | 2250 | 291 | 3239 | 493 | 989 | 158 to 1819 |
| Control | 2504 | 447 | 2959 | 497 | 455 | –706 to 1616 |
| Societal | N = 227 | |||||
| Intervention | 7961 | 584 | 9665 | 668 | 1704 | 569 to 2838 |
| Control | 8450 | 709 | 10,038 | 759 | 1588 | 3 to 3174 |
| Outcomes | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up period | Follow-up – baseline | ||||
| Mean | SE | Mean | SE | Mean | 95% CI | |
| EQ-5D-5L-participant | N = 229 | |||||
| Intervention | 0.774 | 0.016 | 0.766 | 0.017 | –0.008 | –0.038 to 0.021 |
| Control | 0.774 | 0.016 | 0.766 | 0.017 | –0.008 | –0.038 to 0.021 |
| EQ-5D-5L-proxy | N = 257 | |||||
| Intervention | 0.569 | 0.013 | 0.539 | 0.013 | –0.031 | –0.057 to –0.004 |
| Control | 0.575 | 0.018 | 0.517 | 0.016 | –0.057 | –0.089 to –0.025 |
| Costs | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up period | Follow-up – baseline | ||||
| Mean | SE | Mean | SE | Mean | 95% CIb | |
| Health and social cared | N = 243 | |||||
| Intervention | 2257 | 295 | 3639 | 538 | 1381 | 409 to 2360 |
| Control | 2502 | 40 | 3185 | 526 | 683 | –547 to 1913 |
| Societal | N = 241 | |||||
| Intervention | 8037 | 600 | 10,051 | 696 | 2013 | 640 to 3387 |
| Control | 8536 | 691 | 10,397 | 735 | 1860 | 273 to 3449 |
| Outcomes | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up period | Follow-up – baseline | ||||
| Mean | SE | Mean | SE | Mean | 95% CI | |
| EQ-5D-5L-participant | N = 243 | |||||
| Intervention | 0.767 | 0.016 | 0.752 | 0.017 | –0.015 | –0.043 to 0.012 |
| Control | 0.807 | 0.018 | 0.808 | 0.015 | 0.001 | –0.026 to 0.027 |
| EQ-5D-5L-proxy | N = 266 | |||||
| Intervention | 0.567 | 0.014 | 0.524 | 0.013 | –0.064 | –0.095 to –0.033 |
| Control | 0.573 | 0.018 | 0.509 | 0.015 | –0.043 | –0.07 to –0.017 |
FIGURE 13.
Cost-effectiveness acceptability curve: institutionalisation-free days and total costs at the 104-week follow-up.
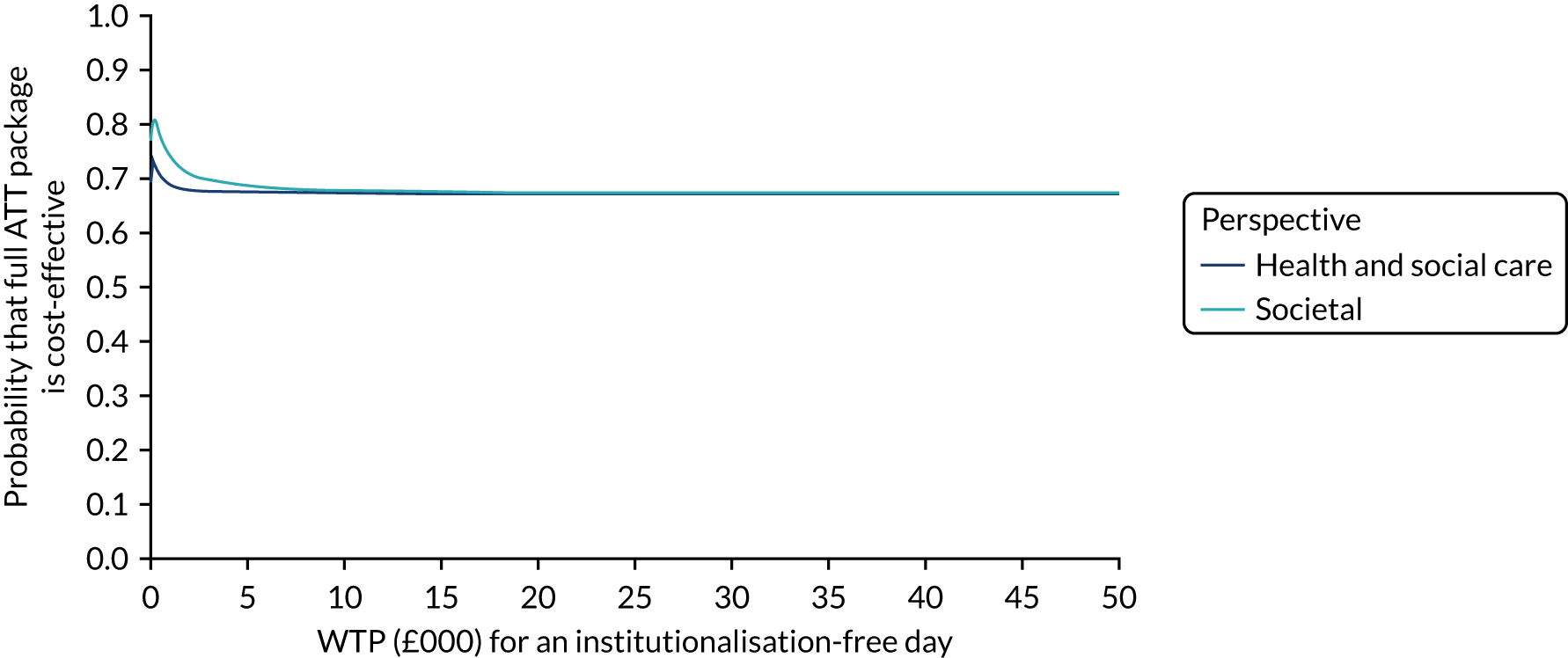
FIGURE 14.
The CEAC at the 24-week follow-up: EQ-5D-proxy index scores and health and social care costs. MCID, minimal clinically important difference. MCID = 0.74.
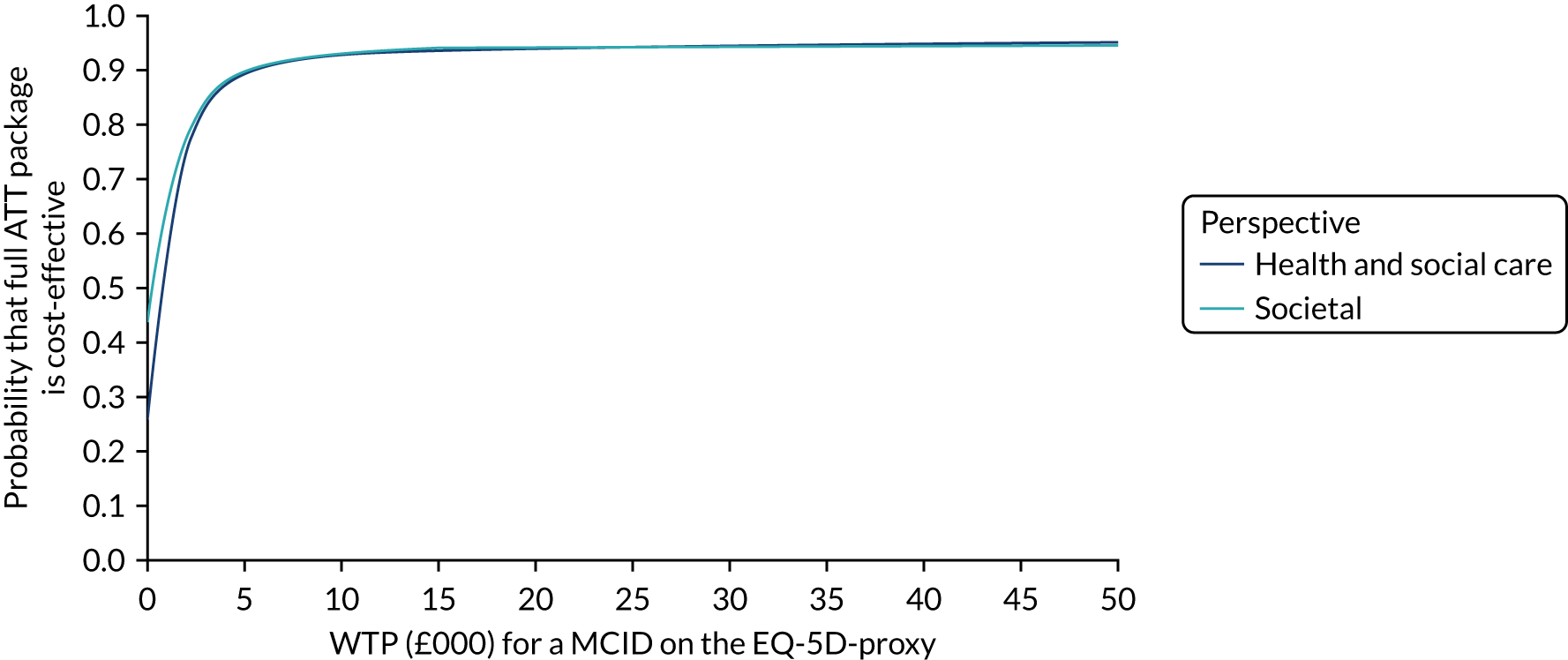
FIGURE 15.
The CEAC at 52-week follow-up: EQ-5D-proxy index scores and health and social care costs. MCID, minimal clinically important difference. MCID = 0.74.
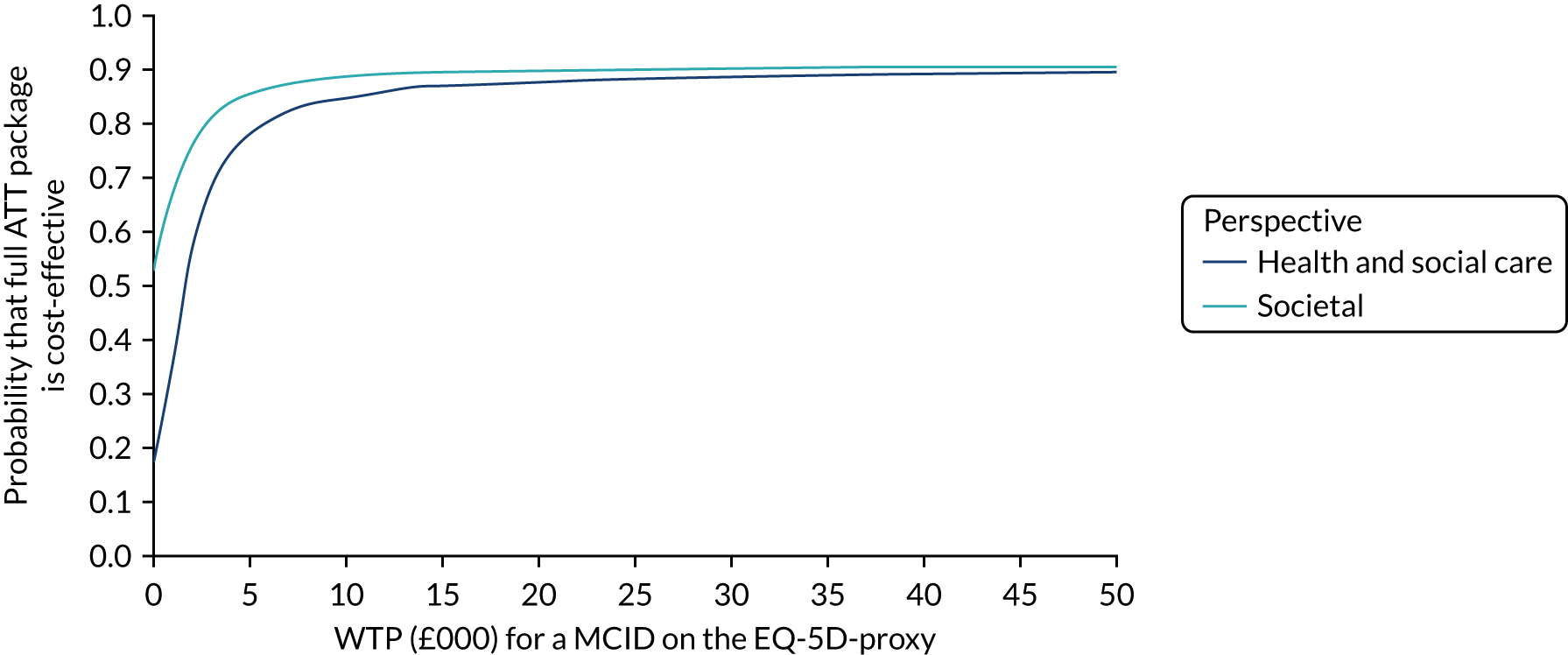
FIGURE 16.
The CEAC at 104-week follow-up: EQ-5D-proxy index scores and health and social care costs. MCID, minimal clinically important difference. MCID = 0.74.
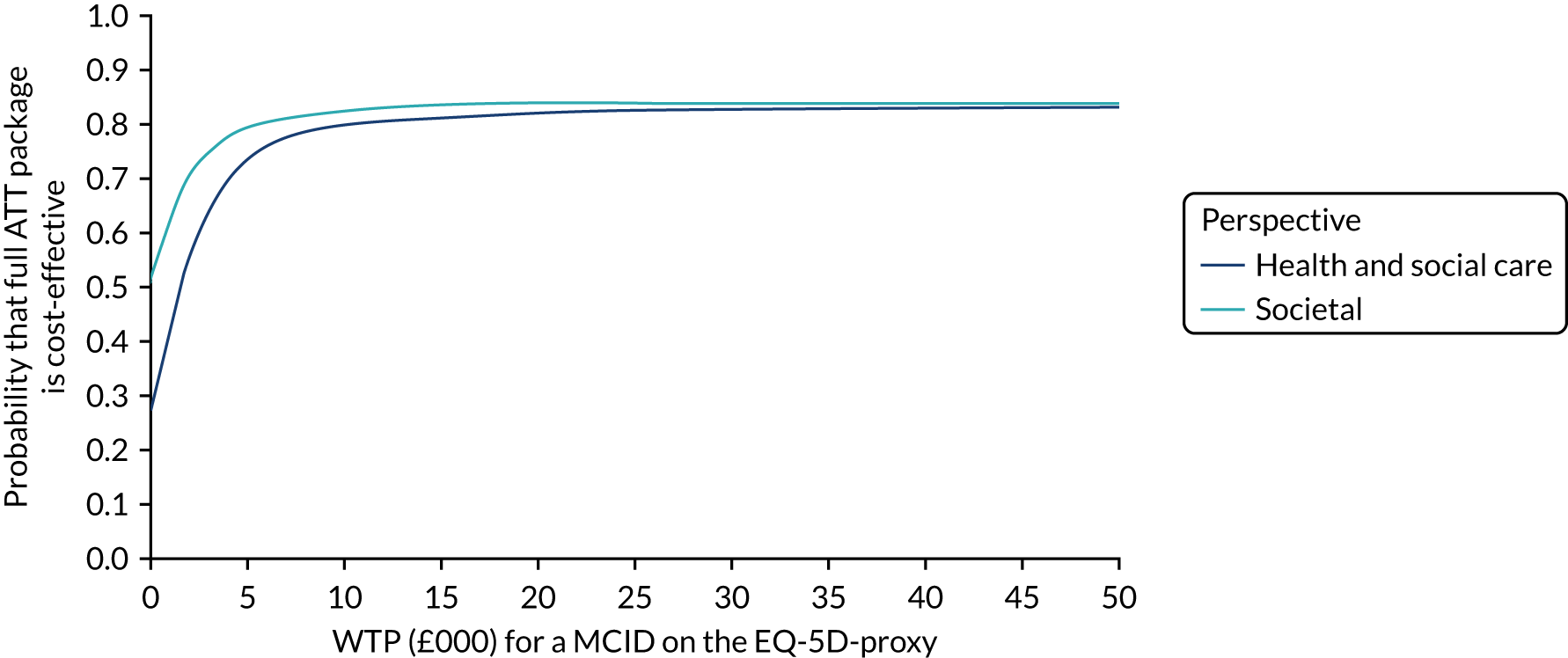
FIGURE 17.
Cost-effectiveness acceptability curve: person with dementia QALY derived from the EQ-5D-proxy and total costs at the 24-week follow-up.
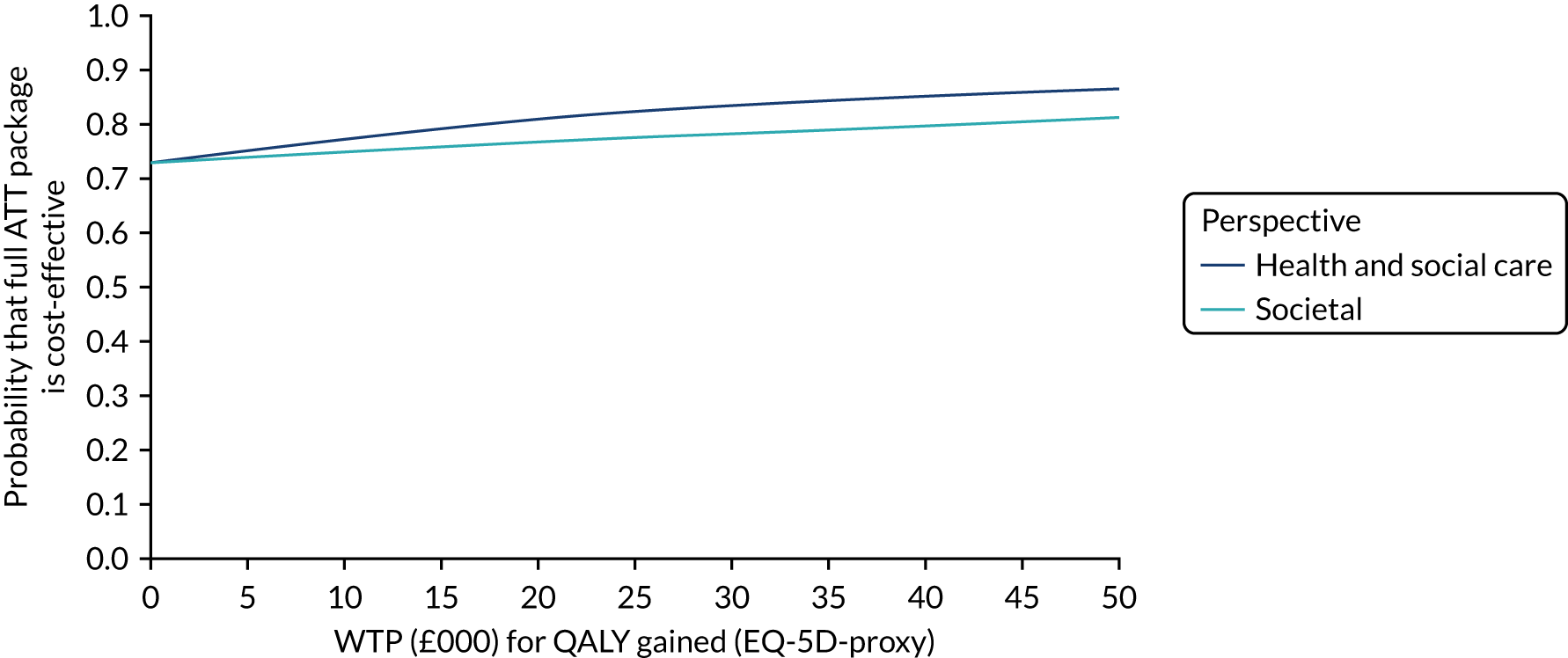
FIGURE 18.
Cost-effectiveness acceptability curve: person with dementia QALY derived from the EQ-5D-proxy and total costs at the 52-week follow-up.
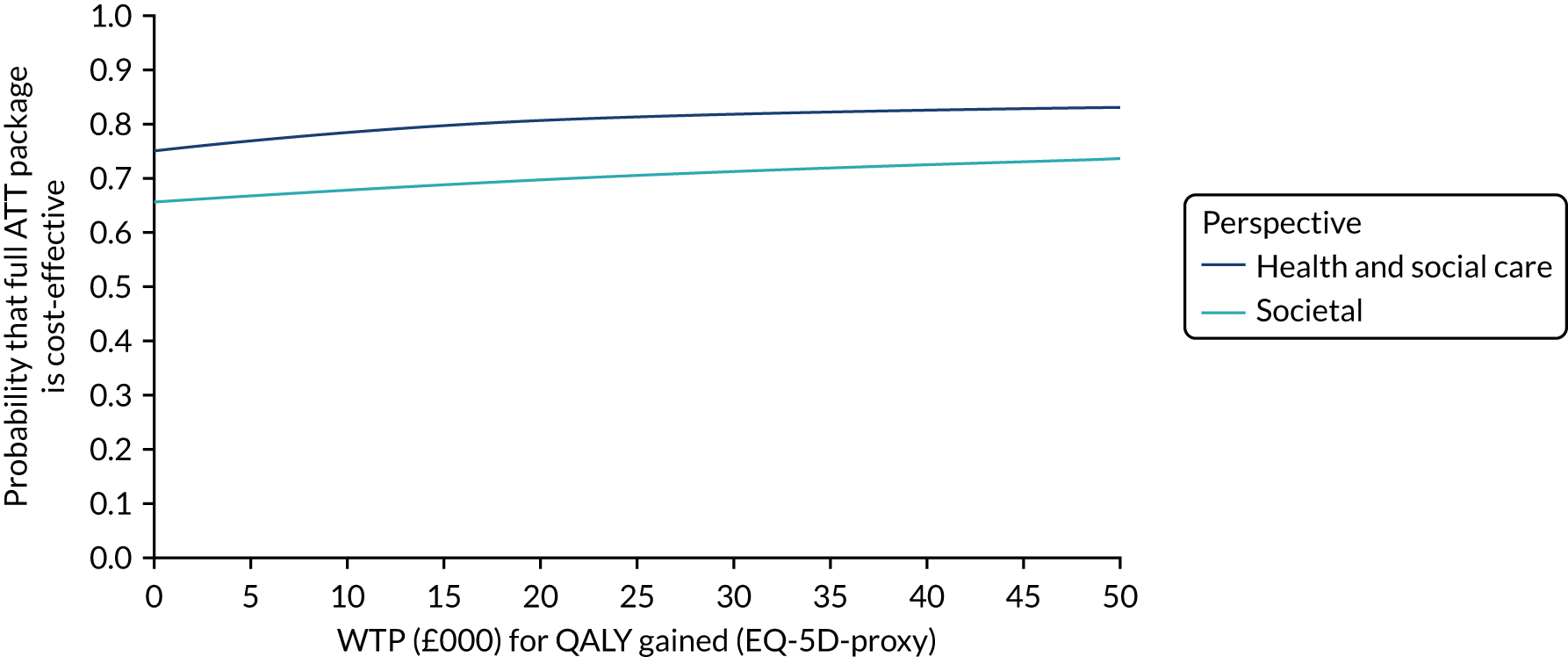
FIGURE 19.
Cost-effectiveness acceptability curve: person with dementia QALY derived from the EQ-5D-proxy and total costs at the 104-week follow-up.
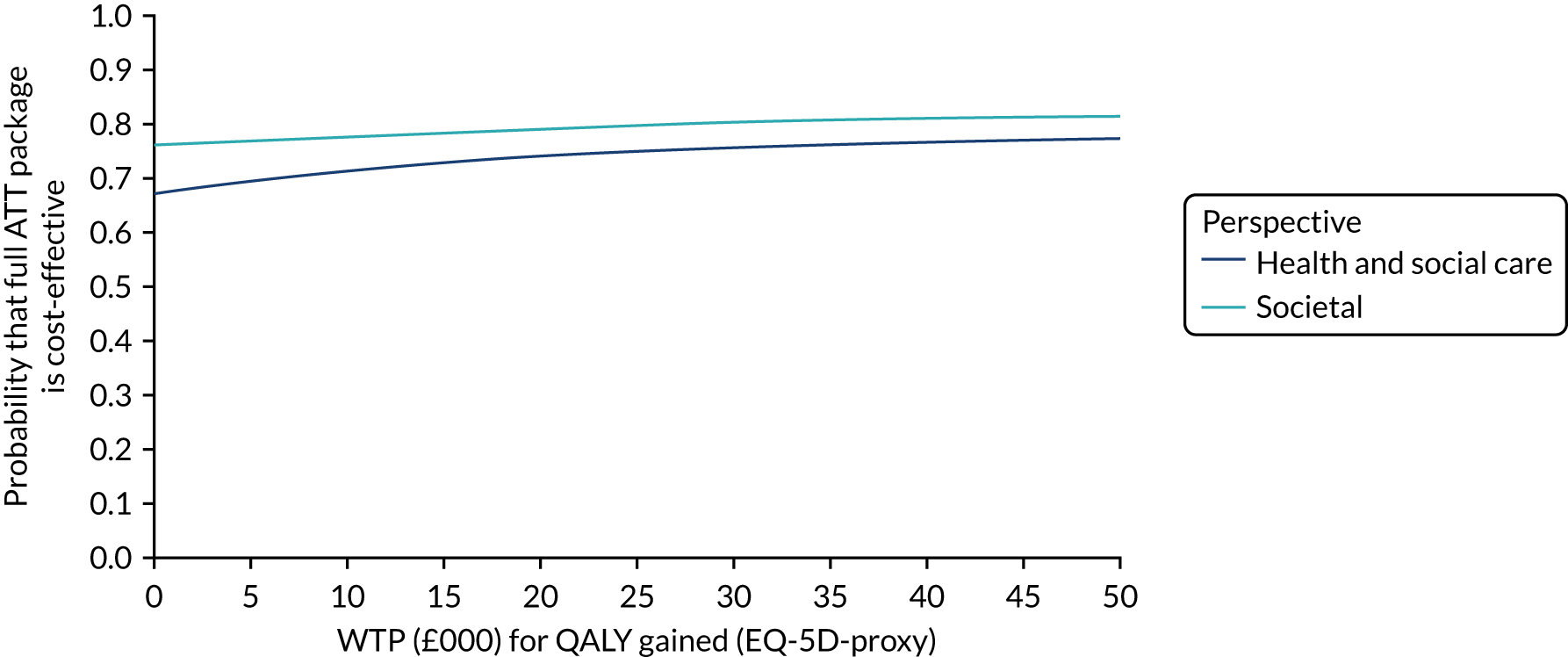
| Cost | Trial arm | Intervention – control | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| n | Mean | SE | n | Mean | SE | Mean difference | 95% CI | |
| Baseline | Expected = 229 | Expected = 224 | ||||||
| Unpaid carea | 217 | 18,270 | 1240 | 202 | 20,106 | 1334 | –1836 | –5410 to 1739 |
| Intervention + societalb | 203 | 20,502 | 1285 | 188 | 23,102 | 1493 | –2600 | –6457 to 1256 |
| Week 12 | Expected = 189 | Expected = 188 | ||||||
| Unpaid carea | 186 | 19,802 | 1332 | 183 | 22,229 | 1511 | –2427 | –6384 to 1530 |
| Intervention + societalb | 181 | 22,569 | 1371 | 160 | 25,187 | 1696 | –2618 | –6869 to 1632 |
| Week 24 | Expected = 178 | Expected = 168 | ||||||
| Unpaid carea | 175 | 21,685 | 1528 | 168 | 22,830 | 1602 | –1144 | –5497 to 3208 |
| Intervention + societalb | 170 | 24,769 | 1575 | 151 | 25,719 | 1716 | –951 | –5527 to 3625 |
| Week 52 | Expected = 150 | Expected = 139 | ||||||
| Unpaid carea | 147 | 20,117 | 1517 | 136 | 25,180 | 1791 | –5063* | –9661 to –464 |
| Intervention + societalb | 143 | 24,582 | 1633 | 128 | 28,512 | 1914 | –3930 | –8854 to 995 |
| Week 104 | Expected = 96 | Expected = 90 | ||||||
| Unpaid carea | 93 | 21,337 | 2035 | 89 | 23,652 | 2103 | –2316 | –8088 to 3457 |
| Intervention + societalb | 92 | 27,125 | 2527 | 84 | 29,122 | 2161 | –1998 | –8620 to 4624 |
| Difference | 95% CIa | ICERb (difference in costs/MCID) | |
|---|---|---|---|
| Person with dementia-reported | N = 241 | ||
| EQ-5Dc | –0.019 | –0.06 to 0.017 | 316/–0.262 = –1209 |
| Societald | 316 | –3457 to 3978 | |
| Person with dementia proxy-reported | N = 266 | ||
| EQ-5D-proxyc | 0.021 | –0.022 to 0.06 | –288/0.281 = –1024 |
| Societald | –281 | –3930 to 3249 |
| Trial arm | Intervention – control | Cost per institutionalisation-free daya | |||||
|---|---|---|---|---|---|---|---|
| Interventiona | Controla | ||||||
| Mean | 95% CIb | Mean | 95% CIb | Mean difference | 95% CIb | ||
| Societal costs (£) | 131,847 | 119,111 to 146,973 | 133,781 | 119,333 to 149,963 | –1934 | –19,986 to 16,892 | –1934/7.898 = –245 |
| Institutionalisation-free days | 597.075 | 572.464 to 620.939 | 589.177 | 563.373 to 614.062 | 7.898 | –26.438 to 42.425 | |
| Trial arm | Intervention – control | Cost per QALY | |||||
|---|---|---|---|---|---|---|---|
| Interventiona | Controla | ||||||
| Mean | 95% CIb | Mean | 95% CIb | Mean difference | 95% CIb | ||
| Societal costs (£) | 131,847 | 119,111 to 146,973 | 133,781 | 119,333 to 149,963 | –1934 | –19,986 to 16,892 | |
| QALY – EQ-5D-participant | 1.201 | 1.127 to 1.271 | 1.306 | 1.234 to 1.376 | –0.105* | –0.204 to –0.007 | –1934/–0.105 = 18,371 |
| QALY – EQ-5D-proxy | 0.828 | 0.762 to 0.894 | 0.798 | 0.733 to 0.861 | 0.030 | –0.058 to 0.117 | –1934/0.030 = –63,587 |
| Negative appraisal of caring | Adequacy as a care partner | Care partner burden and strain |
|---|---|---|
| zarit12_rg.1 | zarit20_rg.1 | zarit18_rg.1 |
| zarit8_rg.1 | zarit21_rg.1 | zarit5_rg.1 |
| zarit2_rg.1 | zarit15_rg.1 | zarit9_rg.1 |
| zarit17_rg.1 | zarit19_rg.1 | |
| zarit11_rg.1 | zarit16_rg.1 | |
| zarit14_rg.1 | zarit4_rg.1 | |
| zarit3_rg.1 | zarit22_rg.1 | |
| zarit1_rg.1 | ||
| zarit6_rg.1 |
Item loadings
| Item | Item code | Component | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Do you feel your social life has suffered because you are caring for the person you care for? | zarit12_rg.1 | 0.823 | ||
| Do you feel that the person you care for is dependent on you? | zarit8_rg.1 | 0.715 | ||
| Do you feel that because of the time you spend with the person you care for that you do not have enough time for yourself? | zarit2_rg.1 | 0.710 | ||
| Do you feel that you have lost control of your life since the illness/disability of the person you care for? | zarit17_rg.1 | 0.597 | ||
| Do you feel that you do not have as much privacy as you would like because of the person you care for? | zarit11_rg.1 | 0.595 | ||
| Do you feel that the person you care for seems to expect you to take care of him/her as if you were the only one he/she could depend on? | zarit14_rg.1 | 0.576 | ||
| Do you feel stressed between caring for the person you care for and trying to meet other responsibilities for your family or work? | zarit3_rg.1 | 0.425 | 0.313 | |
| Do you feel that the person you care for asks for more help than he/she needs? | zarit1_rg.1 | 0.424 | ||
| Do you feel that the person you care for currently affects your relationships with other family members or friends in a negative way? | zarit6_rg.1 | 0.404 | ||
| Do you feel your health has suffered because of your involvement with the person you care for? | zarit10_rg.1 | 0.387 | –0.378 | |
| Do you feel you should be doing more for the person you care for? | zarit20_rg.1 | 0.855 | ||
| Do you feel you could do a better job in caring for the person you care for? Values | zarit21_rg.1 | 0.806 | ||
| Do you feel that you do not have enough money to take care of the person you care for in addition to the rest of your expenses? | zarit15_rg.1 | 0.361 | 0.415 | |
| Are you afraid what the future holds for the person you care for? | zarit7_rg.1 | 0.340 | 0.346 | |
| Do you wish you could leave the care of the person you care for to someone else? | zarit18_rg.1 | –0.758 | ||
| Do you feel angry when you are around the person you care for? | zarit5_rg.1 | –0.713 | ||
| Do you feel strained when you are around the person you care for? | zarit9_rg.1 | –0.674 | ||
| Do you feel uncertain about what to do about the person you care for? | zarit19_rg.1 | –0.613 | ||
| Do you feel that you will be unable to take care of the person you care for much longer? | zarit16_rg.1 | –0.602 | ||
| Do you feel embarrassed over the behaviour of the person you care for? | zarit4_rg.1 | –0.584 | ||
| Overall, how burdened do you feel in caring for your relative? | zarit22_rg.1 | –0.576 | ||
| Do you feel uncomfortable about having friends over because of the person you care for? | zarit13_rg.1 | 0.369 | –0.391 | |
| F-value | df | p-value | ||
|---|---|---|---|---|
| 1 | 2 | |||
| Total score | ||||
| Time | 1.233 | 2 | 24754846 | 0.291 |
| Group | 0.646 | 1 | 561897 | 0.422 |
| Interaction | 0.466 | 2 | 89862616 | 0.628 |
| Component 1: negative appraisal | ||||
| Time | 1.552 | 2 | 1562062 | 0.212 |
| Group | 0.504 | 1 | 483265 | 0.478 |
| Interaction | 0.816 | 2 | 6865985 | 0.442 |
| Component 2: adequacy as a care partner | ||||
| Time | 0.582 | 2 | 407414 | 0.559 |
| Group | 0.111 | 1 | 92008 | 0.739 |
| Interaction | 0.849 | 2 | 195724 | 0.428 |
| Component 3: burden and strain | ||||
| Time | 2.027 | 2 | 608124 | 0.132 |
| Group | 1.237 | 1 | 1260074 | 0.266 |
| Interaction | 0.838 | 2 | 1370133 | 0.433 |
| Time point | Trial arm | Mean | SE | 95% CI |
|---|---|---|---|---|
| Total score | ||||
| Baseline | Control | 25.0 | 2.84 | 19.4 to 30.5 |
| ATT intervention | 25.1 | 2.92 | 19.4 to 30.8 | |
| Week 12 | Control | 25.5 | 2.91 | 19.8 to 31.2 |
| ATT intervention | 26.3 | 2.98 | 20.5 to 32.2 | |
| Week 24 | Control | 25.2 | 2.93 | 19.4 to 30.9 |
| ATT intervention | 27.1 | 2.98 | 21.2 to 32.9 | |
| Component 1: negative appraisal | ||||
| Baseline | Control | 11.6 | 1.34 | 9.0 to 14.2 |
| ATT intervention | 11.6 | 1.38 | 8.9 to 14.3 | |
| Week 12 | Control | 12.3 | 1.38 | 9.6 to 15.0 |
| ATT intervention | 11.8 | 1.41 | 9.0 to 14.5 | |
| Week 24 | Control | 11.9 | 1.40 | 9.2 to 14.7 |
| ATT intervention | 12.8 | 1.41 | 10.0 to 15.5 | |
| Component 2: adequacy as a care partner | ||||
| Baseline | Control | 3.5 | 0.53 | 2.4 to 4.5 |
| ATT intervention | 3.5 | 0.54 | 2.5 to 4.6 | |
| Week 12 | Control | 3.2 | 0.54 | 2.1 to 4.2 |
| ATT intervention | 3.8 | 0.55 | 2.7 to 4.8 | |
| Week 24 | Control | 3.3 | 0.55 | 2.2 to 4.4 |
| ATT intervention | 3.4 | 0.55 | 2.4 to 4.5 | |
| ZBI Component 3: Burden and strain | ||||
| Baseline | Control | 6.1 | 1.08 | 4.0 to 8.2 |
| ATT intervention | 6.3 | 1.10 | 4.1 to 8.4 | |
| Week 12 | Control | 6.3 | 1.11 | 4.2 to 8.5 |
| ATT intervention | 7.2 | 1.13 | 5.0 to 9.4 | |
| Week 24 | Control | 6.0 | 1.12 | 3.8 to 8.2 |
| ATT intervention | 7.0 | 1.13 | 4.8 to 9.2 | |
| F-value | df | p-value | ||
|---|---|---|---|---|
| 1 | 2 | |||
| STAI-6 | ||||
| Time | 1.388 | 2 | 25730363 | 0.250 |
| Group | 2.290 | 1 | 6245543 | 0.130 |
| Interaction | 0.489 | 2 | 15591551 | 0.613 |
| CES-D-10 | ||||
| Time | 0.246 | 2 | 503980 | 0.782 |
| Group | 0.016 | 1 | 370269 | 0.899 |
| Interaction | 0.159 | 2 | 916269 | 0.853 |
| Time point | Trial arm | Mean | SE | 95% CI |
|---|---|---|---|---|
| STAI-6 | ||||
| Baseline | Control | 37.5 | 2.51 | 32.6 to 42.5 |
| ATT intervention | 39.2 | 2.55 | 34.2 to 44.2 | |
| Week 12 | Control | 38.7 | 2.61 | 33.6 to 43.9 |
| ATT intervention | 40.0 | 2.64 | 34.8 to 45.2 | |
| Week 24 | Control | 38.4 | 2.65 | 33.2 to 43.6 |
| ATT intervention | 41.9 | 2.64 | 36.7 to 47.1 | |
| CES-D-10 | ||||
| Baseline | Control | 8.8 | 1.05 | 6.7 to 10.9 |
| ATT intervention | 8.3 | 1.07 | 6.2 to 10.4 | |
| Week 12 | Control | 8.7 | 1.08 | 6.6 to 10.8 |
| ATT intervention | 8.1 | 1.10 | 6.0 to 10.3 | |
| Week 24 | Control | 8.6 | 1.09 | 6.5 to 10.8 |
| ATT intervention | 8.5 | 1.10 | 6.4 to 10.7 | |
| F-value | df | p-value | ||
|---|---|---|---|---|
| 1 | 2 | |||
| Total score | ||||
| Time | 0.372 | 2 | 74615089 | 0.690 |
| Group | 0.007 | 1 | 404895 | 0.931 |
| Interaction | 0.150 | 2 | 119338508 | 0.861 |
| Component 1: negative appraisal | ||||
| Time | 0.816 | 2 | 7177464.9 | 0.442 |
| Group | 0.019 | 1 | 1066716 | 0.890 |
| Interaction | 1.038 | 2 | 20626935 | 0.354 |
| Component 2: adequacy as a care partner | ||||
| Time | 0.755 | 2 | 456838.93 | 0.470 |
| Group | 0.251 | 1 | 136990.12 | 0.617 |
| Interaction | 0.767 | 2 | 236804.1 | 0.465 |
| Component 3: burden and strain | ||||
| Time | 1.491 | 2 | 523331.74 | 0.225 |
| Group | 0.237 | 1 | 1210269 | 0.626 |
| Interaction | 0.672 | 2 | 2791857 | 0.511 |
| Time point | Trial arm | Mean | SE | 95% CI |
|---|---|---|---|---|
| Total score | ||||
| Baseline | Control | 31.7 | 1.69 | 28.4 to 35.1 |
| Intervention | 31.3 | 1.76 | 27.8 to 34.7 | |
| Week 12 | Control | 32.5 | 1.76 | 29.0 to 35.9 |
| Intervention | 31.8 | 1.82 | 28.2 to 35.4 | |
| Week 24 | Control | 32.0 | 1.79 | 28.5 to 35.5 |
| Intervention | 32.2 | 1.83 | 28.6 to 35.8 | |
| Component 1: negative appraisal | ||||
| Baseline | Control | 15.5 | 0.801 | 13.9 to 17.1 |
| Intervention | 15.5 | 0.832 | 13.9 to 17.1 | |
| Week 12 | Control | 16.3 | 0.843 | 14.6 to 17.9 |
| Intervention | 15.3 | 0.871 | 13.6 to 17.0 | |
| Week 24 | Control | 15.9 | 0.864 | 14.2 to 17.6 |
| Intervention | 16.1 | 0.877 | 14.3 to 17.8 | |
| Component 2: adequacy as a care partner | ||||
| Baseline | Control | 3.5 | 0.29 | 2.9 to 4.1 |
| Intervention | 3.6 | 0.30 | 3.0 to 4.2 | |
| Week 12 | Control | 3.4 | 0.31 | 2.8 to 4.0 |
| Intervention | 3.7 | 0.32 | 3.1 to 4.3 | |
| Week 24 | Control | 3.6 | 0.32 | 2.9 to 4.2 |
| Intervention | 3.4 | 0.32 | 2.7 to 4.0 | |
| Component 3: burden and strain | ||||
| Baseline | Control | 7.9 | 0.62 | 6.7 to 9.1 |
| Intervention | 7.6 | 0.64 | 6.4 to 8.9 | |
| Week 12 | Control | 8.1 | 0.65 | 6.8 to 9.3 |
| Intervention | 8.4 | 0.67 | 7.1 to 9.7 | |
| Week 24 | Control | 7.7 | 0.66 | 6.4 to 9.0 |
| Intervention | 8.1 | 0.67 | 6.8 to 9.4 | |
| F-value | df | p-value | ||
|---|---|---|---|---|
| 1 | 2 | |||
| CES-D-10 | ||||
| Time | 1.668 | 2 | 941283.6 | 0.189 |
| Group | 0.977 | 1 | 1667711 | 0.323 |
| Interaction | 1.564 | 2 | 1978748 | 0.209 |
| STAI-6 | ||||
| Time | 2.583 | 2 | 52141891 | 0.076 |
| Group | 3.061 | 1 | 6614194 | 0.080 |
| Interaction | 0.986 | 2 | 13038219 | 0.373 |
| Time point | Trial arm | Mean | SE | 95% CI |
|---|---|---|---|---|
| CES-D-10 | ||||
| Baseline | Control | 11.1 | 0.63 | 9.9 to 12.4 |
| Intervention | 10.9 | 0.65 | 9.6 to 12.2 | |
| Week 12 | Control | 10.9 | 0.67 | 9.6 to 12.3 |
| Intervention | 10.6 | 0.69 | 9.2 to 11.9 | |
| Week 24 | Control | 10.7 | 0.69 | 9.3 to 12.0 |
| Intervention | 11.6 | 0.69 | 10.2 to 12.9 | |
| STAI-6 | ||||
| Baseline | Control | 41.1 | 1.45 | 38.3 to 43.9 |
| Intervention | 42.4 | 1.49 | 39.4 to 45.3 | |
| Week 12 | Control | 41.8 | 1.57 | 38.7 to 44.8 |
| Intervention | 42.7 | 1.60 | 39.6 to 45.9 | |
| Week 24 | Control | 41.7 | 1.63 | 38.6 to 44.9 |
| Intervention | 45.5 | 1.63 | 42.3 to 48.7 | |
List of abbreviations
- ACCOMODATE
- A Collaborative COMMunity-based ethnography Of people with Dementia using Assistive technology and Telecare at home in England
- ADL
- activities of daily living
- AE
- adverse event
- ASCD
- Adult Social Care Department
- ATT
- assistive technology and telecare
- ATTILA
- Assistive Technology and Telecare to maintain Independent Living At home for people with dementia
- BADLS
- Bristol Activities of Daily Living Scale
- CASSR
- council with adult social services responsibilities
- CCTV
- closed-circuit television
- CEAC
- cost-effectiveness acceptability curve
- CES-D-10
- 10-item Center for Epidemiological Studies Depression Scale
- CI
- confidence interval
- CSRI
- Client Service Receipt Inventory
- DHSC
- Department of Health and Social Care
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- GPS
- Global Positioning System
- ICER
- incremental cost-effectiveness ratio
- ICH-GCP
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use – Good Clinical Practice
- ICT
- information and communications technology
- LMM
- linear mixed modelling
- MOHOST
- Model of Human Occupation Screening Tool
- NHC
- Northern Housing Consortium
- NICE
- National Institute for Health and Care Excellence
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SMMSE
- Standardised Mini-Mental State Examination
- STAI-6
- State–Trait Anxiety Inventory-6 items
- TECS
- Technology Enabled Care Services
- TIDieR
- Template for Intervention Description and Replication
- WSD
- Whole System Demonstrator
- WTP
- willingness to pay
- ZBI
- Zarit Burden Interview
